Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 02/02/01. The contractual start date was in October 2003. The draft report began editorial review in March 2012 and was accepted for publication in May 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Steve Halligan reports non-financial research and development evaluation work provided for iCAD Inc., which develops CAD for computed tomographic colonography. This author also provides expert witness testimony on matters relating to radiological diagnosis of colorectal cancer and holds patents/patent applications for computed tomography imaging technology (2010; International PCT no. PCT/GB2011/050448 ‘Apparatus and Method for Registering Medical Images Containing a Tubular Organ’). Wendy Atkin reports funds to support this work were also obtained from Imperial College London, London, UK.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Halligan et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Along with lung cancer, colorectal cancer (CRC) is one of the most commonly diagnosed cancers in the developed world, and around 1.2 million cases were diagnosed worldwide in 2008. 1 Roughly 40,000 new cases are reported in the UK each year2 and, with an ageing population, this number is increasing. Since 1 July 2000, the Department of Health has made recommendations that any patients with suspected cancer should be seen by a specialist within 2 weeks of referral from their general practitioner (GP). 3 This has led to the development of guidelines for referral of patients with suspected CRC, taking account of factors such as age (patients over 60 years are considered to be at increased risk) and symptoms including change in bowel habit, rectal bleeding and anaemia. 4 However, as these symptoms are non-specific and common in the general population, most patients who are investigated will not have the disease. 5,6 This places a considerable burden on diagnostic services and highlights the need for diagnostic tests that are not only sensitive and specific, but widely available, safe and acceptable to patients.
The currently established tests for examining the whole colon are colonoscopy and barium enema (BE). Colonoscopy is generally considered to be the most accurate examination for the detection of CRC and has the advantage of allowing biopsies to be taken in order to confirm the presence of cancer or other abnormalities within the bowel, and enabling the complete removal of precancerous polyps. However, colonoscopy is not without limitations. It is an invasive and technically demanding procedure and carries a small risk of serious adverse events, including bowel perforation and bleeding. 7 A study of 53,220 outpatients undergoing colonoscopy found that the risk of perforation and bleeding increased with age,8 yet older people constitute the majority of those with symptoms. Sedation is also usually required and this conveys additional risk. 9
Barium enema is safer than colonoscopy in elderly patients, but has been found in audits to miss a greater proportion of cancers in routine practice. 10 This has led to recommendations that its use be reduced. 11 However, for patients with a low index of suspicion for serious disease, avoidance of colonoscopy may be desirable, particularly in the elderly for whom the risks of sedation are greatest. BE is inexpensive, there is considerable experience with its use and it remains widely available, with an estimated 4 million procedures performed worldwide in 2009 (Mr Maurizio Franchini, Bracco International, 2009, personal communication).
Computed tomographic colonography (CTC) or ‘virtual colonoscopy’ is a relatively new health technology, potentially combining the sensitivity of colonoscopy with the safety of BE.
Existing research on the new technology
Computed tomographic colonography was first described in 1994. 12 The examination consists of a helical computed tomography (CT) scan of the cleansed and gas-distended large bowel, with evaluation of the resulting images by a radiologist. Complex image analysis techniques are used to aid interpretation, including three-dimensional (3D) rendering that simulates the colonoscopist’s endoluminal view of the colon.
Computed tomographic colonography has already received considerable attention in the context of CRC screening, as there is evidence that it is safer than colonoscopy13,14 and it does not require the patient to be sedated. It has a similar high sensitivity for cancer and large polyps in asymptomatic populations examined by experienced radiologists. 15–17 However, a role for symptomatic diagnosis has been relatively ignored.
One disadvantage of CTC when compared with colonoscopy is that it requires an additional endoscopic procedure to biopsy or remove any significant lesions detected, increasing inconvenience and cost. CTC may also detect small incidental lesions within the colon that are unlikely to be the cause of symptoms but which, once identified, will require endoscopic removal. Any patients with such lesions will not avoid colonoscopy and will need to undergo a second bowel preparation (unless colonoscopy can be performed on the same day, which is rare in normal clinical practice). However, the majority of patients having CTC will not need colonoscopy and there is evidence that CTC may be more acceptable to patients. 18–20
Because of its high sensitivity for CRC, it has been suggested that CTC should replace BE as an alternative to colonoscopy. 21 However, few studies have directly compared BE and CTC,22,23 and until now there have been no randomised trials. As a result, robust data to guide health policy have been lacking.
As CTC and colonoscopy have similar sensitivity for cancer and large polyps,15–17 the choice between these two procedures is likely to depend on other factors. For example, it is not known what proportion of patients presenting with symptoms of CRC require subsequent colonic investigation to verify findings at CTC, compared with colonoscopy. This is an important consideration if CTC is to be regarded as an alternative diagnostic test.
In the NHS it is possible that CTC will find a role in cancer detection in elderly patients, in whom the risks of colonoscopy-related adverse events (oversedation, colonic perforation) are greatest and the risks of exposure to ionising radiation less important. Unlike colonoscopy or BE, CTC can also image organs outside the colon, which may aid diagnosis in patients whose symptoms are extracolonic in origin. However, this also results in incidental findings24 with associated medical, psychological and financial consequences. If an extracolonic abnormality is identified it may prompt further investigation without any ultimate benefit to the patient, so careful evaluation is required. 25,26
We felt that such equipoise was the ideal point at which to conduct a trial,27 so that recommendations could be evidence based and any future implementation would be sensible, balanced and informed.
Objectives of the Special Interest Group in Gastrointestinal and Abdominal Radiology trials
Our aim was to examine the diagnostic efficacy, acceptability and cost of CTC compared with BEs or colonoscopy, by carrying out two parallel randomised trials. In the trial of CTC compared with BEs, this comparison was based primarily on detection rates for significant neoplasia, whereas in the trial of CTC compared with colonoscopy, it was based on rates of referral for a confirmatory diagnostic procedure, because the similar sensitivities of CTC and colonoscopy would have made a trial powered on detection rates impractical (see Chapter 2, Sample size). We also compared other outcomes, including miss rates for CRC and rates of serious adverse events.
The acceptability to patients of CTC, BEs and colonoscopy was assessed by giving psychological questionnaires to a sample of participants in the study, documenting their experiences on the day after the test and at 3 months after the test (see Chapter 5).
Finally, an economic analysis was undertaken to estimate the costs and cost-effectiveness of the three procedures (see Chapter 6).
Chapter 2 Methods
Study design
The study consisted of two multicentre randomised trials (International Standard Randomised Controlled Trials Number 95152621), conducted in accordance with the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Guideline for Good Clinical Practice. 28 Ethics approval was obtained from the Northern and Yorkshire Multi-Centre Ethics Committee in January 2004 and subsequently from all participating hospitals. The trial was supervised by an independent Data Monitoring Committee and a Trial Steering Committee. All patients gave informed written consent.
Patients with symptoms suggestive of CRC were initially seen in clinic and referred for either colonoscopy or a BE (the ‘default’ examinations) by the clinician seeing the patient, depending on whether they preferred radiological or endoscopic investigation in normal clinical practice for the particular patient in question. This decision depended on factors such as expectation of cancer, perceived frailty and locally available diagnostic resources. BEs and colonoscopy are not considered clinically equivalent in normal clinical practice. To design a three-way randomised controlled trial (RCT) of BE compared with colonoscopy compared with CTC would have been unethical because of a lack of equipoise between BE and CTC, and would have suffered from poor recruitment. As a result, the study was split into two separate trials: one for CTC compared with BE (the ‘BE trial’) and one for CTC compared with colonoscopy (the ‘colonoscopy trial’).
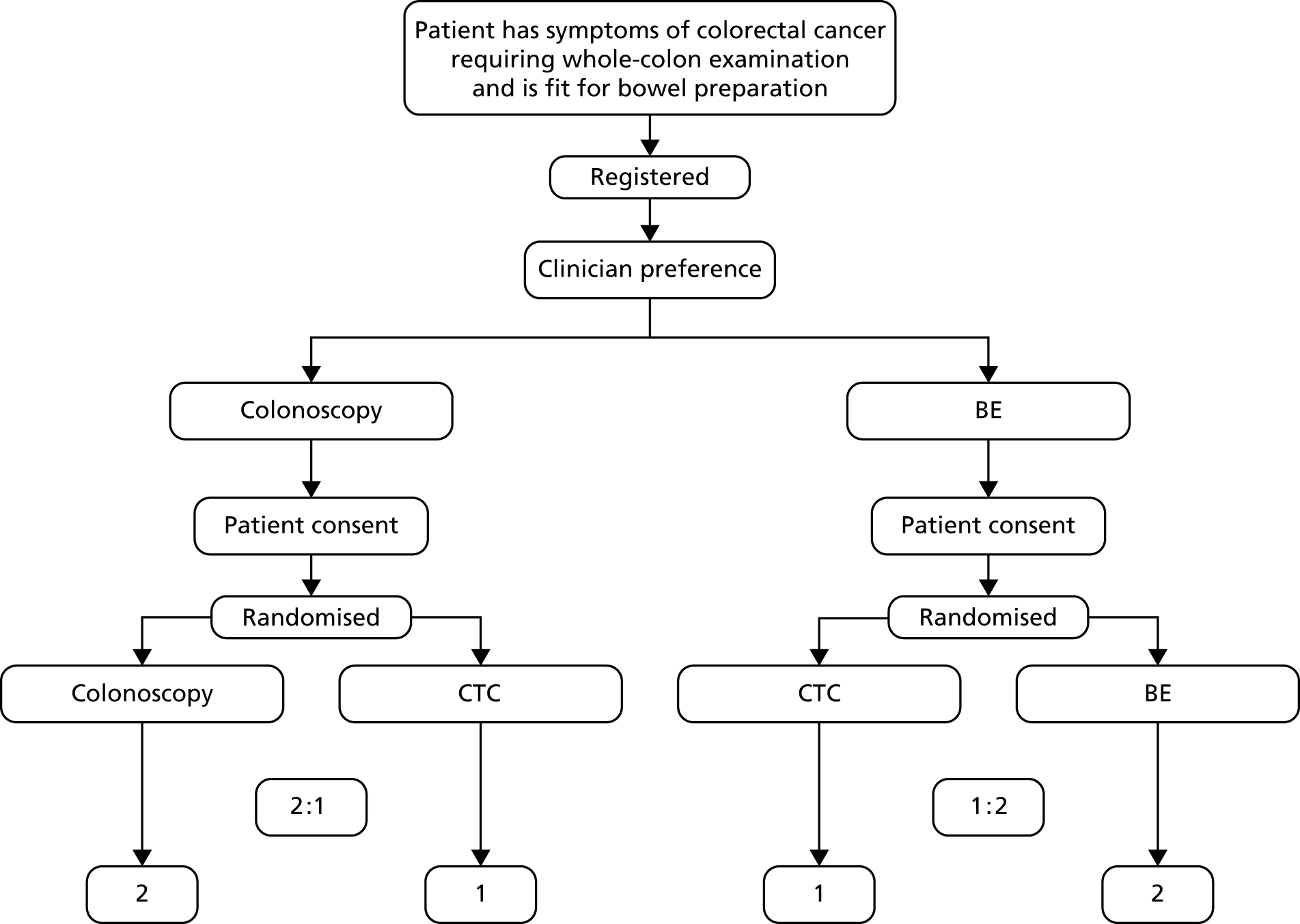
Within each trial, patients were randomised in a 2 : 1 ratio in favour of the ‘default’ whole-colon examination, in accordance with the algorithm shown in Figure 1. A 2 : 1 ratio was chosen in order to maximise recruitment within the constraints of provision for the new technology and the study was powered accordingly.
FIGURE 1.
Study design. Lower boxes refer to randomisation ratio numbers.
Centres
We recruited patients from 21 NHS hospitals in England. To increase generalisability, both teaching and general hospitals were included. Participating centres were expected to have an established and efficient fast-track referral system for patients with symptoms of CRC, usually an identifiable diagnostic clinic, to facilitate recruitment. Each centre had to have a named colorectal nurse specialist or researcher attached to the clinic who would take responsibility for recruitment. Centres were also required to nominate a lead clinician who would supervise the work of the colorectal nurse specialist and a lead radiologist [and a Special Interest Group in Gastrointestinal and Abdominal Radiology (SIGGAR) member] who was willing to undergo central training in CTC according to accreditation guidelines issued by the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) (see Computed tomographic colonography training). A final criterion for selection was agreement by the participating site that use of CTC as a primary diagnostic test would only be offered to eligible patients as part the trial.
Participating hospitals were chosen from interested centres via a ‘sham randomisation’ that identified centres likely to achieve a monthly recruitment target of at least 18 patients. 29 Over a 2-month period each centre was asked to identify patients who satisfied the trial eligibility criteria and to enter simple demographics (age, sex, symptoms, route of referral and type of whole-colon investigation requested) on a secure, password-protected online database. No patients were approached directly, but this ‘sham randomisation’ provided an estimate of how each centre might perform once the trial was in progress.
Participants
Following referral from their GP, suitable patients were identified from clinics and procedure waiting lists by the colorectal nurse specialist.
Inclusion criteria
Patients were eligible for the study if they were:
-
experiencing symptoms suggestive of CRC (this included both patients who fulfilled a 2-week wait criterion and those considered less urgent)
-
aged ≥ 55 years
-
clinically judged to need a whole-colon examination
-
clinically judged fit to undergo full bowel preparation
-
able to give fully informed consent.
Exclusion criteria
Patients could not be included in the study if they had:
-
a known genetic predisposition to cancer, for example familial adenomatous polyposis or hereditary non-polyposis CRC
-
a known diagnosis of ulcerative colitis or Crohn’s disease
-
undergone a previous whole-colon examination in the past 6 months
-
been referred for whole-colon examination to follow up a previously diagnosed CRC.
Flexible sigmoidoscopy
It became clear during piloting that many patients were being given a flexible sigmoidoscopy (FS) examination prior to their randomised procedure. Excluding these patients would have made it difficult to recruit adequately to the study, as many hospitals make use of FS and this is likely to increase in future. Patients having FS were therefore eligible for the study if a strong clinical suspicion of right-sided cancer remained and if BE or colonoscopy would usually be the next test.
Interventions
Barium enema
Technical parameters
Exams were performed after full bowel preparation, with all centres using sodium picosulphate and magnesium citrate (Picolax®, Ferring Pharmaceuticals) as the primary laxative. An intravenous spasmolytic was administered routinely, usually 20 mg of hyoscine butylbromide (Buscopan®, Boehringer Ingelheim Ltd) unless contraindicated. Air or carbon dioxide was used for insufflation. Digital fluoroscopic images of the double-contrasted colorectum were obtained to the caecum, supplemented by overcouch decubitus films. A minimum 512 × 512 matrix was required for all images.
Interpretation
Scans were interpreted by radiologists with a subspecialty interest in gastrointestinal (GI) radiology or by fully trained radiographic technicians. Radiologists had to be performing an average of three or more BEs each week in routine clinical practice and were required to have performed at least 50 enemas unaided. Radiographers had to have completed an accredited course in double-contrast BE techniques and since that time to have performed unaided an average of at least three enemas per week for at least 6 months. Radiographers’ practice had to be kept under regular audit.
Reporting
In total, 82 practitioners (including radiologists and fully trained radiographic technicians) interpreted BE scans in the study. Examinations could not be reported solely by a trainee radiologist without a subspecialty interest in GI radiology, so as to maintain parity with the CTC and colonoscopy groups. However, dual reporting was allowed, provided that one of the reporting clinicians fulfilled the criteria described above. In practice, all reports were either written or verified by a radiologist, except in a single centre, where dual reporting by senior radiographers was sometimes used. Radiologists or radiographers issued a report in accordance with normal clinical practice and completed a case report form (CRF) (see Appendix 1), which made it possible to capture additional information that would not routinely be mentioned in the report. Items recorded on the CRF included estimated size (mm) and location of detected lesions, the presence and site of diverticulosis, time taken for interpretation, technical difficulties (e.g. incontinence to barium or gas), quality of visualisation and any adverse events. Procedures were assessed as ‘very easy’, ‘quite easy’, ‘quite difficult’ or ‘very difficult’. The quality of the bowel preparation was rated as ‘excellent’, ‘good’, ‘adequate’ or ‘poor’. Visualisation of the six major large bowel segments (rectum, sigmoid, descending colon, transverse colon, ascending colon and caecum) was rated as ‘excellent’, ‘good’, ‘adequate’, ‘poor’ or ‘not seen’. The presence of diverticulosis in each segment was graded as ‘none’, ‘mild’, ‘moderate’ or ‘severe’. If a polyp or cancer was visualised, confidence for its presence was rated as ‘excellent’, ‘good’, ‘adequate’ or ‘poor’.
Colonoscopy
Technical parameters
Colonoscopy was performed after full bowel preparation using video endoscopes. Sedation (usually 1–5 mg of midazolam) and analgesia (usually 50 µg of fentanyl or 25–50 mg of pethidine) were administered as judged clinically necessary. Examinations were carried out according to usual practice and any detected lesions were measured and either biopsied or excised where indicated.
Interpretation
A total of 217 gastroenterologists or colorectal surgeons who had satisfied criteria for competence performed colonoscopies in the study. After the trial obtained funding, the government announced three national training centres for endoscopy, one of which is the Wolfson Unit at St Mark’s Hospital in Harrow, UK, where the trial office was originally based. The aim of the national centres is to establish baseline and widespread competence in colonoscopy, and the training and accreditation procedures for the national centre were developed by trial collaborator Dr Brian Saunders. Assessment criteria for the GI endoscopists participating in the SIGGAR study were based on the accreditation procedures in place at the national training centres.
Reporting
A report was issued in line with normal clinical practice and an additional CRF completed, recording maximal diameter (mm) and location of detected lesions, information on any biopsies taken, presence and site of diverticulosis, depth of intubation and details of any adverse events. Procedures were assessed as ‘very easy’, ‘quite easy’, ‘quite difficult’ or ‘very difficult’. The quality of the bowel preparation was rated as ‘excellent’, ‘good’, ‘adequate’ or ‘poor’. The presence of diverticulosis in each segment was graded as ‘none’, ‘mild’, ‘moderate’ or ‘severe’.
Computed tomographic colonography
Technical parameters
Computed tomographic colonography was performed in accordance with international guidelines for good practice,30,31 after full bowel preparation. Most centres used Picolax as the primary laxative, although one used macrogol (Klean-Prep®, Norgine Pharmaceuticals Ltd) and one used diatrizoate (Gastrografin®, Bayer Healthcare). In general, ‘dry’ preparations (e.g. Picolax) are better suited to CTC than wetter preparations, which are generally used for colonoscopy and can impair interpretation of CTC because lesions may be obscured by excess residual fluid. 32 Positive faecal tagging could be used if this was local practice or preferred. Examinations were performed following administration of intravenous spasmolytic, usually 20–40 mg of Buscopan, unless contraindicated. Insufflating gas could be either room air or carbon dioxide, according to local preference, and an automatic insufflator could be used. Intravenous contrast was administered at the discretion of the supervising radiologist, depending on local practice, their interpretation of the clinical circumstances of the patient and the probability that symptoms were due to extracolonic pathology.
Multidetector row machines were required, with a minimum capacity of four detector rows and individual slice collimation not exceeding 2.5 mm. A pitch that allowed abdominal coverage (40 cm) within a single breath hold (20 seconds at most) was used. Scans were usually obtained in two patient positions, normally prone and supine, but occasionally one of these plus lateral decubitus if the patient found it difficult to lie prone. In particularly frail patients, a single position could suffice if the supervising radiologist or CTC radiographer felt satisfied that distension was adequate to exclude a carcinoma.
Interpretation
In total, 46 radiologists registered by the Royal College of Radiologists and subspecialising in GI radiology interpreted CTC procedures in the trial. All were members of SIGGAR (now the British Society of Gastrointestinal and Abdominal Radiology), so as to reflect the type of radiologist likely to report CTC if implemented widely in UK practice (i.e. a subspecialist with an interest in GI imaging). All had prior experience of CTC, supplemented by a 2-day course for those who were judged to be relatively inexperienced (< 100 prior cases) and for more experienced radiologists who desired additional training (see Computed tomographic colonography training). The reading platform was determined by local preference but a minimum standard was primary analysis of the two-dimensional (2D) axial prone and supine CTC data sets, with volume-to-surface rendering for problem-solving. Visualisation software was provided [Voxar Colonscreen (Barco, Edinburgh, UK) and V3D (Viatronix, High Wycombe, UK)], but commercial alternatives were acceptable. Readers used 2D and 3D visualisation as required. Computer-assisted detection was also available (Medicsight PLC, London, UK). The primary focus was on identification/exclusion of significant colorectal neoplasia, defined as CRC or any polyp measuring ≥ 10 mm in maximal diameter, using electronic callipers on the 2D or 3D image, according to local preference.
Reporting
Radiologists issued a report and completed a CRF recording the same information as that for reporting BE examinations (see Barium Enema, Reporting). For CTC, the CRF included an additional section in which the radiologist could record details of extracolonic findings, including recommendations for any follow-up investigations that might be needed. Technical details of the scan were also recorded, including slice collimation (mm), number of detector rows, patient positioning (usually prone and supine), use of carbon dioxide or air for colon insufflation, use of mechanical insufflators, use of intravenous contrast or oral labelling, reading platform used and the proportion of 2D to 3D reading for interpretation.
Computed tomographic colonography training
Computed tomographic colonography is a new health technology with a steep learning curve. It is generally accepted that somewhere between 30 and 100 studies need to be analysed to achieve competence. In the UK, at the time recruitment began, relatively few radiologists possessed the requisite skills, although these skills are probably over-represented in the SIGGAR membership. ESGAR asked one of the present authors (SH) to formally assess the levels of training needed for competent CTC interpretation via a multicentre pan-European trial. 33 What is clear from the ESGAR study is that competence is variable and that some individuals can attain (and occasionally surpass) the median competence of very experienced readers after training on 50 endoscopically validated cases. Although many readers in the SIGGAR trial were very competent, it was clear from testing that others needed additional training, some of which was delivered by a 2-day course in June 2005 and some by one-to-one instruction at individual centres.
Recruitment
Suitable patients were identified from outpatient clinics and procedure waiting lists by the colorectal nurse specialist, who was responsible for checking the details of the referral and establishing that each patient met the trial entry criteria. Patients were then seen by the consulting physician, who assessed their need for a whole-colon examination and decided whether this should be a BE or colonoscopy. No patients could participate in the trial without the consultant’s consent. If consultant consent was given, the nurse specialist met the patient to explain the purpose of the study and describe the tests involved. Patient information sheets relating to the trial into which the patient was to be randomised (i.e. CTC vs. BE or CTC vs. colonoscopy) were given to the patient (see Appendix 2). All patients who wished to participate were given a consent form to complete. Patients recruited from outpatient departments were asked to complete the form in clinic, to ensure they could receive an appointment for their investigation on the day they were consented, and so would not be disadvantaged by having to return to the hospital to sign the form and arrange the procedure. For patients recruited from BE or colonoscopy waiting lists, the consent form and relevant patient information sheet were sent with an explanatory letter, inviting the patient to participate in the study. This was possible only if there was sufficient time for the consent form to be sent by post and returned, and (if necessary) for the patient’s procedure to be changed from a BE or colonoscopy to CTC, depending on the outcome of randomisation.
Once the consent form had been signed, the colorectal nurse specialist telephoned the trial office to randomise the patient. The patient’s name, sex, date of birth and the procedure for which they were originally referred (BE or colonoscopy) were recorded on the trial database and the randomised procedure was allocated and disclosed to the nurse specialist, who then booked the patient’s appointment. The trial office also sent an explanatory letter to the patient’s GP, informing them of the patient’s participation in the study.
Patients retained a copy of their consent form and the relevant patient information sheet and were given the telephone number of the trial office in case they had any further questions. They were informed that they could withdraw from the study at any time, in which case the consent form would be destroyed by the nurse specialist and the patient allocated their original test.
Data collection
Demographic and baseline clinical information was collected on all potentially eligible patients, including those ultimately not randomised (the term ‘registered patients’ will be used here to refer to all patients who were registered as eligible for the trial but not subsequently randomised). Data at initial recruitment were recorded on a specially designed CRF (see Appendix 1), recording information such as the patient’s sex and date of birth, symptoms at presentation, urgency of the referral, details of the outpatient clinic, investigation requested and whether or not sigmoidoscopy was performed.
Details of the main trial procedures – BE, colonoscopy and CTC – were also recorded on CRFs, as were flexible sigmoidoscopies, surgical procedures and details of any outpatient appointments (see Appendix 1). Copies of the relevant radiology and endoscopy reports were also requested, along with copies of any pathology reports relating to endoscopy or surgery. Data on any other relevant procedures such as abdominal CT or gastroscopy were obtained by requesting a copy of the hospital report. Copies of patients’ discharge letters were also requested, to assist in determining their final diagnosis from the trial.
All documentation was collected by the local colorectal nurse specialist and sent to the trial office by post or fax. Forms were entered on a bespoke Oracle database (Oracle UK, Reading, UK) and any missing fields queried with the centre, to make the data as complete as possible.
Follow-up
After the randomised procedure, patients were referred for additional tests as judged clinically necessary, taking account of the patient’s status and symptoms, findings from the randomised procedure, and local policy. If cancer was detected by CTC or BE, options included referral for endoscopy and subsequent histological confirmation, or direct referral for staging examinations and/or surgery if the diagnosis of cancer was felt to be certain (which occurs more often in the case of CTC, as it has the capability to visualise the extramural extent of the cancer or to detect metastases outside the colon). Patients with lesions ≥ 10 mm at radiology exams were usually referred to endoscopy for excision and histological diagnosis. The decision to refer patients with smaller lesions was left to the clinician in charge, taking account of the patient’s age, wishes, nature of symptoms and the overall quality of the radiological examination. Further colonic investigation (usually endoscopy) was also requested where diagnostic uncertainty persisted following the randomised procedure, either owing to poor visualisation of one or more segments of the bowel, or when no cause for the patient’s symptoms had been found. Extracolonic lesions detected by CTC were also investigated if considered significant by the clinician in charge and details of these additional procedures were obtained.
If cancer was histologically confirmed at colonoscopy, patients could be referred directly for staging examinations and surgery. However, in cases for which lesions could not be histologically confirmed,if the examination was incomplete or if there was persistent clinical uncertainty, patients might be referred for additional tests.
If any adverse events occurred during or shortly after the randomised procedure, they could be reported by radiologists or endoscopists on the relevant trial CRF, or by patients themselves on a questionnaire completed the following day. Details of unplanned hospitalisations and deaths within 30 days of the randomised procedure were also collected, using hospital records and the NHS Information Centre (NHSIC), respectively. These were reviewed independently by a gastroenterologist, a radiologist and a surgeon, who each gave their opinion on whether or not the hospitalisation could be attributed to the patient’s randomised procedure (reviewers were blinded to the procedure type).
In order to identify any cancers missed by the trial procedures (including extracolonic cancers), all patients in the study were identified on the NHS Central Register (NHSCR) and details of new cancer diagnoses and deaths were obtained from NHSIC. Patients were also matched with national data from Hospital Episode Statistics (HES) to reduce the time lag between cancer diagnosis and the time of notification. Cancers were confirmed using pathology and imaging reports obtained from the hospital where the cancer was diagnosed.
Outcomes
The primary outcome in the BE trial was the detection rate of CRC or large polyps (≥ 10 mm), confirmed histologically where possible. In a small number of cases, cancers were not confirmed histologically, for example because the presence of distant metastases was confirmed at CTC, or when staging scans showed the tumour to be unresectable. Secondary outcomes were referral rates for additional colonic investigation, time to diagnosis, miss rates for CRC, diagnoses of extracolonic cancer within 3 years, all-cause mortality and serious adverse events. Extracolonic findings at CTC were also analysed.
Evidence suggests that CTC and colonoscopy are similarly sensitive for the detection of cancer and polyps ≥ 10 mm,17 so a RCT powered on detection rates would be unfeasibly large (see Sample size). The primary outcome in the colonoscopy trial was therefore the proportion of patients undergoing additional colonic investigation following the randomised procedure, an important consideration if CTC is to become a suitable alternative to colonoscopy. Secondary outcomes were detection rates of CRC or large polyps, time to diagnosis, miss rates for CRC, other colorectal diagnoses, diagnoses of colonic and extracolonic cancer within 3 years, all-cause mortality and serious adverse events. Extracolonic findings at CTC were also analysed.
Sample size
In the BE trial, the sample size was initially calculated assuming a detection rate for cancers or large polyps of 15% for CTC and 10% for BE. With 2 : 1 randomisation in favour of BE, a sample size of 2160 gave 90% power to detect a significant difference in detection rates at 0.05 alpha (two-tailed). The 2 : 1 ratio was chosen so as not to overwhelm facilities for CTC and results in only a small loss of statistical power compared with the more usual 1 : 1 ratio.
In December 2005, an interim analysis performed for the external Data Monitoring Committee showed that the prevalence of significant neoplasia was lower than expected. It had been anticipated that this would differ between the two trials, as high-risk patients are more likely to be referred for colonoscopy (and, therefore, more likely to enter the colonoscopy than the BE trial). However, this difference was even larger than expected, with a prevalence of 12% in the colonoscopy trial and only 5% in the BE trial. The sample size, therefore, had to be increased and the power was dropped at the same time from 90% to the more conventional 80%. The revised calculation assumed detection rates for cancers and large polyps of 5% for BE and 7.5% for CTC. 34 With randomisation in a 2 : 1 ratio in favour of BE, a sample size of 3402 gave 80% power to detect a significant difference in detection rates at 0.05 alpha (two-tailed).
Powering the colonoscopy trial on detection rates would not have been practical. Assuming a detection rate of 15% for colonoscopy and a sensitivity of 93% for CTC relative to colonoscopy, with an inferiority margin equating to approximately 87% sensitivity, such a trial would require a total of 39,000 patients. The colonoscopy trial was, therefore, powered instead on the proportion of patients having additional colonic investigation following the randomised procedure. Assuming that symptomatic patients would need additional colonic tests in 20% of cases following CTC and 14% following colonoscopy, and with randomisation in a 2 : 1 ratio in favour of colonoscopy, a sample size of 2160 gave 90% power to detect a significant difference in referral rates at 0.05 alpha (two-tailed). However, as recruitment to the colonoscopy trial was proceeding at a lower rate than expected, it was decided to lower the power to 80% at the same time that this was done for the BE trial. Keeping all other assumptions as before, the new sample size required was 1430. The external Data Monitoring Committee approved all modifications.
Randomisation
The randomisation codes were generated by a programmer unconnected with the study and kept concealed until interventions were assigned. Randomisation was performed in blocks of six, stratified by centre, sex and diagnostic pathway (BE or colonoscopy). Details of the randomisation blocking, etc. were concealed from participating centres by excluding them from the study protocol for distribution.
Implementation
Once patients had agreed to take part in the study and signed the consent form, the colorectal nurse specialist contacted the trial office by telephone and gave the patient’s name, sex, date of birth and the procedure recommended by the clinician (BE or colonoscopy). These details were entered onto the trial database and the patient’s allocated test was then revealed.
Blinding
Given the nature of the interventions involved, there could be no blinding for either patients or medical staff.
Statistical methods
In the BE trial, both intention-to-treat and per-protocol analyses were performed for the primary outcome. Intention-to-treat analyses considered the 2527 and 1277 patients randomised to BE and CTC, respectively, excluding those who withdrew consent. Per-protocol analyses considered the 2300 and 1206 patients who had their randomised procedure (BE and CTC respectively). Secondary outcomes were analysed only on a per-protocol basis, except for extracolonic cancers and overall mortality, which were analysed by intention to treat.
In the colonoscopy trial, both intention-to-treat and per-protocol analyses were performed for the primary outcome, as well as for the secondary outcome of the detection rate of CRC or large polyps. All other secondary outcomes were analysed only on an intention-to-treat basis, except for CRC miss rates, adverse events and time to diagnosis, which were analysed by on a per-protocol basis. Intention-to-treat analyses considered the 1047 and 533 patients randomised to colonoscopy and CTC, respectively, excluding those who withdrew consent. Per-protocol analyses considered the 967 and 503 patients who had their randomised procedure (colonoscopy and CTC, respectively).
In patients who had FS prior to the randomised procedure, it was impossible to be certain whether or not the radiologist performing the randomised procedure was aware of the FS results. If any such lesions were seen again at the randomised procedure, we therefore had to assume that the radiologist was aware of the FS findings and these lesions were not counted as being detected at the randomised procedure. As a result, lesions found at sigmoidoscopy prior to randomisation were excluded from all analyses. Lesions found at FS between randomisation and the randomised procedure were included in the intention-to-treat analysis because these lesions were found in patients who were part of the randomised group, but were excluded from per-protocol analyses.
Detection rates were analysed on a per-patient basis, using the most advanced colonic lesion diagnosed. Lesions identified on successive procedures were matched based on size and location. The size measured at endoscopy was used as a reference standard unless it was unavailable or was exceeded by a measurement at pathology or surgery, in which case the latter was used. As in previous studies,15,16 lesions seen at randomised and subsequent procedures were considered to be the same if they were in the same or an adjacent colonic segment and the size was within 50% of the endoscopic measurement. For lesions not meeting these criteria, a consensus was reached by members of the research team.
When patients had a cancer or large polyp found during the trial, the date of diagnosis was defined as the date of the examination at which histological confirmation was first obtained (the date of first sighting on radiology was used for cancers that were not histologically confirmed). In the case of patients in whom no cancer or large polyp detected, the date of the final colonic examination was used (the date of the randomised procedure in patients with no subsequent referrals).
A CRC was defined as missed if it was identified through the NHSCR or the HES database within 36 months of randomisation but was not detected at the randomised procedure or mentioned in the patient’s final discharge letter.
Included extracolonic cancers were all reported primary malignant neoplasms, excluding CRCs [International Classification of Diseases and Related Health Problems, Tenth Edition (ICD-10) site codes C18–C20] and non-melanoma malignant neoplasms of the skin (C44), diagnosed within 36 months of randomisation. The expected number of extracolonic cancers was calculated by applying age- and sex-specific cancer incidence rates for the general population to our cohort, having adjusted for reported mortality. Incidence rates were compared assuming a Poisson distribution.
Comparisons of categorical outcomes were made using Pearson’s chi-squared test or Fisher’s exact test, as appropriate. Relative risks (RRs) or risk differences with 95% confidence intervals (CIs) were calculated to estimate differences between groups. RRs by age group (< 65 years and ≥ 65 years) and sex were illustrated using forest plots and tests of interaction (Mantel–Haenszel) were used to identify significant differences. Trial participants were randomised individually but we expected some natural clustering by centre. To check whether or not clustering by trial centre affected results, we also analysed the primary outcomes using random-effects logistic models allowing for heterogeneity in the outcome and intervention effects by centre (odds ratios were compared). All tests were two-tailed, with significance assigned at 5%. Analysis was performed using Stata 9.1 (StataCorp LP, College Station, TX, USA).
Chapter 3 Results
This chapter contains information reprinted with permission from Elsevier, The Lancet, 2013, vol. 381, Halligan S, Wooldrage K, Dadswell E, Kralj-Hans I, von Wagner C, Edwards R, et al. Computed tomographic colonography versus barium enema for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): a multicentre randomised trial, pp. 1185–93;35 and The Lancet, 2013, vol. 381, Atkin W, Dadswell E, Wooldrage K, Kralj-Hans I, von Wagner C, Edwards R, et al. Computed tomographic colonography versus colonoscopy for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): a multicentre randomised clinical trial, pp. 1194–202. 36
Patient recruitment and randomisation
Patient recruitment began in March 2004 and was completed in December 2007, by which time the number of patients randomised in each trial had exceeded the target sample size. In total, 8484 patients were registered to the trial from 21 centres. Table 1 shows registration and randomisation by centre (centres ordered by date of joining the trial). The proportion of registered patients varied significantly by centre and may have been due to inadequate reporting of all eligible patients at some centres. In addition, centres relying heavily on recruitment from procedure waiting lists may have had lower rates of registration as they were only identifying patients who had already been referred for a whole-colon examination.
| Centre | Total registered | Registered only, excluded from randomisation | Randomised | Randomised within the BE vs. CTC trial | Randomised within the colonoscopy vs. CTC trial | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % of centre total | n | % of centre total | n | % of centre total | n | % of centre total | |
| St Mark’s | 1721 | 20 | 1012 | 59 | 709 | 41 | 216 | 13 | 493 | 29 |
| Birmingham | 441 | 5 | 25 | 6 | 416 | 94 | 310 | 70 | 106 | 24 |
| Bradford | 766 | 9 | 217 | 28 | 549 | 72 | 433 | 57 | 116 | 15 |
| Oldham | 454 | 5 | 270 | 59 | 184 | 41 | 151 | 33 | 33 | 7 |
| Portsmouth | 882 | 10 | 316 | 36 | 566 | 64 | 524 | 59 | 42 | 5 |
| Cornwall (Truro) | 536 | 6 | 73 | 14 | 463 | 86 | 366 | 68 | 97 | 18 |
| Lancaster | 451 | 5 | 84 | 19 | 367 | 81 | 358 | 79 | 9 | 2 |
| Nottingham City Hospital | 251 | 3 | 138 | 55 | 113 | 45 | 95 | 38 | 18 | 7 |
| Bath | 477 | 6 | 38 | 8 | 439 | 92 | 344 | 72 | 95 | 20 |
| Nottingham Queen’s Medical Centre | 410 | 5 | 186 | 45 | 224 | 55 | 210 | 51 | 14 | 3 |
| Crewe | 655 | 8 | 224 | 34 | 431 | 66 | 413 | 63 | 18 | 3 |
| Charing Cross | 256 | 3 | 115 | 45 | 141 | 55 | 5 | 2 | 136 | 53 |
| Plymouth | 250 | 3 | 16 | 6 | 234 | 94 | 33 | 13 | 201 | 80 |
| Hammersmith | 47 | 0.6 | 3 | 6 | 44 | 94 | 0 | 0 | 44 | 94 |
| Withington | 179 | 2 | 49 | 27 | 130 | 73 | 119 | 66 | 11 | 6 |
| Wythenshawe | 32 | 0.4 | 12 | 37 | 20 | 63 | 20 | 63 | 0 | 0 |
| Furness | 112 | 1 | 24 | 21 | 88 | 79 | 73 | 65 | 15 | 13 |
| Frimley Park | 59 | 0.7 | 18 | 31 | 41 | 69 | 17 | 29 | 24 | 41 |
| Oxford | 162 | 2 | 13 | 8 | 149 | 92 | 147 | 91 | 2 | 1 |
| Paddington (St Mary’s) | 271 | 3 | 164 | 61 | 107 | 39 | 0 | 0 | 107 | 39 |
| North Tees | 72 | 0.8 | 39 | 54 | 33 | 46 | 4 | 6 | 29 | 40 |
| Total | 8484 | 100 | 3036 | 36 | 5448 | 64 | 3838 | 45 | 1610 | 19 |
Baseline patient characteristics
Of the 8484 patients considered potentially eligible for the trial, 3036 were ultimately not randomised. Reasons why these patients were not included are given in Table 2. In most cases (72%), it was the clinician in charge of the patient’s care who made the decision not to enter the patient into the trial, usually because the clinician felt that a specific examination was needed and, therefore, could not allow the patient to be randomised. A smaller group of patients (7% of those excluded) met the eligibility criteria but were judged by the clinician to be unfit for a whole-colon examination. In 27% of cases it was the patient who declined consent, usually because they wanted to have a specific procedure, for example because they felt colonoscopy was too invasive or they were attracted by the possibility of extracolonic imaging at CTC.
| Reason | n | % |
|---|---|---|
| Clinician reasons for declining consent | ||
| Colorectal or other cancer already diagnosed | ||
| CRC diagnosed | 56 | 1.8 |
| Other cancer diagnosed | 69 | 2.3 |
| Specific procedure requested | ||
| Colonoscopy | 731 | 24.1 |
| CT | 303 | 10.0 |
| FS | 230 | 7.6 |
| Oesophagogastroduodenoscopy | 218 | 7.2 |
| BE | 19 | 0.6 |
| Ultrasonography | 16 | 0.5 |
| Magnetic resonance imaging | 5 | 0.2 |
| Unknown | 39 | 1.3 |
| Clinical situation too urgent or waiting list too long | 52 | 1.7 |
| Patient unfit for whole-colon examination | 215 | 7.1 |
| Patient unable to give informed consent | 75 | 2.5 |
| No reason given | 148 | 4.9 |
| Total where clinician declined consent | 2176 | 71.7 |
| Patient reasons for declining consent | ||
| Patient wanted a specific procedure | ||
| Colonoscopy | 15 | 0.5 |
| CT | 3 | 0.1 |
| BE | 2 | 0.07 |
| Unknown | 128 | 4.2 |
| Patient did not want a specific procedure | ||
| CT as claustrophobic | 13 | 0.4 |
| CT for other reasons | 2 | 0.07 |
| Colonoscopy | 1 | 0.03 |
| BE | 1 | 0.03 |
| Patient had difficulty comprehending | 84 | 2.8 |
| Patient died before consent obtained | 2 | 0.07 |
| No reason given | 583 | 19.2 |
| Total where patient declined consent | 834 | 27.5 |
| Reason for exclusion unknown | 26 | 0.9 |
| Total excluded | 3036 | 100.0 |
Table 3 shows the baseline characteristics of patients in the BE trial compared with those patients who were not randomised in either trial. The proportion of women in the BE trial was significantly higher than in the non-randomised group. There was a significant difference in the age profile of the two groups, with patients in the BE trial being younger overall. There were also significant differences in all symptoms between the two groups, with patients in the BE trial more likely to present with a change in bowel habit or abdominal pain and less likely to present with rectal bleeding, anaemia or weight loss. The BE trial contained a significantly lower proportion of 2-week-wait patients than the non-randomised group.
| Randomised within BE vs. CTC trial (n = 3838) | Excluded from both BE vs. CTC trial and colonoscopy vs. CTC trial (n = 3036) | p-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | |||||
| Male | 1483 | 39 | 1251 | 41 | 0.031 |
| Female | 2355 | 61 | 1785 | 59 | |
| Age (years) | |||||
| 55–64 | 1253 | 33 | 802 | 26 | < 0.001 |
| 65–74 | 1498 | 39 | 1045 | 34 | |
| 75–84 | 980 | 26 | 930 | 31 | |
| 85 + | 107 | 3 | 259 | 9 | |
| Symptoms | |||||
| Change in bowel habit | 2909 | 76 | 1926 | 63 | < 0.001 |
| Harder, less frequent | 490 | 13 | 297 | 10 | |
| Looser, more frequent | 1557 | 41 | 1049 | 35 | |
| Variable frequency | 355 | 9 | 180 | 6 | |
| Unspecified | 507 | 13 | 400 | 13 | |
| Rectal bleeding | 1167 | 30 | 1169 | 39 | < 0.001 |
| Abdominal pain | 1235 | 32 | 574 | 19 | < 0.001 |
| Anaemia | 476 | 12 | 620 | 20 | < 0.001 |
| Weight loss | 523 | 14 | 500 | 16 | 0.001 |
| Other symptoms | 431 | 11 | 585 | 19 | < 0.001 |
| Route of referral | |||||
| Outpatient clinic | < 0.001 | ||||
| Colorectal surgical clinic | 2964 | 77 | 2733 | 90 | |
| Gastroenterology | 462 | 12 | 192 | 6 | |
| Geriatrics | 5 | 0.1 | 6 | 0.2 | |
| Other clinic type | 59 | 2 | 46 | 2 | |
| Unknown clinic type | 9 | 0.2 | 4 | 0.1 | |
| GP | 237 | 6 | 32 | 1 | |
| Other | 93 | 2 | 21 | 0.7 | |
| Unknown | 9 | 0.2 | 2 | 0.1 | |
| Urgency of referral | |||||
| 2-week wait | 1682 | 44 | 1851 | 61 | < 0.001 |
| Urgent | 649 | 17 | 511 | 17 | |
| Soon | 484 | 13 | 179 | 6 | |
| Routine | 686 | 18 | 315 | 10 | |
| Not recorded | 337 | 9 | 180 | 6 | |
A comparison of the baseline characteristics of patients in the colonoscopy trial and those who were not randomised is given in Table 4. The colonoscopy trial had a significantly lower proportion of female patients than the non-randomised group. There was also a significant difference in the age of patients, with those in the colonoscopy trial being younger overall. The colonoscopy trial contained a higher proportion of patients presenting with a change in bowel habit, rectal bleeding, or abdominal pain and a lower proportion with anaemia. There was no significant difference in the proportion of patients with weight loss as one of the presenting symptoms.
| Randomised within colonoscopy vs. CTC trial (n = 1610) | Excluded from both BE vs. CTC trial and colonoscopy vs. CTC trial (n = 3036) | p-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | |||||
| Male | 729 | 45 | 1251 | 41 | 0.0075 |
| Female | 881 | 55 | 1785 | 59 | |
| Age (years) | |||||
| 55–64 | 609 | 38 | 802 | 26 | <0.001 |
| 65–74 | 576 | 36 | 1045 | 34 | |
| 75–84 | 372 | 23 | 930 | 31 | |
| 85 + | 53 | 3 | 259 | 9 | |
| Symptoms | |||||
| Change in bowel habit | 1175 | 73 | 1926 | 63 | <0.001 |
| Harder, less frequent | 194 | 12 | 297 | 10 | |
| Looser, more frequent | 635 | 39 | 1049 | 35 | |
| Variable frequency | 182 | 11 | 180 | 6 | |
| Unspecified | 164 | 10 | 400 | 13 | |
| Rectal bleeding | 686 | 43 | 1169 | 39 | 0.0066 |
| Abdominal pain | 357 | 22 | 574 | 19 | 0.0081 |
| Anaemia | 208 | 13 | 620 | 20 | <0.001 |
| Weight loss | 240 | 15 | 500 | 16 | 0.17 |
| Other symptoms | 280 | 17 | 585 | 19 | 0.12 |
| Route of referral | |||||
| Outpatient clinic | <0.001 | ||||
| Colorectal surgical clinic | 1401 | 87 | 2733 | 90 | |
| Gastroenterology | 106 | 7 | 192 | 6 | |
| Geriatrics | 0 | 0 | 6 | 0.2 | |
| Other clinic type | 12 | 0.7 | 46 | 2 | |
| Unknown clinic type | 0 | 0 | 4 | 0.1 | |
| GP | 62 | 4 | 32 | 1 | |
| Other | 29 | 2 | 21 | 0.7 | |
| Unknown | 0 | 0 | 2 | 0.1 | |
| Urgency of referral | |||||
| 2-week wait | 963 | 60 | 1851 | 61 | <0.001 |
| Urgent | 333 | 21 | 511 | 17 | |
| Soon | 94 | 6 | 179 | 6 | |
| Routine | 105 | 7 | 315 | 10 | |
| Not recorded | 115 | 7 | 180 | 6 | |
Comparing patients randomised in the BE trial with those randomised in the colonoscopy trial (Table 5), patients in the BE trial were older and more likely to be female. They were less likely to present with rectal bleeding and more likely to present with abdominal pain and change in bowel habit. There was no significant difference between the two trials in the proportion of patients presenting with anaemia or weight loss.
| Randomised within BE vs. CTC trial (n = 3838) | Randomised within colonoscopy vs. CTC trial (n = 1610) | p-value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | |||||
| Male | 1483 | 39 | 729 | 45 | < 0.001 |
| Female | 2355 | 61 | 881 | 55 | |
| Age (years) | |||||
| 55–64 | 1253 | 33 | 609 | 38 | 0.001 |
| 65–74 | 1498 | 39 | 576 | 36 | |
| 75–84 | 980 | 26 | 372 | 23 | |
| 85 + | 107 | 3 | 53 | 3 | |
| Symptoms | |||||
| Change in bowel habit | 2909 | 76 | 1175 | 73 | 0.029 |
| Harder, less frequent | 490 | 13 | 194 | 12 | |
| Looser, more frequent | 1557 | 41 | 635 | 39 | |
| Variable frequency | 355 | 9 | 182 | 11 | |
| Unspecified | 507 | 13 | 164 | 10 | |
| Rectal bleeding | 1167 | 30 | 686 | 43 | < 0.001 |
| Abdominal pain | 1235 | 32 | 357 | 22 | < 0.001 |
| Anaemia | 476 | 12 | 208 | 13 | 0.60 |
| Weight loss | 523 | 14 | 240 | 15 | 0.21 |
| Other symptoms | 431 | 11 | 280 | 17 | < 0.001 |
| Route of referral | |||||
| Outpatient clinic | < 0.001 | ||||
| Colorectal surgical clinic | 2964 | 77 | 1401 | 87 | |
| Gastroenterology | 462 | 12 | 106 | 7 | |
| Geriatrics | 5 | 0.1 | 0 | 0 | |
| Other clinic type | 59 | 2 | 12 | 0.7 | |
| Unknown clinic type | 9 | 0.2 | 0 | 0 | |
| GP | 237 | 6 | 62 | 4 | |
| Other | 93 | 2 | 29 | 2 | |
| Unknown | 9 | 0.2 | 0 | 0 | |
| Urgency of referral | |||||
| 2-week wait | 1682 | 44 | 963 | 60 | < 0.001 |
| Urgent | 649 | 17 | 333 | 21 | |
| Soon | 484 | 13 | 94 | 6 | |
| Routine | 686 | 18 | 105 | 7 | |
| Not recorded | 337 | 9 | 115 | 7 | |
Flexible sigmoidoscopies
Among randomised patients, the proportion of patients having FS before recruitment differed in the two groups, with the highest rate in the BE trial [25.1% (963/3838) vs. 9.1% (147/1610) for the colonoscopy trial; p < 0.0001]. This was probably as a result of variations in the use of FS between centres because the proportion of patients from each centre varied substantially between the two trials, as shown in Table 1.
Barium enema trial
Numbers analysed
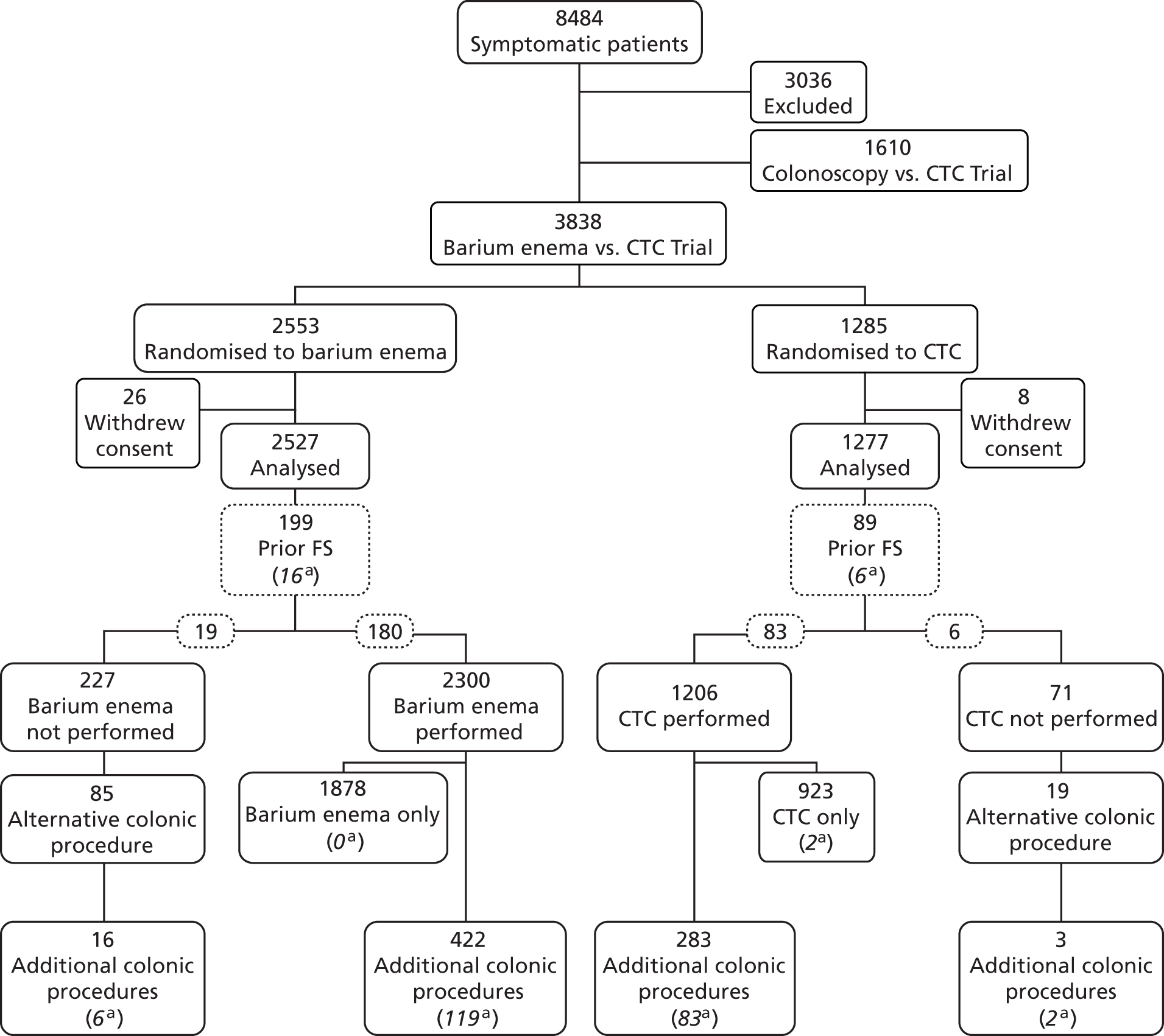
As seen in Figure 2, a total of 5448 patients were randomised in the study, with 1610 entering the colonoscopy trial and 3838 entering the BE trial, who were randomised between BE (n = 2553) and CTC (n = 1285). Thirty-four patients withdrew consent [26 (1%) in the BE and eight (1%) in the CTC arm], leaving 3804 for analysis (2527 BE and 1277 CTC). The proportion of patients withdrawing consent did not differ significantly between the two procedures.
FIGURE 2.
Participants’ progress through the BE vs. CTC trial and selected outcomes. a, Number of patients with cancers or large polyps diagnosed at that procedure.
Centres varied in the number of patients randomised to the BE trial [median 151, interquartile range (IQR) 33–358]. There were no significant differences in the demographic or clinical characteristics of patients randomised to BE or CTC (Table 6) (this is also true if patients who withdrew consent are included). The median age of patients was 69 years (IQR 62–75 years), and 61% were women. The most frequent presenting symptoms were change in bowel habit (76%), abdominal pain (32%) and rectal bleeding (30%); patients could report more than one symptom.
| Characteristic | Randomised to BE (n = 2527) | Randomised to CTC (n = 1277) | Total (n = 3804) | Excluded (n = 3036) | p-valuea | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Sex | |||||||||
| Male | 983 | 39 | 490 | 38 | 1473 | 39 | 1251 | 41 | 0.0371 |
| Female | 1544 | 61 | 787 | 62 | 23311 | 61 | 1785 | 59 | |
| Age (years) | |||||||||
| 55–64 | 826 | 33 | 416 | 33 | 1242 | 33 | 802 | 26 | < 0.0001 |
| 65–74 | 993 | 39 | 494 | 39 | 1487 | 39 | 1045 | 34 | |
| 75–84 | 640 | 25 | 330 | 26 | 970 | 25 | 930 | 31 | |
| 85 + | 68 | 3 | 37 | 3 | 105 | 3 | 259 | 9 | |
| Symptomsb | |||||||||
| Change in bowel habit | 1910 | 76 | 975 | 76 | 2885 | 76 | 1926 | 63 | < 0.0001 |
| Harder, less frequent | 321 | 13 | 166 | 13 | 490 | 13 | 297 | 10 | |
| Looser, more frequent | 1007 | 40 | 535 | 42 | 1557 | 41 | 1049 | 35 | |
| Variable frequency | 240 | 9 | 113 | 9 | 355 | 9 | 180 | 6 | |
| Unspecified | 342 | 14 | 161 | 13 | 507 | 13 | 400 | 13 | |
| Rectal bleeding | 767 | 30 | 388 | 30 | 1155 | 30 | 1169 | 39 | < 0.0001 |
| Abdominal pain | 819 | 32 | 406 | 32 | 1225 | 32 | 574 | 19 | < 0.0001 |
| Anaemia | 319 | 13 | 153 | 12 | 472 | 12 | 620 | 20 | < 0.0001 |
| Weight loss | 331 | 13 | 185 | 14 | 516 | 14 | 500 | 16 | 0.0008 |
| Other symptoms | 289 | 11 | 138 | 11 | 427 | 11 | 585 | 19 | < 0.0001 |
The number of patients who went on to have their randomised procedure is shown in Table 7, along with reasons why the procedure did not occur. A lower proportion of patients randomised to BE had their randomised procedure [91.0% (2300/2527) for BE vs. 94.4% (1206/1277) for CTC; p = 0.0002]. The main reason for this is that a larger proportion of patients were judged by the clinician to be unable to tolerate the examination (2.2%), which was rare for CTC (0.3%). There were also a substantial proportion of patients who did not want to have their randomised procedure, but this did not differ significantly between groups (3.2% for BE vs. 2.5% for CTC). Of those patients randomised to BE who did not have their assigned procedure, 37% (85/227) had an alternative procedure, usually CTC (62%, 53/85) or colonoscopy (21%, 18/85). Of those randomised to CTC who did not have their procedure, 27% (19/71) had an alternative examination usually BE (74%, 14/19) or colonoscopy (11%, 2/19).
| Status | BE (n = 2527) | CTC (n = 1277) | p-value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Occurrence of randomised procedure | |||||
| Occurred | 2300 | 91.0 | 1206 | 94.4 | 0.0002 |
| Did not occur | 227 | 9.0 | 71 | 5.6 | |
| Reasons did not occur | |||||
| Patient’s decision | |||||
| Patient refused randomised procedure | 81 | 3.2 | 32 | 2.5 | 0.27 |
| Patient did not attend scheduled procedure | 27 | 1.1 | 13 | 1.0 | |
| Total | 108 | 4.3 | 45 | 3.5 | |
| Medical decision | |||||
| Patient unable to tolerate randomised procedure | 55 | 2.2 | 4 | 0.3 | < 0.0001 |
| Finding at prior FS | 10 | 0.4 | 2 | 0.2 | |
| Consultant requested alternative procedure | 29 | 1.0 | 11 | 0.9 | |
| Patient became too ill | 16 | 0.6 | 1 | 0.1 | |
| Patient’s symptoms resolved | 4 | 0.2 | 2 | 0.2 | |
| Patient died | 4 | 0.2 | 3 | 0.2 | |
| Total | 118 | 4.7 | 23 | 1.8 | |
| Other reasons | |||||
| Equipment failure | 1 | 0.03 | 3 | 0.2 | 0.11 |
| Total | 1 | 0.03 | 3 | 0.2 | |
Prior FS was performed in 199 patients (7.9%) in the BE arm and 89 (7.0%) in the CTC arm (p = 0.32) (see Figure 2). The performance of prior FS did not affect the occurrence of the randomised procedure (91.3% in those who had prior FS, vs. 92.2% in those who did not; p = 0.58).
Performance of the examinations
A greater proportion of BE examinations were judged to be difficult to perform, with 24.1% (554/2300) rated as ‘quite’ or ‘very’ difficult, compared with 9.0% (109/1206) for CTC (p < 0.0001). Similarly, in a significantly higher proportion of BE examinations there was at least one segment that was not seen or for which the radiologist rated visualisation as ‘poor’: 22.3% (514/2300) for BE and 16.1% (194/1206) for CTC (p < 0.0001). In the left colon, there was no significant difference in the quality of visualisation between BE and CTC. However, poor visualisation in the right colon was more than twice as likely to be reported at BE (12.1%) as at CTC (4.9%) (p < 0.0001).
Outcomes
Detection of colorectal cancer and large polyps
Among 2527 patients randomised to BE, a CRC or large polyp was diagnosed in 141: in 119 following BE, in 16 at FS prior to BE and in six patients who had an alternative procedure (see Figure 2). Among 1277 patients randomised to CTC, a CRC or large polyp was diagnosed in 93: in 85 following CTC, in six at prior FS and in two patients having an alternative whole-colon investigation. The 141 lesions diagnosed in patients randomised to BE included 86 CRCs (including one carcinoid tumour and five non-pathologically confirmed cancers) and 55 large polyps (51 adenomas, two hyperplastic polyps, one juvenile polyp and a polyp ≥ 10 mm which was excised but not retrieved). The 93 lesions diagnosed in patients randomised to CTC included 47 CRCs (including two non-pathologically confirmed cancers) and 46 large polyps (41 adenomas, one hyperplastic polyp, one serrated adenoma and three polyps ≥ 10 mm which were excised but not retrieved). Analysing by intention to treat, the overall detection rate of CRC or large polyps was 7.3% (93/1277) in the CTC arm and 5.6% (141/2527) in the BE arm (p = 0.0390) (Table 8). The difference was mainly as a result of a higher detection rate of large polyps in the CTC arm (3.6% vs. 2.2%; p = 0.0098). There was no significant difference in detection rates of CRC (3.7% vs.3.4%; p = 0.66). Analysing per protocol, a cancer or large polyp was diagnosed in 7.0% (85/1206) of patients having CTC and 5.2% (119/2300) having a BE (p = 0.0243).
| Analysis | BE group | CTC group | Comparison of detection rates between procedures (CTC vs. BE) | ||||
|---|---|---|---|---|---|---|---|
| Intention to treat | N = 2527 | N = 1277 | |||||
| n | % | n | % | RR | 95% CI | p-value | |
| CRC | 86b | 3.4 | 47c | 3.7 | 1.08 | 0.76 to 1.53 | 0.6600 |
| ≥ 10-mm polypd | 55e | 2.2 | 46f | 3.6 | 1.66 | 1.13 to 2.43 | 0.0098 |
| CRC or ≥ 10-mm polypd | 141b,e | 5.6 | 93c,f | 7.3 | 1.31 | 1.01 to 1.68 | 0.0390 |
| Per protocol | N = 2300 | N = 1206 | |||||
| n | % | n | % | ||||
| CRC | 73b | 3.2 | 42c | 3.5 | 1.10 | 0.76 to 1.59 | 0.6300 |
| ≥ 10-mm polypd | 46e | 2.0 | 43f | 3.6 | 1.78 | 1.18 to 2.69 | 0.0051 |
| CRC or ≥ 10-mm polypd | 119b,e | 5.2 | 85c,f | 7.0 | 1.36 | 1.04 to 1.78 | 0.0243 |
The difference in detection rates between BE and CTC is probably due to the greater sensitivity of CTC for small lesions. BE and CTC have similar sensitivity for cancer, but most cancers in the trial were larger than 30 mm in size (Table 9). CTC was significantly better at detecting small lesions; the proportion of patients for whom the largest confirmed lesion was < 10 mm was 2.5% (58/2300) for BE and 4.3% for CTC (52/1206) (p = 0.0039) (see Table 9).
| Type of lesion and size | BE (N = 2300) | CTC (N = 1206) | ||
|---|---|---|---|---|
| n | % | n | % | |
| CRCs | ||||
| ≥ 30 mm | 62 | 2.7 | 33 | 2.7 |
| 20–29 mm | 9 | 0.4 | 6 | 0.5 |
| 15–19 mm | 1 | 0.04 | 1 | 0.08 |
| 10–14 mm | 1 | 0.04 | 2 | 0.2 |
| Total | 73 | 3.2 | 42 | 3.5 |
| Large polyps | ||||
| ≥ 30 mm | 7 | 0.3 | 7 | 0.6 |
| 20–29 mm | 5 | 0.2 | 6 | 0.5 |
| 15–19 mm | 13 | 0.6 | 9 | 0.7 |
| 10–14 mm | 21 | 0.9 | 21 | 1.7 |
| Total | 46 | 2.0 | 43 | 3.6 |
| Small polyps | ||||
| 6–9 mm | 16 | 0.7 | 15 | 1.2 |
| ≤ 5 mm | 42 | 1.8 | 37 | 3.1 |
| Total | 58 | 2.5 | 52 | 4.3 |
| No polyps or cancers detected | 2123 | 92.3 | 1069 | 88.6 |
Comparing results from models ignoring clustering to those controlling for clustering by trial centre showed that odds ratios were very similar in size and significance (Table 10).
| Analysis | No clustering | With clusteringa | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p-value | Odds ratio | 95% CI | p-value | |
| Intention-to-treat analysis | 1.33 | 1.01 to 1.74 | 0.0390 | 1.44 | 1.04 to 2.01 | 0.0302 |
| Per-protocol analysis | 1.39 | 1.04 to 1.85 | 0.0243 | 1.47 | 1.01 to 2.14 | 0.0455 |
There was a significant difference by age in the relative detection rate following CTC compared with BE (p = 0.0159); in younger patients, the detection rate following CTC was double that for BE, whereas in older patients the RR did not differ from that found overall (age < 65 years: RR 2.32, 95% CI 1.36 to 3.94; age ≥ 65 years: RR 1.10, 95% CI 0.82 to 1.47) (Figure 3). There was no significant difference in relative detection rates between men and women (p = 0.66).
FIGURE 3.
Relative risks for CRC or large polyps (CTC vs. BE) by sex and age group in the BE vs. CTC trial.

Further colonic investigation
The proportion of patients undergoing a second colonic investigation was significantly higher in the CTC group [23.5% (283/1206)] than in the BE group [18.3% (422/2300); p = 0.0003] (Table 11 and see Figure 2). This was true whether the referral was for a suspected cancer or large polyp (11.0% vs. 7.5%; p = 0.0005) or for suspected smaller polyps (7.2% vs. 2.3%; p < 0.0001). Conversely, a lower proportion required further investigation because of an inadequate examination or clinical uncertainty (5.2% vs. 8.5%; p = 0.0005).
| Reason for referral | BE performed (N = 2300) | CTC performed (N = 1206) | Comparison of referral rates between procedures (CTC vs. BE) | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | RR | 95% CI | p-value | |
| Cancer or large (≥ 10 mm) polyp suspected | |||||||
| Cancer | 86 | 3.7 | 68 | 5.6 | 0.0005 | ||
| Polyp ≥ 10 mm | 87 | 3.8 | 65 | 5.4 | |||
| Total with cancer or large (≥ 10 mm) polyp suspected | 173 | 7.5 | 133 | 11.0 | 1.47 | 1.18 to 1.82 | |
| Smaller polyp suspected | |||||||
| Polyp 8–9 mm | 18 | 0.8 | 18 | 1.5 | < 0.0001 | ||
| Polyp 6–7 mm | 12 | 0.5 | 34 | 2.8 | |||
| Polyp ≤ 5 mm | 24 | 1.0 | 35 | 2.9 | |||
| Total with smaller polyp suspected | 54 | 2.3 | 87 | 7.2 | 3.07 | 2.20 to 4.28 | |
| Clinical uncertainty (no polyps seen) | |||||||
| Inadequate examination | 116 | 5.0 | 34 | 2.8 | 0.56 | 0.38 to 0.81 | 0.0020 |
| Adequate examination | 79 | 3.4 | 29 | 2.4 | |||
| Total with clinical uncertainty | 195 | 8.5 | 63 | 5.2 | 0.62 | 0.47 to 0.81 | 0.0005 |
| Total having second procedure | 422 | 18.3 | 283 | 23.5 | 1.28 | 1.12 to 1.46 | 0.0003 |
Of the 422 patients referred following BE, 368 (87%) had colonoscopy (complete or limited as appropriate), 29 had a radiological procedure and 25 were referred straight to surgery. Of the 283 patients referred following CTC, 259 (91%) had colonoscopy, six had a radiological procedure and 18 were referred straight to surgery.
The probability of diagnosing a cancer or large polyp at a subsequent procedure (positive predictive value) was similar for patients referred because of findings at CTC or BE (29% vs. 28%, respectively) (Table 12). Among patients referred because of a suspected cancer or large polyp, the proportion in whom the lesion was confirmed was also similar for CTC and BE (56% vs. 62%). Of those referred because of smaller lesions at the randomised procedure, important end points were diagnosed in 10% referred after CTC (9/87; one cancer, eight polyps ≥ 10 mm) and 7% referred after BE (4/54; one cancer, three polyps ≥ 10 mm). Of 195 patients who had a second procedure because of clinical uncertainty after BE, four were found to have cancers and four had large polyps. No cancers or large polyps were detected in the 63 patients referred because of clinical uncertainty after CTC. Among patients not referred for an additional procedure, there remained a number of patients with incomplete or inadequate examinations: in BE 18.2% (341/1878) and in CTC 13.2% (122/923).
| Randomised procedure undertaken | BE | CTC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Second colonic procedure undertaken | Lesions diagnosed at second procedure | Second colonic procedure undertaken | Lesions diagnosed at second procedure | |||||||||||
| CRC | Polyp ≥ 10 mmb | Cancer or polyps ≥ 10 mmb | CRC | Polyp ≥ 10 mmb | Cancer or polyps ≥ 10 mmb | |||||||||
| Reason for referral | N | n | % | n | % | n | % | N | n | % | n | % | n | % |
| Cancer or large (≥ 10 mm) polyp suspected | ||||||||||||||
| Cancer | 86 | 64c | 74 | 7 | 8 | 71 | 83 | 68 | 36 | 53 | 2 | 3 | 38 | 56 |
| Polyp ≥ 10 mm | 87 | 4 | 5 | 32 | 37 | 36 | 41 | 65 | 3 | 5 | 33 | 51 | 36 | 55 |
| Total with cancer or large (≥ 10 mm) polyp suspected | 173 | 68c | 39 | 39 | 23 | 107 | 62 | 133 | 39 | 29 | 35 | 26 | 74 | 56 |
| Smaller polyp suspected | ||||||||||||||
| Polyp 8–9 mm | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 1 | 6 | 5 | 28 | 6 | 33 |
| Polyp 6–7 mm | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 34 | 0 | 0 | 2 | 6 | 2 | 6 |
| Polyp ≤ 5 mm | 24 | 1 | 4 | 3 | 13 | 4 | 17 | 35 | 0 | 0 | 1 | 3 | 1 | 3 |
| Total with smaller polyp suspected | 54 | 1 | 2 | 3 | 6 | 4 | 7 | 87 | 1 | 1 | 8 | 9 | 9 | 10 |
| Clinical uncertainty (no polyps seen) | ||||||||||||||
| Inadequate examination | 116 | 4 | 3 | 2 | 2 | 6 | 5 | 34 | 0 | 0 | 0 | 0 | 0 | 0 |
| Adequate examination | 79 | 0 | 0 | 2 | 3 | 2 | 3 | 29 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total with clinical uncertainty | 195 | 4 | 2 | 4 | 2 | 8 | 4 | 63 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total having second procedure | 422 | 73c | 17 | 46 | 11 | 119 | 28 | 283 | 40 | 14 | 43 | 15 | 83 | 29 |
Time to diagnosis
Among patients who had their randomised procedure, the median time from the date of randomisation to the date of diagnosis was 23 days (IQR 14–40 days); the time was significantly longer for patients having CTC (n = 1206, median 28 days, IQR 16–47 days vs. BE n = 2300, median 21 days, IQR 14–37 days; p < 0.0001). The time to the randomised procedure was slightly longer for CTC (median 22 days, IQR 15–34 days vs. BE: median 18 days, IQR 12–28 days; p < 0.0001), but for subjects who had an additional referral after the randomised procedure there was no difference between procedures in the time from the randomised procedure to the second procedure [CTC (n = 283 patients) median 50 days, IQR 28–94 days vs. BE (n = 422 patients) median 49.5 days, IQR 27–94 days; p = 0.84]. For patients who had a diagnosis of cancer or a large polyp, the time to diagnosis for those having CTC was significantly longer [CTC (n = 93 patients) median 64 days, IQR 38–99 days vs. BE (n = 141 patients) median 47 days, IQR 30–72 days; p = 0.0045].
Extracolonic findings following computed tomographic colonography
At least one previously unknown extracolonic finding was reported in 58% (673/1161) of patients having CTC who did not have CRC diagnosed during the trial (patients with CRC were excluded from the analysis because it is not always possible to be sure that extracolonic findings are unrelated to the CRC and the presence of CRC will also influence the rate of subsequent investigation for extracolonic findings that are considered less important). Eighty-seven of these (7.5%) were referred for additional procedures, leading to diagnosis of extracolonic malignancy in 13 patients (Table 13). A more detailed analysis of extracolonic findings is given in Chapter 4.
| CTC (n = 1277) | BE (n = 2527) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Month of diagnosis | Found by CTC | Total | Month of diagnosis | Found by BE | |||||
| 1–12 | 13–24 | 25–36 | 1–12 | 13–24 | 25–36 | |||||
| All extracolonic cancersa | 78 | 28 | 31 | 19 | 11b | 131c | 66 | 37 | 28 | 4 |
| Person-years of follow-upd | 3663 | 1259 | 1219 | 1185 | 7275 | 2489 | 2424 | 2362 | ||
| Incidence (per 1000 person-years) | 21.3 | 22.2 | 25.4 | 16.0 | 18.0 | 26.5 | 15.3 | 11.9 | ||
| Cancer type (ICD-10) | ||||||||||
| Stomach (C16) | 2 | 2 | 10 | 5 | 4 | 1 | ||||
| Small intestine (C17) | 0 | 3 | 2 | 1 | ||||||
| Hepatobiliary system (C22, C24) | 2 | 2 | 1 | 3 | 2 | 1 | ||||
| Pancreas (C25) | 5 | 3 | 2 | 2 | 9 | 7 | 1 | 1 | ||
| Digestive organs, other and ill defined (C26) | 0 | 1 | 1 | |||||||
| Bronchus and lung (C34) | 16 | 5 | 5 | 6 | 1 | 23 | 9 | 9 | 5 | |
| Mesothelial and soft tissue (C45, C46, C48) | 3 | 3 | 1 | 5 | 2 | 1 | 2 | 1 | ||
| Breast (C50) | 14 | 3 | 6 | 5 | 14 | 6 | 5 | 3 | ||
| Cervix uteri (C53) | 1 | 1 | ||||||||
| Ovary (C56) | 2 | 2 | 1 | 4 | 4 | |||||
| Prostate (C61) | 7 | 4 | 1 | 2 | 1 | 20 | 10 | 7 | 3 | 2 |
| Kidney (C64, C65) | 6 | 3 | 2 | 1 | 3 | 5 | 2 | 1 | 2 | |
| Bladder (C67) | 4 | 1 | 1 | 2 | ||||||
| Lymphoid or haematopoietic tissue (C81, C82, C83, C85, C90, C91, C92) | 7 | 2 | 5 | 12 | 6 | 4 | 2 | 1 | ||
| Primary site unknown (C80) | 5 | 2 | 3 | 1 | 3 | 3 | ||||
| Othere | 9 | 1 | 3 | 5 | 14 | 6 | 2 | 6 | ||
Cancers diagnosed during 3 years’ follow-up
When analysis was performed in June 2012, registration was reported to be 97% complete for all cancers diagnosed until December 2010 (at which point all patients had been followed up for at least 36 months) and all deaths until December 2011 had been registered. By that time (median follow-up for deaths 5.4 years, IQR 4.7–6.0 years), 400 patients (15.8%) assigned to BE and patients 201 (15.7%) assigned to CTC patients had died (p = 0.94), and three patients (two BE and one CTC) who did not have their randomised procedure had been diagnosed with CRC. A further 12 CRCs were diagnosed following apparently normal BE (five distal – rectum or sigmoid colon – and seven proximal) and three following CTC (one distal and two proximal). In one of the CTC patients, a 6-mm caecal polyp was detected at CTC, but colonoscopy was not performed and a 12-mm caecal adenocarcinoma was diagnosed 28 months later. The miss rate among patients having their randomised procedure was, therefore, 6.7% for CTC (45 cancers diagnosed, of which three were missed) and 14.1% for BE (85 cancers diagnosed, of which 12 were missed) (difference –7.5%, 95% CI –17.8% to 2.9%; p = 0.21). Combining the two procedures, this gives a miss rate of 7.8% for distal cancers (6/77) and 17.0% for proximal cancers (9/53).
During the 3-year follow-up of the trial cohort, 78 primary extracolonic cancers were diagnosed in the CTC group and 131 in the BE group [21.3 per 1000 person-years in the CTC group vs. 18.0 per 1000 person-years in the BE group; incidence rate ratio (IRR) 1.18, 95% CI 0.89 to 1.57; p = 0.24]. In the first year, rates of primary extracolonic cancer diagnosis in the trial cohort were nearly twice as high as expected (IRR 1.88, 95% CI 1.33 to 2.65; p = 0.0002), but rates did not differ significantly between the CTC and BE groups (IRR 0.84, 95% CI 0.54 to 1.30; p = 0.43). CTC detected 11 (39%) of the 28 extracolonic cancers that were diagnosed within the first year in the CTC group and BE detected 4 of 66 (6%).
Adverse events
An unplanned hospital admission within 30 days of the randomised procedure occurred in 14 patients following CTC and in 25 following a BE. Reasons for admission were reviewed independently by a gastroenterologist, radiologist and surgeon, who concluded that five were possibly attributable to the randomised procedure (reviewers were blinded to the procedure type). One patient was admitted directly after CTC with a possible perforation and was treated conservatively. Four hospitalisations occurred after a BE (reasons for admission were cardiac arrest, abdominal pain, rectal bleeding and patient collapsed after procedure). Three patients died within 30 days of a BE, one at 5 days (cardiac failure), one at 25 days (liver failure) and one at 28 days (perforated viscus), and one following CTC at 30 days (obstructive pulmonary disease).
Colonoscopy trial
Numbers analysed
Of the 1610 patients in the colonoscopy trial, 1072 were randomised to colonoscopy and 538 to CTC. Thirty patients withdrew consent. The proportion of patients withdrawing consent differed between the two arms of the trial, just reaching the level of statistical significance [25 (2.3%) in the colonoscopy arm and 5 (0.9%) in the CTC arm; p = 0.0496].
The number of randomised patients at each site varied (median 37.5, IQR 16.5–106.5). There were no significant differences in the demographic or clinical characteristics of patients randomised to colonoscopy or CTC (Table 14). The median age of patients was 68 years (IQR 61–75 years) and 55% were women. The most frequent presenting symptoms were change in bowel habit (73%), rectal bleeding (43%) and abdominal pain (22%).
| Characteristic | Colonoscopy (N = 1047) | CTC (N = 533) | Total (N = 1580) | Trial participants excluded (N = 3036) | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | p-valuea | |
| Sex | |||||||||
| Male | 476 | 45 | 240 | 45 | 716 | 45 | 1251 | 41 | 0.0074 |
| Female | 571 | 55 | 293 | 55 | 864 | 55 | 1785 | 59 | |
| Age (years) | |||||||||
| 55–64 | 384 | 37 | 217 | 41 | 601 | 38 | 802 | 26 | <0.0001 |
| 65–74 | 377 | 36 | 186 | 35 | 563 | 36 | 1045 | 34 | |
| 75–84 | 253 | 24 | 113 | 21 | 366 | 23 | 930 | 31 | |
| 85 + | 33 | 3 | 17 | 3 | 50 | 3 | 259 | 9 | |
| Symptomsb | |||||||||
| Change in bowel habit | 772 | 74 | 383 | 72 | 1155 | 73 | 1926 | 63 | <0.0001 |
| Harder, less frequent | 126 | 12 | 66 | 12 | 192 | 12 | 297 | 10 | |
| Looser, more frequent | 410 | 39 | 214 | 40 | 624 | 39 | 1049 | 35 | |
| Variable | 124 | 12 | 54 | 10 | 178 | 11 | 180 | 6 | |
| Unspecified | 112 | 11 | 49 | 9 | 161 | 10 | 400 | 13 | |
| Rectal bleeding | 432 | 41 | 240 | 45 | 672 | 43 | 1169 | 39 | 0.0080 |
| Abdominal pain | 227 | 22 | 124 | 23 | 351 | 22 | 574 | 19 | 0.0077 |
| Anaemia | 140 | 13 | 60 | 11 | 200 | 13 | 620 | 20 | <0.0001 |
| Weight loss | 155 | 15 | 82 | 15 | 237 | 15 | 500 | 16 | 0.19 |
| Other symptoms | 172 | 16 | 102 | 19 | 274 | 17 | 585 | 19 | 0.11 |
The number of patients who had their randomised procedure is shown in Table 15, along with reasons the procedure did not occur. There was no significant difference between the two arms in the proportion of patients having their randomised procedure [92.4% (967/1047) for colonoscopy and 94.4% (503/533) for CTC; p = 0.14]. Significantly more patients refused their procedure or did not attend among those randomised to colonoscopy than CTC [6.0% (63/1047) vs. 3.0% (16/533); p = 0.0093]. Of those patients randomised to colonoscopy who did not have their assigned procedure, 20% (16/80) had an alternative procedure, which was usually CTC (8/16, 50%). Of those randomised to CTC who did not have their procedure, 37% (11/30) had an alternative examination, which was usually colonoscopy (10/11, 91%).
| Status | Colonoscopy (N = 1047) | CTC (N = 533) | p-value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Occurrence of randomised procedure | |||||
| Occurred | 967 | 92.4 | 503 | 94.4 | 0.1373 |
| Did not occur | 80 | 7.6 | 30 | 5.6 | |
| Reasons did not occur | |||||
| Patient’s decision | |||||
| Patient refused randomised procedure | 48 | 4.6 | 12 | 2.3 | |
| Patient did not attend scheduled procedure | 15 | 1.4 | 4 | 0.7 | |
| Total | 63 | 6.0 | 16 | 3.0 | 0.0093 |
| Medical decision | |||||
| Patient unable to tolerate randomised procedure | 5 | 0.5 | 1 | 0.2 | |
| Consultant requested alternative procedure | 4 | 0.4 | 8 | 1.5 | |
| Patient became too ill | 5 | 0.5 | 2 | 0.4 | |
| Patient’s symptoms resolved | 2 | 0.2 | 0 | 0.0 | |
| Patient died | 1 | 0.1 | 2 | 0.4 | |
| Total | 17 | 1.6 | 13 | 2.4 | 0.26 |
| Other reasons | |||||
| Equipment failure | 0 | 0.0 | 1 | 0.2 | |
| Total | 0 | 0.0 | 1 | 0.2 | |
As expected, no patients randomised to colonoscopy had FS performed prior to the randomised procedure, but eight patients randomised to CTC had prior FS (see Figure 2). Randomised CTC procedure occurred in 75.0% (6/8) of patients following FS, whereas CTC occurred in 94.7% (497/525) of patients without prior FS.
Performance of the examinations
A greater proportion of colonoscopy examinations were judged to be difficult, with 27.4% (265/967) rated as ‘quite’ or ‘very’ difficult, compared with 8.3% (42/503) for CTC (p < 0.0001). A total of 12.2% of patients (118/967) had an incomplete colonoscopy and 16.1% (81/503) of patients had an inadequate CTC, defined as at least one segment not seen or for which visualisation was rated as ‘poor’.
Outcomes
Further colonic investigation
Of the 1047 patients randomised to colonoscopy, 86 had a further colonic investigation; these included 83 of 967 who had an additional procedure following colonoscopy and 3 of 16 who had an alternative whole-colon examination (Figure 4). Of the 533 patients randomised to CTC, 160 had a further colonic examination; these included 159 of 503 having CTC and 1 of 11 having an alternative procedure. Thus, additional colonic investigation was performed in 30% (160/533) randomised to CTC, compared with 8.2% (86/1047) randomised to colonoscopy (RR 3.65, 95% CI 2.87 to 4.65; p < 0.0001) (Table 16).
FIGURE 4.
Participants’ progress through the colonoscopy vs. CTC trial and selected outcomes. a, Number of patients with cancers or large polyps diagnosed.
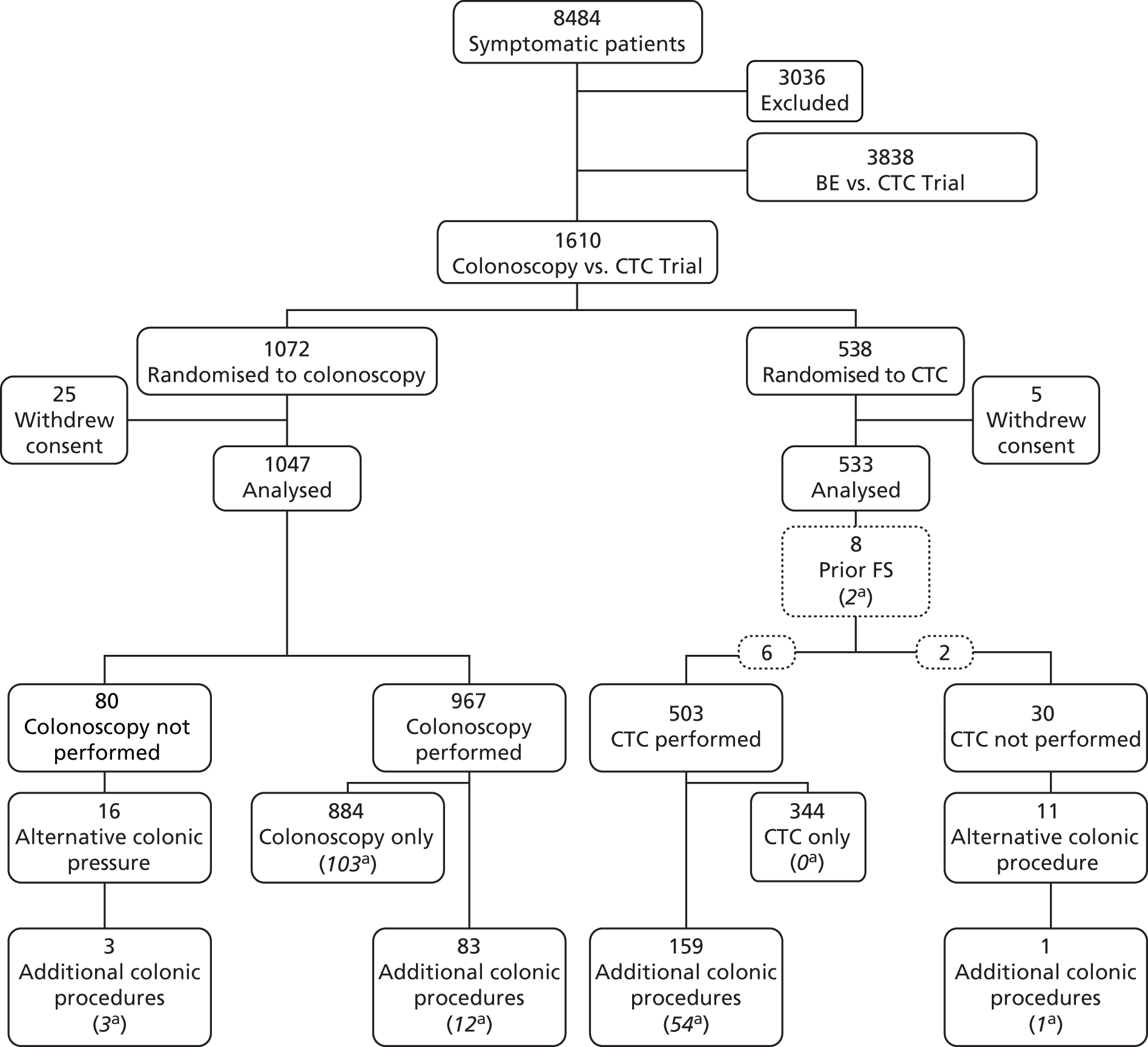
| Reason for referral | Randomised procedure | RR (CTC vs. colonoscopy) | Colonoscopy (N = 1047) | CTC (N = 533) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colonoscopy (N = 1047) | CTC (N = 533) | Men (n = 476) | Women (n = 571) | Men (n = 240) | Women (n = 293) | ||||||||||
| n | % | n | % | RR | 95% CI | p-value | n | % | n | % | n | % | n | % | |
| Cancer or large (≥ 10-mm) polyp suspected | |||||||||||||||
| Cancer | 10 | 1.0 | 47 | 8.8 | 4 | 0.8 | 6 | 1.0 | 25 | 10.4 | 22 | 7.5 | |||
| Polyp ≥ 10 mm | 2 | 0.2 | 36 | 6.8 | 0 | 0.0 | 2 | 0.4 | 20 | 8.3 | 16 | 5.5 | |||
| Total with cancer or large (≥ 10 mm) polyp suspected | 12 | 1.1 | 83 | 15.6 | 13.59 | 7.48 to 24.66 | <0.0001 | 4 | 0.8 | 8 | 1.4 | 45 | 18.7 | 38 | 13.0 |
| Smaller polyp suspected | |||||||||||||||
| Polyp 8–9 mm | 1 | 0.1 | 14 | 2.6 | 1 | 0.2 | 0 | 0.0 | 8 | 3.3 | 6 | 2.0 | |||
| Polyp 6–7 mm | 0 | 0.0 | 22 | 4.1 | 0 | 0.0 | 0 | 0.0 | 14 | 5.8 | 8 | 2.7 | |||
| Polyp ≤ 5 mm | 0 | 0.0 | 13 | 2.4 | 0 | 0.0 | 0 | 0.0 | 5 | 2.1 | 8 | 2.7 | |||
| Total with smaller polyp suspected | 1 | 0.1 | 49 | 9.2 | – | <0.0001 | 1 | 0.2 | 0 | 0.0 | 27 | 11.2 | 22 | 7.5 | |
| Clinical uncertainty (no polyps seen) | |||||||||||||||
| Inadequate examination | 72 | 6.9 | 18 | 3.4 | 21 | 4.4 | 51 | 8.9 | 10 | 4.2 | 8 | 2.7 | |||
| Adequate examination | 1 | 0.1 | 10 | 1.9 | 1 | 0.2 | 0 | 0.0 | 5 | 2.1 | 5 | 1.8 | |||
| Total with clinical uncertainty | 73 | 7.0 | 28 | 5.3 | 0.75 | 0.49 to 1.15 | 0.19 | 22 | 4.6 | 51 | 8.9 | 15 | 6.3 | 13 | 4.4 |
| Total having second procedure | 86 | 8.2 | 160 | 30.0 | 3.65 | 2.87 to 4.65 | <0.0001 | 27 | 5.7 | 59 | 10.3 | 87 | 36.2 | 73 | 24.9 |
Of the 86 patients in the colonoscopy group who had an additional diagnostic procedure, 63 (73%) had radiological investigation, 16 (19%) had a second colonoscopy (complete or limited as appropriate) and seven were referred straight to surgery without prior histological diagnosis. Of the 160 patients in the CTC group who had an additional investigation, 150 (94%) had colonoscopy and 10 had surgery. Table 17 describes reasons for referral: 1% of patients (12 cases) randomised to colonoscopy were referred because of a suspected cancer or large polyp, when biopsies either had not been taken or were inadequate for histological confirmation; 16% of patients randomised to CTC were referred to investigate a suspected cancer or large polyp and 9% to investigate a smaller polyp. There was no significant difference between the procedures in the proportion of patients referred because of clinical uncertainty (7% for colonoscopy vs. 5% for CTC; p = 0.19). All cancers identified at colonic investigations following CTC were in patients in whom a cancer or large polyp had been suspected at CTC (see Table 17) and three large polyps were found in people in whom smaller polyps had been suspected. Of the 28 patients who had a second procedure because of clinical uncertainty after CTC, one was found to have a large polyp. Of 73 patients who had a second procedure because of clinical uncertainty after colonoscopy, three were found to have cancers and one had a large polyp.
| Randomised procedure group | Colonoscopy | CTC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Findings at first procedure | Subsequent colonic procedure undertaken | Lesions seen at subsequent procedure | Subsequent colonic procedure undertaken | Lesions seen at subsequent procedure | ||||||
| CRC | Polyp ≥ 10 mmb | Cancer or polyps ≥ 10 mmb | CRC | Polyp ≥ 10 mmb | Cancer or polyps ≥ 10 mmb | |||||
| N | n | n | n | % | N | n | n | n | % | |
| Cancer or large (≥ 10 mm) polyp suspected | ||||||||||
| Cancer | 10 | 9 | 0 | 9 | 90 | 47 | 27 | 3 | 30 | 64 |
| Polyp ≥ 10 mm | 2 | 0 | 1 | 1 | 50 | 36 | 2 | 19 | 21 | 58 |
| Total | 12 | 9 | 1 | 10 | 83 | 83 | 29 | 22 | 51 | 61 |
| Smaller polyp suspected | ||||||||||
| Polyp 8–9 mm | 1 | 0 | 0 | 0 | 0 | 14 | 0 | 2 | 2 | 14 |
| Polyp 6–7 mm | 0 | 22 | 0 | 0 | 0 | 0 | ||||
| Polyp ≤ 5 mm | 0 | 13 | 0 | 1 | 1 | 8 | ||||
| Total | 1 | 0 | 0 | 0 | 0 | 49 | 0 | 3 | 3 | 6 |
| Clinical uncertainty (no polyps seen) | ||||||||||
| Inadequate examination | 72 | 3 | 1 | 4 | 6 | 18 | 0 | 1 | 1 | 6 |
| Adequate examination | 1 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 |
| Total | 73 | 3 | 1 | 4 | 5 | 28 | 0 | 1 | 1 | 4 |
| Total having subsequent colonic procedure | 86 | 12 | 2 | 14 | 16 | 160 | 29 | 26 | 55 | 34 |
Comparing results for the primary outcome from models ignoring clustering with those controlling for clustering by trial centre showed that the odds ratios were quite similar in size and significance (Table 18).
| Analysis | No clustering | With clusteringa | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p-value | Odds ratio | 95% CI | p-value | |
| Intention-to-treat analysis | 4.79 | 3.59 to 6.39 | < 0.0001 | 4.43 | 3.03 to 6.46 | < 0.0001 |
| Per-protocol analysis | 4.92 | 3.67 to 6.60 | < 0.0001 | 4.40 | 2.90 to 6.67 | < 0.0001 |
There was a significant difference in relative referral rates between men and women. Men were more than six times as likely to have an additional examination after CTC as after colonoscopy, while women were just over twice as likely to do so (men: RR 6.39, 95% CI 4.27 to 9.56; women: RR 2.41, 95% CI 1.76 to 3.30; p = 0.0002) (Figure 5). These differences arose because men were more likely to require a second examination following CTC (36% of men vs. 25% of women), largely owing to detection of polyps (see Table 16), and women were more likely to require a second examination following colonoscopy (10% of women vs. 6% of men), usually because colonoscopy was incomplete. There was no significant difference in relative referral rates between the two procedures by age group (< 65 years and ≥ 65 years; p = 0.32) (see Figure 5).
FIGURE 5.
Relative risks for referral for additional colonic procedure (CTC vs. colonoscopy) by sex and age group in the colonoscopy vs. CTC trial.
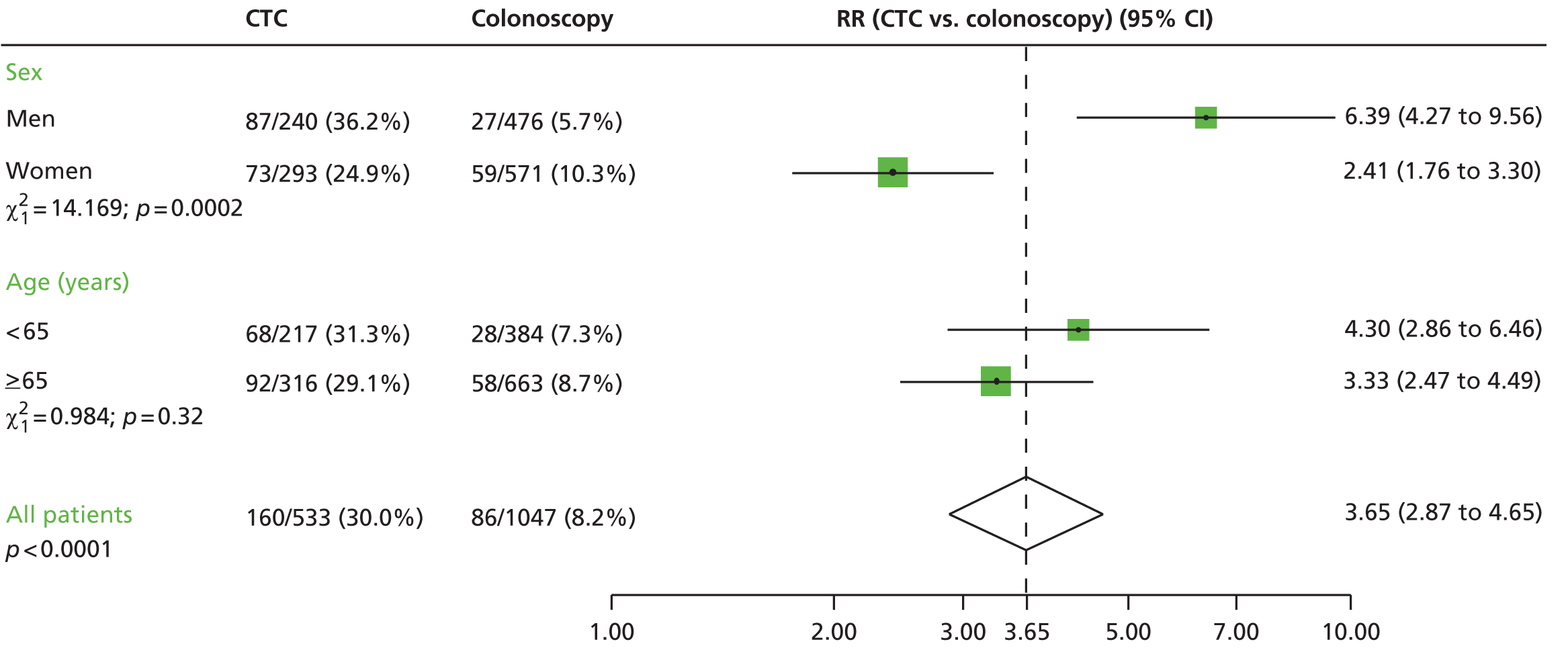
Detection of colorectal cancer and large polyps
Among 1047 patients randomised to colonoscopy, a CRC or large polyp was diagnosed in 119: in 103 at initial colonoscopy, in 13 at subsequent colonic investigation and in three patients who had an alternative procedure (see Figure 4). Among 533 patients randomised to CTC, a CRC or large polyp was diagnosed in 57: in 54 following CTC, in two at FS prior to CTC and in one a patient undergoing an alternative whole-colon investigation. The 119 lesions diagnosed in patients randomised to colonoscopy comprised 58 CRCs and 61 large polyps (52 adenomas, four hyperplastic polyps, three serrated adenomas, one juvenile polyp and one polyp which was excised but not retrieved). The 57 lesions diagnosed in patients randomised to CTC comprised 30 CRCs and 27 large polyps (24 adenomas and three hyperplastic polyps). The overall detection rate of CRC or large polyps did not differ between groups: 10.7% (57/533) for CTC compared with 11.4% (119/1047) for colonoscopy (RR 0.94, 95% CI 0.70 to 1.27; p = 0.69). There was no difference in detection rates when cancers (p = 0.94) and large polyps (p = 0.53) were analysed separately. Similar results were obtained when the analysis was restricted to patients having the randomised procedure (per-protocol analysis): 10.7% (54/503) for CTC compared with 12% (116/967) for colonoscopy (RR 0.89, 95% CI 0.66 to 1.21; p = 0.47) and no difference in detection rates when cancers (p = 0.92) and large polyps (p = 0.38) were analysed separately (Table 19).
| Analysis | Colonoscopy group | CTC group | Comparison of detection rates between procedures (CTC vs. colonoscopy) | ||||
|---|---|---|---|---|---|---|---|
| N = 1047 | N = 533 | RR | 95% CI | p-value | |||
| n | % | n | % | ||||
| Intention to treat | |||||||
| CRCb | 58 | 5.5 | 30 | 5.6 | 1.02 | 0.66 to 1.56 | 0.94 |
| ≥ 10-mm polypc | 61d | 5.8 | 27e | 5.1 | 0.87 | 0.56 to 1.35 | 0.63 |
| CRC or ≥ 10-mm polypc | 119d | 11.4 | 57e | 10.7 | 0.94 | 0.70 to 1.27 | 0.69 |
| N = 967 | N = 503 | ||||||
| n | % | n | % | ||||
| Per protocol | |||||||
| CRC | 55 | 5.7 | 28 | 5.6 | 0.98 | 0.63 to 1.52 | 0.92 |
| ≥ 10-mm polypc | 61d | 6.3 | 26e | 5.2 | 0.82 | 0.52 to 1.28 | 0.38 |
| CRC or ≥ 10-mm polypc | 116d | 12.0 | 54e | 10.7 | 0.89 | 0.66 to 1.21 | 0.47 |
Colorectal diagnoses other than cancer or polyps were analysed. Diverticular disease was detected in significantly more patients having CTC than colonoscopy [54% (287/533) and 35% (366/1047) respectively; p < 0.0001]. Diagnoses that were significantly more frequent in patients having colonoscopy included inflammatory bowel disease [1% (4/533) vs. 3% (934/1047); p = 0.0022] and anal findings [2% (13/533) vs. 7% (73/1047); p = 0.0002]. Other findings occurred in numbers too low to be analysed.
Time to diagnosis
Among patients who had their randomised procedure or an alternative procedure, the median time from date of randomisation to date of diagnosis was 21 days (IQR 12–43 days); the time was longer for subjects randomised to CTC than for those randomised to colonoscopy [CTC (n = 514 patients) median 24 days, IQR 13–49 days; colonoscopy (n = 983 patients) median 20 days, IQR 11–41 days; p = 0.0001]. The time to the randomised procedure was similar for the two procedures [CTC (n = 514 patients) median 16 days, IQR 11–27 days; colonoscopy (n = 983 patients) median 17 days, IQR 11–35 days; p = 0.0133], but for subjects who had an additional referral after the randomised procedure, the time from the randomised procedure to the second procedure was longer for CTC [CTC (n = 160 patients) median 43 days, IQR 25.5–83 days; colonoscopy (n = 86 patients) median 25 days, IQR 12–42 days; p < 0.0001]. For patients who had a diagnosis of cancer or a large polyp, the delay for those having CTC was considerably longer (CTC median 42 days, IQR 29–79 days; colonoscopy median 15 days, IQR 11–41 days; p < 0.0001).
Extracolonic findings following computed tomographic colonography
At least one previously unknown extracolonic finding was reported in 61% (287/474) of patients having CTC who did not have CRC diagnosed during the trial. Forty-eight of these (10%) were referred for additional procedures, leading to diagnosis of extracolonic malignancy in nine patients (Table 20). A more detailed analysis of extracolonic findings is given in Chapter 4.
| CTC (N = 533) | Colonoscopy (N = 1047) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Month of diagnosis | Found by CTC | Total | Month of diagnosis | Found by colonoscopy | |||||
| 1–12 | 13–24 | 25–36 | 1–12 | 13–24 | 25–36 | |||||
| All extracolonic cancersa | 27 | 16 | 3 | 8 | 9 | 56b | 33 | 9 | 14 | 1 |
| Person-years of follow-upc | 1536 | 523 | 512 | 501 | 2992 | 1030 | 994 | 968 | ||
| Incidence (per 1000 person-years) | 17.6 | 30.6 | 5.9 | 16.0 | 18.7 | 32.0 | 9.1 | 14.5 | ||
| Cancer type (ICD-10) | ||||||||||
| Stomach (C16) | 4 | 2 | 2 | 1 | 3 | 1 | 1 | 1 | ||
| Small intestine (C17) | 1 | 1 | 1 | 1 | ||||||
| Hepatobiliary system (C22, C23) | 1 | 1 | 4 | 1 | 2 | 1 | ||||
| Pancreas (C25) | 2 | 1 | 1 | 1 | 7 | 6 | 1 | |||
| Bronchus and lung (C34) | 2 | 2 | 1 | 12 | 8 | 2 | 2 | 1d | ||
| Mesothelioma (C45) | 1 | 1 | ||||||||
| Breast (C50) | 2 | 2 | 7 | 4 | 1 | 2 | ||||
| Ovary (C56) | 1 | 1 | ||||||||
| Prostate (C61) | 3 | 2 | 1 | 10 | 4 | 2 | 4 | |||
| Kidney (C64, C65) | 5 | 5 | 4 | 4 | 3 | 1 | ||||
| Bladder (C67) | 2 | 1 | 1 | |||||||
| Lymphoid or haematopoietic tissue (C82, C85, C90) | 2 | 2 | 2 | 3 | 1 | 2 | ||||
| Othere | 2 | 1 | 1 | 4 | 4 | |||||
Cancers diagnosed during 3-years’ follow-up
When analysis was performed in June 2012, registration was reported to be 97% complete for all cancers diagnosed until December 2010 (at which point all patients had been followed up for at least 36 months) and all deaths until December 2011 had been registered. By that time (median follow-up for deaths 5.2 years, IQR 4.6–5.9 years), 154 patients (14.7%) assigned to colonoscopy and 63 patients (11.8%) assigned to CTC had died (p = 0.11). Sixty-one patients in the colonoscopy group had a CRC diagnosis, including three who had refused colonoscopy; there were no CRC diagnoses after discharge in patients who had their colonoscopy. Thirty-one patients in the CTC group had a CRC diagnosis, including one patient who was diagnosed 15 months after an apparently normal CTC; the miss rate among patients with CTC performed was 3.4% (1/29).
During the 3-year follow-up of the trial cohort, 27 extracolonic cancers were diagnosed in the CTC group and 56 in the colonoscopy group (17.6 per 1000 person-years in the CTC group vs. 18.7 per 1000 person-years in the colonoscopy group; IRR 0.94, 95% CI 0.59 to 1.49; p = 0.79). In the first year, rates of primary extracolonic cancer diagnosis in the trial cohort were more than twice as high as expected (IRR 2.33, 95% CI 1.40 to 3.89; p = 0.0007), but rates did not differ significantly between the CTC and colonoscopy groups (IRR 0.95, 95% CI 0.53 to 1.73; p = 0.88) CTC detected 9 of 16 (56%) extracolonic cancers diagnosed within the first year in the CTC group, while colonoscopy detected one extracolonic cancer in the colonoscopy group (a lung primary diagnosed via a colonic metastasis).
Adverse events
An unplanned hospital admission within 30 days of the randomised procedure occurred in 12 patients following colonoscopy and in six following CTC. Reasons for admission were reviewed independently by a gastroenterologist, a radiologist and a surgeon, who concluded that three were possibly attributable to the randomised procedure (reviewers were blinded to the procedure type). All three followed colonoscopy [reasons for admission were abdominal pain (n = 1), rectal bleeding (n = 1), and diarrhoea and vomiting (n = 1)]. Another patient had polyps detected at CTC and experienced a possible perforation after polypectomy at colonoscopy; the patient was treated conservatively and discharged the following day. There was one death within 30 days of a patient who underwent surgery for a CRC seen at CTC.
Chapter 4 Extracolonic findings
An updated and more recent analysis of the data is available in Halligan et al. 37
Introduction
Symptoms suggestive of CRC include a change in bowel habit, rectal bleeding, anaemia, weight loss and abdominal pain. However, these symptoms are non-specific and (with the likely exception of rectal bleeding) may originate from pathology outside the colon. 38 A potential advantage of CTC for investigating symptomatic patients is that it can examine both the large bowel and other abdominopelvic organs at a single examination. A study of 1077 CTC examinations performed in frail elderly patients (median age 80 years), with symptoms suggestive of CRC, found extracolonic disease in 106 patients (10%) that was ultimately believed to be the cause of symptoms. 38 Another study of 400 symptomatic patients aged ≥ 70 years found extracolonic abnormalities in 67%, including 23 malignancies. 39 The authors concluded that CTC should replace a BE because it is a more comprehensive investigation.
However, although CTC can detect extracolonic pathology in patients with suspected CRC, it is uncertain if this is always beneficial. Although some patients will have clinically important abnormalities outside the colon, in other cases the detection of extracolonic lesions can precipitate follow-up investigations that increase morbidity and anxiety, are costly and ultimately offer no clinical benefit. A systematic review of 3488 patients from 17 studies found that 14% underwent further investigation, leading to detection of an extracolonic cancer in 2.7% overall. 40 An economic analysis of 225 patients by the same group found that the average cost incurred to investigate extracolonic pathology exceeded the cost of the initial CTC examination. 26 Researchers have assessed the clinical impact of extracolonic findings via chart review,24,39,41 but this may introduce bias as the decision on whether or not a detected lesion is important is made retrospectively. In addition, the largest studies have been performed in asymptomatic individuals being screened for CRC. 42,43 Until now there has been no prospective, randomised study examining the consequences of detecting extracolonic lesions in symptomatic patients having CTC. We collected data on the frequency and nature of extracolonic pathology detected at CTC, subsequent investigations and resource use and ultimate clinical outcome.
Methods
Data collection
Radiologists interpreting the procedures issued a report in line with normal clinical practice and completed a specially designed trial CRF (see Appendix 1), noting any suspected colonic lesions (e.g. cancers or polyps) that might explain a patient’s presenting symptoms. Space was also provided on the CRF for radiologists to describe any significant extracolonic lesions identified during their interpretation.
Follow-up
Extracolonic lesions recorded on the CTC report were investigated if considered clinically relevant by the clinician in charge, in light of the patient’s presenting symptoms, status and personal wishes. Details of subsequent procedures requested to investigate or treat potential extracolonic pathology were collected from trial centres, as described in Chapter 2.
In order to identify cancers (both intracolonic and extracolonic) that were missed by the procedures, all participating patients were identified on the NHSCR and information on new cancer diagnoses and deaths was obtained from the NHSIC. Trial patients were also matched with national HES to reduce the time lag between cancer diagnosis and the time of notification. Cancers were confirmed using pathology and imaging reports obtained from the hospital where the cancer was diagnosed.
Statistical analysis
The analysis of extracolonic lesions was prespecified in the trial protocol. Patients with proven CRC were excluded from this analysis as it is not always possible to be sure that such patients’ extracolonic findings were unrelated to their CRC and referral patterns for follow-up of extracolonic lesions are likely to be different for patients in whom CRC is also present.
A data manager examined the radiology reports from CTC examinations, extracting references to extracolonic lesions and placing them in a spreadsheet. Each extracolonic finding was subsequently assigned an Extracolonic Reporting And Data System (E-RADS) score44 by a radiologist (SH) blind to study arm, patient, reporting radiologist, centre and ultimate clinical diagnosis. E-RADS scores categorise the perceived clinical importance of an extracolonic finding detected at CTC as follows: E-RADS category 1, normal examination or anatomic variant (E1) – ‘normal exam or anatomic variant’; E-RADS category 2, clinically unimportant finding (E2) – ‘clinically unimportant finding’; E-RADS category 3, likely unimportant finding, incompletely characterised (E3) – ‘likely unimportant finding, incompletely characterised’; and E-RADS category 4, potentially important finding (E4) – ‘potentially important finding’. 44 Patients’ E-RADS scores were then matched with their demographic data, presenting symptoms and details of their subsequent diagnostic/therapeutic procedures. In cases for which a potential extracolonic abnormality was conclusively diagnosed, a data manager assigned it a code according to the International Classification of Diseases and Related Health Problems (ICD) classification. A senior surgeon, gastroenterologist and radiologist independently reviewed these diagnoses by ICD code, together with the presenting clinical symptoms in patients assigned that code, noting for each symptom whether or not they thought it could be related to the final diagnosis. When there was any difference in opinion, a majority vote was used to reach a final decision on whether or not the symptom was likely to be related.
Results
Patient flow through the trial is shown in Figure 6. Of 8484 patients, 3100 were excluded (Table 21 shows the reasons), 3804 were randomised within the BE trial and 1580 were randomised within the colonoscopy trial. There were 1297 CTC examinations performed in the BE trial and 545 performed in the colonoscopy trial. Fifty-six patients and 38 patients in the BE trial and the colonoscopy trial, respectively, were excluded because of a diagnosis of CRC, leaving 1241 CTC studies for analysis of extracolonic lesions in the BE trial and 507 in the colonoscopy trial.
FIGURE 6.
Patient flow through the trial.
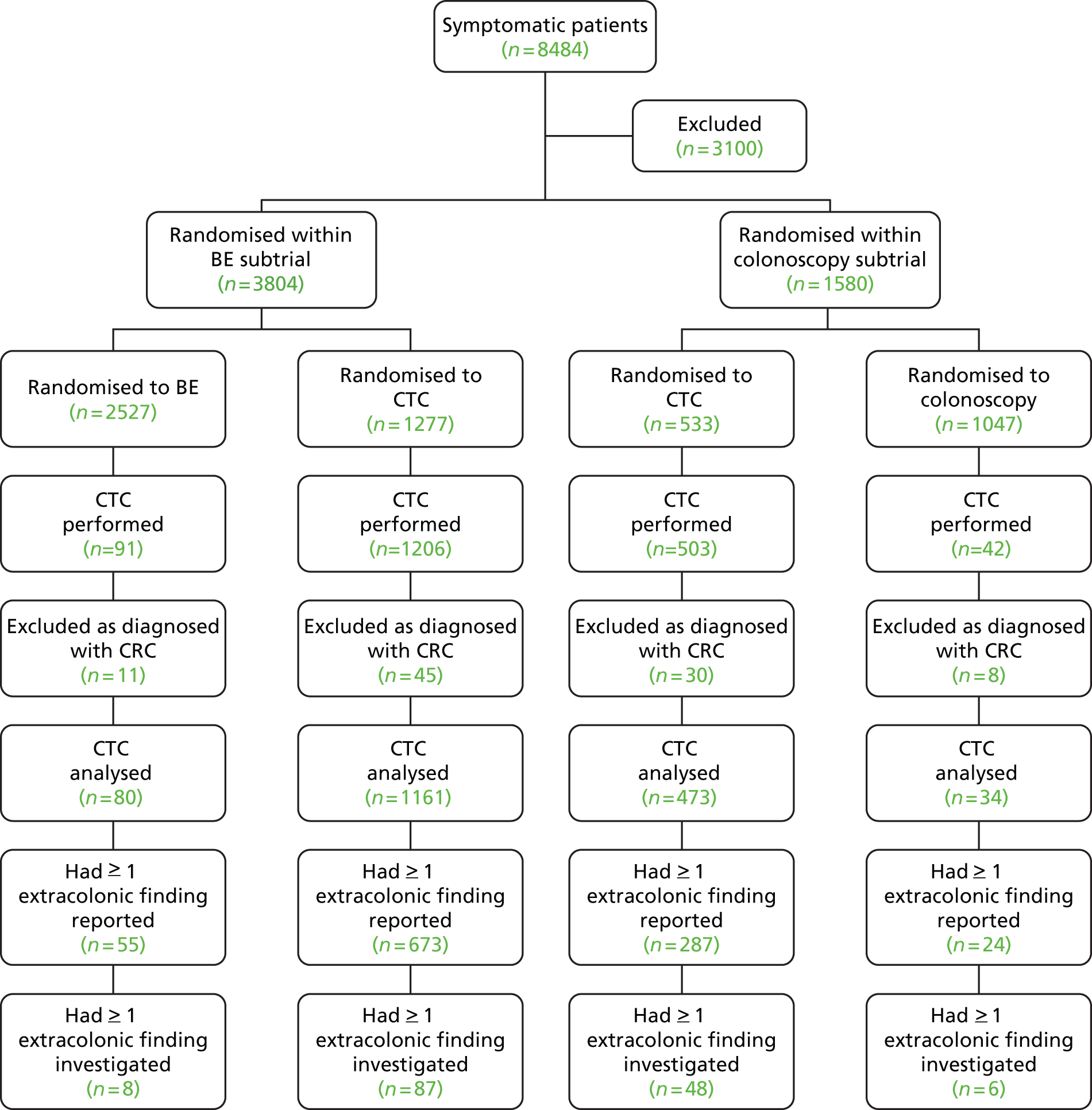
| Reason | n | (%) |
|---|---|---|
| Clinician reasons for declining consent | ||
| Colorectal or other cancer already diagnosed | ||
| CRC diagnosed | 56 | 1.8 |
| Other cancer diagnosed | 69 | 2.2 |
| Specific procedure requested | ||
| Colonoscopy | 731 | 23.6 |
| CT | 303 | 9.8 |
| FS | 230 | 7.4 |
| Oesophagogastroduodenoscopy | 218 | 7.0 |
| BE | 19 | 0.6 |
| Ultrasonography | 16 | 0.5 |
| Magnetic resonance imaging | 5 | 0.2 |
| Unknown | 39 | 1.3 |
| Clinical situation too urgent or waiting list too long | 52 | 1.7 |
| Patient unfit for whole-colon examination | 215 | 6.9 |
| Patient unable to give informed consent | 75 | 2.4 |
| No reason given | 148 | 4.7 |
| Total for which clinician declined consent | 2176 | 70.2 |
| Patient reasons for declining consent | ||
| Patient wanted a specific procedure | ||
| Colonoscopy | 15 | 0.5 |
| CT | 3 | 0.1 |
| BE | 2 | 0.06 |
| Unknown | 128 | 4.1 |
| Patient did not want a specific procedure | ||
| CT as claustrophobic | 13 | 0.4 |
| CT for other reasons | 2 | 0.06 |
| Colonoscopy | 1 | 0.03 |
| BE | 1 | 0.03 |
| Patient had difficulty comprehending | 84 | 2.7 |
| Patient died before consent obtained | 2 | 0.06 |
| No reason given | 583 | 18.8 |
| Total for which patient declined consent | 834 | 26.9 |
| Reason for exclusion unknown | 26 | 0.8 |
| Patient withdrew consent following randomisation | 64 | 2.1 |
| Total excluded | 3100 | 100.0 |
Reporting of extracolonic findings
In both trials, change in bowel habit was the most common presenting symptom (76.1% of all patients analysed), followed by abdominal pain (BE trial) and rectal bleeding (colonoscopy trial) (Table 22). Of the 1748 CTC studies analysed overall, 1039 (59.4%) had at least one extracolonic finding described by the reporting radiologist; 728 (58.7%) from the BE trial and 311 (61.3%) from the colonoscopy trial (see Figure 6). There was no association between sex and reporting of an extracolonic finding; however, patients were significantly more likely to have an extracolonic finding reported if they were older (p < 0.0001). In both trials, the proportion of patients with at least one extracolonic finding increased with age, rising in the BE trial from 48% for those aged 55–64 years to 74% for those aged ≥ 85 years, and from 55% to 91%, respectively, in the colonoscopy trial (see Table 22).
| Characteristic | CTC performed and analyseda | At least one extracolonic finding | Referred | BE trial | Colonoscopy trial | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTC performed and analyseda | At least one extracolonic finding | Referred | CTC performed and analyseda | At least one extracolonic finding | Referred | ||||||||||
| n | n | % | n | % | n | n | % | n | % | n | n | % | n | % | |
| Total | 1748 | 1039 | 59 | 149 | 9 | 1241 | 728 | 59 | 95 | 8 | 507 | 311 | 61 | 54 | 11 |
| Sex | |||||||||||||||
| Male | 689 | 414 | 60 | 61 | 9 | 465 | 279 | 60 | 39 | 8 | 224 | 135 | 60 | 22 | 10 |
| Female | 1059 | 625 | 59 | 88 | 8 | 776 | 449 | 58 | 56 | 7 | 283 | 176 | 62 | 32 | 11 |
| Age (years) | |||||||||||||||
| 55–64 | 618 | 313 | 51 | 47 | 8 | 407 | 196 | 48 | 27 | 7 | 211 | 117 | 55 | 20 | 9 |
| 65–74 | 655 | 384 | 59 | 52 | 8 | 478 | 281 | 59 | 32 | 7 | 177 | 103 | 58 | 20 | 11 |
| 75–84 | 421 | 300 | 71 | 45 | 11 | 313 | 219 | 70 | 32 | 10 | 108 | 81 | 75 | 13 | 12 |
| ≥ 85 | 54 | 42 | 78 | 5 | 9 | 43 | 32 | 74 | 4 | 9 | 11 | 10 | 91 | 1 | 9 |
| Symptoms/signsb | |||||||||||||||
| Change in bowel habit | 1330 | 787 | 59 | 108 | 8 | 960 | 553 | 58 | 70 | 7 | 370 | 234 | 63 | 38 | 10 |
| Rectal bleeding | 599 | 352 | 59 | 50 | 8 | 373 | 215 | 58 | 24 | 6 | 226 | 137 | 61 | 26 | 12 |
| Abdominal pain | 513 | 293 | 57 | 46 | 9 | 400 | 227 | 57 | 32 | 8 | 113 | 66 | 58 | 14 | 12 |
| Anaemia | 193 | 138 | 72 | 24 | 12 | 142 | 102 | 72 | 15 | 11 | 51 | 36 | 71 | 9 | 18 |
| Weight loss | 253 | 163 | 64 | 28 | 11 | 179 | 115 | 64 | 19 | 11 | 74 | 48 | 65 | 9 | 12 |
| Other | 228 | 135 | 59 | 20 | 9 | 133 | 77 | 58 | 10 | 8 | 95 | 58 | 61 | 10 | 11 |
A total of 1945 individual extracolonic findings were reported: 22 (1.13%) findings were categorised as E1 (‘normal exam or anatomic variant’), 1472 (75.7%) were categorised as E2 (‘clinically unimportant’), 362 (18.6%) were categorised as E3 (‘likely unimportant, incompletely characterised) and 89 (4.6%) were categorised as E4 (‘potentially important’). The proportion of findings falling into each category was similar in the BE and colonoscopy trials (E2: 37% vs. 42%; E3: 16% vs. 15%; E4: 5% vs. 5%, respectively).
Rate and nature of subsequent investigation
A total of 149 patients (8.5% of those analysed) underwent subsequent procedures to investigate and/or treat extracolonic findings. Three of these patients had two unrelated findings investigated (i.e. there were 152 individual findings in total). In the E-RADS categories, 26 findings were classified as E2, 60 as E3, and 66 as E4, and the proportion investigated in each category was 1.8% for E2, 16.6% for E3 and 74.2% for E4. In total, 97 of the 149 patients (65%) underwent a single procedure and 52 (35%) underwent multiple procedures, with 31 patients having two procedures, 14 having three procedures, four having four procedures and one having five procedures.
The follow-up procedures undertaken to investigate or treat extracolonic findings are shown in Table 23. Follow-up was most frequently by non-invasive imaging (80 cases). There were 68 invasive follow-up procedures, 32 of which were surgical, including some that combined investigation and therapy (e.g. excision biopsy, vascular repair). There was a positive correlation between E-RADS category and invasiveness of follow-up procedure, with most surgical procedures performed to investigate and/or treat E4 findings whereas most radiological procedures were performed to investigate E2 findings (see Table 23). E3 detections were intermediate, with most procedures in this category invasive but non-surgical (e.g. endoscopy). The type and frequency of surgical and non-surgical procedures is shown in Table 24. The most frequently performed surgical procedure was nephrectomy (nine cases), followed by salpingo-oophorectomy and/or hysterectomy (eight cases). The most common invasive, non-surgical procedure was transvaginal ultrasonography (nine cases) followed by upper GI endoscopy (six cases).
| Most invasive procedure performed as a result of finding | E-RADS category of finding | Total (n = 152) | |||||
|---|---|---|---|---|---|---|---|
| E4 (n = 66) | E3 (n = 60) | E2 (n = 26) | |||||
| n | % | n | % | n | % | ||
| Surgical | 23 | 34.8 | 7 | 11.7 | 2 | 7.7 | 32 |
| Invasive, non-surgical | 15 | 22.7 | 17 | 28.3 | 4 | 15.4 | 36 |
| Non-invasive imaging | 25 | 37.9 | 36 | 60.0 | 19 | 73.1 | 80 |
| Otherb | 3 | 4.5 | 0 | 0.0 | 1 | 3.8 | 4 |
| Procedure | Frequency |
|---|---|
| Surgical procedure | |
| Radical nephrectomy | 9 |
| Oophorectomy with or without salpingectomy with or without hysterectomy | 8 |
| Aneurysm repair (three endovascular, two open) | 5 |
| Laparotomy | 3 |
| Whipple procedure | 1 |
| Inguinal hernia repair | 1 |
| Laparoscopic cholecystectomy | 1 |
| Right upper lobectomy | 1 |
| Splenectomy | 1 |
| Adrenalectomy | 1 |
| Video-assisted thoracoscopy | 1 |
| Total surgical procedures | 32 |
| Non-surgical procedure | |
| Ultrasound transvaginal | 9 |
| Oesophagogastroduodenoscopy | 6 |
| Hysteroscopy | 4 |
| Ultrasound-guided biopsy | 3 |
| Bronchoscopy | 2 |
| Lymph node biopsy | 2 |
| CT-guided biopsy | 1 |
| Endoscopic retrograde cholangiopancreatography | 1 |
| Endoscopic ultrasound of pancreas | 1 |
| Extracorporeal shock wave lithotripsy | 1 |
| Flexible cystoscopy | 1 |
| Fluid aspiration and culture of uterus | 1 |
| Prostate biopsy | 1 |
| Renal biopsy | 1 |
| Ultrasound-guided drainage of ascites | 1 |
| Ultrasound-guided fine-needle aspiration of pancreas | 1 |
| Total non-surgical, invasive procedures | 36 |
Final extracolonic diagnosis, predictive value of presenting symptoms and missed extracolonic cancers
An extracolonic diagnosis was reached in 133 of the 149 referred patients (89%). A list of the final diagnoses is given in Table 25. In total, 79 extracolonic neoplasms were diagnosed, 29 of which were malignant. Extracolonic neoplasms were therefore diagnosed in 4.5% of those having CTC overall (excluding those diagnosed with CRC) and an extracolonic malignancy was diagnosed in 1.7%. The most frequent extracolonic primary malignancies were renal (nine cases), pancreatic (four cases) and ovarian (three cases). The most frequent extracolonic benign tumour was ovarian (16 cases). A total of 26 of the 29 malignancies (90%) had been assigned to the E4 category and the remaining three were E3. In all, 21 of the 50 benign tumours (42%) were assigned an E4 rating, 24 (48%) were E3 and 5 (10%) were E2. The most frequent non-neoplastic diagnosis was abdominal aortic aneurysm (14 cases).
| ICD code | Pathology | Frequency | Number of patients with ≥ 1 symptom related to diagnosis |
|---|---|---|---|
| Malignant neoplasms | |||
| C64 | Malignant neoplasm of the kidney, except renal pelvis | 8 | 5 |
| C25 | Malignant neoplasm of the pancreas | 4 | 4 |
| C56 | Malignant neoplasm of the ovary | 3 | 3 |
| C78 | Secondary malignant neoplasm of respiratory and digestive organs | 2 | 2 |
| C34 | Malignant neoplasm of the bronchus and lung | 2 | 1 |
| C61 | Malignant neoplasm of the prostate | 2 | 2 |
| C16 | Malignant neoplasm of the stomach | 1 | 1 |
| C17 | Malignant neoplasm of the small intestine | 1 | 1 |
| C22 | Malignant neoplasm of the liver and intrahepatic bile ducts | 1 | 1 |
| C65 | Malignant neoplasm of the renal pelvis | 1 | 1 |
| C77 | Secondary and unspecified malignant neoplasm of lymph nodes | 1 | 1 |
| C80 | Malignant neoplasm without specification of site | 1 | 1 |
| C82 | Follicular (nodular) non-Hodgkin’s lymphoma | 1 | 1 |
| C90 | Multiple myeloma and malignant plasma cell neoplasms | 1 | 1 |
| Total | 29 | 25 | |
| Benign neoplasms | |||
| D27 | Benign neoplasm of the ovary | 16 | 7 |
| D18 | Haemangioma and lymphangioma, any site | 10 | 0 |
| D14 | Benign neoplasm of the middle ear and respiratory system | 9 | 0 |
| D25 | Leiomyoma of the uterus | 5 | 4 |
| D30 | Benign neoplasm of urinary organs | 4 | 0 |
| D35 | Benign neoplasm of other and unspecified endocrine glands | 2 | 0 |
| D36 | Benign neoplasm of other and unspecified sites | 2 | 0 |
| D13 | Benign neoplasm of other and ill-defined parts of the digestive system | 1 | 1 |
| D44.1 | Adrenal neoplasm of uncertain or unknown behaviour | 1 | 1 |
| Total | 50 | 13 | |
| Other diagnoses | |||
| D50.9 | Iron deficiency anaemia, unspecified | 1 | 1 |
| B90.9 | Sequelae of respiratory and unspecified tuberculosis | 1 | 1 |
| I71.9 | Abdominal aortic aneurysm, without mention of rupture | 14 | 7 |
| I72.3 | Aneurysm of the iliac artery | 1 | 0 |
| J92 | Pleural plaque, with presence of asbestos | 3 | 0 |
| J20.8 | Acute bronchitis due to other specified organisms | 1 | 0 |
| J98.9 | Respiratory disorder, unspecified | 1 | 0 |
| K83.5 | Biliary cyst | 7 | 0 |
| K44.9 | Diaphragmatic hernia without obstruction or gangrene | 4 | 1 |
| K80.2 | Calculus of the gallbladder, without cholecystitis | 3 | 0 |
| K80.1 | Calculus of the gallbladder, with other cholecystitis | 1 | 1 |
| K86.1 | Other chronic pancreatitis | 1 | 1 |
| K50.0 | Crohn’s disease of small intestine | 1 | 1 |
| K91.5 | Post-cholecystectomy syndrome | 1 | 1 |
| K76 | Fatty liver, not elsewhere classified | 2 | 0 |
| K40.9 | Inguinal hernia, without obstruction or gangrene | 1 | 1 |
| N28.1 | Cyst of the kidney, acquired | 6 | 0 |
| N71.9 | Inflammatory disease of the uterus, unspecified | 1 | 0 |
| N20 | Calculus of the kidney | 1 | 0 |
| N13 | Hydronephrosis with ureteropelvic junction obstruction | 1 | 1 |
| N13.5 | Kinking and stricture of the ureter without hydronephrosis | 1 | 0 |
| Q60 | Renal agenesis, unilateral | 1 | 0 |
| R59 | Localised enlarged lymph nodes | 2 | 0 |
| Total | 56 | 16 | |
Twenty-five of 29 patients (86%) with an extracolonic malignancy had presenting symptoms that were attributable to the tumour (see Table 25), compared with 13 of 50 patients (26%) with a benign extracolonic tumour. Sixteen of the 56 patients (29%) with a non-neoplastic extracolonic finding had symptoms attributable to this.
Overall, 3% of patients having CTC had presenting symptoms attributable to an extracolonic finding (Table 26). This proportion was equal for males and females and for all ages except patients aged ≥ 85 years (of whom only five were referred). The positive predictive value of individual symptoms for an extracolonic diagnosis was low; for example, of the 1330 patients presenting with altered bowel habit, only 13 (1%) had an extracolonic finding to which this symptom could be attributed (see Table 26). Abdominal pain had the highest positive predictive value and rectal bleeding the lowest (7% and 0.3% of patients, respectively, had an extracolonic finding that explained their symptoms).
| Characteristic | Patients with CTC performeda | Patients referred for further investigations | Patients with an extracolonic diagnosis made | Patients with at least one symptom attributable to extracolonic diagnosis | |||
|---|---|---|---|---|---|---|---|
| N | n | % | n | % | n | % | |
| Total | 1748 | 149 | 9 | 133 | 8 | 54 | 3 |
| Sex | |||||||
| Male | 689 | 61 | 9 | 55 | 8 | 20 | 3 |
| Female | 1059 | 88 | 8 | 78 | 7 | 34 | 3 |
| Age (years) | |||||||
| 55–64 | 618 | 47 | 8 | 42 | 7 | 19 | 3 |
| 65–74 | 655 | 52 | 8 | 45 | 7 | 20 | 3 |
| 75–84 | 421 | 45 | 11 | 41 | 10 | 11 | 3 |
| ≥ 85 | 54 | 5 | 9 | 5 | 9 | 4 | 7 |
| Symptoms/signsb | |||||||
| Change in bowel habit | 1330 | 108 | 8 | 96 | 7 | 13 | 1 |
| Rectal bleeding | 599 | 50 | 8 | 45 | 8 | 2 | 0.3 |
| Abdominal pain | 513 | 46 | 9 | 44 | 9 | 34 | 7 |
| Anaemia | 193 | 24 | 12 | 22 | 11 | 7 | 4 |
| Weight loss | 253 | 28 | 11 | 26 | 10 | 8 | 3 |
| Other | 228 | 20 | 9 | 17 | 7 | 7 | 3 |
Analysis of registry data showed that there was no significant difference in the proportion of patients diagnosed with an extracolonic cancer within 3 years of randomisation, regardless of the original randomised procedure. In the BE trial, 34 of 2427 patients (1.4%) randomised to BE were diagnosed with an extracolonic cancer within 3 years, compared with 20 of 1226 (1.6%) randomised to CTC (p = 0.689). In the colonoscopy trial, 17 of 983 patients (1.7%) randomised to colonoscopy were diagnosed with an extracolonic cancer, compared with 9 of 501 (1.8%) randomised to CTC (p = 0.926) (Table 27). For all randomised procedures, the greatest proportion of extracolonic tumours was diagnosed within the first 6 months, compared with subsequent 6-month blocks (see Table 27). There was also no significant difference by randomised procedure in the prevalence of extracolonic malignancies potentially detectable by CTC: in the BE trial, 35 of 2441 patients (1.4%) randomised to BE compared with 21 of 1230 (1.7%) randomised to CTC (p = 0.523); in the colonoscopy trial, 17 of 986 patients (1.7%) randomised to colonoscopy compared with 9 of 502 (1.8%) randomised to CTC (p = 0.924). Registry data revealed that 14 patients had an extracolonic abdominopelvic cancer diagnosed within 3 years of CTC that had not been detected by the procedure: seven prostate, three pancreatic, two renal, one ovarian and one jejunal.
| BE trial (patients with missed CRC also excluded) | BE | CTC | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 2427 | Months to diagnosis | Cancer death | Found at CTC | N = 1226 | Months to diagnosis | Cancer death | Found at CTC | |||||||||
| n | % | 0–6 | 6–12 | 12–24 | 24–36 | n | % | 0–6 | 6–12 | 12–24 | 24–36 | |||||
| C17 Malignant neoplasm of the small intestine | 3 | 0.1 | 2 | 0 | 1 | 0 | 2 | 1 | 0.1 | 1 | 0 | 0 | 0 | 0 | 1 | |
| C25 Malignant neoplasm of the pancreas | 8 | 0.3 | 5 | 1 | 1 | 1 | 6 | 1 | 5 | 0.4 | 3 | 0 | 2 | 0 | 4 | 2 |
| C56 Malignant neoplasm of the ovary | 4 | 0.2 | 4 | 0 | 0 | 0 | 3 | 1 | 2 | 0.2 | 1 | 1 | 0 | 0 | 2 | 1 |
| C61 Malignant neoplasm of the prostate | 16 | 0.7 | 4 | 5 | 5 | 2 | 2 | 7 | 0.6 | 3 | 1 | 2 | 1 | 0 | 2 | |
| C64 Malignant neoplasm of the kidney, except the renal pelvis | 3 | 0.1 | 2 | 0 | 0 | 1 | 1 | 1 | 5 | 0.4 | 2 | 0 | 2 | 1 | 2 | 4 |
| C65 Malignant neoplasm of the renal pelvis | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | ||
| Total | 34 | 1.4 | 17 | 6 | 7 | 4 | 14 | 3 | 20 | 1.6 | 10 | 2 | 6 | 2 | 8 | 10 |
| Colonoscopy trial | Colonoscopy | CTC | ||||||||||||||
| N = 983 | Months to diagnosis | Cancer death | Found at CTC | N = 501 | Months to diagnosis | Cancer death | Found at CTC | |||||||||
| n | % | 0–6 | 6–12 | 12–24 | 24–36 | n | % | 0–6 | 6–12 | 12–24 | 24–36 | |||||
| C17 Malignant neoplasm of the small intestine | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 | 0 | 1 | 0 | 0 | 0 | ||
| C25 Malignant neoplasm of the pancreas | 8 | 0.8 | 4 | 2 | 1 | 1 | 6 | 2 | 0.4 | 1 | 0 | 0 | 1 | 2 | 1 | |
| C56 Malignant neoplasm of the ovary | 1 | 0.1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | |
| C61 Malignant neoplasm of the prostate | 7 | 0.7 | 2 | 1 | 2 | 2 | 0 | 2 | 0.4 | 0 | 0 | 2 | 0 | 0 | ||
| C64 Malignant neoplasm of the kidney, except the renal pelvis | 1 | 0.1 | 0 | 0 | 0 | 1 | 0 | 3 | 0.6 | 3 | 0 | 0 | 0 | 0 | 3 | |
| C65 Malignant neoplasm of the renal pelvis | 0 | 0.0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 | 1 | 0 | 0 | 0 | 1 | 1 | |
| Total | 17 | 1.7 | 8 | 3 | 3 | 4 | 7 | 1 | 9 | 1.8 | 5 | 1 | 2 | 1 | 3 | 5 |
Six patients died within 60 days of a follow-up procedure for an extracolonic finding: four from metastatic disease, one following a Whipple procedure for pancreatic carcinoma and one following open repair of an aortic aneurysm. There was no significant difference by randomised procedure in the proportion of patients dying within 3 years of randomisation (excluding those with CRC): in the BE trial, 158 of 2441 patients (6.5%) randomised to BE compared with 96 of 1230 (7.8%) randomised to CTC (p = 0.13); and in the colonoscopy trial, 66 of 988 patients (6.7%) randomised to colonoscopy compared with 26 of 503 (5.2%) randomised to CTC (p = 0.25).
Chapter 5 Health psychology assessment
This chapter contains information reproduced from Von Wagner C, Ghanouni A, Halligan S, Smith S, Dadswell E, Lilford RJ, et al. Patient acceptability and psychologic consequences of CTC compared with those of colonoscopy: Results from a multicenter randomized controlled trial of symptomatic patients. Radiology 2012;263:723–3145 with permission from the Radiological Society of North America; and information reproduced with kind permission from Springer Science + Business Media: European Radiology, Patient acceptability of CTC compared with double contrast barium enema: results from a multicentre randomised controlled trial of symptomatic patients, vol. 21, 2011, 2046–55, von Wagner C, Smith S, Halligan S, Ghanouni A, Power E, Lilford RJ, et al. 46 © European Society of Radiology 2011. The final publication is available on http://link.springer.com.
Introduction
If any test is to become widely adopted for the diagnosis of CRC, its acceptability to patients is an important consideration. Existing research suggests that CTC may be more acceptable than BE in both symptomatic18,47 and asymptomatic patients,48 and that it is possibly more acceptable than colonoscopy. 20,49 However, most evidence is based on intraindividual comparisons in which patients experience both CTC and another test. Acceptability judgements are, therefore, relative rather than absolute, which could exaggerate differences in patient experience. To date, studies comparing acceptability of each of the procedures alone have been small and typically non-randomised, and have compared very different samples. 18 In comparing CTC and colonoscopy, there are additional complications because any lesions detected at colonoscopy can be biopsied or removed at the same examination, whereas for CTC (like BE) an additional endoscopic procedure is required. Patients may view these additional referrals negatively. Prospective preference studies have investigated attitudes towards hypothetical referral rates,50 but there is no available evidence on patients’ retrospective appraisals of follow-up investigations. Researchers have also highlighted important differences regarding result delivery: face to face at the time of the procedure (which is typical for colonoscopy) is perceived by patients to be superior to communication later (as is usually the case for BE or CTC). 51 This has not been assessed quantitatively. Finally, little is known about whether or not intertest differences are associated with longer-term psychological outcomes.
To address these limitations, we aimed to determine the acceptability of BE, colonoscopy and CTC by carrying out a post-examination survey of reported experiences for a sample of patients in the SIGGAR study. This enabled differences in test acceptability, post-test complications and both short- and longer-term experiences to be assessed in a sizeable population for whom the impact of clinician preference on outcomes had been reduced by randomisation. Moreover, as patients in the study follow normal clinical pathways after the primary randomised procedure (i.e. receive usual care), the results are more likely to reflect experience in everyday practice than designs in which all patients undergo CTC and another test.
Methods
The psychological evaluation took place during the last 12 months of recruitment to the SIGGAR study, after a series of qualitative interviews – used to design the questionnaires – had been conducted and analysed. 51 Questionnaire administration began at 18 hospitals on 1 December 2006 and at a further three hospitals on 12 February 2007.
Questionnaires
Copies of the psychological questionnaires can be seen in Appendix 3.
Post-test questionnaire
The post-test questionnaire was either given directly to patients by a research nurse or sent by post, according to centre preference. Patients were asked to complete the questionnaire at home on the morning after their test and to return it in a prepaid envelope. A reminder was sent by post if the patient had not responded within 14 days of the randomised procedure.
Patients were asked to rate the acceptability of the bowel preparation (‘how acceptable did you find the bowel preparation?’) using a four-item scale ranging from ‘not at all acceptable’ (score 1) to ‘very acceptable’ (score 4). The questionnaire also asked patients to identify the least acceptable part of their investigation: ‘bowel preparation’, ‘bowel test’, or ‘other – please specify’. Acceptability of the randomised procedure was assessed using an adapted version of a previously validated scale to assess satisfaction with colonoscopy. 52 There were 29 items, each rated on a 7-point scale anchored at the end point (e.g. ‘felt out of control’ to ‘felt in control’). Several items were added to make the scale more relevant for participants having a BE or CTC (see bold items in Table 28). In accordance with previous research,18 we divided the scale into three subcomponents – satisfaction, worry and physical discomfort – each of which met the standard threshold for adequate internal reliability (Cronbach’s alpha= 0.84, 0.72 and 0.87, respectively, for patients in the BE trial, and 0.85, 0.72 and 0.85, respectively, for patients in the colonoscopy trial). Higher scores reflected greater degrees of the construct being measured by each subscale. The questionnaire also assessed patients’ experience in the 24 hours following the test with respect to the following eight complaints: ‘abdominal pain/cramps’, ‘nausea/vomiting’, ‘faint feeling or dizziness’, ‘wind’, ‘bottom soreness’, ‘soiling’, ‘sleep difficulties’ and ‘anxiety’. Four response options ranged from ‘none’ to ‘severe’.
| Satisfaction | |
|---|---|
| Dissatisfied | Satisfied |
| Staff were not interested in me | Staff were interested in me |
| I was not pleased with how it went | I was pleased with how it went |
| Staff were cold | Staff were warm |
| Staff were not informative | Staff were informative |
| Undignified | Dignified |
| I was not interested | I was interested |
| Not confident in staff | Confident in staff |
| Not enough privacy | Enough privacy |
| Loss of modesty | No loss of modesty |
| Worry | |
| Worried | Not worried |
| Agitated | Calm |
| I was worried about what they would find | Not worried about what they would find |
| Did not understand what was happening | Understood what was happening |
| I felt puzzled | I did not feel puzzled |
| I was confused | I was not confused |
| Discomfort | |
| Painful | Not painful |
| I’d have preferred to been less awake | I’d have preferred to be more awake |
| Uncomfortable | Comfortable |
| A bad experience | A good experience |
| Felt out of control | Felt in control |
| Soreness | No soreness |
| Afraid of making a fool of myself | Not afraid of making a fool of myself |
| Bloated afterwards | Not bloated afterwards |
| Intrusive | Not intrusive |
| Hard to cope with | Easy to cope with |
| Tired afterwards | Not tired afterwards |
| Claustrophobic | Not claustrophobic |
Follow-up questionnaire
A further questionnaire was sent to patients by post, 3 months after the randomised procedure, excluding any patients who had died or received a diagnosis of cancer. Reminders were posted to all patients who did not respond within 14 days.
Follow-up questionnaires asked patients to indicate when they had received their test results (10 options ranging from ‘during the test’ to ‘I have not had the results yet’), how results had been relayed (‘face to face’, ‘on the phone’, ‘in a letter’, ‘can’t remember’) and from whom (‘the hospital’, ‘my GP’, ‘can’t remember’). Patients were asked to rate satisfaction with the way results had been explained (using a 4-point scale from ‘very satisfied’ to ‘very dissatisfied’). Patients indicating that they had been referred for a follow-up colonic examination were asked ‘did you mind having the follow-up test?’ (using a 4-point scale ranging from ‘not at all’ to ‘very much’). Details of follow-up tests were obtained from the main trial data set and referrals precipitated by extracolonic findings were excluded.
Positive psychological consequences of the diagnostic episode were assessed using a six-item adapted version of the Psychological Consequences of Screening Questionnaire positive emotional subscale. 53 Items were measured on a 4-point scale with higher scores indicating superior outcomes. One item regarding anxiety reduction related to bowel cancer included an option of ‘did not feel anxious’. Negative affect was assessed using 10 items from the Positive and Negative Affect Schedule,54 rated on a 5-point scale ranging from ‘very slightly or not at all’ to ‘extremely’.
Statistical analysis
Basic patient information (randomised procedure, age, sex, post code) was obtained from data recorded in the main trial data set. Each patient’s post code was used to derive a marker of area-based socioeconomic deprivation,55 the Index of Multiple Deprivation (IMD). The IMD uses census-derived indicators of income, education, employment, environment, health and housing at a small-area level to generate a scale from 0 (least deprived) to 80 (most deprived). We grouped IMD scores into tertiles of lower, medium and higher levels of deprivation.
Full data were available for patient age, sex, post code and procedures. Missing values in the patient acceptability scales were imputed for individuals who had completed at least 50% of the scale using the expectation maximisation method. 56 This uses regression analyses to estimate missing values based on individuals with complete data and other significant variables (chosen by the researcher).
Initial analysis revealed that responses were skewed towards the upper end of the distribution (indicating high levels of acceptability), therefore non-parametric methods were used to test for differences between groups and for any influence of patient characteristics, including age (< 70 years vs. ≥ 70 years), sex and level of patient-reported acceptability of bowel preparation (acceptable vs. unacceptable). The Mann–Whitney U-test was used to compare two groups and the Kruskal–Wallis test was used to examine differences between three or more groups (i.e. deprivation tertiles). Post-test complications were reported using frequency statistics and analysed using Pearson’s chi-squared test.
Results
Post-test questionnaire
During the period in which the questionnaires were administered, a total of 1018 patients were randomised in the BE trial (675 BE and 343 CTC) (Figure 7). Ninety-seven patients were excluded because they did not undergo the randomised procedure (n = 84) or because they had withdrawn consent (n = 13). The final sample therefore comprised 921 patients (606 BE, 315 CTC), of whom 674 responded to the questionnaire (61% female, median age 68 years), giving a response rate of 73% (450 randomised to BE and 224 to CTC).
FIGURE 7.
A Consolidated Standards of Reporting Trials (CONSORT) diagram of participant flow through the BE vs. CTC trial. a, Total number analysed varied by measure.
In the same period, 595 patients were randomised in the colonoscopy trial (397 colonoscopy and 198 CTC). Forty-eight were excluded because they did not undergo the randomised procedure (n = 33), withdrew consent (n = 13) or died before the procedure (n = 2). Therefore, the final sample comprised 547 patients (362 colonoscopy and 185 CTC), of whom 388 responded to the questionnaire (55% female, median age 67 years), giving a response rate of 71% (258 randomised to colonoscopy and 130 to CTC).
In both trials, patients were more likely to return the post-test questionnaire if they were from a less socioeconomically deprived area (BE trial, IMD median score = 13.9 for responders vs. 16.6 for non-responders, p = 0.004; colonoscopy trial, IMD median score = 17.5 for responders vs. 21.0 for non-responders, p = 0.002). Response status was unaffected by age, sex or randomised procedure. Patients who failed to complete at least 50% of the satisfaction, worry and discomfort scales were excluded from imputation (16 in the BE trial and nine in the colonoscopy trial).
Acceptability of bowel preparation
In both trials, the median score for tolerability of bowel preparation was 3, indicating a response of ‘fairly acceptable’. A substantial proportion of patients rated bowel preparation as either ‘not at all acceptable’ or only ‘slightly acceptable’ (BE trial, 23% of patients having a BE and 17% of patients having CTC; colonoscopy trial, 23% of patients having colonoscopy and 18% having CTC).
Patient satisfaction, worry and discomfort
Individuals having a BE were slightly less satisfied than those having CTC (median 61, IQR 54–67 vs. median 64, IQR 56–69, respectively; p = 0.003). Two individual items on this scale reached significance – ‘dignity’ and ‘modesty’ – with patients having a BE responding less favourably than those having CTC (Table 29). Similarly, individuals having colonoscopy were slightly less satisfied than those having CTC (median 61, IQR 55–67, vs. median 64, IQR 58–70; p = 0.008). The two individual items that reached significance were ‘staff were interested in me’ and ‘confident in staff’, with patients having colonoscopy responding less favourably (Table 30).
| Item | BE (n = 436) | CTC (n = 222) | p-value | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Satisfaction scale | |||||
| Satisfied | 7 | 5–7 | 7 | 5–7 | 0.131 |
| Staff were interested in me | 7 | 6–7 | 7 | 7–7 | 0.279 |
| I was pleased with how it went | 7 | 6–7 | 7 | 6–7 | 0.087 |
| Staff were warm | 7 | 6–7 | 7 | 7–7 | 0.290 |
| Staff were informative | 7 | 6–7 | 7 | 6–7 | 0.343 |
| Dignified | 4 | 2–6 | 5 | 3–7 | 0.000 |
| I was interested | 7 | 6–7 | 7 | 6–7 | 0.111 |
| Confident in staff | 7 | 7–7 | 7 | 7–7 | 0.629 |
| No loss of modesty | 5 | 3–7 | 6 | 4–7 | 0.001 |
| Enough privacy | 7 | 6–7 | 7 | 6–7 | 0.692 |
| Worry scale | |||||
| Worried | 3 | 1–5 | 4 | 1–5 | 0.984 |
| Agitated | 2 | 1–4 | 2 | 1–4 | 0.127 |
| I was worried about what they would find | 4 | 3–6 | 4 | 2–7 | 0.850 |
| Did not understand what was happening | 1 | 1–2 | 1 | 1–2 | 0.749 |
| I felt puzzled | 1 | 1–2 | 1 | 1–2 | 0.494 |
| I was confused | 1 | 1–2 | 1 | 1–2 | 0.567 |
| Physical discomfort scale | |||||
| Painful | 3 | 1–4 | 2 | 1–4 | 0.005 |
| I would have preferred to be less awake | 4 | 2–4 | 4 | 2–4 | 0.739 |
| Uncomfortable | 4 | 3–6 | 4 | 2–5 | 0.003 |
| A bad experience | 4 | 2–4 | 3 | 1–4 | 0.000 |
| Felt out of control | 4 | 1–4 | 3 | 1–4 | 0.071 |
| Soreness | 2 | 1–4 | 1 | 1–4 | 0.000 |
| Afraid of ‘making a fool of myself’ | 2 | 1–4 | 2 | 1–4 | 0.012 |
| Claustrophobic | 1 | 1–2 | 1 | 1–2 | 0.206 |
| Bloated afterwards | 5 | 3–7 | 4 | 2–7 | 0.031 |
| Intrusive | 3 | 1–5 | 3 | 1–4 | 0.004 |
| Hard to cope with | 2 | 1–4 | 2 | 1–3 | 0.000 |
| Difficult to do what was required | 2 | 1–3 | 1 | 1–2 | 0.000 |
| Tired afterwards | 3 | 1–5 | 2 | 1–5 | 0.135 |
| Item | Colonoscopy (n = 251) | CTC (n = 128) | p-value | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Satisfaction scale | |||||
| Satisfied | 7 | 5–7 | 7 | 6–7 | 0.122 |
| Staff were interested in me | 7 | 6–7 | 7 | 7–7 | 0.038 |
| I was pleased with how it went | 7 | 6–7 | 7 | 6–7 | 0.123 |
| Staff were warm | 7 | 6–7 | 7 | 7–7 | 0.076 |
| Staff were informative | 7 | 6–7 | 7 | 6–7 | 0.335 |
| Dignified | 4 | 3–7 | 5 | 4–7 | 0.157 |
| I was interested | 7 | 6–7 | 7 | 6–7 | 0.263 |
| Confident in staff | 7 | 6–7 | 7 | 7–7 | 0.019 |
| No loss of modesty | 6 | 4–7 | 6 | 4–7 | 0.224 |
| Enough privacy | 7 | 6–7 | 7 | 6–7 | 0.066 |
| Worry scale | |||||
| Worried | 4 | 2–6 | 3 | 1–5 | 0.017 |
| Agitated | 2 | 1–4 | 2 | 1–4 | 0.579 |
| I was worried about what they would find | 5 | 4–7 | 4 | 2–6 | < 0.0005 |
| Did not understand what was happening | 1 | 1–2 | 1 | 1–2 | 0.727 |
| I felt puzzled | 1 | 1–2 | 1 | 1–2 | 0.610 |
| I was confused | 1 | 1–2 | 1 | 1–2 | 0.160 |
| Physical discomfort scale | |||||
| Painful | 2 | 1–5 | 2 | 1–4 | 0.010 |
| I would have preferred to have been less awake | 4 | 4–6 | 4 | 2–4 | < 0.0005 |
| Uncomfortable | 4 | 2–6 | 4 | 2–5 | 0.931 |
| A bad experience | 4 | 2–4 | 3 | 1–4 | 0.003 |
| Felt out of control | 4 | 1–5 | 3 | 1–4 | 0.175 |
| Soreness | 2 | 1–4 | 2 | 1–4 | 0.149 |
| Afraid of making a fool of myself | 2 | 1–4 | 2 | 1–4 | 0.235 |
| Claustrophobic | 1 | 1–2 | 1 | 1–1 | 0.609 |
| Bloated afterwards | 3 | 1–5 | 4 | 2–5 | 0.318 |
| Intrusive | 3 | 1–4 | 2 | 1–4 | 0.241 |
| Hard to cope with | 2 | 1–4 | 2 | 1–3 | 0.001 |
| Hard to do what was required | 1 | 1–3 | 1 | 1–2 | 0.103 |
| Tired afterwards | 3 | 1–6 | 2 | 1–5 | 0.003 |
There were no significant differences between BE and CTC with regard to reported worry overall (median 15, IQR 10–20, vs. median 15, IQR 9.75–19, respectively; p = 0.617); nor were there significant differences between BE and CTC on any of the individual items (see Table 29). There were, however, significant differences in reported worry between colonoscopy and CTC (median 16, IQR 12–21 vs. median 15, IQR 9–19; p = 0.007). The two individual items that reached significance were ‘worried’ and ‘worried about what they would find’, with patients having colonoscopy responding less favourably (see Table 30).
Overall, physical discomfort was rated as significantly worse by patients having a BE than by those undergoing CTC (median 40, IQR 29–52 vs. median 35.5, IQR 25–47, respectively; p < 0.001). Significant differences were observed for nine individual items on the discomfort subscale – ‘painful’, ‘uncomfortable’, ‘a bad experience’, ‘soreness’, ‘afraid of “making a fool of myself” ’, ‘bloated afterwards’, ‘intrusive’, ‘hard to cope with’ and ‘difficult to do what was required’ – with patients having a BE responding less favourably (see Table 29). Physical discomfort was also rated as significantly worse by patients having colonoscopy than by those undergoing CTC (median 39, IQR 29–51, vs. median 35, IQR 24–44; p = 0.001). The five individual items for which significant differences were observed for were ‘painful’, ‘would have preferred to be less awake’, ‘a bad experience’, ‘hard to cope with’ and ‘tired afterwards’, with patients having colonoscopy responding less favourably (see Table 30).
Regarding patient demographics, only age was associated with differences in patient experience in the BE trial; there was no effect of sex or IMD. Specifically, among participants randomised to CTC, older patients reported less physical discomfort and worry than younger patients. No such differences were found for patients randomised to BE (Table 31). In the colonoscopy trial, only sex was associated with differences in patient experience. Among participants randomised to colonoscopy, women reported less satisfaction, more worry and more physical discomfort. No such differences were found for patients randomised to CTC (Table 32).
| BE (n = 436) | CTC (n = 222) | |||||
|---|---|---|---|---|---|---|
| Satisfaction score (median, IQR) | Worry score (median, IQR) | Discomfort score (median, IQR) | Satisfaction score (median, IQR) | Worry score (median, IQR) | Discomfort score (median, IQR) | |
| Age (years) | ||||||
| < 70 | 60, 54–66 | 15, 10.5–20 | 41, 31–53.5 | 63, 55–68 | 16, 11–20 | 37, 27–50 |
| ≥ 70 | 62, 55–68 | 15, 10–21 | 38, 28–52 | 65, 57–70 | 13, 8–18 | 33, 23–45 |
| p-value | 0.057 | 0.889 | 0.130 | 0.138 | 0.017 | 0.009 |
| Sex | ||||||
| Male | 62, 54–66 | 14, 10–20 | 38.5, 29–49.3 | 63, 55.8–69 | 14, 9–19 | 32.5, 22–45 |
| Female | 61, 54–67 | 15, 10–21 | 41, 29–56 | 64, 57.3–69 | 16, 10–19 | 37, 26–48 |
| p-value | 0.859 | 0.491 | 0.104 | 0.343 | 0.544 | 0.066 |
| IMD | ||||||
| Low | 61, 54–67 | 15, 10.5–20 | 41, 29.5–54 | 63, 56–68 | 14.5, 9–19 | 35.5, 25.3–47 |
| Mid | 60.5, 54–66 | 15, 10–20.3 | 40, 30–52.3 | 64, 58.3–68 | 15, 10–19.8 | 37, 26–48.8 |
| High | 63, 57–67.5 | 15, 10–21 | 39, 28–51.5 | 65, 55.8–70 | 17, 9–19 | 33.5, 22–44.5 |
| p-value | 0.254 | 0.841 | 0.883 | 0.518 | 0.825 | 0.194 |
| Colonoscopy (n = 251) | CTC (n = 128) | |||||
|---|---|---|---|---|---|---|
| Satisfaction (median, IQR) | Worry (median, IQR) | Discomfort (median, IQR) | Satisfaction (median, IQR) | Worry (median, IQR) | Discomfort (median, IQR) | |
| Age (years) | ||||||
| < 70 | 61, 54–67 | 17, 12–21 | 41, 30–53 | 63, 58–70 | 16, 11–19 | 36, 24–45 |
| ≥ 70 | 63, 58–69 | 15, 12–20 | 39, 25–48 | 67, 59–70 | 11, 9–20 | 33, 23–44 |
| p-value | 0.256 | 0.224 | 0.059 | 0.253 | 0.324 | 0.595 |
| Sex | ||||||
| Male | 63, 58–69 | 14, 11–19 | 36, 25–45 | 64, 59–70 | 12, 9–18 | 34, 24–41 |
| Female | 61, 53–66 | 18, 13–22 | 44, 33–57 | 65, 57–70 | 16, 9–20 | 36, 24–48 |
| p-value | 0.008 | 0.001 | < 0.0005 | 0.606 | 0.232 | 0.350 |
| IMD | ||||||
| Low | 61, 54–67 | 15, 11–20 | 40, 30–51 | 65, 58–70 | 12, 8–18 | 33, 23–43 |
| Mid | 62, 56–68 | 17, 13–21 | 38, 28–48 | 65, 56–69 | 16, 11–21 | 36, 26–47 |
| High | 61, 53–68 | 17, 12–21 | 44, 30–58 | 63, 59–70 | 15, 11–20 | 37, 24–44 |
| p-value | 0.651 | 0.505 | 0.300 | 0.747 | 0.212 | 0.643 |
Post-test complications
In the BE trial, patients in both groups reported post-test complaints: ‘wind’ {92% for BE vs. 84% for CTC; chi-squared [1 degree of freedom (df), n = 647 patients included] = 11.15; p = 0.001}, ‘abdominal pain/cramps’ [68% vs. 57%; chi-squared (1 df, n = 645 patients included) = 7.61; p = 0.007], ‘bottom soreness’ [57% vs. 37%; chi-squared (1 df, n = 646 patients included) = 21.81; p < 0.001], ‘nausea/vomiting’ [16% vs. 8%; chi-squared (1 df, n = 636 patients included) = 6.72; p = 0.009), and ‘soiling’ [31% vs. 23%; chi-squared (1 df, n = 639 patients included) = 4.67; p = 0.034] were all significantly more common for BE than CTC (Table 33). The severity of post-procedural wind was greatest for BE [27% reporting ‘severe’ as opposed to 15% for CTC; chi-squared (1 df, n = 647 patients included) = 11.47; p = 0.001]. No other symptoms differed significantly between the groups. In the colonoscopy trial, only one symptom differed significantly: ‘faint feeling or dizziness’ was significantly more common after colonoscopy than after CTC (82/246 vs. 28/122; p = 0.039). Rates of other post-test complaints are shown in Table 34.
| Symptom | Test | None (%) | Mild (%) | Moderate (%) | Severe (%) |
|---|---|---|---|---|---|
| Abdominal pain/cramps | BE | 31.8 | 26.6 | 29.3 | 13.2 |
| CTC | 42.7 | 29.5 | 18.6 | 9.1 | |
| Nausea/vomiting | BE | 84.4 | 8.9 | 4.8 | 1.9 |
| CTC | 91.7 | 6.4 | 1.8 | 0.0 | |
| Faint feeling or dizziness | BE | 76.3 | 16.1 | 7.1 | 0.5 |
| CTC | 73.9 | 18.8 | 6.9 | 0.5 | |
| Wind | BE | 7.7 | 22.8 | 42.3 | 27.2 |
| CTC | 16.3 | 37.6 | 30.8 | 15.4 | |
| Bottom soreness | BE | 43.5 | 28.2 | 21.9 | 6.4 |
| CTC | 62.9 | 24.0 | 9.5 | 3.6 | |
| Soiling | BE | 68.6 | 15.2 | 12.1 | 4.0 |
| CTC | 76.7 | 16.4 | 4.6 | 2.3 | |
| Sleep difficulties | BE | 72.4 | 14.0 | 11.7 | 1.9 |
| CTC | 78.2 | 12.6 | 6.0 | 3.2 | |
| Anxiety | BE | 61.8 | 21.8 | 13.5 | 2.8 |
| CTC | 67.7 | 18.6 | 10.0 | 3.6 |
| Symptom | Test | n | None (%) | Mild (%) | Moderate (%) | Severe (%) | Any severity (%) | p-valuea |
|---|---|---|---|---|---|---|---|---|
| Abdominal pain/cramps | Colonoscopy | 246 | 48 | 36 | 13 | 4 | 53 | 0.507 |
| CTC | 121 | 51 | 26 | 18 | 5 | 49 | ||
| Nausea/vomiting | Colonoscopy | 241 | 85 | 7 | 7 | 1 | 15 | 0.072 |
| CTC | 121 | 92 | 5 | 3 | 1 | 8 | ||
| Faint feeling or dizziness | Colonoscopy | 246 | 67 | 24 | 7 | 2 | 33 | 0.032 |
| CTC | 122 | 78 | 16 | 6 | 1 | 22 | ||
| Wind | Colonoscopy | 248 | 20 | 34 | 35 | 12 | 80 | 0.562 |
| CTC | 123 | 23 | 34 | 32 | 11 | 77 | ||
| Bottom soreness | Colonoscopy | 243 | 58 | 27 | 12 | 2 | 42 | 0.609 |
| CTC | 124 | 56 | 21 | 19 | 5 | 44 | ||
| Soiling | Colonoscopy | 240 | 81 | 13 | 5 | 2 | 19 | 0.629 |
| CTC | 122 | 79 | 16 | 5 | 0 | 21 | ||
| Sleep difficulties | Colonoscopy | 236 | 75 | 13 | 11 | 1 | 25 | 0.224 |
| CTC | 122 | 80 | 11 | 5 | 4 | 20 | ||
| Anxiety | Colonoscopy | 241 | 72 | 18 | 8 | 3 | 28 | 0.520 |
| CTC | 124 | 69 | 27 | 4 | 1 | 32 |
Least acceptable aspects of the patient experience
The majority of respondents stated that bowel preparation was the least acceptable aspect of the experience (BE trial, 69% for BE and 74% for CTC; colonoscopy trial, 66% for colonoscopy and 78% for CTC). A smaller proportion rated the test itself as the least acceptable aspect (BE trial, 24% for BE and 14% for CTC; colonoscopy trial, 25% for colonoscopy and 14% for CTC). The remaining patients cited other aspects such as the waiting time after arriving for their appointment.
Follow-up questionnaire
Follow-up questionnaires were analysed only for patients in the colonoscopy trial, to look for any longer-term differences in experience between patients having radiological or endoscopic procedures.
A total of 337 patients responded to the follow-up questionnaire (59% female, median age 67 years), which was a response rate of 62% (230 colonoscopy, 107 CTC). As with the post-test questionnaires, patients were significantly more likely to respond if they were from less socioeconomically deprived areas (IMD median score = 17.2 for responders vs. 20.5 for non-responders; p = 0.015). Women were also significantly more likely to respond to the follow-up questionnaire than men (p = 0.003). Response was unaffected by age or procedure.
Receiving results
Patients having colonoscopy were significantly more likely to receive their results on the same day than at a later time (65% vs. 17% for CTC; p < 0.0005). Colonoscopy patients were also more likely to receive results via a face-to-face conversation than by telephone or post (85% vs. 50%; p < 0.0005) and more likely to receive them from the hospital than from their GP (94% vs. 80%; p = 0.001). Patients having colonoscopy were significantly more satisfied with the way results were conveyed than were those having CTC (median 4, IQR 3–4, vs. median 3, IQR 3–3; p < 0.0005).
Referral for follow-up investigations
Significantly more responders to the follow-up questionnaire had additional colonic tests following CTC than colonoscopy [33% (37/107) vs. 7% (17/230); p < 0.0005]. Among patients referred for colonic follow-up testing and responding to the appropriate question, two out of eight (25%) in the colonoscopy group reported that they did mind being referred, compared with 9 out of 23 responding patients (39%) referred following CTC. No statistical analysis was attempted because of the small sample size.
Psychological outcomes
At 3 months, there was no significant difference between CTC and colonoscopy in positive psychological consequences of the diagnostic episode (p-values ranged from 0.153 to 0.844 for all six items) (Table 35). A trend was observed towards higher levels of negative affect for patients having colonoscopy than those having CTC (median 12, IQR 10–17, for colonoscopy vs. median 11, IQR 10–15, for CTC). However, this did not reach significance (p = 0.050).
| Item (scored 1–4) | Colonoscopy (n = 224) | CTC (n = 102) | p-value | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Given me a sense of reassurance that I do not have bowel cancer | 4 | 3–4 | 4 | 3–4 | 0.486 |
| Made me feel more able to do the things that I normally do | 3 | 1–3 | 3 | 1–4 | 0.326 |
| Made me more hopeful about the future | 3 | 2–4 | 3 | 2–4 | 0.653 |
| Made me get on better with those around me | 2 | 1–3 | 3 | 1–3 | 0.153 |
| Given me a greater sense of well-being | 3 | 2–4 | 3 | 2–4 | 0.844 |
| Made me feel less anxious about bowel cancera | 3 | 3–4 | 3 | 3–4 | 0.676 |
Chapter 6 Health economic assessment
Introduction
The SIGGAR study aimed to provide a detailed comparison of CTC with BE and with colonoscopy for diagnosis of CRC or large polyps in symptomatic patients. To provide a complete picture of the relative advantages of the three procedures, and to assess the feasibility of more widespread implementation of CTC within the NHS, an economic analysis is an important part of the study.
The advantage of the SIGGAR randomised trial design, over most previous studies in which patients receive both CTC and colonoscopy, is that it provides extensive data on referral rates for additional tests after BE, colonoscopy or CTC in normal clinical practice and on the nature of the follow-up tests undertaken. This allows us to calculate the cost of each procedure, taking account of the full series of tests it will typically give rise to.
This chapter is divided into two parts. In part 1, previous economic studies of diagnostic and screening tests are reviewed systematically. In part 2, we model costs and effects based on trial data that cover a minimum follow-up period of 3 years and a median follow-up duration of 5.2 years. In this section, we first model costs from resource-use data and then combine this with detection rates to calculate incremental costs per case detected for large polyps or cancer and for colon cancer alone. In subsequent work, we have modelled the consequences of polyp and colon cancer detection over the remaining lifespan to calculate incremental cost–utility ratios (submitted for publication).
Part 1: literature review on economic studies of computed tomographic colonography
Screening and diagnosis
Several systematic reviews of economic studies relating to methods for detecting CRC have been published in recent years. The majority of the literature has focused on screening rather than diagnosis in symptomatic patients. The results of economic evaluations of screening programmes cannot be translated directly into cost-effectiveness measures of the same procedures when used for diagnosis in symptomatic patients. First, the prevalence of CRC and large polyps is likely to differ between the two settings. Second, cancers identified in screening populations are likely to be systematically different (e.g. growing less rapidly) to those identified in a symptomatic setting. These factors will influence predictive values and may also influence sensitivity and specificity. Furthermore, compliance is likely to be lower in a screening programme than in a symptomatic setting. Consequently, the relative cost-effectiveness of competing detection methods in a screening setting does not necessarily reflect their relative cost-effectiveness when used as diagnostic tests in symptomatic patients. Although this limits the relevance of much of the available literature related to screening for CRC, it is not entirely inapplicable to our study. The screening literature provides information on costs and outcomes of various clinical pathways downstream of the detection of colonic lesions. Therefore, our review included both screening and symptomatic diagnosis of CRC.
Cost-effectiveness: literature
We carried out a systematic review using the following search strategy:
Economic evaluations were identified by searching PubMed for economic evaluations of CRC diagnosis or screening, in which CTC was compared with colonoscopy. The search included the keywords ‘colorectal cancer’, ‘diagnosis’, ‘screening’, ‘CT-colonography’, ‘computerized tomography colonography’, ‘virtual colonoscopy’, ‘colonoscopy’ and ‘cost-effectiveness’, ‘cost-utility’, ‘cost benefit’, ‘life years’, ‘quality life year’. The search was restricted to articles published between July 1999 and July 2013, and in the English language.
This revealed a recent systematic review of 16 studies dealing with screening tests for colon cancer. 57 We proceeded as follows:
-
We extracted data dealing specifically with CTC compared with colonoscopy from the above systematic review. Fourteen papers satisfied this criterion. 58–71
-
We updated the above systematic review of screening tests finding one more paper dealing with CTC compared with colonoscopy;72 therefore, we analysed 15 papers on this topic.
-
We also repeated the search to include papers comparing CTC with colonoscopy in symptomatic diagnostic studies, finding one such paper. 73
Cost-effectiveness: findings
The systematic review of screening tests included 14 studies comparing CTC with colonoscopy. Twelve of these 14 studies were based on Markov models58–61,63,65–71 and two on microsimulations. 62,64 Eleven studies58–63,66–68,70,71 provided outcomes in terms of life-years saved, while two64,69 derived quality-adjusted life-years (QALYs) and one65 reported both life-years and QALYs. In six studies,61–64,69,70 colonoscopy dominated CTC. In the only study that concluded CTC dominated colonoscopy, the effects of CTC in detecting extracolonic lesions were considered. 59 The incremental cost-effectiveness ratios (ICERs) among the remaining seven studies58,60,65–68,71 varied from US$2144 to US$498,668, with a tendency for more recent studies to yield more favourable ratios. The results were sensitive to a number of assumptions, including relative unit costs of the two tests and differences in screening uptake rates by test. They were somewhat insensitive to performance characteristics (sensitivity and specificity) within the plausible range. However, they were highly influenced by whether or not extracolonic lesions were included in the model – a point to which we will return. Lee et al. 71 reported that colonoscopy appeared marginally more effective than CTC but was more expensive in the UK setting, with an ICER of £34,002 per QALY (this was one of the many papers that did not consider extracolonic lesions). Knudsen et al. 62 used microsimulation models (MISCAN, SimCRC and CRC-SPIN) to project the natural history of polyp growth and cancer development. They concluded that CTC was dominated by colonoscopy. The additional paper by Vanness et al. 72 adopted the above simulation models but populated them with data from the ACRIN national CTC trial to compare three screening options: CTC, colonoscopy and flexible sigmoidoscopy plus faecal occult blood test or faecal immunochemical testing. 71 They reported that CTC is more costly and less effective than colonoscopy and was thus dominated by it.
As stated above, results were strongly influenced by the relative unit costs of CTC and colonoscopy. This may explain much of the difference in findings across the various studies. For example, the study by Lee et al. 71 was one to find that CTC was cost-effective in comparison with colonoscopy (albeit marginally so), and in this study CTC was also considerably less expensive (£128) than colonoscopy (£488). By contrast, Knudsen et al. 62 reported results in favour of colonoscopy despite similar assumptions regarding effectiveness and here the unit costs of the tests were reversed.
Extracolonic lesions
One of the important features of CTC is that it can detect lesions outside the colon. Hassan et al. 59 investigated the potential benefits of incidental detection of aortic aneurysms and extracolonic cancers, finding that CTC was dominant over colonoscopy59 when these were included in the model.
Only one study compared the use of CTC with colonoscopy in symptomatic patients (i.e. for diagnostic purposes). 73 This study adopted the Markov screening model produced by the School of Health and Related Research at the University of Sheffield, UK,74 and assumes that CTC and colonoscopy provide comparable accuracy for diagnosis of clinically significant polyps and CRC. They found that CTC (compared with colonoscopy) was a marginally cost-effective option for primary colonic imaging of symptomatic patients (ICER of £23,000).
Another study26 investigated resource use and costs associated specifically with incidental extracolonic findings from CTC, based on a retrospective cohort study. The authors found that resources consumed as a result of extracolonic findings in symptomatic patients approximately doubled the costs of CTC. The authors modelled the potential payback from detection of extracolonic lesions and we will return to this model later.
Conclusion
Previous cost-effectiveness studies of CTC have focused on screening for CRC rather than symptomatic diagnosis. The results of cost-effectiveness analyses favour colonoscopy over CTC, but there is very wide variance in the results. To a substantial extent, this reflects difference in the unit costs of each test – a surprisingly variable quantity. More problematic still, most studies ignored the potential benefits or harms of detecting extracolonic findings at CTC (a point discussed more fully in Part 2: health economic analysis within the trial). Finally, we found no studies comparing the cost-effectiveness of CTC with BE, perhaps because the latter test is becoming obsolete in high-income countries.
Our economic analysis is designed to evaluate costs and cost-effectiveness of CTC compared with BE and of CTC compared with colonoscopy, for diagnosis of CRC and large polyps (≥ 10 mm) in older symptomatic patients.
Part 2: health economic analysis within the trial
Introduction
In this section, we analyse the economic data over the trial follow-up period (median 5.2 years). We calculate costs contingent on following the diagnostic pathways compared in the two trials. However, few patients would be expected to die from cancer over the follow-up period. Therefore, in this section, our analysis is based on cost per case detected. We analyse cost per case of cancer or a large polyp and also the cost per case of cancer alone.
Methods
Perspective and time horizons
The economic analysis was conducted in the context of NHS secondary care (i.e. only costs relevant to secondary care were included). The time horizon was set at 5.2 years, which is the median follow-up for patients in the trial.
Trial data used in the analysis
The outcome measures were based on primary clinical end points observed in the trials. We collected data on detection rates of cancer and large polyps and the proportion of patients referred for further investigation or treatment. The number and nature of extracolonic findings were recorded for patients undergoing CTC.
Estimating the costs
Resources considered
Resource usage included the initial assigned procedures (BE, colonoscopy or CTC), medications to prepare a patient for the procedure and any subsequent procedures required to make a diagnosis. Downstream activities resulting from detection of colonic lesions included radiological investigations, surgical procedures, outpatient attendances, treatment for cancers or large polyps and hospitalisations as a result of major adverse events. Downstream activities to investigate and treat extracolonic lesions found at CTC were also recorded.
Resource usage on medications
Information was collected on medications and dosages used during the procedures. The main medications included pethidine, midazolam, fentanyl, Buscopan and glucagon. Doses recommended by the British National Formulary (BNF) were used if data were missing.
Information on medications used for bowel preparation was not collected individually during the trial. However, all centres were asked to provide details of the standard bowel preparation used for BE and CTC during the period the trial was in progress. Ultimately, we assumed that every patient having a procedure was given two sachets of Picolax, which was the preparation used in most centres.
Resource usage on diagnostic procedures
Details of all diagnostic and follow-up procedures were recorded. For colonoscopy, this included information on whether or not any biopsies were taken or polyps removed.
Resource usage on surgery for colonic lesions
Information was collected on surgery related to colonic findings. Almost all surgical procedures were carried out for the treatment of cancer. Detailed information was recorded on the type of operation performed, reasons for the operation, length of hospital stay and whether or not there were any complications during or following the procedure. Costs were based on the type of operation and length of stay.
Resource usage on adverse events
Adverse events were classified as major or minor. Major adverse events were defined as those involving unplanned hospitalisation or death. The costs of hospitalisation were estimated based on the actual length of stay and the specialty concerned. Costs were not attributed to minor adverse events.
Resource usage on outpatient appointments
A trial pro forma (see Appendix 1) was completed to capture data on outpatient attendances resulting from initial investigation of the colon. This form included a record of any referrals for additional investigation, such as a flexible sigmoidoscopy or CT scan.
Resource usage on extracolonic findings
All procedures relating to follow-up of extracolonic abnormalities detected by CTC were collected. This included the type of procedure(s) performed and the date on which they occurred. Information was collected on operations performed. However, patients were not followed up in the longer term. For example, we did not collect information on surgery for aneurysms that might have enlarged to the point at which surgery was indicated under surveillance initiated as a result of the study. In addition, data on the investigation of extracolonic lesions that manifested in the non-CTC arms of the trials were not collected. We will try to make good estimates of these deficiencies by staging outcomes using HES data.
Unit costs
The unit costs of medications were obtained from data published by the BNF in September 2011. 75 Table 36 reports this information. The unit costs of BE, CTC, colonoscopy and flexible sigmoidoscopy were obtained from NHS national reference costs at 2010–11 prices. 76,77 This information is presented in Table 37. The costs of colonoscopy and flexible sigmoidoscopy are classified according to whether or not a biopsy was taken or a polyp removed during the procedure. There is no specific national cost for CTC; instead we used the cost of a CT scan as an approximation (the 2010–11 reference cost: RA08Z-RA14Z Computerised Tomography Scan, more than three areas).
| Drug name | Preparation | Cost (£) | Standard dose for BE/CTC/colonoscopy |
|---|---|---|---|
| Buscopan (injection) | 20 mg/ml, 1 ml | 0.22 | Intravenous injection 10–40 mg |
| Glucagon (injection) | 1 mg | 11.52 | Intravenous injection 0.5–1 mg |
| Pethidine (injection) | 50 mg/ml, 1 ml | 0.43 | Intravenous injection 25–50 mg |
| Midazolam (injection) | 1 mg/ml, 2 ml | 0.50 | Intravenous injection 1–2 mg |
| Fentanyl (injection) | 50 µg/ml, 2 ml | 0.30 | Intravenous injection 25–100 µg |
| Picolax | 10 mg/sachet × 2 | 3.39 | Two sachets before the procedure |
| Intervention | Value (£) | Low range (£) | High range (£) | HRG4 codes | Description |
|---|---|---|---|---|---|
| Colonoscopy alone | 330 | 287 | 395 | FZ51Z | Diagnostic colonoscopy ≥ 19 years |
| Colonoscopy with biopsy | 385 | 305 | 450 | FZ52Z | Diagnostic colonoscopy with biopsy ≥ 19 years |
| Colonoscopy with polyps or CRC removed | 450 | Used the high range of colonoscopy with biopsy | |||
| FS alone | 220 | 150 | 253 | FZ54Z | Diagnostic FS ≥ 19 years |
| FS with biopsy | 253 | Used the high range of flexible sigmoidoscopy alone | |||
| FS with polyps or CRC removed | 450 | Assumed the same as the high range of colonoscopy with biopsy | |||
| BE | 135 | 91 | 162 | RA17Z | Contrast fluoroscopy procedures 20–40 minutes |
| CTC | 160 | 89 | 186 | RA14Z | CT scan, more than three areas |
Analysis
The total costs per patient were calculated as the sum of the products of resources used and their unit costs.
The costs of diagnosis associated with each investigative pathway were evaluated according to the original randomised group and the differences in costs compared. The following cost comparisons were carried out:
-
total costs contingent on assigned procedure, excluding the costs of investigation and treatment for incidental extracolonic lesions
-
the cost per case detected for colon cancer or a large polyp and also for colon cancer alone.
Initially, we had intended to compare all the costs contingent on each diagnostic strategy. This would have included the costs of investigating and treating incidental extracolonic lesions across all groups. However, these costs were collected only for patients having CTC. It could not be assumed that there were no similar additional costs in the BE and colonoscopy arms, even within the time scale of the study. Pending further investigation using national HES data, we have presented the costs of following up extracolonic lesions after CTC in separate tables. Failure to include these costs for patients having other procedures will exaggerate the cost difference between CTC and these procedures. However, failure to collect information on surgery for extracolonic lesions in the longer term will lead to an underestimate in CTC costs. Given these uncertainties, we decided to analyse the costs and benefits of detecting extracolonic lesions separately from those of detecting colonic lesions.
The cost per additional cancer or large polyp detected by CTC was compared with that for the other two strategies. In addition, the mean intervals between presentation and diagnosis were compared for each modality. Total costs for each patient, contingent on their final diagnosis, were calculated for the trial period, with the caveat that a separate calculation was carried out for the cost of following up extracolonic lesions.
All analyses were based on the principle of intention to treat. Bootstrap methods were used to estimate costs and cost differences. ICERs with their CIs and a scatterplot were produced based on 1000 replicates. The analyses were conducted in SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Costs over diagnostic sequence (excluding extracolonic lesions)
In terms of the costs of the procedures themselves (unit costs), BE is costed at £135, CTC at £160 and colonoscopy at £330. However, the total costs associated with each procedure are higher (by over 100%) as they take into account the various contingent downstream costs such as repeat procedures, complications and operations. These total costs do not take account of those arising from detection of incidental extracolonic lesions. In the BE trial, the mean total cost per person was £460 for BE and £532 for CTC. In the colonoscopy trial, it was £739 for colonoscopy and £674 for CTC. Details of costs by procedure are presented in Table 38. Colonoscopy is more expensive than CTC (despite the considerably higher requirement for follow-up investigations after CTC), but the difference is £65 per patient and well within the limits of statistical precision. BE is less expensive than CTC, with a cost difference of £72 per patient, also within the limits of statistical precision. The higher costs of CTC in the colonoscopy trial than in the BE trial can be attributed to the higher risk of patients, on average, in the colonoscopy trial (see Chapter 2).
| BE trial | Colonoscopy trial | |||
|---|---|---|---|---|
| BE (n = 2527) | CTC (n = 1277) | Colonoscopy (n = 1047) | CTC (n = 533) | |
| Diagnostic procedure | 184 (178 to 189) | 231 (222 to 240) | 368 (360 to 375) | 266 (250 to 281) |
| Colonic surgery | 223 (179 to 266) | 242 (178 to 306) | 295 (218 to 373) | 323 (212 to 434) |
| Other examinations | 1 (1 to 2) | 1 (0 to 1) | 1 (0 to 2) | 0 (0 to 1) |
| Outpatient administration | 48 (46 to 51) | 54 (50 to 58) | 71 (67 to 76) | 86 (79 to 92) |
| Adverse event | 4 (–1 to 10) | 4 (–4 to 12) | 4 (–2 to 10) | 0 |
| Total | 460 (415 to 506) | 532 (465 to 599) | 739 (660 to 819) | 674 (559 to 790) |
Incremental cost-effectiveness ratio (excluding extracolonic lesions)
Here we consider the differences in cost per case detected as a result of the initial diagnostic sequence. CTC and colonoscopy detected a similar proportion of both cancers and large polyps, but with a slight (non-significant) trend in favour of colonoscopy, which detected an additional 6.8 such lesions per 1000 cases. Based on this difference in point estimates, the incremental cost per significant lesion detected by colonoscopy was £9543. Cancer detection rates differed by only 1 in 10,000 so the incremental cost-effectiveness is large (£650,000 per case detected).
The difference in detection rates for significant lesions in the BE trial was statistically significant and in this case CTC detected more lesions, with a difference in the point estimate of 17 significant lesions per 1000 cases. Scatter plots of differences in costs and detection rates of cancer or large polyps for CTC compared with BE are shown in Figure 8. The figure shows the positive correlation between increased costs and increased detection rates for CTC compared with BE. The incremental cost per neoplasm detected by CTC was £4235 (95% CI £395 to £9656).The corresponding ratio for detection of an additional three cases of colon cancer per 1000 patients (a statistically non-significant difference) was £24,000 per cancer.
FIGURE 8.
Scatter plot of difference in costs vs. difference in detection rate of cancer or large polyps for CTC compared with BE.
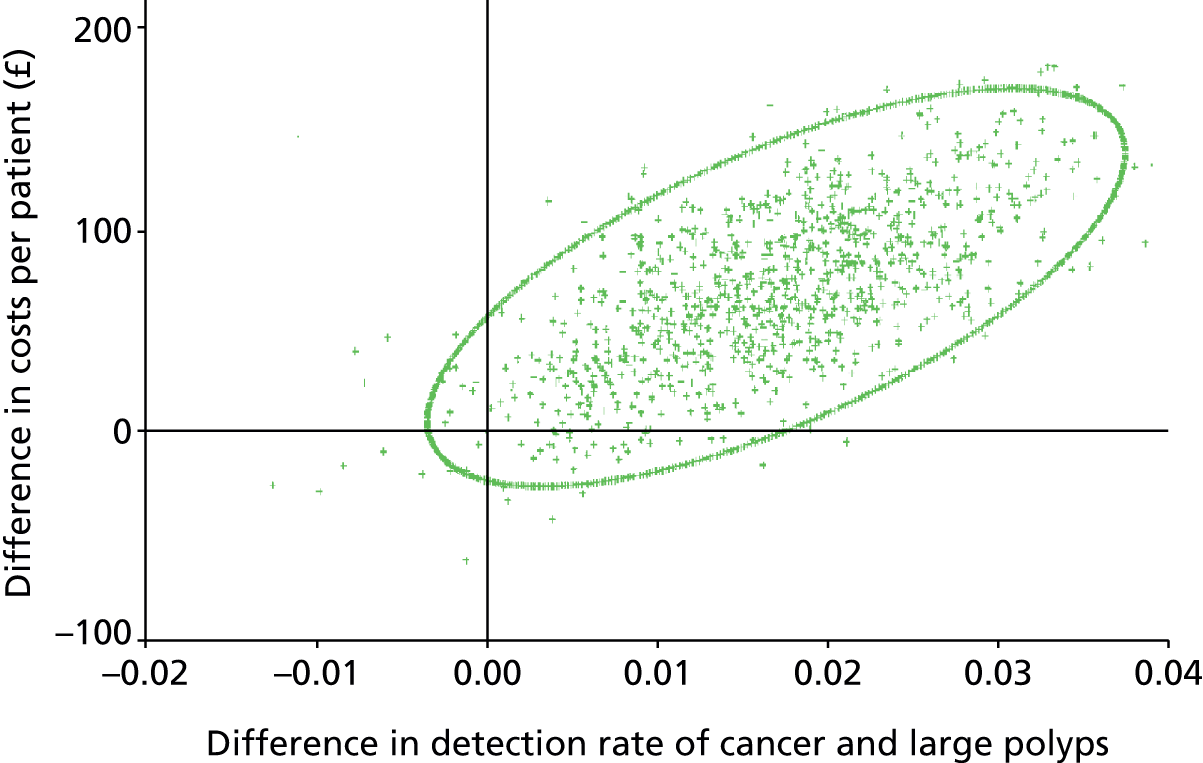
Costs contingent on finding extracolonic lesions
The diagnosis and treatment costs for following up extracolonic lesions (both trials) are shown in Table 39. The primary cancers and aneurysms are the most costly items and costs would be higher if longer-term effects were included. We have not accounted for costs in the counterfactual groups and the figures given are an overestimate the marginal gains from CTC. A summary of incremental costs per case detected for CTC and BE is given in Table 40.
| Lesion type | Number of patients | Cost per patient (£) | |||
|---|---|---|---|---|---|
| Mean | Lower quartile | Median | Upper quartile | ||
| Primary cancer | 23 | 2094 | 99 | 483 | 5175 |
| Secondary cancer | 5 | 470 | 105 | 276 | 816 |
| Haematological | 2 | 137 | 63 | 137 | 211 |
| Aneurysm | 13 | 1901 | 53 | 95 | 6609 |
| Minor cases | 89 | 449 | 53 | 142 | 794 |
| Other | 17 | 268 | 53 | 109 | 361 |
| Gains in detection per 1000 cases | Incremental cost per case detected (£) |
|---|---|
| CRCa | 24,000 |
| CRC + polypsa | 4235 |
| Extracolonic lesions (primary cancers)b | 6628 |
| Extracolonic lesions (primary and secondary cancer, haematological and aneurysms)b | 3545 |
Chapter 7 Discussion
Barium enema trial
This is the first randomised trial to compare the performance of CTC and BE for diagnosis of CRC and large polyps in symptomatic patients. The pragmatic design allowed us to compare referral rates for further colonic investigation and positive predictive values for the two tests when used in normal clinical practice. We found that CTC detected significantly more cancers or large polyps, confirming its higher sensitivity for significant lesions. However, referral rates for a second colonic procedure were significantly higher following CTC because of higher detection rates of both large and small polyps. The probability of diagnosing a cancer or large polyp at a second examination did not differ between the two procedures.
The diagnostic yield of CRC was relatively low in this cohort (3.5%) compared with the parallel CTC compared with colonoscopy trial (5.6%) and the detection rate for cancer in this trial did not differ significantly between the procedures (3.7% for CTC vs. 3.4% for BE). The cancer miss rate was lower for CTC than BE (6.7% vs. 14.1%), but not significantly so; however, the study was not powered to find a difference based on cancer detection alone, as the required sample size would be unfeasible, even in a symptomatic cohort. We did find a significantly higher detection rate of large polyps by CTC (3.6% vs. 2.2%), suggesting that it is more sensitive for detection of these lesions. We used large polyps as a surrogate for cancers, as it is rare to find cancers smaller than 10 mm. Therefore, our findings suggest that CTC is superior to BE for detecting smaller cancers. This is important as a recent preference study78 found that two-thirds of participants considered sensitivity the single most important attribute for a diagnostic bowel test, with even modest improvements valued highly.
The observed CRC miss rate for BE in the present study (14%) is similar to those reported in several large audits. Defining a missed cancer as one diagnosed within 3 years of a negative procedure, a large Canadian audit found a miss rate in a hospital setting of 21%. 79 A similar audit of 20 US hospitals found a miss rate of 15%10 and a national audit of UK hospital radiology departments examining CRCs diagnosed within 12 months of BE found an average miss rate of 15%, with variation from 0% to 50%. 80 In the present trial, 9 of the 12 cancers missed at BE were diagnosed more than 12 months after the randomised procedure, so it is likely that the prior UK audit underestimated the actual BE miss rate. There are no comparable data on miss rates following CTC in routine clinical practice. Meta-analysis of published data17,81 suggests that its sensitivity for cancer is around 96% (95% CI 91% to 99%), but the data are derived mainly from small studies from specialist centres.
We could not determine the miss rate of large polyps at CTC or BE, as colonoscopy was not performed as a reference standard. In the only study that has compared both BE and CTC with colonoscopy, 613 high-risk patients underwent all three procedures with a per-patient sensitivity for detection of any lesion ≥ 10 mm of 48% for BE and 59% for CTC. 82 A similarly low sensitivity for CTC of 55% was reported in a study83 of 600 patients undergoing CTC prior to clinically indicated colonoscopy. 83 These results conflict with other published data, mainly from asymptomatic screened populations, in which sensitivity for lesions ≥ 10 mm is around 90%. 15,17 Differences in radiologist expertise may account for these divergent findings.
Randomisation and a pragmatic design allowed us to observe patient pathways reflecting normal clinical practice, which is impossible in within-patient comparisons for which all participants receive colonoscopy after a radiological procedure, regardless of findings. We were therefore able to determine the proportion of patients undergoing a second diagnostic procedure either to confirm a lesion seen at the randomised procedure or to exclude pathology for which no lesion had been seen but diagnostic uncertainty persisted, for example owing to poor visualisation. Referral rates were significantly higher after CTC than BE (23% vs. 18%). Previous screening studies have reported referral rates after CTC of 8–16% using detection of a polyp ≥ 6 mm as a threshold. 15,83–85 Although referral rates might be higher in our symptomatic cohort because of the increased prevalence of cancers and large polyps, they may also indicate lack of confidence in radiological findings. Even in patients who had FS prior to their randomised procedure (and ignoring all referrals arising from the sigmoidoscopy findings), referral rates were still high (11% after BE and 18% after CTC). The routine use of prior FS in some centres might reflect the referring clinician’s judgement that CTC and BE are less reliable in the distal colon. However, the Canadian audit suggests that the miss rate of cancers following BE is higher in the right than the left colon. 79 Our findings corroborate this as 8% (6/77) of distal and 17% (9/53) of proximal cancers were missed by BE or CTC.
Significantly more referrals occurred following CTC because of suspected large polyps (11.0% vs. 7.5%) and also because of suspected smaller polyps (7.2% vs. 2.3%). Careful management of patients with suspected lesions < 10 mm is required, as the majority of these lesions have low malignant potential and are unlikely to be the cause of symptoms. However, of the significant lesions found at colonoscopy following detection of small polyps at CTC, the single cancer and five of the six large polyps were in patients with lesions measured as 8–9 mm at CTC, indicating a possible benefit of lowering the threshold for referral to 8 mm. Following detection of small polyps at BE, all four of the significant lesions found at colonoscopy (one cancer and three large polyps) were in patients with lesions measured as ≤ 5 mm at BE. This could be a result of measurement errors at BE, particularly when dealing with sessile lesions, or the lesions seen at BE and colonoscopy may not have been the same.
Factors other than sensitivity will influence test choice for both doctors and patients. BE is more physically demanding because multiple patient positions are required, while CTC requires only two (usually prone and supine). This makes CTC more suitable for frail elderly patients, who account for many of those with symptoms. Radiation dose for the two procedures is similar overall, but can be lowered for CTC if intravenous contrast is omitted. 86 The symptomatic perforation rate has been calculated at 0.03% for CTC13 and 0.0004% for BE. 87 In the present trial, serious adverse events were rare for either procedure: four attributed to BE and one to CTC.
Patients consider the detection of extracolonic lesions to be one of the advantages of CTC. 51 We found that 1.7% of people undergoing CTC as their randomised procedure had an extracolonic lesion that was subsequently confirmed as malignant (4.5% had a neoplasm). However, in both symptomatic and asymptomatic populations, extracolonic findings are reported for the majority of CT colonographies and many precipitate unnecessary investigations with associated psychological and physical morbidity, and increased financial cost. 25,40
Another potential advantage of CTC is that patients can be referred for same-day colonoscopy to remove any significant lesions identified in the bowel. This is impossible following BE because of residual barium suspension, so patients require a further clinic attendance and second bowel preparation. However, this advantage of CTC can be exploited only if there is prompt reporting of CTC findings and adequate spare capacity in endoscopy departments, which may limit its feasibility. In the present trial, only one patient had a follow-up colonoscopy on the same day as CTC.
A limitation of studies of all radiological colonic examinations is the requirement that lesions be confirmed at colonoscopy and it is impossible to be certain that a negative colonoscopy following a positive radiological examination is not a false negative. Furthermore, when lesions are detected at both procedures, matching them is problematic because of discrepancies in location and size. Among the polyps that were measured at both colonoscopy and radiology, size was underestimated at radiology in 23% of those identified at BE and 37% at CTC, and overestimated in 47% at BE and 41% at CTC, using colonoscopy as a reference standard. For small lesions, in particular, it was difficult to be certain that a lesion visualised by the randomised procedure was the same lesion identified subsequently at colonoscopy. The potential to introduce bias when matching lesions is therefore considerable, although less so for large lesions.
In conclusion, in patients with symptoms of CRC in whom a radiological examination is indicated, we show that CTC is superior to BE for detection of cancers and lesions ≥ 10 mm and there was a trend towards fewer missed cancers. CTC also offers potential benefits such as diagnosis of extracolonic disease, although this, along with its ability to detect small lesions in the colon, can lead to unnecessary follow-up tests for findings that are not clinically important. If CTC is to replace BE as the preferred radiological test, it must be implemented with a system of training and continuous audit for radiologists and rigorous guidelines on patient referral, to maximise the potential benefit of this technology.
Colonoscopy trial
We compared referral rates for additional colonic procedures following colonoscopy or CTC in patients investigated for symptoms suggestive of CRC. The observed referral rate of 30% after CTC was 3.6 times higher than that after colonoscopy (8.2%). CTC also identified extracolonic lesions, leading to further investigation in 10% of patients. CTC showed a high sensitivity similar to colonoscopy for detection of CRC; only one CRC was missed by CTC and none by colonoscopy, although there were three later cancer diagnoses in patients who had refused colonoscopy. Extracolonic cancers were diagnosed by CTC in nine patients.
In powering this study we assumed that CTC is equivalent to colonoscopy in terms of sensitivity for cancer, as has been confirmed in recent meta-analyses, so sensitivity was not the main focus of the study. Instead, we aimed to determine the proportion of patients having additional colonic tests after CTC compared with colonoscopy, which is an important consideration if CTC is to become widely used. We anticipated that the rate of additional colonic investigation would be higher after CTC than after colonoscopy because lesions detected at CTC require histological confirmation, but expected this would be offset by a lower rate of referral because of incomplete examinations, as CTC is not subject to the technical difficulties of navigating an endoscope through the colon and is better tolerated than colonoscopy. A survey undertaken prior to this study concluded that more than 20% of colonoscopies undertaken in routine practice in the UK were incomplete. 88 In response to this finding, a national quality improvement programme for colonoscopy was initiated89 and the low rate of completion examinations observed in this trial suggests that standards, at least among the unselected clinicians performing colonoscopy in this trial, were much better than previously observed.
Although the rate of further colonic examination after colonoscopy was lower than expected, it was much higher than expected after CTC, based on the very few observational studies that had compared rates. 84,85 Most previous evaluations of CTC and colonoscopy have been within-patient comparisons in which all patients receive CTC followed by colonoscopy15,16 and further examination rates are not observed directly but are estimated from detection rates of lesions larger than the threshold size for referral. In our trial, the rate of further colonic investigation following CTC was double that reported in a retrospective analysis of an asymptomatic screening population of a similar age. 84 In that study, 10% of patients were referred for colonoscopy for investigation of lesions ≥ 10 mm and a further 5% for 6–9-mm lesions. 84 In our cohort, 15% of patients were referred for investigation of a suspected cancer or polyp ≥ 10 mm and a further 16% for smaller polyps or clinical uncertainty. In the last group, no cancers and only three large polyps were diagnosed. The observed high referral rate following CTC demonstrates both the higher yield of potentially significant lesions and the more cautious approach of radiologists and referring clinicians when dealing with findings in a symptomatic population. Small polyps generally have low malignant potential and, with few exceptions, their removal offers no clinical benefit in this symptomatic group, but contributes to the patient and economic burden caused by the demand for additional tests following CTC. A careful assessment of patients’ clinical status and risk factors is therefore needed when choosing whether or not to refer in such cases.
There were significant differences in rates of referral by sex, with men more than six times as likely to be referred after CTC than colonoscopy, while women were only twice as likely to be referred. The higher referral rate after CTC in men arose because they had more polyps detected at CTC that required investigation. Conversely, in women, colonoscopy was more likely to be incomplete. These findings confirm results from other studies that show that women do not tolerate colonoscopy as well and have fewer polyps than men. 90,91
The ability of CTC to detect abnormalities outside the colon was also investigated. Extracolonic findings were reported in around 60% of patients undergoing CTC in both this and the parallel trial of CTC compared with BE. In 10% of cases in this trial and 8% in the parallel trial, additional investigations were deemed necessary. These rates are similar to those reported in two retrospective UK studies of symptomatic patients26,39 and in an older asymptomatic US cohort. 24 The financial cost of investigating extracolonic lesions at CTC has been estimated at approximately £150 per patient. 26 Whether or not this expenditure is worthwhile depends on the clinical importance of the lesion detected. In this trial, nine patients had an extracolonic cancer detected by CTC; however, the proportion of patients with an extracolonic cancer diagnosed within 3 years was similar following CTC and colonoscopy (4.6%). Around half of these cancers were diagnosed within the first 6 months after the randomised procedure, indicating that the diagnoses resulted from ongoing investigation of the symptoms for which patients were initially referred. This suggests that even patients having colonoscopy must undergo additional investigation to identify an extracolonic cause of their symptoms. As this usually involves referral to a different specialty, we were not able to capture this information during the course of the study. Thus, clinicians are faced with a choice: to perform CTC which permits examination of extracolonic regions of the abdomen but results in additional investigation for lesions of uncertain importance, or to perform a specific colonic investigation such as colonoscopy and then refer for additional investigation if there is persistent concern about symptoms (which may similarly lead to detection of unimportant lesions).
Our study bears out previous evidence that adverse events are more often associated with colonoscopy than CTC. In this and the parallel BE trial, there was only one hospitalisation attributed to CTC and six to colonoscopy. 7,13 However, it is important to note that endoscopy-related adverse events may be less frequent but are not avoided if CTC is followed by a referral for colonoscopy, as happened in 30% of patients having CTC in this trial.
Our study has limitations. Since recruitment finished there have been significant improvements in CTC, which may have considerably reduced the proportion of patients requiring a subsequent investigation to confirm or exclude cancer or large polyps. For example, faecal tagging (the use of oral contrast to label residual stool) was not generally used in our study, although it is now widespread in clinical practice to improve specificity. 92 Its use, together with a more restrictive policy on referral for removal of small polyps, would reduce the proportion of patients needing subsequent endoscopy. The combination of dietary restriction with faecal tagging also means that full bowel preparation can be avoided altogether. Although this will diminish sensitivity, it has been shown to increase compliance in screening studies. 93
With our sample size it was impossible to compare CTC and colonoscopy on the basis of sensitivity for cancer or large polyps, as meta-analyses suggest that the sensitivity of CTC is around 90%. We calculate that a trial powered to do this would require a sample of around 40,000 patients and suggest that the best way to compare sensitivity of the two procedures for cancer would be to implement a national audit programme for both CTC and colonoscopy using national administrative data sets. However, we did follow all patients for 3 years to identify cancers that were missed by either procedure. Combining data from both this and the BE trial, we found that CTC missed 4 out of 74 CRCs (5.4%), while none (0 out of 33) was missed in patients having colonoscopy, although there were three CRCs diagnosed in patients who refused colonoscopy and one in a patient who refused CTC.
The sensitivity and specificity that can be achieved with CTC depends on the expertise of the radiologists reporting the examination. Those in the current study were all experienced in interpreting CTC and their performance probably represents the best that could be achieved in older symptomatic patients at the time the study was performed. A recent study points out that CTC generally tends to be performed by a limited number of radiologists in each hospital who may, therefore, be more experienced. 94 More widespread implementation could result in a decrease in sensitivity if it is not accompanied by a thorough programme of audit and training. 95
In conclusion, CTC may be a desirable alternative to colonoscopy if symptoms are vague, if patients are frail or elderly, and in women because they generally have fewer polyps and a higher rate of incomplete colonoscopy. The results of our trial lend weight to previous studies indicating that CTC has comparable sensitivity to colonoscopy for cancer and appears to be more acceptable to patients. However, CTC is associated with a significantly higher rate of referral for additional tests, potentially increasing inconvenience and overall cost, and – in patients referred for colonoscopy – mitigating any advantage that comes from avoiding an endoscopic examination. Despite this, for the majority of patients who are not referred for additional colonic tests, CTC offers a straightforward, non-invasive, lower-risk alternative to colonoscopy. Close attention to referral criteria and continued emphasis on radiologist training and assessment are needed if CTC is to become an effective tool for investigation of symptomatic patients.
Extracolonic findings
It was Hara et al. 24 who first highlighted the importance of detecting extracolonic lesions at CTC, finding that 30 of 264 consecutive patients (11%) at high risk of CRC had potentially important extracolonic lesions reported, 18 of whom underwent subsequent investigation. The authors concluded that ‘the evaluation of extracolonic structures with CTC can help detect clinically important disease’. In the USA, CTC is promoted for CRC screening, with the ability to simultaneously image extracolonic organs cited as an advantage. A retrospective review of 10,286 CTC screening examinations found CRC in 22 patients (0.21%) and extracolonic cancers in 36 patients (0.35%), the most common of which was renal. 42 However, others have argued that detection of extracolonic lesions is unhelpful. A 2009 commentary concluded that CTC screening would generate a ‘deluge’ of incidental extracolonic findings that would inevitably cause subsequent anxiety, morbidity and mortality as well as increase costs. 96
Detection of extracolonic lesions in symptomatic patients is perceived as less controversial because standard abdominopelvic CT is used to search for an extracolonic cause of symptoms in elderly patients from whom a reliable history cannot be obtained owing to debilitation or confusion,97 helping to reassure clinicians that they are not missing occult disease. CTC combines intracolonic and extracolonic investigation in an acceptable format and is easier for older patients to tolerate than colonoscopy or BE. 39
The pragmatic design of our study meant that we were in a unique position to examine how the reporting of extracolonic lesions by radiologists influenced patients’ subsequent diagnostic pathway and ultimate diagnosis. We found that most CTC reports (59.6%) mentioned an extracolonic abnormality; a figure very similar to the 63% quoted by a US study of patients at high and average risk of CRC. 98 We found that the frequency of extracolonic findings rose significantly with age, also in keeping with the results of other studies: one in the USA found that extracolonic abnormalities were reported in 185 out of 250 patients (74%) aged ≥ 65 years, compared with 113 out of 204 younger patients (55.4%). 99 A UK series of 400 consecutive symptomatic patients > 70 years found that 268 (67%) had extracolonic lesions. 39 The E-RADS classification44 was devised to clarify management of extracolonic findings by ascribing an estimate of likely clinical significance. In common with researchers working in an asymptomatic setting, we found that the highest categories (E3, E4) were least frequently reported: a US screening study of 2277 patients found that 46% had at least one extracolonic finding, but this was rated as E3 or E4 in only 250 patients (11%). 43 The combined rate for E3 and E4 detections in our study was higher (21%), likely reflecting the symptomatic nature and greater age of our cohort.
The E3 and E4 detections are important because they represent lesions that may warrant further investigation by the clinician in charge of the patient’s care. Interestingly, although 365 patients (21%) were assigned these scores, only 150 (8.6%) were subsequently investigated. This difference may reflect a clinical decision that the finding is unlikely to be the cause of symptoms and is therefore not worth pursuing. It is also possible that patients may decline further investigation or are too frail to undergo it. As expected, E4 lesions were most frequently investigated but 15% were not, despite being ‘potentially important’. We also found that investigation of extracolonic lesions was not straightforward: one-third of investigated patients needed two or more procedures. Although most investigation was non-invasive, a substantial minority underwent invasive procedures including surgery, usually for E4 findings.
We attempted to address the question of whether or not extracolonic findings were responsible for patients’ presenting symptoms rather than being incidentally found, as is the case with screening. When the relationship between presenting symptoms and extracolonic findings was analysed, 2.9% of patients in the BE trial and 3.6% of patients in the colonoscopy trial had symptoms that were ultimately ascribed to extracolonic pathology. These figures are similar to the prevalence of CRC in the trials (3.5% and 5.6% respectively; excluded from the present analysis). It can be concluded that the predictive value of individual symptoms for both intracolonic and extracolonic disease is low. The chance of an extracolonic lesion being the cause of presenting symptoms was highest in the group aged ≥ 85 years, supporting the use of CTC in this group, although the number of such patients in our study was small (54 people). We had thought that we might be able identify symptoms associated with extracolonic disease, but anaemia was the only one significantly associated with reporting of an extracolonic finding. When the likelihood that individual symptoms could be attributed to an extracolonic finding was taken into account, abdominal pain had the strongest association with extracolonic pathology. However, abdominal pain was a common presenting complaint, therefore, clinical usefulness on a per-patient basis was limited.
We had hypothesised that the comprehensive nature of intracolonic and extracolonic diagnosis possible by CTC would lead to earlier diagnosis of intra-abdominal or pelvic extracolonic cancer than either BE or colonoscopy, especially as some cancers may be chance detections in patients whose symptoms originated elsewhere. We found extracolonic malignancy in 1.7% of patients having CTC (excluding those with CRC). However, we were surprised to find that there was no difference by randomised procedure in the incidence of newly diagnosed primary extracolonic cancers at 3 years. Furthermore, the rate of extracolonic cancer was highest within the first 6 months after the randomised procedure for all three modalities. We believe that these data suggest that many patients randomised to colonoscopy or BE will eventually undergo comprehensive abdominopelvic imaging if no colorectal cause for their symptoms is found and that this often occurs relatively soon after the initial referral. We collected details of follow-up procedures for each patient until discharge from the colorectal clinic, but do not have data on resource use beyond this, so cannot confirm this hypothesis at the time of writing.
Our study does have limitations. In all, 46 different radiologists participated and their personal thresholds for reporting extracolonic lesions will differ, especially for lesions perceived as being of lower importance. Differences may also arise depending on CTC technique, for example the use of intravenous contrast. We did not analyse centre-to-centre variation, which will form the basis of a subsequent report. However, the pragmatic nature of the study in terms of setting and radiologist experience means that our findings are generalisable to daily practice with symptomatic patients. Another limitation arises from the use of the E-RADS categories to classify the potential importance of extracolonic findings. We used this system because it is well established,44 but there are no comprehensive classification tables linking individual findings to E-RADS scores. Therefore, the category assigned to an individual finding could vary depending on the individual making the assessment. Further, E-RADS is based on asymptomatic patients. There is a need to develop similar guidelines for symptomatic patients and to describe which scores should be assigned to the entire range of CTC findings.
Patients with proven CRC were excluded from the present analysis because it is not always possible to be certain that extracolonic findings are unrelated to the CRC and its presence will also influence the rate of subsequent extracolonic investigation. It is possible that some patients had both CRC and an important extracolonic lesion, although the number will be small.
We collected data on resource use until discharge but cancer registry data suggest that extracolonic examination was ultimately performed on a proportion of patients whose primary randomised procedure was either BE or colonoscopy. Collection of resource use by individual patients for the whole 3 years of registry follow-up is beyond available resources. We are also planning a modelling exercise that will extrapolate data beyond the trial to determine the impact of extracolonic detections in terms of lives ultimately saved compared with morbidity/mortality owing to unnecessary investigation. This will include non-neoplastic diagnoses such as abdominal aortic aneurysm (14 cases). A prior cost-effectiveness analysis of asymptomatic patients suggests that CTC is ‘highly cost-effective’ when used to screen for aortic aneurysm in combination with CRC. 67
In summary, extracolonic findings are commonly identified by radiologists reporting CTC in patients referred with a suspicion of CRC and their frequency rises with patient age. A small proportion of patients are investigated subsequently. Approximately 3% of patients presenting with symptoms of CRC have extracolonic pathology that is likely to be the cause of their presenting symptoms and in approximately half of these cases, this will be owing to extracolonic malignancy. However, when used in normal clinical practice, CTC does not appear to hasten the diagnosis of extracolonic intra-abdominal or pelvic malignancy compared with either BE or colonoscopy.
Health psychology assessment
This study reports the experience of symptomatic patients randomised to undergo CTC, BE or colonoscopy in order to diagnose or exclude significant colorectal neoplasia. This is the first time a randomised design has been used rather than cohort studies in which patients undergo all tests under investigation. A randomised design is advantageous because it increases generalisability and allows us to obtain a more valid representation of patient experience in daily clinical practice.
We found that patient experience differed significantly according to the diagnostic test administered, with patients having a BE or colonoscopy reporting less satisfaction and greater discomfort than those having CTC. These data support previous findings from non-randomised cohort studies. 20,47,48 The fact that CTC is more acceptable than BE while also offering superior diagnostic sensitivity indicates that it should replace BE as the standard radiological whole-colon investigation. 100
In the colonoscopy trial, patients having colonoscopy reported higher scores on the ‘worry’ subscale of the satisfaction measure than those having CTC. It is known that women perceive colonoscopy as more painful than men,18 a finding confirmed in our study in women having colonoscopy. No significant differences were found between men and women having CTC. It was reassuring that most patients having CTC did not report claustrophobia, which had been identified by previous research as a potential drawback. 78 Our findings also support previous evidence suggesting that bowel preparation is the worst aspect of patients’ overall experience.
Patients having CTC rated staff interactions significantly more favourably. This was surprising because a previous qualitative study suggested that patients regard CTC as more impersonal than colonoscopy, with less interaction. 51 However, that research was conducted in a single tertiary referral unit. The present multicenter design (including both teaching and community hospitals) is likely to better reflect day-to-day practice.
An important prespecified aim was to assess patients’ experiences of receiving their test results, because delays following radiological procedures may cause uncertainty and anxiety. 51 We confirmed that patients having CTC were less likely to receive their results on the day of the test or face to face; a consequence of the fact that CTC is usually reported by a radiologist some time after the scan takes place, while colonoscopy is interpreted during the procedure itself. As a result, patients having CTC were less satisfied with the reporting of results than patients having colonoscopy. Timely delivery of results may be particularly important if CTC is used for screening, as patients will expect a standard of care equivalent to colonoscopy. The situation is more complex for symptomatic work, for which diagnosis of cancer is much more likely. Immediate delivery of a cancer diagnosis is inappropriate in radiology departments because the required support services (i.e. psychological support and discussion of treatment options, implications and prognosis) are usually unavailable. However, some units have the facility to call such services to the radiology department and immediate delivery of results face to face may be feasible in such circumstances. It should also be borne in mind that most symptomatic patients will receive a negative examination and consideration should be given to making this information rapidly available, perhaps via a subsequent telephone call to the patient.
Our study has limitations. Although a randomised design offers the opportunity to observe patient experience in normal clinical practice, it also has some drawbacks. Perhaps most importantly, patients were aware that two tests were being compared and a description of each was inevitably part of the consent process. Therefore, it is possible that a prerandomisation preference, perhaps arising from the perceived advantages of a new technology (CTC), may have biased patients who were subsequently randomised to BE or colonoscopy and impacted negatively on their reported experience.
Patients rated their experience without comprehensive information regarding relative test sensitivity. Previous research has found sensitivity to be important when patients form preferences for colorectal investigations, both in symptomatic78 and screening contexts. 101–103 It is possible that participants would rate CTC less positively if aware that it may be less sensitive than colonoscopy. 15 Observations may also have been influenced by centre-to-centre variation in bowel preparation, sedation/analgesia and gas insufflation. We did not stipulate that carbon dioxide or air be used for colon insufflation, as both gases are used for both procedures in daily practice. However, we did stipulate that ‘full bowel preparation’ was used for both procedures, so the experience would be comparable despite the use of different pharmacological agents to achieve this. Although full purgation is necessary for BE, CTC is an evolving technology and it is possible that bowel preparation will be reduced or abandoned altogether in future. 104,105 As a result, the acceptability of CTC will likely improve beyond the level documented in our study.
Although it is long-established that carbon dioxide is more comfortable than air for patients having a BE,106 we were surprised that nine centres were still using air (indeed seven were using air for CTC). This may impact on physical discomfort (the item for which we would expect the gas used to have most influence) but, at the same time, it is important that our data are generalisable to daily practice.
A number of important questions arise from our findings that warrant future research. An evaluation of the extent to which patients prefer a more convenient or comfortable test (e.g. one that avoids bowel preparation) if this means trading off some degree of diagnostic sensitivity would be of interest to both health-care professionals and policy makers. Despite preliminary qualitative evidence suggesting unwillingness to sacrifice any level of sensitivity,78 more extensive research is required.
In conclusion, patients with symptoms suggestive of CRC perceive CTC as a more acceptable test than patients having a BE or colonoscopy. In conjunction with clinical efficacy data for CTC, our findings support the wider implementation of CTC for diagnosis of symptomatic colorectal disease.
Health economic assessment
Computed tomographic colonography compared with barium enema trial
The comparison of CTC with BE showed that CTC was superior in terms of significant colonic lesions detected. However, BE is less expensive because it has lower unit cost and is less likely to lead to follow-on investigation. For CTC, the incremental cost per case detected was nearly £4235 per significant lesion (large polyp or cancer) detected, rising to £24,000 per CRC. The estimate for significant neoplasms was significant albeit a wide confidence limits (95% CI £395 to £9656). Costs per cancer detected were unsurprisingly higher given the small number of cases, but not statistically significant. Costs vary from place to place and over time. In addition, not all costs can be included because the data were not collected, such as a change in primary care consultation rates for example. The overall cost-effectiveness of CTC relative to BE requires extrapolation beyond the trial necessarily requiring many assumptions. Such a model will be submitted for publication.
Computed tomographic colonography compared with colonoscopy trial
According to systematic reviews, CTC and colonoscopy have approximately equal sensitivity for large polyps and cancers. Therefore, the comparison between them depends on other issues. We compared costs and costs per case detected.
Computed tomographic colonography has a lower unit cost, but 30% of patients require a further test before a firm diagnosis can be made, compared with 8% of patients having colonoscopy. CTC turns out to be marginally less expensive overall, despite the need for a further test (usually colonoscopy) in nearly one-third of patients. However, costs are closely balanced and a very small increase in unit costs attributed to CTC would alter the above conclusion. In that case, colonoscopy would dominate CTC. As in the case of CTC compared with BE, the confidence limits are relatively wide, especially as far as cancer is concerned. There are further factors to be considered, such as patient acceptability (see Chapter 5). On the negative side, we found a slightly increased time to diagnosis for CTC compared with colonoscopy, presumably as a result of the need to refer for colonoscopy to biopsy suspicious lesions.
Extracolonic lesions
One stark difference between CTC and both colonoscopy and BE relates to the detection of extracolonic lesions. Although CTC detected CRC in 5.2% of cases (across both trials), it detected aortic aneurysms or extracolonic cancers in 2.4% – almost half as many. Primary extracolonic cancers were found in 1.3% of cases. Many of these patients would have had a poor prognosis despite detection and others would have had as good a prognosis even if their cancer had gone undetected. However, there are likely to be cases for which earlier detection would make a material difference to patient outcomes. A calculation of the net benefit (or harm) of detecting extracolonic lesions would require complex modelling and this is planned as part of a subsequent follow-on study by the investigators. Xiong et al. 40 calculated that if the mean cost of investigating extracolonic lesions was £150, then detecting such lesions would be cost-effective if it resulted with a gain of one life-year every 3000 CTC examinations. Whether or not this is the case is scientifically unproven at this point.
Conclusion
Neither CTC nor colonoscopy shows clear superiority in terms of either cost or cases detected. However, CTC detects more cases than BE, but at higher total cost. Extracolonic lesions remain an enigma as far as health economics of CTC is concerned. In the meantime, individual patients will have personal utilities driving individual decisions and there is an argument that leaving the decision to individual discretions maximises societal utility gain in such split choice scenarios. 107
Conclusions
Implications for health care
-
Computed tomographic colonography detects more cancers and large polyps than BE, misses fewer cancers and improves patient experience, but also precipitates more follow-up investigations. CTC could replace BE for large bowel investigation.
-
Computed tomographic colonography is a safe alternative to colonoscopy in symptomatic patients, with similar sensitivity and improved patient experience in the short term. The way in which the results are conveyed (i.e. quicker and face to face) favours colonoscopy. CTC precipitates significantly more follow-up examinations – which, in a limited sample, did not adversely impact on patient experience – but criteria for subsequent referral are needed.
-
Most patients have extracolonic findings reported at CTC and 8.5% undergo further investigation for these. Approximately 2% overall have an extracolonic malignancy detected. Offering CTC as the primary procedure did not significantly alter the proportion of patients diagnosed with extracolonic malignancy at 3 years compared with colonoscopy or BE.
-
When compared with BE, CTC detected one extra serious colonic neoplasm for approximately £4000. However, the detection rates were similar for CTC compared with colonoscopy and costs were similar such that there was little evidence on which a firm recommendation should be based.
Recommendations for further research
-
The benefits of CTC observed in both trials will improve if referrals for clinically unnecessary subsequent investigations are diminished; there is a need to develop evidence-based guidelines for referral after CTC.
-
No difference was found between tests regarding the proportion of extracolonic cancers detected within 3 years. The reasons for this are unclear but it appears that many patients having a BE or colonoscopy have subsequent extracolonic investigation in cases where no colonic abnormality was found. How and why this happens merits further research to clarify the clinical effectiveness and cost-effectiveness of CTC.
-
The combined benefits of detecting intracolonic and extracolonic pathology by CTC should be modelled beyond the trial data set over an extended time horizon to estimate if CTC is cost-effective compared with colonoscopy overall. This is not a trivial undertaking.
-
Research is needed to guide implementation of CTC, especially the training needed for competent interpretation.
-
The acceptability to patients of increased referrals following CTC needs further investigation.
-
Implementation of CTC in the National Bowel Cancer Screening Programme warrants investigation. Detection characteristics for significant neoplasia were good in the present study but lesions are likely to be smaller in a screening group and, therefore, more difficult to detect.
Acknowledgements
Contributions of authors
Steve Halligan designed the parallel RCTs, secured funding from Health Technology Assessment (HTA), was primarily responsible for drafting Chapters 1, 2, 3 and 4 and editing the article, and acts as a guarantor for this monograph.
Edward Dadswell was responsible for recruitment, data collection and trial management, and primarily responsible for assembling the initial draft of the monograph.
Kate Wooldrage analysed the data from the RCTs and helped draft the monograph.
Jane Wardle designed the parallel RCTs, secured funding from HTA and was primarily responsible for analysis and drafting of Chapter 5.
Christian von Wagner was primarily responsible for analysis and drafting of Chapter 5.
Richard Lilford designed the parallel RCTs and secured funding from HTA and was primarily responsible for analysis and drafting of Chapter 6.
Guiqing L Yao was primarily responsible for analysis and drafting of the Chapter 6.
Shihua Zhu was primarily responsible for analysis and drafting of Chapter 6.
Wendy Atkin designed the parallel RCTs, secured funding from HTA and was responsible for recruitment, data collection and trial management; she analysed the data from the RCTs, was primarily responsible for drafting Chapters 1, 2, 3 and 4 and editing the monograph, and acts as a guarantor for this monograph.
All authors helped interpret data from all studies and reviewed and approved the final version of the monograph for submission.
The Special Interest Group in Gastrointestinal and Abdominal Radiology investigators
Data Monitoring and Ethics Committee
Professor DG Altman (chairperson), Professor R Steele and Dr A Walker.
Trial Steering Committee
Professor A Dixon (chairperson), Mrs L Faulds-Wood, Professor T Marteau and Dr R Valori.
Consultant Clinicians
Dr Julian Teare (Consultant Gastroenterologist) and Mr Omar Faiz (Clinical Senior Lecturer in Colorectal Surgery), Imperial College Healthcare NHS Trust.
Trial Office staff
Mr E Dadswell (trial manager), Ms K Wooldrage (statistician), Mrs R Kanani (trial manager), Mrs P Rogers (statistician), Dr Rob Edwards (statistician), Mrs U Shah (senior data manager), Dr I Kralj-Hans (senior data manager), Mr A Ghanouni (data clerk), Mr K Pack (data clerk), Miss J Waddingham (data clerk), Miss A Thomson (data clerk), Ms A Verjee (projects manager), Dr C Monk (projects manager), Dr L J Turner (projects manager) and Mrs R Howe (projects administrator).
Research nurses/radiographers/local co-ordinators
Mrs R Kanani, Mr E Dadswell, Mr A Ghanouni and Mrs J Muckian (St Mark’s Hospital, Harrow, UK).
Mrs N Gibbons, Mrs D Bastable and Ms L Coni (Queen Alexandra Hospital, Portsmouth, UK).
Mrs J Martin, Mrs S Stephenson, Ms C Jackson and Mr A Wormald (Bradford Hospitals NHS Trust, UK).
Dr D Beech (principal investigator for study centres), Ms C Lynn and Ms C Bloor (Royal Cornwall Hospital, Truro, UK).
Mrs S Wilkinson, Mrs L Pickles, Mrs J Scothern, Mrs A Hennedy, Mrs T Larkin, Mrs P Pearson, Mr S Preston, Mrs L Smith and Mrs L Wright (Royal Lancaster Infirmary/Furness General Hospital, UK).
Dr J Blackstock, Ms R Thomas and Ms L Young (Royal United Hospital, Bath, UK).
Mrs J Butler-Barnes, Mrs V Adamson, Mrs T Larcombe, Mrs V Bradshaw, Mrs S Chapman, Mr M Slater, Mrs J Stylan and Mr D Wood (Leighton Hospital, Crewe, UK).
Mrs J Bradbury, Mr J Breedon, Mrs M Coakes, Mrs L Crutch, Mrs A Leyland, Mrs W Pringle, Mrs L Rowe and Mrs M White (Queen Elizabeth Hospital, Birmingham, UK).
Ms A Worley and Dr M K Neelala (principal investigator for study centres) (Nottingham City Hospital/Queens Medical Centre, UK).
Mrs M Gandy and Mrs J Pascoe (Derriford Hospital, Plymouth, UK).
Mr J Wood, Mrs W Cook and Ms Y Memory (Royal Oldham Hospital, Oldham, UK).
Mrs M Avery, Miss K Pearson, Mrs D Shivapatham, Mrs S Thomas, Mr C Ong and Ms B Poppinga-Scholz (Charing Cross Hospital, London/Hammersmith Hospital, London, UK).
Mrs B Shanahan (Oxford Radcliffe Hospital, Oxford).
Ms L Turner, Ms K Fellows, Ms A Duffy, Ms A Owen and Ms A Usansky (South Manchester University Hospital, Manchester, UK).
Mr F Naim, Mr V Bohra and Mr S Prabhudesai (St Mary’s Hospital, London, UK).
Ms M Hayes, Ms N Lancelotte, Ms T James, Miss S Johnston and Miss J Stevenson (Frimley Park Hospital, Frimley, UK).
Mrs D Whetter (University Hospital North Tees, Stockton-on-Tees, UK).
Radiologists
Professor C Bartram, Dr D Burling, Dr A Gupta, Professor S Halligan, Dr M Marshall and Dr SA Taylor (St Mark’s Hospital, Harrow, UK).
Dr A Higginson (principal investigator for study centres) and Dr J Atchley (Queen Alexandra Hospital, Portsmouth, UK).
Dr C Kay (principal investigator for study centres) and Dr A Lowe (Bradford Hospitals NHS Trust, UK).
Dr G F Maskell (Royal Cornwall Hospital, Truro, UK).
Dr A Taylor, Dr E Tan and Dr J McGregor (Royal Lancaster Infirmary/Furness General Hospital, UK).
Dr S Hayward, Dr A Philips and Dr M Noakes (Royal United Hospital, Bath, UK).
Dr S Zaman (Leighton Hospital, Crewe, UK).
Dr P Guest, Dr I McCafferty, Dr P Riley and Dr D Tattersall (Queen Elizabeth Hospital, Birmingham, UK).
Dr C Jobling (principal investigator for study centres) and Dr R Dhingsa (Nottingham City Hospital/Queens Medical Centre, UK).
Dr S A Jackson (principal investigator for study centres), Dr B M Fox and Dr J Shirley (Derriford Hospital, Plymouth, UK).
Dr M K Neelala (Royal Oldham Hospital, Oldham, UK).
Dr D Blunt (principal investigator for study centres) and Dr M Roddie (Charing Cross Hospital, London, UK).
Dr A Slater (principal investigator for study centres) (Oxford Radcliffe Hospital, Oxford, UK).
Dr S A Sukumar (principal investigator for study centres) (South Manchester University Hospital, Manchester, UK).
Dr N Hughes (principal investigator for study centres) (Frimley Park Hospital, Frimley, UK).
Dr P Woolfall (principal investigator for study centres) (University Hospital North Tees, Stockton-on-Tees, UK).
Surgeons/endoscopists
Dr B Saunders and Professor JMA Northover (St Mark’s Hospital, Harrow, UK).
Dr P Goggin and Mr D O’Leary (Queen Alexandra Hospital, Portsmouth, UK).
Mr J Ausobsky, Dr C Beckett, Mr J Davies, Mr J Griffith and Mr M Steward (Bradford Hospitals NHS Trust, UK).
Mr PJ Arumugam (Royal Cornwall Hospital, Truro, UK).
Mr I Crighton (principal investigator for study centres), Miss C Bronder, Dr C Brown, Dr A Higham, Dr R Lea, Dr C Meaden, Mr W Morgan, Mr P Patel and Mr G Nasmyth (Royal Lancaster Infirmary/Furness General Hospital, UK).
Mr M Williamson (principal investigator for study centres) (Royal United Hospital, Bath, UK).
Mr D Cade (principal investigator for study centres) (Leighton Hospital, Crewe, UK).
Professor D Morton (principal investigator for study centres) (Queen Elizabeth Hospital, Birmingham, UK).
Professor J Scholefield (Nottingham City Hospital/Queens Medical Centre, UK).
Mr K Hosie and Dr E Whitehead (Derriford Hospital, Plymouth, UK).
Dr P Conlong, Dr B Rameh, Mr A Rate and Mr D Richards (Royal Oldham Hospital, Oldham, UK).
Dr D Bansi, Mr G Buchanan, Mr P Dawson, Dr J Martin, Dr G Smith, Mr N A Theodorou, Dr A Thillainayagam (Charing Cross Hospital, London, UK).
Mr C Cunningham and Dr S Travis (Oxford Radcliffe Hospital, Oxford, UK).
Dr G M Hyde, Mr D J Jones and Miss S T O’Dwyer (South Manchester University Hospital, Manchester, UK).
Mr P Ziprin (principal investigator for study centres) (St Mary’s Hospital, London, UK).
Col D Edwards, Miss S Burton, Dr P Fabricius, Mr M Gudgeon and Mr I Jourdan (Frimley Park Hospital, Frimley, UK).
Dr M Rutter (University Hospital North Tees, Stockton-on-Tees, UK).
Other
The investigators are grateful to:
Barco (Edinburgh, UK) and Viatronix (Stony Brook, NY) for providing visualisation software.
Medicsight (Hammersmith, London, UK) for computer-assisted detection software.
Bracco (High Wycombe, UK) for the insufflators.
Publication
Halligan S, Wooldrage K, Dadswell E, Shah U, Kralj-Hans I, von Wagner C, et al. Identification of extra-colonic pathologies by computed tomographic colonography in symptomatic patients. Gastroenterology 2015;149:89–101.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- World Health Organization . Globocan 2008 n.d. http://globocan.iarc.fr (accessed 29 July 2011).
- Cancer Research UK . CancerStats. Incidence – UK 2008. http://info.cancerresearchuk.org/prod_consump/groups/cr_common/@nre/@sta/documents/generalco (accessed 25 September 2014).
- The New NHS: Modern, Dependable. London: Department of Health; 1997.
- Thompson M, Atkin W, Coles C, Ellis B, Wood LF, Heath I, et al. ACPGBI Referral guidelines for colorectal cancer. Colorectal Dis 2002;4:287-97. http://dx.doi.org/10.1046/j.1463-1318.2002.00348.x.
- Hamilton W, Lancashire R, Sharp D, Peters T, Cheng K, Marshall T. The risk of colorectal cancer with symptoms at different ages and between the sexes: a case-control study. BMC Med 2009;7. http://dx.doi.org/10.1186/1741-7015-7-17.
- Svendsen RP, Støvring H, Hansen BL, Kragstrup J, Søndergaard J, Jarbøl DE. Prevalence of cancer alarm symptoms: a population-based cross-sectional study. Scand J Prim Health Care 2010;28:132-7. http://dx.doi.org/10.3109/02813432.2010.505412.
- Levin TR, Zhao W, Conell C, Seeff LC, Manninen DL, Shapiro JA, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med 2006;145:880-6. http://dx.doi.org/10.7326/0003-4819-145-12-200612190-00004.
- Warren JL, Klabunde CN, Mariotto AB, Meekins A, Topor M, Brown ML, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med 2009;150:849-57. http://dx.doi.org/10.7326/0003-4819-150-12-200906160-00008.
- Singh H, Poluha W, Cheung M, Choptain N, Baron KI, Taback SP. Propofol for sedation during colonoscopy. Cochrane Database Syst Rev 2008;4.
- Rex D, Rahmani E, Haseman J, Lemmel G, Kaster S, Buckley J. Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice. Gastroenterology 1997;112:17-23. http://dx.doi.org/10.1016/S0016-5085(97)70213-0.
- Fletcher RH. The end of barium enemas?. N Engl J Med 2000;342:1823-4. http://dx.doi.org/10.1056/NEJM200006153422409.
- Vining D, Gelfand D, Bechtold R, Scharing E, Grishaw E, Shifrin R. Technical feasibility of colon imaging with helical CT and virtual reality. Am J Roentgenol 1994;162.
- Burling D, Halligan S, Slater A, Noakes MJ, Taylor SA. Potentially serious adverse events at CT colonography in symptomatic patients: national survey of the United Kingdom. Radiology 2006;239:464-71. http://dx.doi.org/10.1148/radiol.2392051101.
- Sosna J, Blachar A, Amitai M, Barmeir E, Peled N, Goldberg SN, et al. Colonic perforation at CT colonography: assessment of risk in a multicenter large cohort. Radiology 2006;239:457-63. http://dx.doi.org/10.1148/radiol.2392050287.
- Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med 2008;359:1207-17. http://dx.doi.org/10.1056/NEJMoa0800996.
- Pickhardt P, Choi J, Hwang I, Butler J, Puckett M, Hildebrandt H, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 2003;349:2191-200. http://dx.doi.org/10.1056/NEJMoa031618.
- Halligan S, Altman DG, Taylor SA, Mallett S, Deeks JJ, Bartram CI, et al. CT colonography in the detection of colorectal polyps and cancer: systematic review, meta-analysis, and proposed minimum data set for study level reporting. Radiology 2005;237:893-904. http://dx.doi.org/10.1148/radiol.2373050176.
- Taylor SA, Halligan S, Saunders BP, Bassett P, Vance M, Bartram CI. Acceptance by patients of multidetector CT colonography compared with barium enema examinations, flexible sigmoidoscopy, and colonoscopy. AJR Am J Roentgenol 2003;181:913-21. http://dx.doi.org/10.2214/ajr.181.4.1810913.
- Svensson MH, Svensson E, Lasson A, Hellström M. Patient acceptance of CT colonography and conventional colonoscopy: prospective comparative study in patients with or suspected of having colorectal disease. Radiology 2002;222:337-45. http://dx.doi.org/10.1148/radiol.2222010669.
- van Gelder RE, Birnie E, Florie J, Schutter MP, Bartelsman JF, Snel P, et al. CT colonography and colonoscopy: assessment of patient preference in a 5-week follow-up study. Radiology 2004;233:328-37. http://dx.doi.org/10.1148/radiol.2331031208.
- Ferrucci JT. Double contrast barium enema: use in practice and implications for CT colonography. AJR Am J Roentgenol 2006;187:170-3. http://dx.doi.org/10.2214/AJR.05.0900.
- Taylor SA, Halligan S, Burling D, Bassett P, Bartram CI. Intra-individual comparison of patient acceptability of multidetector-row CT colonography and double-contrast barium enema. Clin Radiol 2005;60:207-14. http://dx.doi.org/10.1016/j.crad.2004.07.006.
- Halligan S, Marshall M, Taylor S, Bartram C, Bassett P, Cardwell C, et al. Observer variation in the detection of colorectal neoplasia on double-contrast barium enema: implications for colorectal cancer screening and training. Clin Radiol 2003;58:948-54. http://dx.doi.org/10.1016/S0009-9260(03)00317-9.
- Hara AK, Johnson CC, MacCarty RL, Welch TJ. Incidental extracolonic findings at CT colonography. Radiology 2000;215:353-7. http://dx.doi.org/10.1148/radiology.215.2.r00ap33353.
- Fletcher RH, Pignone M. Extracolonic findings with computed tomographic colonography: asset or liability?. Arch Intern Med 2008;168:685-6. http://dx.doi.org/10.1001/archinte.168.7.685.
- Xiong T, McEvoy K, Morton DG, Halligan S, Lilford RJ. Resources and costs associated with incidental extracolonic findings from CT colonography: a study in a symptomatic population. Br J Radiol 2006;79:948-61. http://dx.doi.org/10.1259/bjr/58438178.
- Lilford RJ, Jackson J. Equipoise and the ethics of randomisation. J R Soc Med 1995;88:552-9.
- The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) n.d. www.ich.org/LOB/media/MEDIA482.pdf (accessed February 2013).
- Halligan S, Lilford RJ, Wardle J, Morton D, Rogers P, Wooldrage K, et al. Design of a multicentre randomized trial to evaluate CT colonography versus colonoscopy or barium enema for diagnosis of colonic cancer in older symptomatic patients: the SIGGAR study. Trials 2007;8. http://dx.doi.org/10.1186/1745-6215-8-32.
- Guidelines for the Training, Appraisal and Assessment of Trainees in GI Endoscopy. London: Royal College of Physicians; 2004.
- Barish MA, Soto JA, Ferrucci JT. Consensus on current clinical practice of virtual colonoscopy. AJR Am J Roentgenol 2005;184:786-92. http://dx.doi.org/10.2214/ajr.184.3.01840786.
- Taylor S, Halligan S, Goh V, Morley S, Atkin W, Bartram C. Optimizing bowel preparation for multidetector row CT colonography: effect of Citramag and Picolax. Clin Radiol 2003;58:723-32. http://dx.doi.org/10.1016/S0009-9260(03)00187-9.
- Halligan S, Burling D, Atkin W, Bartram C, Fenlon H, Laghi A, et al. Effect of directed training on reader performance for CT colonography: multicenter study. Radiology 2007;242:152-61. http://dx.doi.org/10.1148/radiol.2421051000.
- Johnson CD, MacCarty R, Welch TJ, Wilson LA, Harmsen WS, Ilstrup DM, et al. Comparison of the relative sensitivity of CT colonography and double-contrast barium enema for screen detection of colorectal polyps. Clin Gastroenterol Hepatol 2004;2:314-21. http://dx.doi.org/10.1016/S1542-3565(04)00061-8.
- Halligan S, Wooldrage K, Dadswell E, Kralj-Hans I, von Wagner C, Edwards R, et al. Computed tomographic colonography versus barium enema for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): a multicentre randomised trial. Lancet 2013;381:1185-93. http://dx.doi.org/10.1016/S0140-6736(12)62124-2.
- Atkin W, Dadswell E, Wooldrage K, Kralj-Hans I, von Wagner C, Edwards R, et al. Computed tomographic colonography versus colonoscopy for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): a multicentre randomised clinical trial. Lancet 2013;381:1194-202. http://dx.doi.org/10.1016/S0140-6736(12)62186-2.
- Halligan S, Wooldrage K, Dadswell E, Shah U, Kralj-Hans I, von Wagner C, et al. Identification of extra-colonic pathologies by computed tomographic colonography in symptomatic patients. Gastroenterology 2015;149:89-101.
- Ng CS, Doyle TC, Courtney HM, Campbell GA, Freeman AH, Dixon AK. Extracolonic findings in patients undergoing abdomino-pelvic CT for suspected colorectal carcinoma in the frail and disabled patient. Clin Radiol 2004;59:421-30. http://dx.doi.org/10.1016/S0009-9260(03)00342-8.
- Tolan DJM, Armstrong EM, Chapman AH. Replacing barium enema with CT colonography in patients older than 70 years: the importance of detecting extracolonic abnormalities. AJR Am J Roentgenol 2007;189:1104-11. http://dx.doi.org/10.2214/AJR.07.2026.
- Xiong T, Richardson M, Woodroffe R, Halligan S, Morton D, Lilford RJ. Incidental lesions found on CT colonography: their nature and frequency. Br J Radiol 2005;78:22-9. http://dx.doi.org/10.1259/bjr/67998962.
- Hellström M, Svensson M, Lasson A. Extracolonic and incidental findings on CT colonography. Am J Roentgenol 2004;182:631-8. http://dx.doi.org/10.2214/ajr.182.3.1820631.
- Pickhardt PJ, Kim DH, Meiners RJ, Wyatt KS, Hanson ME, Barlow DS, et al. Colorectal and extracolonic cancers detected at screening CT colonography in 10 286 asymptomatic adults. Radiology 2010;255:83-8. http://dx.doi.org/10.1148/radiol.09090939.
- Veerappan GR, Ally MR, Choi JH, Pak JS, Maydonovitch C, Wong RK. Extracolonic findings on CT colonography increases yield of colorectal cancer screening. AJR Am J Roentgenol 2010;195:677-86. http://dx.doi.org/10.2214/AJR.09.3779.
- Zalis ME, Barish MA, Choi JR, Dachman AH, Fenlon HM, Ferrucci JT, et al. CT colonography reporting and data system: a consensus proposal. Radiology 2005;236:3-9. http://dx.doi.org/10.1148/radiol.2361041926.
- Von Wagner C, Ghanouni A, Halligan S, Smith S, Dadswell E, Lilford RJ, et al. Patient acceptability and psychologic consequences of CT colonography compared with those of colonoscopy: results from a multicenter randomized controlled trial of symptomatic patients. Radiology 2012;263:723-31. http://dx.doi.org/10.1148/radiol.12111523.
- von Wagner C, Smith S, Halligan S, Ghanouni A, Power E, Lilford RJ, et al. Patient acceptability of CT colonography compared with double contrast barium enema: results from a multicentre randomised controlled trial of symptomatic patients. Eur Radiol 2011;21:2046-55. http://dx.doi.org/10.1007/s00330-011-2154-y.
- Bosworth HB, Rockey DC, Paulson EK, Niedzwiecki D, Davis W, Sanders LL, et al. Prospective comparison of patient experience with colon imaging tests. Am J Med 2006;119:791-9. http://dx.doi.org/10.1016/j.amjmed.2006.02.013.
- Gluecker TM, Johnson CD, Harmsen WS, Offord KP, Harris AM, Wilson LA, et al. Colorectal cancer screening with CT colonography, colonoscopy, and double-contrast barium enema examination: prospective assessment of patient perceptions and preferences. Radiology 2003;227:378-84. http://dx.doi.org/10.1148/radiol.2272020293.
- Thomeer M, Bielen D, Vanbeckevoort D, Dymarkowski S, Gevers A, Rutgeerts P, et al. Patient acceptance for CT colonography: what is the real issue?. Eur J Radiol 2002;12:1410-15. http://dx.doi.org/10.1007/s003300101082.
- Marshall DA, Johnson FR, Kulin NA, Ozdemir S, Walsh JME, Marshall JK, et al. How do physician assessments of patient preferences for colorectal cancer screening tests differ from actual preferences? A comparison in Canada and the United States using a stated choice survey. Health Econ 2009;18:1420-39. http://dx.doi.org/10.1002/hec.1437.
- von Wagner C, Knight K, Halligan S, Atkin W, Lilford R, Morton D, et al. Patient experiences of colonoscopy, barium enema and CT colonography: a qualitative study. Br J Radiol 2009;82:13-9. http://dx.doi.org/10.1259/bjr/61732956.
- Salmon P, Shah R, Berg S, Williams C. Evaluating customer satisfaction with colonoscopy. Endoscopy 1994;26:342-6. http://dx.doi.org/10.1055/s-2007-1008988.
- Cockburn J, Luise TD, Hurley S, Clover K. Development and validation of the PCQ: a questionnaire to measure the psychological consequences of screening mammography. Soc Sci Med 1992;34:1129-34. http://dx.doi.org/10.1016/0277-9536(92)90286-Y.
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988;54:1063-70. http://dx.doi.org/10.1037/0022-3514.54.6.1063.
- Noble M, Wilkinson K, Whitworth A, Barnes H, Dibben C. The English Indices of Deprivation 2007. London: Department for Communities and Local Government; 2008.
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. Boston, MA: Allyn and Bacon; 2007.
- Hanly P, Skally M, Fenlon H, Sharp L. Cost-effectiveness of computed tomography colonography in colorectal cancer screening: a systematic review. Int J Technol Assess Health Care 2012;28:415-23. http://dx.doi.org/10.1017/S0266462312000542.
- Hassan C, Hunink MG, Laghi A, Pickhardt PJ, Zullo A, Kim DH, et al. Value-of-information analysis to guide future research in colorectal cancer screening. Radiology 2009;253:745-52. http://dx.doi.org/10.1148/radiol.2533090234.
- Hassan C, Pickhardt PJ, Laghi A, Kim DH, Zullo A, Iafrate F, et al. Computed tomographic colonography to screen for colorectal cancer, extracolonic cancer, and aortic aneurysm: model simulation with cost-effectiveness analysis. Arch Intern Med 2008;168:696-705. http://dx.doi.org/10.1001/archinte.168.7.696.
- Hassan C, Zullo A, Laghi A, Reitano I, Taggi F, Cerro P, et al. Colon cancer prevention in Italy: cost-effectiveness analysis with CT colonography and endoscopy. Dig Liver Dis 2007;39:242-50. http://dx.doi.org/10.1016/j.dld.2006.09.016.
- Heitman SJ, Manns BJ, Hilsden RJ, Fong A, Dean S, Romagnuolo J. Cost-effectiveness of computerized tomographic colonography versus colonoscopy for colorectal cancer screening. CMAJ 2005;173:877-81. http://dx.doi.org/10.1503/cmaj.050553.
- Knudsen AB, Lansdorp-Vogelaar I, Rutter CM, Savarino JE, van Ballegooijen M, Kuntz KM, et al. Cost-effectiveness of computed tomographic colonography screening for colorectal cancer in the medicare population. J Natl Cancer Inst 2010;102:1238-52. http://dx.doi.org/10.1093/jnci/djq242.
- Ladabaum U, Song K, Fendrick AM. Colorectal neoplasia screening with virtual colonoscopy: when, at what cost, and with what national impact?. Clin Gastroenterol Hepatol 2004;2:554-63. http://dx.doi.org/10.1016/S1542-3565(04)00247-2.
- Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, Boer R, Wilschut J, Habbema JD. At what costs will screening with CT colonography be competitive? A cost-effectiveness approach. Int J Cancer 2009;124:1161-8. http://dx.doi.org/10.1002/ijc.24025.
- Regge D, Hassan C, Pickhardt PJ, Laghi A, Zullo A, Kim DH, et al. Impact of computer-aided detection on the cost-effectiveness of CT colonography. Radiology 2009;250:488-97. http://dx.doi.org/10.1148/radiol.2502080685.
- Pickhardt PJ, Hassan C, Laghi A, Zullo A, Kim DH, Morini S. Cost-effectiveness of colorectal cancer screening with computed tomography colonography: the impact of not reporting diminutive lesions. Cancer 2007;109:2213-21. http://dx.doi.org/10.1002/cncr.22668.
- Pickhardt PJ, Hassan C, Laghi A, Kim DH. CT colonography to screen for colorectal cancer and aortic aneurysm in the Medicare population: cost-effectiveness analysis. AJR Am J Roentgenol 2009;192:1332-40. http://dx.doi.org/10.2214/AJR.09.2646.
- Sonnenberg A, Delcò F, Bauerfeind P. Is virtual colonoscopy a cost-effective option to screen for colorectal cancer?. Am J Gastroenterol 1999;94:2268-74. http://dx.doi.org/10.1111/j.1572-0241.1999.01304.x.
- Telford JJ, Levy AR, Sambrook JC, Zou D, Enns RA. The cost-effectiveness of screening for colorectal cancer. CMAJ 2010;182:1307-13. http://dx.doi.org/10.1503/cmaj.090845.
- Vijan S, Hwang I, Inadomi J, Wong RK, Choi JR, Napierkowski J, et al. The cost-effectiveness of CT colonography in screening for colorectal neoplasia. Am J Gastroenterol 2007;102:380-90. http://dx.doi.org/10.1111/j.1572-0241.2006.00970.x.
- Lee D, Muston D, Sweet A, Cunningham C, Slater A, Lock K. Cost effectiveness of CT colonography for UK NHS colorectal cancer screening of asymptomatic adults aged 60–69 years. Appl Health Econ Health Policy 2010;8:141-54. http://dx.doi.org/10.2165/11535650-000000000-00000.
- Vanness DJ, Knudsen AB, Lansdorp-Vogelaar I, Rutter CM, Gareen IF, Herman BA, et al. Comparative economic evaluation of data from the ACRIN National CT colonography Trial with three cancer intervention and surveillance modeling network microsimulations. Radiology 2011;261:487-98. http://dx.doi.org/10.1148/radiol.11102411.
- Gomes M, Aldridge RW, Wylie P, Bell J, Epstein O. Cost-effectiveness analysis of 3-D computerized tomography colonography versus optical colonoscopy for imaging symptomatic gastroenterology patients. Appl Health Econ Health Policy 2013;11:107-17. http://dx.doi.org/10.1007/s40258-013-0019-z.
- Tappenden P, Chilcott J, Eggington S, Patnick J, Sakai H, Karnon J. Option appraisal of population-based colorectal cancer screening programmes in England. Gut 2007;56:677-84. http://dx.doi.org/10.1136/gut.2006.095109.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- NHS Reference Costs 2010–11. London: Department of Health; n.d.
- NHS Reference Costs. London: Department of Health; n.d.
- von Wagner C, Halligan S, Atkin WS, Lilford RJ, Morton D, Wardle J. Choosing between CT colonography and colonoscopy in the diagnostic context: a qualitative study of influences on patient preferences. Health Expect 2009;12:18-26. http://dx.doi.org/10.1111/j.1369-7625.2008.00520.x.
- Toma J, Paszat LF, Gunraj N, Rabeneck L. Rates of new or missed colorectal cancer after barium enema and their risk factors: a population-based study. Am J Gastroenterol 2008;103:3142-8. http://dx.doi.org/10.1111/j.1572-0241.2008.02199.x.
- Tawn DJ, Squire CJ, Mohammed MA, Adam EJ. National audit of the sensitivity of double-contrast barium enema for colorectal carcinoma, using control charts: for the Royal College of Radiologists Clinical Radiology Audit Sub-Committee. Clin Radiol 2005;60:558-64. http://dx.doi.org/10.1016/j.crad.2004.09.014.
- Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection – systematic review and meta-analysis. Radiology 2011;259:393-405. http://dx.doi.org/10.1148/radiol.11101887.
- Rockey DC, Paulson E, Niedzwiecki D, Davis W, Bosworth HB, Sanders L, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005;365:305-11. http://dx.doi.org/10.1016/S0140-6736(05)17784-8.
- Cotton P, Durkalski V, Pineau B, Palesch Y, Mauldin P, Hoffman B, et al. Computed tomographic colonography (virtual colonoscopy): a multicentre comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA 2004;291:1713-19. http://dx.doi.org/10.1001/jama.291.14.1713.
- Kim DH, Pickhardt PJ, Hanson ME, Hinshaw JL. CT colonography: performance and program outcome measures in an older screening population. Radiology 2010;254:493-500. http://dx.doi.org/10.1148/radiol.09091478.
- Kim DH, Pickhardt PJ, Taylor AJ, Leung WK, Winter TC, Hinshaw JL, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med 2007;357:1403-12. http://dx.doi.org/10.1056/NEJMoa070543.
- Liedenbaum MH, Venema HW, Stoker J. Radiation dose in CT colonography – trends in time and differences between daily practice and screening protocols. Eur Radiol 2008;18:2222-30. http://dx.doi.org/10.1007/s00330-008-0994-x.
- Blakeborough A, Sheridan MB, Chapman AH. Complications of barium enema examinations: a survey of UK consultant radiologists from 1992 to 1994. Clin Radiol 1997;52:142-8. http://dx.doi.org/10.1016/S0009-9260(97)80108-0.
- Bowles C, Leicester R, Romaya C, Swarbrick E, Williams C, Epstein O. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow?. Gut 2004;53:277-83. http://dx.doi.org/10.1136/gut.2003.016436.
- NHS Modernisation Agency . Improving Endoscopy Services: Meeting the Challenges 2004 n.d. www.thejag.org.uk/downloads/A-National Policies and Reports/Meeting the Challenges 2004.pdf (accessed February 2013).
- Shah HA, Paszat LF, Saskin R, Stukel TA, Rabeneck L. Factors associated with incomplete colonoscopy: a population-based study. Gastroenterology 2007;132:2297-303. http://dx.doi.org/10.1053/j.gastro.2007.03.032.
- Brenner H, Altenhofen L, Hoffmeister M. Sex, age, and birth cohort effects in colorectal neoplasms. Ann Intern Med 2010;152:697-703. http://dx.doi.org/10.7326/0003-4819-152-11-201006010-00002.
- Lefere PA, Gryspeerdt SS, Dewyspelaere J, Baekelandt M, Van Holsbeeck BG. Dietary fecal tagging as a cleansing method before CT colonography: initial results polyp detection and patient acceptance. Radiology 2002;224:393-40. http://dx.doi.org/10.1148/radiol.2241011222.
- Stoop EM, de Haan MC, de Wijkerslooth TR, Bossuyt PM, van Ballegooijen M, Nio CY, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol 2011;13:55-64. http://dx.doi.org/10.1016/S1470-2045(11)70283-2.
- Fisichella VA, Hellström M. Survey update on implementation, indications, and technical performance of computed tomography colonography in Sweden. Acta Radiol 2010;51:4-8. http://dx.doi.org/10.3109/02841850903292735.
- McFarland EG, Fletcher JG, Pickhardt P, Dachman A, Yee J, McCollough CH, et al. ACR Colon Cancer Committee white paper: status of CT colonography 2009. J Am Coll Radiol 2009;6:756-72.e4.
- Berland LL. Incidental extracolonic findings on CT colonography: the impending deluge and its implications. J Am Coll Radiol 2009;6:14-20. http://dx.doi.org/10.1016/j.jacr.2008.06.018.
- Fink M, Freeman AH, Dixon AK, Coni NK. Computed tomography of the colon in elderly people. BMJ 2004;308. http://dx.doi.org/10.1136/bmj.308.6935.1018.
- Yee J, Kumar NN, Godara S, Casamina JA, Hom R, Galdino G, et al. Extracolonic abnormalities discovered incidentally at CT colonography in a male population. Radiology 2005;236:519-26. http://dx.doi.org/10.1148/radiol.2362040166.
- Macari M, Nevsky G, Bonavita J, Kim DC, Megibow AJ, Babb JS. CT colonography in senior versus nonsenior patients: extracolonic findings, recommendations for additional imaging, and polyp prevalence. Radiology 2011;259:767-74. http://dx.doi.org/10.1148/radiol.11102144.
- Stevenson G. Colon imaging in radiology departments in 2008: goodbye to the routine double contrast barium enema. Can Assoc Radiol J 2008;59:174-82.
- Marshall DA, Johnson FR, Phillips KA, Marshall JK, Thabane L, Kulin NA. Measuring patient preferences for colorectal cancer screening using a choice-format survey. Value Health 2007;10:415-30. http://dx.doi.org/10.1111/j.1524-4733.2007.00196.x.
- Nayaradou M, Berchi C, Dejardin O, Launoy G. Eliciting population preferences for mass colorectal cancer screening organization. Med Decis Making 2010;30:224-33. http://dx.doi.org/10.1177/0272989X09342747.
- Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care 2008;46:S10-16. http://dx.doi.org/10.1097/MLR.0b013e31817d932e.
- Keeling AN, Slattery MM, Leong S, McCarthy E, Susanto M, Lee MJ, et al. Limited-preparation CT colonography in frail elderly patients: a feasibility study. Am J Roentgenol 2010;194:1279-87. http://dx.doi.org/10.2214/AJR.09.2896.
- Jensch S, Bipat S, Peringa J, de Vries AH, Heutinck A, Dekker E, et al. CT colonography with limited bowel preparation: prospective assessment of patient experience and preference in comparison to optical colonoscopy with cathartic bowel preparation. Eur Radiol 2010;20:146-56. http://dx.doi.org/10.1007/s00330-009-1517-0.
- Taylor PN, Beckly DE. Use of air in double contrast barium enema – is it still acceptable?. Clin Radiol 1991;44:183-4. http://dx.doi.org/10.1016/S0009-9260(05)80866-9.
- Girling A, Lilford RJ, Kattan MW. Encyclopedia of Medical Decision Making. Thousand Oaks, CA: Sage Publications; 2009.
- Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al. Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10450.
- NICE . Update to the NICE Technology Appraisals Methods Guide 2013. http://www.nicedsu.org.uk/NICE-Methods-Guide-updates(1985333).htm (accessed 9 December 2014).
Appendix 1 SIGGAR forms
Appendix 2 Patient information sheets
Appendix 3 Psychological questionnaires
Appendix 4 Economics tables
Data accessed at the UK Government websites76,77 which contain public sector information licensed under the Open Government Licence v3.0 URL: www.nationalarchives.gov.uk/doc/open-government-licence/version/3.
| Other diagnostic procedures | |||||
|---|---|---|---|---|---|
| Procedure | Value (£) | Low range (£) | High range (£) | Procedure codes used to identify unit costs | Description; other sources of cost information (if not from NHS reference cost 2010/11) |
| CT abdomen | 95 | 73 | 106 | RA08Z | CT scan, one area, no contrast |
| CT abdomen and pelvis | 151 | 116 | 177 | RA13Z | CT scan, three areas with contrast |
| CT chest and abdomen | 151 | 116 | 177 | RA13Z | CT scan, three areas with contrast |
| CT chest, abdomen and pelvis | 151 | 116 | 177 | RA13Z | CT scan, three areas with contrast |
| Contrast enema | 135 | 91 | 162 | RA17Z | Contrast fluoroscopy procedures 20–40 minutes |
| MRI pelvis | 165 | 108 | 188 | RA01Z | MRI scan, one area, no contrast |
| Outpatient | 106 | 104 | Outpatient: colorectal surgery | ||
| Rigid sigmoidoscopy | 180 | 132 | 212 | FZ57Z | Diagnostic or therapeutic rigid sigmoidoscopy for patients ≥ 19 years |
| Surgical procedures | |||||
| Abdominoperineal resection | 6411 | 4892 | 7255 | FZ08B | Complex large intestine procedures without major CC |
| Abdominoperineal resection | 6411 | 4892 | 7255 | FZ08B | Complex large intestine procedures without major CC |
| Altemeier operation | 3723 | 3003 | 4387 | FZ11B | Large intestine – major procedures without major CC |
| Anterior resection | 5954 | 4802 | 6902 | FZ10B | Distal colon procedures without major CC |
| Anterior resection and loop ileostomy | 5954 | 4802 | 6902 | FZ10B | Distal colon procedures without major CC |
| Anterior resection and loop ileostomy and splenectomy | 9969 | 7831 | 11,542 | FZ10B and GA07B | Distal colon procedures without major CC and hepatobiliary procedures category three without CC |
| Anterior resection/sigmoid colectomy | 5954 | 4802 | 6902 | FZ10B | Distal colon procedures without major CC |
| Colonoscopy polypect (general anaesthetic) | 1424 | 1008 | 1777 | FZ50Z | Intermediate large intestine procedures ≥ 19 years |
| End ileostomy/mucous fistula | 3695 | 2869 | 4337 | FZ67B | Major small intestine procedures without CC |
| Evacuation | 1424 | 1008 | 1777 | FZ50Z | Evacuation |
| Extended right hemicolectomy | 5260 | 4359 | 5942 | FZ09B | Proximal colon procedures without major CC |
| Hartmann’s procedure | 5954 | 4802 | 6902 | FZ10B | Distal colon procedures without major CC |
| High anterior resection | 5954 | 4802 | 6902 | FZ10B | Distal colon procedures without major CC |
| High anterior resection/sigmoid colectomy | 5954 | 4802 | 6902 | FZ10B | Distal colon procedures without major CC |
| Ileotransverse bypass | 3723 | 3003 | 4387 | FZ11B | Large intestine – major procedures without major CC |
| Lap subtotal colectomy and ileostomy | 6411 | 4892 | 7255 | FZ08B | Complex large intestine procedures without major CC |
| Laparoscopic-assisted low anterior resection with loop ileostomy | 6265 | 5042 | 7247 | FZ10B; cost adjusted | Distal colon procedures without major CC; reference cost adjusted by a factor of 1.05 (inflated 5%) for laparoscopic procedure based on Murray et al.108 |
| Laparoscopic-assisted right hemicolectomy | 5523 | 4577 | 6239 | FZ09B; cost adjusted | Proximal colon procedures without major CC; reference cost adjusted by a factor of 1.05 (inflated 5%) for laparoscopic procedure based on Murray et al.108 |
| Laparoscopic-assisted anterior resection | 6265 | 5042 | 7247 | FZ10B; cost adjusted | Distal colon procedures without major CC; reference cost adjusted by a factor of 1.05 (inflated 5%) for laparoscopic procedure based on Murray et al.108 |
| Laparoscopic sigmoid colectomy | 6265 | 5042 | 7247 | FZ10B; cost adjusted | Distal colon procedures without major CC; reference cost adjusted by a factor of 1.05 (inflated 5%) for laparoscopic procedure based on Murray et al.108 |
| Laparoscopy and repair of umbilical hernia | 1969 | 1548 | 2346 | FZ18C; cost adjusted | Inguinal umbilical or femoral hernia repairs ≥ 19 years without CC; reference cost adjusted by a factor of 1.05 (inflated 5%) for laparoscopic procedure based on Murray et al.108 |
| Laparotomy and right oophorectomy | 4066 | 3295 | 4628 | MA06Z | Open major upper and lower genital tract procedures |
| Laparotomy only | 3177 | 2217 | 3968 | FZ12F | General abdominal – very major or major procedures without CC |
| Left hemicolectomy | 5954 | 4802 | 6902 | FZ10B | Distal colon procedures without major CC |
| Left hemicolectomy and high anterior resection | 5954 | 4802 | 6902 | FZ10B | Distal colon procedures without major CC |
| Left hemicolectomy and low anterior resection and loop ileostomy | 5954 | 4802 | 6902 | FZ10B | Distal colon procedures without major CC |
| Left hemicolectomy/left oophorectomy and small bowel resection | 6411 | 4892 | 7255 | FZ08B | Complex large intestine procedures without major CC |
| Local excision other sites | 3723 | 3003 | 4387 | FZ11B | Large intestine – major procedures without major CC |
| Low anterior resection | 5954 | 4802 | 6902 | FZ10B | Distal colon procedures without major CC |
| Pelvic clearance | 5510 | 2952 | 6986 | FZ12B | General abdominal – very major or major procedures without CC/with major or intermediate CC |
| Perianal excision | 1498 | 1186 | 1715 | FZ22A | Intermediate anal procedures in patients ≥ 19 years |
| Right hemicolectomy | 5260 | 4359 | 5942 | FZ09B | Proximal colon procedures with/without major CC |
| Right hemicolectomy and anterior resection | 6411 | 4892 | 7255 | FZ08B | Complex large intestine procedures without major CC |
| Sigmoid colectomy | 5954 | 4802 | 6902 | FZ10B | Distal colon procedures with/without major CC |
| Sigmoid colectomy and appendectomy | 6411 | 4892 | 7255 | FZ08B | Complex large intestine procedures without major CC |
| Sigmoid colectomy and small bowel resection | 6411 | 4892 | 7255 | FZ08B | Complex large intestine procedures without major CC |
| Sigmoid colectomy with en bloc right hemicolectomy | 6411 | 4892 | 7255 | FZ08B | Complex large intestine procedures without major CC |
| Spare | 3723 | 3003 | 4387 | FZ11B | Large intestine – major procedures without major CC |
| Subtotal colectomy | 6411 | 4892 | 7255 | FZ08B | Complex large intestine procedures with/without major CC |
| 5260 | 4359 | 5942 | FZ09B | Proximal colon procedures without major CC | |
| Transverse colectomy | 2078 | 1417 | 2396 | FZ21Z | Major anal procedures |
| Transanal endoscopic microsurgery | 2078 | 1417 | 2396 | FZ21Z | Major anal procedures |
| Transanal procedure | 6411 | 4892 | 7255 | FZ08B | Complex large intestine procedures without major CC |
| Procedure | Value (£) | Low range (£) | High range (£) | Procedure codes used to identify unit costs | Description; other sources of cost information (if not from NHS reference cost 20010/11) |
|---|---|---|---|---|---|
| Surgical procedures | |||||
| Adrenalectomy | 5033 | 3432 | 6366 | KA04Z | Adrenal procedure |
| Bilateral oophorectomy | 2561 | 2067 | 2896 | MA08Z | Upper genital tract laparoscopic/endoscopic major procedures |
| Bilateral salpingo-oophorectomy | 2561 | 2067 | 2896 | MA08Z | Upper genital tract laparoscopic/endoscopic major procedures |
| Unilateral salpingo-oophorectomy | 2561 | 2067 | 2896 | MA08Z | Upper genital tract laparoscopic/endoscopic major procedures |
| Hysterectomy and bilateral salpingo-oophorectomy | 3175 | 2632 | 3688 | MA07D | Upper genital tract major procedures without major CC |
| Endovascular aneurysm repair | 6940 | 4499 | 8375 | QZ01B; cost adjusted | Aortic or abdominal surgery without CC; reference cost was adjusted by a factor of 1.05 (inflated 5%) based on estimates in aNICE guideline 2010109 |
| Open-tube graft repair | 6609 | 4285 | 7976 | QZ01B | Aortic or abdominal surgery without CC |
| Laparotomy | 3177 | 2217 | 3968 | FZ12F | General abdominal – very major or major procedures ≥ 19 years without CC |
| Laparoscopic cholecystectomy | 2326 | 1821 | 2653 | GA10D | Laparoscopic cholecystectomy with length of stay ≥ 1 day without CC |
| Inguinal hernia repair | 1875 | 1475 | 2234 | FZ18C | Inguinal umbilical or femoral hernia repairs ≥ 19 years without CC |
| Partial nephrectomy | 5175 | 4009 | 6040 | LB02C | Kidney major open procedure ≥ 19 years without CC |
| Radical nephrectomy | 5175 | 4009 | 6040 | LB02C | Kidney major open procedure ≥ 19 years without CC |
| Right upper lobectomy | 6043 | 4515 | 7051 | DZ02C | Complex thoracic procedures without CC |
| Splenectomy | 4015 | 3029 | 4640 | GA07B | Hepatobiliary procedures category three without CC |
| Whipple procedure | 8424 | 5704 | 10493 | GA03B | Hepatobiliary procedures category seven |
| Other diagnostic procedures | |||||
| Bronchoscopy | 1081 | 377 | 1198 | DZ07A | Fibre optic bronchoscopy ≥ 19 years |
| Flexible cystoscopy | 1107 | 865 | 1287 | LB14E | Bladder intermediate endoscopic procedure ≥ 19 years |
| Hysteroscopy | 206 | 157 | 248 | MA21Z | Diagnostic hysteroscopy |
| ERCP | 1091 | 722 | 1335 | GB06D | ERCP category two with length of stay ≤ 2 days |
| Bone scan | 181 | 131 | 214 | RA36Z | Nuclear medicine – category two |
| Dimercaptosuccinic acid scan | 181 | 131 | 214 | RA36Z | Nuclear medicine – category two |
| Endoscopic ultrasonography | 168 | 72 | 155 | GB03B | Endoscopic/radiology category two without CC |
| Positron emission tomography scan lung | 354 | 118 | 478 | RA39Z | Nuclear medicine – category five |
| Renal ultrasonography | 53 | 39 | 61 | RA23Z | Ultrasonography scan < 20 minutes |
| Barium meal | 92 | 47 | 120 | RA16Z | Contrast fluoroscopy procedures < 20 minutes |
| Lymph node biopsy | 211 | 137 | 292 | WA24Z | Procedures on the lymphatic system |
| Prostate biopsy | 200 | 147 | 212 | LB27Z | Prostate or bladder neck minor endoscopic procedure – male |
| Renal biopsy | 191 | 155 | 199 | LB04B | Kidney major endoscopic procedure without CC |
| Blood test | 99 | 31 | 149 | WA21Y | Other procedures and health-care problems without CC |
| Extracorporeal shock wave lithotripsy | 140 | 140 | 142 | LB36Z | Extracorporeal lithotripsy |
| Fluid aspiration and culture | 1162 | 777 | 1300 | MA19A | Vacuum aspiration with cannula – < 14 weeks’ gestation |
| Intravenous urography | 87 | 40 | 103 | RA26Z | Ultrasonography mobile scan/intraoperative procedures 20–40 minutes |
| Oesophagogastroduodenoscopy | 747 | 391 | 881 | FZ60Z | Diagnostic endoscopic procedures on the upper GI tract 19 years and over |
| Prostate-specific antigen test | 200 | 147 | 212 | LB27Z | Prostate or bladder neck minor endoscopic procedure – male |
| Stent insertion | 281 | 101 | 381 | QZ15C | Therapeutic endovascular procedures without CC |
| Surveillance | 99 | 31 | 149 | WA21Y | Other procedures and health-care problems without CC |
| Urine test | 99 | 31 | 149 | WA21Y | Other procedures and health-care problems without CC |
| Video-assisted thoracic surgery | 3576 | 2349 | 4684 | DZ04B | Intermediate thoracic procedures without CC |
| CT endovascular aortic repair protocol | 6940 | 4499 | 8375 | QZ01B; cost adjusted | Aortic or abdominal surgery without CC; reference cost was adjusted by a factor of 1.05 (inflated 5%) based on estimates in aNICE guideline 2010109 |
| CT abdomen | 95 | 73 | 106 | RA08Z | CT scan, one area, no contrast |
| CT abdomen and pelvis | 112 | 90 | 124 | RA11Z | CT scan, two areas without contrast |
| CT aorta | 95 | 73 | 106 | RA08Z | CT scan, one area, no contrast |
| CT chest | 95 | 73 | 106 | RA08Z | CT scan, one area, no contrast |
| CT chest and abdomen | 112 | 90 | 124 | RA11Z | CT scan, two areas without contrast |
| CT chest abdomen and pelvis | 151 | 116 | 177 | RA13Z | CT scan, three areas with contrast |
| CTC | 160 | 89 | 186 | RA14Z | CT scan, more than three areas |
| CT pancreas | 95 | 73 | 106 | RA08Z | CT scan, one area, no contrast |
| CT renal | 95 | 73 | 106 | RA08Z | CT scan, one area, no contrast |
| CT surveillance | 95 | 73 | 106 | RA08Z | CT scan, one area, no contrast |
| CT-guided biopsy | 95 | 73 | 106 | RA08Z | CT scan, one area, no contrast |
| MRI liver | 165 | 108 | 188 | RA01Z | MRI scan, one area, no contrast |
| MRI abdomen | 165 | 108 | 188 | RA01Z | MRI scan, one area, no contrast |
| MRI abdomen and pelvis | 211 | 131 | 258 | RA04Z | MRI scan, two–three areas, no contrast |
| MRI adrenal glands | 165 | 108 | 188 | RA01Z | MRI scan, one area, no contrast |
| MRI liver | 165 | 108 | 188 | RA01Z | MRI scan, one area, no contrast |
| MRI pancreas | 165 | 108 | 188 | RA01Z | MRI scan, one area, no contrast |
| MRI pelvis | 165 | 108 | 188 | RA01Z | MRI scan, one area, no contrast |
| MRI spine | 165 | 108 | 188 | RA01Z | MRI scan, one area, no contrast |
| MRI thoracic spine | 165 | 108 | 188 | RA01Z | MRI scan one area, no contrast |
| Ultrasonography abdomen | 53 | 39 | 61 | RA23Z | Ultrasonography scan < 20 minutes |
| Ultrasonography abdomen and pelvis | 66 | 50 | 73 | RA24Z | Ultrasonography scan > 20 minutes |
| Ultrasonography aorta | 53 | 39 | 61 | RA23Z | Ultrasonography scan < 20 minutes |
| Ultrasonography biliary | 53 | 39 | 61 | RA23Z | Ultrasonography scan < 20 minutes |
| Ultrasonography jejunal mesentery | 53 | 39 | 61 | RA23Z | Ultrasonography scan < 20 minutes |
| Ultrasonography liver | 53 | 39 | 61 | RA23Z | Ultrasonography scan < 20 minutes |
| Ultrasonography pelvis | 53 | 39 | 61 | RA23Z | Ultrasonography scan < 20 minutes |
| Ultrasonography renal | 53 | 39 | 61 | RA23Z | Ultrasonography scan < 20 minutes |
| Ultrasonography surveillance | 53 | 39 | 61 | RA23Z | Ultrasonography scan < 20 minutes |
| Ultrasonography transvaginal | 53 | 39 | 61 | RA23Z | Ultrasonography scan < 20 minutes |
| Ultrasound-guided biopsy | 53 | 39 | 61 | RA23Z | Ultrasonography scan < 20 minutes |
| Ultrasound-guided drainage | 53 | 39 | 61 | RA23Z | Ultrasonography scan < 20 minutes |
| Ultrasound-guided fine-needle aspiration | 53 | 39 | 61 | RA23Z | Ultrasonography scan < 20 minutes |
| Radiography chest | 95 | 73 | 106 | RA08Z | CT scan, one area, no contrast |
| Radiography humerus | 21 | RA08-RA11 | CT scan; Tariff 2010/11 | ||
| Radiography pelvis | 21 | RA08-RA11 | CT scan; Tariff 2010/11 | ||
| Radiography skeleton | 21 | RA08-RA11 | CT scan; Tariff 2010/11 | ||
| Radiography surveillance | 21 | RA08-RA11 | CT scan; Tariff 2010/11 | ||
List of abbreviations
- 2D
- two-dimensional
- 3D
- three-dimensional
- BE
- barium enema
- BNF
- British National Formulary
- CI
- confidence interval
- CRC
- colorectal cancer
- CRF
- case report form
- CT
- computed tomography
- CTC
- computed tomographic colonography
- df
- degrees of freedom
- E1
- E-RADS category 1
- E2
- E-RADS category 2
- E3
- E-RADS category 3
- E4
- E-RADS category 4
- E-RADS
- Extracolonic Reporting And Data System
- ESGAR
- European Society of Gastrointestinal and Abdominal Radiology
- FS
- flexible sigmoidoscopy
- GI
- gastrointestinal
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- ICD
- International Classification of Diseases and Related Health Problems
- ICD-10
- International Classification of Diseases and Related Health Problems, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- IRR
- incidence rate ratio
- NHSCR
- National Health Service Central Register
- NHSIC
- National Health Service Information Centre
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk
- SIGGAR
- Special Interest Group in Gastrointestinal and Abdominal Radiology