Notes
Article history
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as award number 16/138/17. The contractual start date was in November 2020. The draft manuscript began editorial review in October 2023 and was accepted for publication in April 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Sherlaw-Johnson et al. This work was produced by Sherlaw-Johnson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Sherlaw-Johnson et al.
Chapter 1 Introduction and overview
Background and context
Introduction
The 2019 NHS Long Term Plan set out an ambitious programme to fundamentally redesign outpatient services in England. 1 This was in part a response to the steady rise in outpatient attendances in the UK over the previous decade, which outstripped population growth and was not matched with a commensurate increase in workforce or system capacity. 2 The number of outpatient appointments increased by two-thirds between 2008–09 and 2019–20 to 125 million a year. 3 This was the largest increase in activity of any hospital service and, given the context of chronic financial and workforce constraints and system pressures, long wait times, delayed appointments, and rushed consultations, have all become increasingly common, frustrating patients and staff alike. 4
After a drop during the COVID-19 (coronavirus disease discovered in 2019) pandemic, total volumes of outpatient activity have recovered to approximately where they were beforehand,5 but challenges have been exacerbated with the number of patients waiting for consultant-led elective care rising to 7.6 million by June 2023. 6,7
Traditional outpatient service models have relied on face-to-face consultations, which can require repeat hospital visits that prolong uncertainty and potentially waste patient and staff time. 2 As the number of appointments has grown, so too has the number of those that are unattended without prior cancellation [‘did not attend’ (DNA)] which account for nearly 8 million booked appointments each year, which is 6% of all appointments. 8 The high rate of missed appointments has led clinics to overbook, exacerbating problems of poor patient experience.
These inefficiencies have made improving the value of outpatient care a key priority for the NHS. In 2018, the Royal College of Physicians declared that the ‘traditional model of outpatient care is no longer fit for purpose’ and that the NHS must change how it commissions and delivers the service if it is to be sustainable over the long term. 2 It also aims to create a more ‘holistic’ service for patients who do not need to attend hospital, by making better use of primary, secondary and community services, and helping people to manage their own health. 9 As part of its outpatient redesign programme, the NHS Long Term Plan sought to avoid one-third of face-to-face outpatient appointments by 2024 – estimating that this would save the NHS £1.1bn per year (and patients 30 million visits to hospital) by streamlining service delivery through expanded technology at each stage of the pathway. Similar strategies have also been rolled out in Wales and Scotland to expand digital access and shift more care to community settings. 10,11 More recently, NHS England’s (NHSE) 2023–24 Operational Planning Guidance asked health systems to reduce follow-up outpatient attendances by 25% from 2019 to 2020 levels by March 2024. 12
Across the outpatient care pathway, a broad range of innovations have and are being pursued to better manage outpatient care and reduce unnecessary appointments.
Such innovations include referral optimisation (specialist triage of outpatient referrals), Advice and Guidance, Patient-Initiated Follow-Up (PIFU) and video consultation.
Through Advice and Guidance, general practitioners (GPs) can access specialist advice about a patient, thereby allowing a timely response to patients and reducing the need for referral to outpatient services. Expanding primary care access to specialist Advice and Guidance is a central part of NHSE’s delivery plan for tackling elective care backlog. Referral optimisation of outpatient referrals (e.g. rapid triage) involves GPs accessing specialist advice to discuss the most appropriate care with the aim to triage outpatient appointments more effectively, get the right support and reduce ‘unnecessary’ appointments. PIFU aims to give more flexibility and choice to patients over the timing of their care and allow them to book appointments as and when they need them, rather than follow a fixed or standard schedule (e.g. every 6 months).
Understanding which interventions are most effective and the factors that contribute to their success is limited by several factors: (1) innovations being delivered in conjunction with other service changes; (2) differences in delivery across specialties and conditions; (3) the precise components of the interventions often not being reported and (4) variation in the outcomes studied to judge effectiveness.
However, available evidence shows that digital innovations such as teleconsultations and virtual appointments can be effective in reducing missed appointments and lead to shorter consultation times. 13–15 Remote monitoring services have been found to be related to fewer outpatient visits across several specialties. 16–18 Innovations such as rapid triage services (e.g. telephone assessment clinics) have been found to reduce referral waiting times. 19,20 Similarly, direct or rapid access clinics have been shown to reduce waiting times and can alleviate requirement for face-to-face clinic attendances. 21–23 The introduction of non-specialist clinics and expanding of clinical support roles (e.g. advanced care practitioners, nurse practitioners) has also been linked to reduced waiting times24 and increased appointment availability. 25 Other innovations, such as clinic administration and scheduling systems, show variable outcomes, as these tend to be linked to the context in which they have been implemented.
The aims of outpatient transformation efforts have been varied but coalesce around several common themes, including:
-
Making better use of clinical space and staff time.
-
Making better use of wider professional teams.
-
Integrating primary, secondary and tertiary care.
-
Co-ordinating care for people with multiple health conditions.
-
Increasing patient satisfaction, empowerment and convenience.
-
Reducing unnecessary in-person appointments.
-
Increasing savings for the NHS and improving cost effectiveness.
-
Reducing greenhouse gases and other pollution through reduced travel.
-
Decreasing waiting times for patients and their carers.
Study aims
This study aimed to identify innovations in outpatient services implemented in recent years in the English NHS and to carry out a rapid evaluation of one of the most important but little studied recent innovations: PIFU. The identification of innovations included a review of published literature to understand the breadth of system innovations and their potential impacts. This was followed by detailed data analysis of national outpatient activity to identify hospital trusts or clinical specialties where there were notable and recent changes in activity. The evaluation of PIFU was a rapid mixed-methods study investigating its implementation and impact on the use of hospital services and on patients and staff.
The specific aims were:
-
To understand the scope and breadth of interventions being pursued to improve efficiency in outpatient service delivery, and to understand key evidence gaps and future research needs.
-
To identify trusts and/or specialties where there is quantitative evidence of a notable change to outpatient activity, for example, a reduction in the numbers of attendances, or a substitution between different modes of attendances (e.g. from face-to-face consultation to teleconsultation).
-
To undertake interviews of selected trusts and specialties thus identified, to investigate whether changes in their outpatient activity were the result of specific innovations in care management.
-
To conduct a mixed-methods evaluation of PIFU, considering its implementation, impact and the experiences of patients and staff.
Research questions
The evaluation included four workstreams associated with each of the four aims with the following research questions (RQs):
-
RQ1. What interventions to improve efficiency and effectiveness of outpatient care have been studied? Where are the greatest gaps in evidence? (workstream 1)
-
RQ2. Can we identify from routine NHS data any healthcare organisations or specialties that have exhibited significant and sustained changes in outpatient activity that might suggest the influence of service innovations? (workstream 2)
-
RQ3. Can any identified changes in outpatient activity be linked to specific service innovations? (workstream 3)
-
RQ4. What is the current evidence relating to the implementation and impact of PIFU? What is the impact of PIFU on measures of outpatient activity? What are the experiences of patients and staff? (workstream 4)
Further, more detailed RQs were developed for the PIFU evaluation in workstream 4, which are stated separately in Aims and objectives.
Motivation for the study
This study was carried out by members of the National Institute for Health and Care Research (NIHR)-funded Rapid Service Evaluation Team (RSET) and arose following recommendations from the RSET Stakeholder Advisory Board (SAB). The initial aim for workstream 4 (RQ4) was to evaluate one of the promising innovations identified in workstreams 2 and 3. However, as the project progressed, we were approached independently by the Outpatient Transformation and Recovery Programme team at NHSE to undertake an evaluation of PIFU. Moreover, it was already becoming clear from the earlier workstreams that PIFU was the most prominent innovation in outpatient care within the English NHS. For these reasons we devoted workstream 4 to an evaluation of PIFU.
Report structure
This report has four main chapters. Chapter 1 is an overview of the clinical and policy context for outpatient services in the English NHS together with the project aims and a broad overview of the methods employed, including use of patient involvement and the approach to equality and diversity. Chapter 2 combines workstreams 1, 2 and 3, describing the work to identify innovations in outpatient care. Chapter 3 focuses on the methods and findings from the evaluation of PIFU (workstream 4), with an evaluation guide described in Chapter 4 and a discussion in Chapter 5. In Chapter 6 we outline recommendations for decision-makers and further research arising from all workstreams.
Methods overview
Study design
This study employed mixed methods and was divided into four workstreams (see Background and context). Workstream 1 was designed as a literature review to provide context for the rest of the study, workstream 2 was designed in parallel as a quantitative analysis of routine patient data. Findings from workstream 1 informed the final analysis in workstream 2 leading to engagement with healthcare providers in workstream 3. Workstream 4 arose from a combination of findings from workstreams 1 and 3, alongside engagement with external stakeholders. These links between the different workstreams are illustrated in Figure 1.
FIGURE 1.
How the different workstreams interact. The arrows represent the links between the different workstreams.
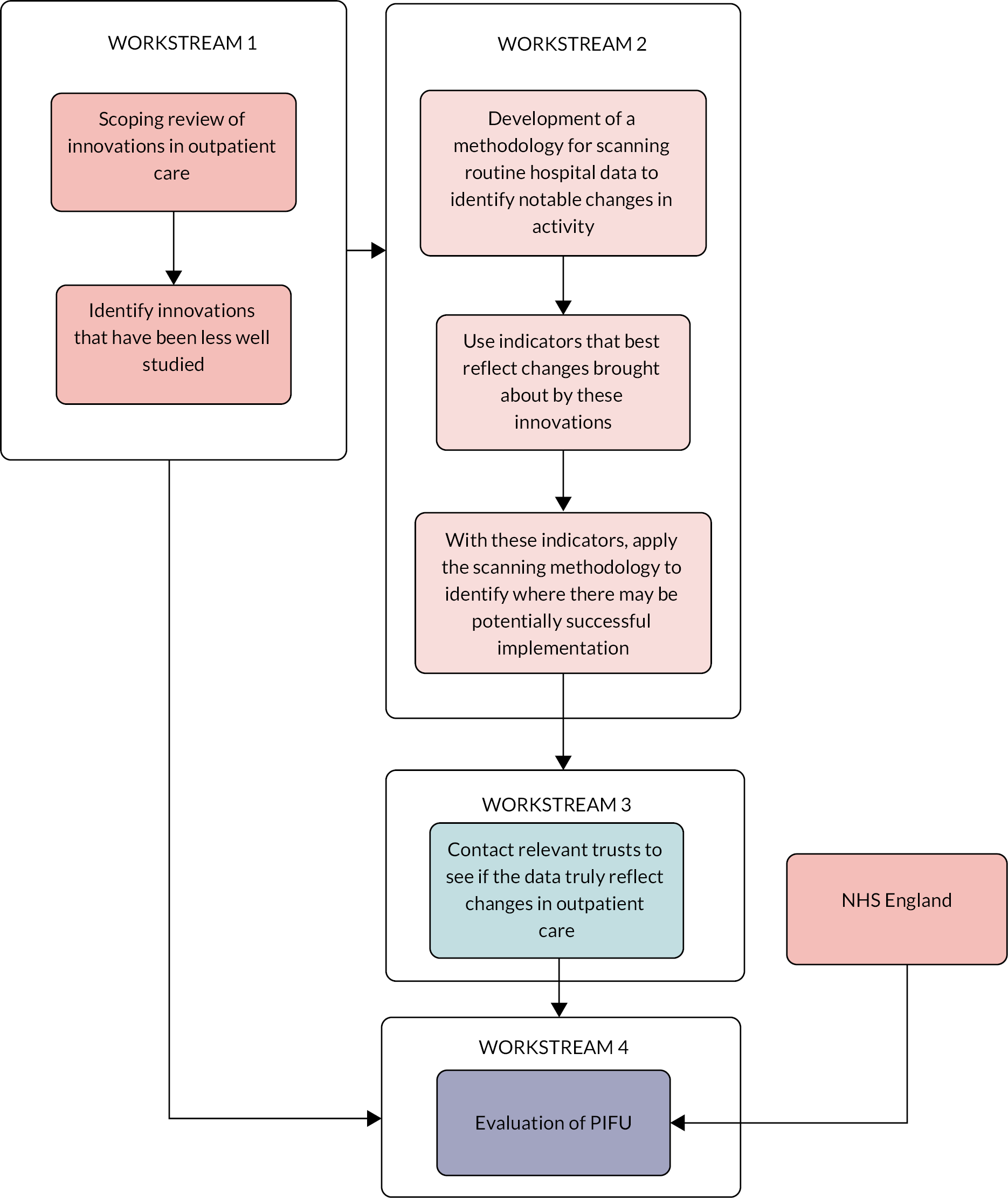
Workstream 4 was designed separately as a mixed-methods evaluation with an evidence review and two phases, with phase 1 providing interim findings that informed how we approached phase 2. For further details on the designs and methods for workstream 1, see Rapid scoping review of outpatient innovations. For workstreams 2 and 3, see The development and use of a methodology to detect, rank, and investigate positive changes in outpatient activity, and for workstream 4 see Methods for the evaluation of Patient-Initiated Follow-Up.
Contact with stakeholders and advisers
The initial idea for evaluating outpatient services was put forward by the NIHR RSET SAB. This group comprised health and social care professionals, patient representatives, data analysts, voluntary service representatives and service managers. The SAB reviewed progress at our annual board meetings and made suggestions for taking forward.
Distinct from the SAB, a formal Project Advisory Group (PAG) was established to oversee progress. This group reviewed the protocol (including amendments) and provided constructive feedback on all the outputs during the course of the project. They met by video conference once at the start of the project and then at intervals as appropriate, including at key points where decisions needed to be taken by the research team. Where meetings were not possible due to other commitments, we engaged with the group by e-mail.
The group included members with various types of experience and responsibilities, including clinical and academic expertise. The group also included one patient representative (see Patient and public involvement and engagement).
The responsibilities of the group included:
-
providing context, advice, and challenges to the research team, based on knowledge of outpatient service policy and delivery
-
helping the team to prioritise in addressing the RQs
-
advising on service issues around outpatient activity and related data collection
-
guiding the research team in seeking further expert advice
-
helping to interpret outputs, for example, activity patterns by specialty or the long list of trusts, with potential innovations from workstream 2
-
supporting the research team in scoping workstream 4.
Workstream 4 was shaped in partnership with the Outpatient Recovery and Transformation Policy and Strategy team at NHSE, with whom we had regular meetings to report ongoing findings. The team also provided advice, particularly about the data and engagement with the service.
The identity of the study sites was not revealed to any of the stakeholders or advisors.
Patient and public involvement and engagement
Patients and the public were actively involved in:
-
project design
-
project management
-
developing participant information resources
-
contributing to writing up and sharing findings.
Patient and public involvement and engagement (PPIE) benefited the project in the following ways:
-
ensuring the study focused on issues of importance to service users
-
ensuring that this focus was reflected in the aims, objectives and RQs
-
ensuring that these were operationalised suitably in the approach to data collection and analysis, and
-
ensuring that the findings were disseminated effectively and in a manner that is meaningful to patients, carers and the public.
Patient and public representatives reviewed the protocol and contributed to the design of this study. For example, two patient representatives from the NIHR RSET (PPIE) panel reviewed the initial protocol in November 2020 following a virtual meeting in which they were given an overview of the project and the opportunity to ask questions.
We recruited a patient representative with lived experience of outpatient care to be a member of the PAG to provide perspectives on their experiences of care and different system innovations to guide the work.
The patient representative [Jenny Negus (JN)] participated in all PAG meetings and commented on study documents, such as the protocol’s Plain language summary. They were offered appropriate training and support, for example, on how to effectively participate in meetings. The team budgeted to support them in all these activities. To support effective participation, the team ensured that documents relating to meetings and events were distributed in a timely fashion (e.g. a week in advance). Also, a member of the team [Pei Li Ng (PLN)] was identified as the primary contact with whom the patient representative could raise any issues or concerns.
Patient and public involvement and engagement feedback contributed significantly to several aspects of the project. This included the development of the patient interview materials (including the participant information sheet, consent form, and topic guide) which were submitted as part of the ethics application. We also conducted a mock patient interview with a member of the PPIE group, which informed the approach. Prior to publication, the PPIE representative reviewed and provided feedback on project outputs (e.g. PIFU evidence review and explainer,26 slide packs for NHSE).
Equality, diversity and inclusion
Many outpatient innovations would have important implications for equality, diversity and inclusion (EDI), for example, if they rely on engaging with technology or patient activation.
For the analysis in workstream 2 (see The development and use of a methodology to detect, rank, and investigate positive changes in outpatient activity), we were analysing changes in outcomes within providers over time, so we decided that corrections for patient characteristics between or within different populations did not need to be applied. Where notable changes in outcomes are driven by changes in population characteristics it is not appropriate to adjust for different patient groups, otherwise they would remain undetected.
The PIFU evidence review (see Rapid Patient-Initiated Follow-Up scoping review) sought to understand outcomes and experiences of PIFU across different patient groups, such as age, gender, ethnicity, and socioeconomic status; however, many studies did not report these findings. We identified further understanding of the impact of PIFU across different population groups as a crucial area for further research.
In phases 1 and 2 of the qualitative work in workstream 4, we aimed to sample sites based on several criteria in order to get a diverse sample. This included the levels of ethnic diversity and deprivation relative to the average population and other demographic factors such as age.
We identified staff interviewees primarily by role and aimed to include a variety of perspectives including clinical, operational, and administrative at different levels of the organisation (specialty and trust-wide). We considered the EDI implications for PIFU in the design of the topic guides and included questions which focused on understanding the opportunities and barriers for PIFU in access for different patient groups.
For the patient interviews, we intended to sample patients from a range of backgrounds to ensure we had a representative sample and to identify whether there were differences in experience depending on patient demographics. Initially we aimed to sample patients by age, gender, ethnicity, and socioeconomic status. We included optional demographic questions at the end of the patient interviews. Due to numerous challenges in setting up the patient interviews, the approach became more pragmatic and we sampled primarily based on specialty. Although we did capture demographic details of the interviewees we spoke to, given the low number of patient interviews (four) we were unable to comment on differences by patient background.
Methods for the evaluation of Patient-Initiated Follow-Up contains further details of how EDI influenced the selection of study sites and patient interviewees.
To evaluate EDI implications of PIFU in the quantitative analysis, we asked for specific data from local sites since the national data were not going to allow us to look at these aspects. Further details are provided in What is the impact of Patient-Initiated Follow-Up on health inequalities and how is this being measured?.
The research team consisted of researchers from two organisations [the Nuffield Trust and University College London (UCL)]. The team was multidisciplinary in nature and team members differed in seniority. The team comprised a mix of backgrounds in relation to gender and ethnicity. When members of the team had to leave, due to a variety of commitments, we ensured, as far as possible, they overlapped with their replacements so they could receive the necessary support during the handover.
Differences from the project protocol
Any changes from the original protocol were discussed with the advisory group.
The final conduct of workstream 1 differed from the project protocol in the following ways:
-
We stated that we would review studies published between 2010 and 2022. However, to manage scope and ensure we were focusing on more current innovations, we changed the start year to 2015. This is explained further in Methods overview.
-
We also stated we would summarise existing evidence of the impact of outpatient interventions, identify factors that might support or hinder the implementation and identify gaps in evidence. The final scope of the review did not include this. The revised aims and RQ are described in more detail in Rapid scoping review of outpatient innovations.
The final conduct of workstream 4 differed in the following ways:
-
We stated we would analyse local patient-level data from hospitals that can identify patients on PIFU pathways to compare their outcomes with patients from hospitals that have not applied PIFU within the same specialties. None of the hospitals we engaged with were able to supply such data, either because they could not identify PIFU patients within their electronic patient administration systems (PAS) or because of the local practicalities of preparing such data. We considered that a more practical way of using local data was to analyse differences in the use of and engagement with PIFU between different individual characteristics where we could use aggregated data that could be easily prepared by the local sites.
-
We stated that we would hold a workshop with three to four ‘late-adopter’ sites that show limited evidence of moving patients on to PIFU pathways. Due to challenges with engagement, the focus of the workshop was broadened to focus more generally on the barriers and facilitators to PIFU implementation. Sites represented at the workshop were therefore at varying stages of PIFU implementation. We considered this an advantage since focusing on late adopters assumes that these issues are specific to such organisations.
-
We stated that we would interview patient advocacy groups, such as Healthwatch and National Voices, but this did not take place as we thought it best, given the time available, to focus on interviewing individual patients who had a relationship with the services we had selected for staff interviews.
Chapter 2 Identifying innovations in outpatient care
Introduction
This section of the report describes the research into identifying innovations in outpatient care and covers project workstreams 1–3. The links between these workstreams are illustrated above in Methods overview (see Figure 1).
Rapid scoping review of outpatient innovations describes the scoping review of the literature to investigate what innovations have been previously studied and for what purpose. The development and use of a methodology to detect, rank, and investigate positive changes in outpatient activity describes the methods we used for scanning routine hospital outpatient data to identify changes in activity that might indicate innovations in service design and the subsequent follow-up with selected acute trusts with most notable changes.
Although the methods described in The development and use of a methodology to detect, rank, and investigate positive changes in outpatient activity are for more general application, we specifically applied them to an indicator that better reflects one of the innovations that we found from the scoping review to be less well studied.
Rapid scoping review of outpatient innovations
What this section adds
This section describes a rapid scoping review of the published literature on innovations in outpatient care that was carried out in the early stages of the project. Adopting an evidence-mapping approach, the review identified which innovations have been studied, their purposes and potential benefits. This has helped us identify where there were gaps in the evidence and inform the choice of innovation to study in workstream 4.
Introduction
In this scoping review we sought to address RQ1:
-
What interventions to improve efficiency and effectiveness of outpatient care have been studied? Where are the greatest gaps in evidence?
Across the outpatient care pathway, a broad range of innovations have and are being pursued to better manage outpatient care and reduce unnecessary appointments, but there is limited understanding of which interventions are most effective and what factors contribute to their success.
The purpose of this review was to provide context for the wider study of outpatient services and, in particular, help us to identify innovations that would be suitable to evaluate in workstream 4. Assessing the effectiveness of innovations in improving outpatient service delivery or examining outcomes were beyond its scope.
Methods
We adopted an evidence-mapping approach,27–29 followed a pre-defined protocol and, where applicable, have reported the methods and findings according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 30
Eligibility criteria
The review included published studies conducted between January 2015 and July 2021 that evaluated the effect of an outpatient service innovation on outpatient or secondary care activity and resources (e.g. waiting times, referral rates, attendances, missed appointments, costs). Due to the rapid timescale, the review was limited to published articles (grey literature and commentaries were not included). See Appendix 1 for full details of the inclusion and exclusion criteria.
Search strategy
We identified studies by searching the online databases MEDLINE, EMBASE, Social Sciences Citation Index (SSCI) and Health Management Information Consortium (HMIC). To manage scope, and to capture innovations most relevant to current health services, we limited the review to papers published between January 2015 and July 2021 (search conducted August 2021) and from comparable health systems to the NHS.
Search terms were developed by conducting scoping work for relevant studies. Terms related to the setting, the innovation, and the outcome of interest (see Appendix 1).
Data collection, extraction and synthesis
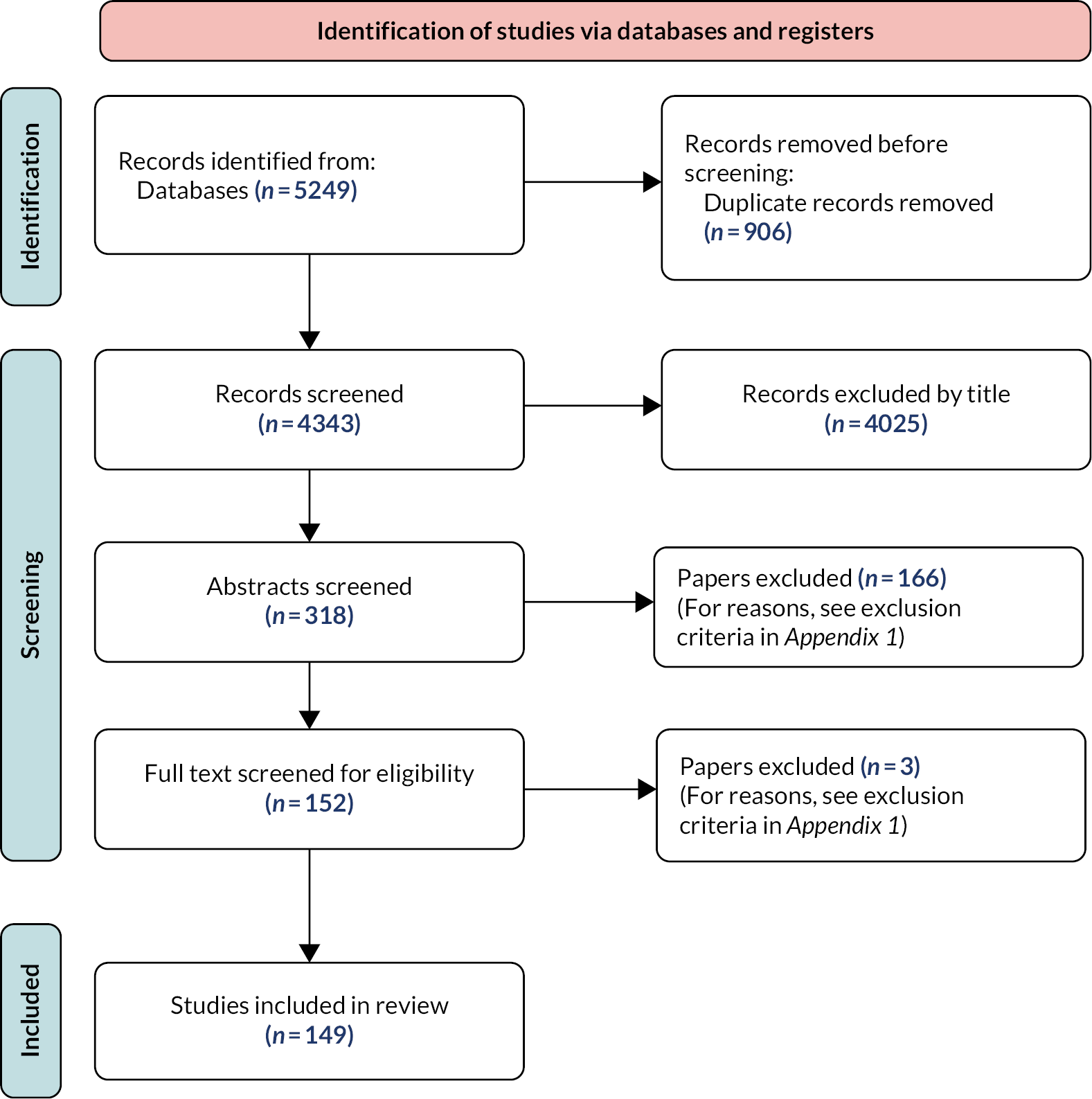
All references retrieved were exported into EndNote referencing software (v20) and duplicates removed. After removing duplicates, papers were screened in two stages by two researchers [Sarah Reed (SR) and Nadia Crellin (NC)], based firstly on the title and abstract and secondly on the full text. Papers were excluded at each stage if they did not meet the eligibility criteria. For those papers that did meet the eligibility criteria, data were extracted independently by two researchers (SR and NC). See Appendix 1 for details.
We conducted a narrative synthesis, organised by intervention and outcome using a framework that helped identify and classify interventions by type (e.g. virtual appointments, administrative/booking systems) and by their anticipated benefits (e.g. how they might affect outpatient activity or resource use), including optimising referral and booking, modernising consultations and personalising follow-up. See Appendix 1 and Table 1 for further details of the framework categories. The framework was developed in consultation with the PAG prior to undertaking the review. The focus of the review was to report the range of interventions and outcomes that have been explored but not to assess how well the different interventions worked.
| Intervention type | Total studies per intervention type | Potential benefits evaluated by each intervention within the studies | ||||||
|---|---|---|---|---|---|---|---|---|
| Optimising referral and booking | Modernised consultations | Fewer and/or more personalised follow-up | ||||||
| Reduce referral volume and improve appropriateness of referrals | Book patients into all available time slots and reduce missed appointments | Using face-to-face only when it adds value | Optimising skill mix and staff mix | Reducing unnecessary administration, optimising clinical time per consultation | Appointments scheduled based on clinical need, streamlining number of appointments | Improving patient support and understanding of when to seek care | ||
| Teleconsultations/virtual appointments (incl. remote monitoring) | 42 | 2 | 1 | 39 | 4 | 4 | 10 | 14 |
| Management tools, administrative systems, and processes to support appointment scheduling (incl. patient reminders and pre-consultation communication) | 44 | 6 | 25 | 2 | 6 | 28 | 10 | 2 |
| New roles, non-specialist-led clinics, including task-shifting | 26 | 4 | 2 | 6 | 25 | 7 | 6 | 9 |
| Drop-in clinics, direct-access clinics, and rapid triage | 24 | 7 | 1 | 2 | 2 | 12 | 20 | 1 |
| Advice and Guidance, shared care models, and models that move care from secondary to primary care settings | 21 | 21 | 0 | 1 | 13 | 1 | 4 | 2 |
| Personalising follow-up and open booking | 7 | 0 | 0 | 2 | 2 | 0 | 7 | 6 |
| Other (e.g. intensive outpatient care clinic) | 9 | 1 | 1 | 0 | 4 | 4 | 3 | 1 |
Quality assessment
Due to the evidence mapping approach, rapid timescale and broad scope of the review, a quality assessment of included studies was not undertaken.
Findings
The initial searches identified 5249 papers, of which 149 were included for review (Figure 2). A summary of the characteristics of these studies is provided in Appendix 1, Table 13. A full list of studies is provided in Report Supplementary Material 1.
FIGURE 2.
Flowchart of study selection.
What innovations have been studied and what are the potential benefits?
We grouped the purposes of innovations into three main areas: (1) optimised referral and booking; (2) modernised consultations and (3) fewer and/or more personalised follow-ups. Within each of these we mapped the potential benefits evaluated by each of the 149 selected studies. This mapping is shown in Table 1 with the innovations specified in the second row and the mapped benefits in the third. For example, innovations with the purpose of modernising consultations could lead to the benefits of only using face-to-face where it adds value or optimising skill and staff mix. The number of studies that addressed each combination of innovation and benefit are also shown in Table 1. Further details on innovation purpose and benefits for each study are provided in Report Supplementary Material 1.
The most studied interventions were management tools or administrative processes to support appointment scheduling (n = 44) and teleconsultations and virtual appointments, including remote monitoring (n = 42). For the former, interventions included pre-consultation questionnaires,31 automated patient reminders (e.g. SMS or telephone),32 predictive models, and electronic booking systems,33 with the key aims of booking patients into all available slots and reducing missed appointments (n = 25) and reducing unnecessary administration to free up more time for appointments (n = 28). Teleconsultations and virtual appointments were most often aimed at modernising consultations, particularly only using face-to-face appointments when it added value (n = 39). 13,34,35
Only seven studies investigated different approaches to personalising follow-up, including more patient-directed or other forms of open access or open booking approaches. This represented the biggest gap in evidence in the existing literature. All seven of these studies evaluated whether personalising follow-up better matched appointment availability with clinical need and all but one evaluated whether it improved patient support and their ability to self-manage and know when to seek professional care.
Other studied innovations included those involving non-specialist-led clinics or expanding of clinical support roles, such as advanced care or nurse practitioners and advanced allied health practitioner roles,36 and patient navigator programmes. 37,38
Other interventions included direct access clinics,39 drop-in clinics and rapid triage services, such as telephone assessment clinics, active triage19 (i.e. referrals for advice only), and electronic triage. 40 Further interventions included shared care models (e.g. between primary and secondary care), for example, integrated, community-based primary-secondary shared care,41,42 and shared care models substituting hospital visits with primary care support. 43
Discussion
Key findings
There are a variety of innovations in outpatient care that have been implemented and evaluated within different health systems. These include changing how appointments are delivered, changing administration and support systems, better engagement with primary care and personalised follow-up. These meet several purposes, ranging from making booking systems more efficient, optimising use of resources to improving the clinical value of follow-up appointments. Virtual appointments and administration and scheduling systems are the most evaluated innovations and only a few studies investigated personalised follow-up.
Strengths and limitations
We are not aware of any previous studies that have reviewed the range and purpose of outpatient innovations implemented internationally. We have tried to be inclusive in the selection of studies, including many from other developed countries. We have also worked with external stakeholders to create a typology of innovations and purposes which was established before undertaking the review.
Because this was a rapid study and to capture innovations most relevant to current health services, we decided not to consider studies published before 2015, and so would have missed further evidence of specific innovations that may have been under-represented within the sample. Due to the scope of the review, we were not able to assess the quality of individual studies and are therefore not able to make any conclusions about the quality of evidence.
While the evidence-mapping approach has provided a good understanding of innovations that have been more or less well studied, it was beyond the final scope of the review to assess the relative effectiveness of the identified innovations to improve outpatient service delivery and summarise the evidence relating to outcomes, such as waiting times for outpatient care, the number of inappropriate appointments and patient health.
Implications and future research
There is currently a focus in the NHS in England and Wales on implementing PIFU, yet the scoping review has shown personalised follow-up and open booking has been little studied. This was one of the motivations for choosing to undertake an evaluation of PIFU within workstream 4 (see Chapters 3–5).
While the focus in the following workstreams is on PIFU, further work is needed to examine the effectiveness of the less well-studied outpatient innovations identified in the review (e.g. rapid triage, Advice and Guidance, models of shared care, direct access) to understand their potential impact. Work should also look to identify factors that might support or hinder the implementation and impact of these innovations, as well as whether and how such innovations might affect health inequalities.
Conclusions
The NHS has been facing significant problems in recent years, including a backlog of elective activity and waiting times for outpatient appointments. To meet these and similar challenges, in other countries health services have been reconsidering the value of outpatient appointments and how they could better meet the needs of patients while making more efficient use of available resources. A range of innovations have been tried out, the most commonly studied being virtual appointments and improved booking systems. Less frequent are studies of innovations that focus on personalised follow-up (e.g. PIFU), as well as rapid triage, Advice and Guidance, models of shared care, and direct access. Given that these innovations are an important component of outpatient transformation in the English NHS, we recommend further study of their impact.
The development and use of a methodology to detect, rank and investigate positive changes in outpatient activity
What this section adds
-
Indicator saturation (IS) methods can be successfully applied to large numbers of multiyear, clinical service-level time series derived from national-scale hospital data sets.
-
These methods simplify complex time series into relatively few best-fit lines and can identify change or break points: points in time where performance on an activity measure appeared to change.
-
The identification and prioritisation of exceptional potentially positive changes was more challenging, in part due to the wide scope of the aim.
-
We nevertheless found a small number of units where potentially positive changes in performance may have been tied to specific service changes.
-
Investigation with trusts raised questions about data quality and highlighted the importance of local data validation.
-
With more tightly focused study aims, IS methods could be more widely used to detect signals of changed performance within large health service data sets.
Introduction
National appointment-level outpatient data have been collected in England since 200344 and is made available for research and other secondary uses as Hospital Episode Statistics (HES) and Secondary Uses Service data (typically the latter for NHS organisations, the former for researchers and other users). After 20 years, while there are concerns about aspects of data quality,45,46 the data set has proven to be a substantial resource for service evaluation and research, although one which could be used more extensively.
One challenge comes from its size. In the most recent financial year 2022–3, it reported on 123.8 million appointments for 23.3 million people, in 170 clinical specialties, across 295 acute care providers (mostly NHS hospital trusts).
Rapid scoping review of outpatient innovations outlined approaches to reduce rates of unnecessary attendances, make better use of virtual consultations, and reduce the number of DNAs among other strategies to improve outpatient services. In 2019, the SAB recommended that we might prioritise evaluating innovations in outpatient service delivery as an important policy area with which secondary services were grappling.
One innovation of NIHR RSET has been the approved access to HES outpatient (HES OP) data collection for NIHR RSET programme work. This offered an opportunity to use data-led techniques to identify potential innovations that might benefit from evaluation.
In theory, where an innovative service change had been implemented, and the innovation had a direct or indirect positive effect on a measure of outpatient activity, it is possible that we might be able to detect the change retrospectively in HES OP data with an appropriate analytical methodology.
In this workstream we aimed to develop such a methodology. The anticipated outcome was a small list of clinical units with exceptional positive recent performance. We would then attempt to contact the relevant units to investigate potential reasons for the detected changes. Where innovative service changes were plausibly suggested as having caused or contributed to the detected changes, we would consider one or two units for more detailed mixed-methods evaluation work.
Hence, we aimed to implement the ‘positive deviance’ approach to improving quality of health care. This methodology, as described by Bradley et al. ,47 aims to identify exceptional performance that already exists within healthcare organisations, and follows this with detailed investigation, testing, and finally dissemination of evidence about best practice.
The aim was that the methodology should be able to prioritise between a potentially very large number of signals of positive change in HES OP data, covering different outpatient activity measures, and multiple clinical specialties within English NHS hospital trusts.
However, methods to achieve the above are not well defined. So, as well as being a potential prelude to evaluation, we hoped that this workstream would help to define new methods that might be applied perhaps not just to outpatient data, but more generally.
We took as a starting point the approach of Walker and colleagues. 48 In their 2019 paper, they outlined a novel approach to automatically detect the timing and magnitude of changes apparent in prescribing data. Noting that previous work to quantitatively assess implementation of new practices ‘has typically relied on measuring change at the level of a whole population, using techniques such as interrupted time series analysis or static measures of variation in care at one point in time’, they presented indicator saturation (IS) techniques as robust, systematic methods to find meaningful changes in data over many institutions, over any general time. These methods – adapted from econometric modelling – were able to detect ‘structural changes’ or ‘breaks’ in time series data ‘without imposing an intervention or change date a priori.’
Ultimately, in part due to the decision to focus the evaluation efforts on PIFU, we did not achieve all the aims. The analysis involved was complex, and we were not fully able to test the consequences of choices made within the context of a rapid evaluation study. However, we learnt several valuable lessons and discuss these at the conclusion of this section.
Aims
We aimed to develop a methodology to produce a ranked list of units (a unit being a clinical specialty at an English NHS hospital trust) where there was evidence of apparent exceptional positive improvement made in performance on an outpatient activity measure. We also aimed to identify whether positive changes identified may have been linked to specific service changes in trusts.
We addressed RQs 2 and 3 (see Background and context):
-
Can we identify from routine data any trusts or trust specialties that have exhibited significant and sustained changes in outpatient activity that might be indicative of the impact of innovations in service design? (RQ2)
-
Can any of these identified changes in outpatient activity be linked to specific innovations in care? (RQ3)
Methods
General approach
Figure 3 provides a summary of the approach. The analysis aimed to act as a filter and prioritisation tool to select approximately ten time series from upward of 20,000.
FIGURE 3.
Stages of analysis.
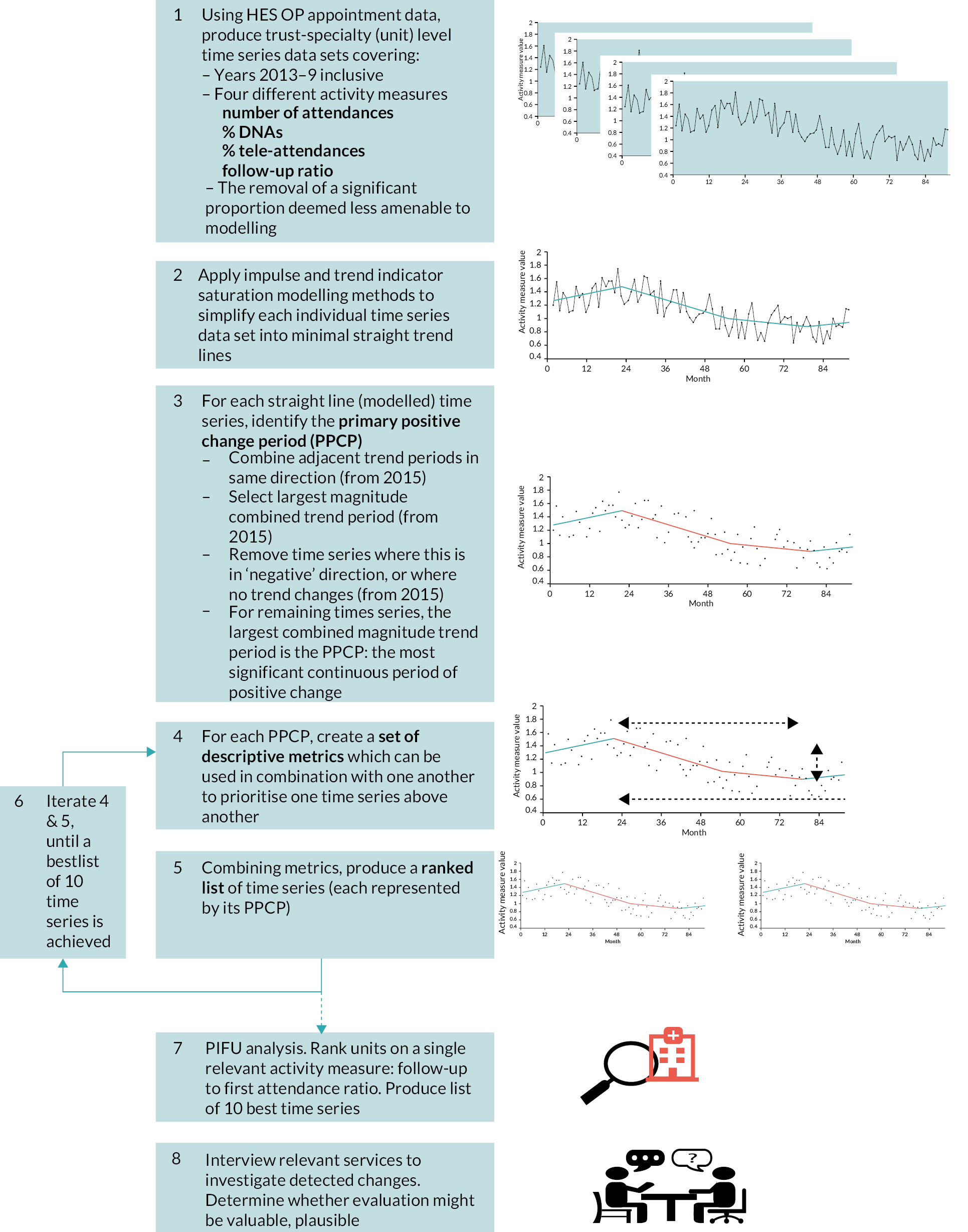
Data
For the main analysis, we used HES OP data from 12 December 2012 to 31 December 2019 (see below for explanation of the time periods used). Within this period, we searched for positive changes during the 5 years from 7 January 2015 to 31 December 2019 (referred to as the ‘search’ period), with the 2 earlier years 12 December 2012 to 6 January 2015 used to provide baseline information (referred to as the ‘baseline’ period).
We purposefully stopped the search period at the end of 2019, before the COVID-19 pandemic affected hospital services. The impacts of COVID-19 were significant and sudden, especially compared to pre-pandemic changes that might have reflected the impacts of innovations. 49 However, before the final selection of units at the latter stages of analysis, we used HES OP data to document follow-up and first attendance data to July 2021 (to test whether improvements had been sustained into the pandemic period).
Construction of time series of outpatient activity measures
From the appointment-level data, we created a large number of time series data sets (over 20,000 in total).
These included data describing four different measures of outpatient activity over time:
-
Attendances: count of all attendances.
-
DNAs: percentage of appointments not attended.
-
Tele-attendances: percentage of attendances that were tele-attendances/virtual consultations.
-
Follow-up to first attendance ratio: the number of follow-up attendances divided by the number of first attendances.
Each of these measures was simple to define, could be constructed with relatively few data quality concerns and was of a status whereby a change in the measure might plausibly have reflected positive impacts of active service change.
For attendances, DNAs, and the follow-up to first attendance ratio, a fall in measure values over time was considered to be broadly in line with the intended outcome of national strategies, while for tele-attendances, rising values were important.
Each activity measure was calculated for every English NHS hospital trust and clinical treatment specialty combination (each trust-specialty combination referred to in this section as a ‘unit’). For trusts which had closed or merged with another since 2012, we included historic activity against the relevant succeeding trust code reported in the NHS Organisation Data Service at May 2021. 50
In creating time series from appointment-level data, we aggregated into periods of 28 days, based on sets of 4 consecutive Wednesday to Tuesday weeks (ending Tuesday, 31 December 2019). This was a pragmatic choice: each time series would be constructed from a reasonable 92 points (there being 92 28-day periods between 12 December 2012 and 31 December 2019), and each time unit would contain the same number of days and the same number of working days (outpatient activity being relatively scarce on weekends). 49 We were not particularly interested in activity measure changes on scales shorter than 28 days. We focused development work on relatively high-volume units where it would be more likely that changes in activity would be found to reach statistical significance and also be of practical relevance. We made a series of exclusions of units where there were relatively few attendances and a further set of exclusions specific to DNA, tele-attendance, and follow-up ratio measures (see Appendix 2, Section 1).
Data extraction was carried out in Statistical Analysis System (SAS) v9.4 (SAS Institute, North Carolina, US). Time series construction and exclusions were carried out in R (tidyverse) (The R Foundation for Statistical Computing, Vienna, Austria). 51
Application of indicator saturation to time series – detecting break points, and outliers
We applied impulse and trend IS modelling methods to all remaining time series (see Appendix 2, Section 2 and Appendix 2, Figures 21 and 22).
The trend-IS modelling had the effect of simplifying each time series into a relatively small number of best-fit trend lines, with break points between adjacent trend lines. The impulse-IS allowed for the handling of outlying data points.
Before applying to all time series, we needed to determine the specific implementation of impulse- and trend-IS appropriate to the four outpatient data measures, making choices about form of the models fitted and various modelling parameters. We started with attendances and expanded to the other measures. The approach – which took as its starting point the method used by Walker et al. 48 – was broadly as follows:
-
A ‘fully saturated’ model was set up (see Appendix 2, Section 2).
-
For attendances we added two dummy variables: one to signal 28-day periods containing Christmas Day, and another to count bank holidays. These were not necessary for the three other measures, which were ratios of activity.
-
The models specified in formulae (1) and (2), as appropriate, were estimated.
-
We chose a level of significance of p = 0.000001 to ensure a low false-positive rate and a ‘block size’ of 15, to ensure relatively few break periods per time series.
| Attendances | Yt= | μ | + βbh count + βchristmas | +∑j=1nγj1{t=j} | +∑j=1nδj1{t−j}(t−j) | +ut | (1) |
| DNAs Tele-attendances follow-up ratio |
yt= | μ | +∑j=1nγj1{t=j} | +∑j=1nδj1{t−j}(t−j) | +ut | (2) | |
| Explanation of terms >> | Rate | Intercept | Bank holiday and Christmas indicators (attendances only) | Impulse indicator | Trend indicator | Error term |
Given the large number of time series we were modelling, we were not looking to specify an approach that described every time series optimally, rather we required that the large majority of those we viewed had a reasonable fit when assessed empirically with relatively few, but practically significant, trend lines fitting the underlying data.
The modelling was carried out in R, with IS modelling applied using GETS package (version 0.21). 52
All subsequent analyses were carried out in R.
Selecting each time series’ primary positive change period
With IS methods applied, each time series was now represented by relatively few best-fit trend lines (typically fewer than four). From this point onward in the methodology, formal, well-defined statistical methods had relatively little part to play, and the approach was determined iteratively and pragmatically.
The first task was to define, for each time series, what we called the primary positive change period (PPCP) during the search period. This would be used to represent the time series in the subsequent ranking.
The PPCP would exist for time series where: (1) there was at least one break point (trend line change) in the search period and (2) where the largest magnitude trend period change (this might be a series of adjacent trend periods in the same direction, treated as one) was in the ‘positive’ direction. Where both conditions were true, the largest magnitude positive change was defined as the PPCP.
Time series without a PPCP were not eligible for subsequent ranking.
Classifying units via their primary positive change periods: creating summary metrics and ranking
Summary ranking metrics
For the remaining time series, the PPCP now primarily represented the unit/activity measure for the purpose of ranking.
We created a series of ‘summary metrics’ to describe each PPCP, and to help us to compare one unit with another, following the approach of Walker et al. 48 (Note: we use ‘metrics’ to refer to values used for ranking, vs. measures which are the underlying outpatient activity measures or data. See Glossary).
For example, two metrics we defined were the magnitude of change and the length of change. The former measured the total extent of the positive change in the activity measure during the PPCP (e.g. the total fall in attendances per 28-day period from the start of the PPCP to the end). The latter measured the time over which the change occurred (e.g. the PPCP might have been 56 days long, or 280 days or some other length).
The process we used to define summary metrics was iterative. We were guided by considering pairs of theoretical time series with small but realistic differences, in each case asking which we would prioritise above the other. Appendix 2, Section 3 and Appendix 2, Figure 23 give an example of the considerations.
It should be emphasised that these metrics were aimed at summarising and comparing, in general, different types of activity measure with one another (e.g. changes in attendance rates vs. changes in follow-up to first attendance ratios). This increased the complexity of the ranking approach development. This also meant that we used transformations for each metric, typically rescaling values on to a 0 (neutral/poor performance) to 1 (good performance) scale.
Combining metrics: ranking
The ranking process started simply – we calculated an overall ranking score for each unit by summing the transformed descriptive metrics, weighted for the perceived importance of each metric. We were not overly concerned about starting weights as we intended for there to be multiple iterations.
Two researchers [Jonathan Spencer (JS) and Theo Georghiou (TG)] reviewed a selection of units at the top, in the middle and at the bottom of the ordered list of all ranked units. We reflected on factors including the mix of measures (did the ranking seem to be penalising or promoting one measure above others?) and understanding units which, for example, had been promoted to the top on the basis of changes that were clearly not likely to be related to service changes (e.g. where large changes were most likely to be data errors). Specific additional metrics were developed in the context of ranking iterations to solve some of the problems identified.
Focus on Patient-Initiated Follow-Up
Selecting time series for a single activity measure: follow-up to first attendance ratio
As the study’s focus shifted to the implementation of PIFU, the task became analytically simpler. We aimed to identify 10 or so units with reductions in numbers of follow-up attendance relative to first attendances. This was considered to be a plausible and desired impact of effective implementation of PIFU. 53
We used modelled time series and descriptive metrics produced up to this point rather than designing and carrying out a new analysis, and the process for final unit selection relied on a significant amount of manual researcher input, rather than a purely numerical ranking. This was driven by the research schedule, but it was enabled by the significant reduction in the number of time series being considered and the removal of the need to balance across different activity measures.
We first imposed new sets of filters to exclude a large proportion of follow-up to first attendance ratio time series. Two researchers (JS and TG) subjectively ranked several hundred remaining time series.
The top-rated 36 were then reduced to nine priority candidates by three researchers [JS, TG and John Appleby (JA)]. To prioritise these final units, we considered the trends prior to the PPCP, what had happened to the other activity measures (including the volume of activity) for the trust/specialty and we additionally considered whether the improvements had been sustained into the pandemic period (to summer 2021).
Service interviews
We contacted the nine selected hospital trusts by e-mail, in each case targeting chief executives and directors or managers of service transformation. The e-mail informed the recipient that an analysis of national data had identified that their trust – in a specific-named specialty – had been found to have made significant reductions in numbers of follow-up attendances relative to first attendances, starting at a specific year. We requested a meeting to present findings and to ask questions about how the service might have changed in the relevant period. Where we received no reply, we followed up with up to two e-mails.
Three trusts welcomed a meeting. To these we sent a brief (three-page) data pack, succinctly outlining trust- and clinical specialty-specific findings on the ratio of follow-up to first attendances over time, and we also included performance against national trends. We posed three questions in preparation for the discussion:
With respect to the reduction in follow-up to first attendance ratios between 2015 and 2020 [and with a special focus on changes from (specific year)]:
-
To what extent has this reduction been recognised by clinical and managerial leaders in the specialty and trust? If yes, in which forums and with whom was it discussed? What actions did it lead to, if any?
-
Does this reflect something you tried to achieve?
-
Are you able to identify specific initiatives or activities that might have contributed to these reductions? What are these?
Semistructured interviews were held virtually in January and February 2022 and were 45 minutes in length, each attended by two researchers. Interviews were carried out with a prepared topic guide outlining pre-set questions about the interviewee, the service and reflections on the trends, including questions around whether any specific initiatives or innovations might have contributed to the trend.
Results
Summary of implementation of approach
The flow chart in Figure 4 summarises the implementation approach of the analysis, through numbers excluded at each stage.
FIGURE 4.
Flow chart summarising numbers of time series and exclusions. The stages are grouped under three categories, depending on which outpatient activity measures were relevant (left-hand side).
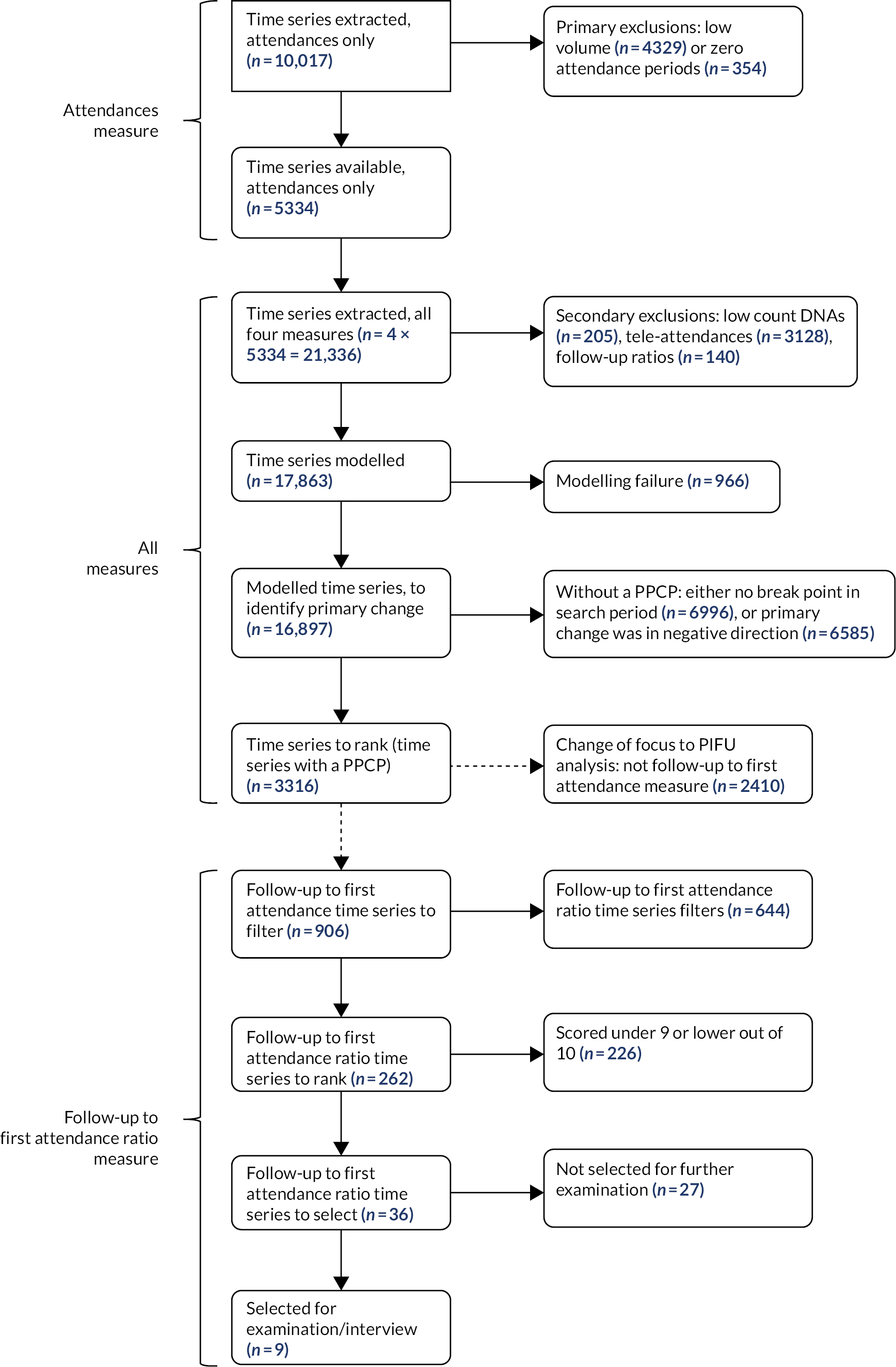
We extracted outpatient activity attendance time series data for 10,017 units covering 190 trusts and 153 treatment specialties (Table 2). We excluded 4329 (43.2%) units with low activity volumes (fewer than 100 mean attendances per 28-day period in the most recent calendar year 2019). A further 354 units (3.5%) were excluded as they had one or more 28-day periods (between 2017 and 2019) with zero attendances.
| Summary of stage of time series and exclusions | Units excluded | % | Units remaining | % |
|---|---|---|---|---|
| Total units initially extracted | – | – | 10,017 | (100%) |
| Primary exclusions (based on attendances) | ||||
| Low recent volumes (< 100 per 28-day period) | 4329 | (43.2%) | 5688 | (56.8%) |
| Zero attendance 28-day period | 354 | (3.5%) | 5334 | (53.2%) |
| Total after primary exclusions | 4683 | (46.8%) | 5334 | (53.2%) |
| Secondary exclusions (measure-specific) | ||||
| Attendances: (no further exclusions) | 0 | (0%) | 5334 | (100%) |
| DNAs: < 5 per 28-day period | 205 | (3.8%) | 5129 | (96.2%) |
| Tele-attendance: < 5 per 28-day period | 3128 | (58.6%) | 2206 | (41.4%) |
| Follow-up to first attendance ratio: <5 per 28-day period | 140 | (2.6%) | 5194 | (97.4%) |
| After primary and secondary exclusions: sum of four measures for modelling | – | – | 17,863 | – |
There were 5334 units (53.2%) remaining for potential modelling. For each of these units we constructed three additional time series, one for each of the other outpatient activity measures (DNA, tele-attendance, follow-up to first attendance ratio).
Secondary exclusions were made, such that 17,863 time series were finally taken forward for modelling. The tele-attendance time series were the most likely to be removed at this point: only 2206 time series were taken forward for modelling (vs. over 5000 each for each of the other three time series).
Application of indicator saturation to time series – detecting break points and outliers
Indicator saturation modelling was successfully applied to 16,897 of 17,863 time series. This was the case for 100% of attendance, 95.7% of DNA, 97.4% tele-attendance and 86.8% of the follow-up ratio time series.
Table 3 summarises characteristics of the resulting IS-modelled time series. DNA rates generally fell over the 7-year period. The opposite was the case for tele-attendances, and follow-up to first attendance ratios were stable.
| Activity measure | |||||
|---|---|---|---|---|---|
| Attendances | DNA | Tele-attendance | Follow-up to first attendance ratio | ||
| Number time series, modelling attempted | 5334 | 5129 | 2206 | 5194 | |
| Number time series, successfully modelled (% of attempted) | 5334 (100%) | 4908 (95.7%) | 2148 (97.4%) | 4507 (86.8%) | |
| Mean value start baseline period (12 December 2012) (interquartile range) | 746 (133–919) | 8.6% (5.3–11.1%) | 2.5% (0–9.0%) | 3.7 (1.3–3.8) | |
| Mean value start search period (7 January 2015) (interquartile range) | 1145 (246–1370) | 7.9% (4.9–10.0%) | 3.6% (0.0–3.3%) | 3.5 (1.3–3.7) | |
| Mean value end search period (31 December 2019) (interquartile range) | 1077 (244–1300) | 7.2% (4.4–9.0%) | 10.1% (1.8–14.7%) | 3.6 (1.2–3.7) | |
| Mean number of changes (break points) during baseline and search period (12 December 2012 to 31 December 2019) | 2.14 | 1.83 | 1.68 | 1.72 | |
| Mean number of changes (break points) during search period (7 January 2015 to 31 December 2019) | 1.46 | 1.27 | 1.46 | 1.18 | |
| Number of series (%) with N changes (break points) during search period (7 January 2015 to 31 December 2019) | N = 0 | 1955 (36.7%) | 2160 (44.0%) | 696 (32.4%) | 2185 (48.5%) |
| N = 1 | 1010 (18.9%) | 683 (13.9%) | 433 (20.2%) | 638 (14.2%) | |
| N = 2 | 1440 (27.0%) | 1330 (27.1%) | 676 (31.5%) | 1072 (23.8%) | |
| N = 3 | 452 (8.5%) | 426 (8.7%) | 174 (8.1%) | 288 (6.4%) | |
| N = 4 | 200 (3.7%) | 137 (2.8%) | 98 (4.6%) | 157 (3.5%) | |
| N = 5 + | 277 (5.2%) | 172 (3.5%) | 71 (3.3%) | 167 (3.7%) | |
| Number with any outliers during search period (2015–9) (%) | 168 (3.1%) | 177 (3.6%) | 329 (15.3%) | 345 (7.7%) | |
| Mean number of outliers during search period (2015–9) for time series with any outliers | 4.0 | 2.9 | 3.5 | 2.8 | |
The IS modelling resulted in 1.7–2.1 break points on average (depending on activity measure) during the 7 years of the entire period, and 1.2-1.5 break points during the 5 years of the search period.
During the 5-year search period many time series (up to almost half, depending on activity measure) had no modelled breaks (and so were modelled as a single trend line). The next most likely outcome was two breaks (three trend lines). A minority of time series (3.3―5.2%) had five or more break points (six or more trend lines).
Modelled outlying points were common for tele-attendance time series (15.3% had at least one), but less so for the other activity measures (e.g. only 3.1% of attendance time series had at least one).
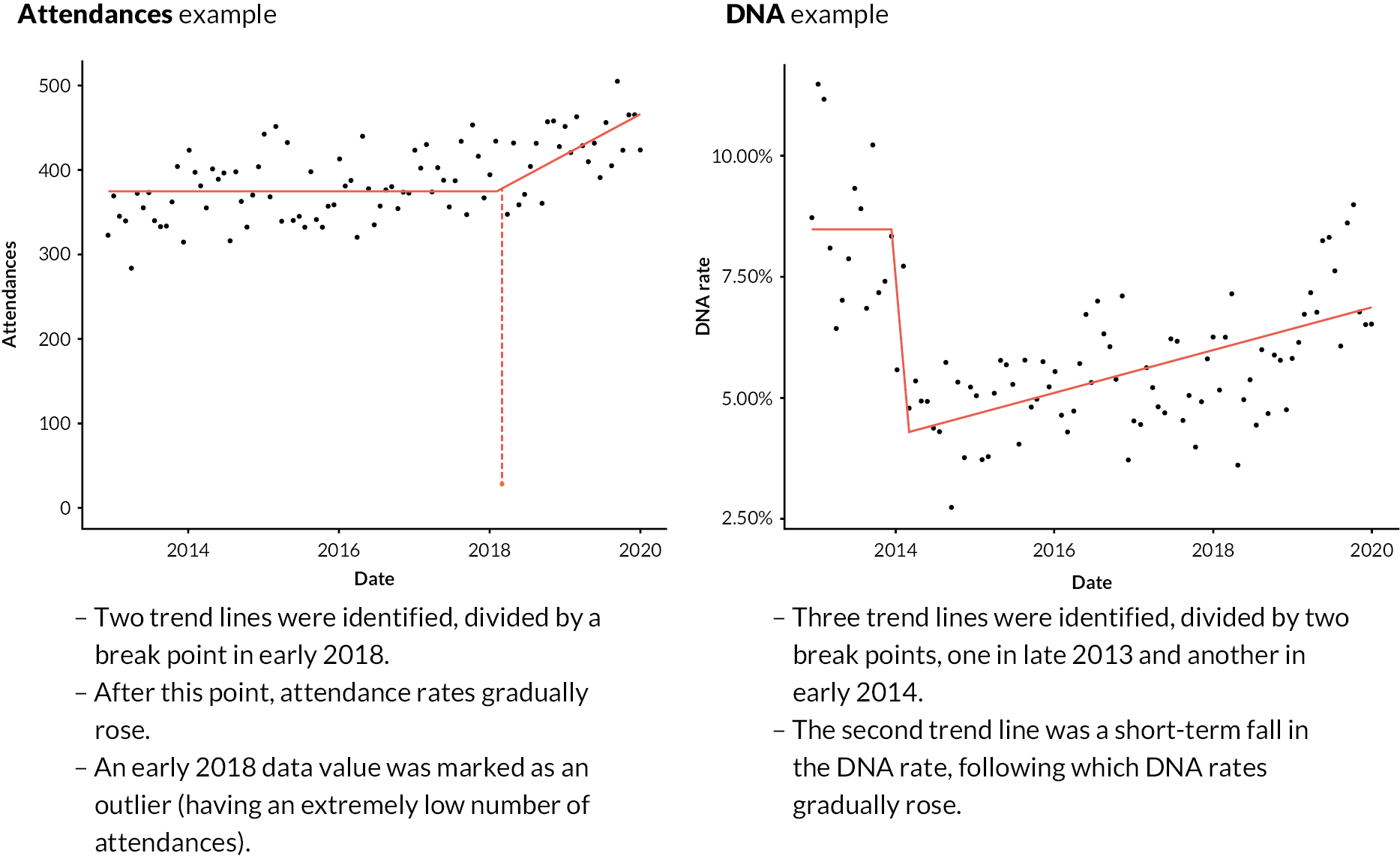
Figure 5 shows trend lines for two example time series (one for attendances, the other DNAs), overlaid on the data points, including a brief description of the key features of each. Appendix 2, Figure 24 includes further examples (two for each activity measure).
FIGURE 5.
Two examples of IS-modelled time series, with key features noted. Points are 28-day period values during the baseline and search periods (late 2012 to end-2019). Red lines are IS-modelled trend lines. Outlying data points are shown in orange, attached to a hashed vertical line (attendances example).
Selecting each time series’ primary positive change period
Across the four activity measures, 16,897 time series were successfully IS-modelled. During the search period, 6996 (41.4%) had no change and were excluded from ranking. This left 9901 (58.6%) time series with at least one change in the search period. For these, where adjacent trend periods were in the same direction, we effectively merged trend lines and treated them as a single trend line.
We calculated the largest magnitude trend change for each time series as the ‘primary change period’. This was done over the merged sets of trend lines and only from the start of the search period 7 January 2015 if the trend started in the baseline period. Of these time series, 3316 (19.6% of the original 16,897 time series) had primary changes in the ‘positive’ direction; these were those that were eligible for subsequent ranking, with the primary change period redefined as the PPCP. Table 4 gives numbers of these time series by measure.
| Attendances | DNA | Tele-attendance | Follow-up to first attendance ratio | |
|---|---|---|---|---|
| Number with a PPCP (% of all with a PPCP) | 660 (19.9%) | 772 (23.3%) | 978 (29.5%) | 906 (27.3%) |
The remaining 5188 had primary changes in the negative direction; these were not eligible for ranking but were used to calculate some ranking metrics.
Appendix 2, Section 5 and Appendix 2, Figure 25 outline with example time series the choices we made about selecting PPCPs.
Classifying units via their primary positive change periods – creating summary metrics and ranking
Summary ranking metrics
There were 3316 time series to rank, based on each series’ PPCP. Figure 6 summarises the ranking metrics we developed. The figure includes for each metric a description of transformations made and the aims for each.
FIGURE 6.
Summary of ranking metrics developed. Examples are given with respect to a measure where a fall in value is ‘positive’. PPCPs are shown as bold lines.
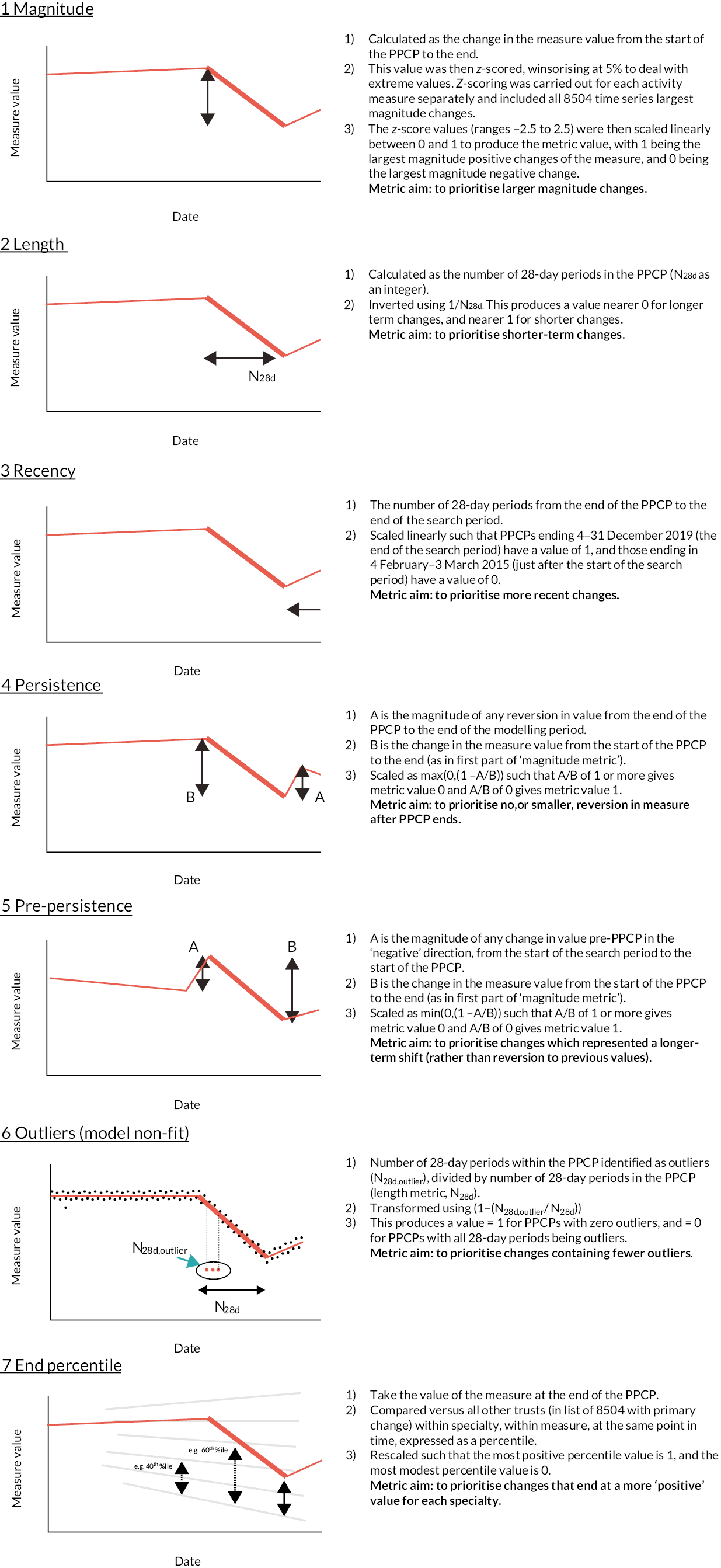
Many of the metrics we created included linear transformations (i.e. linearly scaling minima to maxima – or vice versa – on to a 0-to-1 scale), but for the length metric we used an inverse function and this risked overpromoting very short length changes. The implications of this are visible in the following section but is mentioned here to make the point that the metrics outlined in this section were a work in progress, and may have been changed in subsequent iterations.
Combining metrics: ranking
We ranked time series by combining sets of metrics. Appendix 2, Section 6 outlines a series of iterations of ranking attempts we made. At the time the study’s focus moved to the evaluation of PIFU, we were testing a relatively simple ranking approach, combining four metrics (magnitude, length, persistence, and end percentile) as a simple sum, with equal weights. Development of the general approach to ranking stopped at this point. Figure 7, however, shows a representation of the ranking outcome at this point. It shows 400 (of 3316) units as ranked by the third approach: 200 from the top of the list, 100 from the middle, and 100 from the very bottom.
FIGURE 7.
Representation of a subset (400 of 3316) unit time series from top, middle and bottom of ranked list (based on third-attempt method). Key included for activity measure and ranking and for metric score colour scales (deeper colours being better). The first four metrics listed were those used in the final approach ranking.
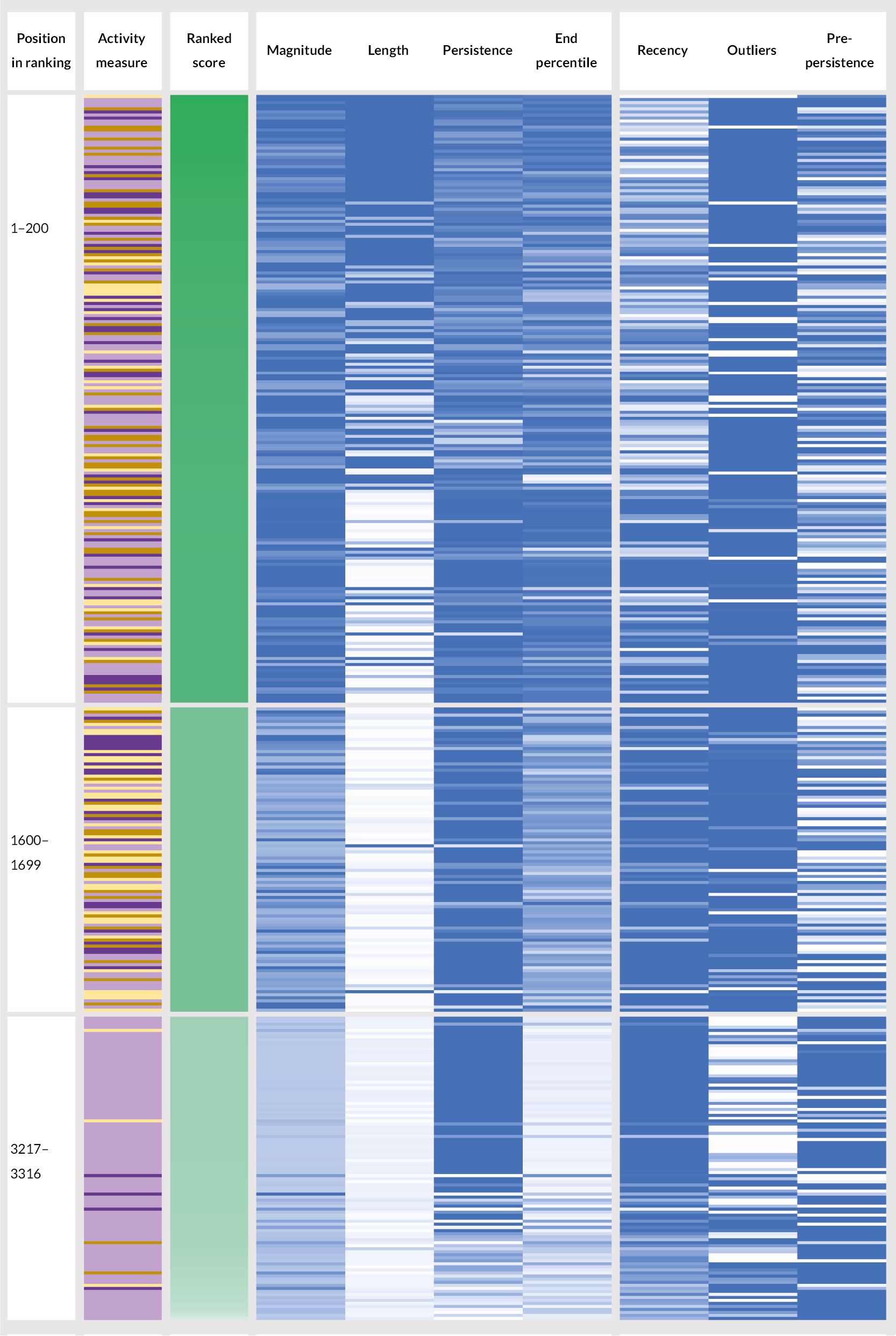
One observation is that there was not an equal distribution of the four activity measures across the ranking. For example, while making up 27% of all time series being ranked, only 14% of the top 200 were follow-up to first attendance ratio time series, and 42% in the top 200 were tele-attendance time series (which made up 29.5% overall). At the bottom of the ranking, 91 out of 100 time series were for tele-attendance measures. We would not necessarily expect a completely even distribution of activity measures throughout the ranking, but we might have investigated these discrepancies before the next iteration had there been one.
Briefly, other observations include:
-
The impact of having a non-linear length metric transformation is clear. Length contributed relatively little to the ranking for the majority of time series, except for the very shortest lengths (these were one or two 28-day periods), which were typically to be found promoted to the top of the ranking.
-
The correlations between recency and persistence are visible, with similar patterns of light/dark occurring in each.
Focus on Patient-Initiated Follow-Up
Selecting time series for a single activity measure: follow-up to first attendance ratio
The starting point was 906 time series describing the follow-up to first attendance ratio activity measure and their metrics, developed for the general ranking approach. The focus on a single activity measure greatly simplified the process of finding a small number of specially performing units.
We decided to implement a specific set of filters to reduce the number of time series further. These are outlined in the flow chart Figure 8. The reason for these filters was to prioritise more sustained improvements, better modelling performance and better relative performance versus other trusts in the clinical specialty. Applying these filters left us with 262 follow-up to first attendance ratio time series.
FIGURE 8.
Numbers of follow-up to first attendance ratio time series available and excluded in filtering and final time series selection.
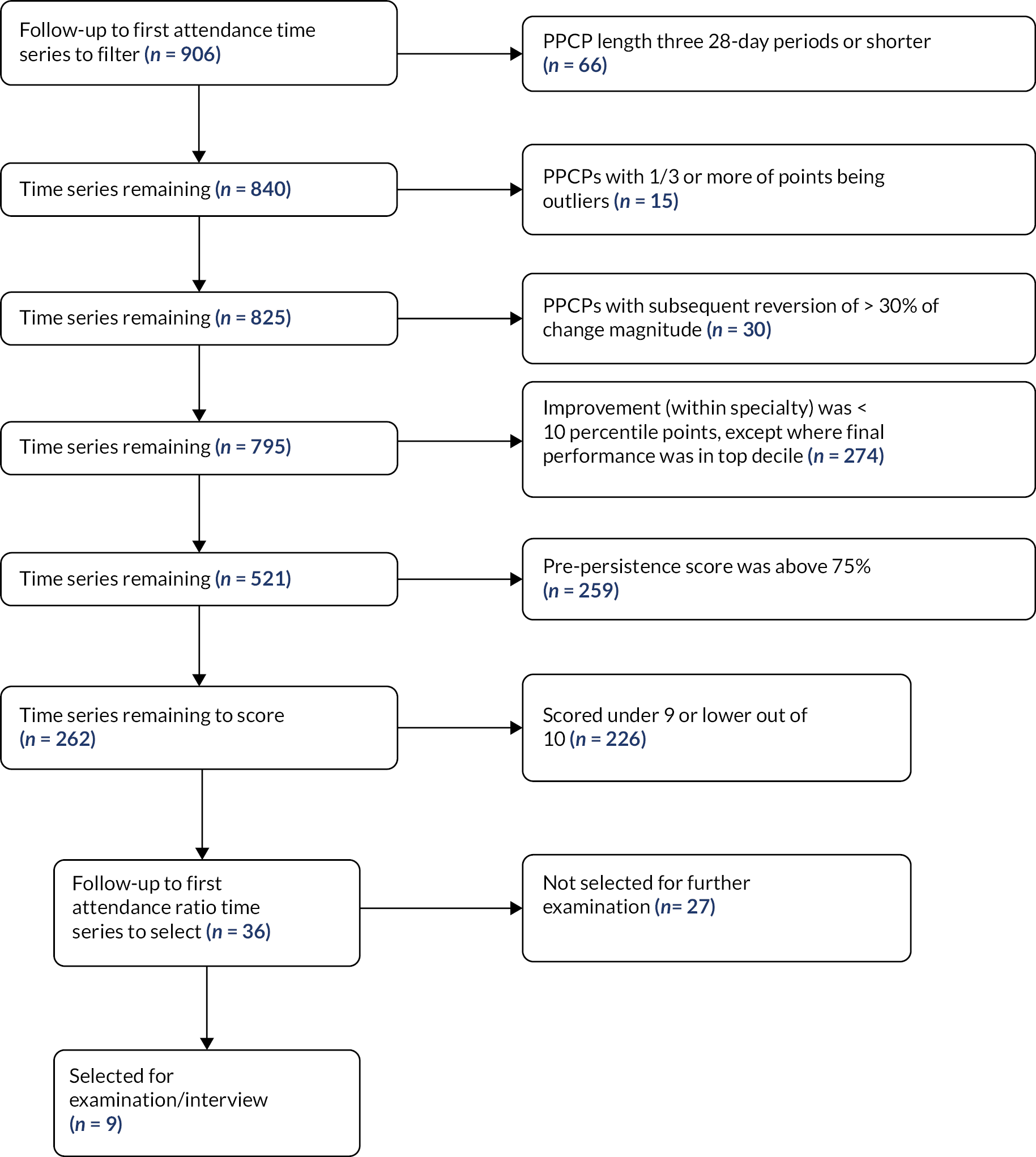
With the number reduced so considerably, we manually ranked the subjective quality of the identified change on a scale of 1–10, and were left with 36 time series that scored 10.
Three researchers (JS, TG and JA) further considered the top 36, and manually selected 9 of these that we considered to be most promising as potentially being the result of a service change or innovation. We considered, among other things:
-
The pattern of activity before the PPCP (whether the time series had been flat, rising or jumped upwards).
-
The volume of activity in the service, compared to others.
-
The context in terms of the other activity measures for the unit.
-
How sustained the changes had been into 2020 and 2021.
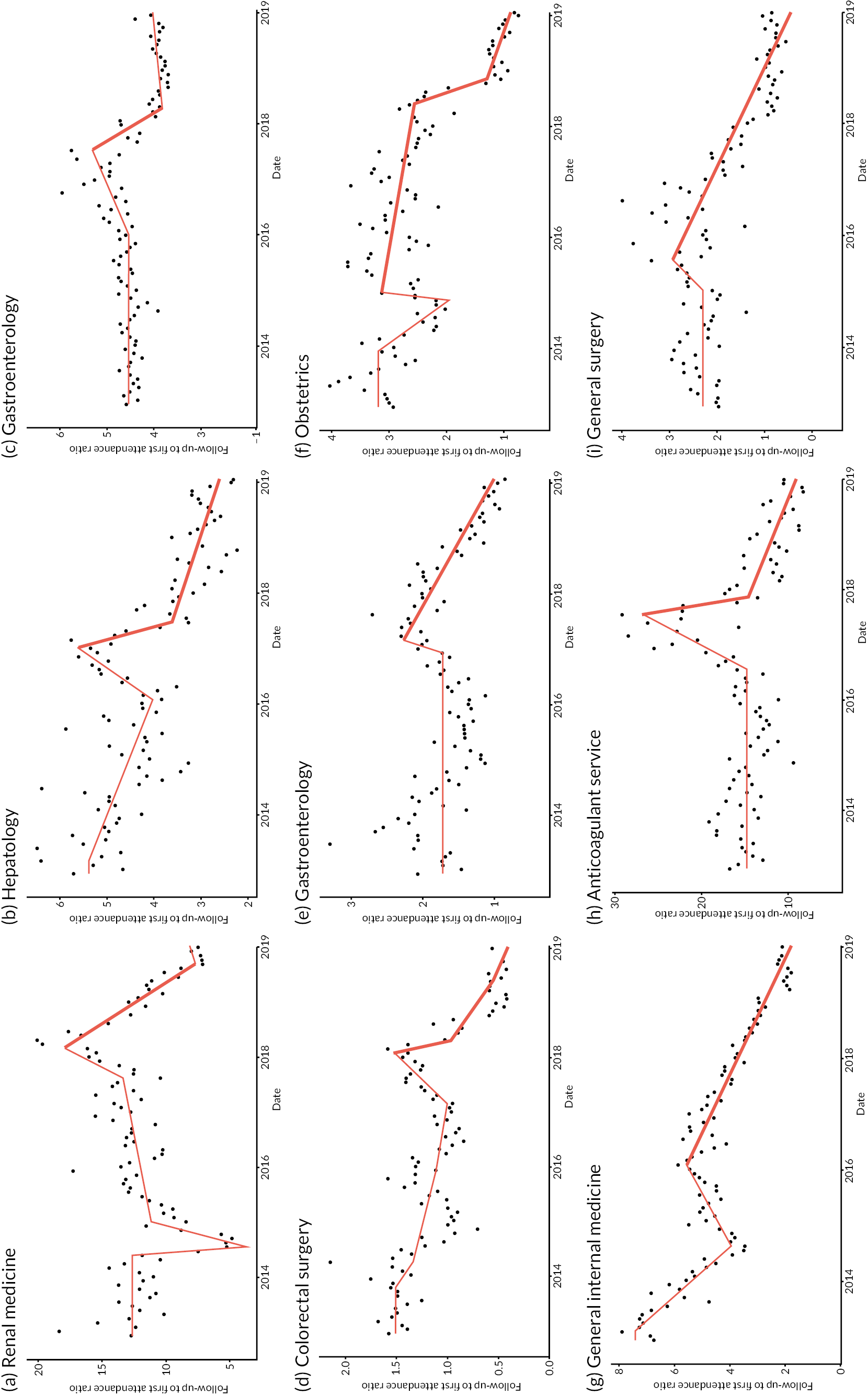
Figure 9 shows the time series of the nine services that we identified. All services were from different NHS trusts.
FIGURE 9.
The selected nine services with significant falls in follow-up to first attendance ratios [each from a different NHS trust, labelled (a)–(i)]. Red lines are IS-modelled trend lines. The PPCP is shown in bold.
Service interviews
We received five responses from nine hospital trusts contacted. One trust was unable to participate due to pandemic-related service pressures. A second – an anticoagulation service – responded that the finding had been recognised and understood locally and was likely due to a change to anticoagulant drugs which no longer needed regular blood test monitoring. This change had taken place in 2017, broadly matching the start of the changes identified but was out of scope as an innovation for further study. Note that we had been made aware of this change by one of the advisory group members, and we had made specific attempts to reduce the relative ranking of anticoagulant services in the earlier general ranking methodology (see Appendix 2, Section 6).
Three trusts welcomed a call and suggested one or more relevant members of staff who would be able to talk with us.
The outcome of the interviews was mixed. Table 5 summarises key reflections from the three unit interviews.
| Unit 1 | Unit 2 | Unit 3 | |
|---|---|---|---|
| Unit clinical specialty | Gastroenterology | Obstetrics | General internal medicine |
| Organisation type, location | Teaching hospital trust, Midlands | Multi-hospital trust, South East | Teaching hospital trust, London |
| Interviewee role | Consultant gastroenterologist (in position > 8 years) |
|
Head of transformation (in position < 3 years) |
| Change in follow-up to first attendance ratio found and discussed | Reduction from 2.3 to 1, from 2017 to 2020 (Figure 9e) | Reduction from 3.1 to 1, from 2015 to 2020 (with accelerated fall in 2018) (Figure 9f) | Reduction from 5.5 to 2, from 2016 to 2020 (Figure 9g) |
| Trend recognised at trust? | Yes, and reflects a change in practice | Not picked up at the trust | No. Did not reflect trust’s own data; no such large falls known in any medical service |
| Suggested main cause of change | Clinical assessment service, introduced in 2017. Aimed to refer patients directly to tests without initial consultation. A modest trial had shown a large fall in consultations before discharge to general practice | No clear explanation identified. Suggestion of possible recording artefact, especially given declining birth rate (and yet first attendances more than doubling according to HES OP) | N/A |
| Additional notes | Other initiatives also noted:
|
In only one of the three units, a gastroenterology service, did the interviewee recognise the finding we presented of falling numbers of follow-up attendances compared to first attendances over time. This was cautiously attributed to a clinical assessment service that started in 2017 (and which a local trial had shown to reduce numbers of consultations before discharge).
Interviewees of the other two units were more surprised by the findings; indeed one (from a general internal medicine service) consulted internal trust data during the call and could find no such large fall in ratios in any medical specialty during the relevant period. This remains unexplained. The other unit interviewees (from an obstetric service) suggested that there were data recording problems, given that locally declining birth rates were at odds with rising numbers of first attendances as recorded in HES OP data.
No unit discussed PIFU as an intervention, and the investigation into these sites ended.
Discussion
The aim of this section’s work was twofold. Firstly, to develop a quantitative methodology to apply to national hospital outpatient data, the output of which would be a small, ranked list of clinical units where there was evidence of outstanding improvements in an aspect of outpatient activity data, potentially indicating the impact of a service change. Secondly, to investigate a small number of the detected improvements, with a view to identifying one or two potentially innovative service changes that we might evaluate.
The wider study changed course as the analytical work was in progress, and as a result we did not achieve all the aims. The work changed to support an evaluation of the implementation of PIFU, and this halted work on a generalised quantitative methodology for identifying positive changes in outpatient data. The final process had more manual – and subjective – input than was envisaged, and arguably might have been better supported with more well-established quantitative and statistical methods, given the increased simplicity of the analytical aim.
Of a small number of clinical units, we identified as having unusually large falls in numbers of follow-up attendances versus first attendances (an impact we expected to be connected to successful implementation of PIFU), we were able to interview three. Two did not recognise the data findings and had no explanation for the apparent improvements we found. The third recognised the changes and attributed them to a non-PIFU-related service change. A fourth (not interviewed) identified the fall as having been connected to a change in medication use. While these results are a potential measure of success for half of the units from which we received further information, numbers are too small to generalise, and we cannot exclude the possibility that there was no direct connection between data findings and the suggested causes.
Nevertheless, this work did not therefore feed into the PIFU evaluation.
We reflect below on the analytical work in the context of other studies, and its strengths and limitations within the setting of a rapid evaluation study.
How this work relates to previous research
Walker and colleagues successfully implemented methods to detect meaningful changes in prescribing practice in English general practices. 48 The scale of their work was not dissimilar in one respect to the present study; they analysed upward of 8000 practices over 5 + years, compared to 10,000 hospital ‘units’ over 7 years. However, their analysis was more focused. They aimed to study changes in practice with respect to two common medication treatments, at the time of a patent expiry, and new guidance and financial incentives. The same group has subsequently adapted similar methods for analysis of changes in statin prescribing connected with changes in national guidance54 and to analyse impacts of new policies to reduce opioid over-prescribing. 55 In the latter study, there was an element of ranking based on practices’ and commissioning groups’ changes in prescribing rates, however this was on a single measure of activity.
The present analysis lacked the tight focus of these studies. The aim was more ambitious: to cover multiple different aspects of activity data for an entire health service type – outpatient services – and to define methods to not only find meaningful changes, but importantly – given the potentially vast amount of data – to prioritise between these changes. It is perhaps not surprising that we have been less successful at achieving the full aims.
With the exception of the above-noted studies and a COVID-19 modelling study,56 limited use appears to have been made of these methods within research relevant to health services.
The present work had clear parallels with ‘positive deviance’ methodologies. Bradley et al. 47 defined these as having two goals: the identification of units that have had exceptional performance followed by promotion of good practice derived from these units. A 2015 systematic review of positive deviance approaches within healthcare organisations57 analysed studies against Bradley et al. ’s four-stage process for adopting positive deviance approaches in healthcare organisations. The four stages were: identification of positive deviants using routinely collected data; qualitative study of the positive deviants to generate hypotheses about the strategies used to succeed; quantitative testing of these hypotheses in larger samples of units; and dissemination of evidence of positive strategies. They found that research quality was generally low, that there was wide variation in how positive deviants were identified (some appearing to lack validity/replicability) and that the final two stages were often absent.
The quantitative analysis addressed Bradley and colleagues’ first stage and interviews began to address the second stage. Detailed mixed-methods investigation (further second stage) and dissemination (fourth stage) would have followed as part of the intended evaluation workstream had we identified plausible candidates for investigation. The third stage may have been covered as part of the mixed-methods evaluation, dependent on data availability but was not an explicit aim (however we note that Bradley et al. were the only authors to have ‘truly test[ed]’ positive deviant hypothesises within a larger sample of units). 57
With respect to positive deviance studies that used quantitative approaches to identify positive deviants within healthcare organisations, these typically rely on analysing improvements (or single period performance) on single (or small numbers of) measures of performance over fixed time scales. 47,58–60 The analysis appears to be an outlier in terms of its complexity, particularly around the ambition to prioritise between multiple different types of change and over undefined time periods (within a maximum 5-year window).
Strengths and limitations
Strengths
The NIHR RSET has a remit to innovate in its approach to carrying out rapid evaluations. One well-established innovation of the team was its data sharing agreement with NHSE (NHS Digital) which outlined broad permissions to use national hospital data in NIHR RSET evaluations. With guidance from the SAB to prioritise evaluation of service innovations in outpatients – a vast area of clinical activity – we identified an opportunity to innovate and test the use of data-driven methods to identify candidates for evaluation (alongside the scoping review of appropriate literature). In doing so, this work involved adaptation of new methods to a very large and complex data set, taking further Walker and colleagues’ application of methods previously more widely used in econometrics than to the setting of national hospital data.
We made rapid progress by being pragmatic at numerous stages of the work, with the aim not being perfection but rather outcomes that were considered empirically reasonable given the large amount of data. To keep up the pace of progression we adapted to changes in study focus (i.e. to evaluation of PIFU) by making use of what we had already produced but reassessing how the existing outputs and data were to be used.
Limitations and lessons
The ambition we had for the quantitative analysis was ultimately not met. The task we set ourselves may have been too complex within the scope of a rapid evaluation setting. Factors that impeded progress included the activity measures we used and how we used them together, pandemic impacts, questions around data quality, and the cumulative impact of numerous analytical choices we made. We have expanded on these points in Appendix 2, Section 7. We were limited in the number of clinical units we were able to interview to test quantitative findings, and so we did not find innovations to evaluate via this methodology. More importantly, we were not able to confidently validate the outputs of the methodology. Nevertheless, the IS methods that we adapted and applied have the potential to be more widely used to find potential evidence of exceptional improvements (and, indeed, the opposite).
These are more likely to be successful where aims are drawn to be more tightly focused. For example, studies might focus on single types of service change that are local or national priorities or analyse specific pathways [e.g. Getting It Right First Time (GIRFT) pathways61]. Or they might focus on specific priority patient groups, where these are able to be derived from available, linked data sets (e.g. Core20PLUS). 62
In each case, before any analysis takes place it will be beneficial to define more explicitly what good looks like and then design the modelling and ranking to be as simple as possible. For example, while we searched for changes over a 5-year period, we then prioritised later changes above earlier ones. It may have been better to have simply limited the search period to a final year. While we may have missed some interesting earlier changes, this would have been counterbalanced by a significant reduction in complexity.
Conclusion
This section outlines an ambitious and complex attempt to use data-driven methods to target evaluation efforts. While we successfully implemented several stages of the analysis, we did not achieve the overall aims: to define a method to detect and prioritise for investigation among different types of positive changes in outpatient services. Interviews with a small number of services highlighted the need for local scrutiny of data findings to verify signals found in national data sets. The methods we adapted show promise for more extensive use in quantitative health services research but with more tightly focused aims.
Chapter 3 Evaluation of Patient-Initiated Follow-Up
Introduction
Background
This section of the report covers workstream 4 of the overall outpatient study and is a mixed-methods evaluation of Patient-Initiated Follow-Up (PIFU) in the English NHS.
In Background and context we described some of the issues and challenges facing outpatient care within the English NHS. These include the large increase in volumes over recent years, long waiting times exacerbated by the COVID-19 pandemic and the potential overuse of patient and staff time. Part of the problem is that under standard pathways, patients with long-term conditions or following surgery are automatically called back for outpatient appointments at regular intervals (e.g. every 6 months). These timings are not necessarily decided by clinical need or when a patient wants extra support. This means that when follow-ups do occur, they can fail to lead to further investigation or any meaningful change in patient management. 63 Conversely, when a patient’s symptoms or circumstances do change, they may experience a long wait for an appointment as capacity has been devoted to routine follow-up.
NHS leaders have called for an ‘industrial’ drive to cut the number of unnecessary outpatient appointments and better prioritise clinical time where it adds the most value – setting an ambition to reduce outpatient follow-ups by 25% against 2019–20 activity levels by March 2024. 12 To deliver this, NHSE has been encouraging hospitals to move some of their outpatients to PIFU pathways. 64
PIFU aims to give more flexibility and choice to patients over the timing of their care and allow them to book appointments as and when they need them, rather than follow a fixed or standard schedule (e.g. every 6 months). Figure 10 shows how the process might change under a typical PIFU pathway when follow-up appointments are initiated by the patient or carer compared to a traditional fixed or standard schedule pathway. In some specialties, similar services have been operating for some years, referred to by terms, such as ‘open access’. One of the key goals of PIFU is to better match clinical resources with patient need – an ever-growing challenge in the NHS as the number of patients waiting for specialist care continues to grow. NHSE has produced guidance to support trusts to implement PIFU, including advice on which specialties and cohorts PIFU is suitable for. 53 This also outlines three quality standards that must be met (reproduced from public sector information 40 licensed under the Open Government Licence v3.0. www.nationalarchives.gov.uk/doc/open-government-licence/version/3/):
FIGURE 10.
Fixed follow-up outpatient schedule (standard pathway) vs. personalised follow-up schedule (PIFU pathway). A typical outpatient pathway.
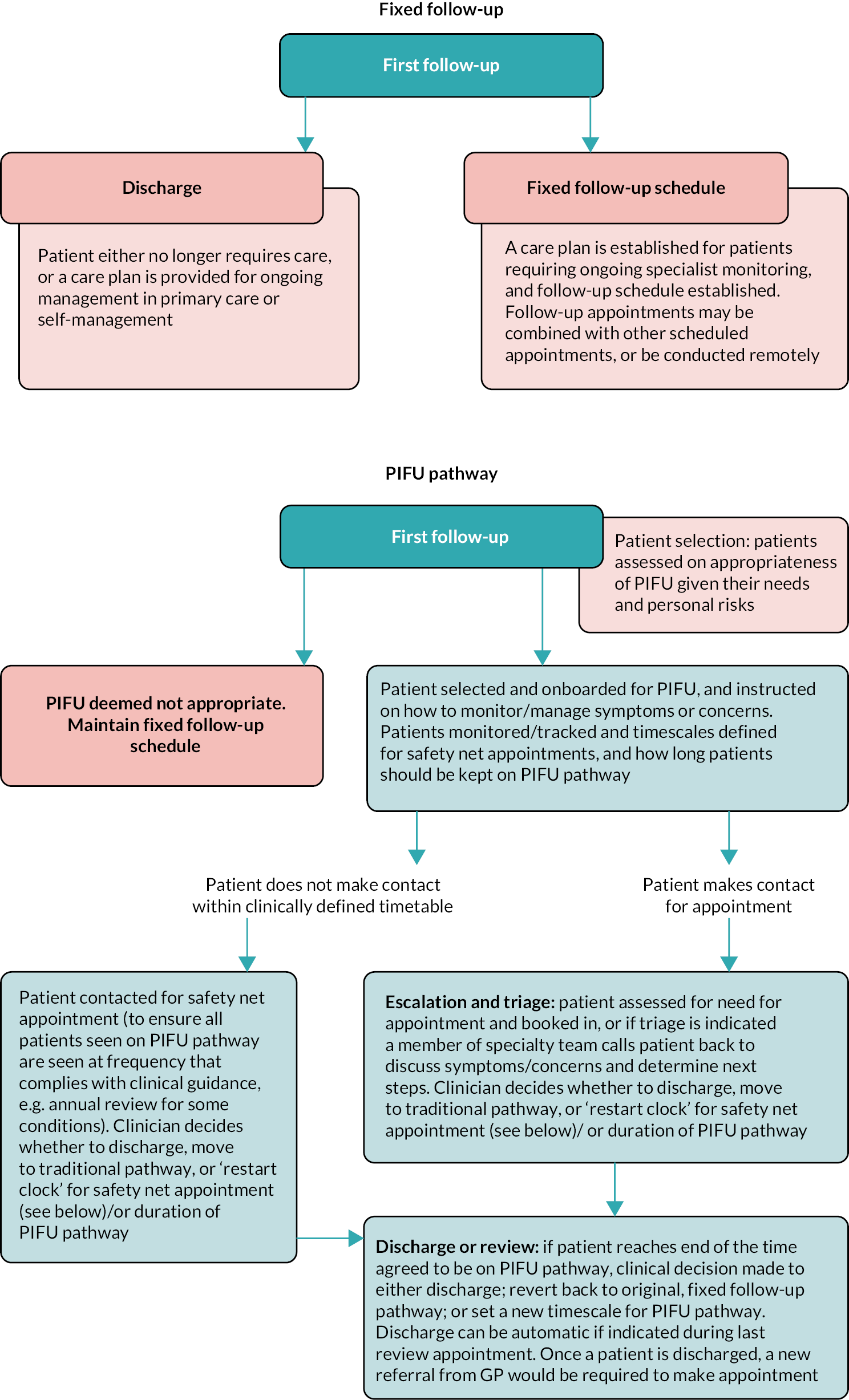
-
All patients and/or carers should have PIFU explained to them and the opportunity to ask questions and raise concerns. If they do not understand how or when to trigger an appointment, PIFU should not be used (see information on shared decision-making).
-
A standard operating procedure (SOP) that includes patient safety nets should be in place.
-
All patients moved to a PIFU pathway should be logged and tracked on the organisation’s information technology (IT) system, and the service able to report on key metrics including the number of patients who are on a PIFU pathway.
The requirement to meet these standards aims to distinguish PIFU from other less-formalised approaches to personalised follow-up (such as open access). Additional guidance has been produced to support PIFU implementation in particular specialties, such as adult Trauma and Orthopaedic pathways. 65 These consider specific factors relevant to implementation in these specialties, such as which patients are clinically suitable and the duration of a PIFU pathway for different conditions or injuries.
Although the guidance provides support, it recognises that implementing PIFU is a decision for individual services who should ‘develop their own guidance, criteria and protocols on when to use PIFU and be clear that it should not be used as a substitute for the appropriate timely discharge of patients’. Several factors are stated as influencing how long it will take to implement PIFU including ‘the size of the service, current service demand, maturity of any existing PIFU, staff engagement, IT system set up and the time and capacity the team leading this work has’. Alongside this guidance, NHSE has produced a more detailed PIFU implementation plan and pre-implementation checklist.
However, as the NHS seeks to expand PIFU further, there are several questions about how well it works, and for whom. These include:
-
The evidence that PIFU reduces unnecessary appointments and frees up clinical capacity for patients who need it the most.
-
How patients experience PIFU, and what risks might it involve.
-
How PIFU might affect health inequalities and different patient groups.
-
What differences there are in how patients are selected for PIFU.
While there are potential benefits of PIFU to patients, clinicians and the NHS, there are relatively few studies exploring them. 26 Where evidence does exist, results are mixed both in terms of impact on patient experience and satisfaction, as well as the ability to reduce volumes of appointments. 26 Modelling carried out in December 2020 by the Outpatient Recovery and Transformation Policy and Strategy team at NHSE (based on two trusts for a number of specialties) suggested that PIFU could reduce follow-up patient attendances by between 0.8 and 1.8 follow-ups per patient depending on the specialty. 53 Further evidence of the impact of PIFU on, for example, outpatient activity, waiting lists, the potential diversion of activity to other services, patient and staff experience, and variations in access and use, would help in refining its design and implementation.
Aims and objectives
The RQs for this evaluation were:
-
3.1 How is PIFU being implemented? What components and processes does it involve? What is the involvement of technology in the pathway?
-
3.2 What are the relevant outcomes for evaluating the impact of PIFU services? To what extent can we measure the different impacts of these services? How are the data being used to monitor the progress against these outcomes and how can the data be used?
-
3.3 What are the unintended consequences (e.g. on other services) of PIFU and how are potential risks being managed? What strategies have been adopted to address potential inequalities along the PIFU pathway? What data are being collected to understand potential disparities? Is there variation in how different patient populations access and engage with PIFU?
-
3.4 What are patient experiences of engaging with PIFU services? What is the level of patient engagement?
-
3.5 What are staff experiences of delivering PIFU? What is the impact on staff satisfaction, workload, and capacity across different roles?
After describing the methods (see Methods for the evaluation of Patient-Initiated Follow-Up), we present findings from a rapid evidence review of evaluations of personalising outpatient follow-up (see Rapid Patient-Initiated Follow-Up scoping review). This is then followed by sections describing the findings relating to implementation (see How is Patient-Initiated Follow-Up being implemented?), impact on outpatient and other hospital activity (see Measuring the impact of Patient-Initiated Follow-Up), health inequalities (see What is the impact of Patient-Initiated Follow-Up on health inequalities and how is this being measured?), experiences and views of staff (see Staff experiences), and of patients (see What is the impact of Patient-Initiated Follow-Up on patient engagement and experience?). In Chapter 4 we present an evaluation guide to assist with ongoing and future evaluation of PIFU services and, finally, key findings and implications are described in the Discussion (see Chapter 5). Recommendations for policy-makers and further research are summarised in Chapter 6.
Methods for the evaluation of Patient-Initiated Follow-Up
Design
The evaluation of PIFU was designed as a convergent mixed-methods study in two phases preceded by a rapid evidence review. The two-phase approach allowed the team to understand many of the important issues about data, implementation and experiences that would inform more in-depth investigation at a later stage. It also allowed the team to provide early feedback to NHSE to inform evolving policy.
Phase 1 was a 4-month rapid evaluation using national data sets alongside qualitative interviews with local and national stakeholders. Phase 1 took place between June and September 2022. The qualitative aspects focused primarily on how PIFU was being set up within specialties where it was most widespread or established. This included high-volume specialties like Physiotherapy or Orthopaedic surgery where pathways tend to be short term or time-limited in nature. Quantitatively, we analysed national data to assess the impact of implementing PIFU on a range of outcomes relating to patients and use of hospital services.
Phase 2 took place between January and July 2023 and built on the insights from phase 1. The qualitative part of this phase consisted of in-depth interviews with both patients and staff and a workshop to discuss the challenges and enablers of PIFU uptake. Compared to phase 1, the purpose was to investigate issues in greater depth and across a wider range of specialties and conditions. This included a focus on how PIFU was being implemented within sites and applied to patients with longer-term conditions that tended to involve more complexity and variation in practice. It also included gathering patient experience data and quantitative analysis of both local and national data.
The design was agreed with the advisory group including the PPIE representative.
Evidence review
For the first stage of the evaluation, we conducted a rapid scoping review which investigated the available evidence to understand what was already known about the impact of personalised follow-up initiatives for outpatient care on service use, patient experience and patient outcomes. This also enabled the team to gain a better understanding of key implementation issues (see Rapid Patient-Initiated Follow-Up scoping review).
Analysis of the implementation and impact of Patient-Initiated Follow-Up at national level
Outcome measures
There are several potential outcomes of PIFU affecting, for example, patients, staff workload and use of health service resources,26 not all of which are readily quantifiable. We selected a range of outcomes that were identified in the evidence review by national stakeholders and the advisory group. These included numbers of outpatient attendances and follow-ups, time to follow-up, numbers of appointments where the patient failed to attend (DNAs) and attendance at the emergency department (ED). The likely impact of PIFU on these measures was not clear and it could be hypothesised that PIFU could reduce outpatient attendance where follow-ups are not needed and increase attendance where some routine follow-ups are still in place. If patients are choosing whether to have an appointment, then the impact may be a reduction in DNAs. If patients are being seen when they most need to then PIFU may reduce ED attendance. On the other hand, if certain clinical signs are missed because a patient has not fixed an appointment, then there may be a knock-on increase in ED attendance.
Data collection
We obtained patient-level data on outpatient attendances and DNAs from the HES OP data set and data for ED attendance from the Emergency Care Dataset (ECDS). Reported use of PIFU was obtained from the Provider Elective Recovery Outpatient Collections (P-EROC) which is national data on PIFU activity submitted by most trusts and reported by month and clinical specialty. P-EROC submissions are collated by NHSE and access was granted through a data-sharing agreement. We obtained P-EROC data from the month it was first submitted (September 2021) to March 2023. We had access to HES and ECDS data up to March 2023 under the terms of a separate ongoing data-sharing agreement with NHSE. More details on the data sources are provided in Measuring the impact of Patient-Initiated Follow-Up.
Data analysis
Because HES or ECDS do not report whether someone has been put onto a PIFU pathway, we analysed the relationship of each outcome to the amount of PIFU being reported by each clinical specialty within each NHS trust. Since the P-EROC data are a new collection, we also assessed its quality by looking for internal inconsistencies and discrepancies with HES.
We carried out descriptive analyses of the data to view patterns of implementation of PIFU over time and within different trusts and specialties. Alongside providing useful context, this also helped to inform decisions as to which sites to select for phase 2.
Statistical models were developed to test the association between the amount of PIFU implemented by each trust specialty with each outcome. Further details of this analysis are described in Measuring the impact of Patient-Initiated Follow-Up.
Analyses of outcomes and implementation were carried out during both phases of the project. The initial analysis in phase 1 enabled us to report early findings and helped us to understand the potential of the national data we had available.
Analysis of the implementation and impact of Patient-Initiated Follow-Up at a local level
To understand how PIFU is being implemented at a local level as well as gain views and experiences of staff and patients, we selected five study sites. Three sites were included in phase 1 [one integrated care system and two acute NHS trusts] and two sites in phase 2 (two acute NHS trusts). A COREQ checklist for the staff and patient interviews has been completed and is available as Report Supplementary Material 2.
Study design and ethics
Phase 1 of the study and the phase 2 staff interviews component were categorised as service evaluation by the Health Research Authority (HRA) decision tool and UCL/University College London Hospital (UCLH) Joint Research Office.
The phase 2 patient interviews element and the plans for sharing local quantitative data were reviewed and given favourable opinion by the London – Chelsea Research Ethics Committee (REC reference: 23/LO/0143, IRAS: 319517) on 12 April 2023.
Site sampling
The local sites were recruited, across both phases, using a sampling framework that sought to achieve balance across a range of variables. This is shown in Appendix 3, Table 14.
The aim was to use a two-stage process to identify specialties of interest. We began by creating a list of specialties which had greater scope for PIFU in terms of a medium to high volume of outpatient activity and which related to longer-term conditions. We used the national P-EROC data to identify sites that had a range of PIFU uptake across these selected specialties (i.e. high and low volumes of PIFU uptake in the conditions of interest). We then sampled sites across a range of factors including geography (rural or urban), size of hospital, patient demographics, length of time delivering PIFU and volumes of PIFU. We did not recruit sites according to the models of PIFU they were using because within each trust multiple approaches to PIFU coexist and vary by clinical condition.
When deciding on which specialties to focus on in phase 2, we began by identifying a shortlist of long-term clinical specialties which had greater variation in the levels of uptake between trusts according to the national data. This included Gynaecology, Paediatrics, Dermatology, Gastroenterology and Neurology.
However, in practice, the approach became more pragmatic. This was, firstly, to account for discrepancies between the P-EROC data and what we heard from sites as to which specialties had high uptake, and secondly to account for staff availability. Furthermore, we found that the focus on ‘specialty’ at a high level masked the variation of how PIFU was being implemented at a more granular level in conditions within the specialty. This meant, for example, that national data showing high uptake for some specialties could be driven by high amounts of PIFU activity in one particular condition or clinical pathway. As a result, the specialties we investigated were chosen through conversations with staff responsible for implementing PIFU within the case study sites which would illustrate variation in how PIFU is implemented in different services.
In phase 1, the specialties we looked at within sites included Physiotherapy, Trauma and Orthopaedic Surgery and Community Paediatrics. In phase 2, we looked at Gynaecology, Gynae-oncology, Breast Care, Paediatrics, Respiratory and Neurology.
Documentary analysis
In both phases, we conducted documentary analysis at a national and site level of key information related to national policy and how PIFU was being implemented in different specialties. This included site and specialty-specific SOPs and guidance produced by NHSE.
Staff interviews
Recruitment
Recruitment at each study site began with a purposive approach but evolved into predominantly convenience sampling due to challenges around staff availability. We asked site-wide staff (such as outpatient managers and clinical leads) to recommend clinical and operational staff within the specialties of interest and, due to the team’s understanding of the limitations of national level data, also welcomed others they identified as suitable for fulfilling the research aims, based on their personal experiences of PIFU implementation within the site. We also used snowball sampling to identify relevant individuals within specialties. All individuals were informed about the purpose of the study and invited to participate via e-mail.
Qualitative data collection and analysis
Across the 2 phases, we conducted 36 semistructured interviews with staff from 5 case study sites (see Appendix 4, Table 15 for a detailed breakdown). In phase 1, 18 staff were sent a formal invitation to interview, and 13 attended. In phase 2, 32 staff were sent a formal invitation to interview, and 23 attended. Some staff who declined to participate or did not participate after providing consent cited capacity issues, while others did not provide a reason. The majority lasted between 30 and 60 minutes. Across the two phases, staff interviewed in local sites represented a range of roles and perspectives. This included operational, administrative, and clinical roles at a site and specialty level. Since sampling was based solely on staff role and specialty, demographic data was not collected for staff participants. In phase 1, we undertook two national level interviews to understand perceptions of national PIFU strategy and in phase 2, we also interviewed three primary care physicians to understand their perceptions of the implications, opportunities, and risks of PIFU for general practice.
All interviewees were provided with a participation information sheet (see Report Supplementary Material 3) which outlined the purpose of the study and interview. Either written or verbal consent was obtained before conducting the interview. Interviewees were asked about a range of topics including (but not limited to):
-
Their perceptions of the aims and objectives of PIFU.
-
The typical patient pathway within the trust or specialty.
-
Enablers and barriers to PIFU implementation.
-
Impact on staff, patient and service.
-
Risks and opportunities for PIFU.
Topic guides were created in consultation with the broader project team and PAG. A sample topic guide can be found in Report Supplementary Material 4. All interviews took place over Microsoft Teams and were audio- and video-recorded, with audio transcription using the platform’s own software. No relationship between any team members and any participants had been established prior to the study. Interviews were mostly conducted by two members of the research team [Cyril Lobont (CL), Rachel Hutchings (RH), NC, Camille Oung (CO) and SR], who discussed key reflections and themes after each interview. Due to the rapid nature of the study, some interviews were conducted by one member of the research team who then discussed findings with the wider team afterwards. Nobody except the interviewer(s) and participant(s) were present in all instances. All participants were only interviewed once.
Given the short time frame of this rapid evaluation, the study team adopted a pragmatic approach to the comprehensive analysis of the data, with a shorter timescale than traditional qualitative analysis. Data collection and analysis of interview data were carried out in parallel and facilitated using rapid assessment procedure (RAP) sheets in MS Word and MS Excel. 66 RAP sheets were developed for each study site to facilitate cross-case comparisons (i.e. to make comparisons between subgroups both within and between sites, such as across staff roles or between specialties), see Report Supplementary Material 5. The categories used in the RAP sheets were structured in accordance with the interview topic guide (drawing on the scoping review and earlier interviews), maintaining flexibility to add categories as the data collection proceeded. We also drew on the Non-adoption and Abandonment of technologies by individuals and the challenges to Scale-up, Spread and Sustainability of such technologies in health and care systems (NASSS) framework to support with the analysis. 67 The research team used both inductive and deductive thematic analysis, and the RAP sheets were adapted to take account of emerging themes and identify new ones.
These categories were the same in both phases and included the PIFU model (including components, context and use of technology), implementation factors such as barriers and facilitators, impact of the service, staff experiences of delivering the service, patient engagement with the service, opportunities, and risk. The interviewers completed site-specific RAP sheets following each interview, noting key points from the data under the agreed categories and consolidating notes where multiple interviewers were present. The researchers held weekly meetings to discuss emerging learning and themes, and relayed this learning to other members of the study team during weekly team meetings throughout the duration of the study. Data saturation was an ongoing consideration for the team, and it became apparent that it would be highly unlikely to reach it within the scope of this evaluation, due to the variation in PIFU implementation at both site and specialty level, and the small number of interviews and case study sites.
Patient interviews
Following ethical approval, local site confirmation and capacity approval was obtained for each site in May and June 2023 respectively. The intention had been to interview up to 15 patients from each phase 2 case study site (total = 30).
Sampling and recruitment
We worked with case study sites to determine the most appropriate approach for recruiting patients. In both phase 2 sites, eligible patients were contacted by telephone and asked whether they would consent for their contact details to be passed to the research team. In one site this was done by clinical staff and the other by administrative staff.
Patients were eligible if they were over 18 and had capacity to consent. We focused on people who had been placed on a PIFU pathway since January 2023 to capture recent experiences. We originally intended to speak to patients who had consented to be on a PIFU pathway and who had been offered but declined, however neither site recorded this information in their data. We began with a purposive approach, and intended to sample patients from across the specialties we had focused on in the phase 2 staff interviews (i.e. Gynaecology, Gynae-oncology, Breast Care, Ophthalmology, Neurology, and Respiratory), as well patients from a range of demographic backgrounds. However, given the low response rate the approach became more pragmatic.
Data collection and analysis
Contact details were shared with the research team through a secure data transfer portal. In total, 14 patients’ details were received by the study team from Respiratory, Breast Care, and Gynaecology. The first details were received on Friday, 14 July. All patients were contacted a minimum of three times at different times of the day up until Monday, 7 August. From this, four patients consented to take part in an interview, one was unable to take part in the time frame and three declined to take part. No response was obtained from the other contacts.
Patients who agreed to participate were provided with a copy of a participant information sheet, which informed them of the purpose of the study (see Report Supplementary Material 6). A consent form and written consent was obtained. We received input from the PPIE members of RSET on the design of the patient-facing materials.
A total of four interviews were conducted between July and August 2023, lasting approximately 30–60 minutes. Only the interviewer and participant were present in each. All interviews were carried out by one team member (RH). Three patients were on the Breast Care pathway and one on Gynaecology. Patients were asked about how they were introduced to PIFU, the information they were provided with, their experience of seeking support and overall reflections. A copy of the patient topic guide is in Report Supplementary Material 4 which was developed and piloted with a PPIE member of RSET. All participants were only interviewed once. Patients were informed that they could contact the research team for further information about the evaluation, and to see or withdraw the data held about them.
All interviews were conducted via telephone and were audio-recorded and transcribed using a secure device and transcription service. The researcher also took notes during the interview. Two members of the research team (RH, CL) coded the data and conducted a thematic analysis using NVivo software (version 20, QSR International, Warrington, UK). We coded the data inductively into themes related to patient experiences of engaging with PIFU and different aspects of the pathway, such as information provided and seeking support. Given the small number of interviews, we were unable to reach data saturation.
Response rate
The total number of interviews was low. There could be several reasons for this. First, the delays in receiving ethical and local site approvals, alongside staff turnover meant that we did not receive details until mid-July 2023, and the window for contacting patients and organising interviews was limited. It was also compounded by taking place over the summer period. The number of patients who agreed to be contacted by the research team was also lower than anticipated.
PIFU is an intervention that requires minimal or no engagement from patients unless they have a reason to contact the service, particularly following short-term interventions. It may be that patients considered they had little to say about being on a PIFU pathway. Given what we heard in staff interviews, it may also be that awareness and understanding of PIFU among patients was low, which could have impacted their engagement.
Analysis of local site data
The decision to acquire local data for phase 2 was informed by the assessments of available national data in phase 1. In particular, we were not able to identify individuals on PIFU pathways. Therefore, we acquired quantitative data from one acute NHS hospital trust in order to assess the individual characteristics of patients on PIFU and who were more likely to activate a PIFU follow-up. This site was different to the ones selected for interviews. The data were aggregated by age and ethnicity. Further details on this analysis are provided in What is the impact of Patient-Initiated Follow-Up on health inequalities and how is this being measured?.
Workshop on challenges and enablers for Patient-Initiated Follow-Up
In phase 2, in addition to the interviews with case study sites, we held a workshop with NHS staff to explore perspectives and experiences of PIFU. The workshop took place on Tuesday, 6 June 2023 and lasted two-and-a-half hours. Attendees were staff from NHS trusts who were invited to attend the workshop through an open invitation on the future NHS platform. People were asked to express their interest in attending to share experiences of PIFU implementation and put forward two representatives from their trust to cover both operational and clinical roles. In total, 22 external participants attended the workshop representing 13 trusts from both clinical and operational roles. All attendees were provided with a participant information sheet in advance.
The workshop was structured around three separate discussion topics which covered how participants viewed the aims and objectives of PIFU, challenges they were experiencing with implementation and factors which would encourage an enabling environment for PIFU implementations. These discussions were facilitated by members of the research team. All discussions were recorded and content captured through a combination of note-taking and virtual whiteboards before being fed into a RAP sheet (see Report Supplementary Material 5).
Findings from the workshop were summarised in a short document provided to NHSE.
Combining results
We triangulated the qualitative findings by data source and combined the results from the patient and staff interviews, and workshop to identify common themes in relation to the RQs. 68 Quotations are used throughout the report. Staff were identified by whether they had a trust or specialty level role, and whether their role was operational or clinical. Patients were identified according to the specialty.
Evaluation guide
We developed an evaluation guide for adoption by local services to facilitate their own ongoing evaluation of PIFU. The development of the guide was informed by the findings from all phase 1 and phase 2 workstreams. The guide includes both quantitative and qualitative elements. It covers planning and design, data collection and analysis, and reflecting on and reporting of results. Further details on the methods adopted for this guide are described in Chapter 4.
Rapid Patient-Initiated Follow-Up scoping review
Overview
What was already known
-
PIFU is being increasingly adopted by health services as a potential solution to capacity challenges faced in outpatient care.
-
PIFU aims to give more flexibility and choice to patients over the timing of their care and allow them to book appointments when they need them, to better align need and access.
-
There is limited evidence of the impact of PIFU on service use, and the evidence that is available is restricted to a narrow range of specialties and clinical conditions.
What this section adds
-
Provides an understanding of the available evidence for PIFU, its impact on service use and patient outcomes across a broader range of specialties and conditions. The review found mixed results relating to whether PIFU might lead to fewer overall outpatient appointments. The impact of PIFU on wider health service use and costs is unclear due to a lack of evidence. PIFU has little or no impact on patient quality of life, clinical outcomes, or satisfaction.
-
Increases understanding of the range of models of PIFU that have been studied and components involved and discusses key implementation considerations. We found substantial variation in the way that PIFU was implemented across studies (e.g. safety-netting appointments, education sessions, booking processes, and triaging).
-
Discusses limitations in the available evidence and identifies gaps where further evaluation is needed – in particular, the impact PIFU is likely to have on patients, outcomes across patient groups and potential inequalities in access or engagement, the range of PIFU models that have been delivered and how components might be related to effectiveness, and staff experiences.
Introduction
Following the scoping review of outpatient innovations, we carried out a rapid review to examine what we know from the available evidence about the impact of personalising follow-up. This review has been published as a Nuffield Trust report. 26 Below we provide a summary.
Aims
The review aimed to examine the available evidence to understand how personalising follow-up impacts service use (including outpatient activity, wider health service use, and costs), patient experience and outcomes (including satisfaction, quality of life, and clinical outcomes). We also sought to identify gaps in the available evidence. We aimed to examine how PIFU has been implemented across a range of contexts and conditions and to understand key implementation considerations that might impact its effectiveness.
Methods
The rapid PIFU scoping review emerged from the wider scoping review (described in Rapid scoping review of outpatient innovations) conducted to understand the evidence that exists relating to different outpatient service innovations.
We adopted an evidence-mapping approach27,29,69 to provide timely information to inform the later stages of the evaluation and we followed recommendations from similar evidence mapping reviews. Where feasible, we have reported the methods and findings according to PRISMA guidelines. 30 See Appendix 5 for a PRISMA checklist.
The review search was conducted (initially in August 2021 and renewed in July 2022) across four databases: MEDLINE, EMBASE, SSCI and HMIC for studies published between January 2015 and June 2022. The review was limited to published articles (grey literature and commentaries were not included).
To be eligible, studies had to examine the effects of patient-initiated approaches to follow-up on service use (e.g. waiting times, referral rates, attendances, missed appointments, costs) as a primary or secondary outcome. We also report on patient outcomes, such as experience and clinical outcomes (where reported). The broader search strategy of the initial scoping review (see Rapid scoping review of outpatient innovations) was supplemented with PIFU-related terms adapted from Whear et al. 70 The quality of included studies was assessed using a modified version of the Mixed Methods Appraisal Tool (MMAT). 71
Further details on eligibility, search strategies and quality assessment are described in Appendix 5.
Findings
We identified 17 studies that examined the effects of PIFU on service use, 7 also reported on costs, and 14 on patient experience and/or clinical outcomes (see Appendix 5, Figure 26). See Report Supplementary Material 7 for details of all included studies and quality assessment.
The review found promising evidence that PIFU might result in fewer overall outpatient appointments, however results across studies were mixed (Table 6). The impact of PIFU on wider health service use and costs is unclear due to a lack of evidence. PIFU seems to have little or no impact on patient quality of life or clinical outcomes but might have a small beneficial impact on patient satisfaction.
| Outcome of interest | Summary of findings |
|---|---|
| Outpatient activity | Eight of the 15 studies that examined the impact of PIFU on outpatient activity reported a statistically significant reduction in the number of outpatient appointments (7 showed no difference). However, effect size varied widely and there was considerable variation in the outcomes used |
| Wider health service use | Mixed results, variation in outcomes and limited evidence meant it was not possible to determine whether PIFU might be related to reduced primary care use or broader hospital activity or whether PIFU might shift activity to other areas of the health system |
| Health service cost | Some studies show a large reduction in costs while others show no impact or an increase in expenditure associated with PIFU. Evidence of the impact of PIFU on costs is mixed and muddied by differences in study design, quality and length of follow-up, differences in PIFU model, and clinical condition |
| Patient outcomes | Evidence shows PIFU has little or no effect on clinical outcomes, patient satisfaction, and quality of life. PIFU, therefore, has the potential to impact outpatient activity without adverse effects on patient experience or outcomes |
| Implementation of PIFU | There was substantial variation in the way that PIFU was implemented across studies, such as whether safety-netting or standard review appointments were offered, whether education sessions were provided, booking processes, and triaging of appointment requests, and the use of remote monitoring. Often, information reported by studies provides only limited understanding of PIFU components and how they were delivered. From the available evidence, it is not possible to determine which PIFU components are associated with effectiveness |
Discussion
This review not only provides an update of the available evidence, but also extends the evidence by including a broader range of study designs and synthesising evidence across a wider range of specialties and conditions. The review is novel in its focus on implementation and understanding the models of components of PIFU.
Despite some promising findings, available evidence is limited and often lacks generalisability. In many cases results across studies were mixed. Only a small number of studies have been conducted across a few specialties, few studies were rated as good quality, there is uncertainty about the clinical significance of statistical findings, and design issues were common. Only a small proportion of studies were conducted within the NHS and many PIFU models involved other innovations and service changes. Most studies involved a short follow-up duration and there was substantial variation in which outcomes were measured.
The review identified several key gaps in the available evidence where further work is needed: in particular, the impact PIFU is likely to have on patients, outcomes across patient groups and potential inequalities in access or engagement, the range of PIFU models that have been delivered and how components might be related to effectiveness, and staff experiences of delivering PIFU.
How is Patient-Initiated Follow-Up being implemented?
Overview
What was already known
-
PIFU is an NHS priority, but existing evidence on how it is being implemented is limited. 26
-
Where personalised follow-up has been studied, models vary widely in terms of the approach to monitoring patients, how they initiate and attend appointments, and how care is provided remotely. 26
-
NHSE has developed guidance to support trusts with implementation which acknowledges the impact of local service factors and the need to adapt PIFU to suit particular conditions or specialties. 53
What this section adds
-
Since September 2021, the recorded numbers of patients who were either moved or discharged to PIFU pathways across the English NHS had approximately tripled by March 2023 to about 185,000 per month.
-
Use of PIFU between NHS trusts and specialities varies considerably.
-
Several trusts were already using patient-initiated approaches to follow-up before September 2021, although it not being captured in national or local data.
-
In the NHS, PIFU is most commonly being used in short-term pathways (e.g. Physiotherapy or following surgery) although there are several examples where it is being used for people with long-term conditions.
-
Staff perceived several benefits of PIFU for patients, staff, and the wider service. This included reducing unnecessary contacts and better aligning capacity with patient need.
-
Models of PIFU vary widely between trusts and clinical areas, with a significant degree of variation in the approach to patient selection, monitoring, and discharge.
-
The nature of the condition was a key factor in how PIFU was implemented.
-
Where PIFU had been implemented successfully, enablers included conditions where symptoms and deterioration were easy to identify, clinical engagement, supporting guidance, champions, dedicated staff capacity and flexible recording systems.
-
Barriers to successful implementation included patients not being aware they were on PIFU, perceptions of challenges accessing care, staff resistance, competing priorities and limited capacity to dedicate to PIFU, a lack of engagement with primary care, and challenges updating electronic patient record (EPR) systems to record PIFU activity.
-
Staff saw several opportunities around PIFU, including supporting their service to become more efficient and patient centred. They also saw opportunities for wider adoption of the innovation and better use of technology to facilitate delivery.
-
However, staff also pointed to several potential risks with PIFU, especially where patients do not or cannot access the service when they should. They also raised potential inequality issues for patients for whom PIFU does not work well, and risks for service capacity within and beyond outpatient departments.
Introduction
In this section, we present evidence that addresses RQ 3.1 for the PIFU evaluation:
-
How is PIFU being implemented? What components and processes does it involve? What is the involvement of technology in the pathway?
We begin by providing a national overview of how PIFU is being implemented in the NHS before discussing the key components of a PIFU pathway with reference to examples from the case study sites. We then summarise how staff in the interviews perceived the aims and objectives of PIFU and the factors that have been considered to enable and hinder PIFU implementation. Finally, we summarise what staff perceived to be the key opportunities and risks associated with PIFU.
The findings on staff and patient experience are explored in more detail in Staff experiences and What is the impact of Patient-Initiated Follow-Up on patient engagement and experience? respectively.
Methods
Findings in this section draw on:
-
semi-structured interviews with clinical and operational staff across five case study sites
-
analysis of national and local site documentation
-
a workshop with clinical and operational staff representing 13 NHS trusts
-
analysis of national data.
Triangulating data from the different sources, it was clear that there was significant overlap between the themes identified in the interviews and workshop discussions, so we present these as one data source. However, where quotes are provided, we specify the source.
Further details on methods and data sources can be found in Methods for the evaluation of Patient-Initiated Follow-Up.
What components and processes are involved in a Patient-Initiated Follow-Up pathway?
Figure 10 (see Introduction) illustrates a typical PIFU pathway. Each stage involves different design and implementation choices and the approach taken will depend on a range of contextual factors, including the condition, patient need and local site processes. 53
Table 7 illustrates some of the key design considerations and choices available at each stage of the PIFU pathway and distinguishes between higher and lower intensity input from services in implementation. It is drawn from the evidence review and phase 1 interviews.
| Key design considerations | Range of approaches across sites/specialties | ||
|---|---|---|---|
| Lower-intensity input from services | Higher-intensity input from services | ||
| Patient selection |
|
Individual clinical judgement/no standardised criteria for patient selection | Standardised clinical protocols and selection criteria by condition/clinical area |
| Patient education/induction |
|
PIFU by default – for example, all patients automatically placed on PIFU and notified via letter, unless they have clear clinical exemption | In-person consent followed up by patient letter/written educational material on PIFU and their condition |
| Patient booking, appointment management, and triage |
|
No designated booking system – for example, patients use same contact as other outpatients. No protected or reserved capacity for PIFU patients. PIFU appointments allocated on a first-come, first-served basis without triage |
Designated booking line/mechanism for PIFU patients. Reserved PIFU appointment slots/designated PIFU clinic. Designated triage nurse available to field PIFU requests and determine need for appointment |
| Safety-netting, patient monitoring and discharge |
|
No form of monitoring – patients make contact only when they detect change or fluctuation in their own condition or have concern. Patients automatically discharged if no contact made |
Routine patient questionnaires, lab testing or remote technology used to monitor changes in patients’ condition. Patients proactively contacted if acceptable period of time lapses before making contact Patients clinically reviewed before discharged from PIFU |
National overview: how is Patient-Initiated Follow-Up being implemented across the National Health Service?
Since reporting of PIFU activity began in September 2021, the recorded numbers of times patients were either moved or discharged to PIFU pathways across the English NHS had approximately tripled by March 2023 (Figure 11) to about 185,000 per month. In the same month, the total number of reported outpatient attendances was about 7.4 million, which means that reported movements to PIFU pathways is about 2.5% of this. Since not all submissions of PIFU activity data to P-EROC are recorded as ‘complete’, this number is likely to be an underestimate.
FIGURE 11.
Total numbers of times patients were reported to be moved or discharged to PIFU between September 2021 and March 2023. Source: P-EROC.
Patient-Initiated Follow-Up is most commonly used in the Trauma and Orthopaedics and Physiotherapy specialties (Figure 12), which together have 35% of all reported patient transfers to PIFU pathways between September 2021 and March 2023. However, these two specialties also treat relatively high volumes of outpatients. Measuring numbers transferred to PIFU as a proportion of all outpatient activity over the most recent 6 months in the data (from October 2022 to March 2023), of these specialties, PIFU is most commonly used in Orthotics with a proportion of approximately 11.5% (see Figure 12). Proportions in Trauma and Orthopaedics and Physiotherapy are still comparatively high at 6–7%, whereas 12 of these specialties rates below 5%.
FIGURE 12.
Total numbers of times patients were reported to be moved or discharged to PIFU between September 2021 and March 2023 (only trusts that record PIFU activity in P-EROC for these specialties are included). ENT, Ear, Nose and Throat.
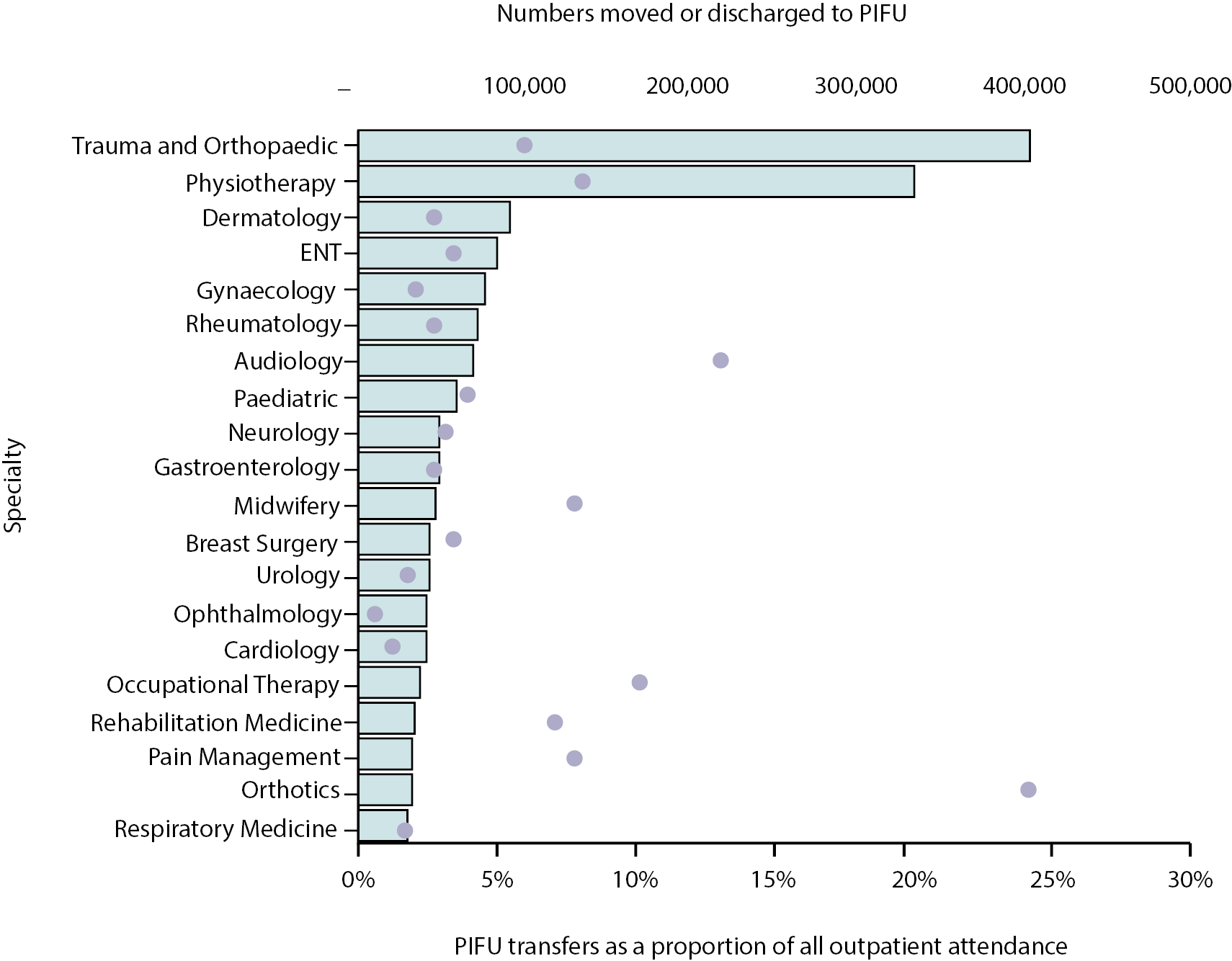
There is a large amount of variation between trusts within the same specialties. Across the 10 specialties where most patients have been moved or discharged to PIFU in the 6 months between October 2022 and March 2023, proportions of numbers moved or discharged to PIFU per outpatient attendance for each trust are shown in Figure 13. Although the majority of these proportions are below 10%, there is some notable variation, with two proportions over 50%.
FIGURE 13.
Variation in proportions of numbers of patients moved or discharged to PIFU per outpatient attendance for October 2022 to March 2023. The top 10 specialties for volumes moved or discharged to PIFU over the same period. ENT, Ear, Nose and Throat. Note: The mean value is marked by an ‘X’ for each specialty, while the median value is marked by a horizontal line across the box. Quartiles were calculated excluding the median. Outliers were defined using the IQR (inter-quartile range) x 1.5 threshold. Only trusts that record at least 100 outpatient attendances a month and PIFU activity in P-EROC for these specialties are included.
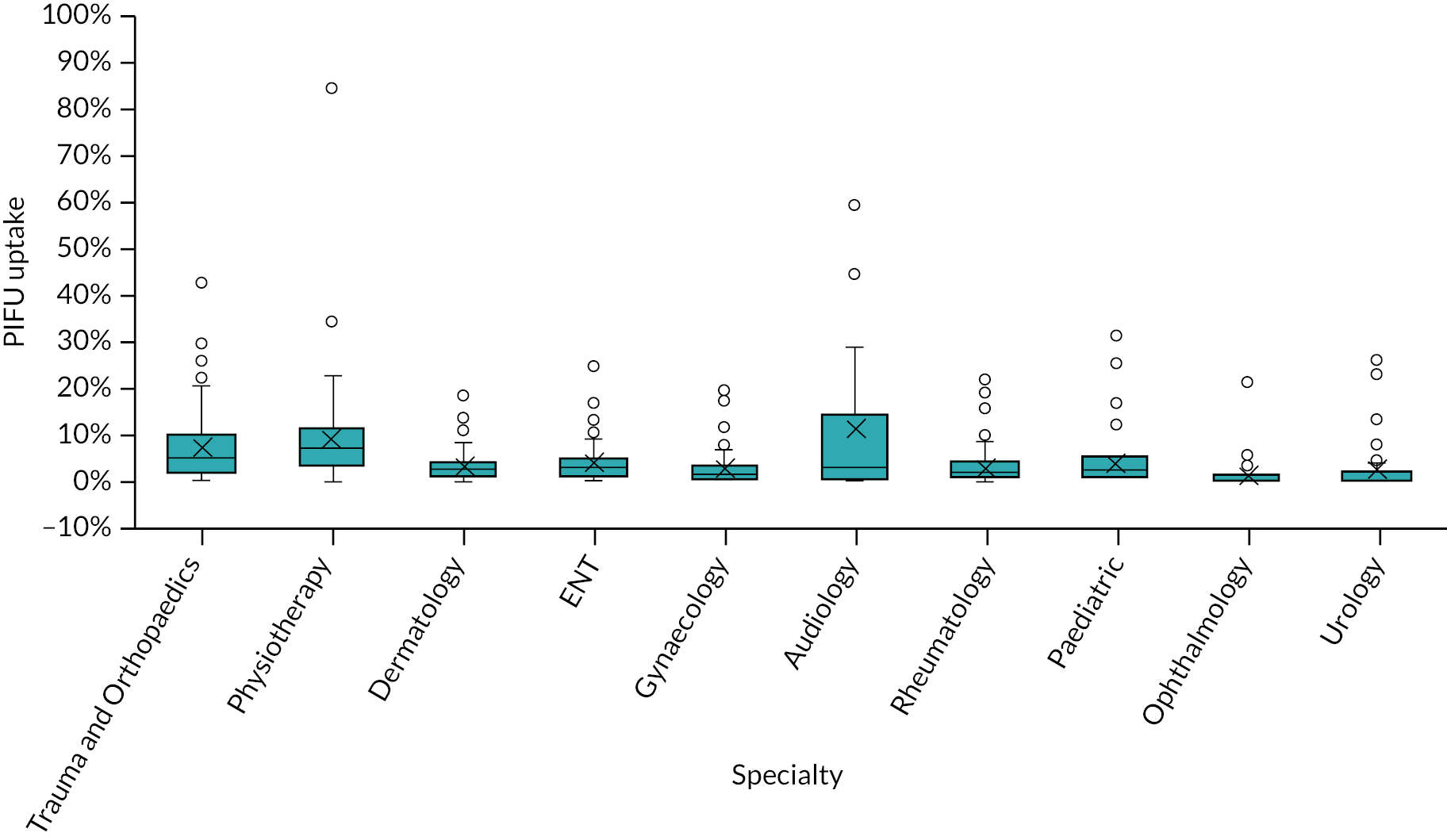
Local perspective: how is Patient-Initiated Follow-Up being implemented within National Health Service trusts?
The following section explores how PIFU was being implemented locally across NHS trusts in England during the study period (June 2022–July 2023). It draws on interviews with staff from five case study sites and the workshop with staff from a wider range of sites. As illustrated by the quantitative analysis, hospital trusts in England are at different stages of PIFU implementation. Also, PIFU pathways vary widely in their design. This was observed both from the workshop and the case study sites.
The differences we observed in how PIFU was being implemented included:
-
Whether implementation was staggered specialty by specialty or whether it was done across the trust in one go.
-
The extent to which PIFU (or similar approaches) had been in use previously within the site.
-
The influence (and level of emphasis placed on) the NHSE ambitions (including original 5% target) on adoption of PIFU.
-
Who led the implementation (e.g. whether it was done by a central outpatient team, or whether it was by individual clinicians) and the level of support they provided.
-
The extent to which PIFU implementation was given allocated or dedicated staff time (e.g. dedicated PIFU leads or outpatient transformation leads).
-
The electronic PAS in use and number of changes required to record PIFU activity.
-
The involvement of technology to support PIFU implementation and delivery.
-
The development and use of standardised materials such as SOPs.
-
The types of short-term and long-term PIFU pathways in use (e.g. PIFU with or without clinical review, discharge to PIFU or PIFU with fixed recall).
-
How sites approach PIFU in relation to discharge.
-
The priorities of sites (e.g. codifying existing activity or increasing the use of PIFU in long-term follow-up pathways).
We also observed differences in how PIFU is implemented across clinical areas. Table 8 illustrates a sample of four PIFU pathways we heard about from phase 2 of the data collection, where we looked in more detail at two case study sites to understand how they are implementing PIFU across clinical areas. These were selected to illustrate the range of approaches that we heard about in the research. It is important to note that given the wide variation in how pathways are designed, these may not be representative of how PIFU is being used more widely within the NHS.
| Patient selection | Patient onboarding and information | Booking appointments | Safety-netting and discharge | |
|---|---|---|---|---|
| Breast care | ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| Epilepsy (neurology) | ||||
|
|
|
|
|
|
|
|
|
|
|
||||
| Bronchiectasis (respiratory) | ||||
|
|
|
|
|
|
|
|
||
|
||||
|
||||
| Gynaecology | ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
We now describe the key stages of these pathways in more detail.
Patient selection
Sites varied in the degree to which patient selection criteria for PIFU were formalised across the trust. More generally, we observed differences between a highly personalised approach and one where people were placed on PIFU routinely, unless they actively objected.
Defined clinical protocols existed in most services. Alongside this, subjective criteria about a patient’s ability to manage their own condition or ability to initiate contact typically came down to a clinician’s discretion and knowledge of the patient. Specialty-specific factors were also considered, for example, in paediatric care, safeguarding was a key consideration.
We’ve said to all our clinicians, it’s a patient-by-patient decision. You know, even if a patient fits […] the clinical criteria, it’s got to be right for that patient. So in general it works really well with patients who understand their condition. They are empowered to […] know what to look out for.
Operational, trust-wide – Site 2.1
It’s important for the clinician to be conscious when using it about whether it’s appropriate for this particular family or not – it’s not just a routine thing.
Clinical, specialty level – Site 2.2
Patient information and induction
National Health Service England guidance states that the decision to put someone on PIFU should be shared, with patients fully involved. 53 We found that even when there is officially a standardised trust-wide approach, there is variation within organisations by clinical pathway in how PIFU is communicated to patients and the degree of shared decision-making involved.
For shorter-term pathways like physiotherapy or after orthopaedic surgery, some patients were automatically placed on PIFU as that is already the standard of care for these conditions in many organisations, and no fixed follow-up alternative is offered. For longer-term conditions, we observed broad variation, with some sites requiring a face-to-face conversation between patients and their clinician about PIFU and for patients to provide consent to the pathway. In other sites or clinical areas, patients were made aware they were placed on PIFU without a formal discussion (such as by letter) and told to contact their clinical team with concerns.
Within the pathways we observed, patients were provided with information about PIFU in several ways, such as patient information sheets, leaflets, and wallet cards which detail symptoms to look out for and how to get in touch with the service. Sometimes this is included alongside patient letters, which often include details of the PIFU pathway and how long a person is placed on PIFU.
Patient booking, appointment management and triage
There are different approaches to managing PIFU patient requests and appointments. In some services, the way a patient initiates an appointment did not change under PIFU – they would still use the same booking telephone or e-mail alongside patients on fixed follow-up and would be allocated an appointment on a first-come, first-served basis. Waiting times for PIFU appointments also varied – some services had specified windows (such as within 2 weeks). However, in one gynaecological pathway a wait for an appointment could be up to 6–9 months, a similar length of time to those waiting for an initial post-referral appointment.
Others reported a more structured approach where PIFU patients had a designated route to request an appointment, which included a choice of options, such as a phone number, e-mail address or trust website contact form. In most pathways we studied, clinicians triaged requests before allocating appointments, although in some cases initial contacts were managed by administrative staff who reviewed requests and clinicians would triage. In one pathway for patients with breast cancer, a designated PIFU nurse had been hired to do this and assess the need for an appointment, amongst other responsibilities supporting the PIFU pathway. Patients were then provided with the support they required which could include a virtual or face-to-face appointment with a nurse or consultant, a scan or blood test, or just a conversation involving patient education or referring to further resources. In other pathways, especially for shorter-term conditions, any PIFU patient requesting an appointment would get one without triage.
How appointments are scheduled also varied and features of the clinics and the ability of services to be flexible with their capacity influenced how this was managed. We heard examples where designated slots for PIFU patients were reserved in clinics (and filled if there were no PIFU requests). Other services had set up specific clinics just for patients on PIFU which were nurse-led and addressed a variety of issues which people had approached the service about. How much capacity was required was largely estimated rather than based on a data-driven understanding of patient need and demand. Some patients on PIFU also had a fixed appointment scheduled at some point in the future but were able to contact the service in the interim if they had any concerns.
Safety-netting, patient monitoring, discharge
Pathways varied in the level of safety-netting. (Safety-netting refers to the processes, safeguards, and information provided to patients with uncertain conditions to identify deterioration.) In some pathways, particularly for cancer or patients taking biologic medicines (e.g. Rheumatology or Breast Care), routine screening and monitoring is an embedded part of the pathway where patients are still seen at regular intervals. Some pathways (such as one Respiratory example) had a built-in review, whereby people approaching the end of the pathway are reviewed and contacted by a consultant to see how they are getting on, based on other information (such as whether they had been in touch with the service). In some pathways, there was minimal or no proactive monitoring and patients were automatically discharged if they made no contact with the service within an agreed time frame. This tended to be more common in shorter-term pathways, such as after a surgical procedure, where a patient would be automatically discharged, for example, after 6 months in the absence of any contact.
Most sites and services were limited in the degree to which they used technology to support remote monitoring or safety-netting, though several interviewees identified this as a key area of development and future opportunity. While not yet widespread, some sites have been piloting patient portals to issue routine questionnaires or surveys to monitor fluctuations in a patient’s condition or symptoms, the results of which would offer reassurance or could trigger an appointment if needed.
Long-term pathways also varied in their approach to discharge. The duration of the pathway tended to be clinically dependent but could be 2–5 years, or in some cases 10 for conditions like epilepsy that are life-long and can fluctuate over time. Although most of the PIFU pathways we explored had a set time limit, there was also an acknowledgement that if someone gets in touch slightly after that time frame, they would probably be seen without needing to be re-referred by their GP.
What factors influence Patient-Initiated Follow-Up implementation?
Interviewees and workshop participants were asked to describe the enablers and barriers they had experienced with regards to PIFU implementation. In this section we summarise these findings in relation to PIFU implementation using the structure of the NASSS framework. 67 This framework notes that innovation adoption is affected by several factors including those relating to the intervention itself, the condition in which it is being used, those using the innovation (staff, patients and carers), the organisation in which it is being implemented and the influence of the wider system. These categories reflect those present in other analytical frameworks, such as the Consolidated Framework for Implementation Research. 72
Below we consider the findings considering each of these elements.
Value proposition
Within the NASSS framework, ‘value proposition’ refers to the value of the technology as viewed from the perspective of the healthcare system and patients. 67 We have used it to explore the aims and objectives of PIFU from the perspective of staff in relation to the system, service, and patients. How the value or purpose of the innovation is viewed is a factor in how it is perceived and implemented.
Multiple staff, both in clinical and non-clinical roles, spoke of disparities in perception of the aims of PIFU at different levels of the organisation. Table 9 outlines how staff in the interviews perceived the benefits of PIFU for patients, staff and the service.
| For patients |
|
|
|
|
|
|
|
| Quite often what happens at the moment is that they have a planned follow-up in 2 years’ time, they come in. They’re feeling absolutely fine. It’s 5-minute appointment they’ve travelled miles to get here, paid for parking and the whole lot. And they don’t really need to be seen, but a couple months later they’re feeling really unwell. They tried to be seen and we’ve got no capacity or, you know, and then they might end up coming in through the emergency route. So actually, it’s about giving the patients the opportunity to be seen when they need to be seen.Operational, trust-wide – Site 2.1 | |
| For staff |
|
|
|
| I think it makes the whole clinic process a lot more efficient in that we see the patients when they need our specialist input rather than the traditional rolling appointment once or twice a year ... this felt a little bit more modern.Clinical, specialty level – Site 2.1 | |
| For the system and service |
|
|
|
|
|
| For years some clinicians have had a system of ‘call me when you need me’ with a designated admin line for when they had a flare-up, but lacked governance or any systematic process or shared decision around it – so PIFU creates more structure, safety-netting, and patient engagement.Clinical, trust level – Site 2.2 |
Nature of the condition
The nature of the particular condition, specialty or pathway appears to be a key factor affecting PIFU implementation. PIFU seems to be common or more straightforward in conditions with lower complexity, easily detectable fluctuations, or changes in symptoms. Where people had multiple complex conditions requiring different levels of input from different services, PIFU was considered less straightforward.
They come in, we sort them out, operate on them and then hopefully we’ve done what we need to do and we can say goodbye to them, whereas I understand within medical specialties you’ve got people [with] long-term health conditions and I completely understand that that is a whole different ball game. But from our perspective it was quite an easy thing to implement.
Operational, specialty level – Site 2.2
In most cases, condition-specific criteria shaped how PIFU was implemented, and each specialty established their own set of standards and principles for choosing patients for PIFU. In some areas, trust-wide protocols have also been developed that consider factors such as a patient’s ability to self-manage, carer support and other risk factors. Although most specialties within the case study sites had set criteria, it did not appear that they were routinely communicated more widely. For example, interviewees in primary care were unclear as to how patients were selected for PIFU and there was a perception that it was based on the judgement of an individual clinician.
Condition-specific guidance, criteria, and examples areas were also thought to be beneficial and where national guidance exists, this was seen to facilitate support for PIFU. This has already been developed in some specialties (such as Gynae-oncology). 73
National guidelines are really important because I think clinicians need that backup of ‘This is the right thing to do. This is the evidence. This is who we think are the right patients from a technical point of view. These are not the right patients.’ So I think national guidelines are really, really key.
Clinical, specialty level – Site 2.2
There are a range of conditions or specialty areas that are perceived to be of higher risk, where PIFU tends to be less well established and where more guidance would be beneficial (e.g. conditions where it may be less easy for patients to identify symptoms for deterioration or deterioration is asymptomatic). Work to identify conditions within specialties where PIFU could be used most effectively was considered valuable.
We’ve done a lot of work both within our trust looking at what you could do within the PIFU envelope and looking at each specialty in isolation ... let’s look at everything and then think about the ones we could.
Clinical, specialty level – Site 2.1
Characteristics of the specialty, condition or clinical pathway may also be relevant. Within Gynae-oncology, interviewees noted that the likelihood of identifying recurrence of some gynaecological cancers from a follow-up routine physical examination alone is low (and that recurrence usually occurs with symptoms), so the value for both staff and patients in attending such appointments is more limited. Another interviewee pointed to the extent to which some conditions are also managed in primary care, which may affect how patients (and staff) feel about PIFU because they are more aware of alternative places to get support.
Specialties like Ophthalmology, which don’t have any primary care, there is that concern that you’re just going to abandon people. And I think specialties with lots of good primary care don’t have to worry about that.
Clinical, specialty level – Site 2.1
PIFU was perceived from those in primary care to work well with conditions with ‘protocol-driven pathways’ with clearly defined stages and treatments (such as certain cancers). However, pathways which involved investigations and treatment for symptoms, where cancer had been ruled out, introduced complexity and uncertainty not only for the patient but also in determining the role of the specialist and GP. This may influence who patients approach (or think they should be approaching) for different aspects of their condition. One GP described this as follows:
The challenge is when you’ve got patients with conditions like inflammatory bowel disease, where most GPs would be able to manage straightforward flare-ups and we know what to do, but there’s a bit of a grey area between the level of expertise that the GP would have, and perhaps the very latest evidence-based specialist intervention for using immunological drugs, etc. So, I think sometimes that grey area creates a bit of confusion and sometimes patients do cycle around the system a bit.
GP
Adopters
Staff
Staffing factors were a key issue in supporting or hindering implementation. Importantly, staff considered that PIFU should be seen as a clinically led change and not an operational one and clinical champions have been valuable. Where pathways had been implemented effectively, individual clinicians have often pushed it forward, in some cases joining trusts from previous organisations where PIFU was more common. Conversely, we heard that some clinicians want to see that it works before implementing it themselves. This seems to have been particularly relevant in specialties traditionally seen as inappropriate for PIFU, where sharing learning and best practice about implementation has been desirable.
Not everyone wants to be a pioneer.
Workshop participant
Clinical autonomy – [it’s] important for surgeons in general but also our team specifically. People less keen were more reassured they didn’t have to accept something they didn’t want.
Clinical, specialty level – Site 2.1
Know your early adopters, then link those people with other teams nationally who have done this elsewhere, get them enthused. If people can see the benefit and see the impact on their waiting list and can see they can still manage their patients they’ll want to do this. You need to demonstrate positive impact.
Clinical, trust-wide – Site 2.1
If you can identify one clinician within the specialty to lead on it, it helps because if no one takes ownership of it, it’s a bit like herding cats.
Operational, trust-wide – Site 2.1
Access to specialist nurses or support workers also play a role in PIFU implementation. Often, they have existing relationships with patients, are used to answering questions and providing support and provide additional capacity. There was a perception from interviewees in services who did have access to these roles, that specialties without them may find it harder to integrate PIFU.
In our service the clinical nurse specialists [are] always there for the patients anyway because it’s cancer. But I think in other services, it has been a lot harder. For instance, in colorectal for their inflammatory bowel disease patients, there are nurses, but they’re not quite the same. So they don’t have the same ability for patients to call up and that adds to their workload a lot.
Clinical, specialty level – Site 2.2
Dedicated support has also been an asset. One clinical nurse specialist interviewed had taken on a PIFU-specific role (with dedicated funding) in response to challenges the team had in managing PIFU alongside other responsibilities. They were able to manage the vast majority of PIFU-related workload on behalf of the department, including patient induction, receiving patient contacts, triage, dealing with requests and managing patient prescriptions.
At the same time, factors relating to staff could also be a barrier to implementation and concerns around staff resistance were noted as a key barrier throughout the study (findings on staff experience are explored more in Staff experiences). This was felt to be driven by a combination of factors including a clinician’s pre-existing values and lack of confidence in the PIFU pathway.
Lack of confidence was in part driven by a fear of patients getting lost in the system due to inadequate administrative infrastructure. Interviewees also considered that clinicians with more transient patient populations, who have less of an understanding about their patient’s life circumstances, may be less confident or likely to consider PIFU as an option and would be more likely to default to a fixed follow-up schedule because they may not be confident that patients would contact the service when they needed to. Alternatively, PIFU works well when clinicians have a long-term relationship with patients and a good understanding of their clinical and personal situation. This may also be related to levels of clinical experience, with a feeling that less experienced clinicians may be more likely to default to fixed follow-up and were also less confident to discharge people fully. Some services discussed patients’ eligibility for PIFU within their multidisciplinary team meetings which provided an opportunity to discuss any concerns, and this was considered a useful way to not only support staff but embed PIFU within standard clinical practice.
Throughout [the] journey we’ve built a good relationship with patients so they know if they do contact us, we will get back to them, help and support them – they feel quite secure knowing that they know who you are and will get back to them.
Clinical, specialty level – Site 2.2
There were challenges getting engagement from staff who were unclear how PIFU was different to what they were already doing (e.g. open access or open appointments). In this case, resistance was more to the need to formalise, standardise and record PIFU. This was especially true for clinicians for whom PIFU (or something similar) is already routine, who felt that they were being asked to formalise and spend more administrative time just to record something they are already doing. PIFU was therefore perceived as a tick box exercise to achieve a national target. Staff appeared far less enthusiastic about PIFU if it was framed as an exercise in meeting targets and reducing backlogs. Those in trust-wide operational roles responsible for implementing PIFU reflected that their approach to clinician engagement focused more on the benefits for patients, rather than the need to reach a specific target.
Some clinicians also appeared resistant to PIFU because of the potential increases to workload (impact on staff workload is explored further in Staff experiences). For short-term pathways this seems to mostly not have materialised, although (as discussed further in What is the impact of Patient-Initiated Follow-Up on patient engagement and experience? on patient engagement) information on who is getting in touch and why remains a gap for some services.
Patients
The extent to which patients and carers are engaged has been a crucial factor in PIFU implementation (enablers and barriers to patient engagement are also explored in What is the impact of Patient-Initiated Follow-Up on patient engagement and experience?). This depends on several factors, such as how patients are provided with information about PIFU. At a minimum, staff felt that patients should receive clear and consistent information detailing how and when to contact the service. Some sites are developing educational material to ensure that each patient receives the same level of information. Adequate time to explain PIFU and go over any questions and concerns with patients is also key. However, not all staff were confident in the extent to which this information is shared in a consistent and timely way with patients. Several interviewees reported anecdotal feedback that patients were unaware they were on PIFU and that the terminology is unfamiliar and inaccessible.
The ability to contact a service is a key factor in the success of PIFU. This means patients trust it and feel confident they would be able to speak to someone if needed. It is important to maintain multiple channels for patient contact and not solely relying on electronic systems so as not to exclude anyone from PIFU who may be less digitally capable or confident, especially if technology is used more in the future (see What is the impact of Patient-Initiated Follow-Up on health inequalities and how is this being measured?). In some pathways, creating PIFU-specific roles helped support patient engagement because patients had a contact who could respond to requests quickly, and offer reassurance without need for an appointment.
Having a dedicated resource makes such a difference, because [the triage nurse] can reassure people and have those initial conversations with the patient to tell them about PIFU and what it is ... so the patient feels really valued and not like they’re being fobbed off to a service that could be unreliable.
Operational, trust-wide – Site 2.1
Perceptions of access to care are also a factor in how patients may engage with the service (patient engagement and experiences are explored in more detail in What is the impact of Patient-Initiated Follow-Up on patient engagement and experience?). Staff in secondary care suggested that the current context around long waits and visible pressures on NHS services may make patients more resistant to PIFU in the first place or leave them less inclined to get in contact if they needed to. This was in part for fears of being abandoned or being reluctant to give up a fixed appointment in case they cannot get an appointment when they need one. We also heard concerns from staff that people may not contact a service for fear of ‘bothering’ them.
Getting patients to call us [has been] really tricky – [I] have had instances of writing to a patient at review with no evidence of anything and say everything seems fine and they come back and say it’s been awful, but they’ve not been wanting to bother the doctor.
Clinical, specialty level – Site 2.1
Some staff seemed less concerned about this and noted that even if people did not contact the PIFU service, they would receive care somewhere (such as primary care or ED).
They’ll end up somewhere, so it’s not ideal, is it? But, from a patient safety point of view, I don’t think it’s unsafe. Because they’ll see somebody in health care and get something sorted. But it goes back to everybody knowing they’re on PIFU.
Clinical, specialty level – Site 2.1
However, we also heard another perspective from primary care interviewees who referred to feedback they were hearing from patients about not being able to get hold of hospitals and specialist teams (this applied to people on PIFU but also people trying to access hospital care more generally).
Internal factors: the organisation
Digital infrastructure
Patient-Initiated Follow-Up implementation was more easily facilitated in those sites with the digital infrastructure to deliver the pathway. Where interviewees had been able to make this work well, being clear with their supplier about the patient safety implications for having a robust infrastructure was considered vital. One site talked about implementing PIFU with support from digital leads from the beginning to ensure that the relevant digital systems could be piloted and refined.
However, PIFU implementation is being hindered by limited and variable digital infrastructure and maturity across the system. Organisations are experiencing challenges making the necessary amendments to their EPR/PAS, which record PIFU. This included not being able to set up alerts to remind clinical staff when someone is due a review and not being able to record the appropriate PIFU outcome for patients (such as when people are on a PIFU pathway but also have a fixed appointment). This means that trusts are also struggling to obtain data they could use to monitor and evaluate their performance and compounded concerns about patients getting ‘lost’ in the system (this is explored in further detail below in risks). These barriers were particularly acute in trusts where digital suppliers seemed to be more rigid in their response to the need to make amendments to digital infrastructure or where there were competing digital priorities.
In some sites, progress had been halted until new processes could be set up to record and track patients on PIFU. Some people noted that this is an area where further support and guidance from NHSE would be beneficial.
The main barrier was not having access to an IT system that would safely track the patients. We needed faith that we could suddenly recall everyone on PIFU.
Clinical, specialty level – Site 2.1
Practically, interviewees noted that primary care electronic records do not have the capability to record PIFU, which compounds the challenges created by a lack of integrated, linked technology between primary and secondary care.
Support and engagement within the organisation
Appropriate time and resources as well as leadership support is crucial. Funding for dedicated transformation leads (across outpatients more broadly), administrative support to manage and design pathways, rather than absorb PIFU into existing workflows, and support from project management roles to implement within individual specialties, were all noted as valuable. Where people did have support from these roles, they supported clinicians with implementation, ensured consistency across the trust and maintained the focus on PIFU through regular engagement and conversations between operational and clinical teams.
Leadership support for PIFU is also important. One site noted that even when there was strong specialty-level support for PIFU, progress has been limited because of lack of support at a board level, who were more focused on other trust priorities.
Service characteristics
Specific organisational and service factors have also enabled implementation. Some services had implemented PIFU because they were experiencing significant backlogs in follow-up appointments and they recognised that they did not have capacity to see everyone. PIFU had provided an opportunity to implement a different way of working. A key step had been identifying the appropriate capacity within services needed for PIFU (e.g. for appointments or for conducting reviews). Some clinics created designated PIFU slots on each day or ran a designated PIFU clinic (knowing they could always fill those appointments if they went unused). More broadly, teams have adapted the format of clinics, such as by reducing the number of follow-up slots and increasing the numbers of appointments available for new patients or fast-track appointments (in the case of cancer).
If we get enough people and the demand for reviews, which we should do, then we will have to have some hot slots in clinics as in face-to-face ones and [...] can put rules in to say if it’s not filled within 48 hours, then fill it with anything so that you don’t waste the slots cause that’s really critical, isn’t it?
Clinical, specialty level – Site 2.1
For clinics booked up a long time in advance, giving someone on PIFU an appointment sooner was more challenging whereas in specialties where appointments were more ‘fluid’, accommodating someone on PIFU could be done more quickly. Flexibility helps not only with allocating appointments but in putting people on to PIFU in the first place. Services which can be flexible with their approach may lead to more effective implementation of PIFU.
Why it works is because we have the flexibility and availability in staff and clinic space – even if [the PIFU] clinic space ends up full, we’ll just see them in a different clinic – it wouldn’t work if you can’t be flexible.
Clinical, specialty level – Site 2.1
Complexity could also arise with the staff involved in delivering the service, particularly if appointment booking is done by centralised rather than specialty-specific teams who may be less familiar with the process of allocating appointments to those on PIFU pathways.
So if you have a central bookings team who do bookings for lots of different specialties, who don’t know your specialty particularly well, they wouldn’t necessarily know that you’re ring-fencing appointments and that they have to then book those appointments. You might waste slots.
Clinical, specialty level – Site 2.2
Complexity
Organisational complexity has also been a factor, for example, where hospitals have different EPR systems, approaches to outpatient management and PIFU. This had resulted in specific issues, such as patients receiving multiple, disjointed communications from different parts of the same trust. From a primary care perspective, multiple trusts within an integrated care board (ICB) can also have different approaches to PIFU (and wider treatment pathways) creating additional complexity for themselves and their patients when trying to navigate the system.
It’s hard to impose consistency between trusts – there are very different pathways and they are run so differently ... this lack of consistency makes implementation tricky. Not better or worse just different.
GP
External factors: the wider system
National Health Service England activity
Patient-Initiated Follow-Up implementation has also been affected by factors in the external environment. NHSE has taken steps to support the implementation of PIFU by producing guidance,53 identifying regional leads for PIFU for different specialties and making use of the FutureNHS platform to share learning and practice. 74 Given that many of the challenges are shared across the system these forums were considered vital for spreading learning. However, it was less clear whether these are happening within trusts themselves and between specialties that are already doing PIFU who could share their learning.
For some of the case study sites, we observed that the now discarded NHSE target to move or discharge 5% of outpatient attendances to PIFU by March 2023 acted as a catalyst to a more formal trust-wide rollout. 75 However, interviewees were mixed in their views as to whether these targets had been helpful. Although a target can help create focus and buy-in from senior leadership, there was concern that the target did not support clinician engagement, who were more likely to be influenced by the value of PIFU for their patients and service. It also contributed to cynicism that PIFU was seen as a way of reducing activity and not something that was desirable for staff and patients. Participants questioned the rationale behind the 5% figure and were concerned that this displayed a lack of understanding surrounding how PIFU affects different specialties differently.
So, what is PIFU? Is PIFU a personalised approach to outpatient that enables empowers patients [to self-care] and makes the most use of our resources by making sure that appointments are appropriate and needed and patients get seen the best time that suits them or as the national drive seems to be going ahead now, is this a means to reduce people on waiting lists as part of the elective recovery? It should be the first, but it’s being repurposed.
GP
People think PIFU is just going to go away. If it’s just a target, people aren’t interested. If it’s enabling [them] seeing who they need to see when they need to see them, then they are. People don’t generally feel this. Need to focus on it and communicate well. Giving it targets and acronyms makes the process more complicated than it actually is.
Clinical, trust level – Site 2.1
Primary care
Clear and consistent engagement with other parts of the system (such as primary care) is required for PIFU to work successfully. Staff in the interviews and workshop noted that there also does not seem to be a clear way to record in primary care that a person is on PIFU. This could lead to several issues. Staff in secondary care recalled examples of receiving re-referrals from GPs suggesting that they (and/or the patient) were unaware the patient was on PIFU and able to get back in touch with the service directly. From the primary care perspective, we heard of challenges where letters arrived late, or it was unclear when the pathway started, meaning GPs were unaware whether the person was still on a PIFU pathway.
PIFU itself as an acronym [I’ve] not seen, I have seen ‘open access’. It might say for example, ‘this person’s IBD [inflammatory bowel disease] is now stable and they are on this drug, no follow-up but can contact us.’
GP
So I think we have done some engagement in terms of putting information out, but I think missing primary care out of conversations and things being very secondary care-led is a mistake because there is always an impact on primary care because I might see these people who have been missed ... how do we make sure that as a practitioner that I’m aware that people are on a PIFU pathway?
GP
We’ve had some conversations on who’s going to ‘own’ the patient, and how does it impact primary care or pharmacy?
Operational, trust-wide – Site 2.2
Healthcare access more generally is challenging. Primary care staff said that patients frequently reported not being able to contact hospital services, and secondary care staff reported the same for primary care.
Participants considered that better engagement with primary care about the management and implications for patients on PIFU would improve communication between services and continuity for patients on PIFU pathways, particularly those with long-term conditions. Some workshop participants perceived an ‘us and them’ mentality between primary and secondary care. Several consultants noted that they were experiencing an increase in referrals from primary care which was exacerbating their own workload, although it was felt this was partly a result of concerns accessing services following the pandemic and a perceived changing dynamic between generalist and specialist care (and perception of patients on this).
The problem with [the] modern NHS is need is so high and specialists focusing Aon niche area and role has become narrower – primary care is expected to run with everything else.
GP
I think patients find it difficult to see the GP. They’re often waiting for longer. By the time they get their GP appointments, they’re less likely to kind of go, ‘we’ll just watch this for a little while’ ... And I think a lot of the press about missed cancers and all these patients who’ve now got cancers and don’t know about it in the community has played into this [narrative of] ‘well I can’t be reassured until I’ve seen a specialist in hospital and I won’t take it just from my GP that actually breast pain is fine and not a sign of breast cancer’.
Clinical, specialty level – Site 2.1
Capacity, time and resources
More generally, staff (particularly from secondary care) are being asked to implement PIFU at the same time as other transformation projects, when headspace and capacity is stretched, and staff are fatigued. This has been due to several factors including the need to respond to winter pressures, recovery from COVID-19, competing targets and industrial action. This not only affected the administrative and IT support available, but also engagement with PIFU, with some questioning why they needed to prioritise PIFU over other things. More generally, it was felt that any transformation within outpatients was often deprioritised when other things happened. Even when system goals are mutually reinforcing, delivery requires time to set up new systems and approaches to make progress.
Anything that isn’t directly related to ED and patient flow isn’t a priority at the moment. [The] main issue with outpatients is it’s always one of the easiest things to drop because other things are more urgent.
Operational, trust-wide – Site 2.2
We’ve been in an operational quagmire for quite a long time now. On occasion we’ve had meetings to progress things but they’ve had to be stood down because there will be a major incident or other critical incident that draws in all clinicians – so non-clinical things often get stood down. This has happened a number of times over the last few months – it makes it really difficult to move things forward.
Outpatient, trust-wide – Site 2.1
In many sites, administrative and operational staff needed to implement PIFU on top of existing work, limiting the pace at which teams could progress. Administrative teams with a high turnover and reliance on locum or temporary staff may be under additional pressure.
COVID-19
Participants considered the impact of the COVID-19 pandemic on PIFU to be mixed. While there was some suggestion that it helped because people became more used to alternative approaches to care (such as remote care or having less frequent contact with the hospital), others were concerned that the ongoing impact it was having on waiting lists and access to care (particularly primary care) was affecting patients’ willingness to either be put on a PIFU pathway or contact services once they were on that pathway.
We have accumulated huge new patient follow-up backlog as a result of the pandemic. Introducing PIFU would have been done better in a vacuum without that additional burden.
Operational, trust-wide – Site 2.2
During COVID a lot of things have happened, people have become isolated, follow-ups haven’t happened. I think we’re still feeling the impact of trying to chase that.
Clinical, specialty level – Site 2.1
What do staff consider the opportunities and risks associated with Patient-Initiated Follow-Up?
We asked staff what they considered to be the greatest opportunities and risks associated with PIFU going forward. The various issues raised are laid out in detail in Appendix 6, Table 16. Opportunities included expanding the use of PIFU to other suitable clinical areas and making greater use of technology. They also saw PIFU as creating opportunities for reimagined ways of working within organisations and as well disposed to better collaboration and information sharing between organisations. However, concerns were raised around the implications for patient wellbeing and safety if they did not or could not get in touch with the service when needed. Some staff were also concerned that PIFU could disadvantage patients for whom it did not work as well or were not offered the opportunity of PIFU when it could work for them. In addition, there were worries that PIFU could increase demands on staff capacity, both within outpatient teams and elsewhere in the system.
Summary of findings
In the NHS, PIFU is most commonly being used in short-term pathways (e.g. physiotherapy or following surgery) although there are several examples where it is being used for people with long-term conditions. Several trusts were already using patient-initiated approaches to follow-up before December 2021, although it was unclear how this was being captured in national or local data. Models of PIFU vary widely across trusts and clinical area, with a significant degree of variation in the approach to patient selection, monitoring, and discharge.
Where PIFU had been implemented successfully, enablers included clinical engagement, supporting guidance and champions, dedicated staff capacity and flexible recording systems. Barriers to implementation included patients not being aware they were on PIFU, staff resistance, competing priorities and limited capacity to dedicate to PIFU, a lack of engagement with primary care and challenges amending EPR systems to record PIFU activity. More generally, factors relating to how the benefits of PIFU were perceived by staff and the nature of the condition were also relevant.
Measuring the impact of Patient-Initiated Follow-Up
Overview
What was already known
-
While there are potential benefits of PIFU to patients, clinicians and the NHS, there are relatively few studies exploring the impact of PIFU on patient outcomes and healthcare resources.
-
Previous studies have provided evidence that PIFU might result in fewer overall outpatient appointments, however results were mixed.
What this section adds
-
Increasing PIFU rates appear to be associated with less frequent outpatient attendance and rates of patient DNA, particularly within certain clinical specialties. However, within some specialties increased PIFU rates seem to be associated with more frequent visits.
-
This complements findings from interviews with staff and the workshop in that the variety of ways PIFU is implemented can lead to different impacts.
-
We found no practically significant association between PIFU rates and frequency of emergency department (ED) visits overall (results were statistically significant but of negligible effect size), but a small number of specialties appeared to have increasing PIFU rates associated with less-frequent ED visits.
-
Staff at study sites described limitations of their PIFU data for monitoring outcomes locally.
-
Existing data is not currently able to capture wider consequences, such as the impact on primary care.
-
These findings need to be interpreted with the understanding that HES does not currently record which patients are on PIFU pathways and uncertainty about the completeness of the available PIFU data.
Introduction
In this section we address RQ 3.2 for the PIFU evaluation:
-
What are the relevant outcomes for evaluating the impact of PIFU services? To what extent can we measure the different impacts of these services? How are the data being used to monitor the progress against these outcomes and how can the data be used?
Methods
We describe the general approach and the data we have analysed in Methods for the evaluation of Patient-Initiated Follow-Up. In this section we provide more detail of the methods we used.
Data sources
We obtained patient-level data on outpatient attendances and patients who DNAs from the HES OP data set and data for ED attendances from the ECDS. Reported use of PIFU was obtained from the P-EROC. P-EROC data are submitted by English NHS trusts each month and records numbers of patients moved or discharged to PIFU pathways over the month within each clinical treatment specialty. Patients who are on more than one PIFU pathway in different specialties will be counted multiple times, once for each specialty. Trusts also report whether their submissions are complete or partially complete. For some months, there may be no data submitted for a particular trust specialty even where submissions had previously been made or where outpatient data exist for that trust specialty within HES. Interpretation of such missing data is ambiguous and could either mean that nobody was put on to a PIFU pathway that month, or that patients were, but the data were not submitted.
Selection of National Health Service trusts
In the analyses we included any NHS trust that was reporting P-EROC data between September 2021 and March 2023. Where trusts merged over the period, we combined data before the mergers into what would be the new merged entity.
Measurement of Patient-Initiated Follow-Up activity
We defined the PIFU rate in an NHS trust and specialty as the number of people moved to and discharged to PIFU, divided by the number of attendances over the relevant period (different for each specific analysis). For sensitivity analysis we tested an alternative denominator as the number of unique patients attending the NHS trust within each specialty.
Completeness of reporting, missing data and other uncertainty
Following advice and testing of various options (see Appendix 7, Section 1 and Appendix 7, Table 17), we used data from NHS trusts reporting both partial and/or complete P-EROC submissions over the relevant periods of analysis. For the baseline analysis, any missing data (e.g. where HES reported activity within a specialty but no P-EROC data were reported) were assumed to be unknown and therefore not used (see Appendix 7, Section 2). Due to uncertainty as to how to interpret missing data, we analysed the data under different scenarios to see how robust the findings would be to different assumptions.
Two analytical approaches
We used two approaches to analyse the relationship between PIFU rates and outcomes. Firstly, a Poisson regression76 to analyse impact at NHS trust and specialty level. Secondly, a survival (or ‘time to next event’) analysis77 to investigate the relationship between the PIFU rate for each trust, specialty and month, and the time to the next outcome at an individual attendance level.
The reason for applying two analytical approaches was so that we could handle different assumptions that we needed to make due to the fact we were not able to identify those individuals on PIFU pathways in the data. The survival analysis could use individual patients as subjects, adjusting for individual patient factors. The Poisson regression approach used trust and specialty combinations as subjects and was easier to interpret, but relied on assumptions about the time between implementing PIFU and observing impact and the amount of PIFU-type activity that was already happening before data were being collected.
We analysed only the 30 specialties with highest PIFU volumes according to the P-EROC data (accounting for 88% of all PIFU recorded between September 2021 and March 2023 and around two-thirds of all outpatient attendances). Analyses were carried out for individual specialties separately and by pooling together multiple specialties.
Identifying outpatient attendances more likely to be affected by Patient-Initiated Follow-Up
We implemented an algorithm to classify outpatient attendances by function. The algorithm was originally developed by the NHS Strategy Unit,78 but we applied a modified approach following advice from NHSE (see Appendix 7, Section 4). Applying the classification, we identified so-called ‘structured review’ attendances. These attendances (which accounted for approximately half of all follow-ups) were characterised by not being any of the following types of attendance: treatment or diagnostic attendances, urgent, pre or post operation, or post-non-elective admissions. We hypothesised that these would be more likely to be impacted by PIFU.
Analysis of outcomes at National Health Service trust and specialty level (Poisson regression)
Outcomes
The outcomes we analysed with the Poisson regression analysis are shown in Table 10 (definitions in Appendix 7, Section 3). For single-specialty models, as baseline analyses, we have focused on follow-up attendances identified as structured review per patient. The results for other outcome measures are presented in Appendix 7, Section 2.
| Measure | Numerator (dependent variable) | Denominator (offset variable) |
|---|---|---|
| Outpatient attendances | Outpatient attendances each period by trust and specialty | Number of unique patients within each period, trust, and specialty |
| Outpatient attendances recorded as ‘follow-up’ | Outpatient attendances recorded in HES as ‘follow-up’ each period by trust and specialty | Number of unique patients within each period, trust, and specialty |
| Follow-up attendances identified as ‘structured review’ per patient | Outpatient attendances identified as ‘structured review’ each period by trust and specialty | Number of unique patients within each period, trust, and specialty |
| Proportion of outpatient attendances that are reported as ‘structured review’ | Outpatient attendances identified as ‘structured review’ each period by trust and specialty | Total outpatient attendances each period by trust and specialty |
| Proportion of missed appointments (DNAs) | Total reported DNAs each period by trust and specialty | Total outpatient attendances plus total DNAs |
Time periods
We identified two periods in the analysis over which we measured outcomes. The first period (September 2019 to February 2020) was selected to occur before the COVID-19 pandemic and for which we assumed only negligible use of PIFU. The later period (October 2022 to March 2023) was selected as a time when more patients were put on to PIFU pathways. We assumed that any impact of putting patients on to PIFU pathways would not be immediate, so assumed a 6-month lag between the measured PIFU rate and outcome. Because we assumed only negligible use of PIFU during the first period, the PIFU rate was only measured in relation to the second period and estimated between April and September 2022.
For sensitivity analysis we tested a further option without a lag and two annual periods: March 2019 to February 2020 (pre-COVID-19 period) and April 2022 to March 2023, the PIFU rate being measured over the second period.
Model design
For each specialty we used the same basic model. The dependent variable was the numerator for each outcome measure (see Table 10), the exposure variable was the denominator and independent variables were the time period and the PIFU rate in each period (always zero in the earlier period). Each NHS trust was included as a repeated measure. Further details on these models are provided in Appendix 7, Section 2.
For multispecialty models we also added specialty as a categorical independent variable.
Selection of specialties
As noted above, multispecialty included 30 specialties with highest-volume PIFU counts. For single-specialty Poisson regression models, to obtain sufficient sample sizes we further restricted specialties to those where data were reported for at least 30 NHS trusts.
Sensitivity analysis
Due to uncertainties and potential inconsistencies in data coding, we ran the models under a range of different assumptions to see what influence changes in these assumptions have on the results. As explained above, we tested different assumptions around missing data, reported completeness, time periods, and denominators for measuring PIFU rate.
Analysis of time to next event (survival analysis)
Time periods and data inclusion
We used outpatient and emergency care data from 1 September 2021 to 31 March 2023. We selected outpatient attendances in this period as index attendances, applying the following inclusion criteria:
-
We included 142 NHS trusts that had submitted P-EROC data.
-
We included activity from 30 treatment specialties with the highest total PIFU counts between September 2021 and March 2023.
-
We excluded activity where the person’s sex was recorded as being neither female nor male, and where age was not between 0 and 120.
-
We additionally only included activity where the NHS trust had explicitly submitted a PIFU count for the specific treatment specialty in the specific month of attendance (this could include counts of zero PIFU).
Where a person had multiple attendances in the period, all attendances were included as separate index attendances. Where a person had two or more attendances in 1 day, in the same clinical specialty and in the same trust, we allowed this to be represented only as a single index admission.
Outcomes
For every index attendance, we looked for subsequent activity for the same patient. For outpatient events this had to occur within the same treatment specialty and NHS trust, for emergency care this could be to any trust, for any reason.
The full set of outcomes were as follows (see Appendix 7, Sections 3 and 4 for definitions):
-
Next attended appointment (‘attendance’).
-
Next follow-up attendance.
-
Next structured review follow-up attendance.
-
Next not-attended appointment (specifically DNA).
-
Next ED visit.
Analytical approach
Using HES OP data, for each index attendance, we recorded the patient’s sex and age, attendance date, and treatment specialty. PIFU rates were calculated as noted above for every calendar month and attached to the month of the index attendance. We also attached another variable derived from the P-EROC data – whether the submission that month was complete or partial.
For each outcome we developed a proportional hazards regression model,77 to analyse the time to the next outcome event. We carried out multispecialty (i.e. all specialties together) and single-specialty models. Independent variables included age band interacted with sex, a two-monthly time period variable (based on attendance month), a flag to denote complete P-EROC submissions, treatment specialty (for multispecialty models only) and the PIFU rate. Models were stratified by NHS trust. For each outcome we used a specific approach to censoring (see Appendix 7, Section 5 for details).
Note that the survival analysis approach allowed us to test for subsequent events for as long as we had data. For index attendances in September 2021, we were able to scan 18 months after the attendance (to 31 March 2023), while for index attendances in February 2023 we had only a month’s worth of data to scan forward.
For single-specialty models we focused primarily on time to next structured review attendance, however, other outcomes were also analysed, with results presented in Appendix 7, Section 2.
Staff views on measuring the impact of Patient-Initiated Follow-Up
As part of the interviews with operational and clinical staff, we asked their views on the impact of PIFU on use of healthcare resources and patient outcomes, and their experiences of the data being collected on the use of PIFU. We triangulated findings from these interviews with our own understanding and analysis of the data.
Results
Table 11 gives a sense of the scale of outpatient activity and PIFU activity for all specialties, and for the 30 we have focused on. PIFU counts in the period represent < 2% of all attendances.
| Attendances | Follow-ups | Structured reviews | DNA | PIFU activity counts | |||||
|---|---|---|---|---|---|---|---|---|---|
| All specialties | 151,535,137 | 103,138,750 | (68.1%) | 49,011,423 | (32.3%) | 12,690,444 | (8.4%) | 2,126,678 | (1.4%) |
| Top 30 PIFU specialties | 97,501,400 | 65,781,477 | (67.5%) | 30,581,159 | (31.4%) | 8,600,951 | (8.8%) | 1,862,855 | (1.9%) |
When P-EROC data were first collected in September 2021, half of NHS trusts reported complete data and 85% complete or partial data. These proportions had increased to 58% and 98%, respectively, by March 2023 (Figure 14).
FIGURE 14.
Proportions of trusts reporting complete or partial data within P-EROC, by month.
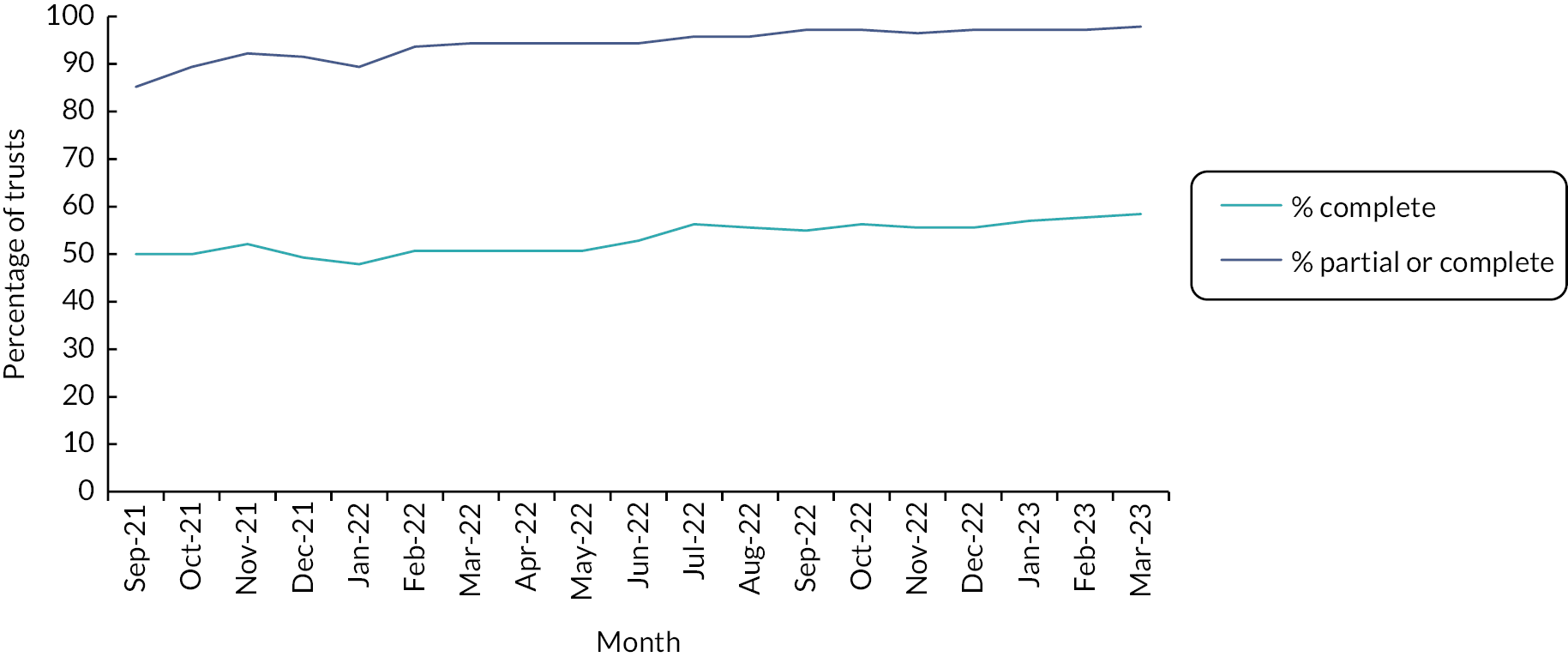
Multispecialty analyses
Analysis at National Health Service trust and specialty level (Poisson regression)
A total of 116 trusts had complete or partial data reported for each month of the period over which PIFU rate was measured (April–September 2022).
We found no statistically significant relationship between the PIFU rate and each measured outcome (Figure 15). These findings were consistent across a range of different assumptions tested in the sensitivity analysis (see Appendix 7, Tables 18–23).
FIGURE 15.
Relationship between the PIFU rate and outpatient activity across the 30 specialties with the highest volume of numbers moved/discharged to PIFU. Change in outcomes associated with a 5% increase in PIFU rate. Error bars show 95% confidence intervals.
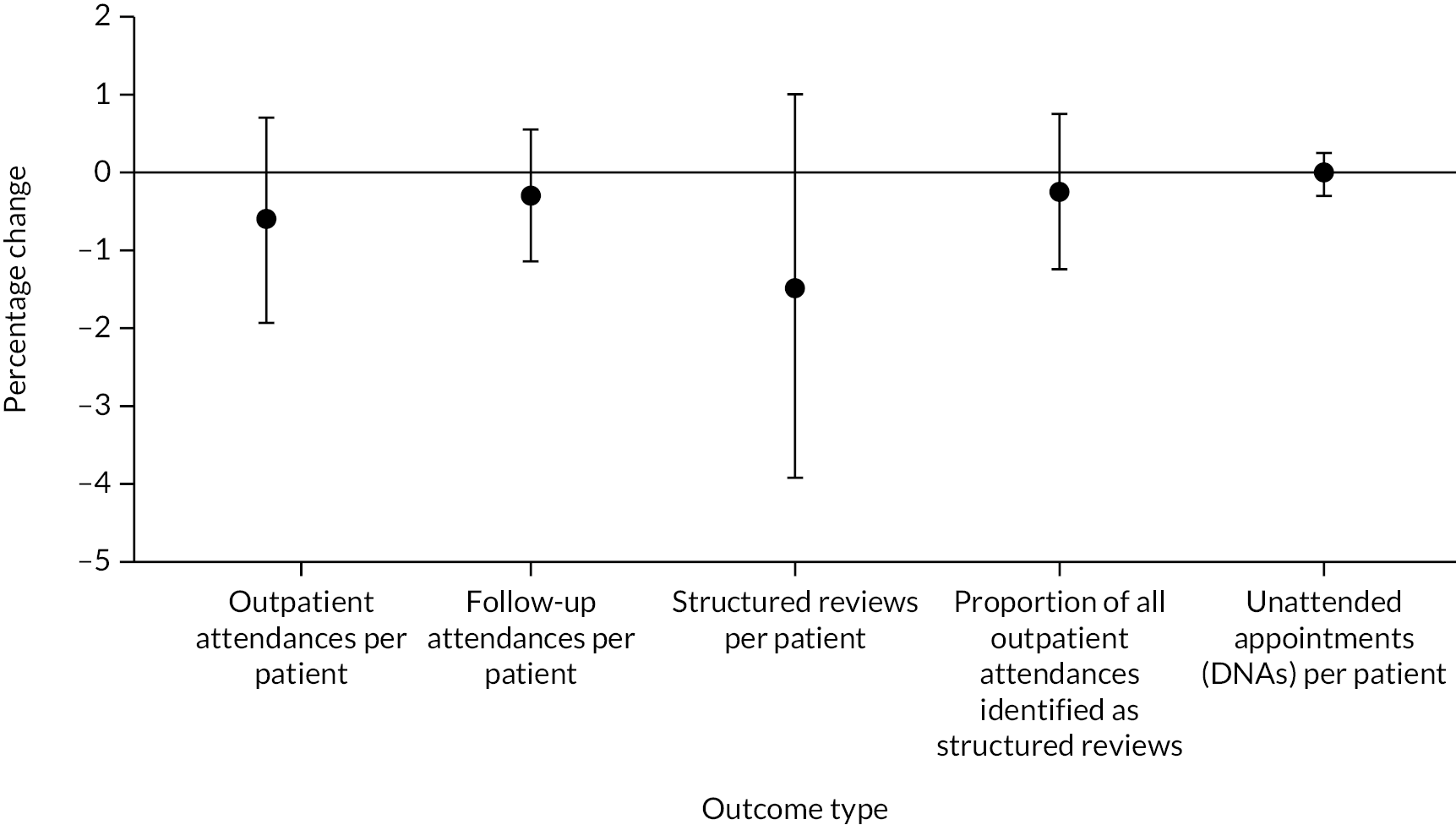
Analysis of time to next event (survival analysis)
We included 56.7 million attendances in the multispecialty models (details of the exclusions we have made are described in Appendix 7, Figure 27). Figure 16 shows adjusted hazard ratios (HRs) for five outcomes (four outpatient-related and one ED-related). The HRs are expressed for each five-percentage point increase in the PIFU rate.
FIGURE 16.
Adjusted HRs for five outcomes. HR expressed for each five-percentage point increase in PIFU rate, with 95% CIs. Note: HR = 1 (bold line) signifies no association between PIFU rate and time to next outcome. HR < 1 signifies that higher PIFU rates are associated with a longer time to next outcome (i.e. less frequent events).
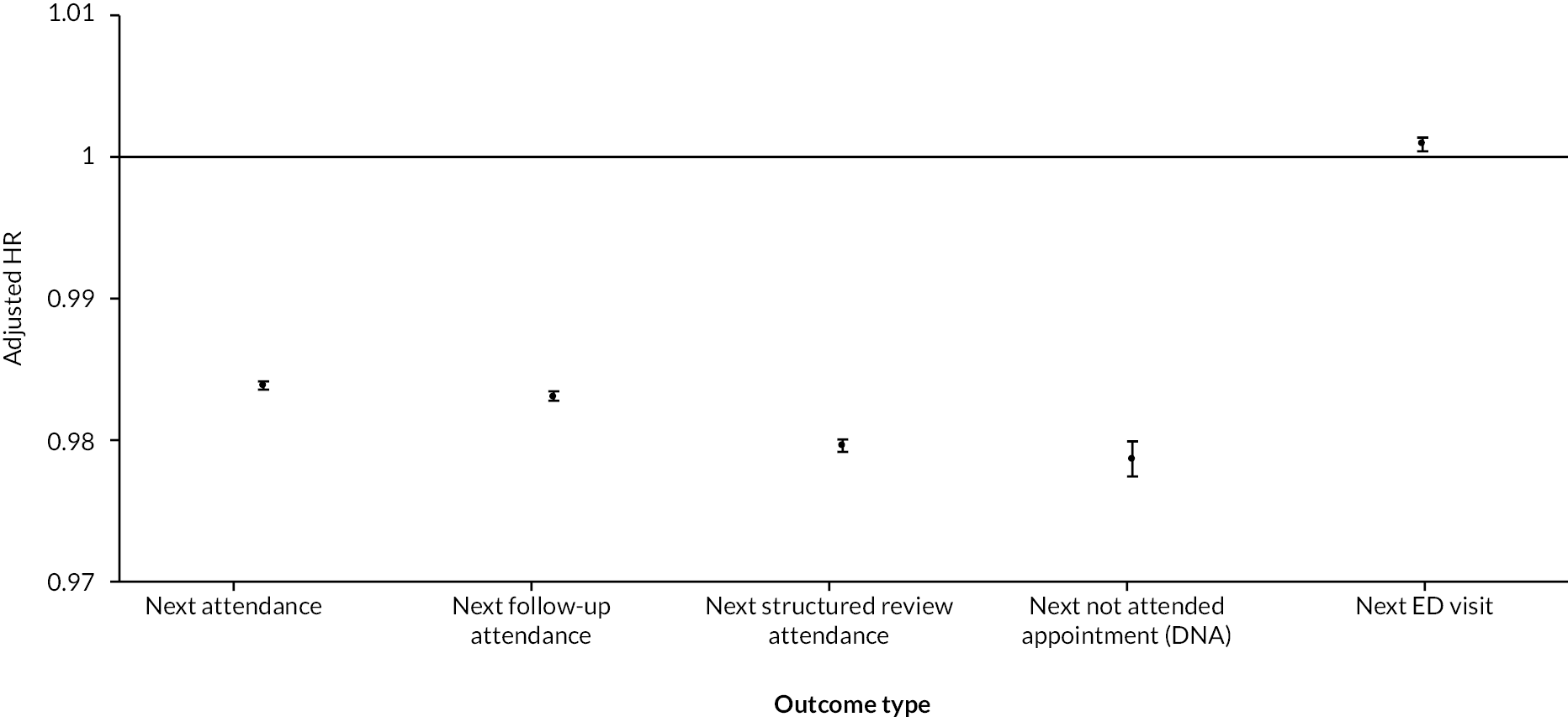
An example interpretation of the HRs follows. We found that for each five-percentage point increase in PIFU rate the adjusted HR for the structured review attendance outcome (‘time to next structured review’) was 0.980 [95% confidence interval (CI) 0.978 to 0.980]. This was statistically significantly below 1, suggesting an association between increasing PIFU rates and decreasing frequency of structured review attendances (or longer time to next structured review). However, the effect size was modest: for each five-percentage point increase in PIFU rate, the probability of a structured review on any given day (for individuals yet to have one) was 2% lower (1 – 0.980 = 0.020 = 2.0%). It is important to note that this effect is not cumulative over time. However, given that this is impact measured over the whole outpatient population, this may correspond to a larger effect on those patients on PIFU pathways.
All four outpatient outcomes showed statistically significant results on the same scale – with HRs between 0.984 (next attendance) to 0.979 (next not attended/DNA).
The adjusted HR for next ED visit was 1.001 (95% CI 1.000 to 1.001), statistically significantly greater than 1, but in practical terms only marginally so.
Single-specialty analyses
Of the top 30 specialties by volume of PIFU activity, one was only reported at a single trust and therefore not included in any of the single-specialty analyses.
Analysis at National Health Service trust and specialty level (Poisson regression)
Fifteen of the 29 specialties had data reported by 30 or more NHS trusts and therefore were included in our single-specialty Poisson regression models carried out at trust and specialty level. (Numbers of trusts that were included for each specialty are shown in Table 12.) As illustrated in Figure 17 (left-hand side), 10 out of 15 specialties reported a percentage reduction in structured reviews per patient for every 5% increase in PIFU rate. Of these, as individual statistical tests, the relationship is significant for Breast Surgery (18.7% lower attendances for every 5% increase in PIFU rate, 95% CI 2.4% to 32.3%; p = 0.026) and Ear, Nose and Throat (ENT) (18.9% lower attendances for every 5% increase in PIFU rate, 95% CI 5.7% to 30.2%; p = 0.006). However, neither of these results become statistically significant after adjusting for multiple testing (using the Bonferroni correction – revised p-value threshold = 0.003).
FIGURE 17.
Structured review attendances – model results for individual specialties. Two model variants: (a) Poisson regression model (showing percentage change in attendances per patient) and (b) survival model (showing adjusted HRs, for subsequent events), both per five-percentage point increase in PIFU rate. Ninety-five per cent CIs included. Specialties are ordered by decreasing volume of PIFU. For survival model * denote statistically significant results, having adjusted for multiple testing.
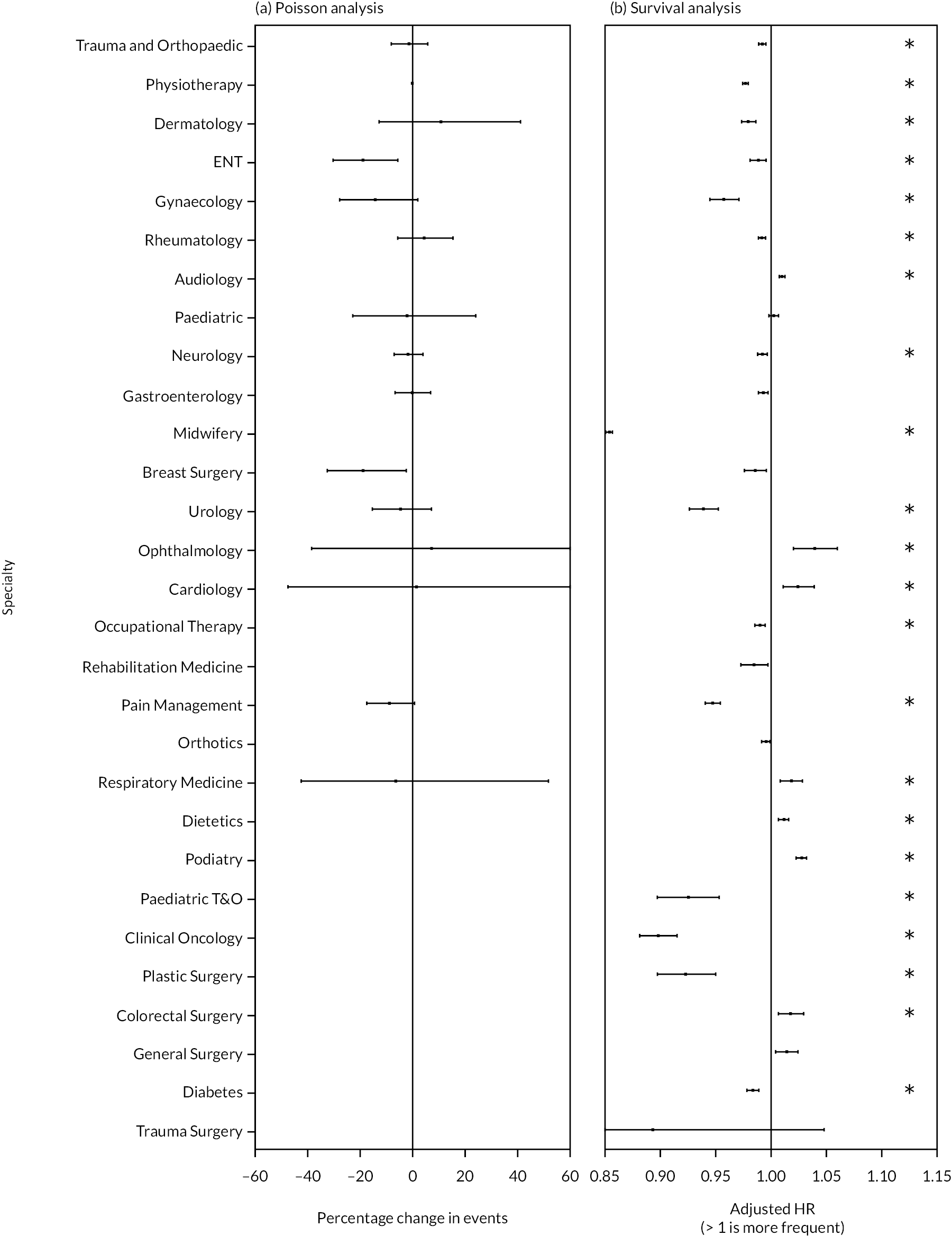
| Specialty | Number of trusts |
|---|---|
| Urology | 45 |
| Breast Surgery | 38 |
| Trauma and Orthopaedics | 69 |
| ENT | 58 |
| Ophthalmology | 39 |
| Pain Management | 36 |
| Gastroenterology | 49 |
| Cardiology | 44 |
| Dermatology | 52 |
| Respiratory Medicine | 44 |
| Neurology | 40 |
| Rheumatology | 55 |
| Paediatric | 42 |
| Gynaecology | 63 |
| Physiotherapy | 46 |
With regard to other outcomes, Breast Surgery and ENT did not report a significant reduction in total outpatient attendances, follow-ups or unattended appointments. However, for Urology there was a significant reduction in overall attendances per patient (−4.2%, 95% CI −6.8% to −1.5%; p = 0.002), follow-ups per patient (−3.4%, 95% CI −5.3% to −1.6%; p < 0.001) and DNAs (−15.4%, 95% CI −24.2% to −5.6%; p = 0.003). We found no significant relationship in Urology with structured reviews per patient or proportions of attendances that were structured reviews. For Respiratory Medicine there was also a significant reduction in overall attendances per patient (−15.4%, 95% CI −23.8% to −6.1%; p = 0.002). Further details are described in Appendix 7, Section 2.
With the sensitivity analysis, under different assumptions regarding the denominator to use for measuring PIFU rates and the lag between PIFU rates and outcomes, results were similar. Using assumptions that treat missing PIFU data as no PIFU, more specialties became eligible for inclusion within the analysis. Under these assumptions, we found significant reductions in structured reviews associated with increased PIFU use in Paediatric Trauma and Orthopaedic, significant reductions in DNAs within Dermatology and Midwifery, and significant increases in DNAs within Rehabilitation Medicine, Podiatry and Clinical Oncology (see Appendix 7, Tables 18–23).
Analysis of time to next event (survival analysis)
We were able to analyse single-specialty models for 29 specialties using the same data as for the multi-specialty model (56.7 million attendances in total). Figure 17 also shows adjusted HRs for the time to next structured review attendance by specialty (right-hand side).
After adjusting for multiple testing (revised p-value threshold of 0.0017), of the 29 specialties, 15 had statistically significant results with adjusted HR < 1 signifying a longer time to next structured review (i.e. less frequent attendances).
However, the effect sizes were generally modest. For the two largest PIFU specialties Trauma and Orthopaedic and Physiotherapy, the adjusted HRs were 0.992 (95% CI 0.989 to 0.995) and 0.977 (95% CI 0.975 to 0.979) respectively. In the former, each five-percentage point increase in the PIFU rate was associated with a reduction in the probability of a structured review on any given day of 0.8%.
The effect size was largest for Midwifery attendances, where the adjusted HR was 0.854 (95% CI 0.851 to 0.857; each five-percentage point increase in PIFU was associated with reduction in the probability of a structured review attendance by nearly 15%). Gynaecology, Urology and Pain Management were specialties with adjusted HRs of about 0.95 (a 5% reduction in the probability of a structured review for every five-percentage point increase in PIFU rate).
Some specialties (7 out of 29, having adjusted for multiple testing) appeared to show more frequent structured review attendances associated with increasing PIFU rates. This was the case in, for example, Ophthalmology (adjusted HR 1.039, 95% CIs 1.020 to 1.059) and Cardiology (adjusted HR 1.024, 95% CIs 1.011 to 1.038).
Regarding other outcomes, findings for time to next attendance and time to next follow-up were similar to those described for structured reviews, although with slightly fewer specialty results reaching levels of statistical significance. Time to next DNA results were less likely to be statistically significant. Only 4 specialties of 29 (Trauma and Orthopaedics, Physiotherapy, Midwifery and Pain Management) had adjusted HRs < 1 (with p-value < 0.0017), and a single specialty – Podiatry – had HR > 1 (so more frequent DNAs associated with increasing PIFU rates). Time to next ED visits results were also significant for four specialties (Trauma and Orthopaedics, Gynaecology, Dietetics and Clinical Oncology) with increasing PIFU rates associated with less frequent ED visits. Further details are shown in Appendix 7, Figures 28 and 29.
Staff views on measuring the impact of Patient-Initiated Follow-Up
In the interviews and at the workshop, staff highlighted limitations and challenges with quantitative data. A general view was that there was a lack of data, with most NHS trusts focusing on what is needed for the P-EROC collection. Data availability was also dependent on the capacity of the trusts’ EPR system and competing internal priorities. We heard examples where teams aspired to collect more data but strains on the service meant all capacity was diverted towards meeting demand. Concerns about data quality were raised by several interviewees, including instances of misreporting (often exacerbated by complex recording processes).
When asked, most staff did not have a clear understanding of the impact that PIFU was having (either in their service or across the trust as a whole) and this should be considered within the context that for most trusts and specialties fewer than 10% of outpatient appointments result in someone either moved or discharged to PIFU (see How is Patient-Initiated Follow-Up being implemented?). Most staff assumed PIFU would result in a reduction in outpatient attendances and DNAs, and some mentioned individual specialties or services that had undertaken small-scale audits of impact with positive effects, including a podiatry service in one of the study sites. Where a service was already using open appointments, it was difficult to observe what additional impact PIFU was making.
In terms of service capacity, while some staff noted that using PIFU had resulted in them doing fewer follow-up appointments, it was difficult to identify any changes in overall volumes of appointments given the pressures from other parts of the system (such as the increase in new referrals). Some staff mentioned that if PIFU was being used in instances where patients could be safely discharged, this could have an adverse impact on capacity but would be difficult to isolate in the data. We also learnt that the capacity impact of reductions in outpatient attendances could be partly offset by increased use of telephone conversations with nurses or non-clinical staff.
Some staff had concerns that unintended consequences could occur without being adequately understood, such as whether demand was merely being shifted to other parts of the system, such as general practice and ED.
It hasn’t released anything necessarily, but I think it’s definitely helped patients not having to keep coming back to the hospital all the time.
Administrative, specialty level – Site 2.2
PIFU certainly hasn’t helped our backlog yet in terms of outstanding outpatient appointments. I think it will be difficult to measure the impact of PIFU on system because there are so many variables, e.g. referral rates, Advice and Guidance rates, discharge rate, long-term follow-up rate, etc. that will affect the number of patients on the waiting list or the amount of appointments that are happening. Things might go up and down, but it will be hard to say what’s being driven by PIFU.
Operational, trust-wide– Site 2.2
Sometimes like comparing apples with pears – before PIFU took off, lots of people called it open appointments – come back whenever you like (no documentation/who to call) – when started introducing it across [the region] some people were unclear how to differentiate with an open appointment.
Clinical, specialty level – Site 2.2
Discussion
Introduction
In this section we discuss the key findings relating to the measuring impact of PIFU and strengths and limitations of the approaches we have used. Wider implications are discussed in Chapter 5.
Key findings
We assessed the impact of PIFU across 30 specialties with highest volumes of PIFU patients (accounting for nearly 9 in 10 of total PIFU volumes and two-thirds of all outpatient attendances). We found statistically significant associations between increased PIFU rates and a lower frequency of outpatient attendances and DNAs when analysed using survival analysis at an individual attendance level. However, we found no correspondingly significant reductions in volumes per patient when we used trust specialties as the units of analysis, and this result was consistent across a range of different modelling assumptions. We found no practically significant impact on the frequency of ED attendances overall (increased PIFU rates were statistically significantly associated with higher frequency of visits – but the scale of the effect was negligible).
Within specialties, using survival analysis, and restricting the analyses to follow-up attendances that were more likely to be affected by PIFU, we found significant reductions in attendance frequencies for 15 specialties, and significant increases for 7. Fewer specialties showed significant findings for DNA rates and four showed significant reductions in ED attendance. Again, however, using trust specialties as the unit of analysis, we found no statistically significant relationship between PIFU use and these types of follow-up attendance under the baseline assumptions. However, we found a significant reduction in DNAs among Urology patients. Using different measures of attendance and different assumptions about handling missing data a mix of other specialties did show significant associations. However, it is unclear how reliable the results are when we assume missing PIFU data reflects no PIFU activity.
Where we found significant associations in the multispecialty models, the implied effect was small. For example, a five-percentage point increase in PIFU rate was associated with a 2% lower probability of having a structured review on any given day. However, such small observed impacts may reflect that most patients included in the analysis would not have been on PIFU pathways and mask the impact at the individual patient level.
The survival analysis model – analysing times between attendances – estimates outcomes at an individual attendance level. As such, it has narrower CIs, and using this approach we may be seeing impacts that are not discernible with the trust specialty-level Poisson regression models.
Staff at study sites and attending the workshop believed PIFU should be having an impact on patient attendance and DNAs but described limitations of their electronic systems for monitoring their outcomes. In some specialties they did not expect to see much change given that they had been operating an open-appointment system for some years. Some consequences are hard to measure, such as increased workload for other hospital staff contacting patients by telephone and the impact on the wider system, such as the impact on primary care.
Strengths
To help mitigate some of the uncertainties associated with the data and its interpretation we applied two different modelling approaches and tested the consequences of making different assumptions with sensitivity analysis. The two approaches complemented each other and, as anticipated, we have seen more precise effects with the analysis at the individual patient level.
Many types of outpatient attendance would be unaffected by PIFU, for example, pre-operative check-ups, visits for treatment or diagnostic purposes. We have therefore applied an algorithm to identify types of attendance that are more likely to be affected and thus giving us a greater chance of detecting impacts that may exist.
Limitations
We have grouped patients into specialties because that is how the P-EROC data are reported. This means we have not been able to focus on patients with particular conditions within specialties for whom practice may be varied.
We have had to make several assumptions about how to interpret missing data, the relative value of ‘complete’ and ‘partially complete’ data, lags between starting a PIFU pathway and its impact and how to measure the use of PIFU within a trust specialty. Although we have mitigated the resulting uncertainty by running different scenarios and adjusting for some of these factors where possible, variable data quality and reporting consistency may still have an impact.
The PIFU rate was measured as a ratio of the number of people moved or discharged to PIFU divided by the number of outpatient attendances over the same period. In the sensitivity analysis we considered a different definition, replacing outpatient attendances with unique patients as a denominator. These values estimate the proportion of outpatient attendances that led to a transfer to a PIFU pathway and are not necessarily precise measures of the extent of PIFU activity. For example, in some trusts, decisions to transfer patients to PIFU pathways do not always happen during an outpatient appointment, and some of these appointments might be for patients already on PIFU pathways.
There are also other issues relating to the data which could have a bearing on the findings:
-
Open appointments have been operating in some trust specialties, such as Physiotherapy, for several years. This would affect the analyses of these specialties using the Poisson models with the possibility of masking any potential impact of PIFU. However, this would not affect the results from the survival analysis.
-
We use aggregated data on numbers of patients put on to PIFU pathways. Given that most patients for whom we are observing outcomes are not on PIFU pathways, there is a risk that some true effects are undetected.
-
Discharges to PIFU could include patients who in other circumstances may have been discharged and taken off a care pathway. Thus, the group of patients on PIFU may be in lesser need of clinical time or input than the cohort of patients that PIFU is intended for who have been actively placed on a care pathway.
-
We have linked P-EROC and HES data by specialty and assumed that specialty coding is consistent between the two data sets.
What is the impact of Patient-Initiated Follow-Up on health inequalities and how is this being measured?
Overview
What was already known
-
There is limited evidence on how impact varies by patient group.
-
Two studies in the review found that factors including patient activation (such as the patient’s knowledge, skills and confidence in self-managing their condition), age, gender and clinical factors were not related to the number of self-referrals or treatment received.
What this section adds
-
There was limited understanding of the impact of PIFU on different patient groups although staff in the research recognised this as a concern. Digital exclusion, demographic characteristics, socioeconomic status and patient characteristics were all thought to be relevant to how patients engage with, or are impacted by, PIFU.
-
Local evaluation of outcomes and inequalities is difficult in many trusts due to the problems of reporting PIFU activity on the local electronic PAS. However, data from one site report 17% of children put on to PIFU pathways have a return visit within 1 year, compared to 11% of adults.
-
It is not clear how much observed differences by ethnic group are due to differences factors, such as age and clinical condition.
Introduction
In this section we present findings in addressing RQ 3.3:
-
What strategies have been adopted to address potential inequalities along the PIFU pathway? What data are being collected to understand potential disparities? Is there variation in how different patient populations access and engage with PIFU?
Methods
Data are drawn from interviews with clinical and operational staff, four interviews with patients and a workshop with staff. Further details on the methods can be found in Methods for the evaluation of Patient-Initiated Follow-Up.
From one acute hospital trust, different to the ones where we conducted interviews, we acquired aggregated data on numbers of patients put on to PIFU pathways and, of those, the number who made a return outpatient visit within 1 year of being put on to the PIFU pathway. These were broken down separately by age band and ethnicity. We then analysed these data to observe if there were any differences in return rates by age and ethnicity.
How are services measuring the impact of Patient-Initiated Follow-Up on different groups of patients?
Most staff interviewees in the study reported limited understanding on the impact of PIFU on different groups of patients and limited insight into how (if at all) this was being measured within their service. However, they did consider that there was a risk that PIFU would have an unequal impact on different groups (see How is Patient-Initiated Follow-Up being implemented? on implementation for further discussion of the risks of PIFU). One ICB lead noted that PIFU had been considered within broad equality impact assessments on outpatient care while others noted that it had not come up at all in these contexts. Although the ICB had produced guidance for trusts on this, there was concern about how consistently this was being applied in practice. There was significant concern that the national attention on targets (in particular, reducing follow-up appointments by 25%) was overshadowing attempts to address the risks around health inequalities.
There’s often a disparity between what is pushed in order to recover from national levels towards what’s right and best for the patient. And they sometimes clash, and sometimes this push is causing harm to these people.
GP/Operational – ICB
At a trust level, although most interviewees recognised that addressing health inequalities as part of the recovery from COVID-19 more generally is an NHSE priority,79 they also recognised the need for a greater understanding of the particular impact of PIFU for people from different backgrounds. The current lack of alignment of demographic data with PIFU data seems to be a barrier to some trusts doing this effectively (see Measuring the impact of Patient-Initiated Follow-Up). Some service leads – especially those working in more rural trusts – felt that their local area was not particularly diverse so their understanding of how PIFU might impact people with different protected characteristics was even more limited.
I don’t think we’ve identified anything related to PIFU, but we certainly have identified issues related to health inequity within outpatient settings. But it’s baby steps at the moment. We need to do so much more.
Operational, trust-wide – Site 2.2
We heard limited examples of specific actions taken to address or mitigate against potential inequalities in the sites we looked at, although, as noted, understanding of this issue is limited. Some felt that a person who was at risk of not engaging with PIFU would not be put on to the pathway in the first place and therefore the issue was less likely to arise. In terms of supporting people who may otherwise struggle to engage with PIFU (such as older people or people with a learning disability), staff pointed to the valuable role of formal and informal carers.
If you’ve got a patient that maybe lives in a care home then a carer would come with them and, [ ... ] is it right to be dragging somebody who’s 88 back to the hospital? No, it’s not appropriate [...] ... Very often, some of these people don’t have a next of kin, so as long as we make it very clear to the care home that [...] if a woman started to bleed again, [...] this is what you need to do [...]. So yeah, we are very careful in the instructions that we give to carers or nursing staff that are looking after patients and care homes.
Administrative, specialty level – Site 2.2
What is the perceived impact of Patient-Initiated Follow-Up on health inequalities?
Although there is limited information on the impact of PIFU on different patient groups, staff described how they thought health inequalities could arise. Although staff interviewed considered that it is important to not make assumptions about someone’s engagement just because of certain characteristics, there was also concern that staff may not be putting people on to PIFU who may benefit. The opposite was also raised, mostly in the context of the concern around using PIFU as part of a waiting list validation exercise, where decisions may be made without proper interaction with patients. This was identified as being particularly likely in areas where the overt focus of PIFU was reducing activity, rather than on supporting patients.
Clinicians shouldn’t make assumptions that because [they] have an older patient they won’t use PIFU or that a younger patient necessarily will use PIFU.
Operational, trust-wide – Site 1.1
When asked about health inequalities, staff delivering services often referred to people of different ages or people who may be digitally excluded. However, they also reflected that because there were alternative options available such as contacting the service by phone (and staff suggested that were patients really at risk they would not put on to PIFU anyway) they felt that this was less of an issue. Regarding digital exclusion, the extent to which this was currently a problem was considered limited given that there are usually non-digital ways to contact the services. As described previously (see How is Patient-Initiated Follow-Up being implemented?), the opportunity to use technology to support PIFU implementation (such as patient portals or apps) was noted by several staff who did acknowledge that this could have the potential to exclude people who may not feel confident using these tools.
In terms of health inequalities, [the] elderly population isn’t comfortable using electronic systems. But since telephone is one of the ways they can get in contact, they are more comfortable with it.
Clinical, specialty level – Site 2.1
Rather than focusing on demographic characteristics, interviewees referred to an individual’s motivation and ability or willingness to advocate for themselves (this was based on anecdotal experience rather than evidence and also is not necessarily specific to PIFU but more generally around engagement with services). Staff felt this was in part linked to socioeconomic status but also the local area and how accepting people are around how outpatient appointments have been previously managed. There was also recognition that people from some backgrounds (e.g. those from lower socioeconomic backgrounds) do not routinely engage with services as much, and this may be a particular concern for PIFU. This concern was raised particularly in the interviews with primary care staff.
If I’m really honest, does it close the gap [on health inequalities], probably not – it probably increases the gap actually because it’s the activated, it’s the motivated, it’s the well-educated who are more likely to navigate the system quickly and effectively.
GP
Interviewees from secondary care suggested that PIFU could be better for people who may, for whatever reason, be unable to engage with a more fixed appointment schedule. For example, people with ‘chaotic lifestyles’, which may affect their ability to engage with healthcare services more generally. PIFU therefore was not only seen as an opportunity for supporting their engagement in their own health care, but also a way to reduce missed appointments. However, there was concern that such people may be least likely to be put on to PIFU, or that they would be put on PIFU without proper discussion or engagement.
I have a gentleman who’s homeless. We put him on PIFU because every appointment we gave to him, he DNA’d. He’s not very good at sticking to set times for appointments, so by giving him the ability to phone when he needs and setting an appointment that will work for him, actually he’s attended.
Clinical, specialty level – Site 2.1
Impact on Patient-Initiated Follow-Up engagement by patient characteristics
For one study site, the proportions of patients on PIFU pathways across all specialties who make a return appointment within a year by ethnic group are shown in Figure 18 and by age group in Figure 19. On average, across all ethnicities, 12.9% of those placed on a PIFU pathway made an appointment within the following year. Variation among reported ethnicities ranged between 10.4% for the Bangladeshi population and 15.4% for people reported as mixed ethnicity. It is not clear from these data whether these results reflect underlying ethnic barriers to engaging with the service after being put on to a PIFU pathway. Differences could result due to different ages and case mixes among different ethnic groups.
FIGURE 18.
The proportion of patients on a PIFU pathway who make a return appointment within a year, by ethnicity. Data provided by one study site.
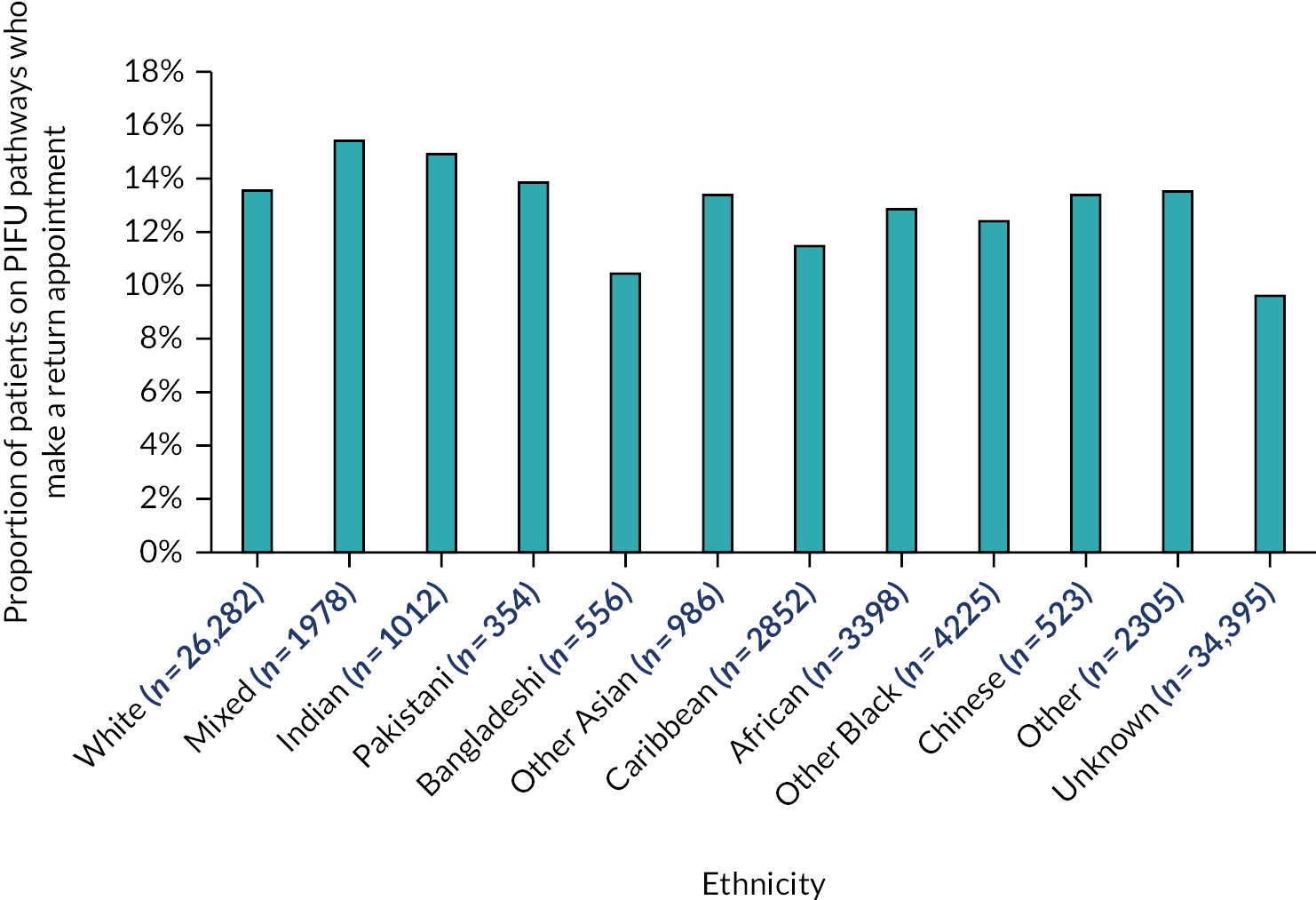
FIGURE 19.
The proportion of patients on a PIFU pathway who make a return appointment within a year, by age group. Data provided by one study site.
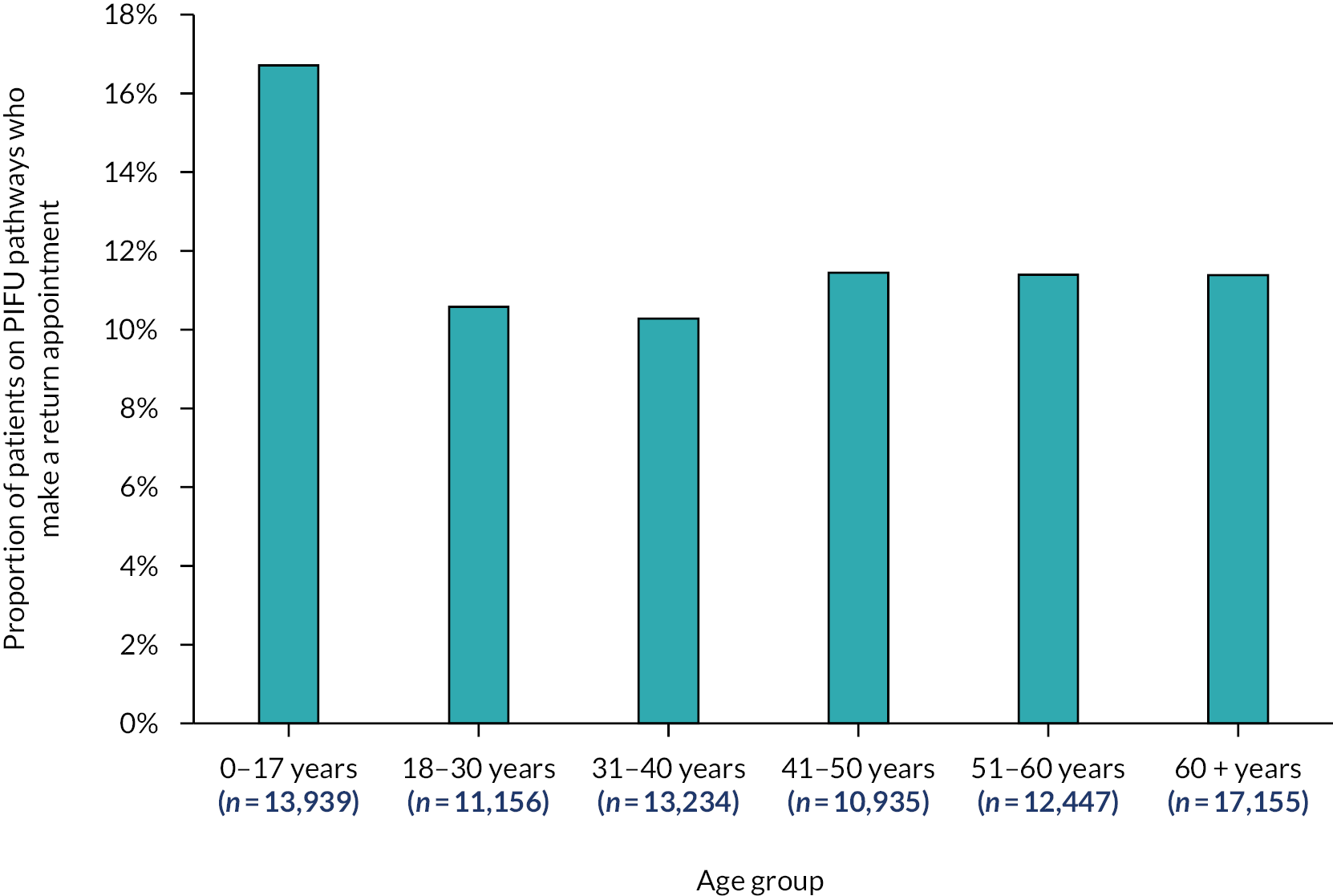
For children and adolescents between the ages of 0 and 17, 16.7% of those placed on a PIFU pathway scheduled a return appointment within a year. This compares with an average of 11% for those aged 18 and above. The distribution of return rates among adults lies within a 2% margin for all age groups. The lack of granular data for individuals aged 60 years and older makes it hard to comment on PIFU engagement for the oldest patients.
Summary of findings
There is limited understanding of the impact of PIFU on different patient groups although staff who participated in the research recognised this as a concern. Digital exclusion, demographic characteristics, socioeconomic status, and patient characteristics were all thought to be relevant to how patients engage with or are impacted by PIFU.
Data from one site indicate some differences in reattendance of patients on PIFU pathways within a year by ethnic group and across adult age groups. It is unclear whether observed variation by ethnic group reflects underlying ethnic barriers or other confounding factors. There are notably higher return rates for children than for adults.
However, these data come from only one trust, and we were not able to analyse multiple characteristics so caution should be made when assessing the generalisability of these findings.
Staff experiences
Overview
What was already known
-
Existing studies of PIFU presented little evidence related to staff experience.
-
Mixed evidence was found on the impact of PIFU on the volume of outpatient appointments. This volume could have an impact on workload for different staff groups.
-
A variety of factors affect the design of PIFU for use in specific contexts, which would lead to different experiences among the staff tasked with implementation.
What this section adds
-
The characteristics of the model of PIFU being used in a service (as outlined in How is Patient-Initiated Follow-Up being implemented?), as well as the extent to which it is a departure from previous practice, significantly impacts how staff experience PIFU.
-
The volume of patients on PIFU affects the extent to which any differences are felt by staff.
-
PIFU can entail significant changes to the roles of clinical staff including taking on significant new responsibilities. It also sometimes leads to the creation of new, PIFU-specific roles.
-
PIFU has the potential to increase staff workload, which is often caused by new administrative burdens and additional processes.
-
The scale of outpatient waiting lists means that, even if PIFU reduces follow-up outpatient attendances, staff are unlikely to see overall demands on their time decrease.
-
The extent to which staff are confident that their service is capable of executing PIFU as intended impacts levels of satisfaction and uncertainty surrounding the innovation.
-
Staff satisfaction with PIFU being introduced in their service is affected by whether they believe the main drivers behind its rollout are related to benefits for patients.
Introduction
In this section, we present evidence that addresses RQ 3.5:
-
What are staff experiences of delivering PIFU? What is the impact on staff satisfaction, workload, and capacity across different roles?
We describe:
-
Any changes to staff roles, such as the assumption of new responsibilities and moving away from historical responsibilities.
-
The variable ways in which different staff groups we spoke to perceived PIFU to have impacted their workloads.
-
Broader satisfaction with PIFU as applied to the areas participants worked in and compared to other models of outpatient care.
Staffing factors which were considered to affect PIFU implementation specifically are discussed in How is Patient-Initiated Follow-Up being implemented?.
Methods
Findings in this section draw primarily on semistructured interviews with clinical and operational staff across five case study sites. They are also informed by analysis of national and local site documentation and discussions at the workshop with NHS staff. Further details on methods can be found in Methods for the evaluation of Patient-Initiated Follow-Up.
When asked about ways in which PIFU had impacted their experience and roles, as well as that of their colleagues, participants provided a diverse set of reflections. Key influencing factors include the extent to which PIFU was implemented within their service, how far it was perceived to be a departure from existing practice, and the volume of patients on PIFU.
It’s not had much of an impact really – it’s what we do for all our patients all the time. Patients get in touch with concerns related to their gynae cancers.
Clinical, specialty level – Site 2.2
Impact of Patient-Initiated Follow-Up on roles
For some staff participants, implementing PIFU entailed distinct new approaches to their clinical practice. Aspects of PIFU, which are discussed in more detail in How is Patient-Initiated Follow-Up being implemented?, place demands on staff which may have not previously existed. One example is patient selection processes, which rely on the judgement of individual clinicians. The need to perform end-of-pathway clinical reviews could also substantially affect day-to-day roles, as could the necessity for more proactive forms of patient monitoring or safety-netting relative to previous practice. There was some concern that these additional activities had not been adequately considered within individual work plans. Administrative demands of PIFU could also impact the roles of clinical staff in terms of their interactions with administrators and EPR.
[Patient selection] might be outlined in the SOP but it would be done very subjectively [on a patient-by-patient basis].
Clinical, specialty level – Site 1.3
Those who are placed on a PIFU list, they might be monitored and certainly when it gets to the end of that PIFU period there should be a form of clinical review.
Administrative, specialty level – Site 1.2
Where PIFU-specific roles, such as nurse specialists (as discussed in How is Patient-Initiated Follow-Up being implemented?), had been instituted, it helped simplify the workload of the individual with the PIFU-specific role, while taking some pressure off colleagues. One nurse specialist interviewed had roles that straddled PIFU and other responsibilities, but the low volume of PIFU patients and the minimal changes in practice brought on by PIFU meant that they had not noticed significant changes in their day-to-day role.
Impact of Patient-Initiated Follow-Up on workload
Changes to workloads were more noticeable for staff in areas which had previously relied on fixed follow-up schedules for outpatients, or in areas where implementing PIFU increased administrative demands compared to previous, more rudimentary, ‘open appointment’ models. Another key variable was the volume of PIFU patients in a specialty or sub-specialty. Where this was very small, it was often difficult for staff working in those specialties to register any substantial impact upon their day-to-day work. Implementing PIFU had varying implications for different roles, which is why impact on each broad category of role is discussed separately.
As discussed in How is Patient-Initiated Follow-Up being implemented?, in many specialties, administrative staff were responsible for acting as intermediaries between PIFU patients and clinicians. This included receiving contacts and booking slots, sometimes with a degree of triage, as well as recording PIFU on EPRs. This exposed them to significant changes in workload. Often administrative teams had to absorb PIFU alongside their existing set of responsibilities, and tasks were especially onerous where trust EPRs were not well adapted to recording PIFU. Additionally, where communication from clinicians regarding placing patients on PIFU was unclear and incomplete, PIFU could be very demanding on administrative staff time.
. . . for non-clinical staff, I think it’s been a nightmare. Because they have to deal with all the outgoing patients, and which pathway and partial booking list [to place patients on]. And also, clinicians are not writing particularly clear letters.
Clinical/Operational, specialty level/region – Site 2.2
Hospital staff also mentioned a variety of experiences of PIFU. Those who managed very few PIFU patients saw little to no change in their workload as a result. We heard of a limited number of examples where PIFU eased some pressure on clinics because they could reduce the amount of time spent on follow-up appointments and dedicated more time to new patients. The impact of PIFU here varied according to the characteristics of services, as discussed in How is Patient-Initiated Follow-Up being implemented?. For example, those in services requiring patients to be seen within a short time frame, such as Oncology, were more likely to see PIFU free up staff time that would not be immediately reassigned to other patients. This is because these services were considered to have greater flexibility to adapt their clinics. However, we heard little from most staff to suggest that their overall workload had been reduced by PIFU, as any capacity which may have been freed up by reducing the number of follow-up appointments would immediately be filled by new patients or those on waiting lists.
Several participants raised the concern that PIFU would mean that more appointments were spent with patients with more complex needs. More high-intensity appointments could in turn increase the overall intensity of a clinician’s workday, and the participants who raised the issue were concerned about the implications this may have for staff stress and burnout. This was because PIFU would reduce the number of routine ‘check-ups’. Additionally, in some cases, PIFU was considered to increase workload because of the amount of time required to do clinical reviews and the capacity required in PIFU clinics to manage requests.
Obviously, seeing a new patient is a lot more time consuming and difficult than seeing a follow-up patient. And so you used to have clinics where every third or fourth patient was oh, hello, nice to see you. How are you? I’m really good. I feel much better. Great. Thank you. Nice to see you. Bye. That doesn’t exist anymore in my clinic.
Clinical, specialty level – Site 2.2
The more patients are placed on to PIFU, the higher proportion of patients will have greater acuity (because those being seen have a particular reason for initiating that appointment, vs. a fixed follow-up), meaning appointments will need to be longer on average, even if there are fewer appointments overall – NHSE/commissioners need to understand this. The patients on PIFU are not going to be the ‘well’ – we need more time. I know from colleagues PIFU patients may take 20 minutes rather than the standard 15, they’re just a higher demand and you know they’re flaring.
Clinical, specialty level – Site 2.1
Although a small sample, none of the GPs we spoke to noticed any reduction in their workload due to PIFU and raised concerns that PIFU may result in a transfer of workload to general practice if patients were struggling to contact outpatient departments. One reported first-hand experience of where patients who had failed to get through to a PIFU service resorted to visiting their practice, which raised concerns about the possible impact (or lack of positive impact) of PIFU on GP workload, particularly if a PIFU service is not accessible or responsive.
Some hospital staff reported that their workload had increased because of PIFU, particularly as it had become more embedded within the service. There were instances raised where high volumes of PIFU could lead to increased demand, thus potentially increasing rather than decreasing follow-up activity. These were more prevalent in long-term pathways, where patients may have a diverse range of reasons to contact the service over a long period of time and require clinical review at the end of their pathway. This contrasts with short-term pathways, such as post-operative, ‘just in case’ pathways, where most patients were acknowledged to recover without issue. The impression that PIFU might increase workload was cited as a key driver of resistance from staff.
PIFU very much built into the role now – [we’ve] increased [the] team further to manage that with addition to two additional consultants doing more varied surgeries which need more space in the PIFU clinic.
Clinical, specialty level – Site 2.1
[Clinical review] almost defeats the object as it puts an additional appointment in for every individual who is put on PIFU. The criticism from some people is well, you’re still forcing me to do further clinical admin. Clinical, specialty level – Site 1.2
This raised a concern that this could intensify if more patients are placed on PIFU in the future. While low volumes of PIFU patients could be managed without major restructuring of how care is delivered in their specialty, high volumes of PIFU would necessitate a more structured approach, incorporating elements, such as safety-netting and revised methods of delivering appointments.
If we start moving thousands of patients on to PIFU that might create a bigger issue. But in terms of admin workload, especially if you’re having to generate questionnaires and monitor patients closely – it could have an impact and we don’t actually know what that looks like yet. But at the moment, there haven’t been any significant changes.
Operational, trust-wide – Site 2.1
Impact of Patient-Initiated Follow-Up on staff satisfaction
We asked staff to provide their reflections on PIFU as an intervention (staff perceptions of the aims and objectives of PIFU are described in detail in How is Patient-Initiated Follow-Up being implemented?).
Several participants in the research were open advocates of PIFU, believing it to be an overwhelmingly beneficial intervention. One key reason for this was that PIFU gave them more confidence to address patient needs as well as possible, by giving those who needed to be seen most a simple route back into the service, while causing less stress upon capacity than the use of fixed follow-up pathways. This was also mentioned as a source of reassurance because it was seen to increase the overall visibility of patients with the most acute need.
Now we have the option of going, ‘if there’s any problems then you can contact us and we can get you an appointment however it’s needed be it face-to-face or telephone.’ So I suppose that the biggest impact for us is knowing that there’s a different way people can contact us if needed.
Clinical, specialty level – Site 2.1
By contrast, staff who did not have confidence in PIFU functioning as intended expressed anxieties that it could be making some patients less able or likely to be seen when needed, to the detriment of their safety. Levels of confidence varied by team, although numerous interviewees reported a range of confidence levels towards PIFU within their own teams.
Additionally, some staff, with various roles, viewed PIFU positively as a welcome step forward, away from antiquated and paternalistic models of care. Many patients on short-term pathways, such as those who had undergone common procedures, did not need any follow-up appointments (or only a small number of people did). This suggested to some that fixed follow-up pathways were less efficient in terms of the time and effort of both staff and patients. PIFU, meanwhile, could account for the needs of those who did experience issues without expending resources on those who did not. Previously mentioned themes, such as patient empowerment, were also raised as contributors to positive attitudes towards PIFU among staff, with the innovation appearing to align with contemporary shifts towards more inclusive models of care. Conversely, attachment to habitual practices was often cited by staff reflecting on colleagues who were more resistant to PIFU.
I’ve been in the NHS a long time and you would find that patients would be brought back every six months, every 12 months, you know it’s just ridiculous amounts of follow-ups that were happening. Because it seemed to be historical, and whoever saw the patient last then did exactly the same thing as what the previous clinician did, and also patients very often just liked the security of having that follow-up appointment in hospital. Well, now we all know that that’s not necessary.
Administrative, specialty level – Site 2.2
Staff satisfaction with the concept of PIFU often varied depending upon what they believed were the primary aims of adopting the intervention in their specialty. As mentioned previously in How is Patient-Initiated Follow-Up being implemented?, staff perceived distinct purposes at a service level and at a staff or patient level, with a framing of PIFU centred upon improving patient outcomes far better received than one based around targets and service recovery. Some participants reported target fatigue and cynicism towards initiatives passed down from the ‘centre’, with concern expressed that PIFU rollout aimed at maximising volume of uptake would result in placing patients on PIFU for whom it would not lead to an improvement in outcomes.
Currently I think they feel it’s something that they [staff] are kind of being forced to do, especially with the ICB asking how many patients are on PIFU pathways all the time. So I guess it’s really about, actually, can we turn the conversation around and not say how many people, but who would this benefit, who would be better off?
Clinical/Operational, specialty-wide/trust level – Site 2.1
Summary of findings
Staff experience of PIFU is not uniform, being heavily influenced both by the specific characteristics of the model of PIFU being employed in their service, as well as by what practice looked like before PIFU. The volume of PIFU patients in a service affects the extent to which any differences from previous practice are felt. Where it does entail significant changes in practice, PIFU can have a notable impact on the roles and responsibilities of staff and can sometimes also lead to the creation of entirely new roles to support implementation. Different staff roles, even within the same specialty, can experience different impacts on their workload, such as high administrative burdens falling on certain staff. There were some concerns about PIFU increasing the average intensity of appointments or even the overall volume of follow-ups. Staff attitudes towards PIFU beyond its impact on their roles specifically were affected by several factors. These included their level of confidence that their service could implement it as intended, their views on existing (pre-PIFU) practices, and their perceptions of the motivations behind its rollout.
What is the impact of Patient-Initiated Follow-Up on patient engagement and experience?
Overview
What was already known
-
Of the 13 studies in the evidence review (see Rapid Patient-Initiated Follow-Up scoping review) that looked at patient experience and quality of life, 5 found that PIFU had a statistically significant beneficial impact for patients although this varied by clinical condition.
What this section adds
-
Although we were able to interview only four patients, those we spoke to were positive about their experiences and liked having the option to contact a specialist when they needed to.
-
Across the case study sites, limited activity had taken place to capture formal feedback from patients, although staff reported that patients were positive about PIFU as an approach and the support they had received.
-
Reasons for patients declining PIFU included a preference for regular interaction, a desire to stick to their routine and a concern that they would be unable to get an appointment if they needed one.
-
Staff were unclear if patients were contacting the services when they needed to. However, when they did, this could be for several reasons not all of which required a face-to-face appointment with a consultant.
-
Enablers to patient engagement include clear routes to getting support, communication and ensuring patients do not feel abandoned by the service.
-
Barriers to patient engagement included a lack of awareness and understanding about PIFU, the wider context on access to services and factors related to specific conditions.
Introduction
In this section we present findings in relating to RQ 3.4:
-
What are patient experiences of engaging with PIFU services? What is the level of patient engagement?
We first present findings from the interviews with patients. We then explore how staff in the case study sites are measuring and capturing patient feedback. We then discuss staff perceptions of patient experience and engagement with PIFU. We also explore the enablers and barriers to patient engagement.
Methods
Data are drawn from interviews with clinical and operational staff, patients, and a workshop with staff. Given the small number of patient interviews, we also drew insight on the perception and experience of patient experience from the staff interviews. Further details on the methods can be found in Methods for the evaluation of Patient-Initiated Follow-Up.
How do patients perceive and experience Patient-Initiated Follow-Up?
We conducted four interviews with patients on PIFU pathways. Three patients we spoke to were on PIFU pathways for Breast Care and one for Gynaecology. All patients had completed their treatment and were on fixed-term pathways of between 6 months and 5 years in length. Although this is a very limited sample, the interviews provided useful insight into how patients experience and engage with PIFU and the factors that affect this.
Individual health situation and characteristics
A person’s attitude to their condition was highlighted by staff as relevant to how someone might engage with PIFU, and this was reflected in the interviews. People spoke about themselves as being pragmatic and motivated by the option of self-care.
I think when you’re discharged – because I’ve had it previously where I’ve had this, but not been offered it [PIFU] and I’ve just felt like oh my God I’m left on my own now. I’ve just got to get on with it. This gives me that bit of reassurance [that] if I’m not 100% sure, I’ve got someone I can really just say can I have a check-up please?
Patient, Gynaecology
One interviewee also referenced the stage they were at in their treatment as influencing her attitude to PIFU.
I would recommend it but there are people who it will [not] work for because they’re not in the same position [cancer free]. They’re not in the same mentality. We’re all so different, I can see why it wouldn’t work for others.
Patient, Breast Care
Introduction to Patient-Initiated Follow-Up
Patients described how they had been introduced to PIFU. All had first heard about PIFU in an appointment with a consultant.
I just thought it’s like the norm now.
Patient, Gynaecology
In the Breast Care pathway, patients were also provided with an information pack following their last mammogram appointment. All patients reported being clear they could contact the team if they had any questions or concerns and on how to do this. However, they could not recall what information was provided in detail, reflecting on the significant amount of information they received throughout their care.
I can’t remember the wording of the letter that said that I wouldn’t necessarily be having regular appointments, but I would still have access [to the nurse].
Patient, Breast Care
In terms of how PIFU was described to them, patients talked about being able to contact the service directly, often contrasting PIFU with needing to go back via their GP, rather than with a fixed routine schedule.
They just said it was patient led. So if we needed it we could just ring.
Patient, Gynaecology
I took away from that information that I was basically signed off, to the fact that I didn’t need regular appointments. But if I have any concerns whatsoever that may be [...] related to the cancer that I was to call the nurse on the number I’d got and that she would instigate the right person to call me back, meet me, whatever it might be needed.
Patient, Breast Care
Patients we spoke to did not report any initial concerns about the approach, but this initial perception was influenced by where they were in their treatment.
I’ve fundamentally finished my treatment ... and therefore I go into the next phase which is where I don’t need to be seen on a regular basis. I will be called over the next five years for a mammogram on a yearly basis. But in the meantime, I’m not to go through my GP, you know if I’ve found a lump ... and I have a feeling it might be cancer, instead of going to my GP and all that, I can fast track it through directly.
Patient, Breast Care
Experience of contacting service
Three of the four patients we spoke to had contacted the service already, and the other planned to do so. Reasons for contacting the service and the actions taken as a result included:
-
requesting information and being pointed towards appropriate resources
-
a clinical issue requiring a telephone appointment with a nurse followed by antibiotics
-
a clinical reason requiring an appointment with a consultant
-
a general check-up and request for reassurance.
Patients we spoke to, who had requested further support, were positive about the response they had received and emphasised the responsiveness of the service as being key to their positive experience.
When I wanted them, they were there for me.
Patient, Breast Care
I don’t feel like I’m ringing an answer machine ... But these guys are really busy. As long as I’m not forgotten, I’m fine about it. I put my trust in them and at the moment it’s working, that they will understand the urgency or non-urgency and will manage it in the way that they know best and all of them know far more than I do. Also they all know what their resources are like.
Patient, Breast Care
For safety-netting, in the Gynaecology pathway, a self-report questionnaire was included in the letter which patients were required to complete at the end of the 6 months if they had not been in touch with the service, although this had not been done by the patient we spoke to. Patients on the breast care pathway were also required to attend annual mammograms.
Discharge and post Patient-Initiated Follow-Up
All those we spoke to understood that the service would be available to them for a set amount of time. For those on the breast care pathway, this corresponded with the amount of time they would be required to attend annual mammograms or take specific medication. Patients understood that after that point, the difference would be that they needed to go back through the GP if they had any concerns.
This did cause some anxiety due to concerns about being able to access GP appointments. They were particularly concerned because their cancer had been a type which had a high risk of recurrence.
I must admit that is something that stays in your mind because I’ve no idea [what happens at the end of the pathway]. At the moment I feel that I’ve got the support if I need it. In two years’ time when you fall off the list altogether it will be back to square one ... trying to get an appointment with the GP. Will they be available then? I don’t know. And then starting the waiting list to go to see a consultant and then, waiting to get whatever treatment. Yes, that is a real concern.
Patient, Breast Care
Another hoped that if they went back to the GP for the same issue, they would be seen more quickly.
I just need to go back to my GP and explain this is what’s happened, and they will refer me again. I didn’t think I would have to wait as long because it’s already on record.
Patient, Gynaecology
Overall experience and views of Patient-Initiated Follow-Up
We asked patients to describe their overall experience of PIFU and whether they would recommend it to a friend in a similar position.
All of the patients we interviewed were positive about their own experience of PIFU. They pointed to several benefits including being able to ask questions when they needed to, being able to access a specialist directly rather than through a GP and to help them feel supported and not abandoned. Although most said they would recommend it to a friend, this was caveated with the view that it might not work for everyone and would be dependent on the individual’s situation and attitude.
I think if you’re coming to the end of your treatment or your time and they go, oh we think we’ve done whatever we can do. I think it’s a good option to have the open appointment because it’s down to you if something goes wrong, or you’re not 100% sure about something, like myself. I have that option to ring.
Patient, Gynaecology
Definitely, [I would recommend to a friend] because that way you don’t have to go through your GP, you go direct to them ... I’ve always been told to go direct to them, don’t go to your GP, go direct to them.
Patient, Breast Care
The patient-initiated system should work for everyone but some people won’t trust it and I think that’s going to be the problem.
Patient, Breast Care
Comments from patients regarding improvements often related to the general need for improved communication and co-ordination between different services, rather than the PIFU service specifically. Indeed, some contrasted the positive experiences they had had with the PIFU pathway compared to other services. One patient reflected that having the opportunity to ask questions easily after the initial appointment would be valuable:
I think you want to ask loads of questions, but at the time you don’t think of them. I think it would be nice to have an option to be able to perhaps have an email address just to ... say I forgot to ask this, I forgot to ask that. Because at the time when you’re sat there, everything just goes by and they’re giving you so much information and sometimes you don’t retain it all.
Patient, Gynaecology
We also heard general feedback about the challenge of accessing healthcare services. All patients in the interviews expressed concern about accessing GP services, particularly once the PIFU pathway had ended.
How do services measure patient experience and engagement?
Most staff we interviewed had not conducted any formal research to capture patient feedback and were not aware of any work that had been done across their trust. Some had done short surveys within specialties or engaged with broader patient groups about PIFU (and more generally around alternative approaches to outpatient care).
We did a patient forum at the start. Patients liked [the] concept of PIFU – coming in when they needed the service – but didn’t like the name. Some patients struggle to understand what PIFU is.
Operational, trust-wide – Site 2.1
Several staff interviewees mentioned that capturing more formal patient feedback was something they were planning or wanted to do. Existing approaches (such as the Friends and Family Test) were considered too generic. As such, most staff reported on anecdotal experience based on whether patients had agreed to being on a PIFU pathway and how they had subsequently engaged with the service.
Perceptions of patient experience were sometimes based on a lack of contact or complaints rather than positive comments (adopting a ‘no news is good news’ approach). One person in a trust-wide role reported that problems related to PIFU had not been raised as a concern in ICB-wide meetings and this was interpreted as there not being any significant issues. This was also reflected in interviews with individual service leads.
I can’t remember a complaint that has ever come in that said you’ve discharged me and I didn’t want to be. So I think they’re very well informed when they have that last consultation. So as far as I’m aware, patients are happy with it, plus they don’t have to keep coming back to hospital. And I think some of them quite like that.
Operational, trust-wide – Site 2.2
Where patient feedback had been collected, a common theme was that patients were not aware they were on PIFU (discussed previously in How is Patient-Initiated Follow-Up being implemented? and see Appendix 6, Table 16). This suggested that patients were not being properly informed about PIFU when it was introduced to them. One reason given for this was that the time pressure clinicians are under means they may not engage in the conversations in the way that they should. For central outpatients teams, this pointed to the importance of communicating how PIFU should work consistently across the trust and in particular distinguishing it from previous approaches.
We’ve done surveys, but [the] feedback was all about trust communications – so [I’m not sure] whether clinicians are having shared decision-making conversations with patients ...
Operational, trust-wide – Site 2.2
It’s about making sure that the same conversations and equal conversations are happening across the trust and with every member of your team, particularly when you have a transient workforce coming through like registrars and locums.
Operational, trust-wide – Site 2.1
So, if PIFU takes a long time to explain if you’re in a hurry because you’ve had a couple of urgent patients and they’ve got something really complex to do, are you going to spend all the time you need to do to explain?
Clinical, specialty level – Site 2.1
How do staff perceive patient understanding and engagement of Patient-Initiated Follow-Up?
We asked staff in the interviews how they thought patients perceived and engaged with PIFU and what the impact on patient experience was. Perceptions of patient experience often related to how staff perceived the aims and objectives of PIFU for patients, such as increasing convenience and access to health care when people need it (see How is Patient-Initiated Follow-Up being implemented?). Several interviewees mentioned that PIFU was more convenient for patients and meant they did not need to travel a long way or struggle to find parking. This was particularly the case in trusts where patients were spread across a wide geographical area and may need to travel to sites where there is limited public transport. Then, in some cases, travelling a long way for a short appointment only to find that everything is fine. In this way, PIFU was considered a more desirable option than fixed follow-up.
Staff perceptions of patient views of Patient-Initiated Follow-Up
None of the case study sites captured data on the numbers of patients who declined the offer of a PIFU pathway. Staff did however report their views on the reasons why patients preferred to keep to a fixed schedule. This included:
-
Having the security, structure and routine of a follow-up appointment
-
Patients with epilepsy tend to have a poorer memory and lots of people like the reassurance knowing they’ll get sent an appointment.Clinical, specialty level – Site 2.1
-
The reassurance of attending an appointment where everything is fine because that is how their condition had always been managed.
-
Initially when you explain PIFU to patients, [they] seem quite keen on it. You either have a patient who will be very accepting of it and think it’s a good idea or you’ll have that patient that wants to know that there is an appointment on the system for them. Clinical, specialty level – Site 2.1
Staff also reported on the reasons why patients want to use PIFU. In the context of one breast care pathway we observed, clinical staff talked about how attending hospital for regular appointments triggered significant anxiety for patients because it brought back memories of needing to attend for treatment.
Staff also compared PIFU to discharge and suggested that, from a patient perspective, PIFU is a more desirable approach both to ensure the patient does not feel abandoned and to prevent the need for people to go back to their GP. This appears to be a particular issue for people with long-term conditions which may be managed across both primary and secondary care.
It’s great having another option so you don’t just discharge somebody and say, OK, we know you’re stable, we’re not [going to] change your medication. We can refer you back to primary care and any problems, get the GP to write to us again. Now we have the option of going if there’s any problems then you can contact us and we can get you an appointment however it’s needed, be it face-to-face or telephone. So I suppose the biggest impact for us is knowing that option is there.
Clinical, specialty level – Site 2.1
Engagement with the Patient-Initiated Follow-Up service
We asked staff how and why patients in their service had engaged with PIFU (in terms of contacting the service). Although most pathways did not formally capture data on this, staff reported that anecdotally this was for a wide range of reasons.
Given this information is not consistently captured, it is difficult to know whether patients are accessing the service at times that are clinically appropriate, although it was important to note that there were a wide range of reasons that people were contacting the service. This included the need for reassurance about symptoms, medication management or requests for information. A lot of these issues could be managed by other staff members (such as nurses) within the service, without requiring an appointment with a consultant.
Where these reasons had been captured, staff had been able to identify unmet needs. For example, in the breast care service, people on the PIFU pathway were often getting in touch with concerns about medication side effects and the team then provided further information and support to patients about this. Another interviewee however pointed out that this may also reveal challenges to accessing health care in other parts of the system and a lack of clarity from patients about who they should be contacting for different things.
We are getting a lot of requests from patients where an appointment isn’t needed – it’s more issues of not being able to get a hold of their GP, medication has run out, etc. So, we need to understand the reasons for triggering much better – and I suspect this is the case for all long-term conditions.
Clinical/Operational, specialty level/trust-wide – Site 2.1
That said, views were mixed about whether patients were contacting the service when they needed to or not. As discussed earlier, the lack of data and information on patient contact meant there was limited reliable understanding of patient engagement with the service.
Some staff interviewees considered if patients were unhappy about PIFU they would contact the service, although others were concerned that not hearing from patients did not necessarily mean good news. It may instead have meant that patients were either unaware of, or unwilling to contact the service even if they needed to. They were unsure whether patients who required the service were using it.
It’s a situation of known unknowns – if someone is on PIFU and they don’t trigger an appointment, you don’t know if they’re running into trouble until you see them. If they are triggering an appointment, is it because they truly need a clinical appointment, or is it something else?Clinical/Operational, specialty level/trust-wide – Site 2.1
We know it worked because patients who did have recurrence and called up with symptoms [knew] there was a clear pathway for them to get back in and they did call us ... I’ve picked up a few patients who are not on a PIFU pathway, i.e. they are on routine follow-up every six months and one particular woman had an appointment in six months’ time and had symptoms of recurrence and waited because they know they’ve got an appointment. Operational, trust-wide – Site 2.2
Another issue that was raised was that if patients are not getting in touch with the PIFU service, it was not clear whether that was because they were receiving support elsewhere (e.g. in primary care). Staff views were mixed about whether this was a good thing or not. On the one hand, it suggested that people were unaware they were on a PIFU pathway and were struggling to access care when they could have just gone to their specialist. On the other, it was felt that if those patients were getting support from somewhere, it did not matter where this was.
The interviews with primary care staff – although limited – do suggest that patients are not always able or willing to contact the PIFU service. People we spoke to reported hearing challenges from patients regarding getting through to hospitals and specialty services.
PIFU was supposed to be beneficial to acute trust and patients but from patient point of view it’s still tricky – ideally there shouldn’t be any impact on primary care but because lines of communication aren’t there it’s coming back to primary care because [GPs] need to initiate contact or help patients to initiate contact. GP
Enablers to patient engagement
Staff reported that patient engagement was enabled by clear communication and providing confidence that people would be able to speak to someone and get an appointment. This included ensuring that patients were provided with information both written and in face-to-face conversations (as described in How is Patient-Initiated Follow-Up being implemented?). Within the Gynae-oncology and Breast Care pathways, we heard from interviewees that PIFU was usually mentioned in several conversations with patients so that they were more aware of it when the pathway began. The importance of this long-term relationship has already been discussed as an enabler to successful PIFU implementation (see How is Patient-Initiated Follow-Up being implemented?) and this was reflected by staff when referring to how patients engaged with the PIFU service. As discussed in How is Patient-Initiated Follow-Up being implemented?, ensuring there are clear avenues in place for people to get back in touch (and this was properly resourced) was also seen a key enabler for PIFU.
Barriers to patient engagement
Lack of clarity and understanding about the PIFU process itself was noted consistently by staff as a barrier to patient engagement. Some teams mentioned confusion among patients about what conditions they can or cannot be seen for when on a PIFU pathway. Staff mentioned instances of patients attempting to initiate care for issues (unrelated to the reason they were on PIFU), even though the pathway is limited to follow-ups for concerns related to what they had already been seen for, or their primary diagnosis. Staff from some sites also referenced examples of patients being unclear about when they would be discharged and not realising that after a certain end point they would no longer be able to access secondary care without a new referral.
Sometimes when PIFU doesn’t go well it is because patients don’t understand they are going to be discharged after a period of time and they will phone up after being discharged thinking they have an open appointment – so it’s really important they understand this. It is also tricky if we are not the person/service to be able to address their concern. Clinical, specialty level – Site 2.2
Several interviewees also referred to the wider impact that COVID-19 has had on patient confidence in healthcare access, and one also referred to patients’ changing expectations. This was highlighted in the context of people who have been used to a particular approach (i.e. fixed follow-up schedules) for a long time and a shift to PIFU introduced a change in routine.
Trying to engage people with PIFU especially during Covid has been difficult – [there’s been a] loss of faith in services and people feel like they’ve been dropped off the radar. [It] puts fear into people that they won’t be able to get an appointment. Clinical, specialty level – Site 2.1
They remember how it was ten years ago and they’re frustrated that it’s no longer as quick and as efficient as it was. GP
How patients engage with PIFU may also be dependent on their approach to their condition. One example was given in the context of epilepsy, where people may be less willing to engage in managing their condition because of the impact it can have on other aspects of their life.
Epilepsy is a difficult condition. There’s an impact in every aspect of life with epilepsy. So if you have seizures, you can’t drive, so that might affect employment, finances, family life, socially ... If some people don’t face their conditions and it’s not there if they don’t tell people, then it’s not happening ... Clinical, specialty level – Site 2.1
Summary of findings
We conducted a small number of interviews with patients on PIFU pathways at the case study sites. Patients liked having the option to contact a specialist when they needed to, rather than needing to be re-referred via their GP. All either planned to or had already contacted the service and were positive about their experience.
Across the case study sites, limited activity had taken place to capture formal feedback from patients, although staff reported that anecdotally patients were positive about PIFU as an approach and the support they had received. Reasons for patients declining PIFU included a preference for regular interaction, a desire to stick to their routine and a concern that they would be unable to get an appointment if they needed one.
There appears to be a gap in understanding about whether patients are contacting the services when they need to. However, when they do, this can be for several reasons, not all of which require a face-to-face appointment with a consultant. Enablers to patient engagement include clear avenues of access, support and communication and ensuring patients do not feel abandoned by the service.
Chapter 4 Evaluation guide for Patient-Initiated Follow-Up
Overview
What was already known
-
A rapid evaluation of PIFU with a handful of study sites would not be able to study the impact of the variety of modes of implementation across all different clinical specialties – particularly as PIFU evolves over the long term. Therefore, there is a need for local evaluations to improve the depth of evidence available at a specialty level, which in turn can inform improvement in PIFU services.
-
To support local evaluators, there is a need for guidance on how to analyse and learn from PIFU services.
What this section adds
-
We developed a guide to support teams to evaluate the outcomes and impacts of their PIFU services at a specialty level, thus helping address research gaps and encouraging the sharing of learning.
-
A version of the full evaluation guide is available in Report Supplementary Material 8.
Introduction
New policies, such as a nationwide push towards introducing PIFU pathways in outpatient services and decreasing unnecessary outpatient activity, typically move through a cyclical policy process. First, the policy is formulated by central policy-makers (despite in this case PIFU existing in various forms at local levels before becoming a national policy), then implemented by health and care services and the final step for most policies is an evaluation. Policy evaluation involves the results of policies being monitored, which often leads to the rethinking of policy problems and solutions and the start of a new iteration of the cycle. 80
To evaluate PIFU as a policy, RSET undertook the national level evaluation under workstream 4. But as we describe in the Results sections of How is Patient-Initiated Follow-Up being implemented? and Measuring the impact of Patient-Initiated Follow-Up, undertaking this evaluation has been challenging because of the variability of PIFU services and the data that are available at a national level. For example, national data are not able to describe who is on PIFU pathways. Furthermore, during the evaluation, it was difficult to obtain access to relevant local level data because local IT systems, at the time of writing, are not yet set up to enable evaluations. But that is expected to change with planned improvements to data recording processes enabled by actions taken by NHSE and IT suppliers. An important next step in understanding how and why PIFU works requires providing local evaluators with appropriate, easy-to-use tools and encouragement to undertake and share findings of local evaluations that match their available capacity.
While policy-makers are keen to understand the impacts of PIFU-related policies, there is also a need for local services to understand what is (and is not) going well in their service. They should also know how to improve PIFU delivery, demonstrate the value of PIFU pathways and their trade-offs and, if appropriate, how to expand PIFU pathways to other outpatient services. Furthermore, they need to engage with patients to ensure PIFU’s aims of improving personalisation of services are being realised from patients’ perspectives.
Evaluation of PIFU services across a range of specialties is particularly important considering the variability in how PIFU is being implemented and the mixed evidence of PIFU’s effect on health outcomes and costs. 26
Aims of the guide
The aim of the guide is to encourage and support local evaluators to focus on examining and sharing learning on the outcomes and impacts of their PIFU pathway at a specialty level. The production of the resource aligns with the ‘democratisation of evaluation’ movement described in the evaluation literature,81 which encourages researchers to share evaluation tools and resources to increase evaluation capabilities in health and care services.
Audience for the guide
Recognising the variability in who is responsible for delivering PIFU across services, the evaluation guide is for anyone who is interested in evaluating PIFU services locally. In most cases, this will include operational or outpatient managers at a trust-wide level or clinical or operational leads within specialties who would ideally be supported by a range of specialists working in their hospitals on IT, data analysis and patient engagement. Where resources are not available within a provider organisation for an evaluation, the guide might also be helpful to evaluators in local health systems.
Methods
The PIFU evaluation guide was developed by adapting existing guidance on evaluation. 82 Their approach is both theory- and practice-led and supported by a range of citations from the evaluation literature. However, their approach was designed for trained evaluators and would not have suited the target audience who have specialist clinical and operational management skills and may be relatively new to evaluations. We did not change the core concepts of their approach but instead focused on adaptations, such as modifying language, focusing more on evaluation rather than on both monitoring and evaluation, and by decreasing recording and reporting requirements.
To use the guide, evaluators need to have a clear understanding of how their local level PIFU service works – and to be able to identify its similarities and differences from the national PIFU policy agenda. To support them in doing this, we developed a set of national objectives and a national programme theory drawing on all relevant guidance available on the FutureNHS platform.
To ensure the guide met the needs of commissioners and end users, we shared an initial draft of the programme theory with NHSE for editing. After producing a complete first draft, we presented the guide at an Action on Outpatients: Improving Access to PIFU online event webinar on 1 August 2023, where we asked anyone interested in giving feedback to contact the RSET team. People responsible for leading or managing PIFU services agreed to look at the guide, and we received comments from five. We additionally met with a group of service-embedded evaluators in a region in England and received comments from disease-specific alliances carrying out their own evaluation. The most significant comments received were that the guide was too long, which might be intimidating, and its asks were too resource heavy, which might not be realistic for most providers in the current climate. Suggestions for overcoming these barriers were to have a message upfront that encourages users of the guide to take time out and work through the steps, alongside messages that sell why providers might want to evaluate PIFU (not just because PIFU policies at a national level have been framed as a national target).
We took on board this main piece of advice (among a range of other points of clarification raised), resulting in an executive summary with the key aspects of evaluation which time- and resource-poor end users will want to consider, as well as the detailed draft we shared with reviewers. We disseminated the guide using the varying routes described below, including sharing the revised version with the reviewers ahead of publication.
Key sections of the guide
A version of the full evaluation guide is available in Report Supplementary Material 8. The guide includes an executive summary slide, an introduction to the differences between monitoring and evaluation and why PIFU service leads might want to evaluate. The background section also provides a clear description of how and why PIFU services work (drawn from PIFU policy documents) and what outcomes and impacts PIFU can lead to. The seven steps of evaluation are provided sequentially, and at each relevant step the guide encourages local evaluators to think about whether the vision produced at the national level makes sense for their local context and to make adaptations to the expected aims and objectives, the outcomes and assumptions that underlie their PIFU service. The appendices cover an evaluation terminology glossary, examples of data collection tools and an evaluation planning document template.
The main message emphasised throughout is that it is important to plan carefully and thoughtfully for an evaluation and engage the right people throughout. We also stress that time and headspace are needed to plan, execute, and report an evaluation, and encourage users of the guide to seek out strategic partnerships and help with signposting from NHSE regional leads to ensure evaluations are carried out with the widest support network possible and learning is shared.
Step 1: build an evaluation working group
The initial step focuses on the composition of the evaluation working group. Considering that evaluations demand a significant amount of time, data access and potential regulatory approvals, it is advisable to involve 6–10 senior leaders, managers and patients. The guide provides a list of 12 questions that the evaluation working group should discuss and agree on, some of which are practical, such as ‘Does everyone in the working group have a role and know what they’re doing? Is there anyone missing?’ But also, theoretical questions such as, ‘Are there any unintended consequences (positive or negative) that would be worth measuring in an evaluation?’. By working through the 12 questions with the right people in the (potentially virtual) room and capturing what the group agrees in writing, the guide becomes a resource that can be shared among the group and with wider stakeholders to communicate the scope of the evaluation.
Step 2: decide the aims of Patient-Initiated Follow-Up in your specialty
The second step entails determining which national level objectives for PIFU policy most align with the local values, aims and objectives (Figure 20), such as ‘empowering patients to take ownership over the timing of their care’ or ‘reducing unnecessary appointments’. The emphasis in this section lies in the working group identifying and prioritising those objectives that most closely align with local initiatives and, where possible, working with other stakeholders such as hospital leaders and patients to seek out their opinions.
FIGURE 20.
National programme aim and objectives.

Step 3: describe how and why your Patient-Initiated Follow-Up service works
The third step is designed to assist local evaluators in articulating the functionality and rationale behind their service by employing a logic model template (see Report Supplementary Material 8, p. 13). While the prospect of creating a logic model (i.e. a visual description of the components of the planned change which describes how and why PIFU works) may be daunting for some, we provided them with a pre-existing model describing NHSE policy team’s national PIFU logic model, which they can edit to ensure alignment with local plans.
Step 4: agree on evaluation questions
The fourth step urges evaluators and their evaluation working group to contemplate which evaluation questions hold the greatest significance and importance to their aims and objectives. The guide groups questions by outcome area, such as patient experience or effectiveness. In most outcome areas, the guide provides multiple possible evaluation questions to show local evaluators the different ways in which they can approach their evaluation. While the guide does not provide advice on how many questions a local evaluation should include, it suggests that each new question adds time and resource implications for evaluators.
Step 5: select indicators and outcomes
On having developed a local-level logic model and identified evaluation questions, the fifth step involves reviewing the diverse array of PIFU-related indicators possible and suggests collaborating with business analysts and information teams to assess whether any existing data could inform these indicators or whether new data are needed. While there is currently a lack of much existing administrative data, this is set to change, and the guide makes clear that using existing data can be easier than collecting new data.
Step 6: collect and analyse data
In the sixth step, the guide offers valuable advice regarding the various types of qualitative and quantitative data that evaluators can collect to supplement any pre-existing information. These data collections will serve to enrich evaluators’ understanding and address the evaluation questions effectively.
Step 7: reflect and report on your findings
The final step of the guide provides practical advice and illustrative examples on how to disseminate evaluation findings to different audiences, ranging from patients to trust boards. This comprehensive approach aims to ensure that evaluation outcomes are effectively communicated and contribute to informed decision-making.
Dissemination
The guide was made available on the FutureNHS platform to all NHS staff who were members in April 2024.
Discussion
We developed this guide to support anyone interested in carrying out an outcomes evaluation of a PIFU pathway at a service level. Its development supports the ‘democratisation of evaluation’ movement described in the evaluation literature by encouraging and guiding local researchers to address gaps in knowledge about PIFU services.
The guide is also a direct response to Lamont et al. ’s81 call to action for researchers to support evaluation democratisation by ‘working with service leaders to articulate the goals and describe the components of planned change; to synthesise helpful evidence on related interventions; to identify key stakeholders, appropriate methods, and outcome measures; to test early findings with target audiences; and to consider the best ways to share results’. The guide does this by encouraging local evaluators to work with all relevant stakeholders, adapt national goals and plan change to best suit their local plans, leverage existing data, and offer findings to stakeholders in varied ways.
Overall, the guide encourages local evaluators to plan their evaluation carefully and thoughtfully at the start of a project and engage the right people throughout, which should pay dividends regardless of whether a simple patient satisfaction survey or complex mixed-method evaluation.
Conclusions
We have developed a detailed but accessible evaluation guide for managers of PIFU services that guides them through a specialty-level outcome evaluation in seven steps. The guide builds on existing guidance for evaluators by following their key steps and combines it with policy guidance on PIFU, resulting in a guide that enables novice evaluators to adapt national objectives and data collection tools for their local context. The guide will support the evaluation of PIFU as a policy and provide valuable evidence to inform current research gaps identified in the scoping review and the national evaluation, as well as hopefully help local services improve their PIFU offer based on the local challenges and successes they identify in their evaluations. However, the use of the guide depends on how much time and resource is made available to evaluators and their teams.
Chapter 5 Evaluation of Patient-Initiated Follow-Up: discussion, impact and learning
Introduction
There is recognition that NHS outpatient services need a major transformation that changes how outpatients are managed and moves away from a purely hospital focus by further integrating primary, secondary, community and social care. Initiatives that have been implemented across high-income countries include establishing new roles for staff so that clinics are led by nurses or non-specialist roles, the sharing of care delivery between secondary and primary care, and patients initiating their own appointments as and when they need them. Within the English NHS, the latter has been formalised as Patient-Initiated Follow-Up (PIFU) and has been rolled out within nearly all hospital providers with outpatient services.
The evaluation of PIFU sought to understand how it has been implemented in outpatient services in the NHS in England, the factors that supported or hindered implementation, and the impact on secondary care use, staff experience, patient experience, and health inequalities. This rapid evaluation presents findings from a mixed-methods analysis across five case study sites and national data.
Summary of findings
Patient-Initiated Follow-Up implementation
-
The recorded numbers of times patients were either moved or discharged to PIFU pathways across the English NHS approximately tripled between September 2021 and March 2023, to about 185,000 per month.
-
PIFU is most commonly being used in short-term pathways (e.g. physiotherapy or following surgery) although there are several examples where it is being used for people with long-term conditions.
-
There is a large amount of variation in how PIFU is implemented between trusts within the same specialties. This includes the extent to which adopting PIFU is a departure from existing practice, given that some specialties were already using a system like PIFU (such as open appointments).
-
Models of PIFU vary widely between trusts and clinical area, with a significant degree of variation in the approach to patient selection, safety-netting, and discharge. Also, the nature of the condition was a key factor in how PIFU was implemented.
-
Where PIFU had been implemented successfully, enablers included factors related to the nature of the condition, specialty or pathway, organisational and service context, staff and patient factors and the wider system. This included clinical engagement, supporting guidance and champions, dedicated staff capacity and flexible recording systems.
-
Barriers to successful implementation included patients not being aware they were on PIFU, staff resistance, competing priorities and limited capacity to dedicate to PIFU, a lack of engagement with primary care, and challenges amending trust IT systems to record PIFU activity.
Impact on service use
-
In 15 of 29 clinical specialties, we found evidence that greater use of PIFU is associated with reduced frequency of outpatient attendance for individual patients, most notably in Midwifery, Gynaecology, Urology and Pain Management. For seven specialties we found evidence that greater use of PIFU is associated with increased frequency of attendance, most notably in Ophthalmology. Within some specialties, although there were statistically significant changes, the practical effect was small. However, since most patients were not on PIFU, we may not expect to see a large impact across a whole specialty.
-
Similar significant findings were not replicated when we analysed outpatient attendance per patient aggregated at the trust-specialty level, which may be because such an approach, using aggregated outcomes, would be less sensitive.
-
These findings correspond to views from the study sites where impact on attendance is seen to be variable depending on how the PIFU pathways are implemented.
-
We found no practically significant association between PIFU rates and frequency of ED visits overall (results were statistically significant but of negligible effect size), but for four specialties number of specialties appeared to have increasing PIFU rates associated with less frequent ED visits.
-
Staff at local sites have noted fewer individual patient appointments within some specialties, whereas in others they expected no reduction because they gave patients on PIFU pathways the opportunity to arrange additional appointments as necessary, while maintaining fixed follow-up.
-
Some local IT systems limit the degree to which services can evaluate their own use of PIFU.
-
It is unclear whether any observed reductions in outpatient attendance are offset by increased use of telephone conversations or GP visits.
Staff experience
-
PIFU was viewed by most staff as a positive intervention to support patient autonomy and self-management and ensure that time was directed towards those patients with the greatest clinical need.
-
The impact of PIFU on individual staff roles and workload was mixed and dependent on the extent to which PIFU was used routinely within the service and considered a departure from previous ways of working.
Patient experience and engagement
-
Across the case study sites, limited activity had taken place to capture formal feedback from patients, although staff reported that patients were positive about PIFU as an approach and the support they had received.
-
Reasons reported by staff for patients declining PIFU included a preference for regular interaction, a desire to stick to their routine and a concern that they would be unable to get an appointment if they needed one. We were not able to speak to people who had declined PIFU as this information was not captured in the case study sites.
-
Although we only spoke to four patients, those we spoke to were positive about their experiences and liked having the option to contact a specialist when they needed to.
-
Staff were unsure whether patients were contacting the services when they needed to. However, when they did, this could be for several reasons not all of which required a face-to-face appointment with a consultant.
-
Enablers to patient engagement included clear avenues of access and support, communication and ensuring patients do not feel abandoned by the service.
-
Barriers to patient engagement included a lack of awareness and understanding about PIFU, the wider context on access to services and factors related to specific conditions.
Inequalities
-
There was limited understanding of the impact of PIFU on different patient groups, although staff in the research recognised this as a concern. Digital exclusion, demographic characteristics, socioeconomic status and patient characteristics were all thought to be relevant to how patients engage with or are impacted by PIFU.
-
Staff perceptions of the impact of PIFU on health inequalities were mixed. Some staff were concerned that PIFU could exacerbate inequalities because those with greater individual motivation and ability to advocate for themselves would be more able or willing to initiate contact when they needed. However, some services have used PIFU as a way to engage with patients who otherwise may not engage (or were more likely to miss appointments) by enabling them to contact the service at a time that was convenient for them.
-
Data from one site indicated some variation in reattendance of patients on PIFU pathways within a year by ethnic group. However, it is unclear whether this reflects underlying ethnic barriers or other confounding factors. The difference in return rates between different adult age groups was small, although we were not able to isolate the oldest patients within the data. For children (ages 0–17), return visits of patients on PIFU pathways was approximately 50% higher than for adults.
How findings relate to previous research
There have been few previous studies on the impact of patient-initiated approaches. 26 Of 15 studies in the evidence review assessing the impact of PIFU on outpatient activity, 8 showed that PIFU led to a statistically significant reduction in the number of outpatient appointments compared with fixed follow-up and 7 showed no difference. 26 Two studies found that a reduction in outpatient visits was replaced with an increase in phone contacts with the service. 83,84
The review also identified a gap in knowledge around the impact of patient-initiated approaches to follow-up for different groups of patients. Previous studies have identified the importance of organisational factors in implementing PIFU, including leadership, capacity, and trust between teams. 85,86 Patient factors were highlighted in other studies, for example, a named contact,87 enough capacity to be responsive to requests88 and patient confidence, convenient and timely appointments, and regular monitoring. 86 One study identified the importance of reliable routes to recall patients if needed and monitoring workload on roles such as nurses or allied health professionals (AHPs) who may pick up displaced activity. 89
Previous evidence on the impact of PIFU on patient experience was limited and although we were only able to conduct four interviews with patients, the study contributes to learning about how patients engage with and experience PIFU. Although previous literature suggested that factors such as patient activation, age, gender, and clinical factors were not related to the treatment people received, the present study suggests that individuals’ health situations and health-seeking behaviours could influence how they engage with PIFU. 90,91
Prior evidence also suggested that a lot of people decline PIFU and that patients who decline to take part might be different from those who accept. For example, one study looking at PIFU services for patients with diabetes found that older patients and those with a longer diabetes duration were more likely to decline PIFU. 83 Other factors that affected acceptance rates included how the pathway was communicated to patients and study design elements (i.e. randomisation), all of which were factors also raised in the study. We were unable to determine how many people decline PIFU within the pathways we studied (and their characteristics) as these data were not routinely collected in the case study sites. However, staff in some services said that they put very few patients on PIFU (either because they were not eligible or individuals preferred to keep to a regular appointment schedule).
The qualitative findings on patient experience were consistent with previous literature. 87,88,92 Although we only identified a few qualitative studies in the review, they suggested that patients are generally satisfied with PIFU and experience the service positively, with patients reporting that it supports them to have more control over their health and fits better with routine management. Studies also report that PIFU has helped address patient needs when symptoms increase or do not respond to treatment.
Impact and implications for policy and practice
Due to existing pressures on the NHS, it may be some years before any impact of PIFU on overall outpatient capacity is realised. This is in part because any capacity which may have been freed up as a result would immediately be filled by new patients or those on waiting lists. Also, with approximately 200,000 people put on to PIFU pathways per month by March 2023, this is small in comparison to the total number of outpatient appointments (7.4 million in the same month). The ultimate impact will also depend on the extent to which PIFU is a departure from previous ways of working. This appears to vary a lot between different trusts and specialties, and even within services depending on previous experiences and approaches of particular consultants.
The impact on workload will largely depend on how PIFU is adopted within services, the numbers of people contacting services and how clinics are configured. Where interactions do occur, they could be more complex and time-consuming (compared to routine follow-up appointments) and may be different to a traditional appointment with a consultant, for example a telephone call with a nurse specialist. It is currently difficult to capture these interactions in the data.
Within different trusts, changes in outpatient service use are determined by several factors, of which PIFU is just one. It is not always the case that, by design, PIFU will reduce outpatient attendances per patient. For example, there are cases where a specialty maintains fixed follow-up appointments and PIFU is offered to patients in case they wish to be seen sooner.
However, there is a need for more patient-centred outcome measures. While visits to ED can reflect an impact on patients, outpatient attendance is more of a measure of activity. There is a case for capturing more patient outcomes than are currently available, such as patient-reported outcome measures or outcomes derived from primary care data and other secondary care data sets. The extent to which services in the case study sites captured information on – for example – the reasons why PIFU patients do initiate and attend appointments is also variable, limiting the ability to understand whether those attendances are clinically required or desirable from the perspective of patients and staff.
There is also a need for better communication and engagement across the system and with primary care in particular, not just in relation to PIFU, but more widely around referral processes, Advice and Guidance, and other new ways of delivering care. There also appears to be a tension between the aims of PIFU to support patients and its perception as a tool for reducing activity or managing the waiting list. However, further evidence on the impact on patient outcomes is crucial.
The study showed that there is wide variation in how PIFU is being implemented. Although it is desirable to ensure that PIFU is implemented in a way that is appropriate to individual specialties and organisations, this has implications for understanding and evaluating how PIFU is being used and its impact. Given the scope of the study we were unable to look in detail at how PIFU is being implemented in every specialty, but we did identify several factors through the qualitative work which were considered to enable PIFU to work well. These were a combination of condition-specific factors, and factors relating to the service, organisation, and wider context.
Despite national guidance, PIFU is sometimes perceived by staff and patients as an alternative to discharge (by both staff and patients). 53 This could be driven by perceived problems accessing appointments in primary care. In the patient interviews, we heard concerns over what would happen when their PIFU pathway had concluded including worries that they would then need to be re-referred through primary care. This could be a particular concern for individuals who had experienced difficulties or long waits for appointments and diagnoses in the first place.
If the number of patients placed on PIFU continues to increase it is likely that (depending on the pathway) additional capacity will be required to manage demand, although what this looks like will depend on the characteristics of individual services. This may be a particular issue for services which have not ring-fenced capacity for PIFU.
That said, PIFU may provide an opportunity for enabling different workforce models. This includes increasing use of nurses, AHPs or support workers to respond to people’s needs.
We have identified reductions in the frequency of a patient’s outpatient attendance within some specialties, but it is not possible to determine the implications for the patient. If any of these relationships are due to PIFU then they could either reflect reductions in unnecessary appointments or that patients are not being seen when they need to. There is a lack of reliable information on the numbers of people engaging with services with no news often taken as positive. However, this may not be the case for every specialty and may mask situations where individuals are not contacting services when they need to or are receiving care elsewhere (such as ED). This raises the importance of safety-netting or standard review appointments to catch instances where people are not contacting the service when they should.
We are also uncertain about the impact of any reduced attendance on the wider health and social care system. With the current appointment backlogs in hospitals, any reduction in visits by individual patients is unlikely to have immediate impact on workload.
There are several caveats related to the data and improved data collection processes will be vital to ensuring robust evaluation of impact is possible. The challenge of recording PIFU activity within local IT systems was a consistent theme in this study.
Despite limitations, the study has highlighted several factors which are important for PIFU to work effectively. This includes trust and confidence that services will be able to respond effectively if patients require appointments. But as already acknowledged, challenges accessing appointments, particularly in primary care, are well known. Public satisfaction with the NHS is currently at its lowest level with waiting times for appointments a key factor,93 which may contribute to engagement and attitudes to PIFU.
It is too early to identify the wider impact that PIFU is having on health inequalities due to a lack of systematic data collection on the issue and the early stage in its rollout. However, this has been raised as an area both of concern and of opportunity. In particular, issues could arise when patients are selected for PIFU if based on existing relationships with clinicians. Pressure on staff time may also mean that staff are unable to engage in detailed conversations about PIFU with patients who may require additional support. While there are opportunities to use technology more for PIFU (such as for sharing information, remote monitoring, and online patient questionnaires), it is important to consider digital exclusion.
It is also important that sites and services undertake their own evaluations of PIFU. To this end we have developed an evaluation guide to support trusts in this undertaking. This should achieve an accurate understanding of how PIFU is being implemented and the wider impact it is having across the system, including on health inequalities.
However, what cannot be addressed in a guide is the need for resources to do evaluation well. There are significant resource implications when undertaking a mixed-methods evaluation. Time and headspace are needed to plan, execute, and report an evaluation. Considering the capacity of most PIFU and operational managers, there is a need to think about strategic partnerships within and beyond local provider organisations to ensure evaluations enable learning and improvements to PIFU services can be carried out.
Strengths
The study team conducted a mixed-methods rapid evaluation, following established methodology and guided by previous evidence of implementation, while engaging iteratively with published literature. This enabled the team to develop a comprehensive understanding of the introduction and rollout of PIFU in NHS outpatient services in England.
The approaches we used have been influenced by the qualitative findings and triangulation across different data sources. For example, the wide variation in implementation has informed us of the importance of analyses for individual specialties. We have focused on outcomes per patient rather than overall volumes, due to the influence of the current backlog in appointments.
Qualitative findings have also helped with the interpretation of outcomes. For example, why attendance frequency may increase in some specialties and informing us of other factors unrelated to PIFU that may be influencing results.
The study benefited from a multisite case study approach which enabled us to examine PIFU across different organisations and clinical areas. Staff interviews included people with clinical and operational roles, at both specialty and trust level, enabling us to capture a range of different perspectives. The workshop also allowed us to reach a wider range of participants. We captured patient feedback on their experiences with PIFU which revealed important considerations. We triangulated the findings across the qualitative data sources to test and confirm the findings.
There are several uncertainties associated with the data and their interpretation. To meet these challenges we have applied two different modelling approaches and tested the consequences of making different assumptions with sensitivity analysis. The two approaches complemented each other and, as anticipated, we have seen more precise effects with the analysis at the individual patient level.
Many types of outpatient attendance would be unaffected by PIFU, for example, pre-operative check-ups, visits for treatment or diagnostic purposes. We have therefore applied an algorithm to identify types of attendance that are more likely to be affected and thus giving us a greater chance of detecting impacts that may exist.
Limitations
Conducting a rapid study of an intervention which is still being rolled out alongside other interventions, where data are limited and at a time when there were significant pressures on NHS staff capacity, introduced considerable challenges. Due to the lack of data on outcomes, we have necessarily focused on changes in resource use and qualitative data.
There are several caveats which must be considered when interpreting the quantitative findings. PIFU is implemented in a variety of ways which may not all have the same impact (such as on the number of follow-up appointments). Within clinical teams there can be inconsistencies in how PIFU is implemented, and some sites are adapting their pathways through experience which means we are not evaluating a stable process.
Due to the many different approaches to PIFU, it is perhaps unsurprising that we found a large variation in outcomes on outpatient attendance between specialties. However, because of differences of approach between trusts within the same specialty, it is possible that the within-specialty analyses are masking a mix of different local impacts.
We were not able to identify individuals on PIFU pathways in the data and had to use aggregate measures of PIFU use within a trust specialty. Also, we were unable to compare the numbers of appointments led by different healthcare professionals (e.g. consultants or nurses) as this was not captured in the data.
The quantitative data we have used to analyse disparities in return rates for patients on PIFU pathways only comes from one trust, and we were not able to analyse multiple characteristics so caution should be made when assessing the generalisability of these findings.
Although the qualitative work involved interviews with a range of staff across five sites, given the variation in how PIFU is being implemented, it may be that their experiences are not representative of PIFU across the NHS. The sample size both in terms of case study sites and interviewees is also small, including 5 sites and 36 interviews across both phases, of which only 3 were from primary care roles. Engagement was more limited than anticipated given the considerable pressures facing NHS sites and staff at the time of data collection. Furthermore, the approach to case study, specialty and interviewee selection was in part influenced by figures from the P-EROC data set, which as discussed above is subject to several caveats when interpreting the data. A focus on specialty at a high level may also have masked nuance within specialties.
The intention was that phase 2 would look in more depth at PIFU’s use in long-term pathways. However, given the challenges outlined above, and the relatively immature adoption of PIFU in long-term conditions in the case study sites, the approach became much more pragmatic.
We experienced significant challenges with patient recruitment and as a result were only able to conduct four interviews with patients within the time frame. Furthermore, three of these were patients from the same service. We therefore drew on the interviews with staff to identify their perception of patient experience and engagement, although given the variation described above and the lack of consistent data that services collect about patients on PIFU, this is an area that requires further research.
The challenges with patient recruitment included:
-
delays in obtaining approval from the REC and Confirmation of Capacity and Capability from the two case study sites (the latter was not obtained until May and June 2023 respectively) which restricted the time available for recruitment
-
variation in how the case study sites capture information about patients on PIFU which affected how they were able to identify patients eligible for the study
-
limited staffing capacity at case study sites to support patient recruitment
-
a lower number of patients consenting to be contacted by the research team than originally anticipated
-
a lack of response from patients once contacted
-
the rapid nature of the study meant we only had a brief window to organise and conduct patient interviews after receiving ethical approval.
Although the insights from these discussions were invaluable and point to several implications, it is not possible to generalise the feedback across other patients, services, or specialties. Although the intention was to sample patients across a range of demographic characteristics, the sample was too small to do this.
We note that there are both advantages and drawbacks of using the NASSS framework to structure this evaluation. This provides a rigorous systematic method of organising findings for evaluations and considers the nature of intervention, stakeholders both outside and within the intervention setting and contextual factors. However, it was designed initially to explore the reasons why technology is not adopted and we recognise that the study team has taken an adapted approach when using the framework. It has been used as a lens to guide the evaluation, rather than a more encompassing application of the framework with reference to each of the numerous subdomains within it.
Learning
Because of some of the limitations of the present PIFU evaluation and planned improvements in data capture and hospital IT systems, we have developed an evaluation guide to facilitate ongoing evaluation at a local level.
During the PIFU evaluation, we have engaged routinely with the Outpatient Transformation Team at NHSE and shared with them interim findings. We have contributed to two Action on Outpatient webinars, organised by NHSE for hospital operational and clinical staff and shared interim findings to the North West Outpatients Pathway Evaluation Oversight Group and a roundtable organised by the NHS Confederation. The PIFU evidence review published by the Nuffield Trust in August 2022 was downloaded 742 times between August 2022 and 10 September 2023. The accompanying Explainer was viewed 2298 times over the same period.
Conclusions
Patient-Initiated Follow-Up is generally perceived positively by staff and patients, but a lot is still unknown about its impact on patient outcomes, health inequalities, patient experience, workload, and capacity. Indeed, with the many different ways PIFU is being delivered in NHS hospitals alongside other initiatives for reforming outpatient care, it is always going to be difficult to obtain a consistent view of its impact.
We found some evidence of relationships between the use of PIFU and frequency of outpatient attendance, but the direction of these relationships varied by specialty. Moreover, we do not know the degree to which these are truly influenced by PIFU. When better data is available, more robust quantitative assessments of impact should become possible.
The success of PIFU is affected by its delivery. Guidance should be targeted towards specialties or patient conditions and the purpose of PIFU and implications for patients should be communicated clearly, consistently and in a way that is accessible to both staff and patients.
Chapter 6 Recommendations
Recommendations for decision-makers
Many of the findings have implications for decision-makers regarding how to organise and monitor outpatient services both at a national and local level. This includes how to communicate with patients, engagement with primary care and monitoring health inequalities, as well as having a better understanding of risks and opportunities. In several areas, further research is needed, and the evaluation guide provides advice on how to decide on appropriate outcomes and data collection for future evaluation of PIFU. Also, the work on identifying innovations in outpatient care in data has implications for following up data signals. Below we set out specific recommendations for national policy-makers:
-
Signals of changes in outcomes in data sets may reflect problems in the data themselves, rather than true performance. Decision-makers ought to be cautious with findings at organisation or service levels, and the first stage of any follow-up should be a local data validation exercise.
-
NHSE has already produced guidance on implementing PIFU and has begun to do this for particular specialties (such as Trauma and Orthopaedics). There would be value in their continuing to engage with different specialties and conditions to support others to implement PIFU in their own service. Furthermore, there would be benefit in NHS services sharing and learning from others who have implemented PIFU through, for example, a community of practice.
-
PIFU provides opportunities to change the way outpatient care is delivered for patients, staff, and services. However, it is just one approach which is being adopted across secondary care services and must be considered as one part of a holistic approach to patient care. This means considering how it interacts with other interventions such as Advice and Guidance and self-referral. Engagement with all parts of the system is an important part of this and should be a focal point of the expected outpatient strategy.
-
Clear, consistent and accessible communication on PIFU and its purpose, to both staff and patients, is key to successful implementation.
-
Data collection within services needs to be improved to enable effective evaluation of PIFU and other outpatient innovations. This includes properly identifying which of their patients are offered, and accept, PIFU pathways and making this information available to all other services providing care for the patient. As services collect better data, they should aim to evaluate the impact on meaningful patient outcomes. Services should also assess whether there are disparities in the abilities of different groups of patients to make use of PIFU services and subsequent impacts on their health and care. Analyses may be challenging at organisation and clinical service levels, so providers might consider whether cross-organisational bodies (e.g. ICBs or commissioning support units) may be able to support this work.
Research recommendations
For further research we recommend:
-
With tightly focused analytical aims, IS methods could be more widely used to detect notable changes in performance in large health service data sets, although it would be beneficial to compare outcomes with other methodologies.
-
Further quantitative evaluation of measurable outcomes of PIFU should be undertaken that includes the impact on patients and staff workload. Analysis of patient outcomes (including analysis of inequalities) will be enhanced when the identification of patients on PIFU pathways is more widely reported within hospital PAS and thus linkable to other secondary care events.
-
More research should be conducted on patient views and experiences of PIFU, in particular, comparing shorter-term and longer-term pathways. Given the challenges we experienced in patient recruitment, it may be that a more targeted approach within specific services would be more appropriate.
-
Further research is needed to understand the reasons why people may decline to use PIFU to understand the perceived and actual barriers and potential action required to address these. Current ability to do this is limited due to a lack of information captured on whether or not patients do decline PIFU in trust systems.
-
More research should be conducted on the views and experiences of staff relating to PIFU, particularly in clinical specialties not covered by our own evaluation. We believe there would be particular value in exploring how PIFU impacts on role and workload for different groups of staff, including GPs.
-
There needs to be better understanding of how different patient populations access, engage with, and experience PIFU. Also, better understanding of how services can be designed and adapted to mitigate against potential inequalities including how services are already adapting or tailoring their services and approach to account for this.
-
There remains a lack of reliable information on how many people are getting back in touch with PIFU services and whether this is appropriate. Although some services can report this anecdotally, it is important for this to be captured systematically to understand whether patients are able to contact and be seen by the service when they need to be. Without this information it is difficult to determine whether patients with a clinical need can access healthcare services.
This rapid evaluation has provided a useful foundation for a substantive NIHR HS&DR trial of PIFU in rheumatology which should address many of these recommendations (https://fundingawards.nihr.ac.uk/award/NIHR156922).
Additional information
Acknowledgements
We are very grateful to all the hospitals, national and local stakeholders, staff, and patients who took part in our interviews and workshops and provided data. We also thank our NIHR RSET public and patient involvement members for their help in shaping the original study protocol and providing feedback on some sections of the final report.
We also thank our Project Advisory Group: Dr John Dean (East Lancashire Hospitals NHS Trust, Royal College of Physicians), Dr Frances Sanderson (Imperial College Healthcare NHS Trust), Dr Laura Coates (Oxford University Hospitals NHS Foundation Trust), Andrew Wilkinson (NHS England), and Vanessa Downes (Guy’s and St Thomas’ NHS Foundation Trust) for their expert support, advice and guidance throughout the project.
Other thanks should go to the Outpatient Recovery and Transformation Policy and Strategy team at NHS England: Rich Watts, Jenny Wood, Sarah Kemp, Mati Caldwell, Stef Dimov, Rachel Harris, Barbara Goss, Michaela Laxton, and Sarah Mole, who have supported our PIFU evaluation, given feedback on preliminary results, and provided data.
Finally, we thank the North West Outpatients Pathway Evaluation Oversight Group for feedback and advice during the project, the University of Birmingham library services for its support with the scoping review and rapid evidence review, Cono Ariti (London School of Hygiene and Tropical Medicine) for statistical advice, Sarah Scobie (Nuffield Trust) for reviewing the report and advice, and Olivia Wallace (Nuffield Trust) for helping to organise external meetings, administer the workshop, and helping to organise and put together this report.
CRediT contribution statement
Chris Sherlaw-Johnson (https://orcid.org/0000-0002-4851-6060): (Senior Fellow) Conceptualisation (equal), Data curation (equal), Formal analysis (equal), Funding acquisition (equal), Investigation (equal), Methodology (equal), Project administration (equal), Software (equal), Supervision (lead), Visualisation (equal), Writing – original draft (lead), Writing – reviewing and editing (lead).
Theo Georghiou (https://orcid.org/0000-0001-9532-876X): (Senior Fellow) Conceptualisation (equal), Data curation (equal), Formal analysis (equal), Investigation (equal), Methodology (equal), Software (equal), Supervision (equal), Visualisation (equal), Writing – original draft (equal), Writing – reviewing and editing (equal).
Sarah Reed (https://orcid.org/0000-0002-0329-9728): (Senior Fellow) Conceptualisation (equal), Data curation (equal), Formal analysis (equal), Investigation (equal), Methodology (equal), Supervision (equal).
Rachel Hutchings (https://orcid.org/0009-0000-6083-7759): (Fellow) Conceptualisation (equal), Data curation (equal), Formal analysis (equal), Investigation (equal), Methodology (equal), Supervision (equal), Writing – original draft (equal), Writing – reviewing and editing (equal).
John Appleby (https://orcid.org/0000-0003-3839-9662): [Chief Economist and Head of Research (retired)] Conceptualisation (equal), Methodology (equal), Project administration (equal), Supervision (lead), Writing – reviewing and editing (equal).
Stuti Bagri (https://orcid.org/0009-0005-1267-2503): (Research Assistant) Data curation (support), Formal analysis (equal), Software (equal), Writing – original draft (equal), Writing – reviewing and editing (equal).
Nadia Crellin (https://orcid.org/0000-0002-9497-5874): (Fellow) Conceptualisation (equal), Data curation (equal), Formal analysis (equal), Funding Investigation (equal), Methodology (equal), Project administration (equal), Writing – original draft (equal), Writing – reviewing and editing (equal).
Stephanie Kumpunen (https://orcid.org/0000-0002-7668-8687): (Senior Fellow) Conceptualisation (equal), Investigation (equal), Methodology (equal), Writing – original draft (equal), Writing – reviewing and editing (equal).
Cyril Lobont (https://orcid.org/0009-0006-0031-2129): (Researcher) Data curation (equal), Formal analysis (equal), Investigation (equal), Writing – original draft (equal), Writing – reviewing and editing (equal).
Jenny Negus (https://orcid.org/0000-0002-7542-0443): (Patient representative) Conceptualisation (equal), Methodology (equal), Writing – reviewing and editing (equal).
Pei Li Ng (https://orcid.org/0000-0001-8411-220X): (Project Manager) Funding acquisition (lead), Project administration (lead).
Camille Oung (https://orcid.org/0009-0007-4504-310X): (Fellow) Data curation (equal), Formal analysis (equal), Investigation (equal), Methodology (equal), Writing – reviewing and editing (equal).
Jonathan Spencer (https://orcid.org/0000-0001-6660-6919): (Researcher) Conceptualisation (equal), Formal analysis (equal), Methodology (equal), Software (equal).
Angus Ramsay (https://orcid.org/0000-0002-4446-6916): (Principal Research Fellow) Conceptualisation (equal), Methodology (equal), Project administration (equal), Writing – reviewing and editing (equal).
Other contributions
Jenny Negus (Patient representative) PPIE contributions.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/VGQD4611.
Primary conflicts of interest: Dr Angus Ramsay is a Trustee of the Health Services Research UK. He was previously an NIHR HS&DR Associate Board Member (2013–7) and an NIHR HS&DR Commissioned Associate Board Member (2013–6).
Chris Sherlaw-Johnson has undertaken work for Health Navigator Ltd from funds paid to Nuffield Trust.
Data-sharing statement
Individual patient-level data and data supplied under specific data-sharing agreements cannot be made available by the study team. Sources for data that are already publicly available are supplied either in the text or the references. All qualitative data that can be shared are contained within the report. The nature of the data means that nothing else can be provided. Further information can be obtained from the corresponding author.
Ethics statement
Workstream 4 phase 1 and phase 2 staff interviews were categorised as a service evaluation by the HRA decision tool and UCL/UCLH Joint Research Office.
Workstream 4 phase 2 patient interviews and local data set analysis were reviewed and given favourable opinion by the London-Chelsea Research Ethics Committee [REC reference: 23/LO/0143 (April 2023)].
The use of HES outpatient data and emergency care data (workstreams 2, 3, and 4) was governed by a data-sharing agreement with NHS England covering NIHR RSET analysis (DARS-NIC-194629-S4F9X). HES/ECDS data are reused with permission of NHS England (Copyright © 2023, NHS England. All rights reserved).
P-EROC data was made available for use in workstream 4, governed by a data-sharing agreement between NHS England and NIHR RSET/Nuffield Trust (dated 7 June 2022).
Information governance statement
The study is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, UCL is the Data Controller for the qualitative data from patient interviews and the Nuffield Trust is the Data Controller for qualitative data from staff interviews. You can find out more about how we handle personal data, including how to exercise your individual rights, and the contact person for the UCL Data Protection Officer is Alex Potts (a.potts@ucl.ac.uk), and for the Nuffield Trust it is Tony Harbon (dataprotection@nuffieldtrust.org.uk).
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the NIHR. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the Health and Social Care Delivery Research programme, or the Department of Health and Social Care.
This monograph was published based on current knowledge at the time and date of publication. The NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Publications
Reed S, Crellin N. Patient-initiated Follow-up: Will It Free Up Capacity in Outpatient Care? The Nuffield Trust; 2022. URL: www.nuffieldtrust.org.uk/research/patient-initiated-follow-up-will-it-free-up-capacity-in-outpatient-care
Reed S, Crellin N. Patient-initiated Follow-up: Does It Work, Why It Matters, and Can It Help the NHS Recover? An Explainer. The Nuffield Trust; 2022. Revised 2024. URL: www.nuffieldtrust.org.uk/resource/patient-initiated-follow-up-does-it-work-why-it-matters-and-can-it-help-the-nhs-recover
Sherlaw-Johnson C, Georghiou T, Spencer J, Reed S, Crellin N, Oung C, et al. Patient-initiated Follow-up: Findings from Phase 1 of a Mixed-methods Evaluation. The Nuffield Trust; 2022. URL: www.nuffieldtrust.org.uk/research/patient-initiated-follow-up-findings-from-phase-1-of-a-mixed-methods-evaluation
Hutchings R. What Could Patient-initiated Follow-up Mean for Patient Care? The Nuffield Trust; 2024. URL: www.nuffieldtrust.org.uk/news-item/what-could-patient-initiated-follow-up-mean-for-patient-care-0
Sherlaw-Johnson C, Georghiou T, Hutchings R, Lobont C, Bagri S, Kumpunen S, et al. An Evaluation of Patient-initiated Follow-up (PIFU) Outpatient Services in the English NHS. The Nuffield Trust; 2024. URL: www.nuffieldtrust.org.uk/research/an-evaluation-of-patient-initiated-follow-up-pifu-outpatient-services-in-the-english-nhs
Preprints
Morris J, Georghiou T, Appleby J. Changes in English NHS outpatient activity during the early COVID-19 period. medRxiv. https://doi.org/10.1101/2021.04.28.21256176
Conference papers and seminars
Reed S. PIFU: emerging findings from a rapid evaluation. In: Harnessing the Value of PIFU in an Evolving Healthcare System. NHS Confederation Events. December 2022.
Hutchings R, Sherlaw-Johnson C, Georghiou T, Spencer J, Reed S, Crellin N, et al. Patient-initiated Follow-up: Findings from a Mixed-methods Evaluation. HSRUK 2023, Birmingham. July 2023. URL: www.youtube.com/watch?v=C-jAanv0JdY
Hutchings R. Patient Initiated Follow-Up – Learning from a Mixed Methods Evaluation. British Sarcoma Group conference, Leeds, February 2024.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care.
References
- NHS England . The NHS Long Term Plan 2019. www.longtermplan.nhs.uk/wp-content/uploads/2019/08/nhs-long-term-plan-version-1.2.pdf (accessed 5 November 2020).
- Royal College of Physicians . Outpatients: The Future – Adding Value Through Sustainability 2018. www.rcplondon.ac.uk/projects/outputs/outpatients-future-adding-value-through-sustainability (accessed 5 November 2020).
- NHS Digital . Hospital Outpatient Activity 2019–20 2020. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-outpatient-activity/2019-20 (accessed 25 September 2023).
- Castle-Clarke S, Edwards N. Rethinking Outpatient Services: Learning from an Interactive Workshop 2018. www.nuffieldtrust.org.uk/research/rethinking-outpatient-services-learning-from-an-interactive-workshop (accessed 4 November 2020).
- Scobie S. Are Strikes by Health Care Staff Impacting NHS Waiting Lists?. The Nuffield Trust; 2023.
- British Medical Association . NHS Backlog Data Analysis 2023. www.bma.org.uk/advice-and-support/nhs-delivery-and-workforce/pressures/nhs-backlog-data-analysis (accessed 18 September 2023).
- The Nuffield Trust . NHS Performance Tracker 2023. www.nuffieldtrust.org.uk/qualitywatch/nhs-performance-summary (accessed 18 September 2023).
- NHS Digital . Hospital Outpatient Activity 2021–22 2022. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-outpatient-activity/2021-22 (accessed 25 September 2023).
- Royal College of Physicians, Royal College of General Practitioners . Rebuilding the NHS: Resetting Outpatient Services for the 21st Century in the Context of COVID-19 2020. https://medicalcare.rcp.ac.uk/media/4qnpugot/4-rebuilding-the-nhs-policy_outpatients_202006_rcp_rcgp_principles_recommendations_0.pdf (accessed 25 September 2023).
- NHS Scotland . The Modern Outpatient: A Collaborative Approach 2017–2020 2017. www.gov.scot/publications/modern-outpatient-collabortaive-approach-2017-2020/ (accessed 5 November 2020).
- Welsh Government . Transforming the Way We Deliver Outpatients in Wales 2020. www.gov.wales/sites/default/files/publications/2020-06/transforming-the-way-we-deliver-outpatients-in-wales--a-three-year-strategy-and-action-plan-2020-2023.pdf (accessed 6 November 2020).
- NHS England . 2023/24/Priorities/and/Operational/Planning/Guidance 2023. www.england.nhs.uk/publication/2023-24-priorities-and-operational-planning-guidance/ (accessed 27 July 2023).
- Akobeng AK, O’Leary N, Vail A, Brown N, Widiatmoko D, Fagbemi A, et al. Telephone consultation as a substitute for routine out-patient face-to-face consultation for children with inflammatory bowel disease: randomised controlled trial and economic evaluation. EBioMedicine 2015;2:1251-6. https://doi.org/10.1016/j.ebiom.2015.08.011.
- Morris J, Campbell-Richards D, Wherton J, Sudra R, Vijayaraghavan S, Greenhalgh T, et al. Webcam consultations for diabetes: findings from four years of experience in Newham. Pract Diabetes 2017;34:45-50. https://doi.org/10.1002/pdi.2078.
- Ma Y, Jones G, Tay YK, Hunter T, Holden D, Rodgers-Wilson S, et al. Post-operative telephone review is safe and effective: prospective study – Monash outpatient review by phone trial. ANZ J Surg 2018;88:434-9. https://doi.org/10.1111/ans.14280.
- Kalafat E, Leslie K, Bhide A, Thilaganathan B, Khalil A. Pregnancy outcomes following home blood pressure monitoring in gestational hypertension. Pregnancy Hypertens 2019;18:14-20. https://doi.org/10.1016/j.preghy.2019.07.006.
- Vestergaard AS, Hansen L, Sorensen SS, Jensen MB, Ehlers LH. Is telehealthcare for heart failure patients cost-effective? An economic evaluation alongside the Danish TeleCare North heart failure trial. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-031670.
- de Jong MJ, van der Meulen-de Jong AE, Romberg-Camps MJ, Becx MC, Maljaars JP, Cilissen M, et al. Telemedicine for management of inflammatory bowel disease (myIBDcoach): a pragmatic, multicentre, randomised controlled trial. Lancet 2017;390:959-68. https://doi.org/10.1016/S0140-6736(17)31327-2.
- Bennett K, de Boisanger L, Moreton F, Davenport R, Stone J. The safety of using active triage to provide advice rather than a face-to-face neurology outpatient appointment. J R Coll Physicians Edinb 2019;49:193-8. https://doi.org/10.4997/JRCPE.2019.305.
- Gregory C. Improving colorectal cancer referrals. BMJ Open Qual 2018;7. https://doi.org/10.1136/bmjoq-2017-000280.
- Klimis H, Thiagalingam A, Altman M, Atkins E, Figtree G, Lowe H, et al. Rapid-access cardiology services: can these reduce the burden of acute chest pain on Australian and New Zealand health services?. Intern Med J 2017;47:986-91. https://doi.org/10.1111/imj.13334.
- Nisar MK. Early arthritis clinic is cost-effective, improves outcomes and reduces biologic use. Clin Rheumatol 2019;38:1555-60. https://doi.org/10.1007/s10067-019-04515-3.
- Sagar A, Mai DVC, Divya GS, Al-Habsi R, Wothers T, Ni Bhroin O, et al. A colorectal straight-to-test cancer pathway with general-practitioner-guided triage improves attainment of the 28-day diagnosis target and increases outpatient clinic capacity. Colorectal Dis 2021;23:664-71. https://doi.org/10.1111/codi.15410.
- Pokorny MA, Thorne PR, Whitfield BC, Lee AC, Wilson WJ. Can an advanced audiology-led service reduce waiting times for paediatric ear nose and throat outpatient services?. J Paediatr Child Health 2021;57:268-72. https://doi.org/10.1111/jpc.15218.
- Hill CE, Thomas B, Amendt A, Lawler M, Kalfin M. A multidisciplinary care model to increase access to neurologic ambulatory care. J Am Assoc Nurse Pract 2020;33:254-9. https://doi.org/10.1097/JXX.0000000000000435.
- Reed S, Crellin N. Patient-initiated Follow-up: Will It Free Up Capacity in Outpatient Care?. The Nuffield Trust; 2022.
- Bragge P, Clavisi O, Turner T, Tavender E, Collie A, Gruen RL. The global evidence mapping initiative: scoping research in broad topic areas. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-92.
- Snilstveit B, Vojtkova M, Bhavsar A, Gaarder M. Evidence Gap Maps – a Tool for Promoting Evidence-Informed Policy and Prioritizing Future Research. World Bank Policy Research Working Paper No 6725; 2013.
- Miake-Lye IM, Hempel S, Shanman R, Shekelle PG. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst Rev 2016;5. https://doi.org/10.1186/s13643-016-0204-x.
- Moher D, Liberati A, Tetzlaff J, Altman DG, ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Anugraha A, Dalal R, Raad M, Patel N, Sugathan H. Preconsultation questionnaires for patients attending elective foot and ankle clinics: is this the way forward in outpatient clinics?. Foot Ankle Spec 2022;15:487-93. https://doi.org/10.1177/1938640020986644.
- Kiruparan P, Kiruparan N, Debnath D. Impact of pre-appointment contact and short message service alerts in reducing ‘Did Not Attend’ (DNA) rate on rapid access new patient breast clinics: a DGH perspective. BMC Health Serv Res 2020;20. https://doi.org/10.1186/s12913-020-05627-2.
- Dusheiko M, Gravelle H. Choosing and booking-and attending? Impact of an electronic booking system on outpatient referrals and non-attendances. Health Econ 2018;27:357-71. https://doi.org/10.1002/hec.3552.
- Hull SA, Rajabzadeh V, Thomas N, Hoong S, Dreyer G, Rainey H, et al. Do virtual renal clinics improve access to kidney care? A preliminary impact evaluation of a virtual clinic in East London. BMC Nephrol 2020;21. https://doi.org/10.1186/s12882-020-1682-6.
- Buvik A, Bergmo TS, Bugge E, Smaabrekke A, Wilsgaard T, Olsen JA. Cost-effectiveness of telemedicine in remote orthopedic consultations: randomized controlled trial. J Med Internet Res 2019;21. https://doi.org/10.2196/11330.
- Creen J, Kennedy-Behr A, Gee K, Wilks L, Verdonck M. Reducing time between referral and diagnosis in paediatric outpatient neurodevelopmental and behavioural clinics. J Paediatr Child Health 2021;57:126-31. https://doi.org/10.1111/jpc.15156.
- Balaban RB, Zhang F, Vialle-Valentin CE, Galbraith AA, Burns ME, Larochelle MR, et al. Impact of a patient navigator program on hospital-based and outpatient utilization over 180 days in a safety-net health system. J Gen Intern Med 2017;32:981-9. https://doi.org/10.1007/s11606-017-4074-2.
- Horny M, Glover W, Gupte G, Saraswat A, Vimalananda V, Rosenzweig J. Patient navigation to improve diabetes outpatient care at a safety-net hospital: a retrospective cohort study. BMC Health Serv Res 2017;17. https://doi.org/10.1186/s12913-017-2700-7.
- Whitelaw L, Hammond K, Cumming M, Mansfield K, Saurman E. The direct access colonoscopy clinic: improving time to colonoscopy for eligible positive faecal occult blood test patients in Broken Hill NSW. Aust J Rural Health 2020;28:81-6. https://doi.org/10.1111/ajr.12569.
- Lindsay JR, Lawrenson G, English S. A service evaluation of e-triage in the osteoporosis outpatient clinic-an effective tool to improve patient access?. Arch Osteoporos 2020;15. https://doi.org/10.1007/s11657-020-0703-1.
- Donald M, Jackson CL, Byrnes J, Vaikuntam BP, Russell AW, Hollingworth SA. Community-based integrated care versus hospital outpatient care for managing patients with complex type 2 diabetes: costing analysis. Aust Health Rev 2021;45:42-50. https://doi.org/10.1071/AH19226.
- Hollingworth SA, Donald M, Zhang J, Vaikuntam BP, Russell A, Jackson C. Impact of a general practitioner-led integrated model of care on the cost of potentially preventable diabetes-related hospitalisations. Prim Care Diabetes 2017;11:344-7. https://doi.org/10.1016/j.pcd.2017.03.009.
- Emery JD, Jefford M, King M, Hayne D, Martin A, Doorey J, et al. ProCare trial: a phase II randomized controlled trial of shared care for follow-up of men with prostate cancer. BJU Int 2017;119:381-9. https://doi.org/10.1111/bju.13593.
- NHS Digital . Hospital Outpatient Activity – 2003–04 2007. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-outpatient-activity/hospital-outpatient-activity-2003-04 (accessed 9 May 2023).
- Keith J, Grimm F, Steventon A. How Better Use of Data Can Help Address Key Challenges Facing the NHS. The Health Foundation; 2022.
- Chaudhry Z, Mannan F, Gibson-White A, Syed U, Ahmed S, Majeed A. Research outputs of england’s hospital episode statistics (HES) database: bibliometric analysis. J Innov Health Inform 2017;24. https://doi.org/10.14236/jhi.v24i4.949.
- Bradley EH, Curry LA, Ramanadhan S, Rowe L, Nembhard IM, Krumholz HM. Research in action: using positive deviance to improve quality of health care. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-25.
- Walker AJ, Pretis F, Powell-Smith A, Goldacre B. Variation in responsiveness to warranted behaviour change among NHS clinicians: novel implementation of change detection methods in longitudinal prescribing data. BMJ 2019;367. https://doi.org/10.1136/bmj.l5205.
- Morris J, Georghious T, Appleby J. Changes in English NHS outpatient activity during the early COVID-19 period. MedRxiv 2021. https://doi.org/10.1101/2021.04.28.21256176.
- NHS Digital . Organisation Data Service (ODS) 2021. https://digital.nhs.uk/services/organisation-data-service (accessed 10 May 2023).
- Wickham H. Tidyverse: Easily Install and Load the ‘Tidyverse’ 2023. https://tidyverse.tidyverse.org/ (accessed 5 July 2023).
- Pretis F, Reade JJ, Sucarrat G. Automated general-to-specific (GETS) regression modeling and indicator saturation for outliers and structural breaks. J Stat Softw 2018;86:1-44. https://doi.org/10.18637/jss.v086.i03.
- NHS England . Implementing Patient Initiated Follow-Up: Guidance for Local Health and Care Systems (Version 1, 17 May 2022) 2022. www.england.nhs.uk/wp-content/uploads/2022/05/B0801-implementing-patient-initiated-follow-up-guidance-1.pdf (accessed 12 July 2023).
- Curtis HJ, Walker AJ, MacKenna B, Croker R, Goldacre B. Prescription of suboptimal statin treatment regimens: a retrospective cohort study of trends and variation in English primary care. Br J Gen Pract 2020;70:e525-33. https://doi.org/10.3399/bjgp20X710873.
- Hopcroft LEM, Curtis HJ, Croker R, Pretis F, Inglesby P, Evans D, et al. Data-driven identification of potentially successful intervention implementations: a proof of concept using five years of opioid prescribing data from over 7000 practices in England. MedRxiv 2023. https://doi.org/10.1101/2023.06.28.23291704.
- Castle JL, Doornik JA, Hendry DF. Short-term Forecasting of the Coronavirus Pandemic. Economics Group, Nuffield College, University of Oxford; 2020.
- Baxter R, Taylor N, Kellar I, Lawton R. What methods are used to apply positive deviance within healthcare organisations? A systematic review. BMJ Qual Saf 2016;25:190-201. https://doi.org/10.1136/bmjqs-2015-004386.
- Taliani CA, Bricker PL, Adelman AM, Cronholm PF, Gabbay RA. Implementing effective care management in the patient-centered medical home. Am J Manag Care 2013;19:957-64.
- Bradley EH, Byam P, Alpern R, Thompson JW, Zerihun A, Abebe Y, et al. A systems approach to improving rural care in Ethiopia. PLOS ONE 2012;7. https://doi.org/10.1371/journal.pone.0035042.
- Kraschnewski JL, Sciamanna CN, Pollak KI, Stuckey HL, Sherwood NE. The epidemiology of weight counseling for adults in the United States: a case of positive deviance. Int J Obes (Lond) 2013;37:751-3. https://doi.org/10.1038/ijo.2012.113.
- NHS England . GIRFT: Getting It Right First Time n.d. https://gettingitrightfirsttime.co.uk/workstreams/ (accessed 24 August 2023).
- NHS England . Core20PLUS5 (Adults) – An Approach to Reducing Healthcare Inequalities n.d. www.england.nhs.uk/about/equality/equality-hub/national-healthcare-inequalities-improvement-programme/core20plus5/ (accessed 24 August 2023).
- Isherwood J, Hillman T, Goddard A. Outpatients: The Future – Adding Value Through Sustainability 2018. www.rcplondon.ac.uk/projects/outputs/outpatients-future-adding-value-through-sustainability (accessed 14 July 2022).
- NHS England . 2021/22/Priorities/and/Operational/Planning/Guidance 2021. www.england.nhs.uk/wp-content/uploads/2021/03/B0468-nhs-operational-planning-and-contracting-guidance.pdf (accessed 25 September 2023).
- NHS England . Guide to Implementing Patient Initiated Follow-up (PIFU) in Adult Trauma and Orthopaedic Secondary Care Pathways 2023. www.england.nhs.uk/long-read/guide-to-implementing-patient-initiated-follow-up-pifu-in-adult-trauma-and-orthopaedic-secondary-care-pathways/ (accessed 21 July 2023).
- Vindrola-Padros C, Chisnall G, Cooper S, Dowrick A, Djellouli N, Symmons SM, et al. Carrying out rapid qualitative research during a pandemic: emerging lessons from COVID-19. Qual Health Res 2020;30:2192-204. https://doi.org/10.1177/1049732320951526.
- Greenhalgh T, Wherton J, Papoutsi C, Lynch J, Hughes G, A’Court C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.8775.
- Carter N, Bryant-Lukosius D, DiCenso A, Blythe J, Neville AJ. The use of triangulation in qualitative research. Oncol Nurs Forum 2014;41:545-7. https://doi.org/10.1188/14.ONF.545-547.
- Snilstveit B, Vojtkova M, Bhavsar A, Gaarder M. Evidence Gap Maps – a Tool for Promoting Evidence-Informed Policy and Prioritizing Future Research 2013. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2367606 (accessed 5 September 2023).
- Whear R, Thompson-Coon J, Rogers M, Abbott RA, Anderson L, Ukoumunne O, et al. Patient-initiated appointment systems for adults with chronic conditions in secondary care. Cochrane Database Syst Rev 2020;4. https://doi.org/10.1002/14651858.CD010763.pub2.
- Hong QN, Pluye P, Fabregues S, Bartlett G, Boardman F, Cargo M, et al. Mixed Methods Appraisal Tool (MMAT) Version 2018: User Guide. McGill University. Registration of Copyright (#1148552), Canadian Intellectual Property Office, Industry Canada; 2018.
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-50.
- Newton C, Nordin A, Rolland P, Ind T, Larsen-Disney P, Martin-Hirsch P, et al. British Gynaecological Cancer Society recommendations and guidance on patient-initiated follow-up (PIFU). Int J Gynecol Cancer 2020;30:695-700. https://doi.org/10.1136/ijgc-2019-001176.
- FutureNHS n.d. https://future.nhs.uk/ (accessed 12 July 2023).
- NHS England . 2022/23/Priorities/and/Operational/Planning/Guidance 2022. www.england.nhs.uk/wp-content/uploads/2022/02/20211223-B1160-2022-23-priorities-and-operational-planning-guidance-v3.2.pdf (accessed 25 July 2023).
- Cameron AC, Trivedi PK. Regression Analysis of Count Data. Cambridge: Cambridge University Press; 2013.
- Collett D. Modelling Survival Data in Medical Research. New York: Chapman and Hall/CRC; 2014.
- Jones A, Wyatt S. Classifying Outpatient Activity by Function: Introducing a Tool to Support Service Reform and Enhance Analyses of Outpatient Care. NHS Strategy Unit; 2021.
- NHS England . Action Required to Tackle Health Inequalities in Latest Phase of COVID-19 Response and Recovery n.d. www.england.nhs.uk/about/equality/equality-hub/national-healthcare-inequalities-improvement-programme/what-are-healthcare-inequalities/action-required-to-tackle-health-inequalities-in-latest-phase-of-covid-19-response-and-recovery/ (accessed 24 August 2023).
- Howlett M, Geist S. Routledge Handbook of Public Policy. London: Routledge; 2012.
- Lamont T, Barber N, de Pury J, Fulop N, Garfield-Birkbeck S, Lilford R, et al. New approaches to evaluating complex health and care systems. Br Med J 2016;352. https://doi.org/10.1136/bmj.i154.
- Markiewicz A, Patrick I. Developing Monitoring and Evaluation Frameworks. Thousand Oaks, CA: SAGE; 2016.
- Drojdahl Ryg N, Gram J, Haghighi M, Juhl CB. Effects of patient-initiated visits on patient satisfaction and clinical outcomes in a type 1 diabetes outpatient clinic: a 2-year randomized controlled study. Diabetes Care 2021;44:2277-85. https://doi.org/10.2337/dc20-3083.
- Poggenborg RP, Madsen OR, Sweeney AT, Dreyer L, Bukh G, Hansen A. Patient-controlled outpatient follow-up on demand for patients with rheumatoid arthritis: a 2-year randomized controlled trial. Clin Rheumatol 2021;40:3599-604. https://doi.org/10.1007/s10067-021-05674-y.
- Kieft E, Day J, Byng R, McArdle P, Goodwin VA. Bridging the second gap in translation: a case study of barriers and facilitators to implementing patient-initiated Clinics into secondary care. Eur J Person Centered Healthcare 2017;5:129-37.
- Child S, Goodwin VA, Perry MG, Gericke CA, Byng R. Implementing a patient-initiated review system in rheumatoid arthritis: a qualitative evaluation. BMC Health Serv Res 2015;15. https://doi.org/10.1186/s12913-015-0837-9.
- Beaver K, Martin-Hirsch P, Williamson S, Kyrgiou M. Exploring the acceptability and feasibility of patient-initiated follow-up for women treated for stage I endometrial cancer. Eur J Oncol Nurs 2020;44. https://doi.org/10.1016/j.ejon.2019.101704.
- Rogers A, Kennedy A, Nelson E, Robinson A. Patients’ experiences of an open access follow up arrangement in managing inflammatory bowel disease. Qual Saf Health Care 2004;13:374-8. https://doi.org/10.1136/qhc.13.5.374.
- Lorenc A, Wells M, Fulton-Lieuw T, Nankivell P, Mehanna H, Jepson M. PETNECK2 Research Team . Clinicians’ views of patient-initiated follow-up in head and neck cancer: a qualitative study to inform the PETNECK2 trial. Clin Oncol (R Coll Radiol) 2022;34:230-40. https://doi.org/10.1016/j.clon.2021.11.010.
- Hovdenak Jakobsen I, Juul T, Bernstein I, Christensen P, Jensen FS, Johansen C, et al. Follow-up after rectal cancer: developing and testing a novel patient-led follow-up program. Study protocol. Acta Oncol 2017;56:307-13. https://doi.org/10.1080/0284186X.2016.1267400.
- Lawes-Wickwar S, McBain H, Brini S, Hirani SP, Hurt CS, Flood C, et al. A patient-initiated treatment model for blepharospasm and hemifacial spasm: a randomized controlled trial. BMC Neurol 2022;22. https://doi.org/10.1186/s12883-022-02603-7.
- Bech B, Lykkegaard JJ, Lundbak T, Schroder HM, Birkeland LM, Schlyter ML, et al. Patient-Initiated Follow-Up (PIFU) as reorganized support for increased patient involvement – focus group discussions among patients’ with inflammatory arthritis. BMC Rheumatol 2020;4. https://doi.org/10.1186/s41927-020-00143-6.
- Morris J, Schlepper L, Dayan M, Jefferies D, Maguire D, Merry L, et al. Public Satisfaction with the NHS and Social Care in 2022. The Kings Fund, The Nuffield Trust; 2023.
- NHS England . Provisional Monthly Hospital Episode Statistics for Admitted Patient Care, Outpatient and Accident and Emergency Data, April 2023–May 2023 2023. https://digital.nhs.uk/data-and-information/publications/statistical/provisional-monthly-hospital-episode-statistics-for-admitted-patient-care-outpatient-and-accident-and-emergency-data/april-2023---may-2023 (accessed 2 August 2023).
- Whiting P, Savovic J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS group . ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol 2016;69:225-34. https://doi.org/10.1016/j.jclinepi.2015.06.005.
- NHS Data Model and Dictionary . CDS V6-3 n.d. www.datadictionary.nhs.uk/data_sets/cds_v6-3.html (accessed 6 September 2023).
Appendix 1 Scoping review search strategy and included studies
Introduction
We adopted an evidence-mapping approach to understand which service innovations have been more or less studied, and where the biggest gaps in understanding may be.
Search terms
Search terms were developed by conducting scoping work for relevant studies. Search terms for the broader review included: (1) setting ‒ outpatients, hospital, secondary, specialist services; (2) the innovation ‒ telemedicine, virtual consultations, remote monitoring, PIFU; and to the (3) outcome of interest ‒ efficiency, productivity, attendance rates, waiting lists, referral rates.
Study eligibility
The review included published studies conducted between January 2015 and July 2021:
-
Written in English.
-
From comparable health systems to the NHS ― that is, the review was limited to studies conducted within the top 20 Organisation for Economic Co-operation and Development (OECD) countries (by gross domestic product), given differences in health system context to improve the chances that the issues relating to outpatient delivery were broadly similar to those within the UK. Countries included Japan, South Korea, New Zealand, UK, France, Canada, Finland, Australia, Belgium, Germany, Sweden, Iceland, Austria, Netherlands, Denmark, USA, Norway, Switzerland, Ireland and Luxembourg.
-
Evaluating an outpatient service and examining the effect on outpatient or secondary care activity and resources (e.g. waiting times, referral rates, attendances, missed appointments, costs). Secondary outcomes of interest included, but were not limited to, access, patient experience and outcomes, or staff experience and outcomes.
Exclusion criteria:
-
Interventions that focused exclusively on patient self-management, education or self-care, or clinical interventions, treatment, or diagnosis.
-
Dissertations/theses.
-
Proceedings.
-
Published abstracts only.
-
Articles with no empirical data/research component (e.g. commentaries, published protocols).
-
Full texts not available.
Procedure (data collection, data extraction and synthesis)
All references retrieved were exported into EndNote referencing software (v20) and duplicates removed. Two independent reviewers (SR and NC) screened the title and abstracts of citations for eligibility. For those identified as relevant, or in ambiguous cases, the full text was sought. A data extraction database (Excel) was developed and piloted independently by two reviewers (SR and NC). Data extracted included type of study, country, sample, intervention, specialty, details of methods including data collection, outcomes, and analysis, and main findings.
We used a pre-specified framework to categorise innovations by type and anticipated benefits (e.g. how they might affect outpatient activity or resource use). Due to the evidence mapping approach, rapid timescale and broad scope of the review, a quality assessment of included studies was not undertaken. Characteristics of the selected studies are summarised in Appendix 1, Table 13.
| Study characteristics | Number of studies (n = 149) | |
|---|---|---|
| Setting | ||
| Country | Australia | 25 |
| Belgium | 1 | |
| Canada | 6 | |
| Denmark | 7 | |
| France | 6 | |
| Germany | 2 | |
| Ireland | 3 | |
| Netherlands | 13 | |
| New Zealand | 2 | |
| Norway | 4 | |
| Not applicable (review) | 5 | |
| Spain | 1 | |
| Sweden | 2 | |
| UK | 27 | |
| USA | 46 | |
| Year | 2015 | 16 |
| 2016 | 12 | |
| 2017 | 22 | |
| 2018 | 30 | |
| 2019 | 23 | |
| 2020 | 27 | |
| 2021 | 19 | |
| Design | ||
| Innovation type | Teleconsultations/virtual appointments (incl. remote monitoring) | 42 |
| Management tools, administrative systems, and processes to support appointment scheduling, incl. patient reminders and pre-consultation communication | 45 | |
| New roles, non-specialist-led clinics, including task-shifting | 25 | |
| Drop-in clinics, direct access clinics, and rapid triage | 25 | |
| Advice and Guidance, shared care models, and models that move care from secondary to primary care settings | 21 | |
| Personalised follow-up and open booking | 7 | |
| Other (e.g. intensive outpatient care clinics) | 9 | |
| Outcome | ||
| Potential benefits | Reduce referral volume and improve appropriateness of referrals | 36 |
| Book patients into all available time slots and reduce missed appointments | 26 | |
| Only using face to face when it adds value | 43 | |
| Optimising skill mix and staff mix (incl. making best use of clinic support roles) | 40 | |
| Reducing unnecessary administration, optimising clinical time per consultation (incl. minimising patient waits in clinic) | 48 | |
| Appointments scheduled based on clinical need, streamlining number of appointments (incl. more effective sequencing of clinical input) | 49 | |
| Improving patient support and understanding of when to seek care | 28 | |
Appendix 2 Workstream 2: additional detail
Section 1: Primary and secondary exclusion of time series
We made a set of primary exclusions of units where there were relatively few attendances. There were two primary exclusion conditions (determined pragmatically based on an initial analysis of the data):
-
where there were fewer than 100 average attendances per 28-day period in 2019; or
-
where there was a least one 28-day period without any attendances between 2017 and 2019 inclusive.
With smaller units removed, we additionally made secondary exclusions for specific activity measures:
-
DNAs: we excluded time series with a mean number of DNAs per 28-day period of below 5.
-
Tele-attendances: we similarly excluded time series with a mean number of tele-attendances per 28-day period of below 5.
-
Follow-up to first attendance ratio: we excluded time series with a mean number of first or follow-up attendances per 28-day period of below 5.
These secondary exclusions were made with reference to the 2017–9 period inclusive. The implication of these latter exclusions was that a unit would still have its ‘attendances’ time series included for modelling (so long as it had passed the threshold of the primary exclusions), but any, or all, of the other three time series may have been excluded from modelling.
Section 2: Impulse and trend indicator saturation modelling
Indicator saturation has been a crucial development in GETS [general-to-specific] modeling to address the distorting influence of outliers and structural breaks (changes in parameters) in econometric models. Such parameter changes are generally of unknown magnitudes and may occur at unknown times. Indicator saturation tackles this challenge by starting from a general model allowing for an outlier or shift at every point and removing all but significant ones using GETS selection. This serves both as a method to detect outliers and breaks, as well as a generalized approach to model mis-specification testing – if the model is well-specified, then no outliers/shifts will be detected. 52
As Pretis and colleagues suggest, Indicator saturation (IS) is a method that can help us to choose between theoretical models to find a model compatible with underlying data. It can be used to detect ‘breaks’ in time series data. Effectively, these are points in time where a change appeared to have occurred: either a step change, a change in the trend, or where there were more isolated outlying data points that did not align with overall trends.
For the purpose of the study, we made combined use of impulse and trend indicator saturation methods. The former to help us deal with outlying data points, the latter to detect trend-line changes in activity measures – ones that we hoped might be associated with the impacts of innovations. We did not model step changes; we considered that changes to outpatient services would not typically be associated with abrupt step changes in measures of outpatient activity.
Implementing impulse and trend saturation modelling has three main steps:
-
A ‘fully saturated’ time series regression model (otherwise known as a General Unrestricted Model) – which includes a full set of impulse and trend variables (see Appendix 2, Figure 21) – is estimated.
-
Using GETS automated methods, the model is reduced in complexity by removing statistically insignificant regressors using backwards elimination and comparing overall fit with an information criterion. This method does not identify a particular order to remove insignificant regressors, and removing these in different orders can lead to different final models. To account for this, the insignificant regressors are removed in different orders, creating multiple paths that lead to potentially different ‘terminal’ models. Terminal models are models where all remaining regressors are significant.
-
Once all paths have been calculated, the best terminal model is selected using an information criterion selection.
FIGURE 21.
Example structure of a ‘saturated’ model impulse and trend variables, for a time series with 100 data points.
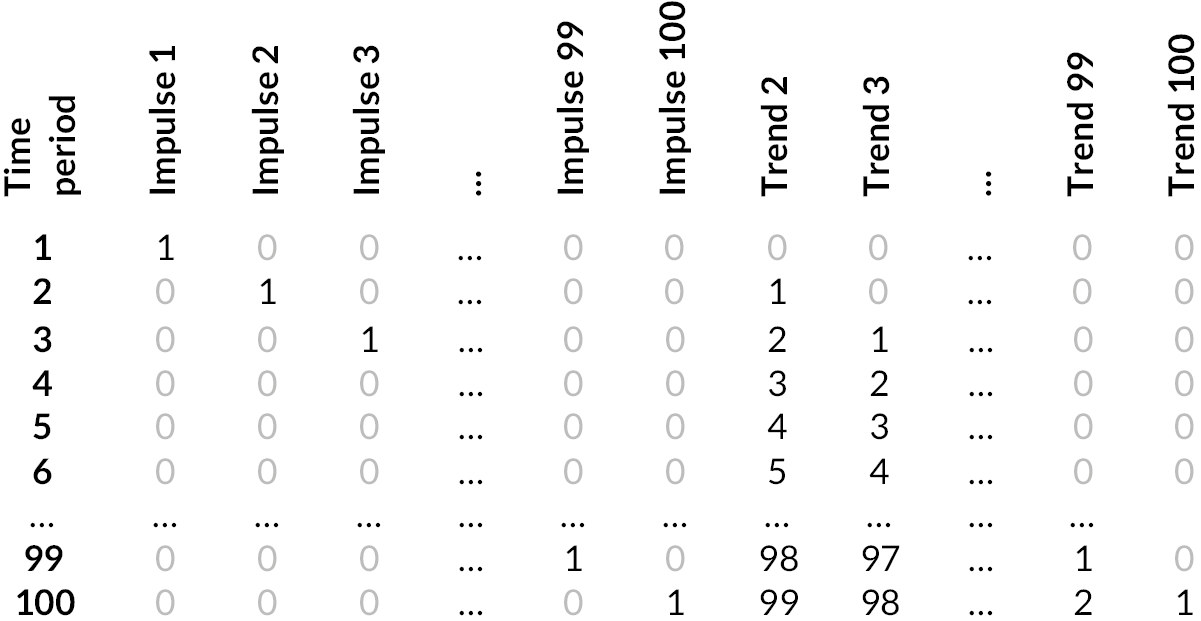
Appendix 2, Figure 22 shows examples of the outputs of IS applied to real-time series. In these examples, 92 data points (each representing a 28-day period) are modelled by 1–6 trend lines (shown in red), with 0 to 5 break points between the trends.
FIGURE 22.
Example time series: attendances in four units over time. Actual 28-day period values (black points) and modelled lines (red). Time period numbers from 1 (period starting 12 December 2012) to 92 (period ending 31 December 2019).
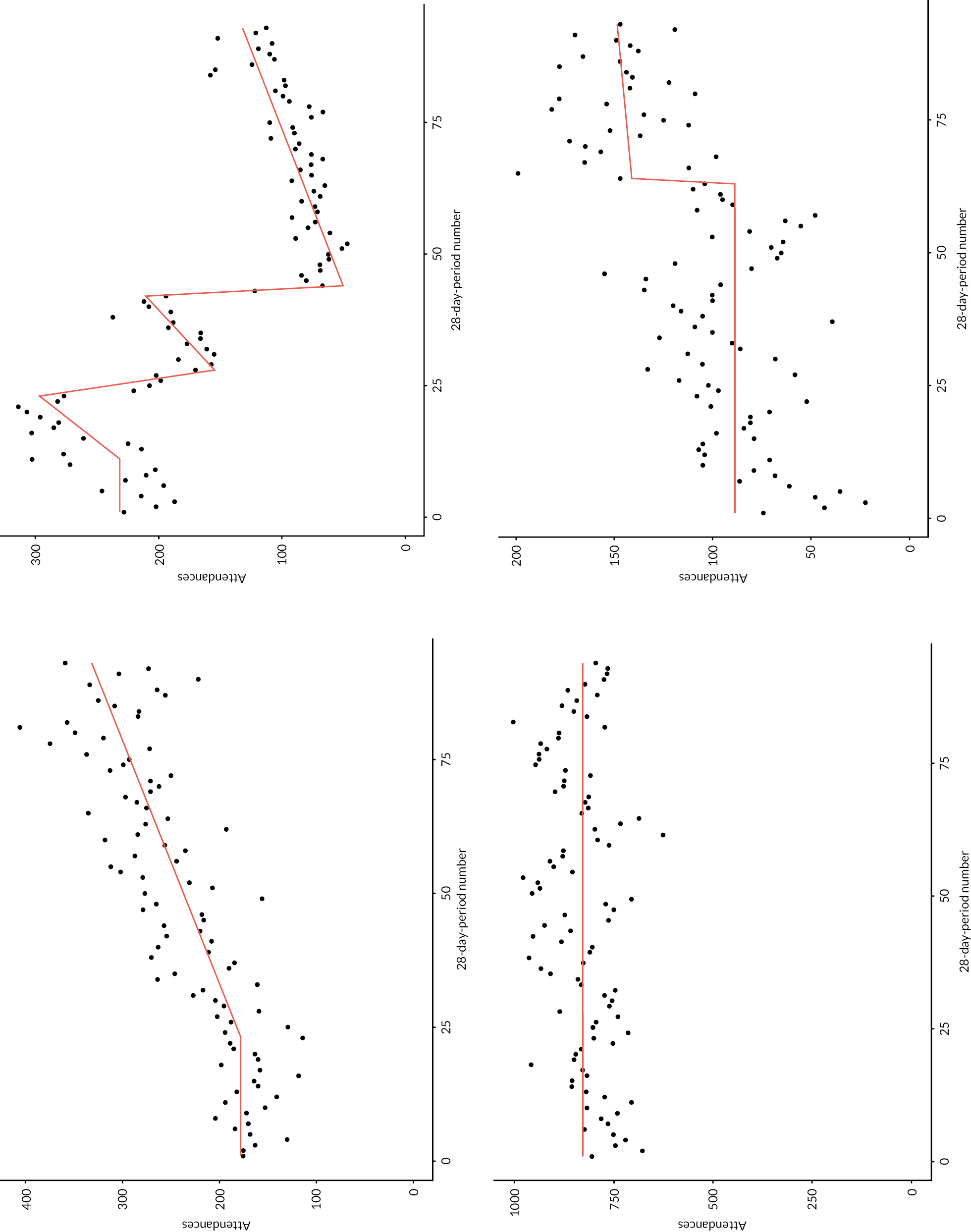
Section 3: Summary ranking metrics; considerations in ‘metric’ development
As an example of the challenge: Appendix 2, Figure 23 shows two example time series – the modelled follow-up to first attendance ratios of two units (one red, the other blue) over time. The PPCPs for each are highlighted as bold lines. Lower follow-up to first appointment values are considered, for the study’s purposes, to be more positive: over time, for every first attendance, there were fewer follow-up attendances.
FIGURE 23.
Two example time series: follow-up to first attendance ratios, with PPCPs highlighted as thicker lines.
There are challenges in deciding which unit’s PPCP we would prioritise in a ranking to identify innovations to evaluate. For example:
-
The magnitude of the fall of the red unit is larger than that of the blue unit – so the red unit had the most significant ‘positive’ direction fall.
-
However, the red unit change started in mid-2016, while the blue unit’s change was more recent, starting in 2018. In practice, the more recent a change, the more likely it might be for us to (a) be able to identify a service change as the cause, and (b) more efficiently carry out an evaluation (for reasons of staff turnover, institutional memory, etc).
-
In addition, the red unit’s follow-up to first attendance ratio fell for a relatively short period, and then reverted to a higher value. The blue unit’s fall was for slightly longer and was still ongoing leading up to the end of the analysis period.
The examples of these descriptive metrics are not exhaustive, and for any single metric consideration – there are potential pitfalls, for example, a large magnitude change might be implausibly large.
Section 4: Examples of application of indicator saturation to time series (detecting break points and outliers)
FIGURE 24.
Indicator saturation modelled time series, two examples for each activity measure. Points are 28-day period values during the baseline and search periods (late 2012 to end 2019). Red lines are IS-modelled trend lines. Outlying data points are shown in orange, attached to a hashed vertical line (see attendances example 1).
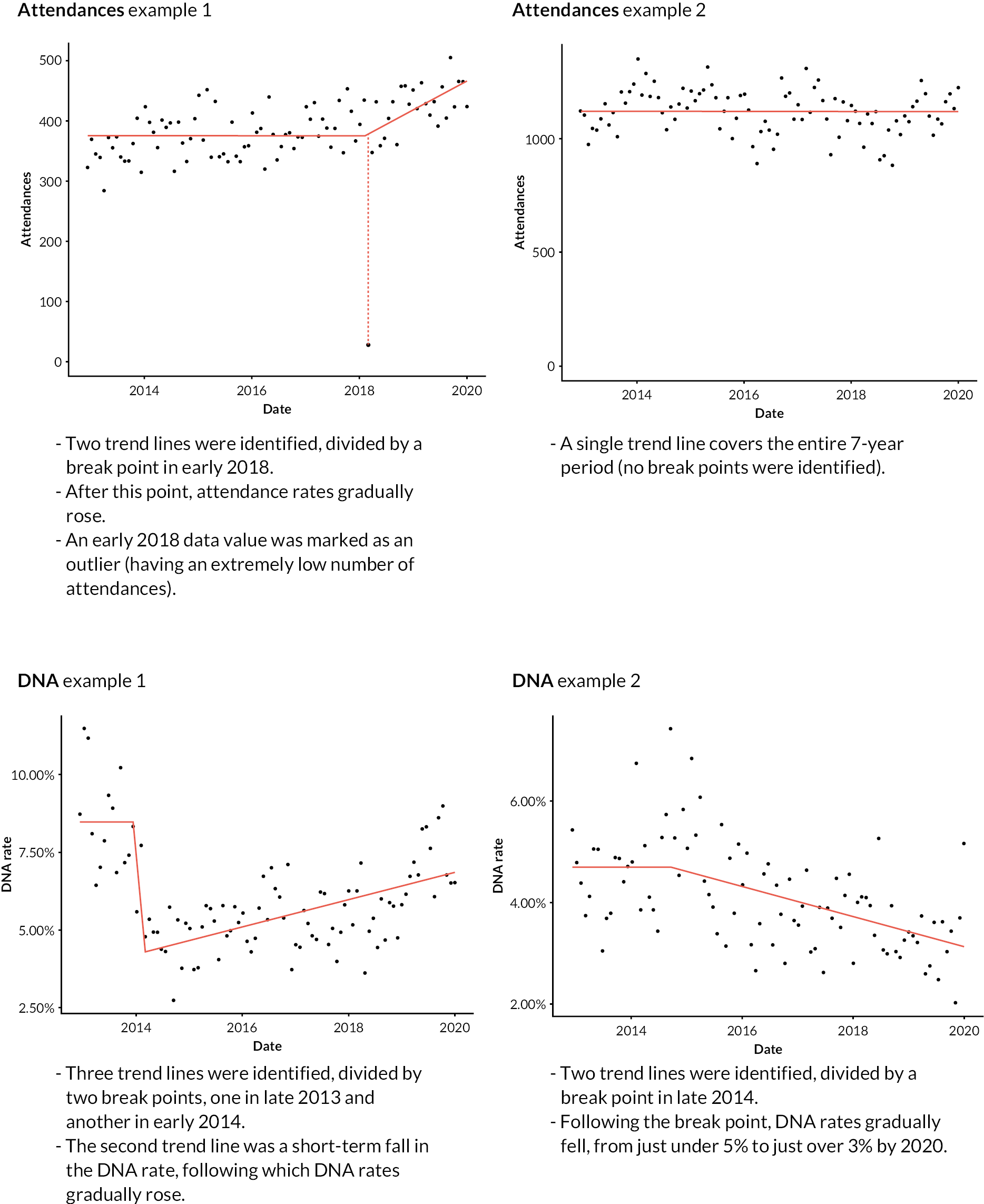
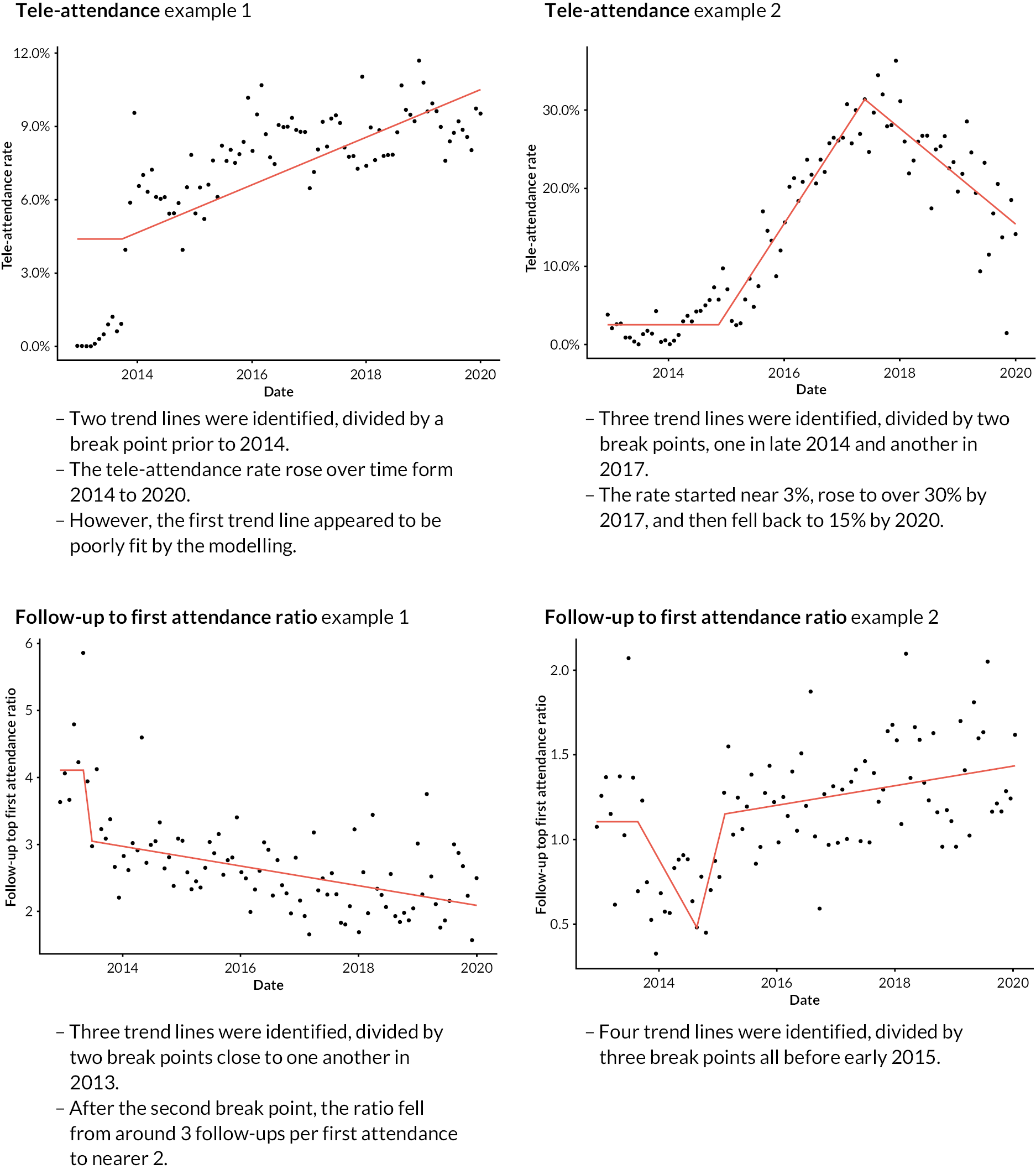
Section 5: Selection of primary positive change periods from modelled time series
Appendix 2, Figure 25 summarises PPCP selection, with example modelled time series. The axes are activity measure rates or ratios, and in these examples, falling trends are ‘positive’ changes. Red lines are IS-modelled time series, with individual trend lines numbered. Broken lines are merged adjacent trend lines. Bold lines are those with the largest magnitude change in the search period in a positive direction (the PPCP).
FIGURE 25.
Primary positive change period selection, summarised with example modelled time series.
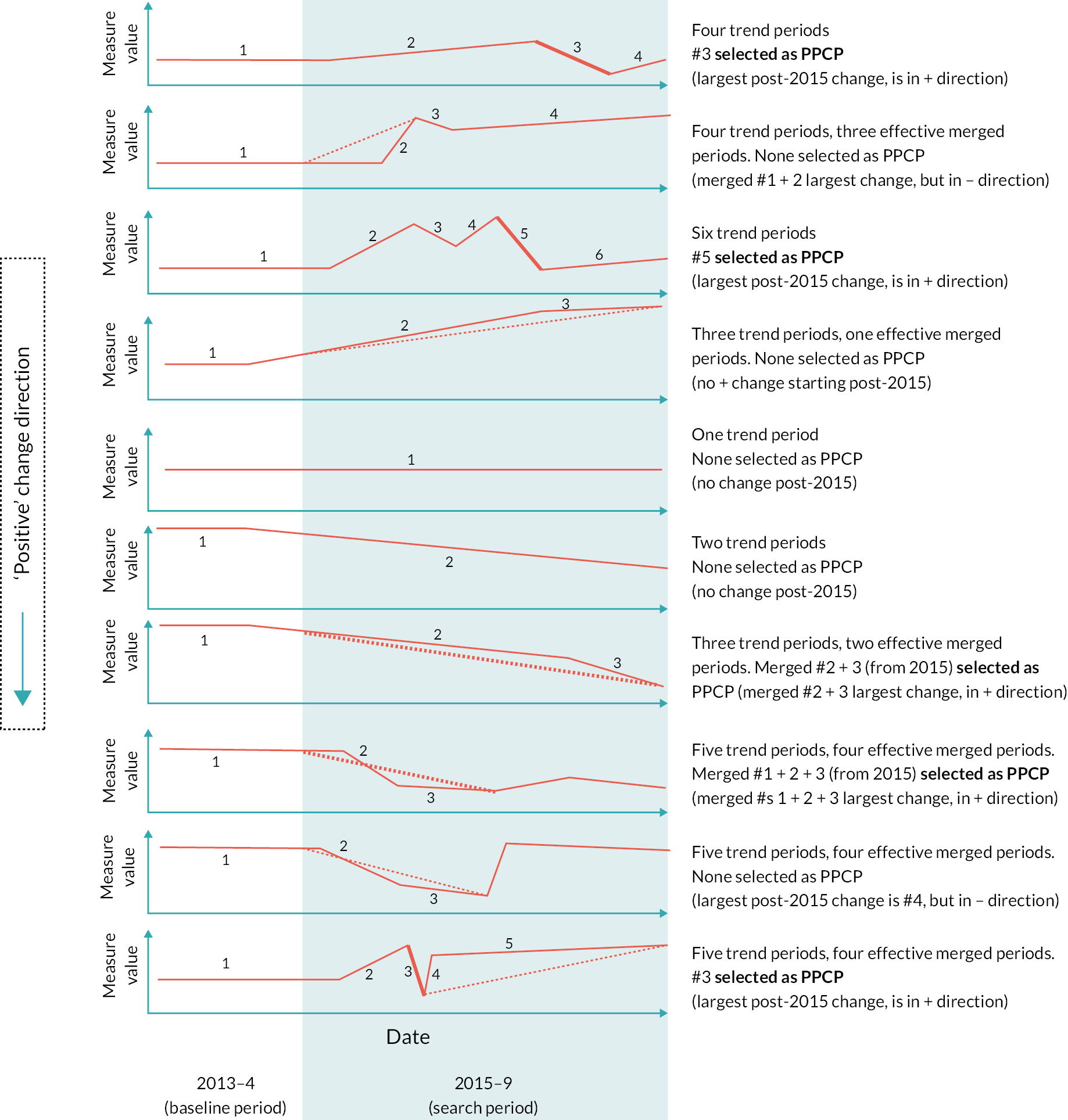
Section 6: Iterations of attempts at ranking
The initial attempt was to rank 3316 time series used 5 metrics, combining as in (1). The weights were chosen pragmatically, based on our judgement of the relative importance of each metric: the magnitude metric was given then highest weighting, and the recency metric the lowest.
Reviewing the outcome of this ranking, we found a number of follow-ups to first attendance time series for a specific specialty (anticoagulant service) highly scored, with very large magnitude falls during the search period. While this was interesting to note, it appeared to reflect widespread changes within the service over many trusts related to medication changes (to newer drugs which no longer needed regular blood test monitoring), rather than seeming to reflect specific innovations we might investigate.
As a result, we introduced the ‘end percentile’ metric, to include a ranking element that placed positive changes in the context of other trusts’ performance for a specific clinical specialty.
With this as an addition, the second attempt to rank the models was a minor update to the first, see (4).
Following this second attempt three researchers (JS, TG, JA) reviewed 100 time series – with a focus on the top of the ranking – to gather some early reflections on the approach.
It was clear that the rankings were not behaving optimally. Two key reflections were that:
-
The magnitude metric appeared to be dominating the ranking, to an unreasonable extent.
-
Recency and persistence had a combining effect: both had maximum positive values for PPCPs that ended at the end of the search period (as many PPCPs did – see Appendix 2, Section 5), over-promoting such time series.
So for the third ranking attempt (5), we moved to a simpler approach, with fewer metrics contributing to the ranking score (keeping persistence at the expense of recency, and also removing outliers, as outliers were relatively rare), and also with the relative weightings removed (an attempt to rebalance other metrics vs. magnitude).
As we were implementing this approach, the wider study’s focus turned to the evaluation of PIFU, and further progress – including reflecting on and responding to the third ranking attempt – stopped.
Section 7: Study limitations – expanded discussion points
We identified a number of areas where the approach had limitations. These are discussed here.
-
Broadness of ambition, especially within a rapid context. We intended the outcome of the quantitative approach to be one where national outpatient time series data would be fed in, and the methodology would take care of removing small or unviable units, and prioritise the remainder. In practice, the complexity of covering, for example, more than a hundred clinical specialties, and different activity measures, made progress challenging. It may have been better to have focused, for example, on a more limited subset of clinical specialities (identified by a combination of data analysis, literature scoping, and external advice), to develop a plausible approach, and to consider more carefully the implications of extending to more services.
-
Analysis of multiple activity measures. We intended to start modestly by including four outpatient activity measures, adding others later if possible. But the four measures highlighted a fundamental difficulty in the overall aim: how were we to prioritise changes in very different measures (e.g. improvements in DNAs vs. improvements in follow-ups)? This was a key challenge. It may be that had we sought more specific input from policy-makers and service managers, we might have made progress resolving this before the study changed its focus.
-
Choice of individual activity measures. In addition, the four outpatient activity measures themselves, and the direction we used to denote positive performance may not have been wholly appropriate. For example, with respect to attendance rates, while there were efforts to reduce specific types of attendances, it was not apparent that success would lead to reduced attendances overall. Instead, it is more likely that capacity would be freed for other – perhaps more necessary – attendances.
-
Substantial pandemic-related changes. In addition, with regard to the tele-attendance measure, the impact of the COVID-19 pandemic was immediate, substantial and apparently long term. From a pre-pandemic rate of 4%, tele-attendances rose to 36% of all attendances in early 2020, and by the 2022–23 year had settled to just under 20% (our own analysis of national data). The search period was entirely pre-pandemic, and any significant positive changes that we found – even where caused by an intentional service change – would have been from the context of a service that had now fundamentally changed. To a lesser extent, follow-up to first attendance ratios were also impacted by the onset of the pandemic: they rose (due to the relatively large fall in first attendances),49 however ratios nationally have broadly settled back to pre-pandemic levels. 94
-
The individual, and cumulative impact of analytical choices. The complexity and ambition of the analysis meant that we pragmatically and rapidly moved through its stages. But each stage of the analysis is open to comment and critique. For example:
-
Initial time series filtering: this removed a substantial number of units from the analysis (nearly half of 10,000), and several thousand additional measure-specific time series.
-
IS modelling: the application of IS modelling was successful, but alternative approaches might have led to different impacts.
-
The PPCP: we chose one change (or series of changes) within a modelled time series to represent the entire time series when ranking, but alternative approaches might have been used. In addition, the implementation of PPCP selection was not always internally consistent (e.g. compare the 6th and 7th time series in Figure 3). As the eligibility of time series for ranking was determined at this stage, this will also have had an impact on ultimate outcomes.
-
Metric creation and ranking approach – this remained very much a work in progress as the study’s focus turned to evaluation of PIFU. While more iterations may have led us closer to an acceptable ranking, it is not clear what an optimal ranking would have looked like. Some of the metrics we created were flawed and would have been priorities to improve in subsequent iterations.
-
PIFU-related analysis. Aided by the smaller amount of data and increased simplicity of dealing with a single activity measure, we decided to focus less on an algorithmic ranking. We imposed additional – somewhat arbitrary, although defensible – sets of filters, and we judged units’ improvements (falls in follow-up to first attendance ratios) ourselves. We chose to use the substantial amount of work already carried out to complete the task, however, if we had started with an aim to analyse a single measure, it is likely that we would have made different analytical choices in prior stages, or indeed, have proposed entirely different, perhaps more widely used, methods.
-
Ultimately, the time and resources needed to fully consider the implications of each analytical stage (through sensitivity and other analyses) were not available. A simpler, better focused analysis would have required fewer choices and would have been easier to test.
-
Understanding data quality. HES OP data constitute a large data set with promise for researchers, but there are concerns about its quality. 45,46 While only a modest sample, that 2 of 3 units interviewed did not recognise data derived from HES OP describing their services should give us pause in how we use outputs without some element of local validation. Extensive local validation input into national analyses is often not feasible, and so researchers and analysts must be cautious. The data owners, meanwhile, should aim to identify and address data quality gaps.
Appendix 3 Site sampling framework
| Variable | Sites (n = 5) |
|---|---|
| Geography | |
| Rural | 2 |
| Urban | 3 |
| Size | |
| Small | 1 |
| Medium (500–850 beds) | 2 |
| Large (> 850 beds) | 2 |
| Academic status | |
| General | 1 |
| Teaching | 4 |
| Deprivation | |
| Higher levels of deprivation | 2 |
| Lower levels of deprivation | 2 |
| Mixed levels of deprivation | 1 |
| Ethnicity | |
| Higher levels of ethnic diversity | 2 |
| Lower levels of ethnic diversity | 3 |
| Length of time delivering PIFU | |
| < 5 years | 3 |
| 5 + years | 2 |
Appendix 4 Breakdown of staff interviews
| Staff | Patients | |
|---|---|---|
| Phase 1 | ||
| Site 1 | 2 × operational (site-wide) | N/A |
| 2 × clinical (specialty level) | ||
| Site 2 | 2 × operational (site-wide) | N/A |
| 2 × clinical (specialty level) | ||
| Site 3 | 1 × operational (site-wide) | N/A |
| 2 × clinical (specialty level) | ||
| National | 1 × operational | N/A |
| 1 × clinical | ||
| Phase 2 | ||
| Site 4 (2.1) | 1 × clinical (site level) | 3 × Breast Care |
| 3 × operational (site level) | ||
| 8 × clinical (specialty level) | ||
| 3 × primary care | ||
| Site 5 (2.2) | 1 × clinical (site level) | 1 × Gynaecology |
| 2 × operational (site level) | ||
| 4 × clinical (specialty level) | ||
| 1 × operational (specialty level) | ||
| Total | 36 | 4 |
Appendix 5 Evidence review methods
The rapid evidence review and findings emerged from the wider scoping review (described in Rapid scoping review of outpatient innovations) conducted to understand the evidence that exists relating to different outpatient service innovations and their impact on outpatient service utilisation (e.g. waiting times, referral rates, attendances, missed appointments, costs, etc.). We followed a pre-defined protocol and adopted an evidence mapping approach in order to provide timely information to inform the later stages of the evaluation. We followed recommendations from similar evidence mapping reviews. Where applicable, we have reported the methods and findings according to the PRISMA guidelines – see end of this appendix for PRISMA checklist. The rapid evidence review has been published as a Nuffield Trust report.
Further details of the search strategy and data extraction process are provided below.
Search strategy
We conducted a search across four databases for relevant studies published in English between January 2015 and June 2022, both in the UK and internationally. These databases were MEDLINE, EMBASE and the SSCI for academic literature and the HMIC for grey literature. The searches were supplemented by hand searches of papers reported by systematic reviews and forward and backward citation searches.
Eligibility criteria
Papers were eligible if they met the following criteria; focused on the effect of patient-initiated or open-access approaches to follow-up on service use (e.g. waiting times, referral rates, attendances, missed appointments, costs, etc.) as either a primary or secondary outcome, given the focus on personalising follow-up to help bring down waiting times and reduce backlogs as part of the NHS’s elective recovery strategy. We also report on patient outcomes and experience, and clinical outcomes (where they were a focus of the included studies), but they were not the primary focus of this review.
Papers were excluded if they met any of the following criteria; non-English language, published pre-2015; dissertations/thesis, conference proceedings, published abstracts, articles with no empirical data/research component (e.g. commentaries or published protocols); studies from outside the top 20 OECD countries (given differences in health system context that contribute to inefficiencies in outpatient delivery); papers focusing on only self-management, self-care, education or clinical treatment.
Search terms
Search terms were developed by conducting scoping work for relevant studies. Search terms for the broader review included (1) setting; outpatients, hospital, secondary, specialist services; (2) the innovation; telemedicine, virtual consultations, remote monitoring, PIFU, and (3) outcome of interest; efficiency, productivity, attendance rates, waiting lists, referral rates. We supplemented the broader search strategy with PIFU-related terms adapted from the Whear et al. 70 Cochrane review (i.e. patient-initiated, patient led, patient access, self-refer, open access, direct access, advanced access, shared care, patient choice/choosing, same day schedule).
Procedure (data collection, data extraction, and synthesis)
All references retrieved were exported into EndNote referencing software (v20) and duplicates removed. Two independent reviewers (SR and NC) screened the title and abstracts of citations for eligibility. For those identified as relevant, or in ambiguous cases, the full text was sought. If there was any uncertainty, final agreement was sought from a third and fourth researcher (TG and JS). See Appendix 5, Figure 26 for details of study selection.
FIGURE 26.
Flow chart of study selection.
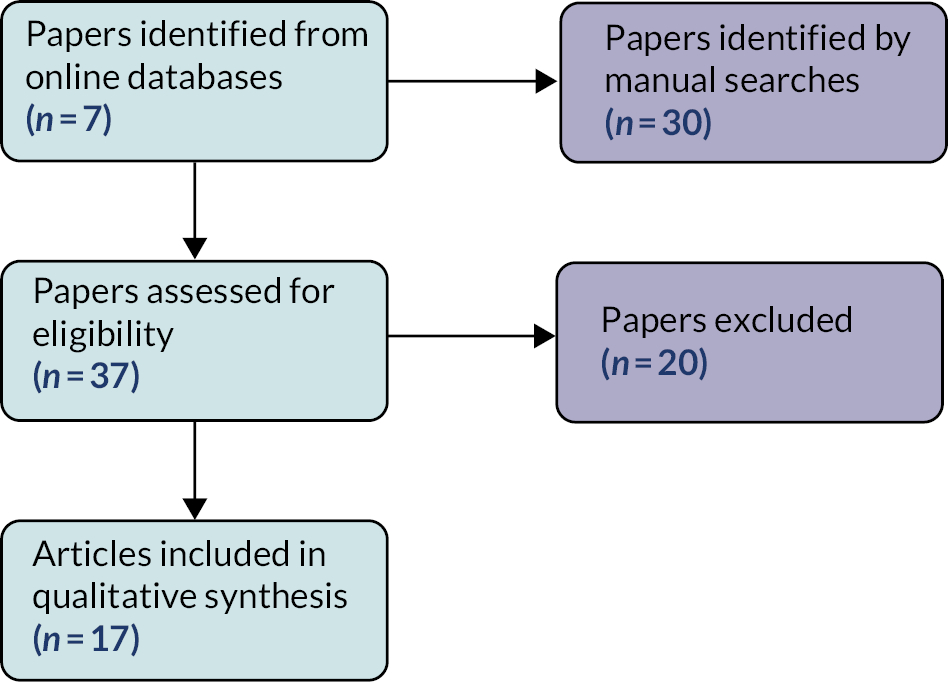
A data extraction database (Excel) was developed and piloted independently by two reviewers (SR and NC). Data extracted included study aims, specialty and study population, country and setting, intervention characteristics, type of study and duration, sample, main outcomes, and findings including any subgroup analyses and quality score (as outlined below). Any uncertainty or queries in the data extraction process were discussed between reviewers (SR and NC).
Outcomes recorded included those related to service use (i.e. number of appointments or visits with outpatient services, telephone contacts, teleconsultations, hospital visits, GP visits, missed visits), clinical outcomes, patient satisfaction, experience, and quality of life (i.e. mental wellbeing, depression and anxiety, fear of recurrence, confidence), and also costs. Other relevant outcomes were recorded if reported, such as change of treatment and safety indicators.
Extracted data were tabulated into a summary matrix of the evidence. Information presented included author, specialty and country, key components of the PIFU intervention (where reported), and findings related to outpatient service use, other health service use, health service costs, clinical outcomes, and patient satisfaction and quality of life. Studies and outcomes were coded according to whether PIFU was found to be associated with a positive, negative or no impact. Data describing the interventions were used to generate a figure of the key characteristics and components of PIFU models, and the review also summarised previously conducted reviews and qualitative work examining PIFU in a table format.
Quality assessment
The quality of the eligible studies was assessed using the modified MMAT, with adapted scoring from the Risk of Bias in Systematic Reviews (ROBIS) tool. The MMAT has been widely used in similar rapid reviews and can be used for a range of study designs. 71 The tool covers five domains, as determined by the study design. For randomised trials domains include: appropriateness of the randomisation; comparability of baseline groups; data completeness; blinding; and adherence to the intervention(s). For non-randomised, observational studies, domains include: relevance of the sampling strategy, whether the sample is representative of the target population; appropriateness of measures; risk of nonresponse bias; and appropriateness of the statistical analysis for the RQ. The scoring was adapted from the ROBIS tool – each domain was scored as yes (1), no (0), or can’t tell (0). 95 Scores were summed across the five domains to produce a total score. A score of 5 was indicative of high quality (low risk of bias), 4 moderate quality (moderate risk of bias), 3 low quality (high risk of bias) and 2 or less very low quality (very high risk of bias). Other quality related considerations were also noted (particularly relevant to the generalisability of findings) including selection bias, number of study sites, study power, length of follow-up.
Preferred Reporting Items for Systemic Reviews and Meta-Analyses checklist
The PRISMA checklist for the review is on the following page. Where deviations from PRISMA guidelines are reported this is due to the evidence mapping approach that was adopted – the aim of the review was primarily to understand and summarise the existing evidence for personalised follow-ups, as well as identify any evidence gaps. We did not set out to synthesise findings beyond the narrative approach or tabulation presented in the reported, nor conduct any quantitative meta-analyses. In addition, given that the review has already been published as a Nuffield Trust report, the reporting requirements and presentation of the review differs from traditional academic literature. We have therefore included further detail of the methods in this appendix and in the NT report appendix itself. 26 The page numbers referred to in the PRISMA checklist relate to the published NT report, and ‘report appendix’ refers to the appendix published alongside the NT report.
Appendix 6 Staff-reported risks and opportunities of Patient-Initiated Follow-Up
| Risk or opportunity | Mechanism(s) | ||
|---|---|---|---|
| Expanding use of PIFU | To a broader range of clinical conditions | Opportunity |
|
|
|||
|
|||
|
|||
| Adapting approaches to PIFU (to increase uptake/facilitate patient engagement) | Opportunity |
|
|
|
|||
|
|||
| Using PIFU on a larger scale | Opportunity |
|
|
|
|||
|
|||
| Ideally, we’d get to a place where we’re able to move more patients from the existing backlog onto PIFU, but we can’t do that because PIFU requires a shared decision. But there are a lot of patients with overdue follow-ups for whom PIFU would work quite well. We currently have a ‘two-tier’ system where PIFU patients can come straight in while others in the backlog are just waiting.Operational, trust-wide – Site 2.1 | |||
| Improved use of technology | Technology to support service delivery | Opportunity |
|
|
|||
| Technology and data to support monitoring processes and understanding of health inequalities | Opportunity |
|
|
|
|||
| Clinicians would then be much happier with patients not being ‘active’ in the system. GPs would also buy into that as well – so they [GPs] wouldn’t think if something goes wrong, patients would just end up knocking on my door. . . [we] need to develop these tools that can sit in the middle and help primary and secondary care feel more confidence and control.Clinical, trust-wide ‒ Site 2.2 | |||
|
|||
| To support new staffing models or ways of working | Creating new roles | Opportunity |
|
|
|||
|
|||
| Expanding or shifting of existing roles | Opportunity |
We’ve been able to move people [clinicians] doing a lot of follow-up to doing mainly fast-track. Demand for new patient appointments has rocketed. It allows us to flex things.Clinical, specialty level ‒ Site 2.1 |
|
|
|||
|
|||
| To support collaboration and linking across services | Increasing opportunities for information sharing and shared care | Opportunity |
|
|
|||
|
|||
| Make sure that all parts of the system affected are represented in the discussions ... You know, active engagement and constructive discussions and making sure that the discharge summaries come back to primary care and the patient on time.GP | |||
|
|||
| Patient care | Patients being ‘lost’ in the system | Risk |
What would be helpful, I think is a better IT system to mean clinicians are completely 100% reassured that these patients are not going to get lost and that they’re going to come back for their appointments when they need them. And that means that you’ll have many more patients that appear to be in a PIFU. Nothing would have changed. It’s just that they’re recorded.Clinical – specialty level ‒ Site 2.2 |
|
|||
Where there [concerns] are specialties like ophthalmology, which don’t have much primary care there is that concern that you’re just going to abandon people. And I think specialties with lots of with good primary care don’t have to worry about that.Clinical, trust-wide – Site 2.1 |
|||
| Patients not initiating contact | Risk |
I think the biggest risk is that patients will not recognise their deterioration.Operational, trust-wide ‒ Site 2.2 |
|
|
|||
|
|||
I guess my main challenge/worry is patients not wanting to bother the doctor. And I wonder whether part of that is around the fact that it’s lungs and they know we got hammered over COVID and they’re just trying to be nice and not bother us.Clinical, specialty level – Site 2.1 |
|||
|
|||
| Health inequalities | Exacerbating inequalities | Risk |
|
Those few people [who are ineligible for PIFU] probably have the highest need for healthcare. So yet again, is this another means by which some people will be disadvantaged? So you’re not put on it because you’re not deemed stable and therefore you wait on a long waiting list.GP |
|||
| Service capacity and demand | Increased demand for appointments | Risk |
Most of the patients that we’re putting on would be patients that would be discharged anyway... so, if anything, you might have an impact in the other direction, increasing demand.Clinical, specialty level ‒ Site 2.1 |
|
|||
| Increased staff workload | Risk |
|
|
|
|||
| Impact on other services | Activity displaced to primary care | Risk |
|
If it works really well you shouldn’t see an impact in primary care because they should go directly back [to outpatient teams], but if not there is a high risk there will be push back into primary care.GP |
|||
|
|||
|
|||
| Activity displaced to urgent and emergency care | Risk |
|
|
Appendix 7 Additional analysis of impact and sensitivity analysis
Section 1: Comparisons of Patient-Initiated Follow-Up uptake between trusts reporting complete and partial submissions into the Provider Elective Recovery Outpatient Collections data
Background
Each month, NHS trusts that submit data on PIFU activity to P-EROC will state whether their submission is complete or partially complete. For March 2023, the latest month in the anthesis, 58.5% of submissions were reported as ‘complete’ and 39.4% reported as ‘partial’ (see Figure 14, Measuring the impact of Patient-Initiated Follow-Up). (The remaining 2.1% were classified as ‘null’ or ‘unknown’.)
From interviews in phase 1 of the PIFU evaluation, and from views of stakeholders, we were told of potential inconsistency in how different trusts interpreted their returns as ‘complete’ or ‘partial’. For example, when trusts submit complete data for the specialties they decide to include but no data for other specialties where there are patients on PIFU, the issue is whether they classify this as ‘complete’, because the data is complete for the submitted specialties, or ‘partial’, because not all PIFU specialties were submitted. For this reason, we decided to compare levels of PIFU reporting between complete and partial submissions and determine if there were any substantial differences. This would then inform the decision about whether to include partial submissions in the analysis.
Methods
We used Poisson regression to compare levels of PIFU reporting between complete and partially complete submissions. The Poisson regression model used the number of people moved or discharged to PIFU each month as a dependent variable with type of submission and month as explanatory variables. The natural logarithm of the number of outpatient attendances each month was the offset variable. Both the provider trust and the treatment specialty were included as repeated measures.
Provider Elective Recovery Outpatient Collections has separate fields for numbers of patients moved to PIFU pathways each month and numbers discharged to PIFU. The sum of these numbers was the dependent variable. If both these numbers were not reported in a given month, the dependent variable was treated as missing. If one of these values was not missing, then that was used as the total. We limited the analysis to the top 30 specialties by numbers of patients moved or discharged to PIFU between September 2021 and March 2023.
Results
Monthly PIFU counts per attendance split by reported data completeness are shown in Appendix 7, Table 17. The mean uptake among partial submissions is approximately 18% compared to 8% for complete submissions.
| Submission type | Number of observations (combinations of provider, specialty, and month) | Mean ratio of numbers of patients moved or discharged to PIFU to total outpatient attendances (standard error) |
|---|---|---|
| Complete | 28,700 | 0.082 (0.009) |
| Partial | 24,087 | 0.184 (0.057) |
Poisson regression analysis accounting for changes over time within provider trust and specialty, showed no significant difference in uptake between trusts reporting complete or partial submissions (8.5% increase – 95% CI 10.1% decrease to 32.2% increase). For this reason, we included data from both complete and partial submissions in the baseline analysis.
Discussion
The findings suggest that there is no difference in the ratios of patients transferred to PIFU each month per outpatient attendance. This provides evidence to support the view that the differences between complete and partial submissions maybe more due to how different organisations account for what specialties are left out of the data rather than the completeness of the specialties that are included.
This analysis has not been carried out within specialties, and there may be differences observed at that level. Also, it may be possible that trusts with more PIFU activity are more likely to report partial submissions, although we have no evidence that this is the case.
Section 2: Additional analyses of impact and sensitivity analysis
Introduction
The Poisson regression models relied on many assumptions relating to:
-
the lag between putting patients onto PIFU pathways and observed impact on attendance
-
whether we include P-EROC submissions that are reported to be ‘partially’ complete
-
how we handle missing data. In particular, where a trust has reported no data for a given specialty in a given month, when we know, either from other P-EROC months or HES that the trust has outpatient activity within that specialty
-
the denominator used for measuring PIFU rate: whether per outpatient attendance over the period or per unique patient.
In this appendix we present the impact these different assumptions have on the findings and so identify which are the more important areas of uncertainty.
The range of assumptions
The different assumptions are listed below. Those labelled ‘baseline’ were applied to our analysis presented in Measuring the impact of Patient-Initiated Follow-Up of the main report.
The lag between putting patients onto PIFU pathways and observed impact
This assumption also influences the choice of periods for counting outcomes as well as use of PIFU. We have applied two options:
-
Option 1. Two annual periods for measuring outcomes: March 2019 to February 2020 (Pre-COVID-19 period), April 2022 to March 2023. PIFU use was measured over the second period.
-
Option 2. Two 6-month periods for measuring outcomes: September 2019 to February 2020, October 2022 to March 2023. PIFU use was measured in the 6 months prior to the second period (April 2022 to September 2022). (Baseline assumption.)
Completeness of P-EROC submission
Again, we have tested two options:
-
Option 1. Include all submissions reported as ‘complete’ or ‘partial’. (Baseline assumption.)
-
Option 2. Include only those submissions reported as ‘complete’.
Missing data options
The P-EROC records numbers of patients moved to PIFU pathways each month within each trust and specialty, and, separately, the numbers discharged to PIFU each month. It also reports the numbers of patients within each trust and specialty who are on PIFU pathways at the start of each month (referred to as ‘PIFU episodes’). Patients who are on more than one PIFU pathway in different specialties will be counted multiple times, once for each specialty.
For the analysis of impact, we developed four options for handling missing data in P-EROC, each progressively more stringent. For those trusts already selected under the option for including complete or partial data, these options are as follows:
-
Option 1. We include all P-EROC data on numbers moved or discharged to PIFU pathways. For any specialty that we know is operating at a trust (either from P-EROC or HES) any missing values in P-EROC are assumed to be zero. If no outpatient attendances are reported in HES for the trust specialty for any month over the period, we are measuring PIFU usage then the trust/specialty combination is excluded.
-
Option 2. We apply option 1. However, if the total numbers moved or discharged to PIFU over the period we are measuring PIFU usage are fewer than the recorded change in PIFU episodes (indicative of incorrect data), then data from that trust/specialty combination are not used.
-
Option 3. We apply option 2. However, if data on both numbers moved and numbers discharged to PIFU are not recorded for at least 1 month over the period we are measuring PIFU usage, the trust/specialty combination is excluded.
-
Option 4. We apply option 3. However, any missing data are assumed to be unknown and therefore not used. (Baseline assumption.)
Measuring PIFU rate
We tested two options for measuring PIFU rate:
-
Option 1. The ratio between the number of people moved or discharged to PIFU pathways over the period and total outpatient attendance over the same period. (Baseline assumption.)
-
Option 2. The ratio between the number of people moved or discharged to PIFU pathways over the period and the number of unique patients attending outpatient appointments within the same specialty over the same period.
Additional outcomes and sensitivity analysis
Multispecialty analysis at trust and specialty level (Poisson regression)
We ran additional Poisson regression models which operated under a different set of assumptions than the ones used in the baseline analysis. Results from these analyses, along with results from the baseline analysis, are shown in Appendix 7, Table 18.
| Assumptions applied (see above for option descriptions) | Measure | % change in outcome (95% CI) | p-value |
|---|---|---|---|
| (Baseline analysis) | Outpatient attendances | −0.6% (−1.9% to 0.7%) | 0.370 |
| Proportion of outpatient attendances that are reported as ‘structured review’ | −0.3% (−1.2% to 0.8%) | 0.496 | |
| Follow-up attendances identified as ‘structured review’ per patient | −1.5% (−3.9% to 1.0%) | 0.239 | |
| Outpatient attendances recorded as ‘follow-up’ | −0.3% (−1.1% to 0.6%) | 0.621 | |
| Proportion of appointments where the patient did not attend (DNA) | 0.0% (−0.3% to 0.3%) | 0.866 | |
| Missing data options = 1 | Outpatient attendances | −0.8% (−2.0% to 0.4%) | 0.165 |
| Proportion of outpatient attendances that are reported as ‘structured review’ | −0.1% (−0.9% to 0.6%) | 0.678 | |
| Follow-up attendances identified as ‘structured review’ per patient | −1.7% (−4.4% to 1.1%) | 0.221 | |
| Outpatient attendances recorded as ‘follow-up’ | −0.2% (−0.6% to 0.3%) | 0.460 | |
| Proportion of appointments where the patient did not attend (DNA) | −1.3% (−4.0% to 1.3%) | 0.318 | |
| Missing data options = 2 | Outpatient attendances | −0.8% (−1.9% to 0.4%) | 0.197 |
| Proportion of outpatient attendances that are reported as ‘structured review’ | −0.2% (−1.0% to 0.7%) | 0.672 | |
| Follow-up attendances identified as ‘structured review’ per patient | −1.7% (−4.4% to 1.1%) | 0.219 | |
| Outpatient attendances recorded as ‘follow-up’ | −0.2% (−0.6% to 0.3%) | 0.458 | |
| Proportion of appointments where the patient did not attend (DNA) | −1.1% (−3.7% to 1.5%) | 0.402 | |
| Missing data options = 3 | Outpatient attendances | −0.5% (−1.6% to 0.6%) | 0.357 |
| Proportion of outpatient attendances that are reported as ‘structured review’ | −0.2% (−1.0% to 0.7%) | 0.650 | |
| Follow-up attendances identified as ‘structured review’ per patient | −1.3% (−3.6% to 1.1%) | 0.281 | |
| Outpatient attendances recorded as ‘follow-up’ | −0.2% (−0.7% to 0.4%) | 0.476 | |
| Proportion of appointments where the patient did not attend (DNA) | −0.7% (−3.0% to 1.6%) | 0.534 | |
| Measuring PIFU rate = 2 | Outpatient attendances | 0.0% (−0.1% to 0.1%) | 0.561 |
| Proportion of outpatient attendances that are reported as ‘structured review’ | 0.0% (−0.1% to 0.1%) | 0.906 | |
| Follow-up attendances identified as ‘structured review’ per patient | 0.0% (−0.2% to 0.2%) | 0.801 | |
| Outpatient attendances recorded as ‘follow-up’ | 0.0% (−0.1% to 0.1%) | 0.474 | |
| Proportion of appointments where the patient did not attend (DNA) | 0.0% (−0.2% to 0.2%) | 0.814 | |
| Completeness of P-EROC submission = 2 | Outpatient attendances | −1.0% (−2.2% to 0.2%) | 0.082 |
| Proportion of outpatient attendances that are reported as ‘structured review’ | −0.9% (−2.7% to 0.9%) | 0.308 | |
| Follow-up attendances identified as ‘structured review’ per patient | −2.2% (−5.3% to 1.0%) | 0.169 | |
| Outpatient attendances recorded as ‘follow-up’ | −0.5% (−1.6% to 0.5%) | 0.301 | |
| Proportion of appointments where the patient did not attend (DNA) | −0.4% (−2.3% to 1.6%) | 0.694 | |
| Period lag = 2 | Outpatient attendances | −1.1% (−2.4% to 0.2%) | 0.093 |
| Proportion of outpatient attendances that are reported as ‘structured review’ | −0.2% (−1.0% to 0.6%) | 0.587 | |
| Follow-up attendances identified as ‘structured review’ per patient | −1.8% (−4.3% to 0.8%) | 0.167 | |
| Outpatient attendances recorded as ‘follow-up’ | −0.2% (−0.8% to 0.3%) | 0.355 | |
| Proportion of appointments where the patient did not attend (DNA) | 0.0% (−0.2% to 0.2%) | 0.743 |
None of the models reported a statistically significant relationship (p < 0.05) between PIFU rate and outpatient activity when calculated across multiple specialties. In this respect, the findings from the additional analyses did not deviate from the baseline findings reported in the main report.
Single-specialty analysis at trust and specialty level (Poisson regression)
The main outcome for this analysis presented in the main report was the number of structured reviews per patient. Associations between PIFU rate and other outcome measures are shown in Appendix 7, Table 19 and Appendix 7, Figure 28. After adjusting for multiple testing (p-value threshold = 0.0033) there were statistically significant findings for a reduction in overall outpatient attendances per patient within Urology and Respiratory Medicine. There were also statistically significant findings within Urology for follow-ups per patient and DNAs per patient. Specialties were only included in this analysis if 30 or more trusts reported activity within the specialty.
| Specialty | Outpatient attendance | Outpatient attendances recorded as ‘follow-ups’ | Proportion of outpatient attendances that are reported as ‘structured review’ | Proportion of appointments where the patient DNA | ||||
|---|---|---|---|---|---|---|---|---|
| % change in outcome (95% CI) | p-value | % change in outcome (95% CI) | p-value | % change in outcome (95% CI) | p-value | % change in outcome (95% CI) | p-value | |
| Urology | −4.2% (−6.8% to −1.5%) | 0.002 | −3.4% (−5.3% to −1.6%) | 0.000 | −0.4% (−9.0% to 8.9%) | 0.926 | −15.4% (−24.2% to −5.6%) | 0.003 |
| Breast Surgery | 0.1% (−2.0% to 2.3%) | 0.926 | −1.0% (−4.6% to 2.7%) | 0.590 | −18.2% (−30.9% to −3.1%) | 0.020 | −8.7% (−17.1% to 0.7%) | 0.069 |
| Trauma and Orthopaedic | −0.4% (−1.9% to 1.1%) | 0.557 | −0.2% (−1.5% to 1.1%) | 0.733 | −0.3% (−4.2% to 3.7%) | 0.859 | 0.1% (−0.4% to 0.6%) | 0.772 |
| ENT | −.02% (−1.8% to 1.5%) | 0.821 | −2.2% (−4.6% to 0.3%) | 0.077 | −18.5% (−29.3% to −6.1%) | 0.005 | −9.7% (−18.9% to 0.6%) | 0.065 |
| Ophthalmology | −2.1% (−15.1% to 13.0%) | 0.774 | −7.1% (−14.7% to 1.2%) | 0.092 | 9.1% (−36.3% to 86.8%) | 0.751 | −20.1% (−37.4% to 1.9%) | 0.071 |
| Pain Management | −2.0% (−6.7% to 2.8%) | 0.405 | −4.2% (−7.6% to −0.5%) | 0.024 | −7.3% (−14.6% to 0.7%) | 0.072 | 5.0% (−2.5% to 13.1%) | 0.193 |
| Gastroenterology | −0.9% (−2.2% to 0.5%) | 0.188 | −0.5% (−4.3% to 3.3%) | 0.776 | 0.6% (−5.1% to 6.7%) | 0.845 | −2.7% (−5.7% to 0.5%) | 0.091 |
| Cardiology | 2.3% (−13.2% to 20.6%) | 0.787 | 20.2% (−3.5% to 49.7%) | 0.101 | −0.9% (−52.5% to 106.9%) | 0.981 | −26.4% (−42.4% to −5.8%) | 0.015 |
| Dermatology | −2.5% (−7.2% to 2.5%) | 0.322 | 1.0% (−2.8% to 4.8%) | 0.623 | 14.2% (−8.4% to 42.3%) | 0.239 | −24.6% (−38.6% to −7.6%) | 0.007 |
| Respiratory Medicine | −15.4% (−23.8% to −6.1%) | 0.002 | −17.6% (−30.2% to −2.8%) | 0.022 | 10.7% (−31.1% to 77.9%) | 0.675 | −4.3% (−24.8% to 21.9%) | 0.725 |
| Neurology | −0.8% (−2.2% to 0.6%) | 0.233 | 0.8% (0.4% to 1.3%) | 0.001 | −0.7% (−4.9% to 3.6%) | 0.740 | −1.1% (−2.7% to 0.6%) | 0.201 |
| Rheumatology | −0.4% (−5.4% to 4.9%) | 0.885 | 0.2% (−1.5% to 1.9%) | 0.837 | 5.3% (−1.8% to 12.9%) | 0.146 | −0.4% (−7.5% to 7.2%) | 0.913 |
| Paediatric | −4.1% (−8.6% to 0.6%) | 0.084 | 0.2% (−8.9% to 10.1%) | 0.975 | 1.9% (−16.3% to 24.2%) | 0.850 | 7.0% (−3.8% to 18.9%) | 0.213 |
| Gynaecology | −1.0% (−5.8% to 4.1%) | 0.699 | −0.2% (−4.0% to 3.6%) | 0.889 | −12.8% (−25.9% to 2.7%) | 0.101 | −3.1% (−12.2% to 7.1%) | 0.542 |
| Physiotherapy | 0.0% (−0.1% to 0.1%) | 0.318 | 0.0% (0.0% to 0.0%) | 0.138 | 0.0% (−0.1% to 0.1%) | 0.764 | 0.0% (−0.1% to 0.2%) | 0.936 |
Results of applying the different assumptions relating to measuring PIFU rates, inclusion of complete submissions and the lag between PIFU rates and outcome are shown in Appendix 7, Table 20 for structured reviews per patient and Appendix 7, Table 21 for DNAs. These findings were all consistent with the results under the baseline assumptions, with no significant relationships within all specialties for structured reviews per patient and a significant reduction in DNAs within Urology. Assuming no lag between PIFU rates and outcome, there was a significant reduction in DNAs within Dermatology.
| Specialty | Assumptions applied (see above for option descriptions) | |||||||
|---|---|---|---|---|---|---|---|---|
| Measuring PIFU uptake = 2 | Completeness of P-EROC submission = 2 | Period lag = 2 | Baseline assumptions | |||||
| % change in outcome (95% CI) | p-value | % change in outcome (95% CI) | p-value | % change in outcome (95% CI) | p-value | % change in outcome (95% CI) | p-value | |
| Urology | −3.3% (−11.1% to 5.1%) | 0.428 | −2.2% (−9.2% to 5.5%) | 0.570 | −4.6% (−15.3% to 7.3%) | 0.926 | ||
| Breast Surgery | −10.8% (−19.7% to −.8%) | 0.035 | −9.5% (−21.7% to 4.8%) | 0.182 | −18.7% (−32.3% to −2.4%) | 0.020 | ||
| Trauma and Orthopaedic | −0.8% (−4.9% to 3.3%) | 0.678 | −2.6% (−14.2% to 10.6%) | 0.684 | −0.4% (−4.3% to 3.6%) | 0.827 | −1.5% (−8.2% to 5.7%) | 0.859 |
| ENT | −12.9% (−21.5% to −3.4%) | 0.009 | −13.5% (−25.5% to 0.5%) | 0.058 | −18.9% (−30.2% to −5.7%) | 0.005 | ||
| Ophthalmology | 2.4% (−21.3% to 33.3%) | 0.859 | −5.6% (−61.1% to 128.8%) | 0.898 | 7.2% (−38.6% to 87.2%) | 0.751 | ||
| Pain Management | −2.9% (−10.1% to 5.0%) | 0.465 | −13.2% (−23.4% to −1.6%) | 0.027 | −8.8% (−17.4% to 0.8%) | 0.072 | ||
| Gastroenterology | 0.1% (−4.5% to 4.8%) | 0.989 | −0.8% (−9.5% to 8.7%) | 0.861 | −0.3% (−6.8% to 6.7%) | 0.845 | ||
| Cardiology | −0.8% (−35.0% to 51.3%) | 0.968 | −11.3% (−41.0% to 33.2%) | 0.563 | 1.4% (−47.3% to 94.8%) | 0.981 | ||
| Dermatology | 11.% (−2.7% to 26.5%) | 0.119 | 1.3% (−22.2% to 31.8%) | 0.924 | 10.9% (−12.7% to 40.9%) | 0.239 | ||
| Respiratory Medicine | −1.5% (−31.5% to 41.6%) | 0.935 | 5.6% (−38.1% to 80.1%) | 0.841 | −6.4% (−42.2% to 51.7%) | 0.675 | ||
| Neurology | −0.8% (−4.3% to 2.7%) | 0.635 | −4.7% (−17.7% to 10.5%) | 0.524 | −1.6% (−6.9% to 4.1%) | 0.740 | ||
| Rheumatology | 7.5% (−2.7% to 18.6%) | 0.153 | 0.8% (−27.8% to 40.6%) | 0.964 | 9.3% (−12.9% to 37.2%) | 0.443 | 4.3% (−5.6% to 15.3%) | 0.146 |
| Paediatric | 0.2% (−14.4% to 17.2%) | 0.984 | 0.9% (−17.6% to 23.5%) | 0.933 | −2.3% (−22.9% to 23.9%) | 0.850 | ||
| Gynaecology | −9.3% (−18.0% to 0.2%) | 0.055 | −12.5% (−26.7% to 4.7%) | 0.144 | −15.3% (−30.0% to 2.6%) | 0.091 | −14.1% (−27.7% to 1.9%) | 0.101 |
| Physiotherapy | 0.0% (0.0% to 0.1%) | 0.498 | −0.1% (−0.4% to 0.3%) | 0.509 | 0.0% (−0.1% to 0.1%) | 0.764 | ||
| Specialty | Assumptions applied (see above for option descriptions) | |||||||
|---|---|---|---|---|---|---|---|---|
| Measuring PIFU uptake = 2 | Completeness of P-EROC submission = 2 | Period lag = 2 | Baseline assumptions | |||||
| % change in outcome (95% CI) | p-value | % change in outcome (95% CI) | p-value | % change in outcome (95% CI) | p-value | % change in outcome (95% CI) | p-value | |
| Urology | −12.0% (−19.0% to −4.4%) | 0.003 | N/A | −6.9% (−10.9% to −2.8%) | 0.001 | −15.4% (−24.2% to −5.6%) | 0.003 | |
| Breast Surgery | −5.6% (−11.0% to 0.0%) | 0.050 | N/A | −3.2% (−6.9% to 0.8%) | 0.110 | −8.7% (−17.1%,.7%) | 0.069 | |
| Trauma and Orthopaedic | 0.1% (−0.5% to 0.7%) | 0.833 | 0.2% (−0.1% to 0.5%) | 0.273 | 0.1% (−0.1% to 0.2%) | 0.565 | 0.1% (−0.4% to 0.6%) | 0.772 |
| ENT | −7.2% (−14.6% to 0.8%) | 0.077 | N/A | −5.6% (−9.2% to −1.8%) | 0.004 | −9.7% (−18.9% to 0.6%) | 0.065 | |
| Ophthalmology | −11.4% (−20.0% to −2.0%) | 0.019 | N/A | −8.5% (−22.0% to 7.3%) | 0.273 | −20.1% (−37.4% to 1.9%) | 0.071 | |
| Pain Management | 5.3% (0.2% to 10.6%) | 0.041 | N/A | 0.6% (−3.1% to 4.4%) | 0.754 | 5.0% (−2.5% to 13.1%) | 0.193 | |
| Gastroenterology | −2.1% (−4.3% to 0.1%) | 0.059 | N/A | −1.3% (−3.1%,.5%) | 0.151 | −2.7% (−5.7% to 0.5%) | 0.091 | |
| Cardiology | −16.9% (−27.0% to −5.4%) | 0.005 | N/A | −4.0% (−14.3% to 7.6%) | 0.484 | −26.4% (−42.4% to −5.8%) | 0.015 | |
| Dermatology | −15.9% (−25.4% to −5.1%) | 0.005 | N/A | −16.2% (−24.2% to −7.4%) | 0.001 | −24.6% (−38.6% to −7.6%) | 0.007 | |
| Respiratory Medicine | −0.3% (−18.2% to 21.5%) | 0.974 | N/A | −12.6% (−24.7% to 1.5%) | 0.078 | −4.3% (−24.8% to 21.9%) | 0.725 | |
| Neurology | −0.9% (−2.3% to 0.5%) | 0.196 | N/A | 0.1% (−1.8% to 2.0%) | 0.937 | −1.1% (−2.7% to 0.6%) | 0.201 | |
| Rheumatology | −0.3% (−7.2% to 7.0%) | 0.924 | −34.4% (−61.2% to 10.8%) | 0.115 | −3.1% (−11.5% to 6.0%) | 0.489 | −0.4% (−7.5% to 7.2%) | 0.913 |
| Paediatric | 3.9% (−5.0% to 13.7%) | 0.398 | N/A | 3.3% (−0.4% to 7.3%) | 0.087 | 7.0% (−3.8% to 18.9%) | 0.213 | |
| Gynaecology | −1.9% (−7.9% to 4.4%) | 0.539 | −2.6% (−13.5% to 9.6%) | 0.662 | −1.1% (−4.8% to 2.8%) | 0.571 | −3.1% (−12.2% to 7.1%) | 0.542 |
| Physiotherapy | 0.0% (−0.1% to 0.1%) | 0.824 | N/A | 0.0% (−0.1% to 0.1%) | 0.953 | 0.0% (−0.1% to 0.2%) | 0.936 | |
The results of alternative interpretation of missing PIFU data on structured reviews per patient are shown in Appendix 7, Table 22 for structured reviews per patient and Appendix 7, Table 23 for DNAs. Because missing data were being interpreted as zero, fewer trusts were excluded for missing data and more specialties became eligible for inclusion in the model. Under these assumptions, we found significant reductions in structured reviews associated with increased PIFU use in Paediatric Trauma and Orthopaedic, significant reductions in DNAs within Dermatology and Midwifery, and significant increases in DNAs within Rehabilitation Medicine, Podiatry, and Clinical Oncology. Again, we adjusted for multiple testing and used a revised p-value threshold.
| Specialty | Assumptions applied (see above for option descriptions) | |||||
|---|---|---|---|---|---|---|
| Missing data option = 1 | Missing data option = 2 | Missing data option = 3 | ||||
| % change in outcome (95% CI) | p-value | % change in outcome (95% CI) | p-value | % change in outcome (95% CI) | p-value | |
| General Surgery | 31.1% (−2.9% to 77.0%) | 0.077 | 27.3% (−8.2% to 76.6%) | 0.148 | 25.3% (−14.9% to 84.4%) | 0.253 |
| Urology | −4.4% (−14.7% to 7.0%) | 0.434 | −1.8% (−8.2% to 5.0%) | 0.588 | −1.0% (−7.2% to 5.7%) | 0.766 |
| Breast Surgery | −7.0% (−20.0% to 8.1%) | 0.345 | −11.7% (−25.8% to 5.0%) | 0.159 | −8.1% (−21.8% to 8.1%) | 0.308 |
| Colorectal Surgery | −7.5% (−24.6% to 13.5%) | 0.455 | −5.1% (−20.9% to 13.8%) | 0.571 | −6.7% (−24.5% to 15.4%) | 0.525 |
| Trauma and Orthopaedic | −0.9% (−5.8% to 4.3%) | 0.728 | −0.2% (−1.8% to 1.5%) | 0.827 | 0.0% (−0.6% to 0.7%) | 0.973 |
| ENT | −16.7% (−26.6% to −5.4%) | 0.005 | −16.5% (−26.5% to −5.1%) | 0.006 | −18.4% (−29.3% to −5.8%) | 0.005 |
| Ophthalmology | 9.2% (−24.6% to 58.1%) | 0.642 | 11.5% (−20.8% to 56.8%) | 0.534 | 7.0% (−29.1% to 61.8%) | 0.746 |
| Plastic Surgery | −18.2% (−51.5% to 37.7%) | 0.449 | −24.1% (−57.5% to 35.5%) | 0.350 | −31.1% (−62.3% to 25.7%) | 0.225 |
| Pain Management | −4.4% (−12.0% to 3.9%) | 0.289 | −4.6% (−12.5% to 3.9%) | 0.277 | −5.1% (−13.5% to 4.0%) | 0.261 |
| Paediatric Trauma and Orthopaedic | −13.6% (−19.9% to 6.9%) | < 0.001 | −12.9% (−19.4% to −5.9%) | 0.001 | −9.0% (−15.7% to −1.8%) | 0.015 |
| Gastroenterology | 0.8% (−4.9% to 6.7%) | 0.796 | 0.5% (−5.5% to 6.8%) | 0.882 | 0.8% (−5.4% to 7.2%) | 0.818 |
| Diabetes | −1.4% (−5.4% to 2.6%) | 0.481 | 0.2% (−3.3% to 3.8%) | 0.939 | 1.5% (−2.5% to 5.7%) | 0.466 |
| Rehabilitation Medicine | 0.2% (−0.3% to 0.7%) | 0.511 | 0.2% (−0.3% to 0.7%) | 0.511 | −0.1% (−0.5% to 0.3%) | 0.689 |
| Cardiology | −23.2% (−48.1% to 13.5%) | 0.186 | −17.6% (−55.9% to 53.8%) | 0.542 | 6.9% (−40.4% to 92.0%) | 0.822 |
| Dermatology | 4.7% (−9.7% to 21.4%) | 0.543 | 4.9% (−10.5% to 22.9%) | 0.558 | −1.9% (−21.1% to 21.8%) | 0.860 |
| Respiratory Medicine | 4.7% (−17.2% to 32.2%) | 0.704 | −12.1% (−35.3% to 19.4%) | 0.408 | −4.3% (−32.4% to 35.7%) | 0.808 |
| Neurology | −0.2% (−3.4% to 3.1%) | 0.907 | −0.4% (−4.1% to 3.4%) | 0.810 | −0.6% (−4.4% to 3.4%) | 0.765 |
| Rheumatology | 2.3% (−3.5% to 8.4%) | 0.454 | 1.9% (−7.7% to 12.6%) | 0.709 | 1.7% (−8.1% to 12.4%) | 0.748 |
| Paediatric | 9.5% (−1.9% to 22.3%) | 0.107 | 9.2% (−2.4% to 22.2%) | 0.124 | 7.3% (−5.7% to 22.0%) | 0.287 |
| Gynaecology | −15.0% (−26.5% to −1.5%) | 0.030 | −12.8% (−24.0% to 0.2%) | 0.052 | −11.2% (−22.7% to 2.2%) | 0.097 |
| Midwifery | −9.2% (−23.3% to 7.4%) | 0.259 | −9.2% (−23.3% to 7.4%) | 0.259 | −4.7% (−23.0% to 17.9%) | 0.655 |
| Physiotherapy | −0.2% (−1.8% to 1.4%) | 0.765 | −0.2% (−1.5% to 1.1%) | 0.734 | −0.5% (−7.7% to 7.2%) | 0.889 |
| Occupational Therapy | −10.5% (−18.6% to −1.5%) | 0.024 | −10.5% (−21.5% to 2.1%) | 0.098 | −14.3% (−26.5% to 0.0%) | 0.050 |
| Podiatry | 0.9% (−3.2% to 5.1%) | 0.672 | 0.4% (−3.7% to 4.7%) | 0.862 | 0.4% (−3.9% to 4.8%) | 0.871 |
| Dietetics | −4.0% (−16.1% to 9.7%) | 0.551 | −3.8% (−16.9% to 11.3%) | 0.601 | −4.6% (−18.5% to 11.5%) | 0.552 |
| Orthotics | 0.2% (−0.2% to 0.6%) | 0.332 | 0.2% (−0.2% to 0.6%) | 0.332 | 0.9% (−2.0% to 3.9%) | 0.547 |
| Clinical Oncology | 11.3% (−15.1% to 45.9%) | 0.437 | 5.2% (−19.1% to 36.8%) | 0.706 | −16.6% (−51.3% to 42.5%) | 0.506 |
| Audiology | −0.8% (−7.3% to 6.0%) | 0.800 | −2.3% (−9.0% to 4.9%) | 0.522 | −2.4% (−9.1% to 4.9%) | 0.508 |
| Specialty | Assumptions applied (see above for option descriptions) | |||||
|---|---|---|---|---|---|---|
| Missing data option = 1 | Missing data option = 2 | Missing data option = 3 | ||||
| % change in outcome (95% CI) | p-value | % change in outcome (95% CI) | p-value | % change in outcome (95% CI) | p-value | |
| General Surgery | −28.8% (−49.1% to −0.5%) | 0.046 | ||||
| Urology | −15.2% (−23.3% to −6.2%) | 0.001 | −23.0% (−39.2% to −2.6%) | 0.030 | −20.2% (−33.6% to −4.1%) | 0.016 |
| Breast Surgery | −9.6% (−22.1% to 4.8%) | 0.181 | −14.1% (−22.1% to −5.4%) | 0.002 | −13.2% (−21.7% to −3.8%) | 0.007 |
| Colorectal Surgery | −6.4% (−16.4% to 4.7%) | 0.247 | −6.3% (−18.1% to 7.2%) | 0.343 | −5.8% (−17.9% to 8.1%) | 0.398 |
| Spinal Surgery | N/A | −5.5% (−14.8% to 4.7%) | 0.278 | N/A | ||
| Trauma and Orthopaedic | −3.9% (−8.4% to 0.8%) | 0.102 | −3.8% (−8.5% to 1.2%) | 0.135 | −1.4% (−6.5% to 3.9%) | 0.587 |
| ENT | −15.1% (−25.5% to −3.2%) | 0.015 | −15.0% (−25.4% to −3.3%) | 0.014 | −14.4% (−24.8% to −2.6%) | 0.018 |
| Ophthalmology | −29.8% (−44.6% to −11.0%) | 0.003 | −27.4% (−42.0% to −9.2%) | 0.005 | −28.7% (−43.9% to −9.4%) | 0.006 |
| Plastic Surgery | −26.8% (−46.1% to −0.5%) | 0.046 | −25.4% (−44.6% to 0.4%) | 0.053 | −26.7% (−46.1% to −0.2%) | 0.049 |
| Pain Management | −0.9% (−6.6% to 5.0%) | 0.749 | −1.0% (−6.7% to 5.0%) | 0.734 | −0.6% (−6.5% to 5.6%) | 0.839 |
| Paediatric Trauma and Orthopaedic | −3.0% (−7.2% to 1.5%) | 0.189 | −3.2% (−7.7% to 1.5%) | 0.175 | −4.2% (−9.3% to 1.3%) | 0.135 |
| Gastroenterology | −3.1% (−6.4% to 0.2%) | 0.063 | −3.0% (−6.1% to 0.2%) | 0.064 | −2.7% (−5.6% to 0.4%) | 0.083 |
| Diabetes | 0.0% (−14.8% to 17.4%) | 0.996 | −1.4% (−15.9% to 15.4%) | 0.857 | −1.5% (−15.9% to 15.4%) | 0.855 |
| Rehabilitation Medicine | 1.0% (0.5% to 1.4%) | < 0.001 | 1.0% (0.5% to 1.4%) | < 0.001 | 0.8% (0.3% to 1.3%) | 0.002 |
| Cardiology | −22.1% (−35.9% to −5.4%) | 0.012 | −19.7% (−36.7% to 2.0%) | 0.073 | −22.7% (−40.4% to 0.3%) | 0.052 |
| Dermatology | −19.8% (−28.7% to −9.7%) | < 0.001 | −20.3% (−29.7% to −9.7%) | < 0.001 | −22.8% (−33.9% to −9.8%) | 0.001 |
| Respiratory Medicine | −13.2% (−28.9% to 5.9%) | 0.163 | −10.0% (−30.4% to 16.4%) | 0.422 | −5.8% (−27.2% to 21.9%) | 0.650 |
| Neurology | −3.5% (−8.4% to 1.7%) | 0.180 | −3.5% (−8.5% to 1.7%) | 0.183 | −3.3% (−7.9% to 1.6%) | 0.180 |
| Rheumatology | −1.5% (−7.5% to 4.9%) | 0.640 | −6.8% (−18.3% to 6.4%) | 0.300 | −5.9% (−17.1% to 6.9%) | 0.349 |
| Paediatric | 1.2% (−8.1% to 11.5%) | 0.803 | 1.3% (−8.1% to 11.6%) | 0.794 | 1.7% (−8.3% to 12.7%) | 0.754 |
| Gynaecology | −10.5% (−21.4% to 1.9%) | 0.094 | −9.7% (−20.6% to 2.6%) | 0.118 | −8.1% (−19.0% to 4.1%) | 0.184 |
| Midwifery | −12.8% (−17.3% to −8.1%) | < 0.001 | −12.8% (−17.3% to −8.1%) | < 0.0001 | −13.4% (−19.2% to −7.1%) | < 0.0001 |
| Physiotherapy | 0.0% (−0.1% to 0.2%) | 0.992 | 0.0% (−0.1% to 0.2%) | 0.957 | 0.0% (−0.1% to 0.2%) | 0.995 |
| Occupational Therapy | −3.8% (−9.7% to 2.5%) | 0.233 | −4.1% (−11.4% to 3.8%) | 0.301 | −3.0% (−10.4% to 5.0%) | 0.453 |
| Podiatry | 4.7% (2.0% to 7.5%) | 0.001 | 5.1% (2.0% to 8.3%) | 0.001 | 5.8% (2.3% to 9.5%) | 0.001 |
| Dietetics | −2.4% (−10.1% to 6.1%) | 0.573 | −1.8% (−10.0% to 7.1%) | 0.679 | −0.7% (−10.0% to 9.6%) | 0.892 |
| Orthotics | −0.9% (−3.0% to 1.3%) | 0.415 | −0.8% (−2.9% to 1.2%) | 0.416 | 0.3% (−2.8% to 3.4%) | 0.868 |
| Clinical Oncology | 35.9% (17.0% to 57.7%) | < 0.001 | 50.8% (31.1% to 73.7%) | < 0.001 | 60.2% (25.5% to 104.5%) | < 0.001 |
| Audiology | −2.8% (−7.0% to 1.7%) | 0.217 | −3.1% (−7.6% to 1.5%) | 0.178 | −3.3% (−7.7% to 1.4%) | 0.164 |
Section 3: Outpatient activity definitions
The Poisson regression and survival analyses included impacts on multiple outcomes. The definitions of these, with respect to standard fields in the HES OP data,96 are given in Appendix 7, Table 24.
| Outcome activity type | Appointments selected where: |
|---|---|
| Outpatient attendances | ATTENDED = 5 or 6 |
| Follow-up attendances | FIRSTATT = 2 or 4 and ATTENDED = 5 or 6 |
| Missed/not attended appointments (DNAs) | ATTENDED = 3 or 7 |
Section 4: Outpatient classification algorithm
An algorithm to classify outpatient activity by function was published by the Strategy Unit in 2021. 78 The algorithm, intended to help enhance analyses of outpatient hospital data by categorising attendances into 1 of 10 types, used information in the outpatient and inpatient data sets linked at the person level.
These categories are as follows:
-
Initial opinion.
-
Structured review.
-
Diagnostic procedure.
-
Discuss results.
-
Treatment.
-
Urgent investigation.
-
Pre-operative (pre-op) assessment.
-
Post-operative (post-op) review.
-
Review after a non-elective admission (Review NEL).
-
Direct Access.
Definitions of these can be found in this publication by the Strategy Unit in 2021. 78
In 2022 NHS England were preparing to publish documentation on a tool to classify outpatient activity by function, based on the Strategy Unit’s approach, although with some changes. NHSE kindly shared draft documentation on their approach, which we implemented using HES inpatient and outpatient data, and supported us in making decisions where these were ambiguous.
At the time of writing, NHSE have yet to publish their tool.
We applied the categorisation to 348.8 million attendances between 1 March 2019 and 31 March 2023. To do this we used HES OP data from 1 April 2017 to 31 March 2023, and HES Admitted Patient Care from 1 September 2018 to 31 March 2023. Appendix 7, Table 25 shows the relative size of each category. As the data were not available in the HES extract, we could not implement the Direct Access category (although, according to documentation from NHSE, these general represented < 1% of activity).
| Outpatient category | Count of attendances | Percentage of all attendances (%) |
|---|---|---|
| Initial opinion | 52,296,547 | 15.0 |
| Structured review | 122,891,223 | 35.2 |
| Diagnostic procedure | 25,440,975 | 7.3 |
| Discuss results | 19,081,240 | 5.5 |
| Treatment | 48,003,128 | 13.8 |
| Urgent investigation | 49,015,286 | 14.1 |
| Pre-operative (pre-op) assessment | 17,000,172 | 4.9 |
| Post-operative (post-op) review | 9,964,977 | 2.9 |
| Review after a non-elective admission (review NEL) | 5,120,834 | 1.5 |
| Direct access | N/A | N/A |
Section 5: Censoring approach in survival analyses
In survival analysis the outcome being analysed has two components: (1) an event that occurs; and (2) the time to the event. The time to an event occurring is called the survival time.
Censoring is necessary because sometimes we do not know the survival time. This could be for several reasons. Firstly, where an event has not yet occurred before the end of the time period we are able to analyse. Secondly, where an individual, for example, is lost to follow-up or withdraws from a study part way through.
In the analyses, the first of these is common (the data ended at 31 March 2023, and so any events after this point would be unknown). However, the second is treated as being not relevant: we assume that the HES OP and ECDS data are complete.
Nevertheless, there are common situations that we did additionally censor.
For example – consider where we have an index (time 0) attendance, and the outcome in which we are interested is time to next DNA. If the next appointment is attended, then the event does not occur, and now cannot occur for the index attendance. So we censor at the time of the intervening attendance, as the ‘data window’ has effectively shut. Note that the event can occur in subsequent appointments, but that event will ‘belong’ to an intervening attendance (when it is the turn of that attendance to be considered as the index attendance).
Each outcome required a particular approach to censoring:
-
Time to next attendance. We censored where no subsequent attendance occurred between the index attendance and the end of the data (31 March 2023). We also censored where there was a DNA at the next appointment date.
-
Time to next follow-up attendance. We censored where no subsequent attendance occurred between the index attendance and the end of the data (31 March 2023). We also censored where there was a DNA, or a first attendance, at the next appointment date.
-
Time to next structured review follow-up attendance. We censored where no subsequent attendance occurred between the index attendance and the end of the data (31 March 2023). We also censored where there was a DNA, or an attendance that was not a ‘structured review’ at the next appointment date.
-
Time to next DNA: We censored where no subsequent attendance occurred between the index attendance and the end of the data (31 March 2023). We also censored where the next appointment was attended.
-
Time to next AE visit: We censored where no accident and emergency visit occurred between the index attendance and the end of the data (31 March 2023).
As noted in the methods, for the outpatient outcomes, all analysis was carried out within the same provider trust and specialty as the index attendance. For the accident and emergency outcome, analysis was carried out to any trust, and for any visit reason.
Section 6: Survival models data inclusion/exclusion
FIGURE 27.
Flow chart describing data inclusion/exclusion for survival model.
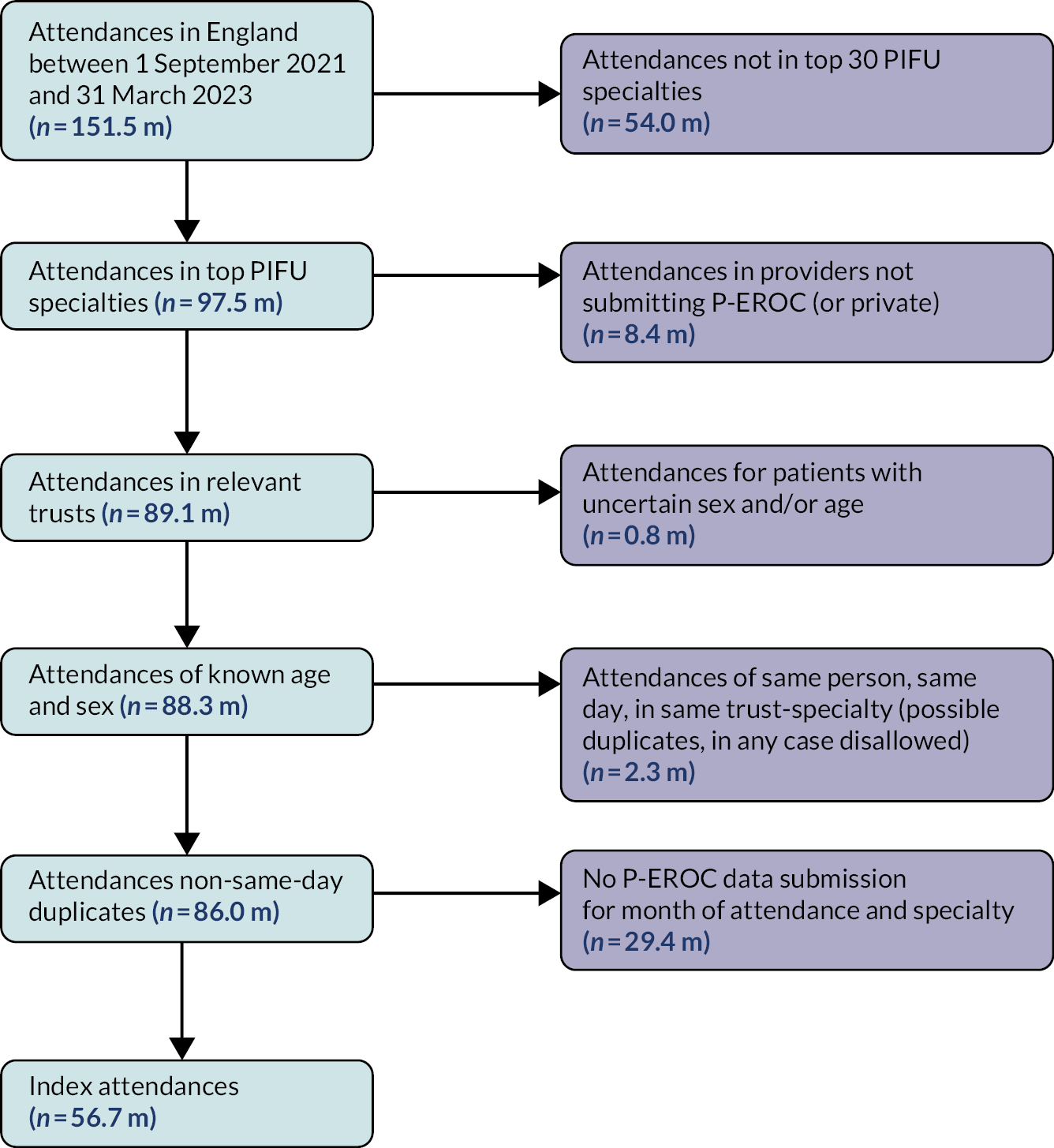
Section 7: Additional single-specialty results
Appendix 7, Figures 28 and 29 show single-specialty results for the other outpatient activity outcomes, and for ED visits (survival analysis only). These supplement the results for structured reviews shown in Figure 17.
FIGURE 28.
Two additional outpatient outcomes: model results for individual specialties. Two model variants: (a) Poisson regression model (showing percentage change in attendances per patient), and (b) survival model (showing adjusted HRs, for subsequent events), both per 5-percentage point increase in PIFU rate. 95% CIs included. For survival model * denote statistically significant results, having adjusted for multiple testing (p < 0.0017). Specialties are ordered by decreasing volume of PIFU. T&O, Trauma and Orthopaedic.
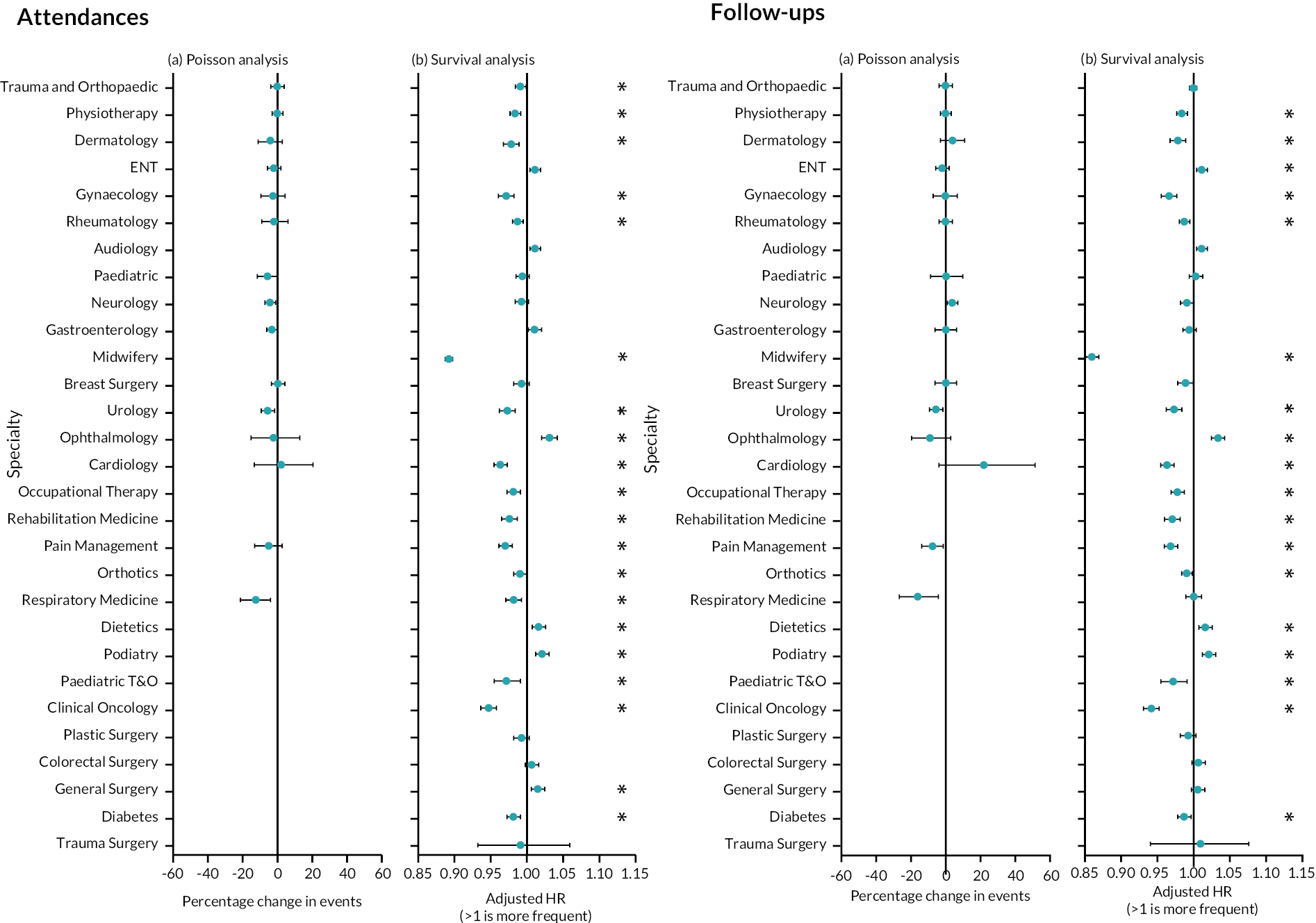
FIGURE 29.
Outpatient DNAs and time to next ED visits: model results for individual specialties. Survival model showing adjusted HRs, for subsequent ED visits, per 5-percentage point increase in PIFU rate. 95% CI intervals included. * denote statistically significant results, having adjusted for multiple testing (p < 0.0017). Specialties are ordered by decreasing volume of PIFU. T&O, Trauma and Orthopaedic.
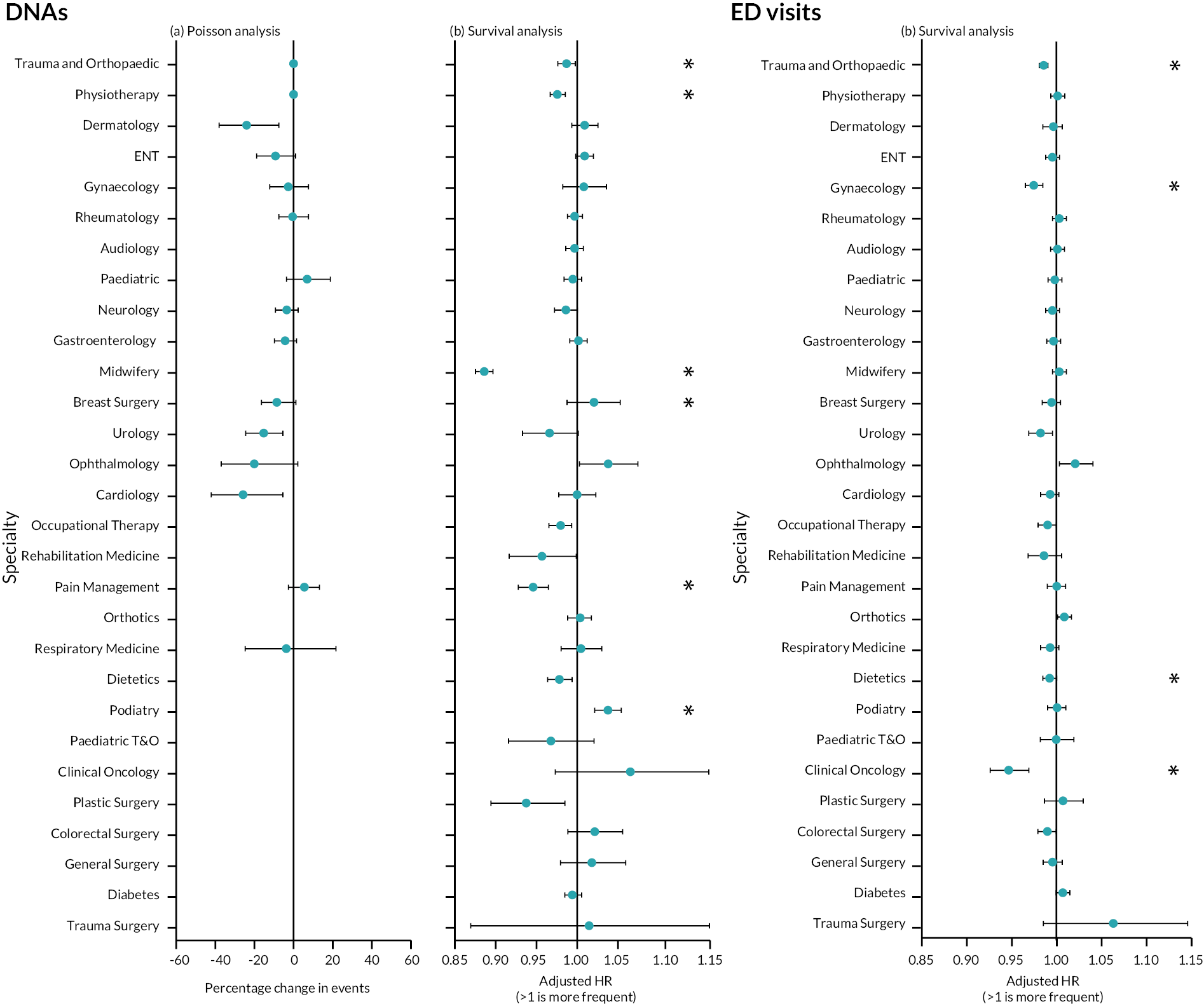
Glossary
Activity measure–See Measure.
Attendance–An outpatient appointment that took place, either in person or via telephone or other virtual means.
Break point–A point in time that separates two modelled contiguous trend lines within a time series.
Dependent variable–The variable hypothesised to be impacted by manipulations made to the independent variable. See Independent variable.
Did not attend–An outpatient appointment that was not attended either because the patient did not turn up, or turned up too late to be seen.
Exposure variable–The period of time or space over which the dependent variable is measured. See Dependent variable.
First attendance–The first attendance in a series of outpatient attendances, or the only attendance if not part of a series.
Follow-up attendance–Any subsequent attendance following a patient’s first attendance.
Healthcare resource–A product or service associated with health care which aids patient care and recovery. It could refer to time with a healthcare professional as an appointment or a conversation, surgery, medication, staying in a hospital bed, etc.
Hospital Episode Statistics Outpatient data–A national data set containing individual records of all outpatient appointments occurring in England, with relevant details.
Independent variable–The variable whose manipulation is hypothesised to have an impact on the dependent variable. See Dependent variable.
Index attendance–For survival analyses, each included attendance is an index attendance. For each index attendance, we search for subsequent activity from the date of that attendance.
Indicator saturation–A methodology that enables us to model a time series data set as relatively few trend lines, with occasional break points between adjacent trend periods. This has the impact of simplifying subsequent analysis.
Measure–Generally, meaning 1 of 4 retrospectively quantified descriptors of an outpatient service at Unit level, as derived from Hospital Episode Statistics Outpatient Data. The four measures are: numbers of attendances; proportion of appointments that were not attended; proportion of attendances that were tele-attendances; and follow-up attendance to first attendance ratios. Occasionally refers to additional properties, derivable from Hospital Episode Statistics. Measure is distinguished from the term Metric.
Metric–We use this term to describe any of the set of indicators we developed to numerically describe each time series, via each time series’ primary positive change period. Metrics were intended to be used in combination with one another to rank time series. Metric is distinguished from the term Measure.
Open access follow-up/open appointments–See Patient-Initiated Follow-Up.
Outpatient activity measure–See Measure.
Patient-Initiated Follow-Up–This is when a patient is able to request an appointment when they need one outside of a fixed follow-up schedule. It has also been known as open access follow-up and open appointments.
Patient-Initiated Follow-Up activity–The number of patients moved/discharged to Patient-Initiated Follow-Up over the relevant period.
Patient-Initiated Follow-Up pathway–This refers to when a patient is placed on to Patient-Initiated Follow-Up within a certain specialty at a certain trust. One patient could be placed on more than one Patient-Initiated Follow-Up pathway if they are seeking treatment under different specialties or at different trusts.
Patient-Initiated Follow-Up rate–The number of people moved to and discharged to Patient-Initiated Follow-Up, divided by the number of attendances, over the relevant period. ‘Moved to PIFU’ and ‘discharged to PIFU’ are given as two separate values in Provider Elective Recovery Outpatient Collections data; we sum these together.
Primary positive change period–In a time series, this is the period of time which contains the most significant change in the positive direction (from the start of the change to the end). Not all time series have a primary positive change period.
Ranking metric–See Metric.
Sensitivity analysis–A sensitivity analysis compares the results obtained when applying different assumptions to answer the same question.
Statistically significant–A statistically significant result is one where the likelihood that the result is obtained by pure chance is low (usually 5%).
Tele-attendance–An attendance that does not take place in person (face to face). This could be by telephone or by videocall, for example.
Time series–A data set describing a service’s performance on a single measure over time.
Trend line–A (modelled) straight line overlaid on a chart segment indicating the general course of underlying data points.
Trend period–A time period during which the modelled trend line has a single slope value.
Unit–Used to mean a clinical specialty at a single National Health Service trust. For example, Cardiology at Trust A, Cardiology at Trust B and Urology at Trust B are considered to be three separate units.
List of abbreviations
- AHP
- allied health professional
- COVID-19
- coronavirus disease discovered in 2019
- DNA
- did not attend
- ECDS
- Emergency Care Dataset
- ED
- emergency department
- EDI
- equality, diversity and inclusion
- ENT
- Ear, Nose and Throat
- EPR
- electronic patient record
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HES OP
- Hospital Episode Statistics outpatient
- HMIC
- Health Management Information Consortium
- HRA
- Health Research Authority
- ICB
- integrated care board
- IS
- indicator saturation
- IT
- information technology
- MMAT
- Mixed Methods Appraisal Tool
- NASSS
- Non-adoption and Abandonment of technologies by individuals and the challenges to Scale-up, Spread and Sustainability of such technologies in health and care systems
- NHSE
- National Health Service England
- NIHR
- National Institute for Health and Care Research
- OECD
- Organisation for Economic Co-operation and Development
- PAG
- Project Advisory Group
- PAS
- patient administration systems
- P-EROC
- Provider Elective Recovery Outpatient Collections
- PIFU
- Patient-Initiated Follow-Up
- PPCP
- primary positive change period
- PPIE
- patient and public involvement and engagement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RAP sheets
- rapid assessment procedure sheets
- REC
- Research Ethics Committee
- ROBIS
- Risk of Bias in Systematic Reviews
- RQ
- research question
- RSET
- Rapid Service Evaluation Team
- SAB
- Stakeholder Advisory Board
- SAS
- Statistical Analysis System
- SOP
- standard operating procedure
- SSCI
- Social Sciences Citation Index
- UCL
- University College London
- UCLH
- University College London Hospital
Notes
-
Consolidated criteria for reporting qualitative research checklist
-
Evaluating the impacts of your PIFU programme: a guide to evaluating Patient Initiated Follow-Up services
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/VGQD4611).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
