Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/50/14. The contractual start date was in April 2012. The draft report began editorial review in October 2016 and was accepted for publication in March 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
All authors report grants from the National Institute for Health Research (NIHR) during the course of the study. David A Richards reports grants from the European Science Foundation. David A Richards and Rod S Taylor have received funding support from NIHR Collaborations for Leadership in Applied Health Research and Care. David A Richards reports NIHR Clinical Development and Senior Clinical Fellowship and Senior Investigator Panel memberships. Rod S Taylor reports membership of NIHR Health Technology Assessment (HTA) programme themed call, NIHR HTA Efficient Study Designs Board and NIHR Health Services and Delivery Research Commissioning Boards. Simon Gilbody reports membership of the NIHR HTA Evidence Synthesis Board and NIHR HTA Efficient Study Designs Board. Willem Kuyken reports fees from Guilford Press for book royalties and Collaborative Case Conceptualisation.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Richards et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
This chapter uses material from Open Access articles previously published by the research team (see Rhodes et al. 1 and Richards et al. 2). © Rhodes et al. ;1 licensee BioMed Central Ltd. 2014 This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated and © The Author(s). 2 Published by Elsevier Ltd. This is an Open Access article under the CC BY license.
Scientific background and review of current literature
Clinical depression is one of the most common and debilitating of the psychiatric disorders. It accounts for the greatest burden of disease among all mental health problems, and is the second largest cause of global disability. 3 Lifetime prevalence has been estimated at 16.2% and rates of comorbidity and risk for suicide are high. 4–6 Depression is often recurrent, and without treatment many cases become chronic, lasting > 2 years in one-third of individuals. Over three-quarters of all people who recover from one episode will go on to have at least one more. 7 In the UK, depression and anxiety are estimated to cost the economy £17B in lost output and direct health-care costs annually, with a £9B impact on the Exchequer through benefit payments and lost tax receipts. 8 Globally, the economic impact of depression on aggregate economic output is predicted to be US$5.36 trillion between 2011 and 2030. 9
Reducing these substantial costs is a key objective for low-, medium- and high-income countries alike. Antidepressant medication (ADM) and cognitive–behavioural therapy (CBT) are the two treatments with most evidence of effectiveness, both of which are recommended by the National Institute for Health and Care Excellence (NICE). 10 Problems with ADM include side effects, poor patient adherence and relapse risk on ADM discontinuation. Service user organisations and policy think tanks advocate greater availability of psychological therapies, which many people prefer. 11 CBT, which is of similar efficacy to ADM,12 has several advantages: (1) it reflects the desire of many service users for non-pharmacological treatment; (2) it has no physical side effects; and (3) it modifies the illness trajectory in that benefits continue after the end of treatment, preventing recurrence. 13 However, CBT has several disadvantages: (1) its complexity makes it difficult to learn to implement in a competent fashion; (2) its efficacy is dependent on the skill of the individual practitioner; (3) patients are required to learn quite high-level skills; and (4) the high cost of training and employing sufficient therapists limits access to CBT.
As a consequence of the disadvantages above, many people do not receive adequate treatment, and, even when treatment is given, many respond only partially or not at all. 14 Despite the recent government initiative in England – ‘Improving Access to Psychological Therapies’ (IAPT; URL: www.iapt.nhs.uk/) – no more than 15% of people with depression will receive NHS-delivered CBT, and only 50% receiving CBT will recover. 15 It is therefore important to continue to test promising new treatments, especially if there are indications that such treatments reduce the risk of symptom return, are applicable to a wide range of depressed people including those with severe disease, are easy to implement in clinical practice and are therefore potentially more accessible,16 and are a cost-effective use of resources.
Globally, health services require effective, easily implemented and cost-effective psychological treatments for depression that can be delivered by less specialist health workers in order to close a treatment gap that can be as much as 80–90% in some low-income countries. 17 The English NHS, in order to meet public and professional expectations, requires a simple, equivalently effective, easily implemented psychological treatment for depression which can be delivered by less specialist (albeit appropriately competent) junior mental health workers (MHWs) to treat many more people with depression in a more cost-effective manner.
Rationale for the research
Behavioural activation (BA) is a psychological treatment based on behavioural theory that alleviates depression by focusing directly on changing behaviour. 18–20 This theory states that depression is maintained by avoidance of normal activities. As people withdraw and disrupt their basic routines, they become isolated from positive reinforcement opportunities in their environment. The combination of increased negative reinforcement with reduced positive reinforcement results in a cycle of depressed mood, decreased activity and avoidance which maintains depression. 19 BA systematically disrupts this cycle, initiating action in the presence of negative mood, when people’s natural tendency is to withdraw or avoid. 21,22 Although CBT incorporates some behavioural elements, these focus on increasing rewarding activity and initiating behavioural experiments to test specific beliefs. In contrast, BA targets avoidance from a contextual, functional approach not found in CBT (i.e. BA focuses on understanding the function of behaviour and replacing it accordingly). BA also explicitly prioritises the treatment of negatively reinforced avoidance and rumination. Furthermore, the BA rationale is easier to understand and operationalise for both patients and MHWs than CBT, which also focuses on increasing activity, but primarily on changing maladaptive beliefs. 23 Moreover, there is some evidence that CBT is less effective when delivered by less competent therapists. 12,24
In the UK, CBT is delivered by professionally qualified senior MHWs (mainly clinical psychology, nursing, occupational therapy, social work or counselling), who have obtained a further 1-year, full-time postgraduate qualification in CBT. Their training is long and expensive and their employment grade is costly compared with junior MHWs, who deliver much of the routine mental health care in the UK. The relative simplicity of BA treatment may make it easier and cheaper to train junior MHWs in its application than CBT, the argument of ‘parsimony’ first advanced by one of the early proponents of this approach, Neil Jacobson. 19 However, this is appropriate only if BA delivered in this way is as effective as, and more cost-effective than, CBT.
Limitations of previous trials
A number of systematic reviews have attempted to address the more general question of BA effectiveness compared with CBT. 10,25–28 All have commented on the relatively poor quality of component studies. We conducted a meta-analysis of randomised controlled trials (RCTs) of BA,25 and identified 12 studies with a total of 476 patients. At the primary end point we found no difference between the groups on depression symptom level [Hedges’ g = 0.102, 95% confidence interval (CI) −0.122 to 0.326; I2 = 29%; p = 0.372] (Figure 1). At follow-up we found no difference between the groups on depression symptom level (Hedges’ g = 0.395, 95% CI −0.032 to 0.822; I2 = 61%; p = 0.070).
FIGURE 1.
Meta-analysis of pre-COBRA (Cost and Outcome of BehaviouRal Activation) trial BA vs. CBT primary outcome point, all included studies. AT, automatic thoughts; LS, low severity.
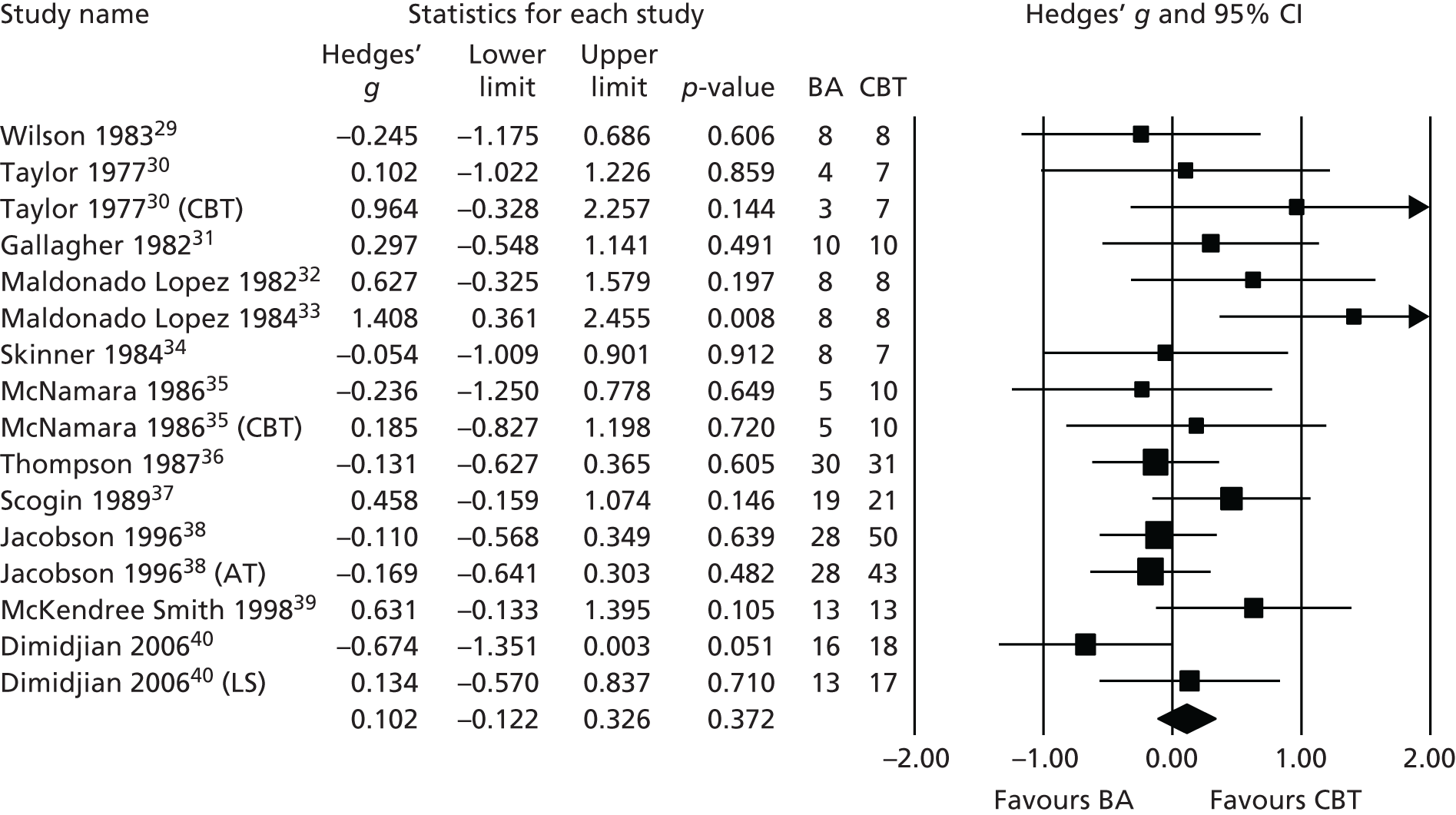
In a subsequent update of this review26 we found no additional trials comparing BA with CBT. Many of the trials included in our review were of limited methodological quality, all were underpowered for comparing treatments, and most did not utilise diagnostic interviews for trial inclusion. Treatments in many cases did not conform to modern clinical protocols for BA. Long-term outcomes were rarely reported, with average follow-up only to 4 months. These results have been replicated in two recent Cochrane reviews of behavioural therapies,27,28 which concluded that there was only low- to moderate-quality evidence that behavioural therapies and other psychological therapies were equally effective and called for ‘Studies recruiting larger samples with improved reporting of design and fidelity to treatment to improve the quality of the evidence’. 27
Most significantly, NICE10 reviewed the same evidence and regarded only a small subset of three trials31,38,40 as of sufficient quality to be able to contribute evidence of effect (Figure 2). In those studies no difference was found between BA and CBT at primary end point (Hedges’ g = 0.139, 95% CI –0.4.00 to 0.122; I2 = 1%; p = 0.296) or at follow-up (Hedges’ g = 0.135, 95% CI –0.456 to 0.186; I2 = 0%; p = 0.409).
FIGURE 2.
Meta-analysis of pre-COBRA (Cost and Outcome of BehaviouRal Activation) trial BA vs. CBT, subset of high-quality included studies in NICE review. AT, automatic thoughts; LS, low severity.
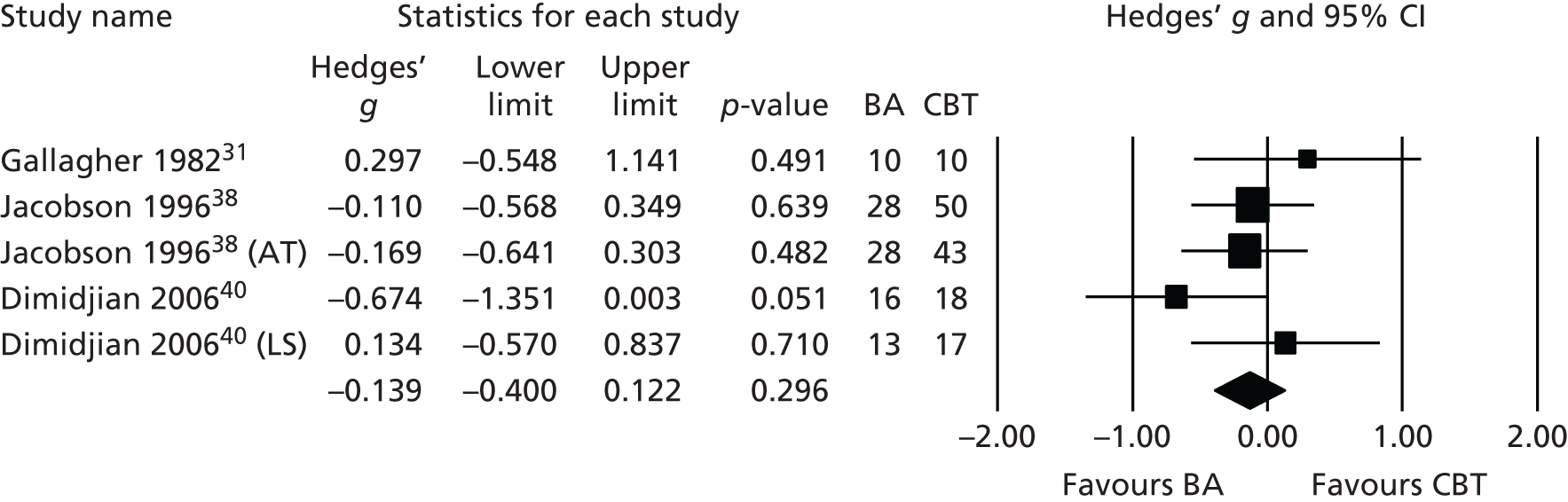
The conclusion of the NICE Guideline Development Group was that that the evidence base for BA was not ‘sufficiently robust’ for it to be recommended as an alternative to CBT. It was suggested that BA could be an option for clinicians, but the limited evidence base should be considered when making this treatment choice. 10 Consequently, NICE made a clear research recommendation ‘to establish whether behavioural activation is an effective alternative to CBT’ using a study which is ‘large enough to determine the presence or absence of clinically important effects using a non-inferiority design’ (p. 256). 10
Pilot work preceding this trial
In order to test uncertainties around our main objectives, we piloted BA in a Phase II RCT to examine whether or not MHWs without previous specialist training in psychological therapy can effectively treat depressed people using BA. 41 We compared BA against usual care. Relatively junior NHS MHWs (‘band 5’ – equivalent to a basic grade, qualified mental health nurse) with no previous formal training or experience in psychotherapy delivered BA. These workers received 5 days’ training in BA and, subsequently, 1 hour of clinical supervision, fortnightly, from a clinical nurse consultant or trained psychotherapist. Intention-to-treat (ITT) analyses indicated a difference in favour of BA of −15.79 points (n = 47, 95% CI −24.55 to −7.02 points) on depression (as measured via the Beck Depression Inventory-II), an effect size of −1.15 standard deviation (SD) units (95% CI −0.45 to −1.85 units). We also found a quality-adjusted life-year (QALY) difference in favour of BA of 0.20 points (95% CI 0.01 to 0.39 points; p = 0.042), incremental cost-effectiveness ratio (ICER) of £5756 per QALY and a 97% probability that BA is cost-effective at a threshold value of £20,000. 41
Conclusion
From our literature reviews and pilot work we concluded that BA was a potentially viable treatment for depression when delivered by junior MHWs, but that, as NICE had suggested, a non-inferiority trial of BA versus CBT was required to test whether or not BA was non-inferior to CBT and if BA could be a potentially cost-effective alternative to CBT for depression. We now report the results of this randomised trial to determine if BA is non-inferior to CBT in the treatment of patients with depression. This report is divided into chapters detailing the methods and results of our primary clinical effectiveness and cost-effectiveness questions followed by a chapter for our process evaluation. We conclude with a discussion chapter summarising our results and considering their implications for the treatment of depression in the UK and internationally.
Chapter 2 Methods
This chapter uses material from Open Access articles previously published by the research team (see Rhodes et al. 1 and Richards et al. 2). © Rhodes et al. ;1 licensee BioMed Central Ltd. 2014 This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated and © The Author(s). 2 Published by Elsevier Ltd. This is an Open Access article under the CC BY license.
Research objectives
-
To assess the clinical effectiveness of BA compared with CBT for depressed adults in terms of depression treatment response at 12 and 18 months.
-
To assess the cost-effectiveness of BA compared with CBT in terms of QALYs at 18 months.
In addition, we undertook a secondary process evaluation to investigate the moderating, mediating and procedural factors in BA and CBT that influence outcome, the methods for which are covered in Chapter 4.
Study design
We undertook a research assessor-blinded, multicentre, two-arm, non-inferiority, patient-level RCT for people with depression, to test the effectiveness of a psychological intervention for depression, BA, against the current gold standard, evidence-based treatment, CBT. We included clinical, economic and process evaluations. The rationale for a non-inferiority trial is that we needed to establish whether or not the clinical effectiveness of BA is not substantially inferior to CBT. Therefore, we powered our trial on the basis of clinical non-inferiority, and analysed our data accordingly. 42,43
Patient and public involvement
We involved patient and public involvement (PPI) representatives at all stages of the project. A PPI advisor (NR) was a full member of the Trial Management Group (TMG). He attended all meetings of the TMG and advised on patient-facing materials, including ethics materials and participant therapeutic manuals, and on the conduct of the trial including project management, questionnaire development, data collection and project dissemination. There was a PPI representative on the Trial Steering Committee (TSC) from a depression consumer advocacy group who provided important checks and balances as part of the independent TSC oversight of the trial.
All sites had excellent local PPI mechanisms. We followed national good practice guidance for researchers on public involvement in research and the paying of PPI representatives actively involved in research. 44 We also worked with our PPI representatives to ensure that our dissemination strategies were inclusive and accessible to other people who use services. In addition, the trial was co-ordinated from the University of Exeter’s Medical School. The Medical School operates within a culture of PPI – guided by published theories of participation, empowerment and engagement – through the National Institute for Health Research (NIHR) Collaborations in Leadership in Applied Health Research and Care for the Peninsula Public Involvement Group.
Setting and participants
We recruited participants over a 20-month period from September 2012 to April 2014. Potential participants were identified by clinical studies officers (CSOs) or practice staff from the electronic case records of primary care and psychological therapy services in Devon, Durham and Leeds, indicating that the person had been identified as currently depressed at least once during the previous 2 months. Practices or services contacted patients to seek permission for researcher contact. The research team interviewed those who responded, provided detailed information on the study, took informed consent and assessed people for eligibility.
Inclusion and exclusion criteria
Inclusion
People aged ≥ 18 years with a major depressive disorder (MDD) as assessed by the Structured Clinical Interview for DSM Disorders (SCID) and Diagnostic and Statistical Manual for Mental Disorders-Fourth Edition (DSM-IV) were eligible to take part in the study. 45 Researchers were trained to administer the SCID using established training and inter-rater reliability procedures in use at the University of Exeter for all of our trials.
Exclusion
People who were alcohol or drug dependent, acutely suicidal or cognitively impaired, had bipolar disorder, psychosis or psychotic symptoms, ascertained at baseline by research interviews, were excluded. We also excluded people currently undergoing psychological therapy.
Randomisation, concealment of allocation and blinding
Following interview, participants were allocated in a 1 : 1 ratio to either the BA or CBT arm stratified according to their symptom severity on the Patient Health Questionnaire-9 (PHQ-9)46 (PHQ-9 of < 19 vs. ≥ 19 points), ADM use (currently using ADM or not) and recruitment site. The registered Peninsula Clinical Trials Unit (PenCTU) allocated participants remotely after the researchers had collected and entered baseline data into a computer database to ensure researcher blinding and allocation concealment. Investigators were not informed of participants’ allocations. The computer-based system allocated the first 20 participants to each arm on a truly random basis. For subsequent participants, allocation was minimised to maximise the likelihood of balance in stratification variables across the two study arms. Concealment was ensured by the use of a password-protected trial website and retaining a stochastic element to the minimisation algorithm. The computer-based allocation and website were set up and maintained by PenCTU, independent of the trial. The participant’s details were then sent to the relevant MHW to alert them to contact this person and begin treatment. The general practitioner (GP) was then informed of their patient’s involvement in the study.
In this type of trial, in which interventions are complex and clearly different from each other, it is not possible to blind participants or clinicians, so our procedures focused on helping to keep research workers blind to participant allocation and by protecting the study against assessment interpretation bias through the use of self-report measures. All research measures were applied to both groups, and researchers were instructed to maintain blindedness by reminding participants at follow-up of the need not to discuss their treatment with the researcher. We recorded instances where researchers were unblinded by patients disclosing their treatment during interviews.
Sample size calculation
We estimated the non-inferiority margin for the primary outcome (PHQ-9) using two potential approaches with reference to (1) the effect size of historical trials comparing BA versus control; and (2) the published minimum clinically important difference for the primary outcome (PHQ-9) of 2.59 to 5.00 points. 47 Based on our meta-analysis, BA was superior to control in depression score by a mean of 0.7 SD units (95% CI 0.39 to 1.00 SD units) or 3.8 PHQ-9 points (95% CI 2.1 to 5.4 PHQ-9 points) (assuming a SD of 5.4 from Lowe et al. ). 47 It has been proposed that non-inferiority margins be taken as ≈0.5 × mean control effect size (i.e. 0.5 × 3.8 = 1.90 points) or as the lower 95% limit of the control effect size (i.e. 2.1 points). 48,49 To ensure the adequacy of this trial to test non-inferiority between BA and CBT, we therefore examined a number of potential scenarios taking into account the potential uncertainty in the non-inferiority margin for the primary outcome.
We selected a conservative non-inferiority margin of 1.90 points and power of 90%. As a consequence, we needed to recruit a total of 440 participants to detect a between-group non-inferiority margin of 1.90 points in PHQ-9 at one-sided 2.5% alpha, allowing for 20% attrition caused by dropouts and protocol violators. Furthermore, although previous trials of CBT have shown little or no effects of clustering in outcome by therapists, even when delivering group CBT,50,51 if we were to assume a small therapist clustering effect (i.e. intracluster correlation coefficient of 0.01), this sample size would still have 80% power for a non-inferiority margin of 1.90 points on the PHQ-9 at one-sided 2.5% alpha, allowing for 20% attrition.
Our sample size was inflated by 20% for participant dropout to take account of participants who might exit the trial and refuse follow-up assessment, although our experience in running large primary care trials of depression treatment is that attrition rates would be less than this. Therefore, we planned to recruit 440 participants to the trial, 220 per arm. A summary of the sample size calculations is provided in Table 1.
| Approach | MCID (points) | Power (%) | Attrition rate (%) | Sample size per groupa |
|---|---|---|---|---|
| 50% BA control effect size | 1.90 | 90 | 20 | 220 |
| 50% BA control effect size | 1.90 | 80 | 20 | 160 |
| LCI BA control effect size | 2.10 | 90 | 20 | 180 |
| LCI BA control effect size | 2.10 | 80 | 20 | 135 |
| Lower MCID | 2.59 | 90 | 20 | 120 |
| Lower MCID | 2.59 | 80 | 20 | 90 |
Recruitment
Randomised controlled trials are vulnerable to selection bias and threats to external validity if there are systematic differences in behaviour between referring clinicians. We minimised this potential bias by recruiting participants through searching general practice records and referral logs from primary care to local depression and anxiety treatment services rather than by direct referral from GPs. We identified suitable participants by examining electronic case records for all patients in each general practice or treatment service. The search was conducted by practice staff or Clinical Research Network CSOs, identifying people with at least one identification code for depression recorded against their name in the last 2 months. In primary care practices we searched for the codes most widely used by GPs to classify participants as depressed. The list of potentially suitable participants was reviewed by GPs to identify any patients whom had known exclusion criteria. The remaining patients were written to, inviting them to take part in the study. For patients already referred to local psychological therapies services, we contacted those on the waiting list. Letters were sent with a short participant information sheet, stamped and addressed envelope and a ‘Permission for Researcher to Contact’ form to allow a researcher to contact them. If potential participants did not return the form, they were contacted by telephone by practice staff or practice-based Clinical Research Network CSOs to check that they had received the letter and asking them if they wished to participate in the Cost and Outcome of BehaviouRal Activation (COBRA) trial. Potential participants identified were interviewed by researchers on the telephone to confirm the presence of depressive symptoms and to explain the trial fully. If positive on the screen, potentially eligible participants were interviewed face to face by researchers to confirm eligibility, take consent, conduct a diagnostic interview and collect baseline measures. Eligible, fully informed and consenting participants were then entered into the study and randomised.
From our experience of previous trials, we calculated that 37% of potential participants interviewed at baseline would be likely to decline participation, would not meet our inclusion criteria or would meet one of the exclusion criteria. Therefore, we were required to interview 700 potential participants in order to induct our planned sample size of 440 eligible participants into the trial. Following random allocation of 440 participants, a maximum of 20% attrition would lead to our target sample size of 366 participants.
In order to identify 700 people for baseline interview, we planned to contact around 3400 potential participants through letter and/or telephone to inform them of the trial and offer them the chance to participate. In order to do so, we needed to identify 5300 potential participants from a sensitive coded search of practice case note records, as our existing data predicted that 1900 (approximately 36%) of these would be excluded by GPs against known trial exclusion criteria. Identifying 5300 potential participants was expected to generate at least 700 positive replies.
For an average-size practice of 7000 registered patients, we expected that searches would be likely to identify around 37 potentially eligible participants per search. Four searches per practice would, therefore, identify 148 potential participants per practice. Consequently, we planned to recruit at least 36 practices (12 per site) to identify sufficient potential participants to meet our target number of 5300.
Trial interventions
We developed our BA and CBT intervention protocols in line with (a) published treatment protocols,19,21,22,40,53,54 including that developed for BA and CBT in our trials;41,55 (b) advice from national and international collaborators (Martell, Dimidjian, Hollon); and (c) NICE recommendations10 for duration, and frequency, of BA and CBT. To recognise realities of real-world clinical presentations, our protocols included behavioural and cognitive strategies for managing comorbidity, particularly anxiety, where this is present in addition to depression. Therapists were able to provide participants with a maximum of 20 sessions over 16 weeks with the option of four additional booster sessions. 10
Behavioural activation
The overall goal of BA is to re-engage participants with stable and diverse sources of positive reinforcement from their environment and to develop depression management strategies for future use. MHWs delivering BA followed a written treatment manual. Sessions were face to face, of 1 hour duration, with the option of being conducted up to twice weekly over the first 2 months and weekly thereafter. The sessions consisted of a structured programme increasing contact with potentially antidepressant environmental reinforcers through scheduling and reducing the frequency of negatively reinforced avoidant behaviours. The central behavioural technique was a functional analysis of the participant’s problems, based on a shared formulation drawn from the behavioural model in the early stages of treatment, thereafter developed with the patient throughout their sessions. Specific BA techniques included the use of a functional analytical approach to develop a shared understanding with patients of behaviours that interfere with meaningful, goal-oriented behaviours and included self-monitoring, identifying ‘depressed behaviours’, developing alternative goal-orientated behaviours and scheduling. In addition, the role of avoidance and rumination was addressed through functional analysis and alternative response development incorporating recent trial evidence. 56
We selected MHWs from NHS Agenda for Change (AfC) band 5 staff, such as mental health nurses and psychological well-being practitioners (PWPs),52 who received 5 days’ training in BA. In line with the programme developed and tested in our Phase II trial,41 training focused on the rationale and skills required to deliver the BA protocol for depression and included sections on behavioural learning theory and its application to depression, developing individualised BA formulations and specific techniques used in sessions. Training was a mix of presentation and role-play with repeated practice and feedback. Workers were competency-assessed at the end of training using standardised marking criteria consistent with the BA protocol and further training was given if competency was not demonstrated in practical clinical exercises. BA workers received 1 hour of clinical supervision, fortnightly, from the three site leads or other members of the trial team who were clinically qualified in BA.
Cognitive–behavioural therapy
The overall goal of CBT is to alter the symptomatic expression of depression and reduce risk for subsequent episodes by correcting the negative beliefs, maladaptive information processing and behavioural patterns presumed to underlie the depression. Therapists delivering CBT followed a written treatment manual. Sessions were face to face, of 1 hour duration, with the option of being conducted up to twice weekly over the first 2 months and weekly thereafter. The sessions consisted of a structured, collaborative programme. Treatment began with agreeing a problem list and goals for therapy, participants learning the CBT model, behavioural change techniques, and moved on to identifying and modifying negative automatic thoughts, maladaptive beliefs and, if indicated, underlying core beliefs. In later sessions, learning was translated to anticipating and practising the management of stressors that could provoke relapse in the future. Specific CBT techniques included scheduling activity and mastery behaviours, the use of thought records and modifying maladaptive beliefs and rumination content. The behavioural elements in CBT focused on increasing activity with practical behavioural experiments to test specific cognitive beliefs. CBT did not take the contextual, functional analytical approach of the BA trial arm.
Cognitive–behavioural therapy was delivered by NHS AfC band 7 senior MHWs with a specialist postgraduate diploma in ‘high-intensity’ CBT from an accredited university course. The CBT therapists also received a 5-day orientation training to the specific COBRA trial CBT protocol, including its adaptation for comorbidities, cognitive theory of depression, developing individualised cognitive formulations and specific techniques used in sessions. Therapists were competency-assessed at the end of training using standardised marking criteria consistent with the CBT protocol and further training was given if competency was not demonstrated. CBT therapists also received a subsequent 1 hour of clinical supervision, fortnightly, from established supervisors in the three sites with advice from other members of the trial team who were clinically qualified in CBT.
Outcomes
An overview of our data collection timings is presented in Table 2.
| Data | Source of data | Timing of data collection | Months after baseline | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Gender, age, ethnic origin, education level, employment, marital status, number of children, presence or absence of ADM treatment, previous history and age at onset of depression, duration of any ADM treatment, and presence of any comorbid anxiety disorder | CRF | Baseline | |||||
| Primary outcome | PHQ-9 | CRF | Baseline | 6 | 12 | 18 | ||
| Secondary outcomes | DSM-IV depression status | CRF | Baseline | 6 | 12 | 18 | ||
| Number of depression-free days | CRF | 6 | 12 | 18 | ||||
| SF-36 | CRF | Baseline | 6 | 12 | 18 | |||
| Economic data | EQ-5D-3L | CRF | Baseline | 6 | 12 | 18 | ||
| AD-SUS | CRF | Baseline | 6 | 12 | 18 | |||
| Process evaluation data | Age at depression onset | CRF | Baseline | |||||
| Number of previous depression episodes | CRF | Baseline | ||||||
| BADS | CRF | Baseline | Therapy session 4 | Therapy session 7 | 6 | |||
| DAS | CRF | Baseline | Therapy session 4 | Therapy session 7 | 6 | |||
| RRS | CRF | Baseline | Therapy session 4 | Therapy session 7 | 6 | |||
| SHAPS | CRF | Baseline | Therapy session 4 | 6 | 12 | 18 | ||
| Acceptability of BA and CBT | Qualitative interviews | Patients: on completion of therapy Clinicians: on completion of trial involvement |
||||||
| Treatment mechanisms and impact | Qualitative interviews | Patients: on completion of therapy Clinicians: on completion of trial involvement |
||||||
Baseline information
We collected demographic data at baseline through a purposely designed form. We recorded data on gender, age, ethnic origin, education level, employment, marital status, number of children, presence or absence of ADM treatment, previous history and age at onset of depression, duration of any ADM treatment and presence of any comorbid anxiety disorder.
Clinical data
We conducted follow-up assessments at 6, 12 and 18 months post-baseline assessment. Our primary outcome was self-reported depression severity and symptomatology, as measured by the PHQ-9,46 at 12 months. The PHQ-9 is a nine-item questionnaire that records the core symptoms of depression with established excellent specificity and sensitivity characteristics in a UK population. 57 Our secondary outcomes were DSM-IV MDD status and number of depression-free days between follow-ups, assessed by the SCID,45 anxiety assessed by the Generalised Anxiety Disorder-7 (GAD-7) questionnaire58 and health-related quality of life assessed by Short Form questionnaire-36 items (SF-36). 59 We also assessed the relative proportions of participants meeting criteria for ‘recovery’ (proportions of participants with PHQ-9 scores of ≤ 9 points) and ‘response’ (50% reduction in scores from baseline) on the PHQ-9. We also recorded the presence of DSM-IV anxiety disorders at baseline and follow-ups.
Economic data
We took the UK NHS and Personal Social Services (PSS) perspective consistent with the UK NICE reference case. 60 A broader societal perspective was explored in sensitivity analysis to capture the effects of productivity loss as a result of time off work due to illness, as depression is known to impact on an individual’s ability to work and can result in substantial losses in the workplace. 61 In addition, the use of complementary therapies was included in a further sensitivity analysis following advice from the clinical team that such therapies are commonly used by adults with depression. Narrower perspectives of intervention and mental health care were also examined in sensitivity analyses.
We collected participants’ use of BA and CBT from clinical records, with additional resource information (e.g. training, supervision and other non-face-to-face activities) collected from therapists and trainers. We measured all other health and social care services used, including medication prescription using the adult service use schedule (AD-SUS), designed on the basis of previous evidence of service use in depressed populations. 62 We measured productivity losses using the absenteeism and presenteeism questions of the World Health Organization’s Health and Work Performance Questionnaire. 63 The AD-SUS and the Health and Work Performance Questionnaire were completed by patients in interviews with a research assessor at baseline and at the 6-, 12- and 18-month follow-ups. At baseline, participants were asked to report service use over the previous 6 months. At all follow-up points, participants were asked to report service use since the last interview, to capture service use for the entire period from baseline to follow-up, even if participants had missed intermediate interviews.
We measured effectiveness for the economic evaluation in terms of QALYs calculated using the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), a non-disease-specific measure for describing and valuing health-related quality of life, at baseline and at the 6-, 12- and 18-month follow-ups. 64 The EQ-5D-3L consists of five dimensions in the domains of mobility, self-care, usual activities, pain/discomfort and anxiety/depression, each scored on three levels (no problems, some problems or extreme problems), and classifies individuals into one of 243 health states. Health states are converted into a single summary index utility score by applying weights to each level in each dimension derived from the valuation of EQ-5D-3L health states in adult general population samples. 65 QALYs were calculated as the area under the curve defined by the utility values at baseline and each follow-up. It was assumed that changes in the utility score over time followed a linear path. 66
Process data
In addition to information on age at depression onset and number of previous episodes collected using the SCID,45 we also collected data on changes in specific behaviour [as assessed via the Behavioural Activation for Depression Scale (BADS)],67 changes in beliefs [as assessed via the Dysfunctional Attitudes Scale (DAS)],68 rumination [as assessed via the Ruminative Response Scale (RRS)],69 hedonic tone [as assessed via the Snaith–Hamilton Pleasure Scale (SHAPS)],70 acceptability of BA and CBT for participants and clinicians (assessed with qualitative methods), and per protocol (PP) treatment adherence (from therapist case records). We collected qualitative data via semistructured interviews to access participants’, BA MHWs’ and CBT therapists’ accounts of the mechanisms and impacts of treatment. Interviews focused on acceptability, views of the role of cognitive and behavioural change strategies and broader impacts of treatment in participants’ lives.
Intervention fidelity
We assessed the quality of, and adherence to, BA and CBT clinical protocols using audiotapes and written records of therapy sessions, which MHWs and therapists were instructed to take, with participant permission, for each clinical session. A random sample of tapes, stratified by therapist, therapy session and intervention, were sent to independent experts in both treatments for competency rating using the Cognitive Therapy Scale-Revised (CTS-R)71 (range 0–78, competency cut-off score = 36) for CBT and the Quality of Behavioural Activation Scale72 (range 0–84, competency cut-off score = 42) for BA. Independent rating of recorded therapy sessions was undertaken by the Oxford Cognitive Therapy Centre (Oxford Health NHS Foundation Trust) and Dr Christopher Martell of the University of Wisconsin–Milwaukee, for CBT and BA, respectively.
Therapy ratings were used for several different purposes:
-
to assess each therapist’s competency, at the start of the trial, to deliver the interventions by rating the first two therapy sessions undertaken
-
to monitor whether or not levels of competence/adherence were maintained throughout the trial.
We asked all therapists in both treatment groups to record their in-session activity by completing specially designed therapy record sheets. These sheets included a list of therapeutic techniques specific to each type of therapy, with a tick box against each element. Therapists indicated which of the techniques they had used in each session.
Safety and adverse events
For adverse events (AEs), we recorded deaths from whatever cause, and all self-harm and suicide attempts. The independent Data Monitoring Committee (DMC) reviewed all AEs and made relevant trial conduct recommendations as a consequence.
Data analysis
We analysed and report primary and secondary outcomes in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines for non-inferiority and equivalence trials. 43 All analyses were carried out using an a priori statistical analysis plan prepared in the first 6 months of the trial and agreed with the TMG, TSC and the DMC.
Equivalence of baseline characteristics and outcomes in the two groups were assessed descriptively. As differences between randomised groups at baseline could have occurred by chance, no formal significance testing was conducted. We also undertook a descriptive analysis of the baseline patient characteristics according to the recruitment method (recruitment from psychological therapies waiting list vs. GP case note review).
We undertook both ITT and PP analyses. PP analysis provides some protection for any theoretical increase in the risk of type I error (erroneously concluding non-inferiority). 73 The ITT population was defined as all randomised patients in the groups to which they were allocated with observed outcome data at follow-up. The PP population was predefined by the TMG as those patients who met the ITT definition and received eight or more treatment sessions for both groups. Although the CONSORT guidelines recommend a PP approach (i.e. analysis according to actual treatment received) as the conservative non-inferiority analysis option, given the potential biases of both PP and ITT analyses,43 we took the approach of the European Medicines Agency, that security of inference depends on both PP and ITT analyses demonstrating non-inferiority of the primary outcome. 74 We, therefore, checked for non-inferiority in both the PP and ITT populations. In order to check the security of inference of non-inferiority, sensitivity analysis for the primary outcome was undertaken for the ITT imputed population and PP analyses based on different definitions of adherence/protocol adherence.
The TMG predefined our PP population. We also conducted sensitivity analyses using different definitions of PP adherence. We included varying proportions of PP participants in these sensitivity analyses populations, depending on how much of each therapy they had received, ranging from 40% to 100% of planned therapy sessions. Our analysis plan specified that, if non-inferiority was consistently shown by these analyses, we would proceed to assess superiority of CBT compared with BA (i.e. the CI lower bound lies above 0). If we found that conclusions were inconsistent across analyses, we planned to revert back to primacy of the PP analysis to confirm or refute the non-inferiority hypothesis.
Our primary analysis compared observed primary and secondary outcomes between BA and CBT groups at 12 months after randomisation using linear regression models that adjusted for baseline outcome values and stratification/minimisation variables (symptom severity, site, ADM use). Although we initially planned to include therapist as a random-effects variable in our models, given the low levels of observed clustering we took a parsimonious approach and fitted our models without inclusion of therapist. We also checked that there was no difference in inference with and without the inclusion of a random-effects therapist term.
We estimated that the one-sided 97.5% CI for the between-group difference and non-inferiority of BA compared with CBT was accepted (in a 0.025 level test) if the lower bound of the 97.5% CI lay within the non-inferiority margin of –1.90 points in PHQ-9 score. We checked for non-equivalence of the primary outcome at all follow-up points using the same approach. We extended the primary analysis models to fit interaction terms to explore possible differences in treatment effect in baseline symptom severity and ADM usage.
We undertook secondary analyses to compare groups at follow-up across 6, 12 and 18 months using a mixed-effects, repeated measures regression approach. We also ran sensitivity analyses for both primary and secondary analyses to assess the likely impact of missing data using multiple imputation models. We also calculated the relative proportions of participants meeting criteria for ‘recovery’ (proportions of participants with PHQ-9 scores of ≤ 9 points) and ‘response’ (50% reduction in the PHQ-9 scores from baseline). Between-group differences are presented for continuous outcomes as CBT versus BA (i.e. CBT minus BA) and for binary outcomes as BA relative to CBT (i.e. BA divided by CBT).
No interim inferential analyses were undertaken. However, the DMC requested (October 2013) a check of the statistical power of the trial for the PP analyses. This calculation used the assumptions of our original power calculation and was based on the observed level of attrition of the primary outcome at 12 months and the proportion of patients who fulfilled the PP definition (eight or more treatment sessions; as of January 2014). All analyses were undertaken using Stata v.14 (StataCorp LP, College Station, TX, USA).
Economic analysis
Although studies designed to test equivalence of effects are considered to be a legitimate situation in which a cost minimisation analysis (where costs alone are compared given equal outcomes) may be appropriate,75 the same may not be true for non-inferiority designs. Even in situations where equivalence or non-inferiority are demonstrated, exploration of the joint distribution of costs and effects in a cost-effectiveness analysis is recommended to represent uncertainty75 and to help interpret the economic results. 76 For these reasons, we prespecified that we would undertake a cost-effectiveness analysis irrespective of whether or not non-inferiority in the primary clinical outcome was demonstrated. We assessed cost-effectiveness in terms of QALYs using the net benefit approach. 77 We explored Bosmans et al. ’s methods76 for economic evaluations alongside equivalence or non-inferiority trials, which requires specification of non-inferiority margins for both costs and effects. However, as our prespecified method of economic evaluation was cost–utility analysis, using QALYs, rather than cost-effectiveness analysis, using the PHQ-9, which was the measure on which the hypothesis of non-inferiority is based, no non-inferiority margin for economic effects was specified. In addition, there is a general lack of guidance on how to define an economically unimportant difference in costs with which to estimate an appropriate non-inferiority margin for costs.
We compared the costs and cost-effectiveness of BA and CBT at the final, 18-month, follow-up to capture the economic impact of events, such as relapse with unit costs from the 2013–14 financial year. 78,79 We discounted costs and QALYs in year 2 at 3.5%. 60 We used complete-case analysis, with missing data explored in a sensitivity analysis using multiple imputation by chained equations (MICE). Our primary analysis took the NHS/PSS perspective preferred by NICE. 80 The impact of productivity losses as a result of time off work, known to be a substantial cost in depression,81 were explored in sensitivity analysis. In addition, narrower cost perspectives were tested (e.g. an intervention perspective and a mental health-care perspective), to ensure that the NHS/PSS perspective had not captured irrelevant costs that may hide the true impact of BA and CBT on service use.
For each participant, a unit cost was applied to each item of service use reported to calculate the total cost for the duration of the trial. All unit costs are summarised in Table 3.
| Service | Unit | Cost (£) |
|---|---|---|
| BA | Per hour | 67.80 |
| CBT | Per hour | 86.20 |
| Medication | Per daily dose | Various |
| Inpatient | Per night | 527.17–602.52 |
| Outpatient | Per appointment | 49.00–411.00 |
| Accident and emergency | Per attendance | 108.96–266.85 |
| Ambulance | Per attendance | 231.00 |
| GP surgery | Per minute of patient contact | 2.90 |
| Practice nurse | Per minute of face-to-face contact | 0.73 |
| Case manager | Per home visit minute | 2.39 |
| Community occupational therapist | Per minute of face-to-face contact | 0.68 |
| Social worker | Per minute | 2.65 |
| Advice service | Per minute | 1.05 |
| Chiropractic/osteopathy | Per minute | 1.42 |
| Homeopathy | Per minute | 1.67 |
| Acupuncture | Per minute | 1.33 |
| Massage therapy | Per minute | 1.13 |
We estimated intervention costs using the bottom-up costing approach set out by the Personal Social Services Research Unit at the University of Kent. 82 We based BA MHW costs on NHS AfC salary band 5 (salary range: £21,909–28,462; US$31,662–41,130; €27,726–35,993) and NHS AfC band 7 (salary range: £31,383–41,373; US$45,350–59,786; €39,738–52,388) for CBT therapists, including employer’s National Insurance and pension contributions plus capital, administrative and managerial costs. 79 We calculated a cost per hour using standard working time assumptions,79 weighted to account for time spent on non-patient facing activities, which was estimated based on the results of a survey of trial therapists.
We costed hospital services using unit costs from the NHS Reference Costs 2013–14. 78 Unit costs for NHS primary care and social care services were taken from nationally applicable published sources. 79 Costs for complementary services were taken from the NHS Choices website. 83 The costs of medications were calculated based on averages listed in the British National Formulary84 for the generic drug and using daily dose information collected using the AD-SUS.
We valued productivity losses as a result of time off work due to illness using the human capital approach, which involves multiplying the individual’s salary by reported days off work due to illness. 85
We report differences in use of services between randomised groups descriptively as the mean by group and as a percentage of the group who had at least one contact. We tested for differences in mean costs per participant between groups using standard parametric t-tests, with the results confirmed using bias-corrected, non-parametric bootstrapping. 86 This is the recommended approach, despite the skewed nature of cost data, as it allows inferences to be made about the arithmetic mean. 87
We explored cost-effectiveness using ICERs – the difference in mean cost divided by the difference in mean effect88 – and cost-effectiveness planes constructed to show the probability that BA is more or less effective and more or less costly than CBT. As ICERs are calculated from four sample means and are therefore subject to statistical uncertainty, the planes were generated using 1000 bootstrapped resamples from regression models of total cost and outcome by treatment group. These were then used to calculate the probability that each of the treatments is the optimal choice, for different values a decision-maker is willing to pay for a unit improvement in outcome (the ceiling ratio, λ). Cost-effectiveness acceptability curves (CEACs) are presented by plotting these probabilities for a range of possible values of λ to explore the uncertainty that exists around estimates of mean costs and effects, and to show the probability that BA is cost-effective compared with CBT. 89
All analyses were controlled for the following covariates: site, ADM use, symptom severity and baseline measurement of the variables of interest. Additionally, data have been truncated to exclude influential outliers (i.e. cases with total costs in the 99th percentile that make a significant difference to the results). 90 Between-group differences for costs are presented for continuous outcomes as BA versus CBT (i.e. BA minus CBT).
We carried out a number of independent sensitivity analyses to test assumptions made in the analysis:
-
the impact of including the use of complementary therapies
-
the impact of including productivity losses as a result of time off work as a result of illness
-
the impact of missing data, considered using MICE
-
the impact of taking an intervention perspective
-
the impact of taking a mental health-care perspective.
Process data analysis
A full description of the methods and analyses of our process data, including qualitative data, is presented in Chapter 4. Based on recent reviews,91 exploratory analyses examined baseline variables that might moderate outcome at multiple time points (6, 12 and 18 months) across the two treatments, including depression severity, age at depression onset, number of previous episodes, and baseline levels of cognitive and behavioural dysfunction, using the approach set out by Kraemer et al. 92 Although the power to detect moderate subgroup interactions was low, we were primarily interested in exploring the possibility of large interactions that could inform subsequent clinical decision-making regarding treatment allocation.
Mediational analyses investigated the hypothesised mechanisms of change (for BA, changes in specific behaviour such as reduced avoidance and rumination, learned capacity to apply behavioural principles to modify the environment; for CBT, changes in beliefs and underlying information processing style), pretreatment to mid-treatment, mid-treatment to post treatment across the trial arms using approaches to testing mediation that allow multiple mediators in one model. 92 The effects of the mediators on outcome at 12 and 18 months were modelled. This approach to examining mediation ensures that changes in putative mediators temporally precede changes in the primary outcome and allow baseline to post-treatment change in symptoms to be statistically controlled, necessary to rule out reverse causality. 93
Qualitative data analysis
Participant and therapist interviews were analysed using a framework approach94 combining deductive themes from the topic guides and inductive themes emerging from the data. Some interviews were coded independently to assess the reliability of coding and meetings were held to discuss and refine emerging themes. 95 Transcripts were examined thematically across the whole data set, as well as in the context of each interview, using constant comparison techniques. 96 Data were indexed and sorted using the identified themes and subthemes, and were summarised in framework matrices. 94 In keeping with the framework approach, we interrogated the data, searching for comparisons and contradictions and keeping interpretive notes. Alternative explanations or negative cases were identified, discussed and a consensus reached. 95
Ethics issues
We conducted the trial in such a way as to protect the human rights and dignity of the participants as reflected in the Helsinki Declaration. 97 Participants did not receive any financial inducement to participate. The study received National Research Ethics Committee (REC) approval from the South West REC in the UK (reference number 12/SW/0029). Local REC and NHS research and development approvals were also given for each recruitment site. To conform with data protection and freedom of information acts, all data have been stored securely and anonymised wherever possible. No published material will contain patient-identifiable information.
Obtaining informed consent from participants
We determined informed consent by a two-phase consent process. Participants received a study information sheet in the post and a form seeking their permission to be contacted by a member of the research team, not at this stage to give consent to trial participation. The information leaflets were produced using current guidelines for researchers on writing information sheets and consent forms98 and informed by our consumer/lived-experience user representatives. Participants who wished to partake in the trial returned their initial written consent to be contacted form to the site research team. Full informed written consent was obtained through an interview by a researcher where the information sheet was fully explained and where the opportunity to ask questions was given. The opportunity to withdraw from the trial was also fully explained. Researchers seeking consent were fully trained and supervised by the chief investigator and site leads. Communication and recording systems were set up to enable the trial team to monitor and act on participants’ wishes to withdraw from the trial.
Anticipated risks and benefits
All participants received usual GP care and, therefore, no treatment was withheld from participants in this trial. Both arms were active psychological treatments with previously demonstrated efficacy and no known iatrogenic effects. This trial may have in fact benefited individual participants, as CBT is not generally available for the majority of people with depression. By participating in this trial, participants also received an intensive level of monitoring such that any participants with worsening symptoms or who were at suicidal risk were identified and directed to appropriate care. We recorded all instances of AEs as detailed earlier.
Informing participants of anticipated risks and benefits
Participant information leaflets provided potential participants with information about the possible benefits and known risks of taking part in the trial. Participants were given the opportunity to discuss this issue with their GP or the trial manager prior to consenting. The trial manager would have informed the participant if new information came to light that may have affected the participant’s willingness to participate in the trial.
Management of suicide risk
Inherent in the nature of the population under scrutiny is the risk of suicide. We followed good clinical practice in monitoring for suicide risk during all research and clinical encounters with trial participants, developed for our previous trials. 55,99 Where any risk to participants attributable to expressed thoughts of suicide were encountered, we reported these directly to the GP (with the participant’s expressed permission), or if an acute risk was present we sought advice from the GP immediately and followed locally established suicide risk management plans. Systems were put into place to ensure that the chief investigator, trial manager and researchers were informed if there were any risks to the participants’ safety.
Trial Steering Committee and Data Monitoring Committee
A TSC was set up and included an independent chairperson, an academic GP and at least two other independent members, along with the lead investigator and some other study collaborators. The TSC met at least once a year. The DMC was set up and was composed of an independent mental health professional, statistician and clinician. The role of the DMC was to review serious AEs thought to be treatment related and look at outcome data regularly during data collection.
Execution dates
The preparatory period started in March 2012. Recruitment ran from September 2012 to April 2014. Follow-up lasted 18 months after randomisation and was completed by October 2015. Data analysis and reporting were completed 12 months after this (September 2016). The entire study period lasted 54 months (March 2012 to September 2016).
Chapter 3 Results of clinical and economic analyses
This chapter uses material from an Open Access article previously published by the research team (see Richards et al. 2). © The Author(s). 2 Published by Elsevier Ltd. This is an Open Access article under the CC BY license.
Participant flow and retention
Between 26 September 2012 and 3 April 2014, we recruited 440 participants, randomly allocating 221 participants (50%) to the BA group and 219 participants (50%) to the CBT group. We recruited to time and target and the majority of participants were recruited from primary care (87%). Progress over the course of recruitment and achievement of the target is shown in Figure 3. Participant recruitment and retention is shown for both the ITT and PP analyses in the trial CONSORT diagram (Figure 4). There were no protocol deviations.
FIGURE 3.
Trial recruitment. Solid line shows target recruitment and dashed line shows cumulative actual recruitment.

FIGURE 4.
The trial CONSORT flow diagram. (a) 6-month follow-up, (b) 12-month follow-up and (c) 18-month follow-up.

Baseline characteristics of participants
Patient- and trial-level characteristics at baseline were well balanced between groups (Table 4). We also found no evidence of a difference in patient characteristics between recruitment methods (Table 5). The PHQ-9 primary outcome at baseline was negatively skewed, with a high proportion of participants scoring towards the upper end of the distribution (Figure 5), and scores were similar between groups [BA, 17.7 PHQ-9 points (SD 4.8 PHQ-9 points); CBT, 17.4 PHQ-9 points (SD 4.8 PHQ-9 points)] (Table 6).
| Characteristic | Trial arm | All (n = 440) | |
|---|---|---|---|
| BA (n = 221) | CBT (n = 219) | ||
| Trial characteristic | |||
| Method of recruitment, n (%) | |||
| Case notes | 192 (87) | 190 (87) | 382 (87) |
| IAPT | 29 (13) | 29 (13) | 38 (13) |
| Patient characteristics | |||
| Age (years), mean (SD) range | 43.9 (14.1) 18–82 | 43.0 (14.1) 19–84 | 43.5 (14.1) 18–84 |
| Gender, n (%) | |||
| Male | 79 (36) | 71(32) | 150 (34) |
| Female | 142 (64) | 148 (68) | 290 (66) |
| Number of episodes of depression including current | |||
| Mean (SD), n | 7.0 (15.0) 192 | 6.3 (13.8) 192 | 6.7 (14.4) 384 |
| Median (IQR) | 3.0 (1–5) | 2.0 (1–4) | 3.0 (1–5) |
| First depression episode, age at onset (years), mean (SD) | 27.2 (15.0) | 26.3 (13.5) | 26.7 (14.2) |
| Duration of antidepressant treatment (weeks)a | |||
| Mean (SD), n | 106 (210), 157 | 81 (164), 168 | 93 (188), 325 |
| Median (IQR) | 21 (10–71) | 18 (7–51) | 19 (8–66) |
| At least one comorbid anxiety disorder, n (%) | 131 (59) | 141 (64) | 272 (62) |
| Marital status, n (%) | |||
| Single | 68 (31) | 59 (27) | 127 (29) |
| Cohabiting (not married) | 29 (13) | 25 (11) | 54 (12) |
| Civil partnership | 1 (1) | 1 (1) | 2 (1) |
| Married | 84 (38) | 92 (42) | 176 (40) |
| Divorced/separated | 39 (18) | 42 (19) | 81 (18) |
| Number of children, n (%) | |||
| 0 | 74 (34) | 72 (33) | 146 (33) |
| 1 | 35 (16) | 31 (14) | 66 (15) |
| 2 | 67 (30) | 69 (32) | 136 (31) |
| 3 | 31 (14) | 27 (12) | 58 (13) |
| ≥ 4 | 14 (6) | 20 (9) | 34 (8) |
| Level of education, n (%) | |||
| No qualifications | 25 (11) | 30 (14) | 55 (13) |
| GCSEs/O-levels | 36 (16) | 43 (20) | 79 (18) |
| AS/A-levels | 28 (13) | 22 (10) | 50 (11) |
| NVQ or other vocational qualification | 54 (24) | 71 (32) | 125 (28) |
| Undergraduate degree | 44 (20) | 35 (16) | 79 (18) |
| Postgraduate degree | 28 (13) | 14 (6) | 42 (10) |
| Doctoral degree | 2 (1) | 1 (0) | 3 (1) |
| Professional degree (e.g. MD) | 4 (2) | 3 (1) | 7 (2) |
| Ethnicity, n (%) | |||
| White British | 204 (92) | 197 (90) | 401 (91) |
| Other | 17 (8) | 22 (10) | 39 (9) |
| Stratification/minimisation variables | |||
| PHQ-9 points category, n (%) | |||
| < 19 | 118 (54) | 118 (54) | 236 (54) |
| ≥ 19 | 103 (46) | 101 (46) | 204 (46) |
| Antidepressant use, n (%) | |||
| Yes | 172 (78) | 175 (79) | 345 (78) |
| No | 49 (22) | 46 (21) | 95 (22) |
| Site, n (%) | |||
| 1 | 74 (33) | 73 (33) | 147 (33) |
| 2 | 79 (36) | 78 (36) | 157 (36) |
| 3 | 68 (31) | 68 (31) | 136 (31) |
| Recruitment method | Recruitment method | All (n = 440) | |
|---|---|---|---|
| Primary care (n = 382) | IAPT (n = 58) | ||
| Patient characteristics | |||
| Age (years), mean (SD) | 43.6 (14.2) | 42.7 (13.4) | 43.5 (14.1) |
| Gender, n (%) | |||
| Male | 122 (32) | 28 (48) | 150 (34) |
| Female | 260 (68) | 30 (52) | 290 (66) |
| Number of episodes of depression including current | |||
| Mean (SD), n | 6.9 (15.3), 327 | 5.6 (7.2), 57 | 6.7 (14.4), 384 |
| Median (IQR) | 3.0 (1–5) | 3.0 (2–5) | 3.0 (1–5) |
| Age at onset of first depression episode (years), mean (SD) | 27.0 (14.6) | 25.6 (13.2) | 26.7 (14.2) |
| Duration of antidepressant treatment (weeks)a | |||
| Mean (SD), n | 84 (165), 290 | 167 (313), 35 | 93 (188), 325 |
| Median (IQR) | 18 (8–64) | 23 (15–108) | 19 (8–66) |
| At least one comorbid anxiety disorder, n (%) | 246 (64) | 26 (45) | 272 (62) |
| Marital status, (%) | |||
| Single | 112 (29) | 15 (26) | 127 (29) |
| Cohabiting (not married) | 45 (12) | 9 (16) | 54 (12) |
| Civil partnership | 2 (1) | 0 (0) | 2 (1) |
| Married | 149 (39) | 27 (47) | 176 (40) |
| Divorced/separated | 74 (19) | 7 (12) | 81 (18) |
| Number of children, n (%) | |||
| 0 | 123 (32) | 23 (40) | 146 (33) |
| 1 | 58 (15) | 8 (14) | 66 (15) |
| 2 | 121 (32) | 15 (26) | 136 (31) |
| 3 | 49 (13) | 9 (16) | 58 (13) |
| ≥ 4 | 31 (8) | 3 (5) | 34 (8) |
| Level of education, n (%) | |||
| No qualifications | 50 (13) | 5 (9) | 55 (13) |
| GCSEs/O-levels | 71 (19) | 8 (14) | 79 (18) |
| AS/A-levels | 44 (12) | 6 (10) | 50 (11) |
| NVQ or other vocational qualification | 106 (28) | 19 (33) | 125 (28) |
| Undergraduate degree | 66 (17) | 13 (23) | 79 (18) |
| Postgraduate degree | 36 (9) | 6 (10) | 42 (10) |
| Doctoral degree | 3 (1) | 0 (0) | 3 (1) |
| Professional degree (e.g. MD) | 6 (2) | 1 (2) | 7 (2) |
| Ethnicity, n (%) | |||
| White British | 353 (92) | 49 (84) | 402 (91) |
| Other | 29 (76) | 9 (2) | 38 (9) |
| Stratification or minimisation variables | |||
| PHQ-9 category, n (%) | |||
| < 19 points | 200 (52) | 36 (62) | 236 (54) |
| ≥ 19 points | 182 (48) | 22 (38) | 204 (46) |
| Antidepressant use, n (%) | |||
| Yes | 309 (81) | 36 (62) | 345 (78) |
| No | 73 (19) | 22 (38) | 95 (22) |
| Site, n (%) | |||
| Devon | 145 (38) | 2 (4) | 147 (33) |
| Durham | 157 (41) | 0 (0) | 157 (36) |
| Leeds | 80 (21) | 56 (97) | 136 (31) |
FIGURE 5.
Distribution of primary outcome (PHQ-9 score) at baseline.

| Outcome | Trial arm: n, mean (SD or %) | Between-group difference (CBT – BA) at 12-month follow-up: mean (95% CI), p-value | ||
|---|---|---|---|---|
| CBT | BA | Observed data only | Observed and imputed data | |
| Primary outcome | ||||
| PHQ-9 | ||||
| Baseline | 219, 17.4 (4.8) | 221, 17.7 (4.8) | ||
| ITT 12-month follow-up | 189, 8.4 (7.5) | 175, 8.4 (7.0) | 0.1a (–1.3 to 1.5), 0.89 | 0.2a (–1.1 to 1.7), 0.80 |
| PP 12-month follow-up | 151, 7.9 (7.3) | 135, 7.8 (6.5) | 0.0a (–1.5 to 1.6), 0.99 | 0.0a (–1.6 to 1.6), 0.99 |
| Secondary outcomes | ||||
| GAD-7 | ||||
| Baseline | 219, 12.6 (5.1) | 221, 12.7 (5.1) | ||
| ITT 12-month follow-up | 176, 6.3 (6.0) | 161, 6.4 (5.9) | –0.1a (–1.0 to 1.3), 0.82 | 0.0a (–1.3 to 1.4), 0.96 |
| PP 12-month follow-up | 146, 6.0 (5.8) | 129, 5.9 (5.5) | 0.01a (–1.3 to 1.2), 0.95 | –0.4a (–1.7 to 1.0), 0.60 |
| SCID number of depression-free days | ||||
| ITT 12-month follow-up | 160, 129 (58) | 150, 120 (56) | 9a (–3 to 23), 0.13 | 7a (–7 to 20), 0.27 |
| PP 12-month follow-up | 138, 132 (55) | 125, 119 (55) | 13a (0 to 26), 0.06 | 8a (–4 to 21), 0.21 |
| SF-36 v2 PCS | ||||
| Baseline | 65, 50.1 (13.1) | 69, 51.4 (11.9) | ||
| ITT 12-month follow-up | 168, 48.1 (12.2) | 150, 49.9 (11.6) | 1.6b (–1.0 to 4.2), 0.22 | –1.4b (–1.1 to 4.0), 0.27 |
| PP 12-month follow-up | 144, 48.0 (12.2) | 125, 49.9 (12.0) | 1.6b (–1.3 to 4.4), 0.28 | –1.3b (–1.5 to 4.1), 0.36 |
| SF-36 v2 MCS | ||||
| Baseline | 65, 23.2 (9.4) | 69, 22.5 (7.8) | ||
| ITT 12-month follow-up | 168, 41.7 (14.1) | 150, 41.6 (14.0) | 0.0b (–3.0 to 3.0), 0.99 | 0.0b (–2.8 to 2.9), 0.97 |
| PP 12-month follow-up | 144, 42.9 (13.6) | 125, 42.3 (13.3) | 0.5b (–3.7 to 2.7), 0.77 | 0.6b (–3.8 to 2.7), 0.73 |
| n/N (%) | n/N (%) | Odds ratio (BA/CBT) (95% CI), p-value | Odds ratio (BA/CBT) (95% CI), p-value | |
| SCID depression | ||||
| Baseline | 219/219 (100) | 221/221 (100) | ||
| ITT 12-month follow-up | 37/163 (23) | 31/154 (20) | 0.9 (0.5 to 1.6), 0.71 | 0.9 (0.5 to 1.6), 0.70 |
| PP 12-month follow-up | 30/141 (21) | 24/128 (19) | 0.9 (0.5 to 1.7), 0.80 | 0.9 (0.5 to 1.7), 0.75 |
| Recoveryc | ||||
| ITT 12-month follow-up | 124/189 (66) | 115/175 (66) | 1.0 (0.6 to 1.5), 0.96 | 1.2 (0.7 to 1.9), 0.53 |
| PP 12-month follow-up | 104/151 (69) | 94/135 (70) | 1.0 (0.6 to 1.7), 0.96 | 1.2 (0.7 to 2.0), 0.47 |
| Responsed | ||||
| ITT 12-month follow-up | 117/189 (62) | 107/175 (61) | 1.0 (0.9 to 1.1), 0.73 | 0.9 (0.6 to 1.4), 0.75 |
| PP 12-month follow-up | 100/151 (66) | 87/135 (64) | 0.9 (0.9 to 1.0), 0.64 | 0.9 (0.5 to 1.4), 0.55 |
Delivery and receipt of the interventions
Ten MHWs provided BA [median 22 participants each (interquartile range 19–25 participants each)] and 12 therapists provided CBT [median 21 participants each (interquartile range 13–23 participants each)]. MHWs had a mean of 18 months’ mental health experience (SD 11 months’ mental health experience) and CBT therapists had a mean of 22 months’ experience (SD 24 months’ experience) post CBT qualification. We removed one CBT therapist from the trial in the early stages who did not meet acceptable competency.
Participants received a mean of 11.5 BA sessions (SD 7.8 sessions) or 12.5 CBT sessions (SD 7.8 sessions). Three hundred and five participants (69%) completed the PP number of at least eight sessions [BA 147 (67%) participants, mean 16.1 sessions (SD 5.3 sessions); CBT 158 (72%) participants, mean 16.4 sessions (SD 5.4 sessions)]. Participants completing fewer than eight sessions {135 participants (31%) [BA 74 participants (33%) and CBT 61 participants (28%)] completed a mean of 2.5 BA sessions (SD 1.9 sessions) or 2.6 CBT sessions (SD 2.1 sessions)}.
Primary outcome: Patient Health Questionnaire-9 at 12 months
We present primary and secondary outcomes at 12 months in Table 6. We found no evidence of inferiority of PHQ-9 score at 12 months in either the ITT [CBT 8.4 PHQ-9 points (SD 7.5 PHQ-9 points), BA 8.4 PHQ-9 points (SD 7.0 PHQ-9 points); mean difference 0.1 PHQ-9 points, 95% CI –1.3 to 1.5 PHQ-9 points; p = 0.89] or PP [CBT 7.9 PHQ-9 points (SD 7.3 PHQ-9 points), BA 7.8 PHQ-9 points (SD 6.5 PHQ-9 points); mean difference 0.0 PHQ-9 points, 95% CI –1.5 to 1.6 PHQ-9 points; p = 0.99] populations. The non-inferiority of BA to CBT was accepted for both the ITT and PP populations, as the lower bound of the 95% CI (one-sided 97.5% CI) of the between-group mean difference lies within the non-inferiority margin of –1.9 PHQ-9 points (Figure 6). We ruled out superiority of CBT to BA as the lower bound of the 95% CI includes zero for the ITT and PP populations. The inference of non-inferiority was robust to sensitivity analysis across different PP definitions (Table 7). Our predefined stratification variable subgroup analyses (Table 8) showed no ITT or PP between-group difference by depression severity, ADM or site.
FIGURE 6.
Mean difference and two-sided 95% CI for the primary outcome of PHQ-9 at 12 months and non-inferiority margin.

| PP definition | Time point, mean (SD), n | Between-group difference (CBT – BA):a mean (95% CI), p-value | |||
|---|---|---|---|---|---|
| Baseline | 12-month follow-up | ||||
| CBT | BA | CBT | BA | ||
| Standard PP definitionb | |||||
| ≥ 8 sessions attended | 17.4 (4.9), 158 | 17.6 (4.6), 147 | 7.9 (7.3), 151 | 7.8 (6.5), 135 | 0.0 (–1.5 to 1.6), 0.99 |
| Alternative PP definitionsb | |||||
| ≥ 0 sessions attended | 17.4 (4.8), 219 | 17.7 (4.8), 221 | 8.4 (7.5), 189 | 8.4 (7.0), 175 | 0.1 (–1.3 to 1.5), 0.89 |
| ≥ 4 sessions attended | 17.4 (5.0), 176 | 17.7 (4.6), 169 | 8.0 (7.4), 161 | 8.2 (6.8), 150 | 0.1 (–1.4 to 1.7), 0.86 |
| ≥ 12 sessions attended | 17.8 (4.8), 115 | 17.7 (4.8), 109 | 8.6 (7.1), 112 | 7.6 (6.2), 105 | –0.6 (–2.3 to 1.1), 0.52 |
| ≥ 16 sessions attended | 17.8 (4.8), 83 | 18.3 (4.7), 77 | 8.8 (7.0), 81 | 8.6 (6.1), 75 | 0.2 (–1.9 to 2.3), 0.86 |
| ≥ 20 sessions attended | 18.2 (4.7), 58 | 18.5 (4.6), 51 | 9.0 (6.7), 57 | 8.5 (6.1), 49 | –0.4 (–3.3 to 2.5), 0.78 |
| Stratification variable | Population | |||
|---|---|---|---|---|
| ITT | PP | |||
| Between-group difference (CBT – BA):a mean (95% CI) | Interaction coefficient (95% CI), p-value | Between-group difference (CBT – BA):a mean (95% CI) | Interaction coefficient (95% CI), p-value | |
| Depression severity | ||||
| PHQ-9 < 19 points | 0.4 (–1.3 to 2.0) | 1.1 (–1.8 to 3.9), 0.48 | 0.7 (–1.1 to 2.6) | 1.9 (–1.2 to 5.0), 0.23 |
| PHQ-9 ≥ 19 points | –0.6 (–3.5 to 1.8) | –0.9 (–3.5 to 1.7) | ||
| Receiving ADM | ||||
| Yes | 0.2 (–2.7 to 3.1) | 0.2 (–3.2 to 3.7), 0.90 | –0.4 (–3.5 to 2.7) | 0.6 (–4.3 to 3.2), 0.74 |
| No | –0.1 (–1.7 to 1.5) | 0.2 (–1.6 to 2.0) | ||
| Site | ||||
| Exeter | –1.2 (–3.6 to 1.1) | –2.1 (–5.5 to 1.4) | –1.2 (–3.9 to 1.5) | –1.7 (–5.5 to 2.1) |
| Durham | 1.0 (–1.6 to 3.6) | –0.9 (–4.5 to 2.6) | 0.6 (–2.2 to 3.4) | –1.3 (–5.3 to 2.6) |
| Leeds | –0.2 (–2.8 to 2.3) | 0.49b | 0.2 (–2.3 to 3.0) | 0.64b |
Response and recovery at 12 months
Between 61% and 70% of ITT and PP participants in both groups met criteria for recovery or response, with no difference in the proportions of patients in each group who recovered or responded (see Table 6).
Primary and secondary outcomes at all follow-up points
Tables 9 and 10 show the repeated measures comparison of primary and secondary outcomes across time points for both ITT and PP populations. For the primary outcome, there was no evidence of difference between the CBT and BA groups across observed or imputed outcomes over the period of the trial, as indicated by a non-significant time by treatment effect interaction. Although there was some weak evidence (p = 0.06) of a higher number of depression-free days at follow-up with BA than CBT in the ITT analyses, this difference was not apparent in the PP analysis (p = 0.11).
| Outcome | Time point | Between-group comparison, p-valuea,b | |||
|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | 18 months | ||
| Primary outcome | |||||
| PHQ-9, mean (SD), n | |||||
| CBT | 17.4 (4.8), 219 | 9.7 (7.3), 195 | 8.4 (7.5), 189 | 8.5 (7.2), 189 | 0.95 |
| BA | 17.7 (4.8), 221 | 9.8 (6.9), 185 | 8.4 (7.0), 175 | 8.3 (7.1), 176 | |
| Secondary outcomes | |||||
| GAD-7, mean (SD), n | |||||
| CBT | 12.6 (5.1), 219 | 7.5 (6.0), 186 | 6.3 (6.0), 176 | 7.0 (6.2), 167 | 0.32 |
| BA | 12.7 (5.1), 221 | 7.5 (5.8), 176 | 6.4 (5.9), 161 | 6.4 (5.9), 165 | |
| SCID number of depression-free days, mean (SD), n | |||||
| CBT | – | 66 (41), 171 | 130 (58), 160 | 125 (60), 161 | 0.06 |
| BA | – | 70 (47), 164 | 120 (55), 150 | 129 (57), 153 | |
| SF-36 v2 PCS, mean (SD), n | |||||
| CBT | 50.1 (13.1), 65 | 48.4 (11.7), 107 | 48.1 (12.2), 168 | 48.8 (12.5), 167 | 0.64 |
| BA | 51.4 (11.9), 69 | 49.4 (12.1), 111 | 49.9 (11.6), 150 | 49.6 (12.5), 160 | |
| SF-36 v2 MCS, mean (SD), n | |||||
| CBT | 23.2 (9.4), 65 | 39.5 (12.4), 107 | 41.7 (14.1), 168 | 40.7 (14.4), 167 | 0.50 |
| BA | 22.5 (7.8), 69 | 37.5 (13.7), 111 | 41.6 (14.0), 150 | 42.3 (13.8), 160 | |
| Other outcomes | |||||
| Recovery,c n/N (%) | |||||
| CBT | 13/219, 6 | 111/195, 57 | 134/189, 66 | 127/180, 59 | 0.88 |
| BA | 208/221, 6 | 97/185, 52 | 115/175, 66 | 116/176, 66 | |
| Response,d n/N (%) | |||||
| CBT | – | 96/195, 49 | 117/189, 62 | 108/180, 60 | 0.94 |
| BA | – | 91/185, 49 | 107/175, 61 | 108/176, 61 | |
| Outcome | Time point | Between-group comparison, p-valuea,b | |||
|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | 18 months | ||
| Primary outcome | |||||
| PHQ-9, mean (SD), n | |||||
| CBT | 17.3 (4.8), 158 | 9.1 (6.9), 152 | 7.9 (7.3), 152 | 8.0 (7.3), 147 | 0.51 |
| BA | 17.6 (4.6), 147 | 9.7 (6.7), 145 | 7.8 (6.5), 135 | 7.7 (6.7), 137 | |
| Secondary outcomes | |||||
| GAD-7, mean (SD), n | |||||
| CBT | 12.6 (5.2), 158 | 6.9 (5.8), 149 | 6.0 (5.8), 146 | 6.5 (5.9), 141 | 0.45 |
| BA | 12.5 (5.0), 147 | 7.1 (5.6), 140 | 5.9 (5.4), 129 | 6.0 (5.7), 132 | |
| SCID number of depression-free days, mean (SD), n | |||||
| CBT | – | 67 (40), 140 | 132 (55), 138 | 130 (59), 136 | 0.11 |
| BA | – | 68 (46), 137 | 119 (55), 125 | 130 (56), 123 | |
| SF-36 v2 PCS, mean (SD), n | |||||
| CBT | 50.3 (12.4), 51 | 49.9 (10.9), 90 | 48.0 (12.2), 144 | 49.0 (12.6), 141 | 0.60 |
| BA | 50.4 (12.1), 46 | 49.9 (11.8), 87 | 49.9 (12.0), 125 | 49.6 (12.7), 130 | |
| SF-36 v2 MCS, mean (SD), n | |||||
| CBT | 23.4 (9.3), 51 | 40.2 (12.2), 90 | 42.9 (13.6), 144 | 41.6 (14.6), 141 | 0.58 |
| BA | 22.9 (7.8), 46 | 39.0 (13.3), 87 | 42.3 (13.3), 125 | 42.7 (13.8), 130 | |
| Other outcomes | |||||
| Recovery,c n/N (%) | |||||
| CBT | 9/158 (6) | 91/152 (60) | 104/151 (69) | 93/147 (63) | 0.77 |
| BA | 7/147 (5) | 75/145 (52) | 94/135 (70) | 96/137 (70) | |
| Response,d n/N (%) | |||||
| CBT | – | 81/152 (53) | 100/151 (66) | 95/147 (64) | 0.40 |
| BA | – | 67/145 (46) | 87/135 (64) | 88/137 (64) | |
Primary and secondary outcomes and clustering by therapist
There was evidence of a small, negligible clustering of primary and secondary outcome scores at follow-up across therapists overall and within BA and CBT groups (intracluster correlation coefficient of ≤ 0.04).
Missing data
Of the 440 participants recruited, 76 participants (17%) had missing primary outcome data at 12 months’ follow-up. The proportion of missing PHQ-9 data was higher in the BA than in the CBT group [46 (21%) vs. 30 (14%), ITT relative risk 1.15, 95% CI 1.0 to 1.32; p = 0.044]. Drop out from PP treatment was not significantly different (PP relative risk 1.13, 95% CI 0.98 to 1.30; p = 0.84). Imputation of data for primary and secondary outcomes at 12 months showed that, in accordance with the observed data analysis, no difference existed between groups (see Table 6), supporting our conclusion of non-inferiority. The odds of missing PHQ-9 data were higher for patients with increased baseline severity of depression (PHQ-9 ≥ 19, odds ratio 1.6, 95% CI 1.0 to 2.6; p = 0.05), and increasing age (in years) was associated with lower odds of missing PHQ-9 data (odds ratio 0.97, 95% CI 0.96 to 0.99). We found no evidence of an association between missingness and any other baseline characteristic.
Blinding
Outcome assessors reported having been unmasked for 16 (4%) participants [five (2%) in the BA group and 11 (5%) in the CBT group] as a result of participants informing assessors of their treatment allocation.
Safety and adverse events
Two (1%) non-trial-related deaths [one (1%) multidrug toxicity death in the BA group and one (1%) cancer-related death in the CBT group] and 15 depression-related, but not treatment-related, serious AEs (three in the BA group and 12 in the CBT group) occurred in three (2%) participants in the BA group [two (1%) patients who overdosed and one (1%) who self-harmed] and eight (4%) participants in the CBT group [seven (4%) who overdosed and one (1%) who self-harmed].
Intervention quality
Mental health workers and therapists met acceptable competency standards above our set thresholds, as judged by our independently rated tapes: mean Quality of Behavioural Activation Scale score for BA competence was 55 (SD 7.5) and mean CTS-R for CBT competence was 37.9 (SD 10.9).
Results of economic evaluation
Data completeness
At 18 months, full service use data for the entire follow-up were available for 159 (90%) of the 176 participants with outcome data in the BA group and 169 (93%) of the 180 participants with outcome data in the CBT group, which was 75% of the total number randomised. One participant was identified as an influential outlier in the CBT group and removed from the main analysis.
Service use
The use of most health services, social care services and complementary therapies was broadly similar across the randomised groups over the 18-month follow-up. Some services were used only by those in one group (case manager and homeopathy in BA; occupational therapist, community psychiatrist, advice service and acupuncture in CBT), but these were all services used by < 2% of the sample. Service use between baseline and the 18-month follow-up is detailed in Table 11.
| Service | Trial arm | |||||
|---|---|---|---|---|---|---|
| BA (n = 159) | CBT (n = 168) | |||||
| Mean (SD) | Range | % using | Mean (SD) | Range | % using | |
| Inpatient stay (nights), n | 0.55 (2.13) | 0–20 | 25.79 | 0.74 (2.96) | 0–25 | 27.98 |
| Outpatient appointments (contacts), n | 3.45 (5.25) | 0–4 | 66.67 | 3.48 (5.94) | 0–58 | 58.33 |
| Accident and emergency (contacts), n | 0.55 (1.09) | 0–6 | 30.82 | 0.44 (0.96) | 0–7 | 25.00 |
| GP surgery (contacts), n | 6.67 (8.49) | 0–42 | 51.57 | 5.96 (8.18) | 0–55 | 51.19 |
| GP telephone (contacts), n | 0.67 (2.75) | 0–21 | 8.18 | 0.67 (2.49) | 0–17 | 8.93 |
| Practice nurse (contacts), n | 2.02 (8.04) | 0–82 | 18.87 | 1.35 (3.57) | 0–25 | 17.26 |
| Case manager (contacts), n | 0.09 (1.19) | 0–15 | 0.63 | 0.00 (0.00) | 0 | 0.00 |
| Occupational therapist (contacts), n | 0.00 (0.00) | 0 | 0.00 | 0.32 (3.00) | 0–34 | 1.19 |
| Psychiatrist (contacts), n | 0.00 (0.00) | 0 | 0.00 | 0.03 (0.36) | 0–5 | 0.60 |
| Social worker (contacts), n | 0.06 (0.71) | 0–9 | 0.63 | 0.04 (0.54) | 0–7 | 0.60 |
| Advice service (contacts), n | 0.00 (0.00) | 0 | 0.00 | 0.47 (6.10) | 0–79 | 0.60 |
| Chiropractor/osteopath (contacts), n | 0.27 (2.05) | 0–18 | 1.89 | 0.47 (4.70) | 0–59 | 1.79 |
| Homeopath (contacts), n | 0.03 (0.32) | 0–4 | 0.63 | 0.00 (0.00) | 0 | 0.00 |
| Acupuncture (contacts), n | 0.00 (0.00) | 0 | 0.00 | 0.45 (5.86) | 0–76 | 0.60 |
| Massage therapy (contacts), n | 0.97 (4.92) | 0–53 | 8.18 | 1.29 (6.32) | 0–54 | 7.14 |
Medication
The use of prescribed, psychotropic medication is summarised in Table 12. ADM was prescribed most frequently (around 80% of the full sample), followed by medication for anxiety or sleep (around 10% of the full sample). Mood stabilisers and antipsychotics were both prescribed for < 2% of the sample. There were no obvious differences in the proportion of participants in each group prescribed psychotropic medication.
| Medication type | Trial arm, % of sample using | |
|---|---|---|
| BA (n = 159) | CBT (n = 168) | |
| Antidepressants | 79.87 | 82.74 |
| Anxiety/sleep | 10.06 | 11.31 |
| Mood stabilisers | 0.63 | 0.60 |
| Antipsychotics | 0.63 | 1.79 |
Productivity losses
Of those in employment [BA, n = 77 (48%); CBT, n = 71 (42%)], over the 18-month follow-up the mean number of days off work did not differ between the groups (28.94 days in the BA group and 28.46 days in the CBT group).
Total costs
Total costs over the 18-month follow-up period are summarised in Table 13, including a breakdown of costs by service-providing sector. We found a significant difference in mean intervention costs between the two groups, but no differences in other categories of cost or in total cost.
| Service | Trial arm, mean (SD) | BA – CBT, mean differencea | 95% CIa | p-valuea | |
|---|---|---|---|---|---|
| BA (n = 159) | CBT (n = 168) | ||||
| Intervention | 974.81 (475.02) | 1235.23 (610.03) | –262.29 | –381.40 to –143.19 | < 0.0001 |
| Hospital | 860.23 (1509.88) | 927.26 (1975.64) | –75.67 | –451.75 to 300.42 | 0.692 |
| Community health and social care | 644.36 (816.07) | 944.25 (1726.17) | –15.14 | –304.90 to 274.62 | 0.918 |
| Medication | 103.20 (197.92) | 117.64 (265.92) | 2.15 | –39.83 to 44.13 | 0.920 |
| Total | 2596.62 (1846.72) | 3250.74 (3040.99) | –343.24 | –857.62 to 171.13 | 0.190 |
Outcomes
Health-related quality of life
We found that the mean health utility scores (as measured via the EQ-5D-3L) were slightly higher in the BA group than in the CBT group across the entire follow-up period, with resultant QALYs also higher for BA, but the QALY difference was not significant (Table 14).
| Time point/QALY | Trial arm | Mean differencea (BA – CBT) | 95% CIa | p-valuea | |||
|---|---|---|---|---|---|---|---|
| BA | CBT | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| Baseline | 159 | 0.548 (0.307) | 168 | 0.474 (0.317) | |||
| 6 months | 153 | 0.683 (0.310) | 151 | 0.677 (0.310) | |||
| 12 months | 147 | 0.684 (0.341) | 156 | 0.671 (0.348) | |||
| 18 months | 152 | 0.670 (0.311) | 157 | 0.624 (0.335) | |||
| QALYs | 152 | 0.985 (0.422) | 157 | 0.935 (0.433) | 0.050 | –0.145 to 0.046 | 0.308 |
Cost-effectiveness
As observed costs were lower and QALY outcomes better in the BA group than in the CBT group, this generated an ICER (the additional cost of one intervention compared with another divided by the additional effects) of –£6865, suggesting that BA dominates CBT (i.e. BA is both cheaper and more effective). The scatterplot of the bootstrapped cost and effectiveness pairs for BA versus CBT (Figure 7) illustrates dominance of BA over CBT, with the point estimate and two-thirds of the scatter points (66%) falling in the south-east quadrant of the cost-effectiveness plane, where BA replications are cheaper and more effective than CBT ones.
FIGURE 7.
Scatterplot showing the bootstrapped mean differences in costs and effects of BA compared with CBT. NE, north-east; NW, north-west; SE, south-east; SW, south-west.
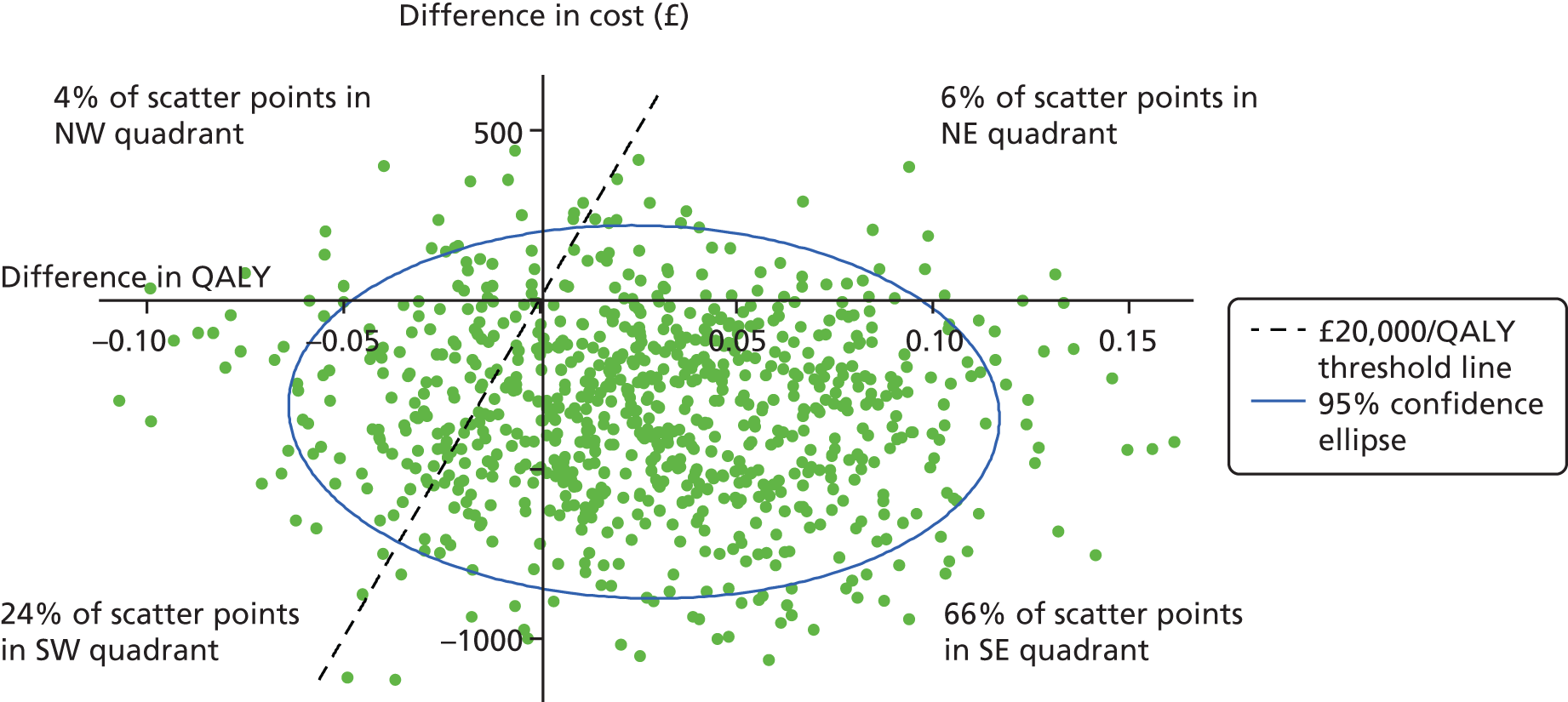
The CEAC showing the probability of BA being cost-effective compared with CBT does not fall < 75% and is closer to 80% at the standard NICE-preferred willingness-to-pay levels of £20,000–30,000 per QALY (Figure 8).
FIGURE 8.
Cost-effectiveness acceptability curve showing the probability that BA is cost-effective compared with CBT for different values of willingness to pay per QALY.

Sensitivity analyses
Inclusion of the costs of complementary therapies and productivity losses (Table 15) increased the overall mean difference in total cost between the two groups, making BA significantly less costly than CBT in both cases (p = 0.039 with the inclusion of complementary therapies and p = 0.003 with the inclusion of productivity losses). Narrower perspectives, focusing only on the cost of the interventions and the mental health service perspective, had the same effect (p < 0.0001 in both cases). Figure 9 shows the CEACs for the inclusion of complementary therapies and productivity losses and Figure 10 shows the CEACs for the two narrower cost perspectives. In all cases, BA continues to have a higher probability of being cost-effective compared with CBT at the NICE threshold of £20,000–30,000 per QALY.
| Analysis type | Trial arm | Mean differencea (BA – CBT) | 95% CIa | p-valuea | |||
|---|---|---|---|---|---|---|---|
| BA | CBT | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| Impact on total costs (£) | |||||||
| Main analysis | 159 | 2596.62 (1846.72) | 168 | 3250.74 (3040.99) | –343.24 | –857.62 to 171.13 | 0.190 |
| Inclusion of complementary therapies | 158 | 2729.54 (2604.00) | 168 | 3367.90 (3530.36) | –535.60 | –1045.28 to –25.92 | 0.039 |
| Inclusion of productivity losses | 152 | 3305.91 (3410.20) | 166 | 4937.92 (6367.92) | –1726.37 | –2870.80 to –581.93 | 0.003 |
| Intervention perspective | 159 | 992.73 (60.54) | 168 | 1255.03 (88.16) | –262.30 | –381.40 to –143.19 | < 0.0001 |
| Mental health-care perspective | 159 | 914.71 (67.86) | 168 | 1253.85 (99.97) | –339.14 | –472.64 to –205.64 | < 0.0001 |
| Imputation of missing data | 221 | 1841.67 (287.97) | 219 | 2282.40 (423.94) | –440.73 | –1007.70 to 126.26 | 0.127 |
| Impact on QALYs | |||||||
| Main analysis | 152 | 0.984 (0.422) | 157 | 0.935 (0.433) | 0.050 | –0.145 to 0.046 | 0.308 |
| Imputation of missing data | 221 | 1.224 (0.043) | 219 | 1.198 (0.061) | 0.026 | –0.058 to 0.109 | 0.546 |
FIGURE 9.
Cost-effectiveness acceptability curve showing the probability that BA is cost-effective compared with CBT for different values of willingness to pay for a QALY, including costs of complementary therapies and productivity losses.
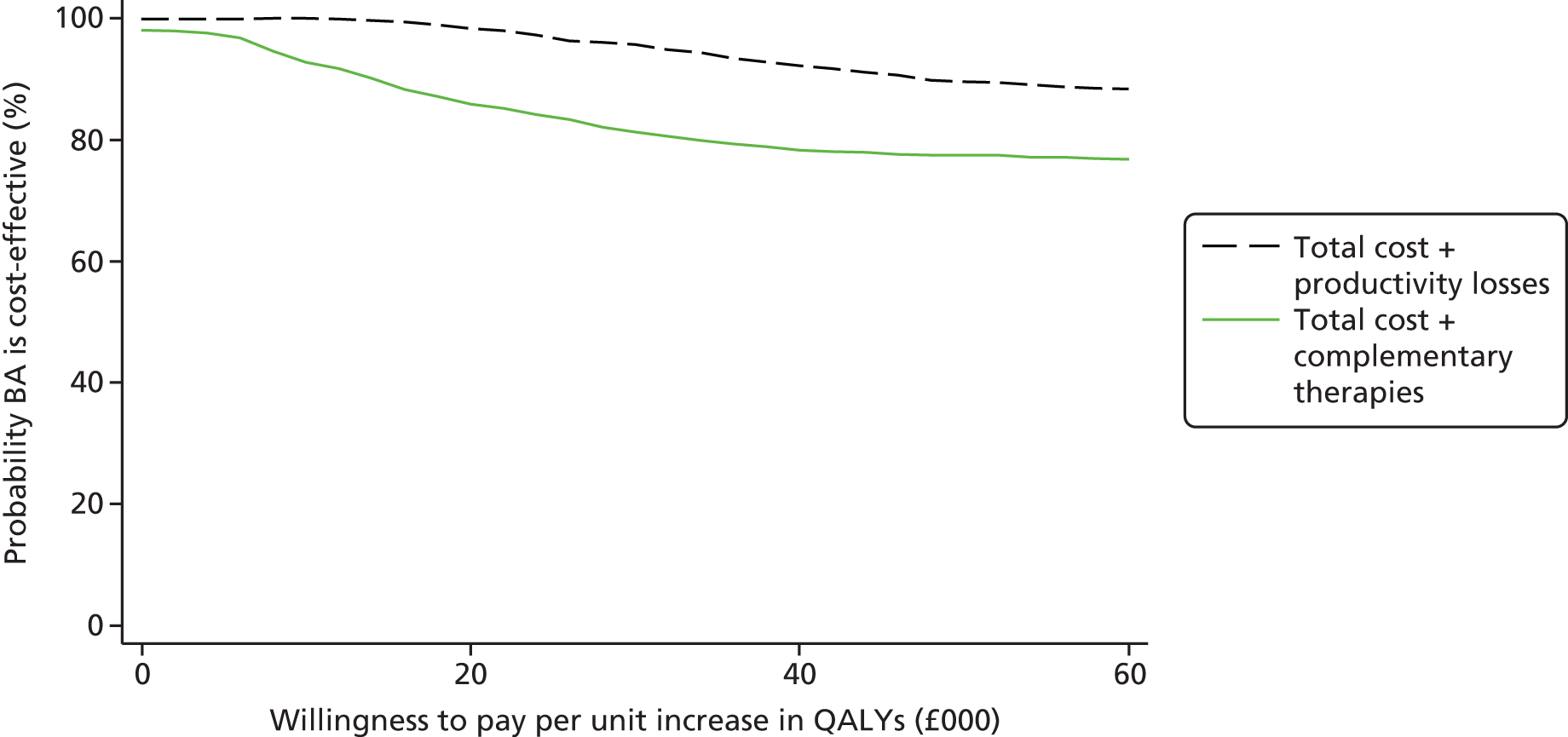
FIGURE 10.
Cost-effectiveness acceptability curve showing the probability that BA is cost-effective compared with CBT for different values of willingness to pay for a QALY, from intervention and mental health-care perspectives.

Imputation of missing data increased the difference in total cost (£440.00 vs. £343.00 in the main analysis), but reduced the difference in QALYs (0.026 vs. 0.050 in the main analysis), increasing the ICER from –£6865.00 to –£16,951.00. Figure 11 shows the CEAC for the missing data analysis, which again supports the likelihood that BA is cost-effective compared with CBT.
FIGURE 11.
Cost-effectiveness acceptability curve showing the probability that BA is cost-effective compared with CBT for different values of willingness to pay for a QALY, including imputed missing data.

Chapter 4 Methods and results of the process evaluation
This chapter uses material from Open Access articles previously published by the research team (see Rhodes et al. 1 and Richards et al. 2). © Rhodes et al. ;1 licensee BioMed Central Ltd. 2014 This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated and © The Author(s). 2 Published by Elsevier Ltd. This is an Open Access article under the CC BY license.
Introduction
Alongside the main clinical and economic evaluation, we undertook a process evaluation to investigate the implementation of the intervention, moderators of outcome and possible mechanisms of effect. This chapter consists of a description of our quantitative and qualitative methods, analyses and results.
Objectives
-
To investigate factors that may moderate the effect of treatment allocation on outcome (PHQ-9 score), and mechanistic and procedural factors that may mediate the effect of treatment on outcome.
-
To obtain a more in-depth understanding of the ongoing mechanisms and impact of treatment from participants and therapists including participants’ views of the role of cognitive and behavioural change strategies and the broader impacts of treatment on participants’ lives.
Quantitative process study
Moderators: identifying patient subgroups who may receive differential treatment effects
Having established no statistically significant difference in treatment effect between CBT and BA at any of the three follow-up points (6, 12 and 18 months), for the PHQ-9 score, a further issue of clinical interest is whether or not there are any specific subgroups of patients who would receive differential treatment effects (e.g. increased benefit from one treatment compared with the alternative treatment). In the event of identifying any such subgroups (defined by covariates that are known as moderators), these patients could be offered the treatment that would confer the strongest benefit, taking into account their own characteristics. To investigate this possibility, we extended the models used for the main analyses, by adding an interaction term between treatment allocation and the potential moderator of treatment effect.
The potential moderators of treatment effect that we investigated include indicators of depression severity: baseline PHQ-9 score, number of previous episodes of depression and age at onset of depression. We also included other psychological variables measured at baseline: total BADS score, DAS score and total RRS score. With regard to investigating whether or not patient preference for a certain treatment acted as a moderator, we used information elicited on whether or not the patient had a treatment preference, and if so whether the patient preferred CBT or BA, to derive three categories: (1) no treatment preference; (2) received preferred treatment; and (3) received non-preferred treatment.
Mediators: investigating potential mechanisms of therapy action
The traditional approach to causal mediation has followed the initial work of Baron and Kenny. 100 This approach is based on the existence of a significant effect of the independent variable on the mediator, and on the dependent variable, and also a significant effect of the mediator on the dependent variable, when the independent variable has been controlled for. However, this approach has received criticism, for example with regard to the possibility of potential confounders between the mediator and the dependent variable. 101 In addition, it has been argued that a significant association between the independent variable and the dependent variable is not required to demonstrate mediation. 102,103
In a more complex situation, including more than one mediator in the structural model, such mediators could be conceptualised as exerting their effect between the independent and dependent variables either in sequence or in parallel to one another. In these analyses, all models that include multiple mediators are structured so that the mediators are acting in parallel. More recent methods for causal mediation analysis, for example using instrumental variables or potential outcomes (counterfactuals), have been developed,101 but are not considered further, because of the difficulty in identifying a suitable instrumental variable.
We used four process measures (PMs) as potential mediators:
-
BADS
-
DAS
-
RRS
-
SHAPS.
The BADS comprises four subscales (activation, avoidance, work impairment and social impairment) that sum to give a total score. The RRS includes a subset of five items that consitute a reflection subscale. The SHAPS revised score (Franken) was used for all analyses, owing to increased granularity compared with the standard score, with each of the 14 items scored from 0 to 3, resulting in an overall score from 0 to 42. Each measure was calibrated such that a higher score indicated a more clinically negative outcome so that each measure was aligned with the directionality of the PHQ-9 primary outcome measure. Each mediation analysis included the baseline score for the relevant mediator(s) in the model.
We used three mediators based on participants’ experience of their treatment:
-
proportion of sessions attended (out of a maximum of 24)
-
basic treatment fidelity
-
overall treatment fidelity.
Basic treatment fidelity was based on the proportion of core topics for the relevant therapy that were covered during all attended sessions. There were six core topics for BA and seven for CBT; hence, the number of topics was linearly rescaled on a score of 0–100. Inevitably, patients who received BA had a propensity for an increased proportion of core topics covered, owing to the smaller number of potential topics. The overall treatment fidelity was derived by linearly rescaling the proportion of mandatory topics covered during all attended sessions (two for BA and four for CBT) on a scale of 0–100, then combining with the score from 0 to 100 for basic quality therapy and dividing by 2 to produce a score from 0 to 100. For the mediation analyses, the scores for proportion of sessions attended and the treatment fidelity variables were reversed so that a low score indicated a more negative outcome. These measures were used as potential mediators for the 12- and 18-month PHQ-9 follow-up only, as 210 participants had not completed their final session at 6 months’ follow-up. Analyses to evaluate the potential mediators of the treatment effect were performed for PHQ-9 measured at 6, 12 and 18 months; the potential mediators for each PHQ-9 time point are set out in Table 16.
| Outcome variable | Mediator variable | Time of measurement of mediator variable |
|---|---|---|
| PHQ-9: 6-month follow-up | BADS total score | PM1, PM2 |
| BADS activation | PM1, PM2 | |
| BADS avoidance | PM1, PM2 | |
| BADS work impairment | PM1, PM2 | |
| BADS social impairment | PM1, PM2 | |
| DAS | PM1, PM2 | |
| RRS total score | PM1, PM2 | |
| RRS rumination score | PM1, PM2 | |
| PHQ-9: 12-month follow-up | BADS total score | PM1, PM2, 6 months |
| BADS activation | PM1, PM2, 6 months | |
| BADS avoidance | PM1, PM2, 6 months | |
| BADS work impairment | PM1, PM2, 6 months | |
| BADS social impairment | PM1, PM2, 6 months | |
| DAS | PM1, PM2, 6 months | |
| RRS total score | PM1, PM2, 6 months | |
| RRS rumination score | PM1, PM2, 6 months | |
| SHAPS | 6 months | |
| Number of sessions | N/A | |
| Basic treatment fidelity | N/A | |
| Overall treatment fidelity | N/A | |
| PHQ-9: 18-month follow-up | BADS total score | 6 months |
| BADS activation | 6 months | |
| BADS avoidance | 6 months | |
| BADS work impairment | 6 months | |
| BADS social impairment | 6 months | |
| DAS | 6 months | |
| RRS total score | 6 months | |
| RRS rumination score | 6 months | |
| SHAPS | 6 months, 12 months | |
| Number of sessions | N/A | |
| Basic treatment fidelity | N/A | |
| Overall treatment fidelity | N/A |
Having established that there is no evidence for a differential treatment effect between BA and CBT at any of the follow-up time points, but that both treatments are equally effective, the purpose of the mediation analyses is to investigate whether or not there are any differences in treatment mechanism between the two therapies.
Methods
Moderation analyses
Potential interaction effects between treatment allocation and baseline PHQ-9 score, age at onset of depression, number of past episodes of depression, baseline scores for BADS, DAS and RRS totals, and treatment were investigated. Participants who reported their age at onset of depression as < 10 years of age had their age at onset raised to 10 years, to bring them into alignment with the overall distribution of age at onset. Participants who reported having experienced > 50 episodes of depression had their number of episodes reduced to 50. The reported data on treatment preference were recombined into a categorical variable with three levels, using ‘no treatment preference’ as the default category, with two comparator categories: ‘had a preference and did receive preferred treatment’, and ‘had a preference and did not receive preferred treatment’. For those participants who indicated a preferred treatment, no distinction was made in the analyses between a preference for CBT and a preference for BA.
Interactions between treatment allocation and each covariate were investigated at 6, 12 and 18 months’ follow-up for PHQ-9. Separate analyses for the ITT and PP populations were performed. For the analyses of PHQ-9 data at 6 months’ follow-up, the PP population was considered to be participants who had completed eight or more therapy sessions before the date of the 6-month follow-up. Only participants with observed data for all covariates included in each model were included within each analysis. A series of models were performed, each model adjusting for the stratification variables, trial site, baseline ADM use and baseline PHQ-9 score. Each model included the specific covariate being investigated as a potential moderator and its interaction with treatment allocation.
Mediation analyses
Mediation population
Mediation analyses were performed on a subgroup of participants that excluded seven participants whose process measure point 1 (PM1) and/or process measure point 2 (PM2) data were collected after 6 months’ follow-up. Otherwise, all analyses were conducted using the ITT population, and included only participants with observed data.
Analysis methods
Unadjusted mean scores were reported for both treatment groups for PHQ-9 at all follow-up times, and for the mediator variables at all recorded follow-up times, for the mediation population. We also produced line plots to show the trajectories of individual participants by treatment group for PHQ-9 and each of the PM variables, and investigated whether or not there was any between-group difference over time for the PMs using a repeated measures model with adjustment for site, baseline PHQ-9 score and baseline ADM use. We also investigated whether or not there was any difference between groups with regard to proportion of sessions attended, basic treatment fidelity and overall treatment fidelity. Post hoc regression models were performed to investigate the effects of basic fidelity and overall fidelity on PHQ-9 score at 12 and 18 months’ follow-up, with adjustment for treatment group, baseline PHQ-9 score, site and baseline ADM use. The interactions between fidelity and treatment group were also investigated.
For the mediation analyses we used a structural equation modelling (SEM; command sem in Stata v.14) approach to evaluate the effect of each individual mediator at each follow-up time, on the primary outcome, PHQ-9 score. We also included all mediators measured at a specific follow-up time in an overall model for each follow-up time for PHQ-9 measurement. All analyses adjusted for the stratification variables, baseline PHQ-9 (measured as a continuous variable rather than dichotomised as for the stratification process), trial site and whether or not the participant was using ADM at baseline. We also adjusted for age at onset of depression, as this was found to be a predictor (p < 0.05) of PHQ-9 score at all follow-up times. It was noted that none of the three potential mediator variables (BADS total, RRS, DAS) examined at baseline as potential predictors or moderators of PHQ-9 at any of the three follow-up times was significant. For the mechanistic mediators (BADS, DAS, RRS and SHAPS), we adjusted all analyses for the baseline score, but not for any other previous scores (if available). This approach takes account of both the participant’s initial state and his/her change in state from baseline to follow-up. We report the total effect and indirect effect for each mediator, with 95% bias-corrected CIs104 derived from bootstrapping with 5000 replications. All analyses were performed using Stata v.14.
Results
Moderation analyses
The results of the analyses investigating interactions between treatment effects and selected covariates are shown in Table 17. Across all three follow-up times, and in both the ITT and PP populations, there were no statistically significant interactions between treatment group and the specified covariates. However, in the PP population, at both 12 and 18 months’ follow-up, there was weak evidence (p < 0.1) for an interaction between treatment group and baseline PHQ-9 score. This interaction was in the direction of participants with higher PHQ-9 scores at baseline, and receiving BA, having lower PHQ-9 scores at follow-up than with participants who received CBT. This treatment moderation effect of baseline PHQ-9 score is shown graphically in Figure 12 for the 12-month follow-up. Weak evidence was found for a moderating effect of baseline total RRS score on the treatment effect for PHQ-9 at 12 months’ follow-up, also in the PP population. However, this effect was not observed at any other follow-up time points.
| Interaction terma | PHQ-9: time point | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6-month follow-up | 12-month follow-up | 18-month follow-up | ||||||||||
| ITT population | PP population | ITT population | PP population | ITT population | PP population | |||||||
| Mean difference (95% CI), n | Global p-value | Mean difference (95% CI), n | Global p-value | Mean difference (95% CI), n | Global p-value | Mean difference (95% CI), n | Global p-value | Mean difference (95% CI), n | Global p-value | Mean difference (95% CI), n | Global p-value | |
| CBT/baseline PHQ-9 score | 0.22 (–0.06 to 0.51), 380 | 0.128 | 0.21 (–0.11 to 0.53), 284 | 0.206 | 0.18 (–0.11 to 0.48), 364 | 0.226 | 0.28 (–0.05 to 0.61), 286 | 0.092 | 0.17 (–0.13 to 0.46), 356 | 0.274 | 0.29 (–0.03 to 0.62), 284 | 0.080 |
| CBT/baseline BADS total score | 0.01 (–0.07 to 0.08), 350 | 0.880 | 0.00 (–0.08 to 0.08), 263 | 0.983 | 0.01 (–0.08 to 0.06), 338 | 0.766 | –0.01 (–0.09 to 0.07), 265 | 0.858 | 0.00 (–0.07 to 0.07), 331 | 0.990 | 0.00 (–0.08 to 0.08), 263 | 0.978 |
| CBT/baseline DAS score | –0.02 (–0.05 to 0.02), 375 | 0.313 | –0.01 (–0.05 to 0.03), 282 | 0.704 | 0.01 (–0.02 to 0.05), 361 | 0.508 | 0.02 (–0.02 to 0.07), 284 | 0.249 | –0.02 (–0.06 to 0.01), 354 | 0.193 | –0.02 (–0.06 to 0.02), 283 | 0.423 |
| CBT/baseline RRS total score | 0.07 (–0.06 to 0.21), 379 | 0.278 | 0.01 (–0.14 to 0.16), 284 | 0.907 | 0.08 (–0.06 to 0.22), 364 | 0.249 | 0.13 (–0.02 to 0.29), 286 | 0.097 | 0.08 (–0.06 to 0.22), 356 | 0.247 | 0.09 (–0.07 to 0.25), 284 | 0.268 |
| CBT/age at onset of depression | –0.01 (–0.11 to 0.08), 371 | 0.774 | 0.01 (–0.09 to 0.11), 278 | 0.909 | –0.03 (–0.13 to 0.07), 357 | 0.572 | –0.07 (–0.18 to 0.04), 281 | 0.194 | –0.02 (–0.12 to 0.07), 349 | 0.625 | –0.05 (–0.15 to 0.06), 279 | 0.373 |
| CBT/number of past episodes of depression | 0.13 (–0.04 to 0.30), 333 | 0.141 | 0.09 (–0.11 to 0.29), 245 | 0.367 | –0.02 (–0.21 to 0.17), 319 | 0.827 | –0.09 (–0.31 to 0.14), 248 | 0.447 | –0.05 (–0.22 to 0.13), 312 | 0.605 | –0.07 (–0.27 to 0.13), 246 | 0.489 |
| Treatment preferenceb | 0.54 (–3.35 to 4.43), 378; 0.09 (–3.28 to 3.46) | 0.963 | 1.70 (–2.35 to 5.74), 283; –0.57 (–4.28 to 3.13) | 0.623 | 0.42 (–3.59 to 4.43), 363; 0.23 (–3.27 to 3.72) | 0.976 | 1.35 (–2.93 to 5.63), 285; –1.28 (–5.16 to 2.60) | 0.589 | 0.39 (–3.57 to 4.36), 353; 0.14 (–3.43 to 3.70) | 0.981 | 0.62 (–3.71 to 4.95), 283; –1.26 (–5.21 to 2.69) | 0.747 |
FIGURE 12.
Margins plot of interaction, with 95% CIs, between the effect of treatment group and baseline PHQ-9 on PHQ-9 at 12 months’ follow-up using the PP observed data sample only (with adjustment for site and baseline antidepressant use). a, Linear predictor of PHQ-9 at 12 months’ follow-up.

Mediation analyses
Table 18 shows the unadjusted scores for PHQ-9 and all mediator variables at all time points at which the variable was recorded. Figures 13–17 show the treatment group mean scores and Figures 18–22 (see Appendix 2) show the individual participant scores, for all time points at which each variable was recorded. For PHQ-9, the greatest reduction in mean scores occurred between baseline and 6 months’ follow-up, with a smaller reduction between 6 and 12 months’ follow-up. Mean scores were stable between the 12- and 18-month follow-ups. On looking at the individual participant trajectories for PHQ-9 between the follow-up times, there was wide variation among participants with regard to their personal PHQ-9 trajectory. Some participants had an evident reduction in PHQ-9 score between baseline and 6 months’ follow-up, which may be sustained in some cases but increased after the 6-month follow-up in others. A similar wide variation in individual participant trajectories was observed across all process mediator variables. No significant between-group difference across time points was found for any of the process mediators (p-values for interaction between group and time point were > 0.1 for all process mediators across all available time points). There was no significant difference between the treatment groups (CBT vs. BA) for percentage of sessions attended (mean difference 4.4 sessions attended, 95% CI –1.7 to 10.5 sessions attended). For basic fidelity (on a scale of 0 to 100), there was evidence that the fidelity for CBT was lower than for BA (mean difference –12.1, 95% CI –17.6 to –6.7), although this may be artefactual because of the different numbers of topics for the two therapies. Among participants receiving BA, 158 out of 220 (71%) completed all six of the core topics, compared with only 70 out of 218 (32%) participants who received CBT completing all seven of the core topics, and only 132 out of 218 (61%) completed at least six out of seven core topics. For overall fidelity, there was no significant difference between the groups (mean difference –4.9, 95% CI –11.0 to 1.1), although again this may be arbitrary as a result of the weighting system used to weight core and mandatory topics.
| Outcome variable | Baseline | PM1 | PM2 | 6-month follow-up | 12-month follow-up | 18-month follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBT | BA | CBT | BA | CBT | BA | CBT | BA | CBT | BA | CBT | BA | |
| PHQ-9: mean (SD), n | 17.39 (4.85), 214 | 17.69 (4.80), 219 | DNC | DNC | DNC | DNC | 9.41 (7.04), 190 | 9.72 (6.88), 183 | 8.20 (7.32), 184 | 8.34 (6.97), 173 | 8.39 (7.12), 176 | 8.32 (7.13), 174 |
| BADS total: mean (SD), n | 88.30 (19.98), 195 | 87.65 (21.32), 206 | 76.35 (24.46), 92 | 75.69 (27.10), 91 | 63.74 (27.76), 78 | 67.15 (27.56), 67 | 55.88 (29.65), 146 | 56.74 (30.18), 144 | DNC | DNC | DNC | DNC |
| BADS activation: mean (SD), n | 29.57 (7.21), 206 | 29.90 (7.67), 214 | 26.73 (7.86), 104 | 26.35 (7.97), 100 | 22.82 (8.28), 85 | 22.78 (9.48), 78 | 21.34 (9.24), 155 | 22.09 (9.90), 151 | DNC | DNC | DNC | DNC |
| BADS avoidance: mean (SD), n | 26.77 (9.47), 209 | 25.86 (9.31), 215 | 23.85 (10.54), 105 | 23.35 (10.03), 106 | 19.66 (10.14), 85 | 20.19 (9.39), 79 | 15.96 (11.25), 155 | 17.37 (10.72), 159 | DNC | DNC | DNC | DNC |
| BADS work impairment: mean (SD), n | 15.78 (6.24), 212 | 16.09 (6.43), 219 | 14.23 (6.38), 105 | 14.15 (6.97), 105 | 11.56 (7.20), 85 | 11.30 (6.07), 81 | 10.09 (7.08), 157 | 9.98 (6.99), 160 | DNC | DNC | DNC | DNC |
| BADS social impairment: mean (SD), n | 15.81 (7.23), 205 | 16.13 (7.77), 211 | 11.91 (7.53), 101 | 13.45 (8.39), 101 | 10.25 (8.14), 84 | 11.28 (8.27), 74 | 8.63 (8.02), 156 | 8.75 (8.06), 154 | DNC | DNC | DNC | DNC |
| DAS: mean (SD), n | 154.23 (38.35), 211 | 152.02 (38.67), 217 | 158.36 (40.04), 109 | 158.72 (40.65), 107 | 150.71 (41.98), 88 | 152.70 (41.38), 84 | 134.66 (41.95), 159 | 135.97 (42.15), 160 | DNC | DNC | DNC | DNC |
| RRS total: mean (SD), n | 60.28 (10.05), 213 | 59.61 (11.18), 219 | 58.29 (11.59), 109 | 58.18 (12.42), 111 | 52.22 (12.28), 88 | 53.45 (13.53), 85 | 48.27 (14.48), 159 | 49.95 (14.24), 160 | DNC | DNC | DNC | DNC |
| RRS reflection: mean (SD), n | 12.30 (3.12), 213 | 12.05 (3.41), 219 | 12.06 (2.99), 109 | 11.86 (3.35), 111 | 11.03 (3.00), 88 | 11.04 (3.59), 85 | 10.38 (3.44), 159 | 10.58 (3.37), 160 | DNC | DNC | DNC | DNC |
| SHAPS: mean (SD), n | 21.11 (2.37), 212 | 20.87 (2.43), 217 | DNC | DNC | DNC | DNC | 21.35 (3.16), 161 | 21.39 (3.02), 161 | 21.08 (2.31), 163 | 20.59 (2.75), 147 | DNR | DNR |
| Proportion (%) of sessions attended:a mean (SD), n | 52 (33), 214 | 48 (33), 219 | ||||||||||
| Basic treatment fidelity (scale 0–100):a mean (SD), n | 73 (31), 213 | 86 (28), 218 | ||||||||||
| Overall treatment fidelity:a mean (SD), n | 68 (31), 213 | 73 (33), 218 | ||||||||||
FIGURE 13.
Mean PHQ-9 scores with 95% CI at baseline and at 6, 12 and 18 months’ follow-up, by treatment group for the mediation population.

FIGURE 14.
Mean BADS total score with 95% CI at baseline, PM1 (session 4), PM2 (session 7) and at 6 months’ follow-up, by treatment group for the mediation population.

FIGURE 15.
Dysfunctional Attitudes Scale total score at baseline, PM1 (session 4), PM2 (session 7) and at 6 months’ follow-up, by treatment group for the mediation population.

FIGURE 16.
Ruminative Response Scale total score at baseline, PM1 (session 4), PM2 (session 7) and at 6 months’ follow-up, by treatment group for the mediation population.
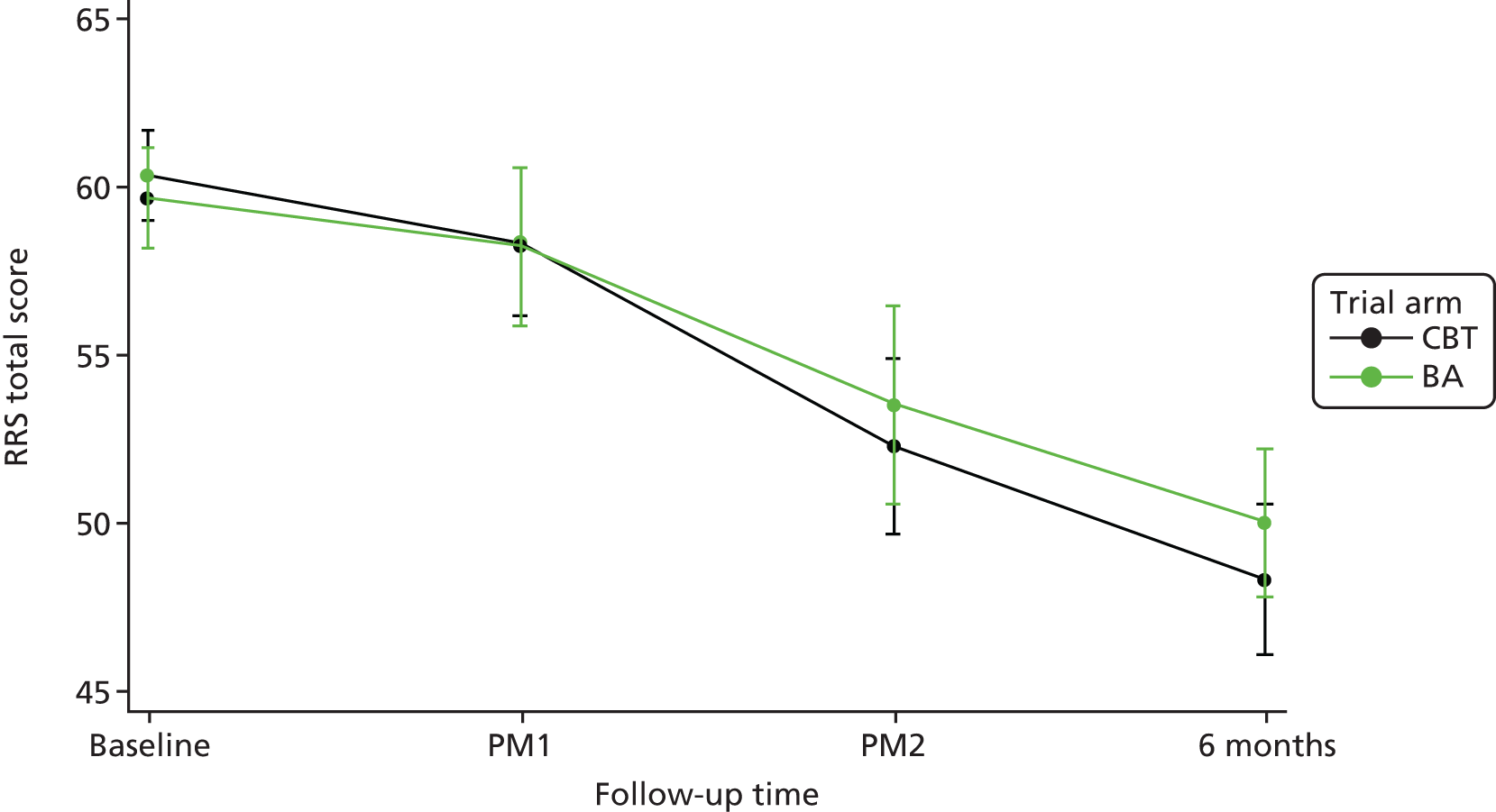
FIGURE 17.
Snaith–Hamilton Pleasure Scale total score at baseline, and at 6 and 12 months’ follow-up, by treatment group for the mediation population.

Using the SEM approach, at the 6-month PHQ-9 follow-up, when considered individually, each mediator measured at PM1 mediated only a small proportion of the total effect of treatment (Table 19). None of the mediators in the single mediator models or in the multiple mediator models was found to have a statistically significant indirect effect of treatment allocation acting via that mediator.
| Mediator | Model | ||||
|---|---|---|---|---|---|
| Single mediator | Including all mediators | ||||
| Indirect effect of treatment allocation acting through mediator (95% CI), n | Total effect of treatment allocation (95% CI) | Proportion of total effect mediated by indirect effect (%) | Indirect effect of treatment allocation acting through mediator (95% CI) | Proportion of total effect mediated by indirect effect (%)a | |
| Mediators measured at PM1 (therapy session 4) | |||||
| Total effect of treatment allocation –1.84 (95% CI –3.79 to 0.04), n = 158 | |||||
| BADS total | 0.18 (–0.34 to 0.91), 163 | –1.52 (–3.43 to 0.34) | –12 | Not included | Not included |
| BADS activation | 0.12 (–0.51 to 0.75), 186 | –1.52 (–3.45 to 0.32) | –8 | 0.13 (–0.11 to 0.81) | 0.13 (–0.11 to 0.81) |
| BADS avoidance | 0.11 (–0.17 to 0.71), 195 | –1.34 (–3.14 to 0.51) | –8 | –0.01 (–0.38 to 0.18) | –0.01 (–0.38 to 0.18) |
| BADS work impairment | 0.10 (–0.45 to 0.69), 198 | –1.45 (–3.24 to 0.42) | –7 | 0.05 (–0.10 to 0.65) | 0.05 (–0.10 to 0.65) |
| BADS social impairment | –0.06 (–0.50 to 0.32), 184 | –1.54 (–3.43 to 0.18) | 4 | 0.04 (–0.12 to 0.52) | 0.04 (–0.12 to 0.52) |
| DAS | 0.13 (–0.26 to 0.71), 202 | –1.73 (–3.46 to 0.16) | –8 | 0.18 (–0.12 to 0.84) | 0.18 (–0.12 to 0.84) |
| RRS total | 0.13 (–0.31 to 0.63), 206 | –1.64 (–3.40 to 0.18) | –8 | 0.06 (–0.14 to 0.64) | 0.06 (–0.14 to 0.64) |
| RRS reflection | 0.04 (–0.09 to 0.39), 206 | –1.60 (–3.36 to 0.21) | –2 | Not included | Not included |
| Mediators measured at PM2 (therapy session 7) | |||||
| Total effect of treatment allocation –2.63 (95% CI –4.87 to –0.32), n = 126 | |||||
| BADS total | –0.23 (–1.45 to 0.89), 129 | –2.52 (–4.94 to –0.35) | 9 | Not included | Not included |
| BADS activation | 0.21 (–0.57 to 1.15), 153 | –1.68 (–3.67 to 0.37) | –13 | –0.01 (–0.47 to 0.19) | 1 |
| BADS avoidance | –0.05 (–0.84 to 0.72), 154 | –2.26 (–4.42 to –0.12) | 2 | –0.01 (–0.48 to 0.30) | 0 |
| BADS work impairment | 0.34 (–0.64 to 1.38), 160 | –2.01 (–4.05 to –0.01) | –17 | 0.04 (–0.35 to 0.80) | –1 |
| BADS social impairment | –0.29 (–1.29 to 0.56), 147 | –2.33 (–4.46 to –0.23) | 13 | –0.19 (–1.19 to 0.19) | 7 |
| DAS | 0.23 (–0.54 to 0.93), 166 | –1.95 (–3.95 to 0.20) | –12 | 0.10 (–0.16 to 0.84) | –4 |
| RRS total | –0.02 (–0.77 to 0.68), 167 | –2.00 (–4.07 to –0.02) | 1 | –0.04 (–0.59 to 0.24) | 2 |
| RRS reflection | 0.11 (–0.42 to 0.71), 167 | –2.04 (–4.06 to –0.02) | –5 | Not included | Not included |
At the 12-month PHQ-9 follow-up, with mediators measured at PM1, again the BADS variables appeared to be the strongest individual mediators, with social impairment mediating 21% of the total effect of treatment, and the total BADS score mediating –18% (Table 20).
| Mediator | Model | ||||
|---|---|---|---|---|---|
| Single mediator | Including all mediators | ||||
| Indirect effect of treatment allocation acting through mediator (95% CI), n | Total effect of treatment allocation (95% CI) | Proportion of total effect mediated by indirect effect (%) | Indirect effect of treatment allocation acting through mediator (95% CI) | Proportion of total effect mediated by indirect effect (%)a | |
| Mediators measured at PM1 | |||||
| Total effect of treatment allocation –0.58 (95% CI –2.61 to 1.59), n = 154 | |||||
| BADS total | 0.09 (–0.78 to 0.81), 158 | –0.49 (–2.55 to 1.41) | –18 | Not included | Not included |
| BADS activation | 0.01 (–0.69 to 0.61), 181 | –0.79 (–2.74 to 1.09) | –1 | 0.08 (–0.27 to 0.69) | –14 |
| BADS avoidance | 0.02 (–0.47 to 0.49), 189 | –0.82 (–2.76 to 1.14) | –3 | 0.01 (–0.20 to 0.45) | –3 |
| BADS work impairment | 0.03 (–0.69 to 0.61), 192 | –0.56 (–2.51 to 1.35) | –5 | 0.08 (–0.26 to 0.79) | –15 |
| BADS social impairment | –0.16 (–0.80 to 0.23), 179 | –0.78 (–2.71 to 1.18) | 21 | 0.00 (–0.24 to 0.24) | 0 |
| DAS | 0.08 (–0.15 to 0.61), 198 | –0.93 (–2.84 to 0.92) | –9 | –0.18 (–0.90 to 0.13) | 32 |
| RRS total | 0.09 (–0.41 to 0.66), 201 | –0.98 (–2.88 to 0.83) | –9 | 0.10 (–0.09 to 0.73) | –17 |
| RRS reflection | 0.08 (–0.17 to 0.54), 201 | –0.95 (–2.84 to 0.87) | –9 | Not included | Not included |
| Mediators measured at PM2 | |||||
| Total effect of treatment allocation –0.84 (95% CI –3.23 to 1.58), n = 125 | |||||
| BADS total | –0.29 (–1.40 to 0.64), 128 | –0.53 (–2.98 to 1.74) | 55 | Not included | Not included |
| BADS activation | –0.09 (–0.73 to 0.84), 150 | –0.27 (–2.29 to 1.69) | –33 | –0.04 (–0.72 to 0.14) | 5 |
| BADS avoidance | –0.10 (–0.74 to 0.48), 150 | –0.37 (–2.36 to 1.66) | 26 | 0.00 (–0.38 to 0.52) | –1 |
| BADS work impairment | 0.14 (–0.76 to 1.10), 156 | –0.47 (–2.58 to 1.67) | –31 | –0.03 (–0.91 to 0.78) | 4 |
| BADS social impairment | –0.33 (–1.35 to 0.43), 145 | –0.58 (–2.78 to 1.52) | 57 | –0.20 (–1.51 to 0.15) | 23 |
| DAS | 0.11 (–0.42 to 0.67), 162 | –0.57 (–2.58 to 1.49) | –19 | –0.04 (–0.73 to 0.21) | 5 |
| RRS total | 0.01 (–0.67 to 0.71), 163 | –0.68 (–2.71 to 1.32) | –2 | –0.03 (–0.63 to 0.43) | 4 |
| RRS reflection | 0.20 (–0.23 to 0.90), 163 | –0.72 (–2.73 to 1.28) | –28 | Not included | Not included |
| Mediators measured at 6 months’ follow-up | |||||
| Total effect of treatment allocation 0.30 (–1.37 to 1.95), n = 250 | |||||
| BADS total | 0.09 (–0.77 to 1.05), 258 | 0.27 (–1.33 to 1.93) | 33 | Not included | Not included |
| BADS activation | –0.05 (–0.82 to 0.68), 281 | –0.22 (–1.73 to 1.31) | 25 | 0.00 (–0.29 to 0.35) | 2 |
| BADS avoidance | –0.40 (–1.13 to 0.37), 285 | –0.06 (–1.52 to 1.42) | 720 | –0.12 (–0.64 to 0.10) | –39 |
| BADS work impairment | 0.24 (–0.46 to 1.04), 294 | –0.26 (–1.82 to 1.17) | –94 | 0.02 (–0.13 to 0.39) | 6 |
| BADS social impairment | 0.26 (–0.52 to 1.09), 282 | –0.06 (–1.58 to 1.53) | –463 | 0.06 (–0.11 to 0.52) | 19 |
| DAS | 0.08 (–0.80 to 0.61), 296 | –0.26 (–1.72 to 1.26) | 33 | –0.02 (–0.36 to 0.11) | –5 |
| RRS total | –0.31 (–0.99 to 0.41), 297 | –0.30 (–1.82 to 1.16) | 105 | –0.11 (–0.59 to 0.19) | –38 |
| RRS reflection | –0.14 (–0.68 to 0.40), 297 | –0.30 (–1.81 to 1.17) | 47 | Not included | Not included |
| SHAPS | 0.02 (–0.04 to 0.22), 298 | –0.09 (–1.70 to 1.41) | –20 | 0.05 (–0.03 to 0.33) | 17 |
| Treatment fidelity mediatorsb | |||||
| Proportion of sessions attended | –0.04 (–0.28 to 0.04), 350 | –0.14 (–1.49 to 1.30) | 29 | 0.01 (–0.12 to 0.24) | 2 |
| Basic treatment fidelity | 0.33 (–0.03 to 0.83), 349 | –0.15 (–1.55 to 1.27) | –216 | 0.07 (–0.79 to 0.76) | 23 |
| Overall treatment fidelity | 0.18 (0.01 to 0.54), 349 | –0.15 (–1.55 to 1.27) | –117 | 0.05 (–0.43 to 0.72) | 17 |
For the overall treatment fidelity, the indirect effect of treatment group acting via this mediator was statistically significant (–0.18, 95% CI –0.54 to –0.01), mediating –117% of the total treatment effect (see Table 20). In the multiple mediator model, however, none of the mediators was found to have a significant indirect effect. BADS avoidance, BADS social impairment, total RRS score, SHAPS, basic treatment fidelity and overall treatment fidelity were found to mediate at least 10% in magnitude of the total treatment effect.
At 18 months’ follow-up, the indirect effect of treatment via basic treatment fidelity in the individual mediator model was statistically significant (observed effect 0.61, 95% CI 0.23 to 1.17) and associated with a proportion of total effect mediated of 610% (Table 21). In the multiple mediators model, with mediator variables measured at 6 months’ follow-up, this effect was not statistically significant, but was associated with a proportion of total effect mediated of 77%. Similarly, the indirect effect via overall treatment quality was statistically significant (observed effect 0.24, 95% CI 0.03 to 0.65) in the individual mediator model, but this significant effect was not seen in the multiple mediators model.
| Mediator | Model | ||||
|---|---|---|---|---|---|
| Single mediator | Including all mediators | ||||
| Indirect effect of treatment allocation acting through mediator (95% CI), n | Total effect of treatment allocation (95% CI) | Proportion of total effect mediated by indirect effect (%) | Indirect effect of treatment allocation acting through mediator (95% CI) | Proportion of total effect mediated by indirect effect (%) | |
| Mediators measured at 6 months’ follow-up | |||||
| Total effect of treatment allocation –0.52 (95% CI –2.16 to 1.13), n = 248 | |||||
| BADS total | 0.06 (–0.76 to 0.96), 255 | 0.64 (–0.91 to 2.27) | 9 | Not included | Not included |
| BADS activation | –0.10 (–0.81 to 0.60), 278 | 0.14 (–1.42 to 1.64) | –75 | 0.00 (–0.15 to 0.22) | 1 |
| BADS avoidance | –0.38 (–1.05 to 0.25), 283 | 0.19 (–1.32 to 1.69) | –196 | 0.02 (–0.13 to 0.37) | 3 |
| BADS work impairment | 0.17 (–0.52 to 0.90), 291 | 0.03 (–1.47 to 1.49) | 653 | 0.11 (–0.07 to 0.64) | 21 |
| BADS social impairment | 0.25 (–0.47 to 1.11), 278 | 0.56 (–0.88 to 2.14) | 45 | 0.12 (–0.29 to 0.71) | 23 |
| DAS | –0.02 (–0.63 to 0.64), 294 | 0.03 (–1.44 to 1.52) | –76 | 0.00 (–0.23 to 0.21) | 0 |
| RRS total | –0.31 (–0.93 to 0.32), 294 | 0.02 (–1.43 to 1.49) | –1600 | –0.06 (–0.47 to 0.08) | –12 |
| RRS reflection | –0.09 (–0.51 to 0.35), 294 | 0.03 (–1.44 to 1.50) | –357 | Not included | Not included |
| SHAPS | 0.00 (–0.11 to 0.08), 293 | 0.01 (–1.50 to 1.48) | –9 | 0.00 (–0.11 to 0.13) | 0 |
| Treatment fidelity mediatorsa | |||||
| Proportion of sessions attended | –0.04 (–0.28 to 0.04), 343 | 0.12 (–1.29 to 1.53) | –36 | 0.04 (–0.08 to 0.45) | 7 |
| Basic treatment fidelity | 0.61 (0.23 to 1.17), 342 | 0.10 (–1.28 to 1.54) | 610 | 0.40 (–0.37 to 1.19) | 77 |
| Overall treatment fidelity | 0.24 (0.03 to 0.65), 342 | 0.10 (–1.28 to 1.54) | 237 | –0.05 (–0.67 to 0.46) | –9 |
| Mediators measured at 12 months’ follow-up | |||||
| SHAPS | 0.04 (–0.10 to 0.29), 291 | 0.34 (–1.16 to 1.96) | 11 | Not included | Not included |
Across the three PHQ-9 time points, the effects of the mediators varied considerably, with the proportions of total effect mediated increasing at later time points, possibly as a result of the mediator variables having a chance to stabilise at later measurement times, and also the PHQ-9 scores becoming more stable on progressing through the follow-up period. The only mediator that was statistically significantly different between the groups was basic treatment fidelity, which was a significant mediator of treatment allocation at 18 months’ follow-up. Using individual regression models, both basic and overall treatment fidelity were associated with the 12-month PHQ-9 score, with poorer fidelity being associated with higher PHQ-9 scores (p-values 0.020 and 0.023, respectively). Similarly, both basic and overall treatment fidelity were significantly associated with the 18-month PHQ-9 follow-up, again with poorer fidelity being associated with higher PHQ-9 scores (p-values 0.001 and 0.020, respectively), the basic fidelity remaining significant when included in a model with overall fidelity (p-value 0.007). No evidence was found for a significant interaction between treatment group and basic or overall fidelity, with regard to either 12 or 18 months’ PHQ-9 follow-up.
Qualitative process study
Methods
Sample and design
At the baseline assessment all COBRA trial participants were asked whether or not they would be willing to complete an additional interview at a later date to discuss their experiences of therapy. Participants for the qualitative study were selected from those providing consent and were purposively sampled to ensure a selection of participants from each recruitment site, both trial arms, some who had fewer than eight sessions of therapy and some who had eight or more. Of those having eight or more sessions, we purposively sampled some who remained depressed and others who were no longer depressed according to the SCID45 at 6 months’ follow-up. Participants were invited to take part in the qualitative study via letter, followed up by a telephone call from a researcher. Interviews were conducted as soon as possible after therapy had ended and aimed to address the following research questions:
-
What are participants’ views on the acceptability of BA and CBT?
-
What are participants’ views on the role of cognitive and behavioural change strategies?
-
What are the broader impacts of BA and CBT on participants’ lives?
In addition, all 22 therapists and MHWs delivering therapy in the trial were invited by e-mail to take part in an interview over the telephone with the trial manager to talk about their experiences of delivering therapy. Those who did not respond to the invitation were not chased further. Semistructured interviews were completed with six CBT therapists and seven BA MHWs.
Participant interviews were conducted over the telephone by Katie Finning (KF), and Rebecca Woodhouse (RW) and Faye Plummer (FP), who were researchers working on the COBRA trial in Devon (KF) and Leeds (RW and FP). All were given in-house training in qualitative interviewing, followed by assessment and feedback on their first interview from David A Richards (DAR), trial chief investigator experienced in qualitative interviewing. Interviews were completed across sites to ensure researcher blinding was maintained for main trial follow-ups (e.g. the researcher in Devon completed interviews with participants in Leeds). Participants therefore had no prior knowledge or relationship with their qualitative interviewer. A semistructured topic guide was developed by DAR and KF based on the study aims and previous literature. Interview topics included general experiences of treatment, acceptability and barriers to therapy, cognitive and behavioural change, and the impact of treatment. Probe questions were used where necessary to help participants elaborate on their responses. The topic guide was pilot tested on two participants and modified to include a question on important parts of therapy, and probe questions were refined.
Therapist interviews were conducted over the telephone by Shelley Rhodes (SR), the COBRA trial manager based in Devon, who was given in-house training in qualitative interviewing. A semistructured topic guide was developed by DAR and SR based on the study aims and previous literature. Interview topics included general experiences of delivering treatment, specific therapeutic strategies used, impact of treatment for participants, perceived reasons for participants who did not improve, how COBRA trial patients compared with patients seen in usual practice and the therapists’ personal experience of taking part in the trial.
Participant and therapist interviews were audio-recorded with participants’ prior consent and transcribed verbatim. Transcripts were not returned to participants or therapists for comment but were double-checked for accuracy by a second member of the research team. Participant interviews were conducted separately from the main trial follow-ups to avoid bias and encourage open communication.
Analysis
Participant transcripts were analysed by KF, Lucy Moore (LM) and DAR using a framework approach,94 with the assistance of QSR International’s NVivo 10 software (QSR International, Warrington, UK). Analysis began with familiarisation with the transcripts and the development of an initial thematic framework, combining deductive themes from the topic guide and inductive themes emerging from the data. KF and LM coded three interviews independently to assess the reliability of coding95 and meetings were held to discuss and refine emerging themes. Transcripts were examined thematically across the whole data set, as well as in the context of each interview, using constant comparison techniques. 96 Data were indexed and sorted using the identified themes and subthemes, and were summarised in framework matrices94 with the original transcripts being frequently revisited to clarify contextual meaning. In keeping with the framework approach we interrogated the data, searching for comparisons and contradictions and keeping interpretive notes. Alternative explanations or negative cases were identified, discussed and a consensus reached. 95 In the final stage of analysis, KF and DAR met to discuss the findings in relation to the research aims and previous literature, focusing on drawing conclusions and synthesising the findings into the overarching themes presented here.
Therapist/MHW transcripts were analysed by KF using the framework approach described above. A second researcher independently coded three interview transcripts and provided an initial thematic framework, which was compared and combined with the framework developed by KF. In the final stage of analysis, KF and DAR met to discuss the findings and relate them to the research aims and previous literature, focusing on drawing conclusions and synthesising the findings into the overarching themes presented here.
Results
Results of participant qualitative interviews
Thirty-six interviews were completed between April 2014 and May 2015. Interviews lasted between 15 and 75 minutes and were completed, on average, 4 months after treatment ended (range 1–17 months). Participant demographics are provided in Table 22 and the distribution of interviews across the purposive sampling frame can be seen in Table 23.
| Participant | Therapy | Number of sessions attended | 6-month depression status | Gender | Age group (years) |
|---|---|---|---|---|---|
| 1 | BA | 1 | N/Aa | Male | 55–64 |
| 2 | CBT | 3 | N/A | Female | 75+ |
| 3 | BA | 14 | Not depressed | Male | 35–44 |
| 4 | BA | 17 | Not depressed | Female | 45–54 |
| 5 | CBT | 24 | Depressed | Female | 45–54 |
| 6 | BA | 3 | N/A | Female | 25–34 |
| 7 | BA | 13 | Depressed | Female | 45–54 |
| 8 | CBT | 22 | Depressed | Female | 35–44 |
| 9 | BA | 9 | Depressed | Male | 35–44 |
| 10 | CBT | 20 | Depressed | Female | 75+ |
| 11 | CBT | 13 | Depressed | Female | 45–54 |
| 12 | CBT | 23 | Not depressed | Female | 55–64 |
| 13 | BA | 12 | Depressed | Male | 55–64 |
| 14 | CBT | 15 | Not depressed | Female | 18–24 |
| 15 | CBT | 14 | Not depressed | Female | 55–64 |
| 16 | BA | 13 | Not depressed | Male | 65–74 |
| 17 | BA | 2 | N/A | Male | 45–54 |
| 18 | CBT | 24 | Depressed | Female | 55–64 |
| 19 | CBT | 14 | Not depressed | Male | 35–44 |
| 20 | BA | 2 | N/A | Male | 45–54 |
| 21 | CBT | 19 | Depressed | Male | 55–64 |
| 22 | BA | 12 | Not depressed | Female | 45–54 |
| 23 | BA | 17 | Depressed | Male | 45–54 |
| 24 | BA | 8 | Not depressed | Female | 35–44 |
| 25 | CBT | 21 | Not depressed | Female | 55–64 |
| 26 | BA | 24 | Depressed | Female | 35–44 |
| 27 | CBT | 22 | Not depressed | Male | 35–44 |
| 28 | CBT | 10 | Not depressed | Female | 55–64 |
| 29 | BA | 24 | Not depressed | Male | 55–64 |
| 30 | CBT | 3 | N/A | Female | 35–44 |
| 31 | BA | 4 | N/A | Female | 35–44 |
| 32 | BA | 5 | N/A | Male | 18–24 |
| 33 | CBT | 1 | N/A | Male | 25–34 |
| 34 | BA | 8 | Depressed | Female | 65–74 |
| 35 | CBT | 10 | Depressed | Female | 35–44 |
| 36 | CBT | 6 | N/A | Female | 25–34 |
| Trial site | Trial arm, number of sessions attended; depression status at 6 months | |||||
|---|---|---|---|---|---|---|
| BA | CBT | |||||
| ≥ 8; depressed | ≥ 8; not depressed | < 8; N/A | ≥ 8; depressed | ≥ 8; not depressed | < 8; N/A | |
| Devon | 3 | 2 | 2 | 4 | 1 | 1 |
| Durham | 1 | 2 | 2 | 2 | 3 | 0 |
| Leeds | 2 | 2 | 2 | 1 | 3 | 3 |
| Total | 6 | 6 | 6 | 7 | 7 | 4 |
Results are presented under three main headings: acceptability of therapy, mechanisms of change and impact of therapy, reflecting our three research questions. Quotes are presented to support analysis and are labelled by participant ID number, therapy received, number of sessions, and for those who received eight or more sessions, depression status at 6-month follow-up (depressed or not depressed). Views were consistent across BA and CBT except where specified.
Acceptability of therapy
Participants’ views about the acceptability of therapy could be understood in terms of three subthemes: elements of therapy, the therapist and barriers to therapy.
Elements of therapy
Many participants enjoyed therapy as an opportunity to learn about depression, themselves, and their thoughts and behaviour. A few participants expressed a preference for one-to-one, face-to-face therapy over alternative modes, and for many the length and regularity of treatment was considered beneficial:
It’s had a lasting effect and I think that may be to do with it being really quite in depth as you’re going for an hour week . . . going once a week is helpful, which had been much better than going once every 3 months for 6 years, it’s like you’re really working on it, like a car.
28 – CBT 10 sessions, not depressed
A small minority felt that therapy did not provide enough opportunity to talk about their feelings or the history behind their depression, but for others not having to focus on the past was considered helpful:
I really loved the fact that I didn’t have to dwell on past experiences . . . I have seen therapists in the past and that but none of it’s ever worked for me ‘cos all they want to do is go over the past and I’ve never wanted to do that.
4 – BA 17 sessions, not depressed
A small number of participants in the BA group made additional comments that were not made by any of those receiving CBT. This included a resistance to the general BA approach, considering it simplistic, superficial and restrictive, and that it was a ‘poorer cousin’ of CBT because of its lack of consideration to thought processes:
I feel like your life’s more complicated or more complex than that . . . I think if you don’t actually kind of go a little bit deeper and underneath things, you’re just sort of tinkering around with some of the superficial stuff on the top and rearranging the furniture.
13 – BA 12 sessions, depressed
In both treatments, experiences of homework were mixed. On the one hand, it could be difficult, bringing therapy into everyday life and having the potential to make mood worse. Some felt a pressure to complete homework, and felt that it could create feelings of fear and failure. But many others considered homework an important part of therapy, providing them with a feeling of owning their depression and gaining control over their feelings, and reported that having things written down was helpful. These views were not always distinct; some participants could recognise the benefits despite finding homework difficult:
People can give you information but you’ve got to put it into practice and act on it even if you might think ‘Oh, this isn’t going to really help me’ . . . you’ve got to go through it and come out the other side, haven’t you, to a certain degree?
7 – BA 13 sessions, depressed
The therapist
For many participants the therapist was a positive part of treatment: someone who was warm, patient and understanding. Participants in both treatments viewed their therapist as an expert who had the skills necessary to help them:
She was a lovely lady she gave me support when I needed it, she pushed me when I needed it . . . she could see when my mind was playing games with me where I was trying to ignore it or move around the situation, so for me she was very good.
4 – BA 17 sessions, not depressed
Some participants reported that the therapist played a particularly important role in helping them overcome difficulties in therapy. Being able to adapt treatment, offering reassurance and not putting pressure on participants were helpful skills when therapy was difficult, and addressing challenges with the therapist was largely seen as a helpful process:
Speaking to the counsellor and just being honest and open about what was, the fears or the barriers . . . because they were quite useful for her to understand, she could then fold that into the treatment as well.
27 – CBT 22 sessions, not depressed
A small minority of BA participants described their therapists as rigid, unauthoritative and lacking in confidence, comments that were not made by any of those receiving CBT. Although discussed only by a few participants in our sample, for those who did so it appeared to be a significant problem and was discussed at length:
It did feel like there was a bit of a confidence issue going on or a lack of confidence from the therapist’s side in some way. ‘Cos I sort of picked up that I needed to kind of make her kind of feel like she was doing a good job with me.
13 – BA 12 sessions, depressed
Barriers to therapy
Work and family were particular features of life that could make therapy difficult. Getting to sessions could be problematic, particularly for those with comorbidities, such as anxiety or chronic pain. A regular routine of appointments was considered to make attendance easier but flexibility was also welcomed (e.g. rearranging sessions or completing them over the telephone if particular barriers arose). There were also emotional challenges to therapy; it could be hard to open up and talk about personal things, especially in the beginning and for those who were not used to expressing their emotions. For some, depression itself made it hard to put things into practice and affected their ability to understand components of therapy:
The whole point about mood which makes it bad is the fact that it’s impacting your ability to do . . . the depression itself is a barrier to doing it. I actually can’t offer a solution that would make it easier, but I believe that it wouldn’t work for everybody.
33 – CBT 1 session
Participants recognised the importance of their own attitude and commitment in helping them overcome barriers to therapy:
Hard as it was I was determined to do it because I knew I had to to make a difference in my life.
4 – BA 17 sessions, not depressed
Mechanisms of change
Participants’ views about mechanisms of change could be understood in terms of three subthemes: behaviour change, cognitive change and talking versus doing.
Behaviour change
Changes to behaviour were considered important by many participants in both treatments, and this included avoidance, triggers, rumination and goal-oriented behaviour. Therapy enabled participants to understand and overcome avoidance behaviours, and this could reduce anxiety:
I felt like a weight had been lifted and I could, I was in a sort of procrastination phase where I couldn’t make decisions and I was just puttin’ things off . . . in gradual steps I started to be able to work my way through problems and being able to prioritise.
19 – CBT 14 sessions, not depressed
Therapy helped participants recognise triggers for low mood and understand the consequences of their response to triggers. Some described being able to choose different behavioural responses, control their feelings with actions and think differently about triggers, and these changes could reduce the power of depression. Therapy helped participants learn to set realistic, achievable goals and use behaviour to improve their mood, as well as encouraging them to reengage with positive activities and act when feeling low, helping to break the cycle of low mood:
When people are depressed I know it’s a circle, you don’t do anything, so you feel terrible, and then you don’t do anything . . . this therapy forced me, pushed me to act, to do something. This is the first time that I’ve actually experienced that somebody tell me ‘Well let’s start doing this and you will feel better’ and it happened.
24 – BA 8 sessions, not depressed
Both therapies encouraged participants to recognise the effect of rumination (i.e. repetitive unproductive thinking, especially about the experience of depression) and, for many, allowed them to manage and reduce time spent ruminating, which could improve mood and make life feel easier:
I care about people, my family and friends, now. As I say I didn’t before, I didn’t want to go anywhere, didn’t want to see anyone, I just wanted to be left alone. And that’s the ruminating time; she got me off that and I feel better for it.
23 – BA 17 sessions, depressed
Cognitive change
Cognitive change was discussed by some participants in both therapies, but was referred to more frequently by those who received CBT. This included having more self-belief, blaming themselves less when things go wrong and reduced beliefs of worthlessness:
It’s given me a different way of looking at things and I suppose that’s the way of believing in things, I have more belief in myself, that has helped a lot.
12 – CBT 23 sessions, not depressed
Participants in both therapies reported a more positive style of thinking, the ability to replace negative thoughts with positive thoughts and changing thoughts before entering a negative spiral. Other changes included a reduced tendency to overthink or ruminate, fewer self-critical thoughts and more balanced thinking. Some participants, particularly those who received CBT, described a sense of increased resilience, such as the ability to reason when things go wrong and taking things less personally:
I used to be a bit like a bull in a china shop if I was upset I would take it all very personally but now I’m more open minded . . . I think you don’t take everything so personally, makes me think more rather than going to it head-long without thinking.
5 – CBT 24 sessions, depressed
Talking versus doing
This subtheme describes two typologies observed across both treatments, either prioritising opportunities to talk or using therapeutic strategies to bring about change. For several participants, having someone to talk to who was unbiased, non-judgemental and emotionally unconnected was the most important part of therapy, and problems from the week could be ‘saved up’ to discuss with the therapist:
Irrespective of what therapy it was, I think just the opportunity for an hour a week to talk about how you’re feeling was in some way therapeutic, irrespective of the specific techniques of the BA.
9 – BA 9 sessions, depressed
In contrast, for many the ‘doing’ side of therapy was critical and the specific strategies of BA and CBT were considered helpful. The therapist was there not just to listen but to offer suggestions, and therapy was perceived to encourage participants to be proactive, finding a way to help themselves:
I think a lot of people need more input than just listening and what I liked about it is that they make very definite suggestions . . . you’d look for evidence and look at what is happening and then make goals to try and work towards.
28 – CBT 10 sessions, not depressed
Impact of therapy
Participants’ views about the impact of therapy could be understood in terms of three subthemes: impact for self, impact for others and impact on the future.
Impact for self
In both therapies participants described no longer feeling depressed, enjoying life more and feeling like their old self again, and for some these improvements were longer lasting than they had experienced with other therapies:
Now I don’t feel so full of despair as I used to be. Sort of, oh it’s like taming the beast, really . . . it’s given me the tools to get through day-to-day life and be more aware of moods and what effect they have on me and how to change that mood.
12 – CBT 23 sessions, not depressed
Other participants described themselves as happier, as a result of therapy, or feeling they have a different relationship with depression, leading to a feeling of acceptance. Several participants described therapy as having enhanced the way they feel about themselves, including increased feelings of self-compassion and improved self-esteem. Participants discussed positive influences of treatment leading to healthier lifestyles such as cooking better meals, exercising or seeking help for other problems such as pain or disability. Treatment was believed by some to have enabled them to get jobs, return to work after a period of being signed off, or perform better at work. For some, therapy enabled them to reduce or stop their ADM, and this could have further perceived benefits such as improved clarity of mind. Even those still meeting diagnostic criteria for depression could perceive a wide-reaching impact of therapy:
I would just say thank you very much for all the help you’ve given me and it’s made an impact on my life that I never would have thought. I thought I was on my own, but evidently I’m not.
23 – BA 17 sessions, depressed
Impact for others
Participants in both treatments discussed ways in which therapy influenced those around them. Many perceived therapy to have helped their relationships and others described being more sociable or behaving more kindly towards others:
We talk, which has never happened before. We talk for like, hours. And we don’t need to watch the telly or listen to music or anything . . . so I’m more interested in what’s going on than the one-eyed god. The television!
23 – BA 17 sessions, depressed
Impact on the future
Many participants described therapy as providing them with a ‘toolkit’ to take away, teaching them skills that have enabled them to deal with life more effectively. For some these skills were becoming automatic as they continued to put them into practice. Relapse prevention work was important, helping participants learn to recognise the signs of depression and knowing how and when to ask for help. Having paper copies of therapeutic tools to take away was considered helpful and many participants revisit these when they feel low:
I think that this will probably be something I’ll do for the rest of my life, ‘cos I’m sure that for the rest of my life I’ll have the ups and downs like everyone else does, but this will stop me going back to those dark places.
11 – CBT 13 sessions, depressed
Results of therapist qualitative interviews
Six CBT therapists and seven BA MHWs were interviewed in November and December 2015. Interviews lasted between 25 and 59 minutes (mean 40 minutes). Therapist demographics are provided in Table 24.
| Therapist characteristic | Number of interviews (n = 13) |
|---|---|
| Recruitment site | |
| Devon | 5 |
| Durham | 3 |
| Leeds | 5 |
| Therapy delivered | |
| BA | 7 |
| CBT | 6 |
| Gender | |
| Male | 3 |
| Female | 10 |
| Mean years since first qualified | |
| BA | 3.0 |
| CBT | 12.4 |
Data are presented under three overarching themes: the therapeutic model, confidence in delivery and the patients. Quotes are presented to support the analysis and are labelled by interview ID number, therapy delivered and recruitment site.
The therapeutic model
This theme illustrates therapists’ views on the model of therapy that was delivered, including particular elements of therapy that were considered beneficial and their experiences of using the treatment manuals that they were trained to follow.
Elements of therapy
Mental health workers confirmed that, as intended, BA was an uncomplicated, logical and simple therapy that they believed made sense to patients, allowing them to make quick gains and encouraging a good therapeutic relationship. The majority of CBT therapists did not discuss the relative simplicity or complexity of therapy, but the one that did expressed a view that CBT is a complicated therapy that is difficult to deliver:
I think the fact that the behavioural activation model was quite simplified, it wasn’t too complicated for people, so that helped with the therapeutic relationship as well. Yeah, it’s an easy therapy to explain to patients.
T10 – BA, Durham
Many therapists, particularly those delivering BA, described working longer term with patients in the COBRA trial protocol than they would in usual practice, and this was seen as beneficial both for therapist and patient, and allowed for a better therapeutic relationship:
It was really good to get the chance to work with people over a much longer period and get to know them better . . . you form more of a relationship with them than you do when you’re working with people for a shorter period of time, like six sessions.
T12 – BA, Leeds
Therapists in both treatments placed high value on relapse prevention. BA MHWs felt that it was useful for patients to reflect on what they had learnt and have a plan to recognise and respond to signs of depression in the future. Some BA MHWs commented that relapse prevention is not given a lot of attention in their usual practice in IAPT, and this was considered an important addition. Likewise, CBT therapists believed relapse prevention to be a core component of treatment that helped patients understand the changes made and encouraged them to continue implementing them. Therapists expressed a view that the aim of CBT is for patients to become their own therapist, and providing them with tools to take forward was important in helping them achieve this:
One of the aims of CBT is for people to become their own therapist . . . it gives people a bit of confidence and sense of hope for the future that there’s stuff within their power and control that they can do to make changes.
T08 – CBT, Devon
For BA MHWs, another component of treatment considered particularly helpful, both for themselves and for patients, was rumination. MHWs believed that rumination is a key problem in depression, and it was therefore a frequently used technique that was considered to be applicable to many patients. It was noted that rumination was not taught when they trained as IAPT PWPs and that it was a helpful addition to their knowledge base, and something they continue to use in their clinical practice now:
Rumination, particularly, I think was quite helpful and very rarely is that used in standard step 2 practice, so I think that would be a really useful addition.
T07 – BA, Devon
Treatment manuals
Both BA MHWs and CBT therapists described the treatment manuals and patient worksheets as good, well structured, clear and concise. CBT therapists commented that it was helpful to have a manual to follow that could be taken off the shelf and used, providing useful and well-needed updates to the CBT model:
The COBRA manual itself was really kind of clear and concise and helpful as well . . . whereas Beck’s original book on depression, it’s very old, it really does need a bit of updating.
T02 – CBT, Devon
Therapists in both treatments described using the manuals flexibly, adapting therapy to meet individual needs. For the BA MHWs, there was a focus on using the optional modules in the manual to achieve this, whereas CBT therapists described a more core therapeutic skill of providing therapy using their own experience to gauge where to take each patient.
Despite recognising the ability of therapy to be adapted for individual needs, some therapists felt restricted by the treatment manuals and confused about how and when they could diverge from it. BA MHWs, in particular, expressed frustration at being unable to address negative thoughts and felt that some patients would have benefited from cognitive work. There was also anxiety about stepping outside the BA model and avoiding ‘therapeutic drift’ towards CBT:
We all had quite a lot of anxiety around following the protocol and making sure we didn’t step outside that and ‘Oh god don’t mention thoughts when you’re doing BA!’
T13 – BA, Durham
There was a view that the treatment manuals are not appropriate for everyone, and therapists could experience difficulty in treating patients whom they considered to have more complex problems, such as comorbid anxiety, personality disorders or a history of abuse, without working outside the treatment manual. This could be particularly challenging when therapists felt that, in usual practice, a patient would have been referred to secondary care or more specialist services:
It impacted on the ability to follow the COBRA-specific protocol because it didn’t feel appropriate if someone’s main thing was anxiety to keep plugging a depression model, so to keep a therapeutic alliance going it was kind of necessary to focus on the anxiety.
T08 – CBT, Devon
Some patients were considered to struggle with the overall approach and structure of BA and CBT, and therapists also recognised that both therapies can be demanding of patients, particularly with regards to the requirement for homework:
Getting people to analyse what they’re doing and getting people not just do more but start to look at activities and behaviours they can start to change. And I guess that’s asking quite a lot of people, especially people on the severe end of depression.
T06 – BA, Leeds
Confidence in delivery
A common theme discussed by many therapists was how confident they felt in delivering COBRA trial therapy. This included discussion on things that were seen to challenge their confidence, as well as remarks about how confidence changed or improved, and the specific role of training and supervision in helping to build confidence.
Challenges to confidence
There was a clear distinction in the way BA and CBT therapists talked about how COBRA trial treatment compared with their usual practice. BA MHWs talked extensively about COBRA trial therapy as a new, different way of working, which could be challenging and overwhelming for them, especially in the beginning. BA was considered a big jump from their previous work and required learning lots of new skills and techniques:
It was quite overwhelming in some respects, I think I went into it thinking that there wouldn’t be that much difference to what I was already doing, but actually once I did the training I realised that there was quite a big difference.
T13 – BA, Durham
A particular area in which BA MHWs lacked confidence was in making the transition from their prior role in guided self-help in which they considered themselves to be a ‘coach’ for patients, to becoming more collaborative in their relationship:
How you make that transition from just doing the kind of coaching style of PWP to this more therapeutic, collaborative, I mean it’s still collaborative at PWP but it’s a bit more in depth in the BA that we were doing, it was expected to be anyway.
T12 – BA, Leeds
For CBT therapists, however, COBRA trial treatment was considered familiar, and matched what they do in their day-to-day clinical work:
It was familiar for any cognitive therapist that’s kind of like bread and butter really, the Beck model.
T02 – CBT, Devon
Other areas where therapists lacked confidence included working with patients with chronic conditions, and a CBT therapist who considered herself to be more of a behavioural worker struggled to work more cognitively.
Improving confidence
Therapists in both treatments felt that they had learned a lot from their involvement in the COBRA trial. BA MHWs described learning new skills and techniques, with particular references to formulation, rumination and functional analysis. They described feeling more capable of delivering behavioural therapy and continuing to use it now as an alternative to cognitive work. For those therapists who struggled with confidence initially, this was considered to have improved over time and with good-quality training and supervision:
We had really good supervision, so I think the training was really good to set you up for what was to be expected and then once you got into it I think my confidence just started to build a lot more with the actual techniques.
T13 – BA, Durham
Cognitive–behavioural therapy therapists, on the other hand, talked about the trial as an opportunity to consolidate old knowledge, build on previous learning and ‘sharpen up’ their skills. Some described feeling more confident in delivering cognitive therapy for depression now, as well as greater confidence in specific areas such as doing better behavioural experiments, dealing with difficult patients or improved flexibility as a therapist:
I’ve got more confidence now in being truly Socratic and curious and flexible in my approach, rather than just chugging along with things . . . it got me to really make sure that I’m connecting with people.
T09 – CBT, Leeds
Role of training and supervision
Training and supervision was discussed extensively by therapists in both treatments. Training was described as great, helpful and comprehensive, but was also hard work. For CBT therapists the training week was seen as a ‘good refresher’, whereas for those delivering BA the training taught them lots of new skills. Having a week of in-depth training on one therapy was considered a positive experience by both BA and CBT therapists:
I learned a lot from the training and just things like learning about rumination, functional equivalence, functional analysis, things like that that I hadn’t learned about from the PWP course . . . having the chance to do a week in-depth about a particular treatment was really useful.
T04 – BA, Leeds
The training week, however, was also considered to be intense and overwhelming, particularly for those delivering BA. One BA MHW thought the training week would have benefited from more attention to core therapeutic skills such as collaborative working, which were considered to be a new way of working for them:
It was meant to be like proper therapy that’s very collaborative and we spend a lot more time doing all that Socratic questioning, trying to get people to get the answer themselves and it’s a different skill . . . maybe could have done more on that side of things in the training.
T12 – BA, Leeds
Supervision was described by therapists in both treatments as helpful, supportive and of high quality. Therapists described the supervisors as knowledgeable ‘experts’ and this was considered to be an invaluable experience which helped to build confidence:
Supervision from experts in the field as well . . . I’ve learned invaluable experience, I’ve sort of changed me as a practitioner as well.
T10 – BA, Durham
The quality of supervision was very good and so for me as a clinician that was very helpful I think, I certainly came out of the trial having felt a lot more confident and that still stays with me in providing CBT for depression.
T05 – CBT, Leeds
Having group supervision with other therapists was described as a beneficial process that allowed therapists to learn from each other. Taking audio-recordings of therapy sessions to supervision was a difficult experience for those in both treatments, but was also recognised as a useful practice from which a lot could be learnt:
In supervision, where our recordings are played out amongst the group of supervisees . . . I did find that difficult . . . but again, you can see how you can work through those things and I learned a hell of a lot in the supervision sessions because of that, and then it was great actually, I loved it in the end.
T01 – BA, Devon
The patients
This theme includes discussion of the patients treated in the trial, including how these patients compared with those they would see in usual practice, perceived cognitive and behavioural change for patients, and the broader impact of therapy on patients’ lives.
Comparisons with usual practice
There were two distinct views about how COBRA trial patients compared with patients treated in usual practice. Therapists located in Durham, whether delivering BA or CBT, did not consider COBRA trial patients to be any different from those treated in usual practice; there was a range of complexity and willingness to engage, which mirrors usual practice:
At step 2 we see more complex people, I don’t think for me there was any change with the COBRA people as such. Because some of them were mild to moderate but some of them were more complex.
T13 – BA, Durham
Therapists in Exeter and Leeds, on the other hand, tended to describe COBRA trial patients as having more complex difficulties and being more difficult interpersonally than patients seen in usual practice. No therapists described COBRA trial patients as being less complex than usual:
They were definitely more complex in terms of their history . . . some of them were quite different, I think, to what I’d usually be working with.
T09 – CBT, Leeds
Cognitive and behavioural change
Behavioural change was discussed extensively by therapists in both treatments. Therapy was believed to have led to significant changes in behaviour, and behavioural work was considered an important part of both BA and CBT. A common theme was that patients had increased their contact with pleasurable activities and re-engaged with the things they value:
You could actually see when they were talking about it how – oh, you know, it was just lovely to feel the water over her skin . . . some patients were very descriptive in explaining that positive reinforcing feeling of going back to an activity that they thought maybe they’d never try again.
T01 – BA, Devon
Cognitive–behavioural therapy therapists described many cognitive changes resulting from treatment, including overall changes to thinking style, less negative thinking and no longer predicting the worst to happen. Therapists described changes to patients’ beliefs about themselves such as reduced feelings of worthlessness, as well as changes to beliefs about others such as believing the world is not out to get them. There were two distinct views from CBT therapists about the role of cognitive change. Some believed this to be a big turning point for many patients, leading to improvements in overall quality of life, and that behavioural change came as a result of cognitive change. The other view, however, was that cognitive work was less important than behavioural work, and one therapist felt it was hard to differentiate cognitive and behavioural change:
The whole treatment is about that really, it’s trying to get people to change the way they think and get out of the kind of depressive thinking styles and then by doing that, that usually makes people more active and less avoidant.
T08 – CBT, Devon
Cognitive change was discussed much less frequently by BA MHWs than by those delivering CBT. For those who did discuss it, a common idea was that cognitive change came as a result of behavioural change:
When people start getting back to doing things that mean something to them and enjoying life, their cognitions naturally change . . . not always straight away, there was often a bit of a cognitive lag but it would catch up.
T06 – BA, Leeds
Patient outcomes
Therapists in both treatments considered therapy to have helped patients in broad and varied domains of life. Both BA and CBT were believed to have helped improve many patients’ mood and other symptoms of depression, including motivation, concentration, energy, sleep and feelings about themselves.
Many therapists felt that therapy had helped improve patients’ health and well-being. There was recognition that depression can have consequences for physical health, and a belief that BA and CBT could have a positive influence in this. Other improvements included patients taking better care of themselves, valuing themselves more and improvements to their overall appearance:
Some people reported feeling physically unwell as well as mentally unwell, and you could see a change in that, people’s energy levels and things.
T06 – BA, Leeds
Both therapies were considered to have helped patients sleep, with behavioural work believed to be particularly important for this. Other improvements included eating better, exercising more and reduced alcohol consumption. Therapists in both treatments described improvements to patients’ home and family lives as a result of therapy, as well as improved relationships with others:
People’s home lives often, you know, depression can take its toll on everybody, so people often reported that things were better at home.
T06 – BA, Leeds
A common theme across both BA and CBT was increased social contact and reduced social avoidance as a result of therapy. Increased social contact was believed to have had a range of positive effects on patients including improving confidence and, for those in CBT, reinforcing positive beliefs about themselves:
For a lot of my patients who I saw they were all – they’ve all been sociable people, they’re all from large families, they’ve enjoyed that and for whatever reason, they’ve lost it. And I think socialising reinforced to them that you are likeable, you do have a lot to contribute.
T11 – CBT, Durham
Therapists in both treatments also considered therapy to have helped patients at work, including getting back to work after a period of being signed off, getting a new job or being more productive at work:
One lady who struggled to get out of the door, over the doorstep, she actually, last year rang me to give her a reference to do some voluntary work . . . and that even now, it makes me feel really – ‘cos it was just fantastic! It’s life changing, isn’t it?
T11 – CBT, Durham
The results of the participant and therapist qualitative interviews will be discussed and interpreted in Chapter 5.
Chapter 5 Discussion and conclusions
This chapter uses material from an Open Access article previously published by the research team (see Richards et al. 2). © The Author(s). 2 Published by Elsevier Ltd. This is an Open Access article under the CC BY license.
Summary of findings
We found that BA for depression is not inferior to CBT in terms of reduction of depression symptoms and is cost-effective compared with CBT against commonly applied decision-maker willingness-to-pay thresholds. We observed our results using both ITT and PP analyses, using a conservative non-inferiority margin. Our economic outcomes were driven by the lower costs of the MHWs who delivered BA, compared with the more experienced psychological therapists who routinely deliver CBT. Our study results, therefore, substantiate the hypothesis that BA is as effective as CBT and that its simplicity renders BA suitable for delivery by junior MHWs with no professional training in psychological therapies. 19
Our process data found that, despite being challenging at times, BA and CBT were acceptable and feasible for participants, MHWs and therapists, and effected changes in people’s specific symptoms and in their lives more broadly. Despite experiencing initial difficulties that could be detected by some participants, with sufficient training, experience and supervision, junior MHWs could feel confident in delivering BA effectively. We found weak evidence for an interaction between treatment and baseline PHQ-9 score on PHQ-9 at 12 and 18 months’ follow-up, indicating that BA may be a better choice of treatment for patients with higher baseline PHQ-9 scores.
Summary of clinical outcomes
Both BA and CBT improved overall depression in the ITT and PP populations. At our primary end point of 12 months post randomisation, the mean PHQ-9 scores were 7.8 points (SD 6.5 points) in the BA group and 7.9 points (SD 7.3 points) in the CBT group, both below the commonly applied PHQ-9 threshold of 10 points associated with a diagnosis of MDD. At baseline, the mean PHQ-9 score for the BA group was 17.7 points (SD 4.8 points) and for the CBT group 17.4 points (SD 4.8 points), both groups being within the moderately severe depression range (15–20 points). Our results demonstrate the unequivocal non-inferiority of BA compared with CBT in both ITT and PP populations, as the between-group mean difference and 95% CIs lie firmly within our a priori non-inferiority margin of –1.9 PHQ-9 points (ITT mean difference: 0.1 PHQ-9 points, 95% CI –1.3 to 1.5 PHQ-9 points; p = 0.89; PP mean difference: 0.0 PHQ-9 points, 95% CI –1.5 to 1.6 PHQ-9 points; p = 0.99). Our results were robust to sensitivity analyses exploring the effect of different PP definitions, predefined subgroups and missing data.
In order to assist with clinical interpretation, we have also presented data that show there were no differences between groups in the proportions of participants responding to treatment or recovering from depression at 12 months post randomisation in either the ITT or PP analyses. In the PP population, 69–70% of trial participants were rated as recovered (PHQ-9 ≤ 9 points) and 64–66% as having responded to treatment (≥ 50% reduction in PHQ-9 score from baseline). The equivalent figures for the ITT population were 66% and 61–62%. These outcomes were maintained at 18 months’ follow-up. We observed very similar patterns for all of our secondary outcomes, including anxiety (as measured via the GAD-7 questionnaire) and health-related quality of life (as measured via the SF-36 questionnaire). We could not, therefore, detect any differential treatment effect on any clinical outcomes between BA and CBT as treatment for depression.
Summary of economic outcomes
We found that resource use and resultant costs were similar for both BA and CBT groups at our primary economic end point of 18 months. The only significant difference between groups was in mean intervention costs between the two groups. There were no significant differences in other categories of cost or in overall total cost. Although health-related quality of life was slightly higher in the BA group than in the CBT group across the entire follow-up period, with resultant QALYs also higher for BA, the QALY difference was not significant. Nonetheless, because observed costs were lower and QALY outcomes better in the BA group than in the CBT group, this generated an ICER of –£6865, suggesting that BA dominates CBT (i.e. BA is both cheaper and more effective). The probability of BA being cost-effective compared with CBT does not fall < 75% and is closer to 80% at standard NICE willingness-to-pay thresholds of £20,000–30,000 per QALY. Once again, these findings were robust to sensitivity analyses using both broader and narrower cost perspectives and analysing the impact of missing data. Our findings are therefore robust in suggesting that BA is cost-effective compared with CBT, driven principally by the lower costs of employing junior MHWs to deliver this simpler treatment.
Summary of process evaluation
Our process analyses indicated a moderating effect (statistically significant interaction) of baseline PHQ-9 score on treatment effect, with regard to PHQ-9 at 12 and 18 months’ follow-up, although the evidence for such an effect was weak in both cases. Our analysis further suggested that BA may be a better choice of treatment for patients with higher baseline PHQ-9 scores. No significant differences were found between the BA and CBT groups with regard to the process mediators, proportion of therapy sessions attended and overall treatment fidelity, although basic treatment fidelity was found to be higher in the BA group. The only statistically significant mediation effects were that overall treatment fidelity mediated the effect of treatment (BA vs. CBT) on PHQ-9 at 12 months’ follow-up, with basic and overall treatment fidelity mediating the effect of treatment on PHQ-9 at 18 months’ follow-up. However, these statistically significant results were only seen in the models that included only one variable acting as a mediator of treatment effect (as opposed to models that included several potential mediators). In terms of the proportion of overall treatment effect mediated, basic treatment fidelity accounted for a high proportion of treatment effect mediated at both the 12- and 18-month follow-ups.
In terms of qualitative data, BA and CBT were considered acceptable by patients, MHWs and therapists. People liked the fact that the therapy offered them someone to talk to, gave them tools and techniques to enable them to help themselves and was an opportunity for them to learn. This is consistent with previous qualitative studies of change processes in cognitive therapy, which have found that patients value both specific cognitive techniques (such as changing negative thoughts), as well as general psychotherapy ingredients (such as a collaborative therapeutic relationship and the opportunity to learn). 105–107 Participants appreciated that therapy was long and regular and that they were not asked to spend a lot of time focusing on the past, although some would have liked more time to talk about their feelings. Homework was considered an important part of treatment for participants, allowing them to gain control of their feelings, but it could also be challenging. This is consistent with previous research in which patients receiving CBT for depression reported finding homework difficult for both emotional and practical reasons, but that they understood its necessity in the therapeutic process. 108 Therapists and MHWs, in particular, appreciated working longer term with patients than in usual IAPT practice and believed this to be beneficial both for themselves and for patients. Aspects of participants’ personal lives, such as work and family, could make therapy challenging, and having other conditions like chronic pain or anxiety were considered by some to be a barrier to treatment. Depression itself could also be a barrier, impacting on participants’ ability to ‘do’ and making it hard to focus or understand therapy.
Clinically, in some cases, both MHWs and patients expressed the opinion that MHW practice could appear to be somewhat rigid and that MHWs might lack confidence in their new BA role, although this had no impact on our main finding of clinical non-inferiority of BA compared with CBT. Furthermore, the finding that treatment fidelity mediated outcome suggests that adherence to the treatment protocol has more beneficial than deleterious effects. Nonetheless, strategies to boost both the appearance and actuality of junior MHW practice will be considered later in this discussion.
Strengths and limitations
The COBRA trial is the largest trial of BA to date and is one of the largest trials of psychological treatments for depression. We followed up participants for 18 months and our economic analysis is one of few in this field. Therapists and MHWs working in three different routine UK care settings delivered treatment, providing evidence of potential generalisability. We assessed therapy quality using independent raters and ensured that treatment in both arms was delivered to the standard recommended in guidelines. Given the nature of the intervention and comparator we could not mask patients or the MHWs or therapists who were delivering the interventions to treatment allocation, but we used self-reported outcome measures and robust outcome assessor-masking procedures to reduce researcher unmasking to < 5%.
At 21% and 14% for BA and CBT, respectively at 12 months, our levels of attrition and outcome loss to follow-up were low at 12 and 18 months, similar to other trials in this area, but are still a limitation. Although we found a between-group difference in attrition for the ITT analyses, this was not the case for the PP analyses, suggesting that any differential attrition was an artefact of the trial and not of the treatments. Furthermore, our between-group inferences were robust to data imputation. Although participants in the PP population attended similar numbers of sessions to those in other CBT trials, 35% of participants overall chose to not attend a minimal number of sessions, a problem well known to routine psychological therapies services.
This pragmatic trial carried out in routine environments means we were unable to quantify or control for the contribution of ADM on outcomes. However, most participants who were taking medication had been doing so for a considerable time before entering the trial, making it unlikely that our results were driven by pharmacological treatment.
In terms of competency ratings, the ratings of our random sample of therapy tapes showed that both CBT therapists and BA MHWs were, on average, performing above competency thresholds. In terms of CBT, our mean sessional competency ratings (37.9) were very similar to the means reported in the CoBalT trial,55 another significant and large recent UK trial of CBT – 38.8, demonstrating that our therapists were achieving competency levels consistent with other similar pragmatic effectiveness studies. However, on this random sample of tapes some therapists were scoring below the threshold of competence for that specific session as assessed by our external raters, although we would stress that all therapists had exceeded the competence threshold at the end of their COBRA-specific protocol training course.
We note that our CBT therapists had received 1 year of postgraduate training in CBT, had passed similar competency tests in order to qualify from these courses, had received an additional specialist training in the COBRA protocol and were supervised by CBT experts. Most importantly, they were NHS employees engaged in routine treatment for patients with depression in the UK NHS IAPT services; indeed they were working for the NHS alongside their trial duties. Finally, the competency ratings from the CBT arm in our trial are consistent with other research findings in the field. 12,40 Therefore, although it may be possible to train CBT therapists to achieve higher competency ratings, we suggest that these levels of competence are those actually seen in routine clinical practice in IAPT services in the UK, results achieved following substantial investment in therapists’ clinical training, and appear unrelated to clinical outcomes.
Furthermore, although the measures of competence for the BA MHWs and the CBT therapists are not directly comparable, we note that the MHWs scored, on average, further above the competency threshold than the CBT therapists on the relevant competency measure. This provides some, albeit weak and indirect, supporting evidence for the proposition that it might be easier to train people to be competent BA workers than CBT therapists, although this is moderated by the difficulties with the unknown psychometric properties of the BA competence measure in particular and we did not set out to test this proposal directly.
The period between baseline and the 6-month follow-up was the period in which the main treatment effect occurred; therefore, it is during this period that any mediation effect would be of greatest interest. However, there may be difficulties in assessing the effect of potential mediators during this period because of the variation in such mediators at the times when recorded during the first 6 months of treatment. Such variation may be attributable to differences in the pace of therapy in terms of how quickly the patients completed their sessions and engaged with the topics relevant to their therapy. At later stages of follow-up, with regard to both the outcome and the mediator variables, it may be the case that the mediator variables had become more stable and therefore better able to facilitate mediation analysis.
The wide variation among patients in their trajectories across time points within the follow-up period, with regard to PHQ-9 and each mediator variable, may also be an impediment to distinguishing possible mediation effects. However, little difference was noted between the CBT and BA groups in terms of the mediating psychological variables, possibly indicating little practical difference in how participants responded to the two forms of therapy. Owing to differences in the structure of therapy, the BA group scored more highly for measures of treatment fidelity, although the proportion of sessions attended was similar across the two treatment groups.
Considering differences in the pattern of mediation across outcome follow-up times, at the earlier follow-up time of 6 months, the indirect effects of the mediators appeared to be accounting for only small proportions of the total treatment effect. This may reflect lack of stability in the early phases of the trial, while many participants were still undergoing therapy, with regard to both PHQ-9 and mediator scores.
In terms of moderators and mediators, COBRA was a large study that incorporated the collection of several psychological variables that were potential mediators of treatment effect; these variables were collected at several time points during the follow-up period of the trial. Also, the primary outcome, PHQ-9, was collected at three time points (at 6, 12 and 18 months’ follow-up). The collection of data for several psychological mediators at multiple time points has facilitated the comparison of how the two treatment groups progressed over the study period, at the group level and at the individual participant level. However, the large number of comparisons performed as a result of this brings about concerns regarding multiple testing and the possibility of finding statistically significant results by chance. With regard to the mediation analyses, only three statistically significant results were found when including a single mediator in each model, one relating to PHQ-9 measured at the 12-month follow-up and two at the 18-month follow-up. These results are consistent with the expectation of significant results occurring by chance. Therefore, these results should be viewed with caution.
Similarly, of the 42 inferential tests for moderation across the ITT and PP populations, and the three follow-up time points, none were found to be statistically significant at a p-value of < 0.05, although three moderation analyses yielded a p-value of < 0.1. In view of the low power to detect interaction effects, and in view of the number of tests performed, these possible interaction effects should also be viewed with caution.
The COBRA trial was the largest trial to date comparing CBT and BA and enabled an in-depth qualitative analysis of patients’ and therapists’/MHWs’ experiences of these treatments alongside the clinical effectiveness, cost-effectiveness and quantitative process analyses. To date, there have been very few qualitative studies of this. Our purposive sampling method for our participant interviews ensured diversity in our sample and we successfully interviewed participants from each recruitment site, both therapies, some who completed the full course/ended treatment early and some who were still depressed/no longer depressed, as well as both male and female participants across a range of ages. Similarly, we interviewed therapists/MHWs from both treatments, from all three recruitment sites and with a range of backgrounds and experience. However, the generalisability of our findings are limited to participants who were both eligible and willing to participate in the COBRA trial, and participants who declined to take part in the qualitative study may also have had different views to those who agreed to be interviewed. Likewise, qualitative interviews were only completed with 13 of the 22 therapists/MHWs involved in the trial, and those who did not respond to the invitation to be interviewed may have had different experiences to those who did respond.
All interviews were carried out over the telephone as a result of the long-distance nature of cross-site interviewing. This maintained researcher blindness for participant quantitative follow-ups and was crucial for the integrity of the COBRA trial. Although some researchers have proposed that telephone interviewing may be less effective than face to face,109 evidence suggests that telephone interviews yield the same number and quality of data as those conducted face to face,110 and some argue that telephone interviewing may even be preferable when participants are discussing sensitive topics. 111 Finally, although we aimed to interview participants as soon as possible after completion of therapy, in practice this was difficult because of delays in obtaining information about therapy end dates, delays in the ability to recruit participants quickly after this time point, and an overall difficulty recruiting participants who had fewer than eight sessions of therapy, resulting in an extension of our qualitative recruitment period. A small number of participants commented that at the time of their interview they found it difficult to remember specific aspects of their treatment, and it is possible that participants who were interviewed soon after therapy completion may have had different reflections on their experiences from those interviewed some time later.
We recruited participants from primary care, rather than specialist settings. Our results are, therefore, applicable to the great majority of patients with depression in these settings. We excluded people who had other major psychiatric diagnoses, people who are most likely to be found in specialist mental health services. We did so because, notwithstanding their depression, people with diagnoses such as addictive disorders, bipolar disorder or psychosis require specialist treatment for these disorders as their primary psychiatric input. We excluded people with these conditions on the understanding that psychological treatment for their depression would not be the first line of treatment offered. Therefore, for this population, our results may not be generalisable.
Implications for health care
Our findings could have substantial implications for the scalability of psychological treatment for depression in the UK and internationally,17 given the greater availability and ease with which a BA workforce could be trained than could a CBT workforce. For many years, CBT has been the foremost psychological therapy recommended by therapists, researchers and policy-makers. Our results challenge this dominance. Although more work needs to be done than has been undertaken so far to find ways to effectively treat the 20–30% of participants whose depression was unchanged by BA or CBT, our findings suggest that BA should be a front-line treatment for depression, with significant potential to improve reach and access to psychological therapy globally.
Our results in both groups compare favourably with a meta-analysis112 of the effects of CBT compared with second-generation ADM. This analysis found no difference between ADM and CBT on rates of remission between 12 and 16 weeks post randomisation (ADM, 40.7%; CBT, 47.9%; risk ratio 0.98, 95% CI 0.73 to 1.32). For comparison purposes, our ITT recovery rates (synonymous with remission in the meta-analysis) at 18 months post randomisation were 66% for BA and 59% for CBT. Equally, in the meta-analysis referred to above, response rates were 44.2% for ADM and 45.5% for CBT (risk ratio 0.91, 95% CI 0.77 to 1.07); for comparison, 61% of our participants receiving BA and 60% of those receiving CBT had responded at 18 months. Thus, the proportion of participants in both treatment arms who experienced long-term positive clinical outcomes was higher in our study than in this recent meta-analysis.
Our cost-effectiveness analyses show the high probability that BA is cost-effective and affordable compared with CBT at standard willingness-to-pay thresholds. Our most striking finding is that BA leads to comparable clinical outcomes for patients with depression, but at a financial saving to clinical providers of 21% compared with the cost of provision of CBT, with no compensatory use of other health-care services by patients.
Driving these savings is the fact that BA can be delivered by inexperienced MHWs with no professional training in psychological therapies, with no lesser effect than that of more highly trained and experienced psychological therapists giving patients CBT. Although training of MHWs is only one of many obstacles to successful dissemination, our findings suggest that health services globally could reduce the need for costly professional training and infrastructure, reduce waiting times and increase access to psychological therapies. 17 Our findings have substantial implications given the increasing global pressure for cost containment across health systems in high-income countries, and the need to develop accessible, scalable interventions in low- and medium-income countries. Such countries might choose to investigate the training and employment of junior workers over expensive groups of psychological professionals. Our results, therefore, offer hope to many societies, cultures and communities worldwide, rich and poor, struggling with the effect of depression on the health of their people and economies.
The results of the moderation analysis did not provide any strong evidence for differential treatment effect across subgroups of participants, although there was some weak evidence for a stronger beneficial effect of BA among participants who were more severely depressed at baseline. This finding is consistent with a previous study,40 and may indicate that, clinically, BA would be the preferred choice of therapy for more severely depressed patients.
The mediation analyses indicated that the quality of therapy, with regard to coverage of core topics, is the strongest mediator of treatment effect. Hence, this may provide guidance to therapists that they should concentrate on including the core topics within their therapy sessions. The mediation effects of the psychological mediators appear to be weaker than those of the mediators related to therapy fidelity, and clear findings with regard to the mediation effects of the psychological mediators were not observed.
Our qualitative findings suggest that, despite BA being non-inferior to CBT in terms of clinical outcomes, junior MHWs delivering BA could feel somewhat overwhelmed by the new skills they were learning and felt an initial lack of confidence in their ability to deliver therapy effectively. This lack of confidence was unique to BA, and was able to be detected by some patients. This is unsurprising given the new role that these workers were being asked to undertake, unlike CBT therapists, who had already been trained and were experienced in delivering CBT. BA delivered by therapeutically naive junior MHWs, therefore, requires good-quality training, time for therapists to build confidence through practice and ongoing supervision from experienced BA practitioners. Indeed, therapists/MHWs in both groups described training and supervision positively, and the model of support provided in the COBRA trial appears to be effective and well regarded by those delivering therapy. In particular, having a week of in-depth training, group supervision with peers and playing out of audio-clips from therapy during supervision were elements of the COBRA trial support model that were regarded as helpful, and should be considered for future implementation of BA and CBT for depression.
For CBT therapists, the COBRA trial treatment manual was generally considered to have been useful, although there was also anxiety about the lack of flexibility permitted. When training therapists to follow a therapeutic manual such as those used in the COBRA trial, therapists should not be too flexible in their clinical practice at the expense of overall treatment fidelity, since our quantitative process analysis demonstrated that fidelity predicts clinical outcomes. In addition, as outcomes for CBT in the COBRA trial were superior to those seen in IAPT services,113 where therapists are not trained to follow a particular depression treatment manual, consideration should be given to whether or not it would be beneficial to routinely use such a treatment manual in IAPT.
Relapse prevention was an element of both treatments that was valued highly by therapists/MHWs and patients. This is important because research has shown that, even when patients make significant clinical improvements during therapy, they expect themselves to remain susceptible to depression and continue to implement techniques learnt in therapy as a way of managing what they consider to be a chronic condition. 114 Previous work has also suggested that the long-term effects of CBT are attributable to patients learning skills that they can continue to implement after therapy has ended. 115,116 Relapse prevention is therefore a crucial component of both therapy protocols, which should continue to be implemented.
Implications for future research
Despite the encouraging nature of both our overall treatment outcomes and the cost-effectiveness and non-inferiority of BA compared with CBT, there remain three issues that should be priorities for research. The first is how to engage the considerable number of people with depression who either do not start, or rapidly drop out of, psychological treatment. Neither treatment in our trial performed better than the other in terms of this engagement and retention. Multiple contextual, specific and common therapeutic factors may contribute to the hypothesis that some patients find psychological therapy either generically undesirable, impractical or specifically inappropriate to their needs. It is unlikely that there is one simple solution – for example, psychological treatments with a different theoretical orientation – that will overcome these diverse factors impeding patient engagement. Nonetheless, research into ways in which patients can be engaged more fully could be considered.
Second, even for the PP population, around 30% of people did not experience a clinically relevant change in their depression symptoms, whether they received BA or CBT. It remains a possibility that matching patients to treatments by specific patient-level moderator variables, as has been hypothesised by some researchers,117 would lead to better outcomes. Unfortunately, in terms of depression severity, although a phenomenon previously observed,40 our study has only provided weak evidence to support the hypothesis that BA has a stronger therapeutic effect for patients who are more severely depressed at the onset of treatment. In terms of potentially enhancing the mediating effect of a range of psychological variables, despite previous studies of CBT55 reporting the mediating effect of changes in dysfunctional attitudes and metacognitive awareness, we were unable to demonstrate any substantial effect of psychological mediators on outcome. We are unable to recommend, therefore, that therapists enhance their therapeutic focus on these areas with any guarantee of improving outcomes for patients. Our only substantive finding that fidelity to a clinical protocol mediates outcome in both BA and CBT merely emphasises the importance for MHWs and therapists of following an evidence-based clinical treatment closely in their work. Much more prospective research using precision medicine approaches such as the Personalised Advantage Index117 to test the potential of matching treatments to individual patient characteristics seems to be warranted.
Third, Kanter and Puspitasari118 have noted that, ‘now that we have support for BA as a treatment that is clinically effective and cost-effective, we can shift our efforts to focus on what is necessary to produce sustainable large-scale BA implementation across diverse geographical and cultural settings’. 118 The central rationale of BA, now supported by our COBRA trial results, is that it is also a simple treatment suitable for widespread dissemination beyond high-income countries. Knowledge for the sustainable dissemination and implementation of BA as an effective health technology to low- and medium-income countries remains suboptimal. Although there have been a number of studies in low- and medium-income countries, including India, Iran and Iraq,119–121 the outcome data are equivocal as these studies either do not use an optimum clinical protocol tested in RCTs, are underpowered, incorporate BA as one part of a multicomponent complex intervention, or focus on very tightly defined populations. Nonetheless, these international studies do provide some evidence for the cross-cultural face validity of BA. 118 We now need to identify the low- and medium-income countries workforce best able and available to deliver this simple treatment. We need to determine if the face validity of BA can go some way to overcoming mental health stigma and people’s reluctance to engage in treatment in different cultures. We need to engage with organisations and cultures unused to the concept of evidence-based mental health therapies. Finally, we need to apply our scientific methods in low and middle income countries contexts, not least our health economic methods, as these data will drive recommendations to implement BA widely or not.
In summary, future research should focus on strategies to improve the initial engagement of depressed people with psychological therapies, should examine ways to personalise and optimise the allocation of a range of evidence-based treatments, and should take an implementation science approach to dissemination and reach outside the high-income countries in which BA in particular has been developed and tested.
Acknowledgements
We would like to thank all participants, NHS services, MHWs, therapists and GPs involved in the study and acknowledge the vital contributions of study researchers and administrators in Devon, Durham and Leeds, the PenCTU and the NIHR Clinical Research Network.
Contributions of authors
Professor David A Richards (Professor of Mental Health Services Research) was chief investigator, designed the study, was responsible for its conduct, and contributed to the writing and editing of the report.
Dr Shelley Rhodes (Trial Manager) was responsible for study management and data collection, and contributed to the writing and editing of the report.
Dr David Ekers (Nurse Consultant Primary Care Mental Health/Senior Visiting Research Fellow) designed the study, was responsible for its conduct, and contributed to the writing and editing of the report.
Dr Dean McMillan (Senior Lecturer) designed the study, was responsible for its conduct, and contributed to the writing and editing of the report.
Professor Rod S Taylor (Professor of Health Services Research) designed the study, was responsible for its conduct, undertook data analysis, and contributed to the writing and editing of the report.
Professor Sarah Byford (Professor of Health Economics) designed the study, was responsible for its conduct, undertook data analysis, and contributed to the writing and editing of the report.
Dr Barbara Barrett (Senior Lecturer, Health Services and Population Research) undertook data analysis, and contributed to the writing and editing of the report.
Katie Finning (Associate Research Fellow) was responsible for study management and data collection, undertook data analysis, and contributed to the writing and editing of the report.
Poushali Ganguli (Research Associate, Health Services and Population Research) undertook data analysis, and contributed to the writing and editing of the report.
Dr Fiona Warren (Lecturer in Medical Statistics) undertook data analysis, and contributed to the writing and editing of the report.
Dr Paul Farrand (Associate Professor) designed the study, was responsible for its conduct, and contributed to the writing and editing of the report.
Professor Simon Gilbody (Director of the Mental Health and Addictions Research Group) designed the study, was responsible for its conduct, and contributed to the writing and editing of the report.
Professor Willem Kuyken (Professor of Clinical Psychology) designed the study, was responsible for its conduct, and contributed to the writing and editing of the report.
Dr Heather O’Mahen (Senior Lecturer in Clinical Psychology) designed the study, was responsible for its conduct, and contributed to the writing and editing of the report.
Professor Ed Watkins (Professor of Experimental and Applied Clinical Psychology) designed the study, was responsible for its conduct, and contributed to the writing and editing of the report.
Dr Kim Wright (Senior Lecturer) designed the study, was responsible for its conduct, and contributed to the writing and editing of the report.
Nigel Reed (PPI representative) provided expert advice on PPI, and contributed to the writing and editing of the report.
Emily Fletcher (Trial Manager) was responsible for study management and data collection, and contributed to the writing and editing of the report.
Professor Steven D Hollon (Gertrude Conaway Vanderbilt Professor of Psychology) provided expert advice on clinical aspects of cognitive–behavioural therapy, and contributed to the writing and editing of the report.
Dr Lucy Moore (Research Fellow) undertook data analysis, and contributed to the writing and editing of the report.
Amy Backhouse (Associate Research Fellow) contributed to data collection, and contributed to the writing and editing of the report.
Claire Farrow (Associate Research Fellow) contributed to data collection, and contributed to the writing and editing of the report.
Julie Garry (Associate Research Fellow) contributed to data collection, and contributed to the writing and editing of the report.
Deborah Kemp (Associate Research Fellow) contributed to data collection, and contributed to the writing and editing of the report.
Faye Plummer (Associate Research Fellow) contributed to data collection, and contributed to the writing and editing of the report.
Faith Warner (Associate Research Fellow) contributed to data collection, and contributed to the writing and editing of the report.
Rebecca Woodhouse (Associate Research Fellow) contributed to data collection, and contributed to the writing and editing of the report.
All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Publications
Rhodes S, Richards DA, Ekers D, McMillan D, Byford S, Farrand PA, et al. Cost and outcome of behavioural activation versus cognitive behaviour therapy for depression (COBRA): study protocol for a randomised controlled trial. Trials 2014;15:29.
Richards DA, Ekers D, McMillan D, Taylor RS, Byford S, Warren FC, et al. Cost and outcome of behavioural activation versus cognitive–behavioural therapy for depression (COBRA): a randomised, controlled, non-inferiority trial. Lancet 2016;388:871–80.
Finning K, Richards DA, Moore L, Ekers D, McMillan D, Farrand PA, et al. Cost and outcome of behavioural activation versus cognitive–behavioural therapy for depression (COBRA): a qualitative process evaluation. BMJ Open 2017;7:e014161.
Data sharing statement
The authors confirm that all data underlying the findings are fully available without restriction. The authors have made the clinical and economic data set available through the University of Exeter’s Institutional Repository – Open Research Exeter (see https://ore.exeter.ac.uk). Access to these data is permitted but controlled through requests made via the repository to the chief investigator (Professor Richards: d.a.richards@exeter.ac.uk). Although use is permitted, this will be on the basis that the source of the data is acknowledged (including the funder) and it includes reference to the data set ‘handle’.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Rhodes S, Richards D, Ekers D, McMillan D, Byford S, Farrand P, et al. Cost and outcome of behavioural activation versus cognitive behaviour therapy for depression (COBRA): study protocol for a randomised controlled trial. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-29.
- Richards DA, Ekers D, McMillan D, Taylor RS, Byford S, Warren FC, et al. Cost and outcome of behavioural activation versus cognitive behavioural therapy for depression (COBRA): a randomised, controlled, non-inferiority trial. Lancet 2016;388:871-80. https://doi.org/10.1016/S0140-6736(16)31140-0.
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet 1997;349:1498-504. https://doi.org/10.1016/S0140-6736(96)07492-2.
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095-105. https://doi.org/10.1001/jama.289.23.3095.
- Singleton N, Bumpstead R, O’Brien M, Lee A, Meltzer H. Psychiatric morbidity among adults living in private households, 2000. Int Rev Psychiatry 2003;15:65-73. https://doi.org/10.1080/0954026021000045967.
- Andrews G, Henderson S, Hall W. Prevalence, comorbidity, disability and service utilisation. Overview of the Australian National Mental Health Survey. Br J Psychiatry 2001;178:145-53. https://doi.org/10.1192/bjp.178.2.145.
- Keller MB. Long-term treatment of recurrent and chronic depression. J Clin Psychiatry 2001;62:3-5.
- Layard R. The Depression Report: A New Deal for Depression and Anxiety Disorders. London: London School of Economics; 2006.
- Bloom DE, Cafiero ET, Jané-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, et al. The Global Economic Burden of Noncommunicable Diseases. Geneva: World Economic Forum; 2011.
- Depression: The Treatment and Management of Depression in Adults (Update) CG90. London: NICE; 2009.
- Bird A. We Need to Talk: The Case for Psychological Therapy on the NHS. London: Mental Health Foundation; 2006.
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry 2005;62:409-16. https://doi.org/10.1001/archpsyc.62.4.409.
- Fava GA, Rafanelli C, Grandi S, Conti S, Belluardo P. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry 1998;55:816-20. https://doi.org/10.1001/archpsyc.55.9.816.
- Harris MG, Burgess PM, Pirkis JE, Slade TN, Whiteford HA. Policy initiative to improve access to psychological services for people with affective and anxiety disorders: population-level analysis. Br J Psychiatry 2011;198:99-108. https://doi.org/10.1192/bjp.bp.109.073650.
- Richards DA, Borglin G. Implementation of psychological therapies for anxiety and depression in routine practice: two year prospective cohort study. J Affect Disord 2011;133:51-60. https://doi.org/10.1016/j.jad.2011.03.024.
- Hollon SD, Munoz RF, Barlow DH, Beardslee WR, Bell CC, Bernal G, et al. Psychosocial intervention development for the prevention and treatment of depression: promoting innovation and increasing access. Biol Psychiatry 2002;52:610-30. https://doi.org/10.1016/S0006-3223(02)01384-7.
- Kohn R, Saxena S, Levav I, Saraceno B. The treatment gap in mental health care. Bull World Health Organ 2004;82:858-66.
- Ferster CB. A functional analysis of depression. Am Psychol 1973;28:857-70. https://doi.org/10.1037/h0035605.
- Jacobson NS, Martell CR, Dimidjian S. Behavioral activation treatment for depression: returning to contextual roots. Clin Psychol Sci Pract 2001;8:255-70. https://doi.org/10.1093/clipsy.8.3.255.
- Lewinsohn P, Friedman RKM. The Psychology of Depression: Contemporary Theory and Research. London: John Wiley and Sons; 1974.
- Martell C, Addis M, Jacobson N. Depression in Context: Strategies for Guided Action. New York, NY: Norton and Co; 2001.
- Addis EMC. Overcoming Depression One Step at a Time. Oakland, CA: New Harbinger; 2004.
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York, NY: Guilford Press; 1979.
- Kuyken W, Tsivrikos D. Therapist competence, comorbidity and cognitive-behavioral therapy for depression. Psychother Psychosom 2009;78:42-8. https://doi.org/10.1159/000172619.
- Ekers D, Richards D, Gilbody S. A meta-analysis of randomized trials of behavioural treatment of depression. Psychol Med 2008;38:611-23. https://doi.org/10.1017/S0033291707001614.
- Ekers D, Webster L, Van Straten A, Cuijpers P, Richards D, Gilbody S. Behavioural activation for depression; an update of meta-analysis of effectiveness and sub group analysis. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0100100.
- Shinohara K, Honyashiki M, Imai H, Hunot V, Caldwell DM, Davies P, et al. Behavioural therapies versus other psychological therapies for depression (review). Cochrane Database Syst Rev 2013;10. https://doi.org/10.1002/14651858.CD008696.pub2.
- Hunot V, Moore TH, Caldwell DM, Furukawa TA, Davies P, Jones H, et al. ‘Third wave’ cognitive and behavioural therapies versus other psychological therapies for depression. Cochrane Database Syst Rev 2013;10. https://doi.org/10.1002/14651858.CD008704.pub2.
- Wilson P, Goldin J, Charbonneau P. Comparative efficacy of behavioral and cognitive treatments of depression. Cognit Ther Res 1983;7:111-24. https://doi.org/10.1007/BF01190064.
- Taylor F, Marshall W. Experimental analysis of a cognitive–behavioural therapy for depression. Cognit Ther Res 1977;1:59-72. https://doi.org/10.1007/BF01173505.
- Gallagher D, Thompson LW. Treatment of major depressive disorder in older adult outpatients with brief psychotherapies. Psychother Theory Res Pract 1982;19:482-90. https://doi.org/10.1037/h0088461.
- Maldonado Lopez A. Behavioural therapy and depression. Rev Psicol General Apl 1982;37:31-56.
- Maldonado Lopez A. Behavioural therapy and depression: an experimental analysis of the interaction between cognitive and behavioural therapies and pharmacological therapy in depressed people. Rev Psicol General Apl 1984;39:517-35.
- Skinner D. Self-control of depression: a comparison of behaviour therapy and cognitive behaviour therapy. Dissertation Abstracts International 1984;45.
- McNamara K, Horan J. Experimental construct validity in the evaluation of cognitive and behavioural treatments for depression. J Couns Psychol 1986;33:23-30. https://doi.org/10.1037/0022-0167.33.1.23.
- Thompson L, Gallagher D, Breckenridge J. Comparative effectiveness of psychotherapies for depressed elders. J Consult Clin Psychol 1987;55:385-90. https://doi.org/10.1037/0022-006X.55.3.385.
- Scogin F, Jamison C, Gochneaur K. Comparative efficacy of cognitive and behavioral bibliotherapy for mildly and moderately depressed older adults. J Consult Clin Psychol 1989;57:403-7. https://doi.org/10.1037/0022-006X.57.3.403.
- Jacobson NS, Dobson KS, Truax PA, Addis ME, Koerner K, Gollan JK, et al. A component analysis of cognitive-behavioral treatment for depression. J Consult Clin Psychol 1996;64:295-304. https://doi.org/10.1037/0022-006X.64.2.295.
- McKendree-Smith N. Cognitive and Behavioural Bibliotherapy for Depression: An Examination of Efficacy and Mediators and Moderators of Change 1998.
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol 2006;74:658-70. https://doi.org/10.1037/0022-006X.74.4.658.
- Ekers D, Richards D, McMillan D, Bland JM, Gilbody S. Behavioural activation delivered by the non-specialist: phase II randomised controlled trial. Br J Psychiatry 2011;198:66-72. https://doi.org/10.1192/bjp.bp.110.079111.
- Jones B, Jarvis P, Lewis JA, Ebbutt AF. Trials to assess equivalence: the importance of rigorous methods. BMJ 1996;313:36-9. https://doi.org/10.1136/bmj.313.7048.36.
- Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJW. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 2006;295:1152-842. https://doi.org/10.1001/jama.295.10.1152.
- Briefing Notes for Researchers: Public Involvement in NHS, Public Health and Social Care Research. Eastleigh: INVOLVE; 2012.
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Löwe B, Unützer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care 2004;42:1194-201. https://doi.org/10.1097/00005650-200412000-00006.
- Snapinn SM. Noninferiority trials. Curr Control Trials Cardiovasc Med 2000;1:19-21. https://doi.org/10.1186/CVM-1-1-019.
- Guideline on the Choice of the Non-inferiority Margin. London: European Medicines Agency; 2005.
- Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psychol 2008;76:966-78. https://doi.org/10.1037/a0013786.
- Baldwin SA, Murray DM, Shadish WR, Pals SL, Holland JM, Abramowitz JS, et al. Intraclass correlation associated with therapists: estimates and applications in planning psychotherapy research. Cogn Behav Ther 2011;40:15-33. https://doi.org/10.1080/16506073.2010.520731.
- Richards DFP, Chellingsworth M. National Curriculum for the Education of Psychological Wellbeing Practitioners (PWPs). London: Department of Health; 2011.
- DeRubeis RJ, Brotman MA, Gibbons CJ. A conceptual and methodological analysis of the nonspecifics argument. Clin Psychol Sci Prac 2005;12:174-83. https://doi.org/10.1093/clipsy.bpi022.
- Beck J. Cognitive Therapy: Basics and Beyond. New York, NY: Guilford Press; 1995.
- Wiles N, Thomas L, Abel A, Ridgway N, Turner N, Campbell J, et al. Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet 2013;381:375-84. https://doi.org/10.1016/S0140-6736(12)61552-9.
- Watkins ER, Mullan E, Wingrove J, Rimes K, Steiner H, Bathurst N, et al. Rumination-focused cognitive-behavioural therapy for residual depression: phase II randomised controlled trial. Br J Psychiatry 2011;199:317-22. https://doi.org/10.1192/bjp.bp.110.090282.
- Gilbody S, Richards D, Barkham M. Diagnosing depression in primary care using self-completed instruments: UK validation of PHQ-9 and CORE-OM. Br J Gen Pract 2007;57:650-2.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. https://doi.org/10.1001/archinte.166.10.1092.
- Ware JESK, Kosinki M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Centre; 1993.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Kessler RC, Barber C, Birnbaum HG, Frank RG, Greenberg PE, Rose RM, et al. Depression in the workplace: effects on short-term disability. Health Aff 1999;18:163-71. https://doi.org/10.1377/hlthaff.18.5.163.
- Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, et al. The effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse/recurrence: results of a randomised controlled trial (the PREVENT study). Health Technol Assess 2015;19. https://doi.org/10.3310/hta19730.
- Kessler RC, Barber C, Beck A, Berglund P, Cleary PD, McKenas D, et al. The World Health Organization Health and Work Performance Questionnaire (HPQ). J Occup Environ Med 2003;45:156-74. https://doi.org/10.1097/01.jom.0000052967.43131.51.
- Kind P, Spilker B. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia, PA: Lippincott-Raven; 1996.
- Dolan PGC, Kind P, Williams A. A Social Tariff for EuroQoL: Results from a UK General Population Survey. York: University of York; 1995.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. https://doi.org/10.1002/hec.901.
- Kanter JW, Mulick PS, Busch AM, Berlin KS, Martell CR. The Behavioral Activation for Depression Scale (BADS): psychometric properties and factor structure. J Psychopathol Behav Assess 2006;29:191-202. https://doi.org/10.1007/s10862-006-9038-5.
- Weissman ANBA. Development and Validation of the Dysfunctional Attitude Scale. Chicago, IL: Association for the Advancement of Behavior Therapy; 1978.
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta Earthquake. J Pers Soc Psychol 1991;61:115-21. https://doi.org/10.1037/0022-3514.61.1.115.
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry 1995;167:99-103. https://doi.org/10.1192/bjp.167.1.99.
- Blackburn I-M, James IA, Milne DL, Baker C, Standart S, Garland A, et al. The revised cognitive therapy scale (CTS-R): psychometric properties. Behav Cogn Psychother 2001;29:431-46. https://doi.org/10.1017/S1352465801004040.
- Dimidjian S HA, Martell CR, Herman-Dunn A, Dobson KS. The Quality of Behavioral Activation Scale Q-BAS. Boulder, CO: University of Colorado; 2012.
- Piaggio G, Pinol AP. Use of the equivalence approach in reproductive health clinical trials. Stat Med 2001;20:3571-7. https://doi.org/10.1002/sim.1078.
- Points to Consider on Switching Between Superiority and Non-inferiority. London: European Agency for the Evaluation of Medicinal Products; 2000.
- Briggs AH, O’Brien BJ. The death of cost-minimization analysis?. Health Econ 2001;10:179-84. https://doi.org/10.1002/hec.584.
- Bosmans JE, de Bruijne MC, van Hout HP, Hermens ML, Ader HJ, van Tulder MW. Practical guidelines for economic evaluations alongside equivalence trials. Value Health 2008;11:251-8. https://doi.org/10.1111/j.1524-4733.2007.00245.x.
- Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 1998;18:68-80. https://doi.org/10.1177/0272989X98018002S09.
- Reference Costs 2013–14. London: Department of Health; 2014.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit; 2013.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- McCrone PDS, Patel A, Knapp M, Lawton-Smith S. Paying the Price: The Cost of Mental Health Care in England to 2026. London: The King’s Fund; 2008.
- Netten A, Knight J, Dennett J, Cooley R, Slight A. A ‘Ready Reckoner’ for Staff Costs in the NHS, Volume I, Estimated Costs. Canterbury: University of Kent; 1998.
- NHS . NHS Choices 2016. www.nhs.uk/pages/home.aspx (accessed 1 July 2016).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2014.
- Koopmanschap MA, Rutten FF. A practical guide for calculating indirect costs of disease. PharmacoEconomics 1996;10:460-6. https://doi.org/10.2165/00019053-199610050-00003.
- Efron B, Tibshirani RJ. An Introduction to Bootstrap. New York, NY: Chapman & Hall; 1993.
- Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed?. BMJ 2000;320:1197-200. https://doi.org/10.1136/bmj.320.7243.1197.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005;187:106-8. https://doi.org/10.1192/bjp.187.2.106.
- Weichle T, Hynes DM, Durazo-Arvizu R, Tarlov E, Zhang Q. Impact of alternative approaches to assess outlying and influential observations on health care costs. SpringerPlus 2013;2. https://doi.org/10.1186/2193-1801-2-614.
- Driessen E, Hollon SD. Cognitive behavioral therapy for mood disorders: efficacy, moderators and mediators. Psychiatr Clin North Am 2010;33:537-55. https://doi.org/10.1016/j.psc.2010.04.005.
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry 2002;59:877-83. https://doi.org/10.1001/archpsyc.59.10.877.
- Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res 2010;19:237-70. https://doi.org/10.1177/0962280209105014.
- Richie J, Lewis J, McNaughton Nicholls C, Ormston R. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage; 2014.
- Mays N, Pope C. Rigour and qualitative research. BMJ 1995;311:109-12. https://doi.org/10.1136/bmj.311.6997.109.
- Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. London: Sage; 1994.
- Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects – Seoul Amendment. Ferney-Voltaire: World Medical Association; 2008.
- Health Research Authority . Consent and Participant Information Sheet Preparation Guidance n.d. www.hra-decisiontools.org.uk/consent/ (accessed 1 November 2012).
- Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, Bower P, et al. Clinical effectiveness of collaborative care for depression in UK primary care (CADET): cluster randomised controlled trial. BMJ 2013;347. https://doi.org/10.1136/bmj.f4913.
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173-82. https://doi.org/10.1037/0022-3514.51.6.1173.
- Dunn G, Emsley R, Liu H, Landau S, Green J, White I, et al. Evaluation and validation of social and psychological markers in randomised trials of complex interventions in mental health: a methodological research programme. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19930.
- Mackinnon DP, Fairchild AJ. Current directions in mediation analysis. Curr Dir Psychol Sci 2009;18. https://doi.org/10.1111/j.1467-8721.2009.01598.x.
- Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr 2009;76:408-20. https://doi.org/10.1080/03637750903310360.
- Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med 2000;19:1141-64. https://doi.org/10.1002/(SICI)1097-0258(20000515)19:9<1141::AID-SIM479>3.0.CO;2-F.
- Gershefski J, Arnkoff D, Glass C, Elkin I. Clients’ perceptions of treatment for depression: I. helpful aspects. Psychother Res 1996;6:233-47. https://doi.org/10.1080/10503309612331331768.
- Clarke H, Rees A, Hardy GE. The big idea: clients’ perspectives of change processes in cognitive therapy. Psychol Psychother Theory Res Pract 2004;77:67-89. https://doi.org/10.1348/147608304322874263.
- Straarup NS, Poulsen S. Helpful aspects of metacognitive therapy and cognitive behaviour therapy for depression: a qualitative study. Cogn Behav Ther 2015;8. https://doi.org/10.1017/S1754470X15000574.
- Barnes M, Sherlock S, Thomas L, Kessler D, Kuyken W, Owen-Smith A, et al. No pain, no gain: depressed clients’ experiences of cognitive behavioural therapy. Br J Clin Psychol 2013;52:347-64. https://doi.org/10.1111/bjc.12021.
- Shuy R, Gubrium JF, Holstein JA. Handbook of Interview Research. Thousand Oaks, CA: Sage; 2001.
- Sturges JE, Hanrahan KJ. Comparing telephone and face-to-face qualitative interviewing: a research note. Qualitative Res 2004;4:107-18. http://dx.doi.org/10.1177/1468794104041110.
- Trier-Bieniek A. Framing the telephone interview as a participant-centred tool for qualitative research: a methodological discussion. Qual Research 2012;12:630-44. https://doi.org/10.1177/1468794112439005.
- Amick HR, Gartlehner G, Gaynes BN, Forneris C, Asher GN, Morgan LC, et al. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ 2015;351. https://doi.org/10.1136/bmj.h6019.
- Fonagy P, Clark DM. Update on the improving access to psychological therapies programme in England. BJPsych Bull 2015;39:248-51. https://doi.org/10.1192/pb.bp.115.052282.
- Glasman D, Finlay WM, Brock D. Becoming a self-therapist: using cognitive-behavioural therapy for recurrent depression and/or dysthymia after completing therapy. Psychol Psychother 2004;77:335-51. https://doi.org/10.1348/1476083041839385.
- Hollon SD, Stewart MO, Strunk D. Enduring effects for cognitive behavior therapy in the treatment of depression and anxiety. Annu Rev Psychol 2006;57:285-31. https://doi.org/10.1146/annurev.psych.57.102904.190044.
- French LRM, Thomas L, Campbell J, Kuyken W, Lewis G, Williams C, et al. Individuals’ long term use of cognitive behavioural skills to manage their depression: a qualitative study. Behav Cogn Psychother 2017;45:46-57. https://doi.org/10.1017/S1352465816000382.
- DeRubeis RJ, Cohen ZD, Forand NR, Fournier JC, Gelfand LA, Lorenzo-Luaces L. The personalized advantage index: translating research on prediction into individualized treatment recommendations. A demonstration. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0083875.
- Kanter JW, Puspitasari AJ. Global dissemination and implementation of behavioural activation. Lancet 2016;388:843-4. https://doi.org/10.1016/S0140-6736(16)31131-X.
- Chowdhary N, Anand A, Dimidjian S, Shinde S, Weobong B, Balaji M, et al. The Healthy Activity Program lay counsellor delivered treatment for severe depression in India: systematic development and randomised evaluation. Br J Psychiatry 2016;208:381-8. https://doi.org/10.1192/bjp.bp.114.161075.
- Moradveisi L, Huibers MJ, Renner F, Arasteh M, Arntz A. Behavioural activation v. antidepressant medication for treating depression in Iran: randomised trial. Br J Psychiatry 2013;202:204-11. https://doi.org/10.1192/bjp.bp.112.113696.
- Bolton P, Bass JK, Zangana GA, Kamal T, Murray SM, Kaysen D, et al. A randomized controlled trial of mental health interventions for survivors of systematic violence in Kurdistan, Northern Iraq. BMC Psychiatry 2014;14. https://doi.org/10.1186/s12888-014-0360-2.
Appendix 1 Qualitative interview topic guides
Appendix 2 Individual participant Patient Health Questionnaire-9, Behavioural Activation for Depression Scale, Dysfunctional Attitudes Scale, Ruminative Response Scale and Snaith–Hamilton Pleasure Scale scores
FIGURE 18.
Individual participant PHQ-9 scores at baseline (0 months) and at 6, 12 and 18 months’ follow-up for each treatment group for the mediation population. (a) CBT and (b) BA.


FIGURE 19.
Individual participant BADS total scores at baseline, PM1, PM2 and 6 months’ follow-up for each treatment group for the mediation population. (a) CBT and (b) BA.
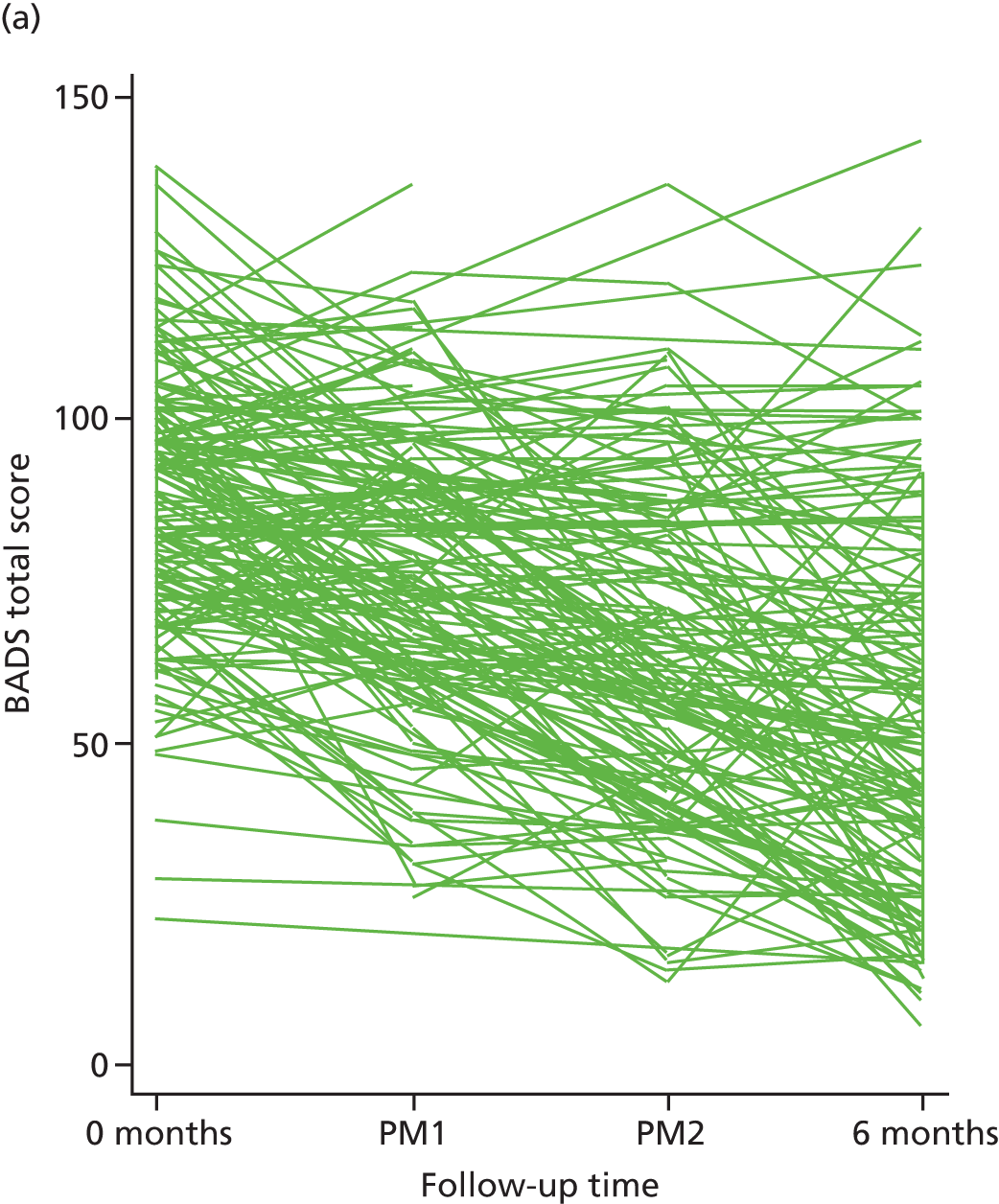
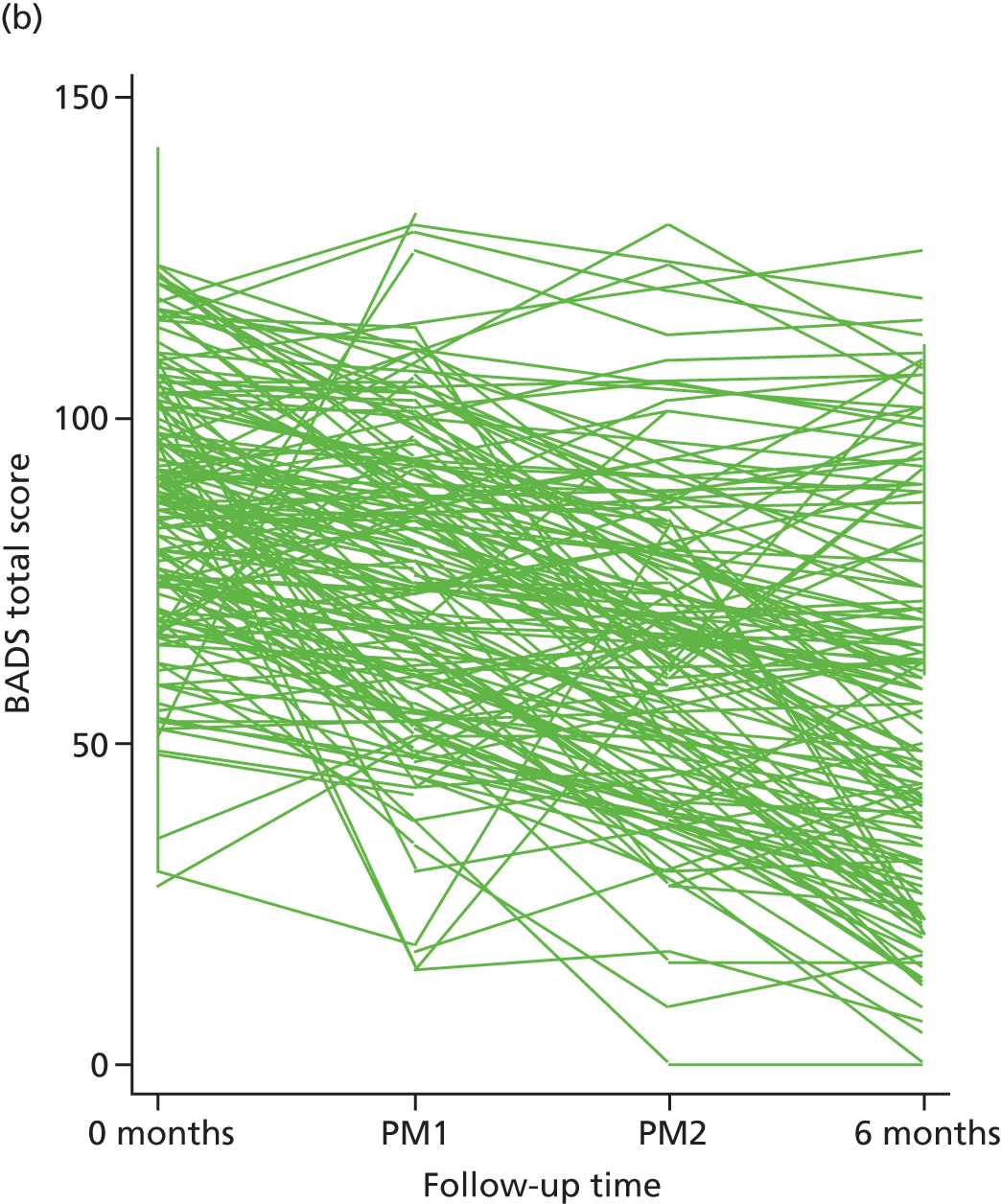
FIGURE 20.
Individual participant DAS scores at baseline, PM1, PM2 and 6 months’ follow-up for each treatment group for the mediation population. (a) CBT and (b) BA.
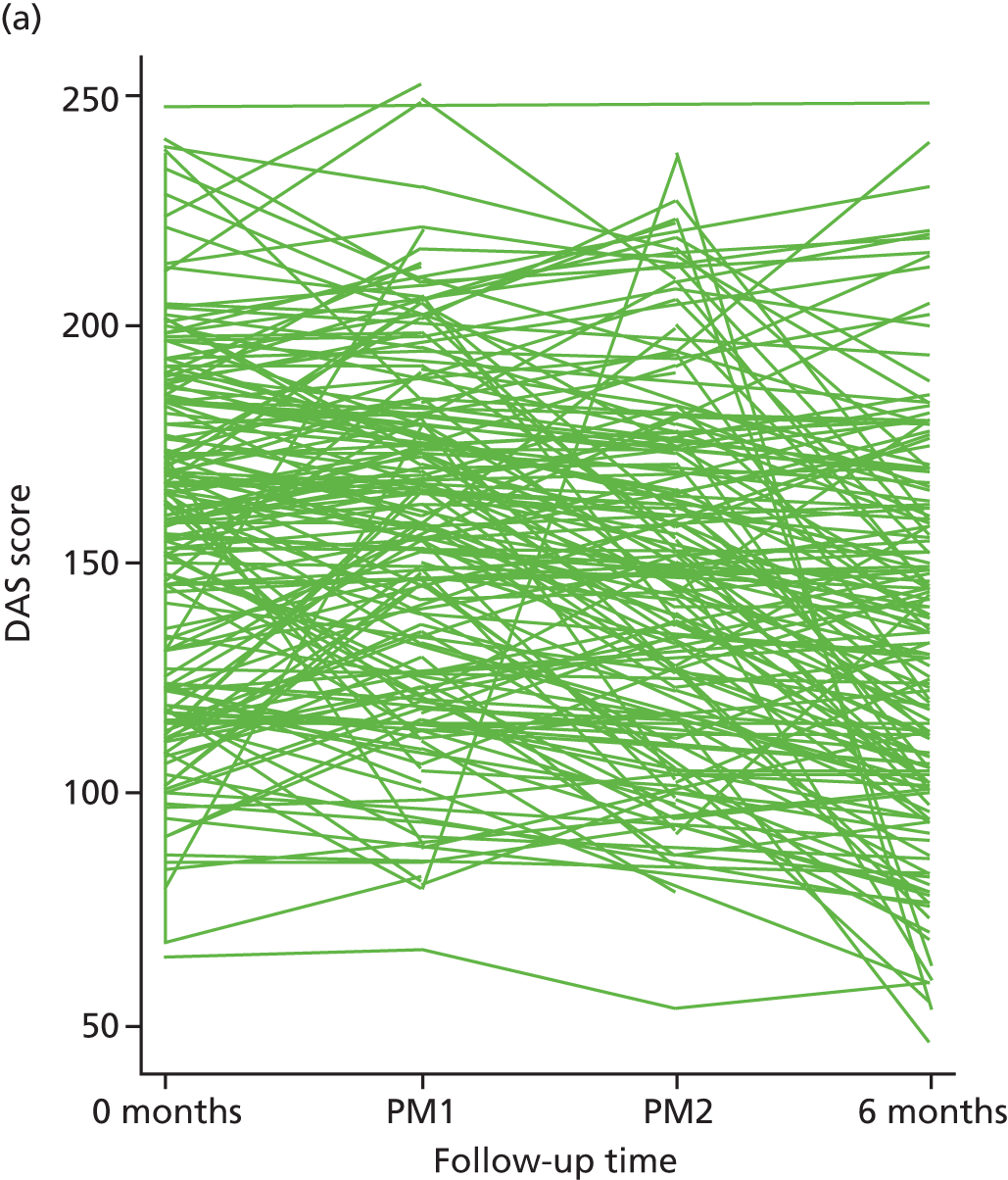
FIGURE 21.
Individual participant RRS total scores at baseline, PM1, PM2 and 6 months’ follow-up for each treatment group for the mediation population. (a) CBT and (b) BA.

FIGURE 22.
Individual participant SHAPS scores at baseline, 6 and 12 months’ follow-up for each treatment group for the mediation population. (a) CBT and (b) BA.

Appendix 3 Ethics documents
Appendix 4 Baseline case report form
Appendix 5 Risk and adverse event documents
Appendix 6 Participant results newsletter
Appendix 7 Behavioural activation clinical practice manual
Appendix 8 Cognitive–behavioural therapy clinical practice manual
List of abbreviations
- AD-SUS
- adult service use schedule
- ADM
- antidepressant medication
- AE
- adverse event
- AfC
- Agenda for Change
- BA
- behavioural activation
- BADS
- Behavioural Activation for Depression Scale
- CBT
- cognitive–behavioural therapy
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- COBRA
- Cost and Outcome of BehaviouRal Activation
- CONSORT
- Consolidated Standards of Reporting Trials
- CSO
- clinical studies officer
- CTS-R
- Cognitive Therapy Scale-Revised
- DAS
- Dysfunctional Attitudes Scale
- DMC
- Data Monitoring Committee
- DSM-IV
- Diagnostic and Statistical Manual for Mental Disorders-Fourth Edition
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- GAD-7
- Generalised Anxiety Disorder-7
- GP
- general practitioner
- IAPT
- Improving Access to Psychological Therapies
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- MDD
- major depressive disorder
- MHW
- mental health worker
- MICE
- multiple imputation by chained equations
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PenCTU
- Peninsula Clinical Trials Unit
- PHQ-9
- Patient Health Questionnaire-9
- PM
- process measure
- PM1
- process measure point 1
- PM2
- process measure point 2
- PP
- per protocol
- PPI
- patient and public involvement
- PSS
- Personal Social Services
- PWP
- psychological well-being practitioner
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RRS
- Ruminative Response Scale
- SCID
- Structured Clinical Interview for DSM Disorders
- SD
- standard deviation
- SEM
- structural equation modelling
- SF-36
- Short Form questionnaire-36 items
- SHAPS
- Snaith–Hamilton Pleasure Scale
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee