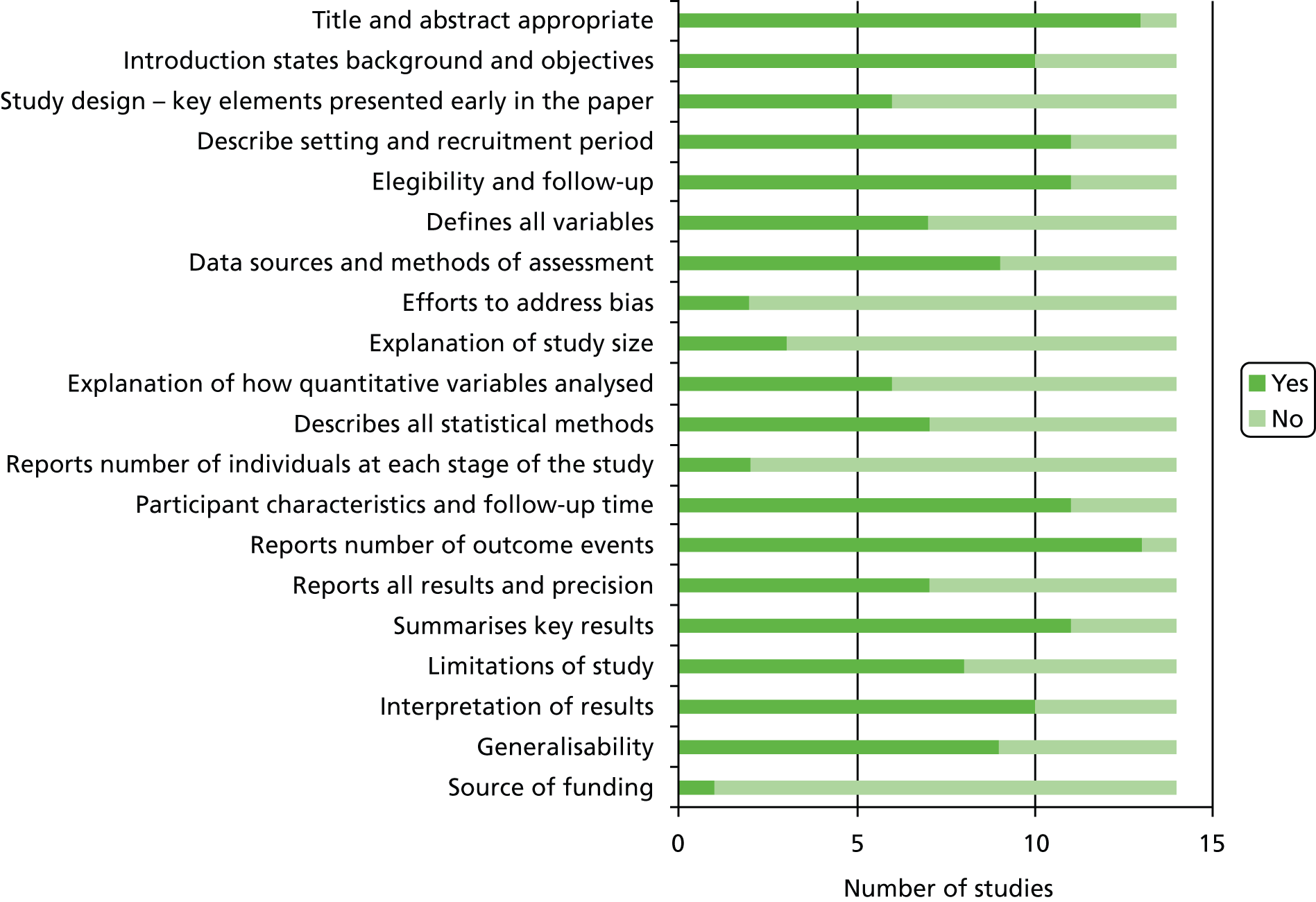
Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/404/84. The contractual start date was in April 2008. The draft report began editorial review in October 2013 and was accepted for publication in February 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Mr Clark reports receiving honoraria for training from Hologic and Ethicon, which make endoscopic instruments [Myosure (Hologic, Marlborough, MA, USA) and Versapoint (Gynecare, Ethicon, Somerville, NJ, USA)] suitable for removing uterine pathologies such as polyps. Since completing the OPT Trial recruitment (but not before completing writing this report) he received £40,000 funding from Smith & Nephew to evaluate a product (TruClear) also suitable for removing uterine polyps and fibroids. Dr Smith reports grants from National Institute for Health Research Grants during the conduct of the study, and grants from Smith & Nephew outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Clark et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Definition of a uterine polyp
Uterine polyps are focal endometrial outgrowths that can occur anywhere within the uterine cavity. They contain a variable amount of glands, stroma and blood vessels, the relative amounts of which influence their visual appearance at hysteroscopy. Polyps may be soft and cystic or firm and fibrous; they may be pedunculated or sessile, single or multiple, and vary in size from small – with minimal uterine cavity distortion – to large, filling the whole cavity.
Aetiology of uterine polyps
Uterine polyps are composed of either functional and/or basal endometrium. They are typically a mixture of dense fibrous tissue (stroma), large and thick-walled vascular channels, and elongated glandular spaces of varying shapes1,2 which protrude into the uterine cavity. The underlying cause of uterine polyp formation remains unclear but is believed to be multifactorial. 3 They are thought to originate as focal areas of stromal and glandular overgrowth. This hyperplasia of the endometrium may occur within an area of mucosa that contains more hormonal receptors, thus making it more sensitive to oestrogenic stimulation. 4,5 The mechanism of uterine polyp development may differ according to menopausal status. In premenopausal women, a decrease in oestrogen and progesterone receptors within polyp stromal cells, although not the glandular component, has been found, which may make polyps less sensitive to cyclic hormonal changes. 6–8 Others have pointed to increased cell longevity, as a result of inhibition of apoptosis7,9 and biomarkers for altered gene expression have been documented within uterine polyps. 10,11
Diagnosis of uterine polyps
For many years the traditional method of diagnosing endometrial polyps was dilatation of the cervix and curettage of the endometrium [dilatation and curettage (D&C)]. However, this approach requires general anaesthesia, is associated with morbidity arising from uterine trauma, and has been shown to have limited diagnostic accuracy, because detecting and removing focal pathologies is notoriously problematic without direct visualisation. 12–15 The advent of convenient imaging of the uterine cavity using ultrasound or endoscopic technologies has largely replaced the D&C for endometrial evaluation; 16 when histological tissue samples are indicated, they can be obtained at the same time utilising blind, miniature outpatient biopsy devices17,18 or, in the case of hysteroscopy, guided biopsy. 19,20
Transvaginal ultrasound scan (TVS) is the least invasive outpatient test to evaluate the endometrium, as it avoids potentially painful instrumentation of the uterine cavity; the ultrasound probe simply sits within the top of the vagina. Uterine polyps typically appear as a hyperechoic lesion with or without cystic spaces, usually with regular contours and surrounded by a thin hyperechoic halo, or the polyp may appear as a non-specific endometrial thickening or focal mass within the endometrial cavity. TVS is often used as the first-line test for evaluating bleeding complaints because of its convenience and acceptability. 21 However, compared with saline infusion sonography (SIS), through which fluid is instilled to expand the uterine lumen and delineate the walls of the uterine cavity, or outpatient hysteroscopy (OPH), which directly visualises the inside of the uterus, TVS has poorer accuracy for diagnosing uterine polyps. 22,23 This is primarily because the sonographic findings are not specific to polyps, and other endometrial abnormalities – such as submucosal fibroids or endometrial irregularity – may have the same features, with the findings often subtle and easily overlooked especially for smaller polyps. 24 The development of ancillary technologies such as three-dimensional imaging and the use of colour or power Doppler may in time help improve the diagnostic accuracy of TVS for detecting focal pathologies within the uterus but evidence to date is lacking. 25,26
The gold standard investigative tools for diagnosing uterine polyps are SIS or OPH. Both tests have good accuracy for both detecting or excluding the presence of polyps within the uterus. 22,27–29 Although SIS is popular in some parts of Europe and North America, OPH is more widely practised in the UK and worldwide. This is because the skills of hysteroscopy are more widely attained by practising gynaecologists and it is safely practised30,31 but also because OPH allows convenient simultaneous removal of polyp (‘see and treat’),32 may be more cost-effective16 and appears to be preferred by women. 33,34 In contrast with OPH, SIS has the advantage of allowing assessment and visualisation of other pelvic structures, and visualisation of potential myometrial and adnexal abnormalities. 35
Prevalence and epidemiology of uterine polyps
The prevalence of uterine polyps in a general adult female population without abnormal uterine bleeding (AUB) is generally estimated to be around 10%. 32 Case series of asymptomatic women are generally small and estimates of prevalence imprecise; after TVS, uterine polyps were detected incidentally in 12% of premenopausal women36 and in 6–11% of infertile women without AUB. 37,38 In asymptomatic postmenopausal women, prevalences of 13%36 and 16%39 have been reported following investigation with ultrasound and hysteroscopy, respectively. However, they are found with increasing frequency in women undergoing investigation for problems with AUB (see Abnormal uterine bleeding and uterine polyps). In addition to AUB, risk factors for uterine polyp development are thought to include obesity, late menopause and the use of the partial oestrogen agonist tamoxifen. 36,40–42 The role of hormone replacement therapy (HRT) on polyp formation is unclear, with some studies supporting an association36,40 and others not. 43,44
Clinical significance of uterine polyps
Once a uterine polyp has been diagnosed, the current clinical consensus is to remove it. 45 The rationale for this approach is based upon (1) a belief that uterine polyps are unlikely to spontaneously resolve; (2) a desire to alleviate AUB symptoms or optimise fertility; and (3) a need to exclude serious endometrial disease. 32
Natural history
The natural history of endometrial polyps is not fully understood. 46 A proportion of small polyps may regress naturally without treatment, but the majority persist; in one series of 45- to 50-year-old asymptomatic women, 27% of 31 polyps regressed spontaneously during a 1-year follow-up. Polyps that regressed tended to be smaller,47 in keeping with an earlier case series. 46
Oncogenesis
The pathogenesis and oncogenic potential of uterine polyps are unclear. However, the vast majority of uterine polyps are benign, and endometrial cancer originating within the polyp is a rare occurrence. Case series of varied populations report a cancer prevalence of approximately 0.5–3%. 48–55 Asymptomatic women are estimated to have a 4- to 10-fold reduced risk of malignancy compared with those women with AUB. 55,56 Outside of AUB, other risk factors for malignancy within uterine polyps appear to include increasing age, postmenopausal status, obesity, diabetes53–55 and an increased polyp diameter. 55,56 The use of tamoxifen appears to increase the risk of atypical hyperplasia and malignancy within uterine polyps. 41,57
Atypical endometrial hyperplasia (EH) is considered to be a premalignant condition58 and the prevalence within polyps ranges between 1% and 3%. 53–56 Non-atypical hyperplasia has been reported to be found in up to 13% of polyps,53 but the oncogenic potential of this condition is generally low. A systematic review of observational series evaluated the prevalence of premalignant and malignant disease within uterine polyps. It reported malignant tissue changes within endometrial polyps in 0–12.9% of included studies, and hyperplastic change in 0.2–23.8% of polyps. They found postmenopausal symptomatic women to have the highest risk of premalignant and malignant tissue changes. 59 A more recent review60 similarly found symptomatic women with AUB bleeding to be at higher risk of premalignancy or malignancy within a uterine polyp; they reported the prevalence of endometrial neoplasia within polyps in women with symptomatic bleeding as 4.2% (195/4697) compared with 2.2% (85/3941) for those without bleeding [relative risk (RR) 1.97; 95% confidence interval (CI) 1.24 to 3.14]. Among symptomatic postmenopausal women with endometrial polyps, 4.5% (88 of 1968) had a malignant polyp in comparison with 1.5% (25/1654) of asymptomatic postmenopausal women (RR 3.36, 95% CI 1.45 to 7.80). The risk of premalignancy or malignancy within a uterine polyp was higher in symptomatic postmenopausal women (5.4%, 214/3946) compared with 1.7% (68/3997) in reproductive-aged women (RR 3.86, 95% CI 2.92 to 5.11).
Abnormal uterine bleeding
Abnormal uterine bleeding affects women of both reproductive (premenopausal women) and postreproductive (postmenopausal women) age. AUB is one of the four most common reasons for consulting a general practitioner (GP), and accounts for 70% of all referrals to hospital gynaecology clinics,61 making this complaint one of the commonest problems in gynaecology. A large proportion of health-care resources in both primary care and hospital settings are used up in managing this condition. 62
In premenopausal women, AUB manifests itself primarily as heavy menstrual bleeding (HMB), which has been defined as ‘excessive menstrual blood loss which interferes with the woman’s physical, emotional, social and material quality of life, and which can occur alone or in combination with other symptoms’. 63 HMB affects one in five women of reproductive age, with 5% of women aged 30–49 years consulting their GP each year because of the condition. 64 The overall prevalence of HMB in England and Wales has been estimated at 1.5 million women. 65 Uterine bleeding may be unscheduled, however, occurring outside of the expected time of the menstrual period. This irregular ‘breakthrough’ bleeding is known as intermenstrual bleeding (IMB) and is also common; a recent survey within primary care found the 2-year cumulative incidence of IMB to be 24% (95% CI 21% to 27%). 66
Postmenopausal bleeding (PMB) is also a common clinical problem in both general practice and secondary care (hospital settings). Women are most likely to present with PMB in the sixth decade of life, for which consultation rates in primary care are 14.3 per 1000 of the population. 67 PMB causes significant alarm and anxiety to women, who recognise vaginal bleeding after their periods have ceased as abnormal. Rapid referral to secondary care for investigation is indicated because between 5% and 10% of women with PMB will have endometrial cancer. 68 Postmenopausal women taking HRT may also develop problematic genital tract bleeding. Women taking sequential HRT regimens may present with either heavy scheduled bleeding or unscheduled, erratic bleeding, whereas women taking continuous, combined ‘no bleed’ HRT preparations present with unexpected bleeding, which is, by definition, unscheduled and abnormal. Similarly, women with breast cancer who are taking the partial oestrogen agonist tamoxifen may present with unscheduled bleeding. 69 Table 1 summarises the types of AUB.
| Bleeding type | Description |
|---|---|
| HMB | Excessive cyclical menstrual bleeding; menses may be frequent, prolonged or irregular |
| IMB | Intermittent or persistent episodes of bleeding that occur between normally timed menstrual periods; the bleeding may be of a regular and predictable or random, following no particular pattern |
| PMB | Any vaginal bleeding occurring after the menopause; in women taking exogenous hormones (HRT) bleeding may be heavy and scheduled (sequential ‘bleed’ HRT regimens) or unscheduled (sequential or continuous combined ‘no bleed’ preparations) |
Abnormal uterine bleeding and uterine polyps
The majority of women with symptomatic polyps present with AUB as described in the preceding section. With the advent of high-resolution pelvic ultrasound and hysteroscopic diagnosis, it has become clear that uterine polyps are highly prevalent during investigation of abnormal bleeding. The reported prevalence of endometrial polyps in general is considered to be between 20% and 30%,43,70,71 the variation reflecting the criteria used to define a polyp, the diagnostic test used, and the type of population studied. Although the prevalence of uterine polyps may be increased after the menopause,36 polyps are found commonly to affect both pre- and postmenopausal women across all age groups. 46 In recognition of the frequency in which uterine polyps are discovered in women of reproductive age, the International Federation of Gynecology and Obstetrics (FIGO) has recently accepted a new classification system for causes of AUB in the reproductive years, based on the acronym ‘PALM-COEIN’ with the ‘P’ denoting a ‘polyp’, i.e. describing AUB associated with the presence of uterine polyps. 72
The improved diagnostic accuracy, has led to the increased use of surgical intervention for the removal of polyps (‘polypectomy’), a procedure that is universally practised to resolve symptoms and to obtain tissue for histological examination. 45
Treatment of uterine polyps
Until recently, inpatient blind uterine curettage (D&C) under general anaesthetic has been the technique routinely used to perform uterine polypectomy. It involves wide dilatation of the cervix and the use of standard surgical polypectomy forceps to explore the uterine cavity. This technique is still used today, although most gynaecologists perform a hysteroscopy beforehand to locate the polyp to direct blind avulsion of the lesion followed by curettage. 45,73 Owing to the need for inpatient hospital admission and general anaesthesia, this approach is associated with heavy use of health-care resources, with over 25,000 inpatient procedures being performed during 2011–12 in the UK, a figure that was up by 4000 on the numbers from 1998 to 1999 confirming a trend towards an increase in the use of inpatient polypectomy [Department of Health (England), Hospital Episode Statistics – 2011/1274].
Expectant management
The observation that polyps are an incidental finding in around 5–15% of women,36–39 the majority of polyps are benign60,75 and some may naturally regress46,47 has led some to question whether removal of uterine polyps is necessary,76 and indeed removal may subject women to unnecessary morbidity and wastage of scarce health service resources. Two RCTs have addressed this issue, randomising women with AUB and uterine polyps to expectant management or surgical removal. 75,76 One trial76 failed to recruit women with PMB because neither doctors nor patients were in equipoise and so were unwilling to participate. This finding is consistent with postmenopausal women having a preference for hysteroscopic diagnosis and treatment when an abnormality is found. 73 The other RCT randomised 150 women with uterine polyps, of which 60% had AUB symptoms. Overall, no reduction in periodic blood loss was demonstrated at 6 months’ follow-up, but IMB symptoms were significantly improved. 75 The findings from this study are limited, given that it was restricted to premenopausal women, the sample size was small (only 60% of the population included were symptomatic), the presenting complaints were heterogeneous and the study length of follow-up was short.
Medical management
Medical management is widely adopted for the treatment of menstrual complaints and includes the use of hormonal contraceptives. Although some of these women may have undiagnosed uterine polyps, evidence for the use of medical therapy is lacking and not recommended. 63 Gonadotrophin-releasing hormone analogues (‘GnRH-a’s) have been used prior to hysteroscopic resection of focal pathologies in premenopausal women77 but the costs and menopausal side effects are difficult to justify for the removal of uterine polyps. This is because polyps are successfully removed in the majority of cases without the need for adjunctive medical preparation, in contrast with submucous fibroids (SMFs). One small series evaluated different HRT regimens to see whether some have a reduced propensity to polyp formation. 78 The use of levonorgestrel-releasing intrauterine system (LNG-IUS) in women taking tamoxifen has been reported to reduce the incidence of endometrial polyps. 79
Surgical management: polypectomy
A UK national survey45 and two subsequent Dutch surveys80,81 confirmed that the vast majority of gynaecologists advocated surgical removal of polyps from the uterus after diagnosis with 854 of 918 (93%),45 455 of 553 (83%)80 and 411 of 585 (91%) respondents performing polypectomy. 81 In the UK, the predominant method for removal of uterine polyps was by blind avulsion or curettage, after hysteroscopic location of the focal lesion under general anaesthesia,45 whereas in the Netherlands removal under direct hysteroscopic vision under general or regional anaesthesia80,81 was the favoured approach.
Blind uterine polypectomy
Blind methods to retrieve focal intrauterine pathology included blind curettage of the endometrium or avulsion with polyp forceps. These approaches can be associated with potential uterine trauma, which can be unrecognised and lead to serious complications from intra-abdominal damage. 12,13 Failure to remove polyps and problems with incomplete removal are well recognised. 13–15,82–84
Hysteroscopic uterine polypectomy
Advances in hysteroscopic technology have enabled polyps to be removed under direct vision. Fine mechanical instruments, such a scissors, biopsy cups, forceps and snares can be used down a 5- or 7-French working channel of a rigid operative hysteroscope and the safety and feasibility of such approaches have been reported. 32,85–87 Potential drawbacks of mechanical instrumentation are the fragility of the instruments, limited manipulation, difficulty with cutting or avulsing large and fibrous pathology, and, in some instances, bleeding. 32,88
The adoption of electrosurgical technologies may help to overcome these difficulties. Large-diameter hysteroscopic resectoscopes that were developed originally to resect the endometrium for the treatment of HMB89 can also be used to resect focal pathologies, such as SMFs77,90 or polyps. 91,92 They have the advantage of speed and manipulation, but the large diameter of the instruments necessitates general anaesthesia, specialised skills are required93,94 and potential serious complications from fluid overload and inadvertent electrosurgical injury can occur. 95
In contrast with firm SMFs of myometrial origin, polyps are generally softer structures that are derived from the underlying endometrium. Thus, it has been recognised that smaller, less-traumatic electrosurgical instruments would suffice. A miniature bipolar electrosurgical system has been developed (Versapoint®, Gynecare, Ethicon, Somerville, NJ, USA) to cut away polyps and the safety, acceptability and feasibility of this approach has been reported. 96–98 However, retrieval of the tissue specimen from the uterine cavity can be problematic, and usually requires the additional use of mechanical instruments to effect. 32 Other technologies have been developed, including monopolar electrosurgical snares,86 and, more recently, morcellation technologies (TRUCLEAR™, Smith & Nephew, Andover, MA, USA) and Myosure (Hologic, Marlborough, MA, USA), which allow simultaneous tissue cutting and extraction. 99,100
Evidence for uterine polyp treatment in abnormal uterine bleeding
In 2006, our group published a systematic review of the efficacy of uterine polypectomy for the treatment of AUB. 87 The review included nine case series (534 patients) and a single controlled observational study comparing setting for the treatment of uterine polyps (58 patients). No randomised controlled trials (RCTs) were identified, or any studies on patient acceptability or cost-effectiveness. A summary of the evidence is given below:
-
Technique Uterine polypectomy was carried out under general anaesthesia utilising hysteroscopic or blind approaches in all studies, although local anaesthetic outpatient approaches were also used in three of these series. The hysteroscopic techniques under general anaesthesia involved use of large-size endoscopes that are associated with the need to perform wide cervical dilatation.
-
Setting A single, non-randomised comparative study of 58 women undertaken at the Birmingham Women’s Hospital97 showed that outpatient removal under local anaesthesia was no worse than inpatient, general anaesthetic treatment [14/18 (78%) vs. 14/16 (88%); p = 0.7], a result that could partly be explained by the possibility of type II error due to small sample size and lack of randomisation.
-
Alleviation of AUB All studies reported an improvement in symptoms of AUB following treatment (range 75–100%) at follow-up intervals of between 2 and 52 months.
-
Influence of type of AUB It was possible to stratify treatment outcome according to type of abnormal bleeding in only one small study of 45 women,101 which could not detect a difference between polypectomy for menstrual dysfunction or PMB (p = 0.2), again partly due to small sample size.
In summary, the evidence from this systematic review suggested that uterine polypectomy was a safe and technically successful procedure for the treatment of AUB. 87 However, randomised effectiveness data comparing treatment approaches or settings were non-existent, as were economic data to examine cost-effectiveness and qualitative data of patient acceptability and preference.
Systematic Review performed during the Outpatient versus inpatient Polyp Treatment Trial
For this report we have undertaken a systematic review of the effectiveness of uterine polypectomy, building on our previously published systematic review,87 using updated methodological advances in search strategies, quality assessment and statistical analysis. 102,103 The objective of the review was to systematically review the literature to evaluate the effectiveness of uterine polypectomy for the treatment of AUB. Our secondary aims were to establish if the type of AUB, setting or technique influenced outcome.
Methods
Search strategy
We performed searches on the general bibliographic databases MEDLINE (1950–2013), EMBASE (1980–2013) and Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1981–2013). Based on published advice, our search term combination for electronic databases was medical subject headings (MeSH) for polyps combined with word variants for endometrium (endometri* OR uter*) and surgical polypectomy (surgery OR curettage OR hysteroscopy OR polypectomy). Furthermore, all of the bibliographies of relevant studies were hand-searched to identify articles that were not captured by the electronic searches.
Study selection
Two reviewers independently selected articles in a two-stage process. First, abstracts obtained by either the electronic database searches or bibliography inspections were reviewed, and articles that could possibly fulfil the following criteria were selected for full text review.
Inclusion criteria
-
Population Women with intrauterine polyps and AUB.
-
Intervention Uterine polypectomy.
-
Outcome Relief of AUB symptoms.*
[*Measured in general terms, e.g. objective, semiobjective or subjective measures of change in AUB; normalisation of bleeding patterns; satisfaction with AUB outcome; change in quality-of-life scores from baseline.]
Once articles were selected both reviewers used specially designed data abstraction forms to collect data on the main outcome measure that was relief of AUB symptoms. Secondary outcomes included technical feasibility, complications and polyp histology. Differences in article and information selection were solved by deliberation. Where a consensus could not be found a third reviewer (TJC) made the final judgement. No language restrictions were applied and translation available where necessary.
The strength of agreement between reviewers taking into account the play of chance was computed using kappa statistic (agreement is considered good if > 0.6 and very good if > 0.8).
Type of study included
All relevant randomised controlled studies were included. Owing to the small number of RCTs, non-randomised studies including both prospective and retrospective observational studies were also included.
Study quality assessment
The 2007 STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist was used to assess the quality of the observational studies,104 whereas the Cochrane risk of bias tool checklist was used to assess the quality of the randomised controlled studies. 102 Two reviewers independently scrutinised the articles against each element of the relevant checklist.
Synthesis of results
Originally, in the absence of heterogeneity, data pooling and meta-analysis was planned. However, owing to a lack of controlled studies this could be performed for only inpatient treatment compared with outpatient treatment. All other extracted data were tabulated to allow qualitative analysis.
Results
Results of search
From the electronic search we obtained 1122 citations and a further two from searching reference lists of relevant articles. At this stage 1075 citations were excluded, based on a review of the abstracts and titles. An attempt was made to retrieve the remaining 49 articles for further scrutiny. One article could not be retrieved either online or via The British Library. Review of these articles showed that 32 did not meet the selection criteria. The characteristics of the excluded articles105 are described in Figure 1. There was a high level of agreement between reviewers for which articles should be retrieved for further scrutiny (kappa agreement = 0.92; p ≤ 0.001).
FIGURE 1.
Study selection process.
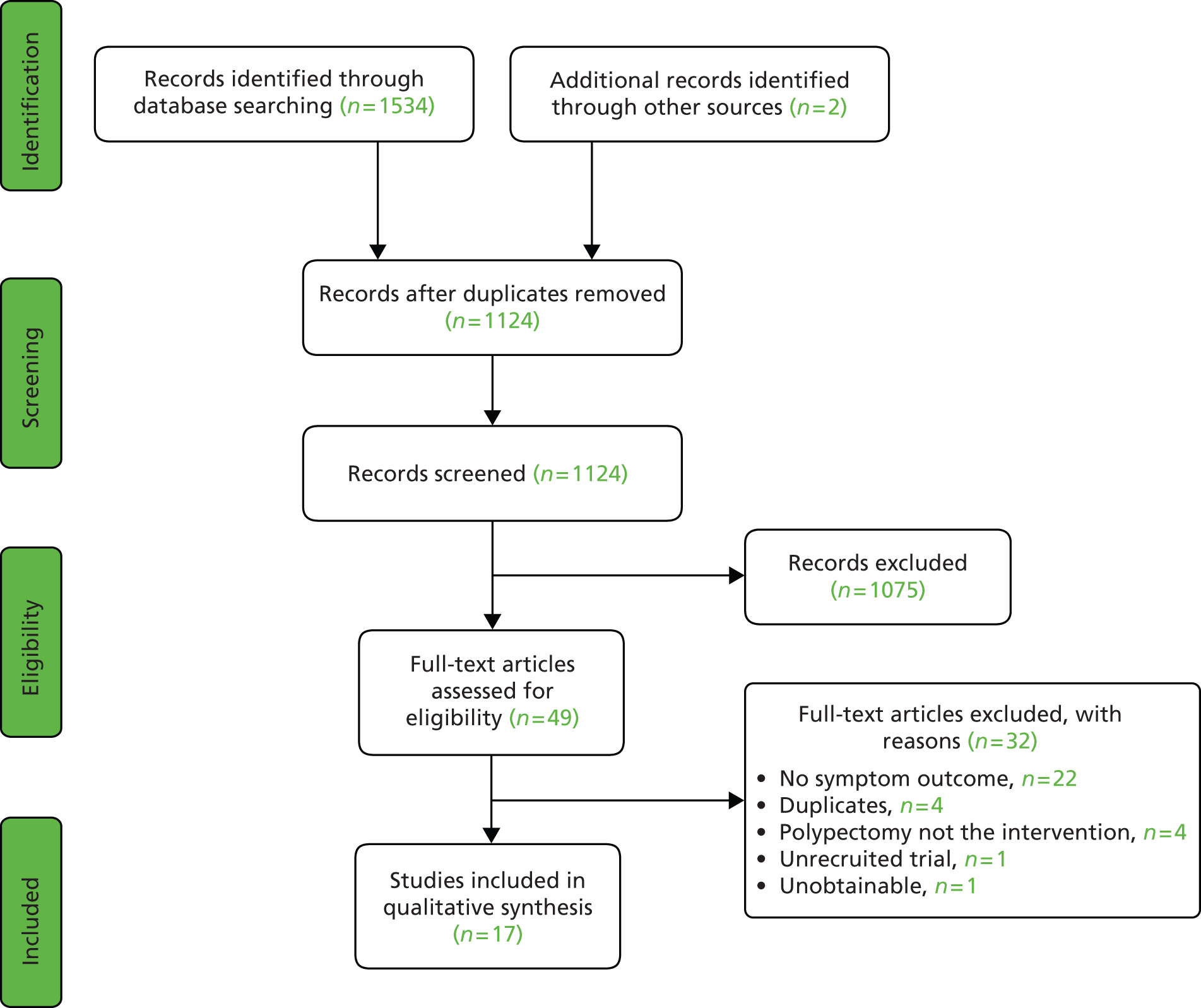
Included studies
The 17 studies23,75,91,97,101,105–114 (including TJ Clark, Birmingham Women’s Hospital, 2013, unpublished) that met our inclusion criteria enrolled a total of 1829 patients between 1989 and 2009. The population size ranged from 8 to 311, with only five studies having a population size of > 100. 12,16,21,22,29
Study quality and design
There were two randomised controlled studies: one comparing inpatient treatment with outpatient treatment (TJ Clark, unpublished), whereas the second compared polyp removal with observation for 6 months. 75 Of the remaining 15 observational studies, only two were controlled: one compared inpatient treatment with outpatient treatment97 and the second compared hysteroscopic morcellation to electrical resection. 105 The remaining 13 articles were uncontrolled observational studies. Only six of the studies were prospective, although the majority of the studies23,75,97,113,114 (plus TJ Clark, unpublished) collected the data consecutively (Figures 2 and 3; Table 2).
| Study author | Methodology, study design | Data collection | Patient Selection | Population: polyps and AUB | Follow-up (%) | |
|---|---|---|---|---|---|---|
| No. | Type (%) | |||||
| Clark TJ, unpublished | RCT | Prospective | Consecutive | 60 | Unspecified menstrual; PMB | 80 |
| Lieng et al.75 | RCT | Prospective | Consecutive | 150 | Intermenstrual (47); menorrhagia (13); irregular (8); discharge (7); asymptomatic (25) | 95 |
| AlHilli et al.105 | Controlled observational | Retrospective | Consecutive | 311 (IUM 139, HSR 172) | Menorrhagia (19.3%), menometrorrhagia (10.6%), PMB (44.4%) | 100 |
| Barisic et al.106 | Observational | Unreported | Unreported | 8 | Unspecified menstrual | 100 |
| Brooks et al.107 | Observational | Unreported | Unreported | 9 | Excessive menstrual | 89 |
| Clark et al.97 | Controlled observational | Prospective | Consecutive | 58 | Unspecified menstrual (10); PMB ± HRT (90) | 58 |
| Cravello et al.108 | Observational | Retrospective | Consecutive | 195 | Unspecified menstrual (60); PMB + HRT (12); tamoxifen (2); PMB (26) | 89 |
| Henriquez et al.109 | Observational | Retrospective | Consecutive | 56 | Unspecified | 100 |
| Nagele et al.101 | Observational | Unreported | Unreported | 33 | Excessive menstrual (47); IMB (13); PMB 40 | 100 |
| Pace et al.110 | Observational | Unreported | Consecutive | 87 | Unspecified menstrual/PMB (86); subfertility (14) | 87; 49 |
| Polena et al.111 | Observational | Retrospective | Consecutive | 367 | Menorrhagia, metrorrhagia, IMB | 83 |
| Preutthipan et al.91 | Observational | Retrospective | Unreported | 155 | Metrorrhagia (31), hypermenorrhea (29), IMB (19), menorrhagia (11) and menometrorrhagia (10) | 100 |
| Stamatellos et al.112 | Observational | Retrospective | Unreported | 83 | Unspecified | 100 |
| Timmermans et al.113 | Observational | Prospective | Consecutive | 49 | PMB | 100 |
| Towbin et al.114 | Observational | Prospective | Consecutive | 14 | Menorrhagia, metrorrhagia; postmenopausal | 100 |
| Tjarks et al.115 | Observational | Retrospective | Unreported | 34 | Unspecified menstrual (64); PMB (36) | 100 |
| Van Dongen et al.23 | Observational | Prospective | Consecutive | 21 | Menorrhagia, metrorrhagia, IMB | 90 |
Participant characteristics
One study looked exclusively at women suffering from PMB,113 seven studies looked at only women who were premenopausal23,75,106,107,109,111,112 and the remaining nine studies91,97,101,105,108,110,114,115 (plus TJ Clark, unpublished) examined mixed populations of women with AUB.
Interventions
When the operative technique was described, polypectomy was performed under direct vision (hysteroscopically) with the exception of two studies (Clark et al. 97 and TJ Clark, unpublished) that had inpatient arms in which blind avulsion was used. 97 There were a variety of techniques described for polyp removal under direct vision including: scissors, polyp forceps, morcellator devices and a variety of bipolar instruments (Table 3).
| Study author | Technique (%) | Anaesthesia (%) | Mean operation time | Polyps | ||
|---|---|---|---|---|---|---|
| No. (%) | Mean size (range) | Histology (%) | ||||
| Clark TJ, unpublished | Outpatient = hysteroscopic; inpatient = blind | Outpatient = local (93), none (7); inpatient = general (100) | Outpatient (consultation time) 29 minutes; inpatient 24 minutes | NR | NR | NR |
| Lieng et al.75 | HSR or observation | General | NR | NR | 16.5 mm (SD 5.3) | EH (1.5); benign (98.5) |
| AlHilli et al.105 | HSR | General | NR | Single (65); multiple (35) | 2.1 cm | EH + ECA (7) |
| Barisic et al.106 | HSR | General | NR | Single (100) | (1.8–3 cm) | EH (13); benign (87) |
| Brooks et al.107 | HSR | General | NR | NR | NR | NR |
| Clark et al.97 | Hysteroscopic (Versapoint) (50); or hysteroscopy + blind avulsion (50) | Local (50); general (50) | NR | NR | 0.9 cm | NR |
| Cravello et al.108 | HSR | General | 19 minutes | Single (90); multiple (10) | 1.4 cm (0.5–4 cm) | EH + A (1); benign (99) |
| Henriquez et al.109 | Hysteroscopic | General or spinal | NR | Single (62); multiple (39) | 17.5 mm (SD 8.3) | NR |
| Nagele et al.101 | HSR or mechanical excision (scissors) | Local (20); general (80%) | NR | Single; ‘some’ multiple | (1–5 cm) | EH + A (2); ECA (2); benign (96) |
| Pace et al.110 | HSR | General | 22 minutes | Single (86); multiple (14) | [< 1.5 cm (45%); > 1.5 cm (55%)] | EH (1); benign (99) |
| Polena et al.111 | HSR | General | NR | Single (81); multiple (19) | NR | ECA (0.05); benign (99.5) |
| Preutthipan et al.91 | HSR | General | 23.1 ± 4.7 minutes, micro scissors; 20.9 ± 3.9 minutes, grasping forceps; 25.2 ± 4.9 minutes, electric probe; 31.9 ± 8.3 minutes | Single (74) | 3.4 ± 0.9 cm premenopausal; 2.5 ± 0.8 cm postmenopausal | EH (3); benign (97) |
| Stamatellos et al.112 | HSR | General or none | NR | Single (41); multiple (59) same as polyp size data | < 1 cm [41]; > 1 cm [59] | NR |
| Timmermans et al.113 | NR | NR | NR | NR | NR | NR |
| Towbin et al.114 | NR | NR | NR | NR | NR | NR |
| Tjarks et al.115 | NR | General | NR | Single (88); multiple (12) | [< 1 cm (13%); > 1 cm (87%)] | NR |
| Van Dongen et al.23 | HSR | General or spinal | NR | Single (38.1); multiple (61.9) | 13.2 mm (SD 4.7) | NR |
Outcomes
There were large differences in the time of follow-up, ranging from 2 months110 to > 9 years91 (Table 4). Only two studies23,97 used a validated tool for measuring efficacy of polypectomy: both studies used a visual analogue scale (VAS). 23,97 The majority of studies defined the primary outcome as an improvement in symptoms of AUB as perceived by the patient. All of the studies23,75,91,97,101,105–115 (plus TJ Clark, unpublished) reported an improvement in symptoms from 60% to 100%. The study looking exclusively at postmenopausal patients presented a survival analysis curve. 113 The seven studies23,75,106,107,109,111,112 looking at premenopausal patients reported improvements in 60–100% of participants. The remaining nine studies91,97,101,105,108,110,114,115 (plus TJ Clark, unpublished) looking at mixed populations reported 65–100% symptomatic improvements. Two of these studies broke down recurrent AUB symptoms for those that were pre- and postmenopausal at treatment. 101,105 One of the studies101 found no significant difference in AUB at 1 year, although the numbers were small (27/34 premenopausal patients vs. 12/12 postmenopausal patients; p = 0.2). Although a larger study105 found that premenopausal women were more likely to have recurrence of symptoms [hazard ratio (HR) 2.42, 95% CI 1.42 to 4.11]. Another study109 broke down symptom outcome by types of AUB in premenopausal women; no differences in outcome were observed for those women complaining of HMB or IMB, although the study population was small (HR 1.29, 95% CI 0.61 to 2.73 and HR 0.42, 95% CI 0.09 to 1.76, respectively).
| Study author | Failure rate/complication rate | Outcome assessment (time) | Outcome measure | Treatment success (%) |
|---|---|---|---|---|
| Clark TJ, unpublished | Outpatient 2/1; inpatient 0/0 | Postal questionnaire (6 months) | Improvement in VAS | Outpatient 18/22 (82); 17/26 (65) inpatient |
| Lieng et al.75 | 0/1 | Postal questionnaire (6 months) | No gynaecological symptom | 68/75 (91) resection; 47/75 (63) observation |
| AlHilli et al.105 | NR | Clinical interview | Recurrence of AUB | 118/139 (85) IUM; 136/172 (79) HSR; 254/311 (81) both |
| Barisic et al.106 | NR/NR | NR (first three menstrual cycles) | Normalisation of AUB | 8/8 (100) |
| Brooks et al.107 | NR/0 | NR (> 3 months) | Improved vs. not improved | 7/8 (88) |
| Clark et al.97 | 0/0 | Postal questionnaire at 6 months | Better vs. not better; satisfied vs. not satisfied | Outpatient 11/12 (92); inpatient: 13/14 (93); outpatient: 14/18 (78); inpatient: 14/16 (88), 156/175 (89) |
| Cravello et al.108 | 0/2 | Telephone interview with patients or referring clinicians (NR) | Normalisation of AUB | 156/175 (89) |
| Henriquez et al.109 | NR | Review of clinical notes | Persistence of AUB requiring medical therapy or surgical intervention | 33/56 (60) 1 year |
| Nagele et al.101 | 0/0 | Clinical interview (3 months); postal questionnaire (5–52 months) | Short-term ‘cure’ of AUB; maintenance of ‘cure’ (no recurrence of AUB) | 44/49 (90); 38/49 (78) |
| Pace et al.110 | 0/1 | Clinical interview (2 months); clinical interview (12 months) | Normalisation of AUB (no ‘relapse’ of symptoms) | 85/87 (98);100 |
| Polena et al.111 | 1/4 (out of total population of 367) | Telephone interview and postal questionnaire | Normalisation of AUB | 91/97 (94) |
| Preutthipan et al.91 | NR/21 | Clinical interview 9 years 2 months | Normalisation of AUB | 144/155 (93) |
| Stamatellos et al.112 | NR/2 | Telephone interview or examination when indicated (3–18 months) | Normalisation of AUB | 76/83 (91) |
| Timmermans et al.113 | NR/NR | Patients self-reported symptoms | Recurrence of PMB | Survival curve |
| Towbin et al.114 | NR | Clinical interview | Recurrence of AUB | 14/14 (100) |
| Tjarks et al.115 | NR/NR | Telephone interview (5–24 months) | Menorrhagia score (scale 0–3); no. of days bleeding/month; satisfied vs. not satisfied | Significant reduction p < 0.05; significant reduction p < 0.05; 23/26 (88) |
| Van Dongen et al.23 | NR/NR | Questionnaire | Improvement of symptoms; menstrual chart score; VAS quality of life | 18/21 (86); improvement p < 0.001; improvement p < 0.001 |
The two studies (Clark et al. 97 plus TJ Clark, unpublished) comparing inpatient to outpatient treatment reported no difference in symptom improvement, although the study sizes were small: 11 of 12 (92%) after outpatient treatment compared with 13 of 14 (93%) after inpatient treatment,97 and 18 of 22 (82%) after outpatient treatment compared with 17 of 26 (65%) after inpatient uterine polypectomy (RR 1.25, 95% CI 0.88 to 1.83). In both studies the outpatient polypectomies were performed under direct vision, whereas inpatient polypectomy was performed ‘blindly’.
There was one other controlled observational study105 and that compared mechanical polyp resection (using a morcellator) compared with electrical resection. Overall, 36 of 172 patients (21%) undergoing electrical resection and 21 of 139 patients (15%) undergoing intrauterine morcellation (IUM) reported recurrence of AUB.
The randomised controlled study comparing resection of polyps with observation for 6 months reported no difference in periodic blood loss using the pictorial blood assessment chart (PBAC) but did report a significant decrease in recurrence of gynaecological symptoms (e.g. IMB and vaginal discharge) in those women having polypectomy [7/75 patients (9.3%) vs. 28/75 control patients (37.3%); p < 0.001]. 75
Discussion
The evidence collated in this review supports the notion that removing uterine polyps is effective at improving symptoms of AUB. However, most of the evidence was derived from observational studies that reported high success rates, but, in general, the quality of the research was poor. The highest-quality studies, the two RCTs (Lieng et al. 75 and TJ Clark, unpublished) reported more modest improvements in symptoms. However, it was unclear whether menopausal status or exact the nature of the presenting AUB complaint influences treatment outcome.
The strengths of this review included the rigorous, systematic approach to literature searching; independent selection of studies and data abstraction in duplicate, and use of recommended study quality assessment tools. 102,104 The included studies were small, however, and many contained heterogeneous populations of women who were both pre- and postmenopausal. In addition, follow-up was often incomplete and short term, such that the strength of any clinical inference possible to draw is limited. Meta-analysis was precluded because of the observed heterogeneity within and between the study populations, as well as variation in follow-up and outcome assessment.
The majority of studies reported hysteroscopic polyp resection under direct vision. However, although hysteroscopic polypectomy is increasing in popularity, a large number of clinicians continue to use blind techniques, such as D&C, affecting the generalisability of the results presented in this review. 45,80,81 To better ascertain the effect of polyp removal on AUB we decided not to include data in which patients had concomitant or subsequent medical or surgical treatments, for example insertion of the LNG-IUS, which may also affect generalisability.
Larger randomised controlled studies are necessary to elucidate if certain groups of patients benefit more from uterine polypectomy. However, recruitment may be hampered by the unwillingness of both gynaecologists and patients to participate in placebo-controlled trials. 76 A further consideration is the increasing move to outpatient polypectomy observed in many units, driven by technological advances in instrumentation, patient expectation and scarcity of health-care resources. 32 Only two randomised studies were identified in this review. Large RCTs comparing conventional inpatient with novel outpatient approaches to polyp treatment are needed to identify best practice before opinion is solidified.
Current practice: surgical method and setting
Outpatient evaluation of the uterus for common gynaecological complaints is now commonplace and has superseded traditional inpatient admission for D&C under general anaesthesia. 21,116 Polyps are highly prevalent and being increasingly identified within the uterus in women presenting with AUB, whether it be HMB, IMB or PMB. 66,70,117–119 Recent technological advances in endoscopy have resulted in improved high-definition optical imaging and digital data capture. Moreover, hysteroscopy systems have become miniaturised, such that they are increasingly portable and easy to insert into the uterine cavity without the need for wide cervical dilatation and blind uterine exploration. Alongside these developments, miniature ancillary instrumentation has been developed, allowing precise targeting of focal pathologies, such as uterine polyps, using a variety of mechanical and electrosurgical equipment32,85–88,96–100 (see Surgical management: polypectomy). These developments have facilitated the concept of ‘see and treat’ hysteroscopy in a convenient outpatient setting. The approach obviates the need for general anaesthesia. Hysteroscopic intervention is not simply restricted to diagnosis, but when pathology amenable to treatment is identified then simultaneous treatment is carried out. 21,32,85,86,88
Despite these technological advances and the apparent safety, convenience and feasibility of outpatient intervention, as well as the high prevalence of uterine polyps associated with AUB, outpatient surgical removal of uterine polyps remains infrequently practised. As described above (see Surgical management: polypectomy), surgical polyp removal is universally practised in the UK45 and elsewhere, although the most prevalent technique differs between the UK and the Netherlands, two countries for which national surveys of practice pertaining to polyp treatment have been conducted. 45,80,81 In the UK the default method for removal of uterine polyps was by blind avulsion or curettage after hysteroscopic location of the focal lesion under general anaesthesia,45 whereas in the Netherlands it was removal under direct hysteroscopic vision under general or regional anaesthesia. 80,81
In 2001 in the UK, removal of polyps under direct vision using hysteroscopic techniques was generally restricted to those gynaecologists with an interest in endoscopic surgery, with such practice being more common in members of endoscopic societies. 45 Although outpatient removal of uterine polyps is possible using blind curettage and/or avulsion, techniques developed for surgery under general anaesthesia, such approaches are associated with increased uterine trauma and discomfort. 12–15,32,82–84 Thus, to set up an outpatient service, the ability to perform hysteroscopic polyp removal under direct vision is a prerequisite. Furthermore, the concomitant removal of focal uterine lesions under direct vision may allow for more complete removal and hence better symptomatic outcomes, in addition to the potential advantages to women and their doctors in terms of increased efficiency, convenience and choice.
Notwithstanding the observed differences in hysteroscopic and blind polyp treatment between the UK and the Netherlands, common to both countries was a propensity among gynaecologists to conduct uterine polypectomy as an inpatient under general anaesthesia. In the UK survey, 19% of respondents performed outpatient uterine polypectomy, but only half of these did so routinely. 45 In the Netherlands, 27% of respondents performed polypectomy in an outpatient setting, but those gynaecologists working in teaching hospitals were twice as likely to undertake such procedures than their colleagues who were practising within non-teaching institutions (39% vs. 19% respectively; p < 0.001). 80 It seems, therefore, that outpatient polypectomy is restricted to those gynaecologists, often working within teaching hospitals, with specific interests or skills in endoscopy. Given the high disease burden associated with uterine polyps,74 the ubiquity of polypectomy and the potential morbidity and resource use associated with inpatient surgery, this difference in practice is unsustainable. It is possible that practice has changed since these surveys were conducted a decade ago, but a subsequent follow-up Dutch survey published last year81 found no such change, confirming again that the vast majority of gynaecologists advocating surgical removal of polyps from the uterus did so using general anaesthesia. Those performing outpatient procedures were again twice as likely to be based within teaching hospitals (43% vs. 19%, respectively; p < 0.001). 81 Within the UK it is also likely that practice is much as it was a decade ago, and this contention is supported by the small proportion of UK centres approached to participate in the Outpatient versus inpatient Polyp Treatment (OPT) Trial who were eligible; the main reason for ineligibility being an absence of a therapeutic OPH service (TJ Clark, Birmingham Women’s Hospital, 2013, personal communication).
The reasons for variation in practice and persistence with inpatient surgical treatment under general anaesthesia are likely to be multiple. The absence of an established outpatient diagnostic hysteroscopy service; no access to, or unfamiliarity with, equipment; lack of necessary surgical skills; and financial considerations restricting the development of new services are likely to play their part to a varying degree. As with any new health technologies, however, evidence of effectiveness and cost-effectiveness is necessary once safety and feasibility of the intervention has been established. The lack of effectiveness and cost-effectiveness data is likely to be a key factor driving the current status quo.
Need for a large simple trial of outpatient uterine polypectomy compared with inpatient uterine polypectomy in abnormal uterine bleeding
Two systematic reviews59,87 (see Systematic review performed during the Outpatient versus inpatient Polyp Treatment Trial) provide evidence which suggests that uterine polypectomy is a safe and technically successful procedure for the treatment of AUB and results in an improvement in AUB symptoms. However, the quality of existing research is poor, introducing a substantial potential for bias, and no RCTs compare their impact upon patient symptoms and other patient-centred outcomes. On this latter point, qualitative data addressing patient factors are also fundamental considerations, such as patient preferences for choice of treatment setting as well as their tolerance and acceptability of interventional hysteroscopic procedures in an outpatient setting without general anaesthesia. OPH is known to be associated with discomfort and can induce anxiety. 32,120 Thus, practitioners and patient fears over inducing pain may also influence the continued use of inpatient admission to hospital and general anaesthesia. Moreover, the relative cost-effectiveness of outpatient polyp treatment (OPT) compared with traditional inpatient approaches remains unclear.
The limitations placed upon intrauterine surgery in the conscious outpatient, which include pain tolerance and problems with access or manipulation of miniature hysteroscopic equipment, may translate into reduced feasibility and poorer clinical outcomes. However, even if this were proven, the advantages to women of outpatient intervention in terms of safety, convenience and efficiency may outweigh any inferiority in clinical effectiveness. In addition to these considerations, the inflated cost of miniaturised technologically advanced equipment required for most outpatient procedures combined with the potential for poorer clinical outcomes may offset the efficiency of outpatient polypectomy, even when it is performed immediately following diagnosis at OPH – the ‘see and treat’ approach. Thus, outpatient polypectomy may remain an attractive and preferred treatment option, even if it were not much worse than, or ‘non-inferior to’, standard inpatient treatment.
In light of these considerations, the traditional RCT objective of establishing superiority of a new treatment, in this case outpatient polypectomy, was thought to be inappropriate. Establishing that outpatient treatment is not unacceptably worse than standard inpatient approaches requires a non-inferiority trial.
Thus further research in the form of an adequately powered RCT between treatment settings (outpatient vs. inpatient), stratified by type (i.e. pattern) of AUB, is required to assess the therapeutic role, patient acceptability, effectiveness and cost-effectiveness of uterine polypectomy in AUB both in the short and longer term. The need for such a trial was evident from publications25,32,87,121,122 and supported by UK consultant gynaecologists. Our national survey in 2001 indicated that 268 of 854 (31%) of gynaecologists performing uterine polypectomy were supportive of a trial comparing inpatient with outpatient uterine polypectomy. 45 This implies that the newer outpatient approach had been introduced in some centres without definite evidence but opinion regarding its use is not yet solidified (i.e. collective equipoise) making the need for a trial even more urgent. Furthermore, a recent practice guideline on the diagnosis and management of endometrial polyps produced by the American Association of Gynecologic Laparoscopists (AAGL) highlighted the ‘paucity of high-quality data in the subject area of endometrial polyps given the common occurrence of this pathology. The following considerations are proposed for future research: 1. Randomized trials of women with abnormal uterine bleeding to evaluate the clinical outcome of polypectomy and 2. Cost comparisons of different methods for hysteroscopic removal of polyps, including outpatient and outpatient locations’. 25
Assessing feasibility for a randomised controlled trial: the Outpatient versus inpatient Polyp Treatment pilot trial
In addition to systematically reviewing the medical literature87 and performing a national survey of practice,45 we undertook two primary clinical studies. The first was an observational cohort study to compare outpatient hysteroscopic polypectomy in the treatment of symptomatic endometrial polyps with inpatient management. 97 This study demonstrated that outpatient treatment was technically feasible and it had the potential to be efficacious and cost-effective. From this we launched an external pilot RCT of 60 patients in a single centre to assess the acceptability of randomisation to patients (TJ Clark, personal communication).
A short summary of the methods used and results are given below.
Objectives
-
To help us to optimise the trial design for a robust large scale, multicentre study with adequate statistical power to evaluate OPT reliably.
-
To demonstrate the acceptability of randomisation.
-
To establish standardised operating procedures for trial management, and piloted questionnaires and consent forms patients.
-
To inform the sample size of a larger study.
Methods
All women with AUB who are referred for a diagnostic OPH between January and July 2000 at the Birmingham Women’s Hospital were approached for consent to participate in this pilot trial. AUB was defined according to four categories: (1) PMB; (2) unscheduled bleeding while on HRT or tamoxifen for > 6 months; (3) IMB in women > 40 years old; and (4) excessive menstrual bleeding refractory to medical therapy. At OPH, those women with a benign uterine polyp [endometrial polyp or pedunculated (grade 0) fibroid] as described by Clark et al. ,32 were randomised to immediate outpatient polypectomy under local anaesthesia or delayed (within 3 months of randomisation) inpatient uterine polypectomy under general anaesthesia as a day case. Women were excluded if hysteroscopic features suggested a malignant lesion or when additional pelvic pathology necessitated hysterectomy. Inclusion and exclusion criteria, in full, are shown below.
Inclusion criteria
-
AUB requiring diagnostic hysteroscopy.
-
Finding of a benign polyp on diagnostic hysteroscopy.
-
No hysteroscopic features suspicious of malignancy.
-
Need for polypectomy or myomectomy.
Exclusion criteria
-
Hysteroscopic features suggesting malignant lesion.
-
Additional pathology necessitating hysterectomy.
Interventions
Outpatient uterine polypectomy was performed under direct hysteroscopic vision using Versapoint® spring tip bipolar electrodes (Gynecare, Ethicon, Somerville, NJ, USA) as previously described. 32,97 Inpatient uterine polypectomy was performed under general anaesthesia by traditional D&C, blind avulsion with or without prior localising hysteroscopy or under direct vision using an operative hysteroscope. In most instances, wide dilatation of the cervical canal was required to accommodate the larger diameter inpatient instruments within the uterus.
Outcomes
Patient completed outcomes, administered by postal questionnaire, were collected at baseline then at 3, 6 and 12 months post surgery. These consisted of:
-
Bleeding response: a 100-mm VAS to measure bleeding in the last month. The scale was anchored at each end by ‘none’ and ‘continuous’. For those women who expected bleeding (premenopausal and those on sequential HRT) we considered a 50% reduction from baseline in bleeding score as a ‘success’. For those women for whom no bleeding was expected (postmenopausal) we considered a score of 0–3 mm a ‘success’.
-
Disease-specific health-related quality of life (HRQL) was evaluated using the disease-specific Menorrhagia Multi-attribute Assessment Scale (MMAS). 123 Scores range from 0 (worst) to 100 (best).
-
Generic HRQL using the generic EuroQol European Quality of Life-5 Dimensions (EQ-5D) instrument. 124 Scores range from –0.59 (worst) to 1.0 (best). Health thermometer scores range from 0 (worst) to 100 (best).
-
Sexual satisfaction and functioning using the Index of Sexual Satisfaction (ISS). 125 Scores range from 0 (best) to 100 (worst).
Randomisation and blinding
Sixty women were randomised. The University of Birmingham Clinical Trials Unit provided third-party randomisation. For the randomisation sequence, variable block size in balanced groups was used to avoid any possibility of foreknowledge. Surgeons and patients could not be blinded in this study (to prevent performance bias and measurement bias) owing to the nature of the comparison, but randomisation was concealed such that the surgeons would be kept unaware of the patient’s group allocation until they had completed the diagnostic OPH, thereby preventing selection bias. In addition, the outcome assessments were conducted by self-administered questionnaires, avoiding possible bias arising from the influence of clinicians’ knowledge of patient’s group allocation at medical follow-up.
Statistical considerations
No formal sample size calculation was made. As many women as possible were randomised over the 6-month period; we considered this length of time to be long enough to achieve our stated objectives.
Summary statistics were produced to describe the demographics of the women randomised. No formal hypothesis testing was attempted on the outcome data, as with this size of sample we could not expect any statistically significant differences; this was not our objective here. Summary statistics for patient completed outcome measures at each time point are presented alongside estimates of uncertainty around point estimates: 95% two-sided CIs for RRs and mean differences between groups. Estimates of differences were adjusted for baseline using analysis of covariance. Analysis was by intention to treat (ITT).
Summary of results
In total, 60 women were randomised to either inpatient or outpatient uterine polypectomy (Figure 4). The questionnaire response rate at 6 months was 54 of 60 (90%), although not all of the components of the questionnaire were always completed.
FIGURE 4.
Pilot OPT Trial profile.
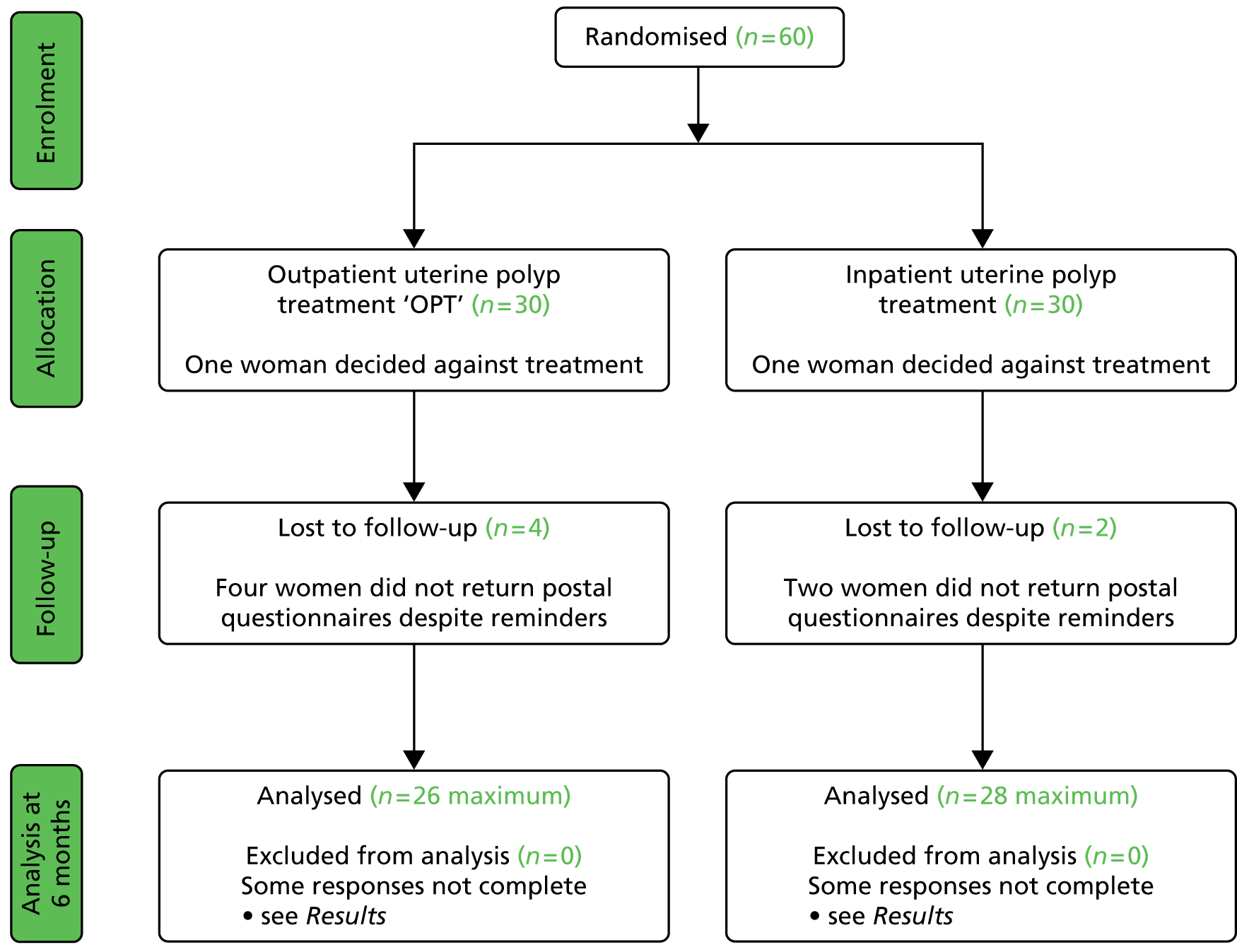
The baseline characteristics of the women randomised are shown in Table 5. Some slight imbalance was seen in some of the parameters that would be expected in a study of this size.
| Patient characteristic | Outpatient (n = 30) | Inpatient (n = 30) |
|---|---|---|
| Age, years | 54 [7.8] | 57 [7.8] |
| Parity: | ||
| 0 | 3 (10) | 2 (7) |
| 1–3 | 20 (67) | 20 (67) |
| > 3 | 5 (16) | 6 (20) |
| Not reported | 2 (7) | 2 (6) |
| Marital status | ||
| Married | 21 (70) | 23 (77) |
| Other | 8 (27) | 5 (17) |
| Not reported | 1 (3) | 2 (7) |
| Sexually active | ||
| Yes | 17 (57) | 21 (70) |
| No | 12 (40) | 7 (23) |
| Not reported | 1 (3) | 2 (7) |
| AUB | ||
| Postmenopausal bleeding | 10 (33) | 9 (30) |
| Unscheduled bleeding on HRT | 13 (43) | 16 (53) |
| IMB | 5 (17) | 4 (13) |
| Excessive menstrual bleeding | 2 (7) | 1 (4) |
| Other uterine pathology | ||
| None | 30 (100) | 27 (90) |
| SMF | – | 1 (3) |
| Not reported | – | 2 (7) |
Bleeding response
Completed responses were available for 45 of 60 (75%) of the women randomised at 6 months. At this time point, successful bleeding response followed outpatient uterine polypectomy in 19/22 (86%) women, compared with 16/23 (69%) after inpatient uterine polypectomy at 6 months (Table 6). Similar proportions were seen at 1 year. Detailed VAS bleeding scores for those women who were expecting bleeding are detailed in Table 7.
| Time from treatment | Outpatient | Inpatient | RR (95% CI)a |
|---|---|---|---|
| 3 months | 13/19 (68%) | 13/22 (59%) | 1.16 (0.73 to 1.84) |
| 6 months | 19/22 (86%) | 16/23 (69%) | 1.24 (0.90 to 1.71) |
| 1 year | 19/24 (79%) | 15/22 (68%) | 1.16 (0.82 to 1.65) |
| Time of assessment | Outpatient, mean (SD) | Inpatient, mean (SD) | Difference between groups, 95% CIa |
|---|---|---|---|
| Baseline | n = 8, 47.6 (33.5) | n = 11, 33.3 (25.6) | – |
| 3 months | n = 10, 22.7 (22.1) | n = 11, 24.9 (35.1) | 0.6 (–26.4 to 28.6) |
| 6 months | n = 11, 17.4 (25.2) | n = 12, 13.5 (22.6) | 3.8 (–15.5 to 23.2) |
| 1 year | n = 12, 21.3 (33.0) | n = 11, 19.1 (26.4) | 4.3 (–22.1 to 30.6) |
Quality of life
Results for the disease-specific (MMAS) and generic quality of life (EQ-5D) scores are given in Tables 8 and 9. Scores generally appeared to improve from baseline. Sexual satisfaction score are given in Table 10; no obvious increasing or decreasing trend was noted here. Low response rates were noted for the MMAS and sexual satisfaction questionnaires.
| Time of assessment | Outpatient, mean (SD) | Inpatient, mean (SD) | Difference between groups (95% CI)a |
|---|---|---|---|
| Baseline | n = 6, 60.0 (28.9) | n = 5, 63.6 (22.3) | – |
| 3 months | n = 5, 68.5 (22.1) | n = 5, 65.3 (19.0) | 8.3 (–20.5 to 37.0) |
| 6 months | n = 6, 77.7 (17.9) | n = 5, 79.4 (26.2) | –2.2 (–31.5 to 27.0) |
| 1 year | n = 7, 76.0 (24.2) | n = 5, 73.6 (30.2) | 2.4 (–31.5 to 36.2) |
| Time of assessment | Outpatient, mean (SD) | Inpatient, mean (SD) | Difference between groups (95% CI)a |
|---|---|---|---|
| EuroQoL EQ-5D | |||
| Baseline | n = 27, 0.75 (0.29) | n = 28, 0.78 (0.21) | – |
| 3 months | n = 25, 0.81 (0.31) | n = 26, 0.86 (0.18) | –0.04 (–0.14 to 0.07) |
| 6 months | n = 26, 0.86 (0.22) | n = 28, 0.88 (0.14) | –0.01 (–0.11 to 0.08) |
| 1 year | n = 27, 0.81 (0.29) | n = 27, 0.86 (0.18) | –0.04 (–0.16 to 0.08) |
| EuroQoL health thermometer | |||
| Baseline | n = 25, 78.1 (18.1) | n = 25, 77.5 (20.6) | – |
| 3 months | n = 23, 78.3 (16.5) | n = 26, 76.4 (18.9) | –0.2 (–9.9 to 9.5) |
| 6 months | n = 23, 80.7 (19.6) | n = 28, 79.0 (16.1) | 0.5 (–8.8 to 9.9) |
| 1 year | n = 26, 74.5 (23.0) | n = 28, 81.1 (15.7) | –3.9 (–14.5 to 6.7) |
| Time of assessment | Outpatient, mean (SD) | Inpatient, mean (SD) | Difference between groups, 95% CIa |
|---|---|---|---|
| Baseline | n = 12, 17.8 (11.3) | n = 16, 23.6 (15.8) | – |
| 3 months | n = 8, 16.9 (13.4) | n = 12, 16.8 (14.5) | –2.8 (–14.2 to 8.5) |
| 6 months | n = 9, 19.8 (9.4) | n = 10, 21.4 (14.7) | –2.1 (–10.2 to 5.9) |
| 1 year | n = 12, 22.1 (17.0) | n = 11, 20.2 (14.8) | 0.4 (–5.3 to 6.1) |
Interpretation
The pilot RCT data demonstrated the acceptability of randomisation, as well as establishing standardised operating procedures for trial management, and piloted questionnaires and consent forms for patients. The accrual rate from a single centre convinced us of the feasibility of undertaking a multicentre trial, especially upon the background of a rapid increase in the provision of OPH facilities within the UK. 45
Impact on planning for a definitive trial
Although AUB may affect the sexual domain of life quality, the response to the ISS was low. This probably reflected the sensitive nature of the questions presented to women of all ages, some of whom may have felt them intrusive or irrelevant. Moreover, the cumulative burden of completing various outcome questionnaires may have been a disadvantage. In view of the low response rate and the concern that potentially inclusion of the ISS may compromise return of the primary outcome in the definitive trial, a decision was made to no longer include the ISS as one of the secondary outcome measures. The response rate to the condition-specific MMAS was also low. This was mainly because it is a condition-specific questionnaire designed for use in women with HMB. However, in the pilot study the MMAS was included within the self-completed outcome booklet that was sent to all women and was not restricted to the minority of women who actually presented with HMB. Consequently, the MMAS was considered irrelevant and ignored by the majority of participants. The use of disease-specific questionnaires, such as the MMAS, has been encouraged as an important and more sensitive patient-reported outcome in trials. 126,127 In light of this we planned to retain the MMAS in the multicentre trial but make it clear within the patient-reported outcome questionnaires that the MMAS should be completed by only those women with regular periods or hormone-induced withdrawal bleeds, even if their presenting complaint is unscheduled bleeding rather than HMB.
Participants found that understanding the concept of the VAS, as applied to amount of bleeding, was difficult. This reflected a lack of familiarity in providing responses on a continuous scale, especially as most women with AUB included in the pilot did not have HMB, so that the nature of the bleeding, not the amount of bleeding, was the primary concern. It was clear therefore that if we were to maintain the relevance and generalisability of our work in a future, larger-scale trial we would have to include all types of AUB found in association with uterine polyps (i.e. HMB, IMB and PMB) and measurement of the primary outcome – successful alleviation of AUB symptoms – needed to be revised.
This pilot RCT appeared to show that outpatient treatment was comparable to inpatient treatment in terms of symptomatic relief (86% vs. 69% respectively). However, given the difficulties with VAS score responses in this population as previously detailed we exercised caution in directly applying these proportions to inform the sample size of the definitive study. Furthermore, it should be noted that they were derived from a small population in a single specialist ambulatory tertiary referral centre. Uncontrolled series included in the original systematic review87 showed symptomatic relief in up to 100% of women treated as inpatients. The only controlled series included in the review reported an 80% vs. 90% satisfaction with treatment outcome in favour of inpatient treatment. Thus, all this evidence was used in combination to inform the sample size calculation for the proposed multicentre RCT.
Clinical factors and prognosis
As outlined above (see Abnormal uterine bleeding), AUB comprises a number of complaints in women of both pre- and postreproductive age. Research to date is limited with regard to the impact of polypectomy upon the type of bleeding complaint: HMB, IMB or PMB. In addition, AUB and polyp formation may be affected by the use of exogenous sex steroid hormones found in HRT or the hormone partial agonist tamoxifen,36,40,41 commonly used in the treatment of breast cancer. Symptomatic outcomes may also be influenced by the location of the polyp within the uterus and its morphology. Fundally sited lesions can be harder to remove because the attachment to the uterine wall is less accessible. 32 Firmer fibrous lesions can be harder to cut through with mechanical instruments and retrieve from the uterine cavity via the narrow cervical canal. In contrast, softer glandular polyps may be more mobile and difficult to stabilise and detach but, because of their compressible consistency, they can be easier to extract from the uterine cavity. These variables were therefore identified a priori as potential prognostic factors.
Design implications for the substantive study
Although outpatient treatment of uterine polyps necessitates in most cases the use of innovative miniature endoscopic equipment, it is the treatment setting rather than the intervention itself that is novel. The limitations of outpatient polypectomy discussed previously could potentially result in reduced feasibility and poorer clinical outcomes. However, even if this were proven, the advantages to women of outpatient intervention in terms of safety, convenience and efficiency may outweigh any inferiority in clinical effectiveness. In addition to convenience, avoidance of inpatient stay and facilities may translate into substantially reduced costs to health services. Thus, outpatient polypectomy may remain an attractive and preferred treatment option, even if it were not much worse than, or ‘non-inferior to’, standard inpatient treatment.
In light of these considerations, the traditional RCT objective of establishing superiority of a new treatment, in this case outpatient polypectomy, was thought to be inappropriate. We therefore framed our primary objective in terms of establishing that outpatient treatment was not unacceptably worse than standard inpatient approaches. Therefore, we designed a non-inferiority trial. Based upon our own experience and consultation with potential collaborating centres, we believed that outpatient would be the treatment of choice even if 25% fewer women (in relative terms) had alleviated symptoms at 6 months. Thus, the margin of non-inferiority was set at 0.75.
The pilot study did not formally collect data on the number of women declining randomisation on the basis of a preference for immediate outpatient treatment under local anaesthesia or delayed inpatient uterine polypectomy under general anaesthesia. However, experience suggested a substantial proportion expressed a strong preference for one or the other setting. Women choosing their treatment setting may tend to report more favourable outcomes, being empowered by having been part of the decision process, or conversely may have high expectations of success and be disappointed with a less-than-complete resolution of their symptoms. Given the potential for interaction between choice, or conversely lack of choice if randomised, and outcome, we planned a parallel patient preference study using the same outcome measures as the RCT.
Objectives of the Outpatient polyp treatment study
In undertaking the OPT study, we aimed to:
-
test the hypothesis that in women with AUB associated with benign uterine polyp(s), OPT achieved as good, or no more than 25% worse (in relative terms), alleviation of bleeding symptoms at 6 months, compared with standard inpatient treatment (principal objective)
-
test the hypothesis that response to uterine polyp treatment differed according to the pattern of AUB and menopausal status by three secondary analyses:
-
premenopausal women compared with postmenopausal women
-
IMB compared with excessive menstruation
-
postmenopausal women on HRT compared with those not on HRT
-
-
explore the variation in the effectiveness of outpatient polyp treatment compared with standard inpatient polyp treatment at different periods of follow-up (12 and 24 months)
-
assess patient acceptability and impact on HRQL
-
perform an economic evaluation for cost-effectiveness.
Chapter 2 Methods of randomised controlled trial and preference study
Objectives
The objectives of the clinical trial and preference study were to:
-
test the hypothesis that in women with AUB associated with benign uterine polyp(s), OPT achieved as good, or no more than 25% worse (in relative terms), alleviation of bleeding symptoms compared with standard inpatient treatment at 6 months (principal objective)
-
test the hypothesis that response to uterine polyp treatment differed according to the pattern of AUB and menopausal status by three secondary analyses:
-
premenopausal women compared with postmenopausal women
-
IMB compared with excessive menstruation
-
postmenopausal women on HRT compared with those not on HRT
-
-
explore the variation in the effectiveness of OPT compared with standard inpatient polyp treatment at different periods of follow-up (12 and 24 months)
-
assess patient acceptability and impact on HRQL
-
investigate how treatment outcomes vary by choice.
Study design
The OPT study (Figure 5) was designed as a pragmatic, multicentre randomised controlled non-inferiority trial of outpatient polypectomy compared with inpatient polypectomy with a concurrent non-randomised cohort of women with a strong preference for treatment setting.
FIGURE 5.
Outpatient polyp treatment flow diagram.
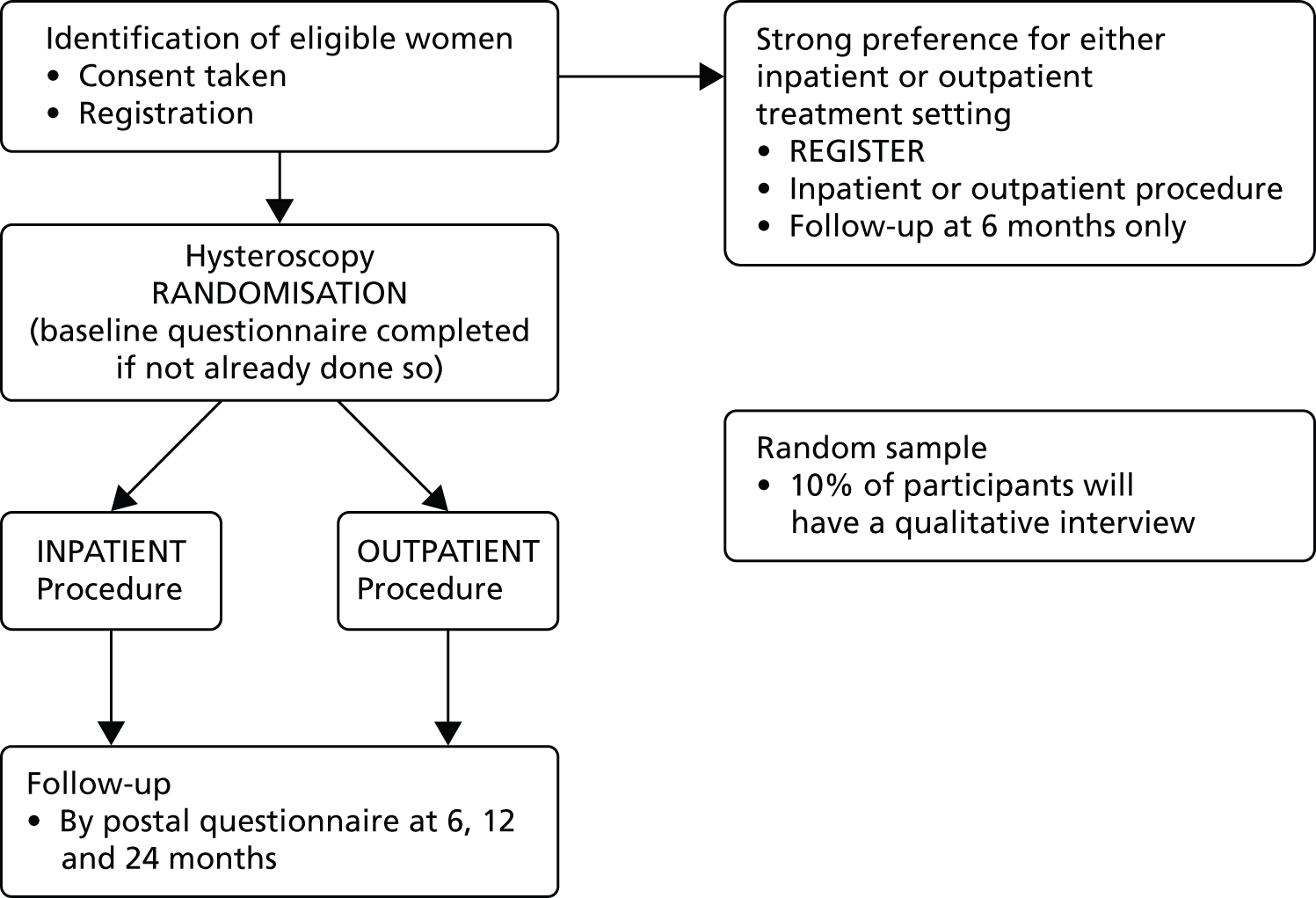
Both the RCT and preference study had the same setting, structure and design, including outcome measures, although the preference study had a shorter follow-up length only up to 6 months; this was to encourage participation, as well as to minimise costs of longer-term follow-up.
Screening and consent prior to outpatient hysteroscopy
All women with AUB seen in hospital outpatient clinics and undergoing a diagnostic OPH were considered for the trial. The trial was introduced to them in the outpatient clinic and a comprehensive, evidence-based patient information sheet was provided, either at the first clinic visit or with the appointment letter for the hysteroscopy. Participant information sheets and consent forms were provided to each centre in English and other languages as appropriate to their local community.
Before the procedure, the women were given the chance to discuss the risks and benefits of uterine polypectomy in the outpatient setting using local anaesthesia and in the inpatient setting under general anaesthesia, the process of randomisation and the follow-up requirements with the consultant gynaecologist and/or gynaecology nurse. It was carefully explained that the final decision about eligibility would be taken during the hysteroscopic examination and would be dependent on the findings; therefore, consent was required before the procedure. Women were informed that the process of randomisation would prolong the diagnostic procedure time by up to 2 minutes. Women were also made aware that if the allocation was outpatient polypectomy then the procedure would be undertaken immediately in most instances and treatment would take an additional 10–15 minutes on average, whereas if the allocation was inpatient polypectomy, the diagnostic hysteroscope would be removed and she would be given another appointment for the inpatient procedure within 8 weeks. Women were also informed that only about one in four women will have a uterine polyp and therefore be eligible for the OPT Trial. Each woman appreciated that if a polyp was not found, appropriate treatment would be offered but she would not be recruited into the trial. In this case, the consent form, baseline questionnaire and randomisation form would be destroyed.
In centres at which a ‘see and treat’ outpatient approach was not used (i.e. outpatient as well as inpatient polypectomies were scheduled for a later date), discussion about participation into the OPT Trial and randomisation could take place after the diagnostic hysteroscopy in eligible women known to have uterine polyp.
Determining eligibility
All women with AUB who provided written informed consent to participate in the OPT study and satisfied the eligibility criteria were included. Those women consenting to the RCT were to be randomised following the diagnostic OPH procedure. Those consenting to participate in the preference study were treated according to their preference. The practitioner inspected the uterine cavity, according to their standard hysteroscopic protocol, to determine the presence of uterine polyp(s), absence of any excluding pathology and technical feasibility for outpatient polypectomy. For the purpose of the OPT Trial a uterine polyp was defined at diagnostic hysteroscopy as:
A discrete outgrowth of endometrium, attached by a pedicle, which moves with the flow of the distension medium.[32] Polyps may be pedunculated or sessile, single or multiple and vary in size (the variable amount of glands, stroma and blood vessels that constitute the polyp will influence their macroscopic appearance [i.e. glandulocystic polyps or firmer, more fibrous polyps (indistinguishable in some instances from grade 0 submucous fibroids)].
The following inclusion/exclusion criteria were applied to assess eligibility.
Inclusion criteria
-
Aged ≥ 16 years.
-
AUB requiring diagnostic hysteroscopy.
-
Finding of a benign polyp or polyps (glandulocystic or pedunculated/grade 0 fibroid) on diagnostic hysteroscopy.
-
Feasible to remove polyp as an outpatient.
-
Need for polypectomy.
-
Ability to perform polypectomy within 8 weeks of diagnosis.
-
Baseline questionnaire completed prior to the hysteroscopy.
-
Written informed consent obtained prior to the hysteroscopy.
Exclusion criteria
-
Hysteroscopic features suggesting malignant lesion.
-
Need for other uterine surgical intervention (i.e. endometrial ablation, resection, myomectomy or hysterectomy).
-
Additional pathology necessitating hysterectomy.
Randomisation
If the woman was found to be eligible for the OPT Trial, the gynaecologist or member of his/her team obtained a randomised allocation during the hysteroscopic examination. Randomisation notepads were provided to investigators and used to collate the necessary information prior to randomisation. Participants were entered and randomised into the trial in a 1 : 1 ratio via a short telephone call to the Birmingham Clinical Trials Unit, or by logging into a secure web-based randomisation system. To avoid any possibility of foreknowledge, the randomiser needed to provide the name and date of birth of the participant, and confirm the eligibility and stratification criteria, whereupon a randomised allocation was provided and a trial number allocated.
Women with strong preference for treatment setting
It was anticipated that some women may be prepared to participate in the OPT study but not wish to be randomised between surgical treatments because they held clear preference for immediate outpatient treatment under local anaesthesia or conversely delayed inpatient uterine polypectomy under general anaesthesia. These women were invited to participate in a preference study and entered the same way as those randomised except that they received the treatment for which they had a preference. These women were followed up for 6 months.
Stratification of randomisation
A minimisation procedure using a computer-based algorithm was used to avoid chance imbalances in important variables. The variables chosen were:
-
premenopausal compared with postmenopausal women
-
postmenopausal women on HRT compared with those not on HRT
-
whether the predominant abnormal bleeding complaint is (excessive) HMB or IMB/unscheduled bleeding
-
history of using tamoxifen (current or previous user vs. those who have never used)
-
location of uterine polyp (fundal vs. non-fundal)
-
type of uterine polyp (endometrial ‘glandulocystic’ vs. fibrous).
For analysis purposes (see Chapter 3), because of the small numbers of the included women taking HRT, or with a history of use of tamoxifen, we narrowed these variables down to a shorter list of three:
-
type of bleeding PMB/HMB/IMB (for definitions, see Table 1)
-
location of uterine polyp (fundal vs. non-fundal)
-
type of uterine polyp (endometrial ‘glandulocystic’ vs. fibrous).
Treatment allocations
Surgical procedures
A named investigator(s), with suitable training and experience in both outpatient and inpatient uterine polypectomy, performed all surgical procedures within participating centres.
Outpatient polypectomy
Outpatient polypectomy was performed immediately after diagnosis at OPH in most instances, although some participants had their outpatient treatment scheduled, depending upon local circumstances, within the following 8 weeks. Polyp removal was carried out under direct hysteroscopic vision using miniature mechanical or electrosurgical instruments, with or without the need for minor degrees of cervical dilatation and local anaesthesia (direct cervical infiltration or paracervical injection). On occasion, blind avulsion with small polypectomy forceps after hysteroscopic localisation may be required to remove the polyp, and this approach was permitted.
Inpatient polypectomy
Inpatient polypectomy was aimed to be performed within 8 weeks of the initial diagnosis at OPH. Inpatient polypectomy was carried out by traditional D&C, blind avulsion – with or without prior localising hysteroscopy – or under direct vision using an operative hysteroscope. In most instances, dilatation of the cervical canal was required to accommodate the larger diameter inpatient instruments within the uterus. General or spinal anaesthesia facilitated major degrees of cervical dilatation and manipulation of these larger diameter instruments within the uterine cavity.
Failure of procedure
Occasionally, complete removal of a uterine polyp under local anaesthetic may not always be possible, usually because of pain or anxiety or because of the technical limitations associated with the miniaturisation of equipment. Successful outpatient polypectomy is possible in the majority of women,97 but the probability of success was not readily predictable. In cases when outpatient treatment had to be abandoned, a second procedure under general anaesthetic was scheduled as soon as possible. Women who required a second procedure were not excluded or withdrawn from the OPT Trial. It was sensitively explained to them that follow-up information is still very important, despite the change in treatment, and unless they wished to be withdrawn completely from the trial they would continue to be followed up. When inpatient treatment failed, further treatment options depended upon the reason for technical failure. Women could be rescheduled for the same procedure or rescheduled using a different surgical approach either as an inpatient or an outpatient.
Concomitant interventions and treatments
It was anticipated that most women presenting with AUB found to be associated with a uterine polyp would require no further intervention other that uterine polypectomy (either as an outpatient or inpatient). However, in some circumstances, particularly those premenopausal women with HMB, additional medical treatments could be considered necessary by the responsible clinician at the time of polypectomy, or subsequently. These could include non-hormonal medical treatments [e.g. non-steroidal anti-inflammatory agents, tranexamic acid (Cyclokapron®, Pfizer)] or hormonal medical treatments [e.g. combined oral contraceptive pill, levonorgestrel-releasing intrauterine system (Mirena®, Bayer), local or systemic HRT]. Surgical interventions in the form of endometrial ablations or hysterectomy may subsequently be necessary and the need for such interventions was to be recorded. However, if the need for additional surgery at the time of polyp diagnosis was indicated then such patients were ineligible to be recruited to the OPT Trial. Patients with AUB associated with polyps were managed as they would be in routine clinical practice after polyp removal, with all therapeutic interventions additional to uterine polypectomy recorded.
Withdrawal from the Outpatient versus inpatient Polyp Treatment Trial
All women who consented to the randomised OPT Trial, or to the preference study, were followed up and asked to complete postal questionnaires, regardless of actual treatment received.
If a woman specifically requested a treatment setting after randomisation then her choices were respected but did not necessitate withdrawal from the trial, nor did failure of the outpatient procedure or subsequent inpatient treatment. In both circumstances, it was sensitively explained to women that follow-up information was still very important and, unless they wished to withdraw completely from the trial, they would be followed up. The OPT Trial office was notified of any withdrawal of consent for further follow-up.
Serious and unexpected adverse events
There may be mortality and morbidity associated with either polypectomy procedure, therefore all serious adverse events (SAEs) were to be reported by fax to the OPT Trial office as soon as possible. This report was to be followed within 2 days by a completed SAE form. For the purposes of this study, SAEs were defined as those that are fatal, life-threatening, disabling or requiring prolonged hospitalisation, and had resulted from the hysteroscopy, the polypectomy procedure, the anaesthetic or postoperative recovery, for example deep-vein thrombosis and hospital-acquired infections.
Other management at discretion of local doctors
Apart from the trial treatments allocated at randomisation, all other aspects of patient management were left entirely at the discretion of the local investigators.
Follow-up and outcome measures
Format
Patient-orientated outcomes were collected using a postal questionnaire, which included a combination of disease-specific and generic measurement instruments, tailored according to the initial symptom at presentation. The postal questionnaires were sent from the OPT Trial office with postage-paid envelopes 2 weeks before the due date. Reminders were sent to patients if the questionnaire was not returned within 1 week of the due date and attempts were made to contact the patient by phone if the questionnaire was not returned by 2 weeks after the due date.
Timing of assessments
The primary time point was 6 months post treatment. In addition, assessments took place at baseline (i.e. time of recruitment and randomisation to OPT Trial), 12 and 24 months post treatment.
Primary clinical outcome measure: treatment success
The woman’s own assessment of bleeding symptoms, using a dichotomous outcome measure, was used to establish if the treatment had been successful. This outcome was to be considered in a non-inferiority framework (see Statistical analysis, below). The question used for this measure was dependent on whether the patient was pre- or postmenopausal, the predominant complaint at randomisation and the type of HRT they were using, if any. This is because successful alleviation of AUB varies according to the type of bleeding associated with the presence of a uterine polyp(s). Eradication of bleeding is not the treatment goal following polypectomy in women with HMB or scheduled withdrawal bleeds on sequential HRT, but rather a reduction in bleeding to acceptable levels. In contrast, persistent unscheduled bleeding or PMB is always considered abnormal. Thus for these categories of AUB, complete cessation of symptoms is the clinical objective as opposed to a reduction in quantity. Further details can be seen in Figure 6. In all cases a ‘yes’ response was defined as a success (outcome measure ‘1’ in the outcome measure boxes, see Figure 6).
FIGURE 6.
Outpatient polyp treatment pathways for outcome measures. LMP, last menstrual period.

Secondary clinical outcome measures
Patient-completed measures:
-
MMAS123 A multiattribute utility, designed to measure the impact of HMB upon HRQL. It has been evaluated for its reliability and face validity (condition-specific instrument). Summary scores range from 0 (severely affected) to 100 (not affected). A modified version of the form was used for women whose symptoms did not specifically relate to the questions on the form (group C in Figure 6). Our objective here was to use the responses from this group to explore and develop the use of a modified questionnaire for those patients for whom bleeding is not expected; results are not presented in this report.
-
EuroQol EQ-5D-3L124 EQ-5D-3L (European Quality of Life-5 Dimensions, three-level version) is a standardised instrument for use as a generic measure of health outcome. Applicable to a wide range of health conditions and treatments, it provides a simple descriptive profile and a single index value for health status. Scores range from –0.59 (health state worse than death) to 1.0 (perfect health state).
-
EuroQol health thermometer124 A VAS score ranging from 0 (worst health state imaginable) to 100 (perfect health state). Scores range from –0.59 (health state worse than death) to 1.0 (perfect health state).
-
Likert bleeding scale All patients were asked how their bleeding had responded to treatment using a Likert scale with four response options: ‘much better’, ‘little better’, ‘same’, ‘worse’.
-
VAS to measure monthly bleeding quantity and duration It is now well established that objective measures of blood loss are not particularly relevant to women’s subjective perception of bleeding symptoms. 126 For those patients with HMB (group A in Figure 6), our pilot work97,128 has demonstrated that improvements in VAS scores correlate very well with improvements in categorical and condition-specific quality-of-life measures. The reliability of VAS has been established in the assessment of chronic gynaecological conditions, such as pain,129 and change in individual VAS scores were considered to have sufficient psychometric strengths to be used in the research of AUB involving large group comparisons. 130 Scores range from ‘0’ (no days bleeding in the last month) to ‘100’ (bleeding every day in the last month), and similarly ‘0’ (no bleeding in the last month) to ‘100’ (heaviest imaginable bleeding in the last month).
-
Perioperative measures Type, location and number of uterine polyps; size of largest polyp; the need for dilatation of the cervix; the use of a vaginal speculum; the technique used to remove the polyp(s) [hysteroscopic (direct vision) or non-hysteroscopic (blind) or combination]; the instruments used to remove the polyp(s) [hysteroscopic (electrosurgery or mechanical or combination) or non-hysteroscopic instrumentation]; the technique used to retrieve the polyp(s) [hysteroscopic or non-hysteroscopic; removal success, defined as complete removal and retrieval of polyp(s) from the uterine cavity]; the time taken to complete the polypectomy; the time in the outpatient room or operating theatre; details of any operative or postoperative complications, including vasovagal reactions (a liberal clinical diagnosis to define vasovagal episodes was used here, i.e. any woman who feels faint requiring her to lie supine or with her head down); and details of any further treatment prescribed for bleeding.
-
Procedure acceptability Questions assessing the patients’ experiences of the procedures focused on pain [VAS; range ‘0’ (no pain) to ‘100’ (worst imaginable pain)] during the procedure (outpatient polypectomy only), at 1 hour after the procedure and on discharge from hospital; acceptability of procedure (totally, generally, fairly acceptable or unacceptable); embarrassment (extremely, moderately, little, none); and ‘yes/no’ answers to questions regarding whether to recommend the procedure to a friend, have the same treatment again and, upon reflection, whether the alternative treatment would have been preferred.
-
Other measures during follow-up Additional medical treatments for bleeding; further polyp removal; gynaecological surgery; use of the following for reasons due to bleeding: outpatient clinic, hospital (day case or inpatient), GP (surgery or at home); days off work; visits to hospital for reasons unrelated to bleeding; visits to gynaecologist (not necessarily related to bleeding).
Statistical considerations
Sample size
The sample size for the RCT part of the study was chosen to give good statistical power to preclude any clinically important inferiority of outpatient polypectomy compared with inpatient treatment.
Outpatient treatment is more convenient for women in that no inpatient stay is required and is also likely to cost substantially less. We believed, therefore, that outpatient would be the treatment of choice, even if 25% fewer women (in relative terms) had alleviated symptoms at 6 months, i.e. the margin of non-inferiority was set at 0.75. Making the assumption that inpatient treatment would be 90% successful (from the primary outcome) and outpatient 80% successful, a sample size of approximately 200 in each arm (400 in total) would be needed to rule out a success rate of < 67.5% in the outpatient arm with 90% power, i.e. not > 25% worse (0.675/0.90 = 0.75). This calculation was based on a conservative two-sided test at the 5% level (equivalent to a one-sided test at the 2.5% level). To also allow for a 15% loss to follow-up, the sample size was inflated to 240 patients in each group (i.e. 480 patients in total).
No target sample size was set for the preference study, as many women as possible were recruited while the randomised study was open to recruitment.
Statistical analysis
Randomised controlled trial
Primary analyses were by ITT but per-protocol (PP) sensitivity analyses were also conducted for the primary outcome, as some protection for any theoretical increase in the risk of type I error (erroneously concluding non-inferiority). 131 The ITT analysis included all randomised patients in the groups to which they were allocated, regardless of whether the women received this, or indeed any, treatment. The PP analysis included only those women who received their allocated treatment at the time of their initial operation.
Point estimates and two-sided 95% CIs from unadjusted risk ratios were calculated for the primary outcome measure using a log-binomial linear model. The trial could declare non-inferiority only if the lower band of the CI was not lower than the prespecified margin of non-inferiority (RR of 0.75). 132 Analysis was performed once all patients had reached their final follow-up time point at 2 years, although 6 months’ follow-up was considered the primary outcome time (see Chapter 1, Objectives of the Outpatient versus inpatient Polyp Treatment study).
Adjusted risk ratios (calculated through the addition of the minimisation parameters to the linear model) were calculated as a sensitivity analysis for the primary outcome measure. Further sensitivity analysis for this parameter included performing the analysis without the following: those who had received a LNG-IUS at the time of operation; those who had gone on to receive a further related procedure; and those outcomes reported at > 3 months after their due date. To examine the possible impact of missing data on the results, sensitivity analysis was also performed, using a multiple imputation approach, on the primary outcome measure. Missing responses were simulated using a Markov chain Monte Carlo (MCMC) method that assumed an arbitrary missing data pattern and a multivariate normal distribution. Variables, including treatment group, the three subgroup variables (see Objectives) and a variable for each time point were included in the model and used to generate 20 simulated data sets. Analysis was then performed on each set, with the results combined using Rubin’s rule to obtain a single set of results (treatment effect estimate and CIs). Best- and worst-case analyses were also performed, assuming that all missing responses were successful or unsuccessful.
Treatment by subgroup interaction parameters were included in the log-binomial linear model used to generate the risk ratios to test for differences in treatment effect for the primary outcome measure between prespecified subgroups [none of these interaction parameters was statistically important (p < 0.05) and so effect sizes within subgroup were not investigated further].
Secondary end points measured on a continuous scale (scores from MMAS, EQ-5D and VAS) were analysed at each time point using a linear model (analysis of covariance) adjusting for baseline score. Further adjusted risk ratios were calculated as a sensitivity analysis for these parameters (as per the sensitivity analysis for the primary outcome calculated through the addition of the minimisation parameters to the linear model). A repeated measures analysis, including all assessment time points, was also performed. 133 Models included parameters allowing for group, time and baseline score. Furthermore, paired t-tests at each time point were used to investigate change scores within groups. Analysis of bleeding scores on a VAS score scale was also performed as a sensitivity analysis following a log transformation to stabilise the variance but this made no difference to outcome and is not presented in this report. Standard tests were used for other outcome measures: Cochran–Armitage test for trend for ordinal responses, t-tests for continuous data with a normal distribution, Wilcoxon signed-rank test for skewed continuous data and chi-squared tests for binary and categorical responses. The non-inferiority hypothesis did not apply for these other secondary end points; 95% CIs and p-values from two-sided superiority tests are presented. 134
The program SAS, version 9.2 (SAS Institute Inc., Cary, NC, USA), was used for analyses. A statistical analysis plan was agreed with the Data Monitoring and Ethics Committee (DMEC) prior to analysis (see Appendix 1).
Preference study
Chi-squared and t-tests were used to assess if there were any systematic differences between the preference groups in terms of their baseline characteristics. Analysis was performed in a similar fashion as per the randomised study with the exception of the repeated measures analysis (due to the fact there was only one follow-up time point here). Where possible, adjusted estimates of differences between groups (for the three variables listed in Objectives) were calculated (and are referred to in the text) to take into account any systematic differences between the groups through known confounders. This was not attempted, although for some outcome measures for which event rates were low (e.g. procedure acceptability) PP subgroup or sensitivity analysis was not attempted for the preference study as it was felt this would add little to the interpretation here. Any comparisons of results or demographics between RCT and preference study are as a result of a combined analysis of both data sets. Indicator variables for the type of study (randomised or preference) were used, with interactions between these and the relevant variables examined through, for example, a logistic regression analysis.
Trial management
Independent Trial Steering Committee
The Trial Steering Committee (TSC) provided independent supervision for the trial, providing advice to the Chief and Co-Investigators and the Sponsor on all aspects of the trial and affording protection for patients by ensuring that the trial was conducted according to the Medical Research Council (MRC) Guidelines for Good Clinical Practice in Clinical Trials (www.mrc.ac.uk/documents/pdf/good-clinical-practice-in-clinical-trials/). The TSC consisted of the following independent members: Professor J Thornton (chairperson, Professor of Obstetrics and Gynaecology, University of Nottingham); Professor P O’Donovan (Professor of Medical Innovation and Gynaecology, University of Bradford); E Nicholls (Birmingham Women’s Hospital, Patient and Public Involvement representative); Professor J Deeks (University of Birmingham, Statistician); and Dr S Petrou (University of Oxford, Health Economist). Professor Deeks stepped down as a TSC member during the study follow-up period after taking up a role as Director as the Birmingham Clinical Trials Unit. He was not replaced on the Committee as the study was shortly due to finish.
Data Monitoring and Ethics Committee: determining when clear answers have emerged
The role of the DMEC was to advise the chairperson of the TSC if, in their view, it had become apparent during the period of recruitment that outpatient treatment was clearly inferior to standard inpatient treatment. Alternatively, new evidence might have emerged from other sources that outpatient polypectomy is definitely less effective than inpatient polypectomy. To protect against this, during the period of recruitment to the study, interim analyses of major end points were supplied, in strict confidence, to an independent DMEC, along with updates on results of other related studies, and any other analyses that the DMEC requested. Pragmatic stopping criteria were used; the DMEC were to advise the chairperson of the TSC if, in their view, any of the randomised comparisons in the trial provided both (a) ‘proof beyond reasonable doubt’ that outpatient treatment is so inferior from inpatient treatment that non-inferiority can never be demonstrated for all, or some, women, and (b) evidence that might reasonably be expected to influence the patient management of many clinicians who were already aware of the other main trial results. The TSC then decided whether to close or modify any part of the trial. Unless this happened, however, the Trial Management Group (TMG), TSC, the investigators and all of the central administrative staff (except the statisticians who supply the confidential analyses) remained unaware of the interim results.
The DMEC consisted of the following independent members: Professor M Lumsden (chairperson, Professor of Obstetrics and Gynaecology, University of Glasgow); Professor S Bhattacharya (Professor of Obstetrics and Gynaecology, University of Aberdeen); and Dr C Cummins (Paediatric Epidemiologist, University of Birmingham and Birmingham Children’s Foundation Trust). They met on three occasions and recommended continuing with the study with no change to the protocol.
Research governance
The trial was conducted according to the principles of the MRC Guidelines for Good Clinical Practice in Clinical Trials (1998) and the UK NHS Research Governance Framework (www.gov.uk/government/uploads/system/uploads/attachment_data/file/139565/dh_4122427.pdf). All Principal Investigators were required to sign an Investigators’ Agreement, detailing their commitment to accrual, compliance, Good Clinical Practice, confidentiality and publication. All of the Trusts hosting the research were required to sign a Clinical Study Site Agreement, detailing the Trust’s responsibilities under the relevant Research Governance Framework and accepting the terms and conditions of the per-patient payments. The sponsor ensured that all researchers not employed by an NHS organisation, who had contact with patients and could have an impact on their quality of care, held a NHS honorary contract for that organisation, or had an honorary contract research passport.
Ethical approval
The Trial had a favourable ethical opinion from South West Multicentre Research Ethics Committee (MREC) approval, determining that the trial design respects the rights, safety and well-being of the participants. Every potential UK centre also obtained Local Research Ethics Committee (LREC) and Trust research and development (R&D) approval.
The final protocol for the OPT Trial is available via the Health Technology Assessment (HTA) website: www.hta.ac.uk/project/1679.asp.
Consolidated Standards of Reporting Trials (CONSORT) recommendations134 were followed for the reporting of this trial (see Appendix 9).
Chapter 3 Results of the randomised controlled trial
Recruitment
There were 1537 women with AUB who were willing to be randomised and assessed for eligibility by hysteroscopy. Of these, 507 women from 31 UK centres were randomised between April 2008 and July 2011 (Table 11 and Figure 7). The trial surpassed its original planned sample size with the permission of the TSC, as recruitment towards the end of the trial was ahead of the anticipated rate. The most common reason for ineligibility was ‘no polyp being present’ (85% of the 1030 not randomised). Of note, 4% (40/1030) of the ineligible women had polyps that were considered by the hysteroscopist as infeasible to remove in an outpatient setting (Figure 8).
| Randomising centre | Frequency (%) |
|---|---|
| Birmingham Women’s Hospital | 168 (33) |
| Royal Infirmary of Edinburgh | 43 (8) |
| Royal Victoria Infirmary, Newcastle | 42 (8) |
| Royal Hallamshire Hospital, Sheffield | 33 (7) |
| Royal Blackburn Hospital | 32 (6) |
| Queen’s Hospital, Romford | 24 (5) |
| Castle Hill Hospital, East Riding | 22 (4) |
| St Mary’s Hospital, London | 21 (4) |
| Bradford Royal Infirmary | 17 (3) |
| Barnsley District General Hospital | 11 (2) |
| Ormskirk & District General Hospital | 9 (2) |
| Sunderland City Hospital | 9 (2) |
| Taunton & Somerset Hospital | 8 (2) |
| Kidderminster General Hospital | 7 (1) |
| New Cross Hospital, Wolverhampton | 7 (1) |
| Liverpool Women’s Hospital | 6 (1) |
| Newham General Hospital | 6 (1) |
| Countess of Chester Hospital | 5 (1) |
| Queen Charlotte’s & Chelsea Hospital | 5 (1) |
| University Hospital of North Staffordshire | 5 (1) |
| Birmingham Heartlands Hospital | 4 (1) |
| Worcestershire Royal Hospital | 4 (1) |
| Bishop Auckland General Hospital | 3 (1) |
| Chelsea & Westminster Hospital | 3 (1) |
| Staffordshire General Hospital | 3 (1) |
| Whiston Hospital, Merseyside | 3 (1) |
| Shotley Bridge Hospital, County Durham | 2 (< 1) |
| Solihull Hospital | 2 (< 1) |
| City Hospital, Birmingham | 1 (< 1) |
| Manor Hospital, Walsall | 1 (< 1) |
| The Royal Oldham Hospital | 1 (< 1) |
| Total | 507 |
FIGURE 7.
Trial accrual.
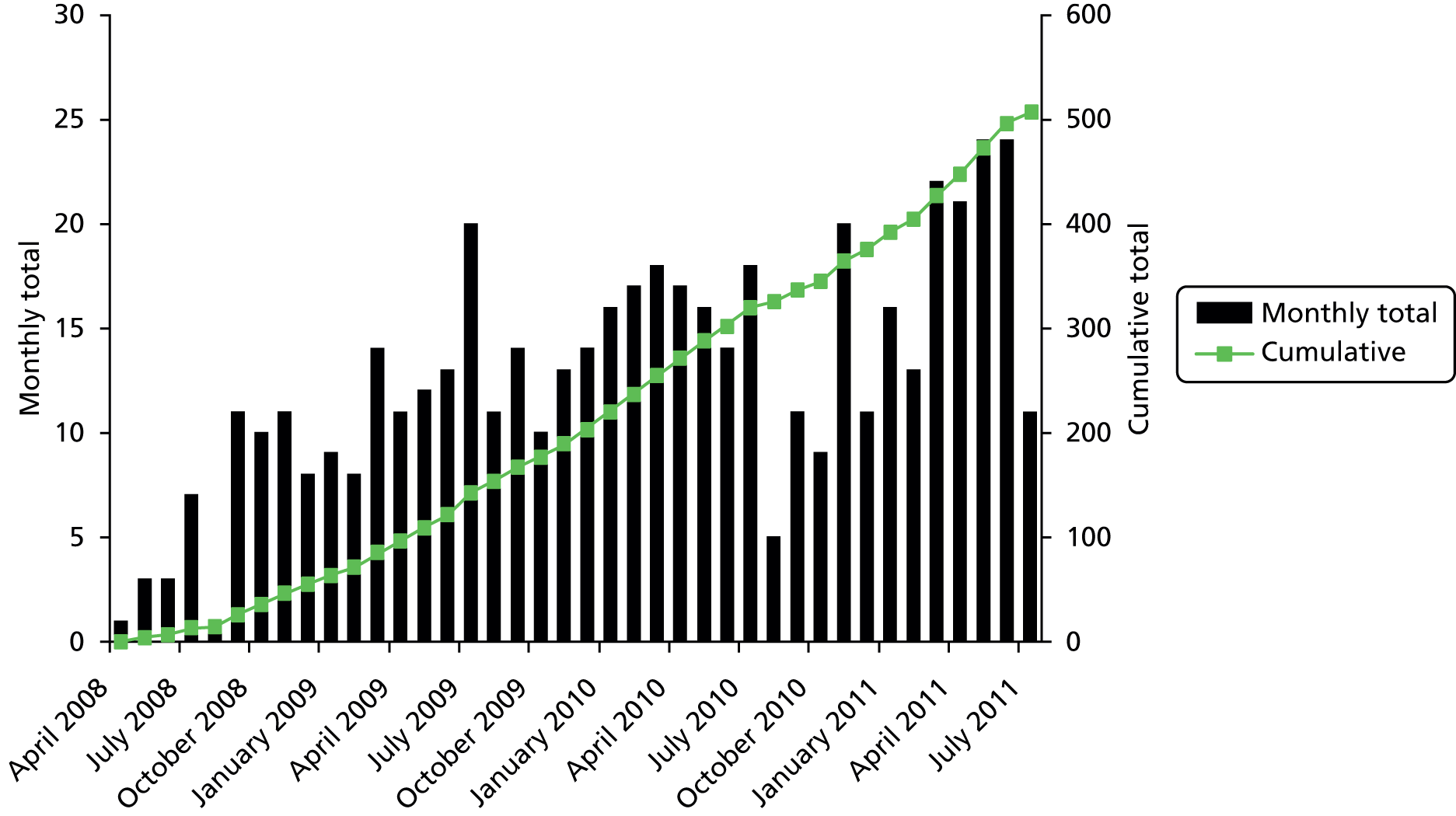
FIGURE 8.
Consolidated Standards of Reporting Trials diagram.

Patients and follow-up
Baseline characteristics of the patients were similar between the two groups. For 45% (227/507) of those randomised, the initial complaint was PMB; 30% (153/507) had HMB and the remaining 25% (127/507) had IMB. The predominant type of polyp was singular (394/507, 78%), non-fundal (309/507, 61%) and glandular (378/507, 75%). The overwhelming majority (501/507, 99%) had no other benign pathology (Table 12).
| Baseline characteristic | Polypectomy | |
|---|---|---|
| Outpatient (n = 254) | Inpatient (n = 253) | |
| Age, years: mean (SD) | 50 (10) | 51 (11) |
| Ethnicity | ||
| White | 207 (88) | 179 (87) |
| Asian | 17 (7) | 16 (7) |
| Black | 11 (5) | 9 (4) |
| Other | 1 (< 1) | 1 (< 1) |
| Not given/not known | 18 | 48 |
| Predominant bleeding complaint at randomisationa | ||
| Postmenopausala | 113 (44) | 114 (45) |
| Heavy menstrualb | 77 (30) | 76 (30) |
| Intermenstrualc | 64 (25) | 63 (25) |
| Site of uterine polypd | ||
| Fundal | 99 (39) | 99 (39) |
| Non-fundal | 155 (61) | 154 (61) |
| Type of uterine polypd | ||
| Glandular | 190 (75) | 188 (74) |
| Fibrous | 64 (25) | 65 (26) |
| No. of polyps | ||
| 1 | 193 (76) | 201 (79) |
| 2 | 40 (16) | 43 (17) |
| ≥ 3 | 21 (8) | 9 (4) |
| Other benign pathology | ||
| None | 251 (99) | 250 (99) |
| SMF/adhesion/septum | – | 1 (< 1) |
| Adhesion/septum | – | 1 (< 1) |
| SMF | 2 (1) | – |
| Septum | 1 (< 1) | 1 (< 1) |
In the outpatient group, 230/254 (91%) women received their randomised allocation compared with 206/253 (81%) in the inpatient group. Twice as many women in the inpatient group had outpatient treatment compared with the other way around (34/253, 13% vs. 14/254, 6%). Similar numbers in both groups ended up not having any polyp removal (see Figure 8).
For 76% [363/478 (29 missing responses)] of the women entering the study, the intention at randomisation was to undergo immediate removal (‘see and treat’) if allocated outpatient polypectomy, with the remaining women to return for treatment at a later date. Upon comparison of randomisation dates with treatment dates for the outpatient group this fell to around 72% [174/242 (12 dates not completed)], which may reflect those patients who ultimately had inpatient polypectomy or did not ultimately have a polyp removal. The median time from randomisation to treatment in the inpatient group was 26 days [interquartile range (IQR) 14–42]. In the outpatient group this was 0 days (IQR 0–14).
Completed primary outcome responses were available from 439 of 507 (87%) participants at the primary outcome time of 6 months (see Figure 8).
Operative results
Table 13 details the operative results. The mean polyp size estimated on visual inspection during hysteroscopy was marginally greater (0.2 cm, 95% CI 0.0 to 0.3; p = 0.04) in the inpatient group than in the outpatient treatment group. Compared with inpatient treatment, outpatient surgery necessitated less use of vaginal instrumentation (RR 0.62, 95% CI 0.54 to 0.71; p < 0.001) and dilatation of the cervix (RR 0.41, 95% CI 0.34 to 0.50; p < 0.001). Removal of polyps under direct hysteroscopic vision was significantly more common in the outpatient surgery setting (RR 1.4, 95% CI 1.2 to 1.6; p < 0.001) and electrosurgery was the most popular method of detaching polyps, being employed in over half of all outpatient cases (RR 1.6, 95% CI 1.3 to 2.0; p < 0.001 compared with inpatient). Similarly, hysteroscopic retrieval of polyp specimen(s) from the uterine cavity was the most common technique in the outpatient setting, whereas blind mechanical extraction was preferred in the inpatient group (RR 2.2, 95% CI 1.7 to 2.9; p < 0.001).
| Operative details | Polypectomy | Mean difference or RR (95% CI);a p-value | |
|---|---|---|---|
| Outpatient | Inpatient | ||
| Largest polyp size, cm: median (IQR), n | 1.0 (0.6–2.0), 230 | 1.2 (1.0–2.0), 217 | –0.2 (–0.3 to 0.0); 0.04 |
| Need for cervical dilatation = yes | 76/241 (32%) | 178/232 (77%) | 0.41 (0.34 to 0.50); < 0.001 |
| Use of vaginal speculum = yes | 126/236 (53%) | 193/224 (86%) | 0.62 (0.54 to 0.71); < 0.001 |
| Use of local anaesthetic = yes | 91/244 (37%) | 15/240 (6%) | 6.0 (3.6 to 10.0); < 0.001 |
| Hysteroscopic removal = yes (vs. blind) | 175/225 (78%) | 122/217 (56%) | 1.4 (1.2 to 1.6); < 0.001 |
| Method used to detach | n = 228 | n = 222 | 1.6 (1.3 to 2.0);b < 0.001 |
| Electrode | 124 (54%) | 75 (34%) | |
| Mechanical | 89 (39%) | 139 (63%) | |
| Combination | 15 (7%) | 8 (4%) | |
| Method of retrieval | n = 227 | n = 223 | 2.2 (1.7 to 2.9);c < 0.001 |
| Hysteroscopic | 127 (56%) | 56 (25%) | |
| Mechanical | 59 (26%) | 147 (66%) | |
| Combination | 4 (2%) | 5 (2%) | |
| None | 37 (16%) | 15 (7%) | |
| Time taken for polypectomy, minutes [median (IQR), n] | 10 (5–14), 223 | 10 (5–15), 186 | –1.5 (–3.0 to 0.0); 0.006 |
| Time in outpatient room/theatre, minutes: median (IQR), n | 25 (18–35), 225 | 27 (20–35), 216 | –1.0 (–3.0 to 1.0); 0.6 |
| Removal success | n = 242 | n = 233 | |
| Complete | 196 (81%) | 215 (92%) | |
| Partiald | 25 (10%) | 15 (6%) | 2.5 (1.5 to 4.1);e < 0.001 |
| Failedd | 21 (9%) | 3 (1%) | |
Partial or failed removals occurred in 46/242 (19%) of the outpatient group compared with 18/233 (7%) in the inpatient group (RR 2.5, 95% CI 1.5 to 4.1; p < 0.001). The most common reason for incomplete removal in the outpatient group was patient discomfort (22/46, 48%). Of the 46 failed outpatient procedures, 25 (54%) were immediately scheduled for subsequent reoperation, usually as an inpatient procedure (23/25, 92%). Of the remainder, all these women were followed up for 2 years and none had a subsequent polyp removal.
Sixteen per cent (74/451) of the women were fitted with a LNG-IUS at the time of operation; numbers were similar in both groups (Table 14).
| Complications and further treatment | Polypectomy | |
|---|---|---|
| Outpatient | Inpatient | |
| Operative complications | ||
| n = 241 | n = 233 | |
| Vasovagal episode | 17 (7%) | 3 (1%) |
| Nausea/pain | 4 (2%) | – |
| Cervical trauma | – | 3 (1%) |
| Uterine perforation | – | 4 (2%) |
| Haemorrhage | – | 3 (1%) |
| Othera | 2 (1%) | 1 (< 1%) |
| Postoperative complications | ||
| n = 232 | n = 223 | |
| Vasovagal episode | 15 (6%) | 3 (1%) |
| Vomiting | 6 (3%) | 4 (2%) |
| Dizziness/nausea | 5 (2%) | 2 (1%) |
| Severe pain | 3 (1%) | – |
| Otherb | 2 (1%) | 2 (1%) |
| Further treatment/procedure given | ||
| n = 229 | n = 222 | |
| Mirena IUS | 31 (14%) | 43 (19%) |
| Tranexamic acid | 12 (5%) | 1 (< 1%) |
| Progestogens | 8 (3%) | – |
| Endometrial destruction | 4 (2%) | 1 (< 1%) |
| Mefenamic acid (Ponstan, Pfizer) | 1 (< 1%) | 1 (< 1%) |
| Hysterectomy | 1 (< 1%) | 1 (< 1%) |
| Goserelin acetate | 2 (1%) | – |
| Missing reason | – | 1 (< 1%) |
| Otherc | – | 1 (< 1%) |
Serious adverse events
Four uterine perforations (4/233, 2%) occurred in the inpatient group and were recorded as SAEs (see Table 13). One of these also involved bowel injury, which required an emergency laparotomy and resection of small bowel with primary re-anastomosis (this required a further five nights in hospital). Of the other three cases, one patient required further hospitalisation for formation of haematoma and infection post perforation, with the other two patients being discharged the same day with antibiotics.
Two other SAEs were recorded: one woman had indwelling catheterisation following an inpatient removal. The sole SAE in the outpatient group involved a woman who was admitted to a high-dependency unit as she had experienced a recent myocardial infarction.
Treatment success: primary outcome
Successful response to surgery was reported by 73% of women at 6 months in the outpatient group compared with 80% of women in the inpatient polypectomy group (ITT RR 0.91, 95% CI 0.82 to 1.02; PP RR 0.92, 95% CI 0.82 to 1.02) (Figure 9). The lower end of the CI shows that outpatient polypectomy is at most 18% (RR 0.82) worse in relative terms than inpatient treatment (same for both ITT and PP analyses), within the 25% margin of non-inferiority we set at the outset of the study. In absolute terms this translates to a risk difference of –0.07 (95% CI –0.16 to 0.03) and a lower bound of the CI for number needed to harm (NNTH) of ‘6’ with outpatient treatment [NNTH 15, 95% CI 6 to number needed to benefit (NNTB) 39].
FIGURE 9.
Primary outcome: comparison with margin of non-inferiority.
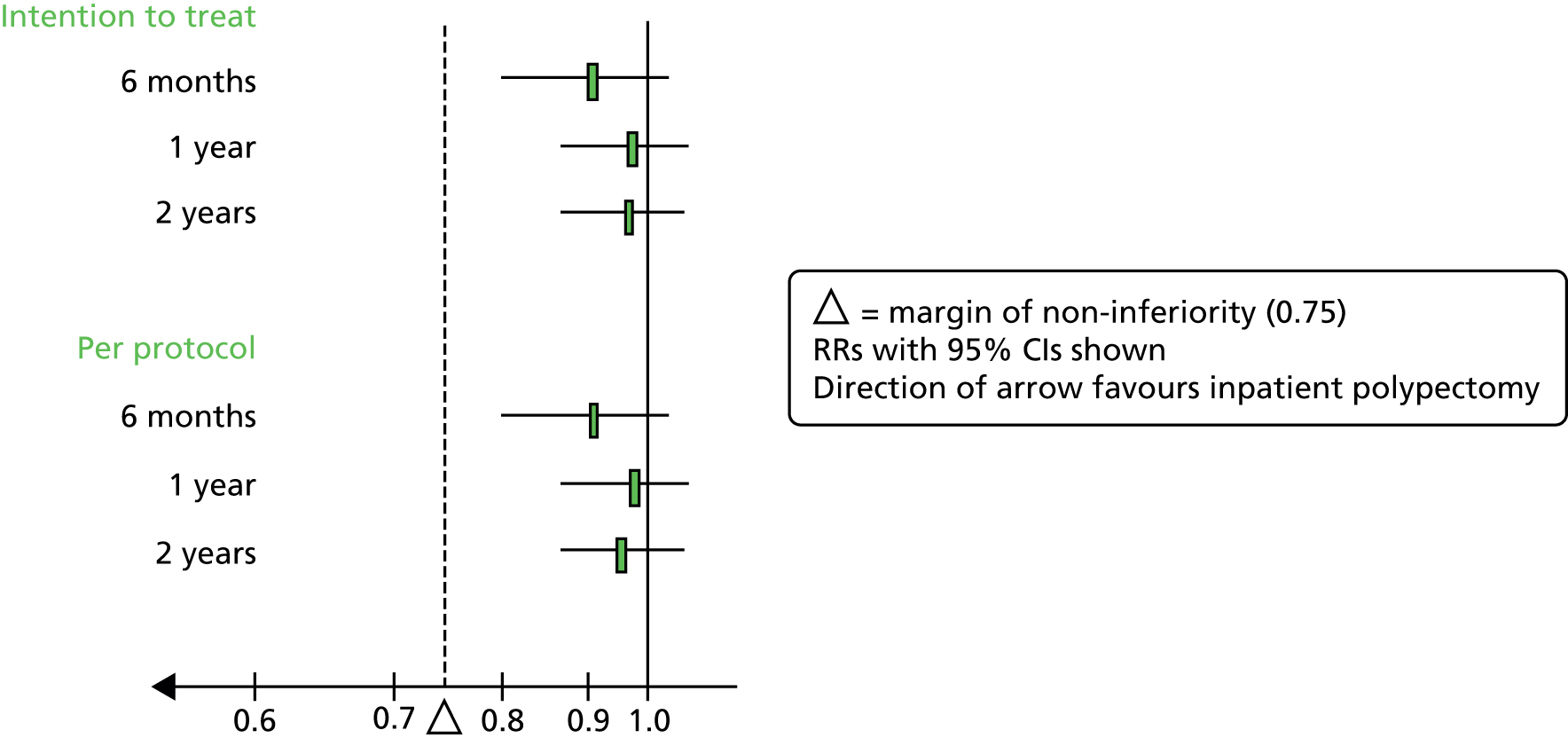
By 1 and 2 years, the corresponding proportions were very similar between groups producing RRs close to unity. Full results can be seen in Table 15, including a further adjusted analysis (see Appendix 1, statistical analyses).
| Time of assessment | Polypectomy | RR (95% CI) | Adjusteda RR (95% CI) | |
|---|---|---|---|---|
| Outpatient | Inpatient | |||
| ITT | ||||
| 6 months | 166/228 (73) | 168/211 (80) | 0.91 (0.82 to 1.02) | 0.93 (0.85 to 1.02) |
| 1 year | 182/225 (81) | 177/214 (83) | 0.98 (0.90 to 1.07) | 0.98 (0.91 to 1.06) |
| 2 years | 179/213 (84) | 169/196 (86) | 0.97 (0.90 to 1.06) | 0.97 (0.91 to 1.05) |
| PP | ||||
| 6 months | 153/210 (73) | 143/180 (79) | 0.92 (0.82 to 1.02) | 0.93 (0.84 to 1.02) |
| 1 year | 168/206 (82) | 153/184 (83) | 0.98 (0.89 to 1.08) | 0.98 (0.91 to 1.06) |
| 2 years | 165/197 (84) | 146/168 (87) | 0.96 (0.89 to 1.05) | 0.97 (0.90 to 1.04) |
The various sensitivity analyses we completed on the treatment success parameter (described in Statistical analysis, above) gave very similar results to this base case and did not change the interpretation (results given in Appendix 10).
Results of a comparison of the primary outcome variable with EuroQol EQ-5D scores are given in Appendix 11; ‘success’ responses were associated with statistically significant higher EQ-5D scores than ‘failure’ responses.
There was no evidence that treatment success differed according to any of the predefined subgroups at any of the time points when treatment by variable interaction parameters were examined (Table 16). Results within subgroups (from the ITT population) are given in Figures 10–12 (error bars indicate 95% CIs).
| Interaction variable examined | 6 months | 1 year | 2 years |
|---|---|---|---|
| Treatment by predominant bleeding complaint | 0.90 | 0.63 | 0.96 |
| Treatment by polyp site | 0.61 | 0.89 | 0.91 |
| Treatment by polyp type | 0.42 | 0.06 | 0.83 |
FIGURE 10.
Primary outcome: by predominant bleeding complaint.
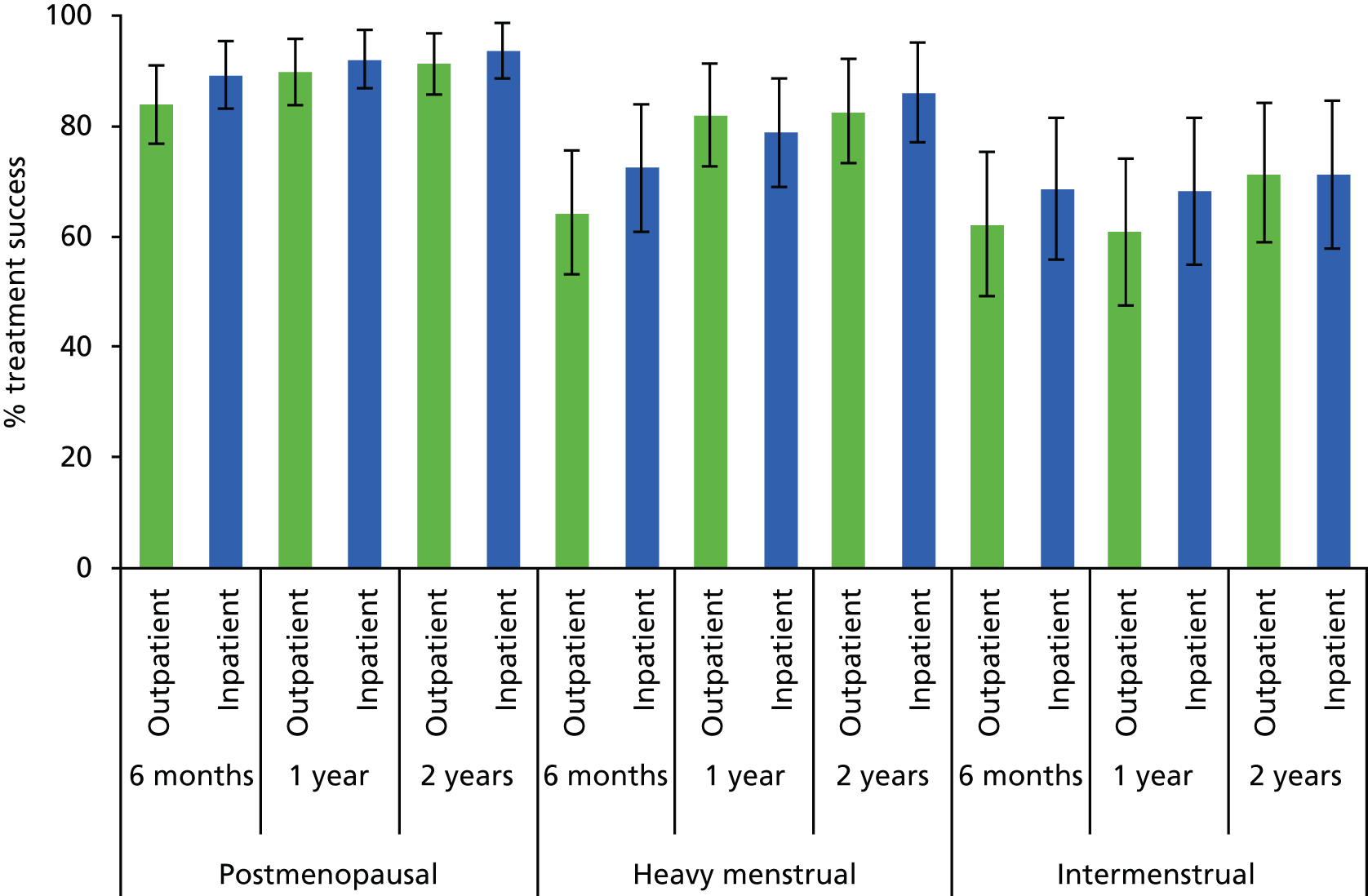
FIGURE 11.
Primary outcome: by site of uterine polyp.
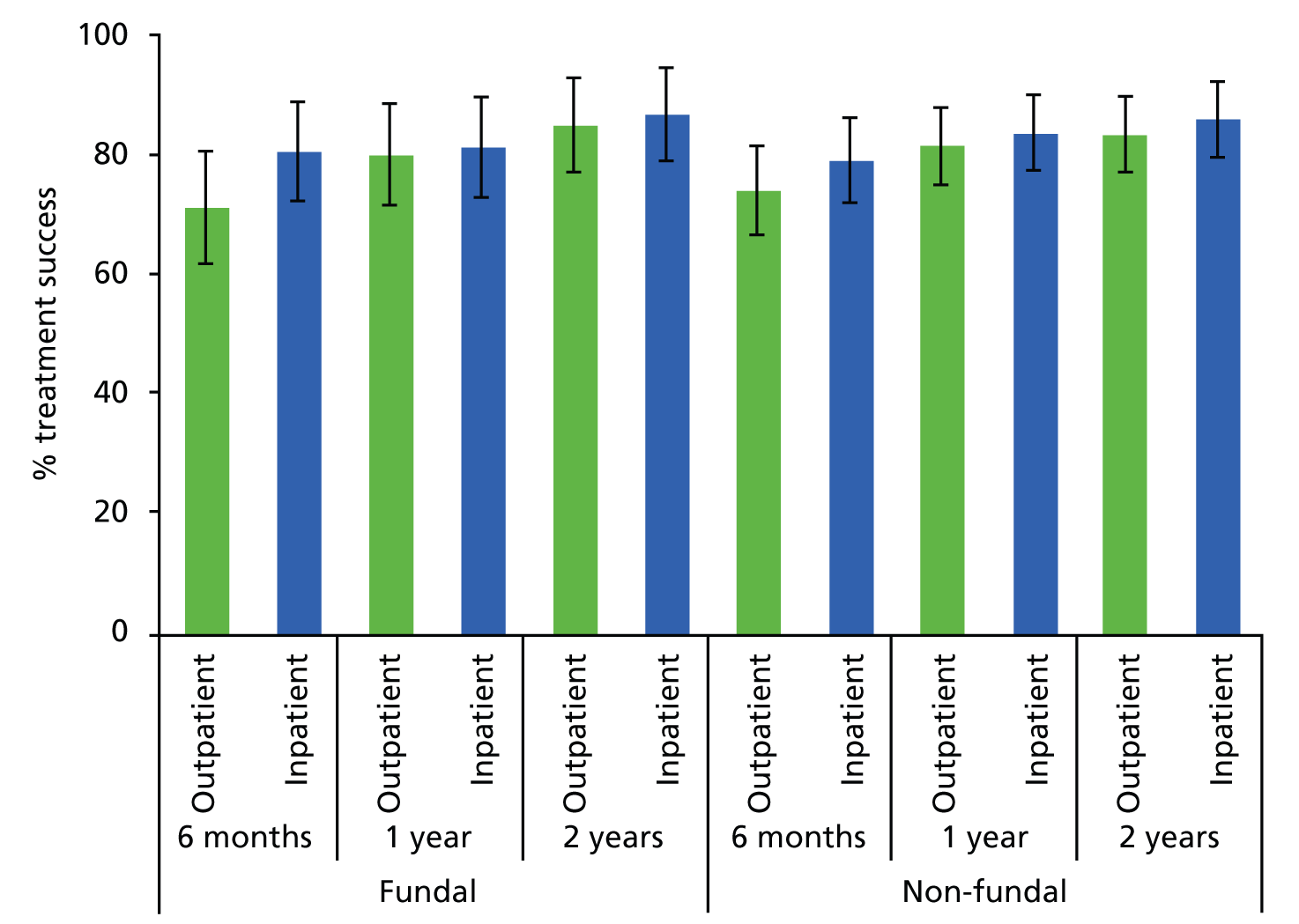
FIGURE 12.
Primary outcome: by type of uterine polyp.
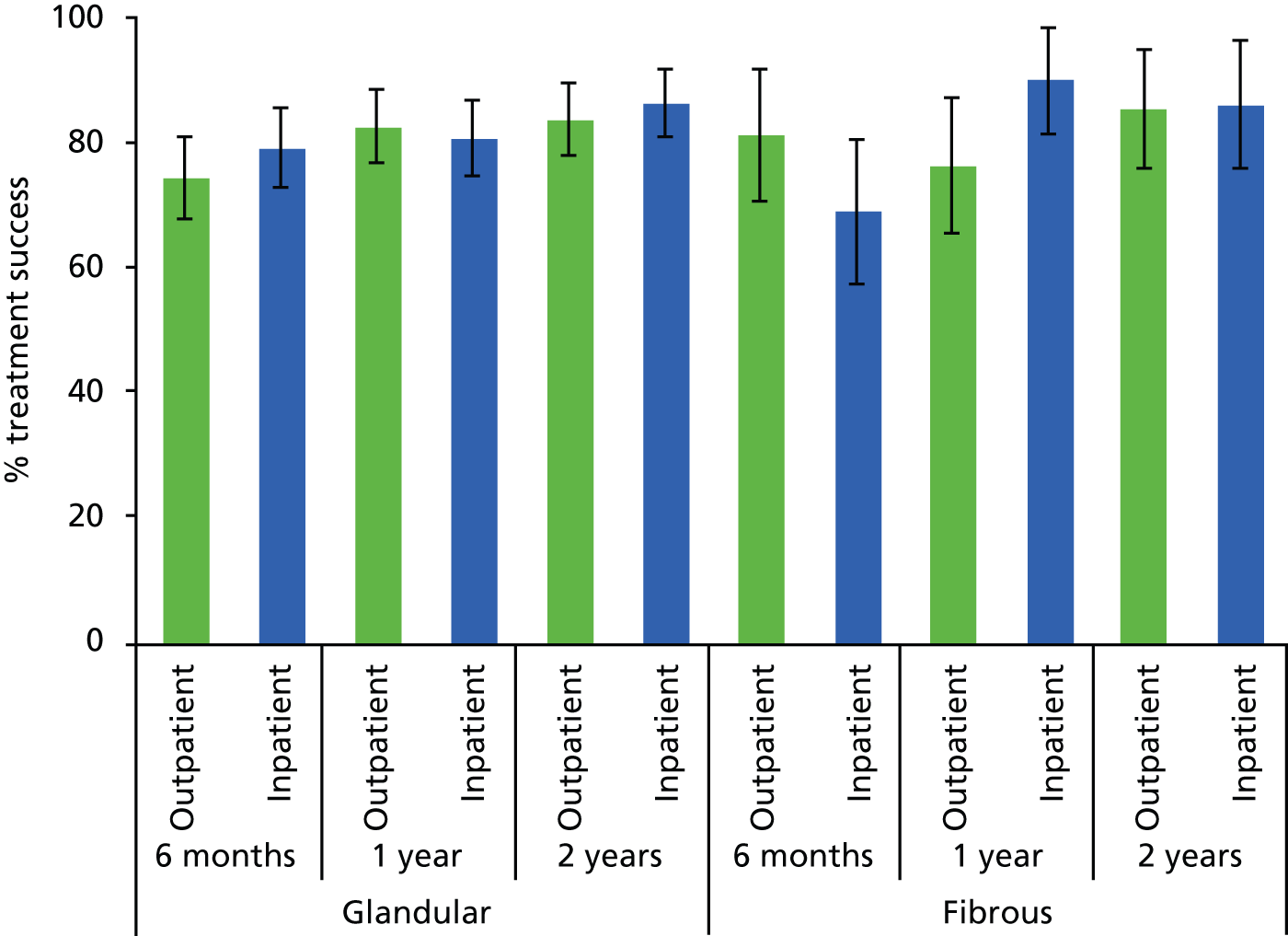
Major secondary outcomes
Responses from the Likert scale question on current bleeding compared with baseline bleeding yielded similar results to the primary outcome (Table 17). Further adjusted analyses as described above (see Statistical analysis) are also given for these outcomes in (see Appendix 12) and are very similar to the unadjusted results.
| Time of assessment | Compared with before your treatment, would you say your bleeding is: | Polypectomy | |
|---|---|---|---|
| Outpatient | Inpatient | ||
| 6 months | Much better | 164 (75%) | 160 (81%) |
| Little better | 24 (11%) | 21 (11%) | |
| Same | 29 (13%) | 12 (6%) | |
| Worse | 3 (1%) | 4 (2%) | |
| Total | 220 | 197 | |
| Test for trend p -value | 0.08 | ||
| RR (95% CI) a | 0.93 (0.87 to 1.00) | ||
| 1 year | Much better | 177 (80%) | 174 (82%) |
| Little better | 18 (8%) | 12 (6%) | |
| Same | 21 (10%) | 21 (10%) | |
| Worse | 5 (2%) | 5 (2%) | |
| Total | 221 | 212 | |
| Test for trend p -value | 0.85 | ||
| RR (95% CI) a | 1.01 (0.94 to 1.08) | ||
| 2 years | Much better | 173 (82%) | 172 (88%) |
| Little better | 17 (8%) | 12 (6%) | |
| Same | 18 (8%) | 10 (5%) | |
| Worse | 4 (2%) | 1 (1%) | |
| Total | 212 | 195 | |
| Test for trend p -value | 0.04 | ||
| RR (95% CI) a | 0.95 (0.90 to 1.01) | ||
Condition-specific and generic quality-of-life scores were significantly improved from baseline at most time points in both groups with no differences between them (Table 18 and Figures 13–17). Similarly, for women with HMB, the overall amount and duration of bleeding was significantly reduced in both groups at all time points post treatment without any difference between groups. Further adjusted analyses, as described in Chapter 2 (see Format), are also given for these outcomes in Appendix 13 and are very similar to the unadjusted results. Appendix 14 describes the results for the VAS score analysis without those prescribed LNG-IUS at the time of operation as a sensitivity analysis; results were very similar to the base case.
| Time of assessment | Polypectomy, mean (SD, n) | Difference (95% CI);a p-value | |
|---|---|---|---|
| Outpatient | Inpatient | ||
| MMASb | |||
| Baseline | 52 (27, 134) | 58 (24, 124) | |
| 6 months | 78 (22, 115)c | 79 (23, 99)c | –1 (–7 to 5); 0.68 |
| 1 year | 82 (23, 110)c | 83 (21, 101)c | –1 (–7 to 5); 0.78 |
| 2 years | 84 (21, 93)c | 85 (21, 83)c | –2 (–8 to 4); 0.47 |
| Overalld | –1 (–6 to 4); 0.65 | ||
| EuroQol EQ-5De | |||
| Baseline | 0.78 (0.25, 242) | 0.78 (0.27, 232) | |
| 6 months | 0.87 (0.23, 230)c | 0.87 (0.20, 211)c | –0.01 (–0.04 to 0.03); 0.70 |
| 1 year | 0.86 (0.25, 227)c | 0.86 (0.24, 219)c | 0.00 (–0.04 to 0.04); 0.85 |
| 2 years | 0.85 (0.25, 213)c | 0.84 (0.27, 196) | 0.03 (–0.02 to 0.07); 0.28 |
| Overalld | 0.00 (–0.03 to 0.03); 0.99 | ||
| EuroQol health thermometerf | |||
| Baseline | 77 (18, 233) | 78 (18, 225) | |
| 6 months | 79 (18, 227)c | 80 (17, 212) | 0 (–3 to 3), 0.89 |
| 1 year | 80 (17, 228)c | 82 (16, 219)c | –1 (–4 to 2), 0.50 |
| 2 years | 79 (18, 207) | 83 (16, 194)c | –2 (–5 to 1), 0.19 |
| Overalld | –1 (–3 to 1), 0.28 | ||
| Bleeding duration VASg | |||
| Baseline | 46 (28, 68) | 53 (28, 67) | |
| 6 months | 35 (30, 64) | 28 (26, 56)c | –10 (–21 to 1), 0.07 |
| 1 year | 18 (21, 58)c | 24 (28, 62)c | 5 (–5 to 14), 0.32 |
| 2 years | 16 (22, 61)c | 15 (25, 53)c | –2 (–12 to 8), 0.65 |
| Overalld | –3 (–10 to 4), 0.39 | ||
| Bleeding amount VASh | |||
| Baseline | 58 (28, 70) | 66 (26, 68) | |
| 6 months | 29 (29, 68)c | 29 (29, 58)c | –1 (–12 to 9), 0.82 |
| 1 year | 23 (26, 66)c | 19 (22, 66)c | –4 (–12 to 5), 0.36 |
| 2 years | 19 (24, 63)c | 18 (27, 57)c | –2 (–12 to 8), 0.66 |
| Overalld | –3 (–10 to 4), 0.40 | ||
FIGURE 13.
Menorrhagia Multi-attribute Assessment Scale scores over time by group (95% CI for mean shown at each time point; higher = better).

FIGURE 14.
EuroQol EQ-5D scores over time by group (95% CI for mean shown at each time point; higher = better).
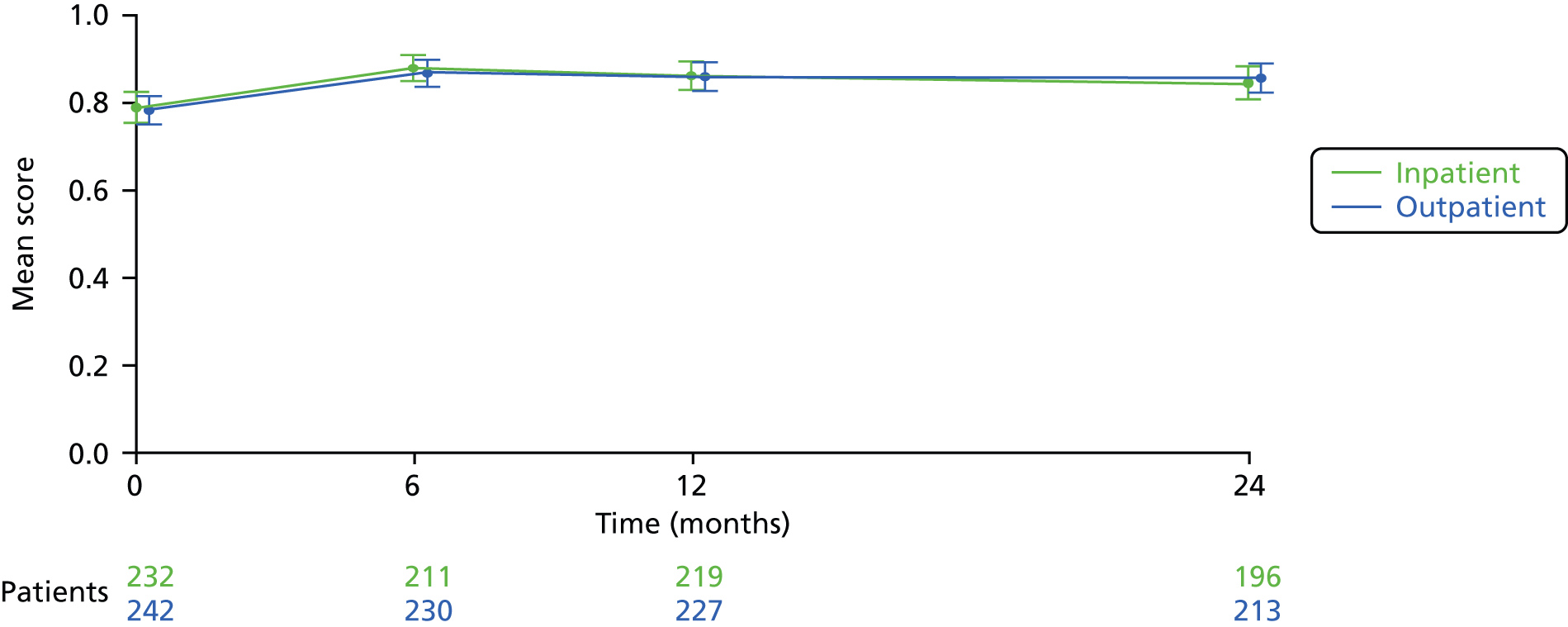
FIGURE 15.
EuroQol health thermometer scores over time by group (95% CI for mean shown at each time point; higher = better).

FIGURE 16.
Visual analogue scale bleeding duration scores over time by group (95% CI for mean shown at each time point; lower = better).

FIGURE 17.
Visual analogue scale bleeding amount scores over time by group (95% CI for mean shown at each time point; lower = better).

Procedure acceptability
Mean pain scores 1 hour following the procedure and upon discharge were higher in the outpatient polypectomy group than in the inpatient group (Table 19), although on average only by 5 (95% CI 0 to 10; p = 0.03) and 8 (95% CI 4 to 12; p < 0.001) points, respectively, out of a possible range of 100 points. Responses to questions on procedure acceptability/recommend to a friend/have the same treatment again were statistically significant in favour of inpatient polypectomy, although the levels of responses were high for both groups; for example, 92% (205/222) of those randomised to outpatient polypectomy would recommend the procedure to a friend and 98% (220/225) found the procedure at least ‘fairly acceptable’. Further adjusted analyses, as described in Statistical analysis above, are also given for these outcomes in Appendix 13, and are very similar to the unadjusted results.
| Patient experience and preference | Polypectomy | Difference or RR (95% CI);a p-value | |
|---|---|---|---|
| Outpatient | Inpatient | ||
| Mean pain score during procedure (SD, n)b | 45 (26, 217) | – | – |
| Mean pain score 1 hour following procedure (SD, n)b | 28 (23, 176) | 23 (22, 191) | –5 (–10 to 0); 0.03 |
| Mean pain score on discharge (SD, n)b | 23 (21, 200) | 15 (17, 186) | –8 (–12 to –4); 0.001 |
| Procedure acceptable? | |||
| n = 225 | n = 197 | ||
| Totally | 136 (60%) | 152 (77%) | |
| Generally | 51 (23%) | 30 (15%) | 0.90 (0.84 to 0.97); 0.001c |
| Fairly | 33 (15%) | 12 (6%) | |
| Unacceptable | 5 (2%) | 3 (2%) | |
| Exposure embarrassing? | |||
| n = 224 | n = 196 | ||
| Extremely | 5 (2%) | 4 (2%) | 1.45 (0.86 to 2.46); 0.24d |
| Moderately | 17 (8%) | 24 (12%) | |
| A little | 79 (35%) | 35 (18%) | |
| No | 123 (55%) | 133 (68%) | |
| Recommend to a friend? | |||
| Yes/total | 205/222 (92%) | 190/196 (97%) | 0.95 (0.91 to 1.00); 0.04 |
| Same treatment again? | |||
| Yes/total | 200/223 (90%) | 186/193 (96%) | 0.93 (0.88 to 0.98); 0.009 |
| Preferred alternative treatment? | |||
| Yes/total | 47/218 (22%) | 39/190 (21%) | 0.95 (0.65 to 1.39); 0.80 |
Other outcomes
Similar numbers of women reported taking additional medical treatments for their bleeding during the 2 years of follow-up (Tables 20 and 21). At 6 months, 15% (31/209) of women were using the LNG-IUS in the inpatient group, compared with 9% (21/228) in the outpatient group. The corresponding figures at 1 and 2 years were 10% (29/219) compared with 8% (19/227) and 11% (21/195) compared with 11% (24/214).
| Time of assessment | Polypectomy | RR (95% CI);a p-value | |
|---|---|---|---|
| Outpatient | Inpatient | ||
| 6 months | 46/228 (20%) | 46/209 (22%) | 1.09 (0.76 to 1.57); 0.6 |
| 1 year | 40/227 (18%) | 38/219 (17%) | 0.98 (0.66 to 1.47); 0.9 |
| 2 years | 38/214 (18%) | 28/195 (14%) | 0.81 (0.52 to 1.27); 0.4 |
| Time of assessment | Medical treatment | Polypectomy | |
|---|---|---|---|
| Outpatient (n = 228) |
Inpatient (n = 209) |
||
| 6 months | LNG-IUS | 21 (9%) | 31 (15%) |
| Tranexamic acid | 4 (2%) | 6 (3%) | |
| Mefenamic acid (Ponstan, Pfizer) | 3 (1%) | 3 (1%) | |
| HRT | 8 (4%) | 1 (< 1%) | |
| Combined oral contraceptive | 3 (1%) | 5 (2%) | |
| Norethisterone (Utovlan®, Pfizer) | 4 (2%) | 0 (–) | |
| Depot medoxyprogesterone acetate (Depo-Provera, Pfizer) | 2 (1%) | 0 (–) | |
| Local oestrogen cream | 1 (< 1%) | 0 (–) | |
| Goserelin (Zoladex®, Astra Zeneca) | 1 (< 1%) | 0 (–) | |
| Missing | 1 (< 1%) | 1 (< 1%) | |
| Total | 48 a | 47 b | |
| n = 227 | n = 219 | ||
| 1 year | LNG-IUS | 19 (8%) | 29 (10%) |
| Tranexamic acid | 3 (1%) | 2 (1%) | |
| Mefenamic acid (Ponstan, Pfizer) | 4 (2%) | 1 (< 1%) | |
| HRT | 10 (4%) | 5 (2%) | |
| Combined oral contraceptive | 3 (1%) | 2 (1%) | |
| Norethisterone | 3 (1%) | 1 (< 1%) | |
| Clomiphene | 1 (< 1%) | 0 (–) | |
| Local oestrogen cream | 0 (–) | 1 (< 1%) | |
| Total | 43 c | 41 d | |
| n = 214 | n = 195 | ||
| 2 years | LNG-IUS | 24 (11%) | 21 (11%) |
| Tranexamic acid | 3 (1%) | 1 (1%) | |
| Mefenamic acid (Ponstan, Pfizer) | 1 (<1%) | 0 (–) | |
| HRT | 7 (3%) | 2 (1%) | |
| Combined oral contraceptive | 0 (–) | 2 (1%) | |
| Norethisterone | 2 (1%) | 0 (–) | |
| Goserelin | 0 (–) | 1 (1%) | |
| Missing | 1 (< 1%) | 1 (1%) | |
| Total | 38 | 28 | |
In total over the 2-year follow-up period, 43/254 (17%) women in the outpatient group and 21/253 (8%) in the inpatient group had at least one further polyp removed (RR 2.0, 95% CI 1.2 to 3.3; p = 0.003) (see Figure 8; note that a small number of women had more than one further removal, which explains the slight discrepancy between total numbers). The total number of gynaecological operations other than polyp removal over the period of follow-up was also higher in the outpatient group [61/230 (27%) vs. 36/219 (16%), RR 1.6, 95% CI 1.1 to 2.3; p = 0.01] (Table 22). If hysteroscopies are ignored, this difference is still statistically significant [39/230 (17%) vs. 20/219 (9%), RR 1.8, 95% CI 1.1 to 3.1; p = 0.01]. All surgery types (hysterectomy, endometrial ablation and others) were more common in the outpatient group.
| Type of surgery | Polypectomy | |
|---|---|---|
| Outpatient | Inpatient | |
| Hysteroscopy | 22/230 (10%) | 16/219 (7%) |
| Hysterectomy | 17/230 (7%) | 10/219 (5%) |
| Endometrial ablation | 12/230 (5%) | 5/219 (2%) |
| Any other | 10/230 (4%)a | 5/219 (2%)b |
| Any gynaecological surgery | 61/230 (27%) | 36/219 (16%) |
General practitioner and hospital use owing to bleeding appeared similar in both groups (Table 23).
| Health service usage, including absenteeism | Polypectomy | |
|---|---|---|
| Outpatient | Inpatient | |
| Outpatient clinic | ||
| 6 months | 12/231 (5%) | 8/211 (4%) |
| 1 year | 15/228 (7%) | 16/219 (7%) |
| 2 years | 15/213 (7%) | 13/196 (7%) |
| Hospital ward day case | ||
| 6 months | 8/231 (3%) | 4/211 (2%) |
| 1 year | 8/228 (4%) | 3/219 (1%) |
| 2 years | 6/214 (3%) | 7/196 (4%) |
| Hospital overnight | ||
| 6 months | 0 (–) | 0 (–) |
| 1 year | 2/228 (1%) | 0 (–) |
| 2 years | 2/215 (1%) | 2/196 (1%) |
| GP surgery | ||
| 6 months | 26/231 (11%) | 23/211 (11%) |
| 1 year | 24/228 (11%) | 21/219 (10%) |
| 2 years | 20/215 (9%) | 17/196 (9%) |
| GP at home | ||
| 6 months | 0 (–) | 1/211 (< 1%) |
| 1 year | 0 (–) | 0 (–) |
| 2 years | 1/215 (< 1%) | 0 (–) |
| Days off work | ||
| 6 months | 14/231 (6%) | 11/211 (5%) |
| 1 year | 6/228 (3%) | 5/219 (2%) |
| 2 years | 7/215 (3%) | 5/196 (3%) |
| Hospital for any other reasons (not bleeding related) | ||
| 6 months | 10/231 (4%) | 8/211 (4%) |
| 1 year | 19/228 (8%) | 9/218 (4%) |
| 2 years | 32/215 (15%) | 21/196 (11%) |
| Gynaecologist for any other reason (not surgery or necessarily bleeding related) | ||
| 6 months | 16/230 (7%) | 9/210 (4%) |
| 1 year | 20/227 (9%) | 19/219 (9%) |
| 2 years | 16/214 (7%) | 13/195 (7%) |
Discussion of the randomised controlled trial
Principal findings
The results of this trial demonstrate that outpatient polypectomy was non-inferior to inpatient polypectomy for the successful alleviation of AUB when compared with our prespecified non-inferiority margin of 25%. Polypectomy successfully treated AUB in 73% (166/228) of the women in the outpatient group, compared with 80% (168/211) in the inpatient group (equivalent to a NNTH of ‘6’ at the lower bound of the 95% CI) at 6 months, with no evidence of interaction of type of bleeding, location or type of polyp with treatment allocation. This treatment success was maintained at both 12 and 24 months, with no differences noted between outpatient and inpatient surgery at any time point. The duration and amount of bleeding were significantly reduced following both outpatient and inpatient treatment and no differences were identified according to treatment allocation. Similarly, a significant improvement in generic and disease-specific HRQL was seen following polypectomy in both treatment groups, which was maintained at 12 and 24 months. Rates of re-referral to a gynaecologist were similar between treatment groups, although the requirement for subsequent gynaecological operative intervention, in the form of endometrial ablation or hysterectomy, was twice as high following outpatient treatment over the 2 years’ follow-up.
Outpatient-based therapy, such as polypectomy, has been facilitated by technological advances in instrumentation, allowing focal pathologies to be removed under direct vision. However, the assumed convenience of avoiding hospital admission and anaesthesia may be diminished if the procedure is infeasible or acceptable in the majority of women. This trial estimated that outpatient polypectomy was successfully completed in four out of five women but the odds of failure to complete polyp removal was two and a half times more likely compared with traditional inpatient treatment. This equates to a NNTH of ‘9’; for every nine outpatient polypectomies performed, an additional one procedure will fail compared with inpatient treatment. Patient discomfort was the main factor predisposing to failure in the outpatient compared with the inpatient setting. Both outpatient and inpatient treatment times were short, i.e. 10 minutes on average. Pain scores were 45 out of 100 on average during outpatient polypectomy, levels which are similar to other commonly adopted intrauterine procedures, such as diagnostic hysteroscopy or endometrial biopsy (M Connor, Royal Hallamshire Hospital, Sheffield, 2013, personal communication). Within 1 hour of treatment, the mean pain scores had reduced to 28 out of 100 implying that pain intensity is moderated quickly. Postoperative pain at 1 hour and on discharge from hospital were significantly higher in the outpatient group than in the inpatient group but the small differences in absolute mean pain scores and their low intensity level suggest that these observations are unlikely to be of clinical significance. Outpatient polypectomy was also statistically less acceptable than inpatient treatment. However, only 2% (5/225) of women found outpatient-based treatment unacceptable and 90% (200/223) of women were prepared to have such treatment again or recommend the outpatient procedure. Nevertheless, with the benefit of hindsight, approximately one in five women in both treatment groups reported a desire to have had the alternative setting for treatment.
Outpatient treatment appeared to be safe; the most common operative complication being vasovagal fainting episodes that occurred in around 1 in 14 (7%, 17/241) women. The majority of polypectomies were conducted hysteroscopically, i.e. polyp detachment under direct vision, but this was done significantly more often in the outpatient group than in the inpatient treatment group (78%, 175/225 vs. 56%, 122/217, respectively). Most polyps (56%, 127/227) were retrieved under direct hysteroscopic vision in the outpatient setting, whereas the converse was true for inpatient therapy, which used traditional blind mechanical extraction in 66% (147/223) of cases. Intra-operative haemorrhage and trauma to the upper genital tract was restricted to a minority of women in the inpatient treatment group. However, four of these traumatic cases were uterine perforations, with one case requiring emergency laparotomy to repair bowel injuries and the other resulting in a pelvic haematoma and subsequent abscess requiring readmission and a period of hospitalisation. These two procedures were conducted blindly. Although traditional blind mechanical instrumentation of the uterus is generally safe, the serious morbidity and mortality that can arise from unrecognised intra-abdominal trauma using such techniques in an anaesthetised patient is well recognised. 12,13
Strengths and limitations of the study
The strengths of our trial include strict randomisation, its size, the multicentre design, the relatively low rates of loss to follow-up, and the tailoring of assessment of outcomes to the primary complaint. Extensive sensitivity analyses were carried out to assess the impact of missing responses and re-intervention on the primary outcome. Some limitations of our study should be noted. These include variation in the practice of outpatient polypectomy, with several techniques and forms of instrumentation being utilised, although the most common approach was bipolar electrosurgery, used in 54% (124/228) of cases. Furthermore, a ‘see and treat’ approach was adopted for outpatient polypectomy in 72% (174/242) of cases, whereas the others were undertaken at a subsequent appointment. PP analyses and a range of further sensitivity analyses did not change our conclusions supporting the robustness of our findings. Furthermore, most women were successfully treated regardless of the type of bleeding complaint with no evidence that relative effectiveness of outpatient compared with inpatient treatment varied by bleeding subtype. Although we could examine the relative efficacy of treatment according to menopausal status, as well as type of bleeding (HMB vs. IMB), we were unable to fulfil one of our original objectives – to compare women with PMB according to whether they were taking HRT. In our pilot study, women with PMB on HRT formed the largest single population subgroup. This contrasted sharply with recruitment to the OPT Trial, for which very few women on HRT and PMB presented, thereby precluding further analysis. This finding coincides with the decline in women taking HRT over recent years following the publication of large cohort studies showing no clear health benefits of long-term HRT use. 135
The overall effect of polypectomy on HMB did not appear to be influenced by the concomitant use of the LNG-IUS, which was fitted immediately post polypectomy in 14% (31/229) of outpatient treatments and 19% (43/222) of inpatient treatments. The type and location of uterine polyp did not influence relative treatment effectiveness.
The pragmatic design of the OPT RCT allowed for the inclusion of women presenting with all types of AUB complaints, enhancing the generalisability of our findings. Successful alleviation of AUB symptoms had to be defined differently according to one of the three categories of AUB: HMB, IMB and PMB. We used the woman’s own assessment of bleeding symptoms, using a dichotomous outcome measure, to establish if the treatment had been successful. We believe that the simplicity of this approach was understandable to women and clinically relevant, but the validity of our chosen outcome has not been tested. Even if women interpret the primary outcome questions differently, the internal validity (outpatient vs. inpatient) should not have been affected. It was reassuring to note that the secondary outcome Likert scale responses for ‘Compared with before your treatment, would you say your bleeding is? (much better to worse)’ were almost identical to the primary outcome measure. In addition, the primary bleeding outcome appeared to be related to an improvement in generic HRQL data measured using EQ-5D scores after treatment.
We also sanctioned the use of any described technique for effecting uterine polypectomy in either setting, but inevitably this led to an imbalance between groups according to treatment method. The outpatient approach to polypectomy was conducted under direct vision using varying technologies in the majority of women, whereas general anaesthesia permitted the use of larger, more traumatic instrumentation and adoption of ‘blind’ techniques in most women. Thus, the technique used may have affected the success of treatment rather than setting alone. However, this is unlikely because polypectomy is a simple procedure and the presumed mechanism of treatment efficacy upon AUB symptoms relates to their removal, however achieved. The study was not powered to determine whether a particular surgical method was more feasible, safe, effective or acceptable.
Implications for practice
Outpatient polyp treatment appears to be safe, feasible, acceptable and effective for the treatment of HMB, IMB and PMB. Outpatient uterine polypectomy should be made routinely available within the NHS in light of these favourable findings and the high disease burden associated with AUB and uterine polyps. Diagnostic OPH facilities are widely available within most NHS hospitals. Diagnostic OPH is familiar to many gynaecologists but additional training would be necessary to become competent in therapeutic procedures such as polypectomy. However, the additional costs and resources required for such training are uncertain. At present only the minority of NHS hospitals are able to provide operative OPH. The ambulatory setting should not be restricted to hospital-based practice, but the establishment of appropriately equipped and staffed community-based services should be explored.
The safety and convenience of outpatient treatment and the equivalent effectiveness in ameliorating AUB symptoms should be weighed against the increased likelihood of failure to complete the procedure, perioperative pain experience and reduced acceptability in the conscious outpatient compared with the anaesthetised inpatient. Women should be made aware of the risk of failure to completely remove the uterine polyp in one in every five outpatient treatments and, as a result, the possible necessity for subsequent inpatient treatment. They should be informed that outpatient intervention is associated with pain, albeit of generally moderate intensity and for a short duration of time. In addition, women should be cognisant of the trend towards reduced acceptability of the overall procedure compared with inpatient approaches, but that at least 8 out of 10 women find the outpatient procedure to be totally or generally acceptable. The thorough counselling of women in this way may present some challenges given that the majority of units effect immediate treatment following polyp diagnosis at hysteroscopy, the so-called ‘see and treat’ approach to management. Current practices for obtaining informed consent should be reviewed and consideration given to providing contemporary written material to women prior to their appointment and verbal information during the pre-hysteroscopy consultation.
Conclusion
We believe that evaluating all types of AUB within our trial has enhanced the generalisability of our findings. However, at present, outpatient uterine polypectomy, with or without the use of local anaesthetic, is not widely available within the NHS and many other health-care systems. Despite this caveat, ‘ambulatory’, ‘outpatient’ or ‘office’-based diagnostic and therapeutic interventions are becoming increasingly prevalent, driven by advances in technology and a desire to enhance recovery. Thus, this trial is timely, novel and relevant to contemporary clinical practice.
Our trial has demonstrated comparable clinical effectiveness of outpatient polypectomy in alleviating gynaecological bleeding complaints at 6, 12 and 24 months. However, despite the convenience and apparent safety of the outpatient treatment setting there were more technical failures reflecting the increased complexity of the procedures in a conscious outpatient compared with an anaesthetised inpatient. The clinical significance of the observations of increased postoperative pain and reduced patient acceptability associated with outpatient compared with inpatient treatment may be inconsequential, given the high overall levels of patient acceptability and relatively low postoperative pain intensity for outpatient polypectomy.
In light of the non-inferiority of outpatient polypectomy and its overall safety, feasibility and acceptability, therapeutic outpatient hysteroscopic services should be established, providing women with a choice of treatment setting. Women should be informed of the relative advantages and disadvantages of either treatment approach, so that they can make an informed decision.
Chapter 4 Results of the preference study
Recruitment
Of the 952 women with AUB who were willing to participate in the treatment preference study and be assessed for eligibility by hysteroscopy, 399 (42%) from 31 UK centres were recruited between April 2008 and July 2011 (Table 24 and Figure 18). Of the 399 women willing to participate, 324 (81%) expressed a preference for outpatient treatment (Figure 19). The most common reason for ineligibility was no polyp being present in 72% of the 553 not recruited. Six per cent (32/553) of the ineligible women had polyps that were considered by the hysteroscopist to be infeasible to remove in an outpatient setting (Figure 20).
| Randomising centre | Frequency (%) |
|---|---|
| Birmingham Women’s Hospital | 129 (32) |
| Royal Hallamshire Hospital, Sheffield | 78 (20) |
| Royal Victoria Infirmary, Newcastle | 37 (9) |
| Barnsley District General Hospital | 29 (7) |
| St Mary’s Hospital, London | 16 (4) |
| Kidderminster General Hospital | 13 (3) |
| Ormskirk & District General Hospital | 11 (3) |
| Castle Hill Hospital, East Riding | 10 (3) |
| Countess of Chester Hospital | 9 (2) |
| Liverpool Women’s Hospital | 9 (2) |
| Worcestershire Royal Hospital | 7 (2) |
| Queen Charlotte’s & Chelsea Hospital | 6 (2) |
| Royal Infirmary of Edinburgh | 6 (2) |
| New Cross Hospital, Wolverhampton | 5 (1) |
| Norfolk & Norwich University Hospital | 5 (1) |
| Queens Hospital, Romford | 4 (1) |
| Taunton & Somerset Hospital | 4 (1) |
| Bradford Royal Infirmary | 3 (1) |
| Newham General Hospital | 3 (1) |
| Bishop Auckland General Hospital | 2 (1) |
| Blackpool Victoria Hospital | 2 (1) |
| Royal Blackburn Hospital | 2 (1) |
| University Hospital of North Staffordshire | 2 (1) |
| Birmingham Heartlands Hospital | 1 (< 1) |
| Chelsea & Westminster Hospital | 1 (< 1) |
| City Hospital, Birmingham | 1 (< 1) |
| Sandwell General Hospital | 1 (< 1) |
| Shotley Bridge Hospital, County Durham | 1 (< 1) |
| Solihull Hospital | 1 (< 1) |
| Whiston Hospital, Merseyside | 1 (< 1) |
| Total | 399 |
FIGURE 18.
Accrual to preference study.
FIGURE 19.
Allocated treatment according to randomisation and preference.

FIGURE 20.
Consolidated Standards of Reporting Trials diagram for preference study.
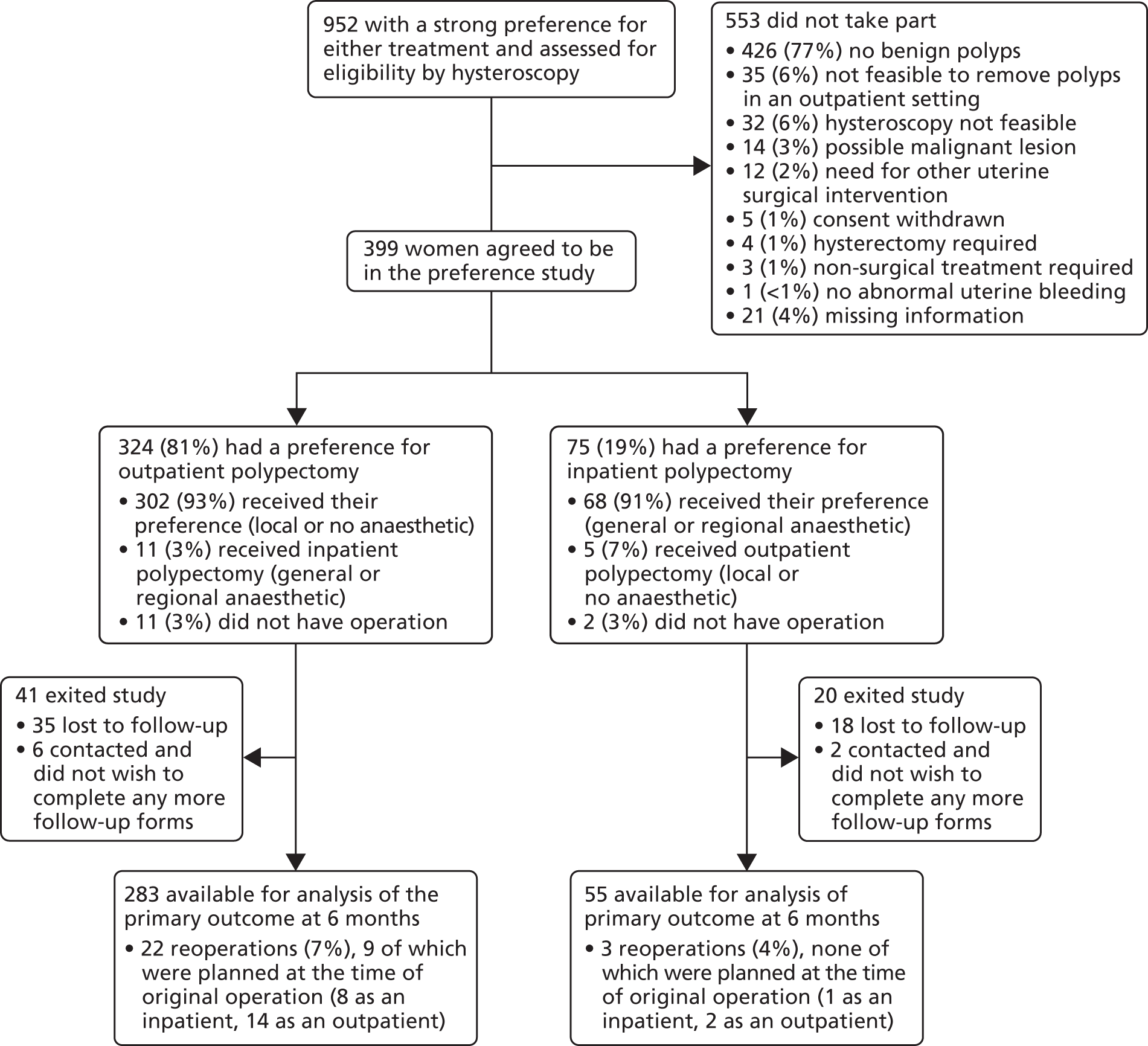
Patients and follow-up
Baseline characteristics of the patients were similar between the two groups (Table 25), with no statistically significant difference seen. For 48% (192/399) of those agreeing to be followed up, the initial complaint was PMB, 25% (98/399) had HMB and the remaining participants had IMB [27% (109/399]. This and the other demographics were similar to those women who were randomised (see Chapter 3, Patients and follow-up) apart from a slightly older overall population here (the average age was 2.3 years more, 95% CI 0.9 to 3.7; p = 0.001).
| Baseline characteristic | Polypectomy | p-value | |
|---|---|---|---|
| Outpatient (n = 324) | Inpatient (n = 75) | ||
| Age, years: mean (SD) | 53 (11) | 51 (12) | 0.2 |
| Ethnicity | |||
| White | 263 (91%) | 54 (93%) | |
| Asian | 12 (4%) | 2 (3%) | |
| Black | 8 (3%) | 1 (2%) | > 0.9 |
| Other | 6 (2%) | 1 (2%) | |
| Not given/not known | 35 | 17 | |
| Predominant bleeding complaint at randomisation | |||
| Postmenopausala | 155 (48%) | 37 (49%) | |
| Heavy menstrualb | 75 (23%) | 23 (31%) | 0.2 |
| Intermenstrualc | 94 (29%) | 15 (20%) | |
| Site of uterine polyp | |||
| Fundal | 118 (36%) | 25 (33%) | 0.6 |
| Non-fundal | 206 (64%) | 50 (67%) | |
| Type of uterine polyp | |||
| Glandular | 229 (71%) | 53 (71%) | > 0.9 |
| Fibrous | 95 (29%) | 22 (29%) | |
| No. of polyps | |||
| 1 | 233 (72%) | 58 (77%) | |
| 2 | 62 (19%) | 11 (15%) | 0.6 |
| ≥ 3 | 29 (9%) | 6 (8%) | |
| Other benign pathology | |||
| None | 318 (98%) | 74 (99%) | |
| SMF/adhesion/septum | – | – | |
| Adhesion/septum | – | – | 0.8 |
| SMF | 5 (2%) | 1 (1%) | |
| Septum | 1 (< 1%) | – | |
In the outpatient group 302 of 324 (93%) women received their treatment preference compared with 68 of 399 (91%) in the inpatient group (see Figure 20). In those women agreeing to take part and with a preference for outpatient polypectomy, 63% [196/312 (87 missing responses)] were to undergo immediate removal following diagnosis (‘see and treat’), with the remaining women returning for treatment at a later date. The median time from randomisation to treatment in the inpatient group was 31 days (IQR 7–55). In the outpatient group this was 0 days (IQR 0–27).
Completed primary outcome responses were available from 338 of 399 (85%) of participants at 6 months (see Figure 20).
Operative results
Table 26 details the operative details. The pattern of results was similar to those reported in the RCT (see Table 13). Compared with inpatient treatment, outpatient surgery necessitated less use of vaginal instrumentation (RR 0.57, 95% CI 0.49 to 0.66; p < 0.001) and dilatation of the cervix (RR 0.45, 95% CI 0.37 to 0.55; p < 0.001). Removal of polyps under direct hysteroscopic vision was significantly more common (RR 1.5, 95% CI 1.2 to 1.8; p < 0.001) in the outpatient surgery setting, and electrosurgery was the most popular method of detaching polyps, being used in over half of all outpatient cases (RR 1.5, 95% CI 1.0 to 2.0; p = 0.02). Similarly, hysteroscopic retrieval of polyp specimen(s) from the uterine cavity was the most common technique in the outpatient setting, whereas blind mechanical extraction was preferred in the inpatient group (RR 2.6, 95% CI 1.7 to 3.9; p < 0.001).
| Operative details | Polypectomy | Mean difference or RR (95% CI);a p-value | |
|---|---|---|---|
| Outpatient | Inpatient | ||
| Largest polyp size, cm: median (IQR), n | 1.1 (0.8–2.0), 286 | 1.0 (0.8–2.0), 57 | 0.0 (–0.2 to 0.2); > 0.9 |
| Need for cervical dilatation = yes | 105/303 (35%) | 52/67 (78%) | 0.45 (0.37 to 0.55); < 0.001 |
| Use of vaginal speculum = yes | 152/301 (50%) | 53/60 (88%) | 0.57 (0.49 to 0.66); < 0.001 |
| Use of local anaesthetic = yes | 132/313 (42%) | 2/73 (3%) | 15.4 (3.9 to 60.8); < 0.001 |
| Hysteroscopic removal = yes (vs. blind) | 246/299 (82%) | 36/64 (56%) | 1.5 (1.2 to 1.8); < 0.001 |
| Method used to detach | |||
| n = 287 | n = 65 | 1.5 (1.0 to 2.0);b 0.02 | |
| Electrode | 155 (54%) | 24 (37%) | |
| Mechanical | 102 (36%) | 35 (54%) | |
| Combination | 30 (10%) | 6 (9%) | |
| Method of retrieval | |||
| n = 292 | n = 62 | ||
| Hysteroscopic | 193 (66%) | 16 (26%) | 2.6 (1.7 to 3.9);c < 0.001 |
| Mechanical | 69 (24%) | 43 (69%) | |
| Combination | 11 (4%) | – | |
| None | 19 (7%) | 3 (5%) | |
| Time taken for polypectomy, minutes: median (IQR), n | 10 (5–15), 290 | 10 (7–15), 52 | –1.5 (3.0 to 0.0); 0.3 |
| Time in outpatient room/theatre, minutes: median (IQR), n | 30 (20–35), 285 | 33 (25–45), 53 | –6.0 (–10.0 to –2.0); 0.003 |
| Removal success | |||
| n = 312 | n = 73 | ||
| Complete | 282 (90%) | 68 (93%) | |
| Partiald | 22 (7%) | 3 (4%) | 1.4 (0.6 to 3.5);e 0.5 |
| Failedd | 8 (3%) | 2 (3%) | |
Partial or failed removals occurred in 30 of 312 (10%) of the outpatient group compared with 5/73 (7%) in the inpatient group (RR 1.4, 95% CI 0.6 to 3.5; p = 0.5). The overall level of failures was lower in the preference study than for those randomised [odds ratio (OR) 0.64, 95% CI 0.42 to 0.99; p = 0.05] but there was no evidence of treatment group interaction with method of study entry (p = 0.3). As with the randomised cohort, the most common reason for incomplete removal in the outpatient group was patient discomfort. Of the failed outpatient procedures (n = 30), nine (30%) were immediately scheduled for subsequent reoperation, usually as an inpatient procedure (6/9, 67%). The most common perioperative complications in the outpatient group were induced vasovagal reactions, affecting 6% of the cohort, a similar proportion to the 7% of such episodes observed in the RCT. No uterine perforations occurred in either treatment group (Table 27).
| Complications and further treatment | Polypectomy | |
|---|---|---|
| Outpatient | Inpatient | |
| Operative complications | ||
| n = 302 | n = 67 | |
| Vasovagal episode | 17 (6%) | – |
| Patient discomfort | 3 (1%) | – |
| Cervical trauma | 1 (< 1%) | 1 (1%) |
| Uterine perforation | – | – |
| Othera | 1 (< 1%) | – |
| Postoperative complications | ||
| n = 301 | n = 67 | |
| Vasovagal episode | 14 (5%) | 2 (3%) |
| Vomiting | 3 (1%) | 2 (3%) |
| Severe pain | – | 2 (3%) |
| Further treatment/procedure given | ||
| n = 292 | n = 64 | |
| Mirena IUS | 42 (14%) | 8 (13%) |
| Tranexamic acid | 9 (3%) | – |
| Progestogens | 3 (1%) | – |
| Endometrial destruction | 2 (1%) | – |
| Local oestrogen cream | 2 (1%) | – |
| Mefenamic acid (Ponstan, Pfizer) | 1 (< 1%) | 1 (2%) |
| Contraceptive pill | 1 (< 1%) | – |
| Missing treatment name | 2 (1%) | 1 (2%) |
Serious adverse events
No treatment-related SAEs were seen. Other operative and postoperative complications not considered SAEs are given in Table 26.
Treatment success
A successful response to surgery was reported in 82% of women at 6 months in both the outpatient group and inpatient polypectomy group (45/55 vs. 231/28, RR 0.99, 95% CI 0.87 to 1.12; p = 0.9). The overall level of success was higher in the preference study than for those randomised (OR 1.4, 95% CI 1.0 to 2.0; p = 0.06), but there was no evidence of treatment group interaction with method of study entry (p = 0.4).
Major secondary outcomes
Responses from the Likert scale question on current bleeding compared with baseline bleeding yielded similar results to the treatment success outcome (Table 28).
| Compared with before your treatment, would you say your bleeding is: | Polypectomy | |
|---|---|---|
| Outpatient polypectomy | Inpatient | |
| Much better | 213 (79%) | 43 (80%) |
| Little better | 24 (9%) | 6 (11%) |
| Same | 24 (9%) | 3 (6%) |
| Worse | 10 (4%) | 2 (4%) |
| Total | 271 | 54 |
| Test for trend ( p -value) | 0.7 | |
| RR (95% CI);a p-value | 0.96 (0.88 to 1.06); 0.5 | |
| Adjusted RR (95% CI); p-value | Not possible to compute | |
Condition-specific and bleeding scores were significantly improved from baseline to 6 months in both groups with no differences between them (Table 29). Generic quality of life was significantly improved in the outpatient group, but not so in the inpatient group. However, we cannot rule out this being due to the small sample size. There was no difference between groups in these quality-of-life parameters.
| Patient-reported outcome measures | Polypectomy, mean (SD, n) | Difference (95% CI);a p-value | Adjusted differenceb (95% CI); p-value | |
|---|---|---|---|---|
| Outpatient | Inpatient | |||
| MMASc | ||||
| Baseline | 63 (26, 163) | 61 (28, 37) | ||
| 6 months | 77 (25, 135)d | 79 (25, 25)d | –3 (–12 to 7); 0.55 | –4 (–13 to 6); 0.45 |
| EuroQol EQ-5De | ||||
| Baseline | 0.79 (0.26, 312) | 0.72 (0.30, 71) | ||
| 6 months | 0.82 (0.25, 289)d | 0.81 (0.30, 56) | 0.00 (–0.07 to 0.06); 0.88 | 0.00 (–0.07 to 0.06); 0.91 |
| EuroQol health thermometerf | ||||
| Baseline | 78 (18, 305) | 75 (21, 71) | ||
| 6 months | 78 (19, 291) | 79 (20, 57) | –2 (–7 to 3); 0.34 | –3 (–8 to 2); 0.28 |
| Bleeding duration VASg | ||||
| Baseline | 39 (26, 74) | 38 (26, 23) | ||
| 6 months | 30 (28, 65)d | 18 (19, 16)d | –13 (–27 to 2); 0.09 | –13 (–28 to 2); 0.09 |
| Bleeding amount VASh | ||||
| Baseline | 59 (28, 75) | 58 (26, 23) | ||
| 6 months | 32 (28, 68)d | 26 (27, 16)d | –4 (–19 to 11); 0.61 | –3 (–19 to 12); 0.67 |
Procedure acceptability
Mean pain scores 1 hour following the procedure and upon discharge were higher in the outpatient polypectomy group compared with the inpatient group (Table 30). The proportions of women responding positively or negatively to the other acceptability questions appeared similar to those in the RCT, but no significant differences were seen here; we cannot rule out this being due to unknown confounding factors and the small size of sample.
| Patient experience and preference | Polypectomy | Difference or RR (95% CI);a p-value | Adjusted difference or RRb (95% CI); p-value | |
|---|---|---|---|---|
| Outpatient | Inpatient | |||
| Mean pain score during procedure (SD, n)c | 42 (26, 296) | – | – | – |
| Mean pain score 1 hour following procedure (SD, n)c | 27 (24, 247) | 20 (24, 60) | –7 (–14 to 0); 0.04 | –7 (–13 to 0); 0.05 |
| Mean pain score on discharge (SD, n)c | 22 (21, 276) | 13 (18, 57) | –9 (–15 to –3); 0.003 | –9 (–15 to –3); 0.003 |
| Procedure acceptable? | ||||
| n = 299 | n = 64 | |||
| Totally | 194 (65%) | 52 (81%) | ||
| Generally | 48 (16%) | 9 (14%) | 0.85 (0.79 to 0.92); 0.003d | Not possible to compute |
| Fairly | 50 (17%) | 3 (5%) | ||
| Unacceptable | 7 (2%) | 0 (–) | ||
| Exposure embarrassing? | ||||
| n = 302 | n = 60 | 1.29 (0.66 to 2.55); 0.45e | 1.28 (0.65 to 2.50); 0.45e | |
| Extremely | 5 (2%) | 2 (3%) | ||
| Moderately | 30 (10%) | 7 (12%) | ||
| A little | 90 (30%) | 8 (13%) | ||
| No | 177 (59%) | 43 (72%) | ||
| Recommend to a friend? | ||||
| Yes/total | 282/302 (93%) | 62/64 (97%) | 0.96 (0.91 to 1.02); 0.28 | Not possible to compute |
| Same treatment again? | ||||
| Yes/total | 283/300 (94%) | 62/63 (98%) | 0.96 (0.92 to 1.00); 0.18 | Not possible to compute |
| Preferred alternative treatment? | ||||
| Yes/total | 36/299 (12%) | 10/63 (16%) | 1.32 (0.69 to 2.51); 0.41 | 1.36 (0.72 to 2.56); 0.35 |
Other outcomes
Similar numbers of women reported taking additional medical treatments for their bleeding during the 6 months of follow-up, with 13% (38/291) and 10% (6/58) using the LNG-IUS in the outpatient and inpatient groups, respectively (Table 31).
| Medical treatment | Polypectomy | |
|---|---|---|
| Outpatient (n = 291) | Inpatient (n = 58) | |
| Mirena | 38 (13%) | 6 (10%) |
| Tranexamic acid | 7 (2%) | 3 (5%) |
| Mefenamic acid (Ponstan, Pfizer) | 5 (2%) | 3 (5%) |
| HRT | 1 (< 1%) | 1 (2%) |
| Progestogens | 1 (< 1%) | – |
| Norethisterone (Utovlan®, Pfizer) | – | 1 (2%) |
| Local oestrogen cream | – | 1 (2%) |
| Thyroxine | – | 1 (2%) |
| ‘Natural’ progesterone cream | 1 (< 1%) | – |
| Total no. of treatments | 53 | 16 |
| Total no. of women | 53 | 14 a |
In total, within the 6-month follow-up period, 22 women in the outpatient group (7%) and three in the inpatient group (4%) had at least one further polyp removed (RR 1.7, 95% CI 0.5 to 5.5; p = 0.5) (see Figure 13). These figures appeared similar to the RCT. The total number of women undergoing operations other than polyp removal was higher with outpatient treatment group (similar to the RCT) but this difference was not statistically significant (RR 1.8, 95% CI 0.6 to 5.7; p = 0.3) (Table 32). Family practitioner and hospital use owing to bleeding also appeared similar (Table 33).
| Type of surgery | Polypectomy | |
|---|---|---|
| Outpatient | Inpatient | |
| Hysteroscopy | 9/293 (3%) | 2/58 (3%) |
| Hysterectomy | 9/293 (3%) | 1/58 (2%) |
| Endometrial ablation | 3/293 (1%) | 0 (–) |
| Any other | 6/293 (2%)a | 0 (–) |
| Any gynaecological surgery | 27/293 (9%) | 3/58 (5%) |
| Resource use | Polypectomy | |
|---|---|---|
| Outpatient | Inpatient | |
| Outpatient clinic due to bleeding | 19/292 (7%) | 4/58 (7%) |
| Hospital ward day case due to bleeding | 3/293 (1%) | 2/58 (3%) |
| Hospital overnight due to bleeding | 1/293 (< 1%) | 0 (–) |
| GP surgery due to bleeding | 31/293 (11%) | 7/58 (12%) |
| GP at home due to bleeding | 0 (–) | 0 (–) |
| Days off work due to bleeding | 11/293 (4%) | 1/58 (2%) |
| Hospital for any other reasons (not bleeding related) | 18/292 (6%) | 1/58 (2%) |
| Gynaecologist for any other reason (not surgery or necessarily bleeding related) | 22/291 (8%) | 3/58 (5%) |
Discussion of the preference study
Principal findings
Forty-four per cent of the 906 women agreeing to participate in the OPT study expressed a strong preference for a particular treatment setting and were unwilling to be randomised. Of these women, 81% (324/399) expressed a preference for outpatient treatment. The results of the preference study demonstrate that there was no difference between outpatient and inpatient polypectomy for the successful alleviation of AUB at 6 months. Over 80% of women reported successful symptomatic treatment regardless of the type of AUB. The duration and amount of bleeding were significantly reduced following both outpatient and inpatient treatment, and no differences were identified according to treatment preference. Similarly, a significant improvement in generic and disease-specific HRQL was seen following polypectomy in both treatment groups. Rates of re-referral to a gynaecologist were similar between treatment groups, as was the need for further medical or gynaecological operative intervention.
The preference study estimated that outpatient polypectomy was successfully completed in 90% (282/312) of women and did not demonstrate any increased likelihood of failure. However, this is likely to be due to unknown confounders and the small sample size because an increased likelihood of failure with outpatient polypectomy was seen in the RCT. Possible explanations for this are the selection of more motivated women and technically less challenging polyps to remove in the outpatient setting. It is also important to note that the power to detect a difference between groups is much smaller in the preference study than in the RCT because the groups are unequal; the inpatient group in the preference study being only 30% of the size of the corresponding group in the RCT. Both outpatient and inpatient treatment times were short, with a mean treatment time of 10 minutes in both groups. Perioperative pain scores for outpatient treatment were similar in the preference study to those observed in the RCT. As with the RCT, mean pain scores at 1 hour following the procedure and upon discharge had diminished but were higher in the outpatient polypectomy group than in the inpatient group, albeit differences were small at low levels of mean pain intensity. Consistent with observations from the RCT, 2% (7/299) of women found outpatient-based treatment to be unacceptable and there was a significant trend towards reduced acceptability with outpatient as opposed to inpatient therapy. However, no differences were observed between groups in the proportion of women who would recommend the procedure to a friend, undergo the same treatment again or have preferred an alternative treatment (again, we cannot rule out this being due to the small sample size).
Outpatient treatment appeared to be safe and in keeping with findings from the RCT; the most common operative complication was self-limiting vasovagal fainting episodes, which occurred in around 1 in 18 (6%, 17/302) women. The majority of polypectomies were conducted hysteroscopically, i.e. polyp detachment under direct vision, but, as observed in the randomised study, this was done significantly more often in the outpatient group than in the inpatient treatment preference group [82% (246/299) vs. 56% (36/64), respectively). Most polyps were retrieved under direct hysteroscopic vision in the outpatient setting, whereas the converse was true for inpatient therapy, which used traditional blind mechanical extraction in 69% (43/63) of cases.
Strengths and limitations of the study
The baseline characteristics of participants – type of bleeding, site, number and type of polyp – were similar to the RCT, apart from a slightly older population. The strengths of our preference study include its size, the multicentre design, the inclusion of participants who were ethnically representative of the UK population, the relatively low rates of loss to follow-up, and the tailoring of assessment of outcomes to the primary complaint. Selection bias inherent in the observational design precludes reliable comparison between treatment groups in contrast with the RCT; however, our analysis did make statistical adjustment for the obvious confounding factors (type of bleeding, site and type of polyp). In contrast with the RCT, failure of complete polyp removal was no different between treatment settings. The overall level of failure over both groups was lower. This finding may reflect the selection of more favourable, better motivated women and technically ‘less challenging’ polyps to surgically remove as an outpatient. There remained a trend towards reduced acceptability with outpatient treatment despite women exerting a preference for this setting, although other measures of acceptability were non-significant in contrast with the RCT, but this may reflect the smaller sample or other unknown confounders. The 4 : 1 preference for outpatient over inpatient resulted in a smaller inpatient cohort for comparison and, as a result, estimates in this group of the study are imprecise. Additional limitations of our preference study include variation in the practice of outpatient polypectomy and a small number of participants failing to get their chosen treatment.
Implications for practice
Outpatient polyp treatment appears to be safe, feasible, acceptable and effective for the treatment of HMB, IMB and PMB in women expressing a preference for such treatment. At present most NHS hospitals are unable to provide operative OPH. However, our study has shown that, where such services exist, and women are offered a choice, most recognise the potential benefits of outpatient treatment. Immediate ‘see and treat’ polypectomy was offered in 63% (196/312) of outpatient treatments. Thus, there is evidence on both clinical and patient preference grounds, to support prioritising the provision of ambulatory gynaecology services, such as outpatient hysteroscopic polypectomy within NHS hospital- and community-based environments. When providing women with a choice for treatment of this common condition, it is imperative that they should be informed that the experience of outpatient intervention could be associated with more pain and reduced acceptability than scheduled inpatient approaches to treatment under general anaesthesia. This information should be tempered by the finding that at least 8 out of 10 women find the outpatient procedure to be totally or generally acceptable. Although this preference study did not demonstrate any difference between technical treatment failures, this is likely to reflect the influence of selection bias and women should be informed of the increased risk of failure, as observed in the RCT, when making their decision as regards treatment setting.
Conclusions
This observational study has shown that the majority of women expressing a treatment preference choose to have outpatient treatment of uterine polyps associated with AUB symptoms. Although the data derived from this observational study are subject to selection bias, the clinical outcomes for all categories of AUB are in keeping with the higher-quality data obtained from the parallel RCT. The current situation of limited and eclectic provision of outpatient or ‘ambulatory’ gynaecological therapeutic gynaecological services for AUB is untenable and needs urgently addressing, based upon the findings of the OPT project. The preferences expressed by women in this study and the current patchy provision of such services imply that within most NHS hospitals women are being denied a choice of treatment setting. Consequently, many women are being subjected to the inconvenience and greater burden of inpatient hospital treatment, which if offered an alternative outpatient treatment option they could avoid.
Chapter 5 Economic analysis of the randomised controlled trial
Introduction
This chapter reports the economic evaluation carried out alongside the OPT Trial. The primary objective of the study was to determine whether outpatient removal of uterine polyps was non-inferior to inpatient polypectomy, which is the standard treatment offered by the NHS. The primary end point was patient-reported improvement in bleeding symptoms at 6 and 12 months.
The aim of this economic evaluation was to determine the cost-effectiveness of OPT compared with standard inpatient treatment for endometrial polypectomy. For the purpose of this evaluation, we compared individuals who were randomised to receive inpatient polypectomy with those who were randomised to treatment in the outpatient department. Patients who had a strong preference for a given treatment setting (i.e. either outpatient or inpatient) were followed up in the preference cohort of the trial. Data from the preference cohort were not included in the economic analysis.
The base-case economic evaluation adopted the perspective of the NHS and took the form of cost-effectiveness and cost–utility analyses (CUAs). Costs were explored from the societal perspective as a part of the sensitivity analyses. The outcomes of interest were patient self-assessment of treatment success at 6 and 12 months, respectively, for the cost-effectiveness analysis (CEA) and quality-adjusted life-years (QALYs) gained at 1 year for the CUA. The reporting of this analysis follows the Consolidated Health Economic Evaluation Reporting Standards (CHEERS). 136
Methods
All analyses were carried out using SAS 9.2, Stata 12® (StataCorp LP, College Station, TX, USA) and Microsoft Excel 2007® (Microsoft Corporation, Redmond, WA, USA). The costs and outcome measures that were incorporated into this economic analysis were collected prospectively during the OPT Trial using forms filled in at the time of polyp treatment and at discharge postoperatively. In addition, in order to explore the societal perspective on costs, the out-of-pocket expenses incurred by the patients when attending for appointments and private time costs, including loss of time from work, were also collected using a separate questionnaire that was administered to the patients at randomisation and again on the day of procedure (if these were not on the same day).
Costs
All costs in the analysis are in UK pounds (£), based on 2011–12 values. 137 An a priori decision was made to use two separate methods to estimate the costs in this analysis. The first method was to use the published standard sources of costs for NHS procedures [NHS reference costs 2011–12138 and Personal Social Services Resource Unit (PSSRU) Costs 2012],137 which was preferred for the base-case analysis as it ensured that the results would be generalisable to all centres in the UK. The second method was to estimate the costs of inpatient and outpatient polypectomy by estimating the costs of the individual components of these procedures (bottom-up costing). These costs were used in a sensitivity analysis to ensure not only the generalisability of the results to centres outside the UK, but also to ensure that the reference costs used in the base case were representative of the ‘real world’ costs of these procedures. Given that there was significant heterogeneity in the types of procedures carried out and in the range of equipment used (see Appendix 16), we estimated resource use and costs for these procedures as carried out at the Birmingham Women’s Hospital (shown in Appendices 20–22). Health and Community Health Services (HCHS) pay and price indices were used to inflate costs, where appropriate. 137
Unit costs (Table 34) were attached to the cumulative resource use in each treatment group in order to calculate costs in both arms of the trial. Outpatient and inpatient polypectomy costs were estimated as per the NHS reference costs138 (Tables 35 and 36). All patients recruited into the trial were assessed in an outpatient clinic and underwent an OPH at the clinic where the uterine polyps were diagnosed. Although most of the patients randomised to an outpatient procedure were treated on their initial visit, 28% were scheduled to attend their treatment in a second clinic. All patients randomised to inpatient were pre-assessed in a nurse-led clinic to ensure that they were suitable to receive general anaesthesia before being scheduled for their day-case inpatient procedure.
| Resource | Unit cost (£) | Details |
|---|---|---|
| Pre-assessment clinic | 118 | Non-consultant led; gynaecological clinic; first attendance; face to face |
| Outpatient clinic attendance | 146 | Gynaecology consultant led; first appointment, not admitted |
| GP clinic appointment | 43 | PSSRU unit costs 2011–12137 |
| Day-case admission cost | 673 | Unit price; day-case admissionb |
| Cost per day/night admission | 502 | Regular day/night admissionb |
| HDU admission | 868 | Adult critical care admission; one organ supported |
| Cost of outpatient procedure | 188 | Outpatient procedure costb |
| OPH | 197 | Outpatient procedure; diagnostic hysteroscopy; currency code: MA21Z |
| Total abdominal hysterectomy | 3288 | Elective inpatient upper genital tract procedure with no complications; currency code: MA07D |
| Excess bed stay after hysterectomy | 353 | Excess bed stay for procedures under MA07D |
| Blood transfusion | 125 | NHS Blood and Transplant summer 2011 prices |
| Resource | Cost (£) | Detailsb |
|---|---|---|
| Initial outpatient clinic | 146 | Consultant gynaecologist-led face-to-face clinic; non-admitted |
| Hysteroscopy | 197 | Initial OPH for diagnosis of polyps; MA21Z |
| Follow-up outpatient clinic | 112 | Gynaecology: follow-up appointment consultant led; face to face; no admission |
| Outpatient polypectomy procedure | 188 | Outpatient procedure; MA12Z |
| Total cost | 643 |
| Resource | Cost (£) | Detailsa |
|---|---|---|
| Initial outpatient clinic | 146 | Consultant gynaecologist-led, face-to-face clinic; non-admitted |
| Hysteroscopy | 197 | Initial OPH for diagnosis of polyps; MA21Z |
| Preoperative assessment clinic | 118 | Gynaecology: first appointment non-consultant led; face to face; no admission |
| Day-case polypectomy procedure | 995 | Day-case procedure; MA12Z |
| Total cost | 1456 |
Outcomes
Outcome data of interest within the trial were patient-reported effectiveness of the procedure and also QALY gains at 6 and 12 months. Within the OPT Trial, the woman’s own assessment of bleeding symptoms was used to establish if the treatment had been successful using a dichotomous outcome measure (see Chapter 2, Primary clinical outcome measure: treatment success). Those patients whose predominant complaint before the procedure was PMB or IMB were determined to have had a successful treatment if their bleeding stopped. In women with HMB, treatment was successful if the patient reported that their bleeding had returned to an acceptable level following the procedure.
All the patients in the trial were asked to complete EQ-5D-3L questionnaires at baseline, 6 months and 12 months, and the responses obtained were used as the basis for the CUA.
Assumptions
It was necessary to make the following pragmatic assumptions before the analysis could be carried out.
-
All of the centres involved in the trial were assumed to have the same expertise and to have followed similar protocols in the management of these patients.
-
Only the related events (REs) that occurred within 1 year of the procedure and were deemed relevant to the polypectomy were included in the analysis. Related events included immediate complications of the procedure, all hysterectomies (irrespective of the indication), endometrial ablation and hysteroscopy/endometrial biopsy procedures within this time frame. Costs relating to further polyp-related procedures, if any, were also estimated and stated separately to the REs.
-
The costs of procurement of the different hysteroscope camera systems used by participating centres in this study [Versascope® (Gynecare, Ethicon, Somerville, NJ, USA); Olympus 5-mm rigid hysteroscope (Olympus, Southend-on-Sea, UK) Storz Bettocchi hysteroscope (Karl Storz – Endoskope, Tuttlingen, Germany] were assumed to be the same (see Appendix 20).
-
The costs obtained from the manufacturers of this equipment varied by around £1000.
Analysis
Cost-effectiveness analysis was carried out at 6 and 12 months, based on the outcomes expressed in natural units, which were estimated using responses obtained from patients to the question regarding effectiveness of the procedure. The results are expressed in terms of cost per additional patient whose symptoms improved with the procedure at 6 months and 12 months, respectively. A CUA was also carried out at 6 and 12 months and the results are expressed as additional cost incurred per QALY gained. Quality-of-life estimates were derived from EQ-5D responses provided by patients at baseline, 6 and 12 months by applying the standard UK tariff values. These estimates were then used to calculate total QALYs over 6 and 12 months for every individual in the study, using standard methods. 139
The presentation of results in QALYs allows comparison of the results with other available and recently published studies. 139 A range of one-way sensitivity analyses were carried out to explore the robustness of the base-case results to plausible variations during the uptake of these procedures in routine NHS use. In addition, a probabilistic sensitivity analysis (PSA) of the base case was carried out to enable the simultaneous exploration of the uncertainties in the cost and outcome data. The results of these analyses are presented in terms of incremental cost-effectiveness ratios (ICERs), which reflect the additional cost per additional outcome of interest of outpatient treatment compared with inpatient treatment. The results of the CUA are presented using cost-effectiveness acceptability curves (CEACs) to reflect sampling variation and uncertainties in the appropriate threshold cost-effectiveness value.
The analysis took the perspective of the NHS following the current recommendation from National Institute for Health and Care Excellence (NICE). 139 A wider societal perspective was also estimated using the patients self-reported out-of-pocket costs and is discussed within the sensitivity analysis within this report. As the time frame of this economic evaluation is only 1 year, discounting was not necessary. Also, given that there was no significant difference in the baseline EQ-5D scores (see Table 5), a baseline adjustment was not performed during the analysis.
Base-case analysis
We carried out two main analyses on the trial data. The base-case analysis was based on the ITT principle. A secondary analysis as per the treatment received by these patients irrespective of randomisation (the ‘PP’ analysis) was also carried out. Deterministic and PSAs were carried out for both of these methods.
Intention to treat
The base-case analysis estimated costs and outcomes as per ITT. In this method, patients are compared within treatment groups to which they were originally randomised irrespective of the treatment received. 140 This method of analysis allows the estimates to follow real-life scenarios in which patients may not always receive the planned treatment. Not using ITT analysis can often exaggerate the benefits of a given intervention, and it is widely acknowledged as the least biased of analysis options. 141
Per-protocol analysis
Within the OPT Trial, equal numbers of patients were randomised to either outpatient or inpatient polypectomy. However, 13% of those randomised to inpatient and 6% of those randomised to outpatient group received the ‘wrong’ treatment within the trial (i.e. outpatient treatment when randomised to inpatient and vice versa). Furthermore, 23 patients did not receive any treatment following randomisation (see Figure 8).
A PP analysis was carried out to look at the effect of treatment received on the outcome estimates. Therefore, in this analysis all patients who received outpatient treatment were compared with those who received inpatient polypectomy irrespective of the treatment to which they were randomised. Those who did not receive any treatment were disregarded for the purposes of this analysis.
Missing data
Within the trial, resource-use and outcome data could not be collected on all randomised patients (reasons outlined in the CONSORT flow chart; see Figure 8). Multiple imputation, a statistical technique that retains overall population variability and the relationship between observations, is considered useful only when > 10% data are missing. 142 Therefore, when > 10% of data were missing within the trial, these were treated as missing at random (MAR) and estimated using MCMC multiple imputation method. In this method we produced five simulated, complete versions of the data set using Stata 12 software. Each of these simulated data sets was analysed using standard methods and the results combined so as to produce estimates and CIs that incorporate missing data uncertainty. Given that the proportion of missing data was > 10% at most of the data analysis points (Tables 37 and 38), multiple imputation was carried out at all data points. The results obtained are summarised in Table 39.
| Cost detaila | Outpatient (£) | Inpatient (£) |
|---|---|---|
| General | ||
| n = 254 | n = 253 | |
| Baseline costs | 343 | 343 |
| Cost of procedure | 280.6 (253.1) | 992.5 (280.6) |
| Total procedure cost | 623.6 (253.1) | 1335.5 (331.5) |
| Cost of related REb (0–6 months) | 116.9 (638.7) | 99.5 (546.1) |
| Cost of related REb (0–12 months) | 156.4 (737.3) | 138.5 (656.3) |
| Between 0 and 6 months | ||
| n = 231 (9%)a | n = 212 (16.2%)a | |
| Cost of additional GP visits | 7.4 (23.9) | 5.5 (17.1) |
| Cost of additional hospital visits | 8.7 (42.2) | 4.2 (21.4) |
| Cost of admissions | 17.4 (103.2) | 11.8 (90.7) |
| Further procedures for polyps | 17.8 (116.3) | 12.0 (98.7) |
| Other related gynaecological procedures | 41.9 (308.0) | 20.9 (227.7) |
| Total additional costs at 6 months (excluding REb) | 93.2 (363.3) | 54.5 (274.6) |
| Overall cost at 6 months | 847.6 (870.9) | 1529.2 (689.8) |
| Between 6 and 12 months | ||
| n = 228 (10.2%)a | n = 219 (13.4%)a | |
| Cost of additional GP visits | 7.4 (25.9) | 5.1 (16.7) |
| Cost of additional hospital visits | 10.3 (44.0) | 10.2 (38.8) |
| Cost of admissions | 17.6 (92.6) | 6.9 (58.5) |
| Further procedures for polyps | 34.8 (173.7) | 18.1 (119.1) |
| Other related gynaecological procedures | 38.0 (309.1) | 19.5 (223.8) |
| Total additional costs for 6–12 months (excluding REb) | 108.2 (434.1) | 59.8 (297.5) |
| Overall cost at 12 months | 978.7 (1060.1) | 1634.8 (835.1) |
| ITT analysis | Outpatient, n = 254 | Inpatient, n = 253 |
|---|---|---|
| Effectiveness | ||
| 6 months (mean; SD) | 0.73; 0.45 (10.2) | 0.80; 0.40 (16.6) |
| 12 months (mean; SD) | 0.81; 0.39 (11.4) | 0.83; 0.38 (15.4) |
| EQ-5D | ||
| Baseline (mean; SD) | 0.78; 0.25 (4.7) | 0.79; 0.27 (8.3) |
| 6 months (mean; SD) | 0.87; 0.22 (9.5) | 0.87; 0.2 (16.6) |
| 12 months (mean; SD) | 0.86; 0.25 (10.6) | 0.86; 0.24 (13.4) |
| ITT analysis | Outpatient,a n = 254 | Inpatient,a n = 253 | Differenceb |
|---|---|---|---|
| 6 months | |||
| Overall cost | 822.1 (832.3) | 1481.6 (680.5) | –659.5 [65.7] |
| Overall QALY | 0.41 (0.09) | 0.41 (0.09) | –0.0006 [0.01] |
| Patient-reported effectiveness | 0.74 (0.44) | 0.81 (0.39) | –0.07 [0.04] |
| ICER (Δ cost/Δ effectiveness) | £9421 per extra patient who feels better with inpatient treatment | ||
| ICER (Δ cost/Δ QALY) | £1,099,167 per QALY gained on the inpatient arm | ||
| 12 months | |||
| Overall cost | 937.6 (971.4) | 1606.3 (861.5) | –668.8 [82.9] |
| Overall QALY | 0.83 (0.19) | 0.84 (0.18) | –0.001 [0.02] |
| Patient-reported effectiveness | 0.82 (0.39) | 0.85 (0.36) | –0.03 [0.31] |
| ICER (Δ cost/Δ effectiveness) | £22,293 per additional patient who feels better with inpatient treatment | ||
| ICER (Δ cost/Δ QALY) | £668,800 per additional QALY gained on the inpatient arm | ||
Skewed data
As the majority of cost and outcome data are skewed, normal parametric methods cannot be used to calculate the differences in means and, subsequently, the ICER. Bootstrapping is a non-parametric approach that can be used to compare arithmetic means without making any assumptions regarding the sampling distributions. In this analysis, 3000 bootstrapping replications were undertaken in order to calculate the 95% CIs around the differences in mean costs and outcomes.
Sensitivity analysis
We carried out both deterministic and PSAs to explore data uncertainty during this economic evaluation.
Deterministic sensitivity analysis (DSA) was carried out to assess the uncertainty associated with input parameters for both analyses. This technique estimates the effect of changing a single parameter (i.e. either cost or effectiveness) on the overall ICER obtained. The point estimates used for all the other parameters remain unchanged. Within the current analysis, the following four options were considered.
Using bottom-up costs for outpatient and inpatient polypectomy (SAE1)
In the initial analyses, we considered only costs that were stipulated in the NHS reference costs. 138 Although these costs are representative of the expenses incurred in the UK, in order to make the results more generalisable we calculated the costs of the procedures by breaking them down to the individual components involved and then adding up all of the costs to obtain an overall cost (i.e. bottom-up costing) and repeated the analyses.
Given the range of drugs and resources used in the trial (see Appendix 16), proportional costs were estimated for local anaesthetic agents used in the outpatient treatment arm (see Appendix 17). Similarly, costs for general anaesthetic agents and equipment were estimated [General anaesthesia (drug costs), see Appendices 18 and 19]. For this analysis, it was assumed that the equipment used during polypectomy was similar for both outpatients and inpatients (see Appendix 20). The estimated ‘bottom-up ‘costs for the outpatient and inpatient procedures are outlined in Appendices 21 and 22, respectively.
Considering the out-of-pocket costs incurred by patients for the treatment (societal perspective) (DSA-2)
As the procedure is a short one and is not expected to result in long-standing illness or sickness absence from work, the human capital approach method was used to estimate the societal costs of inpatient polypectomy compared with outpatient polypectomy. For those patients (and companions) who were in paid employment, the average hourly wage was calculated using the Office for National Statistics (ONS) estimates for the whole UK economy143 and an estimated 38.5 hours of work per week. Estimates used for leisure activities and household work were 42% and 57% of the net wage rate. These were derived from published literature. 144 The cost of looking after relatives was assumed to be the same as that of household work (57% of net hourly wage). When patients had taken paid time off work, these costs would have been incurred by the employers and were therefore included in the analysis. Those who were not involved in housework and were not in active employment (e.g. university students, retired persons) were assumed to incur the same costs as those for leisure activities.
Private travel costs were derived from the Automobile Association (AA). 145 These costs include total standing and running costs (fuel, parking, tolls, depreciation, wear and tear, tyre replacement, servicing, etc.) and depend upon the annual mileage and type of car. 145 For this analysis, it was assumed that the car was a petrol vehicle of middle cost range (£13,000–18,000 for a new car) and averaged about 8430 miles annually. 146
The cost of using public transport and car parking fees (where relevant) were directly obtained from the patients. When time and mileage data were missing, these were assumed to be the same as the average for the entire group. The details of the costs used are shown in Table 40.
| Resource | Cost (£) | Source | |
|---|---|---|---|
| Gross hourly wage | Patient | 12.75 | ONS hourly age for women; August 2013 |
| Companion | 14.75 | ONS average hourly wage; August 2013 | |
| Leisure activity | 5.36 | 42% of gross hourly wage | |
| House work | 7.27 | 57% of gross hourly wage | |
| Cost of private travel (per mile) | 0.59 | AA (AA.com, 2013145) | |
Effect of ‘see and treat’ clinics (DSA-3a and DSA-3b)
Most clinicians who participated in the OPT Trial agreed that one of the advantages of outpatient treatment would be that patients may be reviewed, diagnosed and treated at their first visit. Indeed, a majority of patients included in the outpatient arm (72%) had their polypectomy performed on the day of randomisation. We re-analysed the data assuming 100% and 0% compliance with this rule (i.e. assuming that all patients and no patients were treated on the day of randomisation – DSA-3 and DSA-4, respectively).
Using new tariffs for outpatient polypectomy (DSA-4)
The bottom-up costs for polypectomy are higher than the current NHS tariffs, particularly for outpatient polypectomy (see Appendix 21). There has been some debate about the lack of incentive for ‘see and treat’ clinics, given that the current remuneration is quite low. Therefore, a higher tariff for outpatient polypectomy (at £1000) is being proposed. We have used these costs in a sensitivity analysis to predict the effect of increased charges on the cost-effectiveness of this procedure.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis uses random numbers to generate multiple possible estimates of cost and outcomes from within the probability distributions (i.e. the Monte Carlo principle). A total of 5000 simulations were carried out using the Monte Carlo principle. The distribution around each parameter was specified by its baseline estimate and a bound (upper or lower) of the estimated 95% CI. The advantage of this method is that all parameter uncertainties can be incorporated simultaneously into the analysis. The result of this analysis allowed the generation of a CEAC that demonstrates the probability of an intervention being cost-effective at different willingness-to-pay thresholds.
Results
The results for the base-case (ITT) analysis are presented above (see Table 39). For the base case, we estimated that the point estimates of the mean costs incurred at 6 months on the outpatient and inpatient arm were £822 and £1482 respectively, a cost difference of £660. At the end of 12 months, the costs increased slightly to £938 and £1606 respectively, a cost difference of £669. The results obtained by the PP analysis are outlined in (see Appendix 23). Inpatient costs remained higher than outpatient in the PP analysis (see Appendix 24) at 6 and 12 months, although these were slightly higher than those in the ITT analysis. The differences in cost at 6 and 12 months with the PP analysis were £923 and £941, respectively (see Appendix 25).
The point estimates for mean QALYs in the inpatient and the outpatient groups at 6 months were almost equal in both the ITT and PP analyses. At 12 months, the QALY estimates were slightly different at 12 months in both analyses (see Table 39 and Appendix 25). The point estimates for difference in QALY were 0.0006 and 0.001 at 6 and 12 months, respectively, in the base-case (ITT) analysis. In the PP analysis, QALY estimates for inpatients were slightly higher at 6 months, whereas at 12 months the point estimates for QALY were higher for outpatients (see Appendix 25). The proportion of patients who reported improvement in symptoms following polypectomy were 0.74 and 0.81 at 6 months and 0.82 and 0.85 at 12 months in the outpatient and inpatient arms, respectively, as per the ITT analysis (after imputing for missing data). The corresponding values for difference in effectiveness at 6 and 12 months were 0.07 and 0.03. In the PP analysis, the point estimates for patient-reported effectiveness of inpatient therapy was higher than outpatient therapy at 6 months. However, at 12 months, the point estimate for outpatient treatment was slightly higher. Thus at 6 months in the ITT analysis, we estimated that it cost an extra £9421 per patient who felt better with inpatient treatment and £1,099,167 per additional QALY gained on the inpatient arm (see Table 39). At 12 months, these costs were £22,293 per additional effectively treated patient and £668,800 per additional QALY gained, respectively.
Similar results were obtained using the PP analysis (see Appendix 25), although outpatient treatment dominated inpatient treatment (i.e. it is less expensive while being more effective) at 12 months.
Deterministic sensitivity analysis
Holding the outcome data constant, DSAs were carried out by changing the cost data. As shown in Table 41, inpatient polypectomy remained more expensive than outpatient treatment in all of the scenarios considered. The ICERs for DSA-1 to DSA-3 are similar to those obtained by the base-case analysis, and somewhat lower when the new tariffs are applied for outpatient treatment (DSA-4). The DSA results obtained by PP analyses (see Appendix 26) are also similar to those obtained for the base case. Therefore, we can surmise that the results obtained by this analysis are not sensitive to plausible changes in the costs of these procedures.
| ITT analysis | DSA-1 (bottom-up costs) | DSA-2 (out-of-pocket costs) | DSA-3a (‘see and treat’ at same appointment for all outpatients) | DSA-3b (‘see then treat’ for all outpatients) | DSA-4 (new outpatient tariffs) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 months | 12 months | 6 months | 12 months | 6 months | 12 months | 6 months | 12 months | 6 months | 12 months | |
| Cost difference:a mean difference (standard error of the difference) | –719.8 (68.8) | –664.2 (83.2) | –671.7 (66.2) | –618.6 (84.3) | –673.5 (66.8) | –620.4 (80.0) | –584.5 (65.7) | –531.4 (79.0) | –294.1 (63.0) | –241.0 (76.2) |
| ICERb | 10,282.9 | 22,140 | 9595.7 | 20,620 | 9621.4 | 20,680 | 8350 | 17,713.3 | 4201.4 | 8033.3 |
| Cost/QALY | 1,199,666.7 | 664,200 | 1,119,500 | 618,600 | 1,122,500 | 620,400 | 974,166.7 | 531,400 | 490,166.7 | 241,000 |
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis showed that the likelihood of outpatient polypectomy being effective (when EQ-5D estimates are used as outcome of interest) is similar to that of inpatient treatment as per the ITT analysis (Figures 21 and 22) clearly demonstrate that although inpatient treatment is definitely more expensive than the outpatient treatment, the difference in effectiveness is more uncertain. The mean cost differences between the groups as per the PSA were £661 and £658 at 6 and 12 months, respectively (the inpatient costs are higher). The corresponding values for QALY difference were 0.0002 and 0.0005, respectively, at 6 and 12 months (the inpatient QALY gain is higher). This suggests that the effects of the treatment on costs and QALYs beyond 6 months, as expected, are minimal.
FIGURE 21.
Probabilistic sensitivity analysis: outpatient vs. inpatient treatment – ITT analysis at 6 months.
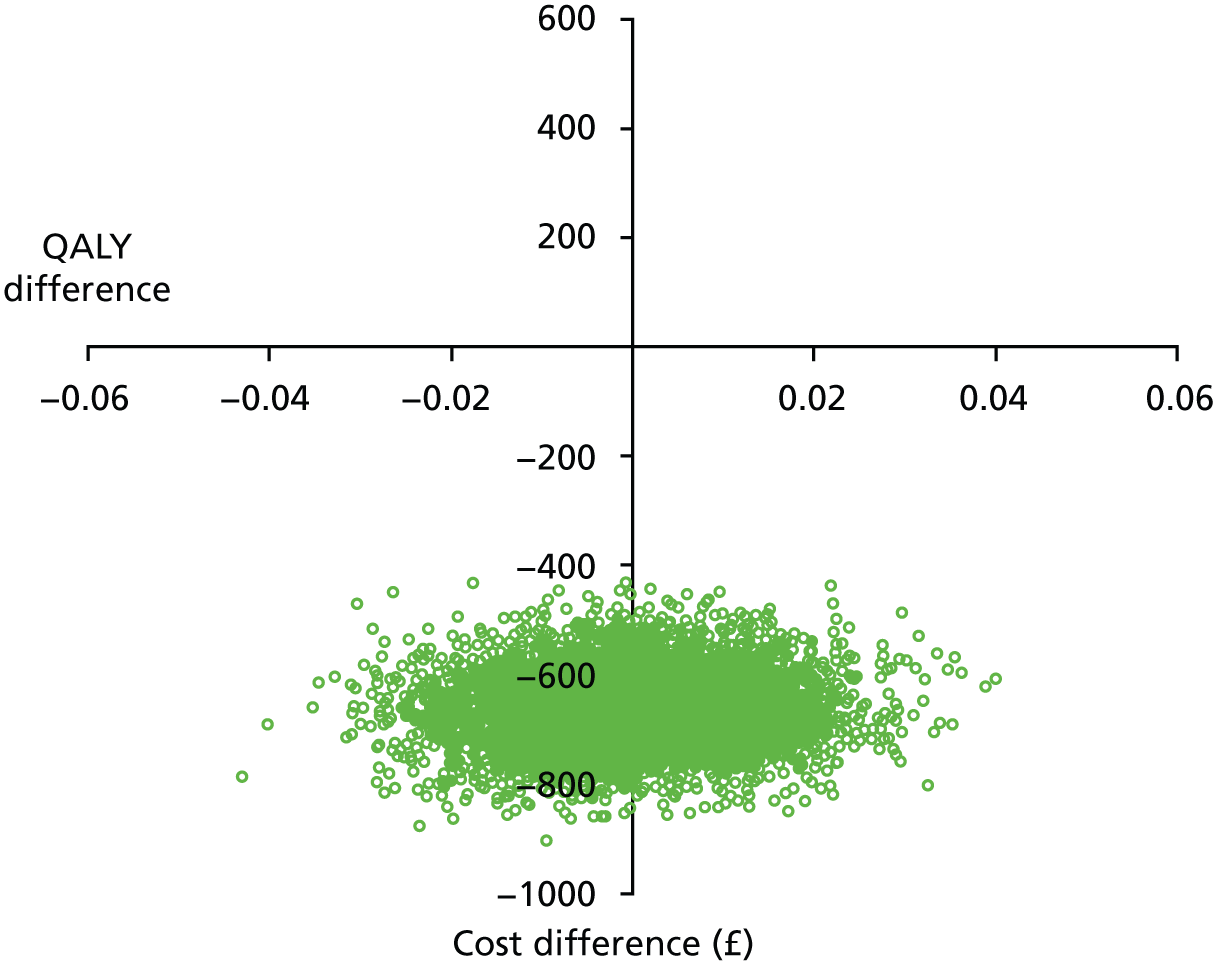
FIGURE 22.
Probabilistic sensitivity analysis: outpatient vs. inpatient treatment – ITT analysis at 12 months.
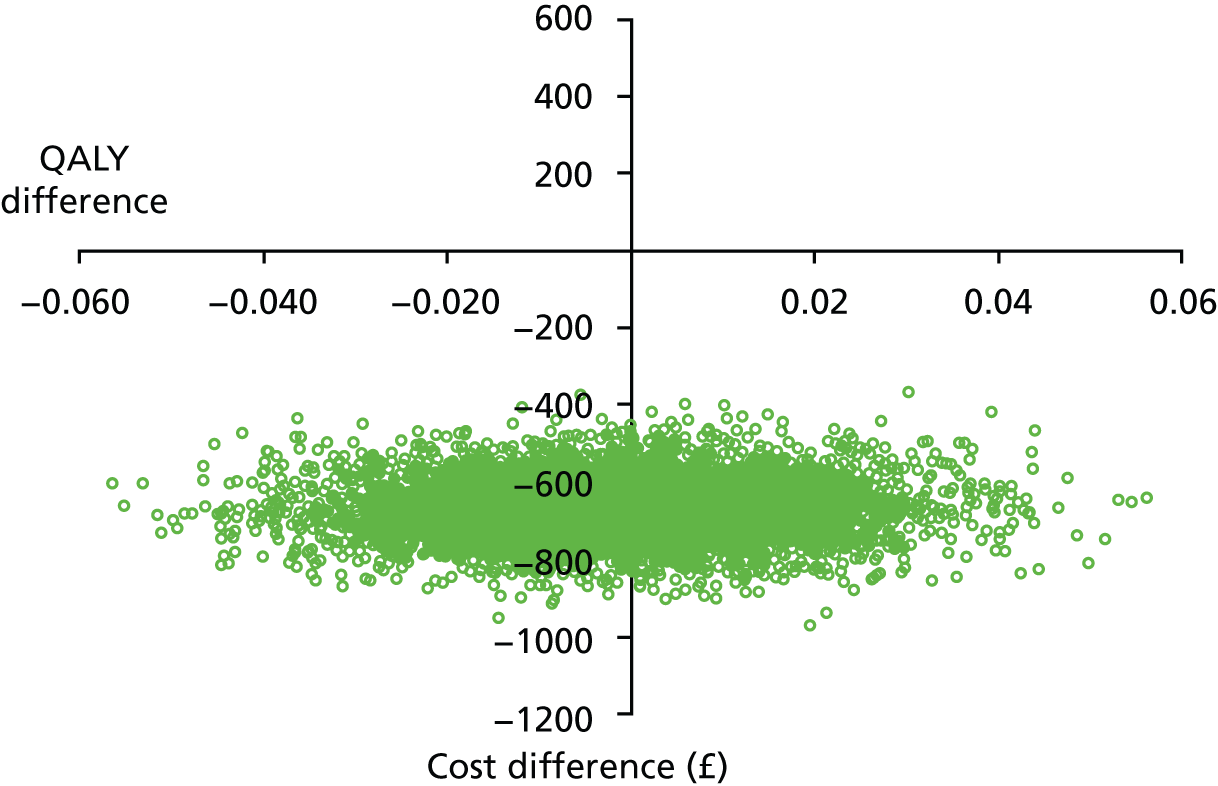
The CEACs shown in Figure 23 demonstrate that given that both treatments are equally effective, the cheaper treatment will be the preferred procedure at lower willingness-to-pay (WTP) thresholds. Indeed, inpatient and outpatient therapy start to become equally cost-effective only at a WTP threshold of £90,000. A similar picture was seen when PSA was carried out using the PP data. The results of this analysis are shown in Appendices 27 and 28. The CEAC derived from the PP analysis is shown in Appendix 29.
FIGURE 23.
Cost effectiveness acceptability curve outpatient vs. inpatient treatment: ITT analysis.
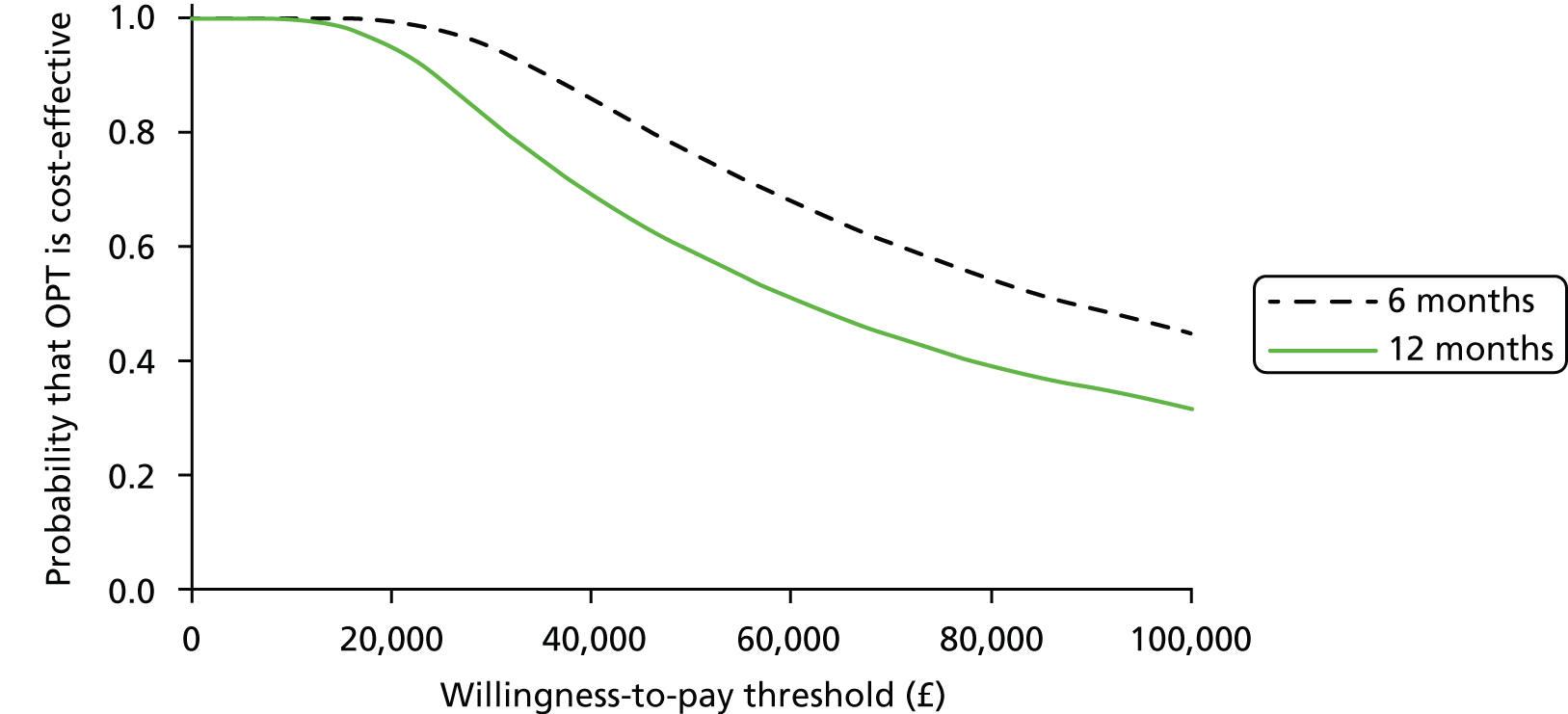
Figures 21 and 22 show PSA, which simultaneously represents uncertainty in cost and QALY values. The x- and y-axes represent the incremental effectiveness and cost of outpatient treatment compared with inpatient treatment respectively. These figures represent the results for ITT analysis at 6 and 12 months. Most of the values fall in the lower-right and lower-left quadrants, demonstrating that inpatient therapy is more expensive than outpatient treatment. The equal distribution between the left and right quadrants suggests that there is considerable uncertainty regarding the effectiveness of one treatment over the other (based on QALY values). In other words, the effectiveness of both treatments is similar.
Figure 23 shows a CEAC illustrating the uncertainty around the cost-effectiveness estimates by demonstrating the likelihood of an intervention being cost-effective at a given cost threshold compared with the proposed alternative. In this case, given that the likelihood of effectiveness of both treatments is roughly the same, the cheaper treatment is considered most cost-effective at baseline.
Discussion
Principal findings
We found that inpatient polypectomy was more expensive than outpatient treatment and marginally more effective, resulting in slightly higher point estimates of self-reported effectiveness and QALY values at 6 and 12 months. The differences in costs and outcomes between these procedures were fairly constant at these time points, reflecting that the treatment has very little long term (i.e. beyond 6 months) implications on health and resource use. The ICERs obtained by cost-effectiveness and CUA were very high, reflecting the equivalence in effectiveness between these procedures. Although the mean estimates of outcomes appear to favour inpatient treatment, it is important to note that there was considerable uncertainty around these point estimates. This was explored further using PSA, clearly demonstrating that although outpatient therapy is definitely cheaper than inpatient treatment, the effectiveness estimates are uncertain, with the likelihood of effectiveness being roughly equal in both groups at 6 and 12 months.
A range of cost variations, which were considered plausible during the implementation of these treatment pathways within the NHS, were considered. However, these did not make a difference to the conclusions from the base-case analysis. It is notable that the bottom-up costs estimated for outpatient and inpatient procedure during this analysis were quite close to the tariffs from the NHS reference costs138 (see Appendices 21 and 22).
Although this was a non-inferiority trial and there was equivalence in effectiveness while costs were different between the groups (the so-called ‘weak dominance’ situation as postulated by Drummond et al. 147), a cost-minimisation analysis was not considered to be appropriate, as this would not provide adequate information regarding the uncertainty in the estimates. This is better estimated by carrying out a full cost-effectiveness and PSAs. 148
Strengths and limitations of the study
The OPT study is the largest randomised prospective study to estimate the costs and effectiveness of inpatient treatment compared with outpatient treatment for uterine polyps in women with AUB. Economic data in this study were collected prospectively alongside clinical outcome data, enabling accurate estimation of the costs and outcomes. In addition, robust techniques were used to account for data uncertainty, missing data and the skewness of data. Although the main analysis is mainly relevant to the UK, the sensitivity analyses enhance the generalisability of the findings of the study.
It was assumed for the purposes of this analysis that all of the participating UK centres had similar expertise with regards to inpatient and outpatient treatments, but it is possible that centres where outpatient therapy has been offered on the NHS for many years perform the procedure better than those with relatively inexperienced clinicians. In the interests of simplicity and clarity, the economic analysis did not explore the differences in costs and outcomes in subgroups of patients considered within the trial (e.g. premenopausal vs. postmenopausal, different age groups, etc.). The clinical effectiveness results for these groups were roughly similar and it was, therefore, felt that no further value could be added by extending the analysis. It should be noted that training costs were not included in this analysis. However, diagnostic hysteroscopy is now routinely performed in the outpatient setting and given this familiarity with the technique, performing an outpatient polypectomy may need relatively little further training. In addition, it is envisaged that all current clinical trainees (and future consultants) may have acquired the skills necessary for this procedure during their training.
This is the first study to estimate prospectively and compare the costs and outcomes of inpatient treatment with outpatient treatment for this condition. 147 A previous analysis was carried out in this area and a CEA was performed but this was a single-centre retrospective audit that included only a small number of patients. 147
Implications for practice
Outpatient polypectomy is a cost-effective procedure and should be recommended as best use of limited NHS resources in patients with AUB due to uterine polyps.
Conclusion
Outpatient polyp treatment was less expensive than traditional inpatient treatment and similarly effective, resulting in slightly lower self-reported effectiveness and QALY values at 6 and 12 months. The differences in costs and outcomes between these procedures were fairly constant at these time points, reflecting that the treatment has very few longer-term implications on health and resource use. The ICERs for inpatient compared with outpatient treatment obtained by cost-effectiveness and CUAs were very high, reflecting the equivalence in effectiveness between these procedures. Sensitivity analyses clearly demonstrated that although outpatient therapy was definitely cheaper than inpatient treatment, there was uncertainty around the effectiveness estimates implying the effectiveness of the two alternatives was broadly similar. Thus, outpatient polypectomy appears to be more cost-effective relative to the current inpatient approaches for polypectomy at current acceptable WTP thresholds for the NHS.
Chapter 6 Patient acceptability and experience of outpatient polyp treatment
Introduction
The qualitative study was undertaken in order to aid interpretation and understanding of the questionnaire data on acceptability of the procedure and to gain insight into women’s experience of undergoing both groups of the trial. Qualitative research is particularly useful when the aim is to understand people’s motivations and experience in the context of their lives through the generation of in-depth data. In a study on the use of qualitative research in urogynaecology, Doshani et al. 149 argued that qualitative research can both inform and complement quantitative research in a number of ways, one of which is the clarification of quantitative findings. Qualitative data also add context to the results by placing them within a woman’s life experience and allowing for individual impact of symptoms or treatments to be considered.
In this research we aimed to assess patient acceptability of both the inpatient and outpatient groups of the OPT Trial using qualitative interviews with a purposive sample of women. We also aimed to explore the motivation of women for agreeing or refusing to take part in the trial.
Little research has been conducted into women’s experience of hysteroscopy, and only one study150 has used a qualitative approach – a study that reported women’s views of outpatient diagnostic hysteroscopy recruited from women who were undergoing the procedure for irregular bleeding or increased endometrial thickness on ultrasound. Of the 29 participants only two said they would prefer to have the hysteroscopy performed under general anaesthetic in the future, one citing pain and the other feeling that it was more convenient to have any problems that were discovered dealt with at the same time as the diagnostic procedure. Other women interviewed spoke of their dislike of anaesthetics, and the convenience of being discharged immediately as motivating factors in their preference for outpatient treatment. Reduced anxiety was associated with having the procedure at the initial clinic appointment, and the psychosocial aspects of care by doctors and nurses was considered to contribute to this. Gupta et al. 120 also studied patient anxiety at a ‘one-stop’ hysteroscopy clinic, using the 20-item State-Trait Anxiety Inventory with 240 women. This found that women attending the ‘one-stop’ clinic had higher anxiety levels than those attending general gynaecology clinics, and also higher levels than those awaiting major surgery. The authors suggest that this may be attributable to expectations that increasingly invasive diagnostic and therapeutic procedures may be carried out in outpatient clinics, but also point out that the presenting disorder may also be a relevant factor as chronic pelvic pain clinics are also associated with high anxiety levels. 120
Literature on participation in clinical trials is largely concerned with serious or complex conditions, such as cancer, and is mainly undertaken from a quantitative perspective. 151 Qualitative work in the field tends to focus on how people make sense of the research study in which they are engaged, their participation within it, and how they manage the tensions within their role as participant. 152 A study that explored the rationale for taking part in a qualitative study on diabetes service provision found that four main themes emerged: recruitment within health contexts (‘the nurse said it would help’), altruism (‘if it can help somebody’), qualitative research being seen as inherently innocuous (‘nothing to lose’) and therapeutic aspects of interviewing (‘getting it off my chest’). 151 A more recent study examined women’s motivation for participating in a trial of a prototype instrumentation with potential for diagnosing breast cancer. Similar themes emerged, which the authors labelled ‘gift and exchange’ relationships; a prescience alignment; and narratives of health care. 152 Altruism is also a feature of research motivation in quantitative research on participation, although research in clinical trials also demonstrates a level of self-interest in that people believe (rightly or wrongly) that they will receive better care by participating. 153
In summary, previous research is limited and suggests that women prefer to have gynaecological procedures carried out under LA, although this is associated with high anxiety levels. Motivation for participation in RCTs is focused around a mixture of altruism and self-interest.
Methodology
The research aim was addressed by undertaking qualitative interviews with a sample of women in each group of the trial in order to collect more in-depth data on both the process and the views of women about their experience than could be collected by quantitative questionnaires. Interviewing allowed women to introduce areas of discussion that were important to them at a time when the experience was still recent but when they had also had the opportunity for reflection.
Sample
The initial intention was to interview a 10% sample of women from the inpatient and outpatient groups of the study, which would give a sample size of 24 patients from each group, although the basic rule of data saturation would have been applied. That is recruitment would have stopped for each group when no new data were being generated on the issues identified as important to many of the women.
This sampling strategy changed when it was realised that a significant number of women were stating a treatment preference, and also that some women who were randomised or stated a preference for outpatient treatment were not treated immediately. It was felt by the team that these factors could affect notions of acceptability, and so recruitment was stratified to include all of these groups. This resulted in greater outpatient than inpatient numbers. Preference patient recruitment was also weighted towards outpatient as 324 of 399 in this cohort chose to be treated as an outpatient.
The final sample (41 women) is detailed in Table 42.
| Randomised patients = 28 (total recruited 507) | ||
|---|---|---|
| Inpatients | 12 | |
| Outpatients | See and treat | 11 |
| Waiting list | 5 | |
| Preference patients = 13 (total recruited 399) | ||
| Inpatients | 4 | |
| Outpatients | See and treat | 6 |
| Waiting list | 3 | |
Women were recruited to the qualitative study during the random allocation process of the trial. They were informed that they had been allocated for interview and consent was taken by the research nurse at the centre. They were then telephoned by one of the qualitative research team to arrange a suitable time for interview, and to answer any questions. Women were recruited from most of the trial sites.
Method
To ascertain issues of importance to women before the main study took place a focus group was conducted with women who had undergone outpatient hysteroscopy in the previous 2 years. Although six women were due to take part in the focus group, only three attended, but it still proved to be a useful exercise, as important issues were raised around pain levels and information received about the procedure, which were included in the interview schedules.
Semistructured interviews were conducted by phone to include a wide range of research sites from across the country in the sample. In a study comparing telephone with face-to-face interviews it was found that telephone interviews tended to be of shorter duration because less detail was given in answers by participants. 154 However, it was acknowledged by the author that there may be good reasons for using telephone interviews, and in the current study the multicentred nature of the trial lead to the adoption of this approach. Interviews took place around 1 week following treatment and took between 15 and 30 minutes. All were recorded with permission. The time interval was chosen in order that women would be able to reflect on the experience with minimum retention bias and to reduce the chance of being influenced in their responses by gratitude to doctors and other hospital staff. Slightly different schedules were developed for randomised and preference patients with questions focusing on the motivation to take part in the research or to choose their treatment method, and their experience of it. Women were also encouraged to raise issues of concern to them that were not asked by the researchers. Interview tapes were transcribed verbatim. To ensure content and face validity, the interview schedule was reviewed after the first three interviews. 155
Data analysis
Data were analysed using thematic analysis, which is used to identify, analyse and report patterns within the data. 156 A staged approach to analysis was undertaken. In the first instance, transcripts were read and data were analysed independently by each researcher, with the subsequent generation of codes and initial themes. The process for carrying out this task was decided individually but involved intensive reading of each transcript, looking for consistency, contradictions and recurring themes. Next the two researchers met to compare codes and themes, and to decide on final themes which reflected the data. Third, an independent qualitative researcher sampled the transcripts and agreed the themes as consistent with the data. In an additional phase to the thematic analysis model used, we also explored inconsistencies within the data. Achieving consensus within the team constituted a form of verification. As the qualitative literature on the subject of hysteroscopy was sparse, verification through embedding this research within it was not possible.
Findings
Most women from all of the categories identified were satisfied with their treatment and with the care they received from staff. There were, however, some differences within the sample, and some minority views.
Characteristics of sample
Women who were recruited to the study suffered from PMB (n = 22), IMB (n = 10) or HMB (n = 9). Women who experienced PMB tended to seek medical advice immediately and were referred to a gynaecologist by their GP without initial investigations or treatment. Those whose main complaint was IMB or HMB were more likely to adopt a ‘wait and see’ approach until symptoms persisted or worsened, and some of these women were sent for scans or treated with medication to reduce bleeding before being referred by their GP.
Participation in a randomised trial
The women who agreed to be randomised all had similar reasons for taking part and these are consistent with previous research. 152 Altruistic reasons around helping other women were common, although a few women also personalised this with comments such as ‘my daughter may need this in the future’ (1388*). Other women gave more abstract reasons to do with research more generally. One randomised inpatient said:
I tend to think that any research for anything is a good thing, and we can only go forward with doing the research.
1116
Two women had concerns about both treatment options, so were happy to have the decision taken for them.
I wasn’t too happy to be put to sleep because basically I’m always sick when I wake up. But then if you look at it the other way I wouldn’t be too happy if they hurt me. So they took the choice from me, so that’s good.
1340
Having been randomised the majority of women declared themselves happy with the group to which they had been assigned. A few women did not really understand the randomisation process, and one thought that the computer was deciding on a treatment, based on clinical factors.
The preference patients had more individual reasons for choosing one treatment option over the other. Most women choosing outpatient treatment wanted it over and done with in one hospital visit, and even although for one of these women the procedure could not be completed she still thought it was the right choice. She commented:
[The doctor] tried her best and it just wasn’t to be. It wasn’t anyone’s fault, it’s just the way it is.
5134
Of the other women, two had a fear of anaesthetics, one had a pre-existing medical condition, one had children to make arrangements for, and one did not want to take time off work. Very few women in the study chose inpatient treatment and the four interviewed for the qualitative study all spoke of a previous bad experience of hysteroscopy or other procedures under local anaesthetic, or embarrassment at being in stirrups, which made them want a general anaesthetic.
In both groups of the study a number of women told of how they had consulted friends and family before attending the clinic, or the nurse in the clinic, in order to make a decision about the procedure, but this had to be weighed up against their personal feelings. For example one woman randomised to outpatient treatment said:
I had a lot of reservations about the procedure because people had told me how painful it was, but I don’t react very well to anaesthetics so for me I was glad it went the way it did.
1019
(*Patient trial no.)
Experience of the procedure
Women were asked about their care during the procedure and whether/how their dignity was maintained. This was an area of importance for women, particularly those in the outpatient group, and, as reported above, it was a factor in the decision of one woman to opt for inpatient treatment.
Most women in the outpatient groups of the trial, whether randomised or preference, were pleased to get the procedure over in one hospital visit, and some expressed surprise that it could all be done without them having to come back. However, those outpatients who had to return for treatment were also satisfied with the process, as there is a general expectation of health services that an initial appointment is followed by a separate appointment for treatment, so they viewed it as normal practice.
Only four women in the outpatient groups reported feeling embarrassed at the physical position they were in and some likened it to childbirth in terms of being exposed in front of strangers. However, most women recognised that there was no alternative, and were resigned to some embarrassment. As one woman commented:
I’ve had four children. It is more undignified than anything else. It’s a bit embarrassing, but it’s got to be done.
1002
The majority of women felt that the staff did as much as possible to maintain their dignity during the procedure, and appreciated having one nurse at their head to talk them through it or to take their mind off it, which may explain the low numbers of women feeling embarrassed during the procedure. There was almost unanimous praise for the sensitive way that the nurses and doctors treated them, putting them at ease and explaining what was happening, and it was implied or directly stated that this influenced their overall satisfaction. A few women felt that the number of people in the room, particularly the presence of staff not directly involved in caring for them, such as medical students or other doctors, added to their embarrassment, and one woman felt that the staff were uncaring.
Most women feel that they were given sufficient time to recover following the procedure, although a small minority felt pressured to leave. Postoperative recovery was generally quick in this group, unless a lot of pain was experienced, which will be discussed below.
For inpatients having a general anaesthetic, dignity was not such an issue, even although they knew that they would be exposed in theatre. Two women felt that they were kept waiting for too long for the operation, as they had been starved, but otherwise all of the women were satisfied with their care. Postoperatively, women in this group reported more fatigue, and took longer to recover as they had had a general anaesthetic, but none reported this as a major drawback. One woman was concerned that no doctor came to the ward to discharge her but, again, this may be to do with patient expectations of how health services operate rather than an actual need to see a doctor. One woman felt that she would have benefitted from staying in hospital overnight (she had a small child) but all of the other inpatients were pleased to go home soon after the procedure.
Pain
Women were all asked about any pain that they experienced during and after the procedure and this was the common factor for all within both groups of the trial, although there was quite a diversity of responses. Pain can be divided into that experienced during the procedure, immediately after and over the following few days.
Obviously pain during the procedure was experienced only by women in the outpatient group. It is noted though that for a small group of patients in the preference group the request for a general anaesthetic was because they reported a very painful previous experience during the OPH. Reports from women in the outpatient group ranged from ‘mild discomfort’ to ‘excruciating pain’ but was reported as difficult to cope with by nearly one-third of all participants treated as outpatients. The preference patients reported less pain than the randomised ones, using descriptions such as ‘uncomfortable’, ‘bearable’ and ‘better than expected’. As one woman described it:
I geared myself up for it being really painful, you know extremely painful cramps, but I haven’t had any of that, touch wood. Actually it was a really good process.
5279
This group also reported little postoperative pain, describing it like wind or period pain, although one woman did get a sharp pain after 2 days, which her GP attributed to thrush.
Randomised patients on the other hand described a wide range of pain experiences both during and after the procedure. Most women who were randomised to an outpatient procedure expected it to be painful, and that was the reality in all but two of the cases. They spoke of cramping pains, a few said that it hurt more than they were expecting and one described it as ‘excruciating’ but for the most part women were quite stoical. They commented that ‘it had to be done’ or ‘it was over in 10 minutes’ and spoke of the trade-off between an element of pain and a quick and convenient procedure. One woman rationalised the amount of pain by her own attitude, stating that she was always very tense during internal examinations and could not relax during the procedure, which had to be abandoned.
I can’t say that’s anything to do with what they were doing, I think a lot was due to me not relaxing properly.
1350
A minority of women felt little or no discomfort during the procedure.
There was a fair bit of oohing and aahing but I am not sure you would call it painful.
1002
I was expecting it to be uncomfortable and it wasn’t. I was almost waiting for something to happen when he said ‘that’s it’.
1029
After the procedure, some women found that the pain disappeared fairly quickly, usually within 2 hours, but others reported that it lasted for a few hours or in four cases even a couple of days, and one woman felt it was worse after the procedure than during it. The main description of pain at this time was that it was crampy, or like a period pain, and was generally short lived. Some women, but not all, had been warned that they may feel like this following the procedure.
Those randomised to inpatient treatment reported postoperative fatigue rather than pain, and tended to experience less postoperative pain than outpatients.
In summary, most women felt some pain either during the procedure (outpatients) and/or in the hours or days following. This was generally a crampy pain that was described as bearable, but for some women it was more uncomfortable. For women randomised to outpatient treatment this was felt to be worth the convenience of a quick procedure but some participants expressed the opinion that the level of pain experienced might be difficult for all women to cope with. Preference patients tended to report less pain overall than those who were randomised.
Information needs
All women should have received the trial patient information sheet before attending the clinic. Some women denied having this and others admitted to not reading it, which affected the level of knowledge of the two treatments. However some of these reported that clinic staff gave detailed information to allow them to make an informed decision on whether to take part.
I think that every point was explained clearly and when I asked questions they gave me the clear answer and support and so I was happy with everything.
1105
Despite receiving information, not all women understood the randomisation process. Three ‘preference’ outpatients thought that they were taking part in the trial, and two said that they did not take part in the trial because they did not understand it.
Most of those women who did agree to be randomised knew what this involved and what the trial groups were. One, however, thought that the computer made the treatment decision on the basis of clinical factors; another that the consultant decided; and there were a few other misconceptions. One woman who was randomised to inpatient treatment did not know that outpatient treatment was carried out without anaesthetic, to which she would not have agreed, as she found the diagnostic hysteroscopy so painful.
Most women were happy with the information that they received preoperatively but there were two suggestions for additional information. One woman said that it should be made clear that after treatment patients would not be able to drive and so should bring someone with them; another that you should be given some idea of how long you would be in the hospital, because of car parking, care responsibilities, etc. Two women who were starved for long periods before inpatient treatment felt that the length of time for starving should be more closely aligned to the operation time.
Three women wanted more specific information regarding pain relief for those having outpatient treatment. This amounted to recommending specific painkillers for the type of pain likely to be experienced (e.g. ibuprofen) and a time to take it for it to be most effective during the procedure.
Women who had inpatient procedures were more likely to feel that they had received adequate information on discharge than those who were outpatients, possibly because they received general discharge information. The type of information that preference and randomised outpatients would like to have had focused on two issues: what to expect in terms of recovery and when they could resume normal activities. Women did not know whether postoperative pain or bleeding was normal and, if so, how long it should last before they should seek medical help. They were also unsure whether to consult the hospital or their GP and felt that a contact for problems could be provided.
Resuming normal activities revolved around issues, such as going back to work, driving and resuming sexual activity.
Most women assumed that the polyp was benign and that once it was taken out that was the end of the matter, but three wondered about the histology and what would happen to the result. Two women reported having had previous gynaecological cancer and were understandably more anxious about receiving a result from the histology but women were generally unaware whether it would be sent to them or not.
Differences between inpatient and outpatient experience
The main difference in experience was that outpatients reported some pain and embarrassment during the procedure, and inpatients had some level of fatigue from the anaesthetic. Only the inpatients reported returning to work before they were really ready but this was an issue for only two patients. Inpatients also had different ideas over the level of medical care they should receive, expecting a doctor to discharge them and follow-up appointments. As discussed above this may be because of expectations of the health care system and being admitted to hospital.
I understand how busy the consultants are but it would have been nice if the consultant or one of the team had come down prior to us going home.
5258
Two inpatients commented on the length of the day, as they were required to be admitted early morning for an afternoon operation. Conversely, outpatients often commented on how quickly they were seen and the procedure carried out, and were pleasantly surprised.
None of the inpatients commented on having to attend an appointment for a hysteroscopy and return for removal of the polyps, but two women who opted for inpatient treatment did cite a painful hysteroscopy as a rationale for their choice.
Women in the outpatient groups balanced the pain of the procedure with the convenience of the ‘one-stop shop’, and often set that within the context of their lives. So, for example, one woman described herself as a workaholic who could not stand the idea of taking sick leave. One visit to the clinic and no anaesthetic allowed her to go back to work the next day. Another woman commented that she did not want to explain details of her health to her employer, and a short appointment allowed her to maintain her privacy. Many women in the outpatient groups felt that the pain and embarrassment of the procedure was compensated by not having to return to the hospital or take time off work and the financial cost that these would incur. Avoiding a general anaesthetic was also a positive aspect, particularly for those who had had previous poor experience of this or who had a complicating medical condition, such as one woman who had epilepsy.
When comparing treatments, another woman randomised to outpatient commented:
[with inpatient treatment] I have to make a lot of arrangements for my daughter and everything so I was quite happy with this.
1098
Three women thought that if they had been asked to go home after the hysteroscopy and return at a later date they may not have done, which they thought was an advantage of a ‘one-stop clinic’. One likened it to the dentist telling you that you need a tooth extracting.
You know that if you go home you won’t come back.
1029
Differences between randomised and preference patients
The rationale for choosing to enter the trial varied, with randomised patients expressing altruistic reasons around helping others or generalised views of the benefits of research, whereas preference patients tended to have more individualised reasons for their choice.
Minority experiences
Two women, both allocated to outpatient treatment, had a negative experience that they found to be very traumatic and which is worth reporting, even although they were a small minority. The first woman had undergone removal of polyps in the past under general anaesthetic but was happy to take part in the trial. She expected some pain during the procedure, as friends had told her it ‘wasn’t very nice’ (1329). However, she described what she went through as horrendous, and something she would never go through again.
It got to the point I think my body went into shock. I was shaking, you know, I couldn’t keep my legs still. They were trying to take my mind off it asking what I considered stupid questions at the time. Obviously they were doing a job and it got to a point where I just couldn’t speak I was in so much pain. I think one of the nurses realised and said are you able to cope and the doctor just said ‘oh, she’ll be alright’.
1329
Later in the interview, she expressed the view that the outpatient treatment should not be offered at all:
It’s torture, it’s barbaric and it shouldn’t be allowed to be done.
1329
This woman’s procedure was completed. However, in the case of the second woman who reported extreme pain and shaking, saying that she was in shock, the procedure was terminated in favour of a later inpatient procedure. This patient also reports that she was in pain and bleeding heavily for several days and would never agree to a similar procedure being done without anaesthetic again. She commented that she had not realised that it would be a one-stop procedure, even although she had agreed to be randomised. She thought that after the hysteroscopy the procedure would be over and she would return for an anaesthetic, as she was in pain from the start. Like the woman above, she describes being in shock and shaking badly due to the pain, but, unlike the first woman, she commented that the clinic staff tried to support and relax her.
Satisfaction with the procedure
There was a high level of satisfaction from the women interviewed, from both groups of the study and from randomised and preference patients.
Women who had outpatient treatment largely felt that the procedure was talked through and they knew what was happening, for most a nurse was with them either explaining or talking to take their mind off it. Women appreciated the presence of a nurse throughout the procedure who was not involved in their clinical care to hold their hand and reassure them. This was a very positive aspect of the study and greatly appreciated by patients. Only one patient (discussed in the minority experiences above) felt that she was not treated with care and empathy.
All appreciated the fact that it was over quickly and they could get back to normal almost immediately.
The fact that it takes 5 minutes and it can be done there and then seems to make perfect sense really.
1029
Although some women did find it painful, this was expected and did not alter their overall satisfaction. One woman randomised to outpatient treatment summed up the views of the majority:
I suppose it was painful but it was more like they explained it a crampy period type pain for the first few hours after, but it wasn’t too bad at all, not like I was expecting . . . I was quite pleased the way it all went and I did go back to work the next day, so I don’t think you can hope for much more than that really.
1019
Women who were admitted for general anaesthetic were also satisfied with their treatment, apart from the long wait experienced by two women. Those who had a lot of pain during the hysteroscopy generally felt pleased to be randomised to inpatient treatment, and it was the reason for two preference patients’ decisions. As with the outpatient group, these women felt that the care they received was good.
Discussion
Principal findings
The most obvious finding of the study was that women expressed satisfaction with their treatment, whatever their preference for inpatient or outpatient treatment, and whether or not they received it. For most of the women their symptoms were an inconvenience rather than a major disruption to their lives, something that they wanted dealing with quickly, and a ‘see and treat’ intervention could be seen as a proportionate response to the problem. The value was that it fitted into their lives, and is consistent with the immediacy and convenience of other aspects of modern living, such as internet banking and 24-hour shopping. A number of women spoke of the advantage of outpatient treatment as being discharged quickly and getting back to normal. However, women who were admitted in a two-stage procedure, whether for outpatient or inpatient treatment, also expressed satisfaction, and for seven women (four preference and three randomised) the idea of invasive treatment while awake was not acceptable, and therefore the option of general anaesthetic is still needed, even although this would seem to be a minority choice. However, this overall finding of satisfaction with the processes and procedures must be weighed against the levels of pain and discomfort experienced by a significant number of the women taking part.
In contrast with findings by Gupta et al. 120 in this study few women expressed feeling anxious preoperatively, although many spoke of expecting some pain during the procedure. This may be because this study was retrospective, whereas Gupta et al. 120 administered the State–Trait Anxiety Inventory pre procedure. It is also possible that as medical procedures are increasingly being carried out on an outpatient basis there is more familiarity and acceptance of them by patients, and indeed some women had previous experience of this for polyps and other conditions.
One finding that is hard to explain is that ‘preference’ patients reported less pain than ‘randomised’ patients. This may be an artefact of a small sample size, or preference patients wanting to justify their choice. There may also be a difference between a population that agrees to be randomised in research and that which does not, and it is important to obtain data from both, particularly in research such as this for which there was a large preference group.
Strengths and limitations of the study
No previous study has used a qualitative methodology to explore women’s experience of OPH, and to compare inpatient and outpatient experience. Undertaking a qualitative study within the OPT Trial has provided an additional perspective on being in a medical trial and has confirmed the overall satisfaction reported in the rating scales. It has also allowed for an expansion of the two minority experiences that women reported. The advantage of the approach was that we were able to explore issues that women raised of concern to them rather than the researcher-defined issues, thereby supplementing quantitative quality-of-life data with more in-depth exploration providing contextualised views. In addition, timing the semistructured interviews one week following treatment provided more perspective to women on their overall treatment.
The main limitations of the study were using telephone interviews, and not being able to obtain respondent validation – issues that may be linked. The disadvantages of telephone interviews have been discussed above, but in a multicentre study the choices are to restrict interviewing to a group of study sites in close proximity or to use the geographical range and to interview by telephone. We made the decision to try to recruit women from a range of centres from across the country, as we considered this to be more reflective of the whole study. Finally, as no previous qualitative study has been conducted on women’s experience of OPH, it was not possible to verify our findings by embedding them within existing literature.
Implications for practice
-
In view of the level of pain and distress experienced by some women, the development of ‘see and treat’ clinics and the option of an anaesthetic should be available if this is patient choice.
-
Patients need information about taking pain relief before the procedure, and the most appropriate analgesia to take. Top-up analgesia should be available during the procedure if necessary.
-
A dedicated rest area could be made available for patients who find the procedure painful and distressing.
-
The presence of a support nurse who is not part of the team carrying out the procedure was unanimously appreciated and should be considered good practice.
-
An information leaflet needs to contain information about what to expect from the procedure (quotes from previous patients are useful), pain relief, who will receive biopsy results, what to expect post procedure in terms of pain and bleeding, and returning to normal activities, work, resuming sexual activity) and who to contact if complications occur.
-
Further qualitative research should be conducted into the experience of undergoing OPH and similar gynaecological procedures.
Conclusion
The value of a qualitative study within a RCT is that it provides an additional type of data, and allows participants the opportunity to raise issues of importance to them, rather than those set by the research team. This research compared two treatment settings and procedures for a condition, uterine polyps, which causes inconvenience rather than major disruption to life. As such for most women a service that offers a timely and sensitive treatment, with a quick return to normal life is the optimum choice. For the majority of women in this study this was provided by ‘see and treat’, but for a minority this was not an acceptable option.
Chapter 7 Discussion
Abnormal uterine bleeding is the commonest presentation in gynaecological practice affecting women of all ages. Symptoms have a significant adverse impact upon HRQL. The high disease burden associated with HMB, IMB and PMB places substantial demands upon health services, utilising scarce health-care resources. Uterine polyps are focal endometrial lesions and, along with fibroids, are the commonest uterine pathologies encountered in association with AUB, and their removal appears to alleviate bleeding symptoms as well as provide tissue for histological assessment. Uterine polyps are being increasingly diagnosed with the advent of better imaging modalities, including the gold standard diagnostic test of OPH, a test that has become widely available within the NHS in recent years with the establishment of ambulatory gynaecological services. In contrast with most uterine fibroids, polyps are amenable to minimally invasive treatment, being easily accessible from within the uterine cavity such that they can be extracted without the need for extensive surgery.
The conventional approach to uterine polypectomy involves scheduled admission to hospital so that formal theatre facilities can be used to conduct the operation under general anaesthesia. Advances in technology, including the development of miniature endoscopic equipment and enhanced imaging, has facilitated the notion of outpatient ‘outpatient-based’ therapeutic surgical interventions. The high prevalence and accessibility of uterine polyps, combined with relative simplicity of uterine polypectomy, has resulted in several hysteroscopy units developing routine outpatient polypectomy among other gynaecological procedures. However, most hospitals restrict OPH services to diagnosis, limiting the technique’s potential utility. The lack of consensus arises from a lack of high-quality evidence to support such service developments. Although it is generally recognised that outpatient intervention is convenient for patients, the avoidance of general anaesthesia means that uterine polypectomy may be very painful and unacceptable to many women. Furthermore, the limitations placed upon intrauterine surgery in the conscious patient, which include noxious pain stimuli and problems with access and manipulation of miniature hysteroscopic equipment, may translate into reduced feasibility and poorer clinical outcomes. However, even if the latter were proven, the advantages to women of outpatient intervention, in terms of safety, convenience and efficiency, may counterbalance any inferiority in clinical effectiveness. In addition to these considerations, the inflated cost of miniaturised technologically advanced equipment required for most outpatient procedures combined with the potential for poorer clinical outcomes may offset the efficiency of outpatient polypectomy even when it is performed immediately following diagnosis at OPH – the ‘see and treat’ approach. Thus, we identified a pressing need to evaluate the effectiveness, acceptability and cost-effectiveness of OPT.
Evidence of effectiveness
The results of this trial demonstrate that outpatient polypectomy was non-inferior to inpatient polypectomy for the successful alleviation of AUB when compared with our prespecified non-inferiority margin of 25%. Polypectomy successfully treated AUB in 73% (166/228) of the women in the outpatient group compared with 80% (168/211) in the inpatient group (equivalent to a NNTH of ‘6’ at the lower end of the 95% CI) at 6 months with no evidence of interaction of type of bleeding, location or type of polyp with treatment allocation.
The OPT study has shown that the outpatient surgical treatment of uterine polyps is non-inferior to traditional inpatient treatment under general anaesthesia for the successful alleviation of AUB. The removal of these focal pathologies was associated with symptomatic control in three-quarters of women at 6 months, and treatment outcomes were maintained at 12 and 24 months. The relative effectiveness of treatment was observed whether the presenting complaint was HMB, IMB or PMB. A significant improvement in generic and disease-specific HRQL was seen after polypectomy in both treatment groups at 6, 12 and 24 months, with no differences observed according to treatment setting.
Outpatient polypectomy was successfully completed in four out of five women, but failure to complete polyp removal was two and a half times more likely in the conscious patient than in traditional inpatient treatment, i.e. for every nine outpatient polypectomies performed an additional one procedure will fail compared with inpatient treatment. Technical failure to remove polyps in the outpatient setting was associated with patient discomfort. It is interesting to note that over the 2-year follow-up period, women treated in the outpatients were twice as likely to have at least one further polyp removal and 1.6 times more likely to have further gynaecological surgery. These observations may imply reduced effectiveness of outpatient compared with inpatient polypectomy. Alternatively, women undergoing treatment as an outpatient may have been more willing to seek referral and treatment for ongoing or new gynaecological problems because of the perceived convenience of their initial experience.
The most common perioperative side effect associated with outpatient polypectomy was induced fainting, i.e. vasovagal reactions arising from cervical stimulation. These affected 7% (17/241) of women but were short-lived and self-limiting. No major complications occurred in the outpatient setting but there were four uterine perforations reported in the inpatient general anaesthetic group, resulting in a pelvic haematoma requiring hospitalisation in one patient and an inadvertent bowel injury necessitating laparotomy, bowel resection and high-dependency unit care in another. Thus, although rare, the potential for trauma remains and the sequelae can be potentially life-threatening. It is for this reason that many recommend polypectomy under direct visualisation using hysteroscopic methods. 32,81,84,107,128 It is possible that the likelihood of unrecognised injury is greater where conventional, more traumatic blind techniques using larger mechanical equipment are adopted in an unconscious inpatient. Such approaches were observed much less frequently in the outpatient treatment group of the OPT Trial.
Evidence of cost-effectiveness
The OPT study showed inpatient polypectomy was more expensive than outpatient treatment and marginally more effective, resulting in slightly higher self-reported effectiveness and QALY values at 6 and 12 months. The differences in costs and outcomes between these procedures are fairly constant at these time points, reflecting that the treatment has very few long-term (i.e. beyond 6 months) implications on health and resource use. The ICERs obtained by cost-effectiveness and CUA were very high, reflecting the equivalence in effectiveness between these procedures.
Although the mean estimates of outcomes appeared to favour inpatient treatment, it is important to note that there was considerable uncertainty around these point estimates. This was explored further using PSA, clearly demonstrating that although outpatient therapy is definitely cheaper than inpatient treatment, the effectiveness estimates are uncertain with the likelihood of effectiveness being roughly equal at 6 and 12 months in both groups. A range of cost variations that were considered plausible during the implementation of these treatment pathways within the NHS were considered. However, these did not make a difference to the conclusions from the base-case analysis.
Outpatient polypectomy is a cost-effective procedure and should be recommended as best use of limited NHS resources in patients with AUB due to uterine polyps.
Evidence of acceptability
The OPT study showed that when women were willing to take part but had a preference for treatment, > 80% (324/299) chose to have the treatment awake as an outpatient. Perioperative pain scores were within the mid-range as measured on a 100-mm scale, consistent with other less invasive common gynaecological diagnostic tests, such as endometrial biopsy or hysteroscopy. Furthermore, outpatient procedures were short, with a mean recorded duration of 10 minutes. Although pain scores were slightly higher postoperatively and on discharge from hospital the clinical significance of this is negligible given that pain intensity was low in both groups, the recorded differences minimal and time to discharge was much quicker in the outpatient treatment group. Procedure acceptability was measured using quantitative and qualitative measures. When asked on a four-point ordinal scale regarding the degree of acceptability, there was a significant trend in favour of conventional inpatient treatment in both the randomised and observational preference studies. However, only 2% (5/225) of women in either group considered the treatment unacceptable. Similarly, although the proportion of women within the RCT willing to recommend their treatment or undergo it again favoured inpatient treatment under anaesthesia, at least 90% (200/223) of women having experienced outpatient polypectomy answered positively to these two questions.
Undertaking a qualitative study enabled us to look beyond the satisfaction data collected by the use of Likert scales and allowed for more nuanced responses. It also provided information on acceptability a week after treatment when reflection on the process and recovery time could be incorporated into their replies. Exploring acceptability and patient experience by semistructured interviews revealed that women valued expeditious treatment and saw the immediacy and convenience of ‘see and treat’ outpatient treatment as a proportionate response to resolving their problem. Women considered the rapid discharge and return to normal activities associated with outpatient treatment as an advantage. Most women expected to experience pain but few women expressed feeling anxious preoperatively. However, some women expressed the view that invasive treatment, although awake, was unacceptable for a minority of women, and choice of treatment setting should be available.
Implications for practice
Polyps are the commonest intrauterine pathology found in association with AUB in women of all ages. The findings from this trial showed that OPT is a safe, feasible, and acceptable setting to treat these focal lesions. Moreover, the effectiveness of outpatient surgical treatment to alleviate the common types of AUB – namely HMB, IMB and PMB – was not inferior to conventional inpatient therapy. The costs of outpatient polypectomy were substantially lower than inpatient treatment so that OPT was highly cost-effective in comparison. Thus, it is self-evident that the current situation – through which the majority of NHS providers of gynaecological services are unable to routinely offer women the choice of outpatient surgical treatment for symptomatic uterine polyps – is unsustainable. Diagnostic OPH facilities are widely available within most NHS hospitals. However, investment in endoscopic technologies including capital outlay for appropriate camera stacks, energy modalities and operative hysteroscopes would be necessary, and provision should be made for purchasing disposable ancillary devices and repairing or replacing relatively fragile, precision instrumentation. Therefore, some financial outlay is likely to be required and outpatient facilities may have capacity constraints but the cost-effectiveness of OPT compared with conventional inpatient approaches should influence future investment in this direction.
Contemporary health service development needs to take into account the views of patients; the demand for the outpatient setting as demonstrated in recruitment to the OPT preference study further supports the clinical and economic argument for change. In addition to developing modern diagnostic and therapeutic ‘ambulatory units’ within hospitals, providers should consider the possibility of setting up or expanding community-based services, which may be more convenient to service users and potentially more cost-effective. Additional training will be necessary for many practitioners to become competent in therapeutic procedures such as polypectomy, but given the familiarity to gynaecologists of diagnostic OPH and the relative simplicity of the procedure, proficiency in uterine polypectomy should be quickly acquired.
The safety and convenience of outpatient treatment and the equivalent effectiveness in alleviating AUB symptoms should be counterbalanced with (1) increased probability of failing to complete the procedure; (2) perioperative pain and (3) reduced acceptability in the conscious outpatient compared with the anaesthetised inpatient. Thus, women should be informed that outpatient intervention is quick but associated with pain of generally moderate intensity. They should be made aware that there is a trend to greater acceptability with inpatient treatment under anaesthesia, although the vast majority of women undergoing the outpatient intervention will consider it acceptable. Women should be offered a choice of treatment setting and practitioners need to recognise that a minority of women will prefer general anaesthesia regardless. We should not lose sight of the fact that technological advancement in instrumentation is ongoing and, indeed, technologies are now available that, for example, simultaneously cut and aspirate polyp tissue. This appears to lessen intraoperative times, reduce pain levels and enhance acceptability (P Smith and TJ Clark, Birmingham Women’s Hospital, 2013, personal communication). Other interventions designed to optimise the patient experience with particular regard to combating pain are under evaluation, such as the use of new topical anaesthetic agents and the role of intrauterine hysteroscopic injection of local anaesthetic. In addition to technical innovations, patient selection, i.e. better identification of women who are more likely to have an adverse experience, should further improve qualitative outcomes.
The results of this work should inform the consenting process so that contemporary written material and counselling is succinct, valid and relevant, and patients acquire realistic expectations of the likely outpatient treatment experience. The provision of timely written and verbal information is therefore of prime importance to allow women to make an informed choice, especially when ‘see and treat’ approaches to diagnosis and treatment are to be offered.
The desire for convenient and rapid outpatient interventions to resolve commonly encountered conditions that currently necessitate traditional inpatient surgery under general anaesthesia within formal operating theatre environments is unlikely to be restricted to AUB complaints in women. In gynaecology and other surgical disciplines, technology and patient expectations will drive the development of outpatient diagnostic and treatment services. Robust health technology assessments such as the OPT Trial should ensure that ambulatory intervention is not developed as a ‘cheap’ alternative to conventional practices but rather as a better way of managing common conditions in terms of effectiveness, convenience and acceptability.
Recommendations for research
Ongoing technological advances in surgical and endoscopic instrumentation are likely to further increase the feasibility and acceptability of ambulatory gynaecological interventions, including outpatient uterine polypectomy. Head-to-head RCTs should be conducted to delineate the optimal surgical approach and identify the best technologies in terms of feasibility, acceptability and effectiveness. Examples may include hysteroscopic morcellation compared with mechanical resection and electrical resection of uterine polyps, and evaluation of specimen retrieval methods, for example polyp snares compared with hysteroscopic graspers and morcellation.
The clinical significance of uterine polyps has been questioned. An estimated 10% of asymptomatic women are found to have uterine polyps. Most polyps are benign and many spontaneously regress. Although there are data compiled from systematic reviews of the published literature showing improvement in AUB symptoms following polypectomy, the primary data sources are generally small observational series. In the case of subfertility, data to support polypectomy to improve fertility outcomes are even scarcer. Thus a RCT comparing uterine polypectomy with expectant management may be warranted for the treatment of abnormal bleeding (stratified by bleeding pattern) and subfertility. Such studies should be designed with adequate assessment and surveillance procedures in the non-treatment groups and preliminary feasibility studies would be needed in light of previously reported problems with recruitment. The aetiology of uterine polyps is unclear but hormonal effects on the endometrium are likely to be involved. Thus, research comparing hormonal regulation/suppression of the endometrium on polyp regression, recurrence and associated bleeding symptoms should be considered, for example a RCT of uterine surgical polypectomy compared with the LNG-IUS in AUB. Prospective, long-term follow-up studies should aid a better understanding of the nature and natural history of uterine polyps in symptomatic and asymptomatic women; outcomes to assess could include polyp recurrence, symptomatic recurrence and risk of premalignancy/malignancy. Outpatient intrauterine surgery for many common, benign gynaecological conditions is limited by perioperative pain, overall patient tolerance and, ultimately, the acceptability of such interventions. Research into further refinements designed to examine and optimise patient experience is required. In addition to RCTs aimed at identifying the best surgical techniques, other trials should be designed to evaluate approaches to minimising pain and enhancing recovery and acceptability. Interventions including the use of targeted, local paracervical or intrauterine anaesthesia, variations in analgesic or sedative regimens and alterations in distension media delivery, for example temperature and pressure, should be undertaken. Examples may include warming saline distension media to body temperature compared with room temperature; vaginoscopic ‘no touch’ hysteroscopic surgery compared with instrumenting the lower genital tract; paracervical compared with intracervical and intrauterine local anaesthesia; inhalational conscious sedation (nitric oxide) compared with placebo; musical distraction compared with standard environment. Furthermore, observational studies are needed to identify clinical factors, for example patient characteristics, and anatomic, surgical and pathological indicators that are predictive of poor patient experience with outpatient surgery.
Inside gynaecological practice and other surgical disciplines, technology and patient expectations will drive the development of convenient and rapid outpatient interventions to resolve commonly encountered conditions that currently necessitate traditional inpatient surgery under general anaesthesia within formal operating theatre environments. Thus, further RCTs, similar to the OPT Trial, should be conducted to evaluate the effectiveness and cost-effectiveness of such practices, for example outpatient hysteroscopic sterilisation compared with day-case laparoscopic sterilisation (gynaecology), and outpatient varicose vein treatments compared with day-case varicose vein surgery (general surgery).
Acknowledgements
We thank all of the collaborating investigators who form part of the OPT collaborating group.
Barnsley District General Hospital, South Yorkshire: C Bonner, K Cannon, KA Farag, K Raychaudhuri, M Reid, A Zahid.
Birmingham Heartlands/Solihull Hospital, Birmingham: Jan Blunn, S Guruswami, S Irani, D Robinson.
Birmingham Women’s Hospital, Birmingham: T Bingham, TJ Clark, N Cooper, J Gupta, S Madari, D Mellers, S O’Connor, C O’Hara, M Pathak, V Preece, E Sangha, M Shehmar, P Trinham, A Wilson.
Bishop Auckland General Hospital, County Durham: J Dent, J Macdonald, P Sengupta.
Blackpool Victoria Hospital, Blackpool: C Brookes, J Davies.
Bradford Royal Infirmary, Bradford: M Jackson, S Jones, H Ludkin, S Riddiough.
Castle Hill Hospital, East Yorkshire: J Allen, T Cathcart, D Cox, S Ford, L Kenny, K Phillips, N Rawal, A Rodgers, J Siddiqui.
Chelsea & Westminster Hospital, London: A Dine-Atkinson, S Kalkur, G Merriner, J Ben-Nagi, A Raza, R Richardson.
City General Hospital, University Hospital of North Staffordshire, Stoke-on Trent: T Bingham, I Hassan.
City Hospital, Sunderland: D Edmundson, J Chamberlain, A Barge, P Wake, D Milford, E Walton.
Countess of Chester Hospital, Chester: S Arnold, M Blake, MJ McCormack, N Naddad, J Hane, S Wood.
Kidderminster Hospital, Worcester: M Labib, T Martin, S Moss, M Pathak.
Liverpool Women’s Hospital, Liverpool: N Aziz, L Harris, D Pattison.
Manor Hospital, Walsall: J Pepper.
Musgrove Park Hospital, Taunton: M Escott, G Fender.
New Cross Hospital, Wolverhampton: M Saeed.
Newham University Hospital, Plaistow: A Antoniou, R Chenoy.
Norfolk and Norwich University Hospital, Norwich: E Morris, M Sule.
Ormskirk & District General Hospital, Southport: A Cope, S Sharma, T Taylor
Queen Charlotte’s & Chelsea Hospital, London: N Panay, C Rothan.
Queen’s Hospital, Romford: A Coker.
Royal Blackburn Hospital, Blackburn: M Abdel-Aty, K Bhatia, S Gardiner, W Myint, M Willett.
Royal Hallamshire Hospital, Sheffield: V Brown, C Bonner, M Connor, S Stillwell.
Royal Infirmary of Edinburgh, Edinburgh: A Horne, S Milne, J Rowan.
Sandwell General Hospital, West Midlands: A Ewies, J Kabukoba.
Shotley Bridge Hospital, County Durham: J Dent, P Sengupta.
St Mary’s Hospital, Manchester: R Biancardi, K Donnelly, L Dwyer, K Naidoo, I Pinton.
Stafford Hospital, Stafford: K Chin, T Harrison, J Roger, D Sirdefield, J Stacey.
Royal Oldham Hospital, Oldham: Z Anjum, N Aziz.
The Royal Victoria Infirmary, Newcastle upon Tyne: J Bainbridge, G Cosgrove, A Desai, J Gebbie, D Koleskas, P Ranka, M Roberts.
Whiston Hospital, Merseyside: C Cunningham, C Nwosu.
We thank the members of the TSC for their assistance throughout the project: Professor J Thornton (Professor of Obstetrics and Gynaecology, University of Nottingham) – chairperson, Professor P O’Donovan (Professor of Medical Innovation and Gynaecology, University of Bradford); E Nicholls (Birmingham Women’s Hospital, Consumer); Professor J Deeks (University of Birmingham, Statistician); and Dr S Petrou (University of Oxford, Health Economist).
We thank the members of the Data Monitoring Committee for their assistance throughout the project: Professor M Lumsden (Professor of Obstetrics and Gynaecology, University of Glasgow) – chairperson, Professor S Bhattacharya (Professor of Obstetrics and Gynaecology, University of Aberdeen); and Dr C Cummins (Paediatric epidemiologist, University of Birmingham and Birmingham Children’s Foundation Trust).
The OPT Trial was coordinated by the Birmingham Clinical Trials Unit and we acknowledge the hard work of all of the staff involved in the study, especially the trial coordinators – Elizabeth Brettell (2008), Laura Gennard, Lisa Leighton and Nicholas Hilken (database programmer) – and Edward Tyler, who designed and developed the on-line randomisation and trial database.
We thank all of the Principal Investigators and the many sites (see Appendix 30) and the Clinical Local Research Network (CLRN)-funded research nurses. A special thank you to Tracy Bingham, trial-funded research nurse (2008–11) for recruiting, activating and supporting collaborating centres.
We thank Professor Richard Grey (Professor of Medical Statistics, Clinical Trial Service Unit, University of Oxford) for his input into the statistical and methodological aspects of the original OPT Trial protocol, and Professor Khalid Khan (Professor of Women’s Health and Clinical Epidemiology Queen Mary’s, University of London) and Professor Janesh Gupta (Professor of Obstetrics & Gynaecology, University of Birmingham) for their input into the design of the pilot RCT and development of the final trial protocol.
We thank the National Institute for Health Research HTA programme for funding the OPT Trial.
We thank the women who took part in the OPT Trial; without their participation none of this work would have been possible.
Contribution of authors
Dr T Justin Clark (Consultant Obstetrician & Gynaecologist and Honorary Reader in Obstetrics & Gynaecology) was the Chief Investigator for the study and was involved in the design, management, analysis of results and overall responsibility for writing the report. He was responsible for writing Chapters 1–4 and 7.
Lee J Middleton (Senior Statistician, Birmingham Clinical Trials Unit) contributed to the design and conduct of the overall study and performed the statistical analysis of the RCT and preference study. He contributed to the drafting and reviewing of all of the sections of the final report.
Dr Natalie AM Cooper (Clinical Research Fellow and Specialist Registrar on Obstetrics & Gynaecology) was the research fellow for the trial (2008–11), managed the trial, was involved in data collection, analysis of results and writing the report.
Dr Lavanya Diwakar (Research Fellow Health Economics) performed the health economics analysis and wrote Chapter 5.
Professor Elaine Denny (Professor of Health Sociology) was the supervisor for the qualitative work, was involved in qualitative data analysis and co-wrote Chapter 6.
Dr Paul Smith (Clinical Research Fellow and Specialist Registrar on Obstetrics & Gynaecology) was the research fellow for the trial (2011–12), and was involved in data collection and writing the report.
Laura Gennard (Trial Coordinator from 2008) had overall responsibility for the management of the trial.
Lynda Stobert (Health Sociology) undertook the patient interviews as part of the qualitative work, was involved in qualitative data analysis and co-wrote Chapter 6.
Professor Tracy E Roberts (Professor of Health Services Research) supervised the health economics research and co-wrote Chapter 5.
Versha Cheed (Trainee Statistician, Birmingham Clinical Trials Unit) helped to carry out the preparation of the data for the RCT and preference study. She performed the statistical analysis of the pilot study.
Tracey Bingham (Research Nurse) was the research nurse for the trial, was involved in data collection and assisted with recruitment.
Dr Sue Jowett (Health Economics) supervised the health economics research and co-wrote Chapter 5.
Elizabeth Brettell (Trial Coordinator, 2008) was responsible for the management of the trial in 2008.
Mary Connor (Consultant Gynaecologist) provided clinical advice, recruited to the trial and was involved in critical revision of the report.
Professor Sian E Jones (Professor of Gynaecology) provided clinical advice, recruited to the trial and was involved in critical revision of the report.
Dr Jane P Daniels (Co-Director Birmingham Clinical Trials Unit) was involved in the trial design and management, and critical revision of the report.
Publications
Cooper NAM, Clark TJ, Middleton L, Diwakar L, Smith P, Denny E, et al. Outpatient versus inpatient uterine polyp treatment for abnormal uterine bleeding: randomised controlled non-inferiority study. BMJ 2015;350:h1398. DOI: http://dx.doi.org/10.1136/bmj.h1398
Diwakar L, Roberts TE, Cooper NAM, Middleton L, Jowett S, Daniels J, et al. An economic evaluation of outpatient versus inpatient polyp treatment for abnormal uterine bleeding. BJOG 2015; in press. DOI: http://dx.doi.org/10.1111/1471-0528.13434
Cooper NAM, Middleton L, Smith P, Denny E, Stobert L, Daniels J, et al. A patient-preference cohort study of office versus inpatient uterine polyp treatment for abnormal uterine bleeding. Gynecol Surg 2015; submitted.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Fox H, Buckley H. Gynaecological and Obstetric Pathology for the MRCOG. London: RCOG Press; 1998.
- Kim K-R, Peng R, Ro JY, Robboy SJ. A diagnostically useful histopathologic feature of endometrial polyp: the long axis of endometrial glands arranged parallel to surface epithelium. Am J Surg Pathol 2004;28:1057-62. http://dx.doi.org/10.1097/01.pas.0000128659.73944.f3.
- Lopes RGC, Baracat EC, de Albuquerque Neto LC, Ramos JFD, Yatabe S, Depesr DB, et al. Analysis of estrogen- and progesterone-receptor expression in endometrial polyps. J Minim Invasive Gynecol 2007;14:300-3. http://dx.doi.org/10.1016/j.jmig.2006.10.022.
- Sant’Ana de Almeida EC, Nogueira AA, Candido dos Reis FJ, Zambelli Ramalho LN, Zucoloto S. Immunohistochemical expression of estrogen and progesterone receptors in endometrial polyps and adjacent endometrium in postmenopausal women. Maturitas 2004;49:229-33. http://dx.doi.org/10.1016/j.maturitas.2004.02.009.
- Lima G, Girão M, Lima GR, Girão MJB, Baracat EC. Ginecologia de Consultório. São Paulo: EPM; 2003.
- Mittal K, Schwartz L, Goswami S, Demopoulos R. Estrogen and progesterone receptor expression in endometrial polyps. Int J Gynecol Pathol 1996;15:345-8. http://dx.doi.org/10.1097/00004347-199610000-00007.
- Taylor LJ, Jackson TL, Reid JG, Duffy SRG. The differential expression of oestrogen receptors, progesterone receptors, Bcl-2 and Ki67 in endometrial polyps. BJOG 2003;110:794-8. http://dx.doi.org/10.1111/j.1471-0528.2003.02098.x.
- Thijs I, Neven P, Van Hooff I, Tonglet, Van Belle Y, De Muylder X, et al. Oestrogen and progesterone receptor expression in postmenopausal endometrial polyps and their surrounding endometrium. Eur J Cancer 2000;36:108-9. http://dx.doi.org/10.1016/S0959-8049(00)00263-X.
- McGurgan P, Taylor LJ, Duffy SR, O’Donovan PJ. Are endometrial polyps from pre-menopausal women similar to post-menopausal women? An immunohistochemical comparison of endometrial polyps from pre- and post-menopausal women. Maturitas 2006;54:277-84. http://dx.doi.org/10.1016/j.maturitas.2005.12.003.
- Vanni R, Dal Cin P, Marras S, Moerman P, Andria M, Valdes E, et al. Endometrial polyp: another benign tumor characterized by 12q13-q15 changes. Cancer Genet Cytogenet 1993;68:32-3. http://dx.doi.org/10.1016/0165-4608(93)90070-3.
- Inceboz US, Nese N, Uyar Y, Ozcakir HT, Kurtul O, Baytur YB, et al. Hormone receptor expressions and proliferation markers in postmenopausal endometrial polyps. Gynecol Obstet Invest 2006;61:24-8. http://dx.doi.org/10.1159/000088018.
- Grimes DA. Diagnostic dilation and curettage: a reappraisal. Am J Obstet Gynecol 1982;142:1-6.
- MacKenzie IZ, Bibby JG. Critical assessment of dilatation and curettage in 1029 women. Lancet 1978;2:566-8. http://dx.doi.org/10.1016/S0140-6736(78)92895-7.
- Bettocchi S, Ceci O, Vicino M, Marello F, Impedovo L, Selvaggi L. Diagnostic inadequacy of dilatation and curettage. Fertil Steril 2001;75:803-5. http://dx.doi.org/10.1016/S0015-0282(00)01792-1.
- Svirsky R, Smorgick N, Rozowski U, Sagiv R, Feingold M, Halperin R, et al. Can we rely on blind endometrial biopsy for detection of focal intrauterine pathology?. Am J Obstet Gynecol 2008;199:115.e1-3. http://dx.doi.org/10.1016/j.ajog.2008.02.015.
- Cooper NAM, Clark TJ. Cost-Effectiveness of Diagnostic Strategies for the Management of Abnormal Uterine Bleeding (Heavy Menstrual Bleeding and Post-Menopausal Bleeding): Systematic Reviews, IPD Meta-Analysis and Model Based Economic Evaluation n.d. www.hta.ac.uk/2145 (accessed 27 September 2013).
- Clark TJ, Mann CH, Shah N, Khan KS, Song F, Gupta JK. Accuracy of outpatient endometrial biopsy in the diagnosis of endometrial cancer: a systematic quantitative review. BJOG 2002;109:313-21. http://dx.doi.org/10.1111/j.1471-0528.2002.01088.x.
- Dijkhuizen FP, Mol BW, Brölmann HA, Heintz AP. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer 2000;89:1765-72. http://dx.doi.org/10.1002/1097-0142(20001015)89:8<1765::AID-CNCR17>3.0.CO;2-F.
- Makris N, Skartados N, Kalmantis K, Mantzaris G, Papadimitriou A, Antsaklis A. Evaluation of abnormal uterine bleeding by transvaginal 3-D hysterosonography and diagnostic hysteroscopy. Eur J Gynaecol Oncol 2007;28:39-42.
- Gimpelson RJ, Rappold HO. A comparative study between panoramic hysteroscopy with directed biopsies and dilatation and curettage. A review of 276 cases. Am J Obstet Gynecol 1988;158:489-92. http://dx.doi.org/10.1016/0002-9378(88)90011-7.
- Clark TJ. Outpatient hysteroscopy and ultrasonography in the management of endometrial disease. Curr Opin Obstet Gynecol 2004;16:305-11. http://dx.doi.org/10.1097/01.gco.0000136491.26463.c2.
- Farquhar C, Ekeroma A, Furness S, Arroll B. A systematic review of transvaginal ultrasonography, sonohysterography and hysteroscopy for the investigation of abnormal uterine bleeding in premenopausal women. Acta Obstet Gynecol Scand 2003;82:493-504. http://dx.doi.org/10.1034/j.1600-0412.2003.00191.x.
- Van Dongen H, Janssen CAH, Smeets MJGH, Emanuel MH, Jansen FW. The clinical relevance of hysteroscopic polypectomy in premenopausal women with abnormal uterine bleeding. BJOG 2009;116:1387-90. http://dx.doi.org/10.1111/j.1471-0528.2009.02145.x.
- Bernard JP, Rizk E, Camatte S, Robin F, Taurelle R, Lecuru F. Saline contrast sonohysterography in the preoperative assessment of benign intrauterine disorders. Ultrasound Obstet Gynecol 2001;17:145-9. http://dx.doi.org/10.1046/j.1469-0705.2001.00336.x.
- American Association of Gynecologic Laparoscopists . AAGL practice report: practice guidelines for the diagnosis and management of endometrial polyps. J Minim Invasive Gynecol 2012;19:3-10. http://dx.doi.org/10.1016/j.jmig.2011.09.003.
- Salim S, Won H, Nesbitt-Hawes E, Campbell N, Abbott J. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol 2011;18:569-81. http://dx.doi.org/10.1016/j.jmig.2011.05.018.
- Jansen FW, de Kroon CD, van Dongen H, Grooters C, Louwé L, Trimbos-Kemper T. Diagnostic hysteroscopy and saline infusion sonography: prediction of intrauterine polyps and myomas. J Minim Invasive Gynecol 2006;13:320-4. http://dx.doi.org/10.1016/j.jmig.2006.03.018.
- De Kroon CD, de Bock GH, Dieben SWM, Jansen FW. Saline contrast hysterosonography in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG 2003;110:938-47. http://dx.doi.org/10.1111/j.1471-0528.2003.02472.x.
- Van Dongen H, de Kroon CD, Jacobi CE, Trimbos JB, Jansen FW. Diagnostic hysteroscopy in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG 2007;114:664-75. http://dx.doi.org/10.1111/j.1471-0528.2007.01326.x.
- Clark TJ, Voit D, Gupta JK, Hyde C, Song F, Khan KS. Accuracy of hysteroscopy in the diagnosis of endometrial cancer and hyperplasia: a systematic quantitative review. JAMA 2002;288:1610-21. http://dx.doi.org/10.1001/jama.288.13.1610.
- Jansen FW, Vredevoogd CB, van Ulzen K, Hermans J, Trimbos JB, Trimbos-Kemper TC. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol 2000;96:266-70. http://dx.doi.org/10.1016/S0029-7844(00)00865-6.
- Clark TJ, Gupta JK. Handbook of Outpatient Hysteroscopy: A Complete Guide to Diagnosis and Therapy. Boca Raton, FL: CRC Press; 2005.
- Van Dongen H, Timmermans A, Jacobi CE, Elskamp T, de Kroon CD, Jansen FW. Diagnostic hysteroscopy and saline infusion sonography in the diagnosis of intrauterine abnormalities: an assessment of patient preference. Gynecol Surg 2011;8:65-70. http://dx.doi.org/10.1007/s10397-010-0649-1.
- Bain C, Parkin DE, Cooper KG. Is outpatient diagnostic hysteroscopy more useful than endometrial biopsy alone for the investigation of abnormal uterine bleeding in unselected premenopausal women? A randomised comparison. BJOG Int J Obstet Gynaecol 2002;109:805-11.
- Widrich T, Bradley LD, Mitchinson AR, Collins RL. Comparison of saline infusion sonography with office hysteroscopy for the evaluation of the endometrium. Am J Obstet Gynecol 1996;174:1327-34. http://dx.doi.org/10.1016/S0002-9378(96)70680-4.
- Dreisler E, Stampe Sorensen S, Ibsen PH, Lose G. Prevalence of endometrial polyps and abnormal uterine bleeding in a Danish population aged 20–74 years. Ultrasound Obstet Gynecol 2009;33:102-8. http://dx.doi.org/10.1002/uog.6259.
- Fatemi HM, Kasius JC, Timmermans A, van Disseldorp J, Fauser BC, Devroey P, et al. Prevalence of unsuspected uterine cavity abnormalities diagnosed by office hysteroscopy prior to in vitro fertilization. Hum Reprod Oxf Engl 2010;25:1959-65. http://dx.doi.org/10.1093/humrep/deq150.
- De Ziegler D. Contrast ultrasound: a simple-to-use phase-shifting medium offers saline infusion sonography-like images. Fertil Steril 2009;92:369-73. http://dx.doi.org/10.1016/j.fertnstert.2008.04.032.
- Fay TN, Khanem N, Hosking D. Out-patient hysteroscopy in asymptomatic postmenopausal women. Climacteric J Int Menopause Soc 1999;2:263-7. http://dx.doi.org/10.3109/13697139909038086.
- Reslová T, Tosner J, Resl M, Kugler R, Vávrová I. Endometrial polyps. A clinical study of 245 cases. Arch Gynecol Obstet 1999;262:133-9.
- Cohen I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol 2004;94:256-66. http://dx.doi.org/10.1016/j.ygyno.2004.03.048.
- Chalas E, Costantino JP, Wickerham DL, Wolmark N, Lewis GC, Bergman C, et al. Benign gynecologic conditions among participants in the Breast Cancer Prevention Trial. Am J Obstet Gynecol 2005;192:1230-7. http://dx.doi.org/10.1016/j.ajog.2004.12.083.
- Bakour SH, Khan KS, Gupta JK. The risk of premalignant and malignant pathology in endometrial polyps. Acta Obstet Gynecol Scand 2002;8:182-3. http://dx.doi.org/10.1034/j.1600-0412.2002.810220.x.
- Perrone G, DeAngelis C, Critelli C, Capri O, Galoppi P, Santoro G, et al. Hysteroscopic findings in postmenopausal abnormal uterine bleeding: a comparison between HRT users and non-users. Maturitas 2002;43:251-5. http://dx.doi.org/10.1016/S0378-5122(02)00272-4.
- Clark TJ, Khan KS, Gupta JK. Current practice for the treatment of benign intrauterine polyps: a national questionnaire survey of consultant gynaecologists in UK. Eur J Obstet Gynecol Reprod Biol 2002;103:65-7. http://dx.doi.org/10.1016/S0301-2115(02)00011-8.
- DeWaay DJ, Syrop CH, Nygaard IE, Davis WA, Van Voorhis BJ. Natural history of uterine polyps and leiomyomata. Obstet Gynecol 2002;100:3-7. http://dx.doi.org/10.1016/S0029-7844(02)02007-0.
- Lieng M, Istre O, Sandvik L, Qvigstad E. Prevalence, 1-year regression rate, and clinical significance of asymptomatic endometrial polyps: cross-sectional study. J Minim Invasive Gynecol 2009;16:465-71. http://dx.doi.org/10.1016/j.jmig.2009.04.005.
- Orvieto R, Bar-Hava I, Dicker D, Bar J, Ben-Rafael Z, Neri A. Endometrial polyps during menopause: characterization and significance. Acta Obstet Gynecol Scand 1999;78:883-6. http://dx.doi.org/10.1080/j.1600-0412.1999.781009.x.
- Savelli L, De Iaco P, Santini D, Rosati F, Ghi T, Pignotti E, et al. Histopathologic features and risk factors for benignity, hyperplasia, and cancer in endometrial polyps. Am J Obstet Gynecol 2003;188:927-31. http://dx.doi.org/10.1067/mob.2003.247.
- Ben-Arie A, Goldchmit C, Laviv Y, Levy R, Caspi B, Huszar M, et al. The malignant potential of endometrial polyps. Eur J Obstet Gynecol Reprod Biol 2004;115:206-10. http://dx.doi.org/10.1016/j.ejogrb.2004.02.002.
- Anastasiadis PG, Koutlaki NG, Skaphida PG, Galazios GC, Tsikouras PN, Liberis VA. Endometrial polyps: prevalence, detection, and malignant potential in women with abnormal uterine bleeding. Eur J Gynaecol Oncol 2000;21:180-3.
- Shushan A, Revel A, Rojansky N. How often are endometrial polyps malignant?. Gynecol Obstet Invest 2004;58:212-15. http://dx.doi.org/10.1159/000080189.
- Antunes A, Costa-Paiva L, Arthuso M, Costa JV, Pinto-Neto AM. Endometrial polyps in pre- and postmenopausal women: factors associated with malignancy. Maturitas 2007;57:415-21. http://dx.doi.org/10.1016/j.maturitas.2007.04.010.
- Gregoriou O, Konidaris S, Vrachnis N, Bakalianou K, Salakos N, Papadias K, et al. Clinical parameters linked with malignancy in endometrial polyps. Climacteric J Int Menopause Soc 2009;12:454-8. http://dx.doi.org/10.1080/13697130902912605.
- Wang J-H, Zhao J, Lin J. Opportunities and risk factors for premalignant and malignant transformation of endometrial polyps: management strategies. J Minim Invasive Gynecol 2010;17:53-8. http://dx.doi.org/10.1016/j.jmig.2009.10.012.
- Ferrazzi E, Zupi E, Leone FP, Savelli L, Omodei U, Moscarini M, et al. How often are endometrial polyps malignant in asymptomatic postmenopausal women? A multicenter study. Am J Obstet Gynecol 2009;200:235.e1-6. http://dx.doi.org/10.1016/j.ajog.2008.09.876.
- Kedar RP, Bourne TH, Powles TJ, Collins WP, Ashley SE, Cosgrove DO, et al. Effects of tamoxifen on uterus and ovaries of postmenopausal women in a randomised breast cancer prevention trial. Lancet 1994;343:1318-21. http://dx.doi.org/10.1016/S0140-6736(94)92466-X.
- Kurman RJ, McConnell TG. Precursors of endometrial and ovarian carcinoma. Virchows Arch Int J Pathol 2010;456:1-12. http://dx.doi.org/10.1007/s00428-009-0824-9.
- Lieng M, Istre O, Qvigstad E. Treatment of endometrial polyps: a systematic review. Acta Obstet Gynecol Scand 2010;89:992-1002. http://dx.doi.org/10.3109/00016349.2010.493196.
- Lee SC, Kaunitz AM, Sanchez-Ramos L, Rhatigan RM. The oncogenic potential of endometrial polyps: a systematic review and meta-analysis. Obstet Gynecol 2010;116:1197-205. http://dx.doi.org/10.1097/AOG.0b013e3181f74864.
- Warner P, Critchley HOD, Lumsden MA, Campbell-Brown M, Douglas A, Murray G. Referral for menstrual problems: cross sectional survey of symptoms, reasons for referral, and management. BMJ 2001;323:24-8. http://dx.doi.org/10.1136/bmj.323.7303.24.
- Coulter A, Long A, Kelland J, O’Meara S, Sculpher M, Song F, et al. Managing menorrhagia. Qual Heal Care 1995;4:218-26. http://dx.doi.org/10.1136/qshc.4.3.218.
- Heavy Menstrual Bleeding. Clinical Guideline No. 44. London: RCOG Press; 2007.
- Coulter A, Noone A, Goldacre M. General practitioners’ referrals to specialist outpatient clinics. I. Why general practitioners refer patients to specialist outpatient clinics. BMJ 1989;299:304-6. http://dx.doi.org/10.1136/bmj.299.6694.304.
- Bhattacharya S, Middleton LJ, Tsourapas A, Lee AJ, Champaneria R, Daniels JP, et al. Hysterectomy, endometrial ablation and Mirena® for heavy menstrual bleeding: a systematic review of clinical effectiveness and cost-effectiveness analysis. Health Technol Assess 2011;15.
- Shapley M, Blagojevic-Bucknall M, Jordan K, Croft P. The epidemiology of self-reported intermenstrual and postcoital bleeding in the perimenopausal years. BJOG 2013;120:1348-55. http://dx.doi.org/10.1111/1471-0528.12218.
- Key Health Statistics from General Practice: Analyses of Morbidity and Treatment Data, including Time Trends, England and Wales. London: ONS; 1998.
- Gull B, Karlsson B, Milsom I, Granberg S. Can ultrasound replace dilation and curettage? A longitudinal evaluation of postmenopausal bleeding and transvaginal sonographic measurement of the endometrium as predictors of endometrial cancer. Am J Obstet Gynecol 2003;188:401-8. http://dx.doi.org/10.1067/mob.2003.154.
- Gardner FJ, Konje JC, Abrams KR, Brown LJ, Khanna S, Al-Azzawi F, et al. Endometrial protection from tamoxifen-stimulated changes by a levonorgestrel-releasing intrauterine system: a randomised controlled trial. Lancet 2000;356:1711-17. http://dx.doi.org/10.1016/S0140-6736(00)03204-9.
- Clevenger-Hoeft M, Syrop CH, Stovall DW, Van Voorhis BJ. Sonohysterography in premenopausal women with and without abnormal bleeding. Obstet Gynecol 1999;94:516-20. http://dx.doi.org/10.1016/S0029-7844(99)00345-2.
- Elfayomy AK, Habib FA, Alkabalawy MA. Role of hysteroscopy in the detection of endometrial pathologies in women presenting with postmenopausal bleeding and thickened endometrium. Arch Gynecol Obstet 2012;285:839-43. http://dx.doi.org/10.1007/s00404-011-2068-6.
- Munro MG, Critchley HOD, Fraser IS. The FIGO systems for nomenclature and classification of causes of abnormal uterine bleeding in the reproductive years: who needs them?. Am J Obstet Gynecol 2012;207:259-65. http://dx.doi.org/10.1016/j.ajog.2012.01.046.
- Timmermans A, Opmeer BC, Veersema S, Mol BWJ. Patients’ preferences in the evaluation of postmenopausal bleeding. BJOG 2007;114:1146-9. http://dx.doi.org/10.1111/j.1471-0528.2007.01424.x.
- Department of Health (DH) . Hospital Episode Statistics – 2011 12 n.d. www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID = 1937&categoryID = 215 (accessed 9 August 2013).
- Lieng M, Istre O, Sandvik L, Engh V, Qvigstad E. Clinical effectiveness of transcervical polyp resection in women with endometrial polyps: randomized controlled trial. J Minim Invasive Gynecol 2010;17:351-7. http://dx.doi.org/10.1016/j.jmig.2010.01.019.
- Timmermans A, Veersema S, van Kerkvoorde TC, van der Voet LF, Opmeer BC, Bongers MY, et al. Should endometrial polyps be removed in patients with postmenopausal bleeding? An assessment of study designs and report of a failed randomised controlled trial (ISRCTN73825127). BJOG 2009;116:1391-5. http://dx.doi.org/10.1111/j.1471-0528.2009.02234.x.
- Vercellini P, Trespidi L, Bramante T, Panazza S, Mauro F, Crosignani PG. Gonadotropin releasing hormone agonist treatment before hysteroscopic endometrial resection. Int J Gynaecol Obstet 1994;45:235-9. http://dx.doi.org/10.1016/0020-7292(94)90248-8.
- Oguz S, Sargin A, Kelekci S, Aytan H, Tapisiz OL, Mollamahmutoglu L. The role of hormone replacement therapy in endometrial polyp formation. Maturitas 2005;50:231-6. http://dx.doi.org/10.1016/j.maturitas.2004.06.002.
- Gardner FJE, Konje JC, Bell SC, Abrams KR, Brown LJR, Taylor DJ, et al. Prevention of tamoxifen induced endometrial polyps using a levonorgestrel releasing intrauterine system long-term follow-up of a randomised control trial. Gynecol Oncol 2009;114:452-6. http://dx.doi.org/10.1016/j.ygyno.2009.06.014.
- Timmermans A, van Dongen H, Mol BW, Veersema S, Jansen FW. Hysteroscopy and removal of endometrial polyps: a Dutch survey. Eur J Obstet Gynecol Reprod Biol 2008;138:76-9. http://dx.doi.org/10.1016/j.ejogrb.2007.05.016.
- Van Dijk LJEW, Breijer MC, Veersema S, Mol BWJ, Timmermans A. Current practice in the removal of benign endometrial polyps: a Dutch survey. Gynecol Surg 2012;9:163-8. http://dx.doi.org/10.1007/s10397-011-0707-3.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol 1989;73:16-20.
- Moghal N. Diagnostic value of endometrial curettage in abnormal uterine bleeding: a histopathological study. J Pak Med Assoc 1997;47:295-9.
- Gebauer G, Hafner A, Siebzehnrübl E, Lang N. Role of hysteroscopy in detection and extraction of endometrial polyps: results of a prospective study. Am J Obstet Gynecol 2001;184:59-63. http://dx.doi.org/10.1067/mob.2001.108332.
- Bettocchi S, Ceci O, Nappi L, Di Venere R, Masciopinto V, Pansini V, et al. Operative office hysteroscopy without anesthesia: analysis of 4863 cases performed with mechanical instruments. J Am Assoc Gynecol Laparosc 2004;11:59-61. http://dx.doi.org/10.1016/S1074-3804(05)60012-6.
- Timmermans A, Veersema S. Ambulatory transcervical resection of polyps with the Duckbill polyp snare: a modality for treatment of endometrial polyps. J Minim Invasive Gynecol 2005;12:37-9. http://dx.doi.org/10.1016/j.jmig.2004.12.014.
- Nathani F, Clark TJ. Uterine polypectomy in the management of abnormal uterine bleeding: a systematic review. J Minim Invasive Gynecol 2006;13:260-8. http://dx.doi.org/10.1016/j.jmig.2006.03.015.
- Garuti G, Centinaio G, Luerti M. Outpatient hysteroscopic polypectomy in postmenopausal women: a comparison between mechanical and electrosurgical resection. J Minim Invasive Gynecol 2008;15:595-600. http://dx.doi.org/10.1016/j.jmig.2008.07.001.
- Magos AL, Baumann R, Lockwood GM, Turnbull AC. Experience with the first 250 endometrial resections for menorrhagia. Lancet 1991;337:1074-8. http://dx.doi.org/10.1016/0140-6736(91)91718-A.
- Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol 1993;82:736-40.
- Preutthipan S, Herabutya Y. Hysteroscopic polypectomy in 240 premenopausal and postmenopausal women. Fertil Steril 2005;83:705-9. http://dx.doi.org/10.1016/j.fertnstert.2004.08.031.
- Cravello L, de Montgolfier R, D’Ercole C, Boubli L, Blanc B. Hysteroscopic surgery in postmenopausal women. Acta Obstet Gynecol Scand 1996;75:563-6. http://dx.doi.org/10.3109/00016349609054672.
- Betjes HE, Hanstede MMF, Emanuel M-H, Stewart EA. Hysteroscopic myomectomy and case volume hysteroscopic myomectomy performed by high- and low-volume surgeons. J Reprod Med 2009;54:425-8.
- Batra N, Khunda A, O’Donovan PJ. Hysteroscopic myomectomy. Obstet Gynecol Clin North Am 2004;31:669-85. http://dx.doi.org/10.1016/j.ogc.2004.06.003.
- Overton C, Hargreaves J, Maresh M. A national survey of the complications of endometrial destruction for menstrual disorders: the MISTLETOE study. Minimally Invasive Surgical Techniques--Laser, EndoThermal or Endorescetion. BJOG 1997;104:1351-9. http://dx.doi.org/10.1111/j.1471-0528.1997.tb11003.x.
- Kung RC, Vilos GA, Thomas B, Penkin P, Zaltz AP, Stabinsky SA. A new bipolar system for performing operative hysteroscopy in normal saline. J Am Assoc Gynecol Laparosc 1999;6:331-6. http://dx.doi.org/10.1016/S1074-3804(99)80071-1.
- Clark TJ, Godwin J, Khan KS, Gupta JK. Ambulatory endoscopic treatment of symptomatic benign endometrial polyps: feasibility study. Gynaecol Endosc 2002;11:91-7. http://dx.doi.org/10.1046/j.1365-2508.2002.00485.x.
- Vilos GA. Intrauterine surgery using a new coaxial bipolar electrode in normal saline solution (Versapoint): a pilot study. Fertil Steril 1999;72:740-3.
- Emanuel MH, Wamsteker K. The Intra Uterine Morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol 2005;12:62-6. http://dx.doi.org/10.1016/j.jmig.2004.12.011.
- Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos J, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol 2008;15:466-71. http://dx.doi.org/10.1016/j.jmig.2008.02.002.
- Nagele F, Mane S, Chandrasekaran P. How successful is hysteroscopic polypectomy?. Gynaecol Endosc 1996;5:137-40.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration; 2008.
- Akers J, Agular-Ibáñez R, Baba-Akbari Sari A, Benyon S, Booth A. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York, NHS Centre for Reviews and Dissemination; 2009.
- Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. http://dx.doi.org/10.1016/j.jclinepi.2007.11.008.
- AlHilli MM, Nixon KE, Hopkins MR, Weaver AL, Laughlin-Tommaso SK, Famuyide AO. Long-term outcomes after intrauterine morcellation vs hysteroscopic resection of endometrial polyps. J Minim Invasive Gynecol 2013;20:215-21. http://dx.doi.org/10.1016/j.jmig.2012.10.013.
- Barisic D, Strelec M, Velimir S. Operative hysteroscopy in patients with abnormal uterine bleeding caused by benign intrauterine growths. Croat Med J 1995;36:275-7.
- Brooks PG, Loffer FD, Serden SP. Resectoscopic removal of symptomatic intrauterine lesions. J Reprod Med 1989;34:435-7.
- Cravello L, Stolla V, Bretelle F, Roger V, Blanc B. Hysteroscopic resection of endometrial polyps: a study of 195 cases. Eur J Obstet Gynecol Reprod Biol 2000;93:131-4. http://dx.doi.org/10.1016/S0301-2115(00)00281-5.
- Henriquez DDCA, van Dongen H, Wolterbeek R, Jansen FW. Polypectomy in premenopausal women with abnormal uterine bleeding: effectiveness of hysteroscopic removal. J Minim Invasive Gynecol 2007;14:59-63. http://dx.doi.org/10.1016/j.jmig.2006.07.008.
- Pace S. Transcervical resection of benign endocavitary lesions. Gynaecol Endosc 1993;2:165-9.
- Polena V, Mergui J-L, Zérat L, Daraï E, Barranger E, Uzan S. Long-term results of hysteroscopic resection of endometrial polyps in 367 patients. Role of associated endometrial resection. Gynécologie Obstétrique Fertil 2005;33:382-8. http://dx.doi.org/10.1016/j.gyobfe.2005.04.021.
- Stamatellos I, Apostolides A, Stamatopoulos P, Bontis J. Pregnancy rates after hysteroscopic polypectomy depending on the size or number of the polyps. Arch Gynecol Obstet 2008;277:395-9. http://dx.doi.org/10.1007/s00404-007-0460-z.
- Timmermans A, van Doorn LC, Opmeer BC, Kroeks MVAM, Duk MJ, Bouwmeester AM, et al. Follow-up of women after a first episode of postmenopausal bleeding and endometrial thickness greater than 4 millimeters. Obstet Gynecol 2008;111:137-43. http://dx.doi.org/10.1097/01.AOG.0000296654.43944.e6.
- Towbin NA, Gviazda IM, March CM. Office hysteroscopy versus transvaginal ultrasonography in the evaluation of patients with excessive uterine bleeding. Am J Obstet Gynecol 1996;174:1678-82. http://dx.doi.org/10.1016/S0002-9378(96)70196-5.
- Tjarks M, Van Voorhis BJ. Treatment of endometrial polyps. Obstet Gynecol 2000;96:886-9. http://dx.doi.org/10.1016/S0029-7844(00)01059-0.
- Clark TJ, Barton PM, Coomarasamy A, Gupta JK, Khan KS. Investigating postmenopausal bleeding for endometrial cancer: cost-effectiveness of initial diagnostic strategies. BJOG 2006;113:502-10. http://dx.doi.org/10.1111/j.1471-0528.2006.00914.x.
- Feng L, Wang W, Zhang H, Zhu Y. Clinical study of hysteroscopic surgery for endometrial polyps. Zhonghua Fu Chan Ke Za Zhi 2003;38:611-13.
- Emanuel MH, Verdel MJ, Wamsteker K, Lammes FB. A prospective comparison of transvaginal ultrasonography and diagnostic hysteroscopy in the evaluation of patients with abnormal uterine bleeding: clinical implications. Am J Obstet Gynecol 1995;172:547-52. http://dx.doi.org/10.1016/0002-9378(95)90571-5.
- Lurie S, Piper I, Woliovitch I, Glezerman M. Age-related prevalence of sonographically confirmed uterine myomas. J Obstet Gynaecol 2005;25:42-4. http://dx.doi.org/10.1080/01443610400024583.
- Gupta JK, Clark TJ, More S, Pattison H. Patient anxiety and experiences associated with an outpatient ‘one-stop’ ‘see and treat’ hysteroscopy clinic. Surg Endosc 2004;18:1099-104. http://dx.doi.org/10.1007/s00464-003-9144-3.
- Clark TJ, Mahajan D, Sunder P, Bingham T, Khan KS, Gupta JK. What research should be done in gynaecological endoscopy? A national questionnaire survey of members of the British Society of Gynaecological Endoscopy. Gynaecol Endosc 2001;10:297-301. http://dx.doi.org/10.1046/j.1365-2508.2001.00465.x.
- Khan KS, Clark TJ, O’Donovan P, McGurgan P. Endometrial Ablation. London: Arnold; 2002.
- Shaw RW, Brickley MR, Evans L, Edwards MJ. Perceptions of women on the impact of menorrhagia on their health using multi-attribute utility assessment. BJOG 1998;105:1155-9. http://dx.doi.org/10.1111/j.1471-0528.1998.tb09968.x.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. http://dx.doi.org/10.3109/07853890109002087.
- Thirlaway K, Fallowfield L, Cuzick J. The Sexual Activity Questionnaire: a measure of women’s sexual functioning. Qual Life Res 1996;5:81-90. http://dx.doi.org/10.1007/BF00435972.
- Clark TJ, Khan KS, Foon R, Pattison H, Bryan S, Gupta JK. Quality of life instruments in studies of menorrhagia: a systematic review. Eur J Obstet Gynecol Reprod Biol 2002;104:96-104. http://dx.doi.org/10.1016/S0301-2115(02)00076-3.
- Jones GL, Kennedy SH, Jenkinson C. Health-related quality of life measurement in women with common benign gynecologic conditions: a systematic review. Am J Obstet Gynecol 2002;187:501-11. http://dx.doi.org/10.1067/mob.2002.124940.
- Clark TJ, Mahajan D, Sunder P, Gupta JK. Hysteroscopic treatment of symptomatic submucous fibroids using a bipolar intrauterine system: a feasibility study. Eur J Obstet Gynecol Reprod Biol 2002;100:237-42. http://dx.doi.org/10.1016/S0301-2115(01)00485-7.
- Revill SI, Robinson JO, Rosen M, Hogg MI. The reliability of a linear analogue for evaluating pain. Anaesthesia 1976;31:1191-8. http://dx.doi.org/10.1111/j.1365-2044.1976.tb11971.x.
- Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain 1999;83:157-62. http://dx.doi.org/10.1016/S0304-3959(99)00101-3.
- Piaggio G, Pinol AP. Use of the equivalence approach in reproductive health clinical trials. Stat Med 2001;20:3571-7. http://dx.doi.org/10.1002/sim.1078.
- Piaggio G, Elbourne DR, Pocock SJ, Evans SJW, Altman DG. CONSORT Group . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308:2594-604. http://dx.doi.org/10.1001/jama.2012.87802.
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer; 2009.
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c869.
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321-33. http://dx.doi.org/10.1001/jama.288.3.321.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f1049.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: PSSRU, University of Kent; 2012.
- Department of Health (DH) . Confirmation of Payment by Results (PbR) Arrangements for 2011–12 2011. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_124356.
- Garside R, Stein K, Wyatt K, Round A, Pitt M. A cost-utility analysis of microwave and thermal balloon endometrial ablation techniques for the treatment of heavy menstrual bleeding. BJOG 2004;111:1103-14. http://dx.doi.org/10.1111/j.1471-0528.2004.00265.x.
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319:670-4. http://dx.doi.org/10.1136/bmj.319.7211.670.
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637-9. http://dx.doi.org/10.1001/jama.1996.03540080059030.
- Barzi F, Woodward M. Imputations of missing values in practice: results from imputations of serum cholesterol in 28 cohort studies. Am J Epidemiol 2004;160:34-45. http://dx.doi.org/10.1093/aje/kwh175.
- Office for National Statistics (ONS) . Gross Weekly Earnings of Full-Time Employees n.d. www.ons.gov.uk/ons/search/index.html?newquery = weekly+earnings (accessed 1 January 2014).
- Ratcliffe J, Ryan M, Tucker J. The costs of alternative types of routine antenatal care for low-risk women: shared care vs care by general practitioners and community midwives. J Health Serv Res Policy 1996;1:135-40.
- Automobile Association (AA) . Motoring Costs for Petrol Cars 2013. theaa.com/resources/Documents/pdf/motoring-advice/running-costs/petrol2013.pdf (accessed 1 January 2013).
- Department of Health (DH) . National Travel Survey 2010. www.gov.uk/government/uploads/system/uploads/attachment_data/file/8940/nts2010–09.pdf (accessed 1 January 2014).
- Drummond MF, Sculpher MJ, Torrance GW, O’Brian BJ. Methods for the Economic Evaluation of Health Care Programme. Oxford University Press; 2005.
- Briggs AH, O’Brien BJ. The death of cost-minimization analysis?. Health Econ 2001;10:179-84. http://dx.doi.org/10.1002/hec.584.
- Doshani A, Pitchforth E, Mayne C, Tincello DG. The value of qualitative research in urogynaecology. BJOG 2009;116:3-6. http://dx.doi.org/10.1111/j.1471-0528.2008.01924.x.
- Morgan M, Dodds W, Wolfe C, Raju S. Women’s views and experiences of outpatient hysteroscopy: implications for a patient-centered service. Nurs Health Sci 2004;6:315-20. http://dx.doi.org/10.1111/j.1442-2018.2004.00202.x.
- Peel E, Parry O, Douglas M, Lawton J. ‘It’s no skin off my nose’: why people take part in qualitative research. Qual Health Res 2006;16:1335-49. http://dx.doi.org/10.1177/1049732306294511.
- Morris N, Schneider M. Volunteer research subjects’ experience of participation in research on a novel diagnostic technology for breast cancer. Qual Health Res 2010;20:81-92. http://dx.doi.org/10.1177/1049732309355592.
- McCann SK, Campbell MK, Entwistle VA. Reasons for participating in randomised controlled trials: conditional altruism and considerations for self. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-31.
- Irvine A. Duration, dominance and depth in telephone and face-to-face interviews: a comparative exploration. Int J Qual Methods 2011;10:202-20.
- Bryman A. Social Research Methods. Oxford: Oxford University Press; 2012.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- British National Formulary. London: BMA and RPS; 2013.
- Lockwood GG, White DC. Br J Anaesth 2001;87:559-63. http://dx.doi.org/10.1093/bja/87.4.559.
- Curtis L. Unit Costs of Health Social Care 2011. Canterbury: PSSRU, University of Kent; 2011.
- Critchley H, Warner P, Lee AJ, Brechin S, Guise J, Graham B. Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8340.
Appendix 1 Statistical analysis plan
Appendix 2 Enrolment form: all participants
Appendix 3 Follow-up forms
Appendix 4 Assessment of inpatient treatment experience
Appendix 5 Assessment of outpatient treatment experience
Appendix 6 Patient cost questionnaire
Appendix 7 Polyp treatment form
Appendix 8 Post-treatment discharge form
Appendix 9 CONSORT checklist (including details with respect to extension for non-inferiority trials)
CONSORT 2010 checklist of information to include when reporting a randomised trial

| Section/topic | Item no. | Checklist item | Reported on page no.: |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomised trial in the title | ii | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance, see CONSORT for abstracts) | iii–iv | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale | 1–33 |
| 2b | Specific objectives or hypotheses | 34 | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 34, 37 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | N/A | |
| Participants | 4a | Eligibility criteria for participants | 36–7 |
| 4b | Settings and locations where the data were collected | 37, 40 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 38 |
| Outcomes | 6a | Completely defined prespecified primary and secondary outcome measures, including how and when they were assessed | 40–3 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | N/A | |
| Sample size | 7a | How sample size was determined | 44 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | 47 | |
| Randomisation: | |||
| Sequence generation | 8a | Method used to generate the random allocation sequence | 37 |
| 8b | Type of randomisation; details of any restriction (such as blocking and block size) | 37 | |
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 37 |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | 37 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | N/A |
| 11b | If relevant, description of the similarity of interventions | 44–6 | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 44–6 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | 44–6 | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome | 53 |
| 13b | For each group, losses and exclusions after randomisation, together with reasons | 53 | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 50 |
| 14b | Why the trial ended or was stopped | 49 | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | 52 |
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | 53, 55–6 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% CI) | 55–68 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | 55 | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing prespecified from exploratory | 55 |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | 55 |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 70–1 |
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings | 71–2 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 69–72 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | iv |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | www.hta.ac.uk/project/1679.asp |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | iv |
Appendix 10 Results of primary end point (treatment success): sensitivity analyses
| Assumption | Time point | RR (95% CI)a |
|---|---|---|
| Using a multiple imputation approach for missing responses | 6 months | 0.92 (0.82 to 1.03) |
| 1 year | 0.98 (0.89 to 1.07) | |
| 2 years | 0.99 (0.91 to 1.07) | |
| Excluding those women who had gone on to have a further related procedure (another polyp removal/endometrial ablation/hysterectomy); responses were excluded from corresponding surgery time onwards | 6 months | 0.92 (0.82 to 1.03) |
| 1 year | 0.97 (0.88 to 1.07) | |
| 2 years | 0.97 (0.88 to 1.07) | |
| Excluding those who returned their forms more than 3 months past their due date (forms returned after the subsequent time point were considered invalid and not used here or in the main analysis) | 6 months | 0.94 (0.84 to 1.05) |
| 1 year | 0.97 (0.89 to 1.07) | |
| 2 years | 0.96 (0.88 to 1.05) | |
| Excluding those who had a LNG-IUS fitted at the time of their original operation | 6 months | 0.91 (0.82 to 1.02) |
| 1 year | 0.96 (0.87 to 1.05) | |
| 2 years | 0.97 (0.89 to 1.05) | |
| Worst-case scenario: all missing responses unsuccessful | 6 months | 0.98 (0.87 to 1.12) |
| 1 year | 1.02 (0.92 to 1.15) | |
| 2 years | 1.06 (0.94 to 1.19) | |
| Best-case scenario: all missing responses successful | 6 months | 0.91 (0.83 to 1.00) |
| 1 year | 0.97 (0.90 to 1.05) | |
| 2 years | 0.97 (0.91 to 1.03) |
Appendix 11 Results of primary end point (treatment success): comparison with European Quality of Life-5 Dimensions score
| Time point | Outcome | Mean (SD, n) | Difference (95% CI)a | p-value |
|---|---|---|---|---|
| 6 months | Treatment ‘success’ | 0.89 (0.21, 332) | 0.06 (0.02 to 0.10) | 0.008 |
| Treatment ‘failure’ | 0.81 (0.23, 105) | |||
| 1 year | Treatment ‘success’ | 0.87 (0.24, 358) | 0.07 (0.01 to 1.12) | 0.01 |
| Treatment ‘failure’ | 0.79 (0.25, 80) | |||
| 2 years | Treatment ‘success’ | 0.87 (0.25, 345) | 0.12 (0.06 to 0.18) | 0.0003 |
| Treatment ‘failure’ | 0.75 (0.31, 61) |
Appendix 12 Results of adjusted analyses for major secondary outcomes
Likert response
| Time of assessment | Compared with before your treatment, would you say your bleeding is: | Polypectomy | |
|---|---|---|---|
| Outpatient | Inpatient | ||
| 6 months | Much better | 164 (75%) | 160 (81%) |
| Little better | 24 (11%) | 21 (11%) | |
| Same | 29 (13%) | 12 (6%) | |
| Worse | 3 (1%) | 4 (2%) | |
| Total | 220 | 197 | |
| Test for trend p -value | 0.08 | ||
| RR (95% CI) a | 0.93 (0.87 to 1.00) | ||
| Adjusted RR (95% CI) b | 0.95 (0.89 to 1.02) | ||
| 1 year | Much better | 177 (80%) | 174 (82%) |
| Little better | 18 (8%) | 12 (6%) | |
| Same | 21 (10%) | 21 (10%) | |
| Worse | 5 (2%) | 5 (2%) | |
| Total | 221 | 212 | |
| Test for trend p -value | 0.85 | ||
| RR (95% CI) a | 1.01 (0.94 to 1.08) | ||
| Adjusted RR (95% CI) b | 1.02 (0.95 to 1.08) | ||
| 2 years | Much better | 173 (82%) | 172 (88%) |
| Little better | 17 (8%) | 12 (6%) | |
| Same | 18 (8%) | 10 (5%) | |
| Worse | 4 (2%) | 1 (1%) | |
| Total | 212 | 195 | |
| Test for trend p -value | 0.04 | ||
| RR (95% CI) a | 0.95 (0.90 to 1.01) | ||
| Adjusted RR (95% CI) b | 0.97 (0.92 to 1.03) | ||
Appendix 13 Results of secondary outcomes: scores
| Patient-reported outcome measures | Polypectomy | Adjusted difference (95% CI);a p-value | |
|---|---|---|---|
| Outpatient: mean (SD, n) | Inpatient: mean (SD, n) | ||
| MMASb | |||
| Baseline | 52 (27, 134) | 58 (24, 124) | |
| 6 months | 78 (22, 115)c | 79 (23, 99)c | –1 (–7 to 5); 0.67 |
| 1 year | 82 (23, 110)c | 83 (21, 101)c | –1 (–7 to 5); 0.79 |
| 2 years | 84 (21, 93)c | 85 (21, 83)c | –2 (–8 to 4); 0.53 |
| Overalld | –1 (–6 to 4); 0.69 | ||
| EuroQol EQ-5De | |||
| Baseline | 0.78 (0.25, 242) | 0.78 (0.27, 232) | |
| 6 months | 0.87 (0.23, 230)c | 0.87 (0.20, 211)c | –0.01 (–0.05 to 0.03); 0.65 |
| 1 year | 0.86 (0.25, 227)c | 0.86 (0.24, 219)c | 0.00 (–0.04 to 0.04); 0.90 |
| 2 years | 0.85 (0.25, 213)c | 0.84 (0.27, 196) | 0.03 (–0.02 to 0.07); 0.28 |
| Overalld | 0.00 (–0.03 to 0.03); 0.95 | ||
| EuroQol health thermometerf | |||
| Baseline | 77 (18, 233) | 78 (18, 225) | |
| 6 months | 79 (18, 227)c | 80 (17, 212) | 0 (–3 to 3); 0.85 |
| 1 year | 80 (17, 228)c | 82 (16, 219)c | –1 (–4 to 2); 0.48 |
| 2 years | 79 (18, 207) | 83 (16, 194)c | –2 (–5 to 1); 0.21 |
| Overalld | –1 (–3 to 1); 0.27 | ||
| Bleeding duration VASg | |||
| Baseline | 46 (28, 68) | 53 (28, 67) | |
| 6 months | 35 (30, 64) | 28 (26, 56)c | –10 (–21 to 1); 0.08 |
| 1 year | 18 (21, 58)c | 24 (28, 62)c | 5 (–5 to 14); 0.36 |
| 2 years | 16 (22, 61)c | 15 (25, 53)c | –3 (–12 to 7); 0.61 |
| Overalld | –3 (–10 to 4); 0.34 | ||
| Bleeding amount VASh | |||
| Baseline | 58 (28, 70) | 66 (26, 68) | |
| 6 months | 29 (29, 68)c | 29 (29, 58)c | –1 (–12 to 10); 0.83 |
| 1 year | 23 (26, 66)c | 19 (22, 66)c | –4 (–13 to 4); 0.34 |
| 2 years | 19 (24, 63)c | 18 (27, 57)c | –2 (–12 to 8); 0.65 |
| Overalld | –3 (–10 to 4); 0.39 | ||
Appendix 14 Results of visual analogue scale bleeding scores sensitivity analysis: without those prescribed levonorgestrel intrauterine system at the time of operation
| Time of assessment | Polypectomy | Difference (95% CI);a p-value | |
|---|---|---|---|
| Outpatient mean (SD, n) | Inpatient mean (SD, n) | ||
| Bleeding duration VASb | |||
| Baseline | 48 (28, 53) | 49 (28, 42) | |
| 6 months | 32 (29, 47)c | 26 (23, 36)c | –8 (–20 to 5); 0.23 |
| 1 year | 21 (22, 44)c | 24 (30, 37)c | 4 (–8 to 17); 0.50 |
| 2 years | 18 (24, 45)c | 16 (23, 31)c | –5 (–17 to 7); 0.41 |
| Overalld | –4 (–12 to 5); 0.38 | ||
| Bleeding amount VASe | |||
| Baseline | 60 (26, 55) | 60 (28, 42) | |
| 6 months | 31 (31, 51)c | 31 (29, 37)c | 0 (–13 to 13); 0.98 |
| 1 year | 28 (27, 50)c | 18 (23, 41)c | –9 (–20 to 2); 0.11 |
| 2 years | 21 (26, 47)c | 22 (30, 35)c | –1 (–14 to 12); 0.93 |
| Overalld | –4 (–13 to 5); 0.33 | ||
Appendix 15 Results of operation acceptability
| Patient experience and preference | Polypectomy | Adjusted difference or RR (95% CI);a p-value | |
|---|---|---|---|
| Outpatient | Inpatient | ||
| Mean pain score during procedure (SD, n)b | 45 (26, 217) | – | – |
| Mean pain score 1 hour after procedure (SD, n)b | 28 (23, 176) | 23 (22, 191) | –5 (–10 to –1); 0.03 |
| Mean pain score on discharge (SD, n)b | 23 (21, 200) | 15 (17, 186) | –8 (–12 to –4); < 0.001 |
| Procedure acceptable? | n = 225 | n = 197 | |
| Totally | 136 (60%) | 152 (77%) | |
| Generally | 51 (23%) | 30 (15%) | 0.92 (0.85 to 0.99); < 0.001c |
| Fairly | 33 (15%) | 12 (6%) | |
| Unacceptable | 5 (2%) | 3 (2%) | |
| Exposure embarrassing? | n = 224 | n = 196 | |
| Extremely | 5 (2%) | 4 (2%) | 1.45 (0.86 to 2.45); 0.24d |
| Moderately | 17 (8%) | 24 (12%) | |
| A little | 79 (35%) | 35 (18%) | |
| No | 123 (55%) | 133 (68%) | |
| Recommend to a friend? | |||
| Yes/total | 205/222 (92%) | 190/196 (97%) | 0.96 (0.91 to 1.02); 0.16 |
| Same treatment again? | |||
| Yes/total | 200/223 (90%) | 186/193 (96%) | 0.95 (0.89 to 1.01); 0.09 |
| Preferred alternative treatment? | |||
| Yes/total | 47/218 (22%) | 39/190 (21%) | 0.95 (0.65 to 1.38); 0.78 |
Appendix 16 Range of procedures and equipment used within the Outpatient versus inpatient Polyp Treatment Trial
| Resource use | Outpatient (n = 254) | Inpatient (n = 253) |
|---|---|---|
| Anaesthesia | ||
| Local | 91 | 15 |
| General | 14 | 201 |
| Regional | 0 | 5 |
| None | 139 | 19 |
| Not specified | 10 | 13 |
| Scope manufacturer | ||
| Olympus 5-mm rigid hysteroscope (Olympus, Southend-on-Sea, UK) | 19 | 46 |
| Storz Bettocchi hysteroscope (Karl Storz – Endoskope, Tuttlingen, Germany) | 57 | 86 |
| Versascope® (Gynecare, Ethicon, Somerville, NJ, USA) | 142 | 44 |
| Wolf (Richard Wolf Medical Instruments Corporation, Vernon Hills, Chicago, IL) | 2 | 18 |
| Other | 4 | 5 |
| None/not specified | 30 | 54 |
| Scope type | ||
| Rigid | 176 | 197 |
| Flexible | 7 | 2 |
| Not specified | 71 | 54 |
| Resectoscope used? | ||
| Yes | 7 | 36 |
| No | 208 | 151 |
| Not specified | 39 | 66 |
| Speculum used? | ||
| Yes | 110 | 193 |
| No | 126 | 31 |
| Not specified | 18 | 29 |
| Instrument for detaching polyp | ||
| Forceps | 68 | 48 |
| Snare | 1 | 2 |
| Scissors | 5 | 1 |
| Curette | 9 | 60 |
| Blind polyp/sponge forceps | 32 | 55 |
| Electrode | 140 | 84 |
| Versapoint spring | 74 | 34 |
| Versapoint twizzle | 56 | 20 |
| Monopolar loop | 1 | 21 |
| Other (bipolar loop) | 9 | 7 |
| Any other instrument? | ||
| Yes | 6 | 4 |
| No | 230 | 224 |
| Not specified | 18 | 25 |
| Instrument to retrieve polyp | ||
| Hysteroscope | 131 | 61 |
| Blind forceps | 49 | 111 |
| Curette | 14 | 55 |
| None | 37 | 15 |
| Not specified | 16 | 29 |
| Further treatment for bleeding? | ||
| No | 170 | 174 |
| Not specified | 23 | 30 |
| Yes | 61 | 49 |
| Mirena | 32 | 43 |
| Progesterone | 7 | 0 |
| Tranexamic acid (Cyclokapron®, Pfizer) | 12 | 1 |
| Mefenamic acid (Ponstan, Pfizer) | 1 | 1 |
| Endometrial ablation | 5 | 2 |
| Hysterectomy | 1 | 1 |
| Other | 3 | 1 |
| Time taken (minutes) | ||
| For polypectomy (mean; SD) | 10.67; 8.21 (88% patients) | 12.1,7.78 (73.5% patients) |
| In outpatient room (mean; SD) | 28.1; 14.62 (81.5% patients) | 24; 9.65 (7% patients) |
| In operating theatre (mean; SD) | 33.78; 14.33 (7% patients) | 29.54; 13.43 (78% patients) |
| Surgeon grade | ||
| Consultant | 172 | 153 |
| Associate specialist | 30 | 15 |
| Staff grade | 7 | 1 |
| Specialist registrar | 28 | 63 |
| Other | 7 | 4 |
| Not specified | 9 | 17 |
Appendix 17 Local anaesthetic use in outpatient polypectomy
| Agent | Average use (ml) | Cost (£)a | Details | Proportional use | Proportional cost (£) |
|---|---|---|---|---|---|
| Lidocaine with adrenaline (2%) | 3.6 | 0.67 | 20 ml of 2% lidocaine with adrenaline = £1.37; (approximately one half vial per patient) | 0.4 | 0.27 |
| Prilocaine with felypressin (3%) | 4.4 | 0.94 | 2.2 ml of 3% prilocaine = £0.47 | 0.16 | 0.14 |
| Mepivacaine (3%) | 6.2 | 1.08 | 2.2 ml of 3% mepivacaine = £0.36 | 0.41 | 0.44 |
| Bupivacaine (3%) | 6 | 0.94 | 10 ml of 0.5% bupivacaine = £0.94 | 0.03 | 0.03 |
| Total cost | 0.88 |
Appendix 18 General anaesthesia (drug costs)
| Parenteral drugs | Details | Cost (£) | Source |
|---|---|---|---|
| Fentanyl (100 μg) | 2-ml ampoule of 50 μg/ml | 0.30 | BNF 2013157 |
| Propofol (200 mg) | 20-ml ampoule of 10 mg/ml | 4.18 | BNF 2013157 |
| Dexamethasone (4 mg) | 1-ml ampoule of 4 mg/ml | 0.91 | BNF 2013157 |
| Ondansetron (4 mg) | 2-ml ampoule of 2 mg/ml | 1.00 | BNF 2013157 |
| Volterol (75 mg) | 2-ml ampoule of 37.5 mg/ml | 4.80 | BNF 2013157 |
| Paracetamol (1 g) | 100-ml ampoule of 10 mg/ml; given in 1 l of normal saline | 4.37 | BNF 2013157 |
| Normal saline (1 l) | 1-l normal saline infusion | 3.12 | Online sourcea |
| Sevoflurane (3%) | For an average of 12 minutesb | 1.93 | BNF 2013157 and Lockwood et al.158 |
| Total cost | 17.49 |
Appendix 19 General anaesthesia (equipment plus staff costs)
| Equipment/staff | Details | Costa (£) | Source |
|---|---|---|---|
| Intravenous cannula | Pink cannula | 1.6 | Online sourcea |
| Dressing | – | 0.2 | Online sourcea |
| Intravenous drug-giving set | – | 1.86 | Online sourcea |
| Laryngeal mask airway | – | 10.74 | Online sourcea |
| Syringes | 2 × 20 ml; 5 × 2 ml | 0.67 | Online sourcea |
| Consultant anaesthetist | 30 minutes | 73.5 | PSSRU 2012137 |
| Nurse (grade 5) | 10 minutes (for patient transfer) | 14.2 | PSSRU 2012137 |
| Porter | 10 minutes (for patient transfer) | 3.5 | PSSRU 2012137 |
| Operating Department Practitioner | 30 minutes | 10.5 | PSSRU 2012137 |
| Total | 116.8 |
Appendix 20 Equipment costs for polypectomy
| Equipment | Procurement cost (£)a | Equipment lifespan (years) | Annuitisation factorb | Annuitised costs (£)c | Times re-used per week | Sterilisation/maintenance per patient (£) | Cost per patient (£) |
|---|---|---|---|---|---|---|---|
| Cervical speculum | 2 | 0 (disposable) | 0 | 2 | 0 | 0 | 2 |
| Grasping forcepsd | 662.5 | 5 | 0.842 | 773.7 | 5 | 0.2 | 0.8 |
| Hysteroscope | 5400 | 10 | 0.71 | 6493 | 5 | 1.5 | 4 |
| Electrode | 212a | 0 (disposable) | 0 | 0 | 0 | 0 | 212 |
| Hysteroscope camera system (Olympus/Versascope/Storz) | 50,500 | 5 | 0.71 | 59,268 | 20 | 14.1 | 25.5 |
| Total cost for equipment | 56,776.5 | 237.5 | |||||
Appendix 21 Outpatient polypectomy cost (bottom-up costing)
| Resource | Cost per patient (£) | Source | Details |
|---|---|---|---|
| Initial clinic | 86 | PSSRU159 | 30 minutes of gynaecological consultant time, including training costa |
| Hysteroscopy | 239.6 | Critchley et al.160 | Inflated to 2011/12 rates from 2004 costsb |
| Nurse (Band 6) | 21.50 | PSSRU | 30 minutes |
| Consultant | 73.5 | PSSRU | As above |
| Procedure costs | 237.5 | Estimated | See Appendix 20 |
| Local anaesthetic costs | 0.35 | Estimated | See Appendix 17; local anaesthetic used in only 40% of outpatient procedures |
| Total cost | 658.45 |
Appendix 22 Inpatient polypectomy cost (bottom-up costing)
| Resource | Cost per patient (£) | Source | Details |
|---|---|---|---|
| Initial assessment | |||
| Initial clinic | 86 | PSSRU159 | 30 minutes of consultant time including training costs |
| Hysteroscopy | 239.6 | Critchley et al.160 | Inflated to 2011/12 rates from 2004 costsa |
| Pre-assessment clinic | 78.8 | PSSRU159 | 45 minutes of Band 6 nurse time – with patient contact |
| Electrocardiography | 61 | NHS reference costs 2011/12138 | Monitoring Electrocardiogram EA47Z |
| Blood tests | 5 | Local NHS laboratory | Full blood count (£2) and urea and electrolytes (£3) |
| Procedure costs | |||
| Day-case admission cost | 673 | NHS reference costs 2011/12138 | Unit price; day-case admission |
| Nurse assessment (Band 6) | 10.75 | PSSRU159 | Assessment for admission; 15 minutes |
| Anaesthetist | 24.5 | PSSRU159 | Consultant; 10 minutes’ preoperative assessment |
| Porter costs | 5.25 | PSSRU159 | Transfer to operating theatre 30 minutes |
| Nurse cost (Band 5) | 17.5 | PSSRU159 | Transfer to operating theatre 30 minutes |
| Equipment cost | 237.5 | Derived | See Appendix 20 |
| General anaesthetic drugs | 17.5 | Estimated | See Appendix 18 |
| General anaesthetic equipment | 116.8 | Estimated | See Appendix 19 |
| Postoperative | |||
| Recovery ward | 52.5 | PSSRU159 | 30 minutes’ nurse time (Band 6); patient contact |
| Porter costs | 5.25 | PSSRU159 | Transfer to ward; 15 minutes |
| Nurse cost | 17.5 | PSSRU159 | Transfer to ward; 30 minutes |
| Discharge | 14.75 | PSSRU159 | Trainee doctor 5 minutes; staff nurse 15 minutes (Band 5) |
| Overall cost | 1663.2 | ||
Appendix 23 Cost incurred (per-protocol analysis)
| Cost detaila | Inpatient (£) | Outpatient (£) |
|---|---|---|
| n = 239 b | n = 248 b | |
| General | ||
| Baseline costs | 343 | 343 |
| Cost of procedure | 1361.3; 26.3 | 854.8; 46 |
| Total procedure cost | 1704.3; 26.3 | 1197.8; 46 |
| Cost of related SAE (0–6 months) | 126.9 (661.2) | 83.6 (507.6) |
| Cost of related SAE (0–12 months) | 168.2 (758.4) | 124.1 (631.9) |
| n = 208 [13%] c | n = 227 [8.5%] c | |
| Total additional cost 0–6 months | 56.3 (277.8) | 79.2 (297.6) |
| Overall cost at 6 months | 1666.8 (754.3) | 734.1 (677.8) |
| n = 217 [9.2%] c | n = 222 [10.5%] c | |
| Total additional cost 0–12 months | 111.5 (404.2) | 478.5 (1139.6) |
| Overall cost at 12 months | 1761.6 (879.8) | 884.3 (1123.2) |
Appendix 24 Outcome data per protocol (complete data set analysis)
| Clinical outcome measures | Outpatient, n = 248 | Inpatient, n = 239 |
|---|---|---|
| Effectiveness | ||
| 6 months | 0.73 (0.45) [15.3] | 0.80 (0.41) [11.7] |
| 12 months | 0.81 (0.39) [9.3] | 0.83 (0.38) [10.5] |
| EQ-5D | ||
| Baseline | 0.79 (0.25) [3.6] | 0.77 (0.28) [6.2] |
| 6 months | 0.87 (0.22) [6.5] | 0.87 (0.2) [13.4] |
| 12 months | 0.86 (0.24) [10.9] | 0.86 (0.25) [9.2] |
Appendix 25 Cost-effectiveness and cost–utility analyses: per protocol
| PP analysis | Outpatienta | Inpatienta | Differenceb |
|---|---|---|---|
| 6 months | |||
| Overall cost | 716.1 (643.4) | 1639.5 (702.6) | –923.4 [59.8] |
| Overall QALY 6 months | 0.41 (0.1) | 0.41 (0.1) | 0.006 [0.01] |
| Effectiveness | 0.76 (0.43) | 0.79 (0.41) | 0.03 [0.04] |
| ICER (Δ cost/Δ effectiveness) | £30,780 per additional patient who feels better with inpatient treatment | ||
| ICER (Δ cost/Δ QALY) | £153,900 per additional QALY gained on the inpatient arm | ||
| 12 months | |||
| Overall cost | 832.4 (818.9) | 1773.3 (877.1) | –941.0 [75.5] |
| Overall QALY 12 months | 0.84 (0.2) | 0.83 (0.2) | 0.006 [0.02] |
| Effectiveness | 0.83 (0.37) | 0.82 (0.38) | –0.01 [0.03] |
| ICER (Δ cost/Δ effectiveness) | Outpatient treatment dominates | ||
| ICER (Δ cost/Δ QALY) | £156,833 per additional QALY gained on the inpatient arm | ||
Appendix 26 Deterministic sensitivity analyses: per protocol
| PP analysis | DSA-1 (bottom-up costs) | DSA-2 (out-of-pocket costs) | DSA-3 (‘see and treat’ at same appointment for all OP) | DSA-4 (‘see then treat’ for all OP) | DSA-5 (new outpatient tariffs) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 months | 12 months | 6 months | 12 months | 6 months | 12 months | 6 months | 12 months | 6 months | 12 months | |
| Cost differencea | –1007.1 [61.1] | –962.6 [77.9] | –944.3 [59.7] | –899.8 [79.6] | –953.5 [62.5] | –909.0 [76.9] | –841.5 [62.5] | –797.0 [76.9] | –476.5 [62.5] | –432.0 [76.7] |
| ICERb | 33,570 | Outpatient dominates | 31,477 | Outpatient dominates | 31,783 | Outpatient dominates | 28,050 | Outpatient dominates | 15,883.3 | Outpatient dominates |
| Cost/QALY | 167,850 | Outpatient dominates | 157,383 | Outpatient dominates | 158,917 | Outpatient dominates | 140,250 | Outpatient dominates | 79,417 | Outpatient dominates |
Appendix 27 Probabilistic sensitivity analysis: patient treatment compared with inpatient treatment – per-protocol analysis at 6 months
Probabilistic sensitivity analysis (see also Appendix 28) examines simultaneously the uncertainty in the cost and outcomes in the PP analysis. The x- and y-axes represent the incremental effectiveness and cost of outpatient treatment compared with inpatient treatment, respectively. Most of the values fall in the lower-right and lower-left quadrants, demonstrating that inpatient therapy is more expensive than outpatient treatment. The equal distribution between the left and right quadrants suggests that there is considerable uncertainty regarding the effectiveness of one treatment over the other (based on QALY values). In other words, the effectiveness of both treatments is similar.
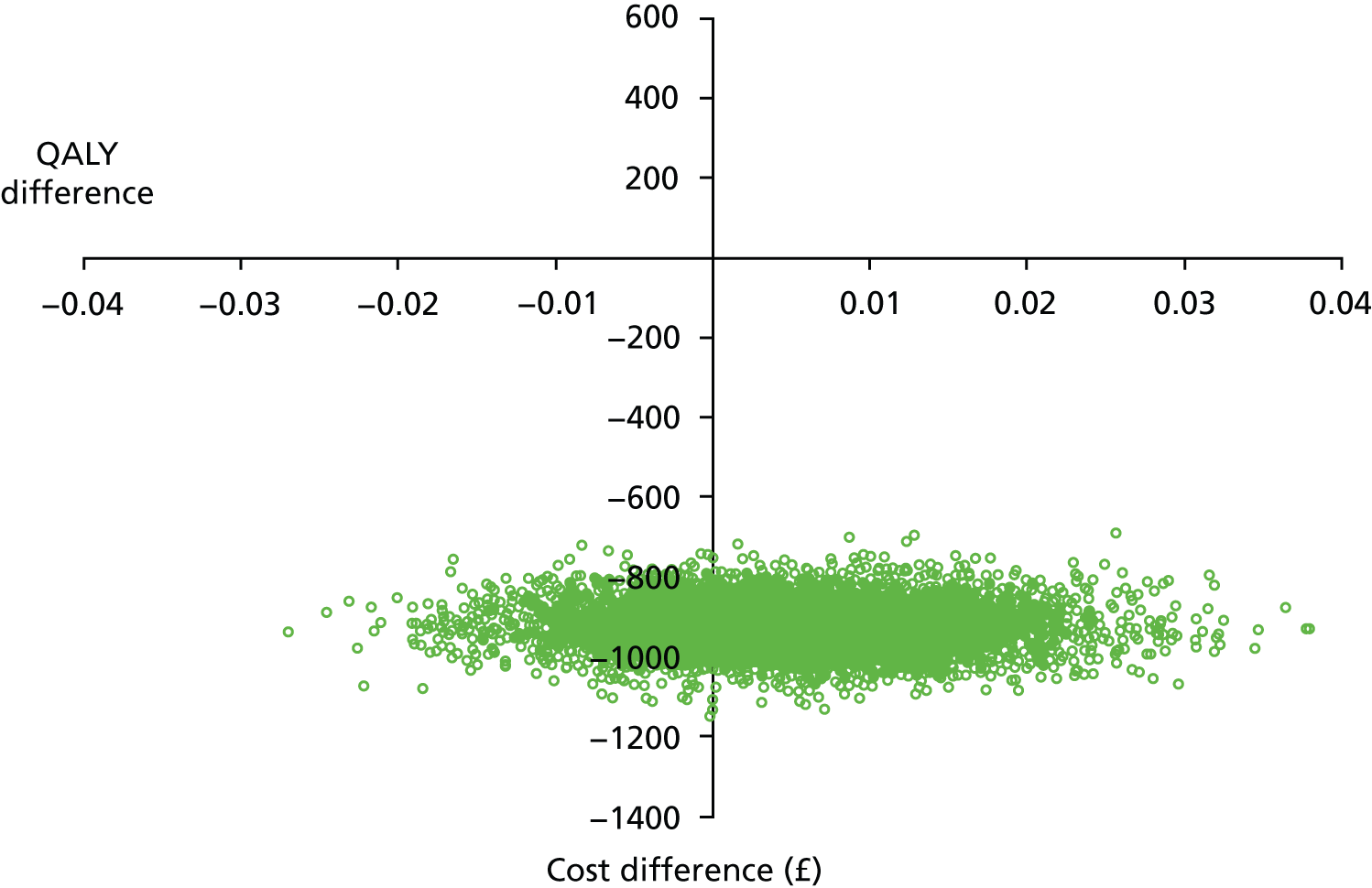
Appendix 28 Probabilistic sensitivity analysis: outpatient treatment compared with inpatient treatment – per-protocol analysis at 12 months
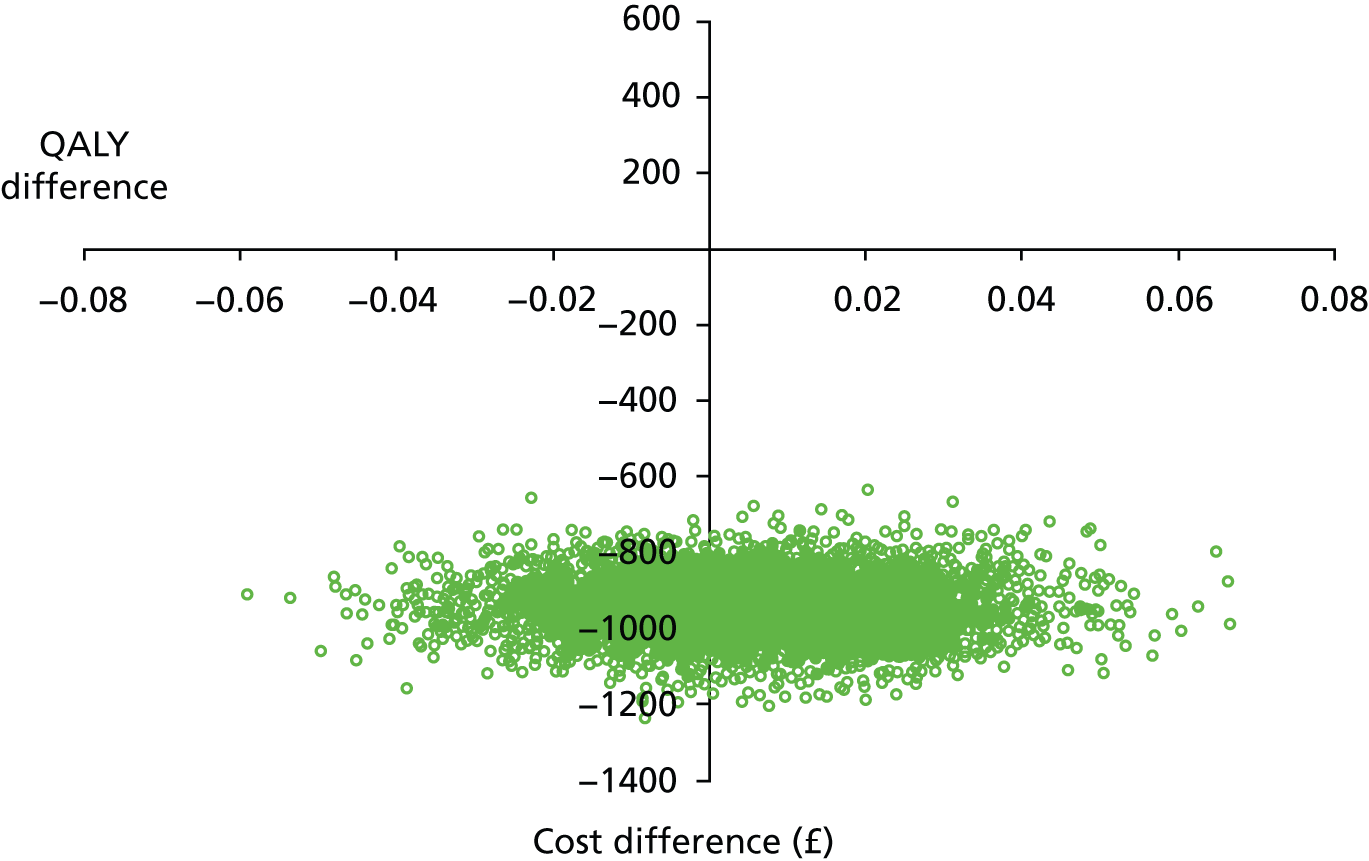
Appendix 29 Cost-effectiveness acceptability curve: outpatient treatment compared with inpatient treatment – per-protocol analysis
A CEAC illustrates the uncertainty around the cost-effectiveness estimates by demonstrating the likelihood of an intervention being cost-effective at a given cost threshold compared with the proposed alternative. In the PP analysis, similar to that in the ITT analysis, outpatient treatment is most cost-effective at baseline, as it is cheaper although being equally effective.
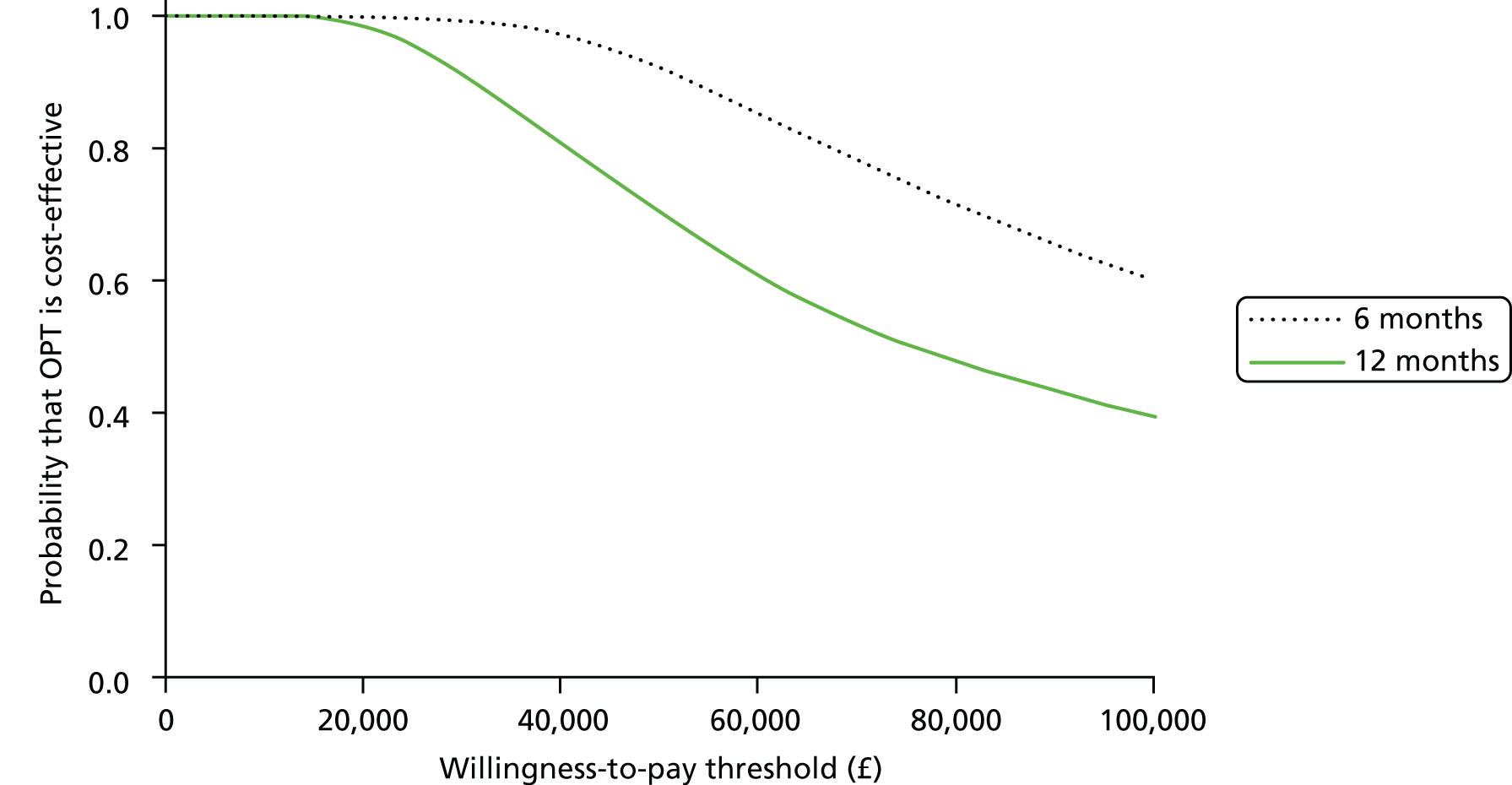
Appendix 30 Outpatient versus inpatient Polyp Treatment Trial collaborators
Barnsley District General Hospital, South Yorkshire: C Bonner, K Cannon, KA Farag, K Raychaudhuri, M Reid and A Zahi.
Birmingham Heartlands/Solihull Hospital, Birmingham: Jan Blunn, S Guruswami, S Irani and D Robinson.
Birmingham Women’s Hospital, Birmingham: T Bingham, TJ Clark, N Cooper, J Gupta, S Madari, D Mellers, S O’Connor, C O’Hara, M Pathak, V Preece, E Sangha, M Shehmar, P Trinham and A Wilson.
Bishop Auckland General Hospital, County Durham: J Dent, J Macdonald and P Sengupta.
Blackpool Victoria Hospital, Blackpool: C Brookes and J Davies.
Bradford Royal Infirmary, Bradford: M Jackson, S Jones, H Ludkin and S Riddiough.
Castle Hill Hospital, East Yorkshire: J Allen, T Cathcart, D Cox, S Ford, L Kenny, K Phillips, N Rawal, A Rodgers and J Siddiqui.
Chelsea & Westminster Hospital, London: A Dine-Atkinson, S Kalkur, G Merriner, J Ben-Nagi, A Raza and R Richardson.
City General Hospital, University Hospital of North Staffordshire, Stoke-on Trent: T Bingham and I Hassan.
City Hospital, Sunderland: D Edmundson, J Chamberlain, A Barge, P Wake, D Milford and E Walton.
Countess of Chester Hospital, Chester: S Arnold, M Blake, MJ McCormack, N Naddad, J Hane and S Wood.
Kidderminster Hospital, Worcester: M Labib, T Martin, S Moss and M Pathak.
Liverpool Women’s Hospital, Liverpool: N Aziz, L Harris and D Pattison.
Manor Hospital, Walsall: J Pepper.
Musgrove Park Hospital, Taunton: M Escott and G Fender.
New Cross Hospital, Wolverhampton: M Saeed.
Newham University Hospital, Plaistow: A Antoniou and R Chenoy.
Norfolk and Norwich University Hospital, Norwich: E Morris and M Sule.
Ormskirk & District General Hospital, Southport: A Cope, S Sharma and T Taylor.
Queen Charlotte’s & Chelsea Hospital, London: N Panay and C Rothan.
Queen’s Hospital, Romford: A Coker.
Royal Blackburn Hospital, Blackburn: M Abdel-Aty, K Bhatia, S Gardiner, W Myint and M Willett.
Royal Hallamshire Hospital, Sheffield: V Brown, C Bonner, M Connor and S Stillwell.
Royal Infirmary of Edinburgh, Edinburgh: A Horne, S Milne and J Rowan.
Sandwell General Hospital, West Midlands: A Ewies and J Kabukoba.
Shotley Bridge Hospital, County Durham: J Dent and P Sengupta.
St Mary’s Hospital, Manchester: R Biancardi, K Donnelly, L Dwyer, K Naidoo and I Pinton.
Stafford Hospital, Stafford: K Chin, T Harrison, J Roger, D Sirdefield and J Stacey.
Royal Oldham Hospital, Oldham: Z Anjum and N Aziz.
The Royal Victoria Infirmary, Newcastle upon Tyne: J Bainbridge, G Cosgrove, A Desai, J Gebbie, D Koleskas, P Ranka and M Roberts.
Whiston Hospital, Merseyside: C Cunningham and C Nwosu.
We thank the members of the TSC for their assistance throughout the project: Professor J Thornton (Professor of Obstetrics and Gynaecology, University of Nottingham) – chairperson; Professor P O’Donovan (Professor of Medical Innovation and Gynaecology, University of Bradford); E Nicholls (Birmingham Women’s Hospital, Consumer); Professor Jon Deeks (University of Birmingham, Statistician); and Dr Stavros Petrou (University of Oxford, Health Economist).
List of abbreviations
- AA
- Automobile Association
- AAGL
- American Association of Gynecologic Laparoscopists
- AUB
- abnormal uterine bleeding
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CUA
- cost–utility analysis
- D&C
- dilatation and curettage
- DMEC
- Data Monitoring and Ethics Committee
- DSA
- deterministic sensitivity analysis
- EH
- endometrial hyperplasia
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-3L
- European Quality of Life-5 Dimensions (three-level version)
- FIGO
- International Federation of Gynecology and Obstetrics
- GnRH-a
- gonadatrophin-releasing hormone agonist
- GP
- general practitioner
- HCHS
- Health and Community Health Services
- HMB
- heavy menstrual bleeding (formerly known as menorrhagia)
- HR
- hazard ratio
- HRQL
- health-related quality of life
- HRT
- hormone replacement therapy
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IMB
- intermenstrual bleeding
- IQR
- interquartile range
- ISS
- Index of Sexual Satisfaction
- ITT
- intention to treat
- LNG-IUS
- levonorgestrel intrauterine system
- LREC
- Local Research Ethics Committee
- MCMC
- Markov chain Monte Carlo
- MeSH
- medical subject heading
- MMAS
- Menorrhagia Multi-attribute Assessment Scale
- MRC
- Medical Research Council
- MREC
- Multicentre Research Ethics Committee
- NICE
- National Institute for Health and Care Excellence
- NNTB
- number needed to benefit
- NNTH
- number needed to harm
- ONS
- Office for National Statistics
- OPH
- outpatient hysteroscopy
- OPT
- outpatient polyp treatment
- OR
- odds ratio
- PBAC
- pictorial blood assessment chart
- PMB
- postmenopausal bleeding
- PP
- per protocol
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Resource Unit
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RCT
- randomised controlled trial
- RE
- related event
- RR
- relative risk
- SAE
- serious adverse event
- SIS
- saline infusion sonography/scan
- SMF
- submucous fibroid
- STROBE
- STrengthening the Reporting of OBservational studies in Epidemiology
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- TVS
- transvaginal ultrasound scan
- VAS
- visual analogue scale
- WTP
- willingness to pay