Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number NIHR128862. The contractual start date was in April 2020. The draft manuscript began editorial review in August 2022 and was accepted for publication in March 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2023 Frederick Crocker et al. This work was produced by Frederick Crocker et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Frederick Crocker et al.
Chapter 1 Background
There were 12.5 million people aged over 65 years in the UK in 2021 (19% of the population), and this number is expected to rise to 15 million (22%) over the following decade. 1,2 Similar growth is expected in most other developed countries, and more rapid population changes are anticipated in developing countries. In response to such demographic changes, policies and initiatives such as the World Health Organization’s Decade of Healthy Ageing emphasise healthy ageing – aiming to increase the number of years lived in good health and to optimise independence and quality of life in the presence of accumulating health conditions. 3,4 The concept of frailty can be used to distinguish between people who have remained in robust health, and those who have accumulated multiple long-term conditions, are at risk of losing independence and are likely to require health and social care resources. 5 In the UK, around 10% of people aged 65 years and over have frailty, rising to around 50% of people aged over 85 years6 and the additional annual cost to the healthcare system per person (in 2013–4) was approximately £550 for mild, £1200 for moderate and £2100 for severe frailty. This equates to a total additional financial cost of £5.6 billion per year across the UK. 7
Health, social care and third-sector organisations provide community services to support healthy ageing. A systematic review and meta-analysis summarised evidence from 89 randomised controlled trials (RCTs) of complex interventions, published up to January 2005 and involving 97,984 participants, aiming to improve physical function and increase independence for community-dwelling older people. 8 The review reported that, in general, complex interventions provided in the community are effective in improving physical function and increasing independence in older people. While this result was encouraging, the review was unable to provide evidence to indicate which of the different service models or intervention components delivered within them were more effective. The review showed that services directed at older people after hospital discharge were significantly effective. It also showed that general untargeted services for older people were also significantly effective, whereas those targeted at older people with frailty were not. However, only one, ill-defined, service model (comprehensive geriatric assessment) was included in this subanalysis, and frailty was not operationalised using valid measurements. Policy-makers, commissioners and service providers require further information about the evidence of effectiveness of services and their components, and the effect that frailty has upon their effectiveness, to guide their decisions about exactly which complex community services for older people to commission and how they should be organised.
We have conducted such an evidence synthesis to update and expand the previous review. This updated meta-analysis includes additional studies of community-based services aiming to sustain the independence of older people in the community published since January 2005. By including information about the nature of the intervention components and the use of network meta-analysis (NMA), this updated review aimed to identify the most effective combinations of components or clusters of interventions. NMA extends traditional pairwise meta-analysis by incorporating evidence regarding the differential effects of multiple types of intervention. The review includes a formal evaluation of certainty in the available evidence. The impact of frailty has been examined in a meta regression analysis. Given the lack of a widely used taxonomy or classification of services or their components, this review has generated and applied a method to distinguish between intervention components and clusters of them.
Research questions and objectives
Research questions
-
Do community-based complex interventions to sustain independence in older people increase living at home, independence and health-related quality of life?
-
Do community-based complex interventions to sustain independence in older people reduce homecare usage, depression, loneliness, falls, hospitalisation, care-home placement, costs and mortality?
-
How should interventions be grouped for NMA?
-
What is the optimal configuration of community-based complex interventions to sustain independence in older people?
-
Do intervention effects differ by frailty level (robust, pre-frailty and frailty)?
Objectives
-
To identify RCTs and cluster RCTs (cRCTs) of community-based complex interventions to sustain independence in older people.
-
To synthesise evidence of their effectiveness for key outcomes (meta-analysis of study-level data).
-
To identify key intervention components and study-level frailty to inform groupings for NMA and meta regression.
-
To compare effectiveness of different intervention configurations (NMA).
-
To investigate the impact of frailty and pre-frailty (meta regression).
Chapter 2 Review methods
Design
Systematic review with NMA of trials evaluating community-based complex interventions to sustain independence in older people (mean age 65 years and over), compared with usual care or another complex intervention meeting our criteria, with follow-up for at least 24 weeks. We followed Cochrane methods,9 evaluated quality of evidence following Grading of Recommendations Assessment, Development and Evaluation (GRADE) NMA guidance10 and reported the review in accordance with preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 and PRISMA NMA guidelines. 11 We prospectively registered the review on PROSPERO (CRD42019162195)12 and the protocol was published before analysis began. 13
Throughout the review we used monthly Project Management Group (PMG) meetings and the quarterly meetings of our established patient and public involvement (PPI) Frailty Oversight Group (FOG) to assure the relevance and appropriateness of day-to-day decisions and resolve disagreements between independent reviewers.
Health technologies being assessed
This review assessed community-based complex interventions for older people that were targeted at the individual and focused on sustaining their independence.
Complex interventions have been defined as interventions with several interacting components (intervention practices, structural elements and contextual factors). 14 They typically attempt to introduce new, or modify existing, patterns of collective action in healthcare or formal organisational settings, with an intention to lead to changed outcomes. 15 We used this definition of complex interventions to inform our eligibility criteria.
For this review, we defined sustaining independence to mean maintaining or improving independence in activities of daily living (washing, dressing, grooming, toileting, walking, preparing meals, doing housework, managing finances, assisting others, etc.), but not only one of these specific activities (e.g. walking only). This was a refinement made during the screening stage of our review, to ensure studies addressed this individually meaningful aim that is less interdependent on the wider healthcare context than other meanings (e.g. care home placement), and therefore more generalisable across trials.
Identification of studies
Information sources
Databases and trial registers
We searched the following databases from inception between 9 and 11 August 2021:
-
Cochrane Central Register of Controlled Trials (CENTRAL) Wiley (1992 to 11 August 2021)
-
MEDLINE Ovid (1946 to 6 August 2021)
-
Embase and Embase Classic Ovid (1947 to 6 August 2021)
-
CINAHL EBSCOhost (1972 to 9 August 2021)
-
APA PsycINFO Ovid (1806 to August Week 1, 2021).
We also searched trial registers from inception on 10 August 2021:
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; to 10 August 2021);
-
World Health Organization International Clinical Trials Registry Platform (https://trialsearch.who.int; to 10 August 2021).
Other sources
We searched for additional reports of all included studies in the reference lists of their reports, by searching Google Scholar (scholar.google.co.uk) with the intervention name, project name and trial register number separately, and, if available, on the study’s website. We scanned the reference lists of included studies to identify potentially eligible trials not already identified (backward citation searching). We exhaustively continued backward citation searching and identification of additional reports, including for all reports and studies included in the review through these same processes.
Search strategy
Search strategies for the database and trial register searches were developed and tested through an iterative process by an experienced medical information specialist in consultation with the review team. The full search strategies for all databases and trial registers are available in Appendix 1, including for CINAHL in Table 23.
The search contained the following concepts:
-
Older people or frailty.
-
Home-based or community interventions.
-
RCT filter.
-
1 AND 2 AND 3.
Restrictions by publication status or language were not used.
Search terms were harvested by exploring three relevant systematic reviews and their included studies. 8,16,17 Their search strategies, as well as words and phrases in title, abstract and subject indexing were reviewed to find relevant search terms for inclusion. These terms were used to develop the initial draft search strategy. Extra search terms were found by reviewing results from that initial strategy. The PubMed PubReMiner (https://hgserver2.amc.nl/cgi-bin/miner/miner2.cgi) word frequency analysis tool was also used to find index terms and keyword terms for inclusion in the search strategy. For the concept ‘home-based or community interventions’, we included both broad and specific search terms as testing showed that this was necessary to capture all relevant interventions. We limited terms about geriatric nursing to community or home settings to increase the relevance of search results. Following testing, we also excluded some specific medical conditions in titles to increase the relevancy of the search results. For our MEDLINE search, we added the Cochrane Collaboration highly sensitive filter to identify randomised trials. 18 This was supplemented with a search filter developed to find Phase Three Trials not found by the Cochrane RCT filter. 19 For the Embase search, we used the filter for finding randomised trials in Embase developed by the Cochrane Collaboration. 20 For the PsycINFO search, we used a sensitive methodological search filter. 21 For the CINAHL search, we developed our own search strategy to identify randomised trials. The strategies were peer reviewed by another information specialist prior to execution using the Peer Review of Electronic Search Strategies (PRESS) checklist. 22
Study selection
Eligibility criteria
Types of studies
Only RCTs and cRCTs were eligible. Where only one unit of randomisation (an individual or cluster) was allocated to an arm of a trial, we excluded the trial as the treatment effect is completely confounded with the unit. We accepted minimisation as a method of sequence generation, in keeping with Cochrane risk-of-bias (RoB) guidance. 23 We did not exclude variants of randomised trials such as stepped-wedge trials. Crossover and waiting-list designs were also eligible, but we only used outcome data from the pre-crossover period because it is likely that the older people’s independence evolves over time and that, were interventions to be effective at modifying outcomes such as activities of daily living (ADL), this would be a long-term modification whose effects may carry over into the subsequent period of the trial.
Types of participants
We included studies involving older people living at home (mean age of study participants 65 years or older). We excluded trials in residential/nursing homes. If not all participants were living at home, we only included the study if data could be extracted specifically for these participants.
Types of intervention
Aligned with our focus on community-based complex interventions, trials were considered eligible if:
-
the intervention was both initiated and mainly provided in the community
-
the intervention included two or more interacting components (intervention practices, structural elements and contextual factors)
-
the intervention was targeted at the individual person, with provision of appropriate specialist care
-
a focus of the intervention was sustaining (maintaining or improving) the person’s independence.
A broad range of interventions was eligible, differing in terms of how the service was organised and what was done to or for the older person. Our criterion of including two or more interacting components could be met in multiple ways. Eligible interventions could include multiple discrete practices, such as exercise sessions and nutritional advice. Alternatively, interventions could include one practice that interacted with other structural elements such as being reliant on general practice or other services; or interaction with contextual factors by being substantially tailored to the person’s physical and social environment such as ‘comprehensive geriatric assessment’24 or rehabilitation interventions.
Interventions that were not eligible for inclusion were those where:
-
the intervention was either not initiated, or not mainly provided, in the community, or neither for example interventions delivered in outpatient, day hospital, inpatient and intermediate (post-acute) care settings
-
the intervention included only one component (intervention practices, structural elements and contextual factors), for example, if any of the following were delivered as single component interventions: a drug; treadmill training; yoga; provision of information; cataract surgery for visual impairment; hearing aid for hearing impairment; medication-review; nutritional supplements
-
the intervention was not targeted at the individual person, with provision of appropriate specialist care for example general staff education (not training in a patient-level intervention), practice-level reorganisation, operational, managerial or information technology (IT) interventions, public health messages
-
the intervention was not focused on sustaining (maintaining or improving) independence; for example, we excluded interventions that primarily addressed cognitive deficits, mood disorders or both, unless they also aimed to improve overall independence
-
disease-focused case management of older people with specific long-term conditions; for example, diabetes, chronic obstructive pulmonary disease or depression
-
interventions in which the primary focus was fall prevention were excluded as the evidence base for fall prevention is well established including via NMA. 25 Nonetheless, falls were an additional outcome of this review.
Initially we intended to only include interventions for which sustaining independence was the main aim, but this was broadened as complex interventions were rarely described as having one main aim.
Comparators
Usual care, ‘placebo’ or attention control or a different complex intervention which met our criteria were eligible comparators.
Outcomes
Studies were only included where outcome data were measured at a minimum 24-week (approximately 6 months) time point. We included studies that met the above criteria whether or not they measured or reported our outcomes of interest (see below).
Study selection process
Records identified from the literature searches were imported to EndNote (vX9.3.3; Clarivate Analytics, Philadelphia, PA, USA) and duplicates were removed. The results were imported into the Rayyan web application (https://rayyan.qcri.org/) (January 2020 search) or Covidence web application (www.covidence.org/) (September 2020 and August 2021 updates). Two researchers independently assessed the title and abstract in each record. Where a record referred to a report of a potentially eligible study, we obtained the full text of the report. Two researchers independently assessed inclusion against our pre-specified criteria, resolving disagreements by consensus with guidance from the PMG. We contacted study authors if further information was required. In cases where there may have been more than one reason to exclude a report, two reviewers reached consensus on a primary reason for exclusion, selecting the first eligibility criterion in our list of eligibility criteria that they were certain was not met. Where studies appeared eligible and to have finished data collection but had not published any results, we contacted the authors to request completed study results. Translation was arranged if necessary throughout the selection process. We excluded as ‘ongoing’ studies that had not been completed, or had finished data collection within 12 months of August 2021 but for which we had no results. 26 The results of the study selection process were imported into and managed within EndNote.
Data collection process
Two independent researchers collected data using a piloted data collection form in a purpose-built database in Microsoft Access (v16.0; Microsoft Corporation, Redmond, WA, USA). Characteristics of included studies tables were produced from this database. The characteristics of excluded studies table was manually produced via our EndNote library.
Study-level data
We sought the following details for each study:
-
study report citations (i.e. authors, date and location of report)
-
sponsorship/funding source(s)
-
country
-
aims and rationale
-
type of RCT design – randomised by individual or cluster, parallel group or crossover. If cluster, level(s) of clustering
-
analysis details (e.g. intention to treat).
We also sought the following characteristics regarding their participants:
-
brief characterisation
-
inclusion and exclusion criteria
-
age (range and/or means)
-
gender percentages
-
frailty status (see Assessment of frailty)
-
health status
-
living arrangement
-
carer presence
-
ethnicity
-
total number of participants (and clusters) randomised into each group.
Trial arm (intervention) level data
-
number of participants allocated (and clusters where appropriate)
-
experimental nature of arm within the study (experimental/control).
Additionally, details of the intervention as specified by the Template for Intervention Description and Replication (TIDieR)27 were coded and summarised in NVivo (see Intervention grouping).
Outcomes of interest
We collected details of the outcomes measured by the included studies and sought to collect quantitative data and results on the following outcomes:
Main outcomes
-
Living at home.
-
Activities of daily living (personal/instrumental).
-
Hospitalisation.
-
Care-home placement.
-
Homecare services (non-healthcare professional) usage.
-
Costs.
-
Cost-effectiveness.
Additional outcomes
-
Health status/health-related quality of life.
-
Depression.
-
Loneliness.
-
Falls.
-
Mortality.
We worked with our PPI FOG to identify key outcomes, and prioritise them as ‘main’ or ‘additional’, from the perspective of older people. Originally (prior to analyses beginning) these outcomes were proposed as one primary outcome (living at home) with the others as secondary outcomes.
We sought binary data for living at home, homecare services usage, hospitalisation, care-home placement, falls and mortality; and continuous data for activities of daily living, homecare services usage, hospitalisation, costs, cost-effectiveness, health status, depression, loneliness and falls. We excluded bespoke metrics without evidence of evaluation of measurement properties, or metrics where significant problems with their use are recognised.
Specifically, for binary outcomes, we sought to extract the total number of participants in each intervention and control group, and the number of outcome events, alongside any reported effect measures and their 95% confidence intervals (CIs), such as risk ratios (RRs) or odds ratios (ORs). For continuous outcomes, we sought to extract the total number of participants in each intervention and control group, the mean and standard deviation (SD) of the outcome in each group at baseline and at follow-up, and the mean and SD of the change score for each group. These estimates were extracted alongside any reported effect measures, such as the mean difference (MD) in final score or change score, or the ANCOVA (adjusted for baseline) result and a measure of variance such as the standard error. Where the above estimates were unavailable within publications, we attempted to recover these using alternate available information so as to avoid selection bias (e.g. using reported elements of the five-number summary, CIs, p-values, 2 × 2 contingency tables etc., to recover mean (SD) or ORs/RRs).
Where living at home was not a reported study outcome but care-home placement and mortality were, we calculated living at home as the remainder (not dead or living in a care home) where it was possible to do so without double-counting participants. Where care-home placement results related to all participants including those who had subsequently died, we could not disaggregate those who had died while living at home and so could not use these results.
Care-home placement as a standalone outcome was only included where it related to care-home residence at that time point, that is the figures excluded mortality during the period of reporting.
Time points
For all outcomes of interest, data were extracted and categorised for three time frames shown in Table 1.
| Label | Target time point | Range |
|---|---|---|
| Short term | 6 months | 24 weeks to 9 months |
| Medium term | 12 months | > 9 months to 18 months |
| Long term | 24 months | > 18 months |
Where more than one time point was reported for an outcome within a range specified above, we used the time point nearest to the target time point.
Because of the large number of outcomes and multiple time frames, we limited analyses of additional outcomes to the medium-term time frame only. We anticipated that the medium term would be of particular interest to commissioners and older people, would be most likely to allow sufficient time for effects to be realised but not washed out, and when most data would be available. We planned to conduct sensitivity analyses and meta regression for the medium-term time frame initially and to extend these to short- and long-term networks in the presence of significant findings.
Throughout the rest of the report we refer to results of interest, which we define here as reported data about a comparison between two arms of a trial for an outcome of interest in one of our analysis time frames.
Other outcomes
We listed all other outcomes measured by included studies but did not collect the results data.
Intervention grouping
We grouped all eligible interventions (including comparators) in preparation for NMA in a three-stage process.
-
We used an extended version of the TIDieR framework to code and summarise reported interventions in NVivo 12 (QSR International Pty Ltd, Hawthorn East, VIC, Australia). 27 The TIDieR framework includes 12 key items (see Table 2). We added a further item regarding the organisational details of the intervention, such as the roles and responsibilities of the intervention providers, means of co-ordination, organisational boundaries and links and financial arrangements. One reviewer coded and summarised each intervention and at least one reviewer (TFC) assessed these and resolved any disagreements by consensus discussion. In the earlier stages, to improve consistency between reviewers, an additional reviewer made an assessment before it was assessed by TFC.
-
We categorised the coding using the principles of content analysis to inform provisional groupings. 28 This categorisation was also considered by at least two reviewers and disagreements were resolved by consensus, involving the PMG where necessary.
-
We developed initial intervention groupings with consideration for service organisation, care processes and specific patient care (e.g. exercise, ADL practice). Through internal discussions involving reviewers and the PMG, including reflections about existing frameworks,29,30 the practical restraints of what was reported and the diversity of interventions, we based our groups on the actions that were intended to be provided to all or almost all participants. These actions determined the components that we used to group an intervention. Interventions that were grouped together were compared and contrasted with subsequent refinement to coding, components and grouping. We presented our provisional groupings to experts including policy-makers, commissioners, older people and carers for open discussion. These led to further revision of our intervention groups and their descriptions (see Chapter 3, Results of the review for details). The intervention groups became the nodes in the NMA.
| TIDieR item | Description |
|---|---|
| 1. Brief name | The brand name of the intervention (if any) and, if not descriptive, a short description. |
| 2. Why | Rationale, theory or goal of the elements essential to the intervention. |
| 3. What (materials) | The physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery/training of intervention providers. |
| 4. What (procedures) | The procedures, activities and/or processes used in the intervention, including any enabling or support activities. |
| 5. Who provided | The expertise, background and any specific training given to each category of intervention provider. |
| 6. How | The modes of delivery (such as face-to-face or by some other mechanism) of the intervention, and whether it was provided individually or in a group. |
| 6b. How organised (additional item) | The roles and responsibilities of the intervention providers, means of co-ordination, organisational boundaries and links and financial arrangements. |
| 7. Where | The types of location where the intervention occurred, and any required infrastructure. |
| 8. When and how much | The amount and intensity of intervention delivered, including number of sessions, their schedule, duration and dose and the overall time frame in relation to triggering events. |
| 9. Tailoring | Details of any individual/group adaptations, personalisation or titration. |
| 10. Modifications | Details of any changes made to the intervention during the course of the study. |
| 11. How well (planned) | Strategies to achieve fidelity or adherence and planned assessment of fidelity. |
| 12. How well (actual) | Extent of intervention fidelity achieved. |
Assessment of frailty
We expected that a range of validated instruments and operationalised measures would be used to identify pre-frailty and frailty in included trial populations of some studies. Examples of such frailty measures include: the use of the Fried phenotype model, cumulative deficit frailty index (FI), the Tilburg Frailty Indicator, Groningen Frailty Indicator, Clinical Frailty Scale, Hebrew Rehabilitation Center for Aged Vulnerability Index, Vulnerable Elders Survey, Brief frailty measure derived from the Canadian Study of Health and Ageing or a formally produced Frailty Index. We classified the trial population in accordance with the frailty measure, so long as it was developed or validated according to the modern meaning of frailty (loss of biological reserves, failure of physiological mechanisms and vulnerability to experiencing adverse outcomes after minor stressor events) and not as a generic term for being old or disabled. We reported methods used for each study, including cutpoints for identification of pre-frailty and frailty.
We also expected that many studies would not formally have described study populations in terms of frailty. In such circumstances two reviewers with extensive clinical academic frailty expertise (AC and JG) independently used the well-validated phenotype model as a framework to categorise study-level frailty profile (robust; pre-frailty; frailty) of trial participants if the relevant variables were reported. 31 The model is based on five characteristics (weight loss; exhaustion; low energy expenditure; slow gait speed; low grip strength). Evidence of ≥ 3 indicates frailty, 1–2 pre-frailty and 0 robust. In the remaining studies where neither a recognised frailty measure nor the variables needed to apply the frailty phenotype categorisation were reported, the two reviewers independently attempted to classify the populations based on trial eligibility criteria and/or reported baseline characteristics closely linked to frailty including gait speed, hand grip strength, mobility, activity or disability levels. Any disagreements were resolved by consensus.
In categorising study level frailty, we recognised that trials included participants across different frailty categories, so as well as ‘robust’, ‘pre-frail’ and ‘frail’, our categories also included ‘robust and pre-frail’, ‘pre-frail and frail’ and ‘all’. Where we were not able to classify the population, we labelled it ‘unclassified’.
We planned for our main analysis of the impact of frailty to only include trials that were categorised according to a validated measure, with subsequent analyses including all categorised trials. However, we categorised insufficient trials according to a validated measure to enable this approach, so our analyses do not distinguish between the methods of categorisation.
Risk-of-bias assessment
Two reviewers independently assessed RoB in each result of interest from each included study, using the Revised Cochrane risk-of-bias tool for randomised trials (RoB 2). 32,33 Disagreements were resolved by consensus between the reviewers or through discussion with the PMG. Our effect of interest was the effect of assignment to the intervention (‘intention-to-treat’ effect). For individually randomised studies, we assessed RoB in five domains:
-
bias arising from the randomisation process
-
bias due to deviations from intended interventions
-
bias due to missing outcome data
-
bias in measurement of the outcome
-
bias in selection of the reported result.
For cRCTs, we used the latest guidance34 to assess identification/recruitment bias, and the other issues such as loss of clusters detailed in section 23.2: Assessing RoB in cluster-randomised trials, of the Cochrane Handbook Version 6. 9 This assessment resulted in two domains of bias in place of domain 1: 1a, bias arising from the randomisation process, and 1b, bias arising from the identification or recruitment of participants. Other details were integrated within the same domains as for individually randomised trials.
For each domain, we made a judgement of high RoB, low RoB or some concerns. We used the signalling questions and algorithms and considered whether to override the result, recording our reasons and supporting evidence. For domain five, in the absence of a pre-specified analysis plan we judged the risk to be low when the result being assessed was very unlikely to have been selected on the basis of the results from multiple eligible outcome measurements or from multiple eligible analyses of the data.
For each assessed result of interest, we summarised our concerns and reached an overall risk-of-bias judgement that was at least as severe as the most severe domain risk. Although we considered whether to upgrade the severity where multiple domains were rated as some concerns (and none as high), we did not do so. We also judged whether a result at high RoB posed serious concerns (only one domain at high risk) or very serious concerns (more than one domain at high RoB or very serious concerns in relation to one domain). These judgements then fed into our GRADE assessment of RoB, consistent with GRADE and Cochrane recommendations regarding the association between RoB in individual results and the results of analyses. 35,36
We used the RoB 2 Excel tools (version 8 for individually randomised studies, version 3 for cluster-randomised studies, available from www.riskofbias.info/welcome/rob-2-0-tool) to manage our assessments and check consistency between reviewers. We resolved any disagreements by consensus. We imported these assessments into our Access database and presented domain level and overall judgements with a summary of the reasons any domain was not rated as low RoB.
Data synthesis
Summary measures
For each trial and each outcome separately, effect estimates and CIs that compared intervention and control groups were either extracted from the trial publication or calculated based on other reported information (e.g. number of outcome events and participants per group; mean and SD of follow-up scores, etc.). For continuous outcomes, we used standardised mean difference (SMD, specifically Hedges’ g) for outcomes with different measures for similar constructs (ADL, depression, self-evaluation of health status) across the trials. Where continuous outcome measures used a mix of measures favouring higher or lower values, the measures in one direction were reversed so that for a particular outcome, all trials measured improvement in outcome in the same direction.
For binary outcomes, we extracted or calculated RRs and ORs. For survival (time-to-event) outcomes, hazard (rate) ratios were extracted. Any details about non-proportional hazards were also extracted. Both adjusted and unadjusted results were extracted, though in practice unadjusted results were used in the synthesis due to a combination of heterogeneity in adjustment factors across studies and a sparsity of evidence to enable synthesis. Where effect estimates were calculated using 2 × 2 contingency tables which contained zero cells, a continuity correction of 0.5 was applied to all cells.
Effect estimates reported from cluster-randomised trials were included directly in cases where the original research had appropriately accounted for the clustered nature of the data in their analyses (e.g. multilevel modelling). Where studies were found to have ignored clustering in their analyses, we adjusted for this by reducing the sample size to the ‘effective sample size’ using the ‘design effect’ as a divisor for the original sample size. For stepped-wedge trials, we only included data from analyses that appropriately accounted for time trends.
Results of outcomes at all time points were recorded and placed into categories (around 6 months, 12 months and 24 months; see Table 1). We conducted meta-analysis separately for each of these three time frames for our main outcomes, and for the medium-term time frame for our additional outcomes as described in time points above.
For care-home placement and hospitalisation, our meta-analysis summarised the odds of this occurring for a participant, rather than counts, for example. We evaluated personal ADL and instrumental ADL as separate outcomes and did not additionally meta-analyse combined personal and instrumental ADL instruments. We did not combine all measures of health status due to differences in the underlying constructs. We chose to meta-analyse single global self-assessments of health status as it is readily interpretable and the most widely available measure. We tabulated results that we did not include in meta-analysis.
Methods of analysis
We meta-analysed the extracted effect estimates using modules within Stata, including metan, mvmeta and network. 37 Random-effects meta-analyses were conducted, to allow for potential between-study heterogeneity in each intervention effect. 38 Restricted maximum likelihood (REML) estimation was used to fit all the models and 95% CIs were calculated using the Wald-based approach, but with inflated variances to account for uncertainty in the estimated variances, similar to the approach of Hartung–Knapp but using a normal approximation instead of a t-distribution. 39,40
Initially, for each outcome separately, we performed a separate meta-analysis for each type of intervention versus control, to provide summary effectiveness results based only on direct evidence. We summarised ORs for binary outcomes, and pooled (standardised) MDs for continuous outcomes. We found insufficient estimates to allow pooling of hazard ratios (HRs) for survival outcomes. We displayed forest plots, with study-specific estimates, CIs and percentage study weights, alongside the summary (pooled) meta-analysis estimates, 95% CI for the summary effect, and (if possible) a 95% prediction interval for the intervention effect in a new study similar to one of those included in the meta-analysis.
Heterogeneity was summarised by the estimate of between-study variance [tau (τ)], the proportion of the total variance due to between-study variance [I-squared (I2)] and 95% prediction intervals, as mentioned above.
Summary ORs were converted to a summary RR and multiple corresponding absolute intervention risks and risk differences; SMDs were re-expressed as the MD of a common measure of the outcome as described in Confidence in cumulative evidence.
Network meta-analysis
A NMA was conducted (for each outcome and time frame separately), using a multivariate random-effects meta-analysis framework (where possible) via the network module in Stata using REML estimation. 37,41 This allowed both direct and indirect evidence to contribute towards each intervention effect (treatment contrast), via a consistency assumption. Where multiple studies were not available for any individual comparison (i.e. no degrees of freedom available to estimate heterogeneity) a common-effect model was fitted. The within-study correlation of multiple intervention effects from the same trial (i.e. in multigroup trials) was accounted for, and a common between-study variance assumed for all treatment contrasts in the network (thus implying a +0.5 between-study correlation for each pair of treatment effects). Summary (pooled) effect estimates for each pair of treatments in the network, with 95% CI, and (if possible) 95% prediction intervals were calculated. For binary outcomes, we chose to synthesise natural log-transformed ORs (ln ORs), as the OR is more portable across different baseline risks than the RR. 42
Based on the results, the ranking of intervention groups was calculated using resampling methods based on the (approximate) posterior distribution of treatment effect, and quantified by the probabilities of being ranked first, second, …, last, together with the mean rank and the Surface Under the Cumulative RAnking curve (SUCRA). Network diagrams, tables split between NMA and pairwise evidence and rankograms were used to graphically display the network set-up, results and rankings.
Certainty assessment
Assessment of inconsistency
The consistency assumption (that direct and indirect evidence were consistent with each other on average) was examined for each treatment comparison where there was direct and indirect evidence (seen as a closed loop within the network plot). This involved estimating direct and indirect evidence, and comparing the two using Wald tests, with a global test across all evidence indicating inconsistency if the p-value was < 0.05. Due to the low power associated with global tests, the node-splitting method of Dias et al. was also used to test for inconsistency separately between each treatment comparison, again p-values < 0.05 indicated the presence of inconsistency. 43,44 If evidence of inconsistency was found, explanations were sought and resolved. Where there were no closed loops in any individual network (i.e. no degrees of freedom to estimate inconsistency), the model was reduced to a so-called ‘consistency’ model whereby the inconsistency parameter in the model was set to zero for all comparisons under the assumption that consistency would hold.
Investigation of small-study effects
We planned that if there were 10 or more studies in a pairwise meta-analysis, funnel plots would be presented to investigate small-study effects (potentially caused by publication bias) and tests of asymmetry performed (Egger’s, Peter’s and Debray’s for continuous, binary and survival outcomes, respectively); however, no pairwise meta-analyses included 10 or more studies. If there were 10 or more studies in an NMA, ‘comparison-adjusted’ funnel plots comparing intervention with control were presented to investigate small-study effects, under the assumption that such effects would apply to the whole field. 45,46
Examination of frailty impact
Meta-analysis results were initially presented for all levels of frailty combined, then for frailty/pre-frailty where reported data permitted. Impact was further examined by extending the standard NMA to a network meta regression, with frailty/pre-frailty as a study-level categorical covariate allowing effects of frailty/pre-frailty to vary for each treatment effect, to quantify if intervention effects varied according to population-level frailty. Where possible, we kept the ‘all’ (robust, pre-fail and frail) category (or the lowest available category) as the reference to calculate the relative effect of frailty using meta regression.
As described above, we planned for our main analysis of the impact of frailty to only include trials that were categorised according to a validated measure, with subsequent analyses including all categorised trials. However, we categorised insufficient trials according to a validated measure to enable this approach, so our analyses do not distinguish between the methods of categorisation.
Additional analyses
Sensitivity analyses were conducted excluding results at the highest RoB (very serious concerns). We chose not to exclude all studies at high RoB while conducting risk-of-bias assessment, when it became apparent we would be able to conduct few sensitivity analyses using this approach. We planned to run additional sensitivity analyses to present results of more recent evaluations, restricted to trials in the last 15 years but decided not to given the volume of networks and their sparsity.
Confidence in cumulative evidence
We used the GRADE framework, adapted for NMA, to rate evidence quality of the results of our NMA. 10,36,47,48 We generated GRADE evidence profiles for our individual intervention groupings in comparison to a reference control for each outcome separately. We initially planned to use GRADE alone and subsequently planned to use the confidence in network meta-analysis (CINeMA) approach to inform our overall GRADE rating. 49 However, the CINeMA software produced errors for many of our analyses due to their sparseness and so we reverted to using GRADE.
As we included RCTs and cRCTs, the starting point was a high-quality evidence rating. We assessed the quality of direct and indirect treatment estimates separately and combined in NMA,48 with a focus on first-order loops for assessment of indirect treatment estimates. We assessed the domains of RoB, inconsistency (between-study heterogeneity), indirectness and publication bias (non-reporting bias) in the direct and indirect evidence, and additionally imprecision and incoherence (direct and indirect estimate inconsistency) for the combined network estimate. We made an overall judgement on whether the quality of evidence for an individual outcome warranted downgrading on the basis of study limitations in each of the domains, aligned with GRADE guidance. 36
For imprecision, we considered the 95% CI in relation to cut points for no effect and very small effect (positive and negative, see below). We also considered the ratio of the 95% CI limits for the OR, and whether the effective sample size was at least 800 for continuous outcomes or sufficient for 400 events for dichotomous outcomes, following the approach of Brignardello-Petersen et al. 47,50
Summary of findings tables were produced using a semi-automated workflow involving Microsoft Excel (v16.0; Microsoft Corporation, Redmond, WA, USA) and Microsoft Word (v16.0; Microsoft Corporation, Redmond, WA, USA) based on recommended formats. 35,51
We described interpretation of the evidence following recent guidance,52 providing an indication of size and direction if the evidence was not very low certainty. We used the terms ‘probably’ and ‘may’ to indicate moderate and low certainty, respectively. 52,53 Where the point estimate was large but the evidence was low certainty, we described only the direction in the text as we were not assessing certainty in the size of the estimate; in these cases, we described the size alongside the statistical summary.
For effect sizes expressed as SMD, we took 0.05 to be the lower bound for a very small effect, 0.16 to be a small effect, 0.38 to be a moderate effect, 0.76 to be a large effect and 1.2 and above to be a very large effect, based on empirical evidence of effect sizes in gerontological research. 54 We re-expressed SMDs as MDs using a pooled SD for a common measure of the outcome from the included studies. 35
We re-expressed ORs (and 95% CIs) as RRs using the median risk in the reference comparator arms, and as absolute effects (corresponding intervention risk and corresponding risk difference) for a high- and low-risk population, using the highest and lowest risk among the reference comparator arms with more than 100 participants as the assumed comparator risks. 35 By reference to other commonly used interventions in fields such as stroke prevention and hypertension, we noted that number needed to treat to benefit (NNTB) for major outcomes was often between 50 and 100, and sometimes larger. 55–57 Based on this, we arbitrarily selected NNTB = 200 as a limit for important difference and used the corresponding risk difference for the high-risk population to define effect sizes as very small (5 per 1000), small (20 per 1000), moderate (40 per 1000), large (60 per 1000) or very large (100 or more per 1000).
Summary of economic evidence
Following the brief economic commentary framework recommended in the Cochrane Handbook, version 6,58 we extracted and summarised brief details of the analytic perspective, time horizon, evaluation type(s), main cost items, currency, price year, any reported details about discounting and sensitivity analysis, the principal findings which applied to this review’s time periods and outcomes of interest, and verbatim text of conclusions of each identified study. We then compared and contrasted the findings from similar interventions (classified in the same intervention group) and between different intervention groups, based on the conclusions of these studies.
We used the definitions stated in the ‘Glossary of Terms for Health Economics and Systematic Review’ from the Campbell and Cochrane Economics Methods Group59 to classify the three full economic evaluation types60 – cost-effectiveness analysis, cost-utility analysis and cost–benefit analysis. We classified analysis of comparative costs for alternative interventions in monetary value of the resources used as cost analysis (partial economic evaluation). 58,61 We identified the cost item categories reported in each of these studies and used these to identify the evaluation perspective. The cost items were classified into four categories: health sector costs, other sector costs, patient and family costs and productivity impacts. 58,61 An evaluation that included the monetary expenses on health and/or social care was regarded as adopting a health and social care system perspective. If non-monetary, intangible resources for care or economic impacts associated with the intervention were also included in the total costs, for example informal care provided by family or friends, productivity loss, the evaluation perspective was classified as societal. Additionally, we extracted the intervention cost items if they were provided separately. We did not further assess the quality of the identified economic evaluations. 58
Chapter 3 Results of the review
Study selection
The results of the search and selection process are summarised in a PRISMA 2020 flow diagram (see Figure 1). 62
FIGURE 1.
PRISMA flow diagram showing identification, selection and inclusion of studies from databases, registers and other sources.
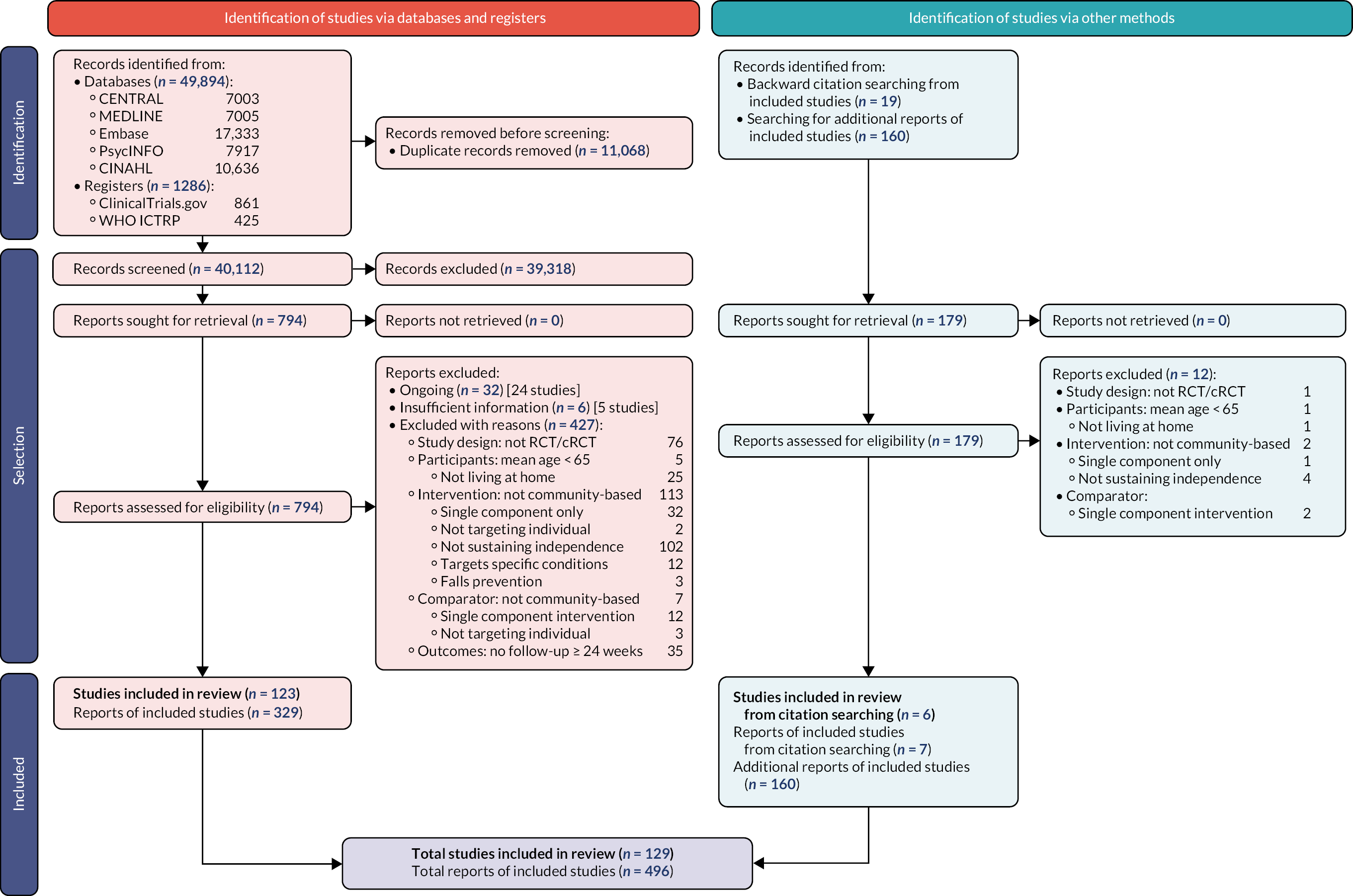
Results of the search and selection process
We found 49,894 records through database searches and 1286 records through trial register searches. After removal of 11,068 duplicates, we screened 40,112 records. We excluded 39,318 records through screening and sought, retrieved and reviewed the full text of the remaining 794 reports. Later, we identified and screened an additional 179 records by backward citation searching of included studies (n = 19) and from searching for additional reports of included studies (n = 160). In total, therefore, we assessed 973 reports for eligibility and excluded 477, finally including 129 studies63–191 consisting of 496 reports (see Figure 1): 123 studies from the electronic searches and six studies from the citation searches. Of these 129 studies, 113 provided results for the outcomes of interest and 90 contributed to the NMAs.
Excluded studies
Details of the studies excluded following assessment for eligibility are provided in Figure 1 above and Report Supplementary Material 2, and are summarised below. Additionally, a brief summary of the interventions excluded (where this was the reason for exclusion) is provided in Report Supplementary Material 2.
We excluded 24 studies192–215 (32 reports) as ongoing because the trial or the analyses were not completed and results were unavailable as of 31 August 2021, according to their respective trial register records. The collective total of their planned sample sizes is approximately n = 9218.
We had insufficient information to confirm the eligibility of five studies216–220 (six reports), despite attempts to contact the authors and searches for additional reports. For these studies, we only found trial register records, protocols or conference abstracts.
We assessed 439 reports as ineligible: 77 did not relate to a RCT or a cRCT; 32 had participants with a mean age < 65 (n = 6) or who were not living at home (n = 26); 271 were ineligible because of the intervention, which was not community based (n = 115), had a single component only (n = 33), did not target the individual (n = 2), did not aim to sustain independence (n = 106), targeted a specific condition (n = 12) or was for fall prevention (n = 3); 24 were ineligible because of the comparator, which was not community based (n = 7), was a single component intervention (n = 14), or did not target the individual (n = 3); and 35 were ineligible because there was no follow-up at 24 weeks or later.
Some excluded studies were part of a larger project from which other studies were included. These projects included PRevention in Older people – Assessment in GEneralists’ practices (PRO-AGE), Community Aging in Place – Advancing Better Living for Elders (CAPABLE) and Assessment of Services Promoting Independence and Recovery in Elders (ASPIRE). PRO-AGE consisted of three RCTs, two of which were eligible,104,164 while the third was ineligible as it was not delivered in a community setting. 221 This third study provided the baseline for a cohort, from which another eligible study was included. 151 We included two studies of the CAPABLE intervention166,167 but excluded a non-randomised study222 and a trial of two implementation strategies of the same intervention. 223 The ASPIRE project consisted of three RCTs, two of which were included146,147 while the third was ineligible because the intervention was partly delivered in residential care facilities. 224
Studies included in the review
Characteristics of included studies are provided as Report Supplementary Material 1; a summary is provided in Table 3.
| Study | Design | Enrolment began | Country | Population frailty | Enrolled | Interventions | Controls | Living at home | IADL | PADL | PADL & IADL | Hospitalisation | Homecare | Care-home placement | Cost | Cost-effectiveness | Health status | Depression | Loneliness | Falls | Mortality | Funding |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alegria 201963,225,226 | RCT | 2015 | USA | P | 307 | exrc & psyc | ac | – | – | – | ▦ | – | – | – | – | – | ◌ | ◌ | – | – | ♦ | NC |
| Arthanat 201964,227,228 | RCT | >2005 | USA | U | 97 | comm | ac | – | – | – | – | – | – | – | – | – | – | – | – | – | ◌ | NC |
| Auvinen 202065,229–231 | RCT | 2015 | FIN | F | 512 | hmcr & med | hmcr | – | ♦ | ♦ | – | ▦ | – | – | – | – | ▦ | ▦ | – | – | ♦ | NC |
| Balaban 198866 | RCT | 1981 | USA | F | 198 | mfa-(w/med) | ac | – | – | ♦ | – | ▦ | – | ◌ | – | – | ▦ | ▦ | – | – | ▦ | NC |
| Barenfeld 201867,232–235 | RCT | 2012 | SWE | all | 131 | educ | ac | – | – | – | – | – | – | – | – | – | ◌ | ◌ | – | ◌ | ♦ | NC |
| Bernabei 199868,236,237 | RCT | 1995 | ITA | F | 200 | hmcr & mfar(w/med) | hmcr | – | ♦ | ♦ | – | ▦ | ▦ | ▦ | ▦ | – | – | ♦ | – | – | ♦ | NC |
| Bleijenberg 201669,238–246 | cRCT | 2010 | NLD | P,F | m:39; 3092 | rsk-mfa-; rsk-mfa- | ac | – | – | ♦ | ▦ | ▦ | – | ◌ | ▦ | ◌ | ▦ | ♦ | – | – | ♦ | NC |
| Blom 201670,245,247,248 | cRCT | 2009 | NLD | all | m:59; 1379 | mfa-(w/med + slfm) | ac | ♦ | ♦ | ♦ | ▦ | ▦ | ▦ | ♦ | ▦ | ▦ | ♦ | ♦ | ▦ | – | ♦ | NC |
| Borrows 201371 | RCT | 2008 | GBR | U | 36 | aids | mfa- | – | – | ▦ | – | – | – | – | – | – | ▦ | – | – | – | ♦ | NC |
| Botjes 201372,249,250 | RCT | 2011 | NLD | U | 218 | mfa- | ac | – | – | – | ◌ | – | – | – | – | – | ◌ | – | ◌ | – | – | NC |
| Bouman 200873,251–255 | RCT | 2002 | NLD | P,F | 330 | mfar(w/med) | ac | – | ♦ | ♦ | ◌ | ♦ | ▦ | ▦ | ▦ | ▦ | ♦ | ♦ | ▦ | – | ♦ | NC |
| Brettschneider 201574,256–259 | RCT | 2007 | DEU | F | 336 | mfar(w/med) | ac | – | ♦ | ♦ | – | – | – | ▦ | ▦ | ▦ | ♦ | ◌ | – | ▦ | ♦ | NC |
| Cameron 201375,260–269 | RCT | 2008 | AUS | F | 241 | exrc & mfar(w/med + slfm) | ac | – | – | ♦ | – | ♦ | – | ▦ | ▦ | ▦ | ♦ | ♦ | – | ▦ | ♦ | NC |
| Carpenter 199076 | RCT | <2006 | GBR | all | 539 | rsk-mfa- | ac | ♦ | – | – | – | ▦ | – | ♦ | – | – | – | – | – | ▦ | ▦ | NC |
| Cesari 201477,270–275 | RCT | > 2005 | FRA | U | ? | mfar(w/med) | ac | – | – | – | ◌ | – | – | – | – | – | ◌ | ◌ | – | – | – | NC |
| Challis 200478,276 | RCT | 1998 | GBR | F | 256 | mfar(w/med) | mfar | ♦ | – | ▦ | – | ▦ | – | ▦ | ▦ | – | – | ▦ | – | – | ▦ | NC |
| Clark 199779,277–281 | RCT | 1994 | USA | R,P | 361 | eng & educ | ac | – | ♦ | ♦ | – | – | – | – | ▦ | ▦ | – | ♦ | – | – | ◌ | Mx |
| Clark 201280,282–287 | RCT | 2004 | USA | U | 460 | eng & educ | ac | – | – | – | – | – | – | – | ▦ | ▦ | ▦ | ▦ | – | – | ▦ | NC |
| Coleman 199981 | cRCT | < 2006 | USA | F | m:9; 169 | educ & mfar(w/med + slfm) | ac | – | – | – | – | ▦ | – | – | ▦ | – | ◌ | ▦ | – | ▦ | ♦ | NC |
| Counsell 200782,288–292 | cRCT | 2002 | USA | U | m:164; 951 | educ & mfar(w/med + slfm) | ac | – | ▦ | ▦ | – | ▦ | – | – | ▦ | – | ▦ | ▦ | – | – | ♦ | NC |
| Cutchin 200983,293 | RCT | 2008 | USA | U | 110 | mfar | ac | – | – | – | ▦ | ◌ | – | ◌ | – | – | ▦ | ♦ | – | – | – | NC |
| Dalby 200084,294 | RCT | < 2006 | CAN | F | 142 | mfar(w/med) | ac | ♦ | – | – | – | ▦ | – | ▦ | – | – | – | – | – | – | ♦ | NC |
| de Craen 200685,295–297 | RCT | 2000 | NLD | all | 402 | mfa- | ac | – | – | – | ▦ | – | ◌ | – | – | – | – | ◌ | ▦ | – | ♦ | NC |
| Dorresteijn 201686,298–301 | RCT | 2009 | NLD | U | 389 | ADL | ac | – | ♦ | ♦ | ▦ | – | – | – | ▦ | ▦ | ▦ | ◌ | – | ▦ | ♦ | NC |
| Dupuy 201787,302 | RCT | > 2005 | FRA | P,F | 32 | hmcr & aids & comm | hmcr | – | ? | – | ▦ | – | – | – | – | – | – | – | – | – | – | NC |
| Fabacher 199488 | RCT | < 2006 | USA | all | 254 | mfar(w/med) | ac | ♦ | ♦ | ♦ | – | ▦ | – | ♦ | – | – | – | ◌ | – | ▦ | ♦ | NC |
| Fairhall 201589,303,304 | RCT | 2013 | AUS | P | 230 | mfar(w/med) | ac | – | – | ◌ | ◌ | ◌ | – | ◌ | – | – | ◌ | ◌ | – | ◌ | ◌ | NC |
| Faul 200990,305 | RCT | – | USA | R,P | 81 | educ & exrc & mfar(w/med + slfm); exrc & mfa-(w/med + slfm) | – | – | ◌ | – | – | – | – | – | – | – | ◌ | ◌ | – | – | – | NC |
| Fernandez-Barres 201791,306,307 | RCT | 2010 | ESP | F | 173 | hmcr & ntr | hmcr | ♦ | – | ♦ | – | – | – | ♦ | – | – | – | ♦ | – | – | ♦ | NC |
| Fischer 200992,308 | RCT | 2004 | DEU | all | 4224 | eng & mfa-(w/slfm) | ac | ♦ | – | – | – | ▦ | – | ♦ | ◌ | – | – | – | – | – | ▦ | ? |
| Ford 197193,309 | RCT | 1963 | USA | P,F | 300 | mfar(w/med) | ac | ♦ | – | ◌ | – | ▦ | – | ♦ | – | – | – | – | – | – | ▦ | NC |
| Fox 199794 | RCT | 1994 | USA | all | 237 | mfar(w/med + slfm) | mfar(w/med) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | NC |
| Fristedt 201995,310 | RCT | 2015 | SWE | F | 62 | hmcr & mfar(w/med) | hmcr | – | – | ◌ | – | ▦ | ◌ | – | ◌ | – | – | – | – | – | ♦ | NC |
| Gene Huguet 201896 | RCT | 2016 | ESP | P | 200 | med & ntr & exrc | ac | – | ♦ | ♦ | – | – | – | – | – | – | – | – | – | – | – | NC |
| Gill 200297,311–314 | RCT | < 2006 | USA | P,F | 188 | ADL & exrc | ac | – | ▦ | ▦ | – | – | – | ◌ | ◌ | – | – | – | – | ▦ | ♦ | NC |
| Giné-Garriga 202098,315–325 | RCT | 2016 | EEE | R | 1360 | exrc | ac | – | – | – | – | – | – | – | – | – | ◌ | ◌ | ◌ | ◌ | – | NC |
| Gitlin 200699,326–336 | RCT | 2003 | USA | P,F | 319 | ADL & aids & exrc | ac | – | ♦ | ♦ | – | – | – | ◌ | ▦ | ▦ | – | ◌ | – | – | ♦ | NC |
| Grimmer 2013100,337 | RCT | 2014 | AUS | U | ? | mfa- | ac | – | – | ? | – | ◌ | – | – | – | – | ◌ | – | – | ◌ | – | ? |
| Gustafson 2021101,338,339 | RCT | 2013 | USA | all | 390 | aids & educ & comm | ac | – | – | – | ▦ | ◌ | – | ◌ | ◌ | ◌ | ▦ | ♦ | ◌ | ◌ | ◌ | Mx |
| Gustafsson 2013102,232,340–347 | RCT | 2007 | SWE | all | 491 | educ & mfa-; educ | ac | – | – | – | – | – | – | – | – | ◌ | ◌ | ◌ | – | ◌ | ♦ | NC |
| Hall 1992103 | RCT | 1986 | CAN | F | 167 | hmcr & mfar(w/slfm) | hmcr & mfar | ♦ | – | – | – | – | – | ♦ | – | – | – | – | ◌ | – | ♦ | ? |
| Harari 2008104,348–362 | RCT | 2000 | GBR | all | 2503 | mfar(w/med) | ac | ♦ | – | – | – | ♦ | – | ♦ | – | – | ◌ | ◌ | – | ◌ | ♦ | NC |
| Hattori 2019105,363 | RCT | 2018 | JPN | P,F | 375 | educ & mfar(w/slfm) | mfar | – | ◌ | – | – | ▦ | – | – | – | – | ◌ | ◌ | – | – | ▦ | NC |
| Hay 1998106,364 | RCT | < 2006 | CAN | U | 619 | mfa- | ac; ac |
♦ | – | – | ▦ | ◌ | – | ♦ | ▦ | – | – | – | – | – | ♦ | NC |
| Hebert 2001107 | RCT | < 2006 | CAN | P,F | 503 | mfar(w/med) | ac | ♦ | – | – | ▦ | – | – | ♦ | – | – | – | – | – | – | ♦ | NC |
| Henderson 2005108,365 | cRCT | 2002 | AUS | R | m:16; 167 | mfar | ac | ♦ | ♦ | ♦ | – | ♦ | – | ♦ | – | – | ▦ | ♦ | – | ▦ | ♦ | NC |
| Hendriksen 1984109,366–368 | RCT | 1980 | DNK | all | 600 | mfar | ac | – | – | – | – | ♦ | – | ▦ | – | – | – | – | – | – | ♦ | NC |
| Hogg 2009110,369–373 | RCT | 2004 | CAN | U | 241 | mfar(w/med) | ac | – | ▦ | – | – | ▦ | – | – | ▦ | ▦ | ▦ | – | – | – | ♦ | NC |
| Holland 2005111,374,375 | RCT | 2001 | USA | U | 504 | educ & exrc & mfar(w/slfm) | ac | – | – | ? | – | ◌ | – | – | – | – | ◌ | ▦ | – | – | ♦ | NC |
| Howel 2019112,376–378 | RCT | 2011 | GBR | all | 755 | wlfr | ac | – | – | – | – | – | ▦ | ◌ | ▦ | ▦ | ▦ | ▦ | – | – | ♦ | NC |
| Imhof 2012113,246 | RCT | 2008 | CHE | all | 461 | mfar | ac | ♦ | ◌ | – | ◌ | ▦ | ◌ | ♦ | – | – | – | ? | – | ◌ | ▦ | NC |
| Jing 2018114 | RCT | 2016 | CHN | F | 80 | psyc; exrc & psyc | – | – | – | – | ▦ | – | – | – | – | – | ▦ | ▦ | ▦ | – | ▦ | ? |
| Jitapunkul 1998115 | RCT | 1993 | THA | U | 160 | rsk-mfa- | ac | – | ♦ | ♦ | – | ▦ | – | – | – | – | – | – | – | ▦ | ▦ | NC |
| Kerse 2014116,379–383 | cRCT | 2008 | NZL | P,F | m:60; 3893 | rsk-mfa- | ac | ♦ | ▦ | – | ▦ | ◌ | ▦ | ♦ | – | – | – | ▦ | – | – | ♦ | NC |
| King 2012117,384–386 | cRCT | 2006 | NZL | P,F | m:21; 186 | hmcr & ADL & mfar(w/slfm) | hmcr | ♦ | ♦ | ♦ | – | ▦ | ▦ | ▦ | – | – | ▦ | ▦ | – | ▦ | ▦ | NC |
| Kono 2016118,387,388 | RCT | 2011 | JPN | P | 360 | mfar(w/med) | mfar | ♦ | ▦ | ♦ | – | ♦ | – | ♦ | ▦ | – | – | ♦ | – | ▦ | ♦ | NC |
| Kono 2004119 | RCT | 2000 | JPN | P,F | 119 | mfar | ac | ♦ | ◌ | ◌ | – | – | – | ♦ | – | – | – | ◌ | – | – | ♦ | NC |
| Kono 2012120,389–391 | RCT | 2008 | JPN | P | 323 | mfar | mfar | ▦ | ▦ | ▦ | – | – | – | ▦ | ▦ | – | – | ▦ | – | – | ▦ | NC |
| Kukkonen-Harjula 2017121,392–396 | RCT | 2014 | FIN | P,F | 300 | ADL & ntr & exrc | ac | ♦ | ◌ | – | – | ▦ | ▦ | ♦ | ▦ | ▦ | ◌ | ◌ | – | ◌ | ♦ | NC |
| Lambotte 2018122,397–404 | RCT | 2017 | BEL | P,F | 871 | mfar | ac | – | – | ? | – | ◌ | – | ◌ | – | – | – | – | – | – | – | NC |
| Leung 2004123,405 | RCT | 2000 | HKG | all | 260 | mfar(w/med) | ac | – | – | – | ? | ▦ | – | ◌ | ◌ | – | – | – | – | – | ◌ | ? |
| Leveille 1998124,406,407 | RCT | 1995 | USA | U | 201 | educ & exrc & mfar(w/med + slfm) | ac | – | – | ▦ | – | ♦ | – | – | ▦ | – | – | ▦ | – | – | ♦ | NC |
| Lewin 2013125,408–410 | RCT | 2005 | AUS | F | 750 | hmcr & educ & mfar | hmcr | ♦ | ? | ? | – | ▦ | ▦ | ♦ | ▦ | – | – | – | – | – | ♦ | NC |
| Liddle 1996126 | RCT | < 2006 | AUS | U | 105 | aids & mfar | ac | ♦ | – | ▦ | – | ◌ | ◌ | ♦ | – | – | ◌ | – | – | – | ▦ | NC |
| Liimatta 2019127,411–413 | RCT | 2013 | FIN | R,P | 422 | exrc & mfa-(w/med) | ac | – | – | – | – | ▦ | ▦ | ▦ | ▦ | ▦ | ▦ | – | – | – | ♦ | NC |
| Loh 2015128,414,415 | cRCT | 2014 | MYS | U | m:8; 256 | ntr & exrc | ac | – | – | – | – | – | – | – | – | – | – | ◌ | – | – | – | NC |
| Lood 2015129 | RCT | 2012 | SWE | R,P | 40 | educ | ac | – | – | – | – | – | – | – | – | – | ◌ | ◌ | ? | – | – | NC |
| Mann J 2021130,416–420 | cRCT | 2018 | AUS | all | m:14; 92 | mfa-(w/med) | ac | – | – | ◌ | – | ▦ | – | – | – | ◌ | ◌ | – | – | – | – | NC |
| Mann WC 1999131 | RCT | < 2006 | USA | F | 104 | hmcr & aids | hmcr | – | ♦ | – | – | ▦ | – | ▦ | ▦ | – | – | – | – | – | ♦ | NC |
| Markle-Reid 2006132,421,422 | RCT | 2001 | CAN | F | 288 | hmcr & mfar(w/med + slfm) | hmcr & mfar | – | – | – | – | – | – | – | ▦ | – | ◌ | ▦ | – | – | ◌ | NC |
| Melis 2008133,423–429 | RCT | 2003 | NLD | F | 155 | mfar(w/med) | ac | – | – | – | ▦ | ▦ | – | ▦ | ▦ | ▦ | – | ▦ | – | – | ▦ | NC |
| Meng 2005134,430–436 | RCT | 1998 | USA | F | 1786 | educ & vchr & mfar(w/med + slfm); educ & mfar(w/med + slfm); vchr |
ac | – | ▦ | ▦ | – | ◌ | – | – | – | – | ▦ | – | – | – | ♦ | NC |
| Messens 2014135,437,438 | RCT | 2011 | EEE | P,F | 208 | aids & cgn & comm & mntr-mfa- | ac | – | – | – | – | ◌ | – | ◌ | – | – | – | ◌ | – | – | ◌ | NC |
| Metzelthin 2013136,245,439–444 | cRCT | 2009 | NLD | F | m:12; 346 | educ & mfar(w/med + slfm) | ac | ♦ | ♦ | ♦ | ▦ | ▦ | ▦ | ♦ | ▦ | ▦ | ▦ | ♦ | – | ◌ | ♦ | NC |
| Moll van Charante 2016137,445–455 | cRCT | 2006 | NLD | all | m:116; 3526 | educ & mfar(w/slfm) | ac | – | – | – | ▦ | ▦ | – | – | – | – | – | ▦ | – | – | ▦ | NC |
| Monteserin Nadal 2008138,456 | RCT | 2004 | ESP | all | 620 | educ & rsk-mfa- | ac | ♦ | ♦ | ♦ | – | – | ▦ | ♦ | – | – | – | ◌ | – | ▦ | ♦ | NC |
| Morey 2006139,457,458 | RCT | < 2006 | USA | all | 179 | exrc; exrc | exrc | – | – | ? | – | – | – | – | – | – | ◌ | – | – | ◌ | – | NC |
| Morey 2009140,459–462 | RCT | 2004 | USA | U | 400 | exrc | ac | – | – | – | ▦ | ◌ | – | – | ◌ | – | ◌ | – | – | ◌ | ♦ | NC |
| Morgan 2019141,463–465 | RCT | 2014 | GBR | P | 51 | exrc | ac | – | ♦ | – | – | ▦ | – | – | – | – | ▦ | ▦ | – | – | ▦ | NC |
| Newbury 2001142,466 | RCT | 1998 | AUS | U | 100 | mfa-(w/med) | ac | ♦ | – | ♦ | – | – | ▦ | ♦ | – | – | ◌ | ♦ | – | ▦ | ♦ | NC |
| Newcomer 2004143,467,468 | RCT | 2001 | USA | U | 3079 | educ & mfar(w/med) | ac | ♦ | – | – | – | ♦ | – | ♦ | ▦ | – | ▦ | – | – | – | ♦ | Mx |
| Ng 2015144,469,470 | RCT | 2009 | SGP | P,F | 246 | cgn & ntr & exrc | ac | – | – | – | – | ♦ | – | ◌ | – | – | – | – | – | ▦ | ♦ | NC |
| Parsons J 2012145,471,472 | cRCT | 2007 | NZL | P,F | m:?; 205 | hmcr & mfar(w/slfm) | hmcr & mfa- | – | – | – | – | – | ◌ | – | – | – | ▦ | – | – | – | ▦ | NC |
| Parsons M 2017146,473–476 | RCT | 2003 | NZL | F | 113 | hmcr & ADL & mfar(w/slfm) | hmcr & mfa- | ♦ | ♦ | ▦ | – | ▦ | – | ▦ | ▦ | ▦ | ▦ | ♦ | – | ▦ | ♦ | NC |
| Parsons M 2012147,473–476 | cRCT | 2003 | NZL | F | m:55; 351 | hmcr & mfar | hmcr & mfa- | ▦ | ♦ | ▦ | – | ▦ | – | ▦ | ▦ | ▦ | ▦ | ♦ | – | ▦ | ♦ | NC |
| Pathy 1992148 | RCT | < 2006 | GBR | all | 725 | rsk-mfa- | ac | – | – | – | ◌ | ▦ | ▦ | ▦ | – | – | ▦ | – | – | – | ▦ | NC |
| Phelan 2007149 | cRCT | 2002 | USA | all | m:31; 874 | mfar(w/med + slfm) | ac | – | – | – | – | ♦ | – | – | – | – | ◌ | ▦ | – | – | ▦ | NC |
| Ploeg 201015.0,477 | RCT | 2004 | CAN | P,F | 719 | educ & mfar(w/med) | ac | ♦ | – | ? | – | ▦ | – | ♦ | ▦ | – | ▦ | – | – | – | ♦ | NC |
| Profener 2016151,478–480 | RCT | 2007 | DEU | F | 553 | educ & mfar | ac | – | – | – | – | – | – | – | – | – | – | – | – | ▦ | ▦ | NC |
| Rockwood 2000152,481 | RCT | < 2006 | CAN | F | 182 | mfa-(w/med) | ac | ▦ | ♦ | ♦ | – | – | – | ▦ | – | – | – | – | – | – | ♦ | NC |
| Romera-Liebana 2018153,482,483 | RCT | 2013 | ESP | P,F | 352 | cgn & med & ntr & exrc | ac | ♦ | ◌ | ◌ | – | ◌ | – | ♦ | – | – | – | – | – | ◌ | ♦ | NC |
| Rooijackers 2021154,484–488 | cRCT | 2017 | NLD | F | m:10; 264 | hmcr & ADL & mfar(w/slfm) | hmcr | ♦ | ♦ | ♦ | ▦ | – | – | ♦ | ◌ | ◌ | ◌ | ♦ | – | ▦ | ♦ | NC |
| Rubenstein 2007155 | RCT | < 2006 | USA | F | 792 | mfar(w/med) | ac | – | ♦ | ♦ | – | ♦ | – | ◌ | – | – | – | ♦ | – | ▦ | ♦ | NC |
| Ryvicker 2011156,489 | cRCT | 2005 | USA | U | m:45; 3290 | hmcr & mfar | hmcr & mfar | – | – | – | – | – | – | – | – | – | – | – | – | – | – | NC |
| Serra-Prat 2017157,490 | RCT | 2013 | ESP | P | 172 | ntr & exrc | ac | – | – | ♦ | – | ◌ | – | ◌ | – | – | ♦ | – | – | ▦ | ♦ | NC |
| Shapiro 2002158 | RCT | 1998 | USA | F | 108 | hmcr & mfar | ac | ♦ | – | – | – | – | – | ♦ | ◌ | – | – | ? | – | – | ♦ | NC |
| Sherman 2016159,491 | cRCT | 2006 | SWE | all | m:16; 583 | mfa-(w/med) | ac | – | – | – | ? | – | – | – | – | – | ? | – | ▦ | – | – | NC |
| Siemonsma 2018160,492,493 | RCT | 2009 | NLD | F | 155 | ADL | mfa- | – | – | – | ▦ | – | – | – | – | – | ▦ | – | – | ◌ | ♦ | NC |
| Stewart 2005161,494,495 | RCT | 2000 | GBR | P,F | 321 | mfa- | mfa- | – | – | ▦ | – | – | – | – | ▦ | ▦ | ▦ | – | – | – | ▦ | NC |
| Stuck 1995162,496–500 | RCT | 1988 | USA | all | 414 | educ & mfar(w/med) | ac | ♦ | ♦ | ♦ | ▦ | ▦ | – | ♦ | – | – | ◌ | ◌ | – | ◌ | ▦ | NC |
| Stuck 2000163,501–504 | RCT | 1993 | CHE | all | 791 | mfar(w/med) | ac | – | – | ◌ | – | ◌ | – | ▦ | ▦ | – | ◌ | ◌ | – | – | ▦ | NC |
| Stuck 2015164,359–362,505 | RCT | 2000 | CHE | R,P | 2284 | educ & mfar(w/med + slfm) | ac | ♦ | – | – | – | ◌ | – | ♦ | ◌ | – | ◌ | ◌ | – | ◌ | ▦ | NC |
| Suijker 2016165,506–511 | cRCT | 2010 | NLD | F | m:24; 2283 | mfar(w/med) | ac | ♦ | ◌ | ◌ | ▦ | ▦ | – | ♦ | ▦ | ▦ | ▦ | – | – | ▦ | ♦ | NC |
| Szanton 2011166,512,513 | RCT | 2010 | USA | P,F | 40 | ADL & aids & educ & exrc & mfar(w/med + slfm) | ac | ♦ | ♦ | ♦ | – | – | – | ♦ | – | – | ▦ | – | – | – | ▦ | NC |
| Szanton 2019167,512,514–523 | RCT | 2012 | USA | P,F | 300 | ADL & aids & educ & exrc & mfar(w/med + slfm) | ac | – | ♦ | ♦ | – | – | – | – | ◌ | – | ♦ | ♦ | – | – | ♦ | NC |
| Takahashi 2012168,524–530 | RCT | 2009 | USA | F | 205 | mntr-mfa- | ac | – | – | ♦ | – | ♦ | – | – | ◌ | – | ♦ | ♦ | – | – | ♦ | Mx |
| Teut 2013169,531 | cRCT | 2009 | DEU | F | m:8; 58 | hmcr & hmnt & exrc | hmcr | – | ◌ | ♦ | – | ▦ | – | – | – | – | – | ♦ | – | – | ♦ | Mx |
| Thiel 2019170,532,533 | RCT | 2017 | DEU | F | ? | exrc & mfar(w/med) | ac | – | – | – | – | ◌ | – | – | – | – | ◌ | ◌ | – | ◌ | – | NC |
| Thomas 2007171 | RCT | 2001 | CAN | P,F | 520 | mfar(w/med); mfar(w/med) |
ac | – | – | – | – | – | ▦ | ♦ | – | – | ♦ | – | – | – | ◌ | ? |
| Tomita 2007172 | RCT | < 2006 | USA | F | 124 | aids | ac | ♦ | ♦ | ◌ | – | ◌ | ◌ | ♦ | – | – | – | – | – | – | ▦ | NC |
| Tulloch 1979173 | RCT | 1972 | GBR | all | 339 | mfar(w/med) | ac | ♦ | – | – | – | ▦ | – | ♦ | – | – | – | – | – | – | ▦ | ? |
| Tuntland 2015174,534–536 | RCT | 2012 | NOR | U | 61 | hmcr & ADL & aids & mfa-(w/slfm) | hmcr & mfa- | – | – | – | – | – | – | – | ▦ | ▦ | ▦ | – | – | – | ▦ | NC |
| van der Pols-Vijlbrief 2017175,537 | RCT | 2013 | NLD | F | 155 | hmcr & ntr & mfar | hmcr | – | – | ▦ | – | – | – | – | ▦ | ▦ | ▦ | – | – | – | ▦ | NC |
| van Dongen 2020176,538–541 | RCT | 2016 | NLD | all | 168 | ntr & exrc | ac | – | – | – | – | ▦ | – | – | ◌ | ◌ | ▦ | – | – | – | – | Mx |
| van Heuvelen 2005177,542 | RCT | 2001 | NLD | P,F | 233 | exrc & psyc | ac | – | ♦ | ♦ | – | – | – | – | – | – | – | ♦ | – | – | – | NC |
| van Hout 2010178,543,544 | RCT | 2002 | NLD | F | 658 | mfar(w/med) | ac | ♦ | – | – | ▦ | ♦ | – | ♦ | ◌ | – | ▦ | ◌ | – | – | ♦ | NC |
| van Leeuwen 2015179,545–549 | cRCT | 2010 | NLD | F | m:35; 1147 | mfar(w/med + slfm) | ac | – | ◌ | ◌ | – | ◌ | – | – | ◌ | ◌ | ◌ | ◌ | – | – | ◌ | NC |
| van Lieshout 2018180,550 | RCT | 2011 | NLD | P,F | 710 | ADL & med & ntr & sst | ac | – | – | – | – | ♦ | – | – | – | – | ▦ | – | – | – | – | NC |
| van Rossum 1993181,551,552 | RCT | 1988 | NLD | all | 580 | mfar | ac | – | ? | ? | – | ♦ | – | ▦ | ▦ | – | ▦ | – | ▦ | – | ♦ | NC |
| Vass 2005182,553–573 | cRCT | 1999 | DNK | all | m:34; 4060 | mfar(w/med) | mfar | – | – | – | – | ▦ | ◌ | ◌ | ▦ | ▦ | – | – | – | – | ♦ | NC |
| Vetter 1984183 | RCT | 1980 | GBR | all | 1148 | mfar | ac | – | – | – | ◌ | – | ◌ | – | – | – | – | ◌ | – | – | ▦ | NC |
| von Bonsdorff 2008184,574–579 | RCT | 2003 | FIN | R | 632 | exrc | ac | – | ▦ | ? | – | ◌ | ▦ | ◌ | – | – | ◌ | ◌ | ? | – | ▦ | NC |
| Wallace 1998185,407 | RCT | < 2006 | USA | all | 100 | exrc & mfar | ac | – | – | – | – | – | – | – | – | – | – | ▦ | – | – | – | NC |
| Walters 2017186,580,581 | RCT | 2015 | GBR | P | 51 | mfar(w/slfm) | ac | – | – | ♦ | – | – | – | – | ▦ | – | ▦ | ▦ | – | ▦ | – | NC |
| Whitehead 2016187,582,583 | RCT | 2014 | GBR | F | 30 | hmcr & ADL & aids & mfa- | hmcr & mfa- | ◌ | ▦ | ▦ | – | ▦ | ▦ | ▦ | ◌ | – | ▦ | – | – | ▦ | ▦ | NC |
| Williams 1992188,584 | RCT | < 2006 | GBR | all | 470 | mfar | mfa- | – | – | – | ▦ | – | – | – | – | – | – | – | – | – | ♦ | NC |
| Wolter 2013189,585–587 | cRCT | 2007 | DEU | F | m:69; 920 | hmcr & mfar(w/med) | hmcr | ♦ | ♦ | ▦ | – | ▦ | – | ♦ | – | – | ▦ | – | – | – | ♦ | NC |
| Wong 2019190,588–591 | RCT | 2016 | HKG | all | 540 | mfar(w/slfm) | ac | ♦ | ? | ◌ | – | ▦ | – | ♦ | ▦ | ▦ | ▦ | ◌ | – | – | ▦ | NC |
| Yamada 2003191 | RCT | 1999 | JPN | P,F | 368 | mfar(w/med) | ac | – | ◌ | – | – | ◌ | – | – | – | – | ▦ | – | – | – | ♦ | NC |
The 129 studies assigned 74,946 participants to an intervention, with a range of 30 to 4224 participants per study {mean 595, median [interquartile range (IQR)] 313 (168–582) participants from 126 studies; 3 studies missing data]. Most were individually randomised but 22 were cluster RCTs; these had a total of 845 clusters [mean 40, median (IQR) 31 (14–55), range 8 to 164 clusters, 1 study missing data}.
Context
The 129 studies enrolled participants between 1963 and 2018 and were published between 1971 and 2021. They took place in 23 countries or regions in four continents: predominantly Europe (64 studies) and North America (41 studies), the rest from Australasia (13 studies) and Asia (11 studies). The most common sources for recruitment were invitations via general practice or a primary care health centre, homecare services, service referrals, health insurance, selections from census or municipal records and community advertisement.
Participants
Sixty-one per cent of participants were women, reported in 123 studies. In 113 of these, most of the participants were women. Forty-six per cent of participants were living alone, reported in 77 studies. Three studies required a consented caregiver to participate with the participant. 82,91,171 Nineteen studies excluded participants with disabilities or who were dependent in activities of daily living. 86,97,99,101,104,108,114,119,124,135,140,141,156,157,164,170,184,185,190
Age
The pooled mean age of participants was 77.3 (SD 7.5) years, reported in 97 studies. Four studies had study populations aged 85 years or over on average (mean or median);74,95,102,113 only one study’s population was < 70 years on average (mean 68.4 years). 66 Most of the studies explicitly targeted older adults, except three where all adults were eligible. 66,71,174 A minimum age was either reported or an eligibility criterion in all but 8 studies: under 65 years in 20 studies (16–18 years: n = 3; 50 years: n = 3; 60 years: n = 14), 65–79 years in 90 studies (65–69 years: n = 46; 70–74 years: n = 20; 75–79 years: n = 24), 80 years or over in 5 studies,74,85,96,102,113 and 6 studies had criteria where minimum age differed in conjunction with ethnicity,116,145–147 medical condition143 or both. 130
Frailty
One-hundred and eight study populations were classified for frailty (21 were insufficiently described to be classified64,71,72,77,80,82,83,86,100,106,110,111,115,124,126,128,140,142,143,156,174): 31 included all frailty levels (robust, pre-frail and frail), 3 populations were robust, 5 were robust and pre-frail, 8 were pre-frail, 25 were pre-frail and frail and 36 were frail. The studies that had populations classified as ‘all’ typically had broad inclusion criteria, although some purposefully sampled multiple risk or frailty groups, and others were selective on health-related criteria but in a way that would include all frailty categories. Ninety-one populations were classified on the basis of characteristics and criteria, while 17 were classified on the basis of a validated measure, of which 2 were classified as all,102,176 4 as pre-frail,89,96,157,186 7 as pre-frail and frail69,121,122,135,144,153,180 and 4 as frail. 75,136,170,179
Excluded groups within included studies
Twelve studies reported on groups of participants which were ineligible for this review and have been excluded from all results. 70,98,103,106,114,121,126,144,149,160,177,183
Hall 1992103 and Siemonsma 2018160 reported about separately recruited, non-randomised observational control groups alongside their trial results, which were excluded from this review. Hay 1998106 and Liddle 1996126 identified participants with problems who were subsequently randomised but also reported on the non-randomised group without identified problems, which were excluded from this review. Similarly, Blom 201670 was a cRCT in which GPs were randomised and only a random subset of the participants with complex problems were intended to receive the intervention. However, they also described the participants without complex problems. Therefore, there were groups without complex problems in each arm and a group with complex problems in the intervention arm who were not individually randomised to receive the intervention, which were excluded from analyses in this review.
Kukkonen-Harjula 2017121 was effectively two parallel RCTs with the same intervention and control groups, one included older people with frailty and the other included older people with recent hip fracture. We only included the trial that recruited older people with frailty.
Seven intervention arms in four studies were excluded from this review because they were considered a single-component intervention: exercise referral scheme in Giné-Garriga 2020;98 Baduanjin training in Jing 2018;114 three arms of Ng 2015144 (nutrition supplementation, physical training and cognitive training); and two arms in van Heuvelen 2005177 (physical activity and psychological training).
Phelan 2007149 was a cRCT in which those recruited first were treated differently. In the intervention arm, participants received assessment and follow-up with geriatric specialists who liaised with their primary care provider, while the control group received usual care. Participants recruited subsequently in the intervention arm only saw the geriatric specialists at request of the primary care provider, with the intention that the interactions regarding those recruited first would improve the primary care provider’s management of subsequent participants. We treated this latter group as an evaluation of staff education and excluded their results from this review.
Vetter 1984183 randomised participants to a visit by a health visitor, usual care or a third, smaller arm who received a questionnaire. No details were provided on whether the results of the questionnaire would be acted upon and no results were provided for this arm. Therefore, we exclude this arm as having insufficient information.
Interventions and comparators
There were 266 eligible intervention arms within the 129 included studies. These are detailed in relation to the TIDieR items in Report Supplementary Material 3 and referenced in the characteristics of included studies (see Report Supplementary Material 1); ineligible intervention arms are described in Excluded groups within included studies. One hundred and twenty-two studies had two eligible intervention arms, 120 of which compared one experimental intervention with one control intervention. Two studies compared two eligible experimental interventions only. 90,114 Six studies had three eligible intervention arms, in three of which there were two experimental interventions and one control intervention,69,102,171 while the other three included one experimental intervention and two control interventions. 79,106,139 One study had four eligible intervention arms, three of which were experimental. 134
Most of the interventions were delivered at the participant’s residence via trained personnel or homecare services; others were delivered in a primary care setting such as a general practice centre, or in leisure or community centres.
Components
We identified 19 separate components that were intended to be delivered to all participants among the 266 interventions. With our PPI group we developed public-facing names and plain language descriptions for these components, and organised them into domains (see Appendix 2). Fourteen components were ‘action’ components (e.g. therapy, support, education) and the remaining five components involved some kind of ascertainment or assessment process with the potential to lead to multiple actions.
The 14 action components, their short (in bold) and abbreviated labels were:
-
formal homecare (hmcr, identified in 41 interventions)
-
physical exercise (exrc, 30 interventions)
-
health education (educ, 26 interventions)
-
ADL training (ADL, 13 interventions)
-
providing aids and adaptations (aids, 12 interventions)
-
nutritional support (ntr, 10 interventions)
-
psychological (mood) therapy (psychology, psyc, 4 interventions)
-
technology for communication and engagement (telecoms, comm, 4 interventions)
-
cognitive training (cgn, 3 interventions)
-
engagement in meaningful-activities (eng, 3 interventions)
-
care voucher provision (vchr, 2 interventions)
-
alternative medicine such as homeopathy and naturopathy (hmnt, 1 intervention)
-
social skills training (sst, 1 intervention) and
-
welfare rights advice (wlfr, 1 intervention).
Homecare involved frequent visits at home by health or care professionals to provide services including support with household tasks, self-care and nursing care. Formal homecare was a component of usual care intended to be received by all participants in 40 of the interventions and any other components typically interacted, or were integrated, with it. In the remaining intervention, formal homecare was part of the programme of ‘early’ interventive social services for older people moderately at risk of becoming unable to remain in their own homes. 158
The five components involving a process of ascertainment or assessment were:
-
multifactorial-action from care planning (mfa, 117 interventions)
-
routine follow-up review (following multifactorial-action from care planning; mfar, 82 interventions)
-
medication-review (med, 4 interventions)
-
monitoring (mntr-mfa-, 2 interventions) and
-
routine risk-screening (rsk-mfa-; 7 interventions).
Multifactorial-action from care planning is the term we gave to a process of individualised multidomain assessment and management intended to lead to subsequent action, where the components of action are tailored to the individual. This did not apply to interventions where the subsequent action was intended to relate to one component only.
Routine review is a process of scheduled, regular follow-ups that follow on from multifactorial-action. This does not include additional contacts that were ad hoc or determined by need. We labelled the remaining 35 interventions with mfa- to denote the absence of routine review (although not necessarily further contact).
Because multifactorial-action encompassed a broad range of approaches and differing patterns of follow-on action, we sought meaningful and identifiable criteria by which to further delineate it. Based on discussion with experts, we identified medication-review (w/med; 54 interventions), and specific psychological strategies to support behaviour change and self-management (w/slfm; 29 interventions) in the assessment and care planning of multifactorial-action, including their combination and absence. The specific self-management strategies included reframing, motivational interviewing, problem-solving, goal setting and action planning,592 but not practical support with adherence (e.g. encouragement phone calls, prompts and reminders), or general comments about encouragement of behaviour change or self-management. This helped to characterise approaches to multifactorial-action that incorporated medical and psychosocial orientations.
We treated medication-review as a component in its own right when present in an intervention that did not include multifactorial-action.
Monitoring and routine risk-screening are two components where participants only receive multifactorial-action from care planning when they meet a particular trigger or threshold. We did not treat risk-screening as an intervention component where it was used solely to identify those eligible for the trial.
Available care was the label we applied to interventions (or conditions) where there was no particular component intended to be delivered to all participants. This acknowledged the availability of a wide range of primary, secondary, tertiary and wider community services that would be accessed by some, but not all, participants without specifying their nature. We also included here actions that were not intended or anticipated to affect an individual’s independence such as attention control, placebo and other minor actions such as giving a leaflet. Additionally, we did not identify trial-specific procedures related with data collection, or safeguarding such as suicide monitoring, as separate components.
Intervention groups
Our intervention groups were formed from the 63 combinations of these components among the 266 interventions, as detailed in Table 4. The largest group is ac (comprised of 98 interventions). Other groups including more than 10 interventions are multifactorial-action and review with medication-review (24 interventions), multifactorial-action and review (15 interventions) and homecare (12 interventions). Eight groups contained two interventions while 37 groups were formed of one intervention only. We have provided TIDieR summaries of the 26 intervention groups with more than 1 intervention in Report Supplementary Material 4.
| Intervention group label | Abbreviated label | Interventions | hmcr | ADL | aids | cgn | comm | educ | eng | exrc | hmnt | ntr | psyc | sst | vchr | wlfr | mfa | mfar | w/meda | w/slfma | med | mntr | rsk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADL | ADL | 2 | ● | ||||||||||||||||||||
| ADL and exercise | ADL & exrc | 1 | ● | ● | |||||||||||||||||||
| ADL, aids and exercise | ADL & aids & exrc | 1 | ● | ● | ● | ||||||||||||||||||
| ADL, aids, education, exercise, multifactorial-action and review with medication-review and self-management | ADL & aids & educ & exrc & mfar(w/med + slfm) | 2 | ● | ● | ● | ● | ● | ● | ● | ● | |||||||||||||
| ADL, medication-review, nutrition and social skills | ADL & med & ntr & sst | 1 | ● | ● | ● | ● | |||||||||||||||||
| ADL, nutrition and exercise | ADL & ntr & exrc | 1 | ● | ● | ● | ||||||||||||||||||
| Aids | aids | 2 | ● | ||||||||||||||||||||
| Aids, cognitive training, telecoms and monitoring | aids & cgn & comm & mntr-mfa- | 1 | ● | ● | ● | ● | |||||||||||||||||
| Aids, education and telecoms | aids & educ & comm | 1 | ● | ● | ● | ||||||||||||||||||
| Aids, multifactorial-action and review | aids & mfar | 1 | ● | ● | ● | ||||||||||||||||||
| Available care | ac | 98 | |||||||||||||||||||||
| Care voucher | vchr | 1 | ● | ||||||||||||||||||||
| Care voucher, education, multifactorial-action and review with medication-review and self-management | educ & vchr & mfar(w/med + slfm) | 1 | ● | ● | ● | ● | ● | ● | |||||||||||||||
| Cognitive training, medication-review, nutrition and exercise | cgn & med & ntr & exrc | 1 | ● | ● | ● | ● | |||||||||||||||||
| Cognitive training, nutrition and exercise | cgn & ntr & exrc | 1 | ● | ● | ● | ||||||||||||||||||
| Education | educ | 3 | ● | ||||||||||||||||||||
| Education and multifactorial-action | educ & mfa- | 1 | ● | ● | |||||||||||||||||||
| Education and risk-screening | educ & rsk-mfa- | 1 | ● | ● | |||||||||||||||||||
| Education, exercise, multifactorial-action and review with medication-review and self-management | educ & exrc & mfar(w/med + slfm) | 2 | ● | ● | ● | ● | ● | ● | |||||||||||||||
| Education, exercise, multifactorial-action and review with self-management | educ & exrc & mfar(w/slfm) | 1 | ● | ● | ● | ● | ● | ||||||||||||||||
| Education, multifactorial-action and review | educ & mfar | 1 | ● | ● | ● | ||||||||||||||||||
| Education, multifactorial-action and review with medication-review | educ & mfar(w/med) | 3 | ● | ● | ● | ● | |||||||||||||||||
| Education, multifactorial-action and review with medication-review and self-management | educ & mfar(w/med + slfm) | 5 | ● | ● | ● | ● | ● | ||||||||||||||||
| Education, multifactorial-action and review with self-management | educ & mfar(w/slfm) | 2 | ● | ● | ● | ● | |||||||||||||||||
| Exercise | exrc | 7 | ● | ||||||||||||||||||||
| Exercise and multifactorial-action with medication-review | exrc & mfa-(w/med) | 1 | ● | ● | ● | ||||||||||||||||||
| Exercise and multifactorial-action with medication-review and self-management | exrc & mfa-(w/med + slfm) | 1 | ● | ● | ● | ● | |||||||||||||||||
| Exercise and psychology | exrc & psyc | 3 | ● | ● | |||||||||||||||||||
| Exercise, multifactorial-action and review | exrc & mfar | 1 | ● | ● | ● | ||||||||||||||||||
| Exercise, multifactorial-action and review with medication-review | exrc & mfar(w/med) | 1 | ● | ● | ● | ● | |||||||||||||||||
| Exercise, multifactorial-action and review with medication-review and self-management | exrc & mfar(w/med + slfm) | 1 | ● | ● | ● | ● | ● | ||||||||||||||||
| Homecare | hmcr | 12 | ● | ||||||||||||||||||||
| Homecare and aids | hmcr & aids | 1 | ● | ● | |||||||||||||||||||
| Homecare and medication-review | hmcr & med | 1 | ● | ● | |||||||||||||||||||
| Homecare and multifactorial-action | hmcr & mfa- | 5 | ● | ● | |||||||||||||||||||
| Homecare and nutrition | hmcr & ntr | 1 | ● | ● | |||||||||||||||||||
| Homecare, ADL, aids and multifactorial-action | hmcr & ADL & aids & mfa- | 1 | ● | ● | ● | ● | |||||||||||||||||
| Homecare, ADL, aids and multifactorial-action with self-management | hmcr & ADL & aids & mfa-(w/slfm) | 1 | ● | ● | ● | ● | ● | ||||||||||||||||
| Homecare, ADL, multifactorial-action and review with self-management | hmcr & ADL & mfar(w/slfm) | 3 | ● | ● | ● | ● | ● | ||||||||||||||||
| Homecare, aids and telecoms | hmcr & aids & comm | 1 | ● | ● | ● | ||||||||||||||||||
| Homecare, alternative-medicine and exercise | hmcr & hmnt & exrc | 1 | ● | ● | ● | ||||||||||||||||||
| Homecare, education, multifactorial-action and review | hmcr & educ & mfar | 1 | ● | ● | ● | ● | |||||||||||||||||
| Homecare, multifactorial-action and review | hmcr & mfar | 6 | ● | ● | ● | ||||||||||||||||||
| Homecare, multifactorial-action and review with medication-review | hmcr & mfar(w/med) | 3 | ● | ● | ● | ● | |||||||||||||||||
| Homecare, multifactorial-action and review with medication-review and self-management | hmcr & mfar(w/med + slfm) | 1 | ● | ● | ● | ● | ● | ||||||||||||||||
| Homecare, multifactorial-action and review with self-management | hmcr & mfar(w/slfm) | 2 | ● | ● | ● | ● | |||||||||||||||||
| Homecare, nutrition, multifactorial-action and review | hmcr & ntr & mfar | 1 | ● | ● | ● | ● | |||||||||||||||||
| Meaningful-activities and education | eng & educ | 2 | ● | ● | |||||||||||||||||||
| Meaningful-activities and multifactorial-action with self-management | eng & mfa-(w/slfm) | 1 | ● | ● | ● | ||||||||||||||||||
| Medication-review, nutrition and exercise | med & ntr & exrc | 1 | ● | ● | ● | ||||||||||||||||||
| Monitoring | mntr-mfa- | 1 | ● | ||||||||||||||||||||
| Multifactorial-action | mfa- | 9 | ● | ||||||||||||||||||||
| Multifactorial-action and review | mfar | 15 | ● | ● | |||||||||||||||||||
| Multifactorial-action and review with medication-review | mfar(w/med) | 24 | ● | ● | ● | ||||||||||||||||||
| Multifactorial-action and review with medication-review and self-management | mfar(w/med + slfm) | 3 | ● | ● | ● | ● | |||||||||||||||||
| Multifactorial-action and review with self-management | mfar(w/slfm) | 2 | ● | ● | ● | ||||||||||||||||||
| Multifactorial-action with medication-review | mfa-(w/med) | 5 | ● | ● | |||||||||||||||||||
| Multifactorial-action with medication-review and self-management | mfa-(w/med + slfm) | 1 | ● | ● | ● | ||||||||||||||||||
| Nutrition and exercise | ntr & exrc | 3 | ● | ● | |||||||||||||||||||
| Psychology | psyc | 1 | ● | ||||||||||||||||||||
| Risk-screening | rsk-mfa- | 6 | ● | ||||||||||||||||||||
| Telecoms | comm | 1 | ● | ||||||||||||||||||||
| Welfare-advice | wlfr | 1 | ● |
Usual care, the standard care provided in that context in the absence of a trial, was often ac or homecare but in some contexts it was multifactorial-action; multifactorial-action and review; aids; or another intervention. Not all trials included a usual care comparator.
Comparisons
The 144 within-trial comparisons between eligible arms formed 80 types of direct comparison (edges or links) between the intervention groups (nodes or points; illustrated in Figure 2). There were 20 comparisons of multifactorial-action and review with medication-review versus ac, 8 comparisons of multifactorial-action and review versus ac and 6 comparisons of risk-screening versus ac. Sixty-one edges were formed of a single within-trial comparison.
FIGURE 2.
Network of 63 intervention groups (nodes) and the 80 types of direct comparison (edges) included in the review (all included studies regardless of availability of results data). ADL, activities of daily living training; aids, provision of aids and adaptions; cgn, cognitive training; comm, technology for communication and engagement; educ, education; eng, engagement in meaningful-activities; exrc, exercise; hmcr, homecare; hmnt, alternative medicine such as homeopathy and naturopathy; med, medication-review; mfa, multifactorial-action; mfar, multifactorial-action routine review follow on; mntr-mfa, monitoring, which may trigger multifactorial-action; ntr, nutritional support; psyc, psychological therapy; rsk-mfa, risk-screening, which may trigger multifactorial-action; sst, social skills training; vchr, care voucher provision; wlfr, welfare rights advice; w/med, with medication-review; w/slfm, with self-management.
Most of the edges had ac as one of their nodes (n = 45). Nine of the edges had formal homecare as one of their nodes. Forty-three nodes were only connected by one edge to the rest of the network, 34 of which were only connected by a single trial’s comparison.
The nodes and edges formed one connected network in principle. However, single studies linked different parts of the network, thus lack of data meant the network was divided in many analyses into two networks: one network of comparisons with ac and one network of comparisons with homecare (hmcr). Additionally, among the nodes with homecare as a component, there were multiple comparisons on the path between the homecare node and the homecare, multifactorial-action and review node, which was connected to ac. This meant there were some analyses where some nodes that included homecare as a component were connected to the ac network but still disconnected from the homecare network.
Nine comparisons formed self-loops, where we had categorised two or more arms of a trial into the same intervention group. These comparisons, therefore, do not form part of the network for meta-analysis. Three of these were in three-arm trials, with a pair of comparisons that still contributed to the analysis. 69,106,171 Morey 2006139 was a three-arm trial that did not report any results of interest where we categorised all three interventions in the same group; all three arms featured a minimal but active intervention, one was a high-intensity alternative and one added an attention control element. The remaining three self-comparisons all included multifactorial-action as a component. Kono 2012120 compared two forms of multifactorial-action and review. Stewart 2005161 compared multifactorial-action from care planning performed by either a social worker or an occupational therapist. Ryvicker 2011156 compared two forms of homecare, multifactorial-action and review.
Outcomes
Instrumental activities of daily living (IADL) outcomes were measured by 51 studies, 38 of which reported results of interest. Personal activities of daily living (PADL) outcomes were measured by 65 studies, 46 of which reported results of interest. Combined PADL and IADL outcomes were measured by 31 studies, 22 of which reported results of interest. Hospitalisation outcomes were measured by 78 studies, 57 of which reported results of interest but only 35 of which reported number of participants hospitalised. Homecare usage was measured by 24 studies but reported by 16. Care-home placement was measured in some form by 71 studies, 55 of which reported results of interest, and 42 of which included a measure of current care-home placement. Health status was measured by 77 studies, 47 of which reported results of interest. Depression outcomes were measured by 72 studies, 41 of which reported results of interest. Loneliness was measured by 12 studies but reported by 6 of them. Falls were measured by 40 studies but reported by 23, 19 of which included a measure of the number of participants who fell. Finally, mortality was reported by 98 studies, often as part of accounting for losses to follow-up.
Follow-up was between 24 weeks166,176 and 8 years. 164 Twenty-seven studies only completed follow-up during the short-term time frame (ignoring any crossover periods), 54 studies completed final follow-up in the medium-term time frame and 48 extended into the long-term time frame.
Funding and conflicts of interest
One hundred and sixteen studies had non-commercial funding, six had both non-commercial and commercial sources of funding (mixed) and funding sources were unclear for seven studies. The authors of 71 studies declared no conflicts of interest without caveats. We did not identify any statement regarding conflicts of interest for 39 studies. Some study authors declared interests including personal ownership of the intervention’s intellectual property and the possibility of commercially exploiting this. Other declarations included funding or donations from an intervention developer. See the characteristics of included studies table in Report Supplementary Material 1 for further details.
Risk of bias
We assessed RoB in results of interest, which were available from 113 studies. Although we assessed RoB for each result of interest, the judgements were the same across results per study in the domains related to allocation and deviations from the intended intervention for all but four studies (described below). Therefore, we have summarised the RoB in these domains here on a per-study basis (see Figure 3).
FIGURE 3.
Summary of study-level RoB for the domains related to allocation and deviations from the intended intervention in the 113 studies with results of interest. Four studies had results at differing RoB in domain 2, so we have used the highest risk in this figure.
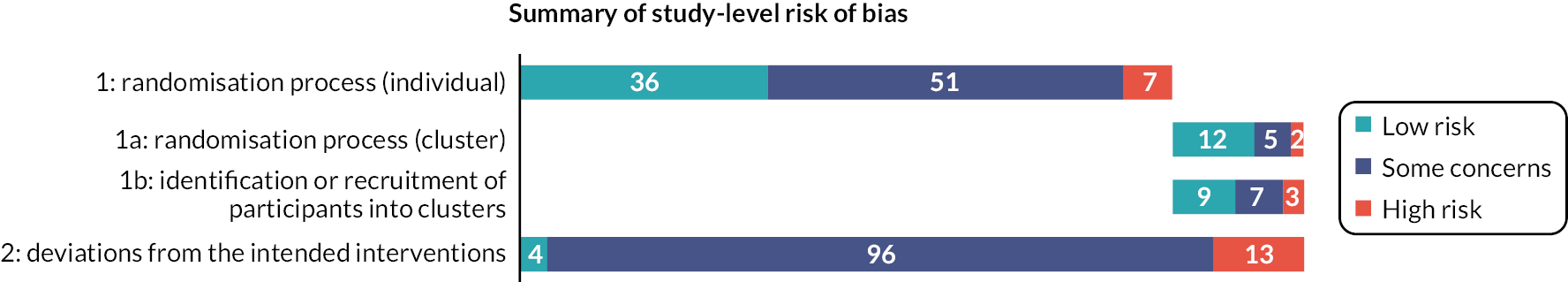
Individually randomised studies
Risk of bias due to the randomisation process (individual)
Among individually randomised studies, we judged the randomisation process to present a low RoB for 36 studies, some concerns for 51 studies and a high risk in 7 studies. Of those at high RoB, we were concerned that allocation was predictable in three due to small-block randomisation,63,79,190 the process was reported to have been subverted in two,66,125 there was unexplained imbalance in baseline characteristics in one172 and participants were allocated prior to recruitment in one. 95
Risk of bias due to deviations from the intended interventions
We judged the RoB due to deviations from the intended interventions to present some concerns in 80 individually randomised studies. Two studies were judged to have low RoB because they investigated whether the control group had deviated from their assigned intervention due to the trial context. Twelve studies presented a high RoB in this domain for at least one result: six due to post-randomisation exclusions,63,106,124,134,158,177 four due to risk or detection of contamination,67,104,126,164 one due to a combined risk of contamination and the reassignment of intervention participants to control following non-engagement125 and one due to substantial modifications in the intervention from what was originally intended. 66
Four of the studies with results at high RoB due to post-randomisation exclusions also reported results for other outcomes for which participants were not excluded, where we could incorporate those excluded in the analysis, or where the number of exclusions was too small to substantially affect the results. 106,124,134,158
Cluster-randomised studies
Risk of bias due to the randomisation process (cluster)
We judged the randomisation process in cluster-randomised trials to present low RoB in 12 studies, some concerns in 5 studies and high RoB in 2 studies. We judged that there was a high RoB in the randomisation process of Bleijenberg 201669 because, despite reportedly being computer-randomised from a complete list stratified by cluster size, there was a substantial and unexplained imbalance in cluster size, education level and socioeconomic status. We reached the same judgement for Blom 2016,70 where the cluster-randomisation process and an additional individual randomisation within the intervention arm were not detailed and there was also substantial and unexplained imbalance in cluster size.
Risk of bias due to identification or recruitment of participants into clusters
We judged the identification or recruitment of participants into clusters to present low RoB in nine studies, some concerns in seven studies and high RoB in three studies. In the three studies at high RoB, participant recruitment took place after cluster allocation; in two studies the recruiters and participants appeared to know the allocation prior to recruitment,108,149 while in Parsons J 2012145 this was unclear and we were concerned by imbalances in participant characteristics.
Risk of bias due to deviations from the intended interventions
We judged the RoB due to deviations from the intended interventions to present low RoB in 2 studies, some concerns in 16 studies and high RoB in 1 study. In the studies at low RoB, it seemed unlikely that there were deviations due to the trial context despite awareness of the intervention. 108,130 Sherman 2016159 was at high risk in this domain because participants who did not receive the intervention were excluded from the analysis.
Risk of bias in results of interest
Risks of bias arising in the other domains varied according to the outcome assessed. For missing outcome data, differences particularly related to whether the outcome was continuous or dichotomous and the proportion of people experiencing the event, with rare, dichotomous outcomes more likely to be at higher risk. For bias in measurement of the outcome, differences largely related to whether the outcome was self-reported or sourced from records and whether the self-reporting was about the individual’s perception (such as depressive symptoms) or memorable, observable events (such as hospitalisation). For bias in selection of the reported result, we very rarely had access to a sufficiently detailed analysis plan, so differences largely related to whether we had access to numbers of events and cases where there were no plausible alternative definitions for the measure such that the same data could be recut into different groups such as mortality as a defined outcome.
Overall risk of bias
Overall, no results of interest were judged to have low RoB; there were some concerns about 28%, with the remaining 72% at high RoB. We further judged those results at high RoB to present either serious concerns (53% of results) or very serious concerns (19% of results). Because there were differences in RoB by outcome, some outcomes were reported by more studies, and different studies report different outcomes, we have not presented a more detailed breakdown here. The RoBs are summarised for each analysis in the text and details are provided in Report Supplementary Material 10. Results were included in analyses regardless of RoB, but we conducted sensitivity analyses excluding results for which we had very serious concerns. We judged whether to downgrade our certainty in the evidence for an effect estimate based on the contributions of results judged as serious concerns or very serious concerns about RoB.
Chapter 4 Results of syntheses
This chapter presents the results of syntheses for clinical and service outcomes; economic evidence is presented in Chapter 5. NMAs were conducted for living at home, IADL, PADL and care-home placement outcomes for three time frames and for hospitalisation, health status, depression and mortality for the medium-term time frame only. Homecare services usage, loneliness and falls were narratively synthesised. Sensitivity analyses and investigation of frailty were only conducted for the medium-term time frame. As described in Interventions and comparators in Chapter 3, the network of comparisons available for analysis was split in two for all outcomes except mortality. Therefore, we present two analyses for all other outcomes and time frames (where possible), one with ac as the reference comparator (ac network) and one with homecare as the reference comparator (homecare network).
The reports of the main results of an analysis present comparisons with the reference comparator that are ordered by certainty first (high to low) and ranking second (first to last). Textual summaries of results indicate uncertainty using the terms ‘probably’ or ‘may’, and effect size as described in Confidence in cumulative evidence in Chapter 2. Results with very low certainty are not summarised in the text but are provided in summary of findings tables.
Characteristics of studies included in each NMA and results are presented in Report Supplementary Material 5. Estimates of the effectiveness of intervention groups for community-based complex interventions are presented in network tables, with direct evidence for pairwise comparisons listed in the upper right triangle of each table and NMA pooled effect estimates listed in the lower left triangle of each table. Most of the networks were small and sparsely connected. Therefore, conclusions regarding comparative intervention effectiveness should be interpreted cautiously, also considering the uncertainty expressed by 95% CIs. The GRADE rating of certainty accounts for uncertainty expressed by the CI as well as other factors such as RoB. The mean rank, 95% CI for the true rank and SUCRA values for intervention groups included across the networks are presented in the rankings table. It should be noted that intervention group rankings are based on intervention group effectiveness (SMDs/ORs) and as such can be susceptible to change if an intervention group is added or removed from small networks.
Additional results not included in NMA are tabulated in Report Supplementary Material 9; RoB judgements for all results are presented in Report Supplementary Material 10.
The following sections describe the results of our analyses for each outcome of interest.
Living at home
Living at home is defined either as a reported trial outcome or the inverse of care-home placement and mortality if reported separately. OR was estimated in the NMA to compare the odds of living at home between two arms. OR > 1 means that the estimated effect favours the experimental intervention group, that is an increased chance of living at home with the intervention. For each time frame, there were two separate networks, one with ac reference comparator and another with homecare comparator, which we describe in turn.
Available-care network
Short-term time frame
The available-care network for living at home in the short term included eight studies (n = 4013) with eight intervention groups (see Table 5 for a summary of findings). 78,113,121,126,136,165,166,190 Each comparison included one study. Two study populations included all frailty categories, two were pre-frail and frail, three were frail and one was unclassifiable. We had some concerns regarding RoB in six study results, with two at high RoB (one serious concerns, one very serious concerns).
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: Living at home Time frame Short term; range of follow-up 24 weeks to 6 months Setting: Community Total studies: 8 Total participants: 4013 Comparator rank: Mean 4.7, 95% CI 2 to 7 |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Relative effect (95% CI) | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | ||||
| Network summary estimate | Calculated RRa | High-risk population (952 per 1000 with ac) | Low-risk population (980 per 1000 with ac) | ||||||
| With intervention | Difference | With intervention | Difference | ||||||
| Multifactorial-action and review (mfar) | OR 1.34 (0.75 to 2.39) Mixed estimate | RR 1.01 (0.99 to 1.02) | 964 per 1000 (937 to 979) | 12 more per 1000 (15 fewer to 27 more) | 985 per 1000 (974 to 992) | 5 more per 1000 (6 fewer to 12 more) | ⊕⊕⊝⊝Lowb | 3.1 (1 to 6) | May result in a very slight increase in chance of living at home |
| ADL, nutrition and exercise (ADL & ntr & exrc) | OR 1.01 (0.25 to 4.13) Mixed estimate | RR 1.00 (0.92 to 1.02) | 953 per 1000 (831 to 988) | 1 more per 1000 (121 fewer to 36 more) | 980 per 1000 (924 to 995) | 0 per 1000 (56 fewer to 15 more) | ⊕⊕⊝⊝Lowb | 4.5 (1 to 8) | May result in little to no difference in chance of living at home |
| Multifactorial-action and review with medication-review [mfar(w/med)] | OR 0.95 (0.58 to 1.55) Mixed estimate | RR 1.00 (0.98 to 1.01) | 949 per 1000 (920 to 969) | 3 fewer per 1000 (32 fewer to 17 more) | 979 per 1000 (966 to 987) | 1 fewer per 1000 (14 fewer to 7 more) | ⊕⊕⊝⊝Lowb | 5.0 (2 to 7) | May result in little to no difference in chance of living at home |
| Education, multifactorial-action and review with medication-review and self-management strategies [educ & mfar(w/med + slfm)] | OR 0.52 (0.13 to 2.06) Mixed estimate | RR 0.98 (0.85 to 1.01) | 912 per 1000 (725 to 976) | 40 fewer per 1000 (227 fewer to 24 more) | 962 per 1000 (867 to 990) | 18 fewer per 1000 (113 fewer to 10 more) | ⊕⊕⊝⊝Lowb | 6.2 (2 to 8) | May result in a reduction in chance of living at home |
| Aids, multifactorial-action and review (aids & mfar) | OR 3.06 (0.31 to 30.42) Mixed estimate | RR 1.02 (0.94 to 1.03) | 984 per 1000 (859 to 998) | 32 more per 1000 (93 fewer to 46 more) | 993 per 1000 (938 to 999) | 13 more per 1000 (42 fewer to 19 more) | ⊕⊝⊝⊝Very lowb,c | 2.2 (1 to 7) | The evidence is very uncertain about the effect on chance of living at home |
| Multifactorial-action and review with self-management strategies [mfar(w/slfm)] | OR 1.34 (0.56 to 3.25) Mixed estimate | RR 1.01 (0.98 to 1.02) | 964 per 1000 (917 to 985) | 12 more per 1000 (35 fewer to 33 more) | 985 per 1000 (965 to 994) | 5 more per 1000 (15 fewer to 14 more) | ⊕⊝⊝⊝Very lowb,d | 3.4 (1 to 7) | The evidence is very uncertain about the effect on chance of living at home |
| ADL, aids, education, exercise, multifactorial-action and review with medication-review and self-management strategies [ADL & aids & ed & ex & mf(w/med + slfm)] | OR 0.18 (0.01 to 3.69) Mixed estimate | RR 0.89 (0.24 to 1.02) | 779 per 1000 (145 to 987) | 173 fewer per 1000 (807 fewer to 35 more) | 897 per 1000 (295 to 994) | 83 fewer per 1000 (685 fewer to 14 more) | ⊕⊝⊝⊝Very lowe | 7.0 (1 to 8) | The evidence is very uncertain about the effect on chance of living at home |
The consistency assumption was found to hold for the network according to both the global Wald test (p = 0.238) and the node-splitting method (all contrasts p > 0.05). As the network contained only a single study measuring each comparison, there was no potential source of heterogeneity and therefore a common-effect model was fitted.
There was low-certainty evidence that multifactorial-action and review may result in a very slight increase in the odds of living at home compared with ac in the short term (OR 1.34, 95% CI 0.75 to 2.39). There was low-certainty evidence that ADL, nutrition and exercise (OR 1.01, 95% CI 0.25 to 4.13); and multifactorial-action and review with medication-review (OR 0.95, 95% CI 0.58 to 1.55) may result in little to no difference in odds of living at home. Education, multifactorial-action and review with medication-review and self-management may result in a reduction in odds of living at home [OR 0.52 (moderate), 95% CI 0.13 to 2.06; low certainty]. Three other comparisons with ac were of very low certainty.
Medium-term time frame
The available-care network for living at home in the medium term consisted of 21 studies (n = 16,937) with 14 intervention groups (see Table 6 for a summary of findings). 70,84,88,103,104,106–108,116,118,119,121,136,138,142,143,150,153,158,165,178 Multifactorial-action and review with medication-review versus ac included data from six studies; two comparisons had data from two studies and the remainder had one. Four study populations included all frailty categories: one was robust, one pre-frail, six pre-frail and frail and six frail. There were some concerns with RoB in 12 of the results; the other 12 were high RoB (6 serious concerns, 3 very serious concerns).
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: Living at home Time: Medium term; range of follow-up 12 to 18 months Setting: Community Total studies: 21 Total participants: 16,937 Comparator rank: Mean 9.9, 95% CI 7 to 12 |
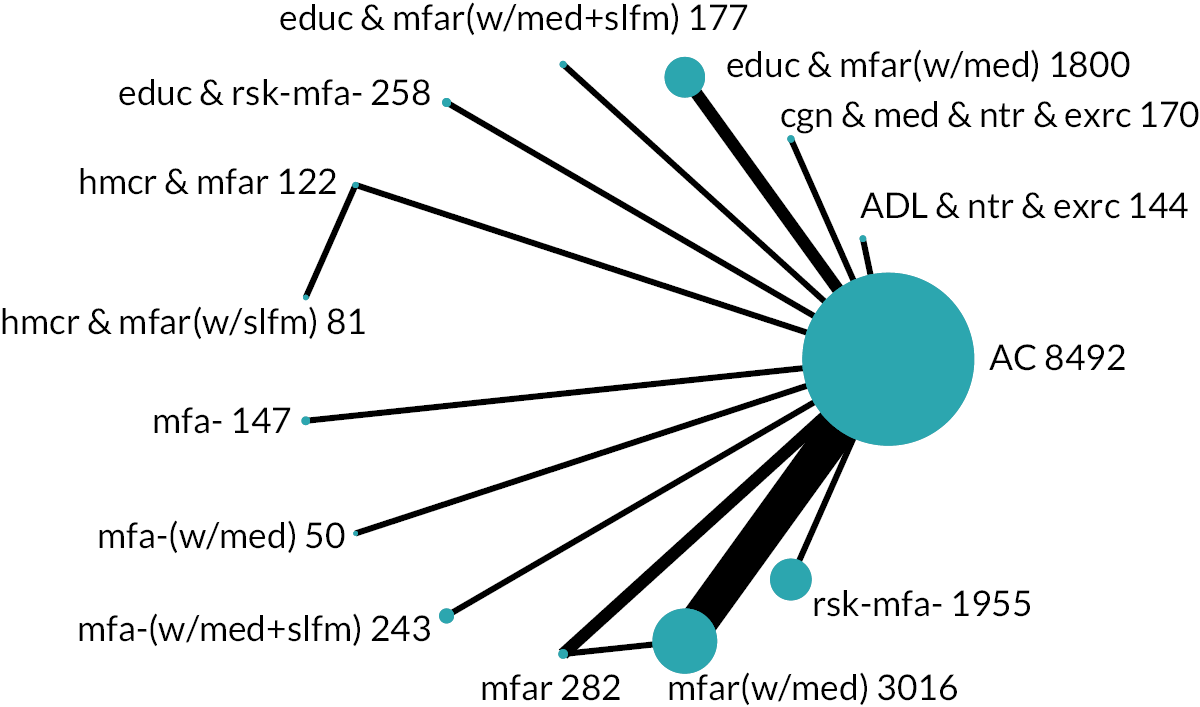
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Relative effect (95% CI) | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | ||||
| Network estimate | Calculated RRa | High-risk population [833 per 1000 with ac] | Low-risk population [981 per 1000 with ac] | ||||||
| With intervention | Difference | With intervention | Difference | ||||||
| Multifactorial-action and review with medication-review [mfar(w/med)] | OR 1.22 (0.93 to 1.59) Mixed estimate | RR 1.01 (1.00 to 1.02) | 859 per 1000 (823 to 888) | 26 more per 1000 (10 fewer to 55 more) | 984 per 1000 (980 to 988) | 3 more per 1000 (1 fewer to 7 more) | ⊕⊕⊕⊝Moderateb | 7.5 (5 to 11) | Probably results in a slight increase in chance of living at home |
| Multifactorial-action with medication-review [mfa-(w/med)] | OR 2.55 (0.61 to 10.60) Mixed estimate | RR 1.04 (0.96 to 1.06) | 927 per 1000 (754 to 981) | 94 more per 1000 (79 fewer to 148 more) | 992 per 1000 (969 to 998) | 11 more per 1000 (12 fewer to 17 more) | ⊕⊕⊝⊝Lowc | 4.6 (1 to 13) | May result in an increase in chance of living at home |
| Cognitive training, medication-review, nutrition and exercise (cgn & med & ntr & exrc) | OR 1.93 (0.79 to 4.77) Mixed estimate | RR 1.03 (0.98 to 1.05) | 906 per 1000 (797 to 960) | 73 more per 1000 (36 fewer to 127 more) | 990 per 1000 (976 to 996) | 9 more per 1000 (5 fewer to 15 more) | ⊕⊕⊝⊝Lowc | 5.3 (2 to 12) | May result in an increase in chance of living at home |
| ADL, nutrition and exercise (ADL & ntr & exrc) | OR 1.79 (0.67 to 4.76) Mixed estimate | RR 1.03 (0.97 to 1.05) | 899 per 1000 (771 to 960) | 66 more per 1000 (62 fewer to 127 more) | 989 per 1000 (972 to 996) | 8 more per 1000 (9 fewer to 15 more) | ⊕⊕⊝⊝Lowc | 5.9 (2 to 13) | May result in an increase in chance of living at home |
| Risk-screening (rsk-mfa-) | OR 0.90 (0.66 to 1.23) Mixed estimate | RR 0.99 (0.97 to 1.01) | 818 per 1000 (768 to 860) | 15 fewer per 1000 (65 fewer to 27 more) | 979 per 1000 (972 to 984) | 2 fewer per 1000 (9 fewer to 3 more) | ⊕⊕⊝⊝Lowc | 11.1 (7 to 13) | May result in a very slight reduction in chance of living at home |
| Education, multifactorial-action and review with medication-review [educ & mfar(w/med)] | OR 0.88 (0.60 to 1.29) Mixed estimate | RR 0.99 (0.96 to 1.01) | 814 per 1000 (749 to 865) | 19 fewer per 1000 (84 fewer to 32 more) | 978 per 1000 (969 to 985) | 3 fewer per 1000 (12 fewer to 4 more) | ⊕⊕⊝⊝Lowc | 11.2 (6 to 14) | May result in a very slight reduction in chance of living at home |
| Education, multifactorial-action and review with medication-review and self-management strategies [educ & mfar(w/med + slfm)] | OR 0.41 (0.14 to 1.17) Mixed estimate | RR 0.91 (0.72 to 1.01) | 670 per 1000 (413 to 854) | 163 fewer per 1000 (420 fewer to 21 more) | 955 per 1000 (879 to 984) | 26 fewer per 1000 (102 fewer to 3 more) | ⊕⊕⊝⊝Lowc | 13.5 (8 to 14) | May result in a reduction in chance of living at home |
| Homecare, multifactorial-action and review with self-management strategies [hmcr & mfar(w/slfm)] | OR 8.89 (1.90 to 41.62) Indirect estimate | RR 1.06 (1.03 to 1.07) | 978 per 1000 (904 to 995) | 145 more per 1000 (71 more to 162 more) | 998 per 1000 (990 to 1000) | 17 more per 1000 (9 more to 19 more) | ⊕⊝⊝⊝Very lowd,e | 1.5 (1 to 4) | The evidence is very uncertain about the effect on chance of living at home |
| Homecare, multifactorial-action and review (hmcr & mfar) | OR 5.71 (1.65 to 19.83) Mixed estimate | RR 1.06 (1.03 to 1.07) | 966 per 1000 (891 to 990) | 133 more per 1000 (58 more to 157 more) | 997 per 1000 (988 to 999) | 16 more per 1000 (7 more to 18 more) | ⊕⊝⊝⊝Very lowf,g | 2.4 (1 to 5) | The evidence is very uncertain about the effect on chance of living at home |
| Multifactorial-action (mfa-) | OR 2.23 (0.63 to 7.91) Mixed estimate | RR 1.04 (0.96 to 1.06) | 917 per 1000 (758 to 975) | 84 more per 1000 (75 fewer to 142 more) | 991 per 1000 (970 to 998) | 10 more per 1000 (11 fewer to 17 more) | ⊕⊝⊝⊝Very lowc,h | 4.9 (1 to 13) | The evidence is very uncertain about the effect on chance of living at home |
| Multifactorial-action and review (mfar) | OR 1.15 (0.60 to 2.18) Mixed estimate | RR 1.01 (0.96 to 1.04) | 851 per 1000 (751 to 916) | 18 more per 1000 (82 fewer to 83 more) | 983 per 1000 (969 to 991) | 2 more per 1000 (12 fewer to 10 more) | ⊕⊝⊝⊝Very lowc,i | 8.4 (4 to 13) | The evidence is very uncertain about the effect on chance of living at home |
| Education and risk-screening (educ & rsk-mfa-) | OR 1.09 (0.60 to 2.01) Mixed estimate | RR 1.01 (0.96 to 1.03) | 845 per 1000 (748 to 909) | 12 more per 1000 (85 fewer to 76 more) | 983 per 1000 (968 to 990) | 2 more per 1000 (13 fewer to 9 more) | ⊕⊝⊝⊝Very lowc,h | 8.9 (4 to 13) | The evidence is very uncertain about the effect on chance of living at home |
| Multifactorial-action with medication-review and self-management strategies [mfa-(w/med + slfm)] | OR 1.00 (0.63 to 1.58) Mixed estimate | RR 1.00 (0.96 to 1.02) | 833 per 1000 (759 to 888) | 0 per 1000 (74 fewer to 55 more) | 981 per 1000 (970 to 988) | 0 per 1000 (11 fewer to 7 more) | ⊕⊝⊝⊝Very lowc,j | 9.8 (5 to 13) | The evidence is very uncertain about the effect on chance of living at home |
The consistency assumption was found to hold for the network according to both the global Wald test (p = 0.796) and the node-splitting method (all contrasts p > 0.05). Between-study variance was estimated to be non-zero, indicating some degree of heterogeneity between studies included in the network (τ = 0.0856).
There was moderate-certainty evidence that multifactorial-action and review with medication-review probably results in a slight increase in the odds of living at home compared with ac in the medium term (OR 1.22, 95% CI 0.93 to 1.59; moderate certainty). There was low-certainty evidence that multifactorial-action with medication-review [OR 2.55 (large), 95% CI 0.61 to 10.60]; cognitive training, medication-review, nutrition and exercise [OR 1.93 (large), 95% CI 0.79 to 4.77]; and ADL, nutrition and exercise [OR 1.79 (large), 95% CI 0.67 to 4.76] may result in an increase in the odds of living at home compared with ac. There was low-certainty evidence that risk-screening (OR 0.90, 95% CI 0.66 to 1.23); and education, multifactorial-action and review with medication-review (OR 0.88, 95% CI 0.60 to 1.29) may result in a very slight reduction in odds of living at home. There was low-certainty evidence that education, multifactorial-action and review with medication-review and self-management may result in a reduction in odds of living at home [OR 0.41 (very large), 95% CI 0.14 to 1.17]. Other comparisons with ac were of very low certainty.
Investigation of small-study effects
The comparison-adjusted funnel plot appeared symmetric, implying no evidence of small-study effects in the network.
Sensitivity analysis for risk of bias
After removing the 3 study results with very serious concerns about RoB, an additional study was disconnected, leaving 17 results (n = 15,457) and 11 intervention groups in the sensitivity analysis. The estimates of effect and CIs were very similar for the comparisons with ac rated as moderate or low certainty in the main analysis. Only the comparison of multifactorial-action and review with ac changed notably, with a greater point estimate and even wider CIs.
Investigation of frailty
All frailty categories were represented in the network and a network meta regression model was fitted including the frailty variable with ‘all (robust, pre-frail and frail)’ set as the reference category. The consistency assumption remained valid (global Wald test p = 0.701, node-splitting method showed all contrasts p > 0.05). Between-study variance remained very small, but non-zero (τ = 3.86 × 10−7). Frailty effects could not be estimated for most contrasts due to collinearity. Where frailty effects could be estimated, the effect was estimated with very large uncertainty as reflected in wide 95% CIs covering a broad range of both beneficial and harmful effects, making interpretation of these results meaningless in practice.
Long-term time frame
The available-care network for living at home in the long term included 13 studies (n = 14,843) with 10 intervention groups (see Table 7 for a summary of findings). 76,92,93,106,116,118,121,136,162,164,165,172,173 Multifactorial-action and review with medication-review versus ac included data from three studies; two comparisons had data from two studies and the remainder had one. Four study populations included all frailty categories, one included the robust and pre-frail categories, three the pre-frail and frail categories, one was pre-frail, three frail and one was unclassifiable. There were some concerns regarding RoB in nine study results and four were high RoB (three with serious concerns, one with very serious concerns).
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: Living at home Time frame: Long term; range of follow-up 24–43 months Setting: Community Total studies: 13 Total participants: 14,843 Comparator rank: Mean 7.5, 95% CI 5 to 9 |
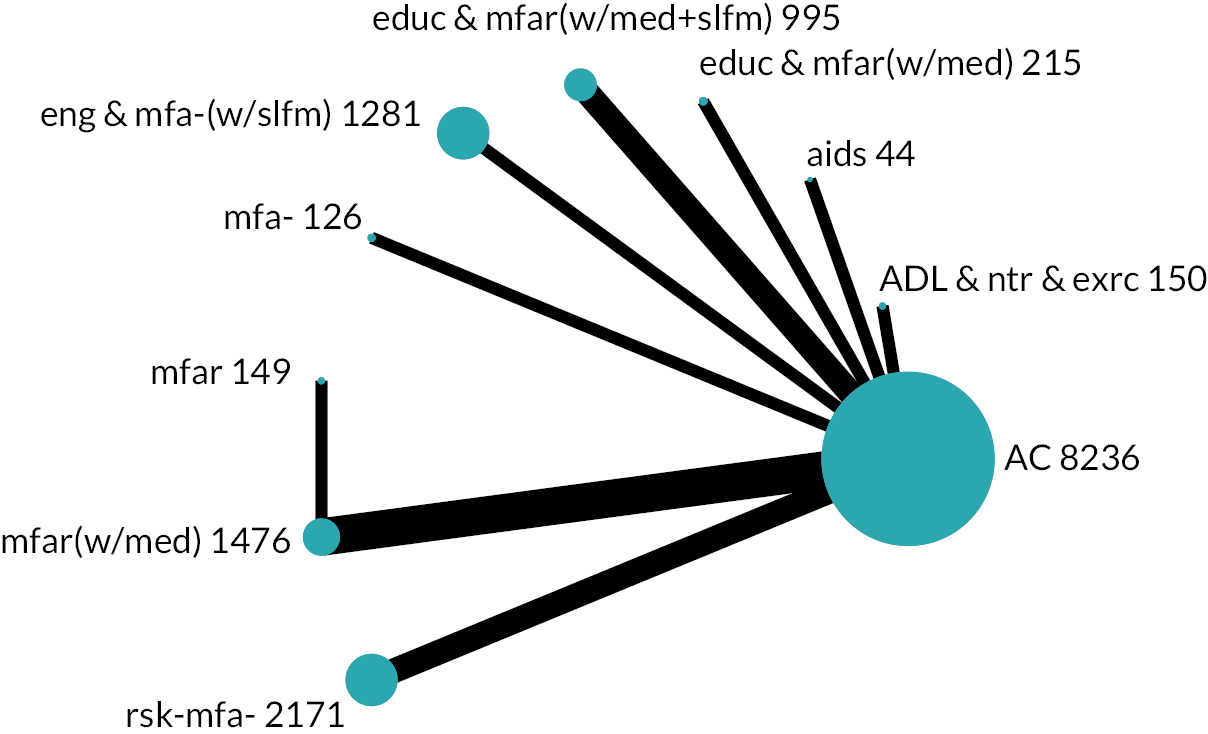
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Relative effect (95% CI) | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | ||||
| Network estimate | Calculated RRa | High-risk population [607 per 1000 with ac] | Low-risk population [932 per 1000 with ac] | ||||||
| With intervention | Difference | With intervention | Difference | ||||||
| Multifactorial-action and review (mfar) | OR 1.29 (0.63 to 2.63) Indirect estimate | RR 1.04 (0.90 to 1.13) | 665 per 1000 (492 to 803) | 58 more per 1000 (115 fewer to 196 more) | 946 per 1000 (896 to 973) | 14 more per 1000 (36 fewer to 41 more) | ⊕⊕⊝⊝Lowb | 5.0 (1 to 10) | May result in an increase in chance of living at home |
| Education, multifactorial-action and review with medication-review [educ & mfar(w/med)] | OR 1.23 (0.72 to 2.10) Mixed estimate | RR 1.04 (0.93 to 1.11) | 654 per 1000 (525 to 764) | 47 more per 1000 (82 fewer to 157 more) | 944 per 1000 (908 to 966) | 12 more per 1000 (24 fewer to 34 more) | ⊕⊕⊝⊝Lowb | 5.2 (2 to 10) | May result in an increase in chance of living at home |
| Multifactorial-action and review with medication-review [mfar(w/med)] | OR 1.17 (0.94 to 1.47) Mixed estimate | RR 1.03 (0.99 to 1.06) | 645 per 1000 (592 to 694) | 38 more per 1000 (15 fewer to 87 more) | 942 per 1000 (928 to 953) | 10 more per 1000 (4 fewer to 21 more) | ⊕⊕⊝⊝Lowb | 5.2 (3 to 9) | May result in a slight increase in chance of living at home |
| ADL, nutrition and exercise (ADL & ntr & exrc) | OR 1.15 (0.64 to 2.05) Mixed estimate | RR 1.02 (0.91 to 1.10) | 639 per 1000 (499 to 760) | 32 more per 1000 (108 fewer to 153 more) | 940 per 1000 (898 to 966) | 8 more per 1000 (34 fewer to 34 more) | ⊕⊕⊝⊝Lowb | 5.8 (2 to 10) | May result in a slight increase in chance of living at home |
| Engagement in meaningful-activities and multifactorial-action with self-management strategies [eng & mfa-(w/slfm)] | OR 1.03 (0.85 to 1.25) Mixed estimate | RR 1.01 (0.97 to 1.04) | 614 per 1000 (567 to 658) | 7 more per 1000 (40 fewer to 51 more) | 934 per 1000 (921 to 945) | 2 more per 1000 (11 fewer to 13 more) | ⊕⊕⊝⊝Lowb | 6.9 (3 to 10) | May result in a very slight increase in chance of living at home |
| Risk-screening (rsk-mfa-) | OR 0.91 (0.77 to 1.07) Mixed estimate | RR 0.98 (0.95 to 1.01) | 584 per 1000 (543 to 624) | 23 fewer per 1000 (64 fewer to 17 more) | 926 per 1000 (913 to 936) | 6 fewer per 1000 (19 fewer to 4 more) | ⊕⊕⊝⊝Lowb | 8.8 (6 to 10) | May result in a slight reduction in chance of living at home |
| Aids (aids) | OR 2.64 (1.02 to 6.88) Mixed estimate | RR 1.13 (1.00 to 1.19) | 803 per 1000 (611 to 914) | 196 more per 1000 (4 more to 307 more) | 973 per 1000 (933 to 990) | 41 more per 1000 (1 more to 58 more) | ⊕⊝⊝⊝Very lowc,d | 1.8 (1 to 7) | The evidence is very uncertain about the effect on chance of living at home |
| Multifactorial-action (mfa-) | OR 2.13 (0.85 to 5.33) Mixed estimate | RR 1.11 (0.97 to 1.18) | 767 per 1000 (568 to 892) | 160 more per 1000 (39 fewer to 285 more) | 967 per 1000 (921 to 986) | 35 more per 1000 (11 fewer to 54 more) | ⊕⊝⊝⊝Very lowb,e | 2.5 (1 to 9) | The evidence is very uncertain about the effect on chance of living at home |
| Education, multifactorial-action and review with medication-review and self-management strategies [educ & mfar(w/med + slfm)] | OR 1.08 (0.78 to 1.49) Mixed estimate | RR 1.01 (0.95 to 1.06) | 625 per 1000 (547 to 697) | 18 more per 1000 (60 fewer to 90 more) | 937 per 1000 (915 to 953) | 5 more per 1000 (17 fewer to 21 more) | ⊕⊝⊝⊝Very lowb,e | 6.3 (3 to 10) | The evidence is very uncertain about the effect on chance of living at home |
The network contained no loops and therefore the consistency assumption could not be tested and a ‘consistency’ model was fitted, setting the inconsistency parameter in the model to zero for all comparisons. Between-study variance was estimated to be very small but non-zero, indicating some degree of heterogeneity between studies included in the network (τ = 9.92 × 10−7).
There was low-certainty evidence that multifactorial-action and review [OR 1.29 (moderate), 95% CI 0.63 to 2.63]; and education, multifactorial-action and review with medication-review [OR 1.23 (moderate), 95% CI 0.72 to 2.10] may both result in an increase in the odds of living at home compared with ac in the long term. Multifactorial-action and review with medication-review (OR 1.17, 95% CI 0.94 to 1.47); and ADL, nutrition and exercise (OR 1.15, 95% CI 0.64 to 2.05) may both result in a slight increase in the odds of living at home. Meaningful-activities and multifactorial-action with self-management may result in a very slight increase in the odds of living at home (OR 1.03, 95% CI 0.85 to 1.25). Risk-screening may result in a slight reduction in odds of living at home (OR 0.91, 95% CI 0.77 to 1.07). Three other comparisons with ac were of very low certainty.
Across time frames
Multifactorial-action and review with medication-review [mfar(w/med)] was estimated to make little to no difference in the short term (low certainty) but result in small (moderate or low certainty) increases in living at home in the medium- and long-term time frames, respectively. Similarly, ADL, nutrition and exercise (ADL & ntr & exrc) may make little to no difference in the short term but result in large or small increases in living at home in the medium- and long-term time frames, respectively (low certainty). Conversely, there was low-certainty evidence that education, multifactorial-action and review with medication-review and self-management [educ & mfar(w/med + slfm)] may result in moderate or very large reductions in living at home in the short- and medium-term time frames, respectively. Risk-screening (rsk-mfa-) may result in a very slight or slight reduction in odds of living at home in the medium and long term, respectively (low certainty). There was contrasting evidence of low certainty that education, multifactorial-action and review with medication-review [educ & mfar(w/med)] may result in a slight reduction or moderate increase in living at home in the medium- and long-term time frames, respectively.
Homecare network
Short-term time frame
The homecare network for living at home in the short term included four studies (n = 704) and four intervention groups (see Table 8 for a summary of findings). 91,117,146,154 The comparison of homecare, ADL, multifactorial-action and review with self-management strategies versus homecare included the data of two studies, with the other two comparisons comprised of one study each. One study population was classified as pre-frail and frail, and three were frail. We had some concerns with RoB in three study results and one was at high RoB (serious concerns).
| Population: Older people Interventions: Community-based complex interventions Comparator: Homecare (hmcr) Outcome: Living at home Time frame: Short term; range of follow-up 6–7 months Setting: Community Total studies: 4 Total participants: 704 Comparator rank: Mean 1.1, 95% CI |
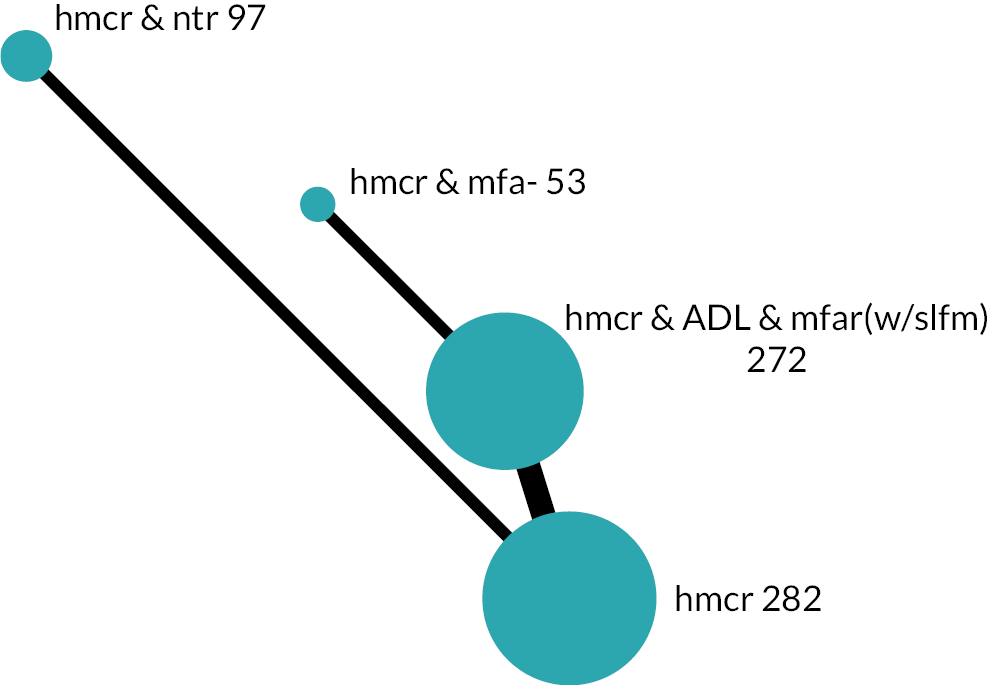
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Relative effect (95% CI) | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | ||||
| Network estimate | Calculated RRa | High-risk population (923 per 1000 with hmcr) | Low-risk population (953 per 1000 with hmcr) | ||||||
| With intervention | Difference | With intervention | Difference | ||||||
| Homecare, ADL, multifactorial-action and review with self-management strategies [hmcr & ADL & mfar(w/slfm)] | OR 0.63 (0.31 to 1.26) Mixed estimate | RR 0.96 (0.86 to 1.02) | 882 per 1000 (789 to 938) | 41 fewer per 1000 (134 fewer to 15 more) | 927 per 1000 (863 to 962) | 26 fewer per 1000 (90 fewer to 9 more) | ⊕⊕⊝⊝Lowb | 2.1 (1 to 3) | May result in a reduction in chance of living at home |
| Homecare and nutrition (hmcr & ntr) | OR 0.34 (0.12 to 0.95) Mixed estimate | RR 0.87 (0.64 to 1.00) | 801 per 1000 (588 to 919) | 122 fewer per 1000 (335 fewer to 4 fewer) | 872 per 1000 (707 to 951) | 81 fewer per 1000 (246 fewer to 2 fewer) | ⊕⊕⊝⊝Lowc | 3.2 (2 to 4) | May result in a reduction in chance of living at home |
| Homecare and multifactorial-action (hmcr & mfa-) | OR 0.26 (0.09 to 0.77) Indirect estimate | RR 0.82 (0.56 to 0.98) | 756 per 1000 (512 to 902) | 167 fewer per 1000 (411 fewer to 21 fewer) | 840 per 1000 (639 to 940) | 113 fewer per 1000 (314 fewer to 13 fewer) | ⊕⊝⊝⊝Very lowd,e | 3.6 (3 to 4) | The evidence is very uncertain about the effect on chance of living at home |
The network contained no loops and therefore the consistency assumption could not be tested and a ‘consistency’ model was fitted. Between-study variance was estimated to be very small but non-zero, indicating some degree of heterogeneity between studies included in the network (τ = 2.85 × 10−7).
There was low-certainty evidence that homecare, ADL, multifactorial-action and review with self-management [OR 0.63 (moderate), 95% CI 0.31 to 1.26]; and homecare and nutrition [OR 0.34 (very large), 95% CI 0.12 to 0.95] may result in reductions in the odds of living at home compared with homecare in the short term. There was very low-certainty evidence regarding homecare and multifactorial-action versus homecare.
Medium-term time frame
Overall characteristics
The homecare network for living at home in the medium term included five studies (n = 1978) and six intervention groups (see Table 9 for a summary of findings). 91,125,146,154,189 Each comparison consisted of one study. All study populations were frail. There were some concerns about RoB in one study and four were at high RoB (three with serious concerns, one with very serious concerns).
| Population: Older people Interventions: Community-based complex interventions Comparator: Homecare (hmcr) Outcome: Living at home Time frame: Medium term; range of follow-up 12–13 months Setting: Community Total studies: 5 Total participants: 1978 Comparator rank: Mean 3.0, 95% CI 1 to 5 |
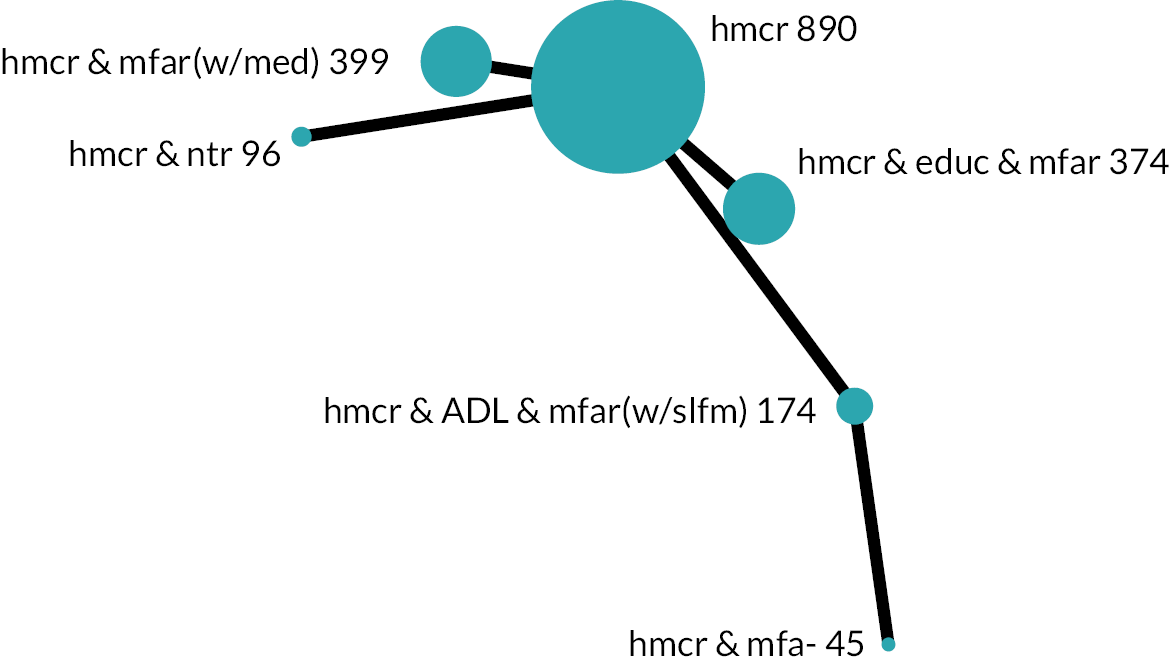
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Relative effect (95% CI) | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | ||||
| Network estimate | Calculated RRa | High-risk population (649 per 1000 with hmcr) | Low-risk population (843 per 1000 with hmcr) | ||||||
| With intervention | Difference | With intervention | Difference | ||||||
| Homecare, ADL, multifactorial-action and review with self-management strategies [hmcr & ADL & mfar(w/slfm)] | OR 0.76 (0.40 to 1.45) Mixed estimate | RR 0.92 (0.72 to 1.09) | 585 per 1000 (426 to 728) | 64 fewer per 1000 (223 fewer to 79 more) | 804 per 1000 (683 to 886) | 39 fewer per 1000 (160 fewer to 43 more) | ⊕⊕⊝⊝Lowb | 3.8 (1 to 6) | May result in a reduction in chance of living at home |
| Homecare, education, multifactorial-action and review (hmcr & educ & mfar) | OR 1.17 (0.85 to 1.59) Mixed estimate | RR 1.04 (0.96 to 1.11) | 683 per 1000 (612 to 747) | 34 more per 1000 (37 fewer to 98 more) | 862 per 1000 (821 to 895) | 19 more per 1000 (22 fewer to 52 more) | ⊕⊝⊝⊝Very lowb,c | 1.8 (1 to 4) | The evidence is very uncertain about the effect on chance of living at home |
| Homecare, multifactorial-action and review with medication-review [hmcr & mfar(w/med)] | OR 1.11 (0.82 to 1.51) Mixed estimate | RR 1.03 (0.94 to 1.10) | 672 per 1000 (601 to 736) | 23 more per 1000 (48 fewer to 87 more) | 856 per 1000 (814 to 890) | 13 more per 1000 (29 fewer to 47 more) | ⊕⊝⊝⊝Very lowb,d | 2.1 (1 to 4) | The evidence is very uncertain about the effect on chance of living at home |
| Homecare and multifactorial-action (hmcr & mfa-) | OR 0.51 (0.17 to 1.49) Indirect estimate | RR 0.80 (0.45 to 1.09) | 485 per 1000 (243 to 734) | 164 fewer per 1000 (406 fewer to 85 more) | 732 per 1000 (483 to 889) | 111 fewer per 1000 (360 fewer to 46 more) | ⊕⊝⊝⊝Very lowb,d | 5.1 (1 to 6) | The evidence is very uncertain about the effect on chance of living at home |
| Homecare and nutrition (hmcr & ntr) | OR 0.50 (0.23 to 1.07) Mixed estimate | RR 0.79 (0.54 to 1.02) | 480 per 1000 (301 to 664) | 169 fewer per 1000 (348 fewer to 15 more) | 729 per 1000 (556 to 852) | 114 fewer per 1000 (287 fewer to 9 more) | ⊕⊝⊝⊝Very lowb,d | 5.2 (2 to 6) | The evidence is very uncertain about the effect on chance of living at home |
Main analysis
The network contained no loops and therefore the consistency assumption could not be tested and a ‘consistency’ model was fitted. As the network contained only a single study for each comparison, there was no potential source of heterogeneity and therefore a common-effect model was fitted.
There was low-certainty evidence that homecare, ADL, multifactorial-action and review with self-management may result in a reduction in the odds of living at home compared with homecare in the medium term [OR 0.76 (large), 95% CI 0.40 to 1.45]. The evidence was very uncertain for four other comparisons with homecare.
Investigation of small-study effects
There were fewer than 10 studies in the network so small-study effects were not investigated.
Sensitivity analysis for risk of bias
After removing the one study for which we had very serious concerns about RoB there remained four studies (n = 1234) and five intervention groups in the sensitivity analysis. The point estimates and CIs were the same in comparisons with ac for the intervention groups that remained in the analysis.
Investigation of frailty
Because all study populations were categorised as frail it was not possible to investigate the effects of frailty for this analysis.
Long-term time frame
There were only two homecare comparisons in the long term (homecare and multifactorial-action vs. homecare, multifactorial-action and review; and homecare, multifactorial-action and review vs. homecare, multifactorial-action and review with self-management), so there was insufficient evidence to conduct an NMA. 103,147
Across time frames
In the short- and medium-term time frames, there was low-certainty evidence that homecare, ADL, multifactorial-action and review with self-management may result in a moderate or large reduction in the odds of living at home compared with homecare alone.
Independence in instrumental activities of daily living
Continuous IADL outcomes were analysed using the SMD. Where necessary, scales were transformed so that greater scores equated to greater independence in IADL. For each time frame there were two separate networks, one with ac reference comparator and one with a homecare comparator, which we describe in turn.
Available-care network
Short-term time frame
A total of six studies and seven intervention groups were analysed for the short-term IADL available-care network (see Table 10 for a summary of findings). 79,99,136,141,152,166 Intervention groups ranged from 595 participants (ac) to 24 (ADL, aids, education, exercise, multifactorial-action and review with medication-review and self-management). The populations were pre-frail, frail or both in all but one study. 79 All results were at high RoB (five: serious concerns, one: very serious concerns).
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: Independence in IADL Time frame: Short term; range of follow-up 24 weeks to 9 months Setting: Community Total studies: 6 Total participants: 1155 Comparator rank: Mean 4.4, 95% CI 2 to 6 |
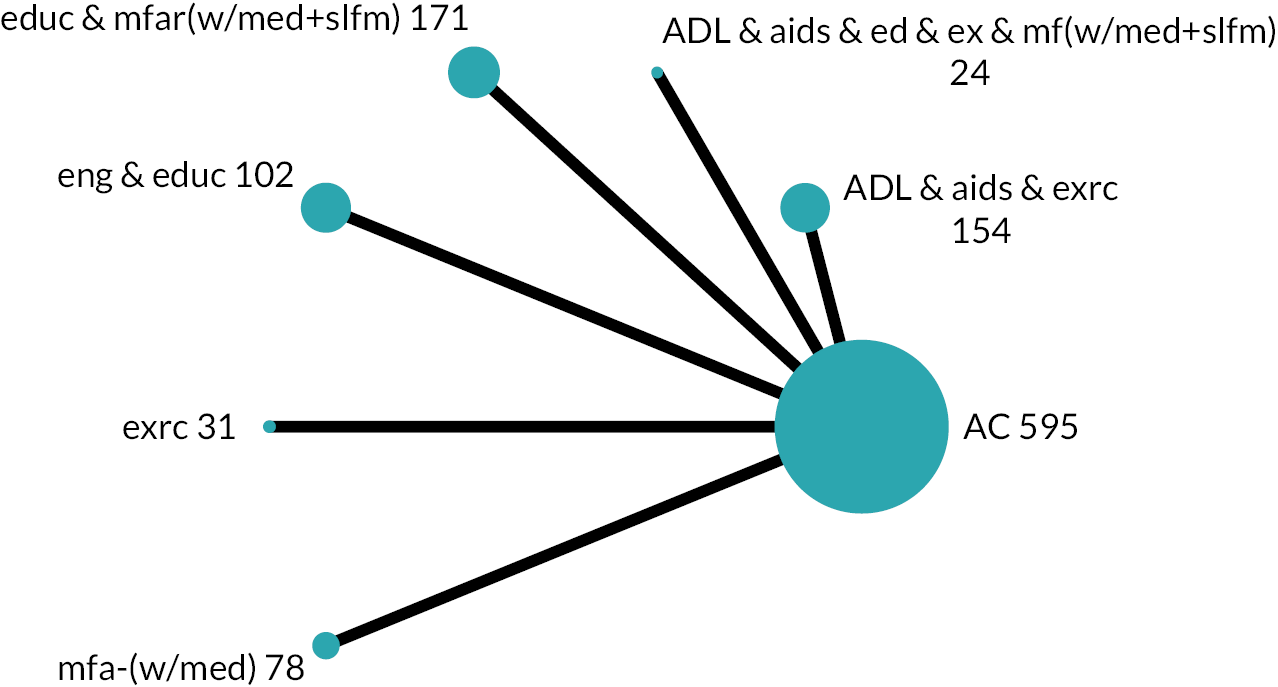
|
||||
|---|---|---|---|---|---|
| Intervention group | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | |
| SMD | MD (Lawton IADL 0–8)a | ||||
| Education, multifactorial-action and review with medication-review and self-management [educ & mfar(w/med + slfm)] | SMD 0.22 lower (0.45 lower to 0.00) Mixed estimate | MD 0.59 lower (1.17 lower to 0.01 lower) | ⊕⊕⊝⊝ Lowb,c |
6.5 (5 to 7) | May result in a slight reduction in IADL independence |
| ADL, aids, education, exercise, multifactorial-action and review with medication-review and self-management [ADL&aids&ed&ex&mf(w/med + slfm)] | SMD 0.38 higher (0.26 lower to 1.01 higher) Mixed estimate | MD 0.98 higher (0.69 lower to 2.66 higher) | ⊕⊝⊝⊝ Very lowb,d |
1.9 (1 to 7) | The evidence is very uncertain about the effect on IADL independence |
| ADL training, aids-adaptations and physical exercise (ADL & aids & exrc) | SMD 0.14 higher (0.09 lower to 0.36 higher) Mixed estimate | MD 0.36 higher (0.24 lower to 0.95 higher) | ⊕⊝⊝⊝ Very lowb,d |
2.8 (1 to 6) | The evidence is very uncertain about the effect on IADL independence |
| Engagement in meaningful-activities and education (eng & educ) | SMD 0.06 higher (0.18 lower to 0.30 higher) Mixed estimate | MD 0.16 higher (0.46 lower to 0.79 higher) | ⊕⊝⊝⊝ Very lowe,f |
3.5 (1 to 6) | The evidence is very uncertain about the effect on IADL independence |
| Exercise (exrc) | SMD 0.00 (0.60 lower to 0.60 higher) Mixed estimate | MD 0.00 (1.58 lower to 1.58 higher) | ⊕⊝⊝⊝ Very lowd,g |
4.2 (1 to 7) | The evidence is very uncertain about the effect on IADL independence |
| Multifactorial-action with medication-review [mfa-(w/med)] | SMD 0.05 lower (0.37 lower to 0.27 higher) Mixed estimate | MD 0.13 lower (0.98 lower to 0.71 higher) | ⊕⊝⊝⊝ Very lowb,d |
4.8 (2 to 7) | The evidence is very uncertain about the effect on IADL independence |
The network contained no loops and, therefore, the consistency assumption could not be tested, and a ‘consistency’ model was fitted. As the network contained only a single study for each comparison, there was no potential source of heterogeneity and therefore a common-effect model was fitted for the short-term IADL available-care network. There was low-certainty evidence that education, multifactorial-action and review with medication-review and self-management may result in a slight reduction in IADL independence (SMD −0.22, 95% CI −0.45 to 0.00). All other short-term estimates were of very low certainty due to imprecision and RoB.
The 95% CI for the true rank was typically wide, encompassing six of the seven ranks for all intervention groups except education, multifactorial-action and review with medication-review and self-management (95% CI 5 to 7).
Medium-term time frame
Overall characteristics
For the medium-term IADL available-care network there was a total of 16 studies (n = 5309) with 14 intervention groups in the network, including the largest node ac (n = 3136; see Table 11 for a summary of findings). 70,73,74,79,86,88,96,99,108,136,138,152,155,167,172,177 Within the network a single study provided a direct comparison for each experimental intervention group, except multifactorial-action and review with medication-review which has four studies. In the network, the number of participants ranged from 34 (aids) to 702 (multifactorial-action and review with medication-review) excluding the comparator ac. The populations of three studies spanned all frailty categories, one study was of a robust population, five a frail population, six studies covered the other categories and one was unclassifiable. There were some concerns regarding RoB in 1 study result, with the other 15 results being at high RoB (10 serious concerns, 5 very serious concerns).
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: Independence in IADL Time frame: Medium term; range of follow-up 10–18 months Setting: Community Total studies: 16 Total participants: 5309 Comparator rank: Mean 7.2, 95% CI 5 to 9 |
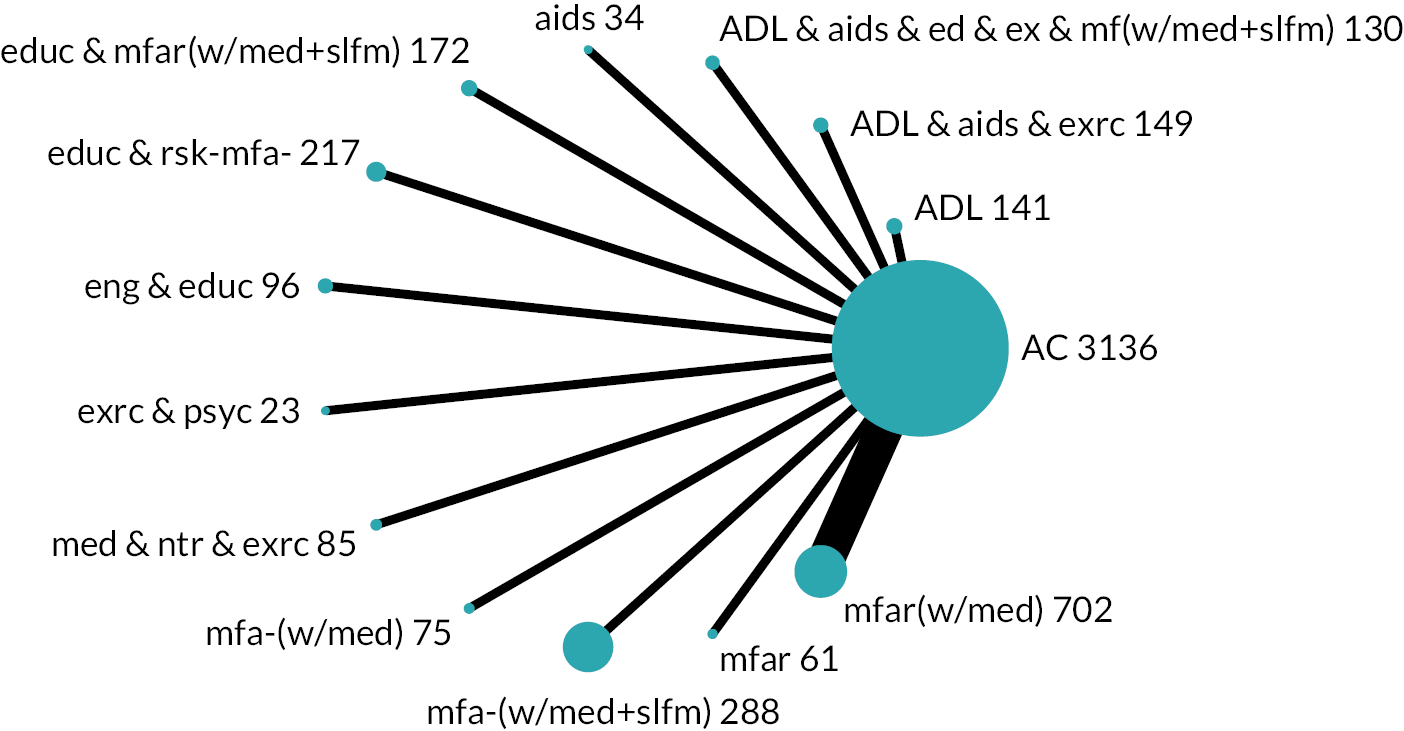
|
||||
|---|---|---|---|---|---|
| Intervention group | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | |
| SMD | MD (Lawton IADL 0–8)a | ||||
| Multifactorial-action and review with medication-review [mfar(w/med)] | SMD 0.11 higher (0.00 to 0.21 higher) Mixed estimate | MD 0.28 higher (0.01 higher to 0.55 higher) | ⊕⊕⊕⊝ Moderateb |
4.4 (2 to 7) | Probably results in a very slight increase in IADL independence |
| ADL, aids and exercise (ADL & aids & exrc) | SMD 0.19 lower (0.42 lower to 0.04 higher) Mixed estimate | MD 0.50 lower (1.11 lower to 0.11 higher) | ⊕⊕⊝⊝ Lowb,c |
11.2 (5 to 13) | May result in a slight reduction in IADL independence |
| ADL, aids, education, exercise, multifactorial-action and review with medication-review and self-management [ADL&aids&ed&ex&mf(w/med + slfm)] | SMD 0.56 lower (0.81 lower to 0.31 lower) Mixed estimate | MD 1.47 lower (2.12 lower to 0.82 lower) | ⊕⊕⊝⊝ Lowb,d |
13.9 (13 to 14) | May result in a reduction in IADL independence |
| Multifactorial-action and review (mfar) | SMD 0.50 higher (0.15 higher to 0.86 higher) Mixed estimate | MD 1.32 higher (0.38 higher to 2.26 higher) | ⊕⊝⊝⊝ Very lowe,f |
1.2 (1 to 4) | The evidence is very uncertain about the effect on IADL independence |
| Medication-review, nutrition and exercise (med & ntr & exrc) | SMD 0.21 higher (0.08 lower to 0.51 higher) Mixed estimate | MD 0.56 higher (0.22 lower to 1.34 higher) | ⊕⊝⊝⊝ Very lowb,g |
3.2 (1 to 10) | The evidence is very uncertain about the effect on IADL independence |
| ADL (ADL) | SMD 0.10 higher (0.12 lower to 0.33 higher) Mixed estimate | MD 0.27 higher (0.31 lower to 0.86 higher) | ⊕⊝⊝⊝ Very lowb,g |
4.9 (2 to 10) | The evidence is very uncertain about the effect on IADL independence |
| Multifactorial-action with medication-review [mfa-(w/med)] | SMD 0.02 higher (0.30 lower to 0.35 higher) Mixed estimate | MD 0.06 higher (0.79 lower to 0.92 higher) | ⊕⊝⊝⊝ Very lowb,g |
6.7 (2 to 13) | The evidence is very uncertain about the effect on IADL independence |
| Education and risk-screening (educ & rsk-mfa-) | SMD 0.00 (0.19 lower to 0.19 higher) Mixed estimate | MD 0.00 (0.50 lower to 0.50 higher) | ⊕⊝⊝⊝ Very lowb,g |
7.1 (3 to 12) | The evidence is very uncertain about the effect on IADL independence |
| Engagement in meaningful-activities and education (eng & educ) | SMD 0.01 lower (0.26 lower to 0.23 higher) Mixed estimate | MD 0.03 lower (0.68 lower to 0.61 higher) | ⊕⊝⊝⊝ Very lowh,i |
7.5 (2 to 13) | The evidence is very uncertain about the effect on IADL independence |
| Exercise and psychology (exrc & psyc) | SMD 0.12 lower (0.60 lower to 0.37 higher) Mixed estimate | MD 0.30 lower (1.58 lower to 0.98 higher) | ⊕⊝⊝⊝ Very lowi,j |
8.9 (2 to 14) | The evidence is very uncertain about the effect on IADL independence |
| Multifactorial-action with medication-review and self-management [mfa-(w/med + slfm)] | SMD 0.07 lower (0.20 lower to 0.06 higher) Mixed estimate | MD 0.19 lower (0.53 lower to 0.15 higher) | ⊕⊝⊝⊝ Very lowi,k |
9.2 (5 to 12) | The evidence is very uncertain about the effect on IADL independence |
| Aids (aids) | SMD 0.15 lower (0.60 lower to 0.30 higher) Mixed estimate | MD 0.39 lower (1.56 lower to 0.78 higher) | ⊕⊝⊝⊝ Very lowi,k |
9.6 (2 to 14) | The evidence is very uncertain about the effect on IADL independence |
| Education, multifactorial-action and review with medication-review and self-management [educ & mfar(w/med + slfm)] | SMD 0.13 lower (0.35 lower to 0.09 higher) Mixed estimate | MD 0.34 lower (0.92 lower to 0.24 higher) | ⊕⊝⊝⊝ Very lowb,g |
10.1 (5 to 13) | The evidence is very uncertain about the effect on IADL independence |
Main analysis
The network contained no loops and therefore the consistency assumption could not be tested and a ‘consistency’ model was fitted. Between-study variance was estimated to be very small but non-zero, indicating some degree of heterogeneity between studies included in the network (τ = 6.91 × 10−8). On average, multifactorial-action and review with medication-review was associated with very slightly increased independence in IADL versus ac (SMD 0.11, 95% CI 0.00 to 0.21; moderate-certainty evidence). ADL, aids and exercise may result in a slight reduction in independence in IADL versus ac (SMD −0.19, 95% CI −0.42 to 0.04; low-certainty evidence). ADL, aids, education, exercise, multifactorial-action and review with medication-review and self-management may result in a reduction in independence in IADL versus ac [SMD −0.56 (moderate), 95% CI −0.81 to −0.31; low-certainty evidence]. Other comparisons with ac were of very low certainty due to imprecision and RoB.
The 95% CI for the true ranking for multifactorial-action and review with medication-review placed it in the top half of the interventions (2–7) and ac was ranked 5th–9th. Multifactorial-action and review was ranked in the top four but note that its comparison with ac was very low certainty.
Investigation of small-study effects
The comparison-adjusted funnel plot appeared symmetric, implying no evidence of small-study effects in the network.
Sensitivity analysis for risk of bias
After removing the 3 study results with very serious concerns about RoB for the medium-term IADL available-care network, 5 studies70,79,108,172,177 were dropped from the network due to very serious concerns of bias leaving 11 studies and 9 nodes. The inconsistency model could not run because there was no source of inconsistency.
The point estimates and CIs for the three comparisons with ac that were not very low certainty in the main analyses remained the same in sensitivity analyses. This was also the case for all other intervention groups that remained in the analysis.
Investigation of frailty
All frailty categories were represented in the network and a network meta regression model was fitted including the frailty variable with ‘all (robust, pre-frail and frail)’ set as the reference category. The network contained no loops and therefore the consistency assumption could not be tested and a ‘consistency’ model was fitted. Between-study variance remained very small, but non-zero (τ = 1.215 × 10−7). Frailty effects could not be estimated for most contrasts due to collinearity. Where frailty effects could be estimated (only multifactorial-action and review with medication-review vs. ac), the effect was estimated with uncertainty, with a 95% CI covering both beneficial and harmful effects (SMD 0.04, 95% CI −0.02 to 0.11).
Long-term time frame
Six studies and five intervention groups were analysed in the long-term IADL available-care network, incorporating 1727 participants (see Table 12 for a summary of findings). 73,115,136,155,162,172 The available-care node was the largest (n = 858) and aids the smallest (n = 34).
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: IADL (higher is better) Time frame: Long term; range of follow-up 24–36 months Setting: Community Total studies: 6 Total participants: 1727 Comparator rank: Mean 3.4, 95% CI 2 to 5 |
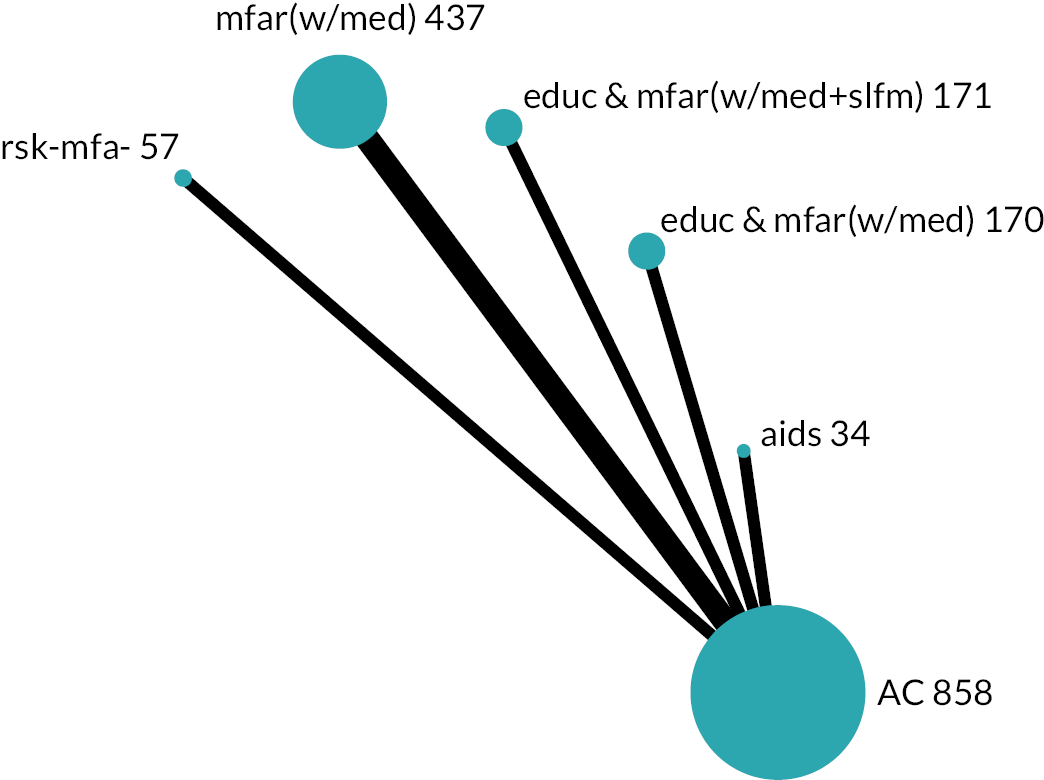
|
||||
|---|---|---|---|---|---|
| Intervention group | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | |
| SMD | MD (Lawton IADL 0–8)a | ||||
| Multifactorial-action and review with medication-review [mfar(w/med)] | SMD 0.08 lower (0.21 lower to 0.05 higher) Mixed estimate | MD 0.21 lower (0.56 lower to 0.13 higher) | ⊕⊕⊕⊝ Moderateb |
4.5 (3 to 6) | Probably results in a very slight reduction in IADL |
| Risk-screening (rsk-mfa-) | SMD 0.23 higher (0.13 lower to 0.60 higher) Mixed estimate | MD 0.61 higher (0.35 lower to 1.57 higher) | ⊕⊕⊝⊝ Lowc |
1.7 (1 to 5) | May result in a slight increase in IADL |
| Education, multifactorial-action and review with medication-review [educ & mfar(w/med)] | SMD 0.14 higher (0.08 lower to 0.36 higher) Mixed estimate | MD 0.37 higher (0.21 lower to 0.95 higher) | ⊕⊕⊝⊝ Lowc |
2.1 (1 to 5) | May result in a very slight increase in IADL |
| Education, multifactorial-action and review with medication-review and self-management [educ & mfar(w/med + slfm)] | SMD 0.21 lower (0.44 lower to 0.01 higher) Mixed estimate | MD 0.56 lower (1.14 lower to 0.02 higher) | ⊕⊕⊝⊝ Lowb,d |
5.5 (4 to 6) | May result in a slight reduction in IADL |
| Aids (aids) | SMD 0.03 lower (0.48 lower to 0.42 higher) Mixed estimate | MD 0.07 lower (1.25 lower to 1.10 higher) | ⊕⊝⊝⊝ Very lowe,f |
3.8 (1 to 6) | The evidence is very uncertain about the effect on IADL |
The network contained no loops and, therefore, the consistency assumption could not be tested and a ‘consistency’ model was fitted. Between-study variance was estimated to be very small but non-zero, indicating some degree of heterogeneity between studies included in the network (τ = 5.31 × 10−6). There was moderate-certainty evidence that multifactorial-action and review with medication-review was associated with a very slight reduction in IADL independence (SMD −0.08, 95% CI −0.21 to 0.05). There was low-certainty evidence that risk-screening (SMD 0.23, 95% CI −0.13 to 0.60); and education, multifactorial-action and review with medication-review (SMD 0.14, 95% CI −0.08 to 0.36) each increased IADL independence (slightly and very slightly, respectively). There was low-certainty evidence that education, multifactorial-action and review with medication-review and self-management (SMD −0.21, 95% CI −0.44 to 0.01) was associated with a slight reduction in IADL independence.
For the long-term IADL available-care network, the 95% CI for the true ranking placed ac 2nd–5th, multifactorial-action and review with medication-review in the bottom four, and education, multifactorial-action and review with medication-review and self-management in the bottom three. For the other three interventions, the 95% CI covered at least five of the six places.
Across time frame
There was moderate-certainty evidence that multifactorial-action and review with medication-review was associated with a very slight increase in IADL independence in the medium term but also a very slight reduction in IADL in the long term. There was low-certainty evidence that education, multifactorial-action and review with medication-review and self-management was associated with a slight reduction in IADL independence in the short and long term.
Homecare network
Short-term time frame
In the short-term IADL homecare network, there were a total of four studies and five intervention groups, with the homecare node having the largest number of participants (n = 301) and homecare, multifactorial-action and review the smallest (n = 121; see Table 13 for a summary of findings). 65,87,117,146,147 Three study populations were frail and one was pre-frail and frail. Two results were at high RoB (serious concerns) and two results were rated as some concerns.
| Population: Older people Interventions: Community-based complex interventions Comparator: Formal homecare (hmcr) Outcome: Independence in IADL (higher is better) Time frame: Short term; range of follow-up 6–7 months Setting: Community Total studies: 4 Total participants: 970 Comparator rank: Mean 2.5, 95% CI 1 to 4 |
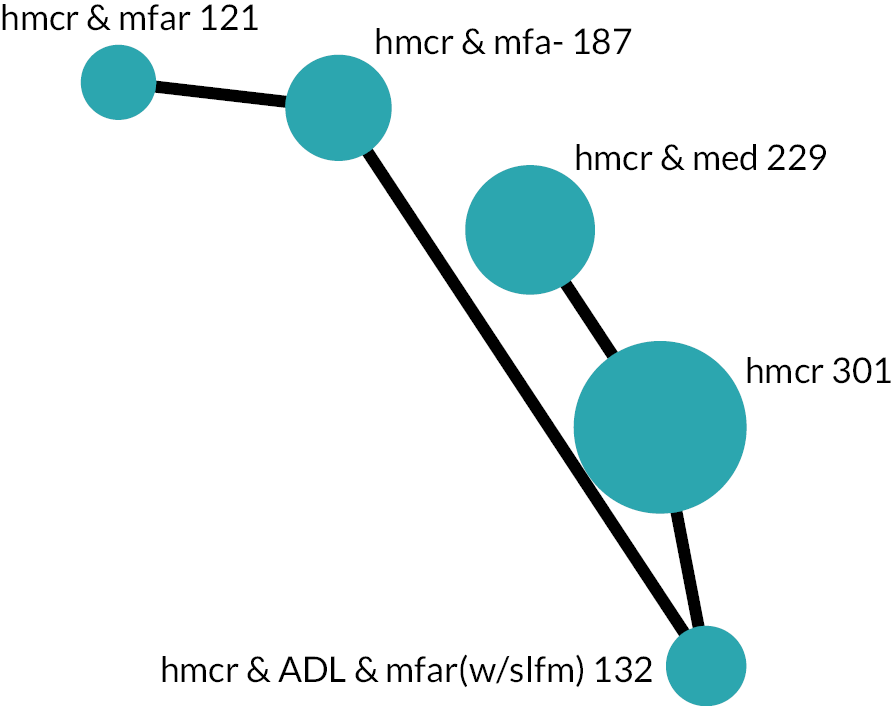
|
||||
|---|---|---|---|---|---|
| Intervention group | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | |
| SMD | MD (Lawton IADL 0–8)a | ||||
| Homecare, ADL, multifactorial-action and review with self-management [hmcr & ADL & mfar(w/slfm)] | SMD 0.02 lower (0.33 lower to 0.29 higher) Mixed estimate | MD 0.06 lower (0.88 lower to 0.76 higher) | ⊕⊕⊝⊝ Lowb |
2.8 (1 to 5) | May result in little to no difference in IADL independence |
| Homecare, multifactorial-action and review (hmcr & mfar) | SMD 0.04 higher (0.51 lower to 0.58 higher) Indirect estimate | MD 0.09 higher (1.33 lower to 1.52 higher) | ⊕⊝⊝⊝ Very lowb,c |
2.4 (1 to 5) | The evidence is very uncertain about the effect on IADL independence |
| Homecare and multifactorial-action (hmcr & mfa-) | SMD 0.07 lower (0.55 lower to 0.42 higher) Indirect estimate | MD 0.18 lower (1.44 lower to 1.09 higher) | ⊕⊝⊝⊝ Very lowb,d |
3.4 (1 to 5) | The evidence is very uncertain about the effect on IADL independence |
| Homecare and medication-review (hmcr & med) | SMD 0.13 lower (0.31 lower to 0.06 higher) Mixed estimate | MD 0.33 lower (0.82 lower to 0.15 higher) | ⊕⊝⊝⊝ Very lowb,e |
3.9 (1 to 5) | The evidence is very uncertain about the effect on IADL independence |
The network contained no loops and therefore the consistency assumption could not be tested and a ‘consistency’ model was fitted. As the network contained only a single study for each comparison, there was no potential source of heterogeneity and therefore a common-effect model was fitted.
One comparison with homecare had low certainty. Homecare, ADL, multifactorial-action and review with self-management may result in little to no difference in IADL independence (SMD −0.02, 95% CI −0.33 to 0.29; low certainty). There were no serious concerns about RoB but the CIs for all estimated effects included benefit and harm.
There was little difference in the mean rank of intervention groups.
Medium-term time frame
Overall characteristics
For the medium-term IADL homecare network there were six studies (n = 1401) and five intervention groups (see Appendix 3, Table 24 for a summary of findings). 68,131,146,147,154,189 The largest node was homecare (n = 489). Within the network the number of participants ranged from 48 (homecare and aids) to 367 (homecare, multifactorial-action and review with medication-review) excluding the homecare comparator. All participant populations were frail. All results were at high RoB (four: serious concerns, two: very serious concerns), primarily due to missing outcome data.
Main analysis
The network contained no loops and therefore the consistency assumption could not be tested and a ‘consistency’ model was fitted. Between-study variance was estimated to be small but non-zero, indicating some degree of heterogeneity between studies included in the network (τ = 0.141). All comparisons with homecare were rated very low certainty due to serious RoB and very serious imprecision.
For the medium-term IADL homecare network, the 95% CI for the true rank covered at least five of the six positions for all intervention groups.
Investigation of small-study effects
There were fewer than 10 studies in the network so small-study effects were not investigated.
Sensitivity analysis for risk of bias
For the medium-term IADL homecare network, Parsons M 2017146 and Rooijackers 2021154 were dropped from the network, leaving four studies and three nodes and leading to a disconnected network.
Investigation of frailty
Because all study populations were categorised as frail it was not possible to investigate the effects of frailty for this analysis.
Long-term time frame
It was not possible to conduct analysis of IADL in the long term for the homecare network as it only had two intervention groups. 146,147
Across Time frames
The only evidence that was not very uncertain was from the short-term time frame.
Independence in personal activities of daily living
Continuous PADL outcomes were analysed using the SMD. Where necessary, scales were transformed so that greater scores equated to greater independence in PADL. For each time frame there were two separate networks, one with available-care reference comparator and one with a homecare comparator, which we describe in turn. We did not analyse combined PADL and IADL measures; study results are provided in Report Supplementary Material 9.
Available-care network
Short-term time frame
A total of eight trials (n = 4075 participants) compared a short-term PADL outcome across nine intervention groups including ac, which was the largest node (see Table 14 for a summary of findings). 69,79,99,136,152,166,168,186 There was a maximum of one trial for a single direct comparison (compared to ac), with the number of participants within any one intervention group in the network ranging from 24 to 1983. All studies were at high RoB (five serious concerns, three very serious concerns).
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: Independence in PADL Time frame: Short term; range of follow-up 24 weeks to 9 months Setting: Community Total studies: 8 Total participants: 4075 Comparator rank: Mean 5.6, 95% CI 4 to 8 |
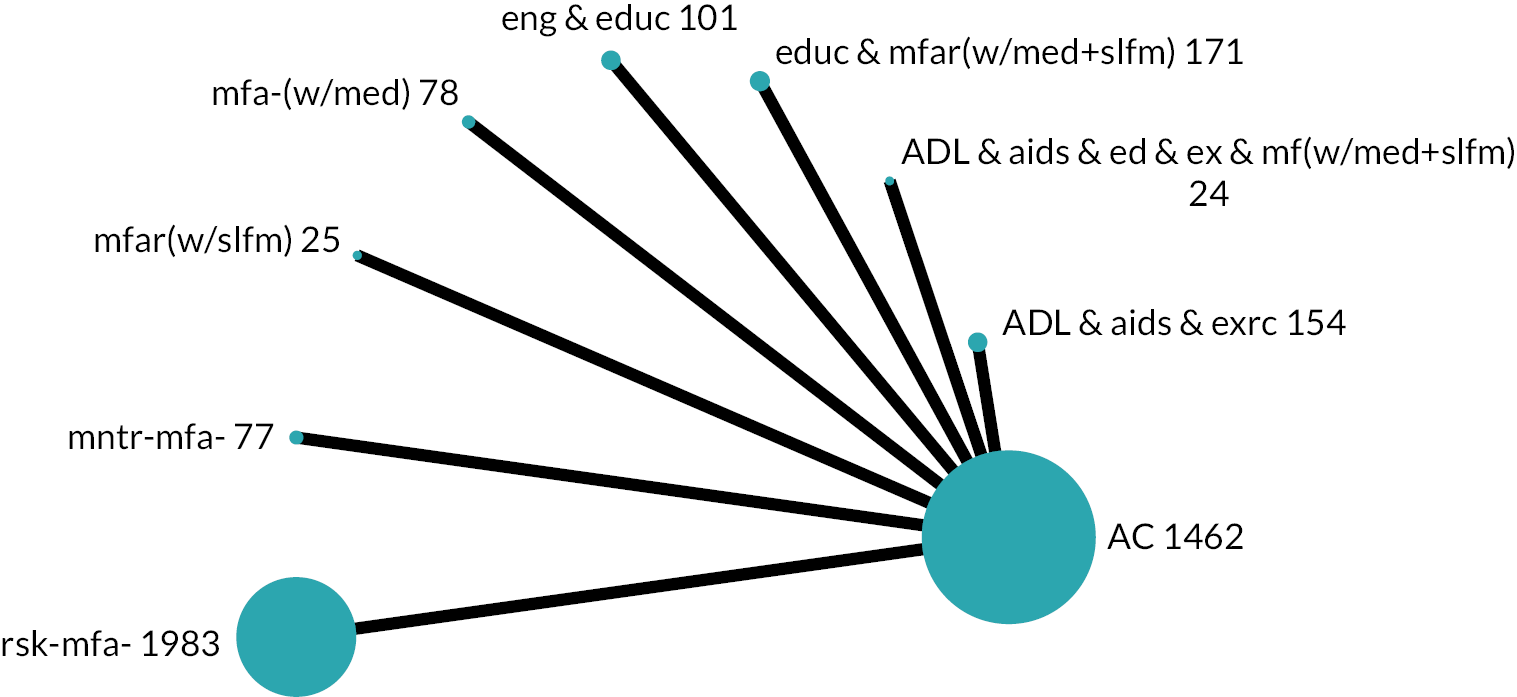
|
||||
|---|---|---|---|---|---|
| Intervention group | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | |
| SMD | MD (Barthel Index 0–100)a | ||||
| Education, multifactorial-action and review with medication-review and self-management [educ & mfar(w/med + slfm)] | SMD 0.25 lower (0.47 lower to 0.03 lower) Mixed estimate | MD 7.34 lower (13.91 lower to 0.76 lower) | ⊕⊕⊝⊝ Lowb,c |
8.4 (6 to 9) | May result in a slight reduction in PADL independence |
| ADL, aids, education, exercise, multifactorial-action and review with medication-review and self-management [ADL&aids&ed&ex&mf(w/med + slfm)] | SMD 0.87 higher (0.21 higher to 1.54 higher) Mixed estimate | MD 25.81 higher (6.17 higher to 45.46 higher) | ⊕⊝⊝⊝ Very lowb,d |
1.4 (1 to 2) | The evidence is very uncertain about the effect on PADL independence |
| Multifactorial-action and review with self-management [mfar(w/slfm)] | SMD 0.67 higher (0.09 higher to 1.26 higher) Mixed estimate | MD 19.94 higher (2.67 higher to 37.21 higher) | ⊕⊝⊝⊝ Very lowe,f |
1.8 (1 to 4) | The evidence is very uncertain about the effect on PADL independence |
| ADL, aids and exercise (ADL & aids & exrc) | SMD 0.14 higher (0.09 lower to 0.36 higher) Mixed estimate | MD 4.03 higher (2.68 lower to 10.74 higher) | ⊕⊝⊝⊝ Very lowb,g |
3.7 (2 to 7) | The evidence is very uncertain about the effect on PADL independence |
| Risk-screening (rsk-mfa-) | SMD 0.03 higher (0.06 lower to 0.11 higher) Mixed estimate | MD 0.75 higher (1.71 lower to 3.21 higher) | ⊕⊝⊝⊝ Very lowh,i |
4.9 (3 to 7) | The evidence is very uncertain about the effect on PADL independence |
| Engagement in meaningful-activities and education (eng & educ) | SMD 0.00 (0.24 lower to 0.24 higher) Mixed estimate | MD 0.00 (7.07 lower to 7.07 higher) | ⊕⊝⊝⊝ Very lowi,j |
5.5 (3 to 9) | The evidence is very uncertain about the effect on PADL independence |
| Monitoring (mntr-mfa-) | SMD 0.09 lower (0.40 lower to 0.21 higher) Mixed estimate | MD 2.76 lower (11.79 lower to 6.28 higher) | ⊕⊝⊝⊝ Very lowb,d |
6.7 (3 to 9) | The evidence is very uncertain about the effect on PADL independence |
| Multifactorial-action with medication-review [mfa-(w/med)] | SMD 0.11 lower (0.44 lower to 0.21 higher) Mixed estimate | MD 3.38 lower (12.94 lower to 6.18 higher) | ⊕⊝⊝⊝ Very lowb,d |
7.0 (3 to 9) | The evidence is very uncertain about the effect on PADL independence |
The network contained no loops and therefore the consistency assumption could not be tested and a ‘consistency’ model was fitted, setting the inconsistency parameter in the model to zero for all comparisons. The network also contained only a single study for each comparison; hence there was no potential source of heterogeneity and, therefore, a common-effect model was fitted.
All but one comparison with ac was judged of very low certainty. Education, multifactorial-action and review with medication-review and self-management may result in a slight reduction in PADL independence (SMD −0.25, 95% CI −0.47 to −0.03; low certainty).
The 95% CI for the true ranking placed ac 4th–8th (of nine); and education, multifactorial-action and review with medication-review and self-management in the bottom four intervention groups. ADL, aids, education, exercise, multifactorial-action and review with medication-review and self-management ranked in the top two and multifactorial-action and review with self-management ranked in the top four, although note that for these intervention groups the evidence from their comparison with ac was very low certainty.
Medium-term time frame
Overall characteristics
A total of 20 trials (n = 8583 participants) compared a medium-term PADL outcome across 16 intervention groups including ac, which was the largest node (see Table 15 for a summary of findings). 69,70,73–75,79,86,88,96,108,118,136,138,142,152,155,157,167,168,177 There was a maximum of four trials for a single direct comparison [multifactorial-action and review with medication-review vs. ac], with the number of participants within any one intervention group in the network ranging from 23 to 4080. The populations of three studies spanned all frailty categories, one study was of a robust population, six a frail population, eight studies covered the other categories and two were unclassifiable. We had some concerns about RoB in two studies with the remainder at high risk of bias (12 serious concerns, 6 very serious concerns).
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: PADL (higher is better) Time frame: Medium term; range of follow-up 10–18 months Setting: Community Total studies: 20 Total participants: 8583 Comparator rank: Mean 9.2, 95% CI 6 to 12 |
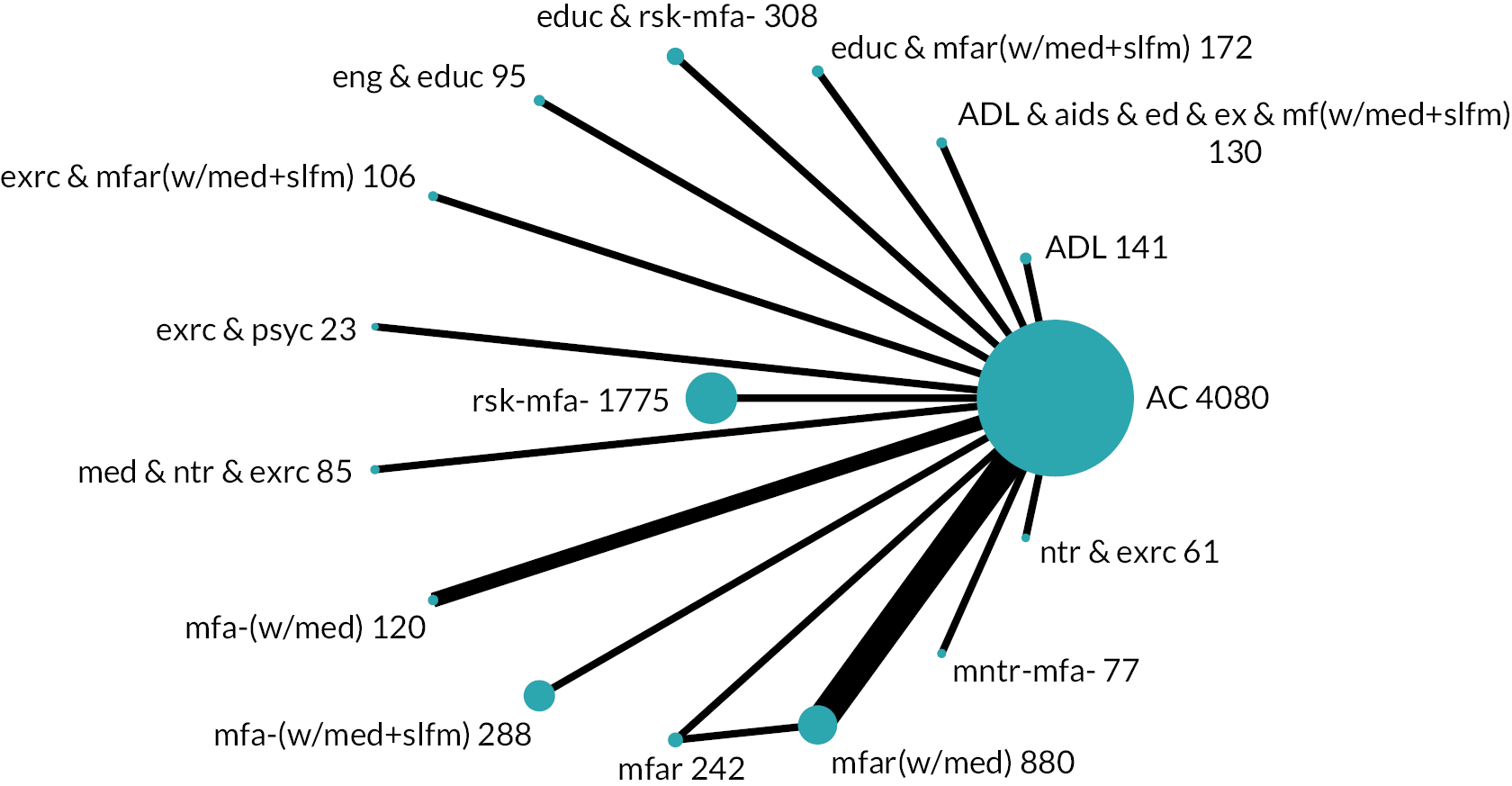
|
||||
|---|---|---|---|---|---|
| Intervention group | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | |
| SMD | MD (Barthel Index 0–100)a | ||||
| Exercise, multifactorial-action and review with medication-review and self-management [exrc & mfar(w/med + slfm)] | SMD 0.16 higher (0.51 lower to 0.82 higher) Mixed estimate | MD 4.68 higher (15.01 lower to 24.37 higher) | ⊕⊕⊝⊝ Lowb |
6.6 (1 to 16) | May result in a very slight increase in PADL independence |
| Multifactorial-action with medication-review [mfa-(w/med)] | SMD 0.51 higher (0.00 to 1.02 higher) Mixed estimate | MD 15.08 higher (0.14 lower to 30.30 higher) | ⊕⊝⊝⊝ Very lowc,d,e |
2.9 (1 to 10) | The evidence is very uncertain about the effect on PADL independence |
| Medication-review, nutrition and exercise (med & ntr & exrc) | SMD 0.31 higher (0.37 lower to 0.99 higher) Mixed estimate | MD 9.27 higher (10.81 lower to 29.36 higher) | ⊕⊝⊝⊝ Very lowb,c |
5.3 (1 to 15) | The evidence is very uncertain about the effect on PADL independence |
| ADL (ADL) | SMD 0.22 higher (0.42 lower to 0.87 higher) Mixed estimate | MD 6.64 higher (12.55 lower to 25.84 higher) | ⊕⊝⊝⊝ Very lowb,c |
6.3 (1 to 15) | The evidence is very uncertain about the effect on PADL independence |
| Risk-screening (rsk-mfa-) | SMD 0.13 higher (0.48 lower to 0.75 higher) Mixed estimate | MD 3.86 higher (14.34 lower to 22.06 higher) | ⊕⊝⊝⊝ Very lowf,g |
7.4 (1 to 16) | The evidence is very uncertain about the effect on PADL independence |
| ADL, aids, education, exercise, multifactorial-action and review with medication-review and self-management [ADL&aids&ed&ex&mf(w/med + slfm)] | SMD 0.07 higher (0.59 lower to 0.73 higher) Mixed estimate | MD 2.07 higher (17.33 lower to 21.47 higher) | ⊕⊝⊝⊝ Very lowb,c |
8.1 (1 to 16) | The evidence is very uncertain about the effect on PADL independence |
| Multifactorial-action and review with medication-review [mfar(w/med)] | SMD 0.05 higher (0.26 lower to 0.36 higher) Mixed estimate | MD 1.40 higher (7.71 lower to 10.51 higher) | ⊕⊝⊝⊝ Very lowb,c |
8.4 (3 to 14) | The evidence is very uncertain about the effect on PADL independence |
| Exercise and psychology (exrc & psyc) | SMD 0.00 (0.78 lower to 0.78 higher) Mixed estimate | MD 0.00 (23.09 lower to 23.09 higher) | ⊕⊝⊝⊝ Very lowg,h |
9.0 (1 to 16) | The evidence is very uncertain about the effect on PADL independence |
| Nutrition and exercise (ntr & exrc) | SMD 0.00 (0.70 lower to 0.70 higher) Mixed estimate | MD 0.00 (20.65 lower to 20.65 higher) | ⊕⊝⊝⊝ Very lowb,c |
9.1 (1 to 16) | The evidence is very uncertain about the effect on PADL independence |
| Multifactorial-action with medication-review and self-management [mfa-(w/med + slfm)] | SMD 0.04 lower (0.66 lower to 0.58 higher) Mixed estimate | MD 1.18 lower (19.60 lower to 17.24 higher) | ⊕⊝⊝⊝ Very lowf,g |
9.5 (2 to 16) | The evidence is very uncertain about the effect on PADL independence |
| Education and risk-screening (educ & rsk-mfa-) | SMD 0.03 lower (0.66 lower to 0.60 higher) Mixed estimate | MD 0.95 lower (19.56 lower to 17.66 higher) | ⊕⊝⊝⊝ Very lowg,i |
9.6 (2 to 16) | The evidence is very uncertain about the effect on PADL independence |
| Engagement in meaningful-activities and education (eng & educ) | SMD 0.05 lower (0.71 lower to 0.61 higher) Mixed estimate | MD 1.42 lower (20.87 lower to 18.03 higher) | ⊕⊝⊝⊝ Very lowg,j |
10.1 (2 to 16) | The evidence is very uncertain about the effect on PADL independence |
| Monitoring (mntr-mfa-) | SMD 0.17 lower (0.85 lower to 0.51 higher) Mixed estimate | MD 5.14 lower (25.30 lower to 15.02 higher) | ⊕⊝⊝⊝ Very lowb,c |
10.9 (3 to 16) | The evidence is very uncertain about the effect on PADL independence |
| Multifactorial-action and review (mfar) | SMD 0.14 lower (0.65 lower to 0.36 higher) Mixed estimate | MD 4.21 lower (19.14 lower to 10.72 higher) | ⊕⊝⊝⊝ Very lowb,c |
11.2 (3 to 16) | The evidence is very uncertain about the effect on PADL independence |
| Education, multifactorial-action and review with medication-review and self-management [educ & mfar(w/med + slfm)] | SMD 0.27 lower (0.92 lower to 0.38 higher) Mixed estimate | MD 7.91 lower (27.09 lower to 11.27 higher) | ⊕⊝⊝⊝ Very lowb,c |
12.5 (3 to 16) | The evidence is very uncertain about the effect on PADL independence |
Main analysis
The consistency assumption was found to hold for the network according to both the global Wald test (p = 0.473) and the node-splitting method (all contrasts p > 0.05). Between-study variance was estimated to be moderate indicating potentially large heterogeneity between studies reporting the same contrasts included in the network (τ = 0.310). One comparison was judged low certainty. Exercise, multifactorial-action and review with medication-review and self-management may result in a very slight increase in PADL (SMD 0.16, 95% CI −0.51 to 0.82). The other comparisons with ac were of very low certainty.
In the available-care network for the medium-term PADL outcome, ac ranked 6th–12th, multifactorial-action with medication-review ranked highest (95% CI 1 to 10). Other rankings had even wider CIs.
Investigation of small-study effects
The comparison-adjusted funnel plot appeared symmetric, implying no evidence of small-study effects in the network.
Sensitivity analysis for risk of bias
In sensitivity analysis, six trials were excluded due to very serious concerns about RoB and this left 14 trials included in the NMA. The network contained no loops as seen in the short-term network, and therefore the consistency assumption could not be tested and a ‘consistency’ model was fitted. Results were very similar as to the main analysis, with most point estimates being identical and the CI marginally wider. None of the changes affected our confidence in the estimates.
Investigation of frailty
A total of 18 trials remained in the NMA that were classifiable for frailty. All frailty categories were represented in the network and a network meta regression model was fitted including the frailty variable with ‘all (robust, pre-frail and frail)’ set as the reference category. The consistency assumption remained valid (global Wald test p = 0.99, node-splitting method showed all contrasts p > 0.05). Between-study variance remained very small, but non-zero (τ = 6.64 × 10−7). Frailty effects could not be estimated for most contrasts due to collinearity. Where frailty effects could be estimated (multifactorial-action and review; multifactorial-action and review with medication-review; and risk-screening, vs. ac), the effects were estimated with large uncertainty, with a 95% CIs covering both beneficial and harmful effects (e.g. for available-care vs. risk-screening SMD −0.031, 95% CI −68.04 to 67.98) making interpretation of these results meaningless in practice.
Long-term time frame
A total of seven trials (n = 2095 participants) compared a long-term PADL outcome across seven intervention groups including ac, which was the largest node (see Table 16 for a summary of findings). 66,73,115,118,136,155,162 There were a maximum of two trials for a single direct comparison (multifactorial-action and review with medication-review compared to ac), with the number of participants within any one intervention group in the network ranging from 40 to 860. There were some concerns about RoB in three study results, the rest being at high RoB (three with serious concerns, one with very serious concerns).
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: Independence in PADL Time frame: Long term; range of follow-up 24–36 months Setting: Community Total studies: 7 Total participants: 2095 Comparator rank: Mean 3.0, 95% CI 1 to 5 |
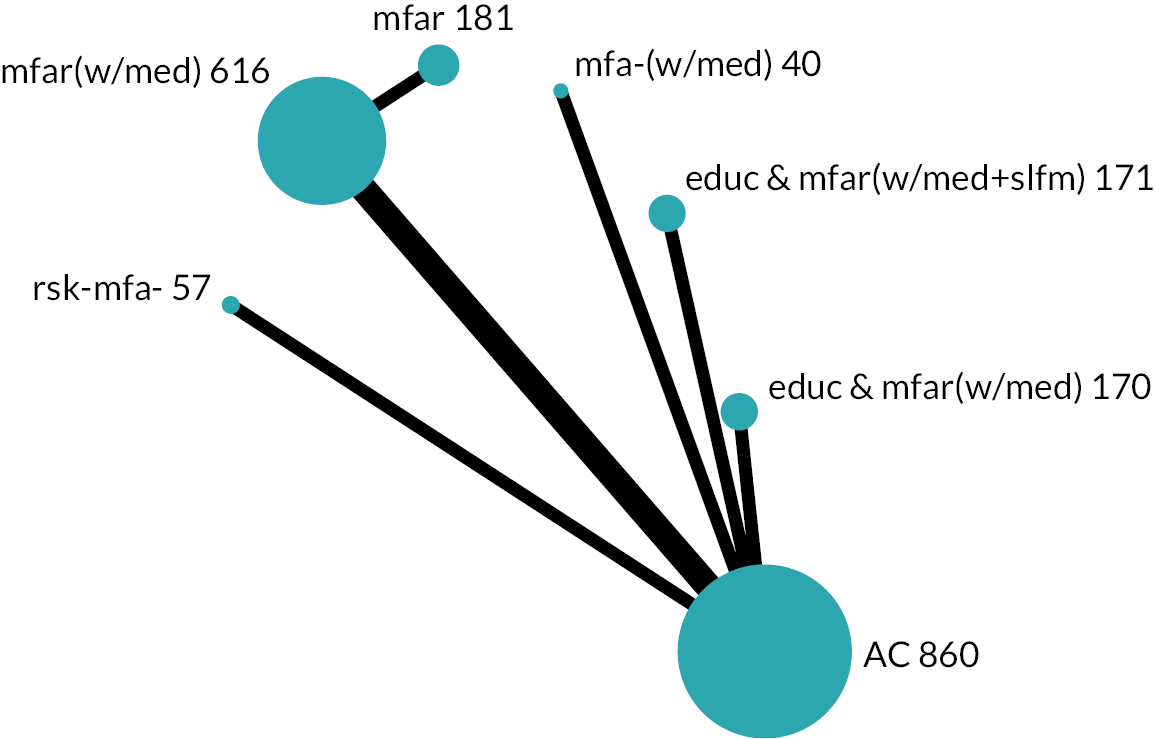
|
||||
|---|---|---|---|---|---|
| Intervention group | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | |
| SMD | MD (Barthel Index 0–100)a | ||||
| Education, multifactorial-action and review with medication-review [educ & mfar(w/med)] | SMD 0.11 higher (0.11 lower to 0.33 higher) Mixed estimate | MD 3.21 higher (3.33 lower to 9.75 higher) | ⊕⊕⊝⊝ Lowb |
1.9 (1 to 5) | May result in a very slight increase in PADL independence |
| Risk-screening (rsk-mfa-) | SMD 0.06 higher (0.30 lower to 0.43 higher) Mixed estimate | MD 1.85 higher (8.92 lower to 12.63 higher) | ⊕⊕⊝⊝ Lowb |
2.5 (1 to 6) | May result in a very slight increase in PADL independence |
| Multifactorial-action and review with medication-review [mfar(w/med)] | SMD 0.03 lower (0.16 lower to 0.10 higher) Mixed estimate | MD 0.79 lower (4.66 lower to 3.08 higher) | ⊕⊕⊝⊝ Lowb |
3.5 (1 to 5) | May result in little to no difference in PADL independence |
| Education, multifactorial-action and review with medication-review and self-management [educ & mfar(w/med + slfm)] | SMD 0.27 lower (0.50 lower to 0.05 lower) Mixed estimate | MD 8.07 lower (14.65 lower to 1.49 lower) | ⊕⊕⊝⊝ Lowc,d |
5.8 (4 to 7) | May result in a slight reduction in PADL independence |
| Multifactorial-action and review (mfar) | SMD 0.37 lower (0.62 lower to 0.13 lower) Indirect estimate | MD 11.06 lower (18.34 lower to 3.79 lower) | ⊕⊕⊝⊝ Lowc,e |
6.5 (5 to 7) | May result in a slight reduction in PADL independence |
| Multifactorial-action with medication-review mfa-(w/med) | SMD 0.17 lower (0.60 lower to 0.25 higher) Mixed estimate | MD 5.09 lower (17.66 lower to 7.48 higher) | ⊕⊝⊝⊝ Very lowf,g |
4.8 (1 to 7) | The evidence is very uncertain about the effect on PADL independence |
The consistency assumption was tested, and no violation of assumption was found (p = 0.736). Between-study variance was estimated to be very small but non-zero, indicating some degree of heterogeneity between studies included in the network (τ = 3.87 × 10−8). The five following comparisons with ac were judged low certainty. Education, multifactorial-action and review with medication-review may result in a very slight increase in PADL independence (SMD 0.11, 95% CI −0.11 to 0.33), as may risk-screening (SMD 0.06, 95% CI −0.30 to 0.43). Multifactorial-action and review with medication-review may result in little to no difference in PADL independence (SMD −0.03, 95% CI −0.16 to 0.10). Education, multifactorial-action and review with medication-review and self-management may result in a slight reduction in PADL independence (SMD −0.27, 95% CI −0.50 to −0.05). Multifactorial-action and review may also result in a slight reduction in PADL independence in the long term (SMD −0.37, 95% CI −0.62 to −0.13).
Available care; education, multifactorial-action and review with medication-review; and multifactorial-action and review with medication-review were all ranked in the top five according to the 95% CI for the true ranking. Education, multifactorial-action and review with medication-review and self-management; and multifactorial-action and review, had 95% CIs of four to seven and five to seven, respectively.
Across time frames
There was low-certainty evidence that education, multifactorial-action and review with medication-review and self-management [educ & mfar(w/med + slfm)] may result in a slight reduction in PADL independence in both the short- and long-term time frames.
Homecare network
Short-term time frame
A total of four trials (n = 775 participants) compared a short-term PADL outcome across five intervention groups including homecare, which was the largest node (see Table 17 for a summary of findings). 65,91,117,169 There was a maximum of one trial for a single direct comparison (compared to homecare), with the number of participants within any one intervention group in the network ranging from 29 to 378.
| Population: Older people Interventions: Community-based complex interventions Comparator: Formal homecare (hmcr) Outcome: Independence in PADL Time frame: Short term; range of follow-up 6–7 months Setting: Community Total studies: 4 Total participants: 775 Comparator rank: Mean 3.4, 95% CI 2 to 5 |
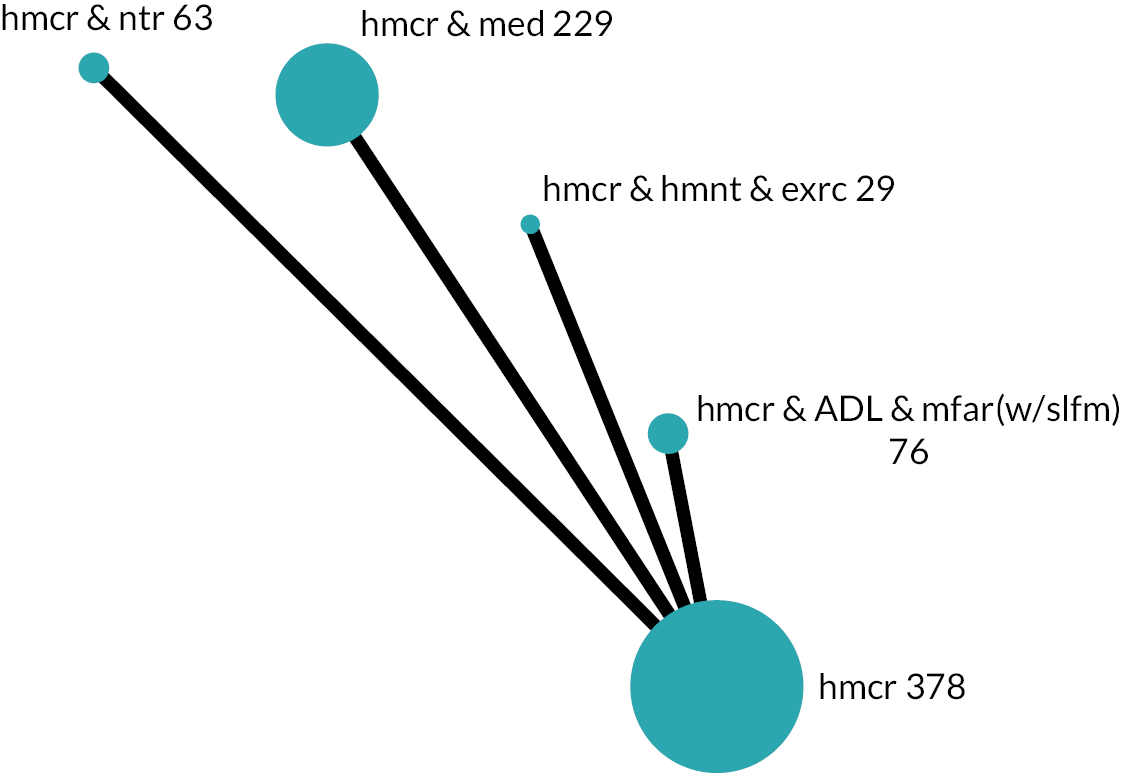
|
||||
|---|---|---|---|---|---|
| Intervention group | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | |
| SMD | MD (Barthel Index 0–100)a | ||||
| Homecare, ADL, multifactorial-action and review with self-management [hmcr & ADL & mfar(w/slfm)] | SMD 0.11 higher (0.20 lower to 0.43 higher) Mixed estimate | MD 3.32 higher (5.95 lower to 12.59 higher) | ⊕⊕⊝⊝ Lowb |
2.4 (1 to 5) | May result in a very slight increase in PADL independence |
| Homecare and nutrition (hmcr & ntr) | SMD 0.13 higher (0.24 lower to 0.51 higher) Mixed estimate | MD 3.92 higher (7.20 lower to 15.05 higher) | ⊕⊝⊝⊝ Very lowb,c |
2.3 (1 to 5) | The evidence is very uncertain about the effect on PADL independence |
| Homecare, alternative-medicine and exercise (hmcr & hmnt & exrc) | SMD 0.03 higher (0.48 lower to 0.55 higher) Mixed estimate | MD 0.91 higher (14.32 lower to 16.15 higher) | ⊕⊝⊝⊝ Very lowb,c |
3.0 (1 to 5) | The evidence is very uncertain about the effect on PADL independence |
| Homecare and medication-review (hmcr & med) | SMD 0.05 lower (0.23 lower to 0.14 higher) Mixed estimate | MD 1.44 lower (6.92 lower to 4.03 higher) | ⊕⊝⊝⊝ Very lowb,d |
3.9 (2 to 5) | The evidence is very uncertain about the effect on PADL independence |
The consistency assumption was tested, and no violation of assumption was found (p = 0.904). Homecare, ADL, multifactorial-action and review with self-management may result in a very slight increase in PADL independence (SMD 0.11, 95% CI −0.20 to 0.43; low certainty). Other comparisons with homecare were rated as very low certainty.
The 95% CI for the true ranking covered at least four of the five places for all intervention groups.
Medium-term time frame
Overall characteristics
Four trials (n = 632 participants) compared a medium-term PADL outcome across five intervention groups including homecare, which was the largest node (see Table 18 for a summary of findings). 68,91,154,169 There was a maximum of one trial for a single direct comparison (compared to homecare), with the number of participants within any one intervention group in the network ranging from 29 to 133, excluding homecare. All study populations were frail and all were at high RoB, primarily due to missing data (three with serious concerns, one with very serious concerns).
| Population: Older people Interventions: Community-based complex interventions Comparator: Formal homecare (hmcr) Outcome: PADL (higher is better) Time frame: Medium term; follow-up at 12 months Setting: Community Total studies: 4 Total participants: 632 Comparator rank: Mean 4.4, 95% CI 3 to 5 |
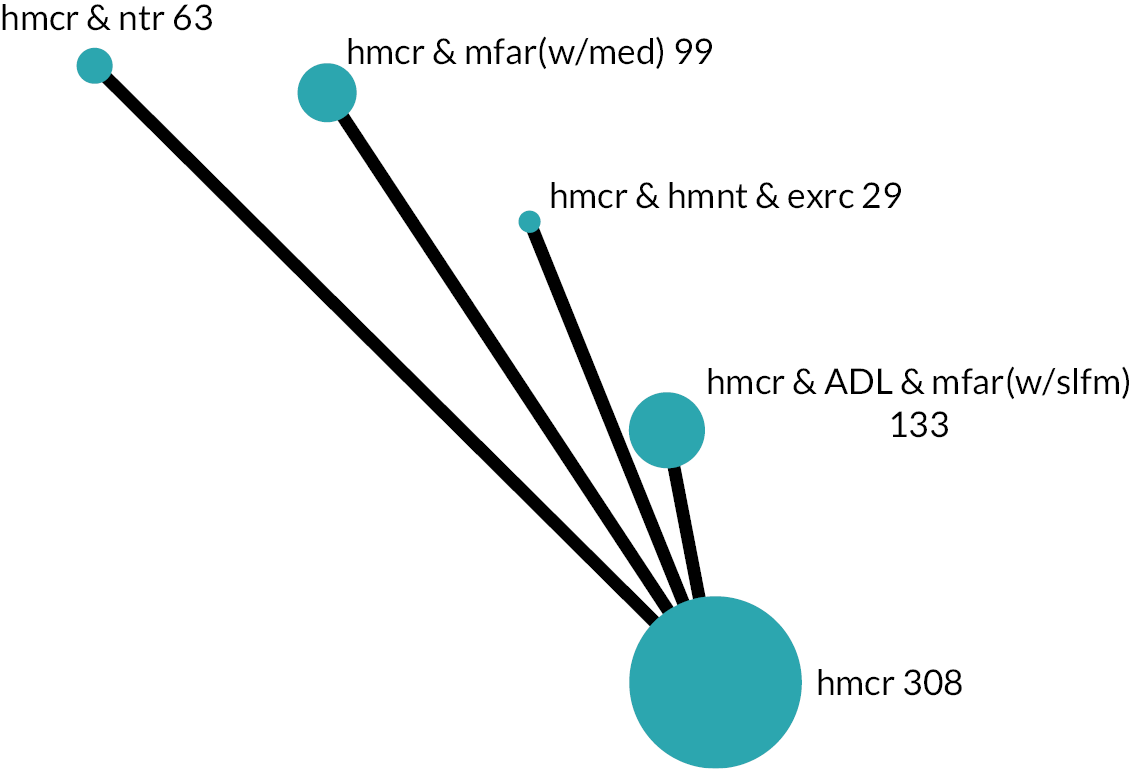
|
||||
|---|---|---|---|---|---|
| Intervention group | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | |
| SMD | MD (Barthel Index 0–100)a | ||||
| Homecare, multifactorial-action and review with medication-review [hmcr & mfar(w/med)] | SMD 0.60 higher (0.32 higher to 0.88 higher) Mixed estimate | MD 17.74 higher (9.32 higher to 26.15 higher) | ⊕⊕⊝⊝ Lowb,c |
1.1 (1 to 2) | May result in an increase in PADL |
| Homecare and nutrition (hmcr & ntr) | SMD 0.23 higher (0.15 lower to 0.60 higher) Mixed estimate | MD 6.70 higher (4.45 lower to 17.85 higher) | ⊕⊝⊝⊝ Very lowb,d |
2.7 (1 to 5) | The evidence is very uncertain about the effect on PADL |
| Homecare, ADL, multifactorial-action and review with self-management [hmcr & ADL & mfar(w/slfm)] | SMD 0.12 higher (0.13 lower to 0.36 higher) Mixed estimate | MD 3.42 higher (3.73 lower to 10.57 higher) | ⊕⊝⊝⊝ Very lowe,f |
3.4 (2 to 5) | The evidence is very uncertain about the effect on PADL |
| Homecare, alternative-medicine and exercise (hmcr & hmnt & exrc) | SMD 0.10 higher (0.42 lower to 0.61 higher) Mixed estimate | MD 2.83 higher (12.41 lower to 18.08 higher) | ⊕⊝⊝⊝ Very lowb,d |
3.4 (1 to 5) | The evidence is very uncertain about the effect on PADL |
Main analysis
The consistency assumption was found to hold for the network according to both the global Wald test (p = 0.407) and the node-splitting method (all contrasts p > 0.05). As the network contained only a single study measuring each comparison, there was no potential source of heterogeneity and, therefore, a common-effect model was fitted. Homecare, multifactorial-action and review with medication-review may result in an increase in PADL [SMD 0.60 (moderate), 95% CI 0.32 to 0.88; low certainty]. All other comparisons with homecare were rated as very low certainty.
In the homecare network for the medium-term PADL outcome, homecare, multifactorial-action and review with medication-review ranked highest (95% CI 1 to 2), and homecare was ranked lowest (95% CI 3 to 5); the ranking of the other three intervention groups had wide CIs.
Investigation of small-study effects
There were fewer than 10 studies in the network so small-study effects were not investigated.
Sensitivity analysis for risk of bias
In sensitivity analysis, one trial was excluded due to very serious concerns about RoB and this left three trials included in the NMA. The consistency assumption was tested and no violation of assumption was found (p = 0.775). Results were very similar to the main analysis, with the same estimate for homecare, multifactorial-action and review with medication-review compared to homecare in both direct and indirect comparisons.
Investigation of frailty
No frailty analysis was possible as the populations in all studies were classified as frail.
Long-term time frame
There were insufficient studies in the homecare network to conduct analyses of long-term PADL outcomes.
Across time frames
There was no high- or moderate-certainty evidence, and low-certainty evidence was identified for one time frame at most for each intervention group.
Hospitalisation
Hospitalisation included hospital inpatient care usage over the follow-up period, which was reported in terms of number of persons admitted, the length of stay in hospital or the frequency of admissions. For the hospitalisation outcome we analysed data on participants hospitalised once or more in the medium term. ORs were estimated in the NMA for the odds of a person having at least one hospitalisation between two intervention groups. An OR smaller than one indicates that the estimated effect favours the experimental intervention group, that is a decrease of odds of any hospitalisation with intervention.
Seventeen studies reported usable data; however, two were in the homecare network and disconnected from the available-care network. 68,125 Therefore, we only present the results from an available-care network.
Overall characteristics
There were 15 studies (n = 9569) and 10 intervention groups (see Table 19 for a summary of findings). 73,75,104,108,109,118,124,143,144,149,155,168,178,180,181 Four studies compared multifactorial-action and review with medication-review versus ac, three studies compared multifactorial-action and review versus ac, the other eight comparisons had data from a single study. Four study populations included all frailty categories, one was robust, one pre-frail, four frail and three a combination of pre-frail and frail categories. We had some concerns with RoB in six study results and nine were judged high RoB (five with serious concerns, four with very serious concerns).
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: Hospitalisation Time frame: Medium term; range of follow-up 12–18 months Setting: Community Total studies: 15 Total participants: 9569 Comparator rank: Mean 4.4, 95% CI 5 to 8 |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Relative effect (95% CI) | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | ||||
| Network estimate | Calculated RRa | High-risk population [520 per 1000 with ac] | Low-risk population [118 per 1000 with ac] | ||||||
| With intervention | Difference | With intervention | Difference | ||||||
| Education, exercise, multifactorial-action and review with medication-review and self-management strategies [educ & exrc & mfar(w/med + slfm)] | OR 0.53 (0.25 to 1.12) Mixed estimate | RR 0.59 (0.30 to 1.09) | 365 per 1000 (213 to 549) | 155 fewer per 1000 (307 fewer to 29 more) | 66 per 1000 (32 to 131) | 52 fewer per 1000 (86 fewer to 13 more) | ⊕⊕⊝⊝ Lowb |
1.4 (5 to 10) | May result in a reduction in chance of being hospitalised |
| Education, multifactorial-action and review with medication-review [educ & mfar(w/med)] | OR 0.92 (0.78 to 1.09) Mixed estimate | RR 0.94 (0.82 to 1.07) | 499 per 1000 (457 to 542) | 21 fewer per 1000 (63 fewer to 22 more) | 110 per 1000 (94 to 127) | 8 fewer per 1000 (24 fewer to 9 more) | ⊕⊕⊝⊝ Lowc |
3.3 (5 to 9) | May result in a slight reduction in chance of being hospitalised |
| Exercise, multifactorial-action and review with medication-review and self-management strategies [exrc & mfar(w/med + slfm)] | OR 1.34 (0.80 to 2.24) Mixed estimate | RR 1.24 (0.84 to 1.75) | 592 per 1000 (465 to 708) | 72 more per 1000 (55 fewer to 188 more) | 152 per 1000 (97 to 231) | 34 more per 1000 (21 fewer to 113 more) | ⊕⊕⊝⊝ Lowd |
7.0 (1 to 8) | May result in an increase in chance of being hospitalised |
| Multifactorial-action and review (mfar) | OR 0.81 (0.62 to 1.06) Mixed estimate | RR 0.85 (0.67 to 1.05) | 467 per 1000 (400 to 535) | 53 fewer per 1000 (120 fewer to 15 more) | 98 per 1000 (76 to 124) | 20 fewer per 1000 (42 fewer to 6 more) | ⊕⊝⊝⊝ Very lowc,e |
2.4 (6 to 10) | The evidence is very uncertain about the effect on chance of being hospitalised |
| Multifactorial-action and review with medication-review [mfar(w/med)] | OR 1.10 (0.95 to 1.28) Mixed estimate | RR 1.08 (0.96 to 1.20) | 544 per 1000 (507 to 581) | 24 more per 1000 (13 fewer to 61 more) | 129 per 1000 (113 to 146) | 11 more per 1000 (5 fewer to 28 more) | ⊕⊝⊝⊝ Very lowc,e |
5.7 (3 to 8) | The evidence is very uncertain about the effect on chance of being hospitalised |
| Multifactorial-action and review with medication-review and self-management strategies [mfar(w/med + slfm)] | OR 1.37 (0.76 to 2.50) Mixed estimate | RR 1.27 (0.80 to 1.86) | 598 per 1000 (450 to 731) | 78 more per 1000 (70 fewer to 211 more) | 155 per 1000 (92 to 251) | 37 more per 1000 (26 fewer to 133 more) | ⊕⊝⊝⊝ Very lowf,g |
6.7 (1 to 9) | The evidence is very uncertain about the effect on chance of being hospitalised |
| Monitoring (mntr-mfa-) | OR 1.39 (0.80 to 2.42) Mixed estimate | RR 1.28 (0.84 to 1.83) | 602 per 1000 (466 to 724) | 82 more per 1000 (54 fewer to 204 more) | 157 per 1000 (97 to 244) | 39 more per 1000 (21 fewer to 126 more) | ⊕⊝⊝⊝ Very lowh,i |
7.0 (1 to 9) | The evidence is very uncertain about the effect on chance of being hospitalised |
| ADL, medication-review, nutrition and social skills (ADL & med & ntr & sst) | OR 1.70 (0.93 to 3.09) Mixed estimate | RR 1.46 (0.95 to 2.09) | 648 per 1000 (503 to 770) | 128 more per 1000 (17 fewer to 250 more) | 185 per 1000 (111 to 293) | 67 more per 1000 (7 fewer to 175 more) | ⊕⊝⊝⊝ Very lowj,k |
8.1 (1 to 7) | The evidence is very uncertain about the effect on chance of being hospitalised |
| Cognitive training, nutrition and exercise (cgn & ntr & exrc) | OR 3.30 (0.63 to 17.30) Mixed estimate | RR 2.16 (0.69 to 3.67) | 781 per 1000 (405 to 949) | 261 more per 1000 (115 fewer to 429 more) | 306 per 1000 (78 to 698) | 188 more per 1000 (40 fewer to 580 more) | ⊕⊝⊝⊝ Very lowh,l |
9.0 (1 to 9) | The evidence is very uncertain about the effect on chance of being hospitalised |
Main analysis
The consistency assumption was found to hold for the network according to both the global Wald test (p = 0.378) and the node-splitting method (all contrasts p > 0.05). Between-study variance was estimated to be very small but non-zero, indicating some degree of heterogeneity between studies included in the network (τ = 1.57 × 10−6).
Three comparisons with ac were rated low certainty. Education, exercise, multifactorial-action and review with medication-review and self-management may result in a reduction in odds of being hospitalised [OR 0.53 (very large), 95% CI 0.25 to 1.12]. Similarly, education, multifactorial-action and review with medication-review may result in a slight reduction in odds of being hospitalised (OR 0.92, 95% CI 0.78 to 1.09). However, exercise, multifactorial-action and review with medication-review and self-management may result in an increase in odds of being hospitalised [OR 1.34 (large), 95% CI 0.80 to 2.24]. The other six comparisons with ac were rated very low certainty.
Investigation of small-study effects
The comparison-adjusted funnel plot appeared symmetric, implying no evidence of small-study effects in the network.
Sensitivity analysis for risk of bias
After removing the four study results with very serious concerns about RoB, there were 11 remaining results (n = 6896) and 8 intervention groups in the sensitivity analysis. Results were very similar to those from the main analysis for the intervention groups that remained in the sensitivity analysis, with no change in certainty of evidence.
Investigation of frailty
All frailty categories were represented in the network and a network meta regression model was fitted including the frailty variable with ‘all (robust, pre-frail and frail)’ set as the reference category. The consistency assumption remained valid (global Wald test p = 0.791, node-splitting method showed all contrasts p > 0.05). Between-study variance remained very small, but non-zero (τ = 1.58 × 10−7). Frailty effects could not be estimated for most contrasts due to collinearity. Where frailty effects could be estimated, the effect was estimated with very large uncertainty as reflected in wide 95% CIs covering a broad range of both beneficial and harmful effects making interpretation of these results meaningless in practice.
Care-home placement
Care-home placement included long-term nursing-home and residential-home admissions. In the NMA, ORs were estimated to compare the odds of care-home placement between two arms. OR smaller than one means the estimated effect favours the experimental intervention group, that is a decrease in risk of care-home placement with intervention. For each time frame, there were two separate networks, one with ac reference comparator and another with homecare comparator, which we describe in turn.
Available-care network
Short-term time frame
The ac network for care-home placement in the short term comprised seven studies (n = 3672) and eight intervention groups (see Appendix 3, Table 25 for a summary of findings). 113,121,126,136,165,166,190 Each comparison had data from a single study only. Two study populations included all frailty categories, two included pre-frail and frail categories, two were frail and one was unclassifiable for frailty. We had some concerns about RoB in one study result, the other study results were judged high RoB (four with serious concerns, two with very serious concerns), primarily due to the proportion of missing outcome data compared with the number of care-home placements.
The network contained no loops and therefore the consistency assumption could not be tested and a ‘consistency’ model was fitted. As the network contained only a single study for each comparison, there was no potential source of heterogeneity and therefore a common-effect model was fitted.
All comparisons with ac were rated as very low certainty. There was very serious imprecision throughout the network as there were data for only 30 care-home placements in total.
Medium-term time frame
The ac network for care-home placement in the medium term comprised 20 studies (n = 16,055) and 14 intervention groups (see Appendix 3, Table 26 for a summary of findings). 70,88,103,104,106–108,116,118,119,121,136,138,142,143,150,153,158,165,178 Five studies compared multifactorial-action and review with medication-review versus ac, two comparisons had data from two studies and the other 11 comparisons had one. Four study populations included all frailty categories, one was robust, one pre-frail, six included the pre-frail and frail categories and five were frail, with three unclassifiable. All study results were judged high RoB, primarily due to the proportion of missing outcome data compared with the number of care-home placements; we had serious concerns regarding 16 and very serious concerns regarding 4.
The consistency assumption was found to hold for the network according to both the global Wald test (p = 0.823) and the node-splitting method (all contrasts p > 0.05). Between-study variance was estimated to be non-zero, indicating some degree of heterogeneity between studies included in the network (τ = 0.244).
All comparisons with ac were rated as very low certainty. In addition to the high RoB, there was very serious imprecision throughout the network as there were data for only 300 care-home placements across the 14 comparisons.
Sensitivity analysis for risk of bias and small-study effects
The results of the sensitivity analysis were very similar to those of the main analysis for the intervention groups that remained in the network.
Investigation of frailty
All frailty categories were represented in the network and a network meta regression model was fitted including the frailty variable with ‘all (robust, pre-frail and frail)’ set as the reference category. The consistency assumption remained valid (global Wald test p = 0.884, node-splitting method showed all contrasts p > 0.05). Between-study variance was very small but remained non-zero (τ = 2.05 × 10−5). Frailty effects could not be estimated for most contrasts due to collinearity. Where frailty effects could be estimated, the effect was estimated with very large uncertainty as reflected in wide 95% CIs covering a broad range of both beneficial and harmful effects making interpretation of these results meaningless in practice.
Long-term time frame
The ac network for care-home placement in the long term comprised 14 studies (n = 13,638) and 10 intervention groups (see Table 20 for a summary of findings). 76,92,93,106,116,118,121,136,162,164,165,171–173 Four studies compared multifactorial-action and review with medication-review versus ac, two comparisons had data from two studies and the others had one. Four study populations included all frailty categories, one included robust and pre-frail categories, one was pre-frail, four included pre-frail and frail categories, three were frail and one was unclassifiable. We had some concerns with RoB in one study; the others were judged high RoB (eleven with serious concerns, two with very serious concerns).
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: Care-home placement Time frame: Long term; range of follow-up 24–48 months Setting: Community Total studies: 14 Total participants: 13,638 Comparator rank: Mean 5.4, 95% CI 3 to 8 |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Relative effect (95% CI) | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | ||||
| Network estimate | Calculated RRa | High-risk population (200 per 1000 with ac) | Low-risk population (7 per 1000 with ac) | ||||||
| With intervention | Difference | With intervention | Difference | ||||||
| Risk-screening (rsk-mfa-) | OR 1.41 (1.06 to 1.88) Mixed estimate | RR 1.39 (1.06 to 1.82) | 261 per 1000 (209 to 319) | 61 more per 1000 (9 more to 119 more) | 10 per 1000 (7 to 13) | 3 more per 1000 (0 to 6 more) | ⊕⊕⊝⊝ Lowb,c |
8.4 (6 to 10) | May result in an increase in care-home placement |
| Multifactorial-action and review (mfar) | OR 0.41 (0.07 to 2.26) Indirect estimate | RR 0.42 (0.08 to 2.16) | 93 per 1000 (18 to 361) | 107 fewer per 1000 (182 fewer to 161 more) | 3 per 1000 (1 to 16) | 4 fewer per 1000 (6 fewer to 9 more) | ⊕⊝⊝⊝ Very lowd,e |
3.1 (1 to 10) | The evidence is very uncertain about the effect on care-home placement |
| Aids (aids) | OR 0.40 (0.04 to 3.97) Mixed estimate | RR 0.41 (0.04 to 3.58) | 90 per 1000 (10 to 498) | 110 fewer per 1000 (190 fewer to 298 more) | 3 per 1000 (0 to 27) | 4 fewer per 1000 (7 fewer to 20 more) | ⊕⊝⊝⊝ Very lowf,g |
3.3 (1 to 10) | The evidence is very uncertain about the effect on care-home placement |
| ADL, nutrition and exercise (ADL & ntr & exrc) | OR 0.79 (0.32 to 1.98) Mixed estimate | RR 0.80 (0.32 to 1.91) | 165 per 1000 (73 to 331) | 35 fewer per 1000 (127 fewer to 131 more) | 6 per 1000 (2 to 14) | 1 fewer per 1000 (5 fewer to 7 more) | ⊕⊝⊝⊝ Very lowb,e |
4.6 (1 to 10) | The evidence is very uncertain about the effect on care-home placement |
| Education, multifactorial-action and review with medication-review [educ & mfar(w/med)] | OR 0.77 (0.25 to 2.33) Mixed estimate | RR 0.78 (0.26 to 2.22) | 161 per 1000 (60 to 369) | 39 fewer per 1000 (140 fewer to 169 more) | 5 per 1000 (2 to 16) | 2 fewer per 1000 (5 fewer to 9 more) | ⊕⊝⊝⊝ Very lowb,e |
4.6 (1 to 10) | The evidence is very uncertain about the effect on care-home placement |
| Multifactorial-action (mfa-) | OR 0.65 (0.07 to 6.34) Mixed estimate | RR 0.66 (0.07 to 5.30) | 140 per 1000 (17 to 613) | 60 fewer per 1000 (183 fewer to 413 more) | 5 per 1000 (0 to 43) | 2 fewer per 1000 (7 fewer to 36 more) | ⊕⊝⊝⊝ Very lowb,e |
4.7 (1 to 10) | The evidence is very uncertain about the effect on care-home placement |
| Multifactorial-action and review with medication-review [mfar(w/med)] | OR 1.08 (0.72 to 1.62) Mixed estimate | RR 1.08 (0.73 to 1.58) | 212 per 1000 (153 to 288) | 12 more per 1000 (47 fewer to 88 more) | 8 per 1000 (5 to 11) | 1 more per 1000 (2 fewer to 4 more) | ⊕⊝⊝⊝ Very lowb,e |
6.2 (3 to 10) | The evidence is very uncertain about the effect on care-home placement |
| Engagement in meaningful-activities and multifactorial-action with self-management strategies [eng & mfa-(w/slfm)] | OR 1.21 (0.79 to 1.86) Mixed estimate | RR 1.20 (0.79 to 1.80) | 232 per 1000 (165 to 317) | 32 more per 1000 (35 fewer to 117 more) | 8 per 1000 (6 to 13) | 1 more per 1000 (1 fewer to 6 more) | ⊕⊝⊝⊝ Very lowb,e |
7.3 (3 to 10) | The evidence is very uncertain about the effect on care-home placement |
| Education, multifactorial-action and review with medication-review and self-management strategies [educ & mfar(w/med + slfm)] | OR 1.26 (0.67 to 2.37) Mixed estimate | RR 1.25 (0.68 to 2.25) | 240 per 1000 (144 to 372) | 40 more per 1000 (56 fewer to 172 more) | 9 per 1000 (5 to 16) | 2 more per 1000 (2 fewer to 9 more) | ⊕⊝⊝⊝ Very lowg,h |
7.4 (3 to 10) | The evidence is very uncertain about the effect on care-home placement |
The network contained no loops and therefore the consistency assumption could not be tested and a ‘consistency’ model was fitted, setting the inconsistency parameter in the model to zero for all comparisons. Between-study variance was estimated to be very small but non-zero, indicating some degree of heterogeneity between studies included in the network (τ = 5.18 × 10−6).
Risk-screening may result in an increase in care-home placement in the long term, in comparison with ac [OR 1.41 (large), 95% CI 1.06 to 1.88; low certainty]. We rated the eight other comparisons with ac as very low certainty.
Risk-screening was ranked in the bottom half of intervention groups according to the 95% CI for the true ranking (6 to 10). Other CIs were wider than this.
Summary across time frames
Proportions of participants placed in a care home were relatively low. Therefore, small numbers of missing outcome data (including mortality) often placed the results at high RoB due to its large proportion with comparison to care-home placement. Low numbers also resulted in wide CIs across the estimates. Only one comparison with ac was not very low certainty, which indicated that risk-screening may result in an increase in care-home placement in the long term (large effect).
Homecare network
There were insufficient data to conduct NMA for homecare interventions in the short- and long-term time frames. We therefore present results for the medium-term time frame only.
Medium-term time frame
The homecare network for care-home placement comprised four studies (n = 1567) and five intervention groups, with each comparison including data from a single study only (see Appendix 3, Table 27 for a summary of findings). 91,125,154,189 All four study populations were frail. We judged each study result to be at high RoB (three with serious concerns, one with very serious concerns).
The network contained no loops and therefore the consistency assumption could not be tested and a ‘consistency’ model was fitted. As the network contained only a single study for each comparison, there was no potential source of heterogeneity and therefore a common-effect model was fitted.
All comparisons with ac were rated very low certainty due to the RoB and very serious imprecision.
Investigation of small-study effects
There were fewer than 10 studies in the network so small-study effects were not investigated.
Sensitivity analysis for risk of bias
The results of the sensitivity analysis were very similar to those of the main analysis for the intervention groups that remained in the network.
Investigation of frailty
Because all study populations were categorised as frail it was not possible to investigate the effects of frailty for this analysis.
Homecare services (non-healthcare professional) usage
Homecare is often provided to enable a person to remain living at home but can be considered costly and a potential limiter of opportunities for activity and agency. 593 Therefore, while some interventions explicitly intended to reduce homecare usage, others consider it an appropriate outcome of assessment and care planning. Sixteen studies reported results regarding the usage of homecare services provided by non-healthcare professionals. Data on two metrics were collected: number of participants using the services at the time, and volume of usage in terms of visits or hours of homecare. These study results are presented in Report Supplementary Material 9. These data were not meta-analysed because of a sparse and disconnected network formed for each of the three time periods. We have summarised results based on direction rather than significance and indicated the RoB in the result. We had very serious concerns over the RoB in the results from two studies; therefore, they are not summarised here.
Available-care comparisons
There was lower usage of homecare for four groups assigned to interventions that were compared with ac: ADL, nutrition and exercise in the medium and long term (no serious concerns);121 education and risk-screening in the medium term (serious concerns);138 exercise in the long term (no serious concerns);184 exercise and multifactorial-action with medication-review in the long term (no serious concerns). 127
There was higher usage of homecare for five groups assigned to interventions that were compared with ac: education, multifactorial-action and review with medication-review and self-management in the long term (serious concerns);136 multifactorial-action with medication-review in the medium term (serious concerns);142 multifactorial-action and review with medication-review in the long term (no serious concerns);73 and two groups assigned to risk-screening in the medium and long term (serious concerns). 116,148
Participants receiving welfare rights advice in Howel 2019112 were less likely to be using homecare in the long term than those in the ac group (serious concerns). However, among those using homecare, the welfare group received more hours of care per week such that hours per participant across the whole sample were very similar between groups.
Thomas 2007171 compared ac with two experimental interventions classified in the same intervention group: multifactorial-action and review with medication-review; one in which advice was given and one in which referrals were offered to the participant. Compared to ac they found higher homecare usage in the group given advice and similar and lower usage in the referrals group in the medium and long term, respectively (serious concerns).
Interventions where all participants received homecare at enrolment
Bernabei 199868 compared homecare, multifactorial-action and review with medication-review to homecare and found lower use in the medium term (serious concerns).
King 2012117 evaluated a form of restorative homecare (homecare, ADL, multifactorial-action and review with self-management). This group received slightly fewer visits per month, but the duration of each visit was slightly longer compared with usual homecare in the short term (no serious concerns).
Whitehead 2016187 compared two 6-week homecare reablement services: homecare, ADL, aids and multifactorial-action versus homecare and multifactorial-action. They found a lower proportion of homecare users in the homecare and multifactorial-action group in the short term, but this was accounted for by mortality (serious concerns).
Summary of results for main outcomes synthesised with NMA
Table 21 summarises the evidence for our main outcomes with moderate- or low-certainty GRADE ratings (there was no high-certainty evidence) for which NMA was conducted.
| Intervention groupa | LAH T1 | LAH T2 | LAH T3 | IADL T1 | IADL T2 | IADL T3 | PADL T1 | PADL T2 | PADL T3 | Hosp T2 | CH T1 | CH T2 | CH T3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADL, aids, education, exercise, multifactorial-action and review (with medication-review and self-management) [ADL&aids&ed&ex&mf(w/med + slfm)] | ⊕⊕⊝⊝ --- |
||||||||||||
| ADL, aids and exercise (ADL & aids & exrc) | ⊕⊕⊝⊝ -- |
||||||||||||
| ADL, nutrition and exercise (ADL & ntr & exrc) | ⊕⊕⊝⊝ ~ |
⊕⊕⊝⊝ ++++ |
⊕⊕⊝⊝ ++ |
||||||||||
| Cognitive training, medication-review, nutrition and exercise (cgn & med & ntr & exrc) | ⊕⊕⊝⊝ ++++ |
||||||||||||
| Education, exercise, multifactorial-action and review with medication-review and self-management strategies [educ & exrc & mfar(w/med + slfm)] | ⊕⊕⊝⊝ ----- |
||||||||||||
| Education, multifactorial-action and review with medication-review [educ & mfar(w/med)] | ⊕⊕⊝⊝ - |
⊕⊕⊝⊝ +++ |
⊕⊕⊝⊝ + |
⊕⊕⊝⊝ + |
⊕⊕⊝⊝ -- |
||||||||
| Education, multifactorial-action and review with medication-review and self-management strategies [educ & mfar(w/med + slfm)] | ⊕⊕⊝⊝ --- |
⊕⊕⊝⊝ ----- |
⊕⊕⊝⊝ -- |
⊕⊕⊝⊝ -- |
⊕⊕⊝⊝ -- |
⊕⊕⊝⊝ -- |
|||||||
| Exercise, multifactorial-action and review (with medication-review and self-management) [exrc & mfar(w/med + slfm)] | ⊕⊕⊝⊝ + |
⊕⊕⊝⊝ ++++ |
|||||||||||
| Engagement in meaningful-activities and multifactorial-action with self-management strategies [eng & mfa-(w/slfm)] | ⊕⊕⊝⊝ + |
||||||||||||
| Multifactorial-action and review (mfar) | ⊕⊕⊝⊝ + |
⊕⊕⊝⊝ +++ |
⊕⊕⊝⊝ -- |
||||||||||
| Multifactorial-action and review with medication-review [mfar(w/med)] | ⊕⊕⊝⊝ ~ |
⊕⊕⊕⊝ ++ |
⊕⊕⊝⊝ ++ |
⊕⊕⊕⊝ + |
⊕⊕⊕⊝ - |
⊕⊕⊝⊝ ~ |
|||||||
| Multifactorial-action with medication-review [mfa-(w/med)] | ⊕⊕⊝⊝ ++++ |
||||||||||||
| Risk-screening (rsk-mfa-) | ⊕⊕⊝⊝ - |
⊕⊕⊝⊝ -- |
⊕⊕⊝⊝ ++ |
⊕⊕⊝⊝ + |
⊕⊕⊝⊝ ++++ |
||||||||
| Homecare, ADL, multifactorial-action and review with self-management strategies [hmcr & ADL & mfar(w/slfm)] | ⊕⊕⊝⊝ --- |
⊕⊕⊝⊝ ---- |
⊕⊕⊝⊝ ~ |
⊕⊕⊝⊝ + |
|||||||||
| Homecare and nutrition (hmcr & ntr) | ⊕⊕⊝⊝ ----- |
||||||||||||
| Homecare, multifactorial-action and review (with medication-review) [hmcr & mfar(w/med)] | ⊕⊕⊝⊝ +++ |
Health status/health-related quality of life
We meta-analysed single global self-assessments of health status using the SMD (unless results were presented as binary data). Health status was treated as a positive outcome, so a positive SMD indicates benefit.
Overall characteristics
There were eight studies and seven intervention groups connected with medium-term self-reported health results with ac as a reference comparator (see Appendix 3, Table 28). 70,73–75,157,167,168,171 A ninth study reported results but was disconnected from the network. There were a total of 2631 participants across this network with ac the largest intervention group (n = 1499) and nutrition and exercise the smallest (n = 61). Three studies compared multifactorial-action and review with medication-review to ac, the other comparisons were populated by one study each. The population of one study covered all frailty levels, one population was pre-frail, three study populations were pre-frail and frail and three were frail. We had some concerns about RoB in the results of one study and the others were high RoB (six with serious concerns, one with very serious concerns), primarily due to missing outcome data.
Main analysis
The network contained no loops and therefore the consistency assumption could not be tested and a ‘consistency’ model was fitted. Between-study variance was estimated to be small but non-zero, indicating some degree of heterogeneity between studies included in the network (τ = 0.0995). There was low-certainty evidence that exercise, multifactorial-action and review with medication-review and self-management strategies may result in little to no difference in self-reported health (SMD −0.01, 95% CI −0.34 to 0.32). The other comparisons with ac were of very low certainty due to RoB and imprecision, but the point estimates of all of these were very small.
For medium-term self-reported health, the 95% CI for the ranking of ac was two to six of seven places and was wider for other interventions.
Investigation of small-study effects
There were fewer than 10 studies in the network so small-study effects were not investigated.
Sensitivity analysis for risk of bias
Only Blom 201670 was identified as being a very serious concern for bias, so it was dropped from the sensitivity analysis. As the network was reduced it still contained no loops and therefore the consistency assumption could not be tested. The between-study heterogeneity estimate remained the same as in the main analysis.
Multifactorial-action and review with medication-review ranked highest (95% CI for true rank 1 to 5), nutrition and exercise ranked second (95% CI for true rank 1 to 6) and monitoring ranked last (95% CI for true rank 1 to 6). No direct or indirect comparison had a statistically significant outcome.
Investigation of frailty
No studies were dropped for the frailty meta regression, however there were no studies categorised as ‘robust’ populations. A network meta regression model was fitted including the frailty variable with ‘all (robust, pre-frail and frail)’ set as the reference category. The network contained no loops and therefore the consistency assumption could not be tested and a ‘consistency’ model was fitted. Between-study variance remained small, but non-zero (τ = 0.032). Frailty effects could not be estimated for most contrasts due to collinearity. Where frailty effects could be estimated (multifactorial-action and review with medication-review; and nutrition and exercise vs. ac), the effects were estimated with large uncertainty, with 95% CIs covering both beneficial and harmful effects (e.g. for ac vs. nutrition and exercise SMD 0.019, 95% CI −248.25 to 248.28) making interpretation of these results meaningless in practice.
Depression
Continuous measures of depression in the medium term were included. Among these we included the five-item Mental Health Index, which is included in the 36-item Short-Form health survey, but preferred more specific and extensive measures of depression where these were also reported. Where necessary, scales were transformed so that lower scores equated to better mental health (or less depressed). There were two separate networks, one with ac reference comparator and one with a homecare comparator, which we describe in turn.
Available-care network
Overall characteristics
A total of 15 trials (n = 7245 participants) compared depression outcome across 13 treatment options including ac as largest node at medium-term follow-up (see Appendix 3, Table 29 for a summary of findings). 69,70,73,75,79,83,101,108,118,136,142,155,167,168,177 There were at most two trials for a single direct comparison (multifactorial-action and review with medication-review vs. ac; and multifactorial-action and review vs. ac), with the number of participants receiving any one treatment in the network ranging from 23 to 3387. Two study populations included all frailty categories, one population was robust, one pre-frail and four frail; five studies included the other categories and two were unclassifiable. There were some concerns about RoB in two study results and the remainder were high RoB, primarily due to missing outcome data (eight with serious concerns, five with very serious concerns).
Main analysis
The consistency assumption was found to hold for the network according to both the global Wald test (p = 0.162) and the node-splitting method (all contrasts p > 0.05). Between-study variance was estimated to be non-zero indicating some degree of heterogeneity between studies included in the network (τ = 0.105).
There was low-certainty evidence that exercise, multifactorial-action and review with medication-review and self-management may result in a very slight reduction in symptoms of depression (SMD −0.11, 95% CI −0.45 to 0.23). The remaining comparisons with ac were of very low certainty.
The CIs for the true ranking were wide, covering at least 11 of the 13 places for all intervention groups except ac (mean rank 7.6; 95% CI of the true rank 5 to 11).
Investigation of small-study effects
The comparison-adjusted funnel plot appeared symmetric, implying no evidence of small-study effects in the network.
Sensitivity analysis for risk of bias
In sensitivity analysis, five trials were excluded due to very serious concerns about RoB and this left ten trials included in the NMA (n = 2893 participants). The consistency assumption was tested, and no violation of assumption was found (p = 0.658). Only comparison of multifactorial-action and review with medication-review versus ac was statistically significant with wide CIs for pairwise analyses (favouring multifactorial-action and review with medication-review vs. ac) as in the main analysis and remained statistically significant after inclusion of indirect evidence in the NMA. This estimate was of moderate certainty with reduced concerns about imprecision compared to the main analysis (very low certainty). Additionally, in NMA, multifactorial-action and review with medication-review versus ac was statistically superior to other treatments.
Investigation of frailty
Thirteen trials were classifiable for frailty. All frailty categories were represented in the network and a network meta regression model was fitted including the frailty variable with ‘all (robust, pre-frail and frail)’ set as the reference category. The consistency assumption remained valid (global Wald test p = 0.99, node-splitting method showed all contrasts p > 0.05). Between-study variance remained very small, but non-zero (τ = 0.005). Frailty effects could not be estimated for most contrasts due to collinearity. Where frailty effects could be estimated (multifactorial-action and review; multifactorial-action and review with medication-review; and risk-screening vs. ac), the effects were estimated with large uncertainty, with 95% CIs covering both beneficial and harmful effects (e.g. for ac vs. risk-screening, SMD 0.026, 95% CI −150.12 to 150.17) making interpretation of these results meaningless in practice.
Homecare network
Overall characteristics
A total of six trials (n = 996 participants) compared depression outcome across seven intervention groups including homecare as largest node at medium-term follow-up (see Appendix 3, Table 30 for a summary of findings). 68,91,146,147,154,169 Each direct comparison included data from only one trial, with the number of participants assigned to an intervention group ranging from 29 to 308. All participant populations were classified as frail. All study results were judged to be at high RoB (four with serious concerns, two with very serious concerns).
Main analysis
The consistency assumption was tested, and no violation of assumption was found (p = 0.254). Comparison of homecare, multifactorial-action and review with medication-review versus homecare was statistically significant with wide CIs in pairwise analysis (favouring homecare, multifactorial-action and review with medication-review), and remained statistically significant (SMD −0.38, 95% CI −0.66 to −0.10; low certainty) after inclusion of indirect evidence in the NMA. The evidence from all other comparisons with homecare was GRADEd as very low certainty.
For the medium-term depression homecare network, homecare, multifactorial-action and review with medication-review ranked first (95% CI 1 to 4) and homecare ranked second last (95% CI 3 to 7). For the other intervention groups, the 95% CI for the true rank covered at least six of the seven positions.
Investigation of small-study effects
There were fewer than 10 studies in the network so small-study effects were not investigated.
Sensitivity analysis for risk of bias
In sensitivity analysis, two trials were excluded due to very serious concerns and one which became disconnected, leaving only three trials included in the NMA (n = 368 participants). The consistency assumption was tested, and no violation of assumption was found (p = 0.793). The NMA found one SMD to be statistically significant in any comparison. As in the main analysis, only comparison of homecare, multifactorial-action and review with medication-review versus homecare, was statistically significant with wide CIs for pairwise analysis and remained statistically significant after inclusion of indirect evidence, with the same effect estimate as in the main analysis.
Investigation of frailty
All trial participants were classified as frail and therefore, no analysis of frailty was possible.
Loneliness
Six studies reported results regarding loneliness; five comparing an experimental intervention with ac70,73,85,159,181 and one comparing two experimental interventions. 114 These data were tabulated in Report Supplementary Material 9 but were not meta-analysed because there were only a maximum of three comparisons, each from a single study, forming a sparse network for each of the three time periods.
Loneliness was self-assessed through questionnaires. Four studies used the 11-item de Jong-Gierveld Scale;70,73,85,181 one study159 used the single item concerning loneliness in the Health Index developed by Hansagi and Rosenqvist;594 one study used a bespoke three-point Likert-type scale114
In the short term, Jing 2018114 found lower loneliness in the exercise and psychology group compared with the psychology group [mean 1.41 (SD 0.68) vs. mean 1.85 (SD 0.70); no serious concerns over RoB]. de Craen 200685 found little difference between the multifactorial-action group and ac (MD change −0.1, 95% CI −0.5 to 0.4; serious concerns over RoB). In the medium term, Bouman 200873 found a small, possibly unimportant reduction in loneliness in the multifactorial-action with medication-review plus self-management group compared to the ac group (MD values 0.44, 95% CI −0.37 to 1.24; serious concerns over RoB). In the long term, de Craen 200685 found little difference between the multifactorial-action group and ac as they did in the short term (MD change 0.0, 95% CI −0.7 to 0.6 serious concerns over RoB). van Rossum 1993181 found a small, possibly unimportant increase in loneliness in the multifactorial-action with review group compared to ac (MD values 0.2, 95% CI −0.2 to 0.6; serious concerns over RoB). We had very serious concerns over RoB in two further results, which are therefore not summarised here. 70,159
Falls
Falls were reported as the proportion of participants who had fallen in a given period and measures of the number of falls per participant by 23 studies. Falls results are tabulated in Report Supplementary Material 9. We synthesised the effect on falls using vote counting based on the direction of effect. 595 In all cases where the same study reported both a proportion of fallers and measure of falls,86,108,146,147,187 or in different time frames,81,118,144,154,155,165 the direction of effect was the same, therefore we have synthesised these together. We had very serious concerns regarding RoB in two results each from three studies which are therefore not reported here. 108,146,147
Comparisons with available care
In 12 studies there was less falling in the group receiving the intervention compared to ac: ADL;86 ADL and exercise;97 cognitive training, nutrition and exercise;144 education, multifactorial-action and review;151 education and risk-screening;138 multifactorial-action with medication-review;142 two groups receiving multifactorial-action and review with medication-review;74,88 multifactorial-action and review with self-management;186 nutrition and exercise;157 and two groups receiving risk-screening. 76,115 In four studies there was more falling in the group receiving the intervention compared to ac: exercise, multifactorial-action and review with medication-review and self-management;75 education, multifactorial-action and review with medication-review and self-management;81 and two groups receiving multifactorial-action and review with medication-review. 155,165
Other comparisons
Two studies compared homecare, ADL, multifactorial-action and review with self-management versus homecare. There was less falling in the intervention group in King 2012117 and the control in Rooijackers 2021. 154 There was less falling in the homecare, ADL, aids and multifactorial-action group than homecare and multifactorial-action in Whitehead 2016187 There was less falling in the multifactorial-action and review with medication-review group than multifactorial-action and review in Kono 2016. 118
Mortality
Mortality was reported as either the number of deaths over a period, or time to death measured from baseline. We analysed data on number of deaths in the medium term. ORs were estimated in the NMA for the odds of a person dying between two intervention groups. An OR smaller than 1 indicates that the estimated effect favours the experimental intervention group, that is a decreased odds of death with intervention. Unlike other NMAs in this review, all comparisons with homecare were connected to the same network as comparisons with ac.
Overall characteristics
Sixty-five studies (n = 38,351 participants) provided mortality data about 41 intervention groups in the medium term (see Table 22 for a summary of findings). 63,65,67–71,73–75,81,82,84–86,88,91,95,97,99,102–104,106–112,116,118,119,121,124,125,127,131,134,136,138,140,142–144,146,147,150,152–155,157,158,160,165,167–169,178,181,182,188,189,191 There was a maximum of 11 trials for a single direct comparison (multifactorial-action and review with medication-review vs. ac) followed by four trials of multifactorial-action and review versus ac, with the number of participants receiving any one treatment in the network ranging from 16 (aids) to 14,557 (ac). The populations of 12 studies included all frailty levels, 1 was robust, 1 robust and pre-frail, 3 pre-frail, 13 pre-frail and frail, 25 frail and 10 were unclassifiable. The RoB in 25 of the study results was judged some concerns, with high RoB in 40 study results (32 serious concerns, 8 very serious concerns). Most notably, we had very serious concerns about the comparison of homecare and multifactorial-action and review versus ac due to missing outcome data and post-randomisation exclusions. Because this linked all other homecare comparisons with ac, there were very serious concerns about their indirect comparison with ac.
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: Mortality Time frame: Medium term; range of follow-up 12–18 months Setting: Community Total studies: 65 Total participants: 38,351 Comparator rank: Mean 26.0, 95% CI 18 to 31 |
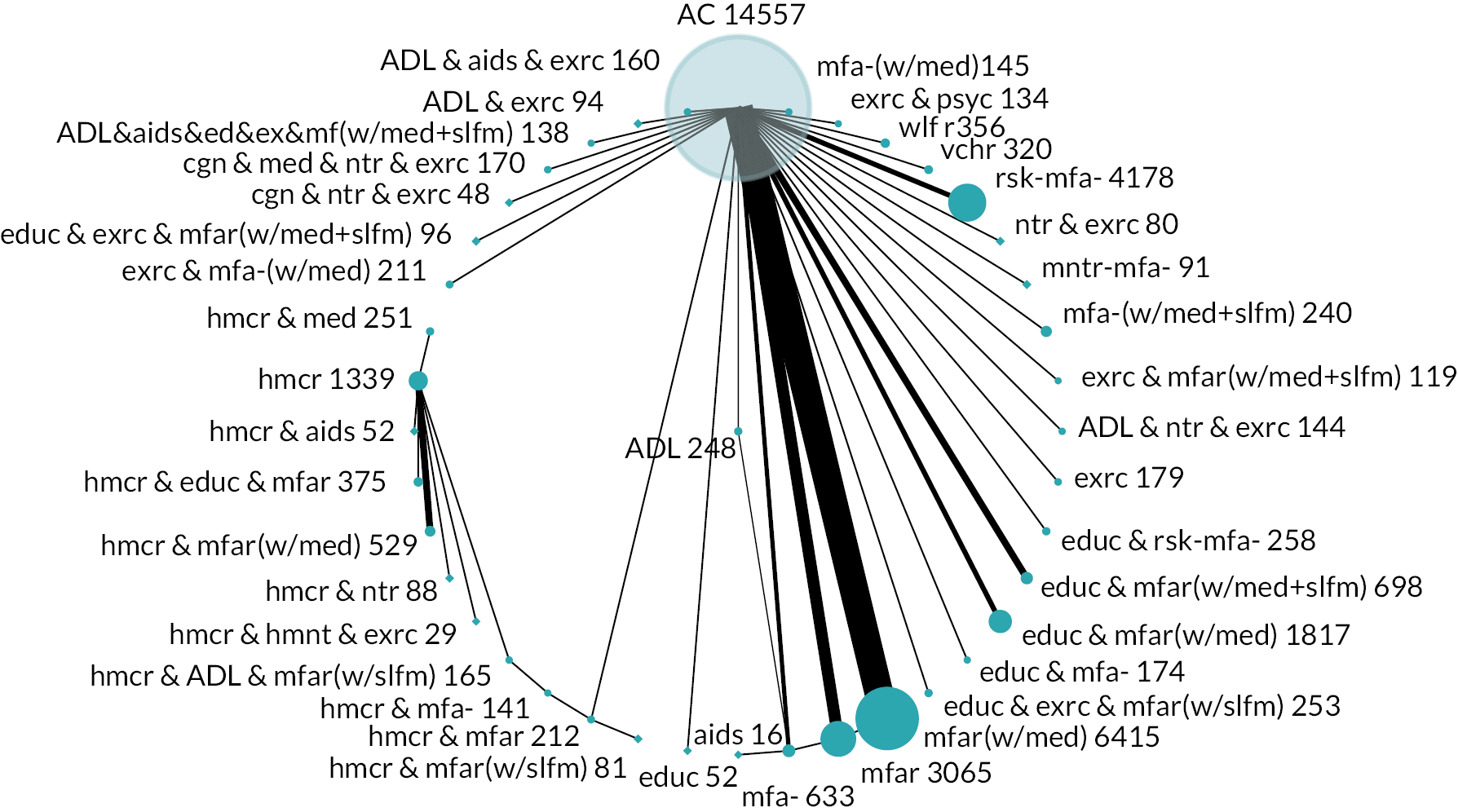
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Relative effect (95% CI) | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | ||||
| Network estimate | Calculated RRa | High-risk population (202 per 1000 with ac) | Low-risk population (7 per 1000 with ac) | ||||||
| With intervention | Difference | With intervention | Difference | ||||||
| ADL, aids and exercise (ADL & aids & exrc) | OR 0.16 (0.03 to 0.71) Mixed estimate | RR 0.17 (0.03 to 0.72) | 39 per 1000 (8 to 152) | 163 fewer per 1000 (194 fewer to 50 fewer) | 1 per 1000 (0 to 5) | 6 fewer per 1000 (7 fewer to 2 fewer) | ⊕⊕⊝⊝ Lowb |
8.6 (1 to 19) | May result in a reduction in mortality |
| Multifactorial-action and review (mfar) | OR 0.88 (0.66 to 1.18) Mixed estimate | RR 0.88 (0.67 to 1.17) | 182 per 1000 (143 to 230) | 20 fewer per 1000 (59 fewer to 28 more) | 7 per 1000 (5 to 9) | 1 fewer per 1000 (3 fewer to 1 more) | ⊕⊕⊝⊝ Lowc |
22.9 (12 to 31) | May result in a slight reduction in mortality |
| ADL (ADL) | OR 1.03 (0.44 to 2.43) Mixed estimate | RR 1.03 (0.45 to 2.29) | 206 per 1000 (100 to 380) | 5 more per 1000 (102 fewer to 179 more) | 8 per 1000 (3 to 18) | 0 per 1000 (4 fewer to 10 more) | ⊕⊕⊝⊝ Lowc |
25.9 (12 to 37) | May result in a very slight increase in mortality |
| Exercise, multifactorial-action and review with medication-review and self-management strategies [exrc & mfar(w/med + slfm)] | OR 1.22 (0.50 to 3.01) Mixed estimate | RR 1.21 (0.51 to 2.77) | 235 per 1000 (112 to 432) | 34 more per 1000 (89 fewer to 230 more) | 9 per 1000 (4 to 22) | 2 more per 1000 (4 fewer to 15 more) | ⊕⊕⊝⊝ Lowc |
28.6 (11 to 38) | May result in a slight increase in mortality |
| Multifactorial-action with medication-review [mfa-(w/med)] | OR 1.23 (0.50 to 3.04) Mixed estimate | RR 1.22 (0.51 to 2.80) | 237 per 1000 (112 to 434) | 35 more per 1000 (89 fewer to 233 more) | 9 per 1000 (4 to 22) | 2 more per 1000 (4 fewer to 15 more) | ⊕⊕⊝⊝ Lowc |
28.8 (14 to 38) | May result in a slight increase in mortality |
| Exercise and multifactorial-action with medication-review [exrc & mfa-(w/med)] | OR 1.51 (0.25 to 9.20) Mixed estimate | RR 1.48 (0.26 to 6.83) | 276 per 1000 (59 to 699) | 74 more per 1000 (142 fewer to 497 more) | 11 per 1000 (2 to 64) | 4 more per 1000 (6 fewer to 57 more) | ⊕⊕⊝⊝ Lowc |
29.7 (8 to 41) | May result in an increase in mortality |
| ADL and exercise (ADL & exrc) | OR 1.53 (0.41 to 5.70) Mixed estimate | RR 1.50 (0.42 to 4.75) | 279 per 1000 (94 to 590) | 77 more per 1000 (108 fewer to 388 more) | 11 per 1000 (3 to 41) | 4 more per 1000 (4 fewer to 33 more) | ⊕⊕⊝⊝ Lowc |
30.9 (11 to 40) | May result in an increase in mortality |
| Homecare and aids (hmcr & aids) | OR 0.07 (0.00 to 1.57) Indirect estimate | RR 0.07 (0.00 to 1.53) | 17 per 1000 (0 to 284) | 184 fewer per 1000 (202 fewer to 82 more) | 1 per 1000 (0 to 12) | 7 fewer per 1000 (7 fewer to 4 more) | ⊕⊝⊝⊝ Very lowd,e |
5.1 (1 to 27) | The evidence is very uncertain about the effect on mortality |
| Homecare, multifactorial-action and review with medication-review [hmcr & mfar(w/med)] | OR 0.10 (0.01 to 1.64) Indirect estimate | RR 0.10 (0.01 to 1.60) | 25 per 1000 (3 to 293) | 177 fewer per 1000 (199 fewer to 91 more) | 1 per 1000 (0 to 12) | 7 fewer per 1000 (7 fewer to 5 more) | ⊕⊝⊝⊝ Very lowd,e |
6.0 (1 to 27) | The evidence is very uncertain about the effect on mortality |
| Homecare, education, multifactorial-action and review (hmcr & educ & mfar) | OR 0.12 (0.01 to 1.93) Indirect estimate | RR 0.12 (0.01 to 1.86) | 29 per 1000 (3 to 328) | 172 fewer per 1000 (199 fewer to 126 more) | 1 per 1000 (0 to 14) | 7 fewer per 1000 (7 fewer to 7 more) | ⊕⊝⊝⊝ Very lowd,e |
7.7 (1 to 30) | The evidence is very uncertain about the effect on mortality |
| Homecare (hmcr) | OR 0.13 (0.01 to 1.97) Indirect estimate | RR 0.13 (0.01 to 1.89) | 32 per 1000 (3 to 332) | 170 fewer per 1000 (199 fewer to 131 more) | 1 per 1000 (0 to 14) | 6 fewer per 1000 (7 fewer to 7 more) | ⊕⊝⊝⊝ Very lowd,e |
8.1 (2 to 29) | The evidence is very uncertain about the effect on mortality |
| Exercise (exrc) | OR 0.17 (0.02 to 1.40) Mixed estimate | RR 0.18 (0.02 to 1.38) | 41 per 1000 (5 to 261) | 160 fewer per 1000 (197 fewer to 60 more) | 1 per 1000 (0 to 10) | 6 fewer per 1000 (7 fewer to 3 more) | ⊕⊝⊝⊝Very lowd,e | 9.6 (1 to 34) | The evidence is very uncertain about the effect on mortality |
| Homecare, multifactorial-action and review (hmcr & mfar) | OR 0.16 (0.02 to 1.58) Mixed estimate | RR 0.17 (0.02 to 1.54) | 39 per 1000 (5 to 285) | 163 fewer per 1000 (197 fewer to 84 more) | 1 per 1000 (0 to 12) | 6 fewer per 1000 (7 fewer to 4 more) | ⊕⊝⊝⊝ Very lowg,h |
9.6 (2 to 28) | The evidence is very uncertain about the effect on mortality |
| Homecare and medication-review (hmcr & med) | OR 0.16 (0.01 to 2.68) Indirect estimate | RR 0.17 (0.01 to 2.50) | 39 per 1000 (3 to 404) | 163 fewer per 1000 (199 fewer to 202 more) | 1 per 1000 (0 to 20) | 6 fewer per 1000 (7 fewer to 12 more) | ⊕⊝⊝⊝ Very lowd,e |
10.8 (2 to 34) | The evidence is very uncertain about the effect on mortality |
| Homecare, multifactorial-action and review with self-management strategies [hmcr & mfar(w/slfm)] | OR 0.18 (0.01 to 2.26) Indirect estimate | RR 0.19 (0.01 to 2.15) | 43 per 1000 (3 to 363) | 158 fewer per 1000 (199 fewer to 162 more) | 1 per 1000 (0 to 17) | 6 fewer per 1000 (7 fewer to 9 more) | ⊕⊝⊝⊝ Very lowd,e |
10.8 (1 to 34) | The evidence is very uncertain about the effect on mortality |
| Nutrition and exercise (ntr & exrc) | OR 0.22 (0.01 to 4.78) Mixed estimate | RR 0.23 (0.01 to 4.12) | 53 per 1000 (3 to 547) | 149 fewer per 1000 (199 fewer to 345 more) | 2 per 1000 (0 to 34) | 6 fewer per 1000 (7 fewer to 27 more) | ⊕⊝⊝⊝ Very lowi |
13.0 (1 to 39) | The evidence is very uncertain about the effect on mortality |
| Homecare, ADL, multifactorial-action and review with self-management strategies [hmcr & ADL & mfar(w/slfm)] | OR 0.22 (0.02 to 3.01) Indirect estimate | RR 0.23 (0.02 to 2.77) | 53 per 1000 (5 to 432) | 149 fewer per 1000 (197 fewer to 230 more) | 2 per 1000 (0 to 22) | 6 fewer per 1000 (7 fewer to 15 more) | ⊕⊝⊝⊝ Very lowd,e |
13.1 (5 to 35) | The evidence is very uncertain about the effect on mortality |
| Cognitive training, nutrition and exercise (cgn & ntr & exrc) | OR 0.32 (0.01 to 8.09) Mixed estimate | RR 0.33 (0.01 to 6.22) | 75 per 1000 (3 to 671) | 127 fewer per 1000 (199 fewer to 470 more) | 2 per 1000 (0 to 57) | 5 fewer per 1000 (7 fewer to 50 more) | ⊕⊝⊝⊝ Very lowe,f |
15.6 (1 to 40) | The evidence is very uncertain about the effect on mortality |
| Homecare and multifactorial-action (hmcr & mfa-) | OR 0.31 (0.03 to 3.46) Indirect estimate | RR 0.32 (0.03 to 3.13) | 73 per 1000 (8 to 466) | 129 fewer per 1000 (194 fewer to 265 more) | 2 per 1000 (0 to 25) | 5 fewer per 1000 (7 fewer to 18 more) | ⊕⊝⊝⊝ Very lowg,h |
15.9 (7 to 37) | The evidence is very uncertain about the effect on mortality |
| ADL, nutrition and exercise (ADL & ntr & exrc) | OR 0.48 (0.16 to 1.46) Mixed estimate | RR 0.49 (0.17 to 1.43) | 108 per 1000 (39 to 269) | 93 fewer per 1000 (163 fewer to 68 more) | 4 per 1000 (1 to 11) | 4 fewer per 1000 (6 fewer to 3 more) | ⊕⊝⊝⊝ Very lowc,f |
16.1 (4 to 33) | The evidence is very uncertain about the effect on mortality |
| Cognitive training, medication-review, nutrition and exercise (cgn & med & ntr & exrc) | OR 0.49 (0.18 to 1.35) Mixed estimate | RR 0.50 (0.19 to 1.33) | 110 per 1000 (43 to 254) | 91 fewer per 1000 (158 fewer to 53 more) | 4 per 1000 (1 to 10) | 4 fewer per 1000 (6 fewer to 3 more) | ⊕⊝⊝⊝ Very lowc,f |
16.3 (4 to 32) | The evidence is very uncertain about the effect on mortality |
| Homecare, alternative-medicine and exercise (hmcr & hmnt & exrc) | OR 0.31 (0.01 to 7.00) Indirect estimate | RR 0.32 (0.01 to 5.58) | 73 per 1000 (3 to 639) | 129 fewer per 1000 (199 fewer to 437 more) | 2 per 1000 (0 to 50) | 5 fewer per 1000 (7 fewer to 42 more) | ⊕⊝⊝⊝ Very lowd,e |
16.8 (2 to 40) | The evidence is very uncertain about the effect on mortality |
| Education, exercise, multifactorial-action and review with medication-review and self-management strategies [educ & exrc & mfar(w/med + slfm)] | OR 0.49 (0.04 to 5.53) Mixed estimate | RR 0.50 (0.04 to 4.64) | 110 per 1000 (10 to 583) | 91 fewer per 1000 (192 fewer to 381 more) | 4 per 1000 (0 to 40) | 4 fewer per 1000 (7 fewer to 32 more) | ⊕⊝⊝⊝Very lowe,f | 17.9 (1 to 40) | The evidence is very uncertain about the effect on mortality |
| Homecare and nutrition (hmcr & ntr) | OR 0.35 (0.02 to 6.49) Indirect estimate | RR 0.36 (0.02 to 5.27) | 81 per 1000 (5 to 621) | 120 fewer per 1000 (197 fewer to 419 more) | 3 per 1000 (0 to 46) | 5 fewer per 1000 (7 fewer to 39 more) | ⊕⊝⊝⊝ Very lowd,e |
17.9 (6 to 39) | The evidence is very uncertain about the effect on mortality |
| Multifactorial-action and review with medication-review [mfar(w/med)] | OR 0.86 (0.71 to 1.05) Mixed estimate | RR 0.87 (0.72 to 1.05) | 178 per 1000 (152 to 210) | 23 fewer per 1000 (50 fewer to 8 more) | 6 per 1000 (5 to 8) | 1 fewer per 1000 (2 fewer to 0) | ⊕⊝⊝⊝ Very lowc,j |
22.1 (13 to 29) | The evidence is very uncertain about the effect on mortality |
| Welfare rights advice (wlfr) | OR 0.80 (0.29 to 2.22) Mixed estimate | RR 0.81 (0.30 to 2.11) | 168 per 1000 (68 to 359) | 34 fewer per 1000 (133 fewer to 158 more) | 6 per 1000 (2 to 16) | 1 fewer per 1000 (5 fewer to 9 more) | ⊕⊝⊝⊝ Very lowc,f |
22.7 (7 to 37) | The evidence is very uncertain about the effect on mortality |
| Multifactorial-action (mfa-) | OR 0.89 (0.56 to 1.43) Mixed estimate | RR 0.89 (0.57 to 1.40) | 183 per 1000 (124 to 265) | 18 fewer per 1000 (78 fewer to 64 more) | 7 per 1000 (4 to 11) | 1 fewer per 1000 (3 fewer to 3 more) | ⊕⊝⊝⊝ Very lowc,f |
23.2 (11 to 34) | The evidence is very uncertain about the effect on mortality |
| Aids (aids) | OR 0.95 (0.05 to 17.30) Indirect estimate | RR 0.95 (0.05 to 10.24) | 193 per 1000 (12 to 814) | 8 fewer per 1000 (189 fewer to 612 more) | 7 per 1000 (0 to 114) | 0 per 1000 (7 fewer to 107 more) | ⊕⊝⊝⊝ Very lowe,k |
24.6 (2 to 41) | The evidence is very uncertain about the effect on mortality |
| Multifactorial-action with medication-review and self-management strategies [mfa-(w/med + slfm)] | OR 1.00 (0.59 to 1.68) Mixed estimate | RR 1.00 (0.60 to 1.63) | 202 per 1000 (130 to 298) | 0 per 1000 (72 fewer to 96 more) | 7 per 1000 (4 to 12) | 0 per 1000 (3 fewer to 5 more) | ⊕⊝⊝⊝Very lowl,m | 25.5 (14 to 35) | The evidence is very uncertain about the effect on mortality |
| Education and risk-screening (educ & rsk-mfa) | OR 1.00 (0.52 to 1.93) Mixed estimate | RR 1.00 (0.53 to 1.86) | 202 per 1000 (116 to 328) | 0 per 1000 (86 fewer to 126 more) | 7 per 1000 (4 to 14) | 0 per 1000 (4 fewer to 7 more) | ⊕⊝⊝⊝ Very lowc,f |
25.9 (11 to 36) | The evidence is very uncertain about the effect on mortality |
| Care voucher provision (vchr) | OR 1.02 (0.59 to 1.79) Mixed estimate | RR 1.02 (0.60 to 1.73) | 205 per 1000 (130 to 311) | 3 more per 1000 (72 fewer to 110 more) | 8 per 1000 (4 to 13) | 0 per 1000 (3 fewer to 6 more) | ⊕⊝⊝⊝ Very lowm,n |
25.9 (14 to 36) | The evidence is very uncertain about the effect on mortality |
| Risk-screening (rsk-mfa-) | OR 1.03 (0.76 to 1.37) Mixed estimate | RR 1.03 (0.77 to 1.35) | 206 per 1000 (161 to 257) | 5 more per 1000 (41 fewer to 55 more) | 8 per 1000 (6 to 10) | 0 per 1000 (2 fewer to 3 more) | ⊕⊝⊝⊝ Very lowc,o |
26.4 (16 to 34) | The evidence is very uncertain about the effect on mortality |
| Education (educ) | OR 1.41 (0.09 to 23.20) Mixed estimate | RR 1.39 (0.09 to 11.96) | 262 per 1000 (22 to 854) | 61 more per 1000 (179 fewer to 653 more) | 10 per 1000 (1 to 148) | 3 more per 1000 (7 fewer to 140 more) | ⊕⊝⊝⊝ Very lowh,p |
27.2 (3 to 41) | The evidence is very uncertain about the effect on mortality |
| Education, exercise, multifactorial-action and review with self-management strategies [educ & exrc & mfar(w/slfm)] | OR 1.19 (0.31 to 4.54) Mixed estimate | RR 1.18 (0.32 to 3.95) | 231 per 1000 (73 to 534) | 29 more per 1000 (129 fewer to 332 more) | 9 per 1000 (2 to 33) | 1 more per 1000 (5 fewer to 25 more) | ⊕⊝⊝⊝ Very lowc,f |
27.8 (8 to 39) | The evidence is very uncertain about the effect on mortality |
| Education, multifactorial-action and review with medication-review [educ & mfar(w/med)] | OR 1.10 (0.73 to 1.67) Mixed estimate | RR 1.10 (0.74 to 1.62) | 217 per 1000 (156 to 297) | 16 more per 1000 (46 fewer to 95 more) | 8 per 1000 (5 to 12) | 1 more per 1000 (2 fewer to 5 more) | ⊕⊝⊝⊝ Very lowc,f |
27.8 (17 to 36) | The evidence is very uncertain about the effect on mortality |
| Education and multifactorial-action (educ & mfa-) | OR 1.32 (0.23 to 7.39) Mixed estimate | RR 1.30 (0.24 to 5.82) | 250 per 1000 (55 to 651) | 48 more per 1000 (147 fewer to 449 more) | 10 per 1000 (2 to 52) | 2 more per 1000 (6 fewer to 45 more) | ⊕⊝⊝⊝ Very lowc,f |
28.4 (7 to 40) | The evidence is very uncertain about the effect on mortality |
| Education, multifactorial-action and review with medication-review and self-management strategies [educ & mfar(w/med + slfm)] | OR 1.15 (0.66 to 2.01) Mixed estimate | RR 1.14 (0.67 to 1.93) | 225 per 1000 (143 to 337) | 23 more per 1000 (59 fewer to 135 more) | 9 per 1000 (5 to 15) | 1 more per 1000 (3 fewer to 7 more) | ⊕⊝⊝⊝ Very lowc,f |
28.4 (16 to 36) | The evidence is very uncertain about the effect on mortality |
| Exercise and psychology (exrc & psyc) | OR 4.06 (0.44 to 37.10) Mixed estimate | RR 3.59 (0.45 to 14.68) | 506 per 1000 (100 to 904) | 305 more per 1000 (102 fewer to 702 more) | 29 per 1000 (3 to 217) | 22 more per 1000 (4 fewer to 209 more) | ⊕⊝⊝⊝ Very lowm,q |
35.9 (15 to 41) | The evidence is very uncertain about the effect on mortality |
| Monitoring (mntr-mfa-) | OR 4.49 (1.41 to 14.30) Mixed estimate | RR 3.91 (1.39 to 9.15) | 531 per 1000 (262 to 783) | 330 more per 1000 (61 more to 582 more) | 32 per 1000 (10 to 96) | 25 more per 1000 (3 more to 89 more) | ⊕⊝⊝⊝ Very lowe,f |
38.5 (32 to 41) | The evidence is very uncertain about the effect on mortality |
| ADL, aids, education, exercise, multifactorial-action and review with medication-review and self-management strategies [ADL & aids & ed & ex & mf(w/med + slfm)] | OR 8.25 (1.01 to 67.40) Mixed estimate | RR 6.31 (1.01 to 17.69) | 676 per 1000 (203 to 944) | 474 more per 1000 (2 more to 743 more) | 58 per 1000 (7 to 335) | 51 more per 1000 (0 to 327 more) | ⊕⊝⊝⊝ Very lowe,f |
38.9 (23 to 41) | The evidence is very uncertain about the effect on mortality |
Main analysis
The consistency assumption was found to hold for the network according to both the global Wald test (p = 0.303) and the node-splitting method (all contrasts p > 0.05). Between-study variance was estimated to be non-zero, indicating some degree of heterogeneity between studies included in the network (τ = 0.0953). Seven comparisons with ac were of low certainty, the remainder were of very low certainty. ADL, aids and exercise may result in a reduction in mortality in the medium term compared with ac although the CIs were very wide, with the direct evidence being based on 14 events [OR 0.16 (very large), 95% CI 0.03 to 0.71; low certainty]. Similarly, the point estimate for multifactorial-action and review was a slight reduction in medium-term mortality but with wide CIs (OR 0.88, 95% CI 0.66 to 1.18; low certainty). ADL may result in a very slight increase in medium-term mortality (OR 1.03, 95% CI 0.44 to 2.43; low certainty). Exercise, multifactorial-action and review with medication-review and self-management strategies (OR 1.22, 95% CI 0.50 to 3.01); and multifactorial-action with medication-review (OR 1.23, 95% CI 0.50 to 3.04) may both result in a slight increase in mortality (low certainty). Exercise and multifactorial-action with medication-review [OR 1.51 (large), 95% CI 0.25 to 9.20]; and ADL and exercise [OR 1.53 (large), 95% CI 0.41 to 5.70] may both result in an increase in mortality (low certainty), though for all of these, CIs were wide (see Table 22).
Of the 41 intervention groups, homecare and aids; and homecare, multifactorial-action and review with medication-review were ranked first and second but with wide CIs (95% CI 1 to 27 each). ADL, aids and exercise was ranked fifth (mean 8.6, 95% CI 1 to 19). Monitoring (mean 38.5, 95% CI 32 to 41); and ADL, aids, education, exercise, multifactorial-action and review with medication-review and self-management (mean 38.9, 95% CI 23 to 41) were the bottom two ranked groups. Other groups had a middling rank, wide CI or both.
Investigation of small-study effects
The comparison-adjusted funnel plot appears symmetric, implying the absence of small-study effects in the network.
Sensitivity analysis for risk of bias
The eight study results with which we had very serious concerns regarding RoB were removed in the sensitivity analysis. This resulted in separate analyses for the ac and homecare networks which became disconnected.
For ac, 46 trials (26 comparisons) were included in NMA (n = 30,425 participants). The consistency assumption was tested, and no violation of assumption was found (p = 0.387). The estimates of effect in the sensitivity analysis were very similar for the seven comparisons with ac with low-certainty evidence in the main analysis.
In sensitivity analysis for the homecare network, 11 trials (10 comparisons) were included in NMA (n = 2479 participants). The consistency assumption was tested, and no violation of assumption was found (p = 0.914).
Investigation of frailty
All frailty categories were represented in the network and a network meta regression model was fitted including the frailty variable with ‘all (robust, pre-frail and frail)’ set as the reference category. The consistency assumption remained valid (global Wald test p = 0.200, node-splitting method showed all contrasts p > 0.05). Between-study variance remained small, but non-zero (τ = 0.149). Frailty effects could not be estimated for most contrasts due to collinearity. Where frailty effects could be estimated, the effect was estimated with very large uncertainty as reflected in wide 95% CIs covering a broad range of both beneficial and harmful effects making interpretation of these results meaningless in practice.
Chapter 5 Health economic evidence
Economic evaluation findings
Among the 129 included studies, 56 (43%) described a plan to conduct an economic evaluation. Thirty-nine studies (30%) compared at least two alternatives in an economic evaluation and reported the findings. We described and summarised the context and principal findings of these 39 reported economic evaluations in Report Supplementary Material 11. Fifteen studies did not report any findings;92,95,101,102,123,130,140,154,158,167,168,176,178,179,187 and two studies detailed the cost items and estimated the experimental intervention costs but did not compare the costs with an alternative. 97,164
The 39 studies included 27,463 older people. All studies were conducted in developed countries or regions: seven countries in Europe (the Netherlands, UK, Finland, Switzerland, Denmark, Germany, Norway; 21 studies), two North American countries (USA, Canada; 13 studies), two countries from Australasia (New Zealand, Australia; four studies) and one country and one region from Asia (Japan, Hong Kong; 3 studies); the Netherlands and USA contributed the most studies (9 each). The analytic price year (assumed to be the trial follow-up period if unclear) ranged from 1988 to 2018. Fifteen evaluations took place in the 2010s, 17 in the 2000s and 9 in the 1990s. Ten currencies were used, including the Dutch guilder (NLG), which was replaced by the Euro in 2002.
Twenty-seven intervention groups (experimental and control) were evaluated in the 39 studies. All studies compared one experimental intervention with a standard intervention or ac (control comparator), except Bleijenberg 201669 and Hay 1998. 106 which compared three interventions. The experimental and standard interventions were grouped into the same intervention group in two studies. 120,161
Analytic perspectives and time horizon
Twenty-nine studies conducted the economic evaluation from the perspective of health and social care system only; nine adopted a societal perspective, which included the health and social care system perspective; one study evaluated the costs from the perspective of health and social care system and the societal perspective separately. All studies included healthcare costs and/or other sector costs. Twelve studies included the patient and family copayments (out-of-pocket expenses) for care. 74,78,86,106,132,136,146,147,161,182,186,190 Eight studies calculated the non-monetary resources for informal care from family and friends into the total costs. 70,74,78,79,86,136,175,186 Only two studies included the productivity impact. 106,132 The commonly reported health sector cost items were primary care (including professional visits), inpatient and outpatient hospital care, emergency department visits, pharmacy costs and rehabilitation. The commonly reported other sector cost items were residential/nursing home admission, respite care, day-care, domestic and/or personal homecare and Meals on Wheels. We have specified reported differences from these items in Report Supplementary Material 11.
The time horizon usually covered the whole trial follow-up period of the study, with length ranging from 6 to 36 months. Results were reported for the whole period except for seven studies, three of them could not follow up all participants for the whole trial follow-up period,146,147,174 and four reported the intervention phase and the post-intervention phase findings separately. 79,82,106,121
Evaluation types
The economic evaluation analytic framework of all 39 studies appeared to be trial-based, but this was not explicitly stated in most studies. The evaluation appeared to be planned and embedded within each study, except for one study which described it as post hoc. 99
All 39 studies reported cost findings. Most studies estimated the total costs of health and social care, and incorporated the costs of experimental intervention and standard intervention or ac into the total costs. Most of the studies described the origins of the cost item prices, for example the national healthcare mean unit cost for a particular year reported by the government, salaries (time and human resources) or materials used during the intervention delivery. Two studies did not collect cost data from the control arm. For conducting the economic evaluation, they assumed that the only difference in cost was that of delivering the experimental intervention. 80,99
Thirteen studies73,75,86,99,110,133,136,146,147,161,165,174,182 conducted cost-effectiveness analysis using health outcomes, for example life years saved, Modified Katz ADL Index. Four of them estimated cost-effectiveness with health outcomes which are not of interest in this review, namely Falls Efficacy Scale-International (FES-I),86 transition out of frailty,75 quality of care110 and Canadian Occupational Performance Measure (COPM) performance scale and satisfaction scale. 174 Fourteen studies70,74,75,79,80,86,112,121,127,136,161,165,175,190 conducted cost-utility analysis using quality-adjusted life-years (QALYs) calculated from the EuroQol Five Dimensions questionnaire (EQ-5D), 12-item Short Form health survey (SF-12), 36-item Short-Form health survey (SF-36) or 15-dimensional measure of health-related quality of life (15D). Twelve studies74,75,99,110,112,133,146,147,174,175,182,190 estimated the cost-benefits, mostly by estimating the probability of willingness to pay a certain amount of additional cost for the health effects. Twenty-three studies70,73–75,82,86,99,110,112,121,127,133,136,146,147,161,163,165,174,175,182,186,190 conducted sensitivity analysis to estimate the uncertainty. These sensitivity analyses included cost-effectiveness plane, cost-effectiveness acceptability curve, alternative values of resources and subgroup analysis.
Economic evaluation results and study conclusion
Based on the conclusion of the 39 studies, we identified the experimental interventions which were clearly concluded as a more cost-effective, lower-cost alternative or recommended by the study authors; were explicitly not recommended; and those that no definite conclusion was reached. The conclusions drawn from full economic evaluations in 22 studies are reported separately from the 17 studies that only conducted cost analysis. In most cases, the conclusions regarding an intervention group for a particular time horizon were only drawn from one study. Therefore, we have only stated when there is more than one relevant study.
Cost-effectiveness findings from full economic evaluations
Short-term time horizon
For the short-term time horizon, the authors of four studies79,80,133,174 concluded that three groups of interventions were more cost-effective in comparison with standard intervention or ac. These intervention groups were evaluated in two similar studies which evaluated meaningful-activities and education versus ac, one adopted a societal perspective79 and the other adopted a health and social care system perspective;80 one study evaluated multifactorial-action and review with medication-review versus ac;133 and one study evaluated homecare, multifactorial-action and review with medication-review and self-management versus homecare, multifactorial-action and review. 174 The latter two studies adopted a health and social care system perspective. 133,174
Two intervention groups were not considered cost-effective by the studies that evaluated them161,175 due to a lack of evidence of clinical effectiveness, in comparison with standard interventions, in the short-term time horizon. Stewart 2005161 evaluated multifactorial-action delivered by an occupational therapist (experimental arm) versus delivery by a social worker (control arm), from a health and social care system perspective. van der Pols-Vijbrief 2017175 evaluated homecare, nutrition, multifactorial-action and review versus homecare, from the societal perspective.
No definite conclusion was drawn for multifactorial-action and review with self-management versus ac for the short-term time horizon. One study190 adopted a health and social care system perspective and concluded that this intervention was not dominant and the probability of cost-effectiveness was conditional on the amount a commissioner was willing to pay. 190
Medium-term time horizon
For the medium-term time horizon, two studies79,86 concluded that their experimental intervention was likely to be cost-effective in comparison with ac. The intervention groups were ADL;86 meaningful-activities and education. 79 Both studies evaluated from a societal perspective.
Three studies concluded that multifactorial-action and review with medication-review in comparison to ac was probably not cost-effective in the medium-term time horizon, mainly based on the high costs or low probability of the intervention being cost-effective at acceptable cost thresholds. 74,110,165 Two studies adopted a healthcare services perspective110,165 and one study adopted a societal perspective. 74 Additionally, one study75 estimated, from a health and social care system perspective, that the probability of saving based on QALY was low for the overall group, frail and very frail subgroups of participants; although between the two subgroups, the probability was approximately twice higher in the very frail subgroup (17.8%) than the frail subgroup (8.2%).
Five studies70,99,121,146,147 reported uncertainty regarding the cost-effectiveness of their experimental intervention in comparison to standard intervention or ac for the medium-term time horizon. They concluded that cost-effectiveness was dependent on the effects and perspectives that were valued. The intervention groups were: ADL, aids and exercise;99 ADL, nutrition and exercise;121 multifactorial-action with medication-review and self-management,70 each compared with ac; and homecare, ADL, multifactorial-action and review with self-management;146 and homecare, multifactorial-action and review,147 each compared with homecare and multifactorial-action. The evaluation of the first intervention adopted a healthcare services perspective; the others adopted a societal perspective.
Long-term time horizon
One study concluded that exercise and multifactorial-action with medication-review dominated ac (i.e. representing additional effects with lower costs) in a long-term time horizon, from the health and social care system perspective. 127
Three studies73,112,136 concluded that their experimental intervention had a low chance of being cost-effective in comparison to ac, mainly based on a lack of treatment effects. These intervention groups were education, multifactorial-action and review with medication-review and self-management;136 multifactorial-action and review with medication-review;73 and welfare-advice. 112 The first intervention was evaluated from a societal perspective,136 the other two were evaluated from a health and social care system perspective. 73,112
Two studies121,182 were uncertain about the cost-effectiveness of their experimental intervention for the long-term time horizon in comparison to a standard intervention or ac, because these interventions were more costly and thus the willingness to pay depended on the decision-makers’ valuation of the health effects. The intervention groups were: ADL, nutrition and exercise versus ac;121 and multifactorial-action and review with medication-review versus multifactorial-action and review. 182 Both were evaluated from a health and social care system perspective.
Consistent and inconsistent findings between studies
Two intervention groups, except standard intervention or ac, were investigated by full economic evaluation in more than one study. We found that each of these groups was only compared as experimental intervention with ac. Meaningful-activities and education was consistently concluded to be more cost-effective than ac in the short- and medium-term time horizons in two studies. 79,80
Multifactorial-action and review with medication-review was evaluated in comparison to ac in five studies across all the three time horizons. It was only concluded to be more cost-effective in the short term,133 but unlikely cost-effective in the medium term74,110,165 and long term. 73 The long-term cost-effectiveness of this intervention was uncertain in comparison with multifactorial-action and review. 182
Cost-analysis findings
Short-term time horizon
For the short-term time horizon, two studies78,132 recommended their experimental intervention based on the small cost difference between arms and the potential benefits from the interventions. One study evaluated multifactorial-action and review with medication-review versus multifactorial-action and review;78 one study evaluated homecare, multifactorial-action and review with medication-review and self-management versus homecare, multifactorial-action and review. 132 Both were evaluated from a societal perspective.
One study186 evaluated the comparative costs of multifactorial-action and review with self-management versus ac, for the short-term time horizon from the two perspectives separately. The authors concluded that whether the intervention was cost-saving depended on who commissioned the services. 186
Medium-term time horizon
Four studies68,69,124,131 concluded that, on average, the medium-term care costs were lower and the potential health effects, for example better preservations of daily function, were likely better in participants who received the experimental interventions compared with those who received ac or homecare only. The two intervention groups compared with ac were risk-screening;69 and education, exercise, multifactorial-action and review with medication-review and self-management. 124 The two intervention groups compared with homecare were homecare and aids;131 and homecare, multifactorial-action and review with medication-review. 68 All were evaluated from a health and social care system perspective.
Two studies106,150 concluded that their respective experimental interventions did not improve costs of services or health outcomes for the medium-term time horizon. The two intervention groups evaluated were education, multifactorial-action and review with medication-review;150 and multifactorial-action,106 each in comparison to ac. The former was evaluated from a health and social care system perspective;150 the latter was evaluated from a societal perspective. 106
Two studies82,143 concluded that their experimental interventions were cost neutral from the health and social care system perspective for the medium-term time horizon. The intervention groups were education, multifactorial-action and review with medication-review;143 and education, multifactorial-action and review with medication-review and self-management,82 each in comparison to ac.
Long-term time horizon
One study concluded that homecare, education, multifactorial-action and review compared with homecare could result in very substantial savings in the long-term time horizon. 125 This conclusion was based on the lower total costs among the participants who received the experimental intervention, evaluated from a health and social care system perspective.
Two studies106,181 concluded that their experimental interventions provided no demonstrable benefits in terms of costs or health status, in the long-term time horizon. The intervention groups were multifactorial-action;106 and multifactorial-action and review,181 each in comparison to ac. The former was evaluated from a societal perspective,106 the latter was evaluated from a health and social care system perspective. 181
Three groups of experimental interventions were compared with a standard intervention or ac with a health and social care system perspective in five studies,81,82,118,120,163 of which the study authors concluded that the care costs or care demand were not constant over the long-term time horizon. They suggested that the experimental intervention arms were more costly during the intervention phase or to the sector paying for the interventions, but total costs appeared lower at a later time or to other sectors. Therefore, at best, the total costs over the long-term time horizon for participants receiving any of the interventions or ac are similar. The intervention groups were: education, multifactorial-action and review with medication-review and self-management compared to ac in two studies;81,82 multifactorial-action and review with medication-review compared to either ac163 or multifactorial-action and review;118 and an alternative version of multifactorial-action and review;120 compared with a standard intervention in the same group.
Discussion and conclusion about the economic evidence
The economic evaluations of the included studies were not critically appraised. Therefore, we have not attempted to compare the methods or findings of these studies directly. Instead, this commentary indicates the available economic information and evidence that may be relevant to the readers and decision-makers in considering whether to implement any of the interventions evaluated in these studies.
We found a wide variation in cost items and perspectives used in the evaluations, reflecting the variations in content in standard interventions and ac for older people between countries. For example, services that are freely available to older people in need and paid for by the health system in one country may require out-of-pocket payments by older people in another country. This potentially influences the care services which the older people may actively acquire and use.
We found only one study that explored the effect of frailty on cost-effectiveness, which was a trial of exercise, multifactorial-action and review with medication-review and self-management. 75 It subgrouped the participants as either frail or very frail and estimated that the probability of cost-effectiveness based on QALY was low in the very frail subgroup and even lower in the frail subgroup.
From the evidence collected from the 22 full economic evaluations, 5 intervention groups appeared promising compared with a standard intervention or ac from an economic perspective. They are ADL (medium-term time horizon), meaningful-activities and education (short- and medium-term time horizon), homecare, multifactorial-action and review with medication-review and self-management (short-term time horizon), multifactorial-action and review with medication-review (short- but not medium- or long-term time horizon), and exercise and multifactorial-action with medication-review (long-term time horizon).
Limitations
We only drew on the verbatim conclusion from each study to summarise the results. The context, such as settings, hypothesised health benefits and assumptions, economic evaluation aims, methods and time horizons, varied between the studies. All included evaluations appeared trial-based and the time horizon was limited to the trial period, making it difficult to infer the cost-effectiveness and costs beyond the measured period without further analyses. The outcomes and their relevance can be influenced by and sensitive to the choices of cost items, whose costs, whose and what values (e.g. quality of life, remaining in community) to focus on and measure.
Some studies showed low intervention cost and reasonable probability of cost-effectiveness, and yet did not recommend the experimental intervention due to the low certainty in health effect gain. 110,112 On the contrary, a study found higher intervention cost and lower probability of cost-effectiveness but suggested that the experimental intervention was worth implementing. 133 Even though some studies suggested benefits and recommended implementation of the intervention, there may be uncertainties and limited evidence in their findings. We considered that each study justified the conclusion according to how the findings best suited the research context, aim and perspectives, which the study had set to investigate and address. Moreover, most intervention groups were only evaluated in a singular study each; and the analytic methodology, such as analysis techniques, varied between the studies. Hence, the variations in all these aspects make direct comparisons between the studies difficult.
Sixteen studies only analysed the costs. Nonetheless, they provided details about the resources required for the experimental intervention, which is useful information for decisions about implementing the interventions. Readers and decision-makers should consider the health and other relevant outcomes reported from the respective randomised controlled trials, especially for the interventions which were only evaluated by cost analysis.
Readers of this review will need to assess the extent to which methods and findings of these identified economic evaluations may apply to their setting; and decide whether economics or merely affordability is the main interest for judgement. The studies used mainly actual costs and evaluated for each treatment arm separately. Therefore, if the evaluation context is applicable, the costs and cost-effectiveness results reported in these studies can be reasonable estimates for real-life settings.
Chapter 6 Discussion
Summary of main results
We have grouped community-based complex interventions to sustain independence in older people and synthesised evidence about their effectiveness but remain uncertain about the optimal configuration of such services or whether their effectiveness is related to the frailty of the population. We included evidence from 129 studies with 74,946 participants allocated in 266 intervention arms, which we placed into 63 intervention groups. We found evidence that multifactorial-action and review with medication-review probably improves some important outcomes slightly, but there was also contradictory evidence in the long term. For some other intervention groups there was low-certainty evidence that they may improve or worsen particular outcomes but for most intervention groups evidence was either absent or very uncertain.
For our main clinical outcomes for interventions compared with ac, there were two findings of moderate certainty of some improvement and one of some worsening. There were 16 low-certainty findings of some improvement, 14 of some worsening and 2 of little to no difference. Comparisons with ac can be conceptualised as the effect of addition of an intervention for a general population of older people, not all of whom were in receipt of any particular form of care, referred for a particular service (e.g. homecare, occupational therapy) nor in receipt of regular check-ups. Among comparisons with homecare, there were only six findings of low certainty, two of some benefit, three of some harm and one of little to no difference. Comparisons with homecare can be conceptualised as the effect of an intervention for a population already in receipt of, or newly referred for, homecare, but where that homecare is not allied with rehabilitation (e.g. reablement) or broader care planning. Although these findings were mostly uncertain and often related to small or very small differences, they did not mostly indicate one direction of effect. Therefore, it does not appear that all community-based complex interventions are necessarily beneficial, and some may worsen these outcomes.
There are several plausible interpretations of the mixed findings, including that for many of these services there is no real effect, that some services do more harm than good or that changes that appear to indicate harm are appropriate outcomes in certain circumstances. The uncertainty in these estimates was primarily driven by CIs that included benefit and harm. It may be that these often-small estimates represent the play of chance around no effect, which appear because so many estimates of effect are being produced. However, it could be that the CIs would narrow around similar point estimates if more data were available. Therefore, it may be that some services produce negative outcomes, despite the best intentions of all involved. Although this was not a finding we anticipated, it is not one that should be dismissed entirely. Plausible mechanisms are dependent on the details of the intervention group but include invoking disengagement with the person’s health or with services, encouraging an individual to take on more than they are capable of or have the resources to effectively manage, or inducing dependency through risk aversion. It can also be the case that certain events, for example care-home placement or hospitalisation, may represent part of the best care strategy for an individual. Even deterioration in ADL may be in response to provision of assistance for tasks that someone finds difficult or painful and otherwise unrewarding and therefore an acceptable trade-off.
The moderate-certainty evidence all related to multifactorial-action and review with medication-review in comparison with ac, finding a probable slight increase in living at home in the medium term and very slight increase in independence in IADL in the medium term, but in contrast also a very slight reduction in IADL in the long term. The finding of probable harm was driven by the results of Rubenstein 2007,154 which also contributed to the beneficial medium-term finding. Low-certainty findings for this intervention group included a slight increase in living at home in the long term, as well as little to no difference in short-term living at home or long-term personal ADL. We also found economic evidence that it can be cost-effective in the short term due to improvements in self-reported mental health, PADL and IADL but also that it was unlikely to be cost-effective in the medium or long term. Clinical findings from other intervention groups that also included multifactorial-action with medication-review (but not self-management) were generally positive.
Among the mixed and uncertain findings, there were some regularities worth noting. The two intervention groups including both nutrition and exercise (ADL, nutrition and exercise; cognitive training, medication-review, nutrition and exercise) were associated with low-certainty findings of increased chance of living at home, and no negative findings. In both these groups the interventions were provided to pre-frail and frail populations following screening. However, the evidence for medium- and long-term cost-effectiveness of ADL, nutrition and exercise was uncertain. Intervention groups including multifactorial-action and review with both medication-review and self-management were generally associated with low certainty findings of worse independence (living at home and ADLs). However, the available economic evidence suggests homecare, multifactorial-action and review with medication-review and self-management may be cost-effective in the short term. It is important to recognise that these are post-analysis observations and therefore apparent patterns may be misleading.
In addition to the findings mentioned above, the summary of economic evidence identified promising intervention groups as ADL (medium term); meaningful-activities and education (short and medium term); and exercise and multifactorial-action with medication-review (long term) based on full economic evaluations.
Our investigations of the impact of frailty and pre-frailty on intervention effects were hampered by a lack of comparisons that contained different frailty populations meaning we were often unable to explore differences. Where we were able to conduct analyses, the results were very uncertain.
Overall completeness and applicability of the evidence
We identified 129 eligible studies despite limiting our criteria to interventions with an explicit focus on independence in ADL, being initiated in the community and excluding falls-specific interventions. Through our intervention categorisation approach, we identified 19 broad components of care and 63 intervention groups among the 266 included interventions. Therefore, a very broad range of interventions have been trialled, but at the same time there are many more possible combinations yet to be trialled. Of those interventions that were trialled, many were not includable in each of our analyses because they had not measured our outcomes of interest or had not reported the results in a way we could include them. For those that we were able to include, we often found the evidence to be of very low certainty, primarily due to insufficient sample sizes that led to wide CIs, and RoB (see Certainty of the evidence below).
The trials were typically pragmatic trials and therefore the results are likely to be applicable to similar contexts. However, given the complexity of the interventions and their interaction with context, it is unclear how broadly applicable the results are to different times and places. The participant groups were often targeted, and this should also be taken into consideration when considering applicability to a general population of community-dwelling older people. However, the frailty meta regressions did not identify significant differences in effect for different populations, although these were typically of low power. Exclusions may have limited the possibility for improvement through ceiling effects. However, only 3 of the 108 populations classified for frailty were robust and a further 13 populations did not include the frail group, meaning 92 populations included people living with frailty. Our approach to intervention grouping also separated out the effect of interventions delivered in conjunction with homecare to participants who already qualified for this support, therefore providing evidence of direct relevance to this particularly vulnerable community.
Equality, diversity and inclusion
The core of this review addresses the equality of providing appropriate services to older people. In focusing on maintaining or improving independence, many of the services are designed for people with disabilities. This review’s evidence originated from a diverse population of older people, mainly from the more developed countries, territories and regions. Population subgroups of various socioeconomic status, frailty levels and ethnicity were included, and the sex ratio seems to reflect that of this age group. Our inclusion criteria did not restrict any of these characteristics; hence our results included studies conducted with a diverse range of population subgroups, for example interventions targeting specific frailty level or all frailty levels, general older population in a community or those classified into lower socioeconomic status.
The included studies were mostly conducted in developed countries; two developing countries are also included, namely China114 and Thailand. 115 Some studies specifically targeted people who were socioeconomically deprived,64,67,82,94,112,134,158,166,167 ethnic minorities or immigrants in the country. 63,67,80,94,122,129
Forty-four studies reported the participant’s ethnicity; three studies only reported participant’s country of origin; one study explicitly restricted eligibility to the local majority ethnic group. 93 Twenty-one studies explicitly restricted the eligibility criteria to only include older people or their caregivers who were able to speak the local languages, and thus may not approach or include people who were not native. White, black, Hispanic, Latino, Māori, Indian, Pacific Islander, Filipino and American Asian were the reported included ethnic groups, and we can infer that Japanese, Chinese and Thai were also included. Most of the studies were conducted in Europe and North America and predominantly included white people; hence white participants are the majority in this review. Studies attempted to address the socioeconomic status of the older people in terms of income, property ownership, education level, last occupation or deprivation index based on home address. However, the methods vary; none of these alone or combined is sufficient to reflect the resources and support available and accessible to the older people who have retired or reduced working hours. A minority of study authors commented on the ethnic groups and socioeconomic subgroups proportions if the study particularly targeted some of these groups, for example lower-income older people; or the authors suggested the limitations in representativeness of the included ethnic groups and generalisability of the study results. Some authors commented that their study population was not as deprived as their respective target population, even though the eligibility criteria and recruitment strategies were intended to achieve this. 64,112,134,186 This suggests that some population groups may be more hesitant in participating in research studies or have difficulties in accessing care. Thoughtful strategies are essential in reaching them and ensuring care accessibility for all with need.
In the majority of studies over 50% of participants were women, while in four US studies in the Department of Veterans Affairs healthcare system, veterans were predominantly men (0.6–3.2% women). 88,139,140,155 The sex ratio among the included studies was approximately 64 men per 100 women, which is similar to recent ratios of over-65-year-olds in the more developed regions in the world (60–74 men per 100 women)596 but lower than the UK’s 2019 ratio (84 men per 100 women). 597 Nevertheless, there may be contextual differences in the willingness to participate in research and seek interventions between men and women.
In common with much historical research, gender and sexuality issues have not been examined here. We do not know if these studies are representative of, or applicable to, the lesbian, gay, bisexual, transgender and queer or questioning (LGBTQ) community.
Participants of all levels of frailty were included. Only a minority of studies targeted older people with no restriction on health condition, for example Howel 2019112 or Vass 2005. 182 Half of the included studies (65 studies) explicitly excluded older people who had cognitive impairment (based on self-report or cognitive screening test) at baseline, which may limit the generalisability of the results to this subgroup of older people. Another common exclusion health reason was terminal illnesses, which is less relevant to this review’s interest. Most of the studies employed secondary (e.g. screening, preventative home visits) or tertiary [e.g. functional task exercise (FTE)] prevention strategies, which targeted older people with symptoms or health conditions. However, even among studies with the oldest populations, people of all frailty levels (robust, pre-frail and frail) were included and the experimental interventions contained health promotion components such as health education,102 nutritional advice and physical exercise. 96
Some interventions were targeted at older people living alone, including provision of community-nurse-based comprehensive assessment and case management (multifactorial-action and review)108 or smart-home aids and adaptations. 172 By contrast, one study investigated integrated nursing care for people living in apartment-sharing communities. 169 Living status can reflect some of the support available to an older person. Some studies required a consented informal caregiver to participate with the older person, thus people who could not identify such a caregiver would have been excluded.
Readers should be aware that the overall diversity included in this review is founded on various narrower subgroups from the individual studies, and therefore the evidence for most intervention groups is based on less diverse population subgroups. When considering the transferability of the review’s findings, readers should specify the target population and their needs, and consider how appropriate the evidence base is for a particular intervention group. The participant characteristics included in this review or in the individual studies may guide the readers and decision-makers, yet cannot clearly predict success or failure. For instance, an intervention already trialled with the local ethnic majorities102 was modified and adapted to suit lower-income ethnic minority migrants to the country, including arrangement of translators and consideration of cultural acceptability in the implementation and delivery. 67 Although the original trial reported postponed decline in health outcomes including self-rated health, the trial of the adapted intervention found no such differences; the authors acknowledged that people with poor language skills and lower education levels were likely to drop out or not be recruited. Hence differences in treatment effects of the same intervention between ethnic groups may be partly explained by the intervention implementation, delivery and uptake. The society’s socioeconomic (e.g. health and social care system, who pays for care), cultural (e.g. lifestyle preference and habits) and environmental conditions (e.g. infrastructure of health and social care, accessible care for all) may be more influential than an individual’s personal characteristics (e.g. age, sex, ethnicity) in the generalisability of interventions involving changes in lifestyle and care provision. 598,599
Certainty of the evidence
Many of the trials appeared to be well conducted under challenging circumstances. Few results were downgraded for high RoB related to randomisation, deviations from the intended interventions, outcome measurement or selection of the reported result. We usually had some concerns (but not serious concerns) about possible deviations from the intended interventions as it was unclear if the trial context may have contributed to changes in ‘usual care’, with participants in the control group seeking alternative interventions as a result of their enrolment and allocation. We typically had some concerns about outcome measurement where our outcome of interest was self-reported: ADLs, self-reported health, depression and loneliness. In most studies participants were likely to be aware of their assignment and we judged there was some risk that their reporting was influenced by this knowledge, but we did not consider the risk to be high. We also typically had some concerns about selection of the reported result as there was no detailed and pre-specified statistical analysis plan available, nor a statement about blinded analysis.
Most results were downgraded due to serious concerns about RoB arising from missing outcome data. This was often inevitable given the combination of a frail population, long timelines, self-reported outcomes and community-based research. It is important to note that many of the risks of within-study bias were not necessarily the risk of finding a false positive but also bias towards no effect or favouring the control group. Therefore, there is a real risk that intervention effects are under-identified here.
No results were downgraded for indirectness. We made a careful selection of appropriate measures for each outcome and limited results to three time frames. Our study inclusion criteria also led to a selection of relatively pragmatic community-based interventions. However, it is difficult to define one precise population and context to which the interventions are intended to apply. We also identified no strong evidence of differences in effect among them. Because most comparisons were populated by only one trial it is unclear if the evidence is applicable to different contexts. If someone was considering implementing one of these interventions, we recommend examining the contexts and populations of the trials in which it was analysed to consider how applicable the evidence may be for a given scenario.
Only two comparisons with ac were not rated down for imprecision: multifactorial-action and review with medication-review for medium-term IADL independence and risk-screening for long-term care-home placement. Most results were downgraded twice for imprecision, usually because their CIs included substantial benefit and substantial harm. This was partly a product of our interest in effects that we labelled ‘very small’ but still considered important. Therefore, an alternative formulation of the review that considered only larger effects to be important may have found greater certainty in more results and that they equated to little or no difference in effect. We believe it was appropriate to seek to identify the relatively small effects that we have, given the importance of these outcomes.
Few results were downgraded for heterogeneity (inconsistency), but this is not because there was uniformity among the studies. There were relatively few studies where pairwise comparisons contained more than one intervention and therefore heterogeneity did not substantially affect most estimates. However, it may be that with the inclusion of more studies we would identify statistical heterogeneity that was not apparent in these analyses.
No results were downgraded for inconsistency (incoherence). It was rare that we were able to assess the difference in direct and indirect estimates as there were few closed loops in our networks. Where we were able to examine this, our assessments were inevitably low powered. Nonetheless the available data and global statistics did not tend to indicate a problem with inconsistency where it could be examined. In one case, we would have considered rating down for inconsistency, but the evidence was already judged very low certainty (multifactorial-action vs. ac for medium-term mortality).
We found no evidence of non-reporting bias (publication bias/small-study effects) through examination of funnel plots although again, it was rare that we were able to investigate this. Some studies did not report usable results for our outcomes of interest, even though it appeared they had measured the outcome, but it was not clear that this was driven by the results as very few of any results reported were statistically significant.
We did not GRADE the ranking of interventions, but CIs were usually wide. Additionally, because the estimates for many interventions were of very low certainty, the meaning of particular rankings was unclear. Therefore, we have placed little emphasis on rankings in our interpretation.
Potential limitations in the review process
The search was complex due to the broad nature of the interventions we were including, which were not limited to an explicit list of intervention types. We built an extensive search strategy and compared results with lists of studies from previous similar systematic reviews; we also included citation searching in our strategy. In screening 40,112 references we cast our net wide to give us the best chance of identifying all relevant studies but, given the lack of specificity, it is likely that some includable studies remained unidentified.
One of the criteria for inclusion was that the intervention aimed to sustain independence in ADL. Aims of interventions (rather than studies) were often not explicitly stated. We included studies where independence in ADL was stated as an aim of the intervention or was measured. We were aware that some interventions that appeared similar in terms of process were excluded, and it is possible that there was selection bias related to non-reporting bias – studies that had measured independence in ADL but failed to report this were excluded – which in turn would be likely to relate to the effects measured. Given that very few studies reported statistically significant findings for ADL it seems unlikely that this was a substantial problem.
We attempted to obtain additional information from many study authors and these attempts were often successful, however, we were not able to be certain of the exclusion of five studies for which we had insufficient information to make a decision.
For sixteen studies, although we had sufficient details to include them in this review, we had no results reports for our outcomes of interest. We contacted the study authors and searched for the reports at least twice.
We developed our grouping of interventions using a data-driven approach with expert guidance. We did so without consideration of the effects reported by studies nor with a particular intervention we were seeking to highlight. However, we acknowledge other syntheses may identify alternative groupings. Indeed, it is easy to imagine many dimensions by which the interventions could be grouped, and these were coded and summarised as part of our work. However, we think some broad considerations of the procedures are likely to be a basic constituent of most classifications of interventions in effectiveness reviews (e.g. Tricco et al. 25). It should also be noted that dividing interventions on other dimensions may have been challenging in practice as other aspects of interest were often not reported consistently, or, in the case of dose/intensity, would be difficult to divide cleanly across such diverse interventions.
Our findings provide an evidence-based and more sophisticated alternative to the ad hoc classification of community-based complex interventions for older people published by Beswick et al. , where five intervention groups were defined (geriatric assessment of older people; geriatric assessment of older people assessed as frail; community-based care after hospital discharge; fall prevention; counselling; and group education and counselling). 8 When we first designed this review, we planned for an expert reference panel to suggest the groupings. However, we revised our plans to conduct the intervention grouping within the research team as we considered there was insufficient tacit expertise on how it is most appropriate to group these interventions for NMA and the volume of data that was produced by our analysis would have been inappropriate for consideration by an expert panel. Having developed groupings, we then sought to refine these based on feedback in open discussions with experts to ensure their clarity and suitability.
The way we grouped interventions, combined with the lack of studies that contributed to most analyses, resulted in sparse networks. In developing the groups, we focused on identifying clinically useful distinctions rather than groups that pooled some number of interventions. We tried to keep components broad but meaningful (e.g. grouping aerobic and resistance exercises but separating these from ADL training). Key to our approach was the principle that the identified components should be intended for all participants. Therefore, differences in groups reflected substantial differences between the interventions (e.g. whether or not everyone was supposed to receive health education). We did not intend or realise how sparse the networks would be until the analyses were conducted. It would have been possible to take steps to regroup the interventions to pool more data. For example, we could have ignored the features of multifactorial-action (self-management strategies and medication-review) by which we further divided that component. We also considered uniting the ac and homecare networks by either ignoring the homecare component in all groups or just grouping homecare with ac. We decided to analyse the interventions consistent with our original groupings given the diversity of multifactorial-action and the potential importance of homecare as a cointervention and delivery mechanism. We also hoped this would produce separate evidence of greater relevance to the social care sector. More radically collapsing the groups, for example to single-domain, multidomain, multifactorial, and multifactorial and multidomain interventions would have produced an analysis with more pooling of evidence, which might have narrowed CIs, but may also have introduced substantial statistical heterogeneity and very abstract groups. By dividing the interventions as we did it is possible that we lost power but gained resolution, which may ultimately be more useful for practical decision-making.
We set out our approach to NMA unaware of how sparse our networks would be. We could have explored a variety of approaches to estimating the effects, which may have given us greater certainty in some of our estimates. 600 Due to the sparsity of both direct and indirect evidence, there was low power to examine heterogeneity and inconsistency in most of the networks.
We were unable to effectively investigate the impact of population frailty on intervention effects. Many intervention groups were unable to be assessed due to collinearity, and all frailty analyses had low power due to the small number of studies included in these analyses. Because we identified homecare as a cointervention for both arms of many studies, we divided our network in two in a way that was highly correlated with frailty, with most homecare populations being frail. This further limited our ability to investigate the effect of frailty.
Although we initially planned to use GRADE to assess certainty, we decided to use CINeMA to make our assessments more systematic and reproducible. However, once we had performed our analyses, we discovered that the CINeMA software could not estimate imprecision, heterogeneity or incoherence for many of our networks. We therefore decided to return to our original plan of using GRADE. Had we used fallback rules for these networks suggested by the authors of CINeMA we would have rated all of the evidence from estimates with no concerns about RoB, indirectness, publication bias or imprecision as very low confidence due to major concerns with heterogeneity and incoherence as they could not be evaluated. By contrast, where we could have implemented CINeMA as intended, we would have rated the evidence for some estimates as high or moderate certainty without manual adjustment that we have rated low or very low certainty using GRADE because the estimates were based on very small amounts of information.
Our summary of economic evidence drew on the verbatim conclusions of the included studies rather than reanalysing their results, therefore it is difficult to examine the comparative strengths of the findings. We did not conduct an additional search for economic evaluations set outside of a trial context. Therefore, further cost-effectiveness information may be available to inform decisions.
Patient and public involvement
This review benefited from the involvement of our established PPI FOG in the Bradford Institute for Health Research. The FOG has a structure that provides connections to the whole spectrum of older people, with a focus on those living with frailty to enable meaningful, public involvement in our research projects. We consulted our FOG throughout the development of the protocol and discussed plans in detail at the group’s quarterly meetings and at our annual consumer research conference. Particular examples of PPI contribution include the selection of important outcomes and their prioritisation as main and additional outcomes. FOG members emphasised a wide range of outcomes were important to older people, with a particular focus on independence in addition to well-being, alongside service-orientated outcomes. We also spent time discussing the intervention components we had identified with FOG members. Through this work we developed and refined our plain language descriptions, public-facing names, and domains in which to organise and explain the components and thus the findings. FOG members also helped draft and revise the plain language summary for our original application and this final report, ensuring this work was clearly and carefully explained. Because this was secondary research there were no participant materials to review, nor current ethical implications to consider.
Agreements and disagreements with other studies or reviews
The systematic review by Beswick et al. 8 identified consistent estimates in favour of community-based complex interventions for living at home, nursing home and hospital admissions and physical function, with little difference between types of intervention, having grouped them as geriatric assessment, community-based care after hospital discharge, fall prevention (excluded from this review) or group education and counselling. By contrast we found almost equivocal evidence of possible benefit and harm from such interventions albeit grouped in a more detailed way. For most estimates in the review of Beswick et al. there was moderate statistical heterogeneity (e.g. I2 = 35% for not living at home for geriatric assessment of general elderly people) suggesting there may have been underlying differences in the effects of pooled interventions. 8 Additionally, that review included many studies excluded from this review, often because they were not clearly aimed at sustaining independence, were initiated in hospital or delivered in an outpatient setting, and therefore the evidence base is different. We also declined to pool as many different measures together, for example limiting to measures of instrumental and personal ADL instead of all measures of physical function.
Whitehead et al. examined the effect of interventions to reduce dependence in personal ADL of homecare users. 601 This is similar to our analyses of interventions compared with homecare as the reference comparator. In addition to RCTs they included non-randomised controlled trials and controlled before-and-after studies. They identified a broad variety of intervention content and concluded there was limited evidence that such interventions reduced dependence in homecare users. We also identified low-certainty evidence of a very slight increase in short-term PADL in the comparison of homecare, ADL, multifactorial-action and review with self-management versus homecare, which drew on a study included in their review. We also found low-certainty evidence of an increase in medium-term PADL due to homecare, multifactorial-action and review with medication-review, which drew on a different literature to that included in the review of Whitehead et al.
Luker et al. examined services to avoid residential aged care admission, including 31 RCTs and pooling 11 studies that they grouped as complex interventions. 602 They found a small, statistically significant reduction in risk in a fixed-effect analysis but substantial heterogeneity (I2 = 78%). They found the reduction was greatest in dementia-specific interventions, which are excluded from this review. In our NMAs there were two statistically significant estimates related to reductions in care-home placement, but we rated them as very low certainty due to their RoB and being based on very few events. We calculated one statistically significant estimate related to increases in care-home placement and rated this evidence as low certainty due to RoB and heterogeneity.
While other reviews of similar literature have identified positive effects, albeit small and with some limitations in the underlying evidence, this review has found apparent evidence of both harm and benefit. While this difference may in part be a product of the different study designs and intervention contexts included in these reviews, it may also relate to the broad pairwise pooling of interventions with different combinations of components, different comparators and heterogeneous effects and time points. These other reviews also did not use GRADE to assess certainty of the evidence or the RoB 2 tool to assess RoB, and may therefore have been less likely to conclude that a statistically significant finding was nonetheless very uncertain.
Recommendations for future research
Despite a huge amount of primary research (129 studies, 74,946 participants) there is low certainty, very low certainty or an absence of evidence for most interventions and comparisons. Although methodological improvements in the primary research could improve some of these findings it is worth considering the scale of effort that would be needed to begin to complete this network of interventions. Therefore, it is unclear whether it would be an effective use of research resources to embark on a programme of large-scale RCTs to examine these interventions further. Value of information analysis may be a helpful next step.
Where RCTs are conducted, the following learning should be considered. The small scale of effects observed suggests future trials should be much larger than most of those included here to provide sufficient power. Alternatively, trials may better focus on more proximal outcomes if exploring small configurational differences in interventions. Comparisons with alternative interventions would be useful to further populate a future NMA, to reduce concerns that control participants sought alternative interventions, but also to provide evidence in comparison with relatively established intervention groups such as multifactorial-action and review with medication-review. Trials should ensure they explore possible effects of the trial context on participant and provider behaviour through process evaluation and consider whether cluster-randomisation would help to minimise the possibilities of contamination. Many results were at high RoB due to missing data, yet others managed to retain participants by providing support and continuing to collect outcomes following care-home placement. Use of routine data and planning to report living at home explicitly would also be beneficial. Furthermore, it is vital that studies report detailed reasons for losses to follow up per group, not overall, to help identify whether losses were balanced. Establishing consensus outcomes and measures, alongside more complete reporting, may enable greater pooling of data in the future.
More generally for intervention research, there is continued need for a focus on the specification of the intervention. While interventions need not be over-specified it is important that there is explicit clarity about what was intended in both arms as well as what happened and the context. In particular, we would like to see greater reporting of the organisational aspects of interventions and their implementation, such as institutional responsibilities, inter-institutional agreements and relations, intervention-deliverer and implementer roles and responsibilities, co-ordination mechanisms such as explicit co-ordinator roles, team meetings, shared information systems and related workflows and responsibilities. For example, it appeared that in some interventions the person conducting multidomain assessment and care planning may have been taking on a co-ordinating role, liaising with other professionals and yet this was entirely implicit. We added an item about organisation to the TIDieR framework as we found this lacking in the original and recommend consideration is given to adopting this more widely. These are important, potentially influential aspects of context and process that should be considered more carefully in complex intervention research. 603
Our approach to intervention grouping ensured our NMA was based on substantial differences in active intervention content, looking beyond the label applied by study authors. The components that defined these groups are similar to the ‘Descriptor subdomains’ in the Prevention of Falls Network Europe (ProFaNE) taxonomy suggesting some applicability beyond the current field. 604 The descriptions of intervention groups in the TIDieR format should be a valuable resource for future intervention development. The components, or our approach to identifying and grouping them, could be adopted by others seeking to conceptualise or synthesise the evidence. It could also be used to consider the components or combinations most in need of evaluation.
We found the RoB 2 tool valuable in differentiating the RoB between outcome results within a study, such as variation in missingness of data between outcomes and time points. On the other hand, this increased the amount of time required to assess the results for each study when compared with our previous experience of using the Cochrane risk-of-bias tool. This review was a very substantial undertaking and thought must be given to reducing the burden of systematic reviews while continuing to strive for excellence.
As described above, we planned to evaluate evidence certainty using GRADE, then informed by CINeMA, and eventually just using GRADE. Through this process we recognised that the differences between CINeMA and GRADE are not just technical but that they are founded on differing principles. Among these, CINeMA typically seeks evidence of the absence of a problem while GRADE does not assume there are serious problems in the absence of evidence. We suggest that reviewers considering using CINeMA are cautious if their network is likely to be sparse. We also suggest they consider adopting GRADE’s recent guidance regarding imprecision to avoid high-certainty ratings for findings based on very little evidence. 50
Future work with this data set could explore whether homecare as a cointervention substantially interacted with the other intervention components and whether the effects of frailty could be better estimated by cancelling out homecare when it is present on both sides of the comparison.
Future reviews of similar interventions for older people could focus on those where there is a process intended to lead to multifactorial-action, among which almost all direct comparisons were found and which may align better with mainstream community services. It would be useful to explore the effectiveness of such interventions regardless of an explicit focus on sustaining independence, which may relate more closely to reporting than the intervention developer’s tacit logic model. With so many participants and such uncertainty in the current review, it may be useful to conduct an individual patient data (IPD) meta-analysis, to better explore the factors that may relate to benefit or harm.
Realist synthesis, to explore the relationships between mechanisms, contexts and outcomes in such a complex field, would provide complementary evidence regarding these interventions, placing an emphasis on questions of for whom and in what circumstances as well as the mechanisms underlying plausible benefit and harm. It seems unlikely that most of these interventions are having no effects on some individuals, and yet they are difficult to identify in these studies. Given the uncertainty that it seems is inherent in the evidence from RCTs in this context, an alternative paradigm that embraces complexity and uncertainty may prove fruitful. This may also inform better targeting of interventions in future evaluations.
Implications for decision-makers
While evidence is far from certain, our best evidence of combinations associated with benefit are service models for older people where there is the potential for multifactorial-action. There is evidence that indicates that medication-review (which usually implies a review of the person’s health conditions and hence what the ideal medications for them should be) is an effective aspect of multifactorial-action, and so services that include access to clinical personnel capable of doing this should be favoured.
On the basis of these findings, services should be favoured in which there is ongoing review. This is a challenge to providers who will find it cheaper to provide services by not providing follow-up, and the more follow-up occurs the more the total size of the caseload of a service will be limited. However, failure to review and follow-up may mean that the service overall is ineffective and thus it may be better to treat a smaller number of people effectively than a larger number ineffectively.
The combination of exercise and nutritional support was a part of two favourable intervention groups within the previously mentioned limits of this review. Given the strong evidence base for the benefit of exercise and optimal nutrition to health and healthy ageing, again it seems prudent to include access to these in multifactorial services (and by inference imprudent not to). 605,606
We found, contrary to prior expectations, that there is an evidence base that some intervention combinations could reduce independence. While this evidence is uncertain, it is nearly as uncertain as the evidence of benefit. This being the case, it seems prudent not to assume that all services are effective and to be aware that some combinations could be harmful.
Finally, reflecting the incompleteness of the results here, we do not wish to imply that the absence of evidence of effectiveness of some interventions and combinations should be taken to imply evidence of ineffectiveness. Some of the risks we identified were that results downplayed true effects or needed additional research to increase certainty. Thus, although we advise that those intervention aspects outlined here should form part of services for older people, we do not mean to imply that only these components should be offered.
In the absence of stronger evidence from elsewhere, evidence-based commissioners, providers and practitioners should aim to align service provision with these most promising intervention combinations.
Conclusions
The available evidence suggests the community-based complex interventions most likely to sustain independence in older people include multifactorial-action from multidomain assessment and individualised care planning, medication-review and routine review of patients. Specifically, such interventions probably increase the chance of living at home slightly and may also increase IADL independence very slightly. The combination of physical exercise and nutritional support may also increase the chance of living at home. There was some positive evidence for multiple other combinations of intervention components. Some combinations may reduce independence, and this deserves further consideration. Studies were diverse and therefore these findings may not apply to all populations or contexts.
Despite an extensive search and a rigorous appraisal and synthesis process, we were unable to identify which community-based complex interventions were the best because the benefits and risks of most types of interventions were unclear. High RoB due to missing outcome data in most results and imprecise estimates due to wide CIs meant that most evidence was low or very low certainty. Few studies contributed to each comparison, which impeded evaluation of inconsistency and frailty, and meant that evidence was absent for many intervention types.
This project has robustly described and categorised the components of the community-based interventions evaluated, illuminating the complexity of the field, improving the granularity with which effects are estimated and providing a substantial resource to inform future service development. Further research is required to explore the mechanisms of action and interaction with context. Large-scale trials and different methods for evidence synthesis may illuminate further.
Additional information
Contributions of authors
Thomas Frederick Crocker (https://orcid.org/0000-0001-7450-3143) (Associate Professor, Applied Health Research). Joint-lead. Chaired the Project Management Group (PMG) and supervised the systematic review. Contributed to all stages of the review.
Natalie Lam (https://orcid.org/0000-0001-8591-444X) (Research Fellow, Public Health). Member of the PMG. Managed study selection and data extraction. Contributed to the design, conducted study selection, data collection, intervention grouping, risk-of-bias assessment, certainty of evidence assessment, economic and narrative synthesis.
Joie Ensor (https://orcid.org/0000-0001-7481-0282) (Associate Professor, Biostatistics). Member of the PMG and supervised the meta-analysis. Contributed to the design, conducted analysis, interpreted findings and prepared results for publication.
Magda Jordão (https://orcid.org/0000-0003-2108-2677) (Research Fellow, Cognitive Ageing). Attended PMG meetings. Contributed to the design, conducted study selection, data collection, intervention grouping, risk-of-bias assessment and PPI liaison.
Ram Bajpai (https://orcid.org/0000-0002-1227-2703) (Lecturer, Epidemiology/Applied Statistics). Attended PMG meetings. Conducted data collection, preparation and analysis.
Matthew Bond (https://orcid.org/0000-0003-0168-7417) (Research Assistant, Medical Statistics). Attended PMG meetings. Conducted data preparation and analysis.
Anne Forster (https://orcid.org/0000-0001-7466-4414) (Professor of Stroke Rehabilitation). Member of the PMG. Contributed to the design and interpretation of the review.
Richard D Riley (https://orcid.org/0000-0001-8699-0735) (Professor of Biostatistics). Member of the PMG. Oversaw analysis plan and implementation. Contributed to the conception, design and interpretation of the review.
Deirdre Andre (https://orcid.org/0000-0003-2059-4662) (Library Research Support Advisor, Information). Developed and executed the database and trial register searches.
Caroline Brundle (https://orcid.org/0000-0003-3412-7280) (Elderly Care Researcher). Conducted data collection and risk-of-bias assessment.
Alison Ellwood (https://orcid.org/0000-0001-8632-1830) (Research Fellow, Ageing). Contributed to intervention grouping and prepared results for publication.
John Green (https://orcid.org/0000-0003-1434-9345) (Research Programme Manager, Rehabilitation). Conducted study selection, data collection and prepared results for publication.
Matthew Hale (https://orcid.org/0000-0003-4056-0304) (Academic Clinical Fellow, Geriatrics). Conducted data collection.
Jessica Morgan (https://orcid.org/0000-0002-4490-5640) (Doctor, Geriatric Medicine). Conducted study selection and data collection.
Eleftheria Patetsini (https://orcid.org/0000-0001-8393-982X) (Research Assistant, Ageing). Attended PMG meetings. Contributed to the design. Conducted study selection and data collection.
Matthew Prescott (https://orcid.org/0000-0001-7397-9422) (Trial Manager, Elderly Care). Conducted risk-of-bias assessment.
Ridha Ramiz (https://orcid.org/0000-0002-3569-8731) (Research Assistant, Ageing). Attended PMG meetings. Conducted study selection and data collection.
Oliver Todd (https://orcid.org/0000-0001-7212-8095) (Academic Clinical Lecturer, Cardiovascular Ageing). Contributed to interpretation. Conducted data collection.
Rebecca Walford (https://orcid.org/0000-0003-2405-5260) (Doctor, Anaesthetics). Conducted study selection and data collection.
John Gladman (https://orcid.org/0000-0002-8506-7786) (Professor of the Medicine of Older People). Member of the PMG. Contributed to the conception, design and interpretation of the review. Conducted frailty assessment of the included studies.
Andrew Clegg (https://orcid.org/0000-0001-5972-1097) (Head of Ageing and Stroke Research, Professor of Geriatric Medicine). Joint-lead. Member of the PMG. Responsible for the delivery of research outputs. Contributed to the conception, design and interpretation of the review. Conducted frailty assessment of the included studies.
Acknowledgements
We are grateful to Nicola Harrison and Lesley Brown who co-ordinated and facilitated meetings with our Frailty Oversight Group (FOG); members of the Frailty Oversight Group, in particular Anne Grice and Marilyn Foster; members of the NIHR Applied Research Collaboration (ARC), Older People with Frailty Theme, Research Implementation Advisory Group; Zubair Arastu and Friederike Ziegler for their contributions to screening reference lists; Lubena Mirza, Andy Mprah and Ismail Patel for their contributions towards data collection; and Jasmin Manik for her support with testing the RoB 2 tool algorithms. We are particularly grateful to all of the authors of studies, both included and excluded, who responded to requests for information.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Ethics statement
Ethics approval was not sought as this systematic review was secondary research using aggregated, anonymised data that is available in the public domain.
Information governance statement
This study did not handle any personal information.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/HNRP2514.
Primary conflicts of interest: Matthew Bond declares NIHR pre-doctoral fellowship; Andrew Clegg declares funding through the NIHR HTA programme, NIHR Programme Grants for Applied Research, NIHR HS&DR programme, NIHR Applied Research Collaboration Yorkshire & Humber and Health Data Research UK. Anne Forster declares NIHR Senior Investigator Award 2017–present, NIHR Programme Grant 10% of salary, NIHR HS&DR grant 8% of salary, HTA grant 5% of salary, National Institute for Health (USA) payment for panel membership 2021, 2022, participation in Programme Steering Committees for NIHR202339 improving the lives of stroke survivors with data, and NIHR202020 research title, Personalised Exercise-Rehabilitation FOR people with Multiple long-term conditions (multimorbidity) – the PERFORM trial, University of Leeds Governor representative on the Governors Board of Bradford Teaching Hospitals NHS Foundation Trust, member of HSDR Researcher-led panel, member of NIHR Doctoral Fellowship Panel member of Policy Research Unit assessment panel. Richard D Riley declares he has received personal payments for training courses provided in-house to universities (Leeds, Aberdeen, Exeter, LSHTM) and other organisations (Roche). He has received personal payments from BMJ and BMJ Medicine as their Statistical Editor. He is a Co-Convenor of the Cochrane Prognosis Methods Group and on the Editorial Board of Diagnostic and Prognostic Research, and Research Synthesis Methods, but receives no income for these roles. He receives personal payment for being the External Examiner of the MSc Medical Statistics, London School of Hygiene and Tropical Medicine, and was previously an External Examiner for the MSc Medical Statistics at University of Leicester. He has written two textbooks for which he receives royalties for sales: Prognosis Research in Healthcare and Individual Participant Data Meta-analysis. He is a lead editor on an upcoming book, Cochrane Handbook for Prognosis Reviews (Wiley, 2025), for which he will receive royalties for sales. He received consulting fees for a training course on IPD meta-analysis from Roche in 2018; the NIHR HTA grant paid for travel to Leeds for one meeting. He is a member of the NIHR Doctoral Research Fellowships grant panel, and the MRC Better Methods Better Research grant panel. For the latter he receives an attendance fee. Matthew Hale declares NIHR Academic Clinical Fellowship. Oliver Todd declares NIHR Academic Clinical Lectureship, Dunhill Medical Trust Doctoral Research Fellowship RTF107/0117.
Publication
The protocol has been published as follows:
Crocker TF, Clegg A, Riley RD, Lam N, Bajpai R, Jordão M, et al. Community-based complex interventions to sustain independence in older people, stratified by frailty: a protocol for a systematic review and network meta-analysis. BMJ Open 2021;11:e045637. https://doi.org/10.1136/bmjopen-2020-045637
Our experience of assessing risk of bias in this review has been published as follows:
Crocker TF, Lam N, Jordão M, Brundle C, Prescott M, Forster A, et al. Risk-of-bias assessment using Cochrane’s revised tool for randomized trials (RoB 2) was useful but challenging and resource-intensive: observations from a systematic review. J Clin Epidemiol 2023;161:39–45. https://doi.org/10.1016/j.jclinepi.2023.06.015
A brief summary of the main findings has been published as follows:
Crocker TF, Ensor J, Lam N, Jordão M, Bajpai R, Bond M, et al. Community based complex interventions to sustain independence in older people: systematic review and network meta-analysis. BMJ 2024. https://doi.org/10.1136/bmj-2023-077764
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Office for National Statistics . Estimates of the Population for the UK, England and Wales, Scotland and Northern Ireland 2022. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (accessed 14 July 2022).
- Office for National Statistics . National Population Projections: 2020-Based Interim 2022. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/bulletins/nationalpopulationprojections/2020basedinterim (accessed 14 July 2022).
- World Health Organization . Decade of Healthy Ageing: 2020–2030 n.d. www.who.int/ageing/decade-of-healthy-ageing (accessed 24 September 2020).
- Government of United Kingdom . The Grand Challenge Missions: Ageing Society 2019. https://www.gov.uk/government/publications/industrial-strategy-the-grand-challenges/missions (accessed 23 September 2020).
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752-62. https://doi.org/10.1016/s0140-6736(12)62167-9.
- Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487-92. https://doi.org/10.1111/j.1532-5415.2012.04054.x.
- Han L, Clegg A, Doran T, Fraser L. The impact of frailty on healthcare resource use: a longitudinal analysis using the Clinical Practice Research Datalink in England. Age Ageing 2019;48:665-71. https://doi.org/10.1093/ageing/afz088.
- Beswick AD, Rees K, Dieppe P, Ayis S, Gooberman-Hill R, Horwood J, et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet 2008;371:725-35. https://doi.org/10.1016/s0140-6736(08)60342-6.
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons; 2019.
- Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, et al. GRADE Working Group . Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol 2018;93:36-44. https://doi.org/10.1016/j.jclinepi.2017.10.005.
- Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777-84. https://doi.org/10.7326/m14-2385.
- Clegg A, Crocker T, Forster A, Gladman J, Riley R, Ensor J, et al. Community-Based Complex Interventions to Sustain Independence in Older People, Stratified by Frailty: A Systematic Review and Network Meta-Analysis 2019. www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019162195 (accessed 23 November 2022).
- Crocker TF, Clegg A, Riley RD, Lam N, Bajpai R, Jordão M, et al. Community-based complex interventions to sustain independence in older people, stratified by frailty: a protocol for a systematic review and network meta-analysis. BMJ Open 2021;11. https://doi.org/10.1136/bmjopen-2020-045637.
- Medical Research Council UK . Developing and Evaluating Complex Interventions: The New MRC Framework 2008.
- May C, Finch T, Mair F, Ballini L, Dowrick C, Eccles M, et al. Understanding the implementation of complex interventions in health care: the normalization process model. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-148.
- Stuck AE, Egger M, Hammer A, Minder CE, Beck JC. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA 2002;287:1022-8. https://doi.org/10.1001/jama.287.8.1022.
- Tappenden P, Campbell F, Rawdin A, Wong R, Kalita N. The clinical effectiveness and cost-effectiveness of home-based, nurse-led health promotion for older people: a systematic review. Health Technol Assess 2012;16:1-72. https://doi.org/10.3310/hta16200.
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2021.
- Cooper C, Varley-Campbell J, Carter P. Established search filters may miss studies when identifying randomized controlled trials. J Clin Epidemiol 2019;112:12-9. https://doi.org/10.1016/j.jclinepi.2019.04.002.
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2021.
- Eady AM, Wilczynski NL, Haynes RB. PsycINFO search strategies identified methodologically sound therapy studies and review articles for use by clinicians and researchers. J Clin Epidemiol 2008;61:34-40. https://doi.org/10.1016/j.jclinepi.2006.09.016.
- McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016;75:40-6. https://doi.org/10.1016/j.jclinepi.2016.01.021.
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA, . Cochrane Handbook for Systematic Reviews of Interventions. n.d.
- Parker SG, McCue P, Phelps K, McCleod A, Arora S, Nockels K, et al. What is Comprehensive Geriatric Assessment (CGA)? An umbrella review. Age Ageing 2018;47:149-55. https://doi.org/10.1093/ageing/afx166.
- Tricco AC, Thomas SM, Veroniki AA, Hamid JS, Cogo E, Strifler L, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA 2017;318:1687-99. https://doi.org/10.1001/jama.2017.15006.
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2022.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Patton MQ. Qualitative Research & Evaluation Methods: Integrating Theory and Practice. Los Angeles: Sage Publications; 2015.
- National Institute for Clinical Excellence . Multimorbidity: Clinical Assessment and Management. 2016. www.nice.org.uk/guidance/ng56 (accessed 16 June 2022).
- Effective Practice and Organisation of Care (EPOC) . EPOC Taxonomy 2015. epoc.cochrane.org/epoc-taxonomy (accessed 20 August 2021).
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56. https://doi.org/10.1093/gerona/56.3.m146.
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366. https://doi.org/10.1136/bmj.l4898.
- Crocker TF, Lam N, Jordão M, Brundle C, Prescott M, Forster A, et al. Risk-of-bias assessment using Cochrane’s revised tool for randomized trials (RoB 2) was useful but challenging and resource-intensive: observations from a systematic review. J Clin Epidemiol 2023;161:39-45. https://doi.org/10.1016/j.jclinepi.2023.06.015.
- Eldridge S, Campbell MK, Campbell MJ, Drahota AK, Giraudeau B, Reeves BC, et al. Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB 2): Additional Considerations for Cluster-Randomized Trials (RoB 2 CRT) 2021. https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/rob-2-for-cluster-randomized-trials (accessed 18 March 2021).
- Schünemann H, Higgins J, Vist G, P G, Akl E, Skoetz N, et al. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2022.
- Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. Updated October 2013. The GRADE Working Group; 2013.
- White IR. Network meta-analysis. Stata J 2015;15:951-85. https://doi.org/10.1177/1536867X1501500403.
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342. https://doi.org/10.1136/bmj.d549.
- Langan D, Higgins JPT, Jackson D, Bowden J, Veroniki AA, Kontopantelis E, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods 2019;10:83-98. https://doi.org/10.1002/jrsm.1316.
- White IR. Multivariate random-effects meta-analysis. Stata J 2009;9:40-56. https://doi.org/10.1177/1536867X0900900103.
- Riley RD, Jackson D, Salanti G, Burke DL, Price M, Kirkham J, et al. Multivariate and network meta-analysis of multiple outcomes and multiple treatments: rationale, concepts, and examples. BMJ 2017;358. https://doi.org/10.1136/bmj.j3932.
- Doi SA, Furuya-Kanamori L, Xu C, Chivese T, Lin L, Musa OAH, et al. The odds ratio is ‘portable’ across baseline risk but not the relative risk: time to do away with the log link in binomial regression. J Clin Epidemiol 2022;142:288-93. https://doi.org/10.1016/j.jclinepi.2021.08.003.
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 2013;33:641-56. https://doi.org/10.1177/0272989x12455847.
- Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. Int J Epidemiol 2013;42:332-45. https://doi.org/10.1093/ije/dys222.
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0076654.
- Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods 2012;3:161-76. https://doi.org/10.1002/jrsm.57.
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380-2. https://doi.org/10.1016/j.jclinepi.2010.09.011.
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. GRADE Working Group . A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349. https://doi.org/10.1136/bmj.g5630.
- Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLOS Med 2020;17. https://doi.org/10.1371/journal.pmed.1003082.
- Brignardello-Petersen R, Guyatt GH, Mustafa RA, Chu DK, Hultcrantz M, Schünemann HJ, et al. GRADE guidelines 33: addressing imprecision in a network meta-analysis. J Clin Epidemiol 2021;139:49-56. https://doi.org/10.1016/j.jclinepi.2021.07.011.
- Yepes-Nuñez JJ, Li S-A, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta-analysis. J Clin Epidemiol 2019;115:1-13. https://doi.org/10.1016/j.jclinepi.2019.04.018.
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE Working Group . GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 2020;119:126-35. https://doi.org/10.1016/j.jclinepi.2019.10.014.
- Glenton C, Santesso N, Rosenbaum S, Nilsen ES, Rader T, Ciapponi A, et al. Presenting the results of Cochrane Systematic Reviews to a consumer audience: a qualitative study. Med Decis Making 2010;30:566-77. https://doi.org/10.1177/0272989x10375853.
- Brydges CR. Effect size guidelines, sample size calculations, and statistical power in gerontology. Innov Aging 2019;3:1-8. https://doi.org/10.1093/geroni/igz036.
- Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non‐valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev 2007. https://doi.org/10.1002/14651858.CD006186.pub2.
- Sudlow CLM, Mason G, Maurice JB, Wedderburn CJ, Hankey GJ. Thienopyridine derivatives versus aspirin for preventing stroke and other serious vascular events in high vascular risk patients. Cochrane Database Syst Rev 2009. https://doi.org/10.1002/14651858.CD001246.pub2.
- Wright JM, Musini VM, Gill R. First‐line drugs for hypertension. Cochrane Database Syst Rev 2018. https://doi.org/10.1002/14651858.CD001841.pub3.
- Aluko P, Graybill E, Craig D, Henderson C, Drummond M, Wilson E, et al. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2022.
- York Health Economics Consortium (YHEC) . Glossary of Terms for Health Economics and Systematic Review n.d. https://methods.cochrane.org/economics/methods-training/glossary (accessed 17 March 2022).
- Shemilt I, Mugford M, Vale L, Craig D, . Searching NHS EED and HEED to Inform Development of Economic Commentary for Cochrane Intervention Reviews. Oxford: Cochrane Collaboration; 2011.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372. https://doi.org/10.1136/bmj.n71.
- Alegria M, Frontera W, Cruz-Gonzalez M, Markle SL, Trinh-Shevrin C, Wang Y, et al. Effectiveness of a disability preventive intervention for minority and immigrant elders: the positive minds-strong bodies randomized clinical trial. Am J Geriatr Psychiatry 2019;27:1299-313. https://doi.org/10.1016/j.jagp.2019.08.008.
- Arthanat S. Promoting information communication technology adoption and acceptance for aging-in-place: a randomized controlled trial. J Appl Gerontol 2019;40:471-80. https://doi.org/10.1177/0733464819891045.
- Auvinen K, Voutilainen A, Jyrkkä J, Lönnroos E, Mäntyselkä P. Interprofessional medication assessment among home care patients: any impact on functioning? Results from a randomised controlled trial. BMC Geriatr 2020;20. https://doi.org/10.1186/s12877-020-01796-1.
- Balaban DJ, Goldfarb NI, Perkel RL, Carlson BL. Follow-up study of an urban family medicine home visit program. J Fam Pract 1988;26:307-12.
- Barenfeld E, Dahlin-Ivanoff S, Wallin L, Gustafsson S. Promoting aging migrants’ capabilities: a randomized controlled trial concerning activities of daily living and self-rated health. AIMS Public Health 2018;5:173-88. https://doi.org/10.3934/publichealth.2018.2.173.
- Bernabei R, Landi F, Gambassi G, Sgadari A, Zuccala G, Mor V, et al. Randomised trial of impact of model of integrated care and case management for older people living in the community. BMJ 1998;316:1348-51. https://doi.org/10.1136/bmj.316.7141.1348.
- Bleijenberg N, Drubbel I, Schuurmans MJ, Dam HT, Zuithoff NP, Numans ME, et al. Effectiveness of a proactive primary care program on preserving daily functioning of older people: a cluster randomized controlled trial. J Am Geriatr Soc 2016;64:1779-88. https://doi.org/10.1111/jgs.14325.
- Blom J, den Elzen W, van Houwelingen AH, Heijmans M, Stijnen T, Van den Hout W, et al. Effectiveness and cost-effectiveness of a proactive, goal-oriented, integrated care model in general practice for older people. A cluster randomised controlled trial: integrated systematic care for older people – the ISCOPE study. Age Ageing 2016;45:30-41. https://doi.org/10.1093/ageing/afv174.
- Borrows A, Holland R. Independent living centre occupational therapy (OT) versus routine community OT. Int J Ther Rehabil 2013;20:187-94. https://doi.org/10.12968/ijtr.2013.20.4.187.
- Botjes E. Methodebeschrijving EigenKrachtWijzer: Databank Effectieve sociale interventies. Report: Movisie; 2013.
- Bouman A, van Rossum E, Ambergen T, Kempen G, Knipschild P. Effects of a home visiting program for older people with poor health status: a randomized, clinical trial in The Netherlands. J Am Geriatr Soc 2008;56:397-404. https://doi.org/10.1111/j.1532-5415.2007.01565.x.
- Brettschneider C, Luck T, Fleischer S, Roling G, Beutner K, Luppa M, et al. Cost-utility analysis of a preventive home visit program for older adults in Germany. BMC Health Serv Res 2015;15. https://doi.org/10.1186/s12913-015-0817-0.
- Cameron ID, Fairhall N, Langron C, Lockwood K, Monaghan N, Aggar C, et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-65.
- Carpenter GI, Demopoulos GR. Screening the elderly in the community: controlled trial of dependency surveillance using a questionnaire administered by volunteers. BMJ 1990;300:1253-6. https://doi.org/10.1136/bmj.300.6734.1253.
- Cesari M, Demougeot L, Boccalon H, Guyonnet S, Vellas B, Andrieu S. The Multidomain Intervention to preveNt disability in ElDers (MINDED) project: rationale and study design of a pilot study. Contemp Clin Trials 2014;38:145-54. https://doi.org/10.1016/j.cct.2014.04.006.
- Challis D, Clarkson P, Williamson J, Hughes J, Venables D, Burns A, et al. The value of specialist clinical assessment of older people prior to entry to care homes. Age Ageing 2004;33:25-34. https://doi.org/10.1093/ageing/afh007.
- Clark F, Azen SP, Zemke R, Jackson J, Carlson M, Mandel D, et al. Occupational therapy for independent-living older adults. A randomized controlled trial. JAMA 1997;278:1321-6. https://doi.org/10.1001/jama.1997.03550160041036.
- Clark F, Jackson J, Carlson M, Chou C-P, Cherry BJ, Jordan-Marsh M, et al. Effectiveness of a lifestyle intervention in promoting the well-being of independently living older people: results of the Well Elderly 2 randomised controlled trial. [Erratum in: J Epidemiol Community Health 2012;66:1079–82]. J Epidemiol Community Health 2012;66:782-90. https://doi.org/10.1136/jech.2009.099754.
- Coleman EA, Grothaus LC, Sandhu N, Wagner EH. Chronic care clinics: a randomized controlled trial of a model of primary care for frail older adults. J Am Geriatr Soc 1999;47:775-83. https://doi.org/10.1111/j.1532-5415.1999.tb03832.x.
- Counsell SR, Callahan CM, Clark DO, Tu W, Buttar AB, Stump TE, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA 2007;298:2623-33. https://doi.org/10.1001/jama.298.22.2623.
- Cutchin MP, Coppola S, Talley V, Svihula J, Catellier D, Shank KH. Feasibility and effects of preventive home visits for at-risk older people: design of a randomized controlled trial. BMC Geriatr 2009;9. https://doi.org/10.1186/1471-2318-9-54.
- Dalby DM, Sellors JW, Fraser FD, Fraser C, van Ineveld C, Howard M. Effect of preventive home visits by a nurse on the outcomes of frail elderly people in the community: a randomized controlled trial. CMAJ 2000;162:497-500.
- de Craen AJ, Gussekloo J, Blauw GJ, Willems CG, Westendorp RG. Randomised controlled trial of unsolicited occupational therapy in community-dwelling elderly people: the LOTIS trial. PLoS Clin Trials 2006;1. https://doi.org/10.1371/journal.pctr.0010002.
- Dorresteijn TA, Zijlstra GA, Ambergen AW, Delbaere K, Vlaeyen JW, Kempen GI. Effectiveness of a home-based cognitive behavioral program to manage concerns about falls in community-dwelling, frail older people: results of a randomized controlled trial. BMC Geriatr 2016;16. https://doi.org/10.1186/s12877-015-0177-y.
- Dupuy L, Froger C, Consel C, Sauzeon H. Everyday functioning benefits from an assisted living platform amongst frail older adults and their caregivers. Front Aging Neurosci 2017;9. https://doi.org/10.3389/fnagi.2017.00302.
- Fabacher D, Josephson K, Pietruszka F, Linderborn K, Morley JE, Rubenstein LZ. An in-home preventive assessment program for independent older adults: a randomized controlled trial. J Am Geriatr Soc 1994;42:630-8. https://doi.org/10.1111/j.1532-5415.1994.tb06862.x.
- Fairhall N, Kurrle SE, Sherrington C, Lord SR, Lockwood K, John B, et al. Effectiveness of a multifactorial intervention on preventing development of frailty in pre-frail older people: study protocol for a randomised controlled trial. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-007091.
- Faul AC, Yankeelov PA, Rowan NL, Gillette P, Nicholas LD, Borders KW, et al. Impact on geriatric assessment and self-management support on community-dwelling older adults with chronic illnesses. J Gerontol Soc Work 2009;52:230-49. https://doi.org/10.1080/01634370802609288.
- Fernandez-Barres S, Garcia-Barco M, Basora J, Martinez T, Pedret R, Arija V. Project ATDOM-NUT group . The efficacy of a nutrition education intervention to prevent risk of malnutrition for dependent elderly patients receiving Home Care: a randomized controlled trial. Int J Nurs Stud 2017;70:131-41. https://doi.org/10.1016/j.ijnurstu.2017.02.020.
- Fischer G, Sandholzer H, Perschke-Hartmann C. Final Report of the Scientific Support of ‘Getting Healthy Elderly (GÄW)’. A Prevention Project of the AOK Lower Saxony. [German] (Abschlussbericht der wissenschaftlichen Begleitung von ‘Gesund Älter Werden (GÄW)’). Unpublished: AOK Niedersachsen; 2009.
- Ford AB, Katz S, Downs TD, Adams M. Results of long-term home nursing: the influence of disability. J Chronic Dis 1971;24:591-6. https://doi.org/10.1016/0021-9681(71)90047-6.
- Fox PJ, Breuer W, Wright JA. Effects of a health promotion program on sustaining health behaviors in older adults. Am J Prev Med 1997;13:257-64.
- Fristedt S, Nystedt P, Skogar O. Mobile geriatric teams – a cost-effective way of improving patient safety and reducing traditional healthcare utilization among the frail elderly? A randomized controlled trial. Clin Interv Aging 2019;14:1911-24. https://doi.org/10.2147/CIA.S208388.
- Gene Huguet L, Navarro Gonzalez M, Kostov B, Ortega Carmona M, Colungo Francia C, Carpallo Nieto M, et al. Pre Frail 80: multifactorial intervention to prevent progression of pre-frailty to frailty in the elderly. J Nutr Health Aging 2018;22:1266-74. https://doi.org/10.1007/s12603-018-1089-2.
- Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med 2002;347:1068-74. https://doi.org/10.1056/NEJMoa020423.
- Giné-Garriga M, Sansano-Nadal O, Tully MA, Caserotti P, Coll-Planas L, Rothenbacher D, et al. Accelerometer-measured sedentary and physical activity time and their correlates in European older adults: the SITLESS study. J Gerontol A Biol Sci Med Sci 2020;75:1754-62. https://doi.org/10.1093/gerona/glaa016.
- Gitlin LN, Winter L, Dennis MP, Corcoran M, Schinfeld S, Hauck WW. A randomized trial of a multicomponent home intervention to reduce functional difficulties in older adults. J Am Geriatr Soc 2006;54:809-16. https://doi.org/10.1111/j.1532-5415.2006.00703.x.
- Grimmer K, Luker J, Beaton K, Kumar S, Crockett A, Price K. TRialing individualized interventions to prevent functional decline in at-risk older adults (TRIIFL): study protocol for a randomized controlled trial nested in a longitudinal observational study. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-266.
- Gustafson DH, Kornfield R, Mares M-L, Johnston DC, Cody OJ, Yang EF, et al. Effect of an eHealth intervention on older adults’ quality of life and health-related outcomes: a randomized clinical trial. J Gen Intern Med 2021;37:521-30. https://doi.org/10.1007/s11606-021-06888-1.
- Gustafsson S, Eklund K, Wilhelmson K, Edberg A-K, Johansson B, Kronlöf GH, et al. Long-term outcome for ADL following the health-promoting RCT – elderly persons in the risk zone. Gerontologist 2013;53:654-63. https://doi.org/10.1093/geront/gns121.
- Hall N, De Beck P, Johnson D, Mackinnon K, Gutman G, Glick N. Randomized trial of a health promotion program for frail elders. Can J Aging 1992;11:72-91.
- Harari D, Iliffe S, Kharicha K, Egger M, Gillmann G, von Renteln-Kruse W, et al. Promotion of health in older people: a randomised controlled trial of health risk appraisal in British general practice. Age Ageing 2008;37:565-71. https://doi.org/10.1093/ageing/afn150.
- Hattori S, Yoshida T, Okumura Y, Kondo K. Effects of reablement on the independence of community-dwelling older adults with mild disability: a randomized controlled trial. Int J Environ Res Public Health 2019;16. https://doi.org/10.3390/ijerph16203954.
- Hay WI, van Ineveld C, Browne G, Roberts J, Bell B, Mills M, et al. Prospective care of elderly patients in family practice. Is screening effective?. Can Fam Physician 1998;44:2677-87.
- Hebert R, Robichaud L, Roy PM, Bravo G, Voyer L. Efficacy of a nurse-led multidimensional preventive programme for older people at risk of functional decline. A randomized controlled trial. Age Ageing 2001;30:147-53. https://doi.org/10.1093/ageing/30.2.147.
- Henderson MJ. In-Home Preventive Health Assessment and Telephone Case Management for Over 75s Living Alone in Independent Living Units: A Cluster Randomized Controlled Trial 2005.
- Hendriksen C, Lund E, Stromgard E. Consequences of assessment and intervention among elderly people: a three year randomised controlled trial. Br Med J (Clin Res Ed) 1984;289:1522-4. https://doi.org/10.1136/bmj.289.6457.1522.
- Hogg W, Lemelin J, Dahrouge S, Liddy C, Armstrong CD, Legault F, et al. Randomized controlled trial of anticipatory and preventive multidisciplinary team care: for complex patients in a community-based primary care setting. Can Fam Physician 2009;55:e76-85.
- Holland SK, Greenberg J, Tidwell L, Malone J, Mullan J, Newcomer R. Community-based health coaching, exercise, and health service utilization. J Aging Health 2005;17:697-716. https://doi.org/10.1177/0898264305277959.
- Howel D, Moffatt S, Haighton C, Bryant A, Becker F, Steer M, et al. Does domiciliary welfare rights advice improve health-related quality of life in independent-living, socio-economically disadvantaged people aged ≥ 60 years? Randomised controlled trial, economic and process evaluations in the North East of England. PLOS ONE 2019;14. https://doi.org/10.1371/journal.pone.0209560.
- Imhof L, Naef R, Wallhagen MI, Schwarz J, Mahrer-Imhof R. Effects of an advanced practice nurse in-home health consultation program for community-dwelling persons aged 80 and older. J Am Geriatr Soc 2012;60:2223-31. https://doi.org/10.1111/jgs.12026.
- Jing L, Jin Y, Zhang X, Wang F, Song Y, Xing F. The effect of Baduanjin qigong combined with CBT on physical fitness and psychological health of elderly housebound. Medicine (Baltimore) 2018;97. https://doi.org/10.1097/MD.0000000000013654.
- Jitapunkul S. A randomised controlled trial of regular surveillance in Thai elderly using a simple questionnaire administered by non-professional personnel. J Med Assoc Thai 1998;81:352-6.
- Kerse N, McLean C, Moyes SA, Peri K, Ng T, Wilkinson-Meyers L, et al. The cluster-randomized BRIGHT trial: proactive case finding for community-dwelling older adults. Ann Fam Med 2014;12:514-24. https://doi.org/10.1370/afm.1696.
- King AII, Parsons M, Robinson E, Jorgensen D. Assessing the impact of a restorative home care service in New Zealand: a cluster randomised controlled trial. Health Soc Care Community 2012;20:365-74. https://doi.org/10.1111/j.1365-2524.2011.01039.x.
- Kono A, Izumi K, Yoshiyuki N, Kanaya Y, Rubenstein LZ. Effects of an updated preventive home visit program based on a systematic structured assessment of care needs for ambulatory frail older adults in Japan: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2016;71:1631-7. https://doi.org/10.1093/gerona/glw068.
- Kono A, Kai I, Sakato C, Harker JO, Rubenstein LZ. Effect of preventive home visits for ambulatory housebound elders in Japan: a pilot study. Aging Clin Exp Res 2004;16:293-9. https://doi.org/10.1007/BF03324554.
- Kono A, Kanaya Y, Fujita T, Tsumura C, Kondo T, Kushiyama K, et al. Effects of a preventive home visit program in ambulatory frail older people: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2012;67:302-9. https://doi.org/10.1093/gerona/glr176.
- Kukkonen-Harjula K, Karmeniemi P, Suikkanen S, Kaaria S, Sipila S, Pitkala K, et al. Long-term home-based physiotherapy for older people with signs of frailty-RCT (NCT02305433) [P-229]. Eur Geriatr Med 2017;8. https://doi.org/10.1016/S1878-7649(17)30179-1.
- Lambotte D, De Donder L, De Roeck EE, Hoeyberghs LJ, van der Vorst A, Duppen D, et al. Randomized controlled trial to evaluate a prevention program for frail community-dwelling older adults: a D-SCOPE protocol. BMC Geriatr 2018;18. https://doi.org/10.1186/s12877-018-0875-3.
- Leung AC-t, Liu C-p, Chow NW-s, Chi I. Cost-benefit analysis of a case management project for the community-dwelling frail elderly in Hong Kong. J Appl Gerontol 2004;23:70-85. https://doi.org/10.1177/0733464804263088.
- Leveille SG, Wagner EH, Davis C, Grothaus L, Wallace J, LoGerfo M, et al. Preventing disability and managing chronic illness in frail older adults: a randomized trial of a community-based partnership with primary care. J Am Geriatr Soc 1998;46:1191-8. https://doi.org/10.1111/j.1532-5415.1998.tb04533.x.
- Lewin G, De San Miguel K, Knuiman M, Alan J, Boldy D, Hendrie D, et al. A randomised controlled trial of the home independence program, an Australian restorative home-care programme for older adults. Health Soc Care Community 2013;21:69-78. https://doi.org/10.1111/j.1365-2524.2012.01088.x.
- Liddle J, March L, Carfrae B, Finnegan T, Druce J, Schwarz J, et al. Can occupational therapy intervention play a part in maintaining independence and quality of life in older people? A randomised controlled trial. Aust N Z J Public Health 1996;20:574-8. https://doi.org/10.1111/j.1467-842x.1996.tb01068.x.
- Liimatta H, Lampela P, Laitinen-Parkkonen P, Pitkala KH. Effects of preventive home visits on health-related quality-of-life and mortality in home-dwelling older adults. Scand J Prim Health Care 2019;37:90-7. https://doi.org/10.1080/02813432.2019.1569372.
- Loh DA, Hairi NN, Choo WY, Mohd Hairi F, Peramalah D, Kandiben S, et al. MultiComponent Exercise and theRApeutic lifeStyle (CERgAS) intervention to improve physical performance and maintain independent living among urban poor older people – a cluster randomised controlled trial. BMC Geriatr 2015;15. https://doi.org/10.1186/s12877-015-0002-7.
- Lood Q, Gustafsson S, Dahlin Ivanoff S. Bridging barriers to health promotion: a feasibility pilot study of the ‘Promoting Aging Migrants’ Capabilities study’. J Eval Clin Pract 2015;21:604-13. https://doi.org/10.1111/jep.12345.
- Mann J, Thompson F, McDermott R, Esterman A, Strivens E. Impact of an integrated community-based model of care for older people with complex conditions on hospital emergency presentations and admissions: a step-wedged cluster randomized trial. BMC Health Serv Res 2021;21. https://doi.org/10.1186/s12913-021-06668-x.
- Mann WC, Ottenbacher KJ, Fraas L, Tomita M, Granger CV. Effectiveness of assistive technology and environmental interventions in maintaining independence and reducing home care costs for the frail elderly. A randomized controlled trial. Arch Fam Med 1999;8:210-7. https://doi.org/10.1001/archfami.8.3.210.
- Markle-Reid M, Weir R, Browne G, Roberts J, Gafni A, Henderson S. Health promotion for frail older home care clients. J Adv Nurs 2006;54:381-95. https://doi.org/10.1111/j.1365-2648.2006.03817.x.
- Melis RJ, van Eijken MI, Teerenstra S, van Achterberg T, Parker SG, Borm GF, et al. A randomized study of a multidisciplinary program to intervene on geriatric syndromes in vulnerable older people who live at home (Dutch EASYcare Study). J Gerontol A Biol Sci Med Sci 2008;63:283-90. https://doi.org/10.1093/gerona/63.3.283.
- Meng H, Friedman B, Wamsley BR, Mukamel D, Eggert GM. Effect of a consumer-directed voucher and a disease-management-health-promotion nurse intervention on home care use. Gerontologist 2005;45:167-76. https://doi.org/10.1093/geront/45.2.167.
- Messens L, Quinn S, Saez I, Cuidad Mas MJ, Squillace P, Laura A-G. Health Monitoring and Social Integration EnvironMent for Supporting WidE ExTension of Independent Life at HOME (Home Sweet Home): Final Trial Evaluation Report No. D7.5. Antwerp: Zorgbedrijf Antwerpen; 2014.
- Metzelthin SF, Van Rossum E, De Witte LP, Ambergen AW, Hobma SO, Sipers W, et al. Effectiveness of interdisciplinary primary care approach to reduce disability in community dwelling frail older people: cluster randomised controlled trial. BMJ 2013;347. https://doi.org/10.1136/bmj.f5264.
- Moll van Charante EP, Richard E, Eurelings LS, van Dalen JW, Ligthart SA, van Bussel EF, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 2016;388:797-805. https://doi.org/10.1016/S0140-6736(16)30950-3.
- Monteserin Nadal R, Altimir Losada S, Brotons Cuixart C, Padros Selma J, Santaeugenia Gonzalez S, Moral Pelaez I, et al. Randomized clinical trial on the efficacy of global geriatric assessment in primary care. [Spanish]. Rev Esp Geriatr Gerontol 2008;43:5-12. https://doi.org/10.1016/s0211-139x(08)71144-2.
- Morey MC, Ekelund C, Pearson M, Crowley G, Peterson M, Sloane R, et al. Project LIFE: a partnership to increase physical activity in elders with multiple chronic illnesses. J Aging Phys Act 2006;14:324-43. https://doi.org/10.1123/japa.14.3.324.
- Morey MC, Peterson MJ, Pieper CF, Sloane R, Crowley GM, Cowper PA, et al. The Veterans learning to improve fitness and function in elders study: a randomized trial of primary care–based physical activity counseling for older men. J Am Geriatr Soc 2009;57:1166-74. https://doi.org/10.1111/j.1532-5415.2009.02301.x.
- Morgan GS, Haase AM, Campbell RM, Ben-Shlomo Y. A pilot randomised controlled trial of physical activity facilitation for older adults: feasibility study findings. Pilot Feasibility Stud 2019;5. https://doi.org/10.1186/s40814-019-0414-9.
- Newbury JW, Marley JE, Beilby JJ. A randomised controlled trial of the outcome of health assessment of people aged 75 years and over. Med J Aust 2001;175:104-7. https://doi.org/10.5694/j.1326-5377.2001.tb143541.x.
- Newcomer R, Maravilla V, Faculjak P, Graves MT. Outcomes of preventive case management among high-risk elderly in three medical groups: a randomized clinical trial. Eval Health Prof 2004;27:323-48. https://doi.org/10.1177/0163278704270011.
- Ng TP, Feng L, Nyunt MS, Feng L, Niti M, Tan BY, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. Am J Med 2015;128:1225-1236.e1. https://doi.org/10.1016/j.amjmed.2015.06.017.
- Parsons J, Rouse P, Robinson EM, Sheridan N, Connolly MJ. Goal setting as a feature of homecare services for older people: does it make a difference?. Age Ageing 2012;41:24-9. https://doi.org/10.1093/ageing/afr118.
- Parsons M, Senior H, Kerse N, Chen MH, Jacobs S, Anderson C. Randomised trial of restorative home care for frail older people in New Zealand. Nurs Older People 2017;29:27-33. https://doi.org/10.7748/nop.2017.e897.
- Parsons M, Senior H, Kerse N, Chen MH, Jacobs S, Vanderhoorn S, et al. Should care managers for older adults be located in primary care? A randomized controlled trial. J Am Geriatr Soc 2012;60:86-92. https://doi.org/10.1111/j.1532-5415.2011.03763.x.
- Pathy MS, Bayer A, Harding K, Dibble A. Randomised trial of case finding and surveillance of elderly people at home. Lancet 1992;340:890-3. https://doi.org/10.1016/0140-6736(92)93294-W.
- Phelan EA, Balderson B, Levine M, Erro JH, Jordan L, Grothaus L, et al. Delivering effective primary care to older adults: a randomized, controlled trial of the senior resource team at group health cooperative. J Am Geriatr Soc 2007;55:1748-56. https://doi.org/10.1111/j.1532-5415.2007.01416.x.
- Ploeg J, Brazil K, Hutchison B, Kaczorowski J, Dalby DM, Goldsmith CH, et al. Effect of preventive primary care outreach on health related quality of life among older adults at risk of functional decline: randomised controlled trial. BMJ 2010;340. https://doi.org/10.1136/bmj.c1480.
- Profener F, Anders J, Dapp U, Minder CE, Golgert S, von Renteln-Kruse W. Acceptance of preventive home visits among frail elderly persons: participants and non-participants in a follow-up after 2 and 4 years within the LUCAS longitudinal study. [German]. Z Gerontol Geriatr 2016;49:596-605. https://doi.org/10.1007/s00391-016-1127-9.
- Rockwood K, Stadnyk K, Carver D, MacPherson KM, Beanlands HE, Powell C, et al. A clinimetric evaluation of specialized geriatric care for rural dwelling, frail older people. J Am Geriatr Soc 2000;48:1080-5. https://doi.org/10.1111/j.1532-5415.2000.tb04783.x.
- Romera-Liebana L, Orfila F, Segura JM, Real J, Fabra ML, Möller M, et al. Effects of a primary care-based multifactorial intervention on physical and cognitive function in frail, elderly individuals: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2018;73:1688-74. https://doi.org/10.1093/gerona/glx259.
- Rooijackers TH, Kempen GIJM, Zijlstra GAR, van Rossum E, Koster A, Lima Passos V, et al. Effectiveness of a reablement training program for homecare staff on older adults’ sedentary behavior: a cluster randomized controlled trial. J Am Geriatr Soc 2021;69:2566-78. https://doi.org/10.1111/jgs.17286.
- Rubenstein LZ, Alessi CA, Josephson KR, Trinidad Hoyl M, Harker JO, Pietruszka FM. A randomized trial of a screening, case finding, and referral system for older veterans in primary care. J Am Geriatr Soc 2007;55:166-74. https://doi.org/10.1111/j.1532-5415.2007.01044.x.
- Ryvicker M, Feldman PH, Rosati RJ, Sobolewski S, Maduro GA, Schwartz T. Improving functional outcomes in home care patients: impact and challenges of disseminating a quality improvement initiative. J Healthc Qual 2011;33:28-36. https://doi.org/10.1111/j.1945-1474.2011.00156.x.
- Serra-Prat M, Sist X, Domenich R, Jurado L, Saiz A, Roces A, et al. Effectiveness of an intervention to prevent frailty in pre-frail community-dwelling older people consulting in primary care: a randomised controlled trial. Age Ageing 2017;46:401-7. https://doi.org/10.1093/ageing/afw242.
- Shapiro A, Taylor M. Effects of a community-based early intervention program on the subjective well-being, institutionalization, and mortality of low-income elders. Gerontologist 2002;42:334-41. https://doi.org/10.1093/geront/42.3.334.
- Sherman H, Soderhielm-Blid S, Forsberg C, Karp A, Tornkvist L. Effects of preventive home visits by district nurses on self-reported health of 75-year-olds. Prim Health Care Res Dev 2016;17:56-71. https://doi.org/10.1017/S1463423614000565.
- Siemonsma PC, Blom JW, Hofstetter H, van Hespen ATH, Gussekloo J, Drewes YM, et al. The effectiveness of functional task exercise and physical therapy as prevention of functional decline in community dwelling older people with complex health problems. BMC Geriatr 2018;18. https://doi.org/10.1186/s12877-018-0859-3.
- Stewart S, Harvey I, Poland F, Lloyd-Smith W, Mugford M, Flood C. Are occupational therapists more effective than social workers when assessing frail older people? Results of CAMELOT, a randomised controlled trial. Age Ageing 2005;34:41-6. https://doi.org/10.1093/ageing/afh230.
- Stuck AE, Aronow HU, Steiner A, Alessi CA, Bula CJ, Gold MN, et al. A trial of annual in-home comprehensive geriatric assessments for elderly people living in the community. N Engl J Med 1995;333:1184-9. https://doi.org/10.1056/NEJM199511023331805.
- Stuck AE, Minder CE, Peter-Wuest I, Gillmann G, Egli C, Kesselring A, et al. A randomized trial of in-home visits for disability prevention in community-dwelling older people at low and high risk for nursing home admission. Arch Intern Med 2000;160:977-86. https://doi.org/10.1001/archinte.160.7.977.
- Stuck AE, Moser A, Morf U, Wirz U, Wyser J, Gillmann G, et al. Effect of health risk assessment and counselling on health behaviour and survival in older people: a pragmatic randomised trial. PLOS Med 2015;12. https://doi.org/10.1371/journal.pmed.1001889.
- Suijker JJ, van Rijn M, Buurman BM, Ter Riet G, Moll van Charante EP, de Rooij SE. Effects of nurse-led multifactorial care to prevent disability in community-living older people: cluster randomized trial. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0158714.
- Szanton SL, Thorpe RJ, Boyd C, Tanner EK, Leff B, Agree E, et al. Community aging in place, advancing better living for elders: a bio-behavioral-environmental intervention to improve function and health-related quality of life in disabled older adults. J Am Geriatr Soc 2011;59:2314-20. https://doi.org/10.1111/j.1532-5415.2011.03698.x.
- Szanton SL, Xue QL, Leff B, Guralnik J, Wolff JL, Tanner EK, et al. Effect of a biobehavioral environmental approach on disability among low-income older adults: a randomized clinical trial. JAMA Intern Med 2019;179:204-11. https://doi.org/10.1001/jamainternmed.2018.6026.
- Takahashi PY, Pecina JL, Upatising B, Chaudhry R, Shah ND, Van Houten H, et al. A randomized controlled trial of telemonitoring in older adults with multiple health issues to prevent hospitalizations and emergency department visits. Arch Intern Med 2012;172:773-9. https://doi.org/10.1001/archinternmed.2012.256.
- Teut M, Schnabel K, Baur R, Kerckhoff A, Reese F, Pilgram N, et al. Effects and feasibility of an Integrative Medicine program for geriatric patients – a cluster-randomized pilot study. Clin Interv Aging 2013;8:953-61. https://doi.org/10.2147/CIA.S45242.
- Thiel C, Braun T, Grüneberg C. Physical training as core component of multimodal treatment of older frail people-study protocol of a randomized controlled pilot study. Z Gerontol Geriatr 2019;52:45-60. https://doi.org/10.1007/s00391-018-1443-3.
- Thomas R, Worrall G, Elgar F, Knight J. Can they keep going on their own? A four-year randomized trial of functional assessments of community residents. Can J Aging 2007;26:379-90. https://doi.org/10.3138/cja.26.4.379.
- Tomita MR, Mann WC, Stanton K, Tomita AD, Sundar V. Use of currently available smart home technology by frail elders: process and outcomes. Top Geriatr Rehabil 2007;23:24-3. https://doi.org/10.1097/00013614-200701000-00005.
- Tulloch AJ, Moore V. A randomized controlled trial of geriatric screening and surveillance in general practice. J R Coll Gen Pract 1979;29:733-40.
- Tuntland H, Aaslund MK, Espehaug B, Førland O, Kjeken I. Reablement in community-dwelling older adults: a randomised controlled trial. BMC Geriatr 2015;15:1-11. https://doi.org/10.1186/s12877-015-0142-9.
- van der Pols-Vijlbrief R, Wijnhoven HAH, Bosmans JE, Twisk JWR, Visser M. Targeting the underlying causes of undernutrition. Cost-effectiveness of a multifactorial personalized intervention in community-dwelling older adults: a randomized controlled trial. Clin Nutr 2017;36:1498-508. https://doi.org/10.1016/j.clnu.2016.09.030.
- van Dongen EJ, Haveman-Nies A, Doets EL, Dorhout BG, de Groot LC. Effectiveness of a diet and resistance exercise intervention on muscle health in older adults: ProMuscle in Practice. J Am Med Dir Assoc 2020;21:1065-1072.e3. https://doi.org/10.1016/j.jamda.2019.11.026.
- van Heuvelen MJ, Hochstenbach JB, Brouwer WH, de Greef MH, Zijlstra GA, van Jaarsveld E, et al. Differences between participants and non-participants in an RCT on physical activity and psychological interventions for older persons. Aging Clin Exp Res 2005;17:236-45. https://doi.org/10.1007/BF03324603.
- van Hout HP, Jansen AP, van Marwijk HW, Pronk M, Frijters DF, Nijpels G. Prevention of adverse health trajectories in a vulnerable elderly population through nurse home visits: a randomized controlled trial [ISRCTN05358495]. J Gerontol A Biol Sci Med Sci 2010;65:734-42. https://doi.org/10.1093/gerona/glq037.
- van Leeuwen KM, Bosmans JE, Jansen AP, Hoogendijk EO, Muntinga ME, van Hout HP, et al. Cost-effectiveness of a chronic care model for frail older adults in primary care: economic evaluation alongside a stepped-wedge cluster-randomized trial. J Am Geriatr Soc 2015;63:2494-504. https://doi.org/10.1111/jgs.13834.
- van Lieshout MRJ, Bleijenberg N, Schuurmans MJ, de Wit NJ. The effectiveness of a PRoactive multicomponent intervention program on disability in independently living older people: a randomized controlled trial. J Nutr Health Aging 2018;22:1051-9. https://doi.org/10.1007/s12603-018-1101-x.
- van Rossum E, Frederiks CM, Philipsen H, Portengen K, Wiskerke J, Knipschild P. Effects of preventive home visits to elderly people. BMJ 1993;307:27-32. https://doi.org/10.1136/bmj.307.6895.27.
- Vass M, Avlund K, Lauridsen J, Hendriksen C. Feasible model for prevention of functional decline in older people: municipality-randomized, controlled trial. J Am Geriatr Soc 2005;53:563-8. https://doi.org/10.1111/j.1532-5415.2005.53201.x.
- Vetter NJ, Jones DA, Victor CR. Effect of health visitors working with elderly patients in general practice: a randomised controlled trial. Br Med J (Clin Res Ed) 1984;288:369-72. https://doi.org/10.1136/bmj.288.6414.369.
- von Bonsdorff MB, Leinonen R, Kujala UM, Heikkinen E, Törmäkangas T, Hirvensalo M, et al. Effect of physical activity counseling on disability in older people: a 2-year randomized controlled trial. J Am Geriatr Soc 2008;56:2188-94. https://doi.org/10.1111/j.1532-5415.2008.02000.x.
- Wallace JI, Buchner DM, Grothaus L, Leveille S, Tyll L, LaCroix AZ, et al. Implementation and effectiveness of a community-based health promotion program for older adults. J Gerontol A Biol Sci Med Sci 1998;53:M301-6. https://doi.org/10.1093/gerona/53a.4.m301.
- Walters K, Frost R, Kharicha K, Avgerinou C, Gardner B, Ricciardi F, et al. Home-based health promotion for older people with mild frailty: the HomeHealth intervention development and feasibility RCT. Health Technol Assess 2017;21:1-128. https://doi.org/10.3310/hta21730.
- Whitehead PJ, Walker MF, Parry RH, Latif Z, McGeorge ID, Drummond AE. Occupational Therapy in HomEcare Re-ablement Services (OTHERS): results of a feasibility randomised controlled trial. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011868.
- Williams EI, Greenwell J, Groom LM. The care of people over 75 years old after discharge from hospital: an evaluation of timetabled visiting by Health Visitor Assistants. J Public Health Med 1992;14:138-44. https://doi.org/10.1093/oxfordjournals.pubmed.a042711.
- Wolter A, Stolle C, Roth G, Rothgang H. Does the resident care assessment instrument improve long-term home care? – results of a nation-wide study in Germany. [German]. Gesundheitswesen 2013;75:29-32. https://doi.org/10.1055/s-0032-1309013.
- Wong AKC, Wong FKY, Chang K. Effectiveness of a community-based self-care promoting program for community-dwelling older adults: a randomized controlled trial. Age Ageing 2019;48:852-8. https://doi.org/10.1093/ageing/afz095.
- Yamada Y, Ikegami N. Preventive home visits for community-dwelling frail elderly people based on minimum data set-home care: randomized controlled trial. Geriatr Gerontol Int 2003;3:236-42. https://doi.org/10.1111/j.1444-1586.2003.00103.x.
- Brazil K, Scott D, Wallace E, Clarke M, Fahey T, Gillespie P, et al. Anticipatory care planning intervention for older adults at risk of functional decline: study protocol for a primary care cluster feasibility randomised trial. Trials 2020;21. https://doi.org/10.1186/s13063-020-4100-2.
- Donelle L, Regan S, Kerr M, Zwarenstein M, Bauer M, Warner G, et al. Caring near and far by connecting community-based clients and family member/friend caregivers using passive remote monitoring: protocol for a pragmatic randomized controlled trial. JMIR Res Protoc 2020;9. https://doi.org/10.2196/15027.
- Falvey JR, Mangione KK, Nordon-Craft A, Cumbler E, Burrows KL, Forster JE, et al. Progressive multicomponent intervention for older adults in home health settings following acute hospitalization: randomized clinical trial protocol. Phys Ther 2019;99:1141-9.
- Ferrat E, Bastuji-Garin S, Paillaud E, Caillet P, Clerc P, Moscova L, et al. Efficacy of nurse-led and general practitioner-led comprehensive geriatric assessment in primary care: protocol of a pragmatic three-arm cluster randomised controlled trial (CEpiA study). BMJ Open 2018;8.
- ISRCTN13927531 . HERO – Home-Based Extended Rehabilitation for Older People 2017. http://isrctn.com/ISRCTN13927531 (accessed 11 May 2020).
- ISRCTN16123291 . Does Personalised Care Planning Improve the Quality of Life of Older People With Frailty and Is It Cost-Effective? 2020. www.isrctn.com/ISRCTN16123291 (accessed 26 August 2021).
- ISRCTN54268283 . Enabling Health and Maintaining Independence for Older People at Home: The ‘HomeHealth’ Trial 2020. http://isrctn.com/ISRCTN54268283 (accessed 26 August 2021).
- Lyndon H, Latour JM, Marsden J, Campbell S, Stevens K, Kent B. The holistic assessment and care planning in partnership intervention study (HAPPI): a protocol for a feasibility, cluster randomized controlled trial. J Adv Nurs 2019;75:3078-87. https://doi.org/10.1111/jan.14106.
- Mohd Suffian NI, Adznam SN, Abu Saad H, Chan YM, Ibrahim Z, Omar N, et al. Frailty Intervention through Nutrition Education and Exercise (FINE). A health promotion intervention to prevent frailty and improve frailty status among pre-frail elderly – a study protocol of a cluster randomized controlled trial. Nutrients 2020;12. https://doi.org/10.3390/nu12092758.
- Mortsiefer A, Wilm S, Santos S, Loscher S, Wollny A, Drewelow E, et al. COFRAIL study group . Family conferences and shared prioritisation to improve patient safety in the frail elderly (COFRAIL): study protocol of a cluster randomised intervention trial in primary care. Trials 2020;21. https://doi.org/10.1186/s13063-020-4182-x.
- NCT03147625 . Salutogenic Healthy Aging Program Embracement (SHAPE) for Elderly-Only Households 2017. https://clinicaltrials.gov/show/NCT03147625 (accessed 11 May 2020).
- NCT03394534 . Comprehensive Geriatric Assessment in Primary Care: A Randomised Feasibility Trial 2018. https://ClinicalTrials.gov/show/NCT03394534 (accessed 11 May 2020).
- NCT03456128 . Reducing Disability Following Hospital Discharge in Vulnerable Older Adults: The CAPABLE Intervention 2018. https://clinicaltrials.gov/show/NCT03456128 (accessed 11 May 2020).
- NCT03591055 . Frailty Assessment and Treatment Strategies in the Elderly at Risk of Functional Decline in the Community 2018. https://ClinicalTrials.gov/show/NCT03591055 (accessed 11 May 2020).
- NCT03649698 . Exercise and Nutrition for Healthy AgeiNg 2018. https://ClinicalTrials.gov/show/NCT03649698 (accessed 11 May 2020).
- NCT03824106 . Frailty Rehabilitation 2019. https://ClinicalTrials.gov/show/NCT03824106 (accessed 11 May 2020).
- NCT03979560 . Clinical and Medico-Economic Evaluation of Taken Care Associating Nutritional Support and Adapted Physical Activity for Malnourished, or at Risk of Undernutrition, Elderly People on Discharge From Hospital 2019. https://clinicaltrials.gov/show/NCT03979560 (accessed 11 May 2020).
- NCT04416815 . Integrating Health Promotion With and for Older People – EHealth 2020. https://clinicaltrials.gov/show/NCT04416815 (accessed 6 November 2020).
- NCT04460742 . CAPABLE Transitions: A Home Health Agency-Based Intervention to Optimize the SNF-to-Home Transition 2020. https://clinicaltrials.gov/show/NCT04460742 (accessed 6 November 2020).
- NCT04500366 . GERAS Frailty Rehabilitation at Home During COVID-19 2020. https://clinicaltrials.gov/show/NCT04500366 (accessed 6 November 2020).
- Peri K, Broad JB, Hikaka J, Boyd M, Bloomfield K, Wu Z, et al. Study protocol: older people in retirement villages. A survey and randomised trial of a multi-disciplinary invention designed to avoid adverse outcomes. BMC Geriatr 2020;20. https://doi.org/10.1186/s12877-020-01640-6.
- Rivas-Ruiz F, Machón M, Mateo-Abad M, Contreras-Fernández E, Güell C, Baro-Rodríguez L, et al. InFrAP investigators . Tackling frailty at primary care: evaluation of the effectiveness of a multicomponent intervention through a randomised controlled trial: study protocol. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-034591.
- Stathi A, Withall J, Greaves CJ, Thompson JL, Taylor G, Medina-Lara A, et al. A community-based physical activity intervention to prevent mobility-related disability for retired older people (REtirement in ACTion (REACT)): study protocol for a randomised controlled trial. Trials 2018;19.
- Teh R, Kerse N, Waters DL, Hale L, Pillai A, Leilua E, et al. Study protocol of a randomised controlled trial to examine the impact of a complex intervention in pre-frail older adults. Aging Clin Exp Res 2019;31:1407-17.
- Denny KG, Tomaszewski-Farias S. A multi-modal intervention to enhance cognitive compensation strategies and promote brain health activities. Alzheimer’s Dementia 2017;13.
- DRKS00024638 . Prevention for More Participation in Older Ages 2021. https://trialsearch.who.int/Trial2.aspx?TrialID=DRKS00024638 (accessed 26 August 2021).
- Fasce GP, Aravena JMC, Araya CO, Bustamante RM, Gonzalez FA, Briceño CR, et al. Domiciliary intervention by occupational therapy after hospital discharge in order to prevent re-admission in the elderly: study protocol for a randomised clinical trial. Rev Esp Geriatr Gerontol 2018;53:337-43.
- Ferreira MM. Efficacy of a primary care geriatric intervention model in reversing frailty-protocol of a randomized clinical trial. Eur Geriatr Med 2018;9. https://doi.org/10.1007/s41999-018-0097-4.
- NCT04574271 . Multidimensional Intervention in Pre-Frail Patients Older Than 70 Years 2019. https://ClinicalTrials.gov/show/NCT04574271 (accessed 26 August 2021).
- Neumann L, Dapp U, von Renteln-Kruse W, Minder CE. Health promotion and preventive care intervention for older community-dwelling people: long-term effects of a randomised controlled trial (RCT) within the LUCAS cohort. J Nutr Health Aging 2017;21:1016-23.
- NCT04076319 . Implementing CAPABLE 2019. https://ClinicalTrials.gov/show/NCT04076319 (accessed 5 November 2020).
- Spoelstra SL, Schueller M, Sikorskii A. Testing an implementation strategy bundle on adoption and sustainability of evidence to optimize physical function in community-dwelling disabled and older adults in a Medicaid waiver: a multi-site pragmatic hybrid type III protocol. Implement Sci 2019;14.
- Senior HEJ, Parsons M, Kerse N, Chen MH, Jacobs S, Hoorn SV, et al. Promoting independence in frail older people: a randomised controlled trial of a restorative care service in New Zealand. Age Ageing 2014;43:418-24.
- NCT02317432 . Building Community Capacity for Disability Prevention for Minority Elders (Positive Minds – Strong Bodies) 2014. https://clinicaltrials.gov/ct2/show/NCT02317432 (accessed 19 May 2020).
- Porteny T, Alegría M, del Cueto P, Fuentes L, Markle SL, NeMoyer A, et al. Barriers and strategies for implementing community-based interventions with minority elders: positive minds-strong bodies. Implement Sci Commun 2020;1:1-13.
- Arthanat S, Vroman KG, Lysack C. A home-based individualized information communication technology training program for older adults: a demonstration of effectiveness and value. Disabil Rehabil Assist Technol 2016;11:316-24.
- Arthanat S, Vroman KG, Lysack C, Grizzetti J. Multi-stakeholder perspectives on information communication technology training for older adults: implications for teaching and learning. Disabil Rehabil Assist Technol 2019;14:453-61.
- Auvinen K, Raisanen J, Merikoski M, Mantyla A, Kumpusalo-Vauhkonen A, Enlund H, et al. The Finnish Interprofessional Medication Assessment (FIMA): baseline findings from home care setting. Aging Clin Exp Res 2019;31:1471-9.
- Merikoski M, Auvinen K, Kumpusalo-Vauhkonen A, Liukkonen T, Lämsä E, Lönroos E, et al. Iäkkäiden Lääkehoidon Moniammatillinen Arviointi (ILMA). Helsinki: Sosiaali-ja Terveysministeriö; 2017.
- NCT02398812 . The Interprofessional Medication Assessment for Older Patients 2015. https://clinicaltrials.gov/ct2/show/NCT02398812 (accessed 28 April 2020).
- Gustafsson S, Berglund H, Faronbi J, Barenfeld E, Ottenvall Hammar I. Minor positive effects of health-promoting senior meetings for older community-dwelling persons on loneliness, social network, and social support. Clin Interv Aging 2017;12:1867-77.
- Arola A, Dahlin-Ivanoff S, Haggblom-Kronlof G. Impact of a person-centred group intervention on life satisfaction and engagement in activities among persons aging in the context of migration. Scand J Occup Ther 2019;1.
- Gustafsson S, Lood Q, Wilhelmson K, Haggblom-Kronlof G, Landahl S, Dahlin-Ivanoff S. A person-centred approach to health promotion for persons 70+ who have migrated to Sweden: promoting aging migrants’ capabilities implementation and RCT study protocol. BMC Geriatr 2015;15.
- NCT01841853 . RCT of Health-Promoting Intervention for Older Foreign-Born Adults 2013. https://clinicaltrials.gov/ct2/show/NCT01841853 (accessed 28 April 2020).
- Coleston-Shields DM. Integration of medical and social services for elderly people in the community reduced costs and use of health services. Evid Based Mental Health 1998;1. https://doi.org/10.1136/ebmh.1.4.106.
- Britian O. Integration of services for elderly people reduced costs and use of health services. Randomized Trial of Impact of Model of Integrated Care and Case Management for Older People Living in the Community. Br Med J 1998;316:1348-51.
- Bleijenberg N, Drubbel I, Ten Dam VH, Numans ME, Schuurmans MJ, de Wit NJ. Proactive and integrated primary care for frail older people: design and methodological challenges of the Utrecht primary care PROactive frailty intervention trial (U-PROFIT). BMC Geriatr 2012;12.
- Bleijenberg N, ten Dam VH, Drubbel I, Numans ME, de Wit NJ, Schuurmans MJ. Development of a proactive care program (U-CARE) to preserve physical functioning of frail older people in primary care. J Nurs Scholarsh 2013;45:230-7.
- Bleijenberg N, ten Dam VH, Drubbel I, Numans ME, Wit NJ, Schuurmans MJ. Treatment fidelity of an evidence-based nurse-led intervention in a proactive primary care program for older people. Worldviews Evid Based Nurs 2016;13:75-84.
- Bleijenberg N, Ten Dam VH, Steunenberg B, Drubbel I, Numans ME, De Wit NJ, et al. Exploring the expectations, needs and experiences of general practitioners and nurses towards a proactive and structured care programme for frail older patients: a mixed-methods study. J Adv Nurs 2013;69:2262-73.
- Laan W, Zuithoff NP, Drubbel I, Bleijenberg N, Numans ME, de Wit NJ, et al. Validity and reliability of the Katz-15 scale to measure unfavorable health outcomes in community-dwelling older people. J Nutr Health Aging 2014;18:848-54.
- NTR2288 . Central Utrecht Elderly Care Project ‘Om U’: ‘Somebody Who Knows what’s Going on and Can See Things from My side’ 2010. https://trialsearch.who.int/Trial2.aspx?TrialID=NTR2288 (accessed 11 May 2020).
- ten Dam VH, Bleijenberg N, Numans ME, Drubbel I, Schuurmans MJ, de Wit NJ. [Proactive and structured care for the elderly in primary care]. [Dutch]. Tijdschr Gerontol Geriatr 2013;44:81-9.
- Metzelthin SF, Bleijenberg N, Blom JW, Imhof L. Primary care strategies to maintain independence of frail older people: looking for evidence across borders. Eur Geriatr Med 2014;5:S31-S2.
- Bleijenberg N, Imhof L, Mahrer-Imhof R, Wallhagen MI, de Wit NJ, Schuurmans MJ. Patient characteristics associated with a successful response to nurse-led care programs targeting the oldest-old: a comparison of two RCTs. Worldviews Evid Based Nurs 2017;14:210-22.
- NL1836 . Integrated Systematic Care for Older PEople (ISCOPE) 2009. www.trialregister.nl/trial/1836 (accessed 28 April 2020).
- van Blijswijk SCE, Blom JW, de Craen AJM, den Elzen WPJ, Gussekloo J. Prediction of functional decline in community-dwelling older persons in general practice: a cohort study. BMC Geriatr 2018;18:1-9.
- ISRCTN66679751 . Implementation of an Internet-Based Instrument, the ‘Eigen Kracht Wijzer’ in Older People 2013. https://doi.org/10.1186/ISRCTN66679751 (accessed 11 May 2020).
- Botjes E. EigenKrachtWijzer. Netherland: Movisie; 2013.
- Nicolaides-Bouman A, van Rossum E, Kempen GI, Knipschild P. Effects of home visits by home nurses to elderly people with health problems: design of a randomised clinical trial in the Netherlands [ISRCTN92017183]. BMC Health Serv Res 2004;4.
- Nicolaides-Bouman A, van Rossum E, Habets H, Kempen GI, Knipschild P. Home visiting programme for older people with health problems: process evaluation. J Adv Nurs 2007;58:425-35.
- ISRCTN92017183 . Effects of Home Visits by Home Nurses to Elderly People With Health Problems 2004. www.isrctn.com/ISRCTN92017183 (accessed 28 April 2020).
- Bouman AIE, Van Rossum E, Ambergen TW, Kempen GIJM, Knipschild PG. House calls to elderly with health problems: effects of a random experiment. [Dutch]. Ned Tijdschr Geneeskd 2009;153:644-50.
- Bouman A, van Rossum E, Evers S, Ambergen T, Kempen G, Knipschild P. Effects on health care use and associated cost of a home visiting program for older people with poor health status: a randomized clinical trial in the Netherlands. J Gerontol A Biol Sci Med Sci 2008;63:291-7.
- Brettschneider C, Luck T, Fleischer S, Roling G, Beutner K, Sesselmann Y, et al. Cost-utility analysis of preventive home visits in older adults. Value Health 2014;17.
- Fleischer S, Roling G, Beutner K, Hanns S, Behrens J, Luck T, et al. Growing old at home – a randomized controlled trial to investigate the effectiveness and cost-effectiveness of preventive home visits to reduce nursing home admissions: study protocol [NCT00644826]. BMC Public Health 2008;8. https://doi.org/10.1186/1471-2458-8-185.
- Luck T, Roling G, Heinrich S, Luppa M, Matschinger H, Fleischer S. Altern zu Hause-Unterstützung durch präventive Hausbesuche-Eine randomisierte kontrollierte Interventionsstudie. Hallesche Beitr Gesundh Pflegewissenschaften 2011;10:1-33.
- NCT00644826 . Growing Old at Home 2008. https://clinicaltrials.gov/ct2/show/NCT00644826 (accessed 28 April 2020).
- ACTRN12608000250336 . Frailty Intervention Trial 2008. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12608000250336 (accessed 8 May 2020).
- ACTRN12608000507381 . Increasing Participation. A Sub-Study of the Frailty Intervention Trial (FIT) 2008. www.anzctr.org.au/ACTRN12608000507381.aspx (accessed 11 May 2020).
- ACTRN12608000565347 . The Impact of a Community Based Multidisciplinary Frailty Intervention for Older People on Informal Carers’ Experience of Caregiving 2008. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=83207 (accessed 12 May 2020).
- Aggar C, Ronaldson S, Cameron ID. Reactions to caregiving during an intervention targeting frailty in community living older people. BMC Geriatr 2012;12.
- Fairhall N, Aggar C, Kurrle SE, Sherrington C, Lord S, Lockwood K, et al. Frailty intervention trial (FIT). BMC Geriatr 2008;8.
- Fairhall N, Sherrington C, Cameron ID, Kurrle SE, Lord SR, Lockwood K, et al. A multifactorial intervention for frail older people is more than twice as effective among those who are compliant: complier average causal effect analysis of a randomised trial. J Physiother 2017;63:40-4.
- Fairhall N, Sherrington C, Kurrle SE, Lord SR, Lockwood K, Cameron ID. Effect of a multifactorial interdisciplinary intervention on mobility-related disability in frail older people: randomised controlled trial. BMC Med 2012;10.
- Fairhall N, Sherrington C, Kurrle SE, Lord SR, Lockwood K, Howard K, et al. Economic evaluation of a multifactorial, interdisciplinary intervention versus usual care to reduce frailty in frail older people. J Am Med Dir Assoc 2015;16:41-8.
- Fairhall N, Sherrington C, Lord S, Susan K, Cameron ID. A multifactorial interdisciplinary intervention reduces frailty, increases function and is cost-effective in older adults who are frail: randomised controlled trial. Physiotherapy 2015;101:e371-2. https://doi.org/10.1016/j.physio.2015.03.588.
- Fairhall N, Sherrington C, Lord SR, Kurrle SE, Langron C, Lockwood K, et al. Effect of a multifactorial, interdisciplinary intervention on risk factors for falls and fall rate in frail older people: a randomised controlled trial. Age Ageing 2013;43:616-22.
- Cesari M, Demougeot L, Vellas B. Multidomain intervention to prevent disability in elders – the MINDED study. Eur Geriatr Med 2013;1:S84-S5.
- Cesari MM. Multidomain Intervention to preveNt Disability in ElDers – MINDED. Agence Nationale de la Recherche; n.d.
- Fougère B, Aubertin-Leheudre M, Vellas B, Andrieu S, Demougeot L, Cluzan C, et al. Clinical research for older adults in rural areas: the MINDED study experience. Age (Dordr) 2016;38.
- Fougère B, Vellas B, Andrieu S, Demougeot L, Cluzan C, Cesari M. Difficulties encountered and solutions provided in the implementation of a multidisciplinary intervention for the prevention of dependency in the older population in rural areas: the MINDED study. Geriatr Psychol Neuropsychiatr Vieil 2015;13:259-64.
- NCT02082171 . Multidomain Intervention to Prevent Disability in Elders 2013. https://ClinicalTrials.gov/show/NCT02082171 (accessed 11 May 2020).
- Zengarini E, Ruggiero C, Mecocci P, Vellas B, Cesari M. Fatigue as a clinical sign of biological aging: exploratory analyses from the MINDED project. Geriatr Gerontol Int 2016;16:533-4.
- Clarkson P, Venables D, Hughes J, Burns A, Challis D. Integrated specialist assessment of older people and predictors of care-home admission. Psychol Med 2006;36:1011-21.
- Azen SP, Palmer JM, Carlson M, Mandel D, Cherry BJ, Fanchiang SP, et al. Psychometric properties of a Chinese translation of the SF-36 health survey questionnaire in the Well Elderly Study. J Aging Health 1999;11:240-51.
- Clark F, Azen SP, Carlson M, Mandel D, LaBree L, Hay J, et al. Embedding health-promoting changes into the daily lives of independent-living older adults: long-term follow-up of occupational therapy intervention. J Gerontol B Psychol Sci Soc Sci 2001;56:P60-3.
- Clark FA, Carlson M, Jackson J, Mandel D. Lifestyle redesign improves health and is cost-effective. OT Practice 2003;8:9-13.
- Hay J, LaBree L, Luo R, Clark F, Carlson M, Mandel D, et al. Cost-effectiveness of preventive occupational therapy for independent-living older adults. J Am Geriatr Soc 2002;50:1381-8.
- Jackson J, Carlson M, Mandel D, Zemke R, Clark F. Occupation in lifestyle redesign: the well elderly study occupational therapy program. Am J Occup Ther 1998;52:326-36.
- Carlson M, Jackson J, Mandel D, Blanchard J, Holguin J, Lai M-Y, et al. Predictors of retention among African American and Hispanic older adult research participants in the Well Elderly 2 randomized controlled trial. J Appl Gerontol 2014;33:357-82.
- Jackson J, Mandel D, Blanchard J, Carlson M, Cherry B, Azen S, et al. Confronting challenges in intervention research with ethnically diverse older adults: the USC well elderly II trial. Clin Trials 2009;6:90-101.
- NCT00786344 . USC Well Elderly Study 2 2008. https://clinicaltrials.gov/ct2/show/NCT00786344 (accessed 21 August 2020).
- Schelly D, Ohl A, Nadres R. Dosage and efficacy in behavioral interventions with community dwelling older adults: lifestyle redesign revisited. J Appl Gerontol 2021;40:1087-95. https://doi.org/10.1177/0733464820911335.
- Seidle JS. The impact of response to stressful events on participation in meaningful activity: a secondary data analysis using the well elderly 2 study. Arch Phys Med Rehabil 2020;101. https://doi.org/10.1016/j.apmr.2020.10.004.
- Sonnenfeld M, Karmarkar A. Cortisol as a biomarker in rehabilitation services for community dwelling older adults. Arch Phys Med Rehabil 2019;100.
- Bielaszka-DuVernay C. Innovation profile: the ‘GRACE’ model: in-home assessments lead to better care for dual eligibles. Health Aff (Millwood) 2011;30:431-4.
- Counsell SR, Callahan CM, Buttar AB, Clark DO, Frank KI. Geriatric Resources for Assessment and Care of Elders (GRACE): a new model of primary care for low-income seniors. J Am Geriatr Soc 2006;54:1136-41.
- Counsell SR, Callahan CM, Tu W, Stump TE, Arling GW. Cost analysis of the Geriatric Resources for Assessment and Care of Elders care management intervention. J Am Geriatr Soc 2009;57:1420-6.
- Iloabuchi TC, Mi D, Tu W, Counsell SR. Risk factors for early hospital readmission in low-income elderly adults. J Am Geriatr Soc 2014;62:489-94. https://doi.org/10.1111/jgs.12688.
- NCT00182962 . GRACE: Geriatric Resources for Assessment and Care of Elders 2005. https://clinicaltrials.gov/ct2/show/NCT00182962 (accessed 21 August 2020).
- NCT00985283 . Feasibility and Effects of Preventive Home Visits for Older Adults 2009. https://clinicaltrials.gov/ct2/show/NCT00985283 (accessed 4 August 2020).
- Dalby DM, Sellors JW, Fraser FD, Fraser C, van Ineveld CH, Pickard L, et al. Screening seniors for risk of functional decline: results of a survey in family practice. Can J Public Health 1999;90:133-7. https://doi.org/10.1007/BF03404117.
- Claus E, Willen A, de Craen A, Knops H, Willems C. Protocol development for individual occupational therapy in the oldest old [article in Dutch]. Ned Tijdschr Ergotherapie 2003;31:83-6.
- De Craen AJM, Westendorp RGJ, Willems CG, Buskens ICM, Gussekloo J. Assistive devices and community-based services among 85-year-old community-dwelling elderly in The Netherlands: ownership, use, and need for intervention. Disabil Rehabil Assist Technol 2006;1:199-203. https://doi.org/10.1080/17483100612331392835.
- NCT00278096 . Randomised Controlled Trial of Unsolicited Occupational Therapy in Community-Dwelling Elderly 2006. https://clinicaltrials.gov/show/NCT00278096 (accessed 11 May 2020).
- NCT01358032 . Evaluating an In-Home Multicomponent Program to Manage Concerns About Falling in Frail Community Dwelling Older People 2009. https://clinicaltrials.gov/ct2/show/NCT01358032 (accessed 11 May 2020).
- Dorresteijn TA, Zijlstra G, Van Haastregt JC, Vlaeyen JW, Kempen GI. Feasibility of a nurse-led in-home cognitive behavioral program to manage concerns about falls in frail older people: a process evaluation. Res Nurs Health 2013;36:257-70.
- Evers S, Dorresteijn TAC, Wijnen BFM, van Haastregt JCM, Kempen G, Zijlstra GAR. Economic evaluation of a home-based programme to reduce concerns about falls in frail, independently-living older people. Expert Rev Pharmacoecon Outcomes Res 2020;20:641-51. https://doi.org/10.1080/14737167.2019.1666714.
- Dorresteijn TA, Zijlstra GA, Delbaere K, van Rossum E, Vlaeyen JW, Kempen GI. Evaluating an in-home multicomponent cognitive behavioural programme to manage concerns about falls and associated activity avoidance in frail community-dwelling older people: design of a randomised control trial [NCT01358032]. BMC Health Serv Res 2011;11.
- Dupuy L, Consel C, Sauzéon H. Self determination-based design to achieve acceptance of assisted living technologies for older adults. Comput Human Behav 2016;65:508-21. https://doi.org/10.1016/j.chb.2016.07.042.
- ACTRN12613000043730 . Pre-FIT: A Multifactorial Interdisciplinary Treatment Program for Older People Who Are Pre-Frail 2013. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613000043730 (accessed 16 November 2020).
- Cameron I, Fairhall N, John B, Lockwood K, Monaghan N, Sherrington C, et al. A multifactorial interdisciplinary intervention in pre-frail older people: randomised trial. J Innov Aging 2017;1.
- Rowan NL, Gillette PD, Faul AC, Yankeelov PA, Borders KW, Deck S, et al. Innovative interdisciplinary training in and delivery of evidence-based geriatric services: creating a bridge with social work and physical therapy. Gerontol Geriatr Educ 2009;30:187-204. https://doi.org/10.1080/02701960903133448.
- Arija V, Martín N, Canela T, Anguera C, Castelao AI, García-Barco M, et al. Nutrition education intervention for dependent patients: protocol of a randomized controlled trial. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-373.
- NCT01360775 . Nutritional Education for Dependant Patients (atdom_nut) 2011. https://clinicaltrials.gov/ct2/show/NCT01360775 (accessed 28 April 2020).
- AOKN . Preventive Home Visits to Seniors The Prevention Program of the AOK Lower Saxony: Getting Healthy Older Qualitative Experience Report [German] (Präventive Hausbesuche Bei Senioren Das Präventionsprogramm Der AOK Niedersachsen: Gesund Älter Werden Qualitativer Erfahrungsbericht) 2010.
- Katz S, Ford AB, Downs TD, Adams M, Rusby DI. The Effects of Continued Care: A Study of Chronic Illness in the Home. Rockville, MA: United States Department of Health, Education, and Welfare; 1972.
- NCT03662945 . Mobile Geriatric Teams: Patient Safety and Healthcare Utilization (MGT) 2018. https://clinicaltrials.gov/ct2/show/NCT03662945 (accessed 6 November 2020).
- Gill TM, Baker DI, Gottschalk M, Gahbauer EA, Charpentier PA, de Regt PT, et al. A prehabilitation program for physically frail community-living older persons. Arch Phys Med Rehabil 2003;84:394-40.
- Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Van Ness PH. A prehabilitation program for the prevention of functional decline: effect on higher-level physical function. Arch Phys Med Rehabil 2004;85:1043-9.
- Gill TM, McGloin JM, Gahbauer EA, Shepard DM, Bianco LM. Two recruitment strategies for a clinical trial of physically frail community-living older persons. J Am Geriatr Soc 2001;49:1039-45. https://doi.org/10.1046/j.1532-5415.2001.49206.x.
- Peduzzi P, Guo Z, Marottoli RA, Gill TM, Araujo K, Allore HG. Improved self-confidence was a mechanism of action in two geriatric trials evaluating physical interventions. J Clin Epidemiol 2007;60:94-102.
- Blackburn NE, Skjodt M, Tully MA, Mc Mullan I, Giné-Garriga M, Caserotti P, et al. on behalf of The SITLESS Group . Older adults’ experiences of a physical activity and sedentary behaviour intervention: a nested qualitative study in the SITLESS multi-country randomised clinical trial. Int J Environ Res Public Health 2021;18. https://doi.org/10.3390/ijerph18094730.
- Coll-Planas L, Blancafort Alias S, Tully M, Caserotti P, Giné-Garriga M, Blackburn N, et al. SITLESS group . Exercise referral schemes enhanced by self-management strategies to reduce sedentary behaviour and increase physical activity among community-dwelling older adults from four European countries: protocol for the process evaluation of the SITLESS randomised controlled trial. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-027073.
- SITLESS Consortium . SITLESS 2021. https://sitless.eu/ (accessed 7 May 2021).
- Deidda M, Coll-Planas L, Gine-Garriga M, Guerra-Balic M, Roque IFM, Tully MA, et al. Cost-effectiveness of exercise referral schemes enhanced by self-management strategies to battle sedentary behaviour in older adults: protocol for an economic evaluation alongside the SITLESS three-armed pragmatic randomised controlled trial. BMJ Open 2018;8.
- Giné-Garriga M, Coll-Planas L, Guerra M, Domingo A, Roqué M, Caserotti P, et al. The SITLESS project: exercise referral schemes enhanced by self-management strategies to battle sedentary behaviour in older adults: study protocol for a randomised controlled trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-1956-x.
- NCT02629666 . Exercise Referral Schemes Enhanced by Self-Management Strategies to Battle Sedentary Behaviour (SitLESS) 2015. https://clinicaltrials.gov/ct2/show/NCT02629666 (accessed 14 May 2020).
- Tully MA, McMullan I, Blackburn NE, Wilson JJ, Bunting B, Smith L, et al. Sedentary behavior, physical activity, and mental health in older adults: an isotemporal substitution model. Scand J Med Sci Sports 2020;30:1957-65. https://doi.org/10.1111/sms.13762.
- Tully MA, McMullan I, Blackburn NE, Wilson JJ, Coll-Planas L, Deidda M, et al. Is sedentary behavior or physical activity associated with loneliness in older adults? Results of the European-wide SITLESS study. J Aging Phys Act 2020;28:549-55. https://doi.org/10.1123/japa.2019-0311.
- Wilson JJ, Blackburn NE, O’Reilly R, Kee F, Caserotti P, Tully MA. Association of objective sedentary behaviour and self-rated health in English older adults. BMC Res Notes 2019;12. https://doi.org/10.1186/s13104-019-4050-5.
- Wilson JJ, McMullan I, Blackburn NE, Skjødt M, Caserotti P, Giné-Garriga M, et al. Associations of sedentary behavior bouts with community-dwelling older adults’ physical function. Scand J Med Sci Sports 2021;31:153-62. https://doi.org/10.1111/sms.13827.
- Wilson JJ, Skjødt M, McMullan I, Blackburn NE, Giné-Garriga M, Sansano-Nadal O, et al. Consequences of choosing different settings when processing hip-based accelerometry data from older adults: a practical approach using baseline data from the SITLESS study. J Meas Phys Behav 2020;3:89-9. https://doi.org/10.1123/jmpb.2019-0037.
- Gitlin LN. Generations (San Francisco, Calif). 2010.
- Gitlin LN, Hauck WW, Dennis MP, Schulz R. Depressive symptoms in older African-American and white adults with functional difficulties: the role of control strategies. J Am Geriatr Soc 2007;55:1023-30. https://doi.org/10.1111/j.1532-5415.2007.01224.x.
- Gitlin LN, Hauck WW, Dennis MP, Winter L, Hodgson N, Schinfeld S. Long-term effect on mortality of a home intervention that reduces functional difficulties in older adults: results from a randomized trial. J Am Geriatr Soc 2009;57:476-81.
- Gitlin LN, Hauck WW, Winter L, Dennis MP, Schulz R. Effect of an in-home occupational and physical therapy intervention on reducing mortality in functionally vulnerable older people: preliminary findings. J Am Geriatr Soc 2006;54:950-5.
- Gitlin LN, Parisi J, Huang J, Winter L, Roth DL. Attachment to life: psychometric analyses of the valuation of life scale and differences among older adults. Gerontologist 2016;56:e21-31.
- Gitlin LN, Winter L, Dennis MP, Hauck WW. Variation in response to a home intervention to support daily function by age, race, sex, and education. J Gerontol A Biol Sci Med Sci 2008;63:745-50.
- Gitlin LN, Winter L, Stanley IH. Compensatory strategies: prevalence of use and relationship to physical function and well-being. J Appl Gerontol 2017;36:647-66. https://doi.org/10.1177/0733464815581479.
- Jutkowitz E, Gitlin L, Pizzi LT, Lee EH, Dennis M. Cost-effectiveness of able a functional program to decrease mortality in community-dwelling older adults. Value Health 2011;14.
- Jutkowitz E, Gitlin LN, Pizzi LT, Lee E, Dennis MP. Cost effectiveness of a home-based intervention that helps functionally vulnerable older adults age in place at home. J Aging Res 2012;2012.
- NCT00249925 . Project ABLE: Advancing Better Living for Elders 2005. https://clinicaltrials.gov/ct2/show/NCT00249925 (accessed 28 April 2020).
- Rose KC, Gitlin LN, Dennis MP. Readiness to use compensatory strategies among older adults with functional difficulties. Int Psychogeriatr 2010;22:1225-39.
- ACTRN12613000234718 . TRialing Individualised Interventions to Prevent FunctionaL Decline in At-Risk Older Adults (TRIIFL): A Nested Randomized Controlled Trial 2013. www.anzctr.org.au/ACTRN12613000234718.aspx (accessed 11 May 2020).
- Gustafson DH S, McTavish F, Gustafson DH, Mahoney JE, Johnson RA, Lee JD, et al. The effect of an information and communication technology (ICT) on older adults’ quality of life: study protocol for a randomized control trial. Trials 2015;16.
- NCT02128789 . Bring Communities and Technology Together for Healthy Aging (ElderTree) 2014. https://clinicaltrials.gov/ct2/show/NCT02128789 (accessed 8 June 2020).
- Behm L, Eklund K, Wilhelmson K, Ziden L, Gustafsson S, Falk K, et al. Health promotion can postpone frailty: results from the RCT elderly persons in the risk zone. Public Health Nurs 2016;33:303-15.
- Behm L, Wilhelmson K, Falk K, Eklund K, Ziden L, Dahlin-Ivanoff S. Positive health outcomes following health-promoting and disease-preventive interventions for independent very old persons: long-term results of the three-armed RCT elderly persons in the risk zone. Arch Gerontol Geriatr 2014;58:376-83.
- Dahlin-Ivanoff S, Eklund K, Wilhelmson K, Behm L, Häggblom-Kronlöf G, Zidén L, et al. For whom is a health-promoting intervention effective? Predictive factors for performing activities of daily living independently. BMC Geriatr 2016;17.
- Dahlin-Ivanoff S, Gosman-Hedstrom G, Edberg AK, Wilhelmson K, Eklund K, Duner A, et al. Elderly persons in the risk zone. Design of a multidimensional, health-promoting, randomised three-armed controlled trial for ‘prefrail’ people of 80+ years living at home. BMC Geriatr 2010;10.
- Gustafsson S, Wilhelmson K, Eklund K, Gosman-Hedstrom G, Ziden L, Kronlof GH, et al. Health-promoting interventions for persons aged 80 and older are successful in the short term-results from the randomized and three-armed elderly persons in the risk zone study. J Am Geriatr Soc 2012;60:447-54.
- NCT00877058 . Support for Frail Elderly Persons – From Prevention to Palliation 2008. https://ClinicalTrials.gov/show/NCT00877058 (accessed 11 May 2020).
- Wilhelmson K, Eklund K. Positive effects on life satisfaction following health-promoting interventions for frail older adults: a randomized controlled study. Health Psychol Res 2013;1.
- Ziden L, Haggblom-Kronlof G, Gustafsson S, Lundin-Olsson L, Dahlin-Ivanoff S. Physical function and fear of falling 2 years after the health-promoting randomized controlled trial: elderly persons in the risk zone. Gerontologist 2014;54:387-97.
- Biddulph JP, Iliffe S, Kharicha K, Harari D, Swift C, Gillmann G, et al. Risk factors for depressed mood amongst a community dwelling older age population in England: cross-sectional survey data from the PRO-AGE study. BMC Geriatr 2014;14. https://doi.org/10.1186/1471-2318-14-5.
- Carmaciu C, Iliffe S, Kharicha K, Harari D, Swift C, Gillmann G, et al. Health risk appraisal in older people 3: prevalence, impact, and context of pain and their implications for GPs. Br J Gen Pract 2007;57:630-5.
- Harari D, Iliffe S, Kharicha K, Stuck AE, Swift CG. Multi-domain health promotion for older people: randomised controlled study of health risk appraisal in primary. British Geriatrics Society: abstracts of papers presented at the Spring Scientific Meeting, 6–7 April 2006. Age Ageing 2006;35.
- Iliffe S, Kharicha K, Carmaciu C, Harari D, Swift C, Gillman G, et al. The relationship between pain intensity and severity and depression in older people: exploratory study. BMC Fam Pract 2009;10.
- Iliffe S, Kharicha K, Harari D, Swift C, Gillmann G, Stuck AE. Health risk appraisal in older people 2: the implications for clinicians and commissioners of social isolation risk in older people. Br J Gen Pract 2007;57:277-82.
- Iliffe S, Kharicha K, Harari D, Swift C, Stuck AE. Health risk appraisal for older people in general practice using an expert system: a pilot study. Health Soc Care Community 2005;13:21-9.
- Iliffe S, Swift C, Harari D, Kharicha K, Goodman C, Manthorpe J. Health promotion in later life: public and professional perspectives on an expert system for health risk appraisal. Prim Health Care Res Dev 2010;11:187-96. https://doi.org/10.1017/S1463423609990442.
- Kharicha K, Iliffe S, Harari D, Swift C, Gillmann G, Stuck AE. Health risk appraisal in older people 1: are older people living alone an ‘at-risk’ group?. Br J Gen Pract 2007;57:271-6.
- Kharicha K, Iliffe S, Harari D, Swift CG, Goodman C, Manthorpe J, et al. Feasibility of repeated use of the health risk appraisal for older people system as a health promotion tool in community-dwelling older people: retrospective cohort study 2001–05. Age Ageing 2011;41:128-31. https://doi.org/10.1093/ageing/afr126.
- Raymond M, Iliffe S, Kharicha K, Harari D, Swift C, Gillmann G, et al. Health risk appraisal for older people 5: self-efficacy in patient – doctor interactions. Prim Health Care Res Dev 2011;12:348-56.
- Tsakos G, Sheiham A, Iliffe S, Kharicha K, Harari D, Swift CG, et al. The impact of educational level on oral health-related quality of life in older people in London. Eur J Oral Sci 2009;117:286-92.
- Blozik E, Wagner JT, Gillmann G, Iliffe S, von Renteln-Kruse W, Lubben J, et al. Social network assessment in community-dwelling older persons: results from a study of three European populations. Aging Clin Exp Res 2009;21:150-7.
- Stuck AE, Elkuch P, Dapp U, Anders J, Iliffe S, Swift CG. Pro-age pilot study group . Feasibility and yield of a self‐administered questionnaire for health risk appraisal in older people in three European countries. Age Ageing 2002;31:463-7. https://doi.org/10.1093/ageing/31.6.463.
- Stuck AE, Kharicha K, Dapp U, Anders J, Von Renteln-Kruse W, Meier-Baumgartner H, et al. Development, feasibility and performance of a health risk appraisal questionnaire for older persons. BMC Med Res Methodol 2007;7. https://doi.org/10.1186/1471-2288-7-1.
- Stuck AE, Kharicha K, Dapp U, Anders J, Von Renteln-Kruse W, Meier-Baumgartner HP, et al. The PRO-AGE study: an international randomised controlled study of health risk appraisal for older persons based in general practice. BMC Med Res Methodol 2007;7. https://doi.org/10.1186/1471-2288-7-2.
- UMIN000031329 . Evaluation of Effectiveness of Preventive Rehabilitation Programs for the Frail Elderly by Physical Therapist 2018. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&recptno=R000035759&type=summary&language=E (accessed 4 May 2020).
- Hay WI, Browne G, Roberts J, Jamieson E. Prospective care of elderly patients in family practice. Part 3: prevalence of unrecognized treatable health concerns. Can Fam Physician 1995;41:1695-704, 1707.
- ACTRN12605000134628 . In-Home Preventive Health Assessment and Telephone Case Management for Over 75s Living Alone in Independent Living Units: A Cluster Randomised Controlled Trial 2005. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12605000134628 (accessed 11 May 2020).
- Hendriksen C. An intervention study among elderly people. Methodological and practical experiences. Scand J Prim Health Care 1986;4:39-42.
- Hendriksen C, Lund E, Strømgård E. Use of social and health services by elderly people during the terminal 18 months of life. Scand J Soc Med 1987;15:169-74. https://doi.org/10.1177/140349488701500308.
- Hendriksen C, Lund E, Stromgard E. Hospitalization of elderly people. A 3-year controlled trial. J Am Geriatr Soc 1989;37:117-22.
- NCT00238836 . Anticipatory &Amp; Preventive Team Care (APTCare): At Risk Patients of Family Health Networks 2005. https://clinicaltrials.gov/ct2/show/NCT00238836 (accessed 28 April 2020).
- Humbert J, Legault F, Dahrouge S, Halabisky B, Boyce G, Hogg W, et al. Integration of nurse practitioners into a family health network. Can Nurse 2007;103:30-4.
- Gray D, Armstrong CD, Dahrouge S, Hogg W, Zhang W. Cost-effectiveness of Anticipatory and Preventive multidisciplinary Team Care for complex patients: evidence from a randomized controlled trial. Can Fam Physician 2010;56:e20-9.
- Fletcher J, Hogg W, Farrell B, Woodend K, Dahrouge S, Lemelin J, et al. Effect of nurse practitioner and pharmacist counseling on inappropriate medication use in family practice. Can Fam Physician 2012;58:862-8.
- Dahrouge S, Hogg W, Lemelin J, Liddy C, Legault F. Methods for a study of Anticipatory and Preventive multidisciplinary Team Care in a family practice. Can Fam Physician 2010;56:e73-83.
- Holland SK, Greenberg J, Tidwell L, Newcomer R. Preventing disability through community-based health coaching. J Am Geriatr Soc 2003;51:265-9.
- Tidwell L, Holland SK, Greenberg J, Malone J, Mullan J, Newcomer R. Community-based nurse health coaching and its effect on fitness participation. Lippincotts Case Manag 2004;9:267-79. https://doi.org/10.1097/00129234-200411000-00006.
- Haighton C, Moffatt S, Howel D, McColl E, Milne E, Deverill M, et al. The Do-Well study: protocol for a randomised controlled trial, economic and qualitative process evaluations of domiciliary welfare rights advice for socio-economically disadvantaged older people recruited via primary health care. BMC Public Health 2012;12.
- Haighton C, Moffatt S, Howel D, Steer M, Becker F, Bryant A, et al. Randomised controlled trial with economic and process evaluations of domiciliary welfare rights advice for socioeconomically disadvantaged older people recruited via primary health care (the Do-Well study). Public Health Res 2019;7. https://doi.org/10.3310/phr07030.
- ISRCTN37380518 . Advice on Welfare Rights for Disadvantaged Older People Recruited Via Primary Health Care 2012. http://isrctn.com/ISRCTN37380518 (accessed 11 May 2020).
- Lin SY, Kerse N, McLean C, Moyes SA. Validation of quality of life and functional measures for older people for telephone administration. J Prim Health Care 2010;2:35-42.
- Wilkinson-Meyers L, Brown P, McLean C, Kerse N. Met and unmet need for personal assistance among community-dwelling New Zealanders 75 years and over. Health Soc Care Community 2014;22:317-27.
- Wham CA, McLean C, Teh R, Moyes S, Peri K, Kerse N. The BRIGHT trial: what are the factors associated with nutrition risk?. J Nutr Health Aging 2014;18:692-7.
- Schäfers A, Martini N, Moyes S, Hayman K, Zolezzi M, McLean C, et al. Medication use in community-dwelling older people: pharmacoepidemiology of psychotropic utilisation. J Prim Health Care 2014;6:269-78.
- McLean C, Kerse N, Moyes SA, Ng T, Lin SY, Peri K. Recruiting older people for research through general practice: the brief risk identification geriatric health tool trial. Australas J Ageing 2014;33:257-63.
- ACTRN12606000256572 . A Cluster Randomised Control Trial to Compare the Effects of a Restorative Home Based Model Versus Usual Care to Improve Wellbeing for Community Dwelling Older People 2006. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=1417 (accessed 21 August 2020).
- King AII. Creating Sustainable Home Care Services for Older People 2010.
- King AII, Parsons M, Robinson E. A restorative home care intervention in New Zealand: Perceptions of paid caregivers. Health Soc Care Community 2012;20:70-9.
- JPRN-UMIN000006463 . Development and Effects of Preventive Home Visit Program Among Ambulatory Frail Older People Living at Home 2011. https://trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000006463 (accessed 11 May 2020).
- Kono A, Izumi K, Kanaya Y, Tsumura C, Rubenstein LZ. Assessing the quality and effectiveness of an updated preventive home visit programme for ambulatory frail older Japanese people: research protocol for a randomized controlled trial. J Adv Nurs 2014;70:2363-72.
- JPRN-UMIN000001113 . Effects of Preventive Home Visits for Ambulatory Frail Elders: A Randomized Clinical Trial in Osaka, Japan 2008. https://trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000001113 (accessed 11 May 2020).
- Kono A, Fujita T, Tsumura C, Kondo T, Kushiyama K, Rubenstein LZ. Preventive home visit model targeted to specific care needs of ambulatory frail elders: preliminary report of a randomized trial design. Aging Clin Exp Res 2009;21:167-73.
- Kono A, Kanaya Y, Tsumura C, Rubenstein LZ. Effects of preventive home visits on health care costs for ambulatory frail elders: a randomized controlled trial. Aging Clin Exp Res 2013;25:575-81.
- Kukkonen-Harjula K, Karmeniemi P, Suikkanen S, Sipil S, Pitkala K, Hupli M. Long-term home-based physiotherapy for older people with signs of frailty or consequent to a hip fracture operation – design of RCT (NCT02305433). Eur Geriatr Med 2016;7.
- NCT02305433 . Effects of Long-Term Intensive Home-Based Physiotherapy on Older People With an Operated Hip Fracture or Frailty (RCT) 2014. https://clinicaltrials.gov/show/NCT02305433 (accessed 11 May 2020).
- Soukkio P, Suikkanen S, Kaaria S, Kautiainen H, Sipila S, Kukkonen-Harjula K, et al. Effects of 12-month home-based physiotherapy on duration of living at home and functional capacity among older persons with signs of frailty or with a recent hip fracture – protocol of a randomized controlled trial (HIPFRA study). BMC Geriatr 2018;18.
- Suikkanen S, Soukkio P, Pitkala K, Kaaria S, Kautiainen H, Sipila S, et al. Older persons with signs of frailty in a home-based physical exercise intervention: baseline characteristics of an RCT. Aging Clin Exp Res 2019;31:1419-27.
- Suikkanen SA, Soukkio PK, Aartolahti EM, Kautiainen H, Kaaria SM, Hupli MT, et al. Effects of home-based physical exercise on days at home and cost-effectiveness in pre-frail and frail persons: randomized controlled trial. J Am Med Dir Assoc 2020;18. https://doi.org/10.1016/j.jamda.2020.06.005.
- Domènech-Abella J, Switsers L, Mundó J, Dierckx E, Dury S, De Donder L. The association between perceived social and physical environment and mental health among older adults: mediating effects of loneliness. Aging Ment Health 2021;25:962-8. https://doi.org/10.1080/13607863.2020.1727853.
- Duppen D, Rossi G, Dierckx E, Hoeyberghs L, De Donder L. D-SCOPE Consortium . Focusing on positive outcomes in frailty research: development of a short well-being instrument for older adults (SWIO). Int Psychogeriatr 2019;31:767-77. https://doi.org/10.1017/S1041610219000401.
- Dury S, Dierckx E, van der Vorst A, Van der Elst M, Fret B, Duppen D, et al. Detecting frail, older adults and identifying their strengths: results of a mixed-methods study. BMC Public Health 2018;18. https://doi.org/10.1186/s12889-018-5088-3.
- NCT03168204 . Evaluating the Efficacy of a Detection and Prevention Program for Frail Community-Dwelling Older Adults (D-SCOPE) 2017. https://clinicaltrials.gov/ct2/show/NCT03168204 (accessed 21 August 2020).
- Smetcoren A-S, Dury S, den Donder L, Dierckx E. D-SCOPE: towards a positive view on prevention in frail elderly [D-SCOPE: naar een positieve kijk op preventie bij kwetsbare ouderen]. Geron 2017;19:35-8. https://doi.org/10.1007/s40718-017-0010-0.
- Switsers L, Dierckx E, Domènech-Abella J, De Donder L, Dury S. Negative old-age life events and well-being in later life: the moderating and mediating role of loneliness. Int Psychogeriatr 2021;33:1265-76. https://doi.org/10.1017/S1041610220004196.
- Van der Elst MCJ, Schoenmakers B, Op het Veld LPM, De Roeck EE, Van der Vorst A, Kempen GIJM, et al. D-SCOPE Consortium . Concordances and differences between a unidimensional and multidimensional assessment of frailty: a cross-sectional study. BMC Geriatr 2019;19. https://doi.org/10.1186/s12877-019-1369-7.
- van der Vorst A, Zijlstra GAR, De Witte N, Vogel RGM, Schols JMGA, Kempen GIJM. D-SCOPE Consortium . Explaining discrepancies in self-reported quality of life in frail older people: a mixed-methods study. BMC Geriatr 2017;17. https://doi.org/10.1186/s12877-017-0641-y.
- Leung AC, Liu CP, Tsui LL, Li SY, Tang GW, Yau DC, et al. The use of the Minimum Data Set. Home Care in a case management project in Hong Kong. Care Manag J 2001;3:8-13.
- Phelan EA, Williams B, Penninx BWJH, LoGerfo JP, Leveille SG. Activities of daily living function and disability in older adults in a randomized trial of the Health Enhancement Program. J Gerontol A Biol Sci Med Sci 2004;59:838-43.
- Phelan EA, Cheadle A, Schwartz SJ, Snyder S, Williams B, Wagner EH, et al. Promoting health and preventing disability in older adults: lessons from intervention studies carried out through an academic-community partnership. Fam Community Health 2003;26:214-20.
- Lewin G, Allan J, Patterson C, Knuiman M, Boldy D, Hendrie D. A comparison of the home-care and healthcare service use and costs of older Australians randomised to receive a restorative or a conventional home-care service. Health Soc Care Community 2014;22:328-36.
- Lewin G, Concanen K, Youens D. The home independence program with non-health professionals as care managers: an evaluation. Clin Interv Aging 2016;11:807-17.
- Lewin G, Vandermeulen S. A non-randomised controlled trial of the Home Independence Program (HIP): an Australian restorative programme for older home-care clients. Health Soc Care Community 2010;18:91-9.
- ACTRN12616001411437 . Evaluation of Preventive Home Visits in Elderly Over 75 Years 2016. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616001411437 (accessed 11 May 2020).
- Liimatta H, Lampela P, Kautiainen H, Laitinen-Parkkonen P, Pitkala KH. The effects of preventive home visits on older people’s use of health care and social services and related costs. J Gerontol A Biol Sci Med Sci 2019;29. https://doi.org/10.1093/gerona/glz139.
- Liimatta H, Lampela P, Laitinen-Parkkonen P, Pitkala KH. Preventive home visits to promote the health-related quality of life of home-dwelling older people: baseline findings and feasibility of a randomized, controlled trial. Eur Geriatr Med 2017;8:440-5.
- ISRCTN22749696 . CERgAS: Multicomponent Exercise and TheRApeutic Lifestyle Intervention to Improve Physical Function and Maintain Independent Living Among Urban Poor Older People 2014. www.isrctn.com/ISRCTN22749696 (accessed 5 November 2020).
- Rosli R, Loh D, Choo W, MohdHairi F, Peramalah D, Kandiben S, et al. Effects of multicomponent exercise and therapeutic lifestyle (CERgAS) intervention on cognitive function in lower income elderly population: a cluster randomised controlled trial. Age Ageing 2017;46. https://doi.org/10.1093/ageing/afx118.26.
- ACTRN12617000198325 . Efficacy and Cost-Effectiveness of a Community Based Model of Care for Older Patients With Complex Needs: A Study Protocol for a Multicentre Randomized Controlled Trial Using a Stepped Wedge Cluster Design 2017. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372235 (accessed 11 May 2020).
- Kinchin I, Jacups S, Mann J, Quigley R, Harvey D, Doran CM, et al. Efficacy and cost-effectiveness of a community-based model of care for older patients with complex needs: a study protocol for a multicentre randomised controlled trial using a stepped wedge cluster design. Trials 2018;19.
- Mann J, Quigley R, Harvey D, Tait M, Williams G, Strivens E. OPEN ARCH: integrated care at the primary–secondary interface for the community-dwelling older person with complex needs. Aust J Prim Health 2020;26:104-8. https://doi.org/10.1071/PY19184.
- Mann J, Thompson F, Quigley R, McDermott R, Devine S, Strivens E. Beyond multimorbidity: primary care and the older person with complex needs. Aust J Prim Health 2021;27:194-201. https://doi.org/10.1071/PY20125.
- Quigley R, Russell S, Harvey D, Mann J. OPEN ARCH integrated care model: experiences of older Australians and their carers. Aust J Prim Health 2021;27:236-42. https://doi.org/10.1071/py20203.
- Markle-Reid M. Frail Elderly Home Care Clients: The Effects and Expense of Adding Nursing Health Promotion and Preventative Care to Personal Support Services. PhD Thesis 2002.
- Markle-Reid M, Weir R, Browne G, Henderson S, Roberts J, Gafni A. Frail Elderly Homecare Clients: The Costs and Effects of Adding Nursing Health Promotion and Preventive Care to Personal Support Services. Ottawa, ON: Canadian Health Services Research Foundation; 2003.
- Houles M. Cost-effectiveness analysis of a multidisciplinary intervention for vulnerable elderly individuals living at home. [French]. Cah De l’Annee Gerontol 2010;2:169-72.
- Houles M. Randomised study of a multi-disciplinary programme focusing on geriatric syndromes in vulnerable, elderly individuals living at home (Dutch EASYcare Study). [French]. Cah De l’Annee Gerontol 2010;2:166-8.
- Melis RJ, Adang E, Teerenstra S, van Eijken MI, Wimo A, van Achterberg T, et al. Cost-effectiveness of a multidisciplinary intervention model for community-dwelling frail older people. J Gerontol A Biol Sci Med Sci 2008;63:275-82.
- Melis RJ, Teerenstra S, Rikkert MG, Borm GF. Pseudo cluster randomization performed well when used in practice. J Clin Epidemiol 2008;61:1169-75.
- Melis RJ, van Eijken MI, Boon ME, Olde Rikkert MG, van Achterberg T. Process evaluation of a trial evaluating a multidisciplinary nurse-led home visiting programme for vulnerable older people. Disabil Rehabil 2010;32:937-46.
- Melis RJ, van Eijken MI, Borm GF, Wensing M, Adang E, van de Lisdonk EH, et al. The design of the Dutch EASYcare study: a randomised controlled trial on the effectiveness of a problem-based community intervention model for frail elderly people [NCT00105378]. BMC Health Serv Res 2005;5.
- NCT00105378 . Dutch EASYcare Study 2005. https://clinicaltrials.gov/ct2/show/NCT00105378 (accessed 28 April 2020).
- Friedman B, Li Y, Liebel DV, Powers BA. Effects of a home visiting nurse intervention versus care as usual on individual activities of daily living: a secondary analysis of a randomized controlled trial. BMC Geriatr 2014;14.
- Friedman B, Wamsley BR, Liebel DV, Saad ZB, Eggert GM. Patient satisfaction, empowerment, and health and disability status effects of a disease management–health promotion nurse intervention among Medicare beneficiaries with disabilities. Gerontol 2009;49:778-92.
- Li Y, Liebel DV, Friedman B. An investigation into which individual instrumental activities of daily living are affected by a home visiting nurse intervention. Age Ageing 2013;42:27-33.
- Liebel DV. Process evaluation of a nurse home visiting intervention that postpones disability worsening in older adults. Diss Abst Int Pt B – Sci &Amp; Eng 2008;68.
- Liebel DV, Powers BA, Friedman B, Watson NM. Barriers and facilitators to optimize function and prevent disability worsening: a content analysis of a nurse home visit intervention. J Adv Nurs 2012;68:80-93.
- Meng H, Friedman B, Dick AW, Liebel D, Wamsley BR, Eggert GM, et al. Impact of a disease management-health promotion nurse intervention on personal assistance use and expenditures. Home Health Care Serv Q 2009;28:113-29.
- Meng H, Friedman B, Dick AW, Wamsley BR, Eggert GM, Mukamel D. Effect of a voucher benefit on the demand for paid personal assistance. Gerontologist 2006;46:183-92.
- Keijser W, Bond R, Saez I, Vandewoude M. Health monitoring and sOcial integration environMent for Supporting WidE ExTension of independent life at HOME (Home Sweet Home): Trial Protocol Version 1.0 No. D1.2. Antwerp: Zorgbedrijf Antwerpen; 2010.
- NCT01218373 . The Clinical Evaluation of Continuous Assistance Offered to Older People Living Independently 2010. https://ClinicalTrials.gov/show/NCT01218373 (accessed 11 May 2020).
- Daniels R, van Rossum E, Metzelthin S, Sipers W, Habets H, Hobma S, et al. A disability prevention programme for community-dwelling frail older persons. Clin Rehabil 2011;25:963-74.
- ISRCTN31954692 . The Reduction of Disability in Community-Dwelling Frail Elderly 2009. http://isrctn.com/ISRCTN31954692 (accessed 11 May 2020).
- Metzelthin SF, Daniëls R, van Rossum E, Cox K, Habets H, de Witte LP, et al. A nurse-led interdisciplinary primary care approach to prevent disability among community-dwelling frail older people: a large-scale process evaluation. Int J Nurs Stud 2013;50:1184-96. https://doi.org/10.1016/j.ijnurstu.2012.12.016.
- Metzelthin SF, Van Rossum E, De Witte LP, Ambergen A, Hobma S, Sipers W, et al. Frail elderly people living at home; effects of an interdisciplinary primary care programme. [Dutch]. Ned Tijdschr Geneeskd 2014;158.
- Metzelthin SF, van Rossum E, de Witte LP, Hendriks MR, Kempen GI, Metzelthin SF, et al. The reduction of disability in community-dwelling frail older people: design of a two-arm cluster randomized controlled trial. BMC Public Health 2010;10.
- Metzelthin SF, van Rossum E, Hendriks MR, De Witte LP, Hobma SO, Sipers W, et al. Reducing disability in community-dwelling frail older people: cost-effectiveness study alongside a cluster randomised controlled trial. Age Ageing 2015;44:390-6.
- Beishuizen CRL, Coley N, Moll van Charante EP, van Gool WA, Richard E, Andrieu S. Determinants of dropout and nonadherence in a dementia prevention randomized controlled trial: the prevention of dementia by intensive vascular care trial. J Am Geriatr Soc 2017;65:1505-13.
- Bussel EF, Richard E, Busschers WB, Steyerberg EW, Gool WA, Moll van Charante EP, et al. A cardiovascular risk prediction model for older people: Development and validation in a primary care population. J Clin Hypertens 2019;21:1145-52.
- ISRCTN29711771 . Prevention of Dementia by Intensive Vascular Care 2006. www.isrctn.com/ISRCTN29711771 (accessed 28 April 2020).
- Ligthart SA, Richard E, van Gool WA, Moll van Charante EP. Cardiovascular risk management in community-dwelling elderly: opportunities for prevention. European Journal of Preventive Cardiology 2012;19:1365-72.
- Ligthart SA, van den Eerenbeemt KD, Pols J, van Bussel EF, Richard E, Moll van Charante EP. Perspectives of older people engaging in nurse-led cardiovascular prevention programmes: a qualitative study in primary care in the Netherlands. Br J Gen Pract 2015;65:e41-8.
- Richard E, Ligthart S, van Charante EM, Van Gool W. Methodological issues in a cluster-randomized trial to prevent dementia by intensive vascular care. J Nutr Health Aging 2010;14:315-7.
- Richard E, Van den Heuvel E, van Charante EPM, Achthoven L, Vermeulen M, Bindels PJ, et al. Prevention of dementia by intensive vascular care (PreDIVA): a cluster-randomized trial in progress. Alzheimer Dis Assoc Disord 2009;23:198-204.
- van Bussel EF, Hoevenaar-Blom MP, Busschers WB, Richard E, Peters RJG, van Gool WA, et al. Effects of primary cardiovascular prevention on vascular risk in older adults. Am J Prev Med 2018;55:368-75.
- van Dalen JW, Moll van Charante EP, Caan MWA, Scheltens P, Majoie C, Nederveen AJ, et al. Effect of long-term vascular care on progression of cerebrovascular lesions: magnetic resonance imaging substudy of the PreDIVA trial (Prevention of Dementia by Intensive Vascular Care). Stroke 2017;48:1842-8.
- van Dalen JW, Moll van Charante EP, van Gool WA, Richard E. Discontinuation of antihypertensive medication, cognitive complaints, and incident dementia. J Am Med Dir Assoc 2019;20:1091-1097.e3. https://doi.org/10.1016/j.jamda.2018.12.006.
- van Dalen JW, Van Wanrooij LL, van Charante EPM, Richard E, van Gool WA, Moll van Charante EP. Apathy is associated with incident dementia in community-dwelling older people. Neurology 2018;90:e82-e9.
- Monteserin R, Brotons C, Moral I, Altimir S, San Jose A, Santaeugenia S, et al. Effectiveness of a geriatric intervention in primary care: a randomized clinical trial. Fam Pract 2010;27:239-45.
- Morey MC, Sloane R, Pieper CF, Peterson MJ, Pearson MP, Ekelund CC, et al. Effect of physical activity guidelines on physical function in older adults. J Am Geriatr Soc 2008;56:1873-8. https://doi.org/10.1111/j.1532-5415.2008.01937.x.
- Peterson MJ, Sloane R, Cohen HJ, Crowley GM, Pieper CF, Morey MC. Effect of telephone exercise counseling on frailty in older veterans: project LIFE. Am J Mens Health 2007;1:326-34.
- Hall KS, Sloane R, Pieper CF, Peterson MJ, Crowley GM, Cowper PA, et al. Long-term changes in physical activity following a one-year home-based physical activity counseling program in older adults with multiple morbidities. J Aging Res 2011;2011. https://doi.org/10.4061/2011/308407.
- Huffman KM, Sloane R, Peterson MJ, Bosworth HB, Ekelund C, Pearson M, et al. The impact of self-reported arthritis and diabetes on response to a home-based physical activity counselling intervention. Scand J Rheumatol 2010;39:233-9.
- Morey MC, Peterson MJ, Pieper CF, Sloane R, Crowley GM, Cowper P, et al. Project LIFE – learning to improve fitness and function in elders: methods, design, and baseline characteristics of randomized trial. J Rehabil Res Dev 2008;45:31-42.
- NCT00435188 . Life 2: Improving Fitness and Function in Elders (Project LIFE) 2007. https://clinicaltrials.gov/ct2/show/NCT00435188 (accessed 20 June 2020).
- Haase AM, Taylor AH, Fox KR, Thorp H, Lewis G. Rationale and development of the physical activity counselling intervention for a pragmatic TRial of exercise and depression in the UK (TREAD-UK). Ment Health Phys Act 2010;3:85-91. https://doi.org/10.1016/j.mhpa.2010.09.004.
- ISRCTN80470273 . The PACE Study: Physical Activity Facilitation for Older Adults 2013. www.isrctn.com/ISRCTN80470273 (accessed 20 June 2020).
- Morgan GS, Haase AM, Campbell R, Ben-Shlomo Y. Physical ACtivity facilitation for Elders (PACE): study protocol for a randomised controlled trial. Trials 2015;16.
- Newbury JW. 75+ Health Assessments: A Randomised Controlled Trial 2001.
- Graves MT, Slater MA, Maravilla V, Reissler L, Faculjak P, Newcomer RJ. Implementing an early intervention case management program in three medical groups. Case Manag 2003;14:48-52. https://doi.org/10.1016/S1061-9259(03)00212-1.
- Maravilla V, Graves MT, Newcomer R. Development of a standardized language for case management among high-risk elderly. Lippincott’s Case Manag 2005;10:3-13. https://doi.org/10.1097/00129234-200501000-00002.
- NCT00973258 . Randomized Controlled Trial of Community-Based Nutritional, Physical and Cognitive Training Intervention Programmes for At Risk Frail Elderly (FIT) 2009. https://clinicaltrials.gov/ct2/show/NCT00973258 (accessed 28 April 2020).
- Ng TP, Ling LHA, Feng L, Nyunt MSZ, Feng L, Niti M, et al. Cognitive effects of multi-domain interventions among pre-frail and frail community-living older persons: randomized controlled trial. J Gerontol A Biol Sci Med Sci 2018;73:806-12.
- ACTRN12608000027314 . Examination of the Effect of a Designated Client Centred Goal Facilitation Tool on Service Provision, Quality of Life and Independence Among Older People 2008. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82511&isReview=true (accessed 8 June 2020).
- Parsons JG, Sheridan N, Rouse P, Robinson E, Connolly M. A randomized controlled trial to determine the effect of a model of restorative home care on physical function and social support among older people. Arch Phys Med Rehabil 2013;94:1015-22.
- Parsons M, Senior HE, Kerse N, Chen MH, Jacobs S, Vanderhoorn S, et al. The Assessment of Services Promoting Independence and Recovery in Elders Trial (ASPIRE): a pre-planned meta-analysis of three independent randomised controlled trial evaluations of ageing in place initiatives in New Zealand. Age Ageing 2012;41:722-8.
- Parsons M, Anderson C, Senior H, Chen X, Kerse N, Jorgensen D, et al. ASPIRE: Assessment of Services Promoting Independence and Recovery in Elders. New Zealand Ministry of Health; 2006.
- Auckland UniServices Limited . An Economic Evaluation of the Assessment of Service Promoting Independence and Recovery in Elders (ASPIRE) – Final Report. 2006. www.health.govt.nz/system/files/documents/pages/aspire-economic-evaluation-report.pdf (accessed 29 May 2020).
- ACTRN12605000140651 . Assessment of Services Promoting Independence and Recovery in Elders 2005. www.anzctr.org.au/ACTRN12605000140651.aspx (accessed 11 May 2020).
- NCT00134836 . Preventive Primary Care Outreach for High Risk Older Persons 2004. https://ClinicalTrials.gov/show/NCT00134836 (accessed 11 May 2020).
- Dapp U, Anders J, von Renteln-Kruse W, Golgert S, Meier-Baumgartner HP, Minder CE. The Longitudinal Urban Cohort Ageing Study (LUCAS): study protocol and participation in the first decade. BMC Geriatr 2012;12. https://doi.org/10.1186/1471-2318-12-35.
- Dapp U, Anders JA, von Renteln-Kruse W, Minder CE, Meier-Baumgartner HP, Swift CG, et al. PRO-AGE Study Group . A randomized trial of effects of health risk appraisal combined with group sessions or home visits on preventive behaviors in older adults. J Gerontol A Biol Sci Med Sci 2011;66:591-8. https://doi.org/10.1093/gerona/glr021.
- von Renteln-Kruse W, Anders J, Dapp U, Meier-Baumgartner HP. Präventive Hausbesuche durch einespeziell fortgebildete Pflegefachkraft bei 60-jährigen undälteren Personen in Hamburg. Z Gerontol Geriatr 2003;36:378-91. https://doi.org/10.1007/s00391-003-0179-9.
- Rockwood K, Howlett S, Stadnyk K, Carver D, Powell C, Stolee P. Responsiveness of goal attainment scaling in a randomized controlled trial of comprehensive geriatric assessment. J Clin Epidemiol 2003;56:736-43.
- NCT01969526 . Effectiveness of a Multifactorial Intervention on Frailty 2013. https://clinicaltrials.gov/ct2/show/NCT01969526 (accessed 27 August 2021).
- Romera L, Orfila F, Segura JM, Ramirez A, Moller M, Fabra ML, et al. Effectiveness of a primary care based multifactorial intervention to improve frailty parameters in the elderly: a randomised clinical trial: rationale and study design. BMC Geriatr 2014;14.
- Metzelthin SF, Rooijackers TH, Zijlstra GAR, van Rossum E, Veenstra MY, Koster A, et al. Effects, costs and feasibility of the ‘Stay Active at Home’ Reablement training programme for home care professionals: study protocol of a cluster randomised controlled trial. BMC Geriatr 2018;18.
- Metzelthin SF, Zijlstra GA, van Rossum E, de Man-van Ginkel JM, Resnick B, Lewin G, et al. ‘Doing with. ’. rather than ‘doing for. ’. older adults: rationale and content of the ‘Stay Active at Home’ programme. Clin Rehabil 2017;31:1419-30.
- NCT03293303 . The Feasibility, Effects and Costs of the ‘Stay Active at Home Programme’ 2017. https://clinicaltrials.gov/ct2/show/NCT03293303 (accessed 28 April 2020).
- Rooijackers TH, Zijlstra GAR, van Rossum E, Vogel RGM, Veenstra MY, Kempen GIJM, et al. Process evaluation of a reablement training program for homecare staff to encourage independence in community-dwelling older adults. BMC Geriatr 2021;21. https://doi.org/10.1186/s12877-020-01936-7.
- Smeets RGM, Kempen GIJM, Zijlstra GAR, van Rossum E, de Man-van Ginkel JM, Hanssen WAG, et al. Experiences of home-care workers with the ‘Stay Active at Home’ programme targeting reablement of community-living older adults: an exploratory study. Health Soc Care Community 2020;28:291-9. https://doi.org/10.1111/hsc.12863.
- Ryvicker M, Marren J, Sobolewski S, Acampora T, Flannery M, Buff E, et al. Spreading improvement strategies within a large home healthcare organization. J Healthc Qual 2008;30:48-5.
- Serra-Prat M. Clinical and Economic Assessment of a Pre-frail Screening Program. NCT02138968. US National Institutes of Health ClinicalTrials.gov; 2014.
- Sherman H. Preventive Home Visits for 75 Years Old Persons by the District Nurse 2012.
- Fleuren MAH, Vrijkotte S, Jans MP, Pin R, van Hespen A, van Meeteren NLU, et al. The implementation of the functional task exercise programme for elderly people living at home. BMC Musculoskelet Disord 2012;13:1-9.
- NTR2407 . Training of the Ability to Live Independently 75+ 2010. www.trialregister.nl/trial/2280 (accessed 8 May 2020).
- Stewart S, Lloyd-Smith W, Poland F. Running a community clinical trial: lessons from the CAMELOT project. Br J Ther Rehabil 2003;10:443-8. https://doi.org/10.12968/bjtr.2003.10.10.13475.
- Flood C, Mugford M, Stewart S, Harvey I, Poland F, Lloyd-Smith W. Occupational therapy compared with social work assessment for older people. An economic evaluation alongside the CAMELOT randomised controlled trial. Age Ageing 2005;34:47-52.
- Alessi CA, Stuck AE, Aronow HU, Yuhas KE, Bula CJ, Madison R, et al. The process of care in preventive in-home comprehensive geriatric assessment. J Am Geriatr Soc 1997;45:1044-50.
- Büla CJ, Alessi CA, Aronow HU, Yubas K, Gold M, Nisenbaum R, et al. Community physicians’ cooperation with a program of in-home comprehensive geriatric assessment. J Am Geriatr Soc 1995;43:1016-20.
- Büla CJ, Bérod AC, Stuck AE, Alessi CA, Aronow HU, Santos-Eggimann B, et al. Effectiveness of preventive in-home geriatric assessment in well functioning, community-dwelling older people: secondary analysis of a randomized trial. J Am Geriatr Soc 1999;47:389-95.
- Cho CY, Alessi CA, Cho M, Aronow HU, Stuck AE, Rubenstein LZ, et al. The association between chronic illness and functional change among participants in a comprehensive geriatric assessment program. J Am Geriatr Soc 1998;46:677-82.
- Rubenstein LZ, Aronow HU, Schloe M, Steiner A, Alessi CA, Yuhas KE, et al. A home-based geriatric assessment, follow-up and health promotion program: design, methods, and baseline findings from a 3-year randomized clinical trial. Aging Clin Exp Res 1994;6:105-20.
- Stuck AE, Gafner Zwahlen H, Neuenschwander BE, Meyer Schweizer RA, Bauen G, Beck JC. Erratum for: Methodologic challenges of randomized controlled studies on in-home comprehensive geriatric assessment: the EIGER project. Aging Clin Exp Res 1995;7:218–23. Aging Clin Exp Res 1995;7.
- Stuck AE, Gafner Zwahlen H, Neuenschwander BE, Meyer Schweizer RA, Bauen G, Beck JC. Erratum for: Methodologic challenges of randomized controlled studies on in-home comprehensive geriatric assessment: the EIGER project. Aging Clin Exp Res 1995;7:218–23. Aging Clin Exp Res 1998;10.
- Stuck AE, Stuckelberger A, Gafner Zwahlen H, Minder CE, Beck JC. A randomised trial of in-home preventive visits with annual comprehensive geriatric assessment: base-line findings of the EIGER project. [French]. Med Hyg (Geneve) 1995;53:2385-97.
- Stuck AE, Gafner Zwahlen H, Neuenschwander BE, Meyer Schweizer RA, Bauen G, Beck JC. Methodologic challenges of randomized controlled studies on in-home comprehensive geriatric assessment: the EIGER project. Aging Clin Exp Res 1995;7:218-23.
- ISRCTN28458424 . Disability Prevention in the Older Population: Use of Information Technology for Health Risk Appraisal and Prevention of Functional Decline 2005. www.isrctn.com/ISRCTN28458424 (accessed 20 May 2020).
- NL2535 . FIT-Study: Maintaining Functionality in Transition 2010. www.trialregister.nl/trial/2535 (accessed 28 April 2020).
- Suijker J, MacNeil-Vroomen J, Van Rijn M, Buurman B, Ter Riet G, De Rooij S, et al. Economic evaluation of nurse-led multifactorial care to prevent or postpone new disabilities in community-living older people: results of a cluster randomized trial. Eur Geriatr Med 2016;7.
- Suijker JJ, Buurman BM, ter Riet G, van Rijn M, de Haan RJ, de Rooij SE, et al. Comprehensive geriatric assessment, multifactorial interventions and nurse-led care coordination to prevent functional decline in community-dwelling older persons: protocol of a cluster randomized trial. BMC Health Serv Res 2012;12.
- Suijker JJ, MacNeil-Vroomen JL, van Rijn M, Buurman BM, de Rooij SE, Moll van Charante EP, et al. Cost-effectiveness of nurse-led multifactorial care to prevent or postpone new disabilities in community-living older people: results of a cluster randomized trial. PLOS ONE 2017;12.
- Suijker JJ, van Rijn M, Ter Riet G, Moll van Charante EP, de Rooij SE, Buurman BM. Minimal important change and minimal detectable change in activities of daily living in community-living older people. J Nutr Health Aging 2017;21:165-72.
- Van Rijn M, Hoogteijling N, Suijker J, De Rooij S, Buurman B, Van Charante EM. Preventive home-visits and nurse-led care coordination: a qualitative study on the experiences, needs and preferences of community dwelling older people. Eur Geriatr Med 2016;7:S103-S4.
- Szanton SL, Leff B, Li Q, Breysse J, Spoelstra S, Kell J, et al. CAPABLE program improves disability in multiple randomized trials. J Am Geriatr Soc 2021;69:3631-40. https://doi.org/10.1111/jgs.17383.
- Szanton SL, Tanner EK, Thorpe RJ, Agree E, Seplaki C, Boyd C, et al. A multi-component pilot to enhance aging-in-place capacity for low-income older adults. Clin Transl Sci 2010;3.
- LaFave SE, Granbom M, Cudjoe TKM, Gottsch A, Shorb G, Szanton SL. Attention control group activities and perceived benefit in a trial of a behavioral intervention for older adults. Res Nurs Health 2019;42:476-82.
- NCT01576133 . Reducing Disability Via a Bundled Bio-Behavioral-Environmental Approach (CAPABLE) 2012. https://clinicaltrials.gov/ct2/show/study/NCT01576133 (accessed 28 April 2020).
- NCT01743495 . CAPABLE for Frail Dually Eligible Older Adults (CAPABLE500) 2012. https://clinicaltrials.gov/ct2/show/NCT01743495 (accessed 28 April 2020).
- Nkimbeng M, Roberts L, Thorpe Jr RJ, Gitlin LN, Delaney A, Tanner EK, et al. Recruiting older adults with functional difficulties into a community-based research study: approaches and costs. J Appl Gerontol 2020;39:644-50. https://doi.org/10.1177/0733464818786612.
- Patadia P, Roberts L, Szanton S. Are difficulties with daily activities related to neighborhood? A geospatial analysis of CAPABLE baseline data. J Am Geriatr Soc 2018;66.
- Smith PD, Becker K, Roberts L, Walker J, Szanton SL. Associations among pain, depression, and functional limitation in low-income, home-dwelling older adults: an analysis of baseline data from CAPABLE. Geriatr Nurs 2016;37:348-52.
- Szanton SL, Alfonso YN, Leff B, Guralnik J, Wolff JL, Stockwell I, et al. Medicaid cost savings of a preventive home visit program for disabled older adults. J Am Geriatr Soc 2018;66:614-20.
- Szanton SL, Wolff JL, Leff B, Roberts L, Thorpe RJ, Tanner EK, et al. Preliminary data from Community Aging in Place, Advancing Better Living for Elders, a patient‐directed, team‐based intervention to improve physical function and decrease nursing home utilization: the first 100 individuals to complete a Centers for Medicare and Medicaid Services innovation project. J Am Geriatr Soc 2015;63:371-4. https://doi.org/10.1111/jgs.13245.
- Szanton SL, Wolff JW, Leff B, Thorpe RJ, Tanner EK, Boyd C, et al. CAPABLE trial: a randomized controlled trial of nurse, occupational therapist and handyman to reduce disability among older adults: rationale and design. Contemp Clin Trials 2014;38:102-12.
- Waldersen BW, Wolff JL, Roberts L, Bridges AE, Gitlin LN, Szanton SL. Functional goals and predictors of their attainment in low-income community-dwelling older adults. Arch Phys Med Rehabil 2017;98:896-903.
- NCT01056640 . Telemonitoring Versus Usual Care 2010. https://clinicaltrials.gov/ct2/show/study/NCT01056640 (accessed 18 May 2021).
- Pecina JL, Hanson GJ, Van Houten H, Takahashi PY. Impact of telemonitoring on older adults health-related quality of life: the Tele-ERA study. Qual Life Res 2013;22:2315-21. https://doi.org/10.1007/s11136-013-0361-5.
- Pecina JL, Vickers KS, Finnie DM, Hathaway JC, Hanson GJ, Takahashi PY. Telemonitoring increases patient awareness of health and prompts health-related action: initial evaluation of the TELE-ERA study. Telemed J E Health 2011;17:461-6. https://doi.org/10.1089/tmj.2010.0213.
- Takahashi PY, Hanson GJ, Pecina JL, Stroebel RJ, Chaudhry R, Shah ND, et al. A randomized controlled trial of telemonitoring in older adults with multiple chronic conditions: the Tele-ERA study. BMC Health Serv Res 2010;10.
- Takahashi PY, Hanson GJ, Thorsteinsdottir B, Van Houten HK, Shah ND, Naessens JM, et al. The impact of telemonitoring upon hospice referral in the community: a randomized controlled trial. Clin Interv Aging 2012;7:445-51. https://doi.org/10.2147/CIA.S36461.
- Upatising B, Hanson GJ, Kim YL, Cha SS, Yih Y, Takahashi PY. Effects of home telemonitoring on transitions between frailty states and death for older adults: a randomized controlled trial. Int J Gen Med 2013;6:145-51. https://doi.org/10.2147/IJGM.S40576.
- Upatising B, Wood DL, Kremers WK, Christ SL, Yih Y, Hanson GJ, et al. Cost comparison between home telemonitoring and usual care of older adults: a randomized trial (Tele-ERA). Telemed J E Health 2015;21:3-8.
- NCT00974506 . Pilot Study: Complementary Therapies in Geriatric Patients 2009. https://clinicaltrials.gov/ct2/show/NCT00974506 (accessed 21 August 2020).
- DRKS00011831 . A Multimodal Intervention Program for Older People With Frailty – A Randomized Controlled Pilot Trial 2017. https://trialsearch.who.int/Trial2.aspx?TrialID=DRKS00011831 (accessed 11 May 2020).
- Ziller C, Braun T, Thiel C. Frailty phenotype prevalence in community-dwelling older adults according to physical activity assessment method. Clin Interv Aging 2020;15:343-55. https://doi.org/10.2147/CIA.S238204.
- Kjerstad E, Tuntland HK. Reablement in community-dwelling older adults: a cost-effectiveness analysis alongside a randomized controlled trial. Health Econ Rev 2016;6.
- NCT02043262 . The Effectiveness of Reablement in Home Dwelling Older Adults. A Randomized Controlled Trial 2012. https://clinicaltrials.gov/ct2/show/NCT02043262 (accessed 28 April 2020).
- Tuntland H, Espehaug B, Forland O, Hole AD, Kjerstad E, Kjeken I. Reablement in community-dwelling adults: study protocol for a randomised controlled trial. BMC Geriatr 2014;14.
- NTR5184 . PROTO-Study: Prevention of Undernutrition in Community Dwelling Older Adults 2015. www.trialregister.nl/trial/5045 (accessed 21 August 2020).
- Dorhout BG, Haveman-Nies A, van Dongen EJI, Wezenbeek NLW, Doets EL, Bulten A, et al. Cost-effectiveness of a diet and resistance exercise intervention in community-dwelling older adults: ProMuscle in practice. J Am Med Dir Assoc 2021;22:792-80. https://doi.org/10.1016/j.jamda.2020.12.036.
- NTR6038 . ProMuscle in Practice: Effectiveness of a Combined Resistance Exercise and Nutrition Intervention to Promote Maintenance of Physical Functioning of Community-Dwelling Elderly in a Real-Life Setting 2016. www.trialregister.nl/trial/5858 (accessed 16 May 2020).
- van Dongen EJI, Doets EL, de Groot LCPGM, Dorhout BG, Haveman-Nies A. Process evaluation of a combined lifestyle intervention for community-dwelling older adults: ProMuscle in Practice. Gerontologist 2020;60:1538-54. https://doi.org/10.1093/geront/gnaa027.
- van Dongen EJI, Haveman-Nies A, Wezenbeek NLW, Dorhout BG, Doets EL, de Groot L. Effect, process, and economic evaluation of a combined resistance exercise and diet intervention (ProMuscle in Practice) for community-dwelling older adults: design and methods of a randomised controlled trial. BMC Public Health 2018;18.
- University of Groningen . Appendix in the Final Report of the Project: ‘The Effects of Physical and Cognitive Interventions on Disability in Older Persons’ No. 014-90-027 2005.
- Van Hout H. The Cost-effectiveness of Systematic Home Visits by Nurses of Frail Elderly Primary Care Patients and Caregivers of Demented Patients. BioMed Central, ISRCTN Registry; 2005.
- van Hout HP, Nijpels G, van Marwijk HW, Jansen AP, Van’t Veer PJ, Tybout W, et al. Design and pilot results of a single blind randomized controlled trial of systematic demand-led home visits by nurses to frail elderly persons in primary care [ISRCTN05358495]. BMC Geriatr 2005;5.
- Hoogendijk EO, van der Horst HE, van de Ven PM, Twisk JW, Deeg DJ, Frijters DH, et al. Effectiveness of a Geriatric Care Model for frail older adults in primary care: results from a stepped wedge cluster randomized trial. Eur J Intern Med 2016;28:43-51.
- Muntinga ME, Hoogendijk EO, van Leeuwen KM, van Hout HP, Twisk JW, van der Horst HE, et al. Implementing the chronic care model for frail older adults in the Netherlands: study protocol of ACT (frail older adults: care in transition). BMC Geriatr 2012;12.
- Muntinga ME, Mokkink LB, Knol DL, Nijpels G, Jansen APD. Measurement properties of the Client-centered Care Questionnaire (CCCQ): factor structure, reliability and validity of a questionnaire to assess self-reported client-centeredness of home care services in a population of frail, older people. Qual Life Res 2014;23:2063-72.
- Muntinga ME, Van Leeuwen KM, Schellevis FG, Nijpels G, Jansen AP. From concept to content: assessing the implementation fidelity of a chronic care model for frail, older people who live at home. BMC Health Serv Res 2015;15.
- NL2043 . The Frail Elderly Person at the Centre of Cohesive Care 2010. https://trialregister.nl/trial/2043 (accessed 11 May 2020).
- NTR3980 . KWIEK. Investigating the Effectiveness on Self-Management and Quality of Life of an Integrated Multidimensional Lifestyle Program in Older Adults 2013. www.trialregister.nl/trial/3814 (accessed 22 May 2020).
- van Rossum E, Frederiks C, Philipsen H, Kil-van Lierop J, Mantel A, Portengen J, et al. Design of a Dutch study to test preventive home visits to the elderly. Nurs Res 1991;40:185-8.
- van Rossum HJL. Effects of Preventive Home Visits to the Elderly 1993.
- Avlund K, Vass M, Hendriksen C. Education of preventive home visitors: the effects on change in tiredness in daily activities. Eur J Ageing 2007;4:125-31.
- Avlund K, Vass M, Kvist K, Hendriksen C, Keiding N. Educational intervention toward preventive home visitors reduced functional decline in community-living older women. J Clin Epidemiol 2007;60:954-62.
- Avlund K, Vass M, Lund R, Yamada Y, Hendriksen C. Influence of psychological characteristics and social relations on receiving preventive home visits in older men and women. Eur J Ageing 2008;5:191-20. https://doi.org/10.1007/s10433-008-0086-4.
- Ekmann A, Vass M, Avlund K. Preventive home visits to older home-dwelling people in Denmark: are invitational procedures of importance?. Health Soc Care Community 2010;18:563-71. https://doi.org/10.1111/j.1365-2524.2010.00941.x.
- Elkjaer E, Poulsen T, Avlund K. Stability and change in physical activity in old age: the role of changes in disability. Eur J Ageing 2006;3:89-97. https://doi.org/10.1007/s10433-006-0025-1.
- Hendriksen C, Vass M. Z Gerontol Geriatr. 2005.
- Jørgensen TSH, Lund R, Siersma VD, Nilsson CJ. Interplay between financial assets and social relations on decline in physical function and mortality among older people. Eur J Ageing 2018;15:133-42. https://doi.org/10.1007/s10433-017-0437-0.
- Kronborg C, Vass M, Lauridsen J, Avlund K. Cost effectiveness of preventive home visits to the elderly: economic evaluation alongside randomized controlled study. Eur J Health Econ 2006;7:238-46.
- Lund R, Nilsson CJ, Avlund K. Can the higher risk of disability onset among older people who live alone be alleviated by strong social relations? A longitudinal study of non-disabled men and women. Age Ageing 2010;39:319-26. https://doi.org/10.1093/ageing/afq020.
- Nilsson CJ, Avlund K, Lund R. Social inequality in onset of mobility disability among older Danes: the mediation effect of social relations. J Aging Health 2010;22:522-41. https://doi.org/10.1177/0898264309359684.
- Nilsson CJ, Lund R, Avlund K. Cohabitation status and onset of disability among older Danes: is social participation a possible mediator?. J Aging Health 2007;20:235-53. https://doi.org/10.1177/0898264307310474.
- Nilsson CJ, Siersma V, Mänty M, Avlund K, Vass M, Lund R. Mobility decline in old age: the combined effect of mobility-related fatigue and socioeconomic position. J Epidemiol Community Health 2014;68:510-5. https://doi.org/10.1136/jech-2013-203060.
- Poulsen T, Elkjaer E, Vass M, Hendriksen C, Avlund K. Promoting physical activity in older adults by education of home visitors. Eur J Ageing 2007;4:115-24. https://doi.org/10.1007/s10433-007-0057-1.
- Poulsen T, Siersma VD, Lund R, Christensen U, Vass M, Avlund K. Educational intervention and functional decline among older people: the modifying effects of social capital. Scand J Public Health 2014;42:295-303. https://doi.org/10.1177/1403494813520353.
- Vass M, Avlund K, Hendriksen C. Randomized intervention trial on preventive home visits to older people: baseline and follow-up characteristics of participants and non-participants. Scand J Public Health 2007;35:410-7.
- Vass M, Avlund K, Hendriksen C, Andersen CK, Keiding N. Preventive home visits to older people in Denmark: methodology of a randomized controlled study. Aging Clin Exp Res 2002;14:509-15.
- Vass M, Avlund K, Kvist K, Hendriksen C, Andersen CK, Keiding N. Structured home visits to older people. Are they only of benefit for women? A randomised controlled trial. Scand J Prim Health Care 2004;22:106-11.
- Vass M, Avlund K, Parner ET, Hendriksen C. Preventive home visits to older home-dwelling people and different functional decline patterns. Eur J Ageing 2007;4:107-13. https://doi.org/10.1007/s10433-007-0059-z.
- Vass M, Avlund K, Siersma V, Hendriksen C. A feasible model for prevention of functional decline in older home-dwelling people – the GP role. A municipality-randomized intervention trial. Fam Pract 2009;26:56-64.
- Vass M, Hendriksen C, Thomsen JL, Parner ET, Avlund K. Preventive home visits to home-dwelling older people and hospital admissions: a municipality-randomised intervention trial. Eur J Ageing 2008;5:67-76.
- Vass M, Holmberg R, Fiil-Nielsen H, Lauridsen J, Avlund K, Hendriksen C. Preventive home visitation programmes for older people: the role of municipality organisation. Eur J Ageing 2007;4:133-40.
- ISRCTN07330512 . Screening and Counseling for Physical Activity and Mobility in Older People 2005. https://doi.org/10.1186/ISRCTN07330512 (accessed 21 August 2020).
- Leinonen R, Heikkinen E, Hirvensalo M, Lintunen T, Rasinaho M, Sakari-Rantala R, et al. Customer-oriented counseling for physical activity in older people: study protocol and selected baseline results of a randomized-controlled trial (ISRCTN 07330512). Scand J Med Sci Sports 2007;17:156-64. https://doi.org/10.1111/j.1600-0838.2006.00536.x.
- Mänty M, Heinonen A, Leinonen R, Törmäkangas T, Hirvensalo M, Kallinen M, et al. Long-term effect of physical activity counseling on mobility limitation among older people: a randomized controlled study. J Gerontol A Biol Sci Med Sci 2009;64A:83-9. https://doi.org/10.1093/gerona/gln029.
- Rasinaho M, Hirvensalo M, Törmäkangas T, Leinonen R, Lintunen T, Rantanen T. Effect of physical activity counseling on physical activity of older people in Finland (ISRCTN 07330512). Health Promot Int 2011;27:463-74. https://doi.org/10.1093/heapro/dar057.
- Sallinen J, Mänty M, Leinonen R, Kallinen M, Törmäkangas T, Heikkinen E, et al. Factors associated with maximal walking speed among older community-living adults. Aging Clin Exp Res 2011;23:273-8. https://doi.org/10.1007/BF03337753.
- von Bonsdorff MB, Leinonen R, Kujala UM, Heikkinen E, Tormakangas T, Hirvensalo M, et al. Effect of physical activity counseling on home care use in older people: letters to the editor. J Am Geriatr Soc 2009;57:571-3.
- Avgerinou C, Gardner B, Kharicha K, Frost R, Liljas A, Elaswarapu R, et al. Health promotion for mild frailty based on behaviour change: perceptions of older people and service providers. Health Soc Care Community 2019;27:1333-43. https://doi.org/10.1111/hsc.12781.
- ISRCTN11986672 . The Development and Feasibility of a New Service to Promote Health and Well-Being in Older People Who Are Starting to Become Frailer: The HomeHealth Study 2015. https://trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN11986672 (accessed 11 May 2020).
- ISRCTN21710246 . Occupational Therapy in Homecare Re-Ablement Services 2014. www.isrctn.com/ISRCTN21710246 (accessed 28 April 2020).
- Whitehead PJ, Drummond AER, Walker MF, Parry RH, McGeorge ID, Latif Z. Occupational Therapy in HomEcare Re-ablement Services (OTHERS): study protocol for a randomized controlled trial. Trials 2014;15.
- Williams EI, Greenwell J, Groom LM. Characteristics of patients aged 75 years and over who are discharged from hospital without district nursing support. J Public Health Med 1992;14:321-7. https://doi.org/10.1093/oxfordjournals.pubmed.a042749.
- Roth G, Wolter A, Stolle C, Rothgang H. The long and bumpy road to outcome-oriented management of long-term care in Germany: implementation of the Resident Assessment Instrument in home-care services. Int J Health Plann Manag 2014;29:316-29. https://doi.org/10.1002/hpm.2186.
- Stolle C, Wolter A, Roth G, Rothgang H. Effects of the resident assessment instrument in home care settings: results of a cluster randomized controlled trial. Z Gerontol Geriatr 2012;45:315-22.
- Stolle C, Wolter A, Roth G, Rothgang H. Improving health status and reduction of institutionalization in long-term care – effects of the resident assessment instrument – home care by degree of implementation. Int J Nurs Pract 2015;21:612-21.
- Wong FKY . Effects of Health-Social Partnership Programme 2014. https://clinicaltrials.gov/show/NCT02286375 (accessed 11 May 2020).
- Wong AKC, Wong FKY. The psychological impact of a nurse-led proactive self-care program on independent, non-frail community-dwelling older adults: a randomized controlled trial. Int J Nurs Stud 2020;110. https://doi.org/10.1016/j.ijnurstu.2020.103724.
- Wong AKC, Wong FKY, So C. Cost-effectiveness of a preventive self-care health management program for community-dwelling older adults: a randomised controlled trial. Age Ageing 2021;50:440-6. https://doi.org/10.1093/ageing/afaa127.
- Wong KC, Wong FKY, Chang KKP. Health-social partnership intervention programme for community-dwelling older adults: a research protocol for a randomized controlled trial. J Adv Nurs 2015;71:2673-85.
- Taylor SJC, Pinnock H, Epiphaniou E, Pearce G, Parke HL, Schwappach A, et al. A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions (PRISMS Practical systematic Review of Self-Management Support for long-term conditions). Health Serv Deliv Res 2014;2. https://doi.org/10.3310/hsdr02530.
- Legg L, Gladman J, Drummond A, Davidson A. A systematic review of the evidence on home care reablement services. Clin Rehabil 2015;30:741-9. https://doi.org/10.1177/0269215515603220.
- Hansagi H, Rosenqvist U. Swedish population norms for the GHRI, HI and STAI-state. Qual Life Res 1993;2:349-56.
- McKenzie JE, Brennan SE, . Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2022.
- United Nations Department of Economic and Social Affairs Population Division . Number of Males Per 100 Females by Broad Age Groups. World Population Prospects 2019, Online Edition. Rev. 1 2019. https://population.un.org/wpp/Download/Standard/Population/ (accessed 6 July 2022).
- Office for National Statistics . Local Authority Ageing Statistics, Sex Ratios for People Aged 65 and Over and 85 and Over 2020. www.ons.gov.uk/datasets/older-people-sex-ratios/editions/time-series/versions/1#id-dimensions (accessed 7 July 2022).
- Whitehead M, Dahlgren G. Policies and Strategies to Promote Social Equity in Health: Background Document to WHO – Strategy Paper for Europe. Stockholm: Institute for Future Studies; 1991.
- Dahlgren G, Whitehead M. The Dahlgren-Whitehead model of health determinants: 30 years on and still chasing rainbows. Public Health 2021;199:20-4. https://doi.org/10.1016/j.puhe.2021.08.009.
- Brignardello-Petersen R, Murad MH, Walter SD, McLeod S, Carrasco-Labra A, Rochwerg B, et al. GRADE Working Group . GRADE approach to rate the certainty from a network meta-analysis: avoiding spurious judgments of imprecision in sparse networks. J Clin Epidemiol 2019;105:60-7. https://doi.org/10.1016/j.jclinepi.2018.08.022.
- Whitehead PJ, Worthington EJ, Parry RH, Walker MF, Drummond AER. Interventions to reduce dependency in personal activities of daily living in community dwelling adults who use homecare services: a systematic review. Clin Rehabil 2015;29:1064-76. https://doi.org/10.1177/0269215514564894.
- Luker JA, Worley A, Stanley M, Uy J, Watt AM, Hillier SL. The evidence for services to avoid or delay residential aged care admission: a systematic review. BMC Geriatr 2019;19. https://doi.org/10.1186/s12877-019-1210-3.
- Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021;374. https://doi.org/10.1136/bmj.n2061.
- Lamb SE, Becker C, Gillespie LD, Smith JL, Finnegan S, Potter R, et al. Taxonomy Investigators . Reporting of complex interventions in clinical trials: development of a taxonomy to classify and describe fall-prevention interventions. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-125.
- UK Chief Medical Officers . UK Chief Medical Officers’ Physical Activity Guidelines 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832868/uk-chief-medical-officers-physical-activity-guidelines.pdf (accessed 4 August 2022).
- Shlisky J, Bloom DE, Beaudreault AR, Tucker KL, Keller HH, Freund-Levi Y, et al. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv Nutr 2017;8:17-26. https://doi.org/10.3945/an.116.013474.
Appendix 1 Electronic search strategies
Cochrane Central Register of Controlled Trials (CENTRAL) via Wiley interface was searched. The database coverage was 1992–present and the database was searched on 11 August 2021.
-
((frail* or prefrailty)):ti,ab,kw (Word variations have been searched) 4037
-
MeSH descriptor: [Aged] explode all trees 213642
-
MeSH descriptor: [Geriatrics] this term only 207
-
(elderly or old* next people* or old* next person* or old* next wom?n* or old* next m?n* or old* next male* or old* next female* or old* next adult* or old* next age* or aging or geriatric* or senior next citizen* or seniors or pensioner* or veteran* or sexagenarian* or septuagenarian* or octogenarian* or nonagenarian* or centenarian*):ti,ab,kw 92534
-
((over Near/2 (‘60’ or ‘61’ or ‘62’ or ‘63’ or ‘64’ or ‘65’ or ‘66’ or ‘67’ or ‘68’ or ‘69’ or ‘70’ or ‘71’ or ‘72’ or ‘73’ or ‘74’ or ‘75’ or ‘76’ or ‘77’ or ‘78’ or ‘79’ or ‘80’ or ‘81’ or ‘82’ or ‘83’ or ‘84’ or ‘85’ or ‘86’ or ‘87’ or ‘88’ or ‘89’ or ‘90’ or ‘91’ or ‘92’ or ‘93’ or ‘94’ or ‘95’ or ‘96’ or ‘97’ or ‘98’ or ‘99’ or ‘100’) Near years)):ti,ab,kw (Word variations have been searched) 3277
-
{or #1-#5} 283983
-
MeSH descriptor: [Independent Living] this term only 544
-
MeSH descriptor: [Community Health Nursing] explode all trees 345
-
(‘Community support services’):ti,ab,kw (Word variations have been searched) 23
-
MeSH descriptor: [Managed Care Programs] explode all trees 502
-
(‘health maintenance organization*’ or ‘health maintenance organisation*’):ti,ab,kw (Word variations have been searched) 627
-
(HMO*):ti,ab,kw (Word variations have been searched) 494
-
MeSH descriptor: [Social Work] this term only 184
-
(social Near/3 services):ti,ab,kw (Word variations have been searched) 1417
-
(‘Voluntary services’):ti,ab,kw (Word variations have been searched) 14
-
MeSH descriptor: [Home Nursing] this term only 282
-
(‘house call*’):ti,ab,kw (Word variations have been searched) 583
-
(home near/5 visit*):ti,ab,kw (Word variations have been searched) 5140
-
(((‘general practice’ or ‘primary care’ or nurse* or group or ‘ambulatory clinic’ or ‘geriatric clinic’) near/3 visit*)):ti,ab,kw (Word variations have been searched) 4731
-
MeSH descriptor: [Geriatric Assessment] this term only 1509
-
(pharmac* near/2 visit):ti,ab,kw (Word variations have been searched) 278
-
((home or house) near/2 appointment*):ti,ab,kw (Word variations have been searched) 24
-
(‘Home Care Services’):ti,ab,kw (Word variations have been searched) 2257
-
MeSH descriptor: [Home Care Services] this term only 1883
-
MeSH descriptor: [Health Services for the Aged] this term only 456
-
MeSH descriptor: [Home Health Nursing] explode all trees 7
-
(‘district nursing’):ti,ab,kw (Word variations have been searched) 115
-
(‘health visit*’):ti,ab,kw (Word variations have been searched) 186
-
(‘community matron’):ti,ab,kw (Word variations have been searched) 4
-
(home Near/3 (intervention* or support* or assessment*)):ti,ab,kw (Word variations have been searched) 4926
-
MeSH descriptor: [Home Health Nursing] this term only 7
-
(((preventive* or preventative*) near/5 medicine)):ti,ab,kw (Word variations have been searched) 781
-
MeSH descriptor: [Preventive Medicine] this term only 121
-
((preventive* or preventative*) near/3 (program* or intervent* or support* or care or service* or approach* or ‘case management’ or measure* or OT or ‘occupational therapy’ or assess*)):ti,ab,kw (Word variations have been searched) 6275
-
{or #7-#34} 25735
-
MeSH descriptor: [Geriatric Nursing] this term only 178
-
(‘geriatric nursing’):ti,ab,kw (Word variations have been searched) 274
-
{or #36-#37} 274
-
MeSH descriptor: [Community Health Services] this term only 1061
-
(community):ti,ab,kw 46478
-
MeSH descriptor: [Community Health Nursing] explode all trees 345
-
MeSH descriptor: [Community Pharmacy Services] this term only 271
-
MeSH descriptor: [Home Care Services] this term only 1883
-
MeSH descriptor: [Aftercare] this term only 661
-
MeSH descriptor: [Primary Health Care] this term only 4388
-
(domiciliary or (‘social support’ and home*) or ((homecare or medical) near/2 home) or (home and package*) or (outreach and home) or ‘(alternative setting’ and home) or ‘home visit*’ or ‘home manag*’ or homecare or ‘home care’ or ‘home therap*’ or (model* adj1 home*) or ‘home program*’ or ‘home monitor*’):ti,ab,kw (Word variations have been searched) 12652
-
(‘home-based’ or homebased or homebound):ti,ab,kw (Word variations have been searched) 7510
-
((live or living or lived or dwell*) near/5 (‘at home’ or ‘own home’ or ‘in home’ or alone or independent*)):ti,ab,kw (Word variations have been searched) 3855
-
(‘Home care’ or ‘primary care’ or ‘primary healthcare’ or ‘primary health care’ or ‘community dwelling’):ti,ab,kw (Word variations have been searched) 31085
-
{or #39-#49} 80654
-
#38 AND #50 103
-
#35 or #51 25779
-
#6 and #52 8010
-
(coronary heart disease or CHD or chronic obstructive pulmonary disease or COPD or kidney failure or CKD or Heart failure or diabetes or asthma or cancer or schizophrenia or severe mental illness*):ti 210929
-
#53 NOT #54 7003
MEDLINE(R) ALL was searched via OvidSP. The database coverage was 1946–present and the database was searched on 9 August 2021.
-
randomized controlled trial.pt. (539556)
-
controlled clinical trial.pt. (94320)
-
randomized.ab. (529280)
-
placebo.ab. (220248)
-
clinical trials as topic.sh. (196870)
-
randomly.ab. (363058)
-
trial.ti. (244962)
-
or/1–7 (1384889)
-
exp animals/not humans.sh. (4870600)
-
8 not 9 [Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity- and precision-maximizing version (2008 revision)] (1274483)
-
Clinical Trial, Phase III/(18797)
-
(‘phase 3’ or ‘phase3’ or ‘phase III’ or ‘P3’ or ‘PIII’).ti,ab,kw. (73139)
-
11 or 12 [search filter for phase three trials to supplement Cochrane HSSS, Cooper 2019] (79735)
-
10 or 13 [final RCT filter] (1318490)
-
(frail* or prefrailty).tw. (25865)
-
exp aged/(3283911)
-
geriatrics/(30590)
-
(elder* or older or old people* or old person* or old wom#n*1 or old m#n*1 or old male*1 or old female*1 or old adult*1 or old age* or aging or ageing or geriatric* or senior citizen* or seniors or pensioner* or veteran* or sexagenarian* or septuagenarian* or octogenarian* or nonagenarian* or centenarian*).tw,kf. (1385083)
-
(over adj2 (‘60’ or ‘61’ or ‘62’ or ‘63’ or ‘64’ or ‘65’ or ‘66’ or ‘67’ or ‘68’ or ‘69’ or ‘70’ or ‘71’ or ‘72’ or ‘73’ or ‘74’ or ‘75’ or ‘76’ or ‘77’ or ‘78’ or ‘79’ or ‘80’ or ‘81’ or ‘82’ or ‘83’ or ‘84’ or ‘85’ or ‘86’ or ‘87’ or ‘88’ or ‘89’ or ‘90’ or ‘91’ or ‘92’ or ‘93’ or ‘94’ or ‘95’ or ‘96’ or ‘97’ or ‘98’ or ‘99’ or ‘100’) adj years).tw. (21451)
-
or/15–19 [older or frail people] (4132930)
-
independent living/(8001)
-
community health services/(32391)
-
community health nursing/(19684)
-
Community support services.tw. (173)
-
exp managed care programs/(40081)
-
(health maintenance organi?ation* or HMO*).tw. (13817)
-
(Social adj3 services).tw. (10694)
-
Voluntary services.tw. (99)
-
*home nursing/(5361)
-
House Calls/(3846)
-
house call*.tw. (656)
-
(home adj5 visit*).tw. (12399)
-
((general practice or primary care or nurse* or group or ambulatory clinic or geriatric clinic) adj3 visit*).tw. (9527)
-
*geriatric assessment/(13906)
-
(pharmac* adj2 visit).tw. (212)
-
((home or house) adj2 appointment*).tw. (52)
-
Home Care Services/(34738)
-
Home care service*.tw. (1913)
-
*health services for the aged/(14001)
-
home health nursing/(364)
-
district nursing.tw. (667)
-
health visit*.ti. or health visit*.ab./freq = 2 (2285)
-
community matron*.ti. or community matron*.ab./freq = 2 (83)
-
(home adj3 (intervention* or support* or assessment*)).tw. (8887)
-
preventive health services/(14024)
-
((preventive* or preventative*) adj5 medicine).tw. (7306)
-
preventative medicine/(11938)
-
((preventive* or preventative*) adj3 (program* or intervent* or support* or care or service* or approach* or case management or measure* or OT or occupational therapy or assess*)).tw. (66507)
-
or/21–48 (283985)
-
geriatric nursing/(13707)
-
geriatric nurs*.tw,kf. (1164)
-
or/50–51 [geriatric nursing] (14118)
-
community.ti,ab,kf. (539116)
-
community health services/or community health nursing/or community mental health services/or community pharmacy services/(74241)
-
‘domiciliary care’/(34738)
-
aftercare/(10404)
-
primary health care/(83064)
-
(domiciliary or (social support and home*) or ((homecare or medical) adj2 home) or (home and package*) or (outreach and home) or (alternative setting and home) or home visit* or home manag* or homecare or home care or home therap* or (model* adj1 home*) or home program* or home monitor*).tw. (58982)
-
((live or living or lived or dwell*) adj5 (‘at home’ or ‘own home’ or ‘in home’ or alone or independent*)).tw. (17479)
-
(home-based or homebased or homebound).tw. (12811)
-
(Home care or primary care or primary health care or primary healthcare).tw. (163820)
-
or/53–61 [interventions in a community or home setting] (808717)
-
52 and 62 [geriatric nursing and interventions in a community or home setting] (2015)
-
49 or 63 [all interventions] (284694)
-
(coronary heart disease or CHD or chronic obstructive pulmonary disease or COPD or kidney failure or CKD or Heart failure or diabetes or asthma or cancer or schizophrenia or severe mental illness*).ti. (1592860)
-
64 not 65 [all interventions excluding specific diseases in title] (267883)
-
14 and 20 and 66 [RCTS and older people and interventions] (7005)
Embase and Embase Classic via OvidSP was searched. The database coverage was 1947–present and the database was searched on 9 August 2021.
-
randomized controlled trial/ (672319)
-
controlled clinical study/ (463974)
-
1 or 2 (860531)
-
random*.tw. (1703521)
-
randomisation/ (91766)
-
intermethod comparison/ (273924)
-
placebo.tw. (332206)
-
(compare or compared or comparison).ti. (574408)
-
((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared)).ab. (2067060)
-
(open adj label).ti,ab. (89661)
-
((double or single or doubly or singly) adj blind).tw. (227285)
-
parallel group$1.tw. (27916)
-
double blind procedure/ (188870)
-
(crossover or cross over).tw. (113362)
-
((assign* or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).tw. (362240)
-
(assigned or allocated).tw. (427304)
-
(controlled adj7 (study or design or trial)).tw. (388612)
-
(volunteer or volunteers).tw. (265628)
-
human experiment/(551078)
-
trial.ti. (343846)
-
or/4–20 (5189451)
-
21 or 3 (5347546)
-
(random* adj sampl* adj7 (‘cross section*’ or questionnaire$1 or survey* or database$1)).tw. not (comparative study/or controlled study/or randomi?ed controlled.tw. or randomly assigned.tw.) (8774)
-
Cross-sectional study/not (randomized controlled trial/or controlled clinical study/or controlled study/or randomi?ed controlled.tw. or control group$1.tw.) (277846)
-
(((case adj control*) and random*) not randomi?ed controlled).tw. (18755)
-
(Systematic review not (trial or study)).ti. (182362)
-
(nonrandom* not random*).tw. (17268)
-
‘Random field*’.tw. (2544)
-
(random cluster adj3 sampl*).tw. (1374)
-
(review.ab. and review.pt.) not trial.ti. (913087)
-
‘we searched’.ab. and (review.ti. or review.pt.) (37761)
-
‘update review’.ab. (119)
-
(databases adj4 searched).ab. (44421)
-
(rat or rats or mouse or mice or swine or porcine or murine or sheep or lambs or pigs or piglets or rabbit or rabbits or cat or cats or dog or dogs or cattle or bovine or monkey or monkeys or trout or marmoset$1).ti. and animal experiment/(1116446)
-
Animal experiment/not (human experiment/or human/) (2346095)
-
or/23-35 (3759577)
-
22 not 36 [Cochrane Highly Sensitive Search Strategy for identifying controlled trials in Embase: (2018 revision); Ovid format (Glanville et al. , 2019b)] (4755948)
-
(frail* or prefrailty).tw. (39809)
-
aged/(3370037)
-
very elderly/(236950)
-
frail elderly/(10922)
-
geriatrics/(39915)
-
(elder* or older or old pele*ople* or old person* or old wom#n*1 or old m#n*1 or old ma1 or old female*1 or old adult*1 or old age* or aging or ageing or geriatric* or senior citizen* or seniors or pensioner* or veteran* or sexagenarian* or septuagenarian* or octogenarian* or nonagenarian* or centenarian*).tw,kw. (1838159)
-
(over adj2 (‘60’ or ‘61’ or ‘62’ or ‘63’ or ‘64’ or ‘65’ or ‘66’ or ‘67’ or ‘68’ or ‘69’ or ‘70’ or ‘71’ or ‘72’ or ‘73’ or ‘74’ or ‘75’ or ‘76’ or ‘77’ or ‘78’ or ‘79’ or ‘80’ or ‘81’ or ‘82’ or ‘83’ or ‘84’ or ‘85’ or ‘86’ or ‘87’ or ‘88’ or ‘89’ or ‘90’ or ‘91’ or ‘92’ or ‘93’ or ‘94’ or ‘95’ or ‘96’ or ‘97’ or ‘98’ or ‘99’ or ‘100’) adj years).tw. (33789)
-
or/38–44 [frail or elderly people] (4516350)
-
independent living/(5523)
-
community care/(61677)
-
community health nursing/(26723)
-
Community support services.tw. (239)
-
(health maintenance organi?ation* or HMO*).tw. (16728)
-
(Social adj3 services).tw. (14016)
-
Voluntary services.tw. (148)
-
home visit/(3712)
-
house call*.tw. (852)
-
(home adj5 visit*).tw. (17275)
-
((general practice or primary care or nurse* or group or ambulatory clinic or geriatric clinic) adj3 visit*).tw. (14158)
-
*geriatric assessment/(6239)
-
(pharmac* adj2 visit).tw. (504)
-
((home or house) adj2 appointment*).tw. (107)
-
Home Care/(66345)
-
Home care service*.tw. (2345)
-
*elderly care/(21267)
-
district nursing.tw. (664)
-
health visit*.ti. or health visit*.ab./freq = 2 (2402)
-
community matron*.ti. or community matron*.ab./freq = 2 (82)
-
(home adj3 (intervention* or support* or assessment*)).tw. (12351)
-
preventive health service/(30244)
-
((preventive* or preventative*) adj5 medicine).tw. (12282)
-
preventive medicine/(29022)
-
((preventive* or preventative*) adj3 (program* or intervent* or support* or care or service* or approach* or case management or measure* or OT or occupational therapy or assess*)).tw. (88643)
-
or/46–70 [specific interventions] (376111)
-
geriatric nursing/(12986)
-
geriatric nurs*.tw,kw. (1405)
-
or/72–73 [geriatric nursing] (13603)
-
community.tw,kw. (686753)
-
community health services/or community health nursing/or mental health service/or ‘pharmacy (shop)’/(144914)
-
aftercare/(8598)
-
primary health care/(70765)
-
(domiciliary or (social support and home*) or ((homecare or medical) adj2 home) or (home and package*) or (outreach and home) or (alternative setting and home) or home visit* or home manag* or homecare or home care or home therap* or (model* adj1 home*) or home program* or home monitor*).tw. (78824)
-
((live or living or lived or dwell*) adj5 (‘at home’ or ‘own home’ or ‘in home’ or alone or independent*)).tw. (24399)
-
(home-based or homebased or homebound).tw. (17773)
-
(Home care or primary care or primary healthcare or primary health care).tw. (217034)
-
or/75–82 [home or community setting] (1060004)
-
74 and 83 [geriatric nursing and home or community setting] (1864)
-
71 or 84 [all interventions] (376832)
-
(coronary heart disease or CHD or chronic obstructive pulmonary disease or COPD or kidney failure or CKD or Heart failure or diabetes or asthma or cancer or schizophrenia or severe mental illness*).ti. (2270105)
-
85 not 86 [all interventions except those mentioning specific diseases] (350036)
-
37 and 45 and 87 [RCT and elderly and Interventions] (17333)
APA PsycINFO via OvidSP was searched. The database coverage was 1806–present and the database was searched on 9 August 2021.
-
(control: or random:).tw. or exp treatment/[sensitive rct psycinfo search strategy Eady et al. , 2009] (1743140)
-
(frail* or prefrailty).tw. (5244)
-
exp aging/(79898)
-
geriatric patients/(13753)
-
geriatrics/(11969)
-
(elder* or older or old people* or old person* or old wom#n*1 or old m#n*1 or old male*1 or old female*1 or old adult*1 or old age* or aging or geriatric* or senior citizen* or seniors or pensioner* or veteran* or sexagenarian* or septuagenarian* or octogenarian* or nonagenarian* or centenarian*).tw. (355903)
-
(over adj2 (‘60’ or ‘61’ or ‘62’ or ‘63’ or ‘64’ or ‘65’ or ‘66’ or ‘67’ or ‘68’ or ‘69’ or ‘70’ or ‘71’ or ‘72’ or ‘73’ or ‘74’ or ‘75’ or ‘76’ or ‘77’ or ‘78’ or ‘79’ or ‘80’ or ‘81’ or ‘82’ or ‘83’ or ‘84’ or ‘85’ or ‘86’ or ‘87’ or ‘88’ or ‘89’ or ‘90’ or ‘91’ or ‘92’ or ‘93’ or ‘94’ or ‘95’ or ‘96’ or ‘97’ or ‘98’ or ‘99’ or ‘100’) adj years).tw. (2391)
-
or/2–7 [frail or elderly people] (371975)
-
Self-Care Skills/(4756)
-
community health/(3653)
-
community services/(17234)
-
social services/(9557)
-
Community support services.tw. (219)
-
exp managed care/(4567)
-
(health maintenance organi?ation* or HMO*).tw. (2449)
-
(Social adj3 services).tw. (11772)
-
Voluntary services.tw. (71)
-
home visiting programs/(1861)
-
home care/(6905)
-
house call*.tw. (106)
-
(home adj5 visit*).tw. (5619)
-
((general practice or primary care or nurse* or group or ambulatory clinic or geriatric clinic) adj3 visit*).tw. (2716)
-
(pharmac* adj2 visit).tw. (23)
-
((home or house) adj2 appointment*).tw. (12)
-
Independent Living Programs/(408)
-
Home care service*.tw. (706)
-
district nursing.tw. (64)
-
health visit*.ti. or health visit*.ab./freq = 2 (342)
-
community matron*.ti. or community matron*.ab./freq = 2 (14)
-
(home adj3 (intervention* or support* or assessment*)).tw. (5172)
-
((preventive* or preventative*) adj5 medicine).tw. (1085)
-
preventive medicine/(2464)
-
((preventive* or preventative*) adj3 (program* or intervent* or support* or care or service* or approach* or case management or measure* or OT or occupational therapy or assess*)).tw. (17107)
-
or/9–33 [interventions] (83270)
-
geriatric nursing.tw. (252)
-
(geriatrics/or geriatric patients/) and nursing/(639)
-
or/35-36 [geriatric nursing] (833)
-
community.tw. (275605)
-
community services/or community health/or community mental health services/or pharmacy/(28713)
-
(community healthcare or community health care).tw. (588)
-
home care/(6905)
-
aftercare/(1121)
-
primary health care/(19284)
-
Public Health Service Nurses/(658)
-
(domiciliary or (social support and home*) or ((homecare or medical) adj2 home) or (home and package*) or (outreach and home) or (alternative setting and home) or home visit* or home manag* or homecare or home care or home therap* or (model* adj1 home*) or home program* or home monitor*).tw. (19096)
-
((live or living or lived or dwell*) adj5 (‘at home’ or ‘own home’ or ‘in home’ or alone or independent*)).tw. (10597)
-
(home-based or homebased or homebound).tw. (5823)
-
(Home care or primary care or primary health care or primary healthcare).tw. (44178)
-
or/38–48 [community or home based] (340141)
-
37 and 49 [geriatric nursing and community or home based] (174)
-
34 or 50 [all interventions] (83378)
-
(coronary heart disease or CHD or chronic obstructive pulmonary disease or COPD or kidney failure or CKD or Heart failure or diabetes or asthma or cancer or schizophrenia or severe mental illness*).ti. (116600)
-
51 not 52 [all interventions except specific diseases in title] (79888)
-
1 and 8 and 53 [RCT filter and elderly and all interventions except specific diseases in title] (7917)
CINAHL via EBSCOhost interface was searched. The database coverage was 1972–present and the database was searched on 9 August 2021.
| # | Query | Limiters/expanders | Last run via | Results |
|---|---|---|---|---|
| S46 | S10 AND S18 and S45 | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
10,636 |
| S45 | S43 NOT S44 | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
106,016 |
| S44 | TI (‘coronary heart disease’ or CHD or ‘chronic obstructive pulmonary disease’ or COPD or ‘kidney failure’ or CKD or ‘Heart failure’ or diabetes or asthma or cancer or schizophrenia or ‘severe mental illness*’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
501,973 |
| S43 | S29 or S42 | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
113,278 |
| S42 | S30 and S41 | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
2217 |
| S41 | S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
687,262 |
| S40 | TX ‘Home care’ or ‘primary care’ or ‘primary health care’ or ‘primary healthcare’ | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
198,977 |
| S39 | TX ‘home-based’ or homebased or homebound | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
9035 |
| S38 | (MH ‘Community Health Services’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
22,541 |
| S37 | TX ((live or living or lived or dwell*) N5 (‘at home’ or ‘own home’ or ‘in home’ or community or alone or independent*)) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
57,710 |
| S36 | TX domiciliary or (‘social support’ and home*) or ((homecare or medical) N2 home) or (home and package*) or (outreach and home) or (alternative setting and home) or home visit* or home manag* or homecare or ‘home care’ or ‘home therap*’ or (model* N1 home*) or ‘home program*’ or ‘home monitor*’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
68,720 |
| S35 | (MH ‘Primary Health Care’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
67,490 |
| S34 | (MH ‘After Care’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
16,366 |
| S33 | (MH ‘Community Health Nursing’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
28,024 |
| S32 | (MH ‘Community Mental Health Services’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
9964 |
| S31 | TX community | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
479,269 |
| S30 | (MH ‘Gerontologic Nursing’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
13,362 |
| S29 | S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
111,533 |
| S28 | TX ((preventive* or preventative*) N3 (program* or intervent* or support* or care or service* or approach* or case management or measure* or OT or ‘occupational therapy’ or assess*)) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
47,492 |
| S27 | (MH ‘Preventive Health Care’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
21,369 |
| S26 | TX (home N3 (intervention* or support* or assessment*)) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
10,880 |
| S25 | TX ‘community matron*’ | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
283 |
| S24 | TX ‘health visit*’ | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
8986 |
| S23 | TX ‘district nursing’ | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
2176 |
| S22 | MM ‘Home Health Care’ | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
17,073 |
| S21 | (MH ‘Health Services for the Aged’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
6819 |
| S20 | (MH ‘Home Visits’) or (MH ‘Community Living’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
23,215 |
| S19 | TX ‘Community support services’ | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
155 |
| S18 | S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
1,209,432 |
| S17 | TX (over N2 (‘60’ or ‘61’ or ‘62’ or ‘63’ or ‘64’ or ‘65’ or ‘66’ or ‘67’ or ‘68’ or ‘69’ or ‘70’ or ‘71’ or ‘72’ or ‘73’ or ‘74’ or ‘75’ or ‘76’ or ‘77’ or ‘78’ or ‘79’ or ‘80’ or ‘81’ or ‘82’ or ‘83’ or ‘84’ or ‘85’ or ‘86’ or ‘87’ or ‘88’ or ‘89’ or ‘90’ or ‘91’ or ‘92’ or ‘93’ or ‘94’ or ‘95’ or ‘96’ or ‘97’ or ‘98’ or ‘99’ or ‘100’) N1 years) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
7999 |
| S16 | TX (aging or ageing or geriatric* or gerontologic* or elderly or ‘senior citizen*’ or seniors or pensioner* or veteran* or sexagenarian* or septuagenarian* or octogenarian* or nonagenarian* or centenarian*) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
523,198 |
| S15 | TX ((older or elder*) N2 (person or people or adult* or patient* or m?n* or wom?n* or female* or male*)) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
194,690 |
| S14 | (MH ‘Aged+’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
878,186 |
| S13 | (MH ‘Geriatrics’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
5708 |
| S12 | TX (frail*) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
18,976 |
| S11 | TX (prefrailty) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
160 |
| S10 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
1,157,497 |
| S9 | AB group or AB groups | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
795,542 |
| S8 | AB trial or AB Trials | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
299,002 |
| S7 | AB randomly | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
96,217 |
| S6 | AB (randomised or randomized) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
221,475 |
| S5 | TX ‘randomised controlled trial*’ | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
26,900 |
| S4 | TX ‘controlled clinical trial*’ | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
10,403 |
| S3 | (MH ‘Clinical Trials’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
177,904 |
| S2 | (MH ‘Randomized Controlled Trials’) | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
117,892 |
| S1 | TX ‘randomized controlled trial*’ | Search modes – Boolean/Phrase | Interface – EBSCOhost Research Databases Search Screen – Advanced Search Database – CINAHL |
To search ClinicalTrials.gov we used the advanced search interface, and searched the Conditions or Disease field using the following search terms: Frail Elderly Syndrome, frailty syndrome, Age-Related Atrophy, Frailty, Old Age; Debility. The search yielded 861 records.
For the International Clinical Trials Registry Platform (ICTRP) we used the advanced search interface, and used the search syntax older or elderly or frail in Title field and community or complex or independent or independence in Intervention field (with synonyms, all recruitment status). The search resulted in 425 records.
Appendix 2 Description of the components and aspects of components used to determine intervention groups, organised by domain
| Domain | Brief name (abbreviation) | Public-facing name | Plain language description |
|---|---|---|---|
| Activities | ADL (ADL) | Practise day-to-day activities | The person is offered support to practise carrying out day-to-day activities, for example dressing or taking the bus. The person may also be offered recommendations on how to carry out day-to-day activities safely or better. For example, this may include using appropriate footwear, removing loose rugs, cords and clutter in walking paths or improvement of lighting. The person may receive an assessment to create a tailored day-to-day activities plan. |
| Activities | Aids (aids) | Get equipment and technology to support day-to-day activities | The person is offered equipment or technology to aid in day-to-day activities. This may include ramps, walking frames, grab rails or a system of sensors that turn on the lights when the person gets up from the bed, for example. The person may receive an assessment to choose specific equipment or technology. |
| Activities | Engagement in meaningful-activities (eng) | Identify and engage in meaningful activities | The person is offered support to identify and participate in activities that they find meaningful. Examples may include leisure activities, crafts, volunteering, but the focus is on the activities being ones that the person finds meaningful. The activities may be organised for the person, be done by the person alone themselves, or be community activities that were already in place, for example. |
| Brain training | Cognitive training (cgn) | Do brain training | The person is offered training in thinking tasks such as memorising, paying attention or planning, among others. The training includes practical exercises and information about strategies to help thinking tasks. |
| Diet/nutrition | Nutrition (ntr) | Get dietary advice and support | The person is offered recommendations about diet and/or food supplements in group sessions or one-to-one. This is different from receiving information about nutrition as part of ‘Find out more information about health’ because there is a greater focus on providing specialised nutrition/dietary advice and related activities. For example, the person may also participate in writing a nutritional diary, cooking certain types of meals and weight monitoring. They may be provided with particular foods or supplements. The person may receive an assessment to create a tailored nutrition plan. |
| Financial support | Care voucher provision (vchr) | Get a health and care voucher | The person is offered a voucher to pay for health and personal care services and support on how to use the voucher. |
| Financial support | Welfare rights advice (wlfr) | Get advice about welfare services with follow-up | The person is offered tailored advice about the welfare services and benefits they can access. This is based on an assessment. Afterwards, the person was offered support in putting the plan in practice and accessing the services and benefits they are entitled to. |
| General health information | Education (educ) | Find out more information about health | The person is offered information about a set of health topics. The topics may include many areas, for example, oral health, nutrition and physical activity. The information may also focus on areas that are more important for the person. The way the information is provided is more structured than the specific advice someone may receive as part of a clinical appointment with a health professional. The person may be offered information in group sessions or on one-to-one contact. |
| Homecare | Homecare (hmcr) | Receive formal home care | The person is offered support services at home by health or care professionals. The services include, for example, nursing care or support with household tasks. |
| Individualised care | Medication-review (med) | Optimise my medication | The person is offered recommendations to change medication. For example, someone may be on too many medicines and be recommended to stop some. The changes to the medication can be provided on their own or as part of a more complete assessment and recommendations (see ‘Take part in individualised care planning based on an assessment’ for more details). |
| Individualised care | Monitoring (mntr) | Get care planning from health monitoring (including providing equipment) | If a health need is identified from monitoring, the person is offered an individualised care plan (see ‘Take part in individualised care planning’ for more details). To check for needs, the person participates in screening and monitoring of their bodily function, for example blood pressure and heart rate. This happens at least weekly. The person is offered equipment to record their bodily function. |
| Individualised care | Multifactorial-action (mfa) | Take part in individualised care planning | The person is offered an individualised care plan that includes recommendations for future action. The care plan is based on an assessment of the person’s needs and preferences and may include a variety of actions (related with physical exercise, diet, mood, etc.). The assessment structure may be set in advance or guided by the experience of a clinician. The person may receive support to carry out actions, for example, with referrals to see certain services. The person may also receive support from a care co-ordinator, who helps to deal with different services and/or professionals. |
| Individualised care | Review [in relation to multifactorial-action] (mfar) | Have regular follow-ups (after individualised care planning) | The person is regularly followed up after receiving an individualised care plan based on an assessment. The follow-up may include encouraging the person to carry out previous recommendations. The person may also be offered a new assessment of their needs and other relevant changes, and an updated individualised care plan. |
| Individualised care | Risk-screening (rsk) | Get care planning following screening for possible health problems | A tool to indicate possible health problems is used routinely and, if indicated, the person is offered an individualised care plan (see ‘Take part in individualised care planning’ for more details). The tool and the results that indicate problems are standardised, such as a questionnaire score or analysis of electronic health records. |
| Individualised care | Self-management [in multidomain assessment and care planning] (slfm) | Do activities to motivate taking care of yourself (when taking part in individualised care planning) | The person is engaged in conversations or activities designed to motivate them to care for themselves. The person may also be offered guided practice in some techniques, for example, to help them set up personal goals and solve problems. |
| Alternative medicine | Alternative medicine (hmnt) | Get alternative medicine | The person is offered alternative medicine such as homeopathic or naturopathic consultation and treatment. |
| Physical exercise | Exercise (exrc) | Do physical exercise | The person is offered support to carry out physical exercise. The exercise may be on their own or in training sessions. This is different from receiving information about physical activity as part of ‘Find out more information about health’ because there is a greater focus on providing specialised physical exercise advice and related activities. Physical exercises are activities done by a person to build up or maintain physical fitness (such as strength, balance, among others). The person may also receive an assessment to create a tailored exercise plan. |
| Social communication | Social skills training (sst) | Practise social interaction | The person is offered information and support to exercise their ability to relate with other people. This may include practising or discussing different ways of communicating. |
| Social communication | Telecoms (comm) | Get comm | The person is offered technology to enable communication with friends, family, neighbours or the community. For example, a tablet, iPad or mobile phone as well as applications such as e-mail or social media. The person will usually receive support in using the applications. |
| Well-being | Psychology (psyc) | Get well-being advice and support | The person is offered support for their well-being in areas like feeling low and dealing with worries. The support includes information about how we usually think and feel, and information and activities to deal with what we think and feel, such as noticing and learning how to overcome unhelpful thoughts. |
Appendix 3 Additional summary of findings tables
| Population: Older people Interventions: Community-based complex interventions Comparator: Homecare (hmcr) Outcome: Independence in IADL Time frame: Medium term; range of follow-up 12–18 months Setting: Community Total studies: 6 Total participants: 1401 Comparator rank: Mean 4.7, 95% CI 2 to 6 |
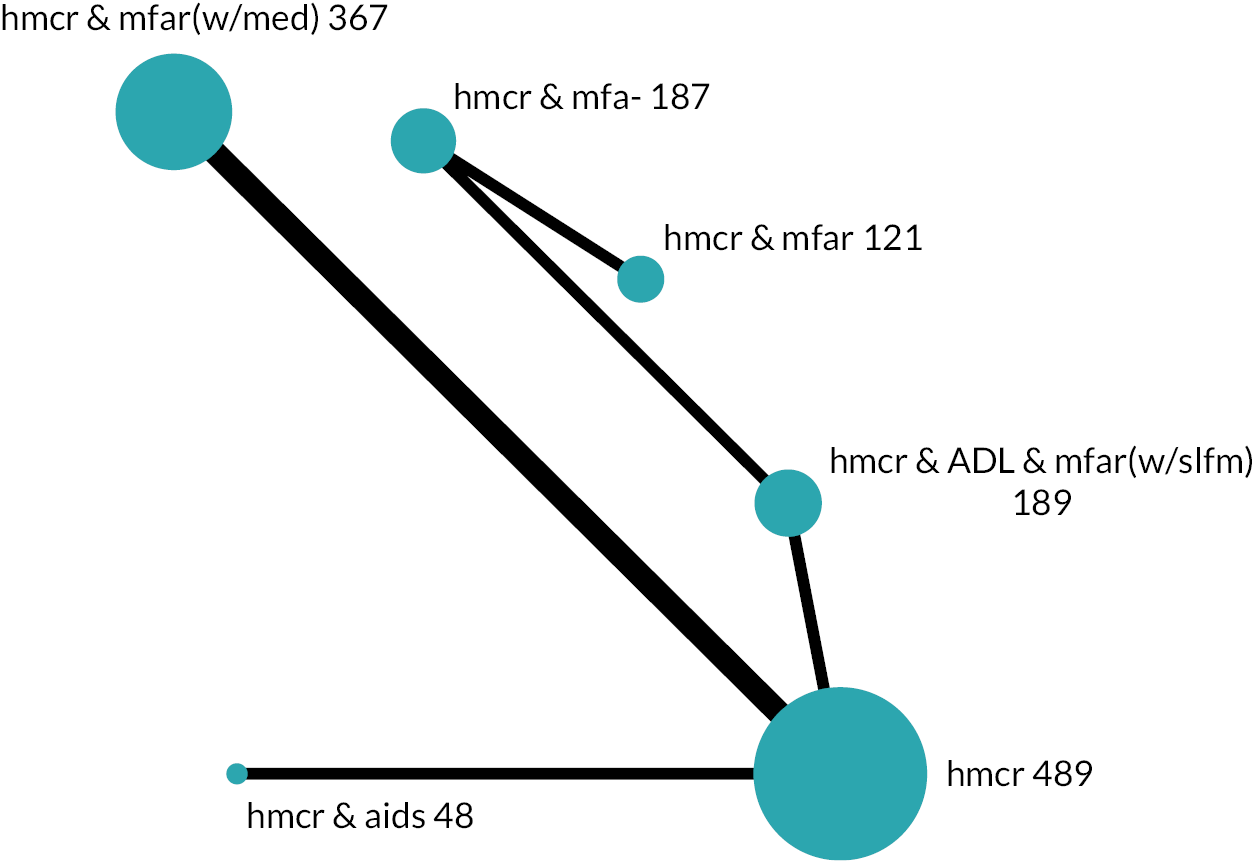
|
||||
|---|---|---|---|---|---|
| Intervention group | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | |
| SMD | MD (Lawton IADL 0 to 8)a | ||||
| Homecare and aids (hmcr & aids) | SMD 0.27 higher (0.23 lower to 0.77 higher) Mixed estimate |
MD 0.71 higher (0.60 lower to 2.02 higher) |
⊕⊝⊝⊝ Very lowb,c |
2.5 (1 to 6) | The evidence is very uncertain about the effect on IADL independence |
| Homecare, multifactorial-action and review (hmcr & mfar) | SMD 0.18 higher (0.52 lower to 0.88 higher) Indirect estimate |
MD 0.47 higher (1.35 lower to 2.30 higher) |
⊕⊝⊝⊝ Very lowd,e |
3.0 (1 to 6) | The evidence is very uncertain about the effect on IADL independence |
| Homecare, ADL training, multifactorial-action and review with self-management [hmcr & ADL & mfar(w/slfm)] | SMD 0.16 higher (0.21 lower to 0.53 higher) Mixed estimate |
MD 0.41 higher (0.55 lower to 1.38 higher) |
⊕⊝⊝⊝ Very lowe,f |
3.1 (1 to 6) | The evidence is very uncertain about the effect on IADL independence |
| Homecare, multifactorial-action and review with medication-review [hmcr & mfar(w/med)] | SMD 0.15 higher (0.11 lower to 0.41 higher) Mixed estimate |
MD 0.38 higher (0.30 lower to 1.06 higher) |
⊕⊝⊝⊝ Very lowb,c |
3.2 (1 to 6) | The evidence is very uncertain about the effect on IADL independence |
| Homecare and multifactorial-action (hmcr & mfa-) | SMD 0.01 lower (0.60 lower to 0.58 higher) Indirect estimate |
MD 0.02 lower (1.57 lower to 1.52 higher) |
⊕⊝⊝⊝ Very lowe,g |
4.5 (2 to 6) | The evidence is very uncertain about the effect on IADL independence |
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: Care-home placement Time frame: Short term; range of follow-up 24 weeks to 6 months Setting: Community Total studies: 7 Total participants: 3672 Comparator rank: Mean 4.6, 95% CI 3 to 7 |
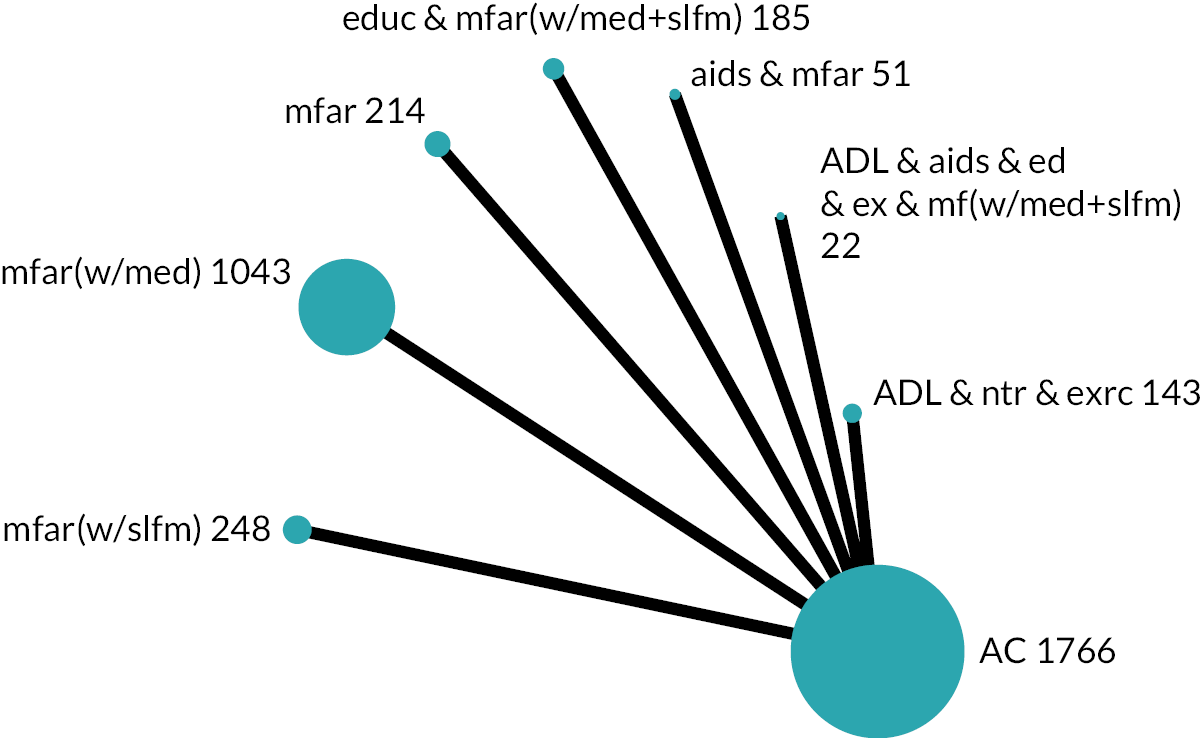
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Relative effect (95% CI) | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | ||||
| Network estimate | Calculated RRa | High-risk population (28 per 1000 with ac) |
Low-risk population (2 per 1000 with ac) |
||||||
| With intervention | Difference | With intervention | Difference | ||||||
| Multifactorial-action and review with medication-review [mfar(w/med)] | OR 0.77 (0.17 to 3.50) Mixed estimate |
RR 0.78 (0.17 to 3.44) |
22 per 1000 (5 to 92) |
6 fewer per 1000 (23 fewer to 64 more) |
2 per 1000 (0 to 7) |
0 per 1000 (2 fewer to 5 more) |
⊕⊝⊝⊝ Very lowb,c |
2.3 (1 to 7) | The evidence is very uncertain about the effect on care-home placement |
| Aids, multifactorial-action and review (aids & mfar) | OR 4.02 (0.18 to 89.76) Mixed estimate |
RR 3.94 (0.18 to 55.36) |
104 per 1000 (5 to 721) |
76 more per 1000 (23 fewer to 693 more) |
8 per 1000 (0 to 152) |
6 more per 1000 (2 fewer to 150 more) |
⊕⊝⊝⊝ Very lowd,e |
3.1 (1 to 8) | The evidence is very uncertain about the effect on care-home placement |
| Multifactorial-action and review (mfar) | OR 2.46 (0.25 to 23.86) Mixed estimate |
RR 2.43 (0.25 to 20.57) |
66 per 1000 (7 to 407) |
38 more per 1000 (21 fewer to 379 more) |
5 per 1000 (1 to 46) |
3 more per 1000 (1 fewer to 44 more) |
⊕⊝⊝⊝ Very lowb,c |
4.0 (1 to 7) | The evidence is very uncertain about the effect on care-home placement |
| ADL, nutrition and exercise (ADL & ntr & exrc) | OR 0.99 (0.34 to 2.87) Mixed estimate |
RR 0.99 (0.34 to 2.83) |
28 per 1000 (10 to 76) |
0 per 1000 (18 fewer to 48 more) |
2 per 1000 (1 to 6) |
0 per 1000 (1 fewer to 4 more) |
⊕⊝⊝⊝ Very lowb,c |
4.6 (1 to 8) | The evidence is very uncertain about the effect on care-home placement |
| Multifactorial-action and review with self-management strategies [mfar(w/slfm)] | OR 0.18 (0.01 to 3.75) Mixed estimate |
RR 0.18 (0.01 to 3.68) |
5 per 1000 (0 to 98) |
23 fewer per 1000 (28 fewer to 70 more) |
0 per 1000 (0 to 7) |
2 fewer per 1000 (2 fewer to 5 more) |
⊕⊝⊝⊝ Very lowe,f |
4.6 (2 to 7) | The evidence is very uncertain about the effect on care-home placement |
| Education, multifactorial-action and review with medication-review and self-management strategies [educ & mfar(w/med + slfm)] | OR 0.33 (0.01 to 8.21) Mixed estimate |
RR 0.33 (0.01 to 7.82) |
9 per 1000 (0 to 191) |
19 fewer per 1000 (28 fewer to 163 more) |
1 per 1000 (0 to 16) |
1 fewer per 1000 (2 fewer to 14 more) |
⊕⊝⊝⊝ Very lowb,c |
6.2 (2 to 8) | The evidence is very uncertain about the effect on care-home placement |
| ADL, aids, education, exercise, multifactorial-action and review with medication-review and self-management strategies ADL & aids & educ & exrc & mfar(w/med + slfm)] | OR 0.99 (0.02 to 50.04) Mixed estimate |
RR 0.99 (0.02 to 37.25) |
28 per 1000 (1 to 590) |
0 per 1000 (27 fewer to 562 more) |
2 per 1000 (0 to 91) |
0 per 1000 (2 fewer to 89 more) |
⊕⊝⊝⊝ Very lowg |
6.6 (2 to 9) | The evidence is very uncertain about the effect on care-home placement |
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: Care-home placement Time frame: Medium term; range of follow-up 12–18 months Setting: Community Total studies: 20 Total participants: 16,055 Comparator rank: Mean 9.2, 95% CI 6 to 12 |
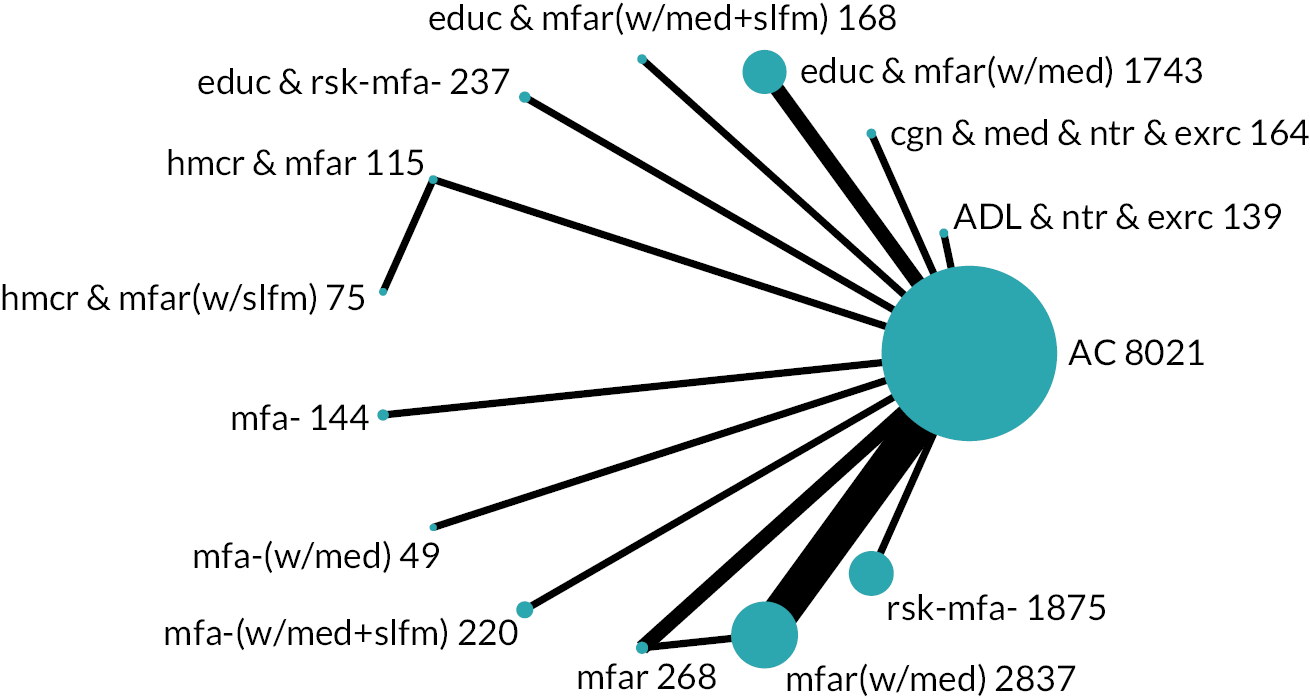
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Relative effect (95% CI) | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | ||||
| Network estimate | Calculated RRa | High-risk population (50 per 1000 with ac) |
Low-risk population (1 per 1000 with ac)b |
||||||
| With intervention | Difference | With intervention | Difference | ||||||
| Homecare, multifactorial-action and review with self-management strategies [hmcr & mfar(w/slfm)] | OR 0.07 (0.01 to 0.53) Indirect estimate |
RR 0.07 (0.01 to 0.53) |
4 per 1000 (0 to 27) |
46 fewer per 1000 (50 fewer to 23 fewer) |
0 per 1000 (0 to 1) |
1 fewer per 1000 (1 fewer to 0) |
⊕⊝⊝⊝ Very lowc,d |
1.6 (1 to 5) | The evidence is very uncertain about the effect on care-home placement |
| Homecare, multifactorial-action and review (hmcr & mfar) | OR 0.18 (0.04 to 0.78) Mixed estimate |
RR 0.18 (0.04 to 0.78) |
9 per 1000 (2 to 39) |
41 fewer per 1000 (48 fewer to 11 fewer) |
0 per 1000 (0 to 1) |
1 fewer per 1000 (1 fewer to 0) |
⊕⊝⊝⊝ Very lowe,f |
2.9 (1 to 7) | The evidence is very uncertain about the effect on care-home placement |
| Multifactorial-action (mfa-) | OR 0.32 (0.02 to 6.48) Mixed estimate |
RR 0.32 (0.02 to 5.81) |
17 per 1000 (1 to 254) |
33 fewer per 1000 (49 fewer to 204 more) |
0 per 1000 (0 to 6) |
1 fewer per 1000 (1 fewer to 5 more) |
⊕⊝⊝⊝ Very lowg,h |
5.3 (1 to 14) | The evidence is very uncertain about the effect on care-home placement |
| Multifactorial-action and review (mfar) | OR 0.53 (0.20 to 1.39) Mixed estimate |
RR 0.53 (0.20 to 1.38) |
27 per 1000 (10 to 68) |
23 fewer per 1000 (40 fewer to 18 more) |
1 per 1000 (0 to 1) |
0 per 1000 (1 fewer to 0) |
⊕⊝⊝⊝ Very lowg,h |
5.7 (2 to 12) | The evidence is very uncertain about the effect on care-home placement |
| Education and risk-screening (educ & rsk-mfa-) | OR 0.59 (0.13 to 2.72) Mixed estimate |
RR 0.60 (0.13 to 2.63) |
30 per 1000 (7 to 125) |
20 fewer per 1000 (43 fewer to 75 more) |
1 per 1000 (0 to 3) |
0 per 1000 (1 fewer to 2 more) |
⊕⊝⊝⊝ Very lowg,h |
6.6 (2 to 14) | The evidence is very uncertain about the effect on care-home placement |
| Cognitive training, medication-review, nutrition and exercise (cgn & med & ntr & exrc) | OR 0.65 (0.10 to 4.17) Mixed estimate |
RR 0.65 (0.10 to 3.91) |
33 per 1000 (5 to 180) |
17 fewer per 1000 (45 fewer to 130 more) |
1 per 1000 (0 to 4) |
0 per 1000 (1 fewer to 3 more) |
⊕⊝⊝⊝ Very lowg,h |
7.2 (1 to 14) | The evidence is very uncertain about the effect on care-home placement |
| Multifactorial-action and review with medication-review [mfar(w/med)] | OR 0.81 (0.42 to 1.57) Mixed estimate |
RR 0.81 (0.42 to 1.55) |
41 per 1000 (22 to 76) |
9 fewer per 1000 (28 fewer to 26 more) |
1 per 1000 (0 to 2) |
0 per 1000 (1 fewer to 1 more) |
⊕⊝⊝⊝ Very lowg,h |
7.7 (4 to 12) | The evidence is very uncertain about the effect on care-home placement |
| Multifactorial-action with medication-review [mfa-(w/med)] | OR 0.91 (0.12 to 7.18) Mixed estimate |
RR 0.92 (0.12 to 6.35) |
46 per 1000 (6 to 274) |
4 fewer per 1000 (44 fewer to 224 more) |
1 per 1000 (0 to 7) |
0 per 1000 (1 fewer to 6 more) |
⊕⊝⊝⊝ Very lowg,h |
8.3 (2 to 14) | The evidence is very uncertain about the effect on care-home placement |
| ADL, nutrition and exercise (ADL & ntr & exrc) | OR 0.96 (0.13 to 7.29) Mixed estimate |
RR 0.96 (0.13 to 6.44) |
48 per 1000 (7 to 277) |
2 fewer per 1000 (43 fewer to 227 more) |
1 per 1000 (0 to 7) |
0 per 1000 (1 fewer to 6 more) |
⊕⊝⊝⊝ Very lowg,h |
8.6 (2 to 14) | The evidence is very uncertain about the effect on care-home placement |
| Multifactorial-action with medication-review and self-management strategies [mfa-(w/med + slfm)] | OR 1.01 (0.40 to 2.58) Mixed estimate |
RR 1.01 (0.40 to 2.49) |
51 per 1000 (21 to 119) |
1 more per 1000 (29 fewer to 69 more) |
1 per 1000 (0 to 3) |
0 per 1000 (1 fewer to 2 more) |
⊕⊝⊝⊝ Very lowi,j |
9.3 (4 to 14) | The evidence is very uncertain about the effect on care-home placement |
| Risk-screening (rsk-mfa-) | OR 1.15 (0.62 to 2.13) Mixed estimate |
RR 1.14 (0.62 to 2.08) |
57 per 1000 (31 to 101) |
7 more per 1000 (19 fewer to 51 more) |
1 per 1000 (1 to 2) |
0 per 1000 (0 to 1 more) |
⊕⊝⊝⊝ Very lowg,h |
10.0 (6 to 14) | The evidence is very uncertain about the effect on care-home placement |
| Education, multifactorial-action and review with medication-review [educ & mfar(w/med)] | OR 1.23 (0.61 to 2.49) Mixed estimate |
RR 1.22 (0.61 to 2.41) |
61 per 1000 (31 to 116) |
11 more per 1000 (19 fewer to 66 more) |
1 per 1000 (1 to 2) |
0 per 1000 (0 to 1 more) |
⊕⊝⊝⊝ Very lowg,h |
10.4 (5 to 14) | The evidence is very uncertain about the effect on care-home placement |
| Education, multifactorial-action and review with medication-review and self-management strategies [educ & mfar(w/med + slfm)] | OR 2.19 (0.39 to 12.29) Mixed estimate |
RR 2.14 (0.40 to 9.93) |
103 per 1000 (20 to 393) |
53 more per 1000 (30 fewer to 343 more) |
2 per 1000 (0 to 12) |
1 more per 1000 (1 fewer to 11 more) |
⊕⊝⊝⊝ Very lowg,h |
12.1 (4 to 14) | The evidence is very uncertain about the effect on care-home placement |
| Population: Older people Interventions: Community-based complex interventions Comparator: Homecare (hmcr) Outcome: Care-home placement Time frame: Medium term; range of follow-up 12–13 months Setting: Community Total studies: 4 Total participants: 1567 Comparator rank: Mean 2.9, 95% CI 1 to 5 |
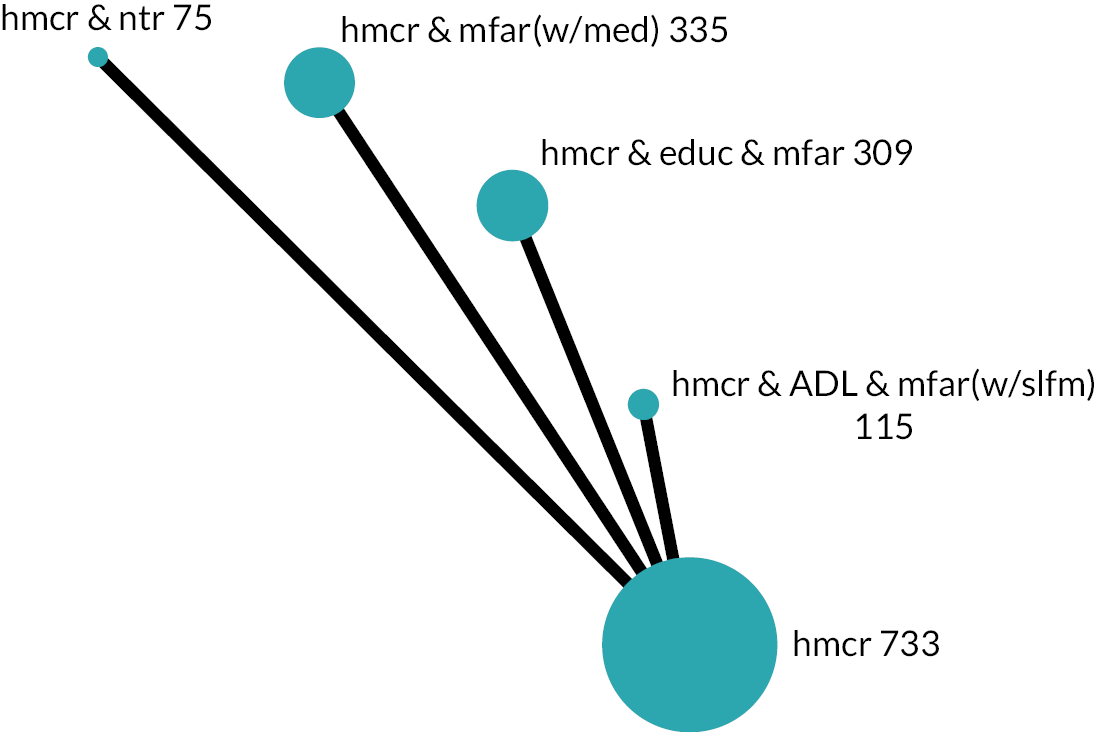
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention group | Relative effect (95% CI) | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | ||||
| Network estimate | Calculated RRa | High-risk population (182 per 1000 with hmcr) |
Low-risk population (85 per 1000 with hmcr) |
||||||
| With intervention | Difference | With intervention | Difference | ||||||
| Homecare, education, multifactorial-action and review (hmcr & educ & mfar) | OR 0.86 (0.55 to 1.35) Mixed estimate |
RR 0.88 (0.59 to 1.29) |
161 per 1000 (110 to 231) |
21 fewer per 1000 (72 fewer to 49 more) |
74 per 1000 (49 to 111) |
11 fewer per 1000 (36 fewer to 26 more) |
⊕⊝⊝⊝ Very lowb,c |
2.2 (1 to 5) |
The evidence is very uncertain about the effect on care-home placement |
| Homecare, ADL, multifactorial-action and review with self-management strategies [hmcr & ADL & mfar(w/slfm)] | OR 0.91 (0.35 to 2.33) Mixed estimate |
RR 0.92 (0.39 to 1.97) |
168 per 1000 (73 to 341) |
14 fewer per 1000 (109 fewer to 159 more) |
78 per 1000 (32 to 178) |
7 fewer per 1000 (53 fewer to 93 more) |
⊕⊝⊝⊝ Very lowd,e |
2.6 (1 to 5) |
The evidence is very uncertain about the effect on care-home placement |
| Homecare, multifactorial-action and review with medication-review [hmcr & mfar(w/med)] | OR 1.12 (0.75 to 1.70) Mixed estimate |
RR 1.11 (0.77 to 1.55) |
200 per 1000 (142 to 274) |
18 more per 1000 (40 fewer to 92 more) |
95 per 1000 (65 to 136) |
10 more per 1000 (20 fewer to 51 more) |
⊕⊝⊝⊝ Very lowd,e |
3.6 (1 to 5) |
The evidence is very uncertain about the effect on care-home placement |
| Homecare and nutrition (hmcr & ntr) | OR 1.37 (0.48 to 3.98) Mixed estimate |
RR 1.31 (0.51 to 2.83) |
234 per 1000 (96 to 470) |
52 more per 1000 (86 fewer to 288 more) |
113 per 1000 (42 to 270) |
28 more per 1000 (43 fewer to 185 more) |
⊕⊝⊝⊝ Very lowd,e |
3.8 (1 to 5) |
The evidence is very uncertain about the effect on care-home placement |
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: Self-reported health (single question) Time frame: Medium term; range of follow-up 12–18 months Setting: Community Total studies: 8 Total participants: 2631 Comparator rank: Mean 4.4, 95% CI 2 to 6 |
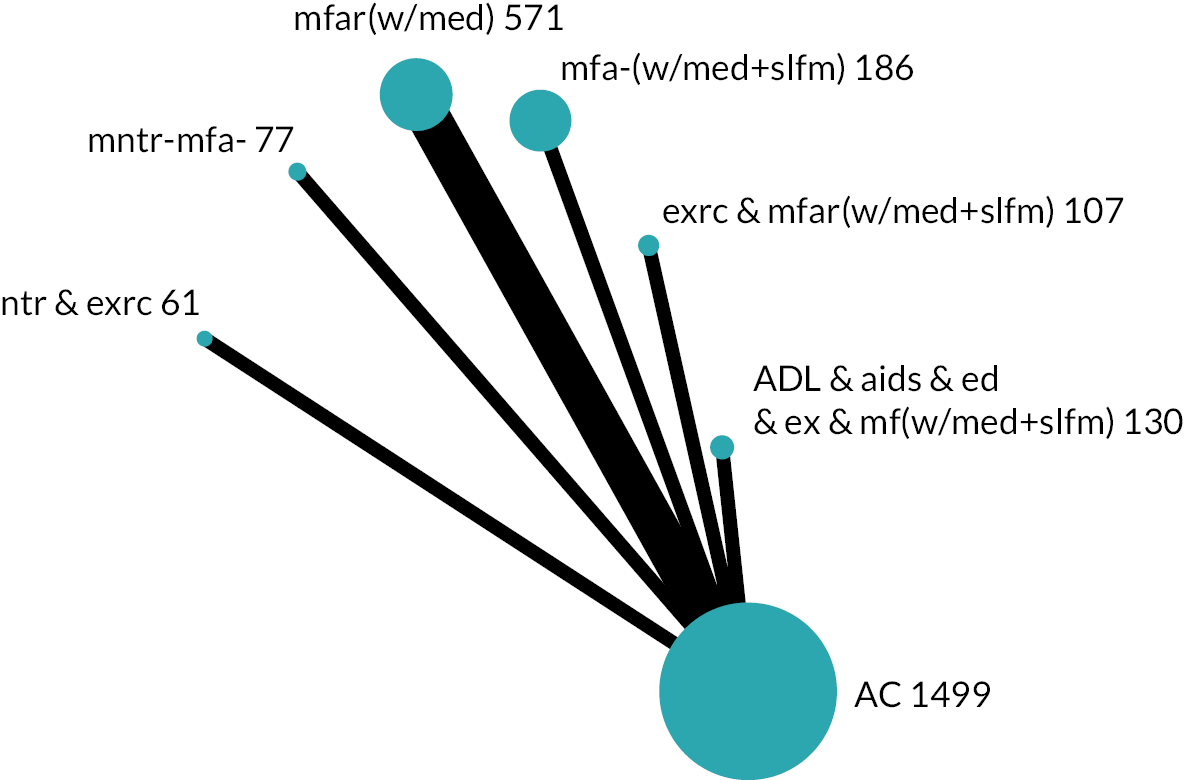
|
||||
|---|---|---|---|---|---|
| Intervention group | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | |
| SMD | MD (EQ-VAS, 0 to 100)a | ||||
| Exercise, multifactorial-action and review with medication-review and self-management strategies [exrc & mfar(w/med + slfm)] | SMD 0.01 lower (0.34 lower to 0.32 higher) Mixed estimate |
MD 0.20 lower (6.94 lower to 6.53 higher) |
⊕⊕⊝⊝ Lowb |
4.3 (1 to 7) | May result in little to no difference in self-reported health |
| Multifactorial-action and review with medication-review [mfar(w/med)] | SMD 0.11 higher (0.06 lower to 0.28 higher) Mixed estimate |
MD 2.24 higher (1.22 lower to 5.71 higher) |
⊕⊝⊝⊝ Very lowb,c |
2.6 (1 to 6) | The evidence is very uncertain about the effect on self-reported health |
| Multifactorial-action with medication-review and self-management strategies [mfa-(w/med + slfm)] | SMD 0.07 higher (0.18 lower to 0.32 higher) Mixed estimate |
MD 1.43 higher (3.67 lower to 6.53 higher) |
⊕⊝⊝⊝ Very lowd,e |
3.2 (1 to 7) | The evidence is very uncertain about the effect on self-reported health |
| Nutrition and exercise (ntr & exrc) | SMD 0.07 higher (0.33 lower to 0.46 higher) Mixed estimate |
MD 1.43 higher (6.73 lower to 9.38 higher) |
⊕⊝⊝⊝ Very lowb,c |
3.4 (1 to 7) | The evidence is very uncertain about the effect on self-reported health |
| ADL, aids, education, exercise, multifactorial-action and review with medication-review and self-management strategies [ADL & aids & ed & ex & mf(w/med + slfm)] | SMD 0.06 lower (0.38 lower to 0.25 higher) Mixed estimate |
MD 1.22 lower (7.75 lower to 5.10 higher) |
⊕⊝⊝⊝ Very lowb,c |
4.9 (1 to 7) | The evidence is very uncertain about the effect on self-reported health |
| Monitoring (mntr-mfa-) | SMD 0.11 lower (0.47 lower to 0.26 higher) Mixed estimate |
MD 2.24 lower (9.59 lower to 5.30 higher) |
⊕⊝⊝⊝ Very lowb,c |
5.3 (1 to 7) | The evidence is very uncertain about the effect on self-reported health |
| Population: Older people Interventions: Community-based complex interventions Comparator: Available care (ac) Outcome: Depression Time frame: Medium term; range of follow-up 44 weeks to 18 months Setting: Community Total studies: 15 Total participants: 7245 Comparator rank: Mean 7.6, 95% CI 5 to 11 |
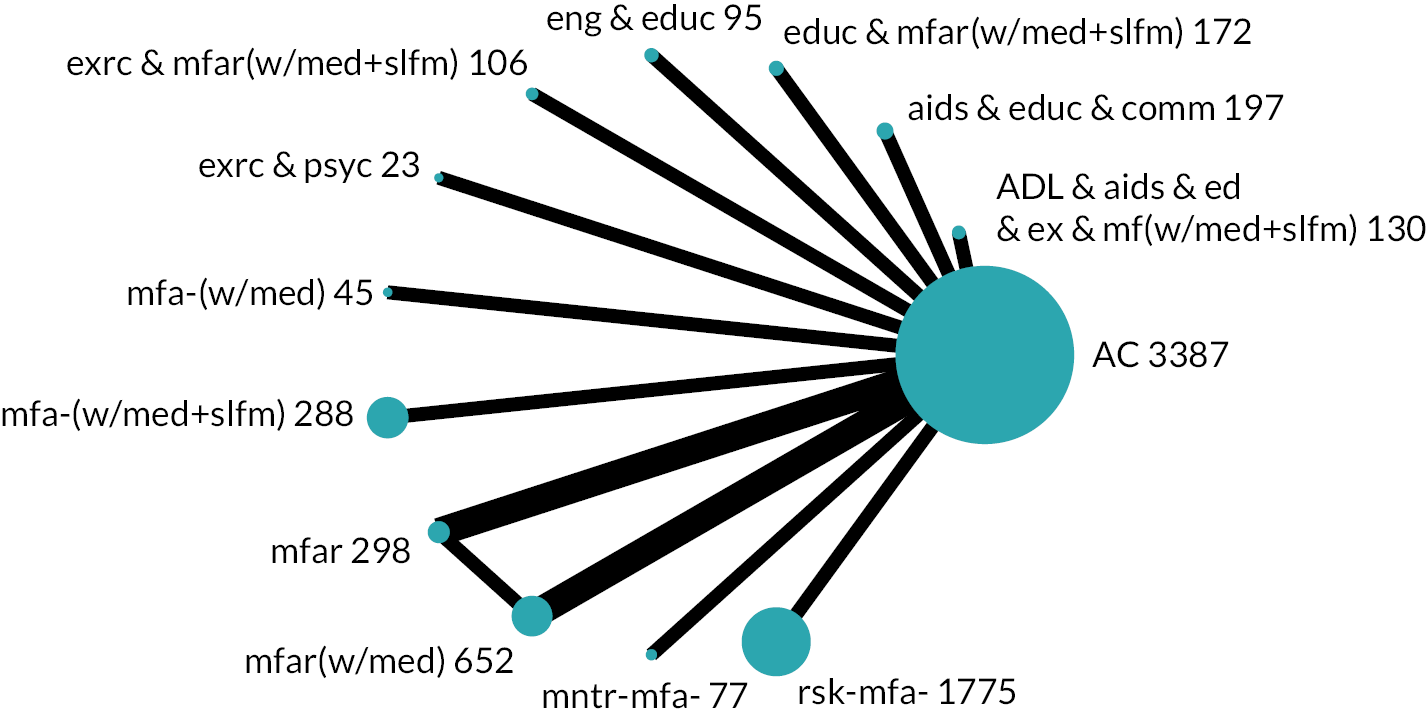
|
||||
|---|---|---|---|---|---|
| Intervention group | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | |
| SMD | MD (GDS 15)a | ||||
| Exercise, multifactorial-action and review with medication-review and self-management strategies [exrc & mfar(w/med + slfm)] | SMD 0.11 lower (0.45 lower to 0.23 higher) Mixed estimate |
MD 0.35 lower (1.41 lower to 0.72 higher) |
⊕⊕⊝⊝ Lowb |
4.9 (1 to 12) | May result in a very slight reduction in symptoms of depression |
| Engagement in meaningful-activities and education (eng & educ) | SMD 0.13 lower (0.46 lower to 0.19 higher) Mixed estimate |
MD 0.42 lower (1.44 lower to 0.59 higher) |
⊕⊝⊝⊝ Very lowc,d |
4.7 (1 to 12) | The evidence is very uncertain about the effect on symptoms of depression |
| Risk-screening (rsk-mfa-) | SMD 0.09 lower (0.31 lower to 0.14 higher) Mixed estimate |
MD 0.28 lower (0.98 lower to 0.43 higher) |
⊕⊝⊝⊝ Very lowd,e |
5.3 (1 to 11) | The evidence is very uncertain about the effect on symptoms of depression |
| Multifactorial-action and review with medication-review [mfar(w/med)] | SMD 0.07 lower (0.25 lower to 0.12 higher) Mixed estimate |
MD 0.21 lower (0.80 lower to 0.37 higher) |
⊕⊝⊝⊝ Very lowb,f |
5.6 (1 to 11) | The evidence is very uncertain about the effect on symptoms of depression |
| Aids, education and telecoms (aids & educ & comm) | SMD 0.05 lower (0.33 lower to 0.24 higher) Mixed estimate |
MD 0.15 lower (1.05 lower to 0.76 higher) |
⊕⊝⊝⊝ Very lowb,f |
6.2 (1 to 13) | The evidence is very uncertain about the effect on symptoms of depression |
| Exercise and psychology (exrc & psyc) | SMD 0.06 lower (0.59 lower to 0.47 higher) Mixed estimate |
MD 0.20 lower (1.87 lower to 1.47 higher) |
⊕⊝⊝⊝ Very lowd,g |
6.2 (1 to 13) | The evidence is very uncertain about the effect on symptoms of depression |
| ADL, aids, education, exercise, multifactorial-action and review with medication-review and self-management strategies [ADL & aids & ed & ex & mf(w/med + slfm)] | SMD 0.01 lower (0.33 lower to 0.31 higher) Mixed estimate |
MD 0.03 lower (1.03 lower to 0.97 higher) |
⊕⊝⊝⊝ Very lowb,f |
7.2 (1 to 13) | The evidence is very uncertain about the effect on symptoms of depression |
| Monitoring (mntr-mfa-) | SMD 0.00 (0.37 lower to 0.37 higher) Mixed estimate |
MD 0.00 (1.16 lower to 1.16 higher) |
⊕⊝⊝⊝ Very lowb,f |
7.3 (1 to 13) | The evidence is very uncertain about the effect on symptoms of depression |
| Multifactorial-action with medication-review and self-management strategies [mfa-(w/med + slfm)] | SMD 0.00 (0.24 lower to 0.24 higher) Mixed estimate |
MD 0.00 (0.77 lower to 0.77 higher) |
⊕⊝⊝⊝ Very lowd,h |
7.5 (2 to 13) | The evidence is very uncertain about the effect on symptoms of depression |
| Multifactorial-action and review (mfar) | SMD 0.04 higher (0.20 lower to 0.28 higher) Mixed estimate |
MD 0.13 higher (0.63 lower to 0.88 higher) |
⊕⊝⊝⊝ Very lowb,f |
8.6 (2 to 13) | The evidence is very uncertain about the effect on symptoms of depression |
| Multifactorial-action with medication-review [mfa-(w/med)] | SMD 0.11 higher (0.35 lower to 0.58 higher) Mixed estimate |
MD 0.36 higher (1.10 lower to 1.82 higher) |
⊕⊝⊝⊝ Very lowb,f |
9.3 (1 to 13) | The evidence is very uncertain about the effect on symptoms of depression |
| Education, multifactorial-action and review with medication-review and self-management strategies [educ & mfar(w/med + slfm)] | SMD 0.17 higher (0.13 lower to 0.47 higher) Mixed estimate |
MD 0.53 higher (0.42 lower to 1.48 higher) |
⊕⊝⊝⊝ Very lowb,f |
10.6 (3 to 13) | The evidence is very uncertain about the effect on symptoms of depression |
| Population: Older people Interventions: Community-based complex interventions Comparator: Homecare (hmcr) Outcome: Depression Time frame: Medium term; follow-up at 12 months Setting: Community Total studies: 6 Total participants: 996 Comparator rank: Mean 5.2, 95% CI 3 to 7 |
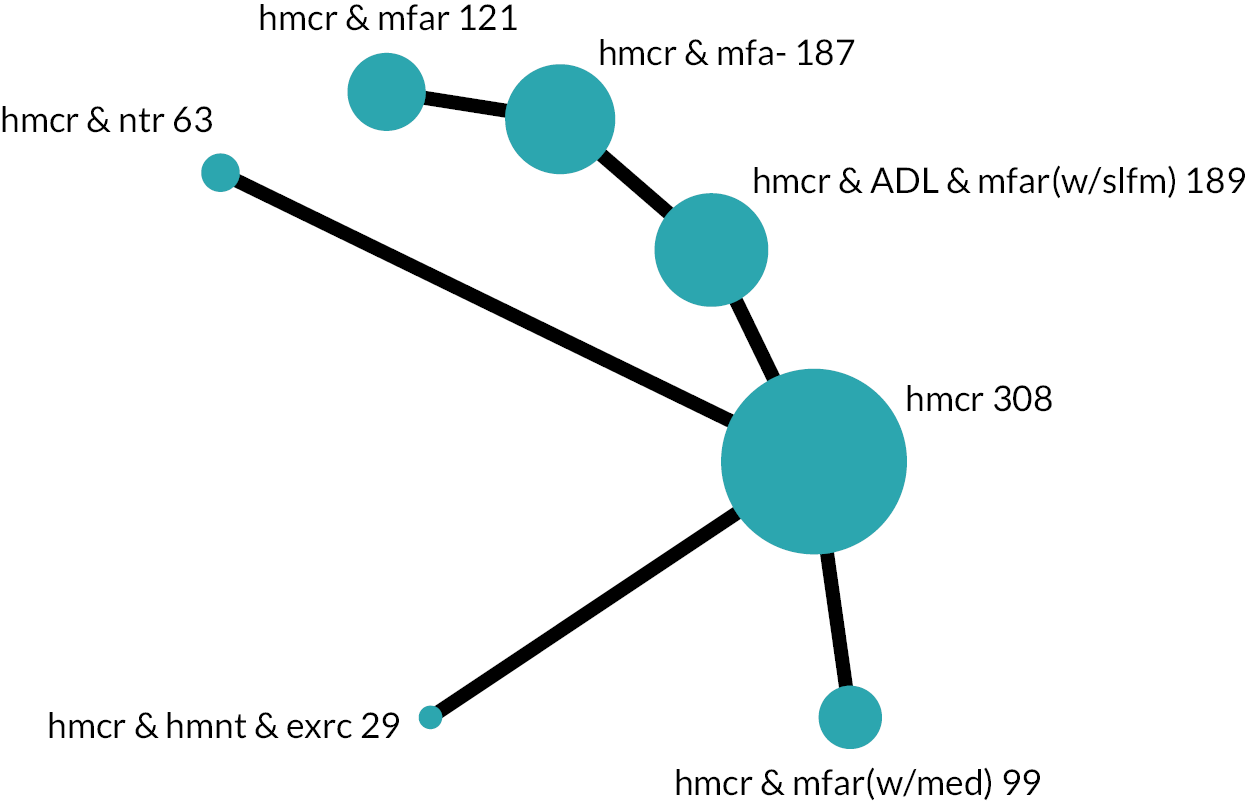
|
||||
|---|---|---|---|---|---|
| Intervention group | Anticipated absolute effect (95% CI) | Certainty of the evidence (GRADE) | Ranking (95% CI) | Interpretation | |
| SMD | MD (GDS 15)a | ||||
| Homecare, multifactorial-action and review with medication-review [hmcr & mfar(w/med)] | SMD 0.38 lower (0.66 lower to 0.10 lower) Mixed estimate |
MD 1.20 lower (2.08 lower to 0.31 lower) |
⊕⊕⊝⊝ Lowb,c |
1.7 (1 to 4) | May result in a slight reduction in symptoms of depression |
| Homecare and nutrition (hmcr & ntr) | SMD 0.24 lower (0.62 lower to 0.14 higher) Mixed estimate |
MD 0.76 lower (1.95 lower to 0.43 higher) |
⊕⊝⊝⊝ Very lowb,d |
2.8 (1 to 7) | The evidence is very uncertain about the effect on symptoms of depression |
| Homecare, ADL, multifactorial-action and review with self-management strategies [hmcr & ADL & mfar(w/slfm)] | SMD 0.09 lower (0.33 lower to 0.16 higher) Mixed estimate |
MD 0.27 lower (1.03 lower to 0.49 higher) |
⊕⊝⊝⊝ Very lowe,f |
4.0 (2 to 7) | The evidence is very uncertain about the effect on symptoms of depression |
| Homecare and multifactorial-action (hmcr & mfa-) | SMD 0.09 lower (0.53 lower to 0.35 higher) Indirect estimate |
MD 0.28 lower (1.67 lower to 1.11 higher) |
⊕⊝⊝⊝ Very lowd,g |
4.0 (1 to 6) | The evidence is very uncertain about the effect on symptoms of depression |
| Homecare, alternative-medicine and exercise (hmcr & hmnt & exrc) | SMD 0.06 lower (0.58 lower to 0.45 higher) Mixed estimate |
MD 0.20 lower (1.82 lower to 1.42 higher) |
⊕⊝⊝⊝ Very lowb,d |
4.4 (1 to 7) |
The evidence is very uncertain about the effect on symptoms of depression |
| Homecare, multifactorial-action and review (hmcr & mfar) | SMD 0.10 higher (0.40 lower to 0.61 higher) Indirect estimate |
MD 0.32 higher (1.27 lower to 1.92 higher) |
⊕⊝⊝⊝ Very lowd,h |
5.9 (2 to 7) | The evidence is very uncertain about the effect on symptoms of depression |
List of abbreviations
- ac
- available care
- ADL
- activities of daily living
- ASPIRE
- Assessment of Services Promoting Independence and Recovery in Elders
- CAPABLE
- Community Aging in Place – Advancing Better Living for Elders
- CI
- confidence interval
- CINeMA
- Confidence in Network Meta-Analysis
- cRCT
- cluster-randomised controlled trial
- FOG
- Frailty Oversight Group
- GRADE
- Grading of Recommendations Assessment, Development and Evaluation
- IADL
- instrumental activities of daily living
- IQR
- interquartile range
- MD
- mean difference
- NMA
- network meta-analysis
- NNTB
- number needed to treat to benefit
- OR
- odds ratio
- PADL
- personal activities of daily living
- PMG
- Project Management Group
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic reviews and Meta-Analyses
- PRO-AGE
- PRevention in Older people – Assessment in GEneralists’ practices
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REML
- restricted maximum likelihood
- RoB
- risk of bias
- RR
- risk ratio
- SD
- standard deviation
- SMD
- standardised mean difference
- SUCRA
- Surface Under the Cumulative RAnking curve
- TIDieR
- Template for Intervention Description and Replication
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/HNRP2514).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
