Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number NIHR128729. The contractual start date was in December 2019. The draft report began editorial review in August 2021 and was accepted for publication in May 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Thomson et al. This work was produced by Thomson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Thomson et al.
Chapter 1 Background
Parts of this report are reproduced or adapted with permission from Thomson et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
Malnutrition (or undernutrition) is very common in older people, affecting > 1.3 million older adults (aged ≥ 65 years) in the UK. 2 Malnutrition contributes to £23.5B per year of health and social care spending in the UK, over half of which is attributed to malnutrition in older adults. 2 Frail older people are much more likely to become malnourished than those who are not frail. 3–6 Malnutrition worsens the health of frail older people, making them more vulnerable to longer stays in hospitals, readmissions, infections and delayed recovery. 4 Finding effective ways of managing malnutrition and reducing its adverse consequences is critical for improving the health of frail older people. Current UK recommendations7 for treating malnutrition are to provide oral nutritional support or artificial nutrition support where clinically indicated. 8 Oral nutritional support strategies include dietary advice (help with meal planning), food fortification and/or prescribed oral nutritional supplements (ONS). As guidelines and evidence reviews have not focused on frail older people, this research set out to examine the effectiveness and cost-effectiveness of ONS in this population, and to understand what effective interventions look like to inform the design of future intervention strategies.
Malnutrition and frailty in older people in the UK
The UK population is ageing rapidly. The proportion of population aged ≥ 65 years is set to increase from 12% in 2016 to 18% by 2041 and further, to 26%, by 2066, with the fastest growth expected in the ≥ 85 years age group. 9 Ageing is associated with increased risk of multimorbidity10 and disability,11 which represents a major challenge for future health and social care service provision and funding. 12 There is a critical need to identify effective interventions to mitigate age-related morbidity in populations who are likely to benefit most. Chronic undernutrition or malnutrition is an important contributor to morbidity and mortality in older adults and is amenable to treatment, thereby providing a potential target for intervention.
Malnutrition is the deficiency of energy, protein, vitamins and minerals that causes weight loss, muscle loss and functional limitations,7 and it is common among older adults aged ≥ 65 years. Although malnutrition affects < 10% of independent community-dwelling older adults,13 prevalence is much greater in settings where there are increased care needs. 14 National surveys have detected malnutrition in 28% of hospital admissions, 27% of residential care home residents and 41% of nursing home residents. 15 Malnutrition has serious adverse consequences, including physical decline, and poorer outcomes of diseases and increased complications, such as infections, delayed recovery, hospital readmissions, increased length of hospital stays, more general practitioner visits, and poor quality of life (QoL) and well-being. 2,16
Frail older people are at a particularly high-risk of malnutrition and are three to four times more likely to be malnourished. 3–6 Frailty is conceptualised as an abnormal health state relating to loss of biological reserves causing increased vulnerability to small environmental or health changes, which can lead to disability, falls, long-term care, hospital admissions and mortality. 17,18 Different tools have been used to measure or operationalise frailty, such as the Fried frailty phenotype and the cumulative deficit model. 19,20 Around 1 in 10 people aged > 65 years and around one-quarter to half of people aged > 85 years are living with frailty. 17 Malnutrition and frailty are closely interlinked. Poor nutritional status and weight loss increase the risk of frailty,18,21 and the presence of malnutrition further worsens the health status of frail older people. 4 Nutrition supplementation is recommended as one of the mainstays of intervention in treating frailty (European Society for Clinical Nutrition and Metabolism); however, much of the evidence is based on short-term protein synthesis studies or micronutrient interventions (e.g. amino acids, omega 3, vitamin D) that have not shown consistent benefits on muscle mass and function. 21 Furthermore, a micronutrient treatment approach is unlikely to benefit malnutrition and broader clinical and functional outcomes that are important in frailty.
Description of current service provision
The National Institute for Health and Care Excellence (NICE) CG32 guidelines recommend that health-care professionals consider oral nutrition support to improve nutritional intake for people who can swallow safely and are malnourished or at risk of malnutrition. 7 The guidelines states that oral nutrition support includes any of the following to improve nutritional intake: food fortified with protein, carbohydrate and/or fat, plus minerals and vitamins; snacks; ONS; altered meal patterns; and dietary advice. Dietary advice is recommended (e.g. meal planning, adding nutrients to meals) for older adults at risk of malnutrition, while powdered or liquid supplements (ONS) can be prescribed to those with existing malnutrition or at high risk of developing malnutrition. 7 The cost-effectiveness of these interventions is also unknown. Evidence from reviews so far suggest that prescribed ONS is effective in reducing malnutrition and its consequences, such as delayed wound healing and infections. 22 ONS is often viewed as a mode of managing malnutrition when it is difficult for individuals to consume energy and/or nutrients from food, for example in the case of acute illness or lack of availability of food. 22 Systematic reviews have also reported the cost-effectiveness of ONS in the management of malnutrition. 23–25 Cost-effectiveness evidence suggests that the use of ONS in community settings can reduce hospital stays and admissions (estimated savings of ≥ £119,200 per 100,000 people). 2 However, a key research gap, highlighted in current guidelines, is evidence specifically among frail older people on oral nutritional interventions that are effective in reducing malnutrition.
Individual study findings are not, however, entirely consistent for clinical outcomes, probably because of differences in the type of ONS evaluated and study methodology. 26 Evidence is mainly derived from small trials conducted in heterogeneous populations and across health-care settings. Some reviews have included only hospital patients post surgery,27,28 whereas others have focused on community-dwelling adults29 and mixed populations;25,30,31 this makes it difficult to draw conclusions about the effectiveness of ONS for high-risk populations such as frail older people.
A further gap in knowledge is whether or not prescribed ONS offer additional benefits above other oral nutrition support strategies such as fortified food or expert dietary advice. Dietary counselling is often the first means of nutritional interventions in practice. 32 This includes supporting older people with planning their diet and making meal plans and is delivered by dietitians in the community or in hospitals. Food fortification, including adding specific nutrients (e.g. vitamins, proteins) to the diet, is another form of oral nutritional support. 33 However, although ONS have also been shown to be cost-effective, the costs of other forms of nutritional support, including dietary advice, food snacks and food fortification, to manage malnutrition remain unclear and need to be elucidated. 22 In addition, reviews so far have mostly compared ONS with routine care (i.e. no nutritional support), not necessarily with dietary advice. 22,24,27
In summary, much of the focus of previous reviews on oral nutritional interventions includes disease-related malnutrition and adult populations aged ≥ 18 years, and not frail older people specifically. 31,34–37 Many of these reviews and studies have mostly looked at interventions to treat malnutrition related to diseases [e.g. cancer or human immunodeficiency virus (HIV)] and after surgery, which will have different underlying mechanisms from malnutrition in frail older people. The evidence in current guidelines is also mostly from studies on disease-related malnutrition. 38,39 As noted by topic experts in the NICE CG32 guidelines,7 there is a lack of emphasis on effective interventions to reduce malnutrition among frail older people.
Determinants of malnutrition in frail older people: understanding factors affecting adherence to and acceptability of interventions
The effective treatment and management of malnutrition should be tailored to meet the needs of frail older people. Malnutrition is multifactorial. In addition to comorbidities, several other factors may affect the nutrition of older people. These include physiological changes with ageing (loss of appetite, poor taste and smell, disability), psychosocial aspects (social support, resilience, lack of knowledge about food) and personal resources (poverty, inability to shop for food). 40–43 These factors then lead to slower eating and lack of diet variety, which in turn lead to poor dietary intake (low energy, protein, and key nutrients such as B-vitamins, vitamin A, vitamin C, iron, calcium, zinc), potential weight loss and, ultimately, a state of malnutrition. 32
Issues of compliance and acceptability also play a crucial role in inadequate nutritional support. 22,32,44 Although ONS have been found to be effective, the uptake of and compliance with them can be poor. The taste, texture, temperature and mode of ONS (liquid, powder) all influence the extent to which ONS are consumed, particularly over prolonged periods of time. For example, change in energy density can improve compliance and uptake of ONS. 22 Similar issues of compliance are also relevant for dietary advice and counselling to ensure that diet plans are acceptable and sustainable over time. The delivery and implementation of nutritional support by clinicians and healthcare professionals can also be very variable. 44 This could be due to lack of consistency in guidelines on whether ONS with or without dietary advice is effective in older people. 44 Clinical practice has been reported by dietitians to be influenced by lack of knowledge, ease of implementation, published research and local departmental protocols. 44 Understanding ways to improve the adoption and implementations of evidence-based nutritional support interventions into routine practice is a particular gap in the existing evidence.
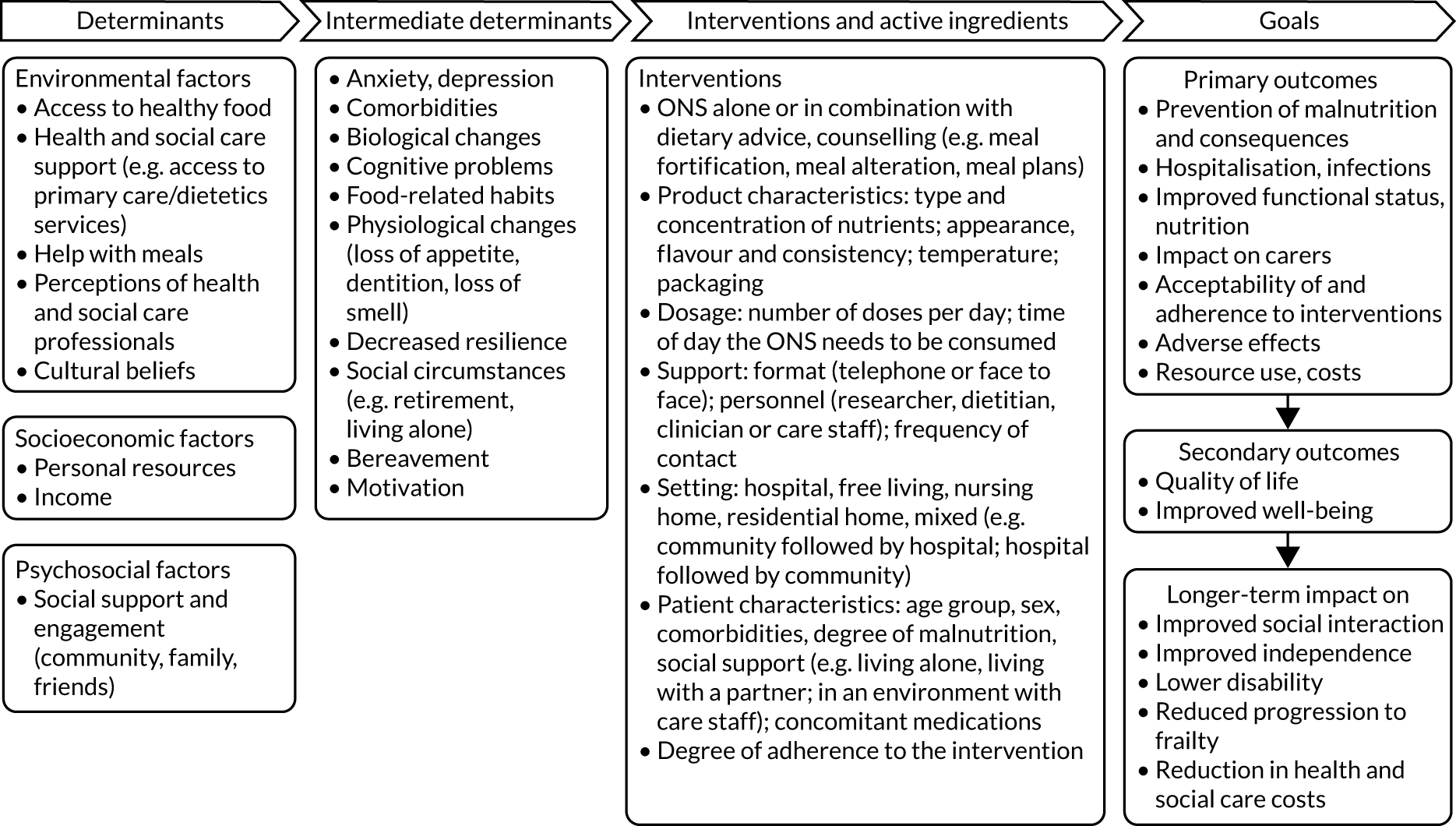
The initial logic model developed prior to the review drew on current evidence and feedback from the preparatory patient and public involvement/engagement (PPIE) work that was undertaken (Figure 1). During the project, the logic model was iteratively refined with emerging findings along with input from stakeholders to produce a final logic model.
FIGURE 1.
Initial logic model.
Aims and objectives
The aim of the study is to evaluate the effectiveness and cost-effectiveness of oral nutritional interventions in frail older people who are malnourished or at risk of malnutrition.
The research objectives are to:
-
systematically review the effectiveness and cost-effectiveness of oral nutritional interventions which include ONS in frail older people who are malnourished or at risk of malnutrition
-
identify components of interventions that are associated with increased effectiveness or adherence
-
systematically review qualitative studies to assess issues related to acceptability of ONS among frail older people who are malnourished or at risk of malnutrition
-
undertake economic modelling to identify the cost-effectiveness of different models of oral nutritional interventions in frail older people who are malnourished or at risk of malnutrition
-
refine and develop a logic model for oral nutritional interventions (including determinants, components and outcomes) to reduce malnutrition in frail older people
-
collate findings and consult with stakeholders to identify (1) recommendations for interventions with potential for testing in future research and (2) implications for practice and policy.
Chapter 2 Methods
The systematic review was registered with PROSPERO (CRD42020170906) and reported in line with PRISMA guidelines. 45 A single search was undertaken for different aspects that this review encompasses, namely effectiveness, adherence and acceptability, and cost-effectiveness.
Search strategy
The search strategy was initially developed in MEDLINE combining the concepts frail older people and nutritional support. Search terms, both text words and subject headings, were identified by an information specialist in conjunction with the project team. Articles previously identified by scoping were also used to identify relevant terms. Population terms included those relating to age, frailty, or care/nursing home settings. Nutritional support included ONS, food fortification, dietary support and malnutrition prevention. Results were restricted to human studies and those published in English. No geographic filters were applied. Publication filters were also not used as a range of publication types were relevant, which allowed the same set of papers to be screened for the cost-effectiveness review.
The searches were run on 26 and 27 February 2020, with updates conducted on 13 September 2021 (see Appendix 1). In total, 11,753 articles were retrieved; these were exported to EndNote (Clarivate Analytics, Philadelphia, PA, USA) reference management software and duplicate records were removed. Following this, 8428 records remained and were exported to Covidence (Melbourne, VIC, Australia) for screening. The databases searched were Ovid MEDLINE® and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, daily and versions®, Ovid EMBASE 1974 to 2020 September 13, EBSCOhost CINAHL, Scopus and Cochrane Library (CDSR and CENTRAL); all databases were searched from inception.
Grey literature searching encompassed a range of sources, including OpenGrey, NHS EED (NHS Economic Evaluation Database), DARE (Database of Abstracts of Reviews of Effects), HTA, IDEAS/REPeC (https://ideas.repec.org), HMIC (Healthcare Management and Information Consortium), ASPEN (American Society for Parenteral and Enteral Nutrition), BAPEN (British Association for Parenteral and Enteral Nutrition), ESPEN (European Society for Clinical Nutrition and Metabolism), European Natural Health Alliance, Canadian Malnutrition Task Force, United Kingdom Malnutrition Task Force, as well as trial registries, conference abstracts, theses and charities (659 unique resources were identified for screening). Finally, reference lists of all included studies and citations including relevant systematic reviews were screened for inclusion.
Inclusion and exclusion criteria
Types of studies
Parallel-arm, crossover and cluster-RCTs, as well as prospective, comparative non-RCTs (e.g. cohort and case–control studies), were included. Single-arm studies and systematic reviews were excluded from the effectiveness review. Mixed-methods and qualitative studies were eligible for the review of adherence and compliance.
For the cost-effectiveness review, we included full economic evaluations whether they were based on a single clinical study or model based. A full economic evaluation was defined as a study that evaluated the costs and outcomes of two or more health-care technologies. 46 Any studies published as abstracts or conference presentations were eligible for inclusion, provided that any outcome data of interest were sufficiently reported. The included lists of systematic reviews published within the last 3 years were checked for any potentially eligible studies that were missed by our searches.
Population
We included studies involving participants who were aged ≥ 65 years (mean age), able to swallow, malnourished or at risk of malnutrition, and considered to be frail. Malnutrition or risk of malnutrition was defined as undernutrition as per NICE guidelines,7 and assessed using standardised tools [e.g. the Malnutrition Universal Screening Tool (MUST), Mini Nutritional Assessment (MNA), MNA-Short Form].
Frailty was defined using any standardised measure, such as Fried’s frailty phenotype, frailty index or the cumulative deficit model. 19,20 In a change from protocol, in discussion with clinical members of the review team we extended the definition of frailty to include the following proxy frailty criteria: participants admitted to hospital for a fall or fracture or emergency orthopaedic surgery, and participants living permanently in a care home. Studies of participants with dysphagia (inability to swallow), immune-nutrition or satiety hormone suppression, or with specific diseases (e.g. cancer, HIV), were excluded. Other conditions (e.g. dementia, stroke or diabetes) were not used as specific exclusion criteria, provided that the participants met the other population inclusion criteria listed above.
Interventions
The intervention of interest was any form of prescribable ONS, with or without dietary advice or counselling. ONS were defined as multinutrient products (e.g. ready-made liquids, puddings or powders to be mixed with fluids) that contained a mix of macronutrients (i.e. protein, carbohydrates and fat) and micronutrients (vitamins and minerals), designed to increase the energy and nutrient intake of individuals with or at risk of malnutrition. Dietary advice included intake modification, food fortification and meal alteration to improve nutritional intake.
We excluded studies evaluating disease-specific ONS (e.g. for renal, liver or critical care patients), non-commercial or home-prepared ONS formulations with only macronutrients, and artificial nutritional support (e.g. delivered through the parenteral or enteral routes).
Comparators
Studies assessing an eligible intervention against any comparator intervention were eligible for the review. Eligible comparators included standard care (SC), dietary advice or counselling. Studies with no comparator (i.e. single-arm studies) were not eligible for the review.
Outcomes
The following outcomes were eligible for the effectiveness review:
-
malnutrition (undernutrition) – change in body weight, change in fat-free muscle mass, change in body mass index (BMI), change in other indicators of nutritional status, change in energy (kcal) and protein (g) levels and change in malnutrition risk (based on NICE guidelines or assessed using screening tools such as MUST or MNA)
-
change in the consequences associated with malnutrition – improvement in wound healing, reduction in hospitalisation, reduction in infections and the reduction in falls.
-
functional status – improvement in Timed-Up and Go (TUG) test, improvement in gait speed test, improvement in walking speed test, increase in hand grip (or other muscle) strength, improvement in activities of daily living (ADL) and improvement in self-reported mobility
-
change in frailty status (e.g. change in Fried’s frailty phenotype, frailty index or cumulative deficit model)
-
quality of life (assessed using tools such as the EQ-5D, SF-36, Health Utilities Index, Short-Form 6 Dimensions and SF-12)
-
mortality
-
morbidity
-
overall adverse event rates
-
serious adverse events (kidney injury, hyperglycaemia, constipation, diarrhoea, nausea, vomiting, refeeding syndrome, micronutrient deficiency).
The following outcomes were eligible for the adherence and acceptability review:
-
barriers to initiating the use of ONS
-
facilitators of initiating the use of ONS
-
proportion of treatment persistence, compliance, adherence and/or acceptance
-
role of carers in delivering the intervention.
The following outcomes were eligible for the cost-effectiveness review:
-
total costs
-
summary health outcomes [e.g. quality-adjusted life-years (QALYs)]
-
incremental cost-effectiveness ratios (ICERs)
-
resource use (e.g. general practitioner, carer or specialist visits, hospital admissions, length of stay).
Deviations from the protocol
In the protocol, ‘change in nutritional intake’ was a measure of malnutrition. However, we changed this to just energy and protein during the extraction process. Similarly, ‘serious adverse events’ were not defined in the protocol but were later defined during the extraction process as kidney injury, hyperglycaemia, constipation, diarrhoea, nausea, vomiting, refeeding syndrome and micronutrient deficiency. In addition, we altered our definition of frailty to encompass more than standard measures, using the following proxy measures: hospitalised for a fall, any fracture or an emergency orthopaedic admission at the time of recruitment to the study; or permanently residing in a care or nursing home.
Selection of studies
Three reviewers (OA, EJ, CM) screened all title and abstracts identified by the search using Covidence. Full texts of potentially eligible studies were sought and then screened. Any disagreements were resolved by a third reviewer (CM, KT, SER). Where multiple reports of the same study were identified, we combined these into a single study to extract and analyse these at study level (see Appendix 2).
For the cost-effectiveness review, one reviewer (WM) screened the title and abstracts of the studies retrieved by the search in Covidence. For studies deemed eligible or for which it was impossible to decide eligibility from the abstract, the full text was retrieved, and two reviewers (WM, SR) independently assessed the full text for inclusion. This was conducted alongside the study selection of effectiveness studies. Two reviewers (SR and WM) made the final selection decisions about the included studies.
Data extraction
A data extraction form was created and piloted on 10% of included studies. Based on this piloting, the form was modified appropriately (e.g. introduction of the TIDieR framework47 for reporting interventions). One reviewer extracted 50% of included studies, with a second extracting the other 50% (OA and EJ). The reviewers then checked each other’s data extraction. Any disagreements between the two reviewers were resolved by arbitration to a third reviewer (LT). For the cost-effectiveness review, one reviewer (WM) extracted 100% of included studies, with a second (SR) checking the data extracted. Any changes suggested by SR were discussed and agreement was reached.
Quality assessment of included studies
The Cochrane RoB 1.0 tool was used to assess parallel-arm, crossover and cluster-RCTs. 48 The following domains were assessed: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and other bias. One review author (of OA or EJ) assessed the risk of bias for each included paper. A second reviewer (either OA or EJ) checked the assessment. Any discrepancies between the two reviewers were adjudicated by a third reviewer (LT). A tool for non-randomised studies was not needed because no studies of this type met the eligibility criteria. The quality of the included cost-effectiveness studies was assessed using the BMJ checklist. 49
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology was used to address the quality of the evidence. 50 Quality of evidence for each outcome was assessed based on study design, risk of bias, imprecision of estimates, inconsistency of results from different studies, indirectness of study results (i.e. lack of applicability) and publication bias. 51 The GRADE approach was used to assess the certainty of evidence for all outcomes (where possible) using the principles outlined in the Cochrane Handbook. 52 Two review authors (EJ and OA) independently assessed the certainty of evidence for each of these outcomes, resolving any disagreements by discussion and, if necessary, through arbitration with a third review author.
Meta-analysis and narrative synthesis
Meta-analysis was undertaken for RCTs with outcomes where at least two studies compared rates of an outcome (for binary variables) or mean values (for continuous variables) between persons receiving ONS (intervention recipients) and those who received SC (the control group). All studies included in the systematic review that had outcome measures that could be combined were included in meta-analyses if they met the following criteria:
-
The required data were reported or calculable (mean and SD for continuous variables, number of events and sample size for binary variables).
-
Trial arms included ONS versus SC as defined by triallists (e.g. the study by Parsons et al. 53 was excluded as it lacked an appropriate ‘SC’ arm).
-
Outcome measures from different studies could be combined (it was deemed that data for quadriceps strength were inappropriate for inclusion in a meta-analysis in which all other studies reported handgrip strength).
Subgroup analysis was implemented on those studies deemed adequately randomised. Adequacy of randomisation was assessed using domain 1 (random sequence generation – selection bias) from the RoB 1.0 tool. The reasons for this approach included the following: the studies were expected to be at varying risk of bias across categories (there was a low expectation of finding studies at low risk of bias across all categories); random sequence allocation and allocation concealment were considered the most important items, especially as most of the outcome measures were not considered to be subjective (ADL is an exception to this); and we sought to minimise the number of sensitivity analyses. The studies included in the adequately randomised sensitivity analysis are not necessarily at low risk of bias.
Studies were deemed to be adequately randomised where a random component (e.g. using a computer random number generator) was used in the sequence generation process. Five out of 11 studies that were included in the meta-analyses were deemed inadequately randomised. 54–57 No meta-regression analyses were conducted to investigate the effect of variation in population characteristics and intervention components across studies due to the small number of studies included in the review for each outcome.
There were a mix of studies reporting final values and change from baseline (CFB) values. CFB outcomes were preferred as they remove a component of between-person variability from the analysis. 58 Sensitivity analyses were also conducted using final values. CFB, final values and standard deviations were calculated where they were not reported. The methods used to determine the standard deviation are described in Appendix 3.
For the binary outcomes (mortality and hospitalisation), we performed an analysis comprising all studies that reported relevant data. A Mantel–Haenszel random-effects meta-analysis was conducted. For continuous outcomes with a uniform measure across studies, an inverse variance random-effects meta-analysis was conducted for the mean difference in outcomes. For continuous outcomes with different measures across studies, standardised mean differences (SMDs) were calculated using the Hedges’ g (adjusted) method. 59 Generic inverse variance random-effect meta-analyses were conducted. In reporting the results, statistical significance was defined at a 95% level of confidence.
As multiple measures of the same outcome were often included in a study (e.g. calf circumference as a measure of fat-free muscle mass), an evidence hierarchy was employed to decide which outcome was preferentially included in the analysis. This is displayed in Table 1.
| Outcome | Analysis method | Outcome hierarchy |
|---|---|---|
| Body weight | MD | Body weight (kg) |
| BMI | MD | BMI (kg/m2) |
| Arm circumference | SMD | Arm circumference (cm) |
| Fat-free muscle mass | SMD | Calf circumference (cm) |
| Lean body mass (kg) | ||
| Energy intake | SMD | Total energy intake (kcal/day) |
| Energy intake (kcal/kg) | ||
| Protein | SMD | Total protein (g/day) |
| Protein (g/kg) | ||
| Albumin | MD | Albumin (g/l) |
| ADL | SMD | ADL score |
| IADL | ||
| Hospitalisation | RR | Readmissions |
| Hospital admissions | ||
| Mortality | RR | Number of deaths |
| Grip strength | SMD | Handgrip strength (kg) |
| Handgrip strength (kPa) | ||
| MNA | SMD | MNA |
| MNA-SF | ||
| Mobility | MD | Improvement in TUG test |
| Improvement in gait speed test |
Data from the longest follow-up time point available in each were included in the meta-analysis so that the longer-term impacts on outcomes could be assessed. In addition, too few studies reported multiple time points, meaning that it would not have been possible to run a meta-analysis for multiple follow-up periods. The degree of heterogeneity was estimated using the I2 statistic, and the p-value of the chi-squared statistic was used to measure the strength of evidence for heterogeneity. I2 values of 0–40% (heterogeneity might not be important), 30–60% (may represent moderate heterogeneity), 50–90% (may represent substantial heterogeneity) and 75–100% (considerable heterogeneity) were used to guide interpretation. 60 Publication bias and other small-study effects were evaluated using Egger’s test and funnel plots if 10 or more studies were included in an analysis. If there were fewer than 10 studies, the power of the test would usually be too low to distinguish real asymmetry from chance. 61 All analysis was conducted in RevMan.
Narrative synthesis methods were used either to analyse outcomes with insufficient data or for those studies that did not meet the criteria for meta-analysis (e.g. cohort studies). Patterns in the data, including statistical significance and direction of effect, are summarised narratively. The results reported are included alongside the meta-analysis outcomes. A narrative synthesis was undertaken for the cost-effectiveness review to describe the similarities and differences in the study questions, methods and results.
Network meta-analysis
There were multiple comparators investigated in the studies included in the systematic review. The effectiveness of ONS compared with these was evaluated using network meta-analysis (NMA). NMA enables direct and indirect evidence of a treatment effect to be combined in the estimation of the effect. For example, if one study (AB) compares A with B, one study (AC) compares A with C and one study (BC) compares B with C, then study AC provides direct evidence for A compared with C, and studies AB and BC provide indirect evidence for A compared with C. NMA also enables an effect to be estimated for A compared with C when only indirect evidence is available.
Only RCTs were included in the NMA. All interventions included in the studies that met the inclusion criteria were included in the NMA, for example different dietary interventions and dietary interventions with exercise. The purpose was to estimate the effectiveness of ONS compared with all of the different comparators found in the review studies. The network diagrams are presented in Appendix 4 (see Figures 19 and 20) and show that there are no cases of both direct and indirect evidence for any one comparison. The purpose of conducting NMAs here is to estimate treatment effects using indirect evidence. The effectiveness of every treatment compared with every other treatment can be estimated. The effect estimates for ONS compared with every other treatment are produced here. The mean and 95% credible interval of the effect estimates are calculated.
A NMA was conducted for an outcome for which there were at least three studies reporting one comparison, generally ONS compared with SC, and there was a connected network of three or more interventions. These conditions were met for two continuous outcomes. One outcome was analysed on the SMD scale and one outcome was analysed on the mean difference scale. 58,62 Analyses were conducted in WinBUGS 1.4.3 (MRC Biostatistics Unit, Cambridge, UK). 63 For the mean difference analysis, the WinBUGS program 5a code for a random-effects analysis with multiarm trials from the NICE Technical Support Document 2 was used. 64 For the SMD analysis, the WinBUGS program 7a code for random-effects analysis with multiarm trials from the NICE Technical Support Document 2 was used. The code requires that the data set include the variance of the baseline treatment in each trial with more than two trial arms. For the SMD analysis, the variance of baseline treatment was approximated as shown in the following equations. The equation presented here for the variance of baseline treatment was not specifically reported in Introduction to Meta-analysis,62 Chapter 4, but it makes use of formulae 4.18, 4.19, 4.20, 4.22 and 4.24 presented there:
µC, SDC, and NC are the mean, standard deviation and sample size of the control group; µT, SDT and NT are the mean, standard deviation and sample size of the intervention group.
A common between-study variance was assumed across treatment comparisons. Multiple studies were reported for only one treatment comparison, ONS compared with SC, so the common between-study variance estimate is determined by those studies. For continuous outcomes, the between-study variance and standard deviation are on the outcome scale. The choice of prior distribution for the between-study standard deviation should be based on the specific scale. Where there are many trials with which to estimate the between-study standard deviation, the upper limit of the uniform prior distribution should be sufficiently high that the upper end of the posterior distribution of the between-study standard deviation is barely, if at all, truncated.
Where there are few studies with which to estimate the between-study standard deviation, the uniform prior distribution can have a significant effect on the posterior distribution. The mean of a uniform prior distribution is (maximum – minimum)/2, and it is not entirely ‘uninformative’. One approach is to identify an informative prior from a published meta-analysis that does not include the same trials as the current study, or to elicit a prior distribution from experts. For the analyses planned, there were four different outcome scales across the analyses and few studies in each analysis. Therefore, a pragmatic decision taken here was to set the upper limit of the uniform distribution for the between-study standard deviation to be the difference between the greatest and smallest effect size for any one comparison in the network (only two analyses were eventually included in the review). For example, comparison A versus B has estimates (–0.4, –0.8, 0.3) and comparison B versus C has estimates (0.6, 0.1). The greatest difference in effect sizes is 0.3 minus –0.8 = 1.1. This is straightforward when there are no comparisons with direct and indirect evidence, as in this study. For networks with direct and indirect evidence, the difference in these estimates would need to be taken into account. The mean (0.55) of the uniform distribution (0 to 1.1) is the maximum possible between-study standard deviation described by the mean effect estimates. But these priors are not as vague as would normally be recommended. Recommended vague priors allow for a huge range of true effect estimates,64 far greater than seen in practice. A review65 of between-study variance estimators reported that a Bayesian approach may overestimate the between-study variance when it is close to zero and when there are few studies.
Convergence was assessed using the Brooks–Gelman–Rubin diagnostic along with a visual inspection of the trace and density plots. 66 The initial 20,000 simulations were discarded, and the results were based on a further sample of 50,000 simulations. As there were no closed loops in the network (no cases of direct and indirect evidence for any one comparison), there was no possibility of inconsistency in the network. The probability that an intervention was most effective was then estimated.
Public and patient involvement/engagement
Public and patient involvement/engagement was undertaken throughout the project, initially helping to develop the proposal and inform the initial logic model, and then scope of the review, discussing results of the review and the implications of findings. In addition, AR (PPIE lead) helped shape the research as part of the project team. The PPIE groups comprised six to eight older people (all of whom were female).
The participants in the PPIE group were members of the Newcastle branch of the Elders Council, a local organisation of older people interested in sharing their views about making the city ‘a great place in which to grow old’. Recruitment to the focus groups was organised by the chairperson of the Elders Council in Newcastle and PPIE lead (AR), and the sessions were facilitated by researchers at Newcastle University. The format of the sessions was a short presentation about review progress to date, followed by open questions to discuss as a group. The online sessions were recorded, and detailed notes were taken by researchers. These notes were subsequently written up and shared with the research team. Key concepts and broad themes were identified and used to complement the data collated in the review.
Following the completion of the review, the findings were presented to a panel of practice or policy partners to allow understanding of how different stakeholders conceptualised the results and their experiences more generally concerning the use of oral nutritional interventions in this population. The main online event comprising eight stakeholders was supplemented with three one-on-one sessions with additional partners to ensure that we collated views from a range of individuals. Geriatricians, dietitians and nurse practitioners were involved in the discussions.
Chapter 3 Results of effectiveness review
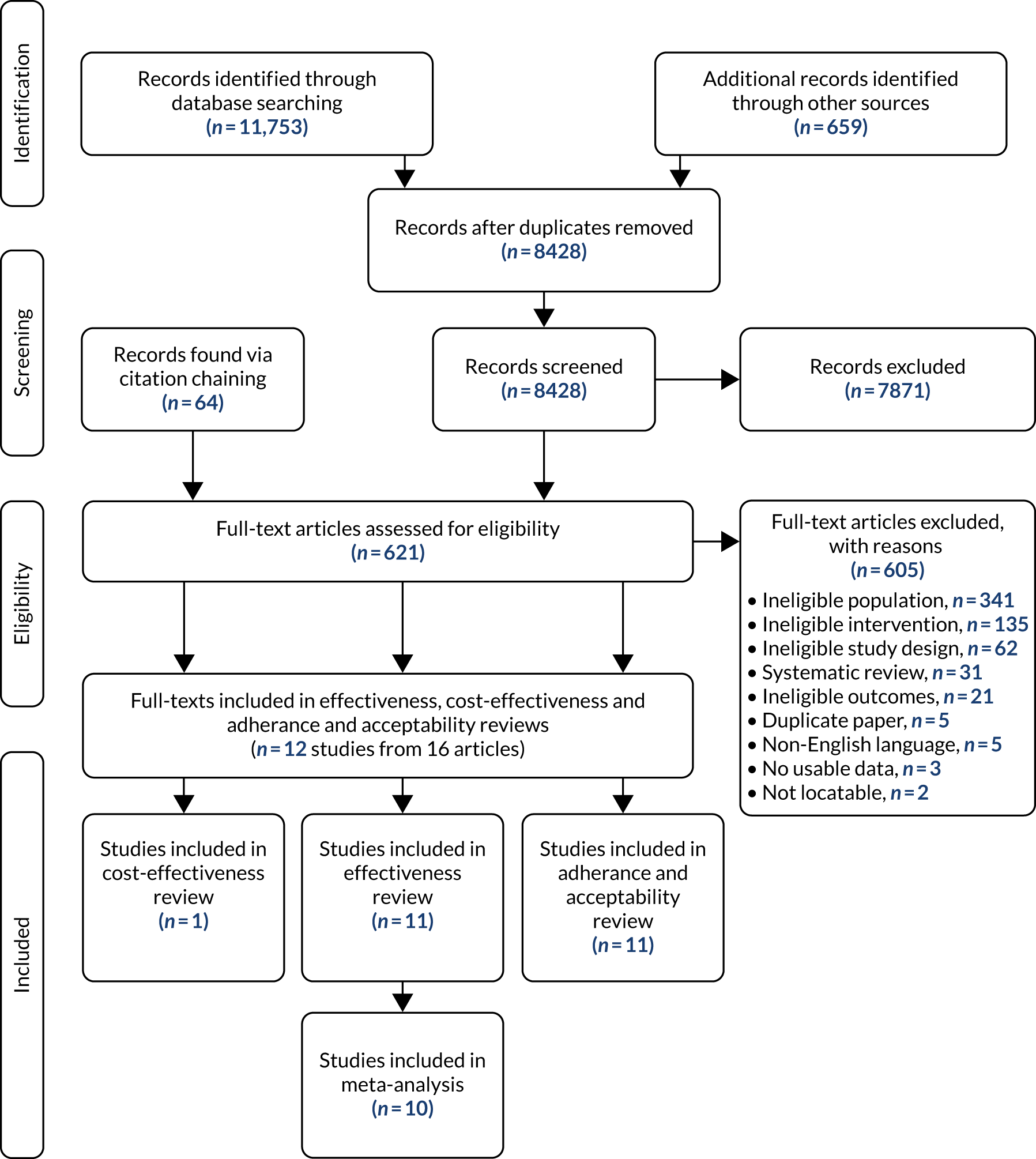
The database searches identified 8428 records after duplicates had been removed. A further 659 additional records were identified and 64 records were found from citation-chaining (Figure 2). In total, 621 papers were screened at full-text level (the reasons for exclusion are detailed in Report Supplementary Material 1). Eleven studies met the inclusion criteria, two of which reported duplicate data (see Appendix 2). 53 In this report, we refer to the paper with the most information gathered from each but reference individual papers where appropriate (see Appendix 5). Included papers were published between the years 2000 and 2017. 53,67 One effectiveness study was included in the cost-effectiveness review. 23
FIGURE 2.
The PRISMA flow chart.
Characteristics of included studies
Eleven studies were included in the effectiveness review, all of which were RCTs. One study was a crossover RCT,56 four studies were multiarm RCTs57,67–69 and the remaining six studies used a parallel-group design with two groups. 53–55,70–73 In total, 882 people were recruited across the 11 studies. The smallest study recruited 39 participants56 and the largest recruited 104. 53 Table 2 describes the characteristics of the included studies in the review.
| Study authors; country; study design | Setting | Number enrolled (withdrawals, % or people)∞ | Duration of intervention (ONS) and follow-up | Intervention | Outcomes | Study funding source/conflicts of interest |
|---|---|---|---|---|---|---|
| Cameron et al.;71 Australia; RCT | Hospital: Hornsby Ku-ring-gai Hospital (a general hospital in Northern Sydney) | 44 (9–56%) |
Treatment duration: 40 days Follow-up duration: 40 days, 4 months |
Liquid high-calorie, high-protein supplement (Novasource/Sustagen Hospital Formula Plus) and diet of choice (n = 23) | Body weight, fat-free muscle mass, BMI,a other indicators of nutritional status, hospitalisations, gait speed, handgrip (or other muscle) strength, ADL, mortality and number of adverse events | Northern Sydney area health service |
| SC – high-protein diet (with high-protein milk) (n = 21) | ||||||
| Lauque et al.;67 France; RCT | Nursing home: eight privately run 80-bed nursing homes in Toulouse | 88 (0–32%) |
Treatment duration: 60 days Follow-up duration: NR |
ONS (Clinutren) – risk of malnutrition (n = 19)b | Body weight, BMI, energy intake (kcal), protein intake, change in malnutrition risk, handgrip (or other muscle) strength and mortality | NR |
| ONS (Clinutren) – malnourished (n = 28)b | ||||||
| No supplementation – well nourished (n = 19) | ||||||
| No supplements – risk of malnutrition (n = 22) | ||||||
| Lee et al.;54 Taiwan (Province of China); RCT | Nursing home: geriatric nursing home | 92c (NR) |
Treatment duration: 24 weeks Follow-up duration: 24 weeks, 1 yeard |
Liquid ONS (n = 47) and all essential micronutrients taken as an afternoon snack | Body weight, mid-arm circumference, fat-free muscle mass, BMI and other indicators of nutritional status | Asia University |
| NR (assumed SC) (n = 45) | ||||||
| Luo et al.;70 Russia; RCT | Hospital | 55b (four or five people) |
Treatment duration: 28 days Follow-up duration: NR |
ONS (Ensure TwoCal) plus standard hospital food (n = 26) | Body weight, serum albumin, protein intake, gait speed, chair-to-bed transfer domain from Modified Barthel Index, number of adverse events in study, nausea and pruritus caused by ONS and compliance | Abbott Nutrition (no details given of the role of industry partner in research) |
| SC including normal hospital food (n = 28) | ||||||
| Miller et al;,68 Australia; RCT | Hospital: orthopaedic wards of Flinders Medical Centre, Adelaide | 100 (3.8–8.3%) |
Treatment duration: 42 days Follow-up duration: NR |
Liquid ONS (Fortisip) (n = 25) plus standard hospital food only for 24 weeks (n = 29) | Body weight, BMI, hospitalisations, gait speed test, handgrip (or other muscle) strength, mortality and QoL | NHMRC Public Health Postgraduate Research Scholarship, Flinders University-Industry Collaborative Research Grant and Nutricia Australia Pty Ltd (no details given of the role of industry partner in research) |
| Exercise – resistance training (n = 25) | ||||||
| Liquid ONS and exercise (n = 24) | ||||||
| SC (general nutrition and exercise advice, usual dietetic and physiotherapy care and onward transfer) (n = 26) | ||||||
| Otten et al.;72 Germany; RCT | After hospital discharge | 71 (NR) |
Treatment duration: 3 months Follow-up duration: NR |
Liquid ONS (n = 42) | QoL | NR |
| ONS with guidance (n = 53) | ||||||
| Parsons et al.;73 UK; RCT | Nursing home: care homes in Hampshire | 104 (NR) |
Treatment duration: 12 weeks Follow-up duration: NR |
ONS (range of Nutricia Ltd products available to choose from) (n = 53) | Body weight, change in nutritional intake, hospitalisations, mortality and QoLe | An unrestricted educational grant from Nutricia |
| Dietary advice (specially designed diet sheet) (n = 51) | ||||||
| Payette et al.;55 Canada; RCT | Community: home | 83 (9.5–9.8%) |
Treatment duration: 16 weeks Follow-up duration: NR |
Liquid ONS (Ensure or Ensure Plus) (n = 41) | Body weight, fat free muscle mass, energy intake (kcal), protein, TUG test, handgrip (or other muscle) strength, QoL | Abbott Laboratories Limited (no details given of the role of industry partner in research) |
| Usual care (n = 41) | ||||||
| Tidermark et al.;69 Sweden; RCT | Community | 59 (two or three people) |
Treatment duration: 6 months Follow-up duration: 6 months, 12 months |
Protein-rich ONS (Fortimel) (n = 20) | Body weight, fat-free muscle mass, other indicators of nutritional status, reduction in infections, handgrip (or other muscle) strength, mortality and QoL | Trygg-Hansa Insurance Company, the Swedish Orthopaedic Association, the Swedish Research Council, the Novo Nordisk Foundation, Nutricia Nordica AB and Nycomed AB (no details given of the role of industry partner in research) |
| Protein-rich ONS (Fortimel) plus nandrolone decanoate (Deca-Durabolin) (n = 19) | ||||||
| SC plus additional calcium and vitamin D for 6 months (n = 20) | ||||||
| Tylner et al.;56 Sweden; crossover RCT | Nursing home: five residential care homes in the southern Stockholm area | 39 (five or six people) |
Treatment duration: 12 weeks Follow-up duration: NR |
Fat emulsion (Calogen Extra) and then SC (6 weeks each) (n = 20) | Body weight, BMI, kcal, protein, other indicators of nutritional status, hospitalisations, handgrip (or other muscle) strength and serious adverse events | Nutricia Nordica AB (no details given of the role of industry partner in research) |
| SC and then fat emulsion (Calogen Extra) (6 weeks each) (n = 19) | ||||||
| Van Wymelbeke et al.;57 France; RCT | Nursing home: eight nursing homes in Burgundy | 87 (12–37%) |
Treatment duration: 12 weeks Follow-up duration: NR |
Liquid high-calorie, high-protein ONS (Fresenius Kabi) and diet of choice (n = 27)f | BMI, kcal, protein, other indicators of nutritional status, change in malnutrition risk, hospitalisations, handgrip (or other muscle) strength and ADL | French government under the FUI (Fonds Unique Interministériel) programme through the project Farineþ |
| Enriched brioche (with similar levels of energy and macro- and micronutrients to the ONS) (n = 35)f | ||||||
| Usual care (normal breakfast) (n = 25) |
Two studies took place in Australia,68,71 two in France,57,67 one in Germany,72 two in Sweden,56,69 and one each in the UK,73 Russia,70 Canada55 and Taiwan (Province of China). 54 Five studies were set in nursing/residential homes,54,56,57,67,73 of which four took place in multiple nursing homes. 56,57,67,73 We acknowledge that definitions of nursing/residential homes vary internationally; however, our groupings were purely for descriptive purposes. Two further studies were set in the community55,69 and three were set in hospital. 68,70,71 One study stated that it had been conducted with patients after they had been discharged from hospital. 72 Reporting on intervention duration and follow-up was often inadequate and lacked detail.
The type of ONS used and the comparisons varied across the studies. Six studies54,55,57,68,71,74 compared ONS with usual care or SC in one of their arms, and two53,72 compared ONS with dietary counselling or advice. The remaining studies contained a number of comparisons; these are detailed in Table 2. One study68 either combined or compared ONS with exercise programmes. The duration of the ONS intervention ranged from 28 days74 to 6 months,69 with a maximum follow-up of 12 months. 69 The timing of follow-up, particularly in relation to the intervention period, was poorly reported and difficult to ascertain from the studies. Of the studies that were included in the effectiveness review, six were either fully funded or part-funded by industry. Of these, four were fully funded (including one with an unrestricted grant) and two were part-funded. A further three were not funded by industry and two studies did not include details of funding/conflict of interests.
Across the studies, participants varied in age, BMI and body weight. Most studies included both men and women, although often more women participated in the studies than men. Two studies included women only. 69,71 Participants’ level of malnutrition at baseline between groups was measured using a variety of tools. Three studies used the MNA54,57,67 and one used the MUST score. 53 One study each used mid-upper arm circumference and albumin levels,71 and a further two studies reported excess weight loss. 55,70 One study stated that most participants were at risk of malnutrition, but it was unclear whether this was assessed using the MNA-SF. 56 Four studies did not report specific levels of malnutrition between groups at baseline. 68–70,72 Further details of the characteristics of participants in the included studies can be found in Appendix 5.
Some studies reported on comorbidities that may contribute to malnutrition (see Appendix 6). Two studies53,67 included participants with dementia, three53,56,68 included participants who had cognitive impairment, and one53 included participants who had cardiovascular disease. No studies reported on participants who had diabetes, stroke or cancer. Only one study57 reported on specific oral health issues that may affect malnutrition and related outcomes. One study57 reported on participants who had complete or partial denture or participants who had no dentures. Two studies54,67 reported on participants’ need for assistance with feeding.
In general, studies did not report clearly on potential social determinants of malnutrition, with the exception of their living arrangements or whether the participants were receiving household or other help (see Appendix 7). No studies reported on the ethnicity of the participants. Of the five studies that did not take place in either nursing or residential care homes, four55,68,71,75 reported the participants’ living situation to some extent (e.g. living along, married). Luo et al. 74 did not describe living arrangements. Tidermark et al. 69 noted that their participants lived at home and were non-institutionalised but did not provide any further details. Otten et al. 72 reported the number of participants living alone, but not by study arm. The interventions, comparisons and modes of delivery across the studies varied and are described in Appendix 8.
Quality assessment of included studies
Quality assessment was conducted using the Cochrane risk-of-bias tool (n = 11) for parallel-arm RCTs, multiarm RCTs, crossover RCTs or cluster-RCTs. A summary of risk-of-bias assessments across all included studies can be seen in Appendix 9.
Randomised controlled trials
We assessed the 11 included studies using the Cochrane risk-of-bias tool. Fewer than half of the RCTs were judged to be at low risk of bias for random sequence generation (45%),55,57,67,69,73 allocation concealment (45%),56,68–70,73 blinding the outcome assessor (45%)54,55,57,68,73 and selective reporting (45%). 55,57,67,69,73 Forty-five per cent of studies were judged to be at high risk of performance bias. 57,67,69–71 Thirty-six per cent of RCTs were judged to be at high risk of attrition bias,56,57,67,70 with 27% of RCTs also judged at unclear risk for this domain. 55,68,72 Most of the included RCTs were at unclear risk of other bias (64%). 56,57,67–69,72,73 Figure 3 shows the assessments across studies for each domain.
FIGURE 3.
Risk-of-bias assessments across studies for each domain.
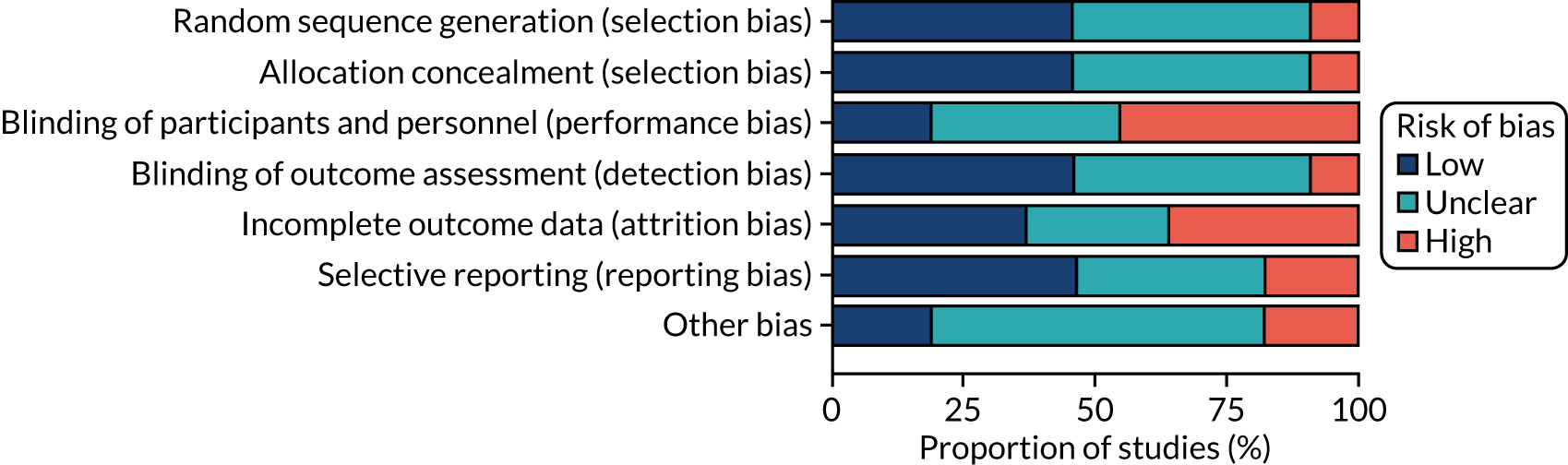
Random sequence generation
One included study67 was assessed as being at high risk of bias for random sequence generation. Five studies54–57,72 did not provide enough detail about their method of randomisation and so were assessed as unclear risk of bias. Five studies53,68–71 were assessed as being at low risk of bias for this domain.
Allocation concealment
One included study71 was rated as being at high risk of bias for allocation concealment. Five studies54,55,57,67,72 were rated as unclear. Five studies53,56,68,69,74 were assessed as being at low risk of bias for this domain.
Blinding of participants and personnel
Five included studies57,67,69–71 were judged to be at high risk of bias for this. Four53,56,68,72 were assessed as being at unclear risk of bias, mainly because the methods of blinding were not clearly reported. Two studies54,55 were assessed as being at low risk of bias for this domain.
Blinding of outcome assessment
One study70 was deemed to be at high risk of bias for this domain. Five included studies56,67,69,71,72 were judged to be at an unclear risk of bias. Five studies53–55,57,68 were assessed as being at low risk of bias for this domain.
Incomplete outcome data
Four included studies56,57,67,70 were deemed to be at high risk of attrition bias. Three studies55,68,72 were judged to be at unclear risk of bias for this domain. Four studies53,54,69,71 were assessed as being at low risk of bias for this domain.
Summary of effectiveness results
Pairwise meta-analyses were undertaken to assess the effects of ONS compared with SC on the outcomes of interest in this review. Ten studies53–57,67–69,71,74 were included in the pairwise meta-analyses (see Appendix 10 for reasons why studies/outcomes were excluded from the meta-analysis). The meta-analysis results are presented alongside a narrative synthesis of the outcomes that were unable to be pooled. As fewer than 10 studies were incorporated into the meta-analyses for any outcome, it was not possible to use funnel plots and other tests for publication bias. Analysis was run using both final values and change of baseline. CFB analysis will be presented here (where possible); final value results are in Appendices 11 and 12.
The outcomes reported below are broadly split into three key categories, which correspond to the period over which the outcomes might be expected to induce a noticeable change. Nutritional intake outcomes and those that relate to visceral protein level (albumin) are presented first; these include total energy, protein and albumin. Following this, body composition outcomes are discussed (body weight, BMI, fat-free muscle mass, lean body mass). Then longer-term outcomes are reported (ADL, grip strength, hospitalisation, MNA, morbidity, mortality, QoL). Finally, other outcomes are narratively synthesised, including adverse events, reduction in falls and compliance. Owing to uncertainty in the duration of follow-up (and the small number of studies identified), meta-analysis was undertaken aggregating all follow-up time points together.
Nutritional intake outcomes
Six studies53,55–57,67,70 reported data on the effect of ONS on nutritional intake outcomes. A meta-analysis was possible for energy and protein intake.
Energy (kcal) intake
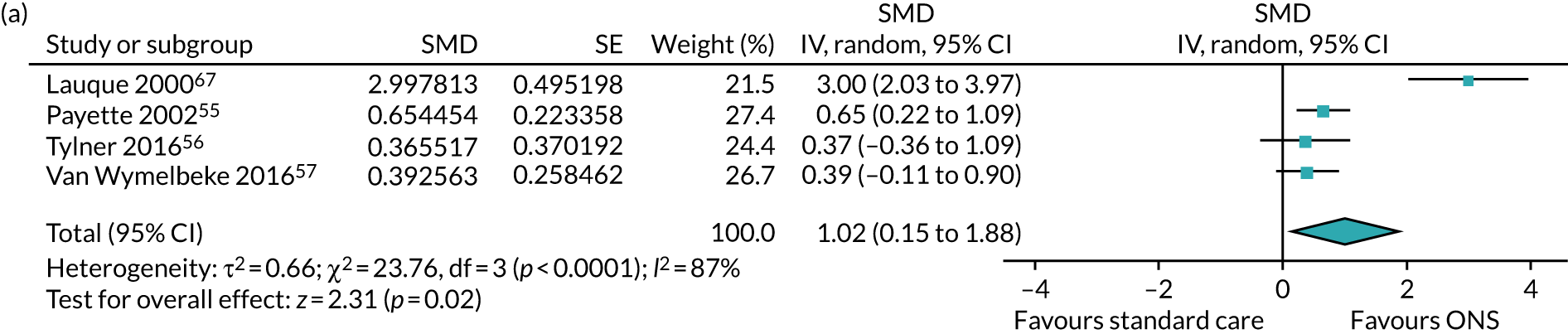
Four studies reported data on the effect of ONS compared with SC on kilocalories (kcal) consumed for a CFB analysis; one55 was undertaken in the community and three56,57,67 were undertaken in care homes. All four studies55–57,67 measured energy intake in kcal, which refers to the energy from food consumption. One study reported data on energy intake at final visit but no data were reported at CFB. 53 The mean and standard deviation could not be calculated as there were insufficient data. This study was not included in the CFB analysis. 53 The follow-up time point, where reported, varied between 6 weeks,56 90 days/3 months,57 16 weeks55 and 60 days. 67 The pooled results of the meta-analysis (Figure 4a) show a positive effect of ONS versus SC on energy intake (SMD 1.02, p = 0.002, 95% CI 0.15 to 1.88). There was evidence of statistical heterogeneity (p < 0.0001, I2=87%). A sensitivity analysis could not be run as there were no adequately randomised studies. GRADE scores showed very low-quality evidence for energy intake (see Appendix 13).
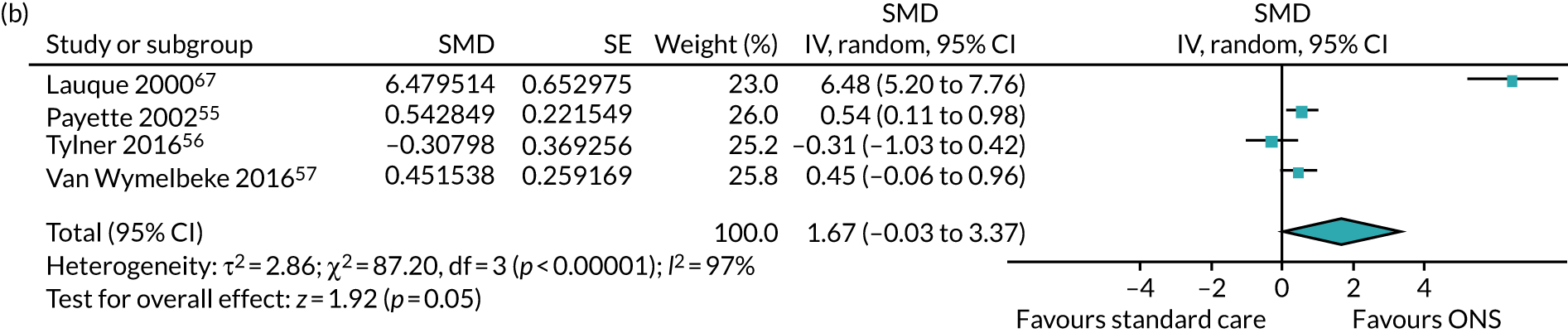
FIGURE 4.
Forest plots of nutritional intake outcomes (CFB). (a) Energy intake; and (b) protein intake.
Protein intake
Four studies55–57,70 reported data on the effect of ONS compared with SC on protein for a CFB analysis. One study53 reported data on protein intake at final visit, but no data were reported on CFB. Insufficient data were reported in this study, and means and standard deviations at CFB could not be calculated. Two studies55,56 measured protein intake in grams (g). Three studies measured total protein intake in grams per day (g/day),55,56,67 while one study measured protein in g/kg. 57 The follow-up period varied: 6 weeks,56 60 days,70 90 days/3 months57 or 16 weeks. 55 The pooled result (see Figure 4b) of the meta-analysis of CFB scores comprising all four studies shows a slightly positive effect of ONS versus SC on protein (SMD 1.67, p = 0.05, 95% CI –0.03 to 3.37). The data show a substantial degree of statistical heterogeneity (p < 0.00001, I2 = 97%). GRADE scores showed very low-quality evidence for protein intake (see Appendix 13).
Visceral protein level
Albumin
Five studies54,56,57,70,71 reported data on the effect of ONS compared with SC on serum albumin, measured using the analysis of serum derived from fasting blood samples in grams per litre (g/l) for a CFB analysis. Two studies70,71 were included in the sensitivity analysis as they had been adequately randomised. The meta-analysis results of the main analysis (Figure 5) show no evidence of effect of ONS versus SC on albumin (MD 1.48, p = 0.13, 95% CI –0.44 to 3.41). There was evidence of statistical heterogeneity (p < 0.00001, I2 = 95%). The pooled results of the sensitivity analysis show a slightly positive effect of ONS versus SC on serum albumin (MD 2.86, p = 0.010, 95% CI 0.69 to 5.03). There was moderate evidence of statistical heterogeneity (p = 0.08, I2 = 68%). GRADE scores showed very low-quality of evidence for albumin (see Appendix 13).
FIGURE 5.
Forest plot of albumin levels (CFB).
Body composition outcomes
Eight studies53–57,69–71 reported data on the effect of ONS on change in body composition outcomes. A meta-analysis was possible for body weight, BMI and fat-free muscle mass.
Body weight
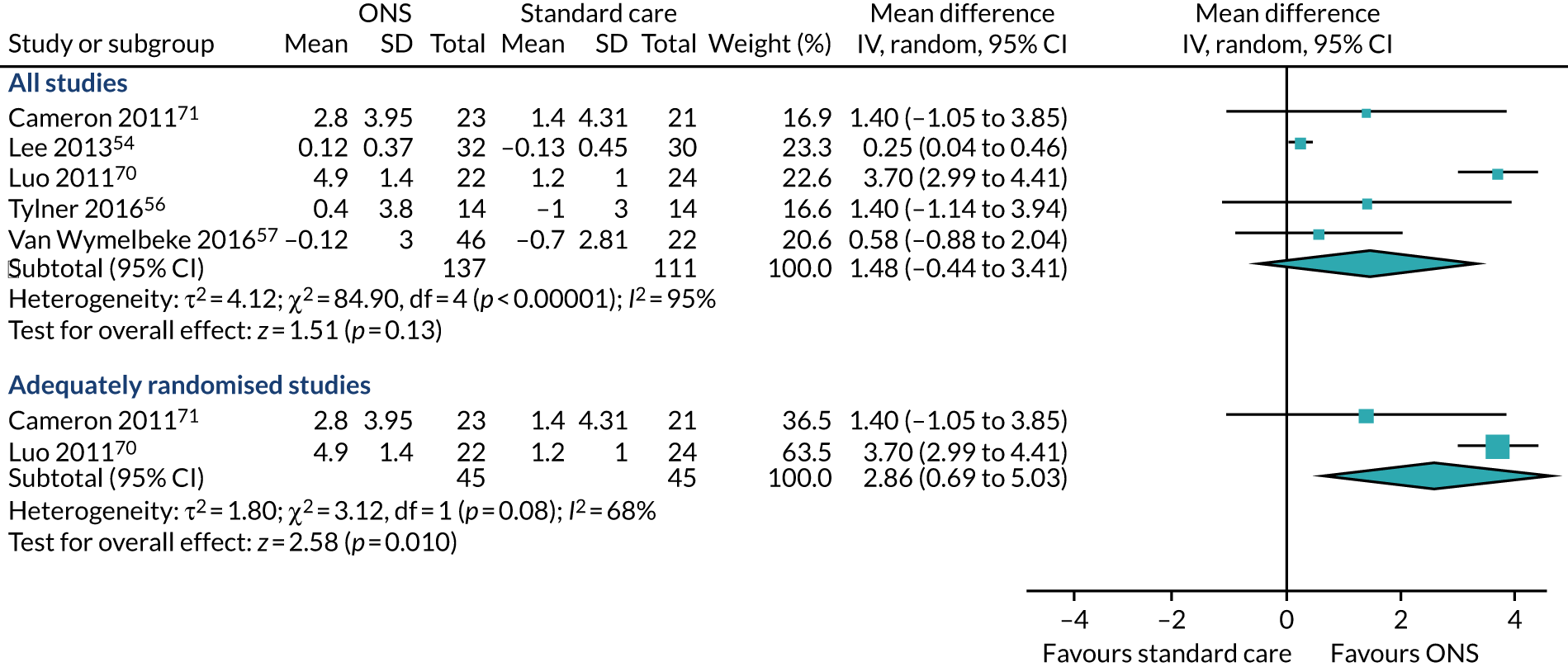
Five studies54,56,69–71 reported appropriate data for inclusion in the meta-analysis of CFB scores between participants receiving ONS versus SC. Three studies69–71 were included in the sensitivity analysis as they had been adequately randomised. The pooled results (Figure 6a) of the main meta-analysis comprising all five studies showed no evidence of effect of ONS versus SC on body weight CFB (MD 1.31, p = 0.06, 95% CI –0.05 to 2.66). Substantial statistical heterogeneity was found (p = 0.004, I2 = 74%) indicating a variation between sample estimates beyond what would be expected by chance when samples are derived from the same population. Three studies69–71 were included in the sensitivity analysis consisting of only adequately randomised studies. The pooled results (see Figure 6a) of the sensitivity analysis also indicate that there was no evidence of effect of ONS compared with SC on body weight (MD 1.28, p = 0.26, 95% CI –0.95 to 3.52). Similar to the main analysis, there was substantial heterogeneity (p = 0.01, I2 = 78%) indicating the presence of a variable confounding factor across the studies. GRADE scores showed very low-quality evidence for body weight (see Appendix 13).
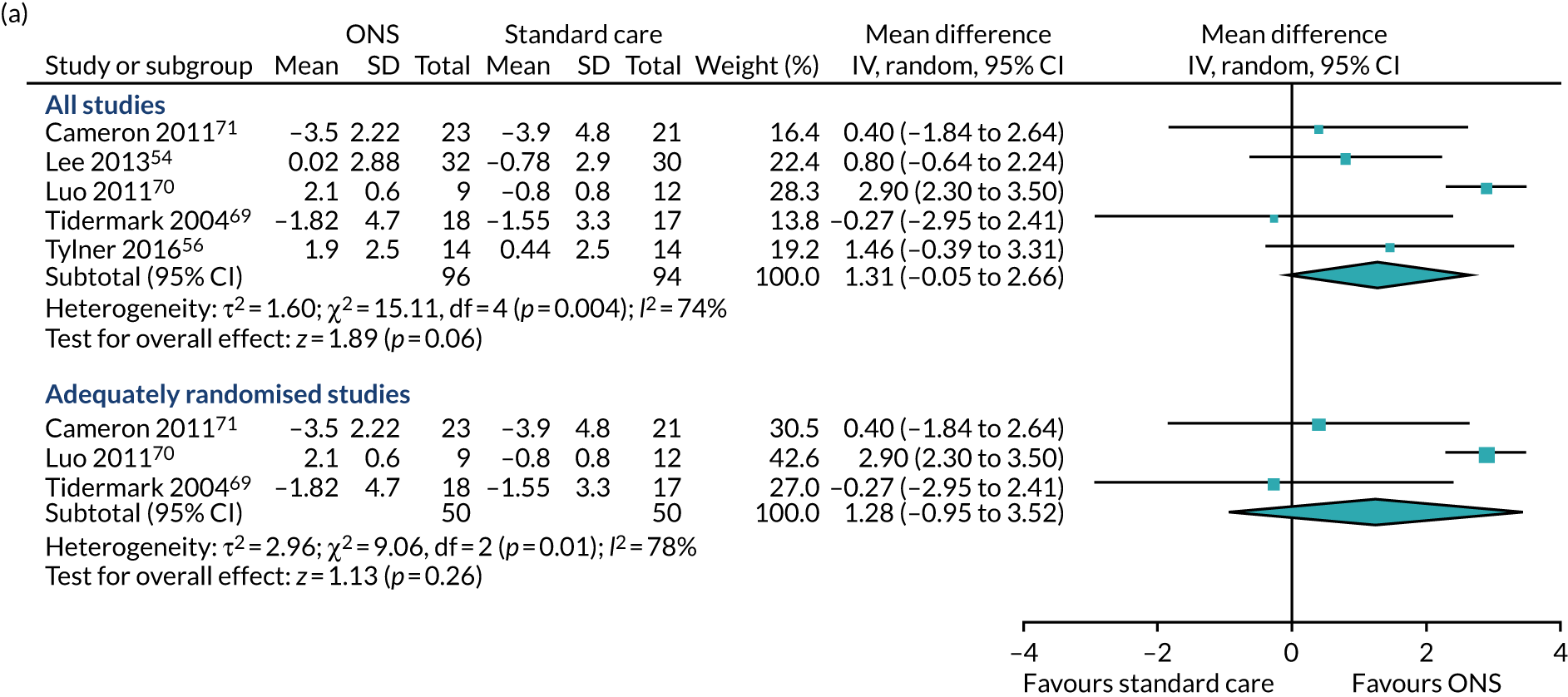
FIGURE 6.
Forest plots of body weight and BMI body outcomes (CFB). (a) Body weight; and (b) BMI.
Body mass index and proxy measures
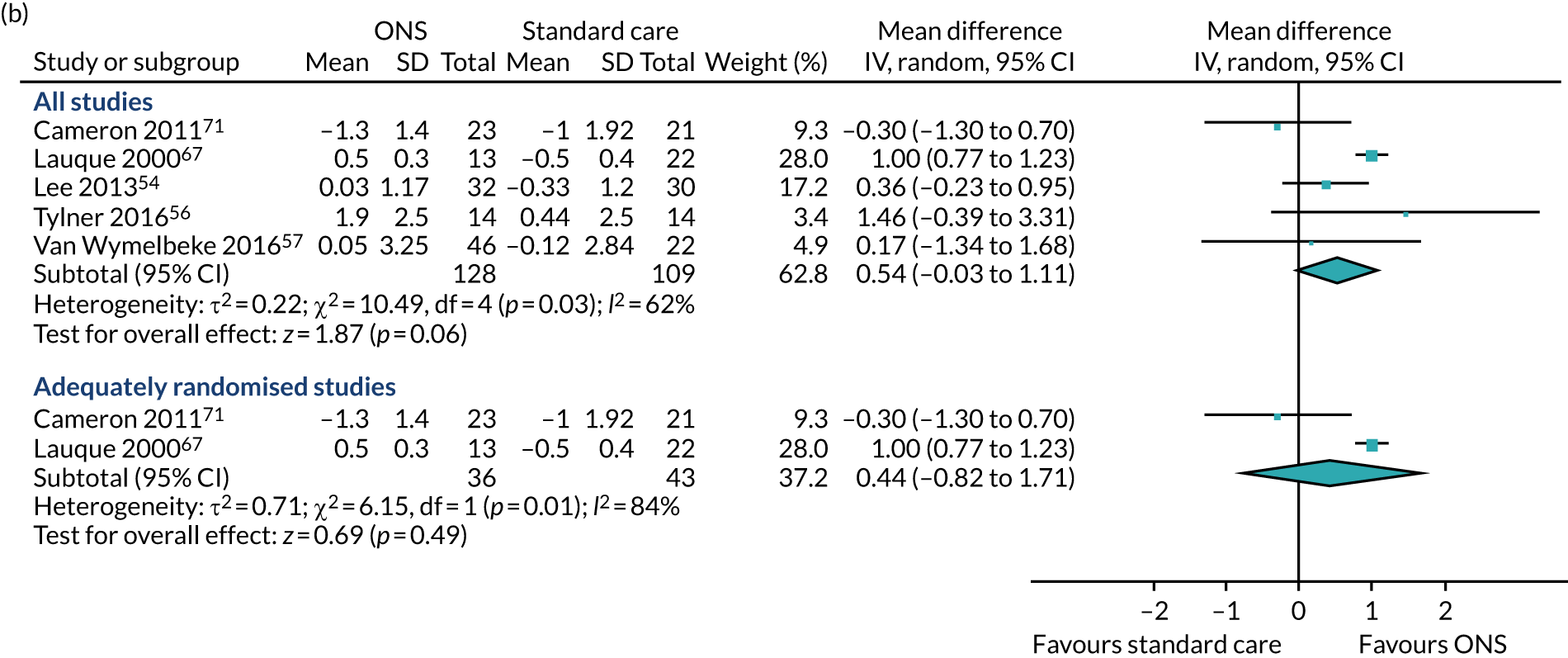
Five studies54,56,57,67,71 reported appropriate data for inclusion in the meta-analysis of CFB scores between participants receiving ONS and those receiving SC. Two studies67,71 were included in the sensitivity analysis as they were adequately randomised. The pooled results (see Figure 6b) of the main meta-analysis comprising all five studies presented no evidence of effect of ONS compared with SC on BMI at CFB (MD 0.54, p = 0.06, 95% CI –0.03 to 1.11). There was evidence of statistical heterogeneity (p = 0.03, I2 = 62%). The pooled results of the sensitivity analysis indicate a mixed effect of ONS compared with SC on BMI (MD 0.44, p = 0.54, 95% CI –0.82 to 1.71). There was significant evidence of heterogeneity (p = 0.01, I2 = 84%), indicating that there may be a variable confounding factor across the studies. GRADE scores showed very low-quality evidence for BMI (see Appendix 13).
One study, by Lee et al. ,54 rated as being at unclear risk of bias for random sequence generation and allocation concealment, assessed the impact of ONS compared with SC on arm circumference, providing data at baseline and post intervention. 54 The authors reported a mean change in mid-arm circumference among people who were malnourished or at risk of malnutrition, at 24-week follow-up, of 0.3 cm in the intervention group and –0.8 cm in the control group. GRADE was unable to be assessed for arm circumference, as meta-analysis was not undertaken.
Fat free muscle mass
Three studies54,55,69 reported data for the effect of ONS versus SC on fat-free muscle mass for a CFB analysis. Calf circumference and lean body mass were the outcomes used to measure fat-free muscle mass. A hierarchy of outcomes was applied, and calf circumference was chosen as the preferred outcome. Two studies54,55 used calf circumference, measured in centimetres (cm), and one study69 measured lean body mass in kilograms using dual energy X-ray absorptiometry.
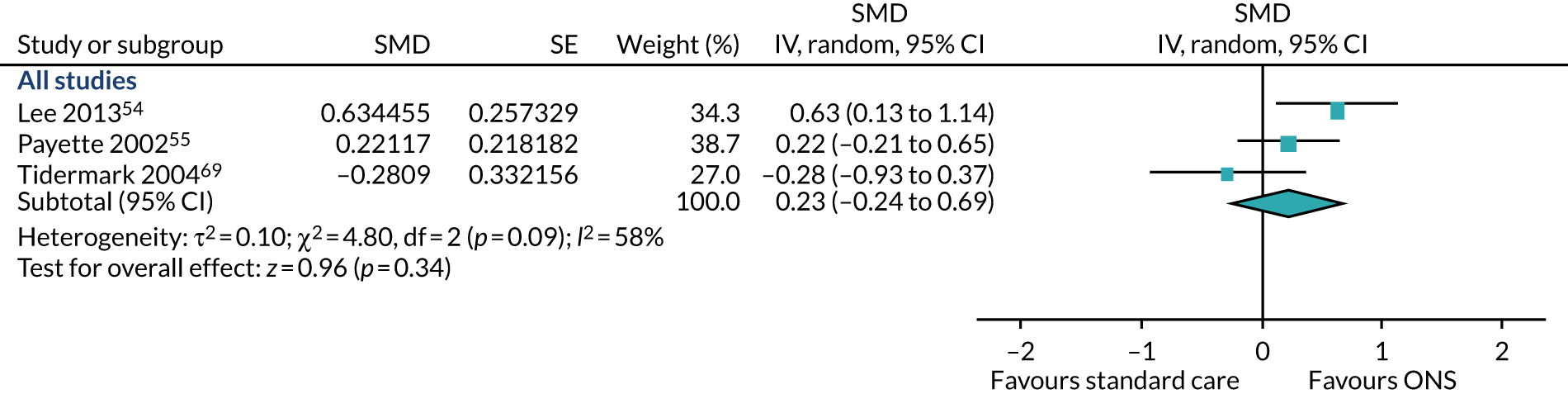
As the studies used different outcome measures, a SMD was calculated using Hedges’ g (adjusted) statistics (a measure of effect size) to standardise the different data across the three studies. Follow-up data were available for 12 weeks54 to 16 weeks55 and 12 months. 69 The pooled result (Figure 7) of the main meta-analysis of CFB scores comprising all three studies showed that the individual study estimates are inconsistent in the direction of effect (SMD 0.23, p = 0.34, 95% CI –0.24 to 0.69). There was evidence of heterogeneity in this analysis (p = 0.09, I2 = 58%). The evidence of this analysis shows that there is a variable confounding factor across studies. A sensitivity analysis could not be conducted as there were no adequately randomised studies. GRADE scores showed low-quality evidence for fat-free muscle mass (see Appendix 13).
FIGURE 7.
Forest plots of fat-free muscle mass (CFB).
Longer-term outcomes
Eight studies53,56,57,67–71 reported data on the effect of ONS on longer-term outcomes related to malnutrition. A meta-analysis was possible for ADL, grip strength, MNA, mobility, hospitalisation and mortality. It was not possible to undertake a meta-analysis for outcomes on QoL; therefore, a narrative synthesis of the results for this outcome was undertaken.
Activities of daily living
Three studies57,71,74 reported data on the effect of ONS compared with SC on ADL, which refers to the ability to perform everyday tasks (or ‘activities of daily living’) as a measure of disability or level of physical functioning. One study71 measured ADL using the Barthel Index, which comprises 10 items in relation to which participants are assigned points, with a higher score indicating an increased ability to perform a task. Luo et al. 74 used a modified version of the Barthel Index. Van Wymelbeke et al. 57 used the Katz score for ADL,57 and compared ADL in participants who received supplements, those who received an alternative dietary intervention (brioche) and those receiving SC. Data from the supplement and brioche groups were combined and compared with the SC group in the analyses presented here. Data from the longest follow-up time available from each study were used in the analyses presented here. This varied across the studies, from 90 days/3 months in Van Wymelbeke et al. 57 to 4 months in the study by Cameron et al. 71 and 24 days in the study by Luo et al. 74
Post-intervention data were used for the meta-analysis, as CFB data could not be calculated for the study by Luo et al. 74 The pooled result of the main meta-analysis comprising all studies (Figure 8a) demonstrated no evidence of an effect of ONS compared with SC on ADL (SMD 0.30, p = 0.55; 95% CI –0.69 to 1.29).
FIGURE 8.
Forest plots of longer-term outcomes including (a) ADL (final values); (b) grip strength; and (c) hospitalisation (CFB). M–H, Mantel–Haenszel.
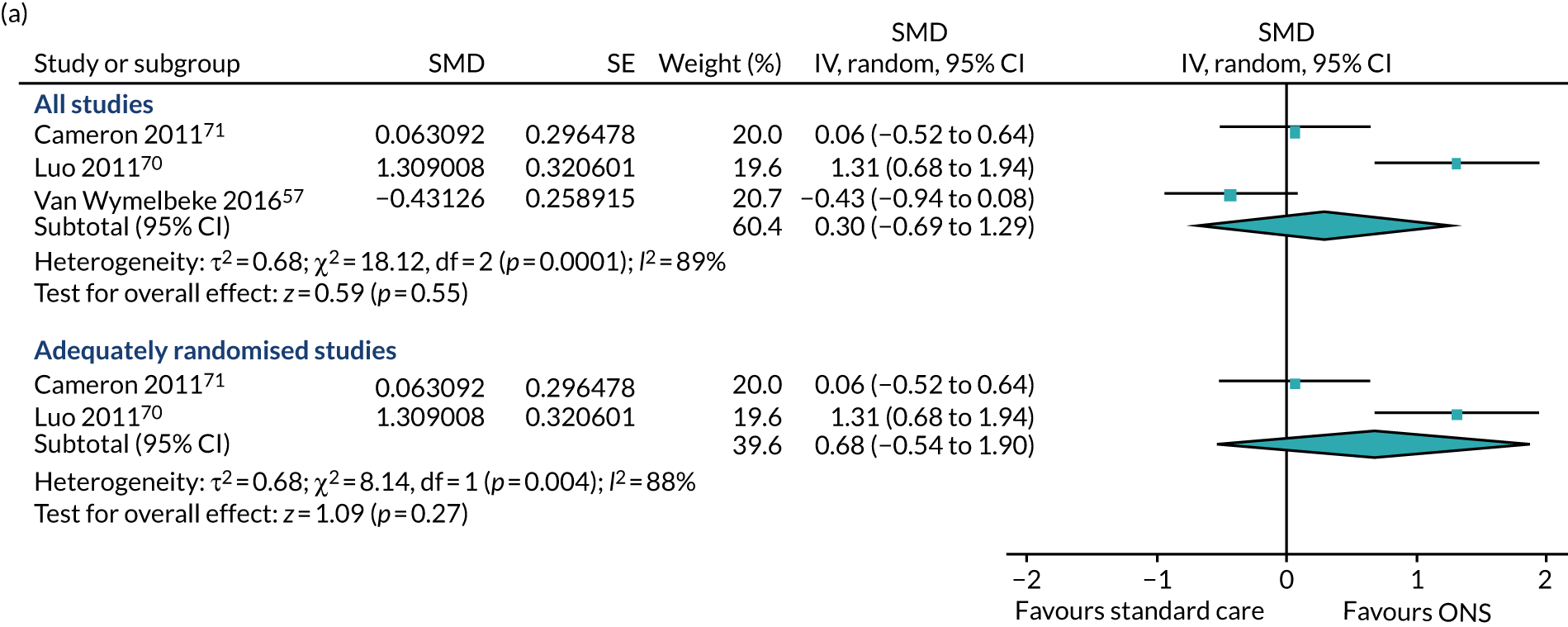
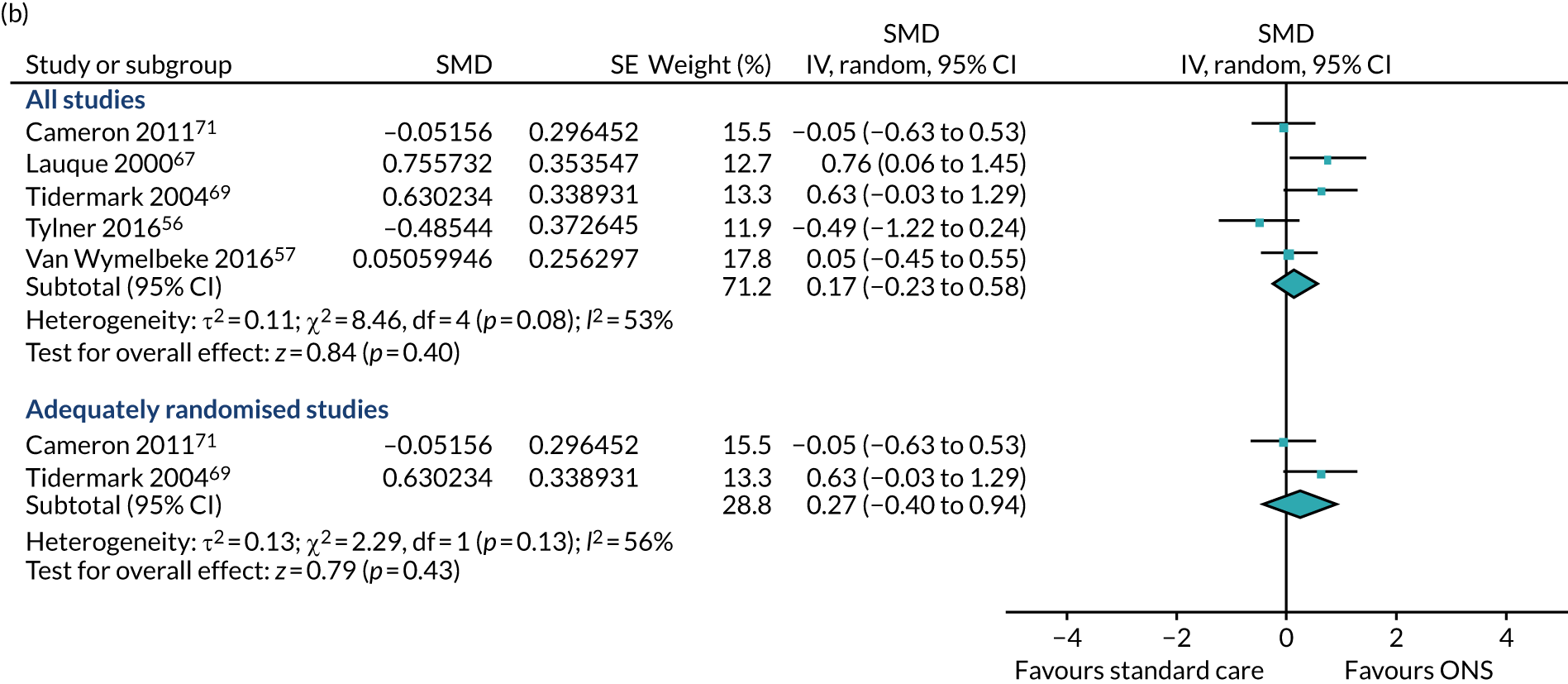
A sensitivity analysis in which the study by Van Wymelbeke et al. ,57 which was not adequately randomised, was omitted also showed no evidence of an effect of ONS compared with SC on ADL (SMD 0.68, p = 0.27; 95% CI –0.54 to 1.90). Substantial heterogeneity was present in the main analysis (I2 = 89%) and in the sensitivity analysis (I2 = 88%). GRADE scores showed very low-quality evidence for ADL (see Appendix 13).
Grip strength
Seven studies55–57,67–69,71 reported data on the effect of ONS compared with SC on grip strength. Five56,57,67,69,71 of these reported data for a CFB meta-analysis. Each of these five studies reported data assessing handgrip strength, our primary outcome measure. Several instruments were used to measure grip strength, including the Jamar Hydraulic Hand Dynamometer57,71 and the Harpenden(R) dynamometer,69 both of which use kilograms as measurement units; and the Martin vigorimeter,56 which measures handgrip strength using kilopascal. Data from two trial arms (one in which participants were provided with ONS and the other in which participants were given brioche) were combined and compared with the SC arm for the study by Van Wymelbeke et al. 57 In the study by Lauque et al. ,67 data were compared between participants who were at risk of malnutrition and received either ONS or SC. The longest follow-up time points across the five studies ranged from 3 to 12 months. The results of the pooled meta-analysis (see Figure 8b) comprising studies using CFB data indicated no evidence of an effect of ONS compared with SC on grip strength (SMD 0.17, p = 0.40; 95% CI –0.23 to 0.58). There was also no evidence of a difference for studies with adequate randomisation (SMD 0.27, p = 0.43; 95% CI –0.40 to 0.94). Substantial statistical heterogeneity was found in both analyses (I2 > 50%), possibly reflecting variation in the follow-up times between studies.
Two studies were not included in the pairwise meta-analyses for this outcome (see Appendix 10). One of these studies from which data could be extracted68 reported an improvement in quadriceps strength (measured in kg using the Nicholas Manual Muscle Tester) among ONS recipients compared with people receiving SC when this was assessed on a non-injured limb (mean CFB scores were 6.5 in the ONS group and 4.8 in the SC group) but not when injured limbs were assessed (mean CFB scores were 2.3 in the ONS group and 2.7 in the SC group). GRADE scores showed very low-quality evidence for grip strength (see Appendix 13).
Hospitalisation
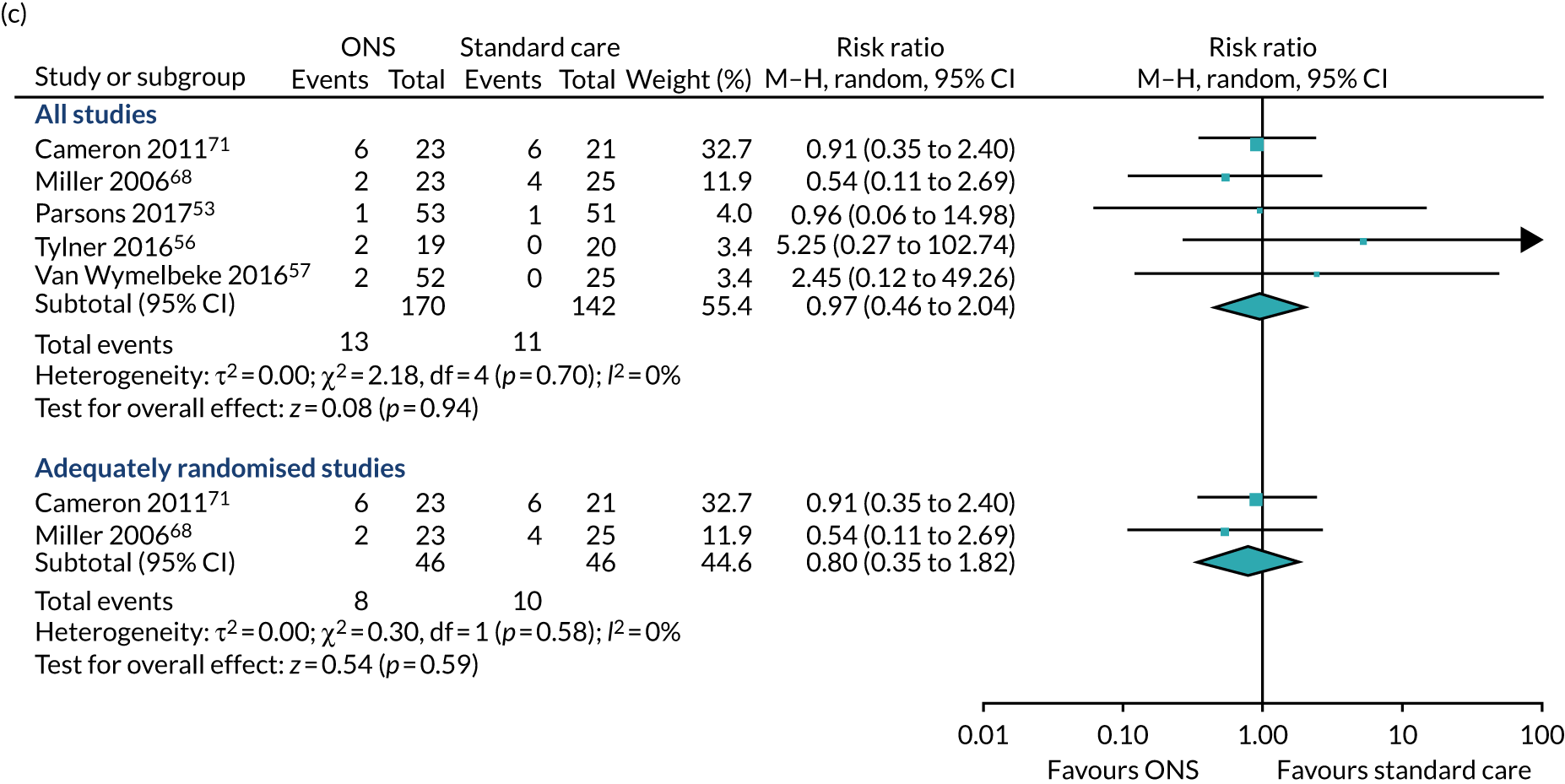
Five studies53,56,57,68,71 considered the impact of ONS on hospitalisation. All five reported data that were suitable for inclusion in the meta-analysis comparing ONS with SC on the number of hospital readmissions,68,71 our preferred measure, or admissions. 56,57 Van Wymelbeke et al. 57 compared hospital admissions between participants who received supplements, brioche or SC. The brioche and SC groups were combined into one intervention arm for the analyses presented here. Follow-up time points, where reported, varied from 6 weeks56 to 90 days/3 months. 57 Only two studies68,71 had been adequately randomised. The pooled result of the main value meta-analysis (see Figure 8c) comprising all five studies showed no evidence of an effect of ONS on hospitalisation (RR 0.97, p = 0.94, 95% CI 0.46 to 2.04). The pooled result of the sensitivity analysis of adequately randomised studies also showed no evidence of an effect of ONS on hospitalisation (RR 0.80, p = 0.59; 95% CI 0.35 to 1.82). Heterogeneity was not detected in either the main or the sensitivity analysis (I2 = 0%). GRADE scores showed very low-quality evidence for hospitalisation (see Appendix 13).
Change in malnutrition
Two studies57,67 reported data for the effect of ONS compared with SC on MNA score, a validated screening tool for the assessment of malnutrition risk. A higher MNA score indicates that a person has a better nutritional status. Both studies reported appropriate data for inclusion in the meta-analysis of post-intervention scores between participants receiving ONS and those receiving SC (Figure 9a). A CFB analysis could not be performed, as one of the studies67 did not report baseline data that are required to calculate CFB scores. The 18-item MNA score was the outcome measure used in both studies. 57,67 Van Wymelbeke et al. 57 assessed MNA at 90 days/12 weeks, whereas Lauque et al. 67 assessed MNA at 60 days/8 weeks. The pooled results of the meta-analysis of post-intervention data provided no evidence of an effect of ONS compared with SC on MNA (SMD –0.36, p = 0.11, 95% CI –0.81 to 0.09). Low heterogeneity was detected between the studies (I2 = 6%). Neither Van Wymelbeke et al. 57 nor Lauque et al. 67 was adequately randomised; therefore, a sensitivity analysis was not undertaken. GRADE scores showed very low-quality evidence for change in malnutrition (see Appendix 13).
FIGURE 9.
Forest plots of longer-term outcomes including (a) MNA (CFB); (b) mobility; and (c) mortality (final values). M–H, Mantel–Haenszel.

Mobility
Three studies68,71,74 reported data for the effect of ONS compared with SC on mobility, assessed using gait speed (in m/second) in two studies,68,71 albeit over different distances, and pace (in seconds/m in one study). 74 The data from the study that measured mobility using pace were converted to speed by dividing the number of metres walked by the average time taken. This ensured that all studies used the same outcome measure, with a larger number indicating a positive outcome, and that MD could be used for the analyses. All three studies were included in the meta-analysis, which analysed post-intervention (final value) scores as CFB data were unavailable. The longest follow-up time points were 4 months in Cameron et al. ,71 12 weeks in Miller et al. 68 and 24 days in Luo et al. 74 The pooled results of the main meta-analysis indicated a positive effect of ONS compared with SC (MD 0.03), which was statistically significant (p < 0.00001, 95% CI 0.02 to 0.04) (see Figure 9b). The results of a sensitivity analysis, including two adequately randomised studies,68,71 demonstrated no evidence of an effect of ONS versus SC on mobility (MD 0.02, p = 0.65, 95% CI –0.06 to 0.09). Statistical heterogeneity was not detected in the main or sensitivity analyses (I2 = 0%). GRADE scores showed very low-quality evidence for mobility (see Appendix 13).
Mortality
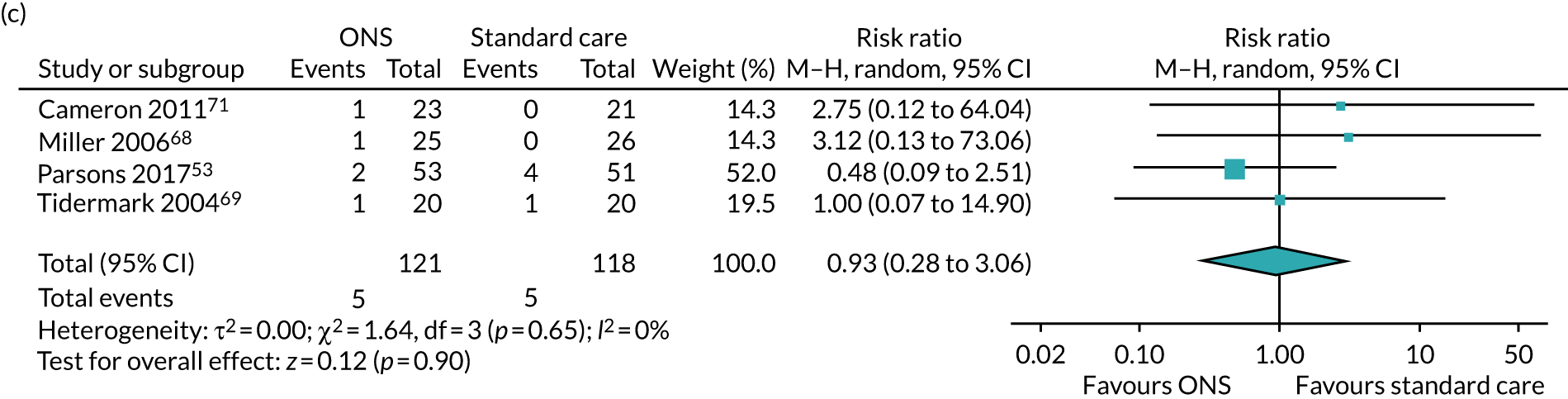
Four studies53,68,69,71 assessed the effects of ONS on mortality and reported data that were suitable for inclusion in the pairwise meta-analysis (see Figure 9c) using final value analysis. Follow-up time points, where reported, varied from 12 weeks53,68 to 4 months71 (follow-up time was not reported in one of the studies69). All four studies had been adequately randomised and, therefore, a sensitivity analysis was not undertaken. The pooled result of the meta-analysis showed no evidence of an effect of ONS on mortality (RR 0.93, p = 0.90, 95% CI 0.28 to 3.06). There was no evidence of statistical heterogeneity (I2 = 0%). GRADE scores showed very low-quality evidence for mortality (see Appendix 13).
Quality of life
Four studies55,68,72,73 reported on the effect of ONS on QoL; two72,73 reported overall QoL scores, and two55,68 reported data from psychological and physical subdomains of quality-of-life tools. Tidermark et al. 69 measured QoL using the EuroQol-5 Dimensions (EQ-5D) but reported only baseline data and so this study is not discussed here further. The results across the four studies reporting on the impact of ONS on overall quality-of-life and physical function domains were mixed,53,55,68,72 although one68 out of two studies55,68 reported a positive effect of ONS on psychological aspects of QoL compared with SC. It was not possible to undertake pairwise meta-analysis using the data from studies reporting overall QoL scores, as none of the studies reported suitable data (see Appendix 10). The reasons varied between studies and included lack of SC group53,68,72,73 and lack of data on comparable QoL tools and domains between studies. 55,68
Parsons et al. 53 compared EQ-5D scores between participants who received ONS and those who received dietary advice (not SC). 53 Their analysis demonstrated higher post-intervention QoL scores, assessed using the EQ-5D time trade-off (TTO) valuation technique and the EQ-5D VAS rescaled tool, among recipients of ONS than among those who received dietary advice. Intention-to-treat (ITT) analysis at week 12 for the ONS and dietary advice groups were 0.496 and 0.364, respectively, on the EQ-5D TTO measure, and 0.535 and 0.457 on the VAS rescaled tool. Mean post-intervention scores from the ITT analysis were higher (indicating increased QoL) among participants who received ONS (mean post-intervention EQ-5D score of 67.4) than among those who received dietary advice (mean post-intervention EQ-5D score of 57.3). Parsons et al. 53 also compared overall QoL between participants who received ONS and those who received dietary advice using the EQ-5D TTO valuation technique. 53 Based on ITT analysis, QoL was significantly higher in the ONS than in the dietary advice group at the 12-week follow-up [EQ-5D TTO scores (mean ± SE) were 0.50 ± 0.04 vs. 0.36 ± 0.05 for the ONS and dietary advice groups, respectively (p = 0.005)]. Otten et al. 72 compared QoL before and after ONS using the EuroQol visual analogue scale (EQ-VAS). CFB data indicated a mean increase in QoL of 10.8 points among ONS recipients after 3 months.
For the studies that reported data on the subdomains of QoL tools, it was not possible to carry out meta-analysis owing to a lack of at least two studies reporting comparable data (pertaining to psychological or physical aspects of QoL) that compared ONS with SC and reported mean and SD values at CFB or post intervention. With regard to psychological aspects of QoL, Payette et al. 55 reported data for the emotional role functioning domain of the 36-item Short Form Survey (SF-36) that showed that the ONS group had a higher post-intervention mean score (better QoL) (84.1, SD 31.4) than the control group (75.4, SD 35.8). Using the mental component score of the 12-item Short Form Survey (SF-12), Miller et al. 68 reported data indicating that participants who received ONS alone had higher (better) scores (post intervention mean 51.4) than those who received exercise (post-intervention mean 51.3), nutrition plus exercise (post-intervention mean 49.8) or SC (post-intervention mean 49.5).
In relation to physical aspects of QoL, Payette et al. 55 reported that, for the physical role functioning domain of the SF-36, mean post-intervention scores were lower among ONS recipients (63.1, SD 35) than among the SC group (69.5, SD 37.7). Miller et al. 68 reported a higher post-intervention mean score for the physical domain of the SF-12 among ONS recipients (post-intervention mean 31.6) than among those who received an exercise intervention (post-intervention mean 31.5), SC (post-intervention mean 30.1) and ONS plus exercise (post-intervention mean 26.9). GRADE was not assessed for QoL as no meta-analysis was undertaken.
Other outcomes
Reduction in infections
Only one study69 reported on reduction in infections. In this study, at 12 months, deep infections engaging the hip joints were not reported in either the group receiving protein-rich supplementation alone or in the group receiving the protein-rich supplementation and nandrolone injection but were reported by 2 out of 17 participants in the control group. Finally, urinary tract infections were seen in 3 out of 18 participants in the protein-rich supplementation alone group, 5 out of 17 participants in the protein-rich supplementation plus nandrolone injection group and 3 out of 17 participants in the control group. This study had a low risk of bias across four of the seven domains reported in the risk-of-bias assessment. GRADE was not assessed for reduction in infections as no meta-analysis was undertaken.
Adverse events
Three studies56,71,74 reported on adverse events, serious adverse events or withdrawals from treatment. Cameron et al. 71 stated that 5 out of 23 participants in the intervention group experienced one or more adverse event, compared with 8 out of 21 in the control group. In the study by Tylner et al. ,56 1 out of 20 participants in the intervention-first group experienced gastrointestinal symptoms at 6 weeks, compared with 2 out of 19 in the control-first group. Luo et al. 74 reported that at 24 days there were 20 adverse effects in the intervention group compared with 24 in the control group. In the intervention arm of that study, 2 out of 22 participants experienced nausea or pruiritis as a result of taking ONS. Cameron et al. 71 reported that three participants (13%) in the intervention group withdrew from treatment. GRADE was not assessed for adverse events as no meta-analysis was undertaken.
Other outcomes not found in the review
Improvement in frailty, morbidity and wound healing and a reduction in falls and admission to long-term care were possible outcomes in the protocol, but no evidence for these was found in the included primary studies. GRADE was not undertaken for these outcomes. Change in frailty status was identified as an outcome of interest, but as no evidence was found it was not possible to assess this.
Summary table of meta-analyses
A complete list of all meta-analysis results for all outcomes is displayed in Table 3.
| Outcome (units) | All studies | Studies with adequate randomisation | ||||
|---|---|---|---|---|---|---|
| n | Statistic | Result (95% CI) | n | Statistic | Result (95% CI) | |
| Consumption outcomes | ||||||
| Energy (kcal/day)/(kcal/kg) | 4 | SMD | 1.02 (0.15 to 1.88)a,b | NA | SMD | NA |
| Protein (g/d)/(g/kg) | 4 | SMD | 1.67 (–0.33 to 3.37)a | NA | SMD | NA |
| Body outcomes | ||||||
| Body weight (kg) | 5 | MD | 1.31 (–0.05 to 2.66)a | 3 | MD | 1.28 (–0.95 to 3.52)a |
| BMI (kg/m2) | 5 | MD | 0.54 (–0.03 to 1.11)a | 2 | MD | 0.44 (–0.82 to 1.71)a |
| Albumin (g/l) | 5 | MD | 1.48 (–0.44 to 3.41)a | 2 | MD | 2.86 (0.69 to 5.03)a,b |
| Fat-free muscle mass (CC cm)/(kg) | 3 | SMD | 0.23 (–0.24 to 0.69)a | NA | SMD | NA |
| MNA scores | 2 | SMD | –0.36 (–0.81 to 0.09)c | NA | MNA score | NA |
| Clinical events | ||||||
| Wound healing | NA | NA | NA | NA | NA | NA |
| Infections | NA | NA | NA | NA | NA | NA |
| Falls | NA | NA | NA | NA | NA | NA |
| Hospitalisation (number/rates) | 5 | RR | 0.97 (0.46 to 2.04)a | 2 | RR | 0.8 (0.35 to 1.82)a |
| Longer-term outcomes | ||||||
| ADL (scores) | 3 | SMD | 0.30 (–0.69 to 1.29)a | 2 | SMD | 0.68 (–0.54 to 1.90)a |
| Mobility (m/second) | 3 | MD | 0.03 (0.02 to 0.04)a,b | 2 | MD | 0.02 (–0.06 to 0.09)a |
| Grip strength (kg)/(kg W)/(kPa) | 5 | SMD | 0.17 (–0.23 to 0.58)a | 2 | SMD | 0.27 (–0.40 to 0.94)a |
| QoL (scores) | NA | NA | NA | NA | NA | NA |
| Mortality (number/rates) | 4 | RR | 0.93 (0.28 to 3.06)c | NA | NA | NA |
Network meta-analysis results
There was a connected network with at least three studies reporting effectiveness for the same comparison for two outcomes: body weight and grip strength. Six studies54,56,67,69,71,74 were included in the body weight analysis and five studies56,57,67,69,71 were included in the grip strength analysis. The effect estimates for ONS and other interventions compared with SC are reported in Table 4. The number of studies with evidence for each comparison is reported. The network diagrams are reported in Appendix 4. The estimates for ONS compared with SC from the meta-analyses are reported for comparison. The estimates of the between-study variance (τ2) from the NMAs and the meta-analyses are also reported for comparison. The probability that each intervention is the most effective is also reported. Values of < 0.7 represent very high uncertainty that the intervention is the most effective. There was convergence for all estimates.
| Comparator | Body weight (kg) | Grip strength (SMD) | ||||
|---|---|---|---|---|---|---|
| n | Mean (95% CrI) | p(best) | n | Mean (95% CrI) | p(best) | |
| ONS | NA | NA | 0.68 | NA | NA | 0.38 |
| SC | 6 | 1.67 (0.12 to 2.93) | 0.01 | 5 | 0.17 (–0.41 to 0.77) | 0.11 |
| ONS + steroid | 1 | 1.05 (–1.99 to 4.10) | 0.32 | 1 | 0.22 (–0.94 to 1.40) | 0.51 |
| τ2 | 1.73 (0.11 to 7.23) | 0.21 (0.00 to 1.24) | ||||
| aSC (pairwise) | 1.35 (0.34 to 2.36) | 0.11 (–0.23 to 0.58) | ||||
| aτ2 (pairwise) | 1.57 | 0.11 | ||||
There is evidence of a difference in effect between ONS and SC for the body weight analysis (1.67, 95% CI 0.12 to 2.93). The NMA confidence intervals for the effect of each outcome were wider than those estimated in the meta-analyses because of greater estimates of the between-study variance. The estimated τ2 from the NMAs is greater than the estimate from the meta-analyses in both analyses, but there is a greater difference in the grip analysis, for which there are fewer than six studies comparing ONS with SC. The estimated τ2 values are particularly high in the grip strength analysis. This is because there are few studies with which to estimate τ2. Although the estimates of τ2 are plausible, it is suspected that they are overestimates when fewer than six studies are in the analysis.
Adherence and acceptability
In addition to the effectiveness outcomes detailed above, we looked for specific evidence regarding factors that may affect adherence to and acceptability of ONS. Specifically, we looked for research detailing barriers and facilitators, determinants and active components that may aid understanding about why some interventions may be more (or less) effective for certain groups of people. Although an inclusive search strategy was used, little relevant information was found in the included studies. The limited information that was collated was derived from the studies included in the effectiveness review. Typically, the data presented, particularly those detailing the acceptability of the ONS, were not assessed through qualitative research methods with patients/health-care professionals but were instead derived from informal observations by the research team. The results are summarised below.
Compliance with ONS was reported in seven studies. 55,57,67,68,70,71 In two studies,67,69 data regarding compliance were reported narratively in brief with no supporting data, making it difficult to draw any firm conclusions about how well participants adhered to ONS. Lauque et al. 67 reported ‘good’ compliance among those at risk of malnourishment who received ONS and those who were malnourished who received ONS but did not give further details. Tidermark et al. 69 measured only compliance with nandrolone injections and not with ONS.
Across the remaining studies,55,57,67,68,70,71 the methods of measuring and reporting compliance were heterogeneous. The lowest level of compliance with ONS was reported in the study by Payette et al. ,55 who assessed adherence in accordance with the number of remaining 250 ml cans of supplement and a mean increase in total energy intake of ≥ 250 kcal per day over the study period. In this study, 23 out of 42 participants (54.8%) were noted to be compliant at 16 weeks. The highest compliance was reported by Miller et al. 68 In this study, 76% of 25 participants in the nutrition -only group adhered to the prescribed volume of nutritional supplement, compared with 66% of 24 participants in the ONS plus exercise group. It may be possible that the differences in compliance between these two studies are related to their setting: Payette et al. 55 was community based, whereas Miller et al. 68 was set in the orthopaedic ward of a hospital. Luo et al. 70 reported that 91–100% of 22 participants in the intervention group consumed their recommended intake of ONS. Van Wymelbeke et al. 57 was the only included study in which it was possible to directly compare compliance with ONS and compliance with another intervention. The study reported that, at 90 days, 74% of 17 participants were consuming all ONS, compared with 83% of 29 participants consuming all the brioche provided.
The precise nature of the interventions included in the effectiveness review was examined closely to understand how the delivery of the intervention may affect its effectiveness. The consistency of reporting on these active components varied across studies. Figure 10 visually displays this information as a rose plot in which the numbers of studies reporting compliance and the energy intake, flavour and frequency of the ONS are shown in blue. Seven studies53–57,68,70 reported frequency of ONS consumption. Five studies53,55–57,67 reported the flavour of ONS. The flavours reported were both sweet and savoury, with strawberry the most common flavour available across all five of the studies. 53,55–57,67 The impact of ONS flavour was not linked with adherence in these five studies. Eight studies53–57,67,70,71 reported the energy intake of the ONS they provided.
FIGURE 10.
Chart showing the level of reporting of various comparators in studies.
Chapter 4 Results of cost-effectiveness review
Characteristics of included studies
One study23 was included in the systematic review of full economic evaluations. This section reviews that study. Tables 5 and 6 present the key study characteristics and results of the study. Appendix 14 details the reasons for excluding studies from the cost-effectiveness review.
| Study author, setting, country and study typea | Intervention and comparator | Effectiveness evidence | Follow-up period | Outcomes measured | Cost-effectiveness results |
|---|---|---|---|---|---|
| Elia et al.23 2017; care homes; UK; cost–utility analysis, single study; linked to effectiveness study (Parsons et al.53) |
Intervention: ONS Comparator: written and verbal dietary advice |
RCT | 12 weeks | QALYs (combination of QoL and mortality) |
Incremental QALYs: ITT 0.0174;b CC 0.018b Incremental cost: ITT £190.50;b CC £217.40b (2016 prices) ICER: ITT £10,941/QALY; CC £11,875 (2016 prices) probability cost-effective: < £20,000/QALY: ITT 0.83, CC 0.80 (2016 prices) Uncertainty: lowc |
| Study | Setting (perspective) | Country | Inpatient | Outpatient/A&E | Community nursing/GP | Nursing home | Specialist | Medication | Social services | Unit costs |
|---|---|---|---|---|---|---|---|---|---|---|
| Elia et al.23 2017 | 55% nursing home, 45% residential home (NHS and PSSa) | UK | Yes | Yes | Yes | Yes | Respiratory | No | No | PSSRU |
Comparators and setting
Elia et al. 23 conducted a cost-effectiveness analysis of ONS. The intervention was described as written and verbal dietary advice. The setting was care homes – roughly half were nursing homes and half were residential homes – and the study was based in the UK. 23 It was not stated if the care homes were privately or publicly owned.
Outcomes and evidence
The measure of benefit in Elia et al. 23 was the QALY. The clinical outcome and resource use evidence came from a single RCT. The follow-up period was 12–13 weeks. An individual patient analysis of clinical study data was conducted. 23 The time horizon of the economic analysis matched the follow-up period of the clinical study. No study perspective was reported; however, it did include costs consistent with the NHS and Personal Social Services (PSS) perspective in the UK. 23
The resource use included in the cost analysis of outcomes and sources of unit costs is reported in Table 6. Hospital inpatient and outpatient, community nursing, nursing home and respiratory care costs were included. 23 Data were collected from patient history and care home records. 23 None of the total intervention costs, the unit cost of ONS or the source of ONS unit cost data were reported,23 but the currency was reported.
Analysis
A cost–utility analysis was conducted. Confidence ellipses were reported, as was the probability of being cost-effective statistics using both bootstrapping and central limit theorem methods. 23
Quality appraisal of included studies
The quality of the included studies was assessed using the BMJ checklist. 49 The completed checklist is reported in Appendix 15. Many of the study design features that would be expected in a well-conducted study were reported in Elia et al. 23 Overall, 30 out of 36 items were assessed as ‘yes’ or ‘not applicable’. Items assessed as ‘no’ included lack of viewpoint and justification for alternatives. Unit costs were not reported, major outcomes were not presented in a disaggregated or aggregated form, and generalisability was not discussed.
Summary of cost-effectiveness results
Summary results from Elia et al. are reported in Table 5. The study found that ONS was associated with greater benefit than the control. ONS was associated with greater QALYs and with higher cost. 23 ONS was cost-effective at a cost-effectiveness threshold of £20,000 per QALY (one of the cost-effectiveness thresholds used by the NICE) with a 0.83 probability. 23
Chapter 5 Development of cost-effectiveness model
The objective of the economic analysis was to evaluate the cost-effectiveness of ONS compared with SC in the studies from the effectiveness review from the perspective of the NHS and PSS. The population was frail older adults in any setting (community, care home, hospital). In addition to ONS and SC, the interventions included in this analysis were those for which evidence of effectiveness on changing BMI was available compared with ONS. The interventions evaluated were ONS and SC.
The systematic review of full economic evaluations of ONS identified one trial-based economic evaluation (see Chapter 4). Although it was not included in the systematic review because the intervention did not meet the inclusion criteria, one study built a decision tree based on the results of the clinical trial. 76 This type of model design could be used to model the direct effect of ONS on longer-term outcomes such as hospitalisation, mortality and QoL. Most studies of ONS reported more immediate outcomes such as the effect on BMI. To use this evidence from the systematic review, a de novo model was developed to evaluate the cost-effectiveness of ONS based on BMI outcome evidence from the systematic review of effectiveness. As described below, the model used evidence from the systematic review on the effect of ONS on change in BMI and modelled the association between BMI and mortality, hospitalisation and EQ-5D.
A cost–utility analysis was conducted that was consistent with the NICE reference case. 77 The cost and QALY outcomes associated with hospitalisation were per episode that occurred over 1 year. The QALY outcomes associated with EQ-5D outcomes were assumed to be over 1 year. The QALY outcomes associated with mortality that occurred within 1 year were life expectancy-related QALYs. The mortality-related QALY outcomes were discounted at a rate of 3.5% per annum. Costs were in Great British pounds (GBP). The price year was 2020.
Model design
The effectiveness of ONS can be measured using a variety of outcomes, and the systematic review of effectiveness investigated many of these. Outcomes researched in the systematic review are presented in Figure 11 in the sequence in which outcomes may occur. The cost-effectiveness of ONS depends on health-related QoL and health-care resource outcomes.
FIGURE 11.
Outcomes related to ONS.
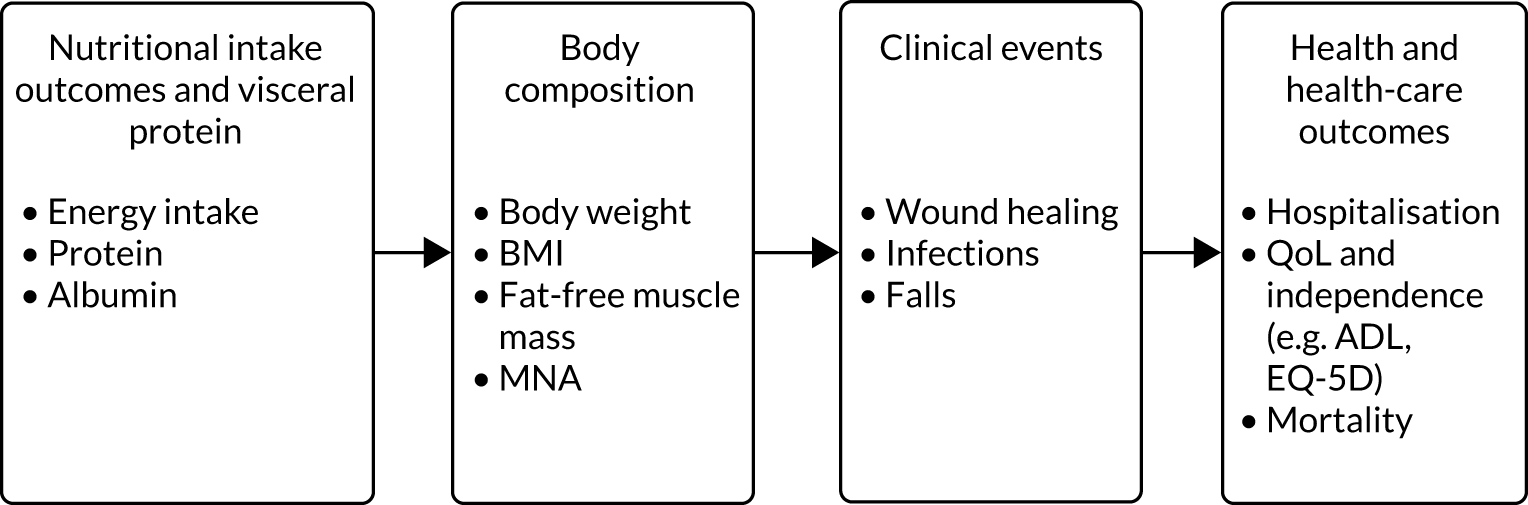
The evidence on outcomes identified in the systematic review was considered for use in two ways to evaluate the cost-effectiveness of ONS. First, the association between BMI and mortality, hospitalisation and EQ-5D was modelled, which was used to estimate the effect of ONS on these outcomes using the effectiveness estimate for ONS on BMI compared with SC and brioche from the systematic review. This approach enabled the cost–utility of ONS to be evaluated for patient cohorts with different BMI values at baseline. BMI was selected as the short-term outcome, as it is commonly used to study the relationship between health status and outcomes, and several included studies in the review reported on BMI using the same scale, meaning that the NMA could be conducted on the original scale. Second, a cost–utility analysis was planned for ONS compared with SC on mortality, hospitalisation and EQ-5D, using the effectiveness evidence from the systematic review. The analysis was to be conducted if there was evidence that ONS might be more effective than SC.
Approach 1 has its limitations. BMI is an imperfect measure of benefit from ONS consumption, as it masks gain in muscle mass versus fat mass, which is a limitation of using this outcome. More accurate measures of malnutrition, such as MNA or MUST scores, muscle mass or functional measures would have been more appropriate; however, these could not be used because of limited evidence from the effectiveness review and limited evidence for the association between MNA or MUST and outcomes such as mortality, hospitalisation and EQ-5D index utility. In addition, estimates of improved longer-term outcomes based on changes in BMI may underestimate the benefit from improved nutrition.
There is a non-linear relationship between BMI and mortality hazard, odds of hospitalisation and EQ-5D. The BMI of the model population is, therefore, very important in determining the cost-effectiveness of ONS. For the base-case analysis, a distribution of BMI values was generated from the data reported in the studies from the effectiveness review (Figure 12). As the cost-effectiveness of ONS can be expected to differ according to baseline BMI values, a number of analyses were conducted with a cohort population with a different baseline BMI value in each cohort.
FIGURE 12.
The base-case BMI distribution at baseline.
Association between body mass index and longer-term outcomes
A focused search of the literature was conducted to identify evidence of the association between BMI and mortality, hospitalisation and QoL, such as EQ-5D and ADL measures in the elderly. The focused search is described in Appendix 16. Only one study with appropriate evidence was found for each of the following outcomes: mortality, hospitalisation and EQ-5D. None of the economic evaluations identified in the review of economic evaluations reported statistics of the association between BMI and these outcomes. Details of these studies are presented in Table 7. The identification of only one study for each outcome meant that it was not possible to estimate the variation in BMI–outcome association that may be found across studies with different characteristics. The uncertainty associated with this could not be captured in the model. No studies estimating the association between BMI and ADL in the population of interest were identified.
| Study | Outcome | Statistic | Population |
|---|---|---|---|
| Nakazawa et al.78 | Mortality | Hazard ratio | Nursing homes, mean age (SD) 84.3 (8.1) years, Japan |
| Ronneikko et al.79 | Hospitalisation | Odds ratio | Home care clients (aged ≥ 63 years), Finland |
| Hunger et al.80 | EQ-5D | Continuous (EQ-5D difference from mean by kg/m2) | People aged ≥ 65 years, Germany |
Mortality
One study was found to provide appropriate evidence for the association between BMI and mortality. 78 The study was a prospective cohort study with a follow-up period of 1 year. The hazard ratio of mortality for each BMI category compared with the reference category is shown in Table 8. The hazard of mortality increases with very low BMI. There is the potential for bias here, as BMI at a certain follow-up time point in a study may not represent BMI status for the period used to estimate mortality risk, and there may be confounding factors. The hazard ratios were modelled on the log scale. In the economic model, linear interpolation was used to convert categorical estimates to hazard ratios for BMI on a continuous scale. A log-hazard ratio was sampled for each individual from the individual-specific normal distribution on the log scale, and this was transformed to the hazard ratio scale.
| BMI (kg/m2) | Hazard ratio of mortality (95% CI) | LN (hazard ratio) | LN (SE) |
|---|---|---|---|
| < 17.3 | 2.4 (1.9 to 3.1) | 0.875 | 0.125 |
| 17.3–19.2 | 1.7 (1.3 to 2.3) | 0.531 | 0.146 |
| 19.3–21.1 | 1.5 (1.2 to 2.0) | 0.405 | 0.130 |
| 21.2–23.5 | 1.2 (0.9 to 1.6) | 0.182 | 0.147 |
| > 23.5 | Reference category | – | – |
The reference hazard of mortality was 0.0518 (0.05 probability over 1 year), taken from Nakazawa et al. 78 The hazard of mortality for each individual was calculated by multiplying the baseline hazard by the estimated hazard ratio for the individual. The probability of dying within the year was derived from the individual’s hazard rate of dying using the formula:
The average life expectancy for a population aged 75–94 years was calculated using life tables from the Office for National Statistics;81 this was 6.29 years.
It was assumed that the EQ-5D index scores for this population, if alive, would be the average in the general population for those aged > 75 years: 0.734. 82 The QALYs at this life expectancy were discounted at a rate of 3.5% per annum.
Hospitalisation
One study79 was found to provide appropriate evidence for the association between BMI and unplanned hospitalisation. This was retrospective cohort study with a follow-up period of 1 year. The odds ratio of hospitalisation for each BMI category compared with the reference category is shown in Table 9. The odds of hospitalisation increase as BMI becomes lower. There is the potential for bias here, as BMI at a certain follow-up time point in a study may not represent BMI status for the period used to estimate hospitalisation risk, and there may be confounding factors. The odds ratios were modelled on the log scale. In the economic model, linear interpolation was used to convert categorical estimates to odds ratios for BMI on a continuous scale. A log-odds ratio was sampled for each individual from the individual-specific normal distribution on the log scale, and this was transformed to the odds ratio scale.
| BMI (kg/m2) | Odds ratio of hospitalisation (95% CI) | ln (odds ratio) | ln (SE) |
|---|---|---|---|
| < 18.5 | 1.09 (0.94–1.27) | 0.086 | 0.077 |
| 18.5–23.9 | Reference category | – | – |
| 24–29.9 | 0.85 (0.78–0.92) | –0.163 | 0.042 |
| ≥ 30.0 | 0.84 (0.76–0.93) | –0.174 | 0.051 |
The reference probability of hospitalisation over 1 year, taken from Ronneikko et al. ,79 was 0.434. The relative risk of hospitalisation the year was derived from the odds ratio of hospitalisation and the reference probability (riskbase) using the formula:
The probability of hospitalisation for each individual was calculated by multiplying the baseline probability by the estimated relative risk for that individual.
A utility decrement of 0.706 for 2 weeks was assumed to be associated with hospitalisation. This was based on the following EQ-5D-3L dimension scores while in hospital: self-care (level 3), mobility (level 3), usual activities (level 3), pain (level 1) and anxiety (level 1). EQ-5D utility was subtracted from the mean for a ≥ 75 years population norm. 82
A cost of £4455 was assumed to be incurred for an admission to hospital. This was the expected cost from the hip fracture non-elective codes HE11A:HE11H. 83
EuroQol-5 Dimensions
One study80 was found to provide appropriate evidence for the association between BMI and unplanned hospitalisation. The study was cross-sectional. The difference in the EQ-5D index from the mean was reported for many different levels of BMI. The 95% CIs were also reported. The data from the published figure were extracted using WebPlotDigitizer version 4.6 (Pacifica, CA, USA) and from the published figure were extracted using WebPlotDigitizer and are reproduced in Figure 13. Using linear interpolation, each individual was assigned an EQ-5D index (difference from mean) normal distribution with mean and standard error.
An increase in BMI is associated with different changes in BMI depending on the baseline BMI. These data are cross-sectional, and there may be some bias in estimating the change in EQ-5D associated with change in BMI from these data. This is particularly the case if the increase in BMI is associated with an increase in protein intake and the increase in weight is fat-free mass. The baseline BMI values assigned to the population in the analyses range from 17 to 23 kg/m2, so change occurs in the upward part of the curve. Increased EQ-5D index utility due to increased BMI was assumed to persist for 1 year.
Effectiveness
No NMA was conducted for the BMI outcome. The effectiveness estimates used in the model were those derived from the BMI meta-analysis. In the base case, the results from all studies were included. Uncertainty in the estimates were modelled using a normal distribution.
Four out of the five studies with evidence for ONS were set in nursing homes, and the remaining study was set in a hospital. Sensitivity analysis using the effectiveness estimate from the meta-analysis included the adequately randomised trials only.
Intervention cost
The incremental cost of ONS compared with SC was calculated for each study included in the NMA of the BMI outcome. For all studies apart from Cameron et al. ,71 the cost of SC was assumed to be zero. For Cameron et al. ,71 SC was high-protein milk, which was costed as a sachet of MCTprocal® per day. Most studies stated the brand of ONS used in the study. The price of the specific product, or as close as possible, was found on online retail sites and in the British National Formulary. 84
The unit costs of each product identified are reported in Table 10 along with the sources. The exchange rates used to convert to GBP, where necessary, were 1 AUD to 0.55 GBP and 1 euro to 0.86 GBP. 85,86
| Resource | Unit cost (foreign currency) | Unit cost (£) | Source |
|---|---|---|---|
| Sustagen® Hospital Formula Active – Neutral, 840 g | AU$23.50 | 12.93 | Pharmacyonline.au87 |
| Novasource 2, 237 ml × 27 pack | – | 238.00 | NineLife88 |
| Enriched brioche bread × 30 | €40.50 | 33.21 | Nutrisens89 |
| Calogen Extra Strawberry, 200 ml | – | 8.50 | Nutridrinks90 |
| Fortisip® Extra, 200 ml | – | 2.43 | BNF91 |
| Fortimel Nutritional Supplement High-Protein High-Energy, 125 ml × 4 | – | 13.20 | Sweetcare92 |
| 1 hour, band 6 hospital-based nurse | – | 45 | PSSRU 201893 |
| Nestle Resource Energy Vanilla, 200 ml × 4 | 11.45 | Nutridrinks94 | |
| MCTprocal® × 30 sachets | 27.42 | BNF85 |
The daily resource use, number of days, per-day cost and total cost of each resource and intervention are reported in Table 11. For most interventions, 1 minute per day of staff time was assumed for delivering the product at normal meal or snack times. In the study by Lauque et al. 67 an average of 2 minutes per day for each patient was costed for a dietitian who visited once per week.
| Study | Intervention | Daily resource/patient | Per day unit cost (£) | Number of days | Total cost (£) |
|---|---|---|---|---|---|
| Cameron et al.71 | ONS | 1× 237 ml sachet Novasource® 2 | 8.81 | 40 | 352.59 |
| Cameron et al.71 | SC | 1× MCTprocal® sachet | 0.91 | 40 | 36.56 |
| Van Wymelbeke et al.57 | ONS | 1× 200 ml Nestle resource energy vanilla | 2.86 | 84 | 240.45 |
| Tylner et al.56 | ONS | 90 ml Calogen® | 3.83 | 42 | 160.65 |
| Lee et al.54 | ONS | 200 ml Fortisip® | 2.43 | 168 | 408.24 |
| Lauque et al.67 | ONS | 300–500 g Clinutren® Nestle | 6.84 | 60 | 410.55 |
| Cameron et al.71 | 1 minute with nurse | 0.75 | |||
| Van Wymelbeke et al.57 | |||||
| Tylner et al.56 | |||||
| Lee et al.54 | |||||
| Lauque et al.67 | ONS | 2 minutes with dietitian | 1.50 |
The total cost per intervention in each study is reported in Table 12. The average cost per type of intervention was included in the analysis: £369.28 for ONS. The assumption was made in the model that ONS is given to the older person for the specific period stated in the studies and no longer. It was also assumed that all of the ONS was used (opened and either consumed or discarded). No information was provided in the studies about unopened products. The settings were nursing homes and hospitals, so it is assumed that the older person continues to be given the ONS during the intervention delivery period.
Incremental cost-effectiveness analysis
The cost-effectiveness of ONS and other comparators was evaluated by estimating the incremental cost-effectiveness ratio (ICER) derived from an incremental cost-effectiveness analysis. The ICER was the incremental cost per QALY gained. This is calculated as the difference in the total discounted cost between the intervention (e.g. ONS) and the comparator (e.g. SC) divided by the difference in the total discounted utility between the intervention and the comparator:
When there are more than two technologies, the ICER for each is compared with the next most cost-effective. A technology is strictly dominated if it costs more and is less effective than a comparator. A technology is dominated by extension if there is a more effective technology with a lower ICER than the next most effective technology. If the ICER of a health technology is less than the accepted cost-effectiveness threshold, then the health technology is considered cost-effective and the decision-maker is willing to adopt the technology. The cost-effectiveness thresholds of £20,000 per QALY and £30,000 per QALY recommended by NICE77 are used as reference cost-effectiveness thresholds in this report.
Analysis of uncertainty
The investigation into how much uncertainty in the evidence influences decision uncertainty, and the uncertainty regarding whether a health-care technology should be adopted, is a key part of an economic evaluation. When evidence is available, we specify probability distributions to represent the uncertainty in the effectiveness estimates. Uncertainty in mortality, hospitalisation and utility outcomes was described in Chapter 5, and uncertainty in the effectiveness estimates was described in Chapter 3. Probabilistic sensitivity analysis was conducted using Monte Carlo simulation, which samples from every distribution n times to produce a joint distribution of the costs and effects of each intervention. The number of iterations was 8000.
The net benefit of adopting a health technology is calculated for different cost-effectiveness thresholds using the following equation:
The proportion of simulation estimates for which the intervention has the highest net benefit represents the probability that the intervention is cost-effective. The probability that an intervention is cost-effective at different cost-effectiveness thresholds is presented in a cost-effectiveness acceptability curve. 95
Base-case and sensitivity analyses
The base-case analysis used the average cost for ONS across the studies included in the BMI NMA. This was £369 per person. The average BMI of 23 kg/m2 of the populations in the included studies evaluating the effect of ONS on BMI was assumed for the model population. The effect estimates from the meta-analysis were used. Sensitivity analysis was conducted based on the meta-analysis result using adequately randomised trials only.
Body mass index–outcomes association
Only one study was identified that detailed the association between BMI and each of the three outcomes in the model: mortality, hospitalisation and EQ-5D. Consequently, sensitivity analyses were conducted exploring the impact on the results of increasing and decreasing the log-hazard ratio, log-odds ratio and difference in EQ-5D index from the mean by 20%.
Cost of oral nutrition supplements and body mass index
The underlying assumption of the meta-analyses of ONS compared with SC is that the different ONS interventions used across the studies have potentially similar effectiveness. There was significant variation in the cost of the ONS intervention across the studies. Sensitivity analysis was conducted varying the cost of the ONS intervention from £200 to £800, while assuming the same effectiveness.
There is a non-linear relationship between baseline BMI and the risk of mortality, and the risk of hospitalisation and the level of EQ-5D index utility. Different analyses were run for baseline BMI values ranging from 17 to 23 kg/m2.
Use of direct evidence of the effectiveness of oral nutrition supplements on longer-term outcomes
A cost–utility analysis was planned using the direct evidence of the effectiveness evidence of ONS on longer-term outcomes (mortality, hospitalisation and EQ-5D outcomes) if sufficient evidence were available and ONS might be more effective than SC.
Chapter 6 Cost-effectiveness results
Base-case results
The incremental cost-effectiveness results for ONS compared with SC in the base-case analysis are presented in Table 13. The cost of ONS intervention was £369 per person and the baseline BMI was 23 kg/m2. The setting for the analysis was assumed to be a care home. Four out of the five studies with BMI effectiveness evidence for ONS were set in nursing homes, and the remaining study was set in a hospital. QALYs and costs were calculated in the model as incremental QALYs and costs compared with SC.
| Intervention | QALYs gained (vs. SC) | Incremental cost (£) (vs. SC) | ICER (£/QALY) | P(CE) £20,000/QALY | P(CE) £30,000/QALY |
|---|---|---|---|---|---|
| SC | – | – | – | 0.72 | 0.64 |
| ONS | 0.0145 | 353 | 24,390 | 0.28 | 0.36 |
Oral nutritional supplements are associated with a greater expected benefit (0.0145 QALYs) and a higher cost (£359) than SC. The ICER for ONS was £24,390. Although this is below the cost-effectiveness threshold of £30,000 per QALY, SC is more likely to be cost-effective than ONS: the probability that ONS is cost-effective is 0.28 at the £20,000-per-QALY threshold and 0.36 at the £30,000-per-QALY threshold. The probability that ONS is cost-effective compared with SC alone is presented as a cost-effectiveness acceptability curve across the threshold range £5000–50,000 per QALY in Figure 14. This is because the incremental net benefit distribution is skewed. The incremental net benefit distribution is presented in Figure 15 and shows that more than 3000 of the 8000 incremental net benefit estimates are slightly negative. The average is £81. The variation in incremental cost estimates is very low, from £320 to £384. The shape of the incremental net benefit distribution is, therefore, driven by the distribution in incremental QALY estimates. The incremental QALY distribution is presented in Figure 16. The incremental QALY value at which the incremental net benefit is zero, the break-even value, is 0.0118. More than 2500 of the 8000 incremental QALY estimates are positive but less than 0.0118.
FIGURE 14.
Cost-acceptability curve for ONS vs. SC.
FIGURE 15.
Base-case incremental net benefit distribution.
FIGURE 16.
Base-case incremental QALY distribution.
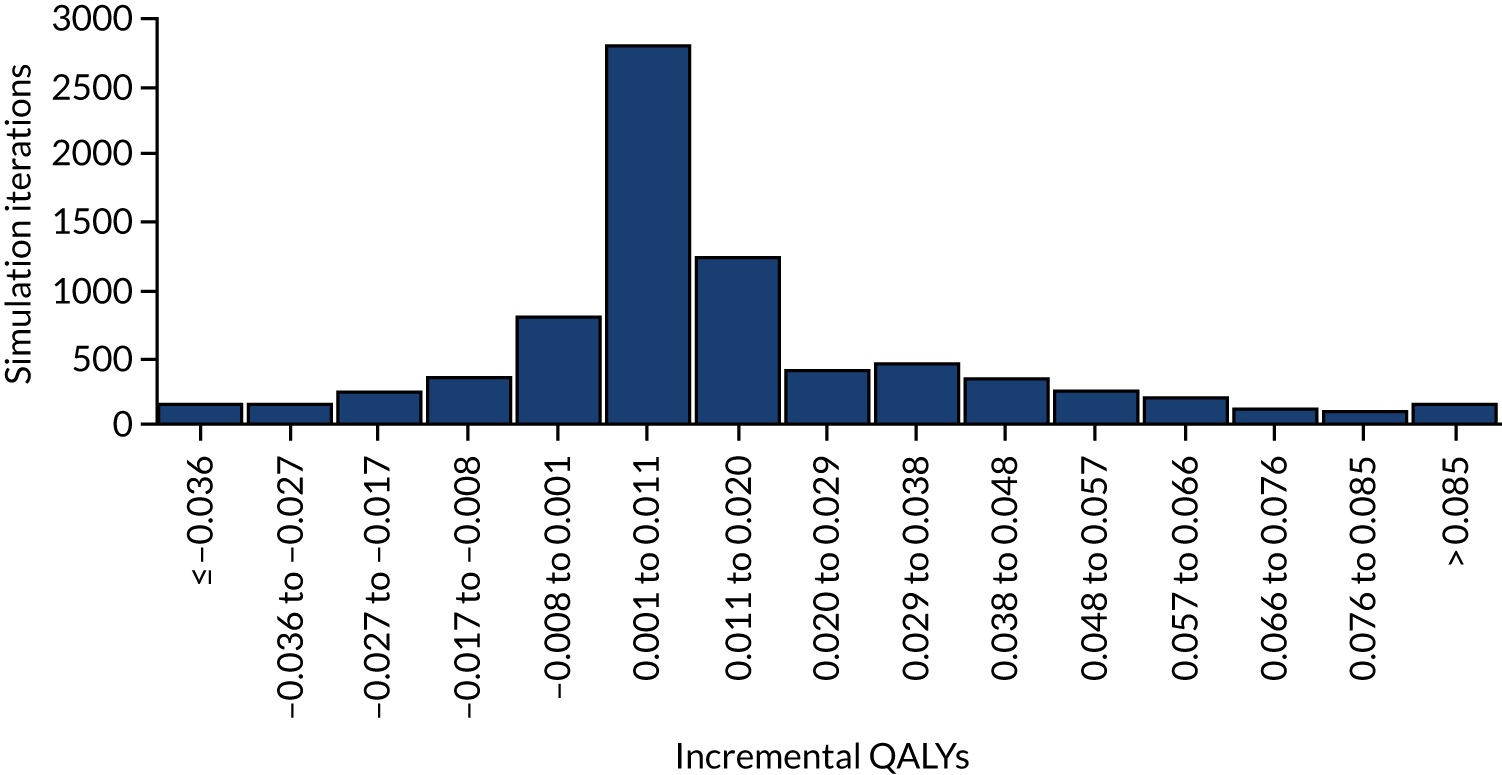
Sensitivity analyses
Body mass index–outcome associations
The evidence for the association between BMI level and mortality risk, hospitalisation risk and EQ-5D index utility showed an increased risk of mortality and hospitalisation at lower levels of BMI. The EQ-5D index utility was lower (utility decrement) at low levels of BMI and at high levels of BMI. The risk of mortality and hospitalisation and the reduction in EQ-5D index utility at lower levels of BMI was reduced by 20% in one sensitivity analysis, reported in Table 14, and increased by 20% in another sensitivity analysis, reported in Table 15. Reducing the benefit from increasing BMI reduces the cost-effectiveness of ONS; the ICER increases (£30,290 per QALY). Increasing the benefit from raising BMI reduces the ICER of ONS (£19,763 per QALY). The probability that ONS is cost-effective at a threshold of £30,000 per QALY remains very low (0.3 to 0.49).
| Intervention | QALYs gained | Incremental cost (£) | ICER (£/QALY) | P(CE) £20,000/QALY | P(CE) £30,000/QALY |
|---|---|---|---|---|---|
| SC | – | – | – | 0.74 | 0.70 |
| ONS | 0.0118 | 356 | 30,290 | 0.26 | 0.30 |
| Intervention | QALYs gained | Incremental cost (£) | ICER (£/QALY) | P(CE) £20,000/QALY | P(CE) £30,000/QALY |
|---|---|---|---|---|---|
| SC | – | – | – | 0.69 | 0.51 |
| ONS | 0.0177 | 350 | 19,763 | 0.31 | 0.49 |
Adequately randomised studies
A sensitivity analysis was conducted using the effectiveness evidence from only the adequately randomised trials (see Chapter 3, Summary of clinical effectiveness results, for the evidence from all trials and from only adequately randomised trials). The magnitude of the effect estimate was less and the uncertainty more in the effectiveness estimate using the only adequately randomised study evidence than when using the effectiveness evidence from all randomised controlled trials. The results are presented in Table 16. The incremental cost-effectiveness ratio increases to £30,466 per QALY, and the probability that ONS is cost-effective at a threshold of £30,000 per QALY is 0.33.
| Intervention | QALYs gained | Incremental cost (£) | ICER (£/QALY) | P(CE) £20,000/QALY | P(CE) £30,000/QALY |
|---|---|---|---|---|---|
| SC | – | – | – | 0.72 | 0.67 |
| ONS | 0.0117 | 356 | 30,466 | 0.28 | 0.33 |
Oral nutritional supplement cost and body mass index levels
Analyses were run for population cohorts assuming BMI values of 17, 19, 21 and 23 kg/m2. The cost of the ONS intervention varied for each of these from £200 per person to £800 per person. The results are presented in Table 17 for the all randomised trials analysis and in Table 18 for the adequately randomised trials analysis.
| Cost (£/person) | BMI (kg/m2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17 | 19 | 21 | 23 | |||||||||
| ICER (£) | P20 | P30 | ICER (£) | P20 | P30 | ICER (£) | P20 | P30 | ICER (£) | P20 | P30 | |
| 100 | 1981 | 0.95 | 0.96 | 3944 | 0.91 | 0.93 | 5158 | 0.90 | 0.92 | 6111 | 0.70 | 0.74 |
| 200 | 4456 | 0.93 | 0.95 | 8301 | 0.81 | 0.88 | 8716 | 0.84 | 0.89 | 12,195 | 0.50 | 0.65 |
| 300 | 6506 | 0.90 | 0.93 | 12,538 | 0.70 | 0.81 | 14,891 | 0.74 | 0.85 | 18,583 | 0.31 | 0.49 |
| 400 | 8805 | 0.84 | 0.90 | 16,930 | 0.57 | 0.74 | 17,052 | 0.60 | 0.77 | 25,984 | 0.27 | 0.33 |
| 500 | 11,408 | 0.78 | 0.87 | 21,587 | 0.43 | 0.66 | 23,950 | 0.46 | 0.70 | 32,698 | 0.25 | 0.30 |
| 600 | 13,639 | 0.72 | 0.85 | 26,011 | 0.30 | 0.57 | 26,339 | 0.31 | 0.60 | 42,993 | 0.20 | 0.26 |
| 700 | 15,727 | 0.65 | 0.81 | 30,335 | 0.20 | 0.48 | 30,582 | 0.19 | 0.50 | 46,185 | 0.19 | 0.25 |
| 800 | 17,913 | 0.57 | 0.77 | 35,118 | 0.11 | 0.38 | 36,727 | 0.10 | 0.40 | 51,861 | 0.16 | 0.24 |
| Cost (£/person) | BMI (kg/m2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17 | 19 | 21 | 23 | |||||||||
| ICER (£) | P20 | P30 | ICER (£) | P20 | P30 | ICER (£) | P20 | P30 | ICER (£) | P20 | P30 | |
| 100 | 2712 | 0.71 | 0.73 | 3892 | 0.68 | 0.71 | 5677 | 0.68 | 0.70 | 6195 | 0.53 | 0.57 |
| 200 | 5280 | 0.70 | 0.72 | 11,172 | 0.59 | 0.64 | 11,045 | 0.63 | 0.66 | 15,955 | 0.38 | 0.47 |
| 300 | 8262 | 0.65 | 0.68 | 16,652 | 0.55 | 0.60 | 16,852 | 0.56 | 0.62 | 24,012 | 0.30 | 0.39 |
| 400 | 11,139 | 0.62 | 0.67 | 22,298 | 0.47 | 0.56 | 19,704 | 0.50 | 0.59 | 33,554 | 0.26 | 0.30 |
| 500 | 14,907 | 0.58 | 0.64 | 26,585 | 0.41 | 0.52 | 26,894 | 0.41 | 0.52 | 41,686 | 0.23 | 0.27 |
| 600 | 18,094 | 0.55 | 0.62 | 31,907 | 0.34 | 0.47 | 31,733 | 0.36 | 0.50 | 52,236 | 0.21 | 0.26 |
| 700 | 20,736 | 0.51 | 0.59 | 38,700 | 0.28 | 0.43 | 39,499 | 0.29 | 0.44 | 60,645 | 0.19 | 0.24 |
Given the evidence informing the model, people with lower BMI will obtain a greater benefit from an increase in BMI. The results in Table 17 are consistent with this and show that ONS are more cost-effective for people with lower BMI. For example, the ICER for a baseline BMI value of 23 kg/m2 and an ONS cost of £200 per person was £12,195 per QALY, whereas the ICER for a baseline BMI value of 17 kg/m2 and an ONS cost of £200 per person was lower, at £4456 per QALY. The corresponding probability that ONS is cost-effective at a threshold of £30,000 per QALY increases from 0.65 to 0.95. The cost-effectiveness of ONS falls as the cost of ONS increases. The results for BMI of 19 kg/m2 and 21 kg/m2 are very similar because they fall within the same BMI category used in the studies that investigated the association between BMI and mortality, hospitalisation and EQ-5D index utility.
In the all-randomised-trials analysis, for the probability of being cost-effective to be > 0.7, the cost of ONS needs to be a maximum of £100 per person for older people with BMI of 23 kg/m2 and a maximum of £400 per person for older people with BMI of 19–21 kg/m2, and could be at least as high as £800 per person for older people with BMI of 17 kg/m2. In the adequately randomised trials analysis, for the probability of being cost-effective to be > 0.7, the cost of ONS needs to be less than £100 per person for older people with BMI of 23 kg/m2 and up to £100 per person for older people with BMI of 19–21 kg/m2, and could be up to £200 for older people with BMI of 17 kg/m2. The lower cost of ONS required for ONS to be cost-effective in the analysis using only the adequately randomised trials compared to the analysis using all trials reflects the greater uncertainty in the effect estimate when using only the adequately randomised trials.
The maximum cost per day of ONS was calculated using different total intervention costs, assuming 1-minute or 4-minute staff time costs per day. The results are presented in Table 19. For example, if the total intervention cost should be, at most, £300 per older person and the targeted duration of ONS provision is 60 days, then the cost of staff time is subtracted from the £300 and the remainder is divided by the number of days to give the maximum ONS cost per day. For 1 minute of staff time per day, this comes to £4.25.
| Total intervention cost (£) | Maximum ONS cost per day (£) | |||||
|---|---|---|---|---|---|---|
| 40 days | 60 days | 80 days | ||||
| 1 minute | 4 minutes | 1 minute | 4 minutes | 1 minute | 4 minutes | |
| 100 | 1.75 | NV | 0.92 | NV | 0.5 | NV |
| 200 | 4.25 | 2.00 | 2.58 | 0.33 | 1.75 | NV |
| 300 | 6.75 | 4.50 | 4.25 | 2.00 | 3.00 | 0.75 |
| 400 | 9.25 | 7.00 | 5.92 | 3.67 | 4.25 | 2.00 |
Three of the ONS interventions were costed at less than £4 a day (1 × 200 ml Nestle resource energy vanilla @ £2.86, 90 ml of Calogen® @ £3.83 and 200 ml of Fortisip® @ £2.43), indicating that there are ONS products that could be cost-effective for older population groups with low BMI. No staff time costs would be incurred daily in the community setting unless there were regular district nurse home visits.
Use of direct evidence of the effectiveness of oral nutritional supplements on longer-term outcomes
A cost–utility analysis was planned using the evidence of effectiveness of ONS on longer-term outcomes (mortality, hospitalisation and EQ-5D outcomes) obtained from the review. However, insufficient evidence was available to suggest effectiveness or enable the analysis.
The mortality effect estimate for ONS compared with SC was RR 0.93 (95% CI 0.28 to 3.06) for all randomised studies. There was no mortality meta-analysis estimate for adequately randomised studies. The hospitalisation effect estimate was RR 0.97 (95% CI 0.46 to 2.04) for all randomised studies and RR 0.8 (95% CI 0.35 to 1.82) for adequately randomised studies. Only one study reported EQ-5D index outcomes, and that study compared ONS with SC. ONS cost more than SC and all of the randomised trial evidence indicates that there is no evidence to support a QALY gain from ONS. The limited evidence means that there is no evidence that ONS are cost-effective from conducting a cost–utility analysis using these longer-term outcome estimates from the systematic review.
Summary of cost-effectiveness results
Two approaches to estimating the cost-effectiveness of ONS based on the systematic review were considered. There was limited evidence of the effectiveness of ONS on longer-term outcomes. Based on the available evidence, there was no evidence that ONS were cost-effective using mortality, hospitalisation and EQ-5D outcome evidence. The cost-effectiveness of ONS and SC was also estimated using the meta-analysis effectiveness evidence for the BMI outcome. The benefit of increased BMI was modelled using evidence from the literature on the association between BMI and mortality, hospitalisation and EQ-5D index utility. This evidence showed that there was a greater benefit of an increase in BMI at lower baseline BMI levels.
Oral nutritional supplements were unlikely to be cost-effective at a threshold of £30,000 per QALY for a population cohort with a baseline BMI of 23 kg/m2. ONS were even less likely to be cost-effective when using only the adequately randomised controlled trial evidence. Using the adequately randomised trial evidence, there was no strong evidence that ONS were cost-effective at any baseline BMI level.
Using the all randomised trial evidence, ONS were cost-effective at a baseline BMI of 19–21 kg/m2 with a high level of certainty when ONS cost no more than £2 per person. It was also cost-effective at a baseline BMI of 17 kg/m2 with a high level of certainty when ONS cost no more than £400 per person.
Chapter 7 Public and patient involvement/engagement
Public and patient involvement/engagement took place throughout the project, from the development of the funding bid to repeated discussions with older people while the review was undertaken. In addition, a stakeholder dissemination event took place at the end of the project to both present the research findings and elicit reflections on the research.
Discussions were conducted with a PPIE group of older people drawn from the Elders Council (Newcastle upon Tyne, UK) at key time points during the review process. Our PPIE team member (AR) liaised on this input. Input was sought into the planning of the review, feedback on the scope of the review and aspects to focus on. One of the main points of feedback was that we needed to assess social factors and issues related to the uptake of ONS in the review; we included these aspects as part of our review. Further discussions were held after data extraction, when the studies included in the review were briefly described and the review team sought feedback on the outcomes that the older people would consider most important. Overwhelmingly, the older people rated QoL and functional outcomes, such as falls, morbidity and wound healing, as most important. The group also highlighted the issue of acceptability of taking ONS. While none of the older people had direct experience of using supplements on a regular basis, they questioned the acceptability of ONS over the short to medium term and hoped that more palatable alternatives would be made available.
Another key time point at which PPIE input was sought was the sharing of our preliminary research findings. The older people were unsure of the relative importance of some of the measures (e.g. albumin) and reiterated that the outcomes most significant to them related to their functional status and QoL. They wanted to see more studies focusing on these outcomes instead of, or in addition to, those relating to more clinical nutritional intake or body composition measures. While the PPIE group did not have much diversity in terms of gender and ethnicity, they highlighted the need for further research that includes different subgroups of older adults, including those in different ethnic groups. The group was also worried about the small and inconclusive evidence base, and consequently would have preferred health-care professionals to exercise caution when prescribing ONS to this population.
The review and economic modelling results were presented in an online workshop to other key stakeholders comprising geriatricians, dietitians and nurse practitioners. The workshop comprised nine stakeholders, and this was supplemented with three individual meetings. In these sessions, discussions considered the experiences of ONS, a presentation of review findings, and group discussions reflecting on the research implications. Three key points arose from the discussion. First, there was the high level of uncertainty about the evidence that this review highlighted. Many stakeholders were surprised that so few studies had been conducted. There was a discussion about the outcomes that appeared to have small positive effects (e.g. energy, mobility); however, the stakeholders questioned the limited clinical significance of these results. The health-care professionals mirrored what the older people considered, namely that functional status was a far more important and relevant outcome than small changes in nutritional intake.
Second, there was widespread concern that there were not enough high-quality studies that compared ONS with good, dietary or ‘food-first’ nutritional support. Although all forms of dietary interventions (e.g. food fortification, advice/counselling) were considered in the review as comparators or without ONS, most studies had limited reporting on these dietary interventions. As a result, the comparators were not given detailed consideration. The feedback was that although ONS might be suitable for a period, other dietary interventions, such as meal fortification, are likely to be more holistic, acceptable and less expensive than having to prescribe ONS to older adults. In addition, stakeholders emphasised that the wider issues underlying malnutrition, such as isolation, loss of appetite, comorbidities and living arrangements, are extremely important in frail older adults. These underlying issues need to be addressed and ONS is only part of a wider suite of nutritional support needed for frail older adults. The stakeholders recognised the heterogeneity of older people, and how nutritional support may vary considerably depending on age, cognitive function, living conditions and other comorbidities. Messaging around what is considered a healthy diet or good nutritional support needs to be more widespread, and, furthermore, studies should consider which (if any) older people would benefit most from the use of ONS.
Chapter 8 Discussion
This systematic review has examined the impact of ONS on frail older people who are malnourished or at risk of malnutrition. Other reviews have been published looking at the impacts on adults more generally31,37 or focusing on specific comorbidities such as cancer,96,97 dementia35 or dialysis therapy98 or following discharge from hospital. 99,100 Additionally, although a cost-effectiveness analysis was undertaken in a previous review, this included children and adults, and the search is now relatively dated, having been completed in March 2014. 24 To better understand the role of ONS in the management of malnutrition in frail older people, a full effectiveness review was combined with a cost-effectiveness review and analysis using the most recent data from published studies.
Summary
Eleven primary studies were identified in the effectiveness review. A summary of characteristics of the included studies and participants, evidence quality and findings can be found in Figure 17. Many of the studies had industry funding. Of the 11 studies identified, six (55%) were either fully (n = 4) or partially funded by industry (n = 2). Three were funded from alternative sources and two studies did not include details of funding/conflict of interests. Given the insufficient information on role of funders and the lack of clarity about independent research, the potential limitation of conflict of interest in reporting findings cannot be ruled out in these studies.
FIGURE 17.
The GOfER (Graphical Overview for Evidence Reviews) diagram of included studies in the effectiveness review.
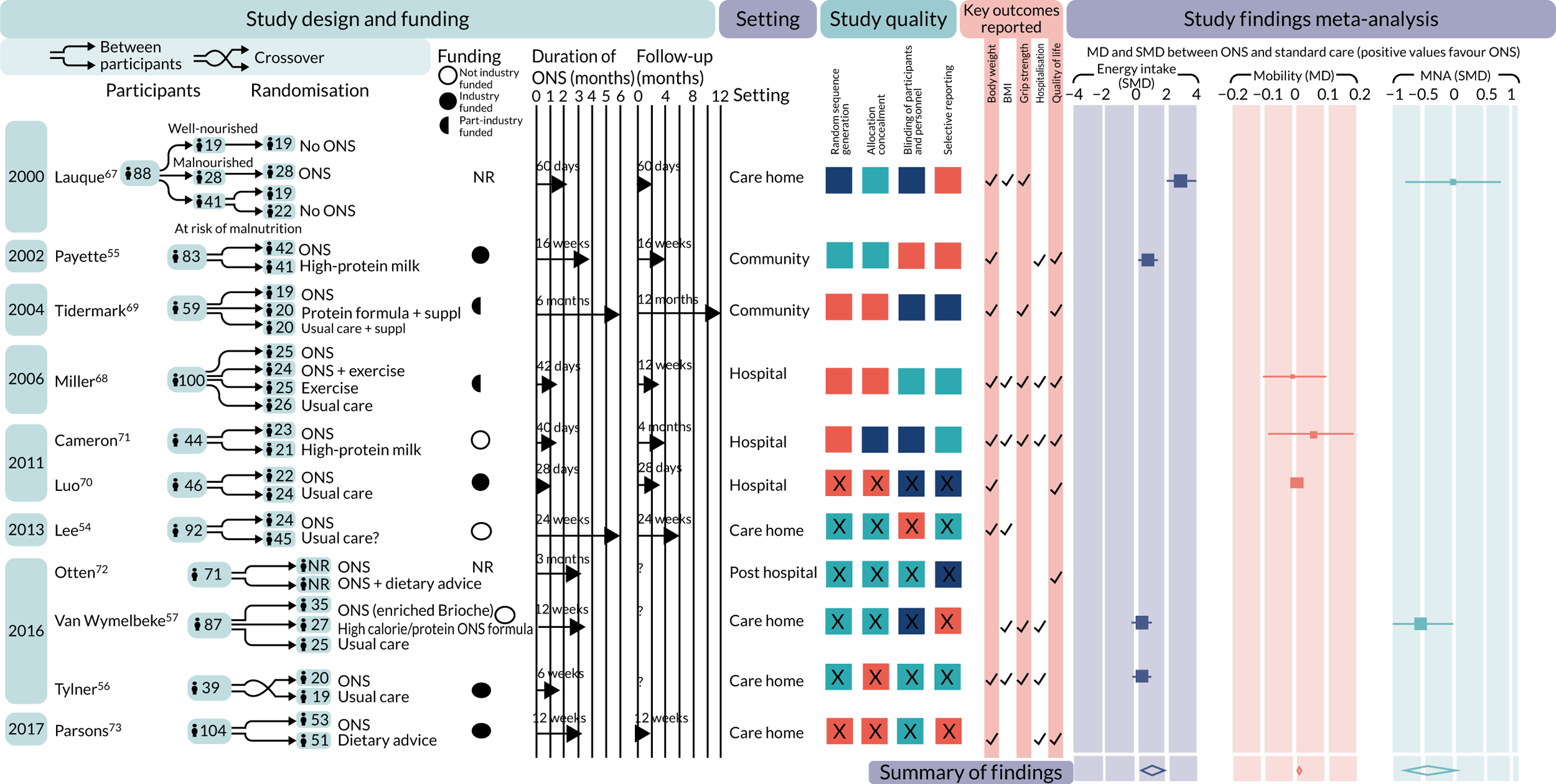
Meta-analyses suggested positive effects of ONS versus SC for energy intake (kcal) (SMD 1.02, 95% CI 0.15 to 1.88; very low-quality evidence) or poor mobility (MD 0.03, p < 0.00001, 95% CI 0.02 to 0.04; very low-quality evidence), and no evidence of an effect for body weight (MD 1.31, 95% CI –0.05 to 2.66; very low-quality evidence) or BMI (MD 0.54, 95% CI –0.03 to 1.11; very low-quality evidence). Pooled results for other outcomes related to malnutrition and its adverse consequences were statistically non-significant. There was mixed narrative evidence regarding the effect of ONS on QoL. All evidence was graded as low or very low quality. NMAs were conducted only for the body weight and grip strength outcomes. The results of the NMA indicated there was evidence of an effect for ONS compared with SC for the body weight outcome only. Study quality was mixed; the method of randomisation was typically poorly reported, and, therefore, all evidence was assessed as low or very low quality using GRADE.
Although the studies included looked at the effectiveness of ONS on all their participants, there was heterogeneity in length and definition of follow-up between the studies. Follow-up spanned from 40 days to 1 year. 54,71 It was difficult to define the length of follow-up in studies, as reports often did not clearly define whether outcome assessments had been undertaken immediately post intervention or after time had elapsed. Additionally, no study reported the impact of the intervention on specific groups (e.g. ethnicity, education or marital status, or by comorbidities). Although demographic information was often reported in the methods section, it was not possible to evaluate the differential impact of ONS in these groups.
Furthermore, there was no systematic reporting in the identified studies of the types or characteristics of ONS that can influence compliance /uptake, such as flavour, and specifically sweetness. Our PPIE group reported that taste was an important factor in whether or not supplements would be consumed. Previous research has reported that age-related changes in taste lead overt sweetness to be one of the major factors contributing to the dislike of ONS. 101 Furthermore, research has demonstrated that the viscosity of the ONS also plays an important role in oral-sensory stimulation and satiety. 102 Den Boer et al. 102 showed that lower thickness of ONS increased intake by one-third without affecting satiation or satiety. Whereas some studies gave a choice of which ONS products could be consumed,53,55,57,67 others either did not report this or used a single brand of ONS.
The studies in the review also lacked any detailed qualitative findings. A single study reported barriers to the use of ONS generally,71 but none examined patient viewpoints in any detail. The discussions with our PPIE group of older people and other stakeholders (e.g. dietitians and clinicians) showed that there was a range of reasons why supplements may work for some people and not others. A lack of reporting on patient experiences in the review is especially surprising, as similar qualitative explorations of the views of both dietitians103 and general practitioners104 views on malnutrition management have already been undertaken.
Compliance data were reported in 7 of the 11 studies. However, there was considerable between-study heterogeneity in how compliance was determined and reported (e.g. aggregated across study arms), which made it difficult to draw firm conclusions. A previous systematic review completed by Hubbard et al. 105 suggested that mean compliance with ONS was 78%; interestingly, compliance was found to be lower in a hospital setting than in the community (67% vs. 81%).
One economic evaluation was identified in the systematic review. This study was conducted in a care home setting. It was well conducted and showed that ONS could be cost-effective in a care home setting when compared with dietary advice.
Two approaches to estimating the cost–utility of ONS using the evidence identified in the effectiveness systematic review were taken. The first approach was to use evidence of the effectiveness of ONS on longer-term outcomes from the systematic review, but there was little or no evidence that ONS was effective using the direct evidence of the effect of ONS on the longer-term outcomes, and so this cost–utility analysis was not conducted. The second approach was to estimate cost-effectiveness using results from the meta-analysis or NMA of effectiveness evidence for BMI in the systematic review. The longer-term consequences of changes in BMI were then modelled using evidence from the literature on the association between BMI and mortality, hospitalisation and EQ-5D index utility. The evidence base for this model design was limited; consequently, the results should be interpreted with caution. This linked evidence showed that there was a greater benefit of an increase in BMI at lower baseline BMI levels. ONS was not likely to be cost-effective at a £30,000 per QALY threshold for a population cohort with a baseline BMI of 23 kg/m2 using all randomised trial evidence. ONS was even less cost-effective when only the adequately randomised control trial evidence was used; there was no strong evidence that ONS was cost-effective at any baseline BMI level. Using the all randomised trial evidence, ONS was cost-effective at a baseline BMI level of 19–21 kg/m2 with a high level of certainty when ONS cost no more than £200 per person. It was also cost-effective at a baseline BMI level of 17 kg/m2 with a high level of certainty when ONS cost no more than £400 per person.
The incremental QALYs in the economic model for ONS versus SC was 0.0145 was slightly lower than the incremental QALYs (0.0174) in the single-study-based economic evaluation by Elia et al. 23 The incremental cost compared with SC (£359) was higher than the incremental cost compared with dietary advice (£191). It is not known if dietary advice is cost-effective. If dietary advice were not cost-effective, then the most appropriate comparator would be SC without dietary advice.
Strengths
This review has many strengths. Our search strategy was broad and wide-ranging and included multiple databases supplemented by searching citations, reference lists of included studies and relevant systematic reviews and comprehensive grey literature sources. We included primary studies that focused on malnourished, frail older people (aged ≥ 65 years). However, we took the pragmatic decision to include studies in which the mean age of participants was ≥ 65 years to ensure that we maximised the evidence base. Two studies53,70 included some participants aged < 65 years. Furthermore, all screening, data extraction and quality assessments were carried out in duplicate to minimise human error. We also included a wide range of outcomes to ensure that the effects of ONS could be investigated across a range of health outcomes, including those hypothesised to respond relatively quickly to ONS (e.g. kcal and protein) and those that may change over a longer period (e.g. hospitalisations, morbidity and mortality). Study authors were contacted when critical missing information was required. Meta-analyses and NMA were undertaken where possible, and the results were compared. For the meta-analyses, both CFB and final values were imputed where one or the other was missing and meta-analyses were conducted for each. A sensitivity analysis was also carried out based on study quality.
A systematic review of economic evaluations of ONS in a frail older population was conducted. Only one study was included, indicating the paucity of cost-effectiveness evidence for ONS in this population. The economic analysis conducted made use of the effectiveness evidence of ONS on BMI from the systematic review. The use of an economic model enabled cost-effectiveness to be explored for different cost assumptions and for different population cohorts defined by BMI. The key uncertainties in the model associated with the effectiveness evidence and the evidence for the association between BMI and outcomes was investigated by conducting sensitivity analyses.
Limitations of the research and deviations from protocol
There are some limitations to this review. The review identified only a small number of included studies focusing on a population of frail older adults who were either malnourished or at risk of malnutrition. This important and growing population, nonetheless, is at high risk of adverse outcomes from malnutrition, and there is limited evidence on the effectiveness of ONS to mitigate malnutrition risk in frail older adults. Therefore, we defined the scope of the review along these lines. A single search was undertaken for both the effectiveness and the cost-effectiveness reviews, which included only studies published in English, which may have excluded some potentially eligible studies. Furthermore, we also deviated from our original protocol in two ways. First, we refined the eligibility criteria with regard to frailty. In the original protocol, we specified that frailty needed to be defined according to a standardised measure such as Fried’s frailty phenotype. However, on screening of the search we found that very few studies described their population as frail in these terms. We therefore decided to expand the eligibility criteria for frailty by using proxy criteria (see Methods). Although these were added after the screening process had begun, to avoid bias we made attempts to prevent being data-driven by asking clinical members of the wider study team for their input into and suggestions about these criteria without indicating the studies and types of data we were encountering. We also updated the searches at a later stage before finalising the results, which will have identified any missing studies related to the extended criteria of frailty. In addition, serious adverse events were defined in discussion among the research team, to only include kidney injury, hyperglycaemia, constipation, diarrhoea, nausea, vomiting, refeeding syndrome and micronutrient deficiency. These were considered the most important serious adverse events, although it is possible that others were not extracted as a result. Finally, we used GRADE to understand the strength of the evidence base, but this was not included in the protocol.
We identified a small evidence base that looked at the impact of ONS on frail, older people specifically. Most studies were based on small samples and the duration of interventions reported was typically ≤ 3 months. The effectiveness of ONS on malnutrition-related outcomes (e.g. grip strength, ADL, hospitalisation) is difficult to establish over a relatively short term. Furthermore, the dose of ONS typically varied across the studies, which could add to the inconsistency observed. Although 11 studies were described as RCTs, the risk-of-bias assessment suggested that there were issues with the allocation sequence generation used and a lack of reporting on how the randomisation was generated, and so forest plots of both all studies and those adequately randomised were reported. 68–71 Additionally, only a few studies reported on certain key outcomes in relation to measures of malnutrition and its consequences (e.g. wound healing, reduction of infections, and falls), and therefore a meta-analysis was not possible. There was also heterogeneity in the measures or scales used for certain outcomes (e.g. ADL, MNA and protein intake). To enable meta-analysis to be undertaken, SMDs were used to aggregate different measures of specific outcomes. As a result, the meta-analysis results are less intuitive to interpret. Another major limitation was the lack of outcome data related to QoL and physical function outcomes, which our PPIE group highlighted as particularly important. Although QoL data were reported in four studies,55,68,72,73 they could not be pooled because of differences in reporting, and, therefore, it was not possible to carry out a meta-analysis. The paucity of robust and consistently reported QoL data highlighted in this review is perhaps not surprising, as measures are often disparate and difficult to combine. The narrative results suggested that the effect of ONS in relation to physical function and overall QoL assessment scores were mixed; however, both studies that reported data for the psychological aspects of QoL reported a positive effect of ONS. The PPIE group questioned the impact of a slight (albeit statistically significant) improvement in energy (for example) and wanted to know whether or not ONS would improve their ability to engage with and be active in their daily lives.
One of the major findings was a lack of data on the effectiveness of interventions by key determinants. Although population characteristics (e.g. socioeconomic status, living arrangement) were reported to some extent at baseline, no studies looked at effectiveness according to these characteristics. As a result, we were unable to examine to whom ONS might be most suited. Similarly, we also set out to investigate what components of the intervention (frequency, length, type, flavour, etc.) led to greater effectiveness. However, owing to both a lack of studies and poor reporting of the nature of the intervention, it was not possible to undertake this analysis. Similarly, there were no qualitative intervention studies identified in the review that examined how patients experienced taking the supplements. Descriptive qualitative studies were identified in this population, but these were not related to a specific intervention and therefore were not eligible for inclusion. 106 Although a wide range of ONS products are available, only five studies gave a choice of flavour or type. 53,55–57,67 Older adults, who are the focus of this review, represent a diverse group, with differences in age and other factors, such as ethnicity and comorbidities. Our PPIE evidence suggested that this was likely to have a strong influence on compliance (and, therefore, potentially on effectiveness), and more research in this area is needed.
Only 10 studies met our inclusion criteria for our meta-analysis. As fewer than 10 studies were included for any outcome, funnel plots could not be used to assess publication bias. This is especially important in this review, as 6 of the 11 studies have a direct link to a company that produces the ONS (through a grant, employees as authors, or free product supply for the trial; see Table 2). Previous research has found evidence between pharmaceutical company sponsorship and results that strongly favour the sponsors’ interest. 107 As a result, there may be trials not published that show negative results. The few studies included in the meta-analyses and the often high level of statistical heterogeneity meant that it was difficult to draw firm conclusions from the results of the meta-analyses.
Sensitivity analysis was conducted on a subset of studies that were assessed as adequately randomised. This was considered to be the most useful sensitivity analysis given the low expectation that studies would be at low risk of bias and that most of the outcome measures were considered to be objective. The exception is the ADL outcome. The classification of the subset of studies in the sensitivity analysis does not imply that the studies included are at low risk of bias and, as expected, no studies were assessed as being at low risk of bias across all categories. Overall, the quality of the reporting and the methods across the included studies was low, and this further limits the conclusions that can be drawn from the results of the meta-analyses.
We included data from the longest follow-up time points available in each study when selecting those for inclusion in the meta-analyses. Too few studies were included in the review to undertake analyses for multiple time points. The reason for choosing the longest follow-up time point was to allow for assessing longer-term impacts on outcomes. Furthermore, as in most meta-analyses, SC was very diverse and varied among the included studies. We made the pragmatic decision to include comparators that we felt were relatively ‘light touch’ or routine and were similar to SC in similar settings (e.g. dietary advice information sheet).
There were also several limitations associated with evaluating cost-effectiveness. There was very limited evidence identified in the systematic review on health-care resource outcomes and outcomes that affect QoL, and this limited the cost-effectiveness conclusions that could be drawn from this evidence. The cost-effectiveness of ONS was also modelled utilising the effectiveness evidence identified in the systematic review focused on linking a short-term measure of malnutrition (i.e. BMI) with longer-term outcomes (i.e. QoL, hospitalisation, mortality). The cost-effectiveness modelling method used, linking BMI effectiveness evidence with outcomes, may underestimate the cost-effectiveness of ONS. This is because change in BMI may be an imperfect proxy for improved nutrition, and the mortality, hospitalisation and EQ-5D index utility outcomes may not capture all of the health-care resource use and health outcomes that may be affected by ONS. The evidence base for the association between change in BMI and QoL and health-care resource outcomes was limited. The use of the cross-sectional study estimating the association of BMI and EQ-5D to model the association between change in BMI and EQ-5D is particularly prone to bias. 80 We also found no information on how the association between BMI and the outcomes might vary by characteristics such as age and setting. Although ONS may not be cost-effective its the average cost, it is possible that it could be cost-effective if a cheaper ONS intervention could be found.
Compared with cost-effectiveness analysis based on effectiveness evidence from a systematic review, the advantage of a trial-based economic analysis is that a comprehensive evaluation of the health-care resource and health outcomes associated with ONS can be undertaken in which all QoL outcomes can be measured using the same preference-based health-related QoL instrument. We conducted a systematic review of the trial-based economic evaluations. There was only one reasonably well-conducted economic evaluation that showed that ONS may be cost-effective in a care home setting. 23 Given the possible variations in settings and older people’s characteristics, additional well-conducted studies may be needed to provide strong evidence of the cost-effectiveness of ONS in a care home. There were no economic evaluations identified conducted in other settings.
Chapter 9 Conclusions
Our review included evidence from different countries and settings and sought to assess the effectiveness and cost-effectiveness of ONS in frail older adults. The current evaluation of studies in frail older people shows that the impact of ONS on most measures related to malnutrition and its adverse consequences is very weak. There was some suggestion of a modest positive effect of ONS on energy and mobility. There was considerable variation in the reporting of ONS and other dietary interventions in studies. Over half of the studies in the effectiveness review were either not randomised or inadequately randomised, and 6 of the 11 studies were fully or partly funded by industry. None of the studies was assessed as being at low risk of bias across all risk-of-bias categories. Furthermore, many did not consistently report functional outcomes that our PPIE group identified as particularly important for older adults. Reporting on intervention duration and follow-up was often inadequate and lacked detail. No studies reported the effectiveness of ONS by determinants. We found no qualitative studies exploring patient experiences of using ONS. There were NMA results for body weight and grip strength outcomes and comparing ONS with SC or ONS with a steroid. The only evidence of an effect for ONS compared with SC was for the body weight outcome.
One study was identified in the cost-effectiveness review. This reasonably well-conducted economic evaluation concluded that ONS may be cost-effective in a care home setting. No studies evaluating the cost-effectiveness of ONS for frail older people in community and hospital settings were identified. The cost-effectiveness analysis conducted here based on the systematic review evidence did not find that ONS was cost-effective, but the evidence base was limited for this analysis. ONS may be cost-effective for older frail people with a BMI of ≤ 21 kg/m2 with cheaper ONS products. However, as there was only one cost-effectiveness study, how the cost-effectiveness of ONS varies across population subgroups defined by, for example, age, independence and BMI is unknown.
The initial logic model developed for the project (see Figure 1) was used throughout as a tool for the researchers to communicate with stakeholders and understand emerging findings from the review on the impact of determinants, pathways and outcomes that relate to the use of ONS in frail older adults. The insights gained from the review and our discussions with older people (PPIE group) and other stakeholders have been used to further refine the logic model presented in Figure 18. The logic model depicts the results (direction of effect) from the review regarding the effectiveness of ONS versus comparators for improving health outcomes and illuminates gaps in the evidence regarding the mechanisms by which ONS exerts its effects. The ‘determinants’ listed in the figure are factors from background literature as well as from stakeholder and PPIE groups. These determinants are believed to influence the context in which ONS would be provided, as well as the feasibility of implementing ONS and factors related to the uptake of this and other dietary interventions. The ‘interventions’ illustrate a variety of approaches that can be used to influence health outcomes related to malnutrition in frail older adults. ‘Active ingredients’ are aspects of the intervention that are likely to influence adherence to ONS and, therefore, the effectiveness of this intervention. As found in our review, there is a need for primary studies to investigate and report the relative impacts of these determinants and active ingredients of dietary interventions on health outcomes to identify pathways with the potential to inform development of ONS and other dietary interventions with maximum effectiveness. The text in italics depicts factors that the PPIE group identified as important, on which data were not necessarily identified in the review findings.
FIGURE 18.
Refined logic model. a, Variable for which baseline data were reported but there was no data regarding its effect on the outcomes; b, factors identified as important by the stakeholders (dietitians). Black text without footnotes or italics indicates that no evidence was identified; blue text indicates interventions for which the effects on health outcomes were assessed in studies that were included in the review; orange text indicates that the outcome favours ONS (based on overall direction of effect); downwards arrows indicate reduction in the outcome; italics indicate factors reported as important by PPIE members.
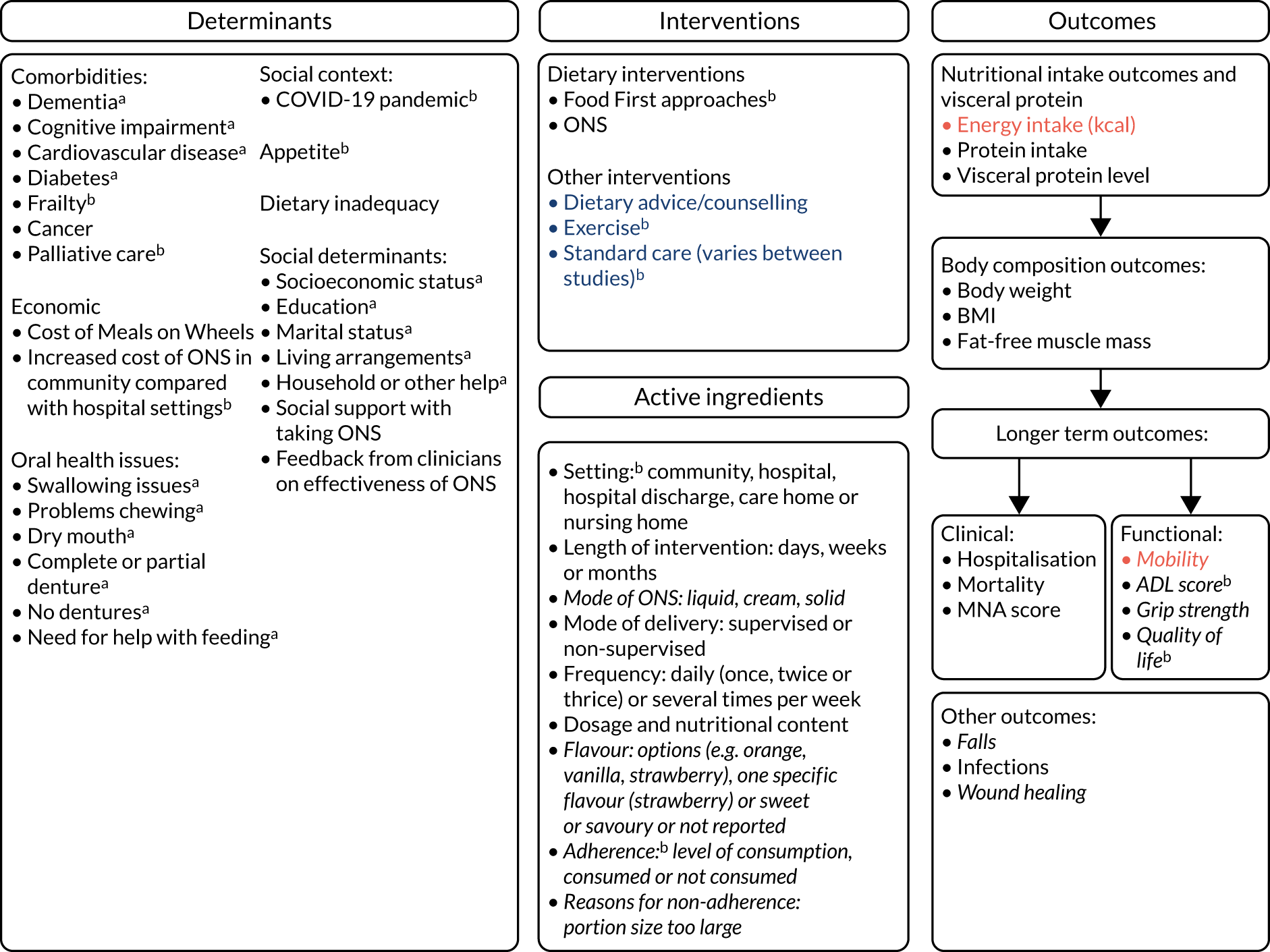
Implications for practice/decision-makers
Insufficient evidence was available to make any firm conclusions regarding the effectiveness and cost-effectiveness of ONS in frail older adults. Overall, there was limited evidence on the effectiveness of ONS with or without other dietary interventions in reducing the risk of malnutrition among frail older adults. There seemed to be positive effects of ONS on energy and mobility, but these were typically small, and the extent to which these are of clinical significance needs to be understood in more detail. Furthermore, it was not possible to make any recommendations about for whom ONS may be best suited or which intervention components (e.g. ONS type, with or without other dietary interventions) lead to more successful outcomes. As the effects of ONS varied greatly between studies, considerable uncertainty remains. ONS are one method of oral nutritional support and are part of a wider toolkit of dietary interventions available to health-care professionals. There remains a need to better understand the role of ONS and the extent to which this works alongside other dietary interventions. Given the limited body of evidence in the review, we were unable to make recommendations for practitioners. Our stakeholder discussions with practitioners (e.g. dietitians, care home staff, clinicians in hospitals) showed a need for further research on dietary interventions (or ‘food-first’ approaches), whereby dietary changes to meals and food are encouraged; and to understand the evidence for ONS in the context of these approaches, along with addressing issues underlying malnutrition in frail older adults (e.g. living conditions, comorbidities).
Implications for research
Considerable uncertainty remains about the effectiveness of ONS in reducing the risk of malnutrition in frail older adults. There is a need for high-quality, adequately powered primary studies that report on short- and long-term health outcomes as well as assess the determinants and participant characteristics that could help understand specific groups for whom ONS might be more (or less) effective.
There was also only one ONS cost-effectiveness trial-based economic evaluation in a care home setting. Further research is need in both this population and others (e.g. in hospital and at home). The cost of ONS varied greatly across studies in terms of both the ONS and staff time preparing and administering ONS. There is a lack of evidence on the relationship between the duration of ONS provision and long-term outcomes such as hospitalisation, mortality and QoL. A primary study that includes both short-term malnutrition outcomes and longer-term outcomes would provide further information about those relationships.
High-quality research with a sufficient sample size should investigate the effect of characteristics such as baseline BMI, MNA, mobility and other comorbidities on the effect of ONS. Primary studies often assume that dietary advice (including the use of ONS) can be followed and will be effective. However, in frail older people, malnutrition can be exacerbated by other factors (e.g. dementia, inability to prepare food, residential status), so there is a need for primary studies that take place in a variety of settings and accurately record (and report by) baseline characteristics. More transparent reporting of the nature of the intervention would also allow effectiveness to be measured against intervention components.
Further research on nutritional support for malnourished, frail older people should aim to close the evidence gaps identified in our review:
-
Outcomes in different subgroups of frail older adults. Older people represent a diverse group. More targeted research is needed from high-quality primary studies on the differential impacts of ONS in older adults representing different groups, for example age (i.e. < 85 or > 85 years), stage of frailty, deprivation, social isolation, place of residence (e.g. at home, care homes), marital status, ethnicity and comorbidities. Future research should also routinely collect and report outcomes in relation to mediators. This would allow the further development of potential tools that could assess the effectiveness of prescribable ONS in particular groups of older adults.
-
Comparison of ONS with other dietary interventions. Our review did not find studies with a wide range of dietary interventions as comparators; for example, no studies had trial arms that promoted energy-dense meals through food enrichment (e.g. choosing full-fat and full-sugar products, nourishing drinks and food enrichment). Future studies should incorporate these dietary approaches compared with, and in combination with, the use of ONS.
-
More evidence on comparisons with multicomponent interventions. While some trial arms incorporated ONS alongside exercise, steroids or dietary advice, further research should examine the impact of combined interventions beyond prescribable ONS, including protein/protein-energy supplementation and exercise.
-
Detailed qualitative research to explore the acceptability and perspectives of patients. There was a lack of detailed qualitative work to discuss the lived experiences of patients prescribed ONS (vs. other treatments). A mixed-methods approach incorporating detailed interviews/focus groups should be added to subsequent trials.
-
Collection of outcome data most relevant to patients. Although a variety of outcomes were reported in the primary studies, our PPIE group members were more concerned with functional or QoL-related outcomes than more clinically driven outcomes. Functional status assessed with either self-report or performance-based measures should be routinely collected alongside clinical outcomes so that patients can realistically assess the likely impact of ONS and other nutritional support interventions on their daily life.
-
Duration of follow-up. The length of follow-up varied greatly across the studies, and few studies looked at the impact of outcomes in the longer term. Specifically, few examined the impact on hospitalisation, morbidity and mortality.
-
Detailed reporting of trial arms. Although the ONS intervention was often described in some depth, too often there was little information on SC (which varied in studies and settings) or the precise nature of dietary counselling/advice. More comprehensive reporting (e.g. using a standard checklist) of standard/usual care should be included in published trial outputs.
-
Cost-effectiveness of ONS interventions across different settings. Only one study evaluated the cost-effectiveness of ONS in a nursing home, and no cost-effectiveness studies were conducted in the home or hospital setting. More primary studies with detailed cost-effectiveness reporting are needed. In addition, there was considerable variation in the cost of ONS across studies. Further research should clarify if there is any difference in effectiveness across types of ONS.
Acknowledgements
We thank the members of the Elders Council of Newcastle who were involved in the PPIE sessions that took place throughout the project.
Contributions of authors
Katie Thomson (https://orcid.org/0000-0002-9614-728X) (Research Associate) co-led the writing of the final report and co-supervised the conduct and write-up of the systematic review. She also oversaw the public engagement and led the stakeholder dissemination workshop.
Stephen Rice (https://orcid.org/0000-0002-6767-0813) (Senior Research Associate, Health Economics) co-led the writing of the protocol and the final report. He also supervised the meta-analysis, conducted the network meta-analysis and led the development of the cost-effectiveness model.
Oluwatomi Arisa (https://orcid.org/0000-0001-8581-6073) (Research Assistant, Evidence Synthesis Group) contributed to the screening of studies for the systematic review, data extraction of the systematic review, meta-analysis and the bias elicitation assessment. She also contributed to the writing of the final report.
Eugenie Johnson (https://orcid.org/0000-0003-3324-7141) (Training Fellow, Evidence Synthesis Group) contributed to the screening of studies for the systematic review, data extraction of the systematic review and the bias elicitation assessment. She also contributed to the writing of the final report.
Louise Tanner (https://orcid.org/0000-0003-2340-8677) (Research Associate, Evidence Synthesis Group) contributed to the data extraction of the systematic review and meta-analysis. She also contributed to the writing of the final report.
Christopher Marshall (https://orcid.org/0000-0002-7970-681X) (Research Associate, Evidence Synthesis Group) led the development of the protocol, was involved in screening and contributed to the writing of the final report.
Tumi Sotire (https://orcid.org/0000-0002-5330-9568) (Research Assistant, Health Economics) contributed to the cost-effectiveness model. He also contributed to the writing of the final report.
Catherine Richmond (https://orcid.org/0000-0002-2940-5197) (Research Assistant, Evidence Synthesis Group) designed and ran the searches for the systematic review, helped manage the references and referencing, and contributed to the writing of the final report.
Hannah O’Keefe (https://orcid.org/0000-0002-0107-711X) (Research Assistant, Evidence Synthesis Group) managed the references and referencing and contributed to the writing of the final report.
Wael Mohammed (https://orcid.org/0000-0003-0370-4903) (Research Assistant, Health Economics) contributed to the screening of studies and to the final report.
Margot Gosney (https://orcid.org/0000-0001-9004-7571) (Elderly Care Consultant and Professor of Elderly Care Medicine) contributed to the design and the writing of the protocol, provided advice on the review and contributed to the final report.
Anne Raffle (https://orcid.org/0000-0003-4684-2230) (Chairperson, Elders Council of Newcastle) contributed to the development of the protocol, facilitated the organisation of the PPIE sessions, provided input into the project and contributed to the final report.
Barbara Hanratty (https://orcid.org/0000-0002-3122-7190) (Professor of Primary Care and Public Health) contributed to the design and the writing of the protocol, provided advice on the effectiveness and cost-effectiveness review and contributed to the final report.
Claire T McEvoy (https://orcid.org/0000-0001-8512-3293) (Senior Lecturer in Nutrition and Ageing Research) contributed to the design and writing of the protocol, provided advice on the effectiveness and cost-effectiveness review and contributed to the final report.
Dawn Craig (https://orcid.org/0000-0002-5808-0096) (Professor of Practice in Evidence Synthesis) contributed to the design and writing of the protocol, provided advice on the effectiveness and cost-effectiveness review and contributed to the final report.
Sheena E Ramsay (https://orcid.org/0000-0002-0391-9704) (Clinical Senior Lecturer) was the principal investigator, and she oversaw the running of the project and provided clinical guidance throughout. She contributed to the design of the protocol, screening and data extraction for the systematic review, the bias elicitation assessment and the writing of the final report.
Publication
Thomson KH, Rice S, Arisa O, Johnson E, Tanner L, Marshall C, et al. Effectiveness and cost-effectiveness of oral nutritional supplements in frail older people who are malnourished or at risk of malnutrition: a systematic review and meta-analysis. Lancet Healthy Longev 2022;3:e654–66.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Thomson KH, Rice S, Arisa O, Johnson E, Tanner L, Marshall C, et al. Effectiveness and cost-effectiveness of oral nutritional supplements in frail older people who are malnourished or at risk of malnutrition: a systematic review and meta-analysis. Lancet Healthy Longev 2022;3:e654-66.
- British Association for Parenteral and Enteral Nutrition . Managing Malnutrition to Improve Lives and Save Money 2018.
- Bollwein J, Volkert D, Diekmann R, Kaiser MJ, Uter W, Vidal K, et al. Nutritional status according to the mini nutritional assessment (MNA®) and frailty in community dwelling older persons: a close relationship. J Nutr Health Aging 2013;17:351-6. https://doi.org/10.1007/s12603-013-0009-8.
- Laur CV, McNicholl T, Valaitis R, Keller HH. Malnutrition or frailty? Overlap and evidence gaps in the diagnosis and treatment of frailty and malnutrition. Appl Physiol Nutr Metab 2017;42:449-58. https://doi.org/10.1139/apnm-2016-0652.
- Verlaan S, Ligthart-Melis GC, Wijers SLJ, Cederholm T, Maier AB, de van der Schueren MAE. High prevalence of physical frailty among community-dwelling malnourished older adults – a systematic review and meta-analysis. J Am Med Dir Assoc 2017;18:374-82. https://doi.org/10.1016/j.jamda.2016.12.074.
- Lorenzo-López L, Maseda A, de Labra C, Regueiro-Folgueira L, Rodríguez-Villamil JL, Millán-Calenti JC. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr 2017;17. https://doi.org/10.1186/s12877-017-0496-2.
- National Institute for Health and Care Excellence (NICE) . Nutrition Support for Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition 2017. www.nice.org.uk/guidance/cg32 (accessed 30 June 2021).
- National Institute for Health and Care Excellence (NICE) . Nutrition Support in Adults 2012. www.nice.org.uk/guidance/qs24/chapter/Quality-statement-2-Treatment (accessed 30 June 2021).
- Office for National Statistics . Living Longer: How Our Population Is Changing and Why It Matters 2019.
- Kingston A, Robinson L, Booth H, Knapp M, Jagger C. MODEM project . Projections of multi-morbidity in the older population in England to 2035: estimates from the Population Ageing and Care Simulation (PACSim) model. Age Ageing 2018;47:374-80. https://doi.org/10.1093/ageing/afx201.
- Guzman-Castillo M, Ahmadi-Abhari S, Bandosz P, Capewell S, Steptoe A, Singh-Manoux A, et al. Forecasted trends in disability and life expectancy in England and Wales up to 2025: a modelling study. Lancet Public Health 2017;2:e307-13. https://doi.org/10.1016/S2468-2667(17)30091-9.
- National Institute for Health and Care Excellence (NICE) . Older People With Social Care Needs and Multiple Long-Term Conditions 2015.
- O’Keeffe M, Kelly M, O’Herlihy E, O’Toole PW, Kearney PM, Timmons S, et al. Potentially modifiable determinants of malnutrition in older adults: a systematic review. Clin Nutr 2018;38:2477-98. https://doi.org/10.1016/j.clnu.2018.12.007.
- Cereda E, Pedrolli C, Klersy C, Bonardi C, Quarleri L, Cappello S, et al. Nutritional status in older persons according to healthcare setting: a systematic review and meta-analysis of prevalence data using MNA®. Clin Nutr 2016;35:1282-90. https://doi.org/10.1016/j.clnu.2016.03.008.
- Russell CA, Elia M. Malnutrition in the UK: where does it begin?. Proc Nutr Soc 2010;69:465-9. https://doi.org/10.1017/S0029665110001850.
- Elia M. The Cost of Malnutrition in England and Potential Cost Savings from Nutritional Interventions (Short Version). Southampton: Malnutrition Action Group of BAPEN and the National Institute for Health and Care Research Southampton Biomedical Research Centre; 2015.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752-62. https://doi.org/10.1016/S0140-6736(12)62167-9.
- Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med 2011;27:1-15. https://doi.org/10.1016/j.cger.2010.08.009.
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56. https://doi.org/10.1093/gerona/56.3.m146.
- Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007;62:722-7. https://doi.org/10.1093/gerona/62.7.722.
- Artaza-Artabe I, Sáez-López P, Sánchez-Hernández N, Fernández-Gutierrez N, Malafarina V. The relationship between nutrition and frailty: effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Maturitas 2016;93:89-9. https://doi.org/10.1016/j.maturitas.2016.04.009.
- Stratton RJ, Elia M. Encouraging appropriate, evidence-based use of oral nutritional supplements. Proc Nutr Soc 2010;69:477-87. https://doi.org/10.1017/S0029665110001977.
- Elia M, Parsons EL, Cawood AL, Smith TR, Stratton RJ. Cost-effectiveness of oral nutritional supplements in older malnourished care home residents. Clin Nutr 2018;37:651-8. https://doi.org/10.1016/j.clnu.2017.02.008.
- Elia M, Normand C, Norman K, Laviano A. A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in the hospital setting. Clin Nutr 2016;35:370-80. https://doi.org/10.1016/j.clnu.2015.05.010.
- Cawood AL, Green C, Stratton RJ. The budget impact of using oral nutritional supplements in older community patients at high risk of malnutrition in England. Proc Nutr Soc 2011;69. https://doi.org/10.1017/S0029665110004131.
- Correa-Pérez A, Abraha I, Cherubini A, Collinson A, Dardevet D, de Groot LCPGM, et al. Efficacy of non-pharmacological interventions to treat malnutrition in older persons: a systematic review and meta-analysis. The SENATOR project ONTOP series and MaNuEL knowledge hub project. Ageing Res Rev 2019;49:27-48. https://doi.org/10.1016/j.arr.2018.10.011.
- Avenell A, Smith TO, Curtain JP, Mak JC, Myint PK. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev 2016;11. https://doi.org/10.1002/14651858.CD001880.pub6.
- Liu Y, Xue X. Systematic review of peri-operative nutritional support for patients undergoing hepatobiliary surgery. Hepatobiliary Surg Nutr 2015;4:304-12. https://doi.org/10.3978/j.issn.2304-3881.2014.12.09.
- de van der Schueren MA, Wijnhoven HA, Kruizenga HM, Visser M. A critical appraisal of nutritional intervention studies in malnourished, community dwelling older persons. Clin Nutr 2016;35:1008-14. https://doi.org/10.1016/j.clnu.2015.12.013.
- Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev 2009;2. https://doi.org/10.1002/14651858.CD003288.pub3.
- Stratton RJ, Hébuterne X, Elia M. A systematic review and meta-analysis of the impact of oral nutritional supplements on hospital readmissions. Ageing Res Rev 2013;12:884-97. https://doi.org/10.1016/j.arr.2013.07.002.
- Nieuwenhuizen WF, Weenen H, Rigby P, Hetherington MM. Older adults and patients in need of nutritional support: review of current treatment options and factors influencing nutritional intake. Clin Nutr 2010;29:160-9. https://doi.org/10.1016/j.clnu.2009.09.003.
- British Association of Parenteral and Enteral Nutrition (BAPEN) . Food FirstFood Enrichment 2016. www.bapen.org.uk/nutrition-support/nutrition-by-mouth/food-first-food-enrichment (accessed 13 January 2022).
- Arnold C, Richter MP. The effect of oral nutritional supplements on head and neck cancer. Int J Radiat Oncol Biol Phys 1989;16:1595-9. https://doi.org/10.1016/0360-3016(89)90968-1.
- Allen VJ, Methven L, Gosney MA. Use of nutritional complete supplements in older adults with dementia: systematic review and meta-analysis of clinical outcomes. Clin Nutr 2013;32:950-7. https://doi.org/10.1016/j.clnu.2013.03.015.
- Collins PF, Stratton RJ, Elia M. Nutritional support in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Am J Clin Nutr 2012;95:1385-95. https://doi.org/10.3945/ajcn.111.023499.
- Cawood AL, Elia M, Stratton RJ. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res Rev 2012;11:278-96. https://doi.org/10.1016/j.arr.2011.12.008.
- Holdoway A, Anderson LM, Ashworth A, Cudby S, Doyle C, Nash L, et al. Managing Adult Malnutrition in the Community 2021. www.malnutritionpathway.co.uk/library/managing_malnutrition.pdf (accessed 17 October 2022).
- NHS England . Guidance – Commissioning Excellent Nutrition and Hydration 2015–2018 2015.
- Shlisky J, Bloom DE, Beaudreault AR, Tucker KL, Keller HH, Freund-Levi Y, et al. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv Nutr 2017;8:17-26. https://doi.org/10.3945/an.116.013474.
- Bloom I, Lawrence W, Barker M, Baird J, Dennison E, Sayer AA, et al. What influences diet quality in older people? A qualitative study among community-dwelling older adults from the Hertfordshire Cohort Study, UK. Public Health Nutr 2017;20:2685-93. https://doi.org/10.1017/S1368980017001203.
- Robinson SM. Improving nutrition to support healthy ageing: what are the opportunities for intervention?. Proc Nutr Soc 2018;77:257-64. https://doi.org/10.1017/S0029665117004037.
- Streicher M, van Zwienen-Pot J, Bardon L, Nagel G, Teh R, Meisinger C, et al. Determinants of incident malnutrition in community-dwelling older adults: a MaNuEL multicohort meta-analysis. J Am Geriatr Soc 2018;66:2335-43. https://doi.org/10.1111/jgs.15553.
- Gibbs M, Drey N, Baldwin C. Oral nutrition support interventions for patients who are malnourished or at risk of malnutrition: a survey of clinical practice amongst UK dietitians. J Hum Nutr Diet 2019;32:108-18. https://doi.org/10.1111/jhn.12599.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372. https://doi.org/10.1136/bmj.n71.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. https://doi.org/10.1136/bmj.313.7052.275.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. https://doi.org/10.1136/bmj.39489.470347.AD.
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401-6. https://doi.org/10.1016/j.jclinepi.2010.07.015.
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Cochrane Handbook for Systematic Reviews of Interventions. Oxford: The Cochrane Collaboration; 2019.
- Parsons EL, Stratton RJ, Cawood AL, Smith TR, Elia M. Oral nutritional supplements in a randomised trial are more effective than dietary advice at improving quality of life in malnourished care home residents. Clin Nutr 2017;36:134-42. https://doi.org/10.1016/j.clnu.2016.01.002.
- Lee LC, Tsai AC, Wang JY, Hurng BS, Hsu HC, Tsai HJ. Need-based intervention is an effective strategy for improving the nutritional status of older people living in a nursing home: a randomized controlled trial. Int J Nurs Stud 2013;50:1580-8. https://doi.org/10.1016/j.ijnurstu.2013.04.004.
- Payette H, Boutier V, Coulombe C, Gray-Donald K. Benefits of nutritional supplementation in free-living, frail, undernourished elderly people: a prospective randomized community trial. J Am Diet Assoc 2002;102:1088-95. https://doi.org/10.1016/S0002-8223(02)90245-2.
- Tylner S, Cederholm T, Faxén-Irving G. Effects on weight, blood lipids, serum fatty acid profile and coagulation by an energy-dense formula to older care residents: a randomized controlled crossover trial. J Am Med Dir Assoc 2016;17:275.e5-11. https://doi.org/10.1016/j.jamda.2015.12.005.
- Van Wymelbeke V, Brondel L, Bon F, Martin-Pfitzenmeyer I, Manckoundia P. An innovative brioche enriched in protein and energy improves the nutritional status of malnourished nursing home residents compared to oral nutritional supplement and usual breakfast: FARINE+ project. Clin Nutr ESPEN 2016;15:93-100. https://doi.org/10.1016/j.clnesp.2016.06.012.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 2021.
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d4002.
- Deeks JJ, Higgins JPT, Altman DG, Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). Cochrane; 2022.
- Page MJ, Higgins JPT, Sterne JAC, Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane; 2022.
- Borenstein M, Hedges LV, Higgins JPT, Rothstein H. Introduction to Meta-Analysis. Chichester: John Wiley & Sons; 2009.
- Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37. https://doi.org/10.1023/A:1008929526011.
- National Institute for Health and Care Excellence (NICE) . NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials 2014.
- Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 2016;7:55-79. https://doi.org/10.1002/jrsm.1164.
- Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434-55. https://doi.org/10.1080/10618600.1998.10474787.
- Lauque S, Arnaud-Battandier F, Mansourian R, Guigoz Y, Paintin M, Nourhashemi F, et al. Protein-energy oral supplementation in malnourished nursing-home residents. A controlled trial. Age Ageing 2000;29:51-6. https://doi.org/10.1093/ageing/29.1.51.
- Miller MD, Crotty M, Whitehead C, Bannerman E, Daniels LA. Nutritional supplementation and resistance training in nutritionally at risk older adults following lower limb fracture: a randomized controlled trial. Clin Rehabil 2006;20:311-23. https://doi.org/10.1191/0269215506cr942oa.
- Tidermark J, Ponzer S, Carlsson P, Söderqvist A, Brismar K, Tengstrand B, et al. Effects of protein-rich supplementation and nandrolone in lean elderly women with femoral neck fractures. Clin Nutr 2004;23:587-96. https://doi.org/10.1016/j.clnu.2003.10.006.
- Luo M, Golubev G, Klyukvin I, Reznik L, Kuropatkin G, Oliver JS, et al. Oral nutrition supplement improved nutritional status in malnourished hip fracture patients: a randomized controlled study. J Sci Res Rep 2015;4:480-9. https://doi.org/10.9734/JSRR/2015/13908.
- Cameron ID, Kurrle SE, Uy C, Lockwood KA, Au L, Schaafsma FG. Effectiveness of oral nutritional supplementation for older women after a fracture: rationale, design and study of the feasibility of a randomized controlled study. BMC Geriatr 2011;11. https://doi.org/10.1186/1471-2318-11-32.
- Otten L, Kiselev J, Franz K, Steinhagen-Thiessen E, Müller-Werdan U, Eckardt R, et al. MON-P021: Effect of a three month post-hospital nutritional intervention on functional performance in frail and malnourished older dults – a randomized controlled study. Clin Nutr 2016;35. https://doi.org/10.1016/S0261-5614(16)30655-0.
- Parsons EL, Stratton RJ, Cawood AL, Smith TR, Warwick H, Elia M. Randomized controlled trial in care home residents shows improved quality of life (QOL) with oral nutritional supplements. Clin Nutr Suppl 2011;6. https://doi.org/10.1016/S1744-1161(11)70075-8.
- Luo M, Golybev G, Klyukvin I, Reznik L, Kuropatkin G, Voss AC. Oral nutritional supplement (ONS) improved nutritional status in malnourished patients receiving hip fracture surgery. Clin Nutr Suppl 2011;6. https://doi.org/10.1016/S1744-1161(11)70389-1.
- de Jong N, Chin A, Paw MJ, de Graaf C, van Staveren WA. Effect of dietary supplements and physical exercise on sensory perception, appetite, dietary intake and body weight in frail elderly subjects. Br J Nutr 2000;83:605-13. https://doi.org/10.1017/S0007114500000775.
- Pouysségur V, Castelli C, Antoine V, Chkair S, Bouvet S. Solid oral supplementation: economic assessment. Economic impact of the introduction of a solid oral nutritional supplement adapted to malnourished older adults with poor dental health. Eur Geriatr Med 2017;8:234-9. https://doi.org/10.1016/j.eurger.2017.04.012.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Nakazawa A, Nakamura K, Kitamura K, Yoshizawa Y. Association between body mass index and mortality among institutionalized elderly adults in Japan. Environ Health Prev Med 2013;18:502-6. https://doi.org/10.1007/s12199-013-0351-9.
- Rönneikkö JK, Mäkelä M, Jämsen ER, Huhtala H, Finne-Soveri H, Noro A, et al. Predictors for unplanned hospitalization of new home care clients. J Am Geriatr Soc 2017;65:407-14. https://doi.org/10.1111/jgs.14486.
- Hunger M, Thorand B, Schunk M, Döring A, Menn P, Peters A, et al. Multimorbidity and health-related quality of life in the older population: results from the German KORA-age study. Health Qual Life Outcomes 2011;9. https://doi.org/10.1186/1477-7525-9-53.
- Office for National Statistics . Life Tables, Principal Projections, UK 2018. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/lifetablesprincipalprojectionunitedkingdom (accessed 5 February 2021).
- Szende A, Janssen B, Cabases J. Self-Reported Population Health: An International Perspective based on EQ-5D. Dordrecht: Springer; 2014.
- NHS England . 2019/20/National/Cost/Collection/Data 2021. www.england.nhs.uk/national-cost-collection/ (accessed 26 July 2021).
- Joint Formulary Committee . BNF 78 (British National Formulary) September 2019 2019.
- National Institute for Health and Care Excellence (NICE) . MCTprocal 2021. https://bnf.nice.org.uk/borderline-substance/mctprocal.html (accessed 10 February 2021).
- Pound Sterling Live . PoundSterling LIVE 2021. www.poundsterlinglive.com (accessed 11 May 2021).
- Pharmacy Online . Sustagen Hospital Formula Active Neutral 840g 2021. www.pharmacyonline.com.au/sustagen-hospital-formula-neutral-840g (accessed 10 February 2021).
- NineLife . Novasource 2 2021. www.ninelife.uk/products/novasource-renal-flavor-vanilla-nestle-nutritional-351100 (accessed 10 February 2021).
- Nutrisens Online Store . Brioche Bread 2021. www.nutrisens.com/shop/en/116-brioche-bread/#/39-flavour-plain/53-packaging-box_of_30 (accessed 10 February 2021).
- NutriDrinks . Calogen EXTRA Strawberry (200ml) 2021. www.nutridrinks.co.uk/calogen-extra-strawberry (accessed 10 February 2021).
- National Institute for Health and Care Excellence (NICE) . Fortisip Extra 2021. bnf.nice.org.uk/borderline-substance/fortisip-extra.html (accessed 10 February 2021).
- SweetCare . Fortimel Compact Nutritional Supplement High-Energy – Nutricia 2021. www.sweetcare.com/nutricia-fortimel-compact-oral-nutritional-supplement-high-energy-p-002599ni (accessed 10 February 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: Personal Social Services Research Unit, University of Kent; 2018.
- NutriDrinks . Nestle Resource Energy Vanilla (4x200ml) 2021. www.nutridrinks.co.uk/nestle-resource-energy-vanilla-4 (accessed 10 February 2021).
- Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005;187:106-8. https://doi.org/10.1192/bjp.187.2.106.
- Baldwin C, Spiro A, Ahern R, Emery PW. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst 2012;104:371-85. https://doi.org/10.1093/jnci/djr556.
- Mello AT, Borges DS, de Lima LP, Pessini J, Kammer PV, Trindade EBSM. Effect of oral nutritional supplements with or without nutritional counselling on mortality, treatment tolerance and quality of life in head-and-neck cancer patients receiving (chemo)radiotherapy: a systematic review and meta-analysis. Br J Nutr 2021;125:530-47. https://doi.org/10.1017/S0007114520002329.
- Liu PJ, Ma F, Wang QY, He SL. The effects of oral nutritional supplements in patients with maintenance dialysis therapy: a systematic review and meta-analysis of randomized clinical trials. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0203706.
- Beck AM, Holst M, Rasmussen HH. Oral nutritional support of older (65 years+) medical and surgical patients after discharge from hospital: systematic review and meta-analysis of randomized controlled trials. Clin Rehabil 2013;27:19-27. https://doi.org/10.1177/0269215512445396.
- Lidder PG, Lewis S, Duxbury M, Thomas S. Systematic review of postdischarge oral nutritional supplementation in patients undergoing GI surgery. Nutr Clin Pract 2009;24:388-94. https://doi.org/10.1177/0884533609332175.
- Kennedy O, Law C, Methven L, Mottram D, Gosney M. Investigating age-related changes in taste and affects on sensory perceptions of oral nutritional supplements. Age Ageing 2010;39:733-8. https://doi.org/10.1093/ageing/afq104.
- den Boer A, Boesveldt S, Lawlor JB. How sweetness intensity and thickness of an oral nutritional supplement affects intake and satiety. Food Qual Prefer 2019;71:406-14. https://doi.org/10.1016/j.foodqual.2018.08.009.
- Liljeberg E, Nydahl M, Lövestam E, Andersson A. A qualitative exploration of dietitians’ experiences of prescribing oral nutritional supplements to patients with malnutrition: a focus on shared tailoring and behaviour change support. J Hum Nutr Diet 2021;34:858-67. https://doi.org/10.1111/jhn.12867.
- Dominguez Castro P, Reynolds CM, Kennelly S, Clyne B, Bury G, Hanlon D, et al. General practitioners’ views on malnutrition management and oral nutritional supplementation prescription in the community: a qualitative study. Clin Nutr ESPEN 2020;36:116-27. https://doi.org/10.1016/j.clnesp.2020.01.006.
- Hubbard GP, Elia M, Holdoway A, Stratton RJ. A systematic review of compliance to oral nutritional supplements. Clin Nutr 2012;31:293-312. https://doi.org/10.1016/j.clnu.2011.11.020.
- den Uijl LC, Kremer S, Jager G, van der Stelt AJ, de Graaf C, Gibson P, et al. That’s why I take my ONS. Means–end chain as a novel approach to elucidate the personally relevant factors driving ONS consumption in nutritionally frail elderly users. Appetite 2015;89:33-40. https://doi.org/10.1016/j.appet.2015.01.016.
- Sismondo S. Pharmaceutical company funding and its consequences: a qualitative systematic review. Contemp Clin Trials 2008;29:109-13. https://doi.org/10.1016/j.cct.2007.08.001.
- Parsons EL, Stratton RJ, Jackson JM, Elia M. OC-039-Oral nutritional supplements are cost effective in improving quality adjusted life years in malnourished care home residents. Gut 2012;61. https://doi.org/10.1136/gutjnl-2012-302514a.39.
- Parsons EL, Stratton RJ, Cawood AL, Jackson JM, Elia M. PP047-SUN oral nutritional supplements are more cost effective than dietary advice in improving quality adjusted life years (QALYS) in malnourished care home residents. Clin Nutr Suppl 2012;7. https://doi.org/10.1016/S1744-1161(12)70099-6.
- Schwarzer G, Carpenter JR, Rücker G. Meta-Analysis with R. Cham: Springer International Publishing; 2015.
- Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. The GRADE Working Group; 2013.
- Edington J, Barnes R, Bryan F, Dupree E, Frost G, Hickson M, et al. A prospective randomised controlled trial of nutritional supplementation in malnourished elderly in the community: clinical and health economic outcomes. Clin Nutr 2004;23:195-204. https://doi.org/10.1016/S0261-5614(03)00107-9.
- Arnaud-Battandier F, Malvy D, Jeandel C, Schmitt C, Aussage P, Beaufrère B, et al. Use of oral supplements in malnourished elderly patients living in the community: a pharmaco-economic study. Clin Nutr 2004;23:1096-103. https://doi.org/10.1016/j.clnu.2004.02.007.
- Seguy D, Hubert H, Robert J, Meunier JP, Guérin O, Raynaud-Simon A. Compliance to oral nutritional supplementation decreases the risk of hospitalisation in malnourished older adults without extra health care cost: prospective observational cohort study. Clin Nutr 2020;39:1900-7. https://doi.org/10.1016/j.clnu.2019.08.005.
- Zhong Y, Cohen JT, Goates S, Luo M, Nelson J, Neumann PJ. The cost-effectiveness of oral nutrition supplementation for malnourished older hospital patients. Appl Health Econ Health Policy 2017;15:75-83. https://doi.org/10.1007/s40258-016-0269-7.
- Freijer K, Nuijten MJC, Schols JMGA. The budget impact of oral nutritional supplements for disease related malnutrition in elderly in the community setting. Front Pharmacol 2012;3. https://doi.org/10.3389/fphar.2012.00078.
- Nuijten M, Mittendorf T. The health economic impact of oral nutritional supplements (ONS) in Germany. Aktuelle Ernahrungsmedizin 2012;37:126-33. https://doi.org/10.1055/s-0032-1304940.
Appendix 1 Search strategies
Searches were designed in Ovid MEDLINE and translated into other databases, as follows.
Date range: inception to date searched.
Dates searched: 26 and 27 February 2020.
The searches were updated 13 September 2021.
MEDLINE (via Ovid)
-
exp Frail Elderly/
-
exp Frailty/
-
frail*.ti,ab,kw,kf.
-
((older or aged) adj (person* or people or patient* or population*)).ti,ab,kw,kf.
-
((geriatric or elder*) adj2 (people or person* or patient* or population*)).ti,ab,kw,kf.
-
exp Nursing Homes/ or exp Homes for the Aged/
-
((residential or nursing or care) adj home*).ti,ab,kw,kf.
-
exp Respite Care/
-
exp Long-Term Care/
-
“home* for the aged”.ti,ab,kw,kf.
-
“old age home*”.ti,ab,kw,kf.
-
“skilled nursing facilit*”.ti,ab,kw,kf.
-
“intermediate care facilit*”.ti,ab,kw,kf.
-
“respite care”.ti,ab,kw,kf.
-
“long term care facilit*”.ti,ab,kw,kf.
-
or/1-15
-
Dietary Supplements/
-
Malnutrition/dh, dt, pc, th [Diet Therapy, Drug Therapy, Prevention & Control, Therapy]
-
Nutritional Support/
-
Food, Fortified/
-
Food, Formulated/
-
“oral nutrition*”.ti,ab,kw,kf.
-
“dietary counselling”.ti,ab,kw,kf.
-
“dietary supplement*”.ti,ab,kw,kf.
-
(food adj2 (fortif* or formulat*)).ti,ab,kw,kf.
-
“nutritional intervention*”.ti,ab,kw,kf.
-
“liquid supplement*”.ti,ab,kw,kf.
-
“sip feed*”.ti,ab.
-
“nutrition* management”.ti,ab,kw,kf.
-
(nutri* adj2 (supplement* or therapy)).ti,ab,kw,kf.
-
(maln* adj2 (prevent* or management or risk factor*)).ti,ab,kw,kf.
-
or/17-31
-
16 and 32
-
exp animal/ not human/
-
33 not 34
-
limit 35 to english language.
EMBASE (via Ovid)
-
exp frail elderly/
-
exp frailty/
-
frail*.ti,ab,kw.
-
((older or aged) adj (person* or people or patient* or population*)).ti,ab,kw.
-
((geriatric or elder*) adj2 (frail* or people or person* or patient* or population*)).ti,ab,kw.
-
exp nursing home/
-
exp home for the aged/
-
((residential or nursing or care) adj home*).ti,ab,kw.
-
exp respite care/
-
long term care/
-
“home* for the aged”.ti,ab,kw.
-
“old age home*”.ti,ab,kw.
-
“skilled nursing facilit*”.ti,ab,kw.
-
“intermediate care facilit*”.ti,ab,kw.
-
“respite care”.ti,ab,kw.
-
“long term care facilit*”.ti,ab,kw.
-
or/1-16
-
dietary supplement/
-
malnutrition/dt, pc, th [Drug Therapy, Prevention, Therapy]
-
nutritional support/
-
fortified food/
-
elemental diet/
-
“oral nutrition*”.ti,ab,kw.
-
“dietary counselling”.ti,ab,kw.
-
“dietary supplement*”.ti,ab,kw.
-
(food adj2 (fortif* or formulat*)).ti,ab,kw.
-
“nutritional intervention*”.ti,ab,kw.
-
“liquid supplement*”.ti,ab,kw.
-
“sip feed*”.ti,ab.
-
“nutrition* management”.ti,ab,kw.
-
(nutri* adj2 (supplement* or therapy)).ti,ab,kw.
-
(maln* adj2 (prevent* or management or risk factor*)).ti,ab,kw.
-
or/18-32
-
17 and 33
-
exp animal/
-
exp human/
-
35 not 36
-
34 not 37
-
limit 38 to english language.
Cochrane
-
#1 MeSH descriptor: [Frail Elderly] explode all trees
-
#2 MeSH descriptor: [Frailty] explode all trees
-
#3 (frail*):ti,ab,kw
-
#4 (((older or aged) NEAR/1 (person* or people or patient* or population*))):ti,ab,kw
-
#5 (((geriatric or elder*) NEAR/2 (people or person* or patient* or population*))):ti,ab,kw
-
#6 MeSH descriptor: [Nursing Homes] explode all trees
-
#7 (((residential or nursing or care) NEAR/1 home*)):ti,ab,kw
-
#8 MeSH descriptor: [Respite Care] explode all trees
-
#9 MeSH descriptor: [Long-Term Care] explode all trees
-
#10 (home* NEXT “for the aged”):ti,ab,kw
-
#11 (“old age” NEXT home*):ti,ab,kw
-
#12 (“skilled nursing” NEXT facilit*):ti,ab,kw
-
#13 (“intermediate care” NEXT facilit*):ti,ab,kw
-
#14 (“respite care”):ti,ab,kw
-
#15 (“long term care” NEXT facilit*):ti,ab,kw
-
#16 (OR #1-#15)
-
#17 MeSH descriptor: [Dietary Supplements] this term only
-
#18 MeSH descriptor: [Malnutrition] this term only and with qualifier(s): [therapy - TH, diet therapy - DH, drug therapy - DT, prevention & control - PC]
-
#19 MeSH descriptor: [Nutritional Support] this term only
-
#20 MeSH descriptor: [Food, Fortified] this term only
-
#21 MeSH descriptor: [Food, Formulated] this term only
-
#22 (oral NEXT nutrition*):ti,ab,kw
-
#23 (“dietary counselling”):ti,ab,kw
-
#24 (dietary NEXT supplement*):ti,ab,kw
-
#25 ((food NEAR/2 (fortif* or formulat*))):ti,ab,kw
-
#26 (nutritional NEXT intervention*):ti,ab,kw
-
#27 (liquid NEXT supplement*):ti,ab,kw
-
#28 (sip NEXT feed*):ti,ab,kw
-
#29 (nutrition* NEXT management):ti,ab,kw
-
#30 ((nutri* NEAR/2 (supplement* or therapy))):ti,ab,kw
-
#31 ((maln* NEAR/2 (prevent* or management or risk factor*))):ti,ab,kw
-
#32 (OR #17-#31)
-
#33 #16 AND #32.
CINAHL (via EBSCOhost)
-
S38 S37 AND LA English
-
S37 S33 NOT S36
-
S36 S34 NOT S35
-
S35 (MH “Human”)
-
S34 (MH “Animals+”)
-
S33 S16 AND S32
-
S32 S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31
-
S31 TI ( (maln* N2 (prevent* or management or risk factor*)) ) OR AB ( (maln* N2 (prevent* or management or risk factor*)) )
-
S30 TI ( (nutri* N2 (supplement* or therapy)) ) OR AB ( (nutri* N2 (supplement* or therapy)) )
-
S29 TI “nutrition* management” OR AB “nutrition* management”
-
S28 TI “sip feed*” OR AB “sip feed*”
-
S27 TI “liquid supplement*” OR AB “liquid supplement*”
-
S26 TI “nutritional intervention*” OR AB “nutritional intervention*”
-
S25 TI ( (food N2 (fortif* or formulat*)) ) OR AB ( (food N2 (fortif* or formulat*)) )
-
S24 TI “dietary supplement*” OR AB “dietary supplement*”
-
S23 TI “dietary counselling” OR AB “dietary counselling”
-
S22 TI “oral nutrition*” OR AB “oral nutrition*”
-
S21 (MH “Food, Formulated”)
-
S20 (MH “Food, Fortified”)
-
S19 (MH “Nutritional Support”)
-
S18 (MH “Malnutrition/DH/DT/PC/TH”)
-
S17 (MH “Dietary Supplements”)
-
S16 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15
-
S15 TI “long term care facilit*” OR AB “long term care facilit*”
-
S14 TI “respite care” OR AB “respite care”
-
S13 TI “intermediate care facilit*” OR AB “intermediate care facilit*”
-
S12 TI “skilled nursing facilit*” OR AB “skilled nursing facilit*”
-
S11 TI “old age home*” OR AB “old age home*”
-
S10 (MH “Long Term Care”)
-
S9 (MH “Respite Care”)
-
S8 TI ( ((residential or nursing or care) N1 home*) ) OR AB ( ((residential or nursing or care) N1 home*) )
-
S7 TI home* for the aged OR AB home* for the aged
-
S6 (MH “Nursing Homes+”)
-
S5 TI ( ((geriatric or elder*) N2 (people or person* or patient* or population*)) ) OR AB ( ((geriatric or elder*) N2 (people or person* or patient* or population*)) )
-
S4 TI ( ((older or aged) N1 (person* or people or patient* or population*)) ) OR AB ( ((older or aged) N1 (person* or people or patient* or population*)) )
-
S3 TI frail* OR AB frail*
-
S2 (MH “Frailty Syndrome”)
-
S1 (MH “Frail Elderly”).
Scopus
( ( TITLE-ABS-KEY ( frail* ) OR TITLE-ABS-KEY ( ( ( older OR aged ) W/1 ( person* OR people OR patient* OR population* ) ) ) OR TITLE-ABS-KEY ( ( ( geriatric OR elder* ) W/2 ( people OR person* OR patient* OR population* ) ) ) OR TITLE-ABS-KEY ( ( ( residential OR nursing OR care ) W/1 home* ) ) OR TITLE-ABS-KEY ( “home* for the aged” ) OR TITLE-ABS-KEY ( “old age home*” ) OR TITLE-ABS-KEY ( “skilled nursing facilit*” ) OR TITLE-ABS-KEY ( “intermediate care facilit*” ) OR TITLE-ABS-KEY ( “respite care” ) OR TITLE-ABS-KEY ( “long term care facilit*” ) ) ) AND ( ( TITLE-ABS-KEY ( “oral nutrition*” ) ) OR ( TITLE-ABS-KEY ( “dietary counselling” ) ) OR ( TITLE-ABS-KEY ( “dietary supplement*” ) ) OR ( TITLE-ABS-KEY ( ( food W/2 ( fortif* OR formulat* ) ) ) ) OR ( TITLE-ABS-KEY ( “nutritional intervention*” ) ) OR ( TITLE-ABS-KEY ( “liquid supplement*” ) ) OR ( TITLE-ABS-KEY ( “sip feed*” ) ) OR ( TITLE-ABS-KEY ( “nutrition* management” ) ) OR ( TITLE-ABS-KEY ( ( nutri* W/2 ( supplement* OR therapy ) ) ) ) OR ( TITLE-ABS-KEY ( ( maln* W/2 ( prevent* OR management OR “risk factor*” OR therapy ) ) ) ) ) AND NOT INDEX ( medline ) AND ( LIMIT-TO ( LANGUAGE , “English” ) )
Appendix 2 Duplicate data reporting across included studies
| Study ID | Paper (Covidence ID) | Study dates | Sample size | Outcomes | Decision |
|---|---|---|---|---|---|
| Parsons 2011 | Parsons 201753 | NR | 104 | Health-related QoL, assessed using EQ-5D-3L and mortality |
Issue: all papers report on specific economic measures. Parsons 201173 and 201753 report on QoL using the EQ-5D Decision: Parsons 201753 was used to report on QoL; Elia 202023 was used to report on economic outcomes |
| Parsons 201173 (3954) | NR | 104 | QoL at baseline and 12 weeks using EQ-5D, including a TTO (range –0.073 to 1) and a VAS (score 0–100) for self-perceived health | ||
| Parsons 2012108 (2744) | NR | 104 | QALYs calculated from QoL (measured using EQ-5D TTO, VAS rescaled and mortality); costs of health-care visits and hospital admissions (3 months prior to and during the RCT) and the interventions calculated using standard unit costs; ICER and probability that one intervention was more cost-effective than the other (cost < £20,000–30,000/QALY gained) were calculated | ||
| Parsons 2012109 (4023) | NR | 104 | QALYs calculated from QoL measured using EQ-5D TTO, VAS and mortalityCosts of healthcare visits and hospital admissions (3 months prior to and during the RCT) and the interventions were calculated using standard unit costs. The incremental cost effectiveness ratio (ICER) and the probability that one intervention was more cost effective than the other (cost < £20,000 30,000/QALY gained) were calculated | ||
| Elia 201723 (249) | August 2007 to March 2010 | 104 | Costs of interventions (dietetic costs and costs of ONS where relevant as specified in the study protocol); number of QALYs gained during the intervention period, calculated using standard procedures based on a combination of QoL and mortality; cost-effectiveness analysis during the 12-week intervention period | ||
| Luo 201570 | Luo 201570 | 2009 to 2010 | 46 | Change in body weight in kg; change in other indicators of nutritional status measured by serum albumin and prealbumin levels; change in nutritional intake measured by the total protein in grams per litre; improvement in gait speed |
Issue: the abstract did not report all outcomes that were measured. The abstract refers to the measurement of morbidity using the Modified Barthel Index, but no data were reported Decision: Luo 201570 was used as it a full text and reports numerous outcomes of interest |
| Luo 201174 | NR | 46 | Change in body weight in kg; change in other indicators of nutritional status measured by serum albumin and prealbumin levels; morbidity measured using chair-to-bed transfer domain from the Modified Barthel Index |
Appendix 3 Final values and change from baseline calculations
Mean CFB values and standard deviations were imputed from the baseline and final values and standard deviations using the following equations:
Mean final values and standard deviations can be imputed from CFB and baseline values and standard deviations using the following equations:
There are two possible solutions for SDfinal, but this equation always produces a real solution and a conservative one, in which a feasible value for the correlation is used.
No studies reported the standard deviation for SDbase, SDchange and SDfinal, so the correlation could not be calculated from reported statistics. Feasible values for the correlation are described by the inequality:
The process for setting the correlation and standard deviation was as follows. In every case, the correlation value was varied until the standard deviation got as close to the targeted standard deviation as possible with the correlation value remaining within the feasible range. The average standard deviation across the treatment groups at baseline, CFB and final values were calculated, and the targeted standard deviation was based on the average of the standard deviations.
Change from baseline
For CFB values, the decision rule was guided by the standard deviation values for studies reporting the same outcome measure. There were two options.
Option A
Select the correlation value where the average estimated standard deviation for change values is closest to, but not higher than, the highest average standard deviation for change values for other studies that reported change values and the same outcome measure
OR
If there are no other studies that report change values, select the correlation value where the average estimated change value standard deviation is closest to but not higher than 50% the value of the average standard deviation of the standard deviations for the baseline values for that study
AND
The lowest plausible correlation value that achieves either of these.
Option B
Select the standard deviation that got as close as possible to the standard deviation of either the baseline or the final values.
Final values
For final values, the lowest correlation value was set that enabled the average standard deviation to get as close to the average standard deviation at baseline as possible. The average standard deviation was always higher due to the conservative solution used for the standard deviation for the final value.
The ratios of the calculated standard deviations to the baseline standard deviations, the ratios of the reported standard deviations to the baseline standard deviations, and the set correlations are reported in Table 20 for each outcome. These include results for a few studies from which data were extracted but were not finally included in the review. The outcomes and context of the excluded studies are still relevant for assessing the plausibility of the imputed values.
| Outcome | Value | Reported | Derived | |||
|---|---|---|---|---|---|---|
| n | SD ratio | n | Correlation | SD ratio | ||
| Body weight | CFB | 7 | 0.14–0.47 | 3 | 0.93–0.97 | 0.37–0.39 |
| FV | 4 | 0.88–1.03 | 6 | 0.9–1 | 1.06–1.30 | |
| Calf circumference | CFB | 2 | 0.46–0.79 | 3 | 0.89–0.94 | 0.34–0.48 |
| FV | 3 | 0.97–1.04 | 2 | 0.91–0.99 | 1.42–1.52 | |
| BMI | CFB | 3 | 0.45–0.54 | 5 | 0.88–0.94 | 0.34–0.50 |
| FV | 5 | 0.81–1.09 | 3 | 0.93–0.98 | 1.27–1.36 | |
| Energy intake | CFB | 4 | 0.13–1.65 | 3 | 0.42–0.43 | 1–1.32 |
| FV | 3 | 0.78–1.27 | 3 | 0.44–0.82 | 1.21–1.65 | |
| Protein | CFB | 5 | 0.13–2.13 | 3 | 0.5–0.7 | 0.98–1 |
| FV | 3 | 0.9–1.32 | 4 | 0.26–1 | 1.13–2.14 | |
| Albumin | CFB | 4 | 0.47–1.86 | 2 | 0.58–0.63 | 0.79–0.99 |
| FV | 3 | 0.8–1.86 | 3 | 0.62–0.95 | 1.06–1.57 | |
| Grip strength | CFB | 3 | 0.69–2.92 | 5 | 0.53–0.65 | 0.79–1 |
| FV | 5 | 0.91–1.28 | 3 | 0.7–0.87 | 1.2–3.18 | |
Appendix 4 Network graphs
The network graphs for the network meta-analyses are presented in Figures 19 and 20. The thickness of the lines represents the relative evidence informing each comparison. The shaded areas represent the presence of at least one multiarm trial. For example, in Figure 19 there are three studies evaluating the effectiveness of ONS compared with SC. Only one of these is a multiarm trial that compares ONS with brioche and with SC. Different shades represent the presence of different multiarm trials with different comparators. These graphs were produced using netmeta in R. 110
FIGURE 19.
Network diagram for body weight.
FIGURE 20.
Network diagram for grip strength.
Appendix 5 Details of linked publications
| Study ID | Primary reference | Other references |
|---|---|---|
| Cameron et al.71 | Cameron ID, Kurrle SE, Uy C, Lockwood KA, Au L, Schaafsma FG. Effectiveness of oral nutritional supplementation for older women after a fracture: rationale, design and study of the feasibility of a randomised controlled study. BMC Geriatr 2011;11:1–671 | |
| Lauque et al.67 | Lauque S, Arnaud-Battandier F, Mansourian R, Guigoz Y, Paintin M, et al. Protein-energy oral supplementation in malnourished nursing-home residents. A controlled trial. Age Ageing 2000;29:51–667 | |
| Lee et al.54 | Lee L-C, Tasi AC, Wang J-Y, Hurng B-S, Hsu H-C, Tsai H-J. Need-based intervention is an effective strategy for improving the nutritional status of older people living in a nursing home: a randomized controlled trial. Int J Nurs Stud 2013;50:1580–854 | |
| Luo et al.70 | Luo M, Golubev G, Klyukvin I, Rexnik L, Kupatkin G, Oliver JS, Voss Anne C. Oral nutrition supplement improved nutritional status in malnourished hip fracture patients: a randomised controlled study. J Sci Res Rep 2015;4:480–8970 | Luo M, Golybev G, Klyukvin I, Reznik L, Kuropatkin G, Voss AC. Oral nutritional supplement (ONS) improved nutritional status in malnourished patients receiving hip fracture surgery. Clin Nutr Suppl 2011;6:15174 |
| Miller et al.68 | Miller MD, Crotty M, Whitehead C, Bannerman E, Daniels LA. Nutritional supplementation and resistance training in nutritionally at risk older adults following lower limb fracture: a randomized controlled trial. Clin Rehab 2006;20:311–2368 | |
| Otten et al.72 | Otten L, Kiselev J, Franz K, Steinhagen-Thiessen E, Müller-Werdan U, Eckardt R, et al. MON-P021: effect of a three month post-hospital nutritional intervention on functional performance in frail and malnourished older adults-a randomized controlled study. Clin Nutr 2016;35:S16172 | |
| Payette et al.55 | Payette H, Boutier V, Coulombe C, Grey-Donald K. Benefits of nutritional supplementation in free-living, frail, undernourished elderly people: a prospective randomized community trial. J Am Dietet Assoc 2002;102:1088–9555 | |
| Parsons et al.53 | Parsons EL, Strattion RJ, Cawood AL, Smith TR, Elia M. Oral nutritional supplements in a randomised trial are more effective than dietary advice at improving quality of life in malnourished care home residents. Clin Nutr 2017;36:134–4253 | Parsons EL, Stratton RJ, Cawood AL, Smith TR, Warwick H, Elia M. PP021-SUN randomised controlled trial in care home residents shows improved quality of life (QOL) with oral nutritional supplements. Clin Nutr Suppl 2011;6:3173 |
| Parsons EL, Stratton RJ, Cawood AL, Jackson JM, Elia M. Oral nutritional supplements are more cost-effective in improving quality-adjusted life-years (QALYs) in malnourished care home residents. Clin Nutr 2012;7:PP047109 | ||
| Elia M, Parsons EL, Cawood AL, Smith TR, Stratton RJ. Cost-effectiveness of oral nutritional supplements in older malnourished care home residents. Clin Nutr 2018;37:651–823 | ||
| Parsons EL, Stratton RJ, Jackson JM, Elia M. OC-039 Oral nutritional supplements are cost-effective in improving quality-adjusted life-years in malnourished care home residents. Gut 2012;61:A17–A108 | ||
| Tidermark et al.69 | Tidermark J, Ponzer S, Carlsson P, Söderqvist A, Brismar K, Tengstrand B, Cederholm T. Effects of protein-rich supplementation and nandrolone in lean elderly women with femoral neck fractures. Clin Nutr 2004;23:587–9669 | |
| Tylner 201656 | Tylner S, Cederholm T, Faxén-Irving G. Effects on weight, blood lipids, serum fatty acid profile and coagulation by an energy-dense formula to older care residents: a randomized controlled crossover trial. J Am Med Direct Assoc 2016;17:275–e556 | |
| Van Wymelbeke et al.57 | Van Wymelbeke V, Brondel L, Bon F, Martin-Pfitzenmeyer I, Manckoundia P. An innovative brioche enriched in protein and energy improves the nutritional status of malnourished nursing home residents compared to oral nutritional supplement and usual breakfast: FARINE+ project. Clin Nutr ESPEN 2016;15:93–10057 |
Appendix 6 Study population characteristics table
| Study ID | Country | Setting | Number enrolled | Intervention | Number in group | Age (years) | Sex, n (%) male | BMI (kg/m2) | Weight (kg) | Malnutrition score | Number of medications taken |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cameron et al.71 | Australia | Hospital: Hornsby Ku-ring-gai Hospital (a general hospital in northern Sydney) | 44 | High-calorie, high-protein supplement and diet of choice | 23 | 83.7 ± 5.6 | 0 (0) | 21.5 ± 2.8 | 50.4 ± 6.0 | MUAC mean 24.2 ± 3.1; albumin (g/l) mean 31.1 ± 5.0 | NR |
| High-protein milk and diet of choice | 21 | 87.1 ± 6.2 | 0 (0) | 21.5 ± 4.0 | 50.2 ± 11.8 | MUAC mean 23.6 ± 2.6; albumin (g/l) mean 31.8 ± 5.4 | NR | ||||
| Lauque et al.67 | France | Nursing home: eight privately run 80-bed nursing homes in Toulouse | 88 | No supplementation (well nourished) | 19 | 87 ± 6 | 1 (8.3) | 25.2 ± 0.8 | 61.0 ± 2.8 | MNA: all ≥ 24 | NR |
| No supplements (risk of malnutrition) | 22 | 87 ± 6 | 1 (6.7) | 21.8 ± 0.9 | 52.5 ± 2.4 | MNA: all 17–23.5 | NR | ||||
| Oral supplements (risk of malnutrition) | 19 | 88 ± 6 | 3 (20) | 22.3 ± 0.7 | 53.9 ± 2.2 | MNA: all 17–23.5 | NR | ||||
| Oral supplements (malnourished) | 28 | 87 ± 7 | 2 (5.7) | 18.5 ± 0.5 | 43.9 ± 1.7 | MNA: all < 17 | NR | ||||
| Lee et al.54 | Taiwan (Province of China) | Nursing home: geriatric nursing home | 92 | ONS | 47 |
65–74: 15 (31.9%) 75–84: 21 (44.7%) ≥ 85: 11 (23.4%) |
19 (40.4) |
20.43 ± 2.5 n = 43 |
48.62 ± 8.02 n = 43 | MNA: 21.4 ± 3.5 | 2.8 ± 1.4 |
| NR | 45 |
65–74: 13 (28.9%) 75–84: 20 (44.4%) ≥ 85: 12 (26.7%) |
20 (44.4) |
20.31 ± 2.61 n = 40 |
48.3 ± 8.47 n = 40 |
MNA: 20.7 +3.9 | 2.9 ± 1.5 | ||||
| Luo et al.70,74 | Russia | Hospitals: hip fracture patients | 55 | Ensure2: nutritionally complete, calorie-dense, high-protein ONS | 26 | 72.4 ± 1.9 | 4 (18) | 25.1 ± 1.4 | 70.5 ± 3.6 | NR | NR |
| Standard hospital food only | 29 | 67.3 ± 2.4 | 7 (29) | 26.7 ± 1.7 | 72.9 ± 4.6 | NR | NR | ||||
| Miller et al.68 | Australia | Hospital: orthopaedic wards of Flinders Medical Centre, Adelaide | 100 | Nutrition | 25 | 83.5 ± NR | 4 (16) | 21.9 + NR | 53 ± NR | NR | NR |
| Exercise | 25 | 84.8 ± NR | 5 (20) | 21.4 ± NR | 52.3 ± NR | NR | NR | ||||
| Nutrition and exercise | 24 | 82.7 ± NR | 7 (29) | 23.2 ± NR | 57.5 ± NR | NR | NR | ||||
| Attention control | 26 | 83.1 ± NR | 5 (19) | 22.1 ± NR | 54.7 ± NR | NR | NR | ||||
| Otten et al.72 | Germany | After hospital discharge | 71 | Dietary counselling | NR | NR | NR | NR | NR | NR | NR |
| ONS | NR | NR | NR | NR | NR | NR | NR | ||||
| Parsons et al.23,53,73,109 | UK | Nursing home: care homes in Hampshire | 104 | ONS with guidance | 53 | 89.6 ± 6.9 | 8 (15.1) | NR | 48.5 ± 9.9 | MUST: medium risk: 22 (41.5%); high risk: 31 (58.5%) | NR |
| Dietary advice | 51 | 87.3 ± 8.7 | 7 (13.7) | NR | 51.1 ± 8.9 | MUST: medium risk: 26 (51%); high risk: 25 (49%) | NR | ||||
| Payette et al.55 | Canada | Community: home | 83 | ONS | 42 | 81.6 ± 7.6 | 12 (29) | 20.1 ± 2.7 | 53.7 ± 8.6 | Excessive weight loss: 5 (12%) |
5.5 ± 2.96 n = 39 |
| Control | 41 | 78.6 ± 6.1 | 12 (29) | 20.1 ± 3.0 | 52.9 ± 9.3 | Excessive weight loss: 5 (12%) |
4.9 ± 3.6 n = 36 |
||||
| Tidermark et al.69 | Sweden | Community | 59 | Protein-rich formula and additional calcium and vitamin D | 20 | 83.5 ± 6.1 | 0 (0) | 20.5 ± 2.4 | 53.7 ± 7.9 | NR | NR |
| Protein-rich formula plus nandrolone decanoate, additional calcium and vitamin D | 19 | 81.1 ± 5.5 | 0 (0) | 19.8 ± 2.2 | 50.0 ± 7.7 | NR | NR | ||||
| SC plus additional calcium and vitamin D | 20 | 84.1 ± 4.3 | 0 (0) | 20.9 ± 2.3 | 56.0 ± 9.9 | NR | NR | ||||
| Tylner 201656 | Sweden | Nursing home: five residential care homes in the southern Stockholm area | 39 | Intervention then SC | 19 |
87.2 ± 5.9 n = 14 |
50% n = 14 |
22.1 ± 3.4 n = 3.4 |
58.6 ± 9.9 n = 14 |
Unclear whether this is MNA-SF |
NR |
| Intervention then SC | 20 |
82.2 ± 7.9 n = 14 |
29% n = 14 |
23.5 ± 4.2 n = 14 |
63.0 ± 14.2 n = 14.2 |
Unclear whether this is MNA-SF |
NR | ||||
| Van Wymelbeke et al.57 | France | Nursing home: eight nursing homes in Burgundy | 87 | Enriched brioche | 35 | 84.2 ± 7.9 | 6 (20.7) | 29.1 ± 7.3 | NR | MNA: 21.1 ± 2.8 | 7.6 ± 3.6 |
| ONS | 27 | 90.3 ± 6.5 | 3 (17.7) | 24.9 ± 6.4 | NR | MNA: 19.9 ± 3.5 | 6.7 ± 2.5 | ||||
| Usual care | 25 | 87.3 ± 8.0 | 5 (22.7) | 28.1 ± 5.8 | NR | MNA: 21.8 ± 2.7 | 6.9 ± 3.4 |
Appendix 7 Comorbidities and social determinants reported in included studies
| Study | Comorbidities | Oral health issues | Social determinants | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dementia | Cognitive impairment | Stroke | Cardiovascular disease | Diabetes | Cancer | Swallowing issues | Problems chewing | Dry mouth | Complete or partial denture | No dentures | Need for help with feeding | Ethnicity | Socioeconomic status | Education | Marital status | Living arrangements | Household or other help | |
| Cameron et al.71 | ✗ | |||||||||||||||||
| Lauque et al.67 | ✗ | ✗ | ✗ | ✗ | ||||||||||||||
| Lee et al.54 | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||||
| Luo et al.70 | ||||||||||||||||||
| Miller et al.68 | ✗ | ✗ | ✗ | |||||||||||||||
| Otten et al.72 | ✗ | ✗ | ||||||||||||||||
| Parsons et al.53 | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||||||
| Payette et al.55 | ✗ | ✗ | ✗ | ✗ | ||||||||||||||
| Tidermark et al.69 | ✗ | |||||||||||||||||
| Tylner 201656 | ✗ | ✗ | ||||||||||||||||
| Van Wymelbeke et al.57 | ✗ | ✗ | ✗ | |||||||||||||||
Appendix 8 Intervention characteristics
| Study | Intervention | Number in group | Materials used | Dosage | Regimen | Delivery details |
|---|---|---|---|---|---|---|
| Cameron et al.71 | High-calorie, high-protein supplement and diet of choice | 23 | One pack of supplement per day and diet of choice |
Novasource per 237 ml: 475 kcal, 21.3 g protein, 51 g carbohydrate, 20.9 g fat, 375 µg vitamin A, 2.5 µg vitamin D, 250 mg calcium, 4.5 mg iron, 250 µg phosphate Sustagen Hospital Plus per 235 ml: 352.5 kcal, 17.6 g protein, 44.2 g carbohydrate, 20.9 g fat, 375 µg vitamin A, 2.5 µg vitamin D, 250 mg calcium, 4.5 mg iron, 250 µg phosphate |
Supplements taken once per day; unclear when they were delivered | The supplement was given once per day from when oral intake was resumed after surgery, or from enrolment for those who did not undergo surgery. They continued usual diet otherwise. If the patient was discharged before the 40-day treatment period ended, they were given the rest of the supplements and instructions to keep drinking them as discussed |
| High-protein milk and diet of choice | 21 | A high-protein diet with high-protein milk and diet of choice | High-protein milk per 150 ml: 194 kcal, 11 g protein, 18.75 g carbohydrate, 8.3 g fat | High-protein milk to be taken once per day; unclear when this was delivered | Given the high-protein diet with high-protein milk because this was the standard practice at the hospital, and it was deemed unethical to withdraw this. When the participants were discharged, they could follow their normal diets | |
| Lauque et al.67 | No supplementation (well nourished) | 19 | NA | NR | NR | NR |
| No supplements (risk of malnutrition) | 22 | NA | NR | NR | NR | |
| Oral supplements (risk of malnutrition) | 19 | Nutritional supplements of 300–500 kcal. Four oral supplementation products (Clinutren, Nestle Clinical Nutrition, Sevres, France) were offered: Clinutren soup, Clinutren Fruit, Clinutren Dessert and Clinutren HP (Hyper-protein) |
300–500 kcal Clinutren soup: 200 kcal and 10 g protein per 200 ml Clinutren Fruit: 120 kcal and 7.5 g protein per 150 ml Clinutren Dessert: 150 kcal and 12 g protein per 150 ml Clinutren HP: 200 kcal and 15 g protein per 200 ml |
‘Given in addition to regular meals’; no other details | The supplements were given in addition to regular meals. They were either liquid or creamy, sweet or savoury and were served hot, warm or cold. They were enriched with proteins, vitamins and minerals and contained high amounts of energy and nutrient in a small volume | |
| Oral supplements (malnourished) | 28 | Nutritional supplements of 300–500 kcal. Four oral supplementation products (Clinutren, Nestle Clinical Nutrition, Sevres, France) were offered: Clinutren soup, Clinutren Fruit, Clinutren Dessert and Clinutren HP (Hyper-protein) |
300–500 kcal Clinutren soup: 200 kcal and 10 g protein per 200 ml Clinutren Fruit: 120 kcal and 7.5 g protein per 150 ml Clinutren Dessert: 150 kcal and 12 g protein per 150 ml Clinutren HP: 200 kcal and 15 g protein per 200 ml |
‘Given in addition to regular meals’; no other details | The supplements were given in addition to regular meals. They were either liquid or creamy, sweet or savoury and were served hot, warm or cold. They were enriched with proteins, vitamins and minerals and contained high amounts of energy and nutrient in a small volume | |
| Lee et al.54 | ONS | 47 | 50 g/day soy protein-based preparation as a ‘warm drink’ | 9.5 g protein, 250 kcal energy and all essential micronutrients | Delivered in the afternoon, daily | 50 g/day soy protein-based preparation; prepared as a ‘warm drink’ as an afternoon snack |
| NR | 45 | NR | NA | NA | NR | |
| Luo et al.70 | Ensure2: nutritionally complete, calorically dense, high-protein ONS | 26 | Standard hospital food plus Ensure TwoCal (Abbott Nutrition, Columbus, OH, USA) – a nutritionally complete, energy- and protein-dense drink including 30 vitamins and minerals | 798 kcal, 34 g protein | Administered three times per day: 100 ml between meals and 200 ml as an evening snack | Two 200 ml containers given three times per day – 100 ml between breakfast and noon meal, 100 ml serving between noon and evening meal and 200 ml as a snack before going to bed |
| Standard hospital food only | 29 | Standard hospital food and SC | NR | NR | NR | |
| Miller et al.68 | Nutrition | 25 | Fortisip (Nutricia Australia Pty Ltd) |
Prescribed to meet 45% individual total energy requirements Fortisip: 6.3 kJ (1.5 kcal)/ml, 16% protein, 35% fat and 49% carbohydrate |
Commenced 7 days after fracture; four doses of equal volume given daily. Weekly visits from weeks 7–12 provided to match participant contact in resistance training | Four equal-volume doses were administered daily. On discharge, those admitted to residential care received the supplement as described. For those discharged, home scheduling was twice per day or more. Weekly visits from weeks 7 to 12 were provided to match the participant contact in the resistance training groups |
| Exercise | 25 | Latex-free resistive elastic bands (REP band, Magister Corporation, Chattanooga, TN, USA) | NR | Commenced 7 days after fracture; took place three times a week for 20–30 minutes | Three times per week, 20–30 minutes per session for 12 weeks. The programme incorporated progressive resistance training (using latex-free bands) of the hip extensors and abductors (supine), knee extensors (supine or sitting), and ankle dorsi- and plantar-flexors (supine or sitting). The frequency and duration of the resistance training programme was determined following a review of the literature that suggested positive outcomes might be achieved through triweekly training between 8 and 15 weeks | |
| Nutrition and exercise | 24 | Combination of groups 1 and 2 above | Combination of groups 1 and 2 above | Combination of groups 1 and 2 above | Combination of groups 1 and 2 above | |
| Attention control | 26 | Home visits | NA | Triweekly visits of equivalent duration in weeks 1–6 and then weekly visits at weeks 7–12 to match the home visits of the active intervention groups | The visits were limited to discussion on general information (e.g. benefits of regular exercise and nutrient-dense meals). All participants encouraged to continue treatments as prescribed during the hospital admission or by their treating health professionals | |
| Otten et al.72 | Dietary counselling | NR | NR | NR | For 3 months | Dietary counselling for 3 months |
| ONS | NR | ONS – details not reported | NR | For 3 months | Dietary counselling for 3 months | |
| Parsons et al.53 | ONS with guidance | 53 | A range of ONS (drinks, soups, puddings, modules), in a range of flavours, volume (125–200 ml), energy density (1.3–4.5 kcal/ml) (Nutricia Ltd, Trowbridge, Wiltshire, UK) | 1.3–4.5 kcal/ml | Daily; no other details | ONS were given so participants could take them according to choice – the majority chose ready-made liquid ONS (1.5–2.4 kcal/ml). They and care staff were given guidance on using ONS and the daily target provision was at least 600 kcal and 16 g protein. However, intake was voluntary and residents remained in the study irrespective of the quantity of ONS or food consumed |
| Dietary advice | 51 | Given a specially designed diet sheet (‘Build yourself up’, Southampton Dieticians, Southampton, UK) | NR | NR | The leaflet encouraged participants to intake high-energy drinks and snacks | |
| Payette et al.55 | ONS | 42 | ONS: Commercial Liquid Formula (Ensure or Ensure Plus) provided by Ross Laboratories (Division of Abbott Laboratories) | 235-ml can | Liquid formula was taken twice per day for a period of 16 weeks; nutritional counselling took place every 2 weeks | Subjects were provided with two 235-ml cans per day of their choice of a commercial liquid. Ensure Plus (vanilla, chocolate and strawberry) was systematically provided to the subjects and Ensure in other flavours such as orange and wild berry was used to minimise flavour fatigueSubjects were clearly instructed not to replace their usual meals with the liquid supplement; rather, they were encouraged to use the supplements and increase overall food intake |
| Control | 41 | ‘Visited each month and given a small gift’; no other details | NR | Visited every month and given a small gift | Did not receive any treatment during this period. Were visited each month and given a small gift | |
| Tidermark et al.69 | Protein-rich formula and additional calcium and vitamin D | 20 | Protein-rich formula (Fortimel) plus additional calcium and vitamin D (400IE) (Calcichew-D3s) |
Fortimel: 200 ml/day, 20 g protein/day Calcichew-D3s: 400IE |
Fortimel plus additional calcium and vitamin D daily | Given daily for 6 months: ‘The care programme was identical otherwise in all three groups’ |
| Protein-rich formula plus nandrolone decanoate, additional calcium and vitamin D | 19 | Protein-rich formula (Fortimel) plus nandrolone decanoate (Deca-Durabols) plus additional calcium and vitamin D (Calcichew-D3s) |
Deca-Durabols: 25 mg i.m. Fortimel: 200 ml/day, 20 g protein/day Calcichew-D3s: 400IE |
Fortimel plus additional calcium and vitamin D daily Deca-Durabols for 3 weeks daily |
Given daily for 6 months:The intramuscular nandrolone injections were given by a research nurse in the home of the patientsThe care programme was identical otherwise in all three groups | |
| SC plus additional calcium and vitamin D | 20 | Standard treatment plus additional calcium and vitamin D (Calcichew-D3s) | Calcichew-D3s: 400IE 1 g calcium | Standard treatment plus additional calcium and vitamin D daily | Daily for 6 months; nature of SC not describedThe care programme was identical otherwise in all three groups | |
| Tylner 201656 | Intervention then SC | 19 | Calogen Extra (Nutricia Advanced Medical Nutrition, Schiphol, The Netherlands), then SC (not described) | Calogen Extra: 360 kcal, 4.5 g protein, approximately 30% recommended micronutrients (including 2.7 µg vitamin D, 201 mg calcium) | Intervention was delivered 3 times daily at the same time as medication (8 a.m., 12 p.m., 8 p.m.). Regimen for SC not described | In intervention phase, each serving was registered during the 6-week intervention period and the daily dose was 90 ml. SC not described |
| Intervention then SC | 20 | First SC (not described) and then Calogen Extra (as described in group 1) | Calogen Extra: 360 kcal, 4.5 g protein, approximately 30% recommended micronutrients (including 2.7 µg vitamin D, 201 mg calcium) | SC regimen not described. Intervention regimen as described for group A | Initial SC not described; intervention regimen for stage 2 as described for group A | |
| Van Wymelbeke et al.57 | Enriched brioche | 35 | Brioche weighed 65 g and was provided by Cerelab (Dijon, France). Designed to provide similar levels of energy and macro- and micronutrients to the ONS | Brioche: 12.8 g protein, 180 kcal, 15.5 g carbohydrate, 4 g sugar, 7.3 g lipids, 0.4 mg vitamin B1, 0.6 mg vitamin B2, 1.2 mg vitamin B6, 183 µg vitamin B9, 1.9 µg vitamin B12, 5 µg vitamin D, 23 µg selenium | One brioche roll per day, taken at breakfast, for 12 weeks | Participants were given one brioche roll per day for 12 weeks. They completed their breakfast with a hot drink, juice, butter, jam and ordinary bread if they wanted to. Three randomised flavours: orange, vanilla and honey |
| ONS | 27 | Supplement: usual bread at breakfast replaced by an ONS. 200 ml carton of Fresenius Kabi (Nestle S.A., Labege, France) | Supplement: 14 g protein and 200 kcal; it also contained 23.6 g carbohydrate, 5.6 g sugar, 5.6 g lipids, 0.3 mg vitamin B1, 0.3 mg vitamin B2, 0.4 mg vitamin B6, 40 µg vitamin B9, 0.2 µg vitamin B12, 1 µg vitamin D, 12 µg selenium | One ONS per day, taken at breakfast, for 12 weeks | Participants received one 200-ml carton of ready-to-use energy-dense liquid per day for 12 weeks. They completed their breakfast with a hot drink, juice, butter, jam and ordinary bread if they wanted to. Three randomised flavours: strawberry, coffee and vanilla | |
| Usual care | 25 | Usual breakfast provided by the nursing homes | NA | NA – usual diet | Participants received their usual breakfast provided by the nursing homes |
Appendix 9 Risk of bias/critical appraisal of included studies in effectiveness review
| Study | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Cameron et al.71 | |||||||
| Lauque et al.67 | |||||||
| Lee et al.54 | |||||||
| Luo et al.70 | |||||||
| Miller et al.68 | |||||||
| Otten et al.72 | |||||||
| Parsons et al.53 | |||||||
| Payette et al.55 | |||||||
| Tidermark et al.69 | |||||||
| Tylner et al.56 | |||||||
| Van Wymelbeke et al.57 |
Appendix 10 Studies excluded from the pairwise meta-analyses, with reasons
| Citation | Outcome(s) | Reason for exclusion from the pairwise meta-analysis |
|---|---|---|
| Lauque et al.67 | Grip strength and MNA | Participants were not randomised to all of the trial arms |
| Parsons et al.53 | Mortality, kcal | No comparison between ONS and SC |
| Hospitalisation, QoL | No comparison between ONS and SC | |
| Body weight, kcal, protein | The data reported were insufficient for a meta-analysis. Mean and standard deviations were unavailable and could not be completed | |
| Payette et al.55 | Grip strength | Data were not reported in an extractable format |
| QoL | This study assessed ‘emotional role functioning’ and ‘physical role functioning’ domains of the SF-36. No other studies reported comparable data; therefore, this study could not be included in the meta-analysis | |
| Miller et al.68 | Grip strength | Quadriceps strength rather than handgrip strength was assessed |
| Body weight | Standard deviation was unavailable and could not be calculated | |
| QoL | This study assessed ‘mental component’ and ‘physical domain’ of the SF-12. No other studies reported comparable data; therefore, this study could not be included in the meta-analysis | |
| Tidermark et al.69 | QoL | Only baseline data were reported for this outcome |
| Otten et al.72 | QoL | No SC group [a within-group comparison was made, before and after the intervention (ONS)] |
Appendix 11 Forest plots for body weight and body mass index outcomes, showing both final values and change from baseline
Forest plots illustrating the difference between the use of final values and CFB are shown for body weight and BMI in Figures 21–24.
Body weight
FIGURE 21.
Body weight: final values.
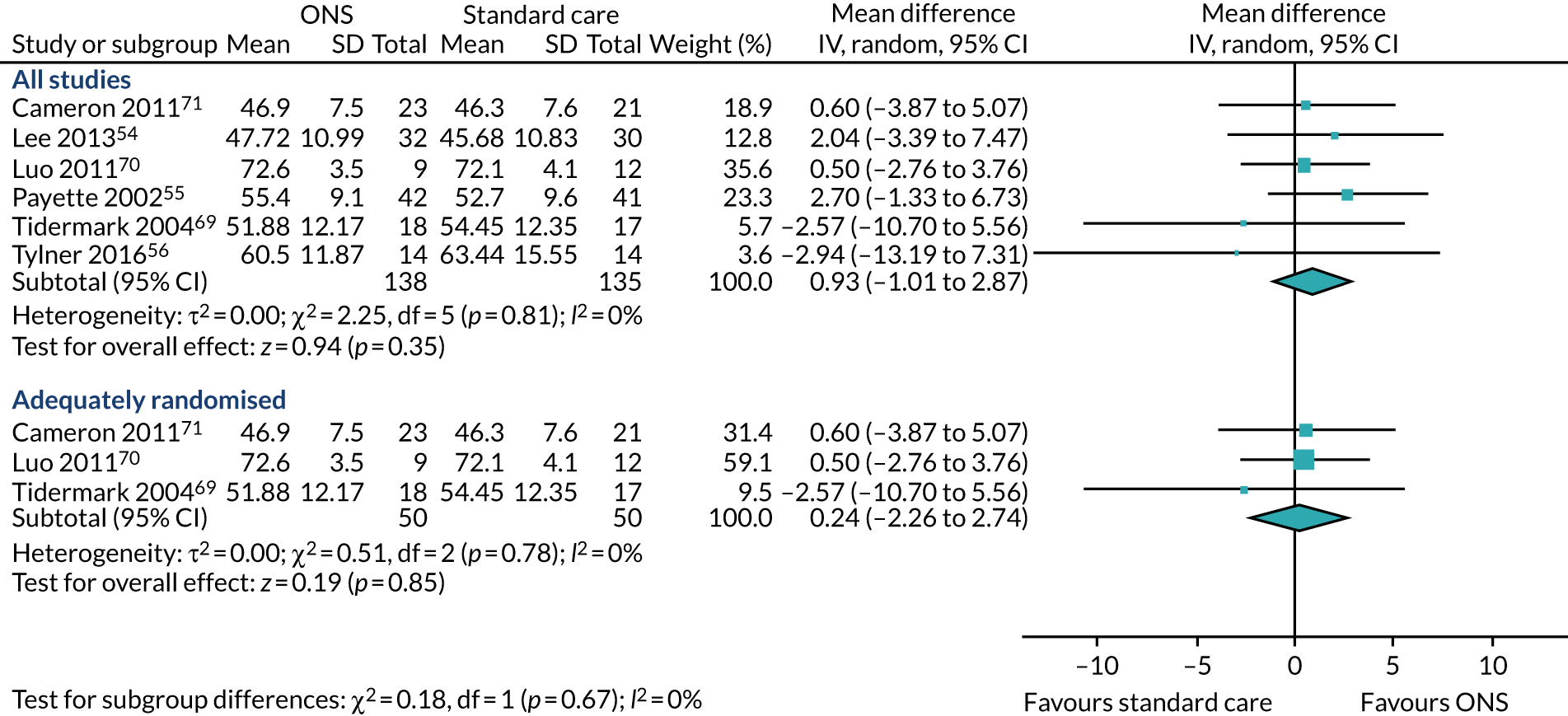
FIGURE 22.
Body weight: CFB.
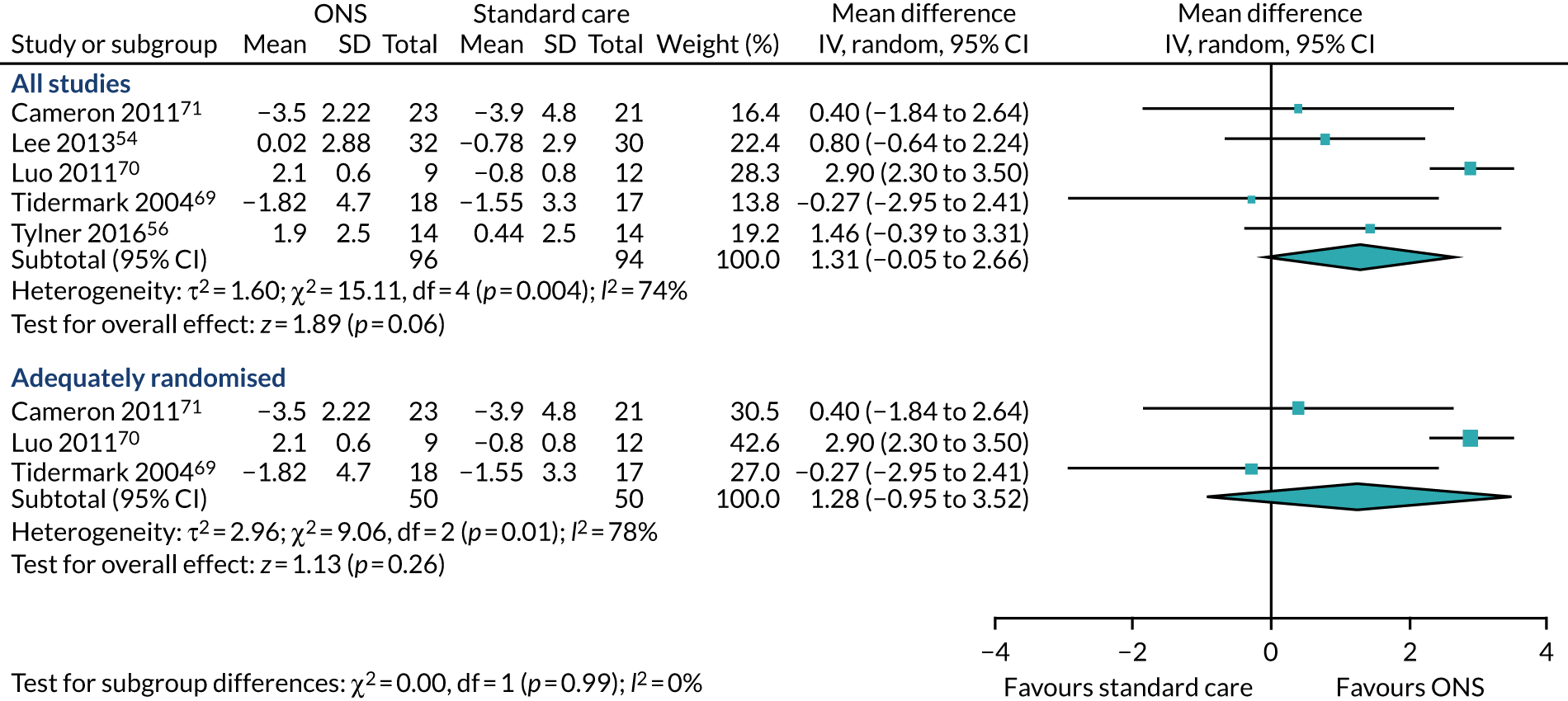
Body mass index
FIGURE 23.
Body mass index: final values.
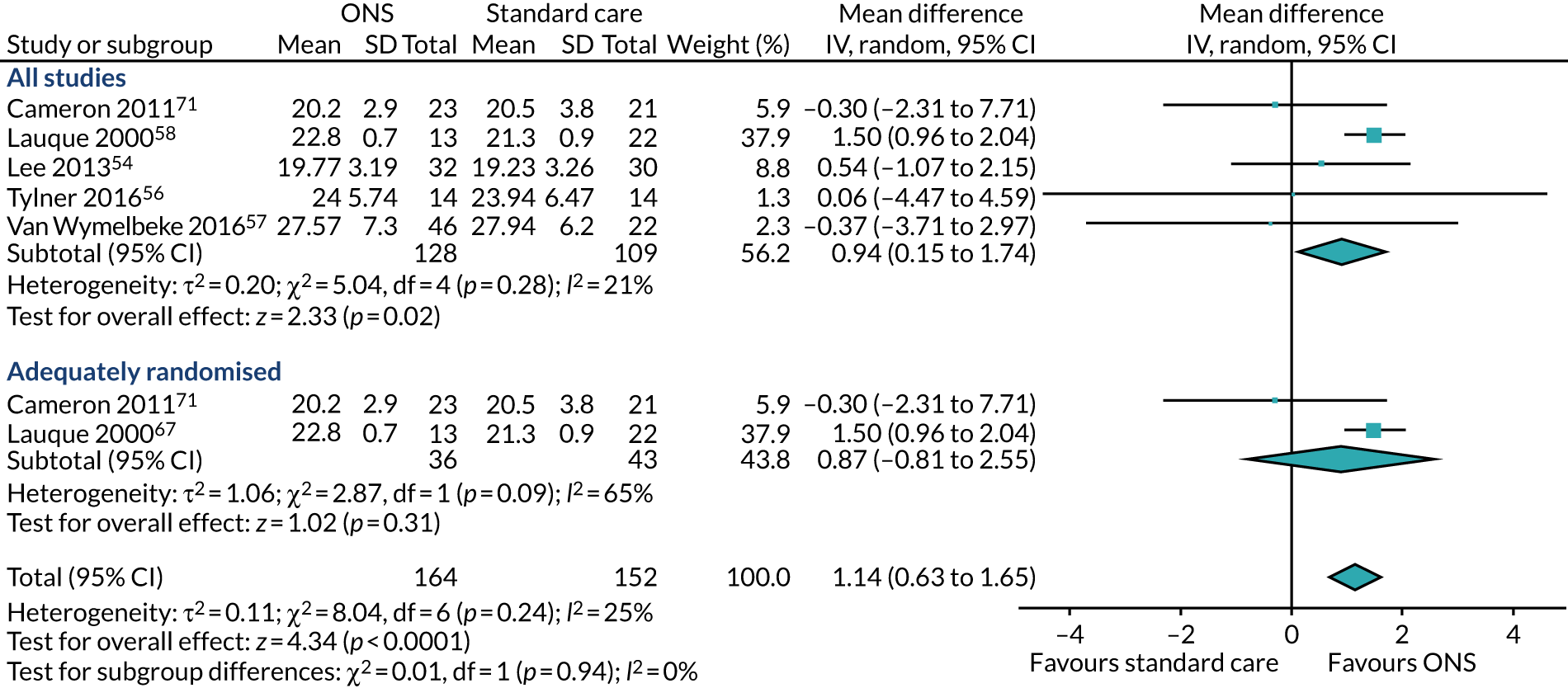
FIGURE 24.
Body mass index: CFB.
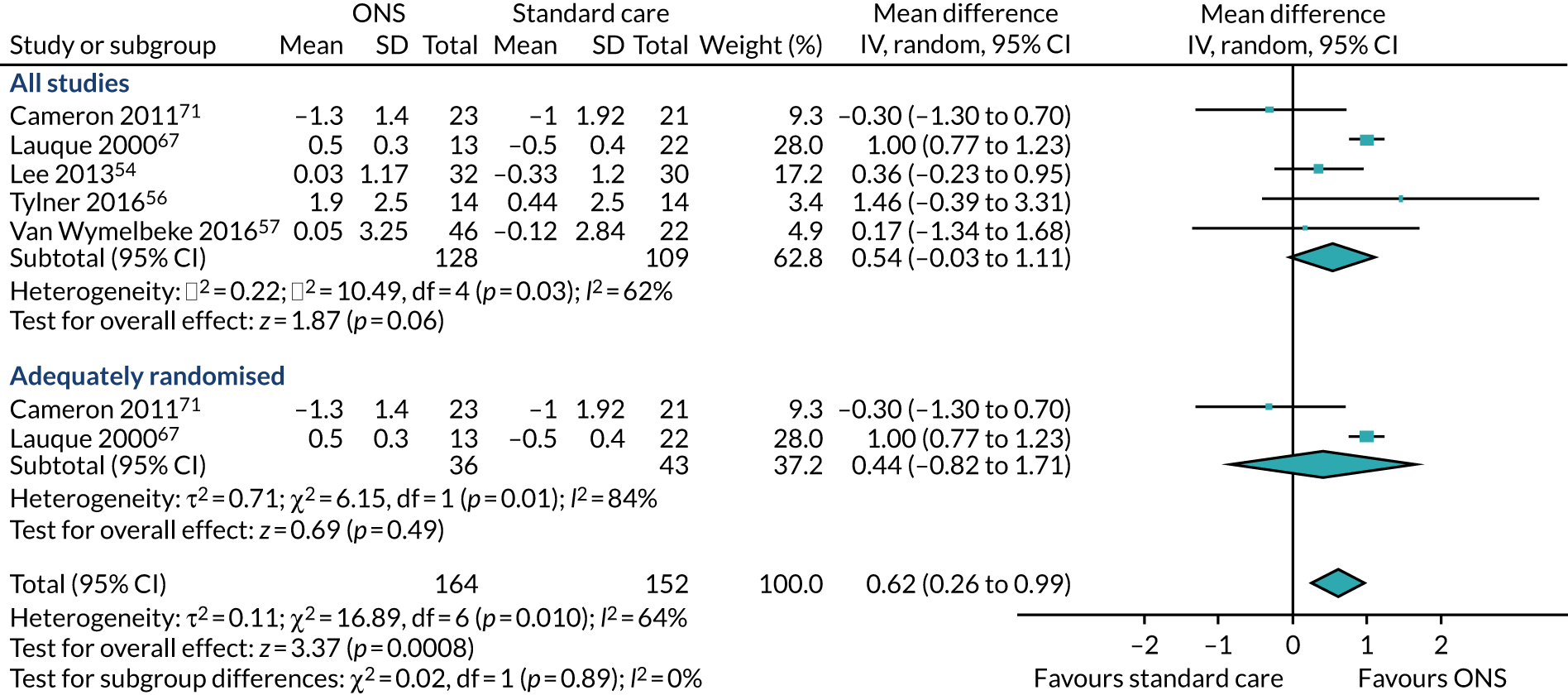
Appendix 12 Meta-analysis results using final values and change from baseline results
| Outcome | Adequate randomisation | All studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unit | CFB | Final values | Unit | CFB | Final values | |||||
| n | Result (95% CI) | n | Mean (95% CI) | n | Mean (95% CI) | n | Mean (95% CI) | |||
| Consumption | ||||||||||
| Energy (kcal/day)/(kcal/kg) | SMD | NA | NA | NA | NA | SMD | 4 | 1.02 (0.15 to 1.88) | 5 | 1.66 (0.40 to 2.93) |
| Protein (g/day)/(g/kg) | SMD | NA | NA | NA | NA | SMD | 4 | 1.67 (–0.03 to 3.37) | 5 | 2.11 (0.48 to 3.73) |
| Body | ||||||||||
| Body weight | kg | 3 | 1.28 (–0.95 to 3.52) | 3 | 0.24 (–2.26 to 2.74) | kg | 5 | 1.31 (–0.05 to 2.66) | 6 | 0.93 (–1.01 to 2.87) |
| BMI (kg/m2) | kg/m2 | 2 | 0.44 (–0.82 to 1.71) | 2 | 0.87 (–0.81 to 2.55) | kg/m2 | 5 | 0.54 (–0.03 to 1.11) | 5 | 0.94 (0.15 to 1.74) |
| Albumin | g/l | 2 | 2.86 (0.69 to 5.03) | 2 | 2.17 (0.00 to 4.33) | g/l | 5 | 1.48 (–0.44 to 3.41) | 5 | 1.04 (–0.63 to 2.71) |
| Arm circumference | NA | NA | NA | NA | NA | SMD | 2 | 0.49 (–0.32 to 1.30) | 2 | 0.16 (–0.28 to 0.60) |
| Fat-free muscle mass | SMD | NA | NA | NA | NA | SMD | 3 | 0.23 (–0.24 to 0.69) | 3 | 0.04 (–0.33 to 0.41) |
| MNA (score) | NA | NA | NA | NA | NA | SMD | NA | NA | 2 | –0.36 (–0.81 to 0.09) |
| Health outcomes | ||||||||||
| Wound healing | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Infections | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Falls | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Hospitalisation | ||||||||||
| Hospitalisation (number/rates) | NA | NA | NA | 2 | 0.8 (0.35 to 1.82) | NA | NA | NA | 5 | 0.97 (0.46 to 2.04) |
| QoL | ||||||||||
| ADL (score) | SMD | NA | NA | 2 | 0.68 (–0.54 to 1.90) | SMD | 2 | –0.14 (–0.76 to 0.49) | 3 | 0.30 (–0.69 to 1.29) |
| Mobility (m/second) | MD | NA | NA | 2 | 0.02 (–0.06 to 0.09) | MD | NA | NA | 3 | 0.03 (0.02 to 0.04) |
| Grip strength (kg/kgW/kPa) | SMD | 2 | 0.27 (–0.40 to 0.94) | 2 | 0.37 (–0.06 to 0.81) | SMD | 5 | 0.17 (–0.23 to 0.58) | 5 | 0.12 (–0.19 to 0.44) |
| QoL (score) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Mortality | ||||||||||
| Mortality (number/rates) | NA | NA | NA | NA | NA | NA | NA | NA | 4 | 0.93 (0.28, 3.06) |
Appendix 13 Summary of findings table
| Outcome | Relative effect | Number of studies | GRADE | Comments |
|---|---|---|---|---|
| Energy (kcal) intake | SMD 1.02 (95% CI 0.15 to 1.88) | 4 | ⨁⊝⊝⊝ very lowa,b,c | |
| Protein intake | SMD 1.67 (95% CI –0.03 to 3.37) | 4 | ⨁⊝⊝⊝ very lowa,b,c | |
| Albumin | MD 1.48 (95% CI –0.44 to 3.41) | 5 | ⨁⊝⊝⊝ very lowa,b,d | |
| Body weight | MD 1.31 (95% CI –0.05 to 2.66) | 5 | ⨁⊝⊝⊝ very lowd,e | |
| BMI | MD 0.54 (95% CI –0.03 to 1.11) | 5 | ⨁⊝⊝⊝ very lowe,f | |
| Arm circumference | – | – | – | Narrative synthesis; GRADE not conducted |
| Fat-free muscle mass | SMD 0.23 (95% CI –0.24 to 0.69) | 3 | ⨁⨁⊝⊝ lowg | |
| ADL | SMD 0.30 (95% CI –0.69 to 1.29) | 3 | ⨁⊝⊝⊝ very lowb,f,g | |
| Grip strength | SMD 0.17 (95% CI –0.23 to 0.58) | 5 | ⨁⊝⊝⊝ very lowaf | |
| Hospitalisations | RR 0.97 (95% CI 0.46 to 2.04) | 5 | ⨁⊝⊝⊝ very lowd,g | |
| Change in malnutrition (MNA score) | SMD –0.36 (95% CI –0.81 to 0.09) | 2 | ⨁⊝⊝⊝ very lowf,g | |
| Mobility (gait speed) | MD 0.03 (95% CI 0.02 to 0.04) | 3 | ⨁⊝⊝⊝ very lowd,e | |
| Mortality | RR 0.93 (95% CI 0.28 to 3.06) | 4 | ⨁⊝⊝⊝ very lowd,g | |
| QoL | – | – | – | Narrative synthesis; GRADE not conducted |
| Reduction in infection | – | – | – | Only one study, no meta-analysis; GRADE not conducted |
| Wound healing | – | – | – | Not reported |
| Reduction in falls | – | – | – | Not reported |
| Improvement in frailty | – | – | – | Not reported |
| Morbidity | – | – | – | Not reported |
| Admission to long-term care | – | – | – | Not reported |
Appendix 14 Additional exclusions in cost-effectiveness review
The initial selection of cost-effectiveness studies was conducted alongside the selection of effectiveness studies. Subsequently, a few studies were excluded by reviewers Stephen Rice and Wael Mohammed. The reasons for exclusion are reported here.
| Study | Reason for exclusion |
|---|---|
| Edington et al.112 | Not a full economic evaluation. No costing of intervention |
| Pouyssegur et al.76 | The intervention was not an ONS intervention |
| Arnaud-Battandier et al.113 | Not a frail population |
| Seguy et al.114 | Not a frail population |
| Zhong et al.115 | Not a frail population |
| Nuitjen et al.116 | Not a frail population |
| Nuitjen et al.117 | Not a frail population |
Appendix 15 Quality assessment in cost-effectiveness review
The result of the completed BMJ checklist for the included study is reported in Table 21.
| Item | Elia et al.23 |
|---|---|
| 1. Was the research question stated? | 1 |
| 2. Was the economic importance of the research question stated? | 1 |
| 3. Was/were the viewpoint(s) of the analysis clearly stated and justified? | 4 |
| 4. Was a rationale reported for the choice of the alternative programmes or interventions compared? | 4 |
| 5. Were the alternatives being compared clearly described? | 1 |
| 6. Was the form of economic evaluation stated? | 1 |
| 7. Was the choice of form of economic evaluation justified in relation to the questions addressed? | 1 |
| 8. Was/were the source(s) of effectiveness estimates used stated? | 1 |
| 9. Were details of the design and results of the effectiveness study given (if based on a single study)? | 1 |
| 10. Were details of the methods of synthesis or meta-analysis of estimates given (if based on an overview of a number of effectiveness studies)? | 2 |
| 11. Were the primary outcome measure(s) for the economic evaluation clearly stated? | 1 |
| 12. Were the methods used to value health states and other benefits stated? | 1 |
| 13. Were the details of the subjects from whom valuations were obtained given? | 3 |
| 14. Were productivity changes (if included) reported separately? | 2 |
| 15. Was the relevance of productivity changes to the study question discussed? | 2 |
| 16. Were quantities of resources reported separately from their unit cost? | 4 |
| 17. Were the methods for the estimation of quantities and unit costs described? | 1 |
| 18. Were currency and price data recorded? | 1 |
| 19. Were details of price adjustments for inflation or currency conversion given? | 1 |
| 20. Were details of any model used given? | 2 |
| 21. Was there a justification for the choice of model used and the key parameters on which it was based? | 2 |
| 22. Was the time horizon of cost and benefits stated? | 1 |
| 23. Was the discount rate stated? | 2 |
| 24. Was the choice of rate justified? | 2 |
| 25. Was an explanation given if cost or benefits were not discounted? | 1 |
| 26. Were the details of statistical test(s) and confidence intervals given for stochastic data? | 1 |
| 27. Was the approach to sensitivity analysis described? | 1 |
| 28. Was the choice of variables for sensitivity analysis justified? | 2 |
| 29. Were the ranges over which the parameters were varied stated? | 2 |
| 30. Were relevant alternatives compared? (That is, were appropriate comparisons made when conducting the incremental analysis?) | 1 |
| 31. Was an incremental analysis reported? | 1 |
| 32. Were major outcomes presented in a disaggregated as well as aggregated form? | 4 |
| 33. Was the answer to the study question given? | 1 |
| 34. Did conclusions follow from the data reported? | 1 |
| 35. Were conclusions accompanied by the appropriate caveats? | 1 |
| 36. Were generalisability issues addressed? | 4 |
Appendix 16 Focused search for association between body mass index and longer-term outcomes
A focused search of the literature was conducted to identify evidence of the association between BMI and mortality, hospitalisation or QoL (e.g. EQ-5D and ADL measures). MEDLINE was searched; the search strategy is reported here.
Search strategy for body mass index outcome: MEDLINE
-
Body Mass Index/
-
BMI.ti,ab,kw,kf.
-
Body Mass Index.ti,ab,kw,kf.
-
MNA.ti,ab,kw,kf.
-
EQ-5D.tib,kw,kf.
-
Barthel Index.ti,ab,kw,kf.
-
Kartz Index.ti,ab,kw,kf.
-
Readmission.ti,ab,kw,kf.
-
Hospital admission.ti,ab,kw,kf.
-
Falls.ti,ab,kw,kf.
-
associat*.ti,ab,kw,kf.
-
or/5-10
-
Statistics as Topic/
-
relationship.ti,ab,kw,kf.
-
statistical.ti,ab,kw,kf.
-
regression.ti,ab,kw,kf.
-
exp Frail Elderly/
-
exp Frailty/
-
frail*.ti,ab,kw,kf.
-
((older or aged) adj (person* or people or patient* or population*)).ti,ab,kw,kf.
-
((geriatric or elder*) adj2 (people or person* or patient* or population*)).ti,ab,kw,kf.
-
exp Nursing Homes/ or exp Homes for the Aged/
-
((residential or nursing or care) adj home*).ti,ab,kw,kf.
-
exp Respite Care/
-
exp Long-Term Care/
-
“home* for the aged”.ti,ab,kw,kf.
-
“old age home*”.ti,ab,kw,kf.
-
skilled nursing facilit*.ti,ab,kw,kf
-
.intermediate care facilit*.ti,ab,kw,kf.
-
respite care.ti,ab,kw,kf.
-
long term care facilit*.ti,ab,kw,kf.
-
or/17-31
-
11 or 13 or 14 or 15 or 16
-
1 or 2 or 3 or 4
-
12 and 32 and 33 and 34
-
limit 35 to (English language and full text and humans and yr=“1980-Current”).
Glossary
- Activities of daily living
- Essential and routine tasks that a healthy individual does on a daily basis without assistance.
- Albumin
- A protein made in the liver. It maintains osmotic pressure of the blood compartment, providing nourishment to the tissues and transporting hormones, vitamins, drugs and other substances such as calcium throughout the body.
- Cost-effectiveness analysis
- An economic analysis that describes the costs of additional health gain and adapts effects into health terms.
- Dietary counselling
- A course by which a health professional with special training in nutrition helps people form healthy eating habits and make healthy food choices.
- Fat-free muscle mass
- Encompasses tissues such as skeletal muscle, brain, heart, kidneys, liver and the gastrointestinal tract organs.
- Meta-analysis
- A statistical technique used to combine the results of two or more studies to obtain a combined estimate of effect.
- Oral nutritional supplements
- Liquid, semisolid or powder preparations, which provide a combination of macro- and micronutrients.
- Publication bias
- The inclination of authors to publish studies with significant results while withholding negative results from publication.
- Quality of life
- Encompasses an individual’s emotional, physical and social well-being and their ability to perform the ordinary tasks of living.
- Quality-adjusted life-year
- A measure of health gain used in economic evaluations, in which survival duration is adjusted or weighted by the patient’s quality of life during the survival period.
List of abbreviations
- ADL
- activities of daily living
- BMI
- body mass index
- CFB
- change from baseline
- CI
- confidence interval
- EQ-5D
- EuroQol-5 Dimensions
- GRADE
- Grading of Recommendations, Assessment, Development and Evaluation
- HIV
- human immunodeficiency virus
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- MD
- mean difference
- MNA
- Mini Nutritional Assessment
- MUST
- Malnutrition Universal Screening Tool
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- ONS
- oral nutritional supplements
- PPIE
- Patient and public involvement/engagement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- risk ratio
- SC
- standard care
- SD
- standard deviation
- SE
- standard error
- SF-12
- 12-Item Short Form Survey
- SF-36
- 36-Item Short Form Survey
- SMD
- standardised mean difference
- TIDieR
- Template for Intervention Description and Replication
- TTO
- time trade-off
- TUG
- Timed Up and Go
- VAS
- visual analogue scale
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/CCQF1608).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.