Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/01/20. The contractual start date was in January 2011. The draft report began editorial review in July 2013 and was accepted for publication in January 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Elaine McColl received grants from Newcastle University, fees and expenses from the NIHR Journals Library Editorial Board and expenses for meeting attendance from the National Institute for Health Research (NIHR) Programme Grants for Applied Research (PGfAR) panel during the course of the study. Luke Vale is a member of the NIHR PGfAR and the NIHR Health Technology Assessment (HTA) Clinical Evaluation and Trials Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Clarke et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Strabismus
Strabismus (sometimes referred to as ‘squint’) is a condition in which the eyes are misaligned, either constantly or intermittently. 1 Intermittent strabismus may progress to constant strabismus. Strabismus may have both socioeconomic and functional consequences for the affected individual. 2–4
The appearance of ocular misalignment may result in discrimination – in both interpersonal relations and employment – in adults,5 and in social exclusion and bullying in children. 6
The functional consequences of strabismus include impairment of three-dimensional (binocular) vision4 and amblyopia (lazy eye),7 which is impairment of visual acuity (VA) in one eye due to the effect, in this case, of ocular misalignment on visual development.
In 2011–12 there were 584,916 hospital appointments for children, between the ages of 0 and 9 years, in children’s eye outpatient departments, including orthoptic departments, in the UK (13% of total UK NHS outpatient appointments for this age group). 8 Ninety per cent of this workload is thought to relate to the management of strabismus and amblyopia. 9 In total, 6205 extraocular muscle surgeries were performed on children aged between 0 and 14 years during the same period.
The evidence base for the treatment of strabismus is poor, and this results in significant variation in the use of health-care resources, which is not easily explained and may not be clinically justified. 10
Intermittent exotropia
Intermittent exotropia [X(T)] is a common type of strabismus in which the eyes are intermittently in a divergent misalignment. 11,12
Intermittent exotropia is the commonest form of divergent strabismus in childhood13,14 and has been associated with later mental illness. 15 The usual age at onset of X(T) is between 12 and 24 months. 11,13 Three-dimensional vision for near viewing is usually within the age-related normal range but may deteriorate if the strabismus progresses from an intermittent to a constant misalignment. 16 X(T) is particularly common in Eastern Asia but is thought to be increasing in prevalence worldwide. 17
The NHS tariff rate for strabismus surgery is £800, and, together with around 100,000 clinic visits annually for review of patients with X(T) (at a tariff cost of £120 per new patient and £60 per review: estimated average new–review ratio of 1 : 8), the total cost to the NHS alone is almost £7.5M annually. With the inclusion of societal and family costs, the management of X(T) is costly.
The underlying cause of X(T) is unknown. The condition is diagnosed on the basis of a parental history of an intermittent ocular misalignment, which may be accompanied by closure of one eye, and on the demonstration of the potential of the eyes to adopt a divergent misalignment when binocular viewing is disrupted by covering one eye (cover test). 1
The frequency of the observed misalignment, or eye closure, and the ease with which the eyes realign following a cover test, is referred to as the control of the strabismus, and is used as clinical indicators of the severity of the condition. 18,19 Other measures of severity include the size of the ocular misalignment at near and distance viewing, and stereoacuity (a measure of three-dimensional vision). 20
Treatment is sought and recommended on the basis of concern about the appearance of the misalignment and the potential for disruption of normal visual development. 21
Treatment may be surgical (eye muscle surgery or botulinum toxin injection);22 non-surgical (glasses, patching, prisms, exercises);23 or a combination of the two. 24,25
Eye muscle surgery for X(T) aims to adjust the tension in the extraocular muscles such that the eyes are placed in a less divergent alignment. This can be achieved by weakening one or both lateral rectus muscles, either alone, or in combination with tightening of one medial rectus muscle.
Around 10–20% of children develop an intermittent or constant convergent strabismus following surgery for X(T), with some requiring further corrective procedures. 20,25,26
There are few data on the efficacy of non-surgical treatments for X(T) but, in general, they appear to be significantly less effective than eye muscle surgery. 24
A recently updated Cochrane review,27 specifically addressing the treatment of X(T), identified only one trial that was eligible for inclusion. This trial showed that unilateral surgery was more effective than bilateral surgery for correcting the basic type of X(T). No trials were identified comparing eye muscle surgery with watchful waiting or active monitoring.
The authors of the review concluded:
The available literature consists mainly of retrospective case reviews, which are difficult to reliably interpret and analyse. The one randomised trial included found unilateral surgery more effective than bilateral surgery for basic intermittent exotropia. However, across all identified studies, measures of severity and thus criteria for intervention are poorly validated, and there appear to be no reliable natural history data. There is therefore a pressing need for improved measures of severity, a better understanding of the natural history and carefully planned clinical trials of treatment to improve the evidence base for the management of this condition.
Another recent paper28 has commented:
To address controversies and improve the evidence base regarding surgical intervention of this condition, randomized controlled trials are needed and justified because the results indicate that it would be relatively safe to randomly allocate patients to groups who could receive differing treatments so as to determine optimum management strategies.
Evidence from randomised controlled trials of the treatment of X(T) is increasing. A randomised controlled trial assessing the relative benefits of different forms of eye muscle surgery is in progress29 and a trial comparing patching (occlusion) to observation has just been published. 30 Although the latter study provides some natural history data neither study addresses the utility of surgical treatment of X(T) per se.
The feasibility of randomised controlled trials (RCTs) of strabismus treatment has been questioned.
A Health Technology Assessment (HTA)-sponsored systematic review of the clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years31 commented, ‘RCTs into the efficacy, effectiveness and efficiency of strabismus treatment are unlikely to be feasible. Ethical considerations in study design prevent complete abstention of treatment, and decisions regarding treatment are often overridden by clinical need’.
We disagree with this statement, and do not see how the subject can make progress in establishing a robust evidence base without such studies.
A previous trial of deferred surgery for a different form of strabismus,32,33 infantile esotropia, demonstrates the potential for recruitment into RCTs of strabismus while highlighting some of the challenges that are inherent in this type of research. 34
We therefore proposed a study to determine the feasibility of a RCT of surgery compared with active monitoring for X(T).
Objectives
The specific objectives of the SamExo study (Surgery vs. Active Monitoring in Intermittent Exotropia), as stated in the original project description, were to:
-
determine whether or not participating centres are likely to recruit a sufficient number of patients to deliver the trial
-
determine whether or not recruited patients will stay within their allocated groups and complete follow-up in sufficient numbers to deliver the trial
-
develop a web-based trial management system to centralise and automate trial processes such as invitation, logging of replies, scheduling of appointments, confirmation of eligibility, randomisation and printing of letters
-
pilot the procedures involved in the trial, including recruitment (giving information and obtaining consent), randomisation, intervention (surgery), masking, and baseline and follow-up data collection
-
monitor potential bias by comparing the demographic and clinical status of patients retained with any withdrawing from the trial, and by comparing those who consent with those eligible but refusing to participate
-
identify through questionnaires and qualitative interviews, where possible, reasons why parents decline permission to participate
-
prepare a detailed protocol and application for funding for such a RCT (if findings from the pilot study indicate that a full-scale RCT is feasible).
Systematic review
The opportunity to apply for further funding for an associated project was offered once the trial was under way. Given the difficulty in synthesising the literature on X(T), and the lack of RCT data highlighted in the Cochrane review, a systematic review of non-randomised studies was proposed and funded, and the output of this work is included in this report.
Chapter 2 Systematic review
Background
Intermittent exotropia [X(T)] is a common form of childhood strabismus (squint) affecting approximately two out of every 100 children before the age of 3 years. 35 This particular ocular misalignment is characterised by an outwards deviation of the eye, which is not constant but is usually present initially on distance fixation or when the child is tired. 20 The natural history of X(T) is poorly understood: the ocular misalignment may worsen or deteriorate into constant exotropia, which adversely affects stereo vision; conversely the misalignment may resolve over time. 36 X(T) is also of concern for psychosocial reasons, as the cosmetic appearance might cause the child to develop social or psychological problems,2 which can impact into adult life, with effects on self-image, work and personal relationships. 37
A range of both conservative and surgical treatment options are available and include observation (watchful waiting), orthoptic exercises/vision therapy, occlusion therapy (patching), minus lens therapy (glasses) and surgery. 38 However, surgery is associated with possible adverse effects, including a risk of overcorrection, which may also adversely impact on stereoacuity. Evidence for the comparative effectiveness of treatment options is limited by the absence of RCT data,27 but there is a much larger literature of observational studies for the various interventions. As a consequence of the absence of robust and reliable effectiveness data on treatment options, and of uncertainty about the natural history of the condition, wide service variation exists (both nationally and internationally) in management of the condition.
Aim
The main aim of the review was to determine the effectiveness of surgical and non-surgical approaches as a means for managing X(T) in childhood. Secondary objectives were to (1) understand the circumstances under which particular interventions are most effective; (2) determine adverse effects associated with particular interventions; and (3) better understand the natural history of X(T).
Methods
The review was conducted following best practice guidelines for the design, conduct and reporting of systematic reviews. 39–41
Criteria for considering studies for this review
Participants
Studies that involved child participants aged up to 18 years were included. Studies including mixed populations (i.e. both adults and children) were eligible if they reported the results for children separately. To satisfy our inclusion criteria, the diagnosis was one of intermittent disease, rather than constant exotropia, and the type was either divergence excess or basic-type exotropia. Convergence insufficiency-type exotropia (misalignment primarily at near fixation) was excluded.
Interventions
A range of both surgical and non-surgical interventions were examined. As well as corrective surgery, we reviewed studies reporting on non-surgical interventions, including minus lenses, prisms, convergence exercises, occlusion therapy, onabotulinumtoxinA (BOTOX®, Allergan) injections and watchful waiting (Box 1). We included studies that made a single comparison (e.g. unilateral vs. bilateral surgery) as well as studies reporting on the effectiveness of multiple therapies (e.g. surgery vs. BOTOX, vs. minus lenses vs. watchful waiting).
Surgery aims to prevent deterioration to constant exotropia, improve distance stereoacuity and improve appearance. 20 The following procedures are commonly offered:
Bilateral lateral rectus recessionThis surgical technique involves weakening of the lateral rectus muscles that control eye movement. Bilateral surgery is undertaken on both eyes, whereas unilateral lateral rectus recession involves one eye only.
Recession/resectionHorizontal rectus surgery (unilateral lateral rectus recession combined with medial rectus resection) is undertaken on the dominant or non-dominant eye to shift the muscular insertion points and alter the balance of forces on the globe. 42
Non-surgical interventions Botulinum toxinThe neurotoxin is used both diagnostically and therapeutically in the management of strabismus. Therapeutically it is used to temporarily paralyse the lateral rectus muscle leading to altered ocular alignment, which returns over time. 43,44
Overminus lensesOvercorrecting minus lenses aim to stimulate convergence of the eyes through the extra effort required to focus. 23
OcclusionOcclusion or patching aims to prevent development of abnormal adaptation to eyes being diverged. 42
Orthoptic exercisesThe objective of orthoptic exercises is to increase fusion, eliminate suppression and improve control in order to reduce the time during which the deviation is manifest. 45
ObservationGiven that the natural history of X(T) is unclear, observation or watchful waiting is another conservative option for management of X(T), particularly in cases of small-angle X(T). 42
Study designs
Given the paucity of high-level evidence in this area, we included RCTs, quasiexperimental studies and comparative observational studies (both prospective and retrospective studies, each with a comparator group). [Based on the Cochrane definition of cohort study as ‘A non-randomised (observational) study in which a defined group of people (the cohort) is followed over time. The outcomes of people in subsets of this cohort who received two or more different interventions are then compared’. This includes ‘routine database followed over time’ prospectively or retrospectively (source: http://bmg.cochrane.org/research-projectscochrane-risk-bias-tool). Accessed 17 December 2014] Case series (defined as chart reviews without a comparison group), qualitative studies and non-empirical, opinion pieces were excluded. Only studies with a follow-up period of at least 6 months were included.
Outcomes
Data on the following outcomes were extracted: angle of deviation, stereoacuity and control. We also sought to record quality of life (QoL) and patient-derived outcomes (e.g. acceptability and adherence), where possible, as well as data on adverse effects. The eligibility criteria used in the review are shown in Box 2.
Participants up to and including the age of 18 years. Where there was a mixed population of adults and children, a study was eligible only if it reported outcomes for children separately.
Divergence excess type [where the deviation is greater (by at least 10 PD at distance than at near], simulated divergence excess type [the deviation is initially greater at distance but after occlusion there is little difference between near and distance measurements (within 10 PD)] or basic type (the deviation is the same at both distance and near) X(T).
Follow-up for at least 6 months.
RCT, quasiexperimental or cohort study with a comparison group.
Exclusion criteriaPopulation aged > 18 years (or data not reported for children separately from adults).
Data unavailable for X(T) separately. In instances when the population included X(T) combined with constant exotropia (or other forms of strabismus), a study was eligible only if disaggregated data were reported for X(T).
Participants with convergence insufficiency type X(T) [the deviation is greater (by at least 10 PD) at near than at distance]. Studies were excluded if they reported outcomes for divergence excess and basic type combined with convergence insufficiency.
Studies with follow-up of < 6 months.
PD, prism dioptres.
Search strategy
We conducted systematic searches using the following databases (abbreviations, host sites and dates searched given in parentheses):
-
MEDLINE (Ovid, 1946 to October week 3 2012)
-
EMBASE (Ovid, 1980 to October week 42 2012)
-
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library via Wiley, issue 7 of 12 August 2012)
-
UK Clinical Research Network Study Portfolio (UKCRN, August 2012)
-
Cochrane Database of Systematic Reviews (CDSR, The Cochrane Library via Wiley, issue 7 of 12 August 2012)
-
Database of Abstracts of Reviews of Effects (DARE, The Cochrane Library via Wiley, issue 7 of 12 August 2012)
-
Health Technology Assessment Database (HTA, The Cochrane Library via Wiley, issue 7 of 12 August 2012)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL, Ovid, 1981 to August 2012)
-
PsycINFO (Ovid, 1967 to August week 2 2012)
-
Science Citation Index (Web of Knowledge, 1970 to August 2012)
-
Conference Proceedings Citation Index (Web of Knowledge, 1990 to August 2012)
-
Latin American and Caribbean Literature on Health Sciences (LILACS, Virtual Health Library; 1982 to September 2012).
Initially a search was conducted which combined synonyms for exotropia with synonyms for under-18-year-olds. Subsequently, we decided to remove the age-related part of the search to capture studies in paediatric journals, which are less likely to specify in the title and abstract that they concern children, and the search was rerun in those databases in which age limits had been used. The search strategy was designed on MEDLINE (Ovid) and translated to other databases. Database-specific thesaurus terms [such as medical subject headings (MeSH)] were used as appropriate for each database. For an example search strategy designed for MEDLINE please see Appendix 1. We supplemented the electronic searches by hand-searching the bibliographies of all included studies for any additional related references, as well as searching the following key organisational websites:
-
Association for Research in Vision and Ophthalmology: www.arvo.org/ – searched meeting abstracts
-
American Association for Pediatric Ophthalmology and Strabismus: www.aapos.org/
-
Royal College of Ophthalmologists: www.rcophth.ac.uk/ – searched 1st & 2nd World Congress of Paediatric Ophthalmology & Strabismus
-
European Paediatric Ophthalmological Society: www.epos-focus.org/ – searched meetings
-
European Strabismological Association: www.esa-strabismology.com/
-
American Society of Certified Orthoptists: www.orthoptics.org/ – searched American Orthoptics Journal
-
American Academy of Ophthalmology: www.aao.org/
-
British Orthoptic Society: www.orthoptics.org.uk/.
We contacted key experts in the field for information about unpublished or in-progress studies and used relevant e-mail lists to issue a request for information about unpublished or ongoing studies that fit the eligibility criteria. We also manually searched the table of contents of key journals (including Journal of Vision; Investigative Ophthalmology and Visual Science) for the past 12 months to identify any papers not yet indexed. For two journals (British Orthoptic Journal and Australian Orthoptic Journal) that are not indexed on PubMed, we hand-searched the table of contents for the past 5 years.
References were managed using EndNote reference management software, version X6 (Thomson Reuters, CA, USA). Reasons for exclusion of studies at full paper sifting stage were recorded. Given the epidemiology of X(T)27 we did not exclude studies on the basis of language, country or publication date.
Selection of studies
Two reviewers (FB and KJ) read the papers’ abstracts independently to consider whether or not the study met the eligibility criteria. Any studies deemed to be potentially relevant by either of the reviewers were sourced in full text and obtained for the second stage of the screening/sifting process. One reviewer (KJ) screened the full papers to exclude any obviously non-relevant papers (specifically letters, commentaries, reviews and qualitative studies); two reviewers (FB and KJ) then applied the eligibility criteria independently to determine inclusion in the review. If there was any disagreement regarding the eligibility of any of the research papers, the two reviewers met to discuss the ambiguous studies in order to come to a definitive decision on whether or not inclusion was warranted. In the situation of non-agreement, a third reviewer (MC or RT) provided input.
Strategy for dealing with foreign-language papers
To eliminate the prospect of language bias we used Google Translate to translate abstracts in order to apply our eligibility criteria. Where we remained unsure we recruited bilingual and multilingual postgraduate students and staff from our institution to support screening of foreign-language titles and abstracts. The process of data extraction of eligible foreign-language papers was then undertaken in collaboration with our bilingual and multilingual volunteers. For two of the papers (written in simplified Chinese) this was repeated with a second volunteer to enhance reliability of the process. This method enabled us to data extract and critically appraise the studies without having to translate the papers verbatim.
Data extraction
Dual independent data extraction was undertaken. To understand the conditions under which interventions are most successful, details around how the intervention was delivered (e.g. for surgical interventions we noted whether or not the aim of surgery was overcorrection), duration of treatment, length of follow-up, and completeness of follow-up, together with any additional contextual data, were recorded on the data extraction form (see Appendix 2). Likewise, detailed information on the study population was extracted to identify what intervention works for whom and at what time point (to include age, time after diagnosis and severity of misalignment). When data were missing or required clarification, we contacted the study authors for further details. We also contacted the study authors of any conference abstracts meeting the eligibility criteria.
Quality assessment
Each study was appraised for quality simultaneously alongside the data extraction process. We used the Cochrane Risk of Bias tool for RCTs and for non-randomised studies we used a tool based on the Critical Appraisal Skills Programme (CASP46) instrument for cohort studies, which includes assessment of both internal and external validity with questions relating to selection bias, study design, confounding, data collection methods, dropout and intervention integrity (see Appendix 3). We neither calculated a quality score nor did we exclude studies on the basis of quality. Any differences in assessment were resolved through discussion between reviewers (KJ and FB) and, if necessary, with other members of the study team (MC and RT).
Data synthesis
We used the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidance and flow chart to support reporting41 (Figure 1). Studies can be pooled only if a number of studies are identified reporting the same primary outcome measure and there is sufficient homogeneity in terms of study design, intervention type and population. We were unable to synthesise any data quantitatively in the form of a meta-analysis due to high levels of study heterogeneity. Differences in study design, population, intervention and outcome measures precluded meta-analysis. In particular, there was a great deal of divergence in defining what constitutes a positive outcome. For example, change in angle of deviation versus with stereoacuity versus degree of control and how success was defined [e.g. deviation of > 20 prism dioptres (PD)] compared with deviation of < 10 PD, which is clearly likely to impact on comparable effectiveness between studies) and at what time point measurements were undertaken. Equally, there were differences in the study populations, for example some studies included only divergence excess type, whereas others grouped basic, true and simulated divergence excess types together.
FIGURE 1.
Flow chart of search process (based on PRISMA guidelines).
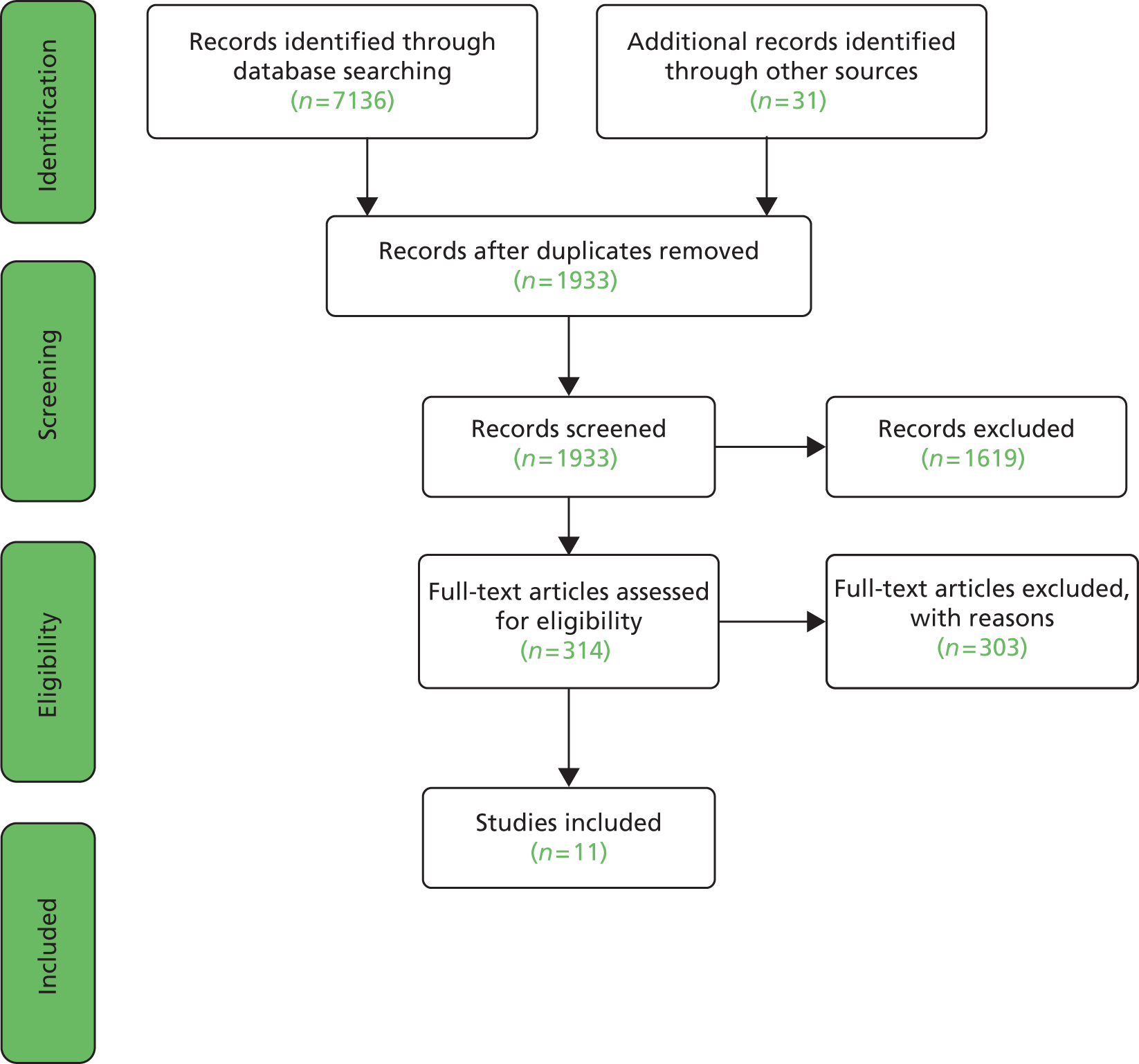
Because heterogeneity between studies precluded meta-analysis, we performed a narrative synthesis and report study findings separately by intervention type. In the narrative synthesis, we describe the main characteristics of the studies included, along with information about study quality and estimates of effect with relevant statistics. To avoid the introduction of bias into the narrative synthesis we have aimed to report the findings of each study judiciously and have made efforts to avoid inappropriate emphasis on any one particular study or author.
Findings
Description of studies
Electronic searches of 12 databases produced 7136 hits, which was reduced to 1902 after removal of duplicates. An additional 31 articles were retrieved through hand-searches, citation follow-up, discussion lists and key expert contact. On the basis of the abstract, these appeared to meet the eligibility criteria but all were excluded after review of the full text. A total of 314 papers were retrieved for full paper analysis but only 11 satisfied our eligibility criteria (see Appendix 4 for reasons for exclusion). 20,42,47–55 A further four conference abstracts were identified as potentially eligible on the basis of abstract alone,56–59 we wrote to the study authors for more information but received a response from the authors of only one of the papers stating that there was no comparison group.
Of the 11 studies that satisfied the inclusion criteria (Table 1), seven examined only surgical interventions,47,49–53,55 whereas four examined either surgery compared with non-surgical interventions or non-surgical interventions alone. 20,42,48,54 Non-surgical interventions included glasses, occlusion therapy, orthoptic exercises, prisms, BOTOX treatment, binocular vision therapy or observation only. Only 2 of the 11 studies were RCTs: one compared bilateral and unilateral surgery47 and the other examined the effectiveness of binocular vision training after surgery compared with no training. 48 The remaining nine studies were non-randomised observational studies involving a comparator group; the majority were retrospective with only two prospective studies. 20,54
| Study | Intervention and type of X(T) | Study design: follow-up | Type of X(T) and age | Final sample size | Outcomes | Summary findings | Adverse effects |
|---|---|---|---|---|---|---|---|
| Buck 201220 | Non-surgical treatment only for X(T) (glasses and/or patches, exercises and/or prism); surgery (bilateral or unilateral) plus BOTOX or further surgery; observation only | Prospective cohort with comparison group; 2-year follow-up for non-surgical; 6 months for surgical | Basic, true divergence excess, simulated divergence excess Aged < 12 years |
n = 371 Observation, n = 195 Conservative intervention, n = 50 Surgery (in some cases plus conservative), n = 58 Surgery plus additional BOTOX or surgery, n = 5 |
Near and distance angle (APCT) Control (NCS61); stereoacuity (Frisby near stereoacuity test) |
Median change in near and distance angle (IQR): Surgery: near –9 (–18 to –1); distance –20 (–30 to –7) Non-surgical treatment: near 0 (–2 to 4); distance 0 (–5 to 0) Vision treatment only: near 0 (–4 to 5); distance 0 (–6 to 5) Observation: near 0 (–4 to 4); distance 0 (–7 to 5) Change in total NCS61 (% improved by ≥ 3): surgery 42%; non-surgical treatment 15%; vision treatment only 8%; observation 10% |
Overcorrection rate for surgery 21% (13/63) Overcorrection defined as the presence of a manifest esotropia (any amount) at 1/3 metre, 6 metres or both at 6 months post surgery |
| Chia 200649 | Surgery (BLR vs. R&R) | Retrospective cohort with comparison group; 1-year follow- up | X(T) with basic or divergence excess type and with divergent strabismus size between 25 and 50 PD; aged < 16 years | N = 118 BLR, n = 64; R&R, n = 54 |
Angle of deviation, success defined as X(T) < 10 PD | Authors report mixed results and conclude that their findings highlight a decline in success over time, resulting in only temporary improvement (with more exotropic drift over time (p = 0.01); subjects with basic type did worse than those with divergence excess at 1-year follow-up BLR: success 42.2%; residual exotropia 56.0%; consecutive ET 2.0% R&R: success 74.2%; residual exotropia 13.0%; consecutive ET 13.0% |
Consecutive esotropia (defined as any esotropia): R&R 13% (7/54), BLR 2% (1/64) |
| Choi 201250 | Surgery (BLR vs. R&R) | Retrospective cohort with comparison group; 24 months’ minimum follow-up |
Basic-type exotropia defined as distance deviation, within 10 PD of the near deviation Age: BLR 6.8 ± 3.4 years; R&R 7.2 ± 2.3 years |
N = 128 BLR, n = 55; R&R, n = 73 |
Angle of deviation (prism and alternate cover test and Krimsky test in some cases) Success was defined as esophoria/tropia ≤ 5 PD to exophoria/tropia ≤ 10 PD |
No statistically significant difference between the groups at 1 day, 1 month, 6 months, 1 year and 2 years postoperatively (p > 0.05); however, with regard to long-term follow-up, the final outcome at mean 3.8 years was significantly different between the groups, demonstrating a higher success rate in the BLR group than in the R&R group (58.2% vs. 27.4%; p < 0.01) | Overcorrection rate (defined as esophoria/tropia> 5 PD): BLR 3.6% (2/55), R&R 4.1% (3/73) |
| Figueira 200642 | Multiple groups: 1. S + Or/Oc; 2. S alone; 3. Or/Oc therapy; 4. O | Retrospective cohort with comparison group; Follow-up at 6 months, 1 year, 2 years and 5 years |
15 PD exodeviation for distance fixation Age (years) mean (SD): S + Or/Oc, 5.98 (2.45); S, 6.92 (3.68); Or/Oc, 4.41 (2.19); O, 4.57 (3.09) |
N = 150 S + Or/Oc, n = 48; S, n = 15; Or/Oc, n = 67; O, n = 20 |
Angle of deviation (mean, PD) Improvement/success was defined as orthophoria or < 10 PD esotropia/exotropia |
Surgery with preoperative orthoptic/occlusion Therapy had the highest success rates. Surgery with orthoptic/occlusion therapy was more effective in reducing exodeviation than surgery only Improvement of X(T) by intervention type in % (natural frequencies): Surgery + Or/Oc group: 6 months 87.50% (42/48); 1 year 85.70% (36/42); 2 years 83.33% (25/30); 5 years 84.62% (11/13) Surgery alone: 6 months 40.00% (6/15); 1 year 42.86% (6/14); 2 years 36.40% (4/11); 5 years 25.00% (1/4)Or/Oc alone: Overminus lenses: 6 months 15.38% (2/13); 1 year 16.67% (2/12); 2 years 11.11% (1/9); 5 years 0.00% (0/5) Occlusion alone: 6 months 6.00% (3/50); 1 year 8.57% (3/35); 2 years 5.26% (1/19); 5 years 0.00% (0/5) Observation alone: 6 months 5.00% (1/20); 1 year 5.26% (1/19); 2 years 9.09% (1/11); 5 years 33.33% (2/6) |
Limited data on over-correction rates One patient overcorrected by 20 PD |
| Kushner 199847 | Surgery (BLR vs. R&R) | RCT, 1-year follow-up (range 12–15 months) | Basic exotropia (randomised to R&R or BLR) Non-randomised simulated divergence excess group (received BLR) 3–18 years in all groups |
N = 36 R&R, n = 17; BLR, n = 19 |
Angle of deviation A satisfactory outcome was defined as between 10 PD of exophoria and 5 PD of esophoria |
R&R found to be more effective than BLR for basic type only: R&R: 14/17 (82%) satisfactory, 1 overcorrected, 2 undercorrected BLR: 10/19 (52%) satisfactory, 2 overcorrected, 7 undercorrected Significant difference (p < 0.2) between two types of surgery favouring R&R Simulated distance exotropia (n = 68): satisfactory 81% (55/68); overcorrected 4% (3/68); undercorrected 15% (10/68) Significant difference compared with basic type receiving BLR (p < 0.05) |
Overcorrection rate (defined as any amount of esotropia manifest or intermittent): BLR 11% (2/19), R&R 6% (1/17) |
| Lee 200151 | Surgery: BLR, vs. R&R | Retrospective cohort with comparison group; 1-year follow-up | Mixed X(T) type: 93 basic type; 10 pseudodivergence excess type 3–17 years with a mean age of 7.1 years |
n = 103 BLR, n = 46, R&R, n = 57 |
Motor alignment classified as follows: overcorrected by 11–20 PD (group I); overcorrected by 1–10 PD (group II); orthotropic (group III); undercorrected with an exotropia of 1–10 PD (group IV) | R&R: success 34/57 (59.6%); undercorrection 17/57 (29.8%); overcorrection 6/57 (10.5%) BLR: success 26/46 (56.5%); undercorrection 17/46 (37%); overcorrection 3/46 (6.5%) There were no significant differences in the success, undercorrection and overcorrection rates between the two surgical procedures after a 1-year postoperative period |
Overcorrection (as defined as > 5 PD esodeviation) BLR 0% (0/8), R&R 10.5% (6/57) |
| Lee 200752 | Surgery: Con or Aug | Retrospective cohort with comparison group; 6 months’ minimum follow-up | Basic type Age mean (SD): aug 8.0 ± 3.6 years; con 7.1 ± 3.9 years |
n = 107 Con = 41, aug = 66 Follow-up: con 21.3 ± 18.4 months (range 6–39 months); aug 23.3 ± 14.5 months (range 6–35) |
Angle of deviation Success defined as 8 PD of exophoria and 8 PD of esophoria |
Last follow-up: success (within 8 PD), significant difference (p = 0.01): con 43.9% (18/41); aug 68.2% (45/66) | Overcorrection rate (as defined as > 8 PD esotropia): con 2.4% (1/41); aug 1.5% (1/66) |
| Maruo 200153 | Surgery: BLR vs. R&R | Retrospective cohort with comparison group; maximum follow-up 4 years, ‘longer’ (mean 11.7 years, range 8–22 years) | No details of type of X(T) Aged < 15 years (no means or ranges given) |
BLR (n = 349); R&R (n = 298) One and four muscle procedures (n = 19) |
Angle of deviation Success defined as ≤ 20 PD heterotropia |
No statistically significant difference between the BLR and R&R techniques, but there is a trend towards the results favouring BLR (success rate 95.2% vs. 80% for R&R) | Overcorrection (as defined as > 5 PD esotropia): BLR 2.4% (5/210); R&R 0% (0/180) |
| aQiu 201048 | Binocular vision training | RCT; 1-year follow-up | No details of type Aged 5–16 years |
n = 121 Intervention n = 61; control n = 60 |
Angle of deviation (regression rate of eye position, defined as > 10 PD) | Regression rate (i.e. failure rates): intervention 7/61 (11.5%); control 21/60 (35%); p < 0.05 | No adverse effect data |
| aWu 200854 | BOTOX | Prospective cohort with comparison group; 6 months’ follow-up | No details of type Range 4–12 years BTXA mean 9.2 ± 2.14 years Surgery mean 7.03 ± 2.48 years |
N = 60 Surgery n = 30; BTXA n = 30 |
Eye alignment; binocular vision Definition of success (deviation must be within ±10 PD) |
After 6 months in the surgical group, 27 of 30 (90%) operations were successful, whereas in the BTXA group 23 of 30 (76.7%) cases were successful; of the seven less successful cases in the BTXA group, four reverted back after 6 months; where deviation was ≥ 20 PD, BTXA injections were repeated | BTXA: one child had diplopia which required patching; seven children had ptosis, six of seven patients improved after 1 month and all patients improved after 3 months Surgery: no adverse effects |
| aYuksel 199855 | Surgery: group 1 = R&R; group 2 = BLR | Retrospective cohort study with comparison group; mean follow-up 2.81 years, with a range of 6 months to 8 years | Basic type: mean (at surgery) 6.5 years; range 2–18 years | Unilateral n = 25; bilateral n = 30 | Deviation (cover and uncover test and prisms) Sensory fusion (Bagolini striated glasses and/or the Worth test) Stereopsis (TNO test and/or Lang stereoacuity test) Optimum outcome = orthotropia; good outcome = < 20 PD |
Immediate postoperative results favour asymmetrical surgery but at long-term follow-up there are no statistically significant differences between outcomes for either technique (p = 0.249) | Overcorrection rate (defined as any consecutive esotropia): R&R 8% (2/25); BLR 3% (1/30) |
Three of the included studies were non-English-language papers written in Chinese (simplified)48,54 and French. 55 Screening of titles and abstracts identified 122 foreign-language papers, of which 37 were reviewed at full-text stage. We collaborated with seven foreign language speakers [Chinese (two native speakers), French, German, Italian, Polish, Russian] to support the translation, data extraction and critical appraisal processes.
Characteristics of setting and participants
Four studies were conducted in Europe, the USA or Australia, whereas seven were conducted in Asian countries, which might be reflective of the epidemiology of X(T), specifically the observation that X(T) is more frequent in Asian populations and latitudes with greater exposure to sunlight. 27 Three studies were conducted in South Korea,50–52 two in China,48,54 one in the USA47 and one each in Australia,42 Belgium,55 Japan,53 Singapore49 and the UK. 20
There was some heterogeneity between studies in terms of the study population examined. Three studies20,49,51 included basic and divergence excess (simulated or true) types of X(T), whereas four studies47,50,52,55 included basic type only and four studies42,48,53,54 did not report on the type of X(T) examined. Only one study20 stated that their sample was recruited from multiple centres; the remainder were single-centre studies.
Risk of bias in included studies
Randomised controlled trials
Two RCTs47,48 met our eligibility criteria: both were judged to have a high or unclear risk of bias in most areas according to the Cochrane risk of bias tool (Table 2). Both trials were unclear in their reporting of randomisation and allocation concealment procedures. One trial47 was judged to have a high risk of selection bias because the three groups were not assigned simultaneously and there were some inconsistencies in reporting of exclusion criteria (patients were also being recruited for two different trials concurrently). The methods in the other RCT48 were poorly reported, specifically with respect to techniques used for randomisation and allocation concealment. Detection bias was a concern in both trials, specifically around masking of outcome assessment. Where details of the intervention were unclear48 (i.e. duration of binocular vision training) we wrote to the study authors for further information but received no response. Both trials were judged to have low risk of bias with respect to attrition bias because one52 reported reasons for exclusions (albeit with uncertainty about the inclusion/exclusion of the patients from the other trial), and the other reported data for all participants at 1 year. 48
Non-randomised studies
Of the nine cohort studies with comparison groups,20,42,49–55 two of the studies20,54 were prospective (Table 3). A strength of the inception cohort study20 was that it recruited from multiple centres with a final sample size of 371 and a loss to follow-up of only 24%, whereas the other prospective cohort54 was a single-centre study and had a smaller sample (n = 60) and short follow-up period (6 months only), although it should be noted that there was no loss to follow-up in this study. Neither of the prospective studies used matched comparison groups. The remaining seven cohort studies42,49–53,55 were retrospective in design.
| Quality criterion | Buck 201220 | Chia 200649 | Choi 201250 | Figueira 200642 | Lee 200151 | Lee 200752 | Maruo 200153 | Wu 200854 | Yuksel 199855 |
|---|---|---|---|---|---|---|---|---|---|
| Was the cohort recruited in an acceptable way (robust inclusion/exclusion criteria or consecutive recruitment)? | Y | Y | Y | Y | Y | Y | U | N | N |
| Was the study prospective? | Y | N | N | N | N | N | N | Y | N |
| Was the intervention conducted in an explicit and standardised manner (i.e. were guidelines or protocol for intervention described)? | N | N | Y | N | Y | Y | Y | U | Y |
| Was the outcome appropriately measured to minimise bias? | Y | Y | Y | Y | Y | Y | N | Y | N |
| Did they identify important confounding factors (e.g. age at intervention, baseline angle of deviation)? | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Did they adjust for confounding factors in the design and/or analysis where necessary? | N | N | N | N | Y | Y | N | Y | Y |
| Were they followed up for at least 12 months? | N | Y | Y | Y | Y | Y | Y | N | Y |
| Are the authors’ conclusions substantiated by the reported data? | Y | Y | N | Y | Y | Y | N | Y | Y |
Of the nine non-randomised studies included,20,42,49–55 three used consecutive recruitment,42,50,52 one study20 identified cases prospectively from multiple cases according to well-defined inclusion criteria, and three studies49,51,53 identified cases retrospectively using well-defined inclusion criteria. In the remaining two studies54,55 details of how patients were recruited is either poorly described or unclear. In at least five of the studies there is a possibility of selection bias associated with determining which patient received which procedure, as many of the studies state that the choice of intervention was at the ophthalmologist’s or parents’ discretion. 20,42,49,50,54 With regard to the possibility of bias incorporated during exposure to the intervention, we assessed whether the interventions were carried out according to guidelines or a standardised protocol. Six studies42,50–53,55 included reference to a standardised protocol or guidelines to determine the amount of surgery performed. In one study it was stated that the amount of surgery was at the discretion of the operating surgeon,49 although this was unclear in two other studies. 20,54 Surgery was performed by three surgeons in one study,49 in six studies42,47,50–52,55 all procedures were performed by one surgeon, and in the remaining three studies48,53,54 details of who performed the surgery were not reported.
Differences in outcome assessment were considerable across the studies with a range of outcome measures being reported including motor alignment (angle of deviation), sensory function (stereopsis), control or a combination of the above. Of those studies reporting motor alignment as the primary outcome, one reported median change in deviation20 and the remainder42,47,49–55 reported success or improvement rates. However, ‘success’ or ‘improvement’ was variously defined so (leaving aside differences in study design, population and follow-up point at which outcomes were assessed) the studies were not suitable for pooling in a meta-synthesis. The diversity in definitions of success used is an important issue here, with some studies conceptualising a successful outcome as anything within 20 PD of orthotropia,53,55 whereas other authors operationalised a much stricter definition (e.g. ≤ 5 PD esophoria/-tropia to ≤ 10 PD exophoria/-tropia,47,50 and various definitions in between (e.g. deviation within ± 8 PD52 or deviation within ± 10 PD42,49,54). In terms of assessment of outcome measures, only one study50 reported carrying out measurements of the angle of deviation on three different occasions; in the remaining studies20,42,49,51–55 minimal detail was reported with regard to when outcomes were measured and how many times measurements were taken.
In two studies there were sparse42 or no data53 reported to assess the possibility of significant baseline differences between the intervention and control groups in terms of age and severity of X(T) at baseline, both of which are important confounding factors. Four studies51,52,54,55 reported no statistically significant differences between the intervention and comparison groups at study outset. However, two studies49,50 reported significant differences between the two groups at baseline, the former in initial angle of deviation and the latter in age, but no adjustments were made for these differences in their analyses.
Surgical interventions (one surgical technique versus another)
Of the seven studies examining the effectiveness of surgery, six compared symmetric with asymmetric surgery [bilateral lateral rectus recession (BLR) vs. unilateral recess resect]. 47,49–51,53,55 One study52 compared effectiveness between augmented and non-augmented forms of symmetric surgery. Only one of the surgical intervention studies47 was described as a RCT, the remainder were retrospective cohort studies with a comparison group.
Bilateral lateral rectus recession compared with unilateral recess resect
One RCT47 and five retrospective cohort studies49–51,53,55 reported on these surgical techniques. The RCT47 was conducted in children aged 3–18 years with basic type X(T), and compared unilateral recession/resection (R&R) (n = 17) with BLR (n = 19). A satisfactory outcome, measured 12 months after surgery, was defined as between 10 PD of exophoria and 5 PD of esophoria; stereoacuity was not assessed. The study47 reported a significantly higher proportion (p < 0.02) of patients with a satisfactory outcome in those receiving R&R procedures (82%,14/17) than in those receiving BLR (52%, 10/19) at least 12 months after surgery (range 12–15 months). The authors also compared their results to a non-randomised ‘control’ group of children with simulated distance exotropia (n = 68) who all received BLR surgery. Successful outcomes were observed in 81% of patients in the control group (55/68); overcorrection and undercorrection rates were 4% (3/68) and 15% (10/68) respectively. The results were significantly different to the patients with basic X(T) receiving BLR (p < 0.05) but similar to those receiving R&R. The study, however, was limited in several respects. First, there was a dearth of information about the randomisation procedures used, making it difficult to assess whether or not the method used to generate the allocation sequence would produce comparable groups and also whether or not the allocation sequence was concealed. Neither participant nor clinician was blinded to the intervention received. Likewise, outcome assessment was unblinded, with the clinician who performed surgery also measuring outcomes at 12 months (little detail is reported on how outcomes were measured, i.e. exact time point and number of measurements). It is noteworthy that the author excluded patients for whom he knew that, at the time of surgery, the referring physician would be conducting follow-up assessment. In addition patients, were excluded because they were enrolled in another in-progress RCT led by the author. The exclusion criteria used raise questions about the fidelity of the randomisation processes and actual study design used, and the possibility of selective reporting. The absence of clear and well-defined criteria for considering a patient for surgery (e.g. size of deviation, control of deviation) also introduces possible bias to the study findings. Finally, the sample size was also small (total n = 36) and the follow-up period may be insufficient to allow generalisability.
A retrospective cohort study49 (n = 118) of children aged < 16 years with either divergence excess or basic type X(T) compared the effectiveness of BLR with R&R undertaken by three different surgeons who determined the type and amount of surgery to achieve orthotropia. It should be noted that one surgeon had a strong preference for R&R, whereas the other two surgeons had a preference for BLR but would undertake R&R if there was a tendency for deviation in one eye or a strong near component. Outcomes were reported at 1 and 3 years postoperatively for both motor alignment (no reporting of number of measurements taken) and control (subjective assessment by an orthoptist). The study reported clear inclusion and exclusion criteria – patients were eligible if they had either basic or divergence excess type X(T) and a deviation of between 25 and 50 PD. A successful outcome was defined as X(T) ≤ 10 PD. At 12 months’ follow-up there was a statistically significant difference in the success rate of surgery between the two groups, with better outcomes in the group receiving the R&R procedure (74.2%) than in the group receiving BLR (42.2%; p < 0.001). However, the authors note that exotropic drift over time was greater in the R&R group, with a significant increase in the mean distance constant exotropia (XT) in the R&R group at 3 years’ postoperative follow-up (p = 0.01). There was no significant change in postoperative deviation for either distance or near measurements in the BLR group. It is noteworthy that the authors report significant differences between the two intervention groups, with the R&R group having a greater mean age (p = 0.052), a higher proportion of children with divergence excess-type X(T) (p = 0.0181) and a smaller mean angle of deviation (p = 0.0005) than the BLR group. These differences were not adjusted for in the analyses, which limits the generalisability of the findings. The authors acknowledge that the wide range in size of X(T) at baseline (up to 50 PD) might also affect interpretation of the study findings. Other limitations include the absence of clear guidelines for choice of surgical procedure (no standard protocol of tables for determining type or amount of surgery).
The comparison between R&R and BLR procedures was also examined in a retrospective study of 128 children. 50 Surgical outcomes were assessed in a cohort of children with basic-type X(T) and at least 2 years’ follow-up (mean 44.2 months for BLR and 47.8 months for R&R). Success was defined as esophoria/tropia ≤ 5 PD to exophoria/tropia ≤ 10 PD) measured on at least three occasions (this is the only study50 that explicitly reported measuring deviation on at least three occasions at baseline and follow-up). The authors found no difference between the two interventions at 2 years postoperatively; however after long-term follow-up (mean 3.8 years) the BLR procedure had a significantly higher success rate than R&R (58.2% vs. 27.4%; p < 0.01). One of the strengths of the study50 is the long follow-up period used, which identified greater recurrence in the R&R group (after mean 3.8 years’ follow-up, recurrence was 68.5% in the R&R group vs. 38.2% in the BLR group). However; it should be noted that ascertainment bias is an issue here, as patients with poorer outcomes might be more likely to be followed for longer. Equally, the inclusion criteria for this study50 were less rigid, with the inclusion of patients with a A or V pattern, dissociated vertical deviation or oblique muscle over-actions that did not require surgery. Further, there were statistically significant differences between the two surgical groups in terms of preoperative deviation and this potential confounding effect was not adjusted for in the analyses. The BLR group had a larger mean angle of deviation at baseline but, even so, had better success rates at longer-term follow-up. The findings are also limited by absence of data on sensory status, specifically stereopsis pre- or postoperatively.
The same surgical comparison was undertaken in a retrospective, single-centre study51 with a 1-year follow-up period. The population of 3- to 17-year-old children (n = 103) was mixed in terms of type of X(T), with 93 basic-type X(T) and 10 pseudodivergence excess-type X(T), and the aim of surgery was deliberate overcorrection. The authors reported no significant differences between the two intervention groups (BLR n = 46 and R&R n = 57) in terms of age or deviation at baseline. Success was defined as no more than 10 PD of exophoria or 5 PD of esophoria (sparse detail was included on how outcomes were measured). The authors reported no statistically significant differences in terms of success rate at 1-year follow-up between the two groups [BLR 56.5% (26/46) vs. R&R 59.6% (34/57); p > 0.05], and age and initial deviation had no significant effect on outcome (p > 0.05). The main objective of this particular study51 was to understand the relationship between motor alignment at day 1 and motor alignment at 1-year follow-up. The authors conclude that optimal results are produced with immediate postoperative overcorrection of 11–20 PD for BLR and 1–10 PD for R&R procedures. In terms of limitations of this study, there is a lack of detail on how choice of surgical procedure was determined, although it is stated that both procedures were performed by the same surgeon.
A large retrospective cohort study53 of children aged 15 years or younger (n = 666) explored long-term outcomes of BLR (n = 349) compared with R&R procedures (n = 298). The study53 reports a paucity of detail with respect to the baseline population, in particular type and severity of X(T); likewise, there is a dearth of information available on the specific protocol for outcome assessment. A further limitation is that the authors fail to explore (and take account of) baseline differences between the surgical groups in terms of age and size of initial deviation. The authors do include, however, guidelines for surgery. Comparative data are reported at 4 years’ follow-up for patients who initially showed orthotropia or minimicrotropia (defined as alignment within 4 PD of orthotropia) at 1 month postoperatively. Of the patients receiving BLR, 66.7% (140/210) retained orthotropia or minimicrotropia, whereas 32.8% who received R&R retained orthotropia or minimicrotropia (with more patients drifting towards exotropia in this group). A subset of 78 patients were followed for between 8 and 22 years but no comparative data for the two surgical techniques were reported. Restoration of normal appearance was also conceptualised as a key indicator of success, although there was no statistically significant difference between procedures in terms of success rates, with 95.2% of patients who received BLR achieving normal appearance compared with 80% of patients who received R&R achieving normal appearance. Caution should be applied when interpreting success outcomes, given that the definition of success is extremely broad (≤ 20 PD of heterotropia).
A retrospective cohort of children (aged 2–18 years) with basic-type X(T) also compared the R&R and BLR procedures. 55 Twenty-five children received R&R surgery on the non-fixating eye, whereas 30 children received BLR surgery; the groups were comparable (i.e. there were no statistically significant differences between the two groups) in terms of age, preoperative deviation and sensory results at baseline. The mean follow-up period was 2.81 years, with a range of 6 months to 8 years; there is limited information about how outcomes were assessed (e.g. use of repeat measurements). There were no statistically significant differences between the two groups in terms of success rates at long-term follow-up (p = 0.249), with 52% of patients achieving an optimal or good outcome (defined as orthotropia or within ± 20 PD of orthotropia, respectively) in the BLR group compared with 57% achieving an optimal or good outcome in the R&R group. In terms of limitations the sample size is small and the findings should be interpreted in light of the retrospective study design.
In summary, the findings of the above studies (one RCT47 and five retrospective cohort studies49–51,53,55 with comparison groups) show that short-term outcomes tend to be better with the R&R procedure, but there is a suggestion of better long-term outcomes with BLR surgery. 47,49–51,53,55
Conventional recession/resection compared with augmented recession/resection
One study52 investigated the effectiveness of augmented surgery compared with conventional surgery, using the symmetric lateral rectus recession procedure that was assessed in a population of 107 children with basic-type X(T) followed for at least 6 months. Conventional surgery was conducted according to Parks formula, whereas augmented surgery was 1.5–2.5 mm more than a conventional lateral rectus recession. Success was defined as between 8 PD of exophoria and 8 PD or esophoria, and follow-up was 6–35 months in the augmented surgery group compared with 6–39 months in the conventional surgery group (sparse detail was included relating to outcome assessment). Conventional surgery was performed in 41 children (mean age 8 ± 3.6 years) and augmented surgery was performed in 66 children (mean age 7.1 ± 3.9 years). Comparison of success rates at the last follow-up visit demonstrated a statistically significant difference favouring augmented surgery (68.2% vs. 43.9%; p = 0.01). One of the key limitations of the study is that, although performed by the same surgeon, the two procedures were conducted at different time points, so, in effect, the control (conventional surgery) was recruited historically (both groups were recruited retrospectively but the control group was recruited earlier than the intervention group). That said, the groups were shown to be comparable, with no statistically significant differences between the groups in terms of mean alignment at baseline (p = 0.23), mean age (p = 0.06) and mean length of follow-up period (p = 0.55). The authors also acknowledge that stricter success criteria might be required, with the recognition that patients with 8 PD of esophoria can complain of diplopia.
Non-surgical interventions
Surgery compared with non-surgical interventions
Four studies20,42,48,54 compared surgical and non-surgical interventions, two of which reported comparative findings on two interventions48,54 while the remaining two studies20,42 considered more than two interventions.
Surgery compared with BOTOX
One prospective cohort study54 investigated the effectiveness of treatment with attenuated botulinum strain A (BTXA) compared with surgery (including both unilateral and bilateral) in a population of children aged 4–12 years. No information about the type of X(T) was reported. The method of recruitment was non-randomised and outcomes were assessed at 2 weeks, 1 month, 3 months and 6 months post intervention. Success was defined as a deviation within ± 10 PD of orthotropia, although limited details relating to outcome assessment (e.g. number of measurements taken) was reported. Although the rate of successful corrections was lower in the BTXA group (23/30, 76.67%) in comparison with the group receiving surgery (27/30, 90.00%), the difference was not found to be statistically significant (p = 0.166). In the BTXA group the undercorrection rate was (7/30, 23.33%). Conversely, in the surgical group one case was undercorrected and two cases were overcorrected. The authors also reported complications for the BTXA group, with one case of double vision (requiring patching); the authors mention that double vision occurred for some of the other patients but not to the extent that it affected daily living; the authors do not state the proportion of patients who experienced these symptoms of double vision. In addition, seven cases of ptosis were reported, all of which resolved after 3 months.
Surgery alone compared with surgery plus binocular vision training
A RCT48 of children, aged 5–16 years, explored the effectiveness of binocular vision training after surgery for X(T). Patients were randomised to either the intervention group (n = 61), which involved a period of binocular vision training, which began 2 weeks post surgery, or the control group (n = 60), which received no training after surgery. Patients in the binocular vision training group completed exercises with red and blue glasses three times per day for a period of 20 minutes on each occasion. There is a dearth of detail regarding the intervention, in particular the period over which the exercises were undertaken and also the type of X(T) under investigation. The primary outcomes were recovery of binocular vision and regression/deterioration rate (defined as deviation of > ± 10 PD); both were assessed at 1 week post surgery and 12 months post follow-up. The authors conclude that binocular vision was better in the training group than the control at 12 months’ follow-up. Likewise the regression rate was worse in the control group than in the intervention, with 21/60 recessing in the control group (35%) compared with 7/61 (11.5%) in the intervention group; this difference was statistically significant (p < 0.05). These findings suggest that additional binocular vision training after surgery might improve outcomes.
Surgery compared with conservative interventions
Two studies20,42 considered surgery versus conservative interventions. Multiple intervention comparisons were explored in a prospective, multicentre cohort study of 371 children aged < 12 years. 20 Previously untreated basic, true and simulated divergence-excess types were included and children were followed for 2 years for each of the non-surgical interventions (n = 50), for observation (n = 195) and for treatment for reduced VA (n = 63), and 6 months postoperatively for the surgical intervention (n = 63). The non-surgical treatment group (n = 50) included the following interventions that aimed to improve control: spectacle lenses (n = 37); occlusion (n = 6); glasses and patching (n = 2); exercises (n = 4); and exercises and prism (n = 1). Outcomes assessed included change in angle of deviation (no detail of whether or not repeated measurements were used), control [Newcastle Control Score (NCS), which incorporates both objective and subjective components: high score = poor control; scores range from 0 to 961,62] and stereoacuity (Frisby Near Stereoacuity Test). The authors conclude that surgery was the only intervention associated with statistically significant improvements in angle of deviation (p < 0.001) and NCS61,62 (mean 60% reduction in both parental and clinic components). However, there was a risk of overcorrection (21% at 6 months) and additional surgery was required in 8% of children (5/63). Non-surgical interventions had no significant effect on angle of deviation, but significant small improvements in control were noted for the non-surgical intervention and observation groups (mean reductions of 20% and 13% in the clinic and parent components, respectively). Another key finding was that few children in the watchful-waiting group showed deterioration to constant exotropia (0.5%); however, follow-up was limited to 2 years. One of the strengths of this study20 is that children were recruited from 26 centres and loss to follow-up was not significant (with 81% of the original cohort being available for final follow-up). The authors also established that there were no significant differences between the final samples and those lost to follow-up. A possible weakness of this study is the absence of robust criteria/protocol for management decisions. However, the study was multicentre and the authors argue that treatment adopted was likely to reflect current practice at the centres involved. A second limitation surrounds comparison of the outcomes of surgery at 6 months compared with non-surgical intervention outcomes at 24 months, which the authors acknowledge may introduce bias in interpretation of the study findings.
A retrospective study of 150 children compared four different treatment options for X(T): (1) surgery only (BLR using recognised guidelines, n = 15); (2) surgery combined with orthoptic/occlusion therapy (n = 67); (3) orthoptic/occlusion therapy alone (48); and (4) observation (n = 20). 42 Within the orthoptic/occlusion group, treatment was divided into the following subgroups: convergence exercises, overminus lens therapy or occlusion therapy. For orthoptic/occlusion therapy before surgery, an additional intervention of preoperative diplopia awareness exercises was undertaken. Children, aged < 15 years, with an exodeviation of 15 PD for distance fixation were included and followed for a maximum of 5 years. Patients were not randomised to the intervention groups; the authors state that treatment method was ‘largely dependent on parent preference’, which might represent a source of bias. Success was defined as orthophoria or < 10 PD esotropia/exotropia (no reporting of protocol for assessment of angle of deviation), good stereoacuity (Lang stereotests) and cosmesis (parental subjective assessment). Comparison of change in deviation for each of the intervention groups revealed that surgery coupled with orthoptic/occlusion therapy produced the greatest mean reduction in exodeviation, the difference was significantly greater than with the other interventions at all follow-up time points (p < 0.001). There were no statistically significant differences in success rates between the subgroups of preoperative orthoptic/occlusion exercises. In terms of study limitations, the authors fail to explore the potential confounding effects of age and severity of X(T) at baseline. From baseline data it appears that the groups were not comparable in terms of mean age, sensory results and mean preoperative deviation, although no statistical assessment of difference across the intervention groups in terms of baseline characteristics is reported.
Adverse effects
Of the 11 included studies,20,42,47–55 1020,42,47,49–55 reported outcomes relating to adverse effects. Nine studies evaluating surgical interventions20,42,47,49–53,55 reported overcorrection rates, which ranged from 1.5%52 to 21%. 20 In four49–51,55 of six studies47,49–51,53,55 comparing BLR and R&R procedures, overcorrection rates were greater in the group undergoing the R&R procedure (overcorrection rates by individual study are shown in Table 1). One of the prospective cohort studies, which examined the effectiveness of BOTOX in comparison with surgery, found that some adverse effects were reported in the BOTOX group: diplopia (which required patching, in one patient) and ptosis (eyelid droop, in 7 of 30 patients – 23%) but these tended to resolve over time (6/7 cases improved after 1 month and all cases improved after 3 months. None of the remaining non-surgical interventions (overminus lenses; occlusion; orthoptic exercises; prisms) was associated with adverse outcomes. 20,42,48
Discussion
Summary of main results
Surgical intervention studies
The review revealed mixed findings when comparing R&R and BLR surgery, with two studies51,55 reporting equivocal results, two studies47,49 favouring R&R surgery, and two studies50,53 reporting more success with BLR surgery at long-term follow-up. R&R surgery produced more successful results, at least in the short term, with two studies47,49 reporting statistically significant results favouring R&R procedures. However, there are reservations around the stability of outcomes for R&R surgery in the long term, with studies49,50 suggesting greater exotropic drift over time. The BLR procedure produced better outcomes at long-term follow-up (at least 3 years) in two studies. 50,53 The reader should be aware that the study populations are different – we are not comparing like with like – some studies examine only basic-type X(T), whereas others include both basic and divergence excess types. Equally the follow-up point for measuring a successful outcome was defined differently, so these comparisons should be interpreted with caution. In terms of adverse effects, the rate of overcorrection was variously reported (range 1.552–21.00%20). The wide range in overcorrection rate is probably due to differences in the follow-up period between studies and the definition of overcorrection applied (e.g. strict definitions, i.e. any esotropia20 vs. looser definitions, e.g. esotropia > 8 PD52).
Non-surgical studies compared with surgical intervention studies
Only four studies20,42,48,54 meeting the design criteria (RCT, quasirandomised study or cohort study with comparison group) were located, each considered different interventions and comparison groups so it is difficult to synthesise results even tentatively. The prospective study comparing surgery, non-surgical interventions and observation found that surgery produced better outcomes in terms of motor alignment and control, but it was associated with a significant risk of overcorrection, with loss of near stereoacuity in some cases. 20 Importantly, the authors also conclude that watchful waiting is not associated with deterioration and progression to constant exotropia within the first 2 years after diagnosis. The RCT of vision training after surgery demonstrated more successful outcomes when compared with surgery alone. 48 The prospective study54 comparing treatment with BTXA with surgery found no statistically significant difference in success rates between the two procedures, but argued that BTXA represented a less invasive option when adverse effects (ptosis and diplopia) are only short lived. A retrospective study of multiple interventions reported orthoptic exercises or occlusion prior to surgery resulted in greater success when compared with surgery alone; there were no significant differences between the success rates of either orthoptic exercises or occlusion. 42
Quality of evidence
The body of evidence retrieved was limited in terms of size and quality. Only two RCTs47,48 were located, both of which had a risk of bias attributed to aspects of the study design (no masking of outcomes assessment and the possibility of selection bias). Both prospective cohort studies20,54 had comparison groups but neither was matched. The main sources of bias in the retrospective studies with comparison groups were small sample sizes, short follow-up periods, absence of a robust protocol for management or allocation to intervention groups, and limited adjustment for known confounders, such as age and initial deviation at baseline. But perhaps more important is the variability in outcome measures used and, specifically, the definition of success applied (e.g. broad vs. narrow thresholds to constitute success). These limitations were coupled with a paucity of detail on study methods, making it difficult to establish how interventions were undertaken (e.g. absence of guidelines or standardised protocol for surgery), how outcomes were assessed (e.g. lack of detail on specific tests used) and whether or not there were any differences between intervention groups at baseline (especially with regard to age and initial deviation).
Comparison with the existing evidence base
Commensurate with the conclusions of the earlier Cochrane review27 we found that there remains a need for further well-designed RCTs to examine questions of effectiveness for different management options of childhood X(T). Given the absence of high-quality evidence, the authors argue for prudence when considering management options given the potential to ‘do harm by correcting the appearance of misalignment but disrupting the ability to maintain binocular stereo vision’,27 p.10. A review of conservative treatment options for X(T)24 found that interventions such as minus lenses, anti-suppression occlusion and orthoptic exercises were effective both as an alternative, and as an adjunct, to surgery but they also highlighted a need for further research to understand the circumstances under which these management strategies were most successful, in particular dosage of antisuppression occlusion therapy. Similarly, a review of non-surgical interventions for X(T)45 underlined the dearth of well-designed intervention studies to examine questions of effectiveness. They highlighted the absence of consensus definitions of success as well as poor reporting of details around the actual intervention delivered and compliance with this. Our review supports each of these calls for further better-designed studies, consensus on outcome measures of success and improved reporting of interventions and outcomes.
Potential biases in the review process
The main limitation of this review is that we are reporting on effectiveness with suboptimal study design. The RCT is the design of choice when addressing questions of effectiveness. Owing to the absence of RCTs in this topic area, we have adopted a pragmatic approach, moving down the hierarchy of evidence to the next level when studies were available (i.e. cohort studies with a comparison group, both prospective and retrospective). By including non-randomised (observational) studies we are aware of the issue of selection bias due to the absence of robust methods of allocation (usually by clinician, which increases the risk of confounding by prognostic factors such as age or severity of the condition at baseline). 40 Far more case series were retrieved in the searches but, owing to the absence of a contemporaneous comparison group, we have excluded this study design. 63 The dearth of high-quality study designs, together with the considerable heterogeneity between studies in terms of population [i.e. type of X(T)], outcomes measured, definition of success and follow-up period used, precluded a meta-analysis that would be the ideal form of synthesis for a review of this nature.
We should also acknowledge that, although searches were conducted across multiple databases and supplemented by hand searches of non-indexed journals and contact with key experts in the field, it is possible that we may not have captured all relevant studies. That said, the search strategy was developed, piloted and refined by a highly experienced information researcher and efforts were made to contact key experts in the field using an established discussion list. In using these research findings, researchers and practitioners alike should be mindful that studies were retrieved using the search strategy presented in Appendix 1 and by applying the strict eligibility criteria set out in Box 2. We excluded studies if they did not report outcomes for children (up to 18 years) with X(T) (either basic or divergence excess types) separately. In other words, studies were excluded if they reported aggregated outcomes for exotropia generically (e.g. constant and intermittent mixed or for children and adults mixed). If time and resource constraints had allowed, we would ideally have contacted study authors of studies with mixed populations (adults and children; XT and X(T); convergence insufficiency type combined with divergence excess) to request disaggregated data.
One of the strengths of our review was the inclusion of foreign-language papers (n = 3), which is particularly important for the topic area given the epidemiology of X(T). 27 It should be noted, however, that the website and hand searches were conducted in English language only. Publication bias might also be an issue here, with studies presenting equivocal or non-significant results not being published.
The review has sought to address questions around effectiveness of interventions for X(T) in children. We included the best available level of evidence (mostly cohort studies with a comparison group) to examine intervention effectiveness. The study design used might not be the most appropriate to address our secondary objectives around the natural history of X(T) and adverse effects. Thus we recommend additional reviews to consider these questions. For example, a review of inception cohort studies would be most appropriate study design to consider evidence of adverse effects associated with different interventions for childhood X(T). Likewise, patient registry studies are suitable to explore the natural history of X(T) in children, observe disease progression and understand long-term outcomes.
Conclusions
Although being mindful of the limitations of observational data to compare the effectiveness of different treatment options, the findings suggest that in some circumstances R&R surgery can produce better outcomes than BLR surgery; however, there is a question about the sustainability of success with the possibility of regression over time. The extent and quality of the evidence have clear limitations that future studies, ideally RCTs or well-designed prospective controlled intervention studies, should address. We look forward to the results of the North American RCTs on efficacy of type of surgery, BLR compared with R&R,29 and observation therapy compared with occlusion therapy for treatment of X(T). 30 The implications of this review for research and practice are summarised in Box 3. To echo the conclusions of Hatt and Gnanaraj,27 consensus is needed as to what constitutes the ideal measure of success, as well as agreement on how this should be measured. Related to this point is the time at which outcomes should be measured post intervention (e.g. 6 months, 1 year, 2 years) as we recognise that surgical outcomes in particular are, to some extent, plastic, with possible drift over time.
On the basis of the findings presented here we tentatively suggest that R&R surgery produces better outcomes in the short term but there is a tendency for deterioration over time.
Surgical outcomes might be improved by coincident additional therapy, such as orthoptic exercises/occlusion prior to surgery or binocular vision training therapy after surgery.
Although non-surgical interventions seem to be less effective in terms of improving angle of deviation, they are rarely associated with adverse outcomes.
We await the results of the ongoing RCTs comparing (1) the effectiveness of BLR and R&R surgery and (2) the effectiveness of occlusion to watchful waiting.
Recommendations for researchThere is a need for well-designed and conducted RCTs to address the question of intervention effectiveness for treatment of divergence excess or basic X(T) in children.
There is a need for a consensus on outcome, how this should be measured and at what time point(s). One approach to address this issue might be to conduct a Delphi survey with experts in the field.
We would encourage authors of future studies to include more detail on how interventions were designed and delivered (e.g. more details on allocation to interventions and measurement of outcomes). Likewise we call for better reporting of methods in future studies.
Acknowledgements
We are very grateful to Fiona Shrive, Omar Hadid, Alan Connor and Umear Ahmed who were involved in the early phases of the review, including contributing to design of the search strategies and screening. Many thanks to Meng Fan, Svetlana Glinianaia, Dominika Kwasnicka, Ibrahim Masri, Lavinia Miceli, Christian Walter and Susie Wong for assistance with translation of non-English-language papers. We would also like to thank Alison Etherington, Ann Payne and Carolyn Wake for administrative support.
Chapter 3 Methods
Trial design
The SamExo trial was a rehearsal pilot RCT to assess the feasibility of a full RCT of the effectiveness of surgical treatment against active monitoring in X(T). The trial was conducted according to recommendations for good practice in pilot studies. 64 Fuller detail about the protocol can be found in our publication. 65
Changes to trial design
Ethical approval
The study was reviewed by the Sunderland Research Ethics Committee (REC) on 18 October 2010 and Mr Michael Clarke, Chief Investigator, and Ms Christine Powell, Co-Investigator, were in attendance. A favourable opinion was given dependent on minor amendments to the Parent Information Sheet and the introduction of a supplementary Parent Information Sheet for parents who declined to take part, which were subsequently agreed. The quality of the documents submitted was commended by the committee.
Amendments to ethical approval
Throughout the course of the study, three substantial amendments were submitted to Sunderland REC for review. All three were granted ethical approval.
Amendment 1 (submitted on 7 July 2011)
The main changes to the protocol were first to the inclusion and exclusion criteria; amendments were made in order to make the criteria clearer to the treatment orthoptists (TOs) and to allow the inclusion of families who did not have English as a first language.
Two new health-economic questionnaires were submitted for REC review. These were used to assess participant’s costs for time and travel, and the use of the health service.
It was found that the original QoL questionnaires were not suitable for children of < 5 years of age, therefore a new QoL questionnaire [Pediatric Eye Disease Investigator Group Intermittent Exotropia Questionnaire (IXTQ)]66 was submitted for review. The IXTQ is a validated three-part, patient-derived health-related quality of life (HRQoL) measure. It has three questionnaires:
-
Parental Asks parents to rate their own HRQoL.
-
Proxy Assesses parental perceptions of the child’s HRQoL.
-
Child For children ≥ 5 years of age, to rate their own HRQoL.
Amendment 2 (submitted on 23 September 2011)
The main change was to the inclusion criteria for ocular alignment evidence. This was to allow the use an alternative test for near stereo acuity (the TNO test for stereoscopic vision) for patients who were unable to cooperate with the Randot test.
The procedure for contacting parents who had expressed interest in the study was also revised. A designated deputy was added to the delegation log in order to assist the TO in contacting the parents by telephone. This was to cover the TO during annual leave.
Amendment 3 (submitted 28 November 2011)
An amendment was submitted in order to ask parents who had declined to allow their child to take part in the study to attend a routine follow-up appointment at 9 months with their child. At this appointment consent would be taken from these parents to allow us to use pseudonymous clinical data collected on their child at the visit, at which they consented, and compare it to the data collected at the 9-month follow-up visit. The aim of this amendment was to help us check that the children who had taken part in the study were representative of all children with X(T) and to find out the proportion in the non-study group who went on to have treatment for control.
A new consent form was developed for this group of people and a new Participant Information Sheet about why we wanted to carry out this substudy was produced.
Research and development approval
As a National Institute for Health Research (NIHR) Portfolio study, the NIHR Coordinated System for gaining NHS permission was applied for via Northumberland Tyne and Wear Comprehensive Local Research Network. No difficulties were encountered with this process.
Participants
Children aged between 6 months and 16 years, who were referred to the clinics with suspected X(T) from community screening, general practice or other health-care professionals, and subsequently diagnosed with X(T), as well as existing patients fulfilling the eligibility criteria, were eligible for the SamExo study. The parent/guardian provided written informed consent for participation in the study prior to any trial-specific procedures.
Data were anonymised by use of a unique trial identification number (ID) assigned by the online trial data management database post consent.
Inclusion criteria
-
Age between ≥ 6 months and ≤ 16 years.
-
Evidence of X(T) on the basis of parental history and clinical examination.
-
No ongoing or planned amblyopia treatment.
-
VA of 0.500 or better on an age-appropriate logMAR (logarithm of minimum angle of resolution) test or, where uniocular testing is not possible, central steady maintained fixation when one eye is occluded.
-
NCS61 of ≥ 3.
-
Minimum of 15 PD misalignment in the distance.
-
Presence of near stereopsis documented using the preschool Randot test if ≥ 3 years of age.
-
If < 3 years old must be able to overcome a base-out prism (10, 15 or 20 PD).
Exclusion criteria
-
Age > 16 years.
-
Previous treatment for X(T).
-
XT (other than microtropia).
-
VA of > 0.500 logMAR in either eye.
-
X(T), where near misalignment is > 10 PDs more than the distance misalignment.
-
Structural ocular pathology.
-
Significant neurodevelopmental delay.
-
Families planning to move out of area.
Study setting
Four secondary ophthalmology care facilities at The Newcastle upon Tyne Hospitals NHS Foundation Trust (co-ordinating site), City Hospitals Sunderland NHS Foundation Trust, Moorfields Eye Hospital NHS Foundation Trust and York Hospitals NHS Foundation Trust, each of which are large centres with specialist paediatric ophthalmology clinics.
The sites (York, Sunderland and Moorfields) were chosen on the basis of their willingness to collaborate with the study and previous experience of collaboration on similar studies.
The patient demographics were significantly different at Moorfields, with a higher variation in ethnicity of the patient population than in the other three sites.
Initial site visits were carried out to each site in order to introduce the study protocol and test procedures. Planned recruitment figures were discussed and any possible issues flagged up early on in the set-up phase of the study.
Feedback from the initial meetings was positive; however, the York and Sunderland sites did express reservations regarding their ability to recruit to target.
Interventions
Identification of potential participants and invitation to participate
(See Figure 2.)
The initial strategy for recruitment was to identify and recruit children newly diagnosed with X(T).
At each site, attempts were made to identify new referrals of children with X(T). In Newcastle, these referrals were booked into specific research clinics. In the other three sites, potential participants in the trial were booked into specific appointment slots within regular clinic sessions.
Apart from general practitioner (GP) referrals, some children were referred on the basis of school vision screening tests, which were carried out in the Sunderland and Newcastle areas.
At the initial appointment, potential participants were screened by the TO and Principal Investigator (PI) at each site for eligibility, and an eligibility screening log was completed to document participants’ fulfilment of the entry criteria. The log also ensured that potential participants were approached only once.
During this visit to the hospital eye service, eligible children were clinically assessed in the normal way and the clinical team introduced the study during the discussion of treatment options and the evidence that was available for each treatment option. Eligible patients were also provided with full study information at this point. Patients who were found not to be eligible resumed normal care.
After at least 24 hours, parents were contacted by the TO or another designated member of the study team by telephone to confirm that they had read and understood the study information, and any questions regarding the study were answered.
All children were then booked into the next available recruitment clinic, which was within 8 weeks of the screening visit.
If consent to further contact about the study was declined then the child entered back into normal care. Reasons for declining to participate in the study were logged by the local PI or delegated deputy. Consent to later contact for interview about reasons for and against participation in the study was also logged, by the local PI or delegated deputy, for those patients who would not be subsequently attending a recruitment clinic. At all stages of this process, it was made clear that consent to participate in the study was entirely voluntary and declining to participate would have no impact on subsequent routine care.
Parents of children who did attend the recruitment clinic, were also asked if they were willing to complete a telephone interview with a qualitative researcher concerning their reasons why they either did or did not want to participate in the study.
Consent was also sought from parents who declined to allow their child to take part in the study but who were willing to attend a routine follow-up appointment at 9 months with their child. At this appointment, pseudonymous clinical data were collected and used in conjunction with previous clinical data collected, in order to assess the extent of participation bias at both recruitment and follow-up.
Clinic appointments
Figure 2 illustrates the schedule of study visits and corresponding assessments for the active monitoring and surgery arm. The assessments involved routine clinical measurements together with the evaluation of QoL using the IXTQ66 (see Appendix 5), a Health Services Use Questionnaire (HSUQ) and a Time and Travel Costs Questionnaire (TTQ) (see Appendix 7). Children in the active monitoring group were offered surgery if a constant strabismus (XT) appeared to be developing or parents requested surgery and the responsible clinical team agreed that this was appropriate. Constant exotropia was defined as NCS 9 with no demonstrable binocular single vision.
FIGURE 2.
Summary of study visits, assessments and interventions. HSUQ, Health Services Use Questionnaire; RO, research orthoptist; TTQ, Time and Travel Costs Questionnaire.

Eye muscle surgery
Surgery was performed by the local PI or delegated deputy, in accordance with agreed surgical formulae tailored to the clinical characteristics of the strabismus and the usual practice of the surgeon. Principles involved in the surgical treatment of children in the study were agreed as follows:
-
general anaesthesia
-
BLR surgery to be performed for true-distance exotropia
-
unilateral recession/resection surgery to be performed for other types of exotropia
-
standard sterile preparation of the operative site
-
conjunctival incisions
-
standard isolation and cleaning of muscle to be operated
-
muscle secured with 6/0 VICRYL® (polyglactin 910) suture (Johnson & Johnson, New Brunswick, NJ, USA)
-
amount of R&R assessed on the basis of the maximum distance angle according to table, modified according to standard practice of surgeon
-
measurement of amount of muscle adjustment to be checked post placement of scleral sutures
-
conjunctival incisions closed with VICRYL sutures
-
topical anaesthetic and antibiotic drops given at end of procedure.
A surgical table was used with modification as appropriate to determine the amount of eye muscle movement to be performed depending upon the size of the angle of exotropia. Surgical technique was carefully recorded and monitored during the pilot, with a view to standardising surgical technique – as far as it was possible to do so – in a full trial and provide a clear description of the intervention in subsequent reports.
Primary outcome visit
The final assessment (9-month outcome) was conducted by a research orthoptist (RO) who was masked to the allocation of the child and was not otherwise in contact with children enrolled in the study. The parent and child were requested not to reveal the group allocation of the child to the RO prior to the assessment. Although children have noticeably red eyes immediately following eye muscle surgery, it is recognised that this redness resolves within 6 weeks when this is a first procedure. Residual scarring of the conjunctiva following eye muscle surgery will be inconspicuous by 6 months.
Parent interviews
We gathered qualitative data (primarily through telephone interviews) from parents to explore their reasons for either accepting or declining participation in the pilot study, their thoughts on randomisation and the study information received, and any ideas they may have had for improving the trial. These data would inform the design of a full RCT. Although the principles of informed consent meant that individuals were not obliged to give a reason for their decision if they did not want to, we invited parents to take part in a telephone interview if they agreed to this level of involvement. Without this information, it is difficult to see how research design can be improved to make it more acceptable. These interviews were conducted by a university researcher who was entirely separate from the clinical team, emphasising that parents’ decisions not to participate have been respected and reassuring that no attempt was being made to change their minds. A total of 48 parents who consented to the telephone call were interviewed, 34 of whom had declined and 14 of whom had agreed participation in the trial. Most (40) were mothers and eight were fathers. Interviews took on average 10 minutes (minimum 5 minutes, maximum 30 minutes). These parents had been approached about the trial between September 2011 and May 2012.
Planned outcome measures
There are three classes of clinical measure that could be used as outcome measures for the treatment of X(T).
-
Stereoacuity This is normally preserved for near fixation in affected children and will generally be unchanged by treatment unless it deteriorates as a result of surgical overcorrection of the development of a constant squint. Stereoacuity at distance may be affected but is not easily measurable and has high test/test variability.
-
Ocular alignment This is measured in PDs for near and distance fixation. It may be measured by a simultaneous prism cover test, which attempts to capture the alignment before binocular vision is disrupted, or on an alternating prism cover test, which is the total misalignment demonstrated by disrupting binocular vision by covering each eye in turn. In practice, binocular vision is easily disrupted in children with the condition and the usual reported measure is the total misalignment on an alternating prism cover test. The limitation of this measure is that alignment may be improved at the expense of deterioration in stereoacuity, if a constant, but small, misalignment is a consequence of treatment. Hence, as an outcome measure, this may overestimate the benefit of treatment by concentrating on the cosmetic outcome at the expense of the functional outcome. Neither does it attempt to measure the frequency with which misalignment occurs, which is a key factor in assessing the success of intervention in a condition in which ocular misalignment is intermittent.
-
Control This is measured using either the NCS61 or the Mayo scale19 for the condition. Both are suited to the assessment of an intermittent misalignment as is seen in the condition, and measure the frequency with which the misalignment occurs and the ease with which realignment occurs. Possible scores range from 0 to 9 and from 0 to 10, respectively; higher scores denote poorer control.
We feel that, because of the limitations of measures of alignment, a combination of measures of control and stereoacuity represent the most appropriate clinical outcome measures for treatment of the condition.
For children with X(T), and their parents, the most relevant outcome from intervention is the restoration of normal eye alignment, with associated cosmetic and functional benefits. The primary outcome in a full-scale trial would be the difference in the cure rate of X(T) between the surgical and actively monitored group; this was also the primary outcome for which data were collected in the current pilot in order to inform sample size calculations for a definitive RCT. Cure was defined as:
-
a control score (NCS61) of 0 (misalignment never noticed by parents, no observable deviation on cover test)
-
demonstrable near stereoacuity in children over 3 years of age.
Secondary outcomes included age-specific QoL assessments, satisfactory control of exotropia assessed by parental report and clinical components of the NCS61 and the Mayo score,19 rates of amblyopia, use of health-care resources, NHS costs, costs to families accessing the treatments being evaluated and incremental cost per cured patient (with cure as defined by the primary outcome) and a cost–consequences analysis based on incremental cost with respect to changes in all relevant outcomes where possible.
With respect to the choice of QoL measure, we know from our previous work with the Pediatric Quality of Life Inventory20 that there is little or no effect of the condition on the scores obtained, so we sought an alternative generic instrument that would capture any visual or psychosocial effect of the condition. We proposed the use of the Health Utilities Index Mark 3 (HUI3) in this study but found it unsuitable for the age of the population involved. In a longer-duration study, where the views of older children and their parents could be captured at outcome, the HUI3 may prove to be an acceptable generic instrument. We therefore used the newly developed condition-specific IXTQ. 66
The key outcomes of this pilot study were:
-
data on the variability of the primary and secondary outcome measures
-
rates of participant recruitment and randomisation
-
nature and extent of participation bias
-
rates of crossover and retention of recruited participants
-
nature and extent of biases arising from crossover or loss to follow-up.
An initial recruitment rate of > 60% and a retention rate of > 70% was considered necessary to indicate feasibility of a full-scale RCT.
Our intention in the full trial would be to conduct a cost-effectiveness analysis based on incremental cost per cured patient (as defined by the primary outcome) and a cost–consequences analysis based on incremental cost with respect to changes in all relevant outcomes where possible, including the QoL measure and different clinical measures. In this study we assessed the ease of collecting information on outcome and costs needed for the health economics analysis.
With respect to collecting costs data, we piloted the TTQ and HSUQ that captured patient costs and the NHS costs. We assessed the response and completion rates of these instruments. Patient costs included travel costs for accessing NHS primary and secondary care; time costs of travelling and attending NHS primary and secondary care; and self-purchased health-care and related management costs. NHS costs comprised use of health-care resources in both primary and secondary care. Total costs consisted of patient costs and the NHS costs compared with the randomised interventions.
Sample size
A formal power calculation was not performed for this feasibility study. It was not powered to detect a clinically or economically meaningful difference in the primary outcome between the surgical and active monitoring groups. Rather, the aim was to provide robust estimates of the likely rates of recruitment and retention, and to yield estimates of the variability of the primary and secondary outcomes to inform power calculations for a subsequent full-scale RCT. We originally estimated that over a recruitment period of 6 months (subsequently extended to 9 months) across four centres we would be able to approach 240 patients who met the entry criteria. From their responses we would be able to determine whether or not the study is acceptable to parents and consequently whether or not it is possible to recruit patients and follow them up. We would also be able to estimate attrition rates. By approaching 240 children/parents we would be able to estimate the recruitment rate with a standard error no larger than 3.3%. Assuming that half of these children were actually recruited we would be able to estimate the 6-monthly attrition rate with a standard error of ≤ 4.3%.
Randomisation
Randomisation was in permuted blocks, stratified by collaborating centre, age and severity of X(T), as measured by the NCS. 61 A blocked allocation (permuted random blocks of variable length) system was used to allocate patients to the two groups in a 1 : 1 ratio to intervention (surgery) and control (active monitoring) groups. Randomisation was administered using a centralised, password-protected, web-based system that was managed by the Newcastle Clinical Trials Unit (NCTU).
As participants in the study were children of ≤ 16 years, consent was taken from the parent or legal guardian; however, every effort was made to include the child in the consent process.
Once consent was obtained, the PI at site, or individual with delegated authority, entered the patient ID, initials and the stratifying variables that then returned the allocation status. Participants were informed of their group allocation and given the appropriate Group Allocation Information Sheet.
Children in the surgery group proceeded to surgical preassessment before undergoing standard eye muscle surgery for X(T). They were then reviewed within 2 weeks of surgery and reviewed as was clinically appropriate, depending on the result of the surgery, before further review at 6 months post recruitment and final review at 9 months post recruitment.
Children in the active monitoring group proceeded to a clinic appointment arranged for 3 months’ time. Further review took place at 6 months post recruitment and then final review at 9 months post recruitment, as for the surgery group. At the final review at 9 months post recruitment (6 months post surgery), clinical outcome measures were obtained for subsequent analysis.
Masking
As surgery was the intervention of interest, it was not possible to mask participants or parents to their group allocation. Masking of investigators was achieved by the designation of a TO and a RO at each site. TOs could not be masked to the group allocation of participants, as they conducted all assessments other than the outcome assessment, and dealt with queries from parents/children during the course of the trial. Clinical examination at the primary outcome visit was carried out by the ROs who were unaware of treatment group allocation. At this final study visit, the success of masking was assessed by asking the outcome assessor: ‘Do you think the patient has had surgery or not? Why do you think this?’ Their responses were recorded on a separate form.
Statistical methods
For primary outcomes Student’s t-tests and Mann–Whitney U-tests were used to compare age and severity of X(T) of those participants retained with patients withdrawing from the trial, and to compare those who consented with those eligible but refused to participate. A Fisher’s exact test was carried out to assess variability of the primary outcome (difference in cure rate at the final assessment between the surgery and active monitoring arm); effect size [95% confidence intervals (CIs)] is also reported.
For secondary outcomes A chi-squared test was used to compare rates of satisfactory NCS61 outcome between groups; odds ratios and 95% CIs are also reported. Rates of development of amblyopia in the two groups were determined by monitoring VA at the 3-, 6- and 9-month assessments. For the QoL measurements, we were primarily concerned with response and completion rates for those instruments in both groups; in addition, their validity was assessed by matching individual pre- and post-treatment scores to the post-treatment primary outcome, using Student’s t-tests.
Statistical significance was set at p < 0.05. Data were analysed with the SPSS statistical package [SPSS (Statistical Package for the Social Sciences) Inc., Chicago, IL, USA], version 19.
Adverse event reporting
Adverse event reporting was undertaken in accordance with the National Research Ethics Service guidelines for adverse event reporting in trials that do not involve investigational medical products.
Definition of serious adverse event
A serious adverse event (SAE) (Table 4) was defined as an untoward occurrence that:
-
resulted in death,
-
is life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity,
-
was otherwise considered medically significant by the investigator.
| Procedure | Adverse event | |
|---|---|---|
| Common and well understood consequences of treatment | Rare events | |
| Perforation of the globe | Occurring within 24 hours | Occurring after 24 hours |
| Intraocular infection | Occurring within 2 weeks | Occurring after 2 weeks |
| Lost or slipped muscle | Occurring within 1 month | Occurring after 1 month |
| Scleritis | Occurring within 1 month | Occurring after 1 month |
| Becoming constant XT | Occurring within 9 months | Occurring after 9 months |
| Persistent overcorrection | Occurring within 9 months | Occurring after 9 months |
Trial management processes
The Trial Management Meeting Schedule for the SamExo Trial included:
-
Trial Management Group Meetings Monthly study management meetings were held between members of the NCTU (Senior Trial Manager and Trial Manager), the Chief Investigator and the core study staff at Newcastle. This meeting reviewed the progress at all sites.
-
Newcastle Study Group Meeting A further monthly study meeting was held between the Trial Manager, the Chief Investigator and the research staff at Newcastle. This meeting reviewed the progress at Newcastle. Both meetings were minuted.
-
Monthly minuted conference calls were held between all PIs (Robert Taylor, York; John Sloper, Moorfields; Peter Tiffin; Sunderland) and the Chief Investigator. TOs could also dial in if they had any issues to discuss.
-
Four Trial Steering Committee (TSC) Meetings were held, chaired by Professor Charlotte Wright.
Good clinical practice training
All staff involved in the study were required to complete Good Clinical Practice (GCP) training. As this study was portfolio adopted, GCP training was provided free of charge through the NIHR.
Site initiation visits
Each site received the Investigator Site File (ISF) with a CD-ROM containing all of the documentation for the Pilot Rehearsal Trial and had a site initiation visit from the Trial Manager and the Co-Investigator, Christine Powell, in advance of patient recruitment. This visit covered training in use of the various logs within the ISF (screening, delegation, etc.), briefing on which paperwork was relevant at each stage and safety reporting using the Serious Adverse Event form and issues such as version control of documents. A ‘typical’ patient route through the study was included, allowing site staff to talk through the practicalities of recruitment. Training was also provided on the electronic Case Report Forms and the web-based randomisation system.
Throughout the Pilot Rehearsal Trial, PIs and TOs were encouraged to contact the Trial team with any queries they had.
Several communication strategies were adopted to encourage retention and fidelity to protocol. These were mainly personal contact through telephone, e-mail and face-to-face meetings. Other methods of communication included the SamExo website (http://research.ncl.ac.uk/samexo/). The website served a dual role of being a point of reference for the study staff and a means of sharing information with the general public. Feedback from the orthoptists, children and parents involved in the trial was very positive.
Facebook was also used as a more novel method of communication and a way to share non-sensitive information with the general public (www.facebook.com/pages/The-SamExo-research-study/279725438765959).
The experiences of the participating sites were captured during a final face-to-face SamExo team meeting after the end of follow-up.
Chapter 4 Results
Site recruitment and retention
All sites that began recruitment of patients were retained throughout the Pilot Rehearsal Trial and all have expressed an interest in continuing with a full trial. A web-based survey of other ophthalmology units in the UK was conducted (via e-mail discussion group PAED-OPHTH-STRABISMUS list: 157 recipients) and responses were obtained from 37 units treating X(T) (see Appendix 6). Of these 37 units, 35 (95%) indicated that they would be willing to participate in a future RCT.
Participant screening and recruitment
As of 29 February 2012, the proposed end of the recruitment period, 183 children had been screened out of an expected 240. Of those screened, 117 (64%) were eligible (expected 228 or 95%) and 29 (25% of eligible) children were recruited [expected 144 (64% of eligible)].
At the TSC meeting on 18 October 2011, the slow pace of recruitment was identified, and it was agreed to make an application for a no-cost extension, to extend recruitment to 31 May 2012, in order to see if strategies to improve recruitment had been effective. This request was granted.
Between 29 February 2012 and 31 May 2012, a further 48 children were screened, of whom 24 (50%) were eligible and 20 (83% of eligible) were recruited. Although this population includes a number of children who were either rescreened for eligibility, or whose parents had expressed a provisional interest in the trial, and was therefore an enriched population in terms of potential recruits, it does indicate that the strategies adopted by the study team to improve recruitment had a significant effect.
In total therefore, by 31 May 2012, 231 children had been screened (expected 240), 138 (60%) of whom were eligible (expected 228: 95%) and 49 (35% of eligible children) were recruited (expected 144: 64% of eligible: Figure 3).
FIGURE 3.
SamExo recruitment graph: expected vs. actual recruitment.

Eligibility
Many more children than predicted did not fulfil the eligibility criteria for the study (10/240 predicted vs. 93/231 observed). Reasons for non-eligibility were determined for 87/93 (94%) and can be summarised as:
-
severity of X(T) insufficient to meet eligibility criteria, 40%
-
inability to demonstrate binocular vision at near fixation, 23%
-
did not have target condition, 19.5%
-
ocular comorbidity, 7%
-
could not be adequately assessed, 4.5%
-
received previous treatment, 3.5%
-
systemic comorbidity, 2%.
The most common reason for children not to meet the eligibility criteria was that their strabismus was not sufficiently severe. This was either a reflection of the size of the misalignment when present (a misalignment of 15 PDs was chosen to reflect usual criteria for surgical intervention) or that the control of the strabismus (the ease with which the eyes were realigned following dissociation) was too good.
Consent to participation in the trial
Brief information on reasons why eligible parents refused participation was available from the screening logs in 80/89 (90%) cases.
These can be summarised as:
-
parents did not feel strabismus severe enough to warrant surgery, 65%
-
parents felt surgery was necessary and did not want to wait, 15%
-
objections to trial processes, for example randomisation, 12.5%
-
other reasons, 7.5%.
Reasons for agreeing to, or declining, participation in the trial were explored in more detail in the qualitative interviews.
Participation bias
Consent was obtained from 56/89 (63%) ‘eligible not recruited’ (ENR) patients to record baseline and 9-month follow-up clinical data. The demographic and clinical characteristics of the ENR group were compared with those who agreed participation in the SamExo trial (Table 5). Those who agreed to take part had poorer control of their exotropia (as assessed by the NCS61) than those who declined; although statistically significant this difference is unlikely to be clinically significant. The mean age of those who agreed was slightly older but not significantly so (see Table 5). The proportion of males and females was almost identical in each arm (34–37% male).
| Characteristics at screening | Agreed trial participation (n = 49) | Refused trial participation (n = 56) | p-value |
|---|---|---|---|
| Median (IQR), NCS61 | 5 (4–6) | 4 (4–5) | p = 0.002a |
| Mean (SD), NCS61 | 5.3 (1.6) | 4.4 (1.1) | p = 0.001b (95% CIs 0.37 to 1.44) |
| Mean (SD) age, years | 4.4 (2.2) | 3.8 (2.0) | p = 0.16b (95% CIs –0.24 to 1.42) |
| % male | 37% | 34% | p = 0.76c [difference between groups = 7.2% (95% CI –42.9% to 57.4%) or RR = 1.08 (95% CI –0.59 to 1.89)] |
Randomisation
The randomisation process was generally smooth, although it was intermittently difficult to access the website. This problem was generally solved through contact with the database team, although occasionally the result of randomisation had to be given to the parents after they had left the clinic.
Randomisation appeared to work well, with 25 participants allocated to surgery and 24 to active monitoring. One parent immediately declined the allocation to surgery, but the remaining 48 participants remained in their allocated group.
Seven children in the active monitoring group, and four in the surgery group, were prescribed glasses at baseline to correct refractive error. No glasses were issued subsequently.
Baseline demographic and clinical characteristics by treatment arm are provided in Table 6; there were no significant differences on any parameter.
| Active monitoring | Surgery | |
|---|---|---|
| Mean (SD) age, years (n = 49) | 4.3 (2.2) | 4.5 (2.3) |
| Female gender (n = 49) | 14 (58%) | 17 (68%) |
| Median (IQR) near stereoacuity (seconds of arc) (n = 40) | 100 (60 to 800) | 100 (60 to 400) |
| Overcome a base-out prisma (n = 9) | 5/5 = yes | 4/4 = yes |
| Mean (SD) VA (n = 47)b | ||
| Right eye | 0.078 (0.11) | 0.057 (0.09) |
| Left eye | 0.072 (0.12) | 0.054 (0.08) |
| ‘Worse’ eye | 0.088 (0.11) | 0.070 (0.08) |
| Median angle (PD) (n = 49) | ||
| Near | 16 (12 to 25) | 20 (11 to 25) |
| Distance | 30 (25 to 35) | 30 (27.5 to 40) |
| Median control score (n = 49) | ||
| Total NCS61 | 5.5 (4 to 6) | 5 (4 to 6) |
| Home control | 2 (1 to 3) | 2 (1 to 2) |
| Clinic control | 3 (3 to 4) | 3 (3 to 4) |
| Mayo score19 | 4 (3 to 4.75) | 4 (3 to 5) |
Adherence to protocol and retention
Two patients received surgery after the proposed 3-month window: one who was awaiting the results of sickle cell investigations and one whose operation was arranged but then cancelled due to chickenpox.
The outcome results for these patients were included in the analysis even although only 4 months’ postoperative follow-up was possible, as opposed to the desired 6 months.
The parent of one patient allocated to surgery immediately declined the allocation. The participant was retained in the trial and the results were analysed on an intention-to-treat basis.
Two patients in the active monitoring group were lost to follow-up despite repeated postal and telephone reminders.
A total of 47/49 (96%) recruited participants attended for the final appointment, scheduled at 9 months post randomisation.
The flow of participants through the trial is shown in the CONSORT (Consolidated Standards of Reporting Trials) diagram (Figure 4).
FIGURE 4.
Consolidated Standards of Reporting Trials diagram.
Adverse events
Unexpected adverse events
There were no serious unexpected adverse events.
Expected adverse events
Active monitoring group
Although using our pre-defined definition no children in the active monitoring arm developed a constant exotropia two (10301 and 46701) appeared to show some reduction in control and/or binocular function as assessed by stereo acuity angle and NCS.
Surgery group
Two children had constant overcorrections following surgery at the outcome visit (10103, 23101); a further five children (10401, 10406, 10408 and 22201, 45501) had intermittent overcorrections at either near or distance fixation at outcome. One child (22103) underwent further eye muscle surgery for an overcorrection within the follow-up period; this child developed a recurrent X(T) following repeat surgery.
In a future full trial consideration would be given to including development of amblyopia and loss of binocular functions as expected adverse events.
Masking
Masking was successfully maintained throughout, in that there were no overt breaches. In the majority of cases, however, the orthoptist assessing outcome correctly guessed which treatment arm the participant was in, except for one case in the surgery arm and two in the active monitoring arm.
Quality-of-life outcomes: Intermittent Exotropia Quality of Life Questionnaires
No parent or child refused to complete this instrument. There were 49 out of 49 expected parent IXTQs at baseline, 45 out of 46 expected proxy IXTQs (one was not administered, in error; the child in the other three cases was too young, i.e. < 2 years) and 23 out of 23 expected child IXTQs (the remainder were not old enough to complete an IXTQ themselves). At follow-up there were 45 out of 47 expected parent IXTQs (two were not administered in error; the remaining two withdrew/were lost to follow-up), 44 out of 47 expected proxy IXTQs (three were not administered, in error; two withdrew/were lost to follow-up) and 29 out of 30 expected child IXTQs (one not administered, in error, and 17 were too young; the remaining two withdrew/were lost to follow-up).
Missing response rates to individual items within the IXTQ were no > 7%. The highest percentage of missing responses was for item 7 on the child-rated IXTQ (‘Does it bother you that you have to shut one eye when it is sunny?’): this was unanswered by 1 out of 23 (4%) of children at baseline and 2 out of 29 (7%) at follow-up. All other items on the child-rated IXTQ were completed 100%. Items 1, 4, 9 and 11 on the proxy-rated IXTQ were missed in 1 out of 45 (2%) cases at baseline; at follow-up, items 3, 5, 7, 9 and 12 were each unanswered in 1 out of 44 (2%) cases. No items were skipped on the parent IXTQ at baseline, and at follow-up items 4, 5 and 15 were missed in only 1 out of 45 (2%) cases.
Higher scores denote better perceived quality of life. At baseline, there were no differences between treatment arm and IXTQ scores on any scale or subscale. There were no statistically significant changes over time in the active monitoring group on any parent IXTQ scales. Significant improvements were found within the surgery arm on all Parent IXTQ scales (total score, and function, psychosocial and surgery subscales, Tables 7 and 8). Mean score on the Proxy IXTQ deteriorated for those in the active monitoring arm and improved for those in the surgery group, but the differences were small and not or only just statistically significant (Table 9). A small, but not statistically significant, improvement was seen in the Child IXTQ scores for each arm (also note the very small numbers) but there was no significant difference between groups (Table 10).
| Group | Baseline | Outcome | Mean change | 95% CIs; p-value |
|---|---|---|---|---|
| Active monitoring (n = 20) | 53.1 (15.9) | 48.4 (22.9) | –4.7 | –14.1 to 4.7; 0.31 |
| Surgery (n = 25) | 51.9 (18.11) | 74.3 (18.0) | 22.4 | 10.7 to 34.0; 0.001 |
Subscale quality-of-life scores
| Group | Baseline | Outcome | Mean change | 95% CIs; p-value |
|---|---|---|---|---|
| Active monitoring (n = 20) | 55.9 (18.4) | 51.3 (25.6) | –4.6 | –13.5 to 4.3; 0.29 |
| Surgery (n = 25) | 51.6 (21.1) | 74.5 (18.0) | 22.9 | 11.3 to 34.4; < 0.001 |
| Group | Baseline | Outcome | Mean change | 95% CIs; p-value |
|---|---|---|---|---|
| Active monitoring (n = 20) | 52.3 (20.6) | 48.4 (27.2) | –3.9 | –15.3 to 7.4; 0.48 |
| Surgery (n = 25) | 54.0 (20.9) | 75.5 (19.4) | 21.5 | 8.6 to 34.4; 0.002 |
| Group | Baseline | Outcome | Mean change | 95% CIs; p-value |
|---|---|---|---|---|
| Active monitoring (n = 20) | 40.0 (24.5) | 36.9 (26.7) | –3.1 | –14.7 to 8.4; 0.58 |
| Surgery (n = 25) | 46.0 (24.9) | 69.5 (24.8) | 23.5 | 10.2 to 36.8; 0.001 |
| Group | Baseline | Outcome | Mean change | 95% CIs; p-value |
|---|---|---|---|---|
| Active monitoring (n = 18) | 77.7 (21.2) | 69.5 (23.9) | –8.2 | –16.9 to 0.5; 0.064 |
| Surgery (n = 23) | 80.9 (14.5) | 87.4 (12.8) | 6.5 | 0.21 to 12.9; 0.043 |
| Group | Baseline | Outcome | Mean change | 95% CIs; p-value |
|---|---|---|---|---|
| Active monitoring (n = 12) | 73.6 (19.8) | 80.7 (13.5) | 7.1 | –5.1 to 19.4; 0.23 |
| Surgery (n = 11) | 73.1 (16.4) | 76.5 (19.7) | 3.4 | –5.5 to 12.3; 0.42 |
Clinical outcomes
Details of the clinical outcomes are provided in Table 11a–d, below.
| ID | Centre | APCT near, baseline | APCT near, 9 months | APCT distance | APCT distance, 9 months | BSV, baseline | Prism, baseline | BSV, 9 months | Prism, 9 months | Composite measure outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 10101 | Newcastle | 12 X | 10 X | 20 X(T) | 25 X(T) | 200 | 100 | Poor | ||
| 10104 | Newcastle | 10 X | 20 X | 25 XT | 30 XT | 1980 | 100 | Poor | ||
| 10301 | Newcastle | 16 X | 20 X(T) | 30 XT | 25 XT | n/u | 15 | n/u | No | Poor |
| 10303 | Newcastle | 12 X | 12 X | 30 XT | 25 X(T) | n/u | 20 | n/u | 20 | Poor |
| 10402 | Newcastle | 14 X | 16 X | 45 XT | 50 XT | 1980 | 400 | Poor | ||
| 10403 | Newcastle | 25 X | 30 X | 35 X(T) | 20 X(T) | 40 | 40 | Poor | ||
| 10405 | Newcastle | 12 X | 10 X | 35 XT | 30 XT | 60 | 100 | Poor | ||
| 10407 | Newcastle | 0 | 2 X | 45 X(T) | 40 X(T) | 40 | – | 40 | Poor | |
| 11401 | Newcastle | 45 X(T) | 45 X(T) | 45 X(T) | 40 X(T) | 100 | 40 | Poor | ||
| 22104 | Sunderland | 16 X | 20 X(T) | 40 XT | 25 XT | n/u | 15 | 100 | Poor | |
| 33701 | York | 6 X | 5 X | 16 X(T) | 12 X | 100 | 100 | Fair | ||
| 33801 | York | 25 X | 22 X | 25 X(T) | 25 X | 200 | 40 | Poor | ||
| 34001 | York | 35 X(T) | 20 X | 30 X(T) | 25 X(T) | 100 | 100 | Poor | ||
| 34501 | York | 25 X(T) | 10 X | 25 X(T) | 18 X(T) | n/u | 15 | n/u | 20 | Poor |
| 34601 | York | 22 X(T) | 20 X(T) | 25 XT | 25 XT | 400 | 100 | Poor | ||
| 45502 | Moorfields | 8 X | 12 X | 25 XT | 18 XT | 60 | 40 | Poor | ||
| 45503 | Moorfields | 6 X | 2 X | 20 X(T) | 18 X(T) | 40 | 200 | Poor | ||
| 45801 | Moorfields | 16 X | 35 X(T) | 35 X(T) | 35 XT | 60 | 60 | Poor | ||
| 45803 | Moorfields | 14 X | 14 X | 35 X(T) | 20 X(T) | 800 | 100 | Poor | ||
| 45806 | Moorfields | 14 X | 12 X | 35 XT | 25 XT | 400 | – | 40 | Poor | |
| 46501 | Moorfields | 20 X(T) | 12 X | 25 XT | 14 X(T) | 40 | 60 | Fair | ||
| 46701 | Moorfields | 30 X(T) | 18 X(T) | 30 XT | 25 XT | 800 | Gross TNO test only | Poor |
| ID | Centre | NCS,61 home, baseline | NCS,61 clinic, baseline | NCS,61 baseline | NCS,61 home, 9 months | NCS,61 clinic, 9 months | NCS,61 9 months | NCS61 of 0 at 9 months | NCS,61 satisfactory outcomea | Mayo,19 baseline | Mayo,19 9 months |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10101 | Newcastle | 1 | 2 | 3 | 1 | 3 | 4 | No | No | 2 | 3 |
| 10104 | Newcastle | 2 | 3 | 5 | 3 | 4 | 7 | No | No | 4 | 5 |
| 10301 | Newcastle | 1 | 3 | 4 | 2 | 6 | 8 | No | No | 4 | 6 |
| 10303 | Newcastle | 2 | 3 | 5 | 2 | 3 | 5 | No | No | 4 | 4 |
| 10402 | Newcastle | 2 | 3 | 5 | 1 | 3 | 4 | No | No | 4 | 4 |
| 10403 | Newcastle | 3 | 3 | 6 | 3 | 5 | 8 | No | No | 4 | 5 |
| 10405 | Newcastle | 1 | 3 | 4 | 1 | 5 | 6 | No | No | 4 | 5 |
| 10407 | Newcastle | 3 | 3 | 6 | 1 | 2 | 3 | No | Yes | 3 | 2 |
| 11401 | Newcastle | 1 | 6 | 7 | 1 | 4 | 5 | No | No | 3 | 4 |
| 22104 | Sunderland | 1 | 3 | 4 | 3 | 3 | 6 | No | No | 8 | 4 |
| 33701 | York | 1 | 2 | 3 | 3 | 2 | 5 | No | No | 4 | 3 |
| 33801 | York | 1 | 2 | 3 | 2 | 3 | 5 | No | No | 2 | 1 |
| 34001 | York | 3 | 3 | 6 | 3 | 1 | 4 | No | No | 1 | 1 |
| 34501 | York | 3 | 4 | 7 | 2 | 3 | 5 | No | No | 1 | 1 |
| 34601 | York | 3 | 5 | 8 | 1 | 6 | 7 | No | No | 4 | 3 |
| 45502 | Moorfields | 1 | 3 | 4 | 2 | 3 | 5 | No | No | 6 | 8 |
| 45503 | Moorfields | 1 | 3 | 4 | 1 | 2 | 3 | No | No | 4 | 5 |
| 45801 | Moorfields | 3 | 3 | 6 | 2 | 6 | 8 | No | No | 3 | 2 |
| 45803 | Moorfields | 2 | 3 | 5 | 1 | 4 | 5 | No | No | 3 | 7 |
| 45806 | Moorfields | 2 | 4 | 6 | 1 | 3 | 4 | No | No | 3 | 5 |
| 46501 | Moorfields | 2 | 6 | 8 | 1 | 4 | 5 | No | Yes | 5 | 4 |
| 46701 | Moorfields | 1 | 6 | 7 | 3 | 5 | 8 | No | No | 8 | 5 |
| ID | Centre | Near APCT, baseline | Near APCT, 9 months | Distance APCT, baseline | Distance APCT, 9 months | BSV baseline | Prism baseline | BSV, 9 months | Prism, 9 months | Composite measure outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 10102 | Newcastle | 20 X | 4 X | 20 X(T) | 6 X | 400 | –ve controlled | 20 | Good | |
| 10103 | Newcastle | 25 X | –25 ET | 25 X(T) | –20 ET | 1980 | n/u | Poor | ||
| 10302 | Newcastle | 8 X | 6 X | 40 XT | 0 | 400 | 200 | 20 | Good | |
| 10304 | Newcastle | 45 X(T) | 45 X(T) | 35 X(T) | 40 X(T) | 1980 | 200 | Poor | ||
| 10401 | Newcastle | 10 X | –30 E | 30 X(T) | –14 E | 60 | 40 | Gooda | ||
| 10404 | Newcastle | 2 X | 0 | 30 X(T) | 8 X | 1980 | 800 | Good | ||
| 10406 | Newcastle | 2 X | –18 E(T) | 30 XT | –1 E | 60 | 40 | Gooda | ||
| 10408 | Newcastle | 30 X | –16 E(T) | 45 XT | –4 E | 200 | 200 | 15 | Gooda | |
| 10409b | Newcastle | 12 X | 45 X | 40 X(T) | 45 X(T) | 40 | 40 | Poor | ||
| 11201 | Newcastle | 45 X | 10 X | 40 XT | 10 XT | n/u | 20 | 400 | 20 | Fair |
| 22102 | Sunderland | 12 X | –4 E | 35 XT | 2 XT | n/u | 20 | n/u | 20 | Good |
| 22103 | Sunderland | 25 X | 40 X | 35 X(T) | 40 X(T) | n/u | 10 | 100 | 15 | Poor |
| 22201 | Sunderland | 10 X | –40 E(T) | 40 X | –30 E | 100 | 100 | Gooda | ||
| 23101 | Sunderland | 25 X(T) | –8 ET | 35 XT | –8 ET | n/u | 10 | n/u | No | Poor |
| 23201 | Sunderland | 20 X(T) | 10 X | 30 XT | 6 X | 40 | 40 | 15 | Good | |
| 33702 | York | 20 X(T) | 20 X(T) | 25 XT | 25 X(T) | 100 | 400 | Poor | ||
| 33703 | York | 15 X(T) | 8 X | 20 X(T) | 4 X | 400 | 200 | Good | ||
| 45501 | Moorfields | 8 X | –12 ET | 25 XT | 6 X(T) | 60 | TNO test, gross | Good | ||
| 45601 | Moorfields | 12 X | 10 X | 20 XT | 12 X(T) | 100 | 100 | Fair | ||
| 45701 | Moorfields | 12 X | 14 X | 45 XT | 20 X | 800 | 200 | Poor | ||
| 45802 | Moorfields | 25 X | 14 X | 35 XT | 18 X | 60 | 100 | Poor | ||
| 45804 | Moorfields | 14 X | 2 X | 30 XT | 0 | 200 | 100 | Good | ||
| 45805 | Moorfields | 20 X(T) | 16 X | 30 X(T) | 20 X(T) | 400 | 200 | Poor | ||
| 45901 | Moorfields | 30 X | 40 X | 50 XT | 30 X | 40 | 40 | Poor | ||
| 46801 | Moorfields | 25 X(T) | 10 X | 30 XT | 16 X(T) | 40 | 40 | Poor |
| ID | Centre | NCS61 home, baseline | NCS61 clinic, baseline | NCS,61 baseline | NCS,61 home, 9 months | NCS,61 clinic, 9 months | NCS,61 9 months | NCS61 of 0 at 9 months | NCS,61 satisfactory outcomea | Mayo,19 baseline | Mayo,19 9 months |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10102 | Newcastle | 1 | 3 | 4 | 0 | 0 | 0 | Yes | Yes | 4 | 1 |
| 10103 | Newcastle | 1 | 2 | 3 | N/A | N/A | N/A | No | No | 2 | –1- |
| 10302 | Newcastle | 2 | 3 | 5 | 1 | 0 | 1 | No | Yes | 4 | 0 |
| 10304 | Newcastle | 3 | 3 | 6 | 0 | 3 | 3 | No | Yes | 3 | 2 |
| 10401 | Newcastle | 1 | 2 | 3 | 0 | 0 | 0b | Yesb | Yesb | 2 | –1- |
| 10404 | Newcastle | 2 | 3 | 5 | 0 | 0 | 0 | Yes | Yes | 4 | 0 |
| 10406 | Newcastle | 2 | 4 | 6 | 0 | 0 | 0b | Yesb | Yesb | 4 | –1- |
| 10408 | Newcastle | 2 | 4 | 6 | 0 | 0 | 0b | Yesb | Yesb | 5 | –1- |
| 10409c | Newcastle | 1 | 2 | 3 | 1 | 4 | 5 | No | No | 4 | 5 |
| 11201 | Newcastle | 3 | 3 | 6 | 1 | 4 | 5 | No | No | 5 | 4 |
| 22102 | Sunderland | 2 | 4 | 6 | 1 | 3 | 4 | No | No | 4 | 1 |
| 22103 | Sunderland | 1 | 2 | 3 | 2 | 3 | 5 | No | No | 1 | 3 |
| 22201 | Sunderland | 2 | 2 | 4 | 0 | 0 | 0b | Yesb | Yesb | 2 | –1- |
| 23101 | Sunderland | 3 | 6 | 9 | N/A | N/A | N/A | No | No | 7 | –1- |
| 23201 | Sunderland | 3 | 5 | 8 | 0 | 0 | 0 | Yes | Yes | 6 | 0 |
| 33702 | York | 1 | 4 | 5 | 1 | 4 | 5 | No | No | 5 | 5 |
| 33703 | York | 1 | 3 | 4 | 0 | 0 | 0 | Yes | Yes | 1 | 0 |
| 45501 | Moorfields | 1 | 4 | 5 | N/A | N/A | N/A | No | No | 4 | –1- |
| 45601 | Moorfields | 2 | 3 | 5 | 1 | 2 | 3 | No | No | 4 | 2 |
| 45701 | Moorfields | 1 | 3 | 4 | 1 | 0 | 1 | No | Yes | 5 | 0 |
| 45802 | Moorfields | 1 | 3 | 4 | 0 | 2 | 2 | No | Yes | 4 | 2 |
| 45804 | Moorfields | 2 | 3 | 5 | 0 | 0 | 0 | Yes | Yes | 4 | 0 |
| 45805 | Moorfields | 2 | 4 | 6 | 2 | 4 | 6 | No | No | 5 | 4 |
| 45901 | Moorfields | 1 | 4 | 5 | 0 | 1 | 1 | No | Yes | 5 | 2 |
| 46801 | Moorfields | 3 | 6 | 9 | 2 | 4 | 6 | No | Yes | 8 | 5 |
In a pilot trial of this nature, which was not set up to provide definitive clinical information about the effectiveness of treatment and was conducted over a short period of follow-up, it would be inappropriate to draw definitive conclusions from a comparison of the clinical outcomes between the intervention and active monitoring arms. Nevertheless, these data are of interest, and one of the primary outcomes of the pilot trial was stated to be a comparison of children achieving a control score (NCS61) of zero between the two groups.
Following discussion at the TSC on 19 June 2012, it was agreed that children who had well controlled overcorrections (esophorias or intermittent esotropias), and in whom measurements of stereoscopic vision had not deteriorated, would be classified as having a clinic control score of zero. Children with constant esotropias following surgery cannot be classified using the NCS61 [which is designed only for the assessment of X(T)] and are referred to as overcorrections.
The management of, and handling of data in relation to, children who developed overcorrections following surgery requires further thought in a future study, with improved standardisation of the management of overcorrections. We think it is reasonable to classify children who have intermittent overcorrections, but have retained near stereo, as a good result but this approach needs to be considered alongside QoL data.
Achievement of a score of zero on the NCS61 requires both that the parents never notice the strabismus (NCS61 home = 0), and that on clinical testing, any deviation is immediately controlled (NCS61 clinic = 0). The strabismus could therefore be regarded as ‘cured’ at this point, however this might not remain the case over the longer term.
Furthermore, this criterion ignores any improvement that might be generated clinically, or in terms of QoL, by improvement, rather than cure, of X(T). As we have seen, however, QoL gains were seen only in relation to parental, rather than perceived or actual child QoL. Although numbers were small, analysis of the QoL data by outcome group indicates that QoL gains were actually greatest in the group for which the control score was improved but not zero (‘cured’), suggesting that ‘cure’, as defined by NCS61 = 0, may not necessarily be the most appropriate outcome measure for a full trial.
It is also known that ‘control’ of strabismus varies from day to day, and this may also be the case for children with a clinic control score of zero. For these reasons, other potential outcome measures were assessed to judge their applicability for further studies.
Measures of control of intermittent exotropia
Newcastle Control Score
In the active monitoring arm, no child had a NCS61 of 0 at the outcome visit (9 months from recruitment). In 11, the score had worsened (by 1 or 2 points in 10), and in nine it had improved (by 1 or 2 points in seven). In two patients, the score was unchanged. Generally, a change in score of ≤ 2 on this instrument would not be regarded as clinically significant. In summary, there was little significant change in control scores over the follow-up period in this group.
In the surgery arm, nine children had a score of 0 at the outcome visit, four of whom had latent or intermittently manifest convergent deviations but with retained or improved stereoacuity.
In 18 children the score was improved: by 1 or 2 points in four, and by > 2 points in 14. In two children there was no change in score, two deteriorated (by 1 or 2 points only) and three were overcorrected. In summary, there were significant changes in this measure of control in the surgical group. The difference in cure rates between the surgery arm [36% (95% CI 18% to 57%)] and the monitoring arm (0%, 95% CI 0% to 15%) was statistically significant (Fisher’s exact test; p = 0.002; difference = 36%, 95% CI 12.4% to 59.5%).
On the measure of a satisfactory NCS61 outcome which we have previously reported (NCS61 ≤ 2 or improvement ≥ 3), 2 out of 22 children achieved this outcome in the active monitoring arm, compared with 15 out of 25 in the surgery arm. The difference in satisfactory NCS61 outcome rates between the surgery arm and the active monitoring arm was statistically significant (60% vs. 9%, χ2 test; p < 0.001; odds ratio 15.0, 95% CI 2.9 to 78.9).
Mayo control score
We also used the scoring system19 for control of X(T) devised by the group at the Mayo Clinic. 18 This is a 10-point scale, based on the presence of exotropia either before or after dissociation of the eyes as determined by a cover test, with timing of recovery of binocular fixation. Five points each are awarded for control at distance and near fixation.
On this instrument, in the active monitoring group, 16 out of 22 children had a score at outcome within ± 1 point of baseline. Two patients improved by ≥ 2 points (3 and 4 points, respectively), and four deteriorated by ≥ 2 points (two patients by 2 points and one patient by 4 points).
In summary, 72% of patients in the active monitoring arm showed no significant change in control using this instrument.
In the surgery group, the Mayo control score19 at outcome was within ± 1 point of the baseline score in eight children, was worse by 2 points in one child, was improved by ≥ 2 points in 13 children, and three were overcorrected.
In summary, 52% of children in the surgery arm showed significant improvement in their control using this instrument.
Measures of ocular alignment
Ocular misalignment is measured clinically by determining the size of prism which, when placed in front of a misaligned eye, abolishes the corrective fixation movement that would otherwise result when the fixing eye is covered. Differences of ≤ 10 PD are generally regarded as within the limits of measurement error, and not considered clinically significant. 67
Measurements of distance misalignment showed that, in the active monitoring arm, four children had improvements of > 10 PD between baseline and follow-up, none had deteriorated, and 18 had measurements at outcome within 10 PD of the baseline measurement.
Measures of distance alignment in the surgery arm showed that 13 children had improvements of > 10 PD, four had intermittent overcorrections, two had constant overcorrections, none had deteriorated and six had measurements within 10 PD of baseline.
In summary, there were significant improvements in distance alignment in 18% of the active monitoring arm and 52% of the surgery arm.
Measurements of near misalignment showed that, in the active monitoring group, three children had improvements of > 10 PD, one child deteriorated by > 10 PD and 18 had measurements at outcome within 10 PD of baseline.
Measures of near misalignment in the surgery arm showed five children had improvements of > 10 PD, two children deteriorated by > 10 PD, 10 had measurements within 10 PD of baseline, five were intermittently overcorrected and three had a constant overcorrections.
In summary, there were significant improvements in near alignment in 14% of the active monitoring arm and 20% of the surgery arm. Of the surgery arm, 32% showed either intermittent or constant overcorrections at near fixation at 6 months postoperatively.
Binocular vision and stereoacuity
A change of two octaves of stereoacuity is generally considered to be clinically significant.
At outcome in the active monitoring arm, of 19 children who could complete near stereoacuity testing at near fixation, two had deteriorated by two or more octaves, nine were within two octaves and eight had improved by two octaves. Of the remaining three, who were too young to perform stereoacuity testing and whose binocular vision was assessed using their ability to demonstrate a fixation movement in response to a prism placed before one eye, two were stable and one had deteriorated.
In summary, the binocular vision of 86% of children in the active monitoring arm was either stable or improved over the follow-up period, and significantly deteriorated in 14%.
In the surgery arm, near stereoacuity deteriorated by more than two octaves in four children, remained within two octaves in 15, and improved by more than two octaves in four. Of two children assessed using prisms, one was stable and one had deteriorated.
In summary, the binocular vision of 80% of children in the surgery arm was either stable or improved, and significantly deteriorated in 20% over the follow-up period.
Eligible not recruited outcome data
Follow-up data were obtained for 49 out of 56 (88%) of the ENR patients who consented to data collection and storage. Mean duration between screening and follow-up was 9.4 ± 2 months, range 4–15 months. In most cases (n = 34), the follow-up data were collected within 10 months from screening. In 12 cases, review was between 11 to 12 months and in 3 cases it was beyond a year from screening (2 at 13 and one at 15 months). However, any treatment reported was received within 9 months from screening; a few were already having treatment for vision prior to screening as indicated in Table 12.
| ENR interventions within 9 months from screening | n | % |
|---|---|---|
| None | 31 | 63.3 |
| Minus lenses for control | 4 | 8.2 |
| Surgery | 7 | 14.3 |
| Glasses for vision (prior to screening) | 5 | 10.2 |
| Glasses for vision (at or after screening) | 1 | 2.0 |
| Occlusion for amblyopia | 1 | 2.0 |
| Total | 49 | 100.0 |
Details of primary and secondary clinical outcomes for ENR patients are provided in Tables 13 and 14.
| NCS60 = 0 at follow-upa | Treatment within 9 months | ||
|---|---|---|---|
| None | Conservative | Surgery | |
| Yes | 0 (0%) | 1 (9%) | 4 (57%)b |
| No | 27 (100%) | 10 (91%) | 3 (43%) |
| NCS61 satisfactorya at follow-up?b | Treatment within 9 months | ||
|---|---|---|---|
| None | Conservative | Surgery | |
| Yes | 1 (4%) | 3 (27%) | 7 (100%)c |
| No | 26 (96%) | 8 (73%) | 0 (100%) |
Chapter 5 Economic analysis
This section presents findings from the health economics component of the pilot study to test the feasibility of a RCT comparing eye muscle surgery against active monitoring for childhood intermittent distance exotropia.
The main aim of the health economics component was to rehearse the methods of data collection and so inform the development of the economic evaluation for a definitive study. We evaluated the ease of data collection (response rate and data completeness) of the different sources, including the case report form (CRF), reference costs documentations and Participant Costs Questionnaires (HSUQ and TTQ).
Collection of data
Data were collected on effects and costs for both study arms. Sources of data collection are shown in the tables in Appendix 8.
Outcome data
Over 50% of the participants were < 5 years old and none of the existing standard QoL instruments used to estimate a utility score is validated for use among children in this age group, hence they were judged to be inappropriate for this study. As an alternative the IXTQ was adopted as a QoL outcome. However, this tool cannot be used to estimate preference-based utility measures, so it is not ideal for incorporation into a cost–utility analysis. Clinical outcomes are obtained and recorded in the CRF. All of those outcomes are collected at baseline and at the final follow-up.
Cost data on the intervention
The main cost is related to the eye muscle surgery in the treatment arm. Surgery-related costs include costs of staff, consumables, capital and overheads, as well as costs due to postoperative complications. Staff costs, overheads and any costs related with postoperative complications. These can be calculated based on the following information recorded on the CRF for every participant in the study:
-
grade of operator
-
grade of anaesthetist
-
grade of assistant staff
-
time of patient entry into and leaving operating room
-
time of patient entry into and leaving recovery room
-
date of admission and discharge
-
postoperative complication.
To calculate total costs of the surgery, data on the use of consumable and reusable equipment required for the surgery would be collected from each participating centre. These data would be collected in a parallel data collection exercise to the participant-level data collection gathered within the trial. This work was not conducted as part of the pilot as the quantities and unit costs are likely to vary between the current pilot study and the end of the definitive study. In addition, the methods required to elicit these costs are well established and hence do not need to be tested as part of this pilot study.
Cost data on the use of NHS health services and patients out-of-pocket expenses
The perspective adopted was that of the NHS and patient. The Participant Costs Questionnaires (HUSQ and TTQ) were designed and piloted to collect information on NHS resource use and patients’ out-of-pocket expenses, as well as the cost of travelling to access care and the time that this takes. As all of the patients were children aged < 12 years, the Participant Costs Questionnaires were completed by their parents.
The Participant Costs Questionnaire was designed to be as extensive as possible and to collect sufficient information but, at the same time, not to over burden the participants. It has two parts: HSUQ (Part A) records information on the level of usage of the health services and the costs of any other self-purchased health care required to manage the condition, and TTQ (Part B) collects information on the time and travel costs of the participants attending each possible type of NHS services. The role of TTQ is to inform the calculation of unit costs of the participants to attend each type of health services, and this will then be combined with the information obtained from HSUQ to derive total costs to the NHS and the patients.
Participants’ self-report data become increasingly unreliable as the period of recall increases. 68 In the pilot study, HSUQ was administered, after surgery, at 2 weeks, 3 months and 6 months for the treatment group, and at 3, 6 and 9 months after randomisation for the control group (considering that it may take up to 3 months for participants in the treatment group to receive surgery) in order to minimise recall bias. As TTQ is used to obtain unit costs, it is necessary to collect it only once, hence it was administered at 3 months from randomisation for the control group and at 3 months after surgery for the treatment group.
In HSUQ the data on NHS resources collected included the use of both secondary and primary care related to the patient’s condition. The use of secondary care services includes non-protocol (protocol visits are those scheduled for the purposes of data collection) outpatient visits and hospital admissions. The use of primary care services includes prescription medications and contacts with primary care practitioners (e.g. GPs, practice nurses and optometrists).
Participant costs comprise three elements: for TTQ, travel costs for accessing NHS primary and secondary care, and time costs of travelling and attending NHS primary and secondary care; and for HSUQ, self-purchased health care and related management costs. The estimation of travel costs requires information from participants about the number of visits to health-care services (collected in HSUQ), and the unit cost of making a single journey to each type of health-care provider (derived from information in TTQ). The parents of the child are asked, in TTQ, for each type of visit, the mode of transport they used and the fare for one way if they travelled by bus, taxi or train, or the number of miles they travelled and parking fees if they used a private car. Participants’ time costs are collected in a similar manner. The parents of the child are asked how long on average they spent travelling to and attending each type of health-care provider. They are also asked what activity they would have been undertaking (e.g. paid work, leisure, housework in the case of parents or carer) had they not accompanied their child to attend the health-care provider. These data are presented in their natural units, for example hours and minutes, and attached to monetary value using standard economic conventions, for example the Department of Transport69 estimates for the value of leisure time. Self-purchased health care includes over-the-counter medications (e.g. eye drops). Private health insurance cost is included if the insurance is purchased for the patient’s eye conditions. Management cost includes parents’ time costs if they are absent from work in order to look after the child due to their eye condition.
Data collection results
As described in the previous section, there are 49 patients in total recruited to this trial, with 24 randomised to the control arm and 25 in the treatment arm; however, one of the patients in the treatment arm changed their mind and asked to be switched to the control arm, so as a result 24 patients in the treatment arm received surgery and 25 patients in the control arm were monitored only of their progress without surgery.
Outcome data
The outcome data collected on the QoL of the patients and their parents have been reported in the QoL results chapter (see Chapter 4), and as we will not be conducting a cost-effectiveness analysis, we do not include this in this chapter.
Data on surgery costs
The completion rate relating to the surgery information on the CRF was extremely high with 100% of core information obtained, including admission and discharge dates, grades of surgeons, and time in and out of the operating theatre. There is a very small number of missing data on the grades of assisting staff, grades of anaesthetists, and time in and out of the recovery room. All of the 24 patients in the treatment arm were admitted as day cases for the surgery. None reported postoperative complications. The vast majority of the participants (96%) were operated on by a consultant (Table 15), with various grades of staff assisting the surgery (Table 16). There are, however, five patients (21%) for whom there is no recorded information on grade of assisting staff but it is unclear whether these data are truly missing or missing because there was no assistant present. Similarly, the majority of the patients (97%) were given anaesthetic by a consultant (Table 17). Only one participant (4%) had no information on the grade of anaesthetist recorded.
| Grade | Observations | % |
|---|---|---|
| Training fellow | 1 | 4.17 |
| Consultant | 23 | 95.83 |
| Total | 24 | 100.00 |
| Grade | Observations | % based only on recorded data (n = 19) | % based on total (n = 24) |
|---|---|---|---|
| Training fellow | 8 | 42.11 | 33.33 |
| Senior house officer | 2 | 10.53 | 8.33 |
| Registrar | 8 | 42.11 | 33.33 |
| Consultant | 1 | 5.26 | 4.17 |
| Total recorded | 19 | – | 79.20 |
| Missing | 5 | – | 20.80 |
| Total | 24 | – | 100.00 |
| Grad | Observations | % based on only recorded data (n = 23) | % based on total (n = 24) |
|---|---|---|---|
| Registrar | 1 | 4.35 | 4.17 |
| Consultant | 22 | 95.65 | 91.67 |
| Total recorded | 23 | – | 95.80 |
| Missing | 1 | – | 4.20 |
| Total | 24 | – | 100.00 |
Based on the information provided on the time in and out of the operating theatre, we calculated the duration of operating time for each participant. The mean operation time was 47 minutes, ranging from 31 minutes to 65 minutes. For the duration of time spent in the recovery room, recorded on the CRF, data were missing for three (13%) participants. The mean time patients spent in the recovery room after surgery is 36 minutes and ranges from 19 to 75 minutes (Table 18).
| Time | Observations | Mean (minutes) | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Length of operation | 24 (100%) | 47.2 | 9.6 | 31 | 65 |
| Length in recovery room | 21 (87.5%) | 36.4 | 14.4 | 19 | 75 |
Participant costs and use of services data
Overall, the response rates for the HSUQ and TTQ have exceeded our expectations (Table 19). For the treatment arm, an average response rate of 97.2% was achieved. For the control arm, the response rate was slightly lower at 84%, which is still much higher than a typical survey response rate in clinical trials of around 50–70%. 70 The high response rate achieved is likely to be because the HSUQ and TTQ were completed on scheduled protocol visits, with participants assisted by a member of the research team. It is not surprising that the response rate in the treatment arm is higher than the control arm, as participants had more engagement with the trial because a physical intervention is carried out.
| Response rate | Control arm (n = 25) | Treatment arm (n = 24) | |
|---|---|---|---|
| Follow-up | 1 | 92% (23/25) | 100% (24/24) |
| 2 | 76% (19/25) | 96% (23/24) | |
| 3 | 84% (21/25) | 96% (23/24) | |
| Average | 84% | 97% | |
Among respondents to the HSUQ and TTQ there were a small number of respondents who did not answer some relevant cases. These missing data can result from a number of reasons, including data input errors, respondents refusing to answer certain questions, respondents’ errors, and questions that were unclear for respondents. For the last three potential reasons for missing data such problems may be alleviated by improving the design of the questionnaires, and, indeed, one of the aims of this pilot study is to test and refine data collection tools and this is returned to below (see Identification of data collection and entry issues with the questionnaire).
Table 20 summarises the health service use collected from the HSUQ (Part A of the Participant Costs Questionnaire), and Table 21 presents patients’ time and travel costs of attending each type of health services collected from the TTQ (Part B). Data collected in this pilot trial can also provide us with information on the pattern of patients’ use of health services, which will be helpful in designing how the relevant data should be collected in the definitive trial. Based on Table 20, on information from the HSUQ, it seems that majority of the service use is concentrated within the time frame of the second follow-up. There is more use of secondary services in the treatment arm than in the control arm, which is not surprising. However, the reported numbers of hospital admissions at the first follow-up time point in the treatment arm may not be an accurate reflection of the service use as a result of the intervention. This may be due to confusion by some patients who may have included their initial surgery as an admission on the Participant Costs Questionnaire despite instructions not to do so (we return to this issue below). This also applies to the prescription medication where they may have also included those that have been prescribed at the hospital following the intervention rather than any additional prescription after the intervention. There appears to be very little use of health services in the control arm overall and very few use of health services in the treatment arm at the third follow-up. Consideration will be given whether or not costs data collection can be restricted to the initial 6 months from randomisation except for critical events (e.g. new or re-interventions) which would also be of interest as a clinical outcome. The TTQ aims to estimate the average costs of patients attending each type of health services. The costs include travel costs as well as time costs of the patients and their accompanying person. With the information recorded, we can calculate the typical costs for an individual to attend the health services, which will then be combined with information collected on the HSUQ to derive a total costs for each individual’s out-of-pocket expenses.
| Cost items collected | Follow-up 1 | Follow-up 2 | Follow-up 3 | |||
|---|---|---|---|---|---|---|
| Control arm (25 participants; 23 questionnaires received) | Treatment arm (24 participants; 24 questionnaires received) | Control arm (25; participants; 19 questionnaires received) | Treatment arm (24 participants; 23 questionnaires received) | Control arm (25 participants; 21 questionnaires received) | Treatment arm (24; participants; 23 questionnaires received) | |
| Contacts with GP | 0 | (1 time) × 1 | (2 times) × 1, 1b | 1b | 0 | (1 time) × 1 |
| Contacts with practice nurse | 0 | 0 | 0 | 0 | 0 | 0 |
| Contacts with optician | 0 | 0 | 0 | 1b | 0 | (2 times) × 1 |
| Outpatient visits | (1 time) × 1, 1b | (1 time) × 4, (5 times) × 1 | (1 time) × 1 | (1 time) × 1, (2 times) × 1 | 0 | 0 |
| Hospital admissions | 0 | (0 night) × 6, (1 night) × 4, 1b | 0 | (0 night) × 3, (2 nights) × 1 | 0 | 0 |
| Prescription medication | 2 | 14 | 4 | 6 | 1 | 1 |
| Antibiotics | (1 time) × 1 | (1 time) × 3 | (1 time) × 2, (2 times) × 1 | (1 time) × 3, (4 times) × 1 | 0 | 0 |
| Steroid | 0 | (1 time) × 4 | 0 | (1 time) × 1, (4 times) × 1 | 0 | 0 |
| Painkiller | 0 | (1 time) × 2 | 0 | (1 time) × 1, (4 times) × 1 | 0 | 0 |
| Other | 0 | (1 time) × 5 | (1 time) × 1 | (1 time) × 1 | 0 | (1 time) × 1 |
| Over-the-counter medicine | 0 | (£0) × 2, (£7) × 1 | 0 | 0 | 0 | 0 |
| Private health care | 0 | 0 | 0 | 0 | 0 | 0 |
| Whether or not took time off work | (2 days) × 1 | (2 days) × 4, (5 days) × 1, (8 days) × 1 | 2b | (3 days) × 3, (5 days) × 1, (30 days) × 1 | 0 | 0 |
| Cost items collected | Control arm, 3 months after randomisation (25 participants; 23 questionnaires received) | Treatment arm, 3 months post operation (24 participants; 23 questionnaires received) | |
|---|---|---|---|
| Hospital admission | Form of transport | (taxi) × 1, (car) × 2, (bus) × 1 | (bus) × 1, (train) × 1, (car) × 5 |
| Cost of fares | (2.2) × 1, (0) × 1, (15) × 1 | (4.2) × 1, (7) × 1 | |
| Miles by car one way | (10) × 1, (3) × 1 | (3) × 1, (6) × 1, (7) × 1, (9) × 1, (10) × 1 | |
| Parking fee | (8) × 1, (1.5) × 1 | ((1.5) × 1, (3) × 1, (4) × 1, (8) × 1, (8.5) × 1 | |
| No. of nights | (0) × 2, (0.5) × 1 | (0.5) × 1, (1) × 5 | |
| Activity would be doing otherwise | (paid work) × 2, (child care) × 1 | (paid work) × 6, (child care) × 1 | |
| No. of hours off work | (0) × 1, (4) × 1, (8) × 1 | (5) × 1, (7) × 1, (9) × 1, (9.5) × 1, (14) × 1, (50) × 1 | |
| Having another accompanying adult | 2 | 5 | |
| No. of visits while in hospital | 0 | (1) × 4 | |
| Outpatient visits | Form of transport | (bus) × 5, (train) × 1, (taxi) × 1, (car) × 9, (walked) × 1 | (bus) × 3, (train) × 1, (car) × 8 |
| Cost of fare | (1.8) × 1, (2.5) × 1, (15) × 1, (26.5) × 1 | (2.3) × 1, (3.7) × 1, (4.2) × 1, (7) × 1 | |
| Miles by car one way | (3) × 1, (5) × 2, (6) × 1, (8) × 1, (10) × 2, (12) × 2, (15) × 1 | (2) × 1, (3) × 1, (6) × 2, (7) × 1, (8) × 1, (9) × 1, (10) × 1 | |
| Parking fee | (1.5) × 1, (2.6) × 1, (3) × 1, (3.1) × 1, (4.7) × 1, (6) × 1, (20) × 1 | (1.5) × 2, (2.5) × 1, (2.6) × 1, (3) × 2 | |
| Length of travel time | (0.1) × 2, (0.15) × 2, (0.2) × 1, (0.25) × 1, (0.3) × 1, (1) × 3, (1.3) × 1, (2) × 1, (4) × 1, (15) × 2 | (0.1) × 1, (0.2) × 3, (0.3) × 4, (0.45) × 1, (10) × 1 | |
| Length of time spent in hospital | (0.25) × 1, (0.3) × 2, (1) × 6, (1.3) × 1, (2) × 1, (2.3) × 1, (3) × 1, (4) × 1, (30) × 1, (45) × 1 | (0.3) × 2, (1) × 5, (1.15) × 1, (1.3) × 1, (2) × 1, (60) × 1 | |
| Activity would be doing otherwise | (paid work) × 9, (housework) × 1, (child care) × 2, (studying) × 1, (other) × 1 | (paid work) × 7, (housework) × 1, (child care) × 1, (leisure) × 2, (other) × 1 | |
| Having another accompanying adult | 8 | 5 | |
| GP/nurse visits | Form of transport | (bus) × 1, (car) × 1 | (car) × 1 |
| Cost of fare | (1) × 1 | 0 | |
| Miles by car one way | 0 | (6) × 1 | |
| Parking fee | 0 | 0 | |
| Length of travel time | (0.1) × 1, (0.3) × 1 | 0 | |
| Length of time spent in GP practice | (0.1) × 1, (0.3) × 1 | 0 | |
| Activity would be doing otherwise | (1) × 2 | 0 | |
| Having another accompanying adult | 1 | 0 | |
| Optician visits | Form of transport | (car) × 2 | (car) × 1 |
| Cost of fare | 0 | 0 | |
| Miles by car one way | (3) × 1, (15) × 1 | (6) × 1 | |
| Parking fee | (2) × 1, (4.7) × 1 | 0 | |
| Length of travel time | (0.2) × 1, (0.4) × 1 | (20) × 1 | |
| Length of time spend at optician | (0.37) × 1, (0.45) × 1 | 0 | |
| Activity would be doing otherwise | (paid work) × 1, (other) × 1 | 0 | |
| Having another accompanying adult | 2 | 0 | |
These data would in the definitive study be presented as average costs for each item. These costs will be estimated by multiplying the number of times each individual’s service use/days off by the number of individuals, then sum them across individuals, which will then be divided by the total number of respondents contributing data (examples are given in the footnote of Table 20). For this study, however, the data within Tables 20 and 21 are presented in their raw form for easier interpretation of responses.
During the follow-up period, any further interventions (e.g. further surgery, BOTOX injection) are also recoded on the CRF. This information is needed as those further interventions will have an impact on the total costs. In this pilot study, there was only one patient who went through a further surgery and no patients had BOTOX injection.
Identification of data collection and entry issues with the questionnaire
Recording of hospital admissions
With respect to questionnaire design, a concern is the confusion of some participants as to whether or not the hospital admission that forms part of the initial surgical intervention should be included when answering questions on use of secondary health services. The response to this question on admission was intended to include only additional admissions during the follow-up period, as details of the initial surgery are already recorded on the CRF. Therefore, inclusion again would introduce an element of double counting that would bias the evaluation against surgical intervention. The version of the questionnaire did include an instruction not to include the initial hospital admission but this might not have been sufficiently clear. For the full trial we will seek to collect information on hospitalisation on the CRF rather than by participant-completed questionnaire to avoid any prospect of double counting. This will increase the burden on researchers but will also reduce the response burden on participants.
We are not aware of any empirical work that has compared 3- and 6-month recall periods on the extent of recall bias. In other studies, we do use a 6-month recall period but in this study we felt that the additional response burden on participants would be worth the additional accuracy gained from more frequent administration of the questionnaires.
The CRF gives a more complete and accurate account of the event regarding hospitalisation; however, we would consider, in a future study, asking all participants about ‘hospitalisation for eye surgery’, as one explicit category and ‘hospitalisation for other reason’ as the next category. That would unblind researchers but, as the randomisation of most was guessed correctly, this might not be an important issue. We would also consider having two categories of hospitalisation in the Participant Costs Questionnaire.
Recording of the mode of transport
It appears that some participants had difficultly completing sections of the TTQ (Part B of the Participant Costs Questionnaire) because they used multiple modes of transport to access care. To maintain an appropriate balance between the level of details collected and the length of the questionnaire, we asked participants to report only the mode of transport that made up the longest part of their journey, but elsewhere we went on to ask the total monetary out-of-pocket costs incurred. This appears to confuse some participants. Therefore, we for the definitive trial will reorganise the layout of the questions asked so that they will become clearer.
Consistency of the coding of data during data entry
Common conventions need to be adopted to convert some participant answers on the time and monetary values when entering data on to the database. The questionnaire was designed for the convenience of respondents to report values in their natural units of hours and minutes (in the case of time) and in pounds and pence (in the case of monetary values) but, at the data input stage, the lack of an agreed framework to convert different units into one common unit leads to confusion and measurement error. Further consideration is needed on how best to handle this in the data trial. Two potential solutions are the development of a data entry manual describing how to respond to data recorded on the questionnaire in a form different to the one intended. A second solution would be for all data to be entered as recorded on the questionnaire and then have common conventions applied to the data at the data cleaning stage, all of which can be formally documented. The former option may still allow variation between data entry clerks to persist and the data entry manual would need revision throughout the study as novel issues arise. The latter would allow clearer documentation of any changes but would require potentially problematic data to be highlighted so that they can be investigated at the data-checking stage.
Summary
This section of the pilot study sought to investigate the feasibility of conducting an economic evaluation as part of a RCT comparing eye muscle surgery against active monitoring for childhood intermittent distance exotropia. Specifically, the health economic component of the study aims to assess the ease of health economics data collection.
Costs data are collected from a number of sources. Information regarding the surgery is recorded on the CRF, and services use following the intervention is collected through the Participant Costs Questionnaire, which also gathers information on patients’ out-of-pocket expenses. Assessing both sources of data collection, a > 80% completion rate was achieved. The high response rate of Participant Costs Questionnaire may be due to the high level of commitment from the participants and the fact that the questionnaires were completed on patients’ study visit. There are, however, some issues regarding questionnaire design, which will require the tool to be refined for the definitive trial.
The use of health services appears to be concentrated within the first 6 months of the trial follow-up for both trial arms. Consideration will be given for a definitive study streamlining the data collection and hence reducing the burden to both the participants and the research team, and potentially also reducing the costs of research to the funder. Overall, the work conducted has shown that it is feasible to collect meaningful health economics data in a definitive trial, subject to adjustment of data collection points and refinement of the data collection tool.
Chapter 6 Discussion
Trial management
The structures that were put in place to manage the trial appeared to be valuable in maintaining regular contact between key stakeholders and ensuring efficiency. Telephone conferences were a useful supplement to face-to-face meetings.
Screening and recruitment
Although screening rates were initially high, as patients had been identified in readiness for the opening of recruitment, there was a lag in recruitment due to the delay in the subsequent appointment for the recruitment clinic.
New referral letters often did not contain sufficient information to make a judgement about whether or not the child had X(T), and many children referred with suspected X(T) did not have X(T) at all. This wasted appointments in the screening clinics and meant that it would have been confusing to provide parents with information about a trial for which their child might not be eligible, so it was decided to reserve providing parents with information leaflets until the initial clinic visit.
Given the age of the children involved, it was not always possible to confirm eligibility for the trial at the initial screening visit, and many children had to be reviewed before eligibility was confirmed. This generated further appointments in the screening clinics, which blocked slots for other potentially eligible children.
Subsequent blockage of appointment slots by children who needed rescreening for eligibility, and parents who wished to take more time to consider recruitment when their child was eligible, contributed to a failure to recruit to target. This was compounded by an unexpectedly high proportion of children who failed to meet the eligibility criteria.
The most common reason for children failing to meet the eligibility criteria was that their strabismus was not sufficiently severe: either the angle of misalignment was too small or their X(T) was too well controlled to meet conventional criteria for surgery for X(T). The NCS61 grading for trial entry was defined as ≥ 3. However, this score could be achieved by a parent who noticed X(T) all the time without any objective evidence that X(T) was present. Although this was not the case for any trial participant, the criteria for trial entry were on the limit of what would be considered an acceptable degree of severity of X(T) to justify intervention, particularly in younger children.
Most surgeons would not be comfortable operating on a child whose X(T) was objectively well controlled. Conventional surgical criteria suggest that a NCS61 of ≥ 2 should be present on distance fixation in order for eye muscle surgery to be appropriate. 18,20 This was not specified in the current study and should be in a future trial.
On the other hand, many children in the active monitoring arm more than fulfilled conventional criteria for surgical treatment, and, by and large, did not deteriorate significantly, with many showing some evidence of spontaneous improvement, particularly in stereoacuity, over the follow-up period.
In a longer duration study, it is uncertain what proportion of parents would be happy to defer intervention for a prolonged period.
Recruitment of children who had failed school vision screening because of X(T) enriched the population of children referred with X(T), but also targeted a group of children whose parents had not presented their children for treatment and who, by implication, were less concerned to have the strabismus treated. It also introduced an age bias to the participants, as those who were recruited from school screening were between 4 and 5 years of age.
For parents and clinicians, the initial screening appointment presented a challenge, in that it had to encompass a diagnosis of the child’s eye condition, an assessment of eligibility for the trial, an explanation of possible treatment options, an explanation of the lack of robust evidence underpinning the timing and effectiveness of these interventions, and an explanation of the trial. It was rarely possible to cover all of these points in a single consultation, not least because of the amount of new information that had to be assimilated by the parents, and the questions they had about treatment and the trial, were often not answerable in the subsequent telephone call. There were also difficulties in contacting many parents by telephone during the working day, and attempts had to be made to contact them outside of normal working hours, which were not always successful.
Furthermore, the explanation of the lack of evidence underlying the effectiveness and timing of intervention served, in many cases, to undermine the parent’s confidence in the treating clinician, and by extension, the trial.
In Newcastle, a research nurse was recruited during the screening and recruitment phase of the trial, and she subsequently gave most of the information about the trial itself. This separation of the role of the treating clinician from the main recruiter to the trial proved extremely beneficial in aiding the process of recruitment. Such use of research nurses in all centres should be considered in a future study.
It became apparent during the process of recruitment that the initial two visits, for screening and recruitment, and the intervening telephone call, often gave insufficient time for parents to fully consider participation in the trial. Many parents wished to observe the progress of the condition before considering treatment, and many were concerned at the prospect of surgery. This often generated further appointments in the recruitment clinics, which blocked further slots for potential recruits.
Some parents had concerns about trial processes, particularly randomisation.
During the screening and recruitment phase, it was decided to not only restrict entry to the trial to newly diagnosed children, but also to provide study information to children who had a recent confirmed diagnosis of X(T) and had not received any treatment, at all sites. These children were then allocated appointments for recruitment clinics if their parents expressed an interest in participating in the trial.
Despite the difficulties outlined above, it is important to state that the rate of refusal of eligible children to participate was similar to that predicted in the initial submission. It was predicted that the refusal to participate rate would be around 33% – in fact, 64% (89/138) of eligible children identified refused to participate.
In summary, the strategies adopted to improve recruitment included improving the efficiency of the utilisation of screening clinic appointments by screening out children who did not have the condition, recruitment of prevalent cases and cases from preschool screening, and the separation of recruitment from clinical care by the use of a research nurse in one centre.
Participation bias
For ethical reasons, it was possible to collect limited information only on children who were eligible for, but declined participation in, the trial. On the basis of the information available to us, there appeared to be differences in age and strabismus control between those participating and those declining participation, with those participating being older and having poorer control. Such differences, if truly present, might affect the generalisability of a longer-term study. QoL was not measured in the group eligible but declining participation, but, given the effect of intervention on parental anxiety, it is tempting to speculate that those parents entering the trial were, on the whole, more anxious about their child’s condition than those who declined participation.
Randomisation
No significant issues were identified in relation to randomisation, which appeared to provide groups that were matched for baseline demographics and clinical severity.
Adherence to group allocation and protocol
There were minor issues in relation to the timing of surgery due to comorbidity.
Adherence to group allocation was excellent, with only one parent declining the allocated group. Adherence to group allocation might be a more significant issue in a longer-term study. In particular, parents who were in favour of surgery for their child’s X(T) would be unlikely to remain in an active monitoring arm in a long-duration study.
Retention of participants for the duration of the study was generally excellent, and is a tribute to the intensive attention paid to this by the study team at all sites.
Adverse events
The major identified risk for participants in the active monitoring arm of the study was that the condition might have a deterioration in their clinical condition. It is challenging to distinguish a permanent deterioration from fluctuation of the condition in this patient group. 62,71,72 However, in spite of some apparent deterioration in the clinical picture in two children (10301, 46701), none developed a constant strabismus.
At outcome, two children in the surgery arm had constant esotropia at near and distance and were classified as over corrected; a further child had surgery for an overcorrection within the follow-up period. In addition, there were four children who had eso deviations for near viewing only, three of which were intermittent and one that was constant. This is in line with previous studies25,26 and permanent overcorrection with loss of stereoacuity remains the most significant complication of surgery. The management of surgical overcorrection should be standardised in a future trial.
Maintaining masking
Although no breaches of the masking to group allocation of the orthoptists assessing outcome at each centre were reported, in almost every case the orthoptist, when asked, correctly guessed the group allocation. It is unlikely that, 6 months after surgery, traces of the surgical procedure itself were still visible, as redness of the eyes following strabismus surgery generally lasts for a maximum of only 6 weeks. In cases of overcorrection, previous surgery would be assumed, as this is not an outcome that occurs spontaneously. Similarly, there may well have been an assumption that cases which had changed little in clinical appearance over the follow-up period were more likely to have been actively monitored. This effect is likely to be lessened, but not abolished, in a study of longer duration.
Qualitative interviews
These indicated that a trial with a preference arm would be more acceptable to parents. In general, the information provided to parents appeared to be of an acceptable standard, and was not a major barrier to trial participation. The way in which the diagnosis and information about the trial was communicated did appear to be an issue, indicating the need for a greater use of research nurses and training in future studies. Some parents were keen to have more details of surgical success rates, indicating the need for a definitive trial.
Quality of life
The IXTQ questionnaires showed no differences between groups for the child-reported questionnaire and the proxy questionnaire, where the parents indicated the impact that they felt that X(T) had on their child. There was a significant difference between the surgery group and active monitoring group for the parental questionnaire, which was spread across all subscales. This portion of the IXTQ mainly enquires about parental anxiety (see Appendix 5) – in fact, all of the questions in this section commence with either the phrase ‘I worry . . .’ or ‘It worries me . . .’.
This suggests that the improvement seen on this instrument mainly reflects the effect of the intervention on parental anxiety, either because ‘something has been done’ or because the effect of the intervention on the condition has reduced parental anxiety, rather than that the child’s QoL has improved, or even the parent’s estimation of the child’s QoL.
This does reflect current clinician experience, where, in the absence of robust evidence of the long-term effectiveness of interventions for X(T), treatment is often driven by parental anxiety and anecdotal or personal experience.
It was not possible to collect IXTQ data on parents of eligible children who did not consent to participation, but it is interesting to speculate that there may have been a bias, with those parents not consenting because they did not wish their child to have surgery being less anxious, and vice versa. It would be interesting to investigate generic measures of anxiety in parents seeking surgery for their child’s X(T) and those wishing to avoid it.
Clinical outcomes
There appeared to be variability in outcomes between the four centres, although the numbers involved were too small for formal analysis. One potential improvement that could be made in a further trial would be greater standardisation of the surgical procedure; however, it is likely that this would not completely eliminate the variation in clinical outcomes. Furthermore, where standardisation – for example photographic documentation with review by a reading centre – has been performed, variations in surgical technique have persisted.
Strabismus control
The inclusion criteria for the study specified a NCS61 of ≥ 3. This figure was chosen to be in line with early work on this instrument, which indicated that few children underwent surgery with scores of < 3. 20,25,61,73
The NCS61 is made up of three components: a home control score (1) that rates the proportion of time that parents see the strabismus, and a clinic score that rates the ease of realignment at near (2) and distance (3) following a cover test. Each portion of the score (home, clinic near and clinic distance) contributes three points, giving a potential total of nine points.
It is possible to obtain a score of 3 from one of the components alone; however, in this study no child was randomised without a home control score of ≥ 1 and a clinic control score of ≥ 2. This is likely to reflect the minimum score at which parents and clinicians are prepared to randomise participants to a RCT of surgery.
There were clear benefits on control from surgery in this study.
Although the NCS61 and the Mayo score19 appeared to correlate well (r = 0.88; p < 0.001), given that the NCS61 incorporates a parent-reported outcome measure, and the difficulties of obtaining a Mayo score19 (which relies on a 30-second period of initial observation and timing to realignment), we would prefer to use the NCS61 in a future trial, while recognising the possible variabilities of clinic control and the potential unreliability of parental reporting.
Ocular alignment
In parallel with the benefits on control, there were clear benefits on ocular alignment from surgery. This needs to be balanced by six participants who were permanently or intermittently ovecorrected at distance, and one only overcorrected at near, at outcome, plus another participant who had further surgery for an overcorrection within the follow-up period.
Four of these seven patients had retained or improved stereoacuity; however, some of these overcorrections were cosmetically significant. Although initial overcorrection has been regarded as a good prognostic indicator of long-term alignment after surgery for X(T)74,75 (although this has been disputed26,76), overcorrection at 6 months following surgery may not resolve, and may require further treatment if cosmetically significant or associated with a reduction in binocular vision or amblyopia.
In summary, there were significant improvements in near alignment in 14% of the active monitoring arm and 20% of the surgery arm; 32% of the surgery arm showed either intermittent or constant overcorrections at near fixation at 6 months postoperatively.
Binocular vision and stereoacuity
Although there were three participants whose binocular vision deteriorated in the active monitoring arm, given the variability in current clinical testing of binocular vision in young children, the clinical significance of this is unclear. The eight participants in whom stereoacuity improved could either be demonstrating a maturational effect or a spontaneous improvement in their condition.
In the surgery arm, two of the participants who were overcorrected at both distance and near, and the one who was overcorrected only at near, had a reduction in binocular vision and stereoacuity, and can be classed as functional overcorrections. The binocular vision or stereoacuity of a further two children deteriorated. These children were not overcorrected, but did show some evidence of deterioration of their X(T). The other four children with intermittent overcorrections did not show any deterioration in their binocular vision.
Chapter 7 Conclusions
We have demonstrated that it is possible to recruit and retain participants to a trial of surgery compared with active monitoring for X(T).
Despite screening, the anticipated number of children with X(T), recruitment levels fell short of those predicted. This can be attributed to two issues.
First, the proportion of children eligible for inclusion was much lower than anticipated. This was primarily due to the proportion of screened children who did not have a severe enough strabismus for inclusion.
Tightening the inclusion criteria to conform with current clinical practice would, while reducing the number of potential recruits overall, increase the proportion eligible.
This has been discussed with the centres involved in the pilot and other potential interested centres.
Children who were eligible but randomised had poorer control of their X(T) than those who were eligible but declined to participate. This does give rise to an important issue about generalisability of a trial of surgery, which we will consider in any future trial. It is clear that we will be able to randomise children to a trial involving surgery only if their parents are prepared to consider surgery as a treatment, implying that the results will be applicable only to this group of patients. Should a trial show that surgery gives more favourable outcomes, this might lead to more parents considering surgery as an option.
Second, given the expressed views of many parents (of children who are eligible for inclusion) regarding their preferences, both for and against, surgical treatment for X(T), the development of a formal RCT should include consideration of a preference arm, which would increase the participation of eligible children.
An alternative strategy that could be considered, particularly if a future trial were to be designed around the benefit of early surgery, would be to randomise children aged < 4 years to surgery or active monitoring, bearing in mind that only parents who would consider early surgery as a treatment would be prepared to be randomised. This would leave open the possibility of later surgery in the active monitoring arm should the condition deteriorate or parents request surgery.
There was little evidence of significant deterioration of X(T) in children in the active monitoring arm, strengthening the case for a formal RCT.
The process of screening and recruitment evolved during the pilot, with an increase in the proportion of eligible children recruited. A more realistic assessment of the time required in clinic, and more astute use of research nurses, would be required for a further study.
The lack of a generic QoL measure that demonstrates the effect of X(T), and the emphasis on parental anxiety in the available disease-specific instrument are of concern. A longer-duration trial would allow the possibility of more children being able to express their own views at outcome, which would enable a more robust estimate of the effect of the condition on QoL.
Chapter 8 Suggestions for further research
Hypothesis generation
The SamExo study was a stand-alone pilot study to assess the feasibility of a full trial of surgery compared with active monitoring for X(T). Although not powered to assess the effectiveness of surgery as an intervention, the clinical outcomes do indicate agreement with previous research, which suggests that, over a short follow-up period, the majority of patients who are actively monitored do not significantly improve or deteriorate, whereas most patients who undergo surgery have, in the short term, improved alignment, albeit with a rate of between 10% and 20% of overcorrection with a deterioration in stereoacuity. The proportion of patients who are ‘cured’, i.e. demonstrate no significant strabismus while having preserved stereoacuity is low, at 36%.
There are two questions that then arise:
1. What are the long-term outcomes of surgery and active monitoring? Most studies indicate that there is a significant rate of recurrence of strabismus in children who have undergone surgery for X(T). 50,77,78 As discussed in the systematic review, one study50 showed that recurrences were most common within 6 months from surgery; however, after that, recurrences occurred continuously in the R&R group and rarely in the BLR group. In a further study, the Kaplan–Meier rate of developing ≥ 10 (delta) of misalignment after the first surgery was 54% by 5 years, 76% by 10 years and 86% by 15 years. 78 This indicates that outcomes from surgery may not be stable, which needs to be taken into account in the design of a future trial.
The data on spontaneous cure rates in children who have not undergone surgery are less clear. The lack of randomised studies means that children who have not undergone surgery will tend to have a less severe strabismus than those who have. Nevertheless, there are studies that suggest that a proportion of children with X(T) will show spontaneous improvement36 or at least no deterioration. 79
This suggests the possibility that the long-term outcomes following strabismus surgery for X(T) may be no better than a strategy of awaiting spontaneous improvement.
We would propose that a future trial would follow up children for at least 5 years from recruitment. Although this might seem onerous, an annual assessment of outcomes might be sufficient, reducing the burden on participants.
Furthermore, a longer-term study would be better able to assess QoL outcomes from a participant perspective, as most of the participants in the pilot study were too young to give their view.
This suggests the need for a formal RCT of surgery compared with active monitoring, which incorporates long-term follow-up of participants in the trial.
2. How can the cure rate of surgery be improved? There are sound theoretical reasons for the view that outcomes from strabismus surgery in children are improved if the surgery is undertaken soon after the onset of strabismus. 80–82 There is evidence of improved outcomes from early surgery in infantile esotropia (convergent strabismus). 83,84 The evidence supporting early surgical intervention in X(T) is less clear. A widely cited study by Pratt-Johnson et al. 85 showed evidence of improved outcomes from surgery for X(T) undertaken in children aged < 4 years.
We have shown in a previous study20 that rates of surgery for X(T) within 2 years of presentation are low in the UK, indicating a reluctance among clinicians in the NHS to adopt a practice of early surgery. Qualitative interviews undertaken by our group (Lecouturier et al. , submitted to BMC Ophthalmology) indicated that the reasons behind this reluctance included:
-
difficulty in assessment of young children, particularly those under 4 years, in whom measurements of stereoacuity and strabismus angle are inaccurate
-
the need for repeat assessment owing to variability of X(T) in order to plan the need for and the amount of surgery
-
concerns about the more severe functional impact of overcorrection of X(T) in younger children, particularly the development of amblyopia in cases where a constant esotropia develops.
Although these concerns are valid, given the low short-term cure rates following surgery undertaken at an older age, and the high rates of recurrence following surgery, there is clearly a need to consider whether or not an alternative treatment strategy would be more effective than deferring surgery until the child is old enough to obtain accurate measurements, by which time the X(T) has usually been present for several years. This suggests the need for a formal RCT that incorporates early surgery as an intervention.
What lessons from the pilot randomised controlled trial should be incorporated into a full randomised controlled trial?
A full RCT of surgical treatment of X(T), testing either the effect of surgery at any age or early surgery against active monitoring, would face a number of challenges, some of which have been highlighted in this pilot study. The principal issue is likely to be the recruitment of sufficient participants. The pilot has clarified that parents are very unlikely to accept the possibility of randomisation to surgery at the presenting visit to an eye clinic. This implies that prevalent cases would have to be included as potential participants. This potentially induces a bias, which would be partially overcome in a trial of early surgery with a limit on the upper age at which participants would be included.
A further issue was the difficulty of the potential conflict between clinical function of the ophthalmologist and recruitment, and we would recommend that research nurses are used for recruitment in all centres in a future trial.
Recruitment to a full trial is also likely to be hampered by strong parental preferences, as demonstrated in this pilot. Although, given enough centres, sufficient participants could be recruited to deliver a full trial, there would be issues about generalisability. There are two possible solutions to this issue: either to incorporate a preference arm or to accept that recruitment will inevitably be restricted to those parents who are prepared to consider surgery as a treatment, with the restrictions on generalisability entailed.
The pilot has also indicated intercentre differences in recruitment and outcome, and in a study with more centres it would be important to further standardise surgery and other treatment, including management of postoperative overcorrections and undercorrections.
We would argue that the IXTQ should be used in a full trial, as a condition-specific QoL measure, but, in addition, a generic measure will be required. A longer-duration study would allow the collection of child-reported QoL information, which has not been possible with many younger patients in this pilot.
What are the risks of not commissioning further research in this area?
The current clinical management of strabismus owes more to art than to science, and is heavily driven by parental preferences, which may, or may not, be clinically appropriate. There is an urgent need to obtain more robust evidence to guide practice for this large group of patients, which has been highlighted by a recent study highlighting variations in clinical practice within the NHS in this area. 10
A recent workshop hosted by the James Lind Alliance for patients and eye health professionals identified the improvement of treatments for exotropia as a priority. 86
A formal RCT of the treatment of X(T) incorporating an active monitoring arm is required to begin to resolve the current uncertainties in the management of this condition.
Acknowledgements
The SamExo Team would like to extend their sincere thanks to all of the children and parents who participated in the study.
The team are indebted to all SamExo ROs and research nurses. The study would not have been as successful without their enthusiasm and commitment.
We would like to thank all members of the TSC who provided expert advice and support throughout the study: Professor Charlotte Wright (Independent Chairperson), Ms Phillippa Cumberland (Independent Statistician), Mr Peter Tiffin (Sunderland PI), Mr Adam Ward (Lay Representative) and Mr Chris Lloyd (Independent Ophthalmologist). We are particularly grateful to Professor Charlotte Wright, the Chair of the TSC, who commented on earlier drafts of this report.
The team would also like to thank the following ROs from the Department of Ophthalmology, Newcastle upon Tyne Hospitals NHS Foundation Trust: Mrs Jackie Younger, Mrs Teresa Saul, Miss Caroline Penrose and Miss Laura Crawford.
Contribution of authors
Mr Michael Clarke (Chief Investigator) conceived the study and contributed to the study design and funding acquisition; was PI at the Newcastle site and oversaw delivery of the intervention; and drafted the report.
Dr Vanessa Hogan (Clinical Trial Manager) co-ordinated local approvals for collaborating sites, and site set-up; monitored conduct of study and site and study close down; and co-ordinated and assisted in drafting the final report.
Dr Deborah Buck (Co-investigator) was data manager and analyst for the SamExo study and final report, with overall responsibility for data quality and analysis; provided expertise in clinical and psychosocial outcomes assessment and qualitative interview techniques, and interviewed parents regarding the acceptability of the study design and conduct; provided a narrative summary of those findings.
Dr Jing Shen (Health Economist) developed the health economics protocol; designed and produced the health economics questionnaires; analysed data; drafted the economic section; and contributed to the final report.
Ms Christine Powell (Co-Investigator) contributed to the study design, protocol development, creation of case report forms and standard operating procedures, ethics committee and sponsor approvals and adoption of the study onto the UKCRN study portfolio.
Mr Chris Speed (Senior Clinical Trial Manager) oversaw study management.
Mr Peter Tiffin (Consultant Ophthalmologist, Sunderland Eye Infirmary) conceived the study; contributed to the study design and funding acquisition; and oversaw delivery of the intervention.
Mr John Sloper (Consultant Ophthalmologist, Moorfields Eye Hospital NHS Foundation Trust) – conceived the study; contributed to the study design and funding acquisition; and oversaw delivery of the intervention.
Mr Robert Taylor (Consultant Ophthalmologist, York Hospitals NHS Foundation Trust) conceived the study, contributed to the study design and funding acquisition, and oversaw delivery of the intervention.
Dr Mahmoud Nassar (Data analyst) analysed the Mayo Control Score data.
Dr Kerry Joyce (Senior Research Associate) contributed to data extraction, quality assessment and synthesis for the systematic review, and wrote up the report.
Mrs Fiona Beyer (Research Associate) devised and carried out the literature searches for the systematic review; wrote up the search methods; contributed to data extraction, quality assessment and synthesis; and commented on the report.
Professor Richard Thomson (Professor of Epidemiology and Public Health) conceived the systematic review (with Michael Clarke), supervised the researchers, participated in discussions about eligibility and quality of the included studies, and critically reviewed and approved the final version of this section of the report.
Professor Luke Vale (Health Foundation Chair in Health Economics) oversaw the health economics element of the study and contributed to the final report.
Professor Elaine McColl (Director Newcastle Clinical Trials Unit, Institute of Health and Society) contributed to the study design and funding acquisition, and supervised the involvement of the Newcastle Clinical Trials Unit.
Dr Nick Steen (Statistician) provided guidance on statistical issue of study design and data analysis.
Publications
Joyce KE, Beyer F, Thomson RG, Clarke MP. A systematic review of the effectiveness of treatments in altering the natural history of intermittent exotropia. Br J Ophthalmol 2014;Epub ahead of print.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Rosenbaum A, Santiago A. Clinical Strabismus Management: Principles and Surgical Techniques. Philadelphia, PA: WB Saunders; 1999.
- Menon V, Saha J, Tandon R, Mehta M, Khokhar S. Study of the psychosocial aspects of strabismus. J Pediatr Ophthalmol Strabismus 2002;39:203-8.
- Archer S, Musch D, Wren P, Guire K, Del Monte M. Social and emotional impact of strabismus surgery on quality of life in children. J AAPOS 2005;9:148-51. http://dx.doi.org/10.1016/j.jaapos.2004.12.006.
- Robaei D, Huynh S, Kifley A, Gole G, Mitchell P. Stereoacuity and ocular associations at age 12 years: findings from a population-based study. J AAPOS 2007;11:356-61. http://dx.doi.org/10.1016/j.jaapos.2006.11.111.
- Olitsky S, Sudesh S, Graziano A, Hamblen J, Brooks S, Shaha S. The negative psychosocial impact of strabismus in adults. J AAPOS 1999;3:209-11. http://dx.doi.org/10.1016/S1091-8531(99)70004-2.
- Horwood J, Waylen A, Herrick D, Williams C, Wolke D. Common visual defects and peer victimisation in children. Invest Ophth Vis Sci. 2005;46:1177-81. http://dx.doi.org/10.1167/iovs.04-0597.
- Rahi J, Logan S, Timms C, Russell-Eggitt I, Taylor D. Risk, causes, and outcomes of visual impairment after loss of vision in the non-amblyopic eye: a population based study. Lancet 2002;360:597-602. http://dx.doi.org/10.1016/S0140-6736(02)09782-9.
- Health and Social Care Information Centre (HSCIC) . Hospital Outpatient Activity: 2011–12 [NS] 2013. http://tiny.cc/5sfyzw (accessed June 2013).
- Stewart C, Moseley M, Stephens D, Fielder A. Treatment dose–response in amblyopia therapy: The monitored occlusion treatment of amblyopia study (MOTAS). Invest Ophthalmol Vis Sci 2004;45:3048-54. http://dx.doi.org/10.1167/iovs.04-0250.
- Chou M, Malik A, Suleman M, Gray M, Yeates D, Goldacre M. Time trends over five decades, and recent geographical variation, in rates of childhood squint surgery in England. Br J Ophthalmol 2013;97:746-51. http://dx.doi.org/10.1136/bjophthalmol-2012-302932.
- Clarke M. Intermittent exotropia. J Pediatr Ophthalmol Strabismus 2007;44:153-7.
- Hutchinson AK. Intermittent exotropia. Ophthalmol Clin North Am 2001;14:399-406. http://dx.doi.org/10.1016/S0896-1549(05)70237-6.
- Mohney B, Huffaker R. Common forms of childhood exotropia. Ophthalmology 2003;110:2093-6. http://dx.doi.org/10.1016/j.ophtha.2003.04.001.
- Govindan M, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood exotropia: a population-based study. Ophthalmology 2005;112:104-8. http://dx.doi.org/10.1016/j.ophtha.2004.07.033.
- Mohney B, McKenzie J, Capo J, Nusz K, Mrazek D, Diehl N. Mental illness in young adults who had strabismus as children. Pediatrics 2008;122:1033-8. http://dx.doi.org/10.1542/peds.2007-3484.
- Hatt S, Mohney B, Leske D, Holmes J. Variability of stereoacuity in intermittent exotropia. Am J Ophthalmol 2008;145:556-61. http://dx.doi.org/10.1016/j.ajo.2007.10.028.
- Chia A, Roy L, Seenyen L. Comitant horizontal strabismus: an Asian perspective. Br J Ophthalmol 2007;91:1337-40. http://dx.doi.org/10.1136/bjo.2007.116905.
- Rosenbaum A, Stathacopoulos R. Subjective and objective criteria for recommending surgery in intermittent exotropia. Am Orthopt J 1992;42:46-51.
- Mohney BG, Holmes JM. An office-based scale for assessing control in intermittent exotropia. Strabismus 2006;14:147-50. http://dx.doi.org/10.1080/09273970600894716.
- Buck D, Powell CJ, Rahi J, Cumberland P, Tiffin P, Taylor R, et al. The improving outcomes in intermittent exotropia study: outcomes at 2 years after diagnosis in an observational cohort. BMC Ophthalmol 2012;12.
- Coffey B, Wick B, Cotter S, Scharre J, Horner D. Treatment options in intermittent exotropia: a critical appraisal. Optom Vis Sci 1992;69:386-404. http://dx.doi.org/10.1097/00006324-199205000-00008.
- Spencer RF, Tucker MG, Choi RY, McNeer KW. Botulinum toxin management of childhood intermittent exotropia. Ophthalmology 1997;104:1762-7. http://dx.doi.org/10.1016/S0161-6420(97)30029-3.
- Watts P, Tippings E, Al-Madfai H. Intermittent exotropia, overcorrecting minus lenses, and the newcastle scoring system. J AAPOS 2005;9:460-4. http://dx.doi.org/10.1016/j.jaapos.2005.04.010.
- Piano M, O’Connor AR. Conservative management of intermittent distance exotropia: a review. Am Orthop J 2011;61:103-16. http://dx.doi.org/10.3368/aoj.61.1.103.
- Buck D, Powell CJ, Sloper JJ, Taylor R, Tiffin P, Clarke MP. Surgical intervention in childhood intermittent exotropia: current practice and clinical outcomes from an observational cohort study. Br J Ophthalmol 2012;96:1291-5. http://dx.doi.org/10.1136/bjophthalmol-2012-301981.
- Pineles SL, Deitz LW, Velez FG. Postoperative outcomes of patients initially overcorrected for intermittent exotropia. J AAPOS 2011;15:527-31. http://dx.doi.org/10.1016/j.jaapos.2011.08.007.
- Hatt SR, Gnanaraj L. Interventions for intermittent exotropia. Cochrane Database Syst Rev 2013;5. http://dx.doi.org/10.1002/14651858.CD003737.pub3.
- Koklanis K, Georgievski Z. Recurrence of intermittent exotropia: factors associated with surgical outcomes. Strabismus 2009;17:37-40. http://dx.doi.org/10.1080/09273970802678750.
- Pediatric Eye Disease Investigator Group . IXT1: A Randomized Trial of Bilateral Lateral Rectus Recession Versus Unilateral Lateral Rectus Recession With Medial Rectus Resection for Intermittent Exotropia 2013 n.d. http://pedig.jaeb.org/Studies.aspx?RecID = 153 (accessed June 2013).
- Pediatric Eye Disease Investigator Group . IXT2 – a randomized clinical trial of observation versus occlusion therapy for intermittent exotropia. Ophthalmology 2014;121:2299-310.
- Carlton J, Karnon J, Czoski-Murray C, Smith K, Marr J. The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation. Health Technol Assess 2008;12. http://dx.doi.org/10.3310/hta12250.
- Meyer K, Breitschwerdt H, Kolling G, Simonsz H. Early vs Late Infantile Strabismus Surgery Study Group. The early vs late infantile strabismus surgery study: do sources for bias exist in this non-randomised trial?. Br J Ophthalmol 1998;82:934-8. http://dx.doi.org/10.1136/bjo.82.8.934.
- Simonsz H, Kolling G, Unnebrink K. Final report of the early vs. late infantile strabismus surgery study (ELISSS), a controlled, prospective, multicenter study. Strabismus 2005;13:169-99. http://dx.doi.org/10.1080/09273970500416594.
- McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ 2002;324:1448-51. http://dx.doi.org/10.1136/bmj.324.7351.1448.
- Pathai S, Cumberland P, Rahi JS. Prevalence of and early-life influences on childhood strabismus: findings from the millennium cohort study. Arch Pediatr Adolesc 2010;164:250-7. http://dx.doi.org/10.1001/archpediatrics.2009.297.
- Romanchuk KG, Dotchin SA, Zurevinsky J. The natural history of surgically untreated intermittent exotropia-looking into the distant future. J AAPOS 2006;10:225-31. http://dx.doi.org/10.1016/j.jaapos.2006.02.006.
- Durnian JM, Noonan CP, Marsh IB. The psychosocial effects of adult strabismus: a review. Br J Ophthalmol 2011;95:450-3. http://dx.doi.org/10.1136/bjo.2010.188425.
- Royal College of Ophthalmologists . Guidelines for the Management of Strabismus in Childhood 2013 n.d. www.rcophth.ac.uk/core/core_picker/download.asp?id = 1291 (accessed August 2012).
- Undertaking Systematic Reviews of Research on Effectiveness. CRD’s Guidance for Carrying Out or Commissioning Reviews. York: CRD; 2001.
- Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley-Blackwell; 2008.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2700.
- Figueira EC, Hing S. Intermittent exotropia: comparison of treatments. Clin Experiment Ophthalmol 2006;34:245-51. http://dx.doi.org/10.1111/j.1442-9071.2006.01199.x.
- Milladot M. Dictionary of Optometry and Visual Science. London: Elsevier; 2004.
- Rowe F, Noonan CP. Complications of botulinum toxin a and their adverse effects. Strabismus 2009;17:139-42. http://dx.doi.org/10.3109/09273970903303860.
- Zhang KK, Koklanis K, Georgievski Z. Aust Orthop J 2007;39:31-7.
- Critical Appraisal Skills Programme (CASP) . Making Sense of Evidence About Clinical Effectiveness. Cohort Appraisal Checklist n.d. www.casp-uk.net/wp-content/uploads/2011/11/CASP_Cohort_Appraisal_Checklist_14oct10.pdf (accessed February 2013).
- Kushner BJ. Selective surgery for intermittent exotropia based on distance/near differences. Arch Ophthalmol 1998;116:324-8. http://dx.doi.org/10.1001/archopht.116.3.324.
- Qiu H, Li XY, Li HY, Wang XL, Zhang JS. Binocular vision training after intermittent exotropia surgery. Int J Ophthalmol 2010;10:1522-3.
- Chia A, Seenyen L, Long QB. Surgical experiences with two-muscle surgery for the treatment of intermittent exotropia. J AAPOS 2006;10:206-11. http://dx.doi.org/10.1016/j.jaapos.2005.11.015.
- Choi J, Chang JW, Kim S-J, Yu YS. The long-term survival analysis of bilateral lateral rectus recession versus unilateral recession-resection for intermittent exotropia. Am J Ophthalmol 2012;153:343-51. http://dx.doi.org/10.1016/j.ajo.2011.06.024.
- Lee S, Lee YC. Relationship between motor alignment at postoperative day 1 and at year 1 after symmetric and asymmetric surgery in intermittent exotropia. Jpn J Ophthalmol 2001;45:167-71. http://dx.doi.org/10.1016/S0021-5155(00)00351-8.
- Lee S-Y, Hyun Kim J, Thacker NM. Augmented bilateral lateral rectus recessions in basic intermittent exotropia. J AAPOS 2007;11:266-8. http://dx.doi.org/10.1016/j.jaapos.2007.02.014.
- Maruo T, Kubota N, Sakaue T, Usui C. Intermittent exotropia surgery in children: Long term outcome regarding changes in binocular alignment. A study of 666 cases. Binocul Vis Strabismus Q 2001;16:265-70.
- Wu X, Li Y. Observation of botulinum toxin a management in childhood with intermittent exotropia. Chung-Hua Yen Ko Tsa Chih 2008;44:967-71.
- Yuksel D, Spiritus M, Vandelannoitte S. Symmetric or asymmetric surgery for basic intermittent exotropia. Bull Soc Belge Ophtalmol 1998;268:195-9.
- Bramante C, Merrill KS, Christiansen SP. Comparison of surgical treatments for basic type intermittent exotropia. ARVO Meeting Abstracts 2009;50.
- Israel MD, Basu R, Choudhury M. Efficacy of hang-back recessions in comparison to conventional recessions. J AAPOS 2011;15:e21-2. http://dx.doi.org/10.1016/j.jaapos.2011.01.083.
- Kamai AM, Abdelhakeem AS, Elhilaly HM. Comparative study between bilateral lateral rectus recession and unilateral recess-resect surgery in managment of basic exotropia. J AAPOS 2008:e7-8.
- Kim MM, Hwang BS, Lim S. The prognostic factors of recurrence in bilateral lateral rectus recession and unilateral recession–resection procedure for intermittent exotropia. J AAPOS 2010;14. http://dx.doi.org/10.1016/j.jaapos.2009.12.087.
- Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7. http://dx.doi.org/10.3310/hta7270.
- Haggerty H, Richardson S, Hrisos S, Strong NP, Clarke MP. The Newcastle Control Score: a new method of grading the severity of intermittent distance exotropia. Br J Ophthalmol 2004;88:233-5. http://dx.doi.org/10.1136/bjo.2003.027615.
- Hatt S, Leske D, Liebermann L, Mohney B, Holmes J. Variability of angle of deviation measurements in children with intermittent exotropia. J AAPOS 2012;16:120-4. http://dx.doi.org/10.1016/j.jaapos.2011.11.008.
- Guyatt GH, Oxman A.D, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. Grade guidelines: 4. Rating the quality of evidence study limitations (risk of bias). J Clin Epidemiol 2011;64:407-15. http://dx.doi.org/10.1016/j.jclinepi.2010.07.017.
- Lancaster G, Dodd S, Williamson P. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. http://dx.doi.org/10.1111/j. 2002.384.doc.x.
- Buck D, McColl E, Powell C, Shen J, Sloper J, Steen N, et al. Surgery versus active monitoring in intermittent exotropia (SamExo): study protocol for a pilot randomised controlled trial. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-192.
- Hatt S, Leske D, Yamada T, Bradley E, Cole S, Holmes J. Development and initial validation of quality-of-life questionnaires for intermittent exotropia. Ophthalmology 2010;117:163-8. http://dx.doi.org/10.1016/j.ophtha.2009.06.038.
- Holmes J, Leske D, Hohberger G. Defining real change in prism-cover test measurements. Am J Ophthalmol 2008;145:381-5. http://dx.doi.org/10.1016/j.ajo.2007.09.012.
- Glick H, Polksy D, Schulman K, Drummond MF, McGuire A. Economic Evaluation in Health Care: Merging Theory with Practice. New York: Oxford University Press; 2001.
- Department for Transport . Values of Time and Operating Costs 2012. www.dft.gov.uk/webtag/documents/expert/unit3.5.6.php (accessed July 2013).
- Van Horn PS, Green KE, Martinussen M. Survey response rates and survey administration in counseling and clinical psychology: a meta-analysis. Educ Psychol Meas 2009;69:389-403. http://dx.doi.org/10.1177/0013164408324462.
- Adams W, Leske D, Hatt S, Holmes J. Defining real change in measures of stereoacuity. Ophthalmology 2009;116:281-5. http://dx.doi.org/10.1016/j.ophtha.2008.09.012.
- Hatt S, Mohney B, Leske D, Holmes J. Variability of control in intermittent exotropia. Ophthalmology 2008;115:371-6. http://dx.doi.org/10.1016/j.ophtha.2007.03.084.
- Buck D, Powell C, Cumberland P, Davis H, Dawson E, Rahi J, et al. Presenting features and early management of childhood intermittent exotropia in the UK: inception cohort study. Br J Ophthalmol 2009;93:1620-4. http://dx.doi.org/10.1136/bjo.2008.152975.
- Ruttum MS. Initial versus subsequent postoperative motor alignment in intermittent exotropia. J AAPOS 1997;1:88-91. http://dx.doi.org/10.1016/S1091-8531(97)90004-5.
- Scott W, Keech R, Mash J. The postoperative results and stability of exodeviations. Arch Ophthalmol 1981;99. http://dx.doi.org/10.1001/archopht.1981.03930020688013.
- Leow P-L, Ko STC, Wu PKW, Chan CWN. Exotropic drift and ocular alignment after surgical correction for intermittent exotropia. J Pediatr Ophthalmol Strabismus 2010;47:12-6. http://dx.doi.org/10.3928/01913913-20100106-04.
- Pineles S, Ela-Dalman N, Zvansky A, Yu F, Rosenbaum A. Long-term results of the surgical management of intermittent exotropia. J AAPOS 2010;14:298-304. http://dx.doi.org/10.1016/j.jaapos.2010.06.007.
- Ekdawi NS, Nusz KJ, Diehl NN, Mohney BG. Postoperative outcomes in children with intermittent exotropia from a population-based cohort. J AAPOS 2009;13:4-7. http://dx.doi.org/10.1016/j.jaapos.2008.06.001.
- Holmes J, Leske D, Hatt S, Brodsky M, Mohney B. Stability of near stereoacuity in childhood intermittent exotropia. J AAPOS 2011;15:462-7. http://dx.doi.org/10.1016/j.jaapos.2011.06.008.
- Blakemore C, Price D. The organization and post-natal development of area 18 of the cat’s visual cortex. J Physiol 1987;384:263-92.
- Fawcett S, Wang Y, Birch E. The critical period for susceptibility of human stereopsis. Invest Ophthalmol Vis Sci 2005;46:521-5. http://dx.doi.org/10.1167/iovs.04-0175.
- Hasany A, Wong A, Foeller P, Bradley D, Tychsen L. Duration of binocular decorrelation in infancy predicts the severity of nasotemporal pursuit asymmetries in strabismic macaque monkeys. Neuroscience 2008;156:403-11. http://dx.doi.org/10.1016/j.neuroscience.2008.06.070.
- Birch E, Wang J. Stereoacuity outcomes after treatment of infantile and accommodative esotropia. Optom Vis Sci 2009;86:647-52. http://dx.doi.org/10.1097/OPX.0b013e3181a6168d.
- Rowe F. Long-term postoperative stability in infantile esotropia. Strabismus 2000;8:3-13.
- Pratt-Johnson JA, Barlow JM, Tillson G. Early surgery in intermittent exotropia. Am J Ophthalmol 1977;84:689-94. http://dx.doi.org/10.1016/0002-9394(77)90385-3.
- James Lind Alliance, Sight Loss and Vision Priority Setting Partnership . Priorities for Refractive Error and Ocular Motility 2013. www.sightlosspsp.org.uk/ (accessed June 2013).
- Abbas S, Said A. Role of concave lenses in the treatment of divergent squint. Bull Ophth Soc Egypt 1975;68.
- Abbasoglu OE, Sener EC, Sanac AS. Factors influencing the successful outcome and response in strabismus surgery. Eye 1996;10:315-20. http://dx.doi.org/10.1038/eye.1996.66.
- Abroms AD, Mohney BG, Rush DP, Parks MM, Tong PY. Timely surgery in intermittent and constant exotropia for superior sensory outcome. Am J Ophthalmol 2001;131:111-16. http://dx.doi.org/10.1016/S0002-9394(00)00623-1.
- Adams WE, Leske DA, Hatt SR, Mohney BG, Birch EE, Weakley DR, et al. Improvement in distance stereoacuity following surgery for intermittent exotropia. J AAPOS 2008;12:141-14. http://dx.doi.org/10.1016/j.jaapos.2007.09.015.
- Akar S, Gokyigit B, Yilmaz OF. Graded anterior transposition of the inferior oblique muscle for V-pattern strabismus. J AAPOS 2012;16:286-90. http://dx.doi.org/10.1016/j.jaapos.2012.01.009.
- Akatsuka T, Kimura H, Oka M, Arai N, Fukai S, Tabuchi A. Examination of eye position after strabismus surgery in kawasaki medical school hospital. Nippon Ganka Gakkai Zasshi 2001;105:781-7.
- Alajbegović-Halimić J. Surgical overcorrection squint angle in extropia related to retinal correspondence relationship. Med Arh 2007;61:94-6.
- Altizer LB. The nonsurgical treatment of exotropia. Am Orthop J 1972;22:71-6.
- Ameri A, Jafari AK, Anvari F, Ahadzadeghan I, Rajabi MT. A new modfied anchored suspension-recession (so-called ‘hang-back’ technique for high risk strabismus surgery. Binocul Vis Strabismus Q 2010;25:21-30.
- Archer SM. The effect of medial versus lateral rectus muscle surgery on distance–near incomitance. J AAPOS 2009;13:20-6. http://dx.doi.org/10.1016/j.jaapos.2008.09.003.
- Arnoldi KA, Reynolds JD. Assessment of amplitude and control of the distance deviation in intermittent exotropia. J Pediatr Ophthalmol Strabismus 2008;45:150-3. http://dx.doi.org/10.3928/01913913-20080501-05.
- Asadi R, Ghasemi-Falavarjani K, Sadighi N. Orthoptic treatment in the management of intermittent exotropia. Iran J Ophthalmol 2009;21:35-40.
- Asjes-Tydeman WL, Groenewoud H, van der Wilt GJ. Timing of surgery for primary exotropia in children. Strabismus 2006;14:191-7. http://dx.doi.org/10.1080/09273970601026193.
- Aslanis D, Follidi V, Constantopoulos I, Spyropoulos G, Paikos P. Surgical results in childhood primary comitant large-angle exotropia. J Fr Ophtalmol 2006;29:37-42. http://dx.doi.org/10.1016/S0181-5512(06)73745-7.
- Attarzadeh H, Zandi A, Nasrollahi K, Mortazavi AA. Two years results of unilateral lateral rectus recession on moderate intermittent exotropia. J Med Sci 2008;13:12-6.
- Awadein A, Gawdat G. Comparison of superior oblique suture spacers and superior oblique silicone band expanders. J AAPOS 2012;16:131-5. http://dx.doi.org/10.1016/j.jaapos.2011.11.011.
- Awadein A, Gawdat G. Bilateral inferior oblique myectomy for asymmetric primary inferior oblique overaction. J AAPOS 2008;12:560-4. http://dx.doi.org/10.1016/j.jaapos.2008.04.013.
- Awaya S, Sugawara M, Miyake S, Torii F, Horibe F. Effect of anti-suppression in exotropia by an on-and-off stimulation with the checkerboard pattern stimulator (author’s translation). Nippon Ganka Gakkai Zasshi 1982;86:264-8.
- Bae HW, Chung SA, Yoon JS, Lee JB. Changes in the interpupillary distance following general anesthesia in children with intermittent exotropia: a predictor of surgical outcomes. J Pediatr Ophthalmol Strabismus 2012;49:49-53. http://dx.doi.org/10.3928/01913913-20110517-01.
- Baker JD. Twenty-year follow-up of surgery for intermittent exotropia. J AAPOS 2008;12:227-32. http://dx.doi.org/10.1016/j.jaapos.2008.02.007.
- Baker JD, Davies GT. Monofixational intermittent exotropia. Arch Ophthalmol 1979;97:93-5. http://dx.doi.org/10.1001/archopht.1979.01020010033007.
- Bansal S, Khan J, Marsh IB. The role of botulinum toxin in decompensated strabismus. Strabismus 2008;16:107-11. http://dx.doi.org/10.1080/09273970802292693.
- Bao J, Tao Y-X. Surgical alignment for comitant exotropia in 59 children. Int J Ophthalmol 2006;6:483-5.
- Barraza RA, McKenzie JA, Diehl NN, Mohney BG. Surgical correction of childhood intermittent exotropia does not influence the development of mental illness. ARVO Meeting Abstracts 2009;50. http://dx.doi.org/10.1016/j.ajo.2014.06.008.
- Basar E, Oguz H, Ermis S. Overcorrecting minus lens application for intermittent exotropia. Ann Med Sci 2000;9:132-4.
- Beneish R, Flanders M. The role of stereopsis and early postoperative alignment in long-term surgical results of intermittent exotropia. Canadian J Ophthalmol 1994;29:119-24.
- Berard PV, Michel-Morris DV, Mouillac-Cambarelli N. Surgical management of divergent strabismus (author’s translation). J Fr Ophtalmol 1979;2:53-61.
- Berard PV, Mouillac-Gambarelli N, Layec-Arnail M, Reydy R. Surgical overcorrections in exotropia. Bull Soc Ophtalmol Fr 1979;79:1009-13.
- Berard PV, Reydy R, Layec M, Marineau S. Treatment of intermittent exotropias. Bull Soc Ophtalmol Fr 1975;75:613-30.
- Berg PH. Low incidence of consecutive eso deviation following surgical correction of exo deviation. Br Orthopt J 1983;40:60-2.
- Berland JE, Wilson ME, Saunders RB. Results of large (8–9 mm) bilateral lateral rectus muscle recessions for exotropia. Binocul Vis Strabismus Q 1998;13:97-104.
- Berrondo P. Sector occlusions for divergent strabismus. Bull Soc Ophtalmol Fr 1980;80:737-8.
- Besharati MR, Lotfi MH, Nourani F. Outcomes and complications of strabismus surgery in yazd, iran. Int J Ophthalmol 2008;8:1104-7.
- Bietti GB. On a technical procedure (recession and fan-shaped oblique reinsertion of the horizontal rectus muscles) for correction of V or A exotropias of slight degree in concomitant strabismus. Boll Ocul 1970;49:581-8.
- Billet E, Freedman M. Surgery of the inferior oblique muscles in V pattern exotropia. Arch Ophthalmol 1969;82:21-2. http://dx.doi.org/10.1001/archopht.1969.00990020023006.
- Binion WW. The surgical treatment of intermittent exotropia. Am J Ophthalmol 1966;61:869-74. http://dx.doi.org/10.1016/0002-9394(66)90927-5.
- Brandner M, Buchberger M, Kaltofen T, Haslwanter T, Hoerantner R, Langmann A. Biomechanical analysis of x-pattern exotropia. Am J Ophthalmol 2011;152:141-6. http://dx.doi.org/10.1016/j.ajo.2011.01.029.
- Brooks DR, Donahue SP, Morrison DG. The efficacy of superior oblique z-tenotomy in the treatment of overdepression in adduction (superior oblique overaction). J AAPOS 2011;15:9-13. http://dx.doi.org/10.1016/j.jaapos.2011.01.012.
- Broniarczyk-Loba A, Nowakowska O. Adjustable sutures surgery of intermittent divergent squint in adolescents and adults. Klin Oczna 2003;105:63-5.
- Broniarczyk-Loba A, Suprunowicz I, Nowakowska O. Squint angle, eyeball length and surgical results in horizontal strabismus. Klin Oczna 1994;96:206-9.
- Caldeira JAF. Some clinical characteristics of v-pattern exotropia and surgical outcome after bilateral recession of the inferior oblique muscle: a retrospective study of 22 consecutive patients and a comparison with v-pattern esotropia. Binocul Vis Strabismus Q 2004;19:139-50.
- Caltrider N, Jampolsky A. Overcorrecting minus lens therapy for treatment of intermittent exotropia. Ophthalmology 1983;90:1160-5. http://dx.doi.org/10.1016/S0161-6420(83)34412-2.
- Capo H, Repka MX, Guyton DL. Hang-back lateral rectus recessions for exotropia. J Pediatr Ophthalmol Strabismus 1989;26:31-4.
- Carlton J. General health-related quality of life in preschool children with strabismus or amblyopia. Evidence Base Ophthalmol 2011;12:212-13.
- Carta A, Pinna A, Aini MA, Carta A, Carta F. Intermittent exotropia: evaluation of results on the basis of different treatments. J Fr Ophtalmol 1994;17:161-6.
- Cassin B, Serianni N, Romano P. The change in ocular alignment between the first day and six weeks following eye muscle surgery. Am Orthop J 1986;36:99-107.
- Castelbuono AC, White JE, Guyton DL. The use of (a)symmetry of the rest position of the eyes under general anesthesia or sedation-hypnosis in the design of strabismus surgery: a favorable pilot study in 51 exotropia cases. Binocul Vis Strabismus Q 1999;14:285-90.
- Castellanos-Bracamontes A, Mozo-Cueto A. Variable angle strabismus and its relation to poor vision and psychomotor retardation. Bol Med Hosp Infant Mex 1990;47:828-32.
- Celebi S, Kukner AS. Large bilateral lateral rectus recession in large angle divergence excess exotropia. Eur J Ophthalmol 2001;11:6-8.
- Chae SH, Chun BY, Kwon JY. The effect of unilateral medial rectus muscle resection in patients with recurrent exotropia. Korean J Ophthalmol 2008;22:174-7. http://dx.doi.org/10.3341/kjo.2008.22.3.174.
- Chang Y-S, Baek S-H, Park J-M, Kwon H-U, Kim Y-R. Effect of muscle relaxants on short-term results of exotropia surgery: a focus on resection procedures. Korean J Ophthalmol 2008;22:246-50. http://dx.doi.org/10.3341/kjo.2008.22.4.246.
- Chang Y-H, Ryu IH, Han S-H, Lee S-J, Lee J-B. Intraoperative adjustment in strabismus surgery under topical anesthesia. Yonsei Med J 2006;47:667-71. http://dx.doi.org/10.3349/ymj.2006.47.5.667.
- Chia A, Seenyen L, Long QB. A retrospective review of 287 consecutive children in singapore presenting with intermittent exotropia. J AAPOS 2005;9:257-63. http://dx.doi.org/10.1016/j.jaapos.2005.01.007.
- Cho YK, Lee S-YL. Relationship of control grade, stereoacuity and surgical success in basic intermittent exotropia. ARVO Meeting Abstracts 2012;53.
- Cho YA, Kim SH. Role of the equator in the early overcorrection of intermittent exotropia. J Pediatr Ophthalmol Strabismus 2009;46:30-4. http://dx.doi.org/10.3928/01913913-20090101-04.
- Cho YA, Kim SH. Surgical outcomes of intermittent exotropia associated with concomitant hypertropia including simulated superior oblique palsy after horizontal muscles surgery only. Eye 2007;21:1489-92. http://dx.doi.org/10.1038/sj.eye.6702585.
- Choi J, Kim SJ, Yu YS. Initial postoperative deviation as a predictor of long-term outcome after surgery for intermittent exotropia. J AAPOS 2011;15:224-9. http://dx.doi.org/10.1016/j.jaapos.2010.12.019.
- Choi DG, Rosenbaum AL. Medial rectus resection(s) with adjustable suture for intermittent exotropia of the convergence insufficiency type. J AAPOS 2001;5:13-7. http://dx.doi.org/10.1067/mpa.2001.111137.
- Chryssanthou G. Orthoptic management of intermittent exotropia. Am Orthop J 1974;24:69-72.
- Chun B-Y, Kim H-K, Kwon J-Y. Comparison of magnitude of astigmatism induced by lateral rectus recession. Optom Vis Sci 2010;87:61-5. http://dx.doi.org/10.1097/OPX.0b013e3181c1d695.
- Chun K-i, Rah S-h. The comparison of outcomes between lateral rectus muscles re-recession and medial rectus muscles resection in recurrent exotropia. Korean J Ophthalmol 2008;22:111-14. http://dx.doi.org/10.3341/kjo.2008.22.2.111.
- Chung SA, Chang YH, Rhiu S, Lew H, Lee JB. Parent-reported symptoms of attention deficit hyperactivity disorder in children with intermittent exotropia before and after strabismus surgery. Yonsei Med J 2012;53:806-11. http://dx.doi.org/10.3349/ymj.2012.53.4.806.
- Chung SA, Kim IS, Kim WK, Lee JB. Changes in exodeviation following hyperopic correction in patients with intermittent exotropia. J Pediatr Ophthalmol Strabismus 2011;48:278-84. http://dx.doi.org/10.3928/01913913-20101217-01.
- Chutter CP. Occlusion treatment of intermittent divergent strabismus. Am Orthop J 1977;27:80-4.
- Ciancia AO, Melek N. New treatment of anomalous correspondence in intermittent exotropias. Archivos De Oftalmologia De Buenos Aires 1969;44:108-19.
- Clark RA, Demer JL. Posterior inflection of weakened lateral rectus path: connective tissue factors reduce response to lateral rectus recession. Am J Ophthalmol 2009;147:127-33. http://dx.doi.org/10.1016/j.ajo.2008.07.029.
- Clarke WN, Noel LP. Surgical results in intermittent exotropia. Canadian J Ophthalmol 1981;16:66-9.
- Cooper J, Selenow A, Ciuffreda KJ, Feldman J, Faverty J, Hokoda SC, et al. Reduction of asthenopia in patients with convergence insufficiency after fusional vergence training. Am J Optom Physiol Opt 1983;60:982-9. http://dx.doi.org/10.1097/00006324-198312000-00007.
- Cooper EL, Leyman IA. The management of intermittent exotropia: A comparison of the results of surgical and nonsurgical treatment. Am Orthop J 1977;27:61-7.
- Dadeya S, Kamlesh. Long-term results of unilateral lateral rectus recession in intermittent exotropia. J Pediatr Ophthalmol Strabismus 2003;40:283-7.
- Dahlmann C, Kaufmann H. Better outcome with early surgery of intermittent exotropia?. Klin Monatsbl Augenheilk 2007;224:546-7.
- Dawson EL, Marshman WE, Lee JP. Role of botulinum toxin a in surgically overcorrected exotropia. J AAPOS 1999;3:269-71. http://dx.doi.org/10.1016/S1091-8531(99)70021-2.
- Debert I, Passos LB, Saraiva PG, Tomikawa VO, Polati M. Simplified intraoperative adjustable suture technique for horizontal strabismus surgery: a study of 153 cases. Arq Bras Oftalmol 2007;70:13-8.
- de Decker W, Baenge JJ. Unilateral medial rectus resection in the treatment of small-angle exodeviation. Graefes Arch Clin Exp Ophthalmol 1988;226:161-4. http://dx.doi.org/10.1007/BF02173308.
- Deitz LW, Pineles SL, Velez FG. Does amount of immediate postoperative esotropia predict later alignment after surgery for intermittent exotropia?. J AAPOS 2011;15:527-31.
- Gomez de Liano Sanchez R, Rodriguez Sanchez JM, Gomez de Liano Sanchez P, De Andres ML, Rodriguez Sanchez J. Botulinum toxin therapy in strabismus operated patients. Arch Soc Esp Oftalmol 1997;72:75-80.
- Demmers ML, Laflamme M. Surgical treatment of divergent strabismus. Union Med Can 1971;100:1616-18.
- Deng HW, Liu CM, Jia HL, Han B, Zhou F. Analyses of visual function recovery and refraction changes of strabismic amblyopia after surgery. Int J Ophthalmol. 2009;9:1797-8.
- Deutsch JA, Nelson LB, Sheppard RW, Burke MJ. Unilateral lateral rectus recession for the treatment of exotropia. Ann Ophthalmol 1992;24:111-13.
- Dong N, Liu LQ, Li PH. Clinical characteristics and surgical management in a-pattern exotropia. Int J Ophthalmol 2006;6:999-1001.
- Donnelly UM, Stewart NM, Hollinger M. Prevalence and outcomes of childhood visual disorders. Ophthalmic Epidemiol. 2005;12:243-50. http://dx.doi.org/10.1080/09286580590967772.
- Dzelkaleia II. Clinical characteristics of divergent concomitant strabismus. Oftalmol Zhurnal n.d.:437-9.
- Edelman PM, Brown MH, Murphree AL, Wright K. Consecutive esodeviation . . . then what?. Am Orthop J 1988;38:111-16.
- Ekdawi NS, Nusz KJ, Diehl NN, Mohney BG. The development of myopia among children with intermittent exotropia. Am J Ophthalmol 2010;149:503-7. http://dx.doi.org/10.1016/j.ajo.2009.10.009.
- El-Defrawi H. A comparatvie study of the role of pre-operative orthoptics and occlusion in the treatment of divergent squint. Bull Ophth Soc Egypt 1970;63:451-5.
- Engel JM, Rousta ST. Adjustable sutures in children using a modified technique. J AAPOS 2004;8:243-8. http://dx.doi.org/10.1016/j.jaapos.2004.01.007.
- Eustace P. Myopia and divergent squint in West Indian children. Br J Ophthalmol 1972;56:559-64. http://dx.doi.org/10.1136/bjo.56.7.559.
- Faridi UA, Saleh TA, Ewings P, Twomey JM. Factors affecting the surgical outcome of primary exotropia. Strabismus 2007;15:127-31. http://dx.doi.org/10.1080/09273970701506086.
- Fastrez-Moutschen A, Paris V. Mini prisms in decompensated exophoria in children. Bull Soc Belge Ophtalmol 1993;247:91-4.
- Feretis D. Large single lateral rectus recession for the treatment of exotropia. Ann Ophthalmol 1999;31:226-30.
- Fiorelli VMB, Goldchmit M, Uesugui CF, Souza-Dias C. Intermittent exotropia: comparative surgical results of lateral recti-recession and monocular recess-resect. Arq Bras Oftalmol 2007;70:429-32. http://dx.doi.org/10.1590/S0004-27492007000300008.
- Friedman Z, Neumann E, Hyams SW, Peleg B. Ophthalmic screening of 38,000 children, age 1 to 2½ years, in child welfare clinics. J Pediatr Ophthalmol Strabismus 1980;17:261-7.
- Friemel E. The operative therapy of strabismus divergenss. Klin Monatsbl Augenheilk 1971;159:96-7.
- Gagnon R, Gohier L. Orthoptic treatment of divergent intermittent strabismus. Union Med Can 1970;99:1490-2.
- Gezer A, Sezen F, Nasri N, Gozum N. Factors influencing the outcome of strabismus surgery in patients with exotropia. J AAPOS 2004;8:56-60. http://dx.doi.org/10.1016/j.jaapos.2003.08.006.
- Gharibyan A, Harutyunyan R, Gevorgyan O. Comparative study of surgical procedures performed on the inferior oblique muscle. ARVO Meeting Abstracts 2012;53.
- Goldrich SG. Optometric therapy of divergence excess strabismus. Am J Optom Physiol Opt 1980;57:7-14. http://dx.doi.org/10.1097/00006324-198001000-00002.
- Goldstein JH. The management of large angle exotropia. Eye Ear Nose Throat Mon 1968;47:480-6.
- Gordon YJ, Bachar E. Multiple regression analysis predictor models in exotropia surgery. Am J Ophthalmol 1980;90:687-91. http://dx.doi.org/10.1016/S0002-9394(14)75138-4.
- Govekar K. Adjustable suture in squint surgery. Afro Asian J Ophthalmol 1993;12:307-10.
- Graemiger A. Reoperation of the internal or external rectus in case of undercorrection (author’s transl). Klin Monatsbl Augenheilk 1979;175:413-17.
- Graf M. Intermittent exotropia surgery in children. Binocul Vis Strabismus Q 2002;17:78-9.
- Guo L, Cai N, Han Y. Research on children’s near stereopsis before and after surgical correction of intermittent exotropia. Int J Ophthalmol 2011;11:1012-14.
- Guo CM, Wang WN, Wang YS, Hu D. Using of supernormal lateral rectus muscle recession in the surgery of large-angle exotropia. Int J Ophthalmol 2009;9:325-7.
- Gupta NC, Narang RK, Khurana AK, Parmar IP, Ahluwalia BK. Exophoria and refractive errors-evaluation of 250 cases. Indian J Ophthalmol 1987;35:204-6.
- Gusek-Schneider GC, Kulzer J, Boss A. How useful is the prescription of glasses in intermittent exotropia and decompensating exophoria?. Klin Monatsbl Augenheilk 2006;223:620-8. http://dx.doi.org/10.1055/s-2006-926500.
- Ha S, Kim SH. Postoperative convergence after recovery from general anesthesia in exotropia surgery. J Pediatr Ophthalmol Strabismus 2011;48:305-10. http://dx.doi.org/10.3928/01913913-20101018-01.
- Hahm IR, Yoon SW, Baek S-H, Kong SM. The clinical course of recurrent exotropia after reoperation for exodeviation. Korean J Ophthalmol 2005;19:140-4. http://dx.doi.org/10.3341/kjo.2005.19.2.140.
- Hamaguchi H, Takahashi H, Sawada T, Miki K. Long-term observations on operated intermittent exotropia. Folia Ophthalmol Japon 1993;44:385-9.
- Hamtil LW, Place KC. Review of surgical results using bilateral lateral rectus recession for the correction of exodeviations. Ann Ophthalmol 1978;10:1731-4.
- Hao HL, Li J, Liu LQ. Operation therapy discussion of the intermittent exotropia. Int J Ophthalmol 2009 n.d.;9:794-5.
- Hardesty HH. Management of intermittent exotropia. Am Orthop J 1983;34:87-91.
- Hardesty HH. Treatment of intermittent exotropia. Arch Ophthalmol 1978;96:268-74. http://dx.doi.org/10.1001/archopht.1978.03910050136006.
- Hatsukawa Y, Nishina S, Sugasawa J, Kimura A, Yagasaki T, Fujikado T, et al. Surgical results of unilateral recession-resection for intermittent exotropia in children: multicenter study in Japan. Nippon Ganka Gakkai Zasshi 2011;115:440-6.
- Hatsukawa Y. The angle of deviation of exophoria: re-evaluating the cure criteria of exotropia. Folia Ophthalmol Japon 1992;43:470-5.
- Hatt SR, Leske DA, Liebermann L, Holmes JM. Predictors of surgery in children with intermittent exotropia. ARVO Meeting Abstracts 2012;53.
- Hatt SR, Leske DA, Mohney BG, Brodsky MC, Yamada T, Karlsson V, et al. Fusional convergence reserve, control, and stereoacuity in children with intermittent exotropia. ARVO Meeting Abstracts 2010;51.
- Hatt SR, Haggerty H, Buck D, Adams W, Strong NP, Clarke MP. Distance stereoacuity in intermittent exotropia. Br J Ophthalmol 2007;91:219-21. http://dx.doi.org/10.1136/bjo.2006.099465.
- Herzau V, Schoser G. The value of the prism adaptation test in determining the degree of squint surgery. Ophthalmologe 1993;90:11-6.
- Hiles DA, Davies GT, Costenbader FD. Long-term observations on unoperated intermittent exotropia. Arch Ophthalmol 1968;80:436-42. http://dx.doi.org/10.1001/archopht.1968.00980050438006.
- Holmes JM, Hatt SR, Mohney BG, Brodsky MC, Leske DA. Near stereoacuity in the natural history of intermittent exotropia. J AAPOS 2010;14:221-6.
- Holtgrave B. A contribution to the question of dosage in the surgery of divergent squint (author’s translation). Klin Monatsbl Augenheilk 1973;163:71-6.
- Hu XD, Chen J, Peng XJ, Yan SM, Yang MD. The effect of digital multimedia visual training applied after intermittent exotropia. Int Eye Sci 2012;12:1362-4.
- Huang YJ, Feng W. Comparison of master eye operation and slave eye operation on patients with concomitant exotropia. Int J Ophthalmol 2010;10:1403-4.
- Hugonnier R, Magnard P, Hugonnier S, Bongard C. Results of the treatment of 253 primary divergent strabismus cases. Bull Mem Soc Fr Ophtalmol 1970;83:203-16.
- Hugonnier R. Anomalous retinal correspondence in divergent strabismus. Documenta Ophthalmologica 1967;23:425-47. http://dx.doi.org/10.1007/BF02550762.
- Hunter DG, Kelly JB, Buffenn AN, Ellis FJ. Long-term outcome of uncomplicated infantile exotropia. J AAPOS 2001;5:352-6. http://dx.doi.org/10.1067/mpa.2001.120175.
- Iacobucci IL, Martonyi EJ, Giles CL. Results of overminus lens therapy on postoperative exodeviations. J Pediatr Ophthalmol Strabismus 1986;23:287-91.
- Inagaki Y, Awaya S, Yagasaki T, Sato M. Effect of the checkerboard pattern stimulator on sensory fusion in exotropia. Folia Ophthalmol Japon 1993;44.
- Ing EB. Delayed adjustable sutures: a multicentered clinical review. Evid Base Ophthalmol 2011;12:90-1.
- Ing MR. Botulinum toxin therapy in exotropia. Ophthalmology 1999;106:1045-6. http://dx.doi.org/10.1016/S0161-6420(99)90282-8.
- Ing MR, Pang S. Surgical alignment for exotropia in children. Am Orthop J 1986;36:108-12.
- Isenberg SJ, Abdarbashi P. Drift of ocular alignment following strabismus surgery. Part 2: using adjustable sutures. Br J Ophthalmol 2009;93:443-7. http://dx.doi.org/10.1136/bjo.2007.136382.
- Jacobi KW. Retinal correspondence in secondary divergent strabismus. Klin Monatsbl Augenheilk 1969;154:193-8.
- Jang JH, Park JM, Lee SJ. Factors predisposing to consecutive esotropia after surgery to correct intermittent exotropia. Graefes Arch Clin Exp Ophthalmol 2012;250:1485-90. http://dx.doi.org/10.1007/s00417-012-1991-y.
- Jeoung JW, Lee MJ, Hwang J-M. Bilateral lateral rectus recession versus unilateral recess-resect procedure for exotropia with a dominant eye. Am J Ophthalmol 2006;141:683-8. http://dx.doi.org/10.1016/j.ajo.2005.11.021.
- Jojic L, Stankov B, Knezevi L. Surgical treatment of divergent strabismus. Fortsch Ophthalmol 1987;84:474-5.
- Jung JH, Choi HY. Comparison of preoperative and postoperative anterior segment measurements with pentacam® in strabismus surgery. J Pediatr Ophthalmol Strabismus 2012;49:290-4. http://dx.doi.org/10.3928/01913913-20120501-02.
- Kang X-l, Wei Y. [The operation time and post-operative target eye alignment of intermittent exotropia. Chung-Hua Yen Ko Tsa Chih 2011;47:964-6.
- Kampanartsanyakorn S, Surachatkumtonekul T, Dulayajinda D, Jumroendararasmee M, Tongsae S. The outcomes of horizontal strabismus surgery and influencing factors of the surgical success. J Med Assoc Thai 2005;88:S94-9.
- Kaszli FA, Neugebauer A, Berger C, Pink U, Russmann W. Comparison of subjective and objective parameters after oculomotor muscle surgery. Ophthalmologe 1997;94:405-11. http://dx.doi.org/10.1007/s003470050134.
- Keenan JM, Willshaw HE. The outcome of strabismus surgery in childhood exotropia. Eye 1994;8:632-7. http://dx.doi.org/10.1038/eye.1994.158.
- Kertesz AE, Kertesz J. Wide–field fusional stimulation in strabismus. Am J Optom Physiol Opt 1986;63:217-22. http://dx.doi.org/10.1097/00006324-198603000-00009.
- Keskinbora KH, Horozoglu F, Gonen T, Sever O. Long-term results of surgery for intermittent exotropia. Turk Klin Tip Bilim 2012;32:638-43.
- Khaier A, Dawson E, Lee J. Traction sutures in the management of long-standing third nerve palsy. Strabismus 2008;16:77-83. http://dx.doi.org/10.1080/09273970802077474.
- Kii T, Nakagawa T. Natural history of intermittent exotropia: statistical study of preoperative strabismic angle in different age groups. Nippon Ganka Gakkai Zasshi 1992;96:904-9.
- Kim C, Hwang JM. ‘Largest angle to target’ in surgery for intermittent exotropia. Eye 2005;19:637-42. http://dx.doi.org/10.1038/sj.eye.6701604.
- Kliuka IV. Classification and diagnosis of divergent strabismus. Oftalmologicheskii Zhurnal n.d.:187-8.
- Koklanis K, Georgievski Z, Zhang K. The use of distance stereoacuity assessment in determining the effectiveness of minus lenses in intermittent exotropia. J AAPOS 2010;14:488-93. http://dx.doi.org/10.1016/j.jaapos.2010.08.010.
- Koo N-K, Lee Y-C, Lee S-Y. Clinical study for the undercorrection factor in intermittent exotropia. Korean J Ophthalmol 2006;20:182-7. http://dx.doi.org/10.3341/kjo.2006.20.3.182.
- Kosaki M. Postoperative observations on exotropia. Nippon Ganka Kiyo – Folia Ophthalmol Japon 1967;18:585-8.
- Kubota N, Maruo T. Pathophysiology, classification and treatment of intermittent exotropia (author’s translation). Nippon Ganka Gakkai Zasshi 1977;81:1140-7.
- Kushner BJ. The occurrence of monofixational exotropia after exotropia surgery. Am J Ophthalmol 2009;147:1082-5. http://dx.doi.org/10.1016/j.ajo.2009.01.005.
- Kushner BJ. Diagnosis and treatment of exotropia with a high accommodation convergence–accommodation ratio. Arch Ophthalmol 1999;117:221-4. http://dx.doi.org/10.1001/archopht.117.2.221.
- Kushner BJ. Does overcorrecting minus lens therapy for intermittent exotropia cause myopia?. Arch Ophthalmol 1999;117:638-42. http://dx.doi.org/10.1001/archopht.117.5.638.
- Kushner BJ. The distance angle to target in surgery for intermittent exotropia. Arch Ophthalmol 1998;116:189-94.
- Kushner BJ, Fisher MR, Lucchese NJ, Morton GV. Factors influencing response to strabismus surgery. Arch Ophthalmol 1993;111:75-9. http://dx.doi.org/10.1001/archopht.1993.01090010079030.
- Kushner BJ, Lucchese NJ, Morton GV. The influence of axial length on the response to strabismus surgery. Arch Ophthalmol 1989;107:1616-18. http://dx.doi.org/10.1001/archopht.1989.01070020694030.
- Kushner BJ. Exotropic deviations: a functional classification and approach to treatment. Am Orthopt J 1988;38:81-93.
- Kutschke PJ, Keech RV. The effect of vertical displacement of horizontal muscles on the deviation in primary position. Am Orthop J 1988;38:117-22.
- Lahlou MG, Rhounimi. [Divergent and convergent strabismus. Bull Soc Ophtalmol Fr 1971;71:457-9.
- Lak D. Conservative treatment of exophoria. Klin Oczna 1997;99:39-41.
- Lange W, Motz S. Concomitant strabismus. Klin Monatsbl Augenheilk 2009;226:R89-106. http://dx.doi.org/10.1055/s-0029-1185648.
- Lange W, De Decker W. Two therapeutic concepts in intermittent divergent squint. Doc Ophthalmol 1993;84:187-200. http://dx.doi.org/10.1007/BF01206253.
- Lee KH, Kang SM. Predictive factors for successful outcome and recurrence with bilateral lateral rectus muscle recession in patients with basic type intermittent exotropia. ARVO Meeting Abstracts 2012;53.
- Lee SJ, Park JM. Factors predisposing to consecutive esotropia after surgery to correct intermittent exotropia. ARVO Meeting Abstracts 2012;53.
- Lee K, Shin KS, Kim Y, Choi MY. Comparison of outcomes of unilateral lateral rectus recession for exotropia between first and second operations. Korean J Ophthalmol 2011;25:329-33. http://dx.doi.org/10.3341/kjo.2011.25.5.329.
- Lee J. Ocular alignment following strabismus surgery. Br J Ophthalmol 2009;93:439-42. http://dx.doi.org/10.1136/bjo.2008.147413.
- Lee J, Kim H, Chung J. A comparison of surgical results between sensory changes in intermittent exotropia. Annals Ophthalmol 1997;29:96-100.
- Lennerstrand G. Effects of surgery on the dominant eye in exodeviations. Acta Ophthalmol (Copenh) 1986;64:391-6. http://dx.doi.org/10.1111/j.1755-3768.1986.tb06941.x.
- Leonardi E, Leonardi A, Filadoro P, Migliorini R, Lombardi C. Observations on the intermittent exotropia treatments. Annal Ottalmol Clin Ocul 1993;119.
- Leonardi E, Grenga R. On the evolution of non-operated intermittent exotropias. Boll Ocul 1970;49:557-68.
- Lew H, Kim C-H, Yun Y-S, Han S-H. Binocular photophobia after surgical treatment in intermittent exotropia. Optom Vis Sci 2007;84:1101-3. http://dx.doi.org/10.1097/OPX.0b013e31815b9dec.
- Lim SH, Hwang BS, Kim MM. Prognostic factors for recurrence after bilateral rectus recession procedure in patients with intermittent exotropia. Eye 2012;26:846-52. http://dx.doi.org/10.1038/eye.2012.55.
- Lim SH, Hong JS, Kim MM. Prognostic factors for recurrence with unilateral recess-resect procedure in patients with intermittent exotropia. Eye 2011;25:449-54. http://dx.doi.org/10.1038/eye.2011.12.
- Litwinska J. Treatment results of squint and incorrect fixation in infants. Klin Oczna 1997;99:107-8.
- Livir-Rallatos G, Gunton KB, Calhoun JH. Surgical results in large-angle exotropia. J AAPOS 2002;6:77-80. http://dx.doi.org/10.1067/mpa.2002.122059.
- Liu SC, Li H, Song SF, Wu YF. Exploration of operative methods in v-pattern exotropia. Int J Ophthalmol 2010;10:160-1.
- Liu S-C, Tao Y-X, Song S-F. Surgical treatment of concomitant extropia in 21 cases. Int J Ophthalmol 2005;5:790-8.
- Lucas E, Bentley CR, Aclimandos WA. The effect of surgery on the ac/a ratio. Eye 1994;8:109-14. http://dx.doi.org/10.1038/eye.1994.21.
- Marrakchi S, Nacef L, Kamoun N, Sebai L, Ayed S. Treatment of divergent intermittent strabismus. Tunis Med 1994;72:571-5.
- Martin LP. Br Orthopt 1989;46:49-58.
- Maruo T. Long term results after strabismus surgery. Binocul Vis Strabismus Q 1988;16:265-70.
- Matsusaka Y, Deguchi M, Yokoyama T, Kawanami K, Matsuda K. A study of exotropia surgery: Multiple regression analysis of the relationship between amount of recession-resection and surgical results. Folia Ophthalmol Japon 1988;39.
- McNeer KW. Observations on the surgical overcorrection of childhood intermittent exotropia. Am Orthopt J 1987;37:135-50.
- McSwain W, Morrison D, Donahue S. The influence of a or v pattern on postoperative drift after surgery for intermittent exotropia. J AAPOS 2011;15. http://dx.doi.org/10.1016/j.jaapos.2011.01.092.
- Melek N. Intermittent exotropia: a study of suppression in the binocular visual field in cases. Binocul Vis Strabismus Q 1992;7:25-9.
- Menon V, Singla MA, Saxena R, Phulijele S. Comparative study of unilateral and bilateral surgery in moderate exotropia. J Pediatr Ophthalmol Strabismus 2010;47:288-91. http://dx.doi.org/10.3928/01913913-20091118-07.
- Metz HS. The use of vertical offsets with horizontal strabismus surgery. Ophthalmology 1988;95:1094-7. http://dx.doi.org/10.1016/S0161-6420(88)33055-1.
- Miller MM, Guyton DL. Loss of fusion and the development of a or v patterns. J Pediatr Ophthalmol Strabismus 1994;31:220-4.
- Mims JL. Exotropia replaces esotropia as most common form of strabismus; HIV and CPEO; ZOO papers; EOM innervation innovation and strabology report of the 37th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus. Binocul Vis Strabolog Q Simms Romano 2011;26:80-9.
- Mims JL. Strabology report of the 34th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus. Binocul Vis Strabismus Q 2008;23:105-19.
- Mims JL, Miller AM, Schoolfield J. The exoshift under anesthesia correlates with probable changes in medial rectus innervation after surgery for infantile esotropia. Binocul Vis Strabismus Q 2008;23:215-26.
- Mims JL. Outcome of 5 mm resection of one medial rectus extraocular muscle for recurrent exotropia. Binocul Vis Strabismus Q 2003;18:143-50.
- Minguini N. A study to determine: should conventional amounts of eye muscle surgery for horizontal binocular deviations be changes when oblique muscle weakening procedures are simultaneously performed?. Binocul Vis Strabismus Q 2005;20:21-5.
- Mitsui Y. Effect of master eye surgery in exodeviations. Jpn J Ophthalmol 1980;24:221-33.
- Mojon DS. A new transconjunctival muscle reinsertion technique for minimally invasive strabismus surgery. J Pediatr Ophthalmol Strabismus 2010;47:292-6. http://dx.doi.org/10.3928/01913913-20091019-07.
- Moore S, Stockbridge L, Knapp P. A panoramic view of exotropias. Am Orthop J 1977;27:70-9.
- Morrison D, McSwain W, Donahue S. Comparison of sensory outcomes in patients with monofixation versus bifoveal fusion after surgery for intermittent exotropia. J AAPOS 2010;14:47-51. http://dx.doi.org/10.1016/j.jaapos.2009.11.015.
- Morrison D, McSwain W, Donahue S. Sensory outcomes after surgery for intermittent exotropia. J AAPOS 2009;13. http://dx.doi.org/10.1016/j.jaapos.2008.12.128.
- Mumma JV. Surgical treatment of exotropia. Am Orthop J 1975;25:96-100.
- Nelson LB, Bacal DA, Burke MJ. An alternative approach to the surgical management of exotropia: the unilateral lateral rectus recession. J Pediatr Ophthalmol Strabismus 1992;29:357-60.
- Nowakowska O, Beben M, Loba P, Broniarczyk-Loba A. In what age should we operate on children with primary exotropia? Indications based on own therapeutic results. Klin Oczna 2009;111:224-8.
- Nusz KJ, Mohney BG, Diehl NN. The course of intermittent exotropia in a population-based cohort. Ophthalmology 2006;113:1154-8. http://dx.doi.org/10.1016/j.ophtha.2006.01.033.
- Oh JY, Hwang JM. Survival analysis of 365 patients with exotropia after surgery. Eye 2006;20:1268-72. http://dx.doi.org/10.1038/sj.eye.6702091.
- Ohtaki A. Statistical analysis of the effects of surgery on exotropia. Folia Ophthalmol Japon 2000;51:697-702.
- Ohtsuki H, Hasebe S, Kono R, Yamane T, Fujiwara H, Shiraga F. Prism adaptation response is useful for predicting surgical outcome in selected types of intermittent exotropia. Am J Ophthalmol 2001;131:117-22. http://dx.doi.org/10.1016/S0002-9394(00)00704-2.
- Ohtsuki H, Hasebe S, Okano M, Furuse T. Comparison of surgical results of responders and non-responders to the prism adaptation test in intermittent exotropia. Acta Ophthalmol Scand 1997;75:528-31. http://dx.doi.org/10.1111/j.1600-0420.1997.tb00143.x.
- Oleszczyska-Prost E. Botulinum toxin a in the treatment of concomitant strabismus in children. Klin Oczna 2004;106:64-7.
- Olitsky SE. Early and late postoperative alignment following unilateral lateral rectus recession for intermittent exotropia. J Pediatr Ophthalmol Strabismus 1998;35:146-8.
- Orlin A, Mills M, Ying G-S, Liu C. A comparison of hang-back with conventional recession surgery for exotropia. J AAPOS 2007;11:597-600. http://dx.doi.org/10.1016/j.jaapos.2007.06.001.
- Owen M, Noonan CP, Al-Khalid M, Rowe FJ. Ketamine and botulinum: a safe combination for the management of childhood strabismus. Strabismus 2010;18:8-12. http://dx.doi.org/10.3109/09273970903496925.
- Paakkala A. Surgical treatment of strabismus: a retrospective investigation of results of treatment of horizontal strabismus. Acta Ophthalmol 1982;156:S1-109.
- Pajakowa J, Kobiela-Krzystkowa K, Mietus-Polakowa B. Results of surgical treatment of divergent squint. Klin Oczna 1973;43:1277-83.
- Paris V. Intermittent exotropia: postoperative prismatic treatment. Bull Soc Belge Ophtalmol 1998;268:187-92.
- Park HJ, Kong SM, Baek SH. Consecutive esodeviation after exotropia surgery in patients older than 15 years: comparison with younger patients. Korean J Ophthalmol 2008;22:178-82. http://dx.doi.org/10.3341/kjo.2008.22.3.178.
- Parkesh P, Garg R, Menon V. Dominant eye surgery in exodeviation. Indian J Ophthalmol 1984;32:467-75.
- Patel GR, Makwana VD. Results of treatment of concomitant squint. Afro-Asian J Ophthalmol 1988;11:94-6.
- Paula JS, Ibrahim FM, Martins MC, Bicas HEA, Velasco e Cruz AA. Refractive error changes in children with intermittent exotropia under overminus lens therapy. Arq Bras Oftalmol 2009;72:751-4. http://dx.doi.org/10.1590/S0004-27492009000600002.
- Pietruschka G, Bostelmann I. Information on frequency, refraction, amblyopia and surgical results in divergent squint (author’s translation). Klin Monatsbl Augenheilk 1973;163:481-6.
- Pineles SL, Rosenbaum AL, Demer JL. Decreased postoperative drift in intermittent exotropia associated with a and v patterns. J AAPOS 2009;13:127-31. http://dx.doi.org/10.1016/j.jaapos.2008.10.013.
- Pratt-Johnson JA, Tillson G. Prismotherapy in intermittent exotropia. A preliminary report. Can J Ophthalmol 1979;14:243-5.
- Qiu H, Shi MY, Li HY, Zhang JS. Eye position influence on far and near stereoscopic vision of intermittent exotropia patients. Int Eye Sci 2012;12:1132-3.
- Rajavi Z, Ghadim HM, Nikkhoo M, Dehsarvi B. Comparison of hang-back and conventional recession surgery for horizontal strabismus. J Pediatr Ophthalmol Strabismus 2001;38:273-7.
- Remy C. Quantified surgery of exotropia. Statistic analysis. Bull Soc Ophtalmol Fr 1990;90:825-6.
- Richard JM, Parks MM. Intermittent exotropia. Surgical results in different age groups. Ophthalmology 1983;90:1172-7. http://dx.doi.org/10.1016/S0161-6420(83)34410-9.
- Rodrigues AC, Nelson LB. Long-term results of hemihang-back lateral rectus recession. J Pediatr Ophthalmol Strabismus 2005;42:296-9.
- Rodrigues AC, Nelson LB. Long-term results of hang-back lateral rectus recession. J Pediatr Ophthalmol Strabismus 2006;43:161-4.
- Rohatgi JN, Singh HK, Siddique AA. The surgical treatment of horizontal concomitant squint – an appraisal. Indian J Ophthalmol 1982;30:275-7.
- Ron A, Merin S. The use of the pre-op prism adaptation test (pat) in the surgery of exotropia. Am Orthop J 1985;38:107-10.
- Roth A, Montard M, Gadelle-Barbier E, Tournier C. Approach to differentiated surgery of primary divergent strabismus. Bull Mem Soc Fr Ophtalmol 1981;93:224-9.
- Rowe FJ. Treatment of intermittent distance exotropia. Br Orthopt J 1990;57:90-3.
- Rutstein RP, Corliss DA. The clinical course of intermittent exotropia. Optom Vis Sci 2003;80:644-9. http://dx.doi.org/10.1097/00006324-200309000-00009.
- Rutstein RP, Marsh-Tootle W, London R. Changes in refractive error for exotropes treated with overminus lenses. Optom Vis Sci 1989;66:487-91. http://dx.doi.org/10.1097/00006324-198908000-00001.
- Santos EA, Lira RPC, Bicas HEA, Gaete MIL. Action of botulinum toxin on isometric contraction of medial and lateral rectus muscles in esotropic or exotropic patients. J Pediatr Ophthalmol Strabismus 2011;48:38-44. http://dx.doi.org/10.3928/01913913-20100420-07.
- Saxena R, Kakkar A, Menon V, Sharma P, Phuljhele S. Evaluation of factors influencing distance stereoacuity on Frisby-Davis distance test (FD2) in intermittent exotropia. Br J Ophthalmol 2011;95:1098-101. http://dx.doi.org/10.1136/bjo.2010.182139.
- Schulz E, Haase W. Technic and results of inferior oblique advancement in incomitant strabismus (the A phenomenon). Fortschr Ophthalmol 1984;81:490-3.
- Schwartz RL, Calhoun JH. Surgery of large angle exotropia. J Pediatr Ophthalmol Strabismus 1980;17:359-63.
- Scott AB, Magoon EH, McNeer KW, Stager DR. Botulinum treatment of childhood strabismus. Ophthalmology 1990;97:1434-8. http://dx.doi.org/10.1016/S0161-6420(90)32390-4.
- Scott AB, Mash AJ, Jampolsky A. Quantitative guidelines for exotropia surgery. Invest Ophthalmol 1975;14:428-36.
- Segal ZI, Rehany U, Rumelt S. Measurements for horizontal extraocular muscle surgery from the suture site: Outcome and influencing factors. Eye 2000;14:879-83. http://dx.doi.org/10.1038/eye.2000.241.
- Self CA, Enzenauer RW. The application of statistical process control to horizontal strabismus surgery. J AAPOS 2004;8:165-70. http://dx.doi.org/10.1016/j.jaapos.2003.09.003.
- Sethi S, Sethi MJ, Hussain I, Kundi NK. Causes of amblyopia in children coming to ophthalmology out patient department Khyber Teaching Hospital, Peshawar. JPMA J Pak Med Assoc 2008;58:125-8.
- Shippman S, Veronneau-Troutman S. The effect of preoperative convergence exercises on the surgical outcome of primary exotropia. Ophthalmology 1979;86:2146-9. http://dx.doi.org/10.1016/S0161-6420(79)35297-6.
- Siatkowski RM, Black BC, Schweers MA. Strabology report of the 36th Annual Meeting of the American asSociation for Pediatric Ophthalmology and Strabismus. Binocul Vis Strabismus Q 2010;25:94-109.
- Singh V, Roy S, Sinha S. Role of orthoptic treatment in the management of intermittent exotropia. Indian J Ophthalmol 1992;40:83-5.
- Smoot CN, Simon JW, Nelson LB. Binocularity following surgery for secondary esotropia in childhood. Br J Ophthalmol 1990;74:155-7. http://dx.doi.org/10.1136/bjo.74.3.155.
- Somer D, Demirci S, Cinar FG, Duman S. Accommodative ability in exotropia: predictive value of surgical success. J AAPOS 2007;11:460-4. http://dx.doi.org/10.1016/j.jaapos.2007.01.123.
- Spielmann A. Adjustable surgery in exotropia. Bull Soc Ophtalmol Fr 1983;83:259-66.
- Spierer O, Spierer A, Glovinsky J, Ben-Simon GJ. Moderate-angle exotropia: a comparison of unilateral and bilateral rectus muscle recession. Ophthalmic Surg Lasers Imaging 2010;41:355-9. http://dx.doi.org/10.3928/15428877-20100430-10.
- Spierer A, Ben-Simon GJ. Unilateral and bilateral lateral rectus recession in exotropia. Ophthalmic Surg Lasers Imaging 2005;36:114-17.
- Spoor DK, Hiles DA. Occlusion therapy for exodeviations occurring in infants and young children. Ophthalmology 1979;86:2152-7. http://dx.doi.org/10.1016/S0161-6420(79)35295-2.
- Stoller SH, Simon JW, Lininger LL. Bilateral lateral rectus recession for exotropia: a survival analysis. J Pediatr Ophthalmol Strabismus 1994;31:89-92.
- Strogal AS. Features of choice of surgical procedure and long-term results of complex treatment of children with concomitant divergent nonaccommodative strabismus. Oftalmologicheskii Zhurnal 1983;38:229-31.
- Stuteville JL, King JD, West RW. Redirecting gaze to improve the cosmetic appearance of strabismus. Optom Vis Sci 2007;84:865-71. http://dx.doi.org/10.1097/OPX.0b013e3181559d82.
- Suh Y-W, Kim S-H, Lee J-Y, Cho YA. Conversion of intermittent exotropia types subsequent to part-time occlusion therapy and its sustainability. Graefes Arch Clin Exp Ophthalmol 2006;244:705-8. http://dx.doi.org/10.1007/s00417-005-0195-0.
- Sun YL, Ren WN, Zhang Y. Clinical analysis of asymmetrical surgery for concomitant strabismus. Int J Ophthalmol 2010;10:1635-9.
- Kim TW, Kim JH, Huang J-M. Long-term outcome of patients with large overcorrection following surgery for exotropia. Ophthalmologica 2005;219:237-42. http://dx.doi.org/10.1159/000085734.
- Tao LJ, Wang P, Wang XL, Yang HL, Guo Y, Xiao ZG. Clinic characteristics and surgical management in v-pattern deviation. Int J Ophthalmol 2008;8:2286-7.
- Tatham A, Amaya L. Immediate post-operative adjustable suture strabismus surgery using a target-controlled infusion of propofol-remifentanil. Ophthalmologica 2009;223:192-5. http://dx.doi.org/10.1159/000200766.
- Thouvenin D, Lesage-Beaudon C, Arne JL. Botulinum injection in infantile strabismus. Results and incidence on secondary surgery in a long-term survey of 74 cases treated before 36 months of age. J Fr Ophtalmol 2008;31:42-50. http://dx.doi.org/10.1016/S0181-5512(08)70329-2.
- Tibbs A. Orthoptic treatment of constant and intermittent exotropia. Am Orthop J 1978;28:117-23.
- Tsuji T, Hayakawa Y, Shiotani N. Assessment of surgical treatment of the Master Eye Department of Ophthalmology, Kurume University School of Medicine. Folia Ophthalmol Japon 1988;39.
- Usui C, Kubota N, Maruo T. Binocular function of intermittent exotropia before and after surgery. Nippon Ganka Gakkai Zasshi 2000;104:584-9.
- Vishnoi SK, Singh V, Mehra MK. Role of occlusion in treatment of intermittent exotropia. Indian J Ophthalmol 1987;35:207-10.
- Wang L, Nelson LB. Outcome study of unilateral lateral rectus recession for small to moderate angle intermittent exotropia in children. J Pediatr Ophthalmol Strabismus 2010;47:242-7. http://dx.doi.org/10.3928/01913913-20091019-12.
- Wang XL, Tao LJ, Yang HL, Wang P, Xiong S. Clinical analysis of binocular vision after operation in patients with concomitant strabismus. Int J Ophthalmol 2009;9:318-20.
- Wang ZH, Qiu Y, Di YL, Huang QM. Therapeutic effects of concomitant exotropia surgery in 79 cases. Int J Ophthalmol 2008;8:422-3.
- Weakley DR, Stager DR. Unilateral lateral rectus recessions in exotropia. Ophthalmic Surg 1993;24:458-60.
- Weston B, Enzenauer RW, Kraft SP, Gayowsky GR. Stability of the postoperative alignment in adjustable-suture strabismus surgery. J Pediatr Ophthalmol Strabismus 1991;28:206-11.
- Wickens R. Results of surgery in distance exotropia. Br Orthopt J 1984;41:66-72.
- Wilson ME, Parks MM. Primary inferior oblique overaction in congenital esotropia, accommodative esotropia, and intermittent exotropia. Ophthalmology 1989;96:950-5. http://dx.doi.org/10.1016/S0161-6420(89)32774-6.
- Windsor CE. Surgery, fusion, and accommodative convergence in exotropia. Int Ophthalmol Clin 1971;11:46-52. http://dx.doi.org/10.1097/00004397-197101140-00008.
- Wu Q-z, Wu X, Lu W, Wang J-h. [Clinical study on binocular vision in intermittent exotropia before and after surgery. Chung-Hua Yen Ko Tsa Chih 2007;43:968-71.
- Wu H, Sun J, Xia X, Xu L, Xu X. Binocular status after surgery for constant and intermittent exotropia. Am J Ophthalmol 2006;142:822-6. http://dx.doi.org/10.1016/j.ajo.2006.06.045.
- Wutthiphan S. Botulinum toxin a in surgically overcorrected and undercorrected strabismus. J Med Assoc Thai 2008;91:S86-91.
- Wygnanski-Jaffe T, Wysanbeek Y, Bessler E, Spierer A. Strabismus surgery using the adjustable suture technique. J Pediatr Ophthalmol Strabismus 1999;36:184-8.
- Xu J, Yu X, Huang Y, Chen J, Yu H, Wang Y, et al. The psychosocial effects of strabismus before and after surgical correction in chinese adolescents and adults. J Pediatr Ophthalmol Strabismus 2012;49:170-5. http://dx.doi.org/10.3928/01913913-20110920-02.
- Yam JCS, Wu PKW, Chong GSL, Wong USF, Chan CWN, Ko STC. Long-term ocular alignment after bilateral lateral rectus recession in children with infantile and intermittent exotropia. J AAPOS 2012;16:274-9. http://dx.doi.org/10.1016/j.jaapos.2012.01.005.
- Yan H, Zhu B-Y, Chen L, Ha W-J, Wang W-N. Surgical treatment of 2 100 strabismus. Int J Ophthalmol 2006;6.
- Yang C-Q, Shen Y, Gu Y-S, Han W. Clinical investigation of surgery for intermittent exotropia. J Zhejiang Univ Sci B 2008;9:470-3. http://dx.doi.org/10.1631/jzus.B0720007.
- Yang SM. Large recession of the external rectus for large-angle exotropia. Chung-Hua Yen Ko Tsa Chih 1984;20:339-41.
- Yao LJ, Yang SM. Restoration of normal binocular vision after successful surgical correction of concomitant exotropia. Chung-Hua Yen Ko Tsa Chih 1993;29:157-9.
- Yazdian Z, Ghiassi G. Re-recession of the lateral rectus muscles in patients with recurrent exotropia. J AAPOS 2006;10:164-7. http://dx.doi.org/10.1016/j.jaapos.2005.11.014.
- Yi JH, Chung SA, Chang YH, Lee JB. Practical aspects and efficacy of intraoperative adjustment in concomitant horizontal strabismus surgery. J Pediatr Ophthalmol Strabismus 2011;48:85-9. http://dx.doi.org/10.3928/01913913-20100518-06.
- Yildirim C, Mutlu FM, Chen Y, Altinsoy HI. Assessment of central and peripheral fusion and near and distance stereoacuity in intermittent exotropic patients before and after strabismus surgery. Am J Ophthalmol 1999;128:222-30. http://dx.doi.org/10.1016/S0002-9394(99)00079-3.
- Yin Y, Ha S, Du J, Xu H, Liu Q, Jian C. A comparison of master eye operation with slave eye operation on patients with large-angle exotropia. Chung-Hua Yen Ko Tsa Chih 2002;38:36-8.
- Yu G. The surgical treatment of intermittent exotropia. Chung-Hua Yen Ko Tsa Chih 1989;25:279-81.
- Zaki HA. A surgery on twenty six thousand and three hundred seventy eight patients with convergent squint (26378), thirteen thousand, one hundred and eighty one patients with divergent squint (13181), one thousand and twenty nine patients of vertical squint (1029), ten thousand and one hundred thirty nine patients of heterophoria (10139) and two thousand sixty one patients of paralytic squint (2061) seen during the past ten years at Giza Ophthalmic Hospital squint clinic from the year 1960 to the year 1970. Bull Ophthalmol Soc Egypt 1972;65:457-70.
- Zhi Y, Long T, Zhao XT, Liang HC. Operations of posterior sclera reinforcement combining with recession of the lateral rectuses. Int J Ophthalmol 2008;8:1709-10.
- Zibrandtsen P, Rindziunski E, Gregersen E. Ten years follow-up of surgery for intermittent exotropia. Acta Ophthalmol (Copenh) 1986;64:374-8. http://dx.doi.org/10.1111/j.1755-3768.1986.tb06938.x.
- Ziegler D, Huff D, Rouse MW. Success in strabismus therapy: a literature review. J Am Optom Assoc 1982;53:979-83.
- Buck D, Clarke MP, Haggerty H, Hrisos S, Powell C, Sloper J. Grading the severity of intermittent distance exotropia: the revised Newcastle Control Score. Br J Ophthalmol 2008;92. http://dx.doi.org/10.1136/bjo.2007.120287.
Appendix 1 Example search strategy
MEDLINE (via Ovid)
-
Exotropia/
-
(divergen$ adj10 (excess$ or strabismus or squint$)).tw.
-
(exotrop$ or IDEX).tw.
-
or/1-3
-
limit 4 to humans
Appendix 2 Data extraction form
| Reviewer | Study ID | Source | Country study conducted | Language |
|---|---|---|---|---|
| Full citation | ||||
| Corresponding study author and contact details | ||||
| Eligibility (yes or no) | |
|---|---|
| Does the study consider exotropia rather than some other form of misalignment? | |
| Does the study address X(T) (rather than permanent exotropia)? | |
| Does the study involve children aged ≤ 16 years? | |
| Does the study design include a comparator group? | |
| Is the sample size > 20 participants? | |
| Methods and participants | |
|---|---|
| Study design | |
| Setting | |
| Intervention(s) | |
| Duration of intervention | |
| Contextual factors | |
| Population (age) | |
| Population [severity of X(T)] | |
| Population (time since diagnosis) | |
| Method of recruitment and control group selection (if appropriate) | |
| Total population | |
| Baseline response (no. and rate) | |
| Time between baseline and follow-up | |
| Follow-up response | |
| Final sample size | |
| Results and outcomes | |
|---|---|
| Primary outcomes assessed | |
| Tools used to assess primary outcomes | |
| Secondary outcomes assessed (include QoL and patient-derived outcomes) | |
| Tools used to assess secondary outcomes | |
| Intervention group: effect sizes with CIs and p-values (or relevant statistics) | |
| Control group: effect sizes with CIs and p-values (or relevant statistics) | |
| Incidence of adverse effects (for inception cohort studies) | |
| Data about natural history of X(T) | |
| Notes/comments (include possible conflict of interest) | |
| Correspondence required (additional study data) | |
Appendix 3 Quality appraisal tools
Cochrane risk of bias tool for randomised controlled trials (http://bmg.cochrane.org/research-projectscochrane-risk-bias-tool)
| Domain | Support for judgement |
|---|---|
| Selection bias | |
| Random sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether or not it should produce comparable groups |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether or not intervention allocations could have been foreseen in advance of, or during, enrolment |
| Performance bias | |
| Blinding of participants and personnel: assessments should be made for each main outcome (or class of outcomes) | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received; provide any information relating to whether or not the intended blinding was effective |
| Detection bias | |
| Blinding of outcome assessment: assessments should be made for each main outcome (or class of outcomes) | Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received; provide any information relating to whether or not the intended blinding was effective |
| Attrition bias | |
| Incomplete outcome data: assessments should be made for each main outcome (or class of outcomes) | Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis; state whether or not attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition/exclusions where reported, and any re-inclusions in analyses performed by the review authors |
| Reporting bias | |
| Selective reporting | State how the possibility of selective outcome reporting was examined by the review authors, and what was found |
| Other bias | |
| Other sources of bias | State any important concerns about bias not addressed in the other domains in the tool[***]If particular questions/entries were prespecified in the review protocol, responses should be provided for each question/entry |
Quality Appraisal Tool for Cohort Studies (adapted from Critical Appraisal Skills Programme)
1. Was the cohort recruited in an acceptable way?
2. Was the study prospective?
3. Was the intervention conducted in an explicit and standardised manner?
4. Was the outcome appropriately measured to minimise bias?
5a. Did they identify all potential important confounding factors?
5b. Did they adjust for confounding factors in the design and/or analysis?
6a. Were they followed up for at least 12 months?
6b. Were at least 80% of participants followed up (for prospective studies)?
7. Are the authors’ conclusions substantiated by the reported data?
Appendix 4 Table of excluded studies after full-text review
| Study (first author, year) | Reason for exclusion |
|---|---|
| Abbas 197587 | No separate data for intermittent vs. constant (says in methods section that there are both); no separate data for children (patients aged 2.5–27 years) |
| Abbasoglu 199688 | No data for X(T), grouped as exotropia only |
| Abroms 200189 | No separate data for children, adults and children mixed (upper age of sample 18.4 ± 16.0 years) |
| Adams 200890 | Fewer than 20 cases and data for children and adults mixed |
| Akar 201291 | Not all X(T) in case series and only 25 cases of exotropia in total |
| Akatsuka 200192 | No separate data for X(T) |
| Alajbegović-Halimić 200793 | No data reported separately for X(T), exotropia only |
| Altizer 197294 | Sample size, 13 conservative intervention + 16 surgical X(T) remainder XT; interventions – occlusion, convergence exercises, prisms |
| Ameri 201095 | Children and adults mixed, also no subgroups so unable to extract X(T) data from other types of squint |
| Archer 200996 | No disaggregated data are available for X(T) |
| Arnoldi 200897 | Not an intervention study |
| Asadi 200998 | Mixed population of adults and children and follow-up only 13.5 weeks |
| Asjes-Tydeman 200699 | Effectiveness data not available separately for X(T) |
| Aslanis 2006100 | No comparison group |
| Attarzadeh 2008101 | No comparison group, case series data only |
| Awadein 2012102 | Sample size fewer than 20 patients (only 7 of 25 with A-pattern strabismus including exotropia and esotropia) |
| Awadein 2008103 | Sample size fewer than 20 patients with X(T) |
| Awaya 1982104 | Sample size fewer than 20 patients with XT [only six patients with X(T)] |
| Bae 2012105 | Outcomes not assessing intervention effectiveness |
| Baker 2008106 | No comparison group, case series data only |
| Baker 1979107 | No comparison group, case series data only |
| Bansal 2008108 | Considers adults, no separate data for children |
| Bao 2006109 | No comparison group, case series data only |
| Barraza 2009110 | Does not consider effectiveness of surgery |
| Basar 2000111 | No comparison group, case series data only |
| Beneish 1994112 | No comparison group, case series data only |
| Berard 1979113 | No comparison group, case series data only |
| Berard 1979114 | No intervention comparison group, looks at overcorrected subset only |
| Berard 1975115 | No comparison group, case series data only; largely a discursive piece on classification and diagnosis |
| Berg 1983116 | Data not presented separately for basic, convergence insufficiency and distance excess |
| Berland 1998117 | Sample size fewer than 20 cases of X(T) |
| Berrondo 1980118 | Does not include empirical data on effectiveness |
| Besharati 2008119 | No data for X(T) |
| Bietti 1970120 | Fewer than 20 cases and no mention of X(T) specifically |
| Billet 1969121 | No comparison group, case series data only (case reports, n = 11) |
| Binion 1966122 | No comparison group, case series data only |
| Bramante 200956 | Conference abstract only and no response from study authors |
| Brandner 2011123 | No outcomes of interest (not an intervention study) |
| Brooks 2011124 | Fewer than 20 patients with exotropia and no mention of X(T) |
| Broniarczyk-Loba 2003125 | Mostly adults, no separation of results for children (n = only 25 in total) |
| Broniarczyk-Loba 1994126 | ET and XT mixed, no data for X(T) separately |
| Buck 201225 | No comparison group, case series data only |
| Caldeira 2004127 | Fewer than 20 cases, [looking into cases in detail, only 13 X(T)] |
| Caltrider 1983128 | No comparison group (case series data only) |
| Capo 1989129 | Constant and intermittent mixed, no separate data for X(T) and n = 24 only in total |
| Carlton 2011130 | No intervention or natural history data (explores prevalence of eye disorders, risk factors and relationships with QoL) |
| Carta 1994131 | Adults and children in sample, age range 0–32 years, no separate data for children |
| Cassin 1986132 | Sample size fewer than 20 patients |
| Castelbuono 1999133 | Not addressing outcomes of interest |
| Castellanos-Bracamontes 1990134 | Descriptive study, not an intervention study |
| Celebi 2001135 | Adults and children mixed, range 4–51 years, no separate data for children |
| Chae 2008136 | Recurrent X(T) |
| Chang 2008137 | Does not address question of interest |
| Chang 2006138 | Data for adults and exotropia generically only (n = 48), no separate data for X(T) |
| Chia 200649 | No comparison group, case series data only |
| Chia 2005139 | Some patients with convergence insufficiency type and outcomes not reported separately |
| Cho 2012140 | No comparison group, case series data only |
| Cho 2009141 | Follow-up only 3 months |
| Cho 2007142 | No comparison group; mean age 11.4 ± 7.2 years; includes subjects > 18 years (5–19 years) |
| Choi 2011143 | No comparison group, case series data only |
| Choi 2001144 | Convergence insufficiency subtype |
| Chryssanthou 1974145 | Adults and children mixed population age range 5–33 years; data for children not reported separately |
| Chun 2010146 | No outcomes of interest |
| Chun 2008147 | Recurrent exotropia |
| Chung 2012148 | No comparison group, case series data only |
| Chung 2011149 | Adults and children mixed, data not reported separately for children |
| Chutter 1977150 | Cannot separate out data for adults and convergence insufficiency type |
| Ciancia 1969151 | Fewer than 20 patients (n = 15) |
| Clark 2009152 | Not X(T) and fewer than 20 patients (n = 8) |
| Clarke 1981153 | No comparison group, case series data only |
| Cooper 1983154 | Convergence insufficiency type and only seven patients |
| Cooper 1977155 | Cannot separate out patients with convergence insufficiency type who represent 11.1% of the sample |
| Dadeya 2003156 | No comparison group, case series data only |
| Dahlmann 2007157 | Groups differ on basis of condition rather than intervention vs. comparison |
| Dawson 1999158 | No intervention comparison group |
| Debert 2007159 | No data reported for X(T), only exotropia in general |
| DeDecker 1988160 | Adults and children mixed and fewer than 20 patients with X(T) |
| Deitz 2011161 | No comparison group, case series data only |
| DeLianoSanchez 1997162 | Results not reported separately for X(T) |
| Demers 1971163 | Includes convergence insufficiency type |
| Deng 2009164 | Fewer than 6 months’ follow-up |
| Deutsch 1992165 | Exotropia generally (n = 30) not X(T) subtype |
| Dong 2006166 | Exotropia generally not X(T) subtype |
| Donnelly 2005167 | Prevalence study with only sparse outcome data for interventions, where n = 10 for exotropia and only five for intermittent divergence excess type |
| Dzelkaleia 1985168 | Not an intervention study |
| Edelman 1988169 | No comparison group |
| Ekdawi 2010170 | Follow-up < 6 months |
| Ekdawi 200978 | Data for adults and children mixed (age at time of intervention > 18 years), no separate data for children |
| El-Defrawi 1970171 | Not possible to identify X(T) outcome separately |
| Engel 2004172 | No separate data for X(T) and no data on initial deviation |
| Eustace 1972173 | Fewer than 20 cases with divergent squint |
| Faridi 2007174 | Adults and children mixed and no data for children presented separately |
| Fastrez-Moutschen 1993175 | Not X(T), exophoric cases only, also case series (no adequate comparison group) |
| Feretis 1999176 | No comparison group, case series data only |
| Fiorelli 2007177 | Data for adults and children mixed, age range 5–55 years and data not reported separately for children |
| Friedman 1980178 | Prevalence study, no intervention |
| Friemel 1971179 | No patient outcomes |
| Gagnon 1970180 | Adults and children mixed, and no comparison group |
| Gezer 2004181 | Outcome data are for intermittent and constant mixed |
| Gharibyan 2012182 | Subjects with vertical component (n = 20 only) |
| Goldrich 1980183 | Data for adults and children mixed, no separate data for children (n = 28); also unclear how many are X(T) |
| Goldstein 1968184 | Sample size fewer than 20 patients and not all X(T) |
| Gordon 1980185 | Age range is up to 20 years; also not examining intervention effectiveness, reports on predictor models for surgery |
| Govekar 1993186 | No data for X(T) specifically |
| Govindan 200514 | Epidemiological study, looking at incidence only; no intervention or follow-up |
| Graemiger 1979187 | No patient outcome data on effectiveness |
| Graf 2002188 | Comment on an important paper (keep for discussion) |
| Guo 2011189 | Case series, no comparison group |
| Guo 2009190 | Case series, no comparison group |
| Gupta 1987191 | No intervention, considers association of various refractive errors in cases of exophoria |
| Gusek-Schneider 2006192 | Children and adults mixed, children and adults (age range 3.1–47.8 years); also minimum range of follow-up period is only 6 weeks |
| Ha 2011193 | Adults in sample and some patients with constant or recurrent XT |
| Haggerty 200471 | No outcome data on intervention effectiveness |
| Hahm 2005194 | Cases of recurrent exodeviation therefore not outcomes from the primary intervention |
| Hamaguchi 1993195 | No comparison group, case series data only |
| Hamtil 1978196 | Sample size fewer than 20 X(T) (mostly constant) |
| Hao 2009197 | No comparison group, case series data only |
| Hardesty 1983198 | No comparison group, case series data only (considers those undercorrected or with a recurrence) |
| Hardesty 1978199 | No comparison group, case series data only |
| Hatsukawa 2011200 | Case series data only, no comparison group |
| Hatsukawa 1992201 | No comparison group, case series data only |
| Hatt 2012202 | No effectiveness data (conference abstract only) |
| Hatt 2010203 | No effectiveness data considers classification only (conference abstract) |
| Hatt 2007204 | Not considering intervention effectiveness (before-and-after data for seven patients only) |
| Herzau 1993205 | Not considering intervention effectiveness |
| Hiles 1968206 | No comparison group, case series data only |
| Holmes 2010207 | No comparison group, case series data only |
| Holtgrave 1973208 | Not intervention effectiveness |
| Hu 2012209 | Adults and children mixed sample, no separate data for children |
| Huang 2010210 | No separate outcome data for X(T) and data for adults and children mixed |
| Hugonnier 1970211 | No comparison group, case series data only |
| Hugonnier 1967212 | No comparison group, case series data only |
| Hunter 2001213 | Fewer than 20 cases [n = 7 X(T)] |
| Iacobucci 1986214 | No comparison group, case series data only |
| Inagaki 1993215 | No comparison group, case series data only and no outcome data to address question of interest |
| Ing 2011216 | No comparison group, case series data only |
| Ing 1999217 | No comparison group, case series data only |
| Ing 1986218 | Constant and intermittent exotropes combined, no separate data for X(T) |
| Isenberg 2009219 | No data for X(T) separately and adults/children mixed population |
| Israel 201157 | Conference abstract only and no response from study authors |
| Jacobi 1969220 | Fewer than 20 cases |
| Jang 2012221 | No comparison group, case series data only |
| Jeoung 2006222 | RCT but outcome data not available for intermittent and constant exotropes separately |
| Jojic 1987223 | Not X(T) |
| Jung 2012224 | Not considering intervention effectiveness, no outcomes of interest and follow-up 3 months only |
| Kamai 200858 | Conference abstract, have written to study authors but no response |
| Kang 2011225 | Only 6-week follow-up |
| Kampanartsanyakorn 2005226 | No data for X(T) specifically, refers to exotropia generically only |
| Kaszli 1997227 | Reporting on outcomes of surgery for exotropia and esotropia generically with no data on subtypes |
| Keenan 1994228 | No comparison group, case series data only |
| Kertesz 1986229 | Data for adults and children mixed (age range 4.5–70 years), no outcomes for children separately |
| Keskinbora 2012230 | Data for adults and children mixed (range 6–25 years), data for children not reported separately |
| Khaier 2008231 | Not X(T) |
| Kii 1992232 | No outcomes of interest |
| Kim 201059 | Conference abstract only and no response from study authors |
| Kim 2005233 | Outcome data not reported separately for adults and children and data for X(T) not reported separately from XT |
| Kliuka 1987234 | Not an intervention study |
| Koklanis 2010235 | No comparison group, case series data only, all types of X(T) included, no effectiveness data |
| Koo 2006236 | No comparison group, case series data only |
| Kosaki 1967237 | Not X(T), constant, also no comparison group, case series data only |
| Kubota 1977238 | No outcomes of interest, focuses on classification |
| Kushner 2009239 | Re-analysis of data from earlier studies |
| Kushner 1999240 | Fewer than 20 cases (n = 16, subset of 304 consecutive case series) |
| Kushner 1999241 | No outcomes of interest (i.e. no postintervention data for angle of deviation) – rather looks at whether or not myopia is a consequence of overminus lens therapy |
| Kushner 1998242 | Does not consider question of interest |
| Kushner 1993243 | Cannot separate data for X(T) and constant |
| Kushner 1989244 | Fewer than 20 patients [only 17 with X(T)] |
| Kushner 1988245 | No comparison group, case series data only |
| Kutschke 1988246 | Fewer than 20 patients |
| Lahlou 1971247 | Fewer than 20 patients (three cases) |
| Lak 1997248 | Deviation is at near for most patients (n = 28) only seven basic type |
| Lange 2009249 | Literature review |
| Lange 1993250 | Also adults and children mixed, outcome data not available separately for adults and children; cannot separate out divergence excess from convergence insufficiency type |
| Lee 2012251 | No comparison group, case series data only |
| Lee 2012252 | No comparison group, case series data only |
| Lee 2011253 | Fewer than 20 patients who had not previously had surgery (n = 17) |
| Lee 2009254 | Not considering intervention effectiveness, no outcomes of interest |
| Lee 1997255 | Sample aged up to 21 years at time of intervention and cannot separate data for children; also minimum range of follow-up period is 2 months and mean is 5.3 months |
| Lennerstrand 1986256 | Cannot separate data for X(T) from constant exotropia |
| Leonardi 1993257 | Adults and children mixed, does not present effectiveness data by age |
| Leonardi 1970258 | No effectiveness data (selective group) |
| Leow 201075 | No comparison group, case series data only |
| Lew 2007259 | Not considering outcomes of interest |
| Lim 2012260 | No relevant comparison group for the intervention |
| Lim 2011261 | No comparison group, case series data only; also data for adults and children not separate (age range at time of intervention 3–43 years) |
| Litwinska 1997262 | Data for exotropia and esotropia not available separately |
| Livir-Rallatos 2002263 | Adults and children mixed, cannot separate outcome data for children, also includes constant as well as symptomatic X(T) |
| Liu 2010264 | No separate outcome data for X(T) |
| Liu 2005265 | Sample size, fewer than 20 cases |
| Lucas 1994266 | Sample size, fewer than 20 cases |
| Marrakchi 1994267 | No comparison group, case series only |
| Martin 1989268 | No comparison group, case series only |
| Maruo 1988269 | No comparison group, case series only |
| Matsusaka 1988270 | No comparison group, case series only |
| McNeer 1987271 | No comparison group, case series only |
| McSwain 2011272 | No comparison group, case series only |
| Melek 1992273 | Not an intervention study |
| Menon 2010274 | Follow-up only 3 months |
| Metz 1988275 | Does not identify X(T) separately; considers cases with vertical squint |
| Miller 1994276 | Considers only cases of consecutive esotropia after surgery |
| Mims 2011277 | Not an intervention study |
| Mims 2008278 | Not an intervention study |
| Mims 2008279 | No outcomes of interest |
| Mims 2003280 | Not the primary intervention |
| Minguini 2005281 | No data for X(T) separately |
| Mitsui 1980282 | No data for X(T) separately |
| Mojon 2010283 | Not examining intervention effectiveness, adults and children in sample and fewer than 20 patients with follow-up |
| Moore 1977284 | No intervention data, largely a descriptive piece |
| Morrison 2010285 | No comparison group, case series data only, only 2-month follow-up |
| Morrison 2009286 | No comparison group, case series data only, only 2-month follow-up |
| Mumma 1975287 | Data mixed for adults and children, cannot separate data for children and X(T) from constant exotropia |
| Nelson 1992288 | No comparison group, case series data only; also data for adults and children mixed, cannot separate data for children |
| Nowakowska 2009289 | No comparison group, case series data only |
| Nusz 2006290 | Follow-up period of < 6 months for some patients |
| Oh 2006291 | Data for intermittent and constant exotropia mixed, also includes some convergence insufficiency types |
| Ohtaki 2000292 | Sample size, fewer than 20 patients with X(T) |
| Ohtsuki 2001293 | Cannot separate data for children, mean age 17.8 years, range 4–56 years and sample includes some convergence insufficiency types (n = 39) |
| Ohtsuki 1997294 | Data for adults and children mixed, cannot separate data for children |
| Oleszczynska-Prost 2004295 | Sample size fewer than 20 cases |
| Olitsky 1998296 | No comparison group, case series only |
| Orlin 2007297 | Data for exotropia in general, no data for X(T) specifically |
| Owen 2010298 | Not assessing intervention effectiveness, considers incidence of adverse effects only |
| Paakkala 1982299 | No comparison group, case series only |
| Pajakowa 1973300 | No comparison group, case series only |
| Paris 1998301 | Sample size fewer than 20 patients |
| Park 2008302 | No data for X(T) specifically, XT only |
| Parkesh 1984303 | Not X(T) |
| Patel 1988304 | Sample size fewer than 20 patients |
| Paula 2009305 | No outcomes of interest |
| Pietruschka 1973306 | No data for X(T) separately |
| Pineles 201126 | Only considers patients with consecutive esotropia |
| Pineles 201076 | No relevant comparison group, also, adults in sample and includes convergence insufficiency type |
| Pineles 2009307 | No relevant comparison for the interventions (groups differ on the basis of condition rather than on basis of intervention); also data for adults and children mixed |
| Pratt-Johnson 1979308 | Follow-up only 1 month |
| Pratt-Johnson 197784 | No comparison group, case series only |
| Qiu 2012309 | Not examining intervention effectiveness in the long term, considers stereo vision pre and only 1 week postoperatively |
| Rajavi 2001310 | Does not report on X(T) specifically (esotropia and exotropia in general) |
| Remy 1990311 | Sample size, fewer than 20 cases |
| Richard 1983312 | No comparison group, case series only |
| Rodrigues 2005313 | Exotropia in general, no specific data for X(T) |
| Rodrigues 2006314 | Exotropia in general, no specific data for X(T) |
| Rohatgi 1982315 | No comparison group, case series data only |
| Romanchuk 200636 | No comparison group, case series data only |
| Ron 1985316 | No separate data for X(T) |
| Roth 1981317 | No comparison group, case series only |
| Rowe 200944 | No separate data for X(T), as data for adults and children mixed (range 1–79 years) |
| Rowe 1990318 | Sample size fewer than 20 patients |
| Rutstein 2003319 | No separate data for children, also outcomes for convergence insufficiency mixed with basic and divergence excess types |
| Rutstein 1989320 | No outcomes of interest and no comparison group (case series data only) |
| Ruttum 199773 | No comparison group, case series data only, also data for adults and children mixed |
| Santos 2011321 | Sample size fewer than 20 patients with exotropia and no mention of X(T) |
| Saxena 2011322 | Adults in sample, no separate data for children only |
| Schulz 1984323 | No comparison group, case series only |
| Schwartz 1980324 | No data for X(T) separately and adults and children mixed population |
| Scott 1990325 | Does not separate X(T) from exotropia in general |
| Scott 1975326 | Sample age fewer than 20 patients |
| Segal 2000327 | Not data for X(T) specifically |
| Self 2004328 | No data for X(T) specifically |
| Sethi 2008329 | No data on intervention effectiveness |
| Shippman 1979330 | Follow-up < 6 months |
| Siatkowski 2010331 | Not an intervention study |
| Singh 1992332 | Children and adults mixed, no separate data for children, also some cases of convergence insufficiency cases included |
| Smoot 1990333 | Sample size fewer than 20 patients, subgroup of overcorrected ET and n = 9 |
| Somer 2007334 | Convergence insufficiency type |
| Spencer 199722 | No comparison group data reported |
| Spielmann 1983335 | Adults and children mixed and no mention of X(T) specifically |
| Spierer 2010336 | No data for X(T) separately |
| Spierer 2005337 | No data for X(T) separately, also adults and children mixed population |
| Spoor 1979338 | Not X(T) |
| Stoller 1994339 | No separate data for X(T) and adults and children mixed |
| Strogal 1983340 | No comparison group, case series only |
| Stuteville 2007341 | No intervention and adults |
| Suh 2006342 | Follow-up < 6 months |
| Sun 2010343 | No data reported for X(T) separately |
| Tae 2005344 | No comparison group data on effectiveness, also adults and children mixed |
| Tao 2008345 | No data for X(T) separately, adults and children mixed |
| Tatham 2009346 | Data for adults and children mixed, also no data for X(T) separately |
| Thouvenin 2008347 | Not X(T) |
| Tibbs 1978348 | Sample size fewer than 20 patients with X(T) |
| Tsuji 1988349 | No separate data for X(T) |
| Usui 2000350 | No comparison group, case series only |
| Vishnoi 1987351 | Adults and children mixed, no separate data for children |
| Wang 2010352 | No comparison group, case series only |
| Wang 2009353 | No comparison group, case series data only |
| Wang 2008354 | No comparison group, case series only |
| Weakley 1993355 | No separate data for X(T) and adults and children mixed |
| Weston 1991356 | Outcomes for XT generally (n = 34), no data for X(T) specifically |
| Wickens 1984357 | No comparison group, case series only |
| Wilson 1989358 | No effectiveness data for X(T) |
| Windsor 1971359 | Follow-up < 6 months |
| Wu 2007360 | No comparison group, case series only |
| Wu 2006361 | No comparison group, case series only |
| Wutthiphan 2008362 | Data for adults and children mixed |
| Wygnanski-Jaffe 1999363 | No data for X(T) specifically only XT and ET generally |
| Xu 2012364 | Not intervention effectiveness, focuses on adults |
| Yam 2012365 | No comparison group, case series only |
| Yan 2006366 | No mention of X(T) specifically |
| Yang 2008367 | No comparison group, case series only |
| Yang 1984368 | Follow-up only 6 weeks |
| Yao 1993369 | No comparison group, case series data only |
| Yazdian 2006370 | Sample size fewer than 20 patients |
| Yi 2011371 | No data for X(T) separately, pools exotropia and esotropia and includes consecutive as well as primary cases |
| Yildirim 1999372 | Adults and children mixed sample, no data for children separately |
| Yin 2002373 | Data for adults and children mixed |
| Yu 1989374 | No comparison group, case series data only |
| Zaki 1972375 | No intervention, descriptive epidemiological data on prevalence of types of XT |
| Zhi 2008376 | No specific data on X(T) discusses XT only |
| Zibrandtsen 1986377 | Sample size fewer than 20 patients with basic or divergence excess type X(T) |
| Ziegler 1982378 | Review, no empirical data |
Appendix 5 Intermittent Exotropia Questionnaire
Appendix 6 Survey of UK ophthalmology units
An e-mail survey was conducted using the paediatric ophthalmology e-mail Listserv PAED-OPHTH-STRABISMUS list (157 recipients, including trainees).
There were 38 respondents, 37 of whom operated on X(T). Of these 37 respondents, 35 (95%) indicated that they would be willing to participate in a future RCT, although one is now practising outside the UK.
Reasons for being unwilling
-
‘Probably unethical.’ (This was the one who did not operate but still answered this question.)
-
‘Probably not rather than absolutely not, 2 reasons: 1. parents probably won’t accept it. 2. I would be concerned about loss of BSV.’
-
‘The decision for surgery is one made by the parents and the surgeon. Hence to randomise to no treatment after this decision I feel will be a major stumbling block.’
Potential barriers
Potential barriers to such a trial succeeding broadly fell into three main themes: loss to follow-up (mentioned by four people), dropout rates (four people) and the unwillingness of parents to wait for surgery (eight people). Other issues included definitions of squint severity and outcomes:
-
Loss to follow-up:
-
‘Long follow-up would increase the DNA rate especially in the surgical cases that were successful.’
-
‘Losing people under follow-up.’
-
‘Loss of patients to follow up.’
-
‘Failure to continue follow-ups.’
-
-
Dropout rates:
-
‘Parents motivation to continue lengthy follow-up period. There will be more dropouts in the observation group in favour of surgery.’
-
‘Parents may decide to go to another centre for surgical treatment.’
-
‘Change of mind by parents.’
-
‘Dropout because of child being teased at school.’
-
-
The desire for surgery/unwillingness to wait:
-
‘Many parents are keen for surgical intervention and it may be difficult to monitor them for 5 years.’
-
‘In case of severe intermittent XT, may be difficult to convince parents for observation alone.’
-
‘Parents desire for surgery.’
-
‘Pressure from parents wanting surgery.’
-
‘Patient/parental pressure for surgery with worsening control.’
-
‘Difficulty convincing parents that it is satisfactory to monitor especially if their child/teacher complains of difficulty seeing the smart board.’
-
‘Once you mention surgery – parents commonly don’t want to wait.’
-
‘Parents may not want to be randomly assigned into one group especially those not wanting surgery.’
-
-
Criteria/definitions:
-
‘Defining the outcome and what constitutes success/failure.’
-
‘Investigators agreeing the definition of “moderate to severe” as the point at which surgery is recommended is so variable across the UK currently.’
-
‘I think the real difficulty would be in trying to rationalise not only the type of surgery, but also the aim of the surgery. Do you leave them XT or aim to prevent outdrift by making them ET and risk amblyopia?’
-
-
Random:
-
‘Culture amongst some orthoptic colleagues of advising parents that child should have operation based on angle or stereo or age. (“I’ll send you through to the consultant so they can add you to the waiting list,” etc.). This may affect parental attitudes to being randomised.’
-
‘Ethics management pressures on workload.’
-
‘Will need to be clear when patients can withdraw if binocularity threatened.’
-
Question: How important do you think it is to conduct a randomised controlled trial of the clinical effectiveness of surgery versus active monitoring in this condition?
Seventeen (46%) felt that it was very important, 17 (46%) quite important and 3 (8%) not very important. (One skipped.)
Question: How important do you think it is to conduct a randomised controlled trial of the cost effectiveness of surgery versus active monitoring in this condition?
Eight (22%) felt it was very important, 20 (54%) quite important and 9 (24%) not very important. (One skipped.)
Other comments
Interesting idea but personally I would prefer to await outcome of such a trial before delaying surgery in children for whom I currently offer surgery.
5 years seems like a long period to monitor. During period or monitoring would you suggest over minus glasses or convergence exercises. What criteria would move a child from monitoring to surgery?
5 years is ambitious – but probably necessary – and I guess with necessary checks (i.e. break code if obviously doing worse)
I think the first trial should be on observation versus surgery in moderate control, as this is more ethical and management for this varies between departments you may even compare with minus lenses.
Are there any plans to include a third arm for BTxA?
Having trained in the UK and now practising in my home university hospital (Thessaloniki, Greece), I wonder if you’d be interested in non-UK participants in your current and future studies. If so, what would be minimum requirements?
This respondent was based in Wales and indicated he would not be willing:
Mike- this is what I feel though I feel for the establishing a natural history it is extremely important but will be difficult to recruit in my practice – but I’m willing to give it a go.
Good luck Mike.
Go for it guys. Answers not found yet!
Appendix 7 Participant Costs Questionnaires
Health Service Use Questionnaire (Part A)
1a. Has your child seen or contacted a GP because of his or her eyes during the last 3 months?
YES
NO
1b. If YES to Question 1a, how many appointments did your child attend with a GP?
1c. If YES to Question 1a, how many times did a GP visit your child at home?
1d. If YES to Question 1a, how many times did you or your child have a telephone conversation with a GP?
2a. Has your child see a practice nurse because of his or her eyes during the last 3 months?
YES
NO
2b. If YES to Question 2a, how many times in total?
3a. Has your child seen a community optician or optometrist during the last 3 months?
YES
NO
3b. If YES to Question 3a, how many times in total?
4a. Has your child seen a hospital specialist (consultant or one of his/her team) because of his or her eyes other than attending planned follow-up visits during the last 3 months?
YES
NO
4b. If YES to Question 4a, how many times in total?
5a. Has your child been admitted to hospital because of his or her eyes during the last 3 months?
YES
NO
5b. If YES to Question 5a, how many nights was your child in hospital (if admitted as a day case, please enter 0)?
6a. Has your child had prescription medicine for his or her eyes during the last 3 months?
YES
NO
6b. If YES to Question 6a, what type of medication has your child been prescribed?
| Name of the medicine | No. of times prescribed |
|---|---|
| Antibiotic | |
| Steroid | |
| Painkillers | |
| Other |
7a. Have you purchased over the counter medicine (e.g. eye drops) for your child’s eyes during the last 3 months?
YES
NO
7b. If YES to Question 7a, how much did you pay in total?
8a. Have you paid for any other private health care for your child because of his or her eyes during the last 3 months?
YES
NO
8b. If YES to Question 8a, what type of care did you pay for?
8c. If YES to Question 8a, how much in total did it cost?
9a. Have you taken time off from work to look after your child because of his or her eye condition other than attending planned follow up visits during the last 3 months?
YES
NO
9b. If YES to Question 9a, how many days in total were you absent from work?
Time and Travel Questionnaire (Part B)
Please note that this questionnaire assumes that for every form of health care your child attended, you have travelled with and accompanied your child. If your child travelled alone, please only include the costs that incurred to your child.
Part 1 – Your child’s most recent admission to hospital because of his or her eyes
If in the last 3 months your child was not admitted to hospital please go to Part 2.
1. Please circle the number that best describes how you and your child travelled. If more than one form of transport was used, please indicate the way you travelled for the main (longest in terms of distance) part of your journey.
Bus_____________________________________________________________________________________1
Train____________________________________________________________________________________2
Taxi_____________________________________________________________________________________3
Private car_______________________________________________________________________________4
Hospital car______________________________________________________________________________5
Ambulance______________________________________________________________________________6
Other (please specify)_____________________________________________________________________7
2. If you and your child travelled by bus, train or taxi to hospital what was the total cost of the (one-way) journey? Please write the cost in the box below. Please put zero if you did not travel by bus, train or taxi at all or if you did not pay a fare.
Cost of (one-way) fare (£) □□–□□ pence
3. If you and your child travelled by private car, about how many miles did you travel one way? Please write the number of miles in the box below. Please put zero if you did not travel by private car at all.
Number of miles one way □□□
4. If you and your child travelled by private car and you or your companion had to pay a parking fee, howmuch did this cost? Please write the cost in the box below. Please put zero if you did not pay aparking fee.
Expenditure on parking fee (£) □□–□□ pence
5. When your child was admitted to the hospital, how many nights did he or she spend there? Please write the number of days in the box below.
Number of nights □□
6. Please circle the number that best describes what you would otherwise have been doing as your main activity if you had not gone with your child to the hospital.
Paid work__________________________________________________________________________________1
Housework_________________________________________________________________________________2
Child care__________________________________________________________________________________3
Caring for someone else_____________________________________________________________________4
Voluntary work_____________________________________________________________________________5
Leisure activities____________________________________________________________________________6
Other (please specify)________________________________________________________________________7
7. If you take time off from paid work (or business activity if self employed) in order to accompany your child to the hospital. Please indicate the number of hours you took off from paid work (or business activity if self employed) in the box below. Please put zero if you did not take time off from paid work (or business activity if self employed) to accompany your child to the hospital.
Number of hours □□
8. Was there another adult person accompanying you and your child to hospital?
YES
NO
9. While your child was in hospital, approximately how many times did you go to visit your child?
Number of times □□
Part 2 – Your child’s most recent outpatient visit because of his or her eyes
If in the last 3 months your child did not have an outpatients appointment please go to Part 3.
1. Please circle the number that best describes how you and your child travelled. If you used more than one form of transport please indicate the way you travelled for the main (longest in terms of distance) part of your journey.
Bus_______________________________________________________________________________________1
Train______________________________________________________________________________________2
Taxi_______________________________________________________________________________________3
Private car_________________________________________________________________________________4
Hospital car________________________________________________________________________________5
Ambulance________________________________________________________________________________6
Other (please specify)________________________________________________________________________7
2. If you and your child travelled by bus, taxi or train to your child’s outpatients appointment what was the total cost of the (one-way) journey? Please write the cost in the box below. Please put zero if you did not travel by bus, train or taxi at all, or if you did not pay a fare.
Cost of (one-way) fare (£) □□–□□ pence
3. If you and your child travelled by private car, about how many miles did you travel one way? Please write the number of miles in the box below. Please put zero if you did not travel by private car at all.
Number of miles one-way □□□
4. If you and your child travelled by private car and you had to pay a parking fee, how much did this cost? Please write the cost in the box below. Please put zero if you did not pay a parking fee.
Expenditure on parking fee (£) □□–□□ pence
5. When you and your child visited outpatients, how long did it take to travel there? Please write the number of hours and minutes in the box below.
Number of hours □□–□□ minutes
6. When you and your child visited outpatients, how long did you spend there? Please write the number hours and minutes in the box below.
Number of hours □□–□□ minutes
7. Please circle the number that best describes what you otherwise would have been doing as your main activity if you had not accompanied your child to the outpatients?
Paid work__________________________________________________________________________________1
Housework________________________________________________________________________________2
Child care__________________________________________________________________________________3
Caring for someone else_____________________________________________________________________4
Voluntary work_____________________________________________________________________________5
Leisure activities_____________________________________________________________________________6
Other (please specify)________________________________________________________________________7
8. Was there another adult person accompanying you and your child to the outpatient visit?
YES
NO
Part 3 – Your child’s most recent GP/nurse appointment because of his or her eyes
If in the last 3 months your child did not have a GP/nurse appointment, please go to Part 4.
1. Please circle the number that best describes how you and your child travelled to your child’s most recent GP/nurse appointment. If you used more than one form of transport please indicate the way you travelled for the main (longest in terms of distance) part of your journey.
Bus_______________________________________________________________________________________1
Train_____________________________________________________________________________________2
Taxi______________________________________________________________________________________3
Private car_________________________________________________________________________________4
Bike______________________________________________________________________________________5
Walk_____________________________________________________________________________________6
Other (please specify)________________________________________________________________________7
2. If you and your child travelled by bus, taxi or train, what was the total cost of the (one-way) fare? Please write the cost in the box below. Please put zero if you did not travel by bus or taxi or if you did not pay the fare.
Cost of (one-way) fare (£) □□–□□ pence
3. If you and your child travelled by private car, about how many miles did you travel one-way? Please write the number of miles in the box below. Please put zero if you did not travel by private car at all.
Number of miles one-way □□□
4. If you and your child travelled by private car and you had to pay a parking fee, how much did this cost? Please write the cost in the box below. Please put zero if you did not pay for parking.
Expenditure on parking fee (£) □□–□□ pence
5. When you and your child visited the GP/nurse, how long did it take to travel there? Please write the number of minutes in the box below.
Number of minutes □□□
6. When you and your child visited the GP/nurse, how long did you spend there? Please write the number minutes in the box below. Please include in your answer the time spent waiting and also the time spent with the doctors and nurses.
Number of minutes □□□
7. Please circle the number that best describes what you otherwise would have been doing as your main activity if you had not accompanied with your child to visit the GP/nurse.
Paid work_________________________________________________________________________________1
Housework________________________________________________________________________________2
Child care_________________________________________________________________________________3
Caring for someone else_____________________________________________________________________4
Voluntary work_____________________________________________________________________________5
Leisure activities____________________________________________________________________________6
Other (please specify)________________________________________________________________________7
8. Was there another adult person accompanying you and your child to the GP/nurse visit?
YES
NO
Part 4 – Your child’s most recent community optician or optometrist visit because of his or her eyes
If in the last 3 months your child did not attend a community optician or optometrist, please return the questionnaire to the interviewer. Thank you!
1. Please circle the number that best describes how you and your child travelled to your child’s most recent community optician or optometrist appointment. If you used more than one form of transport please indicate the way you travelled for the main (longest in terms of distance) part of your journey.
Bus_______________________________________________________________________________________1
Train______________________________________________________________________________________2
Taxi_______________________________________________________________________________________3
Private car__________________________________________________________________________________4
Bike_______________________________________________________________________________________5
Walk______________________________________________________________________________________6
Other (please specify)________________________________________________________________________7
2. If you and your child travelled by bus, taxi or train, what was the cost of the (one-way) fare? Please write the cost in the box below. Please put zero if you did not travel by bus or taxi or if you did not pay the fare.
Cost of (one-way) fare (£) □□–□□ pence
3. If you and your child travelled by private car, about how many miles did you travel one-way? Please write the number of miles in the box below. Please put zero if you did not travel by private car at all.
Number of miles one-way □□□
4. If you and your child travelled by private car and you had to pay a parking fee, how much did this cost? Please write the cost in the box below. Please put zero if you did not pay for parking.
Expenditure on parking fee (£) □□–□□ pence
5. When you and your child visited the community optician or optometrist, how long did it take to travel there? Please write the number of minutes in the box below.
Number of minutes □□□
6. When you and your child visited the community optician or optometrist, how long did you spend there? Please write the number minutes in the box below. Please include in your answer the time spent waiting and also the time spent with the doctors and nurses.
Number of minutes □□□
7. Please circle the number that best describes what you otherwise would have been doing as your main activity if you had not accompanied your child to visit community optician or optometrist.
Paid work__________________________________________________________________________________1
Housework________________________________________________________________________________2
Child care__________________________________________________________________________________3
Caring for someone else_____________________________________________________________________4
Voluntary work_____________________________________________________________________________5
Leisure activities_____________________________________________________________________________6
Other (please specify)________________________________________________________________________7
8. Was there another adult person accompanying you and your child to the community optician or optometrist visit?
YES
NO
If you have any comments about this questionnaire or about the Exotropia trial please use this space to write them:
Appendix 8 Tables for pilot study
| Resource use | Data collection source | |
|---|---|---|
| Intervention | ||
| No. of times | Receiving general anaesthetic | CRF |
| Receiving local anaesthetic | ||
| No. of cases | Day cases | CRF |
| Inpatient cases | ||
| Mean | Operation theatre time | CRF |
| Recovery room time | ||
| No. NHS travel: | Hospital car | Participant Costs Questionnaire |
| Ambulance | ||
| Follow-up resource use: secondary care | ||
| No. of times | Receiving further surgery | CRF |
| Receiving BOTOX® (Allergan) injections | ||
| No. of cases | Outpatient visits | Participant Costs Questionnaire |
| Inpatients, night | ||
| No. of NHS travel | Hospital car | Participant Costs Questionnaire |
| Ambulance | ||
| Follow-up resource use: primary care | ||
| No. of times | GP visits | Participant Costs Questionnaire |
| Nurse visits | ||
| Optometrist visits | ||
| Unit cost of resource use | Data collection source |
|---|---|
| Intervention costs | |
| Consumables | Manufacturers’ price list |
| Reusable | |
| Per general anaesthetic | |
| Per local anaesthetic | |
| Theatre time per minute | PSSRU |
| Recovery room time per minute | |
| Surgeon per minute | |
| Anaesthetist per minute | |
| Registrar group per minute | |
| Other staff per minute | |
| Associate specialist per minute | |
| Theatre staff per minute | |
| Recovery room staff per minute | |
| Per day case | PSSRU |
| Per inpatient stay per night | |
| Hospital car per journey | PSSRU, Participant Costs Questionnaire |
| Ambulance per journey | |
| Follow-up: secondary care costs | |
| Further surgery | Estimate based on the costs of the initial surgery |
| BOTOX injection | Manufacturers’ price list |
| Inpatient stay per night | PSSRU |
| Outpatient visit | |
| Hospital car per journey | PSSRU, Participant Costs Questionnaire |
| Ambulance per journey | |
| Follow-up: primary care costs | |
| GP visit | PSSRU |
| Nurse visit | |
| Optometrist visit | |
Glossary
- Abnormal retinal correspondence
- Subnormal binocular vision generated from images from eyes that are misaligned.
- Amblyopia (synonym: lazy eye)
- A developmental condition, in which there is dysfunction of the processing of visual information, at a retinal and cerebral level, resulting from impaired visual input, to one or both eyes, during a sensitive period of visual development. Common causes include strabismus and/or refractive error, but more severe forms of amblyopia are seen when there is total absence of visual input to one or both eyes, for example in cases of complete congenital cataract. Amblyopia never occurs in isolation, rather it is the effect of another pathological process on the development of vision.
- Binocular vision
- The ability to integrate the images from each eye to generate one percept with information from each image. Generally considered to have three grades: simultaneous perception, fusion and stereopsis, in which a three-dimensional image can be perceived.
- Esotropia/esodeviation
- A convergent (inturning) misalignment of the eyes.
- Exotropia/exodeviation
- A divergent (out-turning) misalignment of the eyes.
- Ophthalmologist
- Clinician specialising in medical aspects of the diagnosis and treatment of eye and visual disorders.
- Optometrist/optician
- Clinician specialising in the correction of refractive error and eye examination. May have an extended role covering the diagnosis and treatment of low-complexity eye disorders.
- Orthoptist
- Paramedical professional specialising in the assessment and treatment of adults and children with strabismus and amblyopia.
- Orthotropia
- The condition of binocular alignment, i.e. no strabismus present.
- Ptosis
- Drooping of upper eyelid.
- Strabismus (synonym: squint)
- A condition in which the eyes are misaligned. Acquired strabismus from late childhood leads to double vision (diplopia); strabismus with onset either from birth or early childhood does not lead to double vision because cerebral plasticity allows central suppression of the image from one eye when both eyes are open.
- Suppression
- Blocking of the image of one eye from conscious perception.
List of abbreviations
- BLR
- bilateral lateral rectus recession
- BTXA
- botulinum strain A
- CASP
- Critical Appraisal Skills Programme
- CI
- confidence interval
- CRF
- case report form
- ENR
- eligible not recruited
- GCP
- Good Clinical Practice
- GP
- general practitioner
- HRQoL
- health-related quality of life
- HSUQ
- Health Services Use Questionnaire (Health Economics Questionnaire Part A)
- HTA
- Health Technology Assessment
- HUI3
- Health Utility Index Mark 3
- ISF
- Investigator Site File
- IXTQ
- Intermittent Exotropia Questionnaire
- NCS
- Newcastle Control Score
- NCTU
- Newcastle Clinical Trials Unit
- NIHR
- National Institute for Health Research
- PD
- prism dioptre
- PI
- Principal Investigator
- QoL
- quality of life
- R&R
- recession/resection
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RO
- research orthoptist
- SamExo
- Surgery vs. Active Monitoring in Intermittent Exotropia
- TO
- treatment orthoptist
- TSC
- Trial Steering Committee
- TTQ
- Time and Travel Costs Questionnaire (Health Economics Questionnaire Part B)
- UKCRN
- UK Clinical Research Network
- VA
- visual acuity
- X(T)
- intermittent exotropia
- XT
- constant exotropia