Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/14/30. The contractual start date was in September 2009. The draft report began editorial review in April 2015 and was accepted for publication in September 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Catherine Sackley is Deputy Chair of the Health Services and Delivery Research Researcher-led Board; Caroline Watkins is on the Health Technology Assessment Commissioning Board; and Keith Wheatley is on the Health Technology Assessment Clinical Evaluation and Trials Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Sackley et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Prevalence of stroke in the UK
The population of the UK and elsewhere is living longer. The average lifespan since 1960 in England and Wales has increased by 10 years for men and 8 years for women. 1 One in six members of the UK population were aged over 65 years at the time of the 2011 census,2 and the over-85-year-old age bracket is the fastest growing sector. 3 It is predicted that, by 2031, 22% of the population will be over 65 years old. 4 The incidence of stroke increases significantly with age. 5 According to British Heart Foundation statistics released in 2012, there are approximately 152,000 strokes in the UK5 and approximately 65,000 people experience their first transient ischaemic attack (TIA) each year. 6
Survival rates following stroke have improved significantly over the last 20 years because of medical advances in acute care and increased public awareness of stroke symptoms. However, owing to the decreasing levels of stroke mortality there is a significant rise in the number of people living with stroke-related disabilities.
In 2012, it was estimated that there are 1.2 million stroke survivors living in the UK. 5 Stroke represents the third most common cause of disability-adjusted life-years worldwide. 7–9 The disabilities experienced as a result of stroke are complex,10 potentially involving a multitude of physical and mental impairments, including difficulties as detailed in Box 1.
Arm and/or leg movement.
Balance.
Walking.
Swallowing.
Spasticity.
Cognition.
Depression.
Bowel control.
Performing personal activities of daily living (e.g. washing, bathing, dressing).
Pain.
Altered sensation.
Speaking.
Understanding the written or spoken word.
Urinary incontinence.
Vision.
Approximately 10% of patients are discharged from hospital directly to a long-term care facility,11,12 and 25% of stroke survivors require long-term institutional care as a result of the brain injury. 13 Clearly, clinically effective and cost-effective health technologies designed to ameliorate disability and improve quality of life for a growing population of older stroke survivors are needed.
Long-term care descriptors
In England, at the end of 2012 there were 4675 care homes with nursing facilities (218,387 beds) registered with the Care Quality Commission, and 12,917 residential care homes without nursing facilities (245,942 beds). 14 The distinction between homes that provide nursing care and those that offer only residential care is dependent on the skills of the care home staff, and not necessarily associated with the level of disability of the residents. Homes that provide nursing care employ qualified nurses, whereas homes that provide residential care are not required to employ qualified health professionals. It is estimated that between 20% and 45% of all people newly admitted to residential care settings in the UK have stroke-related disabilities. 11,15–17 The prevalence of stroke and dementia in the older population suggests a huge demand for long-term care facilities, and the provision of effective health-care technologies within those facilities, both now and in the future. 18
Health-care services within care home settings
Older people with complex health conditions are the main users of health and social care services. 19 From 1990 to 2010 in the UK there was a significant shift in long-term care for older adults away from geriatric hospitals, and more towards care homes. 20 Long-stay hospital wards benefit from established auditing systems which, prior to recent developments in social care provision, care homes did not. As a consequence, patients living in care homes have been described as ‘living on the margins of care’. 21 During this period there have been a number of initiatives, devised by government, in association with the Royal College of Physicians (RCP), that have introduced care standards to regulate, and improve, health care for the older population, and develop a more integrated service in care homes. 3,19,20,22–24
The National Service Framework for old age presented care standards with three themes: dignity in care; joined-up care; and healthy ageing – promoting exercise and activity, independence, well-being and choice. 19 The joined-up care theme outlined reforms to ensure a comprehensive health assessment is conducted prior to admission to a long-term residential facility, to establish individual health-care needs. 3 The National Service Framework listed standards for four main components central to the development of an integrated stroke service in older age: prevention, immediate care, early and continuing rehabilitation, and long-term support. 3,19
In addition to the National Service Framework, the RCP produced a series of guidelines to enhance the health of older adults in long-term care. 20 A component within these guidelines focused on ‘overcoming disability’ from a therapeutic perspective. 20 The guidelines highlighted the importance of the care home environment, and the use of aids, equipment and adaptations to address disability and improve function in long-term care facilities. The philosophy behind these guidelines was that small increases in functional capacity of older people are deemed to impact positively on quality of life and cost of care. 20 The section on ‘overcoming disability’ concentrates on providing access to resources to improve or maintain functioning in primary activities of daily living (ADL).
For the purposes of this trial, primary ADL are referred to as personal or self-care ADL. Personal ADL are defined as:
-
mobility
-
transfers (e.g. from bed to chair and back)
-
using the toilet
-
grooming
-
bathing
-
getting dressed
-
feeding.
These initiatives have instigated considerable progress in raising standards, increasing awareness of health-care issues in older age and lessening the long-standing stigma associated with this population. However, despite this progress, care services for older adults with high support needs, such as stroke survivors residing in care homes, are notoriously inconsistent, and most often dictated by financial constraints at a regional level. 25
More residents with a higher level of dependency and complex care needs are being admitted to care homes than ever before. 26 For residents with high levels of support needs, there is more of an emphasis on providing specialist care for a short period towards the end of life to ease suffering and promote dignity throughout. 16 It is critical to design health services with the needs, circumstances and preferences of the service users in mind. 19,27 Establishing an evidence base for clinically efficacious and cost-effective therapeutic health technologies, suitable for use by the NHS in care homes to promote dignity, joined-up care and increased independence is a research priority.
Occupational therapy
Occupational therapy is the therapeutic intervention that promotes health by enhancing the individual’s skills, competence and satisfaction in daily occupations . . . to act on the environment and successfully adapt to its challenges.
Yerxa et al. 28 p. 6
Activity is essential to health and well-being. 29 In the re-drafted report published by the World Health Organization entitled International Classification of Functioning, Disability and Health (ICF),30 the term ‘disability’ is described in reference to the interaction between an individual’s impairments, activity limitations, participation restrictions and their environment. This definition focuses on the individual’s capacity to engage in functional activity.
Occupational therapists aim to improve the quality of life of their patients by attempting to augment functional activity and increase their capacity to engage in personal ADL. 31 A philosophy of occupational therapy (OT) is that the intervention is most effective when it is integrated into the context of the individual. 31 OT typically applies a patient-centred goal-setting approach, so that the therapy package is individualised for each patient, and the goals of therapy are continually reviewed in relation to progress. 32 The treatment programme is planned around the patient’s goals. The patient is given as much autonomy as possible in maintaining or improving his or her own quality of life. Task-specific training, guidance and supervision is given to reinforce safe and effective practice of personal ADL. 33 Where necessary, training involves the use of adaptive equipment (e.g. adapted cutlery or walking aids) to facilitate an increase in capability, ameliorate activity limitations and provide therapeutic aid. Enabling modifications, tailored to individual needs, can be applied to the environment to promote safe and effective practice of ADL (e.g. the installation of bed levers, grab rails or a raised toilet seat). Particular attention is given to communication, to engender an informal atmosphere that will enable the exchange of ideas, and the offering of peer support. In summary:
Occupational therapy is a complex intervention. Practice includes skilled observation; the use of standardised and non-standardised assessments of the biological, psychiatric, social, and environmental determinants of health; clarification of the problem; formulation of individualised treatment goals; and the delivery of a set of individualised problem solving interventions. 34
Historically, OT services within the NHS have been situated in acute hospital services; however, nowadays therapists also operate as a part of local authority social care services throughout the UK. The ‘joined-up care’ initiative has helped instigate service reform to better suit the needs of users in the local community. 3,19 A review of the effectiveness of OT administered by local authority social services to older people at home has shown high satisfaction levels for the service. 34 It has also been suggested that the provision of adapted equipment to reduce dependency on additional services may be cost-effective. 35
Occupational therapy for stroke rehabilitation
The National Institute for Health and Care Excellence guidelines for stroke rehabilitation recommend that OT should be provided for people after stroke to help ameliorate difficulties with personal ADL. 36 The guidelines also stipulate that stroke survivors should be monitored regularly by occupational therapists with core competencies in this area. 36
Occupational therapy delivered to stroke survivors in their own homes has good evidence of benefit. 34,37 A systematic review and meta-analysis were conducted by members of the research team to determine whether or not OT, focused on promoting increased activity and independence in performing personal ADL, improves recovery for stroke survivors. 34 Analysis of nine trials (1258 participants) found that OT increased personal ADL scores, measured using the Barthel Index of Activities of Daily Living (BI). The standardised mean difference was 0.18 [95% confidence interval (CI) 0.04 to 0.32; p = 0.01] in favour of OT, compared with receiving no intervention or usual care. This equates to a single-point difference (5%) on the BI (20-point scale). Furthermore, for every 100 people who received OT after a stroke, 11 (95% CI 7 to 30) would be spared a poor outcome, defined as death or deterioration in abilities to perform ADL [odds ratio (OR) 0.67, 95% CI 0.51 to 0.87; p = 0.003]. 34 The review concluded that targeted OT should be available to everyone who has had a stroke, to reduce disability and increase independence in performing personal ADL.
Although the systematic review concluded that OT is effective when administered in patients’ homes,34 the clinical efficacy and cost-effectiveness of OT administered to stroke survivors living in a care home setting was not known. Differentiating stroke survivors who live in their own homes from stroke survivors residing in care homes is important. Typically, stroke survivors living in care homes have increased physical and mental limitations as a result of their brain injury, and their functional capacity to perform personal ADL is often restricted. For instance, 78% of residents in a care home have cognitive impairment, 76% need some form of assistance with ambulation and 71% are incontinent. 17 Reduced functional capacity may limit stroke survivors’ ability to engage in, and respond to therapy. As a result, generalisation of results from community studies to care home settings should be treated with caution.
Occupational therapy for care home residents with stroke-related disabilities
In the Netherlands, 93% of care home residents regularly receive some form of OT;38 however, in the UK it is available to as few as 3–6% of residents. 39,40 An audit of over 1000 residents in England found that none had been assessed for OT. 41 The most recent stroke guidelines recommend reducing nationwide variability in rehabilitative care after stroke,42 including the care home setting. 43 Owing to the number of patients transferring directly from hospital to a care home environment following a stroke, as opposed to returning home,13,44 it is necessary for rehabilitation and social care services to achieve equivalent standards, especially for those patients with increased dependence.
Following admission to a care home, stroke survivors’ health state typically follows a downward trajectory. Observational data suggest that care home residents spend 97% of their daytime hours sitting inactive with eyes open or eyes closed. 45 Inactivity in older care home residents can pose further health risks, such as pressure ulcers, joint contractures, pain, incontinence and low mood. 46 The provision of OT as a means of augmenting levels of functional activity may reduce the likelihood of these further health conditions and reduce unnecessary dependence.
Current evidence evaluating the efficacy of OT across the whole care home population, not restricted to residents who have experienced a stroke, has shown conflicting results. 47,48–51 The evidence relating specifically to stroke survivors living in care homes is extremely limited. A systematic review considering the efficacy of providing OT to stroke survivors living in care homes was conducted by our research team. 52 Literature searches were performed within: MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature, the Cochrane Central Register of Controlled Trials, six trials registers and 10 additional bibliographic databases (all searches ended in September 2012). 52 The review process revealed only one relevant randomised controlled trial (RCT) conducted to date; this was the OT intervention for residents with stroke living in UK Care Homes (OTCH) cluster randomised Phase II pilot trial discussed in full in Chapter 2. No firm conclusions of efficacy of OT provided to care home residents with stroke-related disabilities could be drawn from the systematic review. 52
Depression in stroke survivors residing in care homes
Symptoms of depression are common in older residents residing in care homes,53–57 and very common following stroke. 58 Experiences of depression following stroke may be directly attributable to the brain injury or an adverse psychological response to trauma. 58 Communication problems following stroke limit residents’ ability to express feelings of low mood and may be difficult to recognise by unqualified members of staff. 59 Presence of depression in care home residents is associated with poor outcomes and increased mortality. 57 Symptoms of depression include:
-
losing interest in everyday activities
-
finding it difficult to concentrate or make decisions
-
feeling worthless, guilty, helpless, hopeless or in despair
-
changes in appetite. 58
A previous study, assessing the feasibility of a trial evaluating the effectiveness of an OT programme at reducing levels of depression in a care home population, found no significant effects. 60 However, the trial was not powered to evaluate the efficacy of OT in alleviating in symptoms of depression. The OTCH trial sought to assess the influence of OT on levels of depression as a secondary outcome measure to the performance of personal ADL.
Assessing health-related quality of life in stroke survivors residing in care homes
A central philosophy underpinning OT is that the intervention involves engaging in activities that hold meaning for the individual. The personalised meaning behind the activities is thought to help promote increased quality of life for that individual. 31 Health-related quality of life (HRQoL), assessed according to a number of physical and emotional dimensions of interest,61 can be used to measure the perceived impact of a chronic disease. 62 The purpose of including a measure of HRQoL was to provide an additional multidimensional scale that considers both physical and emotional functioning to evaluate potential effects of the OT intervention not captured by the BI.
Training for care home staff
In the UK, the majority of care for older stroke survivors living in long-term care institutions is provided by the staff of those institutions. 63 During the OTCH Phase I stage, a number of care home staff in one area of the UK were interviewed. 41 From the staff responses, it was evident that none of the care homes was providing aids and appliances effective in reducing physical decline, that is there was a difference between policy and practice. It is therefore doubtful whether or not the RCP guidelines on ‘overcoming disability’ could be universally implementable across the UK. 20 The guidelines highlight the importance of the care home environment and the use of aids, equipment and adaptations to address disability and improve function in long-term care facilities. The results from the Phase I interviews were a strong indication that any development of health services in care homes needs to directly involve the staff who provide the majority of residents’ care.
A later report highlighted several aspects of staff involvement deemed fundamental in establishing a more positive culture in care homes. 64 It promoted the importance of staff training to move away from the prevalent model of task-based care system of doing things ‘for’ residents, and more towards a system with a shared commitment (‘doing with’) that includes emotional care. Consequently, the involvement of care home staff in the evaluation of OTCH was regarded as integral, in order to increase awareness of the broad spectrum of stroke-related disabilities and to provide continuity in care practices between staff and visiting therapists.
Aims and objectives of the Occupational Therapy intervention for residents with stroke living in UK Care Homes trial
Disabilities affecting ADL are commonplace for stroke survivors living in UK care homes, and yet access to rehabilitation services, particularly OT, is very restricted. The purpose of the study was to conduct a Phase III RCT to evaluate the effects of a targeted 3-month course of OT (with provision of adaptive equipment, minor environmental adaptations and staff education) for people with stroke sequelae living in care homes.
The primary outcome measure assessed was the capacity to perform personal ADL.
The secondary outcome measures assessed were mobility, depression and health-related quality of life.
An economic evaluation of the intervention was conducted in parallel with the evaluation of clinical efficacy as part of the health technology assessment. Providing an OT service to stroke survivors resident in care homes was compared against usual care. The trial aimed to evaluate whether or not there is sufficient evidence to advocate the routine implementation of OT for all stroke survivors living in care homes.
Chapter 2 Methods
Trial design
The OTCH study was a Phase III, pragmatic, multisite, cluster RCT with economic evaluation, across several regions in England and Wales. The cluster design was justified because of the inclusion of an education component for care home staff and the potential need to apply minor modifications to the care home environment (e.g. install raised toilet seats). Furthermore, cluster randomisation at a care-home level reduced the potential for between-group data contamination during interaction between carers, therapists and residents. The flow diagram for this trial followed the Consolidated Standards of Reporting Trials (CONSORT) extension for cluster randomised trials. 65
Setting
Local care homes for older people in the UK were located in the following 12 trial administration centres (TACs):
-
University of Birmingham
-
Bangor University
-
University of Central Lancashire
-
University of Nottingham
-
Solent Healthcare Primary Care Trust (PCT)
-
Plymouth PCT
-
Wolverhampton PCT
-
Taunton PCT
-
Stoke-on-Trent PCT
-
Coventry & Warwickshire PCT
-
Bournemouth and Poole PCT
-
Dorset PCT.
Recruitment and consent
Care homes
Residential homes with and without nursing care with more than 10 beds, local to the 12 TACs, were identified from the Care Quality Commission website. Homes were identified randomly, to ensure a non-biased selection, using published care home lists. All funding models of care home were included (i.e. private, charitable, not for profit and local authority). Institutions for people with learning disabilities or drug addiction were excluded from the study. Homes were telephoned by a member of the research team and the trial was explained briefly. No homes were actively delivering OT as a component of standard care. A recruitment pack was mailed to interested homes. This included an invitation letter, a leaflet describing OT, study information sheets, designed in collaboration with service users, and a response form. Another telephone call was made to arrange a convenient time for the assessor to visit, and the homes were asked to consider which residents may be eligible. At the visit, the care home managers were given a full verbal explanation of the study and the opportunity to ask questions. If managers were interested in the study, they were asked to sign a written agreement for their home to participate. Following receipt of care home consent, residents were considered individually.
Residents
Care home managers assisted to identify potential participants. To be eligible for entry into the study potential participants were required to be resident in a care home and to have a history of stroke (ischaemic or haemorrhagic) or TIA. The inclusion of residents with a history of TIA was warranted because of the growing evidence that TIA can cause long-term problems. 66,67 All efforts were made to include participants with communication and cognitive impairments to increase external validity of the trial as these symptoms are commonplace following stroke. Residents receiving end-of-life care were excluded. Care home members of staff searched residents’ files to determine a diagnosis of stroke or TIA. If required, the research team sought confirmation of this diagnosis with general practice records [see Appendix 1 for general practitioner (GP) correspondence details]. When a potential participant was identified as being eligible for the study they, and their family (when appropriate),68 were approached by the assessor or a senior member of the care home staff. Prospective participants (and their family) were given a full explanation of the study. This included a discussion of the treatment options in the trial and the method of treatment allocation. Potential participants (and family, if appropriate) were given an invitation pack consisting of an invitation letter, consent form and participant information sheet, designed in collaboration with service users (see Appendices 2–4 for participant information sheets and consent form). For eligible residents needing the assistance of a consultee (family member), a consultee package was mailed, which included a consultee invitation letter, consultee declaration form and consultee information sheet (see Appendices 5 and 6 for consultee information sheet and declaration form, respectively). Residents were given sufficient time to decide (at least 24 hours) whether or not they would like to join the study. A follow-up telephone call was made by the assessor to arrange a return visit to the care home.
Participants and consent
During a second visit to the care home the independent assessors obtained consent from all eligible residents who had indicated interest. If the resident was considered to be incapacitated, according to guidelines listed in the Mental Capacity Act 2005,68 a consultee was approached for consent. If the resident or consultee consented, the participant’s GP was informed in writing of their involvement in the trial. Following the consent procedure, baseline assessments were administered by the assessor. When all participating individuals in a home had completed baseline assessments, the care home was randomised. If a care home had at least one consenting resident, it was eligible for randomisation.
Randomisation, stratification and blinding
Care homes and participants were recruited and consented into the study before the randomisation process commenced to reduce bias. 69,70 Once the study co-ordinator received confirmation from assessors that all residents in a participating home had given their consent and completed a baseline assessment, the homes were grouped and randomised (1 : 1) to receive either the OT intervention or usual care (control). The allocation sequence was generated in software (nQuery Advisor version 7; Statistical Solutions, Saugus, MA, USA) by an independent statistician using blocked randomisation (block size 2) within strata [type of care home (with or without provision of nursing care)] and geographical location of the TAC at the Primary Care Clinical Research and Trials Unit, University of Birmingham, independent from the research team. Blocked randomisation was used to ensure groups were balanced with respect to the stratification variables. The details of the sequence were concealed from the research team, independent assessors and the study co-ordinator. Once the study co-ordinator received notification that all consenting participants in a care home had completed baseline measures and that the strata data had been logged, care homes were randomised. Care home allocation was revealed to the study co-ordinator, who then informed the care home manager and corresponding site therapist. If a care home had been allocated the OT intervention, the site occupational therapist then contacted the manager of the home to make arrangements for them to visit and commence the intervention. Treatment allocation was concealed from the independent assessors, but it was not possible to mask aspects of the intervention from staff or residents.
Procedure
The trial protocol is published elsewhere. 71
Control: usual care
Care homes in the control arm of the study continued to provide their usual care to residents. None of the participating homes provided OT as a component of routine care. After all the final outcome assessments had been conducted at 12 months, the care homes in the control arm were offered a 2-hour group training session for care home staff. The session was led by an occupational therapist and focused on promoting and supporting activity for care home residents after stroke. 72 It was based on the key principles of OT, such as the facilitation of independent daily living and promotion of activity among residents.
Occupational therapy intervention
We developed an OT intervention package for residents in a care home using evidence and expert occupational therapist consensus opinion, details of which are summarised below and presented in full in a previous publication. 73 The OT intervention was delivered by a qualified occupational therapist and/or an assistant, and it was targeted towards maintaining the stroke survivors’ capacity to engage in personal ADL, such as:
-
feeding
-
dressing
-
toileting
-
bathing
-
transferring
-
mobilising.
In order to promote external validity, the therapy a resident received was not dictated by the trial, but decided on by the therapy professional, in collaboration with the resident or consultee.
Patient-centred goal-setting
The OT intervention employed a patient-centred goal-setting approach to establish an individualised treatment plan for each participant. Goal-setting involves establishing mutually agreed targets between patient and therapist that will be aimed for over a specified duration of therapy. 42 In the initial assessment the therapist met with the participant (and/or carer/family member when appropriate) and recorded demographic details, current medication and discussed challenges experienced with daily activities (see Appendices 7–9). Together they agreed a treatment plan that was reviewed after each session. Two examples of the patient-centred goal-setting approach and treatment plan are presented in Table 1.
| Participant ID | Needs identified | Goals | Action |
|---|---|---|---|
| *** | Maintenance of mobility | Check use of walking equipment | Checked ferrules |
| Improve body strength | Checked height of walking aid | ||
| Improve posture and balance | Encourage to mobilise | ||
| Exercises to improve strength and balance | |||
| Discussed armchair | |||
| Difficulties with transfers | Improve bed and toilet transfer technique | To try equipment to assist toilet transfers and bed transfers | |
| To try toilet seat equipment | |||
| Difficulties with personal care | To improve independence in personal care | To try use of perching stool | |
| *** | Difficulties with sit-to-stand transfers | Improve transfer technique | Assess dining room chair and armchair |
| Practice transfers | |||
| Slow, shuffling walking pattern | Improve walking pattern | Check shoes and walking stick | |
| Improve body strength and balance | Implement exercise regime | ||
| Reduce tightness of hamstrings | Advice on walking technique | ||
| Elevate feet on footstool to feel stretch on posterior thigh | |||
| Difficulties keeping food on plate during meal times | Facilitate feeding technique | Assess use of plate guard |
Intensity of occupational therapy (number of visits)
The frequency and duration of the OT was dependent on the resident’s and therapist’s agreed goals (within the framework of the home). In a study that piloted the intervention on a population of care home residents (not limited to stroke survivors), the number of face-to-face sessions ranged from 1 to 25 per resident over a 3-month period (median time 8.5 hours and mean time 4.7 hours), dependent on the individual needs of the resident. 73 The OT intervention included a continuous process of assessment, treatment and reassessment. 73 In line with current evidence on effective treatment, the intervention adopted a task-specific training approach. 33 Treatment logs were developed, and each occupational therapist was required to document the time spent and content of each individual therapy session. An example of the treatment log is given in Appendix 10.
Occupational therapy content
The content of therapy for the OTCH intervention was assigned to categories, and the time spent on each category was documented in the treatment log. Categories of therapy identified in the intervention design were listed as:73
-
assessment/reassessment and goal-setting – involving the assessment of a resident’s current levels of functional activity in personal ADL and mutually identifying functional goals of therapy
-
communication – including listening to residents’ concerns about personal ADL, providing information and guidance to residents, staff or relatives, initiating referrals to other agencies and ordering equipment
-
ADL training (cognitive and functional) – involving techniques to assist with feeding, bathing, using the toilet, getting dressed and grooming
-
transfers and mobility (cognitive and functional) – involving bed mobility, standing, walking and transfers to/from a chair
-
environment – involving environmental adaptations (e.g. adaptive equipment)
-
other – involving treating impairments directly, such as joint contracture.
Equipment provision
Adaptive equipment was provided as part of the study, which included personal items such as adapted cutlery and walking aids. If required, adaptations to the individual’s environment were made, such as provision of chair raisers, bed levers, raised toilet seats or grab rails. The occupational therapist demonstrated to the participant, and care staff, how to use adaptive equipment effectively, while adhering to safety regulations.
Examples of the type of such equipment are listed below:20
-
mobility: wheelchair, walking stick or walking frame
-
transfers: chairs/beds – correct height to suit different needs
-
toilet aids: includes raised seats and rails
-
bathing: grab rails, bath board, specialist baths and showers
-
getting dressed: dressing aids and clothing adaptations
-
washing: adapted taps
-
eating: adapted cutlery.
Involvement of care home staff
The implementation of the intervention required direct involvement of the care home staff to continue therapy practices, and use of adaptive equipment initiated during treatment visits. Therapists wrote in participants’ care plan after each visit. The care plans summarised the therapist’s assessment of the participant and provided recommendations for the staff to implement in order to maximise participants’ levels of functional activity. Examples of care plans left for care home staff are given in Table 2. The care home manager was continually updated on the progress of the intervention, and the research team adapted therapy visits around care home routines (e.g. meal times and leisure activities).
| Participant ID | Activity |
|---|---|
| *** | Mobility |
| Following walking practice over the last few weeks, and provision of a three-wheeled walker by the physiotherapist, Mr x is able to walk to the dining room from his bedroom, as his pain allows | |
| Recommendation: please continue to provide opportunities for Mr x to walk rather than use the wheelchair | |
| Transfers | |
| Mr x is able to manage all his transfers independently, as long as his pain is under control, including sitting up in bed and moving his legs round to the floor, standing from the bed (can be unsteady so supervision needed), standing from a chair, sitting on a chair and getting on and off the toilet | |
| Recommendation: please continue to offer verbal support rather than physical support as much as possible to facilitate Mr x’s transfers | |
| Personal care | |
| When provided with a bowl of soapy water and a flannel, Mr x is able to wash his upper body mainly independently, needing help with his back, bottom, legs and feet. He currently has help shaving, but may be able to manage this himself if provided with a shaving mirror | |
| Mr x is able to take off his nightwear independently with prompting. He is able to put on a vest and shirt by himself. He can manage some buttons himself, but needs some help with this. Mr x can dress in pants and trousers with minimal help to get these past his heels. He needs full assistance to put his socks and shoes on. Mr x is able to put his cardigan on with minimal help | |
| Recommendation: please continue to maximise Mr x’s participation in his personal care by providing verbal prompts and encouragement rather than physical help as much as possible | |
| *** | Eating/drinking |
| Mr x is able to eat and drink independently when positioned correctly in his bed, and with his table positioned to give him best access to his food. The Nursing sister and a member of care staff have been shown how best to position Mr x for eating/drinking | |
| Recommendation: please ensure Mr x is always positioned correctly and his food placed within reach to maximise his independence with eating and drinking | |
| Personal care | |
| Mr x is able to wash his face when provided with a cloth, prompting and encouragement | |
| Recommendation: please continue to enable Mr x to wash his face independently by providing a cloth, verbal prompts and encouragement rather than physical help as much as possible | |
| *** | Personal care |
| When provided with a bowl of soapy water and a cloth, Mrs x is able to wash her face and upper body mainly independently, needing help with her back, bottom, legs and feet | |
| Recommendation: please continue to maximise Mrs x’s participation in her personal care by providing verbal prompts and encouragement rather than physical help as much as possible | |
| Eating | |
| Mrs x has been provided with a ‘Knork’ fork to enable her to cut up food independently | |
| Recommendation: please ensure Mrs x has her adapted cutlery at meal times | |
| *** | Personal care |
| The occupational therapist assessment observed that Mrs x is able to participate in washing her face and top half, needing some help because of her right-side weakness. The occupational therapist supplied a suction cup denture brush to enable Mrs x to brush her dentures independently using one hand. Mrs x likes to have some buttons left undone on her cardigan, so she can spend time over the day doing them up herself | |
| Recommendation: please ensure Mrs x is always given time and opportunity to participate as much as possible in her personal care routine. A member of care staff at time of assessment gave excellent support, enabling Mrs x to do as much for herself as possible. Please ensure the denture brush is stuck to the wash basin so that Mrs x can access it easily to clean her dentures | |
| Seating/positioning | |
| Mrs x sits in a reclining chair. She is not currently adequately supported in a good seating position by this chair, which may be contributing to the high tone in her right leg, as she is having to try to hold herself up on her weak side. Mrs x does not want to try using pillows for support in her chair, but is willing to be assessed for more suitable seating | |
| Recommendation: Mrs x would benefit from assessment for specialist seating, to maximise her comfort and ability to function and minimise the risk of increased tone/contractures | |
| *** | Mobility |
| Mrs x has good movement in her legs, and is able to weight bear enough to use a standing hoist. She is keen to try walking again. As she has not walked for many months, however, this may or may not be possible | |
| Recommendation: Mrs x may benefit from a referral to community physiotherapists for a full assessment with a view to a period of rehabilitation | |
| Personal care | |
| When provided with a bowl of soapy water and a cloth, Mrs x is able to wash her face and upper body mainly independently, needing help with her back, bottom and legs and feet | |
| Recommendation: please continue to maximise Mrs x’s participation in her personal care by providing verbal prompts and encouragement rather than physical help as much as possible |
Training for care home staff
Providing regular staff development exercises in long-term care facilities is recommended within RCP guidelines. 20 A specific training workshop was provided to staff directly involved in the care of the residents receiving the OT intervention. 72 The workshop aimed to increase awareness of stroke, and the range of stroke-related disabilities residents may experience. Risks associated with inactivity were highlighted as well as describing the carer’s role in:
-
supporting mobility (e.g. safe and effective methods of transfer)
-
preventing accumulative problems from poor positioning (e.g. unsuitable armchairs)
-
facilitating resident participation in self-care activities. 72
The workshop promoted strategies for staff to improve residents’ capacity in performing personal ADL. A key message in the workshop was to encourage residents’ functional activity, and to create a suitable enabling environment to achieve maximum independence in performing personal ADL. It was expected that inviting care home staff to engage in further training would facilitate compliance and reduce loss to follow-up. A copy of the training workbook appears in Appendix 11. The training given to care home staff received UK Stroke Forum Education and Training endorsement. Training for staff allocated to the control arm was offered following completion of the 12-month follow-up assessments.
Quality assurance
Training of site assessors and occupational therapists
When a TAC initiated work on the trial, it received a trial set-up visit from a senior member of the research team and a trial occupational therapist. A full explanation of the study protocol was given to the trial assessors and occupational therapists. Details of the information passed on to the regional therapists during training are contained in Appendix 12. The assessors were provided with training on the trial paperwork and assessments. Completed examples of the paperwork were provided, including instructions on coding for missing data. The occupational therapists received training on completing the OT-specific paperwork, and the process of ordering specific therapy equipment.
Site monitoring and communication
Site recruitment rates were monitored, and the data uploaded onto the centralised trial database were reviewed when a home was ready for randomisation. If inadequate data had been entered, the TAC was contacted and asked to collect and enter the missing data. The process of randomisation was delayed if the site had missing data to upload.
Any amendments to the study protocol or processes were communicated directly to the sites after ethical approval was received. Acknowledgement was sought to clarify that research staff comprehended the amendments. Members of the research team were invited to attend study meetings, which were specific to their role in the study (e.g. assessors and occupational therapists). This provided an opportunity to discuss general challenges occurring in the study and clarify the trial protocol in relation to site-specific cases.
Compliance
Compliance was estimated by the number of intervention sessions recorded in the OT treatment logs (see Appendix 10), which also described the focus and content of each therapy session.
Outcome assessments
Assessment schedule
An overview of the assessment schedule is given in Table 3. A description of each of the assessment measures is listed in Appendix 13. Baseline assessments were conducted prior to randomisation to reduce possible recruitment bias. 69 The primary measurement end point was 3 months after randomisation. Additional assessments were conducted at 6 and 12 months after randomisation. Data were collected from participants where they were currently residing. If a participant moved to another home between assessments, then every attempt was made by the assessors to collect follow-up data from the participant. To maximise efficiency in data collection and reduce potential disruption to the care home’s daily routine, assessors visited a particular care home and tried to complete all follow-up assessments for participating residents in a single day. If a participant was unwell, in hospital or unavailable on the day of the assessment, the assessor returned to the care home within a 4-week window. Efforts were made for all participants to complete the primary outcome measure at each time point. However, if it was foreseen that participants would be unavailable, a proxy response was obtained from a member of the care home staff.
| Assessment | Time of administration | |||
|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | |
| Demographics | ✓ | |||
| Sheffield Screening Testa | ✓ | |||
| Mini-Mental State Examination | ✓ | |||
| BI | ✓ | ✓ | ✓ | ✓ |
| RMI | ✓ | ✓ | ✓ | ✓ |
| Geriatric Depression Scale-15 | ✓ | ✓ | ✓ | ✓ |
| EQ-5D-3L | ✓ | ✓ | ✓ | ✓ |
| Resource use log | ✓ | ✓ | ✓ | |
| Adverse event log | ✓ | ✓ | ✓ | |
Demographic data and screening measures
An assessor collected demographic data directly from the participant or consultee, which included age, sex, ethnicity, comorbidities, history of falls and intake of current medication. A member of staff from the care home provided initial information about the participant’s stroke, such as the date, type and location of stroke. If this information was unavailable at the care home or additional clarification was required, the participant’s general practitioner was contacted to obtain the data. At baseline, the assessor administered the Sheffield screening test for acquired language disorders to assess receptive/expressive aphasia74 and the Mini-Mental State Examination (MMSE) to assess cognitive function. 75 These assessments were not used to exclude individuals. The tests provided an indication of the participant’s capacity to understand instructions and directly engage in therapy. The screening tests also informed the research team if consultee assistance was required during recruitment.
Primary outcome measure
The primary outcome measure was the BI,76–78 which is regarded as the gold standard measure for assessing functional capacity in rehabilitation outcomes. 20,36,79 It is commonly used to assess stroke survivors. 80,81 The BI measures specific aspects of self-care targeted by the therapy, such as transfers (e.g. from bed to chair) and grooming, and how much help people need in completing personal ADL. 79 Furthermore, the BI was used in previous studies assessing the efficacy of OT, and the data from our study are suitable to be incorporated into meta-analyses. 34,48,49 For pragmatic reasons, we chose to use the shortened BI scale between 0 and 20. 77 A higher score signifies that a person has more independence in ADL. A 2-point change in score is widely accepted as having clinical meaning. 82 It equates to a change that is perceived by stroke survivors and clinicians as a step change in function. For example, a patient may change from being unable to dress and feed to being able to manage with some help or from being able to manage the toilet with some help to being able to manage alone.
Secondary outcome measures
Secondary outcome measures assessed mobility, mood and HRQoL. The HRQoL measure [European Quality of Life-5 Dimensions, three levels (EQ-5D-3L)] is discussed in Chapter 4, Measuring outcomes. Mobility was assessed with the Rivermead Mobility Index (RMI),83 a 15-item measure of functional mobility. It is scored from 0 to 15 and a higher score denotes better mobility. It was deemed important to assess the RMI alongside the BI to increase responsiveness to change. 79 According to previous research that assessed the sensitivity of the RMI and BI in capturing change,79 both tests measure similar constructs but have the potential for floor and ceiling effects. The BI is geared more towards assessing global function, but the RMI relates specifically to mobility. The RMI is regarded as a more sensitive measure than the BI, but has the potential for floor effects (i.e. high percentage of scores at the low end of the scale). The BI, on the other hand, has the potential to give rise to ceiling effects (i.e. high percentage of scores at the top end of the scale). 79 Attempting to maximise responsiveness to change was a critical issue in the evaluation of the OTCH intervention because small increases in functional capacity of older people are deemed to impact positively on quality of life and cost of care. 20
The Geriatric Depression Scale (GDS) was administered to measure mood. 84 One point is assigned to each answer in accordance with the mark scheme. A higher score denotes more severe depression. The full 30-item version was initially administered with participants. 84 If residents were unable to self-complete or follow the interview process, the consultee version, which consisted of 15 items, was completed. 85 However, during the study it was decided to replace the full 30-item GDS with the shortened 15-item GDS,85 to reduce the burden on the residents and assessors. The protocol amendment occurred in 2010.
Adverse events
An adverse event was defined as an injury attributable to the intervention requiring a visit to a hospital or GP. A risk assessment found that there was a small increased risk of falling as a result of the OT intervention (e.g. because of an increase in use of ambulatory aids). This small increased risk was stated clearly in the participant information sheet. Every effort was made to minimise the risk of falls throughout the treatment of the participant and by training the care home members of staff (see Appendix 14 for the adverse event reporting form).
Sample size
A change of 2 points on the BI is widely accepted as being clinically meaningful. 82 In order to detect a difference of this magnitude between the treatment arms, a sample of 72 participants in each arm was required, based on an estimate of standard deviation (SD) of 3.7 points,47,48,49 90% power and 5% significance level. However, residents in this trial were cluster randomised by care home and, therefore, the sample size was inflated by a factor of 4.6, resulting in an increase to 330 residents per randomisation arm. This design factor was based on an estimate of intracluster correlation coefficient (ICC) of 0.4 and an average of 10 residents per care home observed in previous research. 48,49 The ICC used in the sample size calculation was estimated from data related to several pilot studies from a single site with relatively small numbers of care homes. A larger estimate of ICC was used than the ICC observed during the OTCH pilot trial in the interest ensuring an adequately powered study. 48 Based on the attrition rate of 26% from the OTCH pilot study,48 it was estimated that 45 homes with 10 residents per home would be required in each arm of the study (900 residents in total) to detect a clinically meaningful difference using the BI. The sample size quoted in the original proposal was estimated at 840 residents from 84 care homes; however, this sample size was initially incorrectly inflated for expected attrition (26%). The correct figure should have been 900 residents from 90 homes. The revised estimate was identified at the start of the trial.
Occupational Therapy intervention for residents with stroke living in UK Care Homes pilot study
A pilot study of the OT intervention for residents in care homes with stroke-related disabilities was conducted in Oxfordshire, UK, and published prior to the trial. 48 The pilot study was undertaken to refine trial procedures and ensure the OT intervention was acceptable to residents and deliverable in the intended format. The design was a cluster RCT with the unit of randomisation trial at the care home level. Twelve homes (118 residents) were randomly allocated to either the OT intervention (six homes, 63 residents) or control (six homes, 55 residents). The control group received usual care. Usual care did not include OT. In the intervention group, a 3-month course of individualised one-to-one OT was provided to residents with stroke-related disabilities. The aim of the therapy was to maintain levels of functional activity in self-care tasks, adapt the physical environment (when necessary) and address specific impairments that limit performance in ADL or cause discomfort.
In addition, the OT intervention included training for care home staff directly involved in the care of the residents. Assessments were made at baseline and at 3-month (immediately following the intervention) and at 6-month follow-up. The measures used were the BI and RMI. The trial indicated a potential for the OT intervention to have detectable and lasting effects on morbidity. From baseline to the primary end point at 3 months the mean BI score increased by 0.6 points (SD 3.9 points) in the OT arm, but decreased by 0.9 points (SD 2.2 points) in the control arm. The mean difference between the groups was 1.5 points (95% CI –0.5 to 3.5 points, allowing for cluster design). The between-group difference in BI score was maintained at 6 months (difference of 1.9 points, 95% CI –0.7 to 4.4 points).
The analysis of the data suggested that OT may have a significant influence on maintaining functional independence in personal ADL for residents living with stroke-related disabilities within care homes. However, the analysis was exploratory because of its small sample size. The pilot study demonstrated feasibility of the research design. The method was practical and provided the relevant information to conduct a formal sample size calculation for a subsequent suitably powered definitive trial.
Data management
All pre- and post-randomisation data were initially captured by a blinded assessor on paper forms. The data were then entered manually onto the purposefully designed trial database system, which was located in the Primary Care Clinical Research and Trials Unit at the University of Birmingham. The data entry application server was accessible over the internet via secure remote access. All traffic through the data entry application to the database was automatically encrypted using 128-bit secure sockets layer. The database was only accessible from within the University of Birmingham’s information technology network. Only senior members of the research team had access to the database. The firewall for the database was configured to allow access from specific machines only. The data entry application and database used role-based security controls, which restricted access to parts of the data (i.e. uploading, editing and viewing). The data entry application forms were designed and set up by a data manager and computer programmers in collaboration with members of the research team.
Queries about data completeness were referred by the study statistician to the trial co-ordinator. Any missing data that needed to be clarified were obtained by telephone call with the participant or a care home member of staff. Completed questionnaires were entered onto the trial’s secure database by the assessor or a member of the research team. If changes were required on the paper questionnaires, they were formally documented and subsequently uploaded onto the database. The baseline and follow-up data were validated continuously throughout the trial for correctness and completeness. Furthermore, computerised validation checks were incorporated to minimise errors in the data sets (i.e. ranges, limitations and categorisation). Interim summary reports were generated for the Data Monitoring Committee.
Statistical analysis
Analyses were conducted according to a pre-specified statistical analysis plan. 71
Recruitment
Recruitment rates and cluster size were analysed according to the stratification variables of type of care home (nursing or residential) and geographical location (TAC).
Analysis of baseline assessments
Baseline characteristics of participants were tabulated by treatment arm. Items included demographic details, including age, ethnicity and comorbidities. In addition, the screening measures assessing cognitive function and language impairment were summarised to gauge mean levels of stroke-affected disabilities between treatment arms.
Intention to treat
All analyses were performed using an intention-to-treat approach. All participants, including those who died, withdrew or were lost to follow-up, were analysed according to the intervention to which they were randomised, regardless of whether or not they complied with treatment. Participants who moved care homes during the course of the trial were analysed by the home to which they were originally randomised.
Statistical analysis was carried out using Proc Mixed and Proc Glimmix in SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). Multiple imputation was performed using the ICE command in Stata version 12 (StataCorp LP, College Station, TX, USA).
Primary analysis
Outcome measures were compared at the level of the participant. The primary outcome was the BI score at the 3-month follow-up (immediately after the intervention). Linear mixed model analysis with identity link was used to compare the BI between the two arms. The analysis was adjusted for care home (as a random effect), baseline BI score and stratification factors [TAC (geographical location) and type of care home (nursing or residential)]. It was expected that there would be a significant number of deaths in this study during the follow-up phase; therefore, it was agreed, a priori, that participants who died before their follow-up date would be given a BI score of zero at all subsequent follow-ups. This approach was discussed at Trial Steering Committee (TSC) meetings and agreed by the independent Data Monitoring Committee. A Barthel score of zero represents complete dependency, which was thought to reflect the participant’s health state if they had been alive. This method of analysis has been used previously in a stroke population. 86 The influence this may have on the results was examined via a complete case analysis that did not impute BI scores for participants that died.
In addition, participants were categorised into three outcome groups based on an individual’s change in BI score at 3 months from baseline (below 0 or death, ‘poor’; 0 to 1, ‘moderate’; 2 and above, ‘good’) for a BI composite analysis. A non-linear mixed-effects model with cumulative logit link was used to compare this ordinal outcome between the groups. Adjustments were made for care home as a random effect, and TAC and type of care home as fixed effects.
Secondary analysis
Secondary outcomes: RMI (mobility), GDS (mood) and EQ-5D-3L (HRQoL) measures were compared at the participant level at the 3-month follow-up using mixed modelling with identity link. Adjustments were made by care home as a random effect and baseline score, with type of care home and TAC as fixed effects.
To examine whether or not there was an effect of the intervention on outcomes over the longer term, treatments were compared using a repeated measures mixed model across all 3-, 6- and 12-month time points.
For the continuous measures, adjusted mean differences between the treatment arms are reported with corresponding 95% CIs. Positive mean differences favour the intervention. Results for the categorical outcomes are presented as ORs with 95% CIs when an OR > 1 favours the intervention.
Subgroup analysis
Exploratory subgroup analyses were performed to further evaluate whether or not the effect of the OT intervention on BI differed by participants’ age, the type of care home in which participants’ resided, the severity rating of participants’ BI scores, the level of cognitive impairment (derived from the MMSE screening measure), and whether or not the measures were completed by the participant or a consultee. The subgroups were evaluated by the inclusion of covariate by treatment interactions in the mixed modelling.
Sensitivity analysis
Several sensitivity analyses were carried out to test the robustness of the conclusions.
-
Statistical methodology: to test the validity of the mixed modelling approach when many clusters had only one or two participants, the primary and secondary analyses were repeated excluding clusters with fewer than three participants.
-
Ceiling effects: to test the potential ceiling effect of the BI, participants with a baseline score of 18 or above were excluded from the primary analysis.
-
Missing Barthel data because of death: the primary analysis was repeated without imputing missing BI data following the death of a participant (complete case analysis).
-
Missing data: the effects of missing data were examined using various imputation methods including: best case (last observation carried forward); worst case (zero) and multiple imputation methods. Missing Barthel data following death were imputed with zeros for all imputation methods.
Intracluster correlation coefficient
The ICCs were calculated to quantify the effect of clustering, which arose from the care home effects in the primary outcome. The smaller the ICC, the less effect the clustering had on the precision of parameter estimates.
Falls
The proportion of falls in each treatment group during the first 3 months of the trial were compared using generalised mixed modelling with logit link. The number of falls in each treatment group that occurred in this period were compared using a negative binomial model. This method was chosen rather than a Poisson model because of the over dispersion of the falls data. Adjustments were made for care home, TAC and type of care home as previously described.
Ethical approval
Potential risks/benefits
The intervention itself was not experimental. OT is readily available for stroke survivors and their families in other settings. The intervention has been demonstrated by meta-analysis to be of benefit to stroke survivors and their families in these settings. 34,37 However, OT is not readily available in a care setting, and the efficacy and cost-effectiveness have not been assessed in long-term care. 40 Very few adverse events have been recorded through OT interventions. It is possible that walking aids can have a manufacturing fault, but this risk was carefully monitored and publicised. None of the aids or equipment used in this study was experimental. All equipment was in routine use throughout the NHS and social care services. Ethical approval for this study was obtained from the National Research Ethics Service, Coventry Research Ethics Committee (Reference 09/H1210/88) in October 2009.
Trial registration
This trial was registered as ISRCTN00757750 on 21 October 2009.
Governance
A TSC was established to monitor the governance of the study. The Trial Steering Group comprised the main research team, an independent chairperson, a geriatrician, an occupational therapist, a physiotherapist with expertise in rehabilitation research, a patient representative and a representative from the NIHR. A Data Monitoring and Ethics Committee (DMEC) was also established. This committee was independent of the trial and monitored accrued data at regular intervals to assess ethical, safety and data integrity aspects of the trial. The DMEC consisted of an independent geriatrician as chair, a statistician and an occupational therapist.
Amendments to the study protocol during the trial
The first substantial amendment was submitted on 15 March 2010 to approve changes to (1) the protocol to clarify the recruitment and randomisation process, the statistical analysis and the TACs and (2) the demographic front sheet, MMSE, OTCH resource usage form and the care home invitation letter. Furthermore, the substantial amendment included the addition of the OT treatment log, adverse events reporting form, OT leaflet and consultee Geriatric Depression Scale-15 items (GDS-15) to the study. The first substantial amendment was approved 23 March 2010.
A second substantial amendment was approved on 21 October 2010. This amendment included (1) a clarification of the consultee’s responsibilities and duties in the declaration process; (2) an update of the consent form and participant information sheet with grammatical changes for clarity; (3) a redesign of the resource usage questionnaire to capture the OT data after the 12-month follow-up assessment to ensure assessors remained blinded; (4) new paperwork including a GP letter notifying them that their patient was randomised to the control arm of the study; and (5) care home letters notifying them of the randomisation outcome.
On 17 August 2011, a third substantial amendment was approved and included (1) changes to the participant and consultee information sheets to provide more information regarding the security of the trial’s database; (2) a new covering letter and form to the participant’s GP to ask for confirmation of their patient’s diagnosis; and (3) shortening the GDS from the 30-item version to 15-item version to reduce the burden on participants and assessors, and to bring the questionnaire in line with the consultee GDS. A further minor amendment was submitted to revise the consent and declaration forms for version control, which was approved on 22 August 2011.
A final substantial amendment was approved on 26 July 2013 that confirmed the transfer in sponsorship of the OTCH trial from the University of Birmingham to the University of East Anglia.
Patient and public involvement
Patient and public involvement was an integral part of the OTCH trial. Patients and carers were directly involved as research ‘partners’ and not just as ‘data providers’ (using the INVOLVE guidance; see www.invo.org.uk). A representative from the local stroke community was involved in the study design, listed as a co-applicant on the funding submission, recruited as a member of the TSC and is a co-author of this report. An additional stroke survivor was recruited onto the TSC which convened regularly throughout the course of the trial. Service users (and carers) were directly involved in developing the written material contained in the study protocol and participant information sheets. All support for patient and carer involvement was provided by the Stroke Research Network members. The Stroke Research Network team had expertise in training and supporting service users (and carers) for involvement in NHS, service evaluation and development. A wider patient/carer audience was consulted about the findings and recommendations drawn from the project. This occurred at meetings convened by West Midlands Stroke Research Network, The Stroke Association and Different Strokes.
Chapter 3 Results
The results are summarised, below, in Box 2.
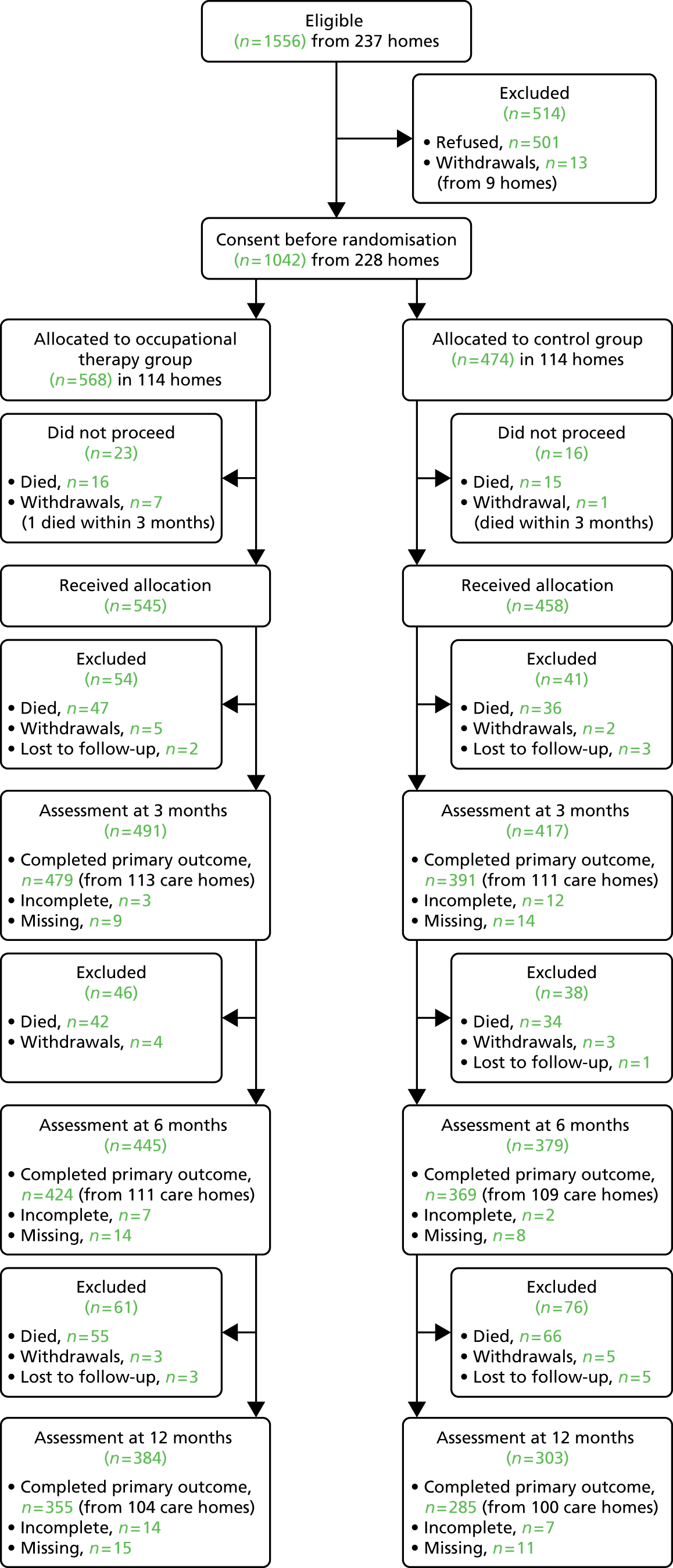
Participating care homes were randomised equally to the two treatment arms (114 in each). The number of participants randomised to the intervention arm was larger than that randomised to the control arm, resulting in 568 residents randomised to the OT group and 474 to the control group. The two groups were comparable with respect to baseline characteristics. The majority of participants recruited were resident in nursing homes (64%). Over 70% of participants were rated as severe or very severe on the BI (primary measure) at baseline.
Primary outcomeAmong surviving participants, similar rates of completion of the BI were observed in each randomisation arm. Overall, 94% of survivors completed the primary outcome measure at 3 months, 94% at 6 months and 88% at 12 months. No significant differences were found in the primary outcome at 3 months. The 95% CI did not contain the clinically important difference of 2 units. The OR for the Barthel composite outcome, which took account of the change in Barthel score from baseline to 3 months, was 0.96. The OR CI at the 95% level crossed the null (0.70 to 1.33). No between-group differences were observed at later end points.
Sensitivity analysisExclusion of scores above 18 or clusters with fewer than three residents did not alter the results. Imputation of missing Barthel scores using three methods (best case, worst case and multiple imputation) did not change the conclusions. None of the exploratory subgroup analyses indicated a significant effect of treatment.
Secondary outcomesNo significant influences of the intervention were found on measures of mobility, depression or HRQoL at any of the follow-up time points.
Adverse eventsNo adverse events attributable to the intervention were reported.
RetentionParticipant retention was good. Among the participants alive at 12 months, 355 out of 407 (87%) in the intervention arm had completed the BI and 285 out of 322 (89%) in the control arm had done so. Lost-to-follow-up data are presented in the CONSORT diagram (see Figure 1). A total of 313 (30%) participants died during the 12-month duration (161 from the intervention arm and 152 from the control arm).
FallsA significantly higher (adjusted) fall rate per resident was reported in the intervention arm (rate ratio 1.74, 95% CI 1.09 to 2.77; p = 0.02). The odds of residents experiencing a fall were not significant between groups at the 0.05 level (OR = 1.55, 95% CI 0.96 to 2.53; p = 0.07).
Sample sizeThe sample size calculation was based on an ICC of 0.4. The unadjusted ICC for the BI in this trial was 0.36 at baseline; however, for BI at 3 months, allowing for the effect of baseline BI score, treatment arm, location and type of care home, the ICC reduced to 0.09.
Randomisation
Participating care homes were randomised between 4 May 2010 and 28 February 2012. Participants were followed up at 3, 6 and 12 months after randomisation.
Recruitment at a cluster level according to stratification factors: geographical location and type of care home
Twelve TACs across England and Wales were involved (with locations at the University of Birmingham, Coventry PCT, Dorset PCT, Wolverhampton PCT, University of Central Lancashire, University of Nottingham, Solent Healthcare PCT, Bournemouth and Poole PCT, Stoke-on-Trent PCT, Taunton PCT, Bangor University and Plymouth PCT).
The distribution of care homes according to the geographical location of the TACs is shown in Table 4. A total of 237 care homes gave consent. The average number of beds in consenting homes was 42 (SD 18.6 beds). Nine homes did not continue to the randomisation stage:
-
One TAC, involving four homes, withdrew prior to randomisation because of administrative problems. As a result, 11 TACs proceeded to the randomisation phase.
-
Four care homes with consent at a managerial level did not provide any consenting participants and were excluded.
-
One home was excluded after the two consenting residents were withdrawn by the care home manager, in order for the participants to receive end-of-life care.
| TAC | Total (%) | Intervention (%) | Control (%) |
|---|---|---|---|
| Bournemouth and Poole PCT | 12 (5) | 7 (6) | 5 (4) |
| University of Central Lancashire | 16 (7) | 8 (7) | 8 (7) |
| Coventry PCT | 14 (6) | 7 (6) | 7 (6) |
| University of Nottingham | 22 (10) | 10 (9) | 12 (11) |
| Plymouth PCT | 14 (6) | 8 (7) | 6 (5) |
| Solent Healthcare PCT | 26 (11) | 13 (11) | 13 (11) |
| Stoke-on-Trent PCT | 10 (4) | 4 (4) | 6 (5) |
| Taunton PCT | 8 (4) | 4 (4) | 4 (4) |
| Bangor University | 17 (7) | 8 (7) | 9 (8) |
| University of Birmingham | 73 (32) | 37 (32) | 36 (32) |
| Wolverhampton PCT | 16 (7) | 8 (7) | 8 (7) |
| Total | 228 | 114 | 114 |
A total of 228 care homes proceeded to the randomisation stage. Care homes were randomised 1 : 1 to receive the intervention or usual care (114 in each arm). The data for the type of care home are presented in Table 5. Of the care homes recruited, 121 (53%) provided nursing care. The distribution of residential and nursing homes was balanced between treatment arms. Retention of care home participation was good throughout the study. Data were collected from 204 homes (89% of homes randomised) at the 12-month end point (104 in the intervention group and 100 in the control group).
| Type of care home | Total (%) | Intervention (%) | Control (%) |
|---|---|---|---|
| Residential | 107 (47) | 53 (46) | 54 (47) |
| Nursing | 121 (53) | 61 (54) | 60 (53) |
Participant recruitment within clusters: sample size
The flow of participants throughout the duration of the trial is depicted in the CONSORT diagram (Figure 1). Within the 237 consenting care homes there were 1556 out of 9840 (16%) eligible residents. The 16% figure represents the prevalence of stroke in the consenting care homes. Of those identified as eligible, 1055 out of 1556 (68%) were registered into the trial and 501 out of 1556 (32%) did not offer consent. A total of 13 participants (nine care homes) did not progress to the randomisation stage, two were withdrawn by the care home manger to receive end-of-life treatment and the remaining 11 participants were excluded because of the withdrawal of the regional TAC described above. Table 6 summarises participant and care home recruitment levels according to regional TACs.
FIGURE 1.
Consolidated Standards of Reporting Trials diagram.
| TAC | Care home recruitment | Participant recruitment | ||||
|---|---|---|---|---|---|---|
| Consenting care homes | Beds in consenting homes | Randomised care homes | Eligible residents screened | Consenting residents | Randomised residents | |
| Bournemouth and Poole PCT | 12 | 434 | 12 | 55 | 48 | 48 |
| University of Central Lancashire | 16 | 954 | 16 | 142 | 86 | 86 |
| Coventry PCT | 14 | 537 | 14 | 107 | 56 | 56 |
| Dorset PCTa | 4 | 179 | 0 | 11 | 11 | 0 |
| University of Nottingham | 22 | 897 | 22 | 175 | 126 | 126 |
| Plymouth PCT | 16 | 556 | 14 | 68 | 40 | 40 |
| Solent Healthcare PCT | 28 | 1006 | 26 | 180 | 110 | 108 |
| Stoke-on-Trent PCT | 10 | 400 | 10 | 76 | 48 | 48 |
| Taunton PCT | 8 | 392 | 8 | 71 | 45 | 45 |
| Bangor University | 17 | 604 | 17 | 119 | 104 | 104 |
| University of Birmingham | 74 | 3098 | 73 | 427 | 323 | 323 |
| Wolverhampton PCT | 16 | 783 | 16 | 125 | 58 | 58 |
| Total | 237 | 9840 | 228 | 1556 | 1055 | 1042 |
Participant recruitment exceeded the original target of 900. More care homes were recruited because the average cluster size was lower than predicted, but comparable between the two arms (intervention mean 5.0 participants, control mean 4.2 participants). The slight over-recruitment of 1042 participants was also because of staggered closure of sites that had already consented residents to the trial. Cluster size information is presented in Table 7. The number of care home residents with a history of stroke was lower than expected. A total of 1042 participants were randomised. More eligible residents resided in clusters randomised to the intervention arm (n = 568) than in clusters randomised to the control arm (n = 474), see Table 8. The disparity in participant numbers between the two treatment arms was a chance occurrence. Consent was obtained prior to randomisation. There were more eligible residents in care homes randomised to the intervention arm than in care homes randomised to the control arm. A larger percentage of participating care homes (53%) provided nursing care (see Table 5), and from these a larger percentage of participants (64%) were recruited (see Table 8).
| Cluster size (number of participants) | Frequency (%) | Randomisation arm | |
|---|---|---|---|
| Intervention, n (%)a | Control, n (%)a | ||
| 1 | 29 (13) | 11 (10) | 18 (16) |
| 2 | 38 (17) | 17 (15) | 21 (18) |
| 3 | 28 (12) | 13 (11) | 15 (13) |
| 4 | 43 (19) | 20 (18) | 23 (20) |
| 5 | 30 (13) | 18 (16) | 12 (11) |
| 6 | 15 (7) | 9 (8) | 6 (5) |
| 7 | 10 (4) | 5 (4) | 5 (4) |
| 8 | 11 (5) | 6 (5) | 5 (4) |
| 9 | 9 (4) | 8 (7) | 1 (1) |
| 10 | 4 (2) | 3 (3) | 1 (1) |
| 11 | 3 (1) | 0 (0) | 3 (3) |
| 12 | 2 (1) | 1 (1) | 1 (1) |
| 13 | 1 (0) | 0 (0) | 1 (1) |
| 14 | 1 (0) | 0 (0) | 1 (1) |
| 15 | 1 (0) | 0 (0) | 1 (1) |
| 19 | 1 (0) | 1 (1) | 0 (0) |
| 21 | 1 (0) | 1 (1) | 0 (0) |
| 23 | 1 (0) | 1 (1) | 0 (0) |
| Total number of care homes | 228 | 114 | 114 |
| Median participants per home (interquartile range) | 4 (2–6) | 4 (3–6) | 4 (2–5) |
| Mean participants per home (SD) | 4.6 (3.3) | 5 (3.7) | 4.2 (3.0) |
| Type of care home | Randomisation arm | |
|---|---|---|
| Intervention (N = 568), n (%) | Control (N = 474), n (%) | |
| Residential | 207 (36) | 166 (35) |
| Nursing | 361 (64) | 308 (65) |
Consent type
The majority of participants (61%) required the assistance of a consultee to offer consent on their behalf,68 indicating a lack of autonomy. The level of need for consultee assistance was similar across treatment arms (Table 9).
| Consenter | Intervention (N = 568), n (%) | Control (N = 474), n (%) | Total (N = 1042), n (%) |
|---|---|---|---|
| Participant | 230 (40) | 174 (37) | 404 (39) |
| Consultee | 338 (60) | 300 (63) | 638 (61) |
Participant characteristics
The demographic information listed in Table 10 indicates that age, sex and ethnicity were balanced across treatment arms. Of the 1042 participants, 962 (92%) were white and 665 (64%) were female. The mean age of all participants was 82.9 years (SD 9.2 years, range 43–102 years). The distribution of age groups was similar across care home type and randomisation arm (Table 11). Stroke or TIA was present in care home notes for all participants; however, confirmation from GP surgeries was received in 721 out of 1042 (69%) cases. The details regarding the distribution of stroke and TIA among participants are given in Table 12.
| Variable | Randomisation arm | |
|---|---|---|
| Intervention (n = 568) | Control (n = 474) | |
| Mean age in years (SD) | 82.8 (9.1) | 83.1 (9.4) |
| Male, n (%) | 203 (35.7) | 174 (36.7) |
| Ethnicity, n (%) | ||
| White | 517 (91.0) | 445 (93.9) |
| Mixed | 2 (0.4) | 3 (0.6) |
| Asian | 10 (1.8) | 5 (1.1) |
| Black | 13 (2.3) | 7 (1.5) |
| Chinese | 1 (0.2) | 1 (0.2) |
| Unknown | 25 (4.4) | 13 (2.7) |
| Age group (years) | Type of care home | |||
|---|---|---|---|---|
| Residential | Nursing | |||
| Intervention (n = 207), n (%) | Control (n = 166), n (%) | Intervention (n = 361), n (%) | Control (n = 308), n (%) | |
| Under 65 | 10 (4.8) | 5 (3.1) | 17 (4.7) | 11 (3.6) |
| 65–69 | 9 (4.4) | 0 (0) | 14 (3.9) | 22 (7.1) |
| 70–74 | 13 (6.3) | 11 (6.6) | 30 (8.3) | 25 (8.1) |
| 75–79 | 28 (13.5) | 25 (15) | 38 (10.5) | 55 (17.9) |
| 80–84 | 43 (20.8) | 26 (15.7) | 85 (23.6) | 55 (17.9) |
| 85–89 | 52 (25.1) | 33 (19.9) | 105 (29.1) | 77 (25) |
| 90–94 | 33 (15.9) | 43 (25.9) | 53 (14.7) | 44 (14.3) |
| ≥ 95 | 19 (9.2) | 21 (12.7) | 17 (4.7) | 19 (6.2) |
| Not known | 0 (0) | 2 (1.2) | 2 (0.6) | 0 (0) |
| Stroke detail | Randomisation arm | |
|---|---|---|
| Intervention (n = 568), n (%) | Control (n = 474), n (%) | |
| Confirmed stroke | 329 (58) | 317 (67) |
| Confirmed TIA | 47 (8) | 28 (6) |
| Suspected stroke/TIA | 73 (13) | 66 (14) |
| Missing | 119 (21) | 63 (13) |
| Locus of stroke in confirmed cases | (n = 318) | (n = 283) |
| Left hemisphere | 161 (51) | 154 (54) |
| Right hemisphere | 148 (46) | 108 (39) |
| Bilateral | 9 (3) | 21 (7) |
Time of stroke and care home length of stay
The median length of stay prior to trial randomisation was 2.35 years (interquartile range 0.96–4.49 years) for the intervention group and 2.16 years (interquartile range 1.04–4.12 years) for the control group.
The exact dates of participants’ stroke obtained from medical records via correspondence with GP surgeries were limited to approximately 50% of all participants. Date of stroke was confirmed for 225 out of 568 (40%) participants in the intervention arm and 250 out of 474 (53%) participants in the control arm. The median duration between residents’ stroke and trial randomisation was 3.17 years (interquartile range 1.30–7.12 years) in the intervention arm and 2.82 years (interquartile range 1.18–5.83 years) in the control arm.
The prevalence of participant comorbidities reported in Table 13 was similar between treatment arms. High percentages of comorbidity were recorded in the cardiovascular, neurological and musculoskeletal categories, as well as in history of falls.
| Comorbidity | Randomisation arm | |
|---|---|---|
| Intervention (n = 568), n (%) | Control (n = 474), n (%) | |
| Cardiovascular disease | 342/530 (64.5) | 278/446 (62.3) |
| Respiratory disease | 90/484 (18.6) | 76/415 (18.3) |
| Hepatic disease | 6/471 (1.3) | 8/406 (2.0) |
| Gastrointestinal disease | 96/485 (19.8) | 78/421 (18.5) |
| Renal disease | 38/461 (8.2) | 51/410 (12.4) |
| Urological disease | 92/475 (19.4) | 80/411 (19.5) |
| Neurological disease | 371/505 (73.5) | 296/424 (69.8) |
| Musculoskeletal problems | 214/474 (45.2) | 199/425 (46.8) |
| Dermatological problems | 86/459 (18.7) | 71/403 (17.6) |
| Fall history | 203/495 (41.0) | 200/427 (46.8) |
Screening measures administered at baseline
Table 14 lists the results from screening measures administered at baseline. The Sheffield Screening test for Acquired Language Disorders was completed by 424 out of 568 (75%) participants randomised to the intervention arm and by 374 out of 474 (79%) participants in the control arm. In total, 458 out of 798 (57%) participants across both randomisation arms scored below 15, indicating significant language impairment. 74 The MMSE screening test was completed by 398 out of 568 (70%) participants in the intervention arm and 362 out of 474 (76%) in the control arm. Overall, 542 out of 760 (71%) participants across both treatment arms scored in the range signifying cognitive impairment. 87 Results from these screening measures were incorporated into the exploratory subgroup analyses.
| Screening measure | Randomisation arm | |
|---|---|---|
| Intervention | Control | |
| Sheffield screening test (0–20) | ||
| n | 424 | 374 |
| Mean (SD) | 10.9 (7.1) | 11.0 (6.9) |
| Median (interquartile range) | 13 (3–17) | 13 (5–17) |
| Language impairment (< 15), n (%) | 245 (57.8) | 213 (57.0) |
| MMSE (0–30) | ||
| n | 398 | 362 |
| Mean (SD) | 13.6 (9.5) | 13.2 (9.0) |
| Median (interquartile range) | 14.5 (4–22) | 14 (6–21) |
| Cognitive impairment (0–20), n (%) | 279 (70.1) | 263 (72.7) |
| Borderline cognitive impairment (21–23), n (%) | 40 (10.1) | 42 (11.6) |
Retention
Retention throughout the trial was good. In the intervention arm, 21 participants moved home during the trial, three died, one was lost to follow-up and the remainder (17) completed outcome assessments at the 12-month end point. In the control arm, 16 participants moved homes, all of whom provided data at the 12-month end point (see Appendix 15).
Withdrawal and lost-to-follow-up data, as well as completion rates for the primary outcome measure at all time points, are presented in Table 15. A total of 313 out of 1042 (30%) participants died during the 12-month trial duration. The percentage of participants that died were balanced between treatment arms at all time points (see Table 14). Two participants (one in each treatment arm) were withdrawn by the care home manager to receive end-of-life care and died within 3 months. These two participants have been included in the number of deaths (see Figure 1 and Table 15).
| Follow-up | BI | Intervention (N = 568), n (%) | Control (N = 474), n (%) |
|---|---|---|---|
| Baseline | Fully completed | 562 (99) | 467 (98.5) |
| Partially completed | 3 (0.5) | 7 (1.5) | |
| Missing | 3 (0.5) | 0 (0) | |
| 3 months | Fully completed | 479 (84.3) | 391 (82.5) |
| Partially completed | 3 (0.5) | 12 (2.5) | |
| Missing | 9 (1.6) | 14 (2.8) | |
| Withdrew/lost | 13 (2.3) | 5 (1.1) | |
| Died | 64 (11.3) | 52 (11.0) | |
| 6 months | Fully completed | 424 (74.6) | 369 (77.8) |
| Partially completed | 7 (1.2) | 2 (0.4) | |
| Missing | 14 (2.5) | 8 (1.7) | |
| Withdrew/lost | 16 (2.8) | 9 (1.9) | |
| Died | 106 (18.7) | 86 (18.1) | |
| 12 months | Fully completed | 355 (62.5) | 285 (60.1) |
| Partially completed | 14 (2.5) | 7 (1.5) | |
| Missing | 15 (2.6) | 11 (2.3) | |
| Withdrew/lost | 23 (4.0) | 19 (4.0) | |
| Died | 161 (28.3) | 152 (32.1) |
Baseline primary and secondary measures
Primary measure
At baseline the BI was completed by 1029 out of 1042 (99%) participants. 76–78 Performance on the BI indicated that 735 out of 1029 (71%) participants in both treatment arms scored in the severe or very severe range, signifying significant disability. Table 16 indicates that levels of impairment were comparable between randomisation arms. The mean BI score for both treatment arms was in the severe range (see Table 16). Across treatment arms, 560 out of 663 (84%) participants resident in care homes providing nursing care scored in the severe or very severe range (Table 17).
| BI score | Randomisation arm | |
|---|---|---|
| Intervention (N = 562), n (%) | Control (N = 467), n (%) | |
| 0–4 (very severe) | 268 (47.7) | 234 (50.1) |
| 5–9 (severe) | 129 (23.0) | 104 (22.3) |
| 10–14 (moderate) | 91 (16.2) | 76 (16.3) |
| 15–19 (mild) | 64 (11.4) | 46 (9.9) |
| 20 (independent) | 10 (1.8) | 7 (1.5) |
| Mean (SD) | 6.5 (5.8) | 6.3 (5.7) |
| Median (interquartile range) | 5 (1–11) | 4 (1–10) |
| BI | Type of care home | |||
|---|---|---|---|---|
| Residential | Nursing | |||
| Randomisation arm | Randomisation arm | |||
| Intervention (N = 204), n (%) | Control (N = 162), n (%) | Intervention (N = 358), n (%) | Control (N = 305), n (%) | |
| Very severe | 62 (30.4) | 31 (19.1) | 206 (57.5) | 203 (66.6) |
| Severe | 42 (20.6) | 40 (24.7) | 87 (24.4) | 64 (21) |
| Moderate | 51 (25) | 49 (30.3) | 40 (11.2) | 27 (8.9) |
| Mild | 42 (20.6) | 35 (21.6) | 22 (6.2) | 11 (3.6) |
| Independent | 7 (3.4) | 7 (4.3) | 3 (0.8) | 0 (0) |
Secondary measures
Rivermead Mobility Index
Baseline RMI scores are presented in Table 18. The measure was completed by 1033 out of 1042 (99%) participants. The mean mobility score was low and comparable between treatment groups.
| RMIa | Randomisation arm | |
|---|---|---|
| Intervention (n = 557) | Control (n = 456) | |
| Mean (SD) | 3.12 (3.81) | 2.85 (3.70) |
| Median (interquartile range) | 1 (0–6) | 1 (0–5) |
Figure 2 displays the baseline RMI data plotted as a function of the baseline BI data. The plot indicates that a high proportion of participants had significant limitations on functional activity at the beginning of the trial. This plot includes data from 1012 out of 1042 (97%) participants who completed both baseline BI and RMI measures. A total of 493 out of 1012 (49%) participants scored below 4 on both the RMI and the BI, suggesting that half of the sampled population had severe limitations on engaging with personal ADL and severe limitations on mobility (see Table 44 in Appendix 16).
FIGURE 2.
The relationship between baseline RMI scores and baseline BI scores (n = 1012). BI 0–20, 20 signifying maximum ability; RMI 0–15, 15 signifying maximum ability.
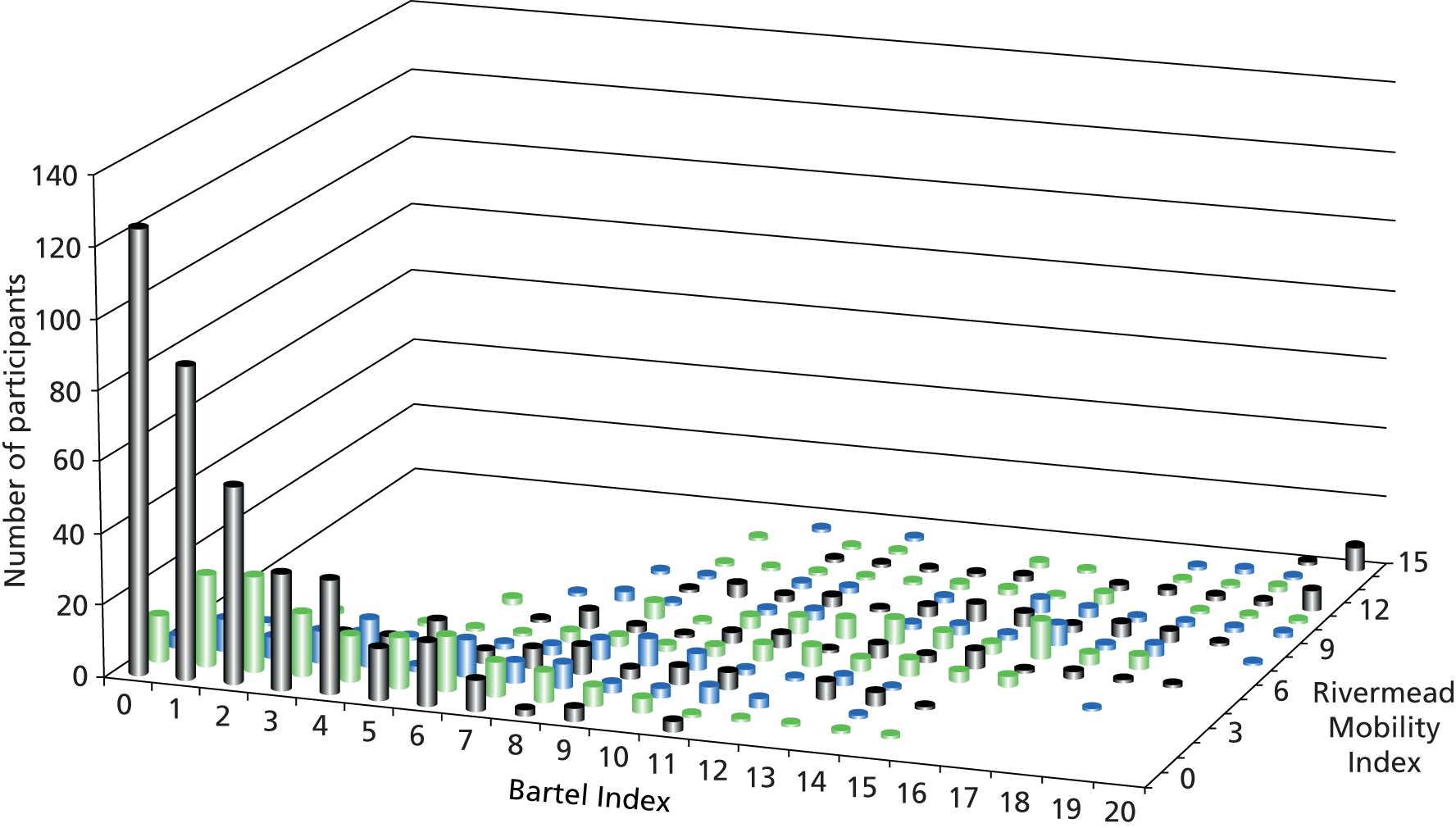
Baseline GDS-15 scores were recorded from 913 out of 1042 (88%) participants (Table 19). The measures of central tendency for the baseline GDS-15 scores were in the range indicative of mild depression, and comparable between randomisation arms. Twenty-four per cent of participants scored in the 10–15 score range, suggesting severe depression.
| GDS-15 score | Randomisation arm | |
|---|---|---|
| Intervention (N = 498), n (%) | Control (N = 415), n (%) | |
| 0–4 (normal) | 157 (32) | 131 (32) |
| 5–9 (mild depression) | 205 (41) | 200 (48) |
| 10–15 (severe depression) | 136 (27) | 84 (20) |
| Mean (SD) | 6.8 (3.9) | 6.4 (3.5) |
| Median (interquartile range) | 6.0 (4–10) | 6.0 (4–9) |
Intervention
Overall, 2538 visits were made to 498 residents in the intervention arm (mean 5.1 visits, SD 3.0 visits). Total therapy time was 1724 hours. Median session duration was 30 minutes (interquartile range 15–60 minutes). Therapy was administered according to categories. Table 20 shows a summary of the time allocated to each category.
| Provision of therapy | Detail | |
|---|---|---|
| Participants | n = 498 | |
| Visits | 2538 | |
| Mean visits per participant (SD, minimum–maximum) | 5.1 (3.0, 0–18) | |
| Median visits per participant (interquartile range) | 5.0 (3–7) | |
| Total duration of therapy | 103,443 minutes (1724 hours) | |
| Mean duration of therapy per participant (SD, minimum–maximum) | 208 minutes (208 minutes, 10–1380 minutes) | |
| Median duration of therapy per participant (interquartile range) | 145 minutes (85–255 minutes) | |
| Mean visit duration (SD) | 41 minutes (34 minutes) | |
| Median visit duration (interquartile range) | 30 minutes (15–60 minutes) | |
| Category of therapy | Duration | % of total |
| Assessment/reassessment and goal-setting: involving the assessment of a resident’s current levels of functional activity and identifying/modifying goals of therapy | 23,683 minutes | 23 |
| Communication: including listening to residents’ concerns about personal ADL, providing information and guidance to residents, staff, or relatives, initiating referrals to other agencies, and ordering equipment | 50,188 minutes | 49 |
| ADL training (cognitive and functional): involving techniques to assist with feeding, bathing, using the toilet, getting dressed and grooming | 7295 minutes | 7 |
| Transfers and mobility (cognitive and functional): involving bed mobility, standing, walking and transfers to/from a chair | 8415 minutes | 8 |
| Equipment and environment: involving environmental adaptations | 7681 minutes | 7 |
| Other: involving treating impairments directly – such as joint contracture | 6181 minutes | 6 |
The 3-month follow-up
Primary outcome measure
Of the participants alive at 3 months, the BI was completed by 479 out of 504 (95%) in the intervention arm, and 391 out of 422 (93%) in the control arm (870 participants in total). The denominator for these calculations includes those participants who withdrew. Participants for whom BI data were missing or incomplete were excluded from the primary analysis. Following imputation of zero for deaths that occurred prior to 3 months, the unadjusted mean for the intervention arm was 5.39 (SD 5.73) and 4.96 (SD 5.51) for the control arm. No significant differences were observed between groups in BI at the primary outcome end point of 3 months. The adjusted mean difference in BI score between groups was 0.19 points higher in the intervention arm (95% CI –0.33 to 0.70; p = 0.48). The mean was adjusted for baseline score, type of care home and TAC as fixed effects and by care home as a random effect to account for effects of clustering. This difference did not represent a significant impact clinically. 82 The composite BI outcome analysis revealed that in the intervention arm 54% had a poor outcome (BI score change of zero or below) and 15% had a good outcome (BI score increase of two or above), compared with 52% and 14%, respectively, in the control arm. The odds of improvement in outcome were not significantly different between the groups (OR 0.96, CI 0.70 to 1.33) (Table 21).
| BI compositea | Randomisation arm | OR (95% CI)b | p-value | |
|---|---|---|---|---|
| Intervention (N = 540), n (%) | Control (N = 436), n (%) | |||
| Poor | 293 (54.3) | 227 (52.1) | 0.96 (0.70 to 1.33) | 0.81 |
| Moderate | 164 (30.4) | 150 (34.4) | ||
| Good | 83 (15.4) | 59 (13.5) | ||
Secondary outcome measures
Unadjusted means at 3-, 6- and 12-month follow-up for measures assessing mobility (RMI) and mood (GDS-15) are presented in Tables 22 and 23. Summary statistics for the HRQoL data are presented in Chapter 4, Economic evaluation results to prevent duplication. The adjusted analyses, comparing mobility, mood and HRQoL, showed no significant influence of the OT intervention at 3 months (Table 24).
| Assessment | RMI score (0–15) | Randomisation arm | |
|---|---|---|---|
| Intervention | Control | ||
| 3 months | n | 472 | 372 |
| Mean (SD) | 2.80 (3.71) | 2.54 (3.65) | |
| Median (interquartile range) | 1 (0–4.5) | 1 (0–4) | |
| 6 months | n | 427 | 363 |
| Mean (SD) | 2.71 (3.69) | 2.51 (3.57) | |
| Median (interquartile range) | 1 (0–5) | 1 (0–4) | |
| 12 months | n | 360 | 285 |
| Mean (SD) | 2.39 (3.51) | 2.46 (3.70) | |
| Median (interquartile range) | 1 (0–3.5) | 1 (0–4) | |
| Assessment | GDS-15 (0–15) | Randomisation arm | |
|---|---|---|---|
| Intervention | Control | ||
| 3 months | n | 415 | 345 |
| Normal (0–4) | 155 (37.3%) | 141 (40.9%) | |
| Mild depression (5–9) | 172 (41.4%) | 118 (34.2%) | |
| Severe depression (10–15) | 88 (21.2%) | 86 (24.9%) | |
| Mean (SD) | 6.3 (3.8) | 6.3 (3.9) | |
| 6 months | n | 363 | 308 |
| Normal (0–4) | 146 (40.2%) | 114 (37%) | |
| Mild depression (5–9) | 124 (34.2%) | 107 (34.7%) | |
| Severe depression (10–15) | 93 (25.6%) | 87 (28.2%) | |
| Mean (SD) | 6.4 (4.0) | 6.7 (4.1) | |
| 12 months | n | 319 | 237 |
| Normal (0–4) | 131 (41.1%) | 101 (42.6%) | |
| Mild depression (5–9) | 115 (36.1%) | 80 (33.8%) | |
| Severe depression (10–15) | 73 (22.9%) | 56 (23.6%) | |
| Mean (SD) | 6.2 (4.1) | 6.2 (3.8) | |
| Outcome | Randomisation arm | Adjusted ICC (95% CI)b | Baseline ICC (95% CI) | Difference in adjusted means (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| Adjusted mean (SE)a | n | Adjusted mean (SE)a | n | |||||
| RMI | 2.74 (0.11) | 465 | 2.73 (0.12) | 382 | 0.04 (0.01 to 0.15) | 0.28 (0.21 to 0.36) | 0.02 (–0.28 to 0.31) | 0.90 |
| GDS-15 | 6.09 (0.21) | 383 | 6.30 (0.22) | 324 | 0.07 (0.03 to 0.17) | 0.11 (0.06 to 0.18) | –0.21 (–0.76 to 0.33) | 0.44 |
| EQ-5D-3Lc | 0.238 (0.018) | 409 | 0.227 (0.019) | 338 | 0.06 (0.02 to 0.17) | 0.25 (0.18 to 0.33) | 0.011 (–0.037 to 0.059) | 0.65 |
The 6- and 12-month follow-up
Primary and secondary outcome measures
At the two additional end points, the BI data showed no significant differences between groups (see Table 24). There was no evidence of any difference between the arms from the BI composite analyses at 6 and 12 months, presented in Table 25. In addition, the results from the secondary outcome measures assessing mobility (RMI, see Table 22), and mood (GDS-15; see Table 23), showed no significant differences between groups, at the 6- and 12-month follow-up time points (Table 26). The BI scores grouped according to severity rating for both treatment arms across all time points are presented in Figure 3.
FIGURE 3.
Barthel Index of Activities of Daily Living severity ratings at all end points across both treatment arms. (The data are given in Table 45, Appendix 16.)

| BI compositea | Randomisation arm | OR (95% CI)b | p-value | |
|---|---|---|---|---|
| Intervention | Control | |||
| 6-month assessment | ||||
| n | 526 | 449 | 0.95 (0.71 to 1.27) | 0.74 |
| Poor, n (%) | 306 (58.2%) | 269 (59.9%) | ||
| Moderate, n (%) | 161 (30.6%) | 122 (27.2%) | ||
| Good, n (%) | 59 (11.2%) | 58 (12.9%) | ||
| 12-month assessment | ||||
| n | 513 | 432 | 0.84 (0.61 to 1.15) | 0.27 |
| Poor, n (%) | 350 (68.2%) | 314 (72.7%) | ||
| Moderate, n (%) | 121 (23.6%) | 77 (17.8%) | ||
| Good, n (%) | 42 (8.2%) | 41 (9.5%) | ||
| Outcome | Follow-up | Randomisation arm | Difference in adjusted means (95% CI)a | p-valueb | Group × time interaction | |||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| Adjusted mean (SE)a | n | Adjusted mean (SE)a | n | |||||
| BIc | 6 months | 4.78 (0.20) | 525 | 4.78 (0.22) | 448 | 0.004 (–0.52 to 0.53) | 0.99 | 0.35 |
| 12 months | 3.93 (0.21) | 512 | 3.77 (0.22) | 430 | 0.16 (–0.40 to 0.72) | 0.58 | ||
| RMI | 6 months | 2.64 (0.11) | 421 | 2.67 (0.12) | 346 | –0.03 (–0.33 to 0.27) | 0.84 | 0.23 |
| 12 months | 2.19 (0.13) | 354 | 2.46 (0.14) | 271 | –0.26 (–0.62 to 0.09) | 0.15 | ||
| GDS-15 | 6 months | 6.20 (0.21) | 338 | 6.68 (0.22) | 284 | –0.48 (–1.04 to 0.09) | 0.10 | 0.57 |
| 12 months | 6.22 (0.22) | 297 | 6.40 (0.25) | 219 | –0.18 (–0.80 to 0.43) | 0.56 | ||
| EQ-5D-3Ld | 6 months | 0.218 (0.017) | 363 | 0.226 (0.017) | 315 | –0.008 (–0.052 to 0.036) | 0.72 | 0.56 |
| 12 months | 0.202 (0.018) | 316 | 0.184 (0.019) | 244 | 0.018 (–0.031 to 0.067) | 0.48 | ||
Sensitivity analyses
Sensitivity analyses were performed on all primary and secondary outcome measures at the primary end point of 3 months that excluded small clusters of size n < 3 residents. Owing to the number of small clusters with one or two participants, this form of sensitivity analysis was suggested (by the TSC) as an approach to test the validity of the mixed modelling approach. Results were consistent with the main analyses, wherein no statistically significant or clinically important differences were observed (Table 27). In addition, a sensitivity analysis excluding clusters of n < 3 was performed on the composite BI outcome at 3 months. Results were similar to the main analysis, no significant differences between the groups were found (Table 28).
| Outcome | Randomisation arm | Difference in adjusted means (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
| Intervention | Control | |||||
| Adjusted mean (SE)a | n | Adjusted mean (SE)a | n | |||
| Primary | ||||||
| BIb | 5.28 (0.21) | 495 | 5.17 (0.22) | 381 | 0.12 (–0.42 to 0.65) | 0.67 |
| Secondary | ||||||
| RMI | 2.62 (0.11) | 427 | 2.64 (0.12) | 333 | –0.02 (–0.31 to 0.28) | 0.91 |
| GDS-15 | 6.09 (0.24) | 346 | 6.51 (0.25) | 278 | –0.42 (–1.03 to 0.19) | 0.18 |
| EQ-5D-3Lc | 0.23 (0.02) | 375 | 0.22 (0.02) | 292 | 0.01 (–0.04 to 0.06) | 0.63 |
| BI compositea | Randomisation arm | OR (95% CI)b | p-value | |
|---|---|---|---|---|
| Intervention (n = 496), n (%) | Control (n = 381), n (%) | |||
| Poor | 276 (55.7) | 197 (51.7) | 1.06 (0.74 to 1.50) | 0.76 |
| Moderate | 147 (29.6) | 132 (34.7) | ||
| Good | 73 (14.7) | 52 (13.7) | ||
The potential ceiling effect of BI was explored by repeating the primary analyses excluding 52 participants with a baseline BI score of 18 or above. The adjusted BI mean score for the intervention group was 4.96 [standard error (SE) 0.19] and for the control group was 4.82 (SE 0.20), with a difference of 0.12 points (95% CI –0.38 to 0.61 points; p = 0.64), indicating no evidence of a difference between the groups.
A complete case analysis, testing the robustness of the BI analysis, by not imputing zero for those with missing data because of death, gave similar results. The adjusted BI mean score for the intervention group was 6.19 (SE 0.19) and for the control group was 6.04 (SE 0.20), with a difference of 0.15 points (95% CI –0.33 to 0.64 points; p = 0.53).
Further sensitivity analyses of missing data were performed using three methods of imputation [best case (last observation carried forward), worst case (zero) and multiple imputation] of missing BI scores at all end points (Table 29). Data imputation did not change the conclusions.
| Follow-up assessmenta | Imputation method | Randomisation arm | Difference in adjusted means (95% CI) | p-value | |
|---|---|---|---|---|---|
| Intervention, adjusted mean (SE)b | Control, adjusted mean (SE)b | ||||
| 3 months | Best case (LVCF) | 5.36 (0.17) | 5.20 (0.18) | 0.16 (–0.33 to 0.66) | 0.52 |
| Worst case (zero) | 5.10 (0.19) | 4.74 (0.20) | 0.35 (–0.20 to 0.91) | 0.21 | |
| Multiple imputation | 5.32 (0.19) | 5.08 (0.21) | 0.24 (–0.31 to 0.78) | 0.39 | |
| 6 months | Best case (LVCF) | 4.79 (0.18) | 4.71 (0.19) | 0.08 (–0.44 to 0.60) | 0.77 |
| Worst case (zero) | 4.28 (0.20) | 4.47 (0.21) | –0.20 (–0.76 to 0.37) | 0.50 | |
| Multiple imputation | 5.13 (0.18) | 4.93 (0.19) | 0.20 (–0.33 to 0.72) | 0.47 | |
| 12 months | Best case (LVCF) | 3.95 (0.19) | 3.77 (0.20) | 0.18 (–0.37 to 0.73) | 0.53 |
| Worst case (zero) | 3.37 (0.20) | 3.35 (0.22) | 0.02 (–0.57 to 0.60) | 0.96 | |
| Multiple imputation | 4.84 (0.19) | 4.64 (0.19) | 0.20 (–0.32 to 0.73) | 0.45 | |
In order to examine potential ceiling effects, a further analysis considered excluding participants with a baseline BI score of ≥ 18. Fifty-two (5%) participants had a baseline BI score of ≥ 18. Exclusion of these cases from the primary analysis did not change the conclusions (difference in adjusted means 0.12, 95% CI –0.38 to 0.61; p = 0.64).
Subgroup analysis
Subgroup analyses were performed according to a predefined list of variables to further evaluate potential influences of the OT intervention. Analyses considered participants’ age, the type of care home in which participants’ resided, the severity rating of participants’ BI scores, the level of cognitive impairment (derived from the MMSE screening measure), and whether the measures were completed by the participant or a consultee. The forest plot (Figure 4) shows no significant findings across all subgroup interactions (Table 30).
FIGURE 4.
Subgroup analysis: comparison of BI at 3 months. Participant numbers for age and MMSE reflect data missing at baseline.
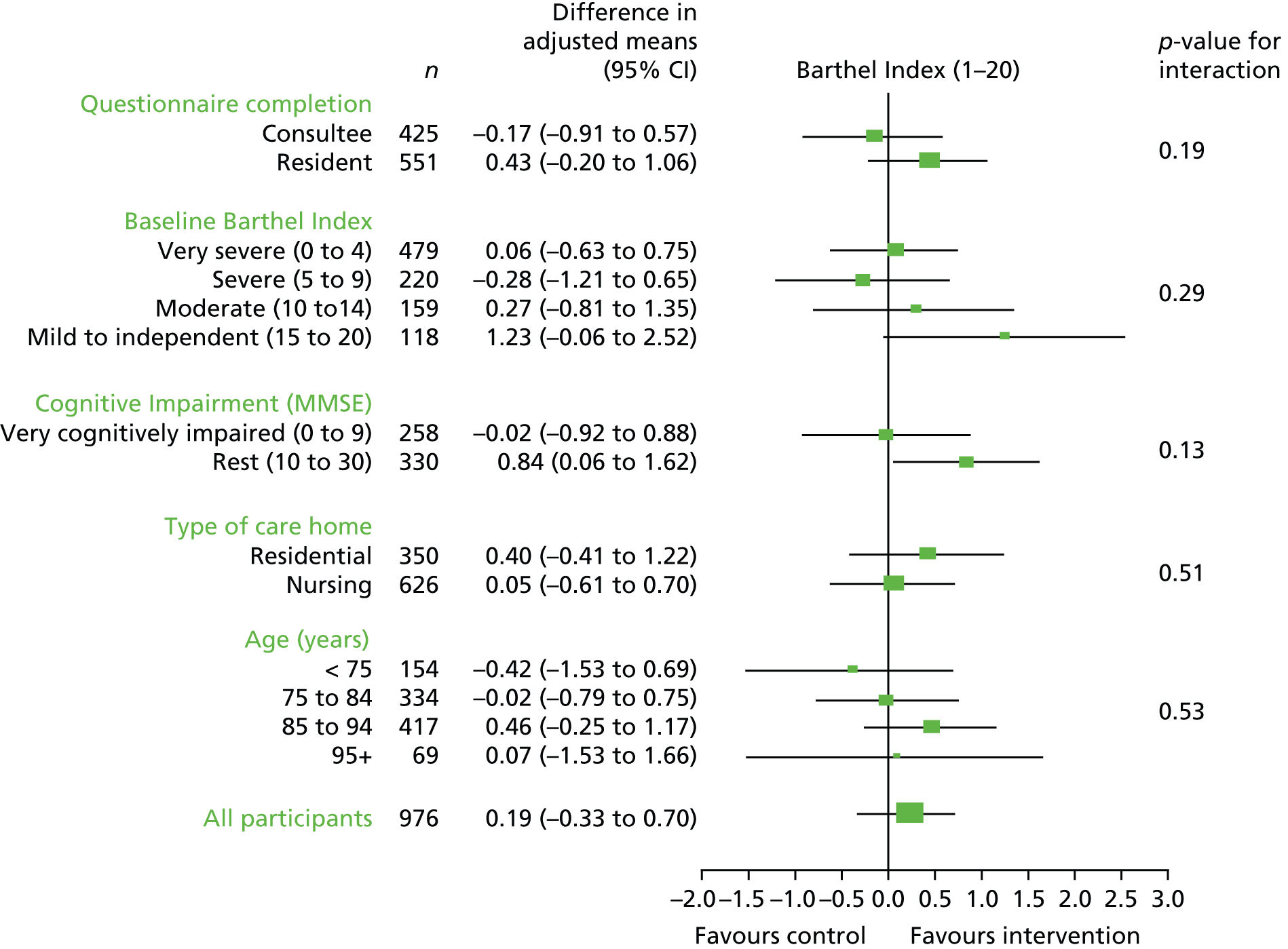
| Outcome | Randomisation arm | Difference in adjusted means (95% CI) | p-value (Interaction) | |||
|---|---|---|---|---|---|---|
| Intervention | Control | |||||
| Adjusted mean (SE)a | n | Adjusted mean (SE)a | n | |||
| Respondent | ||||||
| Consultee | 5.27 (0.30) | 193 | 5.30 (0.32) | 144 | –0.04 (–0.85 to 0.77) | 0.48 |
| Resident | 5.58 (0.23) | 346 | 5.28 (0.33) | 292 | 0.30 (–0.30 to 0.89) | |
| Level of BI | ||||||
| Very severe (0–4) | 5.21 (0.44) | 256 | 5.15 (0.44) | 222 | 0.06 (–0.63 to 0.75) | 0.29 |
| Severe (5–9) | 5.03 (0.33) | 126 | 5.31 (0.37) | 94 | –0.28 (–1.21 to 0.65) | |
| Moderate (10–14) | 5.42 (0.57) | 87 | 5.15 (0.59) | 72 | 0.27 (–0.81 to 1.35) | |
| Mild/independent (15–20) | 7.35 (0.91) | 70 | 6.12 (0.96) | 48 | 1.23 (–0.06 to 2.52) | |
| Level of MMSE | ||||||
| Very cognitively impaired (0–9) | 4.48 (0.36) | 133 | 4.50 (0.36) | 125 | –0.02 (–0.92 to 0.88) | 0.13 |
| Rest (10–30) | 5.49 (0.31) | 175 | 4.65 (0.32) | 155 | 0.84 (0.06 to 1.62) | |
| Type of care home | ||||||
| Residential | 5.70 (0.31) | 197 | 5.30 (0.33) | 153 | 0.40 (–0.41 to 1.22) | 0.50 |
| Nursing | 5.28 (0.24) | 342 | 5.23 (0.26) | 283 | 0.05 (–0.61 to 0.70) | |
| Age group (years) | ||||||
| < 75 | 5.55 (0.38) | 88 | 5.97 (0.44) | 66 | –0.42 (–1.53 to 0.69) | 0.53 |
| 75–84 | 5.41 (0.28) | 189 | 5.44 (0.30) | 145 | –0.02 (–0.79 to 0.75) | |
| 85–94 | 5.55 (0.26) | 228 | 5.09 (0.28) | 188 | 0.46 (–0.25 to 1.17) | |
| ≥ 95 | 5.29 (0.59) | 34 | 5.22 (0.57) | 35 | 0.07 (–1.53 to 1.66) | |
Intracluster correlation coefficient
The sample size calculation was based on an unadjusted ICC of 0.4 from previous pilot studies. 49 The unadjusted ICC for the BI measure in the present trial was 0.36 at baseline and 0.10 for baseline to 3 months. After allowing for the effect of the treatment arm, TACs and type of care home, the ICC for the primary BI measure reduced to 0.09 (95% CI 0.05 to 0.17). Table 31 provides the unadjusted and adjusted ICC results for all measures at the primary 3-month end point.
| Measure | ICC baseline (95% CI) | ICC baseline to 3 months (95% CI) | Adjusteda ICC baseline to 3 months (95% CI) |
|---|---|---|---|
| BI | 0.36 (0.29 to 0.43) | 0.10 (0.06 to 0.18) | 0.09 (0.05 to 0.17) |
| RMI | 0.28 (0.21 to 0.36) | 0.05 (0.02 to 0.15) | 0.04 (0.01 to 0.15) |
| GDS-15 | 0.11 (0.06 to 0.18) | 0.07 (0.02 to 0.16) | 0.07 (0.03 to 0.17) |
| EQ-5D-3Lb | 0.25 (0.18 to 0.33) | 0.10 (0.05 to 0.20) | 0.06 (0.02 to 0.17) |
Adverse events
There were no reported treatment-related adverse events.
Falls
Unadjusted data regarding the number of falls experienced by participants between randomisation arms are presented in Table 32. Comparison of the frequency of falls per resident showed a significantly higher (adjusted) fall rate per resident was reported in the intervention arm [rate ratio 1.74, 95% CI 1.09 to 2.77; p = 0.02] (Table 33). When adjusted for care home, TACs and type of care home there was a suggestion of greater odds of falling in the OT arm (OR 1.55, 95% CI 0.96 to 2.53; p = 0.07) (Table 34).
| Falls | Randomisation arm | |
|---|---|---|
| Intervention | Control | |
| Number of residents with falls data collected | 482 | 408 |
| Number of falls (%) | ||
| 0 | 419 (86.9%) | 373 (91.4%) |
| 1 | 45 (9.3%) | 28 (6.9%) |
| 2 | 12 (2.5%) | 4 (1.0%) |
| 3 | 6 (1.2%) | 3 (0.7%) |
| Mean number of falls per resident (SD) | 0.18 (0.52) | 0.11 (0.40) |
| Number of residents who had a fall resulting in injury and/or medical attention during first 3 months | 63/482 (13.1%) | 35/408 (8.6%) |
| Seen by GP only | 11 | 12 |
| Seen by ambulance staff only | 18 | 4 |
| Seen by GP and ambulance staff | 10 | 4 |
| Not known | 24 | 15 |
| Hospital episode for any reason (including falls) | 107/482 (22.2%) | 90/408 (22.1%) |
| Falls | Randomisation arm | Rate ratioa (95% CI) | p-value | |
|---|---|---|---|---|
| Intervention, adjusted mean (SE) | Control, adjusted mean (SE) | |||
| Number of falls | 0.17 (0.03) | 0.10 (0.02) | 1.74 (1.09 to 2.77) | 0.02 |
| Number of falls | Randomisation arm | ORa (95% CI) | p-value | |
|---|---|---|---|---|
| Intervention, adjusted % (SE) | Control, adjusted % (SE) | |||
| Proportion having a fall | 12.6 (2.2) | 8.5 (1.8) | 1.55 (0.96 to 2.53) | 0.07 |
Process evaluation: summary
A more detailed report of the process evaluation is reported elsewhere. 88 Qualitative evaluation of intervention fidelity was also investigated in semistructured interviews (n = 17) with all occupational therapists, and critical incident reports from the trial (n = 20). These data demonstrated four identified mechanisms through which intervention fidelity was maintained:
-
Occupational therapists described managing a shift from their earlier roles as therapists to their ‘new’ role as experimental occupational therapists within a clinical trial. This was characterised as balancing work in which, over time, they had developed a sense of equilibrium between their professional responsibility as therapists and the tightly defined therapy outlined within the study protocol.
-
A considerable amount of time was spent by some therapists building rapport with care home staff, developing an organisational context more conducive to rehabilitation in general and the trial intervention in particular. This was felt to be important in establishing the foundations on which trial interventions could then be established. One strategy through which this mechanism operated was through therapists ‘mucking in’ and working as a team with members of care home staff.
-
The work focused on re-engineering the personal environments of care home residents. The work that therapists undertook to re-engineer the care home environment around their rehabilitation plans for patients was completed at different degrees of scale, depending on local circumstances. Generally, therapists were careful to propose realistic intervention plans that could be carried out within available resources such as funding, skills availability and access to external services. Through careful assessment and the provision of aids and minor environmental adaptations, the trial interventions intended to provide patients with an environment that best matched their needs.
-
The data clearly demonstrate that therapists were not passive participants in the delivery of the OTCH interventions. Therapists appeared to have learned through their experience of the interventions over time with individual patients and across waves of recruitment within the trial. Therapists modified the way they worked with care homes and patients, adapting to new situations and learning how to carry out their work more effectively. This was important in enabling therapists’ confidence in their work within the trial to grow with time.
These findings from the OTCH process evaluation characterise the real-world nature of fidelity within the OTCH rehabilitation intervention, and specifically the negotiated nature of implementation within clinical settings, and around individual patients’ needs.
Chapter 4 Economic evaluation: methods and results
Overview
To assess the economic viability of delivering OT to stroke survivors in care homes, we conducted a within-trial cost–utility analysis. Costs and quality-adjusted life-years (QALYs) for individuals in the OT intervention group were compared with equivalent data for those receiving usual care. For our base case, we conducted intention-to-treat analysis using ‘complete case’ data. This included participants who died during follow-up, provided that they had returned all costs and outcomes data in the intervention period before their death. As all trial follow-up was completed within 12 months, no discounting was applied to either costs or outcomes.
Estimating costs
In line with the National Institute for Health and Care Excellence methods guide,89 we sought to estimate costs from an NHS and Personal Social Services perspective. This included the cost of the intervention, hospital contacts and community health and social services, and any ADL equipment costs incurred within the intervention or as part of usual care. In deriving costs for the OT intervention, we considered staff training (for therapists who provided the intervention), care home staff training (workshops), participant contact and non-contact time, and equipment and travel costs. Each therapist in the study team completed a ‘Treatment Log’ timesheet (see Appendix 10) to record the number of minutes spent on specific aspects of therapy (such as communication, adaptive equipment or cognitive training) at each session with each participant. To estimate non-contact time spent on the intervention, we also interviewed by telephone two senior occupational therapists who delivered the intervention and training for all aspects of the study. Cost assumptions used here were based on information retrieved in these discussions and confirmed with the principal investigator.
Across all sites, 29 occupational therapists delivered the OTCH intervention. All study staff attended a group training day at the central site (Birmingham). Two such days took place, each run by three senior occupational therapists. Training included adapting OT for the care home environment. Costs were apportioned equally to all participants in the intervention arm. This was estimated as a full working day (7.5 hours) for each of the 29 OT staff (NHS band 6), with average travel to Birmingham estimated as 100 miles. Six days of senior occupational therapist time was also included.
For the workshops attended by nursing and care staff in each care home, it was estimated that the three course co-ordinators (senior occupational therapists) spent one week developing training documents. Although some care homes may have had repeat training (e.g. because of high staff turnover) it was assumed that all care staff from each care home in the intervention arm attended the workshop only once. Seminars lasted 2 hours and were delivered by one senior occupational therapist. Costs were apportioned equally to all participants in the intervention arm. In an additional sensitivity analysis, we included the opportunity cost of time spent by care home staff attending these workshops (assuming workshops would be delivered once every 3 years).
The OTCH intervention was delivered by NHS community occupational therapists, with level of experience ranging from band 5 to 7. In deriving costs, we assumed that the intervention was delivered only by occupational therapists at NHS band 6. 90 For every intervention session spent with each individual participant, the approximate time (in minutes) spent on specific tasks was recorded in the treatment log. As the treatment log recorded only face-to-face time with participants, we ascribed additional time per task for non-contact time – including initial risk assessment for each participant, general administration, liaising with care home staff and collecting and demonstrating the equipment to care home staff. Based on estimates from both of the therapists interviewed and estimates reported in Personal Social Services Research Unit (PSSRU) costs,91 it was estimated that for every hour of contact time there was an additional 40 minutes (67% of contact time) of non-contact time.
Travel time
Although the intervention was delivered to individuals, and not as a group, therapists tried to see all participants in a specific care home on the same day each week. Economies of scale were therefore possible when apportioning travel costs to care homes in which there was more than one participant who received the intervention on the same day. As the date of each OTCH session was recorded, we could monitor how many participants were treated every time a therapist attended a particular care home. Travel costs were ascribed to each care home attendance. These were then divided among each of the participants who were treated in the care home on that date. We estimated each care home visit would entail a 10-mile round trip, at a cost to the NHS of 54p/mile. 92 We also estimated and attributed costs to travel time (30 minutes per round trip).
Finally, within the OTCH intervention, there was provision to fund adaptive equipment and aids to daily living such as cutlery, arm supports or palm protectors. We were unable to retrieve equipment costs from all centres. Instead, we used costs from two larger centres (Birmingham and Nottingham). The total cost of all equipment was estimated for these two centres and then divided by the number of sessions in which equipment provision was noted. Where time spent with adaptive equipment was recorded on the treatment log, this mean equipment cost was added to the cost of that session.
Non-intervention NHS costs
Care home staff were asked to complete Health Resource Use Questionnaires (see Appendix 17) for each resident at months 3, 6 and 12, based on care home records. We did not have access to routine primary care medical records data. Health resource use included visits to GP and other community health-care workers, outpatient appointments, accident and emergency (A&E), inpatient stays and any adaptive equipment paid for by care homes, social services or privately funded which had not been ordered as part of the OT intervention. Unit costs for health professional contacts were obtained from the national health and social care services reference costs,93 and PSSRU for the financial year 2010–11,91 and are reported in Table 35. Unit costs for adaptive equipment were derived from the Nottingham Integrated Community Equipment Service (ICES)/NRS Healthcare catalogue (www.nrs-uk.co.uk), assuming private (non-discounted) prices for all items. When the item listed was ambiguous or unknown, we used mean imputation of all other equipment. We assumed a lifespan of 5 years for all adaptive equipment94 and applied a discount rate of 3.5%. 95
| Item | Estimated unit cost per visit (£) | Source |
|---|---|---|
| GP visit | 36.00 | PSSRU91 |
| District/practice nurse | 13.18 | |
| Physiotherapist | 34.00 | |
| Social worker | 25.16 | |
| Chiropodist | 47.00 | |
| Speech and language therapist | 35.00 | |
| Dietitian | 35.00 | |
| Dentist | 78.00 | |
| Psychiatrist | 209.00 | |
| Clinical psychologist | 112.50 | |
| Community psychiatric nurse | 76.00 | |
| Other health professionals | 27.39 (range 15–115) | |
| A&E visita | 116.90 | NHS Reference Costs 2011–12 93 |
| Outpatient appointment | 104.52 (range 21–205) | |
| Cost per day in hospitalb | 543.55 | |
| Cost per day in hospital (day case)c | 663.68 | |
| OTCH therapist cost per hour (NHS band 6) | 50.00 | PSSRU91 |
Overall costs
Total costs were calculated by combining all intervention costs, non-intervention health resource use costs and the cost of equipment provision. As no one in the control arm received any form of intervention during the trial period, only wider NHS resource use was valued in this group.
Measuring outcomes
Our principal measure of benefit was expressed as QALYs. Health utility was estimated using the EQ-5D-3L,96 a generic measure of HRQoL that generates a single index score. This outcome measure has been successfully applied in previous studies with nursing home residents. 97,98 We applied the Measurement and Valuation of Health Group’s A1 tariff, which used time trade-off data obtained from a sample of approximately 3000 members of the general population in UK. 99 Participants were asked to complete (self-complete or by proxy) the EQ-5D-3L questionnaire at baseline and at 3, 6 and 12 months. Life-years were weighted by these utility scores and linear interpolation was used to generate QALYs per patient using area under the curve methods. If a participant died during the follow-up period, EQ-5D-3L values (and costs) were set to zero at the point of death.
Analysis
The use of regression analysis is advocated to account for potential baseline differences and/or confounders when comparing costs and outcomes between treatment arms, and is essential to formally take account of the cluster-randomised design. 100 We regressed (1) costs and (2) QALYs against treatment arm with covariate adjustment for sex, age and baseline EQ-5D-3L, with cluster-adjustment based on care home. The regression coefficients for costs and outcomes were then used to estimate the incremental cost-effectiveness ratio (ICER), dividing the mean difference in costs by the mean difference in QALYs between the groups. In line with National Institute for Health and Care Excellence guidance, we compared ICERs with a cost-effectiveness threshold (λ) of £20,000 and £30,000 per QALY. 89
Uncertainty and sensitivity analysis
To obtain a graphical representation of the sampling uncertainty of our cost-effectiveness estimates, incremental costs and incremental QALY estimates were generated using bootstrapped output (1000 replications) from our cost and outcome regressions. We plotted our bootstrap samples on a cost-effectiveness plane, showing the spread of incremental costs and incremental effectiveness across four quadrants. Plots on the north-west quadrant are said to be ‘dominated’ (less effective and more costly than usual care), whereas south-eastern plots are said to be dominant (more effective and less costly than usual care). The same bootstrap samples were used to estimate the cost-effectiveness acceptability curve (CEAC) for each group, where the CEAC depicts the probability that an intervention is cost-effective at varying levels of λ. 101
To assess the robustness of the analysis to changes of key input values and assumptions, we considered the following sensitivity analyses:
-
Excluding participants with extremely high costs (> £20,000). The rationale for this is that a small number of very high-cost participants may overinfluence total costs.
-
Including selected societal costs, specifically time spent by care home staff during OTCH training workshops and non-intervention adaptive equipment which may have been privately provided.
Cost-effectiveness of the full data set (n = 1042) following multiple imputation of missing data. Multiple imputation was performed in a single model using the mi impute command in Stata version 12. The model included predictors of total costs (health-care resource utilisation costs at 3 months, 6 months and 12 months), predictors of outcomes (EQ-5D-3L and BI at baseline and at 3 months, 6 months and 12 months), treatment group, care home (cluster), age, sex, death status and withdrawn status. Imputation took place in five cycles, the estimates from which were then pooled and calculated using Rubin’s rules. 102 With complete cost and EQ-5D-3L scores at all four time points, we used area under the curve analysis to generate a QALY value for all participants. This enabled paired cost and outcome data for the entire study population.
Economic evaluation results
All treatment logs were returned and intervention costs were therefore available for all participants in the intervention arm (Table 36). The estimated cost of training occupational therapists to deliver the intervention was £28.62 per participant, while providing the training workshop to care home staff was estimated to cost £36.99 (or £69.83 per participant when also including cost of care home staff who received the training). In total, 2538 therapy sessions were recorded in the OTCH intervention, with an average duration of 41 minutes (SD 34.0 minutes) each. The mean number of sessions per participant was 5.1 (SD 3.0 sessions). The cost of the OTCH intervention per participant varied considerably based on the number of therapy sessions, whether or not other participants were also treated in their care home on the same day and how long each session lasted. Seventy (12.3%) participants in the intervention arm did not receive any treatment, whereas the most intensive treatment involved 18 visits in the 3-month period. A large cost contributor in the intervention was travel costs by therapists to each care home; this ranged from £0.00 to £371.62 per participant. In total, we estimated the mean intervention cost per participant in the active treatment arm to be £398.98 (SD £347.00).
| Resource item | Level of resource | Associated unit cost (UK GBP, 2010–11) | Associated total cost | Per-participant cost (n = 568) |
|---|---|---|---|---|
| OT training | ||||
| Provision of training | 45 hours (2 days – three senior therapists) | £50.00 per hour | £2250.00 | £3.96 |
| Receipt of training | 29 staff × 1 day | £50.00 per hour | £10,875.00 | £19.15 |
| 29 staff × 100 miles to/from Birmingham (£0.54/mile) | £108 per person | £3132.00 | £5.51 | |
| Total OT training | £16,257.00 | £28.62 | ||
| Care home staff training | ||||
| Provision of training | 112.5 hours (5 days – three senior therapists) | £50.00 per hour | £5625.00 | £9.90 |
| OT staff × 2-hour workshop to 118 care homes | £50.00 per hour | £11,800 | £20.77 | |
| OT staff travel to 118 care homes | £5.40 mileage; £25 time | £3587.00 | £6.32 | |
| Total workshops | £21,012.00 | £36.99 | ||
| Receipt of training (sensitivity analysis) | 12 staff per home, training once every 3 yearsa | PSSRU 11.6 = home care worker £18/hour | £18,651.00 | £32.84 |
| Total workshops: (sensitivity analysis) | £39,663.00 | £69.83 | ||
| Intervention | ||||
| Contact time | 1724 hours on treatment log | £50.00 per hour | £86,200.00 | £151.76 (£0.00–1150.00) |
| Non-contact time | 67% × 1724 hours on treatment log | £50.00 per hour | £57,754.00 | £101.68 (£0.00–770.00) |
| Travel time | 10 miles, 17 minutes to/from care home | £10.80 mileage; £28.33 travel time | £33,817.00 | £59.54 (£0.00–371.62) |
| Equipment costs | ||||
| Adaptive equipment | 74 items over two large study centres, mean cost £20.75 | £27.16 per participant, with allocated ‘equipment time’ | £11,787.00 | £20.75 (£0.00–217.28) |
| Intervention costs | Mean cost per participant £398.98 (range £0.00–2267.31) | |||
| Control costs | We did not attribute any intervention costs to the control arm | |||
NHS resource use
A substantial proportion (n = 313) of participants in both treatment arms died during the study period, meaning that their follow-up time was < 1 year. In addition, a smaller proportion of participants did not return their questionnaires at all three time points (Table 37). Health resource use was recorded for 388.5 person-years from 511 respondents in the intervention arm and for 317.25 person-years from 424 respondents in the control arm.
| Time point | Intervention health resource use questionnaires returned (% of 568 randomised) | Control health resource use questionnaires returned (% of 474 randomised) |
|---|---|---|
| 3 months | 469 (83%) | 391 (82%) |
| 6 months | 415 (73%) | 356 (75%) |
| 12 months | 335 (59%) | 261 (55%) |
Resource use was broadly similar in the control and intervention homes (Tables 38 and 39). The provision of OT delivered at care homes was not associated with the level of other health-care use, and the cost of the intervention was not offset by lower costs in terms of GP visits, inpatient stays or contact with other therapists. Participants made frequent visits to GPs, chiropodists and GP practice nurses, whereas visits to A&E, outpatient appointments and inpatient stays were relatively infrequent.
| Type of resource use | Resource use (visits reported) | Mean visits per person | Mean difference between groups | |||
|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Mean difference | 95% CI | |
| GP visits | 2961 | 2354 | 6.13 | 5.76 | 0.37 | –0.49 to 1.21 |
| Practice nurse | 1171 | 1024 | 2.44 | 2.53 | –0.09 | –0.99 to 0.81 |
| Physiotherapist | 740 | 895 | 1.53 | 2.19 | –0.66 | –1.62 to 0.30 |
| Social worker | 213 | 162 | 0.44 | 0.40 | 0.04 | –0.08 to 0.17 |
| Chiropodist | 1518 | 1231 | 3.14 | 3.02 | 0.12 | –0.24 to 0.49 |
| Speech therapist | 155 | 116 | 0.32 | 0.28 | 0.04 | –0.14 to 0.21 |
| Dietitian | 147 | 199 | 0.30 | 0.49 | –0.19 | –0.31 to –0.05 |
| Dentist | 323 | 197 | 0.67 | 0.48 | 0.19 | 0.05 to 0.32 |
| Psychiatrist | 44 | 45 | 0.09 | 0.11 | –0.02 | –0.10 to 0.07 |
| Community psychiatric nurse | 71 | 109 | 0.15 | 0.27 | –0.12 | –0.29 to 0.05 |
| Inpatient bed-daysa | 970 | 913 | 2.01 | 2.24 | –0.23 | –1.06 to 0.60 |
| A&E episode | 109 | 94 | 0.23 | 0.23 | 0.00 | –0.07 to 0.06 |
| Outpatient appointments | 663 | 533 | 1.37 | 1.31 | 0.06 | –0.03 to 0.25 |
| Large adaptive equipment | 22 | 26 | 0.06 | 0.08 | –0.02 | – |
| Health resource cost (total over 12 months) | Intervention mean (£, 2011) | Control mean (£, 2011) | Mean difference (£) | 95% CI of difference (£) |
|---|---|---|---|---|
| OTCH intervention costs | 398.98 | 0.00 | 398.98 | 367 to 430 |
| Health professional visit costs | 482.82 | 497.19 | –14.37 | –122 to 108 |
| Inpatient costs | 1003.27 | 1124.05 | –120.78 | –559 to 323 |
| Outpatient costs | 120.64 | 104.54 | 16.10 | –19 to 68 |
| A&E costs | 25.91 | 26.75 | –0.84 | –8 to 7 |
| Total health resource costs | 2031.61 | 1752.52 | 279.09 | –217 to 714 |
| Care home staff traininga | 32.84 | 0.00 | 32.84 | 33 to 33 |
| Adaptive equipment | 13.87 | 24.47 | –10.60 | –33 to 13 |
| Selected societal costs | 2078.32 | 1777.00 | 301.32 | –187 to 745 |
Quality-adjusted life-years
Outcomes data, namely QALYs based on the EQ-5D-3L, are reported in Tables 40 and 41. Owing to the high mortality rates in both arms, we report the patient-reported outcomes and also the adjusted outcomes when deaths have been included. The inclusion of patients who died lowered the overall QALYs in both treatment arms, but the difference between arms remained negligible.
| Time point | n | Intervention EQ-5D-3L tariff unadjusted mean (SD) | n | Control EQ-5D-3L tariff unadjusted mean (SD) |
|---|---|---|---|---|
| Baseline | 506 | 0.198 (0.38) | 421 | 0.236 (0.36) |
| 3 months | 433 | 0.212 (0.37) | 365 | 0.194 (0.37) |
| 6 months | 392 | 0.196 (0.37) | 341 | 0.200 (0.36) |
| 12 months | 342 | 0.183 (0.37) | 265 | 0.183 (0.34) |
| QALYs gained over 1 year | 285 | 0.233 (0.33) | 221 | 0.235 (0.32) |
| Time point | n | Intervention EQ-5D-3L tariff unadjusted mean (SD) | n | Control EQ-5D-3L tariff unadjusted mean (SD) |
|---|---|---|---|---|
| Baseline | 523 | 0.198 (0.38) | 437 | 0.236 (0.36) |
| 3 months | 497 | 0.176 (0.35) | 417 | 0.168 (0.35) |
| 6 months | 498 | 0.156 (0.33) | 427 | 0.162 (0.33) |
| 12 months | 503 | 0.125 (0.32) | 417 | 0.120 (0.29) |
| QALYs gained over 1 year | 446 | 0.162 (0.30) | 373 | 0.153 (0.27) |
Cost–utility analysis
Excluding participants for whom we did not have complete paired costs and outcomes data, the remaining ‘complete case’ data set, which was used in subsequent regression analyses, consisted of 581 out of 1042 (55.8%) participants (329 out of 568 in the intervention arm; 252 out of 474 in the control arm) in 177 out of 228 care homes (77.6%). The full data set (1042 participants) was considered in subsequent sensitivity analysis, following multiple imputation.
Total costs and QALYs were assessed using ordinary least squares regression, with cluster option to account for randomisation at care home level. This produced a mean incremental cost of £438.78 (95% CI –£360.89 to £1238.46). The mean incremental QALY gain was 0.009, with wide CIs (95% CI –0.030 to 0.048). Neither the mean incremental cost nor the mean incremental QALYs reached an arbitrary 5% significance level. The ICER was estimated to be £49,825.
A summary of costs and effects at base-case and sensitivity analyses, along with cost-effectiveness ratios, is shown in Table 42. In sensitivity analysis, removal of high-cost participants did not change overall cost-effectiveness results, with both incremental costs and incremental QALYs reduced very slightly. Inclusion of care worker time increased intervention costs slightly without affecting outcomes; therefore, the cost-effectiveness of intervention was less favourable.
| Scenarios | Complete case data set (n = 581) | ||||
|---|---|---|---|---|---|
| Incremental cost (£) | 95% CI (£) | Incremental QALY | 95% CI | ICER | |
| Base case (n = 581) | 438.78 | –360.89 to 1238.46 | 0.009 | –0.030 to 0.048 | £49,824.81 |
| Remove high-cost patients (n = 573) | 412.71 | –77.642 to 903.06 | 0.008 | –0.031 to 0.048 | £49,402.53 |
| Include social care costs (n = 581) | 469.23 | –271.25 to 1209.72 | 0.009 | –0.030 to 0.048 | £53,282.71 |
| Imputed data set n = 1042 × 5 iterations | |||||
| Imputed data set (n = 1040) | 339.19 | –133.60 to 811.98 | 0.013 | –0.015 to 0.041 | £26,769.91 |
When we repeated our regression using imputed data sets, incremental costs were slightly lower at £339 (95% CI –£133.60 to £811.98). Incremental QALYs were slightly higher at 0.013 (95% CI –0.015 to 0.041), leading to a slightly more favourable ICER of £26,770. However, this is still higher than the current threshold, and our conclusions regarding cost-effectiveness remained unchanged. In all sensitivity analyses incremental cost and QALY differences were not significantly different between arms.
The bootstrapped replications for costs and QALYs are shown on a cost-effectiveness plane in Figure 5. The majority are located on the north-east quadrant, suggesting the OTCH intervention is associated with better outcomes and higher costs than usual care. However, most of the dots lie very close to both axes, indicating that the cost and outcome differences between groups are very small. The CEAC (Figure 6) shows the probability of the intervention being more cost-effective than usual care at various willingness-to-pay thresholds. At £20,000 per QALY, this probability was 29.2% and at a threshold of £30,000 the probability was 39.2%. The incremental cost is more than £20,000/QALY in all analyses; therefore, based on current cost-effectiveness thresholds,89 we would not endorse the OTCH programme.
FIGURE 5.
Cost-effectiveness plane (base case).
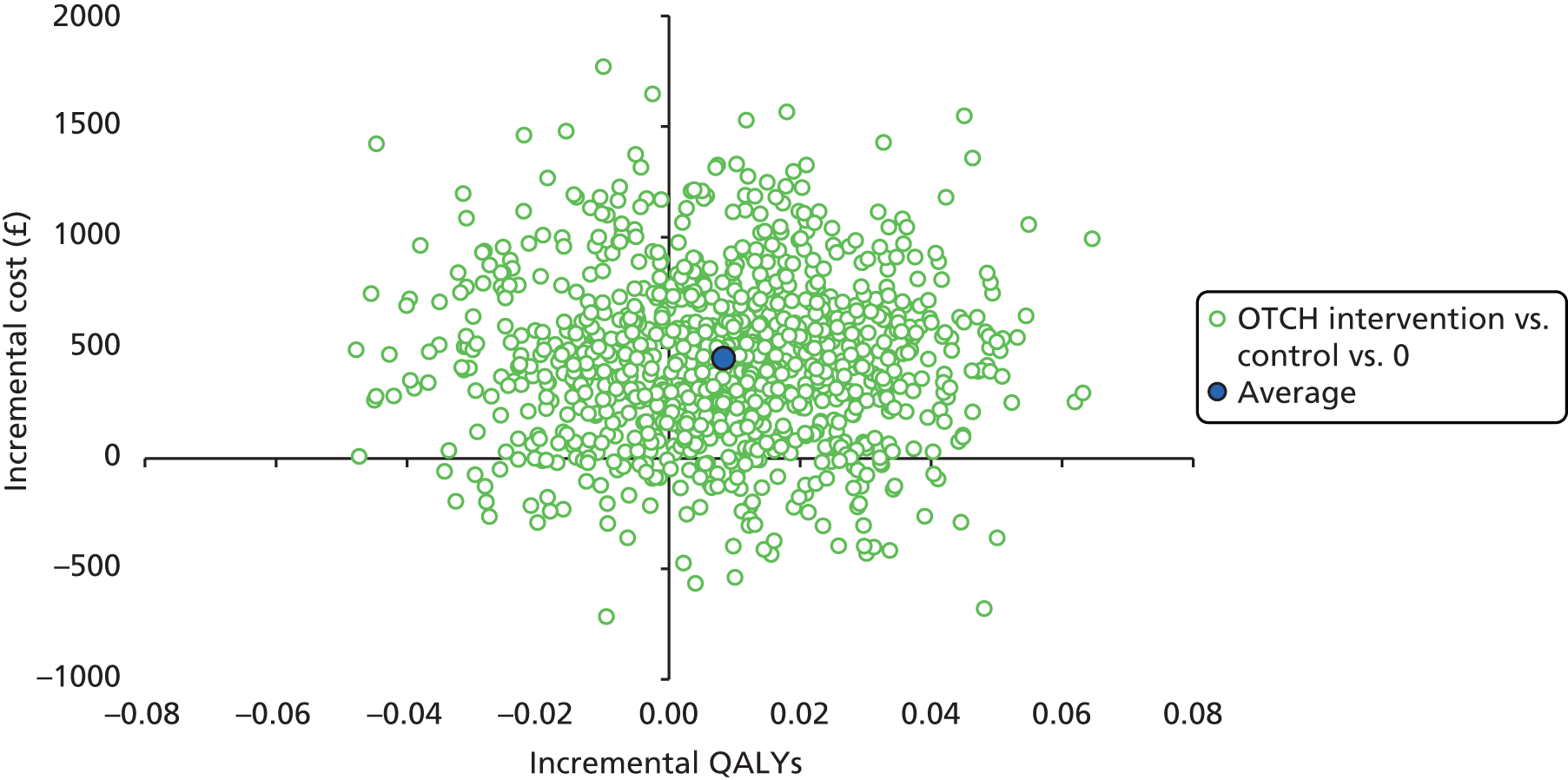
FIGURE 6.
Cost-effectiveness acceptability curve (base case).

Chapter 5 Discussion
Occupational Therapy intervention for residents with stroke living in UK Care Homes trial design summary
The OTCH study was a Phase III pragmatic parallel-group cluster RCT with an economic evaluation. The evaluation assessed the clinical efficacy and economic impact of providing an OT service for stroke survivors living in care homes. The primary research objective was to assess the influence of a 3-month course of OT, according to whether or not it helped participants maintain levels of independence in ADL compared with usual care. Secondary outcome measures assessed the influence of the OT intervention on participants’ mobility, mood and HRQoL.
More care homes were recruited than originally planned because of a larger than expected number of small clusters. The number of eligible residents within each care home with a history of stroke was lower than expected. 11,15–17 All baseline characteristics were similar across randomisation arms in regards to age, sex, ethnicity and comorbidities. A large proportion of the participant population had significant physical disabilities, substantial limitations on activity (see Figures 2 and 3) and increased dependence for personal ADL. Moreover, the assistance of a consultee to consent on the participant’s behalf was needed in 61% of cases,68 indicative of reduced autonomy. There was a high prevalence of cognitive and language impairment: 57% of participants scored below 15 points on the Sheffield screening test, indicative of significant language impairment, and 70% of participants scored in the range signifying cognitive impairment on the MMSE. The participant profile overall is suggestive of significant frailty.
Principal findings
Primary outcome at 3 months
The principal findings are neutral. The 3-month course of individualised OT for care home residents living with stroke, involving patient-centred goal-setting staff training, provision of facilitatory equipment and environmental adaptation, showed no benefit on participants’ capacity to engage in personal ADL at each of the trial end points. Findings observed in the pilot phase were not replicated. 48 OT provision did not help maintain independence in personal ADL compared with usual care. The number of participants with a poor outcome (intervention 54% vs. control 52%), moderate outcome (intervention 30% vs. control 34%) and good outcome (intervention 15% vs. control 14%) at the primary end point was similar between treatment arms.
Exploratory subgroup analyses focused on the primary outcome did not reveal any interactions of interest. The forest plot in Figure 4 indicates that participants’ age, severity on the BI at baseline, type of care home, proxy data and severity of cognitive impairment had no discernible influence on the primary outcome at 3 months. However, there is a suggestion from this exploratory analysis that the OT intervention may be effective at maintaining functional activity for residents that have less severe limitations on activity. Referring stroke survivors on an individual basis as opposed to a routine basis may still be of benefit.
Additional sensitivity analyses which included a complete case analysis, tested the potential ceiling effect, imputed missing data and removed clusters with fewer than three residents, did not change the neutral findings. A process evaluation that examined the fidelity of the OTCH intervention is presented in more depth elsewhere. 88 The process evaluation summary presented here indicates that the intervention was implemented as intended.
Primary outcome assessed at 6 and 12 months
At the 6- and 12-month follow-up stages, the BI data showed no significant differences between groups. The results from the composite BI analyses, employing the change in BI score from baseline at 6 and 12 months, were similar between groups (see Table 26). The data did not support the hypothesis that a 3-month course of OT for care home residents living with stroke-related disabilities will help maintain levels of function activity in self-care tasks over the longer term compared with usual care.
Secondary outcomes
Analyses assessing mobility, mood, and HRQoL showed no benefit of the OT intervention at 3 months (see Table 24). Similarly, the secondary outcome measures at the 6- and 12-month follow-up showed no evidence of benefit of the intervention (see Table 25). Mean mobility scores decreased over the course of the trial in both randomisation arms (see Table 22). The mean mood scores on the GDS-15 remained similar for both treatment arms across all end points (see Table 23). The mean mood scores are indicative of mild depression. 85
Further exploratory analyses
These additional exploratory analyses considered the potential dose effect of OT on depression scores, and the relationship between baseline RMI score and survival. No evidence of an association between OT dose and GDS-15 score was observed. However, there was strong evidence that mobility is associated with survival. Participants with a mobility score of 2 or below at baseline were 1.57 times as likely to die than those with greater mobility over the course of the 12-month trial duration (hazard ratio 1.57, 95% CI 1.15 to 2.15).
Economic evaluation discussion
Based on the mean incremental cost per QALY gain, it is unlikely that the OTCH intervention is cost-effective when compared with usual treatment. Although outcomes were virtually equivalent in both arms, costs were higher in the intervention arm and the intervention did not lead to a reduction in health resource use from other sources.
We acknowledge some limitations in this economic analysis. We requested information on equipment on the assumption that this would have been purchased by the NHS or personal social services. However, it became apparent that some of this equipment may have been purchased by the care home (although who purchased it was not systematically recorded), and that this could be considered outside of the NHS and personal social services perspective. In addition, apart from the training of care home staff, we did not consider all wider social costs, although we considered that, as all participants were in residential care, the burden on community care workers, friends and family would be likely to be less than if the participants lived in their own homes.
Death rates were similar between study arms. We tested the robustness of our estimates by performing multiple imputation for missing data and also running a sensitivity analysis excluding participants who died during follow-up (not reported); our cost-effectiveness conclusions were consistent. 103
Despite these limitations, the cost-effectiveness results were conclusive. At £20,000 per QALY, this probability was 29.2% and at a threshold of £30,000 the probability was 39.2%. Based on the results of this RCT, OT was not a cost-effective routine intervention for stroke survivors living in care homes.
Strengths and weaknesses
This was the largest cluster randomised trial conducted in care homes to date. The potential pitfalls of the cluster design associated with the provision of informed consent, identification of the unit of inference and methods of stratification were addressed in the trial protocol. 71,104 The trial was sufficiently powered to detect a clinically significant change in the BI measure following a 3-month course of individualised OT,82 involving task-related ADL practice, provision of adaptive equipment, adaptations to the individual’s environment and caregiver training. The unadjusted ICC for BI scores was 0.36 at baseline; however, for the change in scores from baseline to 3 months, allowing for the effect of treatment, TACs and type of care home, it decreased to 0.09. The mixed modelling approach has been shown to be a reasonable method of analysis when some cluster sizes are small, allowing for appropriate inferences to be made in relation to fixed effects. 105 There is additional evidence that even with small cluster sizes, mixed modelling is a better approach than an analysis that ignores clustering. 106 Furthermore, results of the sensitivity analysis showed that the conclusions were robust when small clusters were excluded. Results from the complete case analysis, where BI scores were not imputed for participants who died before the primary end point revealed similar neutral results to the primary analysis.
The OT administered to participants was similar to a standard NHS intervention,88 shown to be of benefit to stroke survivors living in their own homes. 34 Compliance over the course of the trial was good, resulting in high completion rates among survivors for all assessments at each end point. On average, participants in the intervention arm were visited on five occasions with visits lasting a median of 30 minutes. Retention of care home participation was good throughout the study, with 204 (89%) homes providing data at the final 12-month end point. The main reason for care home withdrawal was the death of all participating residents. A total of 313 out of 1042 (30%) participants died during the trial. Overall, we regard the findings to be robust and conclusive.
We acknowledge several potential limitations. The percentage of care home residents affected by stroke was less than expected, which resulted in a larger number of small clusters than was originally expected. Previous research suggested the incidence of stroke in care home residents to be approximately 20–40%. 11,15–17 However, this trial observed incidence of stroke in care home residents to be approximately 16% (1556 out of 9840; see Table 6). This figure concurs with results from a recent census of care homes in the UK. 107 In addition, GP surgeries’ response rate to trial correspondence requesting from confirmation of participants’ stroke was low despite multiple attempts.
There were a high proportion of participants with cognitive impairment, and GDS scores were indicative of moderate and severe depression. If these participants had been excluded during recruitment, it would have reduced the external validity of the trial. However, we acknowledge that these participants may have had potentially limited engagement in therapy, as demonstrated by the observed distribution of therapy content (see Table 20). Approximately half of all therapy hours were spent on communication with participants, carers and consultees, as opposed to ADL training (see Table 20). Therapy time attributed to this category was spent on providing information and guidance to residents, staff or relatives on personal ADL, initiating referrals to other agencies and ordering equipment. In the pilot phase the duration of the intervention was also 3 months. 48 The median number of visits per resident per month was 2.7 (interquartile range 1–4.2), and the median duration of therapy time per resident per month was 4.5 hours (interquartile range 2–6.9 hours). 48 This represents a significant difference in the amount of therapy received by residents in the two trials. Residents received more therapy in the pilot phase. In the main trial, the median number of visits was 5 (interquartile range 3–7 visits) over 3 months and the median duration of therapy per resident was 145 minutes (interquartile range 85–255 minutes). The mean duration of therapy listed in Table 20 is 208 minutes (SD 208 minutes; minimum 10 minutes, maximum 1380 minutes). The difference in the amount of therapy administered between the pilot trial and the main trial indicates that the high levels of disability among participants may have prevented engagement in personal ADL training and reduced therapy time as a result.
When viewing these figures it is also important to acknowledge differences in eligibility criteria between the two trials. The pilot trial included 118 residents with a moderate to severe BI score that was between 4 and 15 out of 20. The Phase III definitive trial reported here used a pragmatic approach and included residents with a BI score between 0 and 20. As a consequence the mean baseline BI scores differed between the two trials. In the pilot trial, the mean baseline BI score in the intervention arm was 10.1 (SD 5.7) and 9.5 (SD 5.2) in the control arm. The mean baseline BI scores in both arms in the pilot trial are classed as moderate. The mean baseline scores in the main trial were in the severe range across both arms. Table 16 displays the baseline BI scores. Overall, 48% of participants in the intervention arm had a BI score between 0 and 4, indicating very severe limitations when engaging in personal ADL. It is also important to reflect on the conclusions from the pilot trial. 48 After receiving a higher dose of therapy, participants in the intervention arm showed a tendency for improvement between baseline and the primary outcome time point at 3 months for the Barthel composite measures only (see Table 21 and Table 25 for similar results from this trial). However, participants in the intervention arm deteriorated in a similar way to the control group at all subsequent follow-up time points. 48 Therefore, despite the difference in the amount of therapy administered between the two trials potentially being viewed as a limitation, the main trial has provided a realistic portrayal of the high level of impairment exhibited by care home residents living with stroke-related disabilities throughout England and Wales (see Table 16 and Figures 2 and 3). A realistic conclusion is that the majority of participants may have lacked the capacity to directly engage with structured, repetitious activity-based therapy.
Safety
The OT provided to residents was not an experimental intervention. Therapy given to residents was similar to what stroke survivors living in the community would receive from the NHS. Overall, tolerance of the OT provided to participants was very good, resulting in no adverse events occurring that were attributable to the intervention. There was a higher incidence of falls among participants in the intervention arm. It is a possibility that, by facilitating an increase in functional activity, it may have led to an increased risk of falling. However, according to recently published figures,108–110 the average fall rate of residents in long-term institutional care varies from 1.45 to 2.50 per annum. This suggests the quarterly fall rate of 0.18 recorded in the intervention arm is below the previously observed parameters.
Generalisability
The large sample size, the geographical distribution of different types of care home, the involvement of a large number of qualified therapists and the inclusion of all stroke survivors, regardless of cognitive and communication impairments, increase the potential for generalisability of the observed results across all UK care homes. The baseline participant characteristics, including age, level of comorbidity, cognitive impairment and incidence of depression observed in the present study, are similar to rates recently reported in another large cluster RCT carried out in UK care homes. 111
Research in context
The neutral results observed in the OTCH trial concur with results observed in other RCTs conducted in a care home setting. These trials assessed the benefit of activity-based interventions at reducing levels of depression, and incidence of falls. 111,112 Collectively, these neutral findings suggest that we may be expecting too much from this predominantly frail population with a high prevalence of dementia and depression and with low autonomy to respond to activity-based therapies (see Figures 2 and 3 in relation to levels of severe disability). It seems more appropriate to seek alternative care approaches to ameliorate disability for this very inactive patient group. The established OT intervention has good evidence of efficacy when administered to patients in their own homes. 34 This suggests that the barriers to success are more attributable to the care home environment and the care home population. Furthermore, the suitability and application of a patient-centred goal-setting approach in this population may require further scrutiny. Currently, patient-centred care does not have a universally accepted definition or accepted methods of how it can be measured for adherence. 113
A changing role of care homes is acknowledged in a report from the Centre for Policy and Aging. 16 Residents are being admitted to care homes with a higher level of dependency and more complex care needs than ever before. 26 In the census of UK Bupa (The British United Provident Association Limited) care homes in 2012, 87% of residents were classed as having high-support needs and total dependency. 16 Providing care for residents with high-support needs requires careful consideration of the care home environment. The therapists delivering the OTCH intervention in the participating care homes reported that the use of adaptive equipment and environmental modifications (e.g. the installation of bed levers, grab rails or raised toilet seats) was highly variable.
Future work
In order to advance the level of care currently provided to care home residents, future research should identify applicable criteria to promote an enabling environment where possible. The emphasis in this population is suggested to be about providing care for residents with high support needs for a short period towards the end of life. Further research is needed to demonstrate how the care home environment can be suitably modified to tackle these needs. A key factor in promoting an enabling environment is minimising health-related complications caused by inactivity (such as urinary incontinence and pressure sores). In addition, further work is needed in measuring health-related complications in this population in order to identify problems early.
Conclusions
The neutral results from this Phase III cluster randomised trial are deemed robust and conclusive. The trial was geared towards evaluating whether or not there is sufficient evidence to support an improved NHS provision of OT for care home residents with stroke-related disabilities. The clinical and health economic evidence presented here does not support commissioning a routine OT service in this population of care home residents. There may be benefit for residents with less severe limitations on functional activity; however, these residents were in the minority of the sampled population of stroke survivors. It appears justified to suggest that referrals of care home residents with stroke-related disabilities for OT may be of benefit on an individual basis if left in the hands of the health professional initiating the referral. OT as a service for stroke survivors still able to live in their own home has good evidence of success. 34,37 However, the benefit of this style of therapy as a routine service was not evident for stroke survivors resident in UK care homes.
Acknowledgements
We thank all participants, care home staff, care home managers, owners and providers involved in the trial. The research team also acknowledge the support of the National Institute for Health Research (NIHR), through the Comprehensive Clinical Research Network, in particular, Kate Wilde, Jacqueline Briggs and Rhian Hughes.
Participating centres and collaborative group members
Birmingham
S Bevan, M Feltham, G Bouliotis, G Yao, S Herron-Marx, N Russell, L M Harris, P Bradburn, B Gallivan, T Coles, C Hallam, O Horgan, C Hill, J Anderson, M Banting, C Sexty and J O’Donnell.
Bangor
P Masterson-Algar, E Roberts, H Owen and J Shirley.
Bournemouth
K Saunders, B Longland, A Orpen, J Bell and C Ovington.
Coventry
H Wright and C Randall.
Lancashire
M Auton, D Fawshaw and K Patel.
Norwich
G Sands, K Mortimer, G Barton, E Costello, D Kelly, R Gravelle and P Sharp.
Nottingham
A Moody, C Coole and C Edwards.
Plymouth
L Househam, R Truscott, C Brown and R Newport.
Solent
J Williams, L Burton and C Colwell.
Staffordshire
H Mackey, K Finney, K Townshend and S Lyjko.
Taunton
S Glanfield, L Caudwell, J Homan and S Edwards.
Wolverhampton
J Bisiker, K Preece and S Silvester.
Occupational therapists
A Lake, S Kilmister, V Blakemore, R Parker, B Jenkins, H Nicholls, L Whitfield, J Mackenzie, K Wood, B Lang, S Baker, S Rundle, A McMichael, S Evans, J Shirley, E Paterson, L Hinds, M Sensier, J Cowling, B Jones and M March.
Research networks
Central England Primary Care Research Network, South West Stroke Research Network, West Midlands Stroke Research Network.
Care homes
We are grateful to the staff and residents for their co-operation and enthusiastic participation in the OTCH trial from the following care homes: Abbey Park, Coventry; Abelard, Nottingham; Acacia Care Centre, Nottingham; Acocks Green Nursing Home, Birmingham; Alexandra House Nursing Home, Nottingham; Alexandra House, Solihull; Alexandra Rose Residential Care Home, Portsmouth; Allambie House Care Home, Coventry; Allerton Court, Sandwell; Alston View Nursing & Residential Home, Preston; Alton Manor Ltd, Southsea; Alverstock House Nursing Home, Gosport; Anville Court Nursing Home, Wolverhampton; Arboretum Nursing Home, Walsall; Arborough House Ltd, Southsea; Arden Park Care Home, Leamington Spa; Ardenlea Court, Solihull; Ash Hall Care Home, Stoke-on-Trent; Ash Lodge, Sandwell; Ashleigh Manor, Plymouth; Ashley Lodge Care Home, Birmingham; Atholl House, Compton; Autumn House Nursing Home, Stone; Bearwood Nursing Home, Sandwell; Beaumaris Care Home, Newport; Beech Hill Grange Nursing Home, Sutton Coldfield; Beech Lodge Nursing & Residential Home, Cheadle; Beechcroft Residential Home, Sandwell; Belmont Residential Care Home, Preston; Bentley Court Care Home, Wolverhampton; Birch Green Care Centre, Skelmersdale; Birds Hill Nursing Home, Poole; Birds Hill Starling, Poole; Bloomfield Court, Tipton; Bluebell Nursing Home, Southsea; Bournville Grange, Birmingham; Bridgewood Mews, Tipton; Broadgate Care Home, Nottingham; Brompton Lodge, Rhos on Sea; Brookvale Care Home, Solihull; Burkitt Care Home, Nottingham; Cams Ridge Nursing & Residential Care Home, Fareham; Cann House, Plymouth; Carpenter Place Care Home, Birmingham; Chalgrove Care Home (Edwardian), Poole; Chalgrove Care Home (Tudor), Poole; Chelston Park Nursing and Residential Home, Wellington; Chorley Lodge Residential Care Home, Chorley; Churchfield Christian Care Centre, Nottingham; Churchfield Court Care Home, West Bromwich; Cole Valley Nursing Home, Birmingham; Compton Manor Care Home, Coventry; Cordelia Court Care Home, Coventry; Cosham Court Nursing Home, Portsmouth; Courtfield Lodge Nursing & Residential Home, Ormskirk; Croft Manor Residential Care Home, Fareham; Crossways, Walsall; Crown Meadow Nursing Home, Sandwell; Crystal Hall Residential & Nursing Home, Preston; Cuerden Grange Nursing Home, Preston; Cuerden Grange Rest Home, Preston; Davlyn House, Stoke-on-Trent; Deerwood Grange Nursing Home, Sutton Coldfield; Digby Manor Care Home, Erdington; Down House, Plymouth; Druids Meadow Residential Care, Birmingham; Dunkirk Memorial Home, Taunton; Eastleigh Nursing Home, Minehead; Edgbaston Beaumont Care Home, Birmingham; Edward House, Nottingham; Elizabeth House Care South, Poole; Elm House, Nottingham; Eryl Fryn Nursing & Residential Home, Llandudno; Evedale Care Home, Coventry; Eversleigh, Wolverhampton; Farehaven Lodge, Fareham; Frethey House Care Home, Taunton; Giltbrook House Nursing Home, Nottingham; Godiva Lodge Care Home, Coventry; Greenfield Nursing Home, Preston; Greville House Care Home, Sutton Coldfield; Hallcroft, Nottingham; Hamilton House Care Centre, Portsmouth; Haulfre, Beaumaris; Hawthorne Lodge, Nottingham; Hazel House Nursing Home, Leyland; Heartlands Care Home, Birmingham; Heathlands Care Home, Poole; High Pastures Nursing Home, Deganwy; Holmpark Care Home, Birmingham; Ivy House Care Home, Birmingham; Kelvedon House, Wednesbury; Keresley Wood Care Centre, Coventry; Kingland House Residential Home, Poole; Kinmel Lodge, Kinmel Bay; Knoll House, Wolverhampton; Lake View Nursing Home, Chorley; Landermeads Nursing Home, Nottingham; Langdale Nursing Home, Gosport; Leabrook House Nursing Home, Sandwell; Lilliput House Care Home, Poole; Llys Eilian, Colwyn Bay; Longdean Lodge, Portsmouth; Lucton House Care Home, Birmingham; Lyme Valley House, Newcastle-under-Lyme; Maple Dene Care Home, Birmingham; Marian House Nursing Home, Sutton Coldfield; Mary Street Care Home, Birmingham; Meadow House Residential Home, Portsmouth; Meadowbank Nursing Home, Bamber Bridge; Meadowside/St Francis, Plymouth; Melbourne House Care Home, Coventry; Merafield View, Plymouth; Merry Hall Nursing and Residential Care Home, Fareham; Moorcroft, Llandudno; Moorhaven Care Home, Taunton; Moorlands Nursing Home, Nottingham; Moundsley Hall Nursing and Residential Home, Birmingham; Mountbatten Nursing Home, Taunton; Neville Williams House, Birmingham; New Day Nursing Home, Birmingham; New Milton Nursing Home, Stoke-on-Trent; Northcott House Nursing & Residential Care Home, Gosport; Oakland Grange, Southsea; Oaklands Care Centre, Birmingham; Oakwood Rest Home, Erdington; Olivet Christadelphian Care Homes, Birmingham; Orchard House Nursing Home, Sutton Coldfield; Osborn Manor, Fareham; Park House Nursing Home, Nottingham; Park Manor Care Home, Poole; Parkfields, Wolverhampton; Parkwood House Nursing Home, Plymouth; Parkwood House Residential Home, Plymouth; Patricia Venton Centre, Plymouth; Peel House Nursing and Residential Home, Fareham; Pelsall Hall, Walsall; Perry Locks Nursing Home, Birmingham; Pine Meadows Care Centre, Leek; Plas Gogarth, Llandudno; Plas Gwilym, Caernarfon; Plas Gwynfa Nursing Home, Abergele; Plas y Bryn Nursing Home, Caernarfon; Priestly Rose Care Home, Erdington; Prince of Wales Care Home, Shirley; Priory Grange, Rhos on Sea; Queen Anne Lodge, Southsea; Queenswood Methodist Homes for the Aged, Nottingham; Radnor House Care Home, Birmingham; Rayner House, Solihull; Richmond Hall, Walsall; Robert Harvey House Care Home, Birmingham; Rosemary Lodge Nursing Home, Birmingham; Roxton Nursing Home, Sutton Coldfield; Ruksar, Wolverhampton; Russell Churcher Court, Gosport; Rylands, Newport; Sant Tysilio Nursing Home, Llanfairpwll; Seaview Residential Care Home, Southsea; Selbourne Court Care Home, Coventry; Selly Park Care Centre, Birmingham; Sherwood Court, Preston; Silverbirches, Birmingham; Silverdale Nursing Home, Newcastle-under-Lyme; Solent Cliffs Nursing & Residential Care Home, Fareham; Somerset Abbeyfield, Taunton; South View Lodge, Hesketh Bank; Southern House, Abergele; Sovereign House Nursing Home, Coventry; Springbank Nursing Home, Stoke-on-Trent; Springfield Court Nursing Home, Aughton; Springfield House Nursing Home, Codsall; Springfield Nursing Home, Chorley; Springfield Residential Home, Nottingham; Springholme Care (Anglesey) Ltd, Pentraeth; St Agnes’s Care Home, Erdington; St Bernards Residential Care Home, Solihull; St Catherine’s Residential Home, Sutton Coldfield; St Joseph’s Home, Birmingham; St Martin’s Nursing Home, Sutton Coldfield; St Paul’s Convent, Birmingham; St Ronan’s Nursing and Residential Care Home, Southsea; St Vincent House, Gosport; Staddon Lodge Residential Care Home, Poole; Stennards Leisure Home, Birmingham; Stratford Court Care Home, Birmingham; Sunrise Operations Tettenhall Ltd (Assisted Living), Wolverhampton; Sunrise Senior Living Solihull, Solihull; Sycamore House Care Home, Nottingham; Talbot View, Bournemouth; Tamar House, Plymouth; Templeman House, Bournemouth; Thalassa Nursing Home, Gosport; The Beaufort Care Home, Coventry; The Carlton Care Home, Nottingham; The Field House Care Home, Birmingham; The Firs Nursing Home, Nottingham; The Friendly Inn, Birmingham; The Gables Care Home, Sutton Coldfield; The Grange, Nottingham; The Green Nursing Home, Birmingham; The Grove, Solihull; The Haven Nursing Home, Coventry; The Herons, Nottingham; The Manor House, Stone; The Old Vicarage, Long Eaton; The Orchard, Wednesbury; The Poplars Nursing Home, Sandwell; The Priory Nursing and Residential Home, Shirley; The Priory, Telford; The Regency Nursing Home, Southsea; The Ridings, Birmingham; The Shrubbery, Codsall; Torr Home Nursing, Plymouth; Torr Home Residential, Plymouth; Tudor House Care Home, Birmingham; Uplands Nursing Home, Birmingham; Valley View, Plymouth; Victoria Gardens Care Home, Coventry; Wellesley House Nursing Home, Wolverhampton; Winsor Nursing Home, Minehead; Woodcote Hall, Newport; Woodcroft, Colwyn Bay; Woodland Villa Nursing Care Home, Plymouth; Woodland Villa Residential Care Home, Plymouth; Woodside Grange Care Home, Rhos on Sea; Wrottesley Park House Nursing Home, Wolverhampton; and Wyndeley Grange Nursing, Sutton Coldfield.
Contributions of authors
Catherine M Sackley (Professor of Rehabilitation, University of East Anglia) was the chief investigator, was responsible for developing the intervention and contributed significantly towards the development of the study protocol and drafting the report.
Marion F Walker (Professor of Stroke Rehabilitation, University of Nottingham) was a co-applicant for funding, was responsible for developing the intervention and contributed significantly towards the development of the study protocol.
Christopher R Burton (Senior Research Fellow, Bangor University) was a co-applicant for funding and contributed significantly towards the development of the study protocol.
Caroline L Watkins (Professor of Stroke and Older People’s Care, University of Central Lancashire) was a co-applicant for funding and contributed significantly towards the development of the study protocol.
Jonathan Mant (Professor of Primary Care Research, Institute of Public Health) was a co-applicant for funding and contributed significantly towards the development of the study protocol.
Andrea K Roalfe (Senior Lecturer in Medical Statistics, University of Birmingham) was the trial statistician. She wrote the statistical analysis plan and carried out the statistical analysis.
Keith Wheatley (Professor of Medical Statistics, University of Birmingham) was a co-applicant for funding and contributed towards the development of the study protocol and the final report.
Bart Sheehan (Consultant in Liaison Psychiatry, John Radliffe Hospital, Oxford) was a co-applicant for funding and contributed significantly towards the development of the study protocol.
Leslie Sharp (Dissemination Officer, University of East Anglia) was a co-applicant for funding, a service user and patient and public involvement representative, and contributed towards the development of the study protocol.
Katie E Stant (Study Co-ordinator, University of Birmingham) was the study co-ordinator and was involved significantly in the conduct of the trial.
Joanna Fletcher-Smith (Research Occupational Therapist, University of Nottingham) was involved in the development of the intervention and recruitment for the study and contributed to the final report.
Kerry Steel (Occupational Therapist, University of Birmingham) was involved in the development of the intervention, recruitment for the study, and contributed to the final report.
Garry R Barton (Reader in Health Economics, University of East Anglia) completed the health economic analysis and drafted the health economic evaluation in the report.
Lisa Irvine (Senior Research Associate, University of East Anglia) completed the health economic analysis and drafted the Health Economic evaluation in the report and contributed to the final report overall.
Guy Peryer (Senior Research Associate, University of East Anglia) was involved in data analysis, the creation of figures, wrote the final report and addressed reviewer comments on the final manuscript.
Other members of the trial team
K Lett (University of Birmingham), J Williams (Portsmouth Hospitals NHS Trust), F Rashid (University of Birmingham) and P Masterson-Algar (Bangor University).
Trial Steering Committee (independent members)
Professor H Dawes (independent chairperson, Oxford Brookes University), Professor R Harwood (Nottingham University Hospitals NHS Trust), Dr P Logan (Queen’s Medical Centre, Nottingham), N Phillips (Patient Representative) and Professor M Underwood (University of Warwick).
Data Monitoring Committee (independent members)
Professor D Barer (Newcastle University), Professor G Mountain (University of Sheffield) and Professor J Norrie (University of Aberdeen).
Publications
Fletcher-Smith JC, Walker MF, Cobley CS, Steultjens EM, Sackley CM. Occupational therapy for care home residents with stroke. Cochrane Database Syst Rev 2013;6:CD010116.
Fletcher-Smith J, Walker MF, Drummond A, Sackley CM. Occupational therapy for care home residents with stroke: what is routine practice? Br J Occup Ther 2013;76(Suppl. 1).
Masterson-Algar P, Burton CR, Rycroft-Malone J, Sackley CM, Walker MF. Towards a programme theory for fidelity in the evaluation of complex interventions. J Eval Clin Pract 2014;20:445–52.
Sackley CM, Burton C, Herron-Marx S, Lett K, Mant J, Roalfe A, et al. A cluster randomised controlled trial of an occupational therapy intervention for residents with stroke living in UK care homes (OTCH): study protocol. BMC Neurol 2012;12:52.
Sackley CM, Walker MF, Burton C, Watkins C, Mant J, Roalfe AK, et al. A cluster randomised controlled trial of an occupational therapy intervention for residents with stroke-related disabilities living in UK care homes (OTCH). BMJ 2015;350:h468.
Sands G, Kelly D, Birt L, Fletcher-Smith J, Sackley CM. An occupational therapy intervention for residents with stroke living in UK care homes: a content analysis of occupational therapy records from the OTCH trial. Br J Occup Ther 2015;78:422–30.
The treatment of stroke patients in UK care homes: the potential for occupational therapy. Stroke Matters. The Stroke Association; 2010.
Data sharing statement
Data are available from the chief investigator, Professor Catherine Sackley, at catherine.sackley@kcl.ac.uk
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Mortality in England and Wales: Average Life Span, 2010. London: ONS; 2012.
- The State of Health Care and Adult Social Care In England. London: The Stationery Office; 2013.
- A New Ambition for Old Age – Next Steps in Implementing the National Service Framework for Older People. London: Department of Health; 2006.
- Bray H. Office for National Statistics 2006-based national population projections for the UK and constituent countries. Popul Trends 2008;131:8-18.
- Townsend N, Wickramasinghe K, Bhatnager P, Smolina K, Nichols M, Leal J, et al. Coronary Heart Disease Statistics 2012 Edition. London: British Heart Foundation; 2012.
- Transient Ischaemic Attack (TIA). London: NHS Choices; 2013.
- Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197-223. http://dx.doi.org/10.1016/S0140-6736(12)61689-4.
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245-55. http://dx.doi.org/10.1016/S0140-6736(13)61953-4.
- Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health 2013;1:e259-e81. http://dx.doi.org/10.1016/S2214-109X(13)70089-5.
- Adamson J, Beswick A, Ebrahim S. Is stroke the most common cause of disability?. J Stroke Cerebrovasc Dis 2004;13:171-7. http://dx.doi.org/10.1016/j.jstrokecerebrovasdis.2004.06.003.
- Progress in Improving Stroke Care. London: The Stationery Office; 2010.
- National Sentinel Stroke Clinical Audit 2010 Round 7: Public Report for England, Wales and Northern Ireland. London: Royal College of Physicians; 2012.
- Don’t Forget About Care Home Residents. London: Stroke Association; n.d.
- Care Homes. London: Care Quality Commission; n.d.
- Yap LK, Au SY, Ang YH, Kwan KY, Ng SC, Ee CH. Who are the residents of a nursing home in Singapore?. Singapore Med J 2003;44:65-73.
- A Profile of Residents in Bupa Care Homes: Results from the 2012 Bupa Census. London: Centre for Policy and Aging; 2012.
- Bowman C, Whistler J, Ellerby M. A national census of care home residents. Age Ageing 2004;33:561-6. http://dx.doi.org/10.1093/ageing/afh177.
- Macdonald A, Cooper B. Long-term care and dementia services: an impending crisis. Age Ageing 2007;36:16-22. http://dx.doi.org/10.1093/ageing/afl126.
- National Service Framework for Older People. London: Department of Health; 2001.
- Enhancing the Health of Older People in Long-term Care: Clinical Guidelines. London: Royal College of Physicians; 1998.
- Cowman S, Royston M, Hickey A, Horgan F, McGee H, O’Neill D. Stroke and nursing home care: a national survey of nursing homes. BMC Geriatr 2010;10. http://dx.doi.org/10.1186/1471-2318-10-4.
- Our Health, Our Care, Our Say: A New Direction for Community Services. London: Department of Health; 2006.
- Prioritising Need in the Context of Putting People First: A Whole System Approach to Eligibility for Social Care – Guidance on Eligibility Criteria for Adult Social Care, England 2010. London: Department of Health; 2010.
- Fair Access to Care Services (FACS): Prioritising Eligibility for Care and Support. London: Social Care Institute for Excellence; 2013.
- Older People’s Vision for Long-term Care. London: Joseph Rowntree Foundation; 2009.
- Care Homes Under Pressure - An England Report. Policy Briefing 04/2010. London: Royal College of Nursing; 2010.
- Health Care in Care Homes: A Special Review of the Provision of Health Care to Those in Care Homes. London: Care Quality Commission; 2012.
- Yerxa EJ, Clark F, Frank G, Jackson J, Parham D, Pierce D, et al. Occupational Science: The Foundation for New Models of Practice. New York, NY: The Haworth Press; 1989.
- Activity Provision Benchmarking: Good Practice in Care Homes. London: College of Occupational Therapists; 2007.
- International Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization; 2001.
- From Interface to Integration: A Strategy for modernising Occupational Therapy Service in Local Health and Social Care Communities. London: College of Occupational Therapists; 2002.
- Paterson M, Higgs J, Wilcox S. Developing expertise in judgement artistry in occupational therapy practice. Br J Occup Ther 2006;69:115-23. http://dx.doi.org/10.1177/030802260606900304.
- Duncan PW. Synthesis of intervention trials to improve motor recovery following stroke. Top Stroke Rehabil 1997;3:1-20.
- Legg L, Drummond A, Leonardi-Bee J, Gladman JRF, Corr S, Donkervoort M, et al. Occupational therapy for patients with problems in personal activities of daily living after stroke: systematic review of randomised trials. BMJ 2007;335. http://dx.doi.org/10.1136/bmj.39343.466863.55.
- Boniface G, Mason M, Macintyre J, Synan C, Riley J. The effectiveness of local authority social services’ occupational therapy for older people in Great Britain: a critical literature review. Br J Occup Ther 2013;76:538-47. http://dx.doi.org/10.4276/030802213X13861576675240.
- Stroke Rehabilitation: Long Term Rehabilitation After Stroke. London: NICE; 2013.
- Walker MF, Leonardi-Bee J, Bath P, Langhorne P, Dewey M, Corr S, et al. Individual patient data meta-analysis of randomized controlled trials of community occupational therapy for stroke patients. Stroke 2004;35:2226-32. http://dx.doi.org/10.1161/01.STR.0000137766.17092.fb.
- Sprangers MA, de Regt EB, Andries F, van Agt HM, Bijl RV, de Boer JB, et al. Which chronic conditions are associated with better or poorer quality of life?. J Clin Epidemiol 2000;53:895-907. http://dx.doi.org/10.1016/S0895-4356(00)00204-3.
- Barodawala S, Kesavan S, Young J. A survey of physiotherapy and occupational therapy provision in UK nursing homes. Clin Rehabil 2001;15:607-10. http://dx.doi.org/10.1191/0269215501cr454oa.
- Sackley C, Hoppitt T, Cardoso K, Levin S. The availability and use of allied health care in care homes in the Midlands, UK. Int J Ther Rehabil 2009;16:218-24. http://dx.doi.org/10.12968/ijtr.2009.16.4.41195.
- Sackley C, Gatt J, Walker M. The use of rehabilitation services by private nursing homes in Nottingham. Age Ageing 2001;30:532-3. http://dx.doi.org/10.1093/ageing/30.6.532.
- National Clinical Guideline for Stroke, 4th edition. London: Royal College of Physicians; 2012.
- Dworzynski K, Ritchie G, Fenu E, MacDermott K, Playford ED. Rehabilitation after stroke: summary of NICE guidance. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f3615.
- Brown RD, Ransom J, Hass S, Petty GW, O’Fallon WM, Whisnant JP, et al. Use of nursing home after stroke and dependence on stroke severity: a population-based analysis. Stroke 1999;30:924-9. http://dx.doi.org/10.1161/01.STR.30.5.924.
- Sackley C, Levin S, Cardoso K, Hoppitt T. Observations of activity levels and social interaction in a residential care setting. Int J Ther Rehabil 2006;13:370-3. http://dx.doi.org/10.12968/ijtr.2006.13.8.370.
- Sackley C, Brittle N, Patel S, Ellins J, Scott M, Wright C, et al. The prevalence of joint contractures, pressure sores, painful shoulder, other pain, falls, and depression in the year after a severely disabling stroke. Stroke 2008;39:3329-34. http://dx.doi.org/10.1161/STROKEAHA.108.518563.
- Sackley C, van den Berg M, Lett K, Patel S, Hollands K, Wright C, et al. Effects of a physiotherapy and occupational therapy intervention on mobility and activity in care home residents: a cluster randomised controlled trial. BMJ 2009;339:670-2. http://dx.doi.org/10.1136/bmj.b3123.
- Sackley C, Wade D, Mant D, Atkinson J, Yudkin P, Cardoso K, et al. Cluster randomized pilot controlled trial of an occupational therapy intervention for residents with stroke in UK care homes. Stroke 2006;37:2336-41. http://dx.doi.org/10.1161/01.STR.0000237124.20596.92.
- Sackley C, Rodriguez N, van den Berg M, Badger F, Wright C, Besemer J, et al. A phase II exploratory cluster randomized controlled trial of a group mobility training and staff education intervention to promote urinary continence in UK care homes. Clin Rehabil 2008;22:714-21. http://dx.doi.org/10.1177/0269215508089058.
- Crocker T, Forster A, Young J, Brown L, Ozer S, Smith J, et al. Physical rehabilitation for older people in long-term care. Cochrane Database of System Rev 2013;2. http://dx.doi.org/10.1002/14651858.cd004294.pub3.
- Crocker T, Young J, Forster A, Brown L, Ozer S, Greenwood DC. The effect of physical rehabilitation on activities of daily living in older residents of long-term care facilities: systematic review with meta-analysis. Age Ageing 2013;42:682-8. http://dx.doi.org/10.1093/ageing/aft133.
- Fletcher-Smith JC, Walker MF, Cobley CS, Steultjens EM, Sackley CM. Occupational therapy for care home residents with stroke. Cochrane Database Syst Rev 2013;6. http://dx.doi.org/10.1002/14651858.cd010116.pub2.
- Teresi J, Abrams R, Holmes D, Ramirez M, Eimicke J. Prevalence of depression and depression recognition in nursing homes. Soc Psychiatry Psychiatr Epidemiol 2001;36:613-20. http://dx.doi.org/10.1007/s127-001-8202-7.
- Drageset J, Eide GE, Ranhoff AH. Depression is associated with poor functioning in activities of daily living among nursing home residents without cognitive impairment. J Clin Nurs 2011;20:3111-18. http://dx.doi.org/10.1111/j.1365-2702.2010.03663.x.
- Kramer D, Allgaier AK, Fejtkova S, Mergl R, Hegerl U. Depression in nursing homes: prevalence, recognition, and treatment. Int J Psychiatry Med 2009;39:345-58. http://dx.doi.org/10.2190/PM.39.4.a.
- Mozley CG, Challis D, Sutcliffe C, Bagley H, Burns A, Huxley P, et al. Psychiatric symptomatology in elderly people admitted to nursing and residential homes. Aging Ment Health 2000;4:136-41. http://dx.doi.org/10.1080/13607860050008655.
- Sutcliffe C, Burns A, Challis D, Mozley CG, Cordingley L, Bagley H, et al. Depressed mood, cognitive impairment, and survival in older people admitted to care homes in England. Am J Geriatr Psychiatry 2007;15:708-15. http://dx.doi.org/10.1097/JGP.0b013e3180381537.
- Stroke Association . Depression After Stroke 2012. www.stroke.org.uk/sites/default/files/Depression%20after%20stroke_0.pdf (accessed February 2014).
- Eisses AM, Kluiter H, Jongenelis K, Pot AM, Beekman AT, Ormel J. Care staff training in detection of depression in residential homes for the elderly: randomised trial. Br J Psychiatry 2005;186:404-9. http://dx.doi.org/10.1192/bjp.186.5.404.
- Mozley CG, Schneider J, Cordingley L, Molineux M, Duggan S, Hart C, et al. The care home activity project: does introducing an occupational therapy programme reduce depression in care homes?. Aging Ment Health 2007;11:99-107. http://dx.doi.org/10.1080/13607860600637810.
- Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med 1993;118:622-9. http://dx.doi.org/10.7326/0003-4819-118-8-199304150-00009.
- Patrick DL, Erickson P. Health Status and Health Policy: Quality of Life in Health Care Evaluation and Resource Allocation. New York, NY: Oxford University Press; 1993.
- Enabling Research in Care Homes. Understanding Care Homes. London: National Institute for Health Research; 2013.
- My Home Life: Quality of Life in Care Homes. London: Help the Aged; 2007.
- Campbell MJ. Extending CONSORT to include cluster trials. BMJ 2004;328:654-5. http://dx.doi.org/10.1136/bmj.328.7441.654.
- Daffertshofer M, Mielke O, Pullwitt A, Felsenstein M, Hennerici M. Transient ischemic attacks are more than ‘ministrokes’. Stroke 2004;35:2453-8. http://dx.doi.org/10.1161/01.STR.0000144050.90132.8e.
- van Wijk I, Lindeman E, Kappelle LJ, van Gijn J, Koudstaal PJ, Gorter JW, et al. Functional status and use of healthcare facilities in long-term survivors of transient ischaemic attack or minor ischaemic stroke. J Neurol Neurosurg Psychiatry 2006;77:1238-43. http://dx.doi.org/10.1136/jnnp.2006.089391.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Puffer S, Torgerson DJ, Watson J. Evidence for risk of bias in cluster randomised trials: review of recent trials published in three general medical journals. BMJ 2003;327:785-7. http://dx.doi.org/10.1136/bmj.327.7418.785.
- Giraudeau B, Ravaud P. Preventing bias in cluster randomised trials. PLOS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000065.
- Sackley CM, Burton C, Herron-Marx S, Lett K, Mant J, Roalfe A, et al. A cluster randomised controlled trial of an occupational therapy intervention for residents with stroke living in UK care homes (OTCH): study protocol. BMC Neurol 2012;12. http://dx.doi.org/10.1186/1471-2377-12-52.
- van den Berg M, Lett K, Sackley C. A workshop to maintain residents’ mobility and activity. Nurs Times 2006;102:32-4.
- Sackley C, Atkinson J, Walker M. Occupational therapy in nursing and residential care settings: a description of a randomised controlled trial intervention. Br J Occup Ther 2004;67:104-10. http://dx.doi.org/10.1177/030802260406700302.
- Syder D BR, Parker M, Boddy M. Sheffield Screening Test for Acquired Language Disorders. Windsor: NFER Nelson; 1993.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. http://dx.doi.org/10.1016/0022-3956(75)90026-6.
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61-5.
- Gompertz P, Pound P, Ebrahim S. A postal version of the Barthel Index. Clin Rehabil 1994;8:233-9. http://dx.doi.org/10.1177/026921559400800308.
- Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61-3. http://dx.doi.org/10.3109/09638288809164103.
- Wright J, Cross J, Lamb S. Physiotherapy outcome measures for rehabilitation of elderly people: responsiveness to change of the Rivermead Mobility Index and Barthel Index. Physiotherapy 1998;84:216-21. http://dx.doi.org/10.1016/S0031-9406(05)65552-6.
- Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999;30:1538-41. http://dx.doi.org/10.1161/01.STR.30.8.1538.
- Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol 2006;5:603-12. http://dx.doi.org/10.1016/S1474-4422(06)70495-1.
- Hsieh YW, Wang CH, Wu SC, Chen PC, Sheu CF, Hsieh CL. Establishing the minimal clinically important difference of the Barthel Index in stroke patients. Neurorehabil Neural Repair 2007;21:233-8. http://dx.doi.org/10.1177/1545968306294729.
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. Int Disabil Stud 1991;13:50-4. http://dx.doi.org/10.3109/03790799109166684.
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17:37-49. http://dx.doi.org/10.1016/0022-3956(82)90033-4.
- Sheikh J, Yesavage J. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol 1986;5:165-73. http://dx.doi.org/10.1300/J018v05n01_09.
- Cumming TB, Thrift AG, Collier JM, . Very early mobilization after stroke fast-tracks return to walking: further results from the phase II AVERT randomized controlled trial. Stroke 2011;42:153-8. http://dx.doi.org/10.1161/STROKEAHA.110.594598.
- Folstein MF, Folstein SE, McHugh PR, Fanjiang G. Mini-Mental State Examination: User’s Guide. Lutz, FL: Psychological Assessment Resources; 2001.
- Masterson-Algar P, Burton CR, Rycroft-Malone J, Sackley CM, Walker MF. Towards a programme theory for fidelity in the evaluation of complex interventions. J Eval Clin Pract 2014;20:445-52. http://dx.doi.org/10.1111/jep.12174.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- NHS Staff Earnings Estimates. London: Health and Social Care Information Centre; 2012.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: PSSRU, University of Canterbury; 2011.
- Mileage Allowances. London: NHS Employers; 2012.
- NHS Reference Costs 2011–12. London: Department of Health; 2012.
- Netten A, Netten A, Curtis L. Unit Costs of Health and Social Care. University of Kent, Kent: Personal Social Sciences Research Unit; 2003.
- The Green Book: Appraisal and Evaluation in Central Government: Treasury Guidance. London: The Stationery Office; 2003.
- The EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Borowiak E, Kostka T. Predictors of quality of life in older people living at home and in institutions. Aging Clin Exp Res 2004;16:212-20. http://dx.doi.org/10.1007/BF03327386.
- Underwood M, Lamb SE, Eldridge S, Sheehan B, Slowther A, Spencer A, et al. Exercise for depression in care home residents: a randomised controlled trial with cost-effectiveness analysis (OPERA). Health Technol Assess 2013;17. http://dx.doi.org/10.3310/hta17180.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. York: Centre for Health Economics, University of York; 1995.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. http://dx.doi.org/10.1002/hec.635.
- Little RJ, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley & Sons; 2002.
- Ratcliffe J, Longworth L, Young T, Bryan S, Burroughs A, Buxton M. Assessing health-related quality of life pre- and post-liver transplantation: a prospective multicenter study. Liver Transpl 2002;8:263-70. http://dx.doi.org/10.1053/jlts.2002.31345.
- Donner A, Klar N. Pitfalls of and controversies in cluster randomization trials. Am J Public Health 2004;94:416-22. http://dx.doi.org/10.2105/AJPH.94.3.416.
- Bell BA, Morgan GB, Kromey JD, Ferron JM. The Impact of Small Cluster Size on Multilevel Models: A Monte Carlo Examination of Two-Level Models with Binary and Continuous Predictors. Alexandria, VA: American Statistical Association; 2010.
- Sauzet O, Wright KC, Marston L, Brocklehurst P, Peacock JL. Modelling the hierarchical structure in datasets with very small clusters: a simulation study to explore the effect of the proportion of clusters when the outcome is continuous. Stat Med 2013;32:1429-38. http://dx.doi.org/10.1002/sim.5638.
- Lievesley N, Bowman C, Crosby G. The Changing Role of Care Homes. London: Bupa and Centre for Policy on Ageing; 2011.
- Damian J, Pastor-Barriuso R, Valderrama-Gama E, de Pedro-Cuesta J. Factors associated with falls among older adults living in institutions. BMC Geriatr 2013;13. http://dx.doi.org/10.1186/1471-2318-13-6.
- Rapp K, Becker C, Cameron ID, König H-H, Büchele G. Epidemiology of falls in residential aged care: analysis of more than 70,000 falls from residents of Bavarian nursing homes. J Am Med Dir Assoc 2012;13:187.e1-e6. http://dx.doi.org/10.1016/j.jamda.2011.06.011.
- Whitney J, Close JCT, Jackson SHD, Lord SR. Understanding risk of falls in people with cognitive impairment living in residential care. J Am Med Dir Assoc 2012;13:535-40. http://dx.doi.org/10.1016/j.jamda.2012.03.009.
- Underwood M, Lamb SE, Eldridge S, Sheehan B, Slowther AM, Spencer A, et al. Exercise for depression in elderly residents of care homes: a cluster-randomised controlled trial. Lancet 2013;382:41-9. http://dx.doi.org/10.1016/S0140-6736(13)60649-2.
- Kerse N, Peri K, Robinson E, Wilkinson T, Von Randow M, Kiata L, et al. Does a functional activity programme improve function, quality of life, and falls for residents in long term care? Cluster randomised controlled trial. BMJ 2008;337:912-15. http://dx.doi.org/10.1136/bmj.a1445.
- Helping Measure Person-Centred Care. London: The Health Foundation; 2014.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
Appendix 1 General practitioner’s letter requesting confirmation of participant eligibility
Appendix 2 Occupational therapy information leaflet
What is Occupational Therapy?
Occupational Therapy helps a person to achieve health and well-being through taking part in everyday tasks, such as dressing.
The therapist’s knowledge of adapting activities and surroundings is used to help people achieve the things they want to do and promote safety.
What will it involve?
The Occupational Therapist will be working with residents to help them carry out day-to-day activities such as:
-
Moving around the home
-
Dressing and grooming
-
Washing, bathing or showering
-
Eating and drinking
The OT in Care Homes study
At the start of the study homes will be divided into 2 groups, one group will receive the services of an occupational therapist for about 10 weeks. The other group will continue as before during the study but will receive staff training at the end.
Full details of the study are available from
[contact details removed]
What would happen if I take part?
A plan will be agreed between the resident and the occupational therapist/s. The aim is to help the resident achieve what they want to do. It will not involve anything they do not want to work on. It may include any of the following:
-
Information and advice
-
Ways of managing both for the resident and their carers
-
-
Activity and treatment
-
Relearning ways of doing activities or trying new methods
-
Activities aimed at improving the residents’ abilities, they may be asked to practice between sessions
-
Techniques to Increase/maintain mobility
-
-
Equipment to help them manage
-
Walking aids
-
Equipment to help the resident with activities such as washing and eating.
-
-
Home adaptations
-
Advice on layout of room furniture for ease of use
-
Advice to reduce hazards
-
Supply grab rails
-
-
Wheelchairs
-
Advice on mobility, posture and comfort
-
-
Seating
-
Dining room chair
-
Armchair
-
-
Health and safety promotion
-
Reducing the risk of falls
-
Increasing/maintaining activity levels
-
-
Referrals
-
Referrals can be made to other specialists according to the residents’ wishes and needs
-
Benefits of taking part
It is hoped that the time spent with the therapists will help the residents’ mobility and give them more choice over their day to day activities, in a safe and knowledgeable way.
For further information about Occupational Therapy please contact:
[contact details removed].
Appendix 3 Participant information sheet
Study Title ‘Cluster randomised controlled trial of an occupational therapy intervention for residents with stroke in UK care-homes’
Study Number: REC09/H1210/88
What is the purpose of the study?
This 4 year-long study is being carried out to assess the value of providing a targeted course of occupational therapy to people living in a residential or nursing home after stroke. This service has been found to be of value to people living in their own homes, and to people after a stroke. It has been found to be helpful in terms of improving their independence, their ability to take part in everyday activities, and their mobility. However, occupational therapy is less readily available to people living in residential or nursing homes.
Why have I been chosen?
The service is being provided in the way that it would if it were part of the National Health Service (NHS) or Social Services. Consequently, many of the residents of your home are being invited to take part.
Do I have to take part?
It is up to you to decide whether or not to take part. If you do decide to take part you would be given this information sheet to keep and be asked to sign a consent form. If you decide to take part you are still free to withdraw at any time and without giving a reason. This would not affect the standard of care you receive.
What would happen to me if I take part?
All the homes that participate in the study will either receive the service of an occupational therapist or not for a three month period (on top of any services the home currently receives). However, the computer will randomly decide, as if by the toss of a coin, whether your home receives the therapy.
What do I have to do and what does the therapist do?
If you decide to take part, you will be assessed four times-at 0 months, 3 months, 6 months and finally at 12 months. You may be seen by an occupational therapist, who will deliver therapy according to your needs. The assessments will ask you various questions about your day to day activities. The initial assessments will also look at your communication and clarity of thought.
The therapists providing the service will ask you about your ability to take part in day to day activities and, if the therapist feels that he/she can help you to keep your mobility and or prevent you from losing your independence, they will suggest one of a number of things to help. This may include:
-
Providing a piece of equipment or adapting something (such as raising the height of your chair)
-
Providing advice
-
Providing activities, which he/she will practice with you and ask you to continue to practice between visits
-
Providing exercises for you to practice.
The therapists will arrange a time that is convenient for you and this would not restrict your lifestyle in any way. The therapists providing the therapy would be visiting your place of residence for about 3 months, but as an individual you may only be seen a few times (depending on your needs).
What are the disadvantages or risks of taking part?
The services of an occupational therapist are not thought to put individuals at risk. The therapists would not ask you to do things that you do not want to and you are free to stop at any time. At worst, the services the therapist offers may not have any measurable benefits.
What are the advantages of taking part?
We hope that we can demonstrate that the services of an occupational therapist would be helpful to people living in either residential or nursing homes after a stroke. However, this cannot be guaranteed. The information we get from this study may help us to help people participate in day to day activities more easily and maintain this ability for a longer period of time.
What if new information becomes available?
This is the largest study of its kind and will add to our knowledge, but other studies may be necessary before practice is changed. If information becomes available from other work, it will add to our knowledge, but this study will continue as planned.
What happens when the research study stops?
The occupational therapy service provided by this study will stop when the study finishes. However, the services provided by Social Services and the NHS will continue unaffected.
What if something goes wrong?
If you are harmed by taking part in this research project, there are no special compensation arrangements. If you are harmed due to someone’s negligence, then you may have grounds for a legal action but you may have to pay for it. Regardless of this, if you wish to complain about any aspect of the way you have been approached or treated during the course of this study, the normal NHS complaints mechanisms may be available to you.
What if there is a problem?
If you have a concern about any aspect of this study, you should ask to speak to the researchers who will do their best to answer your questions. If you remain unhappy and wish to complain formally, you can do this by contacting [contact details removed].
Will my taking part in this study be kept confidential?
All information which is collected about you during the course of the research will be kept strictly confidential. Any information about you which leaves the hospital/surgery will have your name and address removed so that you cannot be recognised from it. Your General Practitioner (GP) and other therapists responsible for your care would be notified that you are taking part in the study (with your permission).
What will happen to the results of the study?
The results of the study will be presented to stroke survivors, NHS and Social Services staff and will be published in a scientific journal. Neither the presentations nor publications will identify individuals or homes who participated in the study.
Who is organising and funding the research?
The study is being funded by the National Institute for Health Research, part of the NHS.
Who has reviewed this study?
Coventry research ethics committee has reviewed this study.
Who do I contact for further information?
Please contact [contact details removed] for more information. You will be given a copy of the information sheet and a signed consent form to keep.
Thank you for reading this.
Appendix 4 Participant consent form
Appendix 5 Consultee information sheet
Study Number: REC09/H1210/88
Study Title ‘Cluster randomised controlled trial of an occupational therapy intervention for residents with stroke in UK care-homes (acronym – OTCH).’
Invitation
Your relative (it could also be a friend or someone you care for, but for brevity this document will use the term ‘relative’) is being invited to take part in a research study. Before you decide whether you agree to their participation it is important for you to understand why the research is being done and what it will involve. Please take time to read the following information carefully and discuss it with care home staff, friends, relatives and health care professionals if you wish. Ask us if there is anything that is not clear or if you would like more information. Do take the time to consider this request.
Who can act as a consultee?
Where people cannot take the decision to consent to be involved in a research project then a consultee must be appointed. A consultee can either be ‘personal’ or ‘nominated’. A personal consultee is someone unconnected with the research who knows the potential research participant in a personal capacity and is able to advise on the person’s wishes or feelings. This can be a friend, family member or court appointee. A ‘nominated’ consultee’ is someone unconnected with the research, appointed by the researcher, to advise the researcher about the person’s wishes and feeling in relation to the project. This can be another health-care worker but they must not have any connection with the study. Before a nominated consultee is appointed, the researcher will take all reasonable steps to identify a personal consultee.
What is the role of the consultee?
The consultee advises the researcher on what the participant’s wishes and feelings would be if they were able to consent for themselves, and on whether they should take part. The consultee does not give consent, only advice. The responsibility to decide whether the participant should be entered into the research lies ultimately with the researcher. Consultees will be provided with information about the research project and will be given the opportunity to discuss it and their role as consultee. All consultees must be able to understand their role and be willing to undertake it.
How do I find out more if I am approached to be a consultee?
Further information is available in the Department of Health’s ‘Guidance on nominating a consultee for research involving adults who lack capacity to consent’.
This is also available from the research team, please ask if you would like a copy.
What is the purpose of the study?
This four year-long study is being carried out to assess the value of providing a targeted course of occupational therapy to people living in a residential or nursing home after stroke. This service has been found to be of value to stroke survivors living in their own homes. It has been found to be helpful in terms of improving their independence, their ability to take part in everyday activities, and their mobility. However, occupational therapy is less readily available to people living in residential or nursing homes.
Why has my relative been chosen?
The service is being provided in the way that it would if it were part of the National Health Service (NHS) or Social Services. Consequently, many of the residents of this care home are being invited to take part.
Does my relative have to take part?
We would like you to think very carefully about whether or not this person would have wanted to join the study. If your opinion is that he/she would have decided to take part, you would be given this information sheet to keep and be asked to sign a form indicating your view allowing your relative to participate in the study. If you later decide that he/she no longer wishes to take part, please inform us and he/she will be withdrawn from the study. You do not need to give a reason and it will not affect the standard of care your relative receives.
What will happen to my relative if they take part?
All the homes that participate in the study will either receive the service of an occupational therapist or not for a three month period (on top of any existing services the home receives). However, the computer will randomly decide, as if by the toss of a coin, whether their home receives the therapy.
What does my relative have to do and what does the therapist do?
If you indicate that your relative would like to take part, they will be assessed four times-at 0 months (initial assessment), 3 months, 6 months and finally at 12 months by a researcher.
Your relative may be seen by an occupational therapist, who will deliver therapy according to their needs. The assessments will cover questions about day to day activities. The initial assessments will also look at their communication and clarity of thought.
If your relative’s care home is allocated the service of an occupational therapist, the therapists providing the service will ask them about their ability to take part in day to day activities. Then, if the therapist feels that he/she can help them with their mobility and other day to day activities, they may suggest a number of things that could be helpful. This may include:
-
Providing a piece of equipment or adapting something (such as a non-slip mat to stop their plate moving around the table)
-
Providing advice
-
Providing activities, which he/she will practice with the resident and ask them to continue to practice between visits
-
Providing exercises for your relative to practice.
The therapists will arrange a time that is convenient for your relative and this would not restrict their lifestyle in any way. The therapists providing the therapy would be visiting the care home for about 3 months, but as an individual your relative may only be seen a few times (depending on their needs).
What are the disadvantages or risks of taking part?
There is no evidence that the services of an occupational therapist put individuals at risk. The therapists would not ask your relative to do things that he/she would not want to do. Your relative is free to stop at any time. At worst, the services the therapist offers may not be effective and so your relative would have no benefit from their visits.
What are the advantages of taking part?
We hope that we can demonstrate that the services of an occupational therapist would be helpful to people living in a care home after a stroke. However, this cannot be guaranteed. The information we get from this study may help us to help people participate in day to day activities more easily and maintain this ability for a longer period of time.
What if new information becomes available?
This is the largest study of its kind and will add to our knowledge, but other studies may be necessary before practice is changed. If information becomes available from other work, it will add to our knowledge, but this study will continue as planned.
What happens when the research study stops?
The occupational therapy service provided by this study will stop when the study finishes. However, the services provided by Social Services and the NHS will continue unaffected.
What if something goes wrong?
If your relative is harmed by taking part in this research project, there are no special compensation arrangements. If they are harmed due to someone’s negligence, then they may have grounds for legal action but they may have to pay for it. Regardless of this, if you or your relative wish to complain about any aspect of the way you have been approached or treated during the course of this study, the normal NHS complaints mechanisms may be available to you.
What if there is a problem?
If you have a concern about any aspect of this study, you should ask to speak to the researchers who will do their best to answer your questions. If you remain unhappy and wish to complain formally, you can do this by contacting [contact details removed].
Will their taking part in this study be kept confidential?
All information which is collected about your relative during the course of the research will be kept strictly confidential. Any information about them which leaves the hospital/surgery will have their name and address removed so that they cannot be recognised from it. Your relative’s General Practitioner (GP) and other therapists responsible for their care would be notified that they are taking part in the study (with their permission).
What will happen to the results of the study?
The results of the study will be presented to stroke survivors, NHS and Social Services staff, and will be published in a scientific journal. Neither the presentations nor publications will identify individuals or homes who participated in the study. (If you would like copies of the publications please inform [contact details removed] at the address below).
Who is organising and funding the research?
The study is being funded by the National Institute for Health Research, part of the NHS.
Who has reviewed this study?
Coventry research ethics committee has reviewed this study.
Who do I contact for further information?
Please contact [contact details removed] for more information.
You will be given a copy of the information sheet and a signed consent form to keep.
Thank you for reading this.
Appendix 6 Consultee declaration form
Appendix 7 Demographic front sheet
Appendix 8 Current medication
Appendix 9 Initial participant interview
Appendix 10 Treatment log
At each visit please record the approximate time in minutes spent on each of the areas below:
Appendix 11 Occupational Therapy intervention for residents with stroke living in UK Care Homes training workbook
Appendix 12 Information for occupational therapists delivering interventions for Occupational Therapy intervention for residents with stroke living in UK Care Homes trial
What does the study involve?
This study aims to look at the effects and value of a targeted course of occupational therapy on people living with stroke in nursing and residential homes.
What will my assessment consist of?
Your assessment will be the same as you would conduct on any other patient. You will have access to the baseline outcome measures (Barthel index and Rivermead mobility score) to help inform your assessment and develop a patient specific treatment plan. You can use information from family and carers to inform your assessment if the patient has difficulties in communicating.
What treatments will I be expected to offer?
You will be offering all the usual interventions that you provide as an occupational therapist. The interventions you will be offering will address the performance of a task, the environment in which the task is conducted, and help address any impairments that may limit the performance of the ADL’s.
These interventions could involve:
-
task-specific practice
-
supplying aids/adaptations to the environment/help to simplify the task
-
delivering specific therapeutic interventions
-
educating carers in encouraging/assisting the patient.
Is there anything I shouldn’t do?
The aim of this study is to look at the role of occupational therapy in improving function for people post stroke so some interventions such as reminiscent therapy and relaxation groups are not within the remit of this study.
The outcome measures will highlight the main functional difficulties of the patients so treatment goals can focus on these key areas of functional difficulties.
Does education of carers/family count as a treatment?
Yes, any time spent on this activity counts as part of an intervention. There is space to record this in the treatment log. Discussions with other members of staff or agencies (such as the GP) are also to be included in the treatment log.
How do I record my interventions?
All interventions are split into types and times spent on each intervention needs to be recorded for each contact with the patient.
What if the patients’ needs change during treatment?
The aim is to provide the patients with standard occupational therapy care so, as the patients’ needs change, you can adapt you treatments to address patient specific needs as you would do with any other patient.
What equipment can I supply?
You can supply the adaptive equipment you would supply as an occupational therapist if it will benefit the patient i.e. grab rails, toilet raises, cutlery, mobility aids, seating, etc. There is the possibility of funding small pieces of equipment through the study if it is not available through the normal routes.
Where do I write my notes?
You may be required to write in the patients’ notes at the nursing home.
You will also have your profession specific notes that you will need to keep securely in a locked cabinet at your normal place of work.
How much time can I spend with each person?
This is dependent on the individual goals you have set with the patient and what interventions will best suit their needs. As mentioned previously, the aim of this study is to provide a typical occupational therapy intervention.
What happens if a patient gets hurt?
If a patient falls during a treatment or if the equipment provided fails it is described as an adverse event. All adverse events need to be reported to the study co-ordinator so they can be logged. You may also be required to complete the documentation used by the care home for reporting falls/injuries.
Appendix 13 Screening and outcome measures
Mini-Mental State Examination
The MMSE was used to assess the cognitive function of patients. 75,87 The evaluation uses a series of 12 questions and tests in which patients are assessed in terms of their memory, language, attention and orientation. The test is scored out of 30, 30 being the maximum. A score between 0 and 20 is indicative of cognitive impairment.
Sheffield Screening Test for Acquired Language Disorders
The Sheffield Screening Test for Acquired Language Disorders was used to assess the severity of communication problems in study participants and to identify those with aphasia. 74 The test involves a 10-item questionnaire which is divided into two parts: one assessing receptive skills and one assessing expressive skills. The test is scored from 0 to 20, with 20 signifying no impairment. Participants scoring < 15 are considered to have a language disorder.
Barthel Index of Activities of Daily Living
The BI consists of a questionnaire which scores the patients’ ability to complete 10 activities. 76,77 The assessment consists of questions related to self-care activities (feeding, grooming, bathing, dressing, bowel and bladder care, and toilet use), and mobility-based activities (ambulation, transfers and stair climbing). The results of the questionnaire are added to provide a measure of independence ranging from 0 to 4 (very severe), 5 to 9 (severe), 10 to 14 (moderate), 15 to 19 (mild) and 20 (completely independent).
Rivermead Mobility Index
The RMI was used to evaluate the effectiveness of occupational therapy on the mobility of patients. 79,83 The index is delivered using a 15-item yes/no questionnaire which focuses on ambulation but also assesses the participant’s ability to run, stand unsupported, use stairs, and bathe unsupervised. Positive responses to each question are scored with 1 point, up to a maximum of 15.
Geriatric Depression Scale
The GDS is a screening test for depression in the elderly in which patients answer ‘yes’ or ‘no’ to questions which refer to how they felt over the preceding week, and can be delivered using either a long-form (30 questions) or a short-form (15 questions) questionnaire. 84,85 For the short version with 15 questions, scores of 0 to 4 are considered normal; 5 to 8 indicate mild depression; 9 to 11 indicate moderate depression; and 12 to 15 indicate severe depression.
EQ-5D-3L
The European Quality of Life EQ-5D (EQ-5D-3L) is a standardised measure of HRQoL, developed by the EuroQol Group in order to provide a simple, generic measure of health for clinical and economic appraisal. 95,99,114 The EQ-5D descriptive system comprises the following five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension has three levels of scoring (3L; no problems, some problems, extreme problems) and is expressed as a digit (1, 2 or 3). The digits for the five dimensions are combined in a five-digit permutation describing the respondent’s health state (e.g. 11121 moderate pain and discomfort, 13111 extreme problems with self-care). The study used the EQ-5D-3L measure to conduct an assessment of HRQoL and an economic evaluation.
Appendix 14 Adverse events reporting form
Appendix 15 Summary of participants moving home during the course of the trial
| Time point | Randomisation arm | |
|---|---|---|
| OT | Control | |
| Number of participants moved during trial | 21 | 16 |
| Last follow-up at baseline | 1 (died) | 0 |
| Last follow-up at 3 months | 1 (died) | 0 |
| Last follow-up at 6 months | 2 (1 died and 1 lost) | 0 |
| Last follow-up at 12 months | 17 | 16 |
Appendix 16 Data tables for Figures 2 and 3
Data table for Figure 2
| BI score (0–20) | RMI score (0–15) | Count | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Grand total | |
| 0 | 125 | 13 | 3 | 1 | 142 | ||||||||||||
| 1 | 88 | 26 | 10 | 1 | 1 | 126 | |||||||||||
| 2 | 56 | 27 | 6 | 4 | 2 | 95 | |||||||||||
| 3 | 33 | 18 | 9 | 5 | 1 | 66 | |||||||||||
| 4 | 33 | 13 | 14 | 5 | 1 | 1 | 67 | ||||||||||
| 5 | 15 | 14 | 2 | 11 | 2 | 1 | 2 | 47 | |||||||||
| 6 | 18 | 16 | 11 | 4 | 2 | 1 | 1 | 1 | 54 | ||||||||
| 7 | 9 | 10 | 6 | 6 | 3 | 3 | 5 | 3 | 1 | 46 | |||||||
| 8 | 2 | 9 | 7 | 8 | 6 | 3 | 2 | 5 | 1 | 1 | 1 | 1 | 1 | 47 | |||
| 9 | 4 | 6 | 3 | 3 | 8 | 2 | 1 | 1 | 4 | 1 | 1 | 34 | |||||
| 10 | 4 | 3 | 5 | 5 | 3 | 3 | 4 | 2 | 2 | 2 | 1 | 1 | 1 | 36 | |||
| 11 | 3 | 1 | 5 | 5 | 2 | 5 | 4 | 5 | 3 | 3 | 2 | 1 | 1 | 1 | 1 | 42 | |
| 12 | 1 | 3 | 1 | 6 | 1 | 6 | 1 | 1 | 1 | 21 | |||||||
| 13 | 1 | 5 | 3 | 4 | 4 | 7 | 2 | 3 | 2 | 2 | 1 | 34 | |||||
| 14 | 1 | 1 | 4 | 1 | 5 | 2 | 5 | 3 | 5 | 2 | 2 | 2 | 33 | ||||
| 15 | 1 | 1 | 3 | 5 | 3 | 2 | 4 | 4 | 1 | 1 | 25 | ||||||
| 16 | 3 | 1 | 11 | 6 | 2 | 3 | 3 | 2 | 31 | ||||||||
| 17 | 2 | 3 | 2 | 4 | 2 | 2 | 1 | 1 | 17 | ||||||||
| 18 | 1 | 1 | 4 | 3 | 3 | 2 | 1 | 2 | 1 | 2 | 20 | ||||||
| 19 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 12 | ||||||||
| 20 | 1 | 2 | 1 | 6 | 7 | 17 | |||||||||||
| Count grand total | 386 | 161 | 83 | 68 | 37 | 42 | 33 | 56 | 29 | 33 | 23 | 16 | 20 | 10 | 6 | 9 | 1012 |
Data table for Figure 3
| BI severity rating | BI score (0–20) | Baseline | 3-month follow-up | 6-month follow-up | 12-month follow-up | ||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||
| Very severe | 0 | 85 | 56 | 91 | 63 | 70 | 60 | 67 | 46 |
| 1 | 63 | 65 | 46 | 54 | 63 | 52 | 51 | 50 | |
| 2 | 53 | 44 | 37 | 43 | 39 | 43 | 46 | 28 | |
| 3 | 28 | 38 | 35 | 38 | 31 | 27 | 20 | 20 | |
| 4 | 39 | 31 | 33 | 22 | 24 | 29 | 16 | 24 | |
| Sum | 268 | 234 | 242 | 220 | 227 | 211 | 200 | 168 | |
| % | 47.69 | 50.11 | 50.52 | 56.27 | 53.54 | 57.18 | 56.34 | 58.95 | |
| Severe | 5 | 26 | 22 | 31 | 17 | 24 | 12 | 17 | 10 |
| 6 | 30 | 26 | 23 | 16 | 13 | 12 | 14 | 12 | |
| 7 | 22 | 24 | 17 | 18 | 18 | 22 | 17 | 10 | |
| 8 | 32 | 17 | 19 | 12 | 15 | 12 | 13 | 11 | |
| 9 | 19 | 15 | 17 | 14 | 19 | 10 | 8 | 8 | |
| Sum | 129 | 104 | 107 | 77 | 89 | 68 | 69 | 51 | |
| % | 22.95 | 22.27 | 22.34 | 19.69 | 20.99 | 18.43 | 19.44 | 17.89 | |
| Moderate | 10 | 21 | 15 | 17 | 18 | 17 | 13 | 10 | 9 |
| 11 | 24 | 18 | 12 | 7 | 14 | 8 | 7 | 5 | |
| 12 | 10 | 12 | 12 | 7 | 7 | 8 | 9 | 9 | |
| 13 | 19 | 15 | 12 | 9 | 11 | 10 | 11 | 9 | |
| 14 | 17 | 16 | 11 | 15 | 9 | 15 | 15 | 8 | |
| Sum | 91 | 76 | 64 | 56 | 58 | 54 | 52 | 40 | |
| % | 16.19 | 16.27 | 13.36 | 14.32 | 13.68 | 14.63 | 14.65 | 14.04 | |
| Mild | 15 | 16 | 10 | 19 | 4 | 11 | 10 | 4 | 7 |
| 16 | 21 | 10 | 18 | 10 | 9 | 9 | 8 | 4 | |
| 17 | 11 | 7 | 12 | 7 | 18 | 4 | 13 | 4 | |
| 18 | 11 | 10 | 7 | 7 | 7 | 5 | 6 | 7 | |
| 19 | 5 | 9 | 5 | 7 | 2 | 5 | 1 | 2 | |
| Sum | 64 | 46 | 61 | 35 | 47 | 33 | 32 | 24 | |
| % | 11.39 | 9.85 | 12.73 | 8.95 | 11.08 | 8.94 | 9.01 | 8.42 | |
| Independent | 20 | 10 | 7 | 5 | 3 | 3 | 3 | 2 | 2 |
| Sum | 10 | 7 | 5 | 3 | 3 | 3 | 2 | 2 | |
| % | 1.78 | 1.50 | 1.04 | 0.77 | 0.71 | 0.81 | 0.56 | 0.70 | |
| N | 562 | 467 | 479 | 391 | 424 | 369 | 355 | 285 | |
Appendix 17 Occupational Therapy intervention for residents with stroke living in UK Care Homes health-care resource usage questionnaire: 12 months
Appendix 18 European Quality of Life-5 Dimensions, 3 Levels health profile
| EQ-5D-3L dimension | Level | Assessment point | |||
|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | ||
| Mobility | Level 1 | 11.9 | 11.0 | 10.5 | 11.1 |
| Level 2 | 44.5 | 40.3 | 39.1 | 31.9 | |
| Level 3 | 43.5 | 48.7 | 50.5 | 57.0 | |
| Self-care | Level 1 | 12.6 | 10.9 | 9.4 | 9.6 |
| Level 2 | 29.8 | 29.9 | 28.9 | 27.0 | |
| Level 3 | 57.6 | 59.2 | 61.7 | 63.5 | |
| Usual activities | Level 1 | 16.0 | 18.3 | 23.4 | 18.2 |
| Level 2 | 30.1 | 32.3 | 27.7 | 32.4 | |
| Level 3 | 53.9 | 49.4 | 48.9 | 49.4 | |
| Pain/discomfort | Level 1 | 46.9 | 51.4 | 53.7 | 52.9 |
| Level 2 | 45.7 | 40.4 | 38.1 | 40.9 | |
| Level 3 | 7.4 | 8.3 | 8.1 | 6.2 | |
| Anxiety/depression | Level 1 | 46.5 | 53.0 | 55.3 | 54.4 |
| Level 2 | 45.0 | 36.6 | 36.8 | 37.8 | |
| Level 3 | 8.5 | 10.4 | 7.9 | 7.8 | |
List of abbreviations
- A&E
- accident and emergency
- ADL
- activities of daily living
- BI
- Barthel Index of Activities of Daily Living
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D-3L
- European Quality of Life-5 Dimensions, three levels
- GDS
- Geriatric Depression Scale
- GDS-15
- Geriatric Depression Scale-15 items
- GP
- general practitioner
- HRQoL
- health-related quality of life
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- MMSE
- Mini-Mental State Examination
- NIHR
- National Institute for Health Research
- OR
- odds ratio
- OT
- occupational therapy
- OTCH
- Occupational Therapy intervention for residents with stroke living in UK Care Homes
- PCT
- primary care trust
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCP
- Royal College of Physicians
- RCT
- randomised controlled trial
- RMI
- Rivermead Mobility Index
- SD
- standard deviation
- SE
- standard error
- TAC
- trial administrative centre
- TIA
- transient ischaemic attack
- TSC
- Trial Steering Committee