Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/70/04. The contractual start date was in May 2011. The draft report began editorial review in July 2015 and was accepted for publication in November 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Elaine M McColl is a member of the National Institute for Health Research Journals Library Editorial Group.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Parry et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Falls and fear of falling
Falls are common, frequently devastating events in older people, with between 30% and 62% of older individuals falling per year. 1,2 Falls are responsible for considerable morbidity and mortality, with around 10% of falls resulting in fractures. 3 The economic costs of falls are considerable; as long ago as 2003, the cost of falls to the UK economy was estimated at £981M,4 with more recent data showing that 0.07–0.20 of the gross domestic product and 0.85–1.5% of total health-care expenditure in Western economies was accounted for by falls and their consequences. 5 Adverse consequences of falls are by no means limited to physical injury and escalating levels of dependence. Many older individuals, both fallers and non-fallers, suffer from a variety of adverse psychosocial difficulties related to falling,6–16 including fear, anxiety, loss of confidence and impaired self-efficacy (the self-perception of ability to perform within a particular domain of activities),10,13 resulting in activity avoidance, social isolation and increasing frailty. 6–16 The umbrella term for these problems is ‘fear of falling’, a common and disabling problem in older individuals, found in between 3% and 85% of community-dwelling elders who fall, and up to 50% of those who have never fallen. 8–10,16
Physical treatments for fear of falling
The optimal management strategy for fear of falling and its adverse physical and psychosocial consequences is poorly understood. Clinical and laboratory observations show that concerns about falling have a clear effect on gait patterns in older people. For example, experimental data in older people who are undergoing gait and balance studies on elevated walkways show disproportionately slow walking speeds and other dysfunctional gait adjustments17 alongside abnormalities in postural balance compared with younger subjects. 18 These experimental data and the observation of higher rates of falls and physical frailty in older individuals with fear of falling suggest that physical interventions may help to ameliorate fear of falling. Much of the research focusing on physical treatments includes home- and community-based exercise interventions, t’ai chi and multifactorial interventions aimed at reducing fall rates, with fear of falling reported as a secondary outcome in the majority of these studies. 8 A meta-analysis of nine studies examining t’ai chi in the management of fall prevention, fear of falling, and balance in older adults concluded that insufficient evidence existed to recommend such an intervention in this context. 19,20 A more recent randomised controlled study in 176 elders, randomised to one of three groups (intensive t’ai chi with cognitive–behavioural strategies, t’ai chi alone and a usual care control group) showed improvements in fear of falling as measured by the Falls Efficacy Scale13 (FES) in the cognitive strategies group compared with the other two groups. 21 So, although t’ai chi can help to prevent falls in older adults,22 its role specifically in the management of fear of falling is less clear.
A previous systematic review found 12 high-quality randomised controlled trials (RCTs) that included fear of falling among the outcomes that they assessed,8 but only one of these trials was primarily aimed at reducing fear of falling. 23 The interventions were conducted across a variety of settings – home-based exercise, community t’ai chi and home-based multifactorial interventions – and all improved fear of falling. 8 In a subsequent trial, a geriatric outpatient-based multifactorial intervention study found no such benefit. 24 More recently, a Cochrane review of exercise (three-dimensional exercise, such as t’ai chi and yoga, balance training or strength and resistance training) for reducing fear of falling in older people living in the community found that exercise ‘probably’ reduces fear of falling to a limited extent immediately after intervention, with inadequate evidence of an effect in the longer term. 25 The authors called for further evidence, with priority being given to the establishment of core outcomes, including fear of falling, in all trials of exercise intervention in community-dwelling elders. 25
Psychological treatments for fear of falling
Although such physical interventions may be of benefit in selected populations, the profile of the disorder and its psychosocial complications suggest that well-designed psychological interventions may help ameliorate the fear of falling more definitively. Several studies have examined an explicitly cognitive–behavioural therapeutic approach in fear of falling in community-dwelling elders, or used cognitive–behavioural therapy (CBT) techniques as part of a wider multifactorial intervention strategy. CBT is an evidence-based form of therapeutic intervention, which seeks to alter difficult emotional and physical states by altering the behaviours and cognitions that maintain them. As used in the CBT interventions for fear of falling studies described below, fear of falling is usually conceptualised as an anxiety disorder maintained by negative reinforcement (avoidance behaviour) and fearful cognitions. Tennstedt et al. ’s26 Matter of Balance study assessed the ability of an eight-session, 4-week group cognitive–behavioural approach with exercise instruction to improve fear of falling and related activity restriction. A total of 434 community-dwelling elders aged ≥ 60 years were randomised to intervention and control groups, with significant differences seen in fear of falling as measured by the FES13 and activity during follow-up. The magnitude of improvement in FES scores attenuated over time, prompting the authors to suggest that a booster session should be used in future studies and in clinical practice. 26 Clemson et al. 27 similarly used what they described as a ‘small-group learning environment’ (although, in practice, some of the methods used included cognitive–behavioural techniques) of 12 individuals per group for 2 hours per session over 7 weeks to improve self-efficacy and reduce falls. 27 The intervention incorporated a variety of learning strategies to facilitate behaviour change, including education regarding exercises to reduce falls risks, medication and home environmental review, and medication management. 27 There was a 31% reduction in the number of falls [relative risk 0.69, 95% confidence interval (CI) 0.50 to 0.96; p = 0.025] in the intervention group, although, interestingly, there was no corresponding change in FES scores. 27
More recently, Zijlstra et al. 28 conducted a RCT of a multicomponent cognitive–behavioural group intervention in older community-dwelling elders. Five hundred and forty participants were drawn from a random sample of 7431 individuals sent questionnaires who reported ‘at least some fear of falling’, although the method of assessment was not specified. Following randomisation, the intervention group underwent a structured 2-hour group CBT intervention (CBTi), based on the investigators’ previous work, once weekly for 8 weeks, with a booster session 6 months following the last session. A primary outcome was not specified and, although a power calculation was supplied based on a difference of 2.5 points, the authors fail to specify on which scale. The beginning of the trial predates widespread use of the FES–International version (FES-I)29 (advocated by the same group as the most appropriate measure for such studies),8 instead using a single-item question on fear of falling as well as an unspecified scale, probably the original FES from the description and reference supplied. 28 Other outcomes included perceived control over falling and daily activity as well as falls. There were no measures of physical function despite the prior evidence base suggesting improvement in fear of falling with the exercise-related interventions as described above. All outcomes showed significant differences between control and intervention groups at 2 and 8 months’ follow-up, with between-group differences persisting at 14 months in fear of falling and perceived control over falling, but not in the other outcome measures. There was a 30% attrition rate in the intervention group and 19.6% attrition rate in the control group. 28 The study intervention was carefully developed and grounded in cognitive–behavioural theory, but interpretation of findings is hampered considerably by the lack of clarity on sample size calculation and outcome measures, and the absence of generic quality-of-life measures and measures of physical functioning. The same research group later reported a before-and-after design study20 implementing a similar protocol into routine health care in 125 community-living older people, with significant improvements in concerns about falling.
The relative effectiveness of group-based psychological interventions thus remains uncertain. A theoretical re-examination of models of fear of falling and a recent cohort study of 500 older adults both suggest that the fear of falling population is a complex and heterogeneous one, in which psychosocial and physiological interventions also need to be individualised. 30,31 This suggests that individually based interventions may be more appropriate but, to date, trials have examined only group-level interventions.
What kind of intervention could be implemented in the UK NHS?
Fear of falling is a common, disabling and debilitating condition in older adults, but the current understanding of its management is limited. There is a small evidence base to support the use of some physical therapies to improve the syndrome, and promising early data from a few studies supporting the use of psychological therapies, in particular CBT. The cognitive–behavioural quintet32 of a situation or practical problem (falls, declining mobility, social isolation), altered thinking and emotion, altered physical symptoms with behavioural change, and activity reduction and avoidance is paradigmatic for fear of falling, and offers the hope of a viable therapeutic option. Previous studies are hampered by the factors already described, whereas the issue of the economic viability of such a treatment has yet to be explored. There is a need for many more trained cognitive–behavioural therapists than are currently available; the development of a cognitive therapeutic package for the management of fear of falling that can be delivered routinely by non-specialist staff such as health-care assistants (HCAs) is vital if this common and debilitating condition is to be tackled effectively. CBT can be delivered by suitably trained non-psychotherapist staff33,34 but, to our knowledge, this approach has not been attempted in a randomised controlled study in the context of fear of falling. In addition, only group interventions have been studied so far, with therapy delivered on a one-to-one basis yet to be tested in a fear of falling cognitive–behavioural intervention study despite suggestive evidence that this approach may prove more fruitful.
Any new treatment for potential implementation in the NHS needs to have clinical efficacy, but also needs to be able to be embedded into routine clinical practice. Understanding the dynamics of developing, delivering and trialling a novel cognitive–behavioural intervention by non-specialist staff as a process is useful because it will contribute to understanding the professional and organisational factors that promote or inhibit adherence to treatment protocols and intervention delivery, and how practical and methodological problems are defined, understood and resolved by the project team in the course of the study. The need for understanding the dynamics of complex interventions35 and undertaking process evaluation is now well understood. 36,37 Such work is important to underpin the transportability, workability and integration of interventions into routine clinical practice. In the case of this trial, our aim was to collect longitudinal ethnographic data that would help us to understand the social processes and relationships that lead the intervention and trial to take a particular shape and direction. In earlier studies of trials and other interventions, May and Finch38 developed a robust explanatory model of normalisation processes, which defines psychological and sociological mechanisms of behaviour and action that have been empirically demonstrated to be important in the implementation of complex interventions, and that have been revealed by evaluation in randomised controlled clinical trials. This approach is vital for the understanding and more widespread adoption of any cognitive–behavioural intervention in fear of falling.
Aims
Our aims were to develop a cognitive behaviour-based intervention to be delivered by HCAs on a one-to-one basis to community-dwelling older individuals attending falls services with an excessive or undue fear of falling and then to conduct a pragmatic, patient RCT of this intervention plus usual multidisciplinary care compared with usual multidisciplinary care alone, with integrated health-economic and process evaluations.
Chapter 2 Phase I: intervention development
This chapter reports the development of the CBTi for fear of falling, and the initial process evaluation work that was undertaken to inform the organisation and delivery of the CBTi in preparation for the RCT.
Developing the intervention
The aim of the development work was to develop a CBTi that could be delivered by HCAs (non-specialist, relatively low-paid staff) after training in basic CBT skills. The cognitive–behavioural model32 underpinning the intervention distinguishes between predisposing factors (what made a person vulnerable to a problem), precipitating factors (what triggered the current problem) and perpetuating factors (what is currently maintaining it). The model further distinguishes between physical, emotional, cognitive, behavioural and social factors in each domain. To develop the CBTi appropriately for the client group, assessment interviews with patients with fear of falling were undertaken. The intervention development process also included the preparation of supporting materials (‘manuals’).
Although existing literature on fear of falling indicated some of the likely intervention points, to further refine our intervention we chose to interview people who were affected by the problem. This work took the cognitive–behavioural assessment structure as its starting point, and followed on successful intervention development work that some of our team had previously undertaken in intervention development in other areas, such as medically unexplained symptoms. 34,39
Methods of intervention development
To develop the intervention, we conducted semistructured functional assessment interviews with 15 patients who had significant fear of falling. This number was determined by our previous research in developing psychosocial interventions. These interviews took the form of a standard cognitive–behavioural clinical assessment interview, used to elicit the predisposing, precipitating and perpetuating factors of participants’ problems, be they anxiety or depression (see Interview format). The model makes no assumptions about the content of any of its a priori domains; rather it assumes that there will be an interaction of physiological, cognitive, emotional, behavioural and social factors, which will be implicated, in some way, in the onset and maintenance of any given problem. It is the goal of the interview, then, to ascertain the unique interplay of factors maintaining the problem for each individual.
The patients were recruited from the North Tyneside Falls Prevention Service. These patients were seen as part of normal clinical practice by the multidisciplinary team of physicians, HCA and senior physiotherapist, during which a FES-I29 (see Appendix 1) was administered as routine. The clinical team identified potentially eligible patients on the basis of their age and FES-I scores (see Inclusion and exclusion criteria for patients).
Inclusion and exclusion criteria for patients
Consecutive patients aged ≥ 60 years of both sexes. Those with significant fear of falling, as defined by a FES-I score of > 23,40 were eligible for the study. Patients with cognitive impairment [Mini-Mental State Examination (MMSE) score of < 2441] were excluded from the study, as there is a paucity of data on the use of fear of falling measures in this patient group, and given the difficulties of conducting CBT interventions in those with significant cognitive impairment. Regionally, the population under study had a particularly low proportion of ethnic minorities. Therefore, given the level of communicative nuance needed for the development of a CBT intervention, we excluded those who did not speak English. To avoid participant burden, patients recruited to the CBTi development study were excluded from the Phase I process evaluation work (described later in this chapter) and vice versa.
Sample size
Fear of falling is one of a range of falls-related psychological concerns,42 with emotional and behavioural components including anxiety and activity avoidance, respectively. 43 In the CBTi development study we anticipated interviewing between 10 and 12 patients, although up to 30 were allowed for if data saturation was not reached with fewer numbers. Our rationale for this was that clinically significant anxiety (which fear of falling to be can be a manifestation of) tends to have a fairly narrow range of maintaining factors, which, most usually, are catastrophic beliefs and activity avoidance. The psychological construct of fear of falling as it appeared in the literature suggested that a similar range of maintaining factors was likely to be in operation in our patient group. We therefore expected that the number of patients needed to consistently identify such factors to be relatively small. In fact 15 patients were needed as the construct was more heterogeneous than we anticipated.
Identification, recruitment and consent of participants
The initial approach to patients for the CBTi development study was by a member of staff at the North Tyneside Falls Prevention Service. The staff member provided a brief oral explanation of the research to those patients who met age, FES-I score and cognitive impairment eligibility criteria. Those expressing a preliminary interest were given a Patient Information Sheet (PIS) and an expression of interest (EoI) form (with stamped addressed return envelope addressed to the University) to take home and discuss with family and carers as they felt appropriate. There was no further direct study-related contact with the patients unless they returned the EoI form indicating their willingness to participate in the study. On receipt of an expression of interest form, a telephone call was made by the interviewer (VD) to discuss participation. Questions about the process were actively sought, and if patients required further time for reflection or discussion with family or carers this was encouraged. If patients consented, the interviewer (VD) contacted them by phone and arranged an appointment for the interview. Written consent was obtained prior to the commencement of the interview. These were conducted in the patient’s preferred location, at either at the North Tyneside Falls Prevention Service or, in most cases (14/15 patients), at the patient’s home.
Interview format
The CBTi development interviews were undertaken by VD, a qualified cognitive–behavioural therapist and practitioner health psychologist. The main form of data collection was notes taken by the interviewer as the interview was being conducted, and notes and reflections made in collaboration with the interviewee. All sessions were also audio-recorded in case there should be any need to clarify points from the notes.
The format was a standard CBT assessment, as used by cognitive–behavioural therapists and clinical psychologists. It brings to light which factors are key in having caused the patient’s problem, and which factors are key in keeping the problem going. The structure is often referred to as the ‘three P’s’: what factors predisposed the patient to the problem (such as a history of anxiety); what factors precipitated the current problem (such as a fall or loss of physical capability) and what factors might be perpetuating the problem in terms of cognitions, behaviours, emotional, physical and social factors. Using this model as a template, we investigated the relevant factors in the cause and maintenance of the individuals’ fear of falling.
The interview began with a general open question about the main presenting problem, for example ‘tell me a bit about your concerns about balance and falls’. It then asked more detailed questions about how the problem was affected by different situations, times, people, places and events. Then more detail was sought on what could and could not be done as a result of the problem, and on what made the problem better or worse. Coping strategies and beliefs were then asked about, as well as any current treatments. Next the onset and development of the problem was explored, from what was happening in the interviewee’s life just before they started being concerned about falls, up to the present moment. More general questions were then posed about the interviewee’s current mood and mental state, and about the type of person they perceived themselves to be. This was followed by some questions about any past experiences of emotional or physical problems. Finally, a brief family and social history of family, spouse or partner, carers and social contacts was taken, as well as details of previous and current employment and relationship status as appropriate. The interview ended with the interviewer feeding back to the interviewee some of the information that might help make sense of their fear of falling problem, and with a general discussion of what the interviewee thought of as being the important factors in the cause and management of their problem.
Analysis
The cognitive–behavioural framework (of predisposing, precipitating and perpetuating factors) described above further distinguishes between physical, emotional, cognitive, behavioural and social factors in each domain. Finally, it looks for interactions between the perpetuating factors (such as how anxious thoughts may increase physiological arousal, and vice versa). As a first step of analysis, collected data were sorted into this framework, then their perpetuating factors were identified, and their possible interaction was discussed with participants to attempt an individualised formulation of their fear of falling. This analysis was discussed with them at the end of their interview, and their input was used to develop and refine it. As a last stage of analysis, the interviews were written up as case formulations for each interviewee in terms that attempted to model the onset and maintenance of their condition in terms of the cognitive–behavioural framework. Finally, comparisons were made between individual interviews and a tally of common factors and themes in different domains were made. Factors, and themes and patterns of interaction, were analysed to see if there were recurrent themes between interviewees with significant fear of falling.
Results: understanding fear of falling
The most striking initial finding was how heterogeneous fear of falling was, both in its development and in its maintaining factors. This is what necessitated running to 15 interviews. After this point it became clearer that there were three broad types of fear of falling into which the interviewed participants fell, namely primarily psychological factors, primarily physical factors and ‘the stoics’. To make this clear we will present the three categories with illustrative examples from participants.
Primarily psychological factors
About one-third of the interviewees conformed to the prevailing picture of fear of falling in the medical literature whereby fear, often but not always occasioned by a fall, is maintained by avoidance of activity, leading to loss of confidence, physical weakening and more fear of falling. However even in these cases, the picture was more complicated than this. Interviewee 1, who we will call Amelia, was the closest to the picture that we see in the literature. Her problem had begun 4 years prior to the interview when she had tripped and fallen on her face. Although nothing was broken, she vividly recalled the sense of being out of control and the ‘concrete coming up to meet my face’ (all quotes are directly from participants). Subsequent to this she had become very cautious about walking outside. She altered her walking in that it became much slower and she would almost continually look down. If possible she would ‘link up’, i.e. link arms, with a family member or a friend and increasingly took to either not going out on her own or sticking to areas that she knew well. To refer back to the CBT framework, these behaviours were reinforced and maintained by cognitions. She had come to perceive walking as dangerous, she had the image of the previous fall often in her mind when walking, and she was very aware of the fact that she could no longer ‘stride out’ as she used to (it was Amelia’s story that inspired the name of the intervention). These cognitions and behaviours in turn affected her mood. She was generally anxious when walking, and her mood was low (though she was not depressed) because her life contained much less enjoyment than previously. Her inability to ‘stride out’ and her lack of independence in turn generated a fair amount of frustration about her loss of ability. Physically, she reported being largely OK, but more tired than previously, and specifically mentioned that this type of walking (cautious and slow) was more tiring than how she used to walk, and also much less enjoyable.
As such it was clear from this interview that in Amelia’s case, there was a traumatic precipitating event, although with no obvious predisposing factors. This was the case with most participants, where there was no past history of anxiety disorders. Furthermore, there was a clear interaction of physical, affective, cognitive and behavioural factors that were further reinforced by social isolation (she lived on her own). In CBT literature, the interaction of factors is often presented to the client in the form of a ‘hot cross bun’:44 Amelia’s hot cross bun is presented in Figure 1.
FIGURE 1.
Amelia’s hot cross bun.
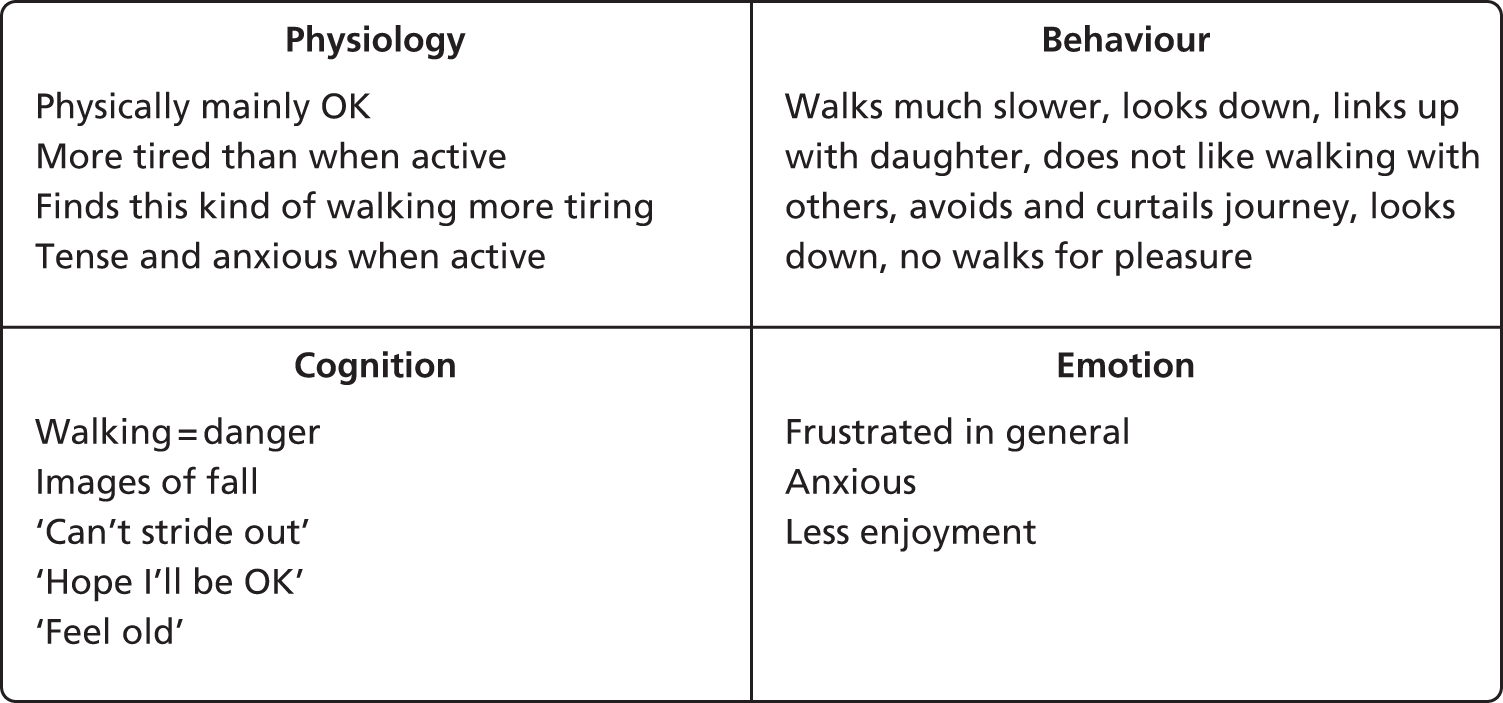
Thus, it was clear that Amelia’s fear of falling was maintained by safety and avoidance behaviours, and by anxiogenic and catastrophic cognitions. As such, the obvious intervention would be to target these cognitions and behaviours with the appropriate evidence-based techniques.
However, she was one of the few participants who so neatly fitted this fear/avoidance model. Even when the factors maintaining the inactivity and avoidance were psychological, they were not always primarily to do with anxiety about falling. For instance, one participant was also largely physically capable, although he benefited from walking with a stick. However, he led a largely sedentary life because he was very self-conscious about being perceived as ‘old’. Although self-deprecatingly dismissing his own concerns as ‘vanity’, he nevertheless was clear that the main barrier to him going outside was that he felt very overlooked by his neighbours, and did not want to be seen as the old man with a stick. He was further restricted by social isolation and lack of companionship, and for him the main thing he thought would help would be someone to walk with. Another participant reported that since losing her best friend 2 years prior to the interview, she no longer saw the point in going out. The interview uncovered an interplay of ongoing grief and low mood, which were the main impediments to her engagement with the outside world.
Primarily physical factors
For another proportion of the interviewees, the primary impediments to walking were physical. For instance, one participant, whom we will call Fred, had multiple physical problems. He was overweight, had angina and breathlessness, a frozen right shoulder and a ‘shattered’ left shoulder (from a previous fall). As a result of pain and arthritis in his knees and ankles, he could walk only with sticks, and then with considerable pain. All of these physical ailments had left him severely limited in his physical capacity. He felt very vulnerable when out walking, as he was aware of being only one fall away from being bedbound or hospitalised. Although this latter anxiety was certainly a barrier to activity, he perceived his principal barriers to be his breathlessness, pain and fatigue. He also reported what emerged as a recurring fear among the cohort – that hospitalisation was a ‘one-way ticket to death’ – ‘once you go in you don’t get out’. Although he worked hard to maintain a social life, his activity was haunted by this fear of loss of independence and ultimate death. His ‘hot cross bun’ is presented in Figure 2.
FIGURE 2.
Fred’s hot cross bun.
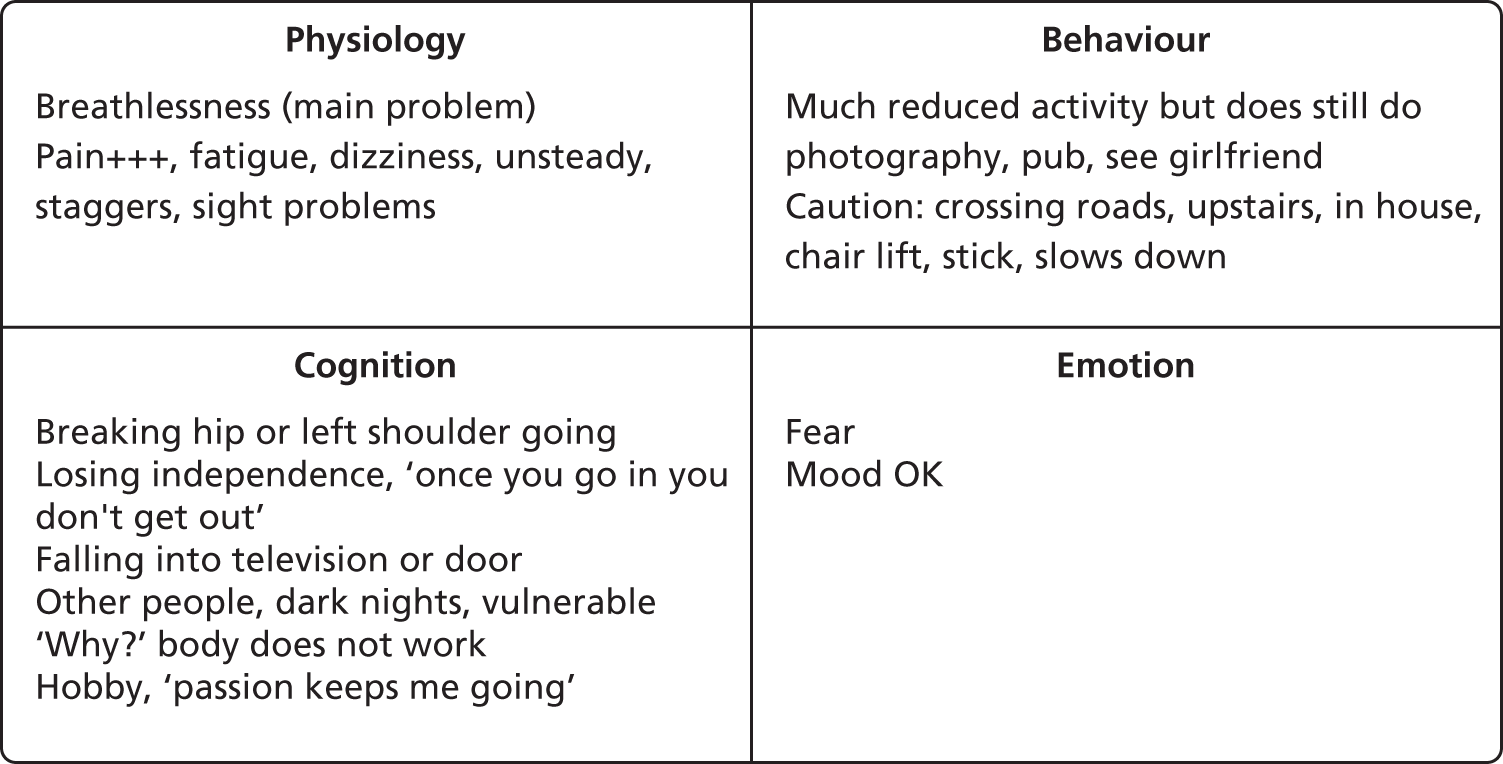
About one-third of the cohort reported similar issues, with pain and fatigue, as a result of other physical problems, being the most commonly reported barriers to activity, rather than primarily fear.
‘The stoics’
The third group were a surprising finding. These were participants who had considerable psychological and/or physical impediments to activity but who, nevertheless, continued to be active. We perceived an element of this in Fred above. Despite considerable physical problems and very real fears, he continued to pursue his hobby of photography, and he saw this as a ‘passion that keeps me going’. Another more marked example comes from a participant whom we will call Violet. She was the oldest, and perhaps the most physically frail of the people interviewed, and had a very unsteady gait. She also highlighted the heterogeneity of fear-of-falling precipitants. She had become aware of her unsteadiness only when her husband of 60 years had died, and she was no longer able to walk arm in arm with him. Now that he was gone she became aware that she would ‘suddenly list’ and ‘stagger unpredictably’. She had also seen her husband decline very rapidly after hospital admission and die there. Like Fred, she had the fear that admission to hospital was a ‘one-way ticket to loss of independence and ultimate death’. Despite both these physical and psychological ‘inducements’ to inactivity, Violet was the most active participant interviewed (Figure 3). Indeed she was difficult to pin down for interview as she was on (solo) holidays to Spain, Italy and the Netherlands during the study period.
FIGURE 3.
Violet’s hot cross bun.
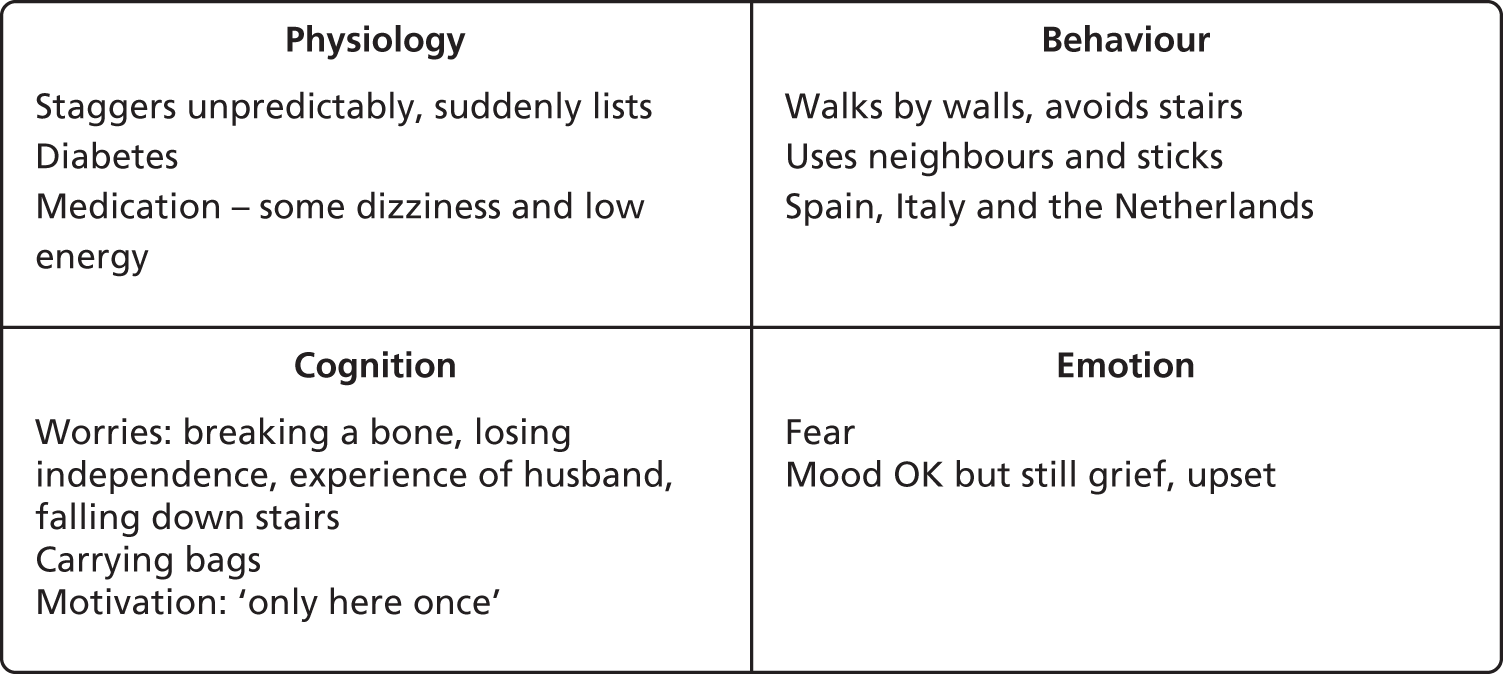
Violet’s experience was not unique. Several other participants reported ‘keeping going’ despite obvious reasons to stop. This provided a new avenue of enquiry for these interviews: ‘What keeps you going?’. For each person it was different. For Violet it was the belief that ‘you are only here once’. She did not believe in God or an afterlife, and so was committed to get the most out of her life while she still could. Seeing her husband decline, although still a source of grief for her, was a further motivation to make the most of the time she had left. Another participant also struggled with both illness and anxiety, but kept going because she was committed to looking after her sisters, both of whom were housebound. She also had a dog to which she was committed to walking daily. In these cases it was clear that there was something that mattered more, a value that superseded the urge to avoid physical or psychological distress, and allowed the latter to be tolerated. We encouraged the interviewees to reflect on this, with the express purpose of helping us to help others with our intervention.
Shaping the intervention and training for the health-care assistants
One of the main conclusions that we drew from these interviews was that fear of falling was a heterogeneous condition and one for which a ‘one-size-fits-all’ intervention would not be appropriate. It also emerged that FES-I scores were, at best, a crude measure of the phenomenology of fear of falling uncovered in the interviews. The interviewer, the co-trainer and intervention supervisor (NSa, a clinical psychologist) and other members of the team (principally SWP and CB) discussed how we could turn these findings into (1) a replicable intervention and (2) a training programme for HCAs. The consensus was that, rather than focus exclusively on behaviour change techniques, we needed to equip the HCAs to arrive at the kind of individualised problem formulations that had emerged from the interviews. These formulations would form the basis of interventions by allowing the HCAs to assess the interplay of factors that were maintaining the fear of falling/activity avoidance in each individual. Only then would the deployment of behaviour change techniques be undertaken, in a tailored fashion. As to the latter, it was clear that there were some key intervention targets, which were fear and avoidance of activity; pain; fatigue; reduced activity; loss of confidence; catastrophic cognitions; grief; social isolation; and shame/self-image. Fear, pain and fatigue were the most commonly reported issues and so it became clear that the HCAs needed clear training for these, and some basic grounding in the evidence-based techniques for tackling the other recurrent issues.
From this discussion, we put together a ‘menu-based’ treatment protocol and designed the training accordingly. We also produced supporting training and client manuals (available on request from the corresponding author of this report, SWP). Treatment was menu based in the sense that once the assessment had been done, and the intervention targets agreed with clients, here would be a range of prescribed interventions to draw on for each of these targets, individualised to the specific client. We then put together the training programme. A detailed description of the intervention using the Template for Intervention Description and Replication (TIDieR) framework45 is provided in Chapter 3 (see Table 6).
Duration of health-care assistant training
The training was delivered by VD and NSa over a period of 5 days in a classroom at the University of Northumbria.
Training content
The training gave an overview of CBT and then looked in more detail at the three P’s (predisposing, precipitating and perpetuating) model. Central to the HCAs’ training were the skills of engagement with the client, and assessment and formulation development. Standard CBT interventions for identified problems were described and practised: graded exposure for anxiety; activity monitoring, graded activity and behavioural activation for pain, fatigue and low mood; and sleep management for fatigue. For other recurrent issues HCAs were taught specific cognitive techniques, such as identifying thoughts; making explicit the links between thoughts, feelings and behaviour; thought diaries; thought challenging; and cost–benefit analysis for catastrophic and otherwise unhelpful cognitions. Furthermore, the HCAs were taught how to structure assessment and treatment sessions by working from formulation through to treatment plan in the first one to two sessions, then to specific treatment strategies in the middle three to four sessions, and then focusing on consolidation and discharge planning in the final sessions. An eight-session format was agreed as being most suitable in terms of intervention replicability, with the aim of weekly delivery.
The treatment plan was guided by end of treatment targets, which were worked out in collaboration with the clients, meaning that each client had specific and measurable goals that they were working towards throughout treatment. These individualised and personally meaningful goals aimed to capture that element of ‘valued activity’ that we found had kept the ‘stoic’ participants going, despite physical and/or psychological distress. The weekly implementation of treatment plans was carried out using specified interventions for specified targets in the form of client homework. Subsequent sessions were structured by homework reviews and problem-solving any additional barriers that had been identified before agreeing further target-setting for the next session. Finally, treatment review, skills embedding, and relapse prevention and preparation for discharge shaped the final sessions (Table 1).
| Session | Content |
|---|---|
| 1 | Assessment and formulation |
| 2 | Goals and target-setting |
| 3, 4, 6 | Continuation |
| 5 | Review |
| 7 | Relapse prevention |
| 8 | Final review |
| 6-month follow-up | Review and recap, goals, setbacks, outcomes |
Training delivery methods
A mixture of chalk and talk, observation, modelling and practice of the above techniques by the HCAs, as individuals and in pairs, formed the bulk of the training sessions. A strong emphasis was placed on ‘real play’, through which the HCAs were encouraged to use their own anxieties and experiences to work on in assessment, formulation and treatment. This was done with consent, and participants agreed that it helped to bring the techniques to life. Real fear of falling cases, derived from the interviews outlined above, suitably anonymised, were used to present the range of issues involved. The content of the training was summarised in both therapist and participant manuals (available from SWP) and the HCAs were encouraged to develop their own assessment and treatment props, which they did (see Appendix 2).
Post-training supervision
In the months following the above training the therapists engaged in both individual and group supervision. The former was of 1 hour every fortnight with NSa within which the focus was on skills development and consistency of intervention delivery. Individual client session audio tapes were shared and scrutinised with the supervisor. Discussion about internal and external challenges to the process of the study were also explored, as were individual issues that were pertinent. Group supervision was led by VD on a bimonthly basis, and focused on cases brought by the therapists for sharing and exploration of practice and interventions.
Acceptability and feasibility of the cognitive–behavioural therapy intervention
The previous section focused on the development of the CBTi and associated training materials; in this section we report the methods and results of Phase I of the process evaluation, which sought to understand the feasibility and acceptability of the CBTi from the perspectives of the different stakeholder groups involved (patients, clinicians and CBTi developers). (Phase II of the process evaluation is reported in Chapter 5, Process evaluation.)
Methods for process evaluation
Interviews with patients and carers
A sample of 14 patients (aged 60–85 years, nine female) and two family members (both female, age not specified) were interviewed. In contrast with the intervention development interviews, these explored patient and family members’ perceptions about the proposed CBTi, practical aspects of the CBTi (including how to describe the intervention, proposed delivery) and perceived relevance of the CBTi to their situation. Patients were recruited from the North Tyneside Falls Prevention Service to reflect inclusion criteria for the RCT, using maximum variation sampling (for variety in age, gender and FES-I score). When appropriate, family members of these patients were invited to take part. Participants were interviewed in their own homes.
Interviews with professionals
The six professionals (three geriatricians, two physiotherapists and one HCA) who constituted the multidisciplinary team at the North Tyneside Falls Prevention Service were interviewed. VD and NSa (hereafter psychologists) were also formally interviewed using a semistructured interview schedule (three interviews). These interviews explored perceptions of key factors that might impede or facilitate the proposed CBTi being effective and workable in practice.
Observation
Clinical sessions at the North Tyneside Falls Prevention Service (n = 5) were observed to gain an understanding of the clinical context of the study. Professionals sought verbal consent from patients and any companions for the observation. No audio or audiovisual recordings were undertaken of these observations.
The regular meetings of the CBTi development team (VD and NSa) were audio recorded and observed by CB (n = 9 meetings). These data were supplemented with field notes that were taken of informal discussions (n = 8) with VD outside these meetings. Other related stakeholder engagement activities, including a briefing meeting for clinic staff, were also observed.
Data analysis
All qualitative data were analysed thematically using the constant-comparative technique. 46 This process allowed for the meaning of the data, and themes represented within them to emerge freely without the constraints that might be imposed on the data if coding to a prespecified coding frame. Themes were identified individually by CB and TF, who developed initial ideas and undertook coding independently, and then came together to discuss and compare ideas. We systematically worked through different data sources (professional interviews, patient interviews, intervention development meetings, interviews and informal discussions with the intervention development researchers, and field notes) identifying themes separately for each type of data prior to producing an overarching coding frame. The team members responsible for intervention development reviewed and commented on the coding frame thus providing some respondent validation. There were no formal opportunities for respondent validation by patients and carers; however, the identification of similar themes in the intervention development interviews conducted by VD, and those conducted as part of the process evaluation, provided some evidence of trustworthiness.
The process evaluation component of the study was designed not merely to evaluate the development of the CBTi, but also to collect data to inform and optimise the CBTi for delivery within the RCT. This was achieved through iterative data collection and feedback loops (that included formal and informal project-related meetings, and face-to-face and e-mail communications with the psychologists and the wider project team) to enable practical resolution of problems as they arose. The impacts of the process evaluation activities on the development and delivery of the CBTi are highlighted below.
Data reported here are attributed using the unique identifier allocated to the participant. Prefixes are as follows: ‘P’ denotes professionals; ‘C’ denotes patients interviewed for the process evaluation; ‘F’ denotes family members (caregivers); and ‘R’ denotes study team members.
Results: acceptability and feasibility
The first section of the results explores how the intervention developers adapted the proposed CBTi to fit with their emerging understandings of fear of falling and achieved an intervention which, from their perspectives, made sense. The second section highlights the concerns expressed by a range of stakeholders over the acceptability and feasibility of the proposed CBTi and RCT, in particular the ‘fit’ of trial structures and processes with older people; the staff who would be delivering the intervention; and the service in which patients were initially to be recruited. We examine the work undertaken by the research team to improve the coherence of the trial to stakeholders.
Based on their initial views that fear of falling could be conceptualised as anxiety based and avoidant, the psychologists had anticipated developing a simple, linear, manualised intervention centred on graded exposure. The initial clinical interviews conducted by VD (described in the initial section of this chapter) revealed that fear of falling was a complex and multidimensional phenomenon with diverse precipitating, perpetuating and predisposing factors. These observations created uncertainty about the feasibility of developing a CBTi that is suitable for all patients:
. . . it may be that there are certain groups within that, those complex dimensions that you’ve already described, that actually this may not be an appropriate intervention with all [. . .] groups [. . .]
R12, intervention development meeting, 22 November 2011
I’d be struggling to know what to do with them, to be honest. You can imagine a little change, but you’re up against the real, as it were.
R4, intervention development meeting, 22 November 2011
Broadening the focus of the CBTi to include work on pain, fatigue and other issues raised issues over the scope of the intervention and fit with the protocol:
So I think there’s a boundary issue about are we doing CBT for fear of falling.
The boundary issue is key.
Or are we doing CBT for fear of falling and actual (R4: and pain and . . .)
[. . .]
. . . in a sense you’ll be working with them with whatever they bring, I mean it might be that you know the pain is a major factor in them not going out or whatever so I think that’s a legitimate piece of work.
Intervention development meeting, 15 February 2012
The decision to focus on individualised formulations also created concerns over the potential mismatch between the complexity of the client group, the wide range of skills required to deliver the intervention (Box 1) and the allotted time frame:
Because you’ve identified very challenging, difficult clients [. . .] in terms of actual CBT, how do we get those relatively inexperienced therapists to actually be able to collect this information and actually then work – develop a relationship – and then work with these people over six sessions or however long it’s going to be. Because it’s such a small period of time.
R12, intervention development meeting, 22 November 2011
At the same time there was an emphasis on being realistic and focusing on the aim of the project:
We’re not training psychological therapists; we’re training some healthcare assistants to deliver a very, very short intervention. So we’ve got to be clear about what’s possible as well.
R12, intervention development meeting, 15 March 2012
Concerns were also raised about the potential generalisability of the intervention. There was an awareness that, in light of the careful selection, training and supervision of HCAs to meet the demands of this complex CBTi, the findings may not be replicable elsewhere:
All of which does raise some questions about generalisability, if we were recruiting the ones who are a bit savvy and we’re giving them a lot of training [. . .] that doesn’t necessarily translate into your ‘Joe Bloggs care assistant’ who is already working in a very busy . . . but we’ll see, I think we have to prove it’s doable first.
R4, intervention development meeting, 13 September 2011
-
Ability to develop a therapeutic relationship.
-
Formulation skills.
-
Managing uncertainty during the process of learning CBT skills.
-
Willingness to practise new skills under scrutiny.
-
Ability to contain potentially distressing emotions.
-
Knowing own limitations and ability to judge when referral to more experience professionals is required.
Achieving coherence in randomised controlled trial structures and processes
The process evaluation interviews that were conducted with patients and clinic staff indicated a number of other issues that needed to be addressed to maximise the feasibility and acceptability of the RCT in which the CBTi was to be evaluated. These issues were iteratively fed back by staff responsible for the process evaluation (CB and TF) to key members of the project team to allow the development and implementation of strategies to address them prior to the start of the RCT.
Perceived acceptability of cognitive–behavioural therapy intervention to older people
A key issue raised by all stakeholders was the extent to which CBT would be acceptable to older people, as illustrated by the following comments:
I imagine that some stoical North Easterners might think that it was wishy washy mumbo jumbo that wasn’t necessarily going to help, you know the idea of going and sort of seeing like, effectively having a sort of a counselling type sessions, some people might not take that seriously.
P2, interview, 6 December 2011
So I think although she would agree to any kind of help I think there’s a part of her that possibly feels she doesn’t need it, and I think it’s getting over that hurdle.
F2, interview, 9 March 2012
A key issue concerned the name of the CBTi. Patients and clinic staff indicated that the use of terms such as ‘psychological’ and ‘psychotherapy’ were potentially alienating to the intended client group:
what does psychotherapy treatment mean to you?
Sitting on a couch with a shrink!
And would you then sign up for that do you think?
I don’t know, I don’t know whether that would help me with the problems I’ve got.
C2, interview, 30 January 2012
All stakeholder groups participating in qualitative interviews were asked for advice and suggestions. It was seen as essential to ensure that the title was positive: avoid the word ‘falling’ (as well as any words beginning with ‘psych’) and to consider including terms such as ‘confidence’, to which people would be able to relate. In addition to being non-threatening, we wanted the name to convey a sense of the purpose and focus of the intervention so that it would not be mistaken for an exercise class. Following discussions, we agreed on STRIDE as the brief study title (Strategies for increasing independence, confidence and energy).
Although there were clear reservations about the concept and name of the CBTi, stakeholders were generally positive about the practical aspects of the CBTi, for example the number, frequency and duration of sessions, the timing of the follow-up session, and the intention to deliver the intervention at home. Patients emphasised the need for flexibility and for the sessions to fit around their other commitments. Several patients suggested group sessions, either instead of, or alongside, individual sessions, and although the likelihood of individual preferences was noted it was agreed that this could not be accommodated in the RCT.
Perceived value of cognitive–behavioural therapy intervention for fear of falling
Clinic staff expressed some reservations about the proposed CBTi for fear of falling, in particular the possibility that, for some patients, fear of falling may actually be protective with regard to falling:
I don’t know whether it’s protective or not which would be my other question, is it a good thing people have a fear of falling, if you take that fear of falling away are they going to fall because their sense of caution has gone?
P3, interview, 7 December 2011
Clinic staff also expressed reservations over patients’ willingness to engage with an intervention that focused primarily on fear of falling:
I’m not sure how receptive they will be to addressing fear of falling as a primary factor in the absence of anything else, any modifications to gait or aids or physical performance, so I don’t know how often it’s a stand-alone problem.
P3, interview, 7 December 2011
As the North Tyneside Falls Prevention Service focused on identifying underlying medical causes for falls, it was not surprising that staff were uncertain about the value of psychological interventions such as CBT. In light of their key role in ‘selling’ the CBTi to eligible patients in Phase I of the study, we organised a briefing meeting for clinic staff in which the intervention developers fed back the results of the interviews, described the intervention and illustrated how it would be relevant to different types of clients. This enabled staff to recognise the similarities between the CBTi and their existing practice:
I mean I’ve used it a lot with working with older people and it’s not actually called CBT but it’s what you do with them from the point of view you’ve gradually got to build their confidence up through very simple increasing their activities and things and the way you talk to them and the way you encourage them and things like that as well, so yes I think it would be very, very helpful actually.
P5, briefing meeting for clinic staff, 17 February 2012
During this briefing meeting, we were able to provide reassurance about the venue of the treatment (usually in patients’ own homes) and also to address other concerns, for example mechanisms for informing patients’ general practitioners (GPs) about their participation.
Recruitment of patients to a randomised controlled trial
Observation of the clinical setting in which the RCT was to take place revealed that the service specialised in fast paced, routinised, structured assessments. Potential barriers to patient recruitment were time pressures and the lack of any routine discussion of fear of falling within the service. To facilitate recruitment in these difficult circumstances, we produced a user-friendly leaflet and a ‘script’ that staff could follow. This was designed to have minimal time implications and to allow staff to follow their existing approach to referring patients for strength and balance classes provided by a local voluntary organisation.
Relevance and acceptability of the primary outcome measure
A final issue that emerged from discussions with clinic staff related to the adequacy of the FES-I as a means of identifying potential participants and the primary outcome measure despite clear published evidence of its validity. 29 These concerns over the outcome measure undermined the perceived validity of the proposed RCT. A key concern was the perceived lack of correlation between apparent fear of falling and FES-I scores:
She’s an agoraphobic and was very, very frightened of falling [. . .] she won’t go to the hairdresser and that sort of thing, but because the FES was so low from that point of view then obviously she wouldn’t fit your criteria then would she?
P5, briefing meeting for clinic staff, 17 February 2012
Staff felt that responses on the FES-I often reflected functional limitations (e.g. arthritis or other pain) that affect mobility rather than fear of falling. Given the extent of clinic staff concerns over the use of the FES-I as the primary outcome measure, we added a visual analogue scale, on which patients could rate their fear of falling, to be included alongside other measures used in the RCT.
Chapter 3 Phase II: randomised controlled trial methods
Objectives of the randomised controlled trial
Primary objective
To determine the effectiveness of a new cognitive–behavioural therapy-based intervention (CBTi) plus usual care compared with usual care alone (the control condition) in reducing fear of falling in community-dwelling older adults attending falls services.
Secondary objectives
To:
-
measure the impact of the intervention compared with the control on fall and injury rates in the trial participants, and its impact on functional abilities
-
measure the effectiveness of the intervention compared with control on anxiety and depression, quality of life, loneliness, social isolation and social participation
-
investigate the acceptability of the intervention for patients, family members and professionals
-
further investigate the professional and organisational factors that promote or inhibit the implementation and integration of the intervention
-
measure the costs and outcomes of the intervention in this setting.
Objectives (c) and (d) relate to the process evaluation, the methods and findings of which are described in Chapter 5; objective (e) relates to the health-economic evaluation, the methods and findings of which are described in Chapter 6.
Summary/overview of trial design
Two-arm, parallel-group patient RCT of a novel CBTi plus usual multidisciplinary care compared with usual multidisciplinary care alone in older patients with significant fear of falling and who are attending multidisciplinary falls services. Patients were randomised in a 1 : 1 ratio.
Trial registration and protocol availability
The trial was registered as ISRCTN78396615 on 17 May 2012. The latest version of the full protocol is available at www.nets.nihr.ac.uk/projects/hta/097004 and a published version is also available. 47 Table 2 summarises key changes to the original STRIDE trial protocol as approved by the research ethics committee (REC).
| Description | Version | Date |
|---|---|---|
| Update to recruitment procedures; administrative updates | 2.0 | 15 March 2012 |
| Update to prospective recruitment of participants to the RCT | 3.0 | 27 November 2012 |
| Revision of the sample size Update of the protocol to incorporate additional recruitment sites |
4.0 | 5 April 2013 |
| Staff member update Update of study duration Update of procedures for follow-up of non-returned self-completion questionnaires and completion of questionnaires via telephone |
5.0 | 6 March 2014 |
Ethics and governance
The Newcastle upon Tyne Hospitals NHS Foundation Trust was the sponsor for the trial. Favourable ethical opinion for Phase II (the RCT) was obtained on 16 February 2012 from the Newcastle and North Tyneside 1 REC (REC reference 12/NE/0006) and subsequent Research & Development and Caldicott approvals were granted by each participating site. Approval was sought and obtained for all substantive protocol amendments.
Trial setting
This trial was conducted in three community-based Falls Services in the North East of England: North Tyneside Falls Prevention Service, Newcastle upon Tyne Hospitals NHS Foundation Trust; Galleries Day Unit, Washington, South Tyneside NHS Foundation Trust; and Falls and Syncope Service, Newcastle upon Tyne Hospitals NHS Foundation Trust. The trial was originally designed as a single-site study at North Tyneside Falls Prevention Service but, as a result of recruitment problems, the other sites were subsequently added (June 2013 for Galleries Day Unit; May 2013 for the Falls and Syncope Service).
The North Tyneside Falls Prevention Service was a community falls prevention service focusing on early identification of patients at risk of falls. The Galleries Day Unit was a nurse-led community falls service providing assessment and rehabilitation. The Falls and Syncope Service was a specialist centre serving both local and national communities. Key characteristics of each site are summarised in Table 3.
| Service specifications and characteristics | North Tyneside Falls Prevention Service | Galleries Day Unit | Falls and Syncope Service |
|---|---|---|---|
| Year established | 2009 | 2007 | 1991 |
| Aim | To investigate, diagnose and help manage syncope (blackouts/loss of consciousness) and falls | To provide assessment and rehabilitation to patients who are either known fallers or are at risk of falling | To investigate, diagnose and help manage syncope and falls |
| Staff | HCA; physiotherapist; consultant physician | HCA; physiotherapist; nurse | Nurse; physiotherapist; consultant physician |
| Age group, years | 60+ | 60+ | 16+ |
| Catchment area from which patients drawn | All GP practices within a geographically defined area | All GP practices within a geographically defined area | Primarily local (Newcastle, North Tyneside and Northumbria), but with regional and national referrals |
| Method of access of patients to service | Screening questionnaire administered by GP practice to patients aged 60+ years to identify patients at risk of falling, referrals from GPs | Health or social care professional (including the ambulance service) | Referrals from GPs, ambulance service, A&E department; tertiary referral centre |
| Patients’ living arrangements | Community dwelling | Any | Any |
| Premises | Shared rooms within a health centre | Dedicated rooms within a health centre | Dedicated clinic in a hospital |
| Availability | 2.5 days per week | 5 days per week | 5 days per week |
| Duration of initial appointment (minutes) | 90 | 120 | 90 |
| Typical number of visits | 1 | 8–12 for patients on rehabilitation programme | 3–4 |
| Documentation of care | Electronic care pathway only | Loose-leaf sheets | Care pathway booklet (with separate booklet for physiotherapist documentation) |
The routine (i.e. for patients outwith the STRIDE trial) patient assessment process is summarised in Table 4, using the National Institute for Health and Care Excellence (NICE) guidance on possible content of multifactorial falls assessment. 48 As can be seen there were marked variations between sites.
| Clinical assessments | North Tyneside Falls Prevention Service | Galleries Day Unit | Falls and Syncope Service |
|---|---|---|---|
| Identification of falls history | |||
|
Y | Y | Y |
|
Y | Y | Y |
|
Y | Y | Y |
|
Y | Y | Y |
|
Y | Y | Y |
|
Y | Y | Y |
| Assessment of gait, balance and mobility and muscle weakness | |||
|
PRN | Y | PRN |
|
Y | Y | Y |
|
Y | Y | Y |
|
Y | Y | PRN (physio) |
|
Y | Y | Y |
|
Y | Y | Y |
|
Y | Y | PRN (physio) |
|
Y | Y | Y |
|
Y | Y | Y |
| Assessment of osteoporosis risk | FRAX | FRAX | FRAX |
| Assessment of perceived functional ability and fear relating to falling | |||
|
Y | Y | Y |
|
PRN | Y | PRN |
|
Y | Y | PRN (physio) |
|
Y | Y | PRN (physio) |
|
N | Y | N |
| Assessment of visual impairment | |||
|
Y | Y | Y |
| Assessment of cognitive impairment and neurological examination | |||
|
Y | PRN | PRN |
|
Y | Y | Y |
|
PRN | PRN | PRN |
|
N | N | PRN |
| Assessment of urinary incontinence | PRN | Y | PRN |
| Assessment of home hazards | PRN | Y/PRN | PRN |
| Cardiovascular examination and medication review | |||
|
Y | Y | Y |
|
Y | Y | Y |
|
Y | Y | Y |
|
Y | Y | Y |
|
N | N | PRN |
|
N | N | PRN |
| Other | |||
|
Y | Y | Y |
|
Y | Y | Y |
|
Y | Y | Y |
|
PRN | PRN | PRN |
|
Y | N | N |
|
Via GP | PRN | PRN |
|
N | PRN | Y |
|
PRN | PRN | PRN |
A key characteristic of the North Tyneside Falls Prevention Service was that all recording was carried out electronically during consultations; as each professional completed their assessment with an individual patient, the colour of the patient’s electronic record changed so that their colleagues could see that the assessment was completed and could access the notes. The consultant saw patients last, and, following detailed explanation of findings, printed out a summary of the assessment at the end of the consultation for patients to take home with them; a hard copy was also sent to the patient’s GP.
A key difference between the Falls and Syncope Service and the other two sites was that patients were not routinely assessed by the physiotherapist; this reflects the more varied patient group seen in the Falls and Syncope Service, a significant proportion of whom were younger patients who were referred for dizziness and blackouts rather than falls. The physiotherapist within this service therefore operated like an outpatient clinic, with patients being referred for assessment when required. Patients who were referred to the physiotherapist had an initial assessment of 60 minutes, with review appointments of 30 minutes. In contrast with the physiotherapy assessments in the other two sites, the dynamic gait index was performed, for which patients are asked to walk at different speeds, turn their head horizontally and vertically when walking, turn and stop, and to negotiate obstacles while walking. Other aspects of the physiotherapy assessment were similar to those observed in the North Tyneside Falls Prevention Service and Galleries Day Unit. The Falls and Syncope Service also performed the Epley manoeuvre for patients with benign positional paroxysmal vertigo (BPPV), whereas patients attending the North Tyneside Falls Prevention Service and Galleries Day Unit had to be referred to Ear, Nose and Throat services for this treatment.
The care pathways and interventions offered by the different sites post initial assessment also varied (Table 5). In the North Tyneside Falls Prevention Service, following assessment, patients were referred:
-
back to their GP for medication or other review
-
to secondary care, day hospital, community physiotherapy and occupational therapy as appropriate
-
for bone densitometry at the local hospital, and/or
-
to targeted strength and balance training classes run in conjunction with the voluntary sector (Age UK) in North Tyneside.
Patients were also signposted patients to local exercise classes, such as t’ai chi run by Age UK. In the Galleries Day Unit, the main outcome of initial assessment was referral to the in-house rehabilitation programme, although other options were available including home exercises or in-house t’ai chi and referral to the lead consultant geriatrician and falls specialist at Sunderland Royal Hospital for ongoing investigation. Arrangements for follow-up and management, post initial assessment, were more fluid in the Falls and Syncope Service; this related to the range of investigations available within the service (e.g. carotid sinus massage, tilt table), as well as the options for physiotherapy intervention (within the service, community physiotherapy, day hospital, community strength and balance training via the Staying Steady project, etc.).
| Intervention | North Tyneside Falls Prevention Service | Galleries Day Unit | Falls and Syncope Service |
|---|---|---|---|
| Referral to community exercise | Strength and balance classes | Yes (Age UK classes or gym membership) | Staying Steady intervention |
| In-house physiotherapy | No | No | Yes (typically prescribed home exercises and reviewed four to five times) |
| In-house rehabilitation programme | No | Yes (typically 11–12 sessions) | No |
| In-house exercise/t’ai chi | No | Yes | No |
| Home exercises | Yes (sheets provided) | Yes (sheets provided and HCA could visit patients at home to provide 1 : 1 exercises where appropriate) | If referred to in-house or community physiotherapist, home exercises might be provided PRN (sheets provided) |
The in-house rehabilitation programme provided at the Galleries Day Unit comprised six stages, although most patients started at stage 4. Rehabilitation sessions typically lasted 15–20 minutes. The patients were reviewed by the physiotherapist typically before moving on to stage six when a decision was made whether or not to continue, repeat some of the earlier sessions or discharge the patient.
In the Falls and Syncope Service, patients requiring physiotherapy intervention were often given an individual exercise programme to follow at home and reviewed after about 6 weeks. Patients who were frailer and required more input tended to be referred to a day hospital where more support and dedicated transport were available. Many of the patients requiring intervention began with home exercises and were later referred to Staying Steady – evidence-based community exercise groups which ran over 36 weeks, alternating periods of exercise classes and home exercises. However, access to this intervention varied according to where patients lived and there were significant waiting lists in some areas of the city. In the North Tyneside Falls Prevention Service, approximately 25% of patients were referred to strength and balance training classes run in conjunction with Age UK, with tailored exercise programmes for up to 10 individuals provided weekly for 10 weeks, following which participants were encouraged to attend follow-on exercise classes.
Study participants
Participants were community-dwelling older patients aged ≥ 60 years, of both sexes, attending one of the above falls services with excessive or undue fear of falling as assessed by the FES-I29 score of > 23. The cut-off score of > 23 was informed by a comprehensive longitudinal study of the clinical and psychometric properties of the FES-I in 500 community-dwelling elders, which suggested this threshold as indicating high levels of concern about falls. 40
As described below, patients provided written informed consent for participation in the study prior to any study-specific procedures.
Inclusion criteria
-
FES-I score of > 23.
-
Aged ≥ 60 years.
Exclusion criteria
-
Cognitive impairment [Mini Mental State Examination (MMSE) score of < 24/30]. 41
-
Life expectancy < 1 year or unlikely for any other reason to be unable to complete 1-year follow-up
-
Requiring psychosocial interventions that are unrelated to fear of falling.
-
Current involvement in other investigational studies or trials, or involvement within 30 days prior to study entry.
-
Patients who had taken part in Phase I (i.e. qualitative work to develop the intervention) of the study. A small number of these patients acted as ‘pilot clients’ and received the intervention (as part of the training of the HCAs) but did not complete any of the outcome measures.
During the course of the study, it became apparent that some couples both fulfilled study eligibility criteria. To avoid contamination, it was decided that only one member of each household should be randomised. Therefore, if a spouse or partner of an otherwise eligible individual was already participating in the study, the second individual to be identified was excluded at the second stage of screening by research assistants (RAs) (see below).
Interventions
The control group received usual care for the falls services in which they were recruited, as described above. The intervention group received usual care as detailed above plus a CBTi. The rationale for this choice of intervention and the theoretical basis for it are described in greater detail in Chapter 2 (see Phase I: intervention development) and intervention materials (therapist and patient manuals) can be obtained from the corresponding author of this report (SWP). The CBTi was developed by our team in Phase I of this research; the development process is described in Chapter 2 (see Phase I: Intervention development). It was delivered face to face, on a one-to-one basis, by one of three trained HCAs, at participants’ homes, the North Tyneside Falls Prevention Service or community centres or facilities (e.g. Age UK offices in North Shields), Newcastle University or the Royal Victoria Infirmary, Newcastle upon Tyne (RVI), as per patient choice. HCAs were trained in basic CBT assessment, formulation and treatment skills by a qualified cognitive–behavioural therapist and practitioner health psychologist (VD) and a clinical psychologist (NSa) (hereafter ‘psychologists’). Assessment and formulation skills allowed them to identify, with the patient, the unique set of beliefs, behaviours, emotions and physical factors that were maintaining the fear of falling in that individual. Treatment sessions focused on targeting these beliefs and behaviours. CBTi sessions lasted approximately 45 minutes, with 15 minutes’ preparation time, and were based on an individualised formulation that identified and targeted the beliefs and behaviours maintaining fear of falling for that individual. The planned course of treatment was once per week for 8 weeks, with a single reinforcement session 6 months after the last CBTi session. The intervention is summarised using the Template for Intervention Description and Replication (TIDieR) framework45 in Table 6.
| Item no. | Item | Explanation |
|---|---|---|
| 1 | Brief name: Provide the name or a phrase that describes the intervention | STRIDE |
| 2 | Why: Describe any rationale, theory, or goal of the elements essential to the intervention | Based on the cognitive–behavioural model32 |
| 3 | What materials: Describe any physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery or in training of intervention providers. Provide information on where the materials can be accessed (such as online appendix, URL) | Patient manual; therapist manual; training materials |
| 4 | Procedures: Describe each of the procedures, activities, and/or processes used in the intervention, including any enabling or support activities | Formulation, tailored intervention |
| 5 | Who provided: For each category of intervention provider (such as psychologist, nursing assistant), describe their expertise, background, and any specific training given | HCAs Five-day training programme. Two recent psychology graduates: one with experience as HCA managing patients with falls; one physiotherapist |
| 6 | How: Describe the modes of delivery (such as face to face or by some other mechanism, such as internet or telephone) of the intervention and whether or not it was provided individually or in a group | Individual face-to-face sessions |
| 7 | Where: Describe the type(s) of location(s) where the intervention occurred, including any necessary infrastructure or relevant features | Patients’ homes (or convenient clinic if preferred) |
| 8 | When and how much: Describe the number of times the intervention was delivered and over what period of time including the number of sessions, their schedule, and their duration, intensity or dose | Planned as eight sessions over a period of 8 weeks, with each session lasting about 1 hour; behavioural and cognitive homework (e.g. graded exposure to avoided situations, fearful thoughts recorded and challenged) to be completed between sessions. One review session scheduled at 6 months after completion of initial intervention |
| 9 | Tailoring: If the intervention was planned to be personalised, titrated or adapted, describe what, why, when, and how | Intervention to be determined by individual ‘hot cross bun’ formulations, as described in Chapter 2 (see Results: understanding fear of falling) |
| 10 | Changes: If the intervention was modified during the course of the study, describe the changes (what, why, when, and how) | No formal modifications were made although the intervention evolved during the study (see Box 2) |
| 11 | How well – planned: If intervention adherence or fidelity was assessed, describe how and by whom, and if any strategies were used to maintain or improve fidelity, describe them | Intervention delivery was monitored through individual and group supervision, which included listening to recordings of therapy sessions (see Table 37) |
| 12 | How well – actual: If intervention adherence or fidelity was assessed, describe the extent to which the intervention was delivered as planned | See Chapter 4 |
Other therapeutic interventions were permitted in both intervention and usual care arms of the study per routine clinical care. None was prohibited.
Outcomes
Primary outcome measure
There is considerable variation in the literature on what constitutes fear of falling and how it should be measured. 12,25,43 Single-item questions are sometimes used, but have been criticised for their inability to measure variability in degrees of fear of falling. 12 We included a single-item, 11-point rating scale of fear of falling (see next section) in our battery of outcomes, but felt that a multi-item scale would be a more appropriate primary outcome measure. A number of scales have been developed following Tinetti’s initial FES,13 but its modified version, the FES-I29 has gained currency as a measure of fear of falling because of its simplicity, ease of administration and widespread validation across patient groups49–52 and cultures. 40,52,53 Tinetti’s original FES13 measured purely self-efficacy, an individual’s belief in his or her own capabilities in performing specific activities,54 but the FES-I modification (see Appendix 1) more explicitly measures concerns about falling. 29,55 We re-examined this issue in 200 older people using a more detailed principal factor-analytic approach than the original validation study,29 and found that the FES-I more robustly measures concerns about falling than had previously been found. 56 An integrative review of tools to measure fear of falling, considering papers published between 1982 (when the concept of fear of falling was first defined) and 2010, concluded that the FES-I is the best assessment tool for assessing fear of falling in community-dwelling elders. 57
Secondary outcome measures
Numeric rating scales for pain and fear of falling when walking
As summary measures of pain and fear of falling, participants were asked to rate these constructs on a 11-point scale, ranging from 0 (no concern/no pain) to 10 (as bad as it could be); a similar approach has been used in a number of studies as summarised by Scheffer et al. 10
Falls
-
Number of patients falling.
-
Number of falls.
-
Fractures and significant soft tissue injuries.
The close relationship between fear of falling and falls6,8–16,23,24,26,27,29,58,59 means that measurements of falls and their adverse consequences are vital. Falls research is often hampered by poor definitions and hence difficult interpretation of falls outcome data. In line with consensus guidelines,60 falls were defined as ‘an unexpected event in which the participant comes to rest on the ground, floor, or lower level’.
Anxiety and depression
Hospital Anxiety and Depression Scale
Although fear of falling was the primary outcome measure, the inter-related nature of fear of falling and anxiety mean that change in anxiety was clinically highly relevant. In addition, it was considered vital to assess depression in this population. 11 The Hospital Anxiety and Depression Scale (HADS), although widely used, was originally validated in a population with a maximum age of 65 years. 61 However, a large-scale UK study in four centres across England, Wales and Scotland has shown that the scale’s psychometric constructs are intact and applicable to community-dwelling older people. 62
Generic quality of life
-
World Health Organization Quality of Life questionnaire – older adults module (WHOQOL-OLD). 63
-
European Quality of Life-5 Dimensions [EQ-5D (5L)], the five-level version. 64
-
Short Form six-dimension health survey [Short Form questionnaire-6 Dimensions (SF-6D), derived from the Short Form questionnaire-36 items (SF-36)]. 65,66
The 24-item WHOQOL-OLD is a module of the World Health Organization’s broad measure of quality of life, the WHOQOL, which was designed for adults of all ages. The WHOQOL-OLD includes additional items that specific to older people. The EQ-5D (5L) is a generic quality-of-life measure that has the added benefits of enabling cost–utility analysis (CUA) (see Chapter 6), as does the SF-6D.
Social participation and social isolation
Social Disconnectedness and Perceived Isolation Scales67,68
Avoidance of social contact and social isolation are crucial co-factors both caused by, and contributing to, fear of falling. Cornwell and Waite67,68 have shown that the Social Disconnectedness and Perceived Isolation scales, which measure both social isolation and participation, may be particularly useful in evaluating interventions in older people. The Lubben Social Network Scale–6 (LSNS-6)69 is a questionnaire that is designed to gauge social isolation in older adults by measuring perceived social support from family and friends. It consists of 10 items, which are used to measure size, closeness and frequency of contacts of a respondent’s social network. The De Jong Gierveld Loneliness Scale70 is a well-validated and reliable 11-item scale that measures loneliness, including two subscales measuring emotional loneliness and social loneliness.
Impact on physical function
The Short Physical Performance Battery (SPPB) is a well-validated set of lower limb performance tests,71,72 which measures walking speed, a series of chair stands to assess muscle power, plus a test of balance, and has been used in major surveys such as the Women’s Health and Aging study. 73 Performed by a trained person, the SPPB takes 10–15 minutes to complete, with a composite score being derived by summing the category scores for each of the three tests. Better (higher) scores on the SPPB have been related to slower functional decline on the physical subscale of the Medical Outcomes Study SF-36. 74 Others have reported that SPPB scores are related to fatigue in older persons living in the community. 75 Functional decline and fatigue are related to SPPB scores, yet injurious falls were not related to total SPPB scores. 76 Injurious falls were related to taking ≥ 16.7 seconds to complete the five-times chair rise test and a history of falling. 76 Silva et al. 77 compared the World Health Organization Disability Assessment Schedule 2.0 to the SPPB, and noted that the self-report and SPPB were not related. Both physical and self-report measures may measure two different, but important, constructs.
The functional reach test is a good indicator of confidence in balance and increased risk of having a fall,78,79 whereas the measurement of maximum isometric handgrip strength (using a dynamometer) has functional relevance for supporting weight (e.g. holding on to a stair rail). Grip strength has been included in numerous large-scale surveys, and is predictive of both disability and mortality. 80–82 Functional reach and handgrip strength were measured three times at each protocol visit and the mean of the three values was calculated.
Table 7 summarises the measures used and the time points at which data were collected.
| Outcomes | Visit: | ||||
|---|---|---|---|---|---|
| 1. Initial screening | 2. Baseline visit confirmation of eligibility and randomisation | 3. End of CBTi intervention 8 weeks (visit window ± 1 week) | 4. 6 months post randomisation (visit window ± 2–4 weeks) | 5. 12 months post randomisation (visit window ± 2–4 weeks) | |
| Study discussed/PIS given | ✗ | ||||
| Informed consent | ✗ | ||||
| Randomisation (post eligibility checked and baseline tests) | ✗ | ||||
| Numeric rating scale for pain when walking | ✗ | ✗ | ✗ | ✗ | |
| Numeric rating scale for fear of falling when walking | ✗ | ✗ | ✗ | ✗ | |
| HADS | ✗ | ✗ | ✗ | ✗ | |
| MMSE | ✗ | ✗ | ✗ | ||
| FES-I | ✗ | ✗ | ✗ | ✗ | ✗ |
| Resource utilisation form | ✗ | ✗ | |||
| WHOQOL-OLD | ✗ | ✗ | ✗ | ✗ | |
| EQ-5D (5L) | ✗ | ✗ | ✗ | ✗ | |
| SF-36 | ✗ | ✗ | ✗ | ✗ | |
| De Jong Gierveld Loneliness Scale 11-item | ✗ | ✗ | ✗ | ✗ | |
| LSNS-6 | ✗ | ✗ | ✗ | ✗ | |
| Social Participation Questionnaire | ✗ | ✗ | ✗ | ✗ | |
| SPPB | ✗ | ✗ | ✗ | ✗ | |
| Functional reach | ✗ | ✗ | ✗ | ✗ | |
| Handgrip strength | ✗ | ✗ | ✗ | ✗ | |
| Adverse events | ✗ | ✗ | ✗ | ||
| Falls diaries | ✗ (monthly) | ||||
All outcomes were assessed and recorded by RAs/interviewers (unless specified otherwise below) at baseline and at 8 weeks (the anticipated end of the intervention period), 6 months and 12 months post randomisation; the later follow-up time points were selected to measure whether or not any immediate benefits of the intervention were sustained over time, with the 12-month assessment of FES-I comprising the primary outcome. Over the course of the study, five RAs/interviewers contributed to data collection and all were trained in the study procedures. Physical measures training was performed via Skype™ (Microsoft Corporation, Redmond, WA, USA) with one of the investigators (SLW) twice. The first session was designed to train the associates to collect the physical performance data and in patient safely, as there is always a risk of falling when asking older adults to perform balance tasks. The research associates were trained to guard the subjects closely and also to set up the environment so that the study participants felt safe during the testing. The group also practised timing each of the measures until the stopwatch measures were ± 0.5 seconds of each other. A subsequent Skype session and e-mails followed up session 1 in order to answer specific questions that arose in the home during testing. Whenever possible, all interviews with a given participant were undertaken by the same individual. The assessments took place in participants’ homes, the North Tyneside Falls Prevention Service or community centres or facilities (e.g. local Age UK premises), Newcastle University or the RVI, in accordance with participant preference.
Structured, weekly returned, postage-paid falls diaries were used to capture contemporaneous details of falls (see Appendix 3). Participants and carers were given verbal and written instructions to ‘. . . record each day any fall including a slip or trip in which you lost your balance and landed on the floor or ground or lower level’. We have previously used this technique successfully in similar populations, with published data showing in excess of 90% return rates. 83,84
The HADS, WHOQOL-OLD, EQ-5D (5L), SF-36 and LSNS were compiled into a questionnaire booklet and sent to patients for self-completion at the appropriate time points (see Table 7), prior to the planned visit date by the RA/interviewer, with a reply-paid envelope for return of the completed questionnaire direct to the research team. If participants had not returned this self-completion questionnaire within 2 weeks of the corresponding follow-up visit by the RA, the participant was contacted by a member of the research team and prompted to return it. If the participant had misplaced the questionnaire, a covering letter, replacement self-completion questionnaire and reply-paid envelope were provided. If the participant was unable to self-complete the questionnaire then the data were captured via the telephone by a RA. In these circumstances, the questions were asked in the following order to obtain the key measures in the event the participant did not wish to complete the full questionnaire (the HADS was not included, as it was considered too difficult to administer by telephone):
-
EQ-5D (5L)
-
SF-36
-
WHOQOL-OLD
-
LSNS-6.
At baseline, prior to randomisation, the RA administered the numeric rating scale for pain when walking. Participants were asked to rate their pain when walking by selecting a number on a scale from 0 to 10 (11-point scale) by circling the number that best described the level of pain they experienced. 85 Pain on walking, dichotomised as ‘no’ (score of 0) compared with ‘yes’ (scores of 1–10) formed one of the stratification variables in randomisation.
At each of the visits, the RA administered (in order) the FES-I, numeric rating scale for fear of falling when walking, the De Jong Gierveld Loneliness Scale and the Social Participation Questionnaire, SPPB, functional reach and handgrip strength tests. A standard chair was used in assessing muscle power, to ensure consistency. If it was not possible to arrange an in-person visit, but the participant was willing to provide outcome data, the FES-I, numeric rating scale for concern about falling, Social Participation Questionnaire, De Jong Gierveld Loneliness Scale and resource utilisation form were administered via telephone by a RA.
Harms
The anticipated potential risks associated with this trial were few. The main anticipated issue centred on the potential for patients to gain confidence and lose fear of falling in a way that was inconsistent with their improvement (or lack thereof) in physical function. In other words, patients who had hitherto considerably limited activity through fear of falling might have the potential to increase activity levels through amelioration of the condition with the CBTi before any physical interventions had been able to take effect. In practice, we felt this to be unlikely, given the prolonged course of the CBTi.
All adverse events were elicited and recorded by falls diaries and RAs/interviewers at all visits, and were categorised as to expectedness, relatedness and severity. All serious adverse events, excluding any pre-planned hospitalisations (e.g. elective surgery) not associated with clinical deterioration, routine treatment or monitoring of the studied indication (i.e. fear of falling), not associated with any deterioration in condition and elective or scheduled treatment for pre-existing conditions that did not worsen during the study, were likewise recorded throughout the study. Seriousness of adverse events was defined in accordance with Good Clinical Practice as follows:
Serious adverse event An untoward occurrence (whether expected or not) that:
-
results in death
-
is life-threatening (refers to an event in which the subject was at risk of death at the time of the event; it does not refer to an event that, hypothetically, might have caused death if it were more severe)
-
requires hospitalisation, or prolongation of existing hospitalisation
-
results in persistent or significant disability or incapacity
-
consists of a congenital anomaly or birth defect
-
is otherwise considered medically significant by the investigator.
Participant timeline
The schedule of patient visits is summarised in Table 7 and in Figure 4. Patient recruitment took place between 3 July 2012 and 28 February 2014. The first site, North Tyneside Falls Prevention Service, started screening on 28 May 2012, and the first patient was recruited from this site on 3 July 2012; the Falls and Syncope Service started screening on 25 May 2013, and the first patient was recruited from this site on 19 July 2013; the Galleries Day Unit started screening on 5 June 2013, and the first patient was recruited from this site on 11 July 2013. Study interventions were delivered between 27 July 2012 and 17 November 2014. The last patient/last visit was on 31 January 2015.
FIGURE 4.
Trial flow chart.

Sample size calculation
As indicated above, the primary outcome was change (from baseline) in fear of falling, as measured by the FES-I, at 12 months. The estimated standard deviation (SD) of change scores was 12.5. 29 The target difference in group means that we wished to be able to detect was four points (per clinical judgement, in combination with observed effects in a range of studies27,40 corresponding to a standardised effect size of 0.32). Initially, sample size was calculated for 90% power, 5% significance level. Slow recruitment led to a review of the power calculation and agreement – by the funder, Trial Steering Committee (TSC) and Data Monitoring Committee – that power could be reduced to 80%. Accordingly, the number required to provide full outcome data (80% power, 5% significance level) was calculated as 154 per group, or 308 in total. We estimated a 25% dropout rate on the basis of previous experience with older fallers in RCTs83 and data from a previous study of CBT in fear of falling in which 30% of the intervention group and 25% of the usual care group failed to complete the study. 28 Accordingly, to allow for 25% dropouts, we aimed to recruit 412 participants.
Methods for participant recruitment
Identification and screening of participants
Potential participants with significant fear of falling (FES-I score of > 23) were identified prospectively and retrospectively by staff at the participating community falls services, as described in greater detail below; the majority were identified and approached prospectively. When the FES-I was not administered routinely at a particular site, consent was taken prior to administering this measure. At the majority of sites, all other screening assessments were part of routine clinical practice; when a screening measure was not part of routine clinical practice, consent was taken prior to administration of that measure.
A screening log was kept at each site to document details of subjects who were invited to participate in the study. For subjects who declined participation, this documented any reasons that were available for non-participation. The log also ensured that potential participants were approached only once.
Phase I participants who screened eligible and had expressed an interest in the CBTi intervention were offered the opportunity to receive the CBTi, but were not randomised and did not complete any outcome measures.
Recruitment procedures
Prospective recruitment of participants
Original plans for prospective recruitment at North Tyneside Falls Prevention Service and thereafter at the Galleries Day Unit were to send an invitation letter and summary participant information leaflet providing details of the STRIDE study with the routine appointment letter inviting patients to attend the falls services. However, the organisations providing these services felt that this strategy might impact adversely on clinic attendance, and therefore this approach was abandoned at those sites. Instead, a member of the research team (STRIDE HCA or interviewer) or a clinical trial associate (CTA) from the Clinical Research Facility (CRF) was present in the clinic waiting area at North Tyneside to discuss the STRIDE study with patients who were waiting for their appointment and those who had attended their appointment. This person explained to patients that their eligibility would be confirmed by the clinic staff following the patient’s appointment. In the Galleries Day Unit, potentially eligible patients were invited to participate by staff members (nursing and/or medical) during clinic visits and the study was explained to them by this staff member. In the Falls and Syncope Service, potentially eligible patients were invited to participate by staff members (nursing and/or medical) or clinical research network research associates during clinic visits, and the study was explained to them by the relevant individual.
In all sites if, following the procedures described above, the patient expressed an initial interest in participating in the study, a detailed Participant Information Sheet was provided for the patient to take home, along with an expression of interest form, to be forwarded directly to the research team at Newcastle University. If the patient expressed an interest in taking part while still in clinic, the member of clinic staff completed a ‘consent to release contact details’ form, which was passed on to the research team. Those returning a positive expression of interest form, or for whom we had a ‘consent to release contact details’ form, were contacted, usually within 1 week, by a RA/interviewer, to discuss further their participation and arrange a convenient time to meet to take consent and complete the baseline assessments. If the patient had not returned the expression of interest form to the STRIDE office at Newcastle University within 1 week of their clinic appointment, a member of the clinical team from the relevant falls service contacted the patient by telephone to establish whether or not they had any further questions about the study and to ask if they would like to participate. A RA/interviewer then contacted the patient by telephone to discuss the study further and answer any questions. Arrangements were then made to meet those patients who were willing to proceed, in a place of their choice, to take formal consent and administer the baseline measures.
Retrospective recruitment of participants to the randomised controlled trial
Eligible patients who had attended the Galleries Day Unit in the 3 months preceding the study opening in that site were identified, retrospectively, by a member of clinic staff prior to prospective recruitment commencing at that site. The initial retrospective sample was approached following initial poor recruitment to the study, and following ethical, sponsor, funder, Trial Steering Group and Data Monitoring Committee review and approval. Patients were routinely asked at this clinic to give consent for their details to be used for research, and only those who had given this permission were considered. Identified patients were sent an invitation letter, detailed Participant Information Sheet, expression of interest form and a prepaid envelope in which to return the expression of interest form to the STRIDE office at Newcastle University. A member of clinic staff telephoned those patients who had not returned the expression of interest form after 1 week to check that they had received the letter and to explore their views on participation. If the patient expressed an interest in taking part, the member of clinic staff completed a ‘consent to release contact details’ form, which was passed on to the research team. A RA/interviewer then contacted the patient by telephone to discuss the study further and to answer any questions. Arrangements were then made to meet those patients who were willing to proceed, in a place of their choice, to take formal consent and administer the baseline measures.
Consent procedures
Informed consent discussions were undertaken by trained RAs/interviewers, with opportunity for participants to ask any questions. Following receipt of information about the study, participants were given reasonable time (minimum of 24 hours) to decide whether or not they wished to participate. Those wishing to take part provided written, informed consent by signing and dating the study consent form, which was witnessed and dated by the RA/interviewer. Written informed consent to study-specific procedures/investigations was obtained prior to randomisation. The original signed consent form was retained in the Investigator Site file, with a copy placed in the clinical notes and another copy provided to the participant. Participants specifically consented to their GP being informed of their participation in the study.
Interpreters were available for all visits of patients who required them, either for verbal translation or for deaf subjects wishing to take part in the study, via local NHS arrangements. In practice, no patients required an interpreter or signer.
Randomisation
A computer-generated blocked allocation (programmed in Awk, Version 4.1; GNU; Free Software Foundation, Inc., Boston, MA, USA) was used to allocate patients in a 1 : 1 ratio to intervention and control groups (permuted random blocks of variable length, block size was not disclosed to the investigators). Randomisation was stratified by site, patient gender, baseline score on the numeric rating scale for pain when walking (0 vs. 1–10) and whether or not the patient had been referred for strength and balance training.
Randomisation was administered centrally via the Newcastle Clinical Trials Unit’s internet-accessed secure web-based system to ensure concealment of allocation. The Chief Investigator, or other individual with delegated authority, accessed the web-based system once patient eligibility had been confirmed and informed consent obtained. Patient screening ID, initials and stratifying variables were entered into the web-based system, which returned the allocation status. Participants were informed of their allocated treatment group immediately after randomisation.
Blinding
Owing to the nature of the intervention, it was not possible to blind patients or care providers. The research team, including those collecting outcome data and the statistician analysing data, were also unblinded.
Statistical methods
Analyses were conducted using IBM SPSS Statistics release 22 (IBM Corporation, Armonk, NY, USA) and Stata release 12 (StataCorp LP, College Station, TX, USA).
Primary analysis
The difference between FES-I at the end of treatment (12 months) and FES-I immediately before treatment (baseline) in two arms was analysed using analysis of covariance. The dependent variable was the FES-I score at 12 months; baseline FES-I was included as a covariate. Stratification variables (site, gender, pain when walking, and referral for strength and balance training) were included as fixed effects. A two-tailed test was performed, with significance level set at 5% (95% CIs of parameters of interest). The primary analysis was on the basis of intention to treat (ITT) with patients included in the group to which they were randomised.
Secondary analysis of primary outcome measure
The above analyses were extended to include intermediate measures of FES (8 weeks and 6 months). Within a multilevel model both variation between occasions within an individual participant and variation between participants were included as random effects (assumed to have a Gaussian distribution). The hypothesis to be tested was that fear of falling would be lower in the intervention group at 8 weeks (i.e. on completion of therapy) and the difference between groups would be maintained until the end of follow-up at 12 months.
If > 25 patients in the intervention group fail to complete the full course of CBT we planned to investigate whether or not there was an association between the effectiveness of the intervention and the number of CBT sessions attended. We indicated that this would be achieved by adding the number of sessions of therapy as a patient-level covariate to the above model.
Secondary outcomes
Each of the remaining health status and quality-of-life measures took the form of a score that was treated as a continuous variable and analysed using analysis of covariance with an assumed normal error structure. The dependent variable was the response observed at 12 months. Baseline response was included as a covariate; differences between randomisation strata were included as fixed effects (a binary indicator of pain when walking, gender, referral for strength and balance training, and treatment centre). Finally, the difference between groups was fitted as a fixed effect. A 95% CI for the estimated impact of CBTi is presented. Differences between strata were then removed from the model and unadjusted estimates of the impact of CBTi are presented for comparison purposes.
Change over time in these outcomes was investigated using a sequence of multilevel models with both variation between occasions within an individual participant and variation between participants included as random effects (assumed to have a Gaussian distribution). Differences between strata were included as fixed effects. An initial model assumed a common linear trend over time in each of the study groups. In a second model, a separate trend was fitted for each study group; 95% CIs are presented for each estimate of trend and for the difference between them.
Analyses were designed to:
-
measure the impact of the intervention compared with control on fall and injury rates in the trial participants and its impact on functional abilities
-
assess the effectiveness of the intervention compared with control on anxiety (HADS), quality of life [EQ-5D (5L), SF-6D, WHOQOL-OLD], social isolation and social participation at 6 and 12 months post randomisation, through analysis of covariance
-
summarise the acceptability of the intervention for patients, family members and professionals (see Chapter 5)
-
investigate further the professional and organisational factors that promote or inhibit the implementation and integration of the intervention (see Chapter 5)
-
measure the costs and outcomes of the intervention in this setting (see Chapter 6).
For all analyses, examination of residual plots was used to check model assumptions.
Scoring of questionnaires and handling of missing data
The FES-I scores were calculated in accordance with the authors’ instructions:29 if data were missing one more than four items, the data were declared as missing; if no more than four of the items were missing, the score was calculated by adding the responses to all completed items, dividing by the number of items completed and multiplying the value thus obtained by 16, and then rounding to the nearest whole number.
In respect of secondary outcome data, quality-of-life scores were calculated in accordance with their authors’ instructions. 61,64,67–70,86 For the SF-36, scoring software provided by the developers and distributors of the instrument was used (www.optum.com/optum-outcomes/what-we-do/health-surveys.html, accessed 1 October 2015). In the absence of any authors’ rule for handling missing data, simple imputation was used, provided that at least half of the items in any scale had been completed (imputed missing value = mean value of non-missing items). Secondary outcomes were analysed using mixed models that made use of all data available.
To address differential dropout in the two groups, in both the protocol and statistical analysis plan, we originally proposed to use the method of joint modelling described by Henderson et al,87 based on the assumption that time to dropout would be predictive of final outcome. The rationale behind this approach is that there is some underlying characteristic of the participant, usually referred to as ‘frailty’, which is associated both with dropout and outcome.
In practice, dropout did not occur steadily through this study but rather was triggered by particular activities arising from participation in the study. A large proportion of the dropout occurred prior to week 8; a smaller proportion of dropout accrued over the remaining 44 weeks. The fitting of a Cox proportional hazards model to ‘time to drop out’ would appear to be very questionable, given the pattern of missing data within the two arms of the trial. Instead, to assess the impact of missing visits, we adopted an approach based on multiple imputation. 88
Multiple imputation was used to attribute missing values for the FES-I score at each of the follow-up time points, in those cases when the instrument was not completed at all, or could not be scored. The imputation model included the following variables measured at baseline:
-
age
-
sex
-
gait and balance score
-
handgrip strength
-
functional reach
-
falls efficacy (FES score)
-
concern about falling (0–10 scale)
-
pain when walking (0–10 scale)
-
participation score
-
loneliness score
-
site.
In addition, the number of sessions of CBT completed was included as a predictor variable.
Four sets of imputation were undertaken. The FES score at 1 year was estimated using a regression model with the above variables included as predictors. Then FES scores at 8 weeks, 6 months and 1 year were estimated simultaneously, assuming a multivariate normal model.
Each of these methods was undertaken:
-
separately for each study group (intervention and control)
-
with both groups together (with group added as a predictor variable).
In each case, 50 data sets were imputed.
Imputation was carried out using the ‘mi impute’ procedure in the Stata statistical package. Two different multivariate data imputation models were used. In both cases, missing values for all of the specified variables were imputed simultaneously. The first method used a regression model approach (‘mi impute regress’ in Stata); the second method assumed a multivariate normal distribution (‘mi impute mvn’ in Stata). These methods were applied using two different approaches. First, the imputation was carried out using all participants; second, the imputation was done separately for each group. Thus there were four sets of imputed data. In each case, 50 imputations were undertaken, giving a total of 4 × 50 = 200 imputed data sets. Inference on each of the four sets of 50 data sets was carried out using the ‘mi estimate’ procedure in Stata. Coefficients and standard errors are adjusted for the variability between imputations according to the combination rules set out by Rubin. 89
Safety (harms)
The aim of the analysis of harms was to estimate the difference in the rate of adverse events experienced by the two treatment groups. For serious adverse events, we determined 95% CI for the hazard ratio (i.e. risk of a serious adverse event) for a patient randomised to CBTi plus usual care compared with a patient randomised to usual care.
Compliance
The number of CBT sessions attended was documented and the date of each session was captured. Descriptive analysis focused on the number of sessions attended and the time interval over which the first eight sessions were completed. We also analysed the distribution of time between completion of the first eight sessions of CBT and the scheduled 8-week and 12-month follow-up assessment of outcomes.
Data archiving
Study documents and data will be archived for 10 years, in line with sponsor policies.
Patient and public involvement
Our group was fortunate to have powerful clinical and research links to North Tyneside Age UK. The North Tyneside Falls Prevention Service [for which the principal investigator (SWP) was clinical lead] was in partnership with Age UK to provide targeted strength and balance training classes, developed jointly between the Service and Age UK. These links allowed us the opportunity of early and comprehensive involvement of the Age UK executive team, staff, volunteers and clients in the development and implementation of the study. Indeed, AC, Chief Executive of Age UK North Tyneside, was a co-applicant for the STRIDE study, and her knowledge and expertise added immeasurably to these processes. The Health and Wellbeing leads for Age UK North Tyneside (AW and SG) were active members of our TSC, adding expertise and critical faculty regarding the client group and the conduct of the trial to this key Committee.
In developing the proposal, in addition to more general information and constructive advice and criticism of the study methods and recruitment processes, Age UK staff, volunteers and clients gave invaluable advice on appropriate inclusions in the study, the choice of the primary outcome measure, its clinical relevance to older people, and what changes in score would be relevant to older people with fear of falling. They were similarly involved in focus groups informing likely recruitment rates, while these groups also reviewed study literature to ensure readability and enhance understanding by participants and their families and carers. Focus groups and staff and client views were also key in providing information on what duration and frequency of CBT would be acceptable to older people prior to the commencement of the study.
Our proposal was thus rooted developmentally and during the conduct of the study in the locality and the ecology of its elder population in a way that would otherwise have been impossible, adding immeasurably to our understanding of what would was acceptable to this group of older people and so to the successful design and conduct of the study, and ultimately to the research question we eventually answered.
A specific example of how our links to Age Concern UK informed trial design and conduct relates to review and refinement of the patient recruitment process. One member of the research team (CB) met with the Health and Wellbeing lead for Age UK North Tyneside (SG) to discuss possible changes to this process. Presented with a range of options, SG immediately recommended having a STRIDE therapist or researcher at the clinic to make the first approach to potential participants. This approach had been used successfully by one of her colleagues to recruit people to the ‘Fit as a fiddle’ initiative. SG suggested that we could cite Age UK when making the case for a face-to-face approach in clinic, and that this approach could be described as having been ‘tried, tested, used and endorsed by Age UK’. She also emphasised the need to make sure that patients could subsequently opt out in order to manage any potential sense of coercion. These suggestions were taken on board.
Chapter 4 Phase II: randomised controlled trial results
Introduction
In line with Consolidated Standards of Reporting Trials (CONSORT) guidance,90 in this chapter we present the results of the Phase II of the STRIDE study, the RCT of the CBTi with usual care compared with usual care. The sections below detail patient flow, screening, eligibility and recruitment data and a comprehensive overview of usual care in the three recruitment sites, and then baseline and follow-up data for the primary and secondary outcome measures, including harms.
Participant flow
Screening within the sites
Overall, 2355 patients were initially screened (using information recorded in clinic databases) by the clinic staff across the three recruitment sites (Table 8). Of those screened, 1346 were considered to be eligible for the trial (57.1%). Some eligible patients explicitly declined the initial study information that was available at the clinic.
| Site | Total screened | Ineligible | Eligible | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total eligible | Declined information | Provided with information | |||||||
| n | %a | n | %a | n | %b | n | %b | ||
| Falls and Syncope Service | 679 | 300 | 44.2 | 379 | 55.8 | 131 | 34.6 | 248 | 65.4 |
| Galleries Day Unit | 239 | 66 | 27.6 | 173 | 72.4 | 90 | 52.0 | 83 | 48.0 |
| North Tyneside Falls Prevention Service | 1437 | 647 | 45.0 | 790 | 55.0 | 161 | 20.4 | 629 | 79.6 |
| Total | 2355 | 1013 | 43.0 | 1342 | 57.0 | 382 | 28.4 | 960 | 71.3 |
Rates of eligibility were similar between the Falls and Syncope Service and the North Tyneside Falls Prevention Service, but were markedly higher at the Galleries Day Unit. Rates of decline of study information by those who were deemed to be eligible varied significantly across the three sites, with patients at the Galleries Day Unit most likely to decline information.
One reason for ineligibility was recorded for each patient within the screening log. The reasons for non-eligibility at initial screening are shown in Table 9; most commonly these were FES-I score of ≤ 23 (78.4%), MMSE score of < 24 (17%) and patients aged < 60 years (1.6%). There were marked differences between sites in the stated reasons for ineligibility. This in part reflected inter-site differences in patient case mix and variations in practice (see Chapter 3) with respect to routine administration of the FES-I and MMSE, and therefore availability of these data in assessing eligibility.
| Description | Site | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Falls and Syncope Service | Galleries Day Unit | North Tyneside Falls Prevention Service | ||||||
| n | % | n | % | n | % | n | % | |
| FES-I score of ≤ 23 | 265 | 88.3 | 13 | 19.7 | 516 | 79.8 | 794 | 78.4 |
| Cognitive impairment (MMSE score of < 24) | 13 | 4.3 | 39 | 59.1 | 120 | 18.5 | 172 | 17.0 |
| Aged < 60 years | 0 | 0.0 | 9 | 13.7 | 7 | 1.1 | 16 | 1.6 |
| Care home resident | 11 | 3.7 | 0 | 0.0 | 0 | 0.0 | 11 | 1.1 |
| No reason provided | 0 | 0.0 | 5 | 7.6 | 2 | 0.3 | 7 | 0.7 |
| Patient requires psychosocial interventions unrelated to fear of falling | 2 | 0.7 | 0 | 0.0 | 2 | 0.3 | 4 | 0.4 |
| Current involvement in other investigational studies or trials, or involvement within 30 days prior to study entry | 8 | 2.7 | 0 | 0.0 | 0 | 0.0 | 8 | 0.8 |
| Life expectancy of < 1 year | 1 | 0.3 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 |
| Total number of patients considered ineligible | 300 | 100.0 | 66 | 100.0 | 647 | 100.0 | 1013 | 100.0 |
Screening prior to randomisation
The RAs made a further check on eligibility by reviewing each of the inclusion and exclusion criteria with patients who returned an expression of interest form. This was to ensure that their eligibility had not changed prior to randomisation. Some additional participants were excluded at this stage, the reasons for exclusion are shown in Table 10.
| Description | Falls and Syncope Service | Galleries Day Unit | North Tyneside Falls Prevention Service | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | %a | n | %a | n | %a | n | %a | |
| Returned expression of interest form | 245 | 99 | 371 | 715 | ||||
| Unable to contact | 13 | 5.3 | 2 | 2 | 18 | 4.9 | 33 | 4.6 |
| Reason for ineligibility on RA screening | ||||||||
| FES-I score of ≤ 23 | 4 | 1.6 | 2 | 2 | 14 | 3.8 | 20 | 2.8 |
| Patient has cognitive impairment (MMSE score of < 24) | 7 | 2.9 | 3 | 3 | 0 | 0 | 10 | 1.4 |
| Patient requires psychosocial interventions unrelated to fear of falling | 4 | 1.6 | 0 | 0 | 2 | 0.5 | 6 | 0.8 |
| Aged < 60 years | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0.1 |
| Current involvement in other investigational studies or trials, or involvement within 30 days prior to study entry | 4 | 1.6 | 2 | 2 | 5 | 1.3 | 11 | 1.5 |
| Spouse randomised | 1 | 0.4 | 1 | 1 | 5 | 1.3 | 7 | 1 |
| Total ineligible | 20 | 8.2 | 9 | 9.1 | 26 | 7.1 | 55 | 7.7 |
| Total declining | 86 | 35.1 | 38 | 38.4 | 87 | 23.5 | 211 | 29.5 |
| Consented but died shortly thereafter | 1 | 0.4 | 0 | 0 | 0 | 0 | 1 | 0.1 |
| Total non-participation (including unable to contact) | 120 | 49 | 49 | 49.5 | 131 | 35.3 | 300 | 42 |
| Consented and randomised | 125 | 51 | 50 | 50.5 | 240 | 64.7 | 415 | 58 |
Rates of non-contact did not vary significantly between the three sites. Among the 682 patients contacted, rates of eligibility were 91.4% for the Falls and Syncope Service, 90.7% for the Galleries Day Unit and 92.6% for North Tyneside Falls Prevention Service. A total of 627 of those contacted were deemed eligible, of whom 211 (33.7%) did not consent to randomisation. Rates of non-consent were 40.6% for the Falls and Syncope Service, 43.2% for the Galleries Day Unit, and 26.6% for the North Tyneside Falls Prevention Service; pairwise comparisons indicated that the rate of non-consent at the North Tyneside Falls Prevention Service was significantly different from the rates at the other two sites. There appeared to be some variation across sites in the reasons for declining consent, but this may be due, in part, to inconsistency between RAs/interviewers in how the reasons offered were categorised (Table 11).
| Description | Falls and Syncope Service | Galleries Day Unit | North Tyneside Falls Prevention Service | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | %a | n | %a | n | %a | n | %a | |
| Felt no intervention needed | 6 | 7.0 | 0 | 0.0 | 9 | 10.3 | 15 | 7.1 |
| Felt CBTI ‘not for me’ | 25 | 29.1 | 15 | 39.5 | 11 | 12.6 | 51 | 24.2 |
| Wanted only strength and balance training | 2 | 2.3 | 0 | 0.0 | 6 | 6.9 | 8 | 3.8 |
| Too busy | 14 | 16.3 | 8 | 21.1 | 15 | 17.2 | 37 | 17.5 |
| Illness | 23 | 26.7 | 11 | 28.9 | 17 | 19.5 | 51 | 24.2 |
| No reason given | 16 | 18.6 | 4 | 10.5 | 29 | 33.3 | 49 | 23.2 |
| Total | 86 | 38 | 87 | 211 | ||||
The overall participation rate (i.e. number consenting and randomised as a percentage of those returning an expression of interest form) was 58%, with site-specific rates of 51.0%, 50.5% and 64.7% for the Falls and Syncope Service, Galleries Day Unit and North Tyneside Falls Prevention Service, respectively. Pairwise comparisons showed that the participation rate for the North Tyneside Falls Prevention Service was significantly different from the rates at the other two sites. When reasons for non-participation were grouped as ‘no contact’, ‘ineligible’, ‘declined consent’ and ‘died post randomisation’, there were no significant differences between sites.
Randomisation
The screening, recruitment, randomisation and trial follow-up are summarised in the CONSORT diagram shown in Figure 6.
FIGURE 6.
The STRIDE CONSORT diagram.

Recruitment by strata
A total of 415 participants were randomised; 240 (57.8%) came from the North Tyneside Falls Prevention Service, the first site to open; 125 (30.1%) came from the Falls and Syncope Service; 50 (12.1%) came from the Galleries Day Unit). In addition to recruitment site, the sample was stratified by three other variables: gender, pain when walking (dichotomised as yes/no from ratings on a 11-point scale) and referral to strength and balance training (yes/no) (Table 12). Overall, 124 (29.9%) of those randomised were men (95% CI 25.5% to 34.5%). Two hundred and one participants (48.4%; 95% CI 43.5% to 53.4%) reported that they suffered pain when walking; 288 participants (69.4%; 95% CI 64.7% to 73.8%) had been referred for strength and balance training.
| Description | Falls and Syncope Service | Galleries Day Unit | North Tyneside Falls Prevention Service | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | %a | n | %a | n | %a | n | % | |
| Total randomised | 125 | 30.1 | 50 | 12.1 | 240 | 57.8 | 415 | 100.0 |
| n | % b | n | % b | n | % b | n | % b | |
| Male | 35 | 28.0 | 19 | 38.0 | 70 | 29.2 | 124 | 29.9 |
| Pain on walking | 52 | 41.6 | 29 | 58.0 | 120 | 50.0 | 201 | 48.4 |
| Referral to strength and balance training | 124 | 99.2 | 48 | 96.0 | 116 | 48.3 | 288 | 69.4 |
Strata were included as fixed effects in analyses of covariance. To inform this approach, the level of collinearity in these potential explanatory variables was examined.
There were systematic differences between the three sites only with respect to the percentage of participants referred for strength and balance training, reflecting variation in ‘usual care’ between these sites, as described in Chapter 3.
Overall, 50.8% of men and 47.4% of women reported pain on walking [relative risk (RR) 1.04, 95% CI 0.02 to 1.18]. Of those referred for strength and balance training, 50.3% had pain on walking, 49.7% did not [RR for an individual referred for strength and balance training having pain on walking = 1.09 (95% CI 0.87 to 1.36)]. Across all sites, men were more likely to be referred for strength and balance training than women (77.4% vs. 66.0%, RR 1.17, 95% CI 1.03 to 1.32).
Recruitment by arm
Two hundred and five patients (49.4%) were randomised to the control arm; 210 patients (50.6%) were randomised to receive CBTi (Table 13).
| Site | Trial arm | Total | |||
|---|---|---|---|---|---|
| Control | Intervention | ||||
| n | % | n | % | ||
| Falls and Syncope Service | 62 | 49.6 | 63 | 50.4 | 125 |
| Galleries Day Unit | 23 | 46.0 | 27 | 54.0 | 50 |
| North Tyneside Falls Prevention Service | 120 | 50.0 | 120 | 50.0 | 240 |
| Total | 205 | 49.4 | 210 | 50.6 | 415 |
Baseline data
Stratification was successful and study arms were balanced with respect to site, gender, pain on walking (yes/no) and referral for strength and balance training (Table 14).
| Characteristic | Control | Intervention | Study population | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | 95% CI | |
| Male | 60 | 29.3 | 64 | 30.5 | 124 | 29.9 | 25.6 to 34.6 |
| Pain on walking | 101 | 49.3 | 100 | 47.6 | 201 | 48.4 | 43.5 to 53.4 |
| Referred for strength and balance training | 143 | 69.8 | 145 | 69.0 | 288 | 69.4 | 64.7 to 73.6 |
Falls Efficacy Scale–International version
Completion of the individual items within the FES-I at baseline was excellent. Four participants did not complete the item ‘Going to the shop’. Greatest concern was expressed about ‘Walking on slippery surface’; least concern was expressed about ‘Preparing simple meals’ (see Appendix 4). The scale was internally consistent; Cronbach’s alpha, based on 402 complete cases, was 0.89. The total FES-I score could be computed for all 415 participants (Figure 7). FES-I score was not associated with age (correlation –0.034 (95% CI –0.130 to 0.070)].
FIGURE 7.
The FES-I scores at baseline.
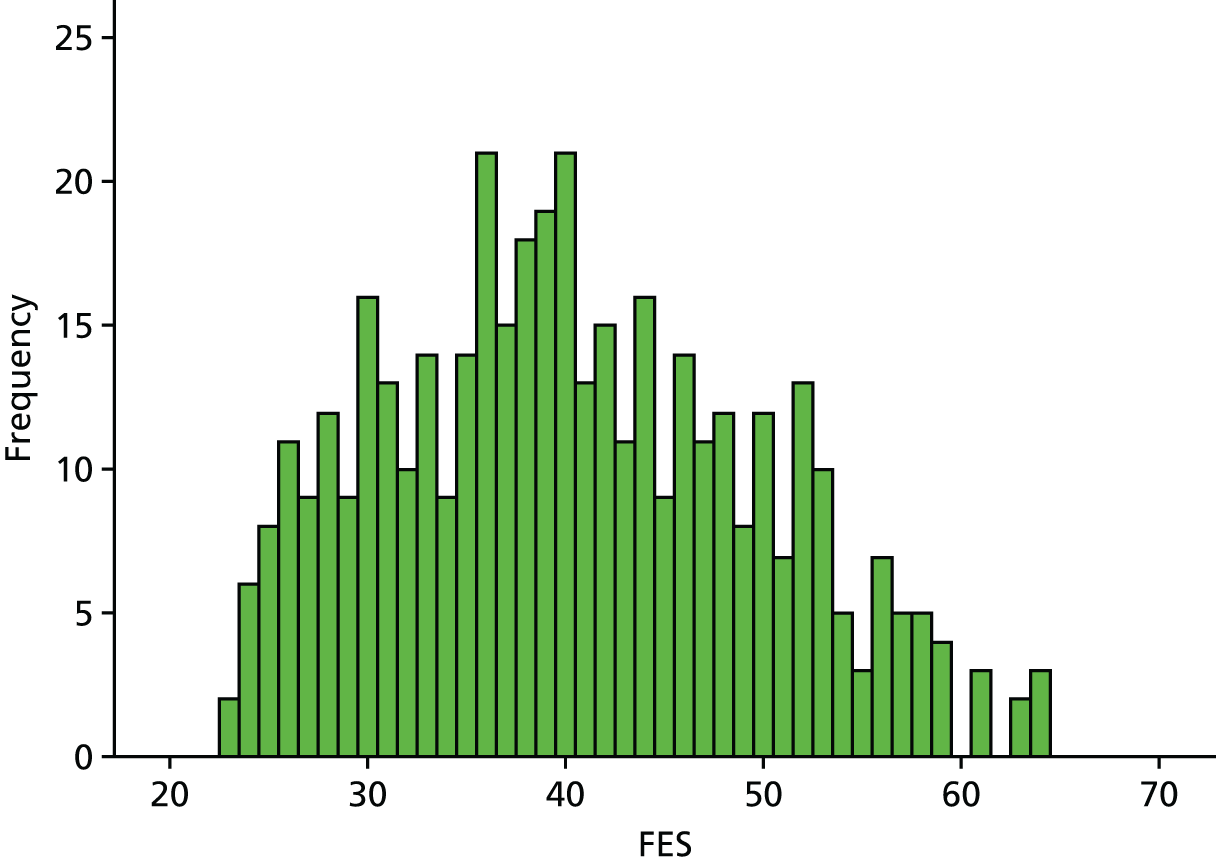
Fear of falling 11-point scale
In the baseline interview, participants were asked to rate their concerns about falling on an 11-point scale, for which ‘0’ meant ‘no fear of falling’ and ‘10’ meant ‘fear of falling as bad as it could be’. All 415 participants responded to this item (Figure 8). The majority of participants scored between 5 and 10 The correlation between FES-I scores and fear of falling numerical rating was 0.53 (95% CI 0.46 to 0.60) (CI based on bootstrap procedure with 1000 replications). There is clearly a strong association between FES-I scores and fear of falling, as assessed by the rating scale method (see Appendix 4 for a graphical presentation of this association).
FIGURE 8.
Distribution of fear of falling, 11-point scale numerical rating.
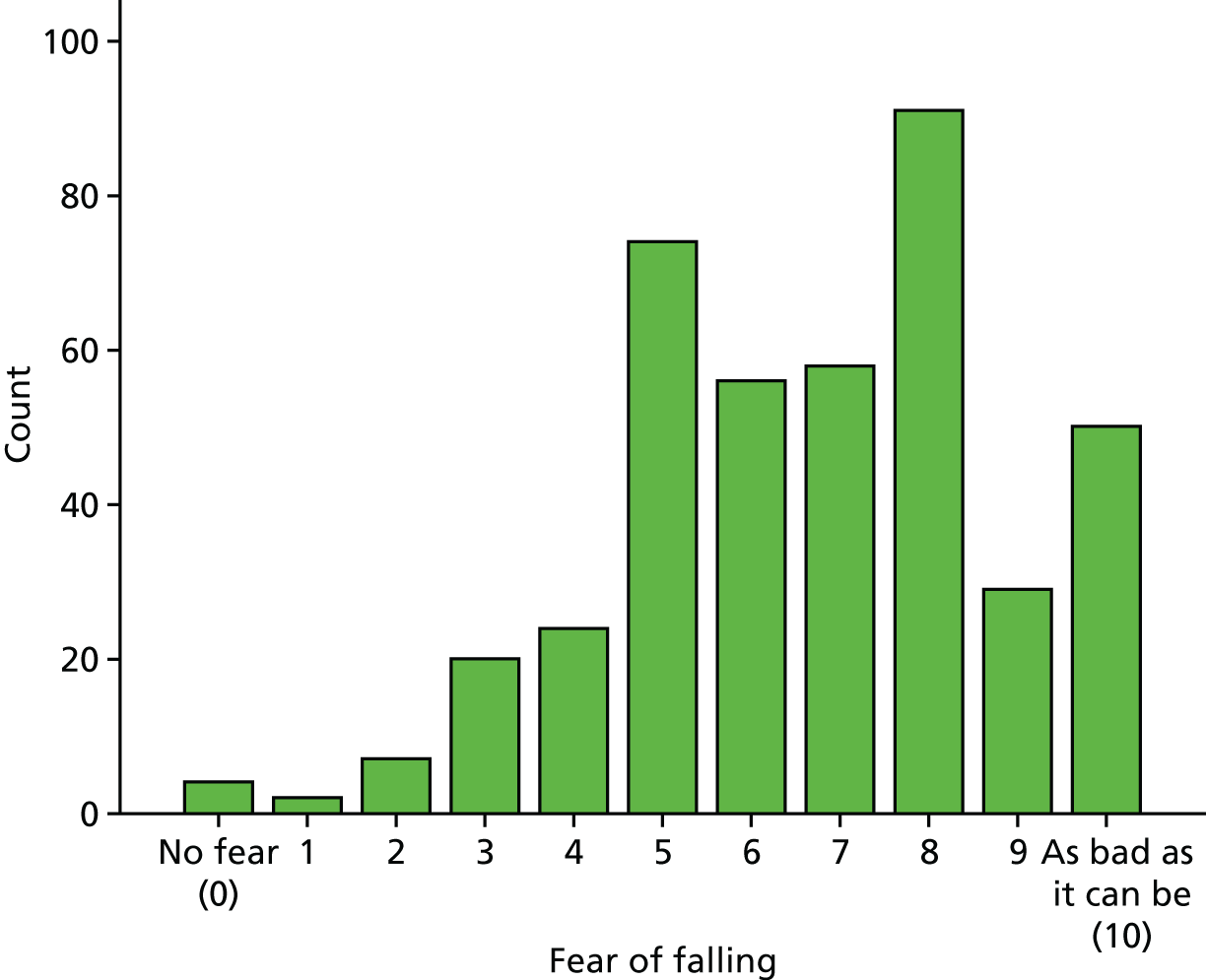
Pain when walking, 11-point scale
In the baseline interview, participants were asked to rate their pain on walking on a 11-point scale, for which zero ‘0’ meant ‘no pain’ and ‘10’ meant pain that was ‘as bad as it could be’ (Figure 9). This variable was dichotomised as ‘no pain’ (0) versus ‘pain’ (1–10) for purposes of stratification. ‘Pain when walking’ ratings were weakly but positively correlated with FES-I scores [ρ = 0.35 (95% CI 0.27 to 0.44)] (see also Appendix 4) and with fear of falling numerical rating scales [ρ = 0.32 (95% CI 0.22 to 0.41)].
FIGURE 9.
Distribution of pain when walking, 11-point scale numerical rating.
Social participation and social isolation scale score
Total participation scores ranged from 0 to 37, with a mean of 9.49 and a median of 7 (Figure 10). Internal consistency, measured by Cronbach’s alpha, was poor at 0.27. Total participation score was not correlated with age [ρ = –0.006 (95% CI –0.101 to 0.084)].
FIGURE 10.
Distribution of social participation and social isolation scale score at baseline.
De Jong Gierveld Loneliness Scale
High levels of loneliness were reported by participants. Scores ranged from 0 to 11, with a mean of 8.20, median of 9 and mode of 11 (Figure 11). Internal consistency of this scale was poor, with a Cronbach’s alpha (α) = 0.42. Loneliness score was not correlated with age [ρ = –0.035 (95% CI –0.145 to 0.070)] but was positively, albeit weakly, correlated with total participation score [ρ = 0.148 (95% CI 0.054 to 0.235)].
FIGURE 11.
Distribution of De Jong Gierveld Loneliness Scale scores at baseline.

Short Physical Performance Battery (gait, balance and sit to stand)
Short physical performance battery scores ranged from 0 to 12, with a mean of 5.66 (Figure 12). Lower scores are associated with poorer physical performance, with higher scores reflecting better physical condition in relation to these activities. The mean for our participants represents an average performance for this patient group.
FIGURE 12.
Distribution of SPPB scores at baseline.

Handgrip strength and functional reach
Data on handgrip strength were not available for 13 participants, with reasons for non-completion recorded for eight (one refused; in two cases the test was not attempted because the participant felt unsafe; in one case the participant attempted the test but could not hold the position unaided; and in four cases, the participant attempted the test but failed). Among those completing the test, scores over three attempts ranged from 0.3 kg to 54.4 kg, with a mean of 16.5 kg (Figure 13). The mean is low compared with normative data for older patients, with an overall mean for this age group of 31 kg. 91
FIGURE 13.
Distribution of mean handgrip strength (kg) at baseline.
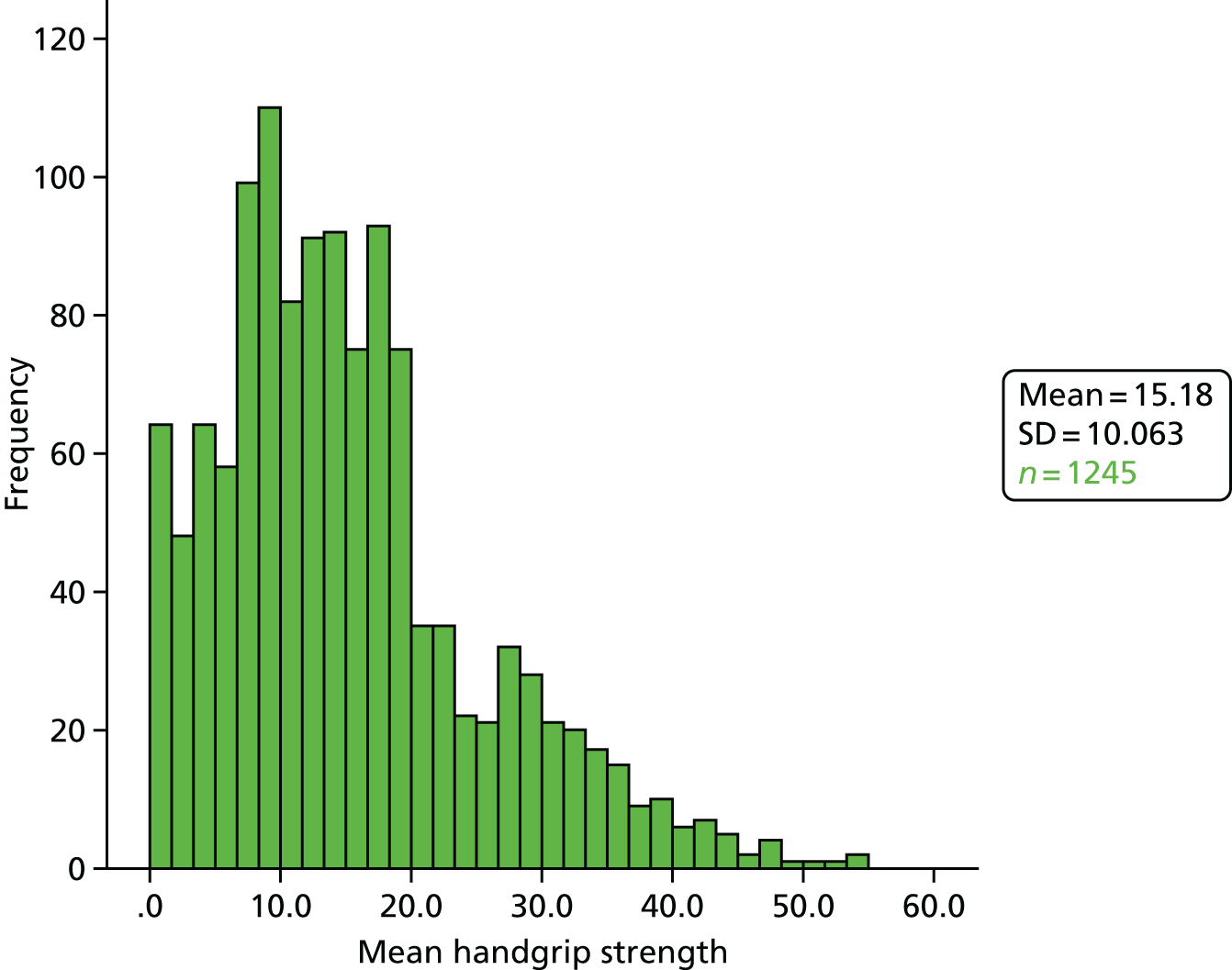
Data on functional reach were not available for 40 individuals, with reasons recorded for 39 of these (four participants refused; one participant could not understand the instructions; in sixteen cases the participant felt unsafe, and in five cases the RA felt that it would be unsafe; in five cases the participant attempted the test but could not hold the position; in five cases, the participant attempted the test but failed; other reasons were given for three participants). Among those completing the test, scores averaged over three attempts ranged from 1.3 cm to 51.1 cm, with a mean of 22.8 cm (Figure 14), with > 22 cm representing the average normal value for older patients. 92
FIGURE 14.
Distribution of mean functional reach (cm) at baseline.
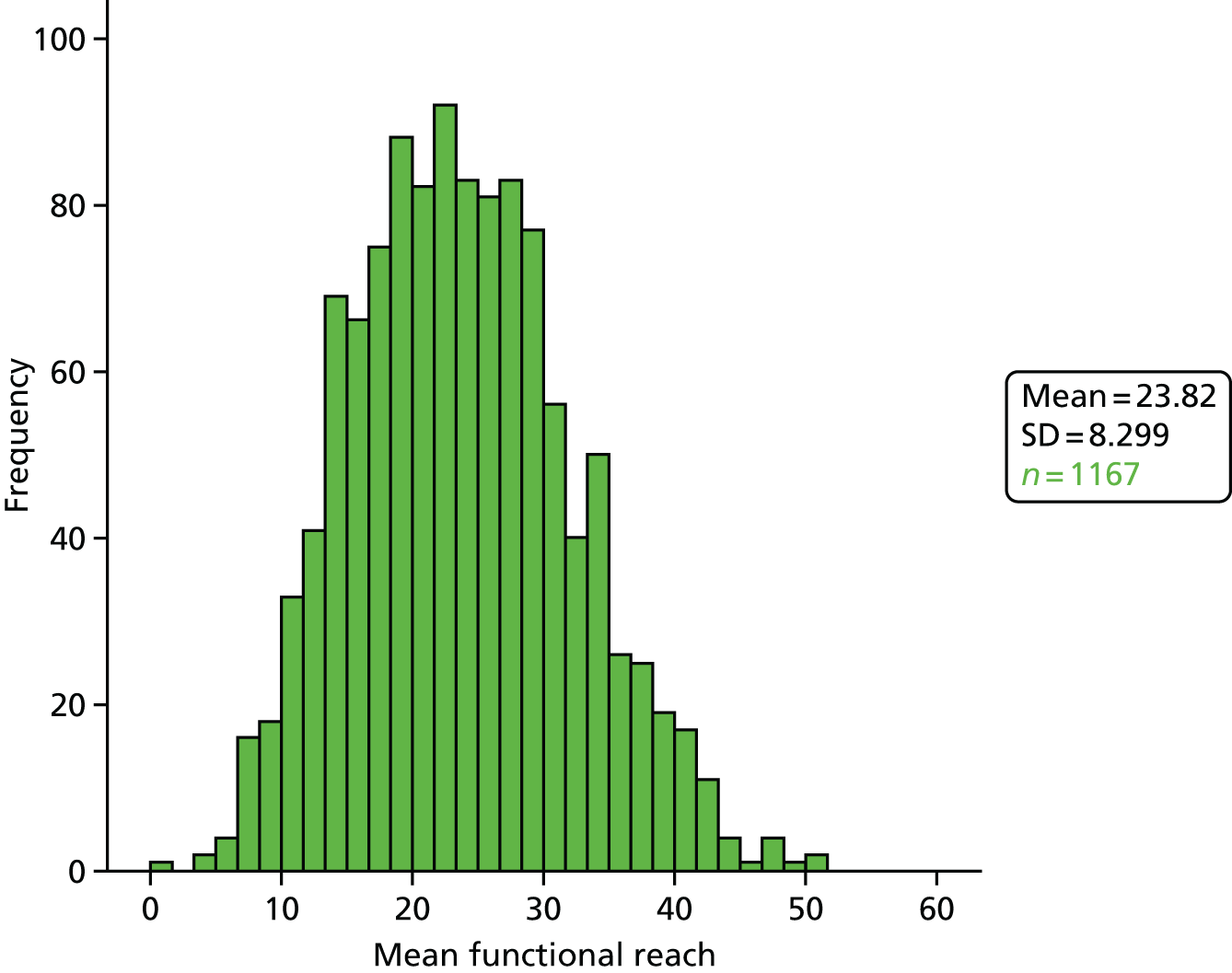
Self-completion questionnaires
Baseline self-completion questionnaires were returned by 323 participants (77.8%). A minority of respondents (20.8%, 95% CI 16.5% to 25.5%) reported that they had help completing the questionnaire; the percentage requiring assistance was similar across the two arms (Table 15).
| Help provider | Control | Intervention | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| RA | 24 | 14.2 | 25 | 14.5 | 49 | 14.3 |
| Friends/family | 9 | 5.3 | 13 | 7.5 | 22 | 6.4 |
| None/not stated | 136 | 80.5 | 135 | 78.0 | 271 | 79.2 |
| Total | 169 | 100 | 173 | 100 | 342 | 100 |
Lubben Social Network Scale–6
Lubben Social Network Scale scores ranged from 0 to 30, with a mean of 14.69 (Figure 15). Internal consistency of this scale was adequate, with a Cronbach’s α = 0.83.
FIGURE 15.
Distribution of LSNS-6 scores at baseline.

European Quality of Life–5 Dimensions (5 Level)
The EQ-5D (5L) ratings of ‘Health today’ ranged from 5 to 100, with a mean of 58.6 (median 60, mode 50; Figure 16). Applying UK tariff values, EQ-5D (5L) ‘Index values’ ranged from –0.20 (a state worse than death) to 1.0 (perfect health), with a mean of 0.51 (median 0.56; Figure 17). Internal consistency of the five items in this scale was adequate, with Cronbach’s α = 0.73.
FIGURE 16.
Distribution of EQ-5D (5L) ratings of ‘health today’ at baseline.
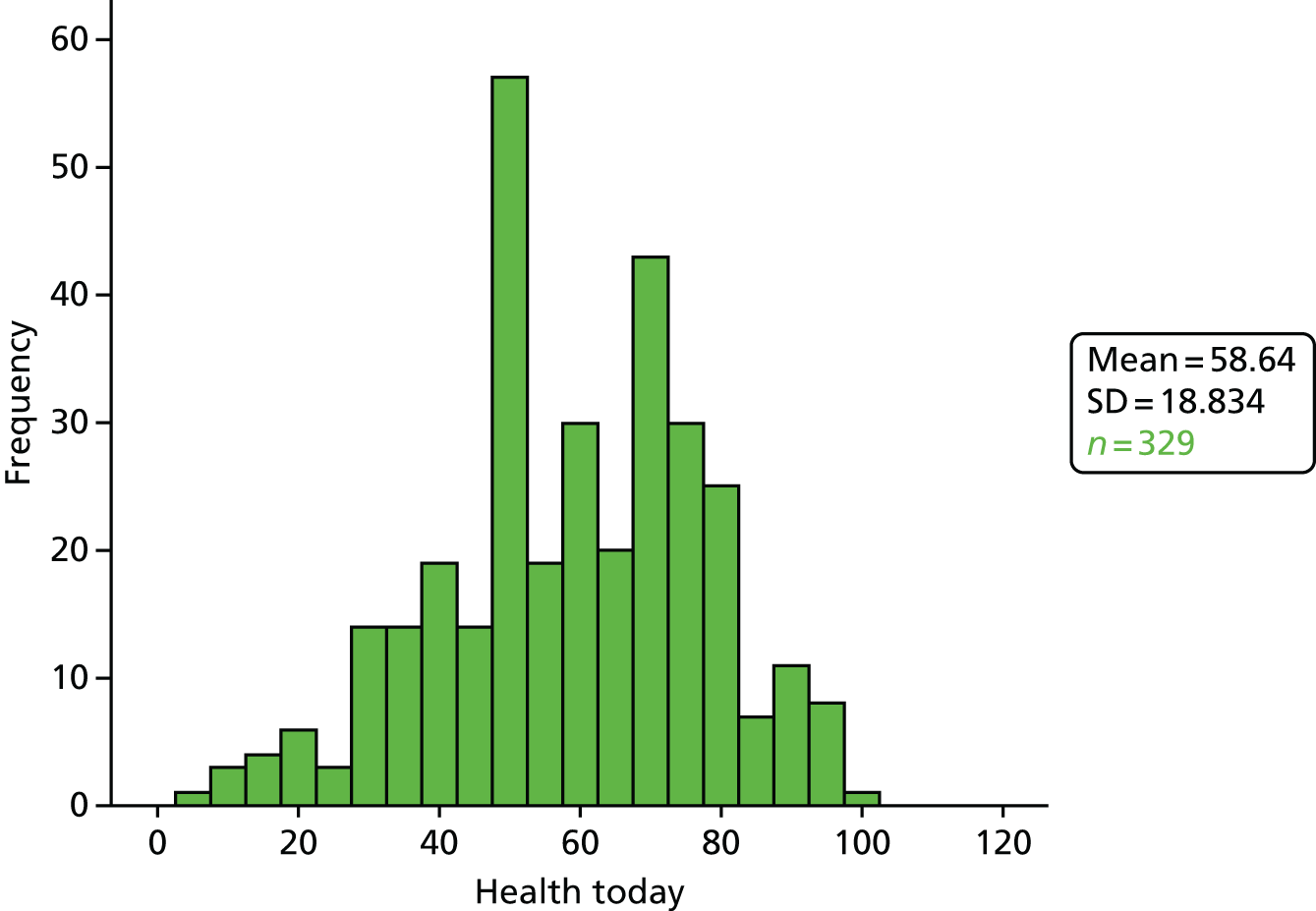
FIGURE 17.
Distribution of EQ-5D (5L) index values at baseline.
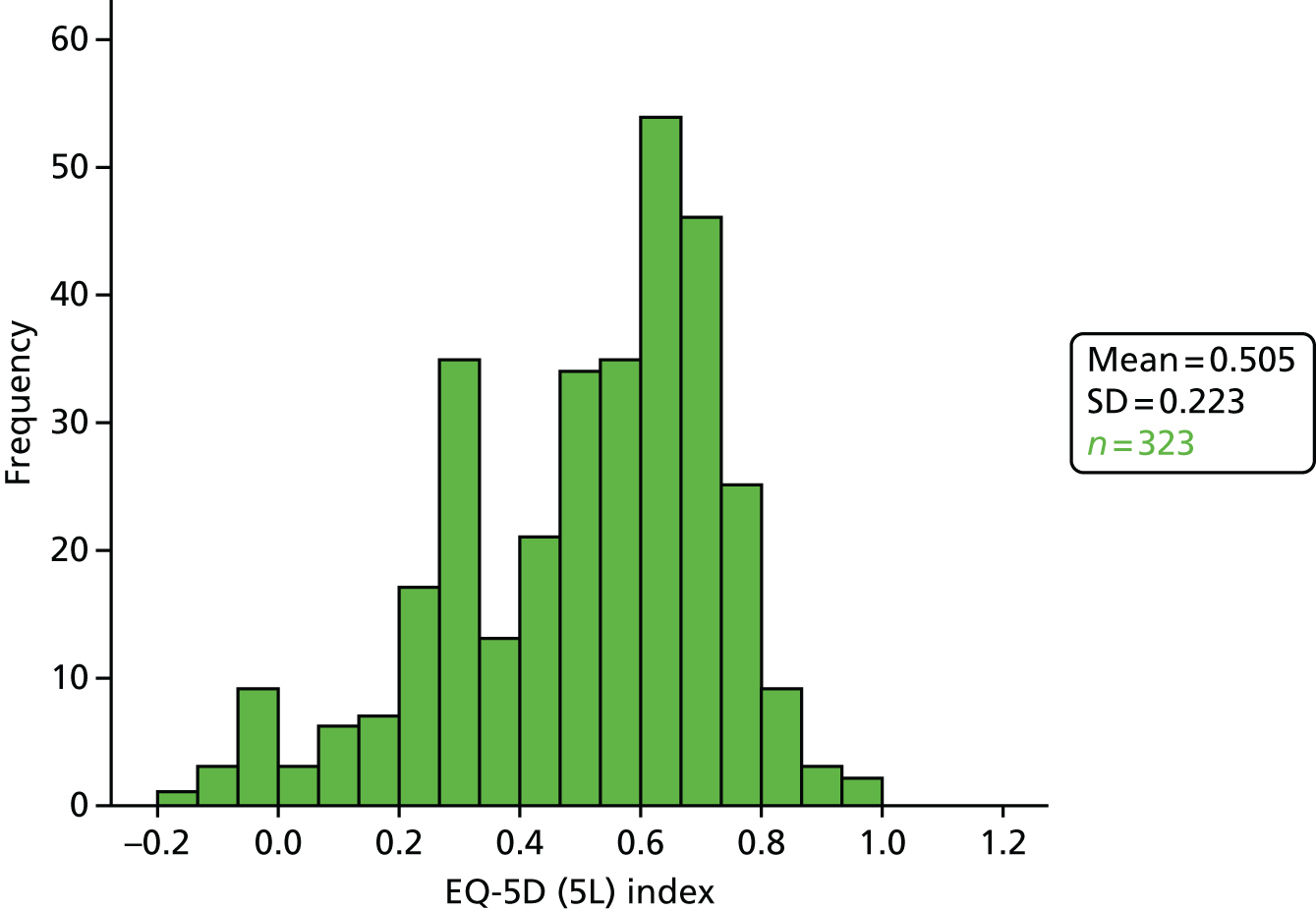
Hospital Anxiety and Depression Scale
The HADS Anxiety scores ranged from 0 to 21, with a mean of 8.2 (median 8.0, mode 7.0; Figure 18). Scores of ≤ 7 are considered ‘normal’ and 45.5% of participants had scores in this range at baseline; percentages with ‘mild’ (8–10), ‘moderate’ (11–14) and ‘severe’ anxiety were 23.7%, 23.9% and 6.9%, respectively. HADS Depression scores ranged from 0 to 17, with a mean of 6.8 (median and mode both 7.0; Figure 19). Scores of ≤ 7 are considered ‘normal’ and 62.8% of participants had scores in this range at baseline; percentages with ‘mild’ (8–10), ‘moderate’ (11–14) and ‘severe’ depression were 22.1%, 12.6% and 2.5%, respectively. Internal consistency of both scales was adequate, with Cronbach’s α = 0.85 for HADS Anxiety and 0.77 for HADS Depression.
FIGURE 18.
Distribution of HADS Anxiety scores at baseline.

FIGURE 19.
Distribution of HADS Depression scores at baseline.
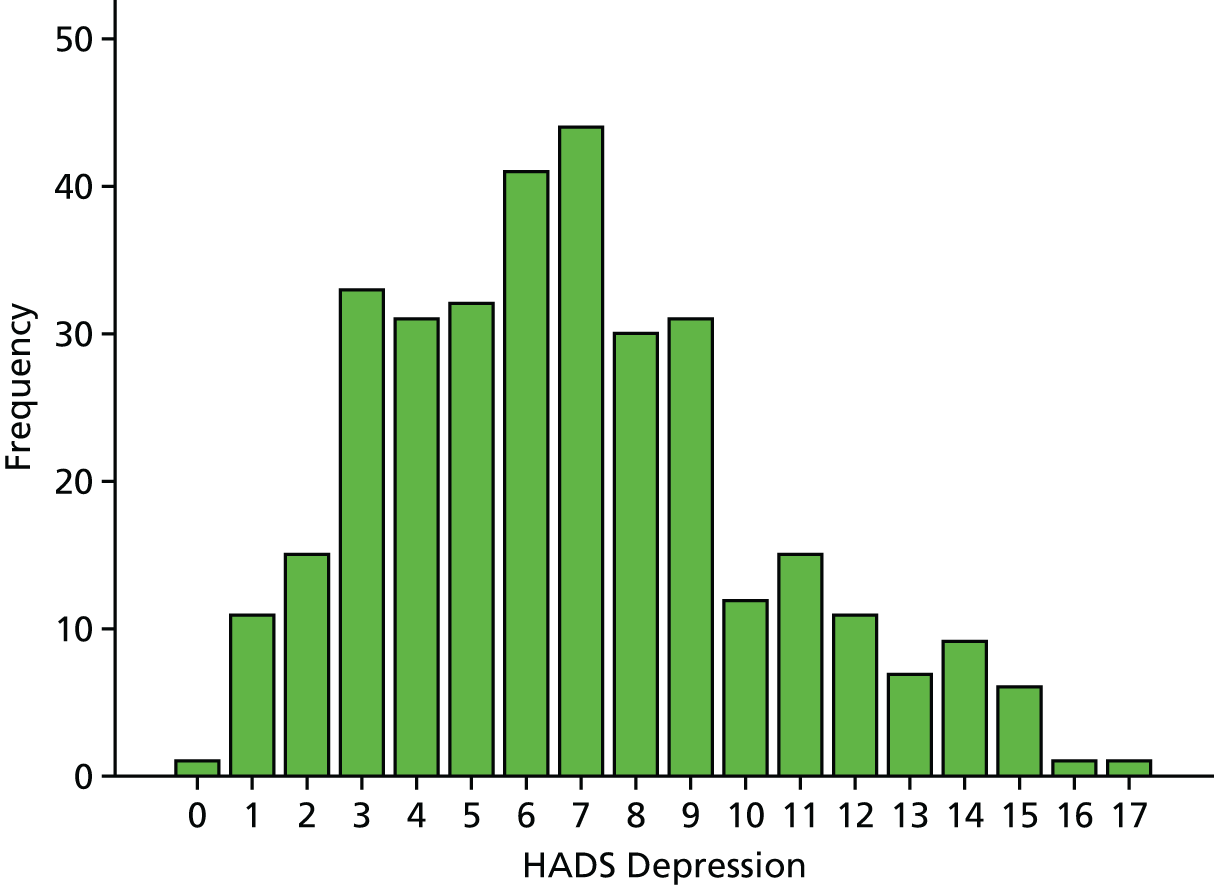
World Health Organization Quality of Life questionnaire – older adults module
The WHOQOL-OLD total scores ranged from 8.3 to 88.5 with a mean of 57.2 (Figure 20). Internal consistency for the whole scale was adequate, with a Cronbach’s α = 0.86. Cronbach’s alpha for the individual subscales ranged from 0.66 for ‘Autonomy’ to 0.95 for ‘Intimacy’.
FIGURE 20.
Distribution of WHOQOL-OLD scores at baseline.
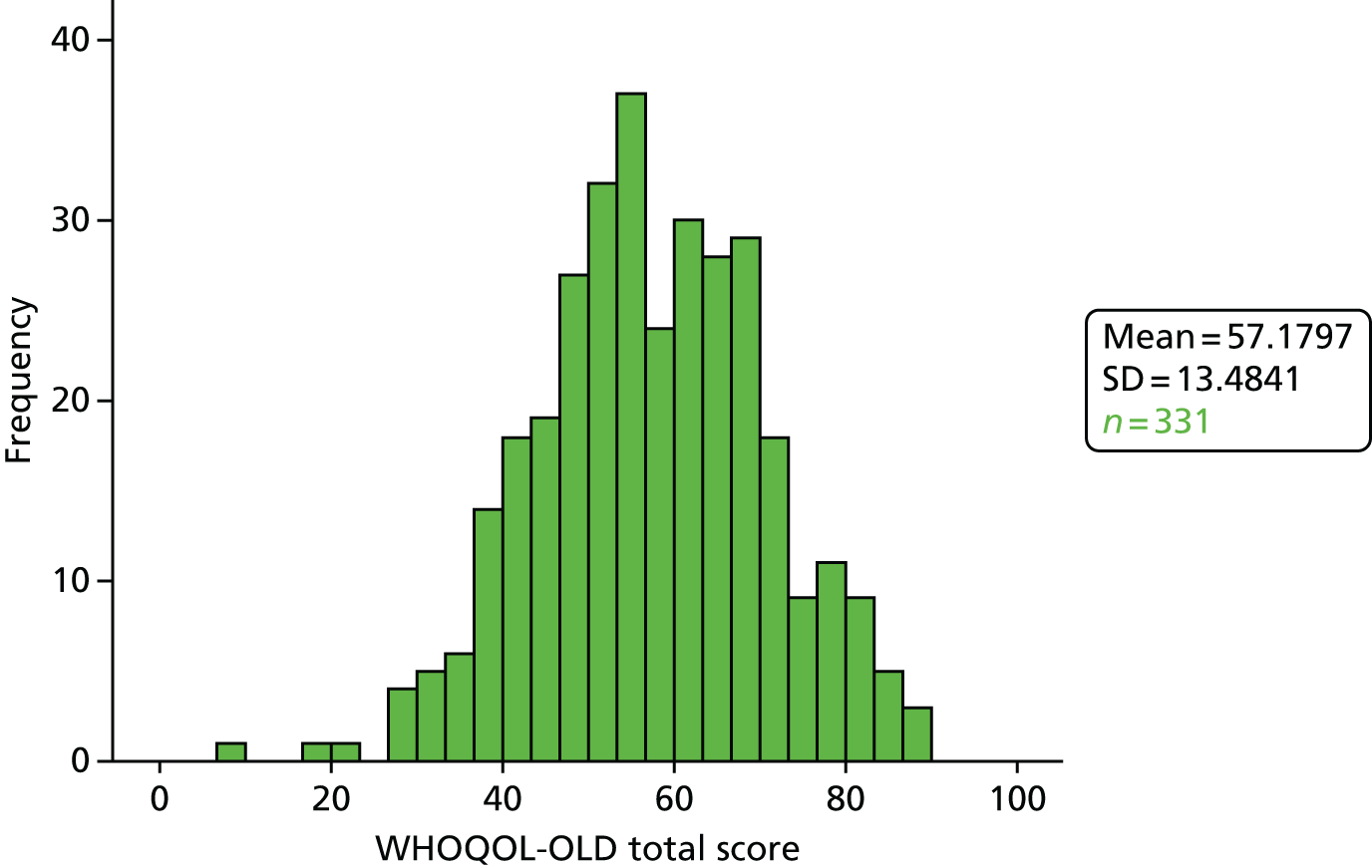
Short Form questionnaire-36 items
Internal consistency of the seven multi-item domain scores was adequate, with Cronbach’s alphas ranging from 0.72 (General Health Perception) to 0.93 (Role-Emotional). SF-36 Physical Component Scores (PCSs) ranged from 8.4 to 63.8 with a mean of 32.1 (Figure 21). SF-36 Mental Component Scores (MCSs) ranged from 17.2 to 69.7, with a mean of 45.2 (Figure 22). PCSs and MCSs are norm based, with a mean of 50 and SD of 10 in a general population. The observed mean scores for this study population indicate poor physical health relative to a general population, with the mean score almost two SDs below the norm of 50. MCSs were also below the norm, but to a lesser extent.
FIGURE 21.
Distribution of SF-36 PCSs at baseline.

FIGURE 22.
Distribution of SF-36 MCSs at baseline.

Baseline comparability
The study arms were well balanced at baseline (Table 16).
| Outcome | Control | CBTI | Combined sample | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | µ | σ | n | µ | σ | n | µ | 95% CI | σ | 95% CI | |
| Age, years | 205 | 75.3 | 8.6 | 210 | 75.8 | 8.5 | 415 | 75.5 | 74.7 to 76.4 | 8.55 | 8.14 to 8.94 |
| FES-I score | 205 | 40.9 | 9.9 | 210 | 39.8 | 8.9 | 415 | 40.3 | 39.4 to 41.2 | 9.37 | 8.83 to 9.88 |
| Fear of falling 11-point scale | 205 | 6.7 | 2.1 | 210 | 6.7 | 2.3 | 415 | 6.7 | 6.5 to 6.9 | 2.17 | 2.02 to 2.31 |
| Total social participation/isolation score | 205 | 9.4 | 7.5 | 210 | 9.6 | 7.9 | 415 | 9.5 | 8.8 to 10.3 | 7.69 | 7.12 to 8.33 |
| Gait and balance score | 203 | 6.0 | 3.0 | 208 | 5.8 | 2.9 | 411 | 5.9 | 5.6 to 6.2 | 2.96 | 2.78 to 3.10 |
| Mean handgrip strength | 200 | 15.9 | 9.5 | 202 | 17.1 | 10.6 | 402 | 16.5 | 15.5 to 17.5 | 10.08 | 9.28 to 10.91 |
| Mean functional reach | 186 | 22.6 | 8.5 | 189 | 23.0 | 8.3 | 375 | 22.8 | 21.9 to 23.6 | 8.40 | 7.81 to 8.99 |
| HADS Anxiety | 167 | 8.4 | 4.3 | 171 | 8.0 | 3.9 | 338 | 8.2 | 7.8 to 8.7 | 4.13 | 3.85 to 4.41 |
| HADS Depression | 167 | 6.7 | 3.5 | 172 | 7.0 | 3.3 | 339 | 6.9 | 6.5 to 7.2 | 3.41 | 3.16 to 3.64 |
| WHOQOL-OLD total score | 166 | 56.9 | 14.1 | 173 | 57.4 | 12.9 | 339 | 57.1 | 55.7 to 58.6 | 13.47 | 12.43 to 14.45 |
| LSNS-6 | 167 | 14.6 | 5.4 | 172 | 14.9 | 5.8 | 339 | 14.7 | 14.1 to 15.3 | 5.63 | 5.25 to 5.98 |
| EQ-5D (5L) Health today | 166 | 57.6 | 19.7 | 171 | 59.6 | 17.6 | 337 | 58.6 | 56.7 to 60.7 | 18.70 | 17.34 to 20.15 |
| EQ-5D (5L) Index | 165 | 0.50 | 0.23 | 166 | 0.51 | 0.21 | 331 | 0.50 | 0.48 to 0.53 | 0.22 | 0.20 to 0.24 |
| SF-36 PCS | 157 | 32.3 | 9.1 | 164 | 31.9 | 8.0 | 321 | 32.1 | 31.1 to 33.0 | 8.5 | 7.7 to 9.3 |
| SF-36 MCS | 157 | 45.3 | 10.6 | 164 | 45.0 | 10.5 | 321 | 45.2 | 44.0 to 46.4 | 10.5 | 9.7 to 11.2 |
The relationship between baseline scores on the FES-I and stratification variables was examined (see Appendix 4 for detailed graphs and tables). In general, the difference in mean scores between the strata across a range of health status and quality-of-life measures was fairly small.
The FES-I scores at baseline were higher at the Galleries Day Unit (indicating that participants have a higher level of concerns about falling) than at the other two sites (one-way analysis of variance indicated that differences between sites was statistically significant (F2,412 = 8.69; p < 0.001). Similarly, numerical ratings of fear of falling appeared to differ between sites. The mean fear of falling 11-point numerical rating scores were 6.64 for participants recruited by the Falls and Syncope Service, 7.62 for participants recruited by the Galleries Day Unit, and 6.51 for participants recruited by the North Tyneside Falls Prevention Service; patients from the Galleries Day Unit reported a higher fear of falling per the 11-point scale than patients from the other two sites, a finding consistent with that reported for FES-I scores above. This difference between sites persisted even on allowing for a difference between sites in the proportion of patients reporting pain when walking. For other measures, participants recruited at the Galleries Day Unit tended to have poorer health status and reported poorer quality of life at baseline than patients from the other two sites.
The FES-I scores at baseline were similar for men and women. As expected, men were observed to have greater handgrip strength and greater functional reach than women. Otherwise, differences between men and women were fairly small.
There was a strong positive association between pain on walking and FES-I score at baseline. Scores on the fear of falling numerical rating scale were also positively associated with pain when walking at baseline. The mean fear of falling rating scale scores were 5.99 for those with no pain when walking and 7.42 for those reporting pain when walking, a difference of 1.43 (95% CI 1.03 to 1.83); participants who suffered pain with walking reported significantly greater fear of falling than other participants. Of all of the four randomisation strata ‘pain when walking’ was most highly correlated with the health status of the participant; those who reported pain when walking tended to report poorer health status and quality of life.
As already noted, referral for strength and balance training was confounded with site. There was no evidence of an association between FES-I score at baseline and referral for strength and balance training. Patients who were referred for strength and balance training actually had higher handgrip strength and greater functional reach than those who were not referred. Other outcome measures were not associated with referral for strength and balance training.
Numbers analysed
Table 17 shows the rate of data completion at each time point.
| Visit | RA interview | FES-I questionnaire | Self-completion questionnaire | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | CBTi | Control | CBTi | Control | CBTi | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Baseline | 205 | 100 | 210 | 100 | 205 | 100 | 210 | 100 | 169 | 82.4 | 173 | 82.4 |
| 8 weeks | 180 | 87.8 | 164 | 78.1 | 180 | 87.8 | 164 | 78.1 | 155 | 75.6 | 146 | 69.5 |
| 6 months | 161 | 78.5 | 151 | 71.9 | 163 | 79.5 | 151 | 71.9 | 138 | 67.3 | 114a | 54.3 |
| 1 year | 163 | 79.5 | 151 | 71.9 | 163 | 79.5 | 151 | 71.9 | 145 | 70.7 | 141 | 67.1 |
Falls Efficacy Scale–International version completion
The figures in the green boxes indicate the number of participants who completed the FES-I sufficiently to allow it to be scored; the figures in the unshaded boxes indicate the number of participants for whom no FES-I items have been scored. We have responses at all four visits for 297 participants (Figure 23).
FIGURE 23.
Number of participants visited by RA and asked to complete the FES-I.

Attrition was slightly greater in the group that was randomised to CBTi (Table 18).
| Visit | Study arm | Relative difference between study arms | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Control | CBTi | |||||||
| n | %a | n | %a | RR | 95% CI | n | %a | |
| Baseline | 205 | 210 | 415 | |||||
| 8 weeks | 180 | 87.8 | 164 | 78.1 | 0.89 | 0.82 to 0.97 | 344 | 82.9 |
| 6 months | 163 | 79.5 | 151 | 71.9 | 0.90 | 0.81 to 1.01 | 314 | 75.7 |
| 1 year | 163 | 79.5 | 151 | 71.9 | 0.90 | 0.81 to 1.01 | 314 | 75.7 |
Completion of falls diaries
The majority of diaries (3288/3309) were completed, as anticipated, on paper. The number of diaries that were returned fell over the period of follow-up. In general, participants in the control arm were better at completing and returning falls diaries than participants who were randomised to CBTi (Table 19).
| Month | Proportion of sample providing data (%) | Risk ratio | ||||
|---|---|---|---|---|---|---|
| Control | CBTi | Difference | 95% CI | Risk ratio | 95% CI | |
| 1 | 75.1 | 71.4 | –3.7 | –12.2 to 4.8 | 0.95 | 0.85 to 1.07 |
| 2 | 76.1 | 63.3 | –12.8 | –21.5 to –4.0 | 0.83 | 0.73 to 0.95 |
| 3 | 72.7 | 64.3 | –8.4 | –17.3 to 0.5 | 0.88 | 0.78 to 1.01 |
| 4 | 66.8 | 58.6 | –8.3 | –17.5 to 1.0 | 0.88 | 0.76 to 1.02 |
| 5 | 64.9 | 57.1 | –7.7 | –17.1 to 1.6 | 0.88 | 0.76 to 1.03 |
| 6 | 66.3 | 59.0 | –7.3 | –16.6 to 2.0 | 0.89 | 0.77 to 1.03 |
| 7 | 66.8 | 56.2 | –10.6 | –19.9 to –1.3 | 0.84 | 0.72 to 0.98 |
| 8 | 67.3 | 55.2 | –12.1 | –21.4 to –2.8 | 0.82 | 0.70 to 0.96 |
| 9 | 63.9 | 53.3 | –10.6 | –20.0 to –1.1 | 0.84 | 0.71 to 0.98 |
| 10 | 63.9 | 54.3 | –9.6 | –19.0 to –0.2 | 0.85 | 0.72 to 1.00 |
| 11 | 62.9 | 51.9 | –11.0 | –20.5 to –1.6 | 0.83 | 0.70 to 0.98 |
| 12 | 59.5 | 50.5 | –9.0 | –18.6 to 0.5 | 0.85 | 0.71 to 1.01 |
| 13 | 50.7 | 43.8 | –6.9 | –16.5 to 2.7 | 0.86 | 0.70 to 1.06 |
Sixty-eight participants (16.4%) returned no falls diaries at all (Figure 24); 106 participants (25.5%) returned all 13 diaries. The mean number of diaries returned was 7.97 (95% CI 7.44 to 8.49). On average, participants randomised to the CBTi returned 1.18 (95% CI 0.20 to 2.11) fewer diaries than participants in the control arm.
FIGURE 24.
Distribution of the number of diaries returned.

Delivery of the cognitive–behavioural therapy intervention
One hundred and thirty-nine (66.2%) participants who were randomised to the CBTi completed nine sessions of therapy (Table 20). Fifty-six (26.7%) participants either failed to complete any sessions or withdrew after the first one or two sessions. Participants from the North Tyneside Falls Prevention Service were significantly more likely (76.7%) to complete nine sessions of therapy than those from the Falls and Syncope Service (52.4%) or the Galleries Day Unit (51.9%) (χ2 = 13.73; p = 0.001). A total of five patients received the initial eight sessions of CBTi but withdrew prior to the 6-month top-up session.
| Number | Falls and Syncope Service | Galleries Day Unit | North Tyneside Falls Prevention Service | Total | % |
|---|---|---|---|---|---|
| 0 | 8 | 10 | 9 | 27 | 12.9 |
| 1 | 7 | 1 | 10 | 18 | 8.6 |
| 2 | 6 | 1 | 4 | 11 | 5.2 |
| 3 | 1 | 0 | 0 | 1 | 0.5 |
| 4 | 2 | 0 | 0 | 2 | 1.0 |
| 5 | 3 | 0 | 3 | 6 | 2.9 |
| 7 | 0 | 1 | 0 | 1 | 0.5 |
| 8 | 3 | 0 | 2 | 5 | 2.4 |
| 9 | 33 | 14 | 92 | 139 | 66.2 |
| Total | 63 | 27 | 120 | 210 | 100.0 |
Case load varied across HCAs, with one (full-time) HCA seeing 47.1% of participants, and the other two (part-time) HCAs seeing 27.1% and 25.6%, respectively.
Scheduling issues, particularly participant availability, caused delays between randomisation and delivery of the first session of CBTi (Figure 25). For similar reasons, only 33% of participants completed the initial course of eight sessions within 8 weeks; 79% completed within 12 weeks, but for a minority of participants the initial course extended over a longer period, to a maximum of 27 weeks (Figure 26). The mean duration of therapy was just under 11 weeks. Elapsed time from the eighth visit to the ‘6-month’ top-up session ranged from 21.4 to 44.4 weeks, with a mean of 27.2 weeks.
FIGURE 25.
Days from randomisation to first CBTi session.

FIGURE 26.
Duration of initial eight sessions of CBTi.
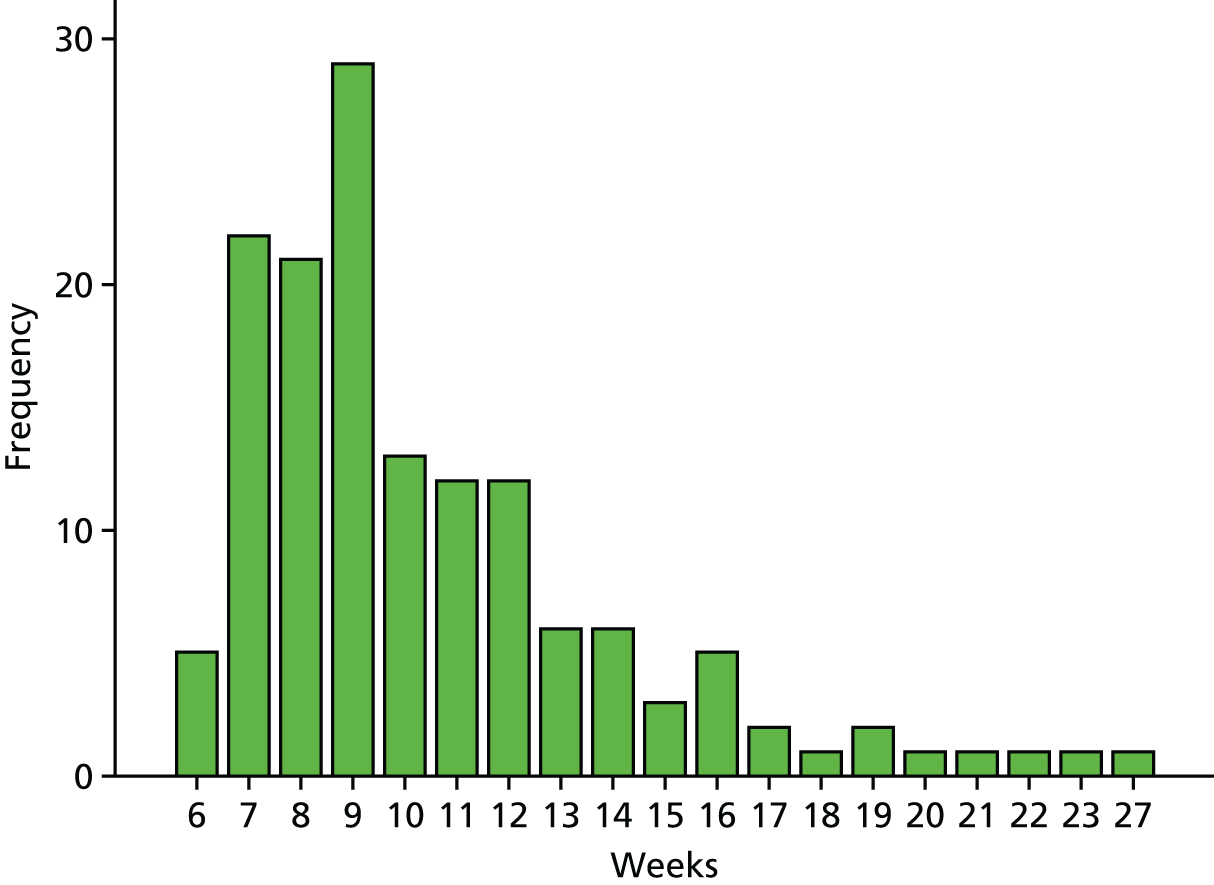
A smaller proportion of those patients who, at baseline, reported pain when walking completed the intervention (Table 21) although the 95% CI for the relative risk included one (RR of completion = 0.84, 95% CI 0.69, 1.02).
| Completed nine sessions of CBTi? | Pain when walking? | Total | ||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| n | % | n | % | n | % | |
| No | 31 | 28.2 | 40 | 40.0 | 71 | 33.8 |
| Yes | 79 | 71.8 | 60 | 60.0 | 139 | 66.2 |
| Total | 110 | 100 | 210 | |||
Participants who did not complete their course of CBTi had lower FES-I scores at baseline, indicative of fewer concerns about falling (Table 22).
| Completed nine sessions of CBTi? | Difference | ||||
|---|---|---|---|---|---|
| No | Yes | ||||
| µ | 95% CI | µ | 95% CI | µ | 95% CI |
| 41.7 | 39.6 to 43.8 | 38.8 | 37.4 to 40.3 | –2.97 | –5.47 to –0.50 |
Participants who withdrew early from CBTi were more likely to be lost to follow-up for data collection (Table 23); only 14 (19.7%) of the 71 participants who did not complete all nine sessions of CBTi provided data on the primary end point at 12 months.
| Visit | Control | Intervention | ||||||
|---|---|---|---|---|---|---|---|---|
| Completed nine sessions of CBTi? | Total | |||||||
| No | Yes | |||||||
| n | % | n | % | n | % | n | % | |
| Baseline | 205 | 100.0 | 71 | 100.0 | 139 | 100.0 | 210 | 100.0 |
| 8 weeks | 180 | 87.8 | 31 | 43.7 | 133 | 95.7 | 164 | 78.1 |
| 6 months | 162 | 79.0 | 17 | 23.9 | 134 | 96.4 | 151 | 71.9 |
| 1 year | 162 | 79.0 | 14 | 19.7 | 137 | 98.6 | 151 | 71.9 |
Timing of randomisation and follow-up data collection relative to cognitive–behavioural therapy intervention delivery
Eighty-five per cent (95% CI 81.2% to 88.4%) of participants were randomised on day of the baseline assessment. The week 8 visit took place within 44 and 120 days of randomisation. For those randomised to the CBTi, the interval from the eighth CBTi session to the week 8 visit could be calculated for 137 individuals (in the remaining instances, one or both dates were missing either because the participant had withdrawn from the CBTi or did not have a week 8 visit, or both). In 124 (90.5%) of these cases, the week 8 visit took place on or before the date of the eighth CBTi session; the delay ranged from zero (one participant) to 141 days, with a mean of 31 days, median of 26 days.
Although there was a wide visit window observed at each follow-up point the difference in the mean time between date of randomisation and date of visit between control and intervention participants was not statistically significant (Table 24). At 1 year, the mean difference in timing of ascertainment of primary end point, vis-à-vis randomisation date, was 1.9 (95% CI –3.7 to 6.8) days.
| Visit | Study arm | Difference | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | CBTi | |||||||
| n | µ | 95% CI | n | µ | 95% CI | µ | 95% CI | |
| 8 week visit | 180 | 59.2 | 57.6 to 61.0 | 164 | 59.6 | 58.1 to 61.1 | 0.4 | –1.8 to 2.7 |
| 6 month visit | 163 | 196.0 | 192.3 to 199.5 | 151 | 195.4 | 192.7 to 198.5 | –0.5 | –4.7 to 4.2 |
| 1 year visit | 163 | 371.8 | 368.7 to 375.6 | 151 | 373.7 | 369.9 to 377.4 | 1.9 | –3.7 to 6.8 |
Primary outcome: Falls Efficacy Scale–International version
The primary outcome was the total FES-I score at 12 months.
The number of missing responses on each FES-I questionnaire was calculated. A total of 1387 questionnaires were completed across all time points. All 16 items were completed for 1310 [94.4% (95% CI 93.1 to 95.6%)] of FES-I questionnaires. The number of missing items ranged from 1 to 11, and the rate of missing items did not vary between the arms of the trial. Following the developers’ instructions, the total FES-I score was imputed provided that there were fewer than five missing items. Consequently, data imputation was undertaken for a total of 95 items spread across 75 questionnaires. A total score could not be determined for 2 of the 1387 questionnaires.
The distribution of FES-I scores at each time point is displayed graphically in Figure 27. The upper and lower edges of each box correspond to the 75th and 25th percentiles, respectively; the horizontal line through the centre corresponds to the median, and the whiskers show the upper and lower limits of the distribution. The median values decrease over time (corresponding to reduced concern about falling) and the difference between study arm median values appears to get larger.
FIGURE 27.
Box-and-whisker plots of FES-I scores by visit by study arm.

Analysis of Falls Efficacy Scale–International version scores at 1 year (the primary end point)
The difference between FES-I at the end of treatment (12 months) and FES-I prior to treatment (baseline) in two arms was analysed using analysis of covariance. The dependent variable was the FES-I score at 12 months; baseline FES-I was included as a covariate. Stratification variables (site, gender, baseline pain when walking and referral for strength and balance training) were included as fixed effects.
This was achieved by fitting a simple linear regression model. The regression coefficients are given in Table 25. FES-I at 1 year was highly correlated with baseline FES-I.
| Variable | Regression coefficients | Significance | ||
|---|---|---|---|---|
| B | 95% CI | t | p-value | |
| Constant | 9.26 | 3.33 to 15.20 | 3.07 | < 0.001 |
| Baseline FES-I | 0.74 | 0.62 to 0.85 | 12.79 | < 0.001 |
| Strataa | ||||
| Galleries Day Unit | 4.46 | 0.75 to 8.17 | 2.36 | 0.02 |
| North Tyneside Falls Prevention Service | –0.70 | –3.25 to 1.85 | –0.54 | 0.59 |
| Presence of pain when walking | 2.78 | 0.75 to 4.81 | 2.69 | 0.01 |
| Referred for strength and balance training | –2.83 | –5.28 to –0.37 | –2.26 | 0.02 |
| Gender (female) | 0.00 | –2.13 to 2.14 | 0.00 | 1.00 |
| Impact of CBT (study arm) | –4.02 | –5.95 to –2.10 | –4.11 | < 0.001 |
There were significant differences between sites, with participants at the Galleries Day Unit having higher FES-I scores (reflecting poorer falls-related self-efficacy and fear of falling) than participants from the other two sites. Participants who reported pain when walking had higher FES-I scores, whereas participants who had been referred for strength and balance training had lower FES-I scores. The difference between men and women was not significant. Finally, the difference between study arms was statistically significant; the estimated impact of CBTi on FES-I scores was a reduction in mean score of 4.02 (95% CI 2.10 to 5.95).
The baseline SD of FES-I was 9.37. The reduction in mean score of 4.02 can be expressed as a standardised effect size by dividing by this SD, yielding a point estimate of effect size of 0.43 and an interval estimate of between 0.22 and 0.64.
The change in R2 on adding the effect of therapy to the model including baseline FES-I scores and the effects corresponding to each of the strata is shown in Table 26. R2 increased from 0.45 to 0.48.
| Model | R 2 | Adjusted R2 | Change statistics | ||||
|---|---|---|---|---|---|---|---|
| R2 change | F change | df 1 | df 2 | Significant F change | |||
| 1 | 0.447 | 0.436 | 0.447 | 41.146 | 6 | 306 | < 0.001 |
| 2 | 0.476 | 0.464 | 0.029 | 16.877 | 1 | 305 | < 0.001 |
Plots of standardised residuals suggest assumption of a normal error distribution for the residuals is reasonable.
Impact of non-response on primary outcome measure
As previously described (see Chapter 3), multiple imputation was used to impute missing values for the FES-I score at each of the follow-up time points, in those cases where the instrument was not completed at all, or could not be scored. A pooled estimate of the impact of the intervention at 1 year across data sets was based on an analysis of covariance model with adjustments for baseline FES and randomisation strata. The parameter estimates are given in Table 27.
| Method | Imputation model | |||
|---|---|---|---|---|
| Regression model | Multivariate normal model | |||
| µ | 95% CI | µ | 95% CI | |
| By arm | –3.84 | –6.52 to –1.17 | –2.89 | –5.21 to –0.57 |
| Complete sample | –3.05 | –5.32 to –0.77 | –2.51 | –4.76 to –0.27 |
Adjusting for missing data reduces the ITT estimate of the effect of the CBTi; all methods of data imputation result in a smaller estimated impact of the CBTi on fear of falling. This is consistent with the observations that (1) those patients who withdrew from the CBTi arm had lower FES-I scores at baseline and (2) in the analysis of imputed data we have a much greater proportion of patients in the CBTi arm who did not receive the intervention than in the complete case analysis. Point estimates of standardised effect sizes, adjusting for differential loss to follow-up, range from 0.27 to 0.41.
For comparison purposes we can undertake an ‘as treated’ analysis using the imputed data sets. Here we replace the study arm variable with a CBTi variable that ranges from 0 (no sessions of CBTi) to 1 (nine sessions of CBTi). This yields an estimate of the effect of the CBTi for those patients who received nine sessions (Table 28).
| Method | Imputation model | |||
|---|---|---|---|---|
| Regression model | Multivariate normal model | |||
| µ | 95% CI | µ | 95% CI | |
| By arm | –3.65 | –5.84 to –1.46 | –4.19 | –6.32 to –2.06 |
| Complete sample | –4.27 | –6.45 to –2.09 | –4.37 | –6.52 to –2.22 |
The ‘as treated’ analysis suggests a mean improvement in FES-I score of approximately ‘4’ for those patients who complete the CBTi. The results of the ‘as treated’ analysis are very similar to those obtained from the ‘complete case’ analysis, which, perhaps, reflects the poor outcome completion rate among those failing to complete the planned CBTi. Point estimates of standardised effect sizes for this ‘as treated’ analysis range from 0.39 to 0.47. These results are consistent with there being a subgroup of patients who benefit from the CBTi but when we analyse on an ITT basis the average impact across the target population is somewhat reduced (from around a mean change of ‘4’ to a mean change of ‘3’ in FES-I score).
Longitudinal analysis of Falls Efficacy Scale–International version
To provide a more detailed picture of how FES-I scores changed over time, a further analysis, utilising data from all four time points (baseline, 8 weeks, 6 months and 12 months) was conducted. The distribution of time to completion is shown in Figure 28.
FIGURE 28.
Time to completion of FES-I.
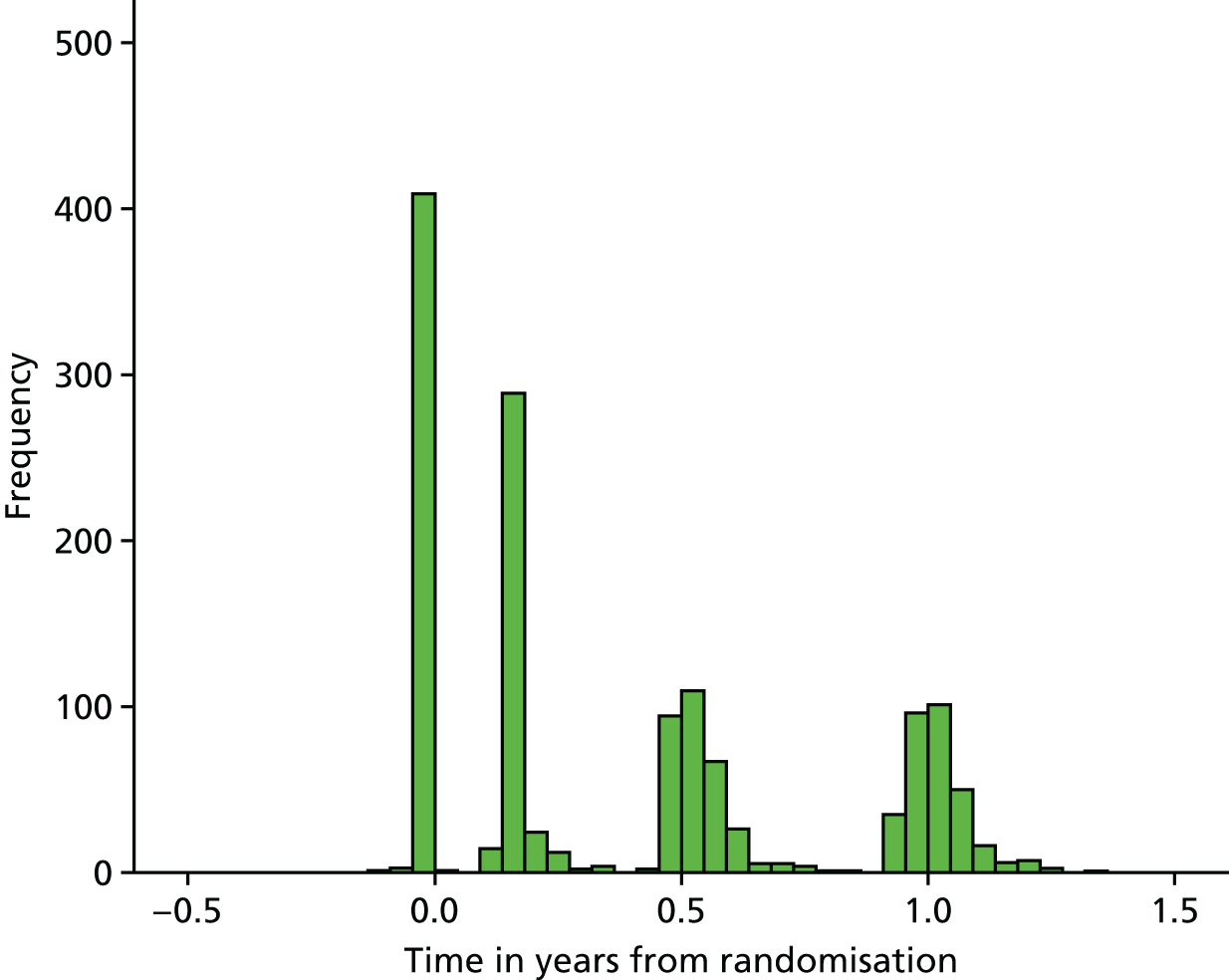
The four distinct CBTis of bars correspond to visits at baseline and 8 weeks, 6 months and 1 year post randomisation. Time in years was included in this longitudinal analysis as a continuous variable.
-
Scores at the follow-up visits were analysed using a mixed-model approach. All models included two random effects, variation between:
-
participants
-
follow-up visits within participants.
-
Model 1 included the following fixed effects:
-
baseline FES-I scores
-
pain when walking (stratification variable, binary indicator)
-
referral for strength and balance training (stratification variable, binary indicator)
-
gender (stratification variable, binary indicator)
-
site (two binary indicators: Galleries Day Unit and North Tyneside Falls Prevention Service)
-
trend over time
-
study arm (an impact of the CBTi).
Additionally, model 2 included an interaction between study arm and time (equivalently to fitting a separate trend over time for intervention and control patients).
The parameter estimates corresponding to model 1 are given in Table 29.
| Effect | Coefficient | Test of difference from zero | 95% CI | |
|---|---|---|---|---|
| z | p-value | |||
| Constant | 9.32 | 4.14 | 0.000 | 4.91 to 13.74 |
| Baseline FES-I | 0.71 | 16.82 | 0.000 | 0.63 to 0.79 |
| Strata | ||||
| Pain when walking | 2.23 | 2.90 | 0.004 | 0.72 to 3.73 |
| Strength and balance training | –1.66 | –1.75 | 0.080 | –3.52 to 0.20 |
| Female | 0.43 | 0.53 | 0.595 | –1.14 to 2.00 |
| Galleries Day Unit | 2.03 | 1.57 | 0.116 | –0.50 to 4.56 |
| North Tyneside Falls Prevention Service | –0.45 | –0.46 | 0.642 | –2.34 to 1.44 |
| Annual trend | –0.78 | –1.51 | 0.131 | –1.80 to 0.23 |
| CBTi | –3.54 | –4.87 | 0.000 | –4.96 to –2.12 |
| Random effects | ||||
| Sigma_u | 5.80 | 5.24 to 6.43 | ||
| Sigma_e | 5.64 | 5.33 to 5.96 | ||
Sigma_u and sigma_e are the estimated SD of the between-participant and within-participant random effects, respectively. This model suggests that the annual trend does not differ significantly from zero. The annual change in FES-I score is –0.78 (95% CI –1.80 to 0.23). In this model the estimated impact of the intervention corresponds with an average difference in FES-I scores between study arms over the three follow-up time points; this was –3.54 (95% CI –4.96 to 2.12).
In model 2, we fit separate trends over time for patients in the control and intervention arms. The parameter estimates corresponding to this model are given in Table 30.
| Effect | Coefficient | Test of difference from zero | 95% CI | |
|---|---|---|---|---|
| z | p-value | |||
| Constant | 9.37 | 4.14 | 0.000 | 4.93 to 13.81 |
| Baseline FES-I | 0.71 | 16.82 | 0.000 | 0.63 to 0.79 |
| Strata | ||||
| Pain when walking | 2.23 | 2.90 | 0.004 | 0.72 to 3.74 |
| Strength | –1.66 | –1.75 | 0.080 | –3.52 to 0.20 |
| Female | 0.42 | 0.53 | 0.597 | –1.15 to 1.99 |
| Galleries Day Unit | 2.03 | 1.57 | 0.116 | –0.50 to 4.56 |
| North Tyneside Falls Prevention Service | –0.44 | –0.46 | 0.645 | –2.33 to 1.45 |
| Trend control | –0.68 | –0.94 | 0.348 | –2.09 to 0.73 |
| Trend CBTi | –0.90 | –1.20 | 0.229 | –2.36 to 0.57 |
| CBTi | –3.64 | –4.21 | 0.000 | –5.33 to –1.95 |
| Random effects | ||||
| Sigma_u | 5.80 | 5.24 to 6.43 | ||
| Sigma_e | 5.64 | 5.33 to 5.96 | ||
The estimated annual trend in the control arm is –0.68 (95% CI –2.09 to 0.73) and the estimated annual trend in the intervention arm is –0.90 (95% CI –2.36 to 0.57). The difference in these trends is –0.22 (95% CI –2.26 to 1.81) and does not differ significantly from zero. There is no evidence that rate of change in FES-I differed between control and intervention patients. In this model the CBTi coefficient corresponds with the difference between the study arms at a single point in time (because we have non-parallel regression lines corresponding to the two study arms). The trend variables have been coded in such a way that this coefficient represents the difference between arms 12 months post randomisation.
As the interaction between time and CBTi is not significant, applying the principle of parsimony, model 1 is the preferred model. The mean impact of the CBTi over the period of follow-up is a reduction of 3.54 (95% CI 2.12 to 4.96).
Falls
Numbers of falls and injuries sustained
The number of falls and related injuries is summarised in Table 31.
| Arm | Number of falls and related injuries | ||||
|---|---|---|---|---|---|
| Fallers | Falls | Soft tissue injuries | Soft tissue injuries requiring medical intervention | Fractures | |
| Control | 99 | 480 | 48 | 2 | 7 |
| CBTi | 97 | 335 | 50 | 2 | 3 |
| All | 196 | 815 | 98 | 4 | 10 |
The distribution of the number of falls reported by participants was positively skewed (Figure 29).
FIGURE 29.
Distribution of falls.

Of the 347 participants who returned at least one diary, 154 (44.4%; 95% CI 39.1% to 50.0%) reported no falls at all. One participant reported a total of 73 falls.
Sixty-one per cent of men and 53% of women reported at least one fall (relative risk = 1.13; 95% CI 0.93 to 1.38). Fifty-eight per cent of those reporting pain when walking at baseline and 54% of those reporting no pain when walking reported at least one fall (relative risk = 1.06; 95% CI 0.88 to 1.28). Fifty-eight per cent of those not referred for strength and balance training and 55% of those who were referred for strength and balance training reported at least one fall (relative risk = 1.06; 95% CI 0.87 to 1.29). The proportion of patients reporting a fall was broadly similar across the three sites (Falls and Syncope Service 55.3%, Galleries Day Unit 47.4%, North Tyneside Falls Prevention Service, 57.3%).
The number of falls was analysed using a negative binomial regression model adjusted for randomisation strata (site, pain when walking, referral for strength and balance training, and gender) and the number of diaries returned (as an exposure variable). The reference model is shown in Table 32.
| Variable | Regression coefficients | Significance | ||
|---|---|---|---|---|
| IRR | 95% CI | z | p-value | |
| Constant | 3.78 | 1.70 to 8.42 | 3.25 | < 0.001 |
| Strataa | ||||
| Galleries Day Unit | 0.91 | 0.47 to 1.73 | –0.30 | 0.77 |
| North Tyneside Falls Prevention Service | 0.84 | 0.51 to 1.39 | –0.67 | 0.50 |
| Presence of pain when walking | 1.01 | 0.69 to 1.49 | 0.07 | 0.95 |
| Referred for strength and balance training | 0.80 | 0.49 to 1.31 | –0.89 | 0.38 |
| Gender (female) | 0.88 | 0.57 to 1.36 | –0.58 | 0.57 |
| Impact of CBT (study arm) | 0.73 | 0.51 to 1.06 | –1.63 | 0.10 |
The estimated impact of the CBTi on the likelihood of falling corresponds to an incidence risk ratio of 0.88 (95% CI 0.61 to 1.27). The point estimate of 0.88 suggests a slight reduction in the risk of falling but the 95% CI includes one; the difference between arms is not statistically significant. One patient reported 73 falls. Excluding this patient from the analysis changed the estimated impact of CBT to an incidence risk ratio of 1.02 (95% CI 0.71 to 1.46).
The above analysis is based on the 347 participants who returned at least one diary. To look at the potential impact of missing diaries, the analysis was repeated making the assumption that for those patients who returned at least one diary that they had no falls for the missing months. The corresponding estimate of the incidence rate ratio (IRR) was 0.74 (95% CI 0.51 to 1.06).
Likelihood of a participant reporting at least one fall
The likelihood of a participant reporting at least one fall was analysed using logistic regression. The modelling strategy (adjusting for strata and the number of diaries returned) is as reported above.
The estimated impact of the CBTi based on the 347 participants who returned at least one diary adjusted for site, gender, pain when walking, referral for strength and balance training, and the number of diaries returned corresponded with an odds ratio (OR) of 1.17 (95% CI 0.75 to 1.81).
Sensitivity analysis, accounting for missing data, provided the following estimates. Assumption that:
-
there were no falls corresponding to the missing diaries for the 347 participants who returned at least one diary. OR 1.10 (95% CI 0.72 to 1.69)
-
patients who did not return any diaries at all did not fall OR 0.92 (95% CI 0.62 to 1.36)
-
patients who did not return any diaries at all did fall OR 1.29 (95% CI 0.86 to 1.92).
Options (2) and (3) represent the most extreme scenarios. In each case, the 95% CI includes ‘1’; there is no evidence that the CBTi had an impact on the likelihood of reporting one or more falls.
A total of 97 falls resulted in a reported soft tissue injury. The number of soft tissue injuries reported by individual participants ranged from zero to four, with the vast majority (80%) of participants reporting no such injuries. Only four (4.1%; 95% CI 1.1% to 10.2%) of these injuries required medical intervention. A small number of falls (10) resulted in a reported fracture. One participant reported three falls resulting in a fracture; a second participant reported two falls, both of which resulted in a fracture. Five participants reported a single fracture resulting from a fall. The CBTi had no impact on the incidence of injurious falls.
Secondary outcomes
The distribution of secondary study outcomes at each visit are displayed graphically, and the change from baseline for each visit for each of these outcomes is tabulated in Appendix 4.
For each of the secondary outcomes, change from baseline to 12 months was analysed using analysis of covariance. Estimates of the impact of the intervention, adjusted for randomisation strata, are given in Table 33. The corresponding unadjusted results are presented in Appendix 4.
| Measure | R 2 | Baseline response | Impact of CBTia | ||
|---|---|---|---|---|---|
| B | 95% CI | B | 95% CI | ||
| FES-I score | 0.48 | 0.74 | 0.62 to 0.85 | –4.02 | –5.95 to –2.10 |
| Fear of falling 11-point numerical scale | 0.24 | 0.52 | 0.38 to 0.65 | –1.01 | –1.55 to –0.46 |
| Total social participation/isolation score | 0.33 | 0.58 | 0.48 to 0.67 | 1.03 | –0.44 to 2.50 |
| Gait and balance score | 0.06 | 0.70 | 0.33 to 1.07 | 0.90 | –1.06 to 2.87 |
| Mean handgrip strength | 0.62 | 0.65 | 0.54 to 0.75 | –0.22 | –1.82 to 1.38 |
| Functional reach | 0.37 | 0.50 | 0.38 to 0.61 | 0.91 | –0.85 to 2.66 |
| De Jong Gierveld Loneliness Scale score | 0.48 | 0.78 | 0.68 to 0.88 | 0.19 | –0.28 to 0.65 |
| HADS Anxiety | 0.56 | 0.80 | 0.70 to 0.89 | –0.70 | –1.42 to 0.03 |
| HADS Depression | 0.48 | 0.73 | 0.63 to 0.83 | –0.97 | –1.62 to –0.33 |
| WHOQOL-OLD total score | 0.47 | 0.67 | 0.58 to 0.77 | 0.85 | –1.56 to 3.26 |
| LSNS-6 | 0.62 | 0.83 | 0.74 to 0.91 | 0.74 | –0.16 to 1.65 |
| EQ-5D (5L) Health today | 0.24 | 0.44 | 0.32 to 0.55 | 3.07 | –1.06 to 7.21 |
| EQ-5D (5L) index | 0.54 | 0.72 | 0.61 to 0.82 | 0.01 | –0.04 to 0.05 |
| SF-36 PCS | 0.48 | 0.67 | 0.55 to 0.79 | 0.99 | –0.91 to 2.9 |
| SF-36 MCS | 0.34 | 0.59 | 0.47 to 0.72 | 1.17 | –1.51 to 3.84 |
Considering the 12-month data in isolation, there was a reduction FES-I score, as described above, and a reduction in fear of falling (as measured by the 11-point numerical rating scale) of –1.42 points (95% CI –1.87 to 1.07 points) in participants who were randomised to the CBTi relative to participants in the control arm. There is some evidence of a reduction in depression. The estimated impact of the CBTi is a reduction in mean HADS Depression score of 1 unit (on a 21-point scale) (95% CI 0.3 to 1.6 units). The CBTi had a much smaller impact on the other variables of interest.
Longitudinal analyses, utilising data collected at all time points available for the secondary outcomes, was conducted using the same approach as for the analysis of FES-I scores, described in the previous section. The estimated trend(s) and difference between study arm are shown in Appendix 4. There is evidence that fear of falling, anxiety and depression fell more in participants who were randomised to the CBTi than in control participants. These were the only variables for which there was a significant study arm-by-time interaction.
Harms
Serious adverse events
Forty-eight participants (11.6%, 95% CI 8.6% to 15.0%) experienced one or more serious adverse events. A total of sixty-nine serious adverse events were recorded. Thirty-four participants experienced a single event; 10 participants, two events; three participants, three events; and one participant experienced six events. Most of the events (89.9%) were categorised as either ‘moderate’ or ‘severe’ (Table 34).
| Severity | Study arm | Total | ||||
|---|---|---|---|---|---|---|
| Control | CBTi | |||||
| n | % | n | % | n | % | |
| Mild | 3 | 7.3 | 1 | 3.6 | 4 | 5.8 |
| Moderate | 21 | 51.2 | 16 | 57.1 | 37 | 53.6 |
| Severe | 17 | 41.5 | 8 | 28.6 | 25 | 36.2 |
| Unknown | 0 | 0.0 | 3 | 10.7 | 3 | 4.3 |
| Total | 41 | 28 | 69 | |||
Just over 20% of serious adverse events related to a fall suffered by the participant. Three of the adverse events culminated with the death of the participant and these events had the following descriptions:
-
acute intestinal obstruction and incarcerated incisional hernia: raised number of white blood cells, raised level of C-reactive protein, low calcium and magnesium levels, and very raised creatinine level
-
pneumonia and pulmonary oedema – died in hospital
-
stroke – rushed to hospital but died in Casualty at 12.40.
The first of the events listed pertained to a participant who was randomised to CBTi but happened prior to any of the scheduled CBTi sessions. The other two events involved participants in the control arm. None of these deaths is believed to be causally related to participation in the study.
There is no evidence that the serious adverse event rate differed between study arm (Table 35).
| Event type | Study arm | Risk ratio | |||||
|---|---|---|---|---|---|---|---|
| Control | CBTi | ||||||
| n | % | n | % | Risk ratio | 95% CI | ||
| Any reported serious adverse event? | 27 | 13.2 | 21 | 10.0 | 0.76 | 0.44 | 1.30 |
| Any non-fall-related serious adverse event? | 22 | 10.7 | 15 | 7.1 | 0.67 | 0.36 | 1.25 |
The mean number of reported events were:
-
0.20 (95% CI 0.12 to 0.30) for participants in the control arm
-
0.13 (95% CI 0.08 to 0.20) for participants the CBTi arm.
The difference in means between study arms = –0.07 (95% CI –0.18 to 0.04). Adjusting for differences between strata using negative binomial regression, the estimated IRR = 0.61 (95% CI 0.32 to 1.16); there is no evidence of a difference in the likelihood of reporting serious adverse events.
One hundred and seventy-five participants (42.2%, 95% CI 37.3% to 47.1%) reported at least one adverse event. A total of 514 adverse events were recorded. Most of the events (90.9%) were either ‘possibly’ or ‘definitely’ fall related. None of the events was deemed to be ‘possibly’, ‘probably’ or ‘definitely’ attributed to the receipt of CBT. Sixteen events (3.1%) were classified as ‘severe’; two-thirds of the events were classified as ‘mild’.
The mean number of reported non-serious adverse events were:
-
1.30 (95% CI 0.94 to 1.71) for participants in the control arm
-
1.18 (95% CI 0.86 to 1.54) for participants in the CBTi arm.
The difference in means between study arms = –0.12 (95% CI –0.60 to 0.42). Adjusting for differences between strata using negative binomial regression, the estimated IRR = 0.91 (95% CI 0.63 to 1.33); there is no evidence of a difference in the likelihood of reporting adverse events.
Considering all adverse events (both serious and non-serious) the corresponding IRR was 0.88 (95% CI 0.63 to 1.23). CBTi does not appear to be a particularly high-risk intervention.
Chapter 5 Process evaluation
Introduction
Process evaluation is important not only for providing insight into the reasons why a trialled intervention has been shown to be clinically effective (or not), but also is integral to understanding issues concerning transportability, workability and integration of interventions into routine clinical practice. 36,93 Although process evaluations in fear of falling are limited, there is some evidence to suggest that group-based CBT for fear of falling is feasible for participants and facilitators,94 and fits with regular care. 95 However, previous process evaluations have not been integrated throughout the study and have therefore not considered the process of intervention development. Furthermore, they have relied on data from questionnaires94 or audit,95 rather than a more robust framework. In this study, we drew on normalisation process theory (NPT)38 to explore psychological and sociological mechanisms of behaviour and action that have been empirically demonstrated to be important in the development, planning and implementation of complex interventions. 96 NPT focuses on understanding the collaborative ‘work’ in four key areas: coherence (ways in which an intervention ‘makes sense’ and has clear purpose and objective); cognitive participation (willingness and ability to commit time and energy needed to make it work); collective action (the resources, arrangements, skills, etc. required to make an intervention work); and reflexive monitoring (formal and informal mechanisms for appraising the process and outcomes of an intervention).
Phase I of the process evaluation, the refinement of the intervention, has been reported in Chapter 2. In Phase II, the process evaluation aimed to identify, describe and explain the professional and organisational factors that promoted or inhibited the implementation and integration of the CBTi. Specifically the objectives were to:
-
understand the factors that influence the delivery of the CBTi by non-specialist HCAs
-
explore the acceptability of the CBTi
-
describe factors influencing the enactment of the RCT.
Within this chapter we have used the terminology used by the psychologists and the HCAs delivering the intervention. The term ‘clients’ is used to denote participants who were randomised to CBTi; the HCAs are referred to as ‘STRIDE therapists’, as this was the term agreed on completion of the training to describe their role and was key to developing a sense of identity. The only exceptions to this are in quotations, in which we have used the terms used by the speaker.
Methods
Data collection and consent
Data collection involved ethnographic methods, including semistructured interviews, informal discussions and non-participant observation (captured in field notes). Therapy sessions (notes summaries and audio recordings) provided data to assess the content and delivery of the CBTi. Participants in the process evaluation included staff who were working in sites from which clients were recruited (hereafter ‘professionals’); STRIDE therapists who were involved in delivering the CBTi; clients who were participating in the intervention arm of the trial and family members (hereafter ‘caregivers’); and members of the study team and TSC. The data collected for the process evaluation are summarised in Table 36. The average duration of each episode of data collection was 49 minutes for interviews, 14 minutes for informal discussions, 143 minutes for observations and 49 minutes for meetings. Overall, 75% of data collected was recorded (although not all was transcribed). Field notes were also made to support analysis (75 pages in Phase I and 268 pages in Phase II). All data collection for the process evaluation was undertaken by CB, with the exception of one focus group with the STRIDE therapists, which was carried out by a student as part of her master’s dissertation.
| Data collection type | Phase I | Phase II | Total |
|---|---|---|---|
| Interviews | |||
| Clients | 14 | 20 | 34 |
| Caregivers | 2 | 3 | 5 |
| Professionals | 5 | 4 | 9 |
| STRIDE therapists | NA | 7 | 7 |
| Team members | 9 | 12 | 21 |
| Total interviews | 30 | 46 | 76 |
| Informal discussions | |||
| Professionals | 0 | 1 | 1 |
| STRIDE therapists | NA | 15 | 15 |
| Team members | 11 | 39 | 50 |
| Other | 0 | 1 | 1 |
| Total informal discussions | 11 | 56 | 67 |
| Observation | |||
| Clinic observation | 3 | 7 | 10 |
| Therapy sessions | NA | 5 | 5 |
| STRIDE therapist training | NA | 5 | 5 |
| STRIDE therapist supervision | NA | 11 | 11 |
| Other | 0 | 3 | 3 |
| Total observation | 3 | 31 | 34 |
| Meetings | |||
| Intervention development | 9 | 0 | 9 |
| Team meetings | 7 | 17 | 24 |
| TSC | 0 | 6 | 6 |
| Office meetings | NA | 11 | 11 |
| Other | 0 | 11 | 11 |
| Total meetings | 16 | 45 | 61 |
Semistructured interviews
Interviews with all participants explored perceptions of key factors that might impede or facilitate the CBTi being effective and workable in practice. The topic guides were loosely structured around NPT,38 with ample scope for participants to raise issues that they saw as important (see Appendix 5 for an overview of topic guides).
Professionals were interviewed about the service provided, their views of the CBTi, and their experiences of identifying and recruiting clients to take part in the study. Two professionals interviewed in Phase I of the study were interviewed again towards the end of the study to assess whether or not their perceptions of the CBTi had changed.
Each STRIDE therapist was interviewed two or three times to explore how their interpretations and evaluations of the intervention evolved across the duration of the study. For efficiency, their reflections towards the end of the study were obtained through two focus groups: one conducted by CB and VD, and the other by a master’s student supervised by VD.
In Phase II (the RCT; see Chapters 3 and 4), interviews with clients and caregivers focused on their experiences of the intervention, the extent to which the techniques had been embedded into daily life, and the ‘fit’ of the intervention with other services (such as strength and balance training or the in-house rehabilitation programme provided by site 2 (see Table 5). Phase II clients were recruited from the database of trial participants using ethics committee approved recruitment and consent processes (see below). Maximum variation sampling was used (based on gender, age, degree of fear of falling, STRIDE therapist seen, and STRIDE therapist’s perception of key factors influencing activity restriction). Caregivers were recruited via clients who were interviewed.
Members of the study team were interviewed to aid understanding of the conduct of the study, or to explore key issues as they emerged. Some key team members were interviewed multiple times.
Informal discussion
Informal discussions had not been included in early versions of the protocol (but emerged as a key source of data for the study). These discussions typically arose in response to an event or issue arising in the conduct of the study. These contemporaneous discussions helped us to understand and manage emerging issues. They also allowed a more in-depth exploration of issues that were raised in formal meetings from different perspectives.
Non-participant observation
Routine practice was observed at participating sites to gain an understanding of the fit between the CBTi and existing service provision. This included observation of initial assessments, follow-up appointments and interventions provided directly by, or through, the service (see Tables 4 and 5).
A range of activities involving the STRIDE therapists was also observed, including initial training; individual and group supervision; and a small number of sessions with clients. Finally, meetings focusing on intervention development, study management and ad hoc meetings to discuss specific issues (e.g. client recruitment) were also observed with appropriate consent (see Consent). Observation was supplemented with recordings and documentation (e.g. training materials) when available.
Content and delivery of the cognitive–behavioural therapy intervention
The STRIDE therapists summarised their work with each client who completed the study on a structured form (see Appendix 6 for three completed examples). This included details of the formulation (predisposing, precipitating and perpetuating factors); perceived barriers to activity; focus of the intervention; goals; planned treatment strategies; a rating of client engagement with the CBTi; perceived outcomes of the intervention from the perspective of the STRIDE therapist; and a narrative summary. Further data on the content and delivery of the CBTi were obtained through non-participant observation of therapy sessions. Additionally, CB listened to, and made field notes on, a purposive sample of recorded therapy sessions (including some with clients who dropped out of the CBTi).
Consent
All participants in interviews, informal discussions and meetings were provided with a Participant Information Sheet and asked to provide written informed consent prior to participation. Verbal consent was sought prior to each subsequent episode of data collection. The consent form that clients completed for the RCT included permission to audio-record the CBTi sessions. Professionals sought verbal consent from clients and any companions for (non-recorded) observation of clinic appointments and interventions.
Variations to protocol
Some variations to the process evaluation protocol (versions 1–4) were required. Fewer follow-up interviews to assess changes in perceptions over time were conducted than intended. In terms of professionals, this was because the addition of extra study sites necessitated interviews with a larger sample of professionals across the three sites. In relation to clients, early follow-up interviews indicated that they yielded few additional data to justify the additional respondent burden that these posed. Most clients were therefore interviewed once, but at different points along the intervention trajectory. We interviewed fewer caregivers than anticipated, as their involvement in the CBTi proved to be the exception rather than the rule.
Data management and analysis
Details of all data collected for the process evaluation were recorded in a Microsoft Access® version 2010 (Microsoft Corporation, Redmond, WA, USA) database and stored in a password-protected network that was accessible only to the process evaluation team. Interviews and informal discussions were generally audio-recorded, transcribed, checked and anonymised. Field notes were anonymised.
All qualitative data were analysed thematically using the constant-comparative technique,46 as described in Chapter 2. In Phase II, the volume of data collected and the variety of sources meant that analysis was conducted in a series of rounds of purposively sampled data sources, adapting an approach that previously used to manage a substantial data set. 97 In each round, CB and TF agreed a selection of five data sources to cover a variety of time points (across the study), perspectives (participant groups) and data types (interviews, field notes, meetings, etc.), and independently read the data to identify key themes. These were then discussed and a preliminary coding frame was agreed. A further set of (five) data sources was identified for the next round of analysis. In each subsequent round, possible themes were compared with those initially identified, and revised or re-organised as appropriate. This iterative process of reviewing new data items and modifying the emerging coding frame continued until saturation was reached, with few new themes being identified.
Data from the STRIDE therapist summaries were extracted using a coding frame that was developed through discussions with the STRIDE therapists and psychologists. The data were analysed descriptively using IBM SPSS Statistics release 21 (IBM Corporation, Armonk, NY, USA).
Data reported here are attributed using the unique identifier allocated to the participant. Prefixes are as follows: ‘P’ denotes professionals; ‘ST’ denotes STRIDE therapists delivering the intervention; ‘C’ denotes clients (‘PC’ for pilot clients recruited from Phase I of the study); ‘F’ denotes caregivers; and ‘R’ denotes study team members. When quotations are from joint interviews or focus groups, the speaker is indicated in bold. The focus group conducted by CB and VD is denoted as focus group 1; the one facilitated by the student as focus group 2. All STRIDE therapists are referred to as female to prevent identification of the single male STRIDE therapist. Within this chapter, participating sites have been anonymised as sites 1–3 (the order is unrelated to the order of presentation of the sites in other chapters).
Results
The development of the CBTi delivered in the present study was described in Chapter 2; here, we focus on how the principles and techniques for CBTi were transmitted to the STRIDE therapists via training and supervision, and the factors that influenced the ways in which the STRIDE therapists enacted the CBTi with clients. We also examine the acceptability of the CBTi to clients and professionals working in participating sites. The CBTi was embedded within a RCT and we review how the broader study activities impacted on the delivery of CBTi and describe some of the challenges of implementing this trial. We conclude the chapter by framing the findings in the NPT framework and drawing out the implications for both the CBTi and RCTs.
Delivery of cognitive–behavioural therapy intervention by non-specialist STRIDE therapists
A key aim of the study was to explore the feasibility of training HCAs to deliver the CBTi to older people. This section describes the training and supervision provided. The recruitment of the STRIDE therapists is described later (in the section on conduct and delivery of the RCT); briefly, the posts were advertised and 32 people applied, most of whom were graduates. Three people were appointed to share two full-time posts, two of whom had a background in health care.
Training and supervision of STRIDE therapists
The organisation, content and aims of training and supervision are summarised in Table 37 (further details of methods of delivery and content are provided in Chapter 2) and the manuals supporting the training are available from SWP). This section provides a reflective description of the training from the perspectives of the STRIDE therapists, psychologists (NSa and VD) and process evaluator (CB). In addition to the formal individual and group supervision provided as part of the study, the STRIDE therapists met informally for peer support and had a separate NHS line manager.
From the perspectives of the psychologists, the initial training achieved the stated aim of equipping the STRIDE therapists with enough skills to start their work with clients:
In general I’m more confident about the trial now than I was at – than I have been at any point. I think it’s got a real chance of working if we‘ve got a – if we can give them the support and they’ve got the time to deliver the intervention as we’ve trained them to.
R4, informal discussion, 5 July 2012
Although the STRIDE therapists reported having been concerned about the duration of the training, once they started seeing clients, they felt that the training had provided them with the necessary skills:
To be honest it’s weird because at the time we were like ‘this isn’t enough time’. But then looking back it’s like ‘well I actually learned all of the stuff and I am implementing it’.
ST1, interview, 23 August 2012
| Item | Training | Individual supervision | Group supervision |
|---|---|---|---|
| Frequency | One 2-week period at the outset of the study | Fortnightly (reducing towards the end of the study; average 35 sessions per STRIDE therapist) | Variable (fortnightly immediately after training; bimonthly later in the study; 21 sessions in total) |
| Duration | 5 days spread over 2 weeks | 60 minutes | 60–90 minutes |
| Content | Overview of CBT Engagement with the client Assessment and formulation Standard CBT interventions (behavioural and cognitive techniques) Relapse prevention Discharge planning |
Overview of all clients Plans for intervention Feedback on recordings of sessions Practical issues Group dynamics |
Training Case-based discussion Intervention fidelity Practical issues Group dynamics |
| Main aim | To equip the STRIDE therapists with enough skills to begin working with clients | Embedding training and monitoring intervention delivery | |
One issue raised by the STRIDE therapists related to the extent to which the training was appropriate for non-specialist trainees. The STRIDE therapists with psychology degrees questioned whether or not non-specialist staff with a less relevant background would have been able to manage the volume of information and skills covered in the training:
It’s not something that you can just learn in five days if you have never done anything like this. I think it also helped for me that I had done a counselling course when I was at University [. . .] if I hadn’t had done that I think that I might have struggled because empathy is kind of a hard thing to grasp if you have never really been in an empathetic role before. It’s not something that you learn overnight.
ST3, interview, 4 September 2012
A separate concern that was raised about the training related to the relationship between the CBTi and the RCT. The training focused on the CBTi, without exploring how it was embedded within the RCT:
Personally I would have preferred an introduction. Right this is where we’re at; this is where it’s evolved from; this is what we’re planning to do [. . .] I would have preferred to know what the patients knew about us and it would give us a better understanding of ‘Right so the patients know this, this is their understanding. This is how we’re going to introduce ourselves to the patients.’ That would’ve been really useful.
ST2, interview, 27 June 2012
From the perspectives of the STRIDE therapists, the training (and documentation) focused too heavily on assessment and formulation and they would have valued more time to practise treatment strategies. This was highlighted both in early interviews and in the focus group towards the end of the study when the STRIDE therapists acknowledged that treatment strategies had been covered but that:
We weren’t practised at them.
Especially –
So it was more hypothetical?
We didn’t know how to use them.
Focus group 1, 2 July 2014
Although ‘real play’ was used successfully to practise assessment and formulation skills, the STRIDE therapists suggested that it could also have been used in relation to treatment strategies as a way of increasing familiarity and confidence with these techniques. The STRIDE therapists intuitively grasped some treatment strategies (e.g. graded exposure) but were less certain of others (e.g. cognitive work). Techniques such as graded exposure also seemed more congruent with client expectations of CBTi (especially as some clients confused CBTi with strength and balance training). In contrast, clients had difficulty in distinguishing between behaviours and thoughts that tended to increase the STRIDE therapists’ uncertainty about using thought diaries as a way of approaching cognitive work:
You try to explain it [ST3: yes], they don’t understand it and they would write in ‘I had lunch’. Do you know what I mean? [Laughter]
I thought it was tasty.
Focus group 1, 2 July 2014
Regular supervision was seen as an essential adjunct to consolidate the initial training. Listening to recordings of CBTi sessions in supervision reassured the psychologists that the STRIDE therapists had embedded the training to some extent and were applying their new skills. Supervision also allowed new information and techniques to be introduced, when relevant, to current work with clients. For example, information on obsessive–compulsive disorder and post-traumatic stress disorder was provided when STRIDE therapists were working with clients whose difficulties seemed to be consistent with these conditions. Aspects of training and supervision that helped the STRIDE therapists to embed the CBTi are summarised in Table 38.
| Theme | Description | Example |
|---|---|---|
| Setting realistic expectations | STRIDE therapists were being trained to develop useful interventions for clients with fear of falling rather than as CBT therapists Embedding the skills for CBTi was seen largely as taking place through practice Having clear expectations helped manage STRIDE therapist anxiety about learning new skills |
The psychologists made it clear that they did not expect the STRIDE therapists to be fully up to speed by the end of the training and that it would probably not be until they had seen 20 or 30 clients [approximately 10% of the anticipated number who would receive CBTi in the trial] that their skills would be fully developedField notes of training, day 2, 1 June 2012 |
| Managing STRIDE therapist anxiety | Learning new skills creates anxiety Anxiety can interfere with the ability to absorb new information Tendency to revert to familiar roles and ways of interacting when under stress |
Before ST1 started the assessment, R4 reminded her not to worry about getting it wrong, to which ST1 commented ‘I’m well past that point!’ At this point, ST2 also commented that they should ‘embrace the wrongness’. This seemed a significant shift from the start of the day when they had both been very anxious about getting it right and not making mistakesField notes of training, day 2, 1 June 2012Because it sounds like you are having successes and actually learning from the ones that are going well is something that might be worth doingR4, group supervision, 16 January 2013 |
| Use of ‘real play’ (training technique where STRIDE therapists used CBT to explore their own anxieties e.g. fear of flying) | Embedding new skills Integrating interpersonal and technical skills Providing insight into client experience |
Doing the role play felt really clunky and as if you’re more concentrating on the pro forma and getting that right. But doing the real play we could concentrate more on like the personal, the empathy and it felt more natural to do thatST2, interview, 27 June 2012 (author‘s emphasis in bold text) |
| Encouraging reflexive practice (including using CBT to understand their own experience of learning CBT) | Embedding new skills Providing insight into the STRIDE therapists’ own learning process Providing opportunities for the STRIDE therapists to experience ‘what it’s like to be a client’ (R12 interview, 2 October 2012) Increasing awareness of how the STRIDE therapists’ styles of interacting might impact on their work with clients Managing STRIDE therapist anxiety (and increasing confidence in CBT techniques) |
I was quite nervous about actually doing this. I was using this stuff for myself – basically like rationally – ‘well I’ve only had 5 days of training’; ‘it’s my first patient ever’; ‘it’s practice, if it goes wrong then I’ll just do it next week, the week after’; ‘this is all a learning thing, I can’t change it. I know as much as I can do at this time’. ‘I just rationally, I just used it on myself basically seeing that the thoughts were wrong and all that, so the training actually helped me manage myself within the whole scheme of things’ST1 interview, 23 August, 2012 |
| Encouraging ownership of the CBTi | Providing a sense of agency in the context of learning new skills Ensuring a sense of identity in the STRIDE therapists’ new role |
ST2 comments that they were having difficulty working out how to introduce themselves without having a term. R4 suggests ‘STRIDE researchers?’ R12 suggests ‘STRIDE clinicians’ then R4 suggests ‘STRIDE therapists’ and then asks ‘is therapists pushing too far? But people talk about physiotherapists and occupational therapists’. ST1 seems to agree and then R12 agrees ‘yes, STRIDE therapists?’ ST1 makes a comment about how to respond if people say ‘what’s that?’ R4 and R12 comment that they have been trained in how to give people back their confidence. There seems to be a consensus and [. . .] R4 comments ‘I can see that working, ‘one of the STRIDE therapists will be calling you . . .Field notes of training, day 5, 15 June 2012 |
| Managing boundaries | Developing a sense of what fell within the remit of the CBTi Distinguishing between aspects of life for which therapeutic intervention was relevant and those that were part of life |
ST1 felt that reducing the checks was the only area she felt she could address with the client. R12 asked whether she saw this as part of her responsibility; ST1 commented that there was nothing else to work on since the client had no fall anxiety or any fatigue or pain. So in terms of the usual STRIDE activities she felt there was nothing she could do apart from working on the anxietyField notes of ST1/R12 supervision, 15 May 2013If you are having thoughts that are getting in the way of your walking, and ‘if I go out I’ll break my back’ that’s clearly not a useful thought. But if you’re actually having those feelings in the evening, you know, ‘I miss my wife’ you know, that’s notR4, group supervision, 16 January 2013 |
Although modelling interpersonal and technical skills that were relevant to the delivery of the intervention was used extensively during training and supervision, the extent to which the STRIDE therapists were aware of modelling and/or learned from this approach is unclear. None of them mentioned the value (or otherwise) of modelling during the interviews and informal discussions that were conducted for the process evaluation. The psychologists rarely explicitly drew attention to their use of modelling, and this may have limited the opportunities for the STRIDE therapists to learn and embed these skills in their own practice. The following example is unusual in that the psychologist explicitly refers to the use of modelling and illustrates that not all of the STRIDE therapists had recognised this:
R4 then conversationally asked ST2 about each of these areas and then commented ‘I just did a FIND [a technique used in assessment to explore the extent of the problem by exploring Frequency, Intensity, Number and Duration] on you, did you notice?’ [. . .] ST3 had noticed that R4 had used the FIND questions as part of his questioning but it was less obvious to the others.
Field notes of group supervision, 25 July 2012
The supervision provided by the psychologists was valued by the STRIDE therapists. Based on their experience, the STRIDE therapists suggested that access to a multidisciplinary team would have been helpful. Although they were able to access the expertise of the STRIDE therapist with a physiotherapy background, and were sometimes able to discuss specific physical issues with physiotherapists based at participating sites, more formal arrangements for future CBTi delivery were seen as essential:
The access to physios, because like ST1 was saying just that the ability to go and check with someone who knows. So if someone develops a new pain as happened with one of ST1’s clients, is it because of the walking that they’ve been doing? Is it safe to continue walking? Having that validation from an expert means you can go back
Informal discussion, R4 and ST1, 17 December 2013
Peer support
Peer support was identified by the STRIDE therapists as a key adjunct to the formal supervision that was provided by the psychologists. This tended to be on an ad hoc basis, although the three STRIDE therapists aimed to meet weekly, usually for 2–3 hours, particularly at the beginning of their work. None of these meetings was observed, but the STRIDE therapists described them as covering a wide range of areas, including self-audit; discussing concepts; talking through alternative strategies for use with specific clients; and general issues relating to the conduct of the research. The process of self-audit was illustrated through a discussion captured as part of a group supervision session:
We did like a mini thing between the three of us one day, where we analysed each other’s assessment to see if we came up with the same formulation as was written down . . . In all three, we all came up with pretty much the same rationale from the information provided on the assessment.
ST1, group supervision, 23 January 2013
The frequency of peer support varied throughout the study depending on workload, working arrangements and team dynamics. Different STRIDE therapists were seen as having different strengths, for example the STRIDE therapist with a physiotherapy background was seen as having particular skills and knowledge that was relevant to clients who had difficulties in managing pain.
In addition to support relating to working with clients, the meetings also focused on establishing and owning the infrastructure to facilitate delivery of the CBTi. The psychologists highlighted the extent to which the STRIDE therapists gained a sense of involvement and ownership of the CBTi:
They were doing so much problem solving during those meetings. They were thinking locked briefcases, transport, continuation sheets, forms, referral. They were thinking through all these things as – as a group in a way we should’ve thought through. But they were coming up with it on the hoof and taking ownership of how they were going to do it. And that – and again that helped them bond.
R4, informal discussion, 5 July 2012
Factors affecting the delivery of the cognitive–behavioural therapy intervention
A formal evaluation of intervention fidelity was not included in the process evaluation; however, insights into the delivery of the intervention were provided through available data. Through supervision, the psychologists were confident that the intervention was being delivered as intended, and identified the competencies and skills that each STRIDE therapist brought to her work with clients:
ST1’s behavioural work is good in terms of exposure [. . .] we talked in detail last time about it, about a client who had a real phobia about going out, there were some issues to do with mobility but again she worked that very well.
R4/R12, discussion, 19 June 2013
ST2’s very good at breaking stuff down [. . .] and selling that to those people, so she’s using the knowledge and skills that she has in a good CBT way which is great.
R4/R12, discussion, 19 June 2013
I think ST3’s got a lovely way with the clients, a lovely style, there’s humour, she’s able to listen, she’s able to contain people and you know, this is good, very good therapy that’s how it sounds.
R4/R12, discussion, 19 June 2013
There were a number of subtle shifts in the emphasis of the CBTi over the period of the study (Box 2). The CBTi evolved in these ways as a result of a range of factors (Table 39); these included challenges to delivering the CBTi as planned and increased insight and understanding of fear of falling as the STRIDE therapists gained more experience.
-
Exploration of barriers to activity (rather than fear of falling).
-
Emphasis on falls preparation (rather than falls prevention).
-
Emerging advocacy role.
-
Emergence of supportive role (with clients with no experiential avoidance).
-
Shift to exercise-based goals.
| Challenge | Components | Impacts |
|---|---|---|
| Multidimensionality of fear of falling | Clients identified a range of barriers to activity Some clients with fear of falling have no experiential avoidance |
Delays in embedding skills after training (initially) Shift to barriers to activity rather than fear of falling Emergence of supportive role |
| Working with clients with complex and unmet medical needs | Medical issues Managing unmet needs |
Impact on client motivation and restrictions on feasible goals Shift to falls preparation Emergence of advocacy role |
| Fit of CBTi with existing interventions | Conflicts between medical advice and CBTi Ethos of CBTi and existing interventions Timing of interventions |
Willingness of clients to engage and faith in STRIDE therapists and CBTi Potential burden for clients; restrictions on feasible goals |
| STRIDE therapist uncertainty over underlying rationales and specific techniques | STRIDE therapist anxiety Difficulties in transmitting principles of CBTi to clients Difficulties tuning into client motivation Uncertainties in enacting key skills |
Tendency to revert to familiar/established ways of working Varied understandings of the intervention Extent to which principles embedded by clients varied Dependency on STRIDE therapists More didactic style by STRIDE therapists Problems with setting SMART goals Shift to exercise-based goals |
| External factors relating to the RCTa | Viability of the study Issues relating to practical organisation and management |
Delays in embedding skills as a result of slow recruitment rate Involvement of STRIDE therapists in recruitment Source of anxiety; distraction from CBTi |
The factors were interrelated, for example low recruitment rates to the RCT led to the addition of two new sites, which raised issues concerning the fit of the CBTi with existing interventions and increased the number of clients with complex and unmet medical needs. These shifts in the focus of the CBTi raised questions over the identity of the CBTi:
So, was what you were doing still CBT? [4 second pause]
Yes [ST2/ST3: Yes].
What made it CBT and not physio?
Well, it’s how you de– well, possibly semantics I don’t know, because it’s graded activity [ST2/ST3: Yeah] that you were doing.
It’s their goals that they’ve come up with or you’ve worked out together.
Focus group 1, 2 July 2014
Later in the same focus group, however, the STRIDE therapists clarified the differences between the CBTi delivered in the present study and CBT:
It wasn’t CBT, it was supportive therapy we were providing.
Sort of a stripped back, simplified version.
Focus group 1, 2 July 2014
We’re not CBT therapists. We weren’t trained to do that, and now reading back about what they should be has just highlighted the difference.
ST3, ST focus group 1, 2 July 2014
These comments partly reflect the timing of the focus group which met shortly after two of the STRIDE therapists had received additional CBT training towards the end of the study and also after they had revisited the majority of clients for the 6-month follow-up. The perceived ability of clients to sustain changes contributed to the STRIDE therapists questioning the relationship between the CBTi they had delivered and CBT:
I think after the 8 weeks they probably, for the vast majority of them we probably have made a difference but come their 6-month follow-up or their 12-month certainly, it’ll be gone.
ST3, focus group 1, 2 July 2014
At this point in the study, the STRIDE therapists’ impressions of the CBTi seemed at odds with those of the psychologists, who were confident that the intervention had been delivered as planned.
Multidimensional nature of fear of falling
The Phase I interviews to inform the development of the CBTi indicated that fear of falling was a more complex construct than had previously been recognised and three distinct types of clients were identified (see Figures 1–3). Through the delivery of the CBTi to clients, the multidimensional nature of fear of falling was further revealed and even clients with apparently similar presentation responded differently to the CBTi:
There’s a lot more issues coming out than what we first thought I think, we had the pain, the fear and the ones that were just getting on with it. I know we had that to begin with but it’s much more complicated than that because you could have two patients with pain issues but completely different and they can do completely differently.
ST3, interview, 4 September 2012
As part of the initial formulation, the STRIDE therapists identified the factors that were thought to have precipitated fear of falling. A total of 391 precipitating factors were identified by 132 clients (four identified no precipitating factors and data were missing for two clients). After recoding the data to avoid double counting of similar factors, 317 unique factors were identified (a mean of 2.3 per client); 31% of clients identified three precipitating factors and 11% identified four or more.
The most common precipitating event was a fall, blackout or problems with balance (reported by 81% of clients). Other common precipitating factors were bereavement (21%) and life events, such as family disputes, retirement and caring responsibilities (17%). Clients also identified a wide range of other medical problems that contributed to their current difficulties (these are described below).
Clients therefore presented with a range of challenging medical and psychosocial conditions (some examples are provided in Box 3). The psychologists acknowledged that it would be challenging for any therapist to work with the complex clients recruited to the study, many of whom were described as experiencing ‘existential narrowing’ (R4) as a result of poor health, bereavement and social isolation. In this context it proved difficult for the STRIDE therapists to embed their newly acquired skills:
It can be really quite difficult when you’re actually trying to implement something that you think you’ve got the hang of now but actually the clients don’t fit that kind of you know they’re not doing what they’re supposed to you know kind of they’re not saying what they’re supposed to, they’re not presenting as we’ve been told they were going to be, and that can be quite, well very unsettling.
R4/R12, discussion, 24 October 2012
-
Stroke; quadruple heart bypass; wife died in last year; BPPV; diabetes; blackouts.
-
Hysterectomy last year; husband died 5 months ago (was primary carer); falls; heart attack 15 years ago; son died; second son brain damaged after car crash; bladder cancer 3 years ago.
-
Fell and fractured shoulder; osteoarthritis of knees; raised blood pressure; breast cancer.
-
Couple of falls; heart issues; total knee replacement and total hip replacement some years ago; wife died in 2003; anxiety attacks.
-
Whiplash injury in car crash in 1990s; recent death of husband; two falls in last 2 years; poor kidney function; neuropathy in feet; frozen shoulder.
-
Fell and broke two ribs 6 years ago; husband died 4 years ago; lots of falls over last 4 years; mini stroke in cerebellum; pain in left foot.
Faced with this complexity, it was crucial for the STRIDE therapists to be flexible and able to adapt their approach. Understandably, this was challenging at the outset of the study, although the STRIDE therapists grew in confidence over time. As it was clear that the factors restricting activities were rarely limited to fear of falling, the STRIDE therapists shifted from a focus purely on fear of falling to a broader focus on the range of barriers that led to clients limiting their lives and avoiding certain situations or activities:
It started off with like, fear of falling, that’s what we originally aimed at looking, but it started becoming like more, what is causing you to avoid different things [. . .].
And it didn’t matter so much as what necessarily caused the avoidance if we could reverse that cycle and that improved that situation.
Focus group 2, 17 June 2014
Working with clients with complex and unmet medical needs
Although site 3 aimed to prevent falls by reviewing and addressing risk factors of patients identified through screening in primary care, patients attending sites 1 and 2 typically had more complex medical histories and were perceived as more frail (as suggested by the difference in some of the baseline characteristics reported in Table 12). Precipitating factors relating to medical problems were recoded into three groups: musculoskeletal problems (including knee and hip replacement, arthritis; reported by 51% of clients); cardiovascular and respiratory problems (23% of clients); and other medical problems (e.g. diabetes, cancer; 38% of clients). As would be anticipated with an older client group, only a minority of clients (20%) did not describe medical problems in any of these categories, and 29% identified problems across two or three of these categories. STRIDE therapists and clients agreed that the CBTi was most effective after any medical issues had been resolved, as it enabled clients to maximise the benefits of their improved health by tackling any residual loss of confidence and encouraging them to try new activities:
If you work on that physical strength, definitely the confidence comes back. But [. . .] the variety of applications of that confidence is quicker because you’re getting different ideas of how to apply it.
C5969 interview, 29 January 2014
Professionals in sites 1 and 2 similarly suggested that greater integration of different interventions would be beneficial:
I just think it’s a missed opportunity, probably for interventions to be joined up [. . .] if somebody’s having ongoing medical intervention it may be that, that that’s not as joined up.
P20 interview, 24 March 2014
Not all complex medical conditions were optimally managed, with some clients having significant unmet needs. The STRIDE therapists were frustrated by the lack of appropriate care provided to some clients, particularly when they felt that this was creating dependency and reducing quality of life. This led to the emergence of an advocacy role to expedite appropriate interventions; this usually involved encouraging clients to seek further medical help but sometimes the STRIDE therapists intervened on a client’s behalf:
We’re signposting patients before they go into the GP practices and telling them what they need to tell the GPs because in seven minutes they’re getting nothing [. . .] we’re doing a lot of like almost skills rehearsal, ‘right the GP needs to know this’.
ST2, interview, 19 June 2013
The advocacy role occasionally taken by the STRIDE therapists was valued by staff in participating sites, as it enabled them to address any deterioration or emerging problems promptly.
The multiple medical issues faced by many clients led to a growing recognition by the STRIDE therapists that further falls were (often) inevitable. This in turn prompted a subtle shift to a more explicit focus on falls preparation whereby the STRIDE therapists aimed to minimise the psychological and physical impact of falls by making clients more robust, increasing positive safety behaviours (e.g. carrying a mobile phone) and providing advice on getting up after a fall:
Well I’m still worried about it [falling] because I’ve got Parkinson’s and I shake a lot but I was like given a lot of confidence when I actually did fall just by what she said ‘well just sit there and take some deep breaths and then try to get up and make sure you’re all right’ and that sort of thing. I’m still confident with that.
C4465, interview, 26 June 2014
Fit of cognitive–behavioural therapy intervention with existing interventions
All sites either provided in-house interventions or referred clients to appropriate community resources (e.g. Staying Steady or strength and balance classes; see Table 5). Given the varied nature of existing interventions available to clients, we explored the fit between the CBTi and these interventions. Clients interviewed for the process evaluation generally valued all of the interventions that they received. The physiotherapist at site 1 similarly saw the CBTi as being complementary to existing provision:
. . . quite often what we’re able to do is very clinic based. So I can give people exercises to do. I can do a certain amount of goal setting with them, but I can’t go out and see them at home [. . .] So I think it might be quite a nice opportunity to make some of the clinic-based stuff a little bit more meaningful.
Interview with P20, 24 March 2014
However, other professionals sometimes saw the CBTi as potentially overlapping or conflicting with existing interventions. This was particularly the case when goals related to deconditioning and clients were doing activities, such as sit to stand exercises and practising going up and down a single step. Although the STRIDE therapists considered the exercises as graded activities, as these movements were an integral component towards achieving the broader goal, clients typically perceived them as ‘exercises’ and described them as such when asked about the CBTi by professionals. Unsurprisingly, professionals at site 2 were concerned that the STRIDE therapists were giving patients additional exercises that were outside the scope of their rehabilitation programme. The study team tried to address this by arranging for a STRIDE therapist to attend a team meeting at site 2 but this did not entirely dispel their concerns about the CBTi:
So that’s why we went over there and talked to them, and told them what we were doing and how we’d gone about it a different way, and for a lot of them they were very open in that, in that meeting and for some of them, you got the impression they were sceptical. [. . .] I was very careful not to give exercises. I asked about ‘Have you been given exercises from your knee classes? What are you doing with the falls clinic? Could you do them a little bit more? Could that feed into what we’re doing?’ But a lot of it was breaking down say, sitting to standing, or people wanting to get on and off the floor to do their garden. I could break it down, because of my movement background and they might see that as being – it’s exercise but it was functional. It was very much functional, but then you did feel as though you were sort of treading a line.
ST2, focus group 1, 2 July 2014
A more general issue for the STRIDE therapists was how to manage conflicting advice. This arose most frequently in relation to the use of walking aids and care after a hip or knee replacement. All of the STRIDE therapists worked with clients who had internalised advice given after hip or knee replacement surgery and changed their behaviour permanently as a result (e.g. by never kneeling or by holding on to bannisters on the stairs). The STRIDE therapists were aware of the potential conflict between the CBTi and advice given by clinicians:
They are being told by the NHS ‘don’t do this any more; and don’t do that any more’ and ‘that would be dangerous’.
And we come in.
And we’re coming in saying ‘no, try it’.
Informal discussion ST2/ST3, 7 March 2014
The extent to which clients were willing to try the STRIDE therapists’ advice partly depended on the extent to which it was congruent with their own wishes and preferences. For example, one client who was dependent on a walking stick following a stroke wanted to return to the running track (having been a runner all of his life). The initial goal set with the STRIDE therapist was to walk round the track once or twice using his stick:
Anyway, the first time I went round, I walked round twelve times. I just put my stick down, that was it, walking round. Not just walking, speed, speed walking [. . .] The first two laps, I walked gently round. Then I’d, you know, get in my STRIDE, get faster and faster and faster. Then I’ll do ten fast laps, roughly three, four hundred metres in three, three and half minutes, or three and a quarter minutes. Which is what I’ve been doing all my life, this. Done running all my life. I felt, God, that’s great. So from that day on, I had the stick with us, all the time but I never, ever use it.
C4458 interview, 2 July 2014
Although this is an extreme example, it illustrates the ways in which clients could underestimate their physical abilities through becoming dependent on walking aids. Strategies that the STRIDE therapists might use to deal with potentially conflicting advice were explored in a group supervision session:
R4 asked whether they had tried to change clients’ behaviour. Although the STRIDE therapists had sometimes tried to tackle the issue, ‘but they’ve still got that ‘the doctor has said’. R4 suggested that one approach would be to say ‘ok your doctor was right, but that was for the acute period’. ST3 said that she had tried this approach and said that it had seemed to work. R4 elaborated that they could continue by saying: ‘that’s for the recovery period, it is absolutely the right thing to do, but in fact what you might be doing now is inadvertently keeping your knees a little bit weak . . . so agree with the doctors’ advice, so you’re not setting up an opposition between you and the doctor but just translate it, point out the difference between acute and ongoing’.
Field notes of group supervision, 19 September 2013
Other tensions between the CBTi and existing interventions related to the underlying ethos of services. This was most evident in site 2, which seemed more risk averse than the other sites. Their emphasis on safety was evident in the assessment documentation (which recorded whether or not a risk assessment was needed in relation to a range of areas, including moving and handling, medication, mental status); observations of interactions between staff and patients; and comments made by clients in therapy sessions and informal discussions and meetings with the STRIDE therapists:
. . . at the end of the assessment, a member of staff commented that it was ‘really, really safe here’. She then elaborated that two people usually walk patients down to the rehabilitation room. If patients are unsteady and are with just one member of staff, they will use a wheelchair to get them from the waiting room to the rehabilitation room.
Field notes of observation at site 2, 29 October 2013
But they tell you at the falls clinic never do exercises where you’re stretching yourself, only do them within your own limits.
C4661, session 2 with ST2, 10 December 2013
STRIDE therapist uncertainty over underlying rationales and specific techniques
The complexity of the clients inevitably challenged the STRIDE therapists in the light of their relatively limited training. Three specific areas in which the STRIDE therapists’ skills could have been developed further were the ability to transmit CBT principles to clients; tuning into client motivation; and setting SMART (specific, measurable, achievable, realistic and time-limited) goals. The psychologists anticipated that the STRIDE therapists would revert to existing patterns of interacting with clients in situations in which they were anxious or uncertain about new techniques. Two examples of such patterns were being directive when clients did not seem to be taking an active role in the CBTi, and focusing on exercise-based goals (particularly for the STRIDE therapist with a background in physiotherapy, as this was her area of expertise).
Transmitting principles of cognitive–behavioural therapy intervention to clients
Although the STRIDE therapists could clearly articulate the overall purpose of the CBTi, they had difficulties in transmitting this information to clients. A recurrent theme in discussions with the STRIDE therapists was the extent to which clients ‘got’ the intervention. The STRIDE therapists’ discussions of client understandings of CBTi often implied that clients were responsible for understanding the intervention. Although, towards the end of the study, one of the STRIDE therapists acknowledged her responsibility for facilitating client understanding, she still felt ill equipped to do so:
I feel it’s down to how I present a reiteration of how I worked with it. I didn’t do it effectively enough; because they should be able to get it if I’ve explained it well.
How would you do it more effectively now?
I don’t know. I still don’t know.
Focus group 1, 2 July 2014
Observation of CBTi sessions (and listening to recordings) indicated that the language used by the STRIDE therapists could sometimes be inaccessible (e.g. terms used included deconditioning, generic, premise, functional) and that there could be a lack of fluency, with repetitions and hesitations, in explaining the CBTi, which would exacerbate client difficulties in making sense of the intervention. The STRIDE therapists had very different approaches to explaining the intervention (Box 4) and also adapted their approach to individual clients. Consequently, it is difficult to identify which approaches were more successful in facilitating client understanding.
. . . the core with cognitive behavioural therapy is [. . .] you can work on one area and it will help with the others. And it’s called cognitive behavioural because you work on those two; of the four you work on how you think and what you’re doing.
ST1/C6285, session 1, 7 February 2014
. . . so the pain and dizziness affect your sleep which creates fatigue and life has become more limited, you feel more stressed in busy public places and as a consequence you possibly don’t go out in those busy places so it’s all closed in a little bit. So what I’d like you to do is, we have to come up with, one of the areas, what STRIDE’s about is increasing your confidence and your independence and your energy levels.
ST2/C2018, session 1, 4 December 2012
So what we could do through this treatment is we could look at your balance, look at a few simple exercises you could do to try and improve that a little bit, support you to try and increase your activity levels a little bit more, get you out walking maybe a little bit more to obviously improve your balance, lose a little bit of weight, get your confidence.
ST3/C5359, session 1, 15 October 2013
Although the psychologists highlighted the importance of ensuring that clients understood the rationale for CBT, limited attention was paid during the training to how this might be achieved in practice. Useful ideas for explaining the intervention were often embedded in other activities, for example when practising formulation skills one of the psychologists commented on:
. . . the need to be explicit about what CBT could offer clients; R4 advised the STRIDE therapists that the intervention was not going to ‘cure’ people, or ‘magically make it better’, but would just give them a bit more control over their life.
Field notes of training, day 3, 13 June 2012
However, the extent to which the STRIDE therapists absorbed these ideas to use in their own introductions was unclear, and the training provided few opportunities for them to rehearse how they might introduce the CBTi to clients.
The psychologists frequently emphasised that the STRIDE therapists would embed the skills and principles of the CBTi through practice and success with clients. This process also applied to clients, particularly in relation to relapse prevention. The STRIDE therapists found that relapse prevention sessions made more sense to clients, and helped to embed skills, if they were delivered at the time as a client had a fall or other setback, rather than being done in the abstract:
I had one patient recently that I did much better with relapse prevention because something happened during therapy. [ST2: Mmm.]
It makes a difference.
When something happens it’s so much easier to talk about it, than prep what if something happens. [ST2: Yes.]
Focus group 1, 2 July 2014
The varied client understanding of CBTi was confirmed by the client interviews conducted for the process evaluation, which showed that clients conceptualised the CBTi in a number of ways (see Table 40).
Beyond further training, suggestions for improving understanding of the CBTi focused around better preparation of clients, for example by a pre-assessment session or by providing materials, such as the client manual, in advance:
A lot of the clients will be, yes they’ll have signed up, in a sense, and accepted it, [. . .] but in terms of actually their understanding and you know something like you know them having access to the manual or something beforehand or whatever might be one way of then people’s, ‘Oh right so it’s about this and these are the kind of things’ you know. So it was a sense of actually what they’re being expected to perhaps being expected to do. So I think some pre-interview might actually be helpful with that.
R12, interview, 2 October 2012
The STRIDE therapists similarly suggested that a pre-assessment session would be useful to introduce the ideas and ‘socialise’ the clients into the idea of the CBTi. A separate session would enable the STRIDE therapists to provide a more leisurely introduction to the CBTi, and help clients to understand what was required of them if they were to get the most benefit from the CBTi. As the assessment and formulation session was perceived as off-putting to clients, a pre-assessment session could also help to build rapport in preparation for this session. Although the patient information leaflet provided an explanation of the study, a personal explanation, which could be tailored to the individual, was seen as essential.
Tuning into client motivation
Client investment in the process of CBTi was perceived as a key factor affecting the success of the intervention. Some clients preferred adapting their environment to changing themselves, others were thought to have ‘given up’ and accepted the limitations on their lives:
They move things, like instead of using upstairs cupboards they’ll put it on lower shelves so they don’t have to reach, things like that, just little things like that and gradually it deconditions them.
And then finally the mind-set of ‘they’re old and want to give up on living’ because we‘re seeing a lot of that.
ST2/ST3, informal discussion, 7 March 2014
Working with clients with low motivation is always challenging, even for experienced therapists. 98 It is therefore not surprising that the STRIDE therapists sometimes found working with these clients frustrating and were not always sure how to address the issue of motivation:
I think the ones that are going well; they have been engaged with the process. So it’s been like their personal, it’s been nothing that I could control; they’ve just been interested right from the beginning.
ST2, group supervision, 16 January 2013 (author‘s emphasis in bold text)
The STRIDE therapists developed a range of strategies to try to tap into client motivation, including negotiating, persuading, giving examples of other people in similar situations, exploring barriers, exploring benefits of change, exploring values and trying to encourage them to make even small changes:
Like I say you pick your battles, like some people yeah they don’t want to go out and do things but they might be willing to do something in the house so you encourage them doing that, they might have an exercise bike which might be the only thing you can encourage them to do so it’s just a case of supporting them to do what they’re willing to do [. . .] and the other thing is look at the parameters, so like the lady I just mentioned about her excuse is always ‘oh well it depends on the weather, I’ll only go out if the weather is nice’, so I might be looking around ‘well what do you class as nice weather’ and trying to shift it so instead of it being well blue skies, sunny, warm, changing it to well it might be a little bit cloudy but the threat of rain isn’t there so it’s like kind of making their willingness a bit bigger, if that makes sense.
ST3, interview, 20 May 2013
When these strategies were not successful, the STRIDE therapists tended to take a more directive role and suggest exercise-based activities and goals:
But if you go in and you say, ‘Maybe if you try sitting to standing a few more times?’ and they come in and do that, that might be their level of engagement and for some of them, that sometimes is all they need to do.
ST2, focus group 1, 2 July 2014
The feasibility of delivering CBTi successfully with clients with low motivation was questioned by one of the psychologists and the STRIDE therapists, and introducing further eligibility criteria or a pre-assessment session that explicitly explored motivation to change, were suggested as possible ways of targeting the CBTi on clients who were most likely to benefit:
I think probably there needs to be a sophisticated vetting process for the client [. . .] the FES thing, then that doesn’t, I think it doesn’t really tap into people’s motivation to change. And I mean that’s, you know you can say that for any therapeutic intervention, but I really think that’s critically important if you want to maximise your resources and so on, then you do need to be clever about who gets the intervention. It’s not for everybody.
R12, interview, 2 October 2012
As a result of discussions about the importance of client motivation and engagement, the STRIDE therapists were asked to rate each client’s engagement with the intervention on a 7-point scale. Of the 138 clients for whom ratings were available, mean level of engagement was 5.2 (SD 1.5), with only 9 (6.7%) rated as having low engagement. (This is similar to the proportion of clients who ‘rarely’ or ‘never’ completed homework assignments in a study of group CBT.)94 This suggests that discussions focused disproportionately on those clients with low motivation. Nevertheless a number of suggestions were made which could help to focus the CBTi on clients who were motivated to change, for example, by requiring clients to take responsibility for arranging at least the initial session (while this was not feasible in the context of a RCT, it may be appropriate if the intervention is rolled out in practice) or by formally assessing motivation to change, for example, by using measures such as Suitability for short term Cognitive therapy. 99
Setting specific, measurable, achievable, realistic and time-limited goals
Although the identification of valued goals that could be worked towards during the initial eight sessions was a central component of the CBTi, the ability of the STRIDE therapists to work collaboratively with clients and identify SMART goals was varied. The STRIDE therapists had no difficulties in explaining the principle of breaking down goals:
ST3 then reiterates that next time ‘we’ll come up with some goals that you want to achieve over the eight weeks’. She explains that they will ‘break them down into bite-sized chunks, or baby steps that we’ll work on over the weeks to build up to the long term goals’. She draws a parallel with running a marathon, developing a training plan and building up the distance over time.
Field notes of observation of session 1, ST3/C1521, 9 September 2013
As in the above example, the example of running a marathon was frequently used by the STRIDE therapists; more appropriate examples may have been more helpful (particularly as client feedback on the manual indicated that they valued examples with which they could identify). The importance of setting SMART goals and exploring obstacles was emphasised in supervision:
R4 highlighted the importance of defining clear homework goals; and signposted the STRIDE therapists to work on implementation intentions which indicated that people were more likely to follow through if there was a clear plan in terms of when, how often etc. [. . .] R4 went back to the issue of verbally negotiating the goal and ensuring that goals were SMART; he also suggested that they should explore potential obstacles to the goal.
Field notes of group supervision, 19 September 2012
These principles, however, were not always put into practice, and the STRIDE therapists could not always see how to break a goal into tasks:
Sometimes it’s helpful breaking these up, but some things, things don’t apply to it. So, because it’ll be like, you know, they’re like, ‘I want to arrange . . . you know, I want to go to the theatre and stuff’, okay, so it’s like, ‘Book a seat at the theatre’.
ST1, interview, 11 September 2013
In the above example, exploring implementation intentions and potential obstacles could have helped to identify specific tasks that the client could have worked on (e.g. anxieties about travel, difficulties hearing on the telephone, worries about delaying people in adjoining seats if they are not able to negotiate their way out of the seats quickly).
Identifying goals with clients who were ‘stoics’ (i.e. clients with fear of falling but no experiential avoidance; see Figure 3) was particularly difficult as:
They were really pretty much doing the things, they just needed that bit of support and guidance really in to what they wanted to do. And there’s only so much you can do with those people.
ST1, interview, 23 August 2012
This led to the emergence of a ‘supportive’ role, for which the STRIDE therapists primarily encouraged clients to continue with their existing activities rather than identifying new goals and applying CBT techniques.
Despite the strong emphasis in the training on working collaboratively with clients, as already described, when faced with clients who did not seem to ‘get’ the intervention or who seemed to lack motivation, the STRIDE therapists tended to become more directive and suggest goals. These were typically informed either by the process of assessment and formulation or through observation:
As she walked to the door I said ‘you’re touching all the walls’. I said ‘maybe we could look at creating a little programme where you improved your balance in the house’.
ST2, group supervision, 16 January 2013
Goals were also circumscribed by the physical abilities of clients and this contributed to the shift towards more exercise-based goals. The physiological knowledge of the STRIDE therapist with a background in physiotherapy was valued by the psychologists, as it complemented their knowledge base:
By nature of our training we think everything is psychological, so we forget that if you have been stuck at home for five years you’re probably not that fit, so when you do go out the house your heart rate is going to go up a lot because you’re not fit and you’re going to interpret that as an anxiety and you’re just going to start the whole panic cycle, so actually having that kind of physiological complement to that where you address the physiological deconditioning in tandem with that.
R4/R12 discussion, 7 May 2014
Reflecting the varied health and social circumstances of the clients, there was wide variation in the scope of the goals identified. For example, goals ranged from ‘picking something up off the floor’ (C1514) to ‘going to live in London for a month to learn a foreign language’ (C5072). Review of the goals recorded in the STRIDE therapist summaries highlights the non-specific nature of many goals. A total of 234 goals were identified by 129 clients (seven clients had no goals and data were missing for two clients). Of the 234 goals, context was specified for half (49%), duration for 14% and frequency for 8%. Reviewing goals and homework tasks recorded in case notes during supervision might have helped the STRIDE therapists to refine their ability to set SMART goals. The goals were coded into nine categories (Figure 30); walking-related goals were most common, with relatively few goals relating to social activities.
FIGURE 30.
Client goals for CBTi.

As part of the CBTi, it was intended that clients would grade the perceived difficulty of their goals at the outset of therapy, approximately midway through the sessions and in the eighth session. These ratings would indicate the extent to which the CBTi enabled clients to meet their individual goals and supplement the formal outcome measures. There was, however, significant variability between the STRIDE therapists in the extent to which these ratings were completed (the percentage of clients having ratings for at least one goal at all three time points ranged from 25% to 71%; p< 0.01; chi-squared). The STRIDE therapists found it difficult to elicit ratings from some clients, as they often preferred to describe their progress or difficulties verbally rather than attaching a numeric value. For the 118 goals for which ratings were available at the start of therapy and in the eighth session, the majority were rated as having reduced in difficulty (78.8%), with only a minority either remaining at the same level of difficulty (14.4%) or being more difficult (6.8%). The difficulties in consistently obtaining ratings from clients suggest that further training and discussion of eliciting ratings was needed.
Acceptability of the cognitive–behavioural therapy intervention
This section focuses primarily on the perspectives of clients receiving the CBTi and the professionals from participating sites. Where relevant, however, the perspectives of other stakeholders are also included. This allows an integrated perspective on each theme or issue rather than presenting the views of different stakeholder groups in isolation. In exploring acceptability we have focused on client views on the CBTi and the STRIDE therapists; the perceived benefits of CBTi; the practical organisation of CBTi; and professional views of the CBTi.
Client perspectives of cognitive–behavioural therapy intervention and the STRIDE therapists
Client interviews suggested that they conceptualised the intervention in terms of four domains: increasing self-awareness; learning practical techniques; companionship; and ‘not for me’ (Table 40). Although some clients perceived the intervention to focus exclusively on a single domain, others recognised two or more of these domains. Their perspective on the intervention also reflected the extent to which they took an active or passive role in the CBTi (see Table 40).
Within the category of self-awareness there was a distinction between clients who viewed the CBTi as an opportunity to learn more about themselves and gain insight into the factors contributing to their fear of falling and those who took a more passive role and relied on the STRIDE therapist to ‘make you think about it’ (C4158). In terms of practical techniques, some clients understood the intervention in terms of identifying targets and breaking these into manageable activities, whereas others perceived the CBTi as essentially exercise based. The latter clients, together with those who conceptualised the CBTi as primarily about companionship, tended to take a more passive role.
| Understanding of CBTi | Example of when clients were taking: | |
|---|---|---|
| A more active role | A more passive role | |
| Self-awareness | I think for once you get to be me, you get to have you time and analyse yourself and find out your own strengths and weaknessesC3028 | She saw which way I was thinking and she pointed us down the right road how to think in that directionC4158 |
| Practical techniques | I’m always seeing that long end target and what I picked up with talking with ST2 and from here [the manual] you know it’s to tackle things in small bite-size chunksPC07 | There’s one exercise she was telling me – just stand behind that settee, grab a hold and go down on my, balance myself and go down; I couldn’t do that before she startedC4465 |
| Companionship | – | We talk about what’s going on. She asks me what I’ve been doing, and I tell her what I’ve been doing, we have a chat about it and she’s good companyC0506 |
| ‘Not for me’ | I appreciate what you’re trying to do but I’ve got a feeling probably that what I’m doing myself is as good as it’s going to beC5367 | I cannot see the point, I get depressed, I get, waste of timeC5074 |
A final group of clients perceived the CBTi as ‘not for me’; these clients tended to withdraw either from CBTi or from the RCT altogether. Previous studies of falls prevention strategies have highlighted that older adults may perceive strategies as ‘better for others than for me’. 100 Clients who were already managing their fear of falling by continuing to be active despite their fear of falling (‘stoics’) tended to see the CBTi as superfluous, as they had no experiential avoidance. Although some of these clients continued with the intervention, with the STRIDE therapist taking a supportive role, others chose to drop out (see Table 40). A small number of clients did not identify as having lost confidence and therefore did not see the CBTi as relevant (this is consistent with dropout rates being associated with lower FES-I scores; see Table 22):
So we got to the rationale part and I said well ‘it sounds as if you’ve lost a little bit of your confidence’, ‘no I’m a very confident person, I don’t think this is for me’, really shut down, and I was like ‘OK well this is not for everybody, do you want to keep hold of the manual?’ ‘No, no I’m not interested’.
ST3, group supervision, 5 September 2013
Other clients who thought the intervention was ‘not for me’ tended to have a wider sense of resignation about their lives and capabilities and were not interested in pursuing change:
It’s no good doing things that are out of your reach; you’ve got to be feasible.
C5371/ST3, withdrew after session 3, 23 January 2014
Finally, a number of clients withdrew from the CBTi for reasons unrelated to the intervention including life events (e.g. bereavement), poor health and ongoing medical investigations or treatment:
She ended up withdrawing from the CBT side of things because it wasn’t appropriate for her at that time because she had far too much that needed to be done before true CBT could be carried out.
ST1, focus group 2, 17 June 2014
Dropout rates provide some indication of acceptability of the CBTi. Overall, 65.7% of clients allocated to CBTi completed the study (all of whom completed all nine sessions of the CBTi); 8.1% withdrew from the intervention but continued to complete outcome measures and 26.2% fully withdrew. Of those who withdrew from CBTi, 12.9% did so before their first CBT session, and 21.4% during the intervention after attending an average of 3.0 (SD 2.5) sessions.
Relatively few caregivers were directly involved with the CBTi; when they provided support to clients this mainly took place outside formal sessions. The involvement of caregivers centred on providing encouragement:
I have fell a couple of times like I told ST3 and it did knock us back and my husband after reading the manual opened the front door, put us out, closed the front door and locked it and said ‘you’ve done it before, go 3 doors up and come back’ and that’s the way I did it. I started from the beginning again.
PC14, interview, 13 September 2012
Independent of their understandings of the CBTi, clients consistently valued their interactions with the STRIDE therapists and the small number of caregivers interviewed similarly emphasised the interpersonal skills of the STRIDE therapists. In describing these interactions, comments related to four domains: the development of a therapeutic alliance; negotiation; support and challenge; and technical skills/advice (Table 41). The development of a therapeutic alliance is key to the success of psychological interventions101 and the STRIDE therapists proved skilled at developing positive relationships with clients. The challenges in working collaboratively with clients to identify goals was highlighted earlier in this chapter; while most clients reported that the process of developing goals and identifying tasks was shared, some of the phrasing used suggests that the STRIDE therapists sometimes took a more directive approach:
She [STRIDE therapist] used to spend an hour just doing exercises and talking about how you feel, how your confidence feels, setting yourself goals, like going to a football match. I used to be right into that and she said ‘well get one of your sons to take you’.
C4465 interview, 26 June 2014 (author‘s emphasis in bold text)
I can truly say that if she set me an objective the first week, we would discuss it the next time. She came and discussed it but she never let it go, until she could write it off and I knew that, so I did have to try.
C5969 interview, 29 January 2014 (author‘s emphasis in bold text)
| Domain | Example |
|---|---|
| Building a therapeutic alliance | She’s got such a pleasant manner and not overbearing or condescending or anything like thatC2042 interview, ST1, 28 January 2014I wish it would go on and on because I love her coming. I look forward to her coming, I think she’s good company and she’s got a lovely nature and she is the right type of person for the job I thinkC3009 interview, ST2, 22 October 2012She was very understanding with what I was saying and trying to say and she understood how I felt about going out and about the anxiety I felt about falling and she was really very understandingPC14 interview, ST3, 18 June 2013 |
| Negotiation | It was more like two friends meeting over a coffee and suddenly you think ‘oh I need an answer to this’ and then as you’re talking the answer comesC3028 interview, ST1, 8 November 2013We would just sit and chat and she would maybe suggest it or I would or maybe I would say something and she would say ‘oh, we could use that as . . .’ you know, for such and such or she would be talking about something and I’d say ‘oh that’s a good idea’. So it was, sort of, just between usC5960 interview, ST2, 28 January 2014C:It was a two-way thing all the time, she wasn’t sort of saying all the things, she was saying a little bit and then I would put my bit in and what I thought, but I can’t thinkF:Well like you discussed together targets that you might useC:That’s right, that’s rightF:. . . to help, didn’t you? But when she discussed them with you, she was finding out what you thought you could manage, rather than what she thought you could manageC3020 and daughter interview, ST3, 11 June 2013 |
| Support and challenge | With her being friendly and not sort of ‘oh, you must do it’ or ‘you’ve got to try harder’, it gives you more encouragement to try to please, you know, and reach that goal. As I say, I’m really looking forward to seeing her face when I say ‘I’ve made it!’C2042 interview, ST1, 28 January 2014Encouragement, encouragement; telling us I could do itC4458 interview, ST2, 2 July 2014I had to go to Sainsbury’s, so I goes out, lovely day, walks up the hill and everything, gets up to Sainsbury’s, does me shopping, coming out of Sainsbury’s the man at the top of the street says ‘Are you going straight home [client’s name]?’ I says ‘yes’. ‘Come on pet, I’ll give you a lift’. So I did, I got the lift back. She said ‘we’re not counting that because you’ve got to go both ways – up the hill and down again’C5969 interview, ST3, 29 January 2014 |
| Technical skills/advice | About the middle of her sessions, she took me down to the metro station and showed me just how the ticket machine operated, which seems stupid, but you know you get frightened of these things and in fact it had changed quite a bit since the last time I’d used itC5377 interview, ST1, 30 June 2014She put me in touch with bereavement people and everything, which was very goodC4465 interview, ST2, 26 June 2014She came with some really good ideas to do thingsC5969 interview, ST3, 29 January 2014 |
It was clear, however, that clients did not find this approach oppressive or too directive. This suggests that the STRIDE therapists modified their style in terms of working collaboratively or being more directive according to the extent to which clients took an active role in identifying goals and/or tasks. The support/challenge model highlights the importance of judging the optimal levels of both support and challenge. 98 The interviews indicated that clients felt able to modify tasks that seemed too challenging, and were fully supported by the STRIDE therapists in doing so. Some challenges seemed (from the perspective of the process evaluator) too great; for example, one client reported having been physically sick prior to going out in the dark for the first time for years; another client had been terrified during a 10-minute walk down a hill (which took her 45 minutes to complete). Both of these clients, however, reported feeling an immense sense of pride and achievement on successfully completing these challenges.
Although professionals in participating sites received relatively little feedback from patients about the intervention, any comments tended to focus on the interpersonal aspects of the CBTi and were consistent with the interview data:
I mean people have come and said, you know, ‘That nice ST3 comes out to see me’ or, ‘Isn’t ST1 lovely, she comes to see me.’ And, and so they’ll tell me more about, I think, the interaction that they’ve had, the interpersonal side of things.
P20 interview, 24 March 2014
Perceived benefits of cognitive–behavioural therapy intervention
A range of positive outcomes from the intervention were identified by clients, caregivers and STRIDE therapists (Table 42). Although some clients reported specific changes, others found it difficult to explain the outcomes of the CBTi:
All I know is how I feel and I feel different. I feel good and I don’t think ‘oh I’d like to go to such as such – Oh I couldn’t manage that’; now ‘I’d like to go such and such, right we’ll go’. It is nebulous, it’s very difficult to explain.
C3028 interview, ST1, 8 November 2013
No negative outcomes of the CBTi were reported, even when clients had fallen during the intervention period. Caregivers generally identified similar outcomes, although some felt the client was exaggerating the benefits. Some caregivers had directly benefited from the CBTi, either because of reduced anxiety or concern about the client, or because of improvements in the client’s mood (see Table 42).
| Domain | Example |
|---|---|
| Increased confidence | She just told us I could do it. I just, I felt I would never be able to do anything again. And when ST2 told us to try, I went and said, ‘Maybe I can do it’C4458 interview, ST2, 2 July 2014Certainly I’m very much more confident than I was before it startedC5377 interview, ST1, 30 June 2014 |
| Decreased anxiety | I was getting I didn’t want to go out, you know? And I thought, ‘Oh I could go out today and I might fall’, you know? And it was on my mind all the time, where now, I don’t even think about itC3514 interview, ST3, 30 June 2014I’ve always had a thing about coming down these stairs, I’ve always stopped at the top and thought ‘right, now I’ve got to get down these stairs’, now I don’t, I just come out of the room and go downC3028 interview, ST1, 8 November 2013 |
| Increased independence | Well my husband’s over the moon. Yeah because I used to rely on him a lot to do a lot of things and now, I mean if I needed a birthday card he used to have to go and get it because I wouldn’t even venture up to [place 33] on me ownPC14 interview, ST3, 13 September 2013 |
| Improved mood, enjoyment | I’m much more cheerful and outgoingC3028 interview, ST1, 8 November 2013When I was going out it was lovely, it was nice well summer is nice anywhere when you’ve got lovely early mornings, I felt like I was grabbing the new day, it uplifted me [. . .] it gave me an energy boost, it felt goodPC07 interview, ST2, 1 November 2012I mean, with her getting frustrated she’ll yell at me for the least thing [. . .] I’m calling it depression but I would say frustration might be a better word. And she couldn’t do it and if things went wrong, if she dropped something or whatever and couldn’t. . . ‘Aargh,’ type of thing, you know? But now since we’ve had the five weeks with ST3 and different things is happening, getting better, getting more relaxed againC3029 and husband interview, ST3, 11 June 2013 |
| Increased activity | Just by doing the tasks, and setting myself little tasks as well. Going somewhere, and saying, ‘Well, I’ll go here.’ So I used to sit, planning, and when ST3 went away, I said, ‘What can I do? What am I doing? Where am I going?’ Get my days planned out, which meetings I’ve got to go to, and then other things, into the town [. . .], I often go down there, just shopping. Having a look around the shopsC0506 interview, ST3, 22 October 2012 |
| Increased self-acceptance | I’ve got over the feeling that it [falling] is totally embarrassing and it’s not right to fall, it’s not shame to fall flat or whatever, you know. And now I think if I fell I would be all right. Yes, I would have the confidence to say, ‘Well you either help me or you don’t’C5969 interview, ST3, 29 January 2014I know I cannot do the marathon now, you know. I know, like, me limitations a bit better and accepting them, that’s the thing I thinkC4158 interview, ST2, 29 January 2014 |
| Improved walking/balance | I used to leave a bruise on her [sister’s] arm. She used to have a bruise every time she went home. She used to say, ‘Do you know you’ve got my arm black and blue,’ ‘cause honestly I used to cling on for grim death.’ And she used to say, ‘For goodness’ sake, can you not leave go?’ And now I don’t need her armPC14 interview, ST3, 18 June 2013We’re getting people off their sticks, I’ve have a few people that don’t use their stick as much – either stopped using it in the house or just carry when they’re out and about, which I think makes a big differenceInformal discussion ST2/ST3, 7 March 2014 |
| Increased motivation | I’m more confident about doing other things, and getting on with them, instead of sitting and saying, ‘Oh, I’ll do it tomorrow. I’ll do it tomorrow, or the next day.’ I’ll tend now to get on with itC0506 interview, ST3, 22 October 2012 |
Professionals working in participating sites occasionally gained some insight into client experiences of the intervention during follow-up appointments:
The ones that have been in the treatment arm all seem to be, they have engaged I think and they’ve, all the ones that come back anyway that see us, they seem to be very positive, they’ve all been doing a bit of exercise as well as whatever the CBT aspect is and most of them seem to have benefited, I haven’t had anybody who has said it’s been a negative experience at all.
P4 interview, 19 June 2014
Internalising the principles of cognitive–behavioural therapy intervention
A key aim of the intervention was to equip clients with the skills to apply CBTi principles to new contexts and become, in a sense, their own therapists. Although available data provide only limited insight into the extent to which the principles were internalised and the factors that facilitated or inhibited clients’ abilities to generalise the CBTi to new challenges, it was clear that clients varied in the extent to which they had embedded the underlying principles. For some clients, the role of the STRIDE therapist in providing encouragement seemed crucial and, when such support was withdrawn at the end of the eight sessions, they were not necessarily able to sustain changes on their own:
There are patients where you’ve gone back and it’s been like, ‘So how have you done?’ ‘Well, I did it for a bit.’ ‘So what stopped you? What curtailed it?’ ‘Oh, well I just stopped doing it as much.’ I think it’s – and they’re like, ‘Well you weren’t here’.
ST1, focus group 1, 2 July 2014
Although some clients continued with the exercises suggested by the STRIDE therapist, they did not always have the ability to identify new goals and break these into manageable (and relevant) steps. For example, when one client was asked in an interview whether he had any current goals, his response was ‘to be able to go out by myself and maybe have a pint in the pub’ (C4465, ST2). When asked about how he planned to achieve this, he explained:
. . . just actually getting around in the house and being more independent, our [name of son] still comes every morning and me other son comes at teatime and before I go to bed one of them will ring us.
C4465 interview, ST2, 26 June 2014
When the interviewer tried to clarify how this related to the goal, the client explained:
Oh I’ve got miles to go yet; it’s in [village], it’s about a mile and a half away, 2 mile, so I don’t think I’ll ever achieve that one.
C4465 interview, ST2, 26 June 2014
Applying the principles of the CBTi to going out during the winter was challenging. Some clients simply reported that they would not venture outdoors in poor weather, but one client explained how she would use the techniques learned:
If it’s just snowing I’m fine. But once it starts getting a little bit slippy, I just refuse to go out, you know? I just haven’t got that confidence for that yet, you know?
C3514 interview, ST3, 10 June 2013
I would judge on the day how I feel, what the weather is like, but I’d also take into account now I’ve had these sessions with ST2 ‘well really wait a minute how can we get around this and make it better, what if it’s going to be icy for a week you’re not going to get out, I’ve got to get out, how am I going to get out?’ So yes, I would be starting to think again ‘what’s the best way?’ Like my footwear I’ve got special things I put on over my shoes and walking you know I look where I’m going. I mean even when it’s been really bad [. . .] I’m thinking you cannot be wrapped up in cotton wool all the time, you cannot. So yes it has made a difference because I would break it down again.
PC07 interview, ST2, 1 November 2012
The manual was a valued resource for some clients who referred back to it to enable them to maintain progress despite setbacks:
The way I did it was I sort of like cut my brain in half and had a conversation with the left side of my head and the right side of my head. When the left side of my head said ‘no you were going to fall’ I took on board, if it makes sense to you, the manual and convinced myself I wasn’t going to fall because the manual said positive thinking and that’s what I did and I used to have this conversation in my head and I mean I was telling my sister and she looked at us as though I was going crackers but it does work, it does.
PC14 interview, ST3, 13 September 2012
Increased awareness of the ways in which fear of falling (often alongside physical impairments and ill health) had limited their activities often helped motivate clients to maintain activities:
I do sometimes think, ‘I’m not going back there.’ And the coffee morning, not last Saturday but the Saturday before, was that wet day and I’m sitting there [. . .] and I’m thinking, ‘I’m not going. Oh I’m not going to go. I’m not going to get soaking wet and not go. No, I’m not going to bother.’ And then I just thought, ‘Aha, get yourself organised. Go in the car.’ And that was it, so I went.
C5969 interview, ST3, 29 January 2014
Practical organisation of cognitive–behavioural therapy intervention
The perspectives of all stakeholders are included in this section. Few quotations are included in this section due to space limitations; instead an overview of the advantages/disadvantages of existing arrangements and suggested changes is provided in Table 43.
Several suggestions made by clients were already in place. For example, the STRIDE therapists occasionally accompanied clients with tasks; this was seen as a legitimate activity by the psychologists, particularly in the initial stages:
I think if people can do it on their own that’s you know it’s even better but we want safe experiences, people to have safe experiences, we don’t want failure experiences, so if it means going out with a STRIDE therapist then I would certainly encourage them.
R4/R12, discussion 19 June 2013
Although some clients suggested more flexibility in the timing and duration of sessions, this was already offered by the STRIDE therapists, as and when needed. Although the therapy nominally comprised eight sessions at weekly intervals, in reality the sessions were scheduled to fit in with clients’ other commitments (reflected in the average duration of the initial eight sessions; see Chapter 4). This meant that the 8-week assessment, designed to capture immediate postintervention outcomes, frequently took place before the CBTi was complete (see Chapter 4). The duration of sessions was also flexible; some clients (typically those who were well motivated and/or for whom the STRIDE therapists had a supportive role) had sessions of only 15–20 minutes once the intervention was under way. The flexibility in delivering the CBTi reflects the constraints of working in a ‘real world’ setting. Many of our clients were active, but many more were unwell with multiple comorbidities, and hospital and GP appointments, and hence found it difficult to commit to a rigid timetable of weekly sessions.
There was a strong preference for home visits among clients and, despite the additional costs in terms of travelling time, the STRIDE therapists also thought home visits were appropriate for this client group (particularly at the beginning of the intervention):
Well initially one of the things that you’re fighting against is going out, so you wouldn’t go to a clinic [. . .] it would be an easy cop out not to go. There’s no way would you not be in for somebody coming to your home. But you would use any excuse [not to go to a clinic], the cold, the rain, too sunny, too hot. You would use any excuse.
C5969 interview, ST3, 29 January 2014
| Aspect | Benefits of existing arrangements | Drawbacks of existing arrangements | Suggested changes |
|---|---|---|---|
| Number, duration and frequency of sessions | Generally seen as appropriate | Eight sessions was not seen as adequate to work successfully with clients with multiple barriers Clients seen during the summer had little chance to apply principles to icy weather |
More flexibility Shorter, more frequent sessions Seasonal booster session to prepare clients for the winter |
| ‘Homework’ | Generally seen as helpful | Varied compliance | STRIDE therapists to accompany clients with initial tasks |
| Home visits | Convenience Clients felt relaxed STRIDE therapists could see the home environment Appropriate for clients with fear of falling who often struggled to go out (or to use public transport) and who admitted that they might not have attended clinic appointments |
Time-consuming for STRIDE therapists Home environment could be distracting |
Use clinic appointments when congruent with client goals (or when preferred by clients) |
| Timing of follow-up session | Gives opportunity to put skills into practice | Easy to lose incentive or forget techniques Long time to wait if the client has a setback shortly after the initial sessions |
More flexibility so support can be accessed in the event of a setback More gradual withdrawal after initial eight sessions (e.g. follow-up at 1, 3 and 6 months) |
| Individual sessions | Opportunity for in-depth discussions Intervention could be tailored to individuals |
No opportunities to compare strategies and provide a sense of solidarity (or competition) | Addition of a group session to explain CBTi at the beginning Addition of a group session at the end/after the 6-month follow-up to maintain impetus and provide support |
| Client manual | The presentation, content and language used were acceptable to clients Clients valued the examples, particularly when they could identify with them |
Some clients did not engage with written materials | Production of a simplified version |
From the STRIDE therapists’ perspectives, offering an intervention in primary care seemed more appropriate than basing a service in secondary care or specialist falls services. This would allow the early identification of clients with fear of falling (or barriers to activities) and could avoid some of the problems of implementing CBTi at the same time as addressing other medical problems:
Or even offer a service within a GP practice – if you have any concerns about your confidence, about your walking kind of book an appointment with dadadadada. You know, that may be the best way to start as such to actually having someone within your primary as supposed to your tertiary and secondary’s and stuff. Or even have sort of more person bouncing between GP practices to GP practice spending a day in each one.
ST1, focus group 2, 17 June 2014
As highlighted in previous sections, other suggested changes included a pre-assessment interview, assessment of client motivation to change, ensuring that any medical conditions were optimally managed prior to the start of the CBTi and better integration with existing interventions, in particular combining CBTi with specialist input relating to exercises.
Ideally you should do them [rehabilitation and CBTi] combined; doing one before the other doesn’t quite work as well I would have thought. Obviously we didn’t have the opportunity to really do it properly but I think if you combined some of the stuff we did in CBT with like physio rehab on the patient . . .
Like strength and balance classes.
. . . would work really well.
Focus group 2, 17 June 2014
Professionals’ views of cognitive–behavioural therapy intervention
None of the sites offered specific interventions targeting fear of falling. The views of professionals in site 3 on the CBTi have already been described (see Chapter 2). Senior staff in site 2 hoped that the CBTi would complement their existing rehabilitation programme by directly addressing fear of falling:
Fear of falling wasn’t really changing or was increasing so that’s where this comes in because we were hoping that the addition of the talk through the CBT would possibly help us.
P21/P22 interview, 28 November 2013
Although at the outset of the study, some professionals subscribed to the view that fear of falling would automatically decrease with improved physical strength, their views seemed to have changed by the end of the study:
There are some people still I see with very severe fear of falling and it’s a great shame that there doesn’t seem anything to do other than offer them physiotherapy in the vain hope that this is somehow going to improve their confidence.
P4 interview, 19 June 2014 (author‘s emphasis in bold text)
Despite the lack of robust feedback, staff at sites 1 and 2 expressed an interest in providing CBTi after the end of the study, for example by training existing staff to deliver CBT alongside their existing rehabilitation programme or by employing one of the STRIDE therapists on a sessional basis:
I think the useful part is thinking about our own healthcare assistants being trained to do it; they do a lot of the rehab and so they build up quite a relationship with patients so in that way I think it would be useful, very useful.
P21/P22 interview, 28 November 2013
In site 1, where there were perceived to be ‘pockets’ of CBT within the Trust, it was suggested that a ‘generic CBT team’ could be developed who could work into different hospital departments and clinics as needed:
. . . that means that you’re not just relying on one person so if they then leave or go somewhere [. . .] you’ve got a bit of succession planning and you’ve got a bit of a group.
P4, interview, 19 June 2014
Conduct and delivery of the randomised controlled trial
Managing a perpetual state of flux
The study faced a number of challenges throughout, including delays with research passports, disagreements over the funding and banding of the HCAs to deliver the intervention, threats to the continued funding of the initial (single) site and recruitment difficulties which resulted in a threat to the continued funding of the study. In addition, as with most studies, changes to the protocol and sites resulted in a number of ethical amendments. The STRIDE therapists also had to cope with a number of practical challenges including not having an office or access to computers when they were first appointed. The timing of these various events meant that the study team was faced with an almost continuous stream of challenges; although these challenges are by no means unique, the number and pace of them was particularly relentless within the present study. Although morale generally remained high among core staff (i.e. those most heavily involved in the study on a day-to-day basis), there were moments at which morale dipped either individually or (less frequently) collectively:
I think there was a real loss of morale 2 weeks ago, everybody was thinking about different jobs, they were all thinking everything was ‘going down the pan’ as they put it, so even if they were invested in the therapy, they weren’t invested in the study because they didn’t believe it was working.
R4, informal discussion, 3 May 2013
As with previous research,102 delays with research passports impacted on the study either by delaying data collection by staff already in post or by delaying the appointment of temporary staff to facilitate recruitment. The process of adding the study to CB’s existing research passport took 7 months, meaning that fieldwork and analysis relating to Phase I of the study had to be condensed into a very short period. Even when additional staff were recruited to temporary posts to facilitate recruitment and/or baseline assessments, some took up other posts because of delays in obtaining their research passports. Similar difficulties were experienced in providing support with recruitment in site 2:
R30 had been keen to help on a voluntary basis with recruitment in site 2 but had not been able to start until December despite having agreed to help out in August. These delays had occurred despite having supportive human resources and research and development departments.
Field notes of TSC meeting, 16 January 2014
The recruitment of the STRIDE therapists proved more complex than anticipated. The planned grading of the posts (band 2) was contested and following extensive consultations the posts were advertised at band 4 because of the relative clinical independence of the posts. Owing to factors outside the control of the study team, the posts were advertised as CTAs based within the CRF. A generic CTA job description was used with the only permitted modification being the addition of a single sentence (on p. 5) explaining that the purpose of the post was to deliver a CBT intervention to older people. As a result, one candidate assumed that CBT would just be one part of the role and that the post would be based ‘. . . in a clinic, possibly taking blood’ (ST2 interview, 27 June 2012) and a second candidate thought ‘it was going to be like a drugs trial or something like that’ (ST3 informal discussion, 2 July 2012).
The implications for the study and the generalisability of the findings relate to whether the candidates applying for a CTA post differ systematically from those who would have applied for a HCA post (as originally intended). Several professionals queried the extent to which the STRIDE therapists were typical of HCAs:
Is ST1 a typical representative of a Band 4? I don’t know, it seems to me she’s quite exceptional [. . .] she’s got great, far greater skills I think, personal skills and engagement and again professionalism and reliability and not off sick [. . .] you didn’t really have typical people.
P4, interview, 19 June 2014
Despite their reservations, professionals recognised that it was becoming increasingly common for people with degrees to work as HCAs in the current economic climate. Indeed nearly all of the applicants for the posts were graduates:
The health-care assistants I’ve come across in previous, in private physio practices are almost all graduates with things like sports science degrees [. . .] that might just be the current demographic where there’s a lot of people who want to go into medicine or physio or sort of health-related sciences and can’t get a vocational training job.
Given the nature of the post – delivering a CBT intervention – it is likely that the post would have attracted recent psychology graduates who were interested in pursuing a career in health care. The fact that two of our STRIDE therapists were from this background suggests that the people whom we appointed were not untypical. The third STRIDE therapist had a background in physiotherapy and was studying for a master’s degree in clinical research.
The study was initially funded as a single site study. The funding of this site came under threat on three occasions during the lifetime of the study and, eventually, funding was withdrawn and the clinic closed at the end of the study recruitment period. Negotiations with the commissioners meant that we succeeded in delaying the closure of the site by reducing clinic hours for the last few months of the study. The recruitment of two further sites had significant implications for the team in terms of research governance, travelling time and in understanding how the intervention ‘fitted’ with the service already provided at each of the three sites.
Recruitment to the trial
Low recruitment rates led to a monitoring meeting with the funders in February 2013 (month 9 of the 32-month data collection period) and the potential withdrawal of study funding. The level of the core team’s investment in the study was highlighted by the intense efforts made to reach recruitment targets to ensure continued funding. This took its toll on team members both physically and emotionally and led to reallocation of resources to ensure the continued viability of the study. An agreement by the funder that the power of the study could be reduced from 90% to 80% enabled the required sample size to be reduced from 580 to 412. Even with this reduced target, the issue of recruitment remained challenging until the end of the study when we succeeded in achieving the recruitment target the day before recruitment closed. A range of factors contributed to our difficulties in meeting our recruitment target, none of which is unique to the present study. 103 An overview of the challenges and strategies implemented to address these is provided in Table 44. The strategies used to enhance recruitment were informed by Patient and Public Involvement (PPI) and client interviews.
| Stage of recruitment | Challenges | Strategies |
|---|---|---|
| Determine eligibility | Additional work for professionals in sites 1 and 2 Lack of research experience in site 2 in maintaining screening log Lack of systems in site 2 for recording whether or not patients had been approached and/or the outcome of an approach Difficulties in operationalising eligibility criteria |
Limited administrative support provided in site 1 by one member of staff who facilitated recruitment Trial manager provided some support/training Senior staff introduced a more robust system for monitoring contacts, but implementation continued to vary Re-checked at consent visit by RAs using eligibility checklist |
| Provide with study details and expression of interest form | Professionals in participating sites had only a vague understanding of the CBTi Extent to which professionals were willing and able to introduce the study varied The lack of focus on fear of falling within participating services created genuine difficulties in introducing the study, as it did not readily flow from existing routines and interactions Insufficient time to explain the study to patients in a way that was tailored to their individual circumstances |
Briefing meetings held with professionals to provide more information about the CBTi External staff (STRIDE therapists, CTAs or volunteer) were provided to approach patients ‘Script’ or bullet points provided to help professionals give a brief introduction |
| EoI form returned to study team | EoI form was easily ‘lost’ in the routine paperwork provided (particularly at site 3) PIS seen as inaccessible to clients Process of opting in seemed too burdensome for the client group, many of whom had low levels of motivation |
By involving external staff, paperwork relating to the study was given separately to that relating to the clinic PIS revised and shorter version produced Process simplified to reduce client burden |
| Contacted by RA, eligibility confirmed, consented, baseline assessment completed and randomised | Difficult to engage clients in meaningful discussion of study over the telephone Concerns of RAs who were responsible for recruitment over pressurising clients into participation Difficulties in explaining CBTi to clients in an accessible way Perception that the study was not relevant to clients |
Emphasis on arranging a visit to discuss the study in person Recruitment meeting to explore concerns; further support provided via office meetings Discussion of strategies; STRIDE therapists trained external staff involved in recruitment Review of common concerns raised by clients and ways to respond to these |
| Obtaining complete outcome measures at all time points | Perceived burden of diaries and outcome measures Some clients found questions in self-completion measures off-putting |
Any missing data identified and RAs checked for non-returned data (self-completion questionnaires and falls diaries) at final visit Focus on key outcome measures Telephone administration of FES-I and key data within questionnaires if client unwilling to have a final home visit Telephone reminder for non-returned self-completion questionnaires introduced Clients advised to exclude any questions with which they did not feel comfortable Self-completion questionnaires completed by RA at 6- and 12-month follow-up visits where possible |
Although using external staff provided by the CRF to introduce the study to eligible clients was successful, the study team was not always informed which clinics were being covered by CRF staff or when CRF staff were on leave. This resulted in incomplete coverage of clinics. Clinic staff were similarly unaware of when CRF cover was being provided and so were unsure whether or not they should discuss the study with patients. Improved communication enabled most of these issues to be resolved. Successful changes to the recruitment strategies resulted in an influx of work and backlogs in clients waiting to be contacted by the RAs for consent and baseline assessment, which required further shifts in resources to enable clients to be seen and randomised with minimal delay.
In order to meet recruitment targets, the STRIDE therapists became involved in recruitment. This had a number of benefits – chief among them the successful recruitment of the required number of clients for the study. Other benefits for the STRIDE therapists were an increased awareness of participating sites and more insight into the client pathway into the study. There were also wider benefits for the study, as the direct contact with the STRIDE therapists increased professionals’ confidence in the intervention:
So I knew that they were nice, nice isn’t the word, professional people, so that makes a difference because if you have somebody who is going to see somebody who is disinterested or not bothered or whatever then you don’t, then that would have an effect on their perception of our unit, but I didn’t, I knew that they weren’t like that and I knew that they you know they’re always good with their appointments and you know they turn up, they’re reliable aren’t they?
P4, interview, 19 June 2014
Capturing the outcomes of cognitive–behavioural therapy intervention
While there is a comprehensive evidence base surrounding the use of the FES-I in the evaluation of fear of falling,29,31,56,104 professionals in all sites expressed concerns about the use of the FES-I as the primary outcome measure for the trial. Sites 2 and 3 had before-and-after FES-I scores for patients who had completed the in-house rehabilitation programme, and strength and balance training, respectively. Neither of these interventions had resulted in an improvement in FES-I scores, leading staff to question whether or not the FES-I would capture any changes resulting from the CBTi. One professional additionally queried the interpretation of any changes in FES-I score:
They’re grading how confident they feel on a range of activities which may or not be important to them. So I think it’s very difficult to say, ‘Well I’ve gone from four to two on sweeping the floor’ you know, that’s good, but what does it actually mean to that person?
P20, interview, 24 March 2014
One problem for the RCT was the need to specify the outcome measures in the bid prior to the preliminary therapeutic interviews which provided an understanding of the factors involved in fear of falling and the likely outcomes of an intervention:
We need to know what sorts of things they think they are likely to change before we choose
R4, informal discussion, 13 July 2011
The interviews with clients confirmed that their underlying fear of falling might not have changed, despite a range of other positive self-reported outcomes (see Table 44). The psychologists and STRIDE therapists similarly identified a wide range of outcomes that might not be captured by the FES-I:
Some people’s lives have certainly blossomed as a consequence of it and you know that may only be the fact that you know they’re obviously walking up the stairs now within their house, or they’re actually able to walk to the kitchen and so on without you know to going out you know independently around town on you know on metros and are walking on the promenade or whatever.
R4/R12, interview, 7 May 2014
Reflections on the process evaluation
In terms of the models of qualitative work in trials, the present process evaluation was an example of an ‘integral-in-practice’ model105 for which senior qualitative expertise (TF) was incorporated throughout the study; the qualitative researcher (CB) was valued as a ‘full’ member of the team;105 the process evaluation was adequately resourced and the team were committed to acting on the findings. The role of the process evaluator was facilitated by the openness of the research team to a degree of observation and self-reflexivity exceeding that which would normally be expected in such studies. This proved a key strength of this study which has allowed the capture of important data concerning the processes (and challenges) of delivering a CBTi to older people with fear of falling. Although colleagues recognised the challenges of the process evaluation, they were willing to be involved:
I can understand it’s fascinating. I think God that must be such a slippery place to be in terms of data and all that kind of stuff. I think God I’m not sure that’s something I would want to get involved in doing it. But it’s interesting that it’s actually being done and I think it’s a really good – a great opportunity.
R12 interview, 14 November 2011
The close working relationship between the process evaluator and one of the psychologists was key to the success of the process evaluation in terms of providing support, ongoing discussion of data and exploration of emerging issues and reflecting with a colleague with a therapeutic, rather than a qualitative, perspective. In commenting on drafts of this chapter, one of the psychologists reflected on the difficulties CB was having in writing about how the STRIDE therapists and clients made sense of the CBTi:
The struggle that you are having is a mirror of the struggle that both STRIDE therapists and clients will have had in that process of negotiating a shared understanding of what it was they had found themselves doing together in the room!
R12, e-mail, 19 March 2015
The challenges for the process evaluation were largely concerned with resource management and prioritisation. Many different tasks and situations competed for attention, with the process evaluator even becoming involved in recruitment at times to ensure that the study continued. Some of the difficulties were common to any process evaluation – in particular the inability to know at the time of data collection which factors might emerge as central in explaining the trial results. This tended to result in a focus on data collection at the expense of analysis. It was sometimes difficult to know whether or not and how to use sensitive information. Responsibility for enacting decisions was not always clear and this could result in agreed actions either never happening or being delayed. A separate challenge related to disentangling effects; for example, the shift to more exercise-based interventions reflected multiple factors and understanding cause and effect in this context was impossible.
Despite the challenges, our experience has confirmed the value of undertaking in-depth process evaluation alongside the development and delivery of a RCT,106 in an iterative manner that allows for timely feedback to optimise processes where necessary.
Understanding the findings of the process evaluation using normalisation process theory
The process evaluation for the STRIDE study aimed to identify, describe and explain the professional and organisational factors that promoted or inhibited the implementation and integration of the CBTi. This should facilitate the transfer and integration of the CBTi into routine clinical practice. We have highlighted the complexity of delivering the CBTi and the ways in which the STRIDE therapists and psychologists sought to adapt the intervention to meet the varied clientele. We have also reported factors affecting the conduct and organisation of the trial, and shown how core team members and the STRIDE therapists were highly committed to the study and made strenuous efforts to ensure its successful completion. In the following discussion, NPT is used as a framework to explain the factors which influenced the extent to which the CBTi was embedded in practice. NPT focuses on understanding the collaborative ‘work’ that is needed to support the embedding of a new practice, and in this study various stakeholder groups – STRIDE therapists, clients, professionals working in participating sites and the research team – were key to this process. Our main emphasis here is on the embedding of the CBTi, rather than the trial itself, although these are inextricably linked and, for some groups (e.g. research team), the work of embedding the CBTi was primarily through the study processes. Implications and recommendations for future delivery of the CBTi are presented and discussed in Chapter 7.
Making sense of cognitive–behavioural therapy intervention
For stakeholders to invest the necessary time and effort in making the CBTi ‘work’, the intervention needed to have coherence, that is to ‘make sense’ to the different stakeholders involved in the study. The achievement of this varied both across and within stakeholder groups. For the STRIDE therapists the work of ‘making sense’ of CBTi was initially hindered by the greater than anticipated diversity of clients and slow recruitment. Through the process of enacting CBTi over time, supported by the initial training and ongoing supervision, the STRIDE therapists’ understandings of the intervention developed, although they remained less confident with some aspects of the CBT approach. For clients achieving coherence at a conceptual level could be difficult and they had varying understandings of what the intervention involved. For some, the intervention came to make sense through the process of ‘doing’ CBTi; others, however, never ‘got’ the intervention, which could limit their ability to internalise and generalise the underlying principles of CBTi. Some professionals were uncertain about the relevance of psychological therapy to clients attending for falls assessment and the team had to work to address these concerns during the intervention development phase to ensure that staff would be comfortable recruiting clinic patients into the trial (see Chapter 2). This process was more challenging in site 2, where no members of the study team were directly involved in service provision and there was a risk averse culture. The research team shared a general understanding of the CBTi and the study as a whole, although at times different understandings about study processes (e.g. data collection) among team members became apparent, and needed to be resolved.
Investing in cognitive–behavioural therapy intervention
Engagement and commitment to making the CBTi ‘work’ – cognitive participation – was generally high. The STRIDE therapists were employed to learn the skills required for delivering the CBTi, but thoroughly embraced their role and became central to problem-solving. The training gave therapists legitimacy, despite raising their awareness of challenges in working with the client group. Over time, the therapists became increasingly engaged in the practical organisation of therapy, ‘taking ownership’ of the CBTi, as well as some study processes and documentation. Clients themselves engaged variably with the CBTi. Although the STRIDE therapists utilised a range of strategies to promote clients’ engagement with therapy, their efforts were not successful with all clients. The engagement of professionals, who had a key role in recruiting participants into the trial, was variable across the sites. The core research team were highly motivated to complete the study, solving problems as they presented, and actively engaging in additional roles and tasks where required to achieve study milestones (e.g. recruitment).
Enacting cognitive–behavioural therapy intervention
The collective action work around conducting the CBTi, and the trial as the supporting structure for it, presented challenges for all stakeholders. The successful conduct of both the CBTi and the study were dependent on the resources, arrangements, relationships and skills that were required to make the intervention work. For the STRIDE therapists, a series of practical problems (e.g. not having dedicated office space, logistical barriers to travelling to appointments) initially impacted on their capacity to efficiently complete their work but were resolved over time. Their work was facilitated by the support provided by the team (and each other) for ongoing skill development, and by the high level of confidence from the team in the work they did with clients. The extent to which clients were actively involved with the therapy varied widely; although some enthusiastically set themselves challenges and made significant progress in a short space of time, others relied on the STRIDE therapist to provide impetus. This variation seemed to reflect differences in personal approaches and motivation, perceived benefits of the CBTi and the complex and multiple comorbidities that they experienced. Professionals contributed variably to recruiting participants to the trial, for a range of reasons relating to both individual (e.g. attitudes towards therapy) and structural/cultural (e.g. risk averse) factors. The core research team worked hard to address problems that arose in conducting the study, and although highly motivated, experienced challenges in resolving some issues
Appraising and adapting cognitive–behavioural therapy intervention
A final NPT mechanism, key to understanding the embedding of the CBTi, is the capacity to appraise the value of the intervention and scope for making improvements and adapting it the local contexts – reflexive monitoring. The STRIDE therapists demonstrated a high degree of reflectiveness and adaptation throughout the delivery of the CBTi, to the extent that some changes in the focus of the intervention raised questions over the identity of the CBTi. The extent to which clients internalised the skills that they had learned and applied these to new situations following the completion of therapy was variable. For some clients, increased self-awareness, together with an understanding of basic CBT techniques (such as graded exposure) enabled them to sustain changes. Others, however, found it difficult to generalise CBT principles to new situations without the support of the STRIDE therapists. Professionals at the sites had much more limited capacity to make appraisals of any impact of the CBTi, as there were no formal mechanisms established for staff to receive feedback about clients following therapy. Some received informal feedback from patients who were clients in the study, at times this was positive but in some instances this generated professionals’ anxiety about increased risk of falling. As noted already, the core research team were continually engaged in problem-solving and ‘troubleshooting’ as the study progressed. However, a higher degree of anticipation of problems and forward planning may have facilitated the conduct of the study, and reflections on learning from the process to apply in further studies have been made by the team.
Summary of findings
-
To deliver the CBTi, HCAs needed to learn complex new skills. Although the training was generally felt by the psychologists and the HCAs to be adequate, further adaptation for a non-specialist audience is recommended. The HCAs’ ability to embed and develop their CBTi skills was facilitated by individual and group supervision sessions and the HCAs’ self-initiated peer support sessions.
-
The HCAs’ delivery of the CBTi was evaluated positively by the psychologists, although some skills could be further developed. The delivery of the CBTi was shaped over time by their growing experience of the client group, the complexity of fear of falling and the tendency to revert to existing skill sets.
-
The value of the CBTi for this client group was recognised by some professional staff providing usual care. Senior professionals expressed interest in the ongoing delivery of the CBTi services, and suggested scope for improved integration of the CBTi with existing interventions. There were, however, issues over the ‘fit’ of CBTi with the underlying ethos of one participating site.
-
Client engagement with and understandings of the CBTi varied. Nevertheless, clients valued their interactions with the HCAs and perceived a range of benefits from the CBTi including improvements in confidence, independence, mood, activity levels, walking/balance, self-acceptance, and motivation, and reductions in anxiety.
-
The organisation and delivery of the CBTi in terms of the materials, session content, frequency and duration, was generally acceptable to clients although more flexibility over the follow-up session was suggested. There was a strong preference for delivery in the client’s home.
-
The study was threatened by numerous and varied challenges, including threats to clinic funding, sub-optimal staff recruitment processes, delays in access approvals for research staff, and difficulties meeting recruitment targets. Continual investment in troubleshooting, resource management and teamwork was required to successfully complete the study. These processes were facilitated by the embeddedness of the process evaluation within the study.
-
The study has highlighted the extent to which older people’s lives are curtailed by medical and psychosocial events; very few of the clients who received the CBTi would have considered or been eligible for help from existing psychological services.
Chapter 6 Health-economic evaluation
Introduction
The aim of the economic evaluation was to determine whether or not the novel CBTi plus usual multidisciplinary care is cost-effective compared with usual multidisciplinary care alone for patients – with significant fear of falling – attending a community falls service.
The economic evaluation comprised both a within-trial cost-effectiveness analysis (CEA) and a within-trial CUA. The perspective (i.e. whose costs and benefit are considered relevant) of the evaluation is the health service provider (NHS), but a broader analytical perspective incorporating NHS costs and those of informal caregivers is also considered.
Methods
The primary measure of cost-effectiveness was the incremental cost per unit reduction in FES-I at 12 months. The analysis followed an ITT approach. In addition, as estimates of the incremental cost per unit reduction in FES-I might be difficult to interpret according to standard decision-making criteria within the NHS,107 a CUA was also performed, with the incremental cost per quality-adjusted life-year (QALY) being estimated at 12 months. As the duration of the study was 12 months, discounting of future costs and benefits was not required.
As part of the within-trial economic evaluation the following outcomes were reported:
-
costs to the NHS at 12 months
-
costs to the NHS, and patient’s informal caregivers at 12 months
-
FES-I at 12 months
-
QALYs over 12 months, based on responses to the EQ-5D (5L) and SF-6D questionnaires administered at baseline (pre-randomisation), 8 weeks, and 6 and 12 months.
For the purpose of the analysis, data on costs and effects of the interventions were combined to obtain an incremental cost-effectiveness ratio (ICER). The ICER summarises the cost-effectiveness of a health-care intervention. It is defined by the difference in costs between the intervention and control group, divided by the difference in effect between the two groups. It represents the average incremental costs of one additional unit of the effect. The ICER can be estimated as:
where C1 and E1 are the cost and effect in the intervention arm, and C0 and E0 are the cost and effect in the control group. Costs are described in monetary units, whereas effects can be measured in terms of health status (FES-I) or another outcome of interest, such as the QALY.
This provides the cost per additional unit of effectiveness (e.g. unit reduction in FES-I or QALYs) gained. The ICER can be used to aid decision-making regarding resource allocation. If a decision-maker can establish a willingness-to-pay (WTP) value for the outcome of interest, it is possible to use this as a threshold. If, for a given intervention, the ICER is above this threshold then it will be deemed too expensive and thus should not be funded, whereas if the ICER lies below the threshold then the intervention can be judged to be cost-effective. The results are displayed on the cost-effectiveness plane from which a cost-effectiveness frontier was generated. The cost-effectiveness frontier allows us to determine the treatment option that maximises net benefits at our chosen ceiling ratio. For example, in a CUA NICE typically adopted a threshold value for society’s WTP of £20,000 per QALY. However, it is unclear what the ceiling ratio is for cost per a unit reduction in FES-I. Prices were reported in 2013–14. All statistical analyses were performed using IBM SPSS Statistics release 22 and Microsoft Excel® version 14 (Microsoft Corporation, Redmond, WA, USA). Statistical significance was at p-values of < 0.05.
Identification and measurement of resource use
Cognitive–behavioural therapy intervention costs
The costs of the intervention (CBTi) were microcosted and estimated on a per-patient basis for those in the intervention arm of the trial. The intervention costs comprised two components: the costs of (1) training and supporting three HCAs who delivered the CBTi and (2) its delivery. The costs of training the HCAs who delivered the intervention included a 5-day programme that was delivered by two psychologists (costed as clinical psychologists). During the delivery of the intervention, each HCA received a 1-hour fortnightly one-to-one supervision meeting (with NSa) plus a 1-hour monthly team meeting (with VD). The costs of training were apportioned to each individual taking part in the intervention arm of the trial.
Valuation of NHS and informal care giving resource use
With the exception of the CBTi intervention, patient-level resource use relating to falls and mobility, including all NHS and informal caregiver costs, were identified and measured using a resource utilisation form (see Appendix 7) self-completed by participants in both arms of the trial at 6 and 12 months after randomisation. The use of the resource utilisation form allowed us to divide NHS resource use into secondary care, primary care, community care and ambulance journeys. It also allowed the collation of informal care giving time. Secondary care resources included inpatient stays, accident and emergency (A&E) visits and outpatient visits. Primary and community care resources included general practice (GP) and home visits, practice nurse visits at the surgery, and district nurse visits in the home. With respect to the use of ambulances, the number of trips was recorded. Informal caregiving time comprised the average time per week spent by family and/or friends who were helping participants with activities that they would have usually been able to undertake if they had not experienced problems with mobility or a fall.
For each trial participant, all components of treatment costs stratified by category of resource use were computed by multiplying units of resource use by their unit costs. These were then summed over all resource-use categories to obtain a total annual cost for each participant both from a NHS perspective and a NHS and caregiver perspective. This was then used to generate the average cost per patient in each arm of the trial.
Unit cost sources included NHS reference costs and Unit Costs of Health and Social Care. 108 The unit costs utilised for each category of resource use can be found in Tables 45 and 46 and are expressed in 2013–14 UK pounds sterling (£). 108
Secondary care costs
The use of secondary care resources related to falls or mobility problems included numbers of A&E visits, inpatient admissions, outpatient visits and inpatient length of stay. We assumed that each hospital admission incurred a tariff relating to inpatient care following a fall. 109 The impact of the use of this tariff was tested in the sensitivity analysis. In general, the frequency of use of these health-care resources was multiplied by the national tariff to get the total follow-up cost per patient. Table 45 provides a detailed description of the unit costs used and where they were sourced from.
| Resource | Unit | Cost (£) | Source |
|---|---|---|---|
| Inpatient falls without specific cause, with CC score of 2–3 | Per hospital admission | 2672 | NHS tariff WA23B |
| Consultant-led outpatient geriatric medicine visit | Per attendance | 207 | NHS tariff WF02B |
| A&E attendance | Per attendance | 169 | NHS tariff VB05Z |
| Non-elective long-stay excess bed-day | Per bed-day | 242 | NHS tariff WA23B |
In addition to the secondary care costs, ambulance journey costs were included for those who had reported a frequency of use on the resource utilisation form. The unit cost of this was £192 per journey and was estimated from North East Ambulance Service data.
Primary and community care costs
The health-care resources available to participants at a primary care setting include GPs and health-care workers including practice nurses and district nurses. We assumed that a visit to the health-care practice to see a health-care worker was an appointment with a practice nurse, and a visit to the home was made by a district nurse, with average costs to the NHS estimated per guidance. 108 As GP visits can occur at the health-care practice or at the participant’s house, we distinguished between the different locations of each consultation to account for the different costs associated with each consultation. Table 46 provides a description of the unit costs used to estimate primary and community care; all unit costs are based on Curtis’s Unit Costs of Health and Social Care 2014. 108
| Health Professional | Unit | Unit cost (£) |
|---|---|---|
| GP – at practice | Per visit | 46 |
| GP – at home | Per visit | 92 |
| Practice nurse | Per visit | 11 |
| District/community nurse – at home | Per visit | 39 |
Informal care giving costs
Data were also collected on the amount of informal care giving time from family or friends that participants had received on average on a weekly basis. This was then extrapolated over the 12-month follow-up period and multiplied by an hourly cost of informal caregiver time. The national minimum wage of £6.50110 was taken as a proxy for the value of informal caregivers’ time.
Identification and measurement of outcomes: Falls Efficacy Scale–International version and quality-adjusted life-years
The primary economic analysis mirrored the analysis of effects by focusing on FES-I and estimating the incremental cost per a unit change in FES-I at 12 months. As previously discussed, the FES-I is a validated instrument for assessing fear of falling, which itself is defined as an ongoing concern about falling which ultimately limits activities of daily living. 29 It is a 16-item questionnaire using a 4-point scale (1 = not at all concerned, 4 = very concerned) and was administered at baseline, 8 weeks, and 6 and 12 months’ follow-up.
In line with NICE recommendations,107 outcomes in the economic analysis were also identified and measured using QALYs. Data used to estimate QALYs were collected using two instruments: the EQ-5D (5L) and SF-6D. These were used to collect information about participant’s health-related quality of life (HRQoL) at baseline and 8 weeks, and 6- and 12-month follow-up periods. Both instruments allow HRQoL to be valued on a scale on which perfect health and death are ‘1’ and ‘0’, respectively. The values for each health state were obtained from the respective UK population valuation sets for the two instruments.
The EQ-5D (5L)111 is increasingly being used in preference to the earlier EQ-5D-3L tool to assess the quality of life for participants with different medical conditions. The EQ-5D (5L) questionnaire describes health status in five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each of these dimensions has five levels, ranging from the best possible situation to the worse possible situation. The number of dimensions and levels within each dimension provide 3125 possible health states in which participants could be at any point in time. The responses to the EQ-5D (5L) questionnaire were transformed using a standard algorithm to produce a health-state utility score at scheduled intervals during the follow-up for each participant in each of the treatment groups. 112 Although the EQ-5D (5L) is the most commonly used instrument to measure HRQoL, there are concerns about ceiling effects that it may not be capable of distinguishing between health states that are close to full health. 113 For this reason we used version 2 of the SF-36,114 which contains questions regarding eight domains of HRQoL compared with the five contained in EQ-5D (5L). Utility values for the states described by the SF-36 are obtained using the SF-6D algorithm. 65
Mean QALY differentials between the groups were generated from the utility values derived from responses to the EQ-5D (5L) and SF-6D during the follow-up period using the regression approach, controlling for baseline utility. 115 This area under the curve approach puts a time weight on to each utility score, which allows us to generate QALY values for each participant. Furthermore, stratification variables (pain when walking, referral to strength and balance training classes and gender) were included as fixed effects.
Data analysis
Within the economic analyses, the mean volume of health-care resources and informal care giving time associated with each arm were estimated and reported together with their SDs. Mean differences and 95% CIs were calculated using non-parametric bootstrapping. Mean costs were estimated and reported together with their SDs for each trial arm. In order to account for the skewed nature of the cost data, mean cost differences and CIs were estimated using non-parametric bootstrapping.
In order to inform whether or not the intervention is cost-effective, methods recommended by NICE technology assessment guidance107 have been adopted to report and present the results of the incremental costs and outcomes (FES-I and QALYs) of CBTi compared with usual care. The incremental costs and outcomes were combined within an ICER for six scenarios:
-
NHS costs and FES-I
-
NHS and caregiver costs and FES-I
-
NHS costs and EQ-5D (5L) QALYs
-
NHS and caregiver costs and EQ-5D (5L) QALYs
-
NHS costs and SF-6D QALYs
-
NHS and caregiver costs and SF-6D QALYs.
For the purpose of the analysis, data on costs and effects of the interventions were combined to obtain an ICER. This was performed by calculating the mean difference in costs between the intervention and control group, divided by the difference in effect between the intervention and control group. This provides the cost per additional unit of effectiveness (e.g. unit reduction in FES-I or QALYs) gained. The results are displayed on the cost-effectiveness plane, from which a cost-effectiveness frontier was generated. The cost-effectiveness frontier allows us to determine the treatment option that maximises net benefits at our chosen ceiling ratio. For example, in a CUA NICE typically adopted a threshold value for society’s WTP of £20,000 per QALY. However, it is unclear what the ceiling ratio is for cost per a unit reduction in FES-I. Prices were reported in 2013–14. All statistical analyses were performed using IBM SPSS Statistics release 22 and Microsoft Excel® version 14. Statistical significance was at p-values of < 0.05.
Uncertainty surrounding the cost-effectiveness ratios was addressed using the bootstrapping technique, utilising 5000 bootstrapped samples. The results of the bootstrapping simulations were presented on the ‘cost-effectiveness plane’, which highlights the preferred treatment option. If the results lie in the north-west or south-east quadrants the preferred treatment is clear, as one option dominates the other (i.e. is less costly and more effective). If the results lie in the north-east or south-west quadrants, the decision as to which is the preferred treatment is less clear (i.e. one option may be less costly but also less effective, or more effective but at greater cost); the ICER may aid this decision. The bootstrapping was also used to estimate CIs for both costs and effects of the two arm comparison. A cost-effectiveness acceptability curve (CEAC) based on the net benefit approach116 was also used to present the probability of a treatment being cost-effective, based on a range of values for society’s WTP for a unit of outcome. Base-case results used data with complete information, the ‘complete cases’ for both costs and effects.
Sensitivity analysis
In addition to the stochastic sensitivity analysis, which was undertaken to allow presentation of the level of variance around outcome measures included in the CEA and CUA, sensitivity analysis was also performed to gauge the impact of varying key assumptions and/or parameter values in the base-case analysis. Specifically the cost of an inpatient admission was varied. This was conducted to more accurately capture the impact of length of stay on total costs as a few participants had very high lengths of stay. For this analysis, rather than use a single tariff for inpatient admissions, we separated the hospitalisation costs and the treatment costs by utilising the relevant non-elective long stay reference cost for length of stay then multiplying this by the average length of stay for the national tariff. These costs were then subtracted from the national tariff to estimate the treatment costs without any length of stay component. Total costs per patient were calculated by multiplying the treatment cost by the number of hospital admissions and multiplying their length of stay by the non-elective long-stay reference cost relevant to the national tariff.
Missing data
Missing data can be separated into:
-
Item-level missingness Data missing as a result of blank returns on particular items of questionnaires that had been returned partially completed.
-
Missing because of questionnaire non-response Data missing as a result of dropout.
Item level missingness for resource use and costs was dealt with by using a simple imputation which involved imputing the lower and upper interquartile range for participant’s use of each item of the resource utilisation form when there were missing data. We used this range as the mean value was very skewed because of a small number of participants who used very high quantities of medical services and the majority of participants who responded to the questionnaires had little resource use. We used a simple imputation method to interpolate missing HRQoL data at 8 weeks and 6 months by utilising the weighted average of each participants’ adjacent observations at each time point.
Missing cost and QALY data resulting from dropout were imputed using multiple imputation to allow for comparison and test the robustness of the base-case results. The regression analyses used to impute missing data included the following variables measured at baseline: age, sex, gait and balance score, handgrip strength, functional reach, falls efficacy, concern about falling (0–10 scale), pain when walking, participation score, loneliness score and site. In addition, the number of sessions of the CBTi completed was included as a predictor variable. The QALYs and costs were imputed at 12 months using regression models with the above variables included as predictors. Each of these methods was undertaken separately for each study group and both groups together (with group added as a predictor variable). In each case, 50 data sets were imputed and a pooled estimate of the impact of the intervention at 1 year across data sets was based on an analysis of covariance model with adjustments for baseline (with QALY data) and randomisation strata.
Results
Data completeness
Data were missing either because questionnaires were not fully completed at all time points or because of patient dropout, as presented in Chapter 4. A total of 415 participants were randomised: 205 to the control arm and 210 to the intervention arm. A participant was included in the deterministic cost analysis and categorised as complete data if they had returned either a 6- or 12-month resource utilisation form or both of these. This gave the maximum numbers for analysis. The number of participants included for analysis was 169 (82%) in the control arm and 155 (74%) in the intervention arm. Thus, a total of 324 (78%) participants who had returned information on resource use either at 6 or 12 months’ follow-up were included in the analysis. Missing data within the resource-use questionnaire varied across questions (4.6–9.9%). Complete EQ-5D (5L) data to estimate QALYs for which patients completed the questionnaires at each of the four time points were available for 112 (61%) of patients in the control arm and 85 (46%) of patients in the intervention arm. The number of responses returned with completed EQ-5D (5L) questionnaires by trial arm are shown in Figures 31 and 32.
FIGURE 31.
Completion of EQ-5D (5L) data at baseline and follow-ups in the intervention arm.
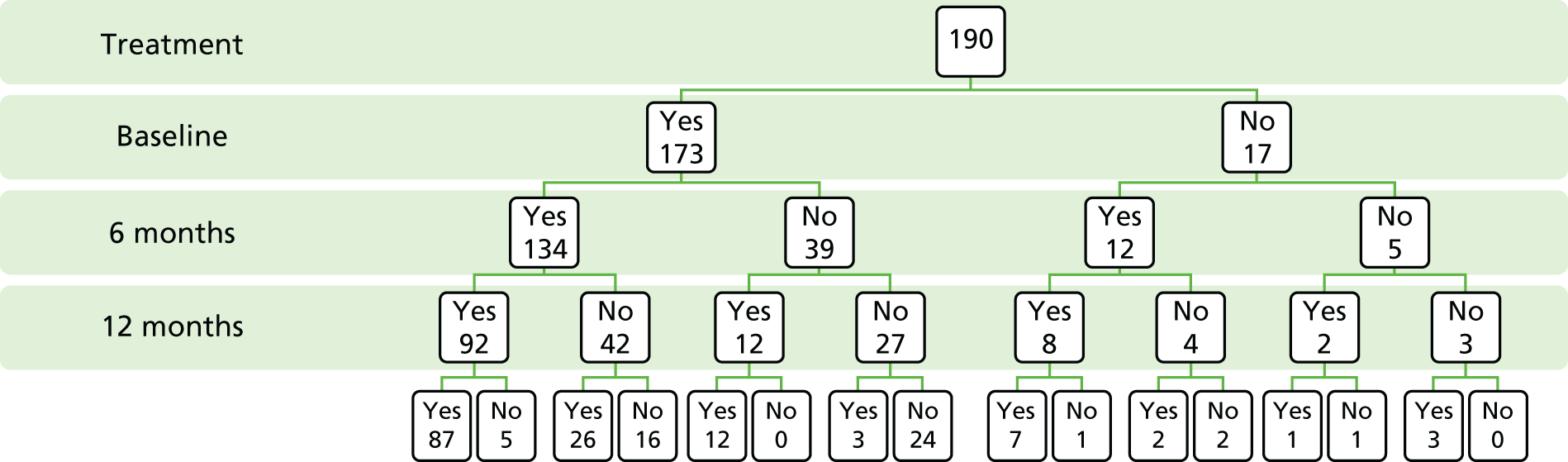
FIGURE 32.
Completion of EQ-5D (5L) data at baseline and follow-ups in the control arm.
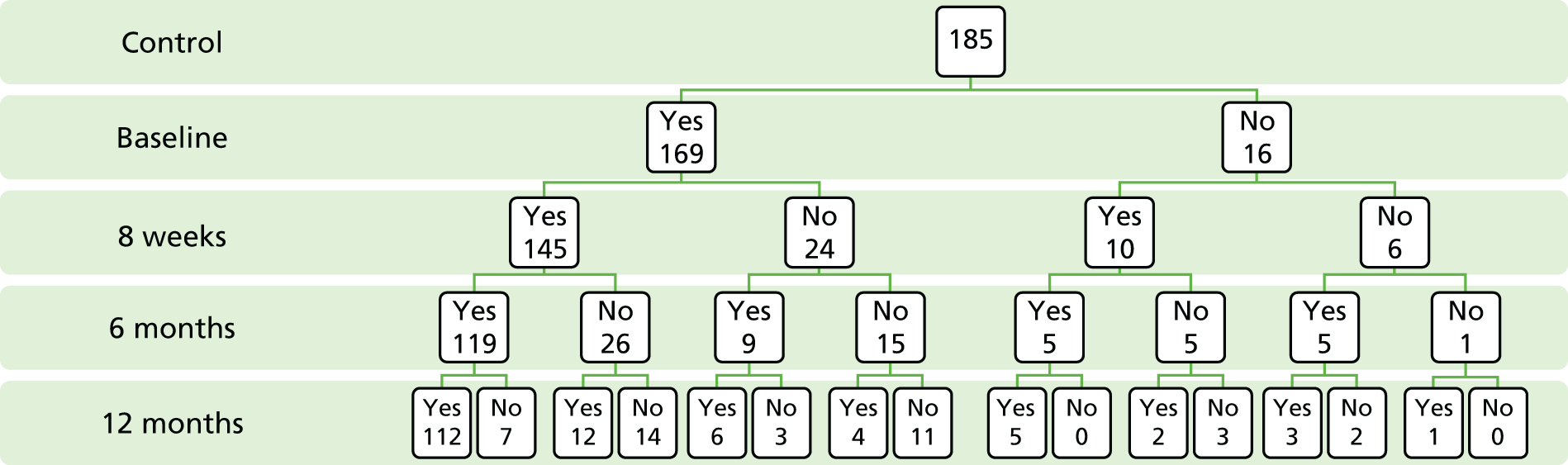
The figures suggest that the loss to follow-up in the intervention arm was greatest at 8 weeks and 6 months compared with the control arm. Those who completed the EQ-5D (5L) questionnaires at 6 months, in the intervention arm, appeared to be no less likely to be lost to follow-up than those who completed the 6-month follow-up in the control arm.
Complete SF-6D data to estimate QALYs for which participants completed the SF-36 at each of the four time points were available for 84 (45%) of participants in the control arm and 69 (36%) of participants in the intervention arm. The number of responses returned with completed SF-36 questionnaires by trial arm is shown in Figures 33 and 34. These completion rates are lower than those for the EQ-5D (5L), which might be expected given the higher response burden associated with the SF-36.
FIGURE 33.
Completion of SF-36 data at baseline and follow-ups in the intervention arm.
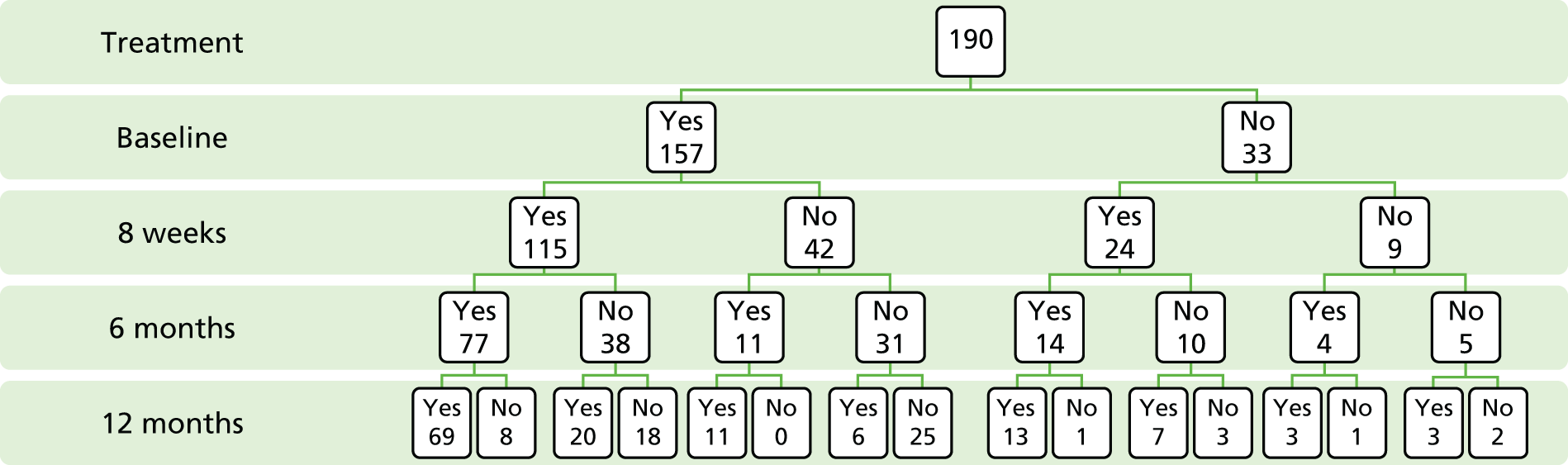
FIGURE 34.
Completion of SF-36 data at baseline and follow-ups in the control arm

As in the case of EQ-5D (5L), the majority of cases were lost to follow-up before month 12, with more losses occurring in the intervention arm.
Complete cost and FES-I outcome data were available for 310 (75%) patients: 159 (78%) in the control arm and 151 (72%) in the intervention arm. Complete cost and EQ-5D (5L) QALY data were available for 187 (45%) patients: 79 (39%) in the control arm and 108 (51%) in the intervention arm. Complete cost and SF-6D QALY data were available for 152 (37%) of patients: 69 (34%) in the control arm and 83 (40%) in the intervention arm. The base-case cost-effectiveness analyses used complete cost and outcome data (n = 310 for FES-I; n = 187 for EQ-5D (5L) QALYs; n = 152 for SF-6D QALYs), but we also include results for all available data, where possible, to maximise transparency of results.
Costs and resource use
Initial intervention cost
The total cost of providing the intervention was calculated and the average intervention cost per patient was calculated by dividing the total costs by the number of patients in the intervention arm of the trial (n = 210). The costs of providing the intervention are detailed in Table 47.
| Resource use | Quantity | Cost (£) |
|---|---|---|
| Training: psychologists’ time, £138 per hour108 | ||
| Block training days with psychologists | 10 days | 10,350 |
| Fortnightly supervision meetings with each HCA | 107 hours | 14,766 |
| Monthly group meetings | 21 hours | 2898 |
| Training: HCA time, £7.04 per hour108 | ||
| Block training time | 112.5 hours for three HCAs | 792 |
| Fortnightly supervision meetings for each HCA | 107 hours | 753 |
| Monthly group meetings | 21 hours | 444 |
| Delivery of intervention, £7.04 per hour108 | ||
| HCA staff time costs delivery of intervention | 9.5 hours per patient | 67 |
Resource use
Inspection of the histograms and associated tests for normality of the distribution of the data (Kolmogorov–Smirnov test and Shapiro–Wilk test) showed that the resource utilisation and cost data were not normally distributed (p < 0.05). Further analysis revealed the data to be right skewed and truncated at zero because of the small numbers of high resource-use patients and the number of participants who incurred no costs. Table 48 shows mean resource use and the bootstrapped 95% CIs. Visits to A&E and visits to the home by GPs and district nurses, along with caregiver time, were higher on average in the intervention arm than in the control arm. However, as the CIs illustrate, there was no evidence of a difference between randomised groups.
| Follow-up resource use 12 months | Intervention CBTi | Control usual care | Mean difference (95% CI bootstrapped difference between means) | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Number of visits to A&E | 155 | 0.16 (0.64) | 169 | 0.14 (0.58) | 0.02 (–0.10 to 0.16) |
| Number of inpatient admissions | 155 | 0.07 (0.44) | 169 | 0.07 (0.33) | 0.00 (–0.08 to 0.09) |
| Number of outpatient attendances | 155 | 1.18 (2.68) | 169 | 1.50 (4.31) | –0.32 (–1.14 to 0.44) |
| Number of ambulance journeys | 155 | 0.71 (0.83) | 169 | 1.13 (1.30) | –0.42 (–1.33 to 0.29) |
| Number of GP contacts at practice | 155 | 1.55 (2.96) | 169 | 1.71 (3.74) | –0.16 (–1.00 to 0.54) |
| Number of GP home visits | 155 | 0.12 (0.60) | 169 | 0.06 (0.34) | –0.04 (–0.04 to 0.18) |
| Number of practice nurse contacts | 155 | 0.18 (0.82) | 169 | 0.22 (0.88) | –0.04 (–0.22 to 0.15) |
| Number of district/community nurse visits at home | 155 | 0.37 (2.18) | 169 | 0.22 (1.71) | –0.25 (–0.25 to 0.62) |
| Number of hours of caregiver time | 155 | 249.60 (572.76) | 169 | 235.31 (428.60) | 14.29 (–100.88 to 130.68) |
Costs of NHS services and caregivers’ time
For each trial participant, all components of treatment costs stratified by category of resource use were computed by multiplying units of resource use by their unit costs. These were then summed over all resource-use categories to obtain a total annual cost for each participant both from a NHS perspective and a NHS and caregiver perspective. This was then used to generate the average cost per patient in each arm of the trial. Table 49 shows the average costs per resource-use category and average cost differences bootstrapped 95% CIs.
Table 49 shows the average costs per participant for each cost category at follow-up and the mean differences in average costs for all cost categories. With the exception of the cost of the intervention itself, the CIs illustrate there was no evidence of a difference between randomised groups although the width of the CIs suggests that there may be economically important differences for many of the cost categories that could favour either intervention.
| Cost item | Intervention CBTi | Control usual care | Mean differences, £ (95% CI bootstrapped) | ||
|---|---|---|---|---|---|
| n | Mean, £ (SD) | n | Mean, £ (SD) | ||
| Cost of visits to A&E | 155 | 27 (108) | 169 | 23 (98) | 3 (–21 to 25) |
| Cost of inpatient admissions | 155 | 174 (1170) | 169 | 176 (887) | –2 (–214 to 244) |
| Cost of outpatient attendances | 155 | 243 (556) | 169 | 310 (892) | –67 (–238 to 83) |
| Cost of ambulance journeys | 155 | 137 (158) | 169 | 218 (250) | –80 (–232 to 52) |
| Cost of GP contacts at practice | 155 | 71 (136) | 169 | 79 (172) | –7 (–43 to 23) |
| Cost of GP home visits | 155 | 11 (56) | 169 | 6 (32) | 5 (–4 to 16) |
| Cost of practice nurse contacts | 155 | 2 (9) | 169 | 2 (9) | 0 (–2 to 2) |
| Cost of district/community nurse visits at home | 155 | 14 (85) | 169 | 9 (67) | 6 (–12 to 23) |
| Cost per week of caregiver time | 155 | 1622 (3723) | 169 | 1530 (2786) | 93 (–658 to 876) |
| Cost of intervention | 155 | 139 (0) | 169 | 0 (0) | 139 (139 to 139) |
Table 50 shows the average total costs per participant for each arm of the trial from both a NHS perspective and a NHS and caregiver perspective. Costs from the NHS perspective include the intervention costs and all NHS costs during follow-up. Costs from the NHS and caregiver perspective include intervention costs, all NHS costs during follow-up, and the informal care giving costs during follow-up.
| Cost | Intervention CBTi | Control usual care | Mean cost difference, £ (95% CI bootstrapped) | ||
|---|---|---|---|---|---|
| n | Mean, £ (SD) | n | Mean, £ (SD) | ||
| Total cost NHS perspective | 155 | 689 (1453) | 169 | 616 (1483) | 73 (–239 to 421) |
| Total cost NHS and informal caregiving perspective | 155 | 2102 (3982) | 169 | 1874 (3044) | 228 (–512 to 1075) |
The mean cost per participant from the NHS perspective was £689 for the intervention group and £616 for the control group. The difference in mean cost between the intervention and control groups was £73 (bootstrapped 95% CI –£239 to £422). The mean cost per participant from the NHS and informal caregiver perspective was £2102 for the intervention group and £1874 for the control group. The difference in mean cost between the intervention and control group was £228 (bootstrapped 95% CI –£512 to £1075). As the CIs illustrate, there was no evidence of a difference between randomised groups although the width of the CIs suggests that there may be economically important differences from both cost perspectives that could favour either group.
Effectiveness: quality-adjusted life-years
The QALY gains for CBTi compared with usual care only are presented with utility values from both EQ-5D (5L) and SF-6D. As noted above, the results presented in this section are based on only those participants with complete HRQoL responses at all four time points (n = 197 for EQ-5D (5L); n = 153 for SF-6D).
The model used to estimate a 1-year QALY was two-twelfths of any utility gain (or loss) made between baseline and 8 weeks plus four-twelfths of any gain (or loss) between 8 weeks and 6 months plus six-twelfths of any gain (or loss) between 6 and 12 months (see Equation 1, above). In each case sex, study site and baseline utility were controlled for.
where u0,i is baseline utility for the ‘i th’ participant; u2,i, u6,i and u12,i are the utilities at 8 weeks, 6 months and 12 months; s1i and s2i are the dummy variable for the different sites; fi is the dummy representing the gender of the ‘i th’ participant; and CBTi is the intervention dummy.
European Quality of Life–5 Dimensions (5 Level)
The analysis of EQ-5D (5L) and SF-6D QALYs gained at 12 months is shown in Table 51. The changes in utility have been calculated using an area under the curve method including patients who (1) completed responses at each time point and (2) completed baseline and 12 months responses and one interim response. In the latter, the missing item interim response was interpolated using a weighted average of the prior- and post-utility score. Weighting was by time to adjacent observation and effect of intervention estimated by regression, controlling for sex, site and baseline HRQoL for both EQ-5D (5L) and SF-6D.
| Variable | Unstandardised coefficients | Significance | 95% CI | Unstandardised coefficients | Significance | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| B | Standard error | B | Standard error | |||||
| (1) Change in EQ-5D (5L) utility values | (1) Change in SF-6D utility values | |||||||
| (Constant) | 0.015 | 0.010 | 0.160 | –0.006 to 0.035 | 0.043 | 0.11 | 0.000 | 0.022 to 0.064 |
| Falls and Syncope Service | –0.002 | 0.007 | 0.811 | –015 to 0.012 | 0.002 | 0.004 | 0.624 | –0.005 to 0.009 |
| Galleries Day Unit | –0.006 | 0.014 | 0.692 | –0.033 to 0.022 | 0.002 | 0.008 | 0.772 | –0.013 to 0.018 |
| Intervention | –0.004 | 0.006 | 0.521 | –0.015 to 0.008 | 0.002 | 0.003 | 0.630 | –0.005 to 0.008 |
| Sex | –0.010 | 0.007 | 0.139 | –0.023 to 0.003 | –0.003 | 0.004 | 0.484 | –0.010 to 0.005 |
| Baseline EQ-5D (5L) tariff | –0.006 | 0.013 | 0.668 | –0.032 to 0.021 | –0.071 | 0.017 | 0.000 | –0.105 to –0.037 |
| (2) Change in EQ-5D (5L) utility values | (2) Change in SF-6D utility values | |||||||
| (Constant) | 0.019 | 0.009 | 0.051 | 0.000 to 0.037 | –0.022 | 0.018 | 0.235 | –0.057 to 0.014 |
| Falls and Syncope Service | –0.007 | 0.006 | 0.212 | –0.019 to 0.004 | –0.007 | 0.006 | 0.307 | –0.019 to 0.006 |
| Galleries Day Unit | –0.008 | 0.011 | 0.462 | –0.031 to 0.014 | –0.018 | 0.012 | 0.141 | –0.041 to 0.006 |
| Intervention | –0.001 | 0.005 | 0.920 | –0.011 to 0.010 | 0.002 | 0.006 | 0.673 | –0.009 to 0.013 |
| Sex | –0.006 | 0.006 | 0.307 | –0.017 to 0.005 | 0.000 | 0.006 | 0.944 | –0.012 to 0.013 |
| Baseline SF-36/SF-6D tariff | –0.015 | 0.012 | 0.209 | –0.039 to 0.009 | 0.037 | 0.028 | 0.189 | –0.019 to 0.093 |
The results for EQ-5D (5L) show that both groups experienced an increase in QALYs estimated by the constant; however, the intervention arm gained less (with a negative estimate for the intervention variable) but with a CI including ‘0’, indicating that there was no evidence of a difference between groups. The results for SF-6D QALYs show a similar pattern in terms of both groups gaining QALYs; however, the intervention group gained more than the control group but, again, with no evidence of a difference between groups.
Incremental cost-effectiveness
These are based on a data set of complete cases both in terms of costs and outcomes (n = 310 for FES-I; n = 187 for EQ-5D (5L) QALYs; n = 152 for SF-6D QALYs).
Incremental cost-effectiveness ratio based on Falls Efficacy Scale–International version
The results show that, using FES-I as an outcome, on average, CBTi was more costly and more effective than usual care alone (Table 52). The incremental cost per unit reduction in FES-I for CBTi compared with usual care, only from a NHS perspective, was £24. The incremental cost per reduction in FES-I for CBTi compared with usual care, from a NHS and caregiver perspective, was £71.
| Intervention | Cost (£) | Comparison of FES-I at 12 months adjusted for baseline | Incremental cost per unit reduction in FES-I (£) | Probability that the intervention is cost-effective for different threshold values for different threshold values for society’s WTP for a unit reduction in FES-I: | ||
|---|---|---|---|---|---|---|
| £0 | £1000 | £10,000 | ||||
| NHS perspective | ||||||
| CBTi | 704 | 5.67 | 24 | 0.33 | 1 | 1 |
| Usual care only | 624 | 2.28 | 0.67 | 0 | 0 | |
| NHS and caregiver perspective | ||||||
| CBTi | 2154 | 5.67 | 71 | 0.29 | 1 | 1 |
| Usual care only | 1915 | 2.28 | 0.71 | 0 | 0 | |
The deterministic results do not illustrate the imprecision surrounding estimates but these can be illustrated using the results of the bootstrapping. The estimated likelihood of treatment, akin to the 1 – p-value in a one-sided statistical test produced from the results of the bootstrapping, are also shown in Table 52.
Graphically, the results of bootstrapping are shown for FES-I (Figure 35) and cost-effectiveness of CBTi being cost-effective compared with usual care only. This figure illustrates the statistical imprecision surrounding estimates of costs but the majority of iterations produced from the bootstrapping analysis are in the north-east and south-east quadrants, which illustrates that CBTi has a more beneficial impact on FES-I than usual care alone. The CEAC presented in Figure 36 is the graphical presentation of the data presented in Table 52 on the likelihood that each treatment is cost-effective. This figure highlights that as society’s WTP for a unit reduction in FES-I increases, there is an increase in the likelihood that CBTi and usual care would be considered cost-effective.
FIGURE 35.
Cost-effectiveness plane showing bootstrapped replicates of the ICER for NHS costs and FES-I.

FIGURE 36.
Cost-effectiveness acceptability curve for NHS costs and FES-I, showing the probability that the intervention is cost-effective at different WTP thresholds.

Figures 37 and 38 show the analysis from the NHS and caregiver perspective. These figures illustrate shows broadly similar results to those when the perspective was of the NHS alone.
FIGURE 37.
Cost-effectiveness plane showing bootstrapped replicates of the ICER for NHS and caregiver costs and FES-I.

FIGURE 38.
Cost-effectiveness acceptability curve for NHS and caregiver costs and FES-I showing the probability that the intervention is cost-effective at different WTP thresholds.

Incremental cost per quality adjusted life-year gained based on European Quality of Life-5 Dimensions (5 Level) responses
The results in Table 53 show that, using the EQ-5D (5L) QALY, from the NHS perspective, CBTi was, on average, more costly and less effective than usual care alone. From this cost perspective, CBTi was dominated, on average, by usual care. However, from a NHS and caregiver perspective, CBTi was less costly but less effective than usual care. Thus, the incremental cost per QALY gain for usual care compared with CBTi and usual care from a NHS and caregiver perspective was £3250. The difference in finding is caused by differences between the two analyses in which participants were able to contribute complete data.
| Intervention | Cost (£) | Change in EQ-5D (5L) from baseline | Incremental cost per QALY (£) | Probability that the intervention is cost-effective for different threshold values for different threshold values for society’s WTP for a QALY: | ||
|---|---|---|---|---|---|---|
| £0 | £1000 | £30,000 | ||||
| NHS perspective | ||||||
| CBTi | 764 | –0.001 | Dominated | 0.36 | 0.35 | 0.24 |
| Usual care only | 670 | 0.003 | 0.64 | 0.65 | 0.76 | |
| NHS and caregiver perspective | ||||||
| CBTi | 2017 | –0.001 | 0.49 | 0.49 | 0.40 | |
| Usual care only | 2030 | 0.003 | 3250 | 0.51 | 0.51 | 0.60 |
Figures 39 and 40 graphically show the results of the stochastic analysis from a NHS perspective. Figure 39 illustrates that the majority of iterations produced from the bootstrapping analysis are in the north-west and south-west quadrants, and that CBTi is likely to have has a less beneficial effect on QALYs than usual care alone. The corresponding CEAC is presented in Figure 40. As this figure illustrates, there is, approximately, a 70% chance that usual care alone would be considered cost-effective over the range of threshold values for society’s WTP for a QALY presented.
FIGURE 39.
Cost-effectiveness plane showing bootstrapped replicates of the ICER for NHS costs and EQ-5D (5L) QALYs.
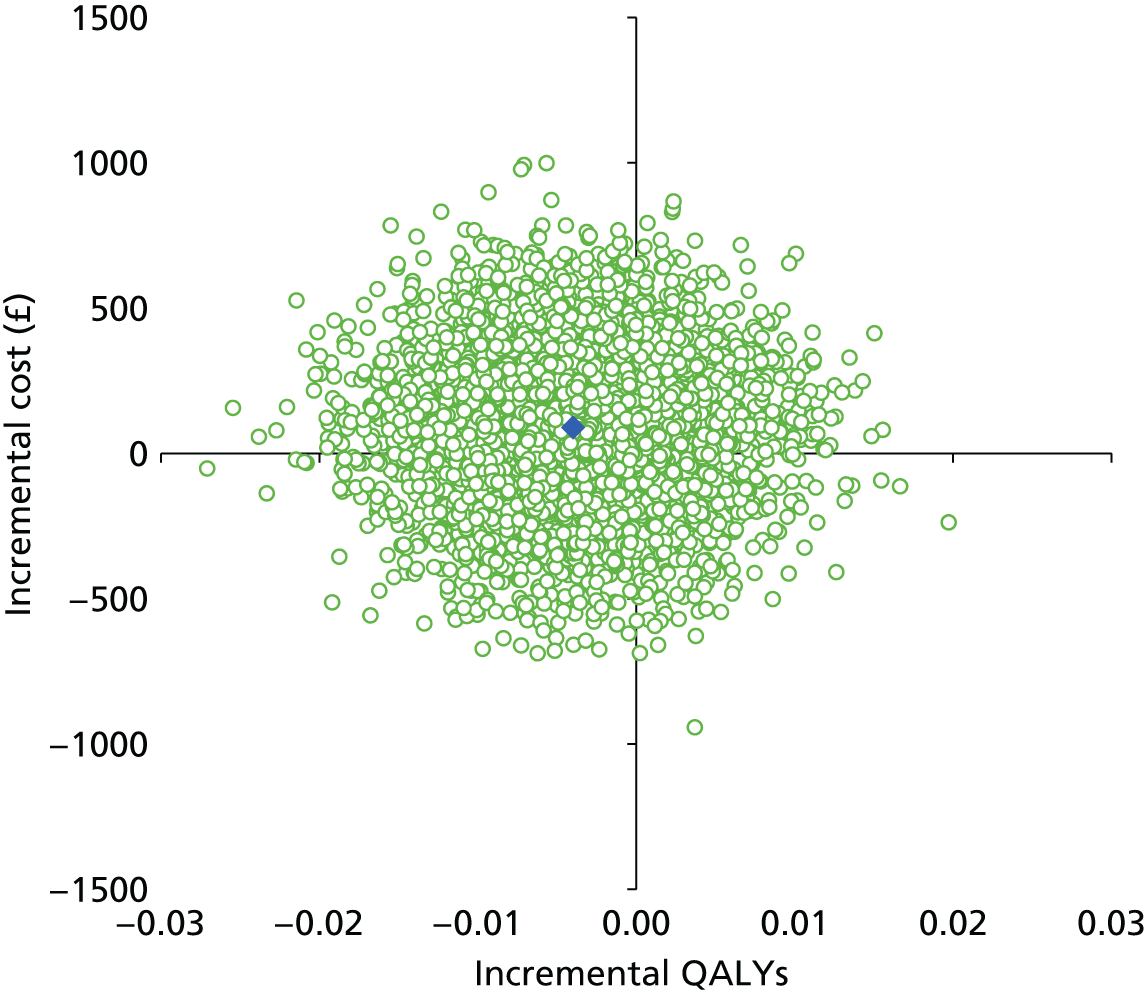
FIGURE 40.
Cost-effectiveness acceptability curve for NHS costs and EQ-5D (5L) QALYs, showing the probability that the intervention is cost-effective at different WTP thresholds.
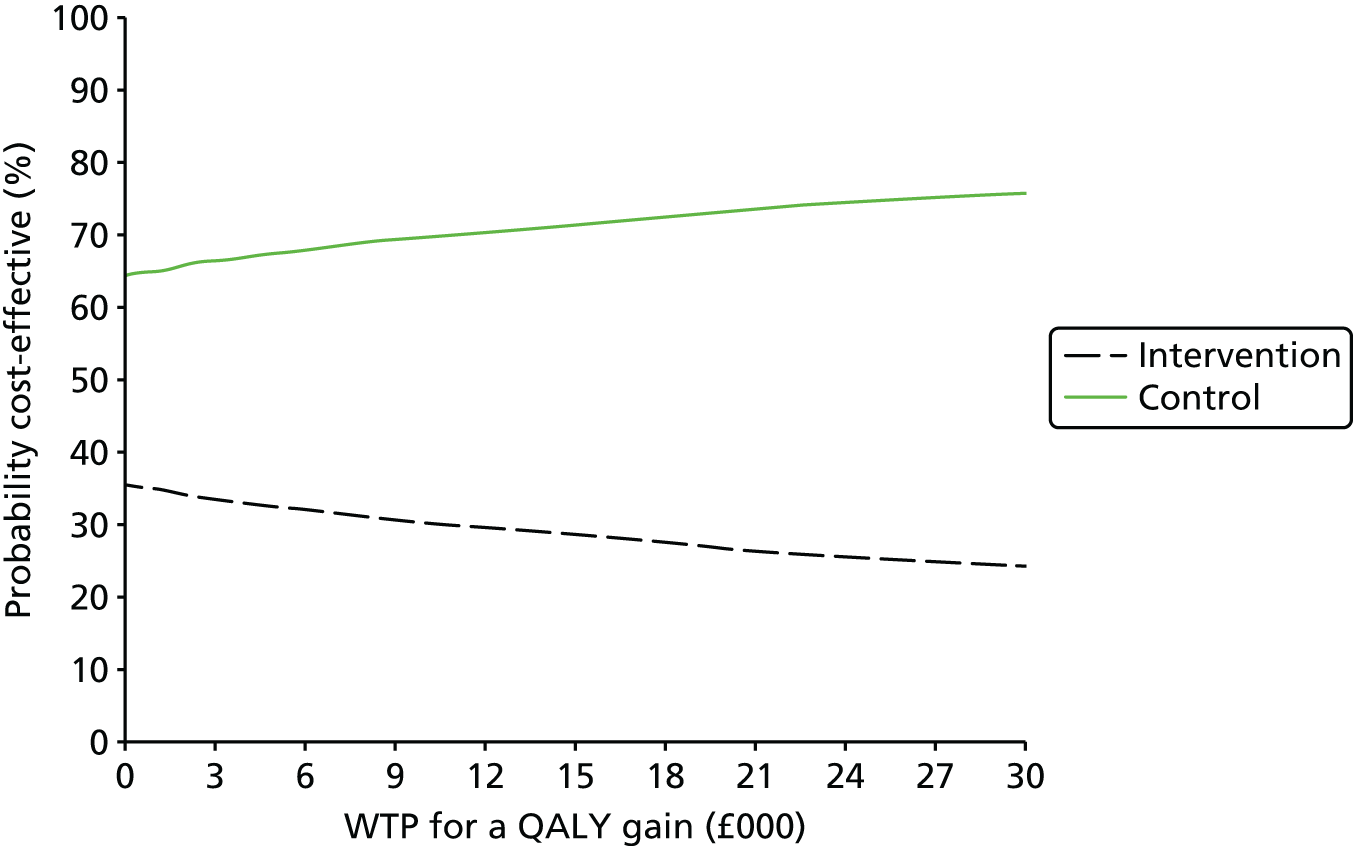
Figures 41 and 42 show the same analysis from the NHS and caregiver perspective. Figure 41 illustrates the statistical imprecision surrounding estimates of costs but the majority of iterations produced from the bootstrapping analysis are in the North West and South West quadrants and this clearly illustrates that CBTi is likely to be less effective compared with usual care alone. The CEAC presented in Figure 42 highlights the increase in the likelihood that usual care alone would be considered cost-effective as society’s WTP for a QALY gain increases.
FIGURE 41.
Cost-effectiveness plane showing bootstrapped replicates of the ICER for NHS and caregiver costs and EQ-5D (5L) QALYs.

FIGURE 42.
Cost-effectiveness acceptability curve for NHS and caregiver costs and EQ-5D (5L) QALYs showing the probability that the intervention is cost-effective at different WTP thresholds.

Incremental cost per quality-assisted life-year gained-based Short Form questionnaire-6 Dimensions data
The results in Table 54 show that, when using SF-6D QALYs as an outcome, and from a NHS perspective, CBTi was, on average, more costly and more effective than usual care alone. The point estimate of the incremental cost per QALY for CBTi compared with usual care only from a NHS perspective was £38,000. Table 54 also shows that from the NHS perspective and caregiver perspective, CBTi was, on average, less costly and more effective using the QALY than usual care alone. From this cost perspective, usual care alone was on average therefore dominated by CBTi and usual care.
| Intervention | Cost (£) | Change in QALYs | Incremental cost per QALY (£) | Probability that the intervention is cost-effective for different threshold values for different threshold values for society’s WTP for a QALY: | ||
|---|---|---|---|---|---|---|
| £0 | £1000 | £30,000 | ||||
| NHS perspective | ||||||
| CBTi | 771 | 0.021 | 38,000 | 0.40 | 0.40 | 0.39 |
| Usual care only | 695 | 0.019 | 0.60 | 0.60 | 0.61 | |
| NHS and caregiver perspective | ||||||
| CBTi | 1913 | 0.021 | 0.67 | 0.67 | 0.66 | |
| Usual care only | 2155 | 0.019 | Dominated | 0.33 | 0.33 | 0.34 |
The statistical imprecision surrounding NHS costs, QALYs and incremental cost per QALY is illustrated in Figure 43, which shows that the majority of iterations are in the north-east and north-west quadrants, meaning that the intervention group is likely to be more expensive than usual care alone. The CEAC presented in Figure 44 highlights, as society’s WTP for a QALY, the likelihood usual care alone would be considered cost-effective over a range of threshold values.
FIGURE 43.
Cost-effectiveness plane showing bootstrapped replicates of the ICER for NHS costs and SF-6D QALYs.
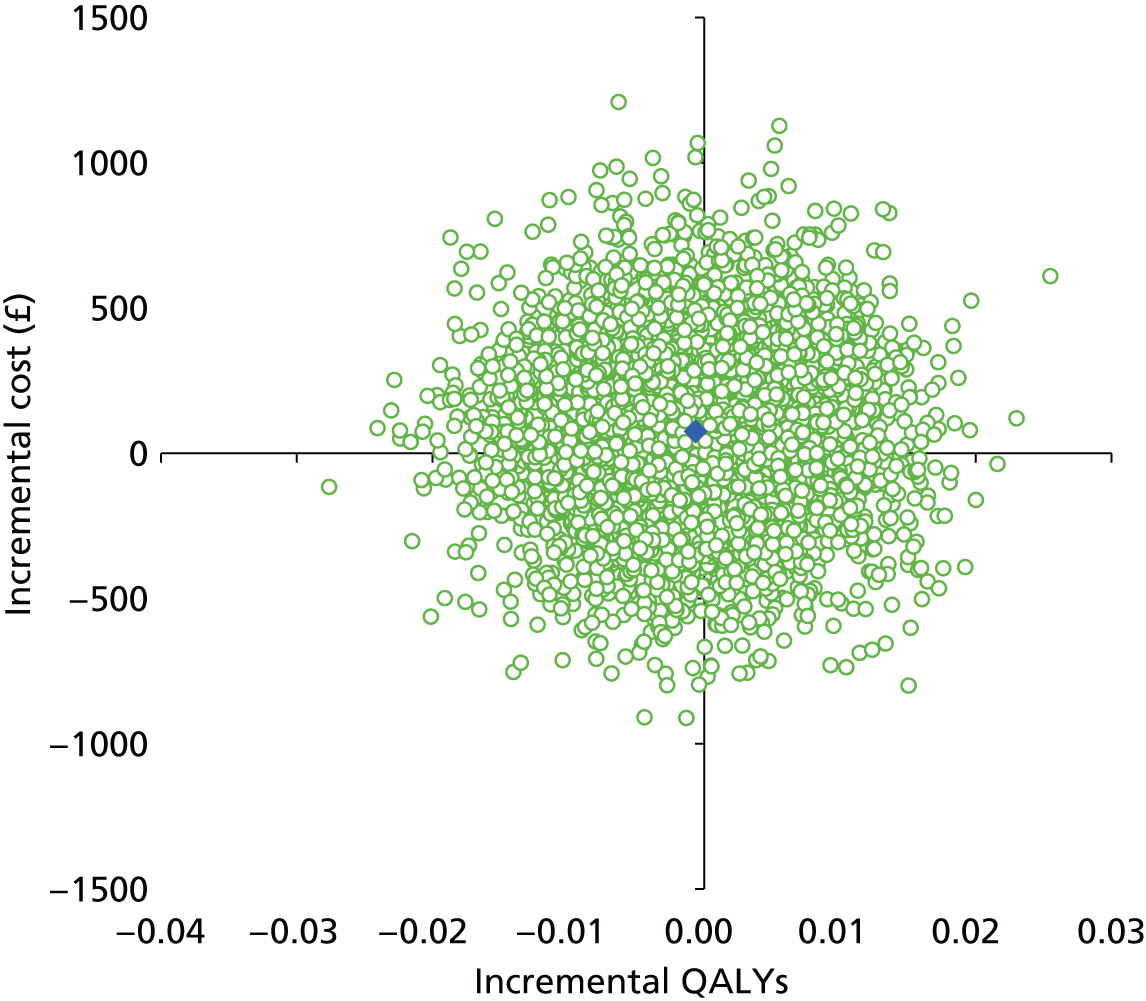
FIGURE 44.
Cost-effectiveness acceptability curve for NHS costs and SF-6D QALYs, showing the probability that the intervention is cost-effective at different WTP thresholds.

The results for the NHS and caregiver perspective are shown in detail in Figures 45 and 46. These results show that usual care is unlikely to be cost-effective over the range of threshold values for society’s WTP for a QALY presented.
FIGURE 45.
Cost-effectiveness plane showing bootstrapped replicates of the ICER for NHS and caregiver costs and SF-6D QALYs.

FIGURE 46.
Cost-effectiveness acceptability curve for NHS and caregiver costs and SF-6D QALYs, showing the probability that the intervention is cost-effective at different WTP thresholds.
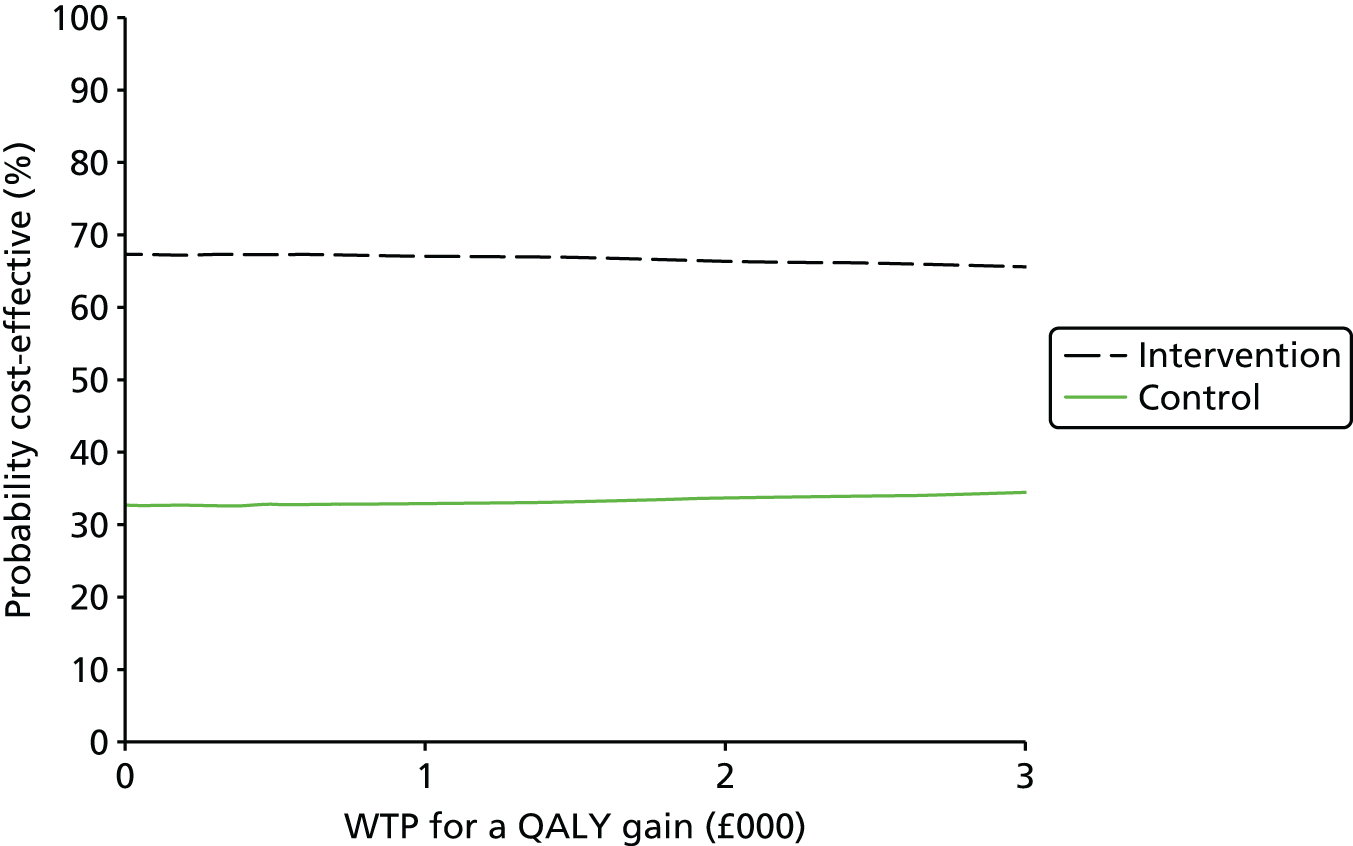
Allowance for uncertainty
In conjunction with our stochastic sensitivity analyses, we also conducted deterministic sensitivity analyses to explore uncertainty in the base-case analysis.
Hospital admission costs not on tariff
The impact of using a treatment cost and non-elective bed-day cost instead of a national tariff for hospital admission for complete cases for NHS costs and FES-I (n = 310), EQ-5D (5L) QALYs (n = 187) and SF-6D QALYs (n = 152) is shown in Table 55.
| Cost | Total NHS costs Intervention CBTi | Total NHS costs control usual care | Mean cost difference, £ (95% CI bootstrapped) |
|---|---|---|---|
| Mean, £ (SD) | Mean, £ (SD) | ||
| Complete NHS and FES-I data | 702 (1524) | 672 (1802) | 30 (–335 to 381) |
| Complete NHS and EQ-5D (5L) data | 627 (942) | 734 (1937) | –106 (–549 to 285) |
| Complete NHS and SF-6D data | 615 (980) | 796 (2169) | –181 (–766 to 289) |
The results for the complete NHS and FES-I cases were insensitive to change. However, for NHS costs and EQ-5D (5L) and SF-6D QALYs, the intervention group became, on average, less costly than the control. The CIs still included zero, indicating that there was insufficient evidence of a difference but these results highlight the potential importance of changes in the cost of hospitalisation (and because the impact would be the same changes in the duration and incidence of hospitalisations).
Implications of missing data
Item level missingness
Resource-use data and costs
The analysis of costs in the base case was based on complete cases using all available cost data. The assumption behind this was that item-level missingness arose as a result of questions on the resource utilisation form not being relevant to some participants. In order to explore the impact of this assumption, we imputed missing data using the lower and upper interquartile range for participants’ use of each item of the resource utilisation form. Table 56 shows the average total costs per participant for each arm of the trial from both a NHS perspective and a NHS and caregiver perspective for the lower and upper interquartile range, respectively (n = 324).
| Cost | Intervention CBTi | Control usual care | Mean difference, £ (95% CI bootstrapped) |
|---|---|---|---|
| Mean, £ (SD) | Mean, £ (SD) | ||
| Total cost NHS perspective | |||
| 25th percentile | 689 (1453) | 632 (1532) | 57 (–290 to 373) |
| 75th percentile | 945 (1594) | 1002 (1492) | –57 (–402 to 282) |
| Total cost NHS and informal caregivers’ perspective | |||
| 25th percentile | 2340 (4075) | 2280 (3518) | 60 (–725 to 890) |
| 75th percentile | 2665 (4108) | 2803 (3490) | –138 (–891 to 683) |
For the 25th centile imputations, the average total cost from the NHS perspective and from the NHS and caregiver perspective was higher in the intervention arm than in the control arm yet the CIs included zero. These results are consistent with our base-case analysis. However, when we imputed resource use at the 75th centile, although the CIs still indicate no evidence of a difference between randomised groups, the results on average favour the intervention as being the least costly. Further analysis showed that this result was due to a skewed high number of ambulance journeys reported in the control arm for one participant. Excluding this individual from analysis resulted in the average total cost being higher in the intervention arm than in the control arm, from a NHS perspective, with a mean difference of £79 (bootstrapped 95% CI –£238 to £407). These imputed results overall were consistent with our base-case analysis in which average total costs in the intervention arm were higher than in the control arm but with no evidence of difference between the groups despite these seeming economically meaningful.
Health-related quality-of-life scores and quality-adjusted life-years
We used a simple imputation method to interpolate missing HRQoL data at 8 weeks and 6 months by utilising the weighted average of adjacent observations at each time point. These interpolated results therefore include those individuals who had completed questionnaires at baseline and 12 months and had missing data at time points in between. This increased the sample size from 199 to 262. The results suggest that neither method of estimating greatly alters the base-case analysis results (Table 57).
| Variable | No interpolated EQ-5D (5L) values | Interpolated EQ-5D (5L) values | ||
|---|---|---|---|---|
| Unstandardised coefficients: B (standard error) | 95% CI | Unstandardised coefficients: B (standard error) | 95% CI | |
| (Constant) | 0.015 (0.010) | –0.006 to 0.035 | 0.019 (0.009) | 0.000 to 0.037 |
| Falls and Syncope Service | –0.002 (0.007) | –015 to 0.012 | –0.007 (0.006) | –0.019 to 0.004 |
| Galleries Day Unit | –0.006 (0.014) | –0.033 to 0.022 | –0.008 (0.011) | –0.031 to 0.014 |
| Intervention | –0.004 (0.006) | –0.015 to 0.008 | –0.001 (0.005) | –0.011 to 0.010 |
| Sex | –0.010 (0.007) | –0.023 to 0.003 | –0.006 (0.006) | –0.017 to 0.005 |
| Baseline EQ-5D (5L) tariff | –0.006 (0.013) | –0.032 to 0.021 | –0.015 (0.012) | –0.039 to 0.009 |
Using the same approach, and obtaining interpolated values for HRQoL using the SF-6D, resulted in an extra 65 observations (Table 58). As with the EQ-5D (5L) data, neither method identifies any QALY difference associated with the intervention.
| Variable | No interpolated SF-6D values | Interpolated SF-6D values | ||
|---|---|---|---|---|
| Unstandardised coefficients: B (standard error) | 95% CI | Unstandardised coefficients: B (standard error) | 95% CI | |
| (Constant) | 0.043 (0.11) | 0.022 to 0.064 | –0.022 (0.018) | –0.057 to 0.014 |
| Falls and Syncope Service | 0.002 (0.004) | –0.005 to 0.009 | –0.007 (0.006) | –0.019 to 0.006 |
| Galleries Day Unit | 0.002 (0.008) | –0.013 to 0.018 | –0.018 (0.012) | –0.041 to 0.006 |
| Intervention | 0.002 (0.003) | –0.005 to 0.008 | 0.002 (0.006) | –0.009 to 0.013 |
| Sex | –0.003 (0.004) | –0.010 to 0.005 | 0.000 (0.006) | –0.012 to 0.013 |
| Baseline SF-6D tariff | –0.071 (0.017) | –0.105 to –0.037 | 0.037 (0.028) | –0.019 to 0.093 |
Missingness due to questionnaire non-response
Impact of non-response on costs and quality-adjusted life-years
A joint modelling approach to missing data was undertaken based on the assumption that time to drop out would be predictive of final outcome. The rationale behind this approach was that there is some underlying characteristic of the participant, usually referred to as ‘frailty’, which is associated both with dropout and outcome. As demonstrated in Chapter 4, the dropout did not occur regularly through the study, but was triggered by particular activities arising from participation in the study. A large proportion of the dropout occurred prior to week 8; a smaller proportion of dropout accrued over the remaining 44 weeks. Consequently, the impact of missing data was assessed using multiple imputation. The results of the multiple imputation on the cost data are presented in Table 59.
| Method | Imputation model: equality of means | |
|---|---|---|
| Mean difference | Bootstrapped 95% CI | |
| NHS costsa | 66 | –363 to 495 |
| NHS and caregiver costsb | 367 | –555 to 1293 |
Adjusting for missing data resulted in a decrease in NHS costs in the intervention arm and a smaller reduction in the control arm, explaining a smaller mean difference between the two arms of the trial. However, as in the base case, although costs were higher in the intervention group, there was no evidence of a difference between groups. Adjusting for missing data resulted in an increase in NHS and caregiver costs in the intervention arm and a small reduction in the control arm. Consistent with the base case – with higher costs in the intervention arm – there was no evidence of a difference between the two arms of the trial.
The impact of multiple imputation on QALYs is presented in Table 60. In the case of EQ-5D (5L), the reduction in QALY gain was greater once missing data were accounted for by multiple imputations. For SF-6D, the small positive effect of the intervention was reversed, with the control group experiencing the greater QALY gain. Following the multiple imputation the estimated QALY gains in each arm were presented (see Table 61).
| Method | Imputation model-based analysis | |||
|---|---|---|---|---|
| Regression model, EQ-5D (5L) | Regression model, SF-6D | |||
| Unstandardised coefficient for CBTi | 95% CI | Unstandardised coefficient for CBTi | 95% CI | |
| By group | –0.005 | –0.018 to 0.007 | 0.002 | –0.005 to 0.012 |
| Complete sample | –0.007 | –0.019 to 0.006 | 0.002 | –0.004 to 0.009 |
| Complete case analysis | ||||
| Regression model, EQ-5D (5L) | Regression model, SF-6D | |||
| m | 95% CI | m | 95% CI | |
| –0.004 | –0.015 to 0.008 | 0.002 | –0.009 to 0.013 | |
In summary, no method of allowing for lost data showed any difference between groups in terms of QALY gains.
Incremental cost-effectiveness ratio with costs and Falls Efficacy Scale–International version following multiple imputation
The results of the stochastic sensitivity analysis using all available complete case costs and FES-I data (see Figure 23) from the multiple imputation analysis are presented in Table 61. The results show that CBTi was more costly and more effective using FES-I as an outcome than usual care alone, the same as the base-case analysis. The incremental cost per unit reduction in FES-I for CBTi compared with usual care only from a NHS perspective was £22. The incremental cost per reduction in FES-I for CBTi compared with usual care from a NHS and caregiver perspective was £121.
| Intervention | Cost (£) | Change in FES-I | Incremental cost per unit reduction in FES-I (£) | Probability that the intervention is cost-effective for different threshold values for society’s WTP for a unit reduction in FES-I: | ||
|---|---|---|---|---|---|---|
| £0 | £1000 | £30,000 | ||||
| NHS perspective | ||||||
| CBTi | 653 | 2.79 | 22 | 0.39 | 1 | 1 |
| Usual care only | 587 | 5.84 | 0.61 | 0 | 0 | |
| NHS and caregiver perspective | ||||||
| CBTi | 2201 | 2.79 | 121 | 0.28 | 1 | 1 |
| Usual care only | 1832 | 5.84 | 0.72 | 0 | 0 | |
Incremental cost-effectiveness ratio with costs and European Quality of Life–5 Dimensions (5 Level) quality-adjusted life-years following multiple imputation
The results of the stochastic sensitivity analysis using costs and EQ-5D (5L) QALY data following multiple imputation care presented in Table 62. The results show that, using EQ-5D (5L) QALYs as an outcome, CBTi was more costly (from both cost perspectives) and less effective than usual care alone.
| Intervention | Cost (£) | Change in EQ-5D (5L) from baseline | Incremental cost per QALY (£) | Probability that the intervention is cost-effective for different threshold values for society’s WTP for a change in QALYs: | ||
|---|---|---|---|---|---|---|
| £0 | £1000 | £30,000 | ||||
| NHS perspective | ||||||
| CBTi | 653 | 0.002 | Dominated | 0.38 | 0.37 | 0.43 |
| Usual care only | 587 | 0.007 | 0.62 | 0.63 | 0.57 | |
| NHS and caregiver perspective | ||||||
| CBTi | 2201 | 0.002 | Dominated | 0.22 | 0.22 | 0.37 |
| Usual care only | 1832 | 0.007 | 0.78 | 0.78 | 0.63 | |
Incremental cost-effectiveness ratio with costs and Short Form questionnaire-6 Dimensions quality-adjusted life-years following multiple imputation
The results of the stochastic sensitivity analysis using costs and SF-6D QALY data following multiple imputation care are presented in Table 63. The results show that, using SF-6D QALYs as an outcome, CBTi was more expensive (from both cost perspectives) and slightly less effective than usual care alone. The intervention was therefore dominated and would not be considered cost-effective.
| Intervention | Cost (£) | Change in SF-6D from baseline | Incremental cost per QALY (£) | Probability that the intervention is cost-effective for different threshold values for society’s WTP for a change in QALYs: | ||
|---|---|---|---|---|---|---|
| £0 | £1000 | £30,000 | ||||
| NHS perspective | ||||||
| CBTi | 653 | 0.081 | Dominated | 0.38 | 0.38 | 0.3 |
| Usual care only | 587 | 0.083 | 0.62 | 0.62 | 0.7 | |
| NHS and caregiver perspective | ||||||
| CBTi | 2201 | 0.081 | Dominated | 0.21 | 0.21 | 0.19 |
| Usual care only | 1832 | 0.083 | 0.79 | 0.79 | 0.81 | |
Summary of cost-effectiveness results
Using FES-I as an outcome measure, the base-case analysis suggests that CBTi is more costly and more effective than usual care alone. Stochastic sensitivity analysis suggests that when society’s WTP for a unit change in FES-I was > £20, the probability of CBTi and usual care being cost-effective would approach ‘1’. The analogous threshold for NHS and caregiver perspective was £58. These base-case results were insensitive to changes explored in all of the sensitivity analysis, including multiple imputation.
When QALYs derived from EQ-5D (5L) were taken as the outcome measure, the results from a NHS costing perspective suggest that CBTi was, on average, more expensive and less effective than usual care alone. CBTi is therefore dominated by usual care and cannot be considered as the most cost-effective treatment option. However, from a NHS and caregiver costing perspective, CBTi was cheaper than, but less effective than, usual care. However, stochastic sensitivity analysis suggested that the intervention is unlikely to be cost-effective over a range of threshold values for society’s WTP for a QALY. These base-case results were insensitive to the sensitivity analyses, including the multiple imputation, for which the intervention is dominated by usual care from both costing perspectives.
When QALYs were based on the SF-6D scores, from a NHS perspective costing perspective the intervention was more costly but more effective than the control. From a NHS and caregiver perspective, although the intervention was more effective, it was less costly than the control. However, results from the CEAC suggest that it is unlikely that CBTi would be cost-effective at typical values that society might be willing to pay from a NHS costing perspective. Only when costed from a NHS and caregiver perspective could the intervention be considered to be cost-effective. The results of the sensitivity analyses, and, in particular, the multiple imputation, suggested that CBTi was dominated by usual care and cannot be considered as the most cost-effective treatment option.
Chapter 7 Discussion and conclusions
Developing a novel cognitive–behavioural therapy intervention for fear of falling in older adults
The development of the CBTi followed established principles in CBT, but required in-depth exploration of the issues relevant to older people with fear of falling and the development of a training approach that would equip non-specialist HCAs to deliver the CBTi in the trial. The initial assessment interviews conducted to inform the intervention development opened up and challenged notions of the concept of ‘fear of falling’, uncovering both its heterogeneity (diversity of views and experiences) and complexity (in terms of associations between falls, appraisals of these, and related behaviours). In developing the intervention it was necessary to acknowledge and accommodate the importance of the network of comorbid physical complaints, interpersonal relationships and/or social isolation revealed as central to fear of falling. This was addressed by introducing individualised formulations that added to the complexity of the intervention and created uncertainty over the feasibility of successful delivery of the CBTi by relatively inexperienced HCAs.
The complexity of fear of falling highlighted here reflects recent arguments for a deeper understanding of fear of falling that have developed out of analysis of associations of structured measures relating to the concept. 30 Our findings also support guidance for the adaptation of CBT for older populations, particularly the recommendation to avoid approaches relying on rigid manualisation. 117
The process evaluation complemented the intervention development work by highlighting the work required to develop an intervention that would ‘make sense’ to key groups whose participation was expected and/or required. For the CBTi for fear of falling, this included addressing key issues such as determining what to call it, developing participants’ understandings of what it would involve, and what benefits it might have for patients.
Summary of main findings of the Phase II randomised controlled trial
The results of the STRIDE study are clear. A novel CBTi that was delivered by HCAs in addition to usual care in community-dwelling elders who were attending falls services resulted in a significant reduction in fear of falling, as measured by the FES-I, compared with those receiving usual care alone. The estimated impact of the CBTi on FES-I at 12 months, based on an analysis of covariance model in which we adjusted for baseline FES-I score and randomisation strata (prespecified primary end point and method of analysis), was a mean change of –4.02 (95% CI –5.95 to 2.1), with reductions in score reflecting improvements in fear of falling. This was from a mean baseline FES-I of 40.9 in the CBTi group and 39.8 in the control group. Similar improvements in FES-I scores to those at 12 months were seen at 8 weeks and 6 months, despite the absence of intervention for the final 6 months of follow-up, and our study was powered on the basis of a 4-point reduction in FES-I scores. Although there are no definitions of minimal clinically significant changes in FES-I or any other fear of falling measure,25 our FES-I effect size is among the highest reported, with recent randomised studies on interventions in fear of falling using the FES-I reporting significant decreases in scores ranging from 0.53 to 3.7. 118–120
Dropouts from the study were at variable time points and tended to be triggered by activities arising from the study (e.g. example the CBTi in the intervention group). Even allowing for such differential dropouts between groups using multiple imputation with ITT, and the most conservative set of assumptions, there was still a mean change in FES-I score of –3.65 (95% CI –5.84 to 1.46). Furthermore, an ‘as-treated analysis’ based on the proportion of CBT sessions completed showed a mean change of –4.4 (95% CI –6.5 to 2.2). It would thus appear that the primary analysis was robust. In addition, fear of falling as measured by the single question numerical rating scale from 0 to 10 was highly correlated with FES-I scale scores, with an mean estimated impact of CBTi of –1.0 (95% CI –1.6 to 0.5), supporting the validity of our main finding.
There were significant improvements in HADS Depression scores in the CBTi group, an important finding given the high incidence of depression in older people, in particular those with fear of falling. 40,43 Depression may have shifted as a result of the similarity of the evidence-based treatment for depression and the STRIDE intervention. ‘Behavioural activation’, encouraging people to re-engage with avoided pleasurable and challenging tasks, is the main behavioural intervention for low mood, and was also a key component of the STRIDE intervention.
There were no differences in social participation, loneliness, quality of life or physical function on a variety of measures; the fact that many trial participants suffered a multitude of comorbid physical difficulties and adverse life events that could not be addressed by the CBTi may explain the lack of responsiveness in these measures. The estimated impact of the CBTi on the likelihood of falling corresponds to an incidence risk ratio of 0.88 (95% CI 0.61 to 1.27). The point estimate of 0.88 suggests a slight reduction in the risk of falling but the 95% CI includes one; the difference between groups is not statistically significant. There were no significant harms associated with the CBTi, with lower fall and fracture rates in the intervention group, although these were not statistically significant.
Understanding the delivery of cognitive–behavioural therapy intervention by health-care assistants: insights for NHS delivery from the process evaluation
One of the key components of the STRIDE study was the delivery of the CBTi by HCAs, given the high prevalence of the problem and the difficulties for the NHS in either finding or funding sufficient trained CBT therapists and clinical psychologists to satisfy this need. The HCAs’ delivery of the CBTi was evaluated positively by the psychologists, although some skills could be further developed as discussed below. Delivery of the CBTi was shaped over time by the HCAs’ growing experience of the client group, the complexity of fear of falling and the tendency to revert to existing skill sets.
The value of the CBTi for this client group was recognised by some professional staff providing usual care. Senior professionals expressed interest in the ongoing delivery of the CBTi services, and suggested scope for improved integration of the CBTi with existing interventions. There were, however, issues over the ‘fit’ of CBTi with the underlying ethos of one participating site.
Client engagement with, and understandings of, the CBTi varied. Nevertheless, clients who persisted with the CBTi valued their interactions with the HCAs and perceived a range of benefits from the intervention, including self-reported improvements in confidence and independence.
The organisation and delivery of the CBTi, in terms of the materials, session content, frequency and duration, was generally acceptable to clients although more flexibility over the follow-up session (here scheduled for 6 months after the end of the initial course of therapy) was suggested, and there was a strong preference for delivery in the client’s home.
These observations highlight the crucial part played by the recruitment, training and supervision of HCAs in any attempt to translate our findings into routine clinical practice and will be explored in more detail below.
Recruitment
The level of skill and autonomy required by the HCAs meant that employment at NHS Band 4 or above would be vital, with recruitment taking into account understanding and experience of the client group, empathy, team working and communication and problem-solving skills.
Training
Health-care assistants needed to learn complex new skills in order to deliver the CBTi. Initial training was short, with ongoing supervision by the psychologists. The training was generally felt to be adequate by the psychologists and the HCAs, and could usefully be adopted as core training for potential HCAs delivering the CBTi. Key areas to address in future training include an emphasis on the rationale for the CBTi before moving on to more detailed techniques to ensure that trainees understand the intervention in a more holistic way rather than focusing on a more protocol-driven, technical approach. One of the most difficult skills for the HCAs to master was how to introduce CBT to clients, many of whom were sceptical of ‘talking treatments’. Greater emphasis on modelling, role playing and practising the introduction of CBT should facilitate client understanding of, and engagement with, such an intervention at the earliest stages of treatment.
The use of ‘real play’, for which trainees use their own issues and problems to explore CBT techniques, was key in helping trainees start embedding their skills while providing insight into the experience of being a client at the start of their training. This approach further facilitates learning, with the use of CBT techniques during training enabling trainees to address their own anxieties and thereby increasing their confidence in the intervention. Soft skills, such as empathy and collaborative working should be more explicitly worked on, alongside training on the support and challenge model. Working towards valued goals identified by patients is an important part of effective and collaborative therapy. Although goal setting was covered in the initial training, data suggest that detailed review of patient goals in supervision would help to embed the principles of SMART goal setting and ensure genuinely individualised therapy.
Finally, top-up training should be available to trainees to address any skill gaps and provide opportunities to practise skills at the point at which they are being used. Initial training provides an excellent grounding, but there is a great deal of new material delivered in a relatively short space of time; reinforcement over time is vital.
Supervision
The HCAs’ ability to embed and develop their CBTi skills was facilitated by individual and group supervision sessions and the HCAs’ self-initiated peer support sessions. The ‘real play’ techniques, highlighted above, are powerful tools in the training process and need to be appropriately resourced and supervised.
Consideration of the role and availability of additional multidisciplinary support is helpful for trainees, given the complex needs of many of their client group. Very few of the clients who received the CBTi would have considered or been eligible for help from existing psychological services despite the NHS IAPT (Improving Access to Psychological Therapies) programme. This was exemplified by our HCAs’ growing health advocacy role, through which individual clients’ new or worsening intercurrent illnesses (e.g. increasing confusion, blackouts, chest pain, injurious falls) required medical and other input that would otherwise have been left to a much later stage. The study has graphically highlighted the extent to which older people’s lives are curtailed by medical and psychosocial events that are frequently unreported to health and social care professionals.
Health-economic analysis
Given the greater reduction in FES-I score in the intervention arm, there is evidence that the intervention is effective and could be considered cost-effective in reducing FES-I between groups. However, such effectiveness is not reflected when using QALYs. Although multiple imputation on the cost-effectiveness data attempted to overcome potential biases from missing data, there was still no evidence that the intervention was cost-effective using QALYs. In support of this finding, the ICER in relation to incremental costs per unit reduction in FES-I and the associated CEAC were insensitive to change following multiple imputation. Furthermore, stochastic sensitivity analysis in base-case analysis for NHS costs and QALY types suggests that the intervention would not be considered cost-effective relative to usual care.
Reported resource-use and associated costs were greater on average in the intervention arm relative to the control arm; however, the CI for the difference includes zero and is sufficiently wide to include economically important differences in favour of either group. In terms of costs (from both perspectives) per improvement in FES-I, it is difficult to assess the relative cost-effectiveness according to standard decision-making criteria within the NHS,107 as WTP for a unit reduction in FES-I is not known.
Strengths and limitations
The conduct and reporting of the development of the intervention development and subsequent RCT have followed Medical Research Council complex interventions,93 TIDieR45 and CONSORT90 recommendations. The study used a web-based randomisation system to conceal allocation and conducted primary outcome analyses using multiple imputation and ITT techniques, with a further ‘as treated’ analysis to account for actual CBTi sessions completed according to a predefined statistical analysis plan agreed with the TSC.
A high proportion of those eligible for study inclusion were recruited, with 43% of eligible patients eventually included in the study, 18% of those screened (see Figure 4). Baseline comparability of the CBTi and control groups was excellent, with balance achieved for the prespecified strata (gender, pain with walking, referral to strength and balance training classes). The recruitment-driven progression from a single-centre study to a three-centre study became a strength in terms of the differing nature of the three falls services and hence the generalisability of our findings. Our dropout rate was 24.6%, almost exactly the anticipated 25% dropout rate based on previous experience with this client group and published data from group CBT studies in fear of falling (see Chapter 3). Sixty-nine patients dropped out of the CBTi arm of the study, along with 43 control subjects; although many of these continued in the study for data collection purposes, for those who did not, multiple imputation and an ‘as-treated’ analysis (see Chapters 3 and 4) were utilised to take statistical account for this. Those dropping out of CBT tended to have lower FES-I scores at baseline, suggesting a degree of self-selection in terms of need for intervention. The original FES13 was not designed to provide a summary numerical scale with diagnostic cut-offs to diagnose fear of falling; rather it was intended as a tool to explore factors affecting patients’ perceptions of their falls’ self-efficacy. In a comprehensive longitudinal study of the clinical and psychometric properties of the FES-I in 500 community-dwelling elders, Delbaere et al. ,40 using receiver operating curve cut-offs, suggested a score of > 23 as indicating high levels of concern about falls. Scores around this level can be generated by minor degrees of apparent concern that more accurately reflect physical issues rather than fear of falling, as highlighted during the intervention development phase noted above and in Chapter 2. As with any questionnaire-based diagnostic tool, cut-offs will occasionally include cases in which there is none and vice versa. In the absence of any competing diagnostic information, we used the > 23 FES-I score as our inclusion criterion for fear of falling, but are mindful that this level may have included those who did not identify themselves as having such issues. However, the repeated assessment by the RAs of FES-I before confirming participant eligibility and the relatively high mean baseline FES-I scores at this repeated assessment (with an average score of 40 in both intervention and control groups) suggests that the study group, as a whole, suffered significant fear of falling. These considerations have important implications for any future use of the FES-I in this respect.
It is possible that respondent burden, related to the volume of outcomes assessed, may have contributed to loss to follow-up. However, we judged that the range of outcome measures utilised were necessary for a comprehensive assessment of the impact of the study intervention.
Performance bias refers to systematic differences between groups in the care that is provided, or in exposure to factors other than the interventions of interest. Theoretically, asking a patient to travel to a centre (for CBT) may have had an impact on falls efficacy unrelated to the CBT provided.
Detection bias refers to systematic differences between groups in how outcomes are determined. The primary outcome was a self-completion instrument. Theoretically, being told that they were in receipt of an intervention may have been an impact on patients’ FES scores (relative to being told that they were in the control group).
Recruitment difficulties during the course of study resulted in attempts to improve recruitment, including revision of our power calculation with TSC, funder, REC and sponsor approval, as described in Chapter 3. Although clearly not a desirable change, our subsequent recruitment to revised time and target with study completion and appropriately comprehensive analyses mitigate this change in power. It is important to note that a further strength was our involvement with Age UK. The local Age UK executive team, staff, clients and their carers were a key component of the study’s development and execution, and were instrumental in many of the changes to recruitment practices and materials, without which the study would not have successfully completed.
The method of data imputation used in the trial analysis assumes that the data are missing at random (MAR). For the primary outcome we assume that the likelihood that a value is missing does not depend on the actual value of FES-I. If this assumption is incorrect then the results of the analyses on the imputed data sets must be treated with caution. However, we believe that any association between likelihood of a value being missing and the actual value is probably due to other factors, such as the frailty of the patient. A number of variables that we believe are likely to capture these factors have been included in the imputation model. We therefore believe that the investigation of the impact of missing data on our estimates of the impact of CBT are robust.
The embedded process evaluation fostered a culture of reflection and open communication, which enabled the early identification of procedural and interpersonal issues, and facilitated the conduct of the study. The role of the process evaluator was facilitated by the openness of the research team to a degree of observation and self-reflexivity exceeding that which would normally be expected in such studies. This proved a key strength of this study, which has allowed the capture of important data concerning the processes (and challenges) of delivering a CBTi to older people with fear of falling.
Our health-economic analysis had several limitations. We used a number of methods to investigate the impact of missing data on the results. Simple imputation methods were used in the first instance on both costs and utility values, and found no sensitivity to the base-case results. Simple imputation methods rely on the assumption that the data are MAR, such that the probability of having missing data depends only on the observed data and not on any unobserved data. 88 The plausibility of the MAR assumption can be improved by including as many covariates as possible that can help predict the missing data, for example patient characteristics, observed cost or resource-use data, measures of effectiveness and any reasons for the ‘missingness’. 121 Inferences from multiple imputation are preferable to single imputation, as they properly reflect the uncertainty in the predictions of the missing values, while preserving the distributions and relationships in the data. 122 We therefore used multiple imputation to overcome these issues.
The average cost of the CBTi was estimated, based on the numbers of participants in the trial and the number of HCAs delivering it. It could be argued that these numbers would not be representative if the CBTi were to be adopted in practice. In routine practice, if more patients are seen then this would have the potential of reducing the average cost, which, in turn, could reverse the relative cost difference between the two arms of the trial. If increased numbers of patients are seen or costs of training HCAs fall and bring down the average cost of the patient to < £73 then this may reverse the increased cost of the intervention relative to control, such that the control is more expensive.
Other methodological issues
The FES-I scores in older adults with fear of falling tend to worsen over time,40 so it is interesting to note that scores in our control group fell slightly at 12 months’ follow-up (–2.2, 95% CI –3.49 to 1.05). The RAs performing outcome measures were highly empathic individuals with excellent communication skills, and although they had no knowledge or training in the CBTi, there may have been an occult Hawthorne effect in operation, which may have masked more significant differences between intervention and control groups in FES-I scores.
Contemporaneous falls reporting is a key component of any study examining fall rates, and we have successfully used falls diaries in this respect in previous trials,83,84 with return rates of up to 95%. Sixty-eight participants (16.4%) did not return any of their monthly falls diaries at all, and the mean number of returns was eight, so there may have been bias in either direction from under-reporting, although reports to RAs and the HCA therapists, and the fact that falls tend to be actively reported, suggest that this is relatively unlikely.
Implications for health care
-
We can be confident in concluding that our novel CBTi improves fear of falling (as measured both by the FES-I and 11-point fear of falling rating scale) in community-dwelling older adults who are attending falls services. There is also evidence that the intervention may improve depression in such individuals. The fact that the intervention was successfully delivered by HCA-grade staff has important implications for its adoption into routine practice.
-
When CBTi is integrated into existing services, it is important to ensure that the intervention is aligned with existing practices, as there is a potential for conflicting messages that can confuse patients and potentially confound the effects of the intervention. PPI-based ‘scripts’ for introducing the CBTi to patients with fear of falling is important, as our process evaluation work showed clearly that clients conceptualise the intervention in various ways, which are highly dependent on how it is presented to them. This, in turn, profoundly influences engagement with the CBTi and the internalisation of its principles and techniques.
-
Although our study did not set out to demonstrate which subset of individuals with fear of falling would benefit most from the intervention, it was clear that those with the lowest FES-I scores, and those who least considered themselves to suffer from fear of falling, were most likely to drop out of the study. This may have resource and clinical implications for targeting of patients for the CBTi.
Future research implications
-
Our work shows clearly the heterogeneous nature of fear of falling and the importance of physical factors in its genesis and promotion. There are some small studies examining the role of a joint CBT and physical training approach in managing fear of falling,123,124 but these need to become larger scale, appropriately powered RCTs using findings such as ours to inform power calculations.
-
Research into targeting of CBTi in older people with fear of falling will aid more rational use of scarce health service resources. As we discussed above, there is some evidence from our study of differential uptake and completion of CBTi but these factors need to be more explicitly explored.
-
Previous studies have examined group CBT for fear of falling, whereas our intervention was individually based. From both individual effectiveness and resource perspectives it would be useful to trial a mix of approaches.
-
This study highlighted the value of an embedded reflexive component to the design and conduct of any large trial. The presence of a qualitative component fostered a culture of reflection and open communication, which enabled the early identification of procedural and interpersonal issues and facilitated the conduct of the study.
-
Stakeholders identified a number of potential service models for the future delivery of CBTi, for example in primary care and/or in generic CBT teams working into a range of departments in secondary care.
-
Future research could explore the feasibility and cost-effectiveness of alternative service provision of CBTi; in addition, it is vital that future research addresses the minimally important difference in FES-I scores to appropriately inform and interpret CEA.
Acknowledgements
The authors would like to acknowledge the contributions of the following: John O’Brien and Alma Caldwell for study design and funding application; Ruth Pearce, Pat McCue and Helen Walker for the design of the case report forms and other study materials; Ruth Pearce for her careful review of social isolation measures; Ruth Pearce, Pat McCue, Emma McLellan and Christie Morley for data collection; the STRIDE therapists (David Green, Victoria Strassheim and Charlotte Dunkel) for their contribution to the design and implementation of CBTI materials and methods; Julie Doughty and Alaa Abouhajar for database design and data management; and Helen Walker, Debbie Laird and Leanne Cork for administrative support.
We would also like to acknowledge the contribution of staff at the participating falls services and Age UK North Tyneside, and to Mehda Singh, Arthur Affleck, Louise Sutcliffe, Sandra Davies and Aaron Jackson for their assistance with recruitment. In addition, we are grateful to the TSC and Data Monitoring and Ethics Committee for their support, important suggestions and constructive criticism in equal measure.
Contributions of authors
Steve W Parry was Chief Investigator for the study; he led the application for funding, was actively involved in all stages of the research and co-ordinated the authorship of the final report, including authorship of Chapters 1 and 7. He is the guarantor for the study.
Claire Bamford was jointly responsible for the process evaluation (with TF), including conducting and analysing interviews, and coauthored Chapter 5 of this report.
Vincent Deary was a coapplicant for funding; he provided CBT expertise to this study, including intervention development, training and supervision of the CBTi therapists and authorship of Chapter 2 of this report.
Tracy L Finch was a coapplicant for funding; she was jointly responsible for the process evaluation (with CB) and coauthored Chapter 5 of this report.
Jo Gray contributed to the health-economic evaluation, including coauthoring Chapter 6 of this report.
Claire MacDonald was the trial manager for this study; she coauthored Chapters 3 and 4 of this report.
Peter McMeekin was a coapplicant for funding; he led the health-economic evaluation and coauthored Chapter 6 of this report.
Neil J Sabin was a coapplicant for funding; he provided clinical psychology expertise to this study, including intervention development, training and supervision of the CBTi therapists.
I Nick Steen was a coapplicant for funding and the study statistician; he coauthored Chapters 3 and 4.
Sue L Whitney was a coapplicant for funding; she provided critical oversight and guidance for the study.
Elaine McColl was a coapplicant for funding; she provided expertise in trial design and conduct, and coauthored Chapters 3 and 4.
All authors provided critical comments on drafts of the final report. With the exception of SW, all authors were members of the Trial Management Group.
Publications
Parry SW, Finch T, Deary V. How should we manage fear of falling in older adults living in the community? BMJ 2013;346:f2933.
Finch T, Bamford C, Deary V, Sabin N, Parry SW. Making sense of a cognitive behavioural therapy intervention for fear of falling: qualitative study of intervention development. BMC Health Serv Res 2014;14:436.
Parry SW, Deary V, Finch T, Bamford C, Sabin N, McMeekin P, et al. The STRIDE (Strategies to Increase confidence, InDependence and Energy) study: cognitive behavioural therapy-based intervention to reduce fear of falling in older fallers living in the community: study protocol for a randomised controlled trial. Trials 2014;15:210.
Data sharing statement
Anonymised study data will be prepared for data sharing in line with National Institute for Health Research (NIHR) policies, with the study team having exclusive access to data for the first 24 months following study completion. Study documents and data will be archived for 10 years in line with sponsor policies; all available data can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Painter JA, Elliott SJ, Hudson S. Falls in community-dwelling adults aged 50 years and older: prevalence and contributing factors. J Allied Health 2009;38:201-7.
- British Geriatrics Society . Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc 2011;59:148-57. http://dx.doi.org/10.1111/j.1532-5415.2010.03234.x.
- American Academy of Orthopedic Surgeons Panel on Falls Prevention . Guideline for the Prevention of Falls in Older Persons. J Am Geriatr Soc 2001;49:664-72. http://dx.doi.org/10.1046/j.1532-5415.2001.49115.x.
- Scuffham P, Chaplin S, Legood R. Incidence and costs of unintentional falls in older people in the United Kingdom. J Epidemiol Community Health 2003;57:740-4. http://dx.doi.org/10.1136/jech.57.9.740.
- Heinrich S, Rapp K, Rissmann U, Becker C, Konig HH. Cost of falls in old age: a systematic review. Osteoporos Int 2010;21:891-902. http://dx.doi.org/10.1007/s00198-009-1100-1.
- Vellas BJ, Wayne SJ, Romero LJ, Baumgartner RN, Garry PJ. Fear of falling and restriction of mobility in elderly fallers. Age Ageing 1997;26:189-93. http://dx.doi.org/10.1093/ageing/26.3.189.
- Murphy SL, Williams CS, Gill TM. Characteristics associated with fear of falling and activity restriction in community-living older persons. J Am Geriatr Soc 2002;50:516-20. http://dx.doi.org/10.1046/j.1532-5415.2002.50119.x.
- Zijlstra GA, van Haastregt JC, van Rossum E, van Eijk JT, Yardley L, Kempen GI. Interventions to reduce fear of falling in community-living older people: a systematic review. J Am Geriatr Soc 2007;55:603-15. http://dx.doi.org/10.1111/j.1532-5415.2007.01148.x.
- Zijlstra GA, van Haastregt JC, van Eijk JT, van Rossum E, Stalenhoef PA, Kempen GI. Prevalence and correlates of fear of falling, and associated avoidance of activity in the general population of community-living older people. Age Ageing 2007;36:304-9. http://dx.doi.org/10.1093/ageing/afm021.
- Scheffer AC, Schuurmans MJ, van Dijk N, van der Hooft T, de Rooij SE. Fear of falling: measurement strategy, prevalence, risk factors and consequences among older persons. Age Ageing 2008;37:19-24. http://dx.doi.org/10.1093/ageing/afm169.
- Van Haastregt JC, Zijlstra GA, van Rossum E, van Eijk JT, Kempen GI. Feelings of anxiety and symptoms of depression in community-living older persons who avoid activity for fear of falling. Am J Geriatr Psychiatry 2008;16:186-93. http://dx.doi.org/10.1097/JGP.0b013e3181591c1e.
- Jorstad EC, Hauer K, Becker C, Lamb SE. ProFaNE Group . Measuring the psychological outcomes of falling: a systematic review. J Am Geriatr Soc 2005;53:501-10. http://dx.doi.org/10.1111/j.1532-5415.2005.53172.x.
- Tinetti ME, Richman D, Powell L. Falls efficacy as a measure of fear of falling. J Gerontol 1990;45:P239-43. http://dx.doi.org/10.1093/geronj/45.6.P239.
- Reelick MF, van Iersel MB, Kessels RP, Rikkert MG. The influence of fear of falling on gait and balance in older people. Age Ageing 2009;38:435-40. http://dx.doi.org/10.1093/ageing/afp066.
- Deshpande N, Metter EJ, Lauretani F, Bandinelli S, Guralnik J, Ferrucci L. Activity restriction induced by fear of falling and objective and subjective measures of physical function: a prospective cohort study. J Am Geriatr Soc 2008;56:615-20. http://dx.doi.org/10.1111/j.1532-5415.2007.01639.x.
- Austin N, Devine A, Dick I, Prince R, Bruce D. Fear of falling in older women: a longitudinal study of incidence, persistence, and predictors. J Am Geriatr Soc 2007;55:1598-603. http://dx.doi.org/10.1111/j.1532-5415.2007.01317.x.
- Delbaere K, Sturnieks DL, Crombez G, Lord SR. Concern about falls elicits changes in gait parameters in conditions of postural threat in older people. J Gerontol A Biol Sci Med Sci 2009;64:237-42. http://dx.doi.org/10.1093/gerona/gln014.
- Carpenter MG, Adkin AL, Brawley LR, Frank JS. Postural, physiological and psychological reactions to challenging balance: does age make a difference?. Age Ageing 2006;35:298-303. http://dx.doi.org/10.1093/ageing/afl002.
- Logghe IH, Verhagen AP, Rademaker AC, Bierma-Zeinstra SM, van Rossum E, Faber MJ, et al. The effects of Tai Chi on fall prevention, fear of falling and balance in older people: a meta-analysis. Prev Med 2010;51:222-7. http://dx.doi.org/10.1016/j.ypmed.2010.06.003.
- Zijlstra GA, van Haastregt JC, Du Moulin MF, de Jonge MC, van der Poel A, Kempen GI. Effects of the implementation of an evidence-based program to manage concerns about falls in older adults. Gerontologist 2013;53:839-49. http://dx.doi.org/10.1093/geront/gns142.
- Huang TT, Yang LH, Liu CY. Reducing the fear of falling among community-dwelling elderly adults through cognitive–behavioural strategies and intense Tai Chi exercise: a randomized controlled trial. J Adv Nurs 2011;67:961-71. http://dx.doi.org/10.1111/j.1365-2648.2010.05553.x.
- Sherrington C, Tiedemann A, Fairhall N, Close JC, Lord SR. Exercise to prevent falls in older adults: an updated meta-analysis and best practice recommendations. N S W Public Health Bull 2011;22:78-83. http://dx.doi.org/10.1071/NB10056.
- Cameron ID, Stafford B, Cumming RG, Birks C, Kurrle SE, Lockwood K, et al. Hip protectors improve falls self-efficacy. Age Ageing 2000;29:57-62. http://dx.doi.org/10.1093/ageing/29.1.57.
- Vind AB, Andersen HE, Pedersen KD, Joergensen T, Schwarz P. Effect of a program of multifactorial fall prevention on health-related quality of life, functional ability, fear of falling and psychological well-being. a randomized controlled trial. Aging Clin Exp Res 2010;22:249-54. http://dx.doi.org/10.1007/BF03324804.
- Kendrick D, Kumar A, Carpenter H, Zijlstra GA, Skelton DA, Cook JR, et al. Exercise for reducing fear of falling in older people living in the community. Cochrane Database Syst Rev 2014;11. http://dx.doi.org/10.1002/14651858.cd009848.pub2.
- Tennstedt S, Howland J, Lachman M, Peterson E, Kasten L, Jette A. A randomized, controlled trial of a group intervention to reduce fear of falling and associated activity restriction in older adults. J Gerontol B Psychol Sci Soc Sci 1998;53:P384-92. http://dx.doi.org/10.1093/geronb/53B.6.P384.
- Clemson L, Cumming RG, Kendig H, Swann M, Heard R, Taylor K. The effectiveness of a community-based program for reducing the incidence of falls in the elderly: a randomized trial. J Am Geriatr Soc 2004;52:1487-94. http://dx.doi.org/10.1111/j.1532-5415.2004.52411.x.
- Zijlstra GA, van Haastregt JC, Ambergen T, van Rossum E, van Eijk JT, Tennstedt SL, et al. Effects of a multicomponent cognitive behavioral group intervention on fear of falling and activity avoidance in community-dwelling older adults: results of a randomized controlled trial. J Am Geriatr Soc 2009;57:2020-8. http://dx.doi.org/10.1111/j.1532-5415.2009.02489.x.
- Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005;34:614-19. http://dx.doi.org/10.1093/ageing/afi196.
- Hadjistavropoulos T, Delbaere K, Fitzgerald TD. Reconceptualizing the role of fear of falling and balance confidence in fall risk. J Aging Health 2011;23:3-23. http://dx.doi.org/10.1177/0898264310378039.
- Delbaere K, Close JC, Heim J, Sachdev PS, Brodaty H, Slavin MJ, et al. A multifactorial approach to understanding fall risk in older people. J Am Geriatr Soc 2010;58:1679-85. http://dx.doi.org/10.1111/j.1532-5415.2010.03017.x.
- Williams C. A cognitive–behavioural therapy assessment model for use in everyday clinical practice. Adv Psychiatr Treat 2002;8:172-9. http://dx.doi.org/10.1192/apt.8.3.172.
- Kennedy T, Jones R, Darnley S, Seed P, Wessely S, Chalder T. Cognitive behaviour therapy in addition to antispasmodic treatment for irritable bowel syndrome in primary care: randomised controlled trial. BMJ 2005;331. http://dx.doi.org/10.1136/bmj.38545.505764.06.
- Daniilidou P, Carding P, Wilson J, Drinnan M, Deary V. Cognitive behavioral therapy for functional dysphonia: a pilot study. Ann Oto Rhinol Laryn 2007;116:717-22. http://dx.doi.org/10.1177/000348940711601002.
- Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F, et al. Designing and evaluating complex interventions to improve health care. BMJ 2007;334:455-9. http://dx.doi.org/10.1136/bmj.39108.379965.BE.
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J. RIPPLE Study Team . Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413-16. http://dx.doi.org/10.1136/bmj.332.7538.413.
- Moore G, Audrey S, Barker M, Bond L, Bonell C, Cooper C, et al. Process evaluation in complex public health intervention studies: the need for guidance. J Epidemiol Community Health 2014;68:101-2. http://dx.doi.org/10.1136/jech-2013-202869.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. http://dx.doi.org/10.1177/0038038509103208.
- Deary V, Chalder T, Sharpe M. The cognitive behavioural model of medically unexplained symptoms: a theoretical and empirical review. Clin Psychol Rev 2007;27:781-97. http://dx.doi.org/10.1016/j.cpr.2007.07.002.
- Delbaere K, Close JC, Mikolaizak AS, Sachdev PS, Brodaty H, Lord SR. The Falls Efficacy Scale International (FES-I). A comprehensive longitudinal validation study. Age Ageing 2010;39:210-16. http://dx.doi.org/10.1093/ageing/afp225.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. http://dx.doi.org/10.1016/0022-3956(75)90026-6.
- Moore DS, Ellis R. Measurement of fall-related psychological constructs among independent-living older adults: a review of the research literature. Aging Ment Health 2008;12:684-99. http://dx.doi.org/10.1080/13607860802148855.
- Hughes CC, Kneebone II, Jones F, Brady B. A theoretical and empirical review of psychological factors associated with falls-related psychological concerns in community-dwelling older people. Int Psychogeriatr 2015;27:1071-87. http://dx.doi.org/10.1017/S1041610214002701.
- Padesky CA, Mooney KA. Presenting the cognitive model to clients. Int Cogn Ther Newsletter 1990;6:13-4.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348.
- Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago: Aldine Publishing Company; 1967.
- Parry SW, Deary V, Finch T, Bamford C, Sabin N, McMeekin P, et al. The STRIDE (Strategies to Increase confidence, InDependence and Energy) study: cognitive behavioural therapy-based intervention to reduce fear of falling in older fallers living in the community: study protocol for a randomised controlled trial. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-210.
- Falls: Assessment and Prevention of Falls in Older People. Manchester: NICE; 2013.
- Van Vliet R, Hoang P, Lord S, Gandevia S, Delbaere K. Falls efficacy scale-international: a cross-sectional validation in people with multiple sclerosis. Arch Phys Med Rehabil 2013;94:883-9. http://dx.doi.org/10.1016/j.apmr.2012.10.034.
- Hauer KA, Kempen GI, Schwenk M, Yardley L, Beyer N, Todd C, et al. Validity and sensitivity to change of the falls efficacy scales international to assess fear of falling in older adults with and without cognitive impairment. Gerontology 2011;57:462-72.
- Hauer K, Yardley L, Beyer N, Kempen G, Dias N, Campbell M, et al. Validation of the Falls Efficacy Scale and Falls Efficacy Scale International in geriatric patients with and without cognitive impairment: results of self-report and interview-based questionnaires. Gerontology 2010;56:190-9. http://dx.doi.org/10.1159/000236027.
- Azad A, Hassani Mehraban A, Mehrpour M, Mohammadi B. Clinical assessment of fear of falling after stroke: validity, reliability and responsiveness of the Persian version of the Fall Efficacy Scale-International. Med J Islam Repub Iran 2014;28.
- Kwan MM, Tsang WW, Close JC, Lord SR. Development and validation of a Chinese version of the falls efficacy scale international. Arch Gerontol Geriatr 2013;56:169-74. http://dx.doi.org/10.1016/j.archger.2012.10.007.
- Bandura A, Madden J. Neurobiology of Learning, Emotion and Affect. New York, NY: Raven Press; 1991.
- Kempen GI, Yardley L, van Haastregt JC, Zijlstra GA, Beyer N, Hauer K, et al. The Short FES-I: a shortened version of the Falls Efficacy Scale-International to assess fear of falling. Age Ageing 2008;37:45-50. http://dx.doi.org/10.1093/ageing/afm157.
- Hill H, McMeekin P, Parry SW. Does the falls efficacy scale international version measure fear of falling: a reassessment of internal validity using a factor analytic approach. Age Ageing 2014;43:559-62. http://dx.doi.org/10.1093/ageing/afu059.
- Greenberg SA. Analysis of measurement tools of fear of falling for high-risk, community-dwelling older adults. Clin Nurs Res 2012;21:113-30. http://dx.doi.org/10.1177/1054773811433824.
- Zijlstra GA, van Rens GH, Scherder EJ, Brouwer DM, van der Velde J, Verstraten PF, et al. Effects and feasibility of a standardised orientation and mobility training in using an identification cane for older adults with low vision: design of a randomised controlled trial. BMC Health Serv Res 2009;9. http://dx.doi.org/10.1186/1472-6963-9-153.
- Murphy SL, Dubin JA, Gill TM. The development of fear of falling among community-living older women: predisposing factors and subsequent fall events. J Gerontol A Biol Sci Med Sci 2003;58:M943-7. http://dx.doi.org/10.1093/gerona/58.10.M943.
- Lamb SE, Jorstad-Stein EC, Hauer K, Becker C. Development of a common outcome data set for fall injury prevention trials: the prevention of falls network Europe consensus. J Am Geriatr Soc 2005;53:1618-22. http://dx.doi.org/10.1111/j.1532-5415.2005.53455.x.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Gale CR, Allerhand M, Sayer AA, Cooper C, Dennison EM, Starr JM, et al. The structure of the hospital anxiety and depression scale in four cohorts of community-based, healthy older people: the HALCyon program. Int Psychogeriatr 2010;22:559-71. http://dx.doi.org/10.1017/S1041610210000256.
- Power M, Quinn K, Schmidt S, Group W-O. Development of the WHOQOL-old module. Qual Life Res 2005;14:2197-214. http://dx.doi.org/10.1007/s11136-005-7380-9.
- The EuroQol Group . EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. http://dx.doi.org/10.1016/S0167-6296(01)00130-8.
- Ware JE. SF-36 health survey update. Spine 2000;25:3130-9. http://dx.doi.org/10.1097/00007632-200012150-00008.
- Cornwell EY, Waite LJ. Social disconnectedness, perceived isolation, and health among older adults. J Health Soc Behav 2009;50:31-48. http://dx.doi.org/10.1177/002214650905000103.
- Cornwell EY, Walte LJ. Measuring social isolation among older adults using multiple indicators from the NSHAP Study. J Gerontol B Psychol Sci Soc Sci 2009;64:i38-46. http://dx.doi.org/10.1093/geronb/gbp037.
- Lubben J, Blozik E, Gillmann G, Iliffe S, von Renteln Kruse W, Beck JC, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist 2006;46:503-13. http://dx.doi.org/10.1093/geront/46.4.503.
- De Jong Gierveld J, Kamphuls F. The development of a Rasch-type loneliness scale. Appl Psychol Meas 1985;9:289-99. http://dx.doi.org/10.1177/014662168500900307.
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85-M94. http://dx.doi.org/10.1093/geronj/49.2.M85.
- Bandinelli S, Lauretani F, Boscherini V, Gandi F, Pozzi M, Corsi AM, et al. A randomized, controlled trial of disability prevention in frail older patients screened in primary care: the FRASI study. Design and baseline evaluation. Aging Clin Exp Res 2006;18:359-66. http://dx.doi.org/10.1007/BF03324831.
- Simonsick EM, Guralnik JM, Volpato S, Balfour J, Fried LP. Just get out the door! Importance of walking outside the home for maintaining mobility: findings from the women’s health and aging study. J Am Geriatr Soc 2005;53:198-203. http://dx.doi.org/10.1111/j.1532-5415.2005.53103.x.
- Bindawas SM, Al Snih S, Ottenbacher AJ, Graham J, Protas EE, Markides KS, et al. Association between lower extremity performance and health-related quality of life in elderly Mexican Americans. J Aging Health 2015;27:1026-45. http://dx.doi.org/10.1177/0898264315572115.
- Soares WJ, Lima CA, Bilton TL, Ferrioli E, Dias RC, Perracini MR. Association among measures of mobility-related disability and self-perceived fatigue among older people: a population-based study. Braz J Phys Ther 2015;19:194-200. http://dx.doi.org/10.1590/bjpt-rbf.2014.0091.
- Ward RE, Leveille SG, Beauchamp MK, Travison T, Alexander N, Jette AM, et al. Functional performance as a predictor of injurious falls in older adults. J Am Geriatr Soc 2015;63:315-20. http://dx.doi.org/10.1111/jgs.13203.
- Silva AG, Queiros A, Sa-Couto P, Rocha NP. Self-reported disability: association with lower extremity performance and other determinates in older adults attending primary care. Phys Ther 2015;95:1628-37. http://dx.doi.org/10.2522/ptj.20140323.
- Duncan PW, Studenski S, Chandler J, Prescott B. Functional reach: predictive value in a sample of elderly male veterans. J Gerontol 1992;47:M93-8. http://dx.doi.org/10.1093/geronj/47.3.M93.
- Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol 1990;45:M192-7. http://dx.doi.org/10.1093/geronj/45.6.M192.
- Keevil VL, Wijndaele K, Luben R, Sayer AA, Wareham NJ, Khaw KT. Television viewing, walking speed, and grip strength in a prospective cohort study. Med Sci Sports Exerc 2015;47:735-42. http://dx.doi.org/10.1249/MSS.0000000000000453.
- Dodds RM, Roberts HC, Cooper C, Sayer AA. The epidemiology of sarcopenia. J Clin Densitom 2015;18:461-6. http://dx.doi.org/10.1016/j.jocd.2015.04.012.
- Cooper AJ, Simmons RK, Kuh D, Brage S, Cooper R. NSHD scientific and data collection team . Physical activity, sedentary time and physical capability in early old age: British birth cohort study. PLOS One 2015;10. http://dx.doi.org/10.1371/journal.pone.0126465.
- Parry SW, Bexton RS, Steen N, Kenny RA. Pacing in elderly recurrent fallers with carotid sinus hypersensitivity (PERF-CSH): a randomised, double-blind, placebo controlled cross-over trial. Heart 2009;95:405-9. http://dx.doi.org/10.1136/hrt.2008.153189.
- Kenny RA, Richardson DA, Steen N, Bexton RS, Shaw FE, Bond J. Carotid sinus syndrome: a modifiable risk factor for nonaccidental falls in older adults (SAFE PACE). J Am Coll Cardiol 2001;38:1491-6. http://dx.doi.org/10.1016/S0735-1097(01)01537-6.
- Mannion AF, Balague F, Pellise F, Cedraschi C. Pain measurement in patients with low back pain. Nat Clin Pract Rheum 2007;3:610-18. http://dx.doi.org/10.1038/ncprheum0646.
- WHOQOL-OLD Manual. Copenhagen: WHO; 2006.
- Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostatistics 2000;1:465-80. http://dx.doi.org/10.1093/biostatistics/1.4.465.
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York, NY: Wiley; 1987.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: J Wiley & Sons; 1987.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c332.
- Massy-Westropp NM, Gill TK, Taylor AW, Bohannon RW, Hill CL. Hand grip strength: age and gender stratified normative data in a population-based study. BMC Res Notes 2011;4. http://dx.doi.org/10.1186/1756-0500-4-127.
- Geriatric Examination Tool Kit . Summary of Available Reference Values and Predictive Values, for Some Instruments Found in This Exam Tool Kit 2012. http://geriatrictoolkit.missouri.edu/indexnorm.htm (accessed 1 July 2015).
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a1655.
- Van Haastregt JC, Zijlstra GA, van Rossum E, van Eijk JT, de Witte LP, Kempen GI. Feasibility of a cognitive behavioural group intervention to reduce fear of falling and associated avoidance of activity in community-living older people: a process evaluation. BMC Health Serv Res 2007;7. http://dx.doi.org/10.1186/1472-6963-7-156.
- Ullmann G, Williams HG, Plass CF. Dissemination of an evidence-based program to reduce fear of falling, South Carolina, 2006–2009. Prev Chronic Dis 2012;9. http://dx.doi.org/10.5888/pcd9.110093.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. http://dx.doi.org/10.1186/1741-7015-8-63.
- Strong PM. The Ceremonial Order of the Clinic: Parents, Doctors, and Medical Bureaucracies. London: Routledge and Kegan Paul; 1979.
- Hawton K, Salkovskis PM, Kirk J, Clark DM. Cognitive Behavioral Therapy for Psychiatric Problems: A Practical Guide. Oxford: Oxford University Press; 1989.
- Safran JD, Segal Z. Interpersonal Process in Cognitive Therapy. London: Jason Aronson; 1990.
- Haines TP, Day L, Hill KD, Clemson L, Finch C. ‘Better for others than for me’: a belief that should shape our efforts to promote participation in falls prevention strategies. Arch Gerontol Geriatr 2014;59:136-44. http://dx.doi.org/10.1016/j.archger.2014.03.003.
- Waddington L. The therapy relationship in cognitive therapy: a review. Behav Cogn Psychoth 2002;30:179-91. http://dx.doi.org/10.1017/S1352465802002059.
- Academy of Medical Sciences . A New Pathway for the Regulation and Governance of Health Research 2011. www.acmedsci.ac.uk/policy/policy-projects/a-new-pathway-for-the-regulation-and-governance-of-health-research/ (accessed 1 July 2015).
- Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al. Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11480.
- Kempen GI, Todd CJ, Van Haastregt JC, Zijlstra GA, Beyer N, Freiberger E, et al. Cross-cultural validation of the falls efficacy scale international (FES-I) in older people: results from Germany, the Netherlands and the UK were satisfactory. Disabil Rehabil 2007;29:155-62. http://dx.doi.org/10.1080/09638280600747637.
- O’Cathain A, Goode J, Drabble S, Thomas K, Rudolph A, Hewison J. Getting added value from using qualitative research with randomized controlled trials: a qualitative interview study. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-215.
- O’Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Hewison J. What can qualitative research do for randomised controlled trials? A systematic mapping review. BMJ Open 2013;3. http://dx.doi.org/10.1186/1745-6215-14-s1-o52.
- Guide to the Methods of Technology Appraisal 2013. Process and Methods Guides 9. Manchester: NICE; 2013.
- Curtis LA. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- National Tariff Payment System 2014/15. London: NHS England; 2014.
- GOV.UK . National Minimum Wage Rates 2014. www.gov.uk/national-minimum-wage-rates (accessed 1 July 2015).
- Devlin NJ, Krabbe PF. The development of new research methods for the valuation of EQ-5D-5L. Eur J Health Econ 2013;14:1-3. http://dx.doi.org/10.1007/s10198-013-0502-3.
- Van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. http://dx.doi.org/10.1016/j.jval.2012.02.008.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. http://dx.doi.org/10.1007/s11136-011-9903-x.
- Ware JE, Gandek B. Overview of the SF-36 Health survey and the international quality of life assessment (IQOLA) project. J Clin Epidemiol 1998;51:903-12. http://dx.doi.org/10.1016/S0895-4356(98)00081-X.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Zethraeus N, Johannesson M, Jonsson B, Lothgren M, Tambour M. Advantages of using the net-benefit approach for analysing uncertainty in economic evaluation studies. Pharmacoeconomics 2003;21:39-48. http://dx.doi.org/10.2165/00019053-200321010-00003.
- Evans C. Cognitive–behavioural therapy with older people. Adv Psychiatr Treat 2007;13:111-18. http://dx.doi.org/10.1192/apt.bp.106.003020.
- Iliffe S, Kendrick D, Morris R, Masud T, Gage H, Skelton D, et al. Multicentre cluster randomised trial comparing a community group exercise programme and home-based exercise with usual care for people aged 65 years and over in primary care. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18490.
- Olsen CF, Bergland A. The effect of exercise and education on fear of falling in elderly women with osteoporosis and a history of vertebral fracture: results of a randomized controlled trial. Osteoporos Int 2014;25:2017-25. http://dx.doi.org/10.1007/s00198-014-2724-3.
- Freiberger E, Blank WA, Salb J, Geilhof B, Hentschke C, Landendoerfer P, et al. Effects of a complex intervention on fall risk in the general practitioner setting: a cluster randomized controlled trial. Clin Interv Aging 2013;8:1079-88. http://dx.doi.org/10.2147/CIA.S46218.
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York, NY: Wiley; 2002.
- Schafer JL. Analysis of Incomplete Multivariate Data. New York, NY: Chapman and Hall; 1997.
- Liu YWJ, Tsui CM. A randomized trial comparing Tai Chi with and without cognitive-behavioral intervention (CBI) to reduce fear of falling in community-dwelling elderly people. Arch Gerontol Geriatr 2014;59:317-25. http://dx.doi.org/10.1016/j.archger.2014.05.008.
- Vendrely A, Messmer E, Moseley J. Integration of cognitive-behavioral therapy with gait training for a 58-year-old male with a fear of falling: a case report. Physiother Theory Pract 2012;28:232-7. http://dx.doi.org/10.3109/09593985.2011.598221.
Appendix 1 Falls Efficacy Scale–International version
| Now we would like to ask some questions about how concerned you are about the possibility of falling. Please reply thinking about how you usually do the activity. If you currently do not do the activity (e.g. if someone does your shopping for you), please answer to show whether you think you would be concerned about falling IF you did the activity. For each of the following activities, please tick the box which is closest to your own opinion to show how concerned you are that you might fall if you did this activity. | |||||
| Not at all concerned 1 | Somewhat concerned 2 | Fairly concerned 3 | Very concerned 4 | ||
|---|---|---|---|---|---|
| 1 | Cleaning the house (e.g. sweep, vacuum or dust) | 1 † | 2 † | 3 † | 4 † |
| 2 | Getting dressed or undressed | 1 † | 2 † | 3 † | 4 † |
| 3 | Preparing simple meals | 1 † | 2 † | 3 † | 4 † |
| 4 | Taking a bath or shower | 1 † | 2 † | 3 † | 4 † |
| 5 | Going to the shop | 1 † | 2 † | 3 † | 4 † |
| 6 | Getting in or out of a chair | 1 † | 2 † | 3 † | 4 † |
| 7 | Going up or down stairs | 1 † | 2 † | 3 † | 4 † |
| 8 | Walking around in the neighbourhood | 1 † | 2 † | 3 † | 4 † |
| 9 | Reaching for something above your head or on the ground | 1 † | 2 † | 3 † | 4 † |
| 10 | Going to answer the telephone before it stops ringing | 1 † | 2 † | 3 † | 4 † |
| 11 | Walking on a slippery surface (e.g. wet or icy) | 1 † | 2 † | 3 † | 4 † |
| 12 | Visiting a friend or relative | 1 † | 2 † | 3 † | 4 † |
| 13 | Walking in a place with crowds | 1 † | 2 † | 3 † | 4 † |
| 14 | Walking on an uneven surface (e.g. rocky ground, poorly maintained pavement) | 1 † | 2 † | 3 † | 4 † |
| 15 | Walking up or down a slope | 1 † | 2 † | 3 † | 4 † |
| 16 | Going out to a social event (e.g. religious service, family gathering or club meeting) | 1 † | 2 † | 3 † | 4 † |
Appendix 2 Cognitive–behavioural therapy intervention assessment and formulation documentation
Appendix 3 Falls diary
Appendix 4 Additional statistical material
Baseline data
The primary outcome, FES-I, comprises 16 items. Tables 64 and 65 show the distribution of responses to each item at baseline.
| FES-I item | Not concerned at all | Somewhat concerned | Fairly concerned | Very concerned | Total | Missing | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | |||
| 1 | Cleaning the house (e.g. sweep, vacuum, dust) | 105 | 25.3 | 150 | 36.1 | 99 | 23.9 | 61 | 14.7 | 415 | 0 |
| 2 | Getting dressed or undressed | 190 | 45.8 | 135 | 32.5 | 62 | 14.9 | 27 | 6.5 | 414 | 1 |
| 3 | Preparing simple meals | 244 | 58.8 | 91 | 21.9 | 55 | 13.3 | 24 | 5.8 | 414 | 1 |
| 4 | Taking a bath or shower | 76 | 18.3 | 122 | 29.4 | 113 | 27.2 | 103 | 24.8 | 414 | 1 |
| 5 | Going to the shop | 71 | 17.1 | 136 | 32.8 | 103 | 24.8 | 101 | 24.3 | 411 | 4 |
| 6 | Getting in or out of a chair | 141 | 34.0 | 171 | 41.2 | 77 | 18.6 | 24 | 5.8 | 413 | 2 |
| 7 | Going up or down stairs | 42 | 10.1 | 114 | 27.5 | 101 | 24.3 | 157 | 37.8 | 414 | 1 |
| 8 | Walking around in the neighbourhood | 43 | 10.4 | 157 | 37.8 | 92 | 22.2 | 121 | 29.2 | 413 | 2 |
| 9 | Reaching for something above your head or on the ground | 34 | 8.2 | 136 | 32.8 | 134 | 32.3 | 111 | 26.7 | 415 | 0 |
| 10 | Going to answer the telephone before it stops ringing | 194 | 46.7 | 120 | 28.9 | 71 | 17.1 | 30 | 7.2 | 415 | 0 |
| 11 | Walking on a slippery surface (e.g. wet or icy) | 2 | 0.5 | 34 | 8.2 | 87 | 21.0 | 292 | 70.4 | 415 | 0 |
| 12 | Visiting a friend or relative | 133 | 32.0 | 140 | 33.7 | 78 | 18.8 | 62 | 14.9 | 413 | 2 |
| 13 | Walking in a place with crowds | 62 | 14.9 | 131 | 31.6 | 102 | 24.6 | 120 | 28.9 | 415 | 0 |
| 14 | Walking on an uneven surface (e.g. rocky ground, poorly maintained pavement) | 5 | 1.2 | 59 | 14.2 | 110 | 26.5 | 241 | 58.1 | 415 | 0 |
| 15 | Walking up or down a slope | 21 | 5.1 | 89 | 21.4 | 130 | 31.3 | 175 | 42.2 | 415 | 0 |
| 16 | Going out to a social event (e.g. religious service, family gathering or club meeting) | 132 | 31.8 | 131 | 31.6 | 82 | 19.8 | 70 | 16.9 | 415 | 0 |
| Fear of falling | Control group | Intervention group | Study population | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Median | SD | n | Mean | Median | SD | n | Mean | 95% CI | |
| When cleaning the house | 205 | 2.27 | 2 | 0.97 | 210 | 2.29 | 2 | 1.04 | 415 | 2.28 | 2.19 to 2.37 |
| Getting dressed or undressed | 204 | 1.98 | 2 | 0.95 | 210 | 1.67 | 1 | 0.85 | 414 | 1.82 | 1.74 to 1.91 |
| Preparing simple meals | 204 | 1.73 | 1 | 0.96 | 210 | 1.60 | 1 | 0.87 | 414 | 1.66 | 1.57 to 1.76 |
| Taking a bath or shower | 205 | 2.62 | 3 | 1.04 | 209 | 2.55 | 2 | 1.07 | 414 | 2.59 | 2.49 to 2.70 |
| Going to the shop | 204 | 2.64 | 3 | 1.00 | 207 | 2.50 | 2 | 1.08 | 411 | 2.57 | 2.46 to 2.68 |
| Getting in and out of a chair | 204 | 2.04 | 2 | 0.89 | 209 | 1.89 | 2 | 0.85 | 413 | 1.96 | 1.87 to 2.05 |
| Going up or down stairs | 205 | 2.91 | 3 | 1.03 | 209 | 2.89 | 3 | 1.03 | 414 | 2.90 | 2.80 to 3.00 |
| Walking around the neighbourhood | 204 | 2.69 | 2.5 | 1.02 | 209 | 2.72 | 3 | 0.99 | 413 | 2.70 | 2.61 to 2.80 |
| Reaching for something above or on ground | 205 | 2.77 | 3 | 0.97 | 210 | 2.78 | 3 | 0.90 | 415 | 2.78 | 2.68 to 2.87 |
| Going to answer telephone before it stops | 205 | 1.89 | 2 | 0.99 | 210 | 1.80 | 2 | 0.92 | 415 | 1.85 | 1.76 to 1.93 |
| Walking on slippery surfaces | 205 | 3.66 | 4 | 0.63 | 210 | 3.56 | 4 | 0.68 | 415 | 3.61 | 3.55 to 3.67 |
| Visiting friend or relative | 205 | 2.20 | 2 | 1.05 | 208 | 2.13 | 2 | 1.04 | 413 | 2.17 | 2.07 to 2.27 |
| Walking in crowds | 205 | 2.63 | 3 | 1.06 | 210 | 2.71 | 3 | 1.04 | 415 | 2.67 | 2.57 to 2.77 |
| Walking on uneven surface | 205 | 3.41 | 4 | 0.78 | 210 | 3.42 | 4 | 0.77 | 415 | 3.41 | 3.34 to 3.49 |
| Walking up or down a slope | 205 | 3.09 | 3 | 0.94 | 210 | 3.12 | 3 | 0.89 | 415 | 3.11 | 3.01 to 3.20 |
| Going to a social event | 205 | 2.32 | 2 | 1.11 | 210 | 2.11 | 2 | 1.02 | 415 | 2.22 | 2.11 to 2.32 |
In Figures 47 and 48, the association between FES-I scores at baseline and patient ratings on 11-point scales of their fear of falling and pain on walking at this time point are examined graphically. The upper and lower edges of each box correspond to the 75th and 25th percentiles, respectively; the horizontal line through the centre of each box corresponds to the median of the distribution, and the whiskers show the upper and lower limits of the distribution.
FIGURE 47.
Distribution of baseline FES-I scores by rating on 11-point fear of falling scale.
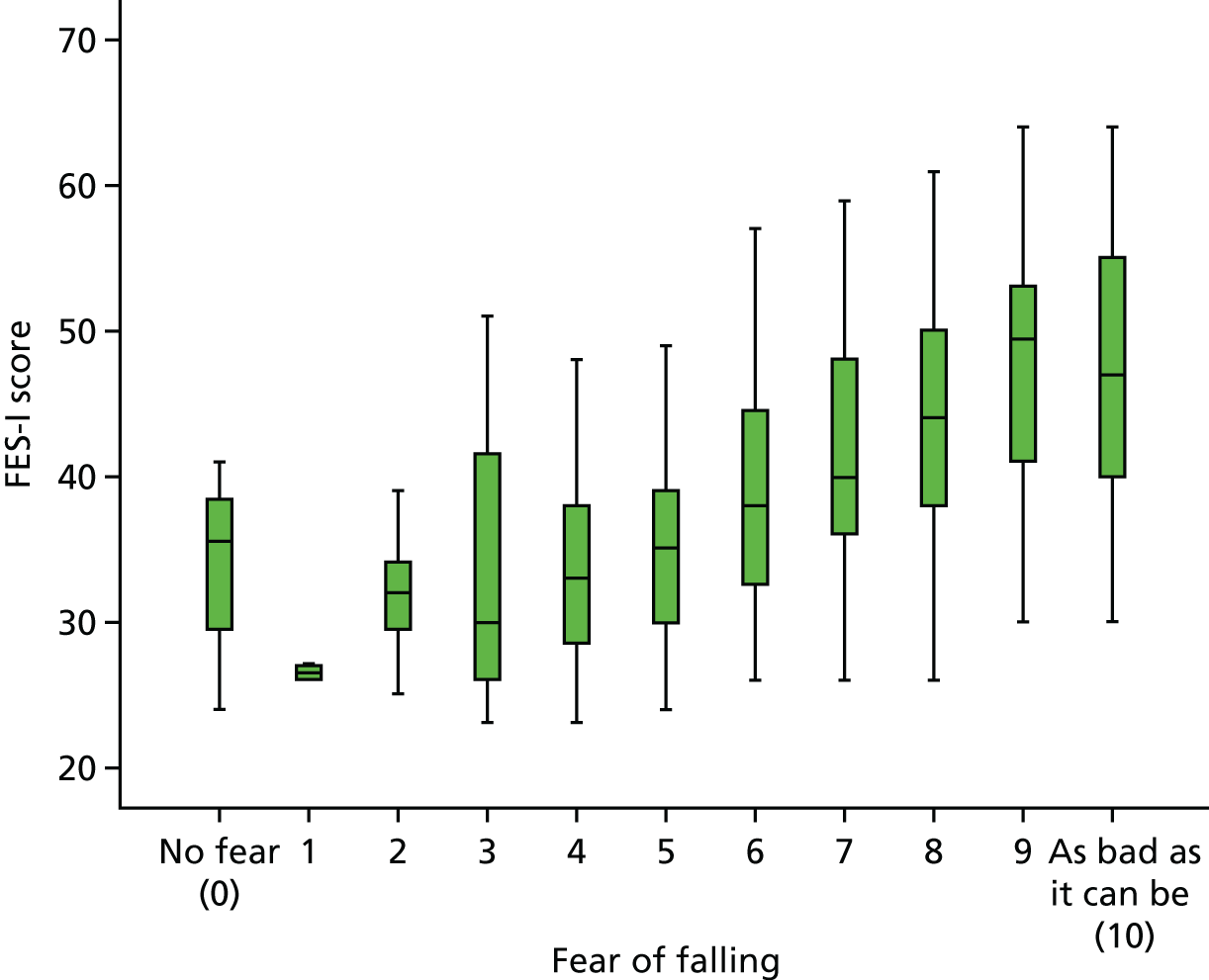
FIGURE 48.
Distribution of baseline FES-I scores by rating on 11-point pain on walking scale.
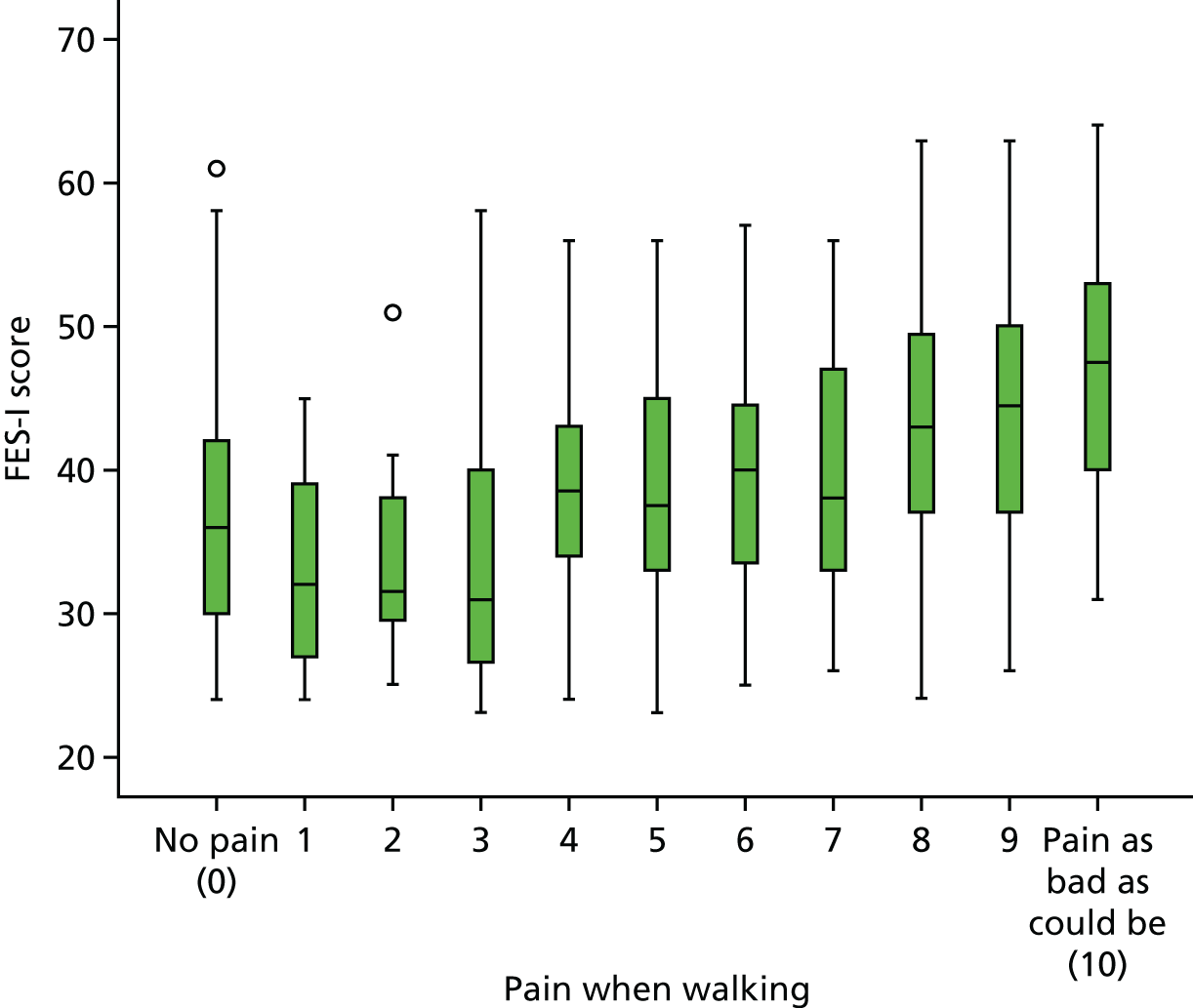
It is good statistical practice to investigate the level of collinearity in any potential explanatory variables so that outcomes can be adjusted to take into account difference between strata. Figures 49–52 provide a graphical presentation of the primary outcome, FES-I, broken down by each of the stratification variables [site, gender, pain on walking (no/yes), referral for strength and balance training (no/yes)]. Tables 66–69 provide summary statistics for all primary and secondary outcome measures, grouped by these stratification variables.
FIGURE 49.
Distribution of baseline FES-I scores by site.
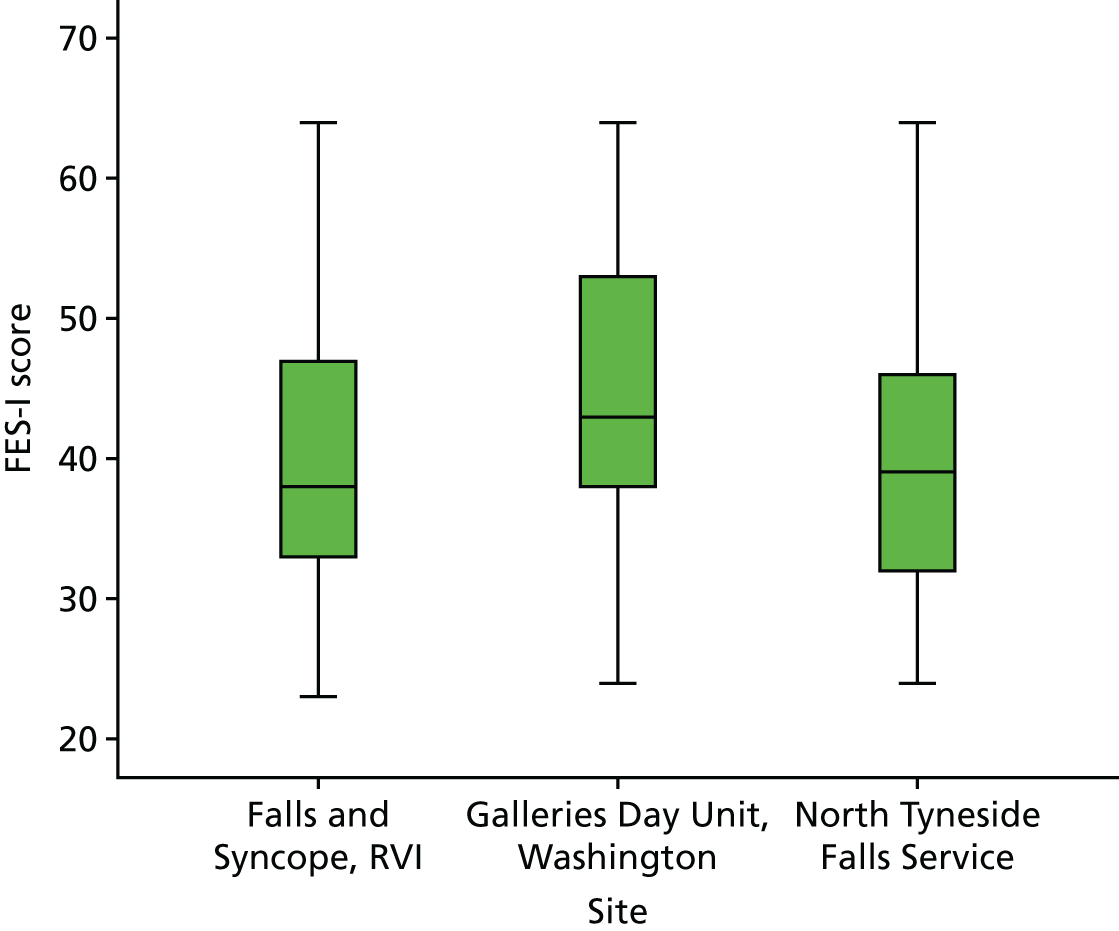
FIGURE 50.
Distribution of baseline FES-I scores by gender.
FIGURE 51.
Distribution of baseline FES-I scores by pain on walking (yes/no).

FIGURE 52.
Distribution of baseline FES-I scores by referral for strength and balance training (yes/no).

| Outcome | Site | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Falls and Syncope Service, RVI | Galleries Day Unit, Washington | North Tyneside Falls Service | ||||||||
| n | µ | 95% CI | n | µ | 95% CI | n | µ | 95% CI | ||
| FES | 125 | 39.6 | 38.1 to 41.2 | 50 | 45.4 | 42.5 | 48.3 | 240 | 39.6 | 38.6 to 40.7 |
| Fear of falling rating scale | 125 | 6.6 | 6.2 to 7.1 | 50 | 7.6 | 7.0 | 8.2 | 240 | 6.5 | 6.3 to 6.7 |
| Total participation score | 125 | 9.5 | 8.3 to 10.8 | 50 | 10.2 | 7.7 | 13.0 | 240 | 9.3 | 8.4 to 10.3 |
| Gait and balance score | 124 | 6.0 | 5.5 to 6.5 | 49 | 3.6 | 2.9 | 4.3 | 238 | 6.3 | 5.9 to 6.7 |
| Mean handgrip strength | 123 | 17.3 | 15.7 to 18.9 | 47 | 17.5 | 14.6 | 20.2 | 232 | 15.9 | 14.5 to 17.3 |
| Mean functional reach | 114 | 22.8 | 21.1 to 24.5 | 36 | 20.2 | 17.6 | 22.9 | 225 | 23.2 | 22.2 to 24.3 |
| De Jong Gierveld Loneliness Scale | 125 | 8.4 | 8.0 to 8.8 | 50 | 7.6 | 6.9 | 8.3 | 240 | 8.2 | 7.9 to 8.6 |
| HADS Anxiety | 95 | 8.2 | 7.4 to 9.0 | 33 | 8.0 | 6.8 | 9.3 | 210 | 8.3 | 7.7 to 8.8 |
| HADS Depression | 95 | 6.4 | 5.8 to 7.1 | 34 | 7.4 | 6.3 | 8.6 | 210 | 7.0 | 6.5 to 7.4 |
| WHOQOL-OLD total score | 95 | 58.1 | 55.3 to 61.0 | 33 | 54.9 | 50.3 | 59.1 | 211 | 57.0 | 55.4 to 58.7 |
| LSNS-6 | 95 | 15.0 | 13.8 to 16.0 | 33 | 16.2 | 14.1 | 18.4 | 211 | 14.4 | 13.6 to 15.3 |
| EQ-5D (5L) Health today | 93 | 59.6 | 55.4 to 63.5 | 34 | 56.3 | 48.9 | 63.3 | 210 | 58.5 | 56.1 to 61.1 |
| EQ-5D (5L) Index | 93 | 0.56 | 0.51 to 0.60 | 31 | 0.40 | 0.33 | 0.47 | 207 | 0.50 | 0.47 to 0.53 |
| SF-36 PCS | 88 | 34.0 | 32.1 to 35.9 | 32 | 28.7 | 26.1 | 30.9 | 201 | 31.8 | 30.7 to 33.0 |
| SF-36 MCS | 88 | 45.3 | 43.0 to 47.5 | 32 | 44.4 | 40.7 | 48.4 | 201 | 45.2 | 43.8 to 46.7 |
| Outcome | Gender | Difference | ||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||
| n | µ | 95% CI | n | µ | 95% CI | µ | 95% CI | |
| FES | 124 | 40.3 | 38.5 to 42.0 | 291 | 40.3 | 39.3 to 41.3 | 0.03 | –2.06 to 2.01 |
| Fear of falling rating scale | 124 | 6.6 | 6.2 to 7.0 | 291 | 6.7 | 6.5 to 7.0 | –0.08 | –0.56 to 0.41 |
| Total participation score | 124 | 10.4 | 8.9 to 12.0 | 291 | 9.1 | 8.3 to 10.0 | 1.30 | –0.30 to 3.02 |
| Gait and balance score | 124 | 5.9 | 5.3 to 6.4 | 287 | 5.9 | 5.6 to 6.3 | –0.04 | –0.67 to 0.62 |
| Mean handgrip strength | 122 | 25.8 | 23.9 to 27.7 | 280 | 12.5 | 11.7 to 13.2 | 13.29 | 11.28 to 15.35 |
| Mean functional reach | 111 | 26.2 | 24.6 to 27.7 | 264 | 21.3 | 20.4 to 22.2 | 4.86 | 2.86 to 6.62 |
| De Jong Gierveld Loneliness Scale | 124 | 8.2 | 7.8 to 8.6 | 291 | 8.2 | 7.9 to 8.5 | –0.04 | –0.56 to 0.48 |
| HADS Anxiety | 103 | 7.4 | 6.6 to 8.2 | 235 | 8.6 | 8.1 to 9.2 | –1.22 | –2.20 to –0.30 |
| HADS Depression | 103 | 7.2 | 6.6 to 7.8 | 236 | 6.7 | 6.3 to 7.2 | 0.44 | –0.31 to 1.23 |
| WHOQOL-OLD total score | 101 | 56.2 | 53.7 to 58.7 | 238 | 57.5 | 55.8 to 59.4 | –1.31 | –4.41 to 1.54 |
| LSNS-6 | 103 | 14.7 | 13.6 to 15.7 | 236 | 14.7 | 14.0 to 15.4 | –0.07 | –1.43 to 1.25 |
| EQ-5D (5L) Health today | 102 | 55.4 | 51.8 to 59.0 | 235 | 60.0 | 57.5 to 62.2 | –4.60 | –8.57 to –0.37 |
| EQ-5D (5L) Index | 103 | 0.46 | 0.42 to 0.50 | 228 | 0.52 | 0.49 to 0.55 | –0.06 | –0.11 to –0.01 |
| SF-36 PCS | 97 | 30.5 | 28.9 to 32.1 | 224 | 32.7 | 31.6 to 33.8 | –2.23 | –4.18 to –0.42 |
| SF-36 MCS | 97 | 45.5 | 43.2 to 47.7 | 224 | 45.0 | 43.6 to 46.4 | 0.42 | –2.32 to 2.94 |
| Outcome | Pain on walking? | Difference | ||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| n | µ | 95% CI | n | µ | 95% CI | µ | 95% CI | |
| FES | 214 | 37.3 | 36.2 to 38.5 | 201 | 43.7 | 42.1 to 44.8 | –6.13 | –7.92 to –4.41 |
| Fear of falling rating scale | 214 | 6.0 | 5.7 to 6.3 | 201 | 7.4 | 7.1 to 7.7 | –1.43 | –1.81 to –1.04 |
| Total participation score | 214 | 10.3 | 9.3 to 11.4 | 201 | 8.7 | 7.7 to 9.7 | 1.62 | 0.26 to 3.12 |
| Gait and balance score | 213 | 6.4 | 6.0 to 6.8 | 198 | 5.4 | 5.0 to 5.8 | 1.00 | 0.42 to 1.55 |
| Mean handgrip strength | 208 | 16.1 | 14.7 to 17.5 | 194 | 17.0 | 15.5 to 18.5 | –0.89 | –3.00 to 0.93 |
| Mean functional reach | 199 | 22.8 | 21.6 to 24.2 | 176 | 22.7 | 21.5 to 24.0 | 0.09 | –1.67 to 1.76 |
| De Jong Gierveld Loneliness Scale | 214 | 8.6 | 8.3 to 8.9 | 201 | 7.8 | 7.4 to 8.1 | 0.81 | 0.36 to 1.32 |
| HADS Anxiety | 182 | 7.5 | 6.9 to 8.0 | 156 | 9.1 | 8.4 to 9.8 | –1.67 | –2.61 to –0.74 |
| HADS Depression | 182 | 6.2 | 5.8 to 6.6 | 157 | 7.6 | 7.1 to 8.2 | –1.46 | –2.16 to –0.73 |
| WHOQOL-OLD total score | 181 | 59.2 | 57.5 to 61.0 | 158 | 54.8 | 52.4 to 56.9 | 4.42 | 1.61 to 7.52 |
| LSNS-6 | 183 | 14.9 | 14.1 to 15.6 | 156 | 14.5 | 13.6 to 15.5 | 0.35 | –0.87 to 1.55 |
| EQ-5D (5L) Health today | 181 | 64.3 | 61.8 to 66.7 | 156 | 52.0 | 49.2 to 55.0 | 12.31 | 8.40 to 16.06 |
| EQ-5D (5L) Index | 180 | 0.6 | 0.6 to 0.6 | 151 | 0.4 | 0.4 to 0.4 | 0.18 | 0.14 to 0.23 |
| SF-36 PCS | 170 | 35.6 | 34.4 to 36.8 | 151 | 28.1 | 27.1 to 29.1 | 7.42 | 5.74 to 9.10 |
| SF-36 MCS | 170 | 46.9 | 45.4 to 48.3 | 151 | 43.2 | 41.4 to 45.0 | 3.63 | 1.41 to 5.92 |
| Outcome | Referred for strength and balance training? | Difference | ||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | |||||||
| n | µ | 95% CI | n | µ | 95% CI | µ | 95% CI | |
| FES | 127 | 41.2 | 39.7 to 42.7 | 288 | 39.9 | 38.9 to 41.0 | 1.23 | –0.62 to 3.06 |
| Fear of falling rating scale | 127 | 6.6 | 6.3 to 6.9 | 288 | 6.7 | 6.5 to 7.0 | –0.12 | –0.56 to 0.31 |
| Total participation score | 127 | 8.9 | 7.7 to 10.2 | 288 | 9.8 | 8.8 to 10.7 | –0.84 | –2.41 to 0.80 |
| Gait and balance score | 126 | 5.4 | 5.0 to 5.9 | 285 | 6.1 | 5.7 to 6.5 | –0.67 | –1.21 to –0.10 |
| Mean handgrip strength | 121 | 13.8 | 12.1 to 15.6 | 281 | 17.7 | 16.4 to 18.9 | –3.86 | –5.77 to –1.69 |
| Mean functional reach | 118 | 21.0 | 19.8 to 22.3 | 257 | 23.6 | 22.5 to 24.7 | –2.60 | –4.28 to –0.87 |
| De Jong Gierveld Loneliness Scale | 127 | 8.2 | 7.7 to 8.6 | 288 | 8.2 | 7.9 to 8.5 | –0.04 | –0.54 to 0.54 |
| HADS Anxiety | 114 | 8.2 | 7.5 to 8.9 | 224 | 8.2 | 7.6 to 8.8 | –0.01 | –0.95 to 0.92 |
| HADS Depression | 114 | 6.9 | 6.4 to 7.5 | 225 | 6.8 | 6.4 to 7.3 | 0.09 | –0.69 to 0.80 |
| WHOQOL-OLD total score | 115 | 56.4 | 53.9 to 58.8 | 224 | 57.5 | 55.7 to 59.4 | –1.16 | –4.30 to 1.80 |
| LSNS-6 | 115 | 14.5 | 13.5 to 15.5 | 224 | 14.8 | 14.1 to 15.7 | –0.37 | –1.59 to 0.96 |
| EQ-5D (5L) Health today | 114 | 60.2 | 57.3 to 63.2 | 223 | 57.8 | 55.2 to 60.5 | 2.34 | –1.66 to 6.21 |
| EQ-5D (5L) Index | 112 | 0.51 | 0.47 to 0.54 | 219 | 0.50 | 0.47 to 0.54 | 0.00 | –0.05 to 0.05 |
| SF-36 PCS | 110 | 31.9 | 30.6 to 33.3 | 211 | 32.1 | 30.9 to 33.3 | –0.19 | –2.01 to 1.74 |
| SF-36 MCS | 110 | 45.7 | 43.8 to 47.6 | 211 | 44.9 | 43.4 to 46.2 | 0.74 | –1.56 to 3.19 |
Distribution of secondary outcomes over time
The distribution of all secondary outcomes by visit and by group to which randomised are shown graphically in Figures 53–66. The change from baseline for each visit is given for each outcome in Table 70.
FIGURE 53.
Fear of falling 11-point rating scale by visit and study arm.

FIGURE 54.
Total participation score by visit and study arm.
FIGURE 55.
Gait and balance score by visit and study arm.

FIGURE 56.
Mean handgrip strength by visit and study arm.

FIGURE 57.
Mean functional reach by visit and study arm.

FIGURE 58.
De Jong Gierveld Loneliness Scale by visit and study arm.

FIGURE 59.
Hospital Anxiety and Depression Scale Anxiety by visit and study arm.
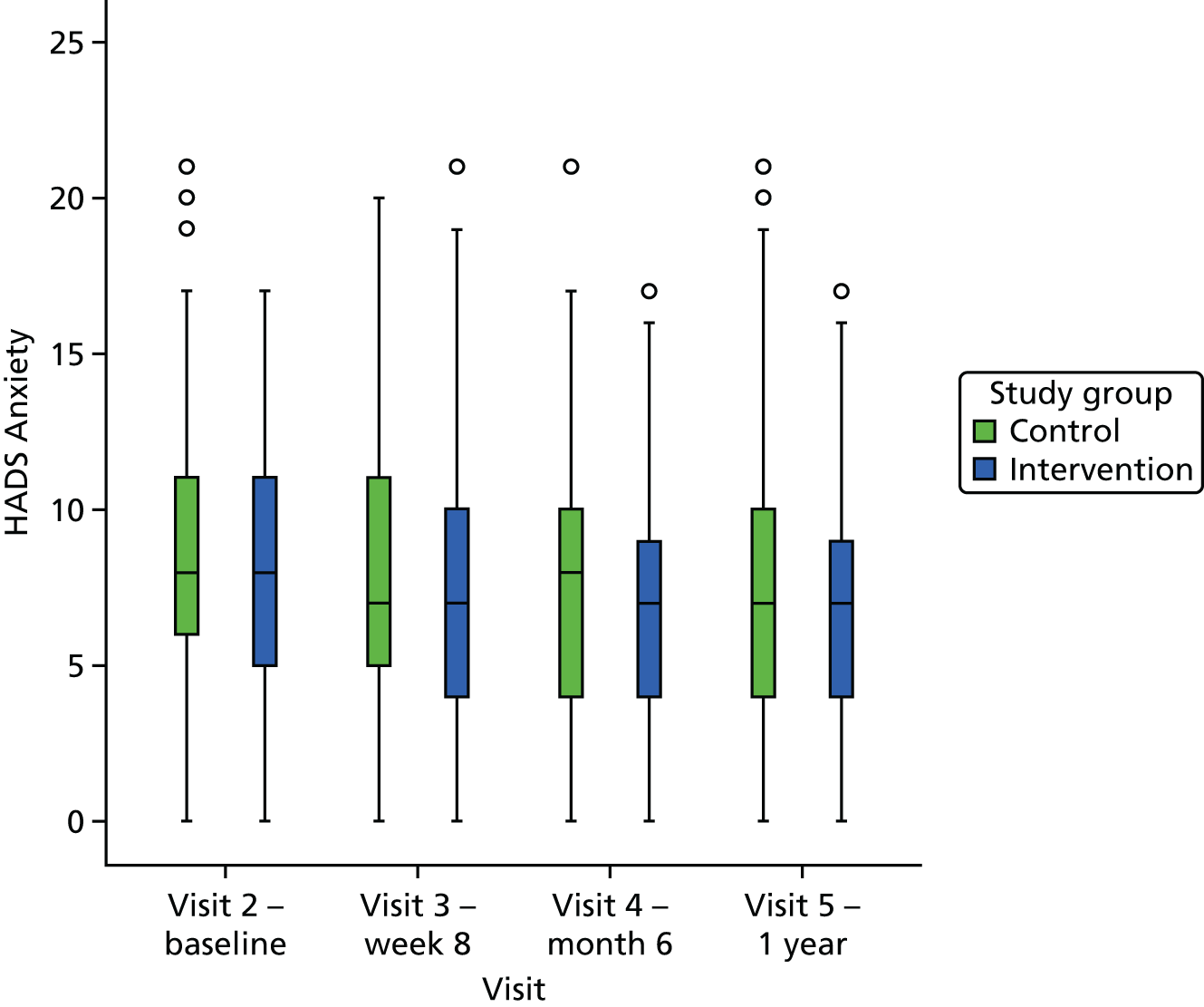
FIGURE 60.
Hospital Anxiety and Depression Scale Depression by visit and study arm.

FIGURE 61.
World Health Organization’s Quality of Life questionnaire – older adults module total score by visit and study arm.
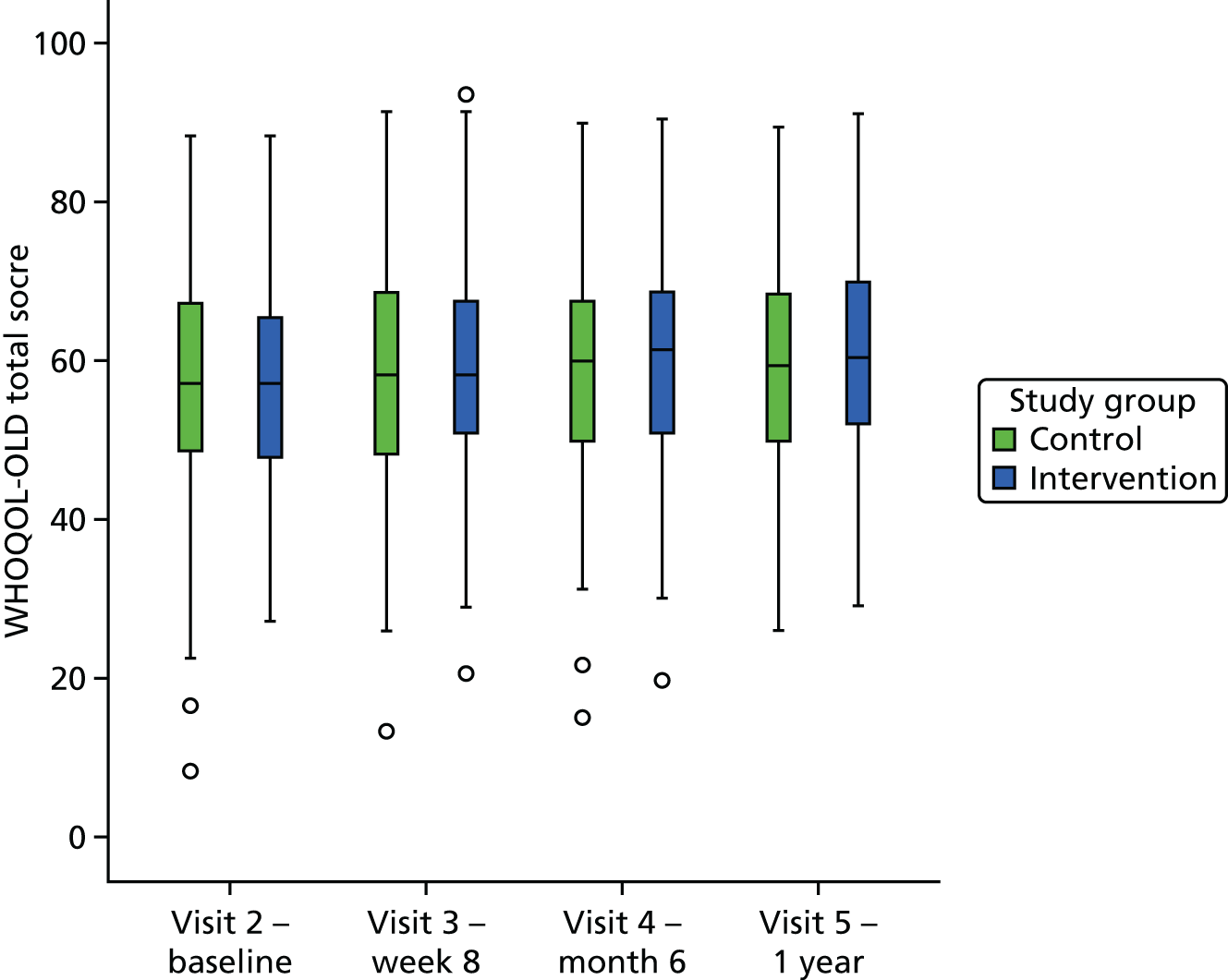
FIGURE 62.
Lubben Social Network Scale–6 by visit and study arm.

FIGURE 63.
European Quality of Life-5 Dimensions (5 Level) Health today by visit and study arm.
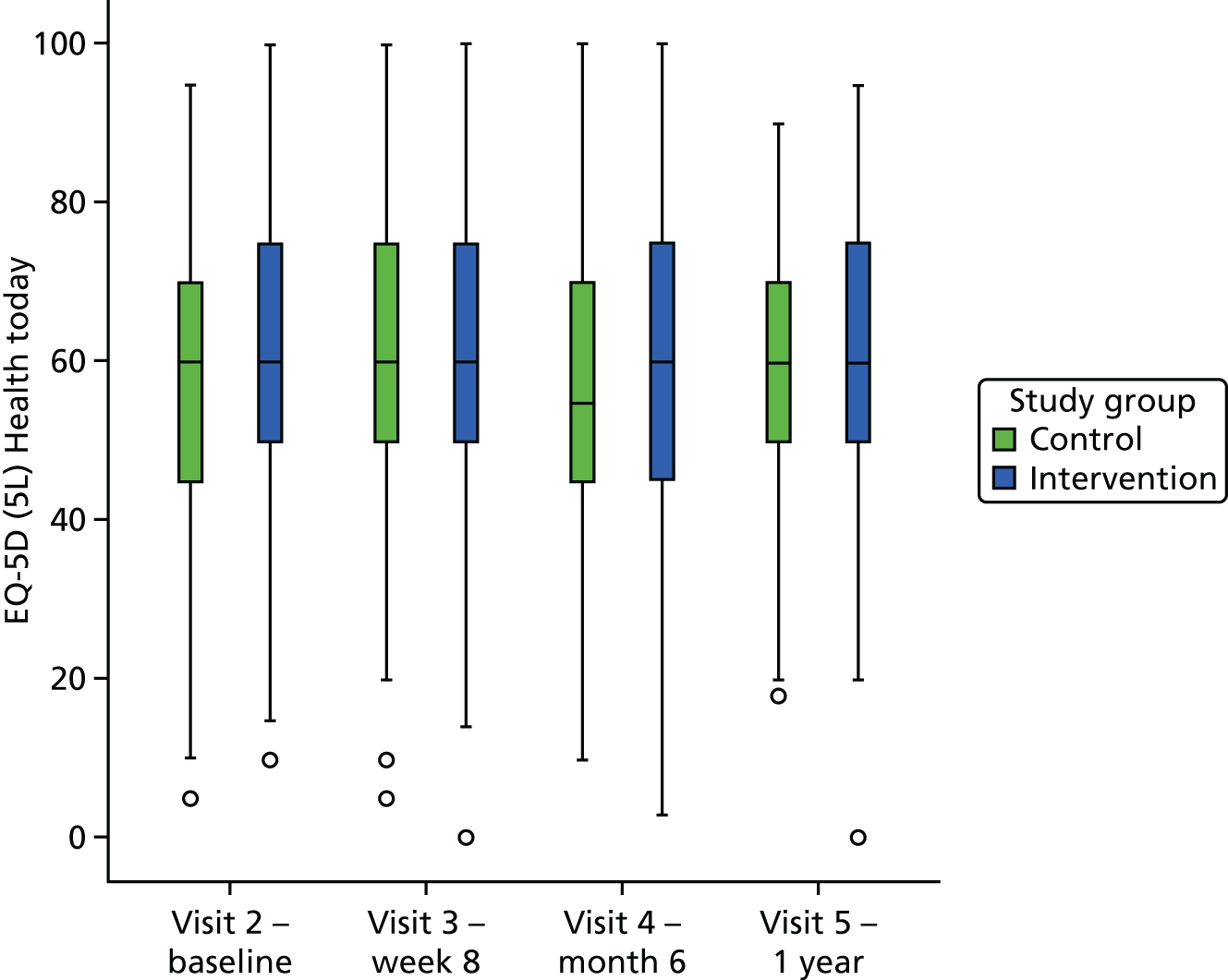
FIGURE 64.
European Quality of Life-5 Dimensions (5 Level) index by visit and study arm.
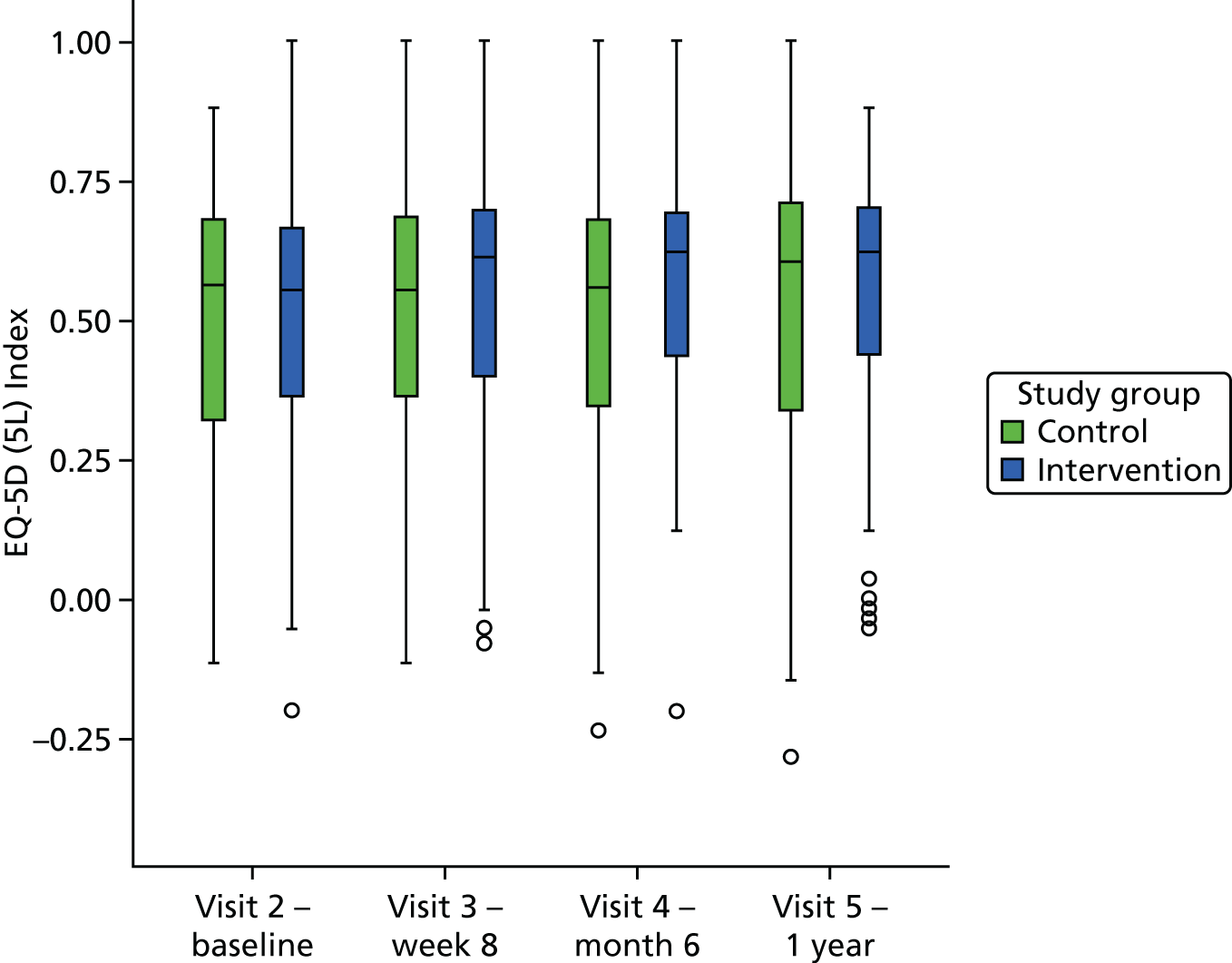
FIGURE 65.
Short Form questionnaire-36 items PCS by visit and study arm.

FIGURE 66.
Short Form questionnaire-36 items MCS by visit and study arm.
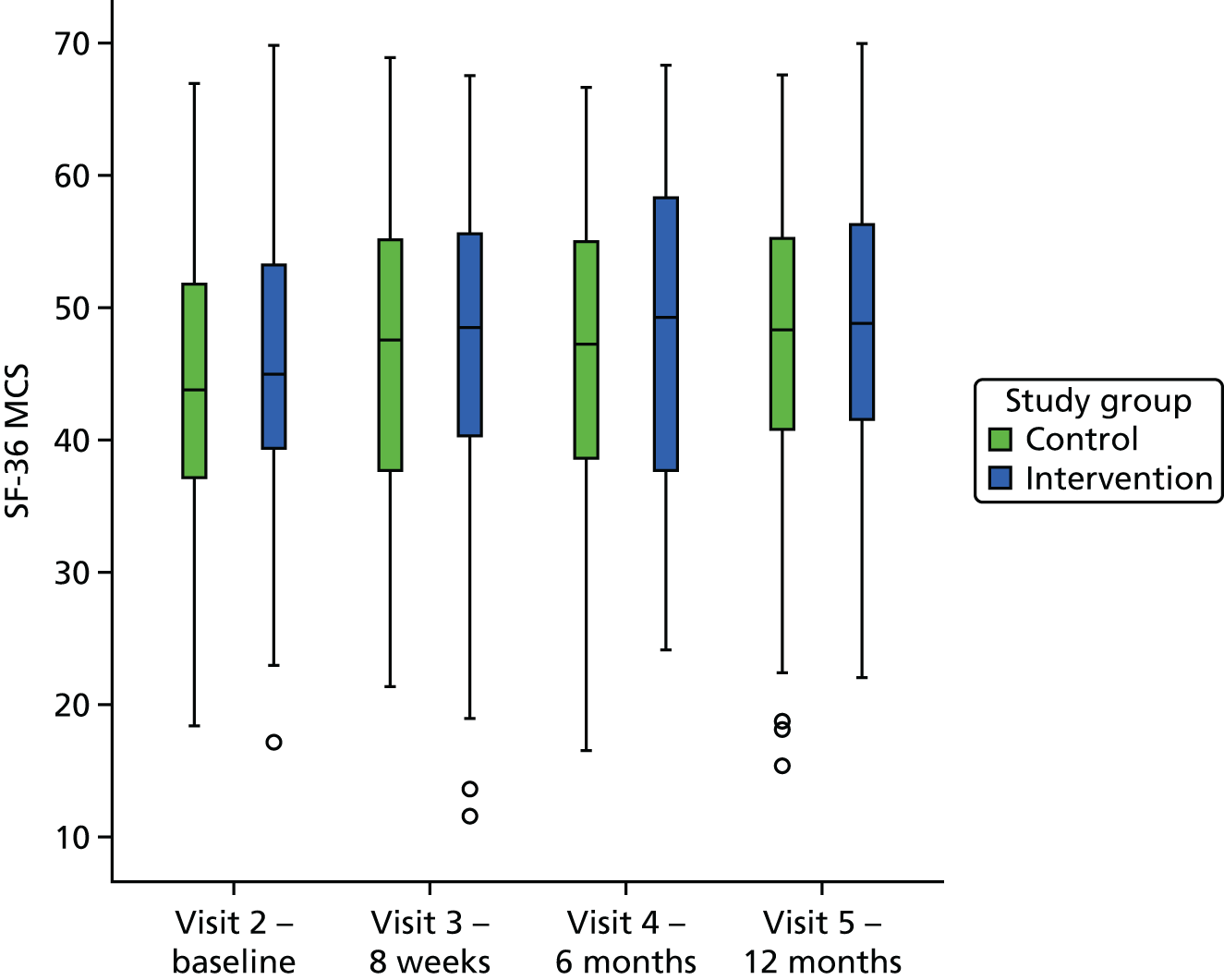
| Outcome | Visit | Study arm | |||||
|---|---|---|---|---|---|---|---|
| Control | CBTi | ||||||
| Mean | Change from baseline | Mean | Change from baseline | ||||
| µ | 95% CI | µ | 95% CI | ||||
| FES-I | 8 weeks | 40.68 | –1.37 | –2.61 to –0.20 | 39.38 | –4.76 | –5.88 to –3.57 |
| 6 months | 40.99 | –2.83 | –4.30 to –1.30 | 39.08 | –5.56 | –6.90 to –4.18 | |
| 1 year | 40.72 | –2.22 | –3.49 to –1.05 | 38.86 | –5.68 | –7.30 to –4.08 | |
| Fear of falling rating scale | 8 weeks | 6.67 | –0.63 | –0.91 to –0.36 | 6.77 | –1.12 | –1.48 to –0.76 |
| 6 months | 6.63 | –0.54 | –0.83 to –0.23 | 6.74 | –1.46 | –1.87 to –1.07 | |
| 1 year | 6.60 | –0.40 | –0.76 to –0.03 | 6.66 | –1.42 | –1.85 to –0.97 | |
| Total participation | 8 weeks | 9.57 | –0.89 | –1.80 to 0.03 | 9.80 | –0.44 | –1.51 to 0.58 |
| 6 months | 9.97 | –1.66 | –2.85 to –0.55 | 10.37 | –0.30 | –1.41 to 0.90 | |
| 1 year | 9.91 | –1.26 | –2.35 to –0.12 | 10.37 | –0.41 | –1.61 to 0.88 | |
| Gait and balance | 8 weeks | 6.03 | 0.16 | –0.11 to 0.45 | 5.93 | 0.35 | –0.01 to 0.68 |
| 6 months | 6.13 | 0.03 | –0.37 to 0.39 | 6.18 | 0.21 | –0.24 to 0.60 | |
| 1 year | 6.40 | 0.37 | –0.59 to 1.91 | 6.33 | 1.11 | 0.07 to 2.64 | |
| Mean grip strength | 8 weeks | 15.84 | –0.62 | –1.37 to 0.10 | 17.20 | –1.48 | –2.42 to –0.68 |
| 6 months | 15.65 | –1.89 | –2.80 to –0.93 | 17.23 | –1.67 | –2.52 to –0.77 | |
| 1 year | 15.48 | –2.29 | –3.53 to –1.08 | 17.29 | –2.83 | –4.08 to –1.69 | |
| Mean functional reach | 8 weeks | 23.24 | 1.20 | 0.07 to 2.37 | 23.01 | 0.94 | –0.14 to 1.99 |
| 6 months | 23.77 | 0.81 | –0.47 to 2.14 | 23.64 | 1.56 | 0.43 to 2.76 | |
| 1 year | 23.60 | 0.30 | –0.94 to 1.46 | 23.48 | 1.29 | –0.34 to 2.83 | |
| De Jong Gierveld loneliness | 8 weeks | 8.29 | 0.04 | –0.22 to 0.29 | 8.16 | 0.31 | 0.04 to 0.58 |
| 6 months | 8.30 | 0.16 | –0.17 to 0.50 | 8.24 | 0.13 | –0.26 to 0.49 | |
| 1 year | 8.31 | –0.01 | –0.31 to 0.30 | 8.23 | 0.20 | –0.17 to 0.55 | |
| HADS Anxiety | 8 weeks | 8.35 | –0.55 | –0.99 to –0.14 | 7.94 | –0.49 | –0.99 to 0.04 |
| 6 months | 8.39 | –0.61 | –1.07 to –0.16 | 8.28 | –1.21 | –1.82 to –0.61 | |
| 1 year | 8.33 | –0.77 | –1.24 to –0.30 | 8.12 | –1.42 | –1.99 to –0.86 | |
| HADS Depression | 8 weeks | 6.48 | 0.15 | –0.23 to 0.51 | 6.83 | –0.55 | –1.04 to –0.05 |
| 6 months | 6.52 | 0.10 | –0.31 to 0.54 | 6.81 | –1.16 | –1.73 to –0.63 | |
| 1 year | 6.36 | 0.30 | –0.13 to 0.73 | 6.82 | –0.80 | –1.30 to –0.34 | |
| WHOQOL-OLD total score | 8 weeks | 56.98 | 0.99 | –0.50 to 2.66 | 58.24 | 1.39 | –0.40 to 3.14 |
| 6 months | 56.84 | 1.51 | –0.14 to 3.11 | 58.86 | 1.24 | –1.21 to 3.52 | |
| 1 year | 57.44 | 1.39 | –0.56 to 3.46 | 58.41 | 1.81 | 0.12 to 3.48 | |
| LSNS-6 | 8 weeks | 14.52 | 0.01 | –0.57 to 0.66 | 14.59 | –0.03 | –0.64 to 0.61 |
| 6 months | 14.69 | 0.20 | –0.32 to 0.79 | 14.93 | 0.09 | –0.72 to 0.80 | |
| 1 year | 14.68 | 0.06 | –0.52 to 0.63 | 14.88 | 0.75 | 0.04 to 1.54 | |
| EQ-5D (5L) Health today | 8 weeks | 58.38 | 1.01 | –1.84 to 3.74 | 60.50 | 2.24 | –0.83 to 5.34 |
| 6 months | 58.32 | –0.83 | –3.94 to 2.22 | 59.71 | 0.38 | –3.65 to 4.58 | |
| 1 year | 59.72 | –2.13 | –5.42 to 1.00 | 60.35 | 0.58 | –2.95 to 4.22 | |
| EQ-5D (5L) Index | 8 weeks | 0.51 | 0.02 | –0.01 to 0.04 | 0.52 | 0.02 | 0.00 to 0.05 |
| 6 months | 0.50 | –0.01 | –0.04 to 0.03 | 0.53 | 0.02 | –0.01 to 0.05 | |
| 1 year | 0.51 | 0.01 | –0.02 to 0.04 | 0.54 | 0.01 | –0.02 to 0.04 | |
| SF-36 PCS | 8 weeks | 32.40 | –0.88 | –2.9 to 1.15 | 34.96 | –2.41 | –4.23 to –0.53 |
| 6 months | 32.75 | –1.23 | –3.35 to 0.89 | 34.84 | –2.29 | –4.45 to –0.15 | |
| 1 year | 32.89 | –1.37 | –3.5 to 0.76 | 34.26 | –1.72 | –3.6 to 0.16 | |
| SF-36 MCS | 8 weeks | 46.34 | –1.78 | –4.26 to 0.71 | 47.22 | –1.49 | –3.9 to 0.91 |
| 6 months | 46.24 | –1.68 | –4.32 to 0.97 | 47.73 | –2.01 | –4.57 to 0.55 | |
| 1 year | 47.45 | –2.89 | –5.44 to –0.35 | 48.14 | –2.41 | –4.73 to –0.08 | |
Outcomes at 12 months
Change from baseline to 12 months was analysed using analysis of covariance. Estimates of the impact of the intervention are given in Table 71:
-
unadjusted for randomisation strata (as previously presented in Chapter 4)
-
adjusted for randomisation strata.
| Measure | M | R 2 | Baseline response | Impact of CBTi | Randomisation strata | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain when walking | Female | Strength and balance referral | Galleries Day Unit | North Tyneside Falls Prevention Service | ||||||||||||
| B | 95% CI | B | 95% CI | B | 95% CI | B | 95% CI | B | 95% CI | B | 95% CI | B | 95% CI | |||
| FES-I | 1 | 0.45 | 0.81 | 0.71 to 0.92 | –3.81 | –5.77 to –1.85 | ||||||||||
| 2 | 0.48 | 0.74 | 0.62 to 0.85 | –4.02 | –5.95 to –2.10 | 2.78 | 0.75 to 4.81 | 0.00 | –2.13 to 2.14 | –2.83 | –5.28 to –0.37 | 4.46 | 0.75 to 8.17 | –0.70 | –3.25 to 1.85 | |
| Fear of falling rating scale | 1 | 0.21 | 0.56 | 0.43 to 0.69 | –0.99 | –1.54 to –0.45 | ||||||||||
| 2 | 0.24 | 0.52 | 0.38 to 0.65 | –1.01 | –1.55 to –0.46 | 0.48 | –0.09 to 1.06 | –0.22 | –0.83 to 0.38 | –0.10 | –0.79 to 0.59 | 1.13 | 0.08 to 2.17 | 0.40 | –0.32 to 1.12 | |
| Total participation score | 1 | 0.31 | 0.57 | 0.47 to 0.66 | 1.04 | –0.43 to 2.51 | ||||||||||
| 2 | 0.33 | 0.58 | 0.48 to 0.67 | 1.03 | –0.44 to 2.50 | –0.31 | –1.79 to 1.18 | –0.44 | –2.09 to 1.20 | 0.61 | –1.25 to 2.46 | –1.58 | –4.48 to 1.32 | 1.94 | 0.01 to 3.88 | |
| Gait and balance score | 1 | 0.05 | 0.64 | 0.29 to 0.98 | 0.71 | –1.23 to 2.66 | ||||||||||
| 2 | 0.06 | 0.70 | 0.33 to 1.07 | 0.90 | –1.06 to 2.87 | 0.48 | –1.55 to 2.51 | 1.20 | –1.01 to 3.41 | 0.34 | –2.20 to 2.88 | 2.36 | –1.61 to 6.34 | –0.72 | –3.41 to 1.98 | |
| Mean handgrip strength | 1 | 0.58 | 0.76 | 0.68 to 0.85 | –0.12 | –1.76 to 1.53 | ||||||||||
| 2 | 0.62 | 0.65 | 0.54 to 0.75 | –0.22 | –1.82 to 1.38 | 0.03 | –1.59 to 1.64 | –4.20 | –6.47 to –1.93 | 0.92 | –1.07 to 2.91 | 0.17 | –3.26 to 3.60 | 2.63 | 0.49 to 4.77 | |
| Functional reach | 1 | 0.32 | 0.57 | 0.46 to 0.68 | 0.94 | –0.85 to 2.74 | ||||||||||
| 2 | 0.37 | 0.50 | 0.38 to 0.61 | 0.91 | –0.85 to 2.66 | –0.61 | –2.40 to 1.19 | –1.29 | –3.37 to 0.79 | 4.02 | 1.77 to 6.27 | –0.01 | –3.82 to 3.81 | 3.62 | 1.20 to 6.03 | |
| De Jong Gierveld Loneliness Scale | 1 | 0.46 | 0.79 | 0.69 to 0.88 | 0.19 | –0.28 to 0.65 | ||||||||||
| 2 | 0.48 | 0.78 | 0.68 to 0.88 | 0.19 | –0.28 to 0.65 | –0.20 | –0.67 to 0.28 | –0.05 | –0.56 to 0.47 | 0.29 | –0.30 to 0.88 | –0.88 | –1.78 to 0.02 | 0.47 | –0.14 to 1.09 | |
| HADS Anxiety | 1 | 0.55 | 0.79 | 0.70 to 0.88 | –0.69 | –1.42 to 0.03 | ||||||||||
| 2 | 0.56 | 0.80 | 0.70 to 0.89 | –0.70 | –1.42 to 0.03 | –0.09 | –0.85 to 0.67 | 0.33 | –0.51 to 1.16 | 0.04 | –0.86 to 0.94 | 1.95 | 0.40 to 3.51 | 0.73 | –0.25 to 1.71 | |
| HADS Depression | 1 | 0.47 | 0.75 | 0.65 to 0.84 | –0.98 | –1.62 to –0.34 | ||||||||||
| 2 | 0.48 | 0.73 | 0.63 to 0.83 | –0.97 | –1.62 to –0.33 | 0.44 | –0.22 to 1.11 | 0.50 | –0.23 to 1.23 | –0.09 | –0.88 to 0.71 | 0.67 | –0.67 to 2.02 | –0.11 | –0.98 to 0.75 | |
| WHOQOL-OLD total score | 1 | 0.46 | 0.67 | 0.58 to 0.76 | 0.74 | –1.66 to 3.14 | ||||||||||
| 2 | 0.47 | 0.67 | 0.58 to 0.77 | 0.85 | –1.56 to 3.26 | 1.10 | –1.39 to 3.59 | –1.07 | –3.82 to 1.67 | 1.45 | –1.55 to 4.45 | –2.59 | –7.72 to 2.55 | –1.76 | –5.00 to 1.48 | |
| LSNS-6 | 1 | 0.61 | 0.83 | 0.75 to 0.92 | 0.73 | –0.18 to 1.63 | ||||||||||
| 2 | 0.62 | 0.83 | 0.74 to 0.91 | 0.74 | –0.16 to 1.65 | –0.28 | –1.20 to 0.64 | –0.15 | –1.19 to 0.88 | 0.71 | –0.42 to 1.83 | 0.42 | –1.52 to 2.37 | –0.11 | –1.33 to 1.11 | |
| EQ-5D (5L) Health today | 1 | 0.22 | 0.47 | 0.36 to 0.58 | 3.05 | –1.09 to 7.19 | ||||||||||
| 2 | 0.24 | 0.44 | 0.32 to 0.55 | 3.07 | –1.06 to 7.21 | –3.81 | –8.19 to 0.57 | 0.69 | –4.00 to 5.39 | 0.89 | –4.24 to 6.02 | –6.17 | –14.83 to 2.50 | 1.98 | –3.57 to 7.53 | |
| EQ-5D (5L) Index | 1 | 0.50 | 0.79 | 0.69 to 0.89 | 0.01 | –0.04 to 0.05 | ||||||||||
| 2 | 0.54 | 0.72 | 0.61 to 0.82 | 0.01 | –0.04 to 0.05 | –0.08 | –0.12 to –0.03 | –0.05 | –0.09 to 0.00 | 0.03 | –0.02 to 0.08 | –0.07 | –0.16 to 0.02 | –0.02 | –0.08 to 0.04 | |
| SF-36 PCS | 1 | 0.47 | 0.70 | 0.59 to 0.81 | 1.05 | –0.88 to 2.9 | ||||||||||
| 2 | 0.48 | 0.67 | 0.55 to 0.79 | 0.99 | –0.91 to 2.9 | –1.48 | –3.54 to 0.58 | –0.9 | –3.09 to 1.3 | 0.72 | 1.55 to 2.3 | –3.22 | –7.5 to 1.1 | –0.6 | –3.12 to 1.9 | |
| SF-36 MCS | 1 | 0.39 | 0.59 | 0.47 to 0.71 | 1.05 | –1.56 to 3.66 | ||||||||||
| 2 | 0.34 | 0.59 | 0.47 to 0.72 | 1.17 | –1.51 to 3.84 | 0.58 | –2.13 to 3.28 | –0.48 | –3.56 to 2.6 | 0.61 | –2.58 to 3.8 | –0.23 | –6.29 to 5.83 | –0.39 | –3.96 to 3.18 | |
Longitudinal analysis
The estimated trend(s) and difference between groups for secondary outcomes are given in Table 72.
| Variable | Model 1 | Model 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annual trend | Impact of CBT | Annual trend | Impact of CBT at 1 year post randomisation | |||||||||
| Control group | CBT group | Difference | ||||||||||
| Coefficient | 95% CI | Coefficient | 95% CI | Coefficient | 95% CI | Coefficient | 95% CI | Coefficient | 95% CI | Coefficient | 95% CI | |
| Fear of falling rating | –0.01 | –0.30 to 0.29 | –0.71 | –1.07 to –0.34 | 0.28 | –0.12 to 0.68 | –0.31 | –0.73 to 0.10 | –0.59 | –1.17 to –0.01 | –0.97 | –1.42 to –0.53 |
| Participation | –0.03 | –0.93 to 0.87 | 0.95 | –0.04 to 1.95 | –0.19 | –1.44 to 1.07 | 0.14 | –1.17 to 1.44 | 0.32 | –1.48 to 2.13 | 1.10 | –0.19 to 2.38 |
| Gait and balance | –0.20 | –0.53 to 0.13 | 0.35 | –0.05 to 0.74 | –0.52 | –0.97 to –0.06 | 0.14 | –0.33 to 0.61 | 0.66 | 0.00 to 1.31 | 0.65 | 0.15 to 1.15 |
| Grip strength | –1.93 | –2.70 to –1.16 | –0.08 | –1.02 to 0.87 | –2.02 | –3.10 to –0.94 | –1.83 | –2.92 to –0.75 | 0.19 | –1.34 to 1.72 | 0.02 | –1.19 to 1.22 |
| Functional reach | –0.20 | –1.16 to 0.76 | 0.36 | –0.83 to 1.55 | –0.74 | –2.10 to 0.63 | 0.33 | –1.02 to 1.68 | 1.07 | –0.85 to 2.99 | 0.87 | –0.63 to 2.38 |
| Loneliness | –0.14 | –0.41 to 0.13 | 0.16 | –0.17 to 0.49 | –0.15 | –0.53 to 0.23 | –0.13 | –0.52 to 0.26 | 0.02 | –0.53 to 0.57 | 0.17 | –0.24 to 0.58 |
| HADS Anxiety | –0.90 | –1.20 to –0.59 | –0.26 | –0.61 to 0.10 | –0.58 | –1.00 to –0.15 | –1.23 | –1.66 to –0.80 | –0.65 | –1.25 to –0.05 | –0.66 | –1.18 to –0.15 |
| HADS Depression | –0.23 | –0.50 to 0.03 | –0.56 | –0.88 to –0.25 | 0.24 | –0.13 to 0.61 | –0.72 | –1.09 to –0.34 | –0.96 | –1.49 to –0.43 | –1.16 | –1.61 to –0.70 |
| WHOQOL-OLD | 1.33 | 0.28 to 2.38 | 0.30 | –0.91 to 1.51 | 1.29 | –0.18 to 2.77 | 1.36 | –0.13 to 2.86 | 0.07 | –2.03 to 2.17 | 0.34 | –1.43 to 2.12 |
| Social network | 0.46 | 0.06 to 0.85 | 0.15 | –0.28 to 0.58 | 0.13 | –0.42 to 0.69 | 0.79 | 0.23 to 1.35 | 0.66 | –0.13 to 1.45 | 0.56 | –0.09 to 1.21 |
| EQ-5D (5L) Health today | –1.18 | –3.12 to 0.75 | 1.64 | –0.30 to 3.59 | –1.72 | –4.44 to 0.99 | –0.63 | –3.38 to 2.12 | 1.09 | –2.77 to 4.96 | 2.32 | –0.76 to 5.40 |
| EQ-5D (5L) Index | 0.00 | –0.02 to 0.02 | 0.01 | –0.01 to 0.03 | 0.00 | –0.02 to 0.03 | 0.00 | –0.02 to 0.03 | 0.00 | –0.04 to 0.04 | 0.01 | –0.02 to 0.04 |
| SF-36 PCS | –0.40 | –1.32 to 0.52 | 1.15 | –0.01 to 2.3 | 0.29 | –0.99 to 1.58 | –1.12 | –2.45 to 0.19 | –1.42 | –3.26 to 0.42 | 0.52 | –0.88 to 1.93 |
| SF-36 MCS | 0.43 | –0.98 to 1.84 | 1.14 | –0.61 to 2.88 | 0.57 | –1.41 to 2.55 | 0.28 | –1.73 to 2.3 | –0.29 | –3.11 to 2.54 | 1.01 | –1.13 to 3.15 |
Appendix 5 Process evaluation topic guide
Rather than produce multiple topic guides with considerable overlap between them, we have instead provided a single list of questions (and related them to the relevant construct within normalisation process theory) and indicated to which groups of respondents each question is applicable. The questions will be framed appropriately for different groups of respondents. The phrasing of the questions will also be modified as appropriate at different time points.
| Professionals | STRIDE therapists | Clients | Family members | Team members | |
|---|---|---|---|---|---|
| How do you think the study/intervention is going so far? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Professionals | STRIDE therapists | Clients | Family members | Team members | |
|---|---|---|---|---|---|
| Coherence | |||||
| How confident are you in the intervention? | ✓ | ✓ | ✓ | ✓ | ✓ |
| What reservations do you have about the intervention? | ✓ | ✓ | ✓ | ✓ | ✓ |
| What kinds of patients do you think would benefit most from this type of intervention? Are there patients for whom it would not be useful? | ✓ | ✓ | ✓ | ||
| Have there been any patients who met the inclusion criteria but you felt were not appropriate for the study? | ✓ | ✓ | |||
| Based on your experience of the CBT sessions, who do you think might benefit from this approach? | ✓ | ✓ | ✓ | ||
| Cognitive participation | Professionals | STRIDE therapists | Clients | Family members | Team members |
|---|---|---|---|---|---|
| How interested do patients seem to be in the intervention? | ✓ | ✓ | |||
| What do you think of the name of the study? | ✓ | ✓ | ✓ | ✓ | ✓ |
| How likely are you to finish this series of CBT sessions? | ✓ | ✓ | |||
| In this study, HCAs are delivering the intervention. From your perspective, what are the advantages and disadvantages of using HCAs? | ✓ | ✓ | ✓ | ||
| From your perspective, what are the facilitators and barriers to getting people/patients engaged in the study? | ✓ | ✓ | ✓ |
| Collective action | Professionals | STRIDE therapists | Clients | Family members | Team members |
|---|---|---|---|---|---|
| Who takes the lead in explaining the study to patients and how do you (they) go about it? | ✓ | ||||
| What impact is recruiting patients for the study having on the day to day running of the clinic? | ✓ | ||||
| Which aspects of the CBT intervention do you feel most confident with? | ✓ | ✓ | ✓ | ||
| Which aspects of the CBT intervention do you find most challenging? | ✓ | ✓ | ✓ | ||
| What do you think about the suitability of the venue for the CBT sessions? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Do you feel you have the support you need to recruit patients to/deliver the study/intervention? | ✓ | ✓ | |||
| How easy or difficult has it been to put what you have learned in the CBT sessions into practice? | ✓ | ✓ | |||
| From your perspective, what are the facilitators and barriers to implementing the intervention? | ✓ | ✓ | ✓ |
| Reflexive monitoring | Professionals | STRIDE therapists | Clients | Family members | Team members |
|---|---|---|---|---|---|
| What sense do you have of how useful the intervention is? | ✓ | ✓ | ✓ | ✓ | ✓ |
| Have you had any feedback from patients or GPs about the intervention? | ✓ | ✓ | |||
| What opportunities have you had to discuss the value of the intervention with your colleagues at the clinic? | ✓ | ✓ | |||
| Do you anticipate continuing to offer CBT to patients with fear of falling after the end of the study? On what basis would you like to continue to offer this treatment? | ✓ | ||||
| How important is it to continue to offer the CBT sessions to people like yourself? | ✓ | ✓ | |||
| How much scope do you feel there is for adapting your approach to the CBT intervention? | ✓ | ||||
| To what extent do you feel the sessions were tailored to your individual needs? | ✓ | ✓ | |||
| From your perspective, what are the facilitators and barriers to evaluating the acceptability/cost-effectiveness/efficacya of the CBT intervention? | ✓ | ✓ | ✓ |
Appendix 6 Example client summaries
Summary for client 5377 (ST1)
| Basic formulation: | Predisposing: | BPPV | |
| Precipitating: | Four falls in garden | ||
| Perpetuating: | Bodily sensations Dizziness |
Doing Planning Checking Avoiding |
|
| Thinking I’m going to fall |
Feeling Anxiety |
||
| Intervention focus (target): |
|
| Outcome: |
|
| Goals | Initial difficulty rating | Review rating | End rating |
| Improve confidence when out | 7 | 0 |
Therapist rating:
In your opinion how engaged with the treatment was the patient?
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Never did any work | Constantly completed all work, often did extra |
Brief overview of therapy and progress
From the start, the client was highly engaged and, as he had a background of educational psychology, he understood all of the basics before we even started. His main problem lay with new areas, whether walking or using public transport. The first visit ended with us testing his beliefs around using the ticket machines at the metro. We rated his perceived level of anxiety, then went out and tested the theory, and when back in the house rated again. This proved to be an effective start to all subsequent steps challenged. We graded different degrees of new situations and periodically worked through them all. On the sixth session he had reported a nasty fall, which gave the opportunity to discuss relapse prevention. This went very well with him getting back on his feet much faster than the last time he fell. His confidence had not suffered and it made him more determined to soldier on. By the eighth session he had very few worries and was very positive for the next 6 months. On my return 6 months later he was still very confident and stated that he had no anxieties about going out or tackling new situations. He felt relief not being so worried about anything any more.

Summary for client 4458 (ST2)
| Basic formulation: | Predisposing: | Had always been active and interested in health | |
| Precipitating: | Brain tumour Loss of wife Dizziness |
||
| Perpetuating: HCB | Bodily sensations Panic attacks |
Doing Mood Avoidance |
|
| Thinking I need to leave situation I feel pressure Cannot do it like I used to |
Emotions Unwell Frustrated |
||
| Intervention focus (target): |
|
| Outcome: |
|
| Goals | Initial difficulty rating | Review rating | End rating |
| To go to running track | 3–4 | 0 | 0 |
Therapist rating:
In your opinion how engaged with the treatment was the patient?
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Never did any work | Constantly completed all work, often did extra |
Brief overview of therapy and progress
This gentleman had experienced a very fitness-orientated lifestyle; he had been an active competitive runner and a lot of his confidence had had come from that. Once he’d been told to start using a stick and be careful, he felt old and that everyone was looking at him, which led to panic attacks in his local shopping mall. He wanted to do all of the things he’d always done and was almost looking for permission to try. He had a lot of social anxiety regarding his bowling past time which he’d recently taken up. He treated it in a similar way to his running career. He had to do his best and push himself the entire time. If he played poorly he found it difficult to look anyone in the eye. We spent a lot of time talking through and challenging his thoughts, and trying to get him to see his situation from a different view point. By the end of the sessions, he was back at the running track walking significant distances. He was going to the gym two to three times per week and attending his bowls without the anxiety. At the 6-month follow-up this gentleman was continuing to play bowls regularly and without the performance anxiety that he had experienced. He had stopped going to the running track in favour of walking around the cricket pitch as it was more picturesque. He was having problems with his back that might require surgery, but there seemed to be a reluctance to do anything or push any further.


Summary for client 3514 (ST3)
| Basic formulation: | Predisposing: | Smoker | |
| Precipitating: | Knee replaced Falls before knee replacement but not since Anaemic |
||
| Perpetuating: HCB | Bodily sensations Knee pain and stiffness Dizzy spells Low energy |
Doing Less active, Less walking Stopped getting bus |
|
| Thinking Will my knee hold my weight? |
Feeling Anxious Fear of falling Low confidence Low mood |
||
| Intervention focus (target): |
|
| Outcome: |
|
| Goals | Initial difficulty rating | Review rating | End rating |
| Get the bus (×2) to daughters | 6 | 2 – done | 0 – done |
| Go up and down the stairs | 7 | 2 – done | 2 – done |
| To walk up to the shop and back | 7 | 2 – done | 0 – done |
Therapist rating:
In your opinion how engaged with the treatment was the patient?
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Never did any work | Constantly completed all work, often did extra |
Brief overview of therapy and progress
Prior to having the knee replaced, she suffered from a lot of pain, stiffness and weakness, and it would often give out. This resulted in a number of falls, lack of confidence and increased anxiety levels. This was hampered by dizzy spells and low energy levels. All of which resulted in a lack of activity, less walking and low mood. The treatment therefore focused on graded exposure for walking and activity. She engaged very well right from the beginning. She started to walk a little more after the first session and even had potential goals ready for the beginning of the second session. She did all of the homework, and more, each week, and really felt the benefit early in the treatment. Her mood improved quickly, along with her energy levels and appetite. She also recorded her sleeping pattern and we went through ways to improve it, which also helped. By the end of the treatment she was getting out nearly every day, getting the bus regularly without any problems, walking more often and further, kneeling again, and getting to the shops without any issues. Her confidence and independence had both increased and she was no longer afraid to put weight on her knee and felt less frightened. She felt like she was ‘back to how I used to be’. However, by the 6-month follow-up she had experienced a major setback. She was diagnosed with lung cancer and had it operated on. When I visited her she was still in the early stages of recovery. She was very weak, out of breath and could not walk more than a few feet. On top of this she had a lot of family disputes occurring between her daughters, which made things a lot worse. The 6-month visit consisted of an update of her health issues and support for her family issues. The 6-month session sheet was not fully completed, as a lot of the questions were not applicable, and she asked for it not to be audio recorded, as her ex-husband was also in the STRIDE study and she felt uncomfortable talking to me about the family issue with the recorder on.
Appendix 7 Resource utilisation form
List of abbreviations
- A&E
- accident and emergency
- BPPV
- benign positional paroxysmal vertigo
- CBT
- cognitive–behavioural therapy
- CBTi
- cognitive–behavioural therapy intervention
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness/utility acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- Clinical Research Facility
- CTA
- clinical trial associate
- CUA
- cost–utility analysis
- EoI
- expression of interest
- EQ-5D (5L)
- European Quality of Life–5 Dimensions (5 Level)
- FES
- Falls Efficacy Scale
- FES-I
- Falls Efficacy Scale–International version
- GP
- general practitioner/general practice
- HADS
- Hospital Anxiety and Depression Scale
- HCA
- health-care assistant
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IRR
- incidence rate ratio
- ITT
- intention to treat
- LSNS-6
- Lubben Social Network Scale–6
- MAR
- missing at random
- MCS
- Mental Component Score
- MMSE
- Mini Mental State Examination
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPT
- normalisation process theory
- OR
- odds ratio
- PCS
- Physical Component Score
- PIS
- Patient Information Sheet
- PPI
- public and patient involvement
- QALY
- quality-adjusted life-year
- RA
- research assistant
- RCT
- randomised controlled trial
- REC
- research ethics committee
- RR
- relative risk
- RVI
- Royal Victoria Infirmary, Newcastle Upon Tyne
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SMART
- specific, measurable, achievable, realistic and time-limited
- SPPB
- Short Physical Performance Battery
- STRIDE
- Strategies to Increase Confidence, Independence and Energy
- TIDieR
- Template for Intervention Description and Replication
- TSC
- Trial Steering Committee
- WHOQOL-OLD
- World Health Organization Quality of Life questionnaire – older adults module
- WTP
- willingness to pay