Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/164/01. The contractual start date was in November 2011. The draft report began editorial review in January 2016 and was accepted for publication in June 2016. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Henry Kitchener is chairperson of the Advisory Committee for Cervical Screening, but views expressed here are those of the author and not those of Public Health England. Emma Crosbie is a National Institute for Health Research (NIHR) Clinician Scientist and has received funding from the NIHR, Medical Research Council, Wellbeing of Women, Wellcome Trust and Central Manchester University Hospitals NHS Foundation Trust for research projects unrelated to the submitted work. She is an executive scientific editor for the British Journal of Obstetrics and Gynaecology.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Kitchener et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Cervical screening based on exfoliative cytology has provided the means of reducing cervical cancer incidence. It is widely recognised that a high population coverage is necessary for cervical screening to be effective. A coverage of around 80% in the UK has been associated with a falling death rate for cervical cancer over the past 25 years. 1 The identification and treatment of premalignant lesions through cervical screening prevents the development of cervical cancer. These lesions are called cervical intraepithelial neoplasia (CIN) and this strategy is one of secondary prevention. Confirmation in the 1990s that high-risk human papillomavirus (HPV) was responsible for all cervical cancer led to the successful development of prophylactic vaccines. These vaccines, composed of virus-like particles, are highly effective in preventing HPV type-specific infection and, as a result, the UK government initiated a programme of prophylactic HPV 16/18 vaccination among adolescent females in 2008. This strategy of primary prevention will have a very significant effect on the incidence of high-grade CIN, more so if the nonavalent vaccine covering five additional high-risk types replaces the currently used quadrivalent vaccine, which covers HPV16/18 as well as HPV 6 and 11 responsible for genital warts. Cervical screening will, however, continue to be required as the currently used vaccines directed against type 16/18 will not prevent all cervical cancers.
NHS Cervical Screening Programme
The organised national Cervical Screening Programme was launched in 1988. It has developed into an internationally renowned programme based on quality assurance throughout and high coverage. The programme has now been delegated to the devolved nations within the UK and some differences currently exist. In England, women are screened every 3 years from the age of 25 to 49 years and 5-yearly from 50 to 64 years. In Scotland, women are screened 3-yearly from the age of 20 years, although this about to change to 25 years, and screening stops at 60 years. Cervical screening currently relies on liquid-based cytology, but HPV triage is used to determine which women with low-grade changes should be referred to cytology. The National Screening Committee has recommended a switch to primary HPV screening (with cytology to triage HPV-positive women for onward referral) on the basis of greater sensitivity and the opportunity for extended screening intervals.
Coverage
There has been a small drop in population coverage (5 years since last test) in England, from 80.6% to 77.8%, in the decade 2004–14. 2 Among 25- to 29-year-olds, however, coverage in 2013/14 (< 3.5 years since last test) was only 63%. This has been accompanied by a worrying increase in the incidence of invasive cancer in the 25- to 34-year-old group. Although lifestyle factors such as smoking may be involved, an increased prevalence of high-risk HPV infection and falling participation in screening are probably factors. 1
Barriers to screening
The reasons for falling participation in cervical screening by young women are incompletely understood, particularly given a NHS programme that offers free screening to all eligible women. Two recent systematic reviews3,4 pointed to a lack of randomised trials that address falling screening uptake and the need to look beyond reminders and instead evaluate new ways to overcome particular barriers. In a qualitative study from London,5 a number of reasons for non-attendance were highlighted. A younger subset from this population of non-attenders said they intended to be screened but raised practical barriers to this actually being achieved.
Considerations in designing a study to address non-participation
Strategies to improve uptake of cervical screening in young women are hampered by several factors. Our incomplete understanding of the reasons for non-attendance has already been mentioned, but for different women convenience, fear, dislike of gynaecological procedures, anxiety, inertia and not feeling at risk are all relevant. Effective contact with large numbers of hard-to-reach individuals with whom academic investigators are unable to contact directly also represents a challenge. Developing novel interventions and implementing them on a large scale is also challenging. Finally, there is the issue of capturing accurate uptake data. Encouraging those who would attend and those who have not attended requires different strategies. Clearly any intervention that looked to be effective would need to be cost-effective too, requiring detailed work on costs and models of screening outcomes.
Our hypothesis was that different interventions would appeal to different women and, therefore, we wished to test several to determine which, if any, would prove most effective and cost-effective. We aimed to recruit a large cohort, and to conduct a randomised trial of interventions over two phases: phase 1 for all invitees; and phase 2 for those who had not attended, using a different set of interventions to phase 1 because we judged that women who had just been invited would differ from those who had not yet taken up the invitation.
We made the decision to conduct the study in two sites: Greater Manchester, where prompt uptake of cervical screening (< 6 months from invitation) is only 25%, and Grampian, Scotland, where it would be possible to gain insight into the effect of prior HPV vaccination on participation. Selection of interventions took into account our hypothesis that ‘one size would not fit all’ (i.e. for different women there were different issues). We considered that for newly invited women, a lack of interest in the lengthy factual national information sheet, and the inconvenience of booking a test could be disincentives. Therefore, we used a prior focus group of young women to inform the development of a more concise leaflet that specifically addressed the key issues highlighted. Indeed, pre-notification for colorectal screening has been associated with a small but significant increase in uptake in both Scotland6 and Australia. 7 Online booking has become the norm for many activities and we felt that this would help overcome any inconvenience engendered by the need to book an appointment to attend for cervical screening.
With regard to non-attenders by 6 months, we felt that there were several disincentives that interventions could address. Dislike of a gynaecological examination could be overcome by self-sampling, which had been shown to be a feasible and reliable means of testing for high-risk HPV. 8,9 Again, the inconvenience of having to book could be addressed by self-testing and by timed appointments, which have been used effectively for other purposes in some general practices. The potential fear of what is involved and a poor understanding of the rationale and potential benefits and harms of cervical screening could be addressed by use of a nurse navigator (NN), a term coined in the USA, where this role has been evaluated in a number of areas of health. 10 Finally, we considered that offering a choice between two different interventions, NNs and timed appointments would be attractive to some women who might wish to exert a preference.
The aim of the trial was to increase participation in cervical screening among women receiving their initial invitation. The objectives were to evaluate the effectiveness and cost-effectiveness of different interventions in a randomised manner in an adequately powered trial. We also wished to determine the impact of prior vaccination on screening uptake. Another objective was through a discrete choice experiment (DCE), to determine what aspects of cervical screening were most highly valued among those who had not yet attended, in order to improve our understanding.
Chapter 2 Clinical effectiveness
Objectives
The overall aim of STRATEGIC was to determine whether or not uptake of cervical screening could be improved among 25-year-old women receiving their initial invitation. The trial was embedded in the NHS Cervical Screening Programme and conducted in two phases with the following objectives.
Phase 1 objectives
-
To determine, in an English cohort of 24.75-year-old women in Greater Manchester and a Scottish cohort of 20-year-old women in Grampian, the clinical effectiveness and cost-effectiveness of a pre-first invitation leaflet (pre-leaflet), which had been designed to increase receptivity of young women to cervical screening. The development of this leaflet had been completed prior to the trial, and was based on focus group work with young women, targeting the issues that influence them when thinking about attending for cervical screening.
-
To determine the feasibility, acceptability, clinical effectiveness and cost-effectiveness of offering online booking as a convenient alternative to telephoning the general practice as a means of making an appointment at first invitation. This was available only to the women covered by Manchester Primary Care Trust (PCT) (now redesignated a Clinical Commissioning Group).
-
To determine, among the 20-year-old women in Grampian, whether prior HPV vaccination in the catch-up campaign was associated with an increase or decrease in participation.
Phase 2 objectives
To determine, among those women in the cohort who had not attended after 6 months, the feasibility, acceptability, clinical effectiveness and cost-effectiveness of several novel strategies:
-
the offer of requesting a self-sampling kit (SSK) for HPV status as a determinant of the need for cervical cytology (HPV-negative women would not require cytology)
-
SSKs sent unsolicited to women
-
a specialist NN to help a woman overcome her barriers to screening
-
timed appointments for cervical screening to encourage women to attend
-
a choice of requesting a HPV SSK or the NN.
In addition, a health economic study (see Chapter 3) would be performed to determine the costs and cost-effectiveness of these interventions and a DCE (see Chapter 4) would be performed to determine the importance non-attending women attach to different aspects of the screening experience.
Methods
Phases 1 and 2 both comprised a cluster randomisation of general practices (Figure 1). All women invited for their first screen were eligible. Concurrent with phase 1, a pilot study tested the feasibility of the planned interventions to be evaluated during phase 2. A pre-leaflet had been developed prior to the STRATEGIC trial (see Appendices 1–3), based on focus group work with young women, and addressed issues that influenced their views. 11 These included the relationship between cervical cancer and HPV, the sexually transmitted nature of HPV and why young women should be screened. The pre-leaflet was sent, by the screening agency in Greater Manchester and by the research team in Grampian, 6 weeks before their standard invitation was sent out. Phase 1 also included the offer of online booking, which provided women in randomised practices restricted to Manchester PCT the option to choose from a selection of set appointments online, as an alternative to telephoning the general practitioner (GP). The phase 2 interventions, which were introduced after the pilot study, were sent out only to women without a cytology test recorded 6 months after their test due date. These included SSKs, either sent unsolicited or requested, timed appointments to have a cervical sample taken, access to a NN and a choice between the NN or a SSK. The primary outcomes were uptake of phase 1 interventions 3 months after the routine invitation and of phase 2 interventions 12 months after the invitation. The cervical screening process within the STRATEGIC trial can be seen in Figure 2. Data were obtained from the NHS screening agency, which also facilitated mailings.
FIGURE 1.
The STRATEGIC study cluster randomisation: Greater Manchester and Grampian. a, Synchronous pilot studies on known non-attenders (age 25.5 years) to ensure the feasibility of HPV self-sampling, NNs and timed appointments; b, in Manchester PCT only.
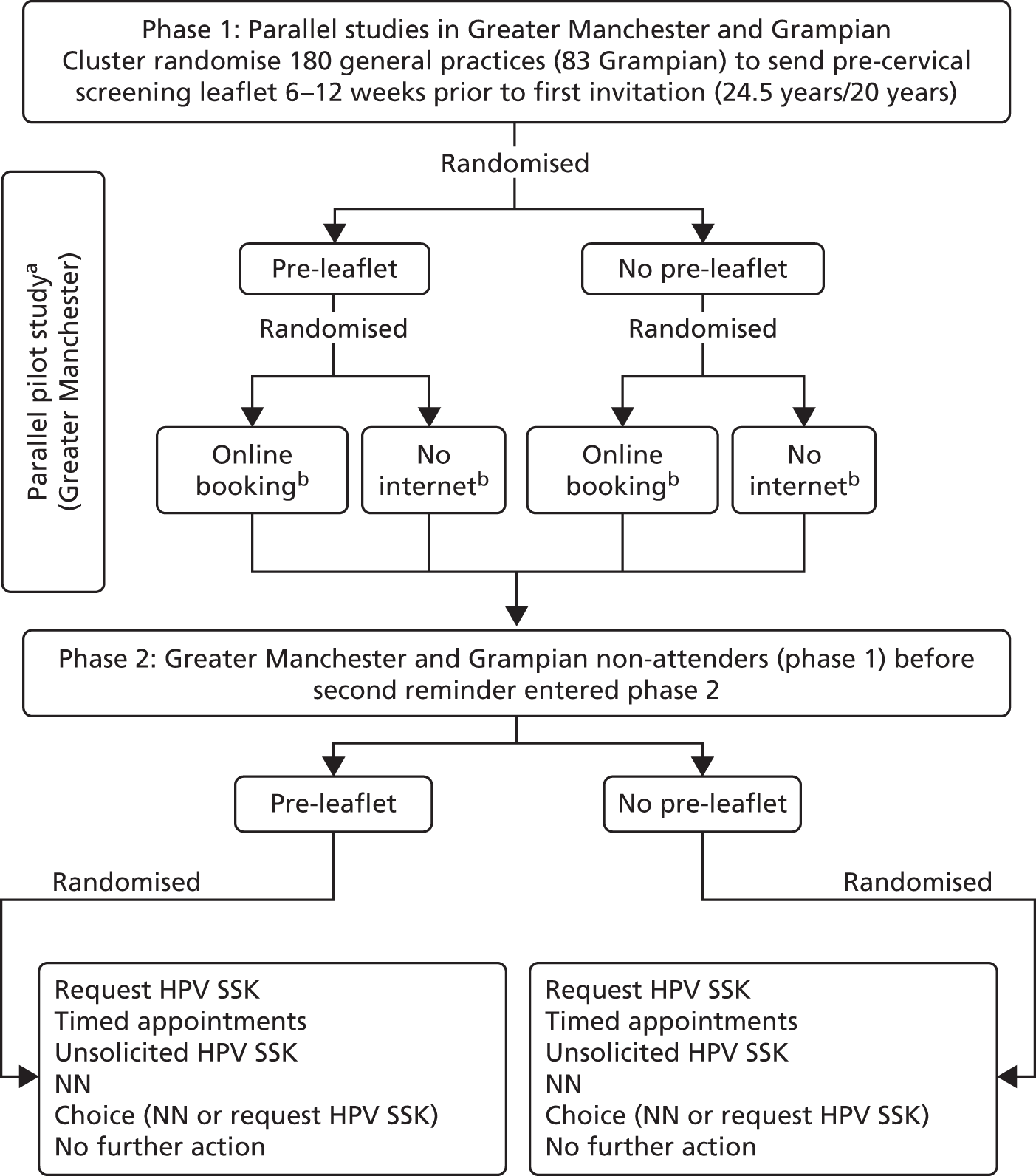
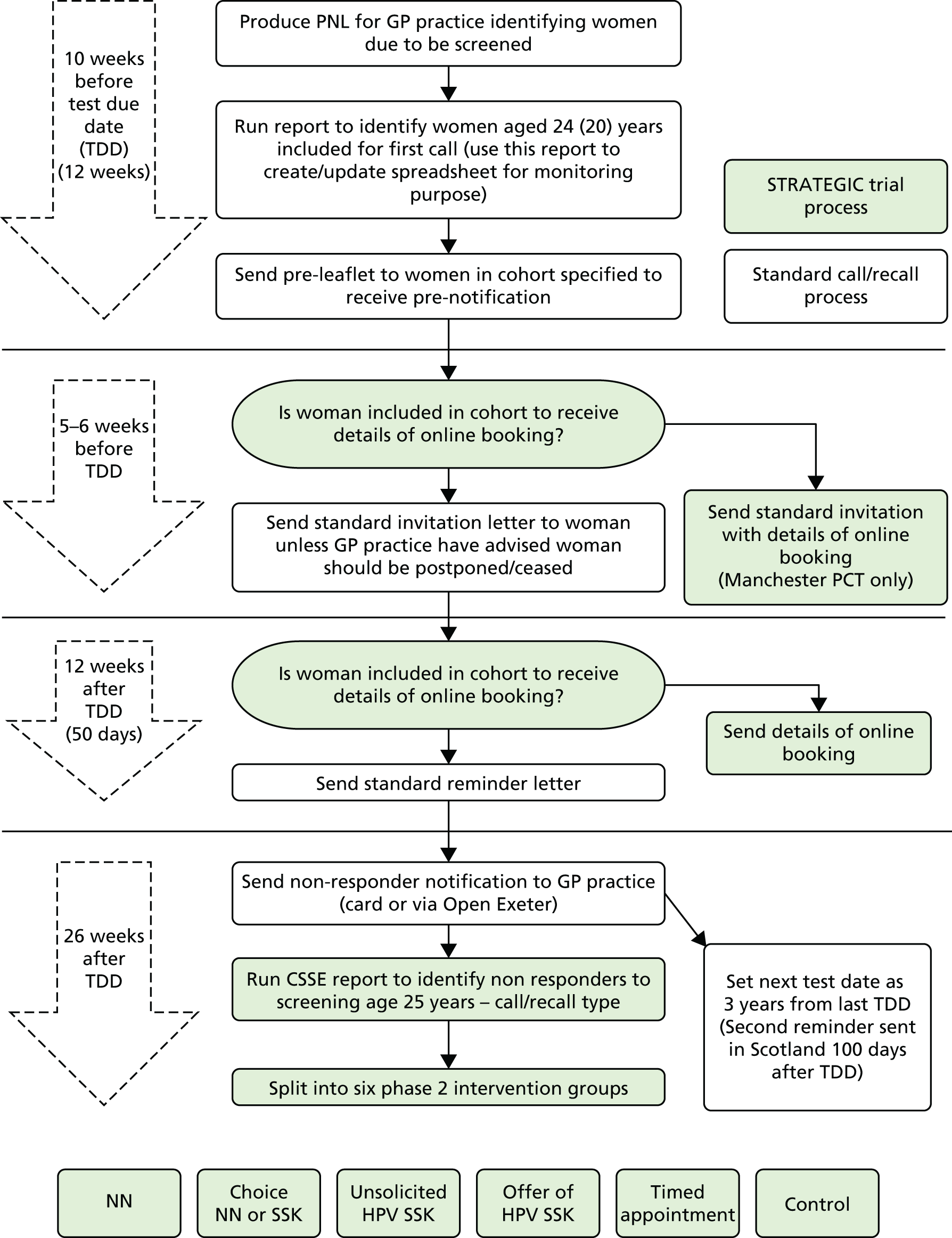
FIGURE 2.
Cervical screening process with STRATEGIC trial processes embedded (Scotland). This excludes pre-leaflet and includes online booking that was not done in Scotland. PNL, patient notification list.
Interaction with the screening agency
The STRATEGIC trial procedures were conducted differently in Greater Manchester and Grampian because of differing amounts of support from the screening agencies.
Greater Manchester
Phase 1
The mailing of the phase 1 pre-leaflet took place weekly, and each week the screening agency recorded and returned a summary of these data to the trial team in Manchester. This summary included the number of pre-leaflets sent that week and the number of participants allocated to the control group that week. This enabled the trial team to keep track of the study numbers for the duration of the trial. It also enabled the trial team to estimate the number of pre-leaflets and pre-paid envelopes the screening agency would require in future weeks and months.
In Manchester, for general practices allocated to the online booking arm, the screening agency inserted extra text into the standard invitation to screening and reminder letters. The extra text invited women to book a screening appointment at one of the community and sexual health (CaSH) clinics in Manchester.
Phase 2
Phase 2 interventions were sent by the screening agency to women who had no cytology test recorded 6 months after their test due date. The screening agency mailed out offers from the study team for the different interventions to eligible women. The screening agency provided a summary of interventions sent weekly to the trial team in Manchester. This was important, as they required the trial team in Manchester to order and maintain stock and supplies of SSKs and the stamps required to post them. Unusual activity prompted a query to the screening agency to ensure that the numbers were accurate.
Grampian
Phase 1
Because the screening agency in Grampian did not have the provision to assist the STRATEGIC trial team in the way the screening agency in Greater Manchester did, the team in Grampian were required to send the phase 1 interventions directly on a weekly basis. In Grampian, ATOS, the information technology company that developed and maintains the Scottish Cervical Call/Recall System (SCCRS), provided the trial team with a data extract of eligible women.
This extract was run weekly by the principal investigator in Grampian and the GP practices were then randomised to the phase 1 intervention. The pre-leaflets were posted by a member of the research team. This was subsequently recorded weekly in a Microsoft Excel® 2003 (Microsoft Corporation, Redmond, WA, USA) format. The data were stored on a secure drive, in line with data protection policy. The vaccination status was extracted from the SCCRS, along with the screening outcome data transferred to the SCCRS by linkage with the Scottish Immunisation Record System. Data recorded include type of vaccine, number of doses and dates of doses administered.
Phase 2
A similar procedure was followed as in phase 1; however, the practicalities were more complicated. ATOS provided a data extract from the SCCRS of women eligible for phase 2. The data were downloaded using the extract by the Aberdeen principal investigator and were used during the preparation of data outcomes as a source document. In order to transform this data extract into a format that could be used by the trial team, a macro was developed to format the data extract into a tabulated layout. This included all women who were potentially eligible for phase 2, filtered to exclude those women with a cytology test result. This left a list of women who were eligible for a phase 2 intervention. A look-up query was added in order to establish which phase 2 intervention their GP practice had been randomised to, and the interventions were sent out.
All the steps above have been followed and records maintained, again stored on a secure drive, in line with University of Aberdeen data protection policies. This procedure was monitored by the trial co-ordinator based in Manchester and visits were made to the centre in Grampian in order for quality checks.
Interaction with general practices
Phase 1
Initial contact was made with the GPs by the trial team in order to introduce them to the trial. The research nurse attended the clinical commissioning group meetings prior to the STRATEGIC pilot study starting and provided a synopsis of the trial. Representatives from general practices were given the opportunity to make the trial team aware of any potential issues that they could foresee with the trial. Next, a standard letter was sent to the general practice informing them of their trial arm allocation to a pre-leaflet or no pre-leaflet.
Phase 2
The next point of contact made to the general practice by the trial team was prior to the phase 2 interventions when the trial team informed them of the intervention to which their practice had been randomised. The timed appointments required the screening agency to send a standard letter to the general practice asking them to send eligible women an offer letter, detailing a time and date for them to attend for a cytology sample. The general practice then returned the letter to the agency with the date given to the woman. Once the trial team received a list of confirmed timed appointments, the general practice invoiced the trial team for reimbursement of £5 per letter.
Operationalising the interventions
Phase 1
Pre-leaflet
The development of the pre-leaflet is described in Appendix 1. Once a general practice was randomised, the research team in Manchester produced a mail merge informing each general practice of the intervention that they had been randomised to. Included in the letter was the contact details of the trial team, and the general practice were encouraged to contact the research team with any questions. Once this initial contact had been made with the general practices, and phase 1 was ready to begin, the research team arranged a meeting with the screening agency in order to discuss the implementation of phase 1. The screening agency (as detailed previously), produced a weekly mailing for women eligible to take part in the STRATEGIC study; the intervention received by each woman depended on the intervention to which their general practice had been randomised. These figures were reported back to the trial team in Manchester on a weekly basis.
Online booking
Young women are more accustomed to using online services, so the provision of a web link that enabled women to book their screening test online could have been popular. It was not possible to offer this in general practice because of the large number of practices involved, but online booking was implemented for the CaSH clinics in the Manchester area. Online booking was not available in Salford/Trafford or in Grampian. A standalone online appointment scheduling system was used (www.supersaas.com/), and appointments were available throughout the day and at clinics across Manchester.
In order to offer this online booking service consistently and effectively, the trial team in Manchester implemented and a followed a strict standard operating procedure, and worked closely with the central CaSH clinic. The offer of booking an appointment for cervical screening online was sent by the screening agency. If a woman chose to respond, she would access the online system to select an appointment, with a choice of three clinics and selecting her preferred date and time, then leaving a contact name and telephone number. Each Friday, a member of the trial team would access the online system in order to remove the empty slots for the next week and inform the CaSH clinic of any booked appointments for the next week. Reminders were set for the day before each booked appointment as a reminder text message was sent by a member of the trial team, via the secure NHS.net system.
Pilot study
The pilot study was performed in Greater Manchester concurrently with phase 1 involving a slightly older cohort than the STRATEGIC cohort, and determined the feasibility of HPV self-sampling, both sent and requested, as well as the NNs and timed appointments being offered to known non-attendees in Manchester. The piloted interventions were tested in women who were already aged 25.5–27.5 years, who had previously not taken up their invitation for their first cervical screen, despite reminders. Although the primary purpose of this pilot was to evaluate the feasibility of the intervention (e.g. arranging pre-booked appointments), as well as practicability such as arrangements for self-testing, it would also provide estimates of the likely effect on uptake of screening as a result.
Phase 2
Nurse navigator
Nurses offer support and guidance to patients, and offering a trained cervical screening nurse with whom women could discuss concerns or raise questions confidentially about screening may help alleviate their fears and ‘navigate’ women over barriers to attending for screening. The NN would be able to discuss the woman’s perceived barrier(s) or difficulty accepting her invitation for cervical screening and advise how these could be overcome. She could also answer questions and assist the woman in booking an appointment if necessary.
Six months after their test due date, if no test date was recorded, women in general practices randomised to receive the NN intervention received a letter describing the trial along with a second reminder letter from the screening agency as per standard procedure. This letter (see Appendix 4) included the ways in which the women could contact the NN (via e-mail, telephone or text message), and the suggested ways in which the NN could offer advice or help. Once contact had been made with the NN, the role of the NN could be far-reaching, providing advice on the importance of cervical screening and how to attend a cervical screening appointment at their general practice, at a sexual health clinic or by sending them a HPV SSK. Written consent for any follow-up was sought by the NN at the time the woman made contact, and with her permission the consent forms and an information sheet were mailed out (see Appendix 5). The consent enabled the trial team to check for the presence of a cytology test result on the national screening database, allowing our researchers to monitor compliance with screening after contact. Women were also offered a follow-up call from the NN to discuss whether or not they had arranged/attended for screening and to provide further advice or support.
Human papillomavirus self-sampling
Cervicovaginal self-testing for high-risk HPV triaged with cytology for the purpose of colposcopy referral is as sensitive in the detection of CIN as practitioner-obtained cytology at detecting CIN. This strategy accepts that a negative self-sample would allow a woman to be considered as having had a negative screen, whereas a positive result would indicate the need for practitioner-obtained cytology in order to achieve the required level of specificity for colposcopy referral. Women were offered a self-sample test for HPV to attract those who preferred not to attend their GP for a cytology sample.
There were two HPV self-sampling interventions; the first was a letter offering the opportunity to request a SSK, and the second an unrequested SSK was sent directly to the home. The SSK comprised the following: a SSK [either Delphi lavage (Rovers Medical Devices BV, Oss, the Netherlands) or The Rovers® Evalyn-Brush (Rovers Medical Devices BV, Oss, the Netherlands)]; and packaging, to return their sample, compliant with transport regulation UN3373 for category B biological substances. 12
An information sheet explained the purposes of the test, how the results would be communicated to the woman and her GP, the implications of the results and instructions for taking the sample and returning it to the virology laboratory. A consent form for processing the sample and providing the results was included, giving her the option of receiving her result by a telephone call, text message, e-mail or letter. The consent also obtained permission for the research team to check the Exeter screening database (SCCRS in Grampian) for subsequent cytology samples in the event of a positive result. After the women collected the sample it was sent to the virology laboratory at Central Manchester University Hospitals NHS Foundation Trust for processing. The team at the laboratory sent the results and the consent forms to the trial team in Manchester University. The trial research nurse provided the HPV result as per the women’s preference (letter/telephone call/text/e-mail).
Choice between nurse navigator and human papillomavirus self-sampling
The rationale of using an intervention involving choice was to provide some women with a degree of control over what intervention they may prefer. Access to a nurse to provide advice and access to a SSK were felt to be sufficiently different to provide a meaningful choice. A letter was sent to women offering them either having access to a NN or a SSK on request.
Timed appointments
Timed appointments for non-responders have shown promise in other screening settings. 13,14 Timed appointments mean that a woman does not have to contact the practice to book a cervical screening appointment herself. Agreement to set up timed appointments was achieved through contact with general practices. As with the implementation of the other STRATEGIC study interventions, the research team in Manchester sent a sample invitation letter to the screening agency it then sent this onto the general practices so that they could populate the letters with the time, date and women’s details, in order to offer them a timed appointment. In these letters, the general practices were asked to send women an invitation letter detailing a time and date for them to attend for a cervical screening appointment (see Appendix 6). Only three general practices were unable to facilitate these appointments.
Patient and public involvement
We had planned to convene a panel of young women who had chosen not to attend for screening, in order to provide insight and advice regarding our interventions and the responses to these. We were not, however, able to use this proposed panel of users during the study. The principal reason for this was that we found it very difficult to engage with non-attenders for the DCE (see Chapter 4), which was required to be conducted at arm’s length in the sense that such women were contacted initially through the screening agency. We considered that a face-to-face panel or, indeed, a more indirect form of communication would have been impossible to facilitate.
The DCE can be considered a form of public engagement because it targeted young non-attenders, as defined by the protocol, but who were completely independent of the study. These individuals were not involved in the study itself, but did provide relevant views regarding preferences with respect to cervical screening.
Data
In addition to monitoring the weekly interventions, the trial team requested intermittent previews of blinded data from the screening agency during the trial to ensure that data outcomes were not being missed. Three such requests were made during the trial.
Data outcomes in Grampian were collated by the trial team and not by the screening agency, as was the case in Greater Manchester. In order to prepare the final data outcomes for analysis, we merged the mailings recorded for phase 1 and phase 2, linking the two mailings on the woman’s Community Health Index, the unique patient identifier containing date of birth and gender. In order to be assured that the files had merged successfully, two members of the research team checked 10% of these data and could be confident that the files had been merged successfully. In addition to this, extensive cleaning was performed on the data outcomes from Scotland by the research co-ordinator and the trial statistician.
Statistical methods
Randomisation of general practices
In this trial the interventions were randomised to practices for both the phase 1 and phase 2 interventions. Because all practices in a PCT entered the trial at the same time it was possible to use a minimisation procedure for cluster randomised trials, as described by Raab and Butcher. 15 In this, allocations of interventions to practices were generated using a random number generator and implemented accordingly. For each allocation the imbalance between intervention arms is calculated and the allocations are then sorted according to the magnitude of the imbalance, before selecting an allocation with good balance. We controlled for the cervical screening uptake rate and size of this cohort of women in each practice prior to randomisation based on standard reports of these data provided by the screening agencies involved. We chose the allocation that was the fifth centile of the distribution of imbalance from 10,000 possible allocations. This procedure was carried out separately for each PCT with the names and location of the practices concealed. In phase 2, allocation was also balanced for allocation in phase 1.
Sample size
Phase 1
The pre-leaflet was tested across Manchester, Salford and Trafford PCTs, which had, respectively, 100, 55 and 46 general practices (the total of general practices had altered by July 2013, the current totals are 97, 47 and 36), with an average practice size of 4900 patients, conservatively suggesting that approximately 40 women per GP practice would become eligible for the screening programme over a 12-month period. Data from Manchester PCT had suggested that the initial response to the first invitation was < 30%. A modified pre-leaflet was tested in women who had been offered HPV vaccination as part of the catch-up component of the Scottish vaccination programme (Grampian cohort). The primary outcome was the absolute increase in screening uptake by 3 months (phase 1) and 12 months (phase 2) following standard invitation, compared with controls. Owing to a potential adverse effect of vaccination on attendance, a larger intervention effect was expected. With an intracluster correlation coefficient (ICC) of 2.6%, and with 38 intervention and 39 control practices (leaflet sent to 1520 women), the trial had a power > 95% to detect a 10% increase in attendance. The power of a cluster randomised trial designed depends on the ICC, the number of clusters, the cluster sizes and variation in cluster size. Jensen et al. 16 suggest an ICC for a similar outcome of 0.026.
With 92 practices randomised to pre-leaflet (leaflet sent to around 4000 women) and 88 control practices, the trial had a power of 89% to detect an uplift in attendance, assuming an ICC of 0.026 and an average cluster size of 40. This calculation assumed that the variance in the cluster size was equal to the mean cluster size.
The online booking intervention was tested in Manchester PCT only. With 49 practices randomised to online booking and 48 to control, the trial had a power of 93% to detect a 7.5% improvement in attendance by 3 months, assuming an ICC of 0.026 and an average cluster size of 40. Given that the online booking intervention was introduced on a different occasion to the pre-leaflet, any interaction between the two interventions was considered unlikely, so that a factorial design was justified.
The effect of online booking could be cumulative over the follow-up period, as access to online booking continued to be available. A total of 63 Greater Manchester practices received no intervention. These had a power of 94% to detect a 10% increase due to access to online booking. The planned allocation of practices in phase 1 and 2 is summarised in Tables 1 and 2, respectively.
| Study site | Intervention | Total | |
|---|---|---|---|
| Pre-leaflet | Control | ||
| Manchester | |||
| Online booking | 24 | 25 | 49 |
| Online booking control | 24 | 24 | 48 |
| Salford | 26 | 21 | 47 |
| Trafford | 18 | 18 | 36 |
| Total (north-west of England) | 92 | 88 | 180 |
| Grampian | 38 | 40 | 78 |
| Study site | Intervention | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-leaflet | Online booking | SSK sent | SSK offered | NN | NN and SSK offered | Timed appointment | Control | ||
| Manchester | Yes | Yes | 3 | 4 | 3 | 4 | 2 | 8 | 24 |
| Yes | No | 3 | 3 | 4 | 3 | 2 | 9 | 24 | |
| No | Yes | 4 | 3 | 3 | 3 | 3 | 9 | 25 | |
| No | No | 2 | 3 | 2 | 3 | 4 | 10 | 24 | |
| Salford | Yes | – | 6 | 5 | 6 | 6 | 5 | 16 | 44 |
| Trafford | No | – | 4 | 5 | 4 | 4 | 5 | 17 | 39 |
| Grampian | Yes | – | 4 | 5 | 6 | 3 | 5 | 15 | 38 |
| No | – | 5 | 6 | 5 | 5 | 6 | 13 | 40 | |
| Total | 31 | 34 | 33 | 31 | 32 | 97 | 258 | ||
Pilot study
The interventions in phase 2 were expected to increase attendance among those who had not attended by between 10% and 20%. In proof-of-concept studies, a larger significance level and increased power is appropriate so that potential beneficial treatments are not rejected. 17 With 120 women in each arm, the study had a power of 90% to reject the null hypothesis of no increase in uptake with a one-sided significance level of 25%.
Phase 2
It was estimated that the standard first reminder might increase response by 5%. At 6 months (second reminder), we estimated that at least 50% would not attend, that is a mean of 20 women per practice. In phase 2, the following five interventions were tested: (1) SSK offered, (2) SSK sent, (3) NN, (4) choice between NN and SSK offered and (5) timed appointments. Statistical analysis compared each intervention with the control.
In order to improve the power in phase 2 of the study, we planned for a larger control group sample than with the phase 2 interventions. It has been shown that the optimal method of choosing the size of the control arm is to multiply the estimated sample size for an intervention arm by the square root of the number of interventions. 18 Our adopted intervention to control group sample size ratio of 1 : 3 was deemed suitable to compare five interventions.
We assumed that a further 5% would attend without further intervention by the time of next recall, and that a follow-up intervention would increase uptake by an additional 5%. Among the 50% of women who we estimated would not have attended by 6 months, this corresponded to a difference of between 10% and 20%. A study with 30 practices in each of the five intervention arms (SSK sent and offered, NN, timed appointments, NN or SSK offered) and 100 control practices would have a power of > 80% to detect this difference, provided that the ICC does not exceed 0.07. This calculation included a Bonferroni correction of the significance level to allow for five comparisons with the control and an allowance for additional variation in cluster sizes as a result of the effect of the phase 1 intervention by assuming a cluster size variance of 40.
Self-sampling kits were sent by the trial centre. It was estimated that about 1200 SSKs would be sent out. So that the acceptability of the two kits can be compared, women were randomised to receive either kit type using randomisation stratified by site with a random block size of 4, 6 or 8. In each study site, the screening centre assigned kits in order according to a pre-prepared list.
Statistical analysis
A detailed statistical analysis plan based on an intention-to-treat (ITT) protocol was prepared and agreed with the Trial Steering Committee at the end of the pilot study. Because of the nature of the primary outcome, we envisaged complete data being available on all women in the trial regarding attendance, via the Exeter system in Greater Manchester and SCCRS in Grampian.
Initially, descriptive analysis tabulated the rate of attendance and rate differences by intervention group followed by formal inferential statistical analysis accounting for clustering of patients within practices. 19 In short, this was accomplished by fitting a generalised estimating equation (GEE) model in the form of a population average logistic regression,20 with the intervention group adjusted for the covariates, uptake rate for each practice and location (Manchester/Salford and Trafford/Grampian).
The primary outcome
The primary outcome for phase 1 was uptake of cervical screening 3 months following the standard invitation, at which time point a reminder would be sent by the screening agency. The primary outcome for phase 2 was uptake of cervical screening at 12 months following the standard invitation. Uptake rates were also calculated at 6 and 18 months to assess any long-term effects. Each primary outcome was calculated using a provided ‘date of test’ variable that related either to cytology test only (phase 1) or to cytology or HPV test (phase 2). In each case a generated binary variable was defined as ‘completed’ (1) and ‘not completed’ (0) by the allotted follow-up time point (e.g. 3 months). A missing date of first test indicated if a test had not been completed by the end of the follow-up period. The data can therefore be considered to be a complete data set for all individuals. Outcome variables were then created by identifying a test present (yes/no) for 3, 6, 12 and 18 months since the standard call.
Secondary outcomes were (1) differential uptake of screening among vaccinated and unvaccinated women in Grampian by 6 months after invitation and (2) uptake 12 months following phase 2 interventions.
Data exclusion and data cleaning criteria
Participants were excluded from the phase 1 data and the subsequent analysis if:
-
the cytology test was prior to the allotted pre-leaflet intervention date (approximately 6 weeks prior to standard invite) assigned to both the intervention and control group participants
-
all intervention dates are missing.
Women were excluded from the phase 2 data and the subsequent analysis if:
-
time in study was > 9 months from when phase 2 interventions started (Greater Manchester)
-
test date was prior to phase 2 interventions start
-
subject had a ‘left trial date’ (i.e. moved home/GP prior to proposed phase 2 intervention sent)
-
subject’s first invitation date was sent outside the recruitment period.
Baseline cervical uptake screening rates
In Greater Manchester, the phase 1 baseline rate for each practice was determined for a baseline data cohort consisting of women for 12 months between October 2010 and September 2011 prior to the trial. The data extraction methodology was the same as that used for the outcome measure. In Grampian, the equivalent cohort was not available and so routinely reported uptake rates for women aged 20–24 years at each practice was used instead. The difference between the methods of determination of the baseline rate is accounted for in the analysis by adding an interaction term between region and baseline rate.
Estimation method
The effect of interventions was estimated using a logistic regression model with covariates for intervention group, baseline rate, PCT (region) and an interaction between baseline rate and PCT/region to account for differences in methods of collection of baseline rate. To account for the clustering effect of practice, a GEE model was used with an exchangeable covariance structure and robust standard errors. This analysis was performed using the Stata procedure xtgee (version 13, StataCorp LP, College Station, TX, USA).
Phase 1 analysis
Phase 1 determined the effect of a pre-leaflet/no pre-leaflet and online/no online booking on uptake of any test, either HPV or cytology. The pre-leaflet was run in Salford, Trafford and Grampian, whereas both the pre-leaflet and online booking was run within a factorial design in the Manchester PCT only. Both interventions were analysed in an ITT analysis, where response was compared between the two groups, as offered, irrespective of whether the subject received the pre-leaflet or the method employed to book the test. A test for evidence of interaction between the pre-leaflet and online booking was performed. Descriptive statistics and response rates were calculated for each intervention and the GEE model estimated the treatment effect. Odds ratios (ORs) would indicate if a significant change in response between the control group and the interventions had occurred (p < 0.05). An OR is expressed on the logarithmic scale meaning a value of 1 indicated no change in response rate, a value > 1 showed an increase in response and a value of 0–1 indicated a decrease in response. Analyses are presented for the uptake at 3 and 6 months post invitation, with the 3-month analysis being the primary end point.
Moderator analyses
Moderator analyses were carried out to test for:
-
an interaction effect between treatment group and the location, with respect to the screening age differential between Scotland and the north-west of England
-
the influence of HPV vaccination status (Grampian PCT only).
Pilot study among non-responders
The pilot study in Manchester was running concurrently with phase 1 in women who had already not taken up their invitation despite reminders. The pilot determined the response rate and effectiveness for the planned interventions for phase 2: HPV self-sampling, choice of two SSKs, NN, choice of SSK or NN, or timed appointments. Each intervention was compared with the control group using the GEE model. Any intervention found to be significant at a one-tailed 25% significance level (i.e. with an OR of an uptake between the intervention and the control larger than 1 with a p-value of < 0.25) would be taken forward into phase 2 of the main study.
Phase 2 analysis
As in phase 1, descriptive statistics in the form of incidence rates described the response to each intervention. Response rates for each intervention were compared with the control response rate, again using a GEE model under an ITT framework. As well as an overall Wald chi-squared test, pairwise tests were carried between each intervention and control. Consistent with the power calculation, a Bonferroni correction of significance levels is used to adjust for multiple testing, with a significant level of 1% in place of 5%. Baseline rates for each general practice used as covariates for the phase 2 analyses were calculated from the uptake rates observed in phase 1. The improved consistency and the direct relationship within general practice of baseline uptake rate in phase 2 compared with phase 1, means an interaction between site and baseline rate should no longer be required.
As the only date available for all women was the standard invitation date, follow-up assessments were timed relative to this. Analyses of phase 2 were carried out at 12 and 18 months after this date, with 12 months post call being the primary end point.
Phase 2 moderator analysis
Moderator analyses were carried out to test for:
-
An interaction effect between the intervention groups and the study location (Greater Manchester vs. Grampian), to investigate the differential screening age and socioeconomic differences between Scotland and the three north-west of England sites.
-
An interaction effect between phase 2 intervention groups and phase 1 intervention groups (pre-leaflet vs. control and online booking vs. control), to determine if a cumulative effect was occurring. The interaction analysis including online booking was performed in the Manchester PCT only.
-
The effect of vaccination status and subsequent interaction effects with phase 2 interventions (Grampian only).
-
Test result was modelled as a binary result (positive equals low or high dyskaryosis) to determine the impact any improved screening may have had on identifying cases.
Table 3 shows the intended comparisons of interventions for each cohort, including any suitable interactions (in bold). The table also outlines the follow-up time points for each comparison to be made. These refer to months since participants’ 25th (20th in Grampian) birthday, and hence allows for a further 6–8 weeks for pre-leaflet intervention.
| Comparisons (interaction)a | Comparison time points (since standard invitation) | |||
|---|---|---|---|---|
| 3 months | 6 months | 12 months | 18 monthsb | |
| Phase 1 | ||||
| Pre-leaflet vs. control | ✗ | ✗ | ✗ | |
| Online booking vs. control | ✗ | ✗ | ✗ | |
| Vaccinated vs. control (Grampian only) | ✗ | ✗ | ✗ | |
| Pre-leaflet (Greater Manchester vs. Grampian) | ✗ | ✗ | ✗ | |
| Pre-leaflet (vaccinated vs. control) | ✗ | ✗ | ✗ | |
| Online booking (pre-leaflet vs. control) | ✗ | ✗ | ✗ | |
| Pilot | ||||
| HPV SSK offer vs. control | ✗c | |||
| NN vs. control | ✗c | |||
| Choice vs. control | ✗c | |||
| Timed appointments vs. control | ✗c | |||
| HPV SSK sent vs. control | ✗c | |||
| Phase 2 | ||||
| HPV SSK offer vs. control | ✗ | ✗ | ||
| HPV SSK sent vs. control | ✗ | ✗ | ||
| NN vs. control | ✗ | ✗ | ||
| Choice vs. control | ✗ | ✗ | ||
| Timed appointments vs. control | ✗ | ✗ | ||
| Phase 2 intervention vs. site | ✗ | ✗ | ||
| Phase 2 intervention vs. phase 1 pre-leaflet | ✗ | ✗ | ||
| Phase 2 intervention vs. phase 1 online book | ✗ | ✗ | ||
| Phase 2 vs. vaccination status | ✗ | ✗ | ||
Results
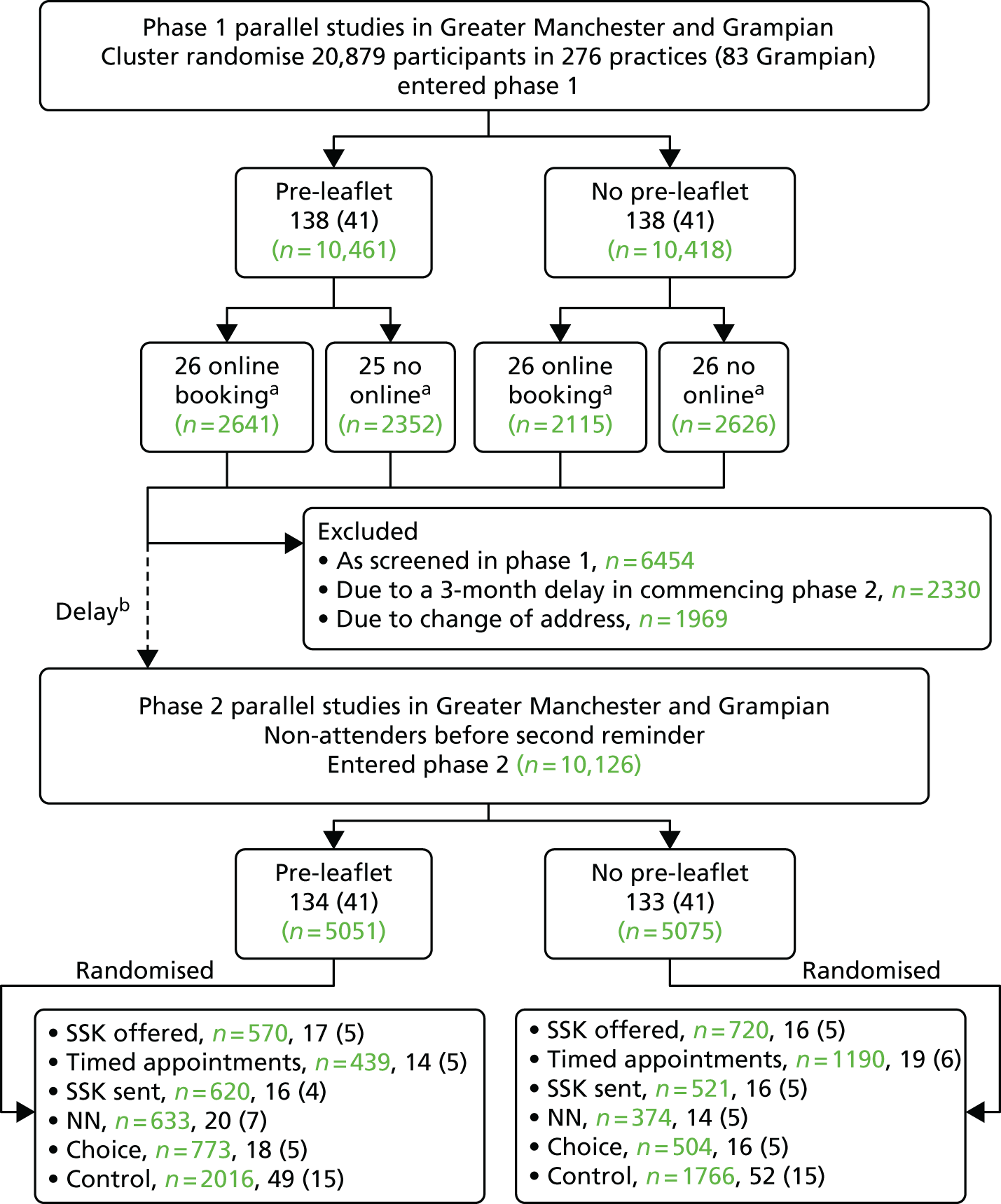
The participant flow diagram is shown in Figure 3. This shows both the number of practices and invited women for phase 1 and the number of practices and non-attenders for phase 2, by intervention. It should be noted that online booking involved just under half of the phase 1 cohort, as it was available only in Manchester.
FIGURE 3.
The Consolidated Standards of Reporting Trials (CONSORT) diagram for the STRATEGIC trial. Note that values in green refer to the number of women invited and numbers in black in brackets refer to the number of general practices. a, In Manchester PCT only; b, delay in starting phase 2 because of a delay in operationalising the interventions.
Phase 1
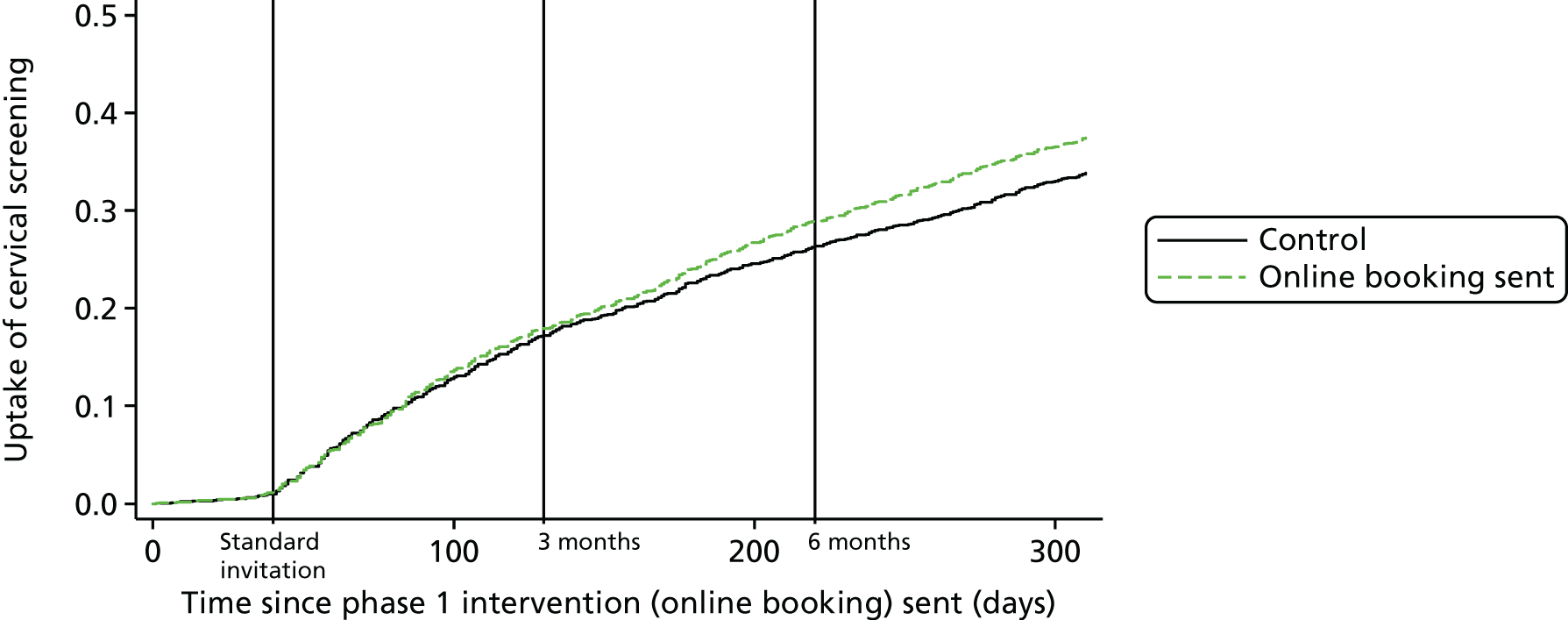
Table 4 shows the number of practices and corresponding number of participants randomised to each phase 1 intervention group. The screening uptake curves over time for the pre-leaflet and online booking, and those of the control practices, are shown in Figures 4 and 5, respectively. The actual percentage uptake at the 3- and 6-month time points following the standard invitation is shown in Table 5. The benefit of pre-leaflet is negligible. Although there was a slight benefit immediately after the standard invite, by 3 months there was no difference. In fact, up until 3 months, both interventions almost overlay the uptake in the controls. At 3 months, a routine reminder was sent out. Between 3 and 6 months the pre-leaflet arm continued to follow the controls, but there was a small increase in the online booking group. Also striking was the steady gradual increase in participation following the invitation and the intervention continuing following the standard reminder at 3 months.
| Study site | Intervention | Total | |
|---|---|---|---|
| Pre-leaflet | Control | ||
| Manchester | |||
| Online booking | 26 (2641) | 26 (2626) | 52 (5267) |
| Online booking control | 25 (2352) | 25 (2115) | 50 (4467) |
| Salford | 26 (1767) | 26 (1972) | 52 (3739) |
| Trafford | 20 (1303) | 19 (1097) | 39 (2400) |
| Total (north-west of England) | 97 (8063) | 96 (7810) | 193 (15,873) |
| Grampian | 41 (2398) | 42 (2608) | 83 (5006) |
| Total combined | 138 (10,461) | 138 (10,418) | 276 (20,879) |
FIGURE 4.
Kaplan–Meier plot showing time to test from standard invitation by pre-leaflet groups (7-month follow-up).
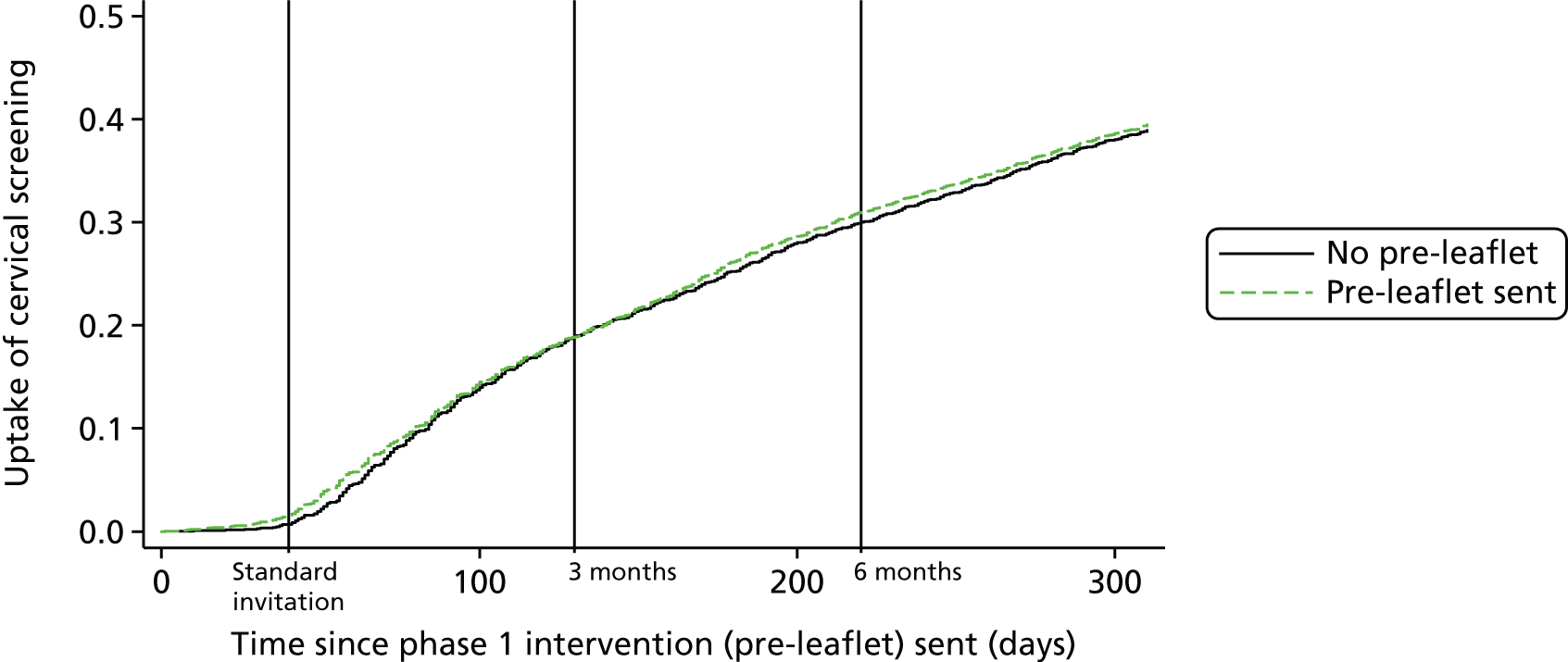
FIGURE 5.
Kaplan–Meier plot showing time to test since standard invitation by online booking groups: Manchester PCT only (7-month follow-up).
| Time | No, % (n/N) | Yes, % (n/N) | Total, % (n/N) | ORa (95% CI) | p-value | ICCb |
|---|---|---|---|---|---|---|
| Pre-leaflet (all sites) | ||||||
| Time from standard invitation | ||||||
| 3 months | 19.2 (2002/10,418) | 18.8 (1970/10,461) | 19.0 (3972/20,879) | 0.967 (0.879 to 1.062) | 0.485 | 0.0099 |
| 6 months | 30.6 (3191/10,418) | 31.1 (3256/10,461) | 30.9 (6447/20,879) | 1.014 (0.928 to 1.109) | 0.747 | 0.0157 |
| Online booking group: Manchester PCT only | ||||||
| Time between intervention and test | ||||||
| 3 months | 17.2 (770/4467) | 17.8 (936/5267) | 17.5 (1706/9734) | 1.021 (0.869 to 1.200) | 0.802 | 0.0090 |
| 6 months | 26.6 (1190/4467) | 28.8 (1518/5267) | 27.8 (2708/9734) | 1.097 (0.939 to 1.282) | 0.242 | 0.0194 |
Considering the percentage uptake (see Table 8), the first observation to be made is that by 3 months 19.2% of the control group had attended, and by 6 months this had increased to 30.6%. Uptake by women sent a pre-leaflet was almost identical at both time points (3 months, OR 0.967; 6 months, OR 1.014). Among the Manchester cohort, uptake by the control group was marginally lower, at 17.2% and 26.6% at 3 and 6 months, respectively. This is not surprising, as Manchester has poorer coverage than the country as a whole. The online booking group showed a very similar uptake by 3 months. Although uptake at 6 months was 2.2% higher, at 28.8%, in the online booking group, this difference was not statistically significant (p = 0.242). It should be noted that 199 women booked an appointment online, but only 127 (63.8%) actually attended that appointment. The absence of data linkage means that it is not possible to determine the precise proportion of women who attended an online-booked appointment before 3 or 6 months, as some of the online-booked appointments may have occurred after 6 months. It follows, therefore, that < 2.4% (127/5267) of women in this arm attended an appointment booked online by 6 months. The interaction between pre-leaflet and online booking at both 3 and 6 months was not significant (p-value is 0.604 at 3 months and 0.912 at 6 months), indicating no synergistic effect of pre-leaflet and online booking.
Table 6 compares, at 3 and 6 months, the overall uptake in Grampian and Greater Manchester. Both the proportion who attended at each location and the proportion attending at each vaccination level were calculated for subjects within the pre-leaflet group, the control group and in total. The OR relates to the baseline group: vaccination none and location Greater Manchester.
| Follow-up | Screening uptake, % (n screened/n invited) | Total rate, % (n screened/n invited) | ORa (95% CI) | p-value | ICCb | |
|---|---|---|---|---|---|---|
| Control | Pre-leaflet | |||||
| Location (all women) | ||||||
| 3 months | ||||||
| Greater Manchester | 19.3 (1503/7810) | 18.3 (1478/8063) | 18.8 (2981/15,873) | – | – | 0.043 |
| Grampian | 19.1 (499/2608) | 20.5 (492/2398) | 19.8 (991/5006) | 1.169 (1.030 to 1.326) | 0.016 | |
| Total | 19.2 (2002/10,418) | 18.8 (1970/10,461) | 19.0 (3972/20,879) | – | – | |
| 6 months | ||||||
| Greater Manchester | 30.1 (2351/7810) | 30.1 (2423/8063) | 30.1 (4774/15,873) | – | – | 0.066 |
| Grampian | 32.2 (840/2608) | 34.8 (833/2398) | 33.5 (1673/5006) | 1.275 (1.133 to 1.435) | < 0.001 | |
| Total | 30.6 (3191/10,418) | 31.1 (3256/10,461) | 30.9 (6447/20,879) | – | – | |
| Vaccination status (Grampian only) | ||||||
| 3 months | ||||||
| None | 9.8 (69/708) | 12.5 (76/607) | 11.0 (145/1315) | – | – | 0.015 |
| Incomplete | 20.1 (30/149) | 16.7 (29/174) | 18.3 (59/323) | 1.404 (0.1030 to 1.914) | 0.032 | |
| Full | 23.1 (398/1724) | 24.2 (383/1583) | 23.7 (781/3308) | 2.074 (1.698 to 2.534) | < 0.001 | |
| Missing | 7.4 (2/27) | 11.8 (4/34) | 9.8 (6/61) | 0.760 (0.402 to 1.438) | 0.399 | |
| Total | 19.1 (499/2608) | 20.5 (492/2398) | 19.8 (991/5007) | – | – | |
| 6 months | ||||||
| None | 16.4 (116/708) | 20.3 (123/607) | 18.2 (239/1315) | – | – | 0.007 |
| Incomplete | 30.9 (46/149) | 29.3 (51/174) | 30.0 (97/323) | 1.555 (1.213 to 1.992) | 0.001 | |
| Full | 39.1 (674/1724) | 41.2 (651/1583) | 40.9 (1325/3308) | 2.571 (2.205 to 2.999) | < 0.001 | |
| Missing | 14.8 (4/27) | 23.5 (8/34) | 19.7 (12/61) | 0.974 (0.541 to 1.754) | 0.93 | |
| Total | 32.2 (840/2608) | 34.8 (833/2398) | 33.5 (1673/5006) | – | – | |
Absolute attendance was similar over time in Greater Manchester and Grampian, although with a slightly greater absolute percentage uptake in Grampian, by 1% and 3.4% at 3 and 6 months, respectively. The ORs of a test for Grampian compared with Greater Manchester were 1.169 [95% confidence interval (CI) 1.030 to 1.326; p = 0.016], and 1.275 (95% CI 1.133 to 1.435; p < 0.001) at 3 and 6 months, respectively. Interaction effects between pre-leaflet and location were found to be not statistically significant, indicating that the effect of location was consistent across intervention groups (a p-value of 0.591 at 3 months and of 0.542 at 6 months).
Table 6 also gives the uptake of screening associated with the level of completeness of vaccination in Grampian. Estimated ORs are given comparing those with ‘full’, ‘incomplete’ or ‘missing’ vaccination status compared with ‘none’. The absolute increase in attendance between ‘none’ and ‘complete’ was 12.62% (23.65 – 11.03%) and 21.96% at 3 and 6 months, respectively. With an approximate doubling of the attendance rate, the ORs for ‘complete’ compared with ‘none’ were 2.074 and 2.571, with both being statistically significant (p < 0.001). Those who were partially vaccinated, that is, with fewer than the three doses, had a smaller but still significant increase in uptake. The three degrees of freedom chi-squared interaction test again indicated that no interaction effect was present between the pre-leaflet group and vaccination status (χ32 p = 0.828 at 3 months and χ32 p = 0.870 at 6 months).
The pilot study of proposed phase 2 interventions
A total of 720 women were targeted, with 120 being offered each of the intervention options, and there was a control group. The piloting required 2–3 months to offer the interventions, 3–4 months of follow-up, and 2 months for analysis.
The piloting interventions were offered as of April 2012 for 3 months; follow-up began in July 2012 for 4 months, ending in November 2012.
The analysis replicated the main study, with the outcome being the performance of a test within 6 months following sent date, compared across interventions. If the cytology date was prior to the intervention, those women were removed from the analysis, as they were duplicates. The following analysis is based on 714 women out of the original 720. The sample sizes per intervention across the north-west of England are summarised in Table 7.
| Intervention | Study site | Total number of women | ||
|---|---|---|---|---|
| Salford | Trafford | Manchester | ||
| Control | 42 (35.3) | 14 (11.8) | 63 (52.9) | 119 |
| Choice | 17 (14.2) | 40 (33.3) | 63 (52.5) | 120 |
| NN | 49 (41.9) | 16 (13.7) | 52 (44.4) | 117 |
| SSK offered | 33 (28) | 17 (14.4) | 68 (57.6) | 118 |
| SSK sent | 22 (18.3) | 34 (28.3) | 64 (53.3) | 120 |
| Timed appointments | 38 (31.7) | 20 (16.7) | 62 (51.7) | 120 |
| Total | 201 (28.2) | 141 (19.8) | 372 (52.1) | 714 |
The pilot demonstrated that each of the interventions could be delivered successfully. The uptake of screening following the different interventions is shown in Table 8. Of the 119 control subjects, 14 (11.8%) attended screening. The greatest rates of uptake were achieved by offering timed appointments (22.5%) and a choice of interventions (18.3%), with the NN intervention actually resulting in a decrease in uptake (7.7%) compared with controls. The effect was striking even in this small sample of women, with 21 out of 22 participants offered a choice actually attending for a routine cytology sample, as did 8 out of 20 women who were sent a SSK. This suggests a ‘nudge’ effect being exerted by these interventions, whereby the intervention indirectly influences the woman to attend for screening.
| Intervention | Test, n (%) | Total number of participants in intervention | ||
|---|---|---|---|---|
| Cytology | HPV | Any | ||
| Control | 14 (11.8) | – | 14 (11.8) | 119 |
| Choice | 21 (17.5) | 2 (1.7) | 22 (18.3) | 120 |
| NN | 9 (7.7) | – | 9 (7.7) | 117 |
| SSK offer | 11 (9.3) | 2 (1.7) | 13 (11) | 118 |
| SSK sent | 8 (6.7) | 13 (10.8) | 20 (16.7) | 120 |
| Timed appointments | 27 (22.5) | – | 27 (22.5) | 120 |
| Total | 90 (12.6) | 17 (2.4) | 105 (14.7) | 714 |
Table 9 reports the OR for each intervention compared with the control group, along with both the 95% and 50% CIs. The 50% CIs are given as the lower limit corresponding to one-tailed 25% significance level test. Timed appointments resulted in a significant increase in uptake, with the odds of any test being administered being almost 2.5 times that of the control group. Choice (1.75) and SSK sent also resulted an increase in uptake, which in both cases was significant at the 25% level. As suggested in Table 8, the NN and offer of a SSK showed a decrease in test uptake, with the NN intervention showing a significant decrease at the 25% level.
| Intervention | OR | p-value (two-tailed) | p-value (one-tailed) | 95% CI | 50% CI |
|---|---|---|---|---|---|
| Control | – | – | – | – | – |
| Choice | 1.75 | 0.177 | 0.089 | 0.78 to 3.95 | 1.32 to 2.31 |
| NN | 0.66 | 0.403 | 0.800 | 0.25 to 1.73 | 0.48 to 0.92 |
| SSK offer | 0.87 | 0.774 | 0.614 | 0.35 to 2.16 | 0.64 to 1.20 |
| SSK sent | 1.67 | 0.231 | 0.115 | 0.72 to 3.87 | 1.25 to 2.23 |
| Timed appointments | 2.37 | 0.034 | 0.017 | 1.07 to 5.29 | 1.80 to 3.13 |
Even among the small sample in this pilot study, timed appointments appeared to result in a significant increase in attendance, along with SSK sent and being offered a choice. The combined effect of both SSK offer and NN appeared to result in a strong increase in attendance, even though each individual intervention resulted in a decrease in attendance. Because choice required both NN and an offer of a SSK, the independent Trial Steering Committee advised that all of the piloted interventions should be offered in phase 2.
Phase 2
Each practice was randomly allocated to one of the five phase 2 interventions or to the control group, again using a cluster randomisation that employed a minimisation algorithm to balance each intervention arm. Study participants were excluded from receiving a phase 2 intervention if they had attended screening or moved practices prior to the phase 2 intervention.
Figure 6 repeats the lower half of the STRATEGIC Consolidated Standards of Reporting Trials (CONSORT) diagram. Of the 20,879 participants who entered the study and participated in phase 1, 2330 were not eligible for phase 2 because of a 3-month delay in implementing the phase 2 interventions. A further 6454 attended for cytology screening within 7.5 months of their standard invitation and a further 1969 moved house or GP also within 7.5 months since their standard invitation was sent. This left 10,126 eligible to participate in phase 2.
FIGURE 6.
The CONSORT diagram for phase 2. Note: black numbers in brackets refer to the number of general practices invited during phase 1. a, Delay in starting phase 2 because of a delay in operationalising the interventions.

Phase 2 results
Table 10 shows the number of practices randomised to each phase 2 intervention. Nine practices were not randomised to phase 2 (leaving 269 practices) because all eligible participants in these practices had been screened, and a further two were effectively lost from phase 2 as all remaining eligible women had moved. This left the 267 practices shown in Table 10, which was still enough for 30 practices for each intervention and 100 control practices, as required to achieve adequate statistical power. It should also be noted that the original power calculation had estimated that 50% of the phase 1 participants would be available as ‘non-attenders’ in phase 2, and, despite the loss of almost 10% of the STRATEGIC study cohort because of a delay in initiating phase 2, 10,126 out of 20,879 (48.5%) participants were available for phase 2. Of those entering phase 2, the median [interquartile range (IQR)] follow-up without attendance was 727 days (IQR 630–860 days) [i.e. 24.2 months (IQR 21–28.6 months)]. Note that the minimum follow-up without attendance was 17.8 months, indicating all but 62 (who were within a few days) were followed for a minimum of 18 months. Those 62 were assumed to have not attended during the last few days and were included in the 18-month follow-up outcome. Of those who did attend screening, the median time to attendance was 358 days (IQR 267–500 days) [i.e. 11.9 months (IQR 8.9–16.7 months)] since standard invitation or 4.4 months (IQR 1.9–9.4 months) since phase 2 interventions were sent.
| PCT | Phase and intervention | Total, n practices (n subjects) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2, n practices (n subjects) | ||||||||
| Pre-leaflet group | Online booking group | SSK sent | SSK offered | NN | Timed appointment | Choice | Control | ||
| Greater Manchester | Yes | Yes | 3 (120) | 4 (109) | 3 (98) | 2 (57) | 4 (294) | 8 (474) | 24 (1152) |
| Yes | No | 3 (196) | 3 (151) | 4 (95) | 2 (72) | 3 (143) | 9 (385) | 24 (1042) | |
| No | Yes | 4 (164) | 3 (135) | 3 (88) | 3 (134) | 4 (175) | 9 (476) | 26 (1172) | |
| No | No | 2 (89) | 3 (204) | 2 (57) | 4 (104) | 3 (139) | 10 (388) | 24 (981) | |
| Manchester total | 12 (569) | 13 (599) | 12 (338) | 11 (367) | 14 (751) | 36 (1723) | 98 (4347) | ||
| Salford | No | – | 3 (51) | 3 (111) | 2 (64) | 4 (283) | 2 (63) | 9 (313) | 23 (885) |
| Yes | – | 3 (132) | 3 (84) | 4 (139) | 3 (62) | 4 (198) | 9 (239) | 26 (854) | |
| Salford total | 6 (183) | 6 (195) | 6 (203) | 7 (345) | 6 (261) | 18 (552) | 49 (1739) | ||
| Trafford | No | – | 2 (53) | 2 (29) | 2 (46) | 2 (80) | 2 (26) | 9 (221) | 19 (455) |
| Yes | – | 3 (58) | 2 (43) | 2 (35) | 2 (77) | 2 (42) | 8 (331) | 19 (586) | |
| Trafford total | 5 (111) | 4 (72) | 4 (81) | 4 (157) | 4 (68) | 17 (552) | 38 (1041) | ||
| Greater Manchester total | 23 (863) | 23 (866) | 22 (622) | 22 (869) | 24 (1080) | 71 (2827) | 185 (7127) | ||
| Grampian | No | – | 5 (164) | 5 (241) | 5 (119) | 6 (589) | 5 (101) | 15 (368) | 41 (1582) |
| Yes | – | 4 (114) | 5 (183) | 7 (266) | 5 (171) | 5 (96) | 15 (587) | 41 (1417) | |
| Grampian total | 9 (278) | 10 (424) | 12 (385) | 11 (760) | 10 (197) | 30 (955) | 82 (2999) | ||
| Overall total | 32 (1141) | 33 (1290) | 34 (1007) | 33 (1629) | 34 (1277) | 101 (3782) | 267 (10,126) | ||
The type of screening test performed in phase 2 participants (i.e. HPV test only, cytology only or both, in which case in which order) is shown in Table 11. The result of the HPV test and whether or not the participant went on to have a cytology test, is shown in the lower part of Table 11. Of the 122 women tested for HPV, 33 tested positive, of whom 20 (60%) then went on to have a cytology test. It is interesting that a large proportion of those screened following self-sampling interventions (i.e. sent or offered as part of a choice) did, in fact, have a practitioner-obtained cytology sample. For example, of the 1149 women who were sent a HPV SSK, 292 (21.3%) were screened in phase 2, although 198 of these women (67%) underwent only a cytology test. Of the 279 women who were screened after being offered a SSK, only 21 had a HPV test, the rest having cytology only. Only 7 of the 314 participants screened after being offered a choice, which included SSK, actually had a HPV test.
| Follow-up | Phase 2 intervention | No test | Type of test | Total number of participants tested | Overall total number of participants in intervention | |||
|---|---|---|---|---|---|---|---|---|
| Single | Both | |||||||
| HPV only | Cytology only | HPV first | Cytology first | |||||
| 12 months | Control | 3169 | 1 | 612 | – | – | 613 | 3782 |
| SSK sent | 898 | 52 | 158 | 32 | 1 | 243 | 1141 | |
| SSK offered | 1081 | 12 | 190 | 7 | – | 209 | 1290 | |
| NN | 861 | – | 145 | 1 | – | 146 | 1007 | |
| Timed appointment | 1306 | – | 323 | – | – | 323 | 1629 | |
| Choice | 1037 | 5 | 233 | 2 | – | 240 | 1277 | |
| Total | 8352 | 70 | 1661 | 42 | 1 | 1774 | 10,126 | |
| 18 months | Control | 2756 | 1 | 1025 | – | – | 1026 | 3782 |
| SSK sent | 799 | 59 | 248 | 34 | 1 | 342 | 1141 | |
| SSK offered | 957 | 12 | 314 | 7 | – | 333 | 1290 | |
| NN | 777 | – | 229 | 1 | – | 230 | 1007 | |
| Timed appointment | 1157 | 1 | 471 | – | – | 472 | 1629 | |
| Choice | 892 | 5 | 378 | 2 | – | 385 | 1277 | |
| Total | 7338 | 78 | 2665 | 44 | 1 | 2788 | 10,126 | |
| HPV result | ||||||||
| 12 months | 8352 | – | 1661 | – | – | 1661 | 10,013 | |
| Invalid | – | 1 | – | – | – | 1 | 1 | |
| Negative | – | 63 | – | 19 | 1 | 83 | 83 | |
| Positive | – | 6 | – | 23 | – | 29 | 29 | |
| Total | 8352 | 70 | 1661 | 42 | 1 | 1774 | 10,126 | |
| 18 months | 7338 | 2 | 2665 | – | – | 2667 | 10,005 | |
| Invalid | – | 1 | – | – | – | 1 | 1 | |
| Negative | – | 67 | – | 19 | 1 | 87 | 87 | |
| Positive | – | 8 | – | 25 | – | 33 | 33 | |
| Total | 7338 | 78 | 2665 | 44 | 1 | 2788 | 10,126 | |
Figure 7 and Table 12 illustrate the proportion of non-attenders in phase 2 who had any screening test (cytology or HPV) associated with each intervention at 12 and 18 months since the standard invitation. Figure 7, a Kaplan–Meier plot, indicates a differential rate of uptake over time since the standard invite for each of the intervention groups. As this cohort did not attend before the first reminder, all curves are flat for the first 6 months. By 12 months post standard invitation (approximately 6 months of phase 2) between 14.5% and 21% of non-attenders had been screened; 6 months later the proportion had reached between 22.8% and 30.1%. The control group demonstrated a similar trend with 16% and 27% of non-attenders in the control group screened at the same time points.
FIGURE 7.
Kaplan–Meier plot showing time to test since standard intervention for women eligible for phase 2 interventions (non-responders at 6 months).

| Phase 2 intervention | Attendance, % (n screened/n invited) | ORa | 95% CI | p-value | ICCb |
|---|---|---|---|---|---|
| 12 months (4.5 months since the start of phase 2 intervention) | |||||
| Control | 16.2 (613/3782) | – | – | – | 0.0083 |
| SSK sent | 21.3 (243/1141) | 1.512 | 1.197 to 1.910 | 0.001 | |
| SSK offered | 16.2 (209/1290) | 1.074 | 0.871 to 1.325 | 0.505 | |
| NN | 14.5 (146/1007) | 0.887 | 0.670 to 1.174 | 0.401 | |
| Timed appointment | 19.8 (323/1629) | 1.408 | 1.141 to 1.738 | 0.001 | |
| Choice | 18.8 (240/1277) | 1.091 | 0.864 to 1.378 | 0.466 | |
| Total (χ52) | 17.5 (1774/10,126) | – | – | < 0.001c | – |
| 18 months (10.5 months since the start of phase 2 intervention) | |||||
| Control | 27.1 (1026/3782) | – | – | – | 0.0211 |
| SSK sent | 30.0 (342/1141) | 1.286 | 1.056 to 1.567 | 0.012 | |
| SSK offered | 25.8 (333/1290) | 1.056 | 0.884 to 1.262 | 0.548 | |
| NN | 22.8 (230/1007) | 0.799 | 0.642 to 0.994 | 0.044 | |
| Timed appointment | 29.0 (472/1629) | 1.191 | 0.975 to 1.456 | 0.087 | |
| Choice | 30.2 (385/1277) | 1.058 | 0.869 to 1.289 | 0.573 | |
| Total (χ52) | 27.5 (2788/10,126) | – | – | 0.008c | – |
Table 12 provides the cumulative uptake following each phase 2 intervention at each time point as a proportion along with their corresponding OR (95% CI) and p-value with respect to the control group. A chi-squared test with 5 degrees of freedom suggests that there was a difference between the six study arms at 12 (p < 0.001) and 18 months (p = 0.008) following the standard invitation. Owing to the multi-intervention study design, the study protocol and power calculation specified a Bonferroni correction of significance levels to adjust for multiple testing with a significant level of 1% in place of 5%. The odds of attending increased significantly (p-value = 0.001) for SSK sent (OR 1.512) and timed appointments (OR 1.408) compared with control at 12 months post standard invitation. Although both continued to suggest an increase, only SSK sent (OR 1.286) was statistically significant (p = 0.012) at 18 months. Of the remaining interventions, SSK offered and choice both resulted in a slight, but non-significant, increase in uptake of any test. The NN intervention actually resulted in a non-significant reduction in uptake at 12 months (OR 0.887), which became a statistically significant reduction at 5% significance but not at the Bonferroni-corrected 1% level at 18 months (OR 0.799; p = 0.044). Table 12 also gives the result of a comparison test of the five interventions. The null hypothesis states that there is no difference between the five intervention effects, here p < 0.01 indicates that the null hypothesis is rejected and that there are significant differences between the five interventions at both 12 and 18 months.
Phase 2 planned moderator and predictor analyses
A series of moderator analyses were specified in the statistical analysis plan. By adding a factor by phase 2 treatment interaction, we examined the moderating effect of phase 1 intervention (intervention pre-leaflet vs. control and online booking vs. control), location (Greater Manchester vs. Grampian) and, in Grampian only, prior vaccination status (none, incomplete and complete). The predictive effect of these factors was considered using a main-effects model without interaction terms. There was no evidence that the pre-leaflet intervention moderated the effect of phase 2 interventions at either 12 (χ52 p = 0.740) or 18 months (χ52 p = 0.216) since the standard invitation (see Appendix 7, Table 43). There was no effect of the pre-leaflet during the phase 2 intervention period at either 12 (p = 0.760) or 18 months (p = 0.875) post call, see Appendix 7, Table 43). Similarly, there was no evidence that the online booking intervention moderated the effect of phase 2 interventions at either 12 (χ52 p = 0.594) or 18 months (χ52 p = 0.321) post call (see Appendix 7, Table 44), nor was there any effect of online booking at 12 (p = 0812) or 18 months (p = 0.263). When the effect of location was considered (Greater Manchester vs. Grampian), there was no effect on phase 2 interventions at 12 months post call (χ52 p = 0. 141), but this was significant at a 5% level at 18 months (χ52 p = 0.020) (Table 13). Inspection of the pairwise interaction of each phase 2 intervention against control suggests that the effect of the NN intervention differed between Grampian and Greater Manchester compared with other interventions. The negative effect of this intervention at 18 months (see Table 12) appeared to be moderated in Grampian. Figures 8 and 9 show the uptake rate at 12 and 18 months post call by intervention and location. With the exception of the NN intervention, we see that the uptake rate is higher in Greater Manchester than in Grampian, whereas the NN intervention attendance was higher in Grampian than in Greater Manchester. When a model was fitted without the interaction terms (see Table 13), there was evidence of an overall higher uptake in Greater Manchester than Grampian during phase 2 at both 12 (p < 0.001) and 18 months (p < 0.001) post call. This contrasts with the equivalent analysis during phase 1, which found that uptake in Grampian was higher than in Greater Manchester (see Table 6).
| Variable | Comparison | Time point | |||||
|---|---|---|---|---|---|---|---|
| 12 months | 18 months | ||||||
| ORa | 95% CI | p-value | ORa | 95% CI | p-value | ||
| Interaction model | |||||||
| Phase 2 (Greater Manchester only) | SSK sent vs. control | 1.52 | 1.163 to 1.986 | 0.002 | 1.247 | 0.988 to 1.574 | 0.064 |
| SSK offered vs. control | 1.103 | 0.854 to 1.424 | 0.454 | 1.111 | 0.895 to 1.378 | 0.34 | |
| NN vs. control | 0.779 | 0.548 to 1.107 | 0.164 | 0.684 | 0.523 to 0.894 | 0.006 | |
| Timed appointment vs. control | 1.420 | 1.099 to 1.835 | 0.007 | 1.108 | 0.892 to 1.376 | 0.355 | |
| Choice vs. control | 1.184 | 0.914 to 1.535 | 0.201 | 1.085 | 0.876 to 1.343 | 0.456 | |
| Location | Grampian vs. Greater Manchester | 0.711 | 0.560 to 0.902 | 0.005 | 0.59 | 0.470 to 0.740 | < 0.001 |
| Interaction | Location × SSK sent | 0.977 | 0.583 to 1.637 | 0.928 | 1.097 | 0.721 to 1.670 | 0.664 |
| Location × SSK offered | 0.910 | 0.568 to 1.460 | 0.697 | 0.796 | 0.538 to 1.180 | 0.256 | |
| Location × NN | 1.473 | 0.859 to 2.524 | 0.159 | 1.636 | 1.104 to 2.425 | 0.014 | |
| Location × timed appointment | 1.000 | 0.649 to 1.541 | 0.999 | 1.310 | 0.866 to 1.980 | 0.201 | |
| Location × choice | 0.573 | 0.347 to 0.946 | 0.029 | 0.791 | 0.491 to 1.276 | 0.337 | |
| Interaction test | 0.141 | 0.02 | |||||
| 6-month rate | 1.028 | 1.021 to 1.035 | < 0.001 | 1.031 | 1.024 to 1.037 | < 0.001 | |
| ICCb | 0.0082 | 0.0158 | |||||
| Main-effects model | |||||||
| Phase 2 | SSK sent vs. control | 1.460 | 1.116 to 1.909 | 0.006 | 1.222 | 0.957 to 1.559 | 0.107 |
| SSK offered vs. control | 1.036 | 0.803 to 1.336 | 0.787 | 1.003 | 0.795 to 1.266 | 0.979 | |
| NN vs. control | 0.868 | 0.647 to 1.163 | 0.343 | 0.772 | 0.613 to 0.973 | 0.028 | |
| Timed appointment vs. control | 1.425 | 1.120 to 1.812 | 0.004 | 1.231 | 0.988 to 1.535 | 0.065 | |
| Choice vs. control | 1.103 | 0.831 to 1.466 | 0.497 | 1.069 | 0.841 to 1.360 | 0.585 | |
| Location | Grampian vs. Greater Manchester | 0.723 | 0.611 to 0.855 | < 0.001 | 0.665 | 0.578 to 0.765 | < 0.001 |
| ICCb | 0.021 | 0.027 | |||||
FIGURE 8.
Percentage attendance at 12 months for phase 2 interventions split by location. GM, Greater Manchester; GR, Grampian.
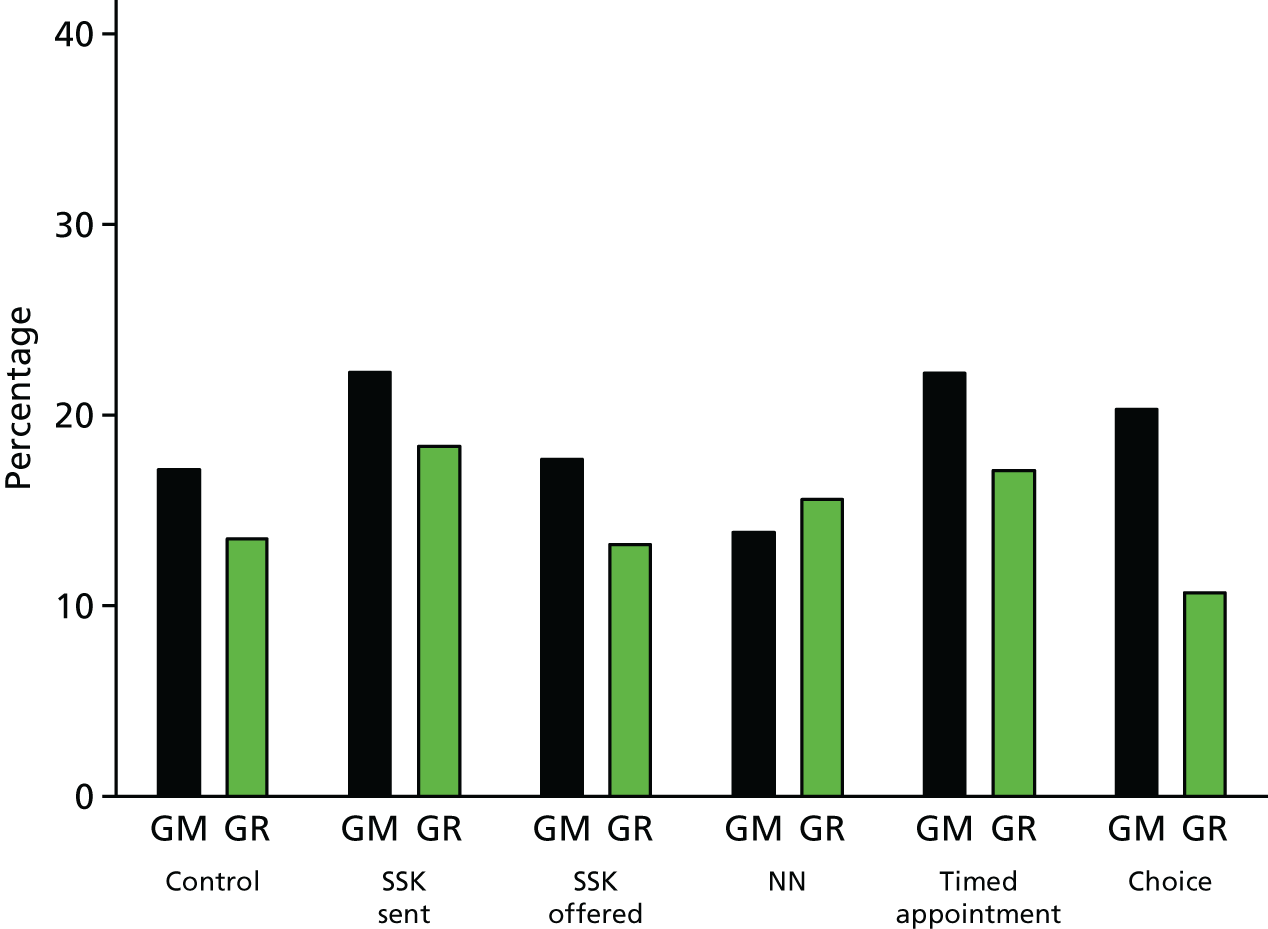
FIGURE 9.
Percentage attendance at 18 months for phase 2 interventions split by location. GM, Greater Manchester; GR, Grampian.

Finally, we considered the relation of vaccination to uptake during phase 2. There was no evidence that intervention effects were moderated at either 12 (χ52 p = 0.147) or 18 months (χ102 p = 0.122) post call (see Appendix 7, Table 45). As with the analysis of phase 2, both ‘incomplete’ and ‘complete’ vaccination status were associated with improved uptake at 12 and 18 months (see Appendix 7, Table 45). As with phase 1, those participants who had experienced some form of vaccination compared with those with none were approximately twice as likely to attend screening in phase 2, with ORs of 2.109 and 2.169 for incomplete and complete, respectively, at 12 months post call, with both effects being statistically significant (p < 0.001).
Adherence with randomisation during phase 2
In both phase 1 and 2, interventions were randomised to practices. The analyses presented above have been by ITT, irrespective of whether or not the intended intervention was actually delivered. The data available do not allow us to determine if a participant could receive or actually received the intended intervention. For example, some women may have changed address, but this information could not be provided. However, the date at which a phase 2 intervention was sent was recorded by the screening agencies for each type of intervention for each woman. These data are summarised in Table 14, by intervention. The column Correct date refers to the number of women recorded as having been sent the randomly allocated intervention for the practice with which they were registered. The column Incorrect date gives the number recorded as having been sent the incorrect intervention for their practice. The percentage of women sent the incorrect intervention ranged from 0.2% for NN to 3.8% for SSK sent. Among the control group, 1.3% received a phase 2 intervention in error. For the timed appointments intervention, 27.6% (449/1629) had no date recorded, largely because one large practice was unable to implement timed appointments. For the other four interventions, between 83% and 89% of women appear to have been sent the correct intervention.
| Phase 2 intervention | Status of phase 2 intervention date, n (%) | Overall total number of participants in intervention | ||
|---|---|---|---|---|
| No date | Correct date | Incorrect date | ||
| Control | 3734 (98.7) | 0 (0) | 48 (1.3) | 3782 |
| SSK sent | 152 (13.3) | 954 (83.6) | 35 (3.1) | 1141 |
| SSK offered | 169 (13.1) | 1072 (83.1) | 49 (3.8) | 1290 |
| NN | 144 (14.3) | 861 (85.5) | 2 (0.2) | 1007 |
| Timed appointments | 449 (27.6) | 1174 (72.1) | 6 (0.4) | 1629 |
| Choice | 136 (10.6) | 1137 (89) | 4 (0.3) | 1277 |
Impact of phase 1 and phase 2 interventions on detection of low- and high-grade cytology
The cervical screening result was available for women who attended for a test. Table 15 gives the rates of women invited by the standard invitation who were tested and found to have low- or high-grade cytology overall, and broken down by phase 1 intervention group and follow-up assessment point. Note that the rates are based on numbers of women randomised and not numbers tested. After 3 months’ follow-up the percentage of women invited for screening and found to have cytology of ‘low grade or above’ or ‘high grade’ was 4.24% and 0.44%, respectively. By 6 months’ follow-up the percentage of women invited for screening and found to have cytology of ‘low grade or above’ or ‘high grade’ cytology was 7.82% and 0.64%, respectively. Rates are given broken down according to intervention. Adjusted ORs were estimated using the same methods and covariates as the analysis of screening uptake. Neither pre-leaflet nor online booking had a significant effect on a low grade or above outcome, or high grade, which is to be expected, as neither intervention had an effect on uptake. The largest, although still non-significant, effect (p = 0.130) occurred for online booking at 6 months (OR 1.57, 95% CI 0.88 to 2.80) for high-grade cytology. It should also be noted that the CIs are now rather wider, which is an indication that the trial is underpowered for plausible effect sizes for these outcomes.
| Interval since standard invitation | Grade | Overall total number of participants in intervention | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low or above cytology | High cytology | ||||||||||
| n (%) | ORa | 95% CI | p-value | ICCb | n (%) | ORa | 95% CI | p-value | ICCb | ||
| Pre-leaflet | |||||||||||
| 3 months | |||||||||||
| No | 456 (4.38) | – | – | – | 0.002 | 47 (0.45) | – | – | – | 0.001 | 10,418 |
| Yes | 430 (4.11) | 0.986 | 0.851 to 1.144 | 0.855 | 45 (0.43) | 0.989 | 0.648 to 1.511 | 0.961 | 10,461 | ||
| Total | 886 (4.24) | – | – | – | 92 (0.44) | – | – | – | 20,879 | ||
| 6 months | |||||||||||
| No | 803 (7.71) | – | – | – | 0.004 | – | – | – | 0.001 | 10,418 | |
| Yes | 830 (7.94) | 1.065 | 0.942 to 1.205 | 0.316 | 1.218 | 0.845 to 1.756 | 0.290 | 10,461 | |||
| Total | 1633 (7.82) | – | – | – | – | – | – | 20,879 | |||
| Online booking | |||||||||||
| 3 months | |||||||||||
| No | 251 (5.62) | – | – | – | 0.003 | – | – | – | 0.000 | 4467 | |
| Yes | 293 (5.57) | 0.969 | 0.781 to 1.202 | 0.774 | 1.170 | 0.597 to 2.293 | 0.647 | 5267 | |||
| Total | 544 (5.59) | – | – | – | – | – | – | 9734 | |||
| 6 months | |||||||||||
| No | 401 (8.98) | – | – | – | 0.005 | – | – | – | 0.001 | 4467 | |
| Yes | 516 (9.80) | 1.071 | 0.885 to 1.298 | 0.481 | 1.565 | 0.877 to 2.795 | 0.130 | 5267 | |||
| Total | 917 (9.42) | – | – | – | – | – | – | 9734 | |||
Table 16 gives the corresponding result by phase 2 interventions. Of this cohort, 5.1% and 7.6% were detected as having low grade or above at 12 and 18 months post call, with 0.3% and 0.5% of the cohort being high grade. At 12 months post call the intervention groups differed in the proportion of low-grade cytology detected (p = 0.043). Of the phase 2 interventions, timed appointments showed a significant increase (p = 0.001) in low grade or above (OR 1.57, 95% CI 1.21 to 2.03) at 12 months that persisted with a borderline significant increase at 18 months (OR 1.24, 95% CI 0.98 to 1.57; p = 0.074). SSKs sent showed a borderline increase at 12 months (p = 0.065). Inference from these data should be viewed with caution because of the possibility of multiple testing artefacts. The significant increase because of timed appointment, and to a lesser extent for SSK sent, nevertheless reflects the increased uptake rates of these interventions (see Table 12).
| Phase 2 intervention and time point | Grade | Overall total number of participants in intervention | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low or above | High | ||||||||||
| n (%) | ORa | 95% CI | p-value | ICCb | n (%) | ORa | 95% CI | p-value | ICCb | ||
| 12 months | |||||||||||
| Control | 177 (4.68) | – | – | – | 0.002 | 11 (0.29) | – | – | – | 0.002 | 3782 |
| SSK sent | 68 (5.96) | 1.345 | 0.981 to 1.844 | 0.065 | 5 (0.44) | 1.543 | 0.464 to 5.125 | 0.479 | 1141 | ||
| SSK offered | 65 (5.04) | 1.171 | 0.871 to 1.574 | 0.297 | 5 (0.39) | 1.427 | 0.505 to 4.029 | 0.502 | 1290 | ||
| NN | 44 (4.37) | 1.027 | 0.671 to 1.57 | 0.904 | 2 (0.20) | 0.649 | 0.143 to 2.954 | 0.576 | 1007 | ||
| Timed appointment | 93 (5.71) | 1.570 | 1.211 to 2.034 | 0.001 | 4 (0.25) | 0.819 | 0.225 to 2.981 | 0.762 | 1629 | ||
| Choice | 66 (5.17) | 0.967 | 0.726 to 1.287 | 0.816 | 3 (0.23) | 0.809 | 0.212 to 3.086 | 0.756 | 1277 | ||
| Total (χ52) | 513 (5.07) | – | – | 0.043 | 30 (0.30) | – | – | 0.757 | 10,126 | ||
| 18 months | |||||||||||
| Control | 290 (7.67) | – | – | – | 0.004 | 24 (0.63) | – | – | – | 0.003 | 3782 |
| SSK sent | 88 (7.71) | 1.048 | 0.816 to 1.346 | 0.714 | 5 (0.44) | 0.723 | 0.239 to 2.188 | 0.565 | 1141 | ||
| SSK offered | 101 (7.83) | 1.126 | 0.912 to 1.392 | 0.270 | 9 (0.70) | 1.160 | 0.539 to 2.495 | 0.704 | 1290 | ||
| NN | 70 (6.95) | 0.996 | 0.752 to 1.319 | 0.977 | 4 (0.40) | 0.619 | 0.217 to 1.765 | 0.370 | 1007 | ||
| Timed appointment | 126 (7.73) | 1.239 | 0.980 to 1.568 | 0.074 | 6 (0.37) | 0.612 | 0.224 to 1.671 | 0.338 | 1629 | ||
| Choice | 97 (7.60) | 0.868 | 0.681 to 1.106 | 0.253 | 6 (0.47) | 0.752 | 0.266 to 2.120 | 0.589 | 1277 | ||
| Total (χ52) | 772 (7.62) | – | – | 0.170 | 54 (0.53) | – | – | 0.727 | 10,126 | ||
Conclusions
Compared with control, no evidence was found to suggest a statistically significant effect for either of the phase 1 interventions: pre-leaflet or online booking. With pre-leaflet only a slight increase in attendance was observed 6 months after the standard invitation. Online booking did indicate an increase in attendance from 3 months that continued out to 6 months. Vaccination status appears to have the largest (and most significant) influence on screening attendance, with fully vaccinated subjects approximately twice as likely to attend screening as unvaccinated women, who had very low participation.
The results indicated that the phase 2 interventions SSK sent and timed appointments exerted a statistically significant effect on attendance compared with the control practices in both Greater Manchester and Grampian. In an underpowered analysis this appeared to lead to increased detection of low-grade cytology. Women in Grampian appeared to be significantly less likely overall to attend in phase 2, yet showed mostly no differences in behaviour with respect to the influence of the phase 2 interventions with the exception of NN and choice. As seen previously in phase 1 vaccination status, in Grampian participants, appears to indicate an increase in the likelihood of attendance and phase 1 interventions failed to have an effect in these non-responders. We can be sure that of those randomised to an intervention, on average, 81% of women were at least sent the correct intervention, 17.6% were sent no intervention and only 1.4% were recorded as being sent the incorrect intervention.
Chapter 3 Cost-effectiveness
Objectives
The economic analysis had three components.
-
Calculating the costs of each intervention. For the leaflet, this included development, printing and distribution costs. For online booking the costs included programming and information technology changes required to implement the system. For the requested and unrequested HPV SSK, the costs included SSKs, staff time to prepare them and distribution. For the specialist NN, the calculated costs included all letters, telephone calls and publicity material, as well as the average time involved in providing advice and facilitating access.
-
Calculating other health-care costs. These included attendances at screening clinics, consultations, screening-related tests and diagnostic procedures. These were obtained from a combination of trial sources and literature.
-
Calculating cost-effectiveness. This was expressed in two ways: (1) the incremental lifetime cost per screened woman for each of the strategies being examined compared with standard practice and (2) lifetime cost utility. Lifetime results were obtained by combining the cost and attendance results of the study with published estimates of the lifetime costs and effects of participating in cervical cancer screening programmes.
Methods
The economic analysis complied with the methodological guidelines issued by the National Institute for Health and Care Excellence (NICE)21 and followed the reporting standards of the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. 22
Measurement and valuation of costs
Intervention costs
Full details of the STRATEGIC study interventions have been described in Chapter 2, Operationalising the interventions, but specific details of the interventions that are relevant to the cost analysis are given in Appendix 8.
All resources required to develop and implement each intervention were identified and recorded by two researchers at the University of Manchester, and providers of the interventions then cross-checked the list to ensure completeness and accuracy. The list included items such as labour time, printing and distribution of information materials, laboratory kits, rent for meeting rooms, training of professionals, information and communications, and programming. Labour time costs were based on gross monthly income corresponding to the salary grade of the staff involved in an activity. A detailed list of the items for each intervention, and associated costs, is presented in Appendix 9. For each intervention, the intervention costs per attendee were calculated by dividing the total intervention costs by the number of women who attended cervical screening.
Other screening-related costs
Data on cytology tests and HPV SSKs were collected from the screening agencies in Greater Manchester and Grampian. The number of colposcopies was estimated by using information about the colposcopy referral rate for the relevant age group, the attendance rate and the different types of colposcopy derived from the national Cervical Screening Programme, categorised into outpatient diagnostic procedures, outpatient diagnostic procedures with biopsy and therapeutic colposcopy.
Unit costs of screening tests
Unit costs of HPV tests and cytology tests were obtained from previous studies23,24 and included staff time in screening centres and laboratories, equipment and consumables. Any costs arising from non-attendance are not included, in line with previous studies. Unit costs of different types of colposcopic procedures were obtained from the NHS Reference Costs 2013–2014. 25 All unit costs were inflated to 2014 prices using the Hospital and Community Health Services inflation index.
Measures of effects and follow-up
The primary within-trial outcome measure for all interventions was attendance for cervical screening. This outcome was measured at 3 and 6 months post invitation for phase 1 interventions (i.e. pre-leaflet and online booking) and at 12 and 18 months post invitation for the interventions provided in phase 2. The primary end point for phase 1 interventions was uptake of screening at 3 months and for phase 2 interventions at 12 months post invitation. These end points were used in the main economic evaluation and the secondary end points (6 months for phase 1 interventions, 18 months for phase 2) in a secondary economic analysis.
Lifetime costs and quality-adjusted life-years of screening attendance
A systematic literature review was performed to obtain information about the lifetime costs and quality-adjusted life-years (QALYs) of women who attended cervical cancer screening and those who did not. We developed the literature search strategy based on the PICOS (population, intervention, comparator, outcomes, study design) framework, in order to retrieve economic evaluations of screening strategies. These included no screening as a comparator and reported lifetime costs and outcomes in the form of life-years and/or QALYs for women eligible for cervical cancer screening. Following the recommendations of the Centre for Reviews and Dissemination26 and Cochrane Central Register of Controlled Trials, the following computerised bibliographic databases were searched on 15 May 2016: MEDLINE, MEDLINE In-Process, EMBASE, EconLit and the NHS Economic Evaluation Database. Letters, editorials, historical articles, animal studies, studies published before 1995 and studies published in languages other than English were excluded from the search.
A two-stage selection process was followed. The title and abstract of the retrieved studies were scanned for relevance and the full text was then accessed if a paper was judged to be an economic evaluation related to cervical cancer screening and had employed a disease progression model with a lifetime time horizon. Where exclusion based on the titles/abstracts was not possible, the full publication was retrieved and evaluated. When all relevant full texts were obtained, they were assessed for eligibility against the PICOS criteria (Table 17).
| PICOS criterion | Inclusion criteria |
|---|---|
| Patient population |
|
| Intervention |
|
| Comparators |
|
| Outcomes |
|
| Study design |
|
The reviewing process was documented in Microsoft Excel, and reasons for inclusion and exclusion detailed to facilitate updates of the review. The websites of agencies including NICE, the Medical Research Council and National Cancer Screening Programmes were also scanned for relevant reports. A template was developed and used to extract the information from the most relevant studies to the STRATEGIC trial. The review was performed in May 2015.
The quality of the selected studies from the literature review was assessed using a checklist of good practice in decision-analytic modelling studies developed by Philips et al. 27 and the CHEERS statement for reporting economic evaluation. 22 In this way, the selected studies were assessed both on specific modelling and on broader evaluation grounds. In case of disagreement between the assessors, the mean assessment score was assigned. In studies where more than one cancer screening strategy was compared with no-screening strategy, we used a selection process to retrieve lifetime costs and outcomes of screening compared with no screening. In this process, the next more-costly strategies to no screening as well as strategies that had similar population (i.e. age groups), interval of screening and discount rates (i.e. 3.5%) to the UK were preferred. Where results from cancer screening in different countries were reported, we selected the UK-based estimates.
A meta-analysis was performed to pool the lifetime costs and outcomes reported in the selected studies. A random-effects model was specified to pool the estimates assuming real differences in treatment effects because of heterogeneity in screening strategies, population and other factors. 28 The study quality score was used in this model to weight each study’s contributed information to the pooled estimate. The NICE appraisal studies were assigned the highest possible weight on the grounds that they were of high relevance to our study and of high methodological quality.
Costs and quality-adjusted life-years
Costs were inflated from each study’s price reference year to 2014 using consumer price inflation rates for each study’s country of origin, as reported by the Organisation for Economic Co-operation and Development (OECD)29 and were converted to UK sterling using average exchange rates for the year 2014 as suggested by the UK Government. 30 QALYs retrieved from the selected studies were adjusted to make them comparable and relevant to the UK context. To do this, the risk rate of life expectancy between the screening and control cohorts in the selected studies was applied to the mean age of women in the trial. For example, if the reported life expectancy of screening was 28.71 years and the reported life expectancy of no screening was 28.69 years, the risk rate of life expectancy would be 1.001. This was then applied to the mean remaining life expectancy of women in the trial (62.29 years) to derive a remaining life expectancy for screened women of 62.35 years (62.29 × 1.001). Quality-adjusted life expectancy and QALY differences were then calculated by weighting these 62.29 and 62.35 years by the utility of women in each age band in the general population from the mean age of women in the trial up to 85 years of life.
Cost-effectiveness and cost–utility analyses
A decision model was constructed in Microsoft Excel to calculate the lifetime costs and outcomes of each intervention. Each of the six interventions was compared with the control group. The probabilities of attendance in each intervention and control group were informed by the trial results. Costs in this model included the intervention costs, screening costs during the study, and lifetime screening and disease-specific costs (which were informed by the meta-analysis described in Lifetime costs and quality-adjusted life-years of screening attendance).The incremental cost-effectiveness ratios (ICERs) were expressed as incremental costs per women attending a screening test and incremental costs per QALY gained taking the NHS perspective.
Uncertainty and scenario analysis
Probabilistic sensitivity analysis was performed to address the uncertainty in the ICERs by performing 5000 draws of all cost and effect parameters using pre-specified distributions, and recording incremental costs and incremental QALYs from each draw. These results were then plotted on cost-effectiveness planes. Mean costs and outcomes, as well as their standard errors as reported by the trial’s statistical analysis, were used to define distributions for the attendance rate. The reported mean estimated and standard errors of lifetime costs and QALYs from the meta-analysis were used to estimate the distribution of these parameters. The intervention costs per attendee, unit costs and probabilities of having a HPV test, cytology test and colposcopy were also included in the probabilistic sensitivity analysis. Cost-effectiveness acceptability curves were derived from these results, to express the uncertainty in these cost-effectiveness estimates in graphical form. This was done by displaying the probability that each intervention is cost-effective as the ceiling ratio for the maximum acceptable ICER varies from £0 to £75,000 per QALY gained.
Two univariate sensitivity analyses were also performed. First, the impact of using secondary end points (6 months instead of 3 months for phase 1 and 18 months instead of 12 months in phase 2) on the results of the economic evaluation was tested. In another univariate analysis, the study quality scores based on Phillips et al. 27 that were used to weight the pooled estimates in the meta-analysis were replaced with the study quality scores based on the CHEERS statement.
A scenario analysis was also performed to explore the adoption of these interventions for a population of 365,087 women in phase 1 interventions, which is approximately the total number of women annually invited for cervical screening for the first time in England, Scotland and Wales. A similar scenario analysis was performed for phase 2 interventions applied to a population of 255,561 women, that is, assuming that 70% of women do not attend phase 1 interventions. In these analyses, intervention costs were categorised into fixed one-off costs, semifixed scalable costs, which are incurred in steps as the scale increases, and variable costs. It was assumed that large-scale purchasing of materials and equipment would allow a cost discount to be obtained; this is set at 20% in the sensitivity analysis. In these scenario analyses, therefore, only the components of total cost that are not variable will affect the cost per woman and the ICERs.
Results
Figure 10 shows that 3766 studies were screened based on title and abstract and 30 studies were screened based on full text. A total of eight studies24,31–37 met the inclusion criteria and were therefore included in the final review. The papers were fully evaluated and relevant information was extracted.
FIGURE 10.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.
An overview of the papers included and the summary results extracted from these papers showing that quality scores were variable across the identified studies can be seen in Appendix 10.
Table 18 shows summary results from the meta-analysis, indicating that participation in a screening programme increased lifetime quality-adjusted life expectancy by 0.0947 QALYs, at an additional lifetime cost of £566.30. More details of the meta-analysis are reported in Appendix 10.
| Variable | Coefficient | Standard error | p-value | 95% CI |
|---|---|---|---|---|
| Lifetime costs no screening | 304.6262 | 96.60469 | 0.002 | 115.2845 to 493.9679 |
| Lifetime costs screening | 870.9233 | 247.2858 | 0.000 | 386.252 to 1355.595 |
| Lifetime QALYs no screening | 57.39219 | 0.0166141 | 0.000 | 57.37411 to 57.429219 |
| Lifetime QALYs screening | 57.48689 | 0.0354359 | 0.000 | 57.41744 to 57.55635 |
Parameters used in the decision model
Table 19 summarises all main probability parameters used in the analysis and Table 20 summarises the cost parameters used.
| Parameter | Deterministic | Standard error | Distribution | Alpha | Beta | Source |
|---|---|---|---|---|---|---|
| Treatment | ||||||
| % screened women | ||||||
| Pre-leaflet | 0.190 | 0.005 | Beta | 1106.31 | 4719.09 | Trial |
| Booking | 0.179 | 0.007 | Beta | 495.64 | 2282.54 | Trial |
| Requested SSK | 0.168 | 0.013 | Beta | 143.74 | 718.86 | Trial |
| Unrequested SSK | 0.220 | 0.018 | Beta | 119.68 | 428.12 | Trial |
| NN | 0.143 | 0.016 | Beta | 67.71 | 412.45 | Trial |
| Timed appointment | 0.208 | 0.015 | Beta | 152.64 | 584.67 | Trial |
| Choice SSK or NN | 0.170 | 0.015 | Beta | 109.80 | 542.14 | Trial |
| Control | ||||||
| % screened women | ||||||
| Pre-leaflet | 0.196 | 0.006 | Beta | 987.33 | 4064.74 | Trial |
| Booking | 0.176 | 0.009 | Beta | 296.71 | 1395.73 | Trial |
| Requested SSK | 0.158 | 0.007 | Beta | 405.13 | 2165.97 | Trial |
| Unrequested SSK | 0.158 | 0.007 | Beta | 405.13 | 2165.97 | Trial |
| NN | 0.158 | 0.007 | Beta | 405.13 | 2165.97 | Trial |
| Timed appointment | 0.158 | 0.007 | Beta | 405.13 | 2165.97 | Trial |
| Choice SSK or NN | 0.158 | 0.007 | Beta | 405.13 | 2165.97 | Trial |
| Probability of having a test by phase 2 trial arm | ||||||
| Requested SSK: HPV test | 0.091 | Beta | 19.00 | 190.00 | Trial | |
| Requested SSK: cytology | 0.943 | Beta | 197.00 | 12.00 | Trial | |
| Unrequested SSK: HPV test | 0.350 | Beta | 85.00 | 158.00 | Trial | |
| Unrequested SSK: cytology | 0.786 | Beta | 191.00 | 52.00 | Trial | |
| NN: HPV test | 0.007 | Beta | 1.00 | 145.00 | Trial | |
| NN: cytology | 1.000 | Beta | 146.00 | 0.00 | Trial | |
| Timed appointment: HPV test | 0.000 | Beta | 0.00 | 323.00 | Trial | |
| Timed appointment: cytology | 1.000 | Beta | 323.00 | 0.00 | Trial | |
| Choice SSK or NN: HPV test | 0.029 | Beta | 7.00 | 233.00 | Trial | |
| Choice SSK or NN: cytology | 0.979 | Beta | 235.00 | 5.00 | Trial | |
| Control: HPV test | 0.002 | Beta | 1.00 | 612.00 | Trial | |
| Control: cytology | 0.998 | Beta | 612.00 | 1.00 | Trial | |
| Follow-up tests | ||||||
| Proportion of HPV triage after cytology (age 20–24 years) | 0.096 | Beta | 1231.00 | 11,561.00 | PHE’s Health Economics Report for Primary HPV Pilot38 | |
| Colposcopy referral rate (cytology only and after HPV triage; age 20–24 years) | 0.117 | Beta | 1503.00 | 11,289.00 | PHE’s Health Economics Report for Primary HPV Pilot38 | |
| Colposcopy attendance rate | 0.767 | Beta | 188,775.57 | 57,346.43 | Table W. Cervical Screening Programme, England. Statistics for 2013–201439 | |
| Colposcopy, outpatient procedure | 0.387 | Beta | 73,072.18 | 115,744.82 | Table X. Cervical Screening Programme, England. Statistics for 2013–201439 | |
| Colposcopy with biopsy, outpatient procedure | 0.482 | Beta | 91,009.79 | 97,807.21 | Table X. Cervical Screening Programme, England. Statistics for 2013–201439 | |
| Therapeutic colposcopy, outpatient | 0.131 | Beta | 24,735.03 | 164,081.97 | Table X. Cervical Screening Programme, England. Statistics for 2013–201439 | |
| Parameter | Deterministic | Standard error | Distribution | Alpha | Beta | Source |
|---|---|---|---|---|---|---|
| Treatment | ||||||
| Intervention costs per woman attending | ||||||
| Pre-leaflet | 4.27 | 0.01 | Gamma | 428,748 | 0.00 | Trial |
| Booking | 2.80 | 0.01 | Gamma | 121,682.18 | 0.00 | Trial |
| Requested SSK | 1.71 | 0.01 | Gamma | 93,762.18 | 0.00 | Trial |
| Unrequested SSK | 52.20 | 0.18 | Gamma | 83,310.51 | 0.00 | Trial |
| NN | 0.99 | 0.00 | Gamma | 51,411.28 | 0.00 | Trial |
| Timed appointment | 23.94 | 0.08 | Gamma | 93,601.84 | 0.00 | Trial |
| Choice SSK or NN | 5.73 | 0.02 | Gamma | 82,240.54 | 0.00 | Trial |
| Lifetime costs | 870.92 | 247.29 | Gamma | 12.40 | 70.21 | Meta-analysis |
| Lifetime QALYs | 57.49 | 0.04 | Normal | Meta-analysis | ||
| Control | ||||||
| Lifetime costs | 304.63 | 96.60 | Gamma | 9.94 | 30.64 | Meta-analysis |
| Lifetime QALYs | 57.39 | 0.02 | Normal | Meta-analysis | ||
| Unit costs, all women attending | ||||||
| Cytology test | 36.37 | 1.66 | Gamma | 478.97 | 0.08 | Kim (2010)40 based on Sherlaw-Johnson and Philips (2004) |
| HPV test | 29.01 | 8.56 | Gamma | 11.48 | 2.53 | Kim (2010)40 based on Sherlaw-Johnson and Philips (2004) |
| HPV (only laboratory costs) | 8.00 | NHS Reference Costs 2013–14 25 | ||||
| Colposcopy, outpatient procedure | 169.56 | NHS Reference Costs 2013–14 25 | ||||
| Colposcopy with biopsy, outpatient procedure | 219.51 | NHS Reference Costs 2013–14 25 | ||||
| Therapeutic colposcopy, outpatient | 229.86 | NHS Reference Costs 2013–14 25 | ||||
Further details of the interventions and their costs are provided in Appendices 8 and 9. It can be seen that the intervention costs per woman attending varied from < £1 for the NN intervention, to > £52 for the unrequested SSK. The relatively high cost of the latter is because those attending are bearing the costs of sending SSKs to all women in GP practices.
Cost-effectiveness analysis
Table 21 shows the results of the analysis pertaining to the pre-leaflet intervention. The probability of attending was slightly higher in the control group than in the intervention group. Intervention costs totalled £8496, but screening costs were slightly higher in the control group because of the higher attendance, resulting in an additional cost for intervention and screening of £0.51 per woman in the intervention group. Lifetime costs were also slightly higher in the control group because of increased participation in screening, and so the lifetime cost per woman invited was £2.46 higher in the control group. Lifetime QALYs were slightly higher in the control group as a higher proportion of women received the benefits of being screened. In consequence, the pre-leaflet intervention was less costly but also less effective than control, and the ICER of £4953 can be interpreted as indicating that opting for the pre-leaflet intervention would result in a loss of 1 QALY for every £4953 saved.
| Variable | Intervention | Difference | |
|---|---|---|---|
| Treatment (n = 10,461) | Control (n = 10,418) | ||
| n attending | 1991 | 2038 | |
| Total intervention costs (£) | 8496 | 0 | 8496 |
| Total screening costs (£) | 114,142 | 116,810 | –2668 |
| Total intervention and screening costs (£) | 122,638 | 116,810 | 5828 |
| Total intervention and screening costs per woman (£) | 11.72 | 11.21 | 0.51 |
| Lifetime costs (£) | 4,314,254 | 4,327,515 | –13,261 |
| Total costs (£) | 4,436,892 | 4,444,325 | –7433 |
| Total cost per woman (£) | 424.14 | 426.60 | –2.46 |
| Total cost per woman attending (£) | 2228.35 | 2181.10 | 47 |
| Lifetime QALYs | 600,568 | 598,105 | 2463 |
| Lifetime QALYs per woman | 57.4102 | 57.4107 | –0.00050 |
| Cost per QALY gained (£) | 4953 | ||
Online booking
The results of the analysis pertaining to the online booking intervention are shown in Table 22. The probability of attending was slightly higher in the intervention group than in the control group. Intervention costs totalled £2382 and screening costs were slightly higher in the intervention group because of higher attendance, resulting in an additional cost for intervention and screening of £0.67 per woman in the intervention group. Lifetime costs were also slightly higher in the intervention group resulting from increased participation in screening, and so the lifetime cost per woman invited was £2.38 higher in the intervention group than the control group. Lifetime QALYs were slightly higher in the intervention group as a higher proportion of women received the benefits of being screened. In consequence, the online booking intervention was more costly but also more effective than the control group, with an ICER of £8344 per QALY gained.
| Variable | Intervention | Difference | |
|---|---|---|---|
| Treatment (n = 4756) | Control (n = 4978) | ||
| n attending | 850 | 875 | |
| Total intervention costs (£) | 2382 | 0 | 2382 |
| Total screening costs (£) | 48,750 | 50,168 | –1417 |
| Total intervention and screening costs (£) | 51,133 | 50,168 | 965 |
| Total intervention and screening costs per woman (£) | 10.75 | 10.08 | 0.67 |
| Lifetime costs (£) | 1,930,385 | 2,012,014 | –81,629 |
| Total costs (£) | 1,981,518 | 2,062,182 | –80,664 |
| Total cost per woman (£) | 416.64 | 414.26 | 2.38 |
| Total cost per woman attending (£) | 2330.08 | 2356.42 | –26 |
| Lifetime QALYs | 273,038 | 285,781 | –12,743 |
| Lifetime QALYs per woman | 57.4091 | 57.4088 | 0.00028 |
| Cost per QALY gained (£) | 8344 | ||
Human papillomavirus self-sampling kit offered
The results of the analysis pertaining to the HPV SSK on request intervention are shown in Table 23. The probability of attending was higher in the intervention group than the control group. Intervention costs totalled £369, and screening costs per woman were higher in the intervention group because of higher attendance, resulting in an additional cost of £0.42 for intervention and screening per woman in the intervention group. Lifetime costs were also slightly higher in the intervention group resulting from increased participation in screening, and so the lifetime cost per woman invited was £5.93 higher in the intervention group than the control group. Lifetime QALYs were higher in the intervention group as a higher proportion of women received the benefits of being screened. In consequence, the HPV SSK on request intervention was more costly but also more effective than the control group, at an ICER of £6436 per QALY gained.
| Variable | Intervention | Difference | |
|---|---|---|---|
| Treatment (n = 1290) | Control (n = 3782) | ||
| n attending | 216 | 597 | |
| Total intervention costs (£) | 369 | 0 | 369 |
| Total screening costs (£) | 11,273 | 32,542 | –21,269 |
| Total intervention and screening costs (£) | 11,642 | 32,542 | –20,900 |
| Total intervention and screening costs per woman (£) | 9.02 | 8.60 | 0.42 |
| Lifetime costs (£) | 515,422 | 1,490,276 | –974,854 |
| Total costs (£) | 527,064 | 1,522,818 | –995,755 |
| Total cost per woman (£) | 408.58 | 402.65 | 5.93 |
| Total cost per woman attending (£) | 2437.44 | 2550.02 | –113 |
| Lifetime QALYs | 74,056 | 217,114 | –143,057 |
| Lifetime QALYs per woman | 57.4081 | 57.4071 | 0.00092 |
| Cost per QALY gained (£) | 6436 | ||
Human papillomavirus self-sampling kit sent
The results of the analysis pertaining to the unrequested HPV SSK intervention are shown in Table 24. The probability of attending was higher in the intervention group than in the control group. Intervention costs totalled £13,157 and screening costs per woman were higher in the intervention group because of higher attendance, resulting in an additional cost of £13.01 for intervention and screening per woman in the intervention group. Lifetime costs were also slightly higher in the intervention group, resulting from increased participation in screening, and so the lifetime cost per woman invited was £48.68 higher in the intervention group. Lifetime QALYs were higher in the intervention group as a higher proportion of women received the benefits of being screened. In consequence, the unrequested HPV SSK intervention was more costly but also more effective than the control group, at an ICER of £8161 per QALY gained.
| Variable | Intervention | Difference | |
|---|---|---|---|
| Treatment (n = 1141) | Control (n = 3782) | ||
| n attending | 252 | 597 | |
| Total intervention costs (£) | 13,157 | 0 | 13,157 |
| Total screening costs (£) | 11,509 | 32,542 | –21,033 |
| Total intervention and screening costs (£) | 24,665 | 32,542 | –7877 |
| Total intervention and screening costs per woman (£) | 21.62 | 8.60 | 13.01 |
| Lifetime costs (£) | 490,304 | 1,490,276 | –999,973 |
| Total costs (£) | 514,969 | 1,522,818 | –1,007,849 |
| Total cost per woman (£) | 451.33 | 402.65 | 48.68 |
| Total cost per woman attending (£) | 2043.27 | 2550.02 | –507 |
| Lifetime QALYs | 65,508 | 217,114 | –151,605 |
| Lifetime QALYs per woman | 57.4131 | 57.4071 | 0.00596 |
| Cost per QALY gained (£) | 8161 | ||
Nurse navigator
Table 25 shows the results of the analysis pertaining to the NN intervention. The probability of attending was higher in the control group than in the intervention group. Intervention costs totalled £142 and screening costs per woman were higher in the control group because of higher attendance, resulting in an additional cost of £0.66 for intervention and screening per woman in the control group. Lifetime costs were also slightly higher in the control group, resulting from increased participation in screening, and so the lifetime cost per woman invited was £9.34 higher in the control group. Lifetime QALYs were higher in the control group as a higher proportion of women received the benefits of being screened. In consequence, the NN intervention was less costly but also less effective than the control, and the ICER of £6435 can be interpreted as indicating that opting for the NN intervention would result in a loss of 1 QALY for every £6449 saved.
| Variable | Intervention | Difference | |
|---|---|---|---|
| Treatment (n = 1007) | Control (n = 3782) | ||
| n attending | 144 | 597 | |
| Total intervention costs (£) | 142 | 0 | 142 |
| Total screening costs (£) | 7838 | 32,542 | –24,704 |
| Total intervention and screening costs (£) | 7979 | 32,542 | –24,563 |
| Total intervention and screening costs per woman (£) | 7.92 | 8.60 | –0.68 |
| Lifetime costs (£) | 388,063 | 1,490,276 | –1,102,213 |
| Total costs (£) | 396,042 | 1,522,818 | –1,126,776 |
| Total cost per woman (£) | 393.29 | 402.65 | –9.36 |
| Total cost per woman attending (£) | 2758.48 | 2550.02 | 208 |
| Lifetime QALYs | 57,808 | 217,114 | –159,306 |
| Lifetime QALYs per woman | 57.4057 | 57.4071 | –0.00145 |
| Cost per QALY gained (£) | 6449 | ||
Timed appointments
Table 26 shows the results of the analysis pertaining to the timed appointments intervention. The probability of attending was higher in the intervention group than in the control group. Intervention costs totalled £18,145 and screening costs per woman were higher in the intervention group because of higher attendance, resulting in an additional cost of £7.79 for intervention and screening per woman in the intervention group. Lifetime costs were also higher in the intervention group, resulting from increased participation in screening, and so the lifetime cost per woman invited was £36.66 higher in the intervention group than in the control. Lifetime QALYs were higher in the intervention group as a higher proportion of women received the benefits of being screened. In consequence, the timed appointments intervention was more costly but also more effective than the control, at an ICER of £7593 per QALY gained.
| Variable | Intervention | Difference | |
|---|---|---|---|
| Treatment (n = 1629) | Control (n = 3782) | ||
| n attending | 340 | 597 | |
| Total intervention costs (£) | 8145 | 0 | 8145 |
| Total screening costs (£) | 18,557 | 32,542 | –13,985 |
| Total intervention and screening costs (£) | 26,702 | 32,542 | –5840 |
| Total intervention and screening costs per woman (£) | 16.39 | 8.60 | 7.79 |
| Lifetime costs (£) | 688,936 | 1,490,276 | –801,340 |
| Total costs (£) | 715,638 | 1,522,818 | –807,180 |
| Total cost per woman (£) | 439.31 | 402.65 | 36.66 |
| Total cost per woman attending (£) | 2103.08 | 2550.02 | –447 |
| Lifetime QALYs | 93,524 | 217,114 | –123,590 |
| Lifetime QALYs per woman | 57.4120 | 57.4071 | 0.00483 |
| Cost per QALY gained (£) | 7593 | ||
Choice of nurse navigator or human papillomavirus self-sampling kit offered
Table 27 shows the results of the analysis pertaining to the combination intervention offering a choice of NN or HPV SSK. The number and probability of attending was higher in the intervention group than the control group. Intervention costs totalled £1243 and screening costs per woman were higher in the intervention group because of higher attendance, resulting in an additional cost of £1.47 for intervention and screening per woman in the intervention group. Lifetime costs were also slightly higher in the intervention group, resulting from increased participation in screening, and so the lifetime cost per woman invited was £8.21 higher in the intervention group than in the control group. Lifetime QALYs were higher in the intervention group as a higher proportion of women received the benefits of being screened. In consequence, this combination intervention was more costly but also more effective than the control, at an ICER of £7290 per QALY gained.
| Variable | Intervention | Difference | |
|---|---|---|---|
| Treatment (n = 1277) | Control (n = 3782) | ||
| n attending | 217 | 597 | |
| Total intervention costs (£) | 1243 | 0 | 1243 |
| Total screening costs (£) | 11,628 | 32,542 | –20,914 |
| Total intervention and screening costs (£) | 12,871 | 32,542 | –19,671 |
| Total intervention and screening costs per woman (£) | 10.08 | 8.60 | 1.47 |
| Lifetime costs (£) | 511,791 | 1,490,276 | –978,486 |
| Total costs (£) | 524,662 | 1,522,818 | –998,156 |
| Total cost per woman (£) | 410.86 | 402.65 | 8.21 |
| Total cost per woman attending (£) | 2419.83 | 2550.02 | –130 |
| Lifetime QALYs | 73,310 | 217,114 | –143,803 |
| Lifetime QALYs per woman | 57.4083 | 57.4071 | 0.00113 |
| Cost per QALY gained (£) | 7290 | ||
Uncertainty concerning cost-effectiveness results
Tables 21–27 report only the point estimates for all results. To capture the full uncertainty around the parameter values, probabilistic sensitivity analysis was performed (see Methods for details), and Figures 11–17 show cost-effectiveness planes for each intervention, in which 5000 cost–effect pairs derived with all parameters varying are displayed. It is immediately evident from the cost-effectiveness planes that timed appointments and unrequested SSK are the two interventions that are clearly more effective than the control intervention, with virtually all cost–effect pairs to the right of the y-axis. Both are also almost certain to cost more than control.
FIGURE 11.
Cost-effectiveness planes for pre-leaflet compared with control (lifetime difference in costs and QALYs, outcomes assessed at 3 months for phase 1 interventions).

FIGURE 12.
Cost-effectiveness planes for online booking compared with control (lifetime difference in costs and QALYs, outcomes assessed at 3 months for phase 1 interventions).

FIGURE 13.
Cost-effectiveness planes for SSKs on request compared with control (lifetime difference in costs and QALYs, outcomes assessed at 12 months for phase 2 interventions).

FIGURE 14.
Cost-effectiveness planes for SSKs sent unrequested compared with control (lifetime difference in costs and QALYs, outcomes assessed at 12 months for phase 2 interventions).

FIGURE 15.
Cost-effectiveness planes for NNs compared with control (lifetime difference in costs and QALYs, outcomes assessed at 12 months for phase 2 interventions).
FIGURE 16.
Cost-effectiveness planes for timed appointment compared with control (lifetime difference in costs and QALYs, outcomes assessed at 12 months for phase 2 interventions).
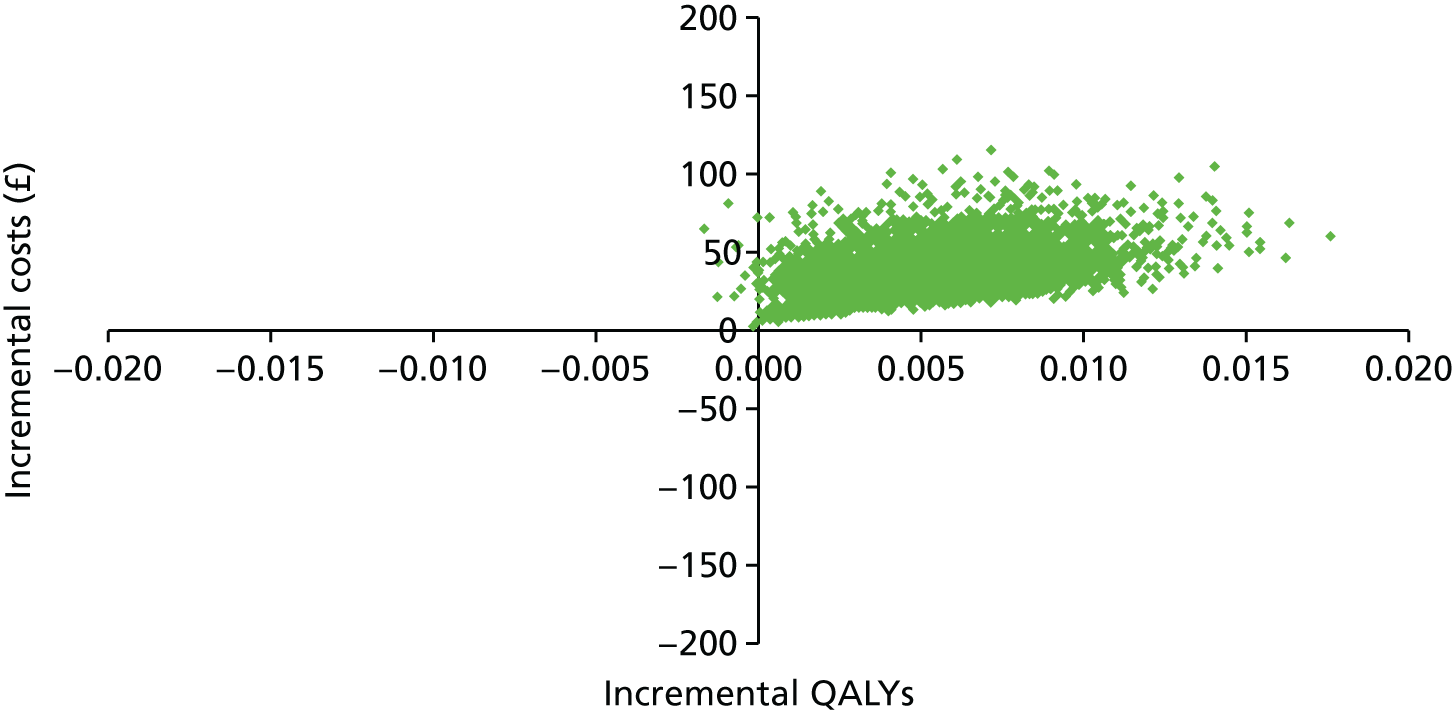
FIGURE 17.
Cost-effectiveness planes for choice of a NN or requested SSK compared with control (lifetime difference in costs and QALYs, outcomes assessed at 12 months for phase 2 interventions).
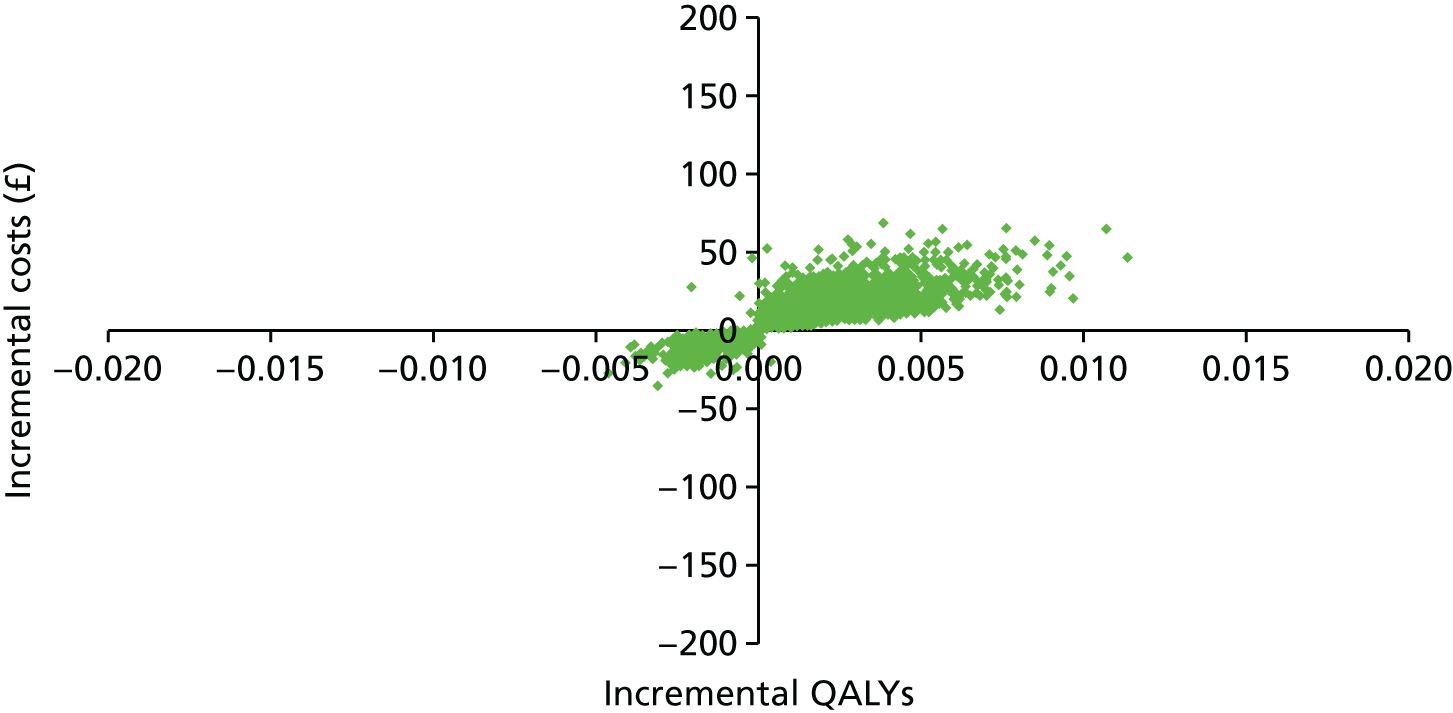
The probability of each intervention being cost-effective at different willingness-to-pay values for 1 QALY is presented in Figure 18. The pre-leaflet intervention is characterised by uncertainty in costs and effectiveness, and so the probability of this intervention being cost-effective is very low. A similar pattern is displayed by the NN intervention. Similarly, there is no clear evidence that online booking is effective, and so the probability that this intervention is cost-effective never rises above 60%. The SSK on request intervention has a slightly higher probability of being cost-effective, but this is still no higher than 71% at conventional ceiling ratios of willingness to pay for 1 QALY; a similar pattern is displayed by the combination intervention, which includes SSK on request. For timed appointments the probability that the intervention is cost-effective at a ceiling ratio of £20,000 per QALY gained is 94%, and for unrequested SSK is 93% (see Figure 18).
FIGURE 18.
Cost-effectiveness acceptability curves for all interventions (outcomes assessed at 3 months for phase 1 interventions and at 12 months for phase 2).

Results from the univariate sensitivity and scenario analyses
The results from the sensitivity analysis, in which the results are based on the secondary end points (outcomes assessed at 6 months for phase 1 interventions and at 18 months for phase 2), are presented in Table 28. The ICERs for each intervention from the main analysis are shown in the final column to facilitate comparison. For most interventions the differences are small, but the cost per QALY gained of the pre-leaflet intervention has risen from < £5000 to > £9000, primarily because the adjusted OR for this intervention has changed from 0.967 at 3 months to 1.014 at 6 months, suggesting that the intervention may have a small effect in increasing attendance, which results in better health outcomes but increased lifetime costs per woman.
| Intervention | Incremental cost per woman (£) | Incremental cost per woman (main analysis) (£) | Incremental QALYs per woman | Incremental cost per QALY gained (£) | Incremental cost per QALY gained (main analysis) (£) |
|---|---|---|---|---|---|
| Pre-leaflet | 2.77 | –2.46 | 0.00030 | 9311 | 4953 |
| Online booking | 12.22 | 2.38 | 0.00178 | 6867 | 8344 |
| Requested SSK | 6.49 | 5.93 | 0.00101 | 6440 | 6436 |
| Unrequested SSK | 41.18 | 48.68 | 0.00486 | 8465 | 8161 |
| NN | –25.25 | –9.36 | –0.00387 | 6516 | 6449 |
| Timed appointment | 26.78 | 36.66 | 0.00333 | 8052 | 7593 |
| Combination | 7.70 | 8.21 | 0.00105 | 7344 | 7290 |
Figure 19 reports the cost-effectiveness acceptability curves for all interventions using the secondary end points, and it can be seen that, for example, the probability that timed appointments are cost-effective at a £20,000 ceiling has fallen to 87% from 94% in the primary analysis, while the probability that the pre-leaflet or online booking interventions are cost-effective has increased. Appendix 11 shows the accompanying cost-effectiveness planes for each intervention.
FIGURE 19.
Cost-effectiveness acceptability curves for all interventions (outcomes assessed at 6 months for phase 1 interventions and at 18 months for phase 2 interventions).
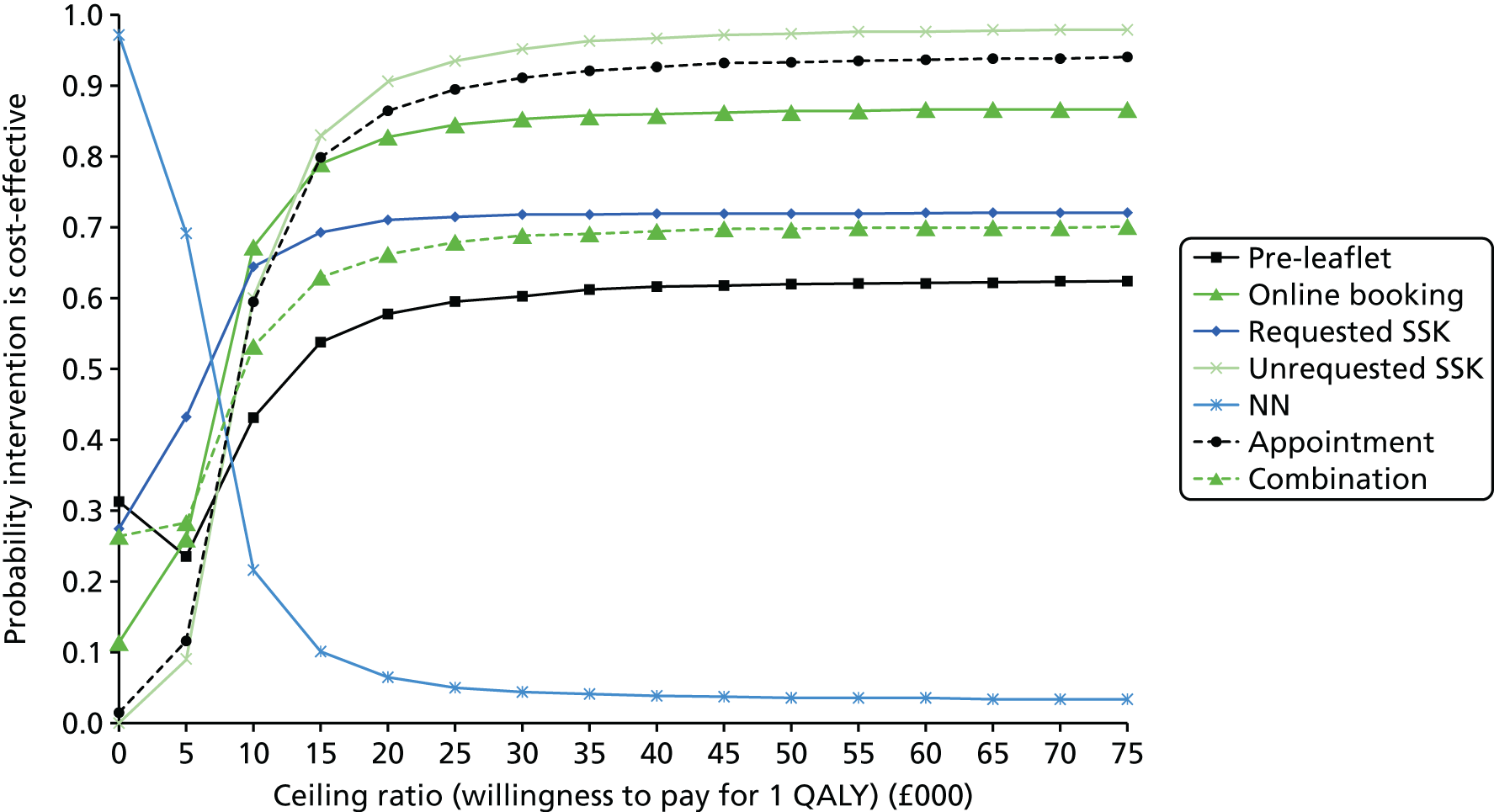
Table 29 reports the results for each intervention when the studies used to derive estimates of lifetime benefits and costs of screening are given quality weights using the CHEERS study criteria. This change has relatively little effect on the cost-effectiveness results.
| Intervention | Incremental cost per woman (£) | Incremental cost per woman (main analysis) (£) | Incremental QALYs per woman | Incremental cost per QALY gained (£) | Incremental cost per QALY gained (main analysis) (£) |
|---|---|---|---|---|---|
| Pre-leaflet | –2.71 | –2.46 | –0.00061 | 4481 | 4953 |
| Online booking | 2.52 | 2.38 | 0.00035 | 7267 | 8344 |
| Requested SSK | 6.39 | 5.93 | 0.00112 | 5699 | 6436 |
| Unrequested SSK | 51.67 | 48.68 | 0.00726 | 7117 | 8161 |
| NN | –10.09 | –9.36 | –0.00177 | 5710 | 6449 |
| Timed appointment | 39.09 | 36.66 | 0.00588 | 6649 | 7593 |
| Combination | 8.77 | 8.21 | 0.00137 | 6401 | 7290 |
Finally, Table 30 reports the results of a scenario analysis in which it is assumed that the relevant population is all women eligible for cervical screening in England (365,087 in 2014). In all cases the incremental costs were lower or savings were higher than in the main analysis because of the economies of scale and amortisation compared with the incremental costs in the main analysis, but the impact of these on the ICERs is not large.
| Intervention | Incremental cost per woman (£) | Incremental cost per woman (main analysis) (£) | Incremental QALYs per woman | Incremental cost per QALY gained (£) | Incremental cost per QALY gained (main analysis) (£) |
|---|---|---|---|---|---|
| Pre-leaflet | –2.69 | –2.46 | –0.00050 | 5412 | 4953 |
| Online booking | 2.18 | 2.38 | 0.00028 | 7654 | 8344 |
| Requested SSK | 5.97 | 5.93 | 0.00092 | 6480 | 6436 |
| Unrequested SSK | 50.33 | 48.68 | 0.00596 | 8438 | 8161 |
| NN | –9.40 | –9.36 | –0.00145 | 6474 | 6449 |
| Timed appointment | 37.38 | 36.66 | 0.00483 | 7741 | 7593 |
| Combination | 8.26 | 8.21 | 0.00113 | 7335 | 7290 |
Conclusion
In this economic analysis we have assessed the costs and cost-effectiveness of each of the interventions considered in the STRATEGIC trial, in each case making comparisons with the relevant control group to estimate the incremental costs and effects. Incremental cost-effectiveness has been calculated in terms of the additional cost per additional woman attending for cervical screening, and the incremental cost per QALY gained over a lifetime. In addition, we have estimated the total lifetime costs and effects of adopting each of the interventions for the entire population of women entering the screening programme each year in England on their 25th birthday.
A full discussion of the economic analysis, results and limitations is presented in Chapter 5. In summary, we find that timed appointments and unrequested SSK have the most compelling cost-effectiveness evidence in favour of adoption: in both cases the incremental cost per QALY gained is well below the generally accepted ceiling values, at £7593 and £8161, respectively, and there is a 94% probability these interventions are < £20,000 per QALY. The uncertainty around the cost-effectiveness of the timed appointments intervention increases slightly if the effectiveness of the intervention is assessed at the 18-month outcome point rather than the primary end point of 12 months, but there is still an 87% probability that it is cost-effective.
The cost-effectiveness results depend heavily on the trial results and on the estimated lifetime costs and benefits of screening, which were estimated from a meta-analysis of published studies. We find that our results are not particularly sensitive to changes in the way evidence from the meta-analysis was synthesised, for example using different checklists to attach quality weights to the studies made little difference overall.
Chapter 4 Evaluating preferences of non-responders to the UK Cervical Screening Programme at first invitation: a stated preference discrete choice experiment
Introduction
The success of any health-care programme depends on acceptability to, and engagement by, its intended users. Understanding the views and preferences of potential users of a new health-care policy is vital for assessing the potential future consequences of the programme. As a means of assessing preferences for policies or programmes on the basis of their inherent factors or characteristics, DCEs offer a credible approach and are increasingly being used in the health-care setting. 41
Discrete choice experiments have been successfully conducted in the area of screening. Watson et al. ,42 for example, used a DCE to examine women’s preferences for characteristics of chlamydia screening, including the location of the test, the type of test and the support available when receiving results. In the field of prostate cancer, de Bekker-Grob et al. 43 used the approach to explore men’s preferences for screening and to quantify the trade-offs between the benefits and costs of screening, including the reduced risk of cancer-related death versus the increased risk of unnecessary biopsy in the event of a false-positive screening test. DCEs have also been used to examine women’s preferences in cervical cancer screening. In 2006, Wordsworth et al. 44 conducted a DCE to explore women’s preferences for process changes to the existing Cervical Screening Programme resulting from the introduction of new guidelines and policies.
An important and upfront aspect of the STRATEGIC study was to evaluate young women’s preferences for the hypothetical interventions to be trialled within phase 2 of the study to increase uptake at first invitation within the context of the existing Cervical Screening Programme. These interventions included pre-specified timed appointments, solicited and unsolicited SSKs, and a NN. For this purpose, a DCE was designed to elicit the preferences of young women in Greater Manchester and Grampian who had not responded to their first invitation to screening.
Methods
Using discrete choice experiments to explore individual preferences
Human beings consciously or subconsciously make choices in their everyday lives. Researchers interested in exploring how individuals make choices between alternatives face methodological, theoretical and practical challenges. It is very complex to find a theoretical model that explains exactly an individual’s decision-making process, as this is highly variable among individuals. 45 Researchers cannot get into people’s minds and observe exactly what happens when a decision is made. Therefore, analysts need to find a strategy for identifying, capturing and using as much as possible the information that an individual considers when processing a scenario that leads to a choice. Because the analyst will never be able to identify all data and information processed by an individual, there is always a margin for error. This error of measurement is an important component of any choice model. There are two main alternatives that have been used to elicit people’s preferences in the economic literature: revealed preferences (we observe the choices made by people) and stated preferences (we directly ask people about their preferences). 46 Revealed preferences can be used only when an individual has made a real decision, and thus far in the context of novel and new therapies its use in health care has been very limited. Stated preferences have so far been found to be a more appropriate strategy to obtain preferences for alternatives that individuals may or may not have experienced in real life. To obtain preferences for different strategies to increase cervical screening uptake by non-responders, we used a stated preference method called a DCE. 47
A DCE is an elicitation technique to obtain individual preferences using a series of hypothetical choices. DCEs are implemented using surveys that require a series of choices to be made between alternatives where each alternative, although described using a set of common attributes (i.e. characteristics or traits), has different values or levels for these attributes. Alternatives are usually presented in pairs known as choice sets. For each choice set, individuals are asked to select which profile they prefer based on the information presented. The response data from a DCE are analysed within a random-utility model using statistical modelling for choice data, such as logit or probit models. A technical summary of the random-utility model and the theoretical framework employed in this DCE study is presented in Appendix 12.
Development of discrete choice experiment attributes
A number of different methods can be used to obtain and develop attributes for DCEs including literature reviews,48 expert reviews49 and focus groups or interviews. 50 Experts in the field do, however, recommend that qualitative work is conducted in order to develop such attributes47 and interviews are considered by Coast et al. 51 as providing the greatest ‘richness’ of attributes. A small qualitative study complemented with the views from the qualitative and quantitative research teams was undertaken to identify the attributes for this DCE exploring young women’s preferences for cervical screening programmes.
Participants and recruitment
A sample of women invited to take part in phase 1 of STRATEGIC was sent a participant information sheet and consent form for this additional qualitative component alongside the main study documentation (see Appendix 14). Those interested in being interviewed were asked to contact the STRATEGIC research team or return a reply slip and consent form directly to the qualitative researchers. The intention was to recruit a subsample of 20–30 women. We planned to use purposive sampling52 to ensure that we included a mix of women who were being offered the three novel interventions (NN, SSK, timed appointments). We also hoped to interview a mix of women who had taken up the offer of the intervention in the pilot study and those who had declined. Over a period of 2 months (August–September 2012), only four women (of approximately 600) responded to the invitation to be interviewed. All had been randomly allocated to receive the unsolicited SSK through the post, although it was unknown prior to interviewing whether or not they had used the kits. A second batch of invitations (to approximately 500 women) was sent out early in 2013, which resulted in one more woman (also allocated to unsolicited SSK) being interviewed.
Data collection
Semistructured telephone interviews were conducted and digitally recorded where consent was given. The first part of the interview explored women’s views on the intervention they had received. For example, whether or not they had used the intervention and, if yes, how had it gone and what did they like/dislike about it. If they had not used the intervention we enquired why that was. The second part focused on what the women felt about important features of a cervical screening programme to encourage attendance, for example, timing, access, cost and who does the screening. These two sets of questions were not considered to be mutually exclusive. It was expected that women’s views on a novel intervention might usefully inform the identification of the attributes for the DCE. The topic guide followed during the interview is provided in Appendix 13.
Data analysis
The audio-recordings were transcribed verbatim, with all personal data anonymised to ensure confidentiality. The interview data were then analysed using deductive content analysis. 53 First, a categorisation matrix was developed in which the interview data were coded to (1) the views of the intervention (initial reaction, understanding the instructions, likes and dislikes, what would encourage use) and (2) features of a cervical screening programme (booking, screening, receiving results, cost). The coded data were then reviewed by the qualitative research team to identify key categories that captured women’s views on the important features of a cervical cancer screening programme. ATLAS-ti software version 6 (Scientific Software Development GmbH, Berlin, Germany) facilitated data management.
Findings
Four key categories emerged, which are presented in Table 31. For the five women interviewed, issues of flexibility, expertise, emotion and normalisation were important issues in encouraging/discouraging them to attend for cervical cancer screening.
| Category | Subcategory |
|---|---|
| Flexibility |
|
| Expertise |
|
| Emotion |
|
| Normalisation/routine |
|
Using the qualitative study findings to inform the development of attributes and levels
Consideration was then given to developing the identified categories and subcategories into attributes for the DCE. Given the small number of interviews conducted, and from only one intervention group, it was considered prudent to use these qualitative findings as the starting point of a series of discussions between the qualitative and quantitative teams to determine the final number of attributes and levels for the DCE.
During initial attempts, we considered up to seven attributes: booking, convenience, flexibility, privacy, expertise, personalised information and cost. Attempting to assign very descriptive labels to the levels of each these attributes to remove any potential ambiguity proved problematic. Seven attributes, each with three or four levels, was felt to be excessive, and in attempting to be so inclusive we found there were some attributes that were inter-related. An example is privacy and expertise – an indication that the test would be performed by a woman herself in private on the privacy attribute would automatically imply that the test would be carried out by the woman herself on the expertise attribute. In addition, using detailed labels for the level meant that the interventions themselves were identified as levels for each attribute, rather than, as DCE methodology dictates, more generic attributes or characteristics that can apply across all study interventions. As a result, the attributes and levels were simplified.
As the rationale for the study interventions was to make the process of screening more acceptable to women, we looked at the way in which the intervention could achieve this. First, there were a variety of approaches for arranging a screen. Simply, these could be classified as those interventions that required some action on the part of the woman to arrange the test (e.g. calling to request a SSK) and those that did not (e.g. a timed appointment is given). It was thought that some women may consider it important whether or not action/effort is required to engage with the screening service. Second, all the interventions offered the potential for the test to be conducted in different locations, namely at a health-care facility, such as a GP practice or some other clinic (routine clinic smear), or in the woman’s own home (SSK). Having just one attribute on location of the test would cover all potential aspects of privacy and expertise that had been shown to be important to women during the interviews.
In addition to these two characteristics that were relevant to all the study interventions, the role of the NN also had to be considered. This role involved discussing screening with women and, if required, facilitating access to a test (whether this be at a clinic or at home). It was felt that the NN could potentially be an adjunctive option with any of the interventions and, therefore, it would be useful to include it as a separate attribute.
Table 32 presents the proposed list of attributes and levels used in the DCE. Each attribute had two levels, with the exception of the attribute relating to the cost of the test, which had four possible values reflecting the approximate costs to the NHS of integrating each of the interventions under investigation into the Cervical Screening Programme. This list was first tested during the pilot phase of the DCE and, where necessary, based on the feedback received, amended for the main DCE study. Details of the pilot and main DCE study phases are provided in First phase (pilot discrete choice experiment data collection) and second phase (main discrete choice experiment data collection).
| Main attributes | Attribute levels |
|---|---|
| Action required by you personally to arrange a test | Yes/no |
| Location of the test | GP clinic or surgery/home |
| Nurse available for discussion or help prior to appointment | Yes/no |
| Cost of your test to the NHS | £8/£20/£25/£40 |
The combination of attributes and levels in Table 32 described potential interventions to increase uptake to the screening programme. The aim of the DCE analysis was, hence, to identify which combination of attributes and associated levels women prefer. The specific combinations of attributes and levels presented in Table 33 identified the interventions under evaluation in the STRATEGIC study. We used these combinations to identify the intervention with a higher probability of being selected after model estimation. For example:
For a solicited SSK:
-
The woman must take some action to request the test.
-
The test is carried out at home.
-
A nurse will not have been available for discussion or help prior to appointment.
-
The cost of this option is approximately £8.00.
For the timed appointment:
-
No action is required on the part of the woman to arrange the screen.
-
The test is carried out at the GP surgery or clinic.
-
A nurse will not have been available for discussion or help prior to appointment.
-
The cost of this option is approximately £20.00.
| Attribute and level | Intervention | |||
|---|---|---|---|---|
| Timed appointment | Solicited SSK | Unsolicited SSK | NN | |
| Action required by you personally to arrange a test | ||||
| Yes? | ✓ | ✓ | ||
| No? | ✓ | ✓ | ||
| Location of the test | ||||
| GP surgery/clinic | ✓ | ✓a | ||
| Home | ✓ | ✓ | ✓b | |
| Nurse available for discussion or help prior to appointment | ||||
| Yes? | ✓ | |||
| No? | ✓ | ✓ | ✓ | |
| Cost of your test to the NHS | ||||
| £8 | ✓ | ✓ | ||
| £20 | ✓b | |||
| £25 | ✓ | |||
| £40 | ✓a | |||
Experimental design
A full factorial design using the attributes and levels in Table 32 resulted in 2 × 2 × 2 × 4 = 32 possible profiles and 1024 (322) possible pairwise choice sets to select from. Given that it was impossible to ask a respondent all the choice sets from the full factorial design, a selection of choice sets was generated using a D-optimal method in Ngene 1.1.2 software (ChoiceMetrics, Sydney, NSW, Australia). 54 This method is designed to provide stated choice experiments with optimal statistical properties and to elicit the maximum information from respondents to inform the estimation of model parameters. 55–57 We based the choice of the D-optimal approach on recent guidance about constructing experimental designs for DCEs. 58 Only main effects were considered, with non-linearities and interactions assumed negligible. Table 34 shows the D-optimality levels for DCE designs with different numbers of choices. The figures indicated that the marginal benefit of increasing the number of choices above 16 choice sets was minimal. We found little guidance about the maximum number of choice sets that a person could endure in a single DCE survey, but a recent study suggested that the number of choice sets depends on the complexity of the DCE study and the context of the research questions. 59 In our case, 16 choices might have been a large number to ask our population as they are not taking up the offer of cervical screening. In addition, the qualitative team already had struggled to recruit participants for their interviews and it was felt that non-responders (the target population for this DCE) were a ‘hard-to-recruit’ sample. Therefore, a trade-off between number of choices and D-optimality levels was made and 12 choice sets were selected for the DCE survey. This provided a D-optimality level of 90.86%, which was still considered high. This would ensure that the survey would be quicker to complete and likely to encourage participation and reduce missing responses.
| Number of choices | D-optimality levels (%) |
|---|---|
| 8 | 0.17 |
| 12 | 90.86 |
| 16 | 97.21 |
| 20 | 95.16 |
| 40 | 97.21 |
| 52 | 96.12 |
| 60 | 96.40 |
| 80 | 97.21 |
The final 12 choice sets used in the DCE are presented in Table 35, with an example actual choice set (in this case set number 1 in Table 33) presented to participants shown in Table 36.
| Choice set | Choice | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | |||||||
| Action | Location | Nurse | Cost (£) | Action | Location | Nurse | Cost (£) | |
| 1 | No | Home | No | 40 | Yes | GP | Yes | 8 |
| 2 | Yes | GP | Yes | 20 | No | Home | No | 25 |
| 3 | No | GP | No | 8 | Yes | Home | Yes | 20 |
| 4 | No | Home | Yes | 20 | Yes | GP | No | 25 |
| 5 | No | Home | Yes | 25 | Yes | GP | No | 40 |
| 6 | Yes | Home | Yes | 25 | No | GP | No | 40 |
| 7 | No | GP | No | 40 | Yes | Home | Yes | 8 |
| 8 | Yes | Home | No | 8 | No | GP | Yes | 20 |
| 9 | Yes | GP | No | 25 | No | Home | Yes | 40 |
| 10 | No | GP | Yes | 8 | Yes | Home | Yes | 20 |
| 11 | Yes | Home | No | 20 | No | GP | Yes | 25 |
| 12 | Yes | GP | Yes | 40 | No | Home | No | 8 |
| Feature | Choice | |
|---|---|---|
| 1 | 2 | |
| Action required by you to personally arrange a test | No | Yes |
| Location of your test | Home | GP surgery/clinic |
| Nurse available for discussion or help prior to appointment | No | Yes |
| Cost of your test to the NHS | £40 | £8 |
| Which choice do you prefer? | ||
Questionnaire design and data collection
Participants were given the option of completing a paper-based or online version of the DCE survey. A copy of the paper-based version and the patient information leaflet prepared to accompany the survey are presented in Appendices 14 and 15, respectively. Prior to the piloting phase, and to assess acceptability and ease of completion, the questionnaire was given to female members of staff at the Health Economics Research Centre within the University of Oxford to complete. Minimal changes to the questionnaire wording were made as a result of this exercise. The DCE questionnaire comprised a section containing a number of choice scenarios that incorporate the attributes and levels developed from the qualitative analysis presented above, a section on women’s general views about the value of the current Cervical Screening Programme and a section where women ranked each of the four attributes in order, from most important to least important. The questionnaire also included questions on basic demographic information such as age, education and employment status. Women were also asked about any difficulties encountered when completing the DCE questionnaire and were given the opportunity to feed back any additional comments. The English and Scottish screening agencies, in conjunction with the STRATEGIC research study team, identified non-responding women who were eligible to participate in the DCE study and sent a participant information leaflet, the paper-based version of the survey and a Freepost return envelope. As women had the choice of whether to complete the paper-based version or the DCE online, the web link for the online version was provided in the patient information leaflet and the paper-based version.
A custom-made online survey was developed using the open source application LimeSurvey (www.limesurvey.org). The online version was an exact replica of the paper-based version. The online version was tested thoroughly before, and during, the pilot study phase to minimise any potential problems during the main data collection. The web version complied with the required information governance standards of the University of Manchester and University of Oxford to ensure anonymity of responders and compliance with data protection. To ensure anonymity, a token number was created in LimeSurvey for each potential participant. Participants had to use this token number to access and complete the online survey. When the system recognised that a token had been introduced, it recorded only that the number had been used and not any other information about the participant. The token number was also included in the paper-based version. Once a token was used and a survey was completed, participants were not able to access the survey again.
To encourage participation in the survey, women were offered a £10 high-street voucher as an incentive. In order to claim the voucher, an online form was created and the web address to access the form was included in the patient information leaflet and paper-based survey.
First phase (pilot discrete choice experiment data collection) and second phase (main discrete choice experiment data collection)
In a first phase, a pilot study was conducted from July 2014 to September 2014 to assess the working interface between the English and Scottish screening agencies, and the STRATEGIC study research team. During this period, we also assessed the robustness of the online survey and the likely response rate for the second phase (main data collection). A total of 1000 questionnaires were sent to women in Greater Manchester (n = 650) and Grampian (n = 350) during this period.
The feedback from the pilot suggested that communications between the parties was clear and that the same arrangement could be used with no modifications in the main study. The online survey also worked well and no major issues were identified. The response rate was low (as described in detail in Results) but, overall, women felt that they completed the questionnaire without difficulties. Some women reported that it was not clear when the nurse would be available to help, so the words ‘prior to appointment’ were added to the description of the attribute for the second phase. We consulted a colleague with extensive experience in population surveys about possible additional ideas to improve the response rate during the main data collection. It was clear that there were difficulties in reaching the population of interest and it was recommended that the look of the questionnaire could be improved, ideally with the assistance of a graphic designer. This was undertaken and the final questionnaire is presented in Appendix 14. The research team also decided to maintain the £10 voucher during the main DCE data collection. No other changes to the questionnaire were made between study phases. The data from the pilot were entered once and subsequently checked by the quantitative STRATEGIC study research team.
The main DCE data collection (second phase) was carried out between mid-July 2015 and mid-September 2015, and 3000 questionnaires were circulated to women in Greater Manchester (n = 2000) and Grampian (n = 1000) during this period. Double data entry of paper responses received was used during this phase.
Sample size
Sample size calculations in statistics are traditionally used to minimise biases and ensure generalisability to the population of interest. There are approximately 300,000 women in England and 30,000 women in Scotland who are eligible to be screened for the first time every year. The STRATEGIC study cohort suggested that around 70% of women do not respond to the first invitation, resulting in 210,000 and 21,000 non-responder women in England and Scotland, respectively. With these figures, obtaining a representative sample size of the preferences of women to the Cervical Screening Programme would be very expensive using traditional sampling theories. Therefore, sample size calculations in stated choice experiments take a different angle: to identify the minimum number of choice observations needed to obtain reliable parameter estimates for discrete choice models using stated choice data. The latter approach is described in a recent paper60 and was used in this study. Briefly, the method suggests that, if prior information on the coefficients of a discrete choice model is available, it is possible to identify the theoretical minimum sample size (called S-error) required for a design. This is achieved combining the asymptotic variance-covariance matrix from the experimental design with the available prior information and specifying a significance level that normally corresponds to 5% and a t-ratio of 1.96. We used the information from the pilot phase to estimate the prior coefficients associated to each attribute using a conditional logit without a constant, as suggested by Rose and Bliemer. 60 The S-error was estimated to be 1154, but we also evaluated the minimum sample size requirements for each parameter separately. Figure 20 shows the asymptotic t-ratios for different sample sizes associated with each of the attribute coefficients, with the dashed line indicating a t-ratio of 1.96 (e.g. a coefficient significantly different from 0 at 5% level).
FIGURE 20.
Asymptotic t-ratios for different sample sizes for each parameter in the discrete choice model.

The figure suggested that, with sample sizes > 150, we were able to estimate significant parameters in the action, nurse and cost attributes but not on location. To obtain a significant parameter in the location attribute using our prior information we needed to obtain the sample identified as S-error = 1151. This information was very useful for the team to understand; given the response rate in the pilot study, it still appeared possible to obtain significant coefficients using the data from the second phase. As the prior information was based on a small sample of women, the results from this analysis were used for guidance, but did point out that a minimum sample size of 150 was needed to reliably estimate three attribute parameters. It was uncertain at that time whether or not the parameter for the attribute location was going to be significant with that sample size.
Statistical analysis
Descriptive statistical analyses were used to present participant demographic information, women’s views of cervical screening in general and any feedback about the stated choice questions. Choice data were analysed first using a conditional logit, and the reader is referred to Appendix 12 for its theoretical and formal derivation to analyse DCE data. One of the main criticisms of this model is the assumption of independence of irrelevant alternatives, which states that the ratio of the probabilities of choosing one alternative over another does not change with the introduction of a new alternative or the removal of an existing one. 47 We therefore also estimated a multinomial conditional probit that relaxed the independence of irrelevant alternatives assumption. Conditional logit and probit models are analysed using maximum likelihood estimation methods, but the former assumes a standard logistic distribution of errors, whereas the latter assumes a normal distribution. In addition, the multinomial conditional probit allows for a general covariance structure in the errors and hence does not impose the independence of irrelevant alternatives property present in conditional logistic models. The coefficients from logit and probit models are difficult to interpret directly, but the signs associated to each coefficient have a more straightforward interpretation. A positive coefficient means that an increase in the attribute leads to an increase in the predicted probability of selecting a particular scenario. A negative coefficient means that an increase in the attribute leads to a decrease in the predicted probability. The aim of the choice model was to identify the effect of each attribute level on women’s preferences for potential interventions to increase the uptake to the Cervical Screening Programme. The levels for action, location and nurse were defined using dummies, whereas the cost variable was included as a continuous variable. The ‘no’ level was used as the reference level for the action and nurse attributes and ‘home’ was used as the reference level in the location attribute. Costs were expressed in 2013/2014 UK pounds. A main-effects specification, using attribute levels only and main effects plus women characteristics, was evaluated in the models. The latter model was used to determine any propensity to select a particular scenario in the choice set given the characteristics of the participants. In our unlabelled DCE, it was expected that all these covariates would be non-significant and that the final model would be either the main-effects conditional logit or probit. Characteristics were included as binary dummies (0/1) in the model (i.e. employed vs. other current main activities or higher education vs. other highest level of education). Robust standard errors associated with attribute coefficients were estimated in recognition that each participant completed 12 choice scenarios. The final selected model was based on the model specification that yielded best goodness-of-fit measures (lowest value) based on the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). The coefficients of the final selected model were employed to predict the probability of selecting each of the STRATEGIC study interventions, identified in Table 33, as potential strategies to increase screening uptake. All analyses were carried out in StataMP version 13 (StataCorp, College Station, TX, USA).
Results
Response rate and final sample size
Figure 21 describes the flow of women through the study. The left-hand side presents figures for the pilot DCE data collection and the right-hand side for the main DCE data collection. In total, 4000 questionnaires were sent out. Figure 21 shows that response rates were similar for both phases at around 5.5%, as were the proportion of respondents from Greater Manchester and Grampian. In both phases, the majority of respondents also chose to complete and return the paper-based version of the questionnaire. There was a general concern that using only data from the second phase would risk obtaining a reasonable sample size to estimate the models as indicated in Sample size. Changes made to the DCE from the first to second phase were minor and the same experimental design was used in both phases with the only amendment of adding the words ‘prior to appointment’ to the nurse attribute. Therefore, data from the first and second phases were combined to obtain a final sample size of 222 responses.
FIGURE 21.
Flow diagram of women’s participation through the study.

Characteristics of the sample
Table 37 describes the characteristics of the 222 participants in the stated choice questionnaire study. Almost three-fifths were from England and two-fifths from Scotland, and the mean overall age was 24.6 years. Women from Scotland were, on average, 4 years younger than women from England, reflecting the lower age at which women are first invited for cervical screening in Scotland (20 years vs. 24.5 years). The majority of women (86.4%) were of white ethnicity, with women of Pakistani, Indian and African descent making up a further 9%. Almost half of the respondents had been educated up to a university level, and a further 40% had either studied to the age of 18 years or undertaken vocational qualifications. Just 6% of women had left school aged 16 years and 5% chose not to disclose information on their education levels. In response to the question on main activity, 57% of women reported being in employment, just over one-quarter were undertaking full- or part-time studies, and 8% were homemakers looking after their families. The percentage of women who were unemployed, undertaking voluntary work, or who were long-term sick or disabled was 3.2%, 1.8% and 1.8%, respectively.
| Variable | Frequency |
|---|---|
| Country, n (%) | |
| England | 127 (57.2) |
| Scotland | 92 (41.4) |
| I’d rather not say | 3 (1.4) |
| Age (years), mean (SD) | 24.6 (2.5) |
| England | 26.4 (0.9) |
| Scotland | 22.1 (1.8) |
| Missing | 6 |
| Ethnicity, n (%) | |
| White British/Irish | 174 (79.5) |
| Any other white background | 15 (6.9) |
| Pakistani | 11 (5.0) |
| Other | 9 (4.1) |
| Indian | 4 (1.8) |
| African | 4 (1.8) |
| Bangladeshi | 1 (0.5) |
| I’d rather not say | 1 (0.5) |
| Missing | 3 |
| Highest level of education, n (%) | |
| University | 108 (48.7) |
| Further education to age 18 years | 47 (21.2) |
| Vocational qualifications | 44 (19.8) |
| School leaver at age 16 years | 13 (5.9) |
| I’d rather not say | 10 (4.5) |
| Main activity | |
| Employed | 127 (57.2) |
| Student (full- or part-time) | 57 (25.7) |
| Homemaker looking after the family | 18 (8.1) |
| Unemployed and seeking work | 7 (3.2) |
| I’d rather not say | 5 (2.3) |
| Unpaid voluntary work | 4 (1.8) |
| Long-term sick or disabled | 4 (1.8) |
Women’s views of cervical screening
To gauge women’s views of cervical screening in general, respondents were presented with the statement ‘screening for cervical cancer is important’, and were asked to select one of the following responses: strongly agree, agree, neither agree nor disagree, disagree or strongly disagree. Figure 22 shows that, despite not attending for their screen, almost two-thirds of responding women strongly agreed with the statement that screening for cervical cancer is important, and a further 30% agreed. A total of 4.5% of women appeared indifferent, and just 1.8% disagreed that screening for cervical cancer is important.
FIGURE 22.
Responses to the statement ‘screening for cervical cancer is important’.
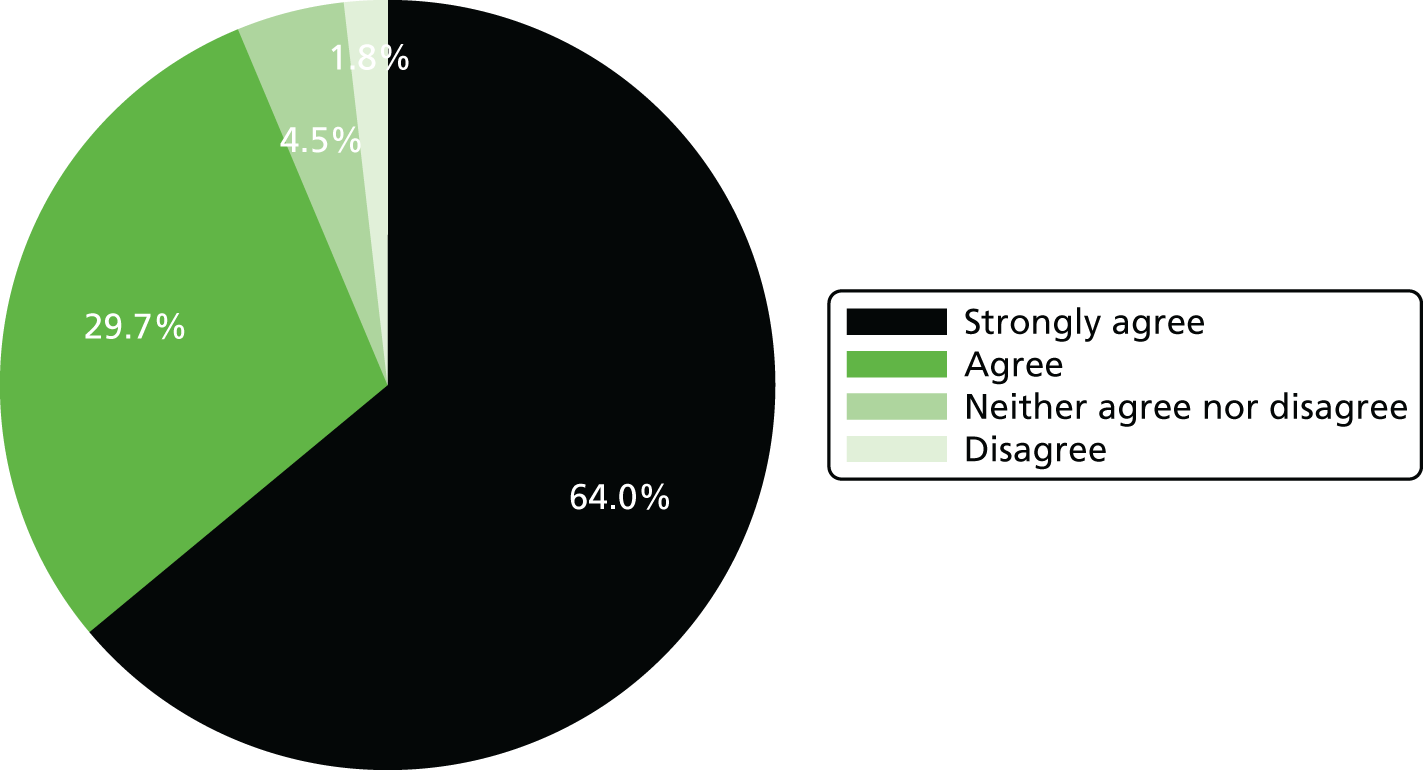
Ranking of attributes
When asked to rank each of the four attributes included in the DCE in order from most important (rank 1) to least important (rank 4). Figure 23 shows that most women identified the location of the test as being most important (93/204, 46%), whereas others were most concerned about whether or not they personally needed to take any action to arrange a test (63/204, 31%) or whether or not a nurse was available as part of the screening process (34/204, 17%). Although a small number of women (14/204, 7%) considered the cost to the NHS to be most important, for most women (91/204, 45%) cost was the least important attribute.
FIGURE 23.
Ranking of four attributes (action required to arrange a test, location of test, nurse available for help and advice, and cost of test) from most (1) to least (4) important. Denominator is 204, as 18 participants did not complete this question in the survey.
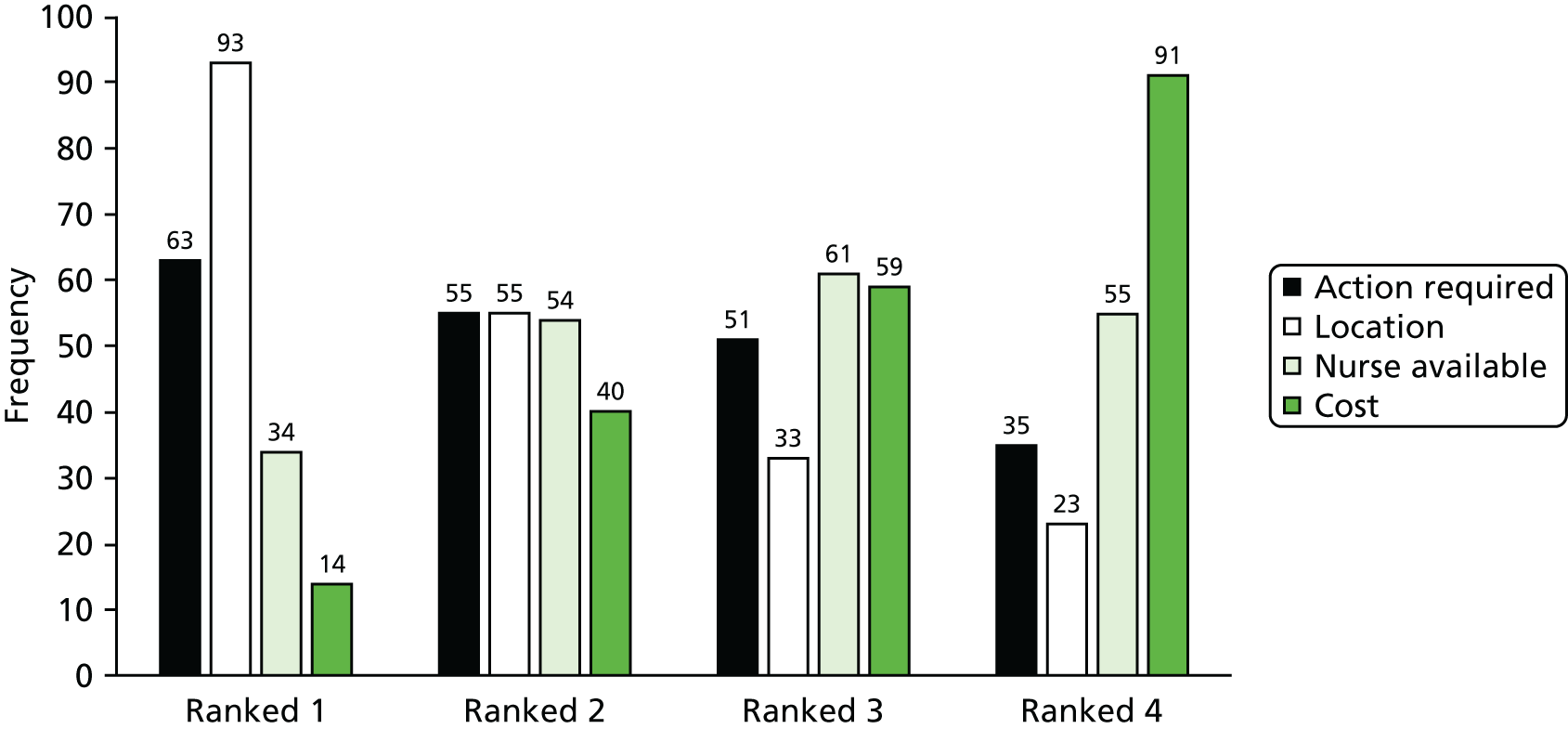
Stated choice data analysis
Table 38 provides a summary of the proportion of participants selecting choice 1 across the 12 choice sets as presented in Table 35. There was a clear trend to select choice 1 in scenarios 4–6 and 10, and a trend to select choice 2 in scenarios 1 and 7. These results provided some insight about the interpretation of the constant in the modelling results in the next table.
| Choice set | Number of participants completing the choice scenario | Proportion selecting choice 1 |
|---|---|---|
| 1 | 221 | 0.276 |
| 2 | 219 | 0.553 |
| 3 | 221 | 0.498 |
| 4 | 220 | 0.8 |
| 5 | 220 | 0.855 |
| 6 | 220 | 0.791 |
| 7 | 222 | 0.176 |
| 8 | 221 | 0.561 |
| 9 | 219 | 0.516 |
| 10 | 222 | 0.712 |
| 11 | 221 | 0.466 |
| 12 | 221 | 0.371 |
The results of the conditional logit and multinomial probit models using main effects and main effects plus selected women characteristics are presented in Table 39. The table reports the coefficients, associated standard error and p-values for each attribute or women characteristic. It also reports information about the goodness of fit to assist in the identification of the final selected model. For both types of models there were marginal improvements in terms of the AIC and BIC using the probit model, although model coefficients were similar between both model types. As expected, including women’s characteristics in the main-effects models did not add any significant information as all demographic coefficients were not significant in both models. Given the lower AIC and BIC, our final selected model for our stated choice data was the multinomial probit model with main effects. The coefficients in the selected model indicate the contribution of each attribute to the probability of selecting a particular scenario (described by a combination of levels for the four attributes). Negative coefficients indicate that women preferred these attributes to take lower values for continuous variables (costs) or preferred the reference attribute for qualitative attributes (action, location and nurse). For example, the signs for the main-effects attributes were significant (p < 0.001), suggesting that women preferred scenarios where minimal personal action was required to arrange a test, the test was done at home, there was a nurse available for discussion or help, and the test was cheaper to the NHS. The constant was statistically significantly different from 0 indicating that potential sources of utility were not captured by the information in the DCE or a potential bias towards selecting always choice 1 in the choice set.
| Covariates | Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Conditional logit | Conditional logit with women characteristics | Multinomial probit | Multinomial probit with women characteristics | |||||
| Coefficient (SE) | p-value | Coefficient (SE) | p-value | Coefficient (SE) | p-value | Coefficient (SE) | p-value | |
| Constant | –0.222 (0.030) | 0.000 | –0.13688 (0.08996) | 0.127 | –0.191 (0.025) | 0.000 | –0.115 (0.076) | 0.132 |
| Main effects | ||||||||
| Action required by you | ||||||||
| No (reference) | ||||||||
| Yes | –0.134 (0.048) | 0.005 | –0.136 (0.048) | 0.005 | –0.116 (0.041) | 0.004 | –0.118 (0.041) | 0.004 |
| Location of test | ||||||||
| Home (reference) | ||||||||
| GP surgery/clinic | –0.256 (0.098) | 0.009 | –0.254 (0.099) | 0.010 | –0.220 (0.084) | 0.009 | –0.219 (0.085) | 0.010 |
| Nurse available | ||||||||
| No (reference) | ||||||||
| Yes | 0.496 (0.055) | 0.000 | 0.494 (0.055) | 0.000 | 0.429 (0.047) | 0.000 | 0.428 (0.047) | 0.000 |
| Cost of your test to NHS | –0.033 (0.003) | 0.000 | –0.032 (0.003) | 0.000 | –0.028 (0.002) | 0.000 | –0.028 (0.002) | 0.000 |
| Covariates | ||||||||
| Women living in England | 0.034 (0.065) | 0.603 | 0.028 (0.055) | 0.609 | ||||
| White British/Irish | –0.067 (0.070) | 0.316 | –0.059 (0.057) | 0.302 | ||||
| Higher education | 0.06 (0.060) | 0.924 | 0.003 (0.051) | 0.960 | ||||
| Employed | –0.082 (0.064) | 0.198 | –0.072 (0.054) | 0.184 | ||||
| Goodness of fit | Value | Value | Value | Value | ||||
| Log-likelihood | –1592.46 | –1575.30 | –1591.96 | –1574.79 | ||||
| AIC | 3194.93 | 3168.61 | 3193.91 | 3167.58 | ||||
| BIC | 3227.80 | 3227.65 | 3226.79 | 3226.63 | ||||
| Number of observations | 5294 | 5222 | 5294 | 5222 | ||||
| Number of women | 222 | 219 | 222 | 219 | ||||
The coefficients from the main-effects probit model were employed to estimate the probability of selecting each of the interventions assessed in the STRATEGIC study based on our choice data and are presented in Table 40. The intervention with a higher probability was the unsolicited SSK, with an estimated figure of 0.263. Although no intervention clearly dominated, strategies involving SSKs accumulated > 75% of the total joined probability across all interventions. This seemed to indicate that women had a clear preference for interventions that include the SSK.
| Timed appointment | Solicited SSK | Unsolicited SSK | NN and SSK | NN and clinic test |
|---|---|---|---|---|
| 0.130 | 0.234 | 0.263 | 0.256 | 0.117 |
Feedback discrete choice experiment survey
Discrete choice experiments have previously been criticised by respondents for being difficult to understand and to complete. To explore if this was the case within the context of the STRATEGIC study DCE, women were presented with each of the three statements shown in the first row of Table 41 and were asked to indicate whether or not they were in agreement, using the same five categories given in the preceding question on the importance of screening.
| Levels of opinions | The questions were easy to understand, n (%) | The difference between the choices on each question was clear, n (%) | It was easy to make a choice on each question, n (%) |
|---|---|---|---|
| Strongly agree | 70 (31.5) | 66 (29.7) | 47 (21.2) |
| Agree | 121 (54.5) | 103 (46.4) | 100 (45.0) |
| Neither agree nor disagree | 20 (9.0) | 42 (18.9) | 43 (19.4) |
| Disagree | 9 (4.1) | 7 (3.2) | 30 (13.5) |
| Strongly disagree | 2 (0.9) | 4 (1.8) | 2 (0.9) |
| Total | 222 (100) | 222 (100) | 222 (100) |
Table 41 shows that 86% of respondents agreed or strongly agreed with the statement that the questions were easy to understand, and just 5% disagreed or strongly disagreed. Over three-quarters of women agreed or strongly agreed that the difference between the choices on each question was clear, although around one-fifth of women appeared unsure, reporting that they neither agreed nor disagreed. In response to the statement about it being easy to make a choice on each question, two-thirds of women agreed or strongly agreed, and around one-fifth again appeared unsure. Almost 15% of women disagreed or strongly disagreed with the statement that it was easy to make a choice on each question.
Women were also given the possibility of writing any comment about the stated choice questions or any other aspect of the questionnaire using a free-text box. The literal qualitative comments received during phases 1 and 2 are presented using themes in Appendix 16.
Discussion
This chapter has described the design, conduct and reporting of a DCE undertaken to elicit women’s preferences for interventions with the potential to be embedded in the current NHS Cervical Screening Programme to increase the uptake of screening among young, non-responding women.
The stated choice experiment aimed to generate information on women’s preferences for each of the interventions under evaluation in phase 2 of the STRATEGIC study, with a view to providing some insight into if they might be effective at improving uptake among non-responders. The results from the modelling exercise suggested that women preferred scenarios where minimal personal action was required to arrange a test, the test was done in the privacy of their own homes, there was a nurse available for discussion or help and that involved less cost to the NHS. The selected probit model predicted that interventions with a SSK were preferred and could potentially result in a higher probability of increasing uptake of screening than with interventions without the SSK. These results were in line with the information obtained in the qualitative phase of the study about potential aspects of privacy and expertise and are briefly considered in light of the trial results in Chapter 5. Comparing stated and revealed preferences is a very interesting issue that has received little attention in the literature.
Despite not attending for their screening test, around 94% of women agreed or strongly agreed with the statement that screening for cervical cancer is important. This finding can be interpreted as meaning that they consider it important to women personally or a more general view that screening is a worthwhile activity for the NHS and the UK population. Although we cannot determine which interpretation is the correct one, either view highlights that screening for cervical cancer from a personal or a societal perspective was highly valued by the participants.
To our knowledge, this is the first attempt to elicit preferences from non-responding women in the Cervical Screening Programme in the UK. Although DCEs in general are becoming increasingly common, few have been conducted in the area of cervical cancer screening. 41 One previous stated choice exercise in the area elicited women’s preferences for changes to the screening programme, which resulted in reduced recall rates and waiting times for results and varying screening intervals by age. 44 That study, however, primarily included women who had previously attended for screening, and consequently the results obtained in the STRATEGIC trial and this stated preference study should be considered as providing the most current evidence about preferences of non-responding women in the Cervical Screening Programme in the UK.
This study attempted to adhere at all times to good practice guidelines for conducting DCEs for both its qualitative and quantitative components, supporting the robustness of the results obtained,47,51,53,58 but there are several limitations worth noting.
During initial discussions about the inclusion of the DCE within the STRATEGIC study, the challenges around attempting to elicit responses from a cohort characterised by their very non-responsiveness, was well recognised. This was solidly verified during the qualitative and quantitative data collection phases. Identifying these women was challenging and could only be facilitated by the English and Scottish Cervical Screening Programmes who also managed the questionnaire mail-out for reasons of patient confidentiality. The study team, therefore, had no access to any individuals’ information at any time. Prior to committing to the main survey, a pilot survey was conducted to test the feasibility of this approach, and showed the screening programme mail-out to work well. Without access to patient information, this approach precluded the use of reminders by the research team, which might have been capable of increasing response rates. The issue of poor response rate was also highlighted in the study by Wordsworth et al. ,44 which targeted a cohort of women of screening age who had or had not previously attended for screening. In that study, although the response rate among women who had previously been screened was 44%, the rate for those who had never been screened was just 8%, despite two reminders being sent. This again highlights the difficulties involved in reaching and engaging with women who choose not to attend for screening.
Other attempts were also made to maximise response rates. A professional design team was involved to improve the look and the attractiveness of the questionnaire, which was also offered to women in both paper and online formats (the thinking being that young women might prefer online submission). In addition, women were also offered an incentive in the form of a £10 shopping voucher. Despite these attempts, the number of respondents was still low, at around 5.5% of the 4000 women mailed. It is therefore necessary to acknowledge that the findings presented in this chapter may not necessarily be generalisable to the population of young, non-responding women as a whole. For example, the educational attainment and social class may be higher than the general population of non-attenders. The findings are, however, intuitive, in line with prior expectations and show clear preferences for the attributes of interventions designed to overcome the well-documented reasons for non-attendance at screening. 4,61–64
An ‘opt-out’ question with the choice ‘not to screen’ was not included as part of the experimental design in the DCE survey. Cervical screening is an area where women can choose not to be screened, as was the case with the target population in this study. Including an ‘opt-out’ question would have made sense if the aim of the study had been to evaluate whether or not cervical screening was a worthwhile activity for the NHS. In this case, women had already stated a preference; they had decided not to attend. The objective of our study was to obtain information about the trade-offs between attributes so that we could gain insight into the most likely combination of attributes that would encourage women to participate, and not if the Cervical Screening Programme itself was a worthwhile activity. Although an opt-out version could have been a realistic alternative in our study design, if selected we would not have known why women had chosen that option. Therefore, in our DCE we excluded an opt-out option forcing a choice but included a question in the survey about views on the Cervical Screening Programme and whether or not interventions such as those described in the survey would encourage personal participation.
The final selected choice model estimated a significant constant indicating the possibility that potential sources of utility were not captured by the information of attributes and levels included in the DCE or a propensity to select choice 1 in the stated choice survey. It is possible that we have excluded additional key attributes for these women but, as described in the qualitative component of this report, a trade-off between an extensive list of attributes and minimising missing responses had to be made. Given the results of the STRATEGIC trial, and those reported here, we are confident that at least the attributes included in our DCE have captured an important proportion of the information processed by women when selecting a particular choice scenario. Related to a potential propensity to select choice 1, it is true that the descriptive analysis showed that women, on average, selected choice 1 in more scenarios than choice 2. Most of these scenarios, however, identified interventions for the SSK and hence this was not surprising. It has been previously recognised that D-optimal designs may result in an experiment with a particular attribute level dominating the exercise. 54 It is therefore possible that our experimental design was not optimal in terms of utility balance (i.e. equally attractive options within choice sets) but optimal in terms of level balance, orthogonality and minimal overlap. 65 In addition, all the estimated models with main effects and women characteristics suggested that none of the covariates (including the constant) was statistically significant. This indicated that, when controlling for respondent’s characteristics, no potential propensity to choose a particular scenario in the choice set was observed.
Chapter 5 Discussion
The STRATEGIC trial has shown that a variety of interventions designed to increase uptake of cervical screening in young women receiving their first invitation exerted little impact when compared with controls. With the exception of sending unrequested SSKs and being given timed appointments, women in practices who were offered these interventions had similar participation in screening when compared with control practices. This applied both to women at the time of their first standard invitation and to those designated as non-attenders 6 months after the invitation.
The pre-leaflet designed to increase preparedness to be screened was ineffective. Online booking, SSK and timed appointments were adopted by a number of women who might otherwise not have been screened. The finding that the control group demonstrated an unexpectedly persistent uptake over time, as well as the data on the type of SSK preferred, were helpful in interpreting the significance of participation following phase 1 and phase 2 interventions.
This was a challenging study in several respects. It relied heavily on a third party (i.e. the NHS screening agency) to provide access to the trial interventions, to women invited for screening in some cases by direct mailing of samplers and in others by mailing additional information. This arm’s-length approach did achieve a realistic operational setting for this type of research and utilised routine data in a large complex clinical trial. The disadvantage was the indirect nature of contact, which resulted in dependence on the goodwill of the screening agency and the inherent interest of women in facilitating the research. Further challenges were that we were trying to reach women in phase 2 who had shown an initial lack of enthusiasm for screening, and operationalising new interventions that had not been fully implemented prior to the study.
Strengths and weakness
The strengths of this study included the following: (1) it was a large study in a real-world context; (2) it tackled a hard-to-reach group and attempted several novel interventions; and (3) it had control practices for comparison.
The weaknesses of the study included the following: (1) the interventions had to be administered at arm’s length by the investigators – administration by primary care staff may have exerted more influence; (2) online booking was not professionally set up and was not as widely accessible as we would have wished; and (3) as acknowledged earlier, the DCE relied on too few survey responders.
A further limitation of the design was that precisely interpreting the effect of the interventions among a broader age range is not possible.
Phase 1: pre-leaflet and online booking
Our expectation from historical screening data was that only 25–30% of invited women of this age would have been screened 6 months following the initial standard invitation. This was indeed what occurred in the STRATEGIC study, with the pre-leaflet control group demonstrating 30.6% uptake at 6 months. The rate of uptake over time shown in Figure 4 shows that women appear to attend for screening over a protracted period, which continued beyond phase 1 and throughout the duration of the study. The lack of impact of the pre-leaflet is striking with the Kaplan–Meier curves for the pre-leaflet and no pre-leaflet group virtually overlying each other (see Figure 4). Pre-leaflets have been shown to be of some benefit in bowel cancer screening. In a randomised study reported from Scotland, a pre-leaflet was associated with increased uptake among both high- and low-uptake groups,7 the authors attributing this effect at least in part to the health belief model,66 with the letter acting as a cue and increasing preparedness to participate in screening. In an Australian study,6 a so-called advanced notification letter ahead of the standard invitation for bowel cancer screening was associated with a 25% greater uptake than the standard invitation alone. These authors cited the transtheoretical model in suggesting that simply increasing awareness may move people from contemplating (screening) to action. Direct comparisons with bowel screening may not be appropriate, however, as the latter involves a much older age group, and the process is a private one. The reason for lack of impact by our pre-leaflet is not clear, despite its content being based on views expressed to us by young women at focus groups on cervical screening. It may be that for a substantial proportion of young women cervical screening is not a priority, while others may appreciate the rationale for screening, and those who intend going for a screen will go eventually. For some, they are at a pre-contemplation stage and proceeding to action depends on a decisional balance. 67 For the woman who does not feel at risk of cancer, the perceived effect size of attending for cervical screening may be insufficient to push them into taking action.
Online booking
We had considered that the facility to book online would appeal to young women who are used to this process in other areas of life. It might have been expected that this would prompt a more rapid response, but it had no such effect. The Kaplan–Meier curve indicates a similar rate of uptake until 3 months, when a routine reminder is sent from the screening agency, and at which point there did appear to be a net uplift in screening among those offered online booking, although only 2.4% actually booked online. The actual absolute difference was 0.53% at 3 months, rising to 2.18% at 6 months. Although this does not achieve statistical significance, it should be borne in mind that this offer through just three family planning clinics was tested only in the Manchester cohort, where the control uptake was actually less than the STRATEGIC study cohort as a whole (26.3% vs. 30.6%). It may also be that some women would feel negatively about attending at a sexual health clinic. It seems possible, therefore, that wider access and more professional presentation could prove a more popular means of booking a screening appointment, but it would not seem an effective way of achieving increased uptake. A number of general practices do offer online booking of appointments, although not necessarily for cervical screening. Aside from the appeal of online booking, it does have the potential to make booking more efficient and avoid the disincentive of booking a practice appointment by telephone, which can be frustrating. Of course, even if online booking cuts out hassle, it does need to offer times that are convenient to women. Bookings were made throughout the day, but only two-thirds of women who made an appointment online actually attended. The one-third non-attendance rate represents a waste of resources.
Effect of prior human papillomavirus vaccination
One of the concerns expressed by professional groups when the HPV vaccination policy was being implemented was that young women may overestimate the degree of protection provided by vaccination and may disregard the benefit of cervical screening, with the effect that fewer women would attend. Although the age of starting cervical screening in Scotland is planned to rise to 25 years, it remains at present 20 years, which provided an opportunity to study uptake among young women in Grampian according to their vaccination status. The vaccinated women in the STRATEGIC study were from the catch-up campaign, which is reflected in the fact that 27.2% had not been vaccinated, a higher proportion than in the schools-based programme for 12- to 13-year-olds. There was, in fact, a very striking difference among those who had no vaccination and those who had been fully vaccinated. Of course, lack of vaccination in this setting could be associated with social deprivation because the catch-up campaign did carry a greater risk of not accessing disadvantaged children than was the case with the schools-based programme for 12- to 13-year-olds. A similar finding68 of greater uptake of cervical screening among vaccinated women was recently reported from Sweden among women aged 25–35 years. A recently published Scottish study69 found that the rate of uptake among unvaccinated women was only 65% of that among vaccinated women. Furthermore, the decline in screening seen between the 1988 and 1993 birth cohorts was compensated for by the increase in screening among vaccinees. Whether or not this observed screening uptake among vaccinees and non-vaccinees will be seen in England, where cervical screening begins 5 years later, remains to be seen. If the pattern is mainly associated with social class, it may well follow the same trend. If it were associated more with increased awareness, this may diminish with longer passage of time. Although the OR was smaller in Sweden, it remained significant after adjusting for socioeconomic status. These data suggest that, rather than making young women complacent about protection from cervical cancer, screening has increased their awareness of the need to be protected. Another recent study in the UK has reported a significant rise in return to screening among mothers of vaccinated daughters, underscoring the positive impact of HPV vaccination on cervical screening uptake. 70 This provides reassurance that uptake among women may increase throughout the UK population, an effect that may be seen in England as early as 2016/17. The increased odds of being screened by 6 months that was noted in Grampian compared with Greater Manchester could be related to a vaccination effect, or it may be related to other factors, such as a higher overall uptake of screening in Grampian than in Greater Manchester.
Phase 1 interactions and moderators
The lack of interaction between pre-leaflet and online booking in phase 1 was not surprising not only because the pre-leaflet appeared to be ineffective, but also because the two interventions are unrelated. The pre-leaflet intervention showed no significant interaction effect with either the location (Grampian vs. Greater Manchester) or the vaccination status, indicating no differential pre-leaflet effect in locations or cumulative effect with vaccination status. It is interesting, however, that the Grampian cohort uptake was significantly greater overall during phase 1 (33.5% vs. 30.1%; OR 1.275, 95% CI 1.33 to 1.44; p ≤ 0.001), suggesting that awareness from the vaccination programme or lifestyle factors of the differing age groups may play a part. The differing location effect should be interpreted with caution, the differing method of acquiring practice baseline attendance rates for Greater Manchester and Grampian meant that the covariate had to be removed from the model. Here, failure to adjust for differential baseline rates may bias the effects.
Phase 2
The phase 2 cohort constituted the non-attenders from the phase 1 cohort, and it might have been expected that they would have been more ‘resistant’ to screening. In fact, 27.5% (2788/10126) of non-attendees in phase 1 had undertaken a screening test by 18 months in phase 2, a proportion very similar to the 30.9% at 6 months post phase 1. This can be seen in the phase 2 Kaplan–Meier curve, which shows a steady and continuous uptake (see Figure 7), almost half of which was achieved in the first 6 months following the interventions, and half subsequently. It is true that the duration of follow-up in phase 2 was longer; nonetheless, it demonstrates that attendance at screening, at least for these young women, is more of a continuous process and less of a prompt response to an invitation. This has not been as clear before, at least following routine invitations. Women who required early recall for mildly abnormal cytology prior to HPV triage were known to respond very variably, often requiring several reminders. It would seem that women do not feel the need to attend promptly, but that they do eventually feel that they should attend. The ‘nudging’ effect of the interventions in phase 2 may have some effect, but does not appear to explain this phenomenon fully because, although some practices in the control group will send their own communication to women, others may not. Additional ‘nudging’ may be provided by GPs who notice when women consult them for unrelated issues that cervical screening is overdue. Other potential nudges are intermittent campaigns run locally. The screening agency does inform GPs of non-attenders, but it is not mandatory for practices to send further reminders after 6 months. We do not have comprehensive data on what general practices do after 6 months’ non-attendance.
As far as the individual phase 2 interventions are concerned, the odds of being screened were significantly greater at both 12 and 18 months with SSKs being sent out unsolicited. SSKs being offered was ineffective either as a stand-alone offer or as part of a choice between it and a NN. The NN intervention performed most poorly and at 18 months was actually detrimental. Timed appointments were associated with increased uptake at 12 and 18 months’ follow-up. SSKs sent and timed appointments appear to emerge as the only interventions that were associated with an increase in uptake, and that uplift was only 5% and 3.6%, respectively, at 12 months and about half of that by 18 months compared with the control group. In terms of the entire population invited for screening this would represent around 1.5% uplift in absolute terms as phase 2 included only 70% of the entire invited cohort.
Another insight into uptake of screening in the STRATEGIC study is that women did not react directly to interventions, in the sense of specifically choosing what was offered. For example, the majority of those who were screened following a SSK being sent, did not actually send in a sample for HPV testing, but simply attended for a cytology sample to be taken. This is a significant finding and does suggest a ‘nudging’ effect.
With regard to timed appointments, although we have data on the actual number of timed appointment requests sent to GPs, we do not know the number that were actually offered to women by GPs, or the number of these that women attended. It is, therefore, not possible to assess what proportion of women who were screened after receiving a timed appointment actually attended that appointment, which is relevant to their cost-effectiveness. Evidence supporting timed appointments comes from a recently published study of breast screening that reported a second timed appointment sent to women who had not attended by 4 months after their invitation timed appointment was taken up by 20% overall. Among those who had received their initial invitation, the uptake was 24%. This translated to a 6% uplift among this age group (< 53 years) overall, and a 1.5% uplift in the overall uptake of the Breast Screening Programme. 71
There is no doubt that the cluster randomised control group in the STRATEGIC study in both phases 1 and 2 has greatly increased the reliability of the findings. Without this, the unexpectedly high uplift in attendance after 6 months could have been attributed directly to the interventions, whereas the control group participants suggest that this is not the case.
Although the STRATEGIC trial was aimed at women receiving their initial invitation, the applicability of these findings to a broader age range is unclear. The large majority of older women will have been screened previously, some not only for cervical disease, but also for breast and bowel cancer. The convenience of timed appointments would apply to women across the full age range as indeed would SSKs for non-attenders. There is no reason, however, to believe that either pre-notification or online booking would be viewed differently.
Cost-effectiveness
The effectiveness of the interventions is driven entirely by two things in our analysis: (1) the observed impact in the trial of the interventions on screening attendance and (2) the lifetime benefits of screening, derived from the meta-analysis, which indicated a lifetime quality-adjusted life expectancy increase of 0.0947 QALYs per woman attending. It was clear from the ORs of the different interventions that the unrequested SSK intervention had the largest statistically significant effect, followed by timed appointment intervention, and this is reflected in the cost-effectiveness results. The total cost of the timed appointments intervention is higher than the control because of the intervention costs and the additional lifetime screening and other health costs arising from participation in the screening programme, and this would amount to just over £37 per woman offered the intervention, or £9.5M over the entire lifetime of a national cohort aged 25 years. The health benefits would be 0.0048 QALYs averaged over a woman offered the intervention, or 1234 for the national cohort aged 25 years. Therefore, the cost per QALY gained of a national programme would be £7741, which is well within the usual cost-effectiveness ceiling of £20,000 per QALY gained used by NICE. As the cost-effectiveness planes and the cost-effectiveness acceptability curves showed, there is a high probability that this intervention is more effective and more costly, and the likelihood that it falls below the £20,000 per QALY ceiling is around 94% (measuring outcome at the primary end point of 12 months). The unrequested SSK intervention was even more effective, but also more costly. Extended to the whole cohort of 25-year-old women in England, the lifetime cost would be £12.9M with 1524 QALYs gained, and a cost-effectiveness ratio of £8438 per QALY gained for a national programme. As with the time appointments, there is a 94% probability that this intervention is below the £20,000 ceiling.
The results for the other interventions were much less favourable. The requested SSK is more likely than not to be effective, as shown in the OR, but this was not statistically significant and when combined with likely costs the probability that this intervention is cost-effective is no more than 70% at conventional cost-effectiveness ceiling ratios. The combination intervention is very similar to requested SSK alone, due almost entirely to the requested SSK component. There is no certainty at all that online booking would be clinically effective or cost-effective. The pre-leaflet and NN interventions might be associated with lower lifetime costs, but only because the OR of attendance for both was < 1, and so both interventions had lower costs but also lower lifetime QALYs than their control groups. In these circumstances, it is possible that decision-makers may wish to consider whether or not a small reduction in health benefits is a price worth paying for a large saving in health-care costs, for example if savings are > £20,000 for every QALY sacrificed. However, here that is not the case; savings would be modest in relation to the loss of health.
The costs of the interventions were based on the costs of delivering them to women participating in the trial. These varied from just £0.14 per woman enrolled in the NN arm of the study to £11.53 per woman enrolled in the unrequested SSK arm. If offered to the entire population there would be some saving on the fixed costs of these interventions (such as development of materials and training of staff), as these could be spread over a larger population and would not have to be incurred each year. In addition, we have assumed that variable costs for things such as printing and posting letters could be reduced if these interventions were scaled up to the national level, and have assumed a reduction of 20% could be obtained. On that basis, the most expensive interventions to provide would be the unrequested SSK, at an annual cost of £3.37M for each new cohort entering the screening programme, and timed appointments at £1.46M.
The health-care costs incurred if women do attend for screening vary between arms only insofar as there was minor variation in the recorded numbers having HPV or cytology tests or both; no information was available on actual colposcopies or other items of health-care use and so the same assumptions were applied to all women. As a result, the cost per woman attending did not vary widely, ranging from £46 for unrequested SSK to £57 for the phase 1 interventions. To estimate the lifetime costs and benefits of attending screening we relied on a meta-analysis of published studies, which reported lifetime costs and outcomes; these suggested that the additional lifetime cost of a screened woman was £566. This is approximately 10 times the within-trial figures quoted above, and so is roughly in line with a woman with a full lifetime attendance record in the English programme and no recalls who would attend 12 times (every 3 years aged 25–49 years, every 5 years aged 50–64 years). The introduction of primary HPV screening could reduce this to seven or eight visits, depending on the duration of extended screening intervals.
If both timed appointments and unrequested SSK were to be independently introduced nationally, the total additional cost would be approximately £22.3M, this would be the intervention and lifetime screening and treatment costs for a 25-year-old cohort, but would approximate to the annual national cost for the entire screened population once a steady-state had been reached. It seems unlikely in the current programme based on cytology that these would be offered simultaneously, and more plausible to think that timed appointments might be offered to all and unrequested SSK to those who did not attend. There is a range of such scenarios in which costs and effects might vary depending on a number of assumptions, these have not been fully explored here, and would require further attention. In the event of primary HPV screening, routine self-sampling for HPV could be feasible and indeed popular with women, and the added expense of sending SSKs would be compensated for by avoiding the need for timed appointments and achieving considerable cost saving in primary care by not requiring practitioner-obtained samples in the large majority.
Among the limitations of the economic analysis was that lifetime costs and effects had to be estimated using average cost and effect results from a meta-analysis; some uncertainty was captured, reflecting variations between studies, but this does not reflect the full uncertainty that might be captured if patient-level simulation had been possible. However, the lack of such data was a consequence of a streamlined and simplified trial design, which otherwise might not have been possible to perform. The implementation costs could also be estimated with more precision, for example by examining the economies of scale obtained in other screening programmes. However, these do not have a major impact on the cost-effectiveness results, which are heavily influenced by the observed effects of the interventions on attendance. Neither has the economic analysis replicated the subgroup analyses performed in the main trial results, comparing vaccinated and unvaccinated women, and comparing the two screening centres in Aberdeen and Manchester; it is unclear how any cost-effectiveness differences arising from these comparisons could be interpreted or used by decision-makers.
The economic analysis used estimates of the costs associated with screening attendance that were derived from previous published studies. These do not explicitly take account of any costs arising from non-attendance or ‘no-shows’. In some health-care settings this may be a significant problem, but in this context it is likely that a no-show rate at a busy general practice or clinic is fairly predictable and does not result in substantial periods of idle staff time; however, further evidence on this issue would be useful.
In conclusion, the economic analysis indicates that the evidence from the STRATEGIC trial strongly supports the conclusion that timed appointments and unrequested SSKs are both highly likely to be cost-effective interventions to improve uptake in first invitees who have failed to attend. Further work is required to establish the optimal way of combining them (e.g. offering them sequentially), as well as the cost-effectiveness of extending them more generally to all women across the screened age range.
Discrete choice experiment
The DCE suggests that, despite being non-attenders, women still clearly understand and agree with the importance of screening for cervical cancer from a personal or societal perspective. This result conflicts with previous evidence from systematic reviews that identified indifference as one of the main barriers to screening uptake. It is possible, nevertheless, that the difference was driven by the social class and education levels of our sample, which indicated that our sample may not be representative for the population of non-attenders. The DCE also demonstrates preferences for screening scenarios where minimal personal action is required to arrange a test, the test is done in the privacy of the home, there is a nurse available for discussion or help, and which are cheaper to the NHS. As these are characteristics of the SSK interventions trialled within phase 2 of the STRATEGIC study, the results from our stated preference exercise provides an important first step in confirming that these interventions are valued by women; a prerequisite for such interventions to be clinically effective and cost-effective. The evidence from the DCE provides an additional layer of information in addition to the clinical effectiveness and cost-effectiveness that should be considered when evaluating which strategy to implement to improve uptake to the Cervical Screening Programme in young women.
Conclusions
Many young women do not respond rapidly to an invitation to cervical screening and uptake appears not to plateau until 18–24 months. Interventions designed to increase uptake should probably be deferred well beyond the 6 months used in the STRATEGIC study. A pre-invitation leaflet was ineffective. Online booking, although not particularly effective, may be more appealing if available through general practices. Two interventions, timed appointments and sending SSKs, showed a statistically significant effect among non-attenders and although the increased uptake was modest, they were shown to have a high likelihood of being cost-effective within accepted thresholds. Self-sampling to test for HPV could be implemented either in the current NHS pilot of HPV primary screening or more widely when the national programme converts to primary HPV testing. There would be a number of operational issues to address. Timed appointments should be more straightforward to implement provided GPs are supportive. Cervical screening is increasingly being viewed as a choice women make, but until the expected herd protection of HPV vaccination exerts its impact over the next 10–20 years, high coverage of cervical screening will remain a necessary measure if cervical cancer incidence is to remain under control.
Implications for health care
Overall, we found that efforts to increase uptake showed either no effect or a modest effect. Evidence from the STRATEGIC trial supports data from other studies that have used broader age ranges, for SSKs being sent to women who have not responded to their initial invitation for cervical screening. The same applies to timed appointments. Six months is probably too soon following the invitation, rather an interval of 18–24 months may achieve a larger effect.
Recommendations for research
We feel that further research should focus on self-sampling:
-
Expanding the potential use to non-attenders throughout the screening age range.
-
Exploring the optimal time to offer a self-sample, as it appears that women chose to attend for screening over a 18–24 month period following their invitation. This is also relevant because self-sampling involves HPV testing and the prevalence of high-risk HPV infection falls significantly in the years between the ages of 25 and 30 years.
-
Exploring the use of urine testing in place of a vaginal samples as a convenient means of obtaining a sample.
Acknowledgements
The STRATEGIC study research team thanks Sara Rodgers and Laura Clark at the University of York for their input conducting qualitative interviews. We are grateful to Maggie Redshaw at the National Perinatal Epidemiology Unit (NPEU) for helpful suggestions to attempt to improve the response rate in our study. We also would like to thank the NPEU design team who significantly improved the look of our final questionnaire, and the NPEU administration team for their assistance preparing the mail-out material. Special thanks are given to our data entry team Sissi Hernandez-Quesada, Jacob Stevens and Pamela White. The authors would also like to thank Zeinab Abbas for her valuable assistance in the economic evaluation. We would like to express our gratitude to the teams at the English and Scottish screening agencies for their willingness to collaborate in this study. Finally, we thank all women who participated and completed the DCE survey. Any errors or omissions are entirely our own.
We are indebted to the support provided by the Lancashire and South Cumbria Agency, without whom we could not have provided the interventions.
We also wish to thank Linsey Nelson for her painstaking work in helping to compile this report.
The STRATEGIC trial study group
Chief investigator
Henry C Kitchener.
Principal investigator
Henry C Kitchener (Greater Manchester) and Margaret Cruickshank (Grampian).
Trial co-ordinators
Rebecca Albrow, Jamie Oughton and Carley Moseley (Institute of Cancer Sciences, University of Manchester) worked sequentially as STRATEGIC trial co-ordinators.
Nurse navigators
Samantha Fletcher and Julie Burnett (Greater Manchester).
Judith Wilson and Diane Ledingham (Grampian).
Statistics
Chris Roberts and Matthew Gittins (Institute of Population Health, University of Manchester).
Virology
Alexandra Sargent (Department of Virology, Central Manchester University Hospitals NHS Foundation Trust).
Economic modelling
Apostolos Tsiachristas and Alastair Gray (University of Oxford).
Discrete choice experiment
Oliver Rivero-Arias, Helen Campbell and Alastair Gray (University of Oxford).
David Torgerson, Cath Jackson and Jude Watson (York Trials Unit, University of York).
The study was conducted under the guidance of a Trial Steering Committee and a Data Monitoring Committee. The independent members are as follows.
Trial Steering Committee membership
Chairperson: Professor Usha Menon (University College London).
Members: Mr Patrick Walker (Royal Free Hospital, London), Dr Wendi Qian (University of Cambridge) and Mr Robert Music (Jo’s Trust).
Data Monitoring Committee membership
Chairperson: Dr Christine Campbell (University of Edinburgh).
Members: Dr Matt Sydes (Medical Research Council Clinical Trials Unit, London) and Mr Pierre Martin-Hirsch (Lancashire Teaching Hospitals Trust).
Contributions of authors
Henry C Kitchener (Professor of Gynaecological Oncology) was the clinical chief investigator for the study and contributed to the design of the study, the analysis and the drafting of the report.
Matthew Gittins (Statistician) was the study statistician and contributed to the drafting of the report.
Oliver Rivero-Arias (Senior Health Economist) was responsible for the study design and statistical analysis of the DCE, and drafted Chapter 4 for the report.
Apostolos Tsiachristas (Senior Researcher) undertook the cost-effectiveness analysis and provided critical comment.
Margaret Cruickshank (Professor of Gynaecology) was the principal investigator in Grampian, and contributed to setting up the study and drafting the report.
Alastair Gray (Professor of Health Economics) contributed to the study design, led the economic analysis and contributed to the DCE and the drafting of the report.
Loretta Brabin (Reader in Women’s Health) contributed to the study design and provided critical comment.
David Torgerson (Professor of Health Services Research) contributed to the study design and provided critical comment.
Emma J Crosbie (National Institute for Health Research Clinician Scientist, Senior Lecturer and Honorary Consultant in Gynaecological Oncology) contributed to the study design and provided critical comment.
Alexandra Sargent (Virologist) was responsible for the HPV testing and provided critical comment.
Chris Roberts (Professor of Biostatistics) contributed to the study design, supervised the statistical analysis and contributed to the drafting of the report.
Data sharing statement
All available data can be obtained from the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Cancer Research UK . Cervical Cancer Incidence Statistics n.d. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer/incidence (accessed 10 December 2015).
- Cervical Screening Programme, England. Statistics for 2013–14. Leeds: Health and Social Care Information Centre; 2014.
- Albrow R, Blomberg K, Kitchener H, Brabin L, Patnick J, Tishelman C, et al. Interventions to improve cervical cancer screening uptake amongst young women: a systematic review. Acta Oncol 2014;53:445-51. http://dx.doi.org/10.3109/0284186X.2013.869618.
- Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J. The determinants of screening uptake and interventions for increasing uptake: a systematic review. Health Technol Assess 2000;4.
- Waller J, Jackowska M, Marlow L, Wardle J. Exploring age differences in reasons for nonattendance for cervical screening: a qualitative study. BJOG 2012;119:26-32. http://dx.doi.org/10.1111/j.1471-0528.2011.03030.x.
- Cole SR, Smith A, Wilson C, Turnbull D, Esterman A, Young GP. An advance notification letter increases participation in colorectal cancer screening. J Med Screen 2007;14:73-5. http://dx.doi.org/10.1258/096914107781261927.
- Libby G, Bray J, Champion J, Brownlee LA, Birrell J, Gorman DR, et al. Pre-notification increases uptake of colorectal cancer screening in all demographic groups: a randomized controlled trial. J Med Screen 2011;18:24-9. http://dx.doi.org/10.1258/jms.2011.011002.
- Gök M, van Kemenade FJ, Heideman DA, Berkhof J, Rozendaal L, Spruyt JW, et al. Experience with high-risk human papillomavirus testing on vaginal brush-based self-samples of non-attendees of the cervical screening program. Int J Cancer 2012;130:1128-35. http://dx.doi.org/10.1002/ijc.26128.
- Szarewski A, Cadman L, Mesher D, Austin J, Ashdown-Barr L, Edwards R, et al. HPV self-sampling as an alternative strategy in non-attenders for cervical screening – a randomised controlled trial. Br J Cancer 2011;104:915-20. http://dx.doi.org/10.1038/bjc.2011.48.
- Ritvo PG, Myers RE, Paszat LF, Tinmouth JM, McColeman J, Mitchell B, et al. Personal navigation increases colorectal cancer screening uptake. Cancer Epidemiol Biomarkers Prev 2015;24:506-11. http://dx.doi.org/10.1158/1055-9965.EPI-14-0744.
- Sadler L, Albrow R, Shelton R, Kitchener H, Brabin L. Development of a pre-notification leaflet to encourage uptake of cervical screening at first invitation: a qualitative study. Health Educ Res 2013;28:793-802. http://dx.doi.org/10.1093/her/cys103.
- UN3373 . Biological Substances Transport 2016. www.un3373.com (accessed 14 July 2016).
- Goldberg D, Schiff GD, McNutt R, Furumoto-Dawson A, Hammerman M, Hoffman A. Mailings timed to patients’ appointments: a controlled trial of fecal occult blood test cards. Am J Prev Med 2004;26:431-5. http://dx.doi.org/10.1016/j.amepre.2004.02.009.
- Torgerson DJ, Garton MJ, Donaldson C, Russell IT, Reid DM. Recruitment methods for screening programmes: trial of an improved method within a regional osteoporosis study. BMJ 1993;307. http://dx.doi.org/10.1136/bmj.307.6896.99.
- Raab GM, Butcher I. Balance in cluster randomized trials. Stat Med 2001;20:351-65. http://dx.doi.org/10.1002/1097-0258(20010215)20:3<351::AID-SIM797>3.0.CO;2-C.
- Jensen H, Svanholm H, Støvring H, Bro F. A primary healthcare-based intervention to improve a Danish cervical cancer screening programme: a cluster randomised controlled trial. J Epidemiol Community Health 2009;63:510-15. http://dx.doi.org/10.1136/jech.2008.077636.
- Schoenfeld D. Statistical considerations for pilot studies. Int J Radiat Oncol Biol Phys 1980;6:371-4. http://dx.doi.org/10.1016/0360-3016(80)90153-4.
- Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc 1955;50:1097-121. http://dx.doi.org/10.1080/01621459.1955.10501294.
- Donner A, Klar N. Design and Analysis of Cluster Randomization Trials in Health Research. London: Arnold; 2000.
- Goldstein H. Multilevel Statistical Models. Oxford: Wiley-Blackwell; 2003.
- Guide to the Methods of Technology Appraisal 2013. London: National Institute for Health and Care Excellence; 2013.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f1049.
- Kim EJ. Modelling the Cost-Effectiveness of Human Pappilomavirus (HPV) Testing for Triage of Women with Low-Grade Abnormal Cervical Smears: A Study within the TOMBOLA Trial. Sheffield: University of Sheffield; 2010.
- Sherlaw-Johnson C, Philips Z. An evaluation of liquid-based cytology and human papillomavirus testing within the UK cervical cancer screening programme. Br J Cancer 2004;91:84-91. http://dx.doi.org/10.1038/sj.bjc.6601884.
- Department of Health . Reference Costs 2013–14 2014. www.gov.uk/government/uploads/system/uploads/attachment_data/file/380322/01_Final_2013-14_Reference_Costs_publication_v2.pdf (accessed 14 July 2016).
- Systematic Reviews – CRD’s Guidance for Undertaking Reviews in Health Care. York: Centre for Reviews and Dissemination, University of York; 2009.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8360.
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d549.
- Organisation for Economic Co-operation and Development (OECD) . OECD Health Statistics 2015 2015. www.oecd.org/unitedkingdom/Country-Note-UNITED%20KINGDOM-OECD-Health-Statistics-2015.pdf (accessed 14 July 2016).
- HM Revenue and Customs . Guidance: Average for the Year to 31 March 2015 2016. www.gov.uk/government/uploads/system/uploads/attachment_data/file/421061/average310315-1.csv/preview (accessed 14 July 2016).
- Bidus MA, Maxwell GL, Kulasingam S, Rose GS, Elkas JC, Chernofsky M, et al. Cost-effectiveness analysis of liquid-based cytology and human papillomavirus testing in cervical cancer screening. Obstet Gynecol 2006;107:997-1005. http://dx.doi.org/10.1097/01.AOG.0000210529.70226.0a.
- Bistoletti P, Sennfält K, Dillner J. Cost-effectiveness of primary cytology and HPV DNA cervical screening. Int J Cancer 2008;122:372-6. http://dx.doi.org/10.1002/ijc.23124.
- Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol 2004;103:619-31. http://dx.doi.org/10.1097/01.AOG.0000120143.50098.c7.
- Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of human papillomavirus DNA testing in the United Kingdom, The Netherlands, France, and Italy. J Natl Cancer Inst 2005;97:888-95. http://dx.doi.org/10.1093/jnci/dji162.
- Vijayaraghavan A, Efrusy MB, Mayrand MH, Santas CC, Goggin P. Cost-effectiveness of high-risk human papillomavirus testing for cervical cancer screening in Québec, Canada. Can J Public Health 2010;101:220-5.
- Voko Z, Nagyjanosi L, Kalo Z. Cost-effectiveness analysis of adding HPV vaccination to cervical cancer screening program in Hungary. Value Health 2010;13:A270-1. http://dx.doi.org/10.1016/S1098-3015(11)71998-2.
- Payne N, Chilcott J, McGoogan E. Liquid-Based Cytology for Cervical Screening. Sheffield: School of Health and Related Research, University of Sheffield; 2000.
- Murphy J, Gray A. Health Economics Report for Primary HPV Pilot 2015.
- Health and Social Care Information Centre . Cervical Screening Programme, England. Statistics for 2013–2014 2014. www.hscic.gov.uk/catalogue/PUB15968/cerv-scre-prog-eng-2013-14-rep.pdf (accessed 14 July 2016).
- Kim E-J. Modelling the Cost-Effectiveness of Human Papillomavirus (HPV) Testing for Triage of Women With Low-Grade Abnormal Cervical Smears: A Study Within the TOMBOLA Trial 2010.
- Clark MD, Determann D, Petrou S, Moro D, de Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. PharmacoEconomics 2014;32:883-902. http://dx.doi.org/10.1007/s40273-014-0170-x.
- Watson V, Ryan M, Watson E. Valuing experience factors in the provision of chlamydia screening: an application to women attending the family planning clinic. Value Health 2009;12:621-3. http://dx.doi.org/10.1111/j.1524-4733.2008.00451.x.
- de Bekker-Grob EW, Rose JM, Donkers B, Essink-Bot ML, Bangma CH, Steyerberg EW. Men’s preferences for prostate cancer screening: a discrete choice experiment. Br J Cancer 2013;108:533-41. http://dx.doi.org/10.1038/bjc.2013.5.
- Wordsworth S, Ryan M, Skåtun D, Waugh N. Women’s preferences for cervical cancer screening: a study using a discrete choice experiment. Int J Technol Assess Health Care 2006;22:344-50. http://dx.doi.org/10.1017/S0266462306051245.
- Hensher DA, Rose JM, Greene WH. Applied Choice Analysis. Cambridge: Cambridge University Press; 2015.
- McIntosh E, Louviere JJ, Frew E, Clarke PM. Applied Methods of Cost-Benefit Analysis in Health Care. Oxford: Oxford University Press; 2010.
- Louviere JJ, Hensher DA, Swait JD, Adamowicz W. Stated Choice Methods: Analysis and Application. Cambridge: Cambridge University Press; 2000.
- Hundley V, Ryan M, Graham W. Assessing women’s preferences for intrapartum care. Birth 2001;28:254-63. http://dx.doi.org/10.1046/j.1523-536X.2001.00254.x.
- Hall J, Kenny P, King M, Louviere J, Viney R, Yeoh A. Using stated preference discrete choice modelling to evaluate the introduction of varicella vaccination. Health Econ 2002;11:457-65. http://dx.doi.org/10.1002/hec.694.
- Phillips KA, Maddala T, Johnson FR. Measuring preferences for health care interventions using conjoint analysis: an application to HIV testing. Health Serv Res 2002;37:1681-705. http://dx.doi.org/10.1111/1475-6773.01115.
- Coast J, Al-Janabi H, Sutton EJ, Horrocks SA, Vosper AJ, Swancutt DR, et al. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health Econ 2012;21:730-41. http://dx.doi.org/10.1002/hec.1739.
- Ritchie J, Lewis J, McNaughton Nicholls C, Ormston R. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage; 2013.
- Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs 2008;62:107-15. http://dx.doi.org/10.1111/j.1365-2648.2007.04569.x.
- Ngene 1.1.1 User Manual & Reference Guide. Sydney, NSW: ChoiceMetrics; 2012.
- Burgess L, Street DJ. Optimal designs for 2 k choice experiments. Commun Stat Theory Methods 2003;32:2185-206. http://dx.doi.org/10.1081/STA-120024475.
- Street DJ, Burgess L. Optimal and near-optimal pairs for the estimation of effects in 2-level choice experiments. J Stat Plan Inference 2004;118:185-99. http://dx.doi.org/10.1016/S0378-3758(02)00399-3.
- Street DJ, Burgess L, Louviere JJ. Quick and easy choice sets: Constructing optimal and nearly optimal stated choice experiments. Int J Res Marketing 2005;22:459-70. http://dx.doi.org/10.1016/j.ijresmar.2005.09.003.
- Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health 2013;16:3-13. http://dx.doi.org/10.1016/j.jval.2012.08.2223.
- Witt J, Scott A, Osborne RH. Designing choice experiments with many attributes. An application to setting priorities for orthopaedic waiting lists. Health Econ 2009;18:681-96. http://dx.doi.org/10.1002/hec.1396.
- Rose JM, Bliemer MCJ. Sample size requirements for stated choice experiments. Transportation 2013;40:1021-41. http://dx.doi.org/10.1007/s11116-013-9451-z.
- Ackerson K, Gretebeck K. Factors influencing cancer screening practices of underserved women. J Am Acad Nurse Pract 2007;19:591-60. http://dx.doi.org/10.1111/j.1745-7599.2007.00268.x.
- Ackerson K, Pohl J, Low LK. Personal influencing factors associated with pap smear testing and cervical cancer. Policy Polit Nurs Pract 2008;9:50-6. http://dx.doi.org/10.1177/1527154408318097.
- Knops-Dullens T, de Vries N, de Vries H. Reasons for non-attendance in cervical cancer screening programmes: an application of the Integrated Model for Behavioural Change. Eur J Cancer Prev 2007;16:436-45. http://dx.doi.org/10.1097/01.cej.0000236250.71113.7c.
- Tacken MA, Braspenning JC, Hermens RP, Spreeuwenberg PM, van den Hoogen HJ, de Bakker DH, et al. Uptake of cervical cancer screening in The Netherlands is mainly influenced by women’s beliefs about the screening and by the inviting organization. Eur J Public Health 2007;17:178-85. http://dx.doi.org/10.1093/eurpub/ckl082.
- Huber J, Zwerina K. The importance of utility balance in efficient choice designs. J Marketing Res 1996;33:307-17. http://dx.doi.org/10.2307/3152127.
- Rosenstock IM, Glanz K, Lewis F, Rimer B. Health Behaviour and Health Education: Theory, Research, and Practice. San Francisco, CA: Jossey-Bass; 1990.
- Greene GW, Rossi SR, Rossi JS, Velicer WF, Fava JL, Prochaska JO. Dietary applications of the stages of change model. J Am Diet Assoc 1999;99:673-8. http://dx.doi.org/10.1016/S0002-8223(99)00164-9.
- Herweijer E, Feldman AL, Ploner A, Arnheim-Dahlström L, Uhnoo I, Netterlid E, et al. The participation of HPV-vaccinated women in a national cervical screening program: population-based cohort study. PLOS ONE 2015;10. http://dx.doi.org/10.1371/journal.pone.0134185.
- Palmer TJ, McFadden M, Pollock KG, Kavanagh K, Cuschieri K, Cruickshank M, et al. HPV immunisation and increased uptake of cervical screening in Scottish women; observational study of routinely collected national data. Br J Cancer 2016;114:576-81. http://dx.doi.org/10.1038/bjc.2015.473.
- Spencer AM, Roberts SA, Verma A, Patnick J, Elton P, Brabin L. Effect of human papillomavirus vaccination of daughters on the cervical screening uptake of their non-vaccinated mothers. Eur J Public Health 2015;25:1097-100. http://dx.doi.org/10.1093/eurpub/ckv146.
- Hudson S, Brazil D, Teh W, Duffy SW, Myles JP. Effectiveness of timed and non-timed second appointments in improving uptake in breast cancer screening. J Med Screen 2016;23:160-3. http://dx.doi.org/10.1177/0969141315624937.
Appendix 1 Pre-leaflet (development)
The pre-leaflet aimed to motivate women who had not considered taking part in screening to do so, based on the transtheoretical model.
The first step in development was to try and establish or identify what would motivate women to take part and what the barriers to screening were. Focus groups were held in order to achieve this, in total five focus group discussions (FGDs), the groups consisted of a range of ages and demographics, four focus groups were with women aged 17–25 years and one group consisted of practice nurses.
The questions and areas for discussion in the FGDs can be summarised as:
-
What is known about the cervical screening process?
-
What do you know about the screening invitation?
-
What is the purpose of screening?
-
What do you know about the details of the (screening) procedure?
-
Do you feel at risk of cervical cancer?
-
Is screening important?
-
Could a pre-leaflet motivate you to attend?
-
What are the views of cervical screening nurses? (In the practice nurse group only.)
The FGDs were recorded and transcribed verbatim. The main themes that arose in the responses to the above questions were compiled and led to the development of the pre-leaflet used in the STRATEGIC study.
For further details on the development of the STRATEGIC study pre-leaflet, please see Health Education Research. 11
Once the content had been decided, a selection of pre-leaflets were designed and presented to two more focus groups. The proposed leaflets were folded like a card, with various formats, in addition to the final choice of a card with a birthday cake on the front cover – owing to the fact that the women were first called to screening just prior to 25 years of age and 20 years of age in Greater Manchester and Aberdeen, respectively. We also proposed a six-page, folded leaflet with mobile phone text and ‘to-do’ list, an A4 information sheet with coloured border and a six-page, folded leaflet featuring a picture of a bin with the slogan, ‘Don’t throw this away, it might just save your life . . .‘.
The focus groups were asked for their views on design and it was decided that the pre-leaflet should take the form of a birthday card.
Appendix 2 Pre-leaflet (Manchester)
© The University of Manchester 2012. Text written by L. Sadler, R. Albrow and H.C. Kitchener.
Appendix 3 Pre-leaflet (Aberdeen)
© The University of Manchester 2012. Text written by L. Sadler, R. Albrow and H.C. Kitchener.
Appendix 4 Nurse navigator intervention letter
Appendix 5 Nurse navigator patient information and consent form
Appendix 6 General practitioner timed appointments letter
Appendix 7 Additional statistical tables
| Time since intervention | Time point | |||||
|---|---|---|---|---|---|---|
| 3 months | 6 months | |||||
| ORa | p-value | 95% CI | ORa | p-value | 95% CI | |
| Pre-leaflet: all data | ||||||
| Pre-leaflet (control) | 0.967 | 0.485 | 0.879 to 1.063 | 1.015 | 0.747 | 0.928 to 1.109 |
| PCT – Trafford (Salford) | 1.332 | 0.713 | 0.29 to 6.122 | 1.095 | 0.913 | 0.215 to 5.57 |
| PCT – Manchester (Salford) | 1.301 | 0.653 | 0.412 to 4.107 | 0.724 | 0.631 | 0.193 to 2.709 |
| PCT – Grampian (Salford) | 0.886 | 0.881 | 0.179 to 4.37 | 0.653 | 0.587 | 0.14 to 3.036 |
| Baseline rate | 1.039 | < 0.001 | 1.022 to 1.056 | 1.033 | 0.001 | 1.013 to 1.053 |
| PCT(Trafford) × baseline rate | 0.994 | 0.598 | 0.971 to 1.017 | 0.997 | 0.801 | 0.972 to 1.022 |
| PCT(Manchester) × baseline rate | 0.993 | 0.443 | 0.975 to 1.011 | 1.001 | 0.922 | 0.98 to 1.022 |
| PCT(Grampian) × baseline rate | 0.996 | 0.717 | 0.973 to 1.019 | 1.001 | 0.91 | 0.978 to 1.025 |
| ICCb | 0.010 | 0.016 | ||||
| Online booking: Manchester PCT only | ||||||
| Online booking (control) | 1.021 | 0.802 | 0.869 to 1.200 | 1.097 | 0.242 | 0.939 to 1.282 |
| Baseline rate | 1.032 | < 0.001 | 1.023 to 1.041 | 1.033 | < 0.001 | 1.025 to 1.041 |
| ICCb | 0.009 | 0.019 | ||||
| Variable | Comparison | Time point | |||||
|---|---|---|---|---|---|---|---|
| 12 months | 18 months | ||||||
| ORa | 95% CI | p-value | ORa | 95% CI | p-value | ||
| Interaction model | |||||||
| Pre-leaflet (sent) | Pre-leaflet vs. control | 1.064 | 0.856 to 1.321 | 0.577 | 1.018 | 0.850 to 1.219 | 0.850 |
| Phase 2 (control only) | SSK sent vs. control | 1.569 | 1.094 to 2.251 | 0.014 | 1.272 | 0.929 to 1.742 | 0.134 |
| SSK offered vs. control | 1.192 | 0.895 to 1.587 | 0.229 | 1.018 | 0.820 to 1.265 | 0.869 | |
| NN vs. control | 0.833 | 0.490 to 1.414 | 0.498 | 0.743 | 0.505 to 1.093 | 0.132 | |
| Timed appointment vs. control | 1.531 | 1.198 to 1.957 | 0.001 | 1.364 | 1.082 to 1.719 | 0.009 | |
| Choice vs. control | 1.005 | 0.764 to 1.321 | 0.972 | 1.012 | 0.825 to 1.242 | 0.906 | |
| Interaction | Pre-leaflet × SSK sent | 0.930 | 0.583 to 1.485 | 0.761 | 1.011 | 0.684 to 1.494 | 0.957 |
| Pre-leaflet × SSK offered | 0.802 | 0.528 to 1.220 | 0.303 | 1.072 | 0.755 to 1.523 | 0.698 | |
| Pre-leaflet × NN | 1.103 | 0.595 to 2.044 | 0.755 | 1.134 | 0.718 to 1.793 | 0.589 | |
| Pre-leaflet × timed appointment | 0.825 | 0.525 to 1.297 | 0.405 | 0.721 | 0.476 to 1.090 | 0.121 | |
| Pre-leaflet × choice | 1.139 | 0.742 to 1.747 | 0.551 | 1.080 | 0.755 to 1.544 | 0.673 | |
| Test of interaction (χ52) | 0.740 | 0.216 | |||||
| PCT | Trafford vs. Salford | 0.903 | 0.678 to 1.203 | 0.486 | 1.052 | 0.851 to 1.301 | 0.64 |
| Manchester vs. Salford | 0.819 | 0.672 to 0.999 | 0.049 | 0.827 | 0.693 to 0.986 | 0.034 | |
| Grampian vs. Salford | 0.617 | 0.497 to 0.767 | < 0.001 | 0.574 | 0.475 to 0.693 | < 0.001 | |
| 6-month rate | 1.026 | 1.019 to 1.033 | < 0.001 | 1.028 | 1.022 to 1.035 | < 0.001 | |
| ICCb | 0.0076 | 0.016 | |||||
| Main-effects model | |||||||
| Phase 1 pre-leaflet | Pre-leaflet vs. control | 1.022 | 0.887 to 1.179 | 0.760 | 1.010 | 0.896 to 1.138 | 0.875 |
| Phase 2 | SSK sent vs. control | 1.512 | 1.196 to 1.912 | 0.001 | 1.287 | 1.056 to 1.568 | 0.013 |
| SSK offered vs. control | 1.075 | 0.871 to 1.326 | 0.499 | 1.056 | 0.884 to 1.262 | 0.546 | |
| NN vs. control | 0.885 | 0.668 to 1.174 | 0.397 | 0.798 | 0.641 to 0.994 | 0.044 | |
| Timed appointment vs. control | 1.412 | 1.142 to 1.744 | 0.001 | 1.192 | 0.975 to 1.459 | 0.087 | |
| Choice vs. control | 1.089 | 0.863 to 1.373 | 0.472 | 1.058 | 0.869 to 1.287 | 0.576 | |
| PCT | Trafford vs. Salford | 0.893 | 0.670 to 1.191 | 0.442 | 1.041 | 0.835 to 1.298 | 0.718 |
| Manchester vs. Salford | 0.811 | 0.664 to 0.990 | 0.040 | 0.818 | 0.685 to 0.977 | 0.027 | |
| Grampian vs. Salford | 0.611 | 0.491 to 0.760 | < 0.001 | 0.563 | 0.465 to 0.681 | < 0.001 | |
| 6-month rate | 1.026 | 1.018 to 1.033 | < 0.001 | 1.028 | 1.021 to 1.035 | < 0.001 | |
| ICCb | 0.0085 | 0.0213 | |||||
| Variable | Comparison | Time point | |||||
|---|---|---|---|---|---|---|---|
| 12 months | 18 months | ||||||
| ORa | 95% CI | p-value | ORa | 95% CI | p-value | ||
| Interaction model | |||||||
| Phase 1 online booking | Online booking vs. control | 1.148 | 0.803 to 1.64 | 0.449 | 1.131 | 0.829 to 1.543 | 0.438 |
| Phase 2 (control only) | SSK sent vs. control | 2.152 | 1.478 to 3.133 | < 0.001 | 1.521 | 1.064 to 2.176 | 0.022 |
| SSK offered vs. control | 1.115 | 0.800 to 1.555 | 0.52 | 0.967 | 0.673 to 1.389 | 0.857 | |
| NN vs. control | 1.284 | 0.477 to 3.454 | 0.62 | 0.893 | 0.430 to 1.852 | 0.76 | |
| Timed appointment vs. control | 1.649 | 0.943 to 2.884 | 0.079 | 1.341 | 0.841 to 2.139 | 0.218 | |
| Choice vs. control | 1.493 | 1.051 to 2.120 | 0.025 | 1.187 | 0.834 to 1.691 | 0.341 | |
| Interaction | Online booking × SSK sent | 0.712 | 0.407 to 1.248 | 0.236 | 0.734 | 0.455 to 1.183 | 0.204 |
| Online booking × SSK offered | 1.130 | 0.720 to 1.774 | 0.595 | 1.302 | 0.833 to 2.034 | 0.247 | |
| Online booking × NN | 0.570 | 0.189 to 1.720 | 0.318 | 0.859 | 0.372 to 1.986 | 0.723 | |
| Online booking × timed appointment | 1.077 | 0.581 to 1.998 | 0.814 | 1.035 | 0.621 to 1.724 | 0.896 | |
| Online booking × choice | 0.734 | 0.444 to 1.214 | 0.229 | 0.919 | 0.595 to 1.419 | 0.702 | |
| Interaction test (χ52) | 0.594 | 0.321 | |||||
| 6-month rate | 1.036 | 1.025 to 1.046 | < 0.001 | 1.040 | 1.030 to 1.049 | < 0.001 | |
| ICCb | 0.005 | 0.0044 | |||||
| Main-effects model | |||||||
| Phase 1 online booking | Online booking vs. control | 1.025 | 0.836 to 1.257 | 0.812 | 1.102 | 0.930 to 1.305 | 0.263 |
| Phase 2 | SSK sent vs. control | 1.795 | 1.316 to 2.448 | < 0.001 | 1.292 | 0.991 to 1.683 | 0.058 |
| SSK offered vs. control | 1.179 | 0.929 to 1.496 | 0.175 | 1.102 | 0.870 to 1.396 | 0.42 | |
| NN vs. control | 0.926 | 0.535 to 1.605 | 0.785 | 0.813 | 0.555 to 1.192 | 0.289 | |
| Timed appointment vs. control | 1.723 | 1.272 to 2.334 | < 0.001 | 1.367 | 1.065 to 1.754 | 0.014 | |
| Choice vs. control | 1.256 | 0.970 to 1.626 | 0.084 | 1.130 | 0.915 to 1.395 | 0.257 | |
| 6-month rate | 1.034 | 1.024 to 1.045 | < 0.001 | 1.039 | 1.030 to 1.048 | < 0.001 | |
| ICCb | 0.006 | 0.0049 | |||||
| Variable | Comparison | Time point | |||||
|---|---|---|---|---|---|---|---|
| 12 months | 18 months | ||||||
| ORa | 95% CI | p-value | ORa | 95% CI | p-value | ||
| Interaction model | |||||||
| Phase 2 (none only) | SSK sent vs. control | 1.399 | 0.542 to 3.608 | 0.488 | 1.118 | 0.557 to 2.243 | 0.753 |
| SSK offered vs. control | 1.602 | 0.831 to 3.090 | 0.159 | 1.258 | 0.747 to 2.119 | 0.387 | |
| NN vs. control | 1.399 | 0.573 to 3.419 | 0.461 | 0.863 | 0.380 to 1.961 | 0.725 | |
| Timed appointment vs. control | 1.231 | 0.666 to 2.275 | 0.508 | 1.181 | 0.747 to 1.867 | 0.478 | |
| Choice vs. control | 0.714 | 0.232 to 2.201 | 0.558 | 0.712 | 0.276 to 1.838 | 0.483 | |
| Vaccination | Incomplete vs. none | 1.886 | 0.862 to 4.127 | 0.112 | 1.436 | 0.824 to 2.505 | 0.202 |
| Complete vs. none | 2.246 | 1.173 to 4.300 | 0.015 | 1.760 | 1.158 to 2.674 | 0.008 | |
| Interaction (incomplete) | SSK sent vs. control | 3.653 | 0.751 to 17.78 | 0.109 | 2.864 | 0.778 to 10.55 | 0.114 |
| SSK offered vs. control | 1.024 | 0.418 to 2.506 | 0.959 | 1.237 | 0.588 to 2.605 | 0.575 | |
| NN vs. control | 0.723 | 0.217 to 2.406 | 0.597 | 0.838 | 0.300 to 2.339 | 0.736 | |
| Timed appointment vs. control | 0.537 | 0.211 to 1.363 | 0.190 | 0.561 | 0.290 to 1.084 | 0.086 | |
| Choice vs. control | 0.770 | 0.143 to 4.161 | 0.762 | 1.258 | 0.248 to 6.373 | 0.782 | |
| Interaction (full) | SSK sent vs. control | 0.823 | 0.259 to 2.618 | 0.741 | 1.419 | 0.535 to 3.764 | 0.482 |
| SSK offered vs. control | 1.604 | 0.667 to 3.857 | 0.292 | 1.345 | 0.656 to 2.758 | 0.419 | |
| NN vs. control | 1.276 | 0.654 to 2.487 | 0.475 | 1.425 | 0.880 to 2.307 | 0.150 | |
| Timed appointment vs. control | 0.482 | 0.044 to 5.253 | 0.549 | 1.425 | 0.202 to 10.03 | 0.722 | |
| Choice vs. control | 1.159 | 0.352 to 3.813 | 0.809 | 1.391 | 0.553 to 3.499 | 0.483 | |
| Overall test of interaction (χ102) | 0.147 | 0.122 | |||||
| ICCb | 0.001 | –0.001 | |||||
| Main-effects model | |||||||
| Phase 2 | SSK sent vs. control | 1.541 | 1.025 to 2.316 | 0.037 | 1.379 | 0.989 to 1.922 | 0.058 |
| SSK offered vs. control | 1.060 | 0.709 to 1.586 | 0.775 | 0.906 | 0.668 to 1.229 | 0.527 | |
| NN vs. control | 1.192 | 0.789 to 1.800 | 0.404 | 1.137 | 0.868 to 1.49 | 0.352 | |
| Timed appointment vs. control | 1.543 | 1.174 to 2.027 | 0.002 | 1.529 | 1.257 to 1.86 | < 0.001 | |
| Choice vs. control | 0.761 | 0.513 to 1.129 | 0.175 | 0.936 | 0.679 to 1.29 | 0.684 | |
| Vaccination | Incomplete vs. none | 2.109 | 1.388 to 3.204 | < 0.001 | 1.734 | 1.191 to 2.524 | 0.004 |
| Complete vs. none | 2.169 | 1.629 to 2.887 | < 0.001 | 1.966 | 1.508 to 2.565 | < 0.001 | |
| ICCb | 0.0024 | –0.0006 | |||||
Appendix 8 Details of interventions
Pre-leaflet
A pre-leaflet was developed by Sadler et al. 11 and aimed to move women about to be invited for first screening from the ‘pre-contemplation’ to ‘contemplation’ stage based on the transtheoretical model. In Manchester, the screening agency, as part of the standard NHS Cervical Screening Programme, sent the pre-leaflets to women 6 weeks before they were due to receive their first invite to cervical screening. As the screening agency in Scotland did not have the provision to assist the research, in Grampian the Aberdeen-based research team sent the pre-leaflets in weekly batches 4–6 weeks before the women were due to receive their first invite to cervical screening. The team spent a great deal of time working on this and so the costs were somewhat higher than in Manchester; however, if the service was operationalised nationwide, the costs would be more comparable to the process in Greater Manchester.
Online booking
Women in surgeries randomised to receive the online booking intervention received a letter providing them with information on how to make an online booking and a list of participating clinics. This information was sent with their first invitation letter, 6 weeks before the women were due to receive their first invitation to cervical screening, and with the first reminder letter from the screening agency, which was sent to women without a test recorded 3 months after their test due date. The team in Manchester set up an online appointment scheduling system via a free service, SuperSaaS (www.supersaas.com). The team then spent time contacting a range of sexual health clinics across Greater Manchester and agreed on a block of set appointments for women to attend. Women who accessed the service were asked to input only their name and a contact number online, and were not able to see appointments that had been booked by other users. An administrator account was held for the system by the research team, who forwarded on details of booked appointment to an administrator at the clinics using the NHS.net e-mail system at the start of each week. In addition, each woman received a text message confirming the appointment the day before. Family planning staff were subsequently asked to indicate if a woman who had booked online had attended. From the 215 women who accessed the service, 128 attended: 20 cancelled beforehand, 60 did not attend and data were missing on seven.
Human papillomavirus self-sampling
There were two HPV self-sampling intervention arms: the first was a letter sent to the women, offering them to chance to request a SSK; and the second was an unsolicited SSK being sent directly to their home. The kits included a consent form that came back with the sample to the laboratory at Central Manchester Health Care Trust. Once the test had been processed, the team at the laboratory sent the results and the consent form to the research team in Manchester who fed back the results as per the request in the consent form.
Nurse navigator
The offer of the NN service came in the form of a letter sent by the screening agency; it provided contact details for the NN and explained how they may be able to offer help and advice in order to allay any anxieties that they might have regarding attending for a cervical screening test. Women were encouraged to contact the NN via telephone to speak to her directly during daytime hours or to contact the trial office via telephone, e-mail or text messaging to request that the NN calls them at a convenient time. The NN discussed the woman’s perceived barrier(s) to accepting the invitation for cervical screening and assisted the woman in booking an appointment where necessary. If the NN felt online booking or a HPV SSK was appropriate to the woman’s needs, she facilitated access to these interventions. Women were also asked if they would like to receive a follow-up call from the NN to discuss whether or not they had arranged/attended for screening.
Timed appointments
General practitioners were asked to send women an invite letter detailing a time and date for them to attend for a cytology appointment. In this invite letter women were given the option to rearrange for a more convenient time if needed. The service proved difficult to operationalise and some practices were unable to ring-fence enough appointments, these figures have been recorded.
Choice of nurse navigator or request human papillomavirus sample
Choice between NN and SSK offered.
Appendix 9 Intervention unit costs
| Intervention | Cost item | Unit | Unit cost (£) | Units | Costs (£) | Cost type |
|---|---|---|---|---|---|---|
| Pre-leaflet | Researcher time | Hour | 17.22 | 20 | 344.40 | Fixed, one-off |
| Refreshments | Item | 15.00 | 15 | 225.00 | Fixed, one-off | |
| Consumables | Item | 100.00 | 1 | 100.00 | Fixed, one-off | |
| Design | 200.00 | 1 | 200.00 | Fixed, one-off | ||
| Pre-leaflets | Item | 0.16 | 10,461 | 1673.76 | Variable | |
| Postage | Item | 0.39 | 10,461 | 4079.79 | Variable | |
| Envelopes | Item | 0.03 | 10,461 | 334.75 | Variable | |
| Labels | Item | 18.29 | 6 | 109.74 | Variable | |
| Staff time to produce and post | Hour | 16.42 | 87 | 1428.54 | Variable | |
| Total | 8495.98 | |||||
| Online booking | Staff time to set-up online booking system | Hour | 16.42 | 35 | 574.70 | Fixed, one-off |
| Staff time for weekly duties | Hour | 16.42 | 104 | 1707.68 | Variable | |
| Consumables (telephone) | Item | 100.00 | 1 | 100.00 | Variable | |
| Total | 2382.38 | |||||
| HPV SSK | Delphi | Item | 2.85 | 603.5 | 1719.98 | Variable |
| Evalyn (variable charge) | Item | 1.25 | 603.5 | 754.38 | Variable | |
| Evalyn (freight charge) | Item | 125.00 | 1 | 125.00 | Variable | |
| Consumables (clinical) | Item | 1.00 | 1207 | 1207.00 | Variable | |
| Postage Evalyn | Item | 0.70 | 603.5 | 422.45 | Variable | |
| Postage Delphi | Item | 2.60 | 603.5 | 1569.10 | Variable | |
| Staff time (set-up) | Hour | 16.42 | 14 | 229.88 | Variable | |
| Staff time (weekly) | Hour | 20.00 | 315 | 6300.00 | Variable | |
| Staff time (lab) | Hour | 11.72 | 18 | 211.00 | Variable | |
| Laboratory kits [Cobas (Roche Diagnostics, Pleasanton, CA, USA)] | Item | 7.00 | 197 | 1379.00 | Variable | |
| Total | 13,917.78 | |||||
| Total cost per kit | 11.53 | |||||
| Requested SSK | Total costs | 368.99 | ||||
| Unrequested SSK | Total costs | 13,156.74 | ||||
| NN | Nurse training | Item | 150.00 | 1 | 50.00 | Variable |
| Nurse time | Hour | 20.00 | 4 | 80.00 | Variable | |
| Requested HPV kit | Item | 11.53 | 1 | 11.53 | Variable | |
| Total | 141.53 | |||||
| Timed appointments | Appointment letter | Item | 5.00 | 1629 | 8145.00 | Variable |
| Total | 8145.00 | |||||
| Combination | Information letter | Item | 0.16 | 1277 | 204.32 | Variable |
| Envelops | Item | 0.03 | 1277 | 38.31 | Variable | |
| Postage | Item | 0.39 | 1277 | 498.03 | Variable | |
| Requested HPV kit | Item | 11.53 | 21 | 242.15 | Variable | |
| Nurse training | Item | 150.00 | 1 | 100.00 | Fixed, scalable | |
| NN time | Item | 20.00 | 8 | 160.00 | Variable | |
| Total | 1242.81 |
Appendix 10 Literature review results
| Author | Location | Description of target population | Statement of decision problem/objective | Type of model |
|---|---|---|---|---|
| Bidus et al.31 | USA | Active duty women in the US army | To compare the outcomes of several cervix cancer screening strategies in a military population using a model that considers both direct and indirect costs of health care | Markov state transition model |
| Bistoletti et al.32 | Sweden | Women in Sweden from the age of 32 years | To simulate the cost-effectiveness of different conceivable strategies in the setting of an organised screening programme | State transition model |
| Goldie et al.33 | USA | Cohort of women in the USA | To evaluate the cost-effectiveness of HPV DNA testing as a primary screening test in combination with cervical cytology in women aged ≥ 30 years | A state transition mathematical model |
| Kim et al.34 | UK, the Netherlands, France and Italy | Women aged 20–65 years, 30–60 years, 25–65 years and 25–65 years | Assessment of the cost-effectiveness of incorporating HPV DNA testing into existing cervical cancer screening programmes in the UK, the Netherlands, France and Italy | A state transition mathematical model |
| Vijayaraghavan et al.35 | Canada | Cohort of 100,000 women over their lifetimes, beginning at age 13 years | Objective was to evaluate the cost-effectiveness of cervical cancer screening strategies utilising HPV testing | Markov Monte Carlo simulation model |
| Voko et al.36 | Hungary | Women aged 25–64 years, on the basis of participation in regular screening | To compare the cost-effectiveness of two national cervical cancer screening programmes aiming to involve those who do not regularly participate in the screening programme in Hungary | Cohort simulation Markov model |
| Sherlaw-Johnson and Philips24 | UK | Women from the age of 13 years | To evaluate different options for introducing LBC within the UK in terms of cost-effectiveness | A state transition mathematical model |
| Payne et al.37 | NICE appraisal | Women aged between 21 and 95 years | To evaluate the clinical effectiveness and cost-effectiveness of liquid-based cytology for cervical screening compared with conventional smear testing | Cohort macro simulation |
| ID | Author | Screening | CHEERS quality score | HTA quality score | Lifetime cost (£) | Adjusted life expectancy (years) | Adjusted QALYs |
|---|---|---|---|---|---|---|---|
| 0 | Payne et al.37 | Yes | 0.95 | 0.80 | 280.26 | 62.355 | 57.4439 |
| 0 | Payne et al.37 | No | 0.95 | 0.80 | 9.0405 | 62.29 | 57.3922 |
| 1 | Sherlaw-Johnson and Philips24 | Yes | 0.90 | 0.75 | 535.37 | 62.349 | 57.4395 |
| 1 | Sherlaw-Johnson and Philips24 | No | 0.90 | 0.75 | 288.61 | 62.29 | 57.3922 |
| 330 | Bidus et al.31 | Yes | 0.68 | 0.38 | 1138.9 | 62.413 | 57.4908 |
| 330 | Bidus et al.31 | No | 0.68 | 0.38 | 688.48 | 62.29 | 57.3922 |
| 342 | Bistoletti et al.32 | Yes | 0.61 | 0.37 | 353.24 | 63.098 | 58.0328 |
| 342 | Bistoletti et al.32 | No | 0.61 | 0.37 | 754.05 | 62.29 | 57.3922 |
| 1233 | Goldie et al.33 | Yes | 0.82 | 0.51 | 2388.7 | 62.496 | 57.5566 |
| 1233 | Goldie et al.33 | No | 0.82 | 0.51 | 369.38 | 62.29 | 57.3922 |
| 1712 | Kim et al.34 | Yes | 0.82 | 0.55 | 538.33 | 62.351 | 57.4412 |
| 1712 | Kim et al.34 | No | 0.82 | 0.55 | 175.43 | 62.29 | 57.3922 |
| 3448 | Vijayaraghavan et al.35 | Yes | 0.66 | 0.51 | 1627.4 | 62.423 | 57.4984 |
| 3448 | Vijayaraghavan et al.35 | No | 0.66 | 0.51 | 795.35 | 62.29 | 57.3922 |
| 3469 | Vijayaraghavan et al.35 | Yes | 0.86 | 0.66 | 1118.7 | 62.329 | 57.4234 |
| 3469 | Vijayaraghavan et al.35 | No | 0.86 | 0.66 | 252.36 | 62.29 | 57.3922 |
It can be seen that the quality scores were variable across the identified studies.
Appendix 11 Cost-effectiveness planes from the secondary cost-effectiveness analysis
FIGURE 24.
Cost-effectiveness plane for the secondary end points of the pre-leaflet assessed at 6 months.
FIGURE 25.
Cost-effectiveness plane for the secondary end points of online booking assessed at 6 months.
FIGURE 26.
Cost-effectiveness plane for the secondary end points SSKs sent on request assessed at 18 months.
FIGURE 27.
Cost-effectiveness plane for the secondary end points of SSKs sent unrequested assessed at 18 months.
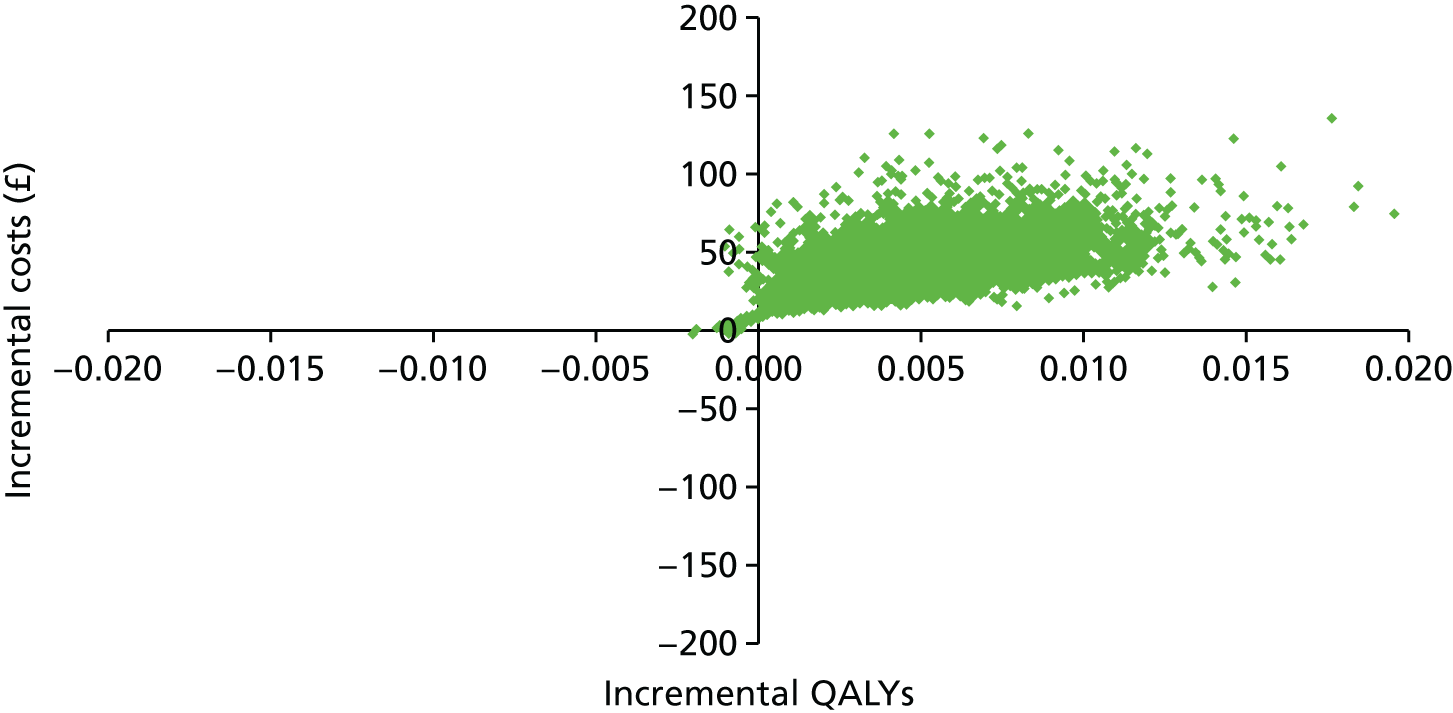
FIGURE 28.
Cost-effectiveness plane for the secondary end points of the NN assessed at 18 months.

FIGURE 29.
Cost-effectiveness plane for the secondary end points of timed appointments assessed at 18 months.
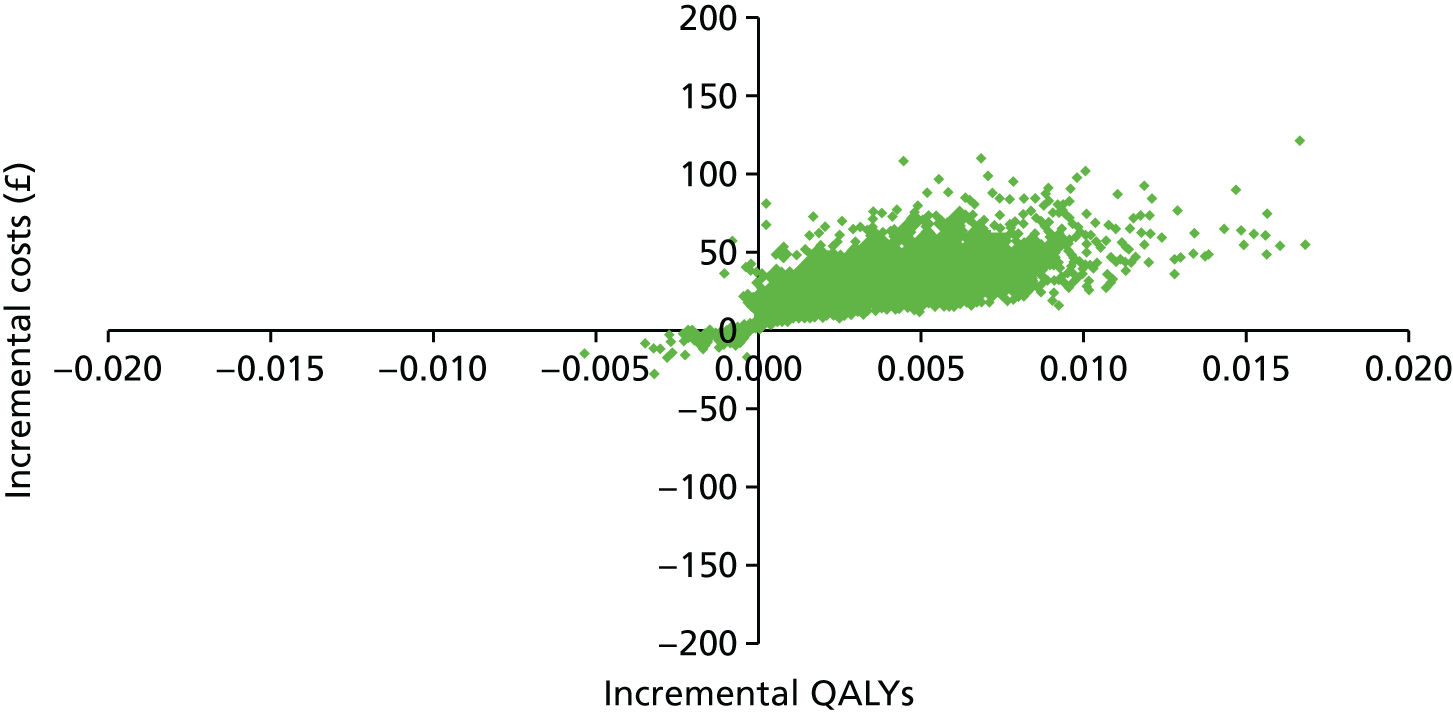
FIGURE 30.
Cost-effectiveness plane for the secondary end points of the choice between NN or SSK sent on request assessed at 18 months.
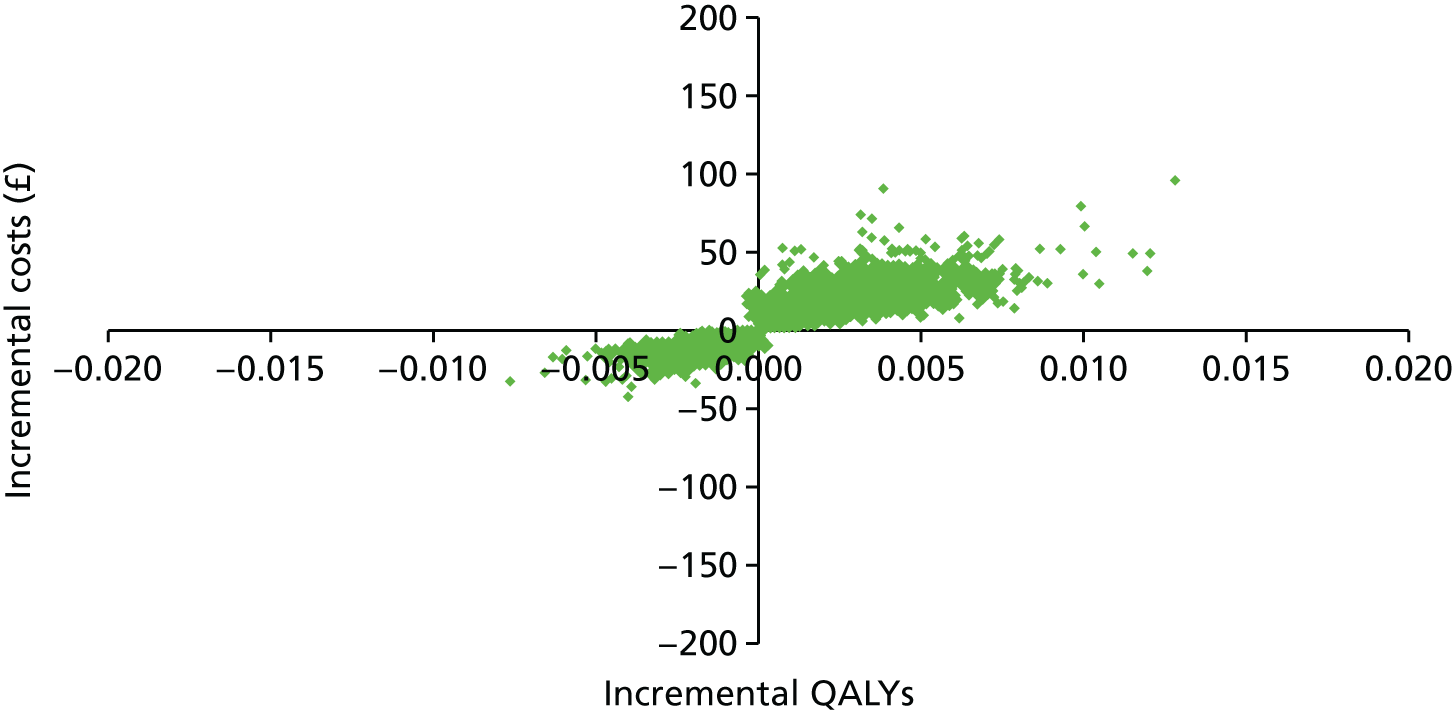
Appendix 12 Theoretical framework of discrete choice experiments
Appendix 13 Topic guide for the STRATEGIC study qualitative interviews
Acceptability of the three novel interventions
-
Which option (NN, SSK, timed appointments) they were offered and their initial reaction to it.
For those in the group who were given the choice of NN or SSK and those in the NN group who were then offered SSK, what was the reason for their choice?
-
If they HAVE used it, what happened (how did it go)?
Prompts for NN
-
How were you contacted by the NN (telephoned directly, via trial team – e-mail, text, telephone)?
-
What was discussed with the NN?
-
Who led the conversation?
-
How long was the conversation?
-
The outcome of the conversation (e.g. referred on to use online booking, SSK).
-
How they felt at the end of the conversation with the NN.
-
What they liked about the NN.
-
What they disliked about NN.
Prompts for SSK
-
How they accessed the SSK (via trial team – e-mail, text, telephone, online).
-
Understanding the instructions about how to use the kit – how easy or difficult.
-
Using the kit – how easy or difficult.
-
Sending back the sample – how easy or difficult.
-
How they felt about using the kit.
-
What they liked about self-sampling.
-
What they disliked about self-sampling.
Prompts for timed appointments
-
How they received the timed appointment.
-
Convenience of the timed appointment they were offered.
-
Understanding of how to confirm/change the timed appointment – how easy or difficult.
-
What they liked about timed appointments.
-
What they disliked about timed appointments.
If they have NOT used it, why was that?
At what point did they decide not to use (explore using the above prompts for each option).
-
How can the NN/SSK/timed appointment options be improved/what would encourage them to use it (again)?
-
Anything else they want to say about NN/SSK/timed appointments?
Identifying the attributes and levels for the discrete choice experiment exploring young women’s preferences for cervical screening programmes
-
What they see as important features of a cervical screening programme – in other words what would encourage them to book and attend for screening.
-
Booking:
How would they prefer to book an appointment?
Prompts
-
Online versus telephone – why is that?
-
Be given an appointment time versus choosing own appointment time – why is that?
What else is important in terms of booking the appointment?
Prompts:
-
Being able to change an appointment.
-
Waiting time for appointment – how long is acceptable?
-
Information in advance – what information?
-
Opportunity to speak to someone about it – who and how (telephone vs. face to face vs. e-mail)?
-
Willingness to pay?
-
Attending screening/doing self-sampling:
How would they prefer to have the screening done?
Prompts
-
Go to an appointment with a health professional versus do a test at home – why is that?
-
What are the advantages of going to an appointment with a health professional? And what are the disadvantages?
-
Who would they want the appointment to be with – why is that?
-
What are the advantages of doing a test at home? And what are the disadvantages?
What else is important in terms of the screening itself?
Prompts
-
Willingness to pay?
-
Accuracy?
-
Getting results and acting on them:
How would they prefer to get the results?
Prompts
-
Letter versus e-mail versus letter versus appointment/telephone call – why is that?
What else is important in terms of getting the results?
Prompts
-
Waiting time for results – how long is acceptable?
-
Accuracy of results – how accurate is acceptable?
-
Confidentiality – who should know?
-
Opportunity to speak to someone about it – who and how (telephone vs. face to face vs. e-mail)?
-
Willingness to pay?
-
Anything else they see as important.
-
Of everything we have discussed, what are the three most important things about the Cervical Screening Programme that would influence whether they attend or not.
-
Anything else that they want to say.
Appendix 14 Discrete choice experiment questionnaire
Appendix 15 Discrete choice experiment patient information leaflet
Appendix 16 Comments
STRATEGIC comments
| Code | Meaning |
|---|---|
| DCE comments | Participant comments on the DCE |
| DCE attributes | Participant comments on attributes |
| DCE process | Participant comments on the process |
| General | General comments on cervical screening |
| Location | Comments on the location of where the cervical screening will take place |
| Action | Comments on the action participants need to take in attending a cervical screening appointment |
| CS-N | Comments on why participants do not attend cervical screening |
| CS-Y | Comments on why participants do attend cervical screening |
| Privacy | Comments on privacy issues relating to cervical screening |
| Time | Comments on time relating to attending cervical screening |
| Risk | Comments on risk perception |
| Cost | Comments on costs to NHS |
| Perception/pain | Comments of perception of pain |
| Nurse | Comments relating to nurses being a part of the cervical screening process |
Code
DCE comments
As a (junior) doctor I think it is important to reiterate the value or cervical screening in SEXUALLY ACTIVE women[.]
As I’ve already written I feel the first set of questions were too much[.]
I am very grateful this questionnaire was sent to me I genuinely forgot/I was too busy to book a cervical smear test and this has encouraged/reminded me to do so Thank you, good luck in your studies[.]
I found the question is a bit confused, but, anyway I tried to answer[e]d it. I hope you understand me[.]
I have decided to take the questio[n]naire, as my case is a bit different, so I don’t know what bracket I fall under[.]
I have now attended my smear thanks to this form, luckily my tests came back negative:)!
I’d encourage more insightful questions regarding the Cervical Screening Process when it comes to overseas students choosing to do the test within the UK[.]
If you are going to hold surveys like this, could you ph[ra]se ‘women who have screenings but have not attended’ better or elaborate further? Not all women have skipped these screenings because they’re afraid, costs or laziness[.]
It would be good to have a section for comments and reasons for peo[p]le to provide for refusing cervical screening test[.]
Obviously the options were not what I would pick overall. The answers were based on what I thought were important if doing the test at home or by GP[.]
Personally I’m not sure your proposals will make me more inclined to have a smear.
Questionnaire does not address my main reason for non-participation in Cervical Screening Programme[.]
Some of the options I didn’t think were viable, and I was asked to choose between two un-realistic options. I think the questions would have been better in a different format, to get peoples opinions on specific options/thoughts of the NHS, instead of making them choose between two options that they would not necessarily choose as an option.//Individual questions would have been better. I do not feel my t[ru]e opinion has been expressed in this questionnaire due to the format[.]
Some of the questions repeat in a way that is confusing, I’m not sure that my answers will be interpreted in a way that reflects my real opinions. The questionnaire in general I found somewhat confusing[.]
Sometimes difficult to make a distinction between questions - variety of questions might be easier to understand.//Otherwise thank you!
The question[n]aire was really easy to complete and understand[.]
The questionnaire has showed me how important cervical cancer screening is I will book my appointment with my GP to do my test. Thanks[.]
The questionnaire indicates I am yet to attend a screening test but does not provide questions asking why women may shy away from undergoing a test. Overall, good questionnaire items[.]
The questionnaire was very easy to follow[.]
This is a great idea [I] am pleased to post my opinions personally[.]
This questionnaire was very easy to answer[.]
[T]his was a fairly complicated questionnaire and can see how some people may become easily confused[.]
DCE attributes
[A]n optional question on whether the participant is sexually active or not can affect your study in terms of the participant possessing adequate knowledge/importance of screening.//
Tweaking the cost etc - would have made more sense to ask people what there options would be i.e. action require by you? ? No// ?Yes.
as much as it gives service users input into methods best used for cervical screening, it doesn’t ask why there was a delay in uptake, which [I] think is also important[.]
It’s great that you are doing a study into this, which may encourage better uptake of cervical screening. However feel it isn’t comprehensive. Clearly you have set parameters which you are working in. But from my point of view the reasons for not undergoing screening are more complex than the 4 issues you are studying. Psychological and cultural factors as well as childhood abuse make it incredibly unlikely that I could allow anyone near me, voluntarily. I see a lack of recognition within the NHS that for some people screening just isn’t for them, and that there are complex, human reasons for this.
The options presented to choose from are based around 4 possible reasons why women not want to undertake cervical screening, but would it not be more pertin[e]nt to ask wo[m]en their own reasons why? My own reasons for not atten[d]ing screening were not covered by these choices. Also, the 4 simple bullet points do not convey the full pros and cons of each choice, and readers may not be aware of them. For example, sending a kit home for patients to take the test themselves may avoid embar[r]assment and time off work to make an appointment, but there are risks that the woman might do the test wrong, or hurt herself, or it may become contaminated. A professional nurse is trained to take the test. These pros and cons might not be immediately obvious to people taking the survey, and may not be taken into account when they are providing their answers.
The questions in section 2 could offer a further option because personally the main reason I chose an option was the location it was carried out although I don’t agree on the higher costs either therefore I wouldn’t want there to be a high cost yet also I wouldn’t want the rest to be carried out at the local surgery therefore I picked out the best option out of the two[.]
DCE process
SITE DID NOT WORK, I DID-NOT GET MY £10 VOUCHER, UNHAPPY WITH.
General
There is no use in screening women who have never been sexually active!
AWARENESS OF THIS SCREENING, NEED IMPROVENING./
Cervical screening (as well as breast examination) is so very important. More and more young adults are finding they have abnormal smears. My Mum and Grandma were diagnosed with carrying the BRAC2 gene.//(I have been tested and luckily I am not a carrier.)//
Cervical Screening age should be lowered to 20. This will save young lives.
Cervical screening is a very intrusive procedure which is not necessary for all women.
I recently went to a ‘lady doctor’s’ appointment, but it was in regards to contraception . . . She had noticed I hadn’t had my smear test so asked if she could do it at the same time - but it messed up the contraception by doing so (the coil-copper coil... the wires were lost).//
I Have Not yet had a smear test, but thinking about it. I feel that starting having smears early is important. I am not sure what age your first test should happen.
I THINK THE TEST AGE SHOULD BE LOWERED TO 16-18[.]
I think you are doing a bril[l]iant job + keep up the hard work, we need more information to be able to tackle this issue head on. Also I think the age for screening should be lower to help save more lives.
I WHOLLY SUPPORT AND ENCOURAGE CERVICAL SCREENING FROM AN EARLY AGE[.]
Your cervical screening records do need to be updated, as I told my doctor I was screened in a different country.
Location
I WOULD GLADLY BE SCREENED IF I HAD THE OPT FOR MY LOCAL SURGERY./
I like the idea of a home test as I feel more women would be more [i]nclined to get tested.
I believe more women, especially younger, would take the test if given the option to take the test at home. Knowing from past experience, some women can feel a bit shy & not vey keen at being poked & prodded.
I feel if testing was to be done at home by individuals it would be a huge succes[s], avoiding embarras[s]ment and time issues. It’s a good idea and something I would use.
I feel many women are at risk due to the screening being carried out by CP [GP] which is embar[a]ssing.
I had a cervical screening, 1 week ago. If there was an option of doing the test at home I would have done it MANY years ago.
I never new that there was even an option being considered where we would be able to do this from home[.]
I think offering a home testing kit as an alternative would encourage women who avoid testing (through embar[a]ssment/forgetting) to test themselves, and could save lives[.]
I WOULD ALWAYS CHOOSE HOME, PROVIDED THAT IT WOULD NOT COST THE NHS TOO MUCH, AND I FEEL GUIDANCE SHOULD ALWAYS BE AVAILABLE - EVEN IF DONE VIA AN ONLINE VIDEO/FAQ [frequently asked questions] SECTION.
However I feel I would also be willing to try a home kit first, before this survey I did not know that was a possible option in the future I hope if becomes available.
Personally I would prefer to take a test at home as I get embarras[s]ed and have a lot of anxiety’s that’s why I have never atten[d]ed for a test to be done.
In summary: I would prefer a home testing kit, that I would have to request (rather than just being sent automatically).
AND I’D PREFER A METHOD THAT IS MORE PRIVATE I.E. THE TEST KIT SENT TO MY HOUSE FOR SELF TESTING.
THE OPTIONS WHEN CHOOSING WHICH CHOICE I WOULD ALWAYS CHOOSE THE OPTION TO DO THE TEST AT HOME.
Therefore, testing myself at home would be 100% better all around.
I believe that sending tests in the post so women can do the test themselves would get a lot more women to do the test.
A LOT of people get put off the idea because of having To go To gp For it but doseNt bother me Either WAY the Leaflets sh[ou]ld be Eas[i]er To understand and [i]f Anyone cAnt mAke it To gp should be offer[e]d To have a home Test sent out if they can do it them self’s.
[I] would be more will to do a test at home if it was automatically posted when it was due.
I would prefer if an appointment was booked for me and I would rather have it at a clinic than do it myself.
Would always say to have screening at surgery.
Action
Young girls are having sexual intercourse at an early age and so I feel they need scree[n]ing and if a test kit could be sent through the post and is not as invasi[ve] as a normal smear, then I think this is the way forward.
Not an excuse but appointment times at a GPs doesn’t help, this problem.
I would highly recommend a time/date being sent to women as this would have definitely given it a higher sense of importance for me personally.
Booking appointments has its pro’s + con’s but if people aren’t going to make an appointment themselves then it’s likely they won’t call to reschedule or simply won’t turn up.//I would prefer an online/SMS [short messaging service] booking process. Allowing me to book an appointment at any time not when the phone line is open.
HOWEVER I DO THINK IF I RECEIVED AN APPOINTMENT ALREADY ARRANGED FOR ME I WOULD HAVE BEEN MORE LIKELY TO ATTEND.// THE REASON TO BE ABSENT FROM WORK WOULD BE COVERED BY A CONFIRED DOCTORS APPOINTMENT.//
A LOT of people get put off the idea because of having To go To gp For it but doseNt bother me Either WAY the Leaflets sh[ou]ld be Eas[i]er To understand and [i]f Anyone cAnt mAke it To gp should be offer[e]d To have a home Test sent out if they can do it them self’s.
I would prefer if an appointment was booked for me and I would rather have it at a clinic than do it myself.
CS-N (why do not attend cervical screening)
The HPV virus is spread by sexual transmission hence if you have never been active sexually it feels like you are being violated. Also the risk is extremely low to warrant a check in my personal opinion.//I did try to be screened by I was unable to let the nurse proceed as it was extremely uncomfortable and violating. This is why I refuse to be screened.
Finding time to attend an appointment is my downfall.
I actually went to my GP surgery to arrange a test, but since I was not at the top of the list to be sent a reminder I could not organise one. I also did not received any invitation to attend, and to date have still not. Thus it has been a bit of a nightmare to actually get a test. If I wasn’t presented with administrative barriers I would have gone ages ago.
I am very grateful this questionnaire was sent to me I genuinely forgot/I was too busy to book a cervical smear test and this has encouraged/reminded me to do so Thank you, good luck in your studies.
I believe more women, especially younger, would take the test if given the option to take the test at home. Knowing from past experience, some women can feel a bit shy & not vey keen at being poked & prodded.
I don’t want anyone to look at my private parts, as they are private . . .
I ignored previous invites to a smear test, as it sounds painful! However in real life, is nothing like the stories! This lack of information . . . is all it was. It’s a painless procedure! Should be a leaflet!// I wrote on the first page. You need a leaflet stating is not scary and it’s painless!
I have not attended screening because I suffer from PTSD [post-traumatic stress disorder] related to sexual traumas so the test would be psychologically traumatic to me. I have never had sex so the chances of cervical cancer are low. [H]aving this test would likely to therefore be painful and unnecessary. I also have been diagnosed with high-functioning autism. I do not cope well with touch. I find many NHS professionals do not make allowances for this.
I can guarantee that most people, certainly my age, don’t go as A. They’re embarrassed and B. We’re always told by others how painful it is which I know is not true.
I think offering a home testing kit as an alternative would encourage women who avoid testing (through embar[a]ssment/forgetting) to test themselves, and could save lives[.]
I think the study needs to look further into having tests done by trained gynaecologist rather than GPs. The reason why I personally haven’t been back to arrange a screening test is because I have previously had a test done (probably before I was 25 and that’s why I didn’t show up in your records) and it was horrible. Having lived abroad up until I was 21, I went for regular smear tests (around every 6 months) as this was the norm for any woman who was on the pill. The tests were done by a gynaecologist, who I knew and trusted, in his clinic. Of course the test isn’t comfortable but I never felt in pain. However, when I had the test done at the GP practice I’m registered at now, it was quite painful and uncomfortable. I didn’t have the impression the doctor was doing this on a regular basis and I didn’t feel she had the right equipment, such as the correct facilities for me to sit on, to make the procedure less uncomfortable. I would much rather prefer to pay privately to have the procedure done by a gynaecologist in their practice, than having this done at my GP, as I don’t think they are equipped for this. Having a test like that done is very personal and I can imagine that a lot of young women are self conscious about themselves when going for such a test, so having this done in the right environment, with the correct equipment and by a doctor who is an expert in that field would, in my opinion, be much more beneficial and effective. (expertise, pain privacy?)
I went to my first smear test just the other week at aged 25. For the sake of a couple of minutes and life. I’m ashamed I’d put it off so long. There need to be much more awareness for how important this is. (time)
HOWEVER, THE REASON WHY I HAVE NOT YET ATTENDED A SCREENING IS LESS TO DO WITH CONVENIENCE OR LOCATION BUT MY PERSONAL CIRCUMSTANCES. I AM A VIRGIN WITH VERY LITTLE EXPERIENCE AND I AM VEY ADVERSE TO BEING TOUCHED BY STRANGERS, TO THE POINT OF PANIC ATTACKS. AN INTIMATE EXAM WITH A COMPLETE STRANGER WOULD, BY ITS NATURE, MAKE ME INCREDIBLY UNCOMFORTABLE AND WOULD LIKELY MAKE SUCH A TEST PAINFUL. THERE ISN’T A LOT OF SUPPORT FOR THIS, AND IF THERE IS, NONE OF IT HAS BEEN OFFERED TO ME. (risk, location perception-pain)
My first experience of a smear was painful, each attempt after (3 attempts) have been painful due to being unable to relax. I found the nurses at the G.P. surgery unsympathetic. Oral sedative (valium) did not help. I feel I would benefit from I.V. [intravenous] [sed]ation (midazolam) as this is also a muscle relaxant, I would be more likely to return for screening if this was an option.
Reason why I have not gone for cervical screening is because I am not sexually active and therefore at low risk of cervical cancer.
The main reason for ignoring these screenings is the pain, and that’s the main reason, so just by having a nurse to help you guide through the process may help the most. A nurse who is encoura[g]ing and not to put you off. Because the pain puts you off!
THE MAIN REASON FOR NOT BOOKING AN APPOINTMENT FOR SCREENING IS THAT IT’S A BIT EMBARASSING[.]
I would like to e[m]phasise that I fully understand the importance of screening, but I find the whole idea of the process far too uncomfortable.
It is mainly embarrassment that has made me put off having the test so far.
The reason I did not accept the invitation to have a cervical screening last year was because I have not yet ever been sexually active. When I am, I will ask for a cervical screening.
The screening Test is A good thing I didn’t go To Mine because I WAS pregnant[.]
I turned 25 in June and have put off going but I will go its just finding the time. I then had the implant put in and have been bleeding for 5 weeks so that[‘]s another reason why I have not been.
While I know that cervical screening is important I always have put off booking an appointment, because I always have other activities I’d put first. I go to the doctors about 4/5 times a year. However, only once in about 20 appointments has the doctor mentioned that I have not had my screen. This, in an inadvertent way reaffirms to me that the fact I have delayed my screen isn’t that important/urgent.
Privacy
I feel if testing was to be done at home by individuals it would be a huge succes[s], avoiding embarras[s]ment and time issues. It’s a good idea and something I would use.
I feel many women are at risk due to the screening being carried out by CP which is embar[ra]ssing.
AND I’D PREFER A METHOD THAT IS MORE PRIVATE I.E. THE TEST KIT SENT TO MY HOUSE FOR SELF TESTING.
Time
I feel if testing was to be done at home by individuals it would be a huge success, avoiding embarras[s]ment and time issues. It’s a good idea and something I would use.
CS-Y (why do attend cervical screening)
I would highly recommend a time/date being sent to women as this would have definitely given it a higher sense of importance for me personally.
I had a cervical screening, 1 week ago. If there was an option of doing the test at home I would have done it MANY years ago. I only got the test one week ago as it was a requirement of the fertility clinic before I could get treatment.
Risk
I find it irritating to be persistently invited for a screening when I am not in an at risk group.
I’ve attended my appointments and was told that was low risk of C.C. [cervical cancer] because I’ve not had sexual intercourse, therefore no screening had to be done. I understand that I have to make my own appointments now but the fact that I am getting letters about me ‘missing’ or ‘refusing’ to go to my screening is getting annoying. I’m sure you don’t mean disrespect you not all women are simply refusing to go, some our us have gone to our appointments but DO NOT need screening!!!
I am a qualified doctor (surgery) & have taken steps to minimise my risk of cervical cancer (HPV vaccination prior to being sexually active, protected sex, small number of sexual partners). I therefore feel my risk of cervi[c]al cancer is low; sufficiently low that the disadvantages of screening (false positives & importantly time off work to attend appoint for screening test) outweighs the benefits in my personal case. I support the cervical screening program[m]e on the whole but based on my personal risk/benefit analysis I have elected not to participate at this stage.//Good luck with your study.
Reason why I have not gone for cervical screening is because I am not sexually active and therefore at low risk of cervical cancer.
I DO BGELIEVE THERE ARE ALOT OF MYTHS AND STORIES WHICH DISCOURAGE ALOT OF PEOLE TO ATTEND A SMEAR TEST.
[I] have heard horrific things about smear tests which has put me off but [. . .]
Cost
But on the other hand if it is gonna cost the NHS a lot more money then I think it should be carried on at your GP by the nurse or the nurse to be there and you do it yourself behind the curtain.
I really don’t want the NHS to invest heavily in this. Although a smear is important there are other areas of the NHS that need investment.
the cost to the NHS may make me change my view if it was vastly more expensive (to the NHS) to send out a home test rather than to have an appoin[t]ment at a clinic.
Perception/pain
The thought of a DIY [do it yourself] kit is quite daunting.
The main reason for ignoring these screenings is the pain, and that’s the main reason, so just by having a nurse to help you guide through the process may help the most. A nurse who is encoura[g]ing and not to put you off. Because the pain puts you off!
Nurse
I would really think if someone was available to discuss the test this would make one comfor[t]able to have it done.
It wouldn’t concern me if I wa[sn’t] able to talk to a nurse PRIOR to the test[.]
The main reason for ignoring these screenings is the pain, and that’s the main reason, so just by having a nurse to help you guide through the process may help the most. A nurse who is encoura[g]ing and not to put you off. Because the pain puts you off!
List of abbreviations
- AIC
- Akaike information criterion
- BIC
- Bayesian information criterion
- CaSH
- community and sexual health
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CI
- confidence interval
- CIN
- cervical intraepithelial neoplasia
- CONSORT
- Consolidated Standards of Reporting Trials
- DCE
- discrete choice experiment
- FGD
- focus group discussion
- GEE
- generalised estimating equation
- GP
- general practitioner
- HPV
- human papillomavirus
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- NICE
- National Institute for Health and Care Excellence
- NN
- nurse navigator
- NPEU
- National Perinatal Epidemiology Unit
- OR
- odds ratio
- PCT
- primary care trust
- PICOS
- population, intervention, comparator, outcomes, study design
- QALY
- quality-adjusted life-year
- SCCRS
- Scottish Cervical Call/Recall System
- SSK
- self-sampling kit