Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/134/02. The contractual start date was in April 2017. The draft report began editorial review in May 2021 and was accepted for publication in June 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Qureshi et al. This work was produced by Qureshi et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – Journals Library, and the DOI of the publication must be cited.
2023 Qureshi et al.
Chapter 1 Introduction
Familial hypercholesterolaemia (FH) is the commonest autosomal dominant disorder with around 1 in 250 individuals (0.4%) affected by the more common heterozygote form. 1,2 However, worldwide it is estimated that < 7% of those with the condition are currently identified. 3,4
Historic data from the time before effective lipid-lowering agents such as statins where available show that, if left untreated, individuals with FH have a dramatically higher risk of coronary heart disease (CHD), with a 100-fold increased mortality risk, than the general population. 5,6 CHD among people with FH can be very effectively prevented by high-intensity lipid-lowering treatment (LLT), with a 48% reduction in CHD mortality. 6 Moreover, 50% of their first-degree relatives and 25% of second-degree relatives will also have the condition and so benefit from intervention.
Improvement in the current low detection rate of FH is urgently needed. More effective cascade testing to identify affected relatives, especially younger relatives, and to initiate early statin treatment to lower low-density lipoprotein cholesterol (LDL-C) will prevent and reduce premature mortality and long-term morbidity. Available service data highlight the major extent of the problem. National audits show that only around one affected relative is identified for each index case. 7
Current national guidelines recommend the early identification and management of patients with FH. Despite recommendations in the 2008 National Institute for Health and Care Excellence (NICE) guidelines,8 the Royal College of Physicians audit in 20109 indicated there were 15,341 FH adults and 1106 FH children known and under care in a UK lipid clinic, suggesting that up to 200,000 individuals with FH were not being treated according to guidelines and would be at elevated risk of CHD. 9
Identification in primary care remains poor and opportunistic. The most recent NICE guidelines advise primary care to search for patients with possible FH based on cholesterol levels. This is followed by referral to specialist care based on Simon Broome or Dutch Lipid Clinic Network (DLCN) criteria. 8 In specialist care, these patients are reassessed using these criteria, or modified versions [e.g. the Welsh-modified Dutch Lipid Clinic Network (WDLCN)10], and FH is confirmed using genetic testing. Subsequent cascade testing of relatives is also suboptimal, partly because of perceived barriers to specialists approaching relatives directly, particularly if the relatives live in different geographies. Moreover, people with FH are mainly managed by specialist care, despite the potential for greater management in primary care.
Existing cost-effectiveness analyses (UK and internationally) have explored whether or not specific protocols for cascading are cost-effective. 11–14 However, commissioners and policy-makers are uncertain about whether current cascade programmes represent the best value for money in practice. 15,16 They have questioned whether or not tighter criteria for cascading could offer better value for money, and whether or not service protocols could do more to maximise the number of relatives tested.
By using robust and multiple data sources and modelling a wide range of possible protocols for cascade testing, this study has identified the most cost-effective protocols for cascade testing for FH in UK clinical practice and the NHS.
The study used economic modelling to evaluate the cost-effectiveness of alternative cascade-testing protocols. Parameters to inform these models were derived from existing published literature and routinely available data in primary and specialist care. In this study, we originally proposed to collect data from three large regional FH services (Wessex, Scotland and Wales), with differing protocols for cascade testing.
The Welsh service, serving a population of 3.2 million since 2011 and co-funded by the British Heart Foundation (BHF), identifies new FH cases using the WDLCN criteria,10 including triglyceride levels. In the service, several modalities of identification are used, including specialist nurses accessing primary care records. Cascade testing of relatives is offered directly (initiated by the clinic/medical professionals) and indirectly (initiated by patients providing information to their family members).
The Wessex service, serving a population of 2.5 million, is one of the first BHF pilots (2014). Cases are identified through primary care-based FH co-ordinators using modified NICE Simon Broome referral criteria with higher LDL-C threshold (> 5.5 mmol/l) and taking account of triglyceride levels. 8 Cascade testing of relatives is usually through indirect contact.
The Scottish service protocol serves a population of 5.5 million since 2008. General practitioners (GPs) use NICE Simon Broome criteria for referring suspected index cases either directly for genetic testing or through a network of 17 lipid clinics. Patients with genetically confirmed FH are referred to genetic services for cascade testing, initially contacting relatives indirectly, but more recently using indirect and direct approaches.
Exploring and identifying the most cost-effective protocols and care pathways and wider implementation will be dependent on their acceptability to a range of stakeholders. Therefore, the experiences, views and attitudes of patients and family members, primary care and specialist health-care providers, and service commissioners are also explored.
Study aim
The aim was to identify the most cost-effective cascade-testing protocol for FH.
Study objectives
-
To determine the yield of cases, treatment patterns, and short- and long-term outcomes for FH patients through routine service data and by new linkage of national FH, primary and secondary care data sets.
-
To evaluate the cost-effectiveness of alternative protocols for cascade testing using data from service protocols in three UK regions, the literature and linkage of national clinical databases.
-
To assess the acceptability of cascade-testing approaches to individuals and families with potential and confirmed FH, and health-care providers.
Objective 1 was addressed by developing an economic model that synthesises evidence on genetic testing to identify index cases and subsequent cascade testing, with evidence on the short- and long-term costs and benefits of identifying and managing FH cases. This was based on data from (1) cascade-testing services, (2) linking UK primary and secondary care data sets describing the management and outcomes of patients with FH (see objective 2) and (3) evidence from the literature and supplied by the Dutch FH service.
Objective 2 described treatment patterns and short- and long-term outcomes of FH patients, by linkage of national clinical databases. Data on cases managed in primary and secondary care were linked to data on LLT use and cholesterol response [from the Clinical Practice Research Datalink (CPRD) and the Simon Broome FH Register)], and to cardiovascular disease (CVD) events [from Hospital Episode Statistics (HES)] and mortality [from the Office for National Statistics (ONS)]. Furthermore, data on the yield of new FH index cases and patterns of contacting and testing of their relatives were captured from routine service data sets. Collectively, these provided a rich source of evidence from which to estimate the impact of identifying and managing relatives with FH, thereby providing more precise and robust parameters for the cost-effectiveness model.
Finally, objective 3 was addressed by qualitatively exploring acceptability, benefits and harms of cascade-testing approaches from patient, health-care practitioner and other stakeholder perspectives.
Modification to original protocol and structure of the report
As the project progressed, we adjusted the protocol to reflect the publication of new studies and data availability.
-
We concluded that a systematic review comparing outcomes among diagnosed patients with outcomes among undiagnosed patients was not required, given the publication of the European Atherosclerosis Society (EAS) consensus statement,17 which reviewed evidence from randomised controlled trials, observational studies and Mendelian randomisation studies. The statement concluded that CVD risk related to raised LDL-C increases over time,17 and, based on the meta-analysis of individual-level data from 28 randomised controlled trials on statin therapy, estimated that a 1-mmol/l reduction in LDL-C leads to a 22% reduction in CVD risk. 18
-
We concluded that a systematic review on the long-term benefits of LLT for paediatric patients was not required given the publication of recent systematic reviews on this topic. 19,20
-
We compared cascade protocols, including alternative ways to select index cases, to contact relatives and to test relatives; we did not compare policies that tested third-degree relatives because the service data did not differentiate between second-degree or more distant relatives to the index case.
-
Aligned with current NICE guidelines, we did not compare policies in which cascade to relatives started from an index in whom a FH mutation was not detected and their relatives are cascaded based on cholesterol alone, because the probability that index cases have an as yet undiscovered FH mutation is low,21 and the probability that relatives are affected is not clear.
-
For the cost-effectiveness model, we analysed the data from the CPRD cohort, a cohort of FH patients with linked NHS and mortality data. However, we were unable to analyse the Simon Broome cohort linked to routine NHS data sets on CVD events and mortality owing to delays with the provision and approval of these data for cost-effectiveness analysis. Furthermore, we applied for linkage of the Simon Broome cohort to the National Institute for Cardiovascular Outcomes Research (NICOR) Myocardial Ischaemia National Audit Project (MINAP) data set 2 months before the start of the study; despite repeated submissions and enquires, after 43 months there was still no progress. Following guidance from the external Study Steering Committee, we discontinued further pursuit of this linkage.
-
Our cost-effectiveness model assumes that diagnosis reduces LDL-C, as observed in the FH cohort, and links those reductions to CVD risk in the long term; it does not account for adherence to LLT explicitly, given the difficulty in linking adherence to changes in LDL-C over time, and its impact on CVD risk.
-
We informed the cost-effectiveness model using data from the Welsh and Wessex FH services. However, we could not use data from the Scottish FH service because it did not allow for the estimation of key parameters (e.g. index cases’ clinical scores, relationship between index cases and relatives).
-
Health resources involved in the cascade process were informed via a review of the literature and expert opinion, due to the lack of data on these in the Welsh, Wessex and Scottish data sets.
-
Given the small number of CVD events experienced by the CPRD cohort, we calculated the NHS cost of CVD events based on the literature, rather than conducting a costing analysis using the CPRD cohort data. This assumes that the costs of CVD events are generalised from a mixed population, a minority of which will have FH, to individuals with FH.
-
Stakeholders were closely involved in the development of the economic models (see Chapter 5). This group comprised consultant lipidologists, FH nurse specialists, public health physicians, commissioners, expert patient and public involvement and engagement (PPIE) representatives, and representatives from relevant charities (HEART UK and the BHF).
The study was completed by three research teams, and is presented accordingly in this report. The systematic reviews were led by the Nottingham team, with the diagnostic accuracy review undertaken by colleagues in Newcastle (see Chapter 2). The description of the included databases and primary epidemiological analysis was completed by the Nottingham team (see Chapters 3 and 4) Economic analyses of the CPRD database were completed by the York economic team (see Chapter 5, Economic analysis of the Clinical Practice Research Datalink database). The York team also completed a descriptive analysis of the PASS (PASS Software, Rijswijk, the Netherlands) Welsh and Wessex FH service data sets (see Section 8.3), and led the development of the two new economic models (see Chapter 5, Service data analysis, and Cost and health benefits of diagnosis of people with familial hypercholesterolaemia in the long term). Finally, the qualitative studies of patients and health professionals were led by the Nottingham qualitative research team (see Chapter 6).
Chapter 2 Systematic reviews
This chapter focuses on the systematic reviews that were proposed to supplement primary data available from clinical data sets to estimate parameters required for inputting into the economic model. The reviews were as follows:
-
effectiveness of cascade-testing protocols among relatives for FH
-
effectiveness of cholesterol-lowering therapies on LDL-C levels and CVD among adults
-
effectiveness of LLTs on LDL-C levels, and the impact of LDL-C levels on CVD and mortality among children with FH
-
diagnostic accuracy of clinical and biochemical criteria for identifying relatives of index cases with confirmed FH.
Review 1: effectiveness of cascade-testing protocols among relatives for familial hypercholesterolaemia
Introduction
The typical pathway for FH identification involves physicians, often the primary care provider, referring individuals with suspected FH to a specialist who confirms the diagnosis, often using genetic testing. The specialist will then arrange testing of relatives of confirmed FH cases, usually by the patient contacting the relatives themselves (indirect cascade testing). The exception could be testing children of affected parents, which may be done directly. In fact, the family are often traced to two or three generations. 8,22 Initially, this usually starts with the affected individuals’ children. 23 Internationally, most cascade testing starts with adult index patients and cascading testing to other relatives including children (‘forward cascade testing’). ‘Reverse’ cascade testing is also under consideration: starting identification from affected children. 24 However, despite being recognised as a cost-effective strategy,25 there are still many patients not being diagnosed, with one of the reasons being the relatively low yield, which could be related, partly, to using the indirect approach. The alternative approach to the indirect approach is direct cascade testing, whereby the genetic specialist contacts the relatives directly.
In 2019, a systematic review found that the proportion of cascade-tested relatives was higher with the direct approach;26 however, as this review did not synthesise the studies quantitatively, the magnitude of the differences between the approaches remains unclear. Therefore, we have performed a systematic review and a meta-analysis to quantify the yield of different approaches (direct, indirect, combination) for cascade testing for FH.
Materials and methods
The protocol for the systematic review was registered in PROSPERO (CRD42019125775). Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines27 were adhered to throughout the conduct and reporting of the systematic review.
The systematic review encompasses relevant study designs, including controlled trials and epidemiological studies, which assessed the effectiveness of cascade testing for FH among relatives. Eligible participants were first- and second-degree relatives of index cases with confirmed FH, determined using a clinical diagnosis [i.e. Simon Broome,5 DLCN,3 make early diagnosis to prevent early death (MEDPED)28 or another criterion appropriate to the population being studied]; LDL-C levels, using age-specific cut-off points; or genetic diagnosis of mutation-positive cases. The protocol for cascade testing was the intervention of interest, which could be conducted via (1) a direct method of contact (whereby the relatives of the index case are contacted directly by the clinic, usually using personalised letters or telephone calls, once consent has been sought from the index case), (2) an indirect method of contact (whereby the index case acts as an intermediary by passing on personalised letters or information to their relatives) or (3) a choice of indirect or direct methods (or a combination of direct and indirect methods). The primary outcome measure was the proportion of relatives of the index cases tested out of those contacted, henceforth referred to as yield. Secondary outcome measures included the proportion of relatives of the index cases with confirmed FH out of those tested, the proportion of relatives of the index cases contacted out of those eligible, the proportion of relatives of the index cases who responded out of those contacted and the proportion of index cases who participated in cascade testing out of those genetically or clinically confirmed with FH.
Comprehensive literature searches of three databases [MEDLINE, from 1946 to May 2020; EMBASE, from 1980 to May 2020; and Cochrane Central Register of Controlled Trials (CENTRAL), from 1966 to May 2020] were performed using a highly sensitive search strategy based on keywords and medical subject heading (MeSH) terms relating to the population (e.g. proband$, index patient$, relative$, family$, patient$) and intervention of interest (e.g. cascade, mass screening, contact tracing) (see Appendix 1 and Table 23, for the MEDLINE search strategy), and other publications were identified through contact with topic experts.
In addition, grey literature was identified from the following sources: the British Cardiovascular Society Annual Conference, the HEART UK Annual Scientific Conference, the European Human Genetics Conference and the EAS Congress, from dates of inception to March 2020, and through hand-searching the Atherosclerosis journal and the HEART UK (www.heartuk.org.uk/) and US Family Heart Foundation (https://thefhfoundation.org/) websites. No language restrictions were applied, and translations were sought when necessary.
Screening and study selection
Following the removal of duplicates, titles, abstracts and full texts of potentially eligible studies were screened independently by two authors (JLB and Ben Young/Kelly Eliman/CB). Disagreements regarding eligibility of a study were resolved through discussion with a third author (NQ). Reasons for exclusion at the full-text stage were documented.
Data extraction and quality assessment
A standardised form, developed by the authors and tailored to this review, was used for data extraction. Data relating to the study characteristics, the methods used, and primary and secondary outcomes were extracted independently by two authors (JLB and CB). When possible, the authors of any studies with missing data were contacted. Two authors (JLB and CB) independently assessed the methodological quality of the included studies using the JBI Critical Appraisal tool. 29 Studies that scored ‘no’ for more than two of the questions were rated as having low methodological quality, high methodological quality was assigned when all the domains were rated as ‘yes’, and the remaining studies were rated as moderate. Discrepancies were discussed between authors, as needed.
Data synthesis and investigations of heterogeneity
For each study, we calculated raw proportions with 95% score-based confidence intervals (CIs) based on the appropriate numerator and denominator for each outcome measure. Variances of the raw proportions were stabilised before pooling using the Freeman–Tukey double arcsine transformation30 to ensure studies that estimated proportions as 100% (standard error = 0) were not excluded from the analysis. The included studies presented outcome data for only one cascade protocol (direct, indirect or combination); therefore, no relative effect measures could be estimated. Thus, pooled proportions for the outcome measures overall and for each cascade protocol were estimated using a random-effects models whereby sufficient studies were included in the meta-analyses to allow for anticipated heterogeneity resulting from inherent biases within the studies. I2 was used to quantify inconsistency (heterogeneity). 31 Analyses were conducted in Stata® version 16.0 (StataCorp LP, College Station, TX, USA).
Results
The searches identified a total of 3742 studies. Following title and abstract screening, 217 studies were assessed for full-text screening (see Figure 1). At the full-text screening, 193 studies were excluded, related to ineligible study design (77 studies), ineligible or duplicate population (35 studies), ineligible or unclear intervention (62 studies) or ineligible outcome reporting (19 studies); therefore, 24 studies were included in the systematic review and meta-analysis32–55 (see Table 1).
FIGURE 1.
The PRISMA flow chart for systematic review of effectiveness of cascade-testing strategies.

| Study (first author and year) | Country | Number of confirmed index cases | Contact method | Format of cascade | Extent of cascading among relatives | FH diagnosis method for | Quality score | |
|---|---|---|---|---|---|---|---|---|
| Index cases | Relatives | |||||||
| Alver32 2019 | Estonia | 27 | Indirect | Forward | Second degree | Genetic | Genetic | Moderate |
| Andersen33 1997 | Denmark | 62 | Direct | Forward | Second degree | Clinical (study-specific) | Clinical (study-specific) | Moderate |
| Bell34 2015 | Australia | 100a | Both | Forward | Third degree | Genetic | Genetic | High |
| Bhatnagar35 2000 | England | 262 | Direct | Forward | First degree | Clinical (SB) | Clinical (SB) | Moderate |
| Breen36 2011 | England | 72 | Indirect | Forward | Not reported | Genetic | Genetic | Moderate |
| Chan37 2019 | Hong Kong | 64 | Indirect | Forward | Second degree | Genetic | Genetic | Moderate |
| Davis38 2016 | USA | 5 | Direct | Forward | First degree | Genetic | Genetic | Moderate |
| Descamps53 2021 | Belgium | 127 | Direct | Forward | Second degree | Clinical (DLCN) | Clinical (MEDPED/DLCN) | Moderate |
| Edwards39 2013 | Wales | 270 | Both | Forward | Not reported | Genetic | Genetic | Moderate |
| Ellis40 2019 | Spain | 755 | Direct | Forward | Not reported | Genetic | Genetic | Moderate |
| Hadfield41 2009 | England | 931 | Both | Forward | Not reported | Clinical (SB) | Clinical (SB) | Moderate |
| Jannes42 2015 | Brazil | 125 | Direct | Forward | Second degree | Genetic | Genetic | Moderate |
| Latkovskis43 2018 | Latvia | 140 | Indirect | Forward | First degree | Clinical (DLCN) | Clinical (DLCN) | High |
| Leren55 2008 | Norway | ≈1300 | Indirect | Forward | Not reported | Genetic | Genetic | Moderate |
| Marks44 2006 | England | 354 | Indirect | Forward | Not reported | Clinical (SB) | Clinical (MEDPED) | Moderate |
| Marteau45 2004 | England | 341 | Direct | Forward | Second degree | Clinical (SB) | Genetic or clinical (SB) | Moderate |
| Muir46 2010 | New Zealand | 76 | Direct | Forward | Not reported | Genetic | Genetic | Low |
| Neuner47 2020 | USA | 2 | Indirect | Forward | Not reported | Genetic | Genetic | Low |
| Raal54 2020 | South Africa | 252a,b | Direct | Forward | First degree | Genetic or clinical (not specified) | Genetic | Low |
| Setia48 2018 | India | 31 | Direct | Forward | Second degree | Genetic | Genetic | Moderate |
| Skovby52 1991 | Denmark | 17 | Direct | Reverse | Not reported | Clinical (study-specific) | Clinical (study-specific) | Moderate |
| Tilney49 2019 | Malta | 9 | Both | Forward | First degree | Clinical (DLCN) | Clinical (DLCN) | Moderate |
| Umans-Eckenhausen50 2001 | The Netherlands | 237a | Direct | Forward | Second degree | Genetic | Genetic | Moderate |
| Webster51 2019 | England | 215 | Both | Forward | Not reported | Genetic | Genetic | Moderate |
Of the 24 included studies, 16 were conducted in Europe (England,35,36,41,44,45,51 Wales,39 Belgium,53,56 Denmark,33,52 Latvia,43 the Netherlands,50 Norway,55 Spain,40 Malta49 and Estonia32), one in Australia,34 three in the Americas (the USA38,47 and Brazil42), one in New Zealand,46 two in Asia (India48 and Hong Kong37) and one in South Africa. 54 All studies used an observational design to assess the outcome measures. The average number of confirmed index cases enrolled in the studies was 242, with sample sizes ranging from 2 to approximately 1300 participants.
The direct method of contact was used in 12 studies,33,35,38,40,42,45,46,48,50,52–54 a further 7 studies used an indirect method,32,36,37,43,44,47,55 and the remaining 5 used a combination of direct and indirect methods. 34,39,41,49,51 Contact could be made via a range of approaches, including postal invitation, telephone, in person or a combination of approaches. Forward cascade testing was used in the majority of included studies (23 studies), with the remaining study using reverse cascade testing. 52 Fourteen of the included studies reported the extent of cascade: the majority (eight studies32,33,37,42,45,48,50,53) cascaded to second-degree relatives, with only five studies cascading to first-degree relatives35,38,43,49,54 and one study cascading to third-degree relatives. 34
The majority of included studies (14 studies32,34,36–40,42,46–48,50,51,55) confirmed FH diagnosis for the index cases using genetic testing; nine studies confirmed FH diagnosis for the index cases using clinical assessment based on Simon Broome (four studies35,41,44,45), DLCN (three studies43,49,53) or study-specific criteria (serum cholesterol of ≥ 8 mmol/l, LDL-C of ≥ 6 mmol/l and family history of hypercholesterolaemia;33 apolipoprotein B: apolipoprotein A-1 ratio > 97th centile or apolipoprotein B > 99th centile, LDL-C > 95th centile and no secondary causes for raised cholesterol52); and one study stated that diagnosis was based on either genetic or clinical criteria, but did not provide additional details. 54 For the relatives, genetic confirmation of FH was used in the majority of studies (15 studies32,34,36–40,42,46–48,50,51,55,57). A further eight studies used clinical assessment based on either Simon Broome,35,41 DLCN,43,49 MEDPED,44 a combination of DLCN and MEDPED,53 or study-specific criteria (serum cholesterol of ≥ 7 mmol/l;33 LDL-C > 95th centile52). The final study used genetic testing or clinical assessment based on Simon Broome criteria depending on which arm of the trial the index case had been randomised to. 45
For the 16 studies using genetic testing for confirmation of FH, testing of only the low-density lipoprotein receptor (LDLR) gene was performed in three studies,46,48,50 testing of the LDLR and apolipoprotein B-100 (APOB) genes was performed in two studies,45 testing of the LDLR, APOB and proprotein convertase subtilisin/kexin type 9 (PCSK9) genes was performed in eight studies,32,34,37,38,40,42,47,51 and testing of the LDLR, APOB, PCSK9, and low-density lipoprotein receptor adaptor protein 1 (LDLRAP1) genes was performed in one study. 54 It was unclear what genes were tested in the remaining two studies. 36,39
The median number of new relatives with FH per known index case was 0.98 (range 0.15–8.6), with the largest medians seen in the studies using the indirect (median 1.39, range 0.22–8.60) and direct (median 1.27, range 0.15–3.86) testing strategies, compared with the combination approach (median 0.72, range 0.26–1.88); however, this is a crude analysis that does not consider the relative contribution of each study.
Quality assessment
The majority of studies were rated as having a moderate risk of bias; only three studies had a high methodological quality score34,43,53 and two studies were rated as having low methodological quality46,47 (see Table 1 and Appendix 1, and Table 24). The reasons for lower methodological quality were primarily related to less clarity regarding consecutive inclusion of participants (question 4) and incomplete inclusion of participants (question 5). Furthermore, many studies scored ‘no’ on clear reporting of the demographics (question 6, 10 studies) and clinical information of the participants (question 7, 10 studies).
Primary outcome measure
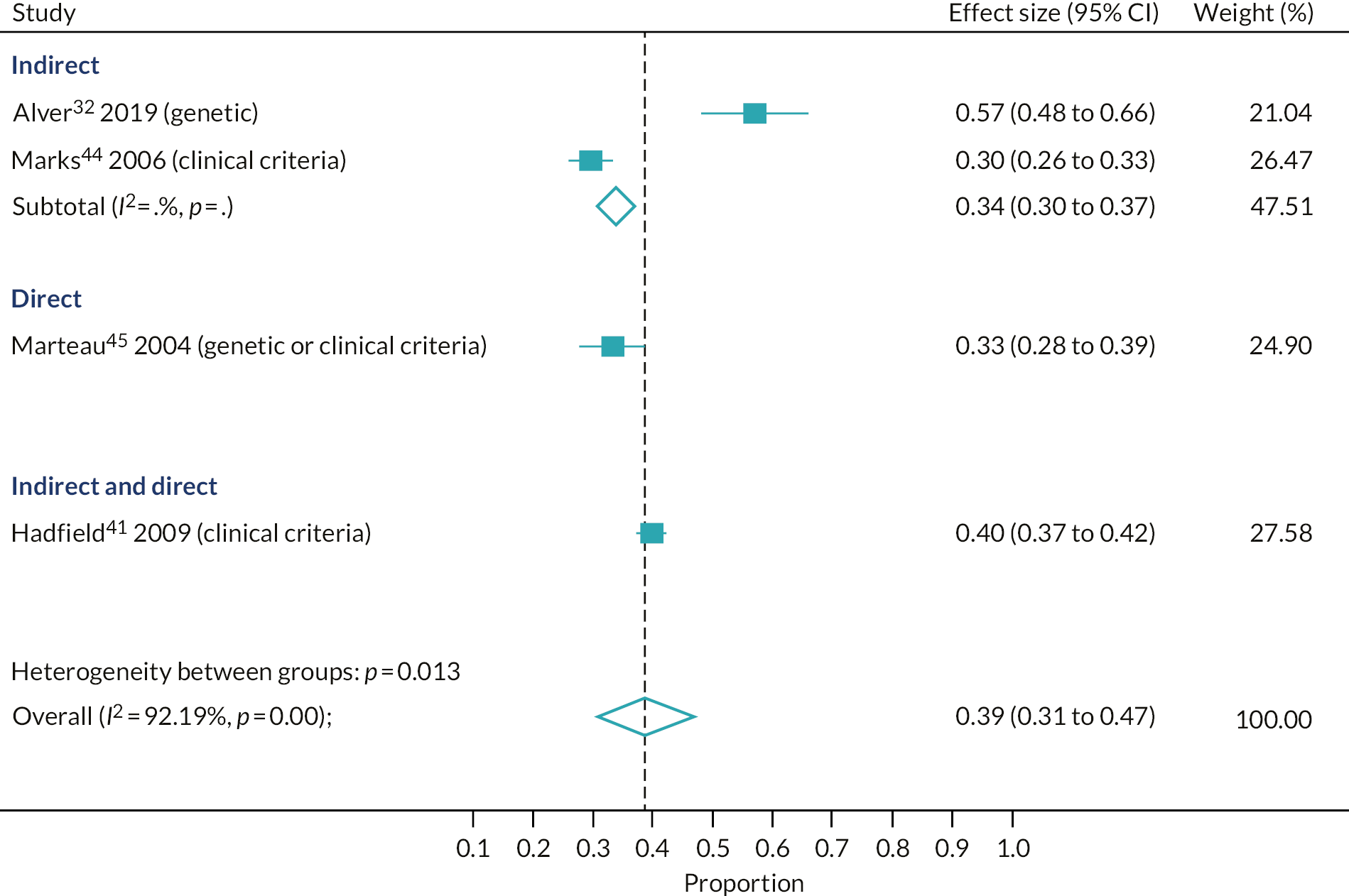
Proportion of relatives of index cases tested for familial hypercholesterolaemia of those contacted
Four studies32,41,44,45 provided data to estimate the primary outcome. On average, 39% of relatives were tested for FH out of those contacted (95% CI 31% to 47%, four studies); however, the estimates varied significantly by the cascade approach used (p-value for subgroup differences, p = 0.01) (see Figure 2). The largest yield was seen in the study conducted in England that used a combination approach (40%, 95% CI 37% to 42%, one study); however, similar, but slightly lower, yields were seen for the direct and indirect strategies [direct: 33%, 95% CI 28% to 39% (one study, conducted in England); indirect: 34%, 95% CI 30% to 37% (two studies, conducted in England and Estonia)], although the results from the last two studies varied considerably (57%32 and 20%44).
FIGURE 2.
Proportion of relatives tested out of those contacted for FH cascade testing, by cascade approach.
Secondary outcome measures
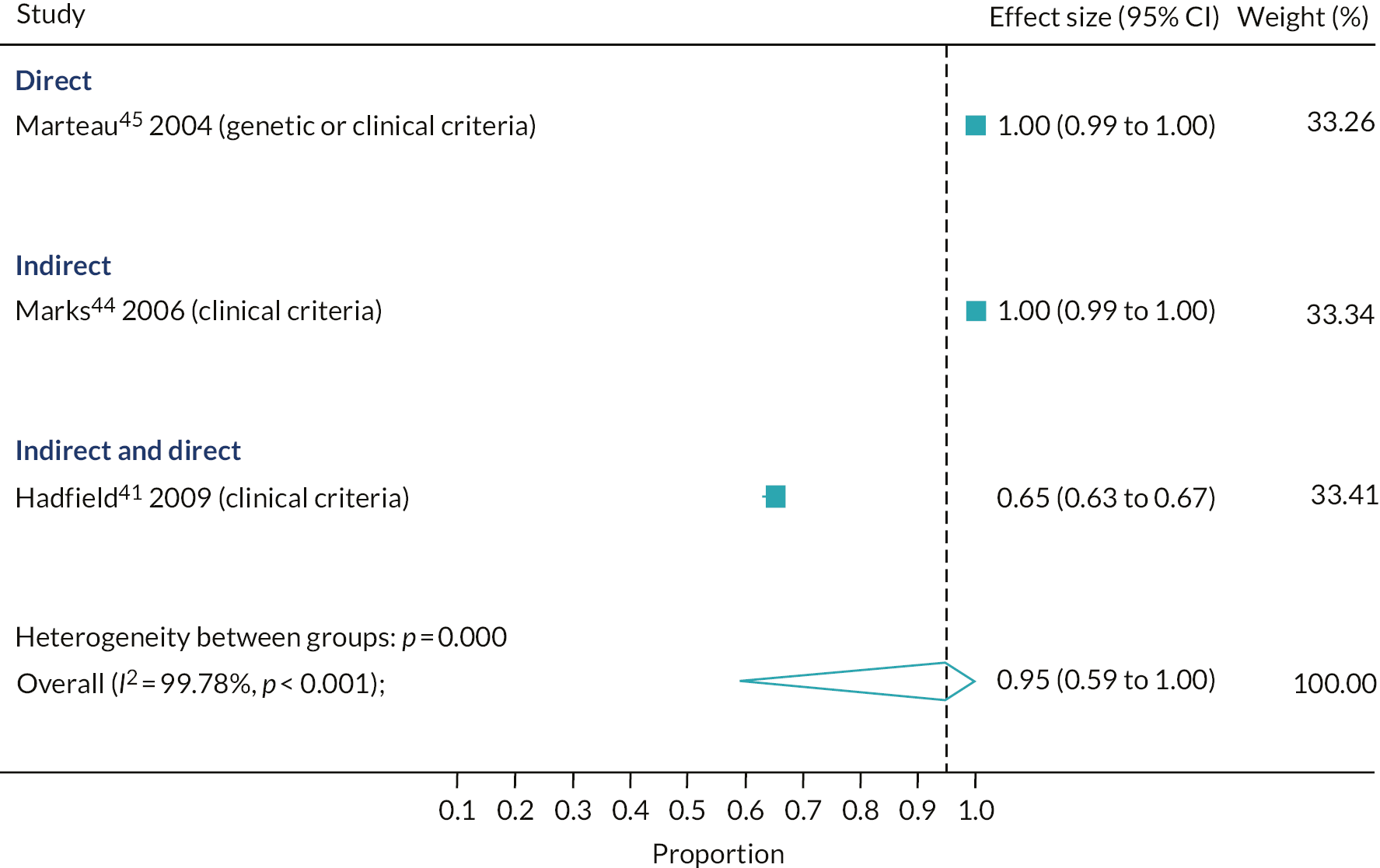
The proportion of relatives contacted for familial hypercholesterolaemia testing out of those eligible
Only three studies reported data to estimate the proportion of relatives contacted for FH testing out of those eligible. 41,44,45 For the studies that reported this outcome, on average, 95% of relatives were contacted out of those eligible (95% CI 59% to 100%, three studies). Using either a direct or an indirect approach resulted in all the relatives who were eligible for testing being contacted (direct: 100%, 95% CI 99% to 100%, one study; indirect: 100%, 95% CI 99% to 100%, one study) (see Figure 3). However, in the single study that used a combination of direct and indirect methods, a significantly lower proportion of relatives were contacted out of those eligible (65%, 95% CI 63% to 67%; p-value for subgroup differences, p < 0.001). However, in this study,41 only 26% of the index cases had a diagnosis of definite FH, with the remaining having a possible diagnosis of FH.
FIGURE 3.
Proportion of relatives contacted out of those eligible for FH cascade testing, by cascade approach.
The proportion of relatives who responded out of those contacted for familial hypercholesterolaemia testing
Three studies reported data on the proportion of relatives who responded to cascade screening out of those contacted;41,44,45 on average, 43% of relatives responded out of those contacted (95% CI 28% to 58%, three studies)
Using a combination of direct and indirect approaches yielded a significantly greater proportion of relatives responding out of those contacted (54%, 95% CI 51% to 56%, one study) than using either an indirect approach (31%, 95% CI 27% to 35%, one study) or a direct approach alone (45%, 95% CI 39% to 51%, one study) (p-value for subgroup difference, p < 0.001).
The proportion of relatives with confirmed familial hypercholesterolaemia of those tested
Twenty-one of the included studies reported data on the proportion of relatives confirmed as having FH out of the number of relatives tested (see Figure 4). On average, 47% of relatives were confirmed to have FH out of those tested (95% CI 42% to 52%, 21 studies). Contact strategies were found to produce similar pooled results (direct: 50%, 95% CI 41% to 58%, I2 = 97%, 10 studies; indirect: 45%, 95% CI 38% to 53%, I2 = 82%, 7 studies; combination: 43%, 95% CI 29% to 58%, I2 = 98, 4 studies; p-value for subgroup differences, p = 0.67).
FIGURE 4.
Proportion of relatives confirmed as having FH out of those tested, by cascade approach.

The proportion of index cases that participated in cascade testing out of those confirmed as having familial hypercholesterolaemia
Seventeen studies reported data on the proportion of index cases who participated in FH cascade testing out of those confirmed as having FH. On average, 89% of index cases participated in cascade testing out of those confirmed with a diagnosis of FH (95% CI 73% to 99%, 17 studies); however, the estimates varied significantly by the cascade approach used (p-value for subgroup differences, p < 0.001). The yield was highest using a direct approach (95%, 95% CI 83% to 100%, I2 = 98%, nine studies), a slightly lower yield was seen using an indirect approach (78%, 95% CI 46% to 99%, I2 = 99%, six studies), and the lowest yield was seen using a combination of direct and indirect approaches (60%, 95% CI 56% to 63%, two studies).
Conclusion
The review provides tentative support for the combination approach to cascade testing, whereby the index case determines which method is used to contact relatives. However, further evidence to support the combination approach requires experimental studies to compare the cascade approaches and/or interrogation of routine data sets and FH registers held on the cascade testing and the modality of contact with relatives.
Review 2: effectiveness of cholesterol-lowering therapies on low-density lipoprotein levels and cardiovascular disease among adults
An overview of reviews was conducted to assess the benefits of cholesterol-lowering therapies on LDL-C and CVD events in the general adult population and among adults with confirmed FH. Owing to the established evidence of the effectiveness of LLTs among people with FH,6 this review was limited to studies assessing the comparative effectiveness of active therapies with each other; thus, placebo and other non-active interventions were excluded. 27
Materials and methods
The protocol for the systematic review adhered to the PRISMA guidelines27 throughout, for both the conduct and the reporting of the review. Eligible participants were adults from the general population and adults with confirmed FH, determined using a clinical diagnosis (e.g. Simon Broome,5 DLCN,3 MEDPED28 or another criterion appropriate to the population being studied); LDL-C levels, with age-specific cut off points; or genetic diagnosis of mutation-positive cases. The eligible interventions and comparators were any licensed LLTs, including statins, ezetimibe, statins with ezetimibe, PCSK9 inhibitors, PCSK9 inhibitors with statins, PCSK9 inhibitors with statins and ezetimibe, or comparisons of different doses of the same treatment. Therefore, placebo or other non-active interventions were not eligible for inclusion as the comparator. The primary outcome measure was LDL-C levels, which could be reported as mean changes from baseline, mean percentage changes, or absolute level at follow-up. The study design included in this overview of reviews was systematic reviews of randomised controlled trials; however, we also included systematic reviews of observational cohort studies when there was a dearth of literature on adults with confirmed FH. We excluded studies that focused solely on index cases with homozygous FH.
Comprehensive literature searches of four databases [MEDLINE, from 1994 to June 2018; EMBASE, from 1994 to June 2018; Cumulative Index to Nursing and Allied Health Literature (CINAHL), from 1994 to June 2018; and CENTRAL, from 1994 to June 2018] were performed using a highly sensitive search strategy based on keywords and MeSH terms relating to the intervention of interest, outcome measures and study design (see Appendix 1 and Table 25, for the MEDLINE search strategy).
Screening and study selection
Following the removal of duplicates, titles, abstracts and full texts of potentially eligible studies were screened independently by two authors (JLB and Jacqueline Mhizha-Murira). Disagreements regarding eligibility of a study were resolved through discussion with a third author (NQ). Reasons for exclusion at the full-text stage were documented.
Data extraction and quality assessment
A MeaSurement Tool to Assess systematic Reviews (AMSTAR) critical appraisal tool58 was used by two reviewers (JLB and JMM) independently to assess the methodological quality of the included studies (e.g. the extent to which the systematic review had minimised the possibility of bias and reporting errors in its conduct) (see Report Supplementary Material 1 for scores on this critical appraisal tool).
Systematic reviews that gained scores of at least 12 on the AMSTAR critical appraisal tool were deemed to be of sufficient methodological quality to be included in the overview. Systematic reviews meeting the methodological quality threshold would have had their data extracted independently by two reviewers (JLB and JMM) using a standardised data extraction form.
Data synthesis and investigations of heterogeneity
A narrative synthesis of the included systematic reviews would have been conducted, whereby the findings from each systematic review would have been grouped based on interventions assessed. The findings would also have been tabulated to identify patterns and reported together with the number of studies that informed the outcome, the number of participants (from the included studies) and the heterogeneity of the results of included studies. When possible, the findings would have been translated using thematic or content analysis to identify areas of commonality between the results of the systematic reviews.
Results
The searches identified 2747 hits from MEDLINE, 1736 hits from EMBASE, 991 hits from CINAHL and 260 hits from CENTRAL, totalling 5734 hits. Following deduplication, 4829 hits were screened by title and abstract. Of the 214 papers identified for full-text screening, 14 papers59–72 were assessed for methodological quality (see Figure 5). The methodological quality of the 14 papers ranged from 1–11; thus, as none of the papers met the threshold of a score of at least 12, no papers were included in the overview of reviews.
FIGURE 5.
The PRISMA flow chart for the review of the effectiveness of cholesterol-lowering therapies.

Conclusion
The search did not identify any high-scoring systematic reviews assessing the effectiveness of head-to-head comparisons of cholesterol-lowering therapies for LDL-C and CVD events in the general adult population or among adults with confirmed FH.
Review 3: effectiveness of lipid-lowering treatments on low-density lipoprotein levels, and the impact of lipid-lowering treatments on low-density lipoprotein levels, cardiovascular disease and mortality among children with familial hypercholesterolaemia
We aimed to assess the effectiveness of LLTs on LDL-C levels and the association between LDL-C levels and CVD events and mortality among children with FH, using two systematic reviews. Two reviews, from 2016 and 2019, found good-quality evidence that LLT reduced LDL-C levels in children and young people. 19,20 However, no randomised controlled trial evidence was found on the direct effects among children of LLT on CVD events in adulthood or on the association between intermediate outcomes in childhood (e.g. atherosclerosis) and CVD events in adulthood. Given the difficulties of conducting these types of studies, we do not anticipate that additional studies will emerge in the next couple of years. Therefore, in the cost-effectiveness modelling, we linked LDL-C reductions to reductions in CVD risk later in life from the published literature based on adult data17 and we assumed that children and adolescents who are treated with LLT achieve the same LDL-C reductions as adults (see Chapter 5, Cost and health benefits of diagnosis of people with familial hypercholesterolaemia in the long term).
For these reasons, we did not consider a further review of paediatric cases to be a high priority and redirected resources to the newly proposed review 4.
Review 4: the diagnostic accuracy of clinical and biochemical criteria and scoring systems for the diagnosis of familial hypercholesterolaemia among relatives
Background
Cascade testing consists of systematically testing individuals who are at risk of a hereditary disease. 73 Cascade testing has long been proposed to diagnose relatives of patients genetically or clinically confirmed to have FH (the initial patient is known as the index case) because, as the inheritance of heterozygote FH is autosomal dominant, the probability of having heterozygote FH is 50% for first-degree relatives, and 25% for second-degree relatives. 74–76 Cascade testing can be conducted with a genetic test for FH mutation or based on the relative’s clinical and biochemical characteristics (e.g. LDL-C level given their age and sex), or a combination of the two (e.g. using LDL-C level to triage relatives before the genetic test). An example of biochemical criteria is using LDL-C cut-off points specific to age and sex, which is recommended by Starr et al. 77 Similarly, the MEDPED criteria use cut-off points that are age-specific for both total cholesterol (TC) and LDL-C. 28
The 2008 NICE clinical guidelines (CGs) recommended cascade testing with a genetic test for relatives of index cases with FH who had an identified mutation and age- and sex-specific LDL-C criteria to diagnose relatives of probands with FH. 8 The 2017 update, however, recommends using only the genetic test to conduct cascade testing. This systematic review aimed to assess the diagnostic accuracy of clinical and biochemical characteristics, including LDL-C and TC, and clinical signs, for the diagnosis of FH among relatives of index case with confirmed FH to inform the parameters for the economic model.
Review method
The systematic review was conducted and reported according to the PRISMA guidelines. 78 The study protocol was published on PROSPERO (CRD42018117445).
Inclusion and exclusion criteria
We included all published and unpublished test-accuracy studies that reported the result of the index test with that of the reference standard, and for which the following criteria were met:
-
population – relative of index case with genetically confirmed FH
-
index test – use of one or more clinical or biochemical characteristics or a clinical scoring system based on these characteristics (e.g. LDL-C, TC)
-
reference standard – genetic confirmation for any mutation in three FH-causing genes (LDLR, APOB, PCSK9)
-
diagnosis of interest – heterozygote FH.
Search strategy
A comprehensive search strategy was developed in MEDLINE by an experienced information specialist in collaboration with the project team (see Appendix 1 and Table 26). We used a combination of thesaurus headings and terms from the title, abstract or keyword fields, and translated the searches to other databases as appropriate. The search was initially conducted in August 2018 and updated in February 2020. The searches were run from 1994, because of the introduction of a government-subsidised cascade-testing scheme in the Netherlands. 79 The following databases were searched: MEDLINE, EMBASE, CINAHL, CENTRAL, Cochrane Database of Systematic Reviews and Science Citation Index (Web of Science). Grey literature was searched via websites of relevant organisations (e.g. FH Foundation, NICE guidelines). We checked the references of included studies. There were no restrictions by language, and translations were sought when necessary.
Study selection
Initially, titles and abstracts were screened independently by two reviewers (RK/Tumi Sotire/Dapo Ogunbayoa/Atefeh Mashayekhi) using a pilot study selection form based on the inclusion criteria for a random sample (10%) of the hits from the searches. The full texts of the potentially eligible studies were screened independently by two reviewers (RK/Tumi Sotire/Dapo Ogunbayoa/Atefeh Mashayekhi). Disagreements were resolved by consensus between the reviewers or using a third reviewer (NQ). 80
Data extraction and risk-of-bias assessment
Two reviewers (RK and Tumi Sotire) independently extracted data from the included studies based on the study methods and setting, index test, reference standard, sample size and participants’ characteristics. Included studies were assessed for risk of bias by two independent reviewers (RK and Tumi Sotire) using the quality assessment of diagnostic accuracy studies-2 (QUADAS-2). 81
Results
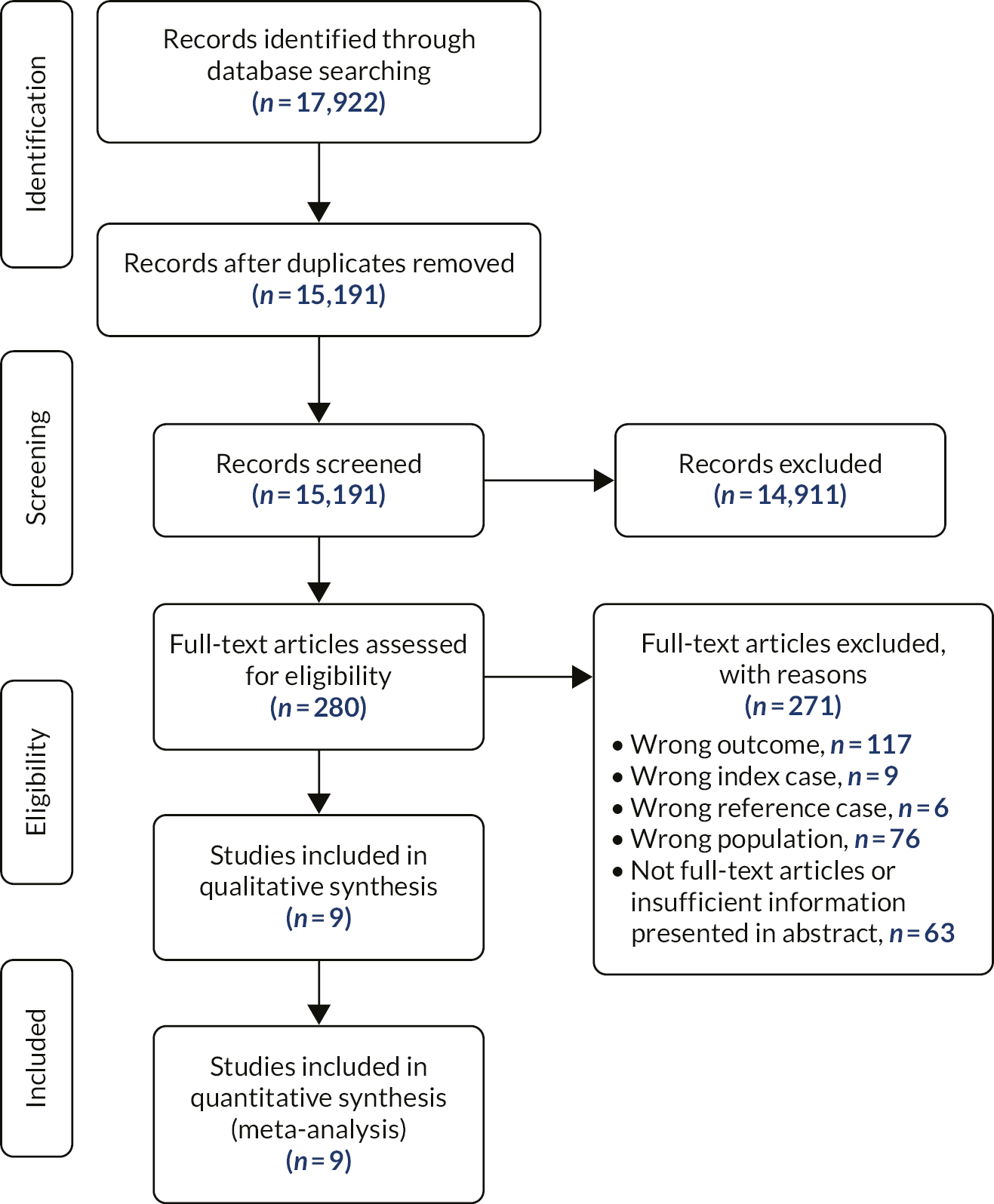
After deduplication, 15,191 titles and abstracts were screened, 280 of which were screened at the full-text stage. Of these studies, 117 reported an ineligible outcome, 9 reported ineligible index cases, 6 reported ineligible reference standards, 76 reported an ineligible population, and 63 were not full-text articles or insufficient information was presented (see Figure 6).
FIGURE 6.
The PRISMA flow diagram of the review of the diagnostic accuracy of biochemical and clinical criteria among relatives.
Nine studies met the inclusion criteria for this systematic review. 37,41,50,55,82–86 The studies were completed in the following countries: Australia,87 Denmark,41 Iceland,85 Japan,83 the Netherlands,41,82 Norway,41,50,55 South Africa86 and Vietnam. 84 Two studies used the same cohort of individuals; however, one assessed the diagnostic potential of TC50 and the other used LDL-C levels (Norwegian cohort). 41 None of the included studies used other index tests.
The nine studies included a total of 40,079 relatives, of whom 14,310 were diagnosed with FH. The number of index cases was 964 across five studies;37,55,84–86 this could not be determined for the remaining four studies. 41,50,82,83 Three studies enrolled only adults (mean age 40 years);55,82,83 one enrolled only children (mean age 13 years);37 two studies enrolled both adults and children,41,84 and three studies did not report the ages of relatives. 50,85,86 The extent of the cascade testing, in terms of the degree of relative, was unclear in the majority of studies (n = 5); however, only first-degree relatives were considered in two studies,37,41 and first- and second-degree relatives were considered in the remaining two studies. 50,83 Eight of the nine studies also collated triglyceride levels. Triglyceride levels among FH individuals were high in one study. 83
The index test used in four studies was LDL-C. 37,41,82,84 A further four studies used TC. 50,55,85 One study assessed both LDL-C and TC as the index test. 83 Starr et al. 77 compared the accuracy of their own age- and sex-specific cut-off points with that of the MEDPED criteria.
The five studies that used LDL-C as a diagnostic criterion reported varying cut-off values. 37,41,82–84 For example, Huijgen et al. 82 reported four statistical models with differing levels of genetic severity in a cohort from the Netherlands. Each analysis used a 90th percentile cut-off point, which is derived from the sample. Starr et al. 77 reported analyses for differing age and sex groups for three different countries (Denmark, the Netherlands and Norway), and presented the results of the MEDPED criteria for comparison. In each cohort, cut-off points varied from 3.01 mmol/l for males and 3.32 mmol/l for females (aged 15–24 years) to 4.31 mmol/l for males (aged 45–54 years) and 4.36 mmol/l for females (aged ≥ 55 years). The Netherlands and Norway cohorts included patients aged 0–14 years, which had cut-off points of 3.11 mmol/l and 3.37 mmol/l for males and females, respectively. 77 Similarly, Truong et al. 84 used different cut-off points for Vietnamese children (3.0 mmol/l) and adults (4.1 mmol/l). The same cut-off point was used for adults of Japanese origin. 83 The final study was conducted only with children, with a cut-off point of 3.5 mmol/l. 37
Five studies used TC as diagnostic criterion. 50,55,83,85,86 There were variations in cut-off values; however, one study, using a Norwegian cohort, did not report the cut-off values used. 55 In a Japanese cohort, a cut-off point of 5.8 mmol/l was assessed. 83 In a South African cohort, a cut-off point of 4.8 mmol/l (80th percentile of sample) was used. 86 The other two studies analysed the 90th and 95th percentiles of the samples, derived from Netherlands and Iceland cohorts, respectively. 50,85
Risk-of-bias overview
Regarding risk of bias, all studies had a low risk of bias for flow and timing; however, only four studies had a low risk of bias regarding the index test,50,55,82,86 whereas the remaining five studies were at either a high risk of bias37,83–85 or an unclear risk of bias41 owing to how the index test was conducted and interpreted. More specifically, in three of these studies, the threshold for the index test was not prespecified. 37,83,84 With regard to the reference standard, the majority of studies had a low risk of bias; however, one study had a high risk of bias related to the interpretation of the index test, because of a lack of prespecified thresholds and a lack of information regarding index test interpretation. 50 For patient selection, a low risk of bias was seen for eight studies; however, one study was rated as having a high risk of bias because of the lack of clarity regarding sampling technique, study design and whether or not any inappropriate exclusions took place. 83
Discussion
The aim of this review was to review the current evidence of the diagnostic accuracy of clinical and biochemical characteristics in identifying relatives with FH, compared with genetic identification. We found nine studies, all of which examined LDL-C and/or TC levels to diagnose FH among relatives of index cases, with one study assessing the diagnostic accuracy of both. 83 The studies varied in terms of geographical location, relatives’ ages, cut-off points (for LDL-C and TC levels) for diagnosis and quality. The small number of studies, coupled with substantial heterogeneity, means that clinical utility of these biochemical tests could not be determined.
Conclusion
Our systematic review on clinical and biochemical characteristics to diagnose FH among relatives of known index cases found nine studies on LDL-C and/or TC. However, the evidence on diagnostic accuracy and appropriate cut-off points for diagnosis was poor, owing to the paucity of studies and high heterogeneity, which we could not investigate quantitatively.
Summary of results and study limitations
The systematic review of the effectiveness of cascade-testing protocols among relatives was limited to four studies of limited quality. The combination approach, which allows the index case to decide how their relatives are contacted, appeared to result in a higher proportion of relatives tested than the direct or indirect approaches, which had similar yields. It was also noted in this study that only 26% of index cases had a definite diagnosis of FH, with the remaining having a probable diagnosis. 41 The UK study using a direct approach was a randomised controlled trial that compared routine clinical diagnosis plus genetic testing with routine clinical diagnosis alone among index cases and their relatives. 45 Therefore, the study design may have had an impact due to it being recruitment to a trial, whereas the participants recruited to the UK study using the indirect approach was part of a cascade-testing programme,44 and therefore probably more generalisable.
The limitations of this and a previous review26 predominantly related to the nature of the studies available. There were no within-study comparisons; therefore, we had to rely on comparing strategies across studies. Hence the differences in yield between the cascade-testing strategies were wholly ascribed to the contact method used. Only approximately half of the included studies reported the extent of cascading to other relatives; therefore, we were unable to explore whether there were differences in yields by cascade approach related to extent of cascading to other relatives. Owing to the limited evidence, particularly the lack of within-study comparison of modalities of contact, the data were not in a format that could be used to parameterise the economic model. However, the Welsh PASS data provided within-study comparison of the impact of the modality of contact (see Chapter 5, Service data analysis).
Although the search of relevant systematic reviews of the effectiveness of cholesterol-lowering therapies on LDL-C levels and CVD among adults identified 14 relevant systematic reviews, none of these met the methodological quality on the AMSTAR critical appraisal tool to be included in the review of reviews.
For the systematic review of the effectiveness of LLTs on LDL-C levels, and the impact of LLTs on LDL-C levels, CVD and mortality among children with FH, a preliminary scoping review identified two recent systematic reviews. 19,20 In particular, Lozano et al. 20 had indicated that there were no studies looking at the (long-term) relationship between LLT in children on CVD events in adulthood or at the association between intermediate outcomes in childhood (e.g. lipid concentrations, atherosclerosis) and CVD events in adulthood. We concluded, owing to the nature of these studies, that such data would not be available.
Finally, the systematic review evaluating the diagnostic accuracy of clinical and biochemical criteria and scoring systems for the diagnosis of FH among relatives found nine studies, all of which examined LDL-C and/or TC levels to diagnose FH among relatives of affected index cases, with one study assessing the diagnostic accuracy of both. The studies varied in terms of geographical location, relatives’ ages, cut-off points (i.e. LDL-C and TC levels) for diagnosis, and quality. The small number of studies, coupled with substantial heterogeneity, means that clinical utility of these biochemical tests could not be determined. However, the search included studies indicating that the Dutch national cascade screening programme for FH had relevant data for our research. 50 Therefore, we contacted the data controllers for this programme, who kindly provided aggregate data on the distributions of LDL-C levels among relatives tested for FH in the Dutch cascade screening programme, which we used to inform the cost-effectiveness analysis.
Chapter 3 Overview of database studies
Several databases were interrogated to identify treatment patterns among FH patients, and their outcomes. This includes a FH primary care data set (CPRD) and the Simon Broome FH specialist register. Both linked to secondary care HES data and national mortality data. Furthermore, yield of cases with FH cascade-testing approaches was to be identified through routine data captured from the Scottish, Welsh and Wessex FH services.
Clinical Practice Research Datalink
The CPRD is a large electronic database of UK patients’ anonymised primary care data. There are data from > 20 million patient lives, with > 5 million patients currently registered and active. It includes information on patient characteristics, clinical diagnoses, symptoms, laboratory test results, medication prescriptions and referrals. 88 Access to the data and ethics approval were granted by the CPRD Independent Scientific Advisory Committee (protocol numbers 16_191R2, 18_143).
From CPRD GOLD, we identified all adults with FH in primary care, including those with documented FH diagnosis, and those with the clinical phenotype of definite FH using the Simon Broome or DLCN criteria. 8 Patients had at least one cholesterol measurement (TC or LDL-C) during the study period (1 January 1999 to 22 July 2016). For each patient with a clinical diagnosis of FH, we randomly identified three patients without FH and individually matched on age, sex and general practice. Those without FH (controls) had cholesterol testing done within 6 months of the start date of their matched FH case and no documented diagnosis of FH. All patients had to be registered in their general practice for at least 1 year before the start of follow-up and they were followed up until their first diagnosis of any cardiovascular outcome. Patients who did not develop CVD were followed up until date of death, transfer out of the practice or study end date, whichever occurred first.
There were 3,936,934 patients in the CPRD with records of either a TC or LDL-C measurement between 1 January 1999 and 22 July 2016. Of these, 14,097 patients had clinical FH, comprising 5152 with documented diagnosis of FH in the electronic health records and 8945 patients who had no documented FH diagnosis but had the clinical phenotype of FH based on the Simon Broome or DLCN diagnostic criteria for definite FH. Of the 5152 patients with a documented diagnosis of FH, 3182 had an eligible linkage to HES. For the epidemiological survival analysis, we utilised all patients regardless of linkage eligibility to HES (5152 documented diagnoses and 8945 patients who had a clinical phenotype and no documented diagnosis). 89,90
Simon Broome familial hypercholesterolaemia disease register
The Simon Broome FH Register comprises 3553 individuals with FH, recruited between 1 January 1980 and 20 December 2010, from 21 participating lipid clinics. Patients were invited to the registry after being referred by either their GPs or hospital specialists. The lipid clinics participating in the Simon Broome Register were in Glasgow, Manchester, Oxford and London.
Information recorded on registration to the Simon Broome Register included demographic and clinical characteristics, such as age, smoking status, alcohol consumption, past medical history, use of lipid-lowering, antihypertensive and diabetic treatments, and family history, and clinical examination findings, such as blood pressure, body mass index (BMI), tendon xanthomas, xanthelasma and arcus cornealis. 90 A fasting blood specimen taken at the registration visit determined serum TC, triglycerides and high-density lipoprotein. 6,88 Serum LDL-C concentrations were calculated using the Friedewald equation. 6 A diagnosis of definite FH was made if (1) TC concentration (either pre treatment or highest on treatment) was > 7.5 mmol/l in adults aged > 16 years or the LDL-C concentration was > 4.9 mmol/l, plus (2) the patient or a first- or second-degree relative had tendon xanthomas. A possible diagnosis of FH required (1) above, plus one of the following: family history of myocardial infarction before age 50 years in second-degree relative or before age 60 years in first-degree relative, or a family history of raised TC concentration above 7.5 mmol/l in a first- or second-degree relative. The presence of tendon xanthoma was determined by examination and palpation of the dorsum of the hands, elbows, pretibial tuberosities, dorsum of the feet and Achilles tendons by the physicians in the participating clinics. 6,88,91 Patients registered in the Simon Broome Register were linked with the NHS Central Register, which is part of the ONS, for ascertainment of death records including underlying cause and date of death, coded using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10). Because of the historical nature of the register, genetic testing for FH had been carried out among only the most recent recruits, with a mutation documented in 570 patients.
Hospital Episode Statistics and Office for National Statistics mortality statistics
The HES contain details on admissions, accident and emergency (A&E) attendances and outpatient appointments at NHS hospitals in England. HES data cover all NHS Clinical Commissioning Groups (CCGs) in England and contain a wide range of information about individual patients, including clinical information about diagnoses and operations, patient information (age, sex, ethnicity), administrative data (dates, methods of admission, discharge) and geographical information (where patients are treated and the area in which they reside).
To explore long-term mortality and morbidity outcomes and effects of treatment, the two data sets (i.e. CPRD and Simon Broome Register) were subsequently linked to three HES data sets (HES Admitted Patient Care, HES Outpatient Care and HES A&E). In total, linkage to HES data sets was possible for 3182 CPRD patients with a clinical diagnosis of FH, and 2997 participants from the Simon Broome Register had a linked record to at least one of the HES data sets. HES Admitted Patient Care covered from March 1997 to April 2018, HES Outpatient Care covered from April 2003 to April 2018 and HES A&E covered from April 2007 to April 2018.
Linkages to HES and to ONS mortality records were approved by NHS Digital (Data Access Request Service reference number: NIC-115405) and the Confidentiality Advisory Group (reference number: 18/CAG/0007).
PASS Wales and Wessex Cascade Service Data
The Wales PASS database comprised 2618 index cases and 1205 relatives who have undergone FH genetic testing. These are patients who have been tested as part of the All Wales FH Service, which commenced in 2010. The data are held within NHS Wales and hosted by Cardiff and Vale University Health Board. PASS data were anonymised by the PASS data guardian (collaborator KH). Patients are from all health boards in Wales and those across English–Welsh border.
The English PASS database contains the Wessex data. These data comprise 1116 index cases and 501 relatives who have undergone FH genetic testing. The University Hospital Southampton NHS Foundation Trust is the data controller for the Wessex PASS data. The Wessex service covered the following CCGs: Fareham and Gosport, Isle of Wight, North and West Reading, North East Hampshire and Farnham, Newbury and District, North Hampshire, Portsmouth, South Eastern Hampshire, South Reading, Southampton, West Hampshire, and Wokingham.
In both the Wales and the Wessex services, index patients are entered into PASS when a referral is received for genetic testing by a FH nurse. This means that both data sets are made up of index patients with pathogenic variants, variants of uncertain significance (VUSs) and patients with no variants identified.
In both services, relatives were added to the pedigrees of index cases where a pathogenic variant is identified. In Wessex, the approach to contacting relatives for cascade testing is indirect, and in Wales the index patient is given a choice of either the direct or the indirect approach. When a relative is referred to cascade testing following direct contact or indirect contact via a letter, they are offered an appointment for genetic testing.
In Wales, segregation testing is offered to families when a VUS is identified in the index patient. This means that, in Wales, the relative data set also includes relatives’ VUS status. Segregation testing has resulted in the VUS of many families being reclassified to either pathogenic or benign. In Wessex, no segregation testing is offered to such families.
Prior to the study commencing, the level of completeness of the PASS clinical data was low. The study funded researchers to manually populate the clinical data both retrospectively and prospectively.
Quality control for mandatory data in PASS was managed by analysing data extractions for missing data. The study data were checked and validated before transferring to researchers, specifically the Welsh DLCN criteria score as it was found to be inaccurate sometimes.
A legacy of the study is that data entry for PASS has continued at a high level, with the FH nurses and FH administrative staff now populating all clinical and genetic data in PASS prospectively. Furthermore, as part of the study, a data extraction script was developed by Kate Haralambos and PASS software so that all the relevant data fields could be extracted in an anonymous format. This script, which took over 1 year to finalise, was vital for the study, but has also been found to be invaluable for future data extractions from PASS as part of evaluation of clinical care.
Scottish familial hypercholesterolaemia service data
The Scottish Lipid Forum, an informal network of 40 health-care professionals from across Scotland who are responsible for the diagnosis of FH and for the provision of care to FH patients (lipidologists, clinical geneticists, genetics counsellors, genetics and biochemistry laboratory scientists) created a sustainable, informally funded genetics cascade-testing programme for FH families in late 2008. Index cases were ascertained by secondary and primary care clinicians largely by the existing network of lipidologists from across Scotland using the Simon Broome criteria. Genetic testing of potential index cases is performed by the genetics laboratory funded by the Aberdeen NHS National Services Division.
NHS Grampian hosts the genetic database for FH screening and gene testing, Genetics Laboratory Information Management Systems (LIMS) funded by NHS Scotland National Services Division. The data cover from 1995 to the first quarter of 2016. Test numbers were low prior to 2009. They then increased by about 250 per year to 2012, and were constant, at just below 1000 tests per year, from 2012 to 2015.
The secure de-identified NHS Safe Haven data linkage system was used to link the NHS Grampian LIMS FH database to NHS Scotland routine electronic data sets, such as National Records of Scotland for births and deaths, Prescribing Information System for dispensed prescriptions and Scottish Morbidity Records/hospital discharge data for cardiovascular outcomes. This was done by the indexing team at NHS National Services Scotland using a probabilistic approach based on Community Health Index numbers (unique patient identifiers). The study period was from 1981 to 2016.
Data access is via the Grampian Data Safe Haven, as the Scottish LIMS FH database and linked data are held by NHS Grampian and the Safe Haven. To comply with information governance procedures and preserve patients’ confidentiality, the Organisation for Economic Co-operation and Development (OECD) guidelines on human biobanks and genetic research databases are followed. The electronic Data Research and Innovation Service (eDRIS) provides pseudo-anonymised data extracts for researchers. Personal identifying information is removed and Community Health Index numbers are replaced by unique study numbers. The security of this process is maintained by different teams handling patient identifiers, study variables and the linkage. 92
This routine LIMS data set, which comprises partial dates of birth and death, sex, reason for test, VUS and mutation, along with linked national data sets, forms the baseline data set. This was further merged with NHS Grampian biochemistry data set (study period 1981–2016), which comprises all cholesterol measurements such as TC, high-density lipoprotein cholesterol, LDL-C and triglycerides, and test date. The total number of patients represented in the baseline data is made up of 4282 index cases and 1065 cascade cases. The data set has been updated since 2016.
Conclusion
The most comprehensive data sets for the cost-effectiveness analysis were the CPRD data set linked to HES and mortality data and the routine data set from the Wales and Wessex FH services, housed on the PASS server. The linked Simon Broome FH Register was also accessible for epidemiological analysis. As mentioned in Chapter 4, Summary of results and limitations, although the Scottish FH data were extracted from the safe haven, this data set did not provide appropriate data to parameterise the economic model. There was very limited recording of the ethnic origin of the FH patients in any of these data sets.
Chapter 4 Epidemiological analysis of longitudinal databases
Parts of this chapter are reproduced with permission from Iyen et al. 89 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/.
Parts of this chapter have also been reproduced with permission from Iyen et al. 90 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/.
Risk of cardiovascular disease among those with familial hypercholesterolaemia, from primary care records
Context
Although the association of FH with premature CHD is well known, the risk of atherosclerotic disease in other vascular regions among patients with FH is less clear, and evidence from previous studies is conflicting. 6,93–95 The work reported in this chapter sought to determine the CVD risk profile of patients with FH in the general population using longitudinal data from patients’ primary care electronic health records. We assessed the incidence and risks of CHD, stroke/transient ischaemic attacks (TIAs) and peripheral vascular disease (PVD) among patients with clinical diagnoses of FH identified in primary care.
Research objective
The objective was to determine the CVD risk profile of patients with FH in the general population.
Methodology
We conducted a retrospective matched cohort study using data from the CPRD. For each patient with a clinical diagnosis of FH, we randomly identified three patients without FH and individually matched on age, sex and general practice. Those without FH had cholesterol testing done within 6 months of the start date of their matched FH case, no documented diagnosis of FH and no pre-existing CVD. Patients who had disease (Read) codes suggesting pre-existing CVD (CHD, stroke, TIA or PVD) prior to study entry were excluded.
Outcomes
Incident CVD was defined as any new clinical diagnosis of CHD, stroke/TIA or PVD. These were identified from patients’ primary care electronic health records during the study period, as were mortality and date of death. Disease codes used for CVD are shown Appendix 4.
Sample size calculation
Based on findings from previous research,96 we estimated a minimum hazard ratio (HR) of 1.2 for overall atherosclerotic CVD risk. To achieve this, a minimum total cohort size of 9450 individuals was required, with an expectation of 1265 CVD events at 90% power and significance level of 0.05 (two-sided test of significance).
Statistical analysis
Baseline descriptive analyses were performed for all patients in the cohort; results are represented as numbers and percentages, means and standard deviations (SDs), and medians and interquartile ranges (IQRs) for categorical, normal continuous and non-normal continuous variables, respectively. Appropriate statistical tests, such as chi-squared tests, t-tests and analysis of variance tests, were used to assess differences between the groups of interest. The incidence rates of CVD were determined for FH and non-FH groups, presented per 1000 person-years at risk. Cox proportional hazards regression was used to derive HRs for the first onset of any CVD and, secondarily, HRs for the various major CVD subtypes (CHD, stroke/TIA and PVD) for patients with and patients without FH. Analyses were stratified on the matched variables and adjusted for individuals’ demographics, lifestyle factors, comorbidities and prescribed medication use, as listed previously. Confounder selection used the change-in-estimate criteria,97 and any covariate that changed the effect size of the univariate exposure–outcome model by 10% was considered an important confounder and included in the fully adjusted model. Statistical tests of the proportional hazards assumption found the Cox proportional hazards regression to be suitable for the analyses. For patients with missing or unrecorded categorical clinical variables, the common assumption was made that these individuals did not have the condition. Multiple imputation techniques98 were used to substitute missing lifestyle data when necessary. All analyses were performed using Stata SE15.
Results
There were 3,936,934 patients in the CPRD with records of either a TC or LDL-C measurement between 1 January 1999 and 22 July 2016. Of these, 14,097 patients had clinical FH and no prior history of CVD at baseline. This comprised 5152 patients with documented diagnosis of FH in the electronic health record, and 8945 patients who had no documented FH diagnosis but had the clinical phenotype of FH based on the Simon Broome or DLCN diagnostic criteria for definite FH. Of these identified patients, 53.3% were females, the mean age at the start of follow-up was 42 years and the mean BMI was 27.3 kg/m2. FH patients were matched with 42,506 non-FH patients. As individuals with and those without FH were matched on age and sex, the distribution of these characteristics were similar across both groups. The median follow-up time for both FH and non-FH patients was 13.8 years (IQR 8.4–17.7 years), and the average follow-up times for patients with and patients without FH were 174,950 and 588,470 person-years, respectively.
The baseline demographic and clinical characteristics of patients with and patients without FH are shown in Table 2. As expected, a significantly higher proportion of FH patients had a family history of premature CHD than non-FH patients (5.1% vs. 2.7%, respectively; p < 0.001), and more FH than non-FH patients were on lipid-lowering medication at the start of the study (19.1% vs. 4.7%, respectively; p < 0.001). Ethnicity records were missing for 85% of patients; for those for whom ethnicity was recorded, 80% were white. BMI records were available for 40% of patients, so multiple imputation with chained equations was used to estimate missing BMI values.
| Risk factor variable | FH patients (n = 14,097) | Non-FH patients (n = 42,506) | p-value |
|---|---|---|---|
| Age (years) at start of study, mean (SD) | 42.5 (11.7) | 41.6 (12.5) | |
| Sex, n (%) | |||
| Male | 6578 (46.7) | 19,843 (46.7) | |
| Female | 7519 (53.3) | 22,663 (53.3) | |
| Follow-up (years), median (IQR) | 12.4 (7.1–16.8) | 14.2 (8.8–17.8) | |
| BMI (kg/m2), mean (SD) | 27.8 (5.4) | 26.2 (5.6) | < 0.001 |
| Alcohol misuse,a n (%) | 265 (1.9) | 712 (1.7) | 0.106 |
| Ever-smoked recordb (yes/no), n (%) | 11,518 (81.7) | 34,395 (80.9) | 0.038 |
| Hypertension, n (%) | 783 (5.6) | 2393 (5.6) | 0.736 |
| Atrial fibrillation, n (%) | 35 (0.3) | 120 (0.3) | 0.503 |
| Chronic kidney disease, n (%) | 57 (0.4) | 127 (0.3) | 0.056 |
| Type 1 diabetes, n (%) | 60 (0.4) | 247 (0.6) | 0.029 |
| Type 2 diabetes, n (%) | 311 (2.2) | 726 (1.7) | < 0.001 |
| Overweight/obesity, n (%) | 525 (3.7) | 1307 (3.1) | < 0.001 |
| Rheumatoid arthritis and/or other inflammatory diseases, n (%) | 100 (0.7) | 285 (0.7) | 0.626 |
| Family history of CHD, n (%) | 712 (5.1) | 1156 (2.7) | < 0.001 |
| HIV, n (%) | 6 (0.04) | 16 (0.04) | 0.797 |
| Antipsychotic use, n (%) | 491 (3.5) | 1165 (2.7) | < 0.001 |
| Oral corticosteroids, n (%) | 313 (2.2) | 920 (2.2) | 0.693 |
| Immunosuppressant drugs, n (%) | 315 (2.2) | 926 (2.2) | 0.694 |
| LLT,c n (%) | 2692 (19.1) | 2007 (4.7) | < 0.001 |
| Lipid profiled (mmol/l) | |||
| TC, mean (SD) | 9.30 (2.6) | 5.98 (1.6) | |
| LDL-C, mean (SD) | 5.72 (2.1) | 3.63 (1.1) | |
| Triglyceride, median (IQR) | 2.10 (1.3–3.5) | 1.30 (0.9–1.9) | |
| Statin potency, n (%) | |||
| Low | 57 (0.4) | 49 (0.1) | |
| Medium | 466 (3.3) | 384 (0.9) | |
| High | 447 (3.2) | 145 (0.3) | |
| Non-statin lipid-lowering medication, n (%) | 1722 (12.2) | 1429 (3.4) | |
For patients on statins or other cholesterol-lowering medication with known potency, the corrected levels of TC were estimated from observed levels, based on estimated percentage reduction in LDL-C with statins of different potencies. 99 As expected, the mean cholesterol concentration was significantly higher among those with FH [9.30 mmol/l (SD 0.02 mmol/l)] than among those without FH [5.98 mmol/l (SD 0.01 mmol/l)]. The median triglyceride concentrations in the FH and non-FH cohorts were 2.10 mmol/l (IQR 1.34–3.52 mmol/l) and 1.30 mmol/l (IQR 0.90–1.92 mmol/l), respectively. See Appendix 2 and Figure 18.
Cardiovascular disease outcomes
There was a total of 6202 incident cases of CVD (CHD, stroke, TIA or PVD) during the period of follow-up. These were identified in 31.7% of individuals with FH and in 4.1% of non-FH individuals. Comparing baseline characteristics of FH and non-FH patients, hypertension and atrial fibrillation were more prevalent among FH patients who developed CVD than among non-FH patients who developed CVD. Although more FH than non-FH patients with CVD had a history of smoking, the differences in prevalence of chronic kidney disease and type 2 diabetes were not statistically significant.
Overall, the incidence rates of CVD among FH and non-FH individuals (per 1000 person-years at risk) were 25.6 and 2.9, respectively. The incidence rate among FH individuals, compared with non-FH individuals, was highest for CHD (incidence rate 20.3 vs. 2.0, respectively), with incidence rates of stroke/TIA and PVD also higher among those with FH than among non-FH individuals (see Appendix 2 and Table 27). The overall mean age at first diagnosis of CHD, stroke/TIA and PVD were 53.3 years, 56 years and 55.5 years, respectively. CVD outcomes were diagnosed approximately 10 years earlier among those with FH than among those without FH.
Table 3 shows the number of CVD events and HRs for all CVD subtypes among FH and non-FH patients. FH patients were more likely to have incident CVD events than non-FH patients (HR 9.14, 95% CI 8.55 to 9.76, p < 0.001). This comprised a higher risk of incident CHD (HR 10.63, 95% CI 9.82 to 11.49, p < 0.001), stroke/TIA (HR 6.74, 95% CI 5.84 to 7.77, p < 0.001) and PVD (HR 7.17, 95% CI 6.08 to 8.46, p < 0.001).
| CVD outcome | Individuals, n (%) | HRa for CVD (95% CI) | ||
|---|---|---|---|---|
| Total (N = 56,603) | FH (n = 14,097) | Non-FH (n = 42,506) | ||
| All CVD outcomes | 6202 (11.0) | 4474 (31.7) | 1728 (4.1) | 9.14 (8.55 to 9.76) |
| CHD | 4718 (8.3) | 3545 (25.2) | 1173 (2.8) | 10.63 (9.82 to 11.49) |
| Stroke/TIA | 1169 (2.1) | 764 (5.4) | 405 (1.0) | 6.74 (5.84 to 7.77) |
| PVD | 887 (1.6) | 592 (4.2) | 295 (0.7) | 7.17 (6.08 to 8.46) |
Adjustment for demographic factors, clinical covariates and the effect of the 2008 NICE guidelines resulted in no major change in these HRs (< 10% change-in-estimate criteria). Stratifying the analysis between FH and various CVD outcomes by sex demonstrated a statistically significant increase in risk of the different cardiovascular end points within the different sexes (p < 0.001 for all end points among males and females). Compared with patients of the same sex, the increased CVD risk among males with FH was markedly higher than the risk increase among females with FH (see Appendix 2 and Figure 19).
Conclusion
Individuals with FH have been shown to have greatly increased risk of a range of cardiovascular outcomes, including not only CHD, but also stroke, TIA and PVD. This has important clinical implications and emphasises the need for improved case identification of clinically recognisable FH in the general population for targeted preventative intervention. The findings also suggest incorporating a broader range of cardiovascular outcomes for economic modelling.
Risk of cardiovascular disease among those with familial hypercholesterolaemia, from secondary care records
Context
Historically, the Simon Broome Register has been linked to ONS data for ascertainment of annual death records, which have provided outcomes related to annual mortality rates (up to 2016). However, much of the participant journey through their life course is missing, including outcomes related to disease morbidity, treatments, procedures and operations, and health resource use. These outcomes have not been previously assessed.
Research objective
The objective was to evaluate the long-term cardiovascular outcomes of individuals with FH.
Methodology
A total of 3553 people in the Simon Broome Register were recruited from participating lipid clinics between 1 January 1980 and 20 December 2010. Of these, 2988 (84%) had linked HES Admitted Patient Care records; these comprised the final study cohort. Individuals without linked HES records had comparable baseline demographic characteristics to those with linked data, but a higher proportion of them had a record of previous history of CVD.
Outcomes
Incident CVD was defined as the first hospital admission recorded in the HES for CHD, myocardial infarction, angina (stable or unstable), stroke, TIA, PVD, heart failure or coronary revascularisation interventions such as percutaneous coronary interventions or coronary artery bypass graft. CVD outcomes were identified from HES using the relevant ICD-10 and Office of Population Censuses and Surveys codes.
Statistical analyses
The baseline characteristics of patients in the Simon Broome Register were assessed, and these were reported as proportions, means and SDs, and medians and IQRs for categorical, continuous normally distributed and continuous non-normally distributed variables, respectively. Appropriate statistical tests, such as chi-squared tests, t-tests and Mann–Whitney U-tests, were used to assess differences in categorical and continuous variables, between males and females. Incidence rates of composite CVD outcomes were assessed for all Simon Broome patients, and within predefined patient subgroups, and Cox proportional hazards models estimated HRs for CVD. We determined the observed number of incident CVD events per person-years of follow-up, stratified by sex and age (< 30 years, 30–50 years and > 50 years). Standardised morbidity ratios (SMbRs) were calculated using indirect standardisation, with age- and sex-specific CVD incidence rates of the UK primary care non-FH population as reference rates. 100 We calculated the expected number of CVD events as the number of person-years of follow-up in the Simon Broome cohort multiplied by the incidence rate for the comparable age group and sex in the reference population. SMbRs were computed as the observed number of CVD events divided by the expected number of events:
The 95% CIs of the SMbR were derived using an error factor, with the equations:
The SMbR was estimated for both composite CVD and constituent cardiovascular outcomes. We conducted the primary analyses on all eligible individuals in the Simon Broome Register, with or without a history of CVD. To evaluate the impact of having a previous history of CVD, we conducted sensitivity analyses by restricting the population to a subset of patients who had no history of previous CVD at the time of registration. Considering that secondary care records in HES became available only after 1 April 1997, further sensitivity analyses were done, restricted to only those individuals with registration dates in Simon Broome on or after 1 April 1997. All analyses were conducted using Stata SE version 15.
Results
The characteristics of the study population, at the time of registration on Simon Broome Register are shown in Table 4. Of the cohort, 1418 (47.5%) were male. Compared with men, women were 5 years older at registration and 4.8 years older at time of commencing LLT. Although women had a slightly lower BMI than men, their mean untreated TC concentration was significantly higher, but median triglyceride concentration was significantly lower. Consumption of alcohol was significantly higher among men than women; significantly fewer women reported ever smoking, whereas the prevalence of current smoking was similar among men and women. Fewer women than men reported a prior history of CVD, with significant difference in myocardial infarction, CHD and previous revascularisation, and women had their first myocardial infarction 8 years later than men. Although significantly more women than men had a history of hypertension, the prevalence of type 2 diabetes was similar among men and women. Overall, women on the Simon Broome Register had a better CVD risk factor profile, but a later age at FH diagnosis and commencement of LLT.
| Characteristic | Male [N = 1418 (47.46%)] | Female [N = 1570 (52.54%)] | p-valuea |
|---|---|---|---|
| Age (years) at registration, mean (SD) | 41.1 (15.0) | 46.1 (16.8) | < 0.0001 |
| BMI (kg/m2) at registration, mean (SD) | 25.17 (4.1) | 24.78 (5.2) | 0.0343 |
| Follow-up (years), median (IQR) | 17.93 (11.17–23.98) | 18.15 (11.59–23.73) | 0.5993 |
| FH diagnosis type, n (%) | N = 1418 | N = 1570 | |
| Definite FH | 770 (54.3) | 814 (51.9) | 0.179 |
| Possible FH | 648 (45.7) | 756 (48.1) | |
| Age started on LLT (years), mean (SD) | 37.5 (14.7) | 42.3 (17.0) | < 0.0001 |
| Pre-treatment cholesterol (mmol/l), mean (SD) | 9.4 (2.8) | 9.7 (2.0) | 0.0136 |
| Pre-treatment triglyceride (mmol/l), median (IQR) | 1.8 (1.2–2.7) | 1.4 (1.0–2.2) | < 0.0001 |
| Pre-treatment lipoprotein (a) (mg/dl), median (IQR) | 29 (10–63), n = 314 | 25 (11–70), n = 339 | 0.9927 |
| Alcohol consumption (units/week), median (IQR) | 10 (1–20) | 2 (0–9) | 0.0001 |
| Cigarette smoke exposure, n (%) | |||
| Ever smoked cigarettes (yes) | 638 (45.0), n = 1418 | 605 (38.6), n = 1568 | 0.001 |
| Current cigarette smoker (yes) | 224 (16.0), n = 1404 | 293 (18.8), n = 1556 | 0.116 |
| History of previous CVD, n (%) | |||
| Angina | 250 (17.8), n = 1403 | 226 (14.5), n = 1554 | 0.091 |
| Myocardial infarction | 187 (13.2), n = 1418 | 99 (6.31), n = 1570 | < 0.0001 |
| CHD (yes) | 352 (24.8), n = 1418 | 276 (17.6), n = 1570 | < 0.0001 |
| Stroke (Yes) | 10 (0.7), n = 1404 | 20 (1.3), n = 1558 | 0.173 |
| TIA | 13 (1.3), n = 1027 | 18 (1.5), n = 1168 | 0.254 |
| History of claudication | 38 (2.7), n = 1402 | 49 (3.2, n = 1556 | 0.79 |
| Previous revascularisation | 174 (17.0), n = 1025 | 96 (8.3), n = 1161 | < 0.0001 |
| Age at first myocardial infarction (years), median (IQR) | 43 (37–49) | 51 (44–58.5) | 0.0001 |
| History of hypertension, n (%) | 111 (10.9), n = 1021 | 196 (16.9), n = 1162 | < 0.0001 |
| History of diabetes, n (%) | 20 (1.4), n = 1418 | 19 (1.2), n = 1570 | 0.718 |
| Use of other medication | |||
| Beta-blockers | 117 (11.4), n = 1028 | 148 (12.7), n = 1168 | 0.644 |
| ACE inhibitors | 39 (6.6), n = 587 | 54 (7.8), n = 697 | 0.61 |
| Antiplatelet medication | 257 (18.1), n = 1418 | 234 (14.9), n = 1570 | 0.01 |
| Anticoagulant medication | 9 (1.5), n = 587 | 10 (1.4), n = 697 | 0.83 |
| Other antihypertensive medication | 49 (4.8), n = 1027 | 74 (6.3), n = 1168 | 0.274 |
| Type of FH mutation, n (%) | N = 295 | N = 304 | |
| LDLR | 178 (60.3) | 193 (63.5) | 0.416 |
| APOB | 10 (3.4) | 13 (4.3) | |
| PCSK9 | 4 (1.4) | 1 (0.3) | |
| None | 103 (34.9) | 97 (31.9) | |
Cardiovascular disease outcomes
Admitted Patient Care records from HES were available from April 1997 to March 2018. The median follow-up time for patients in the Simon Broome Register was 18.1 years (IQR 11.4–23.9 years), constituting 52,000 person-years of follow-up. Over this period, there were 1327 CVD-related hospital admissions. The overall incidence rate for any CVD event among the Simon Broome patients was 25.47 (95% CI 24.14 to 26.88) per 1000 person-years of follow-up. Incidence rates were lower among women, and, compared with men, women had an adjusted HR of 0.65 (95% CI 0.58 to 0.73). As expected, incidence rates and HRs for CVD increased steeply with increasing age, with incidence rates ranging from 6.31 (95% CI 5.12 to 7.77) among those aged < 30 years at registration to 77.35 (95% CI 59.67 to 100.28) among those aged > 70 years. As expected, the CVD incidence rate was 4.5-fold higher among those with a previous history of CVD on registration, with an age- and sex-adjusted HR of 3.45 (95% CI 3.06 to 3.89).
Table 5 shows the observed number of CVD events across different age groups among men and women in the Simon Broome Register, and the number of CVD events that would be expected if these individuals had the same age- and sex-specific CVD incidence rates as the general practice population of individuals without FH. The overall SMbR among individuals with FH in the Simon Broome Register was 7.17 (95% CI 6.79 to 7.56). For both sexes, the SMbR decreased with advancing age such that the highest excess CVD morbidity was among those aged < 30 years, and the lowest was among those aged > 50 years. Women with FH were observed to have larger excess CVD morbidity than men [7.55 (95% CI 6.99 to 8.15) vs. 6.83 (95% CI 6.33 to 7.37), respectively]. There were substantially significant sex differences in SMbR among patients aged 30–50 years and aged > 50 years, as demonstrated in Appendix 2 and Figure 20. These differences were most marked in the group of people aged 30–50 years, such that women had a 50% higher SMbR than men of the same age group [15.04 (95% CI 12.98 to 17.42) vs. 10.03 (95% CI 9.01 to 11.17), respectively]. Among those aged > 50 years, the SMbR was 33% higher for women than for men [6.11 (95% CI 5.57 to 6.70) vs. 4.59 (95% CI 4.08 to 5.15), respectively].
| Sex and age group | Person-years of follow-up | Observed CVD events (n) | Incidence rates per 1000 person-years (95% CI) | Expected CVD eventsa (n) | SMbR (95% CI) |
|---|---|---|---|---|---|
| Males | |||||
| < 30 years | 6942 | 56 | 8.07 (6.21 to 10.48) | 3.47 | 16.13 (12.42 to 20.96) |
| 30–50 years | 12,174 | 331 | 27.19 (24.41 to 30.28) | 32.99 | 10.03 (9.01 to 11.17) |
| > 50 years | 5510 | 282 | 51.18 (45.54 to 57.51) | 61.49 | 4.59 (4.08 to 5.15) |
| Total | 24,627 | 669 | 27.17 (25.18 to 29.30) | 97.96 | 6.83 (6.33 to 7.37) |
| Females | |||||
| < 30 years | 7157 | 33 | 4.61 (3.28 to 6.49) | 2.15 | 15.37 (10.93 to 21.62) |
| 30–50 years | 9546 | 178 | 18.65 (16.10 to 21.60) | 11.84 | 15.04 (12.98 to 17.42) |
| > 50 years | 10,765 | 447 | 41.52 (37.85 to 45.56) | 73.2 | 6.11 (5.57 to 6.70) |
| Total | 27,468 | 658 | 23.96 (22.19 to 25.86) | 87.18 | 7.55 (6.99 to 8.15) |
| Overall | 52,094 | 1327 | 25.47 (24.14 to 26.88) | 185.14 | 7.17 (6.79 to 7.56) |
When the different CVD subtypes were analysed separately, the SMbRs for all subtypes were found to be higher for women than for men. The SMbR for CHD was substantially higher for women than men aged 30–50 years [19.66 (95% CI 16.78 to 23.04) vs. 12.54 (95% CI 11.22 to 14.01), respectively] and aged > 50 years [7.65 (95% CI 6.90 to 8.48) vs. 5.82 (95% CI 5.14 to 6.59), respectively]. Similarly, the SMbR for PVD was higher for women than men aged 30–50 years [16.16 (95% CI 11.85 to 22.03) vs. 8.18 (95% CI 6.26 to 10.68), respectively] and aged > 50 years [8.44 (95% CI 7.02 to 10.14) vs. 4.67 (95% CI 3.68 to 5.93), respectively]. A higher SMbR for stroke was observed for women than for men, but this was only among those aged > 50 years [5.66 (95% CI 4.78 to 6.69) vs. 2.83 (95% CI 2.17 to 3.69), respectively]. In all CVD subtypes, the SMbR for men and women with FH did not differ markedly in those aged < 30 years.
Conclusion
This study finding of significantly higher risk of CVD in all age groups of patients in this registry-based cohort, compared with the general population, emphasises the importance of early diagnosis and treatment of FH. Our study provides confirmatory evidence of higher excess CVD morbidity in younger age groups of patients with FH and, importantly, provides novel insight into sex differences in the diagnosis and management of FH, as well as substantial sex disparities in the excess CVD burden associated with FH. We noted in these patients, cared for in a specialist setting, that excess CVD morbidity is markedly higher among women than men aged 30–50 years and aged > 50 years; this highlights the need for optimisation of lipid lowering and risk factor management for all FH patients, with particular attention to women with FH.
Summary of results and limitations
Both the primary care data set with clinically coded FH and the specialist FH register reconfirmed higher rates of CVD among FH patients, demonstrated by increased incidence rates in the primary care data set and raised SMbRs in the specialist register. Both data sets benefited from linkage to HES data.
The CPRD primary care data set might not have correctly coded all individuals with FH, and the cohort might not be representative of the whole UK FH population.
Furthermore, the analysis of the FH specialist register demonstrated higher risk of CVD in all age groups of patients in this registry-based cohort than in the general population, emphasising the importance of early diagnosis and treatment of FH. The finding, in the specialist setting, where treatment should be optimised, that excess CVD morbidity is markedly higher among women than men aged 30–50 years and aged > 50 years highlights the need for further optimisation of lipid-lowering and risk factor management for all FH patients, with particular attention to women with FH. The limited numbers of patients from ethnic minority backgrounds on this register is also noted.
Chapter 5 Economic evaluation
Overview of the cost-effectiveness workstream
The aim of this workstream was to develop a cost-effectiveness model to evaluate alternative cascade-testing protocols. This cost-effectiveness modelling is reported in Cost-effectiveness of alternative cascade-testing protocols for familial hypercholesterolaemia. To inform this model, we analysed data from the Wales and the Wessex FH cascade-testing services to characterise index cases and relatives and estimate the yield of the cascade. This is reported in Service data analysis. In addition, we analysed the data from a cohort of patients with FH to estimate their risk of CVD events and the impact of diagnosis and treatment on LDL-C, which is reported in Economic analysis of the Clinical Practice Research Datalink database. These data informed a new cost-effectiveness model that compares the long-term health outcomes and costs of patients with FH depending on whether or not they were diagnosed and treated, which is reported in Cost and health benefits of diagnosis of people with familial hypercholesterolaemia in the long term.
Proposed additional data sources to inform the cost-effectiveness model were the systematic reviews on the cascade yield from contacting relatives directly or indirectly (see Chapter 2, Review 1: effectiveness of cascade-testing protocols among relatives for familial hypercholesterolaemia) and on the accuracy of clinical and biochemical characteristics to diagnose relatives (see Chapter 2, Review 4: the diagnostic accuracy of clinical and biochemical criteria and scoring systems for the diagnosis of familial hypercholesterolaemia among relatives).
Economic analysis of the Clinical Practice Research Datalink database
Background and objectives
A number of important papers have characterised long-term outcomes for FH patients in the UK. However, these studies do not provide an ideal basis for economic modelling for a number of reasons. First, these studies have not always included non-fatal CVD events,6,91,94,98 which are important to include in economic modelling as they have significant cost and quality-of-life implications. Second, these studies have not separated out treated and untreated FH patients,101 which makes it challenging to make inferences about the effect of treatment on outcomes, as required when modelling the benefits of diagnosis and treatment. Finally, these studies have not extrapolated risk beyond the censoring time. These approaches are important as they allow prediction of event risk over time horizons beyond the observed data, and can provide risk predictions according to patients’ characteristics. This work seeks to address these limitations, and provide more robust long-term outcome modelling to support cost-effectiveness analyses in FH.
With this work, we wanted to understand the lifetime consequences of diagnosing and treating individuals with FH in terms of both morbidity and mortality. Contemporary UK primary and secondary care data on long-term outcomes in the FH population reflect a group of individuals who are generally under active treatment with LLTs. Therefore, in this section of the economic evaluation chapter, the focus was on appropriately characterising outcomes among these individuals. The objectives of this section were to address two key questions: (1) what is the impact of diagnosis and treatment on LDL-C levels? and (2) what is the long-term risk of major CVD events and mortality for individuals diagnosed with, and treated for, FH?
In Cost and health benefits of diagnosis of people with familial hypercholesterolaemia in the long term, and Cost-effectiveness of alternative cascade-testing protocols for familial hypercholesterolaemia, these findings are used within a long-term cost-effectiveness model to simulate the counterfactual, that is what would have been the long-term outcomes of these individuals had they not been diagnosed with, and consequently treated for, FH. This, in turn, allows the estimation of the benefits of diagnosis and treatment in terms of long-term CVD risks, health-related quality of life, health resource consumption and costs, and mortality.
In Methods, we introduce the utilised data source and the methods used within to achieve our objectives. Results will present the results of the implemented analysis and, finally, Summary of results and study limitations discusses key findings and important limitations.
Methods
Data sources and study population
Data from the CPRD database, linked to HES and mortality data from the ONS, were used. HES provide information on all hospital admissions and the ONS records cause-specific mortality for all deaths in England and Wales up to 2017. From this longitudinal database, all adults with a recorded diagnosis of FH in their primary care records were identified. Of these, 2879 individuals had an eligible linkage to HES and had received their FH diagnosis after their practice was considered to meet CPRD minimum data quality criteria (see Chapter 3, Clinical Practice Research Datalink). 88 This is recorded within the CPRD as the up-to-quality standard (UTS) date. The UTS date is a practice-based quality metric based on the continuity of recording and the number of recorded deaths. The UTS date is calculated for each participating practice, corresponding to the latest date at which practices meet these minimum quality criteria. LDL-C measurements and corresponding dates were available from the CPRD at multiple time points throughout the follow-up period of these individuals, both pre and post FH diagnosis. Similarly, LLT prescribing was available from the CPRD, with prescribed LLT medication and prescribing dates presented in detail.
Current treatment practices and low-density lipoprotein cholesterol response
We focused on estimating the change from baseline to 24 months in LDL-C level (or the closest measure if not available). This was because the relevant literature had a scheduled treatment duration of at least 2 years,18 and so that post 12 months annual medication review LDL-C measurements were captured. 102 The sample of 2879 individuals were classified according to their treatment status before and after FH diagnosis into three subgroups: (1) the ‘untreated’, individuals not receiving treatment before and for 24 months after diagnosis date; (2) the ‘newly treated’, individuals receiving treatment only after FH diagnosis; and (3) the ‘treatment retainers’, individuals treated both before and after FH diagnosis. With these subgroup definitions, we expected their treatment patterns to differ and, thus, also their outcomes. Individuals treated with LLTs were defined as individuals having at least two prescriptions of any LLT within 2 years after their FH diagnosis date. LLTs prescribed included statins, fibrates, ezetimibe and other lipid-lowering drugs, at a variety of intensities. LDL-C missingness was substantial and was tackled with the Friedewald equation and multiple imputation94,103,104 (see Report Supplementary Material 2 for further details). When multiple imputation was used, all outputs were obtained via Rubin’s rules.
Risk of major cardiovascular disease first events and mortality among treated familial hypercholesterolaemia patients from diagnosis
Model structure
A multistate transition model was developed to capture the natural history of FH patients. The model was centred on non-fatal CVD event occurrence, and both CVD and non-CVD mortality. The structure of the model was determined with reference to previous models,8,105–111 expert clinical advice and data availability. Further model information can be found in Cost and health benefits of diagnosis of people with familial hypercholesterolaemia in the long term, which will explain the conceptual model and the choice of health states in more detail. Key information provided by the CPRD cohort relates to time to first major CVD event and time from non-fatal events to any death from diagnosis. These will now be described.
The focus of this analysis was on the treated subgroups of the data set (i.e. the ‘newly treated’ and the ‘treatment retainers’ individuals, n = 2135) as the long-term outcomes model is primarily aimed at predicting benefits of FH diagnosis and treatment. For this purpose, we required estimates of risk that related to treated or untreated individuals, and the treated group were the majority of our sample. We pooled the ‘newly treated’ and ‘treatment retainers’ groups to improve precision as their treatment patterns and LDL-C reductions were similar and generalisable to the broader FH population. For all survival analysis, censoring occurred at the earliest of CPRD last data collection, CPRD transferred-out date, HES last follow-up date or death from non-CVD causes (applies to time to first major CVD event only).
Time to first major cardiovascular disease event
The primary clinical end point assessed was time from FH diagnosis to first major CVD occurrence after diagnosis, that is non-fatal acute coronary syndrome (ACS) (includes unstable angina, unspecified ACS and myocardial infarction), non-fatal stroke or TIA (stroke/TIA) and CVD death combined as first events. CVD events were defined according to the International Statistical Classification of Diseases and Related Health Problems, Ninth Revision (ICD-9), and ICD-10 codes,101,102,112 and sourced from both CPRD and the HES/ONS linkage. Inclusion of these events reflected their severe nature, and the established effects of LLT on their risk of occurring. Only events appearing as a primary diagnosis were evaluated. Any death occurring within 28 days after a first CVD event was deemed a CVD-related death.
Some evidence was found that indicated that the risk of PVD and stable angina is elevated among FH patients. 108,113,150 However, data from the cohorts showed that PVD and stable angina as first events were relatively rare: each event occurred in ≤ 0.5% of the cohort. Therefore, neither PVD nor stable angina were included in the long-term risk modelling. 114 Heart failure was also not included, as evidence from the literature suggests that, although FH patients are at elevated risk of heart failure, this is predominantly an event that occurs following previous CHD (with/without myocardial infarction events). 114
Parametric survival modelling of time to first major cardiovascular disease event
A fully parametric survival modelling framework was adopted. This framework was chosen as it facilitates extrapolations beyond the time range of the data and allows predictions for individuals with different prognostic characteristics, essential features for the succeeding long-term cost-effectiveness model.
The survival distributions that were tested in terms of model fit to the cohort data were the exponential, the Weibull, the Gompertz, the log-normal, the log-logistic, the generalised gamma115 and the Royston–Parmar spline model. 116 Kaplan–Meier curves were initially produced and CVD events were then modelled from FH diagnosis to produce long-term estimates of event rates and disease progression over time for the FH population.
A range of important prognostic variables for the risk of first major CVD event occurrences were considered for the survival modelling. An assessment of available baseline covariates and potential confounding effects was performed. Baseline covariates were selected according to expert clinical advice and reference to previous models,8,105–111 but were mainly influenced by the structure of the cascade model. We note also that, although the CPRD cohort was relatively large, a small number of CVD events of interest were found, which precluded the inclusion of an extensive set of explanatory variables. These were defined to be sex and baseline age (years), baseline clinical history of CVD (yes/no) and pre-treatment low-density lipoprotein cholesterol (PT-LDL-C) level (in mmol/l), with the closest LDL-C measurements prior to treatment initiation being selected. PT-LDL-C level missingness was substantial and was tackled with the Friedewald equation and multiple imputation103 (see Report Supplementary Material 2 for further details).
As described previously, several survival distributions were tested. Following existing guidance on survival analysis for economic evaluations,117 the suitability of each survival model tested was assessed. The choice of distribution is related to the appropriateness of the model in terms of goodness of fit to the observed data, but also whether or not any temporal extrapolation is plausible. The internal validity of the fitted models was assessed through visual inspection of how well the parametric survival models fitted the observed data, through consideration of hazard rates and cumulative hazard rates, but also through the Akaike information criterion test,118 which weighs up model fit and model complexity. Furthermore, quantile survival predictions were obtained to assess how models addressed cohort CVD risk heterogeneity. The external validity of the fitted parametric models was checked through the use of external data from a 2016 publication by Perak et al.;96 see Report Supplementary Material 2 for further details. To check the plausibility of extrapolations presented by each model, Kaplan–Meier curves were overlaid with the different survival distribution curves and by the external data from Perak et al. 96 A similar approach was taken when looking at 1-year conditional survival. 119
Mortality following non-fatal cardiovascular disease events
Other clinical end points were CVD and non-CVD mortality following a non-fatal CVD event, as defined previously. However, insufficient data to support detailed survival modelling existed for these end points; therefore, Kaplan–Meier curves were produced from each non-fatal event type, but no further parametric modelling was considered appropriate.
All statistical analyses were performed using RStudio v1.4.1103 [Posit, PBC (formerly RStudio, PBC), Boston, MA, USA]; multivariate imputation by chained equations (mice)104 and flexsurv120 were the main packages used. Values of p < 0.05 were considered to be statistically significant.
Results
Clinical Practice Research Datalink baseline characteristics
Familial hypercholesterolaemia diagnosis in the data set ranged from February 1991 to June 2016. Baseline characteristics of the CPRD sample are shown in Table 6, split by the listed subsets, with the ‘untreated’ subgroup accounting for approximately only 26% of the sample. The treated subset of individuals (includes ‘newly treated’ and ‘treatment retainers’) was, on average, older and encompassed a larger proportion of males than the ‘untreated’ subset. The prevalence of comorbidities in the treated subset was higher than among untreated individuals. Over 4% of the treated subset had history of CVD (compared with just 1% in the untreated subset) and approximately 16% presented a family history of CHD (compared with 10% in the untreated subset).
| Baseline characteristicsa | Overall (N = 2879) | Untreated (N = 744) | Newly treated (N = 1291) | Treatment retainers (N = 844) |
|---|---|---|---|---|
| Age (years), mean (SD) | 50.4 (13.8) | 47.8 (13.0) | 50. 5 (13.8) | 52.6 (14.1) |
| Sex (male), n (%) | 1197 (41.6) | 273 (36.7) | 536 (41.5) | 388 (46.0) |
| Ethnicity, n (%) | ||||
| Asian/British Asian | 117 (4.1) | 44 (5.9) | 41 (3.2) | 32 (3.8) |
| Black/British black | 26 (0.9) | 10 (1.3) | 10 (0.8) | 6 (0.7) |
| Mixed | 10 (0.3) | 2 (0.3) | 4 (0.3) | 4 (0.5) |
| White | 2302 (80.0) | 582 (78.2) | 1029 (79.7) | 691 (81.9) |
| Other | 33 (1.1) | 17 (2.3) | 12 (0.9) | 4 (0.5) |
| Unknown | 123 (4.3) | 47 (6.3) | 47 (3.6) | 29 (3.4) |
| Missing | 276 (9.6) | 42 (5.6) | 153 (11.9) | 81 (9.6) |
| Deprivation index, n (%) | ||||
| 1 (least deprived) | 854 (29.7) | 206 (27.7) | 394 (30.5) | 254 (30.1) |
| 2 | 687 (23.9) | 182 (24.5) | 312 (24.2) | 193 (22.9) |
| 3 | 565 (19.6) | 138 (18.5) | 261 (20.2) | 166 (19.7) |
| 4 | 474 (16.5) | 139 (18.7) | 195 (15.1) | 140 (16.6) |
| 5 (most deprived) | 298 (10.4) | 79 (10.6) | 128 (9.9) | 91 (10.8) |
| Missing | 9 (0.3) | 0 (0.0) | 6 (0.5) | 3 (0.4) |
| BMI (kg/m2), mean (SD) | 27.2 (5.0) | 26.4 (5.1) | 27.4 (4.8) | 27.5 (5.1) |
| Smoking status, n (%) | ||||
| Current smoker | 616 (21.4) | 143 (19.2) | 283 (21.9) | 190 (22.5) |
| Ex-smoker | 702 (24.4) | 164 (22.0) | 314 (24.3) | 224 (26.5) |
| Never smoked | 1349 (46.9) | 374 (50.3) | 592 (45.6) | 383 (45.4) |
| Unknown | 22 (0.8) | 9 (1.2) | 6 (0.5) | 7 (0.8) |
| Missing | 198 (6.9) | 54 (7.3) | 101 (7.8) | 43 (5.1) |
| Diabetes type 1 and 2, n (%) | 46 (1.6) | 1 (0.1) | 18 (1.4) | 27 (3.2) |
| Hypertension, n (%) | 340 (11.8) | 40 (5.4) | 151 (11.7) | 149 (17.7) |
| Systolic blood pressure (mmHg), mean (SD) | 130.8 (17.0) | 127.8 (17.5) | 132.0 (17.1) | 131.6 (16.0) |
| Other comorbidities,b n (%) | 98 (3.4) | 13 (1.7) | 41 (3.2) | 44 (5.2) |
| Atrial fibrillation, n (%) | 14 (0.5) | 1 (0.1) | 7 (0.5) | 6 (0.7) |
| History of CVD, n (%) | 102 (3.5) | 8 (1.1) | 23 (1.8) | 71 (8.4) |
| Family history of CHD, n (%) | 410 (14.2) | 74 (9.9) | 186 (14.4) | 150 (17.8) |
| QRISK®2 (ClinRisk Ltd, Leeds, UK) score,c mean (SD) | 8.3 (8.5) | 5.9 (7.1) | 9.1 (8.9) | 9.1 (8.6) |
| Antihypertensive, n (%) | 781 (27.1) | 120 (16.1) | 332 (25.7) | 329 (39.0) |
| Polypharmacy,d n (%) | 392 (13.6) | 77 (10.3) | 188 (14.6) | 127 (15.0) |
Treatment practices and low-density lipoprotein-cholesterol response
After applying the Friedewald equation, LDL-C missingness was substantial (overall, 36.4%; ‘untreated’, 34.6%; ‘newly treated’, 29.3%; and ‘treatment retainers’, 49.3%), and was addressed with multiple imputation; see Report Supplementary Material 2 for further details. The results of the analysis on the change from baseline to 24 months in LDL-C levels for the different FH patient subgroups are shown in Table 7. These effects relate to pre and post FH diagnosis analysis of imputed LDL-C levels for the ‘untreated’ and the ‘newly treated’ subgroups and to pre and post first prescription analysis of imputed LDL-C levels for the ‘treatment retainers’ subgroup; see Report Supplementary Material 2 for further details.
| Time point and reduction | LDL-C level (95% CI) | ||
|---|---|---|---|
| Untreated (N = 744) | Newly treated (N = 1291) | Treatment retainers (N = 844) | |
| Baseline LDL-C level (mmol/l) | |||
| Pre imputation | 4.32 (2.10 to 7.06) | 5.39 (3.00 to 8.00) | 5.58 (3.20 to 8.80) |
| Post imputationa | 4.27 (2.07 to 7.10) | 5.44 (2.90 to 8.50) | 5.62 (3.00 to 9.38) |
| Post-baseline LDL-C level (mmol/l) | |||
| Pre imputation | 4.15 (2.10 to 7.09) | 3.46 (1.68 to 6.91) | 3.49 (1.60 to 6.76) |
| Post imputationa | 4.11 (2.01 to 7.70) | 3.49 (1.60 to 6.95) | 3.54 (1.60 to 7.20) |
| Post-imputation reduction from baseline in LDL-C level | |||
| Absolute reduction (mmol/l) | 0.26 (0.17 to 0.34) | 1.94 (1.84 to 2.04) | 2.08 (1.95 to 2.20) |
| Relative reduction (%) | 0.01 (–0.1 to 0.03) | 32.6 (30.9 to 34.3) | 34.4 (32.5 to 36.3) |
Results of the before-and-after analysis show that a small reduction in LDL-C was observed for the ‘untreated’ group, potentially a consequence of lifestyle changes following FH diagnosis. In the ‘newly treated’ and the ‘treatment retainers’ subgroups, similar absolute and relative reductions in LDL-C levels were found to have occurred. These changes were clinically meaningful, with the effect of LLTs implying an average absolute reduction in LDL-C of approximately 2 mmol/l and a relative reduction in LDL-C levels of > 30%, on average, although substantially lower than the 50% reduction target108,121,122 and the absolute target of 2.6 mmol/l for primary CVD prevention123 advocated for FH patients. Figure 7 shows the distribution of proportional LDL-C reduction across the ‘untreated’, ‘newly treated’ and ‘treatment retainers’ subgroups. Indeed, approximately only 30% of the subset of individuals receiving treatment achieve the ≥ 50% LDL-C reduction target. Similar findings were obtained for males and females.
FIGURE 7.
Distribution of proportional LDL-C reductions from baseline to 24 months, by treatment subgroup.
Risk of major cardiovascular first events and mortality among treated individuals
Table 8 shows the number of first events of each type occurring in the treated CPRD cohort after FH diagnosis. The probability of a post-FH diagnosis first major CVD event occurring during the 20 years of follow-up of the CPRD cohort is depicted in Figure 8. The median follow-up time was 4.8 years, with Kaplan–Meier survival estimates of 0.93 (95% CI 0.92 to 0.95), 0.90 (95% CI 0.88 to 0.92) and 0.88 (95% CI 0.84 to 0.92), for 5, 10 and 15 years, respectively. Approximately half of the sample was censored at 5 years, which, together with a low event rate, implied an undefined median survival; see number of FH patients at risk at different time points in Figure 8. A decreasing hazard of the event occurring throughout time is observed.
| Nature of CVD | CPRD treated cohorta (N = 2135) |
|---|---|
| CVD first events included in model, n (%) | |
| ACS | 91 (4.3) |
| TIA/stroke | 35 (1.6) |
| CVD death | 15 (0.7) |
FIGURE 8.
Kaplan–Meier of time to first major CVD event post FH diagnosis in the CPRD cohort, number of individuals at risk and predicted parametric curves. GenGamma, generalised gamma.

An assessment of the suitability of the different survival models fitted was carried out. The Royston–Parmar model with five knots was the model that visually provided the best fit, and had the lowest Akaike information criterion; see Report Supplementary Material 2 for further details. Nonetheless, in terms of internal validity, and when weighing model complexity and model fit, the generalised gamma model was considered the optimal choice as it provided a good fit during the first 12 years of data. The external validity assessment through the Perak et al. 96 data indicated that none of the models fitted particularly well. The external data suggested an increasing hazard over time, and this was the case for all age groups. The available models estimated either decreasing hazards over time or, in the case of the exponential model, a stable hazard over time (see Report Supplementary Material 2 for further details). We therefore included the exponential model as a sensitivity analysis. We return to the appropriateness of the models for long-term extrapolation in the discussion.
The exponential and generalised gamma models’ parameters, pooled using Rubin’s rules for multiple imputed data sets, are provided in Table 9. Both models provided similar results. Being older, male, having a history of CVD and a higher LDL-C level are all factors associated with increased risk of first major CVD events. We highlight that, controlling for age, male FH treated individuals are, on average, two to three times more likely to have a first CVD event than females. Similarly, FH patients with a history of CVD are, on average, 4 to 13 times more likely to have a first major CVD event than individuals with no history of CVD. The heterogeneity in event risk is evident when these risk factors are combined. The average event risk for the generalised gamma model at 5 and 10 years is 0.07 (95% CI 0.05 to 0.10) and 0.11 (95% CI 0.07 to 0.16), respectively. As an example, for a 28-year-old female with a baseline LDL-C level of 5.5 mmol/l and no history of CVD, the predicted event risk at 5 and 10 years is 0.01 (95% CI 0.01 to 0.02) and 0.02 (95% CI 0.01 to 0.03), respectively, whereas, for a male, it is 0.02 (95% CI 0.01 to 0.04) and 0.04 (95% CI 0.03 to 0.07) at 5 and 10 years, respectively. For a 49-year-old female with a baseline LDL-C level of 5.5 mmol/l and a history of CVD, the predicted event risk at 5 and 10 years is 0.16 (95% CI 0.09 to 0.28) and 0.24 (95% CI 0.14 to 0.39), respectively; for a male, it is 0.33 (95% CI 0.19 to 0.49) and 0.48 (95% CI 0.30 to 0.71) at 5 and 10 years, respectively.
| CPRD (N = 2135, events, n = 141) | Exponential survival model | Generalised Gamma survival model | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age at baseline (years) | 1.05 | 1.04 to 1.07 | < 0.001 | 1.08 | 1.05 to 1.10 | < 0.001 |
| Sex (male) | 2.19 | 1.53 to 3.14 | < 0.001 | 3.35 | 1.86 to 6.03 | < 0.001 |
| History of CVD | 4.36 | 2.77 to 6.88 | < 0.001 | 13.13 | 4.14 to 41.65 | < 0.001 |
| PT-LDL-C (mmol/l) | 1.33 | 1.20 to 1.47 | < 0.001 | 1.56 | 1.29 to 1.88 | < 0.001 |
| Mu | – | – | – | 13.19 | 10.54 to 15.84 | – |
| Sigma | – | – | – | 0.79 | 0.19 to 1.40 | – |
| Q | – | – | – | 0.50 | –0.25 to 1.24 | – |
Mortality following non-fatal cardiovascular disease events
Following a non-fatal ACS event, few deaths were observed, with just five (5.5%) and seven (7.7%) FH patients (out of 91) dying for CVD related and non-related reasons, respectively. Similar findings were observed following a non-fatal TIA/stroke event, with two (5.7%) and two (5.7%) FH patients (out of 35) dying for CVD related and non-related reasons, respectively. This reflects both the relatively small number of individuals experiencing non-fatal events and the limited follow-up of these individuals. See Report Supplementary Material 2 for further details.
Summary of results and study limitations
The analysis shows that individuals with a recorded diagnosis of FH in primary care are often not treated with LLT within 2 years of their diagnosis, and of those who do receive LLT, < 30% achieve the recommended reductions in LDL-C. We also noted that, at 10 years of follow-up, the average risk of a first major non-fatal CVD event or CVD-related death was 11% for the treated cohort as a whole, with higher estimates among men than women.
Our analysis used the CPRD cohort, which reflects the treatment and management of FH patients as recorded within primary care in the UK. The use of this data set has its caveats, as it might not have correctly coded individuals with FH, the cohort might not be representative of the UK FH population as a whole or of patients identified via cascade testing who are expected to have genetically confirmed FH rather than a diagnosis based on clinical phenotype. Furthermore, this cohort is unlikely to characterise the current clinical practices in tertiary specialised care, such as lipid clinics, where FH patients are closely followed and managed9 (although some patients in the CPRD may also be managed in lipid clinics). Finally, the CPRD cohort presented a relatively long follow-up of up to 20 years, although event data were sparse from around year 10.
Service data analysis
Background and motivation
Cascade testing offers the opportunity to expand FH diagnoses by systematically testing family members (relatives) of affected individuals (index cases). It has been shown to markedly enhance FH identification and to be cost-effective in the UK health-care setting relative to an absence of cascade testing. 110,124,125
To evaluate the benefits and costs of alternative ways to design a cascade service, it is important to understand how different features of the cascade service affect the yield of FH cases. This chapter reports the analysis of a large sample of index cases and relatives considered for genetic testing within the Welsh and Wessex FH services (see Chapter 3, PASS Wales and Wessex Cascade Service Data, for a description of the services). All available records within PASS for each service were made available for this analysis. Although designed to support the operational needs of FH cascade services, PASS provides a rich source of information on the characteristics of the service and the index cases and relatives engaging with the service. The specific research questions this analysis seeks to inform within a UK context are as follows.
-
What are the characteristics and diagnoses of tested index cases?
-
How well do the clinical criteria used to select index cases for genetic testing perform?
-
How many relatives complete cascade testing, how many relatives could potentially be reached by cascade services and what service or patient factors might explain participation by relatives?
-
What are the characteristics of relatives who complete the cascade and for whom cascade testing offers the opportunity for improved management and outcomes?
Results from this chapter inform the cost-effectiveness model comparing cascade-testing protocols, presented in Cost-effectiveness of alternative cascade-testing protocols for familial hypercholesterolaemia.
Methods
Data sources and study population
Four data extractions from the PASS database in Wales and Wessex informed the current analysis: (1) Welsh index cases, (2) Welsh relative entries, (3) Wessex index cases and (4) Wessex relative entries. Welsh and Wessex extractions comprised all patient records within the Welsh national FH cascade-testing service, and those within the Southampton, Hampshire, Isle of Wight and Portsmouth Wessex FH cascade-testing service at the time of extraction, respectively. Extractions of index cases included patients assessed for genetic testing by their service. Extractions of relatives included every relative case documented by each service.
Index data extraction and inclusion criteria
Index case data were extracted from the PASS system in Wales and the Wessex region of England on 9 October 2019 and 20 November 2019, respectively. The Welsh index case extraction comprised 2717 index cases, and the Wessex extraction comprised 1122 cases. For index cases, data extracted from the PASS system included health board, lower-layer super output area code, number of relatives who have been entered relating to an index, year of birth, sex, date of genetic test request/result, genetic diagnosis (genetically confirmed FH, VUS or neither), FH mutation, CVD event history, LLT, cholesterol level, scoring criteria used for determining eligibility for genetic testing and a family number used to link indexes with relative cases.
To ensure that the analysis sample represented the index population of each service, index cases were excluded if they resided outside the catchment area of the cascade service (henceforth referred to as ‘out of area’, as opposed to ‘within area’).
Relative data extraction and inclusion criteria
Relatives’ data were extracted from the PASS system in Wales and Wessex on 9 October 2019 and 20 November 2019, respectively. The Welsh extraction provided 8577 relative records, and the Wessex extraction provided 6374. Entries without a family number were superseded records and were removed to leave only linkable unique cases. Variables extracted for relatives included health board, lower-layer super output area code, year of birth, sex, genetic test request/result date, genetic diagnosis (FH or not FH), FH mutation, CVD event history, LLT and medication history, cholesterol level, the method used to contact each relative and a family number used to link relatives to their index case. Index patients formed the upper hierarchy of the data structure, which related relatives were nested within. In rare instances when relatives were nested within two index cases, relatives were linked to both index cases, representing the largest feasible number of family members associated to each index case. This resulted in marginal inflation of the number of relatives per index.
Relative-related analyses were conducted using three alternative analysis sets.
-
Analysis set 1 included all relatives who could be linked to a FH-positive in-area index case; it was used to investigate the proportion of relatives who reside outside the catchment area of each service and who may therefore not have access to the cascade process.
-
Analysis set 2 comprised relatives in analysis set 1 who were accessible to cascade service; it was used to assess patient- and service-level predictors of cascade completion. Relatives deemed out of area and/or those who were not contacted by their cascade service were determined to be inaccessible.
-
Analysis set 3 contained only those relatives from analysis set 2 who completed the cascade; it was used to assess the characteristics of relatives who underwent genetic testing.
Variable creation and database assumptions
To best utilise the PASS service data, and generate those variables necessary for our analysis, a variety of database assumptions were required and were informed by discussions with the stakeholder group (including PPIE representatives). FH specialist nurses manually updated scoring criteria, diagnostic, medication, method of contact and clinical event entries in the PASS data set for analysis. A patient’s genetic diagnosis was defined by the corresponding genetic mutation classification used by the services as of 15 April 2020. Details relating to other database assumptions are provided in Report Supplementary Material 3.
Index-related analyses
Index characteristics and test results were compiled descriptively for each service and included age at diagnosis, sex, LDL-C level, genetic diagnosis (FH, VUS or neither) and FH mutation genotype.
Several criteria exist that are designed to select those patients with hypercholesterolaemia who are most likely to have FH genetic mutations. In Wales, FH nurses assess whether or not index patients are eligible for testing according to a scoring system based on a modification of the DLCN scoring criteria10 (the unadjusted WDLCN score). An updated version of the Welsh scoring system includes an age-adjustment factor applied to cholesterol-related scoring (the age-adjusted Welsh DLCN score). The unadjusted WDLCN score and the age-adjusted WDLCN score are henceforth referred to as the Welsh scores. In Wessex, a modified version of the Simon Broome criteria16 is used for patient referral. Patients in Wales with a DLCN score of ≥ 6, and patients in Wessex deemed to have possible or definite FH on Simon Broome criteria, are offered genetic testing. Both services have provided referrals below these thresholds in circumstances in which other individual factors might make FH more likely in a patient (e.g. a family history of early CHD in the absence of smoking or other risk factors). It should therefore be noted that index cases recorded in PASS with scores below service referral thresholds risk being unrepresentative of the population ineligible for testing. For this analysis, unmodified Simon Broome scores were compiled and presented for Wessex index cases, deviating from the criteria used in current practice in Wessex.
As the age-adjusted WDLCN score is relatively new and not available for all patients, it had a high degree of missing data. An inferred age-adjusted WDLCN score was also therefore calculated. For this inferred score, the age-adjusted WDLCN score was used when available; where it was not available, it was inferred using information on the unadjusted WDLCN score and a patient’s LDL-C level. The adjustment used is described in Report Supplementary Material 3.
The rates of FH and VUSs were compared across scores. The sensitivity and specificity of using different clinical thresholds were considered for the Welsh scores. These measures of diagnostic performance reflect the performance of the Welsh scores among individuals who met the screening criteria of having scores of ≥ 6. They therefore reflect the sensitivity and specificity among this subpopulation of index cases only.
Relative-related analyses
To explicate the retention levels achieved between an index case’s pedigree and the final number of relatives eventually cascaded, the average number of relatives per FH index case was calculated in the following descended subsamples: (1) relatives within the pedigree and linked to an index case, (2) within-area relatives, (3) contacted relatives, (4) cascaded (genetically tested) relatives and (5) FH relatives identified.
To better explore patient- and service-level factors that contribute to a relative completing the cascade (i.e. the transition between subsamples 3 and 4), the probabilities of a relative completing the cascade in each cascading service were calculated using logistic regression. Covariate selection was informed by known predictors of cascade completion in the literature and included sex, relative degree and method of contact. 41 Age was not considered as this was typically unavailable for those relatives who did not complete the cascade.
The method used by each service to contact relatives was informed by PASS accounts and, for Wales, a complementary assessment of relative records provided by a FH specialist nurse. The method of cascading is selected on a case-by-case basis by health-care professionals involved in the cascade.
Methods were assigned to one of five categories:
-
indirect contact – mediated by patients who pass on personalised clinic letters/information to identified relatives
-
direct contact – contacted directly by the FH service using telephone calls or letters
-
other contact – contacts to adults made besides direct or indirect methods (e.g. appointments scheduled by a family member or consultant, cases with both direct and indirect contact)
-
paediatric contact – patients aged < 18 years and/or with a contact record involving paediatric services
-
unknown contact – relatives with a contact record denoted as ‘unknown’.
Marginal probabilities of completing the cascade were calculated for each combined sex, relative degree and direct/indirect method of contact profile. Because the Wessex service does not directly contact relatives, probabilities for profiles with direct contact were calculable for the Welsh service only. Given the range of contextual factors relevant to each service, comparisons could be made only within services, not between. Relatives were considered as having completed the cascade if they received a genetic test.
The relative characteristics of those who completed the cascade were assessed, and included age, sex and LDL-C level. Rates of CVD and LLT history prior to the cascade were assessed descriptively by tabulating histories by age group with and without a FH mutation.
All statistical analyses were performed using Stata version 16.1.
Results
Index and relative database exclusions
In the Welsh index extraction, 99 cases were deemed out of area, the majority of which were cases reported from health boards in close proximity to the Welsh border, leaving a primary analysis set with a sample of 2618 Welsh index cases. From the Wessex index extraction, only six cases were deemed out of area, leaving a sample of 1116 within-area index cases (see Figure 9).
FIGURE 9.
Index and relative selection process.

The number of relative entries without a family number totalled 6 in Wales and 101 in Wessex, leaving 8571 and 6273 unique and potentially linkable relative cases in Wales and Wessex, respectively. Of these cases, 3815 Welsh and 2143 Wessex relatives were linked to a FH-positive within-area index and formed analysis set 1. Analysis set 2 comprised 2020 Welsh and 1002 Wessex relatives deemed to have been contacted and within-area from analysis set 1. From analysis set 2, 1205 Welsh and 501 Wessex relatives successfully completed genetic cascading and informed analysis set 3.
Data availability
Age at diagnosis, sex, area and LDL-C level data showed high levels of completion for within-area index cases. Unadjusted WDLCN scores were available for 97% of Welsh indexes, age-adjusted WDLCN scores were available for 38% and inferred age-adjusted WDLCN scores were available for 88%. The Wessex-modified Simon Broome score was available for 91% of Wessex indexes.
Area data were available for 77.1% of Welsh and 66.0% of Wessex relatives in analysis set 1. In analysis set 2, sex, relative degree to index and method of contact showed very high levels of completion. Relatives’ age and sex data were complete for cascaded relatives, whereas other variables were available for only a smaller subsample of cascaded relatives (LDL-C level: Wales 37.0% and Wessex 55.9%; CVD history: Wales 54.4% and Wessex 41.7%; LLT history: Wales 51.6% and Wessex 47.1%).
The analysis presented uses a complete-case approach.
Findings from analysis of index cases
Index cases recorded in Welsh and Wessex services were predominantly female (Wales, 60.3%; Wessex, 64.7%), had elevated mean observed LDL-C levels (Wales, 5.85 mmol/l; Wessex, 5.88 mmol/l), and had an average age of 54.9 years in Wales and 51.3 years in Wessex. Index cases were mostly aged > 40 years in both services (Wales, 88.9%; Wessex, 81.5%); adolescent index cases (aged < 18 years) were rare (Wales, 0.5%; Wessex, 1.7%).
Overall, 552 (21.1%) genetically confirmed FH cases and 103 (3.8%) VUSs have been identified from 2618 index cases in the Welsh service. From 215 initial variants reported as VUSs in Wales, 84 (39.1%) cases were reclassified to FH and 28 (13.0%) as not pathogenic. In the Wessex service, 323 (28.9%) monogenic and 44 (3.9%) VUSs were identified from 1116 indexes. Four reclassifications (8%) were made to FH and two (4%) to not pathogenic (from 50 initial VUS diagnoses). LDLR mutations were the most common in both services (Wales, 86.8%; Wessex, 77.7%) followed by APOB (Wales, 12.0%; Wessex, 19.0%). PCSK9 and apolipoprotein E (APOE) variants were rare (see Table 10).
| Characteristics and diagnosis | Wales (N = 2618) | Wessex (N = 1116) |
|---|---|---|
| Characteristics | ||
| Age (years),a mean (SD) | 54.9 (12.3) | 51.3 (13.3) |
| 0–17, n (%) | 12 (0.45) | 19 (1.70) |
| 18–39, n (%) | 279 (10.7) | 187 (16.8) |
| 40–59, n (%) | 1378 (52.7) | 595 (53.3) |
| ≥ 60, n (%) | 947 (36.2) | 315 (28.2) |
| Female (%) | 60.3 | 64.7 |
| LDL-C level (mmol/l),b mean (SD) | 5.85 (1.9) | 5.88 (1.6) |
| Diagnosis c | ||
| Monogenic FH, N (%) | 552 (21.1) | 323 (28.9) |
| LDLR mutation, n (%) | 479 (86.8) | 251 (77.7) |
| APOB mutation, n (%) | 66 (12.0) | 63 (19.5) |
| PCSK9 or APOE mutation, n (%) | 7 (1.3) | 9 (2.8) |
| VUS, n (%) | 103 (3.8) | 44 (3.9) |
| Negative, n (%) | 1963 (75.0) | 749 (67.1) |
Of the 2531 individuals referred to the Welsh service with an unadjusted WDLCN score, 1988 (78.5%) scored ≥ 6. From the 1005 indexes with age-adjusted WDLCN scores, and 2295 indexes with inferred scores, 772 (76.8%) and 1710 (74.5%), respectively, scored ≥ 6. Of the Welsh index cases who scored ≥ 6 using unadjusted, age-adjusted and inferred age-adjusted WDLCN scores, 505 (25.4%), 306 (39.6%) and 491 (28.7%), respectively, had FH diagnoses. This compared with 32 (5.9%) FH diagnoses among index cases with unadjusted WDLCN of < 6 and 18 (3.2%) FH diagnoses among index cases with inferred age-adjusted WDLCN scores of < 6 (numbers suppressed for the age-adjusted WDLCN score).
In Wessex, the Simon Broome criteria classified 136 (13.4%) genetic referrals as unlikely FH, 853 (84.0%) as probable FH and 26 (2.6%) as definite FH. FH was diagnosed in 8.8%, 29.2% and 57.7% of unlikely, probable and definite FH classifications, respectively.
Figure 10 shows the sensitivity and specificity of unadjusted and age-adjusted WDLCN scores for identifying FH and non-FH cases across alternative referral thresholds. The maximum absolute difference in sensitivity occurred at a threshold score of ≥ 8 (9.2%). The unadjusted score had a higher specificity at thresholds up to 13, and converged with age-adjusted specificities at higher thresholds. Increasing the referral threshold score from ≥ 6 (current) to ≥ 8 for the unadjusted (age-adjusted) score would mean that 56.1% (42.9%) of individuals who do not have FH would be correctly excluded from genetic testing; however, it would mean that 29% (20%) of individuals with FH would be incorrectly excluded from testing and subsequent cascading. The diagnostic accuracy of inferred age-adjusted scores was broadly comparable to observed age-adjusted scores (further details in Report Supplementary Material 3).
FIGURE 10.
Observed data on the accuracy of the Welsh scoring criteria in detecting FH with and without age-adjustments among Welsh service indexes eligible for testing. tnr, true negative rate; tpr, true positive rate.

Relative cases
On average, for each FH index identified in the Welsh and Wessex services, 7.35 and 7.01 relatives were available within PASS (see Table 11). Of the relatives with observed area data (Wales, 69%; Wessex, 66%), approximately 25% of Welsh and 29% of Wessex cases were out of area. If we conservatively assume that cases with missing area data are within area, this brings these proportions down to 16% and 18% for Wales and Wessex, respectively. The number of within-area relatives potentially accessible to each service was 6.17 in Wales and 5.69 in Wessex per FH-positive index case. The Welsh and Wessex services contacted approximately 64.3% and 58.0%, respectively, of relatives not known to be residing out of area. Relatives were not contacted for a variety of reasons, such as index non-participation and second-degree relative whose first-degree relative was found not to have FH. The average number of relatives with evidence of being contacted by the service was 3.97 in the Welsh service and 3.30 in the Wessex service. Of the relatives contacted by the service, 59.7% in Wales and 50.0% in Wessex went on to complete the cascade, corresponding to 2.41 Welsh relatives and 1.66 Wessex relatives per FH index case. In Wales, the final number of FH relatives identified was 666, representing 1.35 cases detected per FH case. The comparable figures for Wessex were 285 relatives identified, representing 0.95 cases per FH case.
| Relatives per FH index case | Relatives, n (%) | |
|---|---|---|
| Wales | Wessex | |
| Relatives registered in PASS per FH indexa | 7.35 (6.60) | 7.01 (5.64) |
| Within-area relatives in PASS per FH indexb | 6.17 (6.31) | 5.69 (5.78) |
| Contacted within-area relatives per FH indexc | 3.97 (4.95) | 3.30 (2.43) |
| Relatives completing cascade per FH indexd | 2.41 (3.60) | 1.66 (2.41) |
| FH relatives identified per FH index | 1.35 (2.13) | 0.95 (1.46) |
Table 12 displays the estimated probabilities for completing the cascade in each cascade service by relative sex, degree and contact profiles; the results of the logistic models are presented in Report Supplementary Material 3.
| Degree of relative and sex | Wales (%) | Wessex (%) | |
|---|---|---|---|
| Direct contact (95% CI) | Indirect contact (95% CI) | Indirect contact (95% CI) | |
| First | |||
| Female | 71.1 (67.0 to 75.1) | 53.7 (48.4 to 59.1) | 43.2 (38.0 to 48.4) |
| Male | 61.3 (56.7 to 66.0) | 42.8 (37.5 to 48.2) | 30.5 (25.5 to 35.5) |
| Second and more distant | |||
| Female | 61.3 (56.2 to 66.4) | 42.9 (37.2 to 48.6) | 47.2 (40.3 to 54.1) |
| Male | 50.6 (45.1 to 56.1) | 32.6 (27.3 to 37.9) | 34.0 (27.6 to 40.4) |
In Wales, sex, degree and the direct method of contact (relative to indirect) were all statistically significant predictors of cascade completion (p < 0.01). Females were 53% more likely to complete the cascade than males, first-degree relatives were 55% more likely than second-degree or further relatives to complete the cascade and relatives contacted directly were 111% more likely to complete the cascade than those contacted indirectly [odds ratio comparing direct with indirect testing 2.11 (95% CI 1.66 to 2.69)]. Paediatric cases were 165% more likely to complete the cascade than adults cascaded indirectly.
Female first-degree relatives were estimated to have a probability of completing the cascade of 71% when directly contacted and 57% when indirectly contacted. In comparison, male first-degree relatives had 62% and 43% probabilities of completion when directly and indirectly contacted, respectively. Female second-degree or further relatives were estimated to have a probability of completing the cascade of 61% when directly contacted and 43% when indirectly contacted, each 10% above the same probabilities for males. No evidence was found to suggest that direct contact was more effective by sex or degree [interaction terms between direct contact and sex, and direct contact and degree, were not statistically significant predictors of cascade completion (p > 0.24)].
In Wessex, sex was a statistically significant predictor of cascade completion (p < 0.01), with contacted females 74% more likely to complete genetic testing than males. First-degree relatives were 15% less likely to complete the cascade than second-degree relatives, although this result was not statistically significant (p = 0.275). Female first-degree relatives were estimated to have a 43% (and males a 31%) probability of completing the cascade when indirectly contacted; female second-degree relatives were estimated to have a 47% (and males a 34%) probability of completing the cascade when indirectly contacted.
Of the relatives who completed cascading, 58.2% in Wales and 60.1% in Wessex were female, the average age was 40.1 years in Wales and 30.9 years in Wessex, and the mean observed LDL-C level was 4.92 mmol/l in Wales and 4.33 mmol/l in Wessex. Table 13 presents the CVD and LLT histories of cascaded relatives prior to genetic testing in each service by FH status and age group (see Report Supplementary Material 3 for further details). In both services, higher rates of CVD history were associated with older age groups and FH status. The probability that individuals had received LLT prior to the cascade was more common among relatives with CVD history and had a positive gradient with respect to age at testing. Relatives without a CVD history were more likely to be treated if they tested positive for FH. Numbers were too small to assess the influence of FH status on the probability of being on LLT among those with a history of CVD.
| Age band (years) | Wales (%) | Wessex (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FH positive | Non-FH and non-VUS | FH positive | Non-FH and non-VUS | |||||||||
| Individuals with CVD history | Individuals with prior LLT | Individuals with CVD history | Individuals with prior LLT | Individuals with CVD history | Individuals with prior LLT | Individuals with CVD history | Individuals with prior LLT | |||||
| CVD history | No CVD history | CVD history | No CVD history | CVD history | No CVD history | CVD history | No CVD history | |||||
| Pooled | 7.2 | 85.7 | 38.5 | 2.3 | 50.0 | 7.9 | 10.0 | 100.0 | 18.9 | 5.1 | 100.0 | 1.8 |
| 0–9 | 0.0 | – | 6.7 | 0 | – | 0.0 | – | – | – | – | – | – |
| 10–17 | 0.0 | – | 15.5 | 0 | – | 0.0 | 0.0 | – | 0 | 0.0 | – | 0.0 |
| 18–39 | 1.9 | 75.0 | 36.3 | 0 | – | 2.5 | 0.0 | – | 8.0 | 0.0 | – | 0.0 |
| 40–59 | 13.3 | 91.7 | 62.4 | 3.7 | 0 | 13.5 | 6.0 | – | 27.3 | 5.3 | – | 0.0 |
| ≥ 60 | 30.9 | 83.3 | 86.2 | 9.4 | 100.0 | 31.0 | 34.6 | 100.0 | 50.0 | 15.0 | 100.0 | 10.0 |
Summary of results and study limitations
This analysis used data from two of the largest FH cascade services in the UK and includes detailed patient-level records on 3839 indexes and 14,951 linked relatives. Analysis of index cases indicated high yields of individuals with FH within both services, with 21% of index cases in the Welsh service and 29% in the Wessex service testing positive for FH mutations. Among index cases meeting the full criteria for genetic testing, rates of FH were even higher, at 25–40% (depending on scoring criteria used) for Wales and 30% for Wessex.
In line with previous studies,10,126–128 we found a strong relationship between the stringency of thresholds used to select index cases for genetic testing and the likelihood that an individual carries a FH mutation. Increasing or decreasing the threshold used to inform which indexes are eligible for genetic testing will have implications for the number of individuals correctly identified as having FH and the number of individuals requiring genetic testing. The cost-effectiveness of alternative thresholds is evaluated in Cost-effectiveness of alternative cascade-testing protocols for familial hypercholesterolaemia.
Detailed analysis of the linked relatives shows that 25–29% of individuals who could potentially be contacted for cascade testing live outside the geographic area covered by the cascade service. The lack of a nationally co-ordinated service may prevent access to appropriate diagnosis and management for these individuals, and lead to duplicate genetic testing. In Cost-effectiveness of alternative cascade-testing protocols for familial hypercholesterolaemia, this information is used to assess the potential for a co-ordinated national service to improve the cost-effectiveness of cascade testing.
As shown by the systematic review in Chapter 2, Review 1: effectiveness of cascade-testing protocols among relatives for familial hypercholesterolaemia, very limited data are available to inform the preferred approach to contacting relatives. The available studies are not comparative in nature, and attempting to make inferences across studies is hampered by heterogeneity. The analysis of the Welsh data represents the first available within-service comparison of direct and indirect contact. This analysis shows that direct contact by the service, rather than contact indirectly via family members, is associated with a significantly higher probability that an individual will complete the cascade process. The cost-effectiveness of direct and indirect contact methods is evaluated in Cost-effectiveness of alternative cascade-testing protocols for familial hypercholesterolaemia. We also show that the probability of completing the cascade is significantly lower among men and among more distant relatives (second-degree or further), although the latter effect was observed in Wales only. Policy measures to enhance engagement among these groups are therefore warranted. Results were not comparable between services given fundamental differences in demography and relevant contextual factors.
We also identified a significant group of relatives who are within area, but not contacted by the service. Detailed information on why these individuals were not contacted was not available from PASS and remains an important priority for future research and data collection.
The strengths of this study lie in the large available sample size, the direct derivation of the data from two services, and the availability of FH specialist nurse input to support the development of additional variables (e.g. by recoding free-text fields within PASS) and provide a detailed understanding of the data.
The limitations of the study relate to its observational nature, the large proportion of missing data and generalisability for some variables. The assessment of unmodified Simon Broome scores in Wessex reduced the representativeness of our findings to the Wessex service. For the comparison of direct and indirect contact methods, we were able to adjust for the degree of relative and relative sex, but other confounding factors that are difficult to measure are also likely to be present. For example, age has been identified as a predictor of cascade success in previous work;41 however, our analysis failed to include age as a predictor because of missingness. As the choice of contact method was made on a case-by-case basis, the indirect method may have been used more frequently when direct contact was challenging because of a lack of information and engagement with the family. This would exert a downwards bias in our estimates of completion for indirectly contacted relatives. However, it is also likely that some indirect contacts that did not lead to further interaction with the service went unrecorded. This would exert an upwards bias in our estimates of completion for indirectly contacted relatives.
All analyses were conducted on a complete-case basis; this may have resulted in biases if the complete cases are not representative of the patients diagnosed by the services. This bias is likely to be particularly acute for the analysis of the characteristics of those who completed the cascade. For this group, CVD history and LLT history were missing for a substantive proportion of patients. This is an important consideration as these results may have significant influence on the results of the cost-effectiveness analysis.
Cost and health benefits of diagnosis of people with familial hypercholesterolaemia in the long term
Introduction
There is widespread consensus that people with FH should be diagnosed and treated early, given that treatment is effective, safe, cost-effective and inexpensive. 8,25,123,129–131 However, the magnitude of health losses and costs due to underdiagnosis, and the magnitude of the benefits from diagnosis and treatment, have had relatively little attention. The cost-effectiveness of diagnosis and treatment has been investigated mainly in the context of cascade testing. 11–13,25,110,130–134 Most studies predicted CVD risk based on data from the general population, adjusted upwards to reflect the increased risk for people with FH, and did not consider the impact of prognostic risk factors on CVD risk or the increased effect on CVD risk of exposure to LDL-C over time (known as ‘LDL-C burden’). The exception is the study by Ademi et al.,131 a cost-effectiveness analysis that compared screening and treatment at 10 years with no screening in the Australian setting, which used historical data from people with FH and accounted for LDL-C burden. However, in Ademi et al.,131 CVD risk depended only on age and results were presented for the entire individual population without exploring differences by prognostic factors.
Our decision model of the benefits of diagnosis and treatment is unique in that it uses data from UK people with FH (CPRD cohort), considers LDL-C burden17 and accounts for the impact of prognostic factors on lifetime risk of CVD events. A model was required because the CPRD cohort comprised diagnosed people who were on LLT and included few younger people, its follow-up did not span to individuals’ expected lifetime and there was no information on health-related quality of life over time.
The objective was to estimate the health benefits and health-care costs of diagnosis and treatment, compared with no diagnosis (and no treatment), to inform the cost-effectiveness analysis of alternative cascade protocols, which is reported in the next section. Our model allows us to capture the differential outcomes of policies that diagnose different individual groups, considering CVD risk factors (e.g. sex, LDL-C level) and age at diagnosis.
Methods
We took the perspective of the UK NHS and used 2019 prices, discounting future costs and health benefits at 3.5% per annum. 135 We validated the model using the Assessment of the Validation Status of Health-Economic decision models (AdViSHE) and the TECHnical VERification (TECH-VER) checklists132,136 (see Report Supplementary Material 4). We built the model in Microsoft Office Excel® 2016 (Microsoft Corporation, Redmond, WA, USA).
Population and subgroups
The population comprises people with heterozygote genetically confirmed FH (henceforth referred to as people with FH). Heterogeneity in CVD risk, and hence in the magnitude of benefit from diagnosis, was reflected where evidence was available and in line with the risk factors considered in the cost-effectiveness analysis of cascade strategies. We defined subgroups according to age, sex, CVD history (with or without a prior CVD event) and PT-LDL-C level, given their influence on the risk of CVD events; age and PT-LDL-C level also influenced the magnitude of treatment effect, as detailed in the Economic analysis of the Clinical Practice Research Datalink database.
So that the individuals’ characteristics aligned with the cascade model, the age subgroups correspond to the same age groups (i.e. 0–9, 10–17, 18–39, 40–59 and ≥ 60 years) and the same PT-LDL-C level bins, assuming that the mid-points represented the entire interval. Overall, we stratified the population into 144 subgroups.
Options
The model estimates long-term health outcomes and costs for two options: diagnosis and management of FH (including monitoring with and monitoring without LLT, depending on age and PT-LDL-C level), and no diagnosis (hence no treatment), so that the results can inform the cost-effectiveness analysis of cascade-testing protocols (see Cost-effectiveness of alternative cascade-testing protocols for familial hypercholesterolaemia).
Model structure
We designed the model based on previous cost-effectiveness models in FH,11–13,25,110,111,130–134 our understanding of the disease and of the impact of diagnosis and treatment, discussions with our stakeholder group, relevant clinical literature and the availability of data to parameterise key aspects of the model. Our conceptual model assumes that people with FH are at risk of CVD events, both fatal and non-fatal. The risk of a CVD event depends on an individual’s characteristics, defined earlier and included in the analysis of the time to the first major CVD event (see Economic analysis of the Clinical Practice Research Datalink database).
Figure 11 shows the model structure. The model is a cohort Markov model. People with FH enter the model in the ‘well’ state, either diagnosed (hence undergoing management of FH) or undiagnosed (hence untreated), depending on the option under evaluation. The age at model entry represents the age at diagnosis if they were diagnosed; the counterfactual assesses what would happen if they were not diagnosed at this same age. In the ‘well’ state, people are at risk of having the first major CVD event since diagnosis (i.e. model entry) and of non-CVD death.
FIGURE 11.
Model structure. Acute coronary syndrome includes unstable angina, myocardial infarction and ACS unspecified. TIA/stroke includes TIAs and ischaemic stroke. The first CVD event represents the first CVD event since model entry, which is the age at diagnosis if the model is evaluating the option in which patients are diagnosed, or the same age but under the counterfactual scenario that patients were not diagnosed.
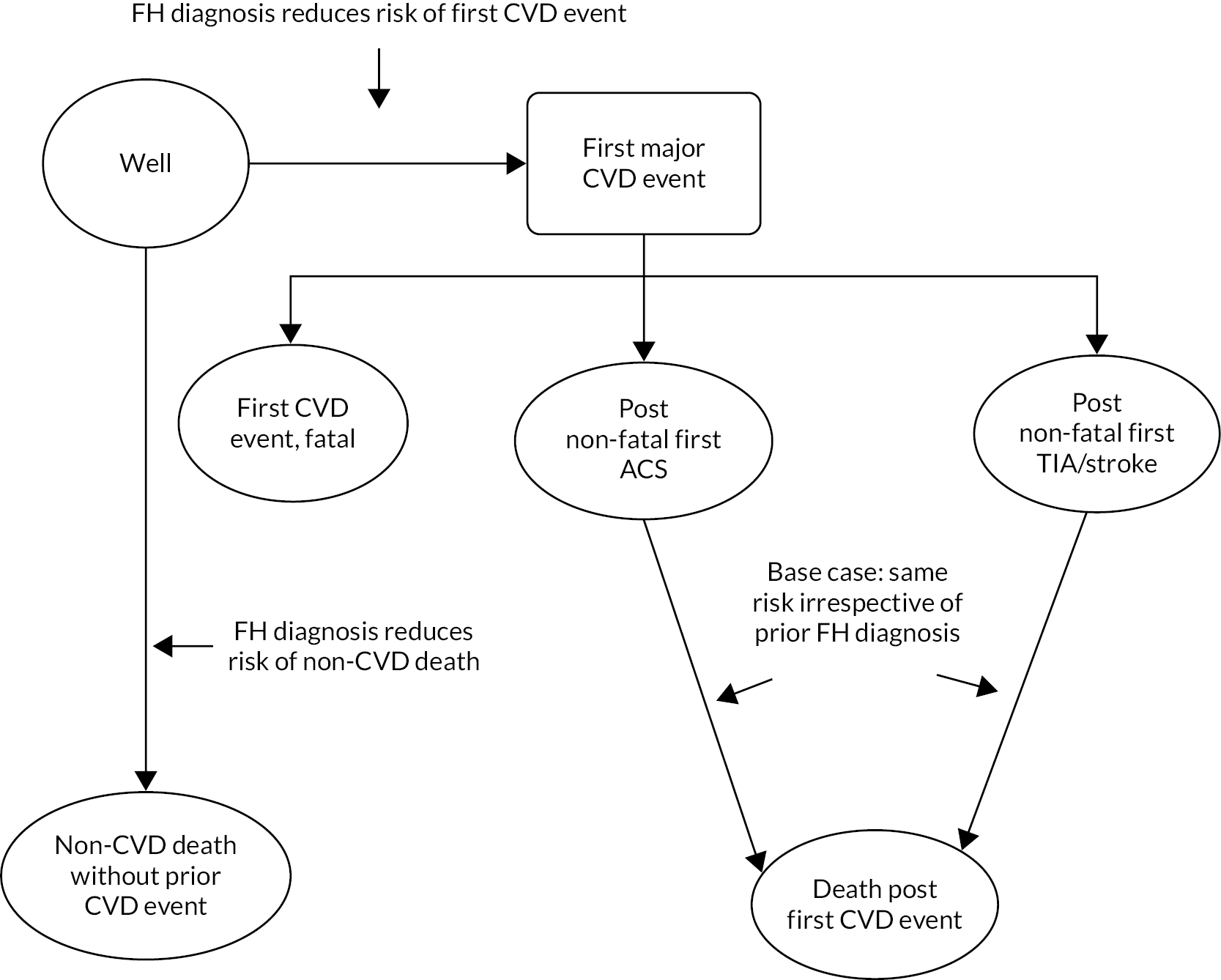
The risk of first major CVD event depends on the age at diagnosis, sex, PT-LDL-C level and prior CVD history, as discussed in Economic analysis of the Clinical Practice Research Datalink database. This first CVD event can be fatal, non-fatal ACS (which includes unstable angina, myocardial infarction and unspecified ACS) or non-fatal ischaemic stroke/TIA. We distinguished between non-fatal ACS and non-fatal ischaemic stroke/TIA given their different lifetime implications to the risk of death, costs and health-related quality of life. The risk of all-cause death following a first non-fatal CVD event depends on the nature of this first major CVD event, either ACS or ischaemic stroke/TIA. The model cycle length is annual, with half-cycle correction. The model structure in Report Supplementary Material 4 justifies the model structure in more detail.
Effect of diagnosis
In the model, diagnosis leads to monitoring with or without LLT initiation (depending on PT-LDL-C level and age), thereby reducing the risk of the first CVD event and of non-CVD death. 17,18 The magnitude of the LDL-C reduction depends on an individual’s PT-LDL-C level, and the effect of a 1-mmol/l reduction in LDL-C level on CVD risk depends on the time from treatment initiation (owing to the effect of LDL-C burden17), as documented in Model inputs. As CVD events reduce health-related quality of life and life expectancy, and lead to greater NHS costs, diagnosis improves quality-adjusted life expectancy and reduces the costs related to CVD events. Conversely, FH diagnosis increases costs because of the costs of treatment, namely acquisition costs of LLT, monitoring costs and costs of managing adverse effects. We did not include the cost of diagnosis, given that this is considered in the cost-effectiveness analysis of cascade protocols, for which these results are inputs (see Cost-effectiveness of alternative cascade-testing protocols for familial hypercholesterolaemia). Our base-case assumption is that the risk of death after a first non-fatal CVD event is the same irrespective of prior diagnosis. This was based on feedback from the stakeholder group that all people in secondary prevention are (or should be) treated with LLT intensely.
Model inputs
Table 14 summarises the model inputs, with details provided in the model inputs section of Report Supplementary Material 4.
| Parameter | Value | Source | Details in (if applicable) |
|---|---|---|---|
| Risk of events | |||
| Reduction in LDL-C due to FH diagnosis (weighted average of reduction in ‘newly treated’ and ‘treatment retainers’) | 33.4% | Analysis of CPRD cohort | Table 7 and Report Supplementary Material 4, Table 1 |
| Risk of first CVD event | Generalised gamma risk equation | Analysis of CPRD cohort | Report Supplementary Material 4, Table 2–4, and Table 9 |
| Distribution of people by type of first CVD event | |||
| Death | 11% | Analysis of CPRD cohort | Report Supplementary Material 4, Table 5 |
| Non-fatal ACS | 65% | ||
| Non-fatal TIA/stroke | 25% | ||
| Risk adjustment to CVD risk (HRs multiplied by hazard rate depending on time from model entry) | |||
| People aged < 40 years | Calculated from Perak et al.,96 figure 2 | Report Supplementary Material 4, Table 6 | |
| 10–20 years vs. 0–10 years | 4.13 | ||
| ≥ 20 years vs. 0–10 years | 6.44 | ||
| People aged ≥ 40 years | |||
| 10–20 years vs. 0–10 years | 1.48 | ||
| 20 + years vs. 0–10 years | 2.36 | ||
| Effect of reducing LDL-C by 1 mmol/l on risk of CVD events | Calculated according to EAS equation | EAS consensus statement,17 table 2 | N/A |
| Effect of reducing LDL-C by 1 mmol/l on the risk of non-CVD death | 0.96 (95% CI 0.92 to 1.01) | Cholesterol Treatment Trialists’ Collaboration,18 webfigure 4A | Report Supplementary Material 4, Table 7 |
| Risk of all-cause death following the first non-fatal CVD event | Constant probability over time by age at event, sex and type of event | Lewsey et al.137 Analysis of CPRD cohort General population mortality statistics138 |
Report Supplementary Material 4, Table 8 |
| Risk of non-CVD death | Depends on age and sex | General population mortality statistics138 | Report Supplementary Material 4, Table 9 |
| Effect of FH diagnosis on costs | |||
| Costs of treatment, per annum | |||
| Cost of LLT, per annum | £21 | Analysis of CPRD cohort Unit costs from the 2019 drug tariff139 |
See Report Supplementary Material 4, Table 10 |
| Adverse effects of LLT: additional costs due to earlier cases of diabetes | |||
| Primary prevention, per annum | £3 | NICE CG181,108 inflated to 2019 prices140 | N/A |
| Secondary prevention, per annum | £6 | ||
| Costs of monitoring, per annum | |||
| Adults in primary care in the year of LLT initiation | £137 | Resource use based on NICE CG181,108 NICE CG71,8 HEART UK consensus statement141 and feedback from the stakeholder group Unit costs obtained from national costs140,142 and NICE CG181,108 inflated to 2019 prices |
See Report Supplementary Material 4, Table 1–14 |
| Adults in primary care in the subsequent years following LLT initiation | £35 | ||
| Adults in secondary care in the year of LLT initiation | £556 | ||
| Adults in secondary care in the subsequent years following LLT initiation | £170 | ||
| Children in secondary care in the year post diagnosis who are not on LLT | £272 | ||
| Children in secondary care in the subsequent years post diagnosis who are not on LLT | £112 | ||
| Children and adolescents in secondary care in the year post diagnosis who were started on LLT | £723 | ||
| Children and adolescents in secondary care in the subsequent years post diagnosis who were started on LLT | £336 | ||
| Other inputs | |||
| Cost of health states, per annum | |||
| Cost of non-fatal ACS year 1 | £8195 | Walker et al.,143 inflated to 2019 prices140 | See Report Supplementary Material 4, Table 15 |
| Cost of non-fatal ACS year 2 and beyond | £2137 | ||
| Cost of non-fatal ischaemic stroke/TIA year 1 | £9244 | ||
| Cost of non-fatal ischaemic stroke/TIA year 2 and beyond | £1990 | ||
| Cost of CVD death | £2300 | ||
| Cost of non-CVD death | £1929 | ||
| Health-related quality of life | |||
| Age and sex adjustments | Regression model | Ara et al.144 | N/A |
| Post first non-fatal ACS year 1 | 0.76 | NICE CG181108 | See Report Supplementary Material 4, Table 16 |
| Post first non-fatal ACS year 2 and beyond | 0.88 | ||
| Post first non-fatal ischaemic stroke/TIA | 0.72 | ||
| N/A, not applicable. | |||
Risk of first major cardiovascular disease event
To inform the risk of the first CVD event among patients who were diagnosed and treated, we took the risk estimated from the CPRD cohort, given that they were all diagnosed and treated, and the observed distribution by type of event (see Economic analysis of the Clinical Practice Research Datalink database). These risk equations predicted that the CVD risk was relatively stable over time, which we thought was unrealistic and did not reflect another study with a longer follow-up and a large sample size. 96 Perak et al. 96 used data from six large US epidemiological cohorts to identify people with a PT-LDL-C level indicating FH over a follow-up of up to 30 years. Using the curves of adjusted 30-year survival free from CHD presented in Perak et al.,96 which we digitised, we estimated a HR associated with longer follow-up by comparing the cumulative hazard rate for 0–10 years with those for 10–20 years and ≥ 20 years. We multiplied this HR by the estimated hazard rate (according to the risk equations estimated in Economic analysis of the Clinical Practice Research Datalink database after 10 years from model entry or being at risk of CVD, whichever was earlier).
To estimate the risk of the first major CVD event among undiagnosed (and untreated) people, we assumed that diagnosis affected the risk of the first CVD event only via LDL-C, and that the effect of LDL-C on CVD risk increases over time (known as ‘LDL-C burden’17,145). The proportional reduction in LDL-C was the same for all patients and corresponded to the average reduction observed among the treated patients in the CPRD cohort (estimated in Economic analysis of the Clinical Practice Research Datalink database), given that the reduction was similar between those treated before and those treated after a formal diagnosis (34.5% vs. 32.6%, respectively). The absolute reduction depended on the subgroup’s PT-LDL-C level. This assumed that (1) the PT-LDL-C level represents the LDL-C level that people would have had if they had not been diagnosed and managed; and (2) the proportional reduction in LDL-C is independent from the pre-treatment levels,99 and it is generalisable to all FH people irrespective of age, sex and PT-LDL-C level.
To account for the effect of LDL-C burden, we estimated the effect of absolute reductions in LDL-C on CVD risk with the equation proposed by the 2017 EAS consensus statement [risk reduction per 1-mmol/l reduction in PT-LDL-C = exp(−0.249 + (number of years of treatment − 5) × (−0.0152)], which relates the number of years in treatment to reduction in atherosclerotic CVD risk. 17 We assumed that the number of years of treatment corresponded to the number of years since diagnosis (i.e. model entry).
Figure 12 shows how the reduction in CVD risk depends on PT-LDL-C level and duration of treatment (i.e. time since model entry and diagnosis) according to the EAS equation. 17 For a 1-mmol/l reduction in PT-LDL-C, the risk reduction ranges from 17% after the first year of treatment to 61% after 50 years of treatment. At 5 years, the risk reduction is 22%, in line with the meta-analysis of trials of statin treatment. 18 Longer treatment durations result in greater risk reductions. For example, 54% risk reduction is achieved after 40 years, which is similar to the risk reduction estimated from Mendelian randomisation studies. 146–149 Greater risk reductions are achieved for larger reductions in PT-LDL-C. For example, a 2-mmol/l reduction results in risk reductions of 31–85%, depending on the time since model entry (or diagnosis).
FIGURE 12.
Relationship between treatment duration and reduction in CVD risk.
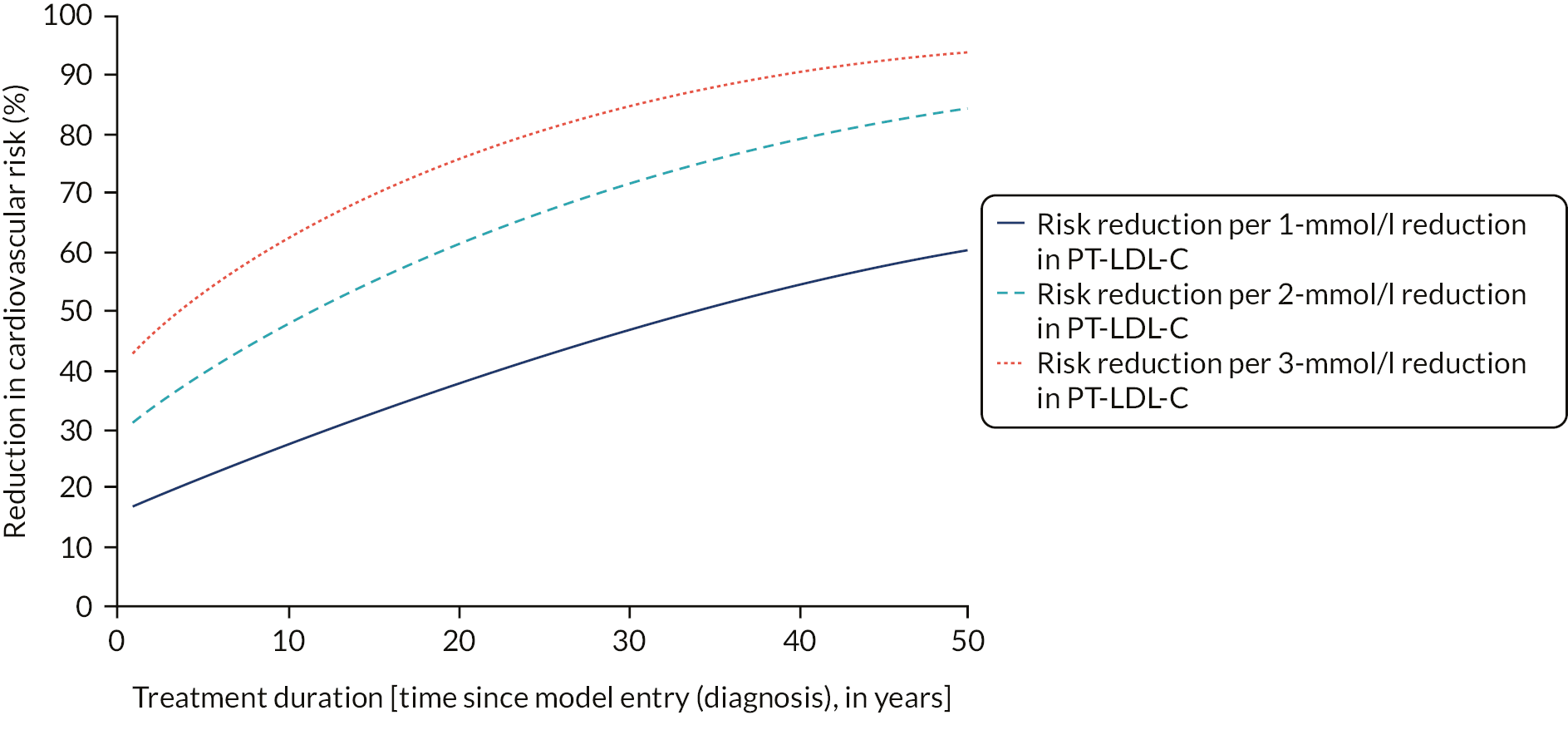
As only 22% of the CPRD cohort were aged < 40 years at the time of FH diagnosis, there is uncertainty in the extent to which the risk equations generalise to younger people. Following feedback from the stakeholder group and given that the youngest age at CVD event recorded in PASS was 25 years, we assumed that people are at risk of a CVD event from 25 years of age.
Risk of non-cardiovascular disease death
We informed the risk of non-CVD death from the UK life tables. 138 We assumed that the LDL-C reduction due to diagnosis reduces the risk of non-CVD death prior to the first CVD event as estimated by the meta-analysis of trials of statins by the Cholesterol Treatment Trialists’ Collaboration. 18
Risk of all-cause death following the first non-fatal cardiovascular disease event
It was not possible to use the data from the CPRD cohort to inform the risk of all-cause death following a first non-fatal CVD event directly because of small numbers (see Economic analysis of the Clinical Practice Research Datalink database). An examination of the literature did not find studies that reported mortality risk by age and sex following a first non-fatal CVD event among people with FH. Therefore, we selected the risk equations by Lewsey et al. 137 because they were based on UK data (the Scottish Heart Health Extended Cohort), with a large sample size (3184 people who had a first non-fatal CVD event), linked to routine data on hospital admissions and death registrations, and long follow-up (median follow-up of 4.8–7.6 years, depending on sex and CVD event group).
We derived a constant probability of death from the first non-fatal CVD event using the Lewsey et al. 137 equations, according to a person’s age at the event, their sex and whether the event was a TIA/stroke or ACS. We adjusted the probability for survival post first non-fatal ACS downwards, given the underprediction of mortality risk compared with the observed survival in the CPRD cohort. In addition, we adjusted with the probability of death in the general population to ensure that the mortality risk was never below the general population mortality. Our approach is described in detail in Report Supplementary Material 4, ‘Survival post-1st major cardiovascular event’.
Effect of familial hypercholesterolaemia diagnosis on costs
We based the frequency and type of appointments and lipid tests on the 2019 NICE CG71,8 the HEART UK consensus statement on care of children and adolescents with FH,141 and feedback from the stakeholder group. We assumed that all people are monitored from diagnosis. People aged ≥ 10 years are actively treated and have the benefits and costs of treatment, irrespective of their PT-LDL-C level. Even though people with a low LDL-C level at diagnosis may be monitored and adopt a healthier lifestyle, and only start LLT when their LDL-C increases, it was not feasible to account for changes in LDL-C over time in the model.
We calculated the acquisition costs of LLT as the weighted average of the acquisition costs of LLT given the most intense LLT within 2 years of treatment initiation observed in the CPRD cohort, by class, and using the cheapest drug in the class. 139
Other inputs
We obtained unit costs from national sources,139,140,142 supplemented by the cost-effectiveness analysis conducted for NICE CG181. 150 We also used this analysis to inform the costs of adverse events due to LLT and the health-related quality of life associated with the health states. We obtained the costs associated with the health states from an analysis of the lifetime health-care use and costs of people with stable coronary artery disease in England, including 94,966 people from 2001 to 2010, using routine health-care records,143 which we inflated to 2019 prices. 140
Analytic methods
We present results in terms of the difference in health outcomes and costs between diagnosing and managing people and not diagnosing people (and no management until the first CVD event). Health outcomes include impact on event-free survival, life expectancy, quality-adjusted life-years (QALYs), costs, and (discounted) net health gains.
We calculated the net health gain from diagnosis as the health gains to people net of the health losses (or gains) elsewhere in the NHS due to the impact on costs. 151,152 A positive net health gain means that an intervention, in this case diagnosis, is cost-effective, whereas a negative net health gain (or net health loss) means that an intervention is not cost-effective. We chose this approach, rather than incremental cost-effectiveness ratios, to make the magnitude of benefit from diagnosis clearer and easier to relate to the results used in the cost-effectiveness analysis of cascade protocols (reported in Cost-effectiveness of alternative cascade-testing protocols for familial hypercholesterolaemia). To calculate the health impact on the NHS, we used the cost-effectiveness threshold used by the UK Department of Health and Social Care (i.e. £15,000 per QALY) and the NICE cost-effectiveness threshold of £20,000 per QALY. 133,153 The £15,000-per-QALY threshold reflects the most recent empirical evidence on the marginal productivity of the English NHS,153 and has also been used as the measure of health opportunity cost in the Department of Health and Social Care’s impact assessments. 154–156
The base-case results are probabilistic over 1000 Monte Carlo simulations,157 with the scenarios run deterministically. We calculated the probability that FH diagnosis and treatment are cost-effective, and the expected value of perfect information. 157–160
Scenario analyses
We conducted 29 scenario analyses related to the following: how diagnosis and management affects LDL-C level, the effect of LDL-C reductions on health outcomes, the management of people with low PT-LDL-C levels, the risk of CVD events over a person’s lifetime, the consequences of the adverse events from LLT, the frequency and setting of monitoring, the source of costs related to post-event care and the source of health-related quality of life weights (details and parameterisation are in Report Supplementary Material 4, ‘Scenario analysis’).
Results
Full results are available in Report Supplementary Material 4.
Gains in health outcomes among diagnosed people
Figure 13 shows the gain in event-free life expectancy due to diagnosis among males (see Figure 13a) and females (see Figure 13b). The gains are greater if diagnosis occurs at a younger age (e.g. approximately a gain of 20.86 years for males aged 0–9 years with PT-LDL-C = 5.5 mmol/l, compared with 5.34 years for males aged 40–59 years with the same PT-LDL-C level). People with higher PT-LDL-C have greater gains (e.g. gains among males aged 40–59 years range between 0.18 and 10.02 years, depending on their PT-LDL-C level). Gains are also more pronounced among people with prior CVD history. The gains among females follow the same pattern. The differences in gains in event-free life expectancy due to diagnosis are driven by differences in the risk of CVD events, the competing risk of non-CVD mortality, the absolute reduction in LDL-C given the same proportional reduction from diagnosis and the increased effect of lowering LDL-C over time. Report Supplementary Material 4, Figure 5, shows the gain in life expectancy. Gains in life expectancy are less pronounced, but still substantial. For example, among males, the gain is 0.08–10.35 years.
FIGURE 13.
Gain in event-free life expectancy due to diagnosis (not discounted, in years). (a) Males; and (b) females.

Figure 14 shows the gain in quality-adjusted life expectancy predicted by the model. This is the ‘QALY gain’, which, after discounting to present values, enters the calculation of cost-effectiveness. Similarly to the gain in event-free life expectancy and overall life expectancy, the differences in QALY gains reflect the different CVD risk and risk reduction given the PT-LDL-C level. The QALY gain ranges between 0.04 and 11.20 QALYs, depending on the subgroup. Once discounted to present values, the QALY gain is lower, at 0.01–2.11 discounted QALYs (see Report Supplementary Material 4, Figure 6).
FIGURE 14.
Gain in quality-adjusted life expectancy from diagnosis (not discounted, in QALYs). (a) Males; and (b) females.

Impact of diagnosis on costs
Figure 15 shows the impact of diagnosis on NHS costs over people’s expected lifetimes (see Report Supplementary Material 4, Figure 7, for discounted results). In subgroups with lower PT-LDL-C levels (< 1.5–3.5 mmol/l, depending on age and prior CVD history), diagnosis increases costs. This is because the risk of CVD events among these people is lower, and the risk reduction due to diagnosis is also lower, as it depends on their PT-LDL-C level. Therefore, the additional costs of LLT and monitoring are not fully offset by the savings in the costs of the avoided CVD events. The cost increase is more pronounced in younger subgroups with lower PT-LDL-C levels because they incur monitoring costs from diagnosis, are treated from 10 years of age, but benefit from CVD events only from 25 years of age. With greater levels of PT-LDL-C, the diagnosis results in larger savings in younger subgroups (than older subgroups), as their CVD risk is higher and diagnosis prevents a larger number of CVD events. The savings are greater in subgroups with prior CVD history because they are at higher risk of CVD events. The additional costs of FH diagnosis are mostly due to monitoring, given the small acquisition cost of statins.
FIGURE 15.
Impact of FH diagnosis on costs (undiscounted). (a) Males; and (b) females.

Impact of diagnosis on net health to the NHS
Figure 16 shows the net health gain of diagnosis at a cost-effectiveness threshold of £15,000 per QALY, discounted to present values (see Report Supplementary Material 4, Figure 8 for results according to the cost-effectiveness threshold of £20,000 per QALY). The net health gain from diagnosis ranged between –0.27 and 2.51 QALYs at the £15,000 per QALY threshold. Diagnosis represents a net health gain for the NHS for most males (see Figure 16a) unless their PT-LDL-C level is < 1.5 mmol/l if aged < 39 years or < 0.5 mmol/l if aged ≥ 40 years or had prior CVD history. The results are similar for females (see Figure 16b), although the net health gains are generally not as large, and diagnosis is not a net health gain for females aged 0–39 years with PT-LDL-C levels of ≤ 2.5 mmol/l. This is because females are at lower risk of CVD events.
FIGURE 16.
Net health gain of FH diagnosis and treatment (compared with no diagnosis and no treatment) results, by PT-LDL-C level, in QALYs per individual, at a cost-effectiveness threshold of £15,000 per QALY. (a) Males; and (b) females. Results are probabilistic. Net health gain = (QALYs if individual group was diagnosed – QALYs if individual group was not diagnosed) – (costs if individual group was diagnosed – costs if individual group was not diagnosed)/ cost-effectiveness threshold at £15,000 per QALY.
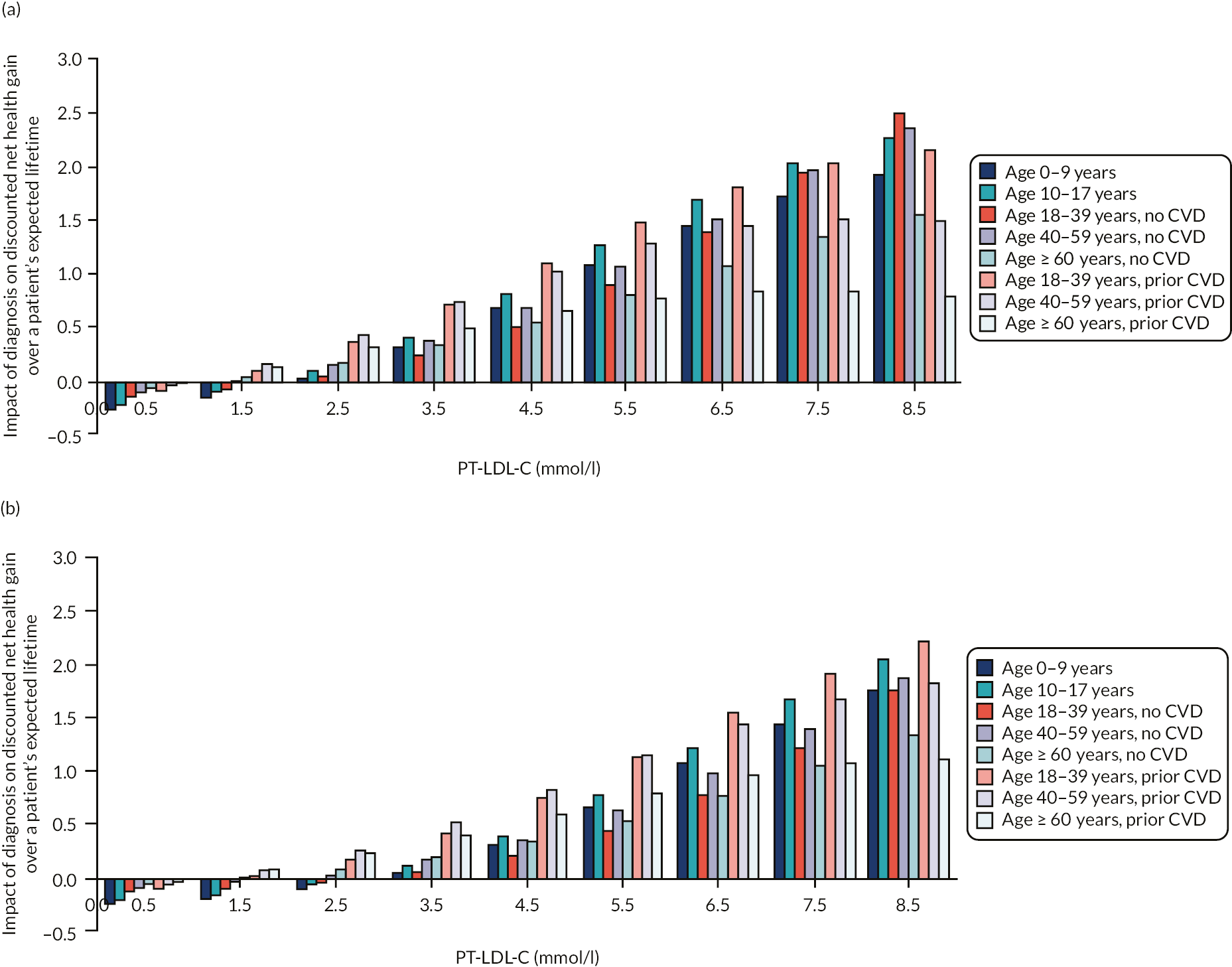
The net health gain increases with higher PT-LDL-C levels. The pattern of net health gain by age is not as clear, because of the effect of discounting and the age at which we assumed people start being at risk of CVD events (25 years of age in the base-case scenario). As a result, people diagnosed younger start incurring costs, but derive benefits only later in life, both of which are discounted to present values at 3.5% per annum.
Uncertainty analysis
The probability that diagnosis is a net health gain to the NHS (i.e. cost-effective) follows a similar pattern (see Report Supplementary Material 4, Figure 9). The probability is close to 1 in most subgroups for which diagnosis is a net health gain, and close to 0 in subgroups for which the diagnosis is a net health loss (e.g. subgroups with PT-LDL-C levels of < 1.5 mmol/l and no prior CVD history).
Table 15 summarises the results of the scenario analyses (details are in Report Supplementary Material 4, Table 28). Diagnosis was a net health gain in the base-case scenario and in all scenarios in most of the subgroups (cells in white). In the subgroups with PT-LDL-C levels ≥ 2.5 mmol/l, diagnosis was a net health gain in the base-case scenario and in most of the additional scenarios (cells in light grey). In the subgroups with no prior CVD and low PT-LDL-C levels (0.5–1.5 mmol/l), diagnosis was not a net health gain in any (cells in black) or most of the scenarios (cells in dark grey).
| Sex and PT-LDL-C (mmol/l) | No prior CVD, age (years) | With prior CVD, age (years) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0–9 | 10–17 | 18–39 | 40–59 | ≥ 60 | 18–39 | 40–59 | ≥ 60 | |
| Males | ||||||||
| 0.5 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 13 |
| 1.5 | 0 | 1 | 3 | 20 | 27 | 28 | 28 | 28 |
| 2.5 | 20 | 25 | 25 | 29 | 29 | 30 | 30 | 30 |
| 3.5 | 26 | 27 | 30 | 30 | 30 | 30 | 30 | 30 |
| 4.5 | 28 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| 5.5 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| 6.5 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| 7.5 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| 8.5 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Females | ||||||||
| 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 1.5 | 0 | 0 | 0 | 1 | 14 | 9 | 28 | 28 |
| 2.5 | 1 | 1 | 3 | 24 | 29 | 30 | 30 | 30 |
| 3.5 | 2 | 26 | 24 | 30 | 30 | 30 | 30 | 30 |
| 4.5 | 26 | 27 | 30 | 30 | 30 | 30 | 30 | 30 |
| 5.5 | 28 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| 6.5 | 29 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| 7.5 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| 8.5 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
The model was most sensitive to scenarios with alternative assumptions about the long-term CVD risk and the effect of LDL-C burden over time, and to alternative assumptions about the benefits and costs of diagnosis. For example, not considering the effect of LDL-C burden on CVD risk (instead assuming that 1-mmol/l reduction in LDL-C reduces CVD risk by 21%, as per the effect observed in trials of statins18) results in diagnosis not being a net health gain in 14 additional subgroups, as the (counterfactual) CVD risk among undiagnosed/untreated people is lower than in the base-case scenario. If the risk of the first CVD event does not increase over time, as suggested by our analysis of the Perak et al. 96 data, diagnosis is not a net health gain in an additional 12 subgroups. Relating to the effect of diagnosis on health outcomes, the scenario with the greatest impact was if diagnosis reduces LDL-C to the EAS targets of 3.5 mmol/l in children and adolescents, 1.8 mmol/l in adults in primary prevention and 1.4 mmol/l in adults in secondary prevention. 123 Under this assumption, diagnosis is not a net health gain in an additional eight subgroups, namely subgroups with PT-LDL-C levels lower than the target, as they are assumed to experience no further reductions, but incur the costs of monitoring. The scenario on the cost implications of diagnosis with the greatest impact was the assumption that 50% of adults are monitored in primary care (vs. 75% in the base-case scenario).
The expected value of perfect information is small, at a maximum of 23.51 and 25.12 QALYs per 1000 people at the £15,000 per QALY and £20,000 per QALY thresholds, respectively (see Report Supplementary Material 4, Figure 10). The small expected value of perfect information suggests that the impact of parameter uncertainty on decision uncertainty is small.
Summary of results and study limitations
Our model predicts that diagnosis leads to large gains in health outcomes, particularly among younger people and people with high PT-LDL-C levels. Diagnosis is predicted to substantially improve event-free survival by up to 30 years and life expectancy by up to 11 years, depending on a person’s age, sex, PT-LDL-C level and prior CVD history. For people with higher PT-LDL-C levels (≥ 2.5–5.5 mmol/l, depending on age and sex), FH diagnosis results in savings to the NHS, given the costs saved as a result of avoided CVD events. In general, the net health gain of diagnosis is greater among males, people with higher PT-LDL-C levels and people with prior CVD history at diagnosis. The pattern is less clear with age because of the effect of discounting health outcomes and costs to their present value, and the interaction between age at diagnosis, which determines the age from which treatment and monitoring costs start to be incurred, and age from which people are at CVD risk, and therefore derive benefit from diagnosis.
In general, and across all scenarios, diagnosis was a net health gain in most subgroups (particularly in subgroups with PT-LDL-C levels ≥ 2.5 mmol/l and/or with prior CVD history). The assumptions with the largest impact were those about the risk of first major CVD event, particularly the effect of LDL-C burden on CVD risk and the increase in CVD risk over time. The effect of LDL-C burden is supported by epidemiological studies,17 although there is uncertainty about the size of the effect and its generalisability to people with FH. Similarly, although the assumption that CVD risk increases with age is uncontroversial, the magnitude of this increase is uncertain.
Uncertainty about the magnitude of net health gain from diagnosis is driven mainly by the structural uncertainty in key assumptions related to the CVD risk over a person’s lifetime and the effect of diagnosis (and management) on LDL-C level. Uncertainty in the parameters had a small impact, as evidenced by the small expected value of perfect information.
By capturing the impact of prognostic factors on health outcomes and costs, the model allows us to understand the impact of policies that target people with different characteristics. An uncertainty that we were unable to explore in the scenario analyses is whether or not the CVD risk observed in the CPRD cohort is representative of the CVD risk experienced by people with FH, given the uncertainty regarding the extent to which the CPRD cohort includes people who do not have FH (e.g. owing to miscoding or misdiagnosis in primary care), as discussed in Economic analysis of the Clinical Practice Research Datalink database.
Another area of uncertainty is the generalisability of the LDL-C reductions observed in the CPRD cohort post diagnosis and treatment to people diagnosed with FH. This relates to the generalisability of the CPRD to people with FH and the variability in their management. Although greater LDL-C reductions may be achieved with more intense management, the costs may increase, as it may require closer clinical management and the use of more effective (and more costly) drugs such as PCSK9 inhibitors. For comparison, the 2010 UK FH survey of lipid clinics found an average reduction in LDL-C after diagnosis of 37%,9 slightly higher than the 33% we observed in the CPRD cohort and assumed in this model. Similarly, if monitoring is less intensive than we assumed for the base-case scenario, LDL-C reductions may be smaller. However, assessing the value of alternative treatment protocols was not the focus.
Other weaknesses of the cost-effectiveness analysis mostly relate to the assumptions required to design and inform the model, which, in turn, relate to the available data on lifetime CVD risk, effect of treatment on LDL-C level and the generalisability of the CPRD cohort to FH patients, as discussed previously. In addition, the costs of monitoring people were based on the national guidelines and stakeholder input, rather than informed by a systematic process (e.g. expert elicitation or review of local guidelines) or empirical data.
Cost-effectiveness of alternative cascade-testing protocols for familial hypercholesterolaemia
Introduction
Cascade screening/testing is recommended by NICE8 and international guidelines,123,161 and has been shown to be cost-effective. 11–13,25,110,130–134 The cascade-testing process can differ in various dimensions, such as method of contacting relatives (directly by the FH service or indirectly via the index case), type of cascade contact pattern (contacting second-degree relatives sequentially only when their first-degree relative was confirmed to have FH or contacting first- and second-degree relatives simultaneously) and testing strategies (e.g. using LDL-C level as a screening test prior to the genetic test); we term the various ways in which cascade testing can be implemented as ‘cascade protocols’. Most cost-effectiveness studies compared cascade screening plus genetic testing with no cascade,12,110,130,131,133 with three studies including testing strategies relying on lipid levels,13,25,134 and no studies, as far as we are aware, comparing protocols with different methods of contact, contact pattern and testing strategies.
This cost-effectiveness analysis compares a wide range of alternative cascade protocols, comprising alternative cut-off points to select index cases to genetic testing and/or the cascade, contacting relatives directly or indirectly, contacting relatives sequentially or simultaneously, and using lipid and genetic tests in combination. The aim was to estimate the short-term and long-term health outcomes, costs and diagnosis yields, and to identify the cost-effective cascade protocols.
We developed a new decision model to simulate cascade protocols, so that we could compare them without requiring their implementation in practice, and to predict long-term health outcomes and costs, which are observable only in a study with very long follow-up. We informed the model with the characteristics of index cases and relatives estimated in the analysis of PASS data from the Welsh and Wessex FH services (the data are described in Chapter 3, PASS Wales and Wessex Cascade Service Data, and the analysis is described in Service data analysis); the long-term health outcomes and costs of diagnosed and undiagnosed FH patients estimated by our cost-effectiveness model, described in Cost and health benefits of diagnosis of people with familial hypercholesterolaemia in the long term; and other sources of data and input from the stakeholder group.
Methods
The cost-effectiveness analysis took the perspective of the UK NHS at a 2019 price base. We discounted future costs and health benefits to their present value at 3.5% per annum. 135
Population
The population comprised patients who were diagnosed with FH based on their clinical characteristics, referred to as index cases, and their relatives.
We considered only the index cases who scored above the threshold for genetic testing used in each FH service. This was the WDLCN score of ≥ 6 in the Welsh PASS data and possible or definite FH according to the Wessex-modified Simon Broome criteria in the Wessex FH PASS. The index cases may or may not have FH. Index cases for whom the genetic test identified a FH mutation have monogenic FH (the genetic test is perfectly specific), and index cases for whom the genetic test did not identify a FH mutation do not have FH (the genetic test is perfectly sensitive). For those index cases for whom the genetic test identified a VUS, we assumed that a proportion have FH, reflecting that some VUSs are reclassified over time as pathogenic or non-pathogenic as more evidence emerges. Given that FH follows an autosomal dominant inheritance pattern, the prevalence of FH among relatives depends on their kinship: 50% among first-degree relatives of a FH index (and among second-degree relatives of an affected first-degree relative), and 25% among second-degree relatives of a FH index. 8 We did not consider the long-term outcomes of index cases because their selection to the cascade does not affect their management.
The relatives are considered in terms of their true FH status (affected with FH or not affected with FH), age, sex, LDL-C level, prior CVD history and LLT at the time of the cascade. We selected these characteristics because they determine disease status, can be used to inform diagnosis and are prognostic factors for the long-term outcomes. We considered age in terms of five age bands (0–9, 10–17, 18–39, 40–59 and ≥ 60 years), given the NICE and HEART UK consensus of offering LLT to children aged ≥ 10 years,8,141 the transition to adulthood at age 18 years, the eligibility for routine CVD risk assessments in the general population from age 40 years and the age cut-off point of 60 years for considering CVD to be premature. 108
Conceptual model
Figure 17 shows our conceptual model. We interpreted the cascade as a three-stage process: (1) selecting index cases to be cascaded, (2) contacting relatives and (3) testing relatives.
FIGURE 17.
Conceptual model. Relatives are correctly diagnosed if their index is selected to the cascade, the relatives are tested, and the test is correct (full arrows). Relatives are not diagnosed if their index is not selected to the cascade, or if relatives are not tested. FH relatives who are correctly diagnosed are treated with LLT, and hence have lower CVD risk than FH relatives who are not diagnosed. Our base-case scenario conservatively assumes that FH relatives who are on LLT at the time of the cascade do not have additional health benefits or costs from FH diagnosis, and hence do not benefit from diagnosis. This is tested in a scenario analysis, in which we assume that diagnosis reduces LDL-C level by 50% irrespective of prior treatment. Non-FH relatives who are correctly diagnosed have no change in management. We assumed that non-FH relatives who are incorrectly diagnosed incur greater costs (because of the monitoring), but experience no health effects. Even though some non-FH relatives are at high CVD risk (because of other risk factors, e.g. age) and may benefit from LLT, we excluded this effect in the base-case scenario because the management of these patients should be conducted independently from cascade testing. MFH, monogenic familial hypercholesterolaemia.

Cascade protocols
For each stage, we considered the alternative strategies and selected those that were likely to be feasible for services to implement, and hence should be compared in the cost-effectiveness analysis. The different approaches to each stage are combined within the model to produce overall cascade protocols for evaluation.
Stage 1: index selection strategies
We considered two types of strategies: strategies using clinical scores as a screening tool to select index cases for genetic testing (as per the Welsh and Wessex FH services), and strategies relying on the clinical score without the genetic test. The clinical scores included the WDLCN (base case), the age-adjusted WDLCN and the Simon Broome criteria (both examined in scenario analyses), at various cut-off points. We were unable to consider the standard DLCN score because it is not used by the Welsh and Wessex FH services. We excluded strategies in which cascading to relatives was carried out for indexes with a VUS because testing of relatives for a VUS is part of the research pathway to establish pathogenicity.
Stage 2: contact strategies
We considered two types of cascade contact pattern: sequential cascade, whereby second-degree relatives are contacted only if the first-degree relative is diagnosed, and simultaneous cascade, whereby first- and second-degree relatives are contacted simultaneously. Simultaneous cascade increases the yield. However, it involves testing a greater proportion of non-FH relatives and has ethics implications in that, by diagnosing the second-degree relative, the first-degree relative who links to the index may have their FH status revealed without consent.
We considered two methods of contact for adult relatives: direct, whereby the FH service contacts the relative directly, and indirect, whereby the index (or the relative, as relevant) contacts the relatives, in line with our systematic review (see Chapter 2, Review 1: effectiveness of cascade-testing protocols among relatives for familial hypercholesterolaemia).
Stage 3: testing strategies
We compared five types of strategies to diagnose relatives, from testing all relatives with the genetic test to offering the genetic test depending on relatives’ PT-LDL-C level and whether or not they are on LLT at the time of the cascade, to diagnosing relatives solely on their LLT status and PT-LDL-C level.
We used LLT status as a component of the diagnostic strategies because a large proportion of FH relatives were on LLT at the time of the cascade in the PASS service (see Service data analysis). We used LDL-C level given the differences in its distribution among relatives with and relatives without FH. 77,82,162 We did not include other clinical or biochemical characteristics, given that a systematic review conducted in parallel with this project found no evidence that could be used in addition to LDL-C level (see Chapter 2, Review 4: the diagnostic accuracy of clinical and biochemical criteria and scoring systems for the diagnosis of familial hypercholesterolaemia among relatives). We did not use the LDL-C thresholds proposed by Starr et al. 77 (and recommended in the 2008 version of NICE CG718) because their age groups did not match the age groups in our evaluation. Our approach allows us to compare testing strategies using a wide range of LDL-C thresholds and identify those that result in the highest net health gain.
We excluded strategies involving screening or diagnosis based on LDL-C levels among relatives on LLT at the time of the cascade because we could not reliably estimate individuals’ PT-LDL-C levels. Therefore, we compared two types of testing strategies: one set assumes that relatives on LLT at the time of the cascade are diagnosed with the genetic test, and the other set assumes that relatives on LLT are assumed to have FH without further testing (and accounts for misdiagnosis, as some relatives on LLT may not have FH).
Table 16 shows our approach to strategy naming. For example, strategy N3_H6_L3_T1 refers to a strategy whereby relatives who are not on LLT have a cholesterol test and are assumed to have FH if their PT-LDL-C level is above the higher cut-off point of 6 mmol/l, have the genetic test if their PT-LDL-C is between 3 and 6 mmol/l, and are assumed not to have FH if their PT-LDL-C level is < 3 mmol/l; relatives who are on LLT at the time of the cascade are assumed to have FH. Overall, we compared 68 testing strategies.
| Component | Coding element | Explanation |
|---|---|---|
| Structure of testing strategy | N1 to N5 | N1: relatives diagnosed according to their PT-LDL-C levels (without the genetic test) N2: relatives with PT-LDL-C levels above a specific cut-off point are tested with the genetic test and diagnosed accordingly, whereas relatives with PT-LDL-C levels below this cut-off point are assumed not to have FH N3: relatives with PT-LDL-C levels above a specific cut-off point are assumed to have FH, relatives with PT-LDL-C levels below this higher cut-off point but above a lower cut-off are tested with the genetic test and diagnosed accordingly, and relatives with PT-LDL-C levels below the lower cut-off are assumed not to have FH N4: relatives with PT-LDL-C levels above a specific cut-off point are assumed to have FH, and relatives with PT-LDL-C below this cut-off point are tested with the genetic test and diagnosed accordingly N5: relatives are all tested with the genetic test and diagnosed accordingly |
| Higher cut-off point of PT-LDL-C, when applicable | H1 to H6 | PT-LDL-C level of ≥ 1 mmol/l to ≥ 6 mmol/la |
| Lower cut-off point of PT-LDL-C, when applicable | L1 to L5 | PT-LDL-C level ≥ 1 mmol/l to ≥ 5 mmol/la |
| Approach to relatives who are on LLT at the time of the cascade | T0 or T1 | T0: if relatives are tested with the genetic test T1: if relatives are assumed to have FH |
Model structure
We implemented the model as a series of four linked modules, which map to the cascade process presented in Figure 17. This model structure accounts for the first- and second-degree relatives who may be misdiagnosed or lost to the cascade, given the accuracy of testing strategies, the probability of relatives coming forward for testing and the proportion of index cases selected to be cascaded:
-
For stage 1 (index selection), a decision tree calculated the outcomes and costs of cascading for the index cases selected to the cascade given the clinical score threshold and the genetic test results (if applicable to the strategy).
-
For stage 2 (contacting relatives), a decision tree calculated the outcomes and costs of the relatives per index selected to the cascade depending on the pattern and method of contact and the implications of this for engagement with the cascade.
-
For stage 3 (testing relatives), a decision tree calculated the outcomes and costs per relative who was tested given the testing strategy, using as inputs the long-term health outcomes and costs, depending on whether relatives were t.reated for FH.
-
The fourth module linked the three decision trees together to calculate the outcomes and costs per index family cascaded.
See Report Supplementary Material 5, ‘Model implementation’ for details on the model structure, and ‘Validation’ for details on the validation. We built the model in Microsoft Excel.
Model inputs
Table 17 summarises the model inputs related to patient characteristics and completion of the cascade for the base-case scenario, with the full list of model inputs provided in Report Supplementary Material 5, ‘Model inputs’. For the base-case, we mostly used data from PASS Wales, because of the larger sample size and greater detail recorded in the database. The exception was the proportion of FH relatives on LLT, which we based on PASS Wessex, because we thought it was more generalisable to England than the PASS Welsh data.
| Type | Parameter | Value | Source |
|---|---|---|---|
| Index characteristics | |||
| Proportion of indexes who have FH for each WDLCN score | WDLCN = 6 | 15% | Analysis of PASS Wales data See Figure 10 and Report Supplementary Material 5, Table 1 for more details |
| WDLCN = 7 | 16% | ||
| WDLCN = 8 | 14% | ||
| WDLCN = 9 | 26% | ||
| WDLCN = 10 | 30% | ||
| WDLCN = 11 | 29% | ||
| WDLCN ≥ 12 | 64% | ||
| VUS | Proportion of indexes with a VUS | 4% | PASS Wales and Wessex See Table 11 and Report Supplementary Material 5, Table 1 for more details |
| Proportion of VUSs reclassified to pathogenic out of those reclassified | 75% | ||
| Relatives’ characteristics | |||
| Relatives who were contacted, by age (years) | Proportion of females | 53% | Analysis of PASS Wales data See Table 11 and 12, and Report Supplementary Material 5, Tables 2–4 for more details |
| 0–9 | 9% | ||
| 10–17 | 15% | ||
| 18–39 | 35% | ||
| 40–59 | 26% | ||
| ≥ 60 | 15% | ||
| Odds ratios to calculate the probability that relatives are tested, given that they were contacted | Females vs. males | 1.55 | |
| First degree vs. second degree and more distant | 1.55 | ||
| Direct vs. indirect contact | 2.11 | ||
| Relatives aged < 18 vs. ≥ 18 yearsa | 2.67 | ||
| Number of relatives | Number of first-degree relatives contacted per index selected to the cascade | 2.20 | |
| Number of second-degree relatives contacted per first-degree relative diagnosed with FH | 1.79 | ||
| Probability that FH relatives have prior CVD history, by age (years) | 0–9 | 0% | |
| 10–17 | 0% | ||
| 18–39 | 2% | ||
| 40–59 | 14% | ||
| ≥ 60 | 31% | ||
| Probability that FH relatives with prior CVD history are on LLT (all ages) | 86% | ||
| Probability that FH relatives with no prior CVD history are on LLT, by age (years) | 0–9 | 0% | Analysis of PASS Wessex data See Table 13 and Report Supplementary Material 5, Table 2 for more details |
| 10–17 | 0% | ||
| 18–39 | 8% | ||
| 40–59 | 27% | ||
| ≥ 60 | 50% | ||
| Probability that non-FH relatives have prior CVD history | 0–39 | 0% | Assumption |
| 40–59 | 11% | Health Survey for England 2017,163 calculated from Table 1 | |
| ≥ 60 | 32% | ||
| Probability that non-FH relatives with prior CVD history are on LLT (all ages) | 81% | Steen et al.,164 calculated from Table 2 | |
| Probability that Non-FH relatives with no prior CVD history are on LLT | Age 0 to 39 | 0% | Assumption |
| Age 40–59 | 7% | Health Survey for England 2017,163 calculated from Table 10 | |
| Age 60 + | 35% | ||
| Number of index families assessed within the cascade | 2618 | PASS Wales See Figure 9 |
|
| Years between first and last genetic test of relative recorded in PASS Wales | 14.48 | ||
As the Wales and Wessex services do not collect LDL-C data from relatives systematically, we based the distribution of relatives across PT-LDL-C bands on data from the Dutch FH screening programme. See Report Supplementary Material 5, ‘Cholesterol levels in relatives’, for more details and a comparison between the Dutch and the Welsh and Wessex FH service data on LDL-C levels.
To calculate the costs, we reviewed the costing assumptions made for NICE CG71,8 asked nurses from five FH services across the UK about how their service operated and validated our assumptions with the stakeholder group (including PPIE representation). The costs are summarised in Table 18 and detailed in Report Supplementary Material 5, ‘Cascade costs’. Assuming that the index cases were diagnosed clinically and known to the FH service, we estimated that selecting index cases to be cascaded costed between £29 and £446 per index assessed, depending on the method and whether or not index cases were selected, and that testing relatives costed between £61 and £184 per relative, depending on the strategy and the test results.
| Activity | Resource | Cost (£) |
|---|---|---|
| Selecting index cases to be cascaded | ||
| Appointment with index to assess clinical score | Arranging appointment: 10 minutes, secretarial staff With FH nurse: 20 minutes |
23 |
| Appointment to do the genetic test, if applicable | Arranging appointment: 10 minutes, secretarial staff With FH nurse or genetic counsellor: 60 minutes (includes blood collection) |
61 |
| Test | Genetic test | 305 |
| Communication of results | Telephone or letter Secretarial staff: 10 minutes |
6 |
| Appointment to start the cascade | Arranging appointment: 10 minutes, secretarial staff With FH nurse or genetic counsellor: 50 minutes |
51 |
| Contacting relatives | ||
| Additional cost of contacting relatives directly, per relative | 10 minutes of secretarial staff time Printing and posting letter |
8 |
| Testing relatives | ||
| Appointment with relative | Arranging appointment: 10 minutes, secretarial staff With FH nurse or genetic counsellor: 60 minutes |
61 |
| Blood sample (each) | 4 | |
| Test | Cholesterol test and/or | 1 |
| Genetic test | 108 | |
| Communication of results | Telephone or letter Secretarial staff: 10 minutes |
6 |
Long-term health outcomes and costs
For the long-term health outcomes and costs of managed and unmanaged FH relatives, we used the cost-effectiveness model described in Cost and health benefits of diagnosis of people with familial hypercholesterolaemia in the long term. For the outcomes of non-FH relatives, we used the NICE CG181110 cost-effectiveness model, adapted to include young patients and patients at low risk of CVD. See Report Supplementary Material 5, ‘Long-term outcomes of relatives who do not have monogenic familial hypercholesterolaemia (FH)’, for details.
Analytical methods
We compared the protocols in two steps, given that it was not feasible to compare all possible protocols simultaneously (see Report Supplementary Material 5, ‘Further information on analytical methods’, for details). Throughout, we used the cost-effectiveness thresholds of £15,000 and £20,000 per QALY,135,153–157 as in the analysis in Cost and health benefits of diagnosis of people with familial hypercholesterolaemia in the long term.
We first compared testing strategies and included those with a probability of ≥ 5% of being cost-effective in each age group, in 2000 simulations. We also included ‘harmonised testing strategies’, whereby the same testing strategy is used across age groups, and a strategy in which genetic testing is offered to all relatives, as per the Welsh and Wessex services.
In the main analysis, the selected testing strategies were included with the options for contacting relatives (direct or indirect, concomitant or sequential cascade) and with the options for selecting index cases (using different thresholds for the WDLCN score), and run probabilistically over 2000 simulations. 157 We calculated incremental cost-effectiveness ratios and the cost-effectiveness acceptability curve, which plots the probability that each protocol has the highest net health gain at a range of thresholds. 165 We calculated the expected value of perfect information for the population of index families in the UK, assuming that the average number of index families assessed per year by the FH service in Wales per million population is generalisable.
We termed the cascade protocol with the highest net health gain in the base case the ‘base-case cost-effective protocol’, and the cascade protocol with the highest net health gain using the same testing strategy for all ages the ‘harmonised cost-effective protocol’.
Scenario analyses
We conducted 21 deterministic scenario analyses to understand the sensitivity of the model to our assumptions. In the scenarios in which the protocol with the highest net health gain differed from the base-case cost-effective protocol, we calculated the loss to the NHS if the base-case cost-effective protocol was implemented, but the alternative assumptions are correct. Report Supplementary Material 5, ‘Scenario analysis’, details the scenarios. We conducted an additional scenario in which genetic testing was not available, because there may be times when genetic testing capacity is constrained (e.g. because of demand, due to either FH or other conditions).
We conducted threshold sensitivity analyses to determine the following: the odds ratio for the likelihood that relatives come forward for testing with direct, compared with indirect, contact, given the uncertainty about this effect; the additional cost of contacting relatives directly, given its uncertainty and variability depending on how services are organised; and the cost of genetic testing among relatives, given the feedback by the stakeholder group that it is likely to reduce over time.
Results
Testing strategies taken forward
The following strategies had ≥ 5% probability of generating the highest net health gain at the £15,000- or £20,000-per-QALY threshold (full results are available in the Report Supplementary Material 6):
-
among relatives aged 0–9 years: N3_H5_L3_T1
-
among relatives aged 10–17 years: N3_H5_L3_T1 and N3_H5_L2_T1
-
among relatives aged 18–39 years: N3_H5_L3_T1, N3_H6_L3_T1 and N3_H5_L2_T1
-
among relatives aged 40–59 years: N3_H6_L2_T1, N2_H3_L_T1, N3_H6_L2_T1 and N2_H2_L_T1
-
among relatives aged ≥ 60 years: N3_H5_L1_T1, N3_H6_L2_T1 and N3_H6_L2_T0.
The ‘N3 strategies’ have good diagnostic performance. For example, among first-degree relatives (who have a pre-test probability of 0.5 for FH), the strategy N3_H5_L3_T1 correctly diagnoses 90% of FH relatives (sensitivity) and 88% of non-FH relatives (specificity), at a diagnostic cost of £111 per relative tested. The strategy of genetically testing all (N5_H_L_T0) had perfect sensitivity and specificity, at a diagnostic cost of £180 per relative tested.
For the full cost-effectiveness analysis, we included the aforementioned testing strategies together with harmonised strategies, whereby the same testing strategy is used for all age groups. In total, we compared 54 combination testing strategies and 10 harmonised testing strategies.
Cost-effectiveness of cascade-testing protocols
Table 19 shows the cost-effectiveness frontier comparing the cascade-testing protocols (full results are available in Report Supplementary Material 7 and in Report Supplementary Material 5, ‘Results’). The results reflect all relatives who could potentially be reached by the cascade and are reported per index family considered for cascading. Overall, we compared 1792 cascade protocols.
| Type of index strategy | WDLCN cut-off score | Cascade pattern | Method of contact | Testing strategies, by age (years) | QALYs vs. cheapest | Costs vs. cheapest (£) | ICER: cost per QALY gained (£) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–9 | 10–17 | 18–39 | 40–59 | ≥ 60 | |||||||
| Including all selected strategies | |||||||||||
| Select indexes to the genetic test; cascade relatives from indexes with FH mutation | 12 | Sequential | Indirect | N3_H5_L3_T1 | Not applicable as cheapest protocol | ||||||
| Direct | N3_H6_L3_T1 | 0.010 | 13 | 1262 | |||||||
| 10 | N3_H5_L3_T1 | N2_H3_L_T1 | N3_H6_L2_T0 | 0.011 | 13 | 1930 | |||||
| 9 | Concomitant | N3_H6_L3_T1 | 0.043 | 131 | 3628 | ||||||
| 7 | N3_H5_L3_T1 | N3_H6_L3_T1 | N2_H3_L_T1 | N3_H6_L2_T1 | 0.060 | 201 | 4184 | ||||
| 6 | N3_H6_L3_T1 | 0.083 | 340 | 6000 | |||||||
| N3_H5_L3_T1 | N3_H6_L3_T1 | N2_H3_L_T1 | N3_H6_L2_T1 | 0.110 | 511 | 6183 | |||||
| N3_H5_L3_T1 | N3_H6_L3_T1 | N2_H2_L_T1 | N3_H6_L2_T1 | 0.111 | 522 | 13,996 | |||||
| N3_H5_L3_T1 | N3_H5_L2_T1 | N3_H6_L3_T1 | N2_H2_L_T1 | N3_H6_L2_T1 | 0.113 | 549 | 18,175 | ||||
| N3_H5_L3_T1 | N3_H5_L2_T1 | N3_H6_L3_T1 | N2_H2_L_T1 | N3_H5_L1_T1 | 0.113 | 550 | 19,288 | ||||
| N3_H6_L2_T1 | 0.115 | 601 | 24,342 | ||||||||
| N5_H_L_T1 | N5_H_L_T1 | N5_H_L_T1 | N5_H_L_T1 | N5_H_L_T1 | 0.115 | 625 | 61,589 | ||||
| Including only harmonised strategies | |||||||||||
| Select indexes to the genetic test; cascade relatives from indexes with FH mutation | 12 | Sequential | Indirect | N3_H5_L3_T1 | Not applicable as cheapest protocol | ||||||
| 12 | Direct | N3_H6_L3_T1 | 0.010 | 13 | 1262 | ||||||
| 12 | Concomitant | N3_H6_L3_T1 | 0.019 | 38 | 2896 | ||||||
| 10 | N3_H6_L3_T1 | 0.043 | 131 | 3883 | |||||||
| 9 | N3_H6_L3_T1 | 0.059 | 200 | 4197 | |||||||
| 7 | N3_H6_L3_T1 | 0.083 | 340 | 5972 | |||||||
| 6 | N3_H6_L3_T1 | 0.110 | 510 | 6222 | |||||||
| 6 | N3_H6_L2_T1 | 0.115 | 601 | 19,092 | |||||||
| 6 | N5_H_L_T1 | 0.115 | 625 | 61,589 | |||||||
The protocol with the highest net health gain at the threshold of £15,000 per QALY, the base-case cost-effective protocol, is detailed in Table 20. Per index assessed within the cascade, this protocol diagnoses 52% of FH relatives (0.31 FH relatives) and misdiagnoses 2% of non-FH relatives as having FH, at a diagnosis cost of £536 per index assessed. The harmonised cost-effective protocol, that is the protocol that uses the same testing strategy for all age groups, has similar diagnostic performance, costs and QALYs, with a net health loss of 0.0003 QALYs, compared with the base-case cost-effective protocol.
| Component | Base-case cost-effective protocol | Harmonised cost-effective protocol |
|---|---|---|
| Index strategy | Select index cases with WDLCN score of ≥ 6 to have the genetic test Contact relatives of index cases in whom a FH mutation was detected |
|
| Type of cascade | Simultaneous: contact first- and second-degree relatives | |
| Method of contact | Direct: FH service to contact relatives directly | |
| Relatives on LLT at the time of the cascade | Assume that they have FH | |
| Relatives not on LLT at the time of the cascade | ||
| Aged 0–17 years | Conduct LDL-C test If LDL-C level is ≥ 5 mmol/l, assume they have FH If LDL-C level is < 5 mmol/l and ≥ 3 mmol/l, conduct genetic test If LDL-C level is < 3 mmol/l, assume they do not have FH |
Conduct LDL-C test If LDL-C level is ≥ 6 mmol/l, assume they have FH If LDL-C level is < 6 mmol/l and ≥ 3 mmol/l, conduct genetic test If LDL-C level is < 3 mmol/l, assume they do not have FH |
| Aged 18–39 years | Conduct LDL-C test If LDL-C level is ≥ 6 mmol/l, assume they have FH If LDL-C level is < 6 mmol/l and ≥ 3 mmol/l, conduct genetic test If LDL-C level is < 3 mmol/l, assume they do not have FH |
|
| Aged 40–59 years | Conduct LDL-C test If LDL-C level is ≥ 2 mmol/l, conduct genetic test If LDL-C level is < 2 mmol/l, assume they do not have FH |
|
| Aged ≥ 60 years | Conduct LDL-C test If LDL-C level is ≥ 6 mmol/l, assume they have FH If LDL-C level is < 6 mmol/l and ≥ 2 mmol/l, conduct genetic test If LDL-C level is < 2 mmol/l, assume they do not have FH |
|
The protocol that correctly diagnoses the greatest proportion of relatives is similar to the base-case cost-effective protocol apart from the testing strategy, in that all relatives who come forward for testing receive the genetic test. This protocol correctly diagnoses 56% of FH relatives and has a diagnosis cost of £589 per index assessed. Compared with the base-case cost-effective protocol, this represents a net health loss of 0.003 QALYs per index family assessed within the cascade, owing to its additional costs (i.e. health losses elsewhere in the NHS outweigh the health gains associated with improved diagnosis).
The usual protocol of contacting relatives indirectly and sequentially, and testing with the genetic test, does not lie on the cost-effectiveness frontier. The model predicts that the usual protocol diagnoses 36% of the FH relatives at a cost of £482 per index assessed; compared with the base-case cost-effective protocol, it results in a net health loss of 0.045 QALYs per index family.
Scenario: genetic testing is not available
If genetic testing is not available, the protocol with the highest net health gain at the £15,000-per-QALY threshold is to select index cases with a WDLCN score of ≥ 6, directly contact their first- and second-degree relatives simultaneously, and assume that relatives have FH if they are on LLT or if their PT-LDL-C level is ≥ 4 mmol/l (or 5 mmol/l if aged 40–59 years). This protocol diagnoses 41% of FH relatives at a diagnostic cost of £388 per index assessed. Compared with no cascade, this protocol results in a net health gain of 0.07 QALYs per index family, but it is not cost-effective compared with the base-case cost-effective protocol if genetic testing is available (net health loss = 0.02 QALYs per index family). Full results presented in Report Supplementary Material 5, ‘Scenario where genetic testing is not available or its capacity is constrained’.
Expected value of perfect information
If the average number of index cases who were assessed by the FH service in Wales per year (n = 181) is representative of the UK, and generalisable for a period of 10 years, the effective population of index cases who could be assessed over this period is 27,793 (including discounting to present values at 3.5% per annum15). The expected value of perfect information is low, at £225,442 at the £15,000-per-QALY threshold and £368,890 at the £20,000-per-QALY threshold (see Report Supplementary Material 5, ‘Expected value of perfect information’). For comparison, this project funding cost was £840,042. 28
Scenario analysis
The base-case cost-effective protocol was the protocol most often associated with the greatest net health gain in the scenarios, including using the possible Simon Broome criteria to select index cases for genetic testing (see Report Supplementary Material 5, ‘Results of scenario analysis’, for details). When this was not the case, there was typically a small difference in net health gain between the base-case cost-effective protocol and the protocol that had the highest net health gain in the scenario (< 0.0001 QALY).
Two of the scenarios relating to longer-term outcomes had a greater impact. In the scenario including the benefits of misdiagnosis as FH among non-FH relatives, the protocol with the highest net health gain assumes that relatives aged ≥ 60 years are treated as if they had FH, regardless of their other characteristics. This occurs because, under this scenario, misdiagnosis of non-FH relatives who are at high risk of CVD, but who are not on LLT, represents a net health gain.
In the scenario excluding the effect of LDL-C burden, that is assuming that the effect of elevated LDL-C on CVD risk does not increase over time (which is informed by the results of scenario 8 of the cost-effectiveness analysis on long-term outcomes from diagnosis; see Cost and health benefits of diagnosis of people with familial hypercholesterolaemia in the long term), the protocol with the highest net health gain was similar to the base-case cost-effective protocol, although only index cases with a WDLCN score of ≥ 9 are selected for genetic testing, relatives aged 0–9 years and 10–17 years are diagnosed with FH if their PT-LDL-C level is ≥ 6 mmol/l and the PT-LDL-C cut-off points to select older relatives for genetic testing are more stringent. This occurs because, without accounting for LDL-C burden, the CVD risk of undiagnosed individuals is lower, hence the benefits of diagnosis are smaller; as a result, there is less scope to invest in diagnosis.
Threshold sensitivity analysis
The base-case results were robust to reductions in the odds ratio of direct contact on cascade completion down to an odds ratio of 1.02 (base case: 2.11, 95% CI 1.67 to 2.69), whereby the protocol with the highest net health gain switched from direct to indirect contact at the £15,000-per-QALY threshold. For contacting relatives indirectly to be a net health gain, the additional costs of contacting relatives directly would have to be at least £270 per relative at the £15,000-per-QALY threshold (compared with £8 in the base case). The testing strategy with the highest net health gain changed to genetic testing if relatives’ genetic tests cost ≤ £10.80 per relative (10% of the base-case cost of £108) at the £15,000-per-QALY threshold, or if relatives’ genetic tests cost < £32.40 per relative (30% of the base-case cost) at the £20,000-per-QALY threshold.
Implications for service development
Since 2005, the Welsh FH service has assessed 2618 indexes, amounting to 181 indexes per year. Assuming that this number generalises to the UK, and scaling up to account for population size (the entire UK population being ≈18 times larger than the Welsh population), 3229 index families could be assessed per year in the UK. If these indexes were assessed and their relatives cascaded with the base-case cost-effective protocol, 1175 FH relatives would be diagnosed, at a diagnosis cost of £2.06M and requiring 5555 genetic tests. Compared with not conducting cascade, the incremental net health gain is 387 QALYs.
The cost-effective harmonised protocol achieves similar outcomes, with 1153 FH relatives diagnosed, at a diagnosis cost of £2.03M, and requiring 5324 genetic tests. Compared with this protocol, the base-case cost-effective protocol results in an incremental health gain of 1.3 QALYs.
Under the usual protocol of indirectly contacting relatives, only contacting the second-degree relatives following diagnosis of the first-degree relative, and offering genetic tests to all relatives, 808 FH relatives are diagnosed, at a cost of £1.85M and requiring 5447 genetic tests. Compared with this protocol, the base-case cost-effective protocol results in an incremental net health gain of 171 QALYs.
Under the best-yield testing strategy, whereby services directly contact the first- and second-degree relatives simultaneously, and offer genetic tests to all, 1276 FH relatives are diagnosed, at a diagnosis cost of £2.26M, and requiring 7488 genetic tests. Compared with the base-case cost-effective protocol, this protocol results in a net loss of health of 12.2 QALYs.
If there was a UK joined-up service that reached out-of-area relatives, 32% more relatives could be contacted (according to PASS Wales data), tested and diagnosed. Therefore, with the base-case cost-effective protocol, 1553 FH relatives would be diagnosed at a cost of £2.72M.
Summary of results and study limitations
The cascade protocols diagnosing the most relatives and achieving the highest net health gains were those in which index cases were genetically tested if they met the criteria currently used by the Wales and Wessex FH services (i.e. WDLCN score of ≥ 6 or Simon Broome possible or definite criteria) and in which the FH service made direct contact with first- and second-degree relatives simultaneously.
The cascade protocol with the highest net health gain (i.e. the base-case cost-effective protocol) involves diagnosing relatives according to their age, whether or not they are on LLT, their PT-LDL-C level and, if this information provides insufficient diagnostic certainty, conducting genetic testing (see Table 20). Compared with not conducting cascade testing, and at the national level, our model predicts that the base-case cost-effective protocol diagnoses 1175 (52%) FH relatives at a diagnosis cost of £2.06M and represents a net health gain of 387 QALYs, assuming that 3229 index families could be assessed per year in the UK.
The cost-effective protocol using the same testing strategy for all relatives regardless of age (i.e. the harmonised cost-effective protocol) achieves similar outcomes and may be preferable if additional nurse time (unaccounted for here) is required to implement a testing approach that differs according to relatives’ age.
The cost-effective protocols comprise testing strategies that misdiagnose some relatives. Within our model, the FH relatives who are incorrectly diagnosed as not having FH are those with lower PT-LDL-C levels, and hence are at lower risk of CVD and have smaller benefits from diagnosis (see Cost and health benefits of diagnosis of people with familial hypercholesterolaemia in the long term). We accounted for misdiagnosis among first- and second-degree relatives, but we were unable to account for consequences in more distant relatives (from index case).
The threshold analysis suggested that genetic testing all relatives has the highest net health gain if the cost of a relative’s genetic test is a fraction of the cost at the time of the analysis. However, we did not consider that knowing the true disease status of all tested relatives and the genetic mutation may have an inherent value, not only for patients and clinicians, but also to inform future research, which, in turn, may have benefits for patients. Conversely, we did not consider any disbenefits to patients from having confirmed FH.
The same yield at lower diagnosis costs may be achievable if the second-degree relatives are contacted only if their first-degree relative was diagnosed or did not come forward for testing. Testing second-degree relatives, even if first-degree relatives are not available, my give rise to ethics concerns by inadvertently revealing information on the first-degree relatives without their consent. If genetic testing is not available, conducting cascade testing with LDL-C achieves a net health gain compared with not conducting the cascade.
A UK joined-up service could diagnose more relatives (e.g. 1553 FH relatives per year in the UK vs. 1175 FH relatives if only in-area relatives can be cascade-tested), at a cost of £2.72M.
The model considers cascade to the first- and second-degree relatives. Owing to the lack of information in the PASS data about the degree of kinship to the index for non-first-degree relatives, we assumed that all non-first-degree relatives were second-degree relatives and did not explicitly consider cascade protocols contacting third-degree or more distant relatives. The implication is that our model cannot inform the cost-effectiveness of cascade protocols beyond second-degree relatives.
There are a few remaining uncertainties that, despite our extensive scenario analyses, we were unable to explore fully. There are several assumptions on generalisability: first, distribution of LDL-C levels from the Dutch cascade programme to the UK population of relatives and, second, characteristics of index cases and relatives, and the outcomes achieved by the Wessex and Welsh FH services, to the UK if similar FH services were implemented across the country. As with any diagnostic test, the cost-effectiveness of the specific WDLCN score and LDL-C level cut-off points depends on FH prevalence and the distribution of these characteristics among people with and people without FH. Furthermore, the model does not include the relationship between disease severity (e.g. in terms of LDL-C level) among the index cases and relatives, although this is likely to be correlated and may make using lower WDLCN score cut-off points less cost-effective. The limitations mostly relate to the available data to inform the cost-effectiveness model. We were unable to include index selection strategies using lower WDLCN score cut-off points, to include index selection strategies comparing alternative clinical scores or to consider cascade-testing protocols including third-degree and more distant relatives because of the limitations of the PASS data, being an administrative data set not designed to inform research. Given the limited data on lipid levels routinely collected by the Welsh and Wessex services, we requested data from the Dutch cascade screening programme (although a comparison with the available Welsh and Wessex FH service data suggested that they were generalisable; see Report Supplementary Material 5, ‘Cholesterol levels in relatives’). Our estimates of the resources involved in conducting the cascade were based on information from FH nurses, literature and feedback from the stakeholder group, because the PASS data did not include details on the staff time and grade. Furthermore, the cost-effectiveness analysis relies on in silico comparisons of cascade protocols that have not been used in clinical practice, and on the validity of the assumptions about the generalisability of available data, the effect of LDL-C burden and long-term CVD risk; hence the effectiveness and cost-effectiveness of the cascade protocols in clinical practice may differ.
Chapter 6 Acceptability of cascade-testing approaches for familial hypercholesterolaemia: qualitative study of health-care professional and patient experiences
Introduction
The nature and importance of detecting FH, including through cascade testing of undiagnosed relatives, has been discussed previously (see Chapter 2, Review 1: effectiveness of cascade-testing protocols among relatives for familial hypercholesterolaemia, and Chapter 5, Cost-effectiveness of alternative cascade-testing protocols for familial hypercholesterolaemia).
Earlier evidence on cascade testing has focused on differences in contacting relatives with respect to either direct contact (i.e. contact from the specialist clinic) or indirect contact (i.e. mediated informally by patients). For example, contact made directly by practitioners resulted in higher response rates and more relatives coming forward for cholesterol testing. 41 However, it has been suggested that a direct contact approach may be coercive and invasive. 166,167 A recent systematic review of 10 studies found that identification of new cases per index case tended to be greater when direct contact was used. 26
Qualitative work in this context has been relatively limited, with little consensus and mixed views about the approach to cascade testing. Exploration of index patients’ experiences in Scotland favoured indirect cascade testing. 168 However indirect contact by index patients may lead to inadequate counselling and a sense of obligation to be tested in solidarity with other family members. 166 In a more recent Australian study, index patients supported health professionals directly contacting relatives, suggesting that health professionals have greater credibility. 100 van El et al. 169 interviewed six stakeholders, including three health professionals (a lipidologist, a geneticist and a FH nurse), about cascade-testing approaches in the Netherlands as its national FH service transitioned from a direct to an indirect approach. This found that good provision of information and education to index patients was needed to facilitate indirect contact approaches and to ensure positive engagement of relatives. Greater empirical evidence is needed, not only from the index patients and cascade relatives, but also from health professionals.
In the UK, current methods of contacting cascade relatives vary, with some localities or regions adopting a direct, and others an indirect, contact approach. There is a lack of recent evidence on how these approaches are viewed by the patients and relatives who experience them. It is also unclear how these approaches or other factors in service pathways may influence relatives’ engagement with, and uptake of, testing from the perspectives of those health-care professionals who deliver them.
Aims
This qualitative study aimed to explore the acceptability of differing cascade-testing approaches for FH from the perspectives of individuals and their families with potential and confirmed FH, and health-care professionals involved in delivering cascade testing for FH. We explored their experiences and perceptions, including of the benefits or concerns associated with cascade testing, and the ways in which testing and service pathways may be optimised.
Methods
Context
Three regions were chosen to reflect differing current approaches in the UK, including varied methods of contacting relatives for FH cascade testing, different service structures, and varied numbers and types of health-care professionals and appointments involved in cascade-testing pathways. Region A has a mixed urban, semi-rural and rural, predominantly white, population. Region B includes a major city, with a non-white population of 14%, and surrounding semi-rural population. Region C includes two large, predominantly semi-rural counties, one with two major cities that include a non-white population of 15%. These regions are summarised in Box 1 by cascade-testing approach, based on information from service leads and participating professionals, and operating service specifications.
-
People with possible FH identified in primary care and in secondary care (e.g. cardiology) or by a FH specialist nurse screening pathology laboratory data for LDL-C results.
-
Referred to secondary care lipid clinic.
-
FH specialist nurse contacts people referred or those identified on laboratory results either via their GP or face to face via an alert when they have presented as an inpatient in secondary care or A&E.
-
People with possible FH invited to see lipid consultant and FH nurse, either in lipid clinic or locally via outreach clinic at patient’s district general hospital, to discuss and have sample taken for FH genetic testing in the same appointment.
-
Telephone appointment offered with the FH nurse if the patient is unwell or not able to attend to reduce the need to come to hospital for initial consultation. Follow-up appointment arranged with FH nurse, usually in the patient’s locality, to take bloods for FH testing.
-
FH-positive (index) patients invited to see FH nurse to take family pedigree (either face-to-face or telephone appointment).
-
FH nurse contacts first-degree relatives directly by letter (then telephone follow-up) to arrange an appointment to discuss and, if appropriate, have FH testing and further follow-up.
-
People with possible FH identified in primary care and secondary care are referred to FH screening programme based in secondary care lipid clinic within clinical genetics service.
-
Lipid consultant assesses patient in clinic for suitability for FH genetic testing and briefly explains testing to the patient.
-
Lipid consultant sends referral to FH specialist nurse who arranges an appointment with FH nurse through clinic administrator.
-
FH nurse sees the patient to explain and arrange genetic testing, if appropriate; results returned to the FH nurse and lipid consultant.
-
If negative FH test (i.e. no mutation detected), patient is offered further appointment with FH nurse to discuss result, either face to face in the lipid clinic or by telephone.
-
If positive FH test, index patient offered further face-to-face appointment with genetic counsellor to take the family pedigree and discuss family testing issues. Genetic counsellor then refers to FH Family History Co-ordinator, who prepares a letter for relatives.
-
Index patient is then given the letter to distribute to family members for them to take to their GP to be referred to their relative’s local lipid service (or back to the same lipid/genetics service if resident in same region as index patient and steps 2–6 repeated).
-
People with possible FH identified by primary care and secondary care referred to FH screening programme situated in secondary care.
-
FH specialist nurses triage referrals to be seen by them in outreach clinics within the person’s locality.
-
At the local clinic, the FH nurse explains FH and the possible outcomes of genetic testing, takes a family pedigree and, if appropriate, takes blood samples for testing.
-
If positive FH test, index patient is offered follow-up appointment with the FH nurse, locally when possible, to discuss the results, which family members should be contacted, whether to do so directly or indirectly, exploring with patient what may be appropriate for each relative, and discuss nature of family relationships.
-
If family dynamics between index patient and family members are perceived to be positive, then index patient passes on a letter to those family members, explaining FH. For those relatives with whom relationships are not perceived to be positive, the FH nurse will contact these family members directly by letter (with option of telephone follow-up).
-
Using either approach (direct contact or indirectly through index patient), the letter to relatives gives details on how the patient can self-refer directly to the FH service without the need to arrange this referral through their GP.
Sampling and data generation
A purposeful sample of individuals and relatives was chosen to reflect a sociodemographic range of index patients and relatives identified by cascade testing, across the three regions, including differing FH assessment and testing experiences, including contact within or outside an index patient’s area and with different health providers. A purposeful sample of health professionals was similarly sought to include a spectrum of roles and experiences in relation to differing types of FH index and cascade-testing provision in the three regions.
Semistructured one-to-one interviews were conducted by telephone (from October 2018 to December 2019) using a broad topic guide to explore the views and experiences of all respondents, and lasted for an average of 45 minutes. Prior to interview, all participants provided written informed consent.
Data analysis
Interviews were audio-recorded, anonymised, transcribed verbatim and checked for accuracy, and data were organised in NVivo version 11 (QSR International, Warrington, UK). For each set of patient/relative and health professional interviews, transcripts were read and reread to gain familiarity with the data and underwent line-by-line coding following a developed coding framework; similar codes were grouped together to construct themes.
Themes for each set of interviews were revised iteratively until final themes were agreed on. Two researchers, with differing disciplinary backgrounds in health psychology (field researcher/interviewer, LC) and academic primary care (health researcher and practising GP, JK), independently developed the coding and themes. These were developed in collaboration with the study PPIE lead (WR). Data analysis was ongoing alongside data collection until thematic saturation had been reached. Framework analysis170 was then used to chart the data and compare themes across regions in relation to the perceptions, views and concerns of patients and relatives, and health-care professionals involved in different cascade-testing approaches.
In a further stage, member checking171 was used to help enhance validity. All participants who agreed to being approached later in the study were subsequently invited to review and comment on summaries of preliminary findings (from patient/relative or health professional interviews according to whether they were a patient/relative or health professional, respectively) to check for accuracy and resonance with their experience, and to enable opportunity to review, develop or confirm findings.
Findings
Ultimately, 40 participants were interviewed, comprising a purposeful sample of 20 index patients and relatives and 20 health professionals, from across the three settings. All patients and relatives were white and English-speaking, and had experienced cascade testing in some form within the preceding 5 years. Relatives of index patients included children, siblings and parents. Their characteristics are summarised in Table 21. Participating health professionals were in active current practice and their range of roles, including in service pathways, are summarised in Table 22.
| Characteristic | Participants (N = 20) (n) |
|---|---|
| Index patient | 10 |
| Relative (first degree) | 10 |
| Sex | |
| Male | 5 |
| Female | 15 |
| Age (years) | |
| < 18 | 1 |
| 19–29 | 1 |
| 30–39 | 1 |
| 40–49 | 7 |
| 50–59 | 4 |
| 60–69 | 3 |
| 70–79 | 2 |
| ≥ 80 | 1 |
| Highest formal education | |
| Degree-level qualification or above | 7 |
| School and college level (GCSE/O Level/CSE/A Level/equivalent vocational) | 8 |
| No formal qualification | 5 |
| Initial contact method used | |
| Direct | 5 |
| Indirect | 4 |
| Hybrid combination | 11 |
| Cascade-testing counselling (first contact for assessment) | |
| FH specialist nurse (in secondary care) | 3 |
| FH specialist nurse (from secondary care or community outreach) | 13 |
| Genetic counsellor (specialist centre) | 2 |
| Lipidologist (secondary care) | 2 |
| Characteristic | Participants (N = 20) (n) |
|---|---|
| Role | |
| Consultant lipidologist | 7 |
| Paediatric lipidologist | 2 |
| FH specialist nurse | 6 |
| FH service lead (genetic counsellor) | 1 |
| GP | 4 |
| Sex | |
| Male | 9 |
| Female | 11 |
| Setting | |
| Region C | 10 |
| Region B | 5 |
| Region A | 4 |
| Principal roles in FH service pathway | |
| Primary care detection, referral to lipid services, long-term management | 5 |
| Providing virtual lipid clinic advice to requests/referrals | 3 |
| Secondary care lipid clinic (FH specialist nurse/lipidologist) | 15 |
| FH nurse outreach (primary care outreach) | 4 |
| Satellite lipid clinics (specialist FH outreach to local hospitals) | 9 |
Communication about familial hypercholesterolaemia and testing in families: influence on acceptability of indirect and direct approaches
Index patients felt able to have conversations about FH testing with family members if they had close relationships. They described using communication they felt would help, such as focusing on the practicalities of blood testing, or using a jocular or informal tone, rather than attempting to convey detail:
I think it is probably better the more simplified it is...., so if people are just expecting to go for a blood test for screening...., I think people might have maybe less resistance to something which seems relatively harmless, than if they get overwhelmed with the big terms.
Index, male, 27, region A – direct
We kept it tongue in cheek and light-hearted and it was just a case of ‘There is this gene that we have inherited, our suspicions are that it goes back to my grandmother’.
Index, female, 40, region C – hybrid
Across all regions, health professionals found use of a solely indirect contact approach less successful. They were concerned that index patients might not convey letters from their service or explain the importance of getting tested to their relatives. Professionals also felt that they could not be confident what information about FH or testing had been communicated. In particular, success of the approach was beholden to relationships within the family. These factors could all increase opportunity for relatives to disengage from a cascade-testing offer:
If we take the pure indirect method, we have got no way of being really sure that the correct information has been passed on to relatives, so obviously there is a point of weakness there... and of course in some families... they refuse to speak to relatives and then we don’t really get to see those people because the family communication has broken down.
FH nurse, direct contact approach
An indirect approach had worked well for some patients with good family relationships, and for those who chose to informally support a direct approach by communicating with relatives to forewarn them about future correspondence they would receive from the specialist service:
We are a close family; I am close with my brother, so it was very easy for me to speak to him about it and saying ‘look I have had this done, I have got this diagnosis, you are the only one that really it could affect next’.
Index, female, 46, region B – indirect
I was sort of tipping the relatives off that somebody would be getting in touch with them from the hospital, so they knew that I had had this incident [diagnosed with FH] and that they were looking at screening everyone [in the family].
Index, male, 52, region A – direct
However, an indirect approach could often leave index patients feeling pressured, or unable to do this in the context of a negative relationship. They felt that they lacked the necessary information and wherewithal themselves, and that this was better and more appropriately undertaken independent of the family:
My [daughter] is a nightmare and it would be good for her to speak to somebody outside of the family, because us trying to get through to her the seriousness of the condition, she just says ‘oh’, you know, ‘you’re always on at me’.
Relative, female, 42, region C – hybrid
... He [brother] doesn’t want to know, and I don’t have enough to do with him really to thrash that one out with him.
Index, female, 56, region B – indirect
... people maybe don’t want you to contact them. I have fallen out with my sister, so it would be quite hard for me to contact my sister about this. I would rather go for someone else contacting her.
Index, male, 46, region B – indirect
In contrast, index patients spoke of several advantages of a direct approach. Even when close family relationships existed, some were concerned about communicating information to family members appropriately. Some also felt frustrated at being unable to persuade reluctant relatives to engage with testing, and would have opted for health professionals’ direct contact if this had been offered. Contact by service health professionals was seen as carrying more helpful credibility and authority:
... I understand people might not be too grateful that they have been summoned to hospital for something that they might have never heard of... but at the same time I think it might be quite difficult for family members to explain what it is.
Index, male, 27, region A – direct
Receiving a letter from somebody in the medical profession who is an expert in it carries more weight than just me saying you need to get this test done.
Relative, F, 77, region B – indirect
I think if a hospital was to contact you and say you really need to come for this test, I think it would be much more effective than your mum....
Relative, male, 52, region C – hybrid
Corresponding with patients’ perspectives, health professionals found that using a direct approach better ensured that contact with the offer of testing was made to relatives and that information about FH and testing could be conveyed appropriately and accurately. Some also highlighted their concerns about a potential ethics difficulty arising with an indirect approach, that returning to the index patient to determine if or why their relatives had not engaged may breach patient confidentiality. This meant that a solely indirect approach could create a dead end for engaging relatives. Using a direct contact method meant that services were better able to follow up non-response from relatives by telephone or by sending another letter:
I think the one frustrating thing about indirect cascade testing is, if a family doesn’t engage, where can you go from there really? You know what are the ethics in contacting the index again and saying ‘Did they get the letters?’ because you can’t really let the index know that people haven’t come in. [With] the direct method you can send letters again.
FH nurse, hybrid approach
Health professionals perceived, or had found, that a more flexible combined or ‘hybrid’ approach worked more effectively. This was informed by exploring the nature of relationships within families with the index patient, and their views on what would best work for them and their relatives. This entailed negotiating using direct or indirect or both methods for each relative. This might, for example, involve index patients wishing to inform a relative and pass information to them (indirect), but also with direct follow-up contact from the service. Conversely, an index patient might prefer their relative be contacted directly by the service only because of the nature of their relationship or because of insights about how their relative may prefer to be contacted or to hear about FH:
[I]t is just having that freedom for that hybrid approach and getting consent at that point to say ‘right, which relatives can we just go straight to? Which ones do you think need a bit more nurturing?’ Direct isn’t [always] better than indirect, it is a combination of the two that works best for each individual family... that’s another privilege of having [FH nurse-led model] within this service, that [they] have an hour with each patient,... while you draw the pedigree, you’re really finding out what the dynamic is in the family.
FH service lead, hybrid approach
For similar reasons, most index patients and relatives had experienced or perceived this ‘blended’ combination approach to be more acceptable:
... a blended approach, definitely, because I think if you do receive that letter, that can be, in itself, quite worrying, but purely relying on the individual [index], it is entirely dependent on that individual’s ability to convey the message and the seriousness of it.
Index, male, 43, region C – hybrid
Use of a FH nurse-led model appeared well suited to this approach. This was perceived to offer particular strengths in establishing continuity of care for index patients and their relatives, providing a single point of contact for them to maintain engagement with the process, and facilitating a therapeutic relationship and understanding of families’ needs. This was greatly valued by families who had experienced this approach:
... it definitely works better, with the nurse doing the whole thing, I think it is better for the patient and the family because they have got one consistent contact who then knows the dynamics of the family better and also the referral process.
FH nurse, indirect contact approach
The [FH] nurse that does all of the genetic testing and things and it is perfect, she is really, really nice and you don’t feel rushed, your family get to ask all of your questions and she came to my daughter’s first appointment too.
Index, female, 40 region C – hybrid
Communication and being informed about familial hypercholesterolaemia
Having positive interactions with health-care professionals who communicated effectively about FH reassured patients about FH testing and what a positive result would mean for their lives. People who had been able to discuss FH testing with their FH nurse, genetic counsellor or consultant recalled a good understanding of FH and the principles of genetic inheritance for FH:
They explained it to me as trying to find the gene in the human genome is like trying to find a spelling mistake in a novel, but once you know what page and what word is spelt wrong, then it is much easier for them to then see if my boys had the same thing.
Index, male, 43, region C – hybrid
Some health professionals felt that they or other colleagues lacked adequate time during busy appointments to ensure that messages had been understood by the patients, or to discuss family issues appropriately, compromising uptake of testing. This required well-developed communication skills and confidence to address any deeper issues that might act as a barrier to engagement with cascade testing, such as guilt or loss related to FH diagnosis, or sensitivity about inheritance or family issues:
... I think genetics is perceived as being a little bit mysterious, and in a busy medical appointment it is quite difficult to explain that.... I have got plenty of people who don’t turn up to their genotyping appointment and that is possibly my fault because I haven’t had the opportunity to explain in a way that they recognise the value of bothering to get tested.
Consultant lipidologist, indirect contact approach
... we have had issues with paternity come up, you know there are all sorts of potential issues,.... It is that whole perspective of the individual versus the family, but also having the confidence to explore because, if there are issues around bereavement or guilt and blame,....
FH nurse, hybrid approach
Familial hypercholesterolaemia nurses were considered to be well placed to take on this counselling role with support and training, while bringing specialist clinical knowledge and potentially greater time during appointments than medical colleagues.
A strong theme prioritised by health professionals was that communication about testing be focused on FH having effective treatment to prevent major cardiac events, and rather less foregrounded as genetic testing for a genetic disease. Introducing this too early could be too disconcerting and lead to patient disengagement:
When we come from a genetic test perspective, the language is far too alarming and we almost put them off the idea of having a test at all... thinking about this as a cardiac condition which is preventable, I think, is more helpful.
Consultant lipidologist, indirect contact approach
Engaging and communicating with a range of people with different health literacy was seen as an issue in this context. Professionals perceived that those from more socially advantaged and educated backgrounds may access FH services to a greater extent and be better able to assimilate the information presented to them:
... [focusing on genetics] does mean that you lend yourself as a service to people who are better resourced [in understanding], so people who perhaps have got higher levels of education, whose first language is English, etc., and it makes it more challenging for people who aren’t of that background, I think, to engage.
Consultant lipidologist, indirect contact approach
... we see a lot of middle-class people; we do see other people, but we see a lot of people from... the higher your social class and your intellect, the better you tend to be at accessing services.
FH nurse, hybrid approach
An example of a family’s confusion about FH testing emphasised the importance of how FH and its inheritance is communicated to wider family members:
... we traced it [FH] back on my mum’s side but they didn’t offer my dad a test... he is absolutely convinced that he will be [have FH] as well because of his family medical history.
Index, female, 40, region C – hybrid
Some patients and relatives had been signposted towards more formal medical resources, such as journal articles and NICE guidelines, and found this off-putting and overwhelming. They appreciated provision of trusted information they were better able to understand, such as from the charity HEART UK, and also accessed wider NHS and external resources online or in written forms from other sources.
Participants felt that wider awareness raising and public understanding about FH was needed if its detection and successful cascade testing was to occur, including at school and through public media:
I mean, there is no one that goes on Holby City with FH is there?... make [people] look at themselves... we realised my grandma’s dad had a massive heart attack and died, grandma had a triple heart bypass... and my mum run ultra-marathons... yet there is still problems, why is that?... So it’s up to individual families to question it, isn’t it?
Relative, female, 23, region C
Access to and organisation of familial hypercholesterolaemia testing pathway
Access to cascade-testing pathway and interface with primary care
Index patients reported difficulties for relatives getting referred for cascade testing when they lived in other areas of the country. Across all settings, there were mixed experiences of accessing cascade testing indirectly through primary care. Some index patients noted that their GPs were well informed, had proactively queried FH and referred them. However, some relatives were challenged when presenting their contact letters from a FH service. They found that their GPs would not refer them, most commonly because they were young or had cholesterol results that did not reach the usual threshold of concern. This experience was familiar to FH professionals:
... being in X [country], a lot of family members are over the border in Y [country], so that is a whole other barrier, you know, of getting them tested.
Index, female, 46, region B – indirect
... so there is really only our eldest son that we don’t know for sure, but the GP says he can’t have it [referral for testing], because his cholesterol isn’t high.
Index, F, 68, region A
No, he [GP] won’t refer him [younger brother].... the GP said it wasn’t necessary, but I do happen to know that it is within the NICE guidelines....
Index, male, 43, region C
... once you get to a GP, they may say ‘OK... but I have checked your cholesterol and it is fine’... but they may still actually have FH and that mutation is more important to test... they may be batted away at that point, but the biggest barrier as a relative is getting that initial appointment with a GP.
FH service lead, hybrid approach
General practitioner respondents reported the challenges they had experienced in this context. These included a lack of information in relatives’ letters about which service to refer to and a lack of guidance about what to do if there was no appropriate local FH service. These challenges made it more resource intensive for the GPs to support these patients. GPs sought better information and communication from FH services to address these issues. The patchy nature of services for FH testing was also recognised by FH service staff as a challenge for primary care:
As a GP, it is particularly those out-of-area ones where you’re left with a letter that makes no sense in your locality and it can involve you ringing the organisational trust who made the initial diagnosis to say ‘what do you want me to do with this?’. And that takes up huge amounts of time.
GP, indirect contact approach
... the GP maybe hasn’t got anyone for them to refer to or it is a lone lipidologist who hasn’t got access to genetic testing or it is a genetic service that won’t see FH patients or no FH nurses in that area.
FH service lead, hybrid approach
Those relatives provided with the option on their cascade contact letter to self-refer to the FH service found this very helpful in facilitating their uptake of the offer:
[W]e don’t have time to go through our GPs... it is a really long-winded process, you have to wait for the GP’s appointment... by the time you have done that, got your referral and they have contacted you, it has just taken longer. So to have a letter sent out with the number, call this direct line, we will make your appointment for you [for lipid service]. Sorted.
Relative, female, 23, region C
With commissioners’ support, this self-referral process had been implemented with positive experiences in one region, although the requirement for GP referral for relatives outside the index patient’s service region still remains:
... the CCG’s agreed for anybody who is at risk of FH can just automatically make an appointment to see us without having a GP referral first, and that for us has made an absolutely massive difference to the amount of people who we have seen coming through for cascade testing in our area.
FH nurse, hybrid approach
Improving service pathways and patient-centred services
Continuity of care
Regardless of cascade-testing approach or service pathway, acceptability of cascade testing was enhanced when patients had experienced continuity of care for themselves and family members from a well-informed health-care professional. Most were able to name a key health-care professional, their FH nurse or lipid consultant or a genetic counsellor, with whom they had built up trust and rapport and felt that they could ask if they had problems or questions:
She [consultant] has done a good job... she knows my history; she has understood the changes in my medical history and my family’s, so she knows us better than anyone.
Index, male, 27, region A
We’re all seen at the FH clinic, which is really nice because [FH nurse] actually knows us all as a family... I really do think that it’s a good thing, you’re actually treated as a family, an FH family really, and I’m quite happy about that.
Relative, female, 66, region C
She [genetic counsellor] is always so approachable, she is brilliant... I have been going to the clinic for a number of years prior to [cascade testing of child]... I knew she was there and I could speak to her at any time.
Index, female, 46, region B
Streamlining services
Nevertheless, professionals described how cascade-testing services were insufficiently designed around patients and their families, and were instead based on existing organisation of the service. This was difficult for patients to navigate and resulted in their non-attendance. They felt that there were too many different health-care professionals in some cascade-testing pathways. This and waiting for referrals between professionals were experienced as significant points for attrition of relatives engaging with cascade testing:
Some patients are obviously put off by their wait or have been lost to follow-up as well... because the pathway has been split between relatives going to [see] genetic counsellors and probands [index patients] seeing the [FH] nurses.... However, [both] the whole family has the potential to be seen by me.
FH nurse, indirect contact approach
Professions sought to reduce inefficient overlap and duplication across different services (lipid, genetic, cardiac) and professionals involved, to streamline their service pathway. This included reducing the number of clinic appointments necessary, completing both counselling and blood sampling for FH testing in a single appointment, creating more flexibility in appointment times and having more outreach clinics rather than a single centralised hospital location. In addition, it was recognised that people respond differently to receiving a cascade offer and may not engage straight away, thus risking being routinely discharged and lost.
The current lack of a centralised information technology system in the UK for FH and related laboratory results, linking services from across all regions of the UK, was highlighted as a major issue. Professionals anticipated that this would improve better nationwide communication and optimise cascade testing:
Sending letters all across the UK and wider obviously makes cascade testing a little bit more distant and tricky, but as we see more services coming together nationally and communicating, communication improves and, ultimately, I think new systems will develop, meaning information will be more easy to pass from one area to another of who is being notified and contacted and how, and I think a good IT [information technology] system is going to be key there.
FH nurse, direct contact approach
Individual contexts for engaging with familial hypercholesterolaemia testing
Seeking an explanation for family history of cardiac events or premature death, and the desire or strongly felt obligation to protect younger generations of the family from the same, were the most common motivations for engaging with FH testing. Most people had been positive about and unsurprised to receive the offer of FH testing and subsequent diagnosis or its exclusion:
Everybody [in family] has been receptive to it [FH testing] and keen to know if potentially they have it because my mother’s stroke was so sudden and unexpected....
Index, female, 40, region C
Index patients explained why their relatives had not taken up the offer of cascade testing. Some, particularly if only indirectly contacted, were deterred by long waiting times for first appointments, or if several successive appointments with different professionals had been involved. Others noted their relatives’ nature, concerns about impact on lifestyle if diagnosed, having to take statins or similar medication, uncertainties about future employment or obtaining life insurance:
I was fine about it [testing], because the consultant had explained... it is manageable, I was OK about it. My brother wasn’t, to be honest, he went into a bit of a meltdown and started panicking about life, etc., but he is a different character to me.
Index, female, 42, region C
There has been all of this talk as well about the statins being not good... conflicting evidence all of the time... conflicting news reports... I think that doesn’t help people or puts people off.
Relative, male, 52, region A
I can remember having the conversation with my eldest son. He was worried that if he had got [tested], it was going to affect his insurance... that is why he thought he best not know.
Index, female, 68, region A
Parents with younger children
Respondents who were parents of younger children, and who had experience of being tested for FH, spoke about their conflicting feelings when considering testing their children. Considerable uncertainly and anxiety existed for some in not being able to test their children of pre-school age, with a concern that they be able to plan for the future as early as possible:
I understood that they wouldn’t medicate him... but I just wanted to know what I was in for. Every time I had an appointment with the consultant, I said ‘Can he come and get tested now?’, ‘No, he is only 5’. When he was 8, I said ‘I really want to know, he is not that far off being 10’.
Index, female, 46, region B
For older children, the decision could be more straightforward, with respondents feeling empowered by knowledge that could help their children’s future health, and for their child to be similarly informed. Others were more ambivalent and wondered if they had rushed their decision to establish if their child had FH. For example, they reflected on whether or not they should have given more thought to how this could affect their children in later life, particularly around potentially limiting career options and the ability to get life insurance:
... it never even occurred to me not to get them tested. Especially for my son... having such a high chance of heart attacks before the age of 35.
Relative, female, 42, region C
I have a new appreciation, I think, for why you may not want to get your children tested; I went gung hoe and all guns blazing. Now I think maybe I shouldn’t have got him tested, not actually got him a diagnosis, just had his cholesterol treated, if I really had thought it through properly. What have I set him up for in adult life?... his future and job prospects, certain careers, and a mortgage... have I scuppered his chances of getting life insurance?
Index, female, 46, region B
One mother described how she was unable to access psychological support for her teenage daughter following her cascade testing and FH diagnosis, which had amplified anxiety against a background of an existing condition:
My daughter has had a really bad year, she now has quite severe anxiety... You know the problem is her processing the fact that for the rest of her life she has a hole in her heart, plus a faulty gene... it has generated another concern and we feel like we have got nothing and nowhere with it.
Index, female, 40, region C
Overall, however, respondents were positive about their experience following cascade testing of their children, and again underlined the value of having continuity of care with a FH professional who had become known and trusted:
We took the children to the clinic... for blood tests, and I then had a phone call from [my FH nurse] to give me their results.... When my daughter sees the child consultant, [FH nurse] is always there as well.
Index, female, 42, region C
He [young son] has got a positive [FH] diagnosis, but... the main thing for me actually was having my consultant for both of us... he knows my history and you build up a rapport.... I am just so glad he is involved in [son’s] treatment as well.
Index, female, 46, region B
Member checking
Member checking responses were received from 14 participants from among the purposeful sample interviewed, including comments on summaries of findings. These were confirmed as true to participants’ experiences. No reflections contesting or additional to findings presented were made. Examples of their comments are included in Appendix 3.
Summary of results and study limitations
This qualitative study has provided insights into the acceptability of different cascade-testing approaches in practice, and what happens in service pathways that may help or hinder engagement with testing.
For index patients and relatives, family relationships shaped experience of, and preference for, an indirect or a direct cascade approach or a combination of both. Experience of quality of communication about FH, the accessibility and organisation of pathways, and continuity of care further determined acceptability of approach. For index patients, making indirect contact worked less well, creating pressure about engaging relatives unless their relationship was conducive, or concern about communicating appropriate FH information. Direct contact of relatives by a specialist service was preferred as offering an independent, more credible and authoritative approach.
Professionals’ experiences echoed the perspectives of index patients and relatives, reporting more success using a direct approach. Using a solely indirect approach by index patients could work well for some, but was commonly problematic because of the unreliability of invitations being passed on, uncertainty about what may be communicated and ethics issues in following up relatives’ non-response. Deploying a direct approach improved confidence that these issues would be avoided.
Combining approaches by using a flexible ‘hybrid’ model was regarded as effective by both patients and professionals. This involved FH professionals developing an understanding of what was appropriate for individual families in discussion with index patients, to inform whether to use an indirect or a direct approach, or a combination of both, for each relevant family member. A FH specialist nurse-led model providing adequate time for enhanced communication and continuity of care for families from commencement and throughout the cascade-testing pathway was preferred and strongly supported.
To further improve acceptability of cascade testing, professionals emphasised that communication with families should focus on FH as effectively treatable to prevent major cardiac disease, rather than as a forbidding genetic condition. From both professionals’ and patients’/relatives’ perspectives, barriers to uptake of cascade testing could be reduced by removing the conventional requirement for GP referral, enabling self-referral to FH services when possible, and limiting the number of appointments and different health professionals in testing pathways. Having continuity of care with a single FH professional, such as a consultant or a FH nurse, for ongoing family support greatly helped. This was particularly valued by parents with younger children who had been tested.
We acknowledge some limitations, which are challenges for future research to address in this field. Index patient and relative participants were white and English-speaking; this lack of ethnic diversity reflects that seen in FH services, and thus who we were able to access and engage for interview and sampling.
Although some relevant experience was captured through the accounts of those participating, we did not secure direct testimony from relatives who declined FH cascade testing or from second-degree relatives. We were unable to identify participants in these groups willing to be interviewed. We note that more women than men were interviewed, perhaps reflecting women’s commonly greater willingness to participate in research or as parents of index patients. Finally, although genetic counsellors were not directly involved in two FH service settings, those in the third were in the midst of genetic services reconfiguration and did not respond to the invitation to participate.
These qualitative findings must be interpreted with regard to the selected samples as described, which may aid assessment of the relevance of these findings beyond this study’s context.
Chapter 7 Conclusion
To achieve the aim of identifying the most cost-effective protocol for cascade testing for FH, we answered three inter-related objectives:
-
to determine the yield of cases, treatment patterns, and short- and long-term outcomes for FH patients
-
to evaluate the cost-effectiveness of alternative protocols for FH cascade testing using data from services in two UK regions, the literature and linkage of national clinical databases
-
to qualitatively assess the acceptability of cascade-testing protocols to individuals and families with potential and confirmed FH, and to health-care providers.
When a person is identified with FH (index case), testing other relatives in the family is cost-effective, but the best protocol or pathway to complete this cascade testing is unclear. The index case, under current guidelines, will be confirmed by genetic testing. Following this, there are three possible approaches to contact the relatives of the index case: the ‘indirect’ approach, whereby the patient with FH contacts their relatives; the ‘direct’ approach, whereby the FH specialist contacts the relatives directly; and a combination of the two approaches. The approach used may affect how many relatives are contacted and tested (‘yield of cases’). Furthermore, the yield of cases could be affected if all relatives are contacted simultaneously or if first-degree relatives and then second-degree relatives are contacted. To understand the impact of diagnosis on individual long-term quality of life, life expectancy and health-care costs, the CVD outcomes of FH patients also need to be incorporated.
Key findings
Yield of cases
Our systematic review of different cascade protocols included 24 non-comparative studies. Based on four studies, the combined approach to contacting relatives led to a slightly higher yield of relatives tested (40%, 95% CI 37% to 42%) than the direct (33%, 95% CI 28% to 39%) or indirect approach alone (34%, 95% CI 30% to 37%). However, only 26% of the index cases in the combined approach had a confirmed diagnosis. The systematic review to assess the diagnostic accuracy of clinical and biochemical criteria to diagnose relatives included nine studies; no study was found reporting relatives’ characteristics that could inform yield of cases, and hence the cost-effectiveness analysis.
Analyses of the PASS databases identified that 21–29% of index cases have genetically confirmed FH, and women, first-degree relatives and those contacted directly by health professionals were more likely to complete cascade testing (p < 0.01). The Welsh PASS database analysis also showed that direct contact by the service, rather than contact indirectly via family members, is associated with a significantly higher probability that an individual will complete the cascade process, although the probability of completing the cascade is significantly lower among men and among more distant relatives (second-degree or more distant). The database also identified that the yield of cases could be affected by locality, with 25–29% of individuals who could potentially be contacted for cascade testing living outside the geographic area covered by the cascade service.
Treatment patterns, and short- and long-term outcomes
The search for relevant systematic reviews of the effectiveness of cholesterol-lowering therapies on LDL-C levels and CVD among adults identified 14 relevant systematic reviews; none of these met the methodological quality on the AMSTAR critical appraisal tool to be included in the review of reviews. However, the CPRD–HES data set indicated that 26% of patients with coded diagnosis of FH in their primary care records remain untreated after 2 years, and < 30% achieve the reductions in LDL-C recommended by NICE FH guidelines (≥ 50% reduction).
Using the primary care CPRD and specialist FH register data sets, we were also able to explore the lifetime consequences in terms of morbidity and mortality among diagnosed and treated individuals with FH. The CPRD demonstrated substantially increased CVD risk among individuals with FH codes in primary care records, compared with controls (HR 9.14, 95% CI 8.55 to 9.76), and, over 20 years of follow-up, they were more likely to develop CVD if they had previous disease or a higher baseline cholesterol level. Furthermore, we estimate that, at 10 years of follow-up, the average risk of first major non-fatal CVD event or CVD-related death is 11%. History of CVD was identified as a key prognostic variable, with age, sex and (baseline) PT-LDL-C level also playing important roles. A specialist FH register confirmed excessive CVD morbidity among those with FH (SMbR 7.17, 95% CI 6.79 to 7.56), with particularly high morbidity among women and younger patients with FH.
However, identifying evidence of long-term CVD outcomes among children diagnosed with FH was limited. A preliminary scoping review identified two systematic reviews, one from 201620 and one from 2019. 19 In particular, Lozano et al. 20 had indicated no studies looking at the (long-term) relationship between LLT for children and CVD events in adulthood, or the association between intermediate outcomes in childhood (e.g. lipid concentrations, atherosclerosis) and CVD event. We concluded that, owing to the nature of these studies, such data would not be available.
Cost-effectiveness of alternative protocols
The cost-effectiveness analysis involved the development of two new cost-effectiveness models. One model predicted the long-term health outcomes of individuals with FH. The results informed the second model, which compared alternative cascade-testing protocols.
Considering long-term health outcomes and costs, the diagnosis of people with FH generally represents a positive net health gain to the NHS (i.e. it is cost-effective) for most people with a PT-LDL-C level of ≥ 2.5 mmol/l or who have prior CVD history. The net health gain from diagnosis and treatment ranged between –0.27 to 2.51 QALYs at the £15,000-per-QALY threshold. In general, the net health gain of diagnosis is greater among males, people with higher PT-LDL-C levels and people with prior CVD history at diagnosis. The assumptions with the largest impact were those about the risk of first major CVD event, in particular the effect of LDL-C burden on CVD risk and the increase in CVD risk over time.
The cascade-testing protocol with the highest net health gain, the base-case cost-effective protocol, involved diagnosing relatives according to their age, whether or not they were on LLT, their PT-LDL-C level and, when this information provided insufficient diagnostic certainty, conducting genetic testing (as presented in Table 20). Compared with not conducting cascade testing, per index family assessed for cascade, the cost-effective protocol diagnoses 52% (n = 0.31) of relatives with the disease, at a cascade cost of £536, and with an incremental cost-effectiveness ratio of £13,996 per QALY gained. The cost-effective protocol using the same testing strategy for all relatives regardless of age, the harmonised cost-effective protocol, achieves similar outcomes and may be preferable if additional nurse time (unaccounted for here) is required to implement a testing approach that differs according to the age of relatives.
Acceptability of cascade-testing approaches
For index patients and relatives, family relationships shaped experience of, and preference for, an indirect or a direct cascade approach, or a combination approach. Experience related to quality of communication about FH, the accessibility and organisation of pathways, and continuity of care further determined acceptability of approach. For index patients, making indirect contact worked less well, creating pressure about engaging relatives unless their relationship was conducive, or concern about communicating appropriate FH information. Direct contact of relatives by a specialist service was preferred as offering an independent, more credible and authoritative approach.
Professionals’ experiences echoed the perspectives of index patients and relatives, reporting more success using a direct approach. Using a solely indirect approach by index patients could work well for some, but was commonly problematic because of unreliability of invitations being passed on, uncertainty about what may be communicated and ethics issues in following up relatives’ non-response. Deploying a direct approach improved confidence that these issues would be avoided.
From both professionals’ and patients’/relatives’ perspectives, barriers to uptake of cascade testing could be reduced by removing the conventional requirement for GP referral, enabling self-referral to FH services when possible, and limiting the number of appointments and different health professionals in testing pathways. Having continuity of care with a single FH professional, such as a consultant or a FH nurse, for ongoing family support greatly helped.
Relationship to other literature
Yield of cases and acceptability of different cascade-testing approaches
The acceptability of different contact strategies may partly explain their effectiveness. Qualitative interviews with FH patients have demonstrated mixed views about the approach to cascade testing: a 2011 Scottish study favoured indirect cascade testing,88 whereas, in a 2015 Australian study,100 index patients supported health professionals directly contacting relatives, perceiving health professionals to have greater credibility and authority. Furthermore, it has been suggested that indirect contact by index patients may lead to inadequate counselling and a sense of social pressure to be tested in solidarity with other family members. 166 With the autosomal dominant mode of inheritance, 50% of the first-degree relatives of index cases will be affected. This finding was confirmed in our systematic review, with similar proportions seen for each cascade strategy.
Considering other factors that affect yield, age has been identified as a predictor of cascade success in previous work;41 however, our analysis of the PASS data set failed to include age as a predictor as a result of missingness. Furthermore, in the Welsh FH cascade service, as the choice of contact method was made on a case-by-case basis, the indirect method may have been used more frequently in cases where direct contact was challenging because of a lack of information about and engagement with the family. This would exert a downwards bias in our estimates of completion for indirectly contacted relatives. However, it is also likely that some indirect contacts that did not lead to further interaction with the service went unrecorded. This would exert an upwards bias in our estimates of completion for indirectly contacted relatives.
The uncertainty related to mode of contact also arose in the qualitative interview study. The study confirmed that practising health professionals also experience direct contact as a more reliable, certain and successful method to use. This trend in favour of the greater effectiveness of direct approaches is consistent with a previous quantitative audit of FH nurse cascade testing,41 and a 2019 systematic review. 26 In this study, when family connections were strong, some index patients preferred to discuss testing with their relatives indirectly, prior to a direct approach from a health-care professional, to prepare them. As other work has found, communication about genetic screening may happen informally within the family,172 and index patients may have more confidence communicating about FH testing with their families if they are well supported with information and education by a health professional. 169 Nevertheless, using a solely indirect approach, we and others100 have found that some index patients become frustrated trying to persuade relatives to engage with testing; they are fearful of relationship breakdown, and thus would welcome direct specialist approaches to support them.
Treatment patterns, and short- and long term outcomes
The results of the analysis of the LDL-C response to treatment are in line with the FH 2010 audit,9 in which a mean reduction in LDL-C (in mmol/l) of 37% (median 33%) was found. Nevertheless, our estimate of approximately 30% of individuals achieving the ≥ 50% LDL-C reduction target falls short of what the same national audit concluded (44%),9 but is above figures reported elsewhere in 2019 (19.6%173).
As highlighted in Bianconi et al.,174 different traditional predictors of CVD risk have been investigated in the literature. 175,176 LDL-C has been unequivocally associated with a premature atherosclerosis onset and an increased risk of CVD,177–179 and our findings confirmed that. Age is considered one of the strongest independent predictors of CVD, and a debate is still ongoing about whether or not sex is an independent predictor of CVD in this population. Our CVD risk modelling indicates that an age dependency of risk exists in this population, potentially accelerated compared with the general population. The same was observed previously in the Simon Broome Register5 and in Perak et al. 96 A 2017 systematic review180 presented similar findings to ours, with most studies identifying that men had a ≈2.5-fold higher CVD risk than women, although the magnitude of the difference varied by study.
As reported in Bianconi et al.,174 a history of previous CVD events among FH patients may confer a higher CVD risk. Among treated FH patients, lower risk estimates have emerged in the literature for primary prevention groups than for secondary prevention groups, with, for instance, the standardised mortality ratio for CHD ranging from 1.03 among people with FH without previous CVD events (primary prevention) to 5.15 among FH patients with previous CVD events (secondary prevention). 181 Our findings reinforce history of CVD as one of the key predictors of CVD risk.
Cost-effectiveness of alternative protocols
Previous cost-effectiveness analyses of screening have estimated the lifetime costs and health outcomes of diagnosis and treatment. 11–13,25,109,137,138,147–149 As all concluded that cascade is cost-effective, compared with no cascade, we can infer that diagnosis and treatment were found to be cost-effective, which is in line with our results. More specific comparisons are difficult because the previous studies did not consider the same subgroups or compare the same range of cascade protocols as our study. For example, in terms of the selection of index cases to the genetic test, similar to Crosland et al.,130 we found that less restrictive clinical score cut-off points were a net health gain to the NHS. Crosland et al. 130 estimated that the cost of cascade testing relatives with the genetic test was £191 per relative (2016 prices), whereas our model estimated £179–192 per relative tested, depending on the type of cascade (sequential or simultaneous) and the method of contact (direct or indirect) at 2019 prices. In Nherera et al.,25 a testing strategy using only LDL-C to diagnose relatives had a sensitivity of 0.64 and specificity of 0.84 (age-averaged estimate; LDL-C cut-off level not reported), based on an analysis of data from the Dutch cascade programme. This is approximately in line with the results from our cost-effectiveness model, if using a LDL-C cut-off level of 4 mmol/l, with a sensitivity of 0.71 and a specificity of 0.80 if the same cut-off level is used for all relatives.
Strengths and limitations
Overall, as demonstrated by the systematic reviews, the available evidence to parameterise the economic models was limited. Therefore, the cost-effectiveness models mostly relied on the evidence from the administrative/routine service databases included in this study, combined with stakeholder input and some external data (i.e. Dutch FH service) and literature (e.g. relationship between exposure to raised LDL-C over time and CVD risk17). Furthermore, some parameters in the databases were poorly reported or missing (e.g. the data from the Scottish FH service could not be used; in the PASS Welsh and Wessex databases, there were limited data about relatives’ LDL-C levels). However, using multiple data sources and regular stakeholder input, combined with threshold analysis and relevant sensitivity analyses, has improved the robustness of the models. There was little representation of different ethnic groups in the qualitative study and in the data sets we examined in this report, and we are unaware of any published data to address this. This means that we are unable to comment on the extent to which the findings can be extrapolated to FH patients of different ethnic origins and their families.
The CPRD, Simon Broome and PASS data sets provided relatively large sample sizes, but were observational in nature, with concerns about missingness and generalisability for some variables. Confounding is an inherent problem with observational studies. This could be ameliorated if a randomised controlled design was available to assess the impact of different cascade-testing protocols. The following sections consider the strengths and limitations relating to each objective.
Yield of cases
(See Chapter 2, Review 1: effectiveness of cascade-testing protocols among relatives for familial hypercholesterolaemia, Review 4: the diagnostic accuracy of clinical and biochemical criteria and scoring systems for the diagnosis of familial hypercholesterolaemia among relatives, and Chapter 5, Service data analysis) In the systematic review of the effectiveness of cascade testing and a previous review,26 there were very limited data available to inform the preferred approach to contacting relatives. The available studies were not comparative in nature and attempting to make inferences across studies is hampered by heterogeneity. The most robust evidence for comparing the effectiveness of the strategies for cascade testing would have been from studies that made within-study comparisons; however, no studies using such designs were identified. Therefore, we had to rely on comparing strategies across studies. We have assumed that the differences in yield between the cascade strategies can be wholly ascribed to the contact method used; however, it is likely that differences in the setting, approaches, method used to diagnose FH (genetic testing or clinical criteria) and time may all have influenced the yield. Furthermore, only four of the 24 included studies provided information to estimate the proportion of relatives tested out of those contacted for cascade testing. Of the other 20 studies, 18 did not report the number of relatives contacted. With approximately half of the included studies reporting the extent of cascading to other relatives, we were unable to explore whether there were differences in yields by cascade strategy related to extent of cascading to other relatives. In the study assessing combined approach, only 26% of index cases had a definite diagnosis of FH, with the remaining having a probable diagnosis;41 therefore, this was likely to result in a reduction in the efficiency of the cascade programme, compared with restricting cascading to relatives for whom index cases had a definite diagnosis of FH. As a result, the true yield using the combination approach could be substantially greater.
Owing to the limited evidence, particularly lack of within-study comparison of modalities of contact, the data were not in a format that could be used to parameterise the economic model. However, the Welsh PASS data provide the first available within-service comparison of direct and indirect contact. This was enhanced by direct derivation of the data from two services, and the availability of FH specialist nurse input to support the development of additional variables (e.g. by recoding free-text fields within PASS) and provide a detailed understanding of the data. Furthermore, the Wales and Wessex PASS data were derived from two of the largest FH cascade services in the UK and include detailed patient-level records on 3839 indexes and 14,951 linked relatives. The limitation of using the PASS Welsh data to compare contact methods is that there may have been confounding factors that we were unable to adjust for, given the limited data (e.g. age, which has been identified as a predictor of cascade success in previous work41). We note that these databases did identify a significant group of relatives who are within area, but not contacted by the service. Detailed information on why these individuals were not contacted was not available from PASS and remains an important priority for future research and data collection. All analyses were conducted on a complete-case basis; this may have resulted in biases if the complete cases are not representative of the patients diagnosed by the services. This bias is likely to be particularly acute for the analysis of the characteristics of those who completed the cascade. For this group, CVD history and LLT history were missing for a substantive proportion of patients. This is an important consideration as these results may have significant influence on the results of the cost-effectiveness analysis.
Yield of new relatives with FH will be affected by number of index cases identified. The latter would require evaluation of robust methods of identifying FH cases. It was recognised by Health Technology Assessment commissioners and the team that this was outside the remit of this commissioned project. However, we were able to provide some indication of the impact of index case identification using the Welsh PASS data set. In line with previous studies,10,131–133 we found a strong relationship between the stringency of thresholds used to select index cases for genetic testing and the likelihood that an individual carries a FH mutation. Increasing or decreasing the threshold used to inform which index cases are eligible for genetic testing will have implications for the number of individuals correctly identified as having FH and the number of individuals requiring genetic testing. In terms of the selection of index cases for genetic testing, similar to Crosland et al.,130 we found that less restrictive clinical score cut-off points were a net health gain to the NHS.
Treatment patterns, and short- and long term outcomes
(See Chapter 4, and Chapter 5, Economic analysis of the Clinical Practice Research Datalink database.) We were able to take advantage of using long-term data from two large, contemporary populations of FH individuals from the UK (CPRD and Simon Broome data sets). These data sets also benefited from linkage to HES data. Our work allows the quantification of risk according to a patient’s age, sex, CVD history and PT-LDL-C level.
In the CPRD data set, we were able to evaluate the absolute long-term CVD risk in both a diagnosed and a treated cohort of individuals with FH (see Chapter 5, Economic analysis of the Clinical Practice Research Datalink database) and compare the CVD risk profile of individuals with FH with that of the general population (see Chapter 4, Risk of cardiovascular disease among those with familial hypercholesterolaemia, from primary care records). Unlike previous analysis of CVD risk with FH, as well as higher rates of CHD and stroke, the primary care data set demonstrated comparable increased risk of PVD. The findings also suggested incorporating a broader range of CVD risk outcomes for economic modelling. On a cautionary note, PVD is less well phenotyped in primary care records than CHD and stroke.
As previously highlighted, the CPRD primary care data set might not have correctly coded individuals with FH, and the cohort might not be representative of the UK FH population as a whole or of patients identified via cascade testing who are expected to have genetically confirmed FH, rather than a diagnosis based on clinical phenotype. Although the CPRD has a relatively long follow-up of up to 20 years, event data were sparse from around year 10. For validation and extrapolation, it seemed relevant to focus on studies with longer follow-up than this. From a number of studies assessed for this purpose, the Perak et al. 96 study was chosen as the most relevant, but there remained a number of limitations to using this study. We had to make a number of adjustments to make the estimates comparable to those in the CPRD, and this study focused on US patients with elevated cholesterol, but without a diagnosis of FH. Furthermore, the CPRD is unlikely to characterise the current clinical practices in tertiary specialised care, such as lipid clinics, where FH patients are closely followed up and managed9 (although some patients in the CPRD may also be managed in lipid clinics). However, the Simon Broome data set identified many patients who were followed up in lipid clinics.
Cost-effectiveness of alternative protocols
(See Chapter 5.) To our knowledge, this is the first cost-effectiveness analysis of cascade testing for FH that compared a wide range of protocols, comprising strategies to select index cases to the cascade, with and without genetic testing; contact methods (direct or indirect), cascade pattern (sequential or simultaneous) and testing strategies for relatives, combining genetic and cholesterol testing; age; and treatment status. We informed the analysis with routine data from FH services in England and Wales about the characteristics of index cases and relatives, and the probability that relatives come forward for testing. Furthermore, we estimated the long-term outcomes of relatives with and relatives without diagnosis with a new cost-effectiveness model, informed by routinely collected data from a cohort of FH patients in the UK. This new cost-effectiveness model predicts lifetime outcomes conditional on age at diagnosis, sex, PT-LDL-C level and prior CVD event history.
The cost-effectiveness model of long-term health outcomes and costs of individuals with FH was informed by risk equations and LDL-C response to treatment estimated from an analysis of the individual-level data of the CPRD cohort of individuals with FH. The focus of this analysis was only on first events post diagnosis, as the context of the long-term economic model is on improving diagnosis. The primary benefit of this is expected to be among individuals who have not yet experienced a CVD event. Once individuals have experienced a CVD event post FH diagnosis, the role of FH diagnosis is likely to be less important, as individuals with CVD history will generally be in receipt of high-intensity statins. The individual-level analysis focused on treated individuals as, being the majority of individuals in our cohort, we believed that they would be more generalisable to the broader FH population.
The weaknesses of the cost-effectiveness analysis mostly relate to the assumptions required to design and inform the two cost-effectiveness models. These relate to the available data on the characteristics and degree of kinship of relatives in the PASS databases, lifetime CVD risk, effect of treatment on LDL-C level, and the generalisability of the CPRD cohort to FH patients. Given the limited data on lipid levels routinely collected by the Welsh and Wessex services, we requested data from the Dutch cascade screening programme (although a comparison with the available Welsh and Wessex FH service data suggested that they were generalisable). In addition, the costs of monitoring people were based on the national guidelines and stakeholder input, rather than informed by a systematic process or empirical data. Our estimates of the resources involved in conducting the cascade were based on information from FH nurses, literature and feedback from the stakeholder group, because the PASS data did not include details of the staff time and grade. Importantly, the cost-effectiveness analysis relies on in silico comparisons of cascade protocols, many of which have not been used in clinical practice, and on the validity of the assumptions about the generalisability of available data, the effect of LDL-C burden and long-term CVD risk. Therefore, the generalisability of the cost-effectiveness results to clinical practice is uncertain.
Our base case assumes that longer duration of exposure to raised LDL-C increases CVD risk (known as ‘LDL-C burden’), based on the relationship proposed by the 2017 EAS consensus statement. 17 This assumption is supported by epidemiological studies,17 although there is uncertainty about the size of the effect and its generalisability to people with FH. Similarly, although the assumption that CVD risk increases with age is uncontroversial, the magnitude of this increase is uncertain. For these reasons, further research is warranted on the effect of LDL-C burden and age on CVD event risk in the long term, particularly among younger people, who were under-represented in the CPRD cohort. An uncertainty that we were unable to explore in the scenario analysis is whether or not the CVD risk observed in the CPRD cohort is representative of the CVD risk experienced by people with FH, given the uncertainty regarding the extent to which the CPRD cohort includes people who do not have FH (e.g. because of miscoding or misdiagnosis in primary care).
Acceptability of cascade-testing approaches
(See Chapter 6.) To our knowledge, this is the first study to explore the experiences and perceptions of both index patients and relatives, and health professionals, across different settings in the UK, chosen to reflect a range of current FH cascade-testing approaches. The purposeful study sample included people with a diversity of cascade-testing experiences and a range of professionals involved in the cascade-testing service. The analysis of the data was developed by two researchers of different disciplinary backgrounds, and a process of member checking with respondents themselves was also undertaken, confirming interpretation of their experiences.
We acknowledge some limitations, which are challenges for future research to address in this field. Index patient and relative participants were white, predominantly female and English-speaking. The lack of ethnic diversity reflects that seen in FH services, and thus who we were able to access and engage for interview and sampling. Moreover, we did not secure direct testimony from relatives who declined FH cascade testing, second-degree relatives or genetic counsellors.
Policy and practice recommendations
-
Provide evidence base to inform the recommendations in future NICE FH guideline: these findings complement the policy recommendations of the NICE FH guideline of starting cascade testing from genetically tested index cases. The further elaboration on the most effective protocol, specifically testing first- and second-degree relatives simultaneously, may be reviewed with other emerging evidence by a future NICE FH guideline development group.
-
Modality of contact with relatives of index cases: based on the cost-effectiveness analysis and the qualitative study, a direct approach appears to be the preferred approach, improving confidence that contact with relatives has taken place and that information about FH is appropriately communicated, and more readily enabling follow-up of non-response. Using a flexible combined approach (of either or both direct and indirect approaches), case by case, may have greatest acceptability and success, guided by consultation with each individual index patient and assessment of their family relationships.
-
UK-wide co-ordinated service: the cost-effectiveness analysis results support the setting up of a nationally co-ordinated FH specialist nurse-led service. A nationally co-ordinated service could diagnose more relatives (e.g. 1553 FH relatives per year in the UK vs. 1175 FH relatives if only in-area relatives can be cascade-tested), at a cost of £2.72M. Without this, the large proportion of relatives living outside the geographic area covered by the relevant cascade service will not be contacted and tested. This co-ordinated service could also help improve access for underserved groups, such as minority communities, men and more distant relatives (second-degree or more distant). To improve access, limited health literacy needs to be tackled and information needs to be provided in formats and languages that are appropriate to the diverse populations served by these services.
Recommendations for future research
-
Establish a long-term FH cohort with robust measurement of cholesterol levels, treatment and cardiovascular outcomes to quantify LDL-C burden.
-
For definitive evidence, conduct a randomised study directly comparing different approaches to contact relatives.
-
A future qualitative appraisal of FH services should aim to interview a more diverse patient population, including ethnic minorities, males and more distant relatives.
Acknowledgements
First, we would like to thank the project administrators (Stephanie Gallimore and Christina Sheehan) for the meticulous work to keep the project on track. This report would not have been finalised without the support of Natasha Rogers, William Evans, Hasidah Abdul Hamid, Pamela Pepper and Sadaf Qureshi.
We thank Professor Mark Sculphur and Dr Robert Pear for their invaluable support and guidance as co-applicants and advisors throughout the study.
We would like to thank the stakeholder group for kindly taking part in various meetings about the cost-effectiveness work, providing feedback and information, which was invaluable for the successful delivery of this project:
-
Delyth Townsend (FH nurse at South West Wales NHS)
-
Dev Datta (consultant in metabolic medicine, medical lead for the Welsh FH service)
-
Dermot Neely (consultant in clinical biochemistry and metabolic medicine, Royal Victoria Infirmary)
-
Jaimini Cegla (consultant in metabolic medicine, Imperial College Healthcare NHS Trust)
-
Jane Breen (lead FH nurse at Harefield Hospital)
-
Joanne Whitmore (clinical lead for FH at the BHF)
-
Paul Roderick (Professor of Public Health, University of Southampton)
-
Peter Green (Medical Director, NHS Kent and Medway)
-
Phil Rowland (patient representative)
-
Robert Cramb (consultant chemical pathologist, retired)
-
Rob Pears (consultant in public health, Hampshire County Council).
We would also like to thank Professor Erik SG Stroes [Department of Vascular Medicine, Amsterdam University Medical Centers (Amsterdam UMC), and medical director of ‘Landelijk Expertisecentrum Erfelijkheidsonderzoek Familiaire Hart-en Vaatziekten’, Amsterdam, the Netherlands], Professor Gerard K Hovingh (Department of Vascular Medicine, Amsterdam UMC) and Rens F Reeskamp (Department of Vascular Medicine, Amsterdam UMC) for generously providing us with the distributions of LDL-C levels of relatives tested for FH in the Dutch cascade screening programme.
We would like to thank David Wonderling (National Guideline Centre) for kindly sharing the cost-effectiveness model that informed NICE CG181,108 which we adapted to obtain the long-term health outcomes and costs of individuals who do not have FH if they are misdiagnosed.
We would like to thank Jane Breen (Harefield Hospital), Moyra Cather (Northern Ireland FH Regional service), Darren Harvey and Zoë Jayne (both at the Royal Free Hospital), Robert Gingell (North Wales FH service), Catherine Sherman and Angela Cazeaux (both at the Wessex FH service) and Norma Macleod (NHS Western Isles) for helpfully answering our questions about how their services operate cascade testing.
We would like to thank Professor Brian Ference (University of Cambridge) for helpfully answering our questions about LDL-C burden.
We would like to thank Maggie Williams (Bristol Genetics Laboratory) for information on the costs of genetic testing.
We would like to thank Professor Susan Griffin (University of York) for kindly assisting in the design of the health economics analysis.
We would like to thank Manimekalai Thiruvothiyur (University of Aberdeen) for collating Scottish FH data.
We would like to thank Sotire Tumi (University of Newcastle) for work on the systematic review of the diagnostic accuracy of clinical and biochemical criteria to diagnose relatives of index cases with FH.
Participants
We would like to thank all the participants and their families for their willingness to take part in this research study. We are grateful for the time and commitment you gave, which has been critical in the success of this work.
Public engagement
As well as attending monthly study group meetings, external steering group meetings every 6 months and training sessions, our PPI representatives, William Rowlands, Mark Fisher and Henry Fisher, helped prepare the grant application, patient information leaflets and the qualitative interview schedule; helped interpret qualitative interviews and database studies; and helped draft the final report (in particular plain language summary). We would also like to thank HEART UK – The Cholesterol Charity (registered charity number 1003904) for disseminating our study findings to the public and lipid specialists.
Study Steering Committee
We thank Paul Durrington (chairperson), Robert Cramb, David Wonderling, Dermot Neely, Mark Fisher, Rachel Elliott, Tony Wierzbicki, Dan Lasserson, Jules Payne and Joanne Whitmore for their oversight and guidance.
Role of the funding source
The funder appointed independent members to the Study Steering Committee, approved all protocol amendments and monitored study progress against agreed milestones. The funder had no involvement in data interpretation or writing of the report. The corresponding author had full access to all outputs from the data in the study.
Ethics approval and research governance
The protocol was approved by the Health Research Authority (reference number). The trial was funded by the National Institute for Health and Care Research Health Technology Assessment programme (15/134/02). Centre-specific approval was obtained at all recruiting centres.
Contributions of authors
Nadeem Qureshi (https://orcid.org/0000-0003-4909-0644) (Chief Investigator, Clinical Professor of Primary Care) led the writing of the grant application, the protocol development and management of the project; contributed to performing the systematic reviews, acquisition of the databases, conceptualisation of the economic models, clinical interpretation of findings; and led the writing of the final report and revisions.
Bethan Woods (https://orcid.org/0000-0002-7669-9415) (Senior Research Fellow, Health Economics) contributed to the development of the funding application, led the health economics workstream, and contributed to reviewing and editing the final report.
Rita Neves de Faria (https://orcid.org/0000-0003-3410-1435) (Research Fellow, Health Economics) developed the model and conducted the cost-effectiveness analysis of the benefits of diagnosis among patients with FH; developed the model and conducted the cost-effectiveness analysis of the alternative cascade-testing protocols; co-supervised the analysis of the PASS data; collaborated in the analysis of the CPRD data; collaborated on the systematic reviews; and contributed to drafting, reviewing and editing the final report and revisions.
Pedro Saramago Goncalves (https://orcid.org/0000-0001-9063-8590) (Research Fellow, Health Economics) developed, conducted and drafted the chapter on the individual participant-level analysis of the CPRD data; collaborated in the analysis of the PASS data and cost-effectiveness models; and contributed to reviewing and editing the final report and revisions.
Edward Cox (https://orcid.org/0000-0001-8981-0699) (Research Fellow, Health Economics) conducted and drafted the chapter on the individual participant data analysis of the Welsh and Wessex PASS service data, collaborated in the analysis of CPRD data and the cost-effectiveness models, and contributed to reviewing and editing the final report and revisions.
Jo Leonardi-Bee (https://orcid.org/0000-0003-0893-6068) (Professor of Medical Statistics) contributed to the development of the funding application in collaboration with the other co-investigators, led the systematic reviews of cascade testing and LLTs and drafted the related chapters, and contributed to reviewing and editing the final report and revisions.
Laura Condon (https://orcid.org/0000-0002-7039-3095) (Qualitative Research Fellow) contributed to the qualitative studies. She conducted all related fieldwork, including interviews with all participants, and contributed to their analysis, the qualitative literature review and respondent validation.
Stephen Weng (https://orcid.org/0000-0002-5281-9590) (Senior Epidemiologist) contributed to the development of the funding application in collaboration with the other co-investigators; led the acquisition, analysis and interpretation of the databases; and drafted the related chapters.
Ralph K Akyea (https://orcid.org/0000-0003-4529-8237) (Research Assistant, Data Science) and Barbara Iye (https://orcid.org/0000-0001-9720-1180) (Clinical Lecturer in Primary Care) contributed to the analysis and interpretation of the databases and to editing the related chapters and revisions of the report.
Paul Roderick (https://orcid.org/0000-0001-9475-6850) (Professor of Public Health) contributed to the development of the funding application in collaboration with the other co-investigators, and assisted with interpretation of the databases and conceptualisation of the economic models.
Steve E Humphries (http://orcid.org/0000-0002-8221-6547) (Emeritus Professor of Cardiovascular Genetics) contributed to the development of the funding application in collaboration with other co-investigators, and assisted with interpretation of the databases, conceptualisation of the economic models, and reviewing and editing of the final report and revisions.
William Rowlands (http://orcid.org/0000-0001-5107-7292) (study PPIE lead) contributed to the development of the funding application in collaboration with the other co-investigators, and assisted with analysis of qualitative interviews, conceptualisation of the economic models and drafting the plain language summary.
Melanie Watson (http://orcid.org/0000-0002-5140-4086) (Consultant FH Nurse Specialist) contributed to the development of the funding application in collaboration with the other co-investigators, led on accessing the Wessex PASS FH database and contributed to recuitment for the qualitative study and interpretation of the qualitative study results.
Kate Haralambos (http://orcid.org/0000-0001-6075-3125) (FH Service Network Manager) contributed to the development of the funding application in collaboration with the other co-investigators, led on extraction of relevant data from the Welsh and Wessex PASS databases and contributed to editing the related chapter.
Ryan Kenny (http://orcid.org/0000-0001-9743-4259) (Research Associate) led the systematic review on diagnostic accuracy of clinical and biochemical criteria and scoring systems for the diagnosis of FH among relatives and drafted the related chapter.
Dev Datta (https://orcid.org/0000-0001-7856-7363) (Consultant in Metabolic Medicine) contributed to the development of the funding application in collaboration with the other co-investigators, and led on accessing the Welsh PASS database.
Zosia Miedzybrodzka (https://orcid.org/0000-0003-2890-8136) (Professor of Clinical Genetics) contributed to the development of the funding application in collaboration with other co-investigators, led on accessing the Scottish FH database and contributed to conceptualisation of the economic models.
Christopher Byrne (https://orcid.org/0000-0001-6322-7753) (Professor of Endocrinology and Metabolism) contributed to the development of the funding application in collaboration with the other co-investigators.
Joe Kai (https://orcid.org/0000-0001-9040-9384) (Clinical Professor of Primary Care) contributed to co-writing the grant application and protocol development, designed and led the qualitative workstream, supervised fieldwork and conducted the analyses with Laura Condon. He wrote the qualitative study (see Chapter 6), and contributed to reviewing and editing the final report and revisions.
All authors reviewed the final report.
Publications
Iyen B, Qureshi N, Kai J, Akyea RK, Leonardi-Bee J, Roderick P, et al. Risk of cardiovascular disease outcomes in primary care subjects with familial hypercholesterolaemia: a cohort study. Atherosclerosis 2019;287:8–15.
Iyen B, Qureshi N, Weng S, Roderick P, Kai J, Capps N, et al. Sex differences in cardiovascular morbidity associated with familial hypercholesterolaemia: a retrospective cohort study of the UK Simon Broome Register linked to national hospital records. Atherosclerosis 2020;315:131–7.
Leonardi-Bee J, Boateng C, Faria R, Eliman K, Young B, Qureshi N. Effectiveness of cascade testing strategies in relatives for familial hypercholesterolemia: a systematic review and meta-analysis. Atherosclerosis 2021;338:7–14.
Data-sharing statement
The key data used in this study were derived from the CPRD, HES and the Simon Broome Register. The agreements in place for use of these data do not permit further distribution or sharing. Requests for the relevant data sets should be made directly to the CPRD, HES and the Simon Broome Register committee. Further information can be obtained from the corresponding author.
Data requests for qualitative transcripts and routine service data should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Wald DS, Bestwick JP, Morris JK, Whyte K, Jenkins L, Wald NJ. Child–parent familial hypercholesterolemia screening in primary care. N Engl J Med 2016;375:1628-37. https://doi.org/10.1056/NEJMoa1602777.
- Akioyamen LE, Genest J, Shan SD, Reel RL, Albaum JM, Chu A, et al. Estimating the prevalence of heterozygous familial hypercholesterolaemia: a systematic review and meta-analysis. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-016461.
- Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013;34:3478-90a. https://doi.org/10.1093/eurheartj/eht273.
- Public Health England . Familial Hypercholesterolaemia: Implementing a Systems Approach to Detection and Management n.d. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/731873/familial_hypercholesterolaemia_implementation_guide.pdf (accessed 16 May 2022).
- Risk of fatal coronary heart disease in familial hypercholesterolaemia . Scientific Steering Committee on behalf of the Simon Broome Register Group. BMJ 1991;303:893-6.
- Humphries SE, Cooper JA, Seed M, Capps N, Durrington PN, Jones B, et al. Coronary heart disease mortality in treated familial hypercholesterolaemia: update of the UK Simon Broome FH Register. Atherosclerosis 2018;274:41-6.
- Haralambos K, Humphries SE, Whitmore J, Datta D, Cather M, Miedzybrodzka Z, et al. Familial hypercholesterolaemia (FH) genetic testing in the UK. Atheroscler Suppl 2018;34.
- National Institute for Health and Care Excellence . Familial Hypercholesterolaemia: Identification and Management. Clinical Guideline [CG71] n.d. www.nice.org.uk/guidance/cg71 (accessed 16 May 2022).
- Pedersen KMV, Humphries SE, Roughton M, Besford JS. National Clinical Audit of the Management of Familial Hypercholesterolaemia 2010: Full Report 2010.
- Haralambos K, Whatley SD, Edwards R, Gingell R, Townsend D, Ashfield-Watt P, et al. Clinical experience of scoring criteria for familial hypercholesterolaemia (FH) genetic testing in Wales. Atherosclerosis 2015;240:190-6. https://doi.org/10.1016/j.atherosclerosis.2015.03.003.
- Ademi Z, Watts GF, Pang J, Sijbrands EJ, van Bockxmeer FM, O’Leary P, et al. Cascade screening based on genetic testing is cost-effective: evidence for the implementation of models of care for familial hypercholesterolemia. J Clin Lipidol 2014;8:390-40. https://doi.org/10.1016/j.jacl.2014.05.008.
- Marang-van de Mheen PJ, ten Asbroek AH, Bonneux L, Bonsel GJ, Klazinga NS. Cost-effectiveness of a family and DNA based screening programme on familial hypercholesterolaemia in the Netherlands. Eur Heart J 2002;23:1922-30.
- Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HA. Cost effectiveness analysis of different approaches of screening for familial hypercholesterolaemia. BMJ 2002;324.
- Nherera L, Calvert NW, Demott K, Humphries SE, Neil HA, Minhas R, et al. Cost-effectiveness analysis of the use of a high-intensity statin compared to a low-intensity statin in the management of patients with familial hypercholesterolaemia. Curr Med Res Opin 2010;26:529-36. https://doi.org/10.1185/03007990903494934.
- Pears R, Griffin M, Futema M, Humphries SE. Improving the cost-effectiveness equation of cascade testing for familial hypercholesterolaemia. Curr Opin Lipidol 2015;26:162-8. https://doi.org/10.1097/MOL.0000000000000173.
- Pears R, Griffin M, Watson M, Wheeler R, Hilder D, Meeson B, et al. The reduced cost of providing a nationally recognised service for familial hypercholesterolaemia. Open Heart 2014;1. https://doi.org/10.1136/openhrt-2013-000015.
- Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459-72. https://doi.org/10.1093/eurheartj/ehx144.
- Cholesterol Treatment Trialists’ Collaboration . Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019;393:407-15.
- Vuorio A, Kuoppala J, Kovanen PT, Humphries SE, Tonstad S, Wiegman A, et al. Statins for children with familial hypercholesterolemia. Cochrane Database Syst Rev 2019;11. https://doi.org/10.1002/14651858.CD006401.pub5.
- Lozano P, Henrikson NB, Dunn J, Morrison CC, Nguyen M, Blasi PR, et al. Lipid screening in childhood and adolescence for detection of familial hypercholesterolemia: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016;316:645-55. https://doi.org/10.1001/jama.2016.6176.
- Futema M, Whittall RA, Kiley A, Steel LK, Cooper JA, Badmus E, et al. Analysis of the frequency and spectrum of mutations recognised to cause familial hypercholesterolaemia in routine clinical practice in a UK specialist hospital lipid clinic. Atherosclerosis 2013;229:161-8. https://doi.org/10.1016/j.atherosclerosis.2013.04.011.
- Herman K, Van Heyningen C, Wile D. Cascade screening for familial hypercholesterolaemia and its effectiveness in the prevention of vascular disease. Br J Diabetes Vasc Dis 2009;9:171-4.
- Kusters DM, de Beaufort C, Widhalm K, Guardamagna O, Bratina N, Ose L, et al. Paediatric screening for hypercholesterolaemia in Europe. Arch Dis Child 2012;97:272-6. https://doi.org/10.1136/archdischild-2011-300081.
- Brunham LR, Ruel I, Aljenedil S, Rivière JB, Baass A, Tu JV, et al. Canadian Cardiovascular Society position statement on familial hypercholesterolemia: update 2018. Can J Cardiol 2018;34:1553-63.
- Nherera L, Marks D, Minhas R, Thorogood M, Humphries SE. Probabilistic cost-effectiveness analysis of cascade screening for familial hypercholesterolaemia using alternative diagnostic and identification strategies. Heart 2011;97:1175-81. https://doi.org/10.1136/hrt.2010.213975.
- Lee C, Rivera-Valerio M, Bangash H, Prokop L, Kullo IJ. New case detection by cascade testing in familial hypercholesterolemia: a systematic review of the literature. Circ Genom Precis Med 2019;12. https://doi.org/10.1161/CIRCGEN.119.002723.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Williams RR, Hunt SC, Schumacher MC, Hegele RA, Leppert MF, Ludwig EH, et al. Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am J Cardiol 1993;72:171-6.
- Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. JBI Manual for Evidence Synthesis. Adelaide, SA: JBI; 2020.
- Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat 1950;21:607-11.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. https://doi.org/10.1136/bmj.327.7414.557.
- Alver M, Palover M, Saar A, Läll K, Zekavat SM, Tõnisson N, et al. Recall by genotype and cascade screening for familial hypercholesterolemia in a population-based biobank from Estonia. Genet Med 2019;21:1173-80. https://doi.org/10.1038/s41436-018-0311-2.
- Andersen LK, Jensen HK, Juul S, Faergeman O. Patients’ attitudes toward detection of heterozygous familial hypercholesterolemia. Arch Intern Med 1997;157:553-60.
- Bell DA, Pang J, Burrows S, Bates TR, van Bockxmeer FM, Hooper AJ, et al. Effectiveness of genetic cascade screening for familial hypercholesterolaemia using a centrally co-ordinated clinical service: an Australian experience. Atherosclerosis 2015;239:93-100. https://doi.org/10.1016/j.atherosclerosis.2014.12.036.
- Bhatnagar D, Morgan J, Siddiq S, Mackness MI, Miller JP, Durrington PN. Outcome of case finding among relatives of patients with known heterozygous familial hypercholesterolaemia. BMJ 2000;321:1497-500.
- Breen J, Jones J, Barbir M. Genetic screening for familial hypercholesterolaemia in a cardiothoracic tertiary referral centre. Atherosclerosis 2011;218:e1-e12.
- Chan ML, Cheung CL, Lee AC, Yeung CY, Siu CW, Leung JY, et al. Genetic variations in familial hypercholesterolemia and cascade screening in East Asians. Mol Genet Genomic Med 2019;7. https://doi.org/10.1002/mgg3.520.
- Davis T, Andersen R, Andersen LK, Testa H, Ibarra J. Combined cascade screening and patient education for familial hypercholesterolemia: genetic results from a family shared medical appointment pilot study. J Clin Lipidol 2016;10:674-5.
- Edwards R, Townsend D, Gingell R, Haralambos K, Datta BN, McDowell IFW, et al. Implementation of a multidisciplinary approach to diagnosis and management of familial hypercholesterolaemia (FH) in Wales: the role of the FH specialist nurse. Atherosclerosis 2013;231:e1-e10.
- Ellis KL, Pérez de Isla L, Alonso R, Fuentes F, Watts GF, Mata P. Value of measuring lipoprotein(a) during cascade testing for familial hypercholesterolemia. J Am Coll Cardiol 2019;73:1029-39.
- Hadfield SG, Horara S, Starr BJ, Yazdgerdi S, Marks D, Bhatnagar D, et al. Family tracing to identify patients with familial hypercholesterolaemia: the second audit of the Department of Health Familial Hypercholesterolaemia Cascade Testing Project. Ann Clin Biochem 2009;46:24-32.
- Jannes CE, Santos RD, de Souza Silva PR, Turolla L, Gagliardi AC, Marsiglia JD, et al. Familial hypercholesterolemia in Brazil: cascade screening program, clinical and genetic aspects. Atherosclerosis 2015;238:101-7. https://doi.org/10.1016/j.atherosclerosis.2014.11.009.
- Latkovskis G, Saripo V, Gilis D, Nesterovics G, Upena-Roze A, Erglis A. Latvian registry of familial hypercholesterolemia: the first report of three-year results. Atherosclerosis 2018;277:347-54.
- Marks D, Thorogood M, Neil SM, Humphries SE, Neil HA. Cascade screening for familial hypercholesterolaemia: implications of a pilot study for national screening programmes. J Med Screen 2006;13:156-9. https://doi.org/10.1258/096914106778440617.
- Marteau T, Senior V, Humphries SE, Bobrow M, Cranston T, Crook MA, et al. Psychological impact of genetic testing for familial hypercholesterolemia within a previously aware population: a randomized controlled trial. Am J Med Genet A 2004;128A:285-93. https://doi.org/10.1002/ajmg.a.30102.
- Muir LA, George PM, Laurie AD, Reid N, Whitehead L. Preventing cardiovascular disease: a review of the effectiveness of identifying the people with familial hypercholesterolaemia in New Zealand. N Z Med J 2010;123:97-102.
- Neuner J, Dimmock D, Kirschner AP, Beaudry H, Paradowski J, Orlando L. Results and lessons of a pilot study of cascade screening for familial hypercholesterolemia in US primary care practices. J Gen Intern Med 2020;35:351-3. https://doi.org/10.1007/s11606-019-05485-7.
- Setia N, Saxena R, Sawhney JPS, Verma IC. Familial Hypercholesterolemia: cascade screening in children and relatives of the affected. Indian J Pediatr 2018;85:339-43. https://doi.org/10.1007/s12098-017-2589-5.
- Tilney M. Establishing a familial hypercholesterolaemia register – the first year. Atheroscler Suppl 2019;36:24-7.
- Umans-Eckenhausen MA, Defesche JC, Sijbrands EJ, Scheerder RL, Kastelein JJ. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet 2001;357:165-8.
- Webster J, Tuson C, Burton C, Mitchell K, Sutton P, Dunn R, et al. Variants of uncertain significance: higher than average results in Yorkshire and Humberside for familial hypercholesterolaemia. Atheroscler Suppl 2019;38:e2-e5.
- Skovby F, Micic S, Jepsen B, Larsen SO, Hansen B, Tegllund L, et al. Screening for familial hypercholesterolaemia by measurement of apolipoproteins in capillary blood. Arch Dis Child 1991;66:844-7.
- Descamps OS, Rietzschel E, Laporte A, Buysschaert I, De Raedt H, Elegeert I, et al. Feasibility and cost of FH cascade screening in Belgium (BEL-CASCADE) including a novel rapid rule-out strategy. Acta Cardiol 2021;76:227-35. https://doi.org/10.1080/00015385.2020.1820683.
- Raal FJ, Bahassi EM, Stevens B, Turner TA, Stein EA. Cascade screening for familial hypercholesterolemia in South Africa: the Wits FIND-FH program. Arterioscler Thromb Vasc Biol 2020;40:2747-55. https://doi.org/10.1161/ATVBAHA.120.315040.
- Leren TP, Finborud TH, Manshaus TE, Ose L, Berge KE. Diagnosis of familial hypercholesterolemia in general practice using clinical diagnostic criteria or genetic testing as part of cascade genetic screening. Community Genet 2008;11:26-35. https://doi.org/10.1159/000111637.
- Rietzschel ER, De Dutter J, Laporte A, Descamps O. Preliminary findings from the first Belgian familial hypercholesterolemia cascade screening (BEL-CASCADE). J Am Coll Cardiol 2019;73.
- Raal F, Stevens B, du Toit R, Troendle D, Pilcher G, Kelso M, et al. Detection of familial hypercholesterolemia in South Africa via cascade screening: the Wits FIND-FH program. J Am Coll Cardiol 2018;71.
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358. https://doi.org/10.1136/bmj.j4008.
- Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol 2009;8:453-63. https://doi.org/10.1016/S1474-4422(09)70058-4.
- Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al. Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation. Health Technol Assess 2008;12.
- Awad K, Mikhailidis DP, Toth PP, Jones SR, Moriarty P, Lip GYH, et al. Efficacy and safety of alternate-day versus daily dosing of statins: a systematic review and meta-analysis. Cardiovasc Drugs Ther 2017;31:419-31. https://doi.org/10.1007/s10557-017-6743-0.
- Cheng C, Sun S, Zhou Y, Yang X. Efficacy and safety of different doses of evolocumab in reducing low-density lipoprotein cholesterol levels: a meta-analysis. Biomed Rep 2016;5:541-7. https://doi.org/10.3892/br.2016.766.
- Gray J, Edwards SJ, Lip GY. Comparison of sequential rosuvastatin doses in hypercholesterolaemia: a meta-analysis of randomised controlled trials. Curr Med Res Opin 2010;26:537-47. https://doi.org/10.1185/03007990903513980.
- Gudzune KA, Monroe AK, Sharma R, Ranasinghe PD, Chelladurai Y, Robinson KA. Effectiveness of combination therapy with statin and another lipid-modifying agent compared with intensified statin monotherapy: a systematic review. Ann Intern Med 2014;160:468-76. https://doi.org/10.7326/M13-2526.
- Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013;61:440-6.
- Peng W, Qiang F, Peng W, Qian Z, Ke Z, Yi L, et al. Therapeutic efficacy of PCSK9 monoclonal antibodies in statin-nonresponsive patients with hypercholesterolemia and dyslipidemia: a systematic review and meta-analysis. Int J Cardiol 2016;222:119-29.
- Poolsup N, Suksomboon N, Wongyaowarat K, Rungkanchananon B, Niyomrat P, Kongsuwan S. Meta-analysis of the comparative efficacy and safety of pitavastatin and atorvastatin in patients with dyslipidaemia. J Clin Pharm Ther 2012;37:166-72. https://doi.org/10.1111/j.1365-2710.2011.01274.x.
- Schmidt AF, Carter JL, Pearce LS, Wilkins JT, Overington JP, Hingorani AD, et al. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2020;10. https://doi.org/10.1002/14651858.CD011748.pub3.
- Weng TC, Yang YH, Lin SJ, Tai SH. A systematic review and meta-analysis on the therapeutic equivalence of statins. J Clin Pharm Ther 2010;35:139-51. https://doi.org/10.1111/j.1365-2710.2009.01085.x.
- Yee LL, Wright EA. Pitavastatin calcium: clinical review of a new antihyperlipidemic medication. Clin Ther 2011;33:1023-42. https://doi.org/10.1016/j.clinthera.2011.07.011.
- Zhang XL, Zhu QQ, Zhu L, Chen JZ, Chen QH, Li GN, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med 2015;13. https://doi.org/10.1186/s12916-015-0358-8.
- Mueller ZT, Craddock KE, Pitlick JM, Crannage AJ. PCSK9 inhibitors: an emerging class of medications. J Pharm Technol 2016;32:201-9.
- NIH National Cancer Institute . Cascade Screening n.d. www.cancer.gov/publications/dictionaries/genetics-dictionary/def/cascade-screening (accessed 15 November 2022).
- Minhas R, Humphries SE, Qureshi N, Neil HA. NICE Guideline Development Group . Controversies in familial hypercholesterolaemia: recommendations of the NICE Guideline Development Group for the identification and management of familial hypercholesterolaemia. Heart 2009;95:584-7. https://doi.org/10.1136/hrt.2008.162909.
- Watts GF, Lewis B, Sullivan DR. Familial hypercholesterolemia: a missed opportunity in preventive medicine. Nat Clin Pract Cardiovasc Med 2007;4:404-5.
- Hopkins PN, Toth PP, Ballantyne CM, Rader DJ. National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemias: prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol 2011;5:9-17. https://doi.org/10.1016/j.jacl.2011.03.452.
- Starr B, Hadfield SG, Hutten BA, Lansberg PJ, Leren TP, Damgaard D, et al. Development of sensitive and specific age- and gender-specific low-density lipoprotein cholesterol cutoffs for diagnosis of first-degree relatives with familial hypercholesterolaemia in cascade testing. Clin Chem Lab Med 2008;46:791-803. https://doi.org/10.1515/CCLM.2008.135.
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350. https://doi.org/10.1136/bmj.g7647.
- Louter L, Defesche J, Roeters van Lennep J. Cascade screening for familial hypercholesterolemia: practical consequences. Atheroscler Suppl 2017;30:77-85.
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5. https://doi.org/10.1186/s13643-016-0384-4.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Huijgen R, Hutten Barbara A, Kindt I, Vissers Maud N, Kastelein JJP. Discriminative ability of LDL-cholesterol to identify patients with familial hypercholesterolemia. Circ Cardiovasc Genet 2012;5:354-9. https://doi.org/10.1161/circgenetics.111.962456.
- Mabuchi H, Higashikata T, Nohara A, Lu H, Yu WX, Nozue T, et al. Cutoff point separating affected and unaffected familial hypercholesterolemic patients validated by LDL-receptor gene mutants. J Atheroscler Thromb 2005;12:35-40.
- Truong TH, Kim NT, Nguyen MNT, Pang J, Hooper AJ, Watts GF, et al. Homozygous familial hypercholesterolaemia in Vietnam: case series, genetics and cascade testing of families. Atherosclerosis 2018;277:392-8.
- Thorsson B, Sigurdsson G, Gudnason V. Systematic family screening for familial hypercholesterolemia in Iceland. Arterioscler Thromb Vasc Biol 2003;23:335-8. https://doi.org/10.1161/01.atv.0000051874.51341.8c.
- Vergotine J, Thiart R, Kotze MJ. Clinical versus molecular diagnosis of heterozygous familial hypercholesterolaemia in the diverse South African population. S Afr Med J 2001;91:1053-9.
- Pang J, Martin AC, Bates TR, Hooper AJ, Bell DA, Burnett JR, et al. Parent–child genetic testing for familial hypercholesterolaemia in an Australian context. J Paediatr Child Health 2018;54:741-7. https://doi.org/10.1111/jpc.13898.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. https://doi.org/10.1093/ije/dyv098.
- Iyen B, Qureshi N, Kai J, Akyea RK, Leonardi-Bee J, Roderick P, et al. Risk of cardiovascular disease outcomes in primary care subjects with familial hypercholesterolaemia: a cohort study. Atherosclerosis 2019;287:8-15.
- Iyen B, Qureshi N, Weng S, Roderick P, Kai J, Capps N, et al. Sex differences in cardiovascular morbidity associated with familial hypercholesterolaemia: a retrospective cohort study of the UK Simon Broome Register linked to national hospital records. Atherosclerosis 2020;315:131-7.
- Neil HA, Huxley RR, Hawkins MM, Durrington PN, Betteridge DJ, Humphries SE. Simon Broome Familial Hyperlipidaemia Register Group and Scientific Steering Committee . Comparison of the risk of fatal coronary heart disease in treated xanthomatous and non-xanthomatous heterozygous familial hypercholesterolaemia: a prospective registry study. Atherosclerosis 2003;170:73-8.
- Alvarez-Madrazo S, McTaggart S, Nangle C, Nicholson E, Bennie M. Data resource profile: the Scottish National Prescribing Information System (PIS). Int J Epidemiol 2016;45:714-715f. https://doi.org/10.1093/ije/dyw060.
- Neil HA, Hawkins MM, Durrington PN, Betteridge DJ, Capps NE, Humphries SE. Simon Broome Familial Hyperlipidaemia Register Group and Scientific Steering Committee . Non-coronary heart disease mortality and risk of fatal cancer in patients with treated heterozygous familial hypercholesterolaemia: a prospective registry study. Atherosclerosis 2005;179:293-7.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499-502.
- Huxley RR, Hawkins MH, Humphries SE, Karpe F, Neil HA. Simon Broome Familial Hyperlipidaemia Register Group and Scientific Steering Committee . Risk of fatal stroke in patients with treated familial hypercholesterolemia: a prospective registry study. Stroke 2003;34:22-5. https://doi.org/10.1161/01.str.0000047123.14312.3e.
- Perak AM, Ning H, de Ferranti SD, Gooding HC, Wilkins JT, Lloyd-Jones DM. Long-term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia phenotype. Circulation 2016;134:9-19. https://doi.org/10.1161/CIRCULATIONAHA.116.022335.
- Lee PH. Is a cutoff of 10% appropriate for the change-in-estimate criterion of confounder identification?. J Epidemiol 2014;24:161-7.
- Neil HA, Hammond T, Huxley R, Matthews DR, Humphries SE. Extent of underdiagnosis of familial hypercholesterolaemia in routine practice: prospective registry study. BMJ 2000;321. https://dx.doi.org/10.1136/bmj.321.7254.148.
- Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326. https://doi.org/10.1136/bmj.326.7404.1423.
- Hardcastle SJ, Legge E, Laundy CS, Egan SJ, French R, Watts GF, et al. Patients’ perceptions and experiences of familial hypercholesterolemia, cascade genetic screening and treatment. Int J Behav Med 2015;22:92-100. https://doi.org/10.1007/s12529-014-9402-x.
- Weng SF, Kai J, Andrew Neil H, Humphries SE, Qureshi N. Improving identification of familial hypercholesterolaemia in primary care: derivation and validation of the familial hypercholesterolaemia case ascertainment tool (FAMCAT). Atherosclerosis 2015;238:336-43. https://doi.org/10.1016/j.atherosclerosis.2014.12.034.
- Akyea RK, Kai J, Qureshi N, Iyen B, Weng SF. Sub-optimal cholesterol response to initiation of statins and future risk of cardiovascular disease. Heart 2019;105:975-81. https://doi.org/10.1136/heartjnl-2018-314253.
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. https://doi.org/10.1136/bmj.b2393.
- van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. J Stat Softw 2011;45:1-67.
- National Institute for Health and Care Excellence (NICE) . Ezetimibe for Treating Primary Heterozygous-Familial and Non-Familial Hypercholesterolaemia. Technology Appraisal Guidance [TA385] 2016.
- National Institute for Health and Care Excellence (NICE) . Familial Hypercholesterolemia: Costing Report, Implementing NICE Guidance 2009.
- National Institute for Health and Care Excellence (NICE) . Familial Hypercholesterolemia. Quality Standard [QS41] n.d. www.nice.org.uk/guidance/qs41 (accessed 18 October 2015).
- National Institute for Health and Care Excellence (NICE) . Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. Clinical Guideline [CG181] 2014.
- National Institute for Health and Care Excellence (NICE) . Alirocumab for Treating Primary Hypercholesterolaemia and Mixed Dyslipidaemia. Technology Appraisal Guidance [TA393] 2016.
- Kerr M, Pears R, Miedzybrodzka Z, Haralambos K, Cather M, Watson M, et al. Cost effectiveness of cascade testing for familial hypercholesterolaemia, based on data from familial hypercholesterolaemia services in the UK. Eur Heart J 2017;38:1832-9. https://doi.org/10.1093/eurheartj/ehx111.
- Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA 2016;316:743-53. https://doi.org/10.1001/jama.2016.11004.
- Akyea RK, Kai J, Qureshi N, Iyen B, Weng SF. LDL cholesterol response to statins and future risk of cardiovascular disease. Heart 2019;105:1290-1. https://doi.org/10.1136/heartjnl-2019-315461.
- Mohrschladt MF, Westendorp RG, Gevers Leuven JA, Smelt AH. Cardiovascular disease and mortality in statin-treated patients with familial hypercholesterolemia. Atherosclerosis 2004;172:329-35. https://doi.org/10.1016/j.atherosclerosis.2003.11.007.
- Hovland A, Mundal LJ, Igland J, Veierød MB, Holven KB, Bogsrud MP, et al. Increased risk of heart failure and atrial fibrillation in heterozygous familial hypercholesterolemia. Atherosclerosis 2017;266:69-73.
- Collett D. Modelling Survival Data in Medical Research. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2015.
- Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 2002;21:2175-97. https://doi.org/10.1002/sim.1203.
- Latimer NR. Survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making 2013;33:743-54. https://doi.org/10.1177/0272989X12472398.
- Akaike H. Selected Papers of Hirotugu Akaike. New York, NY: Springer New York; 1998.
- Guyot P, Ades AE, Beasley M, Lueza B, Pignon JP, Welton NJ. Extrapolation of survival curves from cancer trials using external information. Med Decis Making 2017;37:353-66. https://doi.org/10.1177/0272989X16670604.
- Jackson CH. flexsurv: a platform for parametric survival modeling in R. J Stat Softw 2016;70.
- Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012;33:1635-701. https://doi.org/10.1093/eurheartj/ehs092.
- Ridker PM, Mora S, Rose L. JUPITER Trial Study Group . Percent reduction in LDL cholesterol following high-intensity statin therapy: potential implications for guidelines and for the prescription of emerging lipid-lowering agents. Eur Heart J 2016;37:1373-9. https://doi.org/10.1093/eurheartj/ehw046.
- Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111-88. https://doi.org/10.1093/eurheartj/ehz455.
- McKay AJ, Hogan H, Humphries SE, Marks D, Ray KK, Miners A. Universal screening at age 1–2 years as an adjunct to cascade testing for familial hypercholesterolaemia in the UK: a cost–utility analysis. Atherosclerosis 2018;275:434-43.
- Wald DS, Bestwick JP. Reaching detection targets in familial hypercholesterolaemia: comparison of identification strategies. Atherosclerosis 2020;293:57-61.
- Benn M, Watts GF, Tybjærg-Hansen A, Nordestgaard BG. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur Heart J 2016;37:1384-94. https://doi.org/10.1093/eurheartj/ehw028.
- Damgaard D, Larsen ML, Nissen PH, Jensen JM, Jensen HK, Soerensen VR, et al. The relationship of molecular genetic to clinical diagnosis of familial hypercholesterolemia in a Danish population. Atherosclerosis 2005;180:155-60.
- Civeira F, Ros E, Jarauta E, Plana N, Zambon D, Puzo J, et al. Comparison of genetic versus clinical diagnosis in familial hypercholesterolemia. Am J Cardiol 2008;102:1187-93, 1193.e1. https://doi.org/10.1016/j.amjcard.2008.06.056.
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168-209.
- Crosland P, Maconachie R, Buckner S, McGuire H, Humphries SE, Qureshi N. Cost–utility analysis of searching electronic health records and cascade testing to identify and diagnose familial hypercholesterolaemia in England and Wales. Atherosclerosis 2018;275:80-7.
- Ademi Z, Norman R, Pang J, Liew D, Zoungas S, Sijbrands E, et al. Health economic evaluation of screening and treating children with familial hypercholesterolemia early in life: many happy returns on investment?. Atherosclerosis 2020;304:1-8.
- Ademi Z, Watts GF, Juniper A, Liew D. A systematic review of economic evaluations of the detection and treatment of familial hypercholesterolemia. Int J Cardiol 2013;167:2391-6. https://doi.org/10.1016/j.ijcard.2013.01.280.
- Oliva J, López-Bastida J, Moreno SG, Mata P, Alonso R. [Cost-effectiveness analysis of a genetic screening program in the close relatives of Spanish patients with familial hypercholesterolemia.]. Rev Esp Cardiol 2009;62:57-65.
- Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HA. Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis. Health Technol Assess 2000;4.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013.
- Büyükkaramikli NC, Rutten-van Mölken MPMH, Severens JL, Al M. TECH-VER: a verification checklist to reduce errors in models and improve their credibility. PharmacoEconomics 2019;37:1391-408. https://doi.org/10.1007/s40273-019-00844-y.
- Lewsey JD, Lawson KD, Ford I, Fox KA, Ritchie LD, Tunstall-Pedoe H, et al. A cardiovascular disease policy model that predicts life expectancy taking into account socioeconomic deprivation. Heart 2015;101:201-8. https://doi.org/10.1136/heartjnl-2014-305637.
- Office for National Statistics . National Life Tables: UK 2020. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables/current (accessed 14 December 2022).
- NHS Business Services Authority, NHS Prescription Services . Electronic Drug Tariff for the National Health Service England and Wales n.d. www.drugtariff.nhsbsa.nhs.uk/#/00766639-DC/DC00766631/Home (accessed 24 May 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent; 2019.
- Ramaswami U, Humphries SE, Priestley-Barnham L, Green P, Wald DS, Capps N, et al. Current management of children and young people with heterozygous familial hypercholesterolaemia – HEART UK statement of care. Atherosclerosis 2019;290:1-8.
- NHS England and NHS Improvement . 2019/20/National/Cost/Collection/Data/Publication n.d. www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication/ (accessed 14 December 2022).
- Walker S, Asaria M, Manca A, Palmer S, Gale CP, Shah AD, et al. Long-term healthcare use and costs in patients with stable coronary artery disease: a population-based cohort using linked health records (CALIBER). Eur Heart J Qual Care Clin Outcomes 2016;2:125-40. https://doi.org/10.1093/ehjqcco/qcw003.
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;13:509-18. https://doi.org/10.1111/j.1524-4733.2010.00700.x.
- Vuorio A, Docherty KF, Humphries SE, Kuoppala J, Kovanen PT. Statin treatment of children with familial hypercholesterolemia – trying to balance incomplete evidence of long-term safety and clinical accountability: are we approaching a consensus?. Atherosclerosis 2013;226:315-20.
- Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol 2012;60:2631-9. https://doi.org/10.1016/j.jacc.2012.09.017.
- Ference BA, Majeed F, Penumetcha R, Flack JM, Brook RD. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: a 2 × 2 factorial Mendelian randomization study. J Am Coll Cardiol 2015;65:1552-61. https://doi.org/10.1016/j.jacc.2015.02.020.
- Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J 2015;36:539-50. https://doi.org/10.1093/eurheartj/eht571.
- Linsel-Nitschke P, Götz A, Erdmann J, Braenne I, Braund P, Hengstenberg C, et al. Lifelong reduction of LDL-cholesterol related to a common variant in the LDL-receptor gene decreases the risk of coronary artery disease – a Mendelian randomisation study. PLOS One 2008;3. https://doi.org/10.1371/journal.pone.0002986.
- National Clinical Guideline Centre . Appendix L: Cost-effectiveness analysis: low-intensity, medium-intensity and high-intensity statin treatment for the primary and secondary prevention of CVD. In Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease n.d. www.nice.org.uk/guidance/cg181/evidence/lipid-modification-update-appendices-243786638 (accessed 16 November 2022).
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:68-80. https://doi.org/10.1177/0272989X98018002S09.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Lomas J, Martin S, Claxton K. Estimating the marginal productivity of the English National Health Service from 2003 to 2012. Value Health 2019;22:995-1002.
- Department of Health and Social Care . 2018 Statutory Scheme – Branded Medicines Pricing. IA No: 9553 n.d. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/732122/2018_Statutory_Scheme_Impact_Assessment_1.pdf (accessed 16 November 2022).
- Department of Health and Social Care . NICE’s Technology Appraisal and Highly Specialised Technology Work Programmes – Charging and Appeals Panels n.d. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/733049/Impact_assessment_-_NICE_consultation.pdf (accessed 14 December 2022).
- Department of Health and Social Care . Accelerated Access Collaborative for Health Technologies. IA No: 13003 n.d. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/663094/Accelerated_Access_Collaborative_-_impact_asssessment.pdf (accessed 16 November 2022).
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64.
- Claxton K, Sculpher M, Drummond M. A rational framework for decision making by the National Institute For Clinical Excellence (NICE). Lancet 2002;360:711-5.
- Fenwick E, Steuten L, Knies S, Ghabri S, Basu A, Murray JF, et al. Value of information analysis for research decisions – an introduction: report 1 of the ISPOR Value of Information Analysis Emerging Good Practices Task Force. Value Health 2020;23:139-50.
- Rothery C, Strong M, Koffijberg HE, Basu A, Ghabri S, Knies S, et al. Value of information analytical methods: report 2 of the ISPOR Value of Information Analysis Emerging Good Practices Task Force. Value Health 2020;23:277-86.
- Sturm AC, Knowles JW, Gidding SS, Ahmad ZS, Ahmed CD, Ballantyne CM, et al. Clinical genetic testing for familial hypercholesterolemia: JACC Scientific Expert Panel. J Am Coll Cardiol 2018;72:662-80.
- Besseling J, Reitsma JB, Gaudet D, Brisson D, Kastelein JJ, Hovingh GK, et al. Selection of individuals for genetic testing for familial hypercholesterolaemia: development and external validation of a prediction model for the presence of a mutation causing familial hypercholesterolaemia. Eur Heart J 2017;38:565-73. https://doi.org/10.1093/eurheartj/ehw135.
- University College London, Department of Epidemiology and Public Health, National Centre for Social Research (NatCen) . Health Survey for England, 2017. [Data Collection] 2021.
- Steen DL, Khan I, Ansell D, Sanchez RJ, Ray KK. Retrospective examination of lipid-lowering treatment patterns in a real-world high-risk cohort in the UK in 2014: comparison with the National Institute for Health and Care Excellence (NICE) 2014 lipid modification guidelines. BMJ Open 2017;7.
- Barton GR, Briggs AH, Fenwick EA. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health 2008;11:886-97. https://doi.org/10.1111/j.1524-4733.2008.00358.x.
- van Maarle MC, Stouthard ME, Marang-van de Mheen PJ, Klazinga NS, Bonsel GJ. How disturbing is it to be approached for a genetic cascade screening programme for familial hypercholesterolaemia? Psychological impact and screenees’ views. Community Genet 2001;4:244-52. https://doi.org/10.1159/000064200.
- Horstman K, Smand C. Genetics from the Laboratory to Society: Societal Learning as an Alternative to Regulation. Basingstoke: Palgrave Macmillan; 2008.
- Hallowell N, Jenkins N, Douglas M, Walker S, Finnie R, Porteous M, et al. Patients’ experiences and views of cascade screening for familial hypercholesterolemia (FH): a qualitative study. J Community Genet 2011;2:249-57. https://doi.org/10.1007/s12687-011-0064-y.
- van El CG, Baccolini V, Piko P, Cornel MC. Stakeholder views on active cascade screening for familial hypercholesterolemia. Healthcare 2018;6.
- Ritchie J, Lewis J, Elam G. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE Publications Ltd; 2003.
- Creswell JW. Qualitative Inquiry and Research Design: Choosing Among Five Approaches. Thousand Oaks, CA: SAGE Publications, Inc; 2007.
- Hallowell N, Foster C, Eeles R, Ardern-Jones A, Murday V, Watson M. Balancing autonomy and responsibility: the ethics of generating and disclosing genetic information. J Med Ethics 2003;29:74-9.
- Duell PB, Gidding SS, Andersen RL, Knickelbine T, Anderson L, Gianos E, et al. Longitudinal low density lipoprotein cholesterol goal achievement and cardiovascular outcomes among adult patients with familial hypercholesterolemia: the CASCADE FH registry. Atherosclerosis 2019;289:85-93.
- Bianconi V, Banach M, Pirro M. International Lipid Expert Panel (ILEP). Why patients with familial hypercholesterolemia are at high cardiovascular risk? Beyond LDL-C levels. Trends Cardiovasc Med 2021;31:205-15.
- Galema-Boers AM, Lenzen MJ, Engelkes SR, Sijbrands EJ, Roeters van Lennep JE. Cardiovascular risk in patients with familial hypercholesterolemia using optimal lipid-lowering therapy. J Clin Lipidol 2018;12:409-16.
- Jansen AC, van Aalst-Cohen ES, Tanck MW, Trip MD, Lansberg PJ, Liem AH, et al. The contribution of classical risk factors to cardiovascular disease in familial hypercholesterolaemia: data in 2400 patients. J Intern Med 2004;256:482-90.
- Yuan G, Wang J, Hegele RA. Heterozygous familial hypercholesterolemia: an underrecognized cause of early cardiovascular disease. CMAJ 2006;174:1124-9.
- Robinson JG, Goldberg AC. National Lipid Association Expert Panel on Familial Hypercholesterolemia. Treatment of adults with familial hypercholesterolemia and evidence for treatment: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol 2011;5:18-29. https://doi.org/10.1016/j.jacl.2011.03.451.
- Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol 2011;5:133-40.
- Kruse G, Kutikova L, Wong B, Villa G, Ray KK, Mata P, et al. PCV32 – Cardiovascular disease risk and risk factors associated with familial hypercholesterolemia: a systematic review. Value Health 2017;20.
- Wong B, Kruse G, Kutikova L, Ray KK, Mata P, Bruckert E. Cardiovascular Disease Risk Associated With Familial Hypercholesterolemia: A Systematic Review of the Literature. Clin Ther 2016;38:1696-709. https://doi.org/10.1016/j.clinthera.2016.05.006.
Appendix 1 Systematic reviews
| 1. exp HYPERLIPOPROTEINEMIAS/ |
| 2. (hyperlipoprotein?emi$ and type I).tw. |
| 3. (hyperlipoprotein?emi$ and type II$).tw. |
| 4. (hyperlipoprotein?emi$ and type IV).tw. |
| 5. (hyperlipoprotein?emi$ and type V).tw. |
| 6. familial hypercholesterol?emi$.tw. |
| 7. familial hyperlipid?emi$.tw. |
| 8. familial lipoprotein lipase defici$.tw. |
| 9. familial hyperchylomicron?emi$.tw. |
| 10. burger grutz.tw. |
| 11. familial hypertriglycerid?emi$.tw. |
| 12. familial hyperlip?emi$.tw. |
| 13. familial hyperbetalipoprotein?emi$.tw. |
| 14. dysbetalipoprotein?emi$.tw. |
| 15. familial hyperprebetalipoprotein?emi$.tw. |
| 16. broad beta disease.tw. |
| 17. broad beta band disease.tw. |
| 18. fused beta band disease.tw. |
| 19. remnant removal disease.tw. |
| 20. familial apolipoprotein C-II defici$.tw. |
| 21. apoprotein C defici$.tw. |
| 22. or/1-21 |
| 23. *Mass Screening/mt [Methods] |
| 24. (Cascade adj3 (test$ or screen$)).ti,ab. |
| 25. ($direct$ adj3 contact).ti,ab. |
| 26. ((clinic or physician or practitioner) adj3 contact).ti,ab. |
| 27. contact tracing.ti,ab. |
| 28. proband$.ti,ab. |
| 29. index patient$.ti,ab. |
| 30. ((famil$ or relativ$ or patient$ or people or at-risk) adj3 (contact$ or trac$ or invit$ or refer$ or approach$ or identif$ or notif$ or communicat$)).ti,ab. |
| 31. or/23-30 |
| 32. 22 and 31 |
Methodological quality of included studies
| Study | Q1a | Q2b | Q3c | Q4d | Q5e | Q6f | Q7g | Q8h | Q9i | Q10j |
|---|---|---|---|---|---|---|---|---|---|---|
| Alver32 2019 | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes |
| Andersen33 1997 | Yes | Yes | Yes | Unclear | Unclear | Yes | Unclear | Yes | Yes | Yes |
| Bell34 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Bhatnagar35 2000 | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes |
| Breen36 2011 | Yes | Yes | Yes | Unclear | Unclear | No | No | Yes | Yes | Unclear |
| Chan37 2019 | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Unclear | Unclear |
| Davis38 2016 | Yes | Yes | Yes | Unclear | Unclear | No | No | Yes | Yes | Unclear |
| Descamps53 2021 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Edwards39 2013 | Yes | Yes | Yes | Unclear | Unclear | No | No | Yes | Unclear | Unclear |
| Ellis40 2019 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Unclear | Yes |
| Hadfield41 2009 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Unclear |
| Jannes42 2015 | Yes | Yes | Yes | Unclear | No | Yes | Yes | Yes | Yes | Yes |
| Latkovskis43 2018 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Leren55 2008 | Yes | Yes | Yes | Unclear | Unclear | No | No | Yes | Yes | Yes |
| Marks44 2006 | Yes | Yes | Yes | Unclear | Unclear | No | No | Yes | Yes | Yes |
| Marteau45 2004 | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| Muir46 2010 | Yes | Yes | Yes | Unclear | No | No | No | Yes | Yes | Yes |
| Neuner47 2020 | Yes | Yes | Yes | Unclear | No | No | No | Unclear | Unclear | Yes |
| Raal54 2020 | Unclear | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Yes | Yes |
| Setia48 2018 | Yes | Yes | Yes | Unclear | Unclear | No | No | Yes | Yes | Yes |
| Skovby52 1991 | Yes | Yes | Unclear | Unclear | Unclear | Unclear | No | Yes | Yes | Unclear |
| Tilney49 2019 | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Unclear | Unclear | Yes |
| Umans-Eckenhausen50 2001 | Yes | Yes | Yes | Unclear | Unclear | No | Yes | Yes | Unclear | Yes |
| Webster51 2019 | Yes | Yes | Yes | Unclear | Unclear | No | No | Unclear | Unclear | Unclear |
| 1 | exp hyperlipidemia/ |
| 2 | hyperlipid*.tw. |
| 3 | hyperlip?emia*.tw. |
| 4 | hypercholesterol*.tw |
| 5 | hypercholester?emia*.tw. |
| 6 | hyperlipoprotein?emia*.tw. |
| 7 | exp Cholesterol/ |
| 8 | cholesterol*.tw. |
| 9 | ((familial or inherited) adj2 hypercholesterol?emia*).tw. |
| 10 | Hyperlipoproteinemia Type II/ |
| 11 | or/1-10 |
| 12 | exp antibodies, monoclonal/ |
| 13 | monoclonal antibod*.tw. |
| 14 | MAB*.tw. |
| 15 | evolocumab.tw. |
| 16 | amg 145.tw. |
| 17 | amg145.tw. |
| 18 | alirocumab.tw. |
| 19 | regn 727.tw. |
| 20 | regn727.tw. |
| 21 | sar 236553.tw. |
| 22 | sar236553.tw. |
| 23 | D05?IgG2.tw. |
| 24 | LGT209.tw. |
| 25 | RG7652.tw. |
| 26 | Bococizumab.tw. |
| 27 | “Pf 04950615”.tw. |
| 28 | pf04950615.tw. |
| 29 | rn 316.tw. |
| 30 | PCSK9 antibod*.tw. |
| 31 | Proprotein Convertases/ |
| 32 | proprotein convertase*.tw. |
| 33 | pro-protein convertase*.tw. |
| 34 | pcsk9*.tw. |
| 35 | serine proteinase*.tw. |
| 36 | exp Hydroxymethylglutaryl-CoA Reductase Inhibitors/ |
| 37 | (statin or statins).tw. |
| 38 | atorvastatin.tw. |
| 39 | cerivastatin.tw. |
| 40 | fluvastatin.tw. |
| 41 | lovastatin.tw. |
| 42 | pravastatin.tw. |
| 43 | simvastatin.tw. |
| 44 | lipitor.tw. |
| 45 | baycol.tw. |
| 46 | lescol.tw. |
| 47 | mevacor.tw. |
| 48 | altocor.tw. |
| 49 | pravachol.tw. |
| 50 | lipostat.tw. |
| 51 | zocor.tw. |
| 52 | mevinolin.tw. |
| 53 | compactin.tw. |
| 54 | fluindostatin.tw. |
| 55 | rosuvastatin.tw. |
| 56 | exp ezetimibe/ |
| 57 | (ezetimibe or ezetimib).tw. |
| 58 | ezetrol.tw. |
| 59 | zetia.tw. |
| 60 | vytorin.tw. |
| 61 | inegy.tw. |
| 62 | (SCH-58235 or SCH 58235 or SCH58235).tw. |
| 63 | or/12-62 |
| 64 | ((brain* or cerebral or lacunar) adj2 infarct*).tw. |
| 65 | exp Cardiovascular Diseases/ |
| 66 | cardio*.tw. |
| 67 | cardia*.tw. |
| 68 | (coronary disease* or coronary*).tw. |
| 69 | angina.tw. |
| 70 | ventric*.tw. |
| 71 | myocard*.tw. |
| 72 | isch?em*.tw. |
| 73 | heart failure.tw. |
| 74 | cardiac failure.tw. |
| 75 | arrhythmi*.tw. |
| 76 | thromb*.tw. |
| 77 | atrial fibrill*.tw. |
| 78 | exp Stroke/ |
| 79 | (stroke or stokes).tw. |
| 80 | cerebrovas*.tw. |
| 81 | cerebral vascular.tw. |
| 82 | or/64-81 |
| 83 | review.ab. |
| 84 | review.pt. |
| 85 | meta-analysis.ab. |
| 86 | meta-analysis.pt. |
| 87 | meta-analysis.ti. |
| 88 | 83 or 84 or 85 or 86 or 87 |
| 89 | letter.pt. |
| 90 | comment.pt. |
| 91 | editorial.pt. |
| 92 | 89 or 90 or 91 |
| 93 | 88 not 92 |
| 94 | 11 and 63 and 82 and 93 |
| 95 | limit 94 to yr=“2008 - 2018” |
| 1. exp hyperlipoproteinemia type ii/ or exp hyperlipoproteinemia type iii/ or exp hyperlipoproteinemia type iv/ or exp hyperlipoproteinemia type v/ or hypoalphalipoproteinemias/ |
| 2. (Hyperlipoprotein?emia* adj (type II or type IIa or type IIb or type 2 or type 2a or type 2b)).mp. |
| 3. familial hypercholesterol*.ti,ab. |
| 4. hyperlipoprotein*.ti,ab. |
| 5. exp cholesterol/ |
| 6. 1 or 2 or 3 or 4 or 5 |
| 7. exp Genetic Testing/ |
| 8. exp Genetic Predisposition to Disease/ |
| 9. (diagnostic genetic test* or predictive genetic test*).ti,ab. |
| 10. (gen* adj3 (susceptib* or test* or disease* or risk* or assess*)).ti,ab. |
| 11. (diagnos* adj3 (performance* or accur* or utilit* or value* or efficien* or effectiveness)).ti,ab. |
| 12. exp MOLECULAR DIAGNOSTIC TECHNIQUES/ or molecular testing.ti,ab. |
| 13. (LDLR* or APOB* or low density lipoprotein receptor or Apolipoprotein B).mp. |
| 14. ((molecular or DNA or mutation) adj (test* or analys*)).mp. |
| 15. 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 |
| 16. ((cascad* adj3 (test* or screen*)) or (contact trac* or mass screen*)).ti,ab. |
| 17. exp Genetic carrier screening/ |
| 18. exp mass screening/ |
| 19. (((first or second degree) adj relative) or (proband* or proposit*)).ti,ab. |
| 20. ((famil* or relativ* or patient* or people or “at risk”) adj3 (contact* or identif* or communicat* or test* or screen* or histor*)).ti,ab. |
| 21. parents/ or grandparents/ or siblings/ |
| 22. 16 or 17 or 18 or 19 or 20 or 21 |
| 23. 6 and (15 and 22) |
| 24. exp animals/ not Humans/ |
| 25. (animal*or mice or mouse or pig).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms |
| 26. exp comment/ or exp letter/ or exp editorial/ |
| 27. 23 not (or/24-26) |
| 28. limit 27 to yr=“1994 -Current” |
Appendix 2 Epidemiological analysis
| CVD outcome | Age (years) at first CVD event, mean (SD) | Number of new events | Rate per 1000 person-years (95% CI) | p-value for CVD rate ratio | |||
|---|---|---|---|---|---|---|---|
| FH subjects | Non-FH subjects | FH subjects | Non-FH subjects | FH subjects | Non-FH subjects | ||
| Any CVD | 4474 | 1728 | 25.6 (24.8 to 26.3) | 2.9 (2.8 to 3.1) | < 0.0001 | ||
| CHD | 50.7 (7.5) | 61.1 (10.9) | 3545 | 1173 | 20.3 (19.6 to 20.9) | 2.0 (1.9 to 2.1) | < 0.0001 |
| Stroke/TIA | 51.9 (8.5) | 63.7 (11.2) | 764 | 405 | 4.3 (4.1 to 4.7) | 0.7 (0.6 to 0.8) | < 0.0001 |
| PVD | 52.4 (8.2) | 61.7 (10.9) | 592 | 295 | 3.4 (3.1 to 3.7) | 0.5 (0.4 to 0.6) | < 0.0001 |
FIGURE 18.
Distribution of TC level (mmol/l) among (a) non-FH people and (b) people with FH.
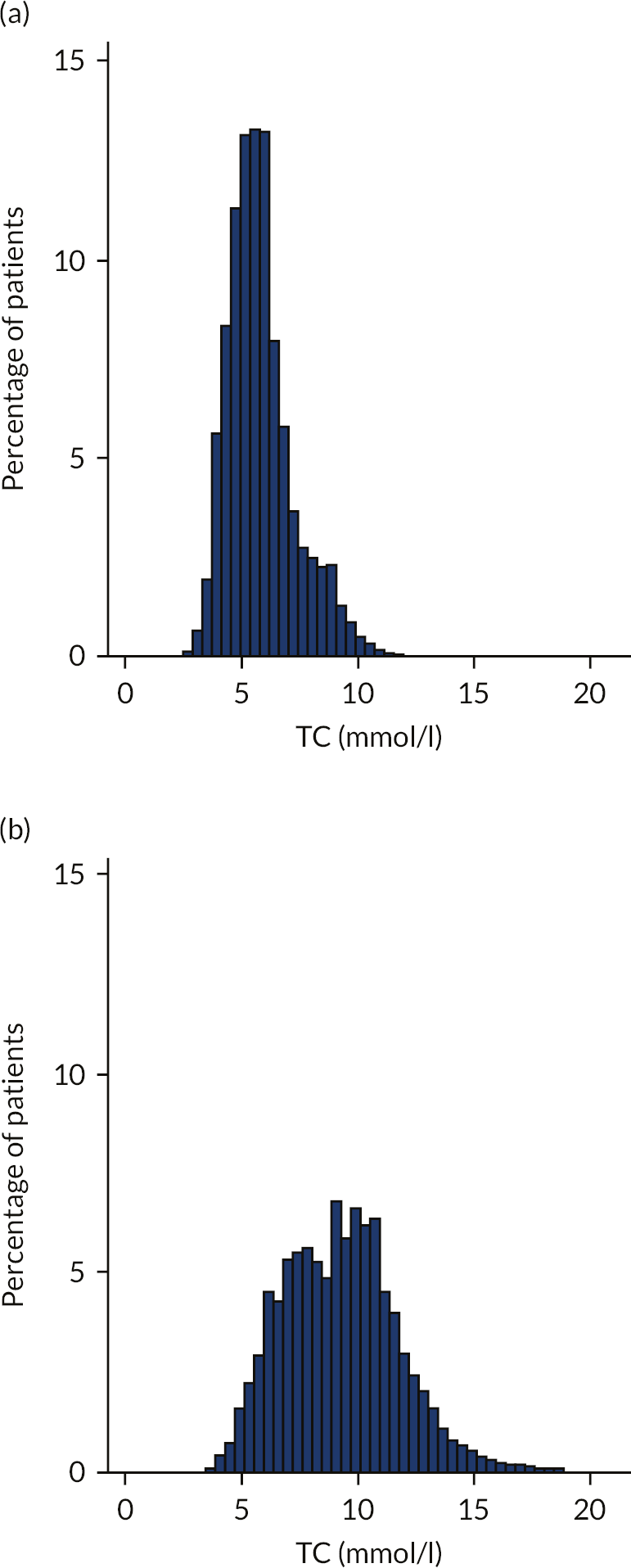
FIGURE 19.
Hazard ratios for CVD outcomes among people with FH, stratified by sex. Reproduced with permission from Iyen et al. 89 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/.
FIGURE 20.
Standardised morbidity ratios for composite CVD among men and women with FH in the Simon Broome Register.

Appendix 3 Examples of comments obtained on member checking
Index patients and relatives
There isn’t anything there that I would disagree with. I can appreciate all the comments made.
I think receiving a letter from the service has more gravitas than hearing from a family member, so may be taken more seriously. I preferred to speak to my [relative] as I have a close relationship.
I experienced a lack of concern from a GP – I think further education/awareness-raising for GPs is likely needed.
Nurse-led service sounds ideal.
Health professionals
This seems in line with my experience. Having had some recent cases where family members appeared utterly surprised by why they were in clinic, including one lad who thought he was there for his toes to be looked at, relying on indirect communication can definitely lead to gaps in the passing on of information. Especially where there may be reduced communication between family members due to physical or emotional distance.
Consultant
Thank you for inviting comments on the summary... I completely agree with all the points made and I am struggling to add anything of value to it.
FH nurse
I absolutely agree that a FH nurse and/or genetic counsellor lead approach is the best; I simply do not have time in clinics to do the job well. Indirect approached via the index case from clinic has not worked for me; relatives do not realise the importance of screening or the relative ease of treating it once discovered if the message comes via the relative.
Consultant
Interesting and accurate. I don’t have anything significant to add.
FH nurse
This looks good. Clearly written and explicable [recognisable] to our audience familiar with this area.
Consultant
Appendix 4 Disease codes used for cardiovascular disease
Coronary artery disease diagnostic codes (Read code lists)
| Medical code | Read code | Read term |
|---|---|---|
| 7783 | 323..00 | ECG: myocardial infarction |
| 26975 | 3233.00 | ECG: anteroseptal infarct |
| 26972 | 3234.00 | ECG: posterior/inferior infarct |
| 55401 | 3235.00 | ECG: subendocardial infarct |
| 52705 | 3236.00 | ECG: lateral infarction |
| 59032 | 323Z.00 | ECG: myocardial infarct NOS |
| 737 | 792..11 | Coronary artery bypass graft operations |
| 18249 | 7920.00 | Saphenous vein graft replacement of coronary artery |
| 11610 | 7920300 | Saphenous vein graft replacement of four or more coronary arteries |
| 7137 | 7920y00 | Saphenous vein graft replacement of coronary artery OS |
| 66236 | 7923200 | Prosthetic replacement of three coronary arteries |
| 5744 | 7927500 | Open angioplasty of coronary artery |
| 2901 | 7928.00 | Transluminal balloon angioplasty of coronary artery |
| 5703 | 7928.11 | Percutaneous balloon coronary angioplasty |
| 18670 | 7928000 | Percutaneous transluminal balloon angioplasty one coronary artery |
| 42462 | 7928200 | Percutaneous transluminal balloon angioplasty bypass graft coronary |
| 41547 | 7928y00 | Transluminal balloon angioplasty of coronary artery OS |
| 732 | 7928z00 | Transluminal balloon angioplasty of coronary artery NOS |
| 33650 | 7929100 | Percutaneous transluminal coronary thrombolysis with streptokinase |
| 8942 | 7929400 | Insertion of coronary artery stent |
| 42304 | 7929500 | Insertion of drug-eluting coronary artery stent |
| 6182 | 7929y00 | Other therapeutic transluminal operation on coronary artery OS |
| 33471 | 792Dz00 | Other bypass of coronary artery NOS |
| 105184 | 792E.00 | Percutaneous coronary intervention |
| 107406 | 792E000 | Emergency percutaneous coronary intervention |
| 43939 | 793G.00 | Percutaneous transluminal balloon angioplasty stenting coronary artery |
| 61208 | 793Gz00 | Percutaneous transluminal balloon angioplasty stenting coronary artery NOS |
| 45960 | 8B27.00 | Antianginal therapy |
| 101121 | 8L40.00 | Coronary artery bypass graft operation planned |
| 101373 | 8L41.00 | Coronary angioplasty planned |
| 240 | G3…00 | Ischaemic heart disease |
| 24783 | G3…11 | Arteriosclerotic heart disease |
| 20416 | G3…12 | Atherosclerotic heart disease |
| 1792 | G3…13 | IHD – ischaemic heart disease |
| 241 | G30..00 | Acute myocardial infarction |
| 2491 | G30..12 | Coronary thrombosis |
| 30421 | G30..13 | Cardiac rupture following myocardial infarction |
| 1677 | G30..15 | Acute myocardial infarction |
| 13571 | G30..16 | Thrombosis – coronary |
| 17689 | G30..17 | Silent myocardial infarction |
| 12139 | G300.00 | Acute anterolateral infarction |
| 5387 | G301.00 | Other specified anterior myocardial infarction |
| 40429 | G301000 | Acute anteroapical infarction |
| 17872 | G301100 | Acute anteroseptal infarction |
| 14897 | G301z00 | Anterior myocardial infarction NOS |
| 8935 | G302.00 | Acute inferolateral infarction |
| 29643 | G303.00 | Acute inferoposterior infarction |
| 23892 | G304.00 | Posterior myocardial infarction NOS |
| 14898 | G305.00 | Lateral myocardial infarction NOS |
| 63467 | G306.00 | True posterior myocardial infarction |
| 3704 | G307.00 | Acute subendocardial infarction |
| 9507 | G307000 | Acute non-Q wave infarction |
| 10562 | G307100 | Acute non-ST segment elevation myocardial infarction |
| 1678 | G308.00 | Inferior myocardial infarction NOS |
| 30330 | G309.00 | Acute Q-wave infarct |
| 32854 | G30B.00 | Acute posterolateral myocardial infarction |
| 29758 | G30X.00 | Acute transmural myocardial infarction of unspecified site |
| 12229 | G30X000 | Acute ST segment elevation myocardial infarction |
| 34803 | G30y.00 | Other acute myocardial infarction |
| 28736 | G30y000 | Acute atrial infarction |
| 62626 | G30y100 | Acute papillary muscle infarction |
| 41221 | G30y200 | Acute septal infarction |
| 46017 | G30yz00 | Other acute myocardial infarction NOS |
| 14658 | G30z.00 | Acute myocardial infarction NOS |
| 27951 | G31..00 | Other acute and subacute ischaemic heart disease |
| 23579 | G310.00 | Post-myocardial infarction syndrome |
| 15661 | G310.11 | Dressler syndrome |
| 36523 | G311.00 | Preinfarction syndrome |
| 4656 | G311.11 | Crescendo angina |
| 1431 | G311.13 | Unstable angina |
| 19655 | G311.14 | Angina at rest |
| 61072 | G311000 | Myocardial infarction aborted |
| 55137 | G311011 | Myocardial infarction aborted |
| 7347 | G311100 | Unstable angina |
| 17307 | G311200 | Angina at rest |
| 34328 | G311300 | Refractory angina |
| 18118 | G311400 | Worsening angina |
| 11983 | G311500 | Acute coronary syndrome |
| 54251 | G311z00 | Preinfarction syndrome NOS |
| 39449 | G312.00 | Coronary thrombosis not resulting in myocardial infarction |
| 9413 | G31y.00 | Other acute and subacute ischaemic heart disease |
| 9276 | G31y000 | Acute coronary insufficiency |
| 68357 | G31y100 | Microinfarction of heart |
| 27977 | G31yz00 | Other acute and subacute ischaemic heart disease NOS |
| 4017 | G32..00 | Old myocardial infarction |
| 1430 | G33..00 | Angina pectoris |
| 20095 | G330.00 | Angina decubitus |
| 18125 | G330000 | Nocturnal angina |
| 29902 | G330z00 | Angina decubitus NOS |
| 11048 | G331.11 | Variant angina pectoris |
| 36854 | G332.00 | Coronary artery spasm |
| 25842 | G33z.00 | Angina pectoris NOS |
| 1414 | G33z300 | Angina on effort |
| 9555 | G33z500 | Post-infarct angina |
| 26863 | G33z600 | New-onset angina |
| 12804 | G33z700 | Stable angina |
| 28554 | G33zz00 | Angina pectoris NOS |
| 28138 | G34..00 | Other chronic ischaemic heart disease |
| 5413 | G340.00 | Coronary atherosclerosis |
| 1344 | G340.12 | Coronary artery disease |
| 3999 | G340000 | Single coronary vessel disease |
| 5254 | G340100 | Double coronary vessel disease |
| 29421 | G344.00 | Silent myocardial ischaemia |
| 34633 | G34y.00 | Other specified chronic ischaemic heart disease |
| 24540 | G34y000 | Chronic coronary insufficiency |
| 23078 | G34y100 | Chronic myocardial ischaemia |
| 35713 | G34yz00 | Other specified chronic ischaemic heart disease NOS |
| 15754 | G34z.00 | Other chronic ischaemic heart disease NOS |
| 18889 | G34z000 | Asymptomatic coronary heart disease |
| 18842 | G35..00 | Subsequent myocardial infarction |
| 45809 | G350.00 | Subsequent myocardial infarction of anterior wall |
| 38609 | G351.00 | Subsequent myocardial infarction of inferior wall |
| 72562 | G353.00 | Subsequent myocardial infarction of other sites |
| 46166 | G35X.00 | Subsequent myocardial infarction of unspecified site |
| 36423 | G36..00 | Certain current complication following acute myocardial infarct |
| 24126 | G360.00 | Haemopericardium/current complication following acute myocardial infarct |
| 23708 | G361.00 | Atrial septal defect/current complication following acute myocardial infarct |
| 37657 | G362.00 | Ventricular septal defect/current complication following acute myocardial infarct |
| 59940 | G364.00 | Rupture chordae tendinae/current complication following acute myocardial infarct |
| 69474 | G365.00 | Rupture papillary muscle/current complication following acute myocardial infarct |
| 32272 | G38..00 | Postoperative myocardial infarction |
| 46112 | G380.00 | Postoperative transmural myocardial infarction anterior wall |
| 46276 | G381.00 | Postoperative transmural myocardial infarction inferior wall |
| 106812 | G383.00 | Postoperative transmural myocardial infarction unspecified site |
| 41835 | G384.00 | Postoperative subendocardial myocardial infarction |
| 68748 | G38z.00 | Postoperative myocardial infarction, unspecified |
| 22383 | G3y..00 | Other specified ischaemic heart disease |
| 1676 | G3z..00 | Ischaemic heart disease NOS |
| 35119 | G501.00 | Post infarction pericarditis |
| 52517 | Gyu3.00 | [X]Ischaemic heart diseases |
| 39546 | Gyu3000 | [X]Other forms of angina pectoris |
| 68401 | Gyu3200 | [X]Other forms of acute ischaemic heart disease |
| 47637 | Gyu3300 | [X]Other forms of chronic ischaemic heart disease |
| 96838 | Gyu3400 | [X]Acute transmural myocardial infarction of unspecified site |
| 109035 | Gyu3500 | [X]Subsequent myocardial infarction of other sites |
| 99991 | Gyu3600 | [X]Subsequent myocardial infarction of unspecified site |
| 40887 | N23yB00 | Ischaemic infarction of muscle |
| ECG, electrocardiogram; NOS, not otherwise specified; OS, otherwise specified. | ||
Peripheral vascular disease diagnostic codes (Read code lists)
| Medical code | Read code | Read term |
|---|---|---|
| 5943 | G73..00 | Other peripheral vascular disease |
| 5702 | G73..11 | Peripheral ischaemic vascular disease |
| 6827 | G73..13 | Peripheral ischaemia |
| 9204 | G732.00 | Peripheral gangrene |
| 105317 | G734.00 | Peripheral arterial disease |
| 38907 | G73y.00 | Other specified peripheral vascular disease |
| 4325 | G73yz00 | Other specified peripheral vascular disease NOS |
| 3530 | G73z.00 | Peripheral vascular disease NOS |
| 1517 | G73z000 | Intermittent claudication |
| 101866 | G73z012 | Vascular claudication |
| 2760 | G73zz00 | Peripheral vascular disease NOS |
| 15302 | G742z00 | Peripheral arterial embolism and thrombosis NOS |
| 73961 | Gyu7400 | [X]Other specified peripheral vascular diseases |
Cerebrovascular accident (stroke) and transient ischaemic attack diagnostic codes (Read code lists)
| Medical code | Read code | Read term |
|---|---|---|
| 569 | G64..12 | Infarction – cerebral |
| 1469 | G66..00 | Stroke and cerebrovascular accident unspecified |
| 1895 | G65z.00 | Transient cerebral ischaemia NOS |
| 2418 | G6…00 | Cerebrovascular disease |
| 3149 | G64z.00 | Cerebral infarction NOS |
| 5184 | G670.11 | Precerebral atherosclerosis |
| 5363 | G64..11 | CVA – cerebral artery occlusion |
| 5602 | G64z.12 | Cerebellar infarction |
| 6116 | G66..13 | CVA – cerebrovascular accident unspecified |
| 6155 | G64..13 | Stroke due to cerebral arterial occlusion |
| 6228 | G68X.00 | Sequelae of stroke, not specified as haemorrhage or infarction |
| 6253 | G66..12 | Stroke unspecified |
| 6960 | G61..11 | CVA – cerebrovascular accident due to intracerebral haemorrhage |
| 8443 | G663.00 | Brain stem stroke syndrome |
| 8837 | G64..00 | Cerebral arterial occlusion |
| 9985 | G64z200 | Left-sided cerebral infarction |
| 10062 | G6z..00 | Cerebrovascular disease NOS |
| 10504 | G64z300 | Right-sided cerebral infarction |
| 11171 | G670.00 | Cerebral atherosclerosis |
| 13577 | G67..00 | Other cerebrovascular disease |
| 15019 | G641.00 | Cerebral embolism |
| 15788 | G65zz00 | Transient cerebral ischaemia NOS |
| 16517 | G640.00 | Cerebral thrombosis |
| 17322 | G664.00 | Cerebellar stroke syndrome |
| 18604 | G61..12 | Stroke due to intracerebral haemorrhage |
| 19354 | G65y.00 | Other transient cerebral ischaemia |
| 23671 | G63y000 | Cerebral infarction due to thrombosis of precerebral arteries |
| 24446 | G63y100 | Cerebral infarction due to embolism of precerebral arteries |
| 27975 | G641000 | Cerebral infarction due to embolism of cerebral arteries |
| 33543 | G6X..00 | Cerebral infarction due to unspecified occlusion or stenosis of cerebral arteries |
| 34117 | G67y.00 | Other cerebrovascular disease OS |
| 36717 | G640000 | Cerebral infarction due to thrombosis of cerebral arteries |
| 37493 | G67z.00 | Other cerebrovascular disease NOS |
| 40053 | G671.00 | Generalised ischaemic cerebrovascular disease NOS |
| 40758 | G6W..00 | Cerebral infarct due to unspecified occlusion/stenosis of precerebral arteries |
| 45781 | G63..00 | Precerebral arterial occlusion |
| 51138 | G68W.00 | Sequelae/other + unspecified cerebrovascular diseases |
| 51311 | G6y..00 | Other specified cerebrovascular disease |
| 51326 | G63y.00 | Other precerebral artery occlusion |
| 51759 | G677000 | Occlusion and stenosis of middle cerebral artery |
| 53745 | Gyu6400 | [X]Other cerebral infarction |
| 57495 | G63..11 | Infarction – precerebral |
| 57527 | G677100 | Occlusion and stenosis of anterior cerebral artery |
| 63746 | Fyu5500 | [X]Other transient cerebral ischaemic attacks + related syndromes |
| 65770 | G677200 | Occlusion and stenosis of posterior cerebral artery |
| 70536 | G671000 | Acute cerebrovascular insufficiency NOS |
| 71274 | G677400 | Occlusion + stenosis of multiple and bilateral cerebral arteries |
| 71585 | G63z.00 | Precerebral artery occlusion NOS |
| 73901 | Gyu6.00 | [X]Cerebrovascular diseases |
| 91627 | Gyu6300 | [X]Cerebral infarction due to unspecified occlusion or stenosis of cerebral arteries |
| 92036 | Gyu6600 | [X]Occlusion and stenosis of other cerebral arteries |
| 94482 | Gyu6G00 | [X]Cerebral infarct due to unspecified occlusion/stenosis of precerebral arteries |
| 98188 | G679.00 | Small vessel cerebrovascular disease |
| 98642 | G633.00 | Multiple and bilateral precerebral arterial occlusion |
| 110337 | Gyu6C00 | [X]Sequelae of stroke, not specified as haemorrhage or infarction |
| 111096 | Gyu6700 | [X]Other specified cerebrovascular diseases |
List of abbreviations
- ACS
- acute coronary syndrome
- A&E
- accident and emergency
- AMSTAR
- A MeaSurement Tool to Assess systematic Reviews
- APOB
- apolipoprotein B-100
- BHF
- British Heart Foundation
- BMI
- body mass index
- CCG
- Clinical Commissioning Group
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CHD
- coronary heart disease
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CPRD
- Clinical Practice Research Datalink
- CVD
- cardiovascular disease
- DLCN
- Dutch Lipid Clinic Network
- EAS
- European Atherosclerosis Society
- FH
- familial hypercholesterolaemia
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems,Tenth Revision
- IQR
- interquartile range
- LDL-C
- low-density lipoprotein cholesterol
- LDLR
- low-density lipoprotein receptor
- LIMS
- Laboratory Information Management Systems
- LLT
- lipid-lowering treatment
- MEDPED
- make early diagnosis to prevent early death
- MeSH
- medical subject heading
- NICE
- National Institute for Health and Care Excellence
- ONS
- Office for National Statistics
- PCSK9
- proprotein convertase subtilisin/kexin type 9
- PPIE
- patient and public involvement and engagement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PT-LDL-C
- pre-treatment low-density lipoprotein cholesterol
- PVD
- peripheral vascular disease
- QALY
- quality-adjusted life-year
- SD
- standard deviation
- SMbR
- standardised morbidity ratio
- TC
- total cholesterol
- TIA
- transient ischaemic attack
- UTS
- up to quality standard
- VUS
- variant of uncertain significance
- WDLCN
- Welsh-modified Dutch Lipid Clinic Network
Notes
-
A MeaSurement Tool to Assess systematic Reviews (AMSTAR) 2 scores
-
Cost and health benefits of diagnosis of individuals with familial hypercholesterolaemia in the long term
-
Net health effects of alternative testing strategies by age group
-
Cost-effectiveness model results for alternative cascade protocols
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/CTMD0148).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
