Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/53/25 and 11/136/108. The contractual start date was in July 2010. The draft report began editorial review in March 2015 and was accepted for publication in November 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jamie Inshaw, Anna Turkova, Nigel Klein, Sarah Bernays, Julia Kenny, Eleni Nastouli, Karen Scott, Lynda Harper, Sara Paparini, Rahela Choudhury, Tim Rhodes, Abdel Babiker and Diana Gibb report grants from the PENTA Foundation during the conduct of the study. Jamie Inshaw, Anna Turkova, Nigel Klein, Julia Kenny, Eleni Nastouli, Karen Scott, Lynda Harper, Rahela Choudhury, Abdel Babiker and Diana Gibb report grants from the European Commission (FP7) during the conduct of the study.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Butler et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Antiretroviral therapy (ART) has dramatically improved the prognosis for human immunodeficiency virus (HIV)-infected children. It has reduced early morbidity and increased survival, with > 80% of children expected to reach adulthood. 1 HIV infection has been transformed from a devastating, rapidly progressive lethal condition into a chronic disease. Now the challenges for the treatment of HIV-infected children are to (1) maximise the benefit of ART, which prevents illness and encourages growth and development, (2) minimise long-term drug toxicity, (3) minimise the development of drug resistance so that children continue to have therapy options as they move through adolescence and into adulthood, and (4) improve quality of life as much as possible for young people on ART.
Following recent results from a randomised controlled trial, in 2008 paediatric ART guidelines advocated starting ART in infancy (< 12 months of age) in all those diagnosed, because of a high risk of disease progression. 2 Subsequent guidelines from the World Health Organization (particularly for African countries) recommended starting ART in all children aged < 2 years (20103) and then in all children aged < 5 years (20134), mainly for programmatic reasons. Even if ART is not started early, vertically infected children face many more years of ART than adults, often given throughout childhood. Therefore, there is a growing population of older children and young people who have already been on ART for many years and are continuing to face the challenge of taking daily medication. 5
A major challenge for young people with HIV infection, as for any chronic illness, is maintaining long-term adherence to treatment regimens. 6–8 The importance of adherence to the long-term success of ART in maintaining virological suppression and preventing the emergence of resistance has been established. 9–16 However, experience with HIV-infected young people suggests that, with current treatment strategies, adherence rates fall far below the 90–95% adherence associated with long-term success. 17–19 Furthermore some studies have demonstrated a decline in adolescent adherence over time associated with duration on therapy. 19 Impediments to adherence for young people have been broadly categorised into two main groups: problems with medication, such as taste and palatability issues, and adherence difficulties related to social situations. 20 Although there have been considerable attempts to improve drug formulations, thus partly addressing the first impediment, the social dimensions are more complex; interference with daily life recurs as a common theme in assessments of poor adherence in young people.
New treatment strategies that promote adherence, minimise the development of resistance and reduce long-term drug exposure while improving quality of life are required for young people ‘burning out’ on daily ART regimens. Approaches to achieve this include (1) simplification of therapy (i.e. minimising the number of pills or swapping from twice-daily to once-daily dosing), (2) treatment interruptions [e.g. based on levels of CD4 as in the Paediatric European Network for Treatment of AIDS (PENTA) 11 trial;21 not currently advocated] and (3) very short treatment interruptions (particularly at inconvenient times for taking medication such as weekends) with the aim of maintaining viral suppression (one such possible strategy is to give therapy during the week but allow a break at the weekend).
Intermittent therapy
CD4-guided treatment interruptions
The large Phase III Strategies for Management of Antiretroviral Therapy (SMART) trial evaluating a CD4-guided strategy in adults of stopping ART when the CD4 count was > 350 cells/mm3 and restarting ART when the CD4 count fell to < 250 cells/mm3 was stopped early because of evidence of increased disease progression and cardiovascular events, albeit at low rates, in the interruption arm. 22 Treatment interruption when viral load (VL) rebound occurs is now not recommended in adults.
The PENTA 11 Phase II trial [a randomised trial that compared CD4-guided planned treatment interruptions with continuous therapy (CT) in children aged 2–15 years] reported no significant increase in clinical progression in children undergoing planned treatment interruptions. 21 Further data on CD4 recovery and VL suppression following reintroduction of continuous ART in all children in this trial are awaited and interruptions using this strategy cannot be currently recommended. However, the initial findings of PENTA 11 provided reassurance for two other paediatric trials to investigate the impact of treatment interruptions {one evaluating interruptions following early limited ART in infants23 and the other evaluating CD4-guided interruptions in 600 older children [the BANA II trial; see www.bipai.org/Botswana/clinical-research.aspx (accessed 6 April 2016)]}.
Fixed-length treatment interruptions
In adults, trials of a fixed-length ART schedule of 1 week on and 1 week off therapy, based on the theory of autoimmunisation,24 showed high virological failure rates in patients following the 1 week on and 1 week off strategy compared with those on continuous or CD4-guided ART. 25,26
Very short treatment interruptions
An alternative approach is to use very short interruptions (short-cycle therapy; SCT) such that viral rebound should not occur, thus minimising the emergence of resistance as well as not compromising antiviral efficacy. This concept is based on the notion that > 95% adherence may not be necessary for virological suppression with all antiretroviral (ARV) regimens and that each ARV combination may have a unique adherence–resistance relationship. 9 Mathematical models of adherence and the emergence of resistance support this and the notion that otherwise strongly adherent patients might miss an acceptable number of doses of selected ARVs before resistance emerges. 27
Short-cycle therapy in adults and adolescents
Two Phase II trials of SCT in adults reported before the start of the BREATHER trial. First, a small single-arm pilot study of 5 days on, 2 days off ART in adults in the USA showed that long-term suppression of VL could be achieved. 28 Two of nine patients on protease inhibitor (PI)-based highly active antiretroviral therapy (HAART) had confirmed virological rebound by 48 weeks compared with one of 10 patients on nevirapine (NVP)-containing HAART and none of eight patients receiving efavirenz (EFV)-based regimens. As a result of this pilot, the randomised FOTO (Five On Two Off) trial in 60 adults with a VL of < 50 copies/ml on tenofovir (TDF)/emtricitabine (FTC)/EFV was conducted comparing daily ART with a strategy of 5 days on, 2 days off treatment. The 24-week results showed that all 25 patients in the FOTO arm and 24 of 28 patients (86%) in the daily arm who reached week 24 had a VL of < 50 copies/ml at this time point. 29 Reasons for not reaching 24 weeks were psychological (n = 2), because of the time burden (FOTO arm) (n = 3), pregnancy (n = 1) and loss to follow-up (daily ART arm) (n = 1); of note, all had a VL of < 50 copies/ml at discontinuation. There were six blips (VL 50–500 copies/ml) in the FOTO arm and nine in the daily ART arm to week 24; there were no instances of virological failure (confirmed VL > 400 copies/ml).
Second, in a trial in Uganda,30,31 146 adults who had a suppressed VL of < 50 copies/ml on a three-drug ART regimen were randomised to receive a week on, week off ART regimen (n = 32; this arm of the study was discontinued early), a 5 days on, 2 days off schedule (SCT) (n = 57) or CT (n = 57). The majority of subjects (94%) received an EFV-based regimen. The trial showed that SCT was not inferior to continuous HAART; there were 11 cases of failure in the CT group (including one death) and six in the SCT group (failure defined as VL > 1000 copies/ml, a decrease in CD4 count from randomisation of > 30% or a CD4 count of < 100 cells/mm3 on two consecutive occasions through the 72 weeks of follow-up; or VL > 400 copies/ml or development of an opportunistic infection at 72 weeks). Levels of resistance were no different between the SCT group and the CT group. For patients on HAART containing stavudine, there was a significant decrease in the incidence of lactic acidosis in the SCT arm compared with the CT arm.
The US-based Adolescent Trials Network conducted the only study of SCT in adolescents prior to the BREATHER trial, which had a non-randomised single-arm design to assess VL suppression (< 400 copies/ml, confirmed) on SCT (4 days on HAART, 3 days off) over 48 weeks. 32 Thirty-two participants aged 12–24 years and on a stable PI-based HAART regimen for at least 12 months were enrolled. Twelve of the 32 (38%) participants had confirmed virological rebound by 48 weeks, seven out of 15 (47%) of those infected before 9 years of age and five out of 17 (29%) of those infected after 9 years of age (p = 0.5). However, 75% of children had been exposed to five or more drugs in the past, with a previous history of virological failure. Overall, adherence was good (88% of participants showed > 90% adherence), with no difference between those with and those without VL rebound (p = 0.6).
Antiretroviral agents, viral load rebound and resistance
The plasma half-life of ARV agents in an ART regimen [or intracellular half-life of nucleoside reverse transcriptase inhibitor (NRTIs)] and their genetic barrier to resistance are important factors that may influence VL rebound and the development of resistance during a strategy of stopping therapy for 2 days every week.
An ART regimen containing three drugs, with relatively similar long half-lives and maintained therapeutic concentrations of > 2 days when treatment is stopped, would be an ideal regimen to avoid risk of VL rebound during the interruption and minimise the development of resistance. The regimen with the most favourable pharmacokinetic profile is TDF/FTC/EFV (median intracellular half-life of 150 hours, median intracellular half-life of 39 hours and plasma half-life of between 36 and 100 hours, respectively). 33 Coformulation of TDF/FTC/EFV34 in a single pill makes this a very attractive combination for older adolescents and young adults. However, in the Ugandan trial,31 EFV was given with either stavudine/lamivudine (3TC) or zidovudine (ZDV)/3TC without evidence of inferior virological performance, even though stavudine, 3TC and ZDV have shorter half-lives than TDF and FTC. PI drugs [e.g. lopinavir/ritonavir (Kaletra®; AbbVie Inc.), the most commonly used PI in children and adolescents] have substantially shorter half-lives and therefore if a regimen containing two NRTIs (with longer half-lives) and a PI (with a shorter half-life) is stopped, ‘functional dual NRTI therapy’ may result, with a risk of VL rebound and the development of resistance.
Data on viral dynamics in the first few days following treatment cessation are scarce. Jacobsen et al. ,24 in a four-arm study of planned treatment interruption ± HIV immunisation, reported viral rebound to > 50 copies/ml following treatment interruption at a median [interquartile range (IQR)] of 15 (8–31) days for those with a prior planned treatment interruption and 21 (13–30) days for those without a prior planned treatment interruption. Harrigan et al. ,35 while assuming a constant rate of viral increase that starts as soon as the patient stops therapy, suggest that many patients stopping therapy (previously suppressed) will have an increase in plasma viral HIV ribonucleic acid (RNA) of about 0.2 log/day and will reach detectable levels (> 50 copies/ml) only within 1–2 weeks of stopping therapy.
A substudy of PENTA 11 evaluated the pharmacokinetics, VL rebound and resistance profiles in 35 children stopping non-nucleoside reverse transcriptase inhibitor (NNRTI)-based ART. 36 In total, 21 children followed a staggered stop strategy whereby the NNRTI (NVP or EFV) was stopped at randomisation and the remaining two NRTI drugs were stopped 7–14 days later, and 14 children followed a replacement strategy whereby the NNRTI was replaced with a PI and all drugs were stopped 7–14 days later. Results of HIV VL testing in eight children following the staggered stop strategy and seven children following the replacement strategy showed that the majority of children still had undetectable HIV RNA 5–8 days after stopping all drugs (minimum 12 days after stopping the NNRTI). No NNRTI resistance mutations were detected in any of the children in the substudy.
Rationale and objectives
Adherence issues tend to worsen for older children and adolescents after they start taking charge of their own medication (after HIV diagnosis is disclosed); self-consciousness and not wanting to be different from peers predominate. There is the additional burden of secrecy around HIV infection and ART,15 and social and family pressures prevent young people from sharing information of their diagnosis or treatment with friends. 37 The result is often worsening adherence with frequently missed doses, particularly at weekends, which are typically times of socialising. Factors contributing to this include alcohol ingestion19 as well as the absence of school and overnight stays with friends at weekends. Therefore, a regimen in which ARVs need be taken only as part of the daily routine during weekdays could be attractive for older children (> 8 years) as well as teenagers and young people (16–24 years) who continue to be followed at paediatric or affiliated adolescent units.
Data from adult studies evaluating the strategy of 5 days on, 2 days off were promising, with low rates of virological rebound seen. However, no randomised trial had been undertaken in older children and adolescents, a population with potentially more to gain in terms of quality of life, long-term adherence to medication and the potential for better treatment options in adulthood. The effect on overall adherence of allowing 2 days off treatment per week was unknown and it was therefore important to first evaluate the strategy in young people who have a history of good adherence, the argument being that offering this strategy could prevent ad hoc missed doses occurring. In view of the relatively shorter half-life of PIs and because both adult trials referred to above were undertaken with EFV-based regimens, enrolment was limited to children who are on, or who are willing to switch to, a regimen containing EFV.
The BREATHER trial aimed to assess whether or not young people with chronic HIV infection undergoing SCT of 5 days on and 2 days off following complete virological response to first-line ART for at least 12 months maintained the same level of VL suppression as those on CT. Importantly, because of insufficient data on short-term VL rebound after stopping ART in this population, the trial incorporated an initial pilot phase to assess the safety of the SCT strategy.
Risks and benefits
The potential risks of the SCT strategy were as follows:
-
The main risk was that the SCT strategy would prove ineffective at maintaining VL suppression, either because VL could not be maintained below detection levels during 2 days off ART or because of non-adherence to the strategy by extending the time off treatment. However, if a raised VL was confirmed on repeat testing (carried out within 1 week) then the participant was placed back on CT.
-
An additional risk was that young people who had been fully adherent to ART before enrolment in the study might extend the permitted very short interruptions and that overall adherence would decline. Of note, there was equipoise about whether adherence would be better or worse in the SCT arm than in the CT arm as young people in the continuous arm may also not take their treatment regularly. All young people were given a diary to record when they had taken ART and were asked to comment about difficulties in remembering to take medication. Rebounding VL comes with the risk of development of resistance (particularly to EFV and 3TC, which have low genetic barriers to resistance), which in turn may limit future therapeutic options. Resistance testing was performed on all young people who lost virological suppression in either arm at the point of loss of suppression (≥ 50 copies/ml), as well as on any subsequent samples with a HIV-1 RNA level of ≥ 1000 copies/ml.
The potential benefits of the SCT strategy were:
-
improvement in quality of life from having weekends free from taking medication
-
improved long-term adherence during the week
-
decreased long-term toxicity of ARV drugs (particularly relevant for some NRTI drugs, e.g. ZDV and TDF)
-
decreased cost, which is important to any health-care service and in particular for many parts of the world where HIV prevalence is highest.
Young people and carers were fully informed of the possible benefits and known risks by means of a patient information sheet as appropriate and this was reinforced by discussions with the study research teams at the individual sites prior to enrolment.
Pilot study
Because of insufficient data on short-term VL rebound after stopping ART in this population, the trial incorporated an initial pilot phase in selected centres. The aim of the pilot phase was to ensure that the SCT strategy did not result in a high proportion of young people with an increased VL (≥ 50 copies/ml) of ART in the first few weeks.
Those enrolled in the pilot and randomised to SCT were permitted to take only Saturday and Sunday off treatment. They received VL testing on Monday, prior to resumption of ART. Recruitment to the main trial commenced only when all pilot participants had completed 3 weeks on the study and the results were reviewed by the Independent Data Monitoring Committee (IDMC) to ensure that there were no safety concerns. Preset criteria for stopping included a HIV-1 RNA level of ≥ 50 copies/ml (validated by a HIV-1 RNA level of ≥ 50 copies/ml on the same sample) at weeks 1, 2 or 3 following weekend interruption and before restarting ART in more than five participants as this would have given evidence that VL suppression rates on a SCT strategy in general were < 90% [10/15 suppressed = 66%, 95% exact confidence interval (CI) 38% to 88%]. Data from the pilot phase were reviewed by the IDMC who identified no safety concerns and recommended that recruitment continue.
Substudies
Plasma and cells were stored for both arms throughout the trial for the virology, inflammatory biomarkers and immunology substudies. The rationale behind the biomarker substudy was to determine whether or not any markers of inflammatory response were increased among children in the SCT arm compared with the CT arm, even if VL suppression was no different. This has been observed in adult trials such as the SMART trial in which interruptions resulted in an increase in VL. 22
There was also a qualitative substudy that aimed to (1) evaluate the acceptability of SCT and of the trial from the perspectives of young people participating; (2) explore whether or not they perceived that SCT facilitated improved adherence; (3) document how young people experience life with ART; and (4) understand how their experiences in adolescence affect their capacity and willingness to adhere to taking their treatment. As well as producing knowledge to better support optimum adherence among adolescents, the study also aimed to (5) compare the HIV treatment and trial experiences of young people in the UK, Ireland, Uganda and the USA.
Chapter 2 Methods
This open, randomised, parallel-group Phase II/III trial was performed in 24 centres worldwide (see Acknowledgements for the different sites). This was a strategy trial.
Trial entry criteria
The trial enrolled HIV-1-infected young people aged 8–24 years inclusive who had been on a stable first-line ART treatment containing at least two NRTIs/NNRTIs and EFV for at least 12 months and who were willing to continue the regimen throughout the study period. Previous dual therapy and/or substitution of NRTIs was allowed providing any changes were not the result of disease progression or immunological or virological failure (with virological failure defined as two successive HIV-1 RNA results of > 1000 copies/ml) subsequent to virological control having been achieved on ART. They must have had viral suppression (HIV-1 RNA < 50 copies/ml) for at least the previous 12 months (at least the last three measurements, including screening). Young people who had experienced a single VL of > 50 copies/ml but < 1000 copies/ml (preceded and followed by a VL of < 50 copies/ml) in the last 12 months could be enrolled. They must have had a CD4 cell count of ≥ 350 × 106/l at the screening visit.
Exclusion criteria were pregnancy or risk of pregnancy in females of childbearing potential, acute illness, receiving concomitant therapy for an acute illness or a creatinine, aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevation of grade 3 or above at screening. Participants could not be on a regimen including NVP or a boosted PI (young people could be switched to an EFV-based regimen) or have had previous ART monotherapy (except for the prevention of mother-to-child transmission).
The age group of 8–24 years was chosen as young people between these ages are likely to undertake independent weekend activities and thus SCT could improve their quality of life by giving them more control over when they take their HIV medication, helping them to maintain their privacy regarding HIV infection and treatment taking while socialising at weekends.
Randomisation and treatment strategies
Participants were randomly assigned 1 : 1 to maintain CT or switch to SCT. Randomisation was performed centrally by the Medical Research Council Clinical Trials Unit (MRC CTU) at University College London (UCL) according to a computer-generated randomisation list, using random permuted blocks stratified by age at randomisation (8–12, 13–17 and 18–24 years) and by African compared with non-African country.
The trial sites enrolled patients and assigned participants to interventions by either accessing the trial randomisation server directly or contacting the relevant trial unit who performed the randomisation on their behalf.
Young people randomised to SCT followed a cycle of 5 days on ART (Monday–Friday or Sunday–Thursday) and 2 days off (Saturday and Sunday or Friday and Saturday). Participants randomised to the SCT arm were able to choose which 2 days off ART they preferred and whichever days were chosen were continued throughout the entire time on SCT within the study. Young people randomised to CT continued their current ART regimen and stopped or switched drugs in their ART regimen only for virological, immunological or clinical failure according to local practice. However, if simplification of the ART regimen or substitution of one drug (not EFV) was deemed necessary for clinical reasons, this was allowed after discussion with the appropriate trials unit.
Clinical examinations
A clinical examination was performed at screening, randomisation and certain follow-up protocol visits. At each visit the following data were recorded: body weight and height and all adverse events (AEs) since the last protocol visit, including in particular haematological abnormalities, pancreatitis, diarrhoea, clinical lipodystrophy, acute illnesses and change in HIV disease stage since the last protocol visit.
A physician assessment of lipodystrophy and Tanner stage was performed at week 0 and repeated every 24 weeks until the end of the study. A pregnancy test was performed for all females of childbearing potential at screening (weeks –4 to –2) and repeated every 24 weeks until the end of the trial and at other time points if required.
Ethnic-origin data were collected because it is known that ethnicity is a factor in the concentration levels of EFV.
Laboratory tests for efficacy and safety monitoring
-
Haematology: haemoglobin, mean corpuscular red blood cell volume (MCV), platelets, white cell count, neutrophil and lymphocyte counts.
-
Biochemistry: creatinine, albumin, total bilirubin, ALT, AST, alkaline phosphatase, calcium, phosphate.
-
Lipids/glucose: triglycerides, cholesterol [total, low-density lipoprotein (LDL), high-density lipoprotein, very-low-density lipoprotein], glucose (participants should have been fasting overnight at randomisation, weeks 24 and 48 and then every 48 weeks during the main trial).
-
Lymphocyte subsets: CD3 (absolute and percentage), CD3 + CD4 (absolute and percentage*), CD3 + CD8 (absolute and percentage*), total lymphocyte count (if measured by immunology laboratory) (*CD45RO/RA if measured).
-
Virology: HIV-1 RNA (VL) using an ultrasensitive assay and resistance testing [locally or on stored samples (when VL was detectable)].
Screening visit
At the screening visit a trial number was assigned and used on all paperwork and labels. A clinical assessment was completed and the participant and/or carer completed an adherence questionnaire according to the young person’s age and knowledge of HIV diagnosis.
Blood was taken for haematological and biochemical investigations, for measurement of T-cell subsets (including RO/RA phenotype when available), for measurement of HIV-1 RNA VL (it was requested to use the same assay at least throughout the pilot phase and ideally throughout the whole trial, although the assays used varied across centres according to clinical practice and management) and for plasma and cell storage (at clinical centres where this was possible).
Randomisation visit
Randomisation (week 0) took place no more than 4 weeks after the screening visit and ideally as soon as possible after eligibility had been confirmed. The eligibility criteria and consent were reconfirmed verbally and noted on the randomisation form. Once a patient was randomised, a clinical assessment was completed, which included measurements of height and weight, presence of AEs, change in HIV disease stage and ethnic origin. The following investigations were performed: haematology, biochemistry, glucose and lipid profiles (fasting) and T-cell subsets (plus RO/RA phenotype if available). Blood was taken for plasma storage (plus cell storage if the participant attended a clinical centre where this procedure was possible or where a courier could be arranged). A quality-of-life questionnaire [Pediatric Quality of Life Inventory; see www.pedsql.org/ (accessed 6 April 2016)] was completed for all participants (and carers) and an acceptability questionnaire was completed for those young people randomised to SCT (and carers). Participants were given a diary to record when they took their ART, which included a reminder to restart therapy after the 2 days off ART in the SCT arm.
Follow-up
Young people were followed until the last randomised participant had completed 48 weeks of follow-up. All young people were seen for clinic visits at weeks –4 to –2 (screening), 0 (randomisation), 4, 12, 24, 36 and 48.
Sample size
This trial planned to enrol a minimum of 160 young people, at least 80 per arm.
Assuming that 90% of young people in the CT arm and in the SCT arm maintained a HIV-1 RNA level of < 50 copies/ml to week 48, 155 young people would have provided at least 80% power to exclude a difference of 12% between the two arms (i.e. to exclude suppression rates of < 78% in the SCT arm) (one-sided alpha = 0.05). 38 A minimum of 160 young people (80 per arm) had to be enrolled to allow for loss to follow-up (in previous PENTA trials loss to follow-up has been < 3%).
The power calculations were based on the assumption that 90% of patients in the CT arm would remain virally suppressed to week 48. Any decrease in this percentage was likely to underpower the study and would lead to an equivocal result, as could other changes to the assumptions.
A sample size of 220 participants would have increased the power of the study by at least 10% for varying levels of suppression. At its meeting in December 2012, the Trial Steering Committee (TSC) recommended that the study should remain open to randomisation until the end of the defined recruitment period, even if the total number of participants enrolled exceeded 160, as this would enhance the power of the study; this was communicated to and agreed by the IDMC. The specified sample size was increased in protocol version 1.7 (dated 24 April 2013) from a recruitment target of 160 to a target of at least 160 but not exceeding 220 participants (see Appendix 1).
The trial was not formally powered to detect differences between CT and SCT, but to exclude substantial virological disadvantages [i.e. HIV-1 RNA suppression rates (< 50 copies/ml) of < 78%] in the SCT arm by following a 5 days on, 2 day off strategy, that is, a non-inferiority trial.
A non-inferiority margin of 12% was chosen to represent a clinically acceptable difference in the rate of virological suppression (HIV-1 RNA < 50 copies/ml) between the two arms and to allow the trial to be adequately powered and feasible to conduct based on estimates of available young people followed in PENTA centres.
Pilot study
The first participants randomised in the study (n = 15 in the SCT arm and n = 17 in the CT arm) were included in the pilot phase and had weekly HIV-1 RNA measurements during the first 3 weeks of the study. Those randomised to the SCT arm and included in the pilot phase stopped taking their ART on Saturdays and Sundays, that is, they followed a cycle of 5 days on ART (Monday–Friday) and 2 days off ART (Saturday–Sunday) during the pilot phase. Recruitment to the CT arm ran concurrently.
Young people in the pilot phase had four additional phlebotomy visits (HIV-1 RNA and blood store only) at weeks 1, 2, 3 and 8. For young people in the SCT arm the blood draws were on the Monday after the first, second and third weekends off ART and before ART recommenced; for young people in the CT arm these blood draws were at any time during the first, second and third weeks.
The IDMC met at the end of the pilot phase to review the interim data (see Appendix 2 for dates of all IDMC meetings).
Management of young people and viral load tests
-
Pilot phase. Polymerase chain reaction (PCR) tests for HIV-1 RNA can occasionally yield spurious results suggestive of low-level viraemia. During the pilot phase only, any HIV-1 RNA measurement detected of ≥ 50 copies/ml at weeks 1, 2 or 3 was repeated on the same sample to ensure that the result was valid and reproducible.
-
SCT arm. Participants with a HIV-1 RNA measurement of ≥ 50 copies/ml had a confirmatory VL measurement taken on a separate sample within 1 week. No further interruptions to ART were undertaken until the repeat test result was obtained. Participants with a confirmed viral rebound of ≥ 50 copies/ml recommenced CT and should not have undergone further interruptions to their therapy. Participants with an isolated HIV-1 RNA measurement of ≥ 50 copies/ml and a subsequent measurement of < 50 copies/ml could remain on SCT. There could be a maximum of three such occurrences during the lifetime of the study. After the third increase, CT was resumed with no further interruptions.
-
CT arm. Participants with a HIV-1 RNA measurement of ≥ 50 copies/ml had a confirmatory VL measurement taken on a separate sample within 1 week. Participants with a confirmed VL of ≥ 50 copies/ml received standard clinical care.
Study duration
Young people were followed until the last randomised participant had completed 48 weeks of follow-up, at which point the main trial was considered complete. Participants who were followed after week 48 were seen every 12 weeks until the main trial was complete. Participants randomised to the SCT arm continued to follow the SCT strategy until the main trial was complete unless the clinician or the family had concerns, which the clinician discussed with the appropriate trials unit.
Following the recommendations of the TSC (December 2013) and the subsequent protocol amendment (version 1.9) and participant consent, ongoing long-term follow-up of the trial for a further 2 years commenced in July 2014 (see Appendix 1). Stable and virologically suppressed young people who were randomised to SCT can opt to continue SCT during the long-term follow-up if they have 12- to 16-week VL monitoring and if agreed by the clinician and family. Management of HIV-1 RNA VL will continue as during the main study period.
Data collection and handling
Sites in the UK and Ireland were managed by the MRC CTU, subsequently the MRC CTU at UCL. Sites in the USA, Germany, Uganda, Thailand (HIV Netherlands Australia Thailand Research Collaboration) and the Ukraine were managed by the MRC CTU at UCL in collaboration with national co-ordinators. Sites in Spain, Belgium, Denmark and Argentina are managed by the French National Institute for Health and Medical Research (INSERM SC10-US19) in collaboration with national co-ordinators. Program for HIV Prevention and Treatment (PHPT) sites in Thailand were managed directly by the PHPT.
Data were recorded on case report forms (CRFs); the completed CRFs were sent to the appropriate trials unit for data entry and a copy kept at the local clinical centre. Data from the CRFs were entered onto databases held at the co-ordinating trials units and exported into Stata 13.1 (StataCorp LP, College Station, TX, USA) for analysis. After completion, adherence and acceptability questionnaires were sent to the appropriate trials unit for data entry.
Data received at each of the trials units were checked for missing or unusual values (range checks) and for consistency within participants over time. If any such problems were identified, a missing data report of the problematic data was sent to the local site by password-protected e-mail for checking and confirmation or correction, as appropriate; any data that were changed were crossed through with a single line and initialled. The amended data were returned to the appropriate trials unit and filed in the notes at the site. The trials units sent reminders to sites under their management for any overdue and missing data.
Interim analysis
The trial was reviewed by the PENTA IDMC. No member of the PENTA Steering Committee or the BREATHER TSC or any clinician (investigator) responsible for the clinical care of trial participants could be a member of the IDMC. The IDMC reviewed all aspects of the trial, including the number of participants recruited.
The IDMC met four times in strict confidence over the course of the trial: 28 October 2011, 5 September 2012, 31 July 2013 and 7 February 2014, with the last meeting being about VL monitoring only. The IDMC was to inform the chairperson of the TSC if, in its view, the results provided either:
-
unequivocal evidence* that was likely to convince a broad range of HIV clinicians, including the study investigators, that one of the two treatment strategies (CT or SCT) was performing poorly for all participants or for a particular category of participants and there was a reasonable expectation that this new evidence would materially influence patient management or
-
good evidence* that CT was superior to SCT in terms of the primary outcome and the non-inferiority of SCT was extremely unlikely to be demonstrated with continued enrolment and/or follow-up.
*The criteria for the strength of evidence could not be defined precisely and were left to the judgement of the IDMC. However, as an example, if in an interim analysis the 99% CI for the hazard ratio for the primary outcome excluded 1, this may be considered as providing good evidence of a difference in risk between the two groups. If the 99.9% CI also excluded 1, this may be regarded as providing unequivocal evidence of a difference between the two groups.
Clinical site monitoring
Trial-related monitoring at trial sites was carried out according to the trial protocol. Trial sites had to agree to provide access to source data/documents. Consent from parents/carers/young people, as appropriate, for direct access to data was also obtained.
In addition to a site initiation (either through a visit or in a teleconference), all clinical centres were monitored at least once during the trial and the following data were validated from source documents:
-
eligibility and signed consent
-
clinical disease progression to new Centers for Disease Control (CDC) C event or death
-
HIV-1 RNA VLs ≥ 50 copies/ml (primary end point only)
-
a random sample of CD4 measurements
-
a random sample of laboratory results
-
a random sample of original records of ARV prescriptions (with batch numbers)
-
a random sample of clinical data
-
all original records of ARV prescriptions (with batch numbers) for all young people participating in the qualitative substudy.
Patient and public involvement
Polly Clayden, a patient advocate, has been an independent member of the PENTA Steering Committee for many years and was closely involved in the discussions about the protocol during its development. She works at i-Base, a treatment activist group, which is developing a young person Community Advisory Committee with the UK Children’s HIV Association (CHIVA). Members of the study team, particularly in the UK and Ireland, are closely involved with CHIVA, which promotes issues relevant to children infected or affected by HIV, including the needs of adolescents and good practice in transitional care. Collaborator Magda Conway is an independent consultant with extensive experience of working directly with young people with HIV. She has been awarded a grant by the Elton John AIDS (acquired immunodeficiency syndrome) Foundation to strengthen networks of young people living with HIV in the UK and facilitated a group discussion with MRC CTU staff and a CHIVA youth group prior to the finalisation of the study protocol. CHIVA was closely involved in developing a communication and dissemination strategy for the main trial results and created a one-page results information leaflet (see Appendix 3) that was sent out to sites to give to their patients.
Protocol changes
See Appendix 1.
Main study statistical methods
Primary end point
The primary analysis was performed on the intention-to-treat population. We used Kaplan–Meier techniques to estimate the proportion of young people failing in each arm by the 48-week assessment, adjusting for stratification factors: age range (8–12, 13–17 and 18–24 years) and recruitment from an African site. Using these proportions, we were able to estimate the difference in the proportion failing between arms and obtain a 90% CI around the difference, using bootstrap standard errors. This CI was used as an indicator of whether or not the results were consistent with the non-inferiority of SCT compared with CT. The non-inferiority margin was prespecified at 12% so that, if the upper bound of the 90% CI of the difference between arms (SCT – CT) was < 0.12, non-inferiority would be demonstrated.
The primary analysis was repeated but without adjusting for stratification factors. Additionally, an analysis was performed investigating the crude proportion of young people who experienced virological failure up to the end of the 48-week assessment.
Secondary end points
All analyses performed on the primary end point were repeated but instead considering the end point as a confirmed VL of ≥ 400 copies/ml.
Immunology, biochemistry and lipids were evaluated at each follow-up visit up to 48 weeks after randomisation by fitting normal regression models, adjusting for randomised arm and baseline value.
Changes in ART from SCT to CT at any point during the first 48 weeks of the trial for those randomised to SCT were summarised by reason for changing strategy. Changes in ART regimen were summarised by arm.
Major HIV-1 resistance mutations39 at the point of VL failure or any time after VL failure were summarised for any VL failures in the first 48 weeks, overall and by drug class. CDC stage B and C events, as well as any deaths, were summarised by arm.
Measuring adherence was an important aspect of the analysis as we were attempting to see whether or not randomised strategies were being adhered to. We measured adherence to the protocol in two ways: first, through adherence questionnaires and, second, through a MEMSCap™ Medication Event Monitoring System (MEMSCap Inc., Durham, NC, USA) substudy on a subset of 61 patients.
Acceptability questionnaires were summarised at baseline and week 48, and the comparison between what young people found difficult before the study and what they found difficult during the study was examined using McNemar’s test.
Safety
Grade 3 and 4 clinical and laboratory AEs were summarised by arm. The difference in rate of grade 3 and 4 AEs between arms per 100 person-years was calculated using Poisson regression (with a random effect for person) and the number of young people with any grade 3 or 4 AE was compared between arms using a Fisher’s exact test. The same analyses were performed for ART-related AEs, treatment-modifying AEs and serious adverse event (SAEs).
Inflammatory biomarkers substudy
Rationale behind methods and markers selected
The blood volume that can be collected in children is limited, which was a major influence on the techniques selected for use in this study. In view of the global recruitment of the PENTA 16 trial, the assay selection was also influenced by its robustness to freezing at –80 °C for variable periods of time.
A panel of 19 biomarkers has been studied in several trials by the immunology laboratory at the UCL Institute of Child Health. Together they cover markers of inflammation (interleukin 1 receptor antagonist, C-reactive protein, tumour necrosis factor alpha, interleukin 10, interleukin 6, interleukin 8), cardiovascular injury (monocyte chemoattractant protein 1, angiopoietin-1 and -2, E-selectin, P-selectin, intercellular adhesion molecule 3, thrombomodulin, serum amyloid A, soluble intercellular adhesion molecule 1, soluble vascular cell adhesion molecule, vascular endothelial growth factor) and disordered thrombogenesis [d-dimer, tissue factor (TF)].
Methods
Blood was collected in ethylenediaminetetra-acetic acid (EDTA) and spun within 4 hours of collection at 1500 g (≥ 2500 rpm) for 15 minutes to separate cells from plasma. The supernatant was removed and placed in aliquots with a minimum of 500 µl of plasma in each cryovial. Samples were frozen at –70 °C immediately. Repeat freeze–thaw cycles were avoided.
All samples were transported to the Institute of Child Health during September 2014 and the assays were run in batches between October and December 2014. All standards were run in duplicate. Initial work within the laboratory showed that the coefficient of variance between duplicate samples was ≤ 10%. Given the small volumes of blood available and the cost of the plates, 95% of samples were run singularly, with 5% duplicated to ensure consistent intra- and inter-assay precision. The lower limit of detection, determined by the mean plus 2 standard deviations of the output signal of 10 blank samples, was calculated for each biomarker.
Meso Scale Discovery technique
A total of 17 biomarkers were analysed using Meso Scale Discovery® (MSD) assays (Gaithersburg, MD, USA). MSD assays use an electrochemiluminescence detection method similar to a sandwich enzyme-linked immunosorbent assay (ELISA) technique. High-binding carbon electrodes in the base of microplates have a 10 times greater binding capacity than polystyrene. Electrochemiluminescent (SULFO-TAG) labels are conjugated to detection antibodies. Within the analyser, electricity applied to the plate electrodes causes light emission by the SULFO-TAG labels. Light intensity is then measured to quantify analytes in the sample. Multiple excitation cycles of each label amplify the signal to enhance light levels and improve sensitivity allowing ultrasensitive assays to be run [see www.mesoscale.com/technical_resources/our_technology/ecl/ (accessed 18 February 2015)].
Five MSD plates were used. Table 1 lists the plates with the biomarkers on each plate. Assays were conducted according to standard manufacturer’s protocols. Samples were read using the QuickPlex SQ analyser (MSD).
| Method | Plate | Biomarker | Units | Lower level of detection |
|---|---|---|---|---|
| MSD | Vascular injury-1 | TM | ng/ml | 0.064 |
| ICAM-3 | ng/ml | 0.064 | ||
| E-selectin | ng/ml | 0.064 | ||
| P-selectin | ng/ml | 0.064 | ||
| Vascular injury-2 | SAA | pg/ml | 10.9 | |
| CRP | pg/ml | 1.33 | ||
| VCAM-1 | pg/ml | 6.0 | ||
| ICAM-1 | pg/ml | 1.03 | ||
| Custom cytokine | IL-6 | pg/ml | 0.192 | |
| IL-8 | pg/ml | 0.132 | ||
| IL-10 | pg/ml | 0.0806 | ||
| MCP-1 | pg/ml | 0.116 | ||
| TNF-α | pg/ml | 0.0798 | ||
| VEGF | pg/ml | 0.229 | ||
| IL-1RA | IL-1RA | pg/ml | 2.44 | |
| Angiopoeitin-1 and -2 | Angiopoietin-1 | pg/ml | 24.4 | |
| Angiopoietin-2 | pg/ml | 2.44 | ||
| ELISA | TF | TF | pg/ml | 0.69 |
| D-dimer | D-dimer | ng/ml | 0 |
Tissue factor
Tissue factor levels were measured using a commercial quantitative sandwich ELISA kit (Quantikine® ELISA Human Coagulation Factor III/Tissue Factor R&D Systems, Minneapolis, MN, USA). A total of 100 µl of assay diluent was added to 96-well microplates precoated with a monoclonal antibody against human TF. Subject plasma was diluted twofold in Calibrator diluent RD5-20 (R&D Systems). The 100-µl standards and then 100-µl diluted subject plasma were added and incubated for 2 hours at room temperature on a horizontal orbital microplate shaker set at 600 rpm. The plate was manually washed four times using wash buffer and excess fluid was removed by tapping the plate on a paper towel before 200 µl of TF enzyme-linked polyclonal antibody conjugate was added. After a further 2 hours’ incubation the wash cycle was repeated and 200 µl of substrate solution [stabilised hydrogen peroxide/chromogen (tetramethylbenzidine)] was added. The plate was then protected from light during a final 30-minute incubation, after which 50 µl of stop solution was added, and optical density was measured within 30 minutes using a microplate reader (Thermo Scientific Multiskan® EX) set to 450 nm. A four-parameter logistic curve fit was calculated using Ascent software 2.6 (Thermo Labsystems Oy, Basingstoke, UK) and the concentration read from the standard curve and multiplied by the dilution factor of two.
D-dimer
The commercial TECHNOZYM® D-dimer ELISA assay (Technoclone, Vienna, Austria) was chosen following a comparison of two ELISA methods with two automated methods used in the NHS coagulation laboratory at Great Ormond Street Hospital. This provided consistent results and required smaller volumes of blood than the automated methods.
Undiluted samples were used after pilot runs showed that most patients had levels of D-dimer below the limit of detection when a twofold dilution was used. A total of 100 µl of calibrator and sample were added to wells precoated with anti-D-dimer monoclonal antibody and were then incubated at 37 °C for 60 minutes. Following manual plate washing three times using wash buffer (pH 7.3), 100 µl of conjugate (monoclonal Anti D-dimer-POX) working solution was added before a further 60-minute incubation at 37 °C. Following a second plate wash, 100 µl of substrate solution (chromogen tetramethylbenzidine) was added. After a 10-minute incubation at room temperature, 100 µl of stop solution (sulphuric acid) was added and the plate read immediately using a microplate reader (Multiskan EX) set to 450 nm. A linear regression curve fit was calculated using Ascent software 2.6 and the concentration read from the standard curve.
Statistical methods
Two linear regression models were fitted for each biomarker, at 48 and 96 weeks, adjusting for baseline biomarker value and randomised arm. However, because of the non-normal distribution of each biomarker, the natural logarithm was used to fit the models.
Immunology substudy
Rationale for an immunology substudy
A study comparing CD4-guided planned treatment interruptions of ART with CT in HIV-1-infected children was performed between 2004 and 2006. 40 The key immunological findings were that (1) there was a rapid fall in CD4 cells that occurred early following treatment interruption; (2) there was a rapid increase in CD8 cells peaking at 8 weeks after the planned treatment interruption; and (3) there were changes in naive and memory cell subsets within both the CD4 and the CD8 cell populations. 41 This study informed our focus on these cell populations to determine whether or not treatment interruption in the BREATHER trial would have an impact on immune dynamics.
Methods
The CD4 and CD8 lymphocyte subsets were quantified locally on fresh samples collected. In some centres, CD45RA and CD45RO subpopulations of CD4 and CD8 cells were also evaluated on fresh samples. In centres able to separate and store cells, additional whole blood was collected in EDTA and peripheral blood mononuclear cells isolated by density gradient centrifugation, divided into aliquots and frozen. A range of established markers was used to quantify naive and memory cells. These varied between laboratories but always included antibodies to detect CD45RA and CD45RO; in some laboratories, CD27 and CD31 were also included.
Statistical methods
Linear models of the CD45RA/CD45RO ratio, adjusting for baseline CD45RA/CD45RO ratio and randomised arm, were fitted at 48 weeks. Similar models were fitted for CD45RA%/CD45RO%, CD8RA/CD8RO and CD8RA%/CD8RO%. Because of non-normal distributions of these ratios, the natural logarithm of each ratio was used to fit the models.
Virology substudy
Statistical methods
We compared the proportion of individuals at week 48 with a VL of ≥ 20 copies/ml with the proportion with a VL of ≥ 50 copies/ml from the main trial analysis. Additionally, we investigated the difference between arms in the proportion of young people with a VL of ≥ 20 copies/ml at week 48 and tested the difference using a Fisher’s exact test.
We examined how many individuals who failed during the first 48 weeks of the main trial had a screening (or baseline if no screening sample available) VL of ≥ 20 copies/ml.
Finally, we compared the proportion of young people in each arm who had a VL of < 20 copies/ml at screening (or baseline if no screening sample available) and a VL of ≥ 20 copies/ml at week 48 using a Fisher’s exact test.
Laboratory methods
Ultrasensitive quantitative HIV-1 ribonucleic acid assay
The quantitative HIV-1 RNA assay used the Qiagen QIASymphony® SP (Manchester, UK) automated nucleic acid extraction procedure for extraction of HIV-1 RNA from 1 ml of plasma sample. An ABI Prism 7500 (Foster City, CA, USA) real-time PCR instrument with Invitrogen (Carlsbad, CA, USA) reverse transcription polymerase chain reaction (RT-PCR) reagents was used for amplification and detection of HIV-1 RNA. The quantification is based on an in-house standard curve calibrated against the World Health Organization hepatitis C virus international standard in IU/ml. The assay uses brome mosaic virus RNA as an internal control, which is introduced at the extraction stage. The multiplex real-time RT-PCR detects both HIV and brome mosaic virus with differently labelled TaqMan® probes.
Quantitative total HIV-1 deoxyribonucleic acid assay
The quantitative HIV-1 deoxyribonucleic acid (DNA) assay used the Qiagen DNA mini blood kit for extraction of DNA. An ABI Prism 7500 real-time PCR instrument with Invitrogen RT-PCR reagents was used for amplification and detection of HIV-1 DNA. The quantification is based on a standard curve using 8E5 cells and carrier RNA (Qiagen lot:139285838). Absolute maximum amount of input DNA in a PCR is 600 ng per reaction. The lower limit of dilution is 10 HIV copies/million cells (patients on HAART and undetectable HIV RNA in plasma usually have a total HIV DNA level of around 100 HIV copies/ml). The assay uses pyruvate dehydrogenase DNA as an internal control. The multiplex real-time RT-PCR detects both HIV and pyruvate dehydrogenase with differently labelled TaqMan probes. Results are reported as copies of HIV per million cells.
Qualitative substudy
Sample
All young people recruited into the BREATHER trial in the UK, Ireland, Uganda and the USA aged 10–24 years were eligible to participate in the qualitative substudy, subject to the appropriate consents and self-awareness of HIV infection (for at least 6 months). Although the trial included children from the age of 8 years, the qualitative study involved trial participants who were aged ≥ 10 years only and aware of their HIV status. Although we appreciate that age can be an inadequate proxy for HIV awareness, this decision was guided by ethical concerns about the extent to which children under the age of 10 years could have achieved the considerable understanding about HIV infection necessary for the in-depth discussions in the qualitative interviews.
In our design we had envisaged that we would adopt a purposive sampling strategy giving primary emphasis to ‘responsibility for medication’ (sole, shared, carer), with secondary dimensions including age (spread as per trial), gender, ethnicity, membership of HIV youth support groups, current domestic situation (living with parent(s), extended kin) and school attendance. However, given the lower than expected levels of recruitment into the trial in the UK and Ireland, we involved anybody who was eligible and willing to participate. In the US site, given the nature and timing of the fieldwork we adopted the same strategy, which meant that we involved all participants who had thus far been recruited into the trial. In Uganda, excluding those who were in the pilot, we initially adopted a similar recruitment strategy as in the other sites and then purposively selected the last 10 in our sample to ensure that we were reflecting a broad range of sample characteristics. The qualitative sample in each site reflects the diversity of the trial population.
Overall, 102 interviews were conducted with 43 young people (Table 2). In total, 26 young people were recruited in Uganda from one clinic (Joint Clinical Research Centre, Kampala), seven were recruited in the UK from three clinics (NHS hospitals in London, Nottingham and Dublin) and 10 were recruited in the USA from one clinic (St. Jude’s Children’s Research Hospital, Memphis, TN).
| Country | Total (n) | Male (n) | Female (n) | On SCT (n) | On CT (n) | Switched or left trial (n) | Age (years), mean | Age (years), range |
|---|---|---|---|---|---|---|---|---|
| Uganda | 26 | 12 | 14 | 14 | 10 | 2 (to CT) | 18 | 11–22 |
| UK (and Ireland) | 7 | 5 | 2 | 4 | 3 | – | 15 | 12–17 |
| USA | 10 | 9 | 1 | 4 | 5 | 1 (from trial) | 21 | 18–22 |
| Total | 43 | 26 | 17 | 22 | 18 | 3 | 17 | 11–22 |
Recruitment response and retention through the repeated phases of the qualitative study was high (around 40%). We had very high rates of participation in the Uganda (26 out of a possible 66 trial participants) and US sites (10/14). Recruitment was most challenging in the UK and Ireland (7/23). Overall, we included 40% of the trial participants who were aged ≥ 10 years. However, the actual proportion of eligible participants recruited is likely to have been higher than this, given that this figure does not account for the participants who would not have been eligible despite being within the age range because of a lack of awareness of their HIV status.
The majority of refusals occurred in the UK sites, with the reasons for refusal varying. Very few participants had taken part in qualitative research before and some of the young people were unwilling to talk to the researchers and were uncomfortable with the idea of the qualitative interviews. Others instead mentioned not wanting to take on any additional clinic attendance and time commitments. This may reflect the amount of overall research being conducted with the relatively small UK cohort, but could also be indicative of the desire that young people have to minimise the time spent in the clinic and focus on other aspects of their lives aside from HIV.
Types of data collected
-
One-to-one interviews with young people living with HIV. In Uganda, participants were interviewed three times over the course of the study (in the early stages of the trial; towards the end of the trial; and during the follow-up period) to explore their experience of adherence and the process of the trial. In the UK and US sites, only the first two interviews were conducted. Interviews lasted approximately 1–2 hours and were audio-recorded, subject to consent. Baseline interviews captured life with HIV on HIV treatment as described by young people and included, but did not focus specifically on, young people’s perceptions of the trial or of SCT. The second interview reconstructed the life and treatment trajectory of participants since the start of the trial, focusing on adherence during this specific time period as well as reflections on intervention and trial acceptability. The third interview in Uganda was conducted as participants moved into the follow-up stage of the study. In these interviews we explored any changes in their treatment experience and their attitudes towards continuing in the intervention or control arm. The third interviews are being conducted in the UK only at trial end to explore participants’ reactions to the outcome of the trial. Reactions to the trial results will be explored informally in Uganda through meeting observations and discussions. It is worth noting that the focus of the third interview has changed from the preliminary study design in response to the changes made in the trial. The third interview was initially planned to explore how participants would adapt had they been required at the end of the trial to switch back from the SCT arm to CT. However, this has not occurred and participants have been retained in the same arm through the follow-up phase. The focus of the qualitative enquiry has shifted to reflect these changes and instead will consider how participants respond to continuing in the same arm and their attitudes towards SCT once the main trial findings have been disseminated.
-
Audio diaries of young people living with HIV. We piloted the use of audio diaries in the UK and Uganda sites. This is an innovative method that we have used before and we consider such diaries to be a valuable method in capturing personal reflections. 42 We wanted to explore whether or not this method would enable participants to generate data at the time and space of their choosing and if this would provide particular insights into the immediacy and variability of treatment and adherence experiences. We therefore offered this method to assess its feasibility and value for research with young people. All participants were offered the chance to keep an audio diary in the Uganda and UK sites. Ethical approval was not given for the audio diaries to be used in the US site. In Uganda, 12 participants agreed to contribute a personal audio diary and in the UK three participants took up the opportunity. We found that there were significant challenges to using audio diaries with this age group in these sites for such a sensitive topic. Despite participants’ initial enthusiasm to use the diaries we found that their lack of privacy within their own home, exacerbated for many (especially those in Uganda) by a lack of physical space, meant that it was difficult for them to first record their diaries with ease and second be confident of securely storing them before returning them to the research team. These conditions undermined the value of the method, as the premise that participants may be able to speak more freely about immediate events with greater convenience could not be realised. Those who did record something mainly recorded public interactions or songs, which were not related to our topic of enquiry. We may conclude from this pilot that use of audio diaries is unlikely to be a feasible method given the restraints of a lack of privacy.
Ongoing research: planned data collection
-
Phase 3 interviews with young people in the trial in the UK post publication of trial findings. In the UK we are conducting a third and final interview which explores attitudes towards SCT in light of the trial findings. Our research focus has been adjusted to reflect the trial plans. It remains rare for participants to be asked about their response to trial findings and we anticipate that this may provide a valuable model for informing the design of any further development of this intervention and future roll-out.
-
Interviews with carers of young people in the trial (Uganda). In Uganda, all participants have been asked to consent to their carers being contacted to take part in an interview. Using thematic sampling criteria, informed by our initial analysis, we are inviting 15 carers to participate in an interview to explore their response to the trial findings. The interview also explores their decision-making process in consenting for their child to participate in the trial, their reflections on how their expectations aligned with their experience of the trial and their perceptions of the barriers to and facilitators of adolescents’ adherence, including the impact of the trial.
-
Focus groups with young people living with HIV not involved in the trial. In Uganda data are being collected in focus groups with up to 25 HIV-positive young people aged 10–24 years who participated in the trial. This is subject to the appropriate consents and self-awareness of HIV infection (for at least 6 months). These focus groups explore young people’s understandings of the results of the trial, their experience in the trial and its influence as their adherence patterns, through peer discussion. We endeavour to ensure that these groups reflect the diversity of the trial population. Data transcription and, where appropriate, translation from these additional sets of data collection are ongoing. Data analysis is being carried out iteratively to inform subsequent data collection and refine questions.
Consent procedures and ethics
Consent was sought from all trial participants aged 10–24 years who had been aware of their status for at least 6 months. Participants were approached in different ways depending on the local procedures of each of the research sites. In the UK, research nurses contacted and consented participants, although the researcher had a number of conversations over the telephone with participants to answer their questions and discuss the topic before they decided whether or not to take part. In the USA, trial staff carried out both the contacting and the consent procedures. In Uganda, the researcher met the carers in the clinic and explained the qualitative study to them and then asked for their consent for the children to participate. This was followed by an ‘assent’ procedure with the children themselves. In all sites, final arrangements for the interviews were organised with the researcher.
In line with Good Clinical Practice principles [see www.ich.org/products/guidelines/efficacy/efficacy-single/article/good-clinical-practice.html (accessed 6 April 2016)], informed consent was treated as a process. This involved reminding participants at each stage of the study what the qualitative study was about, answering any related questions that they may have had, providing information as appropriate and reiterating that participants could withdraw at any time during the process of data collection. We did not need to break confidentiality on any safeguarding issues, although we had plans in place should this have been necessary.
The data that were collected were transcribed verbatim and, when appropriate, were translated into English by the research assistant. Personal identifying details were removed. We have been given an exemption from the mandatory request by the Economic and Social Research Council to archive the Ugandan data given the sensitivity and contextually dense nature of the data collected.
Modes of analysis/interpretation
The study adopted a grounded analytic approach to thematic analysis, using systematic case comparison and negative case analysis throughout. 43 We orientated analyses by themes emerging within/across individual accounts, exploring the acceptability of the trial; the potential value of SCT; and barriers to adherence as it converges with changing priorities during adolescence, as well as to the advancement of social science research and theory on HIV treatment adherence.
All interviews and audio diaries were transcribed verbatim by the data assistants. As discussed above, the limitations in the audio diary data meant that these data have not been integrated into our analysis. In line with our iterative analysis approach, we analysed data as we collected it to inform the direction of subsequent interviews, further coding and case selection. In addition to giving attention to ‘negative cases’ through case comparisons in our analysis, we sought respondent validation on emerging conclusions and maximised internal reliability and reflection through comparing coding between multiple researchers. Coding was undertaken in two linked phases. Our first-level coding drew on a combination of a priori themes reflected in the study topic guide and inductive or in vivo codes. 44 Our second-level coding sought to break down first-level coded data into smaller units, which also involved moving from codes that operate at the level of participant description and meaning to concept-driven categories. This process is similar to moving from ‘open’ to ‘axial’ to ‘selective’ coding in grounded theory. 43 We have maintained an audit trail of the analytical process, including analytical memos and how case comparisons and attention to emerging negative cases have informed ongoing analyses. We have conducted analysis by site and across the study to identify if gender, age and country are significant in how we can disaggregate our findings.
Chapter 3 Results from the main trial
Baseline characteristics
In total, 199 young people were randomised from 11 countries between 1 April 2011 and 28 June 2013 (Table 3). A Consolidated Standards of Reporting Trials diagram for the trial can be found in Figure 1.
| Country (number of sites) | Dates of first and last randomisation | Number randomised | |||
|---|---|---|---|---|---|
| SCT | CT | Total | |||
| Argentina (2) | 13 December 2012 | 28 May 2013 | 5 | 6 | 11 |
| Belgium (1) | 29 April 2013 | 6 May 2013 | 1 | 1 | 2 |
| Denmark (1) | 25 September 2012 | 4 December 2012 | 2 | 1 | 3 |
| Germany (1) | 26 June 2013 | 26 June 2013 | 1 | 2 | 3 |
| Ireland (1) | 11 April 2012 | 9 October 2012 | 3 | 0 | 3 |
| Spain (5) | 5 September 2011 | 4 July 2012 | 6 | 5 | 11 |
| Thailand (3) | 5 July 2011 | 27 June 2013 | 15 | 21 | 36 |
| Uganda (1) | 23 May 2011 | 28 June 2013 | 35 | 35 | 70 |
| UK (7) | 1 April 2011 | 10 June 2013 | 14 | 12 | 26 |
| Ukraine (1) | 21 March 2013 | 17 June 2013 | 10 | 10 | 20 |
| USA (1) | 22 August 2012 | 24 April 2013 | 7 | 7 | 14 |
| Total (24) | 1 April 2011 | 28 June 2013 | 99 | 100 | 199 |
FIGURE 1.
Consolidated Standards of Reporting Trials diagram.
Baseline demographic data were well matched between arms, with 105 (53%) male participants, a median (IQR) age of 14.1 (11.9–17.6) years and a median (IQR) weight of 45.2 (33.8–56.0) kg. In total, 35% of all young people randomised were recruited from an African site; 180 (90%) young people were vertically infected; and 41 (21%) were white, 112 (56%) were black and 37 (19%) were Asian (Table 4).
| Characteristic | SCT | CT | Total |
|---|---|---|---|
| Young people randomised, n | 99 | 100 | 199 |
| Sex, n (%) | |||
| Male | 57 (58) | 48 (48) | 105 (53) |
| Female | 42 (42) | 52 (52) | 94 (47) |
| Age (years) | |||
| Mean (SD) | 14.5 (3.9) | 14.7 (3.9) | 14.6 (3.9) |
| Median (IQR) | 13.7 (11.7–17.7) | 14.4 (12.0–17.5) | 14.1 (11.9–17.6) |
| Range | 8.0–24.2 | 8.3–24.0 | 8.0–24.2 |
| Age range, n (%) | |||
| ≥ 8 to < 13 years | 38 (38) | 39 (39) | 77 (39) |
| ≥ 13 to < 18 years | 39 (39) | 41 (41) | 80 (40) |
| ≥ 18 to < 24 years | 22 (22) | 20 (20) | 42 (21) |
| Weight (kg) | |||
| Mean (SD) | 46.9 (18.2) | 45.6 (14.8) | 46.2 (16.6) |
| Median (IQR) | 45.5 (33.1–56.2) | 45.1 (33.9–55.7) | 45.2 (33.8–56.0) |
| Range | 18.0–114.3 | 20.0–90.1 | 18.0–114.3 |
| Route of infection, n (%) | |||
| Vertical | 90 (91) | 90 (90) | 180 (90) |
| Sexual contact | 7 (7) | 7 (7) | 14 (7) |
| Blood product | 1 (1) | 2 (2) | 3 (2) |
| Unknown | 1 (1) | 1 (1) | 2 (1) |
| Ethnic origin, n (%) | |||
| White | 24 (24) | 17 (17) | 41 (21) |
| Black: African or other | 58 (59) | 54 (54) | 112 (56) |
| Mixed black/white | 0 (0) | 4 (4) | 4 (2) |
| Asian | 15 (15) | 22 (22) | 37 (19) |
| Other | 2 (2) | 3 (3) | 5 (3) |
| Recruited from African site, n (%) | 35 (35) | 35 (35) | 70 (35) |
There was a minor imbalance at baseline in CDC events, with 13% of young people in the SCT arm having had a CDC stage C event compared with 21% in the CT arm. The median (IQR) CD4% at randomisation was 34.0% (29.5–38.5%) and the median (IQR) absolute CD4 count was 735.0 cells/mm3 (575.5–967.5 cells/mm3) (Table 5).
| Characteristic | SCT | CT | Total |
|---|---|---|---|
| Young people randomised, n | 99 | 100 | 199 |
| CDC stage, n (%) | |||
| N | 16 (16) | 10 (10) | 26 (13) |
| A | 25 (25) | 25 (25) | 50 (25) |
| B | 45 (45) | 43 (43) | 88 (44) |
| C | 13 (13) | 21 (21) | 34 (17) |
| Missing | 0 | 1 (1) | 1 (1) |
| CD4% | |||
| Mean (SD) | 34.7 (6.8) | 34.1 (6.4) | 34.4 (6.6) |
| Median (IQR) | 34.5 (29.3–39.0) | 34.0 (29.5–38.1) | 34.0 (29.5–38.5) |
| CD4%, n (%) | |||
| < 30% | 26 (26) | 27 (27) | 53 (27) |
| ≥ 30% to < 40% | 52 (53) | 55 (55) | 107 (54) |
| ≥ 40% | 21 (21) | 18 (18) | 39 (20) |
| Missing | 0 | 0 | 0 |
| CD4 (cells/mm) | |||
| Mean (SD) | 787.3 (297.6) | 798.7 (308.3) | 793.0 (302.3) |
| Median (IQR) | 722.5 (581.0–965.0) | 747.3 (575.3–972.8) | 735.0 (575.5–967.5) |
| CD4 (cells/mm), n (%) | |||
| ≥ 350 to < 1000 | 79 (80) | 79 (79) | 158 (79) |
| ≥ 1000 to < 1500 | 17 (17) | 17 (17) | 34 (17) |
| ≥ 1500 | 3 (3) | 4 (4) | 7 (4) |
| Missing | 0 | 0 | 0 |
Twenty-nine (14.6%) young people had had previous exposure to PIs at randomisation, but none had switched from PIs because of virological failure, and at trial entry all young people were on regimens containing NRTIs and EFV only.
In baseline acceptability questionnaires completed by young people randomised to SCT, 70 out of 80 (87.5%) young people who answered the questionnaires said that they thought that taking weekends off treatment would make things either much easier or a little easier compared with taking ART continuously.
Follow-up
At the end of the trial, the median (IQR) follow-up time was 85.7 (62.0–118.3) weeks. Two young people, both from the CT arm, were lost to follow-up by the end of the trial, one because of leaving the country after the week 24 visit and the other because of transferring to an adult clinic and withdrawing consent after the week 48 visit. Attendance to clinic visits was good, with > 90% of young people still in follow-up attending each visit (Table 6).
| Follow-up | SCT | CT | Total |
|---|---|---|---|
| Participants randomised, n | 99 | 100 | 199 |
| Seen at the following weeks, n (%) [%]a | |||
| 1 | 15 (100)b [100]b | 17 (100)b [100]b | 32 (100)b [100]b |
| 2 | 15 (100)b [100]b | 17 (100)b [100]b | 32 (100)b [100]b |
| 3 | 15 (100)b [100]b | 17 (100)b [100]b | 32 (100)b [100]b |
| 4 | 98 (99) [99] | 98 (98) [98] | 196 (98) [98] |
| 8 | 15 (100)b [100]b | 15 (88)b [88]b | 30 (94)b [94]b |
| 12 | 98 (99) [99] | 99 (99) [99] | 197 (99) [99] |
| 24 | 99 (100) [100] | 100 (100) [100] | 199 (100) [100] |
| 36 | 98 (99) [99] | 97 (97) [98] | 195 (98) [98] |
| 48 | 95 (96) [96] | 94 (94) [96] | 189 (95) [96] |
| 60 | 93 (94) [97] | 89 (89) [98] | 182 (91) [97] |
| 72 | 68 (69) [96] | 70 (70) [99] | 138 (69) [97] |
| 84 | 66 (67) [100] | 56 (56) [97] | 122 (61) [98] |
| 96 | 43 (43) [91] | 44 (44) [96] | 87 (44) [94] |
| 108 | 39 (39) [100] | 38 (38) [97] | 77 (39) [99] |
| 120 | 26 (26) [96] | 26 (26) [100] | 52 (26) [98] |
| 132 | 12 (12) [86] | 17 (17) [106] | 29 (15) [97] |
| 144 | 13 (13) [100] | 13 (13) [93] | 26 (13) [96] |
| 156 | 8 (8) [100] | 7 (7) [100] | 15 (8) [100] |
| 168 | 1 (1) [100] | 0 (0) [0] | 1 (1) [100] |
| Weeks from randomisation to last visit | |||
| Median (IQR) | 86.3 (62.4–118.9) | 84.9 (60.9–117.1) | 85.7 (62.0–118.3) |
| Range | 49.0–169.0 | 25.0–158.0 | 25.0–169.0 |
| Mean | 94.8 | 92.5 | 93.7 |
| Lost to follow-up, n (%) | 1 (1)c | 2c (2) | 3c (2) |
Primary end point
Thirteen young people reached the primary end point by the end of the 48-week assessment, six from the SCT arm and seven from the CT arm. In the primary analysis, the estimated probability (90% CI) of reaching the primary end point was 6.1% (2.1% to 10.2%) for individuals in the SCT arm and 7.3% (2.9% to 11.7%) for those in the CT arm. The estimated difference between arms (SCT – CT) was 1.2% in favour of SCT (90% CI –7.3% to 4.9%) and so the upper bound of the 90% CI was less than the non-inferiority margin of 12% (Figure 2).
FIGURE 2.
Adjusted Kaplan–Meier estimates of time to first detected HIV-1 RNA ≥ 50 copies/ml (confirmed) up to the week 48 assessment.

| Week 48 assessment | ||||
|---|---|---|---|---|
| Treatment arm | Number of events | Person-years at risk | Estimated probability of failinga | 90% CI |
| SCT | 6 | 99.53 | 0.061 | 0.021 to 0.102 |
| CT | 7 | 98.75 | 0.073 | 0.029 to 0.117 |
| Difference (SCT – CT) | –0.012 | –0.073 to 0.049 | ||
Therefore, at the end of the 48-week assessment, the results are consistent with the non-inferiority of SCT compared with CT.
When not adjusting for stratification factors, the estimated probability (90% CI) of reaching the primary end point was 6.1% (3.2% to 11.7%) in the SCT arm and 7.3% (4.0% to 13.1%) in the CT arm. The estimated difference between arms (SCT – CT) was 1.1% in favour of SCT (90% CI –6.8% to 4.6%) and so the upper bound of the CI was less than the non-inferiority margin of 12% (Figure 3). Therefore, non-inferiority was demonstrated in this analysis as well as in the primary analysis.
FIGURE 3.
Kaplan–Meier graph of time to first detected HIV-1 RNA ≥ 50 copies/ml (confirmed) up to the 48-week assessment.

| Week 48 assessment | ||||
|---|---|---|---|---|
| Treatment arm | Number of events | Person-years at risk | Estimated probability of failing | 90% CI |
| SCT | 6 | 99.53 | 0.061 | 0.032 to 0.117 |
| CT | 7 | 98.75 | 0.073 | 0.040 to 0.131 |
| Difference (SCT – CT) | –0.011 | –0.068 to 0.046 | ||
| p-valuea = 0.754 | ||||
The crude proportion (90% CI) of young people experiencing virological failure was 0.061 (0.027 to 0.116) in the SCT arm and 0.070 (0.033 to 0.127) in the CT arm. Therefore, the estimated difference in crude proportion (90% CI) between arms (SCT – CT) was –0.009 (–0.067 to 0.048) (Table 7).
| Treatment arm | Number of events | Estimated proportion | 90% CI | p-valuea |
|---|---|---|---|---|
| SCT | 6 | 0.061 | 0.027 to 0.116 | |
| CT | 7 | 0.070 | 0.033 to 0.127 | |
| Difference (SCT – CT) | –0.009 | –0.067 to 0.048 | 1.000 | |
Secondary end points
Virology
The primary analysis was repeated but with the end point as a confirmed HIV-1 RNA VL of ≥ 400 copies/ml. The estimated difference between arms (SCT – CT) in the proportion of virological failure was 2.1% in favour of SCT (90% CI –6.2% to 1.9%), which is consistent with the non-inferiority of SCT compared with CT (Figure 4).
FIGURE 4.
Adjusted Kaplan–Meier estimates of time to first detected HIV-1 RNA ≥ 400 copies/ml (confirmed) up to the week 48 assessment.
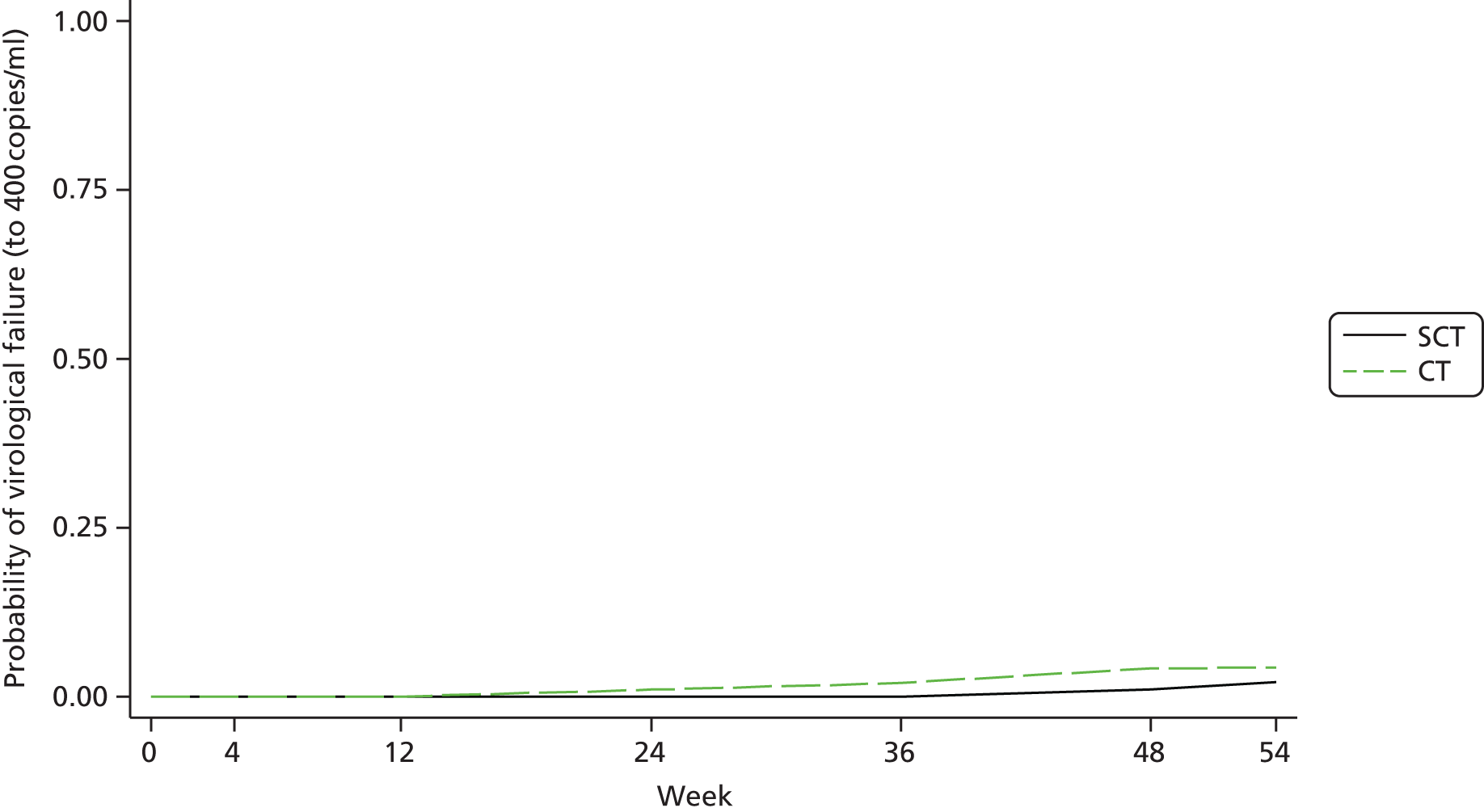
| Week 48 assessment | ||||
|---|---|---|---|---|
| Treatment arm | Number of events | Person-years at risk | Estimated probability of failinga | 90% CI |
| SCT | 2 | 101.35 | 0.021 | –0.000 to 0.044 |
| CT | 4 | 99.72 | 0.042 | 0.010 to 0.075 |
| Difference (SCT – CT) | –0.021 | –0.062 to 0.019 | ||
| Bootstrap p = 0.381 | ||||
The results when analysing without adjusting for stratification factors remained qualitatively unchanged (data not shown).
The crude proportion (90% CI) of young people experiencing virological failure (≥ 400 copies/ml) was 0.020 (0.004 to 0.062) in the SCT arm and 0.040 (0.014 to 0.089) in the CT arm. Therefore, the estimated difference in crude proportion (90% CI) experiencing virological failure between arms (SCT – CT) was –0.020 (–0.060 to 0.020) (Table 8).
| Treatment arm | Number of events | Estimated proportion | 90% CI | p-valuea |
|---|---|---|---|---|
| SCT | 2 | 0.020 | 0.004 to 0.062 | |
| CT | 4 | 0.040 | 0.014 to 0.089 | |
| Difference (SCT – CT) | –0.020 | –0.060 to 0.020 | 0.683 | |
Immunology
There were no significant differences between arms at any clinic visit after randomisation in CD4%, CD4 count, CD4 z-score, CD8%, CD8 count, CD3% or CD3 count (data not shown).
Biochemistry
There were no significant differences between arms at any clinic visit after randomisation in creatinine, bilirubin, alkaline phosphatase, AST, ALT, glucose, triglycerides, very-low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or total cholesterol (data not shown). A significantly higher level of LDL cholesterol was observed in the SCT arm at week 24, but this difference appears to be transient, as there was no significant difference between arms at week 36 or week 48 (Table 9 and Figure 5).
| Weeks since randomisation | SCT | CT | p-value (unadj) | Adjustedb difference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean changea | SE | n | Mean changea | SE | Meanb | SE | 95% CI | p-value (adjb) | ||
| 12 | 20 | 2.9 | 8.0 | 18 | 8.2 | 8.2 | 0.65 | –3.7 | 11.4 | –26.9 to 19.4 | 0.75 |
| 24 | 81 | 4.3 | 1.8 | 83 | –3.8 | 1.8 | 0.00 | 8.4 | 2.5 | 3.4 to 13.4 | 0.00 |
| 36 | 16 | –0.9 | 4.0 | 16 | –3.0 | 3.9 | 0.70 | 5.8 | 5.0 | –4.6 to 16.1 | 0.26 |
| 48 | 89 | 1.3 | 1.7 | 92 | –0.3 | 1.6 | 0.49 | 1.5 | 2.3 | –3.1 to 6.1 | 0.52 |
FIGURE 5.
Mean change in LDL cholesterol (mg/dl) from randomisation to week 48.

Haematology
There were no significant differences between arms at any clinic visit after randomisation in haemoglobin level, white blood cell count, lymphocyte count or neutrophil count (data not shown). However, a significantly lower platelet count was observed in the SCT arm at each week after randomisation (Table 10 and Figure 6). Additionally, a significantly lower MCV was observed in the SCT arm each week after randomisation (Table 11 and Figure 7).
| Weeks since randomisation | SCT | CT | p-value (unadj) | Adjustedb difference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean changea | SE | n | Mean changea | SE | Meanb | SE | 95% CI | p-value (adjb) | ||
| 12 | 95 | –9.8 | 6.0 | 99 | 12.6 | 5.9 | 0.01 | –21.7 | 8.2 | –37.8 to –5.6 | 0.01 |
| 24 | 97 | –13.2 | 5.2 | 99 | 4.3 | 5.1 | 0.02 | –17.2 | 7.3 | –31.6 to –2.9 | 0.02 |
| 36 | 98 | –16.2 | 5.4 | 96 | –1.0 | 5.5 | 0.05 | –15.2 | 7.7 | –30.4 to 0.1 | 0.05 |
| 48 | 95 | –13.4 | 6.5 | 94 | 7.4 | 6.6 | 0.03 | –20.9 | 9.2 | –39.1 to –2.6 | 0.03 |
FIGURE 6.
Mean change in platelet count (per l) from randomisation to week 48.
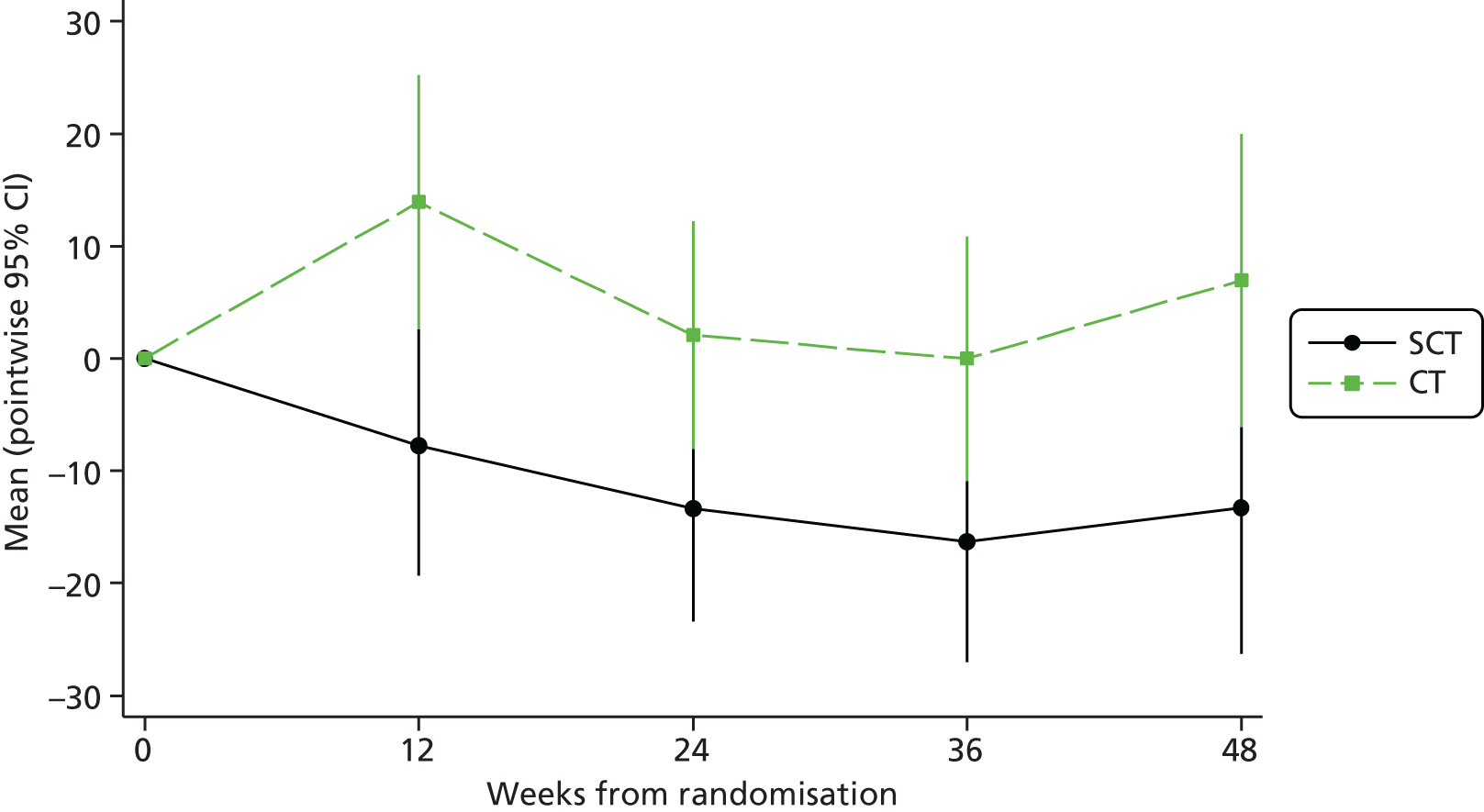
| Weeks since randomisation | SCT | CT | p-value (unadj) | Adjustedb difference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean changea | SE | n | Mean changea | SE | Meanb | SE | 95% CI | p-value (adjb) | ||
| 12 | 92 | –3.5 | 0.6 | 98 | –1.0 | 0.6 | 0.00 | –2.5 | 0.9 | –4.2 to –0.8 | 0.00 |
| 24 | 95 | –3.6 | 0.4 | 97 | –0.9 | 0.4 | 0.00 | –2.7 | 0.6 | –4.0 to –1.5 | 0.00 |
| 36 | 94 | –3.3 | 0.5 | 94 | –1.3 | 0.5 | 0.00 | –2.0 | 0.7 | –3.3 to –0.7 | 0.00 |
| 48 | 94 | –3.6 | 0.5 | 93 | –1.6 | 0.5 | 0.01 | –2.1 | 0.7 | –3.5 to –0.6 | 0.00 |
FIGURE 7.
Mean change in MCV (fl) from randomisation to week 48.

Status of young people randomised to short-cycle therapy
Of the 99 young people randomised to SCT, 91 (91.9%) were still taking weekends off treatment by the end of the week 48 assessment and eight (8.1%) had changed strategy to continuous ART. Of the eight who changed strategy, six did so because they reached the primary end point and therefore they changed as per protocol. One young person changed strategy because of an AE (gynaecomastia), which led him to come off EFV and therefore restart CT as per protocol, and one young person had adherence issues that were noticed by the clinician and she was instructed to return back to CT (Table 12).
| Reason for changing strategy to CT | Other reason details | Week changed to CT | Number of weekends off therapy before changing to CT |
|---|---|---|---|
| Confirmed VL of ≥ 50 copies/ml | 37.9 | 37 | |
| Confirmed VL of ≥ 50 copies/ml | 29.1 | 29 | |
| Confirmed VL of ≥ 50 copies/ml | 9.0 | 9 | |
| Confirmed VL of ≥ 50 copies/ml | 49.6 | 49 | |
| Confirmed VL of ≥ 50 copies/ml | 24.4 | 24 | |
| Confirmed VL of ≥ 50 copies/ml | 50.6 | 50 | |
| Other reason | Patient admitted that she had not been taking her ART for 9–10 months and she was instructed to return to CT at this point | 26.4 | 26 |
| Other reason | Patient returned to CT because of an AEa resulting in EFV being stopped | 35.0 | 32 |
Antiretroviral therapy changes
Twelve young people were on a different ART regimen at week 48 from the regimen that they were randomised to, with a greater number of young people changing regimen in the CT arm (SCT, n = 3; CT, n = 9; Fisher’s exact test, p = 0.13). All changes in ART during the first 48 weeks are presented in Table 13 (including two additional young people who stopped and restarted ART during follow-up but who were back on their baseline regimen at week 48).
| Child | Arm | Week | Regimen before | Regimen after | Reported reason for change |
|---|---|---|---|---|---|
| 1 | SCT | 28.4 | 3TC ABC EFV | 3TC ABC DRV | Return/start |
| 1 | SCT | 52.1 | 3TC ABC DRV | – | Compliance |
| 2 | SCT | 35.1 | FTC TDF EFV | FTC TDF ATA | Switch for toxicityb |
| 3 | SCT | 35.1 | ZDV 3TC EFV | 3TC TDF EFV | Simplification |
| 1 | CT | 3.4 | FTC TDF EFV | DRV | Stop for toxicityc |
| 1 | CT | 16.7 | DRV | FTC TDF EFV | Other |
| 1 | CT | 20.0 | FTC TDF EFV | DRV | Grade 1/2 AEd |
| 2 | CT | 4.0 | ZDV 3TC EFV | 3TC TDF EFV | Simplification |
| 3 | CT | 4.0 | ZDV 3TC EFV | FTC TDF EFV | Switch for toxicitye |
| 4 | CT | 4.4 | ZDV 3TC EFV | – | Temporary break |
| 4 | CT | 5.6 | – | ZDV 3TC EFV | Return/start |
| 4 | CT | 35.7 | ZDV 3TC EFV | 3TC TDF EFV | Switch for toxicitye |
| 5 | CT | 4.0 | ZDV 3TC EFV | FTC TDF EFV | Simplification |
| 6f | CT | 26.9 | FTC TDF EFV | – | Carer request |
| 6 | CT | 29.3 | – | FTC TDF EFV | Carer request |
| 6 | CT | 45.3 | FTC TDF EFV | – | Compliance |
| 6 | CT | 46.3 | – | FTC TDF EFV | Return/start |
| 7f | CT | 27.0 | 3TC ABC EFV | – | Compliance |
| 7 | CT | 33.0 | – | 3TC ABC EFV | Return/start |
| 8 | CT | 30.0 | 3TC ABC EFV | 3TC ABC DRV | Simplification |
| 9 | CT | 48.6 | ZDV 3TC EFV | 3TC ABC EFV | Switch for toxicitye |
| 10 | CT | 50.3 | ZDV 3TC EFV | 3TC TDF LPV | VL failure |
| 11 | CT | 52.7 | FTC TDF EFV | – | Compliance |
Resistance
Resistance tests were performed on all 13 young people who experienced virological failure up to the 48-week assessment. Four of these tests (SCT, n = 3; CT, n = 1) failed to amplify because of a low VL and so resistance results were obtained for nine young people (SCT, n = 3; CT, n = 6). Seven (77.8%) young people (SCT, n = 2; CT, n = 5) had major resistance mutations of any kind. Seven young people (SCT, n = 2; CT, n = 5) had major NNRTI mutations (SCT arm: L100I + Y188C + K103N and K103N; CT arm: E138A + V106M, K103N + V106M, M230L, V106M + K103N and G190S) and two young people (SCT, n = 1; CT, n = 1) also had the M184V mutation (Tables 14 and 15).
| SCT | CT | Total | |
|---|---|---|---|
| Number of children with HIV-1 RNA > 50 copies/ml | 6 | 7 | 13 |
| Number of available sequences | 3b | 6b | 9b |
| Number (%) of children with resistance mutations | |||
| Any class | 2 (66.7) | 5 (83.3) | 7 (77.8) |
| NNRTI only | 1 (33.3) | 4 (66.7) | 5 (55.6) |
| NRTI and NNRTI | 1 (33.3) | 1 (16.7) | 2 (22.2) |
| Number of mutations per child, median (range) | |||
| All classes | 2 (0–3) | 2 (0–2) | 2 (0–3) |
| PI | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| NRTI | 0 (0–1) | 0 (0–1) | 0 (0–1) |
| NNRTI | 1 (0–3) | 1.5 (0–2) | 1 (0–3) |
| Arm | Mutation class | Mutations | ART exposure | Week of virological failure (resistance test) |
|---|---|---|---|---|
| SCT | NNRTI only | L100I K103N Y188C | 3TC d4T EFV ZDV | 48 (48) |
| SCT | NRTI and NNRTI | K103N M184V | 3TC d4T EFV ZDV | 50 (50) |
| CT | NNRTI only | V106M E138A | 3TC ABC NVP EFV | 26 (26) |
| CT | NNRTI only | K103N V106M | ZDV 3TC EFV ABC | 39 (40) |
| CT | NNRTI only | M230L | ZDV 3TC EFV | 21 (21) |
| CT | NNRTI only | K103N V106M | 3TC d4T NVP TDF EFV | 36 (36) |
| CT | NRTI and NNRTI | M184V G190S | ZDV 3TC EFV | 48 (56) |
Centers for Disease Control clinical events and deaths
There were no new CDC C events or deaths during the first 48 weeks of the trial.
There were two new CDC stage B events, one in the SCT arm (grade 2 bronchopneumonia 10 weeks after randomisation) and one in the CT arm (grade 1 bronchitis 0.3 weeks after randomisation).
Adherence
Analysis of questions on compliance to SCT strategy at follow-up visits showed good compliance, with 95% of weekend breaks being taken (99% excluding time after return to CT). Self-reported adherence was similar in both arms, with 7% (29/414) reports in the SCT arm versus 10% (40/409) reports in the CT arm of missing ART in the last week (excluding weekend breaks in SCT) (p = 0.42).
Additionally, a MEMSCap substudy on a subset of 61 young people (n = 31 SCT, n = 30 CT) showed a median (IQR) number of bottle openings per week of five (4–5) in the SCT arm and seven (6–7) in the CT arm. This suggested that there was good adherence to the protocol. When investigating the percentage of bottle openings by day, the SCT arm had < 20% of bottle openings on Saturday and Sunday and > 80% on Monday–Friday, whereas the CT arm had > 80% of bottle openings on each day (Figure 8).
FIGURE 8.
Bar graph comparing the proportion of days MEMSCap opened between arms.
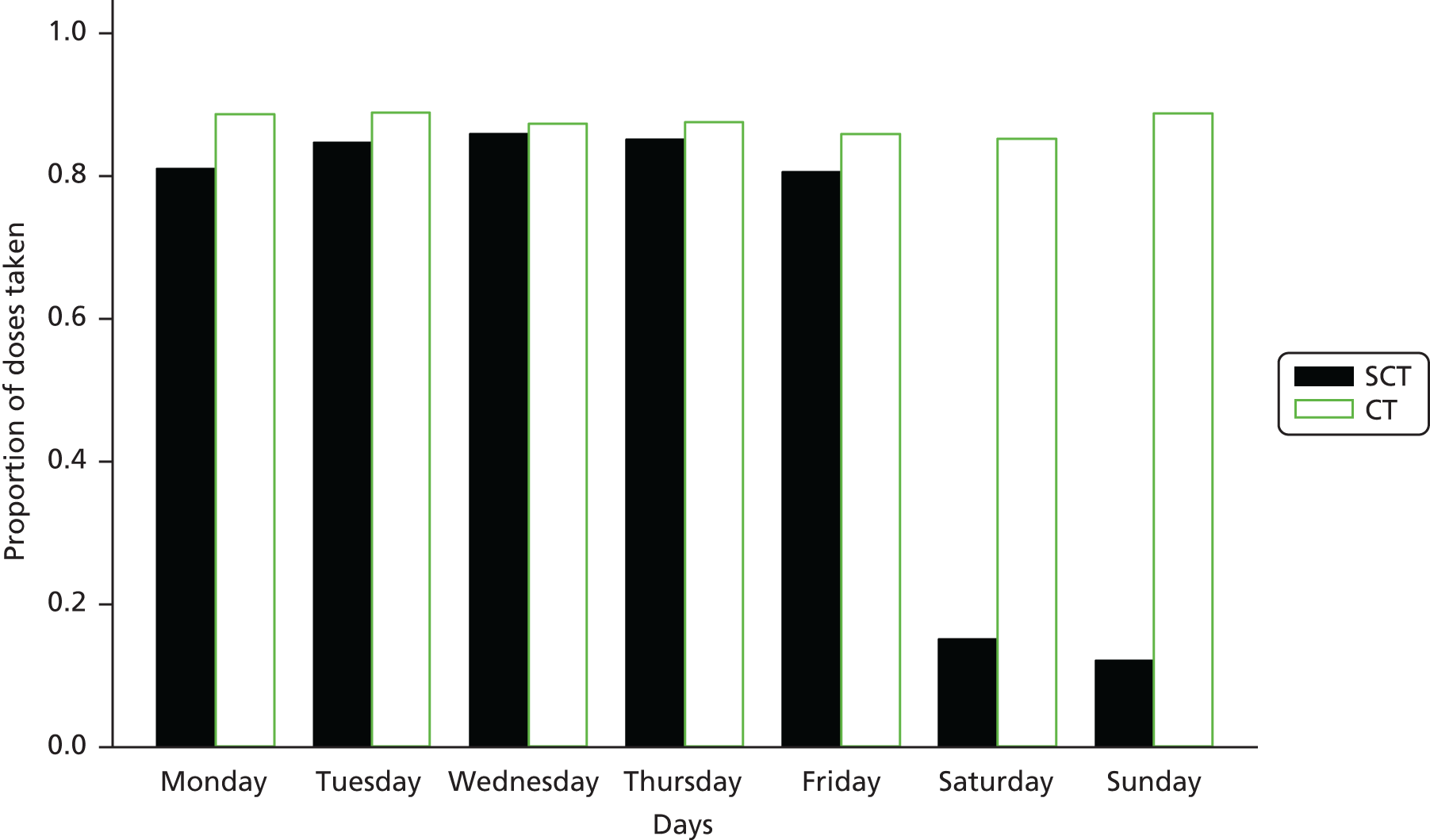
Finally, there was a significantly higher MCV in the CT arm than in the SCT arm at each follow-up visit after randomisation (p ≤ 0.01) (see Table 11 and Figure 7), indicating that young people in the CT arm were taking ART. A raised MCV has been associated with ZDV and, when investigating the differences in MCV between arms in young people without ZDV in their ART regimen, there was no significant difference between arms. This suggests that young people in the CT arm had a higher intake of ART per week than young people in the SCT arm.
Acceptability
Of 61 young people randomised to SCT who completed the end-of-trial acceptability questionnaire, 58 (95.1%) said that they thought that stopping ART at the weekends made things either a little easier or a lot easier than taking continuous ART.
A comparison between what young people randomised to SCT found difficult before the trial and what they found difficult during the trial revealed that young people found it significantly easier to go out with their friends during the trial than before the trial (p = 0.001).
Safety
Twenty children had a total of 27 grade 3 or 4 AEs (SCT: 13 episodes in eight young people; CT: 14 episodes in 12 young people) up to the week 48 assessment. There were five treatment-modifying AEs (SCT, n = 1; CT, n = 4) and two new CDC stage B events (SCT, n = 1; CT, n = 1). There were fewer ART-related AEs in the SCT arm, with two episodes in two young people in the SCT arm compared with 14 episodes in 10 young people in the CT arm. There were 13 SAEs in nine young people: seven episodes in six young people in the SCT arm and six episodes in three young people in the CT arm (Table 16).
| Type of AE | Episodes (young people) | p-valuea | ||
|---|---|---|---|---|
| SCT | CT | Total | ||
| Grade 3 and 4 AEs | 13 (8) | 14 (12) | 27 (20) | 0.480 |
| Treatment-modifying AEs | 1 (1) | 4 (4) | 5 (5) | 0.369 |
| ART-related AEs | 2 (2) | 14 (10) | 16 (12) | 0.033 |
| New CDC stage B events | 1 (1) | 1 (1) | 2 (2) | 1.000 |
| New CDC stage C events | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| SAEs | 7 (6) | 6 (3) | 13 (9) | 0.331 |
The most common type of grade 3 or 4 AE was an abnormal laboratory investigation, of which there were 14 episodes in 14 young people [episodes (young people): SCT 6 (6), CT 8 (8)]. There was no significant difference in the number of young people with any grade 3 or 4 AE (see Table 16), as well as no significant difference in the grade 3 or 4 AE rate ratio, both before (p = 0.886) and after (p = 0.720) adjustment for stratification factors (Table 17).
| Grade 3 and 4 AEs | Episodes (young people) | p-value | ||
|---|---|---|---|---|
| SCT | CT | Total | ||
| Laboratorya | 6 (6) | 8 (8) | 14 (14) | |
| Blood alkaline phosphatase increased | 0 (0) | 1 (1) | 1 (1) | |
| Blood bilirubin increased | 1 (1) | 0 (0) | 1 (1) | |
| Blood calcium decreased | 1 (1) | 0 (0) | 1 (1) | |
| Blood glucose decreased | 1 (1) | 0 (0) | 1 (1) | |
| LDL cholesterol increased | 1b (1) | 1b (1) | 2 (2) | |
| Neutrophil count decreased | 2 (2) | 6 (6) | 8 (8) | |
| Infections and infestations | 3 (2) | 1 (1) | 4 (3) | |
| Appendicitis | 0 (0) | 1 (1) | 1 (1) | |
| Gastroenteritis | 1 (1) | 0 (0) | 1 (1) | |
| Infective exacerbation of bronchiectasis | 1 (1) | 0 (0) | 1 (1) | |
| Measles | 1 (1) | 0 (0) | 1 (1) | |
| Nervous system disorders | 2 (2) | 1 (1) | 3 (3) | |
| Headache | 1 (1) | 0 (0) | 1 (1) | |
| Hemiparesis | 1 (1) | 0 (0) | 1 (1) | |
| Collapse/suspected seizure | 0 (0) | 1 (1) | 1 (1) | |
| Skin and subcutaneous tissue disorders | 0 (0) | 1 (1) | 1 (1) | |
| Lipohypertrophy | 0 (0) | 1 (1) | 1 (1) | |
| Surgical and medical procedures | 0 (0) | 2 (2) | 2 (2) | |
| Contusion | 0 (0) | 1 (1) | 1 (1) | |
| Inguinal hernia repair | 0 (0) | 1 (1) | 1 (1) | |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 1 (1) | 0 (0) | 1 (1) | |
| Kaposi’s sarcoma AIDS related | 1 (1) | 0 (0) | 1 (1) | |
| Psychiatric disorders | 0 (0) | 1 (1) | 1 (1) | |
| Suicidal ideation | 0 (0) | 1 (1) | 1 (1) | |
| Reproductive system and breast disorders | 1 (1) | 0 (0) | 1 (1) | |
| Gynaecomastia | 1 (1) | 0 (0) | 1 (1) | |
| Total grade 3 and 4 AEs | 13 (8) | 14 (12) | 27 (20) | 0.480c |
| Rate per 100 person-years (95% CI) | 12.7 (7.4 to 21.9) | 13.7 (8.1 to 23.1) | 13.2 (9.1 to 19.3) | |
| Rate ratiod (95% CI) | 0.93 (0.36 to 2.43) | 1.00 (–) | – | 0.886 |
| Adjustede rate ratio (95% CI) | 0.84 (0.32 to 2.17) | 1.00 (–) | – | 0.720 |
Fewer young people had an ART-related AE in the SCT arm than in the CT arm (2 vs. 10; p = 0.033). There were fewer ART-related AEs in the SCT arm both before (p = 0.017) and after (p = 0.018) adjusting for stratification factors (Table 18).
| ART-related AEs | Episodes (young people) | p-value | ||
|---|---|---|---|---|
| SCT | CT | Total | ||
| Investigationsa | 0 (0) | 2 (2) | 2 (2) | |
| Transaminases increased | 0 (0) | 2 (2) | 2 (2) | |
| Reproductive system and breast disorders | 1 (1) | 2 (2) | 3 (3) | |
| Gynaecomastia | 1 (1) | 2 (2) | 3 (3) | |
| Nervous system disorders | 0 (0) | 2 (2) | 2 (2) | |
| Dizziness | 0 (0) | 1 (1) | 1 (1) | |
| Headache and syncope | 0 (0) | 1 (1) | 1 (1) | |
| Skin and subcutaneous tissue disorders | 0 (0) | 5 (5) | 5 (5) | |
| Lipodystrophy acquired | 0 (0) | 5 (5) | 5 (5) | |
| Pregnancy, puerperium and perinatal conditions | 1 (1) | 1 (1) | 2 (2) | |
| Abortion spontaneous | 1 (1) | 1 (1) | 2 (2) | |
| Blood and lymphatic system disorders | 0 (0) | 1 (1) | 1 (1) | |
| Neutropenia | 0 (0) | 1 (1) | 1 (1) | |
| Psychiatric disorders | 0 (0) | 1 (1) | 1 (1) | |
| Suicidal ideation | 0 (0) | 1 (1) | 1 (1) | |
| Total ART-related AEs | 2 (2) | 14 (10) | 16 (12) | 0.033b |
| Rate per 100 person-years (95% CI) | 1.96 (0.49 to 7.83) | 13.68 (8.10 to 23.10) | 7.82 (4.79 to 12.77) | |
| Rate ratioc (95% CI) | 0.14 (0.03 to 0.71) | 1.00 (–) | – | 0.017 |
| Adjusted rate ratiod (95% CI) | 0.15 (0.03 to 0.73) | 1.00 (–) | – | 0.018 |
There were no significant differences between arms in how many young people had treatment-modifying AEs, with just five treatment-modifying events in the first 48 weeks (SCT, n = 1; CT, n = 4; p = 0.369) (Table 19).
| AEs | Episodes (young people) | p-valuea | ||
|---|---|---|---|---|
| SCT | CT | Total | ||
| Young people randomised, n | 99 | 100 | 199 | |
| AEs leading to switch in NRTI/NNRTI backbone | 1b (1) | 4 (4) | 5 (5) | |
| AEs leading to break in treatment | 0 (0) | 0 (0) | 0 (0) | |
| AEs leading to change in strategy from SCT to CT | 1b (1) | 0 (0) | 1 (1) | |
| Total AEs leading to treatment modification | 1 (1) | 4 (4) | 5 (5) | 0.369 |
There were no significant differences between arms in how many young people had SAEs (p = 0.331). Additionally, there were no significant differences between arms in the rate ratio, both before (p = 0.836) and after (p = 0.979) adjustment for stratification factors (Table 20).
| SAEs | Episodes (young people) | p-value | ||
|---|---|---|---|---|
| SCT | CT | Total | ||
| Young people randomised, n | 99 | 100 | 199 | |
| SAEs | ||||
| Fatal | 0 (0) | 0 (0) | 0 (0) | |
| Life-threatening | 0 (0) | 1 (1) | 1 (1) | |
| Hospitalisation | 5b (4) | 4 (3) | 9 (7) | |
| Causing disability | 0 (0) | 0 (0) | 0 (0) | |
| Congenital abnormality/birth defect | 0 (0) | 0 (0) | 0 (0) | |
| Other medical conditions | 2 (2) | 1 (1) | 3 (3) | |
| Pregnancya | 1 (1) | 4 (4) | 5 (5) | |
| Total SAEsc | 7 (6) | 6 (3) | 13 (9) | 0.331d |
| SAE rate per 100 person-years (95% CI)c | 6.85 (3.26 to 14.37) | 5.86 (2.63 to 13.05) | 6.36 (3.69 to 10.95) | |
| Rate ratioe (95% CI)c | 1.17 (0.26 to 5.26) | 1.00 (–) | – | 0.836 |
| Adjusted rate ratiof (95% CI)c | 1.02 (0.22 to 4.77) | 1.00 (–) | – | 0.979 |
The results were presented as an oral late breaker presentation at the Conference for Retroviruses and Opportunistic Infections in February 2015 (see Appendix 4). 45 Karina Butler was also interviewed at the conference. 46
Chapter 4 Substudy results
Biomarkers substudy
The results for the linear regression model for each biomarker are presented in Table 21. At 48 weeks, the only inflammatory biomarker that showed a borderline significant difference between the arms was D-dimer (p = 0.048), with lower (favourable) levels seen in the SCT arm at week 48.
| Biomarker | Regression at 48 weeks | Regression at 96 weeks | ||||
|---|---|---|---|---|---|---|
| n | Estimate for SCT relative to CT (95% CI) | p-value | n | Estimate for SCT relative to CT (95% CI) | p-value | |
| CRP | 186 | –0.254 (–0.725 to 0.216) | 0.288 | 85 | –0.051 (–0.738 to 0.636) | 0.882 |
| SAA | 186 | –0.102 (–0.612 to 0.409) | 0.694 | 85 | 0.170 (–0.525 to 0.866) | 0.628 |
| SICAM-1 | 186 | 0.039 (–0.099 to 0.177) | 0.579 | 85 | –0.044 (–0.221 to 0.134) | 0.627 |
| SVCAM | 186 | 0.028 (–0.099 to 0.156) | 0.660 | 85 | –0.016 (–0.182 to 0.150) | 0.845 |
| IL-10 | 185 | –0.073 (–0.254 to 0.109) | 0.431 | 85 | –0.001 (–0.225 to 0.223) | 0.992 |
| IL-6 | 184 | –0.051 (–0.268 to 0.166) | 0.641 | 85 | 0.028 (–0.294 to 0.350) | 0.865 |
| IL-8 | 185 | –0.230 (–0.548 to 0.087) | 0.154 | 85 | –0.125 (–0.606 to 0.356) | 0.607 |
| MCP-1 | 185 | –0.087 (–0.186 to 0.011) | 0.084 | 85 | 0.055 (–0.093 to 0.202) | 0.463 |
| VEGF | 185 | –0.154 (–0.367 to 0.059) | 0.156 | 85 | 0.077 (–0.301 to 0.454) | 0.688 |
| IL-1RA | 186 | 0.002 (–0.200 to 0.204) | 0.985 | 85 | 0.059 (–0.259 to 0.376) | 0.715 |
| E-selectin | 186 | –0.036 (–0.145 to 0.072) | 0.509 | 85 | 0.078 (–0.073 to 0.229) | 0.307 |
| P-selectin | 186 | –0.039 (–0.224 to 0.145) | 0.676 | 85 | –0.033 (–0.281 to 0.215) | 0.792 |
| ICAM-3 | 186 | 0.051 (–0.054 to 0.155) | 0.340 | 85 | –0.025 (–0.189 to 0.140) | 0.765 |
| TM | 186 | 0.034 (–0.051 to 0.119) | 0.432 | 85 | 0.090 (–0.044 to 0.223) | 0.184 |
| Angiopoietin-1 | 186 | –0.064 (–0.364 to 0.237) | 0.677 | 85 | 0.096 (–0.400 to 0.592) | 0.701 |
| Angiopoietin-2 | 186 | 0.037 (–0.056 to 0.130) | 0.434 | 85 | 0.131 (–0.081 to 0.343) | 0.222 |
| D-dimer | 181 | –0.488 (–0.970 to –0.005) | 0.048 | 82 | –0.501 (–1.245 to 0.244) | 0.184 |
| TF | 184 | 0.019 (–0.042 to 0.081) | 0.535 | 85 | 0.035 (–0.080 to 0.150) | 0.545 |
Immunology substudy
CD45 naive and memory cells were collected at baseline and week 48 from 119 individuals (SCT, n = 59; CT, n = 60) and CD8 naive and memory cells were collected at baseline and week 48 from 50 individuals (SCT, n = 24; CT, n = 25). The results from linear regression models of naive/memory cell ratios are provided in Table 22. There were no significant differences between arms at week 48 with respect to the ratios of naive/memory cells. The closest to significance was the ratio of CD8RA/RO (p = 0.135).
| Naive/memory cell ratio | Estimate for SCT relative to CT (95% CI)a | p-value |
|---|---|---|
| Log(CD45RA/RO ratio) | 0.04 (–0.11 to 0.19) | 0.600 |
| Log(CD45RA%/RO% ratio) | 0.03 (–0.12 to 0.18) | 0.658 |
| Log(CD8RA/RO ratio) | 0.20 (–0.06 to 0.46) | 0.135 |
| Log(CD8%/RO% ratio) | 0.19 (–0.08 to 0.45) | 0.162 |
When fitting linear regression models with the outcome variable being naive or memory cells independently and not as part of a ratio, we also found no significant differences between arms (Table 23).
| Naive or memory cells | Estimate for SCT relative to CT (95% CI)a | p-value |
|---|---|---|
| CD45RA | 19.73 (–40.77 to 80.24) | 0.520 |
| CD45RA% | 0.72 (–1.30 to 2.74) | 0.483 |
| CD45RO | 10.88 (–26.20 to 47.96) | 0.562 |
| CD45RO% | –0.17 (–1.61 to 1.26) | 0.809 |
| CD8RA | 76.86 (–30.06 to 183.78) | 0.155 |
| CD8RA% | 3.05 (–1.87 to 7.97) | 0.219 |
| CD8RO | –2.51 (–82.18 to 77.15) | 0.950 |
| CD8RO% | –0.10 (–0.34 to 0.13) | 0.387 |
Virology substudy
In total, 194 young people attended a screening visit and a week 48 visit during the trial and were therefore eligible for this analysis (SCT, n = 98; CT, n = 96). Two young people in the CT arm who had their original blood tests using an assay with a lower cut-off of > 20 copies/ml could not be retested with an ultrasensitive assay, one because there was no sample available and the other because the sample was of an insufficient volume. However, all other individuals had samples tested using an assay with a lower cut-off of ≤ 20 copies/ml (SCT, n = 98; CT, n = 94). Fifteen individuals had a VL of ≥ 20 copies/ml at screening (or week 0 if the screening visit sample was unavailable), seven from the SCT arm and eight from the CT arm. At week 48, 27 (14.1%) young people had a VL of ≥ 20 copies/ml (SCT, n = 13; CT, n = 14); as expected, this is a slightly higher proportion of individuals than had a VL of ≥ 50 copies/ml (Table 24).
| SCT | CT | Total | |
|---|---|---|---|
| Number of individuals with screening (or week 0) and week 48 readings | 98 | 96 | 194 |
| Number of individuals with ultrasensitive assay data available at screening (or week 0) and week 48 | 98 | 94a | 192a |
| ≥ 20 copies/ml at baseline, n (%) | 7 (7.1) | 8 (8.5) | 15 (7.8) |
| ≥ 20 copies/ml at week 48, n (%) | 13 (13.3) | 14 (14.9) | 27 (14.1) |
| Compared with number of individuals with a single VL of ≥ 50 copies/ml at week 48, n/N (%) | 8/98 (8.2) | 8/96 (8.3) | 16/194 (8.2) |
The estimated proportion of individuals with a VL of ≥ 20 copies/ml at 48 weeks was 13.3% in the SCT arm and 14.9% in the CT arm, giving an estimated difference in proportion between arms (SCT – CT) of 1.6% in favour of SCT (90% CI –9.9% to 6.6%; p = 0.836) (Table 25). The upper bound of this difference lies well below the 12% non-inferiority margin (Figure 9), which is interesting to note, although the trial was not powered to investigate the difference in the proportion of individuals with a VL of ≥ 20 copies/ml.
| Treatment arm | Number of young people with a VL > 20 copies/ml at week 48 | Estimated proportion | 90% CI | p-valuea |
|---|---|---|---|---|
| CT | 14 | 0.149 | 0.092 to 0.223 | |
| SCT | 13 | 0.133 | 0.080 to 0.203 | |
| Difference (SCT – CT) | –0.016 | –0.099 to 0.066 | 0.836 | |
FIGURE 9.
Estimated difference between arms in the proportion of young people with a VL of ≥ 20 copies/ml at the week 48 visit.

Of the 15 young people with a baseline VL of ≥ 20 copies/ml, only one (CT arm) developed virological failure during the first 48 weeks of the trial. This indicates that a VL of ≥ 20 copies/ml but < 50 copies/ml at baseline is not predictive of virological failure within 48 weeks.
Finally, a comparison between arms of the number of young people with a VL of ≥ 20 copies/ml at week 48 who were virally suppressed (VL of < 20 copies/ml) at screening (or week 0) showed that 12 out of 91 (13.1%) young people in the SCT arm who were virally suppressed (VL of < 20 copies/ml) at screening (or week 0) had a VL of ≥ 20 copies/ml at 48 weeks, and 12 out of 86 (14.0%) young people in the CT arm who were virally suppressed (VL of < 20 copies/ml) at screening (or week 0) had a VL of ≥ 20 copies/ml at 48 weeks (Table 26).
| VL of < 20 copies/ml at baseline | VL of ≥ 20 copies/ml at baseline | |
|---|---|---|
| SCT arm | ||
| VL of < 20 copies/ml at week 48 | 79 | 6 |
| VL of ≥ 20 copies/ml at week 48 | 12 | 1 |
| CT arm | ||
| VL of < 20 copies/ml at week 48 | 74 | 6 |
| VL of ≥ 20 copies/ml at week 48 | 12 | 2 |
Table 27 and Figure 10 show that the difference between arms (SCT – CT) in the proportion of young people with VLs of < 20 copies/ml at baseline and ≥ 20 copies/ml at week 48 was 0.8% in favour of SCT (90% CI –9.2% to 7.7%; p = 1.000).
| Treatment arm | Number of young people with a VL of ≥ 20 copies/ml at week 48 | Estimated proportion | 90% CI | p-valuea |
|---|---|---|---|---|
| CT | 12 | 0.140 | 0.083 to 0.216 | |
| SCT | 12 | 0.132 | 0.078 to 0.205 | |
| Difference (SCT – CT) | –0.008 | –0.092 to 0.077 | 1.000 | |
FIGURE 10.
Estimated difference in the proportion of young people with a VL of ≥ 20 copies/ml at the week 48 visit who had a VL of < 20 copies/ml at baseline.
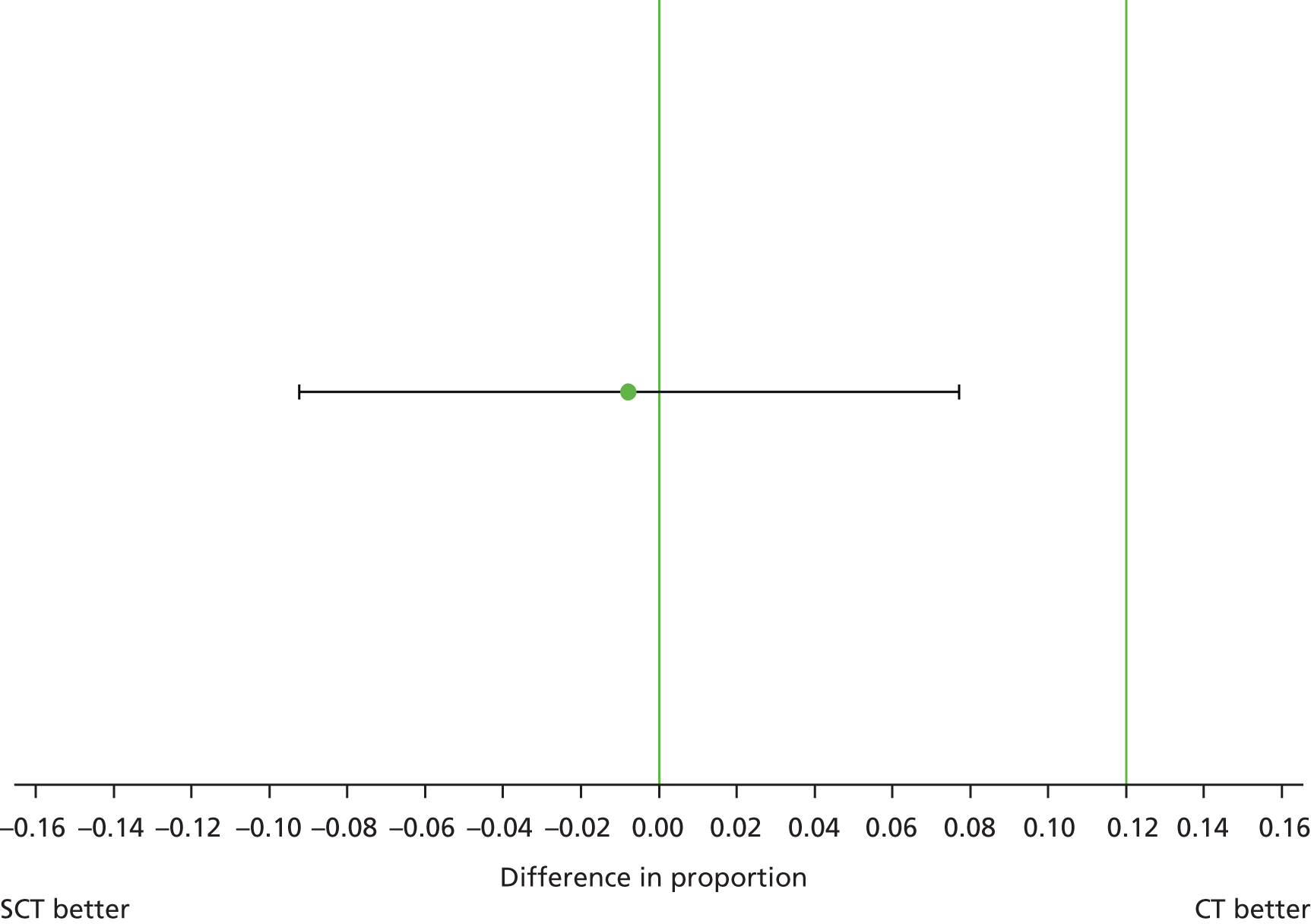
Qualitative substudy
Summary of key findings
The qualitative study was well received with good recruitment overall (although difficult in the UK, as described earlier) and retention of trial participants throughout the longitudinal design. Many of the young people talked at great length during the interviews and mentioned that they enjoyed the opportunity to speak in relative freedom, and in confidence, about topics that they may otherwise be quiet about in their daily lives. These ranged from their relationships with their family, friends and school staff to their communication with clinicians and trial staff. Encouragingly, most participants greatly valued their relationships with their clinicians and appreciated the care and support that they received. However, they did at times feel under pressure to present as ‘an ideal patient’ to their clinicians and this was sometimes an inhibiting factor that restricted their communication in the clinic when they were having difficulties or doubts about their health and treatment.
In relation to the trial overall, participants described a positive SCT experience and a preference for SCT and those on SCT wished to continue with their new regimen, suggesting that SCT was acceptable to most participants in the intervention arm. However, they also described challenges adapting to SCT in the short term. Young people from both arms discussed having initial anxieties about the impact of SCT on their health and adherence patterns, but these concerns decreased over the first few months of the trial. It is important, therefore, to consider how their experiences illustrate some of the risks involved for patients adapting to SCT and the need for tailored support that should accompany any switch to SCT, to which we return throughout this report.
Once young people had adapted to the new routine, SCT encouraged better adherence during the treatment-taking days and eased the pressure to carry and remember medication, thus enabling them to carry out a range of weekend activities. Nonetheless, although described as ‘exemplary adherers’ because of having an undetectable VL, participants did report missing doses occasionally and intermittently, but commonly decided that their stable VL results indicated that it was not necessary to report missed doses or ‘slippages’ to their clinician. Similarly, young people from both randomised arms reported frequent and sometimes disabling treatment side effects that had been difficult to voice in the clinic. Participants also reported that such side effects were not felt on the days when they did not take their medication.
Overall, the findings suggest that SCT has the potential to encourage more candid discussions about adherence ‘slippages’, missed doses and side effects and how treatment demands can be managed alongside young people’s other priorities. In the sections that follow we explore these issues in more detail and illustrate the study results with direct quotations from participants. We have chosen to use only randomised arm and country of origin as identifiers for the quotations. This is to protect the anonymity and confidentiality of the small sample, but also because we did not find significant differences in responses by either gender or sexuality.
In our analysis we found little difference by site or country. Although there is a reasonable distribution of quotations from across the three countries, there are slightly more from the US site. This reflects the difference in linguistic and cultural practices in how ideas are articulated, with the US and UK data lending themselves most easily to short quotations that encapsulate ideas and experiences whereas in the Uganda data a similar idea may emerge over a few pages of a transcript.
‘I was taking a break yet I was not used to that’: pre-trial understandings, fears and hopes
Although participants were keen to take part in the BREATHER trial, throughout the course of the study we found that this did not necessarily equate with an enthusiasm for trying SCT. At the beginning of the study, in the first phase of interviews, there was a vocal minority of participants who told us about their eagerness to take part in the research. A number of them described hoping and praying to be randomised into the SCT arm. One participant explained the appeal of SCT:
It’s like when you are working and you don’t get a break you get tired. OK sometimes I don’t know but it is all about like resting.
Uganda, CT arm
They also reported wanting to help their peers and co-operate with their clinicians in their efforts to find better therapies for them.
However, in later interviews a significant proportion of participants voiced how they had been initially reluctant about the intervention. They explained to us that they had chosen not to express these concerns when they were being consented into the trial. This reticence suggests that they may have been hesitant to talk to anyone about any doubts about taking part in the trial for fear of ‘disappointing’ those in their health-care team. They felt instead that they could be open during the qualitative interviews, as trust had been built throughout the course of the study. They also viewed the qualitative research team as being independent from the clinic and trial teams. Furthermore, their initial concerns had been balanced against the trust that they had in their clinicians and their belief that their health-care workers would not endanger them in any way. In the Ugandan site significant importance was invested by participants in the ‘conclusions’ reached by the pilot component of the trial. Without necessarily discussing this directly with clinicians, many interpreted this to mean that the safety question had already been answered:
I also first said to myself ‘what is going on’ when they tell us to stop drugs . . . until she told me that it was a research they had piloted earlier. This means we are not the first ones since they had done it before.
Uganda, SCT arm
Overall, excellent efforts were made to ensure that participants understood the objectives and methods of the BREATHER trial and all of the young people interviewed understood what SCT involved. However, some of the young people, particularly in the Ugandan site, appeared to have a limited understanding of the study around the time of randomisation and there was also some confusion regarding the distribution of the days (‘on’ vs. ‘off’ treatment) for those randomised to SCT. This signals the importance of repeated explanations for young people in trials that rely on their behavioural change and has implications for the potential future roll-out of SCT, which will be discussed in the concluding section of the report.
Some of the key concerns expressed by participants before randomisation, as well as by those eventually randomised to the SCT arm, included fears that SCT could actually be dangerous for their health and ‘undo’ the efforts that they had put into sustaining good levels of adherence to ART and keeping their VL undetectable. Having been consistently told to always take their HIV treatment every day, SCT disrupted the message of continuous adherence that they had become used to:
I thought that it [SCT] would be harmful . . . Because I was taking a break yet I was not used to that.
Uganda, SCT arm
Some were also worried that SCT could jeopardise their adherence routine as they might forget the days when they were supposed to take their medicines and those when they were meant to stop or they might misunderstand instructions or become confused and make mistakes:
Those on the short cycle arm may continue missing drugs even after the 2 days’ break thinking that they have to miss drugs sometimes.
Uganda, CT arm
Again, they were concerned that mistakes could also cause damage to their health: they were not able to predict how they as individuals would behave or how their bodies would react. These thoughts were common and are illustrated by this participant who shares the reflections he had prior to randomisation:
[It] was bothering me just a little bit to take off two days but then again it wasn’t . . . I might miss an extra day, because then I might be scared that something might go on and my body might change a certain way or something.
USA, CT arm
Nonetheless, participants also talked about their initial hopes for the trial and sometimes juggled both concerns about SCT and excitement about its potentially positive ramifications. They anticipated that SCT would be convenient because it could enable them to engage in various social, study or work activities by relieving them of the pressures of carrying and remembering to take medication. In some cases, however, the relief came from the fact that SCT would make it easier to conceal their treatment taking, hence their HIV status, from others.
This signalled how difficult adherence may be for young people, particularly those who have not disclosed their status beyond their clinic and carers:
I am the only one taking treatment at home . . . Children at home normally ask me why I take treatment day to day and I tell them that I have a sickness. So in case I break they may not know that I am still on treatment.
Uganda, SCT arm
Importantly, for many participants, SCT, and the BREATHER trial more generally, also symbolised the potential for scientific progress in relation to ARVs. Hope was widely felt: the possibility of having 2 days off treatment may herald a foreseeable future when they could take even less medication or when there may be a cure for HIV:
It brings hope that time will come and you stop taking even the one pill and [that one day] completely stop taking drugs.
Uganda, CT arm
Notably, many of the participants could remember a time in their childhood when they were taking larger numbers of pills at different times each day. SCT hence represented a further step along a progressive trajectory towards better HIV therapy, which is particularly valuable for young people who are faced with a lifetime of treatment taking:
The thought of having to take something for the rest of your life for 7 days a week it’s kind of nerve racking, but when you know that you have that break it’s better . . ., it tells the person that there’s hope.
USA, SCT arm
‘If you put your mind to it, anything is possible’: early experiences of the BREATHER trial
Many participants told us that whatever concerns they may have had about taking part in the trial (or being randomised to the SCT arm) eased off as they saw that SCT was working and their blood tests results continued to look promising. As the perceived dangers of the intervention did not materialise, they could ‘relax’ into their new regimen. The increasing confidence that they had in SCT may also be the reason why they felt that it was possible to discuss whatever early anxieties they may have had during the second set of interviews.
Nonetheless, it is important to note that adaptation to SCT presented them with challenges for some time:
Oh, that was hectic . . . when I first started the study, I think the first week I think I took it on a day that I wasn’t supposed to take it because I’m so used to it. But now I’m used to it.
USA, SCT arm
Once again, these challenges are better understood in the context of the emphasis placed so far on the importance of daily treatment:
Because being so used to taking it 7 days a week and then now they’re saying I can take 2 days off, it’s like a slight change and if you don’t get your mind focused . . . basically it’s like when you’re so used to something and then you’re trying to change it, it takes time. But then if you really put your mind to it then you can, anything is possible.
USA, SCT arm
Short-cycle therapy also temporarily affected some of the participants’ autonomy in treatment taking. To adapt to their new treatment schedule they had to reverse the independence that they had gained by managing their own treatment and temporarily ask for the support or supervision of their carers.
Overall, however, all those on SCT reported getting used to their new regimen and even finding that having 2 days off helped them to stick to their medication for the 5 days when they were meant to take it. SCT thus worked both as a reminder and as a reward:
It [SCT] gives you the courage to take your drugs daily other than in the other days. Reason being that you will say that I have missed to take the drugs in these 2 days that means that in the remaining days I have to be vigilant to take the drugs.
Uganda, SCT arm
Importantly, the discussions about any problems that they may have encountered with SCT stimulated participants to talk to us about their adherence issues more generally. From these rich sets of narratives we were able to identify two fundamental challenges that young people living with HIV in all three sites faced within and beyond the trial period: missing doses and medication side effects.
‘I am scared of getting caught up in my lie’: challenges to self-reported adherence
The qualitative study found that there were widespread patterns of missed ARV doses both before and during the trial. Most participants talked about missing doses from time to time, with some describing substantial periods of non-adherence that had occurred at different points in the past. Notably, however, few of those who had missed doses had discussed these instances with their clinician and this was often because participants felt that this could somehow damage their reputation in the clinic as an ‘exemplary adherer’ and harm their relationship with their health-care team.
Compared with the study questionnaire (see Appendix 5), the qualitative study found much higher levels of non-adherence in participants’ accounts. This highlights the importance of in-depth interviews as spaces where participants may be able to disclose missed doses more comfortably as they are able to also explain the challenges that they face in achieving consistent adherence. Additionally, such findings also help us to better contextualise the broader trial results and the methodological implications of this will be discussed further in the discussion section (see Chapter 5).
Many participants talked about how they made their own assessment of the risk involved in missing doses and, crucially, relied on their blood tests results to decide if it was worth mentioning to their clinician that they had skipped their medication. VL results represented the most accurate version of their health and at once functioned as ‘truth’ tests that could reveal their behaviour. Given that young people considered that telling their clinician about any missed doses would inevitably damage their reputation as an excellent patient, they interpreted an undetectable VL result as meaning that making any disclosure of missed doses was an unnecessary risk:
I try not to hide anything, it’s just that I probably feel like a smidge ashamed . . . I don’t like hurting or disappointing anybody . . . because I have told them if they asked, but if they didn’t ask then and I find out my viral load was undetectable then I just let out a sigh of relief and just keep going.
USA, SCT arm
At the same time, despite the trust that young people placed in their VL results, the practice of non-disclosure could be filled with anxiety:
I’m really scared of disappointing them and making them upset so sometimes I will have times . . . where I wouldn’t say anything especially if I know it’s more than the usual . . . I’m scared of getting caught up in my lie and saying ‘oh, well, I haven’t missed any this month’ and my VL and my CD4 comes back terrible, you know?
USA, SCT arm
In addition, being ‘undetectable’ was a source of pride for young people. They described being praised by clinicians and they often valued having a reputation as an ‘exemplary adherer’, which in some ways counteracted the ‘damaged identity’47–49 that often accompanied their HIV status. Although considered to be coping excellently by clinical staff, in the qualitative study some young people revealed that they felt unable to tell their clinicians about the struggles that they had with their treatment or HIV status. Unfortunately, their wish to not damage their reputation could constrain their capacity to access support. It is important here to underscore that many of the study participants were able to talk about their HIV status only in the clinic and, to varying degrees, within their family. Against the background of the centrality that these social spaces hold in their young lives, the fear of disappointing others or being seen as a failure could be particularly acute.
On the other hand, some participants felt that SCT helpfully ‘legitimised’ missing doses, in a structured way. They were reassured by the trial that their medication had continued to be effective even if they had not been able to adhere 100% to their daily regimen:
If you don’t have a break you may forget to take drugs like on a Saturday or Sunday but if you are supposed to have a break it is acceptable.
Uganda, SCT arm
Indeed, the very fact that SCT was being tried in the BREATHER trial was an indicator for participants that their issues with taking treatment everyday were implicitly understood by clinicians and that a solution was being sought to support them with their adherence. This can be seen as a significant benefit in terms of potentially improving communication in the clinic against the background of some of the reputation-related pressures highlighted so far.
‘I just feel like I am not really there’: significance of antiretroviral treatment side effects
The young people in the study also reported common and significant side effects of their EFV-based combination therapy. The main symptoms that they talked about were dizziness, feeling ‘spaced out’ or ‘high’, not being able to concentrate well and not ‘feeling quite themselves’. Some also reported stomach cramps shortly after taking their pills. This appears to be in line with clinical data on EFV50,51 as well as with extant qualitative research on the side effects of EFV,52 although to our knowledge no data have yet been published about the experiences of young people on this particular medication.
Importantly, we found that more treatment side effects were discussed in the qualitative study than may have been reported by participants to their clinician, both before and during the trial. The extent to which side effects affected those young people in the study who reported them in the interviews varied in terms of severity and frequency. Some participants talked about how the intensity of their initial symptoms had diminished some time after having started the EFV-based combination. In addition, not all participants experienced side effects every time they took their medicine and some talked about the side effects decreasing within a few hours of taking the pills.
In most cases, however, side effects interfered with young people’s daily activities at school and work:
Sometimes when I take it I do feel a bit dizzy and if I take it in the morning I have to then walk to college and I’m feeling a bit ill and I’m finding it a bit hard to concentrate during my lessons.
UK, SCT arm
Some participants discussed having to change the time of day when they took their medicines or adapt their day-to-day activities, including school classes and work shifts, to cope with side effects:
Well other than the doses at five I tried switching it up, when I was in school I tried switching it to me taking it in the morning and that wasn’t, I don’t know, I can’t say it wasn’t really such a good idea but it was just that feeling of being high at school was not the best situation, I don’t like that at all because I mean I can concentrate it’s just I just feel like I’m not really there . . . I get more clumsy if I’m under the influence of the medication.
USA, CT arm
One participant described their experience of treatment side effects:
When I asked the doctor she said it was normal and that the drug was strong. That’s why it causes dizziness. I used to take it while at school but I would feel dizzy. After taking it at 9:30 p.m. I could not read at 10, 11 p.m. and would not be able to walk properly yet I didn’t want disturb other children so I would just lie down there . . . because I knew that sometimes I would feel weak that I couldn’t even stand on the bed.
Uganda, SCT arm
On the whole, side effects were commonly discussed and often appeared to be considerably disabling, although many of the young people portrayed them simply as an inevitable part of their lives. This may in part have been shaped by the initial responses of health-care workers, as is the case above.
Worryingly, some of the young people described not having initially identified these symptoms as being side effects of the medications and perceived instead that the drowsiness and dizziness were part of their personality. This is an important finding, warranting further investigation for this cohort of people living with HIV who have been on treatment since before they can remember.
Many may not be able to disentangle the effects of the medication from their own physiological feelings or they may find it confusing to distinguish between the two, as this participant, who is describing how it feels when he inadvertently skips a dose, explains:
And when you miss it for a day does it change, does it influence how you’re thinking about your health or your body?
No, it actually makes me feel a little better sometimes . . . It feels like I don’t have a lot of strain on my body when I don’t take my medicine . . . It’s kind of scary in a way.
So why is it scary?
Well for one reason why because when I take my medicine I feel one way but the nights that I miss it I feel like my regular self . . .
Which do you prefer, which feeling?
I actually prefer the medicine feeling because I, because it’s helping me.
USA, CT arm
We would suggest that some of the participants, especially those who have been coping with these side effects for a while before the trial, might have chosen not to mention these issues to their clinician if they had found ways to get by despite the challenges of these effects. Nonetheless, we do not currently know enough about how such side effects might affect adherence behaviours, considering that young people may be reluctant to discuss both issues.
‘It’s the best days ever’: successful adaptation to short-cycle therapy
Participants on the SCT arm discussed how SCT had enabled their social life to grow. For some of these young people, having the weekends off taking medicines meant that they were able to go and spend a few days with their friends for the first time. SCT allowed them to travel more, sleep at friends’ houses, visit relatives and go out to parties and clubs without the pressure of carrying, concealing or taking treatment. One participant described how they felt being on the SCT arm:
I feel so free. You feel free; I can see you are even smiling [laughter].
Uganda, SCT arm
Short-cycle therapy was a major improvement for many participants:
I do not know what it is about those 2 days, but it’s the best days ever . . . I can go somewhere and not have to worry about taking that pill. Sometimes when I take the pill, my stomach hurts sometimes . . . but I don’t have to worry about that, and I don’t have to worry about taking this big pill, and I don’t got to worry about coming home at a certain time and taking it, I don’t got to worry about getting up and taking it. I’m just free for those 2 days.
USA, SCT arm
Having the weekend off treatment was described not only in terms of having fun and socialising but also as restful and relaxing:
It’s actually very good because I can actually get some time for me and actually not think of the drugs as in today I don’t have to take and tomorrow. I also get a day I am free to do whatever I want at any time I want, go out wherever I want to stay over the weekend and then take them [drugs] when I am back on Monday. So it’s easier and good.
Uganda, SCT arm
Participants emphasised that the benefits of SCT in part stemmed from reducing the visibility of their HIV treatment in social situations at the weekends, which minimised the risk of potential deductive disclosure. This is illustrative of the broader concerns of young people, which may underpin non-adherence on particular days or during social events. As one participant explained:
Saturday and Sunday are not convenient days for taking the drugs . . . At times it becomes tiresome to take the drugs everyday because there are times when you are away from home or amongst people who don’t know about you . . . So whoever sees you becomes eager to know what you are taking or what you are hiding. In my opinion having to rest is good because sometimes you may be amongst people like on a Saturday or Sunday but you are not going to take drugs so no one will get to know about your health. So having to take drugs everyday makes you anxious because you worry about being seen or being amongst people who do not know you.
Uganda, SCT arm
Another important element of such a rest was having a break from side effects. Many of the young people in the study had talked about side effects occurring immediately after taking their pills, hence many similarly reported not feeling any of the side effects on the days when they did not take treatment. This provided a valued window of respite:
I don’t get hot flushes on the weekends and I can stay up a little bit longer, because normally when I take my medicine I get the hot flushes and I get real irritated, where all I want to do is lay down. It’s like your body starts getting woozy and weak and now on the weekends it’s like, I’m just still full of energy. So it’s better, much better.
USA, SCT arm
Another helpful aspect of SCT was the motivation that it brought to being stricter about taking treatment on the 5 days as prescribed. As previously mentioned, participants perceived their weekend off medication as something to look forward to and this encouraged them the rest of the time. Some also talked about making even more sure that they took their medicines during the 5 days ‘on’ for fear of missing too many cumulative doses:
I’d probably have been a bit more cautious because it’s like 3 days you’re missing, three out of seven instead of, because if you took it six times out of seven, it’s fine, but like you’re already missing 2 days so I, you kind of get the impression you can’t really miss another one.
UK, SCT arm
What transpired from many of the interviews was that adhering to SCT involved work and commitment, but that the new therapy regimen was a welcome opportunity that opened up new possibilities. Some participants were thus recalcitrant about going back to CT, pending the outcome of the trial:
If they were to, if God forbid but if it came to a point where I had to go back to taking it 7 days a week I would probably be a little upset. But it would be OK though.
Do you think that would be difficult to adapt back to?
It will, it will.
What would be hard about it?
Just the fact that I can’t, it probably won’t be as difficult, I’ll be able to adjust to it very quickly, but I just won’t have those reward days any more.
USA, SCT arm
However, although a few were clearly enthusiastic about their new treatment schedule, some also talked about it being ‘more of the same’ as they had no previous issues with taking their medication. They saw little difference in the new regimen:
Yeah, definitely, like if it’s, I don’t know if it’s helping or not but it really doesn’t impose anything on me so why not? It’s kind of one of those things, like nothing lost so you might as well . . . It’s just like a small bit of, it’s a small bit better but then I don’t know how much difference it’s making.
UK, SCT arm
‘Why should I take it every day?’: participants’ perspectives regarding short-cycle therapy for other young people
As we have seen, the young people who were randomised to the SCT arm all reported a preference for SCT after an initial period of adaptation, which supports the results of the clinical trial thus far. However, many of the participants in the trial also expressed concerns about SCT being potentially challenging for other young people to manage. They described SCT as ‘better’ rather than ‘easier’ than CT. For those struggling with their adherence, although there may have been benefits of the breaks, it was considered at least initially a more confusing regimen to follow.
Many of the study participants, from both trial arms, discussed how the message about the need for continuous daily adherence at set times had so far been simple and straightforward. Offering young people the opportunity to take weekend breaks through SCT disrupted the clarity of that message and may also inadvertently indicate to young people that missing further days and doses is OK:
It might be [dangerous], it might be because some people might see it as like, why should I take it every day? Maybe I should just go ahead and stop taking every day and skip 2 days or 3 days, just to give me, just to clean me out or something like that.
USA, CT arm
Many were also concerned about what other HIV-positive young people might do once the trial results are made public. They frequently mentioned this as a significant worry as they imagined that other young people might try to ‘do SCT’ on their own, without telling anyone, and cause damage to their health because they had not understood the preconditions for SCT, including having an undetectable VL and being on an EFV-based medication combination.
Participants viewed this as a substantial risk that needed to be mitigated with careful communication and dissemination, as another participant, also in the CT arm, explained:
I do think for some people if they do find out about the information it may be OK if they do it but in the back of my mind I’m still worried because if some people don’t have, if some people aren’t undetectable and they try to do the short-cycle therapy that would really affect them and I get worried about that.
USA, CT arm
Overall, when participants were asked to reflect on what made them good at adherence, perhaps in comparison to others who they knew, they talked about the importance of a supportive environment, in the clinic or within the family, that would help them manage their medication. A supportive environment in this case was characterised by openness and the possibility to report their adherence challenges without fear of being scolded. Therefore, in the view of participants, other young people who may lack these various forms of support could find it problematic to switch to a new way of taking their treatment.
Chapter 5 Discussion
Main study
Children and adolescents find the commitment to taking ART every single day difficult. Previously it has been accepted that, to achieve optimal virological suppression, > 95% adherence to a daily ART regimen is necessary. 53 Many of the data underlying that assumption were generated in the era before the advent of today’s more potent regimens, now often incorporating one or more of the longer-acting ARV agents such as EFV.
We hypothesised that the pharmacokinetic properties of a drug such as EFV might be exploited to permit very short interruptions in daily ART while effectively maintaining viral suppression, in effect allowing a break in pill taking without a real break in therapy. Such a strategy, that is, SCT, could reap several benefits. Allowing breaks in taking medications at particularly difficult times, for example weekends, could help normalise the life experience for young people, reduce the problem of gradual pill fatigue, reinforce adherence during the remainder of the week and obtain similar benefits to CT in terms of virological suppression but with reduced overall drug exposure.
In this study, the non-inferiority of viral suppression in young people on EFV-based first-line ART was demonstrated for SCT compared with CT.
This global, randomised, Phase II/III study recruited 199 young people across 11 countries. With just over one-third of participants from Africa, one-fifth from middle-income countries and the remainder from Europe and the USA, we were able to study the potential utility and applicability of the strategy in diverse geographical settings. The median age of young people enrolled was 14 years and, in total, 21% were young adults (aged 18–24 years). The generalisability of the results therefore extends not only to older children and adolescents but also to the important group of young adults for whom weekend socialising often leads to missed ARV doses.
The baseline characteristics of participants in the two study arms were well matched. The minor imbalance in CDC C events, with a greater number in the CT arm, is unlikely to be meaningful given the equivalence of CD4 counts and percentage at enrolment. A major strength of this study is the excellent follow-up, with only one participant lost to follow-up before the 48-week assessment and the median duration of follow-up being 86 weeks, with > 98% of clinic visits attended.
Assuring adherence to the randomised strategy was critical to the integrity of the trial results. If those randomised to the CT arm elected of their own accord to take breaks in therapy, non-inferiority of SCT and CT might be shown, but with both arms taking breaks and having similar treatment schedules. Four independent indicators of adherence to the assigned strategy provided reassurance in this regard. The results of the self-reported compliance in the SCT arm (95% of weekends off taken), the MEMSCap substudy (median cap openings 5 for SCT vs. 7 for CT), analysis of MCV levels in ZDV recipients and self-reported adherence questionnaires in both arms were all consistent in supporting the concept that young people in the SCT arm had appropriately less ARV exposure than those on CT arm and that young people were adherent to their assigned strategy.
The overall outcome for young people in the study was excellent, with 93% remaining virally suppressed to < 50 copies/ml and 97% remaining virally suppressed to < 400 copies/ml. This may well reflect that this was a selected population, all of whom had a record of good adherence prior to study entry and none of whom had experienced virological failure. The primary study end point, confirmed VL > 50 copies/ml, was reached by only six in the SCT arm and seven in the CT arm, representing a 1.2% difference in probability of failure in favour of SCT. The study hypothesis was thus confirmed: SCT was non-inferior compared with CT for viral suppression over 48 weeks.
Although viral suppression was the primary end point of this study, it was equally important to ensure that there were no other detectable indicators of potential hazard associated with the strategy in terms of immunological compromise, emergence of viral resistance or inflammatory change. No difference in CD4 counts or CD4%, or in the emergence of major resistance mutations was detected. Although viral sequence data were not available for four out of 13 participants meeting the primary end point criteria (n = 3 SCT, n = 1 CT), all four subsequently resuppressed suggesting that the presence of resistance was unlikely.
In a previous CD4-guided long-period interruption trial, the SMART study,22 the unanticipated excess of cardiovascular events highlighted the importance of HIV infection as an inflammatory state with the potential for re-emergence of chronic inflammation in the event of treatment interruption. Incorporation of the biomarker substudy was thus an important component of this trial and provided additional reassurances with regard to the safety of the strategy.
Study limitations
The BREATHER trial included a selected cohort and was carried out in a very controlled manner, which means that the results may not be easily generalisable to real life. Young people eligible for enrolment were those who had never experienced virological failure and who had stable viral suppression, indicative of good adherence prior to enrolment. To confirm the long-term safety and efficacy of this approach a longer follow-up period is needed and, for clinical settings where monitoring is less available, larger pragmatic trials, based on routine clinical practice, are necessary.
Efavirenz was selected based on its pharmacokinetic properties that, despite its low genetic barrier to resistance, render it relatively resilient and forgiving of short interruptions in therapy. The success of the strategy may also relate to the fact that standard dose recommendations for EFV may be higher than is necessary for virological control. The ENCORE 1 study in adults54 demonstrated non-inferiority for EFV dosed at 400 mg/day compared with the standard 600-mg dose, a 30% dose reduction. The BREATHER trial has shown that the weekly EFV dose can be safely reduced by 2 days a week (29%), with standard doses delivered over 5 rather than 7 days. The SCT strategy has not been studied and may not be applicable in situations where lower EFV dosing regimens are used.
Inflammation and immunology substudies
As presented in Chapter 4, with the exception of the D-dimer results, no significant difference was seen between the trial arms in any of the 19 biomarkers. Patients randomised to SCT had slightly lower D-dimer levels at week 48 than at baseline, which is reassuring rather than worrying. The panel of biomarkers selected was comprehensive and these results add further reassurance that SCT is safe.
Similarly there was no evidence that CD4, CD8 or naive and memory subsets were affected by SCT. This contrasts with the findings reported in the PENTA 11 study,41 in which an interruption of 48 weeks was associated with marked changes in these parameters. This indicates that, at least for the duration of the study, there was no apparent inflammatory or immunological impact of SCT using markers that have previously been shown to be perturbed following treatment interruption.
Virology substudy
Recent evidence suggests that the HIV reservoir, which is not currently assessed with available routine diagnostic methods, plays an important role in disease progression. 55 Markers quantitatively evaluating residual viraemia56 and functionally assessing the ability of the reservoir to produce replication-competent virus57 are therefore emerging as additional necessary tools in patient stratification for prognosis and optimal management. The aim of the virological substudy was to evaluate the impact of SCT and CT on the reservoir as assessed by low-level residual viraemia and total HIV-1 DNA levels.
Although there was a slightly higher proportion of individuals with a HIV-1 RNA VL > 20 copies/ml at week 48 than > 50 copies/ml at week 48, reassuringly the number of young people with a HIV-1 RNA VL > 20 copies/ml at week 48 was comparable between arms (n = 14 CT, n = 13 SCT). There was no difference between the arms at baseline when the 20 copies/ml cut-off was used, which in addition was not predictive of virological failure.
These results indicate that young people in the SCT arm were not at risk of increased low-level viraemia at the end of the trial.
Qualitative substudy
The findings from the qualitative substudy indicate that those in the SCT arm, after taking some time to adapt, expressed a preference for taking the weekends off therapy, suggesting that SCT was acceptable to them. Although promising and preferred by those in the trial to CT, SCT may not, however, be a viable option for everyone, as even ‘exemplary adherers’ encountered initial challenges in adapting to the new 5 days on/2 days off routine.
Notably, SCT was preferred by the young people in the study in part because it enabled more effective hiding of their HIV status. As SCT reduces the risk of deductive disclosure through the witnessing of treatment taking, young people were able to engage in social activities that had previously been complicated by their desire to keep their HIV treatment and/or status secret. Although positive in the short term, it is of some concern in the longer term that denial about their HIV status might be perpetuated and disclosure postponed. At some point, these same young people will be encouraged to disclose their status as they transition to adulthood and begin their sexual lives. SCT might thus unintentionally support non-disclosure for a group of people living with HIV who will have to come to terms with disclosure in the not-so-distant future.
The study highlighted patterns of non-disclosure of adherence behaviours that often prevail among young people living with HIV. 58 In addition, our data have illustrated how young people use clinical indicators to interpret whether or not to share information and to justify non-disclosure, which contributes further insights to our current understanding. This relates to the limited reporting of missed doses. The lack of necessity to disclose missed doses appears to be reinforced by the confidence that young people invest in clinical monitoring; they interpret an undetectable VL as a demonstration that recent missed doses are not significant and do not need to be reported.
The impact of medication side effects on well-being were also consistently under-reported to clinicians and possibly within the trial. Some participants had come to perceive these side effects as ‘normal’ and as they considered them to be inevitable they did not consider it worth bringing them up and discussing them further. Once again, this illustrates the significant filtering that might be involved in young people’s conversations with clinic staff. Furthermore, discussion of treatment side effects may also provide valuable insight into the disparity between the common reporting of these side effects in the qualitative study and the rare reporting in the trial, where they may not have been described as unusual or severe.
The study participants clearly do not currently discuss many of their ongoing issues around their treatment and their condition with anyone and try instead to manage by themselves. They gain confidence in not needing to mention missed doses from the trust that they invest in what an undetectable VL signifies. This is particularly problematic in terms of their openness with clinicians, the lack of which might lead to health-related harms if treatment challenges are explored or revealed too late and medical options restricted. Reinforcing the reputation of young people as ‘exemplary adherers’ may paradoxically function as both an encouragement for young people to continue to take their treatment as prescribed and a disincentive to talk about any problem that might arise, including missed doses and side effects, for fear of damaging their reputation.
Importantly, therefore, an opportunity is opened up whereby SCT has the potential to signify the beginning of a change in the conversation around adherence in the clinic, within the family and within the broader social environments inhabited by young people living with HIV. As a therapy that specifically acknowledges the adherence challenges that pertain to childhood and adolescence, if framed thoughtfully health-care staff can use SCT to show a greater contextual understanding about how treatment can be disrupted for complex reasons. By encouraging more open discussions about ‘slippages’ and how these can be managed, SCT might be able not only to support improvements in communication between health-care staff and young people living with HIV but also to lessen the significant blaming and self-blaming that can at times characterise the experience of those who cannot achieve 100% adherence to ARVs.
The study findings suggest that SCT could be used as a ‘reward’ for those who can manage to adhere well and this could sustain them on the days when they are supposed to take their medication. In addition, SCT could also function as an incentive to put more effort into taking treatment and lowering VL for those who would not currently qualify for SCT. Thoughtful planning and framing of SCT to young people is necessary for these potential benefits to be realised.
However, study participants felt that SCT could also pose a significant risk to other young people who might try to take treatment breaks without clinical assessment and monitoring. This is a further reason to support the need for careful dissemination and communication of the results post trial. Recommendations for how the intervention and its results are to be communicated beyond the trial will be discussed in the concluding sections of this chapter.
Most of all, the initial challenges described by so many of the study participants need to be taken into serious consideration when planning any further interventions based on SCT. The findings suggest that the initial adaptation period, although different for different participants, was generally only short-lived but should not be underestimated. Provided that early adjustments are carefully managed and supported, however, the study has shown that SCT can be successfully transformed into a welcome ongoing treatment option for young people living with HIV, with great benefits for children’s and adolescents’ personal and social lives.
Study limitations
A key limitation of the study is the population on which these findings are based; trial participants were arguably initially predisposed to be more accepting of the intervention. However, this is complicated by our finding that many participants initially agreed to participate in spite of, not because of, the intervention.
So far we have not explored the acceptability of SCT among those who refused to take part in the trial or those who were not eligible. It would be valuable to address the question of the acceptability of SCT more broadly and robustly. Although we aim to be able to redress some of these limitations through our post-trial ongoing data collection, it is important to note that, in the UK, this is quite a heavily researched cohort.
In making sense of the participants’ accounts, it is also important to highlight that the experience of being in the trial in itself may have been conducive to better adherence. Some of the findings should therefore be interpreted with particular caution against the background of the increased support and monitoring that the young people experienced during the course of the study, particularly in contexts where there may be little or no access to this kind of monitoring outside the trial period. Equally, it is possible that some of the early anxieties shared by many participants with regard to the safety of SCT would not arise for other young people once the trial and follow-up have ended and the results are fully established and disseminated.
Conclusions: implications for health care and recommendations for research
The qualitative study within the BREATHER trial has uncovered and highlighted a number of important issues that are relevant for the treatment-related support that is needed for this cohort; the trial and possible subsequent interventions based on SCT; and the direction that future clinical and social research with this age group should take so that we can better understand and effectively respond to the challenges at hand.
Implications for supporting adherence among young people living with human immunodeficiency virus
Our findings point to the predominant need to expand and improve conversations around adherence issues that young people have with their clinicians, family and peers. Young people’s narratives about treatment and adherence are characterised by many of the same problematic realities of their adult counterparts. These include, but are not limited to, fear of stigma, lack of motivation, fear of reprimand, treatment side effects and the practical hurdles of adhering to a daily regimen. 59,60
However, young people also present with particular and unique challenges that pertain to youth, for example their more absolute dependency on their carers and clinicians, their at times limited knowledge about their condition and its potential impact on their future lives, but also the acute importance of socialisation and acceptance by their peers, the restrictions on their capacity for self-management in the context of school and the household, the common histories of HIV-related loss in the family and the added pressure of facing a lifetime of treatment from a very young age. 61,62
It is therefore paramount that their problems with adherence should not be underestimated or dismissed as ‘forgetfulness’, ‘irresponsibility’ or a simple lack of awareness of the seriousness of the medical consequences of poor adherence. Findings from this study reveal the opposite: young people think about their health a great deal and adherence challenges thus need to be recognised and discussed transparently, as well as confronted with even more empathy and patience.
We have found that even within this cohort there is a lack of reporting of missed doses, under-reporting of side effects and a general sense that young people have the need to conceal their problems and devise alternative strategies on their own, without seeking medical advice or other help from carers, relatives or peers. These strategies can include relying on VL tests to assess the risks of missing doses, experimenting with skipping doses to see how much they can miss, adjusting the time when they take their pills to fit in around other daily activities and coping through these processes without seeking support and at times while experiencing significant shame about their health-related behaviours and anxieties about their own health.
Equally, the study results indicate that young people really appreciated the VL testing that they received during the clinical trial, which has particular implications for any settings (such as Uganda) where testing frequency may vary significantly between trial and non-trial clinical practice. Furthermore, the convenience and cost saving attached to reduced monitoring should be weighed against the impact that this may have on young people’s adherence, especially against the background of their current lack of reporting of missed doses and the reassurance that many of the participants draw from the test results, which are also important for their own understanding of HIV infection.
This study contributes valuable qualitative evidence about the significance of side effects experienced by patients on EFV-based regimens. This will be explored further in future analyses and publications and will be the subject of a workshop convened in collaboration with CHIVA UK.
Implications for future short-cycle therapy roll-out
The findings from our study emphasise the importance of incorporating a package of interventions to accompany any roll-out of SCT to support young people in adapting to this new routine. These interventions would need to be designed based on the multiple findings from the clinical and qualitative trial components and subsequently evaluated for further roll-out.
Some preliminary suggestions about what the interventions would need to include can already be made by looking at the qualitative study results. From a synthesis of the discussions in the interviews, we would anticipate an adaptation period of 12–18 weeks to be appropriate for young people switching to SCT from CT, possibly preceded by a 4-week preparation period before switching to ensure understanding and alleviate any concerns. This could draw on peer support opportunities, with those already on SCT providing advice and sharing experiences, written case studies or participation in targeted interventions.
Given what we have observed about how young people make decisions about how they engage with their treatment and decide when and how to take it – and also whether or not to report what they are actually doing – it is critical that they have a genuine understanding of SCT: what it is and how long it lasts and continues to work in the body. The provision of education about SCT to young people (and their carers) would thus be central to any successful roll-out.
One aspect of SCT that would need to be carefully explained is why treatment needs to be missed on 2 consecutive days rather than any 2 days in a week. It would also be beneficial for young people to have the option of choosing which 2 days are best for them to miss as some of the participants who had treatment breaks during the weekend mentioned that they may have preferred missing weekdays in light of their work shifts and school classes.
The study found that young people benefit from the assurance that they derive from clinical monitoring, which gives them confidence in their state of health and the efficacy of the way that they are taking their treatment. Hence, we would strongly recommend that a similar practice of VL monitoring to that provided in the trial is made available for some time if SCT is introduced.
Furthermore, as well as a support intervention around the time of introduction of SCT, we would recommend that currently available measures of both adherence and side effects should be improved. This would be of particular importance for young people moving to SCT because of the disruptive potential of introducing a new regimen, coupled with the possibility that young people may under-report missed doses so that they are not placed back on CT.
Additionally, as SCT would be provided using an EFV-based combination only, we would recommend that the significant issues around side effects detailed in this report are taken into greater consideration for all young people on this medication, but especially for those who may be switched to this particular drug to start SCT having been previously being treated with different ARVs. Side effects have been shown in this study to impact on adherence but also on young people’s overall well-being and it is therefore paramount that they are repeatedly encouraged to discuss them and that any AEs are recorded and managed with greater care than our findings would currently suggest.
Implications for further research
The qualitative study with young people living with HIV explored the experiences of study participants during the course of the clinical trial. However, further follow-up research is needed to review responses to the trial findings among the trial population and, especially, to disentangle the effect of the trial on young people’s adherence-related behaviours. This would be important to understand both how trial participation impacts on behaviour in the present and what might be the effects of participation for the long-term management of HIV in young people on SCT. Furthermore, qualitative research may be needed to investigate how young people experience, manage and adapt to being put onto SCT when this is rolled out outside trial conditions. An evaluation of the accompanying support intervention could be integrated into this qualitative study.
At the same time it would be vital to investigate how HIV-positive young people outside the trial may interpret SCT and to explore perceptions, understandings and the potential impact of knowledge about SCT in the wider HIV population. This evidence would be of help to inform the adherence support intervention mentioned above, but would also allow any other issues that may arise to be addressed promptly. Gaining an understanding of initial reactions to SCT would thus aid implementation work that would be conducted to minimise the risks of inappropriate uptake of SCT by those young people for whom it may not be clinically feasible. Research with both trial participants and their peers that analyses responses to and perceptions of SCT would also contribute to the development of any communication/dissemination tools that would be needed to support the roll-out of SCT as well as contributing to the design of an appropriate intervention alongside other programmatic expertise with this population.
We would also recommend that the ways in which qualitative analyses are integrated to inform trial findings could be reviewed. In particular, it will be important to explore novel ways to engage with some of the differences in findings that have emerged from a comparison of the qualitative and quantitative data in the BREATHER trial. Findings about both adherence and side effects differ markedly between the qualitative interviews, the quantitative survey about adherence during the trial and the treatment-related AEs reported in the clinical database. We do not consider these differences in data sets to indicate greater or lower levels of accuracy of the findings generated from each method. Rather, we suggest that these potential differences might be usefully triangulated and integrated in further study designs, including during the course of trials, to obtain a more comprehensive picture of the adherence-related behaviours and experiences of young people on HIV treatment.
Finally, the findings from our study clearly indicate that further and urgent research is needed to improve our understanding of the experiences of young people on EFV-based combination medication. There is a dearth of qualitative research on the side effects of this medication and no current research on how it may affect young people, especially in relation to their self-perceptions and understanding of health and HIV but also in terms of their difficulties with adherence. This research will also have to take into consideration potential forthcoming reductions in the recommended dosing of EFV in light of recent discoveries54 and what the consequences of such changes might be for side effects and for the future of SCT or other interventions involving treatment breaks.
Chapter 6 Conclusions
In conclusion, in the BREATHER trial, a global study involving young people from diverse geographical, ethnic and sociocultural backgrounds, the non-inferiority of SCT compared with CT using EFV-based combination ART was demonstrated.
Overall, 93% of young people remained virally suppressed with a very small difference (1.2%) between the arms favouring SCT. There was no detectable difference in immunological parameters, the development of viral resistance or markers of inflammation, nor was there a difference in the number of AEs between arms; however, a lower number of reported ART-related AEs was observed in the CT arm. Importantly, contrary to pre-study perceptions of some, there was absolutely no evidence of lower adherence in the CT arm than in the SCT arm.
Allowing short weekend breaks in daily pill taking was well accepted by participants, who showed a strong preference for the SCT strategy as it allowed for a more normal weekend lifestyle and had a positive impact on their social activities.
BREATHER was associated with an important reduction in drug exposure for the SCT arm. Lower drug exposure reduces the likelihood of longer-term toxicity and could accrue significant cost savings such that, in resource-limited settings, this could allow more young people to initiate ART (important in view of recent changes in World Health Organization guidelines recommending treatment for all63).
The BREATHER trial represents an important first step in designing therapeutic strategies for young people that, without compromise of virological control or immune function or other hazards, permit normalisation of the adolescent and youth experience. This has relevance to the design of future studies, particularly those involving drugs with longer half-lives and a higher threshold to resistance, increasing numbers of which are becoming available.
The results of this study will be applicable in well-suppressed and adherent young people on a regimen containing a drug with a long half-life, providing that the results of the 2-year long-term follow-up phase of the BREATHER trial confirm that SCT is an effective and a safe strategy. Further pragmatic studies including follow-up under routine clinical conditions in settings with less frequent VL monitoring may be required to confirm the applicability of this strategy in real-life situations. It is also possible that this approach could be undertaken with other suitable long-acting drugs (e.g. tenofovir alafenamide) and/or drugs with a higher barrier to resistance (e.g. dolutegravir).
Acknowledgements
This trial was sponsored by the PENTA Foundation. We thank all of the young people, their families and staff from the centres participating in the BREATHER trial.
PENTA Steering Committee
J-P Aboulker, J Ananworanich, A Babiker, E Belfrage, S Bernardi, R Bologna, D Burger, K Butler, G Castelli-Gattinara, P Clayden, A Compagnucci, T Cressey, M Debré, R de Groot, M Della Negra, A De Rossi, A Di Biagio, D Duiculescu (deceased), A Faye, V Giacomet, C Giaquinto (chairperson), DM Gibb, I Grosch-Wörner, M Hainault, L Harper, N Klein, M Lallemant, J Levy, H Lyall, M Mardarescu, L Marques, MJ Mellado Peña, M Marczynska, D Nadal, E Nastouli, L Naver, T Niehues, C Peckham, D Pillay, J Popieska, JT Ramos Amador, P Rojo Conejo, L Rosado, V Rosenfeldt (deceased), R Rosso (deceased), C Rudin, Y Saïdi, M Sharland, HJ Scherpbier, C Thorne, PA Tovo, G Tudor-Williams, A Turkova, N Valerius, A Volokha, AS Walker, S Welch and U Wintergerst.
BREATHER Trial Steering Committee
JT Ramos Amador, J Ananworanich, A Babiker, K Butler, P Clayden, J Darbyshire, V Leroy, V Musiime and I Weller (chairperson).
BREATHER Trial Management Group
J Ananworanich, A Babiker, S Bernays, T Bunupuradah, K Butler (chairperson), J Calvert, S Chalermpantmetagul, K Chokephaibulkit, R Choudhury, A Compagnucci, T Cressey, C Giaquinto, D Gibb, L Harper, J Inshaw, J Kenny, H Kizito, N Klein, E Menson, S Montero, V Musiime, A Nanduudu, E Nastouli, M Ndiaye, JT Ramos Amador, T Rhodes, Y Riault, Y Saidi, J Seeley, K Scott, S Storey and A Turkova.
Trials units
INSERM SC10-US19, France
JP Aboulker, A Arulananthan, A Compagnucci, S Léonardo, L Meyer, M Ndiaye, Y Riault and Y Saïdi.
Medical Research Council Clinical Trials Unit, UK
A Babiker, J Calvert, R Choudhury, DM Gibb, L Harper, J Inshaw, D Johnson, J Kenny, S Martins, S Montero, C O’Leary, K Scott, J Thompson, A Turkova, S Townsend, S Shidfar and A South.
Program for HIV Prevention and Treatment, Thailand
(L = Laboratory, P = Pharmacy)
TR Cressey, S Chalermpantmetagul, R Peongjakta, K Than-in-at, S Chailert, K Seubmongkolchai, S Thammajitsagul, W Sripaoraya (L), C Kasemrat (L), A Upra (P), G Jourdain, M Lallemant, N Ngo-Giang-Huong and S Le Coeur.
Immunology and Virology Advisory Group
A De Rossi, N Klein, MA Muñoz Fernandez, E Nastouli and N Ngo.
Qualitative Substudy Group
S Bernays, S Paparini, T Rhodes and J Seeley.
Independent Data Monitoring Committee
A Pozniak (chairperson), S Vella, G Chène and T Vesikari.
Endpoint Review Committee
K Butler, DM Gibb and V Musiime.
Communications
Magda Conway, CHIVA.
Recruiting sites
(L = Laboratory, P = Pharmacy, S = Social Scientist.)
Argentina
Hospital Dr JP Garrahan/Fundación Helios Salud, Buenos Aires: R Bologna, J Da Bouza, D Mecikovsky, G Sotera, A Mangano (L) and M Moragas (L).
Belgium
CHU St Pierre (Clinique Pediatrie-Maladies Infectieuses): M Hainaut, E Van der kelen and S Vandenwijngaert (L).
Denmark
Hospital Hvidovre (Pediatric Department): V Rosenfeldt, N Valerius and L Jensen (L).
Germany
JW Goethe University, Frankfurt: C Koenigs, S Schultze-Strasser, R Linde and K Mantzsch.
Ireland
Our Lady’s Children’s Hospital, Dublin: K Butler, P Gavin, R Leahy, A Rochford, M Goode, A Walsh, E Hyland (L) and M O’Connor (P).
Spain
I Garcia Mellado, Hospital 12 de Octubre, Madrid: P Rojo Conejo and MI Gonzalez Tomé; Hospital Sant Joan De Déu, Barcelona: C Fortuny Guasch, A Noguera Julian, P Santin Riba and A Murciano Cabeza (P); Hospital La Fe, Valencia: MD Perez Tamarit, MC Otero Reigada, F Castera Brugada, I Segarra Granell and R Amigo Moreno (L); Hospital La Paz, Madrid: MJ Mellado Peña, M Garcia Lopez Hortelano, MI De José Gomez and L Escosa; Universitario de Getafe, Madrid: S Guillen Martin and LM Prieto Tato; Hospital Clínico San Carlos, Madrid: JT Ramos Amador; Biobanco Gregorio Marañon, Madrid: MA Muñoz Fernandez (L), JL Jimenez Fuentes (L), C Gómez Rico (L) and A Garcia Torre (L).
Thailand
HIV Netherlands Australia Thailand Research Collaboration: T Bunupuradah, J Ananworanich, T Chuanjaroen ka and N Thammajaruk (L); PHPT, Kalasin Hospital: S Srirojana, D Dornngern, N Kunchanarong (P) and L Mongkun (L); Regional Health Promotion Center Region 6, Khon Kaen: S Hanpinitsak, K Aue-apisak,P Kangsavon (P) and M Shevasateanchai (L).
UK
Great Ormond Street Hospital, London: N Klein, J Kenny, A Turkova, D Shingadia, J Flynn, M Clapson, K Parkes, E Howley and L Spencer-Walsh; Evelina Children’s Hospital, London: E Menson, R Cross, C Duncan, V Timms, E Reus, A Callaghan, S Tomlin (P) and E Jones (P); ICH, London: H Poulsom (L), N Klein (L) and L Carter (L); Royal Infirmary, Bristol: J Bernatoniene, A Finn, E Clarke, F Manyika, L Hutchison, H Smee, L Ball (P) and K Stevenson (L); Heartlands Hospital, Birmingham: S Welch, S Hackett, G Gilleran, J Daglish, L Horton (L) and K Gandhi (P); Queen’s Medical Centre, Nottingham: A Smyth, J Smith, A Short, L Fear, S Stafford, S Hodgson (P) and Y Taha (L); Leicester Royal Infirmary: S Bandi, J Philps, J Bwire (P) and J Gardener (L); St George’s Hospital, London: K Doerholt, K Prime, M Sharland, S Donaghy, L Spencer-Walsh, S Storey, O Okolo and D Rolfe (P); London School of Hygiene & Tropical Medicine: S Bernays (S), T Rhodes (S) and J Seeley (S); University College London Hospital: E Nastouli (L), S Kirk (L), P Grant (L) and B Ferns (L).
Uganda
Joint Clinical Research Centre, Kampala: C Kityo, V Musiime, H Kizito, A Nanduudu, A Drasiku, E Kaudha, S Senyonjo, I Obella, M Odera, P Oronon, H Nakyambadde, P Kyobutungi, O Senfuma (L), D Eram (L), J Nkalubo (L), L Nakiire (L), M Nabalaama (L), I Ssewanyana (L), G Pimundu (L), P Segonga (L), B Nakalawa (L), L Mugarura (L), A Kwaga (P), J Kasozi (L), M Ojok (L) and J Namusanje (L); MRC/Uganda Virus Research Institute (URVI), Uganda Research Unit on AIDS, Entebbe: M Ndagire (S) and S Namukwaya (S).
Ukraine
City AIDS Center, Kiev: A Volokha, I Raus, O Mostovenko (P) and N Chentsova (L).
USA
St Jude’s Children’s Research Hospital, Memphis, TN: P Flynn, R Dallas, T Wride, J Utech, S Ost, A Gaur, K Knapp, N Patel, M Shenep, T Culley, M Griffith (L), S Carr (P) and C Longserre (P).
Contributions of authors
Professor Karina Butler (Consultant Paediatrician, Infectious Diseases Specialist and University College Dublin Clinical Professor of Paediatrics, Paediatric Infectious Diseases) devised and conducted the main study.
Jamie Inshaw (Medical Statistician and Research Associate, Statistics) was the delegated statistician for the BREATHER trial and conducted the analyses for the main study and all substudies.
Deborah Ford (Senior Statistician) was a statistician for the BREATHER trial, conducted some analyses for the main study after Jamie Inshaw left the MRC CTU at UCL and contributed to the report.
Sarah Bernays (Lecturer, Medical Sociology) was the Chief Investigator on the qualitative substudy and devised and conducted the qualitative substudy.
Karen Scott (Clinical Trial Manager, Trial Management) managed the main trial and substudies and contributed to the development and production of the report.
Julia Kenny (Senior Clinical Research Fellow and Clinical Advisor, Immunology) devised and conducted the biomarkers substudy.
Nigel Klein (Consultant and Professor, Paediatric Infectious Diseases and Immunology) devised and conducted the immunology substudy and consulted on the immunological aspects of the main study.
Anna Turkova (Senior Paediatrician) contributed to trial management, report writing and follow-up of children at the UK sites.
Lynda Harper (Clinical Project Manager, Trial Management) managed the main trial and substudies, and contributed to the development and production of the report.
Eleni Nastouli, (Consultant Virologist, Virology) devised and conducted the virology substudy and consulted on virological aspects of the main study.
Sara Paparini (Research Fellow, Medical Anthropology) assisted with the data analysis for the qualitative substudy.
Rahela Choudhury (Clinical Trial Manager, Trial Management) managed the main trial and substudies, and contributed to the development and production of the report.
Tim Rhodes (Professor, Public Health Sociology) was the Chief Investigator on the qualitative substudy and codevised the qualitative substudy.
Abdel Babiker (Professor of Epidemiology and Medical Statistics, Statistics and Epidemiology) was the statistician for the BREATHER trial and oversaw all analyses and directed appropriate analyses to be carried out.
Diana Gibb (Professor in Epidemiology and Honorary Consultant Paediatrician, Epidemiology) played a role in all aspects of the trial as the lead of the paediatric programme at the MRC, including design, trial implementation, analysis and dissemination.
Publication
The BREATHER (PENTA 16) Trial Group. Weekends-off efavirenz-based antiretroviral therapy in HIV-infected children, adolescents, and young adults (BREATHER): a randomised, open-label, non-inferiority, phase 2/3 trial. [published online ahead of print 20 June 2016]. Lancet 2016.
Data sharing statement
Data sharing requests can be made to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Gibb DM, Duong T, Tookey PA, Sharland M, Tudor-Williams G, Novelli V, et al. Decline in mortality, AIDS, and hospital admissions in perinatally HIV-1 infected children in the United Kingdom and Ireland. BMJ 2003;327. http://dx.doi.org/10.1136/bmj.327.7422.1019.
- Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Rockville, MD: National Institutes of Health; 2008.
- World Health Organization . Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach 2010. http://apps.who.int/iris/bitstream/10665/44379/1/9789241599764_eng.pdf.
- World Health Organization . Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach 2013. www.who.int/hiv/pub/guidelines/arv2013/download/en.
- Foster C, Judd A, Tookey P, Tudor-Williams G, Dunn D, Shingadia D, et al. Young people in the UK and Ireland with perinatally acquired HIV: the paediatric legacy for adult services. AIDS Patient Care STDS 2008;23:159-66. http://dx.doi.org/10.1089/apc.2008.0153.
- Fiese BH, Everhart RS. Medical adherence and childhood chronic illness: family daily management skills and emotional climate as emerging contributors. Curr Opin Pediatr 2006;18:551-7. http://dx.doi.org/10.1097/01.mop.0000245357.68207.9b.
- Rianthavorn P, Ettenger RB. Medication non-adherence in the adolescent renal transplant recipient: a clinician’s viewpoint. Pediatr Transplant 2005;9:398-407. http://dx.doi.org/10.1111/j.1399-3046.2005.00358.x.
- Smith BA, Shuchman M. Problem of nonadherence in chronically ill adolescents: strategies for assessment and intervention. Curr Opin Pediatr 2005;17:613-18. http://dx.doi.org/10.1097/01.mop.0000176443.26872.6e.
- Bangsberg DR, Kroetz DL, Deeks SG. Adherence–resistance relationships to combination HIV antiretroviral therapy. Curr HIV AIDS Rep 2007;4:65-72. http://dx.doi.org/10.1007/s11904-007-0010-0.
- Casado JL, Sabido R, Perez-Elias MJ, Antela A, Oliva J, Dronda F, et al. Percentage of adherence correlates with the risk of protease inhibitor (PI) treatment failure in HIV-infected patients. Antivir Ther 1999;4:157-61.
- Chesney MA, Morin M, Sherr L. Adherence to HIV combination therapy. Soc Sci Med 2000;50:1599-605. http://dx.doi.org/10.1016/S0277-9536(99)00468-2.
- Deeks SG. Determinants of virological response to antiretroviral therapy: implications for long-term strategies. Clin Infect Dis 2000;30:177-84. http://dx.doi.org/10.1086/313855.
- Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med 2007;146:564-73. http://dx.doi.org/10.7326/0003-4819-146-8-200704170-00007.
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133:21-30. http://dx.doi.org/10.7326/0003-4819-133-1-200007040-00004.
- Reddington C, Cohen J, Baldillo A, Toye M, Smith D, Kneut C, et al. Adherence to medication regimens among children with human immunodeficiency virus infection. Pediatr Infect Dis 2000;19:1148-53. http://dx.doi.org/10.1097/00006454-200012000-00005.
- Wagels T, Amiet R, Battegay M, Guex AC, Opravil M, Vernazza PL. Predictive value of adherence in patients starting highly active antiretroviral treatment for HIV infection. Swiss Med Wkly 2004;134:678-80.
- Gross R, Yip B, Lo Re V III, Wood E, Alexander CS, Harrigan PR, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis 2006;194:1108-14. http://dx.doi.org/10.1086/507680.
- Martin S, Elliott-DeSorbo DK, Wolters PL, Toledo-Tamula MA, Roby G, Zeichner S, et al. Patient, caregiver and regimen characteristics associated with adherence to highly active antiretroviral therapy among HIV-infected children and adolescents. Pediatr Infect Dis J 2007;26:61-7. http://dx.doi.org/10.1097/01.inf.0000250625.80340.48.
- Murphy DA, Belzer M, Durako SJ, Sarr M, Wilson CM, Muenz LR. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med 2005;159:764-70. http://dx.doi.org/10.1001/archpedi.159.8.764.
- Gibb DM, Goodall RL, Giacomet V, McGee L, Compagnucci A, Lyall H. Adherence to prescribed antiretroviral therapy in human immunodeficiency virus-infected children in the PENTA 5 trial. Pediatr Infect Dis J 2003;22:56-62. http://dx.doi.org/10.1097/00006454-200301000-00015.
- Gibb D, Compagnucci A, Green H, Lallemant M, Saidi Y, Ngo-Giang-Huong N, et al. Treatment interruption in children with chronic HIV-infection: the results of the Paediatric European Network for Treatment of AIDS (PENTA) 11 trial. J Int AIDS Soc 2008;11. http://dx.doi.org/10.1186/1758-2652-11-S1-O21.
- El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355:2283-96. http://dx.doi.org/10.1056/NEJMoa062360.
- Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008;359:2233-44. http://dx.doi.org/10.1056/NEJMoa0800971.
- Jacobson JM, Pat Bucy R, Spritzler J, Saag MS, Eron JJ, . Evidence that intermittent structured treatment interruption, but not immunization with ALVAC-HIV vCP1452, promotes host control of HIV replication: the results of AIDS Clinical Trials Group 5068. J Infect Dis 2006;194:623-32. http://dx.doi.org/10.1086/506364.
- Ananworanich J, Nuesch R, Le Braz M, Chetchotisakd P, Vibhagool A, Wicharuk S, et al. Failures of 1 week on, 1 week off antiretroviral therapies in a randomized trial. AIDS 2003;17:F33-7. http://dx.doi.org/10.1097/00002030-200310170-00001.
- Cardiello PG, Hassink E, Ananworanich J, Srasuebkul P, Samor T, Mahanontharit A, et al. A prospective, randomized trial of structured treatment interruption for patients with chronic HIV type 1 infection. Clin Infect Dis 2005;40:594-600. http://dx.doi.org/10.1086/427695.
- Smith RJ. Adherence to antiretroviral HIV drugs: how many doses can you miss before resistance emerges?. Proc Biol Sci 2006;273:617-24. http://dx.doi.org/10.1098/rspb.2005.3352.
- Cohen C, Colson A, Morris A, Alberts L, Sheble-Hall A, Hussey S, et al. The FOTO Study 48 Week Results: Viral Suppression Can Be Maintained When Antiretrovirals Are Taken Five Consecutive Days On, and Two Days Off Each Week n.d.
- Cohen C, Colson A, Pierone G, DeJesus E, Kinder F, Elion R, et al. The FOTO study: 24-week results support the safety of a 2-day break on efavirenz-based antiretroviral therapy. J Int AIDS Soc 2008;11. http://dx.doi.org/10.1186/1758-2652-11-S1-O19.
- Reynolds SJ, Kityo C, Hallahan CW, Kabuye G, Atwiine D, Mbamanya F, et al. A randomized, controlled, trial of short cycle intermittent compared to continuous antiretroviral therapy for the treatment of HIV infection in Uganda. PLOS ONE 2010;5. http://dx.doi.org/10.1371/journal.pone.0010307.
- Reynolds S, Kityo C, Ssali S, Hallahan C, Mwebesa D, Mbamanya F, et al. A Randomized, Controlled, Non-Inferiority Trial of Two Short Cycle Intermittent Regimens Compared to Continuous Antiretroviral Therapy for the Treatment of Chronic HIV Infection in Uganda n.d.
- Rudy BJ, Sleasman J, Kapogiannis B, Wilson CM, Bethel J, Serchuck L, et al. Short-cycle therapy in adolescents after continuous therapy with established viral suppression: the impact on viral load suppression. AIDS Res Hum Retroviruses 2009;25:555-61. http://dx.doi.org/10.1089/aid.2008.0203.
- Taylor S, Boffito M, Khoo S, Smit E, Back D. Stopping antiretroviral therapy. AIDS 2007;21:1673-82. http://dx.doi.org/10.1097/QAD.0b013e3281c61394.
- Frampton JE, Croom KF. Efavirenz/emtricitabine/tenofovir disoproxil fumarate: triple combination tablet. Drugs 2006;66:1501-12. http://dx.doi.org/10.2165/00003495-200666110-00012.
- Harrigan PR, Whaley M, Montaner JS. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. AIDS 1999;13:F59-62. http://dx.doi.org/10.1097/00002030-199905280-00001.
- Cressey TR, Green H, Khoo S, Treluyer JM, Compagnucci A, Saidi Y, et al. Plasma drug concentrations and virologic evaluations after stopping treatment with nonnucleoside reverse-transcriptase inhibitors in HIV type 1-infected children. Clin Infect Dis 2008;46:1601-8. http://dx.doi.org/10.1086/587657.
- Dorrell J. Being Young and HIV Positive: The Experiences of Young People Living With HIV Since Birth: A Research Study in Progress n.d.
- Machin D, Campbell M, Fayes P, Pinol A. Sample Size Tables for Clinical Studies. Blackwell Science; 1997.
- Johnson VA, Calvez V, Günthard HF, Paredes R, Pillay D, Shafer RW, et al. Update of the drug resistance mutations in HIV-1: March 2013. Top Antivir Med 2013;21.
- Paediatric European Network for Treatment of AIDS . Response to planned treatment interruptions in HIV infection varies across childhood. AIDS 2010;24:231-41. http://dx.doi.org/10.1097/QAD.0b013e328333d343.
- Klein N, Sefe D, Mosconi I, Zanchetta M, Castro H, Jacobsen M, et al. The immunological and virological consequences of planned treatment interruptions in children with HIV infection. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0076582.
- Bernays S, Rhodes T, Jankovic Terzic K. Embodied accounts of HIV and hope: using audio diaries with interviews. Qual Health Res 2014;24:629-40. http://dx.doi.org/10.1177/1049732314528812.
- Strauss AL, Corbin JM. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. Newbury Park, CA: Sage Publications; 1990.
- Charmaz K. Constructing Grounded Theory. London: Sage; 2006.
- Butler K. ART With Weekends off Is Noninferior to Continuous ART in Young People on EFV + 2NRTI n.d. www.croiwebcasts.org/console/player/25558?mediaType = slideVideo& (accessed 14 March 2016).
- Butler K. Pediatric Update BREATHER Study n.d.
- Deacon H. Towards a sustainable theory of health-related stigma: lessons from the HIV/AIDS literature. J Community Appl Soc Psychol 2006;16:418-25. http://dx.doi.org/10.1002/casp.900.
- Goffman E. Stigma: Notes on the Management of Spoiled Identity. Englewood Cliffs, NJ: Prentice-Hall; 1963.
- Link BG, Phelan JC. Conceptualizing stigma. Ann Rev Sociol 2001;27:363-85. http://dx.doi.org/10.1146/annurev.soc.27.1.363.
- Cavalcante GI, Capistrano VL, Cavalcante FS, Vasconcelos SM, Macedo DS, Sousa FC, et al. Implications of efavirenz for neuropsychiatry: a review. Int J Neurosci 2010;120:739-45. http://dx.doi.org/10.3109/00207454.2010.520541.
- Raffi F, Pozniak AL, Wainberg MA. Has the time come to abandon efavirenz for first-line antiretroviral therapy?. J Antimicrob Chemother 2014;69:1742-7. http://dx.doi.org/10.1093/jac/dku058.
- Persson A, Newman C. Potency and vulnerability: troubled ‘selves’ in the context of antiretroviral therapy. Soc Sci Med 2006;63:1586-96. http://dx.doi.org/10.1016/j.socscimed.2006.04.001.
- Lima VD, Harrigan R, Murray M, Moore DM, Wood E, Hogg RS, et al. Differential impact of adherence on long-term treatment response among naive HIV-infected individuals. AIDS 2008;22:2371-80. http://dx.doi.org/10.1097/QAD.0b013e328315cdd3.
- Winston A, Amin J, Clarke A, Else L, Amara A, Owen A, et al. Cerebrospinal fluid exposure of efavirenz and its major metabolites when dosed at 400 mg and 600 mg once daily: a randomized controlled trial. Clin Infect Dis 2015;60:1026-32.
- Williams JP, Hurst J, Stohr W, Robinson N, Brown H, Fisher M, et al. HIV-1 DNA predicts disease progression and post-treatment virological control. ELife 2014;3. http://dx.doi.org/10.7554/eLife.03821.
- Palmer S, Wiegand AP, Maldarelli F, Bazmi H, Mican JM, Polis M, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol 2003;41:4531-6. http://dx.doi.org/10.1128/JCM.41.10.4531-4536.2003.
- Laird GM, Eisele EE, Rabi SA, Lai J, Chioma S, Blankson JN, et al. Rapid quantification of the latent reservoir for HIV-1 using a viral outgrowth assay. PLOS Pathog 2013;9. http://dx.doi.org/10.1371/journal.ppat.1003398.
- Kawuma R, Bernays S, Siu G, Rhodes T, Seeley J. ‘Children will always be children’: exploring perceptions and experiences of HIV-positive children who may not take their treatment and why they may not tell. Afr J AIDS Res 2014;13:189-95. http://dx.doi.org/10.2989/16085906.2014.927778.
- Al-Dakkak I, Patel S, McCann E, Gadkari A, Prajapati G, Maiese EM. The impact of specific HIV treatment-related adverse events on adherence to antiretroviral therapy: a systematic review and meta-analysis. AIDS Care 2013;25:400-14. http://dx.doi.org/10.1080/09540121.2012.712667.
- Vervoort SC, Borleffs JC, Hoepelman AI, Grypdonck MH. Adherence in antiretroviral therapy: a review of qualitative studies. AIDS 2007;21:271-81. http://dx.doi.org/10.1097/QAD.0b013e328011cb20.
- Bernays S, Seeley J, Rhodes T, Mupambireyi Z. What am I ‘living’ with? Growing up with HIV in Uganda and Zimbabwe. Sociol Health Illn 2015;37:270-83. http://dx.doi.org/10.1111/1467-9566.12189.
- Bernays S, Jarrett P, Kranzer K, Ferrand RA. Children growing up with HIV infection: the responsibility of success. Lancet 2014;383:1355-7. http://dx.doi.org/10.1016/S0140-6736(13)62328-4.
- World Health Organization . Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV 2015. http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf.
Appendix 1 The BREATHER trial protocol changes taken directly from protocol version 1.9
Appendix 2 The BREATHER trial Independent Data Monitoring Committee and Trial Steering Committee meeting dates
Independent Data Monitoring Committee
-
28 October 2011.
-
5 September 2012.
-
31 July 2013.
-
7 February 2014.
Trial Steering Committee
-
16 December 2010.
-
16 December 2011.
-
16 December 2012.
-
19 December 2013.
-
18 December 2014.
Appendix 3 The BREATHER trial patient results leaflet
Appendix 4 Presentation of the BREATHER trial results at the Conference for Retroviruses and Opportunistic Infections 2015
Appendix 5 Adherence questionnaire
Glossary
- Confirmed viral load
- Viral load tested in a separate blood sample (within a week).
- Reproducible viral load
- Viral load retested in the same blood sample.
List of abbreviations
- 3TC
- lamivudine
- AE
- adverse event
- AIDS
- acquired immunodeficiency syndrome
- ALT
- alanine aminotransferase
- ART
- antiretroviral therapy
- ARV
- antiretroviral
- AST
- aspartate aminotransferase
- CD
- cluster of differentiation
- CDC
- Centers for Disease Control
- CHIVA
- Children’s HIV Association
- CI
- confidence interval
- CRF
- case report form
- CT
- continuous therapy
- DNA
- deoxyribonucleic acid
- EDTA
- ethylenediaminetetra-acetic acid
- EFV
- efavirenz
- ELISA
- enzyme-linked immunosorbent assay
- FOTO
- Five On Two Off
- FTC
- emtricitabine
- HAART
- highly active antiretroviral therapy
- HIV
- human immunodeficiency virus
- IDMC
- Independent Data Monitoring Committee
- INSERM SC10-US19
- National Institute for Health and Medical Research (France)
- IQR
- interquartile range
- LDL
- low-density lipoprotein
- MCV
- mean corpuscular red blood cell volume
- MEMS
- Medication Event Monitoring System
- MRC CTU
- Medical Research Council Clinical Trials Unit
- MSD
- Meso Scale Discovery
- NNRTI
- non-nucleoside reverse transcriptase inhibitor
- NRTI
- nucleoside reverse transcriptase inhibitor
- NVP
- nevirapine
- PCR
- polymerase chain reaction
- PENTA
- Paediatric European Network for Treatment of AIDS
- PHPT
- Program for HIV Prevention and Treatment (Thailand)
- PI
- protease inhibitor
- RNA
- ribonucleic acid
- RT-PCR
- reverse transcription polymerase chain reaction
- SAE
- serious adverse event
- SCT
- short-cycle therapy
- SMART
- Strategies for Management of Antiretroviral Therapy
- TDF
- tenofovir
- TF
- tissue factor
- TSC
- Trial Steering Committee
- UCL
- University College London
- VL
- viral load
- ZDV
- zidovudine