Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/147/03. The contractual start date was in February 2014. The draft report began editorial review in October 2020 and was accepted for publication in July 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Sharples et al. This work was produced by Sharples et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Sharples et al.
Chapter 1 Introduction and background to the ETTAA study
Introduction
An estimated 1 in 10,000 patients per year is diagnosed with chronic thoracic aortic aneurysm (CTAA) of the arch or descending thoracic aorta. 1,2 Between 1999 and 2010, hospital admissions for thoracic aortic aneurysm increased from 4.4 to 9.0 per 100,000 inhabitants. 2
Normal aortic diameter varies by aortic segment, gender, age and body mass index (BMI). An aneurysm is defined as a dilatation to one and a half times the normal size of the vessel. Generally, in the arch or descending thoracic aorta (DTA), aneurysms of diameters of ≥ 4 cm are considered abnormal. Without treatment, aortic aneurysms can continue to expand, usually ‘silently’, giving no symptoms until the point of dissection (when the aortic wall tears) or rupture. Either of these events is immediately life-threatening, and survival depends on timely diagnosis and intervention. A study of Routine Hospital Episode Statistics of patients in England admitted with a new diagnosis of thoracic aortic aneurysm between 2004 and 2011 reported a 6-month mortality rate among treated patients of 17.7% and among untreated patients of 30%. 3
In an era of prevalent computerised tomography (CT) scanning, more cases of CTAA are being diagnosed early, offering the opportunity for planned intervention before any life-threatening event can occur. The Effective Treatments for Thoracic Aortic Aneurysms (ETTAA) study investigates the effectiveness of the current treatments available. This chapter outlines the nature of the condition and describes the available treatments and the evidence that currently guides clinical practice.
Pathology
Most aneurysms develop chronically, over several years. In the majority of patients, these are asymptomatic but occasionally they give rise to episodic chest or back pain. Severe pain or cardiovascular collapse may be a sign of rupture or dissection. Aneurysms can also occur acutely (i.e. within days) in the context of dissection of a normal-sized aorta, infection or trauma, but such situations fall outside the remit of this project.
Anatomical definitions and classifications
As shown in Figure 1, CTAAs are subdivided into ascending aortic, aortic arch and DTA aneurysms. Aneurysms may extend across both thoracic and abdominal segments, in which case they are classified as thoracoabdominal aortic aneurysms. The ETTAA study focuses on CTAAs of ≥ 4 cm in diameter in the arch, descending or thoracoabdominal aorta. Ascending aortic aneurysms are excluded because there is an established body of evidence supporting surgical repair, and no established endovascular treatment for comparison. 4
FIGURE 1.
Diagram illustrating sections of the thoracic aorta. ST, sinotubular.
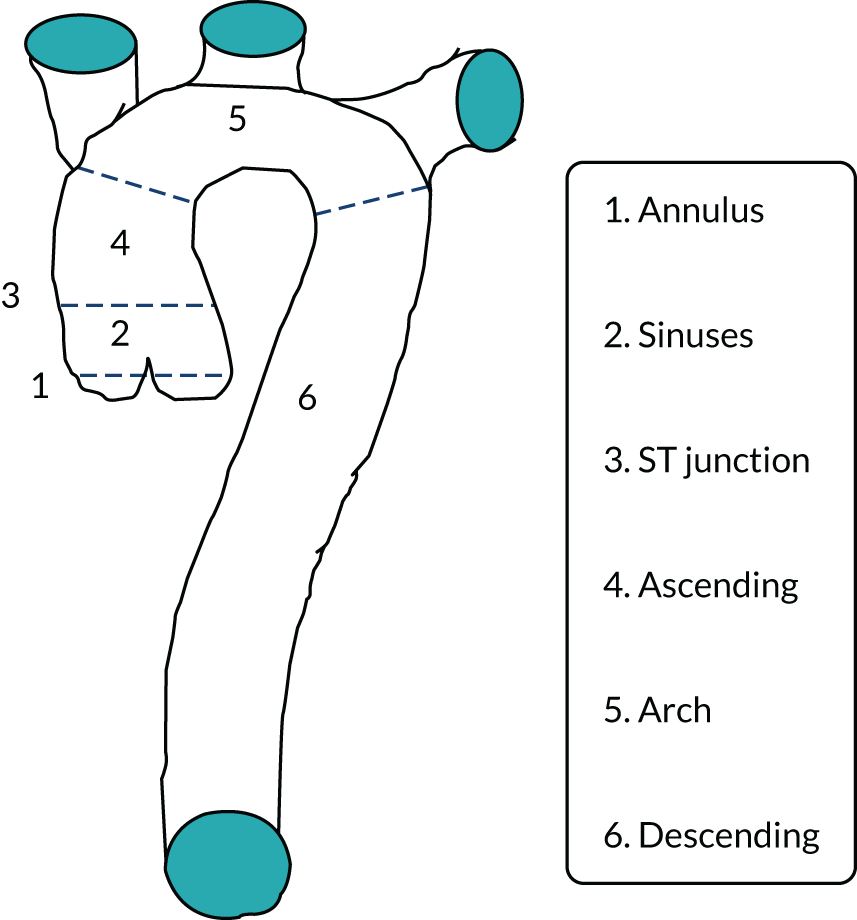
Aneurysms do not always exhibit defined abrupt proximal and distal ends but instead have dilated aortic segments that extend into neighbouring segments. In some cases, most of the aorta or the entire aorta may be dilated to some degree, described as ectatic. Aneurysms in which the aorta has dilated evenly around its circumference are described as fusiform, whereas those in which the dilatation is predominantly on one side of the aorta are described as saccular. Thus, aortic dilatation can have diverse patterns, for various reasons. The different pathological features of the aneurysm influences treatment decisions and, therefore, patient outcomes.
Aetiology
Chronic thoracic aortic aneurysm occurs because the aortic wall is in some way abnormal and cannot withstand the normal stresses exerted by the blood pressure. In approximately 80% of CTAAs, the abnormality in the aortic wall is secondary to atherosclerotic plaques, which in turn are associated with smoking, hypertension, hyperlipidaemia and obesity. 5 In the remaining 20% of cases there is a genetic defect in some structural component of the aortic wall, so that it is weaker and dilates in response to normal stresses. Examples include mutated FBN1 (fibrillin 1 gene), causing Marfan syndrome, and mutated TGFBR2 (transforming growth factor beta receptor 2 gene), causing Loeys–Dietz syndrome. 6,7 The underlying cause of CTAA is important, as this can direct treatment. For example, in cases of genetically mediated aneurysm, current opinion is that the ‘normal’ aortic wall above and below the aneurysm, which are landing zones for the stent graft, will continue to dilate and lead to migration of the stent and endoleak, and so surgery is generally preferred. As individual genetic disorders are rare, we grouped them under the umbrella term ‘connective tissue disorders’ (CTDs).
Presentation and diagnosis
Most aneurysms of the arch or DTA are identified incidentally (on a scan for some other reason), or via screening if CTDs are suspected. Once an aneurysm is detected, monitoring of its progression requires repeated CT imaging or magnetic resonance imaging (MRI), which incurs radiation exposure and a significant cost. Longitudinal surveillance, although limited to small numbers of patients and studies,8,9 suggests that some aneurysms (whatever their size at diagnosis) appear quiescent, without growth for a prolonged period of time, whereas others progress precipitously. Genetic, acquired and pathological features may increase the rate of growth, but the evidence for this is sparse.
Management
Treatment options
In the case of patients with smaller aneurysms, those who are less fit to undergo chest surgery or endovascular intervention or those who reject such interventions, treatment is confined to lifestyle modification advice (smoking cessation and dietary management) and medical management of hypercholesterolaemia and hypertension. In the ETTAA study, these non-intervention patients are described as either watchful waiting (WW) or conservative management (CM) patients (see Chapter 3). WW patients are monitored with serial (usually annual or biennial) CT or MRI scans with a view to future intervention should the aneurysm grow beyond the intervention threshold. CM patients either are considered to be at very high risk of life-threatening complications from intervention, despite having an aneurysm that is above the guideline thresholds for intervention, or have rejected potential interventions. In such patients aneurysm monitoring may decrease or stop altogether.
As aneurysms grow and the risk of fatal complications such as rupture or dissection increases, two main interventions are available: endovascular stent grafting (ESG) and open surgical repair (OSR). 10
Open surgical replacement describes procedures in which the chest cavity is opened in order to replace the diseased aortic segment, usually with woven prosthetic tube grafts. Most cases of OSR require cardiopulmonary bypass to support or reroute blood flow while the aorta is operated on. The arch and DTA give rise to important branch arteries to the head, neck, upper limbs and spine and so OSR carries a risk to these organs when the blood supply is interrupted. This risk is usually mitigated by the use of hypothermia and cardiopulmonary bypass. As the mainstay of treatment for CTAAs for over four decades, OSR has been demonstrated to reduce mortality and can be performed reproducibly in cardiac surgical centres. 11,12 Techniques have improved, but OSR still carries a risk of mortality of around 5% and of paraplegia of around 10%. 13
In ESG, a covered stent or frame (stent graft) is inserted into the arterial system at a peripheral access point (usually the femoral artery in the groin) and guided using X-rays to the aneurysm site. At the target segment, the stent springs open and fixes to the normal aorta above and below the aneurysm, so that the aneurysm is sealed and blood flows through the stent graft. The aneurysm outside the stent graft has no flowing blood and clots; thus, it is excluded from the circulation and the risk of rupture is very low. ESG for CTAAs is a more recent and less invasive technique, with reported risk of in-hospital mortality of 2–10%. It is technically feasible in many patients and excludes the aneurysm from the circulation, with shrinkage or stabilisation of the aneurysm sac in most cases, at least initially. Unfortunately, the procedure cannot be performed in all patients because it has specific anatomical requirements. It is resource intensive, as the stent grafts themselves are expensive, and it requires a hybrid theatre and an appropriate theatre team, but it does lead to a shorter length of stay and usually faster recovery. In the long term, patients who receive ESG need to be monitored as there may be leakage around/between stent components that requires urgent reintervention.
For some patients a hybrid procedure, including both stent components and surgical components, is necessary. For example, a minor surgical procedure may be completed in the patient’s neck to protect the cerebrovascular blood supply after arch stenting, thereby facilitating the endovascular placement of a stent into the arch of the aorta. When the ETTAA study began, these hybrids were most prevalent, and such patients were intended for the ESG arm, as the stent graft was considered (clinically) the predominant intervention. Alternatively, an endovascular stent may be placed into the DTA at the same time as the arch is replaced in an open surgical procedure. During the ETTAA study, these approaches became more prevalent when a hybrid graft became available. The hybrid graft is half surgical prosthesis–half stent and is inserted during open surgery, with the patient usually undergoing median sternotomy. In such cases the surgery is the predominant procedure, so these hybrid patients were placed in the OSR arm of the study.
Treatment decisions
Endovascular stent grafting and OSR are considered for arch/DTA aneurysms when the risk of rupture/dissection or death exceeds the risks associated with intervention. Risk of rupture is mostly determined by aneurysm size, measured using CT or MRI. 14,15
When the ETTAA study launched in 2014, clinical practice was informed by the 2010 American Heart Association guidelines for the diagnosis and management of thoracic aortic disease16 and the 2014 European Society of Cardiology Guidelines on the diagnosis and treatment of aortic diseases. 17 These guidelines were written by international panels of clinical experts, based on the available evidence. The American guidelines advised that operative treatment was reasonable for patients at low operative risk who had an arch aneurysm of > 5.5 cm in diameter. The level of evidence supporting this recommendation is classified as ‘B – multiple non-randomised studies of surgery versus conservative management’ in the hierarchy of evidence. 18 The European guidelines advised that an endovascular procedure ought to be considered in patients for whom it was technically feasible, but that, if surgery (OSR) were the only option, then it should be planned after the aortic diameter reached 6.0 cm. The level of evidence for the European guidelines was classified as ‘C – consensus of opinion of the experts and/or small studies, retrospective studies, registries’ in the hierarchy of evidence. 18 The main body of evidence considered by both committees came largely from data obtained from Yale University publications,19,20 alongside data from the American International Registry of Acute Aortic Dissections (IRAD) database21 and, to a lesser extent, the European German Registry for Acute Aortic Dissection Type A (GERAADA) data set22 and the International Aortic Arch Surgery Study Group's ARCH projects. 23 Analysis of the Yale registry of acute aortic dissections and ruptures indicated that the risk of life-threatening dissection or rupture dramatically increases at a diameter of 6 cm and outweighs the 5–10% risk of death, stroke or paralysis from procedural intervention. 19,20,23
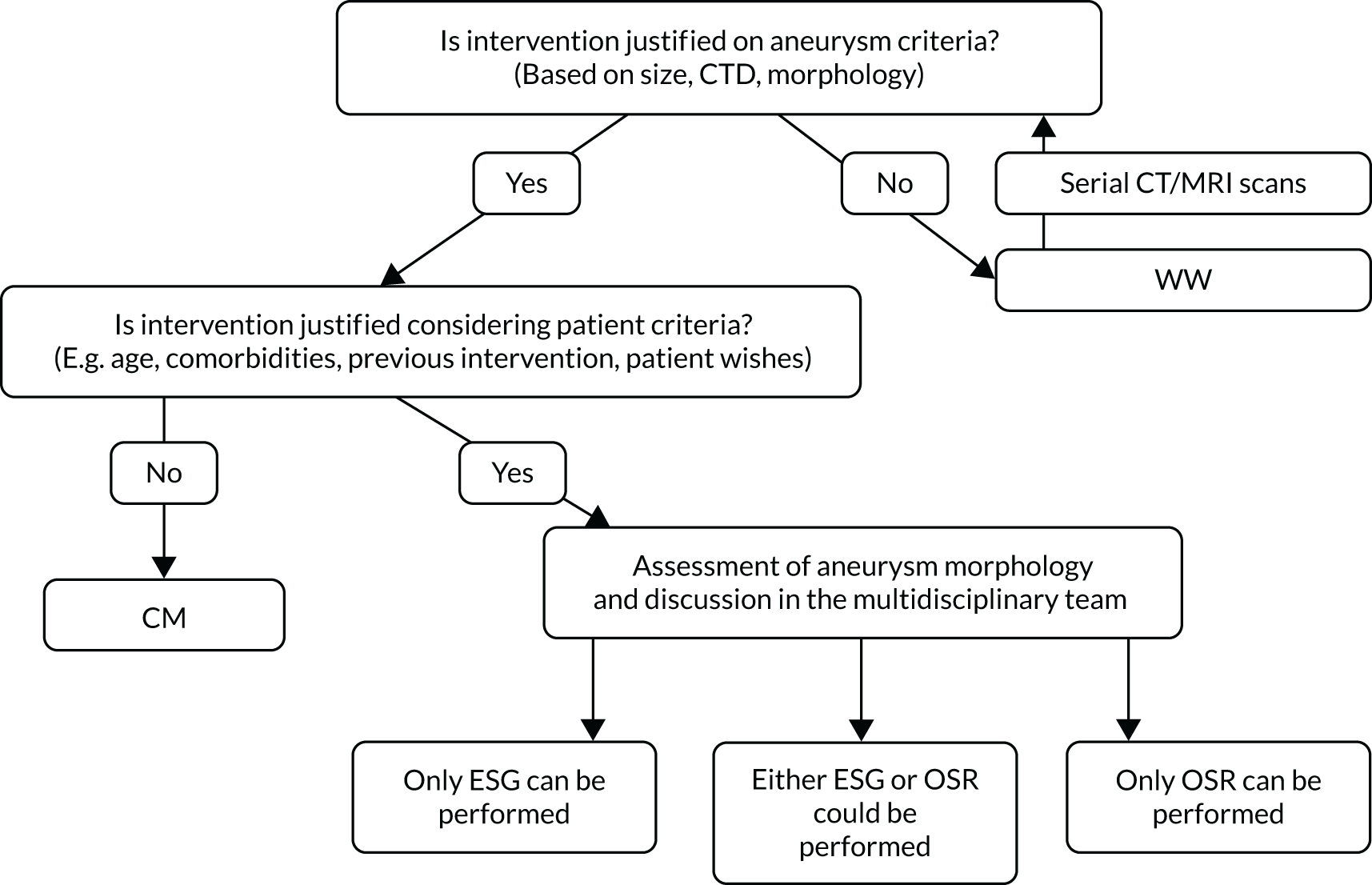
Taking the two guidelines together, and especially considering that many patients requiring arch surgery may not be considered at ‘low surgical risk’, the general practice in the UK in 2014 was to consider intervention with OSR or ESG when an arch/DTA aneurysm became > 6 cm in diameter. 16,17 Clinical opinion at the start of the ETTAA study is explored more comprehensively in Chapter 2, but it is useful to state here that the risks of intervention are influenced by a variety of factors and are different for every patient. At present, there are no national or international guidelines addressing patient selection for WW, CM, ESG or OSR. In the absence of such guidelines, specialist multidisciplinary teams (MDTs) play a key role in assessing the risk–benefit profile of each patient before recommending a treatment pathway (Figure 2). Ultimately, the choice of treatment is made by the patient after appropriate explanation and discussion.
FIGURE 2.
Multidisciplinary team decision-making process for treatment for CTAAs.
Natural history of arch/descending thoracic aorta aneurysms
To determine the benefits and effectiveness of intervention for arch/DTA aneurysms, it is necessary to understand what would happen without intervention. Unfortunately, contemporary studies of the natural history of CTAAs are rare. The most up-to-date analysis of this disease comes from the Yale registry and was published in 2015. 24 With aneurysms of > 6 cm in diameter, the Yale group suggests that the annual risks of dissection, rupture and death are 3.6%, 3.7% and 10.8%. 19 There is, however, increasing evidence that aneurysm diameter is not the only important predictor of risk; a 2011 review of the IRAD database showed that 60% of patients with arch/DTA dissections had aortic diameters of < 5.5 cm at the time of dissection, below the accepted threshold for intervention. 25 After aortic size, presence of CTDs and aneurysm growth rate have been associated with an increased risk of aortic dissection/rupture, along with chronic obstructive pulmonary disease (COPD), hypertension and older age. All of these factors must be considered when judging the relative risks and benefits of management options.
Mean aortic arch growth rates have been reported in the range 0.09–0.56 cm per year, and mean DTA growth in the range 0.12–1.44 cm per year. Rare reports of regression of DTA aneurysms (up to 0.12 cm per year) were confined to cases of chronic dissection where thrombosis in the layers of the aortic wall accounted for the shrinkage. 26–28 Key factors that have been associated with aneurysm growth include aneurysm size, patency and size of the false lumen, number and location of tears around the arch, peak wall stress, comorbidities (hypertension, CTD, COPD), patient characteristics (age, sex, smoking history) and anticoagulant treatment. Owing to a lack of high-quality, consistently-recorded data, no large-scale multivariate regression analysis has been possible and no clear relationship between the risk factors and growth rate has emerged. 8 Two studies have generated prediction models for future aneurysm size using initial diameter and based on single-centre data; neither has been validated in a prospective clinical cohort. 29,30
Outcomes following intervention
Systematic review of outcomes
As part of the ETTAA study, a systematic review was conducted in January 2016 to assess the available evidence regarding the effectiveness of ESG compared with OSR for CTAA in the aortic arch or DTA. The review protocol was registered in the PROSPERO database (CRD42017054565)31 and details can be found in the published article. 32
Briefly, no randomised studies comparing ESG and OSR have been published. Cohort studies and case–control studies matched on key outcomes were included if patients had elective treatment for arch/DTA aneurysms and some attempt had been made to adjust for selection bias. Five comparative cohort studies met the inclusion criteria, reporting a total of 3955 ESG and 21,197 OSR patients. In accordance with the ROBINS-I (Risk Of Bias In Non-randomized Studies – of Interventions) tool,33 one study was judged to be at moderate risk of bias34 and the remaining four were judged to be at severe risk of bias because of the potential for confounding. 35–39 Early mortality rates (30 days or to discharge) ranged from 3.1% to 6.1% after ESG and from 1.5% to 20% after OSR, with extreme rates arising from small studies. The meta-analysis of unadjusted short-term all-cause mortality favoured ESG [odds ratio 0.75, 95% confidence interval (CI) 0.55 to 0.1.03]. Adjusting for heterogeneity between small and large studies, the odds ratio did not change substantially (0.71, 95% CI 0.51 to 0.98). Meta-analysis of long-term all-cause mortality could not be carried out owing to differences in how results were reported. Overall, the long-term mortality was higher for ESG in larger studies and higher for OSR in smaller studies. For example, in von Allmen et al. ’s study36 of 618 patients, the hazard ratio (HR) for ESG relative to OSR up to 5 years, adjusting for age and sex, was 1.45 (95% CI 1.08 to 1.94; p = 0.013). Conversely, the Gore TAG study of 234 patients reported identical survival of 63% to 5 years for the two groups (log-rank p = 0.625),35 and the study of 28 patients by Piffaretti et al. 39 reported higher (non-significant) long-term survival with ESG. Freedom from reinterventions in the long-term also favoured OSR.
Overall, studies reporting short-term non-fatal complications suggested fewer events following ESG, although limited data prevented meta-analysis (Table 1). 35–39 However, Hughes et al. ’s 2014 study38 of 8967 patients reported lower odds of neurological complications (odds ratio 0.48, 95% CI 0.26 to 0.86; p = 0.015), pulmonary complications (odds ratio 0.38, 95% CI 0.21 to 0.67; p = 0.001) and cardiac complications (odds ratio 0.24, 95% CI 0.15 to 0.37; p < 0.001) for ESG patients. The Gore TAG study35 reported a substantial endoleak rate for ESG patients of 8.5%.
| Complication | Number (%) of events | |||||
|---|---|---|---|---|---|---|
| Gore TAG 200735 | Piffaretti et al. 200739 | Hughes et al. 201438 | ||||
| ESG group (N = 140) | OSR group (N = 94) | ESG group (N = 17) | OSR group (N = 11) | ESG group (N = 712) | OSR group (N = 8255) | |
| Neurological | ||||||
| Paraplegia/paraparesis | 4 (3) | 13 (14) | NR | NR | NR | NR |
| Cerebral vascular accident | 5 (4) | 5 (4) | 2 (9) | 1 (12) | NR | NR |
| Neurological: unspecified | NR | NR | NR | NR | 20 (2.8) | 273 (3.3) |
| Respiratory | ||||||
| Respiratory failure | 5 (4) | 19 (20) | NR | NR | NR | NR |
| Pneumonia | NR | NR | 2 (12) | 3 (27) | NR | NR |
| Pulmonary: unspecified | NR | NR | NR | NR | 17 (2.4) | 462 (5.6) |
| Cardiac | ||||||
| Myocardial infarction | 0 (0) | 1 (1) | 0 (0) | 1 (9) | NR | NR |
| Cardiac: unspecified | NR | NR | NR | NR | 28 (2.9) | 1252 (15.2) |
| Other | ||||||
| Endoleaks | 12 (8.5) | NR | NR | NR | NR | NR |
| Peripheral vascular disease | 20 (14) | 4 (4) | NR | NR | NR | NR |
Although this systematic review was, to our knowledge, the first to consider evidence from non-randomised studies directly comparing ESG and OSR for treatment of elective arch/DTA aneurysms in CTAA patients, it identified increasingly dated evidence only, and this was limited by either small size or severe risk of bias. The conflicting evidence reinforced the need for updated evidence on UK practice and comparisons of short- and long-term outcomes of ESG and OSR.
Additional important studies reporting outcomes
Five relatively large, recent cohort studies40–44 reported clinical and cost outcomes but were not eligible for the systematic review because of the inclusion of a heterogeneous cohort. All five studies acknowledged important differences between ESG and OSR cohorts, which resulted in biased comparisons. ESG tended to be chosen for older patients with more comorbidity, many of whom may have been unsuitable for OSR. Despite this, the risk of death, paraplegia or other complications appeared to be lower after ESG than after OSR. Conversely, the need for reintervention was greater after ESG as a result of technical failures of the stent over time, and with each reintervention there was an added risk of complication owing to either the increased complexity of the procedure or the deteriorating health of the patient. One UK44 and one US41 study compared the costs of ESG against those of OSR procedures in the context of CTAAs. Both studies found open surgery to be more expensive by approximately US$6700 and £1650, but they considered in-hospital cost only, excluding reintervention. No formal economic evaluation has been performed. Therefore, there is a lack of economic data to guide decision-makers in allocating the scarce resources available.
Relationship with abdominal aortic aneurysms
Because aneurysms in the abdominal aorta are more prevalent, the evidence base for intervention with both endovascular aneurysm repair (EVAR) and open surgery is stronger for abdominal aortic aneurysms (AAAs). In particular, the EVAR-1 randomised clinical trial (RCT) compared these techniques, initially showing a significant but short-lived benefit for the patients receiving EVAR. At the end of 15 years, the effect was reversed, with a significant advantage in overall survival for those who had received OSR, because of late complications and rupture in the EVAR group. Based on cost-effectiveness analysis of EVAR compared with open repair, draft guidelines for aneurysm repair from the National Institute for Health and Care Excellence (NICE), published in 2018,45 concluded that EVAR is not cost-effective and should not be used in fit or unfit patients with a non-ruptured aneurysm. After unprecedented stakeholder concern and intervention from NICE, the guidelines were revised and finally published. 46 The guidelines state that ‘where open surgical repair can’t be carried out – for example because of medical or anaesthetic risks – EVAR can be considered’46 (© NICE 2020 NICE Publishes its Guideline on the Diagnosis and Management of Abdominal Aortic Aneurysms. Available from www.nice.org.uk/news/article/nice-publishes-its-guideline-on-the-diagnosis-and-management-of-abdominal-aortic-aneurysms. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication). However, cost-effectiveness is clearly an increasingly important issue, given increasing health-care costs, and studies that attempt to find cost-effective improvements are needed.
The ETTAA study
Both ESG and OSR are effective in some patients with CTAAs, but both are associated with significant complications. Currently, there is no consensus on either best management strategy or timing of interventions and there are no UK-specific economic studies that assess outcomes beyond the chosen procedure. Further evidence regarding the cost-effectiveness of ESG and OSR is needed, given the increasing demand for treatment (an ageing population with a rising prevalence of CTAAs)2 and limited NHS resources. The relatively low incidence of aneurysms in the thoracic aorta means that the feasibility of a trial is unclear. Therefore, the ETTAA study was designed as an observational study to document current practice in the management of CTAAs of the arch/DTA in the NHS, and to compare the clinical effectiveness and cost-effectiveness of the available treatment strategies, adjusting for selection bias.
Aims of the ETTAA study
The overall aims of the ETTAA study are to describe the pathways undertaken by current NHS patients who are diagnosed with CTAAs, to estimate the natural history of patients prior to endovascular or open surgical procedures and to compare clinical outcomes and cost-effectiveness between the intervention groups.
Specifically, we aimed to answer the following questions:
-
Without procedural intervention for CTAAs, what is the risk of aneurysm growth, dissection, rupture, permanent neurological injury or death, and how does health-related quality of life (HRQoL) change over time?
-
If a patient receives ESG or OSR, what is the risk of dissection, rupture, permanent neurological injury or death?
-
What factors affect aneurysm growth pre intervention?
-
Can aneurysm- or patient-related predictors of treatment outcomes be determined?
-
What is the most cost-effective strategy in patients eligible for both ESG and OSR?
-
What further research is required?
The report is organised as follows. Chapter 2 reports a study of clinical expert opinion on the current management of CTAAs. Chapter 3 provides an overview of methods employed in the ETTAA study and a description of the resulting cohort. Chapters 4–7 describe the specific methods and results of the analysis of clinical and HRQoL outcomes (see Chapter 4), post-intervention clinical and HRQoL outcomes (see Chapter 5) and bias-adjusted clinical and HRQoL outcomes (see Chapter 6), and of the health economic analysis (see Chapter 7). Chapter 8 provides a discussion of the results and implications for service and future research.
Chapter 2 Expert clinical views at the start of the ETTAA study
Introduction
The ETTAA study was designed to identify the strengths and weaknesses of established practice. An integral consideration when comparing the outcomes in different treatment groups is how patients are selected for treatment. This chapter reports on a consensus study that aimed to understand how aneurysm features and patient characteristics influence treatment decisions in the UK. The main objective was to understand when there is clinician consensus regarding appropriate treatment methods for patients with CTAAs according to predefined criteria, what thresholds for intervention are commonly adopted and when clinicians are in equipoise between different treatment methods and, therefore, further research is required.
Methods
Preparation of resources
An initial design period involved production of study resources, including assembling an expert panel, defining clinical criteria and designing the study questionnaire. Thereafter, the consensus study was carried out in two rounds, combining features of both the Delphi survey technique and the nominal group technique. 47 The Delphi technique uses questionnaires and anonymised responses from experts to identify consensus where it exists. The nominal group technique allows further refinement of consensus in a face-to-face meeting of the panel (the nominal group), where experts discuss reasons for their decisions with the group and have the opportunity to revise their decision. The two rounds are described in greater detail below. The initial Delphi survey was conducted during autumn 2015 and the nominal group technique meeting was held in January 2016.
Assembling the expert panel
Invitations to form an expert panel were sent to cardiothoracic and vascular surgeons, cardiologists, interventional radiologists and anaesthesiologists who participated in thoracic aortic MDTs at 29 UK centres recruiting patients to the ETTAA study. These centres had already been identified as having significant experience in managing patients with arch, DTA and thoracoabdominal aneurysms during recruitment to the ETTAA study. Respondents were specialists who had expertise and significant experience in open or endovascular surgery, or both.
Defining case scenarios
Vascular and cardiothoracic surgeons in the ETTAA team compiled a list of patient and aneurysm factors that they considered influential in making treatment decisions regarding CTAAs. Initially, more than 20 factors were identified. However, to include all possible combinations of factors and levels would generate many millions of case scenarios (cases). Although these combinations (case scenarios) allow granularity of the information obtained by the consensus exercise, the elicitation of all combinations would result in fatigue/disengagement of members of the expert panel and so was not feasible. Thus, comorbidities related to the risk of surgical intervention or the operative fitness of the patient were included under the umbrella term of ‘high, medium or low risk of (open surgery) intervention’. After discussion, five characteristics with two to five levels remained (Table 2).
| Characteristic | Level |
|---|---|
| CTD | Present |
| Absent | |
| Aneurysm location | Aortic arch |
| DTA | |
| Thoracoabdominal aorta | |
| Age (years) | < 65 |
| 65–75 | |
| 76–85 | |
| > 85 | |
| Aneurysm size (maximum orthogonal diameter in cm) | < 5.0 |
| 5.1–6.0 | |
| 6.1–7.0 | |
| 7.1–8.0 | |
| > 8.0 | |
| Risk of open surgery | Low |
| Medium | |
| High |
Questionnaire design
A total of 360 case scenarios were developed in a factorial design of the attributes given in Table 2 and considered a good compromise between granularity and feasibility. These scenarios were grouped into six sections based on aneurysm location and presence or absence of CTD. The order in which experts completed the sections was randomised to minimise bias due to responder fatigue. The questionnaires were e-mailed to participants to be printed, completed and returned by post or e-mail.
Round 1: Delphi survey
For each clinical scenario, participants scored the ‘appropriateness’ of all four management options by indicating how strongly they considered each treatment option to be ‘appropriate’ on a scale of 1–9, where one represented ‘not at all appropriate’, five represented ‘just appropriate’ and nine represented ‘most appropriate’ (see Report Supplementary Material 1 for an example of a completed Delphi survey). Experts were guided to score for all four treatment methods for each clinical scenario and to avoid just marking the most ‘appropriate’. When an expert recorded ‘most appropriate’ for more than one management approach, this suggested equipoise for that expert.
When completing the scores, experts were asked to represent the opinion of their local multidisciplinary team as far as possible and to follow established definitions for clinical attributes. For each scenario, experts could assume that WW, CM, ESG and OSR were available, with no anatomical/morphological contraindications. ‘Hybrid’ interventions that included a component of conventional surgery as well as an endovascular stent graft were classified as OSR if they involved opening a body cavity (e.g. visceral artery bypass, re-implantation of innominate artery origin) and otherwise as endovascular repair (e.g. carotid to subclavian bypass through neck incision). No specific stipulations were given regarding methods of assessing the risk of intervention, but panel members were asked to follow local standard clinical practice.
Data analysis: round 1
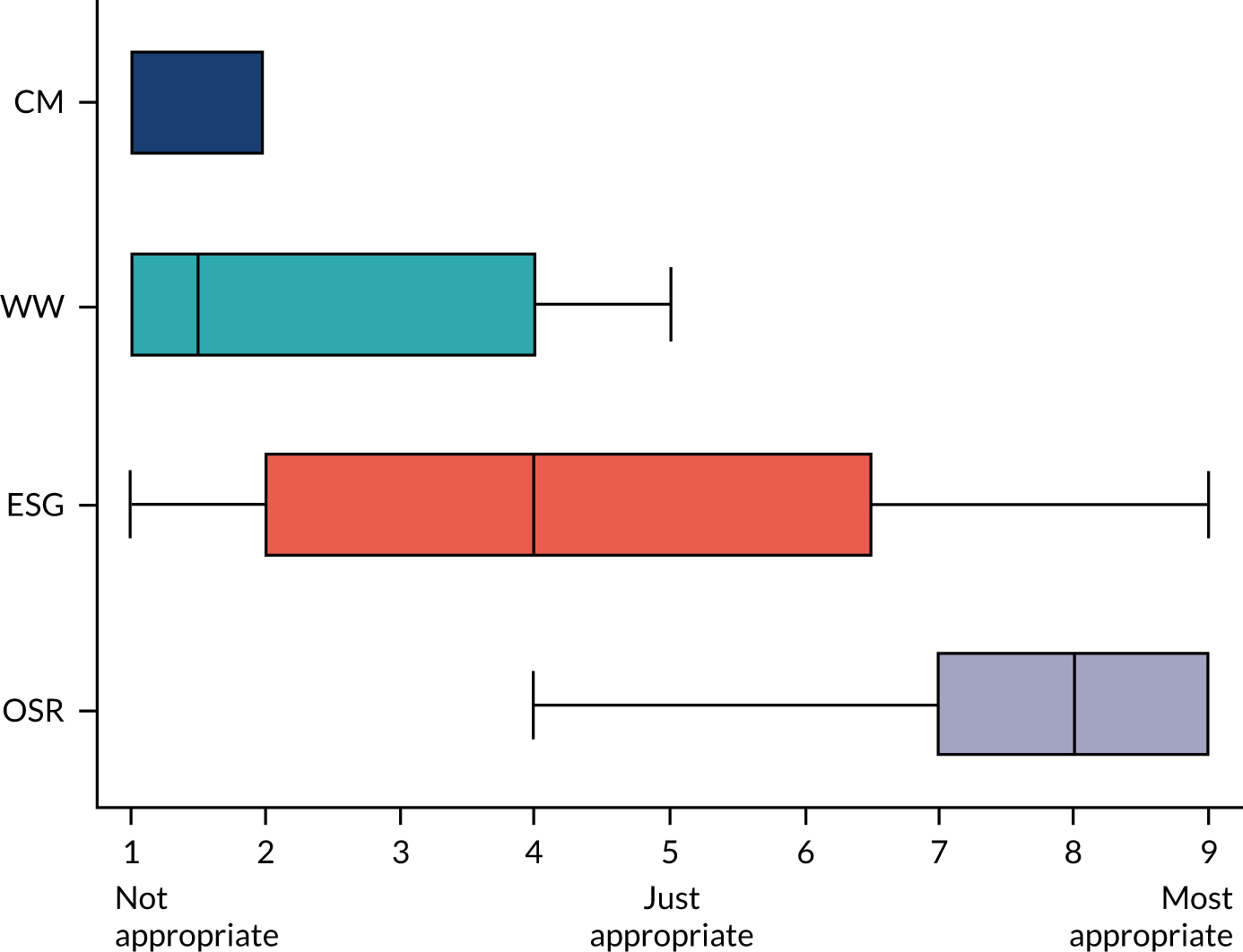
The anonymised results of round 1 were summarised as medians, interquartile ranges and whole ranges using box-and-whisker plots. For example, Figure 3 depicts the scoring for a clinical scenario with a median ‘appropriateness’ score of eight for OSR (range 4–9, interquartile range 7–9).
FIGURE 3.
Example of round 1 results for one clinical scenario: aneurysm size of 7.1–8 cm in diameter, in the aortic arch, in a patient with no connective tissue disorder, aged < 65 years and of low surgical risk.
To assess disagreement and appropriateness (and, thus, define consensus) the Research ANd Development (RAND)/University of California Los Angeles appropriateness method was used. 48 This considers the dispersion of individual scores and identifies scenarios in which expert responses are clustered at either end of the 9-point Likert scale, so that consensus is evident. Fitch et al. 48 argue that in cases when agreement is good, the distribution of responses should be narrow, and in cases where there is disagreement, the distribution should be wider. The width of the distribution is measured by the range between the 30th and 70th percentile, known as the interpercentile range (IPR). However, Fitch et al. 48 found that, in general, the IPR required for disagreement was smaller when responses were symmetrical than when they were asymmetrical, with respect to the middle of the distribution. To overcome this, they developed the asymmetry-adjusted IPR (IPRAS), which includes a correction factor for asymmetry. In this method, disagreement between experts is concluded for the ith scenario if the IPR > IPRAS for that scenario, or, conversely, IPR ≤ IPRAS indicates consensus. Clinical scenarios for which consensus was demonstrated in round 1 were noted and these were not taken into round 2.
Round 2: nominal group technique
Round 2 was completed at a face-to-face meeting of the expert panel moderated by one of the investigators (SRV). For each clinical scenario entering round 2, ‘appropriateness’ scores of the treatment options from round 1 were displayed as box-and-whisker plots, as demonstrated in Figure 3. The expert panel was given 60 seconds to study each summary, after which a brief discussion was held in which individual experts explained the reasons for their treatment choice. The role of the moderator was to clarify ambiguities, ensure a balanced discussion, give everyone a chance to express their opinion to the group and explore reasons for divergent views. It was decided prospectively that each expert would be given a maximum of 60 seconds of uninterrupted time to express opinions. Experts were concise, discussions were constructive and curtailment by the moderator was never required. After each discussion, individual experts were asked to select their single most ‘appropriate’ management strategy or to indicate more than one treatment method if there was equipoise between them.
Data analysis: round 2
In round 2 consensus was defined if the same management was chosen by ≥ 70% of participants; otherwise, there was no consensus. If there was no consensus, and ≥ 33% of participants thought that (the same) two management options were equally effective, equipoise about the choice of management was defined.
Results
Round 1: Delphi survey
Twenty experts from 13 centres returned round 1 scores. The expert panel consisted of an anaesthetist, an interventional radiologist, five cardiac surgeons and 13 vascular surgeons. Among the 360 scenarios considered, consensus was reached in 167 (46%) and the remaining 193 were discussed in round 2. The consensus achieved in round 1 was predominantly that WW was most appropriate for cases involving smaller aneurysms (< 6.0 cm in diameter), OSR was most appropriate for arch aneurysms in low-risk scenarios and ESG was most appropriate for DTA aneurysms in low- or medium-risk patients without CTDs.
Round 2: nominal group technique
Twelve experts, nine vascular surgeons and three cardiac surgeons took part in round 2, during which consensus was reached for a further 80 (22%) scenarios and equipoise between two different treatment modalities was noted for 34 (9%) scenarios, leaving neither consensus nor equipoise for a total of 79 scenarios (22%). Outcomes at the end of round 2 are presented in Tables 3 (aneurysms of ≤ 6.0 cm in diameter) and 4 (aneurysms of > 6.0 cm in diameter).
| Aneurysm site | Aneurysm size (cm) | Patient age (years) | No CTD | CTD | ||||
|---|---|---|---|---|---|---|---|---|
| Low risk | Medium risk | High risk | Low risk | Medium risk | High risk | |||
| Aortic arch | < 5.0 | < 65 | WW | WW | WW | WW | WW | WW |
| 65–75 | WW | WW | WW | WW | WW | WW | ||
| 75–85 | WW | WW | WW | WW | WW | WW | ||
| > 85 | WW | No consensus | CM | WW | No consensus | CM | ||
| 5.1–6.0 | < 65 | WW | WW | WW | No consensus | No consensus | WW | |
| 65–75 | WW | WW | WW | WW | WW | WW | ||
| 75–85 | WW | WW | WW | WW/OSR | WW | WW | ||
| > 85 | WW | WW | No consensus | OSR | OSR | WW | ||
| DTA | < 5.0 | < 65 | WW | WW | WW | WW | WW | WW |
| 65–75 | WW | WW | WW | WW | WW | WW | ||
| 75–85 | WW | WW | WW | WW | WW | WW | ||
| > 85 | No consensus | No consensus | CM | WW | CM | CM | ||
| 5.1–6.0 | < 65 | WW | WW | WW | No consensus | No consensus | No consensus | |
| 65–75 | WW | WW | WW | WW | WW | WW | ||
| 75–85 | WW | WW | WW | WW/OSR | WW/OSR | WW | ||
| > 85 | WW | WW | WW | WW/OSR | WW/OSR | WW | ||
| Thoracoabdominal aortic aneurysms | < 5.0 | < 65 | WW | WW | WW | WW | WW | WW |
| 65–75 | WW | WW | WW | WW | WW | WW | ||
| 75–85 | WW | WW | WW | WW | WW | No consensus | ||
| > 85 | No consensus | CM | CM | WW | No consensus | CM | ||
| 5.1–6.0 | < 65 | WW | WW | WW | WW | WW | WW | |
| 65–75 | WW | WW | WW | WW | WW | WW/CM | ||
| 75–85 | WW | WW | WW | WW | WW | WW | ||
| > 85 | No consensus | No consensus | CM | WW/OSR | WW | CM | ||
| Aneurysm site | Aneurysm size (cm) | Patient age (years) | No CTD | CTD | ||||
|---|---|---|---|---|---|---|---|---|
| Low risk | Medium risk | High risk | Low risk | Medium risk | High risk | |||
| Aortica arch | 6.1–7.0 | < 65 | No consensus | No consensus | CM | No consensus | No consensus | No consensus |
| 7.1–8.0 | OSR | No consensus | CM | OSR | No consensus | No consensus | ||
| > 8.0 | OSR | CM/ESG | CM | OSR | No consensus | ESG/OSR | ||
| 6.1–7.0 | 65–75 | OSR | ESG/OSR | No consensus | OSR | OSR | No consensus | |
| 7.1–8.0 | OSR | ESG/OSR | CM/ESG | OSR | ESG/OSR | CM/ESG | ||
| > 8.0 | OSR | ESG/OSR | CM | OSR | ESG/OSR | CM/ESG | ||
| 6.1–7.0 | 76–85 | OSR | OSR | No consensus | OSR | OSR | No consensus | |
| 7.1–8.0 | OSR | OSR | ESG | OSR | OSR | No consensus | ||
| > 8.0 | OSR | OSR | No consensus | OSR | OSR | No consensus | ||
| 6.1–7.0 | > 85 | OSR | OSR | No consensus | OSR | OSR | No consensus | |
| 7.1–8.0 | OSR | OSR | No consensus | OSR | OSR | No consensus | ||
| > 8.0 | OSR | OSR | ESG/OSR | OSR | OSR | ESG | ||
| DTA | 6.1–7.0 | < 65 | ESG | ESG | CM | No consensus | No consensus | No consensus |
| 7.1–8.0 | ESG | ESG | CM | ESG | No consensus | No consensus | ||
| > 8.0 | ESG | ESG | CM | No consensus | No consensus | No consensus | ||
| 6.1–7.0 | 65–75 | ESG | ESG | No consensus | ESG | ESG | No consensus | |
| 7.1–8.0 | ESG | ESG | ESG | No consensus | No consensus | No consensus | ||
| > 8.0 | ESG | ESG | ESG | No consensus | No consensus | CM/ESG | ||
| 6.1–7.0 | 76–85 | ESG | ESG | ESG | ESG/OSR | ESG/OSR | ESG | |
| 7.1–8.0 | ESG | ESG | ESG | ESG | ESG/OSR | ESG | ||
| > 8.0 | ESG | ESG | ESG | OSR | ESG/OSR | ESG | ||
| 6.1–7.0 | > 85 | ESG | ESG | ESG | OSR | OSR | ESG | |
| 7.1–8.0 | ESG | ESG | ESG | OSR | OSR | ESG | ||
| > 8.0 | ESG | ESG | ESG | OSR | ESG/OSR | ESG | ||
| Thoracoabdominal aortic aneurysms | 6.1–7.0 | < 65 | No consensus | No consensus | No consensus | No consensus | No consensus | No consensus |
| 7.1–8.0 | No consensus | ESG | ESG | No consensus | No consensus | No consensus | ||
| > 8.0 | No consensus | ESG | ESG | OSR | No consensus | No consensus | ||
| 6.1–7.0 | 65–75 | No consensus | No consensus | No consensus | No consensus | No consensus | No consensus | |
| 7.1–8.0 | ESG | ESG | CM | No consensus | No consensus | No consensus | ||
| > 8.0 | ESG | ESG | CM/ESG | No consensus | No consensus | No consensus | ||
| 6.1–7.0 | 76–85 | ESG/OSR | ESG/OSR | ESG | OSR | OSR | No consensus | |
| 7.1–8.0 | ESG/OSR | ESG/OSR | ESG | OSR | OSR | No consensus | ||
| > 8.0 | ESG/OSR | ESG | ESG | OSR | OSR | No consensus | ||
| 6.1–7.0 | > 85 | No consensus | ESG/OSR | ESG | OSR | OSR | CM | |
| 7.1–8.0 | OSR | ESG/OSR | ESG | OSR | OSR | CM | ||
| > 8.0 | ESG/OSR | ESG/OSR | ESG | OSR | OSR | CM | ||
Aneurysms of ≤ 6.0 cm in diameter (144 scenarios; see Table 3)
Watchful waiting was generally the preferred management for patients with aneurysms of ≤ 6.0 cm in diameter (110/144, 76% scenarios), regardless of the presence of CTDs or the location of the aneurysm (arch, DTA or thoracoabdominal). Notable exceptions, mainly for older patients, were:
-
CM was preferred for most high surgical risk patients, aged > 85 years, for all aneurysm sites.
-
OSR was preferred for patients with CTDs, with arch aneurysm of 5.0–6.0 cm in diameter and > 85 years of age, if at low or medium surgical risk.
-
Equipoise was found between WW and OSR for older patients with CTDs and with low surgical risk and aneurysms of 5.1–6.0 cm in diameter.
No consensus was reported for 10% of the clinical scenarios in which aneurysms were of < 6.0 cm in diameter. This was mainly for low- to medium-risk patients aged > 85 years or patients with CTDs aged < 65 years.
Aneurysms of > 6.0 cm in diameter (see Table 4)
For patients with aneurysms of > 6.0 cm in diameter, clinical decisions were influenced by, in order, aneurysm site, surgical risk and age group, rather than by aneurysm size (see Table 4).
Aortic arch
In terms of aneurysms of > 6.0 cm in diameter experts favoured OSR over ESG in the arch, regardless of CTD status, for low- or medium-risk patients aged > 75 years, as well as for most low-risk patients aged ≤ 75 years. Clinicians were in equipoise between OSR and ESG for medium-risk patients aged 65–75 years with arch aneurysms of > 6.0 cm in diameter. There was uncertainty and a general lack of consensus about what to offer patients at high surgical risk, with the exception that experts generally preferred CM for high-risk non-CTD patients aged ≤ 75 years. There was also little consensus among experts on treatment for younger (aged < 65 years) patients at medium surgical risk (with or without CTD) or for younger low-risk patients with aneurysms of 6.1–7.0 cm in diameter.
Descending thoracic aorta
There was consensus that ESG should be offered to non-CTD patients with DTA aneurysms of > 6.0 cm in diameter. CM was recommended only for high-risk patients aged ≤ 65 years. There was little or no consensus on how to treat DTA aneurysms in CTD patients aged ≤ 75 years, although experts agreed that ESG should be recommended for older CTD patients at high operative risk. In general, for CTD patients, there was equipoise between ESG and OSR for those aged 75–85 years at medium surgical risk, and consensus for OSR for those aged > 85 years at low/medium surgical risk.
Thoracoabdominal aneurysms
There was no consensus on treatment for thoracoabdominal aneurysms of 6.1–7.0 cm in diameter for patients aged < 65 years, regardless of CTD status. For younger non-CTD patients (aged ≤ 75 years) with aneurysms of > 7.0 cm in diameter, ESG was often recommended, except for high-risk patients, for whom CM was considered. For older (aged > 75 years) non-CTD patients, ESG was supported for patients at high surgical risk, whereas both ESG and OSR were considered ‘appropriate’ for patients at low or medium surgical risk. For CTD patients aged ≤ 75 years, there was no consensus on ‘appropriate’ treatment. For older (aged > 75 years) CTD patients at low or medium risk, OSR was the treatment of choice, with the oldest high-risk patients considered suitable for CM.
Summary of findings
This chapter reports expert opinion among UK specialists regarding the most ‘appropriate’ management for 360 clinical scenarios relating to thoracic aortic and thoracoabdominal aortic aneurysms. Pathophysiology, natural history of disease, technical aspects relevant to open surgery and ESG are different between the aortic arch, DTA and thoracoabdominal aorta, so they need to be considered separately.
For patients with aneurysms of < 6.0 cm in diameter, there was clear consensus for WW in the majority of patient scenarios, including those in the arch and irrespective of the presence or absence of CTDs. This differs from ascending aortic aneurysms, for which the threshold for surgical intervention in CTD patients is lower (5.0 cm) than for non-CTD patients. This may reflect the fact that surgical repair of the ascending aorta can be offered with much lower operative risks than arch repair.
For larger aneurysms in the aortic arch, OSR was the treatment of choice in older patients provided that operative risk was acceptable, but there was little consensus among experts on the management of younger patients or high-risk patients. Aneurysms of the arch pose particular challenges for ESG as multiple cerebral emboli are associated with the use of endovascular stents and, therefore, there is a high risk of stroke. 49,50 Thus, OSR was preferred in the majority of cases where intervention was considered ‘appropriate’.
Conversely, there was consensus that ESG should be offered to patients with DTA aneurysms of > 6.0 cm in diameter. This is unsurprising given that the risk of paraplegia is significantly lower with ESG than with OSR and that the DTA procedure is more straightforward than ESG in the arch. Experts also recorded consensus for ESG for large aneurysms in the DTA in CTD patients at high operative risk, despite conventional ‘wisdom’ that implanting stents in the intrinsically weak aortic tissue of CTD patients should be avoided. 51–53
For the oldest patients, CTD played an influential role in decisions. Clinicians tended to prefer intervention for smaller aneurysms in patients with CTDs, possibly because of concerns that complications are seen with aneurysms of smaller diameters in this population. The presence of CTDs poses a particular threat to the durability of ESG, compared with absence of CTDs. 54 The consensus reflects a reluctance to use ESG in patients with CTD, particularly in younger and lower-risk cohorts. However, the anatomical features of the DTA conferred a consensus for ESG, even in the presence of CTDs, especially if operative risk was high.
Thoracoabdominal aneurysms are currently treated by ESG in anatomically suitable patients. 55 A consensus for the use of this technique was noted for patients without CTD, with OSR remaining the preferred choice in the majority with CTD.
Unsurprisingly, our findings are in line with recommendations in previously published guidelines,17 but they provide greater detail. They also reflect the importance of aneurysm diameter in the timing of intervention, the perceived benefits of endovascular techniques and the consequences of CTDs.
Our study has some methodological limitations. During study design, we could not identify an objective, widely understood measure of surgical risk. Decisions relied on each participant’s perception of surgical risk category, which may have differed between experts, particularly if they were from different centres or surgical specialties. The methods require us to categorise patient and aneurysm characteristics, but each patient and aneurysm repair might be considered unique, and we were not able to capture all important aspects affecting management decisions. In addition, we did not include aneurysm growth rate as an indication for operation because it would have greatly increased the number of clinical scenarios and growth cannot always be distinguished from random variation in aneurysm measurement. Although we drew participants from as wide a range as possible, all experts practised at UK NHS centres, and worked in multidisciplinary teams that included open surgical and endovascular expertise. Consensus was based on 12–20 participants who may not fully represent their local practice. Analysis of the empirical data from the ETTAA study will demonstrate how closely UK clinical practice aligns with the reported consensus in this study.
One reason for undertaking an early Delphi study was to identify patients for whom clinicians have equipoise between ESG and OSR. Perceived equipoise was found in only a few scenarios by our definition, although we stress that the study is based on practice reported by experts rather than on more objective data. The size of patient groups for which there is equipoise is also unclear. Chapters 3–7 report analysis of CTAA patient management in the NHS, both before and after undergoing a procedure.
Chapter 3 Cohort construction, data and study management and general methods
Introduction
In this chapter we describe the methods for constructing the ETTAA cohort and provide an overview of the design and analysis of planned work packages. The methods for each work package are described in greater detail in the relevant chapters. We also describe the main characteristics of recruited patients and their procedures. We stress here that the ETTAA study was designed as an observational study and we did not intervene in routine practice; rather, we describe management strategies and outcomes for existing patients. As with any observational study, biases can arise from measured and unmeasured confounders, informative dropouts, missing data and other selection strategies; our emphasis is on accommodating biases in the analysis as far as possible and on acknowledging residual bias in results where necessary.
Aims of the project overall
The overall aims of this project are to describe the pathways undertaken by current NHS patients who are diagnosed with CTAA, to compare outcomes between the main treatment groups using modern methods for addressing the biases inherent in non-randomised studies, and to provide inputs for a health economics model.
The specific questions are listed in Chapter 1, along with planned work.
To meet the aims of the ETTAA study, we had the following objectives:
-
to follow patients with CTAA, prospectively recording management, patient characteristics, clinical events, HRQoL and use of health and social services throughout the duration of the study
-
to quantify clinical outcomes in each management cohort (WW, CM, ESG, OSR) in terms of survival, clinical events and quality of life
-
to identify patient-specific or aneurysm-specific features that might predict poor outcome in each treatment group by risk-modelling methods
-
to estimate the clinical effectiveness and cost-effectiveness of competing treatments to define optimal management strategies for patients in whom more than one treatment is considered appropriate.
Methods
Inclusion and exclusion criteria
Inclusion criteria
Included patients were those aged ≥ 18 years presenting to an NHS hospital with an existing or a new CTAA in the arch or DTA of a diameter ≥ 4 cm (including aneurysms secondary to atherosclerotic degeneration, after acute dissection and secondary to aortopathy). Patients were eligible as long as they had not undergone intervention for the index aneurysm. If a patient had already received treatment for an aneurysm on a different part of the aorta (e.g. ascending, abdominal), then that patient was eligible. The arch was defined as between the brachiocephalic artery and the left subclavian artery. The DTA was defined as between the left subclavian artery and the coeliac axis. If the aneurysm of maximum diameter was located in the thoracoabdominal aorta (21 patients), then the patient was included if the index aneurysm in the DTA was ≥ 4 cm in diameter. If the DTA and arch both had aneurysms of the same size, the aortic arch was considered to be the location of the maximal aneurysm size.
Exclusion criteria
Patients were excluded if they were unable or unwilling to give written informed consent, were suffering from acute dissection or malperfusion syndromes (e.g. myocardial infarction, acute stroke or limb ischaemia) or had had a previous intervention for the same aneurysm.
Setting
All NHS hospitals that treat or manage patients with CTAA in a MDT setting or specialist clinic were eligible to participate in the study.
Patient and centre recruitment
Centres were recruited after completion of a feasibility questionnaire confirming that they treated patients with CTAA by ESG, OSR or both. In addition, hospitals that cared for patients using WW or CM were eligible to participate if they referred patients for intervention to a centre also participating in the ETTAA study. The initial intention was to recruit 8–10 large centres but, owing to slow recruitment, it became necessary to open the study to 30 hospitals.
Patient eligibility for the study was assessed either in a MDT setting or in a specialist clinic at participating centres. Eligible patients were enrolled, and consent to collect and retain the patient’s data was taken by local research personnel. Consent was obtained face to face or by post/telephone with the consent form posted to the research team.
Study groups
Patients were divided into four groups, depending on the planned management at the time of recruitment:
-
WW – patients with smaller aneurysms at low risk of rupture who were not expected to undergo a surgical or endovascular procedure as part of the current management plan, but for whom these interventions may be a future option should the aneurysm expand.
-
CM – patients with aneurysms of a size where risk of rupture is significant, who were not expected to undergo a surgical or endovascular procedure as part of the current or future management plan due to patient choice, comorbidities or procedural risk.
-
ESG – patients for whom the risks around intervention were considered lower than the risks of rupture, who were referred to a vascular surgeon for aneurysm repair.
-
OSR – patients for whom the risks around intervention were lower than the risks of rupture, who were referred to a cardiac surgeon for aneurysm repair.
During the study some patients transferred between groups, particularly from WW to active intervention (ESG or OSR), so that the final analysis was based on the numbers of patients in each group at end of the study period, with the exception of the analysis of aneurysm growth rates (see Chapter 4 for more details).
Management and interventions
Watchful waiting
Watchful waiting patients with aneurysms considered ‘below threshold’ for intervention were treated with lifestyle modification advice (smoking cessation and dietary management) and medical management of hypercholesterolaemia and hypertension. Patients underwent surveillance of the aneurysm (by CT or MRI scans at intervals chosen by the local team) and MDT review (as per local practice).
Conservative management
Conservative management included lifestyle modification advice (smoking cessation and dietary management) and medical management of hypercholesterolaemia and hypertension. CM prohibited any endovascular or open surgical procedure. In this group, features of the aneurysm would have normally triggered intervention, but patient-related features (including comorbidities or patient choice) prohibited it; thus, CM is different from WW.
Endovascular stent grafting
Endovascular stent grafting included any endovascular repair of the aneurysm via transluminal introduction of a stent graft under X-ray guidance. It included any primary endovascular procedure comprising a combination of a conventional surgical component and a transluminal repair (described as a hybrid procedure in some publications). It was completed by a vascular surgeon or an interventional radiologist, usually in a ‘hybrid’ theatre equipped with an imaging scanner intensifier (with a fixed C-arm). It could also be performed in a catheter laboratory or surgical theatre with a mobile C-arm. It excluded open procedures via sternotomy or thoracotomy.
Open surgical replacement
Open surgical replacement comprised replacement of the aneurysmal aorta with a prosthetic conduit, requiring sternotomy or thoracotomy with circulatory support. OSR was completed in a surgical theatre by a cardiac or vascular surgeon. It also included cases where an adjacent segment of aorta was stabilised by implanting a stent at the time of surgery, through the surgical incision.
Hybrids
A hybrid treatment means that the intervention has both stent and surgical components (see Chapter 1 for examples). Where surgery involved only a minor incision, for example to guide stent placement, ESG was the predominant procedure, and these patients were included in the ESG group. Where the hybrid graft was half surgical prosthesis-half stent, inserted during open surgery involving median sternotomy, surgery was the predominant procedure and these patients were included in the OSR group.
Populations
Patient group allocation at recruitment
Once consented, patients took one of two typical pathways depending on whether or not an intervention had been planned and a date of procedure fixed. At recruitment, patients were assigned to CM by the recruiting centre if future management was not expected to involve an intervention, or to WW if future management could include an intervention but further imaging was to be completed before any procedure was planned. These patients entered a non-intervention period, during which baseline characteristics, medical history and HRQoL were recorded at the point of consent and at follow-up visits planned at 3, 6, 12, 18, 24, 36 and 48 months while the patient remained in the non-intervention period. Each patient had either a CT or MRI scan to measure aneurysm size at baseline, and this was then repeated according to local management protocols, expected to occur approximately once per year.
Patients who had a known date of intervention at recruitment were assigned to the ESG or OSR group by the recruiting centre. For patients in the OSR or ESG group an additional assessment was undertaken at 1 month post intervention, with all other measurements taken at the same stages as for WW and CM patients. Procedure data, important complications, subsequent hospital admissions and serious adverse events (SAEs) were recorded by the participating centres as they occurred.
Crossover between groups
Owing to the observational nature of the study, patients switched groups according to local centre management protocols. Patients switching from WW to an intervention group were analysed as part of the non-intervention period up to the date of the procedure. Thereafter, patients entered a post-intervention period, with the timing of follow-up reset so that time zero was the date of the procedure to align with those who went straight to procedure.
Planned analyses
In accordance with the protocol, six work packages were planned to:
-
model aneurysm growth in WW and CM patients during the non-intervention period
-
quantify clinical outcomes within each treatment group (CM, ESG, OSR) and to assess the risk factors for each
-
compare propensity score-matched patients from each treatment group to estimate clinical effectiveness for patients in whom more than one treatment is appropriate
-
estimate cost-effectiveness of competing treatments to define optimal management strategies for patients in whom more than one treatment is considered appropriate
-
assess the subjective level of agreement among experts regarding best management for hypothetical patients using a RAND–Delphi exercise (see Chapter 2)
-
analyse aneurysm growth data from Yale University in collaboration with Professor John Elefteriades.
Amendments to the work packages
Work package 6 was not completed as initial analysis showed that the database included only 23 scans in 11 patients who satisfied the ETTAA study inclusion and exclusion criteria. This work package is not discussed further. For other work packages, aneurysm measurements were completed in sufficient numbers at only four locations (ascending, arch, descending thoracic and thoracoabdominal). Other locations are not reported in this monograph. See Report Supplementary Material 2 for a full list of protocol amendments and Report Supplementary Material 3 for a list of departures from the original protocol.
Outcomes
The primary and secondary outcomes for work packages 1–5 are listed briefly below with definitions.
-
Primary: aneurysm diameter in the aortic arch and DTA. Secondary: survival, time to intervention, clinical complications and HRQoL. Exploratory: aneurysm diameter in the ascending aorta and the thoracoabdominal aorta.
-
Primary: survival. Secondary: clinical complications, reinterventions, re-admission and HRQoL.
-
Primary: survival. Secondary: complications, reinterventions, re-admission, length of stay and HRQoL.
-
Primary: incremental cost per QALY gained. Secondary: HRQoL.
-
Primary: expert opinion on best treatment for any given theoretical patient scenario.
Definitions of outcome measurements
Aneurysm diameter
Study centres were asked to provide a copy of the chest CT or MRI radiological scan conducted closest to the time of recruitment to the ETTAA study (baseline scan) and the accompanying report needed to confirm that the patient had an eligible aneurysm of ≥ 4 cm in diameter. Centre co-ordinators were then asked to provide copies of any additional scans during patient follow-up. Note that all CT/MRI scans were conducted as part of routine care and, therefore, neither the timing of the scan nor the scan request itself was determined by participation in the ETTAA study. CT/MRI scans were anonymised and sent on DVD (digital versatile disc) to St George’s Hospital (London) or Royal Papworth Hospital Core Laboratory for the measurement of aortic diameters (in centimetres) to ensure that differences between multiple radiographers, hospitals and measurement techniques were minimised. In the original plan St George’s was to measure all scans but, owing to staffing issues, scans not analysed at the end of recruitment (30 June 2018) were transferred and analysed at Royal Papworth Hospital. A standard protocol was agreed and CT scan measurements at both centres were made using the same 3mensio (Pie Medical Imaging BV, Maastricht, the Netherlands) software (see Report Supplementary Material 4 for the scan measurement protocol). A total of five operators analysed CT scans [two at St George’s Hospital (n = 269 scans) and three at Royal Papworth Hospital (n = 1268 scans)]. All MRI scans were measured at Royal Papworth Hospital by two radiology consultants (n = 125). If neither core laboratory nor co-ordinating centre analysis was available, measurements were taken from the scan results provided by the participating hospital (n = 70). Although published evidence suggested that results from CT and MRI were comparable, statistical analyses of scan diameters were adjusted for scan modality. 56
Survival time in the non-intervention period
This was the time between the date of recruitment and the date of death or censoring. Patients were censored at date of the procedure or withdrawal or the last patient follow-up if any of these preceded death. The date and cause of death were reported by the participating centre.
Survival time in the intervention period
This was the time between the date of the procedure and the date of death or censoring. Patients were censored at withdrawal or on the date of the last post-procedure follow-up if this preceded death. The date and cause of death were reported by the participating centre.
Procedure-related data
For all interventions on the ascending, arch, DTA or thoracoabdominal aorta, the dates of admission, intervention and discharge, details of operative and postoperative care and clinical outcomes between admission and discharge were recorded by local centre staff from hospital medical records.
Time to intervention
This was the time interval between the date of recruitment and the date of the intervention.
Length of hospital stay for the index procedure
This was the interval in days between date of the index procedure and date of discharge from hospital or transfer to a non-hospital setting (e.g. a care home). This was separated into intensive care unit (ICU), high-dependency unit (HDU) and ward stay.
Clinical complications
All clinical complications related to the aneurysm or interventions were collected by centre staff from hospital records during the initial and follow-up hospital admissions, including myocardial infarctions, gastrointestinal, neurological or spinal events, thrombi, infections, vocal cord palsy and return to theatre, as well as requirement for cardiac support, prolonged ventilation and renal support. ESG complications were classified as access vessel injury, stent graft complication, endoleak, fistulae, aneurysm complication and other. In addition, the following were recorded: date of the event, theatre time, relationship to the procedure or treatment (not related, unlikely to be related, possibly related, probably related, definitely related), cause of event and management. Additional complications, which may arise outside the hospital, including vessel injury, endoleaks, aneurysm complications, stent graft complications and fistulae, were reported by participating centres. All complications were reviewed centrally by ETTAA clinicians (PS, SRL). Further details of complications are given in Appendix 1.
Readmissions to hospital
Readmissions after discharge were obtained by centre staff from hospital records. Information on dates of admission, days in ICU, HDU and ward, reason for admission, relationship to aneurysm or treatment and presenting symptoms were recorded. Further interventions on the ascending, arch, descending thoracic or thoracoabdominal aorta were also recorded.
Health-related quality of life
The completion of the EuroQoL-5 Dimensions, five-level version (EQ-5D-5L) was scheduled at baseline, at 3, 6 and 12 months and annually thereafter pre intervention and at 1, 3, 6 and 12 months and annually thereafter post intervention. 57 EQ-5D-5L records mobility, self-care, usual activities, pain/discomfort and anxiety/depression on a five-level Likert scale. Owing to varying times between recruitment and procedure, follow-up was not always synchronised with planned assessment times. See Chapters 4–7 for further details.
Quality-adjusted life-years
The results from the EQ-5D-5L were converted into health state utilities using UK population tariffs58 and used to estimate quality-adjusted life-years (QALYs) using the area-under-the-curve approach. QALYs at 12 months were estimated for patients who completed EQ-5D-5L score in the first year post intervention.
Resource use
Data on resource use from a UK NHS perspective were recorded for the procedure and any subsequent admissions to hospital for aneurysm-related or cardiac-related events. Other resource use was scheduled to be recorded at 1, 3, 6, 12, 18, 24, 36, 48 and 60 months post procedure for the use of primary care and Personal Social Services (PSS). Costs of health-care services were taken from standard sources such as NHS reference costs, Healthcare Resource Group (HRG) tariffs and manufacturer/supplier costs and from the centres themselves. See Chapter 7 for further details of health economic analyses.
Demographic and baseline variables collected
The baseline variables explored as predictors in modelling and for propensity score development are listed below.
Patient related
Sex, age, height, weight, BMI and smoking history.
Aneurysm related
Type of scan (CT or MRI), aneurysm diameter and location, and location of largest aneurysm.
Cardiovascular related
Diagnosis of hypertension, diabetes, left ventricular (LV) function, coronary artery disease, previous cardiac/aortic intervention, extracardiac arteriopathy, valvular heart disease, and medication (antihypertensive, anticoagulant, statin) at baseline and each follow-up visit.
Other markers of comorbidity
Chronic obstructive pulmonary disease (COPD), New York Heart Association (NYHA) classification of heart disease and CTD. Serum creatinine and haemoglobin levels at baseline and follow-up visits, if recorded.
Operative risk related
Logistic EuroSCORE and formal/informal care (as a proxy for frailty).
Health-related quality of life
EQ-5D-5L score.
Data collection
At baseline, medical history was taken by research personnel to identify a patient’s eligibility. Procedure details and related complications, clinical outcome data and EQ-5D-5L questionnaires were collected prospectively until the study ended, either during hospital attendances or by post/telephone and from hospital databases.
Aneurysm imaging using CT or MRI was undertaken in accordance with local practice, and anonymised copies of scans from the time of diagnosis were sent to the study team.
For patients who transferred from WW to ESG or OSR, a reassignment form was completed at the time the clinical decision was made. Following reassignment, assessment visits were scheduled relative to the reassignment date. For patients waiting over 3 months for surgery, pre-procedure follow-up assessments were completed every 3 months.
Follow-up visits had to be conducted within the following windows:
-
1- and 3-month follow-up: ± 1 week
-
6-, 12-, 18- and 24-month follow-up: ± 2 weeks
-
36-, 48- and 60-month follow-up: ± 4 weeks.
Data were transferred into ETTAA’s electronic database by the local principal investigator (PI) or a delegated researcher.
Statistical methods
Original planned sample size and revision in October 2016
Although the ETTAA study was an observational study, we provided sample size estimates based on comparing survival between ESG and OSR. From UK registry data, 360 elective operations and stents were performed each year in the UK for arch and DTA aneurysms. 2 Based on log-rank tests, assuming proportional hazards and uninformative censoring, we calculated the smallest possible effect sizes that would be statistically significant at (two-sided) 5% error rate, with 80% power, assuming a fixed sample size and a range of predicted incidence of events in the OSR group.
Table 5 gives the range of the minimum HRs detectable for a given expected event incidence in OSR. The first set of estimates uses pre-study predictions of the final sample size (ESG, n = 293; OSR, n = 147). The second set uses October 2016 predictions of sample size (ESG, n = 170; OSR, n = 112). With these numbers, moderate to large effects (HR > 0.5) could be detected, providing that the event incidence (e.g. deaths) during the study in the OSR group was at least 30%.
| OSR group probability of observing an event during the study | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50% | 45% | 40% | 35% | 30% | 25% | 20% | 15% | 10% | 5% | |
| HR based on original expected sample sizea | 0.66 | 0.64 | 0.62 | 0.60 | 0.57 | 0.54 | 0.49 | 0.43 | 0.34 | 0.16 |
| HR based on revised expected sample sizeb | 0.59 | 0.57 | 0.55 | 0.52 | 0.49 | 0.45 | 0.40 | 0.33 | 0.22 | 0.02 |
In response to requests from the National Institute for Health Research (NIHR), we also estimated the power of the study to detect the minimum clinically important difference (MCID) in HRQoL. Assuming a MCID of 0.1 in the EuroQoL utility measure,59 with 5% significance and a sample size of 170 ESG and 112 OSR the power was > 90% for either two-sided t-test or Wilcoxon-Mann–Whitney U-test (the latter would account for potential ceiling effects in HRQoL). The more sophisticated modelling methods to be used, adjusting for confounders, mean that 90% is a lower bound for power. Given this number of procedures, we expected 81 CM and 730 WW patients to be recruited during the study.
Data quality assurance
The assessment of data quality was complicated by the observational nature of the ETTAA study and the movement of patients between groups over time. Data completeness was assessed using summaries of individual case report forms (CRFs) returned. Data checks broadly followed recommendations in Kirkwood and Sterne. 60 Outliers in continuous variables were detected using ranges and plotting distributions within each group. Unresolved outliers that were extreme and separated from the distribution of a variable were removed and considered missing. Categorical variables were tabulated and unexpected values were queried with centres. Consistency checks between two or more variables were performed (e.g. bivariate plots, cross-tabulations). Dates were checked against planned timing assessments and interventions, as well as relative to other assessments in the same person. All queries were checked with centres and amended in the ETTAA database.
Data summaries
Detailed methods are provided in each chapter; here we give a brief overview of the descriptive methods. Throughout, variables were summarised as the total participants per group and overall, with means and standard deviations (SDs) if normally distributed, or median and interquartile range otherwise. Categorical data were presented as frequencies and proportion in each level. Time-to-event data were summarised as the actuarial survival probability or incidence during the non-intervention and post-intervention periods using Kaplan–Meier estimates. Post-intervention survival was also calculated separately for deaths within 30 days of an intervention. To assess whether or not patients allocated to different management groups were comparable, baseline variables were compared across the four groups using one-way analysis of variance, Pearson’s chi-squared test or a generalisation of Fisher’s exact test, as appropriate. 61
Multiple-centre issues
For the analysis of aneurysm growth and HRQoL over time, clustering by centre was investigated using normal random effects in a three-level hierarchy (scan within patient within hospital) (see Chapter 4). For time-to-event outcomes in work package 1, gamma-distributed frailty terms for centres were investigated. For all other analyses, between-centre variation in outcomes could not be assessed owing to the small number of patients contributed by most centres.
Subgroup analysis
Sensitivity analysis included the subgroup of patients who were potentially suitable for both OSR and ESG (see Chapter 6).
Missing data
The extent of missing data per variable was quantified as the number of cases divided by the number of patients who were in the study at the point of assessment. All essential variables for work packages 1–3 are expected to be complete or to have low missing rates (< 8%). Variables for which > 25% of data were unavailable or missing were not used in modelling but were summarised and reported.
Missing data patterns were explored (e.g. monotonic, intermittent). Missing data mechanisms, missing at random (MAR) and missing completely at random (MCAR) were investigated using standard statistical tests (log-rank, Student’s t-test, Mann–Whitney U-test, Pearson’s chi-squared test, Fisher’s exact test) to assess the associations between missing variable status (yes/no) and outcomes. To inform imputation models, associations between pairs of covariates and predictors of missingness were assessed using correlations and other standard statistical tests. Missing covariates were analysed together irrespective of the reasons (death, withdrawal, loss to follow-up, test not completed). For the analysis of aneurysm growth and HRQoL (work packages 1 and 2), all patients with at least two measurements were included in random-effects models. No adjustment was made for missing measurements as estimates from such models are unbiased provided that the data are MAR conditional on the observed data. 62 For work packages 2 and 3, the analysis found little evidence against the hypothesis that data were MCAR (see Appendix 2), so the complete-case analysis is presented throughout. Sensitivity analysis assuming MAR used multiple imputation with chained equations (MICE). Imputation models included the outcome variable as well as all important covariates from exploratory analysis. Each imputation model performed predictive mean matching to impute missing data. Values were simulated for each missing variable and the resulting models were combined using Rubin’s rules. 63
Results
Recruitment
Centre recruitment
Between 24 March 2014 and 24 July 2018, 886 CTAA patients were recruited from 30 centres (see Appendix 3 for a list of participating centres). Studies covered the majority of England but did not recruit from the devolved nations (Figure 4). Although some centres specialised in either vascular or cardiac surgery, many centres recruited patients to all four management groups.
FIGURE 4.
Locations of the 30 centres participating in the ETTAA study.
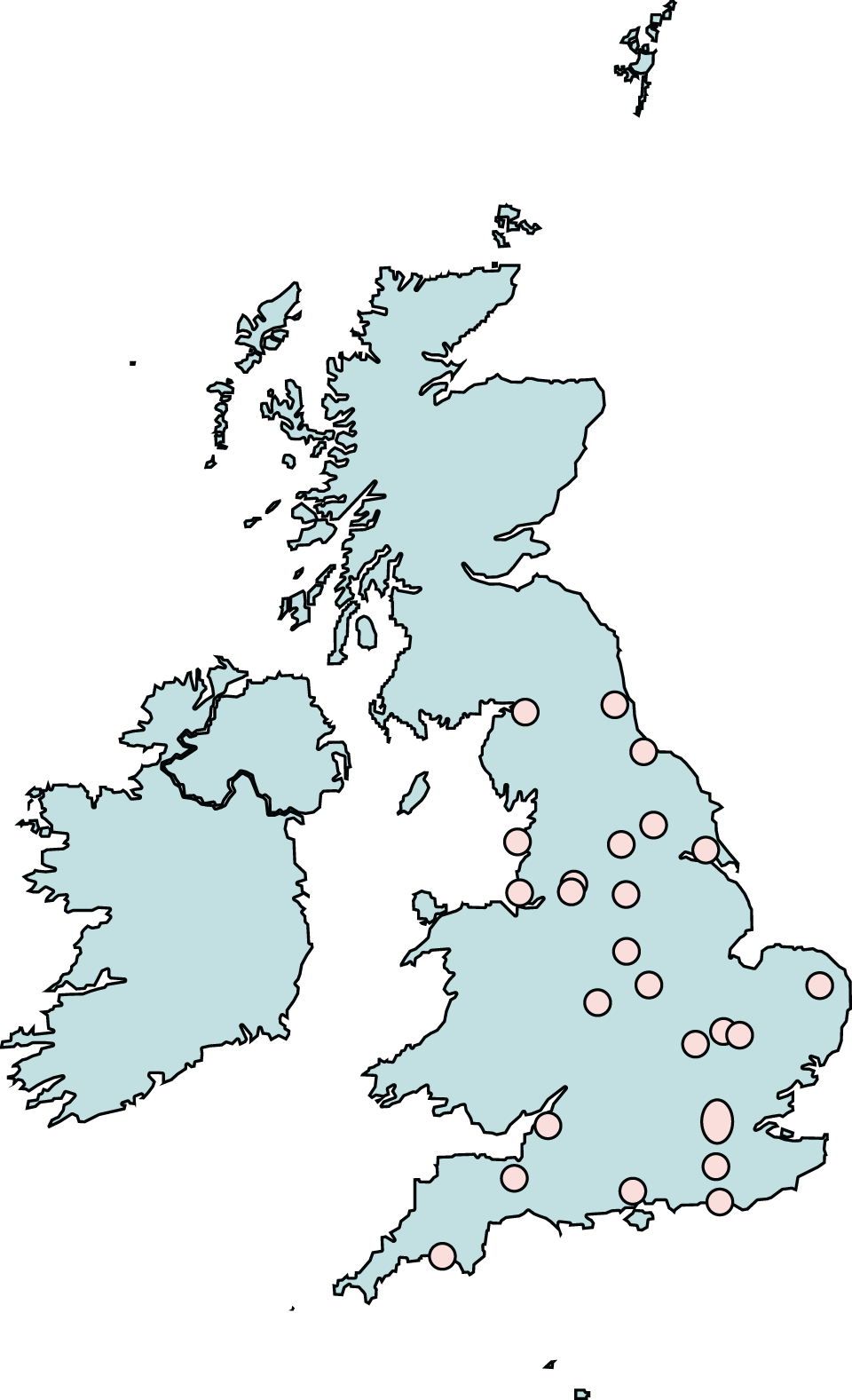
The Consolidated Standards of Reporting Trials (CONSORT)-style flow charts in Tables 6 and 7 show the number of cases assigned to each group pre (see Table 6) and post intervention (see Table 7). A total of 112 (12.6%) patients were assigned to CM, of whom 46 died during follow-up. The remaining 774 (87.4%) patients were eligible and willing to receive the intervention. Of these 774 patients, 150 (19.4%) subsequently received ESG, 135 (17.4%) subsequently received OSR and 83 (10.7%) died before any intervention. Plots of actual against target recruitment are provided in Appendix 4.
| Procedure follow-up relative to date | Number of patients | Percentage of total |
|---|---|---|
| Intervention or WW (N = 774) | ||
| 3 months | ||
| Completed | 525 | 67.8 |
| Died | 12 | 1.6 |
| Procedure | 182 | 23.5 |
| Withdrew | 8 | 1.0 |
| Missing | 47 | 6.1 |
| Censored | 0 | 0.0 |
| 6 months | ||
| Completed | 482 | 62.3 |
| Died | 24 | 3.1 |
| Procedure | 212 | 27.4 |
| Withdrew | 11 | 1.4 |
| Missing | 45 | 5.8 |
| Censored | 0 | 0.0 |
| 12 months | ||
| Completed | 418 | 54.0 |
| Died | 43 | 5.6 |
| Procedure | 244 | 31.5 |
| Withdrew | 15 | 1.9 |
| Missing | 54 | 7.0 |
| Censored | 0 | 0.0 |
| 18 months | ||
| Completed | 323 | 41.7 |
| Died | 58 | 7.5 |
| Procedure | 256 | 33.1 |
| Withdrew | 19 | 2.5 |
| Missing | 51 | 6.6 |
| Censored | 67 | 8.7 |
| 24 months | ||
| Completed | 251 | 32.4 |
| Died | 64 | 8.3 |
| Procedure | 268 | 34.6 |
| Withdrew | 21 | 2.7 |
| Missing | 56 | 7.2 |
| Censored | 114 | 14.7 |
| 36 months | ||
| Completed | 159 | 20.5 |
| Died | 77 | 9.9 |
| Procedure | 278 | 35.9 |
| Withdrew | 23 | 3.0 |
| Missing | 30 | 3.9 |
| Censored | 207 | 26.7 |
| 48 months | ||
| Completed | 66 | 8.5 |
| Died | 83 | 10.7 |
| Procedure | 284 | 36.7 |
| Withdrew | 23 | 3.0 |
| Missing | 29 | 3.7 |
| Censored | 289 | 37.3 |
| 60 months | ||
| Completed | 1 | 0.1 |
| Died | 83 | 10.7 |
| Procedure | 285 | 36.8 |
| Withdrew | 23 | 3.0 |
| Missing | 14 | 1.8 |
| Censored | 368 | 47.5 |
| CM (N = 112) | ||
| 3 months | ||
| Completed | 98 | 87.5 |
| Died | 6 | 5.4 |
| Procedure | 0 | 0.0 |
| Withdrew | 0 | 0.0 |
| Missing | 8 | 7.1 |
| Censored | 0 | 0.0 |
| 6 months | ||
| Completed | 92 | 82.1 |
| Died | 9 | 8.0 |
| Procedure | 0 | 0.0 |
| Withdrew | 2 | 1.8 |
| Missing | 9 | 8.0 |
| Censored | 0 | 0.0 |
| 12 months | ||
| Completed | 85 | 75.9 |
| Died | 15 | 13.4 |
| Procedure | 0 | 0.0 |
| Withdrew | 4 | 3.6 |
| Missing | 8 | 7.1 |
| Censored | 0 | 0.0 |
| 18 months | ||
| Completed | 60 | 53.6 |
| Died | 23 | 20.5 |
| Procedure | 0 | 0.0 |
| Withdrew | 6 | 5.4 |
| Missing | 11 | 9.8 |
| Censored | 12 | 10.7 |
| 24 months | ||
| Completed | 51 | 45.5 |
| Died | 28 | 25.0 |
| Procedure | 0 | 0.0 |
| Withdrew | 7 | 6.3 |
| Missing | 6 | 5.4 |
| Censored | 20 | 17.9 |
| 36 months | ||
| Completed | 28 | 25.0 |
| Died | 40 | 35.7 |
| Procedure | 0 | 0.0 |
| Withdrew | 7 | 6.3 |
| Missing | 2 | 1.8 |
| Censored | 35 | 31.3 |
| 48 months | ||
| Completed | 8 | 7.1 |
| Died | 46 | 41.1 |
| Procedure | 0 | 0.0 |
| Withdrew | 7 | 6.3 |
| Missing | 1 | 0.9 |
| Censored | 50 | 44.6 |
| 60 months | ||
| Completed | 0 | 0.0 |
| Died | 46 | 41.1 |
| Procedure | 0 | 0.0 |
| Withdrew | 7 | 6.3 |
| Missing | 0 | 0.0 |
| Censored | 59 | 52.7 |
| Procedure follow-up relative to date | Number of patients | Percetange of total |
|---|---|---|
| ESG (N = 150) | ||
| 1 month | ||
| Completed | 102 | 68.0 |
| Died | 9 | 6.0 |
| Withdrew | 0 | 0.0 |
| Missing | 39 | 26.0 |
| Censored | 0 | 0.0 |
| 3 months | ||
| Completed | 113 | 75.3 |
| Died | 12 | 8.0 |
| Withdrew | 1 | 0.7 |
| Missing | 23 | 15.3 |
| Censored | 1 | 0.7 |
| 6 months | ||
| Completed | 109 | 72.7 |
| Died | 15 | 10.0 |
| Withdrew | 1 | 0.7 |
| Missing | 21 | 14.0 |
| Censored | 4 | 2.7 |
| 12 months | ||
| Completed | 100 | 66.7 |
| Died | 25 | 16.7 |
| Withdrew | 1 | 0.7 |
| Missing | 13 | 8.7 |
| Censored | 11 | 7.3 |
| 18 months | ||
| Completed | 73 | 48.7 |
| Died | 29 | 19.3 |
| Withdrew | 1 | 0.7 |
| Missing | 18 | 12.0 |
| Censored | 29 | 19.3 |
| 24 months | ||
| Completed | 44 | 29.3 |
| Died | 34 | 22.7 |
| Withdrew | 1 | 0.7 |
| Missing | 13 | 8.7 |
| Censored | 58 | 38.7 |
| 36 months | ||
| Completed | 21 | 14.0 |
| Died | 40 | 26.7 |
| Withdrew | 1 | 0.7 |
| Missing | 11 | 7.3 |
| Censored | 77 | 51.3 |
| 48 months | ||
| Completed | 5 | 3.3 |
| Died | 40 | 26.7 |
| Withdrew | 1 | 0.7 |
| Missing | 2 | 1.3 |
| Censored | 102 | 68.0 |
| OSR (N = 135) | ||
| 1 month | ||
| Completed | 71 | 52.6 |
| Died | 15 | 11.1 |
| Withdrew | 2 | 1.5 |
| Missing | 47 | 34.8 |
| Censored | 0 | 0.0 |
| 3 months | ||
| Completed | 82 | 60.7 |
| Died | 17 | 12.6 |
| Withdrew | 2 | 1.5 |
| Missing | 29 | 21.5 |
| Censored | 5 | 3.7 |
| 6 months | ||
| Completed | 92 | 68.1 |
| Died | 21 | 15.6 |
| Withdrew | 2 | 1.5 |
| Missing | 17 | 12.6 |
| Censored | 3 | 2.2 |
| 12 months | ||
| Completed | 81 | 60.0 |
| Died | 26 | 19.3 |
| Withdrew | 4 | 3.0 |
| Missing | 16 | 11.9 |
| Censored | 8 | 5.9 |
| 18 months | ||
| Completed | 62 | 45.9 |
| Died | 28 | 20.7 |
| Withdrew | 4 | 3.0 |
| Missing | 17 | 12.6 |
| Censored | 24 | 17.8 |
| 24 months | ||
| Completed | 49 | 36.3 |
| Died | 32 | 23.7 |
| Withdrew | 4 | 3.0 |
| Missing | 0 | 0.0 |
| Censored | 50 | 37.0 |
| 36 months | ||
| Completed | 28 | 20.7 |
| Died | 35 | 25.9 |
| Withdrew | 4 | 3.0 |
| Missing | 4 | 3.0 |
| Censored | 64 | 47.4 |
| 48 months | ||
| Completed | 11 | 8.1 |
| Died | 36 | 26.7 |
| Withdrew | 4 | 3.0 |
| Missing | 3 | 2.2 |
| Censored | 81 | 60.0 |
Characteristics of the cohort at recruitment
Both prevalent and incident aneurysms were included in the cohort. Among the 871 patients with a record, the median time between diagnosis and recruitment was 9.1 months (range 4.0 months to 21.6 years).
Baseline predictors
A full breakdown of patient characteristics is given in Appendix 6, with summaries in Tables 8–10. Overall, the cohort comprised 321 (36.2%) women and 565 men and the mean age of the CTAA patients recruited was 70.9 (SD 10.9) years, with CM patients significantly older and OSR patients significantly younger on average (see Table 8). The groups also differed in height, weight (but not BMI) and requirements for additional care, which may relate to the differences in age.
| Characteristic | Patient subgroup (number of patients with a registration scan) | p-value | |||
|---|---|---|---|---|---|
| WW (N = 489) | CM (N = 112) | ESG (N = 150) | OSR (N = 135) | ||
| Age (years) | |||||
| Mean (SD) | 70.8 (10.7) | 76.6 (9.9) | 72.0 (8.6) | 64.9 (11.6) | < 0.0001 |
| Minimum, maximum | 32.3, 92.5 | 26.1, 92.5 | 49.6, 89.2 | 31.6, 83.5 | |
| Sex, n (%) | |||||
| Female | 174 (35.6) | 48 (42.9) | 50 (33.3) | 49 (36.3) | 0.4297 |
| Male | 315 (64.4) | 64 (57.1) | 100 (66.7) | 86 (63.7) | |
| Care, n (%) | |||||
| Formal | 10 (2.0) | 5 (4.5) | 0 (0.0) | 1 (0.7) | 0.0020a |
| Informal | 50 (10.2) | 18 (16.1) | 12 (8.09) | 7 (5.2) | |
| None | 425 (86.9) | 88 (78.6) | 138 (92.0) | 125 (92.6) | |
| Missing | 4 (0.8) | 1 (0.9) | 0 (0.0) | 2 (1.5) | |
| Smoker (current or past), n (%) | |||||
| Yes | 343 (70.1) | 71 (63.4) | 113 (75.3) | 89 (65.9) | 0.1518 |
| No | 142 (29.0) | 40 (35.7) | 36 (24.0) | 45 (33.3) | |
| Missing | 4 (0.8) | 1 (0.9) | 1 (0.7) | 1 (0.7) | |
| Comorbidity | Patient subgroup (number of patients with a registration scan) | p-value | |||
|---|---|---|---|---|---|
| WW (N = 489) | CM (N = 112) | ESG (N = 150) | OSR (N = 135) | ||
| Connective tissue disorder, n (%) | < 0.0001 | ||||
| Yes | 30 (6.1) | 3 (2.7) | 2 (1.3) | 20 (14.8) | |
| No | 459 (93.9) | 109 (97.3) | 148 (98.7) | 115 (85.2) | |
| Coronary artery disease, n (%) | 0.3712 | ||||
| CABG | 26 (5.3) | 10 (8.9) | 7 (4.7) | 8 (5.9) | |
| Medication | 46 (9.4) | 9 (8.0) | 14 (9.3) | 8 (5.9) | |
| No | 377 (77.1) | 85 (75.9) | 123 (82.0) | 116 (85.9) | |
| PCI | 27 (5.5) | 6 (5.4) | 5 (3.3) | 2 (1.5) | |
| Missing | 13 (2.7) | 2 (1.8) | 1 (0.7) | 1 (0.7) | |
| Extracardiac arteriopathy, n (%) | 0.4940 | ||||
| No | 406 (83.0) | 91 (81.3) | 123 (82.0) | 118 (87.4) | |
| Yes | 71 (14.5) | 20 (17.9) | 26 (17.3) | 16 (11.9) | |
| Missing | 12 (2.5) | 1 (0.9) | 1 (0.7) | 1 (0.7) | |
| Valvular heart disease, n (%) | 0.0013 | ||||
| No | 389 (79.6) | 87 (77.7) | 134 (89.3) | 96 (71.1) | |
| Yes | 89 (18.2) | 23 (20.5) | 15 (10.0) | 38 (28.2) | |
| Missing | 11 (2.3) | 2 (1.8) | 1 (0.7) | 1 (0.7) | |
| LV function, n (%) | < 0.0001 | ||||
| Good | 199 (40.7) | 41 (36.6) | 64 (42.7) | 79 (58.5) | |
| Moderate | 30 (6.1) | 14 (12.5) | 13 (8.7) | 19 (14.1) | |
| Poor | 11 (2.2) | 2 (1.8) | 2 (1.3) | 0 (0.0) | |
| Not measured | 241 (49.3) | 55 (49.1) | 70 (46.7) | 36 (26.7) | |
| Missing | 8 (1.6) | 0 (0.0) | 1 (0.7) | 1 (0.7) | |
| Diabetes, n (%) | 0.2350a | ||||
| No | 432 (88.3) | 105 (93.8) | 137 (91.3) | 126 (93.3) | |
| Non-IDDM | 52 (10.6) | 7 (6.3) | 13 (8.7) | 8 (5.9) | |
| IDDM | 2 (0.4) | 0 (0.0) | 0 (0.0) | 1 (0.7) | |
| Missing | 3 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Hypertension, n (%) | 0.7856 | ||||
| Yes | 424 (86.7) | 7 (86.6) | 135 (90.0) | 119 (88.2) | |
| No | 63 (12.9) | 15 (13.4) | 15 (10.0) | 16 (11.9) | |
| Missing | 2 (0.4) | 0 (0) | 0 (0.0) | 0 (0) | |
| COPD, n (%) | 0.1772 | ||||
| Yes | 87 (17.8) | 26 (23.2) | 32 (21.3) | 18 (13.3) | |
| No | 397 (81.2) | 86 (76.8) | 118 (78.7) | 117 (86.7) | |
| Missing | 5 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| NYHA class, n (%) | 0.4187 | ||||
| I | 198 (40.5) | 39 (34.8) | 68 (45.3) | 54 (40.0) | |
| II | 175 (35.8) | 41 (36.6) | 47 (31.3) | 52 (38.5) | |
| III | 86 (17.6) | 27 (24.1) | 20 (13.3) | 17 (12.6) | |
| IV | 16 (3.3) | 3 (2.7) | 4 (2.7) | 3 (2.2) | |
| Missing | 14 (2.9) | 2 (1.8) | 11 (7.3) | 9 (6.7) | |
| Serum creatinine level (µmol/l) | 0.0068 | ||||
| Mean (SD) | 96.0 (32.8) | 104.9 (39.8) | 92.6 (31.9) | 85.7 (27.3) | |
| Minimum, maximum | 45.0, 227.0 | 44.0, 225.0 | 43.0, 200.0 | 32.0, 186.0 | |
| Missing, n (%) | 309 (63.2) | 60.0 (53.6) | 42.0 (28.0) | 48 (35.6) | |
| Haemoglobin level (g/l) | 0.0420 | ||||
| Mean (SD) | 127.5 (19.1) | 128.4 (15.8) | 131.7 (16.2) | 133.6 (17.3) | |
| Minimum, maximum | 76.0, 175.0 | 98.0, 171.0 | 77.0, 176.0 | 90.0, 165.0 | |
| Missing, n (%) | 326 (66.7) | 64 (57.1) | 44 (29.3) | 50 (37.0) | |
| Drug group | Patient subgroup (number of patients with a registration scan), n (%) | p-value | |||
|---|---|---|---|---|---|
| WW (N = 489) | CM (N = 112) | ESG (N = 150) | OSR (N = 135) | ||
| Beta-blocker use | |||||
| Yes | 255 (52.2) | 51 (45.5) | 74 (49.3) | 72 (53.3) | 0.5608 |
| No | 234 (47.9) | 61 (54.5) | 76 (50.7) | 63 (46.7) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Angiotensin-converting enzyme inhibitor use | |||||
| Yes | 116 (23.7) | 39 (34.8) | 45 (30.0) | 40 (29.6) | 0.06342 |
| No | 373 (76.3) | 73 (65.2) | 105 (70.0) | 95 (70.4) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Angiotensin receptor blocker use | |||||
| Yes | 94 (19.2) | 26 (23.2) | 28 (18.7) | 32 (23.7) | 0.5416 |
| No | 395 (80.8) | 86 (76.8) | 122 (81.3) | 103 (76.3) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Calcium channel blocker use | |||||
| Yes | 176 (36.0) | 35 (31.3) | 55 (36.7) | 47 (34.8) | 0.7909 |
| No | 313 (64.0) | 77 (68.8) | 95 (63.3) | 88 (65.2) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Other antihypertensives | |||||
| Yes | 65 (13.3) | 24 (21.4) | 24 (16.0) | 17 (12.6) | 0.1384 |
| No | 424 (86.7) | 88 (78.6) | 126 (84.0) | 118 (87.4) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Any antihypertensive | |||||
| Yes | 412 (84.3) | 94 (83.9) | 131 (87.3) | 116 (85.9) | 0.7900 |
| No | 77 (15.7) | 18 (16.1) | 19 (12.7) | 19 (14.1) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Statins | |||||
| Yes | 283 (57.9) | 72 (64.3) | 106 (70.7) | 51 (37.8) | < 0.0001 |
| No | 204 (41.7) | 40 (35.7) | 44 (29.3) | 84 (62.2) | |
| Missing | 2 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
In addition to age, there were important differences between the management groups in comorbidities/biomarkers (see Table 9) and cardiac medication (see Table 10). Among those with CTDs, only two had ESG, compared with 20 who had OSR. As might have been expected, the CM group were less likely to have good LV function and more likely to have comorbidities such as COPD, higher NYHA classification and higher mean serum creatinine level, although the differences overall were not always statistically significant. Conversely, OSR patients were less likely to have COPD or take statins and more likely to have good LV function. Between 86% and 90% of patients had documented hypertension and were treated.
Some baseline variables were not measured by centres, resulting in large numbers of missing data. For example, LV function was not recorded for approximately half of the WW, CM and ESG groups and for one-quarter of the OSR group because echocardiography was not performed routinely at this stage in all centres. Similarly, serum creatinine and haemoglobin levels at recruitment were missing for 456 (51.5%) and 484 (54.6%) patients, respectively, because these biomarkers were not measured routinely in some centres. Otherwise, the missing data level was < 8% for all baseline variables.
Details of the available scans
Reading of baseline scans was mandated for aneurysms in the arch and DTA, but it was restricted by resources for other locations in the thoracic aorta. Table 11 provides summaries of the measurements at recruitment for the four aortic segments with consistent data collection, along with the largest diameter at any of the four sites. Aneurysms in the arch and those extending into the ascending aorta were smaller in WW and ESG patients than in patients receiving CM and OSR. Overall, aneurysms in the DTA were similar in CM, ESG and OSR and smaller in the WW group, but significantly larger in all groups than those in the arch. Aneurysms that extended into the thoracoabdomen were approximately 2 cm smaller on average.
| Aneurysm location | Patient subgroup (number of patients with a recruitment scan) | p-value | |||
|---|---|---|---|---|---|
| WW (N = 489) | CM (N = 112) | ESG (N = 150) | OSR (N = 135) | ||
| Ascending aorta | < 0.0001 | ||||
| Frequency | 404 | 101 | 134 | 113 | |
| Mean diameter, cm (SD) | 4.0 (0.8) | 4.4 (1.1) | 3.9 (0.5) | 4.7 (1.2) | |
| Minimum, maximum, cm | 2.5, 7.7 | 2.9, 8.9 | 2.8, 5.7 | 2.5, 7.9 | |
| Missing, n (%) | 85 (17.4) | 11 (9.8) | 16 (10.7) | 22 (16.3) | |
| Aortic arch | < 0.0001 | ||||
| Frequency | 472 | 105 | 140 | 126 | |
| Mean, cm (SD) | 3.9 (0.8) | 4.5 (1.3) | 3.7 (0.8) | 4.5 (1.2) | |
| Minimum, maximum, cm | 2.3, 9.4 | 2.5, 10.6 | 2.5, 8.3 | 2.5, 9.5 | |
| Missing, n (%) | 17 (3.5) | 7 (6.2) | 10 (6.7) | 9 (6.7) | |
| DTA | < 0.0001 | ||||
| Frequency | 486 | 111 | 150 | 133 | |
| Mean diameter, cm (SD) | 5.1 (1.1) | 5.9 (1.4) | 6.0 (1.1) | 5.8 (1.4) | |
| Minimum, maximum, cm | 2.8, 9.4 | 2.4, 10.0 | 3.4, 9.7 | 2.5, 9.0 | |
| Missing, n (%) | 3 (0.6) | 1 (0.9) | 0 (0.0) | 2 (1.5) | |
| Thoracoabdominal | 0.0090 | ||||
| Frequency | 388 | 96 | 130 | 100 | |
| Mean diameter, cm (SD) | 3.4 (0.8) | 3.8 (1.1) | 3.5 (1.0) | 3.6 (0.9) | |
| Minimum, maximum, cm | 1.9, 7.2 | 2.3, 6.7 | 1.9, 6.7 | 1.8, 6.4 | |
| Missing, n (%) | 101 (20.7) | 16 (14.3) | 20 (13.3) | 35 (25.9) | |
| Largest aneurysm | < 0.0001 | ||||
| Frequency | 489 | 112 | 150 | 135 | |
| Mean diameter, cm (SD) | 5.3 (1.0) | 6.3 (1.2) | 6.0 (1.1) | 6.3 (1.0) | |
| Minimum, maximum, cm | 2.8, 9.4 | 4.2, 10.6 | 3.7, 9.7 | 4.0, 9.5 | |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Details of the procedures
Between 9 April 2014 and 18 June 2019, 150 patients underwent ESG as the first (index) procedure and 135 underwent OSR. Because the co-ordinating centre is primarily a cardiac surgery centre and opened before other centres, OSR procedure dates began 5 months earlier than ESG procedures. Descriptive data for procedures are shown in Table 12. One ESG recipient had a hybrid procedure involving a carotid-to-carotid bypass; similarly, 12 OSR patients had a combined procedure in which a stent was inserted via a median sternotomy. Procedures were planned to be completed in stages for 39 ESG and 34 OSR patients; of these, 14 ESG and 2 OSR were started prior to recruitment.
| Patient subgroup (number of patients having index procedure) | p-value | ||
|---|---|---|---|
| ESG (N = 150) | OSR (N = 135) | ||
| First procedure date | 12 November 2014 | 9 April 2014 | |
| Last procedure date | 5 June 2019 | 12 June 2019 | |
| Hybrid procedure, n (%) | 1 (0.7) | 12 (9.0) | |
| Staged procedure, n (%) | 39 (26.0) | 34 (25.2) | |
| Index procedure = second/third stage | 14 | 2 | |
| Priority, n (%) | |||
| Elective | 131 (87.3) | 115 (85.2) | 0.0840 |
| Urgent | 13 (8.7) | 19 (14.1) | |
| Emergency | 6 (4.0) | 1 (0.7) | |
| Concomitant procedures, n (%) | |||
| Aortic valve surgery | 0 (0.0) | 12 (8.9) | 0.0005 |
| Aortic valve plus CABG surgery | 0 (0.0) | 2 (1.5) | |
| Aortic valve plus other surgery | 0 (0.0) | 8 (5.9) | |
| CABG | 0 (0.0) | 10 (7.4) | |
| Other surgery | 1 (0.7) | 5 (3.7) | |
| None reported | 149 (99.3) | 98 (72.6) | |
| Operating facilities, n (%) | |||
| Operating room | 24 (16.0) | 125 (92.6) | < 0.0001 |
| Operating room with C-arm | 26 (17.3) | 1 (0.7) | |
| Hybrid theatre | 73 (48.7) | 7 (5.2) | |
| Catheter laboratory | 21 (14.0) | 1 (0.7) | |
| Missing | 6 (4.0) | 1 (0.7) | |
| Surgical incisions required, n (%) | |||
| Sternotomy | 0 (0.0) | 97 (71.9) | < 0.0001 |
| Thoracotomy | 0 (0.0) | 19 (14.1) | |
| Thoracolaparotomy | 0 (0.0) | 15 (11.1) | |
| Other | 0 (0.0) | 4 (3.0) | |
| None | 150 (100.0) | 0 (0.0) | |
| Missing | 0 (0.0) | 0 (0.0) | |
| Reported access site for stenting, n (%) | |||
| Femoral artery | 128 (85.3) | 5 (3.7) | < 0.0001 |
| Iliac artery | 7 (4.7) | 0 (0.0) | |
| Brachial | 4 (2.7) | 0 (0.0) | |
| Other | 2 (1.3) | 4 (3.0) | |
| None recorded | 9 (6.0) | 126 (93.3) | |
| Aortic arch procedures, n (%) | |||
| Repair/replacement | 37 (24.7) | 102 (75.6) | < 0.0001 |
| None | 111 (74.0) | 33 (24.4) | |
| Missing | 2 (1.3) | 0 (0.0) | |
| DTA procedures, n (%) | |||
| Repair/replacement | 139 (92.7) | 82 (60.7) | < 0.0001 |
| None | 11 (7.3) | 51 (37.8) | |
| Missing | 0 (0.0) | 2 (1.5) | |
| Ascending aorta procedures, n (%) | |||
| Repair/replacement | 0 (0.0) | 58 (42.9) | < 0.0001 |
| None | 149 (99.3) | 76 (56.3) | |
| Missing | 1 (0.7) | 1 (0.7) | |
| Thoracoabdominal aorta procedures, n (%) | |||
| Repair/replacement | 14 (9.4) | 10 (7.4) | 0.001 |
| None | 135 (90.0) | 116 (85.9) | |
| Missing | 1 (0.7) | 9 (6.7) | |
| Subsequent procedures: second third | 12 ESG | 24 ESG, 4 OSR 3 ESG | |
Over 85% of patients were treated electively, with a minority having urgent or emergency procedures. OSR was almost always completed in theatre, whereas ESG was completed in theatre, a catheter laboratory or a hybrid theatre combining the traditional operating theatre with an interventional radiology suite. The predominant mode of access for OSR was a median sternotomy, although thoracotomy/thoracolaparotomy was reported for 34 patients. Thirty-seven OSR patients had concomitant procedures, including aortic valve replacements and bypass grafts, which could have been carried out only during open surgery.
A variety of endovascular stent grafts were implanted and, for some patients, branched, fenestrated and scalloped grafts were employed. The site of access for main stent body insertion was the femoral artery in the majority of ESG procedures (see Table 12). In 136 patients in whom the relationship with the left subclavian artery was recorded, 84 (61.8%) were landed distal to the subclavian artery, 36 (26.5%) underwent bypass and 16 (11.8%) had coverage of the left subclavian artery without bypass. One ESG patient had another endovascular procedure but no other details were provided by the time of data lockdown.
There were marked differences in aneurysm site between the two intervention groups. Aneurysms were more likely to be treated with OSR if they were in the aortic arch (102 vs. 37 patients) and less likely to be treated with OSR if they were in the DTA (82 vs. 139 patients). If aneurysms extended into the ascending aorta, then they were invariably treated by OSR. Both procedures were used for aneurysms extending to the thoracoabdominal aorta (see Table 12).
Some patients required reintervention during the study. Twelve patients who received ESG as the index procedure required a second ESG between 0.6 and 35.4 months after the first procedure. In addition, 25 OSR patients had a second procedure (21 ESG and 4 OSR) between 0.4 and 36.1 months after the first procedure; of these, three received a further ESG at 1.1, 11.5 and 11.9 months after the second procedure.
Further details of outcomes of surgical procedures, including complications and NHS resources used, are reported in Chapters 5 and 7.
Summary of findings
This chapter describes the construction of the ETTAA cohort and the classification of patients into management groups depending on their risk of aneurysm-related events and their suitability for open-heart surgery. Although recruitment was lower than expected, we were close to meeting the targets for the ESG and OSR intervention groups, and high levels of baseline data collection were achieved for all but a small number of variables that were not measured routinely at all centres.
Groups differed in their baseline characteristics, reflecting clinician opinions expressed during the Delphi study in Chapter 2. In particular, expert clinicians expressed a preference for OSR for aneurysms in the arch and ESG for aneurysms in the DTA, which was broadly consistent with clinical practice during the study. In practice, the WW group had smaller (on average) aneurysms and lower aneurysm-related risk factors at recruitment; conversely, patients assigned to the CM group had greater risk factors for a poor outcome. Specifically, CM patients were older, more likely to be receiving formal or informal care (a marker of frailty), more likely to be in NYHA class II-IV and less likely to have good LV function than other groups.
It was clear that there were significant differences between the two intervention groups. For some variables the procedure groups overlapped despite important differences; for example, OSR patients were younger, more likely to have good LV function and less likely to be smokers, have statins prescribed or suffer from COPD, but these were not exclusive to OSR patients. Importantly, for some risk factors there was little or no overlap between these two intervention groups. For example, only two CTD patients had ESG, just over one-quarter of OSR patients had concomitant valve or coronary artery surgery which could not have been done in an endovascular procedure and all patients whose aneurysm extended into the ascending aorta had OSR. This lack of overlap between the groups has a major impact on the validity of any direct comparisons between them, which will be discussed in detail in subsequent chapters.
All analysis in this chapter concerned information collected at recruitment or during the interventions. However, there was often a prolonged interval between recruitment and either intervention or the end of the study, during which aneurysms and HRQoL were monitored. Chapter 4 reports changes over time and other clinical outcomes during this non-intervention period.
Chapter 4 Pre-procedure outcomes, aneurysm growth and health-related quality of life
Parts of this chapter have been adapted from Sharples et al. 64 © The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial re-use distribution and reproduction in any medium provided the original work is properly cited. The text below includes minor additions and formatting changes to the original text.
Introduction
This chapter reports on the analysis of data arising prior to any intervention taking place. Specifically, we provide estimates of aneurysm growth and changes in HRQoL over time for different treatment groups, survival, neurological events and incidence of aneurysm-related ruptures and dissections.
Aims of pre-procedure analyses
The aims of this chapter are to:
-
model growth of aneurysms over time in the four sections of the aorta (ascending, arch, DTA and thoracoabdominal) in all patient groups prior to any intervention
-
describe survival patterns in the absence of major interventions by patient group
-
describe the time between diagnosis, recruitment and intervention
-
describe major aneurysm-related events of rupture and dissection
-
model change in HRQoL measured by the EQ-5D-5L utility over time prior to any intervention.
Methods
Population
All patients who were recruited into the study were included in the analysis of pre-intervention outcomes. Patients contributed data from either the date of diagnosis (aneurysm growth) or the date of recruitment (HRQoL, survival, clinical events) until one of the following occurred: first intervention, death, major clinical event, withdrawal or end of the study.
Outcomes
The primary outcome of interest was growth in the CTAA diameter using measurements made at diagnosis and at all subsequent time points prior to intervention.
Secondary outcomes were survival, clinical complications and HRQoL trajectories over time as defined in Chapter 3.
Statistical analysis
Aneurysm growth
All patients with CTAA diameter measurements from either CT or MRI scanning were included in the analysis. The date of the first scan recorded was considered time zero; these scans were completed between 4.0 months and 21.6 years prior to recruitment. Scans up to death, rupture, dissection or surgery (ESG or OSR) were included. Analysis was restricted to the four sections of the aorta that were recorded routinely.
Longitudinal assessments of aneurysm diameters were analysed using linear random-effects models. Centre-specific and patient-specific random effects for the intercept and time variables were explored. Variation in outcomes attributable to centres was close to zero, so this was not included. Heterogeneity in random effects was assessed by allowing the variance components to differ between aneurysm sites. Non-linear growth was assessed by including time-squared in the model, but the small number of cases with more than two measurements precluded more complicated models of growth. Baseline variables were assessed as fixed effects in the model that included time. These were sex, age at scan, height, weight, BMI, hypertension, smoking (current, no, ex-smoker), COPD (yes, no), CTD (yes, no), coronary artery disease, extracardiac arteriopathy, valvular heart disease, type of scan (CT, MRI) and aneurysm location (arch, DTA, ascending aorta, thoracoabdominal aorta). All continuous variables except time of assessment were centred around their mean. A linear term was included for NYHA, with NYHA class rescaled so that class I took the value zero. The rationale for this was to ensure that (1) the overall intercept for the models was interpreted as the mean for patients in NYHA class I (and all other covariates set to zero) and (2) risk increased linearly with each one-class increase in NYHA classification. Treatment group was not included in this analysis of aneurysm growth because the group allocation occurred after time zero.
Initially, exploratory analysis involved plotting empirical distributions and aneurysm trajectories by aneurysm site. The normality of the distributions of baseline and subsequent measurements were confirmed by Q–Q plots and summary statistics. Unresolved extreme values were excluded (one weight/BMI).
Longitudinal random-effects models have two inter-related parts: the covariance structure and the mean structure. 65,66 First, the best covariance structure was investigated after ‘saturating’ the mean part of the model, including all variables and two-way interactions. Then a range of random-effects models, with and without variance heterogeneity, was fitted using restricted maximum likelihood, with nested models compared using likelihood ratio tests. Thereafter, the mean structure was simplified by removing variables sequentially, starting with polynomial and interaction terms, based on z-statistics.
If Yit represents the diameter (in cm) of an aneurysm in an individual i = 1, . . ., n, at time t = 0, 1, . . ., Ti, (measurement time), then the model with best fit had the form:
where:
-
τit is actual time of the measurement for patient i at the tth time point
-
xi is a vector of baseline variables including aneurysm site for patient i
-
(β0, β1, θ) are coefficients for the fixed effects
-
ui|xi,τit∼N(0,σu2) are patient-level random effects
-
εit|ui,xi,τit∼N(0,σε2) are residual errors.
Model fit was assessed informally by histograms and Q–Q plots of standardised residual errors and random effects and by comparing fitted and observed trajectories for individuals. We note that estimates from resulting analyses are unbiased provided that measurements are missing at random conditional on observed measurements in the model. Missing data will not be considered further in longitudinal models.
Health-related quality of life
Patients with EQ-5D-5L utilities at recruitment and at least one subsequent follow-up time were analysed using the same methodology as for aneurysm diameters, with three exceptions: (1) time zero was the date of recruitment to the ETTAA study, (2) fixed age at recruitment rather than age at time of assessment was included and (3) management group was included in the analysis. Again, centre effects were not included in HRQoL models because variation in outcomes attributable to centres was zero. The resulting final model for utilities had the form:
where:
-
τit is the time of the measurement for patient at the tth time point
-
xi is a vector of baseline variables including aneurysm site and intended management for patient
-
(β0, β1, θ) are coefficients for the fixed effects
-
u0i|xi,τit∼N(0,σu02) and u1i|xi,τit∼N(0,σu12) are patient-level random effects for patients on the intercept and time slope respectively, µ0i and µ1i were independent
-
εit|u0i,u1i,xi,τit∼N(0,σε2) are residual errors that vary by management group (level 1 heterogeneity).
Again, estimates from this analysis are unbiased provided that data are missing at random conditional on observed data. Model fit was assessed using fitted and residual plots.
Death, rupture, dissection and neurological events
The numbers of deaths and hospital admissions from recruitment and prior to any intervention were summarised as the number and event rate, with ruptures, dissections and neurological events reported separately. For survival and aneurysm-related survival, the cumulative incidence was summarised using the Kaplan–Meier method. Relationships between baseline risk factors and outcomes were assessed informally from incidence plots and compared using log-rank tests. After assessing the validity of proportional hazards assumptions using Schoenfeld residuals, Cox proportional hazards models were developed to assess the relationship between incidence of events and baseline variables. First, all baseline variables that were significant in exploratory analysis were included in the models, and then removed sequentially, based on z-statistics, until the best parsimonious model was obtained. Centre effects were assessed by including gamma frailty terms in the Cox survival models. The primary analysis was complete case, with main results checked using multiple (m = 12) imputation for missing data. See Chapter 3 and Appendix 2 for details of missing data analysis.
A key assumption of survival analysis is that censoring is independent of risk of death, which is unlikely to hold if high-risk patients are more likely to undergo procedures. In sensitivity analysis we refitted the final model using Fine and Gray’s67 subdistribution hazards model, with ESG and OSR treated as competing risks. In addition, we provided predicted survival rates for the composite outcome of death or intervention with ESG or OSR.
Joint analysis of aneurysm growth and the composite of death, rupture and dissection
Our intention is to investigate whether or not aneurysm growth is related to clinical events (ruptures, dissections, deaths) by developing joint random-effects models for growth and time-to-event processes. 68 As stated in the statistical analysis plan, this was not part of the original application and will be reported separately from this monograph.
Results
Aneurysm growth from first scan
Descriptive analysis
A total of 1789 scans reporting measurements of aneurysm diameter for at least one section of the aorta were returned by 886 patients. After excluding scans after interventions (n = 10), scans using techniques other than CT or MRI (n = 11) and one scan whose follow-up date was over 8 years after the first scan, data from 1767 scans in 882 (99.5%) patients were analysed. These included 6433 measurements of aneurysm diameter: 1537 in the ascending aorta, 1698 in the arch, 1761 in the DTA and 1437 in the thoracoabdominal aorta. The time between first scan and subsequent scans ranged from 3 days to 7.35 years. Mean (SD) diameters at baseline for patients with at least two scans was 4.11 (0.87) in the ascending, 3.98 (0.85) in the arch, 5.26 (1.09) in the DTA and 3.48 (0.81) in the thoracoabdominal aorta. There was little difference between baseline measurements for those with and without repeat scans.
Figure 5 shows diameter measurements at the four aneurysm sites over time. Diameters at the first scan varied substantially between patients at all sites, with DTA aneurysms showing greater average diameter and more variation between patients; thoracoabdominal aneurysms were slightly smaller on average. Trajectories over time are difficult to disentangle, although on average diameters appeared to increase only slightly over time.
FIGURE 5.
Aneurysm diameters over time, by location in the thoracic aorta. (a) Ascending aorta; (b) suprarenal abdominal aorta; (c) descending thoracic aorta; (d) aortic arch.
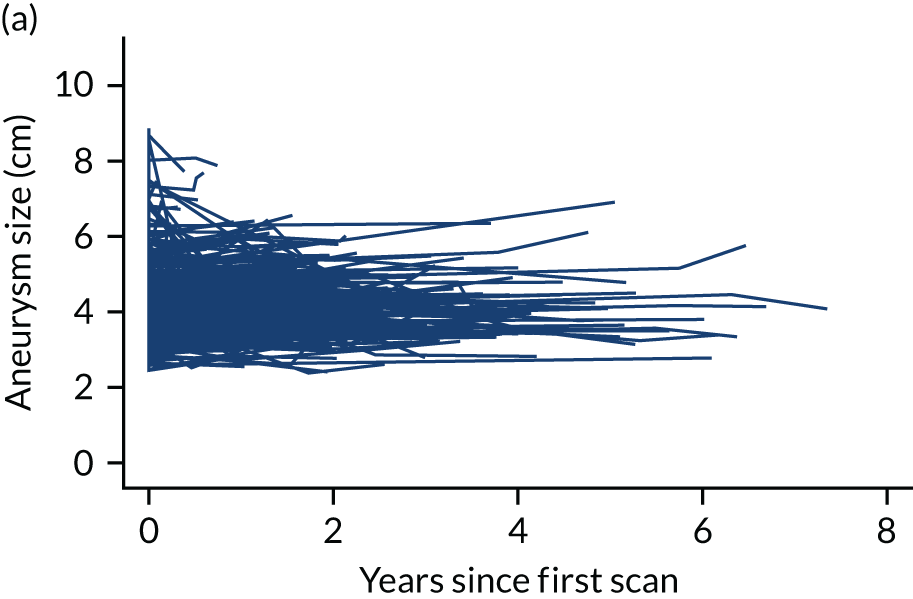
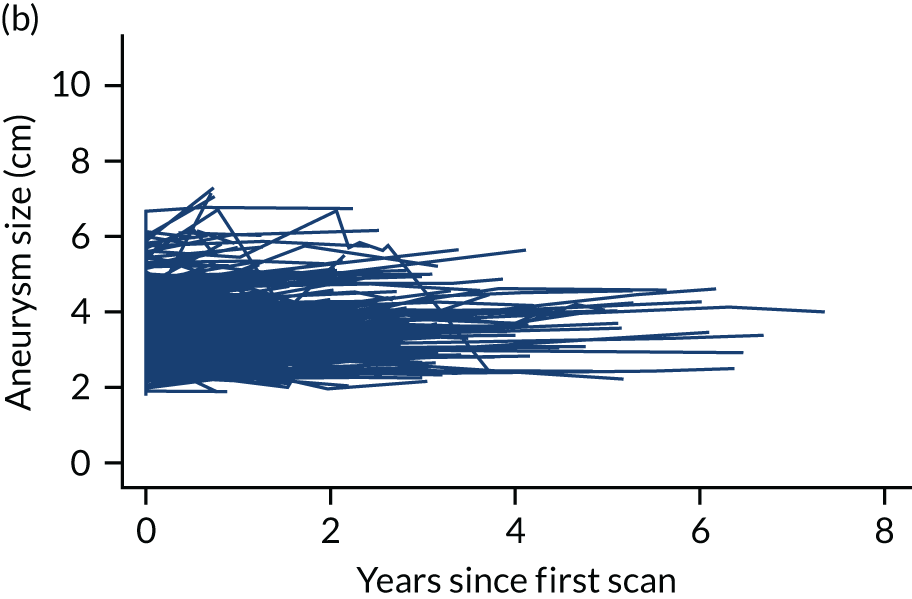
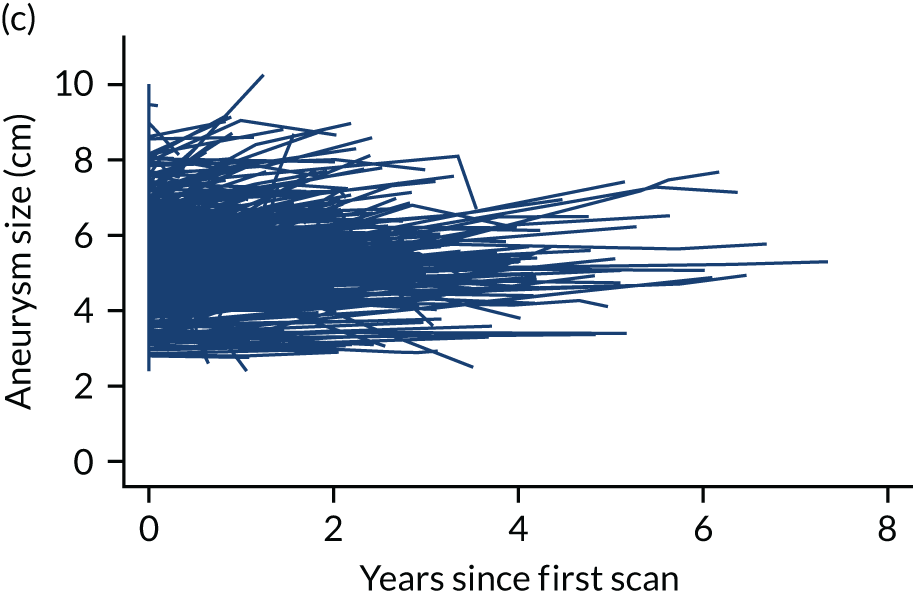
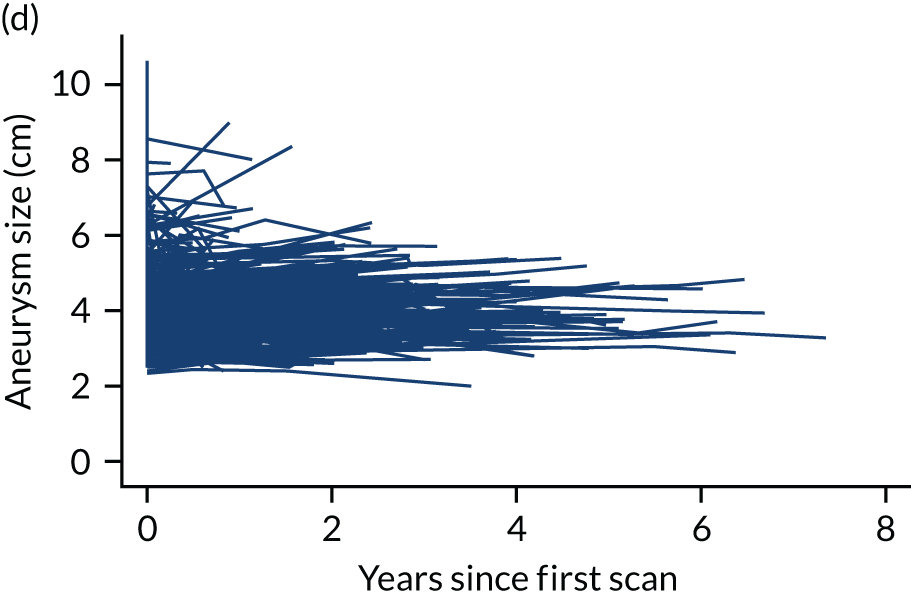
Results of modelling
The final model describing the diameter measurement trajectories in the absence of treatment is shown in Appendix 7; the results are summarised below.
In the final model, at time zero all covariate values are zero if the aneurysm is measured using CT, the patient is of average age (70.9 years) and height (171 cm), has never smoked, and does not have a CTD, COPD or valvular heart disease. For these ‘zero’ aneurysms, the average diameter at first scan (time zero) was 4.14 cm in the ascending aorta, 4.13 cm in the arch, 5.29 cm in the DTA and 3.37 cm in the thoracoabdominal aorta (Table 13, first row).
| Variable | Ascending aorta | Aortic arch | Descending thoracic aorta | Thoracoabdominal aorta |
|---|---|---|---|---|
| All covariates zeroa | 4.14 (4.03 to 4.25) | 4.13 (4.02 to 4.24) | 5.29 (5.18 to 5.40) | 3.37 (3.25 to 3.49) |
| Average age + 10 years | 4.33 (4.20 to 4.46) | 4.22 (4.10 to 4.34) | 5.46 (5.34 to 5.58) | 3.41 (3.28 to 3.54) |
| Connective tissue disorder | 4.12 (3.85 to 4.39) | 4.14 (3.88 to 4.40) | 5.52 (5.26 to 5.78) | 3.93 (3.65 to 4.21) |
| COPD | 4.19 (4.00 to 4.38) | 4.09 (3.91 to 4.27) | 5.56 (5.38 to 5.74) | 3.35 (3.16 to 3.54) |
| Valvular heart disease | 4.39 (4.21 to 4.57) | 4.16 (3.99 to 4.33) | 5.17 (5.00 to 5.34) | 3.35 (3.17 to 3.53) |
| Current smoker | 4.15 (3.95 to 4.35) | 4.04 (3.84 to 4.24) | 5.52 (5.33 to 5.71) | 3.65 (3.45 to 3.85) |
| Ex-smoker | 4.04 (3.90 to 4.18) | 4.01 (3.88 to 4.14) | 5.41 (5.28 to 5.54) | 3.49 (3.35 to 3.63) |
At the time of the first scan (time zero), older age, being taller, having comorbidities such as CTD, COPD and valvular heart disease and being a past or current smoker were all associated with larger aneurysms in one or more aortic sites, although these effects varied between sites. Table 13 shows the average diameters (95% CIs) in these subgroups. For example, at first scan, age was associated with bigger aneurysms in the ascending aorta and DTA, but less so in the arch and only slightly in the thoracoabdominal aorta. Aneurysms in the DTA and thoracoabdominal aorta (but not in the other sites) were larger on the first scan if the patient had CTD than if they did not. DTA aneurysms were larger on the first scan if the patient had COPD, but this effect was not evident in other vessels. Ascending aorta aneurysms on the first scan were larger if the patient had valvular heart disease. Again, this effect was not evident in other sites. Even after adjusting for these baseline variables, there was significant variation in aneurysm size at first scan between patients (random-effects SD 0.54 cm). That is, aneurysm diameters at time zero lie within ± 1.08 cm of the average for that site for 95% of patients, even after adjustment for baseline variables.
On average, aneurysms in the DTA grew by 0.07 cm per year (95% CI 0.03 to 0.12 cm per year), compared with 0.04 cm (95% CI –0.002 to 0.07 cm per year) in the arch and 0.10 cm (95% CI 0.06 to 0.14 cm per year) in the thoracoabdominal aorta. Average growth for aneurysms in the ascending aorta was –0.001 cm per year (95% CI –0.04 to 0.04 cm per year) during the study (see Appendix 7). Figure 6 summarises the average growth trajectories in each site, showing that aneurysms in the DTA were substantially larger throughout, and grew faster than aneurysms in the arch or ascending aorta (all else being equal). There was no evidence that aneurysm growth accelerated or decelerated during this study. At first scan, there was no difference between aneurysm diameters measured by CT and MRI, all other variables being equal. However, over time the average difference between diameters measured by the two modalities increased by 0.11 cm per year, with MRI measurements being smaller (see Appendix 7).
FIGURE 6.
Average model-predicted growth trajectories from first scan by aneurysm site, assuming assessment by CT and all other baseline variables set to zero. Adapted from Sharples et al. 64 © The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial re-use distribution and reproduction in any medium provided the original work is properly cited. The figure includes minor additions and formatting changes to the original figure.
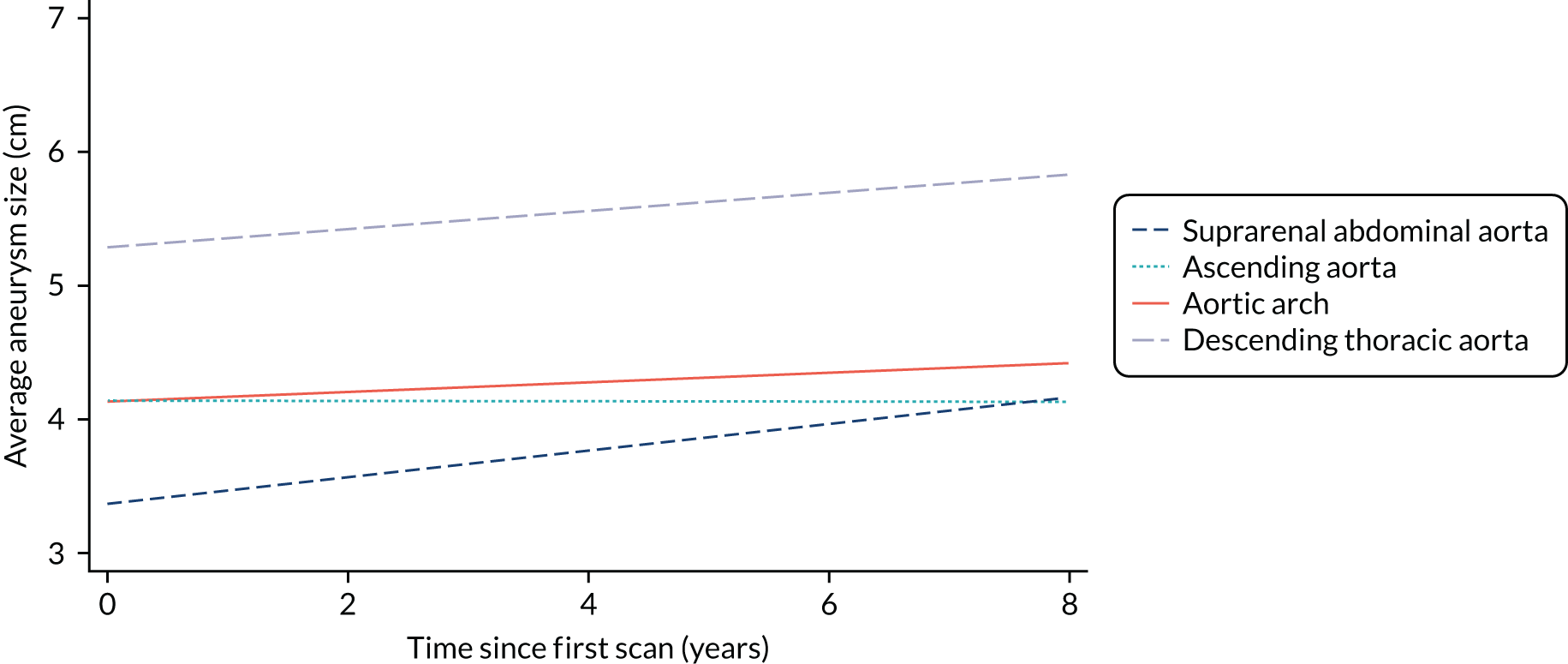
Survival, hospital admissions and aneurysm-related events
Survival: descriptive analysis
In this analysis, follow-up time was from the date of recruitment to the first of death, rupture, dissection, procedure or censoring date. Pre intervention, 129 patients died during a total of 1498.2 patient-years of follow-up, a rate of 8.6% per patient-year (Table 14). Of these, 83 were in the WW group (7.4% of deaths per patient-year) and 46 were in the CM group (20.0% of deaths per patient-year).
| Patient subgroup | ||||
|---|---|---|---|---|
| WW (N = 489) | CM (N = 112) | ESG (N = 150) | OSR (N = 135) | |
| Total time at risk (years) | 1119.8 | 229.7 | 77.1 | 71.4 |
| Deaths (rate per patient-year) | 83 (0.07) | 46 (0.20) | – | – |
| Aneurysm-related deaths (rate per patient-year) | 39 (0.03) | 25 (0.11) | ||
| All admissions, n (rate per patient-year) | 243 (0.22) | 71 (0.31) | 36 (0.46) | 13 (0.18) |
| People with at least one admission, n (%) | 147 (30.1) | 41 (36.6) | 22 (14.6) | 12 (9.0) |
| Admissions, definitely/probably aneurysm related, n (rate per patient-year) | 25 (0.02) | 11 (0.05) | 14 (0.18) | 2 (0.03) |
| Patients admitted, definitely/probably aneurysm related, n (%) | 17 (3.5) | 9 (8.0) | 11 (7.3) | 2 (1.5) |
| Non-fatal ruptured aneurysms | 2 | – | – | – |
| Non-fatal dissected aneurysms | 4 | 1 | 2 | – |
| Non-fatal neurological events | 5 | 1 | – | 2 |
Aneurysm was the primary or contributory cause of death in 64 (49.6%) patients; 45 were ruptured, 11 were dissected, three were ruptured and dissected and five reported aneurysm-related cause of death but no specific event. Thirty-nine aneurysm-related deaths were in the WW group (3.5% per patient-year) and 25 were in the CM group (10.9% per patient-year). One person had a dissected aorta but died from sepsis and aplastic anaemia; this death was not classified as aneurysm related. The results changed only slightly in sensitivity analysis that included this death as aneurysm related (not shown).
The 1- and 3-year cumulative incidence rates for all-cause death were 7.6% (95% CI 5.9% to 9.8%) and 22.4% (95% CI 18.8% to 26.6%), respectively, and for aneurysm-related death were 3.6% (95% CI 2.4% to 5.2%) and 11.7% (95% CI 9.0% to 15.2%), respectively. CM patients had much higher overall and aneurysm-related death rates than patients assigned to WW (Figure 7). Note that 19 patients do not appear in these figures because they were consented on the day of the intervention.
FIGURE 7.
Kaplan–Meier cumulative incidence curves for death from any cause and aneurysm-related deaths by CM and non-CM management (labelled WW). (a) Kaplan–Meier deaths from any cause; and (b) Kaplan–Meier aneurysm-related deaths.
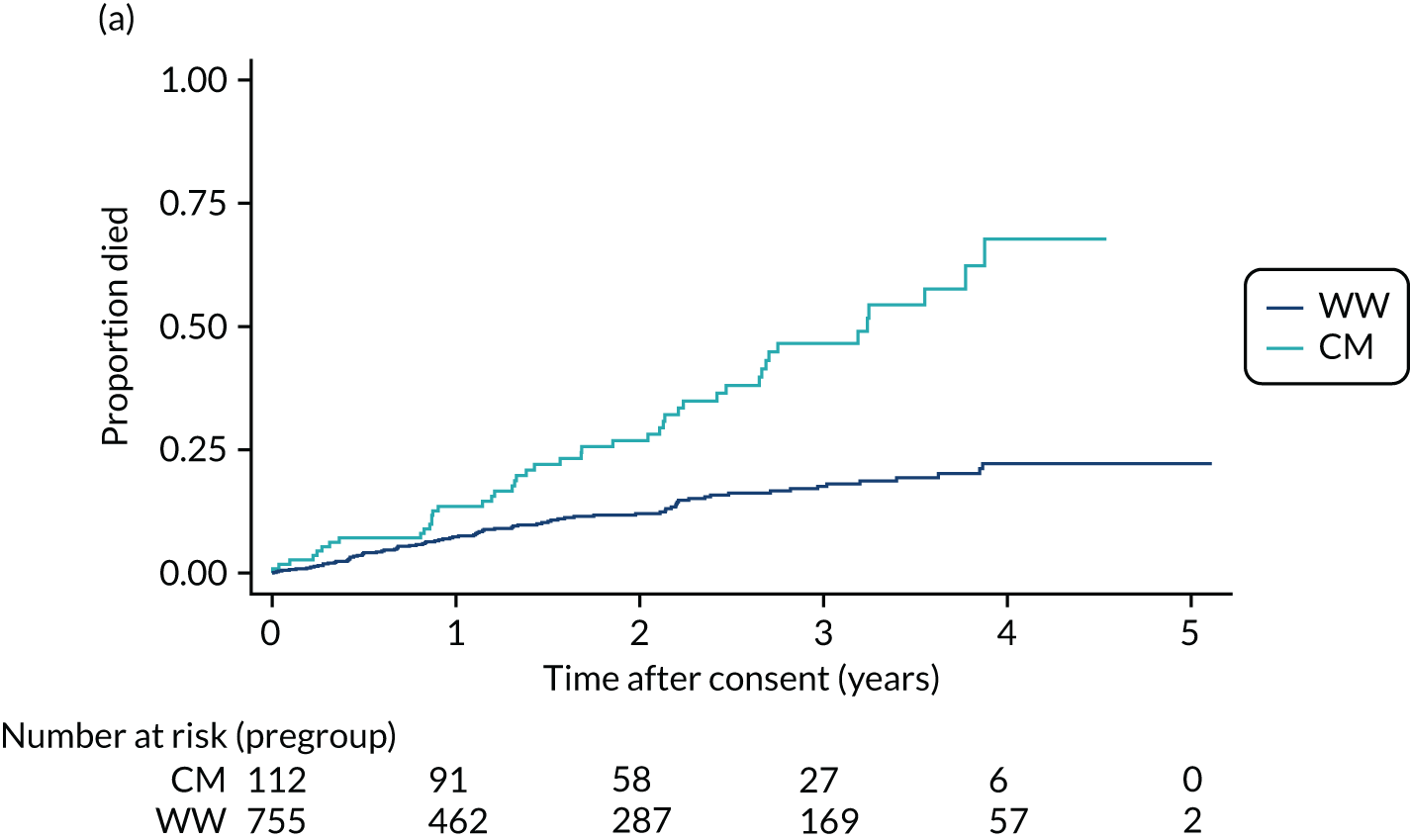
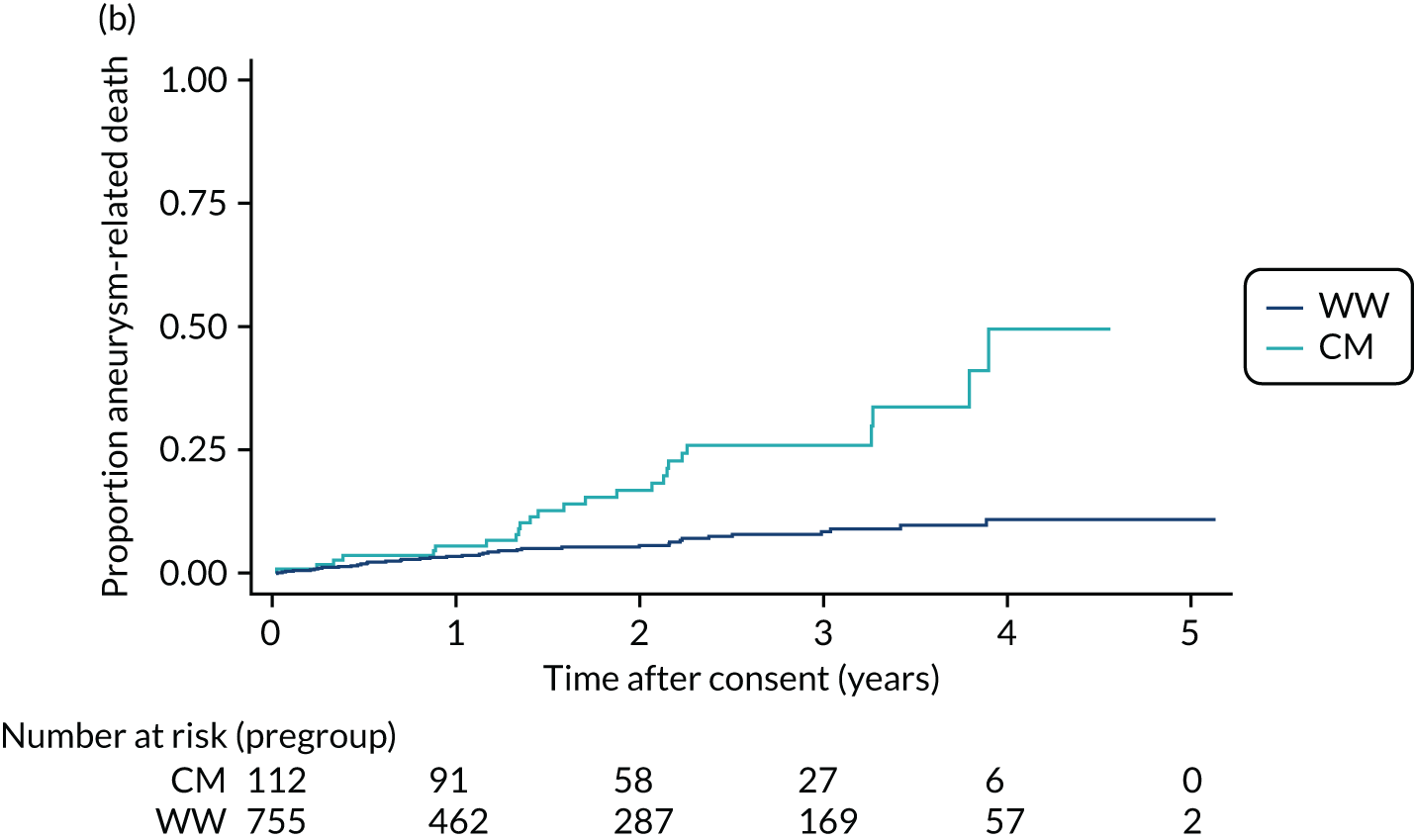
Survival: results of modelling
The variables associated with all-cause and aneurysm-related deaths were similar (Table 15). As expected, in univariable models the hazard for CM was over three times that for WW. Moreover, in univariable models, hazards were significantly higher for women, patients reporting formal/informal care and patients with previous cardiac interventions, COPD, higher NYHA classification, larger aneurysms, older age and smaller frame. The relationship between age and risk of death was not linear, with patients aged > 80 years at particularly high risk, so that age was categorised for analysis. It is likely that the higher risk with lower height and weight measurements reflects a loss of muscle mass due to ageing. Variables not significant at the 5% level in both survival outcomes are not reported.
| Variable | All-cause deaths | Aneurysm-related deaths | ||
|---|---|---|---|---|
| HR (95% CI) | z-test p-value | HR (95% CI) | z-test p-value | |
| CM | 3.05 (2.12 to 4.37) | < 0.001 | 3.55 (2.15 to 5.87) | < 0.001 |
| Female sex | 1.93 (1.36 to 2.72) | < 0.001 | 2.61 (1.59 to 4.29) | < 0.001 |
| Use of formal/informal care | 2.15 (1.40 to 3.29) | < 0.001 | 1.92 (1.02 to 3.61) | 0.042 |
| Previous cardiac interventions CABG/PCI | 2.17 (1.43 to 3.29) | < 0.001 | 1.28 (0.63 to 2.59) | 0.492 |
| COPD | 2.28 (1.56 to 3.34) | < 0.001 | 2.14 (1.24 to 3.69) | 0.007 |
| NYHA per class | 1.47 (1.21 to 1.79) | < 0.001 | 1.35 (1.02 to 1.79) | 0.037 |
| Maximum aneurysm size per cm | 1.94 (1.71 to 2.21) | < 0.001 | 2.16 (1.81 to 2.59) | < 0.001 |
| Age (years) at consent | < 0.001 | < 0.001 | ||
| 61–70 | 2.03 (0.76 to 5.43) | 1.34 (0.42 to 4.29) | ||
| 71–80 | 4.18 (1.68 to 10.41) | 2.49 (0.87 to 7.11) | ||
| > 80 | 8.43 (3.35 to 21.23) | 5.08 (1.75 to 14.74) | ||
| Height per 10 cm | 0.66 (0.56 to 0.77) | < 0.001 | 0.60 (0.48 to 0.76) | < 0.001 |
| Weight per kg | 0.97 (0.96 to 0.98) | < 0.001 | 0.96 (0.94 to 0.98) | < 0.001 |
| BMI per kg/m2 | 0.95 (0.91 to 0.99) | 0.008 | 0.91 (0.86 to 0.97) | 0.002 |
Many of the variables in Table 15 were correlated, and the final multivariable models for all-cause and aneurysm-related deaths are shown in Table 16. Apart from age and sex, aneurysm size was the strongest risk factor for all-cause and aneurysm-related deaths. Figure 8 shows the predicted survival for patients with different aneurysm sizes at baseline. This shows that, for example, the probability of survival to 1 year exceeds 95% for maximum aneurysm diameters of 4–5.5 cm and 90% for diameters up to 6.5 cm. The 1-year risk of death increases rapidly for aneurysms of > 6.5 cm in diamater. The 3-year survival probability exceeds 90% for small (4–4.5 cm) aneurysms and 80% for aneurysms of 5–5.5 cm in diameter. Predicted 3-year survival was 79% for aneurysms of 6 cm in diameter, falling to 42% for aneurysms of 8 cm in diameter.
| Variable | All-cause deaths | Aneurysm-related deaths | ||
|---|---|---|---|---|
| HR (95% CI) | z-test p-value | HR (95% CI) | z-test p-value | |
| Female sex | 1.79 (1.25 to 2.57) | 0.001 | 2.67 (1.61 to 4.42) | < 0.001 |
| NYHA per class | 1.23 (1.00 to 1.52) | 0.052 | ||
| Maximum aneurysm size per cm | 1.90 (1.65 to 2.18) | < 0.001 | 2.19 (1.81 to 2.65) | < 0.001 |
| Age at consent (years) | ||||
| 61–70 | 2.50 (0.76 to 5.43) | < 0.001 | 1.30 (0.41 to 4.14) | 0.0103 |
| 71–80 | 3.49 (1.26 to 9.66) | 1.47 (0.51 to 4.23) | ||
| > 80 | 7.01 (2.50 to 19.62) | 3.36 (1.15 to 9.87) | ||
FIGURE 8.
Predicted overall survival (a) and time to composite event of death, ESG or OSR (b) by maximum aneurysm at baseline, using Cox regression, with all other variables set to their average. Adapted from Sharples et al. 64 © The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial re-use distribution and reproduction in any medium provided the original work is properly cited. The figure includes minor additions and formatting changes to the original figure.
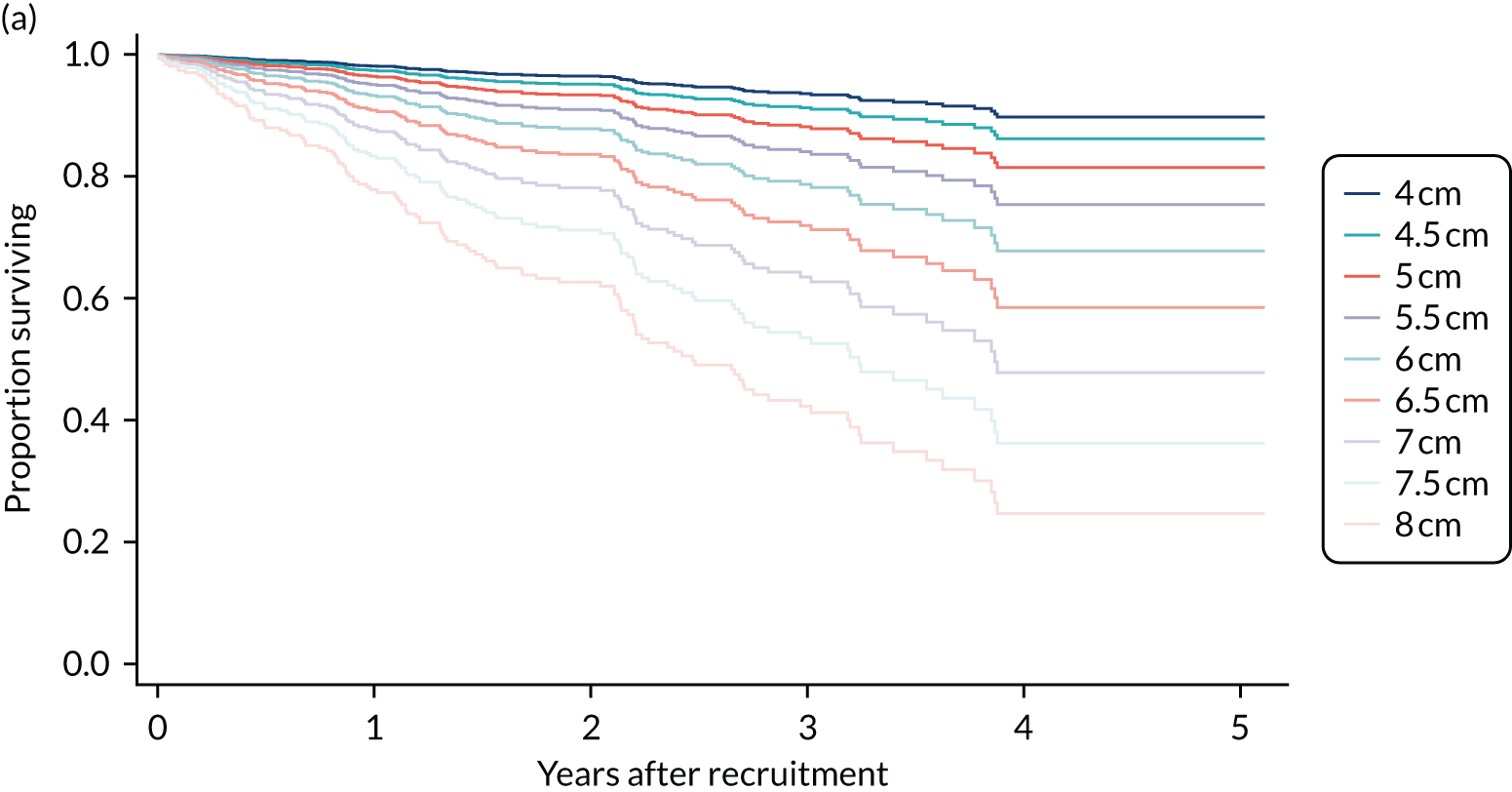
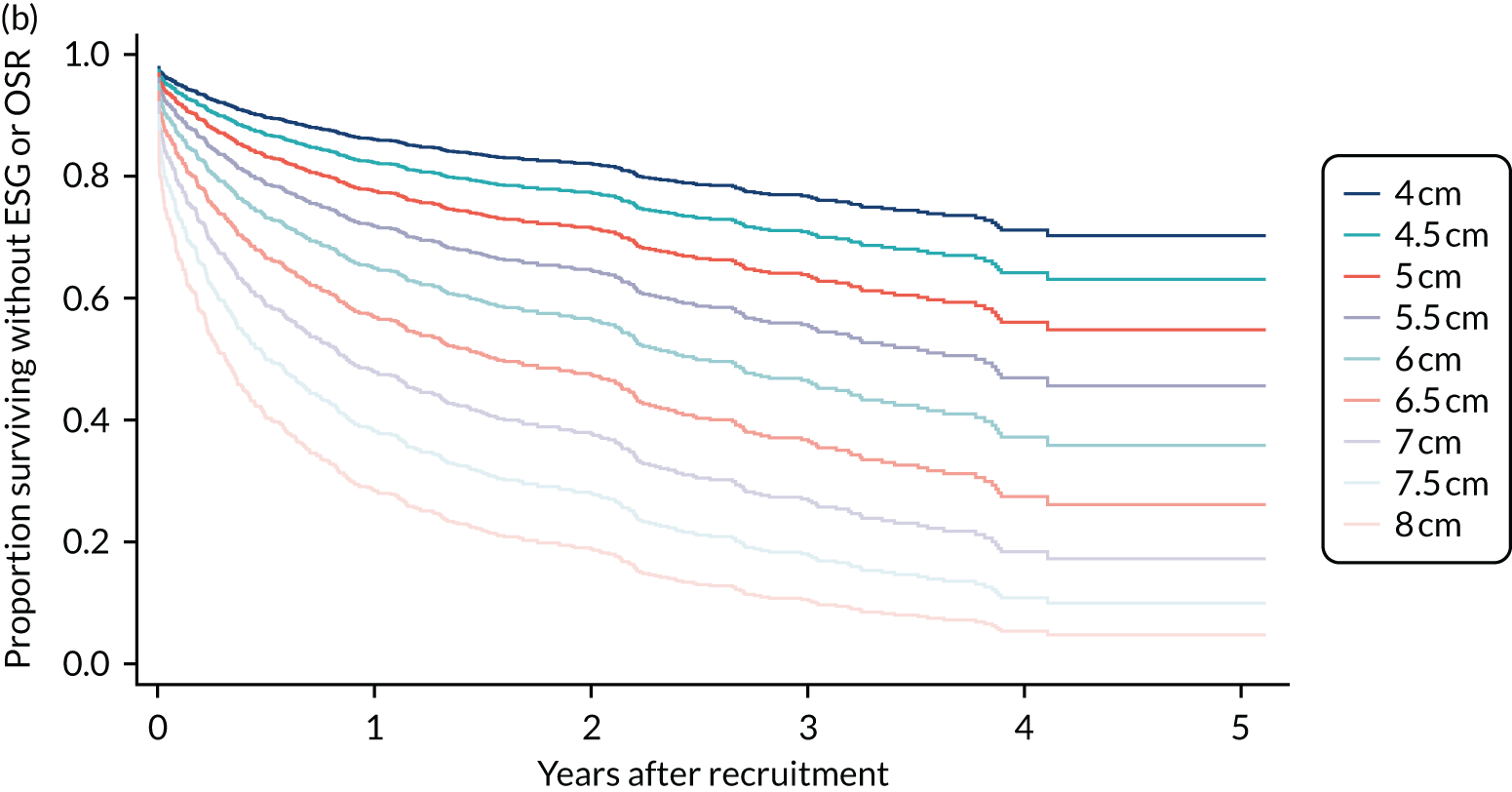
These predictions will underestimate the death rates if people undergoing procedures are at greater risk than people with similar characteristics who do not undergo procedures. Thus, we also provide predicted time to the composite outcome of death or intervention. This provides a worst-case scenario as it assumes that a patient would die on the day of the procedure if this were not performed.
There was weak evidence that increasing NYHA class was associated with increasing risk of all-cause death. In particular, CM and a range of comorbidity markers were not significant in the final model once age, sex, aneurysm size and NYHA class were included. The relatively small number of deaths meant that these models had limited power to detect small to moderate risk factors for death.
Frailty models showed that, given the small number of events, there was no evidence of variation between hospitals in the death rates, so this was not included in the models presented.
All survival models above used complete-case analysis. The results of repeating the analysis using multiple imputation for missing covariates were almost identical (see Appendix 2), showing that the results were not sensitive to missing data, provided that the assumption of MAR conditional on observed covariates holds. If there is some unknown missing data mechanism that depends on characteristics that were not measured in the ETTAA study, some bias in results could result. We consider this unlikely, given the comprehensive covariate adjustment in the imputation process.
We also refitted the final models with ESG and OSR treated as competing risks. This did not affect the main messages from this analysis, but the effect of baseline maximum aneurysm size was lower for both overall survival (HR 1.58, 95% CI 1.37 to 1.82; p < 0.001) and aneurysm-related survival (HR 1.81, 95% CI 1.51 to 2.17; p < 0.001).
Hospital admissions
Hospital admissions and non-fatal clinical events prior to any procedure are reported in Table 14 WW and CM patients respectively, and patients who went on to have interventions after this period. During the pre-procedure period, 363 admissions were reported in 222 patients; WW and patients who subsequently had OSR recorded admission rates of 0.22 and 0.18 per patient per-year of follow-up. Adjusting for age and sex, the difference between groups in overall pre-procedure admission rates was significant (p = 0.016). Taking WW as the reference group, the relative admission rate was 1.31 (95% CI 0.89 to 1.92) for CM patients, 2.10 (95% CI 1.30 to 3.42) for subsequent ESG recipients and 0.90 (95% CI 0.46 to 1.76) for subsequent OSR recipients. This may reflect the greater number of comorbidities in CM and (subsequent) ESG patients.
Fifty-two (definitely or probably) aneurysm-related hospital admissions were recorded in 39 separate patients. The aneurysm-related readmission rate was 0.18 per patient per-year in the patients who subsequently had ESG and was significantly lower in the other three groups (p = 0.0003 likelihood-ratio test, negative binomial regression). Only two non-fatal ruptures and seven dissections were reported. Three of these events were within 1 month of a CT scan and had maximal aneurysm diameters of 5.64 cm, 6.91 cm and 8.29 cm. In the other six events the maximal aneurysm sizes ranged from 4.56 cm to 6.98 cm, but scans had not been carried out within 6 months of the event. Eight non-fatal neurological events were reported (four cerebrovascular accidents and four transient ischaemic attacks).
Health-related quality of life over time from recruitment
Descriptive analysis
During the study, 3732 pre-procedure HRQoL questionnaires were returned by 886 patients. After blank forms (n = 256), duplicate entries (n = 11) and incomplete forms (n = 35) were excluded, 3492 (93.6%) questionnaires remained. Overall, 855 of 886 (96.5%) patients completed between one and nine EQ-5D-5L questionnaires. Of these 855 patients, 179 completed a single questionnaire, leaving 676 (79.1%) who contributed to the longitudinal analysis. Figure 9 shows the HRQoL trajectories over time pre procedure for the four treatment groups. These plots show recognised ceiling effects (maximum health) and very wide variation between patients at recruitment. Patterns over time are difficult to unravel from the plots, but CM patients appear to have lower HRQoL at baseline and there is some sign of a general decline in all groups.
FIGURE 9.
The EQ-5D-5L utilities over time, according to management group: (a) WW; (b) CM; (c) ESG; and (d) OSR.
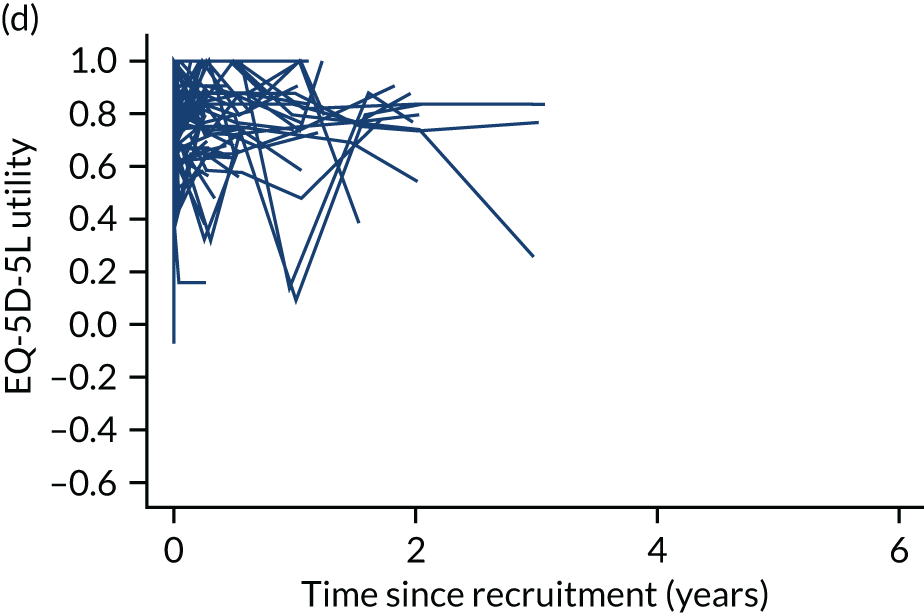
At least two pre-intervention EQ-5D-5L questionnaires were completed by 450 WW, 105 CM, 82 ESG and 64 OSR patients. Mean (SD) utilities at recruitment for these patients were 0.73 (0.23), 0.68 (0.25), 0.77 (0.24) and 0.76 (0.18), respectively. According to Szende et al. ,69 the population average in the UK is 0.785 for people aged 65–74 years and 0.734 for people aged ≥ 75 years, so the ETTAA cohort reported slightly worse HRQoL than the UK population of similar age. Future ESG and OSR patients had similar average HRQoL, despite the 5-year difference in average age. Patients allocated to intervention groups often left the pre-intervention phase before a second assessment had been made; ESG patients were less likely than other patients to complete baseline forms.
Results of modelling
The final model describing HRQoL trajectories in the absence of treatment is shown in the Appendix 7, and a brief summary is provided here.
At time zero, all covariate values are zero if the patient is male, is of average age (70.9 years), is in the WW group, has never smoked, does not receive formal/informal care and is in NYHA class I. For these ‘zero’ patients, average HRQoL at recruitment (time zero) was 0.85 (95% CI 0.82 to 0.88) (Table 17).
| Variable | WW group | CM group | ESG group | OSR group |
|---|---|---|---|---|
| All covariates zeroa | 0.85 (0.82 to 0.88) | 0.83 (0.78 to 0.89) | 0.86 (0.81 to 0.92) | 0.82 (0.76 to 0.87) |
| Formal/informal care | 0.64 (0.58 to 0.70) | 0.71 (0.61 to 0.81) | 0.65 (0.51 to 0.79) | 0.82 (0.68 to 0.96) |
| NYHA class | ||||
| II | 0.76 (0.73 to 0.79) | 0.71 (0.67 to 0.75) | 0.70 (0.65 to 0.75) | 0.76 (0.71 to 0.81) |
| III | 0.67 (0.63 to 0.71) | 0.58 (0.52 to 0.64) | 0.54 (0.46 to 0.62) | 0.71 (0.64 to 0.78) |
| IV | 0.58 (0.53 to 0.63) | 0.45 (0.35 to 0.55) | 0.37 (0.25 to 0.49) | 0.66 (0.54 to 0.78) |
At recruitment (time zero), across all management groups, there was weak evidence that women had lower HRQoL (–0.029, 95% CI –0.55 to –0.003). The average age at recruitment was just under 71 years and HRQoL at recruitment actually increased slightly with age (0.013 per decade, 95% CI 0.000 to 0.025), possibly as a result of selection policies. In terms of patients not requiring formal/informal care, the management groups had similar average HRQoL at recruitment (all else being equal). There was weak evidence that current smokers had worse HRQoL (–0.047, 95% CI –0.091 to –0.004) at recruitment than ex-smokers or never-smokers in all management groups. Reported requirement for formal/informal care had a very large impact on HRQoL at recruitment in WW, CM and ESG patients, but HRQoL was not adversely affected by the reported need for care in OSR patients (see Table 17). In the WW group, HRQoL at recruitment was lower by –0.089 (95% CI –0.11 to –0.069) for each one-class increase in NYHA classification. The interaction between management group and NYHA class shows that the relationship with increasing NYHA class is even stronger in CM and ESG patients than in WW patients, but slightly less strong in OSR patients (see Table 17).
For the relatively fit patients with zero covariates at baseline, HRQoL did not change significantly over time (estimated decrease –0.010 per year, 95% CI –0.022 to 0.003 per year). However, the interaction between follow-up time and age showed that for two patients who differ in age by 10 years, the older patient has a faster decrease in HRQoL of –0.013 (95% CI –0.019 to –0.007) per year (all else being equal). As a result, the higher HRQoL (baseline, 0.013; change in the first year, –0.013) between age groups increased over time thereafter. Moreover, there was reasonably strong evidence that current smokers had a faster decline in HRQoL than non-smokers and ex-smokers (estimated regression parameter –0.034, 95% CI –0.057 to –0.01; p = 0.004) per year. Figure 10 shows the estimated trajectories for variables that affected rate of decline in HRQoL. This shows that, in this cohort, smoking has a much greater influence on HRQoL than a 10-year increase in age.
FIGURE 10.
Model-estimated HRQoL over time by smoking history and age (dark blue is average age of 70.9 years, light blue is average age + 10 = 80.9 years), with all other covariates set to zero (patient is WW, male, no formal/informal care, NYHA class I).
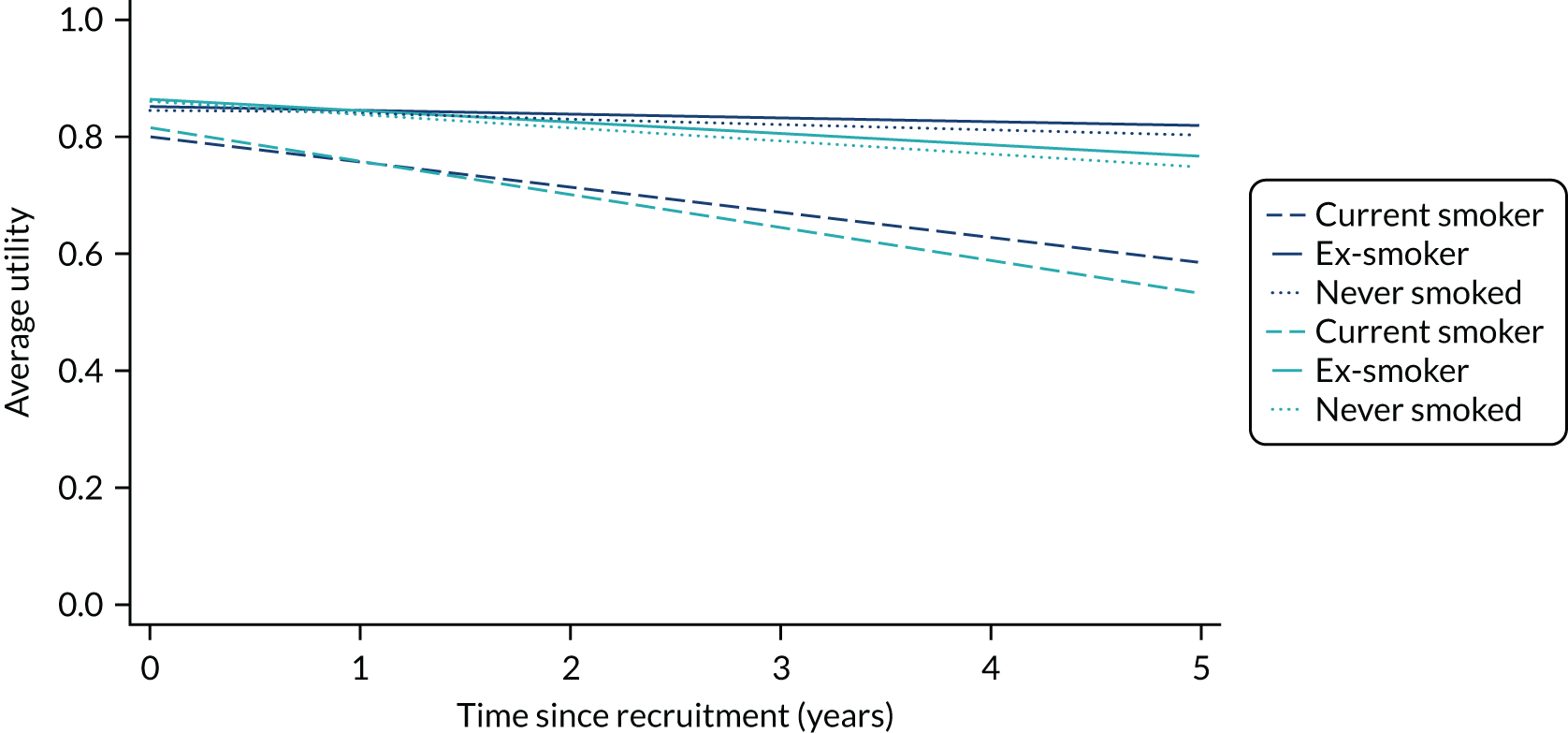
Significant random effects indicated that HRQoL varied significantly between patients both at recruitment and in the rate of decline over time, in addition to the variation that could be attributed to the variables in the model. There were also some differences between the management groups in how much patients varied around the average at recruitment.
Perhaps of greater interest were the variables that did not significantly affect HRQoL, including aneurysm size and comorbidities such as COPD, coronary heart disease and valvular heart disease, although age and the use of formal/informal care (reflecting frailty) were associated with these comorbidities and possibly acted as surrogates.
Summary of findings
This analysis shows that CTAA patients present with widely varying aneurysm sizes. Some of the variation can be explained by patient age, height, smoking history and comorbidities, as well as the site of the aneurysm. In addition, there was variation between aneurysms at presentation that was not explained by the baseline variables recorded in the ETTAA study (significant random intercepts). At presentation, aneurysms in the DTA were significantly larger than in all other sections of the aorta, and they grew faster over time. This may result from the differences in tissue types in different sections of the aorta. Alternatively, the more linear anatomy of this section of the vessel may allow greater expansion before the aneurysm becomes clinically apparent or results in rupture or dissection. We did not observe growth of aneurysms in the ascending aorta. Our inclusion criteria allowed previous intervention for aneurysms in the ascending aorta, and 21.2% of patients had received previous surgery on the ascending aorta or aortic root. A complication in the analysis of this segment is that growth was measured from diagnosis, which may well have preceded surgery in the ascending aorta. A post hoc analysis restricted to measurements in the ascending aorta only showed that there was almost no growth in patients who did not report previous surgery (0.018 cm per year) and a significant decrease in those who did receive surgery (–0.092 cm per year). This suggests that at least some surgery took place between diagnosis (time zero) and entry into the ETTAA study. Unfortunately, the date of previous surgery was not recorded.
The relationship between results from MRI and CT scans over time is difficult to explain. One possible explanation is that, after the initial scan, clinicians may direct patients with slower-growing aneurysms towards MRI and refer patients for CT if growth appears to be accelerating. This potential selection bias induces a difference in aneurysm size over time.
Almost all patients (88%) had hypertension at consent and were treated with one or more antihypertensive medications. Thus, our analysis was not able to identify significant effects of specific antihypertensive drugs. The observational nature of the study may also result in treatment by indication bias, whereby higher-risk patients are treated with more powerful or more expensive drugs, underestimating the treatment effects of the drugs. Such bias is difficult to adjust for unless there are very detailed data on the reasons for the use of single drugs or combination treatments.
The all-cause death rate during this period was relatively high, at 8.6% per patient-year. This compares with the 1-year probability of death for English men aged 71 years of 2.1% and for women 1.4%. 70 Around half the observed deaths in the ETTAA study had a rupture, dissection or other aneurysm-related cause. Comparisons of CM and non-CM patients showed that clinicians successfully identify patients at higher risk from their characteristics and clinical history. As might be expected each 1-cm increase in aneurysm size doubled the hazards of all-cause and aneurysm-related death in the absence of surgical intervention. We note that the relationship between aneurysm size and (log) hazard was linear over the range of aneurysm sizes observed. However, patients leave this analysis when aneurysms become large enough for intervention or through death, so that the pattern of growth outside this range is not known and extrapolation is not valid. The very large and accelerating increase in hazards with each decade increase in age was also expected. The higher risk of death for women in this age group was less predictable and may indicate that women presented at a later stage in their disease or when they also had other comorbidities. There is no evidence that women had longer follow-up after consent than men, so any delays due to (patient or clinician) selection were more likely to have occurred prior to referral to participating MDTs.
We included management group in the analysis of clinical events and HRQoL, even though this was not always decided at baseline. Although this is generally not recommended by statisticians, our rationale was to explore the empirical data rather than establish causal relationships between baseline variables and outcomes. Results should be interpreted with this in mind. From Chapter 3, ESG patients on average were older, had poorer LV function, and were more likely to be current smokers and have COPD. The index aneurysm was also more likely to be in the DTA for this group; on this basis ESG patients were more likely to have faster-growing aneurysms before the procedure. The analysis of pre-procedure clinical events and HRQoL also showed that ESG patients had a faster decrease in HRQoL, especially if they also reported formal/informal care or a high NYHA class, and more admissions to hospital for aneurysm-related and other causes prior to the intervention. These factors will be important and must be taken into account when comparing intervention groups in Chapters 5, 6 (clinical outcomes) and 7 (cost-effectiveness).
Chapter 5 Post-procedure outcomes for intervention groups
Introduction
This chapter provides further details of patients who underwent ESG or OSR and investigates variables associated with post-procedure survival and HRQoL outcomes in each group separately, using traditional regression methods. The Delphi study reported in Chapter 2 showed that there was consensus among clinicians about management options for patients with specific aneurysm and patient-related characteristics. Here we assess whether or not clinician opinion is borne out by clinical practice and summarise differences in outcomes between interventions. This analysis is in preparation for comparisons in Chapters 6 and 7 of outcomes for patients who could undergo both interventions.
Aims of analysis of outcomes following a procedure
The aims in this chapter are to:
-
quantify post-procedure clinical outcomes of survival (primary outcome), complications, reinterventions, readmission and HRQoL within treatment groups ESG and OSR separately
-
assess variables associated with survival and HRQoL
-
compare patient characteristics and clinical histories of ESG and OSR patients for future comparison.
Methods
Population
All patients who underwent at least one ESG or OSR procedure were included in this analysis from the date of hospital admission for the index procedure to death, withdrawal or the end of the ETTAA study. Consistent with the observational study protocol, patients were analysed according to the treatment received, irrespective of management plans at recruitment.
Outcomes
The primary outcome was survival from the date of the index procedure to either death or censoring date. Secondary outcomes were clinical complications, death within 30 days of procedure, reinterventions, readmissions to hospital for aneurysm or cardiac causes, length of stay and HRQoL from procedure to end of follow-up, as defined in Chapter 3.
Statistical analysis
Survival
Owing to the small number of deaths within 30 days, these were summarised with other procedure complications. Overall survival was summarised by Kaplan–Meier incidence plots, and exploratory comparisons used log-rank tests. The association between potential risk factors and survival was modelled by Cox proportional hazards models, after confirming validity of the proportional hazards assumption using Kaplan–Meier plots and Schoenfeld residuals. Initial exploratory analysis identified variables where the z-statistic from the univariable Cox model had a p-value of < 0.2. These variables were considered for the multivariable model using backward selection based on z-statistics. The final model was re-estimated using multiple imputation using chained equations with 30 imputed data sets (see Chapter 3 and Appendix 2).
Complications, reinterventions, readmission and length of stay
Complications following all procedures were pooled and analysed as a binary response for each patient (none/any) and as counts (number of complications per patient). Reintervention included planned and unplanned additional procedures and other reasons for return to theatre. Length of hospital stay was measured from date of procedure to the date of discharge or transfer. Readmissions to hospital for (definitely/probably) aneurysm-related or other cardiac events were summarised as rates per patient-year at risk and confidence intervals for the following time periods: 0–3, 4–6 and 7–12 months and annually thereafter. They included reinterventions (ESG followed by another ESG, or OSR followed by either another OSR or ESG).
Owing to the small number of patients and events, simple tests comparing proportions or event rates were performed for complications, clinical events and readmissions and no modelling was undertaken. For readmissions to hospital the relative rate of readmission was estimated using negative binomial regression to allow for repeated readmissions for individual patients and included time at risk, index procedure, age and sex.
Health-related quality of life
Repeated measures of HRQoL assessed by EQ-5D-5L utilities were analysed using linear mixed models as described in Chapter 4. Continuous time was assumed, with actual rather than nominal times of measurement. Preliminary analysis revealed a temporary decrease in utilities in the OSR group after surgery; to model this, a marker variable for early postoperative assessment (first 6 weeks) was considered, together with an interaction between group and early postoperative assessment. Preoperative patient characteristics and clinical variables measured closest to the index procedure were investigated for this analysis. The final model had the following form.
where:
-
τit is the time (from the procedure) of the tth measurement from patient i and δit is a marker of whether that measurement was in the first 6 weeks
-
xi is a vector of procedure baseline variables including early postoperative assessment, aneurysm site and treatment group for patient i
-
(β0, β1, β1F, β2, β2P, θ) are coefficients for the fixed effects, including interactions between sex and time (β1F) and between procedure and the first 6 weeks (β2P)
-
ui|xi∼N(0,σu2) are random intercepts
-
εit|ui,xi∼N(0,σε2) are residual errors, with σε2 differing between the two intervention groups.
Again, estimates from this analysis are unbiased provided that data are missing at random, conditional on observed data. Model fit was assessed by fitted and residual plots.
Results
Baseline predictors
Baseline variables at consent for the two intervention groups were provided in Tables 8–10. These variables recorded before but closest to the procedure are summarised in the Appendix 8, although changes from baseline were small.
There were important differences between the two groups at the time of their procedures. Excluding missing covariates, compared with OSR patients, ESG patients were older (mean age difference 7.1 years, 95% CI 4.7 to 9.5 years; p < 0.0001), were smaller (mean height difference –3.7 cm, 95% CI –6.3 to –1.2 cm; p = 0.0041 and mean weight difference –5.3 kg, 95% CI –9.2 to –1.4 kg; p = 0.0075) and were more likely to be current or past smokers (75.8% vs 66.4%; p = 0.080). The differences in height and weight were partially explained by the higher proportion of CTD patients in the OSR group, although the differences remained significant when these patients were removed (mean height difference –2.7 cm, 95% CI –5.2 to –0.1 cm; p = 0.0381 and mean weight difference –5.0 kg, 95% CI –9.1 to –0.9 kg; p = 0.0172). ESG patients were more likely to have valve disease (89.9% vs. 71.6%; p < 0.0001), COPD (21.3% vs. 13.3%; p = 0.087) and stage III/IV NYHA (22.3% vs. 16.0%; p = 0.217). Patients with connective tissue disorders almost invariably underwent OSR (14.8% vs. 1.3%; p < 0.0001). A very high proportion of patients (90.7% ESG, 85.2% OSR) had hypertension; there was no difference between groups in the numbers who were prescribed antihypertensive medications (88.7% vs. 85.2%; p = 0.486). ESG patients were more likely to report use of statins (69.3% vs. 42.2%; p < 0.0001). Serum creatinine and haemoglobin measurements were missing for approximately 19% of cases in each group. For those with complete data ESG patients had similar renal function (mean serum creatinine difference –7.1 µmol/l, 95% CI –16.0 to 1.8 µmol/l; p = 0.1191) but lower levels of haemoglobin (mean haemoglobin difference –6.8 g/l, 95% CI –11.2 to –2.4 g/l; p = 0.0026) than OSR patients.
Despite differences in age and other covariates between the groups, mean (SD) HRQoL utilities before the procedures were the same, 0.73 (0.24) for ESG and 0.73 (0.26) for OSR.
Description of outcomes
Outcomes from the procedure
Endovascular stent grafting patients spent a median of 3.15 (quartiles 2.07, 5.08) hours in surgery compared with 8.52 (quartiles 7.25, 9.70) hours for OSR patients (p < 0.0001). Ten (6.7%) ESG and 15 (11.1%) OSR patients died within 30 days of the procedure (p = 0.2107). Two of the ten ESG patients were discharged alive but died at home within 30 days.
Non-fatal complications during the procedure and associated admission are listed in Table 18. In the OSR group one patient had a ruptured aneurysm and one had a dissection. In the ESG group there were 14 intraoperative stent-related complications in 14 different patients recorded during the primary stenting procedure: six endoleaks (three of type I and three of type II), treated either by insertion of an additional stent (n = 2), re-ballooning (n = 2) or conservatively (n = 2); five injuries to the access vessel [two bleeding (one requiring surgery), one pseudoaneurysm, one dissection (treated by insertion of an additional stent) and one ‘peripheral arterial disease – small external iliac artery’] and three complications of the stent graft [one migration, one incomplete procedure (no details given) and one ‘balloon moulding to smooth stent’].
| Patient subgroup (number of patients) | ||
|---|---|---|
| ESG (N = 150) | OSR (N = 135) | |
| Number of deaths within 30 days, n (%) | 10 (6.7) | 15 (11.1) |
| During index procedure | ||
| Number of complications | ||
| Dissection | 0 | 1 |
| Rupture | 0 | 1 |
| Stent complications | 3 | – |
| Stent access vessel injury | 5 | – |
| Endoleak | 6 | – |
| During index procedure admission | ||
| Number of complications | ||
| Myocardial infarction | 9 | 2 |
| Gastrointestinal | 7 | 12 |
| Neurological | 5 | 13 |
| Cerebrovascular accident | 4 | 11 |
| Transient ischaemic attack | 1 | 2 |
| Spinal cord injury | 5 | 4 |
| Paraparesis | 2 | 0 |
| Paraplegia | 3 | 4 |
| Thromboembolic event | 3 | 7 |
| Deep-vein thrombosis | 0 | 3 |
| Pulmonary embolism | 1 | 3 |
| Not recorded | 2 | 1 |
| Infection | 17 | 44 |
| Vocal cord palsy | 2 | 7 |
| Number of patients requiring additional support | ||
| Inotropes/intra-aortic balloon pump | 27 | 79 |
| Prolonged ventilation | 5 | 37 |
| Renal support | 2 | 15 |
| Return to theatre | 16 | 20 |
| Total number of events | 98 | 240 |
| Total number of people with ≥ 1 event (%) | 58 (38.7) | 103 (76.3) |
Post procedure, OSR patients required a significantly longer stay in ICU [median 5 days (quartiles 3, 10 days) vs. median 0.5 (quartiles 0, 3) days; p < 0.0001] and a longer stay in hospital than ESG patients [median 16 days (quartiles 10, 23 days) vs. median 7 days (quartiles 4, 12 days), p < 0.0001]. OSR patients were more likely to have cardiac, gastrointestinal, neurological and infection complications, and to require cardiac, pulmonary and renal support during admission, than ESG patients. Return-to-theatre rates were slightly higher in the OSR group, but not significantly so (see Appendix 9; p = 0.3722). The main reasons for return to theatre were access injuries, aneurysm complications and endoleaks after ESG, and aneurysm injuries and other acute surgical complications after OSR. The total number of complications was much larger in the OSR group (240 vs. 98; relative rate 2.72, 95% CI 2.04 to 3.68; p < 0.0001). Overall, 58 (38.7%) ESG and 103 (76.3%) OSR patients had an adverse event during the index procedure admission (p < 0.0001).
Survival, hospital readmissions and aneurysm-related events
Survival post procedure: descriptive analysis
The primary outcome was overall survival after the procedure. During follow-up, 40 ESG and 36 OSR patients died, of whom 17 and 25, respectively, died from aneurysm-related causes; these included deaths within 30 days (Table 19). The 1-year overall death rate was 17.5% (95% CI 12.2% to 24.8%) after ESG and 20.7% (95% CI 14.6% to 28.9%) after OSR; the 3-year death rate was 34.7% (95% CI 26.1% to 45.1%) after ESG and 31.9% (95% CI 23.7% to 42.2%) after OSR (Figure 11a). For aneurysm-related deaths, the 1-year rate was 10.2% (95% CI 6.2% to 16.6%) after ESG and 12.3% (95% CI 10.9% to 24.1%) after OSR; the 3-year rate was 14.3% (95% CI 8.8% to 22.7%) after ESG and 20.6% (95% CI 14.3% to 29.3%) after OSR (Figure 11b). Despite the slightly higher aneurysm-related death rate for OSR patients in the first 30 days, there was no significant difference between the groups in time to death overall (log-rank p = 0.9918) or in aneurysm-related death (log-rank p = 0.1107).
| Patient subgroup | ||
|---|---|---|
| ESG (N = 150) | OSR (N = 135) | |
| Total time at risk (years) | 265.2 | 253.4 |
| Deaths overall (rate/year) | 40 (0.15) | 36 (0.14) |
| Aneurysm-related deaths (rate/year) | 17 (0.06) | 25 (0.10) |
| All readmissions, n (rate/year) | 111 (0.42) | 87 (0.34) |
| People with at least one readmission, n (%) | 61 (40.7) | 43 (31.9) |
| Readmissions, definitely/probably aneurysm related, n (rate/year) | 40 (0.15) | 23 (0.09) |
| Patients readmitted, definitely/probably aneurysm related, n (%) | 26 (17.3) | 17 (12.6) |
| Non-fatal ruptured aneurysms | 0 | 0 |
| Non-fatal dissected aneurysms | 3 | 1 |
| Non-fatal neurological events | 4 | 0 |
FIGURE 11.
Kaplan–Meier cumulative incidence curves for post-intervention death from any cause and aneurysm-related deaths by ESG and OSR groups. (a) Kaplan–Meier deaths from any cause; and (b) Kaplan–Meier aneurysm-related deaths.
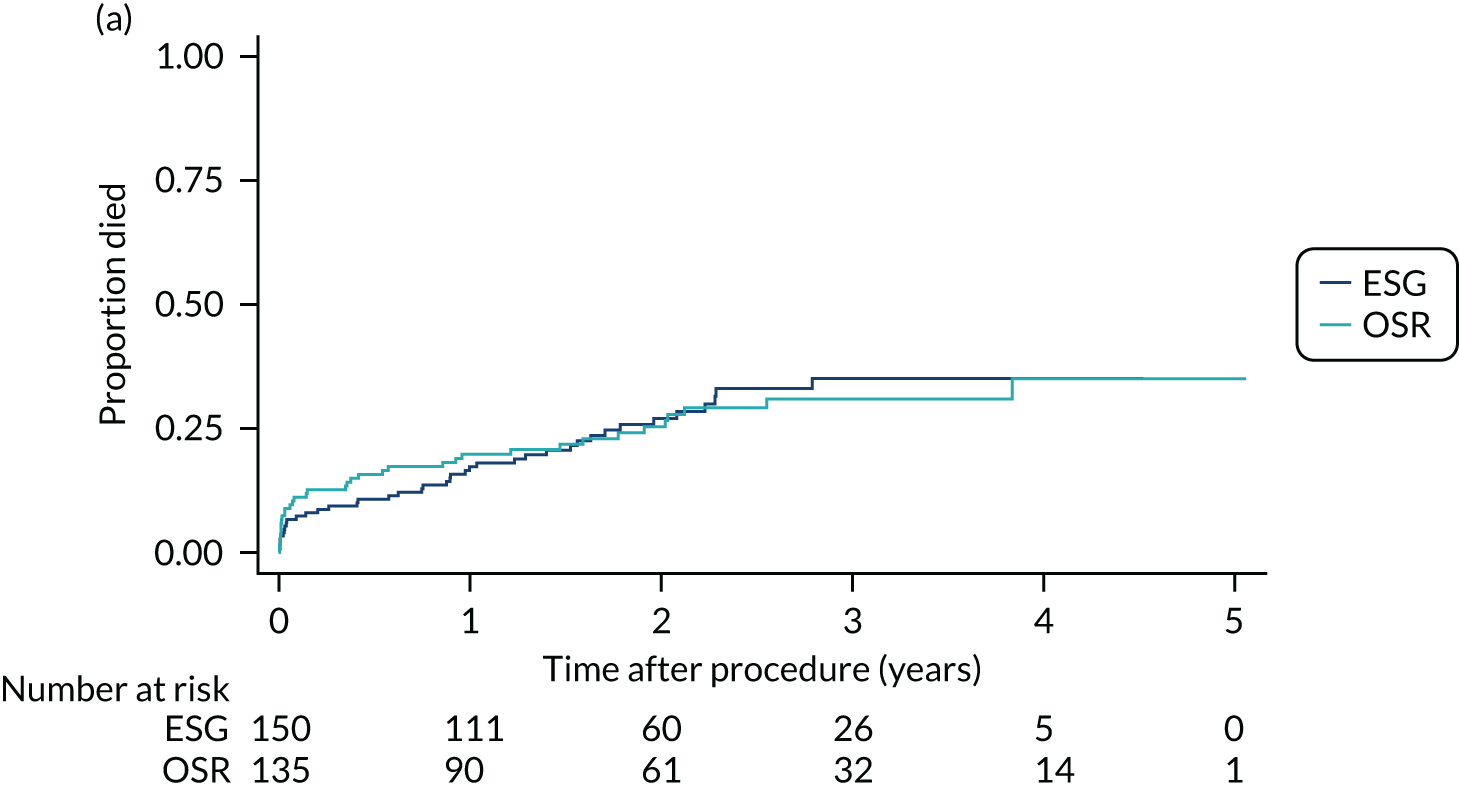
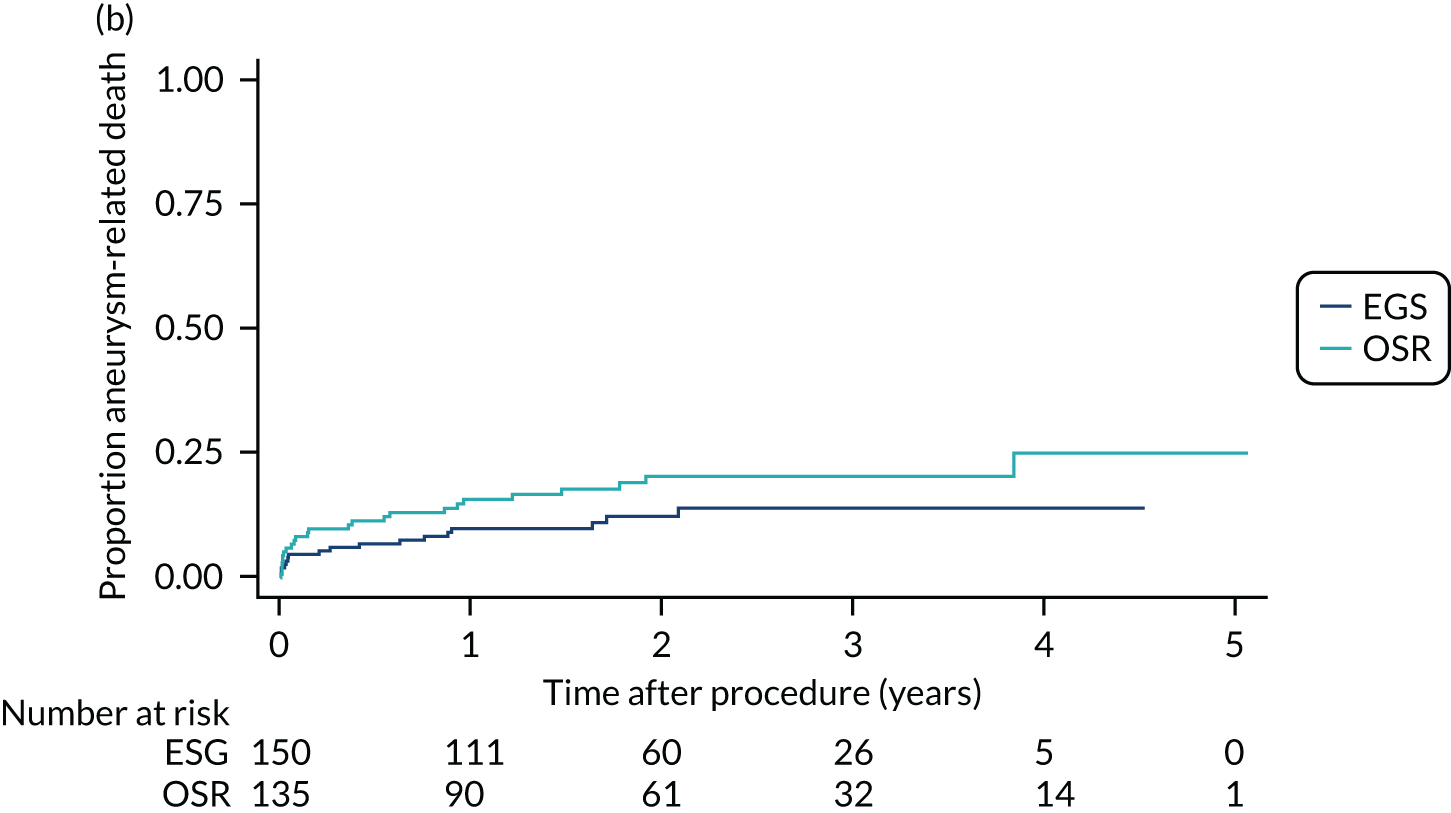
Survival: results of modelling
Table 20 shows the variables for which the p-value of the HR test (H0: HR = 1) was, at most, 0.2 in univariable models for all-cause deaths and aneurysm-related deaths; we also include OSR, despite the higher p-value. For only 2 out of 150 (1.3%) ESG procedures, the location of the maximum aneurysm diameter was the ascending aorta or arch, so these two variables have considerable overlap. Therefore, these were combined into a three-level variable (ESG in the DTA, OSR in the DTA, any procedure in the ascending aorta/arch). Similarly, patients were treated with either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB), but rarely with both, so these were also analysed together as a three-level variable (neither drug, ACE inhibitor only or ARB with or without ACE inhibitor).
| Variable | All-cause deaths | Aneurysm-related deaths | ||
|---|---|---|---|---|
| HR (95% CI) | z-test p-value | HR (95% CI) | z-test p-value | |
| OSR | 1.00 (0.64 to 1.57) | 0.992 | 1.64 (0.87 to 3.04) | 0.115 |
| Maximum aneurysm site DTA/TCAA | 2.00 (0.92 to 4.34) | 0.082 | ||
| Ascending/arch procedure | (Reference category) | 0.134 | (Reference category) | 0.043 |
| OSR in DTA/TCAA | 2.29 (1.01 to 5.23) | 2.70 (0.93 to 7.87) | ||
| ESG in DTA/TCAA | 1.81 (0.81 to 4.06) | 1.33 (0.45 to 3.98) | ||
| Age (per decade) | 1.37 (1.08 to 1.74) | 0.010 | ||
| Female | 1.83 (1.00 to 3.36) | 0.052 | ||
| Weight (per kg) | 0.99 (0.98 to 1.00) | 0.152 | ||
| BMI (per kg/m2) | 0.96 (0.91 to 1.01) | 0.141 | ||
| Per month since recruitment | 1.03 (1.01 to 1.05) | 0.003 | 1.03 (1.01 to 1.06) | 0.010 |
| NYHA per class | 1.41 (1.08 to 1.84) | 0.013 | 1.28 (0.89 to 1.84) | 0.184 |
| COPD | 1.50 (0.89 to 2.55) | 0.131 | ||
| ACE inhibitor | 0.60 (0.35 to 1.05) | 0.071 | ||
| ARB | 1.61 (0.98 to 2.65) | 0.059 | 1.57 (0.80 to 3.06) | 0.190 |
| Neither | (Reference category) | 0.069 | ||
| ACE inhibitor only | 0.64 (0.36 to 1.17) | |||
| ARB ± ACE inhibitor | 1.40 (0.83 to 2.37) | |||
| Any antihypertensive medication | 1.95 (0.85 to 4.49) | 0.116 | ||
In univariable Cox models for all-cause mortality, the two procedures had similar survival probabilities, despite the older age and higher risk profile of ESG patients. There was weak evidence that aneurysms where the maximum diameter was in the DTA/thoracoabdominal aorta conferred a higher risk of death. The strongest associations with all-cause death were age at procedure, higher NYHA class and longer time between recruitment to the ETTAA study and procedure. The median time between recruitment and procedure was longer for OSR patients than for ESG patients (82 vs. 64 days), although this difference was not significant at traditional levels (p = 0.5682).
The smaller number of aneurysm-related deaths meant that only the procedure/location of maximum aneurysm diameter, female sex and time since recruitment were weakly significant in univariable analyses for this outcome.
The final multivariable models for all-cause and aneurysm-related deaths are shown in Table 21. For all-cause mortality, procedures for aneurysms in the ascending aorta/arch (mostly OSR) had lowest risk, whereas OSR for maximum aneurysms in other vessels had highest risk. Age at procedure conferred an increase in risk, with a HR of 1.48 (95% CI 1.12 to 1.94) for each 10-year increase in age, and the hazard was multiplied by 1.39 (95% CI 1.06 to 1.82) for each one-class increase in NYHA class. Owing to the smaller number of aneurysm-related deaths, neither age nor NYHA class was significantly associated with this outcome, but female patients were found to have a higher risk of aneurysm-related death. The length of time in the study before the procedure was associated with higher risk of both all-cause and aneurysm-related death. These associations were almost identical after the imputation of missing covariates (see Appendix 2).
| Variable | All-cause deaths | Aneurysm-related deaths | ||
|---|---|---|---|---|
| HR (95% CI) | z-test p-value | HR (95% CI) | z-test p-value | |
| Ascending/arch procedure | (Reference category) | 0.031 | (Reference category) | 0.041 |
| OSR in DTA/TCAA | 2.82 (1.15 to 6.89) | 2.86 (0.97 to 8.45) | ||
| ESG in DTA/TCAA | 1.61 (0.68 to 3.84) | 1.43 (0.48 to 4.30) | ||
| Age (per decade) | 1.48 (1.12 to 1.94) | 0.005 | ||
| Female | 2.03 (1.10 to 3.75) | 0.024 | ||
| NYHA (per class) | 1.39 (1.06 to 1.82) | 0.018 | ||
| Pre-operation time in study (per month) | 1.03 (1.01 to 1.05) | 0.005 | 1.03 (1.01 to 1.06) | 0.019 |
For clarity it is worthwhile to refit these multivariable models including procedure group but excluding aneurysm site; the HR for OSR was 1.27 (95% CI 0.78 to 2.09; p = 0.332) for overall deaths and 1.59 (95% CI 0.86 to 2.96; p = 0.140) for aneurysm-related deaths. Thus, overall there is a non-significant increase in all-cause and aneurysm-related deaths among OSR patients.
Readmissions after discharge from the index procedure
Table 19 shows the readmissions after discharge from the index procedure. During a total of 265.2 years of follow-up, ESG patients were readmitted 111 times, a rate of 0.42 (0.35, 0.49) per patient-year (i.e. on average 42% of patients admitted to hospital per year of follow-up). This was higher than the rate for OSR patients (0.34, 95% CI 0.27 to 0.41) per patient-year. The relative hospital readmission rate (adjusted for time at risk) was 0.70 (95% CI 0.44 to 1.11; p = 0.133); further adjustment for age and sex increased the relative rate of readmission slightly, to 0.75 (95% CI 0.46 to 1.21; p = 0.237). Furthermore, 40.7% of ESG patients were readmitted during follow-up compared with 31.9% of OSR patients (p = 0.1398). A similar pattern was observed for aneurysm-related readmissions. No ruptures and only one dissection were reported as the reason for aneurysm-related readmissions.
Figure 12 shows the pattern of readmissions over time after procedure for the two groups. In the first 3 months, ESG patients were more likely to be readmitted, and thereafter the readmission rates were similar.
FIGURE 12.
Readmissions to hospital (per patient-year at risk) by group and by time after the index procedure.
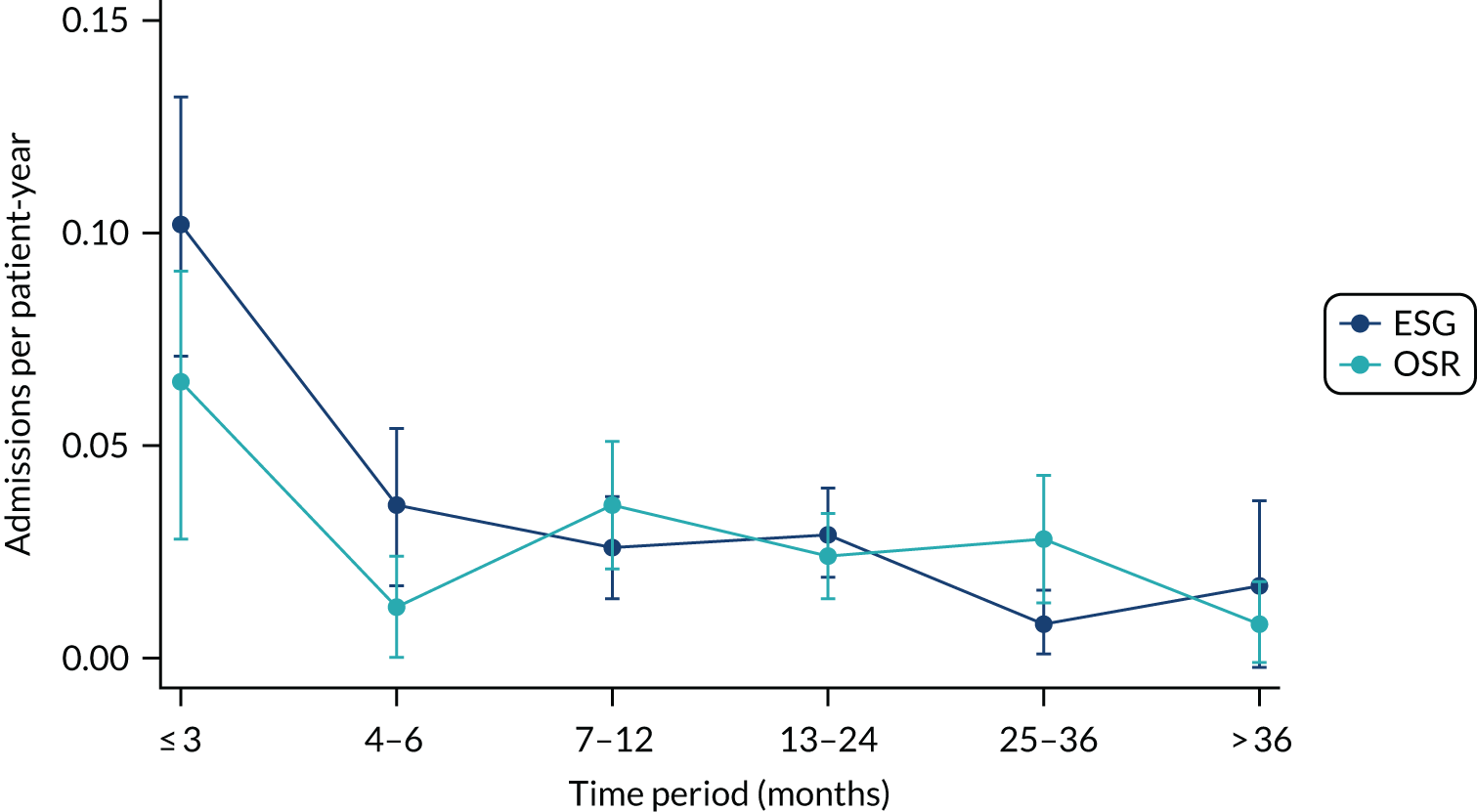
Once readmitted, ESG patients spent a median of 6 days (quartiles 2 to 12) in hospital, compared with 5.5 days (quartiles 2 to 10) for OSR patients (Mann–Whitney U-test p = 0.8236). However, ESG patients were more likely to spend some of the inpatient stay in ICU (25 vs. 7; p = 0.0064).
Table 19 shows that 12 ESG patients underwent a second ESG during the study period at a median of 93.5 days after the index procedure (range 18–1076 days). Similarly, four OSR patients had a second OSR at 58, 149, 217 and 371 days post index procedure. Twenty-one OSR patients had a subsequent ESG; one was in the same admission, and the remaining 20 were a median of 173 days (range 11–1100 days) after the index procedure. Three OSR patients had ESG as a third procedure at 208, 908 and 1399 days after the index procedure. OSR patients had more complications during readmissions. All 40 reinterventions were reported as planned as part of a staged procedure. Complications after second and third procedures are summarised in Appendix 5.
Post-procedure health-related quality of life
Descriptive analysis
For this analysis the preoperative HRQoL assessment nearest to the date of the procedure was included as a potential predictor of postoperative HRQoL. Forty-three patients had no postoperative assessment of HRQoL; the remaining 242 patients completed between 1 and 12 postoperative questionnaires, resulting in a total of 1082 assessments.
Utility measurement trajectories for individual patients are plotted in Figure 13. Again, the ceiling effect at utility = 1 (maximum health) is observed, and there is evidence of wide variation in HRQoL at time zero. Patterns over time were not clear from the trajectories, but initial analysis suggested that both linear and quadratic terms for change over time should be considered for inclusion and that these may differ between the sexes. A marker variable of early postoperative assessment (first 6 weeks) was considered, as was an interaction between group and early postoperative assessment. Again, there was evidence that HRQoL at procedure was more variable in the ESG group.
FIGURE 13.
Post-procedure EQ-5D-5L utilities over time by intervention group. (a) ESG; and (b) OSR.
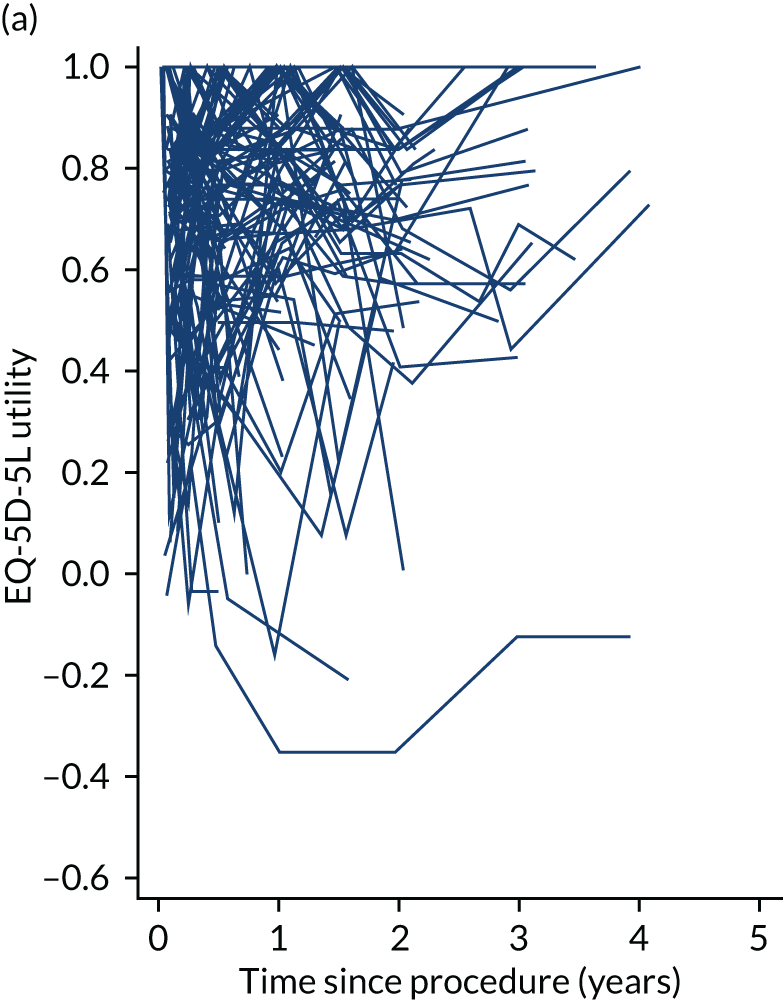
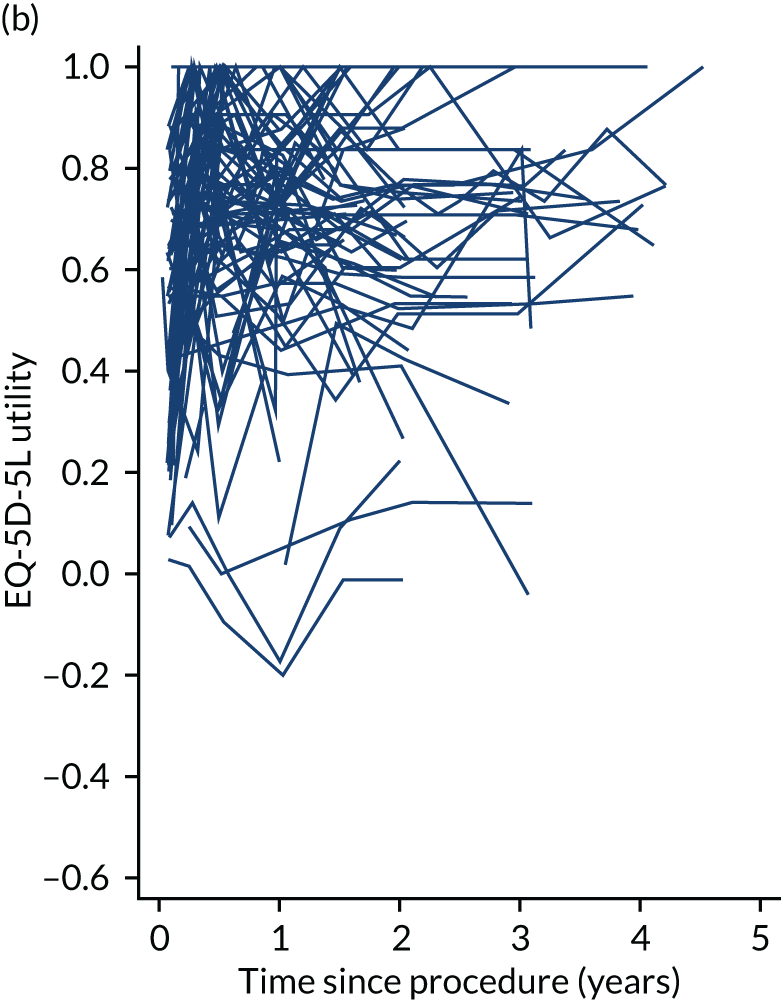
Health-related quality-of-life modelling
The final model for postoperative HRQoL is provided in full in Appendix 10. Briefly, for a male, non-smoking ESG patient, in NYHA class I, with average preoperative utility of 0.73, the average postoperative HRQoL immediately post ESG was estimated to be 0.785 (95% CI 0.725 to 0.844). This decreased very slightly by –0.001 (95% CI –0.012 to 0.013) per year, with little evidence of acceleration or deceleration. There was a slight non-significant dip in HRQoL of –0.017 (95% CI –0.062 to 0.027) in the first 6 weeks. HRQoL between patients showed significantly more variation in the ESG group than in the OSR group. These results did not change substantially if the analysis excluded second and third stages of a staged procedure.
For OSR patients, there was a substantial, significant decrease in HRQoL in the first 6 weeks after the procedure of –0.160 (95% CI –0.199 to –0.121; p < 0.001). Otherwise, the difference between the two procedures during follow-up was not significant (all else being equal). Although female and male OSR patients had the same decrease in HRQoL in the first 6 weeks, the pattern over time was different; women had a slight increase in HRQoL over time, whereas men did not change after the first 6 weeks (Figure 14). Finally, in common with pre-procedure analysis, current smokers and those in higher NYHA classes had significantly lower HRQoL throughout, with decrements of –0.095 (95% CI –0.171 to –0.020) for current smokers and –0.034 per NYHA class (95% CI –0.066 to –0.003).
FIGURE 14.
Average estimated post-procedure EQ-5D-5L utility over time by sex and intervention group.
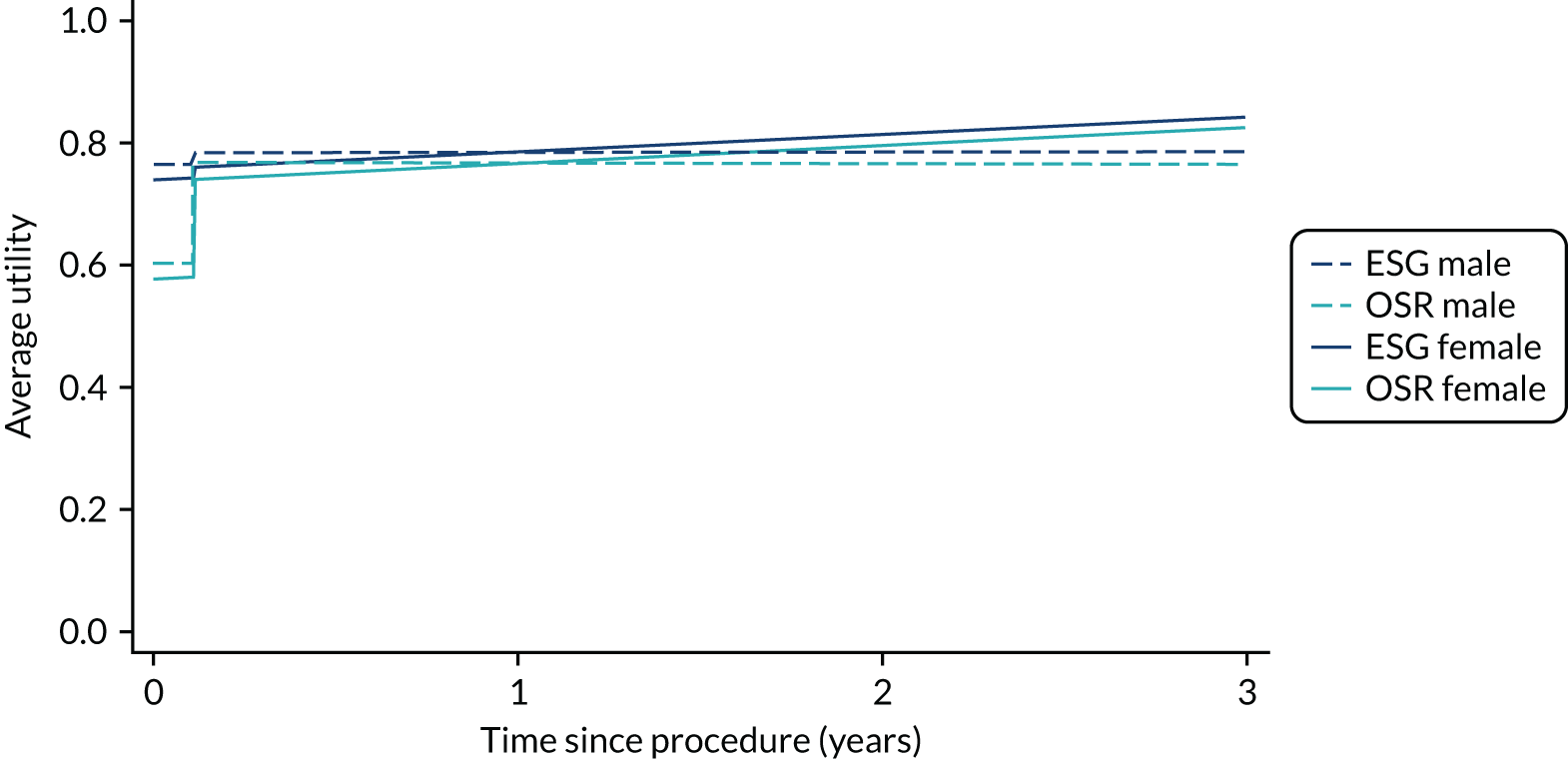
Summary of findings
The main differences in clinical outcomes between interventions were (1) higher readmissions in the short term for ESG patients, and (2) the substantially higher incidence of postoperative complications, longer initial hospital stay and subsequent reinterventions in the OSR group. The latter were predictable differences given the more invasive nature of OSR, and they have implications for the economic analysis reported in Chapter 7.
Chronic thoracic aortic aneurysm patients were at high risk of death, had a range of comorbidities and had somewhat impaired HRQoL measured by EQ-5D-5L utility at the time of the intervention, compared with a UK age- and sex-matched population. Their management was complicated, with most having treatment in more than one segment of the aorta, 40 out of 285 (14.0%) requiring staged procedures and 13 out of 285 (4.6%) undergoing hybrid (open surgery and endovascular) procedures.
As suggested by the Delphi study report in Chapter 2 and initial summaries of the cohort in Chapter 3, there was clear selection of younger patients for the more invasive intervention (OSR). Consistent with older age, ESG was associated with frailty (smaller frame, lower haemoglobin level) and higher levels of comorbidity (valve disease, COPD, higher NYHA class, use of statins). Although some factors were common to both intervention groups (hypertension), there was almost no overlap of others (ascending aorta surgery, CTD), so that the validity of direct comparisons of the interventions is in doubt and unadjusted results should be interpreted cautiously.
Despite this, outcomes for ESG patients were largely comparable to those for OSR patents. The invasive nature of OSR was associated with a slightly higher risk of death within 30 days (11.1% vs. 6.7%) and more complications reported during postoperative hospital stay (240 vs. 98). Although more ESG patients were readmitted to hospital, especially early after discharge, the overall rate of readmission was not significantly greater in this group and fewer ESG patients had reinterventions for aneurysm-related procedures (12 vs. 28). This may be a result of relatively short follow-up post procedure in this study, as well as the stricter selection of older patients for an intervention.
Overall, OSR patients had higher all-cause and aneurysm-related death rates, but the differences were not significant in this study. When the maximum diameter was in the ascending aorta or arch, this was almost invariably treated with OSR. These patients had significantly lower all-cause and aneurysm-related mortality rates than both OSR and ESG patients who had maximal diameter aneurysms in the DTA/thoracoabdominal aorta. The higher mortality rate after OSR of the DTA/thoracoabdominal aorta may have resulted from the selection of OSR for patients with more extensive disease in this segment, including some CTDs, or patients whose aortic anatomy made stent implant difficult. We should highlight that most patients had surgery/stenting in more than one location and these results refer to the location of the maximum diameter. As expected, survival was influenced by age at procedure. Somewhat less predictably, a longer interval between entry to the study and intervention increased the risk of all-cause and aneurysm-related mortality. The timing of intervention is not straightforward, and there may be a tendency for patients with more complicated surgical management requirements to have delayed surgery. For ETTAA patients, post hoc analysis suggested that longer interval was associated with surgery in the DTA/thoracoabdominal aorta (especially OSR in these vessels), staged procedures, current smoking, previous coronary artery bypass grafting (CABG)/percutaneous coronary intervention (PCI) for coronary artery disease and concomitant valve disease. Owing to low power, none of these was a significant risk factor for all-cause or aneurysm-related death individually, but time interval before intervention may capture their combined effects on outcome. In addition, as patients wait longer for the intervention, they may develop both increased aneurysm diameter and length, and increased severity of comorbidities over time.
There was a large initial decrease in HRQoL for OSR patients, a feature reported in other cardiothoracic surgery trials,71,72 including the EVAR 1 trial. 73 This decrease is related to limitations conferred by hospital stay and post-surgical complications. Beyond this period ESG and OSR had similar HRQoL, all else being equal. We should highlight that, in contrast to health economics analyses, we do not impute zeros for patients who have died here. The strongest predictor of postoperative HRQoL was preoperative HRQoL. Both were related to current smoking and NYHA class, which reflects the extent of heart failure by assessing breathlessness, particularly during physical activity. Recommending smoking cessation and optimising the treatment of heart failure may ease these symptoms to some extent, as reported in the OXVASC study,74 and has been shown to improve outcomes after surgery. 75,76
The biggest limitation in these analyses was the small number of patients and events, which caused two main problems; first, there was low power to detect differences in outcomes between intervention groups; and, second, there was limited ability to adjust for confounding in regression models. Moreover, a detailed review of aneurysm scans and patient histories showed that many patients were suitable for only one of the two interventions of interest. To ensure a ‘fair’ comparison between the two intervention groups, it is necessary to exclude any patients who were not suitable for both procedures and apply alternative analysis methods that reduce bias resulting from residual confounding. Propensity score matching or weighting are convenient and relatively straightforward methods, and these are applied in Chapter 6.
Chapter 6 Direct comparison between intervention groups
Introduction
Important differences between the populations undergoing ESG and OSR raised concerns that any comparisons are biased because of unobserved or inadequately controlled confounding. Here we assess the sensitivity of results to alternative analyses that target comparability between groups.
In these analyses it is important to exclude any patients for whom OSR (or ESG) is contraindicated, that is, their probability of receiving OSR (or ESG) is zero (the positivity assumption in propensity methods77). It may be plausible to assume that clinician equipoise exists to some extent in the remaining cohort. Then we can either compare the procedures within this cohort or apply propensity score methods to adjust for any residual confounding.
Aims of comparative analysis of outcomes following a procedure
The aims in this chapter are to:
-
describe how a subset of ETTAA patients who could have had either ESG or OSR was defined; these are referred to as the ‘no-contraindication’ cohort
-
compare the clinical outcomes of survival, readmission and HRQoL for no-contraindication patients in the treatment groups ESG and OSR
-
use propensity score methods to compare the clinical outcomes for no-contraindication patients in the treatment groups ESG and OSR.
Methods
Population
All patients who underwent ESG or OSR procedures as defined in Chapter 5 were eligible. Patients were excluded if they had a zero or close to zero probability of having either of the procedures. Initially, patients were excluded based on variables that were found to be non-overlapping in the exploratory analysis. All remaining ESG patients were then assessed by a cardiac surgeon; anyone aged > 85 years, with BMI < 20 kg/m2 or > 35 kg/m2, with impaired mobility, assessed by the EQ-5D-5L item response severe difficulty walking/self-care, or of NYHA class IV was considered unfit to receive OSR and was excluded. CT/MRI scans for all except seven OSR patients were re-assessed by a vascular surgeon and patients with aneurysms with aortic morphology that could not be managed with ESG were excluded. Seven scans could not be retrieved and analysed by the end of follow-up and these patients were considered suitable for both procedures on the basis of the operation and clinical history CRFs and were included. Finally, because we were interested in the question of whether an open surgical or an endovascular procedure was the better approach for a new patient, any patients for whom the index procedure was the second or third part of a staged procedure were excluded.
Outcomes
Owing to the reduced sample size, outcomes were restricted to all-cause and aneurysm-related mortality, hospital readmissions and HRQoL, as defined in previous chapters.
Statistical analysis
Initially, the final statistical models developed in Chapter 5 were refitted for the comparison cohort only. Then, to reduce any residual bias caused by uncontrolled confounders, we completed a propensity score analysis based on methods described in Leyrat et al. 78 and Mitra and Reiter. 79 Propensity scores were calculated using binary logistic regression on the probability of OSR, including multiple predictors. 80 Theoretically, there is no limit on the number of predictors than can be used to predict propensity, but, owing to the small sample, we restricted the model to all variables related to the main outcomes (mortality and HRQoL) as well as major associates of treatment. The ability of a propensity score to balance the confounding variables between treatment groups was assessed and the model was refined by adding variables to reduce imbalance. 81,82 The final model included age at procedure, sex, height, need for formal/informal care (as a marker of frailty), COPD, NYHA class, diabetes, hypertension, smoking history, maximum aneurysm size, maximum aneurysm location and preoperative time in the ETTAA study. To assess the sensitivity of the results to different methods, we refitted the main outcome analyses by:
-
matching ESG and OSR participants in a 1 : 1 ratio using the nearest neighbour (with replacement) and excluding any patients without a match
-
inverse probability of treatment weighting (IPTW), in which the propensity score was used to weight individual responses as follows: wi = zi/psi + (1 – zi)/(1 – psi), where zi denotes treatment (1 = OSR, 0 = ESG) for individual i
-
IPTW excluding patients with extreme weights (wi > 5) that conferred a large influence on results
-
including the propensity score in regression equations as a covariate. 83
For matching, we used a calliper width of 0.2 SDs of the logit(propensity score).
The analysis was further complicated by incomplete baseline measurements. Following Leyrat et al. 78 our strategy for accommodation of partially complete baseline measurements was as follows:
-
Create 30 complete data sets using multiple imputation.
-
Calculate the propensity score for each complete data set.
-
For predictive mean matching, identify the set of matched patients over all imputed data sets, estimate treatment effects on these matched pairs and combine using Rubin’s rules. 62,63
-
For IPTW or regression adjustment, estimate treatment effects for each imputed data set and combine using Rubin’s rules.
Variables in the imputation models were the same as those included in the propensity score, as well as serum creatinine. We used 30 imputation data sets because of the relatively large number of patients with missing creatinine.
Results
Definition of no-contraindication cohort
Figure 15 shows how the final no-contraindication cohort was derived. Almost all (20/22) CTD patients and all patients who had either surgery in the ascending aorta or concomitant cardiac surgery had OSR. These violated the positivity assumption required for both comparability and propensity score methods (i.e. they were essentially unsuitable for ESG) and were excluded from the analysis before clinician review.
FIGURE 15.
Cohort of no-contraindication ESG and OSR patients.
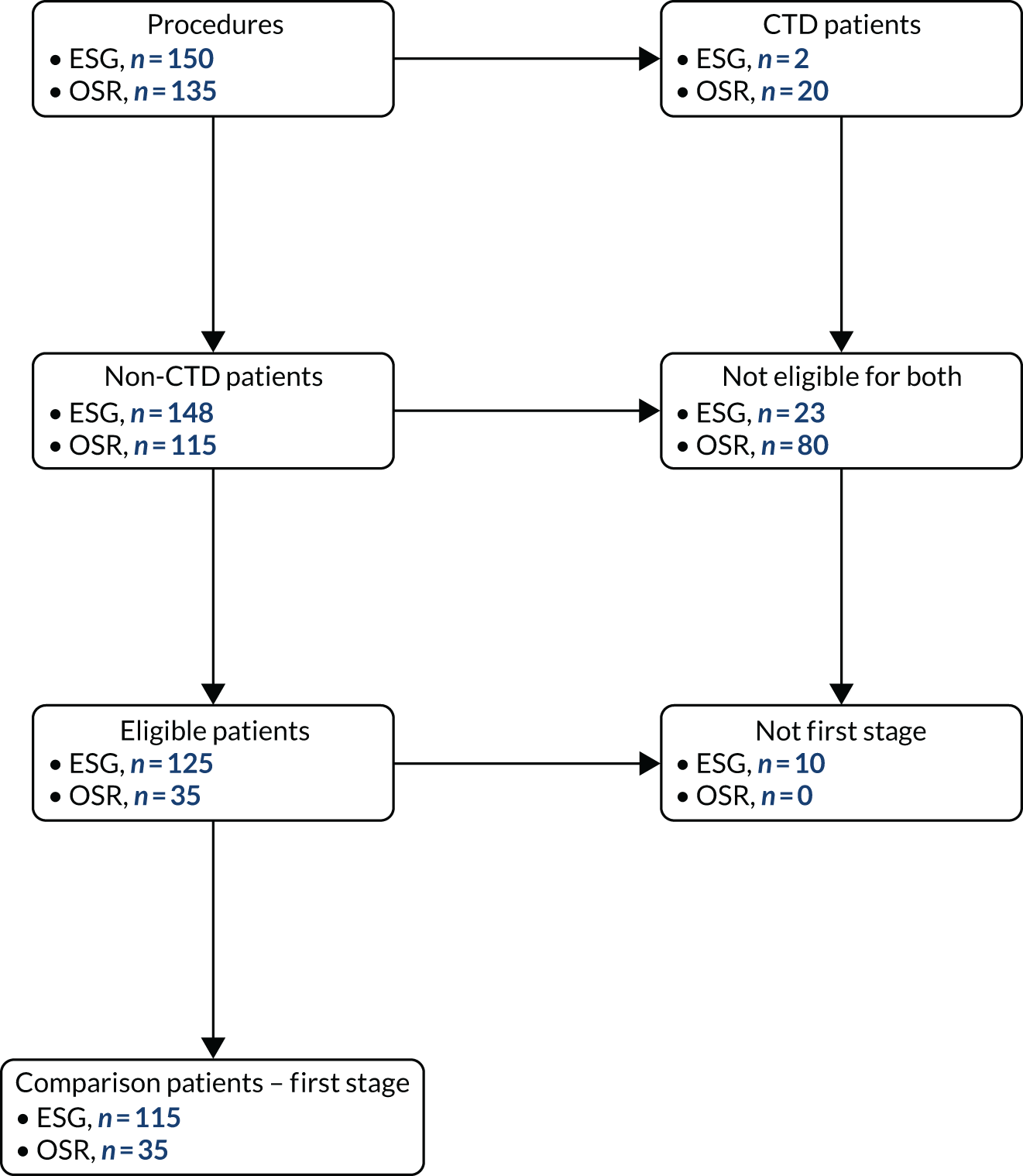
Overall, 35 ESG patients were excluded from the comparison cohort because they had CTDs (n = 2), or the index procedure was stage 2 (n = 8) or 3 (n = 2) of a staged procedure, or the cardiac surgeon considered them unsuitable for OSR (aged > 85 years, n = 1; BMI < 20 kg/m2 or > 35 kg/m2, n = 11; severe mobility problems, n = 9; NYHA class IV, n = 2). The remaining 115 (76.7%) ESG patients were considered suitable for OSR in principle.
Of the 135 OSR patients recruited, 100 were excluded from the comparison cohort because they were not suitable for ESG due to CTDs (n = 20), ascending aorta surgery (n = 57) or concomitant cardiac surgery (n = 7), or based on morphology (n = 16). The remaining 35 (25.9%) were included in the no-contraindication cohort.
Despite the exclusion of patients with contraindications to one or other of the procedures, important differences in baseline variables between the groups remained. The difference between the groups in age at procedure increased to 10.5 years (95% CI 6.9 to 14.1 years), with the mean slightly reduced to 62.6 years for OSR patients and slightly increased to 73.1 years for ESG patients. ESG patients were smaller and were more likely to have diabetes, report a need for informal care and be treated with statins. However, ESG patients were treated quicker, were less likely to have raised serum creatinine and had slightly smaller maximum aneurysm size on average (Table 22).
| Patient subgroup (number of patients in no-contraindication cohort) | p-value | ||
|---|---|---|---|
| ESG (n = 115) | OSR (n = 35) | ||
| Age (years) | < 0.0001 | ||
| Mean (SD) | 73.1 (8.4) | 62.6 (12.2) | |
| Minimum, maximum | 49.8, 85.8 | 33.3, 84.6 | |
| Height (cm) | 0.0205 | ||
| Mean (SD) | 169.5 (10.0) | 174.0 (10.0) | |
| Minimum, maximuma | 149, 188 | 147, 195 | |
| Weight (kg) | 0.0024 | ||
| Mean (SD) | 78.4 (14.5) | 87.8 (18.7) | |
| Minimum, maximumb | 49, 111 | 41, 130 | |
| Care, n (%) | 0.337 | ||
| Informal | 6 (5.2) | 0 (0.0) | |
| None | 109 (94.8) | 35 (100.0) | |
| Diabetes, n (%) | 0.068 | ||
| Non-insulin dependent | 11 (9.6) | 0 (0.0) | |
| None | 104 (90.4) | 35 (100.0) | |
| Statin use, n (%) | 0.016 | ||
| Yes | 82 (71.3) | 17 (48.6) | |
| No | 33 (28.7) | 18 (51.4) | |
| Preoperative time in the ETTAA study (months) | 0.1641 | ||
| Median (quartiles) | 1.8 (0.1, 7.4) | 4.7 (0.1, 13.2) | |
| Minimum, maximum | 0, 49.1 | 0, 36.5 | |
| Serum creatinine | 0.0045 | ||
| Mean (SD) | 86.9 (30.8) | 107.7 (42.9) | |
| Minimum, maximumc | 44, 194 | 54, 221 | |
| Maximum aneurysm size (cm) | 0.0341 | ||
| Mean (SD) | 6.12 (1.20) | 6.62 (1.28) | |
| Minimum, maximum | 3.7, 9.7 | 4.2, 10.3 | |
| Maximum aneurysm site, n (%) | 0.002 | ||
| Ascending aorta/arch | 2 (1.7) | 6 (17.1) | |
| Descending aorta/suprarenal | 113 (98.3) | 29 (82.9) | |
Effect of confounding on propensity score methods
A propensity score was developed for each of the 30 imputed data sets. The residual between-group differences had important implications for propensity score methods; in the 30 imputed data sets, imbalance (difference in propensity scores > 0.1) was observed for two to seven variables. The main variables with residual imbalance between groups were time between consent and procedure (30 data sets), age at procedure (26 data sets) and maximum aneurysm size (24 data sets). This meant that, for propensity score matching, only 46–54 patients formed matched pairs in the imputed data sets, making this method inefficient. IPTW reweights outcomes according to their propensity to be matched and, therefore, included all patients in the no-contraindication cohort, but it can give undue influence to patients with propensity scores close to 0 or 1; IPTW excluding patients with extreme weights is a good compromise and resulted in the loss of only seven to eight patients. Including propensity score in the analysis models retains all patients but may be less effective in adjusting for confounding. 84
Survival and hospital readmissions
During the follow-up of 150 no-contraindication patients, 31 ESG and 8 OSR patients died, of whom 14 and 5, respectively, died from aneurysm-related causes. Eight (7.0%) ESG and three (8.6%) OSR patients died within 30 days. In exploratory analysis (Figure 16), there were no differences between the groups in time to death overall (log-rank p = 0.6608) or aneurysm-related death (log-rank p = 0.7533).
FIGURE 16.
Kaplan–Meier cumulative incidence curves for post-intervention deaths from all-cause and aneurysm-related deaths by ESG and OSR groups (no-contraindication patients only). (a) Kaplan–Meier deaths from any-cause; and (b) Kaplan–Meier aneurysm-related deaths.
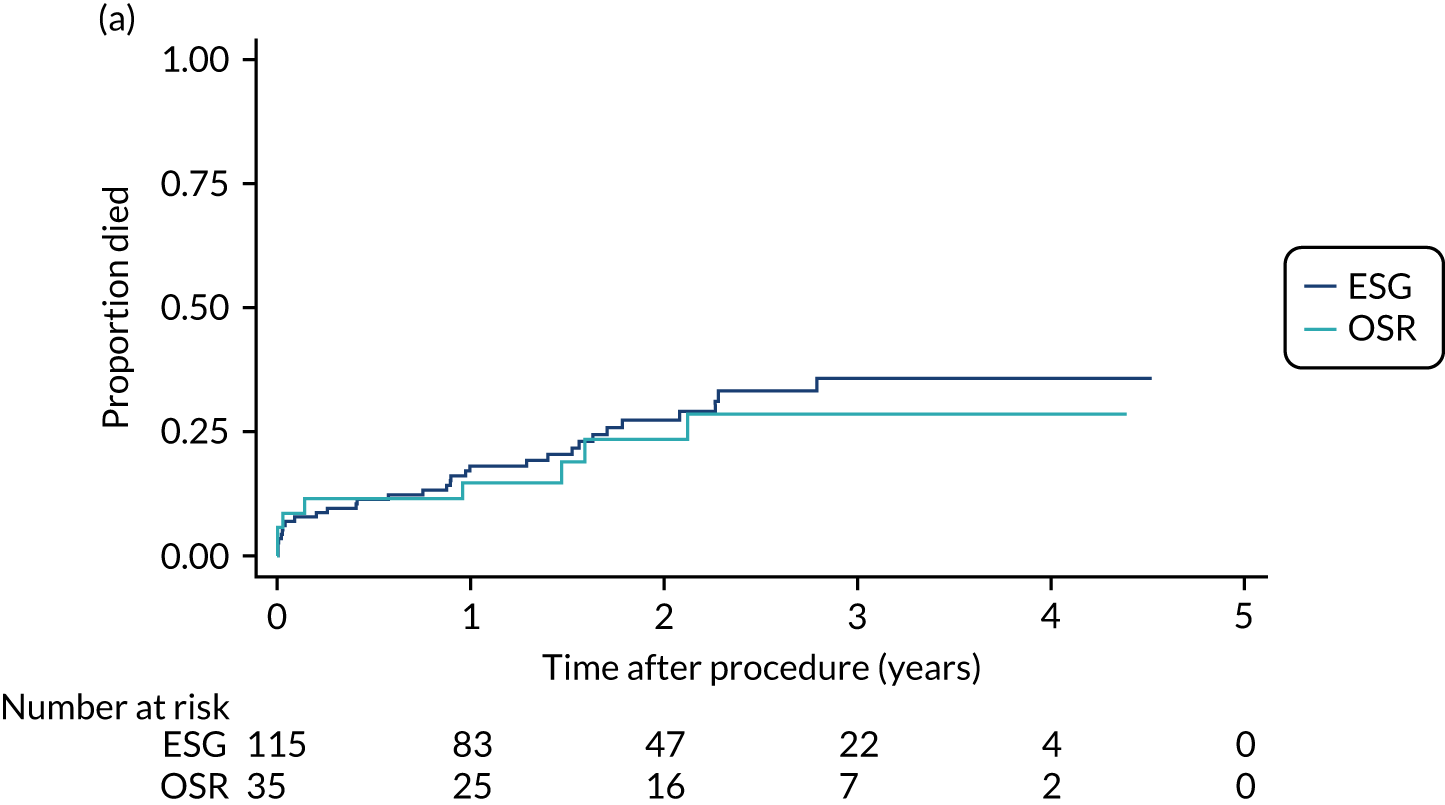
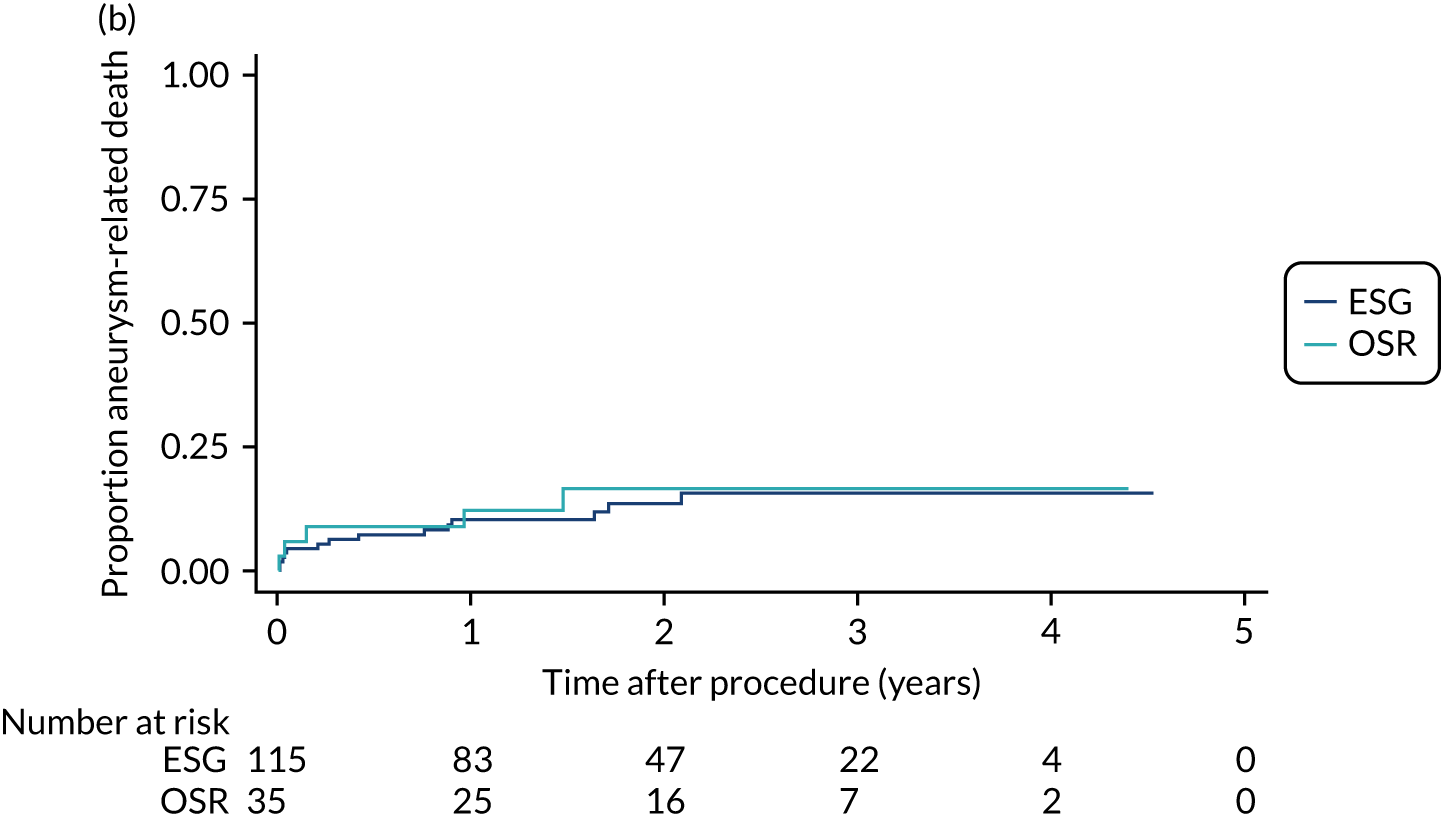
Survival: results of modelling
Table 23 shows the adjusted HRs for OSR relative to ESG for all-cause and aneurysm-related deaths in the no-contraindication cohort. The results for the full data set are included for comparison. For the no-contraindication cohort alone, there was a small decrease in all-cause mortality for OSR, but this may result from inadequate adjustment of the 10-year age difference. Once propensity scores were included, risk of death for OSR patients was generally higher, although estimates of HRs vary and are measured imprecisely; CIs are particularly wide for the propensity matching method because of the very small sample sizes. Adjusting for the propensity score in the no-contraindications cohort results in estimates close to the full data set, but may be subject to residual bias. 84 Despite these differences, the CIs overlap substantially and no clear differences between procedures emerge.
| Model | All-cause deaths (adjusted for age, NYHA class and time from consent to procedure) | Aneurysm-related deaths (adjusted for sex and time from consent to procedure) | ||
|---|---|---|---|---|
| HR (95% CI) | z-test p-value | HR (95% CI) | z-test p-value | |
| All patients: complete-case analysis (n = 264) | 1.27 (0.78 to 2.09) | 0.332 | 1.59 (0.86 to 2.96) | 0.140 |
| All patients: MICE for missing covariates (n = 285) | 1.28 (0.79 to 2.07) | 0.314 | 1.59 (0.86 to 2.96) | 0.140 |
| Patients with no contraindications: complete case (n = 137) | 0.87 (0.36 to 2.09) | 0.754 | 1.20 (0.43 to 3.34) | 0.733 |
| Patients with no contraindications, MICE for missing covariates (n = 150) | 0.91 (0.39 to 2.11) | 0.823 | 1.20 (0.43 to 3.43) | 0.733 |
| Patients with no contraindications: MICE for missing covariates and PS matcheda (n = 48–54) | 1.39 (0.38 to 5.11) | 0.620 | 2.51 (0.32 to 19.6) | 0.379 |
| Patients with no contraindications: MICE for missing covariates and IPTW (n = 150) | 1.43 (0.50 to 4.07) | 0.510 | 1.56 (0.39 to 6.25) | 0.532 |
| Patients with no contraindications: MICE for missing covariates and IPTW excluding extremesb (n = 142–143) | 1.09 (0.40 to 2.96) | 0.865 | 1.54 (0.50 to 4.77) | 0.457 |
| Patients with no contraindications: MICE for missing covariates and PS adjusted (n = 150) | 1.23 (0.47 to 3.24) | 0.679 | 1.58 (0.43 to 5.77) | 0.492 |
Readmissions after discharge from the index procedure
Table 24 reports readmissions after discharge from the index procedure in the no-contraindication cohort. The results are very similar to those for the overall cohort for ESG as most patients remain in the analysis. The subset of OSR patients with no contraindications to ESG have lower readmission rates, which is likely to be due to the younger age distribution. Adjusting for age and sex, the relative readmission rate for OSR compared with ESG is 0.53 (95% CI 0.23 to 1.21; p = 0.132), and restricted to aneurysm-related admissions this is 0.22 (95% CI 0.04 to 1.30; p = 0.094). The number of ESG patients readmitted was greater for any cause (p = 0.0709) and for aneurysm-related causes (p = 0.1627).
| Outcome | ESG group (N = 115) | OSR group (N = 35) |
|---|---|---|
| Total time at risk (years) | 201.9 | 63.4 |
| All readmissions, number (rate/year) | 87 (0.43) | 16 (0.25) |
| People with at least one readmission, n (%) | 47 (40.9) | 8 (22.9) |
| Readmissions, definitely/probably aneurysm related, number (rate/year) | 30 (0.15) | 2 (0.03) |
| Patients readmitted, definitely/probably aneurysm related, n (%) | 19 (16.5) | 2 (5.7) |
| Non-fatal ruptured aneurysms, n | 0 | 0 |
| Non-fatal dissected aneurysms, n | 2 | 0 |
| Non-fatal neurological events (all transient ischaemic attack), n | 3 | 0 |
Post-procedure health-related quality of life
Descriptive analysis
Postoperative HRQoL was available for 129 no-contraindication patients, who completed a total of 548 questionnaires. The results of refitting the final model from Chapter 5 using only these patients are shown in Table 25 (full models can be obtained from the authors). The estimated difference between ESG and OSR in no-contraindication patients within 6 weeks of procedure and after 6 weeks is reported. In common with the full data analysis in Chapter 5, there was a larger decrease in HRQoL for OSR patients within 6 weeks. For this subset of patients, the difference between procedures was much larger both within and beyond the 6-week ‘recovery’ period and this was significant for many of the comparison models fitted. In particular, within 6 weeks OSR patients reported a very large and highly significant decrease in HRQoL in this no-contraindications cohort. After 6 weeks, the difference between ESG and OSR patients decreases, but is still of the order of 0.1–0.15 units and is significant across most models.
| Model | Difference in EQ-5D-5L utility (OSR – ESG) in first 6 weeks | Difference in EQ-5D-5L utility (OSR – ESG) after first 6 weeks | ||
|---|---|---|---|---|
| Estimate (95% CI) | z-test p-value | Estimate (95% CI) | z-test p-value | |
| All 285 patients: complete case | –0.160 (–0.199 to –0.121) | < 0.001 | –0.018 (–0.065 to 0.028) | 0.433 |
| All 285 patients: MICE for missing covariates | –0.167 (–0.223 to –0.102) | < 0.001 | –0.018 (–0.062 to 0.026) | 0.416 |
| No contraindications (n = 150): complete case | –0.255 (–0.371 to –0.139) | < 0.001 | –0.098 (–0.179 to –0.017) | 0.020 |
| No contraindications (n = 150): MICE | –0.261 (–0.371 to –0.151) | < 0.001 | –0.102 (–0.177 to –0.028) | 0.007 |
| No contraindications (n = 150): MICE and PS matched | –0.344 (–0.509 to –0.178) | < 0.001 | –0.091 (–0.200 to 0.017) | 0.100 |
| No contraindications (n = 150): MICE and IPTW | –0.248 (–0.366 to –0.130) | < 0.001 | –0.085 (–0.163 to –0.008) | 0.032 |
| No contraindications (n = 150): MICE and IPTW excluding extremes | –0.307 (–0.428 to –0.185) | < 0.001 | –0.146 (–0.241 to –0.050) | 0.003 |
| No contraindications (n = 150): MICE and PS adjusted | –0.277 (–0.402 to –0.152) | < 0.001 | –0.118 (–0.212 to 0.024) | 0.014 |
Summary of findings
The main finding of this chapter were that (1) ESG patients were more likely to be readmitted to hospital during follow-up and (2) OSR patients had slightly higher risk of death and substantially poorer HRQoL, both early post operation and beyond the first 6 weeks, even though they were 10 years younger on average.
This chapter aimed to identify a cohort of comparable ESG and OSR patients, which might be used to reflect equipoise among clinicians. After excluding contraindications to one of the procedures, the sample size was reduced substantially, particularly for OSR patients, resulting in very limited power for comparisons. Moreover, ESG and OSR groups in the no-contraindication cohort had important differences in age and age-related comorbidities, which suggests that age is an important factor in decisions about which intervention is appropriate in clinical practice.
Although propensity score methods are designed to reduce bias due to confounding in observational studies, they can be unreliable for small samples (in common with many statistical methods). In propensity score matching, only 46 to 54 patients formed matched pairs, resulting in both low power and a more highly selected cohort. IPTW uses all patients and excluding those with very high weights resulted in loss of only seven to eight patients, while adjusting for propensity score in the analysis models retained all. Thus, the weighting and adjustment methods are likely to yield the more reliable results.
From the information available in the ETTAA study, just over half (150/285) of the patients who had an intervention may have had either procedure; we cannot tell from the data whether clinicians were in equipoise, and the decision around ESG or OSR appears to be largely driven by patient age. Our results provide estimates of relative outcomes for these two procedures, which vary between different analysis models, and are imprecisely estimated due to the small samples. The results generally align with the full data analysis (at least qualitatively) from Chapter 5. Concerns about residual confounding remain, but only a randomised trial would provide unbiased comparisons, and this is unlikely to be feasible given the low prevalence of CTAA. Cost-effectiveness analysis will provide further insight into the value of these interventions.
Chapter 7 Health economic analysis
Overall aims of the economic evaluation
This chapter reports the health economic analysis that was performed as part of the ETTAA study using the cohort of patients with no contraindications to either procedure.
Planned analysis
Originally, and according to protocol, the aim of this analysis was to estimate the cost–utility of two alternative surgical methods of treating patients with arch/descending thoracic aortic aneurysm. In the economic evaluation, the costs and effects associated with ESG were to be compared with those associated with OSR to define the optimal management strategies for patients who could be eligible for both treatments and for whom, therefore, there was a choice.
The economic analysis aimed to compare the incremental cost per QALY gained with different threshold values for society’s willingness to pay for a QALY, including those commonly adopted by NICE. 85 Both a ‘within’-study patient-level analysis and a state-transition model to extrapolate findings over patients’ lifetimes were proposed. To facilitate this analysis, data were collected from individual study participants, participating centres and external sources in order to estimate costs. QALYs were to be estimated from serial responses to the EQ-5D-5L.
Resource utilisation and the associated costs were collected from an NHS and PSS perspective and concentrated on the micro-costing of the surgical procedures themselves in secondary care. The utilisation of any subsequent secondary, primary and personal social services during the follow-up period was also recorded. Data on resource use were captured on bespoke CRFs [see Report Supplementary Material 5 for CRFs (use of secondary care services, and incidence and frequency of cost-generating events, e.g. hospital readmissions)]. Further CRFs were completed at 3, 6, 12, 18, 24, 36, 48 and 60 months to capture the use of primary care and PSS. The unit costs of resources were obtained from standard sources such as NHS reference costs, HRG tariffs, manufacturers/suppliers and from the centres themselves. For each participant, measures of resource use were to be combined with unit costs to estimate the total cost for that participant.
In terms of QALY estimation, EQ-5D-5L scores were collected prospectively at baseline and at 1, 3, 6, 12, 18 and 24 months, and then annually until the study ended. The responses for each participant were converted into health state utilities using UK population tariffs via crosswalk mapping58 and used to estimate QALYs using the area-under-the-curve approach. 86
For the within-trial analysis, bootstrapping methods were to be used to estimate the imprecision around estimates of incremental costs, QALYs and incremental cost per QALY. For the model-based analysis, a probabilistic sensitivity analysis was to be conducted. The results of both the within-trial and the model-based analyses were to be presented as plots of incremental cost and QALY and cost-effectiveness acceptability curves.
Revised analysis
To ensure a fair comparison between OSR and ESG as a primary procedure, the cohort of patients who had a surgical procedure (presented in Chapter 6) were assessed by clinical experts to determine whether they would have been eligible to receive both procedures (see Figure 15). Chapter 6 describes reasons that patients who received one procedure would have been ineligible for the other and the numbers of participants excluded for that reason. In brief, the reasons why OSR patients were ineligible for ESG included aneurysm repair extending into the ascending aorta, concomitant cardiac procedures and unsuitable aortic morphology. The reasons why ESG patients were ineligible for OSR included BMI < 20 kg/m2 or > 35 kg/m2; NYHA class IV dyspnoea; or age ≥ 85 years. In addition, 10 ESG patients had primary procedures that were the second or third stage of a planned staged procedure. These patients were excluded as they would have biased the analysis of costs and QALYs. Overall, 115 ESG patients and 35 OSR patients were judged potentially eligible for both procedures.
As there were only 35 no-contraindication participants in the OSR group, the results of a comparative cost–utility analysis were judged to be imprecise and potentially misleading. 87 Therefore, the aims of the economic evaluation were recast to:
-
Provide a non-comparative, descriptive ‘within-study’ analysis of the available cost and QALY data for participants eligible to receive both OSR and ESG as the first (index) procedure, without formally comparing the two procedures statistically. Specifically, the following were estimated –
-
health-care costs of primary surgical procedures (average and median costs per surgical group)
-
health-care and PSS costs that were definitely or probably related to the index procedure/the aneurysm at all follow-ups including average total costs at 12 months by surgical group
-
utilities at all follow-up points and average total QALYs at 12-month follow-up by surgical group.
-
-
Estimate a regression model to explore the predictors of NHS costs for both OSR and ESG that may aid future economic evaluation modelling studies.
-
Estimate the costs and benefits of obtaining further information (about overall cost-effectiveness and costs and QALY individually) based on data obtained from the ETTAA study via value of information (VoI) analyses. 88
Given the potentially misleading results from conducting a comparative cost-effectiveness analysis, none was attempted. 85 Instead, we sought to utilise the limited information from the ETTAA study and conduct a VoI analysis in order to inform proposed future research into the relative cost-effectiveness of OSR compared with ESG and to identify potential sources of uncertainty. However, the results of this analysis should be seen in the context of a lack of a fair comparison in the ETTAA population, and are presented to aid any future research.
All health economic analysis has been designed and conducted to best practice conforming to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS). 89
Within-study descriptive analysis of costs and quality-adjusted life-years methods
Health economic data collection
Identification and measurement of resource use
Resource utilisation was identified and measured using information derived from expert clinical opinion and from data collected in study specific CRFs. Three categories of resource use were considered:
-
Resources necessary to provide the primary procedures including medical devices, surgical equipment, staff and consumables.
-
Resources necessary to provide postoperative care until hospital discharge. This included length of hospital stay during the primary admission, including critical and specialist unit bed-days. Furthermore, all reinterventions associated with the aneurysm during the primary admission, including time in the operating theatre or endovascular suite, devices and consumables, were included in this category.
-
Use of health and personal social care until final follow-up, including readmissions related to the aneurysm. This category also included costs of resource utilisation within primary, community, secondary and PSS settings regardless of whether this was related to the aneurysm or other conditions.
Resource use in the primary surgical procedures
The costs of the two surgical interventions (OSR and ESG) were micro-costed for each participant included in this part of the study. 90,91 Micro-costing attempts to measure costs of a service as accurately as possible. The process involves identifying all of the resources involved in the provision of care, accurately measuring each resource and valuing the resources used. 92 The CRF captured participant-level data on theatre time, type of graft, blood products used and perioperative complications. Other information needed for the micro-costing came by considering what resources would be needed for a typical surgical procedure of each type. These data were collected using an iterative series of resource use capture pro formas (see Report Supplementary Material 5) and expert clinical opinion. Resource utilisation included staffing mix, reusable (e.g. some surgical instruments) and disposable (e.g. grafts) equipment and the overheads (heat, power, light, cleaning) of theatres. For capital and reusable equipment, the equivalent annual costs were estimated based on the life expectancy of the equipment, assuming a 3.5% discount rate per year. 85 The equivalent annual cost was then divided by the expected annual usage to obtain a cost per recipient. Procedure resource use is presented in Appendix 11.
Resource use postoperatively until discharge
Resource use postoperatively until hospital discharge was captured at the patient level using two study-specific CRFs. The post-procedure and discharge CRF (see Report Supplementary Material 5) captured the number of days in hospital, days in an ICU or a HDU, postoperative blood product use, and any adverse events (including cardiac and renal failure). It also captured the use of any diagnostic investigations. If a patient suffered an adverse event requiring a return to theatre, the theatre time and the reason for return to theatre were captured in the return-to-theatre CRF. These events were micro-costed using the same methods as described above for the primary surgical procedure. Postoperative resource use until discharge is presented in Appendix 11.
Use of health and personal social care resources during follow-up
Use of health and personal social services up until final follow-up or the end of the study was collected at the patient level using the study-specific follow-up CRF (see Report Supplementary Material 5) at 3, 6, 12, 18, 24, 36, 48 and 60 months post discharge from the index surgical procedure. This included the use of primary and community care [general practitioner (GP) and nurse visits both at a health care facility and at home, physiotherapy and occupational therapy community visits], secondary care [accident and emergency (A&E) visits, outpatient appointments related to the primary procedure, diagnostic imaging], and formal and informal social care (hours per week) up to patient death, censoring or the end of the study. These data are presented for each resource use category up to each follow-up point in Appendix 11. If a patient was readmitted to hospital for reasons probably or definitely related to aneurysms, a hospital admission CRF captured length of stay by level of care (general ward, HDU and ICU). Details of the number of hospital readmissions and reasons for each surgical procedure are also presented in Appendix 11.
If a patient underwent another procedure during follow-up, this was captured using the same CRFs as used for the primary procedure. This is recorded in Appendix 11 as additional procedures. In addition, following discussion with experts, we assumed that each patient who underwent an ESG procedure had a CT scan and a vascular outpatient visit at 1 month post discharge and annually thereafter. Similarly, it was assumed that a patient who had undergone a primary OSR procedure had a CT scan and a cardiology outpatient appointment at 6 months post discharge and annually thereafter.
Formal and informal caregiving
Data on the provision and number of hours per week of formal care (e.g. social worker, care assistant) and informal care from family or friends were collected on the study CRF. These were then extrapolated for each follow-up period (i.e. each patient’s care was assumed to remain the same across all subsequent time periods) and multiplied by an hourly cost of formal and informal caregiver time.
Valuation of NHS and informal caregiving resource use
Unit costs
Unit costs were obtained from a variety of sources, including national databases93 and published studies94 (see Appendix 11). All unit costs were inflated, where necessary, to 2018–19 prices using the health care and community health services inflation index94 and are reported in Great British pounds.
Primary surgical procedure
The unit costs used to value the required resources utilised in the index procedure are presented in Appendix 11.
Unit costs postoperatively until hospital discharge
The unit costs of a day in a general ward, HDU or ICU were based on the NHS Reference Costs 2017 to 201895 and inflated accordingly. The unit costs of any diagnostic tests and investigations (e.g. X-ray, CT scans) likewise were taken from the NHS Reference Costs 2018 to 2019. 93 Postoperative blood product use was costed from the NHS Blood and Transplant Price list 2018/2019. 96
With the exception of a return to theatre, any adverse events occurring in the admission were not explicitly costed as it was assumed that these would be adequately captured by prolonged length of hospital stay and by the costs of tests and investigations described above. If a patient suffered an adverse event requiring a return to theatre, then the same methods were used to identify the unit costs of resources used. Unit costs post procedure until discharge, return to theatre costs, are presented in Appendix 11.
Unit costs following discharge
The unit costs of NHS primary and community care, NHS secondary care, social service formal care and informal care by family members/friends are presented in Appendix 11.
For use of primary and community care, the unit costs varied according to type of contact (e.g. GP, nurse) and where the contact took place (health-care setting or participant’s home). The unit costs of these were obtained from a standard source collated for use in economic evaluation. 94 For the use of secondary care services, including A&E visits, outpatient appointments and diagnostic imaging related to the aneurysm, the costs came from NHS Reference Costs 2018 to 2019. 93
The unit cost of formal caregiver time was obtained from the PSS Research Unit publication, Unit Costs of Health and Social Care. 94 The national minimum wage of £7.83 was taken as a proxy for the value of informal caregivers’ time. 97
For each of the hospital admissions, a HRG approach was utilised, whereby weighted mean costs for patients for each HRG were derived from the NHS Reference Costs 2018 to 2019 (see Appendix 11). 93
If a patient underwent another full ESG or OSR procedure, then the same micro-costing approach was used as described for the index procedure (these costs are reported separately from hospital admissions).
Estimation of total costs per patient
The cost analysis was divided into three stages (primary surgical procedure, post procedure until discharge and follow-up) based on a chronological sequence of events related to the procedures.
For each study participant, all components of costs stratified by category of resource use were computed by multiplying the units of resource use by their unit costs. These were then summed for each stage of the cost analysis. The primary procedure cost is presented as the average costs for each element of resource use with a subsequent average total cost per participant. The same approach was used to present the costs for the post-procedure-until-discharge stage. These two stages of the costing are based on all of those eligible for inclusion in this comparative analysis (OSR, n = 35; ESG, n = 115).
The follow-up CRFs for costs asked patients to report resource use ‘since the last follow-up’. Therefore, if a CRF was not completed at one planned visit, data could be retrieved at the subsequent visit.
The cumulative follow-up costs are reported at each follow-up period (e.g. at 1, 3, 6, 12, 18, 24, 36, 48, 60 months post discharge) using all data available at each follow-up time point. However, we note that cost estimates for informal care are a concern, as the intensity of care as reported on the CRF may be imprecise (in some instances, for example, patients reported 24-hour care 7 days a week). Hence, although we report costs from an NHS and PSS perspective, the PSS costs may be less reliable.
The costs for the three stages (primary surgical procedure, post procedure until discharge and follow-up) were then summed over all resource use categories to obtain a total annual cost for each participant at 12 months from both an NHS and an NHS and a PSS perspective. This was because the minimum follow-up for the study was 12 months, with variable duration of follow-up thereafter.
Derivation of descriptive cost statistics
For each surgical group, the mean (SD) and median [interquartile range (IQR)] costs are presented for each cost element. The analysis of the costs of the study was conducted using Stata® 15.1 (StataCorp LP, College Station, TX, USA).
Quality of life
The EQ-5D-5L questionnaire was administered at baseline and at 1, 3, 6, 12, 24, 36 and 48 months following a procedure. If a participant died, then they were assigned a zero score from the date of death. For each participant, utilities were collected across three distinct pathways:
-
Pre-procedure follow-ups after consent at 3, 6, 12, 18, 24, 36, 48 months.
-
After the index procedure, follow-up was ‘reset’ and occurred at 1, 3, 6, 12, 18, 24, 36 and 48 months after the index procedure.
-
After each additional procedure (second or third procedure), follow-up was ‘reset’ and occurred at 1, 3, 6, 12, 18, 24, 36 and 48 months after the additional procedure.
In some cases, however, the ‘resets’ did not occur as planned, resulting in misaligned follow-ups. To aid analysis and to use as many available data as possible, the time of all follow-ups was calculated from the date of primary procedure; that is, date of the primary procedure was taken to be time zero. Time of follow-up after the primary (index) procedure was then categorised and aligned to nominal time points 1, 3, 6, 12, 18, 24, 36 and 48 months after the index procedure. For example, 1-month follow-ups were those that actually occurred between 0 and 60 days after the index procedure. In addition, as in the analysis of clinical outcomes in Chapters 5 and 6, baseline was taken as the most recent utility measure prior to the index procedure. Appendix 11 provides the categories of the time post procedure assigned to the different nominal follow-up time points.
The mean (SD) and median (IQR) of the EQ-5D-5L utility scores were calculated at nominal measurement times (i.e. baseline and 1, 3, 6, 12, 24, 36 and 48 months) using the measurements available at those time points. Owing to the small sample size, especially in the OSR group, multiple imputation of missing outcomes was not undertaken.
The mean (SD) and median (IQR) QALYs at 12 months were estimated for each surgical group for those patients who had a follow-up at baseline, 1 month, 12 months and any follow-up point in between, including a score of zero if they had died during follow-up. QALYs for each participant were estimated as the area under the curve, constructed by interpolating between utilities at nominal measurement times. There was no comparison between total QALYs for each surgical procedure because of the limited number of data available. The analysis of the EQ-5D-5L and QALY data was conducted using R, version 6.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Missing cost and quality-of-life data
The data available for comparative purposes were very limited, and there were considerable missing data for study participants (Table 26), particularly after 12 months’ follow-up. Every patient who provided utility data at each follow-up also provided a follow-up CRF for resource use and hence for costs. There was only a very small number of patients (n = 11) who provided resource use and not utility data for some of the follow-up points. Up to 12 months, there was a larger proportion of missing data in the OSR cohort relative to the ESG cohort for both resource use and quality-of-life data. An examination of the data highlighted that patients who were in hospital at 1 month in the OSR group had subsequent missing data points for both resource use and quality of life. The reasons for this are unknown but could include not being sent a questionnaire, being in hospital or being too ill to complete a questionnaire. As expected, there are more censored data at later follow-up points.
| Follow-up period | ESG group | OSR group | ||||||
|---|---|---|---|---|---|---|---|---|
| Complete data | Cumulative deaths | Missing | Censored | Complete data | Cumulative deaths | Missing | Censored | |
| Baseline | 115 | 0 | 0 | 0 | 35 | 0 | 0 | 0 |
| 1 month | 74 | 8 | 33 | 0 | 16 | 4 | 15 | 0 |
| 3 months | 76 | 9 | 29 | 1 | 19 | 4 | 11 | 1 |
| 6 months | 81 | 11 | 19 | 4 | 24 | 4 | 6 | 1 |
| 12 months | 74 | 17 | 14 | 10 | 20 | 4 | 8 | 3 |
| 18 months | 59 | 19 | 10 | 27 | 16 | 5 | 4 | 10 |
| 24 months | 35 | 22 | 14 | 44 | 11 | 7 | 4 | 13 |
| 36 months | 17 | 23 | 12 | 63 | 5 | 7 | 5 | 18 |
| 48 months | 3 | 23 | 6 | 83 | 0 | 7 | 4 | 24 |
Given, the nature and extent of missing data, no imputation was attempted. The exceptions to this were that if the missing data were related to standard resources that are normally used during the treatment pathway, it was assumed that these resources were used and, therefore, costs were added. Furthermore, where length of theatre time and length of stay were missing, averages of similar events were used for each procedure type.
As specified previously, to maximise the data used to estimate EQ-5D-5L QALYs utilities at baseline, 1, 3, 6 or 12 months were utilised.
Results of descriptive analysis
Cost analysis at all time points
The total average cost per recipient by each area of resource use for each of the three stages of the study is presented in Appendix 11. This shows that costs of theatre time and the corresponding staff time are higher on average for the OSR procedure, as are the costs of blood products. Stent costs are much higher on average for the ESG group relative to the average cost of grafts in the OSR group. The mean (SD) total costs of the primary OSR procedure was £17,239 (£8043) and for the primary ESG was £26,536 (£9877). The cost of the ESG procedure was largely driven by the stent costs, which accounted for 79% of the average total cost of an ESG procedure.
Appendix 11 shows the total average costs per patient postoperatively until discharge for each surgical procedure broken down into categories of resource use. With the exception of return-to-theatre costs, average costs were higher in all resource use categories in the OSR group relative to ESG. The largest cost driver for OSR procedures was ICU resource use, which accounted for 71%. Furthermore, a larger percentage of patients in the OSR group were transferred to other hospital settings for further treatment (e.g. rehabilitation).
The average total costs after discharge by follow-up period and resource use category are shown in Appendix 11. The average total NHS costs for the first 12 months following the primary procedure were higher in the ESG group. These were driven by the extra costs of patient admissions to hospital, including the costs of additional procedures (85% of total NHS costs).
There was no discernible pattern in the average total costs of formal and informal care. These costs may also be inaccurate.
The average total costs for the primary procedure, post procedure until discharge and follow-up at 12 months are presented in Table 27 for the participants who were suitable for either procedure. Particularly for the OSR group, mean costs are highly skewed to the right for most categories because of a small number of participants who incurred very high costs.
| Resource use cost (by stage) | ESG group | OSR group | ||
|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| Sample size, n | 115 | 35 | ||
| Primary procedure cost | 26,536 (9877) | 24,733 (19,300–35,173) | 17,239 (8043) | 15,359 (10,350–21,874) |
| Post procedure until discharge cost | 7484 (7848) | 5516 (2873–8526) | 28,636 (23,083) | 13,997 (10,480–28,040) |
| Total NHS cost up to discharge | 34,020 (14,301) | 30,620 (25,180–42,806) | 45,875 (43,023) | 36,488 (23,093–48,724) |
| Follow-up (up to 12 months) | ||||
| Sample size, n | 91 | 24 | ||
| Follow-up cost (NHS resources) | 5206 (11,585) | 696 (495–1387) | 5039 (11,994) | 1105 (784–1821) |
| Formal care | 202 (794) | 0 (0–0) | 9221 (37,547) | 0 (0–0) |
| Informal care | 1234 (3817) | 0 (0–40) | 1729 (3254) | 265 (0–1295) |
| Total follow-up cost | 6642 (11,927) | 825 (506–7958) | 15,989 (38,247) | 2213 (1000–14,326) |
Analysis of quality of life
Owing to the very small numbers of patients reporting utility values at all time points, particularly in the OSR group, utilities [means (SD) and medians (IQR)] are reported for every patient who completed an EQ-5D-5L questionnaire at each time point, or were known to have died by a given time (Table 28). Patient numbers include all those who had completed a utility CRF at each time point, including a zero value for those who died. This excludes those who were censored for any reason. Utility values were lower at all follow-up points for OSR than for ESG. At 1 month post surgery, HRQoL dropped from baseline, with a fall of > 50% in HRQoL in the OSR group. HRQoL also reduced at 1 month in the ESG group, although this drop was not as large (15%). HRQoL in both groups increased until 6 months post procedure in the OSR group and until 12 months post procedure in the ESG group as people recovered from their primary procedure. Average utilities in both groups for the remaining follow-ups then started to decrease. This may be because of the impact of complications, readmissions and deaths (which are assigned a zero score). It must be noted that the sample sizes were small and so estimates were imprecise and potentially unreliable, particularly after the first 12 months. Table 28 shows that the estimated mean QALYs at 12 months were larger in the ESG group than in the OSR group, although this was based on very small (and possibly not representative) numbers of patients who had complete data up to 12 months.
| Time point | EQ-5D-5L utility scores | |||||
|---|---|---|---|---|---|---|
| Patient numbers (n)a | EQ-5D-5L, mean (SD) | EQ-5D-5L, median (IQR) | ||||
| ESG alive (dead) | OSR alive (dead) | ESG | OSR | ESG | OSR | |
| Baselineb | 111 (0) | 35 (0) | 0.75 (0.24) | 0.68 (0.24) | 0.77 (0.68–0.91) | 0.74 (0.64–0.84) |
| 1 month | 72 (8) | 15 (4) | 0.64 (0.32) | 0.33 (0.28) | 0.74 (0.51–0.86) | 0.29 (0.05–0.56) |
| 3 months | 73 (9) | 19 (4) | 0.68 (0.32) | 0.49 (0.30) | 0.79 (0.63–0.87) | 0.56 (0.31–0.70) |
| 6 months | 80 (11) | 22 (4) | 0.65 (0.33) | 0.56 (0.36) | 0.77 (0.50–0.88) | 0.69 (0.34–0.79) |
| 12 months | 73 (17) | 20 (4) | 0.65 (0.37) | 0.49 (0.37) | 0.77 (0.5–1.00) | 0.68 (0.17–0.78) |
| 18 months | 59 (19) | 15 (5) | 0.57 (0.39) | 0.41 (0.33) | 0.72 (0.02–0.88) | 0.56 (0.00–0.65) |
| 24 months | 35 (22) | 11 (7) | 0.45 (0.40) | 0.32 (0.32) | 0.57 (0.00–0.81) | 0.25 (0.00–0.62) |
| 36 months | 17 (23) | 6 (7) | 0.34 (0.42) | 0.27 (0.34) | 0.00 (0.00–0.77) | 0.00 (0.00–0.60) |
| 48 months | 3 (23) | 0 (7) | 0.10 (0.280) | 0 (0) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| Time point | EQ-5D-5L QALYs | |||||
| ESG (n = 65) | OSR (n = 18) | |||||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |||
| 12 months | 0.62 (0.32) | 0.70 (0.47–0.88) | 0.46 (0.35) | 0.62 (0.03–0.73) | ||
Predicting NHS expenditure costs for UK patients receiving open surgical replacement and endovascular stent grafting procedures
Introduction and rationale
The aim of this part of the economic analysis was to identify the key determinants of costs to the NHS of an OSR procedure and, separately, of an ESG procedure up until hospital discharge. Therefore, a separate regression model was used for each surgical procedure. Regression models were used where the dependent variable was the NHS costs for an OSR or an ESG procedure up until discharge. Potential determinants of NHS costs up to discharge were included as independent (explanatory) variables. These analyses are shown to illustrate more fully what the main predictors of these costs were and also to provide a resource for future economic evaluation modelling.
Methods
Planning the analysis
The first step was to examine the distribution of total surgical costs to help decide which type of statistical model would best suit the observed distribution of the data. Based on the observed distribution, a generalised linear model with gamma distribution with an identity link function of the total NHS costs was considered to be the most appropriate. This approach was chosen as cost data are typically heavily skewed to the right and this approach is less influenced by outlying observations.
To identify the main drivers of cost, a long list of variables thought to make the most significant contribution to the total NHS costs of each of the procedures was constructed. Using graphical methods and simple statistical summaries, the relationships between the most important predictive variables (to identify collinearity) and between each variable and the total costs were explored. Thereafter, predictive variables were introduced into each regression model using stepwise forward selection. 98 Variables for entry into the regressions were chosen at each step in accordance with the Akaike information criterion.
Possible explanatory variables and model structure
Appendix 11 presents the explanatory variables that were tested to build the regression model, as well as the reason for their selection. These variables were based on initial assumptions about the factors that might have an impact on the total NHS cost of each surgical procedure up until discharge and were divided into two main categories: patient characteristics and resource use. Given that there were fewer patients in the OSR arm, a more limited regression model was used to avoid overfitting. The initial cost regression models are presented in Equations 4 and 5, but their final forms depended on which model fitted the data and provided robust predictions of costs (i.e. would be calibrated with the observed data):
where COST is the NHS expenditure costs from admission until discharge for the index procedure; AGE is the patient age at the time of the intervention (centred continuous variable), SEX is the patient sex (dichotomous variable: 1 if male; 0 otherwise); UTILITY is the baseline utility for the patient (continuous variable multiplied by 100); DIABETES indicates whether or not patients have any diabetes (dichotomous variable: 1 if diabetic; 0 otherwise); SMOKING current indicates whether or not patients are current smokers (dichotomous variable: 1 if current smoker; 0 otherwise); SMOKING HISTORY indicates if a patient has smoked in the past but is no longer a smoker (dichotomous variable: 1 if smoking history; 0 otherwise); NYHA is the patient’s NYHA score (continuous variable with recalibrated NHYA score of 1 = 0 up to a maximum of 3); HYPERTENSION indicates whether or not patients had hypertension prior to intervention (dichotomous variable: 1 has hypertension; 0 otherwise); PAI indicates whether or not patients had a previous aortic intervention; COPD indicates whether or not patients have COPD (dichotomous variable: 1 has COPD; 0 otherwise).
The regression coefficients describe the direction and magnitude of the relationship between each variable and the NHS cost of each surgical procedure until hospital discharge. All analysis of costs was conducted using the statistical software R, version 6.2.
Results
Current smoking status was the only variable associated with costs in the ESG model. The model estimated that current smokers have an increased cost on average of £10,447 (95% CI £2449 to £20,495) compared with non-smokers/past smokers. In the OSR cost model, there was no evidence of significant predictors. See Appendix 11 for model coefficients.
Value-of-information analysis
Methods
To inform the VoI analysis, we used a subset of patients for whom we had near-complete data about resources and quality of life from baseline to 12 months. Details of this patient subset are shown in Appendix 11 and the data definitions are the same as used throughout this chapter. We also developed a simple model to extrapolate the results to 36 months. This model used annual follow-up costs (ESG, £245; OSR, £337), 12–24 month unadjusted common mortality rate (5%), reintervention rates (yearly 3% OSR and 15% ESG) and 12-month utilities. Utility at 12 months was carried forward into years 2 and 3, and the mean cost of an aneurysm-related admission was estimated at £36,005.
Although we considered that cost–utility data would be of limited use and potentially misleading due to the limited data available for a comparative analysis, the principles of economic evaluation can be used to estimate the expected value of perfect information (EVPI) and the expected value of partial perfect information (EVPPI). Although these estimates suffer from the same limitations as the incremental cost–utility data on which they are based, they do show (for the small sample) the breakdown between the uncertainty associated with health outcomes and resource utilisations. Therefore, they are reported with the caveat that they are based on a small sample, but they may inform future research. EVPI and EVPPI were estimated using a bootstrapping technique whereby subpopulations of study participants were sampled with replacement from the overall study population. It is emphasised that the VoI analysis has been presented using the limited ETTAA data set purely to inform decision-making regarding future research and should not be interpreted as a definitive comparative analysis.
The EVPI for each patient is the net benefit of the decision made with perfect information about the uncertain parameters and the decision made based on existing evidence. For the study population, it is the net benefit of the overall optimal treatment choice across all bootstrap iterations less the net benefit lost when the overall optimal choice was not optimal in a particular iteration. The EVPI at the population level, the expected benefit accruing to all future patients, was calculated by multiplying the individual EVPI by the expected future population where there would be a choice about which of the two interventions to use. The annual population in the UK who might benefit from either treatment was assumed to be 100 (based on a plausible limit of the number of procedures the equipment could be used for in 1 year in a given centre), but sensitivity analyses were undertaken with eligible populations of 50 and 200. Initial analysis assumed that the consequences of intervention would last for 12 months. A second analysis assumed that the consequences would last for 3 years, with these second- and third-year impacts being determined using the data derived in the descriptive analysis presented above but incorporated into a simple Markov model predicting death and reintervention. The EVPPI was calculated to show the value of removing all uncertainty around two groups of parameters, namely costs and QALYs.
All VoI analyses were conducted in Stata 15.1 and used a threshold value of £20,000 per QALY. 85 The analyses used generalised linear models to estimate bootstrapped total costs and QALYs at the time points noted above. The bootstrapping took repeated random sampling with replacement of individuals from the data set and estimating a cost-effectiveness ratio for each sample. These samples capture the uncertainty in the overall estimate of cost-effectiveness and inform the VoI, each representing a possible decision about the marginal cost-effectiveness of the two surgical options.
The perspective of the analysis was that of the NHS (i.e. PSS and patient costs were excluded from this analysis). As the total costs and QALYs estimated extended beyond 1 year, each was discounted at 3.5%. 85 To estimate effect, each bootstrap iteration drew a set of matched patients for the analysis. Using multiple imputation, 45 sets of propensity-weighted patients were created. During each bootstrap iteration of the VoI analysis, the bootstrap sample was merged with a random (1 out of 45) set of propensity scores as estimated in Chapter 6 to estimate marginal costs and QALYs, and hence net benefits and the EVPI and EVPPI.
Results of the value-of-information analyses
For the VoI analyses, the population deemed eligible for either procedure and with complete resource use and EQ-5D-5L up to 12 months was used (OSR, n = 18; ESG, n = 65); 800 bootstraps were used for the estimation of EVPI and EVPPI at the 12-month follow-up, and a further 800 bootstraps were used for estimates of the EVPI and EVPPI at the 36-month follow-up.
The results for the propensity-scored primary analyses are shown in Table 29 and the accompanying 12-month and 36-month cost-effectiveness planes are shown in Figures 17 and 18. Each point on the cost-effectiveness plane shows the average difference in costs and effects from each bootstrap iteration. Points in the north-east and north-west represent circumstances in which OSR is more expensive than ESG, and points in the north-east and south-east quadrants indicate circumstances in which OSR has higher HRQoL compared with ESG.
| 12-month follow-up | 36-month follow-up | |
|---|---|---|
| Cost difference (OSR – ESG) (£), mean (95% CI) | 7870 (–7810 to 22,280) | –710 (–16,390 to 13,670) |
| QALY difference (OSR – ESG), mean (95% CI) | –0.017 (–0.274 to 0.254) | –0.062 (–0.894 to 0.764) |
| Non-parametric incremental cost-effectiveness ratio (£) | ESG dominates OSR | 11,518a |
| EVPI – 100 patients (£) | 84,200 | 493,600 |
| EVPPI QALY – 100 patients (£) | 17,400 | 390,300 |
| EVPPI costs – 100 patients (£) | 72,425 | 328,200 |
FIGURE 17.
Cost-effectiveness plane for the 12-month VoI model. Cost-effectiveness plane OSR – ESG (generalised linear model). Incremental cost-effectiveness ratio: ESG dominates OSR.
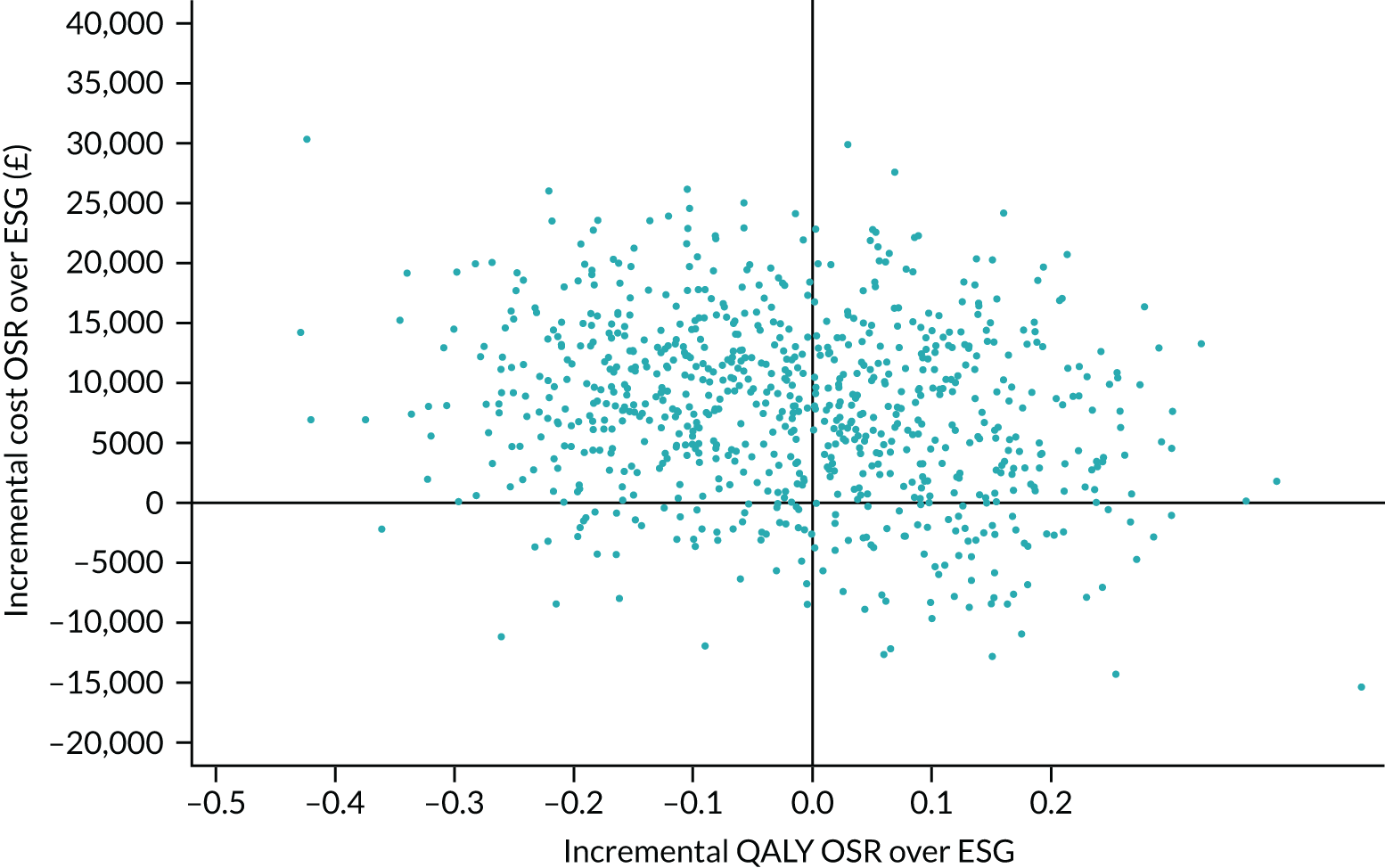
FIGURE 18.
Cost-effectiveness plane for the 36-month VoI model. Cost-effectiveness plane OSR – ESG (generalised linear model). Incremental cost-effectiveness ratio: –£11,518.
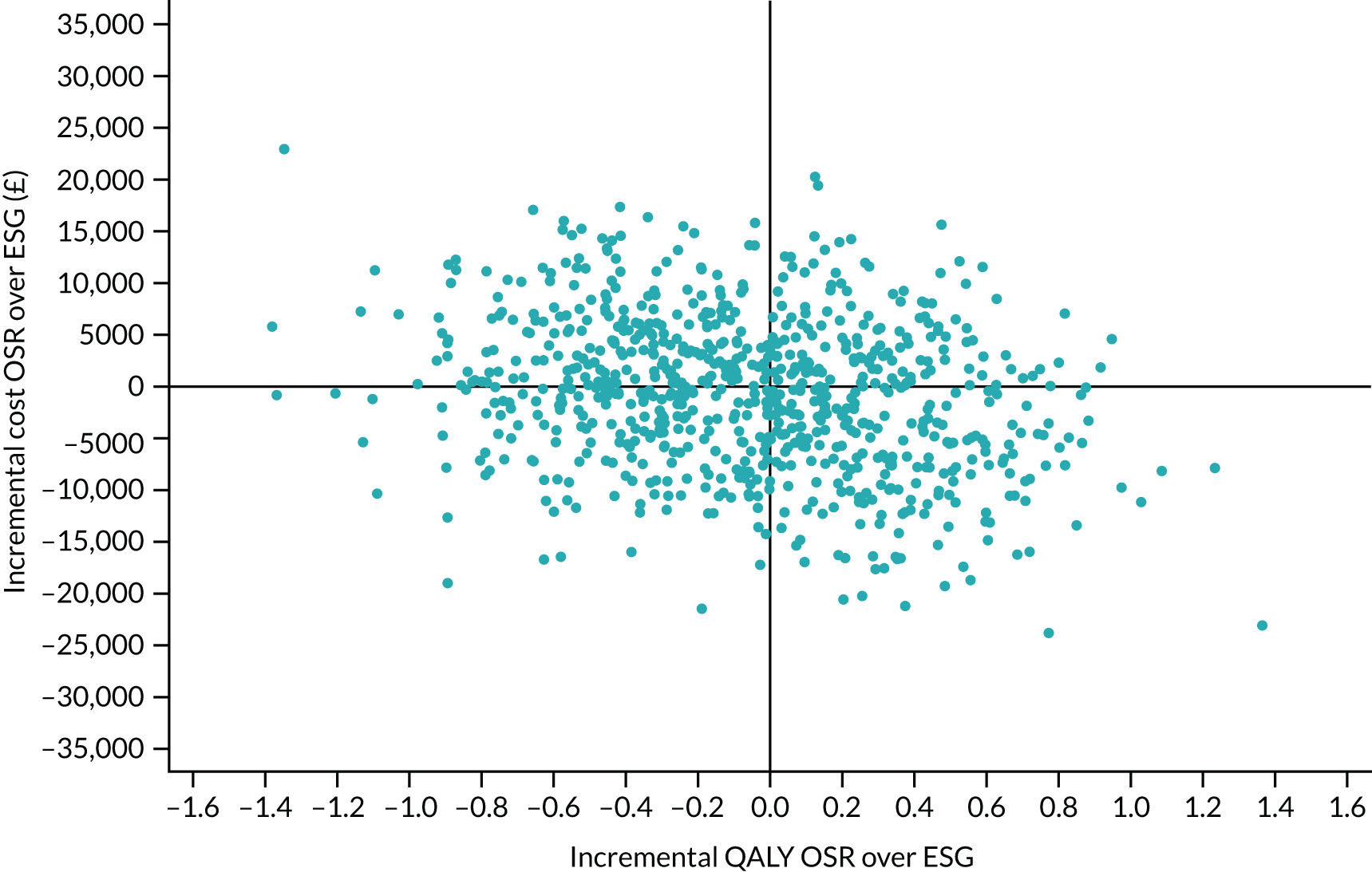
Over 12 months, ESG dominates OSR in this exploratory analysis. It is, on average, associated with higher costs and statistically significantly greater QALYs. The EVPI of £84,200 reflects the distribution of points on the cost-effectiveness plane and that few exist outside the north-west and north-east quadrants. EVPPI confirms that most of the uncertainty derives from uncertainty about costs rather than about QALYs and is, therefore, less sensitive to willingness-to-pay thresholds.
Over 3 years, ESG no longer dominates OSR. OSR is on average less costly but less effective. The incremental cost-effectiveness ratio of £11,518 is the incremental cost per QALY gained for the comparison of the more effective but more costly ESG compared with the less costly and less effective OSR. In this analysis, the VoI increases, with EVPPI estimates for cost parameters increasing the most. Extending the time frame from 12 months to 10 years sees the total EVPI increase to £1,628,800.
Over 12 months, there is considerably more uncertainty around the net benefit associated with resource consequences than with quality of life. However, this relative gap closes in our simple 36-month model as reintervention costs of ESG offset the higher initial costs of OSR.
Summary of findings
The ability to conduct any comparative analysis was limited by the small number of patients in the data set who were eligible for both surgical procedures, particularly OSR. This resulted in much of the analysis being conducted on each surgical group separately.
The primary procedure costs and costs during follow-up were higher for the ESG procedure than for OSR. Seventy-nine per cent of the ESG primary procedure costs were accounted for by stent costs and 85% of the follow-up costs were accounted for by hospital admissions, including additional procedures. Costs post procedure to discharge were higher in the OSR group, with > 70% of these costs accounted for by ICU costs. HRQoL was higher in the ESG group than in the OSR group at all time points, with EQ-5D-5L QALYs higher at the 12-month follow-up.
The regression analysis of costs for both OSR and ESG identified little evidence that patient characteristics were associated with costs. Only current smoking status was associated with costs in the ESG model. The ability of this analysis to identify evidence was limited by the few data available for analysis, and the CIs surrounding the impact of individual characteristics are wide and contain economically important increases or decreases in costs.
The VoI analysis estimated the absolute value of removing all uncertainty from the analysis. The values estimated, regardless of the analyses conducted, were modest. The greatest value would be from removing uncertainty around costs, but our 36-month model suggests that the relative benefits would fall over time. Generally, if the VoI estimates are correct, then further research may not be worthwhile; any further research would be costly and may not exceed any benefits gained from it. However, the analysis conducted makes two critical assumptions. First, it assumes that the data used in the analysis may be imprecise but are not biased. Second, it assumes that the model accurately captures the decision problem. The small sample of patients may be biased by the fact that HRQoL values are imputed only for patients who die, which is likely to introduce bias in both the point estimate and the variance. In addition, the simple, limited-time-frame Markov model used may overly simplify reality and, hence, underestimate decision uncertainty, particularly given the inherent assumptions about previous events.
Chapter 8 Discussion
Summary of research findings
In our study, participating centres collectively referred 886 CTAA patients, with a combined follow-up of 2016.8 patient-years. The incidence of CTAA is expected to rise as the age distribution of the UK population increases and associated comorbidities become more prevalent. When the ETTAA study began, published research on management of CTAA was sparse, consisting of small single-centre studies or larger unselected registry studies (see Chapter 1). All comparisons of treatment options used observational designs with limited adjustment for confounding. The ETTAA study aimed to provide an analysis of service provision in the NHS and to report outcomes for patients diagnosed with CTAA both before and after the intervention.
Without procedural intervention for chronic thoracic aortic aneurysm, what is the risk of aneurysm growth, dissection, rupture, permanent neurological injury or death, and how does health-related quality of life change over time?
Chronic thoracic aortic aneurysm patients were at high risk of death, had a range of comorbidities and had somewhat impaired HRQoL measured with EQ-5D-5L utility at the time of the intervention. Rate of aneurysm growth varied by location but was generally slow in this cohort of patients.
After diagnosis with arch/descending CTAA but before intervention (if planned), the death rate was high (8.6% per patient-year) compared with the general population with similar age distribution in England; for example, in 2015–17, the 1-year probability of death in England among people aged 71 years was 2.1% for men and 1.4% for women. 70 Thus, death rates for ETTAA patients were over four times the death rate for the general population of similar age. Approximately half of the deaths prior to a procedure in the ETTAA study were from aneurysm-related causes. The hazards of death in the absence of surgical intervention were significantly associated with aneurysm size, older age at presentation, female sex and (to a lesser extent) higher NYHA class. Other than aneurysm size and patient sex, these predictors of risk may simply be surrogates for comorbidity. Predictions from fitted models suggested that 3-year survival without treatment is > 90% for patients with small aneurysms (4–4.5 cm in diameter), falling to 88% for aneurysms of 5 cm, 79% for aneurysms of 6 cm, 63% for aneurysms of 7 cm and 42% for aneurysms of 8 cm. Together with the increased risk of poor post-intervention outcomes for longer waiting times, this indicates that intervention should be discussed once aneurysms exceed 6 cm in diameter, provided that operative risk is considered low/moderate.
Consistent with their older age, CM patients and those who went on to have ESG had more hospital admissions, including aneurysm-related admissions. Patients who were managed conservatively were judged to be sicker and less likely to benefit from intervention than other patients, so the higher admission and death rates in this group testify to the judgement of the clinicians. Non-fatal acute ruptures, dissections and neurological injuries were uncommon. Combining fatal and non-fatal cases, there were 69 ruptures or dissections, or both combined, in a combined total of 1489 years of patient follow-up pre intervention, a rate of 4.6% per patient-year at risk. This is broadly consistent with the benchmark study from Yale University, IRAD, GERAADA and reports of the Australasian experience. 19,21–23
Health-related quality of life as measured using the EQ-5D-5L varied substantially at presentation, but, on average, the mean was slightly lower than UK age- and sex-matched population estimates. Heterogeneity at presentation could be partially explained by age, sex, smoking and NYHA class, but there was substantial unexplained variation. Moreover, the decline in HRQoL was faster for older patients and current smokers, but there was also unexplained variation between patients in the rate of change over time. Patients destined for ESG had lower HRQoL at presentation, all other factors being equal, although a few patients waited more than 6 months before intervention, at least partly due to delays in production of custom-made stents.
What factors affect aneurysm growth pre intervention?
The mean aneurysm size at presentation was partly related to known covariates such as age, smoking, CTD and COPD, with only location of the aneurysm related to both baseline diameter and growth rate.
The mean aneurysm size at study entry varied widely as a result of patients being identified at different stages in the evolution of their disease, and there was significant variation that was not explained by the variables measured in the ETTAA study. Almost all patients had documented hypertension, so the impact of hypertension on aneurysm growth (or any other outcome) could not be evaluated; hypertension is, however, an established causal factor in the development and outcome of aneurysms, and treatment should be optimised. 74 Given their older average age, poorer LV function, smoking history, COPD and prevalence of DTA aneurysms, patients who went on to have ESG are likely to have had faster-growing aneurysms before the procedure.
Differences in aortic pathology and morphology in different sections of the aorta meant that aneurysms in the DTA were significantly larger and more variable at presentation and grew faster than those in the arch or thoracoabdominal aorta; no growth was found in the ascending aorta, which largely results from previous interventions in this segment. Mode of surveillance (MRI or CT scans) was the only other variable associated with growth over time, which is likely to be a result of the selection for MRI of patients with slower-growing aneurysms and referral for CT more likely if growth appears to be accelerating. Slower-growing aneurysms are anticipated to require longer-term follow-up and, therefore, clinicians aim to reduce radiation exposure by choosing MRI scans for the patient. However, when an aneurysm is approaching an indication for surgery, CT images are superior for interventional planning. With these exceptions, there was no evidence of measured or unmeasured covariates affecting growth of the aneurysm, which partly results from the short-term follow-up in most patients and possible earlier intervention in patients who have signs of faster growth. Other studies suggested that growth is related to various risk factors including age, smoking, CTD, COPD and hypertension (see Chapter 1). The ETTAA study included surveillance imaging from diagnosis when growth may have been slower, so that ability to detect factors associated with growth was limited. Studies of growth in the infrarenal aorta have also failed to consistently find useful predictors of increased growth rate. 99
Detailed studies of the aneurysm/vasculature (patent or partially patent false lumen, larger false lumen diameter/saccule formation within the false lumen, number and location of tears around the arch, peak wall stress) could not be undertaken in this observational study and so we could not assess their relationship to size or growth. Moreover, treatment by indication bias may have hindered our ability to assess whether or not medical treatments slowed growth. It is important to note that patients leave this analysis when their aneurysms are fast-growing or become large enough for intervention, or when they die, so the pattern of growth outside this range is not known and extrapolation is not justified.
If a patient has endovascular stent grafting or open surgical repair, what is the risk of dissection, rupture, permanent neurological injury or death, and how does health-related quality of life change over time?
Differences in patient populations meant that ESG and OSR outcomes could not be directly compared. ESG patients had lower risk of death within 30 days (6.7% vs. 11.1%) but subsequent survival was comparable; incidences of non-fatal dissection, rupture and neurological injury was rare; and, after an initial dip after OSR, survivors in the two groups had comparable mean HRQoL.
Arch/descending CTAA often required complicated intervention, with most requiring ESG or OSR in more than one segment of the aorta. In addition, 11.2% required concomitant cardiac surgery, 14.0% required staged procedures and 4.6% required hybrid procedures. Although there was clear selection of younger, ‘fitter’ patients for OSR, outcomes for ESG and OSR patients were largely comparable. The invasive nature of OSR was associated with a slightly higher risk of death within 30 days, but few aneurysm-related procedural complications were reported. The main differences in clinical outcomes between interventions were higher readmissions in the short term for ESG patients, and the substantially higher incidence of postoperative complications, longer initial hospital stay and subsequent reinterventions in the OSR group. Although ESG patients were more likely than OSR patients to be readmitted to hospital early after discharge, their overall rate of readmission was not significantly higher, despite the 7- to 10-year age difference. This is at odds with the literature on infrarenal AAA repair, in which the complication rate and subsequent need for reintervention are higher. 100 This perhaps reflects the differences in natural history of the thoracic and abdominal aorta. After excluding patients who had a contraindication to one of the procedures, the results were qualitatively unchanged, although the smaller sample size resulted in more variable estimates of outcomes and low precision.
A large initial decrease in HRQoL followed OSR, a feature reported in other cardiothoracic surgery and vascular surgery trials,71,72 including the EVAR 1 trial,73 and is related to the longer hospital stay and post-surgical complications. Beyond this period, ESG and OSR had similar HRQoL in the full data analysis; conversely, the subset of OSR patients who were also eligible for ESG had significantly poorer HRQoL. The results for patients who were eligible for both ESG and OSR were based on small numbers and the difference between the groups from the full data analysis is more reliable. We also highlight that these analyses include only surviving patients and are not restricted to the nominal planned measurement times.
Can aneurysm- or patient-related predictors of treatment outcomes be determined?
Aneurysm location, age, sex, NYHA class and time before intervention were most closely related to survival after intervention, while previous HRQoL, smoking and NYHA class were related to HRQoL.
Patients receiving OSR had higher all-cause and aneurysm-related death rates overall, and this was more significant for DTA aneurysms than for arch aneurysms. Poorer survival after OSR in the DTA was partly influenced by treatment of CTD patients who have weaker vessel walls in this segment. Similar effects were noted in analyses that excluded patients with CTD (among other contraindications), even after the application of propensity score methods, suggesting that other factors were also in play. In observational studies it is difficult to delineate treatment effects from patient selection effects. As well as age at procedure, post-intervention survival was associated with a longer interval between entry to the study and intervention. The timing of intervention is challenging, our analysis showed that delays were associated with surgery in the DTA/thoracoabdominal aorta (especially OSR in these vessels), staged procedures, current smoking, previous CABG/PCI for coronary artery disease and concomitant valve disease, but most were not significant. It is likely that during the wait aneurysms will continue to grow and patients will continue to deteriorate over time, which may also contribute to this finding. Ultimately, it may be that vasculopathy of the DTA is simply a worse disease than vasculopathy of the arch, given the potential impact of the former aneurysms on spinal, renal, mesenteric and lower limb blood flow.
The strongest predictor of postoperative HRQoL was preoperative HRQoL, both of which were related to current smoking and NYHA grading of breathlessness. Recommending smoking cessation and treating causes of breathlessness may ease these symptoms to some extent and, therefore, optimise HRQoL. 74 This is supported by evidence that smoking cessation prior to other major procedures improves short-term outcomes. 75,76
What is the most cost-effective strategy in patients eligible for both endovascular stent grafting and open surgical replacement?
No comparative cost-effectiveness analysis was feasible. However, detailed micro-costing of ESG and OSR highlights that the ESG procedure itself is more costly than an OSR procedure, with the costs of the stents being the main driver. However, the total costs from admission to discharge on average were higher for the OSR procedure, largely as a result of increased ICU days.
Despite the rigorous work that was undertaken in developing this cohort of patients, relatively few participants were eligible to receive both treatments. We planned to complete a within-study economic evaluation and a sophisticated microsimulation model. However, owing to the limited data available, the analysis was restricted to describing the costs and QALYs for those who had initially received an ESG or OSR in the ‘no contraindication’ cohort, conducting a regression analysis to explore the key drivers of costs, and a VoI analysis. Standard methods of VoI analysis were used (see Chapter 7), and the analysis was based on the assumption that only 100 people would be eligible for both procedures per year.
Over 12 months’ follow-up, mean EQ-5D-5L scores were lower than median scores, indicating that some people in both groups had very low scores. For OSR patients, mean EQ-5D-5L score was much lower 1 month after surgery than at baseline, as patients were still recovering from surgery. Mean EQ-5D-5L scores increased thereafter but, on average, were still lower than baseline scores. This differs from the results of HRQoL trajectories in Chapters 5 and 6 because patients who died were assigned a utility value of zero in the economic analysis and the analysis did not include measurements outside the nominal measurement times. For the ESG group, even at 1 month, EQ-5D-5L scores were not much lower than at baseline. Over a 12-month follow-up period, mean and median QALYs were greater in the ESG group than in the OSR group. For the analysis underpinning the estimates of the EVPI and EVPPI, ESG appears to be the dominant intervention, being both cheaper and more effective than OSR at 12 months and with an incremental cost per QALY of < £12,000 for ESG compared with OSR at 36 months. The value of the EVPI and EVPPI is very modest, given the likely costs of any research project that might be conducted, especially as any research would remove only part of the uncertainty. These results should, however, be treated with caution (see Strengths and weaknesses).
How does this compare with existing literature and what does it add?
To our knowledge, this is the first analysis to scrutinise the clinical effectiveness and cost-effectiveness of elective treatments for chronic arch/descending aneurysms. Previous publications have identified the higher costs but shorter length of stay and reduced morbidity with ESG, but these reports conflated both acute and chronic pathologies. The ETTAA study has identified that clinicians are clear about which patients can benefit most from ESG and which can benefit most from OSR. There are also clear and objective technical parameters that determine whether or not an aneurysm can be treated with ESG at all. Finally, in many important aspects, the results of the ETTAA study are a straightforward extrapolation of the findings of studies of the management of AAAs. 100,101 With this in mind, the ETTAA study has established the costs and clinical outcomes associated with the two interventions and has also demonstrated the futility of directly comparing the two interventions.
The other contribution of the ETTAA study has been to describe the natural history of medically treated arch/DTA CTAA under surveillance. The 8.6% per patient-year risk of death and the 4.6% risk of fatal/non-fatal rupture or dissection are similar to the data from published literature. Having described the patterns of growth and risks associated with medical management, the ETTAA study will facilitate the future planning, location and organisation of specialist aortic centres to cater to the projected increase in demand. This is based on substantial literature showing that high-volume specialist centres improve clinical outcomes in complex aortic interventions. 102,103
Strengths and weaknesses
The main strength of this study was the engagement of 30 centres with specialist aortic aneurysm provision and the diverse and rigorously applied research methods, including a Delphi study, an economic analysis, an analysis of the natural history of aneurysm growth and clinical outcomes before intervention, and the comparison of outcomes after ESG and OSR, using both traditional regression methods and contemporary methods to address bias in patient selection.
The biggest limitations in the comparison of ESG and OSR were the observational nature of the study and the relatively small number of patients, which meant that there was low power to detect differences in outcomes and limited ability to adjust for confounding in regression models using traditional adjustment methods. One of the difficulties with non-experimental studies is that we observe activity based on current clinical mores, so that cause-and-effect relationships are difficult to disentangle. Although we used a variety of methods designed to reduce bias, these can be unreliable for small samples, and analyses were likely to include some level of residual confounding. It is also difficult to compare survival before and after procedures because of the possible selection of higher-risk patients for intervention.
In many situations it appeared that there was little equipoise among clinicians in the choice of patient management. Equipoise was reported for < 10% of scenarios considered in the RAND–Delphi study and around half of the patients who underwent ESG or OSR could not receive the alternative. Even after excluding these patients, clinicians appeared to assign patients largely according to age, fitness for surgery and aneurysm site. It is likely that there was additional selection of patients that was not reflected in the data, particularly as ESG patients, who were 7–10 years older on average, appeared to have similar outcomes to OSR patients, even after adjustment for age and comorbidities. Based on these results, it seems certain that a randomised trial is not feasible in the UK.
A further limitation was the necessity of reporting outcomes for a diverse group of aneurysms (arch and DTA) treated in a number of ways. In general, the treatment of CTAA varies because of significant variation in morphology, and the fact that aneurysms might encompass multiple segments, with very different approaches and risks. In addition, practice and experience vary across centres. The incidence of CTAA is low, so it is impossible to examine the outcomes of a large cohort of patients with morphologically similar CTAAs treated in the same way.
The strength of the economic evaluation is that analyses were based on rigorous and explicit methods that correspond to best practice in terms of identification, measurements and valuation of costs and QALYs. This was hampered by the small number of patients who did not have a contraindication to one or other of the treatments, so the pre-planned economic analysis was not possible. Moreover, estimates of costs and QALYs were made difficult by a complicated data collection schedule that was not always adhered to. More importantly, loss to follow-up was a problem over the longer term, so data past 12 months were very limited. These issues are not uncommon in observational studies, such as the ETTAA study and occurred despite prospective data collection.
A VoI analysis was attempted. The results and their interpretation should be viewed with caution, as the methods rely on the assumption that the data available are imprecise but provide an unbiased estimate of each parameter. Given the small sample sizes available, and the observational nature of the study, the distribution of costs and QALYs observed may not be an accurate representation of the population distribution of costs and QALYs. Therefore, the EVPI and EVPPI may be biased. Furthermore, the EVPI and EVPPI depend, crucially, on the size of population who would be eligible to receive either OSR or ESG. If this were underestimated, then the population EVPI and EVPPI would be underestimated.
Implications for service
Patients with CTAA have complicated management needs and are best managed by specialist centres with the support of MDT meetings. Patients are largely aged between 50 and 90 years (average of 70 years), with a range of cardiac and thoracic comorbidities, and are at an increased risk of death due to rupture, dissection and other aneurysm-related events. Although clinicians expressed reasonably strong agreement about the best management options, the optimal timing of intervention is difficult to define. These factors, together with the relative rarity of CTAA, suggest that care should be delivered by specialist centres with the support of MDT meetings. For optimal patient management it is critical that all patients with a diagnosis of arch/DTA CTAA are discussed in an aortic MDT meeting with cardiac and vascular surgeons, radiologists, interventionalists and cardiologists in attendance, who have experience in treating thoracic disease. The ETTAA data have demonstrated that both ESG and OSR can have good outcomes (at least to 3 years) for some patients and that MDT decision-making is reliable. Thus, there should be a drive towards improved information in primary care about the clinical and HRQoL outcomes of complex aortic interventions.
For small (4–5.5 cm in diameter) aneurysms, current strategies appear to work well, with important aspects being blood pressure management, encouragement to maintain an active lifestyle and smoking cessation. Once aneurysms reach the threshold for intervention (≥ 6 cm in diameter), it is important that this intervention is not delayed, as longer time to intervention is an important modifiable risk factor for a poor outcome. For small aneurysms, an important consideration is the essential value of an imagebank for surveillance scans. Regular imaging, be it CT or MRI, will be essential for choosing, planning and timing the intervention. A national registry of patients with chronic aortic disease tied to an imagebank accessible across the UK would be invaluable for NHS service planning.
Further research
What further research is required?
In priority order:
-
The prediction of aneurysm growth and the timing of intervention is difficult, and aneurysm diameter may not be the best indicator of whether or not rupture will occur. More detailed analysis of the diameter, length and volume of aneurysms, as well as other anatomical features in the ETTAA data set, may help to refine decisions around when and how to intervene. Joint analysis of aneurysm growth and acute clinical events (rupture, dissection and death) would provide valuable information on the timing of interventions.
-
The ETTAA database is now well positioned to describe, from real-world practice, what low, medium and high risk mean in terms of objective variables. This would facilitate a risk–benefit analysis and enable patients to be better informed at consent.
-
Combining post-procedure ETTAA data with longer-term routine electronic data sources would throw light on longer-term survival and hospital admissions to understand whether there is a divergence in survival and reintervention rates, as seen in other studies,36 and with a view to identifying factors that reduce the risk of these events.
-
For quality improvement, and to better understand the drivers of outcome in each group separately, it would be helpful to have a registry that records all CTAA patients, with a wider (but carefully chosen) set of associated variables recorded to the same protocol. This could augment the existing national cardiac and vascular surgical databases (National Institute for Cardiovascular Outcomes Research and National Vascular Registry) and would enable a more reliable assessment of variables affecting outcomes within each intervention group. 104,105 In particular, drug therapies and the value of optimisation prior to intervention could be investigated in a larger group of patients, with more adequate adjustment made for confounders. A registry that is maintained and adopted by the majority of specialist centres will also allow the longer-term follow-up of patients pre and post intervention.
Acknowledgements
The authors would like to thank our funders and the many members of the ETTAA Collaborative Group for their support with this observational study, as detailed below.
Patient and public involvement
Representatives from the Marfan Association UK and Liverpool Aneurysm Support group contributed advice for the grant application and the ETTAA website was designed with their support (ettaastudy.com). Neil Towers, who sits on our Trial Steering Committee, reported last year:
I was present at the first steering committee meeting and have attended the planned meetings ever since. I have been given every opportunity to involve myself in discussions and to make suggestions and comments as appropriate. My opinion has regularly been sought on issues relating to the patient and public point of view and I have on occasions discussed items with other members of Liverpool Heart and Chest Hospital Aortic Support Group and given their feedback to the steering committee. As a former emergency aortic dissection patient I understand the relevance of this research and look forward to further involvement together, reading the report and also sharing the findings with the Liverpool Support Group.
Reproduced with permission from Neil Towers, personal communication, 2021
We are indebted to Neil for his steadfast support for the ETTAA study.
ETTAA Collaborative Group
The ETTAA Collaborative Group includes the ETTAA Working Group members (past and present), the Trial Steering Committee, the Data Monitoring Committee and, from the recruitment centres, the PIs and research co-ordinators.
| ETTAA collaborators contributions by role code | CI; CR; CPM | LST; LHE | HE; ST | RTA | PHC; SM | CRO; STB | PI | RC | TSC | DMC |
|---|---|---|---|---|---|---|---|---|---|---|
| Conceptualisation | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Data curation | ✓ | ✓ | ✓ | |||||||
| Formal analysis | ✓ | ✓ | ✓ | |||||||
| Funding acquisition | ✓ | ✓ | ✓ | ✓ | ||||||
| Investigation | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Methodology | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Project administration | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Resources | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Supervision | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Validation | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Visualisation (presentation) | ✓ | ✓ | ✓ | |||||||
| Writing – original draft | ✓ | ✓ | ✓ | ✓ | ||||||
| Writing – review and editing | ✓ | ✓ | ✓ |
| Group/name | Role in study | Role code |
|---|---|---|
| ETTAA Working Group (present) | ||
| Stephen Large | Chief Investigator | CI/TSC |
| Linda Sharples | Lead Statistician/Lead Author | LST |
| Luke Vale | Lead Health Economist | LHE |
| Priya Sastry | Cardiac Surgeon/Research Fellow | CR |
| Colin Bicknell | Vascular Surgeon/PI | CR |
| Carol Freeman | Clinical Project Manager | CPM |
| Andrew Cook | Public Health Consultant | PHC |
| Yi-Da Chiu | Statistician | ST |
| Andrew McCarthy | Health Economist | HE |
| Jo Gray | Health Economist | HE |
| Peter McMeekin | Health Economist | HE |
| S Rao Vallabhaneni | Vascular Surgeon/PI | CR |
| Nicky Watson | Data Manager | RTA |
| Dilupa Samarakoon | Health Economist | RTA |
| Thomas Devine | Database Manager | RTA |
| Tom Duffy | Papworth Core Laboratory/Administrative Assistant | RTA |
| Victoria Hughes | Senior R&D Manager (Sponsor) | SM |
| ETTAA Working Group (past) | ||
| Linzi Dunham | Administrative Assistant | RTA |
| Steven Frost | Administrative Assistant | RTA |
| Isabel De Val | Administrative Assistant | RTA |
| Anna Godzwa | Administrative Assistant | RTA |
| Liz Hodge | Administrative Assistant | RTA |
| Sharon Allen | Administrative Assistant | RTA |
| Jasmine Hughes | Administrative Assistant | RTA |
| Narain Moorjani | Cardiac Surgeon/Collaborator | CRO |
| Bobby Agrawal | Consultant Radiologist/Core Laboratory MRI | RTA |
| Katherine Tweed | Consultant Radiologist/Core Laboratory MRI | RTA |
| Sally Anne Meakins | Database Manager | RTA |
| Sarah Hopkins | Lead Research Co-ordinator | RTA |
| Siobhan Costello | Lead Research Co-ordinator | RTA |
| Helen Holcombe | Lead Research Co-ordinator | RTA |
| Christopher Smith | Papworth Core Laboratory Manager | RTA |
| Jules Hernandez Sanchez | Statistician | RTA |
| Richard Jackson | Statistician (RAND–Delphi) | STB |
| Ben Patterson | Vascular Surgeon/Core Laboratory CT | RTA |
| Iain Roy | Vascular Surgeon/RAND–Delphi | RTA |
| Trial Steering Committee | ||
| David Crossman | Chairperson (Professor of Clinical Cardiology) | TSC |
| Neil Towers | Patient representative | TSC |
| Belinda Lees | TSC Member | TSC |
| Nicholas Boon | TSC Member | TSC |
| Henry Smithson | TSC Member | TSC |
| Data Monitoring Committee | ||
| Bruce Campbell | Professor of Vascular Surgery | DMC |
| David Taggart | Professor of Cardiovascular Surgery | DMC |
| John Norrie (former member of the NIHR HTA and EME Editorial Board) | Professor of Medical Statistics | DMC |
| Centre | Role code | |
|---|---|---|
| Principal investigators | ||
| Manjit Gohel | Cambridge University Hospitals NHS Foundation Trust | PI |
| Paul Hayes | Cambridge University Hospitals NHS Foundation Trust | PI |
| Arindam Chaudhuri | Bedford Hospital NHS Trust | PI |
| Martin Claridge | University Hospitals Birmingham NHS Foundation Trust | PI |
| Andrew Duncan | Blackpool Teaching Hospitals NHS Foundation Trust | PI |
| Waquar Syed Yusuf | University Hospitals Sussex NHS Foundation Trust. | PI |
| Alan Bryan | University Hospitals Bristol NHS Foundation Trust | PI |
| Cha Rajakaruna | University Hospitals Bristol NHS Foundation Trust | PI |
| David Murray | Manchester University NHS Foundation Trust | PI |
| John Quarmby | University Hospitals of Derby And Burton NHS Foundation Trust | PI |
| Jacek Szostek | University Hospitals of Leicester NHS Trust | PI |
| Rachel Bell | Guy’s and St Thomas’ NHS Foundation Trust | PI |
| Ian Chetter | Hull University Teaching Hospitals NHS Trust | PI |
| Colin Bicknell | Imperial College Healthcare NHS Trust | PI |
| Raghvinder Gambhir | King’s College Hospital NHS Foundation Trust | PI |
| Julian Scott | Leeds Teaching Hospitals NHS Trust | PI |
| Manoj Kuduvalli | Liverpool University Hospitals NHS Foundation Trust | PI |
| Mark Dayer | Taunton and Somerset NHS Foundation Trust | PI |
| Mike Clarke | The Newcastle Upon Tyne Hospitals NHS Foundation Trust | PI |
| Felicity Meyer | Norfolk and Norwich University Hospitals NHS Foundation Trust | PI |
| Leisa Freeman | Norfolk and Norwich University Hospitals NHS Foundation Trust | PI |
| Theo Ojimba | North Cumbria Integrated Care NHS Foundation Trust | PI |
| Pedro Catarino | Royal Papworth Hospital NHS Foundation Trust | PI |
| Jonathon Unsworth-White | University Hospital Plymouth NHS Trust | PI |
| Jason Constantinou | Royal Free London NHS Foundation Trust | PI |
| Rao Vallabhaneni | Liverpool University Hospitals NHS Foundation Trust | PI |
| Graham Cooper | Sheffield Teaching Hospitals NHS Foundation Trust | PI |
| Barney Green | South Tees Hospitals NHS Foundation Trust | PI |
| Geoffrey Tsang | University Hospital Southampton NHS Foundation Trust | PI |
| Ian Loftus | St George’s University Hospitals NHS Foundation Trust | PI |
| Ansuman Saha | University Hospitals Sussex NHS Foundation Trust | PI |
| Charles McCollum | Manchester University NHS Foundation Trust | PI |
| Andrew Thompson | York Teaching Hospitals NHS Foundation Trust | PI |
| Research co-ordinators | ||
| Debbie Read | Cambridge University Hospitals NHS Foundation Trust | RC |
| Danielle Johnson | Cambridge University Hospitals NHS Foundation Trust | RC |
| Beena David | Bedford Hospital NHS Trust | RC |
| Retno Wulandari | Bedford Hospital NHS Trust | RC |
| Carina Galpin | Bedford Hospital NHS Trust | RC |
| Safia Begum | University Hospitals Birmingham NHS Foundation Trust | RC |
| Faye Moore | University Hospitals Birmingham NHS Foundation Trust | RC |
| Deepa Sebastian | Blackpool Teaching Hospitals NHS Foundation Trust | RC |
| Janette Brown | Blackpool Teaching Hospitals NHS Foundation Trust | RC |
| Leonie Benham | Blackpool Teaching Hospitals NHS Foundation Trust | RC |
| Vas Vasudervan | Blackpool Teaching Hospitals NHS Foundation Trust | RC |
| Lauren Thornborough | Blackpool Teaching Hospitals NHS Foundation Trust | RC |
| Conor Wilkinson | Blackpool Teaching Hospitals NHS Foundation Trust | RC |
| Ella Riedal | Blackpool Teaching Hospitals NHS Foundation Trust | RC |
| Admin Team | Blackpool Teaching Hospitals NHS Foundation Trust | RC |
| Laura Ortiz-Ruiz de Gordoa | University Hospitals Sussex NHS Foundation Trust | RC |
| Emma Hopkins | University Hospitals Bristol NHS Foundation Trust | RC |
| Melanie Ridout | University Hospitals Bristol NHS Foundation Trust | RC |
| Rachel Wyatt | University Hospitals Bristol NHS Foundation Trust | RC |
| Alice Panes | Manchester University NHS Foundation Trust | RC |
| Mohammed Nazir | Manchester University NHS Foundation Trust | RC |
| Anam Asif | Manchester University NHS Foundation Trust | RC |
| Anu John | Manchester University NHS Foundation Trust | RC |
| Jessica Nichols | Manchester University NHS Foundation Trust | RC |
| Marie Appleby | University Hospitals of Derby And Burton NHS Foundation Trust | RC |
| Kathleen Holdings | University Hospitals of Derby And Burton NHS Foundation Trust | RC |
| Jane Plume | University Hospitals of Leicester NHS Trust | RC |
| Donna Alexander | University Hospitals of Leicester NHS Trust | RC |
| Federica Francia | Guy’s and St Thomas’ NHS Foundation Trust | RC |
| Josie Hatfield | Hull University Teaching Hospitals NHS Trust | RC |
| Tuong Vi Le-Magowan | Imperial College Healthcare NHS Trust | RC |
| Joanne Smee | Imperial College Healthcare NHS Trust | RC |
| Amanda Henry | Imperial College Healthcare NHS Trust | RC |
| Alison Deslandes | Imperial College Healthcare NHS Trust | RC |
| Ruth Odiase | King’s College Hospital NHS Foundation Trust | RC |
| Thoraya Ammar | King’s College Hospital NHS Foundation Trust | RC |
| Joanne Fletcher | Leeds Teaching Hospitals NHS Trust | RC |
| Nikki Dewhirst | Leeds Teaching Hospitals NHS Trust | RC |
| Susannah Howard | Leeds Teaching Hospitals NHS Trust | RC |
| Merane Todd | Leeds Teaching Hospitals NHS Trust | RC |
| Maureen Morgan | Liverpool University Hospitals NHS Foundation Trust | RC |
| Jacqueline Currie | Liverpool University Hospitals NHS Foundation Trust | RC |
| Helen Mills | Taunton and Somerset NHS Foundation Trust | RC |
| Kate James | Taunton and Somerset NHS Foundation Trust | RC |
| Karen Roberts | Taunton and Somerset NHS Foundation Trust | RC |
| Izabela SrokaLech | The Newcastle Upon Tyne Hospitals NHS Foundation Trust | RC |
| Martin Catterson | The Newcastle Upon Tyne Hospitals NHS Foundation Trust | RC |
| Noala Parr | The Newcastle Upon Tyne Hospitals NHS Foundation Trust | RC |
| Joanna Wright | Norfolk and Norwich University Hospitals NHS Foundation Trust | RC |
| Kerry Brand | Norfolk and Norwich University Hospitals NHS Foundation Trust | RC |
| Toni Calver | Norfolk and Norwich University Hospitals NHS Foundation Trust | RC |
| Mandy Burrows | Norfolk and Norwich University Hospitals NHS Foundation Trust | RC |
| Zoe Sanders | North Cumbria Integrated Care NHS Foundation Trust | RC |
| Toni Wilson | North Cumbria Integrated Care NHS Foundation Trust | RC |
| Katherine Davidson | North Cumbria Integrated Care NHS Foundation Trust | RC |
| Helen Holcombe | Royal Papworth Hospital NHS Foundation Trust | RC |
| Sam Piesley | University Hospital Plymouth NHS Trust | RC |
| Amy Turner | University Hospital Plymouth NHS Trust | RC |
| Tracy Ward | University Hospital Plymouth NHS Trust | RC |
| Natasha Wimshurst | University Hospital Plymouth NHS Trust | RC |
| Yvonne Gleeson | Royal Free London NHS Foundation Trust | RC |
| Claire Harrison | Liverpool University Hospitals NHS Foundation Trust | RC |
| Anna Lowe | Liverpool University Hospitals NHS Foundation Trust | RC |
| Pene Fati | Sheffield Teaching Hospitals NHS Foundation Trust | RC |
| Joann Barker | Sheffield Teaching Hospitals NHS Foundation Trust | RC |
| Cecilia Mason | Sheffield Teaching Hospitals NHS Foundation Trust | RC |
| Emanuel Cirstea | South Tees Hospitals NHS Foundation Trust | RC |
| Maggie Johns | University Hospital Southampton NHS Foundation Trust | RC |
| Kim Golder | University Hospital Southampton NHS Foundation Trust | RC |
| Charlie Merry | University Hospital Southampton NHS Foundation Trust | RC |
| Robert Ingham | St George’s University Hospitals NHS Foundation Trust | RC |
| Sally Collins | University Hospitals Sussex NHS Foundation Trust | RC |
| Jesha Mathews | Manchester University NHS Foundation Trust | RC |
| Jessica Nichols | Manchester University NHS Foundation Trust | RC |
| Stella Normansell | Manchester University NHS Foundation Trust | RC |
| Daniel Cotterell | Manchester University NHS Foundation Trust | RC |
| Becky Tait | York Teaching Hospitals NHS Foundation Trust | RC |
| John Whitwell | York Teaching Hospitals NHS Foundation Trust | RC |
| Sally Gordon | York Teaching Hospitals NHS Foundation Trust | RC |
Contributions of authors
Linda Sharples (https://orcid.org/0000-0003-0894-966X) (Lead Statistician/Lead Author) was responsible for the conceptualisation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, presentation and writing.
Priya Sastry (https://orcid.org/0000-0002-1012-1434) (Cardiac Surgeon/Research Fellow) was responsible for the conceptualisation, data curation, investigation, methodology, project administration, resources, supervision, validation, presentation and writing.
Carol Freeman (https://orcid.org/0000-0001-9751-0784) (Clinical Project Manager) was responsible for the conceptualisation, data curation, funding acquisition, investigation, project administration, resources, supervision, validation, presentation and writing.
Joanne Gray (https://orcid.org/0000-0001-6157-230X) (Health Economist) was responsible for the data curation, formal analysis, methodology, supervision, validation, presentation, writing validation, presentation and writing.
Andrew McCarthy (https://orcid.org/0000-0002-3385-6302) (Health Economist) was responsible for the data curation, formal analysis, methodology, project administration, validation, presentation and writing.
Yi-Da Chiu (https://orcid.org/0000-0002-8014-3399) (Statistician) was responsible for the data curation, formal analysis, methodology, project administration, validation, presentation and writing.
Colin Bicknell (https://orcid.org/0000-0003-0158-1831) (Vascular Surgeon/PI) was responsible for the data curation, investigation, methodology, resources, supervision, validation, presentation and writing.
Peter McMeekin (https://orcid.org/0000-0003-0946-7224) (Health Economist) was responsible for the data curation, formal analysis, methodology, supervision, validation, presentation, writing validation, presentation and writing.
S Rao Vallabhaneni (https://orcid.org/0000-0002-4362-9277) (Vascular Surgeon/PI) was responsible for the conceptualisation, funding acquisition, investigation, methodology, supervision, validation, presentation and writing.
Andrew Cook (https://orcid.org/0000-0002-6680-439X) (Public Health Consultant) was responsible for the conceptualisation, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, presentation and writing.
Luke Vale (https://orcid.org/0000-0001-8574-8429) (Lead Health Economist) was responsible for the conceptualisation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, presentation and writing.
Stephen Large (https://orcid.org/0000-0002-3201-6344) (Chief Investigator) was responsible for the conceptualisation, funding acquisition, investigation, methodology, resources, supervision, validation, presentation and writing.
The contributions of the authors are based on their CRediT contributor roles (for role descriptions, see https://casrai.org/credit/).
Publication
Sharples L, Sastry P, Freeman C, Bicknell C, Chiu YD, Vallabhaneni SR et al. , on behalf of the ETTAA Collaborative Group. Aneurysm growth, survival, and quality of life in untreated thoracic aortic aneurysms: the effective treatments for thoracic aortic aneurysms study [published online ahead of print November 29 2021]. Eur Heart J 2021.
Data-sharing statement
The ETTAA Working Group supports the principles of data sharing. Applications to access data should be submitted via the corresponding author. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Clouse WD, Hallett JW, Schaff HV, Spittell PC, Rowland CM, Ilstrup DM, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004;79:176-80. https://doi.org/10.4065/79.2.176.
- Bridgewater B, Keogh B, Kinsman R, Walton P. The Society for Cardiothoracic Surgery in Great Britain &Amp; Ireland: The Sixth National Adult Cardiac Surgical Database Report 2009.
- Bottle A, Mariscalco G, Shaw MA, Benedetto U, Saratzis A, Mariani S, et al. Unwarranted variation in the quality of care for patients with diseases of the thoracic aorta. J Am Heart Assoc 2017;6. https://doi.org/10.1161/JAHA.116.004913.
- Saliba E, Sia Y. The ascending aortic aneurysm: when to intervene?. Int J Cardiol Heart Vasculature 2015;6:91-100. https://doi.org/10.1016/j.ijcha.2015.01.009.
- Ince H, Nienaber CA. Etiology, pathogenesis and management of thoracic aortic aneurysm. Nat Clin Pract Cardiovasc Med 2007;4:418-27. https://doi.org/10.1038/ncpcardio0937.
- Ostberg NP, Zafar MA, Ziganshin BA, Elefteriades JA. The genetics of thoracic aortic aneurysms and dissection: a clinical perspective. Biomolecules 2020;10. https://doi.org/10.3390/biom10020182.
- Pannu H, Fadulu VT, Chang J, Lafont A, Hasham SN, Sparks E, et al. Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation 2005;112:513-20. https://doi.org/10.1161/CIRCULATIONAHA.105.537340.
- van Bogerijen GH, Tolenaar JL, Rampoldi V, Moll FL, van Herwaarden JA, Jonker FH, et al. Predictors of aortic growth in uncomplicated type B aortic dissection. J Vasc Surg 2014;59:1134-43. https://doi.org/10.1016/j.jvs.2014.01.042.
- Bashir M, Fok M, Hammoud I, Rimmer L, Shaw M, Field M, et al. A perspective on natural history and survival in nonoperated thoracic aortic aneurysm patients. Aorta 2013;1:182-9. https://doi.org/10.12945/j.aorta.2013.13-043.
- Juvonen T, Ergin MA, Galla JD, Lansman SL, McCullough JN, Nguyen K, et al. Risk factors for rupture of chronic type B dissections. J Thorac Cardiovasc Surg 1999;117:776-86. https://doi.org/10.1016/S0022-5223(99)70299-0.
- Patel HJ, Nguyen C, Diener AC, Passow MC, Salata D, Deeb GM. Open arch reconstruction in the endovascular era: analysis of 721 patients over 17 years. J Thorac Cardiovasc Surg 2011;141:1417-23. https://doi.org/10.1016/j.jtcvs.2011.02.020.
- Higgins J, Lee MK, Co C, Janusz MT. Long-term outcomes after thoracic aortic surgery: a population-based study. J Thorac Cardiovasc Surg 2014;148:47-52. https://doi.org/10.1016/j.jtcvs.2013.07.028.
- Thomas M, Li Z, Cook DJ, Greason KL, Sundt TM. Contemporary results of open aortic arch surgery. J Thorac Cardiovasc Surg 2012;144:838-44. https://doi.org/10.1016/j.jtcvs.2011.09.069.
- Clouse WD, Cambria RP. Current status of thoracoabdominal aneurysm repair. Adv Surg 2004;38:197-246.
- Coady MA, Rizzo JA, Hammond GL, . What is the appropriate size criterion for resection of thoracic aortic aneurysms?. J Thorac Cardiovasc Surg 1997;113:476-91. https://doi.org/10.1016/S0022-5223(97)70360-X.
- Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. J Am Coll Cardiol 2010;55:e27-19. https://doi.org/10.1016/j.jacc.2010.02.015.
- Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. https://doi.org/10.1093/eurheartj/ehu281.
- Graves RS. Users’ guides to the medical literature: a manual for evidence-based clinical practice. J Med Library Assoc 2002;90.
- Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg 2002;74:S1877-80. https://doi.org/10.1016/S0003-4975(02)04147-4.
- Elefteriades JA. Indications for aortic replacement. J Thorac Cardiovasc Surg 2010;140:S5-9. https://doi.org/10.1016/j.jtcvs.2010.10.001.
- International Registry of Acute Aortic Dissections (IRAD) . The International Registry of Acute Aortic Dissections n.d. https://iradonline.org/irad.html (accessed 19 October 2020).
- Boening A, Karck M, Conzelmann LO, Easo J, Krüger T, Rylski B, et al. German Registry for acute aortic dissection type A: structure, results, and future perspectives. Thorac Cardiovasc Surg 2017;65:77-84. https://doi.org/10.1055/s-0036-1572436.
- Yan T, Tian DH, LeMaire SA, Misfeld M, Elefteriades JA, Chen EP, et al. The ARCH Projects: design and rationale (IAASSG 001). Eur J Cardiothorac Surg 2014;45. https://doi.org/10.1093/ejcts/ezt520.
- Elefteriades JA, Ziganshin BA, Rizzo JA, Fang H, Tranquilli M, Paruchuri V, et al. Indications and imaging for aortic surgery: size and other matters. J Thorac Cardiovasc Surg 2015;149:10-3. https://doi.org/10.1016/j.jtcvs.2014.07.066.
- Trimarchi S, Jonker FH, Hutchison S, . Descending aortic diameter of 5.5 cm or greater is not an accurate predictor of acute type B aortic dissection. J Thorac Cardiovasc Surg 2011;142:e101-7. https://doi.org/10.1016/j.jtcvs.2010.12.032.
- Cheung K, Boodhwani M, Chan KL, Beauchesne L, Dick A, Coutinho T. Thoracic aortic aneurysm growth: role of sex and aneurysm etiology. J Am Heart Assoc 2017;6. https://doi.org/10.1161/JAHA.116.003792.
- Kuzmik GA, Sang AX, Elefteriades JA. Natural history of thoracic aortic aneurysms. J Vasc Surg 2012;56:565-71. https://doi.org/10.1016/j.jvs.2012.04.053.
- Sueyoshi E, Sakamoto I, Uetani M. Growth rate of affected aorta in patients with type B partially closed aortic dissection. Ann Thorac Surg 2009;88:1251-7. https://doi.org/10.1016/j.athoracsur.2009.06.023.
- Shimada I, Rooney SJ, Pagano D, Farneti PA, Davies P, Guest PJ, et al. Prediction of thoracic aortic aneurysm expansion: validation of formulae describing growth. Ann Thorac Surg 1999;67:1968-70. https://doi.org/10.1016/S0003-4975(99)00435-X.
- Dapunt OE, Galla JD, Sadeghi AM, Lansman SL, Mezrow CK, de Asla RA, et al. The natural history of thoracic aortic aneurysms. J Thorac Cardiovasc Surg 1994;107:1323-32. https://doi.org/10.1016/S0022-5223(94)70054-0.
- Gray J, McCarthy A, McMeekin P, Vale L, Freeman, Sharples L, et al. Effective Treatments for Thoracic Aortic Aneurysms (ETTAA study): Protocol for a Systematic Review 2017. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42017054565 (accessed 24 November 2021).
- McCarthy A, Gray J, Sastry P, Sharples L, Vale L, Cook A, et al. Systematic review of endovascular stent grafting versus open surgical repair for the elective treatment of arch/descending thoracic aortic aneurysms. BMJ Open 2021;11. https://doi.org/10.1136/bmjopen-2020-043323.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355. https://doi.org/10.1136/bmj.i4919.
- Goodney PP, Travis L, Lucas FL, Fillinger MF, Goodman DC, Cronenwett JL, et al. Survival after open versus endovascular thoracic aortic aneurysm repair in an observational study of the Medicare population. Circulation 2011;124:2661-9. https://doi.org/10.1161/CIRCULATIONAHA.111.033944.
- Bavaria JE, Appoo JJ, Makaroun MS, Verter J, Yu ZF, Mitchell RS, et al. Investigators. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg 2007;133:369-77. https://doi.org/10.1016/j.jtcvs.2006.07.040.
- von Allmen RS, Anjum A, Powell JT. Outcomes after endovascular or open repair for degenerative descending thoracic aortic aneurysm using linked hospital data. Br J Surg 2014;101:1244-51. https://doi.org/10.1002/bjs.9568.
- Andrassy J, Weidenhagen R, Meimarakis G, Rentsch M, Jauch KW, Kopp R. Endovascular versus open treatment of degenerative aneurysms of the descending thoracic aorta: a single center experience. Vascular 2011;19:8-14. https://doi.org/10.1258/vasc.2010.oa0256.
- Hughes K, Guerrier J, Obirieze A, Ngwang D, Rose D, Tran D, et al. Open versus endovascular repair of thoracic aortic aneurysms: a nationwide inpatient sample study. Vasc Endovascular Surg 2014;48:383-7. https://doi.org/10.1177/1538574414540484.
- Piffaretti G, Tozzi M, Lomazzi C, Rivolta N, Caronno R, Bacuzzi A, et al. Endovascular repair versus conventional surgery for descending thoracic aortic aneurysms. Ital J Vasc Endovasc Surg 2007;14:279-85.
- Desai ND, Burtch K, Moser W, Moeller P, Szeto WY, Pochettino A, et al. Long-term comparison of thoracic endovascular aortic repair (TEVAR) to open surgery for the treatment of thoracic aortic aneurysms. J Thorac Cardiovasc Surg 2012;144:604-9. https://doi.org/10.1016/j.jtcvs.2012.05.049.
- Gopaldas RR, Huh J, Dao TK, LeMaire SA, Chu D, Bakaeen FG, et al. Superior nationwide outcomes of endovascular versus open repair for isolated descending thoracic aortic aneurysm in 11,669 patients. J Thorac Cardiovasc Surg 2010;140:1001-10. https://doi.org/10.1016/j.jtcvs.2010.08.007.
- Orandi BJ, Dimick JB, Deeb GM, Patel HJ, Upchurch GR. A population-based analysis of endovascular versus open thoracic aortic aneurysm repair. J Vasc Surg 2009;49:1112-16. https://doi.org/10.1016/j.jvs.2008.12.024.
- Dick F, Hinder D, Immer FF, Hirzel C, Do DD, Carrel TP, et al. Outcome and quality of life after surgical and endovascular treatment of descending aortic lesions. Ann Thorac Surg 2008;85:1605-12. https://doi.org/10.1016/j.athoracsur.2008.01.027.
- Narayan P, Wong A, Davies I, Angelini GD, Bryan AJ, Wilde P, et al. Thoracic endovascular repair versus open surgical repair – which is the more cost-effective intervention for descending thoracic aortic pathologies?. Eur J Cardiothorac Surg 2011;40:869-74. https://doi.org/10.1016/j.ejcts.2011.01.010.
- National Institute for Health and Care Excellence . Abdominal Aortic Aneurysm: Diagnosis and Management n.d. www.nice.org.uk/guidance/ng156/chapter/Recommendations (accessed 19 October 2020).
- National Institute for Health and Care Excellence (NICE) . NICE Publishes Its Guideline on the Diagnosis and Management of Abdominal Aortic Aneurysms 2020. www.nice.org.uk/news/article/nice-publishes-its-guideline-on-the-diagnosis-and-management-of-abdominal-aortic-aneurysms (accessed 15 September 2020).
- Delbecq AL, Van de Ven AH. A group process model for problem identification and program planning. J Appl Behav Sci 1971;7:466-92. https://doi.org/10.1177/002188637100700404.
- Fitch K, Bernstein SJ, Aguilar MD, Burnard B, LaCalle JR. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: RAND Corporation; 2001.
- Martin G, Riga C, Gibbs R, Jenkins M, Hamady M, Bicknell C. Short- and long-term results of hybrid arch and proximal descending thoracic aortic repair: a benchmark for new technologies. J Endovasc Ther 2016;23:783-90. https://doi.org/10.1177/1526602816655446.
- Haulon S, Greenberg RK, Spear R, Eagleton M, Abraham C, Lioupis C, et al. Global experience with an inner branched arch endograft. J Thorac Cardiovasc Surg 2014;148:1709-16. https://doi.org/10.1016/j.jtcvs.2014.02.072.
- Ince H, Rehders TC, Petzsch M, Kische S, Nienaber CA. Stent-grafts in patients with marfan syndrome. J Endovasc Ther 2005;12:82-8. https://doi.org/10.1583/04-1415MR.1.
- Geisbüsch P, Kotelis D, von Tengg-Kobligk H, Hyhlik-Dürr A, Allenberg JR, Böckler D. Thoracic aortic endografting in patients with connective tissue diseases. J Endovasc Ther 2008;15:144-9. https://doi.org/10.1583/07-2286.1.
- Pellenc Q, Girault A, Roussel A, De Blic R, Cerceau P, Raffoul R, et al. Optimising aortic endovascular repair in patients with Marfan syndrome. Eur J Vasc Endovasc Surg 2020;59:577-85. https://doi.org/10.1016/j.ejvs.2019.09.501.
- Hicks CW, Lue J, Glebova NO, Ehlert BA, Black JH. A 10-year institutional experience with open branched graft reconstruction of aortic aneurysms in connective tissue disorders versus degenerative disease. J Vasc Surg 2017;66:1406-16. https://doi.org/10.1016/j.jvs.2017.03.451.
- Verhoeven EL, Katsargyris A, Bekkema F, . Editor’s choice – ten-year experience with endovascular repair of thoracoabdominal aortic aneurysms: results from 166 consecutive patients. Eur J Vasc Endovasc Surg 2015;49:524-31. https://doi.org/10.1016/j.ejvs.2014.11.018.
- Frazao C, Tavoosi A, Wintersperger BJ, Nguyen ET, Wald RM, Ouzounian M, et al. Multimodality assessment of thoracic aortic dimensions: comparison of computed tomography angiography, magnetic resonance imaging, and echocardiography measurements. J Thorac Imaging 2020;35:399-406. https://doi.org/10.1097/RTI.0000000000000514.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Fayers PM, Machin D. Quality of Life: The Assessment, Analysis and Reporting of Patient-Reported Outcomes 2015. https://doi.org/10.1002/9781118758991.
- Kirkwood BR, Sterne JAC. Essential Medical Statistics. Malden, MA: Wiley-Blackwell; 2003.
- Mehta CR, Patel NR. A network algorithm for performing Fisher’s exact test in r × c contingency tables. J Am Stat Assoc 1983;78:427-34. https://doi.org/10.1080/01621459.1983.10477989.
- Rubin DB. Inference and missing data. Biometrika 1976;63:581-92. https://doi.org/10.1093/biomet/63.3.581.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley and Sons, Inc.; 1987.
- Sharples L, Sastry P, Freeman C, Bicknell C, Chiu YD, Vallabhaneni SR, et al. ETTAA Collaborative Group . Aneurysm growth, survival, and quality of life in untreated thoracic aortic aneurysms: the effective treatments for thoracic aortic aneurysms study. Eur Heart J 2021. https://doi.org/10.1093/eurheartj/ehab784.
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. College Station, TX: Stata Press; 2012.
- Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. New York, NY: Springer; 2000.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509. https://doi.org/10.1080/01621459.1999.10474144.
- Rizopoulos D. Joint Models for Longitudinal and Time-to-event Data with Applications in R. New York, NY: Chapman and Hall/CRC; 2012.
- Szende A, Janssen B, Cabases J. Self-Reported Population Health: An International Perspective Based on EQ-5D 2014. https://doi.org/10.1007/978-94-007-7596-1.
- Morgan E. National Life Tables: England n.d. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesenglandreferencetables (accessed 25 August 2020).
- Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM. Oxford Vascular Study . Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013;127:2031-7. https://doi.org/10.1161/CIRCULATIONAHA.112.000483.
- Rintoul RC, Ritchie AJ, Edwards JG, Waller DA, Coonar AS, Bennett M, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014;384:1118-27. https://doi.org/10.1016/S0140-6736(14)60418-9.
- Sharples L, Everett C, Singh J, Mills C, Spyt T, Abu-Omar Y, et al. Amaze: a double-blind, multicentre randomised controlled trial to investigate the clinical effectiveness and cost-effectiveness of adding an ablation device-based maze procedure as an adjunct to routine cardiac surgery for patients with pre-existing atrial fibrillation. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22190.
- Brown LC, Powell JT, Thompson SG, Epstein DM, Sculpher MJ, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) trials: randomised trials of EVAR versus standard therapy. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16090.
- Warner MA, Divertie MB, Tinker JH. Preoperative cessation of smoking and pulmonary complications in coronary artery bypass patients. Anesthesiology 1984;60:380-3. https://doi.org/10.1097/00000542-198404000-00022.
- Møller AM, Villebro N, Pedersen T, Tønnesen H. Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet 2002;359:114-17. https://doi.org/10.1016/S0140-6736(02)07369-5.
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41-55. https://doi.org/10.1093/biomet/70.1.41.
- Leyrat C, Seaman SR, White IR, . Propensity score analysis with partially observed covariates: how should multiple imputation be used?. Stat Methods Med Res 2019;28:3-19. https://doi.org/10.1177/0962280217713032.
- Mitra R, Reiter JP. A comparison of two methods of estimating propensity scores after multiple imputation. Stat Methods Med Res 2016;25:188-204. https://doi.org/10.1177/0962280212445945.
- Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 2013;6:604-11. https://doi.org/10.1161/CIRCOUTCOMES.113.000359.
- Williamson EJ, Forbes A. Introduction to propensity scores. Respirology 2014;19:625-35. https://doi.org/10.1111/resp.12312.
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. https://doi.org/10.1002/sim.3697.
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424. https://doi.org/10.1080/00273171.2011.568786.
- Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R, et al. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol 2017;69:345-57. https://doi.org/10.1016/j.jacc.2016.10.060.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2018.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- O’Brien BJ, Drummond MF, Labelle RJ, Willan A. In search of power and significance: issues in the design and analysis of stochastic cost-effectiveness studies in health care. Med Care 1994;32:150-63. https://doi.org/10.1097/00005650-199402000-00006.
- Willan AR, Pinto EM. The value of information and optimal clinical trial design. Stat Med 2005;24:1791-806. https://doi.org/10.1002/sim.2069.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health 2013;16:e1-5. https://doi.org/10.1016/j.jval.2013.02.010.
- Polsky D, Glick H. Costing and cost analysis in randomized controlled trials: caveat emptor. PharmacoEconomics 2009;27:179-88. https://doi.org/10.2165/00019053-200927030-00001.
- Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996;276:1172-7. https://doi.org/10.1001/jama.1996.03540140060028.
- Potter S, Davies C, Davies G, Rice C, Hollingworth W. The use of micro-costing in economic analyses of surgical interventions: a systematic review. Health Econ Rev 2020;10. https://doi.org/10.1186/s13561-020-0260-8.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2018 to 2019 2019.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent; 2019.
- Department of Health and Social Care (DHSC) . NHS Reference Costs 2017 to 2018 2019.
- NHS Blood and Transport (NHSBT) . National Health Service Blood and Transport Price List 2018 19 2018.
- Department for Business, Energy and Industrial Strategy (BEIS) . Department for Business, Energy and Industrial Strategy National Minimum Wage and National Living Wage Rates 2020.
- Henderson DA, Denison DR. Stepwise regression in social and psychological research. Psychol Rep 1989;64:251-7. https://doi.org/10.2466/pr0.1989.64.1.251.
- Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT. UK Small Aneurysm Trial Participants . Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 2004;110:16-21. https://doi.org/10.1161/01.CIR.0000133279.07468.9F.
- Patel R, Sweeting MJ, Powell JT, Greenhalgh RM. EVAR trial investigators . Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 2016;388:2366-74. https://doi.org/10.1016/S0140-6736(16)31135-7.
- Powell JT, Brown LC, Forbes JF, Fowkes FG, Greenhalgh RM, Ruckley CV, et al. Final 12-year follow-up of surgery versus surveillance in the UK Small Aneurysm Trial. Br J Surg 2007;94:702-8. https://doi.org/10.1002/bjs.5778.
- Hudorović N, Vucetic B, Lovricevic I. The evidence for volume-outcome relationships in thoracic aortic surgery. Eur J Cardiothorac Surg 2010;37:248-9. https://doi.org/10.1016/j.ejcts.2009.07.030.
- Karthikesalingam A, Hinchliffe RJ, Loftus IM, Thompson MM, Holt PJ. Volume-outcome relationships in vascular surgery: the current status. J Endovasc Ther 2010;17:356-65. https://doi.org/10.1583/10-3035.1.
- National Institute For Cardiovascular Outcomes Research . NICOR Database n.d. www.nicor.org.uk/ (accessed 17 May 2021).
- Health Quality Improvement Partnership . National Vascular Registry n.d. www.hqip.org.uk/a-z-of-nca/national-vascular-registry/#.YKIjL6hKjIU (accessed 17 May 2021).
- van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Soft 2011;45:1-67. https://doi.org/10.18637/jss.v045.i03.
- Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc 1988;83:1198-202. https://doi.org/10.1080/01621459.1988.10478722.
- NHS Employers . NHS Terms and Conditions Pay Poster 2018 19 n.d. https://www.nhsemployers.org/publications/nhs-terms-and-conditions-pay-poster-201819 (accessed October 2021).
- National Institute for Health and Care Excellence . A to Z of Drugs | BNF Content Published by NICE n.d. https://bnf.nice.org.uk/drug/ (accessed 11 June 2019).
- National Institute for Health and Care Excellence . Perioperative Care in Adults n.d. www.nice.org.uk/guidance/GID-NG10072/documents/evidence-review-12 (accessed October 2021).
- Department of Health and Social Care (DHSC) . National Schedule of NHS Costs 2018 19 2019.
- Balami JS, Coughlan D, White PM, McMeekin P, Flynn D, Roffe C, et al. The cost of providing mechanical thrombectomy in the UK NHS: a micro-costing study. Clin Med 2020;20:e40-e45. https://doi.org/10.7861/clinmed.2019-0413.
- Curtis L., Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- GOV.UK . National Minimum Wage and National Living Wage Rates n.d. www.gov.uk/national-minimum-wage-rates (accessed October 2021).
Appendix 1 Definitions of complications and other clinical events
Observed clinical events during hospital admission
Death
Recorded death by the local centre.
Myocardial infarction
The clinical suspicion of myocardial infarction together with elevated CK-MB (creatine kinase MB isoenzyme) or troponin and/or ECG/echo findings consistent with acute myocardial infarction.
Cardiac support
Support of myocardial pump function either by the use of intravenous/inhaled inotropic agents (e.g. adrenaline, noradrenaline, enoximone, dopamine, nitric oxide) or by the use of an intra-aortic balloon pump.
Prolonged ventilation > 48 hours
Support of respiratory or ventilatory function by means of a mechanical ventilator for more than 48 hours after (a) admission (for conservatively managed WW or CM patients) or (b) procedural intervention by means of ESG or surgery.
Renal support
Temporary
Treatment of acute renal failure* by means of a period of haemofiltration that is confined to the hospital admission and not required after discharge.
*Abnormal kidney function requiring dialysis (including hemofiltration) in patients who did not require this procedure prior to intervention; or a rise in levels of serum creatinine of ≥ 26 µmol/l within 48 hours; or a ≥ 50% rise in levels of serum creatinine known or presumed to have occurred within the past 7 days.
Permanent
Renal dysfunction persisting > 90 days and graded according to estimated glomerular filtration rate, or requirement for haemodialysis sustained for at least 90 days.
Gastrointestinal complications
A new diagnosis of any of the following conditions as determined by the clinical history and standard investigations, interpreted and documented by a qualified physician: upper/lower gastrointestinal bleeding, intestinal ischaemia (small or large bowel), stoma formation, or others including (but not confined to) oesophagitis, duodenal ulcer (perforated or bleeding), erosive gastritis, pancreatitis, liver failure/necrosis and cholecystitis.
Neurological injury
Central nervous system: brain
Any new, temporary or permanent, focal or global neurological dysfunction ascertained by a standard neurological history and examination administered by a neurologist or other qualified physician; or an abnormality identified by surveillance neuroimaging.
Transient ischaemic attack, defined as an acute transient neurological deficit conforming anatomically to arterial distribution cerebral ischaemia, which resolves in < 24 hours and is associated with no infarction on brain imaging (head CT performed > 24 hours after symptom onset; or MRI).
Cerebrovascular accident, defined as a new acute neurological deficit of any duration associated with acute infarction on imaging corresponding anatomically to the clinical deficit, or attributable to intracranial haemorrhage.
Central nervous system: spinal cord
Paraplegia: new onset of impairment in motor and sensory function of the lower extremities after aortic intervention.
Paraparesis: new onset of partial impairment in motor or sensory function of the lower extremities after aortic intervention.
Thromboembolic event (deep-vein thrombosis/pulmonary embolism)
Evidence of a venous thromboembolic event (e.g. deep-vein thrombosis, pulmonary embolism) by standard clinical and laboratory testing.
Infection
Infection pertaining to the operated segment of aorta (including periprosthetic abscess), vascular access site, surgical incision, lungs, pleural/peritoneal cavity or urinary tract; as diagnosed by an appropriately qualified physician according to standard clinical investigations.
Return to theatre
A secondary visit the operating/hybrid theatre for treatment or examination of suspected complications following but during the same admission as the index intervention by ESG or OSR.
Access vessel injury
New-onset intramural haematoma, pseudoaneurysm, dissection, avulsion, disruption, rupture or occlusion of any vessel used to provide vascular access for the delivery of an endovascular stent graft.
Endoleak
Type I
-
Leak at the proximal graft attachment site.
-
Leak at the distal graft attachment site.
-
Leak around a fenestration, branch end point, or branch-occluding plug (e.g. plug occluding a subclavian artery or iliac artery to prevent flow into an aneurysm sac).
Type II
-
Retrograde flow from branch arteries arising from the excluded segment.
Type III
-
Modular disconnect or apposition failure (including branch junctions).
-
Fabric tear.
Type IV
-
Flow through porous fabric (generally resolves within a short time period, typically less than 24 hours).
Type V
-
No detected endoleak, but aneurysm expansion (thus presumed failure to detect the endoleak or presumed pressure transmission through thrombus without blood flow).
Other observed clinical events
Aneurysm complication
Any direct complication localised to the operated segment of aorta, including (but not necessarily confined to) localised rupture, dissection, or pseudoaneurysm formation. This must be diagnosed and documented by an appropriately qualified physician (e.g. vascular/cardiothoracic surgeon or interventional radiologist) according to standard clinical and radiological investigations.
Fistula formation
Defined as an abnormal connection between the operated/stent-grafted segment of aorta and another epithelialised surface, and diagnosed according to standard clinical and radiological investigations.
Reintervention
Any intervention undertaken to preserve or restore the function of an endovascular stent graft (e.g. re-ballooning/additional stent/surgery) or surgically implanted aortic graft.
Appendix 2 Treatment of missing covariates
Exploring missing data patterns
All covariates included in pre- or post-procedure analyses were considered for inclusion in the imputation models. These variables are shown by treatment group in Table 30. With the exception of haemoglobin and serum creatinine levels, the number of missing covariates is relatively small (< 8%) for an observational study. The two biomarkers were more likely to be missing in patients in the WW and CM groups than in those receiving an intervention. Height and weight were slightly more likely to be missing for CM patients. NYHA class at baseline was slightly more likely to be missing for patients in the two intervention groups.
| Missing covariate | Patient subgroup, n (%) | |||
|---|---|---|---|---|
| WW (N = 489) | CM (N = 112) | ESG (N = 150) | OSR (N = 135) | |
| Age | 0 | 0 | 0 | 0 |
| Sex | 0 | 0 | 0 | 0 |
| Height | 19 (3.9) | 9 (8.0) | 4 (2.7) | 3 (2.2) |
| Weight | 22 (4.5) | 8 (7.1) | 5 (3.3) | 3 (2.2) |
| BMI | 24 (4.9) | 9 (8.0) | 6 (4.0) | 3 (2.2) |
| Care | 4 (0.8) | 1 (0.9) | 0 (0.0) | 2 (1.5) |
| Smoker | 4 (0.8) | 1 (0.9) | 1 (0.7) | 1 (0.7) |
| CTD | 0 | 0 | 0 | 0 |
| Extracardiac arteriopathy | 0 | 0 | 0 | 0 |
| Valvular heart disease | 11 (2.3) | 2 (1.8) | 1 (0.7) | 1 (0.7) |
| Coronary artery disease | 13 (2.7) | 2 (1.8) | 1 (0.7) | 1 (0.7) |
| LV function | 8 (1.6) | 0 | 1 (0.7) | 1 (0.7) |
| LV function not measured | 241 (49.3) | 55 (49.1) | 70 (46.7) | 36 (26.7) |
| Diabetes | 3 (0.6) | 0 | 0 | 0 |
| Hypertension | 2 (0.4) | 0 | 0 | 0 |
| COPD | 5 (1.0) | 0 | 0 | 0 |
| NYHA class | 14 (2.9) | 2 (1.8) | 11 (7.3) | 9 (6.7) |
| Beta-blockers | 0 | 0 | 0 | 0 |
| ACE inhibitors | 0 | 0 | 0 | 0 |
| ARBs | 0 | 0 | 0 | 0 |
| Calcium channel blocker use | 0 | 0 | 0 | 0 |
| Other antihypertensives | 0 | 0 | 0 | 0 |
| Any antihypertensives | 0 | 0 | 0 | 0 |
| Statins | 2 (0.4) | 0 | 0 | 0 |
| Serum creatinine | 309 (63.2) | 60 (53.6) | 42 (28.0) | 48 (35.6) |
| Haemoglobin | 326 (66.7) | 64 (57.1) | 44 (29.3) | 50 (37.0) |
| Maximum aneurysm location | 0 | 0 | 0 | 0 |
| Maximum aneurysm size | 0 | 0 | 0 | 0 |
| Pre-procedure variables | ESG (N = 150) | OSR (N = 135) | ||
| Surgical priority | – | – | 0 | 0 |
| LV function | – | – | 113 (75.3) | 68 (50.4) |
| Serum creatinine | – | – | 22 (14.7) | 32 (23.7) |
| Haemoglobin | – | – | 21 (14.0) | 31 (23.0) |
We used the built-in function (missing_pattern) of the R package ‘mice’ to explore the missingness pattern. 106 For the data set used for pre-procedure analysis, there were 33 different patterns of missingness. This showed that 788 (88.9%) patients had complete data, 27 (3.0%) patients had missing NYHA class and were otherwise complete and 23 (2.6%) patients had missing height and weight (and, therefore, missing BMI) but were otherwise complete. All other patterns were observed in small numbers of patients.
For the data set used for post-procedure analysis, the output shows 16 different patterns of missingness. For these analyses, 200 (70.2%) patients had complete data, 44 (15.4%) had missing serum creatinine and haemoglobin levels only and 17 (6.0%) had missing NYHA class only. All other patterns were observed in small numbers of patients.
Missing data mechanisms
We used Little’s107 test to assess whether or not continuous covariates were MCAR. In this test, the means of the covariates are compared between different missing-value patterns. The test is similar to the likelihood-ratio statistic for multivariate normal data; the resulting statistic is asymptotically chi-squared-distributed under the null hypothesis that there are no differences between the means of different missing-value patterns. A p-value of < 0.05 tells us that there is some evidence to conclude that the data are not MCAR. The results in Table 31 suggest that MCAR cannot be safely assumed for either pre- or post-procedure analyses.
| Work package (analysis type) | Variables included | p-value |
|---|---|---|
| Package 1 (pre procedure) | Age, height, weight, BMI, maximum aneurysm diameter, maximum aneurysm sites | 0.0277 |
| Package 2 and 3 (post procedure) | Age, height, weight, BMI, maximum aneurysm diameter, maximum aneurysm sites, creatinine level, haemoglobin level | 0.0073 |
In addition, we used standard statistical tests (log-rank, Student’s t-test, Mann–Whitney U-test, Pearson’s chi-squared test and Fisher’s exact test) to assess the associations between missing variable status (yes/no) and other variables (including outcomes). These analyses showed that survival (either pre or post intervention) was significantly related to missing covariate status for weight, BMI, use of formal/informal care, hypertension and extracardiac arteriopathy. As described above, NYHA class missingness was significantly associated with treatment group. In addition, missing status for specific covariates was related to other measured covariates (data not shown).
Overall, these exploratory analyses suggested that imputation models can be informed by related measured covariates in the data set.
Development of the imputation model
This section provides the technical details of imputation models using MICE.
For analysis of pre-procedure survival, treatment group, pre-procedure survival time from consent, whether or not aneurysm was related to death, death status before the index procedure and all pre-procedure variables were included in the imputation models.
For analysis of post-procedure survival, procedure type (ESG, OSR or hybrid) and procedure priority were included in the imputation models, in addition to the covariates included in the pre-procedure imputation models above (updated to just before the procedure, if appropriate).
In the MICE procedure, we used predictive mean matching for all covariates included in imputation models except BMI. For the imputation of missing BMI, a fixed formula was used.
The percentages of incomplete data for the variables used in imputation models for pre- and post-procedure analysis were 11.1% and 29.8%, respectively. We therefore set the number of imputations in MICE for the two analyses to 12 and 30. During the imputation process, we ran 10 imputation iterations for each imputed variable. The imputed values in the last iteration were used for generating an imputation data set. Those imputed values generated during the process were used for checking whether or not the values converged by inspecting the trajectories.
Results of survival models after multiple imputation
The results of refitting the final models are given in Tables 32 and 33 for pre- and post-procedure survival. These results, which are based on the assumption that data are missing at random conditional on variables included in the imputation models, are almost identical to the complete-case results. It is not possible to tell whether data are missing not at random from the measurements available in the ETTAA study, but the small number of missing data in general (apart from for biomarkers) and the similarity in missing data frequencies between comparison groups suggest that the results will be robust, unless there is an extreme non-random missing data mechanism in play.
| Variable | All-cause deaths | Aneurysm-related deaths | ||
|---|---|---|---|---|
| HR (95% CI) | z-test p-value | HR (95% CI) | z-test p-value | |
| Female sex | 1.79 (1.25 to 2.54) | 0.001 | 2.55 (1.55 to 4.21) | < 0.001 |
| NYHA per class | 1.21 (0.98 to 1.50) | 0.073 | ||
| Maximum aneurysm size per cm | 1.89 (1.64 to 2.17) | < 0.001 | 2.18 (1.80 to 2.63) | < 0.001 |
| Age at consent (years) | < 0.001 | 0.027 | ||
| 61–70 | 2.00 (0.74 to 5.36) | 1.30 (0.41 to 4.15) | ||
| 71–80 | 2.80 (1.12 to 7.01) | 1.53 (0.53 to 4.41) | ||
| > 80 | 5.89 (2.33 to 14.89) | 3.38 (1.15 to 9.92) | ||
| Variable | All-cause deaths | Aneurysm-related deaths | ||
|---|---|---|---|---|
| HR (95% CI) | z-test p-value | HR (95% CI) | z-test p-value | |
| Ascending/arch procedure | (Reference category) | 0.017 | (Reference category) | 0.041 |
| OSR in DTA/SRAA | 2.84 (1.23 to 6.58) | 2.87 (0.97 to 8.46) | ||
| ESG in DTA/SRAA | 1.55 (0.69 to 3.49) | 1.44 (0.48 to 4.31) | ||
| Age (per decade) | 1.50 (1.15 to 1.96) | 0.003 | ||
| Female | 2.03 (1.10 to 3.76) | 0.024 | ||
| NYHA (per class) | 1.39 (1.06 to 1.81) | 0.017 | ||
| Preoperative time in study (per month) | 1.03 (1.01 to 1.05) | 0.009 | 1.03 (1.01 to 1.06) | 0.019 |
Appendix 3 Participating centres
| Centre | Management group, n (%) | |||
|---|---|---|---|---|
| WW (N = 489) | CM (N = 112) | ESG (N = 150) | OSR (N = 135) | |
| Addenbrookes | 11 (2.3) | 8 (7.1) | 8 (5.3) | 2 (1.5) |
| Bedford | 7 (1.4) | 0 (0.0) | 3 (2.0) | 1 (0.7) |
| Birmingham | 3 (0.6) | 0 (0.0) | 1 (0.7) | 0 (0.0) |
| Blackpool | 6 (1.2) | 3 (2.7) | 1 (0.7) | 7 (5.2) |
| Brighton & Sussex | 11 (2.3) | 7 (6.3) | 9 (6.0) | 1 (0.7) |
| Bristol | 13 (2.7) | 3 (2.7) | 11 (7.3) | 8 (5.9) |
| Central Manchester | 14 (2.9) | 4 (3.6) | 4 (2.7) | 3 (2.2) |
| Derby | 12 (2.5) | 0 (0.0) | 0 (0.0) | 1 (0.7) |
| Glenfield | 38 (7.8) | 1 (0.9) | 4 (2.7) | 2 (1.5) |
| Guy’s & St Thomas’ | 12 (2.5) | 1 (0.9) | 9 (6.0) | 5 (3.7) |
| Hull | 1 (0.2) | 1 (0.9) | 0 (0.0) | 1 (0.7) |
| Imperial | 30 (6.1) | 10 (8.9) | 23 (15.3) | 7 (5.2) |
| King’s | 8 (1.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Leeds | 29 (5.9) | 14 (12.5) | 13 (8.7) | 7 (5.2) |
| Liverpool Heart & Chest | 15 (3.1) | 2 (1.8) | 0 (0.0) | 7 (5.2) |
| Musgrove Park | 17 (3.5) | 2 (1.8) | 1 (0.7) | 0 (0.0) |
| Newcastle | 15 (3.1) | 0 (0.0) | 8 (5.3) | 1 (0.7) |
| Norfolk & Norwich | 37 (7.6) | 0 (0.0) | 9 (6.0) | 5 (3.7) |
| North Cumbria | 1 (0.2) | 3 (2.7) | 1 (0.7) | 2 (1.5) |
| Papworth | 72 (14.7) | 12 (10.7) | 2 (1.3) | 39 (28.9) |
| Plymouth | 19 (3.9) | 1 (0.9) | 0 (0.0) | 3 (2.2) |
| Royal Free | 7 (1.4) | 3 (2.7) | 6 (4.0) | 0 (0.0) |
| Royal Liverpool | 13 (2.7) | 0 (0.0) | 5 (3.3) | 0 (0.0) |
| Sheffield | 16 (3.3) | 6 (5.4) | 1 (0.7) | 3 (2.2) |
| South Manchester | 7 (1.4) | 0 (0.0) | 2 (1.3) | 1 (0.7) |
| South Tees | 8 (1.6) | 1 (0.9) | 2 (1.3) | 0 (0.0) |
| Southampton | 32 (6.5) | 12 (10.7) | 9 (6.0) | 27 (20.0) |
| St George’s | 20 (4.1) | 5 (4.5) | 17 (11.3) | 0 (0.0) |
| Surrey & Sussex | 0 (0.0) | 6 (5.4) | 0 (0.0) | 0 (0.0) |
| York | 15 (3.1) | 7 (6.3) | 1 (0.7) | 2 (1.5) |
Appendix 4 Plots of planned against actual recruitment by final management group
FIGURE 19.
Target and actual recruitment over time by management group. (a) WW; (b) CM; (c) ESG; and (d) OSR.
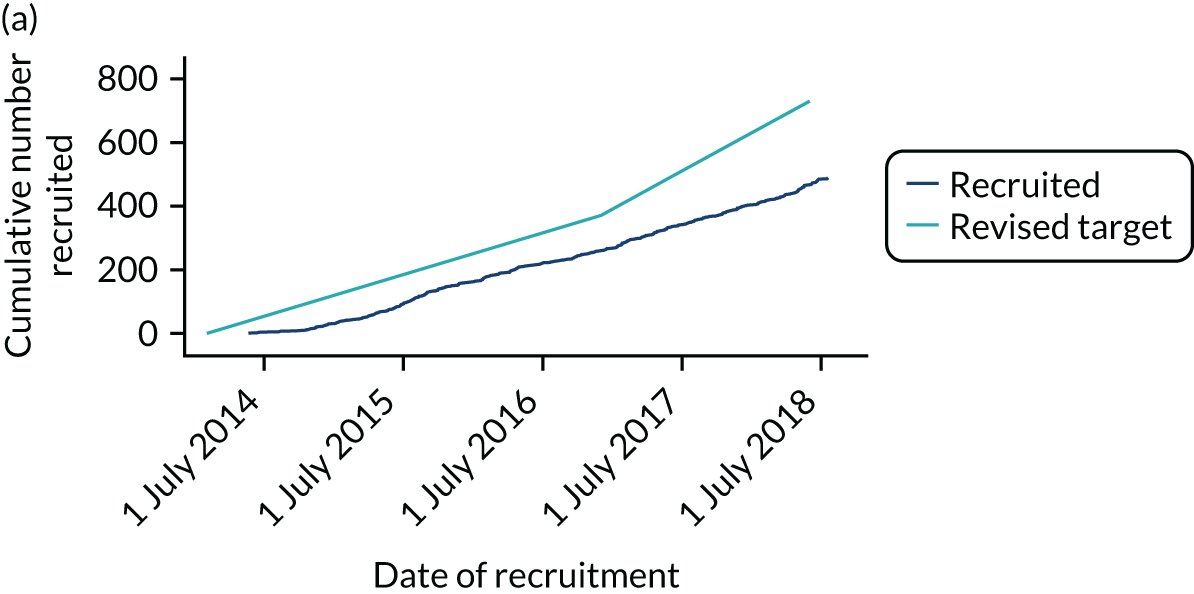
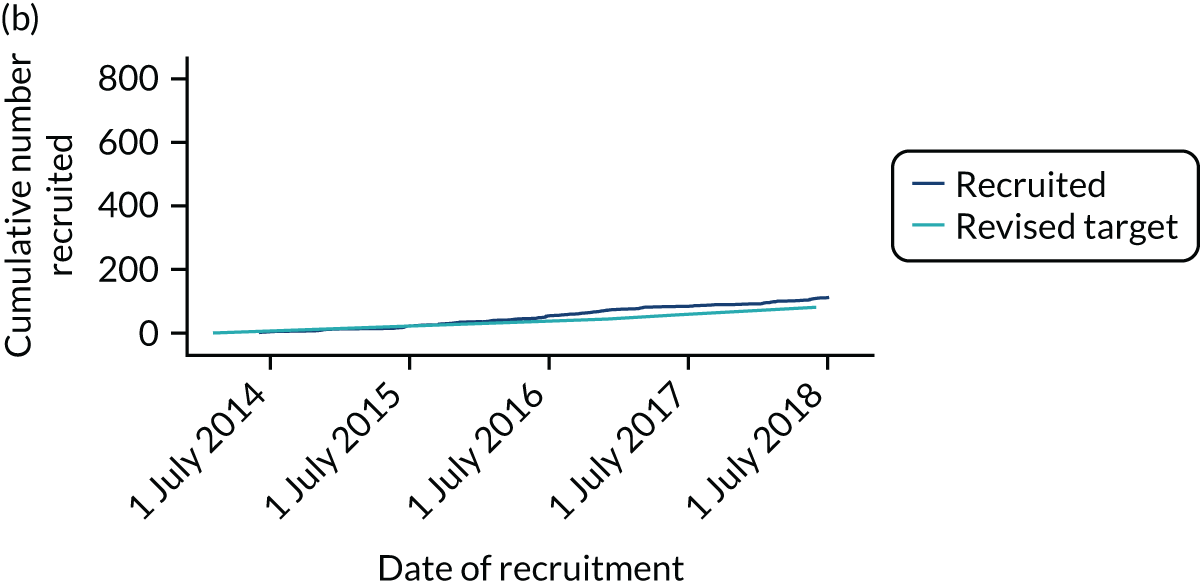
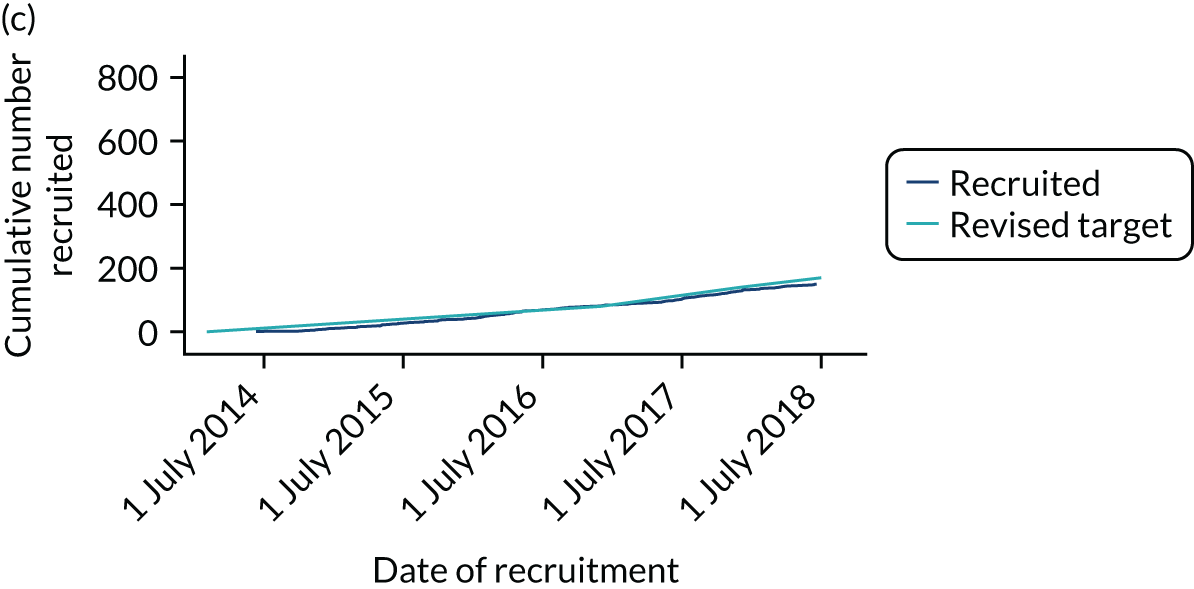
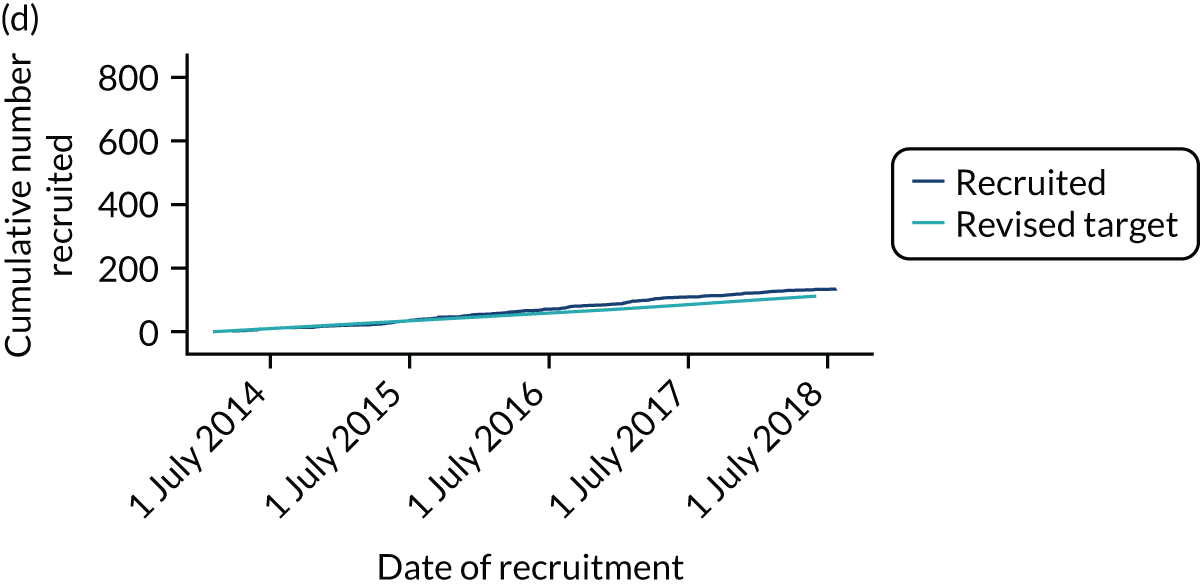
Appendix 5 Complications after second and third procedures
| Procedure/complication | Patient subgroup (number of patients) | |
|---|---|---|
| ESG (n = 12) | OSR (n = 25) | |
| Second procedure ESG/OSR | 12/0 | 21/4 |
| Third procedure ESG/OSR | – | 3/0 |
| Deaths during admission for procedurea | 1 | 2 |
| During additional procedure | ||
| Complications of the procedure | ||
| Endoleak | 2 | 1 |
| During additional procedure admission | ||
| Number of complications | ||
| Gastrointestinal | 0 | 1 |
| Neurological (cerebrovascular accident) | 2 | 0 |
| Spinal cord injury | 0 | 1 |
| Thromboembolic event | 1 | 1 |
| Infection | 0 | 3 |
| Vocal cord palsy | 0 | 1 |
| Inotropes/intra-aortic balloon pump | 1 | 6 |
| Prolonged ventilation | 0 | 3 |
| Renal support | 0 | 1 |
| Return to theatre | 2 | 2 |
| Total number of events | 6 | 19 |
| Total number of people with ≥ 1 event (%) | 4 (33.3) | 10 (40.0) |
Appendix 6 Descriptive summaries of variables at baseline (recruitment to the ETTAA study)
| Patient subgroup (number of patients with a registration scan) | p-value | ||||
|---|---|---|---|---|---|
| WW (N = 489) | CM (N = 112) | ESG (N = 150) | OSR (N = 135) | ||
| Age (years) | < 0.0001 | ||||
| Mean (SD) | 70.8 (10.7) | 76.6 (9.9) | 72.0 (8.6) | 64.9 (11.6) | |
| Median (IQ) | 72.4 (13.1) | 77.8 (9.0) | 74.3 (11.0) | 66.7 (16.1) | |
| Minimum, maximum | 32.3, 92.5 | 26.1, 92.5 | 49.6, 89.2 | 31.6, 83.5 | |
| Sex, n (%) | 0.4297 | ||||
| Female | 174 (35.6) | 48 (42.9) | 50 (33.3) | 49 (36.3) | |
| Male | 315 (64.4) | 64 (57.1) | 100 (66.7) | 86 (63.7) | |
| Height (cm) | < 0.0001 | ||||
| Mean (SD) | 171.3 (10.2) | 167.4 (12.5) | 170.0 (10.0) | 173.7 (11.3) | |
| Median (IQ) | 173.0 (13.0) | 165.0 (18.0) | 170.0 (16.0) | 174.0 (17.0) | |
| Minimum, maximum | 138.0, 205.0 | 132.0, 216.0 | 149.0, 201.0 | 147.0, 210 | |
| Missing, n (%) | 19 (3.9) | 9 (8.0) | 4 (2.7) | 3 (2.2) | |
| Weight (kg) | 0.0001 | ||||
| Mean (SD) | 80.6 (17.2) | 74.2 (17.3) | 78.6 (15.5) | 83.9 (17.5) | |
| Median (IQ) | 79.0 (21.0) | 74.5 (18.5) | 79.0 (21.0) | 85.0 (23.0) | |
| Minimum, maximum | 41.0, 143.0 | 42.0, 146.0 | 44.0, 123.0 | 41.0, 130.0 | |
| Missing, n (%) | 22 (4.5) | 8 (7.1) | 5 (3.3) | 3 (2.2) | |
| BMI (kg/m2) | 0.1880 | ||||
| Mean (SD) | 27.5 (5.0) | 26.5 (4.9) | 27.1 (4.3) | 27.7 (4.6) | |
| Median (IQ) | 26.9 (6.4) | 25.9 (5.7) | 27.3 (6.4) | 27.6 (6.9) | |
| Minimum, maximum | 13.8, 47.5 | 18.1, 43.6 | 18.9, 43.6 | 16.0, 38.0 | |
| Missing, n (%) | 24 (4.9) | 9 (8.0) | 6 (4.0) | 3 (2.2) | |
| Care, n (%) | 0.002a | ||||
| Formal | 10 (2.0) | 5 (4.5) | 0 (0.0) | 1 (0.7) | |
| Informal | 50 (10.2) | 18 (16.1) | 12 (8.0) | 7 (5.2) | |
| None | 425 (86.9) | 88 (78.6) | 138 (92.0) | 125 (92.6) | |
| Missing | 4 (0.8) | 1 (0.9) | 0 (0.0) | 2 (1.5) | |
| Smoker (current or past), n (%) | 0.353 | ||||
| Yes | 343 (70.1) | 71 (63.4) | 113 (75.3) | 89 (65.9) | |
| No | 142 (29.0) | 40 (35.7) | 36 (24.0) | 45 (33.3) | |
| Missing | 4 (0.8) | 1 (0.9) | 1 (0.7) | 1 (0.7) | |
| Patient subgroup (number of patients with a registration scan) | p-value | ||||
|---|---|---|---|---|---|
| WW (N = 489) | CM (N = 112) | ESG (N = 150) | OSR (N = 135) | ||
| CTD, n (%) | < 0.0001 | ||||
| Yes | 30 (6.1) | 3 (2.7) | 2 (1.3) | 20 (14.8) | |
| No | 459 (93.9) | 109 (97.3) | 148 (98.7) | 115 (85.2) | |
| Extracardiac arteriopathy, n (%) | 0.4940 | ||||
| Yes | 71 (14.5) | 20 (17.9) | 26 (17.3) | 16 (11.9) | |
| No | 406 (83.0) | 91 (81.3) | 123 (82.0) | 118 (87.4) | |
| Missing | 12 (2.5) | 1 (0.9) | 1 (0.7) | 1 (0.7) | |
| Valvular heart disease, n (%) | 0.0013 | ||||
| Yes | 389 (79.6) | 87 (77.7) | 134 (89.3) | 96 (71.1) | |
| No | 89 (18.2) | 23 (20.5) | 15 (10.0) | 38 (28.2) | |
| Missing | 11 (2.3) | 2 (1.8) | 1 (0.7) | 1 (0.7) | |
| Coronary artery disease, n (%) | 0.3712 | ||||
| CABG | 26 (5.3) | 10 (8.9) | 7 (4.7) | 8 (5.9) | |
| Medication | 46 (9.4) | 9 (8.0) | 14 (9.3) | 8 (5.9) | |
| No | 377 (77.1) | 85 (75.9) | 123 (82.0) | 116 (85.9) | |
| PCI | 27 (5.5) | 6 (5.4) | 5 (3.3) | 2 (1.5) | |
| Missing | 13 (2.7) | 2 (1.8) | 1 (0.7) | 1 (0.7) | |
| LV function, n (%) | < 0.0001 | ||||
| Good | 199 (40.7) | 41 (36.6) | 64 (42.7) | 79 (58.5) | |
| Moderate | 30 (6.1) | 14 (12.5) | 13 (8.7) | 19 (14.1) | |
| Poor | 11 (2.3) | 2 (1.8) | 2 (1.3) | 0 (0.0) | |
| Not measured | 241 (49.3) | 55 (49.1) | 70 (46.7) | 36 (26.7) | |
| Missing | 8 (1.6) | 0 (0.0) | 1 (0.7) | 1 (0.7) | |
| Diabetes, n (%) | 0.2350a | ||||
| No | 432 (88.3) | 105 (93.8) | 137 (91.3) | 126 (93.3) | |
| NIDDM | 52 (10.6) | 7 (6.3) | 13 (8.7) | 8 (5.9) | |
| IDDM | 2 (0.4) | 0 (0.0) | 0 (0.0) | 1 (0.7) | |
| Missing | 3 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Hypertension, n (%) | 0.7856 | ||||
| Yes | 424 (86.7) | 97 (86.6) | 135 (90.0) | 119 (88.2) | |
| No | 63 (12.9) | 15 (13.4) | 15 (10.0) | 16 (11.9) | |
| Missing | 2 (0.4) | 0 (0) | 0 (0.0) | 0 (0) | |
| COPD, n (%) | 0.1772 | ||||
| Yes | 87 (17.8) | 26 (23.2) | 32 (21.3) | 18 (13.3) | |
| No | 397 (81.2) | 86 (76.8) | 118 (78.7) | 117 (86.7) | |
| Missing | 5 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| NYHA class, n (%) | 0.4187 | ||||
| I | 198 (40.5) | 39 (34.8) | 68 (45.3) | 54 (40.0) | |
| II | 175 (35.8) | 41 (36.6) | 47 (31.3) | 52 (38.5) | |
| III | 86 (17.6) | 27 (24.1) | 20 (13.3) | 17 (12.6) | |
| IV | 16 (3.3) | 3 (2.7) | 4 (2.7) | 3 (2.2) | |
| Missing | 14 (2.9) | 2 (1.8) | 11 (7.3) | 9 (6.7) | |
| Serum creatinine level (µmol/l) | 0.0068 | ||||
| Mean (SD) | 96.0 (32.8) | 104.9 (39.8) | 92.6 (31.9) | 85.7 (27.3) | |
| Median (IQ) | 89.0 (39.0) | 97.5 (56.5) | 88.0 (34.0) | 82.0 (32.0) | |
| Minimum, maximum | 45.0, 227.0 | 44.0, 225.0 | 43.0, 200.0 | 32.0, 186.0 | |
| Missing, n (%) | 309 (63.2) | 60 (53.6) | 42 (28.0) | 48 (35.6) | |
| Haemoglobin level (g/l) | 0.0420 | ||||
| Mean (SD) | 127.5 (19.1) | 128.4 (15.8) | 131.7 (16.2) | 133.6 (17.3) | |
| Median (IQ) | 128.0 (24.0) | 129.0 (23.0) | 133.0 (20.0) | 137.0 (25.0) | |
| Minimum, maximum | 76.0, 175.0 | 98.0, 171.0 | 77.0, 176.0 | 90.0, 165.0 | |
| Missing, n (%) | 326 (66.7) | 64 (57.1) | 44 (29.3) | 50 (37.0) | |
| Patient subgroup (number of patients with a registration scan) | p-value | ||||
|---|---|---|---|---|---|
| WW (N = 489) | CM (N = 112) | ESG (N = 150) | OSR (N = 135) | ||
| Beta-blocker use, n (%) | 0.5608 | ||||
| Yes | 255 (52.2) | 51 (45.5) | 74 (49.3) | 72 (53.3) | |
| No | 234 (47.9) | 61 (54.5) | 76 (50.7) | 63 (46.7) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| ACE inhibitor use, n (%) | 0.06342 | ||||
| Yes | 116 (23.7) | 39 (34.8) | 45 (30.0) | 40 (29.6) | |
| No | 373 (76.3) | 73 (65.2) | 105 (70.0) | 95 (70.4) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| ARB use, n (%) | 0.5416 | ||||
| Yes | 94 (19.2) | 26 (23.2) | 28 (18.7) | 32 (23.7) | |
| No | 395 (80.8) | 86 (76.8) | 122 (81.3) | 103 (76.3) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Calcium channel blocker use, n (%) | 0.7909 | ||||
| Yes | 176 (36.0) | 35 (31.3) | 55 (36.7) | 47 (34.8) | |
| No | 313 (64.0) | 77 (68.8) | 95 (63.3) | 88 (65.2) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Other antihypertensives, n (%) | 0.1429 | ||||
| Yes | 65 (13.3) | 24 (21.4) | 24 (16.0) | 17 (12.6) | |
| No | 424 (86.7) | 88 (78.6) | 126 (84.0) | 118 (87.4) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Any antihypertensive, n (%) | 0.7900 | ||||
| Yes | 412 (84.3) | 94 (83.9) | 131 (87.3) | 116 (85.9) | |
| No | 77 (15.8) | 18 (16.1) | 19 (12.7) | 19 (14.1) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Statins, n (%) | < 0.0001 | ||||
| Yes | 283 (57.9) | 72 (64.3) | 106 (70.7) | 51 (37.8) | |
| No | 204 (41.7) | 40 (35.7) | 44 (29.3) | 84 (62.2) | |
| Missing | 2 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
Appendix 7 Pre-intervention longitudinal models for aneurysm growth and health-related quality of life
| Parameter fixed effects | Coefficient (SE) (95% CI) | p-value (z-test) |
|---|---|---|
| Main effects | ||
| Intercept | 5.29 (0.06) (5.18 to 5.40) | < 0.001 |
| Time per year | 0.07 (0.02) (0.03 to 0.12) | 0.105 |
| Age at scan (per decade) | 0.17 (0.03) (0.11 to 0.22) | < 0.001 |
| Patient height (per 10 cm) | 0.06 (0.02) (0.02 to 0.11) | 0.004 |
| Smoking history | 0.035 | |
| Current smoker | 0.23 (0.10) (0.05 to 0.42) | |
| Ex-smoker | 0.12 (0.06) (0.00 to 0.25) | |
| Site (reference DTA) | < 0.001 | |
| Ascending | –1.15 (0.06) (–1.26 to –1.03) | |
| Suprarenal abdominal | –1.92 (0.06) (–2.04 to –1.80) | |
| Arch | –1.15 (0.06) (–1.27 to –1.04) | |
| MRI relative to CT | 0.00 (0.04) (–0.08 to 0.08) | 0.960 |
| Connective tissue disease | 0.23 (0.12) (–0.01 to 0.47) | 0.056 |
| COPD | 0.27 (0.08) (0.12 to 0.42) | < 0.001 |
| Valvular heart disease | –0.12 (0.07) (–0.26 to 0.02) | 0.096 |
| Interactions | ||
| Time–MRI interaction | –0.11 (0.03) (–0.18 to –0.04) | 0.001 |
| Time–site interaction | < 0.001 | |
| Ascending | –0.07 (0.02) (–0.12 to –0.02) | |
| Suprarenal abdominal | 0.03 (0.03) (–0.02 to 0.08) | |
| Arch | –0.03 (0.02) (–0.08 to 0.02) | |
| Site–age at scan interaction | < 0.001 | |
| Ascending | 0.02 (0.03) (–0.04 to 0.07) | |
| Suprarenal abdominal | –0.13 (0.03) (–0.18 to –0.07) | |
| Arch | –0.08 (0.03) (–0.13 to –0.02) | |
| Site–connective tissue disease interaction | < 0.001 | |
| Ascending | –0.25 (0.13) (–0.51 to 0.01) | |
| Suprarenal abdominal | 0.33 (0.14) (0.06 to 0.59) | |
| Arch | –0.23 (0.12) (–0.47 to 0.02) | |
| Site–COPD interaction | < 0.001 | |
| Ascending | –0.22 (0.08) (–0.38 to –0.06) | |
| Suprarenal abdominal | –0.29 (0.08) (–0.45 to –0.13) | |
| Arch | –0.31 (0.08) (–0.47 to –0.16) | |
| Site–valvular heart disease interaction | < 0.001 | |
| Ascending | 0.37 (0.08) (0.22 to 0.52) | |
| Suprarenal abdominal | 0.10 (0.08) (–0.06 to 0.25) | |
| Arch | 0.14 (0.07) (0.00 to 0.29) | |
| Site–smoking history interaction | < 0.001 | |
| Ascending–current | –0.22 (0.10) (–0.42 to –0.03) | |
| Suprarenal abdominal–current | 0.04 (0.10) (–0.16 to 0.25) | |
| Arch–current | –0.33 (0.10) (–0.52 to –0.13) | |
| Ascending–ex | –0.22 (0.07) (–0.35 to –0.09) | |
| Suprarenal abdominal–ex | 0.00 (0.07) (–0.13 to 0.14) | |
| Arch–ex | –0.25 (0.06) (–0.38 to –0.12) | |
| Random effects | ||
| SD (intercept) | 0.54 | |
| Residual error | 0.81 | |
| Parameter fixed effects | Coefficient (SE) (95% CI) | p-value (z-test) |
|---|---|---|
| Main effects | ||
| Intercept | 0.849 (0.015) (0.819 to 0.879) | < 0.001 |
| Time per year | –0.010 (0.006) (–0.022 to 0.003) | 0.128 |
| Age per decade | 0.013 (0.006) (0.000 to 0.025) | 0.051 |
| Female sex | –0.029 (0.013) (–0.055 to –0.002) | 0.032 |
| Formal/informal care | –0.206 (0.025) (–0.255 to –0.156) | < 0.001 |
| Group (reference WW) | 0.5131 | |
| CM | –0.018 (0.028) (–0.074 to 0.037) | |
| ESG | 0.015 (0.026) (–0.037 to 0.066) | |
| OSR | –0.033 (0.028) (–0.088 to 0.023) | |
| NYHA per class | –0.089 (0.010) (–0.108 to –0.069) | < 0.001 |
| Smoking history | 0.042 | |
| Current smoker | –0.047 (0.022) (–0.091 to –0.004) | |
| Ex-smoker | 0.003 (0.015) (–0.026 to 0.032) | |
| Interactions | ||
| Time–age per decade interaction | –0.013 (0.003) (–0.019 to –0.007) | < 0.001 |
| Time–smoking history interaction | 0.004 | |
| Current smoker | –0.034 (0.012) (–0.057 to –0.010) | |
| Ex-smoker | 0.003 (0.008) (–0.012 to 0.018) | |
| Care–group interaction | 0.009 | |
| CM | 0.086 (0.051) (–0.014 to 0.186) | |
| ESG | –0.007 (0.070) (–0.144 to 0.131) | |
| OSR | 0.239 (0.077) (0.088 to 0.390) | |
| NYHA–group interaction (per class) | 0.004 | |
| CM | –0.037 (0.023) (–0.083 to 0.009) | |
| ESG | –0.075 (0.026) (–0.126 to –0.024) | |
| OSR | 0.036 (0.026) (–0.015 to 0.087) | |
| Random effects | ||
| SD (time slope) | 0.038 | |
| SD (intercept) | 0.160 | |
| Residual error by group | ||
| WW SD (residuals) | 0.121 | |
| CM SD (residuals) | 0.133 | |
| ESG SD (residuals) | 0.122 | |
| OSR SD (residuals) | 0.142 | |
Appendix 8 Descriptive summaries for variables measured just before procedures
| Patient subgroup (number of patients with a registration scan) | p-value | ||
|---|---|---|---|
| ESG (N = 150) | OSR (N = 135) | ||
| Age (years) | < 0.0001 | ||
| Mean (SD) | 72.6 (8.6) | 65.4 (11.6) | |
| Median (IQ) | 74.5 (10.7) | 67.6 (16.1) | |
| Minimum, maximum | 49.8, 89.2 | 31.7, 84.6 | |
| Sex, n (%) | 0.6265 | ||
| Female | 50 (33.1) | 49 (36.6) | |
| Male | 101 (66.9) | 85 (63.4) | |
| Height (m) | 0.005 | ||
| Mean (SD) | 170.0 (10.0) | 173.7 (11.3) | |
| Median (IQ) | 170.0 (15.8) | 174.0 (17.0) | |
| Minimum, maximum | 149.0, 201.0 | 147.0, 210.0 | |
| Missing, n (%) | 4 (2.7) | 3 (2.2) | |
| Weight (kg) | 0.007 | ||
| Mean (SD) | 78.6 (15.5) | 83.9 (17.5) | |
| Median (IQ) | 79.0 (21.0) | 85.0 (22.5) | |
| Minimum, maximum | 44.0, 123.0 | 41.0, 130.0 | |
| Missing, n (%) | 5 (3.3) | 3 (2.2) | |
| BMI (kg/m2) | 0.269 | ||
| Mean (SD) | 27.1 (4.3) | 27.7 (4.6) | |
| Median (IQ) | 27.3 (6.4) | 27.6 (6.9) | |
| Minimum, maximum | 18.9, 43.6 | 16.0, 38.0 | |
| Missing, n (%) | 6 (4.0) | 3 (2.2) | |
| Care, n (%) | 0.3674a | ||
| Formal | 0 (0.0) | 2 (1.5) | |
| Informal | 12 (8.0) | 9 (6.7) | |
| None | 138 (92.0) | 123 (91.1) | |
| Missing | 0 (0.0) | 1 (0.7) | |
| Smoker (current or past), n (%) | 0.1054 | ||
| Yes | 113 (75.3) | 89 (65.9) | |
| No | 36 (24.0) | 45 (33.3) | |
| Missing | 1 (0.7) | 1 (0.7) | |
| Patient subgroup (number of patients with a registration scan) | p-value | ||
|---|---|---|---|
| ESG (N = 150) | OSR (N = 135) | ||
| CTD, n (%) | < 0.0001 | ||
| Yes | 2 (1.3) | 20 (14.9) | |
| No | 148 (98.7) | 115 (85.1) | |
| Extracardiac arteriopathy, n (%) | 0.2567 | ||
| Yes | 26 (17.3) | 16 (11.9) | |
| No | 123 (82.0) | 118 (87.4) | |
| Valvular heart disease, n (%) | 0.0002 | ||
| Yes | 134 (89.3) | 96 (71.1) | |
| No | 15 (10.0) | 38 (28.2) | |
| Missing | 1 (0.7) | 1 (0.7) | |
| Coronary artery disease, n (%) | 0.6640 | ||
| CABG | 7 (4.7) | 8 (5.9) | |
| Medication | 14 (9.3) | 8 (5.9) | |
| No | 123 (82.0) | 116 (85.9) | |
| PCI | 5 (3.3) | 2 (1.5) | |
| Missing | 1 (0.7) | 1 (0.7) | |
| LV function, n (%) | 0.8189 | ||
| Good | 35 (21.3) | 56 (41.5) | |
| Moderate | 5 (3.3) | 10 (7.4) | |
| Poor | 0 (0.0) | 1 (0.7) | |
| Missing | 113 (75.3) | 68 (50.4) | |
| Diabetes, n (%) | 0.6959a | ||
| No | 137 (91.3) | 126 (93.3) | |
| NIDDM | 13 (8.7) | 8 (5.9) | |
| IDDM | 0 (0.0) | 1 (0.7) | |
| Hypertension, n (%) | 0.2141 | ||
| Yes | 136 (90.7) | 115 (85.2) | |
| No | 14 (9.3) | 20 (14.8) | |
| COPD n (%) | 0.1120 | ||
| Yes | 32 (21.3) | 18 (13.3) | |
| No | 118 (78.7) | 116 (86.7) | |
| NYHA class, n (%) | 0.5241 | ||
| I | 62 (41.3) | 55 (40.7) | |
| II | 46 (30.7) | 50 (37.0) | |
| III | 28 (18.7) | 18 (13.3) | |
| IV | 3 (2.0) | 2 (1.5) | |
| Missing, n (%) | 11 (7.3) | 10 (7.4) | |
| Serum creatinine level (µmol/l) | 0.119 | ||
| Mean (SD) | 89.0 (31.1) | 96.1 (37.7) | |
| Median (IQR) | 84.5 (36.2) | 88.0 (33.5) | |
| Minimum, maximum | 43.0, 194.0 | 32.0, 229.0 | |
| Missing, n (%) | 22 (14.7) | 32 (23.7) | |
| Haemoglobin level (g/l) | 0.003 | ||
| Mean (SD) | 124.8 (17.1) | 131.6 (16.8) | |
| Median (IQR) | 127.0 (21.0) | 133.0 (24.0) | |
| Minimum, maximum | 71.0, 168.0 | 86.0, 168.0 | |
| Missing, n (%) | 21 (14.0) | 31 (23.0) | |
| Patient subgroup (number of patients with a registration scan) | p-value | ||
|---|---|---|---|
| ESG (N = 150), n (%) | OSR (N = 135), n (%) | ||
| Beta-blocker | 0.1956 | ||
| Yes | 73 (48.7) | 77 (57.0) | |
| No | 77 (51.3) | 58 (43.0) | |
| Missing | 0 (0.0) | 0 (0.0) | |
| ACE inhibitor | 0.9400 | ||
| Yes | 45 (30.0) | 39 (28.9) | |
| No | 105 (70.0) | 96 (71.1) | |
| Missing | 0 (0.0) | 0 (0.0) | |
| ARB | 0.4511 | ||
| Yes | 29 (19.3) | 32 (23.7) | |
| No | 121 (80.7) | 103 (76.3) | |
| Missing | 0 (0.0) | 0 (0.0) | |
| Calcium channel blocker | 0.3624 | ||
| Yes | 60 (40.0) | 46 (34.1) | |
| No | 90 (60.0) | 89 (65.9) | |
| Missing | 0 (0.0) | 0 (0.0) | |
| Other antihypertensives | 0.2559 | ||
| Yes | 26 (17.3) | 16 (11.9) | |
| No | 124 (82.7) | 119 (88.1) | |
| Missing | 0 (0.0) | 0 (0.0) | |
| Any antihypertensive | 0.4860 | ||
| Yes | 133 (88.7) | 115 (85.2) | |
| No | 17 (11.3) | 20 (14.8) | |
| Missing | 0 (0.0) | 0 (0.0) | |
| Statins | < 0.0001 | ||
| Yes | 104 (69.3) | 57 (42.2) | |
| No | 46 (30.7) | 78 (57.8) | |
| Missing | 0 (0.0) | 0 (0.0) | |
Appendix 9 Reasons for return to theatre
| Patient subgroup (n) | ||
|---|---|---|
| ESG (N = 150) | OSR (N = 135) | |
| Reason | ||
| Access vessel injury | 4 | 2 |
| Aneurysm complication (includes re-exploration for bleeding/tamponade) | 4 | 13 |
| Fistulae | 0 | 0 |
| Reintervention | 0 | 0 |
| Endoleak | 5 | 0 |
| Stent graft complication | 1 | 0 |
| Other acute surgical complication (e.g. tracheostomy, bronchoscopy, laparotomy) | 3 | 12 |
| Planned abdominal aneurysm intervention | 1 | 0 |
| Total number of events | 18 | 27 |
| Total number of people with ≥ 1 event (%) | 16 (10.7) | 20 (14.8) |
Appendix 10 Post-intervention longitudinal models for health-related quality of life
| Parameter fixed effects | Coefficient (SE) | 95% CI | p-value (z-test) |
|---|---|---|---|
| Main effects | |||
| Intercept | 0.785 (0.030) | 0.725 to 0.844 | < 0.001 |
| Time per year | –0.001 (0.006) | –0.012 to 0.013 | 0.913 |
| First 6 weeks | –0.017 (0.023) | –0.062 to 0.027 | 0.440 |
| OSR | –0.020 (0.024) | –0.066 to 0.026 | 0.396 |
| Female sex | –0.028 (0.027) | –0.080 to 0.025 | 0.302 |
| Preoperative HRQoL | 0.473 (0.051) | 0.374 to 0.572 | < 0.001 |
| NYHA per class | –0.034 (0.016) | –0.066 to –0.003 | 0.033 |
| Smoking history | 0.046 | ||
| Current smoker | –0.095 (0.038) | –0.171 to –0.020 | |
| Ex-smoker | –0.031 (0.028) | –0.085 to 0.023 | |
| Interactions | |||
| Time–female interaction | 0.028 (0.013) | 0.003 to 0.054 | 0.029 |
| OSR–first 6 weeks interaction | –0.142 (0.029) | –0.199 to –0.085 | < 0.0001 |
| Random effects | |||
| SD (intercept) | 0.156 | ||
| Residual error by group | |||
| ESG SD (residuals) | 0.161 | ||
| OSR SD (residuals) | 0.126 | ||
Appendix 11 Health economics detailed tables
| Resource or unit intervention | Unit | Mean usage in standard ESG | Mean usage in standard OSR | Resource source |
|---|---|---|---|---|
| Fixed costs: theatre usage overheads | ||||
| Operating room | Average theatre duration | 4 hours 56 minutes | 8 hours 53 minutes | ETTAA study procedure CRF |
| Operating room with C-arm | Average theatre duration | 4 hours 52 minutes | N/A | ETTAA study procedure CRF |
| Catheterisation laboratory | Average theatre duration | 4 hours 5 minutes | N/A | ETTAA study procedure CRF |
| Hybrid theatre | Average theatre duration | 3 hours 19 minutes | N/A | ETTAA study procedure CRF |
| Interventional radiology equipment | Per hour | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Capital equipment | ||||
| Cooling head jacket | Per procedure | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Basic vascular tray | Per procedure | 2 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Cardiac major tray | Per procedure | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Diathermy console | Per procedure | 1 | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Sternal saw | Per procedure | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Defibrillator paddles | Per procedure | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Cell saver machine | Per procedure | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Sternal retractors | Per procedure | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Bypass machine | Per procedure | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| 3M™ Bair Hugger™ System – 3M (Bracknell, UK) | Per procedure | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Rapid transfuser/fluid warmer | Per procedure | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Suction machine | Per procedure | 1 | 1 |
Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Injection pump | Per procedure | 1 | 4 |
Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Staff | ||||
| Consultant surgeon | Per hour | 1 | 1 |
Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Assistant surgeon | Per hour | 1 | 1 |
Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Consultant anaesthetist | Per hour | Included in theatre cost except catheterisation laboratory | Included in theatre cost | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Anaesthetist registrar | Per hour | Included in theatre cost except catheterisation laboratory | Included in theatre cost | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Consultant radiologist | Per hour | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Assistant/registrar radiologist | Per hour | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Radiographer | Per hour | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Surgical care practitioner | Per hour | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Anaesthetic nurse | Per hour | 1 | 1 |
Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Scrub nurse (table) | Per hour | 1 | 1 |
Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Scrub nurse (floor) | Per hour | 1 | 1 |
Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Health-care assistant | Per hour | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Perfusionist | Per hour | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Consumables | ||||
| Central lines | Per item | 1 | 1 |
Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Arterial lines | Per item | 1 | 1 |
Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Nasopharyngeal probe | Per item | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Urinary catheter | Per item | 1 | 1 |
Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Diathermy pad | Per item | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Swabs | Per item | 20 | 60 |
Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Size 15 knife blade | Per item | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Size 10 knife blade | Per item | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Cell saver tubing and fluids | Per item | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Side towels | Per item | N/A | 2 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Vicryl® stay suture (Johnson & Johnson Medical NV, Brussels, Belgium) | Per item | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Cannulae (all sorts) | Per item | N/A | 4 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Axillary cannular | Per item | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Per item | N/A | 10 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 | |
| Prolene® suture (Johnson & Johnson Medical NV, Brussels, Belgium) 5/0 | Per item | 3 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Prolene suture 3/0 | Per item | N/A | 5 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Maxolon ADVANZ Pharma (London, UK) | Per item | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Monocryl suture (Ethicon, Raritan, NJ, USA) | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Vicryl suture 2/0 | Per item | 2 | 2 |
Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Bypass circuit disposable bits | Per item | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Teflon® pledgets/strips (BD, Franklin Lakes, NJ, USA) | Per item | N/A | 10 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 |
| Bair Hugger blanket | Per item | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Haemostatic adjuncts | Per item | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Sternal wires | Per item | N/A | 3 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Biosyn™ (Biosyn Corporation, Carlsbad, CA, USA) | Per item | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Dressings | Per item | N/A | 2 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Argyl drain | Per item | N/A | 2 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Redivac drain | Per item | N/A | 1 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Drain sutures braided nylon | Per item | N/A | 4 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Spinal drain | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Suction tube | Per item | 2 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Sheath | Per item | 2 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Angiography hollow needles | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Pigtail catheter | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| J-wire | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Terumo wire | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Super stiff Meier™ wire | Per item | 2 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Extension for injection pump | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Iodinated contrast | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Injection pump contract syringe | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Measuring pigtail catheter | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| 12F sheath | Per item | 2 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Saline | Per item | 2 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Sterile bowls for coiling wires | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Moulding balloon | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Syringe 20 ml | Per item | 2 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Diathermy forceps | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Blade | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| SURGICEL® | Per item | 1 | N/A | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Blood products | ||||
| Heparin | Per 1000 units/1 ml solution | N/A | 0 | ETTAA study procedure CRF |
| Protamine | Per sulfate 50 mg/5 ml | N/A | 0 | ETTAA study procedure CRF |
| Standard red cells | Per unit | 0.46 | 5.06 | ETTAA study procedure CRF |
| Platelets, pooled | Per unit | 0.07 | 1.63 | ETTAA study procedure CRF |
| FFP | Per unit (275 ml) | 0.10 | 3.51 | ETTAA study procedure CRF |
| Cryoprecipitate, pooled | Per unit (200 ml) | 0.03 | 2.26 | ETTAA study procedure CRF |
| Octaplex® (Octapharma, Manchester, UK) | Per unit (500iu) | N/A | 2 | ETTAA study procedure CRF |
| Beriplex® (CSL Behring, Haywards Heath, UK) | Per unit (500iu) | N/A | 0.06 | ETTAA study procedure CRF |
| Fibrinogen | Per unit (1 g) | N/A | 0.31 | ETTAA study procedure CRF |
| Albumin | Per unit (100 ml of 20%) | N/A | 0 | ETTAA study procedure CRF |
| NovoSeven® (Novo Nordisk Inc., Plainsboro, NJ, USA) | Per unit (2 mg) | N/A | 0.29 | ETTAA study procedure CRF |
| Resource or intervention | Unit | Mean usage in standard ESG | Mean usage in standard OSR | Resource source |
|---|---|---|---|---|
| Type of stay | ||||
| ICU | Per day | 1.85 | 10.66 | ETTAA study, post-procedure form and discharge CRF |
| HDU | Per day | 1.46 | 1.77 | ETTAA study, post-procedure form and discharge CRF |
| Ward | Per day | 6.38 | 8.77 | ETTAA study, post-procedure form and discharge CRF |
| Ward after transfer | Per day | 0.71 | 17.89 | ETTAA study, post-procedure form and discharge CRF |
| Blood products | ||||
| Standard red blood cells | Per unit | 0.58 | 1.66 | ETTAA study, post-procedure form and discharge CRF |
| Platelets, pooled | Per unit | 0.24 | 0.31 | ETTAA study, post-procedure form and discharge CRF |
| FFP | Per unit (275 ml) | 0.07 | 0.66 | ETTAA study, post-procedure form and discharge CRF |
| Cryoprecipitate, pooled | Per unit (200 ml) | 0.03 | 0.11 | ETTAA study, post-procedure form and discharge CRF |
| Albumin | Per unit | 0 | 0.09 | ETTAA study, post-procedure form and discharge CRF |
| Octuplex | Per unit | 0 | 0.06 | ETTAA study, post-procedure form and discharge CRF |
| Pasmalyte | Per unit | 0.01 | 0 | ETTAA study, post-procedure form and discharge CRF |
| Imaging | ||||
| CT | Per investigation | 0.95 | 1.17 | ETTAA study, post-procedure form and discharge CRF |
| MRI | Per investigation | 0.18 | 0.06 | ETTAA study, post-procedure form and discharge CRF |
| X-ray (plain films) | Per investigation | 1.14 | 6.54 | ETTAA study, post-procedure form and discharge CRF |
| TOE | Per investigation | 0.03 | 0.09 | ETTAA study, post-procedure form and discharge CRF |
| TTE | Per investigation | 0.10 | 0.46 | ETTAA study, post-procedure form and discharge CRF |
| Angiography | Per investigation | 0.10 | 0.03 | ETTAA study, post-procedure form and discharge CRF |
| Ultrasound | Per investigation | 0.19 | 0.20 | ETTAA study, post-procedure form and discharge CRF |
| Fluoroscopy | Per investigation | 0 | 0.03 | ETTAA study, post-procedure form and discharge CRF |
| Renography | Per investigation | 0 | 0.03 | ETTAA study, post-procedure form and discharge CRF |
| Echocardiography | Per investigation | 0.02 | 0 | ETTAA study, post-procedure form and discharge CRF |
| Return to theatre | ||||
| Return to theatre | Per event | 0.15 | 0.20 | ETTAA study, return to theatre CRF |
| Resource or intervention | Unit | Mean usage | |
|---|---|---|---|
| ESG | OSR | ||
| Follow-up: 1 month | ESG (n = 74) | OSR (n = 16) | |
| Formal care | Per hour | 2.91 | 0 |
| Informal care | Per hour | 21.76 | 31.58 |
| Nurse visits | Per visit | 0.54 | 0.31 |
| Nurse home visits | Per visit | 0.97 | 0.31 |
| GP visits | Per visit | 0.69 | 0.38 |
| GP home visits | Per visit | 0.14 | 0.25 |
| Physiotherapist visits | Per visit | 0.01 | 0 |
| A&E visits | Per visit | 0.14 | 0.13 |
| CT scans | Per visit | 1 | 0 |
| Outpatient appointments vascular surgery (consultant led) | Per visit | 1 | 0 |
| Outpatient appointments cardiothoracic surgery (consultant led) | Per visit | 0 | 0 |
| Additional procedures | Per event | 0.05 | 0.06 |
| Hospital admissions | Per event | 0.11 | 0 |
| Follow-up: 3 months | ESG (n = 76) | OSR (n = 19) | |
| Formal care | Per hour | 3.97 | 0 |
| Informal care | Per hour | 34.13 | 61.71 |
| Nurse visits | Per visit | 1.46 | 0.68 |
| Nurse home visits | Per visit | 0.24 | 0.16 |
| GP visits | Per visit | 0.88 | 1.32 |
| GP home visits | Per visit | 0.11 | 0.05 |
| Physiotherapist visits | Per visit | 0.13 | 0.63 |
| A&E visits | Per visit | 0.08 | 0.16 |
| CT scans | Per visit | 0 | 0 |
| Outpatient appointments vascular surgery (consultant led) | Per visit | 0 | 0 |
| Outpatient appointments cardiothoracic surgery (consultant led) | Per visit | 0 | 0 |
| Additional procedures | Per event | 0.01 | 0.05 |
| Hospital admissions | Per event | 0.08 | 0 |
| Follow-up: 6 months | ESG (n = 81) | OSR (n = 24) | |
| Formal care | Per hour | 0.67 | 13.63 |
| Informal care | Per hour | 137.18 | 55.23 |
| Nurse visits | Per visit | 1.16 | 1.33 |
| Nurse home visits | Per visit | 0.35 | 2.33 |
| GP visits | Per visit | 1.41 | 2.17 |
| GP home visits | Per visit | 0.1 | 0 |
| Physiotherapist visits | Per visit | 0.36 | 0.54 |
| A&E visits | Per visit | 0.11 | 0.08 |
| CT scans | Per visit | 0 | 1 |
| Outpatient appointments vascular surgery (consultant led) | Per visit | 0 | 0 |
| Outpatient appointments cardiothoracic surgery (consultant led) | Per visit | 0 | 1 |
| Additional procedures | Per event | 0.02 | 0.08 |
| Hospital admissions | Per event | 0.04 | 0 |
| Follow-up: 12 months | ESG (n = 74) | OSR (n = 20) | |
| Formal care | Per hour | 1.15 | 378.83 |
| Informal care | Per hour | 56.81 | 120.25 |
| Nurse visits | Per visit | 1.36 | 2.45 |
| Nurse home visits | Per visit | 2.09 | 7.9 |
| GP visits | Per visit | 1.78 | 2.4 |
| GP home visits | Per visit | 0.04 | 0.1 |
| Physiotherapist visits | Per visit | 0.55 | 1.25 |
| A&E visits | Per visit | 0.26 | 0.35 |
| CT scans | Per visit | 1 | 1 |
| Outpatient appointments vascular surgery (consultant led) | Per visit | 1 | 0 |
| Outpatient appointments cardiothoracic surgery (consultant led) | Per visit | 0 | 1 |
| Additional procedures | Per event | 0.01 | 0 |
| Hospital admissions | Per event | 0.07 | 0 |
| Follow-up: 18 months | ESG (n = 59) | OSR (n = 16) | |
| Formal care | Per hour | 38.1 | 120.44 |
| Informal care | Per hour | 206.68 | 132.59 |
| Nurse visits | Per visit | 1.34 | 2.69 |
| Nurse home visits | Per visit | 1.12 | 12.44 |
| GP visits | Per visit | 1.46 | 3.06 |
| GP home visits | Per visit | 0.02 | 0.38 |
| Physiotherapist visits | Per visit | 0.19 | 0.13 |
| A&E visits | Per visit | 0.15 | 0.56 |
| CT scans | Per visit | 0 | 0 |
| Outpatient appointments vascular surgery (consultant led) | Per visit | 0 | 0 |
| Outpatient appointments cardiothoracic surgery (consultant led) | Per visit | 0 | 0 |
| Additional procedures | Per event | 0 | 0 |
| Hospital admissions | Per event | 0.02 | 0 |
| Follow-up: 24 months | ESG (n = 35) | OSR (n = 11) | |
| Formal care | Per hour | 1.49 | 94.73 |
| Informal care | Per hour | 96.77 | 71.69 |
| Nurse visits | Per visit | 1.03 | 2.73 |
| Nurse home visits | Per visit | 0.11 | 1.27 |
| GP visits | Per visit | 1.63 | 4.82 |
| GP home visits | Per visit | 0.06 | 0.09 |
| Physiotherapist visits | Per visit | 0.4 | 1.09 |
| A&E visits | Per visit | 0.09 | 0.09 |
| CT scans | Per visit | 1 | 1 |
| Outpatient appointments vascular surgery (consultant led) | Per visit | 1 | 0 |
| Outpatient appointments cardiothoracic surgery (consultant led) | Per visit | 0 | 1 |
| Additional procedures | Per event | 0.03 | 0.09 |
| Hospital Admissions | Per event | 0.03 | 0 |
| Follow-up: 36 months | ESG (n = 17) | OSR (n = 5) | |
| Formal care | Per hour | 0 | 0 |
| Informal care | Per hour | 16.77 | 366.17 |
| Nurse visits | Per visit | 0.82 | 1.8 |
| Nurse home visits | Per visit | 0 | 0 |
| GP visits | Per visit | 2.53 | 4 |
| GP home visits | Per visit | 0 | 0 |
| Physiotherapist visits | Per visit | 0 | 1.2 |
| A&E visits | Per visit | 0.24 | 0 |
| CT scans | Per visit | 1 | 1 |
| Outpatient appointments vascular surgery (consultant led) | Per visit | 1 | 0 |
| Outpatient appointments cardiothoracic surgery (consultant led) | Per visit | 0 | 1 |
| Additional procedures | Per event | 0.12 | 0.2 |
| Hospital admissions | Per event | 0.18 | 0 |
| Follow-up: 48 months | ESG (n = 3) | OSR (n = 0) | |
| Formal care | Per hour | 0 | N/A |
| Informal care | Per hour | 34.67 | N/A |
| Nurse visits | Per visit | 2 | N/A |
| Nurse home visits | Per visit | 0 | N/A |
| GP visits | Per visit | 3 | N/A |
| GP home visits | Per visit | 0 | N/A |
| Physiotherapy visits | Per visit | 0 | N/A |
| A&E visits | Per visit | 0 | N/A |
| CT scans | Per visit | 1 | N/A |
| Outpatient appointments vascular surgery (consultant led) | Per visit | 1 | N/A |
| Outpatient appointments cardiothoracic surgery (consultant led) | Per visit | 0 | N/A |
| Additional procedures | Per event | 0 | N/A |
| Hospital admissions | Per event | 0 | N/A |
| Resource or unit intervention | OSR group | ESG group | Cost (£) | Cost source | ||
|---|---|---|---|---|---|---|
| Fixed costs | ||||||
| Operating room for OSR | Yes | N/A | 518.00a | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Operating room for ESGb | N/A | Yes | 550.08a,b | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Operating room with C-armb | N/A | Yes | 550.08a,b | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Catheterisation laboratory | N/A | Yes | 252.08b | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton in 2019 and 2020 | ||
| Hybrid theatre | N/A | Yes | 550.08a,b | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Interventional radiology equipment | Yes | 32.08 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | |||
| Capital equipment costs | OSR group | ESG group | Capital | Annualised cost | Per operating session (253 days) | Cost source |
| Cooling head jacket | Yes | N/A | 12,000 (5 years) | £2657.00 | £11.00 | Capital cost from Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Basic vascular tray | N/A | Yes | 7000 (10 years) | £841.65 | £3.33 | Capital cost from Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Resource or unit intervention | OSR group | ESG group | Capital | Annualised cost | Per operating session (253 days) | Cost source |
| Diathermy console | Yes | Yes | 9000 (5 years) | £1993.00 | £7.88 | Capital cost from Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Sternal saw | Yes | N/A | 7500 (5 years) | £1661.00 | £6.57 | Capital cost from Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Cell saver machine | Yes | N/A | 5000 (5 years) | £1107.00 | £4.38 | Capital cost from Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Sternal retractors | Yes | N/A | 6900 (5 years) | £1528.00 | £6.04 | Capital cost from Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Bypass machine | Yes | N/A | 10,500 (5 years) | £2325.00 | £9.20 | Capital cost from Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Bair Hugger™ (3M™, Bracknell, UK) machine | Yes | N/A | 3750 (1 year) | £3880.00 | £15.35 | Capital cost from Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Rapid transfuser/fluid warmer | Yes | N/A | 15,000 (5 years) | £3322.00 | £13.13 | Capital cost from Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 |
| Suction machine | N/A | Yes | 500 (5 years) | £111.00 | £0.44 | Capital cost from Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Injection pump | N/A | Yes | 2000 (5 years) | £443.00 | £1.75 | Capital cost from Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 |
| Resource or unit intervention | OSR group | ESG group | Cost (£) | Cost source | ||
| Defibrillator paddles | Yes | N/A | 3.00 | Capital cost from Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 | ||
| Cardiac major tray | Yes | N/A | 50.00 | Capital cost from Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 | ||
| Staff costs | ||||||
| Consultant surgeon | Yes | Yes | 109.00 | PSSRU 2018/1994 based on ‘consultant: surgical’ | ||
| Assistant surgeon | Yes | Yes | 47.00 | PSSRU 2018/1994 based on ‘registrar’ | ||
| Consultant anaesthetist (included in theatre cost, except catheterisation laboratory) | Yes | Yes | 109.00 | PSSRU 2018/1994 based on ‘consultant: medical’ | ||
| Anaesthetist registrar (included in theatre cost, except catheterisation laboratory) | Yes | Yes | 47.00 | PSSRU 2018/1994 based on ‘registrar’ | ||
| Consultant radiologist | N/A | Yes | 109.00 | PSSRU 2018/1994 based on ‘consultant: medical’ | ||
| Assistant/registrar radiologist | N/A | Yes | 47.00 | PSSRU 2018/1994 based on ‘registrar’ | ||
| Radiographer | N/A | Yes | 37.00 (band 5) | PSSRU 2018/1994 based on hospital-based ‘scientific and professional staff’ | ||
| Surgical care practitioner | Yes | N/A | 65.00 (band 8a) | PSSRU 2018/1994 based on ‘hospital-based nurses’ | ||
| Anaesthetic nurse | Yes | Yes | 47.00 (band 6) | PSSRU 2018/1994 based on ‘hospital-based nurses’ | ||
| Scrub nurse (table) | Yes | Yes | 38.00 (band 5) | PSSRU 2018/1994 based on ‘hospital-based nurses’ | ||
| Scrub nurse (floor) | Yes | Yes | 38.00 (band 5) | PSSRU 2018/1994 based on ‘hospital-based nurses’ | ||
| Health-care assistant | N/A | Yes | 8.93 (band 2 with 3–4 years’ experience) | NHS Employers website108 2018/19 hourly rate | ||
| Perfusionist | Yes | N/A | 62.35 | PSSRU 2018/1994 based on the average of band 7 and band 8a ‘hospital-based scientific and professional staff’ | ||
| Consumables costs | ||||||
| Central lines | Yes | Yes | 25.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 | ||
| Arterial lines | Yes | Yes | 25.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 | ||
| Nasopharyngeal probe | Yes | N/A | 15.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and 2020 | ||
| Urinary catheter | Yes | Yes | 36.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Diathermy pad | Yes | N/A | 1.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Swabs | Yes | Yes | 0.50 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Size 15 knife blade | Yes | N/A | 10.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Size 10 knife blade | Yes | N/A | 10.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Cell saver tubing and fluids | Yes | N/A | 235.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Side towels | Yes | N/A | 1.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Vicryl® (Johnson & Johnson NV, Brussels, Belgium) stay suture | Yes | N/A | 3.15 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Cannula (all sorts) | Yes | N/A | 0.50 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Axillary cannula | Yes | N/A | 0.50 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| 4/0 Prolene® (Johnson & Johnson NV, Brussels, Belgium) | Yes | N/A | 7.30 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| 5/0 Prolene | N/A | Yes | 7.30 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| 3/0 Prolene | Yes | N/A | 3.15 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Maxalon (ADVANZ Pharma, London, UK) | Yes | N/A | 3.15 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Monocryl (Ethicorn, Raritan, NJ, USA) suture | Yes | Yes | 25.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| 2/0 Vicryl | Yes | Yes | 3.15 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Bypass circuit disposable bits | Yes | N/A | 650.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Teflon pledgets/strips (BD, Franklin Lakes, NJ, USA) | Yes | N/A | 1.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Bair Hugger blanket | Yes | N/A | 6.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Haemostatic adjuncts | Yes | N/A | 25.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Sternal wires | Yes | N/A | 42.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Biosyn | Yes | N/A | 3.15 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Dressings | Yes | N/A | 0.50 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Argyl drain | Yes | N/A | 5.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Redivac drain | Yes | N/A | 5.00 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Drain sutures braided nylon | Yes | N/A | 3.15 | Royal Papworth Hospital NHS Trust based on personal communication with Rosie Thornton between November 2019 and June 2020 | ||
| Spinal drain | N/A | Yes | 413.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Suction tube | N/A | Yes | 1.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Sheath | N/A | Yes | 10.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Angiography hollow needles | N/A | Yes | 2.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Pigtail catheter | N/A | Yes | 10.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| J wire | N/A | Yes | 65.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Terumo wire | N/A | Yes | 5.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Super stiff Meier wire | N/A | Yes | 70.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Extension for injection pump | N/A | Yes | 9.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Iodinated contrast | N/A | Yes | 10.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Injection pump contract syringe | N/A | Yes | 15.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Measuring pigtail catheter | N/A | Yes | 12.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| 12F Sheath | N/A | Yes | 7.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Saline | N/A | Yes | 5.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Sterile bowls for coiling wires | N/A | Yes | 30.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Moulding balloon | N/A | Yes | 300.00 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Syringe 20ml | N/A | Yes | 0.15 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Diathermy forceps | N/A | Yes | 195.80 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Blade | N/A | Yes | 5.89 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Surgicel | N/A | Yes | 55.82 | Imperial College Healthcare NHS Trust based on personal communication with Colin Bicknell between January and June 2020 | ||
| Blood products | ||||||
| Heparin | Yes | N/A | 14.85 | BNF NICE 2018/2019109 | ||
| Protamine | Yes | N/A | 49.55 | BNF NICE 2018/2019109 | ||
| Standard red cells | Yes | Yes | 128.99 | NHS Blood and Transplant Price list 2018/1996 | ||
| Platelets, pooled | Yes | Yes | 185.86 | NHS Blood and Transplant Price list 2018/1996 | ||
| Fresh frozen plasma | Yes | Yes | 28.46 | NHS Blood and Transplant Price list 2018/1996 | ||
| Cryoprecipitate, pooled | Yes | Yes | 177.55 | NHS Blood and Transplant Price list 2018/1996 | ||
| Octaplex (Octapharma, Manchester, UK) | Yes | N/A | 125.00 | Royal Papworth Hospital NHS Trust based on personal communication with Priya Sastry in July 2020 | ||
| Beriplex (CSL Behring, Haywards Heath, UK) | Yes | N/A | 125.00 | Royal Papworth Hospital NHS Trust based on personal communication with Priya Sastry in July 2020 | ||
| Fibrinogen | Yes | N/A | 364.00 | Royal Papworth Hospital NHS Trust based on personal communication with Priya Sastry in July 2020 | ||
| Albumin | Yes | N/A | 42.50 | Royal Papworth Hospital NHS Trust based on personal communication with Priya Sastry in July 2020 | ||
| NovoSeven® (Novo Nordisk Inc., PLainsboro, NJ, USA) | Yes | N/A | 919.00 | Royal Papworth Hospital NHS Trust based on personal communication with Priya Sastry in July 2020 | ||
| Resource or unit intervention | Cost (£) | Unit | Cost source |
|---|---|---|---|
| Type of stay | |||
| ICU | 1417.63 | Per day | From NICE110 inflated using the PSSRU index94 |
| HDU | 724.18 | Per day | From NICE110 inflated using the PSSRU index94 |
| Ward | 416.90 | Per day | From NICE110 inflated using the PSSRU index94 |
| Blood products | |||
| Standard red blood cells | 128.99 | Per unit | NHS Blood and Transplant Price list 2018/1996 |
| Platelets, pooled | 185.86 | Per unit | NHS Blood and Transplant Price list 2018/1996 |
| FFP | 28.46 | Per unit | NHS Blood and Transplant Price list 2018/1996 |
| Cryoprecipitate, pooled | 177.55 | Per unit | NHS Blood and Transplant Price list 2018/1996 |
| Imaging | |||
| CT | 97.00 | Per scan | National Schedule of NHS costs 2018/19.111 Weighted average of codes RD20A, RD21A, and RD22Z to RD27Z |
| MRI | 341.00 | Per scan | National Schedule of NHS costs 2018/19.111 Weighted average of codes RD08Z to RD10Z |
| X-ray (plain films) | 31.00 | Per scan | National Schedule of NHS costs 2018/19.111 Code: DAPF |
| TOE | 257.00 | Per scan | National Schedule of NHS costs 2018/19.111 Code: EY50Z as ‘Complex Echocardiogram’ |
| TTE | 257.00 | Per scan | National Schedule of NHS costs 2018/19.111 Code: EY50Z as ‘Complex Echocardiogram’ |
| Echocardiogram | 64.00 | Per scan | National Schedule of NHS costs 2018/19.111 Code: RD51A as ‘Simple Echocardiogram, 19 years and over’ |
| Ultrasound | 51.00 | Per scan | National Schedule of NHS costs 2018/19. Code: RD47Z ‘Vascular Ultrasound’ |
| Renogram | 209.00 | Per scan | National Schedule of NHS costs 2018/19.111 Code: RN25A ‘Renogram, 19 years and over’ |
| Fluoroscopy | 118.00 | Per scan | National Schedule of NHS costs 2018/19.111 Weighted average of codes RD30Z, RD31Z, RD32Z |
| Angiogram | 150.00 | Per scan | Royal College of Physicians112 |
| Resource or unit intervention | Cost (£) | Units | Source |
|---|---|---|---|
| Primary/community care | |||
| GP visits (surgery) | 39.00 | Per average contact time 9.22 minutes | PSSRU,94 page 120, £39 per surgery consultation |
| GP visits (home) | 100.62 | Cost of home visit (23.4 minutes including travel time) |
Unit Costs of Health and Social Care 2015,113 page 176, average home visit is 11.4 minutes with 12 minutes of travel time. Cost on 23.4 minutes of GP timea |
| Nurse visit (surgery) | 42.00 | Per hour | GP practice nurse, PSSRU,94 page 118, £42 per houra |
| Nurse visit (surgery) | 10.85 | Per contact (15.5 minutes) |
Unit Costs of Health and Social Care 2015,113 page 174, 15.5 minutes for contacta |
| Nurse visit (home) | 16.38 | Per hour |
Unit Costs of Health and Social Care 2015,113 page 176, average home visit is 11.4 minutes with 12 minutes of travel time. a Cost on 23.4 minutes of GP time. Assumed the travel and contact time is the same for a nurse as a GP |
| Physiotherapy/occupational therapy | 58.00 | Per unit | NHS Reference Costs 2018 to 2019,93 assumed physiotherapy (outpatients code 650)b |
| Formal care | 28.00 | Per hour | PSSRU,94 page 134, used home worker and the face-to-face social hours cost per houra |
| Informal care | 7.83 | Per hour | Minimum wage as of 2018/19 tax year114 |
| Secondary care | |||
| A&E visits | 166.00 | Per visit | National Schedule of NHS costs 2018/19.111 Index ‘AE’ |
| Outpatient appointments vascular surgery (consultant led) | 148.00 | Per appointment | National Schedule of NHS costs 2018/19.111 Service code: 107 in ‘total outpatient attendance’ |
| Outpatient appointments cardiothoracic surgery (consultant led) | 241.00 | Per appointment | National Schedule of NHS costs 2018/19.111 Service code: 170 in ‘total outpatient attendance’ |
| Imaging | |||
| MRI | 341.00 | Per investigation | National Schedule of NHS costs 2018/19.111 Weighted average of codes RD08Z to RD10Z |
| CT | 97.00 | Per investigation | National Schedule of NHS costs 2018/19.111 Weighted Average of codes RD20A, RD21A, RD22Z to RD27Z |
| Condition | HRG code | Cost (£) |
|---|---|---|
| Pleurisy | Weighted average of DZ28Z and DZ28B (pleurisy) | 365 |
| Chest pain | Weighted averages of EB12A to EB12C (unspecified chest pain with a CC score range of 0–11+) | 400 |
| Cardiac event | Weighted averages of EB10A to EB10E (actual or suspected myocardial infarction) | 1478 |
| Infection and haematemesis | Weighted average WH07A to WH07b (infections or other complications of procedures without and with single and with multiple interventions) | 1793 |
| Sepsis | Weighted averages of WJ06A to WJ06J (sepsis without intervention, with intervention and with multiple intervention) | 2206 |
| Elective angiography | Weighted average of EY43A to EY43F (standard cardiac catheterisation) | 2401 |
| Angiography | Weighted averages of EY41A to EY41D (standard percutaneous transluminal coronary angioplasty) | 2689 |
| Groin pseudoaneurysm | Weighted averages of YR11A to YR11D (percutaneous transluminal angioplasty of single blood vessel in CC score range) | 2816 |
| Carotid-subclavian bypass | Weighted averages of YQ31A + YQ31B [single open procedure on the carotid artery (CC score of 0–5+)] | 5260 |
| Elective carotid-subclavian bypass | Elective weighted averages of YQ31A + YQ31B [single open procedure on the carotid artery (CC score of 0–5+)] | 5260 |
| Elective complex endovascular repair of AAA | Elective weighted averages of YR66A to YR67B (standard endovascular repair of AAA and complex) | 7321 |
| Endovascular repair of AAA | Weighted averages of YR66A to YR67B (standard endovascular repair of AAA and complex) | 7499 |
| Elective open surgery repair of AAA | Elective weighted average of open repair of AAA single and multiple open procedures | 9141 |
| Endovascular repair of thoracic or thoracoabdomminal aortic aneurysm (fenestrated) | Weighted averages of YR62A + YR62B + YR63A + YR63B | 9314 |
| Complex repair of descending thoracic aorta (fenestrated) | Weighted averages of YR62A + YR62B + YR63A + YR63B | 9314 |
| Endovascular repair of thoracic or thoracoabdominal aortic aneurysm | Weighted average of YR61Z and YR60Z (standard and complex endovascular repair of thoracoabdominal aortic aneurysm using branched stent graft) | 11,856 |
| Elective complex endovascular repair of thoracic or thoracoabdominal aortic aneurysm | Elective weighted average of YR61Z and YR60Z (standard and complex endovascular repair of thoracoabdominal aortic aneurysm using branched stent graft) | 12,493 |
| Follow-up point (months) | Days after index procedure | |
|---|---|---|
| Minimum | Maximum | |
| 1 | 0 | 60 |
| 3 | 61 | 121 |
| 6 | 122 | 244 |
| 12 | 274 | 456 |
| 18 | 457 | 639 |
| 24 | 640 | 821 |
| 36 | 913 | 1278 |
| 48 | 1279 | 1643 |
| 60 | 1644 | 2008 |
| Resource use | ESG group (n = 115) | OSR group (n = 35) | ||
|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| Total staff time costs | 1972 (1399) | 1683 (1022–2471) | 3611 (1082) | 3556 (2885–3915) |
| Total theatre usage costs including capital and consumable equipment | 3517 (1446) | 3085 (2461–4167) | 6088 (1379) | 6018 (5163–6475) |
| Total graft costs | N/A | N/A | 5461 (6696) | 1117 (436–12,500) |
| Total stent costs | 20,966 (9001) | 20,890 (14,734–28,885) | N/A | N/A |
| Total blood products usage costs | 82 (349) | 0 (0–0) | 2079 (3378) | 869 (117–1819) |
| Total costs of index surgical procedure | 26,536 (9877) | 24,733 (19,300–35,173) | 17,239 (8043) | 15,359 (10,350–21,874) |
| Resource use | ESG group (n = 115) | OSR group (n = 35) | ||
|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| Total general ward costs | 2661 (2849) | 1668 (834–3335) | 3657 (2602) | 3335 (1876–4169) |
| Total ICU costs | 2626 (4600) | 0 (0–4253) | 15,108 (20,413) | 8506 (5671–15,594) |
| Total HDU costs | 1067 (2639) | 0 (0–1448) | 1283 (20,413) | 0 (0–2173) |
| Total blood product usage costs | 129 (559) | 0 (0–0) | 322 (625) | 0 (0–315) |
| Total investigations costs | 254 (335) | 128 (31–363) | 499 (547) | 350 (163–659) |
| Total return to theatre costs | 460 (1432) | 0 (0–0) | 310 (1323) | 0 (0–0) |
| Total costs post procedure until discharge | 7216 (7399) | 5451 (2760–8530) | 21,179 (23,083) | 13,997 (10,480–27,846) |
| Total costs of ward days for patients who are transferred on to another care setting | 297 (1683) | 0 (0–0) | 7457 (21,353) | 0 (0–0) |
| Total costs post procedure until discharge including costs for patients who are transferred | 7484 (7848) | 5516 (2873–8526) | 28,636 (23,083) | 13,997 (10,480–28,040) |
| Follow-up point | ESG group | OSR group | ||
|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| 1 month | ||||
| Sample size, n | 82 | 20 | ||
| Primary | 57 (97) | 11 (0–78) | 39 (54) | 27 (0–42) |
| Secondary | 262 (50) | 245 (245–245) | 14 (61) | 0 (0–0) |
| Hospital admissions | 442 (1961) | 0 (0–0) | 0 (0) | 0 (0–0) |
| Additional procedures | 2097 (9362) | 0 (0–0) | 912 (4078) | 0 (0–0) |
| Total cost to the NHS | 2858 (9581) | 284 (245–420) | 964 (4076) | 28 (0–67) |
| Formal care | 74 (570) | 0 (0–0) | 0 (0) | 0 (0–0) |
| Informal care | 154 (399) | 0 (0–0) | 198 (245) | 115 (0–345) |
| Total cost | 3085 (9640) | 323 (245–701) | 1162 (4044) | 201 (0–511) |
| 3 months | ||||
| Sample size, n | 85 | 23 | ||
| Primary | 98 (121) | 55 (0–136) | 113 (135) | 78 (0–158) |
| Secondary | 208 (126) | 245 (245–245) | 30 (100) | 0 (0–0) |
| Hospital admissions | 928 (3125) | 0 (0–0) | 0 (0) | 0 (0–0) |
| Additional procedures | 2504 (10,118) | 0 (0–0) | 1504 (4992) | 0 (0–0) |
| Total cost to the NHS | 3739 (10,514) | 267 (245–500) | 1647 (4982) | 99 (0–283) |
| Formal care | 110 (493) | 0 (0–0) | 0 (0) | 0 (0–0) |
| Informal care | 350 (1434) | 0 (0–0) | 534 (947) | 151 (0–611) |
| Total cost | 4199 (10,589) | 284 (245–1507) | 2181 (5010) | 428 (0–1189) |
| 6 months | ||||
| Sample size, n | 92 | 28 | ||
| Primary | 189 (210) | 133 (32–256) | 237 (282) | 128 (47–279) |
| Secondary | 219 (136) | 245 (245–245) | 372 (95) | 338 (338–338) |
| Hospital admissions | 1073 (3310) | 0 (0–0) | 13 (69) | 0 (0–0) |
| Additional procedures | 2650 (10,099) | 0 (0–0) | 3210 (11,167) | 0 (0–0) |
| Total cost to the NHS | 4130 (10,489) | 384 (245–724) | 3832 (11,153) | 508 (385–1019) |
| Formal care | 168 (731) | 0 (0–0) | 327 (1222) | 0 (0–0) |
| Informal care | 1292 (4535) | 0 (0–177) | 809 (1649) | 0 (0–607) |
| Total cost | 5591 (11,279) | 438 (245–4031) | 4968 (11,100) | 803 (385–3835) |
| 12 months | ||||
| Sample size, n | 91 | 24 | ||
| Primary | 288 (458) | 154 (52–301) | 538 (813) | 300 (77–567) |
| Secondary | 503 (169) | 490 (490–490) | 741 (189) | 676 (676–710) |
| Hospital admissions | 1379 (4738) | 0 (0–0) | 15 (75) | 0 (0–0) |
| Additional procedures | 3036 (10,802) | 0 (0–0) | 3745 (12,013) | 0 (0–0) |
| Total cost to the NHS | 5206 (11,585) | 696 (495–1387) | 5039 (11,994) | 1105 (784–1821) |
| Formal care | 202 (794) | 0 (0–0) | 9221 (37,547) | 0 (0–0) |
| Informal care | 1234 (3817) | 0 (0–40) | 1729 (3254) | 265 (0–1295) |
| Total cost | 6642 (11,927) | 825 (506–7958) | 15,989 (38,247) | 2213 (1000–14,326) |
| 18 months | ||||
| Sample size, n | 78 | 21 | ||
| Primary | 345 (539) | 213 (78–346) | 838 (1207) | 333 (72–1088) |
| Secondary | 487 (206) | 490 (490–490) | 680 (417) | 676 (676–676) |
| Hospital admissions | 1662 (5179) | 0 (0–0) | 145 (588) | 0 (0–0) |
| Additional procedures | 2205 (9589) | 0 (0–0) | 3501 (12,511) | 0 (0–0) |
| Total cost to the NHS | 4699 (10,748) | 737 (490–1413) | 5164 (12,517) | 1287 (676–2440) |
| Formal care | 1042 (5326) | 0 (0–0) | 4372 (11,742) | 0 (0–0) |
| Informal care | 2762 (12,009) | 0 (0–0) | 2499 (4182) | 211 (0–2784) |
| Total cost | 8503 (19,215) | 821 (490–8474) | 12,034 (17,962) | 3099 (676–16,132) |
| 24 months | ||||
| Sample size, n | 57 | 18 | ||
| Primary | 399 (621) | 248 (54–413) | 1020 (1323) | 583 (56–1743) |
| Secondary | 743 (171) | 735 (735–735) | 1075 (434) | 1014 (1014–1014) |
| Hospital admissions | 1431 (3567) | 0 (0–0) | 170 (635) | 0 (0–0) |
| Additional procedures | 3878 (12,686) | 0 (0–0) | 3170 (9880) | 0 (0–0) |
| Total cost to the NHS | 6451 (12,943) | 1017 (735–3355) | 5434 (9855) | 2430 (1033–3149) |
| Formal care | 268 (895) | 0 (0–0) | 6721 (17,328) | 0 (0–255) |
| Informal care | 1885 (5911) | 0 (0–0) | 1912 (3847) | 11 (0–2190) |
| Total cost | 8605 (13,904) | 1370 (796–8663) | 14,068 (22,532) | 3722 (1033–18,851) |
| 36 months | ||||
| Sample size, n | 40 | 12 | ||
| Primary | 360 (669) | 195 (0–327) | 729 (951) | 153 (0–1219) |
| Secondary | 972 (231) | 980 (980–1116) | 1381 (468) | 1352 (1352–1352) |
| Hospital admissions | 1912 (5118) | 0 (0–0) | 0 (0) | 0 (0–0) |
| Additional procedures | 5143 (14,264) | 0 (0–0) | 7080 (13,541) | 0 (0–4560) |
| Total cost to the NHS | 8386 (14,771) | 1173 (980–9047) | 9190 (13,652) | 2145 (1352–8423) |
| Formal care | 319 (1025) | 0 (0–0) | 1958 (6659) | 0 (0–0) |
| Informal care | 1238 (4744) | 0 (0–0) | 2146 (5590) | 0 (0–1236) |
| Total cost | 9943 (15,318) | 1175 (980–10,950) | 13,293 (14,926) | 3682 (1352–24,118) |
| 48 months | ||||
| Sample size, n | 26 | 7 | ||
| Primary | 312 (730) | 117 (0–315) | 470 (846) | 0 (0–551) |
| Secondary | 1231 (193) | 1225 (1225–1225) | 1787 (610) | 1690 (1690–1690) |
| Hospital admissions | 2279 (6051) | 0 (0–0) | 0 (0) | 0 (0–0) |
| Additional procedures | 5349 (15,212) | 0 (0–0) | 2605 (6893) | 0 (0–0) |
| Total cost to the NHS | 9172 (15,498) | 1418 (1225–8906) | 4863 (7714) | 1690 (1690–2483) |
| Formal care | 468 (1260) | 0 (0–0) | 3356 (8708) | 0 (0–196) |
| Informal care | 1885 (5816) | 0 (0–653) | 3 (8) | 0 (0–0) |
| Total cost | 11525 (16,047) | 1891 (1225–16,281) | 8222 (11,177) | 1690 (1690–12,245) |
| Variable number | Explanatory variable | Reason for selection |
|---|---|---|
| Demographic variables | ||
| 1 | Age | The age of the patient can affect the health-care resources needed. Older people have more comorbidities and might not respond as favourably to surgery as someone who is relatively younger |
| 2 | Sex | There are often differences in resources and, therefore, costs depending on the sex of the patient due to biological differences |
| 3 | BMI | BMI outside the healthy range is associated with higher risk factors of surgery |
| 4 | Diabetes | Diabetes is often associated with higher health-care costs. Therefore, this was included in the initial modelling |
| 5 | Smoking | Smoking status (previous or current smoker) has been shown to have an impact on the resource use during surgical procedures for aneurysm repair |
| 6 | NYHA score | NYHA scores are associated with poorer surgical outcomes relative to those with better scores |
| 7 | Hypertension | Higher levels of resource utilisation for surgical repair of aneurysms have been associated with hypertension |
| 8 | Prior aortic intervention | Previous aortic interventions may result in different resource use needed for interventions |
| 9 | COPD | Respiratory function is also associated with poorer clinical outcomes in surgical repair of aneurysms |
| 10 | Utility (baseline) | Utility scores give an estimate of HRQoL prior to surgical intervention. For patients with lower HRQoL, it is expected that they may need to utilise more health care resources and, therefore, have an impact on costs |
| Resource use variables | ||
| 11 | Number of stents | Stent costs in the ESG group accounted for > 70% of the total costs of the primary procedure. Therefore, the number of stents utilised per procedure could directly affect average total costs |
| 12 | ICU days | ICU is associated with sicker patients and a higher level of resource use |
| 13 | HDU days | HDU is associated with sicker patients and a higher level of resource use |
| 14 | Ward days | Longer length of stay in a hospital ward is linked to sicker patients and higher resource use |
| Resource use cost (by stage) | Costs by treatment group (£) | |||
|---|---|---|---|---|
| ESG (n = 65) | OSR (n = 18) | |||
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |
| Primary procedure cost | 26,939 (10,636) | 25,092 (16,118–36,624) | 18,160 (9611) | 16,280 (9692–24,527) |
| Post procedure until discharge cost | 7054 (7555) | 5590 (2699–7549) | 18,295 (16,211) | 12,132 (10,058–22,527) |
| Follow-up cost NHS | 6794 (13,337) | 744 (551–2001) | 6319 (13,691) | 1284 (798–1920) |
| Total costs NHS | 40,788 (18,834) | 38,483 (26,376–52,330) | 42,774 (23,529) | 34,732 (26,083–57,628) |
| ESG group (n = 65) | OSR group (n = 18) | ||
|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) |
| 0.62 (0.32) | 0.70 (0.47–0.88) | 0.46 (0.35) | 0.62 (0.03–0.73) |
| Variable | Coefficient | SE | p-value | 95% CI |
|---|---|---|---|---|
| ESG cost regression | ||||
| Intercept | 32,362 | 1337 | < 0.001 | 29,876 to 35,131 |
| Smoking current | 10,447 | 4535 | 0.023 | 2449 to 2049 |
| OSR cost regression | ||||
| Intercept | 46,323 | 7475 | < 0.001 | 34,303 to 64,687 |
Glossary
- Conservative management
- Management that excludes open surgical or endovascular interventions.
- EuroQol-5 Dimensions, five-level version
- A questionnaire with five dimensions and five levels for each dimension.
- EuroSCORE
- An assessment of cardiac surgical risk developed using logistic regression.
- Hazard ratio
- The ratio of the instantaneous probabilities of an event in two levels of an independent variable.
- Hybrid procedure
- A procedure that involves both open and endovascular surgery in a single admission.
- Index procedure
- In the current study, the first procedure (endovascular stent grafting or open surgical replacement). If more than one procedure was planned, the first stage of that procedure.
- Interquartile range
- The distance between the first and third quartiles of a measurement.
- Missing at random
- The fact that a measurement is missing does not depend on its value, conditional on (adjustment for) observed data.
- Missing completely at random
- The fact that a measurement is missing does not depend on its value.
- Missing not at random
- The fact that a measurement is missing depends on its value.
- Multiple imputation using chained equations
- A method for imputing missing data when missing at random can be assumed.
- Proportional hazard
- An assumption in survival models that the ratio of hazards in different groups is constant through time.
- Quartile
- The values of a variable such that 25% of measurements lie below (Q1) or above (Q3) the value.
- Staged procedure
- A procedure that is planned to take place in more than one theatre session.
- Watchful waiting
- Management during the study that could, but did not, include open surgical or endovascular interventions.
List of abbreviations
- A&E
- accident and emergency
- AAA
- abdominal aortic aneurysm
- ACE
- angiotensin-converting enzyme
- ARB
- angiotensin receptor blocker
- BMI
- body mass index
- CABG
- coronary artery bypass grafting
- CI
- confidence interval
- CM
- conservative management
- COPD
- chronic obstructive pulmonary disease
- CRF
- case report form
- CT
- computerised tomography
- CTAA
- chronic thoracic aortic aneurysm
- CTD
- connective tissue disorder
- DTA
- descending thoracic aorta
- EQ-5D-5L
- EuroQoL-5 Dimensions, five-level version
- ESG
- endovascular stent grafting
- ETTAA
- Effective Treatments for Thoracic Aortic Aneurysms
- EVAR
- endovascular aneurysm repair
- EVPI
- expected value of perfect information
- EVPPI
- expected value of partial perfect information
- GERAADA
- German Registry for Acute Aortic Dissection Type A
- GP
- general practitioner
- HDU
- high-dependency unit
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- ICU
- intensive care unit
- IPR
- interpercentile range
- IPRAS
- asymmetry-adjusted interpercentile range
- IPTW
- inverse probability of treatment weighting
- IQR
- interquartile range
- IRAD
- International Registry of Acute Aortic Dissections
- LV
- left ventricular
- MAR
- missing at random
- MCAR
- missing completely at random
- MCID
- minimum clinically important difference
- MDT
- multidisciplinary team
- MICE
- multiple imputation using chained equations
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NYHA
- New York Heart Association
- OSR
- open surgical repair
- PCI
- percutaneous coronary intervention
- PI
- principal investigator
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RAND
- Research ANd Development
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- VoI
- value of information
- WW
- watchful waiting
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/ABUT7744).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
