Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/56/01. The contractual start date was in January 2010. The draft report began editorial review in October 2014 and was accepted for publication in February 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Willem Kuyken declares that he is a codirector of the Mindfulness Network Community Interest Company.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Kuyken et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Depression is a major public health problem that, like other chronic conditions, tends to run a relapsing and recurrent course,1 producing substantial decrements in health and well-being. 2 The World Health Organization (WHO) predicts that by 2020 depression will be the second leading cause of disability in the world. 3 The cost of mood disorders in the UK has been calculated at 7% of national income with a direct cost to health services of £3B. 4 More than 50% of patients experience at least two episodes of depression. 5 Moreover, without ongoing treatment, people suffering recurrent depression suffer relapse/recurrence at rates as high as 80%, even after successful acute treatment. 5,6 Thus, most of the prevalence, burden and cost of depression is a consequence of relapse/recurrence and the majority of the burden attributable to depression could be offset through interventions aimed at preventing depressive relapse/recurrence. 7
Current treatments for depression in primary care
Currently, the majority of depression is treated in primary care and treatment with maintenance antidepressant medication (m-ADM) is the mainstay approach to preventing relapse/recurrence. 8–11 To stay well the National Institute for Health and Care Excellence (NICE) recommends that people with a history of recurrent depression continue m-ADM for at least 2 years. 8 However, many patients experience unpleasant side effects, rates of m-ADM adherence tend to be low and patients often express a preference for psychosocial interventions. 12–14 Service user organisations, such as Depression Alliance, therefore advocate greater availability of psychosocial therapies. Government advisors Layard and Clark4 recommend that there should be parity of esteem for mental and physical health. That is, today, evidence-based treatment should be as available for mental illness as for physical illness. They provide a compelling narrative that this would be a cost-effective approach to enhancing the mental health of the nation. In line with this, significant government initiatives, such as Improving Access to Psychological Therapies, aim to offer accessible, acceptable and cost-effective psychosocial models of care and the last 10 years has seen significant progress in the accessibility of evidence-based mental health services in the UK. 4,15,16
Psychosocial approaches to prevent depressive relapse/recurrence
There is a strong evidence base for psychosocial treatments for recurrent depression, most notably cognitive–behavioural therapy (CBT) and interpersonal therapy, and promising evidence for several other therapies. 8,17 Policy initiatives, user groups and professional consensus recommend as priorities for future research the development of psychosocial interventions to prevent depressive relapse/recurrence and the use of non-traditional delivery systems, such as group interventions, to maximise accessibility and cost-effectiveness. 18,19 Mindfulness-based cognitive therapy (MBCT) is a psychosocial group-based relapse prevention programme. It was developed from translational research into mechanisms of depressive relapse/recurrence. 20 It is recommended by NICE as a psychological approach to relapse prevention for people who are currently well but who have experienced three or more previous episodes of depression. 8 There is much clinical enthusiasm for MBCT, as evidenced by the high rates of patient engagement, the development of MBCT therapist training programmes in the UK at the Universities of Bangor, Exeter and Oxford and more recently the development of an All Party Parliamentary Group on Mindfulness. 21 Implementation of MBCT throughout the NHS is patchy and variable but becoming more widespread. 22,23 In summary, MBCT shows the potential to contribute significantly to reducing the prevalence of depression in UK primary care settings.
Review of the evidence for mindfulness-based cognitive therapy up to the trial start date
Efficacy and effectiveness
The first two MBCT randomised controlled trials (RCTs) of patients with a history of recurrent depression, currently in remission and not receiving any active treatment, reported in 200024 and 2004. 25 Both found that MBCT plus usual care halved the rate of relapse/recurrence compared with usual care alone over 60 weeks of follow-up [hazard ratio (HR) 0.47, 95% confidence interval (CI) 0.27 to 0.84;25 HR 0.28, 95% CI 0.13 to 0.6024]. In both trials patients were able to seek help as they normally would over the course of the study and rates of treatment update were comparable across both arms of the trial. However, to provide a robust test of the potential effectiveness of MBCT over usual care, both trials included only people not currently receiving antidepressant medication (ADM) at the time of study entry. Moreover, as a first test of MBCT, the comparison was usual care rather than another active treatment.
A systematic review published in 2007 that included these first two trials and two further replications in Europe showed a significant additive effect of MBCT over usual care for patients with recurrent depression, but only for patients who have experienced three or more previous episodes. 26 This is the same evidence that led to NICE’s guidance that MBCT be offered as a relapse prevention approach to patients with three or more episodes of depression.
Exploratory pilot trial
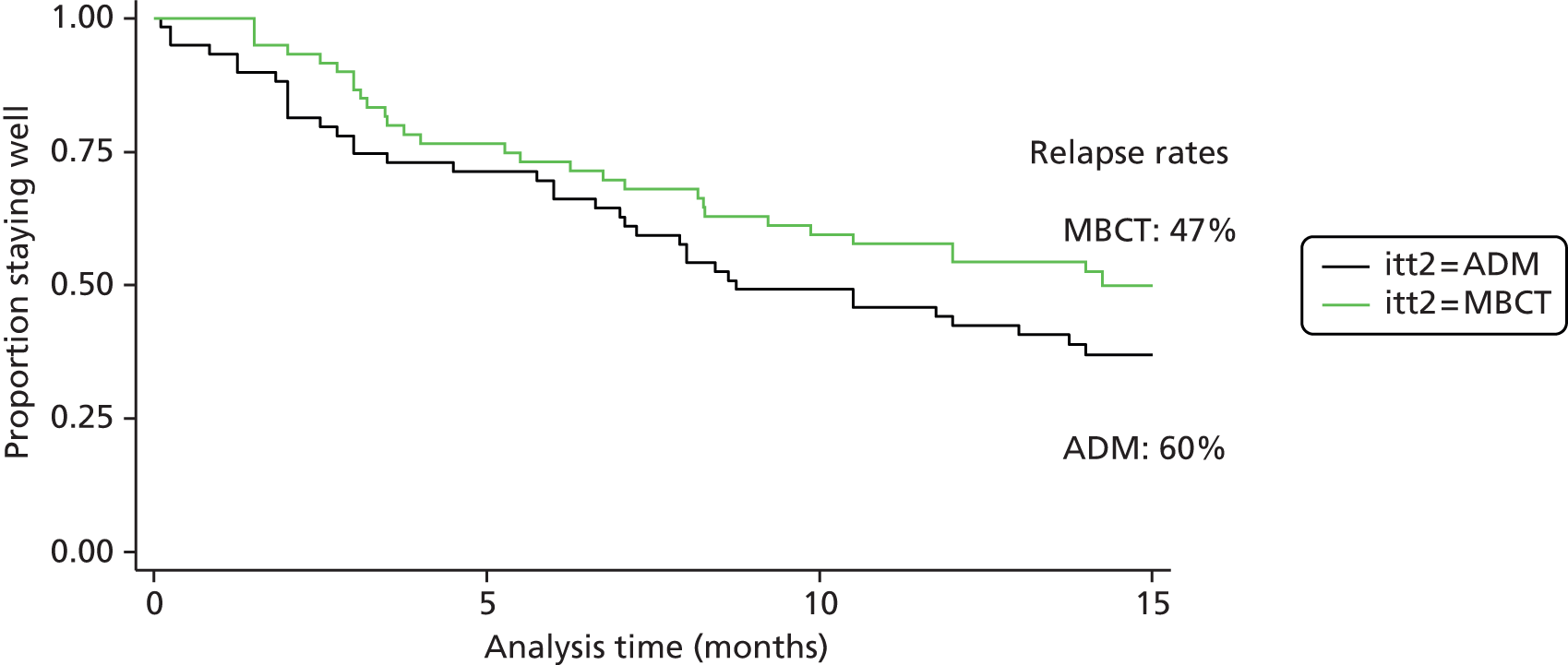
Through a Medical Research Council (MRC)-funded pilot trial27–29 we sought to address the gaps in the evidence base outlined in the previous section by comparing MBCT with the current mainstay approach to relapse prevention, m-ADM. In this pilot trial 123 patients who were currently taking m-ADM to manage recurrent depression were randomised to either continue m-ADM or take part in a MBCT course and then taper and discontinue their m-ADM. The findings suggested that MBCT may not only provide an alternative to m-ADM (relapse/recurrences at 15 months: MBCT 47%, m-ADM 60%), but also, in an adequately powered definitive trial, produce superior outcomes (Figure 1). 28 Finally, the study suggested that MBCT may also be superior to m-ADM in terms of improved quality of life, reduced residual depressive symptoms and reduced psychiatric comorbidity. The study’s stated aims and outcomes were:
-
Examine feasibility. The achievement of all study milestones within budget and on time demonstrated feasibility.
-
Establish recruitment methods. An acceptable and effective recruitment methodology was developed as evidenced by our over-recruitment by 54%.
-
Establish a training model for MBCT therapists. We developed a model of training with input from John Teasdale, one of the developers of MBCT.
-
Cost MBCT. The MBCT intervention was estimated to cost circa £200 per participant,28 which compares favourably with an estimated cost of £750 for individual CBT. 16
-
Elicit service user feedback. At the end of the pilot trial all participants were interviewed and asked about their experience of MBCT as a relapse prevention programme. 30 These studies informed the PREVENT study to maximise MBCT’s acceptability and application to supporting patients taper and discontinue their m-ADM.
FIGURE 1.
Kaplan–Meier survival curve in the exploratory trial. Survival estimates by intention to treat.
Costs and cost-effectiveness
To inform commissioning of care for people with recurrent depression we must establish MBCT’s cost-effectiveness over an adequate follow-up period. In our pilot trial28 we found that the per-person cost for the MBCT group was £274 more than that for the m-ADM group but this difference was not significant. Cost-effectiveness analyses suggested that the additional cost of MBCT may be justified in terms of improvement in the proportion of patients who undergo relapse/recurrence, but only if willingness to pay for such improvements is ≥ £600. In terms of depression-free days, the incremental cost-effectiveness ratio (ICER) of MBCT is comparable with that in similar studies, with a ratio of £30 per depression-free day for total costs and £14 per depression-free day for health service costs. (Please note that these costs were reported in reference 28 in international dollars using a conversion rate of 0.6.) Recent ICERs for collaborative care programmes include £20 in terms of total outpatient costs31 and £12 in terms of total inpatient and outpatient costs. 32 Estimates of £8–14 have been reported for a depression relapse prevention programme. 33 Exploration of costs over time suggested that differences in cost converge and MBCT becomes cheaper than m-ADM over the final 3-month period of the study. If this trend were to continue, the relative cost-effectiveness of MBCT may increase over time. Future studies should consider longer follow-up periods to test this hypothesis.
Process studies to examine acceptability and mechanism of change
As with the cost-effectiveness of MBCT, the evidence for the mechanism of change in outcomes with MBCT therapy is limited. Coelho et al. 26 found no evidence of the ‘specific effectiveness of MBCT, despite this being the logical progression from the current research’ (p. 1004). In other words, no trials had shown whether MBCT works through its specific hypothesised mechanisms and/or through non-specific cognitive–behavioural, psychoeducation and group/therapist support components. They therefore recommended ‘the need for randomised controlled trials to compare MBCT with other non-pharmacological approaches’ (p. 1005) and the inclusion of tests of the specific and non-specific mechanisms of change.
Proposed mechanisms of action
Mindfulness-based cognitive therapy’s theoretical premise is that depressive relapse/recurrence is associated with the reinstatement of negative modes of thinking and feeling that contribute to depressive relapse and recurrence. 34 This ‘reactivated’ network of negative thoughts and feelings can perpetuate into a depressive episode. Laboratory studies support this model by showing that recovered depressed patients revert to a depressive information processing style following a sad mood induction (for a review see Segal et al. 35). Following successful treatment for depression, those patients showing greater reactivation of dysfunctional thinking styles in response to a sad mood provocation are at the highest risk of relapse/recurrence over an 18-month period. 35 Moreover, patients who recovered with CBT showed significantly less cognitive reactivation than those who recovered with ADM. Attenuating the reactivation of dysfunctional thinking styles may therefore represent one mechanism by which CBT helps prevent depressive relapse/recurrence. Mindfulness skills are taught as a means to note distressing thoughts and feelings, hold such experiences in awareness and cultivate acceptance and self-compassion to break up associative networks and offset the risk of relapse/recurrence. 34 This dimension of mindfulness, which involves meeting distressing thoughts and feelings with kindness, empathy, equanimity and patience, is woven into mindfulness-based applications and is thought to be crucial to the change process. 36 Intentional attention is learned in the first three MBCT sessions using a range of core mindfulness practices (the body scan, mindful movement and mindfulness of the breath). As well as developing attention, these early sessions highlight habitual patterns of reactivity that arise during meditation (e.g. intrusive negative thoughts) and the associated aversion and judgements (e.g. ‘I am no good at this, I am just more aware of how badly I feel’). As the person learns mindfulness skills, he or she learns to give less authority to self-judgement and blame – the fuel for depressive thinking – and to respond to these states with compassion, in short to step out of habitual unhelpful patterns of thinking. 36 Elucidating these putative mechanisms of action of MBCT will improve theoretical understanding of how this relatively new treatment works and provide the opportunity to enhance efficacy through emphasis of these mechanisms.
Our previous mechanisms study, which was embedded in the pilot trial, demonstrated that, consistent with MBCT’s theoretical premise, increases in mindfulness and self-compassion across treatment mediated the effect of MBCT on depressive symptoms at 15 months’ follow-up. 37 Furthermore, MBCT changed the relationship between post-treatment cognitive reactivity and depressive outcome. In patients receiving m-ADM, greater reactivity predicted poorer outcome, replicating previous findings. 35 However, following MBCT there was no support for this toxic relationship between reactivity and outcome, with an indication that enhancement of self-compassion had nullified this relationship. These findings were consistent with an evidence synthesis arguing that MBCT works through a ‘retraining of awareness and non-reactivity, allowing the individual to more consciously choose those thoughts, emotions, and sensations, rather than habitually reacting to them’ (p. 569). 38 The results are also in line with data using a self-report measure of cognitive reactivity showing that MBCT attenuates reactivity and its impact on depression. 39 This is reflected in distinct neural responses to sad mood in people who had undergone MBCT, suggesting a neural basis for these findings. 40 The findings suggest that, whereas negative mood may reactivate dysfunctional thinking patterns in people who have participated in a MBCT class, it is their response to these dysfunctional thoughts that is altering their impact at follow-up.
Rationale for the research
In summary, the evidence for MBCT indicates that it is more effective than usual care in preventing depressive relapse/recurrence in people with a history of three or more episodes. Moreover, there is preliminary evidence from our pilot trial to indicate that it may be cost-effective and that it works through its hypothesised mechanism. An editorial published in 2012 in the British Medical Journal41 concluded that key remaining uncertainties include (1) whether or not MBCT provides an alternative for people wishing to discontinue m-ADM, (2) how acceptable MBCT is and (3) what mechanism MBCT works through.
Aims and objectives
The overarching policy aim and research question of the PREVENT trial was to establish whether MBCT with support to taper and/or discontinue antidepressant medication (MBCT-TS) is superior to and more cost-effective than an approach of m-ADM in a primary care setting for patients with a history of recurrent depression followed up over a 2-year period.
The specific objectives of the PREVENT trial were to compare MBCT-TS and m-ADM for patients with recurrent depression in terms of:
-
time to depressive relapse/recurrence over 2 years (primary outcome)
-
depression-free days, residual depressive symptoms and health-related quality of life at 1 month post treatment and 9, 12, 18 and 24 months post randomisation and psychiatric and medical comorbidity at 12 and 24 months post randomisation (secondary outcomes)
-
costs and cost-effectiveness as assessed by incremental cost per relapse/recurrence prevented and incremental cost per quality-adjusted life-year (QALY) at 1 month post treatment and 9, 12, 18 and 24 months post randomisation.
In addition, we asked whether or not an increase in mindfulness skills is the key mechanism of change of MBCT.
To address these policy and explanatory questions patients were recruited through primary care and treated in accessible primary care or community settings. This was a single-blind, parallel RCT examining MBCT-TS compared with m-ADM. The m-ADM was constant over the 2 years of the study and the psychosocial intervention was a front-loaded, 8-week relapse/recurrence prevention programme. Patients in the MBCT-TS arm received support to taper their ADM. The process studies employed mixed methods.
Chapter 2 Mindfulness-based cognitive therapy with support to taper and/or discontinue antidepressant medication
Mindfulness-based cognitive therapy
Mindfulness-based cognitive therapy is an 8-week, group-based programme (12–15 participants per group) designed to teach skills that prevent the recurrence of depression. 20 It is derived from both mindfulness-based stress reduction (MBSR), a programme with demonstrated efficacy in ameliorating distress in people suffering chronic disease,42 and CBT for acute depression,43 a programme with demonstrated efficacy in preventing depressive relapse/recurrence. 44 MBCT is based on theoretical and empirical work showing that depressive relapse/recurrence is associated with the reinstatement of automatic modes of thinking, feeling and behaving that are counterproductive in contributing to and maintaining depressive relapse and recurrence (e.g. self-critical thinking and behavioural avoidance). 45
Participants learn to recognise these ‘automatic pilot’ modes and respond to them in more functional ways by employing complementary cognitive–behavioural and mindfulness practices. This involves recognising early warning signs of depressive relapse/recurrence, being able to ‘turn towards’ them with kindly interest and decentring in ways that ‘nip them in the bud’. The cognitive–behavioural component involves responding to negative thinking and behavioural activation. In the latter stages of the course participants develop a ‘response/action plan’ that sets out strategies for responding when they become aware of early warning signs of relapse/recurrence. The mindfulness component involves extensive practice of mindfulness skills (e.g. meditation practices) designed to improve participants’ attentional control, ability to decentre from negative thinking and emotion regulation and increase self-compassion. There is a movement towards meeting difficulty with curiosity, a quality of allowing and self-compassion before deciding on skilful ways of responding. Participants are encouraged to bring these skills in to all aspects of their lives.
Adaptations made to the mindfulness-based cognitive therapy programme for the PREVENT trial
In the PREVENT trial delivery of MBCT followed the manual as described by Segal, Williams and Teasdale34 with a few adaptations based on (1) the need for therapists to support MBCT participants in tapering their ADM and (2) experience from the pilot trial. 28 Participants attended a one-to-one orientation session with the therapist followed by group sessions lasting 2.25 hours over 8 consecutive weeks. Session content included guided mindfulness practices (i.e. body scan, sitting meditation, movement); inquiry into participants’ experience of these practices; weekly review of home practice (i.e. 40 minutes of mindfulness practice per day with the guidance of a CD, bringing mindfulness into everyday life); and teaching of/dialogue around cognitive–behavioural skills. MBCT-TS patients also received an additional four group reunion sessions during the first year of follow-up to provide ongoing support and rehearse the key components of the interventions. The first of these booster sessions occurred within 3–5 weeks after the end of the 8-week MBCT-TS programme as this was the time when patients would be tapering their ADM and may be in most need of support. Such booster sessions significantly enhance relapse/recurrence prevention46 and were a match for general practitioner (GP) attention, which was part of ongoing clinical management in the m-ADM group. A list of the individual adaptations and when they were made is provided in Table 1.
| Rationale for adaptation | Adaptation | When it came into effect |
|---|---|---|
| Preparation for tapering of medication at session 6 by increasing understanding of relapse/recurrence and ways of taking action/responding at an earlier stage |
|
|
|
|
|
| Involving GPs in the participants’ response plan |
|
|
| Supporting participants around the early stages of tapering |
|
|
| Teaching about depression at an experiential level, allowing participants to track their process with awareness, to illustrate the potential for relapse/recurrence around a drop in mood and how mindfulness may offer the possibility of somewhere else to stand rather than being dragged down the spiral |
|
|
| Allowing more space for working with difficulty |
|
|
Chapter 3 Trial design and methods
Study design
The PREVENT trial was a two-arm, multicentre, single-blind superiority trial randomly allocating patients in a 1 : 1 ratio to receive either MBCT-TS or m-ADM. The trial included a parallel economic evaluation to examine the cost-effectiveness of MBCT-TS compared with m-ADM and a mixed-methods process evaluation that used qualitative methods to assess the acceptability of MBCT-TS from the perspectives of patients and included a quantitative analysis of potential mediators.
Setting, participants and recruitment
We recruited participants from 95 general practices in urban and rural settings in four UK centres: Bristol, Exeter and East Devon, North and Mid Devon and South Devon. Inclusion and exclusion criteria for patient participation were refined through the pilot trial28 to maximise real-world applicability to the population of people in primary care with recurrent depression who are treated with ADM and who are interested in considering a psychological approach to relapse/recurrence prevention. They are also based on our experience of running a Devon NHS Primary Care Trust-commissioned depression service (see www.centres.ex.ac.uk/mood/).
Inclusion criteria
Participants were considered for inclusion if they:
-
had a diagnosis of recurrent major depressive disorder in full or partial remission according to the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV)47
-
had had three or more previous major depressive episodes in which depression was the primary disorder and it was not secondary to substance abuse, bereavement or a general medical condition
-
were aged ≥ 18 years
-
were on a therapeutic dose of ADM in line with the British National Formulary (BNF)48 and NICE guidance8
-
were open either to continue taking antidepressants for 2 years or to take part in a MBCT class and consider stopping their ADM.
Exclusion criteria
Participants were considered unsuitable for inclusion if they:
-
were currently depressed, as assessed using the Structured Clinical Interview for DSM-IV (SCID)47
-
had a comorbid diagnosis of current substance abuse (patients with previous substance abuse were eligible for inclusion as long as they were in sustained full remission)
-
had organic brain damage
-
had current/past psychosis, including bipolar disorder
-
displayed persistent antisocial behaviour
-
engaged in persistent self-injury that required clinical management/therapy
-
were undergoing formal concurrent psychotherapy.
Recruitment procedure
The recruitment strategy for the PREVENT trial built on the protocol used in the pilot trial, which proved acceptable and effective. 29 This is summarised in the following pathway:
-
General practice searches identified patients who had been prescribed ADM at a therapeutic dose in the last 3 months.
-
GPs were then asked to screen this list to exclude any patients who they knew met the exclusion criteria.
-
Letters were sent to the remaining patients enclosing an information pamphlet and reply form.
-
Interested patients were telephoned to discuss the study and a short eligibility screening interview was conducted over the telephone. Information about the study and MBCT was available to help people to begin to make an informed decision about participation. This included the timings and locations of the MBCT groups. The vast majority of exclusions were identified at this stage.
-
Patients who met the telephone screening criteria were invited to attend a face-to-face baseline interview.
-
Consenting patients who met the PREVENT inclusion criteria joined the trial during the baseline assessment.
-
Within a month of the start of the next MBCT-TS group a current GRID-Hamilton Depression Rating Scale (GRID-HAMD)49 score was obtained for each participant so that randomisation could occur.
Although the majority of referrals were through GP surgeries, interested patients were also able to self-refer into the study. We employed a number of different strategies to advertise the trial including placing posters in carefully targeted sites, developing a website, regional media coverage and leaflet dropping in local chemists.
Patients were recruited in cohorts during recruitment ‘time slices’ that corresponded to the 6–8 weeks before the next MBCT-TS group was due to start. Baseline assessments were conducted as close as possible to the start of the MBCT-TS group as residual depressive symptoms are a powerful predictor of relapse/recurrence. 50 On average, each researcher recruited six patients per month (Figure 2).
FIGURE 2.
Summary of the PREVENT recruitment process and follow-up assessments. BDI-ll, Beck Depression Inventory, second edition; EQ-5D, European Quality of Life-5 Dimensions; MSCL, Medical Symptom Checklist; WHOQOL, WHO Quality of Life.

Randomisation and concealment
Patients were randomised in a 1 : 1 ratio to either MBCT-TS or m-ADM using computer-generated random permuted blocks and stratified by recruitment locality (four sites) and symptomatic status (asymptomatic: GRID-HAMD score < 8 vs. partially symptomatic: GRID-HAMD score ≥ 8). Stratification by locality enabled a proportionate workload for research staff and therapists. Residual depressive symptoms are a powerful predictor of relapse/recurrence. 50 Moreover, the pilot trial suggested the importance of this stratification variable in predicting outcome. 27 To ensure concealment randomisation was conducted using a password-protected trial website that was maintained by the Peninsula Clinical Trials Unit (CTU), a UK Clinical Research Collaboration (UKCRC)-accredited CTU. Participants were informed of the outcome of randomisation in a letter sent from the trial administrator. Research assessors were blind to treatment allocation; in cases in which blindness was broken participants were assigned to another research assessor. The fidelity of this masking was moderate, with assessors correctly guessing allocation for 56% of assessments. Given the nature of the interventions, patients and clinicians were aware of treatment allocation.
Patients were randomised in the month before the next MBCT-TS group began. If a participant’s baseline assessment occurred more than a month before the next MBCT-TS group began he or she received a brief ‘randomisation assessment’ before being randomised. During this assessment the patient was asked to give a verbal reaffirmation of his or her wish to take part in the trial and researchers ensured that the patient’s situation had not changed significantly and also obtained a current GRID-HAMD score.
Health technologies assessed
Maintenance antidepressants
The m-ADM relapse/recurrence prevention intervention consisted of maintenance of the ADM treatment that was an inclusion criterion for the trial. Participants were monitored and treated by their physician in a primary care setting. During the maintenance phase, physicians were asked to manage m-ADM in line with standard clinical practice and the BNF. Primary care physicians were asked to meet with patients regularly to review their medication. Changes in medication sometimes occurred during the maintenance treatment stage but physicians and participants were asked to ensure that the dose remained within therapeutic limits. The trial GPs (Dr Richard Byng and Dr David Kessler) and trial psychiatrist (Professor Glyn Lewis) provided materials for all participants and participating GPs on m-ADM and ongoing support as required.
We encouraged participants to adhere to medication for the full length of the trial by sending them letters signed by the chief investigator and their GP after each follow-up, reminding them that the trial was seeking to compare staying on antidepressants for 2 years with taking part in mindfulness classes and stopping ADM. If difficulties with continuation of medication were identified, the trial manager first contacted the participant to understand the difficulty and then whenever appropriate encouraged the participant together with his or her GP to consider maintaining m-ADM in line with standard clinical practice and the BNF. Patients in the m-ADM arm who did not maintain m-ADM in line with BNF and NICE guidelines still remained in the trial and follow-up data were collected as per those who did maintain m-ADM in line with guidelines.
Mindfulness-based cognitive therapy with support to taper and/or discontinue antidepressant medication
A total of 21 MBCT-TS groups were delivered by four therapists in a variety of settings including university campuses, hospital sites and other community-based rooms. Two of the therapists ran seven groups, one ran five and one ran two groups. Two of the groups ran in the evening and the rest were held during the day from Monday to Friday. There were between 4 and 14 participants in each group with an average of 10 per group [standard deviation (SD) 2.6 per group]. The treatment programme involved eight 2.25-hour group sessions, normally over consecutive weeks, with up to four refresher sessions offered in the year following the end of the 8-week programme. In line with previous MBCT trials,24,28,51 an adequate dose of MBCT-TS was defined as participation in at least four of the eight MBCT-TS group sessions. If participants did not receive an adequate dose of MBCT-TS they were not asked to taper/discontinue their m-ADM; however, outcome data were still collected as per those who did receive an adequate dose.
The four MBCT therapists were all mental health professionals (two clinical psychologists and two occupational therapists). They had post-qualification experience ranging from 9 to 30 years, with an average of 19 years (SD 8.9 years). All had extensive training and experience in leading MBCT groups (minimum 4 years) and a long-standing ongoing personal mindfulness practice (minimum 7 years). An independent check on therapist competency was established before therapists progressed to running trial groups. An experienced MBCT therapist independent of the trial rated at least two videotapes of MBCT-TS therapy sessions and, using the Mindfulness-Based Interventions – Teacher Assessment Criteria (MBI-TAC),52 made an overall judgement about whether the therapists were competent. During the trial, all four therapists received supervision every 2 weeks for 3 hours; this was attended once per month by the chief investigator.
All trial groups were videotaped with a digital camera for therapist supervision and checks on therapist competence and adherence. Randomly selected samples of two sessions for each 8-week course (42 sessions in total) were assessed by a MBCT expert independent of the trial team. As for the initial competency checks the MBI-TAC was used and these checks indicated that the MBCT teaching was at and above required levels (Table 2). The mean total adherence score in the trial (23.6, SD 4.30) was at least comparable with those reported in the psychometric evaluation of this scale53 and indicate acceptable adherence to protocol. There were no significant differences between therapists’ total adherence scores as determined by one-way analysis of variance (ANOVA) (F3,37 = 0.64; p = 0.59).
| MBI-TAC domains | Count (and percentage) for each rating across 42 tapes | |||||
|---|---|---|---|---|---|---|
| Incompetent | Beginner | Advanced beginner | Competent | Proficient | Advanced | |
| Coverage, pacing and organisation of the session curriculum | 0 (0) | 0 (0) | 1 (2.4) | 5 (11.9) | 14 (33.3) | 22 (52.4) |
| Relational skills | 0 (0) | 0 (0) | 1 (2.4) | 8 (19.0) | 10 (23.8) | 23 (54.8) |
| Embodimenta | 0 (0) | 0 (0) | 1 (2.4) | 8 (19.5) | 13 (31.7) | 19 (46.3) |
| Guiding mindfulness practices | 0 (0) | 0 (0) | 3 (7.1) | 1 (2.4) | 15 (35.7) | 23 (54.8) |
| Conveying course themes through interactive inquiry and didactic teaching | 0 (0) | 0 (0) | 2 (4.8) | 7 (16.7) | 15 (35.7) | 18 (42.9) |
| Facilitation of the group learning environment | 0 (0) | 0 (0) | 2 (4.8) | 12 (28.6) | 11 (26.2) | 17 (40.5) |
Participants in the MBCT-TS arm were encouraged to taper and discontinue their m-ADM and the study team provided guideline information to physicians and participants about typical tapering/discontinuation regimes and possible withdrawal effects; however, the actual timeline and regime used were determined by physicians and participants. The original MBCT manual34 was adapted to include more work on developing a relapse/recurrence signature and response plan that explicitly included participants considering reduction/discontinuation of m-ADM. Participants in the MBCT-TS arm who experienced a significant deterioration following tapering were encouraged to use the skills developed as part of the treatment. Letters signed by the chief investigator and trial GP were sent to each participant’s GP, copied to the participant, prompting the GP to have a discussion with the participant about a suitable tapering/discontinuation regime after 4–5 weeks of the MBCT-TS group. At the end of the MBCT-TS group another letter was sent reminding the GP to ensure that a tapering/discontinuation regime was in place. We also encouraged participants to taper/discontinue their medication by writing to them and their GP after each follow-up reminding them that the trial was seeking to compare staying on antidepressants with taking part in mindfulness classes and stopping ADM.
If at any time the study team became aware of difficulties with medication tapering/discontinuation, the trial manager first contacted the participant to understand the difficulty and then whenever appropriate encouraged the participant together with his or her GP to once more consider tapering/discontinuing m-ADM. Participants in the MBCT-TS arm who did not taper or discontinue their m-ADM still remained in the trial and follow-up data were collected as per those who did discontinue.
If participants experienced a relapse/recurrence during the course of the trial we encouraged them to discuss the most appropriate treatment with their GP and made no further requests that they consider tapering/discontinuing their medication. However, participants who had relapsed still remained in the trial and further secondary outcome data were collected on the same schedule as per participants who had not relapsed.
Data collection
Baseline assessments did not occur more than 3 months from the start of the next MBCT-TS group, with the start defined as the first orientation session. Following randomisation, participants were assessed at five time points: 1 month after the end of the 8-week MBCT-TS programme (or the equivalent time in the m-ADM arm), which varied between 12 and 24 weeks post randomisation, and 9, 12, 18 and 24 months post randomisation.
Outcomes
Primary outcome
The occurrence of any depressive relapse/recurrence, and time from randomisation to relapse/recurrence, were assessed using the Longitudinal Interval Follow-up Evaluation (LIFE),54 a form of the SCID47 designed for longitudinal studies of depression. A participant was judged to have had a relapse/recurrence if he or she was diagnosed as having a major depressive episode (a score of 5 for 2 consecutive weeks) at any time during the 24-month follow-up period. In conducting the SCID-LIFE interviews, researchers took care to establish the onset of the relapse/recurrence as closely as possible. If the day could not be established they tried to establish the closest week and take the mid-point of that week; if the week could not be established then the closest month or season was established and the mid-point taken.
An experienced clinical psychologist with formal training in the use of the SCID supervised the training of the research staff. To examine inter-rater reliability, we followed the method described in the first MBCT RCT,24 which had the added benefit of guaranteeing that all assessments were carried out blind to treatment condition. For every first actual, borderline or probable relapse/recurrence, a blinded and experienced rater second rated an audio recording of the SCID interview. In total, 198 recordings were second rated, with agreement being recorded on 89.9% of the recordings. The kappa coefficient for agreement between the study interviewer and the blinded rater was 0.62, suggesting good agreement. When there were disagreements between the first and second rater, consensus was reached through discussion. If a relapse/recurrence was considered marginal, a conservative position of no relapse was recorded. Once a judgement about relapse/recurrence was made, the onset of relapse/recurrence was dated from randomisation to the point at which criteria were met.
A subset of 112 SCID diagnoses were also second rated by an experienced rater who was independent of the trial and agreement was recorded on 95.5% of these diagnosis. The kappa coefficient for these ratings was 0.89, suggesting excellent agreement.
Secondary outcomes
Depression-free days were calculated using the SCID interview and residual depressive symptoms were assessed with the observer-rated interviewer-administered 17-item GRID-HAMD49 and a well-established self-report measure, the Beck Depression Inventory, second edition (BDI-II). 55 Psychiatric comorbidity was assessed with the full SCID,47 medical comorbidities were assessed at baseline, 12 and 24 months using the MSCL56 and health-related quality of life status was assessed using the European Quality of Life-5 Dimensions three-level version (EQ-5D-3L)57,58 and the WHO Quality of Life-BREF (WHOQOL-BREF). 59,60
Depression-free days
Depression-free days were calculated from the SCID by first establishing the duration in days of any episodes of depression throughout the follow-up period using the method described earlier. Care was taken to establish the last day of a relapse/recurrence or recurrence of clinically significant symptoms. As before, if the day could not be established then the mid-point of the shortest time period that could be established was used. Each new reported recurrence of depression required that there was a period of 2 months in which remission from the previous episode had been achieved. If there was no remission between two recorded episodes the second episode was regarded as a continuation of the first.
Residual depressive symptoms
The GRID-HAMD is a 17-item modified version of the popular depression rating scale developed by Hamilton in 1960. 61 This scale is an interviewer-administered measure of depressive symptoms with an emphasis on somatic symptoms. Scores range from 0 to 88, with higher scores representing higher levels of depression. The GRID-HAMD was designed to permit the rater to consider the dimensions of intensity and frequency independently for each relevant item in the scale. Symptom intensity is considered on the ‘vertical axis’ and symptom frequency on the ‘horizontal axis’. Symptom intensity, which includes degree of symptom magnitude as well as subjective distress and functional impairment, is rated as absent, mild, moderate, severe or very severe. Symptom frequency is rated as absent, occasional, much of the time or almost all of the time. The GRID-HAMD was administered at every assessment during the 24-month follow-up period.
The vast majority of depression trials fail to measure or report the reliability of their employed instruments, especially secondary outcome measures such as the GRID-HAMD. In the rare circumstances in which reliability is measured, it is carried out in an arbitrary and atheoretical manner. As such, and for the purposes of this trial, we compared the inter-rater reliability of the GRID-HAMD using a subsample of 20 assessments. This sample size was selected in accordance with the method of Walter et al. 62 for calculating the sample size for inter-rater reliability analyses. Walter et al. 62 estimated that a sample size of about 18–20 observations made between two raters is sufficient to obtain an expected intraclass correlation coefficient (ICC) of 0.9, with a minimum acceptable ICC of 0.7. Further, there is evidence which suggests that the reliability of the GRID-HAMD ratings is dependent on the assessor’s experience. 63 Thus, 10 of the 20 observations were randomly selected from assessments completed by an experienced assessor and 10 were selected from assessments completed by a more novice assessor. All 20 assessments from both researchers were obtained from the 18-month follow-up period. The overall ICC for the 20 observations was 0.98, suggesting excellent agreement. The ICC for the 10 assessments completed by the experienced assessor was 0.99, while the ICC for the remaining 10 assessments completed by the novice assessor was 0.86, both suggesting excellent agreement.
The BDI-II55 is a 21-item self-report instrument developed to measure the severity of depression with an emphasis on affective and cognitive symptoms. Higher scores represent greater depression severity (range 0–63) and minimal (0–13), mild (14–19), moderate (20–28) and severe (29–63) symptom severity ranges have been specified. The BDI-II was administered at every assessment during the 24-month follow-up period.
Medical and psychiatric comorbidities
We used the MSCL and the SCID relevant modules at baseline and 12 and 24 months’ follow-up. The MSCL is a list of 115 common medical symptoms and respondents are asked to indicate those that they have experienced as bothersome in the past month. 56 The score is the total number of symptoms checked. Although the reliability and validity of the MSCL have not been evaluated, several studies of MBSR have shown significant reductions in the MSCL score associated with participation in the programme. 64–67
Health-related quality of life
Health-related quality of life status was assessed using the EQ-5D-3L,57,58 a non-disease-specific measure for describing and valuing health-related quality of life. It has particular value as a measure because it is capable of generating a generic preference-based measure of outcome (the QALY) that allows for outcomes and cost-effectiveness to be compared across disease areas. 68,69 The measure includes a rating of own health in several domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) and a rating of own health by means of a ‘thermometer’ (score 0–100). It has been used extensively and thus has large comparative data sets and its psychometric properties are adequate. 57 The EQ-5D-3L was administered at every assessment during the 24-month follow-up period.
Quality of life was assessed using the WHOQOL-BREF,59,60 a generic 26-item measure covering the domains of physical health, psychological health, social relationships and environment. It differs from the EQ-5D-3L in providing a more subjective and holistic assessment of quality of life, rather than a self-report measure of health status. The WHOQOL-BREF is based on a longer version of the original instrument, the WHOQOL-100. 70,71 The WHOQOL-BREF was administered at every assessment during the 24-month follow-up period.
Adverse events
We followed the guidelines laid down by the Medicines and Healthcare products Regulatory Agency (MHRA) for identifying, acting on and reporting adverse events, which were defined as any event that resulted in death, was life-threatening, required hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity or consisted of a congenital anomaly or birth defect.
As soon as a researcher became aware of an adverse event they reported this to the trial manager, who then completed a serious adverse event form (see Appendix 1). Copies of these forms were sent to the Data Monitoring Committee (DMC), the main NHS Research Ethics Committee and the trial sponsor. No adverse reactions were judged by these committees to be suspected unexpected serious adverse reactions or SUSARs.
Sample size
Different relapse prevention interventions with different populations produce different absolute rates of depressive relapse/recurrence. Therefore, we based our sample size on estimated HRs for MBCT-TS compared with m-ADM rather than estimated absolute relapse/recurrence rates. We canvassed service users and clinicians who concurred that a relative reduction in relapse/recurrence of 10% would be clinically important. We used the systematic review of MBCT compared with usual care for patients with recurrent depression that reported HRs of 0.28–0.47 for relapse/recurrence. 26 We applied several conservative assumptions (first row of Table 3). First, even though the pilot trial data suggest that the HR was improving in favour of MBCT as the length of follow-up increased,28 we assumed a HR of 0.63 at 15 months to power the trial at 24-months’ follow-up. Second, even though attrition from MBCT trials to date is consistently < 15%, we assumed an attrition rate of 20% over the 24 months of follow-up. Finally, in spite of evidence to the contrary, we assumed that there may be a small clustering effect (ICC = 0.01). The resultant sample size calculation assumptions and outputs are shown in Table 3, based on 90% power and significance set at 0.05. This led to a total sample size of 420 across the two groups.
| Study | Comparison | Mean HR | ICC | Design factor | Attrition rate | Sample size per groupa |
|---|---|---|---|---|---|---|
| Conservative scenario | MBCT vs. m-ADM | 0.63 | 0.01 | 1.11 | 20% | 210 |
| Kuyken et al.28 | MBCT vs. m-ADM | 0.63 | –0.02 | 1.0 | 7%b | 160 |
| Ma and Teasdale25 | MBCT vs. usual care | 0.28 | –0.008 | 1.0 | 3%c | 14d |
| Teasdale et al.24 | MBCT vs. usual care | 0.47 | –0.04 | 1.0 | 5%c | 41d |
For the secondary outcomes, meta-analyses of generic mindfulness approaches suggest medium effect sizes in terms of changes in depressive symptoms72,73 and our pilot trial suggested medium effect sizes for the secondary outcomes of residual depressive symptoms, psychiatric comorbidity and quality of life. 28 The sample size estimate for our policy question enabled us to detect a medium between-groups effect size (standardised mean difference or Cohen’s d = 0.40) for the main secondary outcomes.
Statistical analysis
Statistical analyses were conducted in accordance with International Conference on Harmonisation (ICH-9) statistical guidelines for clinical trials74 and updated Consolidated Standards of Reporting Trials (CONSORT) guidelines for trials. 75,76 All statistical analyses were undertaken in Stata version 13 (StataCorp LP, College Station, TX, USA) following a predefined analysis plan agreed with the Trial Steering Committee (TSC).
Baseline equivalence was assessed descriptively by comparing the summary baseline characteristics and outcome values in both groups (MBCT-TS and m-ADM). Although there was a difference in gender between groups (see Table 8), as we know of no strong evidence that gender moderates MBCT treatment outcomes28 it was decided not to include and adjust for this variable in statistical models.
The primary analysis model for all primary and secondary outcomes was conducted on an intention-to-treat (ITT) basis, that is, between-group comparison based on the random allocation of patients and using complete data sets. All models adjusted for the stratification variables (centre and severity of depression). For the primary outcome the primary analysis included all patients and censored for missing data at 24 months’ follow-up. To examine the sensitivity of the results to missing data, secondary outcomes models were also run following multiple imputation (using the ‘ICE’ and ‘MIM’ Stata commands and 10 imputation cycles) and based on the assumption that data were missing at random.
The primary outcome (time to relapse/recurrence) was analysed using a Cox regression proportional hazards model including treatment condition (MBCT-TS/m-ADM). We assessed the proportionality of hazards over time by plotting −ln[−ln(survival)] against ln(analysis time) and tested this using Schoenfeld residuals. 23,24 We found no major violations of the proportional hazards assumption. Secondary outcomes were compared using hierarchical repeated measures regression models adjusting for outcome at baseline.
To explore potential treatment moderator effects, interaction terms were included in the Cox regression model for the primary outcome at 24 months for three predefined subgroups: the two stratification variables (centre and severity of depression) and participants in two groups characterised by how abusive their childhood had been. Participants with a more abusive childhood reported experiencing childhood physical or sexual abuse and/or scored above the median score for the Measure of Parenting Scale (MOPS)77 abuse subscale. Participants completed the MOPS at baseline as part of an embedded process–outcome study. 78 The abuse subscale asks participants to indicate how true they felt certain statements about their parents’ behaviour was, for example ‘parent was physically violent or abusive to me’, ‘parent made me feel unsafe’. Participants were categorised either in the lower abusive childhood group (i.e. scored below the median score for the MOPS abuse subscale and did not report childhood physical or sexual abuse) or in the higher abusive childhood group (i.e. scored above the median score for the MOPS abuse subscale or did report childhood physical or sexual abuse).
Given the variable levels of patient adherence to the stated treatment protocol in both the ADM-m and the MBCT-TC groups, we sought to undertake predefined secondary (per-protocol) analyses to examine the potential impact on the primary outcome at 24 months. We first described adherence in the PREVENT trial according to the principles set out by Dodd et al. 79 in their systematic review exploring adherence reporting in RCTs by reporting the rates of adherence in the format displayed in Box 1.
-
The number of participants who remained on a BNF therapeutically stable dose for the duration of the trial.
-
The number of participants who did not remain on a BNF therapeutically stable dose for the duration of the trial.
-
The number of participants who did not initiate MBCT-TS treatment.
-
The number of participants who initiated MBCT-TS treatment.
-
The mean, mode and SD of number of MBCT-TS sessions (0–8) and follow-up sessions attended (0–4).
-
The number of participants who completed MBCT-TS treatment.
-
The number of participants who made no reduction to their ADM dose.
-
The number of participants who reduced their ADM dose.
-
The number of participants who discontinued their ADM.
We then undertook two secondary analyses of the primary outcome comparing groups according to whether participants had (1) received an adequate dose of treatment and (2) adhered to treatment as invited. Definitions of these groups are displayed in Table 4.
| Group | Adequate dose of treatment | Adhering to treatment as invited |
|---|---|---|
| m-ADM | BNF therapeutic dose of ADM throughout follow-up period | BNF therapeutic dose of ADM throughout follow-up period |
| MBCT-TS | Attended four or more classes | Discontinued or reduced ADM and attended four or more classes |
Data management
Missingness within an outcome measure
Data entry and cleaning were overseen by the trial manager and research staff checked each outcome measure for missing data during every assessment and when possible collected missing items at this point. In cases in which ambiguous data were not clarified with the participant we operated a ‘score down policy’, meaning that if two items were checked the item with the lower rating was entered. When < 10% of the total or subtotal items for one outcome were missing, the mean as an integer of the missing items subscale was imputed in place of the missing value. If > 10% of the total was missing then the whole questionnaire was recorded as missing and, if > 10% of any one subtotal was missing, the whole of that subtotal was marked as missing.
Missing assessments
When substantive missing values arose, analyses was undertaken to assess their impact on the findings of the trial. Missing data were assumed to be ‘missing at random’,80 regression-based models were used to assess the relationship between covariates and outcome measure in completers and missing cases were substituted with a predicted outcome value. 81 A sensitivity analysis (with and without imputed data) was undertaken to assess the potential impact of imputation on the trial findings.
Ethical approval and research governance
Multicentre ethical approval for the study was given by the South West Research Ethics Committee (reference number 09/H0206/43) and local research governance approval was obtained for all sites (NHS Devon covering Exeter and Mid and North Devon – PCT0739; NHS Bristol, covering the Bristol site – 2010–004; NHS Plymouth and NHS Torbay, covering the South Devon site – PLY-TOR001). The trial was registered with the International Standard Randomised Controlled Trial Register with the reference number ISRCTN26666654. This trial was classed as a Clinical Trial of an Investigational Medicinal Product (CTIMP) as the MBCT-TS arm randomised patients to alter their standard ADM and as such approval to commence the trial was received from the MHRA (EudraCT number 2009–012428–10). A summary of the changes made to the original protocol is given in Table 5.
| Change to protocol | Date |
|---|---|
| Case note screening not undertaken by research staff and subsequent changes to the patient information sheet | December 2009 |
| Replacement of the follow-up measure SHAP with the DPES | December 2009 |
| Pilot of 3-month follow-up measures on formally depressed individuals who will not be taking part in the main trial | December 2009 |
| Ask pharmacists to insert a short flyer about the trial when dispensing ADM | December 2009 |
| Reimburse reasonable costs incurred when attending MBCT-TS groups for participants who would otherwise be unable to join the trial | July 2010 |
| Pilot qualitative feedback booklets with former NHS patients who have taken part in a previous MBCT group | July 2010 |
| Addition of the FFMQ, SCS, DPES, CERQ and DSC at the 24 months’ follow-up | April 2012 |
| Addition of two follow-ups at 36 and 48 months following an invitation from the National Institute for Health Research HTA programme to submit a bid; however, we were unsuccessful in obtaining the funding and therefore these additional follow-ups did not take place | April 2012 |
| Collaboration with the Mental Health Research Network (MHRN) to give all participants an exit questionnaire at their final follow-up exploring the reasons why they chose to take part in the research | June 2012 |
| Change of principal investigator at the Bristol site because of a move to University College London (Dr David Kessler replaced Professor Glyn Lewis) | June 2012 |
| Permission to ask for consent to conduct further analysis on participants’ previously provided DNA sample | August 2013 |
The University of Exeter sponsored the trial, which was hosted in the Mood Disorders Centre [see www.centres.ex.ac.uk/mood/ (accessed 16 March 2015)], a clinical research setting specialising in translational research hosting current MRC-, Wellcome Trust-, National Institute of Mental Health- and National Institute for Health Research Health Technology Assessment (HTA) programme-funded trials. The trial was overseen by the independent TSC (Chris Leach – Chairperson, Richard Moore and Glenys Parry) and the DMC (Paul Ewings – Chairperson, Andy Field and Joanne MacKenzie).
Informed consent
Our recruitment process gave patients several points at which they could learn about the project, either from reading the patient summary pamphlet and information sheet or through discussion with their GP, research staff or others. Consent was finally given through an interview with a researcher following an opportunity for questions. Research staff were trained and all interviews were recorded and supervised.
Participant welfare
Patients’ GPs approved their participation in the trial and were informed about the outcome of randomisation. The trial psychiatrist (Glyn Lewis) and GPs (Richard Byng and David Kessler) were on hand to offer participants’ GPs further information about the trial and to support GPs in their management of participants’ ADM. At the end of the MBCT-TS group, trial staff wrote to GPs to remind them that ADM tapering should normally have started and to ask them to be mindful of the possibility of relapse/recurrence. Over the follow-up period the MBCT-TS patients were invited to attend reunion sessions every 3 months and the therapists remained available by telephone throughout this period. If symptom exacerbation occurred without a full relapse/recurrence, initial management was encouraged to be within the appropriate treatment arm; however, if either patient preference and/or clinical judgement indicated other interventions, these were pursued.
Confidentiality
All of the information collected was kept strictly confidential and held in accordance with the principles of the Data Protection Act 1998. 82 Each participant was assigned a research number and all data were stored without subject identification. Data were held on a secure database on a password-protected computer at the University of Exeter. Access to data was and continues to be restricted to the research team. To enable follow-up contacts it was necessary to identify patients, but access to contact details (e.g. name and address) was restricted to the key members of the research team who arranged appointments. Any information about patients obtained (with their consent) from their medical records was recorded against their research number. Interviews audiotaped as part of the qualitative study (using a digital voice recorder) were transcribed verbatim and stored securely. Recordings of MBCT-TS sessions were stored securely (indexed by research number only) on a password-protected computer at the University of Exeter.
Disclosure of suicidal thoughts protocol
As the participants in this study have depression, it was appropriate to have a policy in place should any participant disclose suicidal thoughts to a research officer. Research officers assessed participants’ depression status at the study’s intake assessment and at each follow-up, which included questions about suicidal thoughts and plans. If a participant disclosed any suicidal thoughts the research officer followed the TSC-approved suicidal thoughts protocol and completed a suicidal thoughts report (see Appendix 1), which was countersigned by the site clinical lead. The information in this report was forwarded to the participant’s GP to better inform his or her clinical management. The patient information sheet informed the participant that if we were very concerned about his or her safety, or someone else’s safety, we would need to break confidentiality.
Unexpected serious adverse events
We followed the guidelines laid down by the MHRA for identifying, acting on and reporting adverse events [see www.mhra.gov.uk/Howweregulate/Medicines/Licensingofmedicines/Clinicaltrials/Safetyreporting-SUSARSandASRs/index.htm (accessed 16 March 2015)]. We adopted its definitions of adverse events and serious adverse reactions, reporting a total of 10 serious adverse events, none of which were classified as SUSARs by the TSC or the DMC.
Patient and public involvement
The PREVENT trial has benefited from the expertise of many people with lived experience of mental health difficulties including members of a locally organised voluntary group called the Lived Experience Group (LEG). The LEG has assisted the PREVENT trial at every stage of its development and the following sections detail a few examples of the ways in which the PREVENT trial has been shaped by patient and public involvement.
Protocol development
Patient and public involvement was sought in the development of the initial study protocol when developing the proposal and in the finalisation of the protocol prior to starting the trial through co-authorship (Rev. Paul Lanham).
Risk training
We sought the advice of the LEG both on the processes contained in the disclosure of suicidal thoughts protocol and in training and supporting our research team to activate this protocol. This training was particularly valuable as many of our team were anxious about discussing suicidal thoughts and did not feel confident in their ability to explore such thoughts with participants and GPs. The training gave researchers the chance to role-play these discussions, with advice and reassurance from the LEG.
Seeking consent
A member of the LEG provided specific training to our research staff to ensure that the consenting process was coherent and transparent.
Patient information
Given the variability of people’s experiences and the importance of the patient information sheet, we asked a number of different members of the LEG to review the early drafts and combined the suggestions into our final version.
Management and governance of the trial
Sitting on both our Trial Management Group and our TSC is the previous Chairperson of the Board of Trustees for the charity Depression Alliance, the Rev. Paul Lanham. Rev. Lanham has a lifetime history of living with mental health difficulties and his involvement has been key in helping to ensure that the trial is asking questions that are relevant and valid.
Qualitative interviews and feedback
A large component of our trial was asking the research participants about their experiences of the therapies offered (see Chapter 7). We did this predominantly by asking all participants to complete an eight-page feedback booklet and also by conducting in-depth interviews with a subset of participants. Both the feedback booklet and the interview questions were reviewed and then trialled by several members of the LEG, who suggested a number of fundamental changes. The benefit of these changes became apparent as we began the process of analysis and could see the richness of the data that were emerging.
Dissemination of results
It was very important to us that we disseminate the results of our trial to the participants who took part and that this was carried out in the most sensitive and accessible way possible. We organised three separate events across the recruitment sites to disseminate the results of our trial to participants and consulted with a PREVENT trial participant who had since joined the LEG about the format and content of these events. This participant co-organised and chaired the largest of the events.
Chapter 4 Trial results
Participant flow
During the 19 months of recruitment, between 23 March 2010 and 21 October 2011, we identified 28,597 patients from GP practice searches. GPs excluded 8989 patients as unsuitable and invitation letters were sent to the remaining 19,608 patients. Although we asked all GPs to provide reasons for excluding patients, in 56% of cases this information was missing. For the remaining exclusions the most common reasons given were dementia (2%), psychosis (2%) and substance abuse (1%). In total, 3060 patients responded positively to our invitation and a further 89 patients self-referred. Full telephone screens were completed for 2188 patients, which resulted in 498 patients attending for a baseline assessment, of whom 424 patients were randomised. The most common reasons why patients were not eligible for the PREVENT trial were that they had recently stopped taking ADMs or wanted to reduce them (30%), they had not had three previous episodes of depression (19%) or they were currently depressed (17%). The reasons given for not wishing to take part in the PREVENT trial are explored in Chapter 7. Primary outcome data were collected for 90.3% (383/424) of participants and the remaining participants’ data were censored at their last assessment. We retained 86.3% (366/424) of participants over the 24-month follow-up period, with 4.7% (20/424) lost to contact, 8.0% (34/424) withdrawing consent for further follow-up and 0.9% (4/424) dying during the trial; the pattern of missing data was similar across interventions. The flow of participants through the trial is depicted in Figure 3.
FIGURE 3.
The CONSORT flow chart illustrating the flow of participants into the PREVENT trial.

Missing data
The missing data rates for each time point are shown in Table 6.
| Outcome | Number of participants | |||||
|---|---|---|---|---|---|---|
| Baseline | MBCT+1 | 9 months | 12 months | 18 months | 24 months | |
| Primary outcome: days till relapse/recurrence, n (%) | ||||||
| Valid cases | – | 402 (95) | 398 (94) | 395 (93) | 387 (91) | 383 (90) |
| Censored prior to 24 months | – | 22 (5) | 26 (6) | 29 (7) | 37 (9) | 41 (10) |
| Secondary outcomes | ||||||
| Depression-free days, n (%) | ||||||
| Valid cases | – | 402 (95) | 396 (93) | 392 (92) | 377 (89) | 366 (86) |
| Data missing | – | 22 (5) | 28 (7) | 32 (8) | 47 (11) | 58 (14) |
| Residual depressive symptoms, n (%) | ||||||
| BDI-II | ||||||
| Valid cases | 416 (98) | 348 (82) | 293 (69) | 324 (76) | 291 (69) | 336 (79) |
| Individual missing items within valid cases | 16 (0.2) | 2 (0.03) | 3 (0.05) | 0 | 2 (0.03) | 0 |
| Participants who contributed no data | 8 (2) | 76 (18) | 131 (31) | 100 (24) | 133 (31) | 88 (21) |
| GRID-HAMD | ||||||
| Valid cases | 424 (100) | 369 (87) | 352 (83) | 365 (86) | 348 (82) | 366 (86) |
| Individual missing items within valid cases | 0 | 0 | 0 | 0 | 0 | 0 |
| Participants who contributed no data | 0 | 55 (13) | 72 (17) | 59 (14) | 76 (18) | 58 (14) |
| Psychiatric comorbidity: SCID, n (%) | ||||||
| Valid cases | 424 (100) | – | – | 392 (92) | – | 366 (86) |
| Data missing | 0 | – | – | 32 (8) | – | 58 (14) |
| Medical comorbidity: MSCL, n (%) | ||||||
| Valid cases | 416 (98) | – | – | 323 (76) | – | 336 (79) |
| Individual missing items within valid cases | 0 | – | – | 0 | – | 7 (0.02) |
| Participants who contributed no data | 8 (2) | – | – | 101 (24) | – | 88 (21) |
| Quality of life: WHOQOL-BREF, n (%) | ||||||
| Valid cases | 414 (98) | 348 (82) | 292 (69) | 323 (76) | 290 (68) | 336 (79) |
| Individual missing items within valid cases | 36 (0.3) | 15 (0.2) | 8 (0.1) | 5 (0.1) | 4 (0.1) | 3 (0.03) |
| Participants who contributed no data | 10 (2) | 76 (18) | 132 (31) | 101 (24) | 134 (32) | 88 (21) |
| EQ-5D-3L | ||||||
| Valid cases | 413 (97) | 347 (82) | 293 (69) | 324 (76) | 291 (69) | 336 (79) |
| Individual missing items within valid cases | 2 (0.1) | 0 | 0 | 1 (0.1) | 0 | 0 |
| Participants who contributed no data | 11 (3) | 77 (18) | 131 (31) | 100 (24) | 133 (31) | 88 (21) |
Baseline characteristics
Of the 424 randomised participants, 212 were allocated to receive MBCT-TS and 212 were allocated to receive m-ADM. As indicated in Table 7, baseline characteristics were balanced between the two groups with the possible exception of gender. As we know of no strong evidence that gender moderates MBCT treatment outcomes28 we did not add gender to the primary analysis model. It is interesting to note that the psychiatric history of these participants differs in a number of ways from the history of those randomised in the pilot trial (Table 8),28 with PREVENT participants reporting lower BDI scores and fewer comorbidities and a smaller proportion previously attempting suicide. A much larger percentage of the PREVENT participants also previously accessed psychiatric treatment, which is likely to be the result of the recent significant progress made in improving the accessibility of evidence-based mental health services in the UK. 4,15,16
| Characteristic/variable | MBCT-TS (n = 212) | ADM (n = 212) |
|---|---|---|
| Demographic characteristics | ||
| Female, n (%) | 151 (71) | 174 (82) |
| White, n (%) | 210 (99) | 210 (99) |
| Age (years) | ||
| Mean (SD) | 50 (12) | 49 (13) |
| Range | 22–78 | 20–79 |
| Marital status, n (%) | ||
| Single | 42 (20) | 38 (18) |
| Married, cohabiting or civil partnership | 125 (59) | 140 (66) |
| Separated, divorced or widowed | 44 (21) | 33 (16) |
| Missing | 1 (0) | 1 (0) |
| Level of education, n (%) | ||
| No educational qualifications | 10 (5) | 10 (5) |
| Some school qualifications | 36 (17) | 45 (21) |
| High school and/or vocational qualification | 84 (40) | 92 (43) |
| University degree/professional qualification | 77 (36) | 61(29) |
| Missing | 5 (2) | 4 (2) |
| Religion, n (%) | ||
| Christian | 133 (63) | 139 (66) |
| Other | 10 (5) | 4 (2) |
| None | 68 (32) | 68 (32) |
| Missing | 1 (0) | 1 (0) |
| Salary (£ sterling) | ||
| Mean (SD) | 19,930 (13,387) | 18,024 (13,582) |
| Range | 1200–72,000 | 792–80,000 |
| Social class, n (%)a | ||
| Class 0 | 96 (45) | 76 (36) |
| Class 1 | 53 (25) | 52 (25) |
| Class 2 | 22 (10) | 38 (18) |
| Class 3 | 5 (2) | 6 (3) |
| Class 4 | 0 (0) | 2 (1) |
| Class 5 | 35 (17) | 37 (17) |
| Not classified | 1 (0) | 1 (0) |
| Stratification variables | ||
| Depressive symptomology at randomisation, n (%) | ||
| Asymptomatic | 163 (77) | 162 (76) |
| Symptomatic | 49 (23) | 50 (24) |
| Recruitment site, n (%) | ||
| Bristol | 33 (16) | 31 (15) |
| Exeter and East Devon | 72 (34) | 76 (36) |
| North and Mid Devon | 55 (26) | 54 (25) |
| South Devon | 52 (25) | 51 (24) |
| Psychiatric characteristics | ||
| Current depressive symptomology, mean (SD) | ||
| GRID-HAMD score | 4.8 (4.3) | 4.6 (4.3) |
| BDI-II score | 13.8 (10.2) | 14.5 (10.1) |
| Previous major depressive episodes, n (%) | ||
| Fewer than six episodes | 120 (57) | 106 (50) |
| Six or more episodes | 92 (43) | 106 (50) |
| Age (years) at first depression onset, mean (SD) | 24.4 (11.5) | 25.4 (13.3) |
| Time (months) since last depressive episode, mean (SD) | 21.2 (27.0) | 17.1 (23.0) |
| No. of comorbid DSM-IV Axis I psychiatric diagnoses, mean (SD) | 0.5 (0.9) | 0.7 (0.9) |
| Received outpatient psychiatric or psychological treatment, n (%) | 103 (49) | 108 (51) |
| Attempted suicide, n (%) | 48 (23) | 53 (25) |
| No. of previous suicide attempts, mean (SD) | 1.7 (1.1) | 1.9 (1.5) |
| Severity of reported childhood abuse, n (%) | ||
| High | 105 (50) | 111 (52) |
| Low | 105 (50) | 101 (48) |
| Missing | 2 (1) | 0 (0) |
| Quality of life, mean (SD)b | ||
| How would you rate your quality of life? | 3.7 (0.8) | 3.7 (0.8) |
| How satisfied are you with your health? | 2.9 (1.0) | 3.1 (1.0) |
| Physical | 14.5 (6.5) | 14.4 (5.1) |
| Psychological | 12.6 (2.6) | 12.3 (2.6) |
| Social | 13.4 (3.4) | 13.1 (3.4) |
| Environment | 15.0 (2.4) | 15.1 (2.6) |
| Health-related quality of life (EQ-5D-3L tariffs), mean (SD) | 0.760 (0.268) | 0.778 (0.211) |
| Characteristic/variable | MBCT (n = 61) | m-ADM (n = 62) |
|---|---|---|
| Demographic characteristics | ||
| Female, n (%) | 47 (77) | 47 (76) |
| White, n (%)a | 60 (98) | 62 (100) |
| Age (years) | ||
| Mean (SD) | 48.95 (10.55) | 49.37 (11.84) |
| Range | 26–66 | 21–72 |
| Marital status, n (%) | ||
| Single | 4 (7) | 9 (15) |
| Married or cohabiting | 42 (69) | 40 (65) |
| Separated, divorced or widowed | 15 (25) | 13 (21) |
| Level of education, n (%) | ||
| No educational qualifications | 9 (15) | 17 (27) |
| Some school qualifications | 16 (26) | 16 (26) |
| High school and/or vocational qualification | 24 (39) | 15 (24) |
| University degree/professional qualification | 12 (20) | 14 (23) |
| Religion, n (%) | ||
| None | 12 (20) | 16 (26) |
| Christian | 46 (75) | 45 (73) |
| Otherb | 3 (5) | 1 (2) |
| Social class, n (%)c | ||
| Class 1 | 22 (36) | 23 (37) |
| Class 2 | 15 (25) | 12 (19) |
| Class 3 | 7 (11) | 7 (11) |
| Class 4 | 6 (10) | 2 (3) |
| Class 5 | 11 (18) | 17 (27) |
| Psychiatric characteristics | ||
| Depression, mean (SD) | ||
| HRSD score, mean (SD) | 5.62 (4.3) | 5.76 (4.69) |
| BDI-II score, mean (SD) | 18.51 (10.91) | 20.15 (12.86) |
| Depression diagnosis at intake, n (%) | ||
| In full remission | 42 (69) | 41 (66) |
| In partial remission | 19 (31) | 21 (34) |
| Previous episodes of depression | ||
| Mean (SD) | 6.43 (3.04) | 6.35 (2.91) |
| Median | 6 | 6 |
| ≥ 10 episodes, n (%) | 23 (38) | 19 (31) |
| Number of comorbid DSM-IV Axis I psychiatric diagnoses, mean (SD) | 0.83 (0.96) | 1.04 (1.11) |
| Age (years) at first depression onset, mean (SD) | 26.34 (11.7) | 26.11 (12.65) |
| Time (months) since last depressive episode, mean (SD) | 24.20 (27.74) | 18.68 (23.89) |
| Severity of last depressive episode (no. of DSM-IV symptoms recorded), mean (SD) | 7.27 (1.3) | 7.04 (1.35) |
| Attempted suicide, n (%) | 20 (33) | 22 (35) |
| Number of previous attempts | ||
| Mean (SD) | 0.69 (1.37) | 0.66 (1.05) |
| Range | 0–7 | 0–4 |
| Previous psychiatric treatment, mean (SD) | 17 (28) | 13 (21) |
| Quality of life, mean (SD)d | ||
| Physical | 22.64 (5.59) | 23.0 (5.18) |
| Psychological | 17.8 (3.82) | 18.03 (3.63) |
| Social | 9.52 (2.32) | 9.27 (2.65) |
Treatment adherence in each trial arm and the extent to which patients followed invitations to (dis)continue m-ADM are reported in Table 9; > 75% of patients adhered to treatment as intended. Details of the ADM that was taken are provided in Appendix 2.
| Treatment adherence | n (%) |
|---|---|
| m-ADM | |
| Remained on therapeutic dose | 162 (76) |
| Did not remain on therapeutic dose | 50 (24) |
| MBCT-TS | |
| Participants who did not initiate MBCT treatment | 6 (3) |
| Participants who initiated MBCT treatment | 206 (97) |
| Number of sessions attended | |
| Mean | 6 |
| Mode | 8 |
| SD | 2.4 |
| Completed four or more MBCT sessions | 176 (83) |
| ADM use among patients who attended four or more MBCT sessions | |
| No reduction in ADM dose | 23 (13) |
| Reduction in ADM dose | 29 (17) |
| Discontinued ADM | 124 (71) |
Primary outcome
Intention-to-treat analysis
We observed little or no clustering in primary or secondary outcomes by therapist. As model results accounting for clustering by therapist were identical to those obtained for the primary ITT analysis, outcome findings without consideration of therapist clustering are reported.
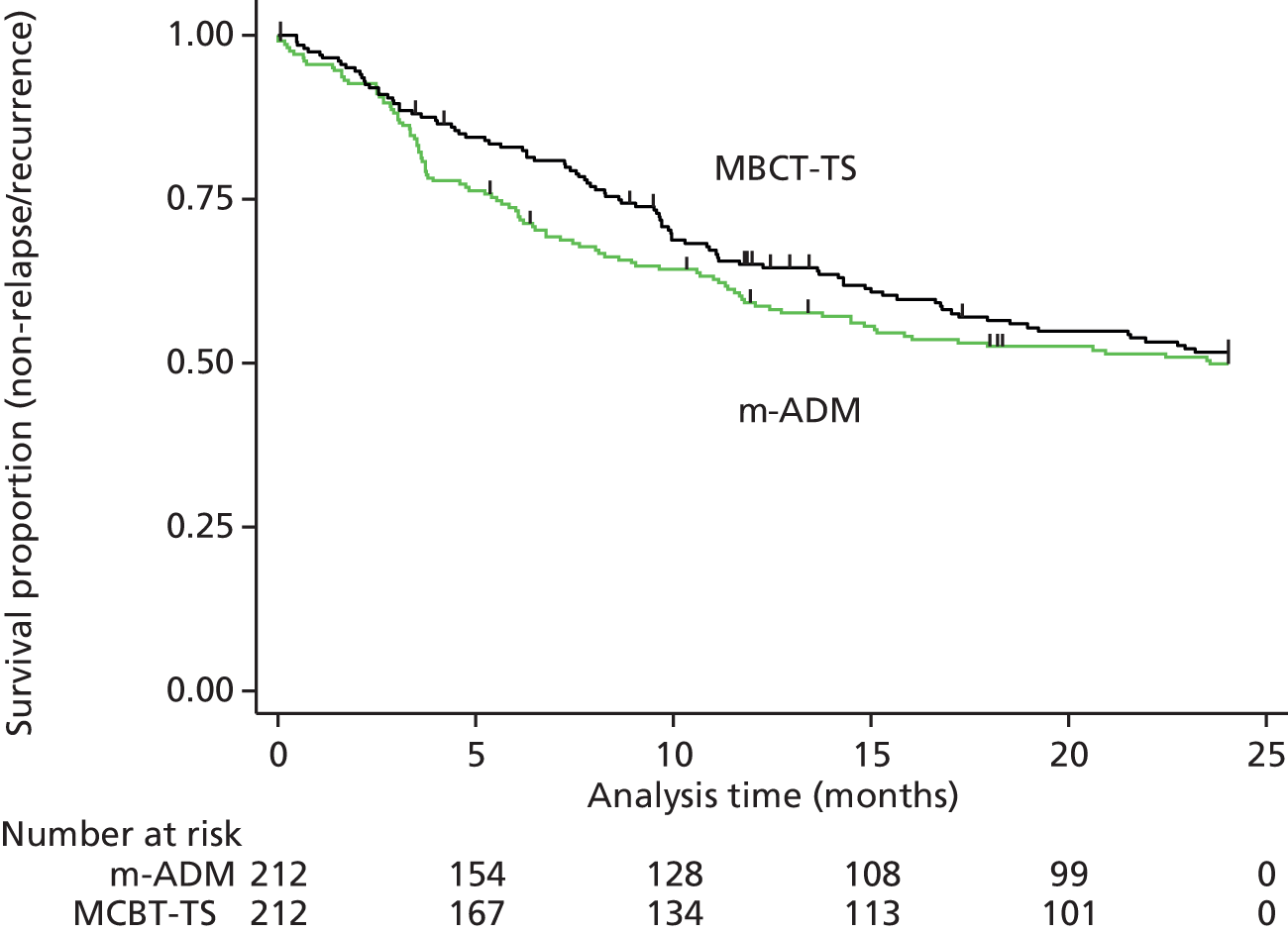
With respect to the primary outcome, the primary ITT analysis showed no evidence of a reduction in the hazard of relapse/recurrence with MBCT-TS compared with m-ADM (HR 0.89, 95% CI 0.67 to 1.18; p = 0.43), with 44% (94/212) of the MBCT-TS patients relapsing compared with 47% (100/212) of the m-ADM patients (Figure 4).
FIGURE 4.
Survival (non-relapse/recurrence) curves comparing relapse/recurrence of major depression for the MBCT-TS and m-ADM groups over the 24-month follow-up period for ITT participants.
Secondary analyses
Two secondary analyses of the primary outcome were undertaken to explore the impact of variations in intervention adherence in the MBCT-TS and m-ADM groups.
There was a non-significant reduction in the hazard of relapse/recurrence with MBCT-TS compared with m-ADM at 24 months in those participants who received an adequate dose of treatment (HR 0.79, 95% CI 0.58 to 1.08; p = 0.14), with 46% (81/176) of the MBCT-TS patients relapsing compared with 49% (80/162) of the m-ADM patients (Figure 5). In this subgroup, the m-ADM group included more women and participants with a greater number of comorbidities than the MBCT-TS group (see Appendix 3).
FIGURE 5.
Survival (non-relapse/recurrence) curves comparing relapse/recurrence of major depression for the MBCT-TS and m-ADM groups over the 24-month follow-up period for those participants who received an adequate dose of treatment.

There was a non-significant reduction in the hazard of relapse/recurrence with MBCT-TS compared with m-ADM at 24 months in participants who followed the invited treatment with respect to ADM use (HR 0.77, 95% CI 0.56 to 1.06; p = 0.10), with 46% (70/153) of the MBCT-TS patients relapsing compared with 49% (80/162) of the m-ADM patients (Figure 6). In this subgroup, the m-ADM group included younger participants, lower earners, more women and participants with a greater number of comorbidities than the MBCT-TS group (see Appendix 4). Given their non-randomised nature, these secondary analyses are prone to selection bias and confounding.
FIGURE 6.
Survival (non-relapse/recurrence) curves comparing relapse/recurrence of major depression for the MBCT-TS and m-ADM groups over the 24-month follow-up period for those participants who followed the invited treatment with respect to ADM use.
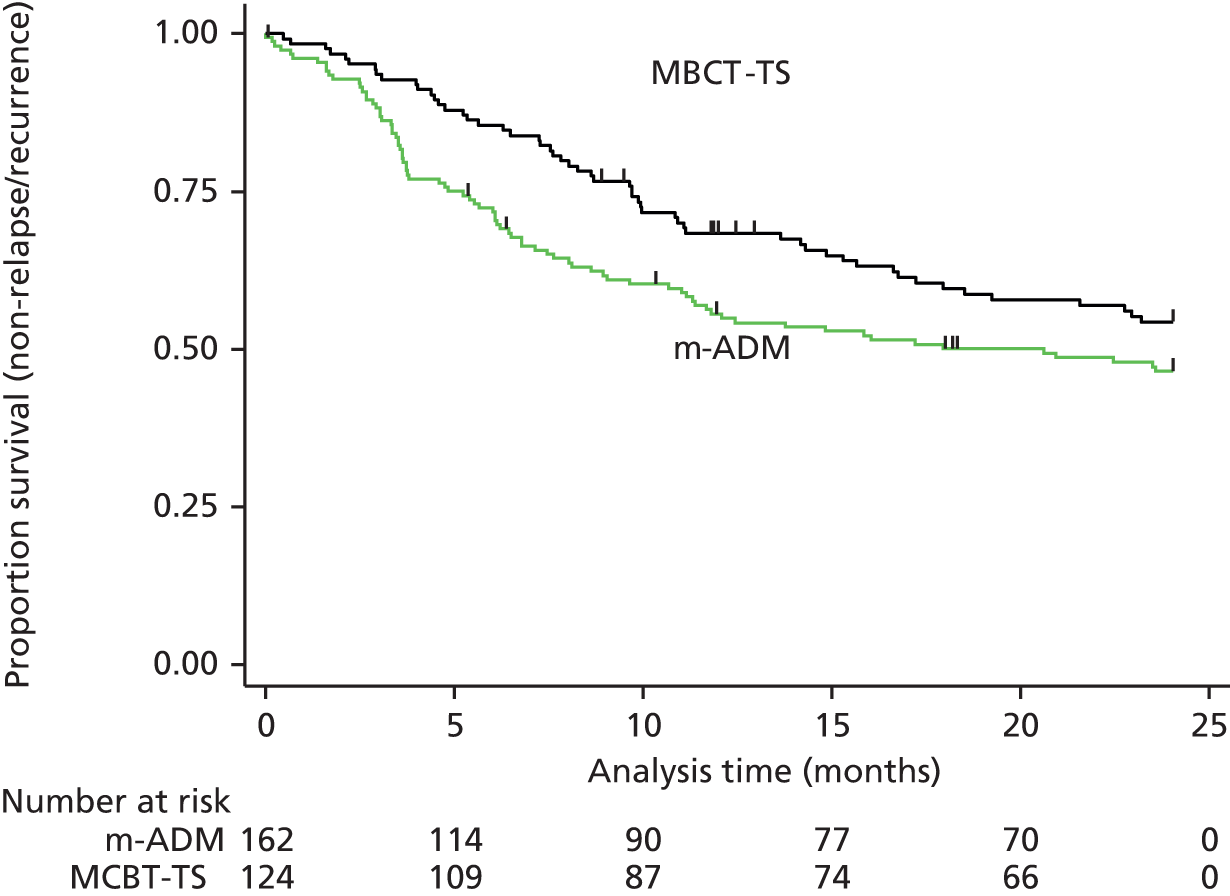
There was no difference in treatment effect on the primary outcome across either stratification variable subgroup (severity of depression at baseline or centre) (Table 10). However, there was evidence of a significant interaction between the severity of reported childhood abuse and treatment group (HR 0.53, 95% CI 0.29 to 0.95; p = 0.03). Specifically, compared with ADM-m, MBCT-TS reduced the risk of relapse/recurrence for participants with a higher severity of reported childhood abuse (MBCT-TS 47% vs. ADM-m 59%) whereas there was a slightly higher risk of relapse/recurrence with MBCT-TS in the lower severity of reported childhood abuse subgroup (MBCT-TS 42% vs. ADM-m 35%) (see Table 10). Comparing the baseline characteristics of those with high and low severities of reported childhood abuse we find a number of differences. Participants who reported a more abusive childhood had had more previous psychiatric treatments, including more hospitalisations, had experienced more previous episodes of depression and made more suicide attempts, had a greater chance of a familial history of both suicide and mental illness and were more likely to smoke (see Appendix 5). Given their non-randomised nature, these secondary analyses are prone to selection bias and confounding
| Subgroup | Stratified HR (95% CI) | Interaction HR (95% CI), p-value |
|---|---|---|
| Depression severity | ||
| Asymptomatic (HRSD score < 8)a | 0.83 (0.60 to 1.15) | 1.27 (0.68 to 2.39), 0.46 |
| Symptomatic (HRSD ≥ 8) | 1.06 (0.62 to 1.18) | |
| Centre | ||
| South Devona | 0.61 (0.33 to 1.13) | 1.75 (0.70 to 4.39) |
| Bristol | 1.60 (0.54 to 2.12) | 1.81 (0.83 to 3.96) |
| Exeter and East Devon | 1.10 (0.68 to 1.81) | 1.37 (0.61 to 3.08), 0.47b |
| North and Mid Devon | 0.84 (0.49 to 1.43) | |
| Childhood abuse | ||
| Lower riska | 1.31 (0.83 to 2.04) | 0.53 (0.29 to 0.95), 0.03 |
| Higher risk | 0.69 (0.47 to 1.00) | |
Secondary outcomes
With respect to the secondary outcomes, there was again no evidence of MBCT-TS’s superiority over m-ADM (Table 11). Furthermore, none of the secondary outcome treatment effects at any follow-up point exceeded a standardised mean difference of 0.4. The health economics outcomes are reported in full in Chapter 5.
| Outcome | Group | Baseline | MBCT+1 | 9 months | 12 months | 18 months | 24 months | p-valuea | p-valueb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | ||||
| Depression-free days | m-ADM | 607.4 (196.4) | 212 | 0.66 | 0.63 | ||||||||||
| MBCT-TS | 607.4 (203.7) | 212 | |||||||||||||
| Residual depressive symptoms | |||||||||||||||
| BDI-II score | m-ADM | 14.4 (10.1) | 206 | 13.9 (10.9) | 174 | 10.5 (9.7) | 142 | 11.3 (9.2) | 157 | 11.3 (10.7) | 149 | 11.9 (10.7) | 167 | 0.18 | 0.21 |
| MBCT-TS | 13.8 (12.4) | 210 | 9.9 (9.7) | 174 | 11.0 (10.5) | 151 | 10.7 (10.0) | 167 | 11.7 (10.6) | 142 | 11.6 (10.9) | 169 | |||
| GRID-HAMD score | m-ADM | 4.6 (4.3) | 212 | 7.4 (6.3) | 183 | 5.6 (6.4) | 175 | 4.7 (5.2) | 181 | 5.3 (6.1) | 174 | 4.7 (5.7) | 183 | 0.76 | 0.55 |
| MBCT-TS | 4.8 (4.3) | 212 | 6.3 (5.6) | 186 | 6.0 (5.5) | 177 | 5.7 (5.7) | 184 | 5.7 (5.7) | 174 | 4.7 (4.8) | 183 | |||
| Psychiatric and medical comorbidity | |||||||||||||||
| Psychiatric comorbidities | m-ADM | 0.7 (1.0) | 212 | 0.1 (0.4) | 196 | 0.3 (0.6) | 183 | 0.91 | 0.90 | ||||||
| MBCT-TS | 0.5 (0.9) | 212 | 0.1 (0.3) | 196 | 0.3 (0.7) | 183 | |||||||||
| MSCL score | m-ADM | 21.7 (13.8) | 206 | 19.3 (13.7) | 156 | 21.7 (16.3) | 167 | 0.42 | 0.43 | ||||||
| MBCT-TS | 22.8 (14.0) | 210 | 21.0 (14.0) | 167 | 22.2 (14.6) | 169 | |||||||||
| Quality of life | |||||||||||||||
| WHOQOL: Q1 overall perception of quality of life | m-ADM | 3.7 (0.8) | 205 | 3.8 (0.9) | 173 | 3.9 (0.8) | 141 | 3.9 (0.9) | 157 | 3.9 (0.9) | 149 | 3.8 (1.0) | 167 | 0.07 | 0.03 |
| MBCT-TS | 3.7 (0.8) | 209 | 3.8 (0.8) | 174 | 3.7 (0.9) | 151 | 3.7 (0.9) | 166 | 3.7 (0.9) | 141 | 3.7 (0.9) | 169 | |||
| WHOQOL: Q2 overall perception of health | m-ADM | 3.1 (1.0) | 205 | 3.2 (1.0) | 173 | 3.2 (1.0) | 141 | 3.3 (1.0) | 157 | 3.3 (1.1) | 149 | 3.2 (1.0) | 167 | 0.97 | 0.90 |
| MBCT-TS | 2.9 (1.0) | 209 | 3.1 (1.0) | 174 | 3.1 (1.1) | 151 | 3.2 (1.1) | 166 | 3.2 (1.0) | 141 | 3.1 (1.0) | 169 | |||
| WHOQOL domain 1: physical health | m-ADM | 12.3 (2.6) | 205 | 14.3 (3.0) | 173 | 14.8 (3.2) | 141 | 14.7 (3.3) | 157 | 14.7 (3.3) | 149 | 14.9 (5.5) | 167 | 0.07 | 0.02 |
| MBCT-TS | 12.6 (2.6) | 209 | 14.3 (3.3) | 174 | 14.2 (3.3) | 151 | 14.1 (3.4) | 166 | 13.9 (3.5) | 141 | 13.9 (3.5) | 169 | |||
| WHOQOL domain 2: psychological | m-ADM | 12.3 (2.6) | 205 | 12.6 (2.8) | 173 | 13.4 (2.7) | 141 | 13.3 (2.7) | 157 | 13.3 (3.0) | 149 | 13.1 (3.0) | 167 | 0.55 | 0.68 |
| MBCT-TS | 12.6 (2.6) | 209 | 13.4 (2.6) | 174 | 13.3 (3.0) | 151 | 13.3 (2.9) | 166 | 12.9 (2.8) | 141 | 13.1 (2.9) | 169 | |||
| WHOQOL domain 3: social relationships | m-ADM | 13.1 (3.4) | 205 | 13.3 (3.4) | 173 | 14.0 (3.4) | 141 | 14.2 (3.3) | 157 | 14.2 (3.4) | 148 | 13.9 (3.5) | 167 | 0.96 | 0.81 |
| MBCT-TS | 13.4 (3.4) | 209 | 13.8 (2.9) | 174 | 13.7 (3.4) | 151 | 13.9 (3.5) | 166 | 14.0 (3.4) | 141 | 13.7 (3.3) | 169 | |||
| WHOQOL domain 4: environment | m-ADM | 15.1 (2.6) | 205 | 15.3 (2.5) | 173 | 15.7 (2.3) | 141 | 15.6 (2.6) | 157 | 15.7 (2.6) | 149 | 15.7 (2.7) | 167 | 0.14 | 0.04 |
| MBCT-TS | 15.0 (2.4) | 209 | 15.21 (2.4) | 174 | 15.4 (2.6) | 151 | 15.2 (2.6) | 166 | 15.3 (2.6) | 141 | 14.9 (2.6) | 169 | |||
| EQ-5D-3L tariff | m-ADM | 0.778 (0.211) | 202 | 0.760 (0.226) | 173 | 0.773 (0.234) | 142 | 0.764 (0.248) | 156 | 0.768 (0.243) | 149 | 0.757 (0.266) | 166 | 0.13 | 0.07 |
| MBCT-TS | 0.760 (0.268) | 209 | 0.727 (0.295) | 174 | 0.735 (0.256) | 151 | 0.721 (0.293) | 167 | 0.723 (0.282) | 142 | 0.715 (0.310) | 169 | |||
Adverse events
A total of 10 serious adverse events were reported and are summarised in Table 12. Following discussion with the centre principal investigators and confirmation from the DMC we concluded that there was no reason to believe that any of the serious adverse events were intervention or trial related.
| Event | Date reported |
|---|---|
| MBCT-TS arm | |
| Attempted suicide | 5 November 2010 |
| Death from lung cancer | 11 September 2012 |
| Death from pancreatic cancer | 14 November 2012 |
| Non-fatal stroke | 27 February 2013 |
| Attempted suicide | 2 July 2013 |
| Non-fatal hemotympanum resulting in a blood clot | 19 July 2013 |
| m-ADM arm | |
| Completed suicide | 15 June 2012 |
| Death from hyperischaemic heart disease | 19 July 2012 |
| Attempted suicide | 8 March 2013 |
| Non-fatal hysterectomy because of cancer and admitted to hospital for 2 nights | 14 May 2013 |
Over the 24 months there was no difference in the total number of suicidal ideations reported between the MBCT-TS group (n = 44 events) and the m-ADM group (n = 46 events) (rate ratio 0.94, 95% CI 0.64 to 1.38; p = 0.75).
Chapter 5 Economic evaluation
Introduction
Aim
The aim of the economic evaluation was to assess the cost-effectiveness of MBCT-TS compared with m-ADM in patients with recurrent major depressive disorder, in full or partial remission, in primary care over 24 months. The economic evaluation followed the methods developed in the pilot trial28 and was carried out alongside the main RCT. 78,83
Methods
Perspective
In the first instance the economic evaluation took a NHS/Personal Social Services (PSS) perspective, which is recommended by NICE84 and covers the use of all hospital, community health and social services. In addition, as depression is known to impact on an individual’s ability to work, resulting in substantial losses in the workplace,85 we also widened our perspective to include productivity losses resulting from time off work and reduced productivity at work because of illness.
Method of economic evaluation
The primary economic analysis is focused on the policy question and is therefore a cost-effectiveness analysis with outcomes expressed as relapse/recurrence prevented. A secondary cost–utility analysis was also undertaken in which outcomes were expressed as QALYs, as recommended by NICE. 84
Outcomes
The primary outcome for the study was time to recurrence of depression and is detailed in Chapter 3. For the purpose of the economic evaluation we created a binary outcome based on whether or not a relapse/recurrence had occurred.
Quality-adjusted life-years were calculated from EQ-5D scores at baseline, 1 month after treatment and 9, 12, 18 and 24 months’ follow-up. The EQ-5D is a non-disease-specific measure for describing and valuing health-related quality of life. 86 The measure includes a rating of own health in five domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) and a rating of own health by means of a visual analogue scale (a ‘thermometer’) (score 0–100). It has been established that the EQ-5D can be used with confidence in economic evaluations for common mental health disorders. 87 The health states from the EQ-5D were given a utility score using responses from a representative sample of adults in the UK. 88 From these, QALYs were calculated using the area under the curve approach as defined by the utility values at baseline and each follow-up. It was assumed that changes in utility score over time followed a linear path. 89 QALYs in the second year were discounted at a rate of 3.5% as recommended by NICE. 84
Calculation of costs
The calculation of costs was separated into the identification, measurement and valuation of relevant resources. All unit costs are for the financial year 2011/12, uprated when necessary using the Hospital and Community Health Services Index. 90 Costs in the second year were discounted at a rate of 3.5% as recommended by NICE. 84
Identification of resources
We identified relevant resources based on the results of the pilot trial28 and in discussion with study clinicians and patient representatives. We collected resource use in the following domains:
-
delivery of the MBCT-TS intervention
-
use of NHS secondary care services:
-
inpatient stays (mental health and all medical specialties)
-
outpatient appointments (mental health and all medical specialties)
-
accident and emergency attendances
-
-
use of NHS primary care services:
-
GP (in surgery, at home and by telephone)
-
practice nurse
-
other community nurse (for example district nurse, health visitor, midwife)
-
support and recovery (STAR) or health-care support worker
-
community psychiatric nurse
-
community psychiatrist
-
community occupational therapist
-
community art/music/drama therapist
-
-
use of medication in the following areas:
-
antidepressants
-
sleeping tablets
-
mood stabilisers/antipsychotic
-
painkiller
-
-
use of social care and voluntary sector services:
-
social worker
-
marriage counselling service
-
advice service, for example Citizen’s Advice Bureau
-
helpline, for example Samaritans/Mind
-
day centre/drop-in centre
-
-
costs to patients and their carers
-
travel to trial MBCT-TS sessions
-
-
productivity costs
-
time off work (absenteeism) and reduced productivity at work (presenteeism).
-
Measurement of resources
Delivery of the MBCT-TS intervention
Throughout the trial, the MBCT-TS therapists recorded details of attendance and non-attendance at group sessions for each study participant and these were used as the basis for the calculation of the total cost of the intervention.
Contact with general practitioners and antidepressant medication
Information on the numbers and types of contact with GPs and on prescriptions for ADM for the 24-month follow-up period was collected through a search of GP records by research assistants blind to randomisation status.
Use of all other health, social and voluntary sector services and out-of-pocket costs
Data on use of all other services included in the study were collected using the Adult Service Use Schedule (AD-SUS; see Appendix 6). The AD-SUS has been developed in several mental health trials and was further modified and successfully employed in the pilot trial. 28 Information about the study participants’ use of services was collected in interviews with a researcher at baseline and at 1 month post treatment and 9, 12, 18 and 24 months’ follow-up. At baseline, information covered the previous 3 months. At each of the follow-up interviews, service use since the previous interview was recorded; in this way the entire period from baseline to the final follow-up was covered. The AD-SUS asks participants for the number and duration of contacts with various services and professionals. In addition to the researcher-completed component of the AD-SUS, we also created a self-complete section that asked participants about their use of non-trial psychological therapies, sleeping tablets, mood stabilisers and painkillers and any out-of-pocket costs associated with their attendance at a MBCT-TS group. The self-complete questionnaire was included to ensure that the researchers maintained blindness to randomisation allocation at each follow-up.
Productivity losses
Information about time off work (absenteeism) and reduced productivity whilst at work (presenteeism) was collected by researchers alongside the AD-SUS using the productivity questions of the WHO Work Performance Questionnaire. 91
Valuation of resources
A unit cost was applied to each resource use to calculate the total cost of resources used by each study participant.
Trial MBCT-TS sessions
We used the approach developed by the Personal Social Services Research Unit (PSSRU) at the University of Kent92 for the calculation of the unit cost of the MBCT-TS group intervention. Trial therapists were on band 7 of the Agenda for Change salary scale and employer’s national insurance, superannuation contributions and overheads were added to the average salary. We collected information from trial therapists on the time that they spent running the MBCT-TS sessions and the time that they spent on other activities and calculated a direct–indirect ratio of 1 : 0.67. MBCT-TS sessions lasted for 2 hours and there were 12 participants allocated to each group. We thus calculated the cost per participant per session at £14 (Table 13). Although there is no clear agreement on how the costs of group interventions should be allocated,93 we decided to calculate the cost of the intervention on the basis of allocation to a MBCT-TS group, regardless of whether or not the individual attended, because if participants did not attend they were not replaced.
| Unit | Unit cost 2011/12 (£ per year) |
|---|---|
| Salary | 37,800 |
| Salary oncosts including employers’ national insurance and superannuation contribution | 9532 |
| Overheads | |
| Management, administration and estates | 9140 |
| Non-staff | 19,865 |
| Capital overheads | 2682 |
| Working time | 42.7 weeks per annum, 37.5 hours per week |
| Ratio of direct face-to-face to indirect time | 1 : 0.67 |
| Length of sessions | 2 hours |
| Total | £49 per hour, £82 per direct contact hour, £165 per session, £14 per participant per session |
Antidepressants and other medication
Medication costs were calculated using daily dose information and the cost of the generic drugs as listed in the BNF. 94
Secondary care services
The unit costs for all hospital services were taken from the National Schedule of NHS Reference costs for 2012. 95 The costs used in the analysis are summarised in Table 14.
| Service | Unit | Cost (£) |
|---|---|---|
| MBCT-TS | Per session | 14.00 |
| Medication | Per daily dose | Various |
| Inpatient | Per night | 496.48–585.58 |
| Outpatient | Per appointment | 60–234 |
| Accident and emergency | Per attendance | 108–157 |
| Ambulance | Per attendance | 214 |
| GP surgery | Per minute of patient contact | 2.78 |
| GP home | Per home visit minute | 3.55 |
| GP telephone | Per minute of patient contact | 2.78 |
| Practice nurse | Per minute of face-to-face contact | 0.75 |
| District nurse, health visitor, midwife | Per home visit minute | 1.05 |
| Community psychiatric nurse | Per minute of face-to-face contact | 1.12 |
| Community psychiatrist | Per minute of patient contact | 1.93 |
| Clinical psychologist | Per minute of patient contact | 2.27 |
| Occupational therapist | Per minute of patient contact | 0.67 |
| Physiotherapist | Per minute of patient contact | 0.50 |
| Counselling | Per minute of patient contact | 1.08 |
| Art/drama/music therapist | Per minute of patient contact | 0.67 |
| Chiropodist | Per minute of patient contact | 0.50 |
| Dietician | Per minute of patient contact | 0.67 |
| Social worker | Per minute of patient contact | 2.60 |
| Support worker | Per minute of patient contact | 0.47 |
| Advice service | Per minute of patient contact | 0.47 |
| Day centre | Per user per session | 37.00 |
Primary care services and social care and voluntary services
For NHS primary care services and social care and voluntary services we used costs contained in Curtis. 90
Costs to patients
The cost of travel to MBCT-TS treatment groups was calculated by multiplying mileage information by estimates of running costs from the UK Automobile Association [see www.theaa.com/motoring_advice/running_costs (accessed 17 March 2015)].
Data analysis
Complete case analysis was used in the economic evaluation.
Resource use
Resource use by study participants is reported as the mean by group and as a percentage of the group who had at least one contact. Differences in the use of services between randomised groups at 24 months’ follow-up are reported descriptively and are not compared statistically to avoid problems associated with multiple testing and because the focus of the economic evaluation was on a quantitative analysis of cost and cost-effectiveness.
Difference in costs
Differences in mean costs between randomised groups at 24 months’ follow-up were analysed using standard parametric t-tests with the validity of the results confirmed using bias-corrected, non-parametric bootstrapping (repeat resampling). 97 Despite the skewed nature of cost data, this approach is recommended to enable inferences to be made about the arithmetic mean. 98
Cost-effectiveness and cost–utility analyses
The primary economic analysis focused on the policy question: MBCT-TS compared with m-ADM at 24 months and assessed cost-effectiveness in terms of cost per relapse/recurrence prevented. A secondary cost–utility analysis was undertaken using QALYs calculated from the EQ-5D measure of quality of life. Initially, ICERs – the difference in mean cost divided by the difference in mean effect – were calculated. 99 Because ICERs are calculated from four sample means and are therefore subject to statistical uncertainty, repeat resampling (bootstrapping) from the cost and outcomes data was used to generate a distribution of mean costs and effects for each of the relapse/recurrence and QALY outcomes. 100 These distributions were used to calculate the probability that each of the treatments is the optimal choice, subject to a range of possible maximum values (the ceiling ratio, λ) that a decision-maker might be willing to pay for a unit improvement in outcome. To explore the uncertainty that exists around estimates of mean costs and effects and the uncertainty regarding the maximum value of λ, cost-effectiveness acceptability curves (CEACs) are presented by plotting these probabilities for a range of possible values of λ. 101,102 CEACs show the probability that MBCT-TS is the more cost-effective of the two options for different values of λ. These analyses were run allowing for stratification variables (recruitment locality and participants’ symptomatic status).
Sensitivity analyses
A number of sensitivity analyses were carried out to test the robustness of the assumptions made:
-
The discount rate was varied from 0% to 6%.
-
The impact of missing data was considered using single imputation of individual missing values using multiple covariates.
Results
Data completeness
At 24 months, full service use data for the entire follow-up period were available for 180 participants in the m-ADM group and 181 participants in the MBCT-TS group, which was 85% of the total number randomised.
Resource use
Trial MBCT-TS sessions
Full details of the MBCT-TS sessions are provided in Chapter 2 and associated costs are summarised in Table 13.
Antidepressants and other medication
For details of the use of ADM see Table 9. The use of other medication is summarised in Table 15, which shows that similar proportions of both the MBCT-TS group and the m-ADM group used painkillers (61%) and sleeping tablets (15–16%).
| Service use | MBCT-TS | m-ADM | ||||
|---|---|---|---|---|---|---|
| Mean | SD | % using at least once | Mean | SD | % using at least once | |
| Inpatient stay (nights) | 0.70 | 3.87 | 14.81 | 0.89 | 2.79 | 20.14 |
| Outpatient appointments (number) | 4.79 | 9.21 | 67.11 | 4.29 | 6.29 | 67.63 |
| Accident and emergency (attendances) | 0.59 | 1.25 | 30.20 | 0.60 | 1.09 | 33.09 |
| Ambulance (calls) | 0.07 | 0.34 | 4.70 | 0.72 | 0.26 | 7.19 |
| GP (contact) | 12.13 | 12.51 | 95.30 | 9.66 | 8.44 | 99.28 |
| Other community health-care services (contact) | 1.27 | 4.43 | 87.92 | 6.99 | 17.22 | 86.33 |
| Social care, local authority and voluntary services (contact) | 1.58 | 4.81 | 22.82 | 2.55 | 7.55 | 18.71 |
| Use of painkillers | 61.26 | 60.85 | ||||
| Use of sleeping tablets | 15.09 | 15.57 | ||||
| Days off work over follow-up | 11.01 | 32.66 | 43.40 | 9.76 | 27.06 | 51.89 |
Secondary care, primary care, social care and voluntary sector services
The use of secondary care, primary care, social care and voluntary sector services was broadly similar across both randomised groups over 24 months’ follow-up and there is no evidence that treatment allocation had any substantial impact on the scope or intensity of service use.
Productivity losses
Over 24 months’ follow-up the mean number of days off work was 9.8 in the m-ADM group and 11.0 in the MBCT-TS group.
Total costs
Changes over time
Changes in total cost per week over follow-up are summarised in Figure 7 (NHS/PSS perspective) and Figure 8 (societal perspective). The charts demonstrate that costs were fairly consistent between groups and over time, suggesting that randomisation to MBCT-TS or m-ADM had little impact on the cost of health and social care services and productivity losses.
FIGURE 7.
Average cost per week at each follow-up using a NHS/PSS perspective.
FIGURE 8.
Average cost per week at each follow-up using a societal perspective.
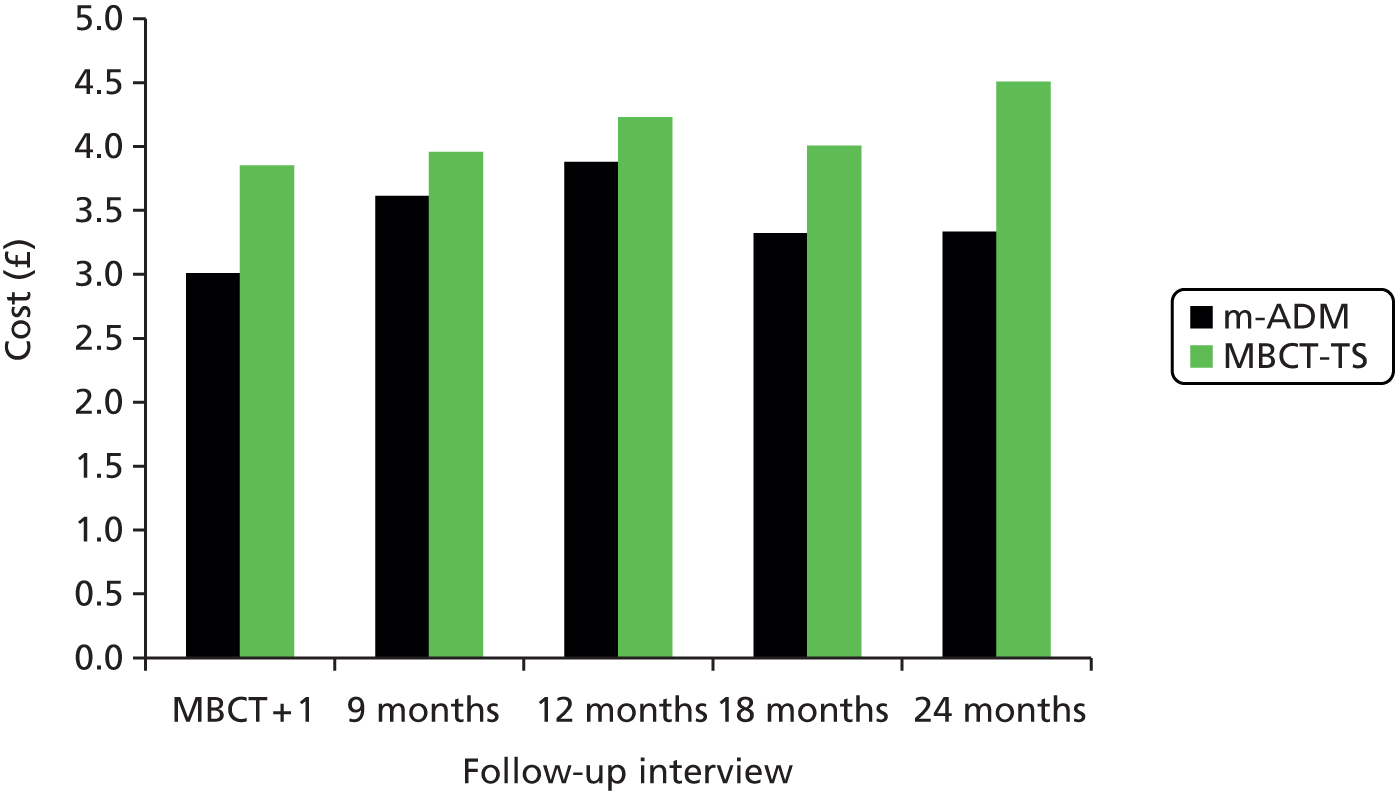
Total costs at follow-up
Total costs over follow-up are summarised in Table 16, including a breakdown of costs by service-providing sector. From a NHS/PSS perspective, the mean total cost over 24 months’ follow-up was £2360 in the m-ADM group and £2485 in the MBCT-TS group; the mean difference in costs was not statistically significant (p = 0.80). From a societal perspective the mean total cost over 24 months’ follow-up was £2755 in the m-ADM group and £3204 in the MBCT-TS group; again, the mean difference in costs of £449 was not statistically significant (p = 0.68).
| Cost category | MBCT-TS (n = 181) | m-ADM (n = 180) | Mean difference | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Mean | Mean | Mean | SD | ||||
| MBCT-TS | 112.00 | 112.00 | 0.00 | 0.00 | |||
| Antidepressants | 40.10 | 72.13 | 69.79 | 168.48 | |||
| Hospital and community services | 2332.43 | 4065.88 | 2290.62 | 4190.65 | |||
| Total health-care costs (NHS/PSS) | 2484.52 | 4077.31 | 2360.41 | 4205.58 | 124.11 | –749.98 to 972.57 | 0.80 |
| Out-of-pocket costs to patients | 56.76 | 168.29 | 83.33 | 283.12 | |||
| Productivity losses (n = 265) | 504.26 | 1881.49 | 310.54 | 761.06 | |||
| Societal costs (n = 252) | 3204.05 | 4011.91 | 2754.92 | 4465.07 | 449.14 | –842.18 to 1286.26 | 0.68 |
| Relapse/recurrence (%) (n = 402) | 47 | 47 | 50 | 50 | –3 | ||
| QALYs (n = 213) | 1.49 | 1.49 | 1.53 | 0.35 | –0.04 | ||
Outcomes
Health-related quality of life
Mean EQ-5D health state scores are summarised in Figure 9. The graph shows that the average health state score was between 0.7 and 0.8 for both groups for the entire period between baseline and 24 months’ follow-up and that there is very little difference between the groups. The resultant QALYs over follow-up are summarised in Table 16; the difference of –0.04 between the groups was not statistically significant (p = 0.42, adjusted by stratification variables).
FIGURE 9.
Mean EQ-5D health state scores over follow-up.
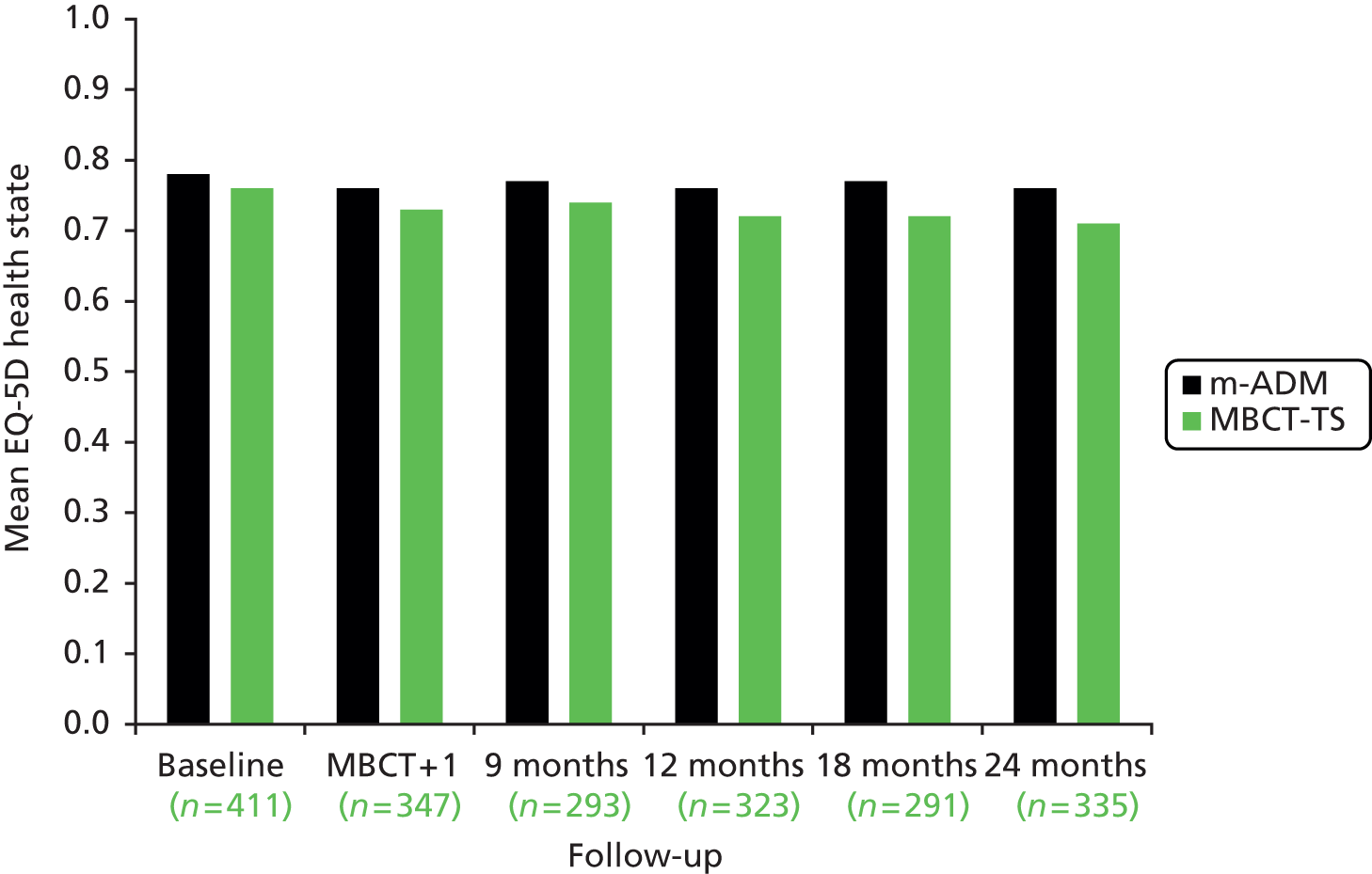
Relapse/recurrence
Detailed information on the relapse/recurrence outcome measure is provided in Chapter 4. For the purpose of the economic evaluation we used the percentage of individuals who relapsed in each of the randomised groups. In the m-ADM group 50% had a relapse/recurrence over follow-up and in the MBCT-TS group this figure was 47% (see Table 16).
Cost-effectiveness
Relapse/recurrence
In terms of relapse/recurrence, costs were higher in the MBCT-TS group and outcomes better, generating an ICER (the additional cost of one intervention compared with another divided by the additional effects) of £4955 from the NHS/PSS perspective and £17,930 from the societal perspective, including productivity losses and patient costs. The ICER indicates that an additional £4955 (from the NHS/PSS perspective) or £17,930 (from the societal perspective) would need to be invested to generate a unit reduction in the percentage of participants who relapse.
Non-parametric bootstrapping from the cost and effectiveness data was used to generate a joint distribution of incremental mean costs and effects for the treatments under comparison to explore the probability that each is the optimal choice, subject to a range of maximum values (λ) that a decision-maker might be willing to pay for improvements in outcomes. Figures 10 and 11 illustrate the scatterplots of the bootstrapped cost and effectiveness pairs for MBCT-TS compared with m-ADM, from the NHS/PSS and societal perspectives respectively. The points in the scatterplot fall in all four quadrants of the cost-effectiveness plane suggesting that there is no clear conclusion to be made regarding cost-effectiveness in terms of relapse/recurrence. It is important to note that, as positive values of relapse/recurrence correspond with worse outcomes, the usual interpretation of the quadrants of the cost-effectiveness plane is reversed with respect to the x-axis so that points that fall to the left of 0 denote better outcomes for MBCT-TS and those that fall to the right denote worse outcomes. Statistical uncertainty around the ICER was explored through the calculation of CEACs, shown in Figure 12, which demonstrate that the probability of MBCT-TS being more cost-effective than m-ADM does not rise above 43%.
FIGURE 10.
Scatterplot showing the bootstrapped mean differences in costs and effects of MBCT-TS compared with m-ADM using relapse/recurrence and service costs.

FIGURE 11.
Scatterplot showing the bootstrapped mean differences in costs and effects of MBCT-TS compared with m-ADM using relapse/recurrence and societal costs.

FIGURE 12.
Cost-effectiveness acceptability curves showing the probability that MBCT-TS is cost-effective compared with m-ADM for different values that a decision-maker is willing to pay for a unit reduction in the percentage of patients who undergo relapse/recurrence.
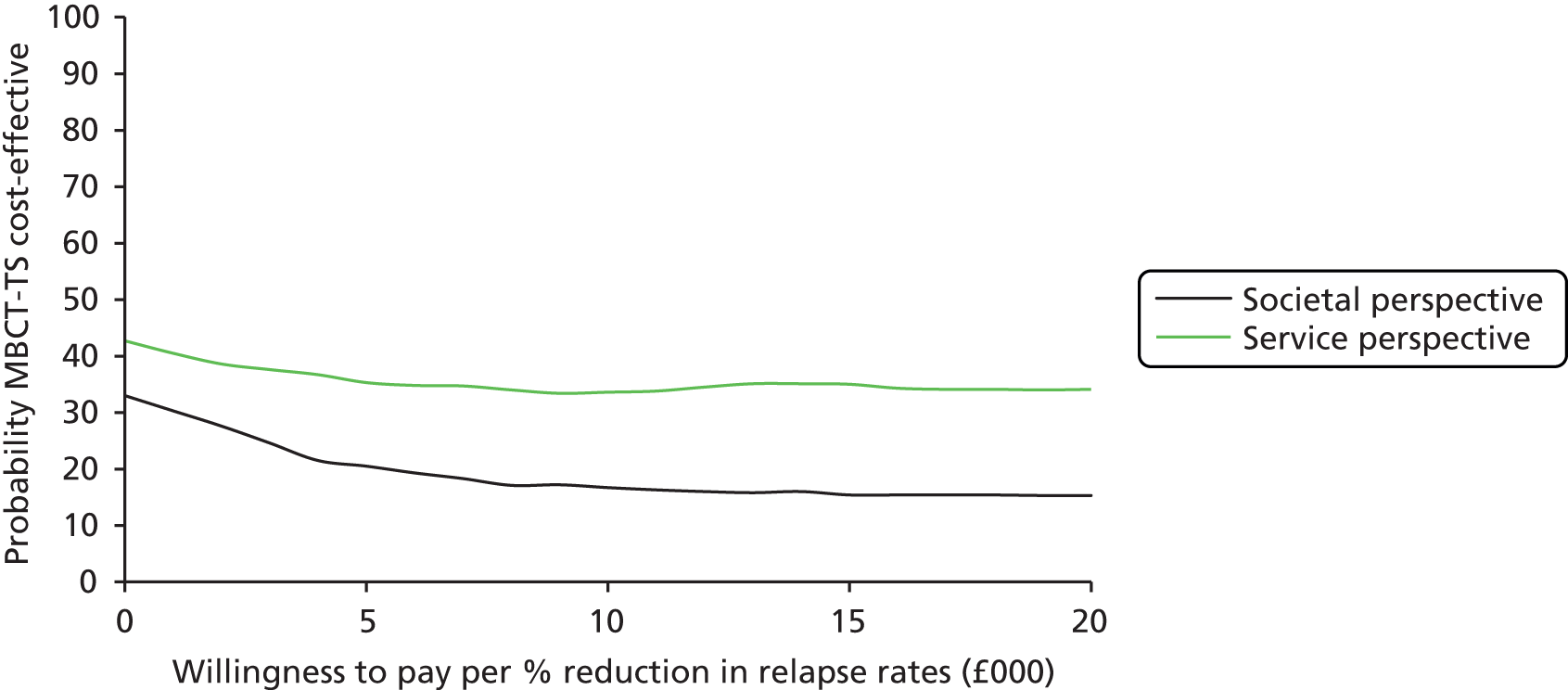
Quality-adjusted life-years
In terms of QALYs, costs were higher in the MBCT-TS group and outcomes slightly worse, suggesting that MBCT-TS was dominated by m-ADM. The scatterplots in Figures 13 and 14 show points falling mainly in the north-west quadrant (outcomes worse and costs higher) and south-west quadrant (outcomes worse and costs lower). The probability that MBCT-TS is more cost-effective than m-ADM, shown in the CEACs in Figure 15, does not rise above 52%.
FIGURE 13.
Scatterplot showing the bootstrapped mean differences in costs and effects of MBCT-TS compared with m-ADM using QALYs and service costs.
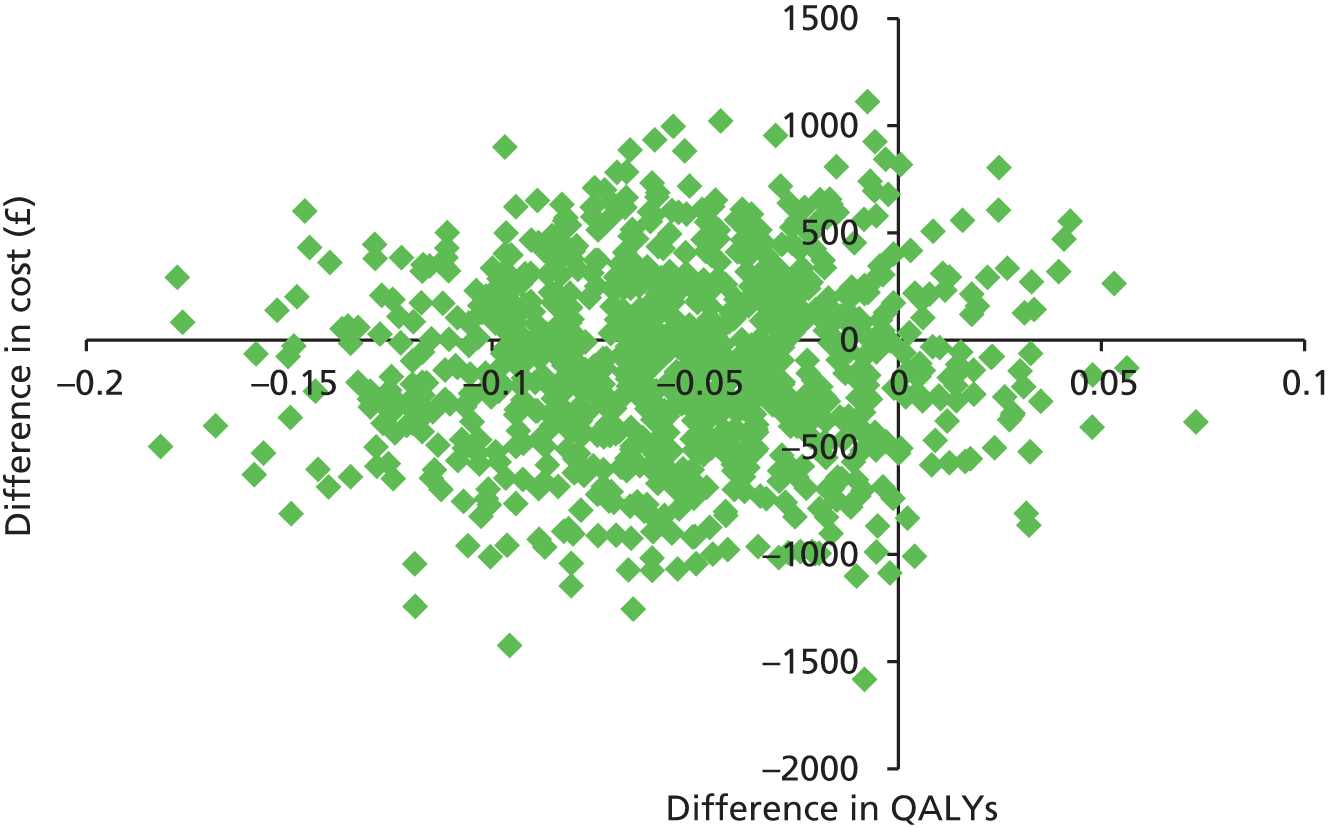
FIGURE 14.
Scatterplot showing the bootstrapped mean differences in costs and effects of MBCT-TS compared with ADM-T using QALYs and adjusted societal costs.
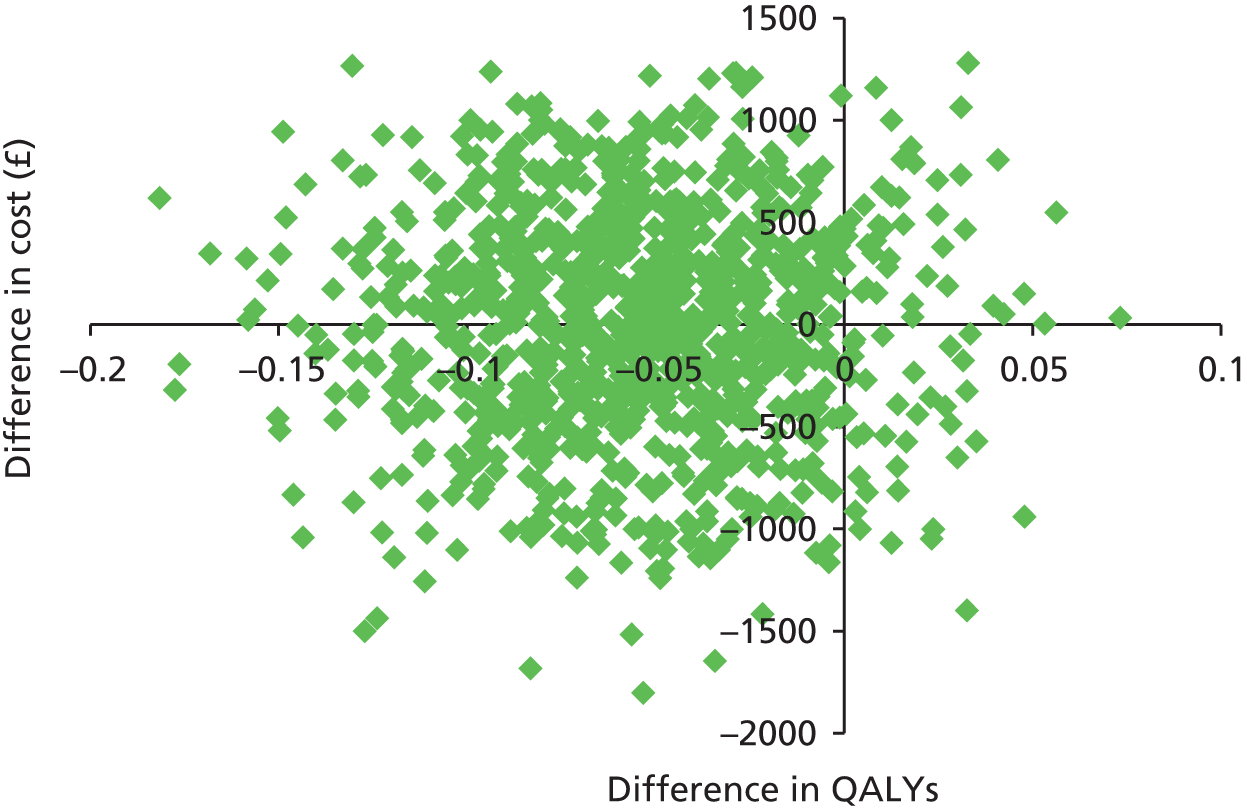
FIGURE 15.
Cost-effectiveness acceptability curves showing the probability that MBCT-TS is cost-effective compared with m-ADM for different values that a decision-maker is willing to pay for a QALY.

Sensitivity analysis
Imputation of missing data and variation in the discount rate from 0% to 6% made no difference to the results, as summarised in Table 17.
| Cost category and discount rate | MBCT-TS (n = 181) | m-ADM (n = 180) | Mean difference | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Total NHS/PSS costs (£), discount rate 0% | 2439.98 | 3992.68 | 2319.75 | 4120.03 | 107.96 | –735.63 to 951.55 | 0.80 |
| Total societal costs (£), discount rate 0% | 3007.99 | 3970.62 | 2747.60 | 4468.00 | 214.72 | –844.97 to 1274.42 | 0.69 |
| Total NHS/PSS costs (£), discount rate 6% | 2516.34 | 4137.95 | 2389.46 | 4267.05 | 113.68 | –760.28 to 987.64 | 0.80 |
| Total societal costs (£), discount rate 6% | 3035.53 | 4041.78 | 2760.14 | 4468.15 | 227.27 | –840.25 to 1294.78 | 0.68 |
| QALYs, discount rate 0% | 1.48 | 0.48 | 1.52 | 0.35 | –0.05 | –0.16 to 0.07 | 0.42 |
| QALYs, discount rate 6% | 1.46 | 0.47 | 1.5 | 0.35 | –0.05 | –0.16 to 0.06 | 0.42 |
| Multiple imputation of missing data, NHS/PSS costs (£) | 2423.68 | 3780.54 | 2348.59 | 3903.41 | 67.13 | –668.39 to 802.65 | 0.858 |
| Multiple imputation of missing data, societal costs (£) | 2934.68 | 3177.99 | 2692.53 | 3595.67 | 229.16 | –417.93 to 876.25 | 0.487 |
Chapter 6 Quantitative process–outcome evaluation
Introduction
Aim
The trial sought to address the following explanatory question: ‘Is an increase in mindfulness skills the key mechanism of change of MBCT?’ We approached this question using embedded process–outcome studies across the trial arms.
An important first step in establishing mechanisms of action is to identify whether or not the selected mechanism variables mediate the effects of the target treatment (MBCT-TS) on outcome. 103 In other words, (1) are those mechanisms differentially altered by the treatment and (2) do those mechanisms explain all or part of the effect of treatment on outcome?
This chapter reports our meditational analyses examining whether changes in mindfulness as measured by the Five Factor Mindfulness Questionnaire (FFMQ)104 from pre treatment (baseline) to post MBCT (1-month post-treatment follow-up point) mediate the effects of treatment arm on clinical outcome over the course of the trial.
Establishing mediation
To establish mediation requires attention to several key aspects of study design103,105,106 that have been instantiated in the PREVENT trial. First, MBCT is compared with a treatment that works but not through the same mechanism of action (m-ADM),107 thus allowing a test of effects specific to MBCT. Second, assessment of change in the hypothesised mediator occurred (1) during MBCT and (2) before the assessment of outcome. For the current approach we assessed change in mindfulness at baseline and immediately following MBCT and depressive relapse/recurrence over 24 months of follow-up was the dependent variable in our mediation analyses. Finally, the design requires that all those in the intervention arm received an adequate dose of the intervention to properly test the hypothesis that MBCT’s impact on the hypothesised mechanism (mindfulness) mediates outcome. Therefore, only patients who attended four or more sessions were included in the mediational analyses.
Hypothesis
Based on our previous work37 and on the theoretical and clinical rationale of MBCT, we predicted that enhanced mindfulness over the active treatment period would significantly mediate outcome, with those participants evidencing larger gains in mindfulness faring better. We did not predict an interaction with treatment arm (see Mediation approach).
Methods
Measurement of mindfulness
As noted, mindfulness in the PREVENT trial was measured using the FFMQ. 104 The FFMQ is a 39-item measure that assesses five facets of a general tendency to be mindful in daily life: observing, describing, acting with awareness, non-reactivity to inner experience and non-judging of inner experience. Items are rated on a 5-point Likert scale, with higher scores indicating a greater tendency to be mindful. The FFMQ was administered at the baseline and 1-month post-treatment assessment points.
Analytic approach
Selection of the sample
Analyses that address mediation of treatment-specific effects require an adequate treatment dose. 103 In the PREVENT trial an adequate dose of MBCT was defined as participation in four of eight MBCT sessions. As relapse/recurrence is the primary outcome it is also necessary that participants have not relapsed prior to the post-MBCT assessment point. Finally, participants need to have full data on mindfulness skills (the FFMQ) at baseline and post treatment as well as full data on GRID-HAMD-assessed depression at baseline and post treatment. Participants satisfying all of these criteria make up the mediation sample.
Mediation approach
We used the mediation analytical framework recommended by Kraemer et al. 105 for RCTs. This comprises a regression approach in which treatment group (T), the mediator (M) and the treatment by mediator interaction term (T × M) are the independent variables. We examined the outcome of relapse/recurrence using Cox proportional hazards regression. 108 Within this regression approach, for M to be a mediator of treatment, M must be an event occurring during or after treatment that is significantly altered by treatment and temporally precedes the outcome. M must also then show a main effect and/or an interactive effect with treatment on outcome, that is, the M and/or T × M terms in the regression should be significant. Treatment need not have a significant overall or main effect on outcome. 105
A main (but not interactive) effect of mediation is therefore when treatment significantly changes the mediator but the effect of the mediator on outcome does not significantly differ across treatment types. In the present analysis, if MBCT-TS differentially improves mindfulness skills and any such improvement translates into a better outcome, but the relationship between improvement and outcome does not differ between MBCT-TS and m-ADM, this would be a main, but not interactive, effect of mediation. In contrast, an interactive effect of mediation is when treatment significantly changes not only the mediator but also the relationship between the mediator and outcome such that it is significantly different for the alternative treatments. In the present study, if treatment significantly affects the acquisition of mindfulness skills, but the relationship between change in mindfulness across treatment and outcome is then significantly different between the m-ADM group and the MBCT group, this would be an interactive effect of mediation.
To ensure that any mediation effects found in the current analyses were present over and above the influence of levels of depression, our regression models included change in depression severity on the Hamilton Rating Scale for Depression (HRSD) from baseline to 1-month post treatment when evaluating mediation, on the first step in the regression analysis.
Calculating change in mindfulness
Development of mindfulness as a potential mediator was computed as change over time, that is, from baseline to 1-month post treatment. We calculated standardised residualised change scores for the mindfulness variables using a simple linear regression model in which time 1 scores predicted time 2 scores. 35,109 The standardised residuals were then used in the mediation analyses. However, we report raw score equivalents when appropriate for ease of comprehension.
Results
Selection of the sample
The full treatment-adherent sample consisted of 388 participants (MBCT-TS n = 176; m-ADM n = 212). However, 68 participants relapsed before the 1-month post-treatment assessment point and were, therefore, not included in these mediation analyses. This reduced the sample to 320 participants (MBCT-TS n = 156; m-ADM n = 164). A further 60 participants did not complete the FFMQ and/or GRID-HAMD at baseline and/or 1-month post treatment, giving a mediation sample of 260 participants (MBCT-TS n = 135; m-ADM n = 125). The baseline characteristics of the mediation sample are provided in Table 18.
| Characteristic/variable | MBCT-TS (n = 135) | m-ADM (n = 125) |
|---|---|---|
| Demographic characteristics | ||
| Female, n (%) | 96 (71) | 102 (82) |
| White, n (%) | 133 (99) | 123 (98) |
| Age (years) | ||
| Mean (SD) | 52 (11) | 50 (13) |
| Range | 25–78 | 20–79 |
| Marital status, n (%) | ||
| Single | 23 (17) | 19 (15) |
| Married, cohabiting or civil partnership | 88 (65) | 90 (72) |
| Separated, divorced or widowed | 24 (18) | 16 (13) |
| Missing | 0 (0) | 0 (0) |
| Level of education, n (%) | ||
| No educational qualifications | 9 (7) | 6 (5) |
| Some school qualifications | 19 (14) | 25 (20) |
| High school and/or vocational qualification | 56 (41) | 57 (46) |
| University degree/professional qualification | 50 (37) | 36 (29) |
| Missing | 1 (1) | 1 (1) |
| Religion, n (%) | ||
| Christian | 85 (63) | 82 (66) |
| Other | 9 (7) | 4 (3) |
| None | 41 (30) | 39 (31) |
| Missing | 0 (0) | 0 (0) |
| Salary (£ sterling) | ||
| Mean (SD) | 21,054 (14,649) | 17,961 (12,910) |
| Range | 1200–72,000 | 1200–75,000 |
| Social class, n (%)a | ||
| Class 0 | 56 (41) | 47 (38) |
| Class 1 | 37 (27) | 29 (23) |
| Class 2 | 14 (10) | 24 (19) |
| Class 3 | 4 (3) | 4 (3) |
| Class 4 | 0 (0) | 0 (0) |
| Class 5 | 24 (18) | 21 (17) |
| Not classified | 0 (0) | 0 (0) |
| Stratification variables | ||
| Depressive symptomology at randomisation, n (%) | ||
| Asymptomatic | 105 (78) | 99 (79) |
| Symptomatic | 30 (22) | 26 (21) |
| Recruitment site, n (%) | ||
| Bristol | 26 (19) | 21 (17) |
| Exeter and East Devon | 48 (36) | 47 (38) |
| North and Mid Devon | 38 (28) | 28 (22) |
| South Devon | 23 (17) | 29 (23) |
| Psychiatric characteristics | ||
| Current depressive symptomology, mean (SD) | ||
| GRID-HAMD score | 4.6 (4.3) | 4.2 (4.1) |
| BDI-II score | 13.0 (10.0) | 13.3 (9.4) |
| Previous major depressive episodes, n (%) | ||
| Fewer than six episodes | 78 (58) | 70 (56) |
| Six or more episodes | 57 (42) | 55 (44) |
| Age (years) at first depression onset, mean (SD) | 26.2 (12.4) | 27.5 (14.2) |
| Time (months) since last depressive episode, mean (SD) | 21.5 (25.3) | 18.2 (23.9) |
| Number of comorbid DSM-IV Axis I psychiatric diagnoses, mean (SD) | 0.4 (0.7) | 0.6 (0.8) |
| Received outpatient psychiatric or psychological treatment, n (%) | 66 (49) | 59 (47) |
| Attempted suicide, n (%) | 23 (17) | 27 (22) |
| Number of previous attempts, mean (SD) | 1.7 (1.0) | 1.5 (0.9) |
| Severity of reported childhood abuse, n (%) | ||
| High | 61 (45) | 55 (44) |
| Low | 74 (55) | 70 (56) |
| Missing | 0 (0) | 0 (0) |
| Quality of life, mean (SD)b | ||
| How would you rate your quality of life? | 3.7 (0.8) | 3.9 (0.7) |
| How satisfied are you with your health? | 2.9 (1.0) | 3.2 (0.9) |
| Physical | 14.4 (5.2) | 14.7 (2.8) |
| Psychological | 12.7 (2.6) | 12.7 (2.4) |
| Social | 13.4 (3.5) | 13.6 (3.1) |
| Environment | 15.2 (2.4) | 15.6 (2.2) |
| Health-related quality of life (EQ-5D tariffs) | 0.786 (0.243) | 0.798 (0.193) |
Mindfulness
Baseline and 1-month post-treatment FFMQ data for the mediation sample are presented in Table 19. The first criterion of mediation is whether treatment significantly changes the mediator. 105 We examined this by entering the FFMQ subscales as dependent variables together in a mixed-model multivariate analysis of variance (MANOVA) with time (baseline, 1-month post treatment) as the repeated measure and group (MBCT-TS, m-ADM) as the between-subjects variable. Multivariate output (broadly reflecting FFMQ total scores) showed significant main effects of group (Wilks’ λ = 0.92, F5,254 = 4.20, p < 0.001, ηp2 = 0.08) and time (Wilks’ λ = 0.67, F5,254 = 24.63, p < 0.001, ηp2 = 0.33), qualified by a significant time × group interaction (Wilks’ λ = 0.88, F5,254 = 7.05, p < 0.001, ηp2 = 0.12), consistent with a greater increase in mindfulness in the MBCT-TS group. Univariate output revealed the same significant interactions for all of the subscales (F-values > 4.12, p-values < 0.05, ηp2 > 0.015).
| Variable | Baseline | MBCT+1 | ||
|---|---|---|---|---|
| MBCT-TS (n = 135) | m-ADM (n = 125) | MBCT-TS (n = 135) | m-ADM (n = 125) | |
| FFMQ Total | 120.12 (18.75) | 119.94 (16.19) | 133.44 (18.26) | 123.57 (16.20) |
| FFMQ Observe | 24.20 (5.46) | 23.66 (5.66) | 28.64 (4.99) | 24.94 (5.45) |
| FFMQ Describe | 26.11 (6.70) | 26.51 (6.72) | 27.81 (6.28) | 26.60 (6.04) |
| FFMQ Awareness | 21.12 (4.92) | 21.58 (4.79) | 22.64 (4.29) | 22.03 (4.22) |
| FFMQ Non-judgement | 25.07 (6.56) | 25.51 (6.07) | 27.93 (6.26) | 26.19 (5.54) |
| FFMQ Non-reactivity | 20.36 (5.01) | 19.42 (4.46) | 23.02 (3.98) | 20.51 (3.96) |
Mediation
To examine mediation effects we included the different FFMQ subscales and the FFMQ total score in separate Cox regression analyses as outlined in the analysis plan. Main effect (M) and interaction (T × M) terms for these mediation analyses are presented in Table 20. There was no support for a relationship between change in mindfulness and risk of relapse/recurrence over 24 months. Following the approach to establishing mediation of Kraemer et al. ,105 there was no support for the hypothesis that change in mindfulness is the key mechanism of action of MBCT-TS.
| Variable | Wald | p-value | Exp (B) |
|---|---|---|---|
| ΔGRID-HRSD | 3.40 | 0.07 | 1.20 |
| Treatment | 1.57 | 0.21 | 1.31 |
| ΔFFMQ Total | 0.03 | 0.87 | 1.02 |
| ΔFFMQ Total × treatment | 0.40 | 0.83 | 0.86 |
| ΔFFMQ Observe | 0.04 | 0.85 | 0.98 |
| ΔFFMQ Observe × treatment | 1.66 | 0.20 | 0.75 |
| ΔFFMQ Describe | 0.07 | 0.79 | 1.03 |
| ΔFFMQ Describe × treatment | 0.01 | 0.93 | 0.98 |
| ΔFFMQ Awareness | 0.03 | 0.86 | 0.98 |
| ΔFFMQ Awareness × treatment | 0.001 | 0.98 | 1.01 |
| ΔFFMQ Non-judgement | 0.70 | 0.41 | 1.10 |
| ΔFFMQ Non-judgement × treatment | 0.32 | 0.57 | 1.13 |
| ΔFFMQ Non-reactivity | 0.72 | 0.40 | 0.91 |
| ΔFFMQ Non-reactivity × treatment | 1.42 | 0.23 | 0.77 |
Follow-up analyses within the MBCT-TS group
To further explore the patterns in the data we performed supplementary analyses focused on only those participants within the MBCT-TS arm (who also met the criteria for inclusion in the mediation sample) (n = 135). We again used Cox proportional hazard regressions, this time without the treatment arm variable on step 1 and without the interactions between treatment and the FFMQ variables on step 3. We therefore examined whether or not changes in FFMQ scores from baseline to 1-month post treatment in the MBCT-TS group significantly predicted relapse/recurrence over 24 months. The results are presented in Table 21. As can be seen, there was no significant relationship between FFMQ changes across treatment and relapse/recurrence, even within the MBCT-TS group.
| Variable | Wald | p-value | Exp (B) |
|---|---|---|---|
| ΔGRID-HRSD | 0.13 | 0.71 | 1.06 |
| ΔFFMQ Total | 0.24 | 0.62 | 0.94 |
| ΔFFMQ Observe | 1.03 | 0.31 | 0.87 |
| ΔFFMQ Describe | 0.00 | 0.98 | 1.00 |
| ΔFFMQ Awareness | 0.10 | 0.75 | 0.96 |
| ΔFFMQ Non-judgement | 0.60 | 0.44 | 0.11 |
| ΔFFMQ Non-reactivity | 2.87 | 0.09 | 0.79 |
Discussion
The analyses presented in this chapter sought to address the following explanatory question: ‘Is an increase in mindfulness skills the key mechanism of change of MBCT-TS?’ We approached this question by examining whether or not change in mindfulness from baseline to 1 month post MBCT-TS (or the equivalent time point in the m-ADM arm), as measured by the self-report FFMQ, was a significant mediator between trial arm (MBCT-TS or m-ADM) and outcome as assessed by depressive relapse/recurrence over 24 months.
We found no support in the data for either main or interactive mediation effects, somewhat contrasting with our earlier finding from the PREVENT pilot trial37 that change in mindfulness (assessed using a different measure) mediated effects on residual symptoms of depression at 12 months.
Supplementary analyses examined whether or not change in mindfulness was significantly associated with clinical outcome (time to relapse/recurrence) within the MBCT-TS group alone. Again, we found no support for this prediction.
Future work will systematically explore whether or not there are any mediating effects of mindfulness on secondary trial outcomes or whether or not any putative mediating effects are moderated by key demographic variables (e.g. exposure to childhood abuse).
Chapter 7 Barriers to participation in the PREVENT trial: a qualitative exploration
Introduction
Qualitative research within RCTs can provide a patient-centred and in-depth perspective on the implementation of complex interventions and on trial processes and outcomes. 110 The PREVENT trial offers an opportunity to build on previous qualitative work on MBCT in three important ways: (1) the follow-up period (2 years) is longer than that in many other studies; (2) participant experiences can be explored across larger numbers than in previous qualitative studies; and (3) these large numbers provide opportunities for purposive sampling. Previous qualitative work on MBCT has included very small numbers111,112 and sociodemographically homogeneous samples. 30 As a consequence, analysis has tended to focus on commonalities, with little exploration of variations in experiences of MBCT. The large number of participants in the PREVENT trial allows exploration of both similarities and variations in their experiences.
One area of focus for the qualitative exploration was the acceptability of MBCT-TS (other qualitative work within the PREVENT trial that is not reported here is listed at the end of the chapter). There are several reasons for interest in acceptability issues: (1) attrition between possible case identification and recruitment in MBCT trials is typically high;28 (2) between 7% and 25% of people do not complete the full MBCT course and a 15% dropout rate from MBCT is typical;24,25,28 and (3) there are wide variations in the extent to which people engage with mindfulness practices that are assumed to be the vehicle of change. 112 Existing evidence about the acceptability of MBCT is limited111 and there is no published evidence on the acceptability of MBCT-TS. Little is known about several elements of acceptability: why people choose not to participate in MBCT; reasons for dropout from treatment; and why people fail to engage with or continue mindfulness practices. The research reported here focuses on the first of these issues, namely barriers to participation in MBCT-TS within the PREVENT trial, at the point of initial invitation into the trial (other acceptability questions are addressed by data not reported here).
Methods
Development of data collection tools drew on the perspectives of both PREVENT MBCT therapists and members of the trial LEG (see Chapter 3). This helped to enhance the relevance and acceptability of data collection to patients and ensured that it was informed by the clinical judgements of MBCT therapists.
Reasons for declining participation at initial contact
Initial letters of invitation to participate in the PREVENT trial sent to potentially eligible patients identified by GP practices provided a return envelope and reply form. This form allowed people who did not want to participate to write their reasons for this, in response to the following prompt:
It helps us to plan research in the future if we know the reasons why this trial did not appeal to you. We would be very grateful if you could write these reasons below, but we want to stress that this is not something you have to do; we respect that this may be a private decision that you do not want to share.
Space for up to four lines of text was provided. Basic demographic information was also collected using this form.
Telephone interviews with non-participants
The reply form referred to in the previous section also asked people who declined to participate in the trial to indicate if they would be willing to conduct a brief telephone interview on their reasons for declining. Of the 2157 individuals who indicated that they were not interested in participating in the trial, 290 (13%) agreed to be interviewed and a sample of 16 was selected. Sampling was based on initial content analysis of the first 50 written responses about reasons for non-participation provided on reply forms. Based on this, we aimed to construct a sample of 16 interviewees using the following criteria: ADM issues – at least four; therapy issues – at least four; lifestyle issues – at least four; others – up to four, including at least one each from ‘research issues’ and ‘symptom issues’. We aimed to recruit from all of the four sites and to ensure that a spread of basic demographic characteristics was included. This strategy ensured that the diversity of the larger sample of people who gave reasons for non-participation was reflected in the sample of people interviewed.
A smaller number of people declined trial participation after the initial telephone screening (n = 187; see Figure 3). We aimed to conduct six to eight semistructured telephone interviews with members of this group, with at least one person from each site in the sample. This subsample allowed exploration of any impact of receiving additional information during a telephone screening interview on people’s reasons for non-participation.
Telephone interviews for both of these groups were conducted within 1 month of the person declining trial participation. They began with an initial open question about reasons for declining participation and subsequently asked about the reasons identified by analysis of written responses provided on reply forms. This included questions on participation in research, randomisation preferences, the nature of MBCT and expectations of ADM use associated with each arm of the trial.
Data analysis
Written responses were transcribed verbatim into Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA). Content analysis was used to classify responses into basic categories of reasons for non-participation. A collaborative approach to analysis was adopted by a small team of researchers who worked together to establish, define and agree on content analysis categories using a subset of data before these categories were used to analyse the complete data set.
Telephone interviews were audio recorded and transcribed, with any identifying information removed. Data were analysed using thematic analysis113 in NVivo 10 software (QSR International, Warrington, UK). The analytical strategy combined inductive and deductive approaches. Reasons for declining that were used to categorise written response data were used as a starting point for data exploration. These were added to as data analysis progressed, and main themes were divided into subthemes to capture complexity and variation. A collaborative approach involving team working among a small group of researchers enhanced the validity of the analytical process.
Results
Reasons for declining participation at initial contact
From a total of 19,608 invitations sent, 2157 people (11%) indicated that they were not interested in participating in the PREVENT trial either by declining any further contact or after further discussion with a member of the research team. Within this sample, 1535 (71%) participants were female and the average age was 58.6 years (range 20–91 years; missing data for 25 cases). The sample was spread across the different geographical areas: South Devon n = 490 (23%); North and Mid Devon n = 788 (37%); Exeter and East Devon n = 580 (27%); and Bristol n = 299 (14%). These characteristics are similar to those of the final sample of PREVENT trial participants (see Table 7) with the exception that the PREVENT trial population was younger on average (mean age 49 years). Education and marital status are not reported as fewer than half of the respondents provided this information.
Because a minority of people gave more than one reason for not participating, responses added up to an overall number of 2402 instances of coding. Of these, 120 codings (5%) were coded not eligible for participation (mainly when participants did not fulfil trial inclusion criteria such as having three episodes of depression or a diagnosis other than depression). Hence their responses were excluded from further analysis. Furthermore, some 1109 (46%) codings did not reveal any reasons, yielding a final number of valid codings of 1173 (49%).
Content analysis produced 10 categories of reasons, the distribution of which within the data is shown in Figure 16. We further categorised these reasons into four broad areas as follows:
-
intervention-related issues – these are about use of ADM, therapy-related issues and the group-based nature of the intervention
-
personal circumstances – three categories of reasons relating to personal circumstances were found: lifestyle issues, time issues and medical issues
-
symptom-related issues – reasons relating to current symptoms and feelings of being in recovery
-
research-related and other issues.
FIGURE 16.
Frequencies of reasons for non-participation provided in written responses on invitation reply forms.

Intervention-related issues
The most commonly cited reasons for non-participation were related to the treatment interventions provided in the PREVENT trial. Together these accounted for 40% of all reasons given. Within this, the largest category related to use of ADM (19% of all responses). Most commonly, people reported that they did not want to stop taking ADM (49% of ADM reasons). Other reasons were that people were no longer taking ADM (24%), were currently coming off ADM (9%) and were happy with their current ADM use (11%). The second most common category of reasons overall related to therapy issues (14% of all responses). In total, 23% of responses in this category related to people having previously tried talking therapies and a further 17% of responses related to reports that past therapy had not helped. Some reported that they were currently receiving a different kind of therapy (19%) and others reported wanting to avoid bringing up bad memories (16% of responses in this category). Related to this, a third category of responses was about reluctance to participate, or anxiety about participating, in group-based therapy (7% of all responses).
Personal circumstances
In total, 35% of responses described personal circumstances as a barrier to participation. The category of lifestyle issues accounted for 13% of responses. Specific reasons within this category included feeling too old (23%), moving house or relocating (17%), transport difficulties (14%) and feeling that participation would mean taking on too much (15%). Health-related barriers were also common (given in 12% of responses overall). Most frequently, people reported their own physical or health difficulties as a reason preventing participation (47%), but caring for others (21%) and illness or death of a family member (15%) were also cited. In total, 10% of responses overall cited lack of time, with one-quarter of these participants specifically stating that they would be unable to take time off work.
Symptom-related issues
Symptom-related reasons for non-participation were cited in 15% of responses. Specific reasons within this included considering that they suffered from anxiety more than depression (18%), feeling that they did not suffer from depression (14%), that they were currently depressed (17%), that they were currently feeling well or recovering (27%) or that they already had strategies in place to manage their depression (12%).
Research-related and other issues
The final 10% of responses referred to research-related issues (3%) and miscellaneous other reasons that could not be categorised in other ways (7%). Just over half of the research-related reasons were that people did not want to be involved in research or had concerns about this. Another common reason was already being involved in research, currently or in the past.
Telephone interviews with non-participants
Telephone interviews were conducted with 16 people who declined to participate having received an initial invitation letter and six people who declined to participate after telephone screening. Demographic information for these subgroups in comparison with that for PREVENT trial participants is provided in Table 22.
| Demographic characteristics | Pre-screen decliners (n = 16) | Post-screen decliners (n = 6) | PREVENT participants (n = 424) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 56.8 (11.1) | 59.0 (11.8) | 49.4 (12.3) |
| Range | 40–72 | 44–77 | 20–79 |
| Missing, n | 1 | 1 | 0 |
| Female, n (%) | 13 (81) | 5 (83) | 325 (77) |
| Recruitment site, n (%) | |||
| Exeter and East Devon | 3 (19) | 1 (17) | 148 (35) |
| North and Mid Devon | 6 (38) | 2 (33) | 109 (26) |
| South Devon | 5 (31) | 2 (33) | 103 (24) |
| Bristol | 2 (13) | 1 (17) | 64 (15) |
| Marital status, n (%) | |||
| Single | 2 (13) | 0 (0) | 80 (19) |
| Married or living together | 10 (63) | 2 (33) | 264 (62) |
| Separated, divorced or widowed | 2 (13) | 1 (17) | 77 (18) |
| Missing | 2 (13) | 3 (50) | 3 (1) |
| Employment status, n (%) | |||
| Employed | 5 (31) | 3 (50) | 240 (57) |
| Unemployed or retired | 9 (56) | 1 (17) | 178 (42) |
| Missing | 2 (13) | 2 (33) | 6 (1) |
Table 22 shows that the main differences between these non-participant samples and the PREVENT trial participants are that there are slightly higher proportions of women in the non-participant samples and the non-participant samples are older on average. The two non-participant subsamples are broadly similar to each other.
Analysis identified nine broad thematic areas within the interview transcripts, with subthemes within each. The frequency of coding across interviews using these themes is show in Table 23.
| Theme | Subthemes | Pre-screen (n = 16) | Post-screen (n = 6) | Total (n = 22) |
|---|---|---|---|---|
| Taking part in a research trial | No concerns | 15 | 5 | 20 |
| Safety and reliability concerns | 4 | 1 | 5 | |
| Wanting to help others | 2 | 2 | 4 | |
| Previously taken part in research | 2 | 1 | 3 | |
| ADM-related issues | Wants to remain on ADM | 14 | 6 | 20 |
| Wants to come off ADM | 7 | 2 | 9 | |
| Does not want to take ADM for 2 years | 1 | 0 | 1 | |
| Not regularly taking ADM | 1 | 0 | 1 | |
| Being randomised to a trial arm | Would like to choose group | 9 | 4 | 13 |
| Would like to be in the ADM group | 7 | 2 | 9 | |
| Would like to be in the therapy group | 2 | 2 | 4 | |
| No worries about randomisation | 3 | 1 | 4 | |
| Time issues | Not the right time | 9 | 4 | 13 |
| 2 years is too long | 2 | 0 | 2 | |
| Working full time | 1 | 1 | 2 | |
| Attitudes to psychological intervention | Had therapy before | 7 | 4 | 11 |
| Previous therapy unhelpful | 3 | 3 | 6 | |
| Previous therapy helpful | 4 | 1 | 5 | |
| Therapy and ADM complementary | 2 | 0 | 2 | |
| MBCT-related issues | Meditation is a good idea | 7 | 4 | 11 |
| Not keen on meditation | 4 | 1 | 5 | |
| Conflicts with religious interests | 1 | 0 | 1 | |
| Being in a group | Unhappy with group therapy | 9 | 1 | 10 |
| No concerns about group therapy | 3 | 2 | 5 | |
| Lifestyle and medical barriers | Too unwell | 3 | 2 | 5 |
| Cannot travel to sessions | 3 | 1 | 4 | |
| Too old | 1 | 1 | 2 | |
| Symptom-related issues | Not depressed enough | 5 | 1 | 6 |
| Depression is contextual | 4 | 3 | 7 | |
| Depression because of chemical imbalance | 2 | 1 | 3 | |
| Worried about relapsing | 4 | 0 | 4 |
Table 23 shows the application of a theme at any point in an interview transcript but does not provide further information on how often or where themes were used in interview transcripts or the relative weights or meanings attached to these. To explore these more complex issues, a distinction was made between reasons for non-participation provided in response to an initial open question at the beginning of the interview (‘Could you tell me the reason or reasons why you are not interested in taking part in this research study’) and reasons provided later in the interview in response to prompts about barriers to participation identified from written replies. It was assumed that reasons given in response to the first open question would usually be the most important for an individual. The distribution of themes and subthemes for first question responses and later parts of the interview and the co-existence of themes within interview transcripts is shown in Appendix 7. Details of each of the thematic areas are reported below in descending order of frequency with which they occur as ‘principal’ reasons for declining participation (i.e. in response to the initial open question). Exploration of these did not detect any obvious differences between the two subsamples of interviewees (those interviewed before telephone screening and those interviewed after). Accordingly, findings from the two subgroups are reported together, yielding a total sample of 22 interviews.
Antidepressant medication-related issues
The most prominent reasons for declining participation were related to ADMs. Concerns about ADMs were raised by all of the interviewees, nearly half of whom (n = 10) discussed ADMs in response to the initial open question. For the majority (18/22), the principal concern was about potentially stopping or reducing their ADM. Most people felt that they needed to take ADM, or were well when they were taking it, and they did not want to jeopardise this or risk relapse/recurrence if randomised to the treatment arm.
When I read through the literature I believe um it w- . . . t’was about stop taking medication and um I wasn’t really prepared to do that because it has taken me a long time to sort of get the balance right um and that would I think that would I didn’t want to stop and jeopardise that was I think that was um what I recall.
2007
For nine people, the concerns around ADMs related to the fact that they would like to eventually come off their medication. For some of these people the risk of being randomised to the m-ADM arm, and feeling that this would entail a loss of choice or flexibility, was a bigger concern.
In agreeing to this I’d have to agree to keep taking my medication for you know the next 2 years. It may be that I wanna come off my medication in a year or so’s time you know so that was an issue. Um you know um so . . . you know I’d feel I’m sort of trapped on medication as it were you know, err, I may not want to be trapped on medication or I might feel that at some point in the fairly near future it might not be a bad idea to come off . . . I have a feeling if I committed to this then that would um that would maybe cramp my options a bit.
2001
Three people described having already started to reduce their ADMs and their hopes that they would be able to stop taking them in the near future.
Time issues
The second most common reasons for non-participation involved time-related issues, with more than half (13/22) mentioning time constraints at some point in the interview and 10 people citing time constraints in response to the initial open question. Reasons related to people’s circumstances and predominantly included not having enough time for the study or it not being the right time in their lives. Two people worked full-time and as such were not able to attend MBCT sessions that were mostly run during working hours. Of those who were employed, nearly all mentioned time constraints as a reason for declining, whereas only half of those who were retired discussed this. Those who were married also tended to give time constraints as a reason for declining. Two people declined as they felt that 2 years was too long a time to be involved in a research study.
Um it is time, it is purely because I just don’t have the time. I’ve taken on so many other new commitments which are quite essential commitments you know jobs and things like that that really need enough time to commit to properly. And rather than start it and give it up, it’s probably best not to start it.
3005
I mean primarily I haven’t got time. Um I work 2 days a week um, I’ve got an elderly mother I look after and I look after my grandson as well so in the literature that you sent out you said about going to group meetings and things like that. I haven’t got time to do it basically.
2005
Lifestyle and medical barriers
Ten people cited practical or medical reasons for non-participation, of whom six gave such reasons in response to the first open question. Five respondents said that they were unable to participate in the PREVENT trial because of physical health problems that meant they felt unable to commit to attending treatment. For four people, travelling to and from sessions was a barrier. Two people felt that they were too old to take part, although the exact reasons for this remained unstated:
No no my general view is that at this stage in my life because of my age I really don’t need to take part.
3008
Being in a group
Ten individuals reported feeling uneasy with the idea of group-based treatment or said that they would feel more comfortable with one-to-one contact. Four of these people gave this reason in response to the first question.
When my depression is really bad . . . and you’re sort of crying out for help um it’s very . . . the thought of . . . being within a group of people you know um is quite daunting in a way, at the best of times. . . . I really didn’t want to be in a group situation with people who were potentially my neighbours or the people who worked in the café or the bank, you know, locally to me.
2014
You know I d- you know I I think there are a lot of people out there that probably feel the same as me, but I realise that and I don’t really think I want to . . . go into a group (.2) you know I, I – just one of those things, I just don’t want to do it really.
2009
However, the group-based nature of MBCT was not a universal barrier and five individuals said that they would not mind taking part in group-based therapy.
But when you’re talking to a stranger sometimes if they can gain your confidence then you feel as though you can talk to them more and tell exactly how you’re feeling. But I think that um group therapy is a good thing actually.
2013
Attitudes to psychological intervention
Half of the individuals interviewed (11/22) had some previous experience of psychological therapy, of whom six had found this unhelpful.
Because I’ve had therapy in the past, counselling sessions actually, the reason I considered this is because the counselling session didn’t actually help, going back over and over again on all the issues and actually why I was upset and feeling unhappy actually just made me feel more upset than happy. So I found that wasn’t particularly beneficial for me, and I had a, you know, sort of aversion to therapy to do with one particular therapy incidence.
3005
Of the six that found psychological therapy unhelpful, three mentioned negative experiences of therapy in the past in response to the first open question. The most common forms of therapy experienced were CBT and counselling. One person had received hypnotherapy and another had seen a psychiatrist.
Mindfulness-based cognitive therapy-related issues
The nature of MBCT treatment was seen as a barrier to participation by some respondents but as a facilitator by a larger proportion of respondents. Half of the individuals interviewed thought that therapy incorporating meditation was a good idea. Some mentioned that they already practised some form of meditative techniques such as breathing exercises, yoga or t’ai chi and so were open to the meditative aspects of MBCT.
Um I think it sounds very good, I’ve tried meditating in the past and you know err breathing techniques and stuff and the idea of it sounds really good.
2007
However, five people expressed concerns about meditation forming a large part of MBCT and appeared sceptical of its benefits.
I’ve tried yoga in the past and tried to meditate. My problem with that is I just I find it very hard and I end up making mental lists of all the different things I have to do and my mind doesn’t switch off.
3006
Only one person cited the nature of MBCT as a significant barrier in response to the first question. This person’s concerns centred on perceived incompatibilities with religious beliefs.
No I think it was to do with somebody like you ringing up and explaining what was going to happen, and I wasn’t happy with it because I’m a Christian and it didn’t hold with my beliefs I think. It was err, I felt it was very based on Eastern religions, Eastern philosophy and that, and that was uncomfortable. I thought the meditation where you’ve got to clear your mind I’m not happy with that anyway, and I just felt it was more like younger based, and I think that’s what concerned me more than anything.
2010
Symptom-related issues
Twelve participants discussed symptom issues as a barrier to participation, although only one person cited this in response to the first question. Six people said that they did not consider themselves to be depressed enough or were no longer suffering from depression. Seven people described their depressive symptoms as contextual or a consequence of specific life stressors. As such, they felt that their symptoms did not warrant psychological therapy.
I’ve always been a person in c- basically in control of myself I mean m- much of my trouble was caused all the work rather than anything else you know? Particularly when I took them [ADMs] before I don’t remember when that was now um but it was caused too much work.
2002
Four people said that they had concerns about relapsing as a consequence of reducing their ADM during MBCT-TS treatment.
I have twice in the past tried to come off the tablets um you know it was quite a long time ago, and after I’d got down to a certain dose I became ill again and you know it’s not something I want to happen, you know me taking 3 weeks off of work, which um is never good for me or the department you know. With a responsible job you can’t um you can’t really do that.
2006
Being randomised to a trial arm
Only one person cited trial randomisation as a barrier to participation in response to the first open question.
I just saw it as an opportunity, and I think if I’d been in a group that wasn’t actually receiving the help, in the other test group [m-ADM] I would find it very disappointing and it may even affect me and I’d feel maybe unfairly treated perhaps. Whereas I should have been looking at it as a research project, um I was probably looking at it as some sort of therapy for me really.
3003
However, when asked later in the interview about being randomised to MBCT or m-ADM, 13 people said that they would prefer to have a choice rather than be randomised. Of these, nine said that they would like to be placed in the m-ADM group and the remainder said that they would choose to receive MBCT. Only four respondents did not mind which trial arm they were placed in.
Taking part in a research trial
In response to a question about taking part in research generally, the majority of respondents (20/22) either had no concerns about taking part in a research study or said that they would be happy to do so if the circumstances were right for them. Four people said that they would take part in research to help other people and three individuals had previously taken part in a trial.
I’m absolutely fine with that, taking part in a research study, I don’t have any problem with that at all.
2007
Five people voiced concerns about the safety and reliability of research. These ranged from concerns about data confidentiality to concerns about the qualifications and professionalism of research staff.
I guess it’s to do with things around privacy and confidentiality of data, stuff to do with that and to do with the uses to which data might be put in the future by persons unknown, as it were, you know, or authorities of note. There is an issue for me with that, but it wouldn’t necessarily put me off taking part in something like this, but it is a nagging issue I feel, personally yeah.
2001
Discussion
Barriers to participation in the PREVENT trial
It is difficult to collect reliable data on why people choose not to participate in research or treatment and hence there is little good evidence on this issue. Our attempts to do so yielded data from < 5% of the total sample who were sent initial invitation letters. It is not possible to judge how representative this subsample is of the larger number of people who were invited to take part, in terms of either their sociodemographic and clinical characteristics or their reasons for not wanting to participate.
Given these sample-related caveats, data from reply forms allowing people to decline participation in the PREVENT trial and provide brief reasons for this suggest that views on the interventions being tested within the PREVENT trial were a principal barrier to participation. Overall, these accounted for 40% of the reasons given at this early stage, with ADM issues – most typically people being reluctant to consider stopping their ADM – being commonly cited (19%). A large proportion of people (21%) also expressed a reluctance to engage with therapy or a group-based intervention (although this was not always about therapy per se as some people reported already receiving another form of therapy). A further 33% of responses cited personal circumstances as barriers to participation. These included health issues, time constraints, caring commitments and transport issues.
Data from telephone interviews allowed more in-depth exploration of reasons for non-participation. Interviewees were carefully selected to be broadly representative of the larger non-participant group according to reasons given on reply forms. Reasons provided in brief written responses were replicated in these data and no new themes were detected. The same four broad clusters of reservations were expressed: intervention-related concerns, personal circumstances, symptom-related issues and research-related concerns. However, a richer understanding of these reasons was obtained and features that were attractive or at least acceptable, as well as those perceived to be barriers to participation, were discussed. This highlights that barriers are not universal. Many respondents had no problems with the prospect of participating in research, psychological therapy and treatment that incorporates meditation practices. Smaller numbers did not object to group-based treatment or being randomised. Interview data also shed light on relationships between these themes. In particular, concerns about randomisation appeared to be primarily motivated by preferences relating to ADM use.
Taking the findings from these two substudies together, the principal barrier to participation in the PREVENT trial at the point of recruitment appeared to be expectations surrounding ADM use. This applies to both arms of the trial. For most people, their concerns centred on being randomised to MBCT-TS, as they did not consider themselves to be in a position to taper their ADM. Although ADM tapering is presented as an invitation rather than a requirement by MBCT-TS therapists, the perceived expectation of this presented a considerable barrier at this point. For a smaller group of people, reluctance to participate related to being randomised to the m-ADM arm, as this carries an expectation of continuing on ADM for 2 years, a prospect that may not be acceptable to many.
Preferences for ADM use appeared to be the principal reason why randomisation within the PREVENT trial was off-putting to many, as it is perceived to remove choice and flexibility. This may be a particularly large barrier within the PREVENT trial, which involved a 2-year follow-up period, and for a participant group with long-term mental health problems who may have developed functional self-management strategies based on experiential knowledge of ADMs that they are reluctant to disrupt. As such, randomisation without taking account of patient preferences runs counter to the promotion of self-management, patient choice and expertise by experience that features in much current policy rhetoric in health and mental health. Randomisation appeared to be the principal barrier to research participation as few other concerns about involvement in research were expressed and the majority of interviewees were positive about participating in research.
Other than ADM-related issues, several identified aspects of the acceptability of MBCT-TS may apply equally to MBCT. The expected time commitment and the group-based nature of treatment appear to be two important perceived barriers at the point when people are deciding whether or not to participate. The considerable time commitment of MBCT participation and home-based practice may not be compatible with many people’s life circumstances. Although the group-based nature of treatment is off-putting to some, previous research on MBCT has found that initial concerns about group work are often replaced by recognising the value of sharing experiences in a safe environment during and following treatment. 111,114 For a significant minority, physical health may be a barrier to participation. Although negative experiences of, or expectations of, talking therapies were cited as reasons for non-participation by some, the nature of MBCT did not seem to be a concern for most at this stage. A small number of interviewees found the prospect of meditation off-putting, but a larger number expressed interest in or positive views of a meditation-based treatment.
There are some suggestions than non-participants may be older than participants (average age of 59 years for written response data participants and 57 years for interviewees compared with 49 years for PREVENT participants). Interestingly, feeling too old as a reason for non-participation appeared in a small number of both written responses and telephone interviews.
Findings can contribute to informing both clinical practice and future research trials on MBCT. The external validity of RCTs can be undermined by low recruitment rates. These data provide a window on reasons for the relatively low recruitment rate in the PREVENT trial (424 from an initial total of 19,608 people invited). Exploring barriers to participation and their impact on the composition and characteristics of trial participant groups is an important aspect of interpreting trial results. Findings also have implications for clinical practice in highlighting features of MBCT-TS and MBCT that may prevent people who are offered this treatment from taking it up. This counters the tendency in previous qualitative research to focus on the benefits of MBCT for those who engage meaningfully with treatment,30 at the expense of exploring the experiences of others who do not engage. Awareness of these barriers may help practitioners to put in place strategies to overcome reluctance to commence therapy and may help to increase the accessibility of mindfulness-based treatments.
Other qualitative studies within the PREVENT trial
As well as the data reported in this chapter, two further sources of qualitative data were collected within the PREVENT trial with the aim of addressing the following research questions:
-
The acceptability of MBCT-TS. We conceptualise acceptability as dynamic and related to various levels of knowledge and experience of MBCT-TS at different stages of recruitment and participation. Further data analysis will focus on barriers to and facilitators of MBCT-TS at later stages of participation, during and after treatment.
-
Mechanisms of change in MBCT. This extends previous qualitative work30 in two ways, first by using larger numbers and purposive sampling and second by drawing on insights from quantitative studies of change mechanisms. 37 Qualitative explorations of processes that have been identified as mediating outcome, such as self-compassion and reactivity, may strengthen understanding of how MBCT works.
-
Participants’ attitudes towards and experiences of ADM with and without MBCT (including ADM tapering and continuation). Monitoring of ADM usage within the PREVENT trial suggests considerable variability in adherence, especially in the MBCT arm (see Chapter 4). No previous qualitative work has been carried out on experiences of combining MBCT with ADM tapering.
The following two sources of qualitative data were collected:
-
Feedback booklets. All trial participants were asked to provide written accounts of their experiences in feedback booklets provided at two time points: 1 month post MBCT-TS (or the equivalent time point for those in the m-ADM arm) and at 24 months’ follow-up. This design helps avoid the influence of memory biases, allows longitudinal comparisons and ensures equipoise. Four feedback booklets were created (1 month post MBCT-TS, 24 months post MBCT-TS, 1 month post m-ADM, 24 months post m-ADM). Members of the LEG provided feedback about the wording of booklet questions, the choice of issues covered and the time needed to complete them. To ensure sensitivity to the range of participants’ experiences, the design of the 24-month booklets was informed in part by responses obtained at the 1-month time point. Booklets covered the following topics:
-
1 month post MBCT-TS: (i) attitudes towards and experiences of taking and reducing ADM; (ii) experiences of taking part in MBCT-TS and MBCT-TS practices; (iii) the impact of MBCT-TS
-
24 months post MBCT-TS: (i) experiences of MBCT-TS reunions; (ii) carrying out mindfulness practices; (iii) the impact of continued MBCT-TS practice; (iv) ongoing experiences of medication use and possible tapering in relation to MBCT-TS impact
-
1 month post m-ADM: (i) acceptability of using ADMs in general; (ii) perceived effectiveness of ADMS; (iii) experiences of ADM continuation within the trial
-
24 months post m-ADM: (i) attitudes to and experiences of continuing to take ADMs during the trial; (ii) deviations from the ADM use protocol during the trial; (iii) reasons for and impacts of any changes.
-
-
End-of-trial interviews. Semistructured interviews were designed to explore experiences during the follow-up period of use of ADMs and MBCT-based techniques, in relation to each other, during periods of wellness and depressive relapse/recurrence and during transitions between them. The research team, informed by responses from a sample of 1-month post-MBCT-TS feedback booklets and feedback from the trial LEG, developed interview topic guides. Interviews were structured around discussion of periods of wellness, early signs of depressive relapse/recurrence (referred to in interviews as ‘wobbles’) and depressive relapses/recurrences. For each of these, questions explored the use and value of mindfulness techniques, use of ADMs and use of a combination of mindfulness techniques and ADMs. The final section of the interview asked about the broader impacts of participation in the trial on other life domains, sense of self and views of depression. A sample of 40 interviewees was constructed according to principles of maximum variation sampling, aiming to access a range of participant positions and characteristics relevant to the research questions. 115 Interviewees were selected evenly across the four study sites and to broadly reflect the sociodemographic characteristics of the full trial sample. In addition, we sampled according to treatment response (depressive relapse/recurrence or not) and medication usage during follow-up (four categories: stopped, stopped and resumed, reduced but never stopped and did not reduce or stop). Face-to-face interviews were conducted in participants’ homes and lasted between 45 minutes and 1 hour.
These data collection strategies will enable a combination of depth of understanding within a purposively selected sample and breadth of data collected from all PREVENT trial participants. The 2-year follow-up period of the PREVENT trial allows exploration of how MBCT-TS impacts on people’s depressive symptoms and lives over a much longer time period than has previously been studied. Careful sampling of respondents for end-of-trial interviews ensures that this data set is genuinely reflective of the range of relapse/recurrence, ADM use and MBCT-TS experiences across trial participants. Analysis of these data sources will be reported in subsequent published papers, adding complexity and further understanding to the trial’s results.
Chapter 8 Discussion and conclusions
Principal findings
The overarching aim of the PREVENT trial was to establish whether or not MBCT-TS provides an effective alternative relapse/recurrence prevention approach to m-ADM in primary care settings for patients with a history of recurrent depression. Specifically, we asked a primary policy research question: ‘Is MBCT-TS superior to m-ADM in terms of a primary outcome of preventing depressive relapse/recurrence over 24 months?’ Secondary outcomes were depression-free days, residual depressive symptoms, psychiatric and medical comorbidities, quality of life and cost-effectiveness. The study was conducted in line with both CONSORT guidance74–76 and our published protocol. 78,83 Participant recruitment, flow and data completeness were above estimated thresholds. We are, therefore, able to answer the key research questions.
There was no evidence for the superiority of MBCT-TS over m-ADM for patients with recurrent depression in terms of either the primary outcome of time to depressive relapse/recurrence over 24 months or any of the secondary outcomes (see Chapter 4). The cost-effectiveness analysis does not support the hypothesis that MBCT-TS is more cost-effective than m-ADM, in terms of either relapse/recurrence or QALYs (see Chapter 5).
In a predefined subgroup analysis,83 we found that time to relapse/recurrence at 24 months for participants with a higher severity of reported childhood abuse was delayed in the MBCT-TS arm, compared with the m-ADM arm.
Finally, the first results from our process studies suggest that, although changes in mindfulness were greater in the MBCT-TS group than in the m-ADM group, they did not mediate time to relapse/recurrence at 24 months (see Chapter 6). In terms of acceptability, the qualitative analyses suggest that people have views about (dis)/continuing their ADM that can serve as a facilitator and barrier to taking part in a trial that requires either continuation for 2 years or discontinuation (see Chapter 7).
Research findings in context
Relapse/recurrence rates in people with three or more previous episodes are as high as 80% over 2 years. 6 Moreover, meta-analyses consistently suggest that m-ADM reduces the relative risk of relapse/recurrence by two-thirds compared with placebo, a halving of the absolute risk. 9 Therefore, it is likely that MBCT would provide benefits over and above either no treatment or pill placebo.
Outcomes were comparatively good across both treatment arms in the PREVENT trial in terms of relapse/recurrence over the 2 years of follow-up compared with those in previous trials (i.e. MBCT-TS 44%, m-ADM 47%). Moreover, PREVENT trial participants reported residual depressive symptoms within the minimal range and quality of life in the range ‘good’ at each follow-up point. This suggests that participants in both arms experienced relatively good mental health and quality of life over the follow-up period.
Before the PREVENT trial, only two small studies had compared MBCT-TS with m-ADM. In our pilot trial with 123 participants, MBCT-TS (n = 62) was compared with m-ADM (n = 61) over 15 months’ follow-up. 28 The relapse/recurrence rate was 47% for MBCT-TS and 60% for m-ADM. In a second study, 84 patients with recurrent depression who had remitted on ADM were randomised to MBCT-TS, m-ADM or pill placebo. 51 Relapse/recurrence rates observed over 18 months of follow-up did not differ between MBCT-TS (28%) and m-ADM (27%; p = 0.93) and both MBCT-TS and m-ADM were superior to placebo (71%; p = 0.007).
Since the start of the PREVENT trial, a key systematic review and meta-analysis has been published that included six RCTs comparing MBCT with usual care, m-ADM or placebo (including the two previously described trials) (n = 593). The pooled estimate from this review shows that MBCT reduces the risk of relapse/recurrence compared with usual care or placebo (risk ratio 0.66, 95% CI 0.53 to 0.82). 116
Since this systematic review/meta-analysis, several additional large-scale trials of MBCT have been published117–119 or are due to be published soon. 120 The Staying Well After Depression (SWAD) trial compared MBCT with cognitive psychoeducation plus usual care and usual care alone in 274 participants currently in remission but reporting at least three previous episodes of depression. 117 The SWAD trial reported no significant effect of treatment on risk of depressive relapse/recurrence over the 12-month follow-up (MBCT vs. psychoeducation: HR 0.88, 95% CI 0.58 to 1.35; MBCT vs. usual care: HR 0.69, 95% CI 0.42 to 1.12). In another recent RCT, 203 non-depressed adults with a history of three or more previous episodes of depression were randomised to MBCT plus depression relapse active monitoring (i.e. training in self-management of depression and monthly monitoring of symptoms) (n = 101) or active monitoring alone (n = 102). 119 The DARE trial found no significant difference between the two treatment arms for the primary outcome of time to first relapse/recurrence over 24 months, although there was some evidence that proportionally fewer people relapsed in the MBCT arm than in the active monitoring alone arm (odds ratio 0.68, 95% CI 0.38 to 1.23). It is noteworthy that the absolute rates of relapse/recurrence in the usual care arms of these two more recent trials are lower than those seen in earlier RCTs. In total, 66% relapsed in the usual care arms in the Piet and Hougaard systematic review116 compared with approximately 50% in the usual care arms in the DARE119 and SWAD117 trials at 12 months’ follow-up.
Economic evaluation found group-based MBCT-TS to be a relatively inexpensive intervention compared with individual therapies. In line with previous results from our pilot study,28 there was little difference in the use of other health and social services between the groups, resulting in only a small difference in the total costs of care over 24 months between the two groups. Coupled with small, non-significant differences in outcome, MBCT-TS failed to demonstrate cost-effectiveness compared with m-ADM.
Consistent with an emergent pattern of findings,117 MBCT may confer most benefit to patients at greatest risk of relapse/recurrence. Earlier trials24,25 and NICE guidance8 suggest that MBCT is indicated for patients who have a history of three or more previous episodes of depression. In the SWAD trial,117 a subgroup analysis compared participants reporting greater or lesser childhood adversity. For participants reporting greater childhood adversity MBCT conferred better protection against relapse/recurrence than both psychoeducation and usual care (MBCT vs. psychoeducation: HR 0.61, 95% CI 0.34 to 1.09; MBCT vs. treatment as usual: HR 0.43, 95% CI 0.22 to 0.87). The PREVENT trial results replicate the SWAD finding and converge with those of earlier studies suggesting that patients at greatest risk of depressive relapse/recurrence may benefit most from MBCT. Trials of other psychosocial approaches have shown that more intensive psychosocial treatments result in greater protection for those most at risk. For example, in a two-arm RCT with 21 months’ follow-up, relapse/recurrence rates were 51% for maintenance CBT and 60% for psychoeducation but, among those at greatest risk, CBT conferred greater protection than psychoeducation. 121 A reported history of abuse and adversity is associated with worse outcomes among people who suffer depression. 122 Perhaps MBCT confers resilience in this group at highest risk because patients learn skills that address some of the underlying mechanisms of relapse/recurrence, a question that we will seek to explore in a subsequent publication from this trial. Future trials of MBCT need to focus on the question of the effectiveness and mechanism of action of MBCT for those at differing risk of relapse/recurrence, with broad and robust measures of risk.
Strengths and limitations
Strengths of the PREVENT trial include the following.
-
The pragmatic main study question is of high relevance to the NHS. The study answered an important clinical question of high relevance to GPs and patients at risk of depressive relapse/recurrence.
-
The trial was a multicentre RCT conducted in accord with both CONSORT guidance and our published protocol/statistical analysis plan.
-
This is the largest trial of MBCT for recurrent depression to date. Good recruitment/retention rates and low levels of missing data mean that the trial was adequately powered and able to answer the primary research question.
-
The study benefited from a comprehensive economic evaluation, providing evidence to support resource allocation decision-making of both clinical and policy relevance.
-
Treatment fidelity with respect to both arms of the trial was designed into the trial and high levels of fidelity ensured high internal validity.
-
The 2-year follow-up allowed assessment of the impact of MBCT-TS over a time period that was comparable to or longer than that in all MBCT trials completed to date.
-
A parallel process evaluation was undertaken to provide contextual understanding and examine the mechanism of action of MBCT.
Limitations of the PREVENT trial include the following.
-
Our recruitment strategy involved searching primary care databases and inviting patients who were currently taking m-ADM rather than recruiting patients who were discussing their options with their GP for preventing relapse/recurrence.
-
The design included neither a usual care nor an attention control arm. The absence of an attention control arm means that any effects of MBCT-TS or m-ADM cannot be inferred to be specific to these treatments.
-
The m-ADM arm included active monitoring of adherence by the research team and in this sense might best be represented as enhanced m-ADM.
The pragmatic nature of the trial resulted in a proportion of patients in both arms not complying with the invitation to (dis)continue ADM. We undertook a per-protocol analysis to examine the impact on the primary outcome inference compared with ITT analysis. This is both a strength (pragmatism and generalisability) and a limitation (the ADM was not completely controlled). Finally, the sample consisted of a group at high risk of depressive relapse/recurrence,123 currently taking ADM, who were open both to considering a group-based psychosocial treatment and to (dis)continuing their ADM. This is both a strength and limitation of the study. The findings of the PREVENT trial are therefore generalisable to only those individuals who are in equipoise about the type of preventative treatment that they choose, that is, m-ADM or switch to psychosocial intervention and reduce their ADM.
Implications for practice
Depression is a major public health problem that tends to run a recurrent course, producing substantial decrements in health and well-being. The cost of mood disorders to the UK economy is substantial. Most of the prevalence, burden and cost of depression is a consequence of recurrent depression and could be offset through providing a range of psychosocial low- and high-intensity treatments. 4
Mindfulness-based cognitive therapy with support to taper/discontinue ADM may confer ongoing protection for patients who would like an alternative to m-ADM. Many patients with recurrent depression may have been on m-ADM for many years and for a variety of reasons would prefer to learn skills that they can use to stay well. The results further suggest that psychosocial treatments such as MBCT and CBT117,121,124 offer added value for patients who need them most, that is, those at highest risk of depressive relapse/recurrence. The investment of effort by these patients in learning the skills taught in psychosocial treatments such as CBT and MBCT pays off. However, for patients at low risk, treatments such as psychoeducation or m-ADM, which require less patient commitment and cost, may be indicated. This has significant potential to improve prevention by maximising the delivery of treatments through stratified approaches, which also have the potential to improve patient choice. As patients with recurrent depression select options to stay well in the long term, these findings taken together with the broader emergent literature suggest a number of alternatives. It is clear that m-ADM is an effective mainstream approach. For patients at low risk of relapse low-intensity interventions may be indicated and for patients at high risk high-intensity treatments such as MBCT may be indicated.
Future research
There are several key areas for future research:
-
Given the completion of the PREVENT trial and the recent publication of other large RCTs, an updated meta-analysis is indicated to re-examine evidence for MBCT as a strategy for the prevention of depressive relapse/recurrence in the context of alternative treatments that include usual care, other active treatments such as m-ADM and additive therapy approaches (e.g. MBCT plus m-ADM). An alternative strategy would be to conduct a large cohort study or effectiveness study to examine the effectiveness of MBCT and m-ADM in various real-world scenarios.
-
Furthermore, an individual patient data meta-analysis of published trials would help address the question of which specific patient subgroup MBCT is best indicated for. There is emerging evidence that MBCT is most effective for those at highest risk of relapse/recurrence and an individual patient data analysis could provide the empirical evidence to address this question.
-
Studies have tended to operationalise risk in somewhat different ways (e.g. early adversity, unstable remission, a greater number of previous episodes, early age of onset) and, although these risk factors overlap, future research should examine how and through what mechanism risk is conferred and resilience learned.
-
In addition to further analysis of the process data from the PREVENT trial and recently completed large MBCT RCTs, future trials need to incorporate process studies and consider dismantling designs to further unpack MBCT’s mechanism of action. This could enhance the specificity and efficacy of MBCT.
-
Given the current NICE guidance that MBCT should be offered to patients with three or more previous episodes of depression, and the limited availability of MBCT within the NHS, research is needed to explore the facilitators and barriers to implementation. This National Institute for Health Research-funded work is in progress. 22
Conclusions
In a large, rigorous yet pragmatic RCT we have demonstrated that MBCT-TS is not superior to m-ADM over 2 years of follow-up for patients with recurrent depression. Benchmarked against epidemiological data, both treatments were associated with enduring positive outcomes in terms of relapse/recurrence, residual depressive symptoms and quality of life. This study provides important evidence that MBCT-TS may confer ongoing protection for patients who would like an alternative to m-ADM. The results further suggest that psychosocial treatments, such as MBCT and CBT,117,121,124 offer added value for patients who need them most, that is, those at highest risk of depressive relapse/recurrence. However, studies have tended to operationalise risk in somewhat different ways (e.g. early adversity, unstable remission, a greater number of previous episodes, early age of onset) and, although these risk factors overlap, future research should examine how and through what mechanism risk is conferred and resilience learned. In the interim, the implication is that, for patients at low risk, treatments such as psychoeducation or m-ADM, which require less patient commitment and cost, may be indicated, whereas for patients at highest risk more intensive treatments such as MBCT may be indicated. This has significant potential to improve prevention by maximising the delivery of treatments through stratified approaches, which also have the potential to improve patient choice.
Acknowledgements
We thank the National Institute for Health Research Health Technology Assessment programme for funding the trial, our programme manager Alexa Cross for her support of the project and Professor Tom Walley for his advice in the early set phase. We are grateful to all of the practitioners and GP surgery staff who took part in this research. We would also like to thank the research workers and therapists who worked on this trial: Claire Brejcha, Jessica Cardy, Aaron Causley, Suzanne Cowderoy, Alison Evans, Felix Gradinger, Surinder Kaur, Paul Lanham, Jonathan Richards, Pooja Shah, Harry Sutton, Rachael Vicary, Alice Weaver, Jenny Wilks and Matthew Williams. AW acted as Acting Trial Manager during RH’s maternity leave. CB, AE, SC and JW were trial therapists. All researchers contributed to data collection. FG, MW, PS and AC all contributed to the qualitative data analysis. We would like to thank Trish Bartley for her input to the MBCT therapist training and MBCT fidelity checks. We would like to thank Beverley Herring, Naomi Gilbert and other service users who brought their personal lived experience of depressive illness to advise on the direction of the trial and provide training for the research assessors. We wish to acknowledge the SCID and GRID-HAMD training that Sandra Kennell-Webb provided to our research staff. We are grateful to members of our TSC (Chris Leach, Richard Moore and Glenys Parry) and DMC (Paul Ewings, Andy Field and Joanne MacKenzie) for their valuable advice and support during the project. We would also like to thank Shadi Beshai who completed some of the final research assessments. Contribution to funding is also acknowledged for RB, WK and RT from the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for the South West Peninsula. The Mental Health and Primary Care Research Networks provided additional support for trial recruitment. We also acknowledge the support provided by the Department of Health and local primary care trusts in meeting the excess treatment and service support costs associated with the trial. Most importantly, we are grateful to the participants for taking part in this trial.
Finally, we would also like to thank the following colleagues who have contributed to the PREVENT study through the recruitment and retention of patients or the provision of administrative support: Rebecca Amey, Miriam Cohen, Alice Garrood, Nora Goerg, Anna Hunt, Sarah Lane, Mary Sharkey, Cara Simmance, Holly Sugg, Lucy Wootton and other undergraduates and researchers who provided support to the trial.
Contributions of authors
Willem Kuyken, Sarah Byford, Richard Byng, Tim Dalgleish, Glyn Lewis, Rod Taylor, Edward Watkins, David Kessler and Nicola Morant were responsible for the original proposal, securing funding for the trial and drafting the original protocol.
Willem Kuyken as chief investigator had overall responsibility for the management of the study and the Exeter site and Glyn Lewis and David Kessler as co-investigators had responsibility for the Bristol site.
Willem Kuyken provided training and supervision for the trial therapists.
Rachel Hayes, Willem Kuyken, Rod Taylor and Sarah Byford wrote the statistical analysis plan.
Rod S Taylor conducted the main analyses and Sarah Byford and Barbara Barrett the health economics analyses. Richard Byng provided support for intervention development and delivery.
Rachel Hayes, Sarah Byford (see Chapter 5), Tim Dalgleish (see Chapter 6), Willem Kuyken, Nicola Morant (see Chapter 6) and Rod Taylor (see Chapter 4) wrote the initial draft of the report.
All authors contributed to, and approved, the final manuscript.
Data sharing statement
Non-identifiable data will be made available following an application to the corresponding author Professor Willem Kuyken.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Judd LL. The clinical course of unipolar major depressive disorders. Arch Gen Psychiatry 1997;54:989-91. http://dx.doi.org/10.1001/archpsyc.1997.01830230015002.
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007;370:851-8. http://dx.doi.org/10.1016/S0140-6736(07)61415-9.
- Murray CJL, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet 1997;349:1498-504. http://dx.doi.org/10.1016/S0140-6736(96)07492-2.
- Layard R, Clark DM. Thrive – the Power of Evidence-Based Psychological Therapies. London: Penguin Group; 2014.
- Frank E, Kupfer DJ, Perel JM, Cornes C, Jarrett DB, Mallinger AG, et al. Three-year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry 1990;47:1093-9. http://dx.doi.org/10.1001/archpsyc.1990.01810240013002.
- Kupfer DJ, Frank E, Perel JM, Cornes C, Mallinger AG, Thase ME, et al. Five-year outcome for maintenance therapies in recurrent depression. Arch Gen Psychiatry 1992;49:769-73. http://dx.doi.org/10.1001/archpsyc.1992.01820100013002.
- Vos T, Haby MM, Barendregt JJ, Kruijshaar M, Corry J, Andrews G. The burden of major depression avoidable by longer-term treatment strategies. Arch Gen Psychiatry 2004;61:1097-103. http://dx.doi.org/10.1001/archpsyc.61.11.1097.
- Depression: the Treatment and Management of Depression in Adults (Update). London: NICE; 2009.
- Geddes JR, Carney SM, Davies C, Furukawa TA, Kupfer DJ, Frank E, et al. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 2003;361:653-61. http://dx.doi.org/10.1016/S0140-6736(03)12599-8.
- Dawe HA. Teaching – social science or performing art. Harvard Educ Rev 1984;54:111-14.
- Katon W, Rutter C, Ludman EJ, Von Korff M, Lin E, Simon G, et al. A randomized trial of relapse prevention of depression in primary care. Arch Gen Psychiatry 2001;58:241-7. http://dx.doi.org/10.1001/archpsyc.58.3.241.
- van Schaik DJF, Klijn AF, van Hout HPJ, van Marwijk HW, Beekman AT, de Haan M, et al. Patients’ preferences in the treatment of depressive disorder in primary care. Gen Hosp Psychiatry 2004;26:184-9. http://dx.doi.org/10.1016/j.genhosppsych.2003.12.001.
- Olfson M, Marcus SC, Tedeschi M, Wan GJ. Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry 2006;163:101-8. http://dx.doi.org/10.1176/appi.ajp.163.1.101.
- Cooper C, Bebbington P, King M, Brugha T, Meltzer H, Bhugra D, et al. Why people do not take their psychotropic drugs as prescribed: results of the 2000 National Psychiatric Morbidity Survey. Acta Psychiatr Scand 2007;116:47-53. http://dx.doi.org/10.1111/j.1600-0447.2006.00974.x.
- Clark DM, Layard R, Smithies R, Richards DA, Suckling R, Wright B. Improving access to psychological therapy: initial evaluation of two UK demonstration sites. Behav Res Ther 2009;47:910-20. http://dx.doi.org/10.1016/j.brat.2009.07.010.
- Layard R. The case for psychological treatment centres. BMJ 2006;332:1030-2. http://dx.doi.org/10.1136/bmj.332.7548.1030.
- Cuijpers P, van Straten A, van Schaik A, Andersson G. Psychological treatment of depression in primary care: a meta-analysis. Br J Gen Pract 2009;59:120-7. http://dx.doi.org/10.3399/bjgp09X395139.
- Hollon SD, Muñoz RF, Barlow DH, Beardslee WR, Bell CC, Bernal G, et al. Psychosocial intervention development for the prevention and treatment of depression: promoting innovation and increasing access. Biol Psychiatry 2002;52:610-30. http://dx.doi.org/10.1016/S0006-3223(02)01384-7.
- Hirschfeld RMA, Keller MB, Panico S, Arons BS, Barlow D, Davidoff F, et al. The National Depressive and Manic–Depressive Association consensus statement on the undertreatment of depression. JAMA 1997;277:333-40. http://dx.doi.org/10.1001/jama.1997.03540280071036.
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression. New York, NY: Guilford Press; 2013.
- The Mindfulness Initiative n.d. www.themindfulnessinitiative.org.uk/ (accessed 8 October 2014).
- Rycroft-Malone J, Anderson R, Crane R, Gibson A, Gradinger F, Griffiths HO, et al. Accessibility and implementation in UK services of an effective depression relapse prevention programme – mindfulness-based cognitive therapy (MBCT): ASPIRE study protocol. Implement Sci 2014;9. http://dx.doi.org/10.1186/1748-5908-9-62.
- Crane RS, Kuyken W. The implementation of mindfulness-based cognitive therapy: learning from the UK health service experience. Mindfulness 2013;4:246-54. http://dx.doi.org/10.1007/s12671-012-0121-6.
- Teasdale J, Segal Z, Williams J, Ridgeway V, Soulsby J, Lau M. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol 2000;68:615-23. http://dx.doi.org/10.1037/0022-006X.68.4.615.
- Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol 2004;72:31-40. http://dx.doi.org/10.1037/0022-006X.72.1.31.
- Coelho HF, Canter PH, Ernst E. Mindfulness-based cognitive therapy: evaluating current evidence and informing future research. J Consult Clin Psychol 2007;75:1000-5. http://dx.doi.org/10.1037/0022-006X.75.6.1000.
- Kuyken W. MRC Final Report: Brain Sciences II Trial Platform ‘Preventing Depression Relapse in NHS Practice Using Mindfulness-Based Cognitive Therapy’ 2007.
- Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psychol 2008;76:966-78. http://dx.doi.org/10.1037/a0013786.
- White K, Holden ER, Byng R, Mullan E, Kuyken W. Under/over-recruitment to mental health trials. Acta Psychiatr Scand 2007;116. http://dx.doi.org/10.1111/j.1600-0447.2007.01061.x.
- Allen M, Bromley A, Kuyken W, Sonnenberg SJ. Participants’ experiences of mindfulness-based cognitive therapy: ‘It changed me in just about every way possible’. Behav Cogn Psychother 2009;37:413-30. http://dx.doi.org/10.1017/S135246580999004X.
- Liu CF, Hedrick SC, Caheny EF, Heagerty P, Felker B, Hasenberg N, et al. Cost-effectiveness of collaborative care for depression in a primary care veteran population. Psychiatr Serv 2003;54:698-704. http://dx.doi.org/10.1176/appi.ps.54.5.698.
- Simon GE, Katon WJ, VonKorff M, Unützer J, Lin EH, Walker EA, et al. Cost-effectiveness of a collaborative care program for primary care patients with persistent depression. Am J Psychiatry 2001;158:1638-44. http://dx.doi.org/10.1176/appi.ajp.158.10.1638.
- Simon GE, Von Korff M, Ludman EJ, Katon WJ, Rutter C, Unützer J, et al. Cost-effectiveness of a program to prevent depression relapse in primary care. Med Care 2002;40:941-50. http://dx.doi.org/10.1097/00005650-200210000-00011.
- Segal Z, Williams J, Teasdale J. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York, NY: Guilford Press; 2002.
- Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Arch Gen Psychiatry 2006;63:749-55. http://dx.doi.org/10.1001/archpsyc.63.7.749.
- Feldman C, Kuyken W. Compassion in the landscape of suffering. Contemp Buddhism 2011;12:143-55. http://dx.doi.org/10.1080/14639947.2011.564831.
- Kuyken W, Watkins E, Holden E, White K, Taylor RS, Byford S, et al. How does mindfulness-based cognitive therapy work?. Behav Res Ther 2010;48:1105-12. http://dx.doi.org/10.1016/j.brat.2010.08.003.
- Chambers R, Gullone E, Allen NB. Mindful emotion regulation: an integrative review. Clin Psychol Rev 2009;29:560-72. http://dx.doi.org/10.1016/j.cpr.2009.06.005.
- Raes F, Dewulf D, Van Heeringen C, Williams JM. Mindfulness and reduced cognitive reactivity to sad mood: evidence from a correlational study and a non-randomized waiting list controlled study. Behav Res Ther 2009;47:623-7. http://dx.doi.org/10.1016/j.brat.2009.03.007.
- Farb NAS, Anderson AK, Mayberg H, Bean J, McKeon D, Segal ZV. Minding one’s emotions: mindfulness training alters the neural expression of sadness. Emotion 2010;10:25-33. http://dx.doi.org/10.1037/a0017151.
- Kuyken W, Crane R, Dalgleish T. Does mindfulness based cognitive therapy prevent relapse of depression?. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e7194.
- Kabat-Zinn J. Full Catastrophe Living: How to Cope with Stress, Pain and Illness using Mindfulness Meditation. New York, NY: Delacorte; 1990.
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York, NY: Guilford Press; 1979.
- Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O’Reardon JP, et al. Prevention of relapse following cognitive therapy vs. medications in moderate to severe depression. Arch Gen Psychiatry 2005;62:417-22. http://dx.doi.org/10.1001/archpsyc.62.4.417.
- Lau MA, Segal ZV, Williams JM. Teasdale’s differential activation hypothesis: implications for mechanisms of depressive relapse and suicidal behaviour. Behav Res Ther 2004;42:1001-17. http://dx.doi.org/10.1016/j.brat.2004.03.003.
- Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves GG, Silver PC. Preventing recurrent depression using cognitive therapy with and without a continuation phase: a randomized clinical trial. Arch Gen Psychiatry 2001;58:381-8. http://dx.doi.org/10.1001/archpsyc.58.4.381.
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2012.
- Williams JBW, Kobak KA, Bech P, Engelhardt N, Evans K, Lipsitz J, et al. The GRID-HAMD: standardization of the Hamilton Depression Rating Scale. Int Clin Psychopharmacol 2008;23:120-9. http://dx.doi.org/10.1097/YIC.0b013e3282f948f5.
- Judd LL, Paulus MP, Zeller P. The role of residual subthreshold depressive symptoms in early episode relapse in unipolar major depressive disorder. Arch Gen Psychiatry 1999;56:764-5. http://dx.doi.org/10.1001/archpsyc.56.8.763.
- Segal ZV, Bieling P, Young T, MacQueen G, Cooke R, Martin L, et al. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psychiatry 2010;67:1256-64. http://dx.doi.org/10.1001/archgenpsychiatry.2010.168.
- Crane RS, Eames C, Kuyken W, Hastings RP, Williams JM, Bartley T, et al. Development and validation of the mindfulness-based interventions – teaching assessment criteria (MBI:TAC). Assessment 2013;20:681-8. http://dx.doi.org/10.1177/1073191113490790.
- Segal ZV, Teasdale JD, Williams JM, Gemar MC. The mindfulness-based cognitive therapy adherence scale: inter-rater reliability, adherence to protocol and treatment distinctiveness. Clin Psychol Psychother 2002;9:131-8. http://dx.doi.org/10.1002/cpp.320.
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, et al. The longitudinal interval follow-up evaluation – a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry 1987;44:540-8. http://dx.doi.org/10.1001/archpsyc.1987.01800180050009.
- Beck AT, Steer RA, Brown GK. The Beck Depression Inventory – Second Edition. San Antonio, TX: Psychological Corporation; 1996.
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation – theoretical considerations and preliminary results. Gen Hosp Psychiatry 1982;4:33-47. http://dx.doi.org/10.1016/0163-8343(82)90026-3.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- EuroQol Group . A new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Harper A, Power M. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med 1998;28:551-8. http://dx.doi.org/10.1017/S0033291798006667.
- Orley J, Harper A, Power M, Billington R. Development of the WHOQOL-BREF quality of life assessment. Qual Life Res 1997;6.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62. http://dx.doi.org/10.1136/jnnp.23.1.56.
- Walter SD, Eliasziw M, Donner A. Sample size and optimal designs for reliability studies. Stat Med 1998;7:101-10. http://dx.doi.org/10.1002/(SICI)1097-0258(19980115)17:1<101::AID-SIM727>3.0.CO;2-E.
- O’Hara MW, Rehm LP. Hamilton Rating Scale for Depression: reliability and validity of judgements of novice raters. J Consult Clin Psychol 1983;51:318-19. http://dx.doi.org/10.1037/0022-006X.51.2.318.
- Kabat-Zinn J. Chapman-Waldrop A. Compliance with an outpatient stress reduction program: rates and predictors of program completion. J Behav Med 1988;11:333-52. http://dx.doi.org/10.1007/BF00844934.
- Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med 1985;8:163-90. http://dx.doi.org/10.1007/BF00845519.
- Kabat-Zinn J, Massion AO, Kristeller J, Peterson LG, Fletcher KE, Pbert L, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry 1992;149:936-43. http://dx.doi.org/10.1176/ajp.149.7.936.
- Williams KA, Kolar MM, Reger BE, Pearson JC. Evaluation of a wellness-based mindfulness stress reduction intervention: a controlled trial. Am J Health Promot 2001;15:422-32. http://dx.doi.org/10.4278/0890-1171-15.6.422.
- Bower P, Byford S, Sibbald B, Ward E, King M, Lloyd M, et al. Randomised controlled trial of non-directive counselling, cognitive-behaviour therapy, and usual general practitioner care for patients with depression. II: cost-effectiveness. BMJ 2000;321:1389-92. http://dx.doi.org/10.1136/bmj.321.7273.1389.
- Konig HH, Bernert S, Angermeyer MC. Measuring preferences for depressive health states: a comparison of the EuroQol instrument, time-trade-off and contingent valuation. Psychiatr Prax 2005;32:122-31.
- Kuyken W, Orley J. Development of the WHOQOL – rationale and current status. Int J Ment Health 1994;23:24-56.
- Kuyken W, Orley J, Power M, Herman H, Schofield H, Murphy B, et al. The World Health Organization Quality of Life Assessment (WHOQOL) – position paper from the World Health Organization. Soc Sci Med 1995;41:1403-9. http://dx.doi.org/10.1016/0277-9536(95)00112-K.
- Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin Psychol Sci Pract 2003;10:125-43. http://dx.doi.org/10.1093/clipsy.bpg015.
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. J Psychosom Res 2004;57:35-43. http://dx.doi.org/10.1016/S0022-3999(03)00573-7.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use . Statistical Principles for Clinical Trials 1998. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf (accessed 20 July 2015).
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. CONSORT group . Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-309. http://dx.doi.org/10.7326/0003-4819-148-4-200802190-00008.
- Schulz KF, Altman DG, Moher D. CONSORT group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med 2010;152:1-15. http://dx.doi.org/10.7326/0003-4819-152-11-201006010-00232.
- Parker G, Roussos J, HadziPavlovic D, Mitchell P, Wilhelm K, Austin MP. The development of a refined measure of dysfunctional parenting and assessment of its relevance in patients with affective disorders. Psychol Med 1997;27:1193-20. http://dx.doi.org/10.1017/S003329179700545X.
- Kuyken W, Byford S, Byng R, Dalgleish T, Lewis G, Taylor R, et al. Study protocol for a randomized controlled trial comparing mindfulness-based cognitive therapy with maintenance anti-depressant treatment in the prevention of depressive relapse/recurrence: the PREVENT trial. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-99.
- Dodd S, White I, Williamson P. Nonadherence to treatment protocol in published randomised controlled trials: a review. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-84.
- Choi SC, Lu IL. Effect of non-random missing data mechanisms in clinical trials. Stat Med 1995;14:2675-84. http://dx.doi.org/10.1002/sim.4780142407.
- Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3-15. http://dx.doi.org/10.1191/096228099671525676.
- Data Protection Act. London: The Stationery Office; 1998.
- Kuyken W, Byford S, Byng R, Dalgleish T, Lewis G, Taylor R, et al. Update to the study protocol for a randomized controlled trial comparing mindfulness-based cognitive therapy with maintenance anti-depressant treatment in the prevention of depressive relapse/recurrence: the PREVENT trial. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-217.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Kessler D, Barber C, Birnbaum HG, Frank RG, Greenberg PE, Rose RM, et al. Depression in the workplace: effects on short-term disability. Health Aff (Millwood) 1999;18:163-71. http://dx.doi.org/10.1377/hlthaff.18.5.163.
- Kind P, Spilker B. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia, PA: Lippincott-Raven; 1996.
- Mulhern B, Mukuria C, Barkham M, Knapp M, Byford S, Soeteman D, et al. Using generic preference-based measures in mental health: psychometric validity of the EQ-5D and SF-6D. Br J Psychiatry 2014;205:236-43. http://dx.doi.org/10.1192/bjp.bp.112.122283.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQoL: Results from a UK General Population Survey. York: Centre for Health Economics, University of York; 1995.
- Richardson G, Manca A. Calculation of quality-adjusted life-years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. http://dx.doi.org/10.1002/hec.901.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Kessler RC, Barber C, Beck A, Berglund P, Cleary PD, McKenas D, et al. The World Health Organization Health and Work Performance Questionnaire (HPQ). J Occupat Environ Med 2003;45:156-74. http://dx.doi.org/10.1097/01.jom.0000052967.43131.51.
- Netten A, Knight J, Dennett J, Cooley R, Slight A. A Ready Reckoner for Staff Costs in the NHS. Canterbury: University of Kent; 1998.
- Barrett B, Byford S, Curtis L. Unit Costs of Health and Social Care 2008. Canterbury: PSSRU, University of Kent; 2008.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2010.
- NHS Reference Costs 2012–2013. London: Department of Health; 2013.
- Koopmanschap MA, Rutten FFH. A practical guide for calculating indirect costs of disease. Pharmacoeconomics 1996;10:460-6. http://dx.doi.org/10.2165/00019053-199610050-00003.
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993.
- Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed?. BMJ 2000;320:1197-200. http://dx.doi.org/10.1136/bmj.320.7243.1197.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219-36. http://dx.doi.org/10.1002/1097-0258(20001215)19:23<3219::AID-SIM623>3.0.CO;2-P.
- Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005;187:106-8. http://dx.doi.org/10.1192/bjp.187.2.106.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. http://dx.doi.org/10.1002/hec.635.
- Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Ann Rev Clin Psychol 2007;3:1-27. http://dx.doi.org/10.1146/annurev.clinpsy.3.022806.091432.
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment 2006;13:27-45. http://dx.doi.org/10.1177/1073191105283504.
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry 2002;59:877-83. http://dx.doi.org/10.1001/archpsyc.59.10.877.
- Murphy R, Cooper Z, Hollon SD, Fairburn CG. How do psychological treatments work? Investigating mediators of change. Behav Res Ther 2009;47:1-5. http://dx.doi.org/10.1016/j.brat.2008.10.001.
- Garratt G, Ingram RE, Rand KL, Sawalani G. Cognitive processes in cognitive therapy: evaluation of the mechanisms of change in the treatment of depression. Clin Psychol Sci Pract 2007;14:224-39. http://dx.doi.org/10.1111/j.1468-2850.2007.00081.x.
- Cox DR. Regression models and life tables. J R Stat Soc Ser B Stat Methodol 1972;34:187-220.
- Mackinnon DP. Introduction to Statistical Mediation Analysis. New York, NY: Lawrence Erlbaum Associates; 2008.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002889.
- Finucane A, Mercer S. An exploratory mixed methods study of the acceptability and effectiveness of mindfulness-based cognitive therapy for patients with active depression and anxiety in primary care. BMC Psychiatry 2006;6. http://dx.doi.org/10.1186/1471-244X-6-14.
- Mason O, Hargreaves I. A qualitative study of mindfulness-based cognitive therapy for depression. Br J Med Psychol 2001;74:197-212. http://dx.doi.org/10.1348/000711201160911.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- Hertenstein E, Rose N, Voderholzer U, Heidenreich T, Nissen C, Thiel N, et al. Mindfulness-based cognitive therapy in obsessive–compulsive disorder – a qualitative study on patients’ experiences. BMC Psychiatry 2012;12. http://dx.doi.org/10.1186/1471-244X-12-185.
- Flick U. An Introduction to Qualitative Research. London: Pine Forge Press; 2006.
- Piet J, Hougaard E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: a systematic review and meta-analysis. Clin Psychol Rev 2011;31:1032-40. http://dx.doi.org/10.1016/j.cpr.2011.05.002.
- Williams JMG, Crane C, Barnhofer T, Brennan K, Duggan DS, Fennell MJ, et al. Mindfulness-based cognitive therapy for preventing relapse in recurrent depression: a randomized dismantling trial. J Consult Clin Psychol 2014;82:275-86. http://dx.doi.org/10.1037/a0035036.
- Shawyer F, Meadows GN, Judd F, Martin PR, Segal Z, Piterman L. The DARE study of relapse prevention in depression: design for a phase 1/2 translational randomised controlled trial involving mindfulness-based cognitive therapy and supported self monitoring. BMC Psychiatry 2012;12. http://dx.doi.org/10.1186/1471-244X-12-3.
- Meadows GN, Shawyer F, Enticott JC, Graham AL, Judd F, Martin PR, et al. Mindfulness-based cognitive therapy for recurrent depression: a translational research study with 2-year follow-up. Aust N Z J Psychiatry 2014;48:743-55. http://dx.doi.org/10.1177/0004867414525841.
- Huijbers MJ, Spijker J, Donders AR, van Schaik DJ, van Oppen P, Ruhé HG, et al. Preventing relapse in recurrent depression using mindfulness-based cognitive therapy, antidepressant medication or the combination: trial design and protocol of the MOMENT study. BMC Psychiatry 2012;12. http://dx.doi.org/10.1186/1471-244X-12-125.
- Stangier U, Hilling C, Heidenreich T, Risch AK, Barocka A, Schlösser R, et al. Maintenance cognitive-behavioral therapy and manualized psychoeducation in the treatment of recurrent depression: a multicenter prospective randomized controlled trial. Am J Psychiatry 2013;170:624-32. http://dx.doi.org/10.1176/appi.ajp.2013.12060734.
- Miniati M, Rucci P, Benvenuti A, Frank E, Buttenfield J, Giorgi G, et al. Clinical characteristics and treatment outcome of depression in patients with and without a history of emotional and physical abuse. J Psychiatr Res 2010;44:302-9. http://dx.doi.org/10.1016/j.jpsychires.2009.09.008.
- Solomon DA, Keller MB, Leon AC, Mueller TI, Lavori PW, Shea MT, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry 2000;157:229-33. http://dx.doi.org/10.1176/appi.ajp.157.2.229.
- Bockting CL, Schene AH, Spinhoven P, Koeter MW, Wouters LF, Huyser J, et al. Preventing relapse/recurrence in recurrent depression with cognitive therapy: a randomized controlled trial. J Consult Clin Psychol 2005;73:647-57. http://dx.doi.org/10.1037/0022-006X.73.4.647.
Appendix 1 Serious adverse event form and suicidal thoughts protocol
Serious adverse event form
Suicidal thoughts protocol
FIGURE 17.
Researcher action required – including suggested scripts.
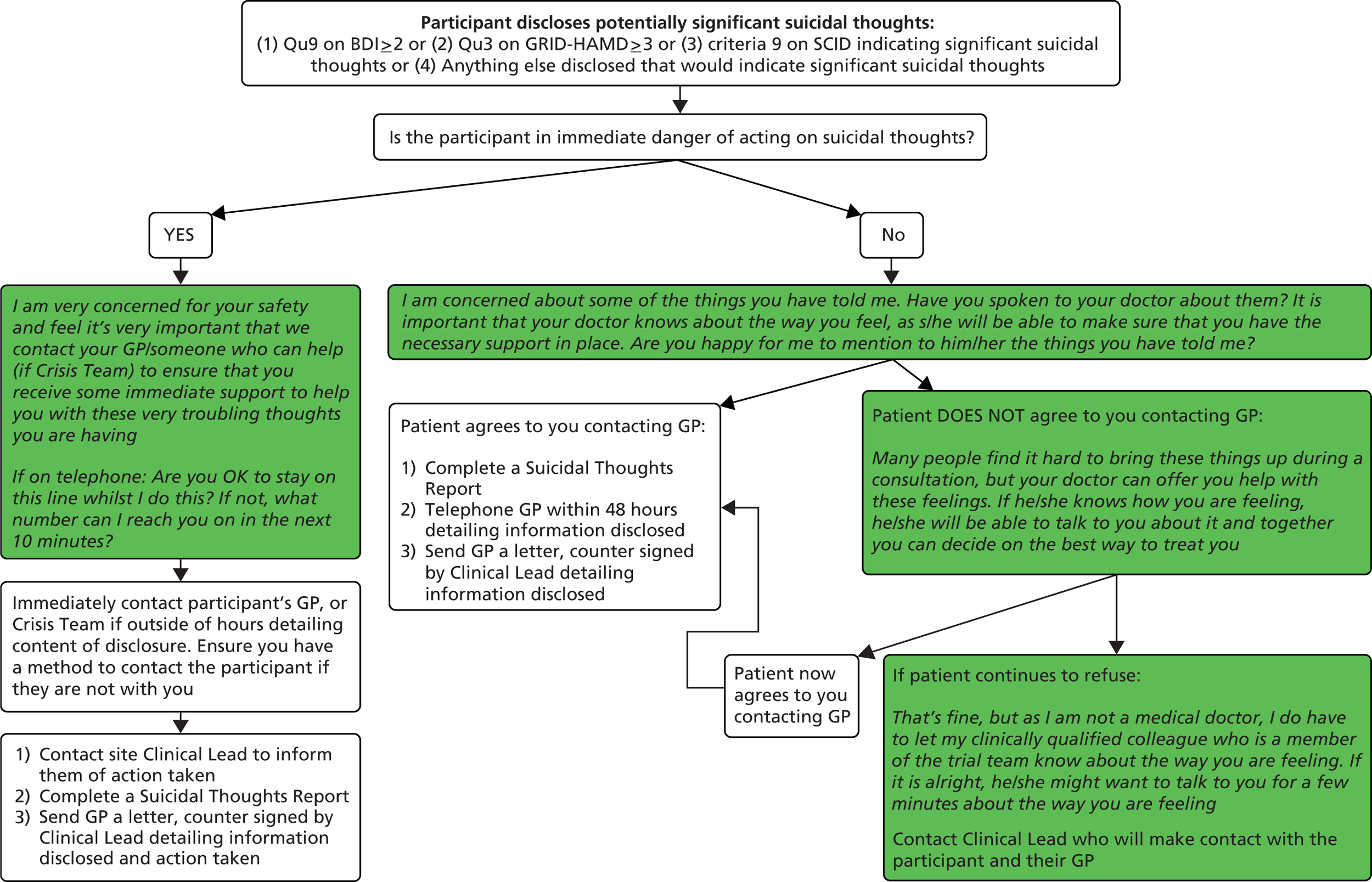
Appendix 2 Antidepressants prescribed
| Antidepressant | m-ADM | MBCT-TS |
|---|---|---|
| Agomelatine | 64 | 0 |
| Amitriptyline | 660 | 350 |
| Citalopram | 7311 | 4258 |
| Clomipramine | 224 | 0 |
| Dosulepin | 212 | 376 |
| Doxepin | 0 | 16 |
| Duloxetine | 222 | 156 |
| Escitalopram | 272 | 128 |
| Fluoxetine | 2915 | 2493.5 |
| Flupentixol | 24 | 0 |
| Fluvoxamine | 0 | 12 |
| Lofepramine | 324 | 224 |
| Mirtazapine | 919 | 622 |
| Moclobemide | 0 | 14 |
| Nortriptyline | 0 | 200 |
| Paroxetine | 394 | 653 |
| Sertraline | 1668 | 1538 |
| Trazodone | 144 | 0 |
| Venlafaxine | 1225 | 1087 |
| Other | 0 | 32 |
Appendix 3 Baseline characteristics of participants who received an adequate dose of treatment
| Characteristic/variable | MBCT-TS (n = 176) | m-ADM (n = 162) | Difference test |
|---|---|---|---|
| Demographic characteristics | |||
| Female, n (%) | 122 (69) | 136 (84) | χ2(1) = 10.00; p = 0.002 |
| Ethnicity, n (%) | χ2(3) = 4.01; p = 0.26 | ||
| White | 174 (99) | 160 (99) | |
| Other (including three categories) | 2 (1) | 2 (1) | |
| Age (years) | t(336) = –1.21; p = 0.23 | ||
| Mean (SD) | 51 (12) | 50 (12) | |
| Range | 22–78 | 24–79 | |
| Marital status, n (%) | χ2(5) = 7.18; p = 0.21 | ||
| Single | 30 (17) | 22 (14) | |
| Married, cohabiting or civil partnership | 107 (61) | 115 (71) | |
| Separated, divorced or widowed | 39 (22) | 24 (15) | |
| Missing | 0 (0) | 1 (1) | |
| Level of education, n (%) | χ2(6) = 8.50; p = 0.20 | ||
| No educational qualifications | 10 (6) | 7 (4) | |
| Some school qualifications | 24 (14) | 36 (22) | |
| High school and/or vocational qualification | 71 (40) | 72 (44) | |
| University degree/professional qualification | 69 (39) | 44 (27) | |
| Missing | 2 (1) | 3 (2) | |
| Religion, n (%) | χ2(7) = 5.04; p = 0.66 | ||
| Christian | 109 (62) | 103 (64) | |
| Other | 10 (6) | 3 (2) | |
| None | 57 (32) | 55 (34) | |
| Missing | 0 (0) | 1 (1) | |
| Salary (£ sterling) | t(190) = –1.92; p = 0.06 | ||
| Mean (SD) | 20,560 (13,707) | 16,884 (12,777) | |
| Range | 1200–72,000 | 792–75,000 | |
| Social class, n (%)a | χ2(5) = 7.39; p = 0.19 | ||
| Class 0 | 72 (41) | 58 (36) | |
| Class 1 | 49 (28) | 38 (23) | |
| Class 2 | 19 (11) | 31 (19) | |
| Class 3 | 5 (3) | 4 (2) | |
| Class 4 | 0 (0) | 2 (1) | |
| Class 5 | 31 (18) | 28 (17) | |
| Not classified | 0 (0) | 1 (1) | |
| Stratification variables | |||
| Depressive symptomology at randomisation, n (%) | χ2(1) = 0.03; p = 0.87 | ||
| Asymptomatic | 136 (77) | 124 (77) | |
| Symptomatic | 40 (23) | 38 (23) | |
| Recruitment site, n (%) | χ2(3) = 0.61; p = 0.90 | ||
| Bristol | 32 (18) | 25 (15) | |
| Exeter and East Devon | 62 (35) | 57 (35) | |
| North and Mid Devon | 45 (26) | 42 (26) | |
| South Devon | 37 (21) | 38 (23) | |
| Psychiatric characteristics | |||
| Current depressive symptomology, mean (SD) | |||
| GRID-HAMD score | 4.7 (4.2) | 4.7 (4.4) | t(336) = –0.14; p = 0.89 |
| BDI-II score | 13.4 (10.3) | 14.3 (9.9) | t(329) = 0.79; p = 0.43 |
| Previous major depressive episodes, n (%) | |||
| Fewer than six episodes | 98 (56) | 76 (47) | |
| Six or more episodes | 78 (44) | 86 (53) | |
| Age (years) at first depression onset, mean (SD) | 24.8 (11.8) | 24.8 (12.3) | t(336) = 0.02; p = 0.98 |
| Time (months) since last depressive episode, mean (SD) | 21.5 (25.0) | 18.6 (25.4) | t(336) = –1.05; p = 0.30 |
| Number of comorbid DSM-IV Axis I psychiatric diagnoses, mean (SD) | 0.5 (0.8) | 0.6 (0.9) | t(336) = 2.06; p = 0.04 |
| Received outpatient psychiatric or psychological treatment, n (%) | 86 (49) | 87 (54) | χ2(1) = 1.58; p = 0.21 |
| Attempted suicide, n (%) | 34 (19) | 42 (26) | χ2(1) = 2.30; p = 0.13 |
| Number of previous attempts, mean (SD) | 1.7 (1.2) | 2.0 (1.6) | t(73) = 0.73; p = 0.47 |
| Severity of reported childhood abuse, n (%) | χ2(1) = 0.12; p = 0.73 | ||
| High | 88 (50) | 84 (52) | |
| Low | 88 (50) | 78 (48) | |
| Missing | 0 (0) | 0 (0) | |
| Quality of life, mean (SD)b | |||
| How would you rate your quality of life? | 3.7 (0.8) | 3.7 (0.9) | t(327) = 0.05; p = 0.96 |
| How satisfied are you with your health? | 2.9 (1.0) | 3.1 (1.0) | t(327) = 1.25; p = 0.21 |
| Physical | 14.3 (4.8) | 13.9 (2.8) | t(327) = –0.90; p = 0.37 |
| Psychological | 12.7 (2.6) | 12.2 (2.6) | t(327) = –1.70; p = 0.09 |
| Social | 13.4 (3.5) | 13.1 (3.4) | t(327) = –0.87; p = 0.39 |
| Environment | 15.1 (2.4) | 14.9 (2.6) | t(327) = –0.81; p = 0.42 |
| Health-related quality of life (EQ-5D tariffs) | 0.773 (0.252) | 0.762 (0.219) | t(325) = –0.43; p = 0.67 |
Appendix 4 Baseline characteristics of participants who did follow invited treatment with respect to antidepressant medication use
| Characteristic/variable | MBCT-TS (n = 153) | m-ADM (n = 162) | Difference test |
|---|---|---|---|
| Demographic characteristics | |||
| Female, n (%) | 105 (69) | 136 (84) | χ2(1) = 10.28; p = 0.001 |
| Race, n (%) | χ2(2) = 2.95; p = 0.23 | ||
| White | 152 (99) | 160 (99) | |
| Other (including two categories) | 1 (1) | 2 (1) | |
| Age (years) | t(313) = –1.85; p = 0.07 | ||
| Mean (SD) | 52 (12) | 50 (12) | |
| Range | 25–78 | 24–79 | |
| Marital status, n (%) | χ2(5) = 6.72; p = 0.24 | ||
| Single | 23 (15) | 22 (14) | |
| Married, cohabiting or civil partnership | 96 (63) | 115 (71) | |
| Separated, divorced or widowed | 34 (22) | 24 (15) | |
| Missing | 0 (0) | 1 (1) | |
| Level of education, n (%) | χ2(6) = 7.10; p = 0.31 | ||
| No educational qualifications | 10 (7) | 7 (4) | |
| Some school qualifications | 22 (14) | 36 (22) | |
| High school and/or vocational qualification | 63 (41) | 72 (44) | |
| University degree/professional qualification | 56 (37) | 44 (27) | |
| Missing | 2 (1) | 3 (2) | |
| Religion, n (%) | χ2(7) = 4.31; p = 0.74 | ||
| Christian | 91 (59) | 103 (64) | |
| Other | 8 (5) | 3 (2) | |
| None | 54 (35) | 55 (34) | |
| Missing | 0 (0) | 1 (1) | |
| Salary (£ sterling) | t(175) = –1.84; p = 0.07 | ||
| Mean (SD) | 20,657 (14,444) | 16,884 (12,777) | |
| Range | 1200–72,000 | 792–75,000 | |
| Social class, n (%)a | χ2(5) = 6.46; p = 0.26 | ||
| Class 0 | 65 (42) | 58 (36) | |
| Class 1 | 39 (25) | 38 (23) | |
| Class 2 | 17 (11) | 31 (19) | |
| Class 3 | 3 (2) | 4 (2) | |
| Class 4 | 0 (0) | 2 (1) | |
| Class 5 | 29 (19) | 28 (17) | |
| Not classified | 0 (0) | 1 (1) | |
| Stratification variables | |||
| Depressive symptomology at randomisation, n (%) | χ2(1) = 0.02; p = 0.88 | ||
| Asymptomatic | 116 (76) | 124 (77) | |
| Symptomatic | 37 (24) | 38 (24) | |
| Recruitment site, n (%) | χ2(3) = 1.68; p = 0.64 | ||
| Bristol | 27 (18) | 25 (15) | |
| Exeter and East Devon | 57 (37) | 57 (35) | |
| North and Mid Devon | 42 (27) | 42 (26) | |
| South Devon | 27 (18) | 38 (24) | |
| Psychiatric characteristics | |||
| Current depressive symptomology, mean (SD) | |||
| GRID-HAMD score | 4.8 (4.2) | 4.7 (4.4) | t(313) = –0.33; p = 0.74 |
| BDI-II score | 13.8 (10.4) | 14.3 (9.9) | t(306) = 0.45; p = 0.65 |
| Previous major depressive episodes, n (%) | |||
| Fewer than six episodes | 84 (55) | 76 (47) | |
| Six or more episodes | 69 (45) | 86 (53) | |
| Age (years) at first depression onset, mean (SD) | 25.3 (12.0) | 24.8 (12.3) | t(313) = –0.37; p = 0.71 |
| Time (months) since last depressive episode, mean (SD) | 20.5 (23.7) | 18.6 (25.4) | t(313) = –0.68; p = 0.50 |
| Number of comorbid DSM-IV Axis I psychiatric diagnoses, mean (SD) | 0.4 (0.7) | 0.6 (0.9) | t(313) = 2.33; p = 0.02 |
| Received outpatient psychiatric or psychological treatment, n (%) | 72 (47) | 87 (54) | χ2(1) = 2.35; p = 0.13 |
| Attempted suicide, n (%) | 30 (20) | 42 (26) | χ2(1) = 1.95; p = 0.16 |
| Number of previous attempts, mean (SD) | 1.8 (1.2) | 2.0 (1.6) | t(69) = 0.50; p = 0.62 |
| Severity of reported childhood abuse, n (%) | χ2(1) = 0.15; p = 0.70 | ||
| High | 76 (50) | 84 (52) | |
| Low | 77 (50) | 78 (48) | |
| Missing | 0 (0) | 0 (0) | |
| Quality of life, mean (SD)b | |||
| How would you rate your quality of life? | 3.7 (0.8) | 3.7 (0.9) | t(305) = –0.14; p = 0.89 |
| How satisfied are you with your health? | 3.0 (1.0) | 3.1 (1.0) | t(305) = 0.86; p = 0.39 |
| Physical | 14.3 (5.0) | 13.9 (2.8) | t(305) = –0.92; p = 0.36 |
| Psychological | 12.7 (2.6) | 12.2 (2.6) | t(305) = –1.69; p = 0.09 |
| Social | 13.6 (3.5) | 13.1 (3.4) | t(305) = –1.29; p = 0.20 |
| Environment | 15.2 (2.5) | 14.9 (2.6) | t(305) = –1.08; p = 0.28 |
| Health-related quality of life (EQ-5D tariffs) | 0.775 (0.256) | 0.762 (0.219) | t(303) = –0.50; p = 0.62 |
Appendix 5 Baseline characteristics of participants who scored high and low on severity of childhood abuse
| Characteristic/variable | Lower severity of childhood abuse (n = 206) | Higher severity of childhood abuse (n = 216) | Difference test |
|---|---|---|---|
| Demographic characteristics | |||
| Female, n (%) | 162 (78.6) | 161 (74.5) | χ2(1) = 0.99; p = 0.32 |
| Ethnicity, n (%) | χ2(3) = 4.23; p = 0.24 | ||
| White | 202 (98.1) | 216 (100.0) | |
| Other (including three categories) | 4 (2.0) | 0 (0.0) | |
| Age (years) | t(420) = 1.02; p = 0.31 | ||
| Mean (SD) | 50.9 (13.2) | 48.9 (11.4) | |
| Range | 20–79 | 21–74 | |
| Marital status, n (%) | χ2(5) = 9.47; p = 0.09 | ||
| Single | 36 (17.5) | 44 (20.4) | |
| Married, cohabiting or civil partnership | 130 (63.1) | 134 (62.0) | |
| Separated, divorced or widowed | 39 (18.9) | 38 (17.6) | |
| Missing | 1 (0.5) | 0 (0.0) | |
| Level of education, n (%) | χ2(6) = 3.27; p = 0.78 | ||
| No educational qualifications | 13 (6.3) | 7 (3.2) | |
| Some O levels/GCSEs | 38 (18.4) | 43 (19.9) | |
| Some AS/A levels | 21 (10.2) | 21 (9.7) | |
| NVQ or other vocational qualification | 61 (29.6) | 72 (33.3) | |
| University bachelor’s degree | 42 (20.4) | 47 (21.8) | |
| University master’s degree | 10 (4.9) | 10 (4.6) | |
| University professional training or PhD | 16 (7.8) | 13 (6.0) | |
| Missing | 5 (2.4) | 3 (1.4) | |
| Religion, n (%) | χ2(7) = 8.63; p = 0.28 | ||
| Atheist | 7 (3.4) | 11 (5.1) | |
| Christian | 142 (68.9) | 130 (60.2) | |
| Muslim | 1 (0.5) | 0 (0.00) | |
| Spiritualist | 3 (1.5) | 1 (0.5) | |
| Buddhist | 1 (0.5) | 1 (0.5) | |
| Other | 3 (1.5) | 4 (1.9) | |
| Agnostic | 5 (2.4) | 3 (1.4) | |
| None | 43 (20.9) | 66 (30.6) | |
| Missing | 1 (0.5) | 0 (0.0) | |
| Smokes, n (%) | χ2(1) = 5.23; p = 0.02 | ||
| Yes | 34 (16.5) | 56 (25.9) | |
| Missing | 3 (1.5) | 0 (0.0) | |
| Salary (£ sterling) | t(231) = –0.26; p = 0.80 | ||
| Mean (SD) | 18,687 (13,938) | 19,147 (13,091) | |
| Range | 792–80,000 | 1200–72,000 | |
| Social class, n (%)a | χ2(5) = 7.86; p = 0.16 | ||
| Class 0 | 82 (39.8) | 89 (41.2) | |
| Class 1 | 43 (20.9) | 62 (28.7) | |
| Class 2 | 38 (18.4) | 22 (10.2) | |
| Class 3 | 6 (2.9) | 5 (2.3) | |
| Class 4 | 1 (0.5) | 1 (0.5) | |
| Class 5 | 35 (17.0) | 37 (17.1) | |
| Not classified | 1 (0.5) | 0 (0.0) | |
| Stratification variables | |||
| Depressive symptomology at randomisation: n (%) | χ2(1) = 0.78; p = 0.38 | ||
| Asymptomatic | 162 (79) | 162 (75) | |
| Symptomatic | 44 (21) | 54 (25) | |
| Recruitment site, n (%) | χ2(3) = 10.38; p = 0.02 | ||
| Bristol | 28 (13.6) | 36 (16.7) | |
| Exeter and East Devon | 76 (36.9) | 72 (33.3) | |
| North and Mid Devon | 64 (31.1) | 45 (20.8) | |
| South Devon | 38 (18.4) | 63 (29.2) | |
| Psychiatric characteristics | |||
| Current depressive symptomology, mean (SD) | |||
| GRID-HAMD score | 4.2 (4.2) | 5.1 (4.3) | t(420) = –2.18; p = 0.03 |
| BDI-II score | 12.9 (9.6) | 15.3 (10.5) | t(413) = –2.44; p = 0.02 |
| Previous major depressive episodes, n (%) | |||
| Fewer than six episodes | 126 (61.2) | 100 (46.3) | |
| Six or more episodes | 80 (39) | 116 (54) | |
| Age (years) at first depression onset, mean (SD) | 28 (13) | 22 (12) | t(420) = 4.31; p < 0.0001 |
| Time (months) since last depressive episode, mean (SD) | 17 (23) | 21 (27) | t(420) = –1.7; p = 0.09 |
| Severity of last depressive episode (no. of DSM-IV symptoms recorded), mean (SD) | 6.5 (1.1) | 7.1 (1.2) | t(420) = –5.67; p < 0.0001 |
| Number of comorbid DSM-IV Axis I psychiatric diagnoses, mean (SD) | 0.5 (0.9) | 0.7 (0.95) | t(420) = –1.72; p = 0.09 |
| Ever been hospitalised for emotional or psychiatric reason? n (%) | χ2(1) = 19.896; p < 0.0001 | ||
| Yes | 11 (5.3) | 43 (19.9) | |
| Missing | 2 (1.0) | 1 (0.5) | |
| Ever received outpatient psychiatric or psychological treatment? n (%) | χ2(1) = 2.78; p = 0.10 | ||
| Yes | 95 (46.1) | 115 (53.2) | |
| Missing | 2 (1.0) | 6 (2.8) | |
| Ever made a suicide attempt? n (%) | |||
| Yes | 30 (14.6) | 70 (32.4) | χ2(1) = 18.12; p < 0.0001 |
| Number of previous attempts, mean (SD) | 1.6 (0.78) | 1.9 (1.4) | t(97) = –1.28; p = 0.21 |
| Family history of mental illness or alcohol or drug abuse, n (%) | χ2(2) = 20.36; p < 0.0001 | ||
| Yes | 77 (37.4) | 121 (56.0) | |
| No | 114 (55.3) | 74 (34.3) | |
| Not sure | 12 (5.8) | 21 (9.7) | |
| Missing | 3 (1.5) | 0 (0.0) | |
| Quality of life, mean (SD)b | |||
| How would you rate your quality of life? | 3.7 (0.8) | 3.6 (0.8) | t(411) = 1.96; p = 0.05 |
| How satisfied are you with your health? | 3.1 (1.0) | 2.9 (1.0) | t(411) = 1.57; p = 0.12 |
| Physical | 14.4 (2.9) | 14.5 (7.6) | t(411) = –0.04; p = 0.97 |
| Psychological | 12.7 (2.6) | 12.2 (2.6) | t(411) = 1.91; p = 0.06 |
| Social | 13.9 (3.2) | 12.7 (3.5) | t(411) = 3.46; p = 0.001 |
| Environment | 15.4 (2.3) | 14.7 (2.6) | t(411) = 2.87; p = 0.004 |
Appendix 6 Adult Service Use Schedule
Appendix 7 Distribution of themes in telephone interviews
| Theme | Subtheme | 2012 | 2006 | 2004 | 2005 | 2007 | 2015 | 3006 | 3008 | 2009 | 2010 | 2001 | 2002 | 2003 | 3005 | 3007 | 2013 | 3004 | 2011 | 3003 | 2008 | 2014 | 2016 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADM issues | Wants to remain on ADM | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||
| Wants to come off ADM | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||||||
| Not wanting to take ADM for 2 years | ✗ | ||||||||||||||||||||||
| Not regularly taking ADM | ✗ | ✗ | ✗ | ||||||||||||||||||||
| Worried about relapse | ✗ | ✗ | ✗ | ||||||||||||||||||||
| Time issues | Not the right time | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||
| Study too long | ✗ | ✗ | |||||||||||||||||||||
| Working full time | ✗ | ✗ | ✗ | ||||||||||||||||||||
| Attitudes to psychological intervention | Had therapy before | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||
| Previous therapy unhelpful | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||||||||
| Previous therapy helpful | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||||||||||
| Therapy and ADM complementary | ✗ | ✗ | |||||||||||||||||||||
| MBCT issues | Meditation is a good idea | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||||
| Not keen on meditation | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||||||||||
| Conflicts with religious views | ✗ | ||||||||||||||||||||||
| Being in a group | Unhappy with group therapy | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||||||
| No concerns about group therapy | ✗ | ✗ | ✗ | ✗ | |||||||||||||||||||
| Lifestyle and medical barriers | Too unwell | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||||||||||
| Cannot travel to sessions | ✗ | ✗ | ✗ | ✗ | |||||||||||||||||||
| Too old | ✗ | ✗ | |||||||||||||||||||||
| Symptom issues | Depression because of chemical imbalance | ✗ | ✗ | ✗ | |||||||||||||||||||
| Not depressed enough | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||||||||||
| Depression is contextual | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||||||||||
| Taking part in research | No concerns | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||
| Safety/reliability concerns | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||||||||||
| Wanting to help others | ✗ | ✗ | ✗ | ✗ | |||||||||||||||||||
| Previously taken part in research | ✗ | ✗ | ✗ | ||||||||||||||||||||
| Being randomised | Would like to choose group | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ||||||||
| Would like ADM arm | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||||||||
| Would like MBCT arm | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | |||||||||||||||||
| No worries about randomisation | ✗ | ✗ | ✗ | ✗ |
List of abbreviations
- AD-SUS
- Adult Service Use Schedule
- ADM
- antidepressant medication
- BDI-II
- Beck Depression Inventory, second edition
- BNF
- British National Formulary
- CBT
- cognitive–behavioural therapy
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CTU
- Clinical Trials Unit
- DMC
- Data Monitoring Committee
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-3L
- European Quality of Life-5 Dimensions three-level version
- FFMQ
- Five Factor Mindfulness Questionnaire
- GP
- general practitioner
- GRID-HAMD
- GRID-Hamilton Depression Rating Scale
- HR
- hazard ratio
- HRSD
- Hamilton Rating Scale for Depression
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- LEG
- Lived Experience Group
- LIFE
- Longitudinal Interval Follow-up Evaluation
- m-ADM
- maintenance antidepressant medication
- MBCT
- mindfulness-based cognitive therapy
- MBCT-TS
- mindfulness-based cognitive therapy with support to taper/discontinue antidepressant medication
- MBI-TAC
- Mindfulness-Based Interventions – Teacher Assessment Criteria
- MBSR
- mindfulness-based stress reduction
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MOPS
- Measure of Parenting Scale
- MRC
- Medical Research Council
- MSCL
- Medical Symptom Checklist
- NICE
- National Institute for Health and Care Excellence
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SCID
- Structured Clinical Interview for DSM-IV
- SD
- standard deviation
- SUSAR
- suspected unexpected serious adverse reaction
- SWAD
- Staying Well After Depression
- TSC
- Trial Steering Committee
- WHO
- World Health Organization
- WHOQOL-BREF
- World Health Organization Quality of Life-BREF