Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 15/06/04. The protocol was agreed in January 2016. The assessment report began editorial review in September 2016 and was accepted for publication in March 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Corbett et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Psoriatic arthritis (PsA) is a chronic autoimmune disease closely associated with psoriasis of the skin and nails, but distinct from rheumatoid arthritis (RA). PsA is one of a family of inflammatory arthritis disorders called spondyloarthritis (or spondyloarthropathy), which also includes ankylosing spondylitis. 1 PsA is closely linked with inflammatory bowel disease, especially the form called Crohn’s disease. 2 Although any joint may be affected, PsA typically affects joints in the hands, feet and spine. Its course may be erratic, with flare-ups and remissions, but it can cause joint damage if it is not treated. Early diagnosis is important to avoid damage to joints. 3 Symptoms of arthritis include inflamed (swollen), stiff and painful joints; and psoriasis symptoms include patchy, raised red areas of inflamed skin with scaling. 4
The symptoms of psoriatic arthritis are similar to those of other forms of arthritis. The difference between PsA and RA is that the pattern of joint involvement is commonly asymmetrical, and involves the distal interphalangeal joints (in the hands and feet) and nail lesions. The following terms are used to present the patterns of PsA: oligoarthritis (four or fewer affected joints; 22–37% of patients); polyarthritis (five or more affected joints; 36–41% of patients); arthritis of distal interphalangeal joints (< 20% of patients); spondylitis (7–23% of patients); and arthritis mutilans (approximately 4% of patients). 5,6 Most patients with PsA will have developed psoriasis first (i.e. joint complications occur around 10 years after initial diagnosis of psoriasis), although joint involvement appears first in 19% of patients and concurrently with psoriasis in 16% of cases. 7
As PsA can affect both skin and joints, it can result in significant quality-of-life impairment, joint deformity and psychosocial disability. 7,8 A recent survey of patients with RA, PsA and axial spondyloarthritis found that disease burden in terms of patient-reported outcome measures was similar in PsA and axial spondyloarthritis patients, but significantly lower for the RA patients. 9 The physical and psychosocial problems experienced by patients affect their ability to perform paid work and everyday tasks; PsA has a substantial economic impact on the UK health-care system as a result of direct health-care costs as well as indirect costs, such as reduced work capacity. 10
Patients with PsA have a 60% higher risk of premature mortality than the general population, with cardiovascular disease being the leading cause of death. 11–13 The estimated reduction in life expectancy for patients with PsA is approximately 3 years,14 with a standardised mortality ratio of 1.62. A Canadian outpatient clinic study reported that mortality due to cardiovascular disease was 30% higher in patients with PsA than that in the general population. 12
Diagnosis
It is difficult to define PsA because there are no precise diagnostic criteria or diagnostic markers. 15 In general, diagnoses are primarily based on patient symptoms and physical examination. In most cases, Moll and Wright’s 1973 criteria16 have been used for diagnosis. Several classification criteria have have been introduced since Moll and Wright’s criteria, but none has been widely accepted or validated. In 2006, the multicentre Classification Criteria for Psoriatic Arthritis (CASPAR) study developed new classification criteria that are simple and have both a high sensitivity and a high specificity; they are currently a preferred method to define cases of PsA (Table 1). 17
| To meet the CASPAR, a patient must have inflammatory articular disease (joint, spine or entheseal) with three points or more from the following five categories17 | |
|---|---|
| 1. Evidence of psoriasis | Current psoriasis,a defined as psoriatic skin or scalp disease present today as judged by a rheumatologist or dermatologist |
| A personal history of psoriasis, defined as a history of psoriasis that may be obtained from a patient, family physician, dermatologist, rheumatologist or other qualified health-care provider | |
| A family history of psoriasis, defined as a history of psoriasis in a first- or second-degree relative according to patient report | |
| 2. Psoriatic nail dystrophy | Typical psoriatic nail dystrophy, including onycholysis, pitting and hyperkeratosis, observed on current physical examination |
| 3. Negative test result for rheumatoid factor | A negative test result for the presence of rheumatoid factor by any method except latex but preferably by an enzyme-linked immunosorbent assay or nephelometry, according to the local laboratory reference range |
| 4. Dactylitis | Either current dactylitis, defined as swelling of an entire digit, or a history of dactylitis recorded by a rheumatologist |
| 5. Radiographic evidence of juxta-articular new bone formation | Defined as ill-defined ossification near joint margins (but excluding osteophyte formation) on plain radiographs of the hand or foot |
Epidemiology
The exact prevalence of PsA is unknown, but estimates vary from 0.3% to 1% of the population. It has been estimated that in England, in 2013, there were around 53,900–161,600 people with PsA. PsA affects men and women equally, in contrast to RA, which is more common in women. 18
Psoriatic arthritis can develop at any time, including childhood,19 but normally it appears between the ages of 30 and 55 years. 18 Its development is a complex process involving both environmental and genetic factors. 20–22 Studies show a stronger genetic or family link to PsA than to other autoimmune rheumatic diseases. Around 40% of people who are diagnosed with PsA and psoriasis also have family members affected by the disease. 2
Measurement of disease
In 2016, the Group for Research and Assessment of Psoriasis and the Psoriatic Arthritis Outcome Measures in Rheumatology Organisation PsA working group updated the core set of domains to be assessed in clinical trials to reflect both patient and physician priorities. The domain set includes musculoskeletal disease activity (which now includes enthesitis, dactylitis and spine symptoms, in addition to peripheral arthritis), skin disease activity, patient global assessment, pain, physical function, health-related quality of life (HRQoL), fatigue and systemic inflammation. Four new items were added to the research agenda: stiffness, independence, treatment burden and sleep. 23
Many trials of PsA have used 20% improvement in the American College of Rheumatology criteria (ACR 20) as the primary outcome;24 the American College of Rheumatology (ACR) criteria were, however, developed to assess RA. The other outcome assessment tools that have commonly been used in clinical trials are:
-
the Psoriatic Arthritis Response Criteria (PsARC), a multidomain measure which has similarities with ACR criteria but which was developed specifically for PsA
-
the Psoriasis Area and Severity Index (PASI), to assess psoriasis
-
the Health Assessment Questionnaire-Disability Index (HAQ-DI), to assess function (activities of daily living)
-
various measures of enthesitis, dactylitis and radiographic progression of disease.
However, there are issues with some assessment tools:
-
HAQ-DI concentrates on physical disability, which may not adequately capture disability in patients with predominantly skin disease. Consequently, there is less change in the context of treatment that has a predominant effect on the skin and not the joints. 25
-
PASI has poor sensitivity to change and responsiveness when skin psoriasis is < 10% of body surface area (BSA) involvement. Furthermore, it has been stated that the correlation with quality-of-life measures is poor. 26 In addition, it is time-consuming and not practically very feasible in daily clinical practice.
-
PsARC identifies only relative changes from baseline and overestimates the number of responders. 27 In general, PsARC placebo response rates are higher than other composite measures. 28
Current service provision
If PsA is not treated early, the inflammation can affect the whole body, which may lead to lasting joint and tissue damage. 2 The clinical management of PsA therefore aims to suppress joint, tendon and ligament inflammation, and to manage the skin symptoms of the disease. Current practice involves early diagnosis and early use of non-steroidal anti-inflammatory drugs (NSAIDs) and/or intra-articular corticosteroid injections. In patients who do not respond to these treatments, disease-modifying antirheumatic drugs (DMARDs) are then used [most commonly beginning with methotrexate (MTX)]. When conventional disease-modifying antirheumatic drugs (cDMARDs) are ineffective, or not tolerated, biologic therapies may be used; for example, anti-tumour necrosis factor (TNF) therapies, such as etanercept [(ETN) ENBREL®; Pfizer Inc., New York City, NY, USA], infliximab [(INF) REMICADE®; Merck Sharp & Dohme, Kenilworth, NJ, USA], adalimumab [(ADA) HUMIRA®; AbbVie Inc., North Chicago, IL, USA] and golimumab [(GOL) SIMPONI®; Merck Sharp & Dohme, Kenilworth, NJ, USA]. These anti-TNFs are approved by the National Institute for Health and Care Excellence (NICE). Anti-TNFs have been shown to slow the progression of joint damage when assessed radiographically. 29,30 Ustekinumab [(UST) STELARA®; Janssen Pharmaceuticals, Inc., Horsham, PA, USA] – a different type of biologic therapy to anti-TNFs [being an interleukin (IL)-12/23 inhibitor] – is also recommended as a possible treatment, specifically when DMARDs have not worked well enough, provided that treatment with anti-TNFs is not suitable, or the patient has had an anti-TNF before. Apremilast [(APR) Otezla®; Celgene Corporation, Summit, NJ, USA], a phosphodiesterase 4 inhibitor, is not currently approved by NICE.
Current NICE guidance relates to the treatment of patients who have had an inadequate response to two or more cDMARDs (administered either individually or in combination). The British Society for Rheumatology (BSR)’s guidelines make a provision for using a biologic after one DMARD in the presence of adverse prognostic factors; these are defined as five or more swollen joints in association with an elevated C-reactive protein (CRP) concentration for more than 3 months and structural joint damage due to disease. 31 Not all patients respond to an initial anti-TNF treatment, and in some patients the response diminishes over time. One observational study showed that one-third of PsA patients had switched to a second anti-TNF because of a lack of efficacy and side effects. 32 NICE does not specifically recommend switching anti-TNFs other than the guidance for UST, and switching decisions may depend on local Clinical Commissioning Group guidelines: in some parts of the country patients are allowed to switch from one anti-TNF to another.
Quite often PsA goes undetected and is sometimes not recognised and diagnosed by dermatologists or general practitioners (GPs). In the UK, rheumatologists manage the majority of patients with PsA, but patients with less severe joint disease may be managed by a dermatologist. However, patients with severe problems with joints and skin will tend to be managed by both rheumatologists and dermatologists.
Description of the technology under assessment
Certolizumab pegol (CZP; CIMZIA®, UCB Pharma, Brussels, Belgium) is a biologic therapy (a monoclonal antibody that targets TNF) that is administered subcutaneously. Anti-TNFs target the activation of tumour necrosis factor alpha (TNF-α) and subsequently activation of downstream inflammatory processes, and as such have the potential to offer symptom control as well as altering disease progression. CZP in combination with MTX has a marketing authorisation in the UK for treating active PsA in adults when the response to previous DMARD therapy has been inadequate. CZP can be given as monotherapy if MTX cannot be tolerated or when continued treatment with MTX is inappropriate.
Secukinumab (SEC; COSENTYX®, Novartis International AG, Basel, Switzerland), which is also administered subcutaneously, is a different type of biologic therapy to CZP, being a monoclonal antibody that targets the IL-17A cytokine molecule (rather than targeting TNF). SEC, alone or in combination with MTX, is indicated for the treatment of active PsA in adult patients when the response to previous DMARD therapy has been inadequate. SEC also has marketing authorisation from the European Medicines Agency for the treatment of ankylosing spondylitis and moderate–severe plaque psoriasis.
Chapter 2 Definition of the decision problem
The decision problem relates to the optimal use of CZP and SEC within their marketing authorisations for treating active PsA in adults for whom DMARDs have been inadequately effective. Evaluations are made at the following points in the treatment pathway:
-
patients who have only received one prior non-biological DMARD
-
patients whose disease has inadequately responded to at least two DMARDs
-
patients whose disease has inadequately responded to both DMARDs and biological therapies.
Previous National Institute for Health and Care Excellence appraisals
There have been no previous NICE technology appraisals (TAs) of CZP or SEC for PsA, although there have been several appraisals of other biologics for PsA: TA19933 (ETN, INF and ADA), TA22034 (GOL) and TA34035 (UST). APR, which is not a biologic, is not currently recommended by NICE.
A number of key areas of uncertainty and potential limitations of the evidence base were identified from the previous appraisals. These include:
-
a lack of direct head-to-head trial evidence evaluating the relative efficacy and safety of the biologics
-
some limitations in the external validity of the trial populations (i.e. the trial populations had some differences from populations seen in routine clinical practice)
-
a lack of patient registry data for PsA
-
the long-term effectiveness of biologics in controlling disease activity
-
the prescription cost of biologics and the cost of treating psoriasis at different levels of severity
-
the progression of (HAQ-DI) score (a measure of patient function) in patients on and off treatment, and the length of time biologics are assumed to be effective
-
long-term progression of PsA with and without biologics
-
a lack of an optimal outcome measure for PsA
-
the rate of treatment withdrawal and the adverse effects associated with the long-term use of biologics
-
a lack of evidence on the efficacy and safety of the sequential use of biologics.
This assessment has considered and attempted to address these limitations and areas of uncertainty using relevant evidence where available.
Overall objective of assessment
To determine the clinical effectiveness and cost-effectiveness within the NHS of CZP and SEC within their marketing authorisations for treating active PsA in adults for whom DMARDs have been inadequately effective.
Chapter 3 Assessment of clinical effectiveness
This report contains reference to confidential information provided as part of the NICE appraisal process. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Methods for reviewing clinical effectiveness
Search strategies
The literature search aimed to identify all relevant randomised controlled trials (RCTs) of CZP and SEC, and the comparators ETN, ADA, INF, GOL, APR and UST for the treatment of PsA.
The searches for CZP and SEC for PsA were not restricted by date. However, as ETN, ADA, INF, GOL, APR and UST for PsA had been subject to previous TAs, updated searches were performed based on the search dates of these previous TAs.
The search strategy was developed in MEDLINE (via Ovid) and then adapted for use in the other resources searched. The strategy included terms for PsA combined, using the Boolean operator AND, with terms for the eight treatments. No language or geographical limits were applied. A study design search filter to limit retrieval to RCTs was used where available.
Search strategies were developed by an information specialist with input from the project team. The MEDLINE search strategy was checked by a second information specialist. The searches were carried out during December 2015 and then updated on 28 April 2016 to capture more recent studies.
The following databases were searched: MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), EMBASE, Health Technology Assessment (HTA) database, PubMed, and the Science Citation Index (SCI).
In addition, the following resources were searched for ongoing, unpublished or grey literature: ClinicalTrials.gov, Conference Proceedings Citation Index – Science (CPCI-S), EU Clinical Trials Register, PROSPERO and the World Health Organization’s International Clinical Trials Registry Platform portal.
As DARE ceased at the end of March 2015, additional searches for systematic reviews were carried out in MEDLINE and EMBASE to ensure that any relevant systematic reviews were identified.
Full search strategies can be found in Appendix 1.
Inclusion criteria
Two reviewers independently screened all titles and abstracts. Full manuscripts of any titles/abstracts that were relevant were obtained, where possible, and the relevance of each study was assessed by two reviewers according to the inclusion criteria, described below. Any discrepancies were resolved by involving a third reviewer. Studies available only as abstracts were also included.
Study design
Randomised or quasi-RCTs were eligible for the review of clinical efficacy and safety. For the eligible interventions (see Interventions), all open-label extension studies of RCTs were included. For the comparators (see Comparators), open-label extensions were identified and listed with the main focus being on those studies that reported data relating to the longest duration of follow-up available for each individual comparator.
To evaluate the adverse effect profiles of the different biologics, the eligible study designs were systematic reviews that covered a range of diseases and large observational studies in patients with PsA.
Prospective registry studies that included PsA patients receiving biologics were eligible to provide data on treatment adherence, treatment withdrawal, and the rates and efficacy of switching to new biologics (i.e. sequential use). Potentially relevant registry studies were sought and identified, with a focus on those deemed to be most clinically relevant and appropriate to the UK setting. This decision was based on an examination of study characteristics and discussion with our clinical adviser.
Studies were also sought on the longer-term natural history of PsA in populations that have not taken a biologic therapy.
Interventions
Certolizumab pegol and SEC were eligible at their licensed doses (see Table 2). Studies comparing these two treatments with each other were also eligible.
Comparators
The relevant comparators were:
-
placebo
-
DMARDs: MTX, sulfasalazine, leflunomide, hydroxychloroquine, azathioprine and ciclosporin
-
biologic therapies: ADA, ETN, GOL, INF and UST, including any licensed biosimilars
-
APR
-
best supportive care (BSC).
Biologics and APR may have been used with or without concomitant DMARDs. Only studies that included treatments used at their licensed dose were eligible. Head-to-head trials of the five biologic comparators (and biosimilars) and APR were eligible, but were anticipated to be rare. Therefore, to allow comparisons of active treatments via network meta-analysis (NMA), the biologic comparators and APR could also have been compared with either placebo or a DMARD.
Participants
For the evaluation of the effectiveness of CZP and SEC, the included studies were of adults with active PsA for whom DMARDs had been inadequately effective.
Outcomes
For CZP and SEC, studies reporting any of the following outcomes were eligible:
-
disease activity, using the following multidomain measures: PsARC, ACR 20, 50% improvement in the American College of Rheumatology criteria (ACR 50) and 70% improvement in the American College of Rheumatology criteria (ACR 70)
-
functional capacity (assessed using HAQ-DI)
-
radiographic assessment of disease progression
-
response of psoriatic skin lesions (assessed using PASI)
-
measures of dactylitis, enthesitis and tendonitis
-
mortality
-
HRQoL, assessed using EuroQol-5 Dimensions (EQ-5D) or Short Form questionnaire-36 items (SF-36)
-
adverse effects of treatment, focusing on the key adverse events (AEs) identified from previous studies of biologics: malignancies, serious infections, reactivation of latent tuberculosis (TB), injection site reactions and withdrawals due to AEs.
Randomised controlled trials of comparators needed to report at least one of the following: PsARC, ACR 20/50/70, PASI 50 (50% reduction in PASI), PASI 75 (75% reduction in PASI), PASI 90 (90% reduction in PASI) or HAQ-DI score.
For patient registry studies, treatment adherence, treatment withdrawal, and the rates and efficacy of switching to new biologics (i.e. sequential use) were the key outcomes of interest, and particularly those which were identified as being useful to inform parameters in the economic model.
Data extraction
For SEC and CZP, data were extracted from published papers and abstracts supplemented by data from the manufacturer submissions (when they were not available from other sources). Data were extracted from previous single technology appraisal (STA) or multiple technology appraisal (MTA) reports for studies of ETN, INF, ADA, GOL, UST and APR. When missing or further information on the trials of these treatments was needed, data were extracted either from the relevant published trial reports or from reviews. 36–39 Some data may have been missing in the original TAs because of commercial- or academic-in-confidence restrictions; and some of these data may have subsequently been published. Data for UST at the 12-week time point were extracted from the full clinical study reports of PSUMMIT (Study of the Safety and Effectiveness of Ustekinumab in Patients With Psoriatic Arthritis) 1 and 2 trials, which were accessed via the Yale University Open Data Access (YODA) project. For APR, although only the Psoriatic Arthritis Long-term Assessment of Clinical Efficacy (PALACE) 1 trial has been published, data from the PALACE 2 and 3 trials were extracted from STA documents on NICE’s website. All data for these treatments were extracted by one reviewer and then checked for any transcription errors by a second reviewer.
For the dichotomous responder outcomes (PsARC, ACR 20/50/70 and PASI 50/75/90), intention-to-treat (ITT) baseline denominators (i.e. the number of patients randomised for each trial arm) were used, with patients assumed to be non-responders where data were missing. This explains why there is a small difference in the ADalimumab Effectiveness in Psoriatic arthritis Trial (ADEPT) denominators used between this current MTA, the previous MTA and the manufacturers’ submissions (the last two used the ‘modified ITT’ data whereby patients had to have received at least one dose of study treatment).
Data on study design, participant characteristics, efficacy outcomes and quality were extracted by one reviewer using a standardised data extraction form and independently checked by a second reviewer for the SEC and CZP trials. Disagreements were resolved through consensus. For the comparator treatments, most of the data were copied (from previous reports) by one reviewer and then checked for any transcription errors by a second reviewer.
Attempts were made, where possible, to contact authors for missing data. Data from studies with multiple publications were extracted and reported as a single study. For the open-label extension studies of comparator treatments, only the data relating to the latest time point were extracted. Data were also extracted from the manufacturers’ submissions when they were not available from other sources.
Quality assessment
The quality of the RCTs was assessed using a modified version of the Cochrane risk-of-bias tool, which incorporated an assessment of baseline imbalance. 40 The assessments of baseline imbalance were made based on evidence from a systematic review of predictors of treatment response to anti-TNFs. 41 The review identified several possible such predictors in patients with PsA, although none was identified as being conclusive owing to the limited number of studies and the heterogeneity of response measures. We looked at baseline CRP concentration, age and sex. The characteristics of young age, male sex and high CRP concentration may be predictive of a better response. Risk-of-bias assessments were performed by one reviewer and checked independently by a second reviewer. Any disagreements were resolved through consensus or by involvement of a third reviewer if necessary. Open-label extension studies were less formally evaluated. This was based on assessing imputation methods, the patient withdrawal criteria used and the clinical relevance of any treatment stopping/changing rules.
Methods of data synthesis
The study characteristics and quality assessment results were tabulated and summarised narratively. Where possible, the clinical effectiveness data for the PsARC, ACR, PASI and HAQ-DI outcomes were synthesised using Bayesian NMA methods (see Chapter 4). For other outcomes, or for studies not included in the NMAs, studies were either summarised narratively or pooled using pairwise meta-analysis methods.
Quantity and quality of the identified evidence
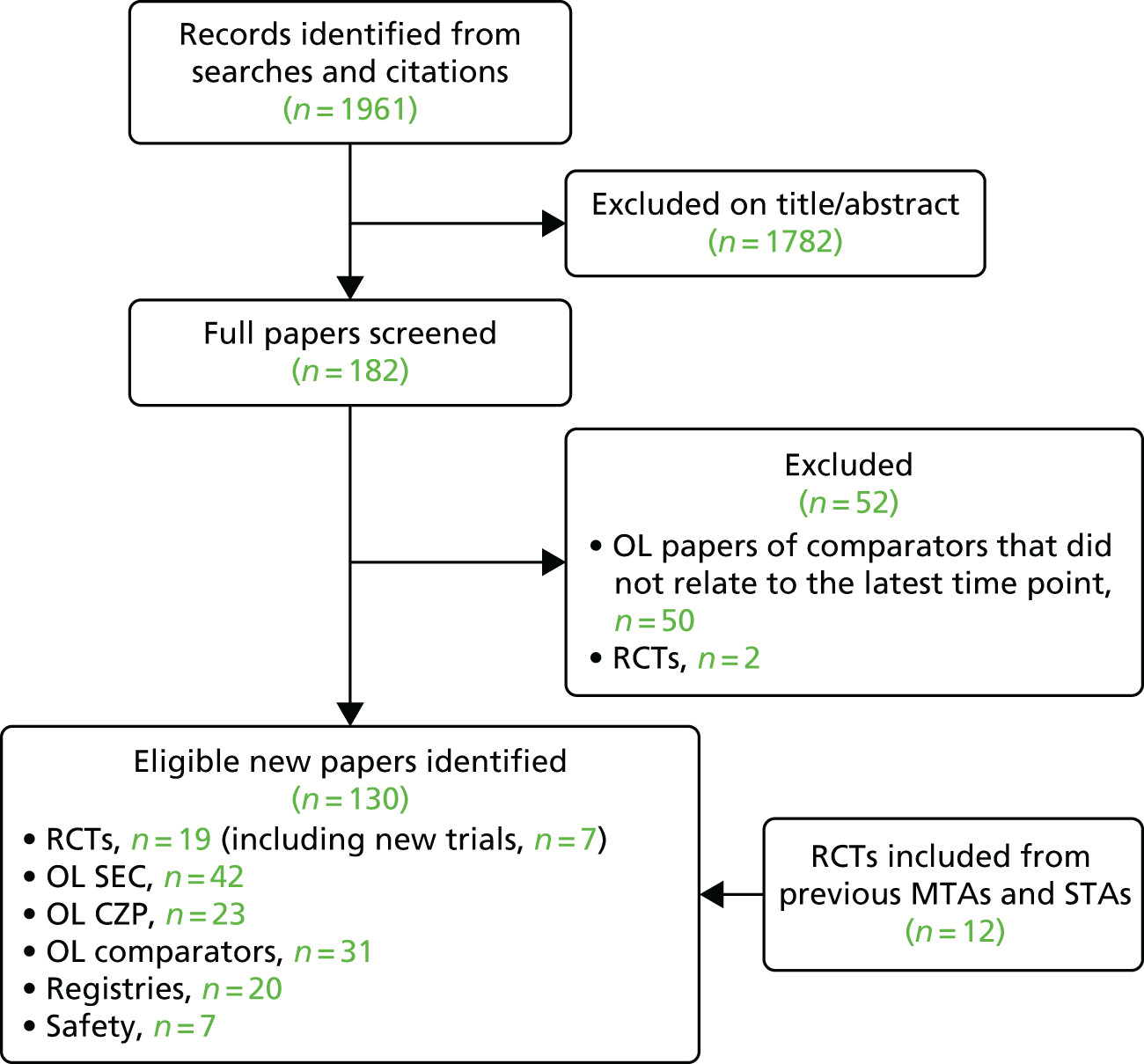
A total of 1761 records were retrieved from the original December 2015 electronic database searches. The searches were updated on 28 April 2016, with a further 200 records available for screening. After screening titles and abstracts, full copies of 182 papers were assessed for inclusion in the review.
Two RCTs were excluded at the abstract stage for using unlicensed dosages (50 mg of ETN twice weekly,42 and 20 and 40 mg of APR43). Two RCTs were excluded at the full-paper stage: one did not report subgroup results for PsA44 and the other included only patients who were naive to MTX. 45 The FUTURE [Efficacy at 24 Weeks and Long Term Safety, Tolerability and Efficacy up to 2 Years of Secukinumab (AIN457) in Patients With Active PsA] 1 trial of SEC was excluded from the RCT short-term efficacy review as it used an unlicensed, very high, loading dose. It was, however, included as an open-label extension study as the impact of the initial high loading dose would probably be negligible at later time points. 46 Fifty open-label studies of comparator treatments were excluded as they did not relate to the latest (longest) duration of follow-up.
Details of the numbers of other eligible full publications or conference abstracts that relate to open-label studies of the included RCTs and patient registry or safety studies are presented in Figure 1.
FIGURE 1.
Flow chart showing the number of studies identified and eligible for inclusion. OL, open label.
Characteristics of the randomised controlled trials included in the systematic review of short-term efficacy
Of the 19 included RCTs, 17 were placebo controlled: one of CZP,47 three of SEC (two of which were reported in one publication),48,49 one of GOL,50 two of INF,51,52 two of ETN,53,54 three of ADA,55–57 two of UST58,59 and three of APR. 60,61 The FUTURE 1 trial of SEC was excluded from the RCT short-term efficacy review as it used an unlicensed, and very high, loading dose. 46
Two trials compared active treatments: one compared SEC with UST62,63 and one compared INF, ETN and ADA. 64
Most studies were conducted mainly in Europe and North America. All but two53,64 were multicentre trials. Details of the trial durations, different phases and the dosing regimens of the main interventions studied are presented in Table 2. Details of all interventions studied are presented in Table 3. For some trials we excluded individual treatment arms from the systematic review (see Table 3). This was as a result of the doses not being licensed or recommended in the populations studied. Some included trials were excluded from the NMAs because of the populations being different from the other trial populations (see Table 3).
| Main study reference and treatments studied | Eligible licensed dosing regimens (with timings) | Duration of truly randomised and blinded phase (before any treatment crossover) | Crossover details | Latest time point with available result data | Anticipated time to response: information from SPC |
|---|---|---|---|---|---|
| FUTURE 2;48 SEC | 150-mg subcutaneous injection at weeks 0, 1, 2 and 3 followed by monthly maintenance dosing from week 4. For patients with concomitant moderate–severe plaque psoriasis or who are anti-TNF inadequate responders, the recommended dose is 300 mg (given as two 150-mg injections) | 16 weeks | Week 16: PNRs (not achieving ≥ 20% improvement from baseline in TJC and SJC) re-randomised to 150 or 300 mg every 4 weeks. Week 24: PRs re-randomised to 150 or 300 mg every 4 weeks | 52 weeks | Clinical response is usually achieved within 16 weeks of treatment. Consideration should be given to discontinuing treatment in patients who have shown no response by 16 weeks of treatment. Some patients with an initial partial response may subsequently improve with continued treatment beyond 16 weeks |
| FIXTURE;49 SEC and ETN | For patients with concomitant moderate–severe plaque psoriasis or who are anti-TNF inadequate responders, the recommended SEC dose is 300 mg | 12 weeks | At 12 weeks PNRs were re-randomised to 150 or 300 mg of SEC | 52 weeks | |
| ERASURE;49 SEC | |||||
| CLEAR;62,63 SEC and UST | SEC: for patients with concomitant moderate–severe plaque psoriasis or who are anti-TNF inadequate responders, the recommended dose is 300 mg UST: 45 mg at week 0, week 4 and every 12 weeks |
52 weeks, but data currently available only for up to 16 weeks | No crossovers | 52 weeks | |
| RAPID-PsA;47 CZP | 200-mg subcutaneous injection Loading dose: 2 × 200 mg at weeks 0, 2 and 4 Maintenance dose: 200 mg every 2 weeks Alternative maintenance dose once clinical response is confirmed can be considered: 400 mg every 4 weeks |
16 weeks | Placebo patients failing to achieve ≥ 10% improvement in both TJC and SJC at both weeks 14 and 16 were re-randomised to 200 or 400 mg at week 16. At week 24 the remaining placebo patients were re-randomised to 200 or 400 mg | 216 weeks | Clinical response is usually achieved within 12 weeks of treatment. Continued therapy should be carefully reconsidered in patients who show no evidence of therapeutic benefit within the first 12 weeks of treatment |
| PALACE 1, PALACE 2 and PALACE 3;60,61,65 APR | 30 mg twice daily, oral tablets | 16 weeks | At week 16, patients without ≥ 20% reduction in SJC and TJC were required to be re-randomised equally to either APR dose if initially randomised to placebo or remained on their initial APR dose. At week 24, all remaining placebo-treated patients were switched to APR | 104 weeks (PALACE 1) | During pivotal trials the greatest improvement was observed within the first 24 weeks of treatment. If a patient shows no evidence of therapeutic benefit after 24 weeks, treatment should be reconsidered. The patient’s response to treatment should be evaluated on a regular basis |
| PSUMMIT 1;58 UST | 45-mg subcutaneous injection followed by a 45-mg dose 4 weeks later, and then every 12 weeks | 16 weeks | At week 16, patients with < 5% improvement in TJC/SJC entered blinded early escape (placebo to 45 mg, 45 to 90 mg, 90 to 90 mg). At week 24, all remaining patients in the placebo group received 45 mg of UST, which they continued at week 28 and every 12 weeks thereafter | 108 weeks for safety and 100 weeks for efficacy evaluation | Consideration should be given to discontinuing treatment in patients who have shown no response up to 28 weeks of treatment |
| PSUMMIT 2;59,66 UST | 45 mg at week 0, week 4, and every 12 weeks | 16 weeks | At week 16, patients with < 5% improvement in TJC/SJC entered blinded early escape (placebo to 45 mg, 45 to 90 mg, 90 to 90 mg). At week 24, all remaining patients in the placebo group received 45 mg of UST | 100 weeks | |
| GO-REVEAL;50 GOL | 50 mg once monthly, subcutaneous injection | 16 weeks | At week 16, patients with < 10% improvement in both TJC and SJC entered blinded early escape (placebo to 50 mg, 50 to 100 mg, 100 to 100 mg). Open label from week 24 (in which all patients were eligible for GOL) | 256 weeks | Clinical response is usually achieved within 12–14 weeks of treatment (after three or four doses). Continued therapy should be reconsidered in patients who show no evidence of therapeutic benefit within this time period |
| ADEPT;55 ADA | 40 mg every other week, subcutaneous injection | 24 weeks | Open label from 24 weeks (in which all patients were eligible for ADA) | 144 weeks | Clinical response is usually achieved within 12 weeks of treatment. Continued therapy should be carefully reconsidered in a patient not responding within this time period |
| SPIRIT-P1;57,67 ADA | 40 mg every other week, subcutaneous injection | NR | NR | NR | |
| Genovese et al., 2007;56 ADA | 40 mg every other week, subcutaneous injection | 12 weeks | Open label from 12 weeks (in which all patients were eligible for ADA) | 24 weeks | |
| IMPACT;51 INF | 5 mg/kg, i.v. infusion followed by additional 5 mg/kg infusion doses at 2 and 6 weeks after the first infusion, then every 8 weeks | 16 weeks | At week 16 patients initially assigned to receive placebo crossed over to receive 5 mg/kg INF | 98 weeks | NR |
| IMPACT 2;52 INF | 5 mg/kg, i.v. infusion followed by additional 5 mg/kg infusion doses at 2 and 6 weeks after the first infusion, then every 8 weeks | 16 weeks | At week 16 placebo patients with < 10% improvement in both TJC and SJC received 5 mg/kg of INF. Open label from 24 weeks (in which all patients were eligible for INF) | 54 weeks | |
| Mease et al., 2004;54 ETN | 25 mg twice weekly, subcutaneous injection | 24 weeks | Open label from 24 weeks (in which all patients were eligible for ETN) | 104 weeks | Clinical response is usually achieved within 12 weeks of treatment. Continued therapy should be carefully reconsidered in a patient not responding within this time period |
| Mease et al., 2000;53 ETN | 25 mg twice weekly, subcutaneous injection | 12 weeks | Open label from 12 weeks (in which all patients were eligible for ETN) | 36 weeks | |
| Atteno et al., 2010;64 INF, ETN and ADA | 5 mg/kg every 6–8 weeks of INF; 25 mg of ETN twice weekly; 40 mg of ADA every other week | 52 weeks (blinding not feasible) | No crossovers | 52 weeks | See details for trials of INF, ETN and ADA |
| Trial | Trialled treatments and doses | Doses included in the review | Dose included in the NMA | Comments |
|---|---|---|---|---|
| FUTURE 248 | 75 mg of SEC; 150 mg of SEC; 300 mg of SEC; placebo | 150 mg of SEC; 300 mg of SEC; placebo | 150 mg of SEC; 300 mg of SEC; placebo | 75 mg is not a licensed dose for PsA |
| ERASURE49 | 150 mg of SEC; 300 mg of SEC; placebo | 300 mg of SEC; placebo | – | The severity of psoriasis seen in the population studied in this trial (> 30% BSA involvement) suggests that the 150-mg arm results have very limited relevance to clinical practice (as these patients are likely to receive 300 mg). Excluded from NMA as baseline PASI and HAQ-DI scores very different from other trials |
| FIXTURE49 | 50 mg of ETN twice weekly; 150 mg of SEC; 300 mg of SEC; placebo | 300 mg of SEC; placebo | – | The severity of psoriasis seen in the population studied in this trial (> 30% BSA involvement) suggests that the 150-mg arm results have very limited relevance to clinical practice (as these patients are likely to receive 300 mg). Excluded from NMA as baseline PASI and HAQ-DI scores very different from other trials. 50 mg of ETN twice weekly excluded as not a licensed dose in PsA |
| CLEAR62 | 300 mg of SEC; 45 or 90 mga of UST | 300 mg of SEC; 45 or 90 mg of UST | – | Baseline characteristics within the subgroup with PsA were not reported, therefore it is not clear how severe the psoriasis is within this subgroup. Excluded from the NMA based on high mean PASI scores in whole-trial population |
| SPIRIT-P157,67 | 80 mg of IXE every 2 weeks; 80 mg of IXE every 4 weeks; 40 mg of ADA; placebo | 40 mg of ADA; placebo | 40 mg of ADA; placebo | IXE is not an eligible treatment for this review |
| RAPID-PsA47 | 200 mg of CZP every 2 weeks; 400 mg of CZP every 4 weeks; placebo | 200 mg of CZP every 2 weeks; 400 mg of CZP every 4 weeks; placebo | 200 mg of CZP every 2 weeks; 400 mg of CZP every 4 weeks; placebo | |
| PALACE 160 | 20 mg of APR; 30 mg of APR; placebo | 30 mg of APR; placebo | 30 mg of APR; placebo | 20 mg is not a licensed dose |
| PALACE 265 | 20 mg of APR; 30 mg of APR; placebo | 30 mg of APR; placebo | 30 mg of APR; placebo | 20 mg is not a licensed dose |
| PALACE 365 | 20 mg of APR; 30 mg of APR; placebo | 30 mg of APR; placebo | 30 mg of APR; placebo | 20 mg is not a licensed dose |
| PSUMMIT 259 | 45 mg of UST; 90 mg of UST; placebo | 45 mg of UST; placebo | 45 mg of UST; placebo | The 90-mg arm was excluded as it was not administered as per the licence for all patients |
| PSUMMIT 166 | 45 mg of UST; 90 mg of UST; placebo | 45 mg of UST; placebo | 45 mg of UST; placebo | The 90-mg arm was excluded as it was not administered as per the licence |
| Atteno et al. 201064 | 25 mg of ETN; 5 mg/kg INF; 40 mg of ADA | 25 mg of ETN; 5 mg/kg INF; 40 mg of ADA | – | Excluded from the NMA – only 1 year of data are available |
| GO-REVEAL50 | 50 mg of GOL; 100 mg of GOL; placebo | 50 mg of GOL; placebo | 50 mg of GOL; placebo | The 100-mg arm was excluded as it was not administered as per the licence |
| Genovese et al., 200756 | 40 mg of ADA; placebo | 40 mg of ADA; placebo | 40 mg of ADA; placebo | – |
| ADEPT55 | 40 mg of ADA; placebo | 40 mg of ADA; placebo | 40 mg of ADA; placebo | – |
| IMPACT51 | 5 mg/kg INF; placebo | 5 mg/kg INF; placebo | 5 mg/kg INF; placebo | – |
| IMPACT 252 | 5 mg/kg INF; placebo | 5 mg/kg of INF; placebo | 5 mg/kg INF; placebo | – |
| Mease et al., 200454 | 25 mg of ETN; placebo | 25 mg of ETN; placebo | 25 mg of ETN; placebo | – |
| Mease et al., 200053 | 25 mg of ETN; placebo | 25 mg of ETN; placebo | 25 mg of ETN; placebo | – |
| Trials excluded from the main review of short-term efficacy | ||||
| FUTURE 141 | 150 mg of SEC; placebo | – | – | Excluded: used unlicensed loading dose. Safety data from the manufacturer submission are eligible though |
| PRESTA42 | 50 mg of ETN twice weekly; 50 mg of ETN once weekly | – | – | Excluded on comparator: not a placebo-controlled trial and 50 mg of ETN twice weekly is not a licensed dose |
| Schett et al., 201243 | 20 mg of APR; 40 mg of APR; placebo | – | – | Excluded: did not include licensed dose (30 mg of APR) |
The design of many trials typically included a fully blinded, placebo-controlled phase followed by an ‘early escape’ crossover phase (from placebo to an active treatment) for non-responders, then finally crossover to active treatment for the remaining placebo participants. Non-response in this context related to failure to achieve prespecified minimum improvements (ranging between 5% and 20%) in tender joint count (TJC) and swollen joint count (SJC). All the trials using an early escape design ran for 16 weeks before patients were eligible for early escape. Trials then entered open-label extension phases (see Long-term effectiveness).
Table 4 describes the population characteristics of the included trials. Where available, this includes subgroup characteristics for patients who had never previously taken a biologic (i.e. biologic-naive populations) and patients who had previously taken a biologic (i.e. biologic-experienced populations). Biologic-experienced patients were available only for the more recent trials (those of SEC, CZP, UST and APR); in the earlier trials such patients were not eligible to participate. Trial sample sizes varied, with earlier trials tending to be smaller than more recent trials. Variation in sample size was also evident within treatments: the two trials of ETN had populations of 60 and 205,53,54 and the three trials of ADA had populations of 100, 207 and 315. 55–57,67 The duration of PsA ranged from 3 to 12 years across trials; the shortest durations (reported as medians) came from the UST PSUMMIT trials58,59,66 and the longest (reported as means) came from the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT). 51,52 The duration of psoriasis ranged from 11 to 23 years, although this information was not available for the FUTURE 248 SEC and RAPID-PsA47 (Certolizumab Pegol in Subjects With Adult Onset Active and Progressive Psoriatic Arthritis) CZP trials. Although not reported in all trials, baseline CRP concentration levels were difficult to interpret as they appeared to have slightly skewed distributions, with means (range 10–31 mg/l) being generally higher than medians (range 7–15 mg/l).
| Trial | Characteristic | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial arm | Number randomised | Age (years), mean (SD) | % male | Duration of PsA (years), mean (SD) | Duration of psoriasis (years), mean (SD) | CRP concentration (mg/l) (SD) | TJC, mean (SD) | SJC, mean (SD) | HAQ-DI, mean (SD) | PASI-evaluable patients ≥ 3% BSA (%) | PASI (0–72), mean (SD) | MTX use at randomisation (%) | |
| FUTURE 2;48 all patients | 150 mg of SEC | 100 | 46.5 (11.7) | 55 | – | – | – | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 58 (58) | 16.2 (14.3) | 44 |
| 300 mg of SEC | 100 | 46.9 (12.6) | 51 | – | – | – | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 41 (41) | 11.9 (8.4) | 44 | |
| Placebo | 98 | 49.9 (12.5) | 40 | – | – | – | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 43 (44) | 11.6 (8.3) | 51 | |
| FUTURE 2;48 biologic experienced | SEC; for pooled doses | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||||||||||
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| FUTURE 2;48 biologic naive | SEC; for pooled doses | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||||||||||
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| aERASURE49,68 | 300 mg of SEC | 57 | 46.1 (12.0) | 58 | – | 19.7 (12.7) | – | – | – | 0.8 (0.8) | 57 (100: subgroup) | 21.4 (8.7) | – |
| Placebo | 68 | 48.4 (12.4) | 63 | – | 22.6 (13.7) | – | – | – | 0.8 (0.6) | 68 (100: subgroup) | 21.3 (10.1) | – | |
| aFIXTURE49 | 300 mg of SEC | 50 | 47.8 (15.3) | 52 | – | 21.7 (15.3) | – | – | – | 0.7 (0.6) | 50 (100: subgroup) | 25.8 (10.9) | – |
| 100 mg of ETN per week | 44 | 46.4 (12.0) | 57 | – | 22.6 (13.0) | – | – | – | 0.7 (0.6) | 44 (100: subgroup) | 21.9 (7.5) | – | |
| Placebo | 49 | 45.7 (11.6) | 55 | – | 20.5 (13.1) | – | – | – | 0.5 (0.6) | 49 (100: subgroup) | 23.7 (8.4) | – | |
| aCLEAR62,63 | 300 mg of SEC | 69 | Baseline data not available for subgroup (the 123 patients with PsA) | ||||||||||
| UST | 54 | ||||||||||||
| SPIRIT-P157,67 | ADAb | 101 | Baseline data not available (trial reported only in conference abstracts) | ||||||||||
| Placebo | 106 | ||||||||||||
| RAPID-PsA;47 all patients | 200 mg of CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 400 mg of CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| RAPID-PsA;47 biologic experienced | Pooled CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| RAPID-PsA;47 biologic naive | Pooled CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| RAPID-PsA;47 biologic experienced (≥ 3% BSA and a PASI score of > 10 units at baseline) | Pooled CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| RAPID-PsA;47 biologic naive (≥ 3% BSA and a PASI score of > 10 units at baseline) | Pooled CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| PALACE 160,69 | 30 mg of APR | 168 | 51.4 (11.7) | 45 | 8.1 (8.1) | 16.50 (12.3) | 8.4 (10.2) | 23.1 (14.5) | 12.8 (7.8) | 1.2 (0.6) | 82 (49) | 9.2 (9.7) | 52 |
| Placebo | 168 | 51.1 (12.1) | 52 | 7.3 (7.1) | 15.7 (13.0) | 11 (14.4) | 23.3 (15.2) | 12.8 (8.8) | 1.2 (0.6) | 68 (41) | 9.1 (9.5) | 54 | |
| PALACE 261,65,69 | 30 mg of APR | 162 | 50.5 (11.2) | 41 | 6.8 (7.6) | 18.7 (14.5) | – | 21.8 (16.8) | 10.3 (8.1) | 1.2 (0.6) | – | 7.8 (7.3) | 70 |
| Placebo | 159 | 51.2 (11.0) | 47 | 7.8 (8.3) | 17.8 (13.9) | – | 18.0 (13.5) | 9.2 (6.6) | 1.2 (0.6) | – | 8.6 (10.0) | 59 | |
| PALACE 361,65,69 | 30 mg of APR | 167 | 49.9 (11.4) | 47 | 7.5 (7.6) | 17.1 (12.1) | – | 20.9 (14.4) | 11.6 (8.7) | 1.2 (0.6) | – | 7.9 (6.3) | 50 |
| Placebo | 169 | 49.5 (11.6) | 46 | 6.8 (6.5) | 17.8 (13.3) | – | 18.3 (14.9) | 11.1 (7.9) | 1.2 (0.6) | – | 7.6 (7.2) | 54 | |
| PSUMMIT 2;59,66 all patients | 45 mg of UST | 103 | 49.0 (40, 56)c | 47 | 5.3 (2.3, 12.2)c | 13.3 (5.0, 24.4)c | 13.0 (4.5, 36.3)c | 22 (15, 33)c | 12 (8, 19)c | 1.4 (0.8, 1.9)c | 80 (78) | 8.6 (4.5, 18.3)c | 52 |
| 90 mg of UST | 105 | 48.0 (41, 57)c | 47 | 4.5 (1.7, 10.3)c | 11.3 (4.5, 21.4)c | 10.1 (4.8, 19.8)c | 22 (14, 36)c | 11 (7, 17)c | 1.3 (0.8, 1.9)c | 81 (77) | 8.8 (4.5, 18.0)c | 50 | |
| Placebo | 104 | 48.0 (38.5–56.0)c | 49 | 5.5 (2.3–12.2)c | 11.4 (6.0–22.0)c | 8.5 (4.6, 22.0)c | 21 (11–30)c | 11 (7–18)c | 1.3 (0.8–1.8)c | 80 (77) | 7.9 (4.5–16.0)c | 47 | |
| PSUMMIT 2;59,66 biologic experienced | 45 mg of UST | 60 | 49.0 (39, 55)c | 38 | 7.3 (4.1, 13.7)c | 15.5 (7.1, 24.7)c | 15.0 (4.9, 37.0)c | 24.0 (16.5, 40.5)c | 14.5 (7.5, 20.5)c | 1.4 (0.8, 2.0)c | – | – | – |
| 90 mg of UST | 58 | 48 (40, 56)c | 38 | 5.7 (2.5, 10.5)c | 12.6 (7.3, 23.4)c | 10.9 (6.9, 26.8)c | 25.5 (17.0, 43.0)c | 12.5 (7.0, 19.0)c | 1.6 (0.9, 1.9)c | – | – | – | |
| Placebo | 62 | 48.5 (37, 55)c | 50 | 7.1 (4.1, 12.5)c | 12.3 (8.3, 22.4)c | 8.7 (4.2, 22.3)c | 24.0 (12.0, 31.0)c | 11.0 (7.0, 17.0)c | 1.3 (0.8, 1.8)c | – | – | – | |
| PSUMMIT 158,66 | 45 mg of UST | 205 | 48.0 (39, 55)c | 52 | 3.4 (1.2–9.2)c | 12.0 (4.1–22.2)c | 10.0 (5.9, 21.1)c | 18 (12–28)c | 10 (7–15)c | 1.3 (0.8–1.8)c | 145 (71) | 7.1 (3.3–15.3)c | 48 |
| 90 mg of UST | 204 | 47.0 (38.5–54.0)c | 57 | 4.9 (1.7–8.3)c | 14.1 (5.4–22.4)c | 12.3 (6.5, 21.7)c | 20 (12–32)c | 10 (7–16)c | 1.3 (0.8–1.6)c | 149 (73) | 8.4 (4.8–14.7)c | 50 | |
| Placebo | 206 | 48.0 (39, 57)c | 52 | 3.6 (1.0–9.7)c | 13.1 (5.3–23.5)c | 9.6 (6.0, 18.6)c | 22 (13–33)c | 12 (8–19)c | 1.3 (0.8–1.8)c | 146 (71) | 8.8 (4.4–14.3)c | 47 | |
| Atteno et al., 201064 | ETN | 36 | 49.3 (13.4) | – | – | – | – | 13 | 4 | 1.2 (0.4)b | – | 26 (18.5)b | 51 |
| ADA | 34 | 47.5 (11.5) | – | – | – | – | 13 | 5 | 1.2 (0.3)b | – | 18 (16.5)b | – | |
| INF | 30 | 48.5 (12.9) | – | – | – | – | 12 | 3 | 1.5 (0.5)b | – | 15 (14.8)b | – | |
| GO-REVEAL50 | 50 mg of GOL | 146 | 45.7 (10.7) | 61 | 7.2 (6.8) | 17.7 (11.9) | 13 (16) | 24.0 (17.1) | 14.1 (11.4) | 0.98 (0.65) | 109 (75) | 9.8 (8.6) | 49 |
| Placebo | 113 | 47.0 (10.6) | 61 | 7.6 (7.9) | 19.0 (12.9) | 13 (16) | 21.9 (14.7) | 13.4 (9.8) | 1.03 (0.55) | 79 (70) | 8.4 (7.4) | 48 | |
| Genovese et al., 200756 | ADA | 51 | 50.4 (11.0) | 57 | 7.5 (7.0) | 18.0 (13.2) | 10 (10) | 25.3 (18.3) | 18.2 (10.9) | 0.9 (0.5) | – | – | 47 |
| Placebo | 49 | 47.7 (11.3) | 51 | 7.2 (7.0) | 13.8 (10.7) | 16 (17) | 29.3 (18.1) | 18.4 (12.1) | 1.0 (0.7) | – | – | 47 | |
| ADEPT55 | ADA | 153 | 48.6 (12.5) | 56 | 9.8 (8.3) | 17.2 (12.0) | 14 (21) | 23.9 (17.3) | 14.3 (12.2) | 1.0 (0.6) | 70 (46) | 7.4 (6.0) | 51 |
| Placebo | 162 | 49.2 (11.1) | 55 | 9.2 (8.7) | 17.1 (12.6) | 14 (17) | 25.8 (18.0) | 14.3 (11.1) | 1.0 (0.7) | 70 (43) | 8.3 (7.2) | 50 | |
| IMPACT 252 | INF | 100 | 47.1 (12.8) | 71 | 8.4 (7.2) | 16.2 (11.0) | 19 (21) | 24.6 (14.1) | 13.9 (7.9) | 1.1 (0.6) | 83 (83) | 11.4 (12.7) | 47 |
| Placebo | 100 | 46.5 (11.3) | 51 | 7.5 (7.8) | 16.8 (12.0) | 23 (34) | 25.1 (13.3) | 14.4 (8.9) | 1.1 (0.6) | 87 (87) | 10.2 (9.0) | 45 | |
| IMPACT51 | INF | 52 | 45.7 (11.1) | 58 | 11.7 (6.6) | 16.9 (10.9) | 22 (27) | 23.7 (13.7) | 14.6 (7.5) | 1.2 (0.7) | 22 (42)d | 5.1 (5.9) | 46 |
| Placebo | 52 | 45.2 (9.7) | 58 | 11 (6.6) | 19.4 (11.6) | 31 (38) | 20.4 (12.1) | 14.7 (8.2) | 1.2 (0.7) | 17 (33)d | 4.2 (5.8) | 65 | |
| Mease et al., 200454 | ETN | 101 | 47.6 | 57 | 9.0 | 18.3 | – | 20.4 (–)b | 15.9 (–)b | 1.1 (–)b | – | – | 45 |
| Placebo | 104 | 47.3 | 45 | 9.2 | 19.7 | – | 22.1 (–)b | 15.3 (–)b | 1.1 (–)b | – | – | 49 | |
| Mease et al., 200053 | ETN | 30 | 46.0 (30–70)e | 53 | 9.0 (1–31)e | 19.0 (4–53)e | 14 (7–28)e | 22.5 (11, 32)b | 14.0 (8, 23)b | 1.3 (0.9, 1.6)b | 19 (63) | 10.1 (2.3–30.0)d | 47 |
| Placebo | 30 | 43.5 (24–63)e | 60 | 9.5 (1–30)e | 17.5 (2–43)e | 12 (8–22)c | 19.0 (10, 39)c | 14.7 (7, 24)c | 1.2 (0.8, 1.6)c | 19 (63) | 6.0 (1.5–17.7)e | 47 | |
Notwithstanding this limited heterogeneity, many key patient characteristics were broadly similar across trials, including mean ages (which ranged from 45 to 51 years), the proportion of male participants (around 50% for most trials), and TJCs and SJCs (TJC, range 18–29; SJC, range 9–18); an exception was the three-arm head-to-head trial, which had notably lower TJC and SJC. 64 The population in this trial, along with the PsA populations from the large SEC psoriasis trials,49 also had markedly higher baseline PASI scores than the other trials (typically around two to three times higher). The FUTURE 2 SEC trial had slightly higher baseline PASI scores than the other trials, most notably in the 150 mg treatment arm. The PsA populations from two of the SEC psoriasis trials49 also had lower baseline HAQ-DI scores (range 0.5–0.8 units) than the other trials (range 0.9–1.6 units). In light of these differences, the characteristics of the PsA patients in the SEC psoriasis trials were deemed to be too dissimilar to the other trials to be included in the NMAs. There were three of these psoriasis trials: Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis (ERASURE), Full Year Investigative Examination of Secukinumab vs. Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis (FIXTURE) and Efficacy of Secukinumab Compared to Ustekinumab in Patients with Plaque-type Psoriasis (CLEAR; baseline data were not available for the PsA patients in CLEAR). To be eligible for the ERASURE, FIXTURE and CLEAR trials, patients had to have moderate–severe psoriasis based on a PASI score of > 12 units and BSA involvement of ≥ 10%. 49 In the trials only of patients with PsA, the proportion of patients with at least moderate psoriasis (i.e. PASI-evaluable patients, defined as a BSA involvement of ≥ 3%) ranged between 41% and 87%.
In the FUTURE 2 (SEC)48 and RAPID-PsA (CZP)47 trials, the biologic-experienced and biologic-naive subgroups were broadly similar except that the biologic-experienced subgroups tended to have slightly higher TJCs and SJCs, and slightly longer durations of PsA.
All the trials of ETN, INF, ADA and GOL and one UST trial58 (nine in total) excluded patients who had previously received an anti-TNF, so their populations comprised entirely biologic-naive patients (Table 5). In the remaining trials, where reported, the proportion of biologic-experienced patients ranged from 15% to 58%. Of the trials that allowed recruitment of biologic-experienced patients, the RAPID-PsA trial47 was more selective than the FUTURE 2,48 PSUMMIT 259,66 and PALACE trials. 60,61,65 RAPID-PsA47 was the only trial that excluded patients with primary failure of a previous anti-TNF (primary failure was defined as no response within the first 12 weeks of treatment with the anti-TNF). (See Appendix 2, which details the eligibility criteria for all trials.) The results for the RAPID-PsA biologic-experienced subgroup may therefore be somewhat inflated when compared with the other trials reporting results for this subgroup.
| Study | Interventions and dose | Number of prior DMARDs, mean | Percentage of patients with numbers of previous DMARDs | Previous biologic therapy | Concomitant treatments during trial (%) | ||
|---|---|---|---|---|---|---|---|
| Corticosteroids | NSAIDs | MTX | |||||
| FUTURE 248 | 150 mg of SEC | – | – | 0 = 63%, 1 = 26%, 2–3 = 11% | 23 | – | 44 |
| 300 mg of SEC | – | – | 0 = 67%, 1 = 16%, 2–3 = 17% | 18 | – | 44 | |
| Placebo | – | – | 0 = 64%, 1 = 16%, 2–3 = 19% | 21 | – | 51 | |
| aERASURE49 | 300 mg of SEC | – | – | 42% had a prior biologic | – | – | – |
| Placebo | – | – | 44% had a prior biologic | – | – | – | |
| aFIXTURE49 | 300 mg of SEC | – | – | 22% had a prior biologic | – | – | – |
| 100 mg of ETN per week | – | – | 18% had a prior biologic | – | – | – | |
| Placebo | – | – | 18% had a prior biologic | – | – | – | |
| aCLEAR62,63 | SEC | – | – | – | – | – | – |
| UST | – | – | – | – | – | – | |
| SPIRIT-P157,67 | ADA | No data available, other than that biologic-experienced patients were excluded from the trial. This trial was reported only as conference abstracts | |||||
| Placebo | |||||||
| RAPID-PsA47 | 200 mg of CZP | – | 1 = 44%, ≥ 2 = 53% | 23% had a prior biologic | – | – | 64 |
| 400 mg of CZP | – | 1 = 53%, ≥ 2 = 45% | 17% had a prior biologic | – | – | 65 | |
| Placebo | – | 1 = 54%, ≥ 2 = 44% | 19% had a prior anti-TNF | – | – | 62 | |
| PALACE 160 | 30 mg of APR | 2% had never received a DMARD | 24% had a prior biologic | – | – | 52 | |
| Placebo | 4% had never received a DMARD | 24% had a prior anti-TNF | – | – | 54 | ||
| PALACE 261,65 | 30 mg of APR | 3% had never received a DMARD | 14% had a prior biologic | – | – | 70 | |
| Placebo | 1% had never received a DMARD | 15% had a prior biologic | – | – | 59 | ||
| PALACE 361,65 | 30 mg of APR | All patients had previously received a DMARD | 26% had a prior biologic | – | – | 50 | |
| Placebo | All patients had previously received a DMARD | 28% had a prior biologic | – | – | 54 | ||
| PSUMMIT 259,66 | 45 mg of UST | – | 14% had never received a DMARD | 180 (58%) had a prior anti-TNF | 20 | 70 | 52 |
| 90 mg of UST | – | 15 | 67 | 50 | |||
| Placebo | – | 13 | 74 | 47 | |||
| PSUMMIT 158,66 | 45 mg of UST | – | 20% had never received a DMARD | Biologic-experienced patients excluded | 18 | 76 | 48 |
| 90 mg of UST | – | 14 | 74 | 50 | |||
| Placebo | – | 16 | 73 | 47 | |||
| Atteno et al., 201064 | ETN | – | – | Biologic-experienced patients excluded | – | – | 51 |
| ADA | – | – | – | – | |||
| INF | – | – | – | – | |||
| GO-REVEAL50 | 50 mg of GOL | – | 0 = 25%, 1–2 = 69%, > 2 = 6% | Biologic-experienced patients excluded | 13 | 75 | 49 |
| Placebo | – | 0 = 25%, 1–2 = 66%, > 2 = 9% | 17 | 78 | 48 | ||
| Genovese et al., 200756 | ADA | 1.7 | All patients had a history of DMARD therapy | Biologic-experienced patients excluded | – | 73 | 47 |
| Placebo | 2.1 | – | 86 | 47 | |||
| ADEPT55 | ADA | 1.5 | – | Biologic-experienced patients excluded | – | – | 51 |
| Placebo | 1.5 | – | – | – | 50 | ||
| IMPACT 252 | INF | – | 0 = 17%, 1–2 = 71%, > 2 = 12% | Biologic-experienced patients excluded | 15 | 71 | 47 |
| Placebo | – | 0 = 24%, 1–2 = 67%, > 2 = 9% | 10 | 73 | 45 | ||
| IMPACT51 | INF | – | 0 = 0%, 1 = 52%, 2–3 = 37%, > 3 = 12% | Biologic-experienced patients excluded | 17 | 89 | 46 |
| Placebo | – | 0 = 2%, 1 = 38%, 2–3 = 48%, > 3 = 12% | 29 | 79 | 65 | ||
| Mease et al., 200454 | ETN | 1.6 | 0 = 27%, 1 = 40%, 2 = 20% | Biologic-experienced patients excluded | 19 | 88 | 45 |
| Placebo | 1.7 | 0 = 21%, 1 = 50%, 2 = 19% | 15 | 83 | 49 | ||
| Mease et al., 200053 | ETN | 1.5 | – | Biologic-experienced patients excluded | 20 | 67 | 47 |
| Placebo | 2.0 | – | 40 | 77 | 47 | ||
Risk-of-bias assessments
The proportion of patients who took concomitant MTX ranged from 44% to 70%; most trials allowed concomitant MTX although the FIXTURE and ERASURE psoriasis trials49 did not. The reporting of data on the number of previous DMARDs used was limited, although it appeared that most patients had tried one or two DMARDs.
The results of the risk-of-bias assessments are presented in Table 6. All except one57,67 of the trials included in the NMAs were judged as being at low overall risk of bias. Only one trial64 was rated as being at high overall risk of bias for all outcomes, which was primarily due to lack of blinding. However, blinding would have been both difficult and impractical as the trial compared INF, ETN and ADA. 64 All the other trials were appropriately blinded. Across the trials the randomisation methods were well reported; only the head-to-head trial had unclear judgements for both sequence generation and allocation concealment. 64 The only chance imbalance of note occurred in the PSUMMIT 2 trial, in which median CRP concentration levels were higher in the 45-mg group (13 mg/l) than in the placebo group (8.5 mg/l). 59 Two of the three SEC trials in patients with psoriasis and PsA had overall judgements as being at unclear risk of bias. 49 This was because PsA subgroup data were being assessed and no details were available on missing outcome data. IMPACT 252 was rated as being at high risk of bias for the PASI 75 outcome, as last observation carried forward (LOCF) was used for missing data (instead of the more conservative non-responder imputation).
| Drug and trial | Risk-of-bias domain | Overall judgement | ||||||
|---|---|---|---|---|---|---|---|---|
| Sequence generation | Allocation concealment | Important baseline imbalance | Blinding of participants and researchers | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | ||
| SEC; FUTURE 248 | ||||||||
| Judgement | Low | Low | Low | Low | Low | Low | Low | Low |
| Support | IVRS used | IVRS used | 15% difference in the proportion of males although this will be a chance imbalance (based on randomisation methods) | Doses were provided in identical prefilled syringes | Doses were provided in identical prefilled syringes | More withdrawals in the placebo group but NRI and LOCF were used for missing data | Results reported for all key outcomes | |
| SEC; FIXTURE (subgroup)49 | ||||||||
| Judgement | Low | Low | Unclear | Low | Low | Unclear | Low | Unclear |
| Support | IVRS used | IVRS used | No data on CRP levels | Adequate blinding (placebo controlled). Double-dummy design used as there was an active comparator arm | Adequate blinding (placebo controlled). Double-dummy design used as there was an active comparator arm | Unclear for the PsA subpopulation | Results reported for all key outcomes | |
| SEC; ERASURE (subgroup)49 | ||||||||
| Judgement | Low | Low | Unclear | Low | Low | Unclear | Low | Unclear |
| Support | IVRS used | IVRS used | No data on CRP levels | Adequate blinding (placebo controlled) | Adequate blinding (placebo controlled) | Unclear for the PsA subpopulation | Results reported for all key outcomes | |
| SEC; CLEAR62,63 | ||||||||
| Judgement | Low | Low | Low | Low | Low | Low | Low | Low |
| Support | IVRS used | IVRS used | In the psoriasis trial as a whole, demographic and disease characteristics were similar between treatment armsa | Treatments looked identical | Treatments looked identical | Dropouts for the subgroup with PsA were not reported. In the psoriasis trial as a whole, there were no imbalances in dropouts between groups | Results reported for key outcomes | |
| ADA; SPIRIT-P157,67 | ||||||||
| Judgement | Unclear | Unclear | Unclear | Low | Low | Low | Low | Unclear |
| Support | Randomisation sequence not reported | NR | NR | Double blind (subject, caregiver, investigator, outcomes assessor) | Double blind (subject, caregiver, investigator, outcomes assessor) | NRI was used for missing data; continuous data of inadequate responders were excluded after 16 weeks | All main outcomes reported | |
| CZP; RAPID-PsA47 | ||||||||
| Judgement | Low | Low | Low | Low | Low | Low | Low | Low |
| Support | IVRS used | IVRS used | Balanced | Blinded prefilled syringes were used | Blinded prefilled syringes were used | NRI and LOCF were used for missing data | Results reported for all key outcomes | |
| APR; PALACE 160,69 | ||||||||
| Judgement | Low | Low | Low | Low | Low | Low | Low | Low |
| Support | IVRS used | IVRS used | Balanced | EMA report states that identical tablets and blister cards were used in the APR psoriasis trialsb | See blinding of participants and researchers cell | NRI and LOCF (for the sensitivity analysis only) were used | All main outcomes reported | |
| APR; PALACE 265,69 | ||||||||
| Judgement | Low | Low | Unclear | Low | Low | Low | Low | Low |
| Support | IVRS used | IVRS used | Data not available for individual trials | As for PALACE 1 | As for PALACE 1 | NRI and LOCF used. Similar withdrawal rates in pooled analysis | All main outcomes reported | |
| APR; PALACE 365,69 | ||||||||
| Judgement | Low | Low | Unclear | Low | Low | Low | Low | Low |
| Support | IVRS used | IVRS used | Data not available for individual trials | As for PALACE 1 | As for PALACE 1 | NRI and LOCF used. Similar withdrawal rates in pooled analysis | All main outcomes reported | |
| UST; PSUMMIT 259 | ||||||||
| Judgement | Low | Low | Unclear | Low | Low | Low | Low | Low |
| Support | IVRS used | IVRS used | Chance imbalance in median CRP levels (placebo, 8.5 mg/l, vs. 45 mg of UST, 13.0 mg/l) | Based on details in table 9 of Craig et al.’s 2013 UST STA66 | Based on details in table 9 of Craig et al.’s 2013 UST STA66 | Low dropout rate. NRI for ACR and PASI and LOCF for change in HAQ-DI score. Otherwise, missing data were not imputed for the rest of the outcomes | All main outcomes reported | But important imbalance, likely due to chance |
| UST; PSUMMIT 166 | ||||||||
| Judgement | Low | Low | Low | Low | Low | Low | Low | Low |
| Support | IVRS used | IVRS used | Balanced | Based on details in table 9 of Craig et al.’s 2013 UST STA66 | Based on details in table 9 of Craig et al.’s 2013 UST STA66 | Low dropout rate. NRI and LOCF used | All main outcomes reported | |
| INF vs. ETN vs. ADA; Atteno et al., 201064 | ||||||||
| Judgement | Unclear | Unclear | Unclear | High | High | Unclear | Unclear | High |
| Support | Study drugs were ‘randomly given’ | Study drugs were ‘randomly given’ | No data on CRP levels | Head-to-head trial of treatments with different regimens | Head-to-head trial of treatments with different regimens | No information on withdrawals nor on imputation methods | No prior registration | |
| GOL; GO-REVEAL50 | ||||||||
| Judgement | Low | Low | Low | Low | Low | Low | Low | Low |
| Support | IVRS used | IVRS used | Balanced | Based on text in Yang et al.’s full STA report70 | Based on text in Yang et al.’s full STA report70 | Although there was insufficient detail on imputation methods, there were few dropouts (and balanced across groups) | All main outcomes reported | |
| ADA; Genovese et al., 200756 | ||||||||
| Judgement | Low | Low | Low | Low | Low | Low | Low | Low |
| Support | Based on details in table 10 of Rodgers et al.33 | Based on details in table 10 of Rodgers et al.33 | Balanced | Based on details in table 10 of Rodgers et al.33 | Based on details in table 10 of Rodgers et al.33 | NRI and LOCF were used for missing data | Results reported for all key outcomes | |
| ADA; ADEPT55 | ||||||||
| Judgement | Low | Unclear | Low | Low | Low | Low | Low | Low |
| Support | Based on details in table 10 of Rodgers et al.33 | NR | Balanced | Based on details in table 10 of Rodgers et al.33 | Based on details in table 10 of Rodgers et al.33 | NRI was used for missing data. In addition, similar levels of dropout across groups and similar reasons | Results reported for all key outcomes | |
| INF; IMPACT 252 | ||||||||
| Judgement | Low | Low | Unclear | Low | Low | High | Low | High, PASI 75; low, other outcomes |
| Support | Based on details in table 6 of Rodgers et al.33 | Based on details in table 6 of Rodgers et al.33 | 20% difference in proportion of males although this will be a chance imbalance (based on randomisation methods) | Based on details in table 6 of Rodgers et al.33 | Based on details in table 6 of Rodgers et al.33 | NRI was used for missing PsARC and ACR 20 data. LOCF used for PASI 75. Unclear for HAQ-DI (appears to be LOCF) | Results for all key outcomes reported | |
| INF; IMPACT51 | ||||||||
| Judgement | Low | Low | Low | Low | Low | Low | Low | Low |
| Support | Based on details in table 6 of Rodgers et al.33 | Based on details in table 6 of Rodgers et al.33 | Mean CRP levels were 31 mg/l for placebo and 22 mg/l for INFc | Based on details in table 6 of Rodgers et al.33 | Based on details in table 6 of Rodgers et al.33 | Very few dropouts | Results for all key outcomes reported | Low |
| ETN; Mease et al., 200454 | ||||||||
| Judgement | Low | Low | Unclear | Low | Low | Low | Low | Low |
| Support | Based on details in table 2 of Rodgers et al.33 | Based on details in table 2 of Rodgers et al.33 | 12% difference in proportion of males although this will be a chance imbalance (based on randomisation methods) | Based on details in table 2 of Rodgers et al.33 | Based on details in table 2 of Rodgers et al.33 | More withdrawals in the placebo group; NRI and LOCF were used for missing data | Results reported for all key outcomes | |
| ETN; Mease et al., 200053 | ||||||||
| Judgement | Low | Low | Low | Low | Low | Low | Low | Low |
| Support | Based on details in table 2 of Rodgers et al.33 | Based on details in table 2 of Rodgers et al.33 | Balanced | Based on details in table 2 of Rodgers et al.33 | Based on details in table 2 of Rodgers et al.33 | Although LOCF was used for missing data (no NRI), there were only four dropouts, all in the placebo group | Results reported for all key outcomes | |
Short-term efficacy of secukinumab
The clinical effectiveness evidence identified for SEC consisted of four Phase III RCTs: FUTURE 2, ERASURE, FIXTURE and CLEAR. 48,49,62,63 The FUTURE 2 trial48 was of patients with PsA and the ERASURE,49 FIXTURE49 and CLEAR trials62,63 were trials of patients with psoriasis and reported subgroup data for patients who also had PsA. The FUTURE 2 trial48 provides the main evidence for SEC. FUTURE 146 studied a non-licensed, very high, loading dose (10 mg/kg) followed by a 150-mg maintenance dose. Although this trial was therefore not eligible to contribute data to the review of efficacy of SEC, nor to be included in the evidence synthesis, it has been used to provide supportive evidence on SEC as, unlike FUTURE 2, it reports data on radiographic progression of joint damage (see Long-term effectiveness). FUTURE 248 and ERASURE49 compared 150 or 300 mg of SEC with placebo; FIXTURE49 compared 150 or 300 mg of SEC with ETN (100 mg/week) and placebo; and CLEAR62,63 compared 300 mg of SEC with 45 or 90 mg of UST (dosing was as per licence, 45 mg in patients weighing ≤ 100 kg and 90 mg for patients weighing > 100 kg).
There are three relevant ongoing trials for which results are not yet available (Table 7).
| Trial name and ClinicalTrials.gov reference | Purpose of trial |
|---|---|
| FUTURE 3;71 NCT01989468 | To provide 24- to 52-week efficacy, safety and tolerability data, as well as up to 3-year efficacy, safety and tolerability data, in subjects with active PsA despite current or previous NSAID, DMARD therapy and/or previous anti-TNF therapy using an autoinjector. Initial data were due to be published in 2016. Estimated primary completion date: January 2018 |
| FUTURE 4;72 NCT02294227 | To provide 16-week efficacy, safety and tolerability data vs. placebo to support the use of 150 mg of SEC by subcutaneous self-administration with or without a loading regimen and maintenance dosing using prefilled syringe and to assess efficacy, safety and tolerability up to 2 years in subjects with active PsA despite current or previous NSAID, non-biologic DMARD or biologic anti-TNF-α therapy. Recruitment closed (nine patients in the UK), but the study is still active. Estimated primary completion date: December 2017 |
| FUTURE 5;73 NCT02404350 | To demonstrate efficacy including effect on inhibition of progression of structural damage, safety and tolerability up to 2 years with primary focus at week 24, to support the use of SEC prefilled syringe by subcutaneous self-administration with or without loading regimen in subjects with active PsA despite current or previous NSAID, DMARD therapy and/or previous anti-TNF therapy. Patient recruitment began in 2015. Estimated primary completion date: May 2019 |
As previously discussed, the baseline characteristics of the ERASURE, FIXTURE and CLEAR49,62,63 subgroup populations were different to the baseline characteristics of the other trials. The patients in these trials had much higher baseline PASI scores and notably lower baseline HAQ-DI scores than the other trials, suggesting that these patients had more severe psoriasis and less severe arthritis symptoms (see Table 4).
The FUTURE 248 and CLEAR62,63 trials were judged as being at a low overall risk of bias with an unclear risk of overall judgements for ERASURE49 and FIXTURE49 (see Table 6).
FUTURE 2 trial
Tables 8 and 9 show FUTURE 2 trial48 results for the key review outcomes for the full-trial population across the 12-, 16- and 24-week time points. Results for the biologic-naive and biologic-experienced subgroups are presented in Tables 10 and 11. The corresponding relative risks (RRs) for the dichotomous outcomes were calculated by the Evidence Review Group (ERG) and are presented in Table 12.
| Population | Drug | Time point (weeks) | Number of patients randomised | Responders, n (%) | HAQ-DI change from baseline (SE) | |||
|---|---|---|---|---|---|---|---|---|
| PsARC | ACR 20 | ACR 50 | ACR 70 | |||||
| All | 300 mg of SEC | 12 | 100 | Confidential information has been removed | 57 (57) | 30 (30) | – | – |
| 150 mg of SEC | 100 | Confidential information has been removed | 56 (56) | 32 (32) | – | – | ||
| Placebo | 98 | Confidential information has been removed | 25 (26) | 5 (5) | – | – | ||
| All | 300 mg of SEC | 16 | 100 | 69 (69) | 57 (57) | 30 (30) | – | – |
| 150 mg of SEC | 100 | 72 (72) | 60 (60) | 32 (32) | – | – | ||
| Placebo | 98 | 41 (42) | 18 (18) | 5 (5) | – | – | ||
| All | 300 mg of SEC | 24 | 100 | Confidential information has been removed | 54 (54) | 35 (35) | 20 (20) | –0.56 (0.05) |
| 150 mg of SEC | 100 | Confidential information has been removed | 51 (51) | 35 (35) | 21 (21) | –0.48 (0.05) | ||
| Placebo | 98 | Confidential information has been removed | 15 (15) | 7 (7) | 1 (1) | –0.31 (0.06) | ||
| Population | Drug | Time point (weeks) | Number of patients with psoriasis affecting ≥ 3% of BSA | PASI 50 | PASI 75 | PASI 90 |
|---|---|---|---|---|---|---|
| All | 300 mg of SEC | 12 | 41 | 34 (83%) | 24 (59%) | 16 (39%) |
| 150 mg of SEC | 58 | 48 (83%) | 31 (53%) | 19 (33%) | ||
| Placebo | 43 | 5 (12%) | 2 (5%) | 2 (5%) | ||
| All | 300 mg of SEC | 16 | 41 | 36 (88%) | – | – |
| 150 mg of SEC | 58 | 48 (83%) | – | – | ||
| Placebo | 43 | 6 (14%) | – | – | ||
| All | 300 mg of SEC | 24 | 41 | – | 26 (63%) | 20 (49%) |
| 150 mg of SEC | 58 | – | 28 (48%) | 19 (33%) | ||
| Placebo | 43 | – | 7 (16%) | 4 (9%) |
| Population | Drug | Time point (weeks) | Number of patients randomised | PsARC | ACR 20 | ACR 50 | ACR 70 |
|---|---|---|---|---|---|---|---|
| Biologic naive | 300 mg of SEC | 12 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Biologic experienced | 300 mg of SEC | 12 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Biologic naive | 300 mg of SEC | 16 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Biologic experienced | 300 mg of SEC | 16 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Biologic naive | 300 mg of SEC | 24 | 67 | – | 39 (58%) | 26 (39%) | 15 (22%) |
| 150 mg of SEC | 63 | – | 40 (63%) | 28 (44%) | 17 (27%) | ||
| Placebo | 63 | – | 10 (16%) | 4 (6%) | 1 (2%) | ||
| Biologic experienced | 300 mg of SEC | 24 | 33 | – | 15 (45%) | 9 (27%) | 5 (15%) |
| 150 mg of SEC | 37 | – | 11 (30%) | 7 (19%) | 4 (11%) | ||
| Placebo | 35 | – | 5 (14%) | 3 (9%) | 0 (0%) |
| Population | Drug | Time point (weeks) | Number of patients with psoriasis affecting ≥ 3% of BSA | PASI 50 | PASI 75 | PASI 90 |
|---|---|---|---|---|---|---|
| Biologic naive | 300 mg of SEC | 12 | 30 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 150 mg of SEC | 36 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Placebo | 31 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Biologic experienced | 300 mg of SEC | 12 | 11 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 150 mg of SEC | 22 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Placebo | 12 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Biologic naive | 300 mg of SEC | 16 | 30 | – | 21 (70%) | 15 (50%) |
| 150 mg of SEC | 36 | – | 23 (64%) | 16 (44%) | ||
| Placebo | 31 | – | 3 (10%) | 3 (10%) | ||
| Biologic experienced | 300 mg of SEC | 16 | 11 | – | 6 (55%) | 3 (27%) |
| 150 mg of SEC | 22 | – | 10 (45%) | 6 (27%) | ||
| Placebo | 12 | – | 0 (0%) | 0 (0%) | ||
| Biologic naive | 300 mg of SEC | 24 | 30 | – | 19 (63%) | 16 (53%) |
| 150 mg of SEC | 36 | – | 20 (56%) | 14 (39%) | ||
| Placebo | 31 | – | 6 (19%) | 3 (10%) | ||
| Biologic experienced | 300 mg of SEC | 24 | 11 | – | 7 (64%) | 4 (36%) |
| 150 mg of SEC | 22 | – | 8 (36%) | 5 (23%) | ||
| Placebo | 12 | – | 1 (8%) | 1 (8%) |
| Treatment | Time point (weeks) | Population | RR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PsARC | ACR 20 | ACR 50 | ACR 70 | PASI 50 | PASI 75 | PASI 90 | |||
| 150 mg of SEC | 12 | All | 1.73 (1.31 to 2.29) | 2.20 (1.50 to 3.21) | 6.27 (2.55 to 15.43) | NR | 7.12 (3.10 to 16.36) | 11.49 (2.91 to 45.42) | 7.04 (1.73 to 28.64) |
| 16 | 1.72 (1.32 to 2.24) | 3.27 (2.09 to 5.11) | 6.27 (2.55 to 15.43) | NR | 5.93 (2.80 to 12.57) | NR | NR | ||
| 24 | Confidential information has been removed | 3.33 (2.01 to 5.51) | 4.90 (2.29 to 10.50) | 20.58 (2.82 to 150.06) | NR | 2.97 (1.43 to 6.14) | 3.52 (1.29 to 9.61) | ||
| 300 mg of SEC | 12 | All | 1.81 (1.38 to 2.38) | 2.23 (1.53 to 3.26) | 5.88 (2.38 to 14.53) | NR | 7.13 (3.09 to 16.45) | 12.59 (3.17 to 49.91) | 8.39 (2.06 to 34.24) |
| 16 | 1.65 (1.26 to 2.16) | 3.10 (1.98 to 4.87) | 5.88 (2.38 to 14.53) | NR | 6.29 (2.97 to 13.33) | NR | NR | ||
| 24 | Confidential information has been removed | 3.53 (2.14 to 5.81) | 4.90 (2.29 to 10.50) | 19.60 (2.68 to 143.24) | NR | 3.90 (1.90 to 7.98) | 5.24 (1.96 to 14.04) | ||
| 150 mg of SEC | 12 | Biologic naive | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 16 | NR | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NR | Confidential information has been removed | Confidential information has been removed | ||
| 24 | Confidential information has been removed | 4.00 (2.20 to 7.28) | 7.00 (2.61 to 18.80) | 17.00 (2.33 to 123.91) | NR | 2.87 (1.32 to 6.23) | 4.02 (1.27 to 12.70) | ||
| 300 mg of SEC | 12 | Biologic naive | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 16 | NR | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NR | Confidential information has been removed | Confidential information has been removed | ||
| 24 | Confidential information has been removed | 3.67 (2.01 to 6.71) | 6.11 (2.26 to 16.53) | 14.10 (1.92 to 103.68) | NR | 3.27 (1.52 to 7.06) | 5.51 (1.79 to 17.00) | ||
| 300 mg of SEC | 12 | Biologic experienced | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 16 | NR | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NR | Confidential information has been removed | Confidential information has been removed | ||
| 24 | Confidential information has been removed | 3.18 (1.30 to 7.77) | 3.18 (0.62 to 10.75) | 11.65 (0.67 to 202.75) | NR | 7.64 (1.11 to 52.56) | 4.36 (0.57 to 33.32) | ||
Efficacy at 12–24 weeks in the full-trial population
For the whole-trial population, SEC was associated with statistically significant improvements in all outcomes at all time points. Patients taking SEC were around six times more likely to be ACR 50 responders – an outcome of particular clinical importance to patients – than patients taking placebo. An increase in RRs is apparent when looking across the PsARC, ACR 20, ACR 50 and ACR 70 columns in Table 12. These increases in RR are likely to be a consequence of the different placebo rates, with higher rates for the lower threshold outcomes (see the placebo rates in Table 8). The lower threshold outcomes (such as PsARC and ACR 20) appear to underestimate efficacy because the RRs tend to be diluted by the high placebo response rates. This association of higher placebo responses with lower relative efficacy was also noted across trials by outcome in the evidence synthesis and is discussed in Chapter 4.
FUTURE 248 trial patients taking 150 or 300 mg of SEC were also around six to seven times more likely to be PASI 50 responders than patients taking placebo. Efficacy was also demonstrated for the higher PASI thresholds (PASI 75 and PASI 90), with the 300-mg group having only slightly higher RRs than the 150-mg group.
All three study arms showed improvements in physical function as assessed using HAQ-DI change from baseline scores; HAQ-DI assesses a patient’s ability to perform eight categories of activities of daily living. Patients taking SEC had greater reductions in HAQ-DI scores than patients taking the placebo (see Table 8). At 24 weeks, the difference when compared with placebo (–0.25 units) was statistically significant for 300 mg (p = 0.004), but the difference of –0.17 units for 150 mg did not quite reach statistical significance (p = 0.055). 48 The manufacturer also submitted HAQ-DI results based on PsARC responder status (Table 13). These results show (confidential information has been removed).
| Population | Time point (weeks) | Group, HAQ-DI change (SE) | |||||
|---|---|---|---|---|---|---|---|
| Placebo | 150 mg | 300 mg | |||||
| Responders | Non-responders | Responders | Non-responders | Responders | Non-responders | ||
| All | 12 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 16 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
Efficacy in the biologic-naive and biologic-experienced subgroups
Table 12 also presents RRs for the subgroups based on patients’ previous use of biologics. These subgroup results are difficult to interpret for several reasons. Some of the subgroup sample sizes were particularly small: there were no placebo responders (PRs) for some outcomes in the biologic-experienced subgroup and the RR confidence intervals (CIs) were therefore extremely wide. The PASI results are effectively based on subgroups (previous biologic status) of a subgroup (patients with psoriasis covering ≥ 3% of BSA). Placebo response rates also differed across subgroups (see Evaluating the secukinumab and certolizumab pegol trial results in comparison with other treatments). Similar subgroup issues were also seen for CZP (see Efficacy in the RAPID-PsA biologic-naive and biologic-experienced subgroups).
The manufacturer also submitted HAQ-DI results based on PsARC responder status for the biologic-naive and biologic-experienced population (Table 14). Again, comparisons between the two subgroups is difficult as (confidential information has been removed).
| Population | Time point (weeks) | Group, HAQ-DI change (SE) | |||||
|---|---|---|---|---|---|---|---|
| Placebo | 150 mg | 300 mg | |||||
| Responders | Non-responders | Responders | Non-responders | Responders | Non-responders | ||
| Biologic naive | 12 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 16 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| 24 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| Biologic experienced | 12 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 16 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| 24 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
Other efficacy results
Efficacy of secukinumab with or without concomitant methotrexate
Just under half of the patients in FUTURE 248 took concomitant MTX. In exploratory post hoc analyses, SEC was found to be similarly efficacious whether or not patients were taking concomitant MTX. 48 For ACR 50, response rates were statistically significantly higher in the 300- and 150-mg groups than in the placebo group for both the concomitant MTX subgroup (p = 0.001 and p = 0.006, respectively) and the no concomitant MTX subgroup (p = 0.007 and p < 0.0001, respectively). Similar statistically significant differences were also reported for the ACR 20 and 70 thresholds. 48
Efficacy of secukinumab in the one prior DMARD subgroup
Data were presented in the manufacturer’s submission at week 24 for efficacy in the one prior DMARD subgroup. (Confidential information has been removed.)
Efficacy in treating dactylitis and enthesitis
At week 24, relative to placebo treatment with both 150 and 300 mg of SEC statistically significantly improved the resolution of both dactylitis (as measured via the Leeds Dactylitis Index; LDI) and enthesitis (as measured via the Leeds Enthesitis Index; LEI) (Table 15).
| Outcome | Trial arm | ||
|---|---|---|---|
| 300 mg of SEC | 150 mg of SEC | Placebo | |
| Resolution of dactylitis at week 24 | Confidential information has been removed; p = 0.0021 | Confidential information has been removed; p = 0.0056 | Confidential information has been removed |
| Resolution of enthesitis at week 24 | Confidential information has been removed; p = 0.0025 | Confidential information has been removed; p = 0.0108 | Confidential information has been removed |
| Dactylitis count at week 16, mean change from baseline ± SD | –2.3 ± 4.0 | –3.1 ± 4.5 | –0.6 ± 2.4 |
| Enthesitis count at week 16, mean change from baseline ± SD | –1.7 ± 1.8 | –1.5 ± 2.0 | –0.9 ± 2.1 |
Health-related quality of life
Up to week 24, improvement in the EQ-5D overall health state (as measured by a visual analogue scale; VAS) was higher in both SEC groups (150 and 300 mg) than in the placebo group. (Confidential information has been removed.)
At week 24, self-reported quality of life and physical functioning, as measured by SF-36 Physical Component Summary score, was found to have improved more in the SEC groups than in the placebo group (SEC 150 mg, 6.39 points; SEC 300 mg, 7.25 points; placebo, 1.95 points).
Mortality
No deaths were reported during the trial.
ERASURE and FIXTURE trials
As the focus of the ERASURE and FIXTURE trials49 was on patient populations with psoriasis (subgroups of which also had PsA), fewer outcomes that were relevant to this assessment were evaluated. Patients recruited into in the ERASURE and FIXTURE trials had more severe psoriasis but lower baseline HAQ-DI scores than the patients recruited into the FUTURE 2 trial and into the other trials included in the systematic review (see Table 4). The FIXTURE trial was one of the very few identified in the systematic review that compared different biologics (SEC with ETN).
Table 16 and Figure 2 (in which data from the ERASURE and FIXTURE trials have been pooled) illustrate SEC’s superiority over placebo for the PASI outcomes. In the FIXTURE trial at 12 weeks, 300 mg of SEC was statistically significantly more effective than 50 mg of ETN twice weekly in terms of patients achieving a PASI 75 response (RR 1.86, 95% CI 1.24 to 2.81) and a PASI 90 response (RR 2.42, 95% CI 1.20 to 4.88). Changes from baseline in the HAQ-DI scores were greater in SEC- and ETN-treated patients in ERASURE and FIXTURE trials than with placebo.
| Trial | Treatment | Number of PsA patients | PASI 50 | PASI 75 | PASI 90 | HAQ-DI change from baselinea |
|---|---|---|---|---|---|---|
| ERASURE49 | 300 mg of SEC | 57 | – | 38 (67%) | 30 (53%) | –0.35 |
| 150 mg of SEC | 46 | – | 32 (70%) | 20 (43%) | –0.18 | |
| Placebo | 68 | – | 3 (4%) | 0 (0%) | –0.08 | |
| FIXTURE49 | 300 mg of SEC | 50 | – | 36 (72%) | 22 (44%) | –0.41 |
| 150 mg of SEC | 49 | – | 29 (59%) | 19 (39%) | –0.19 | |
| 50 mg of ETN | 44 | – | 17 (39%) | 8 (18%) | –0.29 | |
| Placebo | 49 | – | 1 (2%) | 1 (2%) | 0.02 |
FIGURE 2.
Forest plot of the efficacy of 300 mg of SEC vs. placebo for PASI 75 at 12 weeks in PsA patients with moderate–severe psoriasis. 49 df, degrees of freedom; M–H, Mantel–Haenszel.
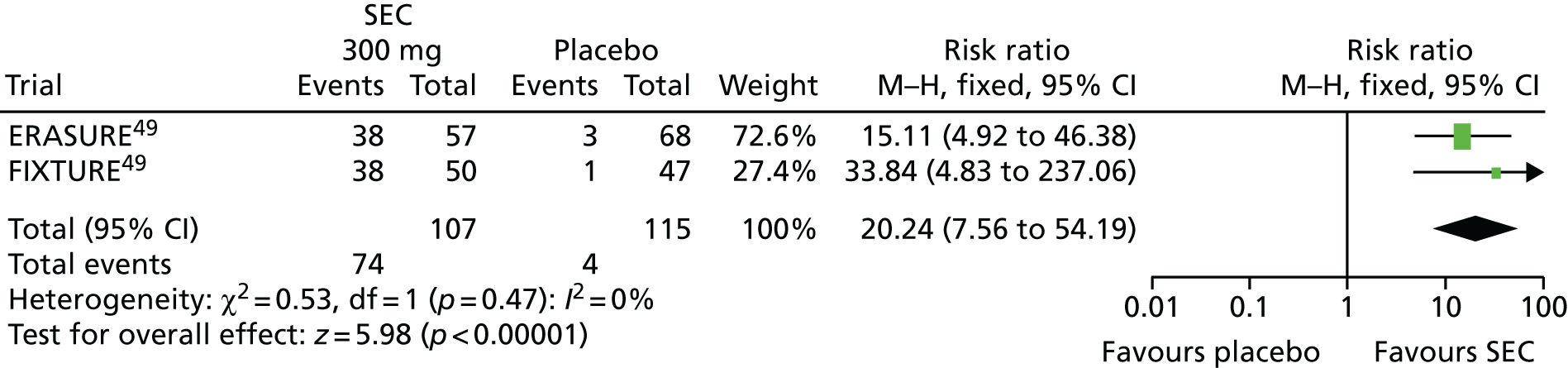
CLEAR trial
The CLEAR trial,62,63 which compared SEC with UST, was similar to the ERASURE and FIXTURE trials49 with respect to the population studied (patients with more severe psoriasis than those recruited into the FUTURE 2 trial) and the limited data assessed and reported (in the CLEAR trial only PASI 90 and HAQ-DI scores were reported for the subgroup of patients with PsA).
At 16 weeks, patients treated with 300 mg of SEC had a better PASI 90 response rate than patients receiving 45 or 90 mg of UST, although the difference was not statistically significant (RR 1.23, 95% CI 0.98 to 1.55; p = 0.08). Patients treated with 300 mg of SEC had a greater improvement in HAQ-DI score than patients receiving 45 or 90 mg of UST (Table 17).
| Treatment | Number of patients randomised | PASI 50 | PASI 75 | PASI 90 | HAQ-DI change from baselinea |
|---|---|---|---|---|---|
| 300 mg of SEC | 69 | – | – | 55 (80%) | –0.29 |
| 45 or 90 mg of UST | 54 | – | – | 35 (65%) | –0.13 |
Summary
The results of the FUTURE 2 trial48 demonstrated the short-term efficacy of SEC in treating PsA. When considering the whole-trial population, SEC was associated with statistically and clinically significant improvements in all key outcomes. Patients taking SEC were around six times more likely to be ACR 50 responders – a key clinical outcome to patients – than patients taking placebo. Clinically important improvements in activities of daily living (assessed using the HAQ-DI) were also evident in patients taking SEC, particularly in patients who were PsARC responders. However, when the trial population was split into subgroups based on previous biologic experience, the resulting RRs for the biologic-experienced subgroup became difficult to interpret. This was attributable to both the low numbers of placebo patients and the differences in placebo response rates across subgroups (see Evaluating the secukinumab and certolizumab pegol trial results in comparison with other treatments). Although SEC is efficacious in both subgroups, it is not possible to make robust conclusions about any difference in the efficacy of SEC across these subgroups. Similar efficacy across the ACR outcomes was evident in subgroups of patients based on presence or absence of concomitant MTX, although limited data and analyses were available specifically for the one prior DMARD group. Treatment with SEC resulted in statistically significant improvements in HRQoL measures and in the resolution of both dactylitis and enthesitis.
Results from the trials of patients with more severe psoriasis demonstrated SEC’s superiority over placebo in terms of psoriasis (as measured by the PASI) and function (as measured by the HAQ-DI) outcomes. SEC was also found to be significantly more effective than ETN in improving psoriasis (assessed using PASI 75 and PASI 90). However, the populations studied in these trials had quite severe psoriasis and less functional impairment (lower baseline HAQ-DI scores) than other trial populations. Their results should not therefore be generalised to more typical PsA populations.
Short-term efficacy of certolizumab pegol
One eligible RCT of CZP was identified. RAPID-PsA47 compared 200 or 400 mg of CZP against placebo up to 24 weeks. The trial was dose blinded to 48 weeks and then open label to 216 weeks. Placebo patients who failed to achieve a 10% improvement from baseline in both swollen and tender joints at week 14 and 16 were re-randomised to active treatment at week 16. At week 24, all the remaining placebo patients were re-randomised to receive 200 or 400 mg of CZP. The RAPID-PsA47 trial was judged as being at low overall risk of bias (see Table 6).
Compared with the other PsA trials, the RAPID-PsA trial was more selective in recruiting biologic-experienced patients; patients with primary failure of a previous anti-TNF were excluded (primary failure was defined as no response within the first 12 weeks of treatment with the anti-TNF).
There are no UCB Pharma-sponsored ongoing studies of CZP in patients with PsA.
Tables 18 and 19 show the RAPID-PsA trial results47 for the key review outcomes for the full-trial population across the 12-, 16- and 24-week time points. ACR 20 results, split into subgroups according to the number of previous DMARDs taken by patients, are presented in Table 20. Results for the biologic-naive and biologic-experienced subgroups are presented in Tables 21–24. The corresponding RRs for the dichotomous outcomes were calculated by the ERG and are presented in Table 25.
| Population | Treatment | Time point (weeks) | Number of patients randomised | Responders, n (%) | HAQ-DI change from baseline (SE) | |||
|---|---|---|---|---|---|---|---|---|
| PsARC | ACR 20 | ACR 50 | ACR 70 | |||||
| All | 200 mg of CZP every fortnight | 12 | 138 | 101 (73) | 80 (58) | 50 (36) | 34 (25) | –0.45 (0.56) |
| 400 mg of CZP once a month | 135 | 89 (66) | 70 (52) | 44 (33) | 17 (13) | –0.39 (0.47) | ||
| Placebo | 136 | 52 (38) | 33 (24) | 15 (11) | 4 (3) | –0.16 (0.36) | ||
| All | 200 mg of CZP every fortnight | 16 | 138 | – | 78 (57) | – | – | – |
| 400 mg of CZP once a month | 135 | – | 73 (54) | – | – | – | ||
| Placebo | 136 | – | 34 (25) | – | – | – | ||
| All | 200 mg of CZP every fortnight | 24 | 138 | 108 (78) | 88 (64) | 61 (44) | 39 (28) | –0.52 (0.66) |
| 400 mg of CZP once a month | 135 | 104 (77) | 76 (56) | 54 (40) | 32 (24) | –0.43 (0.54) | ||
| Placebo | 136 | 45 (33) | 32 (24) | 17 (13) | 6 (4) | –0.17 (0.43) | ||
| Population | Treatment | Time point (weeks) | Number of patients with psoriasis affecting ≥ 3% BSA | PASI 50 | PASI 75 | PASI 90 |
|---|---|---|---|---|---|---|
| All | 200 mg of CZP every fortnight | 12 | 90 | 62 (69%) | 42 (47%) | 20 (22%) |
| 400 mg of CZP once a month | 76 | 48 (63%) | 36 (47%) | 15 (20%) | ||
| Placebo | 86 | 23 (27%) | 12 (14%) | 4 (5%) | ||
| All | 200 mg of CZP every fortnight | 24 | 90 | 67 (74%) | 56 (62%) | 42 (47%) |
| 400 mg of CZP once a month | 76 | 55 (72%) | 46 (61%) | 27 (36%) | ||
| Placebo | 86 | 24 (28%) | 13 (15%) | 5 (6%) |
| Population | Treatment | Number of patients randomised | ACR 20 |
|---|---|---|---|
| Previous use of one DMARD | 200 mg of CZP every fortnight | 61 | 42 (69%) |
| 400 mg of CZP once a month | 72 | 42 (58%) | |
| Placebo | 74 | 22 (30%) | |
| Previous use of two or more DMARDs | 200 mg of CZP every fortnight | 73 | 38 (52%) |
| 400 mg of CZP once a month | 60 | 28 (47%) | |
| Placebo | 60 | 11 (18%) |
| Population | Drug | Number of patients randomised | PsARC | ACR 20 | ACR 50 | ACR 70 | HAQ-DI change from baseline (SE) |
|---|---|---|---|---|---|---|---|
| Biologic naive | CZP combined | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| Biologic experienced | CZP combined | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Population | Drug | Number of patients with psoriasis affecting ≥ 3% BSA | PASI 50 | PASI 75 | PASI 90 |
|---|---|---|---|---|---|
| Biologic naive | CZP combined | 130 | 80 (62%) | 56 (43%) | 25 (19%) |
| Placebo | 66 | 18 (27%) | 11 (17%) | 3 (5%) | |
| Biologic experienced | CZP combined | 36 | 30 (83%) | 22 (61%) | 10 (28%) |
| Placebo | 20 | 5 (25%) | 1 (5%) | 1 (5%) |
| Population | Drug | Time point (weeks) | Number of patients randomised | PsARC | ACR 20 | ACR 50 | ACR 70 | HAQ-DI change from baseline (SE) |
|---|---|---|---|---|---|---|---|---|
| Biologic naive | CZP combined | 24 | 219 | 170 (78%) | 132 (60%) | 91 (42%) | 57 (26%) | –0.45 (0.6) |
| Placebo | 110 | 59 (54%) | 29 (26%) | 16 (15%) | 5 (5%) | –0.2 (0.45) | ||
| Biologic experienced | CZP combined | 24 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Placebo | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Population | Drug | Time point (weeks) | Number of patients with psoriasis affecting ≥ 3% BSA | PASI 50 | PASI 75 | PASI 90 |
|---|---|---|---|---|---|---|
| Biologic naive | CZP combined | 24 | 130 | 89 (68%) | 73 (56%) | 48 (37%) |
| Placebo | 66 | 20 (30%) | 13 (20%) | 5 (8%) | ||
| Biologic experienced | CZP combined | 24 | 36 | 33 (92%) | 29 (81%) | 21 (58%) |
| Placebo | 20 | 4 (20%) | 0 (0%) | 0 (0%) |
| Dose | Time point (weeks) | Population | RR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PsARC | ACR 20 | ACR 50 | ACR 70 | PASI 50 | PASI 75 | PASI 90 | |||
| 200 mg every fortnight | 12 | All | 1.91 (1.51 to 2.42) | 2.39 (1.72 to 3.32) | 3.29 (1.94 to 5.56) | 8.38 (3.06 to 12.39) | 2.58 (1.77 to 3.75) | 3.34 (1.89 to 5.91) | 4.78 (1.70 to 13.41) |
| 16 | NR | 2.26 (1.63 to 3.13) | NR | NR | NR | NR | NR | ||
| 24 | 2.37 (1.83 to 3.05) | 2.71 (1.95 to 3.76) | 3.54 (2.18 to 5.73) | 6.41 (2.80 to 14.64) | 2.67 (1.86 to 3.83) | 4.12 (2.43 to 6.97) | 8.03 (3.33 to 19.33) | ||
| 400 mg every 4 weeks | 12 | All | 1.72 (1.35 to 2.20) | 2.14 (1.52 to 3.00) | 2.96 (1.73 to 5.05) | 4.28 (1.48 to 12.39) | 2.36 (1.60 to 3.49) | 3.39 (1.91 to 6.04) | 4.24 (1.47 to 12.23) |
| 16 | NR | 2.16 (1.55 to 3.01) | NR | NR | NR | NR | NR | ||
| 24 | 2.33 (1.80 to 3.01) | 2.39 (1.71 to 3.35) | 3.20 (1.96 to 5.23) | 5.37 (2.32 to 12.43) | 2.59 (1.80 to 3.74) | 4.00 (2.35 to 6.82) | 6.11 (2.48 to 15.07) | ||
| Combined arms | 12 | Biologic naive | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 24 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
| Combined arms | 12 | Biologic experienced | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 24 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||
Efficacy at 12–24 weeks in the RAPID PsA full-trial population
For the full-trial population, the RRs in Table 25 are for comparisons of the different CZP regimens (200 mg every 2 weeks or 400 mg every 4 weeks) with placebo, across the 12-, 16- and 24-week time points and across the PsARC, ACR and PASI outcomes. For the subgroup analyses (based on previous biologic status), combined data from the two CZP arms were used to calculate RRs.
For the full-trial population, when compared with placebo, CZP was associated with statistically significant improvements in all outcomes at all time points (for which data were available). Patients taking CZP were around three times more likely to be ACR 50 responders than patients taking placebo. Similar to the pattern seen with the SEC FUTURE 2 trial48 results, an increase in RRs is apparent as the outcome thresholds (for achieving a response) increase across the PsARC, ACR and PASI outcomes (see Table 25). Again, these increases are likely to be a consequence of the different placebo rates, with higher rates of placebo response in the lower threshold outcomes.
The RAPID-PsA trial47 patients taking CZP were around two-and-a-half times more likely to be PASI 50 responders than patients taking placebo. Efficacy was also demonstrated in the results for the higher PASI thresholds. Improvements in physical function, as assessed using HAQ-DI change from baseline scores, were also seen, with the difference being reported as being statistically significant (p < 0.001) at 24 weeks. 47 The manufacturer also submitted HAQ-DI results based on PsARC responder status (Table 26). (Confidential information has been removed.)
| Population | Time point (weeks) | Group, HAQ-DI change (SD) | |||||
|---|---|---|---|---|---|---|---|
| Placebo | 200 mg | 400 mg | |||||
| Responders | Non-responders | Responders | Non-responders | Responders | Non-responders | ||
| All | 12 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 24 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
Efficacy in the RAPID-PsA biologic-naive and biologic-experienced subgroups
Table 25 presents RRs for subgroups based on patients’ previous use of biologics. When comparing results for all outcomes across subgroups the efficacy of CZP appears somewhat better in the biologic-experienced subgroup than in the biologic-naive subgroup; this trial evidence is contrary to evidence from large patient registries suggesting that effectiveness may decrease with each new anti-TNF taken (see Drug survival and anti-tumour necrosis factor switching). The differences between subgroups observed in the RAPID-PsA trial47 are likely to have been influenced by two factors. First, there is a problem with sample size, with low numbers of placebo patients and PRs in the biologic-experienced subgroup. There is therefore considerable uncertainty about these estimates, which is reflected in the very wide CIs. Second, there is a notable difference in placebo response rates between the two subgroups (see Table 21 and Evaluating the secukinumab and certolizumab pegol trial results in comparison with other treatments). Furthermore, as detailed previously in Characteristics of the randomised controlled trials included in the systematic review of short-term efficacy, the RAPID-PsA trial excluded patients with primary failure of a previous biologic, so the subgroups were not as different as they could have been (other trials did not exclude primary failures).
The manufacturer also submitted HAQ-DI results based on PsARC responder status for the biologic-naive and biologic-experienced populations (Table 27). (Confidential information has been removed.)
| Population | Time point (weeks) | Group, HAQ-DI change (SD) | |||||
|---|---|---|---|---|---|---|---|
| Placebo | 200 mg | 400 mg | |||||
| Responders | Non-responders | Responders | Non-responders | Responders | Non-responders | ||
| Biologic naive | 12 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 24 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
| Biologic experienced | 12 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| 24 | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |
Other efficacy results
Efficacy of certolizumab pegol with or without concomitant methotrexate
Results were not reported for subgroups based specifically on MTX use, although results were reported based on concomitant use of a DMARD (which was mostly MTX). Concomitant DMARD use did not seem to affect ACR 20 (57% with vs. 50% without) or PsARC (68% with vs. 73% without) response rates to CZP (combined dose) at week 12. 47
Efficacy of certolizumab pegol in the one prior DMARD subgroup
When compared with placebo, at weeks 12 and 24, CZP was associated with statistically significantly better ACR 20 response rates (p < 0.001); 207 patients who had received one prior DMARD were included in the analysis. 47 Data in the manufacturer’s submission showed that (confidential information has been removed).
Efficacy in treating dactylitis and enthesitis
At week 24, patients treated with CZP achieved statistically significant improvements in dactylitis (assessed using the LDI) when compared with placebo-treated patients; statistically significant improvements in enthesitis, as assessed using the LEI, were also seen in the CZP group (Table 28).
| Treatment | Outcome, mean change from baseline at week 24 | |
|---|---|---|
| Dactylitis count ± SD | Enthesitis count ± SD | |
| 200 mg of CZP | –40.7 ± 34.6; p ≤ 0.003 | –2.0 ± 1.8; p < 0.001 |
| 400 mg of CZP | –53.5 ± 69.1; p < 0.001 | –1.8 ± 1.9; p ≤ 0.003 |
| Placebo | –22.0 ± 46.9 | –1.1 ± 1.8 |
Health-related quality of life
At week 12, EQ-5D VAS scores were higher in CZP-treated groups (confidential information has been removed).
In addition, at week 24, there was a significant improvement with pooled CZP in all domains of the SF-36, including both the physical (confidential information has been removed) and mental components (confidential information has been removed), regardless of the dose regimen and prior TNF inhibitor status. (Confidential information has been removed.)
Mortality
Two deaths were reported during the 24 weeks: one was in the 200-mg group and one was in the 400-mg group. The trial investigators considered both deaths to be unrelated to study medication.
Summary
The results of the RAPID-PsA trial47 demonstrated the short-term efficacy of CZP in treating PsA. When considering the full-trial population, CZP was associated with statistically significant improvements in all key outcomes. When the trial population was split into subgroups based on previous biologic experience, the results became difficult to compare (as was seen in the FUTURE 2 trial). The small number of placebo patients in the biologic-experienced subgroup coupled with higher placebo response rates in the biologic-naive subgroup meant that it was not possible to make reliable conclusions about the difference in the efficacy of CZP across these subgroups. Furthermore, patients with primary failure of a previous biologic were excluded from the RAPID-PsA trial, so estimates of efficacy may have been slightly inflated when comparisons were made with other trials that recruited biologic-experienced patients (e.g. FUTURE 248 and PSUMMIT 259,66). Similar efficacy across the ACR and PsARC outcomes was seen in subgroups of patients based on presence or absence of a concomitant DMARD and (confidential information has been removed). Treatment with CZP resulted in statistically significant improvements in HRQoL measures and in the resolution of both dactylitis and enthesitis.
Evaluating the secukinumab and certolizumab pegol trial results in comparison with other treatments
In order to more fully evaluate the clinical efficacy of SEC and CZP, the trial results of these two newer biologics need to be compared with each other and with the results of the older biologics (and APR). However, this is not straightforward for two reasons. First, there is variation across trials with respect to previous biologic use.
-
The populations recruited to clinical trials have changed over time, with earlier trials excluding biologic-experienced patients and later trials including such patients.
-
The RAPID-PsA trial was more selective than the FUTURE 2,48 PSUMMIT 259,66 and PALACE trials60,61,65 in recruiting its biologic-experienced patients: only in RAPID-PsA were patients with primary failure of a previous biologic excluded (see Characteristics of the randomised controlled trials included in the systematic review of short-term efficacy).
Second, placebo response rates have increased markedly over time across the trials included in this review. This issue is key when interpreting RRs because, although RRs are easy to interpret clinically, their ceilings (maximum values) are limited by baseline response rates. For example, in the FUTURE 2 trial48 the placebo response rate for PsARC was (confidential information has been removed) in the biologic-naive subgroup. This high rate meant that the maximum possible RR would be (confidential information has been removed); this maximum result is lower than some of the actual RRs for other biologics presented in Table 29, which compares unadjusted RRs across the trials in the NMAs. Comparisons between treatments using odds ratios (ORs) and that adjust for the varying placebo rates were therefore necessary (see Chapter 4).
| Trial name | Treatment | Time point (weeks) | Population | RR (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PsARC | ACR 20 | ACR 50 | ACR 70 | PASI 50 | PASI 75 | PASI 90 | ||||
| FUTURE 248 | 300 mg of SEC | 12 | All | Confidential information has been removed | 2.23 (1.53 to 3.26) | Confidential information has been removed | NR | 7.13 (3.09 to 16.45) | 12.59 (3.17 to 49.91) | 8.39 (2.06 to 34.24) |
| 150 mg of SEC | 12 | All | Confidential information has been removed | 2.20 (1.50 to 3.21) | Confidential information has been removed | NR | 7.12 (3.10 to 16.36) | 11.49 (2.91 to 45.42) | 7.04 (1.73 to 28.64) | |
| 300 mg of SEC | 12 | Biologic naive | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 6.20 (1.15 to 25.40) | |
| 150 mg of SEC | 12 | Biologic naive | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 5.60 (1.37 to 22.91) | |
| 300 mg of SEC | 12 | Biologic experienced | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 9.78 (0.59 to 162.47) | |
| 150 mg of SEC | 12 | Biologic experienced | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 7.22 (0.44 to 117.84) | |
| SPIRIT-P157,67 | ADA | 12 | All | NR | 1.65 (1.18 to 2.32) | 6.30 (2.54 to 15.59) | 38.82 (2.37 to 635.80) | NR | 5.21 (2.50 to 10.85) | 14.78 (2.01 to 108.77) |
| RAPID-PsA47 | CZP 200 mg | 12 | All | Confidential information has been removed | 2.39 (1.72 to 3.32) | 3.29 (1.94 to 5.56) | 8.38 (3.06 to 22.97) | 2.58 (1.77 to 3.75) | 3.34 (1.89 to 5.91) | 4.78 (1.70 to 13.41) |
| CZP 400 mg | 12 | All | Confidential information has been removed | 2.14 (1.52 to 3.00) | 2.96 (1.73 to 5.05) | 4.28 (1.48 to 12.39) | 2.36 (1.60 to 3.49) | 3.39 (1.91 to 6.04) | 4.24 (1.47 to 12.23) | |
| CZP combined | 12 | Biologic naive | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 5.56 (0.77 to 40.30) | |
| CZP combined | 12 | Biologic experienced | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 4.70 (2.01 to 11.01) | |
| PALACE 160,61 | APR | 16 | All | 1.56 (1.17 to 2.07) | 2.00 (1.39 to 2.89) | 2.70 (1.35 to 5.40) | 3.50 (0.74 to 16.60) | 2.71 (1.50 to 4.91) | 4.98 (1.53 to 16.18) | NR |
| PALACE 261,65 | APR | 16 | All | 1.44 (1.10 to 1.90) | 1.70 (1.15 to 5.52) | 2.09 (0.93 to 4.69) | 1.96 (0.18 to 21.43) | 3.17 (1.69 to 5.96) | 8.17 (1.95 to 34.14) | NR |
| PALACE 361,65 | APR | 16 | All | 1.94 (1.46 to 2.58) | 2.22 (1.54 to 3.20) | 1.81 (0.97 to 3.35) | 1.52 (0.44 to 5.28) | 1.71 (1.10 to 2.64) | 2.83 (1.26 to 6.35) | NR |
| PSUMMIT 259,66 | 45 mg of UST | 12 | Biologic naive | NR | 2.08 (1.01 to 4.28) | 1.63 (0.42 to 6.39) | 2.93 (0.32 to 27.06) | NR | 14.17 (2.00 to 100.35) | NR |
| PSUMMIT 259,66 | 45 mg of UST | 24 | Biologic naive | 1.47 (0.92 to 2.34) | 1.87 (1.08 to 3.26) | 2.93 (0.85 to 10.08) | 1.95 (0.38 to 10.10) | NR | 5.83 (1.93 to 17.67) | NR |
| 45 mg of UST | 12 | Biologic experienced | NR | 2.64 (1.33 to 5.23) | 9.30 (1.21 to 71.19) | 9.30 (0.51 to 169.03) | NR | 15.91 (2.18 to 116.14) | NR | |
| 45 mg of UST | 24 | Biologic experienced | 2.13 (1.32 to 3.44) | 2.53 (1.27 to 5.03) | 2.33 (0.76 to 7.15) | 3.10 (0.33 to 28.98) | NR | 22.73 (3.18 to 162.50) | NR | |
| 45 mg of UST | 12 | All | 1.65 (1.18 to 2.31) | 2.38 (1.44 to 3.91) | 3.53 (1.20 to 10.38) | 7.07 (0.89 to 56.44) | 7.29 (3.52 to 15.07) | 15.50 (3.84 to 62.60) | 16.00 (2.17 to 117.80) | |
| 45 mg of UST | 24 | All | 1.80 (1.28 to 2.52) | 2.16 (1.39 to 3.36) | 2.60 (1.13 to 5.95) | 2.36 (0.63 to 8.86) | NR | 10.25 (3.85 to 27.28) | NR | |
| PSUMMIT 158 | 45 mg of UST | 12 | Biologic naive | 1.62 (1.31 to 2.01) | 1.94 (1.43 to 2.64) | 3.47 (1.83 to 6.60) | 2.68 (0.72 to 9.96) | NR | 4.34 (2.48 to 7.58) | NR |
| 45 mg of UST | 24 | Biologic naive | 1.50 (1.21 to 1.86) | 1.86 (1.38 to 2.50) | 2.85 (1.72 to 4.70) | 5.02 (1.96 to 12.87) | 2.89 (2.06 to 4.05) | 5.22 (3.22 to 8.47) | NR | |
| GO-REVEAL50 | 50 mg of GOL | 14 | All (biologic naive) | 3.45 (2.39 to 4.99) | 5.73 (3.10 to 10.57) | 17.03 (4.22 to 68.75) | 13.93 (1.89 to 102.80) | 6.52 (3.16 to 13.47) | 15.94 (3.98 to 63.84) | 32.67 (2.01 to 530.63) |
| Genovese et al., 200756 | ADA | 12 | All (biologic naive) | 1.86 (1.10 to 3.13) | 2.50 (1.21 to 5.15) | 13.00 (1.77 to 95.73) | 15.00 (0.88 to 255.86) | NR | NR | NR |
| ADEPT55 | ADA | 12 | All (biologic naive) | 2.37 (1.77 to 3.16) | 4.05 (2.71 to 6.06) | 9.53 (4.22 to 21.51) | 31.76 (4.39 to 230.09) | 5.00 (2.77 to 9.03) | 11.33 (3.65 to 35.17) | 43.00 (2.66 to 695.98) |
| IMPACT 252 | INF | 14 | All (biologic naive) | 2.85 (2.03 to 4.01) | 5.27 (2.95 to 9.44) | 12.00 (3.82 to 37.70) | 15.00 (2.02 to 111.42) | 8.91 (4.57 to 17.38) | 27.78 (6.99 to 110.35) | 72.31 (4.50 to 1160.52) |
| IMPACT51 | INF | 16 | All (biologic naive) | 3.55 (2.05 to 6.13) | 6.80 (2.89 to 16.01) | 49.00 (3.06 to 784.91) | 31.00 (1.90 to 504.77) | 33.00 (2.15 to 505.75) | 22.73 (1.46 to 353.35) | 12.47 (0.77 to 201.07) |
| Mease et al., 200454 | ETN | 12 | All (biologic naive) | 2.35 (1.72 to 3.21) | 3.86 (2.39 to 6.23) | 9.78 (3.62 to 26.41) | 23.68 (1.41 to 396.59) | NR | NR | NR |
| Mease et al., 200053 | ETN | 12 | All (biologic naive) | 3.71 (1.91 to 7.21) | 5.50 (2.15 to 14.04) | 15.00 (2.11 to 106.49) | 9.00 (0.51 to 160.07) | 2.00 (0.72 to 5.54) | 11.00 (0.65 to 185.70) | NR |
Examination of the trial baseline characteristics across trials offers no clear reason as to why placebo response rates in biologic trials have increased over time. The PsARC placebo response rates increased most markedly from 2013 onwards, starting with the PSUMMIT trials. 60,61,65 One theory is that patient and clinician expectations have increased over time (i.e. more caution and lower expectations when the first biologics were trialled, and more confidence about the likely benefits in more recent trials). Subjective patient- and clinician-reported outcomes such as PsARC and ACR may be prone to such expectation effects. This theory might also explain why, within trials, higher placebo response rates are observed in the biologic-naive subgroups than in biologic-experienced subgroups, where treatment expectations might be lower. Coupled with this is the trend – beginning with the PSUMMIT trials – for increases in the number of active treatment arms offered in trials: typically there was one active arm in the early trials and two or more active arms in more recent trials (e.g. the FUTURE 2 SEC trial had three active treatment arms: 75, 150 and 300 mg). Patients in the more recent trials might therefore also be more confident and optimistic about the likelihood that they are receiving an active treatment.
Ideally the different treatments would be compared in head-to-head trials. However, only one trial identified in the systematic review compared two or more biologics directly in a PsA population. The Atteno et al. trial64 compared INF, ETN and ADA. It reported that patients on INF and ADA showed the greatest improvement in terms of PASI (statistically significantly better than ETN), whereas patients on ETN showed the greatest improvement in TJC (statistically significantly better than INF and ADA) and HAQ-DI (statistically significantly better than ADA). However, the reliability of this study’s results are limited somewhat by its small size (100 patients were randomised in total). This trial also did not report its methods clearly (see Table 6), and was rated as being at high risk of bias (although blinding would be difficult to achieve in such a trial). Finally, by reporting results only at the 52-week time point, the results of this trial could not be included in our NMAs.
Long-term effectiveness
Open-label extension studies
Long-term efficacy of secukinumab
The Novartis submission to NICE for the appraisal in 2016 reported long-term data for both FUTURE 146 (to 104 weeks) and FUTURE 248 (to 52 weeks) trials. Although the FUTURE 1 trial46 was not eligible for the systematic review of efficacy because it initiated the randomised phase of the study with a non-licensed high loading dose (10 mg/kg), it did use a 150-mg maintenance dose and so can be considered to provide useful long-term data. Importantly, this trial reported radiographic efficacy outcomes (at 2 years); the FUTURE 2 trial48 did not report radiographic efficacy outcomes.
FUTURE 2
Of the FUTURE 2 trial 48 patients originally randomised to 150 or 300 mg of SEC, by week 52, 22 (11%) had withdrawn for any reason, 10 of whom withdrew as a result of an AE or loss of efficacy. In the FUTURE 2 trial,48 most of the dichotomous data reported in the submission used non-responder imputations for missing data; a mixed-effects repeated measures model was used for continuous outcomes. There were no stopping rules up to week 52, so non-responding patients could keep taking SEC thus allowing the possibility of achievement of much later responses than would be viable in the NHS. For time points after week 52, the protocol stated that subjects who are deemed not to be benefiting from the study treatment based on lack of improvement or worsening of their symptoms should discontinue the study. However, results for post-week 52 time points are not yet available. Results for key review outcomes at week 52 are presented in Table 30. The outcomes suggest that SEC continues to be an effective treatment for PsA at this later time point.
| Outcome | Trial arm | |
|---|---|---|
| 300 mg of SEC | 150 mg of SEC | |
| ACR response, n | 100 | 100 |
| % ACR 20 | 64 | 64 |
| % ACR 50 | 44 | 39 |
| % ACR 70 | 24 | 20 |
| PASI response (≥ 3% BSA), n | 41 | 58 |
| % PASI 75 | 73 | 57 |
| % PASI 90 | 56 | 43 |
| PsARC response, n | 100 | 100 |
| % PsARC response | Confidential information has been removed | Confidential information has been removed |
| HAQ-DI, n | 100 | 100 |
| Mean (SD) | –0.56 (0.05) | –0.47 (0.05) |
| SF-36, n | 100 | 100 |
| Mean (SD) | Confidential information has been removed | Confidential information has been removed |
Longer-term efficacy in FUTURE 2 trial patients who were responders at 16 weeks
In the NHS, patients will typically be allowed 16 weeks to achieve a response, after which SEC may be stopped in non-responding patients. The Assessment Group (AG) requested results specifically for patients who are responders at 16 weeks to inform what happens to this group of patients in the longer term. The results (Figures 3 and 4) indicate that for the lower threshold outcomes – such as ACR 20 and PASI 50 – response rates remain high from week 16 to week 52. As the outcome thresholds increase, response rates become more variable over time and there is generally a greater decrease in response rates than the lower threshold outcomes. Around 70% of patients on 150 mg still achieve an ACR 50 response at week 52, and around 55% still achieve an ACR 70 (see Figure 3); the corresponding results for PASI 75 and PASI 90 are around 85% and around 70%, respectively (see Figure 4).
FIGURE 3.
Long-term response rates in the FUTURE 248 trial SEC patients who were (a) ACR 20, (b) ACR 50 or (c) ACR 70 responders at 16 weeks. (Confidential information has been removed.)
FIGURE 4.
Long-term response rates in the FUTURE 248 trial SEC patients who were (a) PASI 50, (b) PASI 75 or (c) PASI 90 responders at 16 weeks. (Confidential information has been removed.)
FUTURE 1
Of the FUTURE 1 trial46 patients originally randomised to receive 75 or 150 mg of SEC or placebo, 15% had withdrawn at week 52 for any reason, of which 6% of withdrawals were the result of an AE or loss of efficacy. 46 At week 104, 79% of patients remained in the study. Here, we report only on the long-term efficacy of 150 mg of SEC. Results at 52 weeks are similar to those seen in the FUTURE 2 trial;48 observed data were also available at 2 years (Table 31).
| Outcome | Time point, 150 mg of SEC | |
|---|---|---|
| 52 weeks | 104 weeksa | |
| ACR response, n | 202 | 153 |
| % ACR 20 | 60 | 74 |
| % ACR 50 | 43 | 46 |
| % ACR 70 | 24 | 28 |
| PASI response (≥ 3% BSA), n | 108 | 82 |
| % PASI 75 | 77 | 83 |
| % PASI 90 | 59 | 70 |
| Dactylitis (LDI), n | 104 | – |
| % resolution of dactylitis | 69 | – |
| Enthesitis (LEI), n | 126 | – |
| % resolution of enthesitis | 66 | – |
| HAQ-DI, n | 202 | 153 |
| Mean (SE) | –0.41 (0.04) | –0.42 (–) |
| SF-36, n | 202 | 152 |
| Mean (SE) | 5.89 (0.54) | 5.94 (–) |
Radiographic progression of joint damage
In the FUTURE 1 trial,46 at week 52 the observed population comprised 189 of the 202 patients randomised to 150 mg; this group had a mean Sharp/van der Heijde change from baseline score of 0.37 points. At 104 weeks, 85% of patients treated with 150 mg of SEC had no radiographic progression – defined as a change in Sharp/van der Heijde score of ≤ 0.5 units – between baseline and week 104. This result was based on the observed population (n = 166).
Long-term efficacy of certolizumab pegol
The UCB Pharma submission reported long-term efficacy data for the RAPID-PsA trial47 at time points up to around 4 years (216 weeks). By week 96, 20% of the 273 patients originally randomised to CZP had withdrawn from the study; 13.5% of the total cohort had withdrawn as a result of an AE or loss of efficacy. Non-responder imputations were used for dichotomous outcomes and LOCF was used for most of the continuous outcomes (except for radiographic progression).
At week 96 the ACR 20, 50 and 70 response rates were 64%, 50% and 35%, respectively,74 and were (confidential information has been removed). PASI 75 and 90 response rates were 53% and 44% at week 96;74 and (confidential information has been removed).
(Confidential information has been removed.) The improvement in HAQ-DI score from baseline was maintained (confidential information has been removed). Efficacy results for the overall population together with the biologic-naive and biologic-experienced subgroups are presented in Table 32.
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
|---|---|---|---|
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
(Confidential information has been removed.)
Longer-term efficacy in patients who were responders at 12 weeks
In the NHS, patients will typically be allowed 12 weeks to achieve a response, after which CZP may be stopped in non-responding patients. The AG requested results specifically for patients who are responders at 12 weeks to inform what happens to this group of patients in the longer term. The response rates at 1 year are similar to those seen with SEC. Later results show that, across outcomes, around two-thirds (of responders at 12 weeks) remain responders at 4 years (Figures 5 and 6).
FIGURE 5.
Long-term response rates in the RAPID-PsA trial47 CZP patients who were (a) ACR 20, (b) ACR 50 or (c) ACR 70 responders at 12 weeks. (Confidential information has been removed.)
FIGURE 6.
Long-term response rates in the RAPID-PsA trial47 CZP patients who were (a) PASI 50, (b) PASI 75 or (c) PASI 90 responders at 12 weeks. (Confidential information has been removed.)
Radiographic progression of joint damage
At week 96, the modified total Sharp score (mTSS) non-progressor rate (non-progression defined as mTSS change from baseline of ≤ 0.5 points) was 87%. This was based on observed data for the combined CZP groups: 218 of the 273 randomised. For patients randomised to CZP (combined group), the mean level of progression was 0.14 points [standard error (SE) 0.09 points], which is below the 0.5-point non-progression cut-off point. Subgroup analyses indicated that patients (randomised to CZP) with a baseline mTSS of > 3.5 points had a slightly greater radiographic progression at week 96 than patients with a baseline mTSS of ≤ 3.5 points [mean 0.24 points (SE 0.19 points) for a mTSS of > 3.5 vs. mean 0.07 points (SE 0.04 points) for a mTSS of ≤ 3.5 points].
Efficacy of other therapies
Methods and result details relating to the latest time point for which long-term data were available for GOL, ETN, ADA, INF, UST and APR are presented in Table 33.
| Original trial name with relevant OL reference(s); treatment and dose; and latest time pointa | Number of patients | Analysis and imputation methods used by the study authors | Main results (ITT data extracted where possible) | Key withdrawal data | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GO-REVEAL;75,76 50 or 100 mg of GOL (at investigator’s discretion); 5 years | Of 405 randomised (placebo, n = 113; 50 mg of GOL, n = 146; 100 mg of GOL, n = 146), 279 (69%) were still on treatment at 5 years | It appeared that LOCF was used except for lack of efficacy discontinuations, where NRI was used and radiographic scores where observed data were used (n = 267) | At 5 years: Trial armModified SHS scoreb (SD)HAQ-DI (SD)ACR 20ACR 50PASI 75cPlacebo/50 mg of GOL0.3 (3.8)0.7 (0.6)71/113 (63%)49/113 (43%)48/79 (61%)50 mg of GOL0.3 (4.2)0.6 (0.6)96/146 (66%)70/146 (48%)67/109 (61%)100 mg of GOL0.1 (2.7)0.6 (0.6)102/146 (70%)74/146 (51%)78/108 (72%) |
Trial arm | Modified SHS scoreb (SD) | HAQ-DI (SD) | ACR 20 | ACR 50 | PASI 75c | Placebo/50 mg of GOL | 0.3 (3.8) | 0.7 (0.6) | 71/113 (63%) | 49/113 (43%) | 48/79 (61%) | 50 mg of GOL | 0.3 (4.2) | 0.6 (0.6) | 96/146 (66%) | 70/146 (48%) | 67/109 (61%) | 100 mg of GOL | 0.1 (2.7) | 0.6 (0.6) | 102/146 (70%) | 74/146 (51%) | 78/108 (72%) | 126/405 (31%) stopped treatment: 50 as a result of an AE and 23 because of lack of efficacy |
| Trial arm | Modified SHS scoreb (SD) | HAQ-DI (SD) | ACR 20 | ACR 50 | PASI 75c | |||||||||||||||||||||||
| Placebo/50 mg of GOL | 0.3 (3.8) | 0.7 (0.6) | 71/113 (63%) | 49/113 (43%) | 48/79 (61%) | |||||||||||||||||||||||
| 50 mg of GOL | 0.3 (4.2) | 0.6 (0.6) | 96/146 (66%) | 70/146 (48%) | 67/109 (61%) | |||||||||||||||||||||||
| 100 mg of GOL | 0.1 (2.7) | 0.6 (0.6) | 102/146 (70%) | 74/146 (51%) | 78/108 (72%) | |||||||||||||||||||||||
| Mease et al., 2004;77 25 mg of ETN twice weekly; up to 2 years | Of 205 randomised (placebo, n = 104; ETN, n = 101), 169 took part in the extended study | Analyses were based on observed populations. All analyses were performed on the subset of patients who had radiographic data for the 2-year assessment (n = 141: placebo/ETN, n = 70; ETN, n = 71) | At up to 2 years: Trial armmTSSbPsARCACR 20ACR 50PASI 75cPlacebo/ETN0.5≈80%63%49%≈38% of 102 patientsETN–0.38≈80%64%44% |
Trial arm | mTSSb | PsARC | ACR 20 | ACR 50 | PASI 75c | Placebo/ETN | 0.5 | ≈80% | 63% | 49% | ≈38% of 102 patients | ETN | –0.38 | ≈80% | 64% | 44% | 44/205 (21%) stopped treatment: 14 in RCT phase, nine in maintenance phase and 21 in OL phase. Three patients withdrew from OL phase because of an AE | |||||||
| Trial arm | mTSSb | PsARC | ACR 20 | ACR 50 | PASI 75c | |||||||||||||||||||||||
| Placebo/ETN | 0.5 | ≈80% | 63% | 49% | ≈38% of 102 patients | |||||||||||||||||||||||
| ETN | –0.38 | ≈80% | 64% | 44% | ||||||||||||||||||||||||
| ADEPT;78 40 mg of ADA every other week, patients without ≥ 20% improvement in TJC and SJC after 12 weeks of OL phase could increase to 40 mg per week; 2 years, 2.75 years for radiographic data | Of 313 randomised (placebo, n = 162; ADA, n = 151), 289 completed 24-week RCT, of which 285 chose to enrol in the extended study | Most analyses were based on a modified ITT population (any patients who had received a dose in either study phase, n = 298) with LOCF imputation | At 2 years (2.75 years for mTSS): Trial armmTSSb (SD)HAQ-DI b (SD)PsARCACR 20ACR 50Placebo/ADA0.9 (6.4), n = 128–0.3 (0.5)188/298 (63%)161/298 (54%)127/298 (43%)ADA0.5 (4.2), n = 115 |
Trial arm | mTSSb (SD) | HAQ-DI b (SD) | PsARC | ACR 20 | ACR 50 | Placebo/ADA | 0.9 (6.4), n = 128 | –0.3 (0.5) | 188/298 (63%) | 161/298 (54%) | 127/298 (43%) | ADA | 0.5 (4.2), n = 115 | 44/285 stopped treatment in the OL phase: 10 as a result of AEs; and 12 because of lack of efficacy | ||||||||||
| Trial arm | mTSSb (SD) | HAQ-DI b (SD) | PsARC | ACR 20 | ACR 50 | |||||||||||||||||||||||
| Placebo/ADA | 0.9 (6.4), n = 128 | –0.3 (0.5) | 188/298 (63%) | 161/298 (54%) | 127/298 (43%) | |||||||||||||||||||||||
| ADA | 0.5 (4.2), n = 115 | |||||||||||||||||||||||||||
| IMPACT;79 5 mg/kg of INF; up to 2 years | 104 patients took part in the RCT. 78 out of the 87 patients who completed the first year continued to enrol in the extended 2-year study | Analyses were based on the 78 patients who entered year 2 (analysed as one group) | At 98 weeks: Trial armModified SHS scoreb (SD)PsARCACR 20ACR 50PASI 75dPlacebo/INF1.2 (8.7), n = 4352/104 (50%)48/104 (46%)35/104 (34%)64% (n = unclear)INF |
Trial arm | Modified SHS scoreb (SD) | PsARC | ACR 20 | ACR 50 | PASI 75d | Placebo/INF | 1.2 (8.7), n = 43 | 52/104 (50%) | 48/104 (46%) | 35/104 (34%) | 64% (n = unclear) | INF | 26 patients withdrew over the 2 years: 12 as a result of AEs; and three because of lack of efficacy | |||||||||||
| Trial arm | Modified SHS scoreb (SD) | PsARC | ACR 20 | ACR 50 | PASI 75d | |||||||||||||||||||||||
| Placebo/INF | 1.2 (8.7), n = 43 | 52/104 (50%) | 48/104 (46%) | 35/104 (34%) | 64% (n = unclear) | |||||||||||||||||||||||
| INF | ||||||||||||||||||||||||||||
| PSUMMIT 1;80,81 45 or 90 mg of UST every 12 weeks; 100 weeks | 615 randomised (placebo, n = 206; 45 mg of UST, n = 205; 90 mg of UST, n = 204) and 598 received at least one dose of UST | Analyses were based on ITT populations using LOCF and NRI for most analyses. Missing radiographic data between week 52 and week 100 were imputed using linear extrapolation (if data were available for two time points), otherwise the median change in the total scores from all patients within the MTX stratification was used | At 100 weeks: Trial armTotal SHS scoreb (SD)HAQ-DI (SD)bACR 20ACR 50PASI 75cPlacebo/45 mg of UST2.3 (12.6), n = 189–0.36 (0.51)111/206 (54%)66/206 (32%)78/136 (57%)45 mg of UST1.0 (3.8)–0.36 (0.56)101/205 (49%)69/205 (34%)87/145 (60%)90 mg of UST1.2 (5.1)–0.45 (0.6)112/204 (55%)81/204 (40%)92/149 (62%) |
Trial arm | Total SHS scoreb (SD) | HAQ-DI (SD)b | ACR 20 | ACR 50 | PASI 75c | Placebo/45 mg of UST | 2.3 (12.6), n = 189 | –0.36 (0.51) | 111/206 (54%) | 66/206 (32%) | 78/136 (57%) | 45 mg of UST | 1.0 (3.8) | –0.36 (0.56) | 101/205 (49%) | 69/205 (34%) | 87/145 (60%) | 90 mg of UST | 1.2 (5.1) | –0.45 (0.6) | 112/204 (55%) | 81/204 (40%) | 92/149 (62%) | By week 88 (last dose), 125 patients (20.3%) had discontinued treatment: 31 as a result of an AE; and 40 because of lack of efficacy |
| Trial arm | Total SHS scoreb (SD) | HAQ-DI (SD)b | ACR 20 | ACR 50 | PASI 75c | |||||||||||||||||||||||
| Placebo/45 mg of UST | 2.3 (12.6), n = 189 | –0.36 (0.51) | 111/206 (54%) | 66/206 (32%) | 78/136 (57%) | |||||||||||||||||||||||
| 45 mg of UST | 1.0 (3.8) | –0.36 (0.56) | 101/205 (49%) | 69/205 (34%) | 87/145 (60%) | |||||||||||||||||||||||
| 90 mg of UST | 1.2 (5.1) | –0.45 (0.6) | 112/204 (55%) | 81/204 (40%) | 92/149 (62%) | |||||||||||||||||||||||
| PALACE 1;61,82 30 mg of APR twice daily, oral tablets; 2 years | 504 patients were randomised (placebo, n = 168; 20 mg of APR, n = 168; 30 mg of APR, n = 168). 101 patients received 30 mg of APR continuously for 2 years (observed population) | Analyses were based on the observed population for the extension period | At 104 weeks: Trial armHAQ-DIbACR 20PASI 75c30 mg of APR–0.43, n = 10167/168 (40%)21/71 (29.6%) |
Trial arm | HAQ-DIb | ACR 20 | PASI 75c | 30 mg of APR | –0.43, n = 101 | 67/168 (40%) | 21/71 (29.6%) | 8.2% discontinued treatment as a result of AEs between weeks 0 and 52 and 1.5% between weeks 53 and 104 | ||||||||||||||||
| Trial arm | HAQ-DIb | ACR 20 | PASI 75c | |||||||||||||||||||||||||
| 30 mg of APR | –0.43, n = 101 | 67/168 (40%) | 21/71 (29.6%) |
The Golimumab – A Randomized Evaluation of Safety and Efficacy in Subjects with Psoriatic Arthritis Using a Human Anti-TNF Monoclonal Antibody (GO-REVEAL) study75 reported results at 5 years using the originally randomised ITT groups. Across the groups the proportion of responders ranged from 63% to 70% for ACR 20, from 43% to 51% for ACR 50 and from 61% to 72% for PASI 75. Mean changes from baseline in the modified Sharp/van der Heijde score ranged from 0.1 to 0.3 units. Clinically important improvements in HAQ-DI scores (a decrease of ≥ 0.3 units) were seen for 52–58% of randomised patients. The use of concomitant MTX at baseline did not affect ACR 20 or PASI 75, but did appear to reduce radiographic progression when a comparison was made with patients who did not use concomitant MTX at baseline. Although some method details were not fully clear, it appeared that the data imputations used were not conservative enough. For example, it seems that LOCF was used for patients who stopped treatment as a result of an AE (so a patient responding well to treatment but who discontinued treatment early in the study as a result of an AE was counted as a responder at 5 years). In addition, it was unclear whether or not there were any stopping rules – such as how long non-responders were allowed to remain on treatment – which raises further uncertainties about the study’s applicability to clinical practice.
The follow-up for the Mease et al. ETN trial54 extended to 2 years and consisted of three phases: the 24-week initial randomised phase, an optional 24-week maintenance therapy phase (according to randomised assignment) and a 48-week open-label phase. Most results were given as percentages and it was not fully clear what the denominator was for some results. Several results were presented only as graphs. Very few data were provided on reasons for withdrawal from the study and HAQ-DI results were not reported. The ACR response results were similar to those seen in the GO-REVEAL trial (at 5 years), although the proportions of PASI 75 responders were markedly lower.
The ADEPT ADA trial78 was extended to 2.75 years for radiographic progression outcomes and to 2 years for other outcomes. The ACR 50 results were similar to those seen for the ETN and GOL open-label studies. PASI 75 results were only presented in a graph; the response was around 60% (n = 128), which is similar to the GO-REVEAL trial’s PASI 75 result at 5 years. Non-responders could increase their dose from 40 mg every other week (the recommended dose) to 40 mg weekly; this occurred in 54 (19%) patients. The use of LOCF imputation for missing data for the ACR, PASI and PsARC outcomes is different (potentially much less conservative) from the imputations used in the placebo-controlled phase, where non-responder imputations were used. This is likely to have inflated the response rates in the open-label phase. The results for HAQ-DI remained very stable throughout the 2 years. These open-label HAQ-DI results are similar to the placebo-controlled, fully blinded 24-week phase in which HAQ-DI scores remained the same between week 12 and week 24 in both the ADA and the placebo groups.
The UST PSUMMIT 1 trial80,81 was extended to 108 weeks, with efficacy data evaluated at 100 weeks. The change from baseline Sharp/van der Heijde radiographic progression scores varied across the three treatment groups. The change from baseline HAQ-DI results ranged between –0.36 and –0.45 units, similar to the ADA study results.
For INF, IMPACT79 was extended to 98 weeks. The data for all patients were summarised as one group (as for the ADA open-label study). At 98 weeks, 46% and 34% were ACR 20 and ACR 50 responders, respectively. The mean change in the modified Sharp/van der Heijde score was 1.2 units, which is similar to the results in the UST PSUMMIT 1. 80,81 However, the result was based on 41% of the initial 104 patients. The authors also acknowledged that the 2-year radiographic progression result may have reflected non-linear progression of damage, with more damage occurring in earlier disease stages. Mean changes from baseline were not available for the HAQ-DI.
For APR, the PALACE 1 trial61,82 was extended to 2 years. There were no separate results for the patients at 104 weeks who were in the placebo group at the beginning of the trial. In the 30-mg group, at 2 years 40% of patients were ACR 20 responders and 30% were PASI 75 responders. The HAQ-DI result may be an overestimate, as it was based on data from patients remaining in the study at 2 years (i.e. observed data). No data were reported on any radiographic progression outcomes.
Summary
The uncontrolled nature of open-label extension studies means that it is often very difficult to determine the magnitude of effects that can be ascribed only to active treatment; results should generally be viewed with much more caution than the results of the earlier randomised controlled study phases. Furthermore, it is not straightforward to compare long-term results across different treatments because of the variation in outcomes and time points reported. There is also variation in the methodological approaches used for analyses and for imputing missing data. Additionally, most studies did not report whether or not there were any treatment stopping rules, and it is likely that the decisions made regarding continuation of treatment were not reflective of those used in the NHS, limiting the applicability of many of these results. For example, in the open-label ADEPT78 non-responders after 12 weeks had their dose doubled – the opposite of what would be expected in practice (when treatment with ADA would have been stopped).
With these caveats in mind, the results relating specifically to those patients who were responders at 12 or 16 weeks appear to be the most clinically relevant and useful (for the dichotomous outcomes), although such data were available only for CZP and SEC (confidential information has been removed).
The available data on disease progression based on radiographic assessments of joint damage indicate that, after 2 years of treatment, CZP effectively reduces disease progression, with results being similar to those observed in the open-label studies of the other anti-TNFs. For SEC, fewer result details were available at 2 years, although the results also indicated effective reduction in radiographic disease progression.
For long-term HAQ-DI results, missing data were often imputed using LOCF, which is not the most conservative of approaches for this outcome. Notwithstanding this, the results suggest that HAQ-DI gains remain stable in PsA patients treated with biologics. The 2-year open-label HAQ-DI results from ADEPT were similar to the placebo-controlled, fully blinded 24-week phase in which HAQ-DI scores remained the same between week 12 and week 24 in both the ADA and the placebo groups. This stability of HAQ-DI scores over time was also seen in the open-label studies of CZP (data up to 4 years) and SEC (data to 1 year).
Withdrawal rates as a result of AEs or loss of efficacy were low in both the FUTURE 248 (5% at 52 weeks) and RAPID-PsA47 trials (around 10% at 52 weeks).
Review of anti-tumour necrosis factor patient registry studies
Drug survival and anti-tumour necrosis factor switching
The database of references, which resulted from the searches for RCTs, was also screened to identify registries containing PsA patients and the corresponding publication output. The results of this search were supplemented by separate searches for the output of the identified patient registries reporting information on their PsA cohorts. A library of 165 potentially relevant studies was assembled and screened fully, from which there were 12 studies83–93 reporting data on drug survival and switching of anti-TNF treatments. The populations of all 12 studies were defined as having clinically diagnosed PsA. These studies are presented in Table 34.
| Publication (first author and year of publication) | Registry name | Number of patients (length of follow-up) | Population | Anti-TNFs included | Drug survival data | Reason for discontinuation | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carmona et al., 200691 | BIOBADASER | 570 (5 years; 963.6 patient-years) | PsA | ETN, ADA and INF | As a proportion, anti-TNF-α survival was 0.88 (95% CI 0.84 to 0.90) at 1 year; 0.81 (95% CI 0.77 to 0.84) at 2 years; and 0.73 (95% CI 0.67 to 0.78) at 3 years | Not split by diagnosis | |||||||||||||||||||||||||||||||
| Chen et al., 201489 | ARAD | 286 (10 years) | PsA | ETN, ADA and INF | Median survival time: ETN (n = 110), 2.62 years (95% CI 1.10 to 4.45 years); ADA (n = 144), 4.21 years; INF (n = 23), 1.92 years (95% CI 0.96 to 2.88 years) | Trial armAny (%)LoE (%)AE (%)ETN4120.58ADA3517.711.5INF7029.611.1 | Trial arm | Any (%) | LoE (%) | AE (%) | ETN | 41 | 20.5 | 8 | ADA | 35 | 17.7 | 11.5 | INF | 70 | 29.6 | 11.1 | |||||||||||||||
| Trial arm | Any (%) | LoE (%) | AE (%) | ||||||||||||||||||||||||||||||||||
| ETN | 41 | 20.5 | 8 | ||||||||||||||||||||||||||||||||||
| ADA | 35 | 17.7 | 11.5 | ||||||||||||||||||||||||||||||||||
| INF | 70 | 29.6 | 11.1 | ||||||||||||||||||||||||||||||||||
| Fagerli et al., 201490 | NOR-DMARD | 439 (3 years; 547 patient-years) | PsA | ETN, ADA, INF, GOL and CZP | The proportion of non-switchers (n = 344) remaining on their first anti-TNF-α after 1 year was 0.83; at 3 years it was 0.71. The 1-year survival for all patients on their first anti-TNF-α was 0.74. The proportion of those patients who switched to a different anti-TNF-α (n = 95) remaining on second treatment for 1 year was 0.56; the 3-year survival was 0.36 | NR | |||||||||||||||||||||||||||||||
| Response rate at 3 months: ResponseNon-switchers (%)Switchers (%)p-value switchers first vs. secondFirstFirstSecondACR 2064.445.822.5NSACR 5040.030.512.5NSACR 7032.223.70.04 |
Response | Non-switchers (%) | Switchers (%) | p-value switchers first vs. second | First | First | Second | ACR 20 | 64.4 | 45.8 | 22.5 | NS | ACR 50 | 40.0 | 30.5 | 12.5 | NS | ACR 70 | 32.2 | 23.7 | 0.04 | ||||||||||||||||
| Response | Non-switchers (%) | Switchers (%) | p-value switchers first vs. second | ||||||||||||||||||||||||||||||||||
| First | First | Second | |||||||||||||||||||||||||||||||||||
| ACR 20 | 64.4 | 45.8 | 22.5 | NS | |||||||||||||||||||||||||||||||||
| ACR 50 | 40.0 | 30.5 | 12.5 | NS | |||||||||||||||||||||||||||||||||
| ACR 70 | 32.2 | 23.7 | 0.04 | ||||||||||||||||||||||||||||||||||
| Fagerli et al., 201484 | BSRBR | 666 (5 years) | PsA | ETN, ADA and INF | After 5 years, 46.8% of patients were still on an initial anti-TNF-α treatment | LoEAEOther/missing35.3%28.8%35.9% | LoE | AE | Other/missing | 35.3% | 28.8% | 35.9% | |||||||||||||||||||||||||
| LoE | AE | Other/missing | |||||||||||||||||||||||||||||||||||
| 35.3% | 28.8% | 35.9% | |||||||||||||||||||||||||||||||||||
| Glintborg et al., 201188 | DANBIO | 764 (9 years; 2135 patient-years) | PsA | ETN, ADA and INF | The proportion of the cohort remaining on the same anti-TNF-α after 1 year was 0.70, and 0.57 at 2 years. The median drug survival was 2.9 years | LoEAE23%12% | LoE | AE | 23% | 12% | |||||||||||||||||||||||||||
| LoE | AE | ||||||||||||||||||||||||||||||||||||
| 23% | 12% | ||||||||||||||||||||||||||||||||||||
| Glintborg et al., 201394 | DANBIO | 1422; 548 switchers (10 years) | PsA | ETN, ADA, INF, GOL, CZP and other non-anti-TNF biologics | The median survival time for patients on their first course of treatment was 2.2 years (95% CI 1.9 to 2.5 years). Second course (n = 548), drug survival was 1.3 years (95% CI 1.0 to 1.6 years). Third course (n = 189), median survival was 1.1 years (95% CI 0.7 to 1.5 years). The median drug survival of first anti-TNF-α among switchers was 0.7 years (95% CI 0.6 to 0.8 years) | Course of treatmentAny (%)LoE (%)AE (%)First62616Second552815Third553314 | Course of treatment | Any (%) | LoE (%) | AE (%) | First | 6 | 26 | 16 | Second | 55 | 28 | 15 | Third | 55 | 33 | 14 | |||||||||||||||
| Course of treatment | Any (%) | LoE (%) | AE (%) | ||||||||||||||||||||||||||||||||||
| First | 6 | 26 | 16 | ||||||||||||||||||||||||||||||||||
| Second | 55 | 28 | 15 | ||||||||||||||||||||||||||||||||||
| Third | 55 | 33 | 14 | ||||||||||||||||||||||||||||||||||
| Glintborg et al., 201492 | DANBIO and ICEBIO | 462 (< 10 years; 1185 patient-years) | PsA (patients on INF) | INF (variable dose) | The 1-year drug survival for INF patients was 59.5% across both registers. Dose did not affect drug survival or treatment response | LoEAE25%29% | LoE | AE | 25% | 29% | |||||||||||||||||||||||||||
| LoE | AE | ||||||||||||||||||||||||||||||||||||
| 25% | 29% | ||||||||||||||||||||||||||||||||||||
| Iannone et al., 201593 | GISEA | 328 (2 years) | PsA | ETN, ADA and INF | The 2-year overall drug survival was 0.67 | NR | |||||||||||||||||||||||||||||||
| Kristensen et al., 200885 | SSATG | 261 (7 years) | PsA | ETN, ADA and INF | Kaplan–Meier graph estimates of drug survival were: Time pointTreatment (proportion of patients remaining on treatment)Anti-TNF-α onlyAnti-TNF-α + MTX1 year0.650.83 years0.550.65 years0.3750.4 |
Time point | Treatment (proportion of patients remaining on treatment) | Anti-TNF-α only | Anti-TNF-α + MTX | 1 year | 0.65 | 0.8 | 3 years | 0.55 | 0.6 | 5 years | 0.375 | 0.4 | Risk of AE lower with concomitant MTX | ||||||||||||||||||
| Time point | Treatment (proportion of patients remaining on treatment) | ||||||||||||||||||||||||||||||||||||
| Anti-TNF-α only | Anti-TNF-α + MTX | ||||||||||||||||||||||||||||||||||||
| 1 year | 0.65 | 0.8 | |||||||||||||||||||||||||||||||||||
| 3 years | 0.55 | 0.6 | |||||||||||||||||||||||||||||||||||
| 5 years | 0.375 | 0.4 | |||||||||||||||||||||||||||||||||||
| Mease et al., 201587 | CORRONA | 497 (7 years) | PsA | ETN, ADA and INF | The median survival time of patients being treated with anti-TNF-α monotherapy was 30.8 months, and for those being treated with combination therapy (anti-TNF + MTX or DMARD) was 32.4 months | NR | |||||||||||||||||||||||||||||||
| Saad et al., 200983 | BSRBR | 566 (3 years) | PsA | ETN, ADA and INF | TreatmentTime point (proportion of patients remaining on treatment)1 year2 years3 yearsTotal first anti-TNF-α0.820.700.59ETN (n = 316)0.860.790.65ADA (n = 88)0.910.700.66INF (n = 162)0.710.520.43Switchers (n = 178)0.740.66– | Treatment | Time point (proportion of patients remaining on treatment) | 1 year | 2 years | 3 years | Total first anti-TNF-α | 0.82 | 0.70 | 0.59 | ETN (n = 316) | 0.86 | 0.79 | 0.65 | ADA (n = 88) | 0.91 | 0.70 | 0.66 | INF (n = 162) | 0.71 | 0.52 | 0.43 | Switchers (n = 178) | 0.74 | 0.66 | – | LoEAE9.5%10% | LoE | AE | 9.5% | 10% | ||
| Treatment | Time point (proportion of patients remaining on treatment) | ||||||||||||||||||||||||||||||||||||
| 1 year | 2 years | 3 years | |||||||||||||||||||||||||||||||||||
| Total first anti-TNF-α | 0.82 | 0.70 | 0.59 | ||||||||||||||||||||||||||||||||||
| ETN (n = 316) | 0.86 | 0.79 | 0.65 | ||||||||||||||||||||||||||||||||||
| ADA (n = 88) | 0.91 | 0.70 | 0.66 | ||||||||||||||||||||||||||||||||||
| INF (n = 162) | 0.71 | 0.52 | 0.43 | ||||||||||||||||||||||||||||||||||
| Switchers (n = 178) | 0.74 | 0.66 | – | ||||||||||||||||||||||||||||||||||
| LoE | AE | ||||||||||||||||||||||||||||||||||||
| 9.5% | 10% | ||||||||||||||||||||||||||||||||||||
| Simard et al., 201186 | ARTIS | 1417 (9 years) | PsA | NR | The Kaplan–Meier graph estimates of survival following treatment with a first anti-TNF-α were 0.75 at 1 year, 0.63 at 2 years, 0.5 at 4 years, 0.37 at 6 years and 0.32 at 8 years | LoEAE9.4%8.2% Within 1 year of treatment initiation |
LoE | AE | 9.4% | 8.2% | |||||||||||||||||||||||||||
| LoE | AE | ||||||||||||||||||||||||||||||||||||
| 9.4% | 8.2% | ||||||||||||||||||||||||||||||||||||
These studies were all retrospective analyses of prospective patient registers from primarily European countries (10 studies83–86,88,90–94), along with one Australian study89 and another from the USA. 87 The majority of patients in each of the registries had been treated with ETN, ADA or INF; two of the studies named other anti-TNF-α treatments, GOL and CZP, but neither had sufficient data to provide individual drug survival information for these.
Drug survival was reported in a number of ways: as the number of patients remaining on treatment at a given time point; as the proportion of patients remaining on treatment at each time point; or as the median duration patients remained on treatment.
Treatment withdrawal rates in patients who had switched anti-TNFs were reported in three studies. 83,94,95 The Danish Database for Biological Therapies (DANBIO) registry94 reported up to three sequential anti-TNFs, with 548 patients who had switched treatment once, and 189 patients who had switched treatment twice. The UK’s British Society for Rheumatology Biologics Register (BSRBR)83 also reported drug survival rates for its population of 178 one-time switchers over 2 years, whereas the 95 switchers in the Norwegian Antirheumatic Drug Register (NOR-DMARD)95 were followed for 3 years.
For the first course of anti-TNF treatment, the proportion of patients remaining on treatment ranged from 60% to 88% at 1 year, from 57% to 81% at 2 years and from 55% to 73% at 3 years. Three studies reported first anti-TNF drug survival rates for ≥ 5 years: (1) the BSRBR study,84 in which 47% of patients were still on the initial anti-TNF treatment at 5 years; (2) the Southern Sweden Antirheumatic Therapy Group study,85 which reported 5-year survival of around 40%; and (3) the study conducted by another Swedish registry, Antirheumatic Therapies In Sweden,86 which reported 6-year first anti-TNF drug survival of 37% and 8-year survival of 32%.
The median first anti-TNF survival time across all anti-TNFs was reported as 2.5–2.9 years. 87,88 One study reported this separately by anti-TNF: ETN, 2.62 years; ADA, 4.21 years; and INF, 1.92 years. 89
Drug survival was consistently lower in patients who switched anti-TNF than in those who did not. The DANBIO94 register had the largest population of switchers; the median drug survival for a first anti-TNF was 2.2 (95% CI 1.9 to 2.5) years, whereas median drug survival for a second anti-TNF was 1.3 years (95% CI 1.0 to 1.6 years) (n = 548), and was 1.1 years (95% CI 0.7 to 1.5 years) (n = 189) for those on a third anti-TNF.
There is some evidence suggesting that drug survival varies between types of anti-TNF; both the Australian Rheumatology Association Database register and the BSRBR study report rates for individual therapies, and both indicate that ADA and ETN are associated with considerably higher survival rates than INF. Two studies reported the impact of concomitant MTX or other DMARDs. 85,87 One reported a small increase in drug survival at 1 year (from 65% to 80%), but this effect was diminished at 3 years (from 55% to 60%) and 5 years (from 37.5% to 40%). 85 The other study reported that median drug survival time for anti-TNF-α monotherapy was 30.8 months, compared with 32.4 months for combination therapy (anti-TNF + MTX or DMARD). 87
Reasons for discontinuation of treatment varied widely between studies, due in part to the inconsistency of observation period duration. Across all registries, between 20% and 35% of patients withdrew from treatment because of a lack of efficacy and, generally, a smaller proportion withdrew as a result of AEs. The frequency of occurrence of AEs was linked to the types of anti-TNF used and whether or not patients received concomitant MTX, which was generally found to reduce AE frequency when MTX subgroups were analysed.
Only one study reported an analysis of response rates; this was based on the 3-month response rates from the NOR-DMARD (n = 439). 90 A retrospective comparison of response rates in switchers and non-switchers found that switchers had a lower response rate to the first anti-TNF: for ACR 50, 30.5% compared with 40%. In addition, the response to the second anti-TNF was lower than to the first: 22.5% (compared with 30.5%, although this difference was not statistically significant). The same pattern was seen for ACR 20 and 70 and, for the latter, the difference reached statistical significance.
In summary, across all relevant studies, those patients who switched treatment had a shorter median drug survival time, also showing a continuously smaller proportion of patients remaining on each subsequent treatment option. This may reflect a lack of improvement in treatment response after switching biologic; however, there are limited direct data on the effect of sequential treatments on relevant outcome measures. The proportion of patients withdrawing from treatment because of a lack of effect also seems to increase with the number of times a patient switches anti-TNF therapy. The registry data suggest that, although patients can benefit from a second (or further) anti-TNF, the expected benefit from anti-TNFs diminishes after switching, with a reduced chance of response and reduced drug survival.
Effect of anti-tumour necrosis factors on radiographic progression and Health Assessment Questionnaire-Disability Index score
Four patient registry studies that provided longitudinal data on the effect of anti-TNFs on HAQ-DI scores were identified, one of which also reported on radiographic progression. The results of these are presented in Table 35.
| Publication (first author and year of publication) | Study description | Findings | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eder et al., 201496 | Up to 4 years of radiographic progression in 65 patients treated with anti-TNF-α compared with 70 patients treated with MTX alone in the University of Toronto cohort. Only patients with bone erosions at baseline were included | At the first assessment after baseline (1–2 years): MTX group, 68% developed a new erosion in at least one joint, 80% of patients exhibited radiographic progression; anti-TNF-α group, 56.4% had a new eroded joint and 58.9% had radiographic progression At the 2- to 4-year assessment: MTX group, 84% developed a new erosion, 88% had radiographic progression; anti-TNF-α group, 75% had a new eroded joint and 61% with radiographic progression Time pointTreatment, HAQ-DI score (units)Anti-TNF-αMTXBaseline0.9 ± 0.70.7 ± 0.71–2 years0.6 ± 0.60.6 ± 0.63–4 years0.6 ± 0.60.7 ± 0.7 |
Time point | Treatment, HAQ-DI score (units) | Anti-TNF-α | MTX | Baseline | 0.9 ± 0.7 | 0.7 ± 0.7 | 1–2 years | 0.6 ± 0.6 | 0.6 ± 0.6 | 3–4 years | 0.6 ± 0.6 | 0.7 ± 0.7 | |||||||||||||||||
| Time point | Treatment, HAQ-DI score (units) | |||||||||||||||||||||||||||||||
| Anti-TNF-α | MTX | |||||||||||||||||||||||||||||||
| Baseline | 0.9 ± 0.7 | 0.7 ± 0.7 | ||||||||||||||||||||||||||||||
| 1–2 years | 0.6 ± 0.6 | 0.6 ± 0.6 | ||||||||||||||||||||||||||||||
| 3–4 years | 0.6 ± 0.6 | 0.7 ± 0.7 | ||||||||||||||||||||||||||||||
| Fagerli et al., 201490 | Analysis of the effect of MTX co-medication in 440 PsA patients in the NOR-DMARD | The study found no difference in treatment response between those on anti-TNF-α monotherapy and those with concomitant MTX. Mean cohort HAQ-DI was recorded as 0.7 units at baseline, 0.39 units at 3 months and 0.38 units at 6 months. Mean change from baseline at 3 months = 0.31 units | ||||||||||||||||||||||||||||||
| Glintborg et al., 201188 | Analysis of long-term anti-TNF treatment response data from the DANBIO register (n = 658). Measured by HAQ-DI over 5 years | Time pointHAQ-DI score (units)Number of patientsBaseline1.06582 weeks0.752756 weeks0.63666 months0.64061 year0.43182 years0.42293 years0.31274 years0.31045 years0.545 | Time point | HAQ-DI score (units) | Number of patients | Baseline | 1.0 | 658 | 2 weeks | 0.75 | 275 | 6 weeks | 0.6 | 366 | 6 months | 0.6 | 406 | 1 year | 0.4 | 318 | 2 years | 0.4 | 229 | 3 years | 0.3 | 127 | 4 years | 0.3 | 104 | 5 years | 0.5 | 45 |
| Time point | HAQ-DI score (units) | Number of patients | ||||||||||||||||||||||||||||||
| Baseline | 1.0 | 658 | ||||||||||||||||||||||||||||||
| 2 weeks | 0.75 | 275 | ||||||||||||||||||||||||||||||
| 6 weeks | 0.6 | 366 | ||||||||||||||||||||||||||||||
| 6 months | 0.6 | 406 | ||||||||||||||||||||||||||||||
| 1 year | 0.4 | 318 | ||||||||||||||||||||||||||||||
| 2 years | 0.4 | 229 | ||||||||||||||||||||||||||||||
| 3 years | 0.3 | 127 | ||||||||||||||||||||||||||||||
| 4 years | 0.3 | 104 | ||||||||||||||||||||||||||||||
| 5 years | 0.5 | 45 | ||||||||||||||||||||||||||||||
| Glintborg et al., 201394 | DANBIO (n = 1422; 548 switchers) (10 years). PsA (ETN, ADA, INF, GOL, CZP and other non-anti-TNF biologics) | Anti-TNF course of treatmentTime point, median HAQ-DI score (units) (IQR)0 months3 months6 monthsFirst (n = 1422)1 (0.5–1.5)0.6 (0.1–1.1)0.6 (0.1–1.0)Second (n = 548)1.1 (0.6–1.6)0.9 (0.4–1.5)0.9 (0.4–1.4)Third (n = 189)1.4 (0.9–2.9)1.0 (0.6–1.5)1.3 (0.5–1.6) | Anti-TNF course of treatment | Time point, median HAQ-DI score (units) (IQR) | 0 months | 3 months | 6 months | First (n = 1422) | 1 (0.5–1.5) | 0.6 (0.1–1.1) | 0.6 (0.1–1.0) | Second (n = 548) | 1.1 (0.6–1.6) | 0.9 (0.4–1.5) | 0.9 (0.4–1.4) | Third (n = 189) | 1.4 (0.9–2.9) | 1.0 (0.6–1.5) | 1.3 (0.5–1.6) | |||||||||||||
| Anti-TNF course of treatment | Time point, median HAQ-DI score (units) (IQR) | |||||||||||||||||||||||||||||||
| 0 months | 3 months | 6 months | ||||||||||||||||||||||||||||||
| First (n = 1422) | 1 (0.5–1.5) | 0.6 (0.1–1.1) | 0.6 (0.1–1.0) | |||||||||||||||||||||||||||||
| Second (n = 548) | 1.1 (0.6–1.6) | 0.9 (0.4–1.5) | 0.9 (0.4–1.4) | |||||||||||||||||||||||||||||
| Third (n = 189) | 1.4 (0.9–2.9) | 1.0 (0.6–1.5) | 1.3 (0.5–1.6) | |||||||||||||||||||||||||||||
| Saad et al., 201097 | Evaluation of the effect of anti-TNF therapies on quality of life and functional status in the BSRBR cohort of 596 PsA patients | Time pointMedian HAQ-DI score (units) (IQR)Number of patientsBaseline1.88 (1.38–2.25)5626 months1.25 (0.63–1.88)42412 months1.38 (0.63–2.00)38218 months1.38 (0.63–2.00)344 | Time point | Median HAQ-DI score (units) (IQR) | Number of patients | Baseline | 1.88 (1.38–2.25) | 562 | 6 months | 1.25 (0.63–1.88) | 424 | 12 months | 1.38 (0.63–2.00) | 382 | 18 months | 1.38 (0.63–2.00) | 344 | |||||||||||||||
| Time point | Median HAQ-DI score (units) (IQR) | Number of patients | ||||||||||||||||||||||||||||||
| Baseline | 1.88 (1.38–2.25) | 562 | ||||||||||||||||||||||||||||||
| 6 months | 1.25 (0.63–1.88) | 424 | ||||||||||||||||||||||||||||||
| 12 months | 1.38 (0.63–2.00) | 382 | ||||||||||||||||||||||||||||||
| 18 months | 1.38 (0.63–2.00) | 344 | ||||||||||||||||||||||||||||||
One study96 reported on radiographic progression; a comparison of anti-TNF and MTX found an inhibitory effect of anti-TNF on radiographic progression over 4 years of observation. Radiographic progression was measured in terms of newly forming erosions and change in a modified Steinbrocker score; radiographic progression according to both measures was significantly more prevalent in the MTX group at each follow-up assessment.
Four studies88,90,96,97 reported on disease progression in terms of HAQ-DI score for between 6 months and 5 years at varying frequency. Eder et al. 96 compared HAQ-DI score change in 70 patients treated with MTX and 65 patients on an anti-TNF, finding no significant difference in HAQ-DI score between the groups at the two assessments at up to 4 years from baseline. The HAQ-DI score was measured in 658 patients receiving anti-TNFs for 5 years in the largest cohort88 (the DANBIO register). The baseline mean HAQ-DI score was 1.0 unit, decreasing to 0.3 units by 3 years, and increasing to 0.5 units at 5 years. This suggests sustained long-term improvement of functional status during anti-TNF treatment, although the number of patients at each time point after the 6-month assessment decreased significantly. Therefore, the trend of improving HAQ-DI scores observed in this study is potentially due to a higher attrition of patients, with greater functional impairment skewing the data. The third study on HAQ-DI change is from the NOR-DMARD,90 and showed an improvement in HAQ-DI score from 0.7 units at baseline to 0.39 units at 3 months, and 0.38 units at 6 months. This study also found no significant difference in HAQ-DI response in patients receiving MTX compared with those on biologics alone. The BSRBR97 study followed an initial cohort of 562 patients on biologics for 18 months. This group of patients appears to have had more advanced disease (12 years since onset) and poorer functional status than those in the other included studies, with a median baseline HAQ-DI score of 1.88 units (95% CI 1.38 to 2.25 units). There is a 0.63-unit decrease in HAQ-DI score between baseline and 6 months of treatment, representing what the authors describe as a clinically important improvement. The median HAQ-DI score then increases to and remains at 1.38 units (95% CI 0.63 to 2.00 units) at both the 12- and 18-month assessments. Disease duration at the time of treatment initiation in the BSRBR study was more than twice that in two of the aforementioned studies on the HAQ-DI, showing that significant improvements in functional status are achievable using anti-TNF therapy in advanced cases of PsA.
Treatment with anti-TNFs appears to yield significant improvement in radiographic progression and long-term HAQ-DI score change in patient registry studies, although it is not clear to what extent the treatment is responsible for the reduction in mean cohort HAQ-DI score over time. Estimation of HAQ-DI score change using measures more robust to attrition bias or profiling those lost to follow-up based on disease severity would have given a truer representation of HAQ-DI score change in these cohorts. The paucity of radiographic data in these registry studies is perhaps surprising given the significance of radiographic damage as a measure of disease progression and treatment effects. This lack of published data may be because few of these registries were set up to record PsA-specific outcomes, and there has historically been little consensus on a method for objectively taking and scoring joint radiographs in this disease. It may be that HAQ-DI was usually preferred as an acceptable and standardised proxy for assessing bone erosion and, as a patient-reported outcome measure, can be cheaply and routinely recorded without the need for specialist assessment.
Review of the natural history of psoriatic arthritis: registry and cohort study data
A total of four publications33,98–100 analysing patterns of natural disease progression in registries or long-term cohort data were found and are shown in Table 36. These were reviewed in order to gain an understanding of the manner in which disease progresses in patients who do not receive anti-TNF therapy, despite being eligible for treatment. Owing to the now ubiquitous nature of anti-TNFs and only recent recognition of PsA as a separate and distinct form of arthritis, information on the long-term uncontrolled progression of the disease is scarce. Two of the studies33,100 found in the literature search were different analyses of the same data set derived from the Norfolk Arthritis Register (NOAR): one was a 2-year prospective cohort study99 and the other a retrospective analysis of a Canadian single-site patient registry. 98
| Publication (first author and year of publication) | Study description | Population characteristics | Findings |
|---|---|---|---|
| Husted et al., 200598 | Analysed long-term change in physical function of PsA patients enrolled in the University of Toronto PsA cohort. Patients were assigned to one of three disability states depending on physical function and transition between states was recorded over time. 341 patients were observed for up to 10 years | Anti-TNF-naive PsA patients: male, n = 201; female, n = 140; age (mean), 45.9 years; duration of PsA (mean), 10.6 years; PASI (mean), 7.1 ± 9.7 units; baseline HAQ-DI score, 0.69 ± 0.67 units | Patients adhered to one of three longitudinal patterns: 46% remained stable [28% of patients remained in the ‘no disability’ state (HAQ-DI < 0.5 units)], 12% ‘moderate’ (0.5–1.5 units), and 6% in ‘severe disability’ (1.51–3 units) throughout the study. 26.7% made a single change to a lower or higher disability group, reflecting steady improvement or deterioration, and 27.3% experienced two or more transitions between states of disability. Mean time between assessments was 1.29 years. Mean change in HAQ-DI between consecutive assessments in deteriorating patients was +0.55 units, and was –0.57 units in improving patients. Greater age was related to slower improvement of HAQ-DI score in the moderate and severe disability groups. Decline in disability was slower in males than in females, and time since diagnosis was related to more frequent transition between disability states. No association was found between PASI score and transition between disability states |
| Kane et al., 200399 | Analysis of 2-year prospective study of 129 PsA patients at St Vincent’s University Hospital Early Arthritis Clinic, Dublin, Ireland | Anti-TNF-naive PsA patients: median PsA symptom duration was 9.9 months and mean age at presentation was 41.2 years. Baseline HAQ-DI score was 0.71 units. 12% of patients were on DMARDS and 11% on corticosteroids | The proportion of patients on DMARDs increased to 59% at the 1-year assessment, and was 56% at 2 years. Mean HAQ-DI score decreased from 0.71 to 0.4 units at both 1- and 2-year assessments and measures of joint swelling also decreased. DMARD-free remission at 1 and 2 years was 12% and 11%, respectively. Measures of radiographic progression all increased from baseline to 2 years and mean Sharp erosion score increased from 1.2 units (SD 2.9 units) at baseline to 3 units (SD 5.2 units) at 2 years |
| Morgan et al., 2007100 | Analysis of HAQ-DI score change over 5 years in 79 patients with inflammatory arthritis plus psoriasis in the NOAR data set | Patients with inflammatory polyarthritis plus psoriasis: Male, n = 36; female, n = 43; age (median), 51.2 years; baseline HAQ-DI score, 0.625 units (IQR 0.25–1.375 units); DMARD use, 16.5% | After 5 years, the median cohort HAQ-DI score had increased from 0.625 to 0.75 units. 54% of the patients had used DMARDs over the observational period |
| Rodgers et al., 201133 | Analysis of HAQ-DI score change over 5 years in the NOAR data set using inclusion criteria specific to eligibility for treatment with biologics (uncontrolled and have tried two or more DMARDs) | Included in the analysis were patients with inflammatory polyarthritis plus psoriasis, three or more tender joints and three or more swollen joints, and previous use of two or more DMARDs | Patients meeting the eligibility criteria at baseline (n = 27) had a HAQ-DI score of 1.55 units and for the first 2 years this changed by –0.060 units per year. Between years 3 and 5, the HAQ-DI score changed by +0.077 units per year in those meeting the eligibility criteria (n = 52) |
The studies explore changes in functional disability in terms of HAQ-DI score and bone erosion as measures of disease activity and progression over time. There is a great deal of variability between the three cohorts under observation in terms of both baseline characteristics and patterns of disease. It should be noted that disease classification of the NOAR cohort99,100 was performed retrospectively and both studies analysing the 79 patients emphasise that they are unlikely to be representative of PsA patients, preferring instead to refer to them as having polyarthritis plus psoriasis. The Morgan et al. 100 study analysed the change in median cohort HAQ-DI score over 5 years in 79 patients, finding an increase of 0.125 units over the observation period, indicating a small increase of 0.025 units in HAQ-DI score every year. The patients in this analysis may or may not have been treated with DMARDs over this period. The analysis in Rodgers et al. 33 includes only those patients who had previously received two or more DMARDs at each time point, finding an annual HAQ-DI score change of –0.060 units per year over the first 2 years (n = 24), and an annual increase of 0.077 units over years 3 to 5 (n = 52). This represents a faster progression of disease than that found in the Morgan et al. 100 study, but is inconsistent and derived from a small cohort of varying size.
A prospective cohort study of progression in early arthritis carried out by Kane et al. 99 found that HAQ-DI score changed from 0.71 units at baseline to 0.4 units at 1 year and remained as such until the end of the 2-year observation period, representing a decrease of 0.31 units. This decrease is likely to be explained by the increase in uptake of DMARDs, as 12% of patients were receiving DMARD treatment at baseline, compared with 59% at 1 year and 56% at 2 years. This was the only study that recorded radiographic progression, finding consistent increases across all measures between baseline and 2 years, despite the simultaneous drop in HAQ-DI score. The Sharp erosion score increased from 1.2 units at baseline to 3 units at 2 years, demonstrating how HAQ-DI score change may not reflect progressive radiographic damage, particularly during early disease.
The study by Husted et al. 98 was the longest and largest study of natural history of PsA, with 341 patients included and observed for up to 10 years. This study found that the patient population exhibited several patterns of disease progression, rather than just universal consistent deterioration over time. Patients were assigned to one of three disability states based on their HAQ-DI score. These were as follows: ‘no disability’ (a HAQ-DI score of < 0.5 units), ‘moderate disability’ (a HAQ-DI score of 0.5–1.5 units) and ‘severe disability’ (a HAQ-DI score of 1.51–3.0 units). The transition of patients between groups was recorded over the course of the observation period to ascertain the direction of change in their symptoms. Forty-six per cent remained in the same disability group over the course of the study, with 28% of these in the no disability state, 12% in the moderate state and 6% in the severely disabled state. A total of 26.7% of patients made a single transition between disability groups, reflecting steady improvement or deterioration, and 27.3% experienced two or more transitions between disability states. Although this methodology may reveal broad patterns of disease progression, it appears to be insensitive to change within groups and weights HAQ-DI score change near thresholds more highly (e.g. a patient with a baseline HAQ-DI score at the lower end of a Markov group can experience a significant worsening of their disability without progressing into the next group). Mean HAQ-DI score change between consecutive assessments was 0.55 units (± 0.32 units) for those moving from a lower to a higher state and –0.57 units (± 0.36 units) for those moving to a lower state, with assessments being on average 1.29 years apart. In those patients who did not move between groups, the mean HAQ-DI score change was –0.002 units (± 0.215 units). A more complete picture of patterns of disease progression would have been possible had there been more Markov states. The mean HAQ-DI change for the majority of patients at any one time was effectively zero, but this may conceal significant within-group changes in either direction. Greater age was associated with a slower improvement in HAQ-DI score in those in the moderate and severe disability groups, and disability worsened more slowly in males than in females. Time since PsA diagnosis was related to more frequent transition between disability states, and there was no association between PASI score and transition between disability states. In summary, this study indicates that functional disability (as measured via the HAQ-DI) in PsA is generally stable over time in the majority of patients, but there are groups who exhibit patterns of more rapidly worsening or improving symptoms at certain periods, with some experiencing fluctuating deterioration and improvement over time.
Owing to the paucity of observational data on natural history of PsA, it is difficult to produce accurate estimates of yearly disease progression rates without anti-TNF therapy. None of the included studies can claim to provide accurate long-term estimates on uncontrolled disease progression. It is clear from the largest cohort that functional disability deteriorates over time, but the course of HAQ-DI progression is not constant or predictable. Therefore, it is unclear if an average rate of HAQ-DI change is a useful statistic, as this change is neither constant nor generalisable to the patient population. The Kane et al. study99 does show that, despite reductions in functional disability in early-stage disease under DMARD therapy, radiographic progression continues to occur, which theoretically will ultimately result in worsening disability in the long term; however, because of the lack of large and long-term observational studies, HAQ-DI change over time in uncontrolled PsA is yet to be properly measured.
Review of adverse effects of certolizumab pegol, secukinumab and comparators
Randomised trials of certolizumab pegol or secukinumab for psoriatic arthritis
Secukinumab: FUTURE 2
During the 16-week placebo-controlled period, AEs were reported in 54% and 58% of patients in the pooled SEC and placebo groups, respectively. The most frequently reported AEs up to 16 weeks in any SEC group (vs. placebo) were upper respiratory tract infection [(confidential information has been removed) vs. 7%], nasopharyngitis [(confidential information has been removed) vs. 8%], headache [(confidential information has been removed) vs. 4%], nausea [(confidential information has been removed) vs. 4%], diarrhoea [(confidential information has been removed) vs. 3%] and urinary tract infection [(confidential information has been removed) vs. 4%]. Rates of infections and infestations were similar across treatment groups (27% on any SEC dose vs. 31% placebo), and no cases of active TB were reported.
The majority of AEs that occurred up to week 16 were mild [(confidential information has been removed) of AEs on any SEC dose and (confidential information has been removed) on placebo] or moderate [(confidential information has been removed) AEs on any SEC dose and (confidential information has been removed) on placebo] in severity. Severe AEs were reported in five patients (1.7% of pooled SEC population), compared with none in patients on placebo. Around 3% of patients in the SEC groups reported non-fatal serious adverse events (SAEs), compared with 2% on placebo. More patients in the placebo group than in the pooled SEC group discontinued study treatment as a result of an AE (confidential information has been removed).
Certolizumab pegol: RAPID-PsA
During the 24-week period, the incidence of drug-related AEs was 26% in the pooled CZP group and 27% in the placebo group and they were mostly of mild intensity (51% pooled CZP vs. 54% placebo) or moderate intensity (30% pooled CZP vs. 36% placebo). The incidence of serious AEs was 6.6% in the pooled CZP group and 4.4% in the placebo group. The incidence of SAEs was 5.8% in the CZP 200 mg group and 9.6% in the CZP 400 mg group.
(Confidential information has been removed.) The most common serious AEs were infections (confidential information has been removed).
Open-label extensions of randomised controlled trials of certolizumab pegol and secukinumab
Secukinumab: FUTURE 2
By the 52-week time point, the most common AEs experienced in patients receiving 300 mg were infection and infestations (79 cases per 100 patient-years), upper respiratory tract infection (18 per 100 patient-years) and nasopharyngitis (14 per 100 patient-years). The rate of discontinuation as a result of AEs in patients who received at least one dose of 150 mg of SEC was 2%. No deaths were reported.
Secukinumab: FUTURE 1
At week 104, 79% of patients remained in the open-label extension study. Infections and infestations were the most common AEs reported, occurring at a rate of 68 per 100 patient-years. Malignant or unspecified tumours occurred at a rate of 0.3 per 100 patient-years, and major adverse cardiac event rates occurred at a rate of 0.7 per 100 patient-years. No cases of active TB or suicide were reported. At week 52 the rate of discontinuation as a result of AEs was 3.9%.
Pooled safety analysis of plaque psoriasis and psoriatic arthritis trials
A conference abstract reported a pooled safety analysis for seven Phase III SEC trials: five plaque psoriasis trials {ERASURE, FIXTURE, SCULPTURE [Efficacy and Safety of Subcutaneous Secukinumab (AIN457) for Moderate to Severe Chronic Plaque-type Psoriasis Assessing Different Doses and Dose Regimens], FEATURE (First Study of Secukinumab in Pre-filled Syringes in Subjects With Chronic Plaque-type Psoriasis: Response at 12 Weeks) and JUNCTURE (Judging the Efficacy of Secukinumab in Patients With Psoriasis Using AutoiNjector: a Clinical Trial Evaluating Treatment Results)} and two PsA trials (FUTURE 1 and FUTURE 2). 101 All trials except FUTURE 2 contributed data up to (at least) 52 weeks; FUTURE had data up to 24 weeks. A total of 3928 patients received at least one dose of SEC (3225 patient-years of exposure; mean exposure 299.8 days for SEC and 105.7 days for placebo). Exposure-adjusted incidence rates per 100 patient-years for SEC and placebo were, respectively, 241 and 329 for AEs, 8 and 10 days for SAEs, and 93 and 94 for infections/infestations. Around 3% of patients treated with SEC discontinued treatment as a result of an AE. Nasopharyngitis and upper respiratory tract infections were the most commonly reported events.
Four deaths occurred in patients treated with SEC (one intracranial haemorrhage, one cardiorespiratory arrest, one alcohol intoxication and one of unknown cause); all the deaths were deemed unrelated to the SEC according to the investigators. There were two (0.05%) cases of suicidality with SEC: one attempted suicide and one case of suicidal ideation.
Certolizumab pegol: RAPID-PsA
In the open-label extension study, 393 patients had been exposed to CZP by week 96 (total exposure 606 patient-years). At week 96, the incidence of overall treatment-emergent AEs was 87.8% (345/393 patients; 330 per 100 patient-years). The rate of SAEs was 17% (67 patients; 14.5 per 100 patient-years). Around 4% of patients reported a serious infection (16 cases; 3.3 per 100 patient-years) and 14.2% of patients reported an upper respiratory tract infection (56 patients; 13.7 per 100 patient-years), with no cases of active TB. Malignancies were reported in 1% of patients (four patients; 0.7 per 100 patient-years).
By 96 weeks, 9.2% of patients had experienced an AE leading to withdrawal and six patients (1.5%) had experienced an AE leading to death (two cardiac disorders, one sudden death, one case of breast cancer, one case of sepsis and one lymphoma). According to the investigator, neither cardiac events was considered to be related to the study medication.
Reviews of safety outcomes for other biologics
Six relevant reviews of AEs were identified from the searches. The key results for three of these reviews33,102,103 have been summarised in a recently published HTA journal publication of a MTA of anti-TNFs for ankylosing spondylitis and non-radiographic axial spondyloarthritis. 104
The Cochrane systematic review and NMA of AEs of nine biologics in adults with any disease (except human immunodeficiency virus infection/acquired immunodeficiency syndrome) used data from 160 RCTs (n = 48,676) and 46 open-label extension studies (n = 11,954). 102 The most frequently studied disease in the included trials was RA. When compared with control treatments, only INF and CZP were statistically significantly associated with AEs. INF was associated with higher rates of total AEs [number needed to harm 13, 95% credible interval (CrI) 8 to 505] and withdrawals because of AEs (number needed to harm 10, 95% CrI 5 to 30). CZP was associated with higher rates of serious infections (number needed to harm 12, 95% CrI 4 to 79) and SAEs (number needed to harm 18, 95% CrI 9 to 162). An individual patient data meta-analysis (n = 22,904 from 74 RCTs) examining short-term cancer risk associated with ETN, INF and ADA found no increase in risk of cancers excluding non-melanoma skin cancer (RR 0.99, 95% CI 0.61 to 1.68) when considering all three anti-TNFs together. 103 However, a doubling in the risk of non-melanoma skin cancer was found, with 332 events per 100,000 person-years in the control group and 655 events per 100,000 person-years in the anti-TNF group [hazard ratio (HR) 2.02, 95% CI 1.11 to 3.95]. NICE TA19933 included a review of studies (including both randomised and non-randomised studies) of the adverse effects of ETN, INF and ADA. The rates of SAEs covered a broadly similar range across the three anti-TNFs. However, all estimates were derived from a highly heterogeneous group of studies in terms of patients, study design and treatment regimens so reliable estimates of the relative rate of SAEs for each anti-TNF could not be made. 33
Of the three more recent reviews identified,105–107 two were reported only as conference abstracts. 105,106 A Danish guideline panel performed a NMA of SAEs from 87 RCTs (n = 27,333) of biologics for inflammatory arthritis (RA, PsA and spondyloarthritis). 105 The conference abstract reported the odds of a SAE to be statistically significantly higher for CZP than for placebo (OR 1.6, 95% CI 1.19 to 2.16). Treatment with CZP was also statistically significantly more likely to result in SAEs than treatment with GOL (OR 2.02, 95% CI 1.26 to 3.25), ETN (OR 1.70, 95% CI 1.15 to 2.51) or ADA (OR 1.44, 95% CI 1.02 to 2.02). The other conference abstract reported a 2014 systematic review and meta-analysis on the safety profile of CZP in patients with an immune-mediated inflammatory disease. 106 The review identified 18 RCTs with 6992 participants; the results, presented in Table 37, also highlight the increased risk of SAEs associated with CZP (compared with ‘control’), particularly the risk of infectious SAEs.
| Type of event | Risk ratio vs. control (95% CI) |
|---|---|
| Overall AEs | 1.07 (1.03 to 1.10) |
| Overall SAEs | 1.58 (1.31 to 1.92) |
| Overall ADRs | 1.20 (1.05 to 1.38) |
| Infectious SAEs | 2.14 (1.34 to 3.43) |
| Injection site reactions | 2.01 (0.95 to 4.29) |
| Neoplasms | 1.18 (0.59 to 2.39) |
| TB | 2.90 (0.73 to 11.43) |
| Withdrawals as a result of AEs | 1.19 (0.96 to 1.47) |
| Fatal AEs | 2.08 (0.83 to 5.17) |
| Infectious AEs | 1.21 (1.09 to 1.34) |
| Upper respiratory tract infections | 1.33 (1.15 to 1.53) |
A review published in 2012 examined the safety of anti-TNFs for treating psoriasis and PsA and focused mainly on data from European patient registries of biologics used across a range of diseases (mostly RA). 107 It was (at least) partly funded by Pfizer, the manufacturer of ETN, and it did not appear to be systematic in its methods of selection, critical appraisal and synthesis of the included studies. It concluded that the safety profile of monoclonal antibodies (INF and ADA) seems generally less favourable than that of ETN, particularly in terms of infections, cancer and hepatotoxicity. The conclusion for infections appeared largely to be based on a BSRBR analysis, specifically on lower respiratory tract infections, even though a previous BSRBR study reported no difference in the risk of infection between ADA, ETN and INF. 108 The conclusion for cancer appeared to be based on an analysis of a small number (38) of lymphomas in a case–control study derived from the French Registry of Infections and Lymphoma in Patients Treated With TNF-α Antagonists (the data were collected between 2004 and 2006). 109 The conclusion for hepatotoxicity was based on a very small number of case reports.
Recent large observational studies
One recent observational study on the safety of biologics in patients with PsA was identified. It was an Israeli retrospective cohort study based on a health services database, which reported on 3128 patients between 2002 and 2013. The study examined the association between traditional DMARDs or anti-TNFs and infection by the herpes zoster virus (shingles). There were 182 cases of herpes zoster infection in 20,096 person-years. The risk of herpes zoster infection significantly increased in patients treated with a combination of an anti-TNF and a traditional DMARD, but did not increase significantly with each of these types of therapy alone. 110
Summary
Safety assessments of new treatments can sometimes be limited in systematic reviews of RCTs because of the small number of trials and relatively short follow-up durations for which data are available. Where available, safety data from trials relating to the same treatment for other indications are therefore sometimes evaluated. For this review, more data from trials of other patient populations were available for CZP than for SEC. The results from three systematic reviews105–107 (which looked specifically at AEs) suggested that CZP was associated with statistically significantly more SAEs and serious infections than placebo. SEC was not included in these systematic reviews of AEs, probably as a result of the limited availability of data at the time. Although the safety data for SEC appear promising, the fairly small number of trials for which data are currently available means there is still some uncertainty regarding its safety.
Chapter 4 Evidence synthesis: relative efficacy of treatments
The effectiveness of SEC and CZP has been summarised in Chapter 3. Results for the main outcome measures, ACR, PsARC, PASI, HAQ-DI and HAQ-DI conditional on PsARC, for all the comparator agents (ETN, ADA, INF, GOL, UST and APR), have also been presented. These data indicate that all these agents demonstrate statistically significant clinical efficacy in PsA. In order to determine the relative efficacy of these agents it would be ideal to have the results from good-quality adequately powered RCTs comparing active treatments with one another. However, as the evidence base is made up almost entirely of comparisons with placebo, statistical methods for making indirect comparisons, such as a NMA, should be considered. NMA enables the comparison of multiple treatments using both direct comparisons of interventions within RCTs and indirect comparisons across trials based on a common comparator. 111 As suggested by the term, NMA needs a ‘network of evidence’ to be established between all of the interventions of interest. The drugs being evaluated here all have a common comparator: placebo. It is this common comparator that allows the network between SEC, CZP and all the active comparators to be established and to provide information on the benefits of these agents relative to placebo and each other. The relevant comparators included in the evidence base are presented in Table 38 and the basic network diagram is presented in Figure 7.
| Treatments, description | Treatments, abbreviation | Class of therapy |
|---|---|---|
| 150 mg of SEC | SEC150 | Anti-IL |
| 300 mg of SEC | SEC300 | Anti-IL |
| 400 mg of CZP | CZP | Anti-TNF |
| 45 mg of UST | UST | Anti-IL |
| 50 mg of GOL | GOL | Anti-TNF |
| 40 mg of ADA | ADA | Anti-TNF |
| 5 mg/kg of INF | INF | Anti-TNF |
| 25 mg of ETN | ETN | Anti-TNF |
| 30 mg of APR | APR | APR |
FIGURE 7.
Network of evidence (not outcome or subgroup specific). PLA, placebo.

Four separate outcomes were considered. Three outcomes were included in the NMA to inform the economic model: PsARC response; change of HAQ-DI score conditional on PsARC response; and PASI 50, PASI 75 and PASI 90 responses. In addition, ACR 20, ACR 50 and ACR 70 responses were analysed, as ACR response is the primary outcome in most of the included trials. Trials with data suitable for the NMA are identified in Table 39. Data from the 12-week time point were used, when available, otherwise data relating to the closest time point after 12 weeks were used (normally 14 or 16 weeks). Not all trials provided data for all of the outcomes analysed.
| Trial | Publication year | Active treatment | Outcome | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PsARC | HAQ-DI score conditional on PsARC | PASI 50, PASI 75 and PASI 90 | ACR 20, ACR 50 and ACR 70 | |||||||||||
| Naive | Experienced | Time point (weeks) | Naive | Experienced | Time point (weeks) | Naive | Experienced | Time point (weeks) | Naive | Experienced | Time point (weeks) | |||
| FUTURE 248 | 2015 | SEC | Yes | Yes | 12 | Yes | Yes | 12 | Yes | Yes | 12 | Yes | Yes | 12 |
| RAPID-PsA47 | 2014 | CZP | Yes | Yesa | 12 | Yes | Yesa | 12 | Yes | Yesa | 12 | Yes | Yesa | 12 |
| PSUMMIT 158,66 | 2013 | UST | Yes | 24 | Yesb | 24 | Yes | 12 | Yes | 12 | ||||
| PSUMMIT 259,66 | 2014 | UST | Yes | Yes | 24 | Yes | 24 | Yes | Yes | 12 | Yes | 12 | ||
| GO-REVEAL50 | 2009 | GOL | Yes | 14 | Yes | 14 | Yes | 14 | Yes | 14 | ||||
| Genovese et al., 200756 | 2007 | ADA | Yes | 12 | Yes | 12 | Yes | 12 | ||||||
| ADEPT55 | 2005 | ADA | Yes | 12 | Yes | 12 | Yes | 12 | Yes | 12 | ||||
| IMPACT 252 | 2005 | INF | Yes | 14 | Yes | 14 | Yes | 14 | Yes | 14 | ||||
| IMPACT51 | 2005 | INF | Yes | 16 | Yes | 16 | Yes | 16 | Yes | 16 | ||||
| Mease et al., 200454 | 2004 | ETN | Yes | 12 | Yes | 12 | Yes | 12 | ||||||
| Mease et al., 200053 | 2000 | ETN | Yes | 12 | Yes | 12 | Yes | 12 | ||||||
| PALACE 160,61 | 2014 | APR | Yes | 16 | Yes | 16 | Yes | 16 | Yes | 16 | ||||
| PALACE 261,65 | 2014 | APR | Yes | 16 | Yes | 16 | Yes | 16 | Yes | 16 | ||||
| PALACE 361,65 | 2014 | APR | Yes | 16 | Yes | 16 | Yes | 16 | Yes | 16 | ||||
| SPIRIT-P157,67 | 2015 | ADA | Yes | 12 | Yes | 12 | ||||||||
Framework of analyses
The evidence synthesis was undertaken using WinBUGS (version 1.4.3; MRC Biostatistics Unit, Cambridge, UK). WinBUGS is a Bayesian analysis software tool that, through the use of Markov chain Monte Carlo methods, evaluates posterior distributions for the parameters of interest given likelihood functions derived from data and prior probabilities (uninformative priors were used throughout). There were few individual studies on each treatment; therefore, fixed-effect models were used across studies in all analyses. Parameter estimates for all functional parameters were reported from the models. These differ by outcome, and further details are presented in Methods. Treatment effects were expressed in relation to placebo. Owing to the sparse evidence imposing a high level of uncertainty over estimates of functional parameters, point estimates are medians throughout. Some models assumed exchangeability across treatments within a class, that is, different treatments of the same class were assumed to be similar, rather than equal. Within such models we reported the relative effectiveness estimates for each treatment (called shrunken estimates), rather than the class means, allowing us to represent any residual differences across treatments.
The validity of a NMA depends on an assumption of homogeneity/exchangeability between all the trials included in the network [i.e. that there are no essential differences between the methods, populations and interventions being studied, and that any differences are a result of chance (as in a standard meta-analysis)]. The lack of homogeneity/exchangeability between studies involving one of the treatments of interest and studies involving the other treatments of interest may generate inconsistency. Checking for consistency in the current network was not possible because of the lack of trials that directly compare active agents. Our examination of the study details and patient characteristics (see Chapter 3, Characteristics of the randomised controlled trials included in the systematic review of short-term efficacy) identified that the trials of the newer agents (SEC, CZP, UST and APR) included biologic-experienced patients as well as biologic-naive patients. Given that it is evident from large observational data sets (see Chapter 3, Review of anti-tumour necrosis factor patient registry studies) that efficacy response rates in biologic-experienced patients are lower than in biologic-naive patients, it was considered inappropriate to conduct an ‘all-patients’ NMA for any outcome, and that, instead, biologic-naive and biologic-experienced patients should be analysed separately. Therefore, separate analyses (separate networks) for treatment-naive and treatment-experienced patients were constructed for each of the four outcomes: one each for PsARC, HAQ-DI conditional on PsARC, PASI 50, 75 and 90, and ACR 20, 50 and 70 responses. A summary of the trials reporting data on each of these outcomes is presented in Table 39. It should be noted that the NICE scope112 for the present appraisal subdivides biologic-naive patients into those who have not responded to one cDMARD and those who have not responded to two cDMARDs. However, sufficient data were not available for these further levels of subgroup analysis.
As discussed in Chapter 3, Evaluating the secukinumab and certolizumab pegol trial results in comparison with other treatments, another important difference between the included trials is the observed results in the placebo arms, particularly for PsARC (see Table 40), PASI outcomes (see Table 50) and ACR (see Table 56). Our investigations on trial designs and patient characteristics did not identify any clear reasons for such differences, other than that placebo response rates appear to have increased over time. This observation (termed ‘placebo creep’) has been made in several other areas of clinical research and its impact on indirect treatment comparisons has been discussed. 113 In the current review, across all trials, the PsARC placebo response rates are high, but are much higher in more recently conducted trials, and this has implications when interpreting unadjusted effect estimates. This is because the ceilings (maximum values) of RRs are limited by baseline response rates. For example, in the FUTURE 2 trial,48 the placebo response rate for PsARC in the biologic-naive subgroup was (confidential information has been removed), which meant that the maximum possible RR would be (confidential information has been removed); this maximum result is lower than some of the actual RRs for other biologics (see Table 40). Higher placebo rates therefore appear to dilute effect estimates somewhat. This is also demonstrated by the examining the RRs moving up the ACR outcome thresholds from ACR 20 to ACR 70, which generally increase (see Table 29). However, it is not clear exactly how these varying placebo rates will affect treatment effects when calculated using ORs. The evidence synthesis – which was based on ORs – therefore explored a potential relationship between baseline risk and relative effectiveness. The NMA explored scenarios where a metaregression on baseline risk (i.e. placebo response) was implemented for PsARC, PASI and ACR outcomes, which imposes an interaction effect between baseline risk and relative effectiveness. 114 Further details of these analyses are presented below. Given that HAQ-DI scores are modelled conditional on PsARC response, such an interaction effect was deemed to be less relevant, and a metaregression model was not implemented on HAQ-DI.
Psoriatic Arthritis Response Criteria response
Subpopulation: biologic naive
Data
For the biologic-naive population, trial-specific PsARC response data were available from 14 trials47,48,50–56,58–61,65,66 of nine active treatments (150 mg of SEC, 300 mg of SEC, CZP, UST, GOL, ADA, INF, ETN and APR), and all treatments were compared with placebo (Table 40).
| Trial | Treatment arm | PsARC response | OR (95% CI) | RR (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment arms | Placebo | ||||||||
| r a | n b | % | r a | n b | % | ||||
| FUTURE 248 | 300 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 3.19 (1.80 to 5.66) | 1.59 (1.17 to 2.15) |
| FUTURE 248 | 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 3.17 (1.77 to 5.67) | 1.59 1.17 to 2.16) | |||
| RAPID-PsA47 | CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 2.98 (2.00 to 4.44) | 1.61 (1.28 to 2.04) |
| PSUMMIT 158 | UST | 115 | 205 | 56 | 77 | 206 | 37 | 2.14 (1.51 to 3.03) | 1.50 (1.21 to 1.86) |
| PSUMMIT 259,66 | UST | 24 | 43 | 56 | 16 | 42 | 38 | 2.05 (0.96 to 4.40) | 1.47 (0.92 to 2.34) |
| GO-REVEAL50 | GOL | 107 | 146 | 73 | 24 | 113 | 21 | 10.17 (6.13 to 16.88) | 3.45 (2.39 to 4.99) |
| Genovese et al., 200756 | ADA | 26 | 51 | 51 | 14 | 51 | 27 | 2.75 (1.29 to 5.86) | 1.86 (1.10 to 3.13) |
| ADEPT55 | ADA | 94 | 153 | 61 | 42 | 162 | 26 | 4.55 (2.97 to 6.97) | 2.37 (1.77 to 3.16) |
| IMPACT 252 | INF | 77 | 100 | 77 | 27 | 100 | 27 | 9.05 (5.39 to 15.20) | 2.85 (2.03 to 4.01) |
| IMPACT51 | INF | 39 | 52 | 75 | 11 | 52 | 21 | 11.18 (5.17 to 24.19) | 3.55 (2.05 to 6.13) |
| Mease et al., 200454 | ETN | 73 | 101 | 72 | 32 | 104 | 31 | 5.87 (3.57 to 9.65) | 2.35 (1.72 to 3.21) |
| Mease et al., 200053 | ETN | 26 | 30 | 87 | 7 | 30 | 23 | 21.36 (8.05 to 56.68) | 3.71 (1.91 to 7.21) |
| PALACE 160,61 | APR | 78 | 168 | 46 | 50 | 168 | 30 | 2.05 (1.35 to 3.10) | 1.56 (1.17 to 2.07) |
| PALACE 261,65 | APR | 78 | 162 | 48 | 53 | 159 | 33 | 1.86 (1.23 to 2.80) | 1.44 (1.10 to 1.90) |
| PALACE 361,65 | APR | 88 | 167 | 53 | 46 | 169 | 27 | 2.98 (1.97 to 4.51) | 1.94 (1.46 to 2.58) |
The nine active treatments were categorised into three classes (anti-TNF, anti-IL and APR). Outcome data for GOL, INF and APR at 14–16 weeks, and for UST at 24 weeks, were included in the analysis and assumed equivalent to outcomes at 12 weeks. The inclusion of the 24-week PsARC data for UST was based on an assumption that they fairly reflected the 12-week results (subgroup results for PsARC at 12 weeks in the PSUMMIT 2 trial59,66 were not available, although 12-week data for the full population were available); this issue is discussed further in Appendix 3, Data used for the ustekinumab (PSUMMIT) trials. The trial-specific data included in the PsARC response analysis are presented in Table 40.
Methods
The NMA implemented separate models for the pooling of treatment effects and of placebo responses. We first implemented a model with independent treatment effects across treatments. Then a number of alternative models were implemented to explore the possibility of placebo response, and, within this, whether or not there was similarity between treatment effects for treatments of the same class.
Exploring placebo response as a treatment effect modifier
An examination of individual trial results suggests that studies presenting higher placebo rates report lower relative effectiveness estimates (see Appendix 3, Detailed methods for the biologic-naive subpopulation). In addition, recent trials, which evaluate newer treatments, also tend to show higher placebo response rates. For example, a recent study on 300 mg of SEC showed a placebo response rate of 46% (the FUTURE 2 trial48), which is much higher than that reported in an earlier study evaluating ETN, of 23% (Mease et al. 53). Our investigations regarding trial designs and patient characteristics did not identify a clear reason for such differences, although placebo response rates appear to have increased over time. We investigated the effect of placebo response as a potential treatment effect modifier. It should be noted that the source of any relationship between placebo response and treatment effect is unclear, and the reader should interpret the results carefully and with caution.
To account for the differences in placebo response rates across the trials, a metaregression was undertaken. The baseline risk estimated for each trial within the synthesis model was used as the adjustment covariate. This allows for uncertainty in the estimation of baseline risk to be considered in the adjustment alongside any correlation with the log-ORs. Note that the baseline risk is expressed as the log-odds of PsARC response in the placebo arm. As typical of metaregression, the relationship between the treatment effect and baseline risk is defined by an interaction term (beta).
Within the independent treatment-effects analysis, beta is estimated by comparing the treatment effects across multiple studies on the same treatment with different placebo response rates. Within the evidence base, not all treatments present with multiple trials. Thus, only a subset of treatments contribute evidence to estimate beta: ADA (ADEPT55 and Genovese et al. 56), ETN (Mease et al. 53,54) and APR (the PALACE 1,60,61 PALACE 261,65 and PALACE 361,65 trials). This limitation in the evidence base meant that the beta had to be assumed to be independent of treatment (i.e. equal for all treatments). Moreover, the evidence base also showed that studies on the same treatment report reasonably similar placebo response rates. This may limit the validity of inferences over beta. For example, the Genovese et al. 56 and ADEPT55 studies report placebo response rates of 27% and 26%, respectively, whereas across the whole set of studies the placebo response range from 21% to (confidential information has been removed).
As inferences on beta are drawn from differences between trials, the smallest difference in placebo rates corresponds to the maximum possible influential difference in reported treatment effects. The two trials on ADA (ADEPT55 and Genovese et al. 56) illustrate this perfectly: the small (1%) difference in placebo response is associated with a 10% difference in response rate in the treatment group (from 51% to 61%). These data are thus influential to estimates of beta. Of the studies that contribute to inferences on beta, two trials have the smallest sample size of the whole set of trials: Mease et al. 53 (ETN) and Genovese et al. 56 (ADA). Given this, a sensitivity analysis excluding both Mease et al. 53 and Genovese et al. 56 was performed and effects on the estimate of beta ascertained (see Appendix 3, Detailed methods for the biologic-naive subpopulation for a more detailed account of the methods). 53,56
Exploring treatment effects as class
In the context of an adjusted model for placebo response, we explored the possibility of there being class effects. Three different class groupings were considered: all treatments as a single class; all biologics as a class with APR separate; and, to reflect the pharmacology, anti-TNFs grouped, ILs grouped and APR separate. Additionally, for the last two groupings, we explored two within-class assumptions: assuming treatments within a class to have equal effectiveness and, alternatively, that treatments within a class have similar (exchangeable) effectiveness. Fixed effects across studies were assumed for all models. We did not consider models assuming exchangeability between classes.
Summary of all treatment effect models explored
All models implemented for the evidence synthesis of PsARC response are presented in Table 41. The models are numbered for ease of reference. Details of the models are presented in Appendix 3, Detailed methods for the biologic-naive subpopulation.
| Sets of analysis | Study | Treatment | Metaregression | Class |
|---|---|---|---|---|
| A1 | FE | Independent | No baseline adjustment | No class effect |
| B1 | FE | Independent | Common interaction term with log-odds of response in placebo arm | No class effect |
| C1 | FE | Equal | class | Common interaction term with log-odds of response in placebo arm | Independent class effect: class = {all treatments} |
| C2 | FE | Equal | class, remaining treatments independenta | Independent class effect: class = APR independent {all remaining biologics} | |
| C3 | FE | Equal | class, remaining treatments independenta | Independent class effect: class = {anti-TNFs, ILs}; APR independent | |
| D1 | FE | Exchangeable | class, remaining treatments independenta | Common interaction term with log-odds of response in placebo arm | Independent class effect: class = APR independent {all other biologics} |
| D2 | FE | Exchangeable | class, remaining treatments independenta | Independent class effect: class = {anti-TNFs, ILs}; APR independent |
Model A1 considers the effectiveness of treatments as independent of each other. Model B1 considers the relative effectiveness of the alternative treatments as independent of each other, but that they all depend on the response in the placebo arm. Models C1, C2 and C3 consider the treatments as equal in terms of their effectiveness within class, but dependent on the effect of the placebo arm. Models D1 and D2 assume the treatments to have a similar, but not equal, effectiveness that is dependent on the effect of the placebo arm; this model introduces more flexibility than assuming treatment effects to be equal (models C2 and C3), but does not fully assume treatments to differ as in model A1. It allows for differences between the effectiveness of treatments that we may not be able to explain but that we should consider.
As stated earlier, sensitivity analysis around the adjustment for placebo response were performed: sets of analyses (models A1, B1, C1, C2, C3, D1 and D2) were conducted for PsARC response, excluding the Mease et al. 53 and Genovese et al. 56 trials.
Network meta-analysis results
Treatment effect models
Table 42 presents results of the treatment effects of PsARC response on the log-odds scale. Results are presented for all the alternative models with measures of goodness of fit. There were no issues with convergence. More detailed results of the models (A1, B1, C1, C2, C3, D1 and D2) are presented in Appendix 3, Detailed results for the biologic-naive subpopulation (ORs as well as log-odds, together with means, medians and 95% CIs are presented).
| Placebo-adjusted metaregression | No | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||
| Treatment | Ind | Ind | = | class | = | class | = | class | ∼ | classa | ∼ | classa | ||||||||
| Class | No | No | {All} | {APR, other} | {IL, TNF, APR} | {APR, other} | {ILs ,TNFs, APR} | ||||||||
| Log-odds placebo | A1 | r | B1 | r | C1 | r | C2 | r | C3 | r | D1 | r | D2 | r | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 300 mg of SEC | –0.16 | 1.178 | 5 | 2.110 | 1 | 1.844 | 3 | 1.833 | 3 | ||||||
| 150 mg of SEC | –0.16 | 1.175 | 6 | 2.104 | 2 | 1.285 | 2 | 1.839 | 4 | 1.822 | 4 | ||||
| UST | –0.51 | 0.758 | 9 | 1.187 | 7 | 1.197 | 8 | 1.174 | 8 | ||||||
| CZP | –0.28 | 1.094 | 7 | 1.837 | 5 | 1.278 | 1 | 1.565 | 1 | 1.722 | 5 | 1.716 | 5 | ||
| GOL | –1.32 | 2.339 | 1 | 1.619 | 6 | 1.692 | 6 | 1.712 | 6 | ||||||
| ADA | –1.02 | 1.401 | 4 | 1.081 | 8 | 1.648 | 1 | 1.201 | 7 | 1.201 | 7 | ||||
| INF | –1.15 | 2.296 | 2 | 1.870 | 4 | 1.853 | 2 | 1.875 | 1 | ||||||
| ETN | –0.99 | 2.043 | 3 | 1.917 | 3 | 1.856 | 1 | 1.872 | 2 | ||||||
| APR | –0.85 | 0.813 | 8 | 0.765 | 9 | 0.756 | 2 | 0.779 | 3 | 0.769 | 9 | 0.771 | 9 | ||
| Beta (mean) | – | –1.471 | –0.498 | –1.692 | –1.061 | –1.264 | –1.225 | ||||||||
| Residual devianceb | 29.9 | 27.2 | 59.24 | 46.8 | 47.5 | 27.8 | 27.9 | ||||||||
| DIC | 193.1 | 190.5 | N/Ac | 203.8 | 199.1 | 190.0 | 190.3 | ||||||||
The unadjusted model A1 indicates an appropriate model fit (with residual deviance close to the number of data points informing the model). The placebo response-adjusted model B1 fits well compared with the unadjusted model A1 [it presents a smaller deviance information criterion (DIC) and residual deviance, but not significantly so, as the difference in DIC is < 5 points]. 115 Model B1 imposes an association between the log-odds of placebo response and treatment effect. The estimated beta implies that a trial with a higher odds of a placebo response is expected to report smaller treatment effects. Consider 300 mg of SEC in unadjusted model A1: the treatment effect is evaluated at 1.178, but the studies on this treatment have a higher log-odds of placebo response than those on other anti-TNFs. The treatment effects reported in the adjusted model assume all treatments were trialled with the same baseline risk. Thus, after adjustment with the placebo response in model B1, the treatment effect estimate for 300 mg of SEC is higher (2.110). This is why the results (and rankings) generated by model B1 are very different from the observed trial results and results generated by the model A1.
Although the assumptions imposed by the placebo-adjusted model may be difficult to justify, or counteract, the limitations in the evidence base that underlie inferences also limit interpretation. First, the distinction between treatment effects and placebo effect is unclear. This is because newer treatments tested under higher placebo response rates show lower treatment effects, whereas older treatments tested under lower placebo response rates show higher treatment effects. There is also limited evidence on the effects of different placebo response rates for the newer treatments (SEC and CZP), as these drugs were studied in a single trial each.
We have further explored treatment effects as class. Model C1, which assumes that all treatments are equal, does not fit well with the existing data as it shows a much increased residual deviance. Models C2 and C3, which assume treatment equal within their class (model C2 separates APR from other drugs and model C3 separates ILs, anti-TNFs and APR), also do not fit well with the existing data, resulting in higher residual deviance and DIC. Models D1 and D2, however, relax the assumption of equality and apply a class effect where treatments within a class are assumed to be similar, not equal. These models fit equally well when compared with model B1 (similar DIC and residual deviance).
In all models exploring treatment effects as a class, the interaction term (beta) is negative. Among the best-fitting models (B1, D1 and D2), the more negative interaction term is observed in model B1. The interaction terms are similar between models D1 and D2.
In sensitivity analyses, we explored the effect of excluding the studies of Genovese et al. 56 and Mease et al. 53 on the placebo interactions (see Appendix 3, Detailed results for the biologic-naive subpopulation for details). The results showed that the beta is still negative, although of lower absolute value. 53,56
Preferred models
The unadjusted model A1 fits the data as well as any of the other models and generates results that reflect the observed results of individual trials. Alternatively, we considered a model adjusted for placebo response. Despite no clear rationale for why placebo response rate should affect the treatment effect, when allowing for such an association (model B1), lower treatment effects are expected with higher placebo response rates. The results (and rankings) attained with model B1 are very different to those evaluated in model A1, and depend on the credibility of the association assumed. Regarding possible class effects, the analyses found that an assumption of equal class effect for the treatments does not produce a better-fitting model (models C1, C2 and C3) than assuming independent treatment effects (models A1 and B1) or similar treatment effects (models D1 and D2). There was little difference in goodness-of-fit statistics (DIC and residual deviance) between models D1 and D2, and we consider the exchangeable class effect model (D2), which utilised two classes (anti-IL and anti-TNF) with APR separate, to be the most clinically plausible. 53,56 Hence, we consider models A1 and D253,56 to be our preferred models for the economic model in Chapter 6. Given the limited effect in sensitivity analysis, the Genovese et al. 56 and Mease et al. 53 studies were included in the preferred models.
A comparison of these analyses with those presented in the company submissions (CSs; Novartis and UCB Pharma) and those in the previous MTA (Rodgers et al. 33) is presented in Appendix 3, Comparison of the network meta-analysis of Psoriatic Arthritis Response Criteria responses in the company submissions (Novartis and UCB Pharma), a previous multiple technology appraisal (Rodgers et al. ) and the current Assessment Group.
Table 43 presents the probability and ORs for PSARC response from these preferred models.
| Treatment | Not adjusted for placebo response, independent treatment (model A1) | Adjusted for placebo response, class effects assumeda (model D2) | ||
|---|---|---|---|---|
| Probability, median (95% CrI) | OR, median (95% CrI) | Probability, median (95% CrI) | OR, median (95% CrI) | |
| Placebo | 0.31 (0.26 to 0.36) | 0.31 (0.26 to 0.36) | ||
| 300 mg of SEC | 0.59 (0.40 to 0.76) | 3.25 (1.56 to 6.89) | 0.73 (0.57 to 0.86) | 6.25 (3.15 to 13.31) |
| 150 mg of SEC | 0.59 (0.40 to 0.76) | 3.24 (1.54 to 6.96) | 0.73 (0.57 to 0.86) | 6.18 (3.10 to 13.30) |
| UST | 0.49 (0.38 to 0.60) | 2.13 (1.49 to 3.07) | 0.59 (0.48 to 0.70) | 3.24 (2.25 to 4.86) |
| CZP | 0.57 (0.44 to 0.69) | 2.99 (1.88 to 4.81) | 0.71 (0.60 to 0.81) | 5.56 (3.59 to 9.11) |
| GOL | 0.82 (0.71 to 0.90) | 10.37 (5.87 to 18.98) | 0.71 (0.58 to 0.81) | 5.54 (3.23 to 9.06) |
| ADA | 0.64 (0.53 to 0.75) | 4.06 (2.70 to 6.21) | 0.60 (0.49 to 0.69) | 3.33 (2.30 to 4.70) |
| INF | 0.81 (0.71 to 0.89) | 9.93 (5.91 to 17.06) | 0.74 (0.63 to 0.83) | 6.52 (4.18 to 10.04) |
| ETN | 0.77 (0.65 to 0.86) | 7.71 (4.53 to 13.58) | 0.74 (0.64 to 0.82) | 6.50 (4.38 to 9.85) |
| APR | 0.50 (0.41 to 0.59) | 2.26 (1.73 to 2.94) | 0.49 (0.41 to 0.57) | 2.16 (1.76 to 2.64) |
The NMA that does not adjust for the placebo response finds that SEC is more effective than CZP, and both are more effective than UST and APR, but both are somewhat less effective than all comparator anti-TNFs. After adjusting for the unexplained increase in placebo rates seen in more recent trials (and, hence, of newer agents), and under a class effect that allows for exchangeability for treatments within each class, the probability of a response with SEC remains slightly higher than with CZP and both remain more effective than UST and APR, but now their probability of response is similar to, or only slightly less than, that of the anti-TNF comparators.
These results indicate that, although SEC and CZP are effective in terms of the PsARC outcome, the relative effectiveness of these biologics compared with ETN, ADA, GOL, UST and INF and with each other, is uncertain. Both agents do seem to be more effective than APR.
Subpopulation: biologic experienced
For the biologic-experienced population, trial-specific PsARC response data were available from three trials for three active treatments (300 mg of SEC, CZP and UST), all compared with placebo. 47,48,59,66 However, the data from the CZP trial were not included in the analysis, as the RAPID-PsA trial excluded patients with primary failures of a prior anti-TNF (i.e. no response within the first 12 weeks of treatment) from being recruited in its biologic-experienced population and so is not comparable to the other two trials. The data included in the NMA for treatment-experienced patients are presented in Table 44.
| Trial | Treatment arm | PsARC response | OR (95% CI) | RR (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment arms | Placebo | ||||||||
| r a | n b | % | r a | n b | % | ||||
| FUTURE 248 | 300 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 5.75 (2.38 to 13.89) | 2.44 (1.38 to 4.31) |
| PSUMMIT 259,66 | UST | 33 | 60 | 55 | 16 | 62 | 26 | 3.51 (1.75 to 7.04) | 2.13 (1.32 to 3.44) |
The NMA conducted for the synthesis of data in the biologic-experienced population is equal to that implemented in the treatment-naive population: treatment effects are assumed to be independent and the model assumed fixed effects across trials. The evidence for the biologic-experienced subpopulation was sparse. The results of the analysis are presented in the Table 45. The result shows that the probability of a PsARC response is higher with SEC than with UST, but the CrIs overlap and the difference is likely to be insignificant. The results are comparable to the observed data (compare Tables 44 and 45) and consistent with those of the biologic-naive subpopulation (compare Tables 43 and 45).
| Treatment | Probability of PsARC response, median (95% CrI) | OR, median (95% CrI) | Treatment effects (log-odds), median (95% CrI) |
|---|---|---|---|
| Placebo | 0.266 (0.19 to 0.36) | –1.013 (–1.48 to –0.58) | |
| 300 mg of SEC | 0.686 (0.41 to 0.88) | 6.033 (2.15 to 18.39) | 1.797 (0.77 to 2.91) |
| UST | 0.566 (0.35 to 0.76) | 3.559 (1.68 to 7.76) | 1.279 (0.53 to 2.07) |
| Residual deviancea | 4.07 | ||
| DIC | 24.62 | ||
Health Assessment Questionnaire-Disability Index changes conditional on Psoriatic Arthritis Response Criteria response/non-response
Subpopulation: biologic naive
Data
For the biologic-naive population, HAQ-DI changes conditional on PsARC responses were available for nine active treatments (150 mg of SEC, 300 mg of SEC, CZP, UST, GOL, ADA, INF, ETN and APR) from 13 trials (see Table 39). 47,48,50–52,54–56,58–61,65,66 The data for HAQ-DI change conditional on PsARC response are presented in Table 46.
| Trial | Treatment | HAQ-DI changes conditional on PsARC response | HAQ-DI changes conditional on PsARC non-response | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment arm | Placebo arm | Treatment arm | Placebo arm | ||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| FUTURE 248 | 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| FUTURE 248 | 300 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||||
| RAPID-PsA47 | CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| PSUMMIT 1 + PSUMMIT 258,59,66 | UST | –0.487 | 0.05 | –0.260 | 0.04 | –0.097 | 0.05 | –0.001 | 0.03 |
| GO-REVEAL50 | GOL | –0.424 | 0.07 | –0.286 | 0.05 | –0.049 | 0.06 | 0.023 | 0.02 |
| ADEPT55 | ADA | –0.500 | 0.05 | –0.313 | 0.08 | –0.120 | 0.05 | 0.026 | 0.04 |
| Genovese et al., 200756 | ADA | –0.423 | 0.08 | –0.177 | 0.06 | –0.150 | 0.09 | –0.057 | 0.05 |
| IMPACT 252 | INF | –0.580 | 0.06 | –0.160 | 0.10 | –0.110 | 0.06 | 0.070 | 0.04 |
| IMPACT51 | INF | –0.650 | 0.09 | –0.270 | 0.14 | –0.200 | 0.09 | 0.020 | 0.05 |
| Mease et al., 200454 | ETN | –0.635 | 0.06 | –0.258 | 0.01 | –0.196 | 0.07 | –0.002 | 0.04 |
| PALACE 160,61 | APR | –0.460 | 0.05 | –0.320 | 0.07 | –0.070 | 0.05 | 0.000 | 0.04 |
| PALACE 261,65 | APR | –0.330 | 0.06 | –0.220 | 0.07 | –0.120 | 0.05 | 0.010 | 0.04 |
| PALACE 361,65 | APR | –0.290 | 0.05 | –0.250 | 0.06 | –0.080 | 0.05 | 0.000 | 0.03 |
Outcome data for GOL and INF at 14–16 weeks, and for UST at 24 weeks, were included in the analysis and assumed equivalent to outcomes at 12 weeks. The rationale for the inclusion of the 24-week data for UST is discussed in Appendix 3, Data used for the ustekinumab (PSUMMIT) trials. The observed data indicate that HAQ-DI changes conditional on PsARC response do vary by treatment, ranging between (confidential information has been removed) (300 mg of SEC, FUTURE 2 trial48) and –0.290 (APR, PALACE 3 trial61,65). The observed HAQ-DI changes conditional on PsARC non-response in treatments range between (confidential information has been removed) (150 mg of SEC, FUTURE 2 trial48) and –0.049 (GOL, GO-REVEAL trial50).
For the placebo arms, the observed HAQ-DI changes conditional on PsARC response and non-response differ between trials [ranging between (confidential information has been removed) (FUTURE 2 trial48) and –0.160 (IMPACT 252) for response, and from (confidential information has been removed) (RAPID-PsA trial47) to 0.070 (IMPACT 252) for non-response].
The observed HAQ-DI changes conditional on PsARC response and non-response with treatments are greater than with placebo in all trials.
Methods
We consider three models to estimate the HAQ-DI changes conditional on PsARC responder or non-responder status. A detailed description of the model and underlying assumptions are presented in Appendix 3, Detailed methods for the biologic-naive subpopulation. The model E1 considers that treatments are independent and considers fixed effects across studies. Models E2 and E3 apply a class effect comprising three groups: anti-TNFs, ILs and APR. This class effect reflects the best-fitting class effect model for PsARC (see Network meta-analysis results). The model E2 assumes that the treatments are similar within class (exchangeable) and considers fixed effects across studies; and model E3 considers that the treatments are equal within class and considers fixed effects across studies.
Network meta-analysis results
The results are presented as absolute changes in HAQ-DI score in relation to baseline (Table 47). More detailed results are presented in Appendix 3, Detailed results for the biologic-naive subpopulation.
| Treatment | Independent treatment | Exchangeable | class {ILs, TNFs, APR} | Equal | class {ILs, TNFs, APR} | PsARC response vs. non-response | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FE | FE | FE | ||||||||||
| E1 | E2a | E3 | ||||||||||
| Study | PsARC response | PsARC non-response | PsARC response | PsARC non-response | PsARC response | PsARC non-response | E1 | rb | E2a | rb | E3 | rb |
| Placebo | –0.26 | –0.26 | –0.25 | –0.26 | 10 | –0.26 | 10 | –0.25 | 4 | |||
| 150 mg of SEC | –0.39 | –0.08 | –0.44 | –0.09 | –0.31 | 8 | –0.35 | 8 | ||||
| 300 mg of SEC | –0.55 | –0.05 | –0.51 | –0.08 | –0.47 | –0.08 | –0.49 | 1 | –0.43 | 3 | –0.39 | 1 |
| UST | –0.49 | –0.10 | –0.48 | –0.09 | –0.39 | 4 | –0.39 | 4 | ||||
| CZP | –0.43 | –0.07 | –0.47 | –0.12 | –0.36 | 6 | –0.35 | 7 | ||||
| GOL | –0.44 | –0.06 | –0.49 | –0.11 | –0.52 | –0.13 | –0.38 | 5 | –0.37 | 5 | –0.39 | 1 |
| ADA | –0.49 | –0.13 | –0.50 | –0.13 | –0.36 | 7 | –0.37 | 6 | ||||
| INF | –0.66 | –0.20 | –0.60 | –0.14 | –0.46 | 2 | –0.46 | 1 | ||||
| ETN | –0.64 | –0.20 | –0.59 | –0.14 | –0.44 | 3 | –0.45 | 2 | ||||
| APR | –0.36 | –0.09 | –0.36 | –0.09 | –0.36 | –0.09 | –0.27 | 9 | –0.27 | 9 | –0.27 | 3 |
| DIC | –126.0 | –133.0 | –131.4 | |||||||||
The model fit statistics (DIC) indicate that neither class effect model (E2 or E3) is a better fit for the data than the unadjusted, independent treatments model (E1). The class effect models had similar fits, but the one that allowed exchangeability within classes (E2) was considered to be the most clinically plausible. For the purposes of the economic model, in Chapter 6, models E1 and E2 were the preferred models.
The results from the two preferred models are similar. The results from the unadjusted independent treatment effects model found that significant reductions in mean HAQ-DI score were achieved with response to all nine treatments and response to placebo. However, patients who responded to placebo achieved a lower level of improvement in the HAQ-DI score than those who responded to active treatment. Furthermore, the improvement in response to placebo is below the minimally important difference for PsA of –0.35. 116
The median conditional on response HAQ-DI change was highest with INF and ETN, followed by 300 mg of SEC, but 150 mg of SEC and CZP were worse than all treatments except for APR.
Subpopulation: biologic experienced
For the biologic-experienced population, HAQ-DI changes conditional on PsARC responses were available for three active treatments (300 mg of SEC, CZP and UST) from three trials. 47,48,59,66 However, the data from the CZP trial were not included in the analysis as the biologic-experienced population in the RAPID-PsA trial is not comparable to that in the other two trials48,59,66 (see Psoriatic Arthritis Response Criteria response, Subpopulation: biologic experienced). The data included in the NMA for treatment-experienced patients are presented in Table 48.
| Trial | Treatment | HAQ-DI changes conditional on PsARC response | HAQ-DI changes conditional on PsARC non-response | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment arm | Placebo arm | Treatment arm | Placebo arm | ||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| FUTURE 248 | 300 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| PSUMMIT 259,66 | UST | –0.315 | 0.11 | –0.146 | 0.09 | 0.007 | 0.13 | 0.010 | 0.05 |
Outcome data at 24-week were included in the analysis and assumed equivalent to outcomes at 12 weeks [see Appendix 3, Data used for the ustekinumab (PSUMMIT) trials]. The observed data indicate that, as in the treatment-naive subgroup, HAQ-DI changes conditional on PsARC response do vary by treatments. The observed HAQ-DI changes conditional on PsARC response and non-response in placebo arms differ between trials. The observed HAQ-DI changes conditional on PsARC response and non-response with treatments are greater than placebo in all trials.
The NMA conducted for the synthesis of data in the biologic-experienced population is equal to that implemented in the treatment-naive population: treatment effects are assumed to be independent and the model assumed fixed effects across trials. No class effect assumption was made for this subgroup analysis. The results are presented as absolute changes in HAQ-DI score in relation to baseline (Table 49). These results are generally comparable with the observed estimates from the primary studies.
| Treatment | HAQ-DI changes in PsARC response in relation to PNR | HAQ-DI changes in PsARC non-response in relation to PNR | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| Placebo/baseline effect | –0.134 | –0.134 | –0.288 to 0.021 | |||
| 300 mg of SEC | –0.385 | –0.385 | –0.624 to –0.145 | –0.431 | –0.430 | –0.880 to 0.014 |
| UST | –0.320 | –0.320 | –0.552 to –0.086 | 0.003 | 0.002 | –0.269 to 0.274 |
| DIC | –8.10 | |||||
The results from the independent treatment effects model found that significant reductions in mean HAQ-DI score were achieved with response to SEC and UST, and response to placebo. As for the biologic-naive patients, those who responded to placebo achieved a lower level of improvement in the HAQ-DI score than those who responded to active treatments. Furthermore, the improvement in responders to placebo is below the minimally important difference for PsA of –0.35. 116
Psoriasis Area and Severity Index response
Subpopulation: biologic naive
Data
For the biologic-naive population, PASI response data were available for nine active treatments (150 mg of SEC, 300 mg of SEC, CZP, UST, GOL, ADA, INF, ETN and APR) from 13 trials47–67 (see Table 2). A brief summary of PASI responses in different trials is presented in Table 50. Outcomes at 14 and 16 weeks were included in the analysis and assumed to be equivalent to outcomes at 12 weeks. Data from the 12-week time point were used for the two PSUMMIT trials. Not all patients who were randomised to trials were eligible for the PASI evaluation, and the proportion of PASI-evaluable patients differed between trials, ranging between 42% and 84% in treatment arms and between 31% and 87% in placebo arms. All trials reported PASI 50 and PASI 75, except the PSUMMIT 2 and SPIRIT-P1 (Study of Ixekizumab in Participants With Active Psoriatic Arthritis) trials,57,59,66,67 which did not report PASI 50. A few trials did not report PASI 90 (i.e. the PALACE trials,60,61,65 Mease et al. 53 and PSUMMIT 259,66).
| Trial | Treatment | PASI evaluated: n (%) of patients randomised to treatment | PASI responses in treatment arm, n (%) | PASI evaluated: n (%) of patients randomised to placebo | PASI responses in placebo arm, n (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| PASI 50 | PASI 75 | PASI 90 | PASI 50 | PASI 75 | PASI 90 | ||||
| FUTURE 248 | 300 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| FUTURE 248 | 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | ||||
| RAPID-PsA47 | CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| PSUMMIT 158 | UST | 145 (71) | 89 (61) | 56 (39) | 28 (19) | 146 (71) | 31 (21) | 13 (9) | 6 (4) |
| GO-REVEAL50 | GOL | 109 (75) | 63 (58) | 44 (40) | 22 (20) | 79 (70) | 7 (9) | 2 (3) | 0 (0) |
| ADEPT55 | ADA | 69 (45) | 50 (72) | 34 (49) | 21 (30) | 69 (43) | 10 (14) | 3 (4) | 0 (0) |
| IMPACT 252 | INF | 83 (83) | 68 (82) | 53 (64) | 34 (41) | 87 (87) | 8 (9) | 2 (2) | 0 (0) |
| IMPACT51 | INF | 22 (42) | 22 (100) | 15 (68) | 8 (36) | 16 (31) | 0 (0) | 0 (0) | 0 (0) |
| Mease et al. 200053 | ETN | 19 (63) | 8 (42) | 5 (26) | NA | 19 (63) | 4 (21) | 0 (0) | NA |
| PALACE 160,61 | APR | 82 (49) | 36 (44) | 18 (22) | NA | 68 (40) | 11 (16) | 3 (4) | NA |
| PALACE 261,62 | APR | 77 (48) | 33 (43) | 17 (22) | NA | 74 (47) | 10 (14) | 2 (3) | NA |
| PALACE 361,65 | APR | 90 (54) | 38 (42) | 20 (22) | NA | 89 (53) | 22 (25) | 7 (8) | NA |
| SPIRIT-P157,67 | ADA | 68 (67) | NA | 23 (34) | 15 (22) | 67 (63) | NA | 5 (7) | 1 (1) |
| PSUMMIT 259,66 | UST | 36 (84) | NA | 17 (47) | NA | 30 (71) | NA | 1 (3) | NA |
Methods
The NMA for PASI utilised a framework of analysis that evaluated the probability of PASI responses in different categories of PASI thresholds (50/75/90) within a single model:117 the single model included all categories of PASI and generated a single effect estimate for each treatment and also probabilities of achieving PASI 50, PASI 75 and PASI 90.
Reflecting the analyses on PsARC, alternative assumptions were tested in two analyses. The first analysis assumed independent treatment effects and did not include any metaregression for placebo effects (model F1). As the number of trials to inform each treatment effect was small, a fixed-effect model was used. In a second analysis, we explored the impact on treatment effects of adjusting for placebo responses [i.e. baseline effects (metaregression model)]. As can be seen in Table 50, there were large differences between trials for PASI responses in the placebo arms, ranging between 0% (in IMPACT51) and 27% (in RAPID-PsA47). The IMPACT51 had a very small sample size and reported 0% response in the placebo arm and 100% response in the treatment arm, which lead to very extreme values for placebo adjustment. Therefore, IMPACT51 could not be included in the metaregression analysis. Unlike the analysis for PsARC, for PASI, we did not assume a class effect as the evidence from individual trials does not support such an assumption. Table 51 presents the key assumptions for the models implemented for the PASI response. The detailed model assumptions are presented in Appendix 3, Detailed methods for the biologic-naive subpopulation.
| Sets of analyses | Study | Treatment | Metaregression | Thresholds (i.e. cut-off points) | Baseline effect for metaregression |
|---|---|---|---|---|---|
| F1 | FE | Independent | No baseline adjustment | FE | – |
| G1 | FE | Independent | No baseline adjustment | FE | – |
| G2 | FE | Independent | Common interaction term with baseline effect | FE | Adjusted with trial-specific baseline effects |
Model F1 considers that treatments are independent of each other and assumes fixed effects on cut-off points/thresholds. Model G1 considers the same assumption as model F1, but IMPACT51 was excluded from the analysis. Model G2 assumes that treatments are independent of each other, but treatment effects are adjusted with the trial-specific baseline effects assuming a common interaction term (beta).
Network meta-analysis results
Table 52 presents the results of the treatment effects for the PASI responses estimated from the three models with measures of goodness of fit. There were no issues with convergence.
| Placebo-adjusted metaregression | No | No | Yes | |||
| Treatments | Independent | Independent | Independent | |||
| Cut-off points | FE | FE | FE | |||
| F1 | r a | G1 | r a | G2 | r a | |
|---|---|---|---|---|---|---|
| Placebo | 1.024 | – | 0.983 | – | 1.015 | – |
| 300 mg of SEC | –1.936 | 2 | –1.932 | 2 | –1.864 | 1 |
| 150 mg of SEC | –1.870 | 3 | –1.865 | 3 | –1.798 | 2 |
| CZP | –0.875 | 7 | –0.873 | 7 | –1.424 | 4 |
| UST | –1.134 | 6 | –1.131 | 6 | –1.342 | 6 |
| GOL | –1.645 | 4 | –1.635 | 4 | –1.141 | 7 |
| ADA | –1.477 | 5 | –1.476 | 5 | –1.422 | 5 |
| INF | –2.412 | 1 | –2.276 | 1 | –1.798 | 2 |
| ETN | –0.798 | 8 | –0.797 | 8 | –0.849 | 8 |
| APR | –0.749 | 9 | –0.748 | 9 | –0.815 | 9 |
| Beta | – | – | –1.310 | |||
| Residual deviance | 76.6b | 62.5c | 58.4c | |||
| DIC | 318.9 | 297.2 | 293.7 | |||
The results of models G1 and F1 are similar, except for a small effect on the estimate of effect for INF; therefore, model F1 is the preferred unadjusted model, as it does not exclude any trial evidence. In model G2, the DIC and residual deviance are lower than in model G1, indicating that the model fits well with the existing data and the data support the assumption of adjustment with baseline effects.
Table 53 shows the probability of achieving PASI 50, PASI 75 and PASI 90 from the preferred treatment-unadjusted and -adjusted model in the biologic-naive population.
| Treatment | Independent treatment, median probability of achieving response (95% CrI) | |||||
|---|---|---|---|---|---|---|
| Unadjusted for placebo response (model F1) | Adjusted for placebo response (model G2) | |||||
| PASI 50 | PASI 75 | PASI 90 | PASI 50 | PASI 75 | PASI 90 | |
| Placebo | 0.153 (0.13 to 0.18) | 0.054 (0.04 to 0.07) | 0.015 (0.01 to 0.02) | 0.155 (0.12 to 0.19) | 0.055 (0.04 to 0.07) | 0.016 (0.01 to 0.02) |
| 300 mg of SEC | 0.819 (0.61 to 0.94) | 0.627 (0.38 to 0.84) | 0.405 (0.19 to 0.67) | 0.801 (0.62 to 0.91) | 0.604 (0.40 to 0.78) | 0.384 (0.21 to 0.58) |
| 150 mg of SEC | 0.801 (0.59 to 0.93) | 0.603 (0.36 to 0.82) | 0.380 (0.18 to 0.63) | 0.783 (0.60 to 0.90) | 0.579 (0.38 to 0.75) | 0.359 (0.19 to 0.54) |
| CZP | 0.441 (0.31 to 0.59) | 0.231 (0.14 to 0.36) | 0.097 (0.05 to 0.18) | 0.657 (0.50 to 0.82) | 0.429 (0.29 to 0.63) | 0.231 (0.13 to 0.41) |
| UST | 0.544 (0.44 to 0.65) | 0.317 (0.23 to 0.42) | 0.149 (0.09 to 0.22) | 0.627 (0.52 to 0.74) | 0.398 (0.30 to 0.52) | 0.207 (0.14 to 0.31) |
| GOL | 0.732 (0.58 to 0.86) | 0.514 (0.35 to 0.68) | 0.297 (0.17 to 0.47) | 0.548 (0.36 to 0.70) | 0.322 (0.17 to 0.48) | 0.154 (0.07 to 0.27) |
| ADA | 0.675 (0.55 to 0.78) | 0.448 (0.32 to 0.58) | 0.242 (0.15 to 0.36) | 0.657 (0.54 to 0.76) | 0.429 (0.32 to 0.55) | 0.231 (0.15 to 0.33) |
| INF | 0.918 (0.84 to 0.96) | 0.789 (0.67 to 0.88) | 0.593 (0.44 to 0.73) | 0.782 (0.61 to 0.88) | 0.578 (0.39 to 0.73) | 0.358 (0.20 to 0.52) |
| ETN | 0.411 (0.15 to 0.72) | 0.209 (0.05 to 0.50) | 0.084 (0.01 to 0.29) | 0.434 (0.20 to 0.69) | 0.227 (0.08 to 0.47) | 0.095 (0.02 to 0.26) |
| APR | 0.391 (0.31 to 0.49) | 0.195 (0.14 to 0.27) | 0.077 (0.05 to 0.12) | 0.420 (0.33 to 0.52) | 0.216 (0.16 to 0.30) | 0.090 (0.06 to 0.14) |
The results of the unadjusted NMA for the PASI, as a single outcome or as separate categorical variables, show that all treatments are more effective than placebo. The difference between treatments is uncertain, with wide CrIs that mostly overlap with each other. The results show that patients taking INF have the highest probability of achieving PASI 50, PASI 75 and PASI 90 responses. However, after adjustment for placebo, 300 mg of SEC has the highest probability of response. The probabilities for CZP changed between the models. It appears to be less efficacious than all other treatments, except APR and ETN, in achieving PASI responses in the unadjusted model. However, in the adjusted model, it appears to be more efficacious than GOL, UST, APR and ETN, and similar to ADA. The estimated probabilities from the analysis reflect fairly closely those from the primary studies, indicating that the model fits the data well.
Subpopulation: biologic experienced
For the biologic-experienced population, trial-specific PASI response data were available for three active treatments (300 mg of SEC, CZP and UST) from three trials,47,48,59,66 but, as for the other outcomes, the data from the CZP trial were not included in the analysis as the biologic-experienced population in the RAPID-PsA trial47 is not comparable to the population in the other two trials48,59,66 (see Psoriatic Arthritis Response Criteria response, Subpopulation: biologic experienced). The data included in the NMA for the treatment-experienced patients are presented in Table 54.
| Trial | Treatment | PASI evaluated: n (%) of patients randomised to treatment | PASI responses in treatment arm, n (%) | PASI evaluated: n (%) of patients randomised to placebo | PASI responses in placebo arm, n (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| PASI 50 | PASI 75 | PASI 90 | PASI 50 | PASI 75 | PASI 90 | ||||
| FUTURE 248 | 300 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| PSUMMIT 259,66 | UST | 44 (73) | NA | 14 (32) | NA | 50 (81) | NA | 1 (2) | NA |
In the FUTURE 2 trial,48 only a small proportion of patients were eligible for the PASI evaluations; 33% in the treatment arm and 34% in the placebo arm. The small sample size and associated lack of events in this placebo arm increase uncertainty in the analysis.
A NMA was conducted under the same specification as used in model F1 (independent treatments, unadjusted biologic-naive analysis). Because the data were sparse, no adjustment was undertaken for this subgroup analysis. The results of the analysis are presented in Table 55.
| Treatment/parameter | Treatment effects on a probit scale, median (95% CrI) | Response, median probability of achieving response (95% CrI) | ||
|---|---|---|---|---|
| PASI 50 | PASI 75 | PASI 90 | ||
| Placebo | 1.354 (0.59 to 2.19) | 0.088 (0.01 to 0.28) | 0.012 (0.00 to 0.06) | 0.002 (0.00 to 0.02) |
| 300 mg of SEC | –2.509 (–4.01 to –1.23) | 0.875 (0.46 to 1.00) | 0.598 (0.23 to 0.89) | 0.365 (0.08 to 0.75) |
| UST | –1.659 (–2.73 to –0.83) | 0.628 (0.29 to 0.89) | 0.279 (0.07 to 0.61) | 0.120 (0.01 to 0.42) |
| PASI 50 | – | |||
| PASI 75 | 0.870 (0.28 to 1.84) | |||
| PASI 90 | 1.484 (0.70 to 2.56) | |||
| Residual deviancea | 5.99 | |||
| DIC | 26.75 | |||
The result shows that the probability of achieving a PASI response in all categories is much higher with SEC than with UST, although the estimates are highly uncertain, with wide CrIs that overlap with each other. The results are fairly comparable with observed data.
American College of Rheumatology response
Subpopulation: biologic naive
Data
For the biologic-naive population, evidence on ACR response was available for nine active treatments (150 mg of SEC, 300 mg of SEC, UST, CZP, GOL, ADA, INF, ETN and APR) from 15 trials. 47,48,50–61,65–67 A brief summary of the ACR responses in the different trials is presented in Table 56. Outcomes at 14 and 16 weeks were included in the analysis and assumed to be equivalent to outcomes at 12 weeks. All 15 trials reported all three categories of ACR response (20/50/70).
| Trial | Treatment | ACR responses | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment arm | Placebo arm | ||||||||||||||
| n a | ACR 20 | ACR 50 | ACR 70 | n a | ACR 20 | ACR 50 | AC 70 | ||||||||
| r b | % | r b | % | r b | % | r b | % | r b | % | r b | % | ||||
| FUTURE 248 | 300 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| FUTURE 248 | 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | |||||||
| PSUMMIT 158 | UST | 205 | 85 | 41 | 38 | 19 | 8 | 4 | 206 | 44 | 21 | 11 | 5 | 3 | 1 |
| PSUMMIT 259,66 | UST | 43 | 17 | 40 | 5 | 12 | 3 | 7 | 42 | 8 | 19 | 3 | 7 | 1 | 2 |
| RAPID-PsA47 | CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| GO-REVEAL50 | GOL | 146 | 74 | 51 | 44 | 30 | 18 | 12 | 113 | 10 | 9 | 2 | 2 | 1 | 1 |
| Genovese et al., 200756 | ADA | 51 | 20 | 39 | 13 | 25 | 7 | 14 | 51 | 8 | 16 | 1 | 2 | 0 | 0 |
| ADEPT55 | ADA | 153 | 88 | 58 | 54 | 35 | 30 | 20 | 162 | 23 | 14 | 6 | 4 | 1 | 1 |
| SPIRIT-P157,67 | ADA | 101 | 52 | 51 | 30 | 30 | 18 | 18 | 106 | 33 | 31 | 5 | 5 | 0 | 0 |
| IMPACT 252 | INF | 100 | 58 | 58 | 36 | 36 | 15 | 15 | 100 | 11 | 11 | 3 | 3 | 1 | 1 |
| IMPACT51 | INF | 52 | 34 | 65 | 24 | 46 | 15 | 29 | 52 | 5 | 10 | 0 | 0 | 0 | 0 |
| Mease et al., 200454 | ETN | 101 | 60 | 59 | 38 | 38 | 11 | 11 | 104 | 16 | 15 | 4 | 4 | 0 | 0 |
| Mease et al., 200053 | ETN | 30 | 22 | 73 | 15 | 50 | 4 | 13 | 30 | 4 | 13 | 1 | 3 | 0 | 0 |
| PALACE 160,61 | APR | 168 | 64 | 38 | 27 | 16 | 7 | 4 | 168 | 32 | 19 | 10 | 6 | 2 | 1 |
| PALACE 261,65 | APR | 162 | 52 | 32 | 17 | 10 | 2 | 1 | 159 | 30 | 19 | 8 | 5 | 1 | 1 |
| PALACE 361,65 | APR | 167 | 68 | 41 | 25 | 15 | 6 | 4 | 169 | 31 | 18 | 14 | 8 | 4 | 2 |
Methods
As ACR is, like PASI, a categorical variable (ACR 20, ACR 50 and ACR 70), the NMA for ACR utilised a similar framework of analysis to that used to estimate the probability of PASI responses: all categories of ACR were within a single model which generated a single effect estimate for each treatment and also probabilities of achieving an ACR 20, ACR 50 and ACR 70.
Analogously to the analyses on PsARC, sets of alternative analyses were conducted for ACR response outcomes. We explored the effect of differences in trial-specific placebo responses on treatment effect by undertaking a metaregression. In the context of an adjusted model for placebo response, we explored the possibility of there being class effects. Three different class groupings were considered: all treatments as a single class; all biologics as a class with APR separate; and, to reflect the pharmacology, anti-TNFs grouped, ILs grouped and APR separate. In addition, we explored two within-class assumptions: assuming treatments within a class to have equal effectiveness and, alternatively, assuming that those treatments within a class have similar (exchangeable) effectiveness. Fixed effects across studies were assumed for all models. We have not considered models assuming exchangeability between classes.
Summary of all treatment effect models explored
All models implemented for the evidence synthesis of an ACR response are presented in Table 57. Detailed coding of the models is presented in Appendix 3, Detailed methods for the biologic-naive subpopulation.
| Sets of analysis | Study | Treatment | Metaregression | Class |
|---|---|---|---|---|
| H1 | FE | Independent | No baseline adjustment | No class effect |
| I1 | FE | Independent | Common interaction term with baseline effect | No class effect |
| J1 | FE | Equal | class | Common interaction term with baseline effect | Independent class effect: class = {all treatments} |
| J2 | FE | Equal | class, remaining treatments independenta | Independent class effect: class = APR independent {all remaining biologics} | |
| J3 | FE | Equal | class, remaining treatments independenta | Independent class effect: class = {anti-TNFs, ILs}; APR independent | |
| K1 | FE | Exchangeable | class, remaining treatments independenta | Common interaction term with baseline effect | Independent class effect: class = APR independent {all other biologics} |
| K2 | FE | Exchangeable | class, remaining treatments independenta | Independent class effect: class = {anti-TNFs, ILs}; APR independent |
Model H1 considers that the treatments are independent of each other. Model I1 considers the relative effectiveness of the alternative treatments as independent of each other, but that they all depend on the response in the placebo arm. Model J1 considers the treatments as equal in terms of their effectiveness, but dependent on the effect of the placebo arm. Models J2 and J3 consider the treatments as equal in terms of their effectiveness within class, but dependent on the effect of the placebo arm. Models K1 and K2 assume the treatments to have a similar, but not equal, effectiveness and to be dependent on the effect of the placebo arm.
Network meta-analysis results
Table 58 presents the results of the treatment effects for ACR responses estimated from the seven models with measures of goodness of fit. There were no issues with convergence.
| Placebo-adjusted metaregression | No | Yes | Yes | Yes | Yes | Yes | Yes | |||||||
| Treatments | Ind | Ind | = | class {all} | = | class {APR, other} | = | class {ILs, TNFs, APR} | ∼ | classa (APR, other) | ∼ | classa (ILs, TNFs, APR) | |||||||
| Cut-off points | FE | FE | FE | FE | FE | FE | FE | |||||||
| H1 | r b | I1 | rb | J1 | r b | J2 | r b | J3 | r b | K1 | r b | K2 | r b | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 0.952 | 0.961 | 0.882 | 0.966 | 0.966 | 0.963 | 0.961 | |||||||
| 300 mg of SEC | –0.914 | 6 | –1.397 | 2 | –1.274 | 2 | –1.236 | 3 | ||||||
| 150 mg of SEC | –0.932 | 5 | –1.415 | 1 | –1.094 | 1 | –1.095 | 1 | –1.283 | 1 | –1.246 | 2 | ||
| UST | –0.570 | 8 | –0.722 | 8 | –0.750 | 8 | –0.732 | 8 | ||||||
| CZP | –0.811 | 7 | –1.265 | 3 | –0.830 | 1 | –1.193 | 5 | –1.176 | 5 | ||||
| GOL | –1.429 | 2 | –0.918 | 7 | –1.010 | 7 | –1.040 | 7 | ||||||
| ADA | –1.072 | 4 | –1.126 | 6 | –0.609 | 2 | –1.121 | 6 | –1.124 | 6 | ||||
| INF | –1.617 | 1 | –1.212 | 5 | –1.246 | 3 | –1.269 | 1 | ||||||
| ETN | –1.362 | 3 | –1.214 | 4 | –1.215 | 4 | –1.228 | 4 | ||||||
| APR | –0.509 | 9 | –0.592 | 9 | –0.610 | 2 | –0.014 | 3 | –0.581 | 9 | –0.576 | 9 | ||
| Beta (mean) | –1.276 | 1.327 | –1.627 | –1.621 | –1.099 | –1.018 | ||||||||
| Residual deviancec | 120.0 | 119.1 | 156.1 | 148.3 | 148.3 | 120.0 | 120.4 | |||||||
| DIC | 482.22 | 480.94 | 511.66 | 503.43 | 503.37 | 480.90 | 481.1 | |||||||
The placebo response-adjusted model I1 fits well compared with the unadjusted model H1 (smaller DIC and residual deviance), but is not significantly better. In addition, the results (rankings) generated by model I1 are very different from the observed trial results. Models J1, J2 and J3 do not fit well with the existing data, resulting in a significantly higher residual deviance and DIC. Both models K1 and K2 fit as well as the unadjusted model H1 (similar DIC and residual deviance).
Among all the placebo response-adjusted models, models I1, K1 and K2 show similar DIC and residual deviance, which means that these three models fit the existing data equally well, although not significantly better than the unadjusted model.
The interaction term (beta) is negative in all models, which means that higher placebo response rates in trials are associated with higher treatment effects, demonstrating that adjustment for heterogeneity in the placebo responses across trials was required. The interaction term varies between models, but is similar between models K1 and K2.
Preferred models
The unadjusted model, H1, fits the data as well as any of the other models and generates results that reflect the observed results. Considering the placebo-adjusted models, model I1-generated results (rankings) are very different from the observed trial results and the results generated by model H1. Using an assumption of equal class effect for the treatments does not produce a better-fitting model (models J1, J2, J3) than assuming independent treatment effects (models H1, I1), or similar (exchangeable) treatment effects (models K1, K2). In addition, there was a little difference in the goodness-of-fit statistics (DIC and residual deviance) between models K1 and K2, and we consider the exchangeable class effect model, which utilised two classes (anti-ILs and anti-TNFs) with APR separate, to be the most clinically plausible. Hence, our preferred models are models H1 and K2. Note that the economic model uses PsARC; thus, these results were not implemented in the economic model in Chapter 6.
Table 59 presents the probabilities of achieving ACR 20, ACR 50 and ACR 70 responses in a biologic-naive population from the preferred models, H1 and K2.
| Treatment | Not adjusted for placebo response, independent treatment (model H1) | Adjusted for placebo response, class effects assumeda (model K2) | ||||
|---|---|---|---|---|---|---|
| ACR 20, median (95% CrI) | ACR 50, median (95% CrI) | ACR 70, median (95% CrI) | ACR 20, median (95% CrI) | ACR 50, median (95% CrI) | ACR 70, median (95% CrI) | |
| Placebo | 0.17 (0.15 to 0.19) | 0.05 (0.04 to 0.06) | 0.01 (0.01 to 0.02) | 0.17 (0.15 to 0.19) | 0.05 (0.04 to 0.06) | 0.01 (0.01 to 0.02) |
| 300 mg of SEC | 0.49 (0.33 to 0.64) | 0.24 (0.14 to 0.38) | 0.09 (0.04 to 0.18) | 0.61 (0.46 to 0.75) | 0.35 (0.22 to 0.50) | 0.16 (0.08 to 0.27) |
| 150 mg of SEC | 0.49 (0.34 to 0.65) | 0.25 (0.14 to 0.39) | 0.10 (0.04 to 0.19) | 0.61 (0.46 to 0.75) | 0.35 (0.22 to 0.51) | 0.16 (0.08 to 0.27) |
| UST | 0.35 (0.27 to 0.44) | 0.15 (0.10 to 0.21) | 0.05 (0.03 to 0.08) | 0.41 (0.34 to 0.49) | 0.19 (0.14 to 0.25) | 0.07 (0.04 to 0.10) |
| CZP | 0.44 (0.34 to 0.55) | 0.21 (0.14 to 0.30) | 0.08 (0.04 to 0.13) | 0.58 (0.49 to 0.69) | 0.33 (0.24 to 0.43) | 0.14 (0.09 to 0.22) |
| GOL | 0.68 (0.55 to 0.80) | 0.43 (0.30 to 0.57) | 0.21 (0.12 to 0.33) | 0.53 (0.40 to 0.66) | 0.28 (0.18 to 0.40) | 0.11 (0.06 to 0.19) |
| ADA | 0.55 (0.47 to 0.62) | 0.29 (0.23 to 0.36) | 0.12 (0.09 to 0.17) | 0.56 (0.50 to 0.63) | 0.31 (0.26 to 0.37) | 0.13 (0.10 to 0.17) |
| INF | 0.75 (0.65 to 0.83) | 0.50 (0.39 to 0.62) | 0.27 (0.18 to 0.38) | 0.62 (0.51 to 0.72) | 0.36 (0.26 to 0.47) | 0.17 (0.10 to 0.24) |
| ETN | 0.66 (0.55 to 0.76) | 0.40 (0.29 to 0.52) | 0.19 (0.12 to 0.29) | 0.61 (0.51 to 0.69) | 0.35 (0.27 to 0.43) | 0.16 (0.11 to 0.21) |
| APR | 0.33 (0.27 to 0.39) | 0.13 (0.10 to 0.17) | 0.04 (0.03 to 0.06) | 0.35 (0.30 to 0.41) | 0.15 (0.12 to 0.19) | 0.05 (0.03 to 0.07) |
The results of the unadjusted NMA for ACR, as a single outcome or as separate categorical variables, show that all treatments are more effective than placebo. The difference between treatments is uncertain, with wide CrIs that mostly overlap with each other. The results show that patients taking INF have the highest probability of achieving ACR 20, ACR 50 and ACR 70 responses. The probabilities for SEC are lower than those for INF, ETN, GOL and ADA. After adjustment for placebo, the probabilities for 300 mg of SEC and 150 mg of SEC increase and are very similar to those for INF. The probabilities of achieving ACR 20, ACR 50 and ACR 70 responses with CZP varied between the models: in the unadjusted model the probabilities were higher than only those for APR and UST, but after adjustment they were also higher than those for GOL, ADA and UST.
Subpopulation: biologic experienced
For the biologic-experienced population, trial-specific ACR response data were available for three active treatments (300 mg of SEC, CZP and UST) from three trials,47,48,59,66 but, as for the other outcomes, the data from the CZP trial were not included in the analysis as the biologic-experienced population in the RAPID-PsA trial is not comparable to the populations of the other two trials. 48,59,66 The data included in the NMA for treatment-experienced patients are presented in Table 60.
| Trial | Treatment | ACR responses | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment arm | Placebo arm | ||||||||||||||
| n | ACR 20 | ACR 50 | ACR 70 | n | ACR 20 | ACR 50 | ACR 70 | ||||||||
| 1.1.1 r | % | 1.1.2 r | % | r | % | 1.1.3 r | % | 1.1.4 r | % | 1.1.5 r | % | ||||
| FUTURE 248 | 2, 300 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| PSUMMIT 259,66 | 3, UST | 60 | 23 | 38 | 9 | 15 | 4 | 7 | 62 | 9 | 15 | 1 | 2 | 0 | 0 |
The NMA model was similar to model H1: independent treatment effects in the biologic-naive subpopulation. Owing to the lack of data, no adjustment was undertaken for this subgroup analysis.
The results of the analysis are presented in Table 61 and show that the probabilities of achieving an ACR response in all categories are slightly higher with UST than with SEC, although the differences are insignificant. The results are fairly comparable to the observed data (compare Tables 60 and 61).
| Treatment/parameter | Treatment effects on a probit scale, median (95% CrI) | ACR response, median probability (95% CrI) | ||
|---|---|---|---|---|
| ACR 20 | ACR 50 | ACR 70 | ||
| Placebo | 1.06 (0.76 to 1.38) | 0.14 (0.08 to 0.22) | 0.03 (0.01 to 0.06) | 0.01 (0.00 to 0.02) |
| 300 mg of SEC | –0.71 (–1.36 to –0.08) | 0.36 (0.19 to 0.57) | 0.11 (0.04 to 0.25) | 0.03 (0.01 to 0.11) |
| UST | –0.85 (–1.34 to –0.37) | 0.42 (0.26 to 0.59) | 0.14 (0.06 to 0.27) | 0.05 (0.01 to 0.12) |
| ACR 20 | – | |||
| ACR 50 | 0.85 (0.62 to 1.13) | |||
| ACR 70 | 1.47 (1.10 to 1.92) | |||
| Residual deviancea | 11.33 | |||
| DIC | 45.85 | |||
Limitations
Data were sparse; there were few studies in each treatment [a maximum of three studies in two treatments (ADA55–57,67 and APR60,61,65)]. For this reason, we were not able to fit random-effect models, especially when considering placebo adjustment. Hence, fixed-effect models were used in all analyses.
Summary of findings of relative efficacy from network meta-analysis
The NMA was conducted to formally investigate the relative efficacy of SEC and CZP and the other active comparators. Analyses were conducted on four outcomes: PsARC, HAQ-DI conditional on PsARC response, PASI and ACR. Analyses were not run for the full-trial populations because of the heterogeneity across trials, but instead were performed separately for the biologic-naive and biologic-experienced subgroups. The data suggest the rate of placebo response to be a potential source of heterogeneity within the biologic-naive population networks, despite there being no clear rationale for such an effect. For this reason, we explored models that adjust for the placebo response, alongside unadjusted models.
Biologic-naive patients
In terms of PsARC response, the results indicated that, although SEC and CZP are effective, the relative effectiveness of these biologics compared with ETN, ADA, GOL and INF, and with each other, is uncertain, although both agents do seem to be more effective than APR.
In terms of HAQ-DI conditional on PsARC response, the results from the preferred adjusted model were similar to the independent treatment effect analysis. The results from the unadjusted independent treatment effects model showed that significant reductions in mean HAQ-DI score were achieved with response to all nine treatments and response to placebo, although the improvement in response to placebo is below the minimum clinically significant threshold for PsA of –0.35. 116 The median HAQ-DI score change was highest with INF and ETN, followed by 300 mg of SEC, but 150 mg of SEC and CZP were worse than all treatments except for APR.
The results of the unadjusted NMA for PASI, as a single outcome or as separate categorical variables, indicated that all treatments were more effective than placebo. The difference between treatments was uncertain, with wide CrIs that mostly overlap with each other. The results showed that patients treated with INF have the highest probability of achieving PASI 50, PASI 75 and PASI 90 responses. However, after adjustment for placebo, 300 mg of SEC has the highest probability of response. The probabilities for CZP changed between the models. It appears to be less efficacious than all other treatments, except APR and ETN, in achieving PASI responses in the unadjusted model. However, in the adjusted model, CZP appears to be more efficacious than GOL, UST, APR and ETN, and similar to ADA.
Similarly, for ACR responses, differences between treatments were uncertain, with wide CrIs that mostly overlapped with each other. The unadjusted results suggested that patients taking SEC or CZP had lower probabilities of a response than those for INF, ETN, GOL and ADA. After adjustment for placebo response, the probabilities of a response for both SEC and CZP increased; those for SEC were very similar to those for INF.
Biologic-experienced patients
The evidence for the biologic-experienced subpopulation is very sparse with only two trials evaluating two treatments. Hence, only two treatments (SEC and UST) could be included in these analyses. The results showed that, across all outcomes analysed, both SEC and UST were significantly more effective than placebo. Most of the results suggested SEC may be better than UST, although the results were uncertain with wide overlapping CrIs.
Chapter 5 Assessment of existing cost-effectiveness evidence
The purpose of this section is to review the existing evidence on the cost-effectiveness of CZP and SEC within their marketing authorisations for treating active PsA in adults for whom DMARDs have been inadequately effective. The review includes published cost-effectiveness studies and the CSs from Novartis (SEC) and UCB Pharma (CZP). The review also includes a broader assessment of published decision-analytic models for relevant comparators. The differences in the model structures and assumptions used across the studies are examined to identify any important differences in approaches and areas of remaining uncertainty. The findings from the review also provide the basis for the development of a new decision-analytic model reported in Chapter 6.
Methods
To identify published economic evidence for CZP and SEC, a broad range of studies was considered for inclusion in the assessment of cost-effectiveness, including economic evaluations conducted alongside trials, modelling studies and analyses of administrative databases. Only full economic evaluations that compared two or more options and considered both costs and consequences (including cost-effectiveness, cost–utility and cost–benefit analyses) were included.
A broader review of economic evidence for the comparator treatments (INF, ETN, ADA, GOL and UST) was also undertaken. The objective was to summarise the modelling approaches, and assumptions, employed in previous studies, and to identify any important differences that may have arisen since the previous MTA (TA19933). As the focus of the broader review related to modelling approaches and assumptions, only decision-analytic modelling studies were included. The broader review also provides an important basis to identify common areas and potential differences between the approaches previously used for the comparator treatments and those employed by UCB Pharma and Novartis for the specific technologies being considered in this appraisal. The broader review also helped inform the conceptualisation of the de novo model presented in Chapter 6.
The following databases were searched for relevant published literature: Cochrane Controlled Trials Register, EMBASE, Health Economic Evaluations Databases, MEDLINE, National Research Register, NHS Economic Evaluation Database (NHS EED), PsycINFO and the SCI. The full details of the main search strategy for this review are presented in Appendix 4. The searches for CZP and SEC for PsA were not restricted by date. The searches for the broader comparator review were date restricted to identify studies published since the previous MTA report for ADA, ETN and INF (TA19933). Additional hand-searching of related TAs (TA199,33 TA220118 and TA34066) was also undertaken. Two reviewers assessed all obtained titles and abstracts for inclusion, with any discrepancies resolved by discussion.
In addition, Novartis and UCB Pharma submitted evidence on the cost-effectiveness of CZP and SEC. These submissions were reviewed and the approaches and findings compared with those found in the review of previously published studies. The quality of the cost-effectiveness studies for CZP and SEC was also assessed according to a checklist updated from that developed by Drummond et al. 119
Results
Identified published studies
No previously published cost-effectiveness studies of SEC for PsA were identified. Two conference abstracts were identified evaluating the cost-effectiveness of CZP for PsA in Greece and Romania. 120,121 Further details were not provided on request from the corresponding authors and so these abstracts were subsequently excluded from further consideration. Given the lack of previously published studies, only the CSs are considered for SEC and CZP.
The systematic search of published literature identified nine studies33–36,66,122–124 that met the inclusion criteria for the cost-effectiveness review for the broader set of comparators. From the nine studies, seven UK studies were identified. 33–36,66,122 Three of the UK studies were reports from the independent AG/ERG for the previous NICE appraisals of ETN, INF and ADA (TA19933), GOL (TA220118) and UST (TA34066). A further three studies were the subsequent journal publications based on the reports for TA199,122 TA22034 and TA340. 35 The final UK study identified was a more recent study that aimed to update the systematic review, synthesis and model previously conducted as part of TA199. 122 This study was funded by Pfizer. 36
Of the two non-UK studies, one evaluated the cost-effectiveness of UST for PsA in Russia123 and the other evaluated the cost-effectiveness of a mixture of biologic treatments to treat moderate–severe PsA in Germany. 124 Both of these studies were available only as conference abstracts. Further details were requested from the authors but were not provided and hence these two studies were excluded from the review.
Review of the existing published cost-effectiveness studies
The review starts with an overview of the seven UK studies identified in relation to the broader set of comparators and then considers the de novo analyses submitted by the companies for SEC and CZP.
Summary of published studies for comparator treatments
Of the seven published studies included in the broader review of comparators,33–36,66,122 six were directly related to three previous NICE TAs: TA199,33 TA220118 and TA340. 66 All of these publications employed a similar modelling approach to that originally proposed by Rodgers et al. 33 for TA199 (hereafter referred to as the ‘York model’). The only study identified that was not directly related to a previous NICE TA was that by Cawson et al. 36 This study also used a very similar approach to the previous York model. Hence, the main differences between these studies lie in relation to the comparators and associated evidence base which have altered since TA199, rather than in terms of major structural differences. As the provenance of the modelling approach used in all these studies can be related back to the York model, only the York model is described in full in the following section. The key differences in the other published studies are subsequently summarised.
Summary of the York model (TA199)
The York model is a cohort Markov model (Figure 8), built using the R software package (The R Foundation for Statistical Computing, Vienna, Austria). The model was developed to estimate the costs and quality-adjusted life-years (QALYs) of three biologics (ETN, INF or ADA), over a lifetime horizon (40 years), compared with palliative care alone. The model adopts the perspective of the UK NHS and Personal Social Services. The price year assumed for costs is 2008/9 and the annual discount rate is 3.5%,125 for both costs and QALYs.
The model structure is based on an understanding of the disease process and how this should be modelled to determine cost-effectiveness. 126 The model is based on a two part structure:
-
initial response period (short-term model used to determine initial response rate and treatment continuation decision)
-
post-response period [longer-term model used to characterise the natural history of the disease (i.e. without biologics) and the impact of biologics while on therapy and when therapy is stopped].
Patients receiving biologics and who meet the response criteria during the initial response period continue on their biologic treatment in the post-response period. Biologics are withdrawn in non-responders and these patients are assumed to move on to palliative care alone. Changes in the HAQ-DI and PASI scores are used to quantitatively model the short- and longer-term cost and quality-of-life implications (estimated using QALYs) of the use of biologics versus palliative care alone.
Initial (primary) response to the drug is defined using PsARC for joints and the PASI 75 for psoriasis, based on BSR127 and British Association of Dermatologists128 guidelines. As two response variables are considered (PsARC and PASI), there are four possible outcomes in the initial response period: (1) skin response only; (2) joints response only; (3) response of both; and (4) response of neither. In the base-case analysis, only joint (PsARC) response is used to determine treatment continuation. Alternative response rules are explored in separate scenarios: skin (PASI 75) response only, and response for both measures (PsARC and PASI 75).
The time point for the assessment of response is assumed to occur at ‘around 3 months’ or between 12 and 16 weeks. Although differences in the recommended time points for assessing initial response were identified by the authors based on the licences and between guideline-making bodies, a common time point was subsequently assumed. This was justified based on the authors’ conclusions that there appeared to be a lack of a clinically meaningful difference in the biologics’ response rates for joint disease or psoriasis between approximately 12 and 24 weeks.
In the decision model, the change in HAQ-DI score compared with baseline is conditional on whether or not a PsARC response was achieved and the specific biologic treatment received. During the initial 3-month response period, the model assumes that patients on biologics have some improvement in their HAQ-DI score, even if they do not reach the PsARC threshold. Patients who do not achieve the required level of response during the first 3 months are withdrawn from therapy, and are assumed to follow the same HAQ-DI score trajectory after withdrawal as patients who had palliative care only.
The model assumes that patients who achieve a PASI 75 response will gain at least a 75% improvement in psoriasis compared with baseline PASI score. Patients who do not achieve a PASI 75 response will also have some proportionate gain in PASI score while they continue taking a biologic, although this will be less than a 75% improvement. The distribution of PASI scores observed in the trials was reflected within the model by utilising the PASI 50, PASI 75 and PASI 90 data to determine the change in PASI score for PASI 75 responders and non-responders.
Following an initial response to biologic therapy, the model assumed that patients maintain the initial improvement in HAQ-DI score for the remaining period of time on that therapy. This assumption was justified based on evidence from an elicitation exercise with clinical experts and supported by data on HAQ-DI and HRQoL from biologics registers and radiographic information supplied by the manufacturers of biologics. It was also assumed that patients maintain the improvement in PASI score while on biologic therapy.
The model assumes that no patients withdraw as a result of AEs in the first 3 months. The authors noted that, as responses in the RCTs are reported on an ITT basis, including withdrawal during the first 3 months would constitute double counting. The model includes an ongoing risk of withdrawal from biologic therapy over the longer term as a result of a lack of continuing efficacy (‘secondary non-response’), AEs or other reasons. The rate of withdrawal after 3 months is assumed to be independent of the HAQ-DI and PASI scores, to be independent of whether the initial response was for both psoriasis and arthritis or just arthritis and to be constant over time.
On withdrawal of a biologic treatment, it is assumed that the mean PASI returns to its initial score at baseline (rebound equal to initial gain). The authors acknowledged that there was more uncertainty about change in HAQ-DI score associated with withdrawal (rebound). In the base-case analysis it is assumed that rebound is equal to initial gain. Other scenarios (rebound less than initial gain and rebound equal to natural history) were explored using sensitivity analyses.
Patient characteristics in the York model
Table 62 shows the baseline characteristics used in the York model. Patients were assumed to fulfil the BSR guidelines and criteria specified for commencing biologics (i.e. that their PsA has not responded to adequate trials of at least two standard DMARDs, administered either individually or in combination).
| Characteristic | Assumed value |
|---|---|
| Age (years) | 47 |
| Weight (kg) | 60–80 |
| Baseline HAQ-DI score (units) | 1.05 |
| Baseline PASI score (units) | 7.5 |
The model cohort is assumed to be aged 47 years at the start of the model, and it is assumed that at least 7 years has passed since the diagnosis of PsA, based on the average characteristics of participants in the included RCTs. The mean baseline HAQ-DI score at the start of the model is assumed to be 1.05 units and patients are assumed to have mild–moderate psoriasis with a PASI score of 7.5 units, based on the average HAQ-DI and PASI baseline scores in the RCTs. The mean body weight is assumed to be between 60 and 80 kg based on the mean adult weight of the general population for men and women.
Alternative subgroups were explored in scenario analyses based on different baseline HAQ-DI and PASI scores:
-
an alternative, more severe HAQ-DI of 1.8 units, which is the mean HAQ-DI score of patients entering the BSRBR83
-
no skin involvement, with a PASI score of 0 units (Smith et al. 128 stated that 50% of patients with PsA starting biologics in clinical practice would have mild or no skin involvement)
-
moderate–severe psoriasis, with a PASI score of 12.5 units (Smith et al. 128 stated that 25% of patients with PsA starting biologics in clinical practice would have a baseline PASI of > 10 units).
Choice of intervention and comparators in the York model
Infliximab, ETN, ADA and palliative care were included, reflecting the licensed biologic treatments available when TA199122 was conducted. Palliative care was assumed to represent conventional care without biologic treatment.
Sequencing of treatments in the York model
In the base-case analysis, patients who are withdrawn from treatment (primary non-response or secondary withdrawal) were assumed to receive palliative care alone. A separate exploratory scenario assessed the cost-effectiveness of a further biologic treatment used as a second line of therapy (biologic experienced), if the first biologic is withdrawn. This scenario considered two subgroups: failure of first biologic as a result of AEs and failure because of efficacy.
In the absence of RCT data on these subgroups, treatment response and withdrawal rates for these subgroups were estimated from observational data for RA patients from the BSRBR. In the case of a patient who failed first-line therapy because of a lack of efficacy, the RR of failing the second-line therapy because of a lack of efficacy increases by 2.7 (95% CI 2.1 to 3.4). If a patient fails first-line therapy because of an AE, then the risk of failing the second-line therapy for AEs increases by 2.3 (95% CI 1.9 to 2.9).
Natural history of psoriatic arthritis in the York model
Psoriatic arthritis is a progressive disease and patients with untreated PsA may have persistent inflammation and progressive joint damage (see Chapter 2). This was reflected in the York model by applying a constant rate of HAQ-DI increase to patients receiving palliative care alone (Figure 9). The increase in HAQ-DI score was applied to characterise the natural history of HAQ-DI (i.e. without biologic treatment) and was estimated as 0.018 units in a 3-month cycle, based on data from the NOAR. Figure 9 graphically shows how the HAQ-DI progression assumptions (on and off treatment) were applied in the York model.
FIGURE 9.
Illustration of the progression of arthritis for a patient successfully maintained on a biologic, a patient without a biologic and a patient who withdraws at 5 years, as implemented in the York model. 33
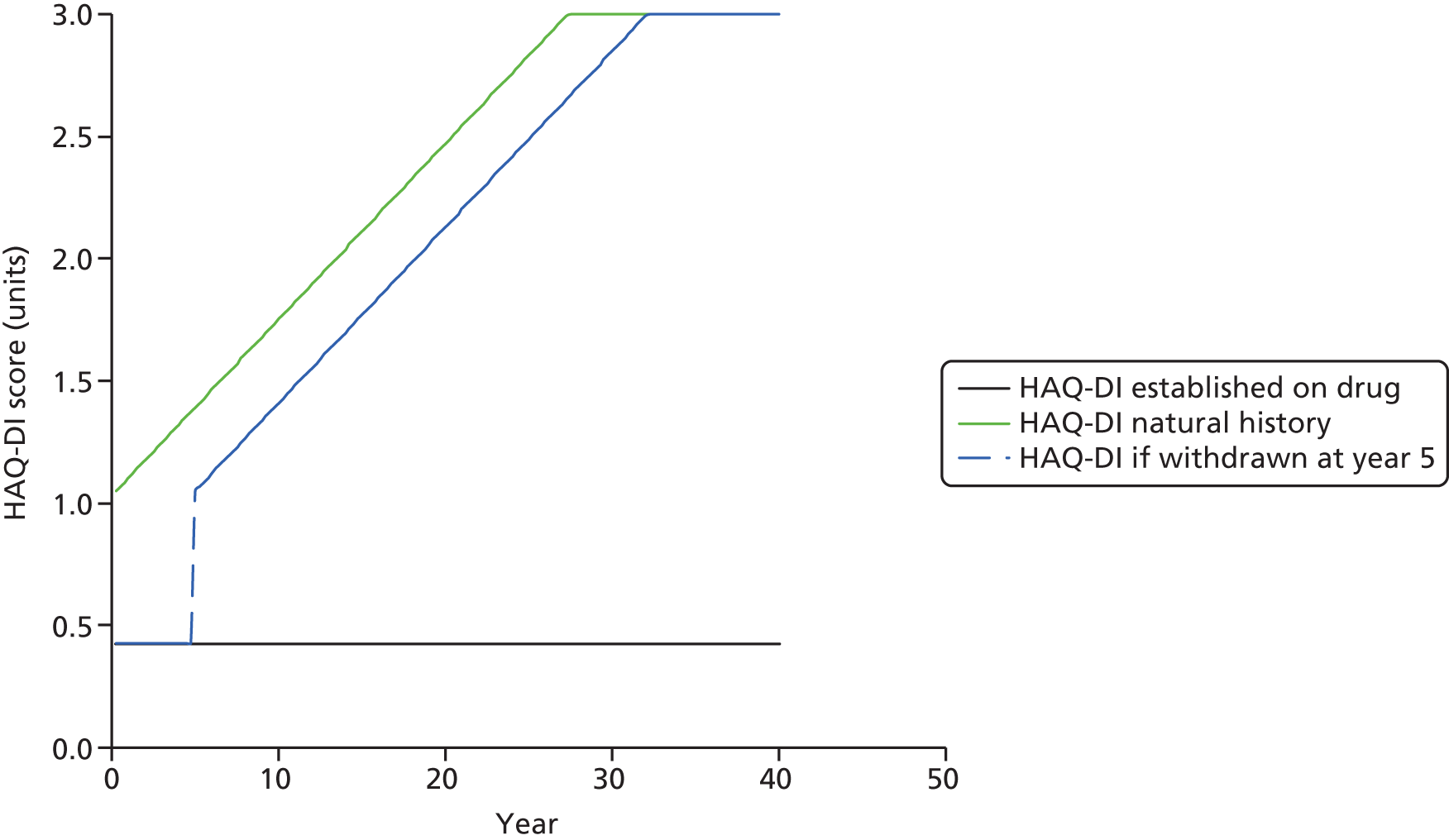
For the psoriasis component of PsA, it was assumed that the PASI score does not worsen over time (off treatment), which was stated to be consistent with clinical evidence.
Sources and synthesis of effectiveness data in the York model
The effectiveness of the alternative treatments was estimated using a NMA. The network of evidence was based on six trials that have a common comparator (placebo). 33 Three different synthesis models were specified to allow relevant outcomes for the economic model to be synthesised: PsARC response at 12–16 weeks; change in HAQ-DI score conditional on a PsARC response; and the probability of achieving PASI 50, PASI 75 and PASI 90 responses.
In the decision model, the change in HAQ-DI score compared with baseline is conditional on PsARC response status. It is uncertain whether the change in HAQ-DI score is the same for all PsARC treatment responders (TRs) or depends on the particular biologic treatment received. In the base case, the change in HAQ-DI score depended on PsARC response and the individual biologic treatment, whereas alternative scenarios (i.e. HAQ-DI score change the same for all PsARC responders) were assessed within the sensitivity analysis.
A placebo or expectation effect, which is the improvement reported for patients in the placebo arms of the RCTs, is uncertain and may not be reproducible in clinical practice. In the base case, the mean change in HAQ-DI score across the placebo arms of the RCTs was discounted from the change in HAQ-DI score for patients using biologics. This was applied in the decision model by deducting the change in HAQ-DI score in the placebo arm, weighted by the PsARC response in that arm, from the HAQ-DI score change in the treatment arm. A similar adjustment is made for the expected change in PASI score. An alternative scenario was conducted assuming that the response rate to treatment in the RCTs is fully generalisable to general practice and, therefore, no adjustment for placebo/expectancy effects is made.
Data on time to withdrawal from first biologic were separately synthesised using a meta-analysis of five European registry studies, one of which was the UK BSRBR. 83 The estimated annual probability of withdrawing from the biologic treatment after the first cycle is 0.165; therefore, patients who achieve an initial PsARC response will, on average, remain on biologic drugs for just over 6 years in the model (1/0.165 = 6.06 years). This was assumed to be identical for all biologics.
The base-case model uses a published estimate of the additional mortality risk in PsA (Wong et al. 12).
Sources of utility data used in the York model
Quality-adjusted life-years were determined by estimating health utilities as a function of HAQ-DI and PASI. The York model used an equation based on an ordinary least squares regression of patient-level data from one of the companies (Wyeth) submitting evidence for TA199. 122 It was stated that similar results were obtained from separate trials across each of the three companies, indicating that the relationship between HAQ-DI, PASI and utility appears stable across independent clinical trials. Equation 1 shows the algorithm used in the base-case analysis of the York model:
Summary of resource utilisation and costs data used in the York model
The costs of acquiring the drugs and of their administration and monitoring were obtained from the BSR guidelines for the use of biologics and national prices and tariffs. The base case assumes that vial sharing is not permitted for INF and, therefore, separate scenarios regarding the use of three or four vials per patient were considered according to different weight assumptions.
Health-care costs increase with severity of both arthritis and psoriasis. Health state costs associated with HAQ-DI were derived from data from a UK-based study by Kobelt et al. 129 including 916 patients suffering from RA and followed up for between 5 and 9 years. Direct health-care resources were collected prospectively for all patients for hospitalisations, surgical interventions and RA medications. Based on this study, Bansback et al. 130 separately applied a linear regression model to estimate the relationship between HAQ-DI score and resource use (Equation 2). The regression estimates were subsequently reduced by 15% to account for expenditure on DMARDs and to avoid double counting other drug acquisition costs which were separately estimated.
As the Kobelt et al. 129 study includes only RA patients, separate costs were estimated for treating mild–moderate psoriasis in patients who do not use biologics, or who do not respond to biologics, from NHS unit costs of phototherapy and a UK RCT. For patients with moderate or severe psoriasis, costs were obtained from a Dutch RCT (see Hartman et al. 131) and adjusted to UK price levels. These costs were assigned to patients based on whether or not a PASI 75 response was achieved (Table 63).
| State | Level of psoriasis, 3-month cost (£) | |
|---|---|---|
| Mild–moderate | Moderate–severe | |
| On anti-TNF-α with PASI 75 response | 16 | 16 |
| On anti-TNF-α without PASI 75 response | 198 | 566 |
| Not on anti-TNF-α therapy | 198 | 566 |
Cost-effectiveness results from the York model
The summary results from the York model are those which are reported in the Final Appraisal Determination document for TA199. 132 The results of the base-case model reported that INF was the most effective strategy taking into account both joint and skin effects (QALYs = 7.3), followed by ETN (QALYs = 7.0) and ADA (QALYs = 6.6). In terms of costs, INF was the most costly treatment (£88,442), followed by ETN (£74,841) and ADA (£68,638). The incremental cost-effectiveness ratio (ICER) for ETN compared with palliative care was £17,853 per QALY. The ICER for INF compared with ETN was around £44,326 per QALY. ADA was extendedly dominated. Of the three biologic therapies, ETN had the highest probability of being cost-effective at a threshold between £20,000 (probability = 44%) and £30,000 (probability = 48%) per QALY.
The results of the subgroup analysis showed that biologics appear slightly less cost-effective if the baseline HAQ-DI score is 1.8 (high), although the ICER for ETN remained below £20,000 per QALY. In patients with a negligible baseline psoriasis (i.e. PASI = 0 units), ETN was the most cost-effective strategy, with an ICER of £18,512 per QALY compared with palliative care. The ICER of INF versus ETN increased to £64,744 per QALY and ADA remained extendedly dominated. However, for a cohort in which the baseline PASI score was moderate to severe (PASI of 12.5 units rather than 7.5 units), ADA was no longer extendedly dominated. The ICER of ADA versus palliative care was £16,310 per QALY. The ICER of ETN versus ADA was £19,319 per QALY and the ICER of INF versus ETN was £27,778 per QALY.
In the scenario considering the cost-effectiveness of biologics, used as a second course of therapy after a first biologic has failed for PsA patients with mild–moderate skin disease, the ICERs depend on which drug was used as first-line therapy and is therefore ineligible for use as second line. For patients failing ETN, ADA has an ICER of < £20,000 and INF is around £25,000 per QALY. The ICERs were reported to be broadly similar for people whose PsA failed to respond to first-line therapy because of adverse effects and those whose disease failed first-line therapy because of inefficacy.
Summary of key differences in modelling approaches from other published studies
As described in Summary of published studies for comparator treatments, following the development of the York model for TA199,33 three further models were developed comparing different sets of interventions. The model developed for TA22070 compared ETN, INF, ADA, GOL and palliative care in a biologic-naive population. The model developed for TA34066 compared ETN, INF, ADA, GOL, UST and palliative care in biologic-naive and biologic-experienced populations. The model developed by Cawson et al. 36 compared ETN, INF, ADA, GOL and palliative care in a biologic-naive population.
The model structure used in each of the three models is broadly the same as the York model. There were some minor variations in the duration of the response period, in particular extending this up to 24 weeks in TA22070 to reflect the longer response period for UST in line with its licence, but generally all models have a similar underlying structure and use PsARC as the main response measure.
One key difference between the models concerns the different sets of interventions that have been compared. The sequence of published studies closely follows the licensing of additional TNF-α inhibitors after TA19933 (GOL) and new biologic alternatives (UST). As a result, the scope of each study has been extended to include these additional licensed treatments. With the exception of the UST (TA34066), the majority of studies have focused on evaluating the relative cost-effectiveness of the alternative TNF-α inhibitors in a biologic-naive population and all have been consistent in assuming that biologics are started only following the failure of at least two DMARDs (individually or in combination, in line with BSR guidelines). However, as one of the RCTs for UST included patients with and without prior exposure to TNF-α inhibitors, the decision problem for TA34066 was subsequently broadened to reflect these different populations. For the TNF-α inhibitor-exposed (experienced) population, UST was compared with conventional management only, because at the time of the submission there were no RCTs of TNF-α inhibitors in this population. Analyses were based on clinical effectiveness evidence from the TNF-α inhibitor-exposed subpopulation of the PSUMMIT 2 trial.
As new interventions have been included, subsequent modelling studies have been based on revised NMAs incorporating new RCT evidence for the interventions being assessed in each appraisal (GOL in TA22070 and UST in TA34066). However, the synthesis approaches and methodologies applied across the studies remains consistent with that applied in the York model. The only exception to this is the comparison of UST with conventional care in the TNF-α inhibitor-exposed subpopulation, which was based on subgroup results from the PSUMMIT 2 trial. For this subpopulation a NMA was not considered feasible because of the lack of RCT evidence for the comparator treatments.
The main approaches to estimating longer-term costs and QALYs employ similar methodologies and assumptions across the studies identified. The main difference in relation to costs concerns the link to PASI. Estimates of PASI costs applied in the GOL and UST appraisals (TA22070 and TA34066) were derived from a clinician survey and used to estimate the expected difference in cost per additional unit change in PASI score. This contrasts with the approach used in the York model, which distinguished costs on the basis of PASI 75 response. Although different utility algorithms have been applied in each of the models (TA22070 and TA34066 used patient-level data from each company’s trials), these have reported similar coefficients for HAQ-DI and PASI to those applied in the York model. All studies have also routinely reported results based on the utility estimates used in the York model in separate scenarios.
With the exception of TA220 (GOL),70 all models have used the same assumptions and data sources to model the natural history and progression of PsA [i.e. assuming a constant PASI score and a linear increase (worsening) of HAQ-DI score over time]. In TA220 (GOL)70 and TA340,66 the annual rate of change per year was derived from an alternative source, the Leeds NESPAR study. However, the estimate is broadly similar to the estimate applied in the York model and other published models (0.0719 per year compared with 0.077 per year in the York model). All published studies have used the same estimate (16.5% per annum) concerning longer-term withdrawal of biologic treatment due to lack of efficacy.
Comparison of cost-effectiveness results from published models
Given the different interventions and effectiveness data utilised in each of the models, it is not surprising that each generates different costs and QALYs, resulting in different ICERs for the various options being compared (Table 64). However, there appeared a number of findings which were consistent across the separate studies. Consistently, ETN appeared to represent the most cost-effective strategy based on fully incremental ICER calculations, with an ICER ranging between £16,426 and £17,853 per additional QALY versus palliative (i.e. conventional) care. In addition, INF was reported to be the most effective and costly strategy with the exception of TA220,70 where INF was reported to have the same effectiveness as ETN. There is greater variation across the studies in terms of the ICERs reported for INF versus palliative care than for other comparisons. The ICERs for INF versus palliative range between £20,789 and £40,943 per QALY. These differences appear largely as a result of differences in assumptions related to dosing for INF based on body weight. In all fully incremental comparisons, treatments other than ETN and INF were reported to be either dominated or extendedly dominated. The majority of studies reported that the ICER for INF versus ETN (the next less effective and non-dominated strategy) ranged between £44,326 and £268,107 per QALY. In contrast, INF was reported to be dominated by ETN in TA220 (i.e. same effectiveness but higher cost). 70
| NICE TA and published studies | |||
|---|---|---|---|
| aTA199:33 Rodgers et al.33 and Bojke et al.122 | aTA220:70 Cummins et al.133 and Yang et al.34 | aTA340:66 Craig et al.66 and O’Connor et al.35 | Cawson et al.36 |
| Only fully incremental ICERs presented. The ICER of ETN compared with palliative care was £17,853 and the ICER of INF compared with ETN was £44,326 per QALY. ADA is extendedly dominated | Pairwise ICERs presented vs. palliative care and fully incremental comparisons presented | Pairwise ICERs presented vs. palliative care and fully incremental comparisons presented. Separate analyses presented for TNF-α inhibitor-naive and TNF-α inhibitor-experienced populations. ERG alternative model estimates presented below including UST PAS | Pairwise ICERs presented vs. palliative care and fully incremental comparisons presented |
| Of the three biologic therapies, ETN has the highest probability of being cost-effective at a threshold between £20,000 and £30,000 per QALY | Pairwise ICERs vs. palliative care (company corrected):
|
Pairwise ICERs vs. palliative care – naive (ERG alternative model including UST PAS):
|
Pairwise ICERs vs. palliative care:
|
Fully incremental ICERs:
|
Fully incremental ICERs – naive (ERG alternative model including UST PAS):
|
Fully incremental ICERs:
|
|
Experienced patients (ERG alternative model including UST PAS):
|
|||
Technology Appraisal 340 included a separate analysis of a biologic-experienced population for UST. In this analysis UST was reported to be cost-effective compared with BSC (ICER around £25,000) in the biologic-experienced/-ineligible population. UST was subsequently approved by NICE for this population, highlighting the importance of considering the impact of broader treatment pathways for PsA for future studies.
Analysis of subgroups, according to psoriasis involvement, has been consistently done via deterministic sensitivity analysis in TA199, TA220 and TA340, specifying a negligible or more severe PASI score.
Critique of company submissions
Two de novo economic models were submitted by the companies (Novartis and UCB Pharma) as part of this TA. The main features of the models are summarised in Table 65 and critiqued in the sections following this. Quality assessment checklists for the two submissions are presented in Appendix 5.
| Feature | CS | |
|---|---|---|
| Novartis | UCB Pharma | |
| Comparators | These are specified according to the subpopulations considered:
|
These are specified according to the subpopulations considered:
|
| Model structure | Short-term (3-month) decision tree, leading into a long-term (40-year) Markov cohort model | Cohort Markov model. Three periods:
|
| Response at 3 months defined using both PsARC and PASI 75. Responders enter the maintenance phase and can switch to SoC as a result of death or withdrawal from treatment | PsARC is used to determine response. Responders enter the maintenance phase and can switch to another treatment as a result of loss of efficacy or for other reasons. Initial non-responders switch to the next line of treatment immediately after the initial period | |
| Disease progression, through PASI and HAQ-DI, are linked to costs and utilities. For patients on treatment, HAQ-DI and PASI scores remain constant from 12 weeks | Disease progression, through PASI and HAQ-DI, is linked to costs and utilities. For patients on treatment, HAQ-DI and PASI scores remain constant. For patients who withdraw from treatment, PASI score rebounds back to the baseline value in the cycle after stopping active treatment, but HAQ-DI score rebounds to a worse position | |
| For patients who withdraw from treatment, PASI and HAQ-DI scores both rebound back to the baseline value in the cycle after stopping active treatment. Patients on SoC experienced a linear increase in their HAQ-DI score of 0.018 units for each cycle | Patients on SoC experienced a linear increase in their HAQ-DI score of 0.018 units for each cycle | |
| Sequencing | Not addressed in the base-case analysis. Included as a scenario in which patients move to a subsequent ‘basket’ of biologics before switching to SoC. This was applied only in the anti-TNF-naive population | Full sequence model of biologics followed by the mix of palliation, the sequence differs based on the subpopulation, ranging from one line to three lines of treatments. Switching can only occur in the first 4 years, after which patients remain on treatment indefinitely, accounting for mortality |
| Patient inputs | Homogeneous cohort using average characteristics from the FUTURE 2 trial:48 baseline HAQ-DI score = (confidential information has been removed); baseline PASI score = (confidential information has been removed). These baseline values were applied to each of the three subpopulations | Homogeneous cohort using average characteristics from the RAPID-PsA trial:47
|
| Sources of effectiveness evidence and synthesis | See Sources and synthesis of effectiveness and Appendix 6 | See Sources and synthesis of effectiveness and Appendix 6 |
| Sources of cost data | MIMS 2016134 and BNF 2015135 for acquisition costs and doses required for treatments. PSSRU136 and NHS Reference Costs 2014 to 2015137 for administration and monitoring costs | MIMS 2016134 and BNF 2015135 for acquisition costs and doses required for treatments. PSSRU136 and NHS Reference Costs 2014 to 2015137 for administration and monitoring costs |
| Health state costs were estimated based on Kobelt et al.129 | Health state costs were estimated based on Poole et al.138 | |
| Utilities | Algorithm derived from patient-level data of FUTURE 248 in which utility is a function of HAQ-DI, PASI, age, sex and anti-TNF response state | Algorithm derived from patient-level data of the RAPID-PsA trial47 in which utility is a function of HAQ-DI and PASI |
| The algorithm from the York model was also applied in a scenario analysis | The algorithm from the York model was also applied in a scenario analysis | |
Model structure and assumptions
The two company models have a similar structure to the York model, reflecting both the initial short-term (response period) and long-term (maintenance) phases (Figures 10 and 11). Within the short-term response period, treatment response is assessed within a decision tree in the Novartis submission, and within a Markov cohort model in the UCB Pharma submission. Both submissions characterise the long-term phase (modelled via changes in HAQ-DI and PASI scores) using a Markov cohort model. This longer-term phase is 40 years in the Novartis model and 50 years in the UCB Pharma model. Both models are built in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA).
FIGURE 10.
Overview of the UCB Pharma model structure. (Confidential information has been removed.)
FIGURE 11.
Overview of the Novartis model structure. (a) Decision tree structure; and (b) Markov model structure (base case). SoC, standard of care.
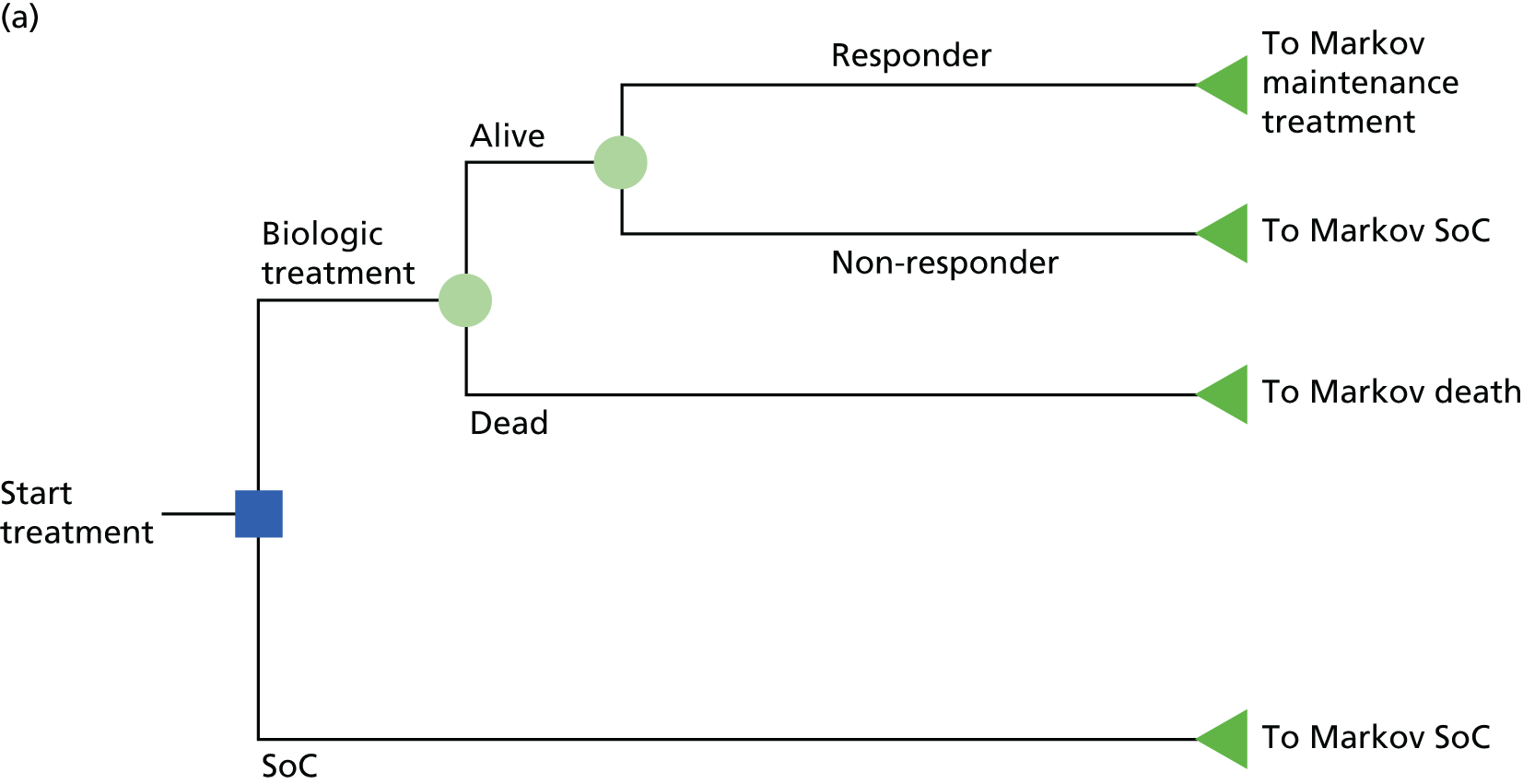

Although both submissions share a similar underlying structure, there are important differences in the base-case approaches of each company in terms of the definition and timing of the response assessment:
-
In the UCB Pharma base-case model, response is defined in terms of PsARC alone. The base case also assumes that PsARC response is assessed at 24 weeks both for CZP and for all other comparators. The use of 24 weeks contrasts with previously published studies reviewed for the comparator treatments, which have consistently assumed that this assessment would occur at around 3 months (12–16 weeks). The main exception in previous studies has been for UST, for which a 24-week time point has been used, in accordance with its marketing authorisation. The justification provided by UCB Pharma for choosing a common time point of 24 weeks for all treatments was based on the European League Against Rheumatism (EULAR) Treat to Target 2013 recommendations, which state that a maximum of 6 months is recommended for reaching the treatment target. However, the submission from UCB Pharma also notes that that the same recommendations also advise that therapy should be adapted earlier than 6 months if no significant reduction in disease activity is observed. The UCB Pharma submission does not explicitly discuss the proportion of patients in whom its therapy would be adapted earlier than the 24-week time point, nor is there any discussion of the potential biases that could arise by assuming that therapy is adapted only after 24 weeks. However, a separate scenario in which the initial response was assessed at 12 weeks both for CZP and for other comparators (including UST) was explored as part of a scenario analysis. Patients are then further stratified according to PASI 75 response/no PASI 75 response. This stratification is not assumed in the base case to alter the decision to continue treatment, but allows alternative cost and utility assumptions to be applied according to PsARC response status.
-
In the Novartis model, patients are defined as responders if both a PsARC and PASI 75 response are achieved at 12 weeks (or 24 weeks for UST). The model also includes additional scenarios in which either PASI or PsARC only is used to determine a patient’s initial response. Although the company notes that the SEC Summary of Product Characteristics recommends a 16-week assessment point, a 12-week time point is assumed for SEC based on consistency with BSR/British Health Professionals in Rheumatology guidelines and previous NICE appraisals.
In both models, HAQ-DI score changes are based on a treatment-specific rate of change conditional on PsARC response status. However, important differences were evident between the companies, in the approaches and assumptions applied in their models:
-
In the UCB Pharma model, HAQ-DI score change for CZP is based on the week 4 data from the RAPID-PsA trial. UCB Pharma justifies this assumption on the basis that the RAPID-PsA trial47 showed minimal further change in HAQ-DI score between weeks 4 and 24. In the absence of HAQ-DI data over time for the other comparators, a similar assumption was made for the comparators. An alternative assumption was explored as part of a scenario analysis in which the highest rate of change (or ‘best’) HAQ-DI score change for the comparators is achieved only at 24 weeks. These assumptions are applied in the UCB Pharma model to the treatment response period (24 weeks in the base case). Beyond 24 weeks, it is also assumed that there is continued improvement in HAQ-DI score up to week 36 post initial response. UCB Pharma justifies this additional period of HAQ-DI score improvement based on continued improvement over this period observed in the RAPID-PsA trial. 47 In the absence of data, a similar assumption is applied to all the comparators. After 36 weeks it is assumed that HAQ-DI score remains constant for patients for the remainder of the period on treatment. Figure 12 illustrates the separate intervals over which different assumptions are applied for patients responding to biological treatment in the UCB Pharma submission.
-
In the Novartis model, HAQ-DI score change data were derived directly from data reported during the 12- to 16-week time period included in its main NMA and were assumed to remain constant from 12 weeks onwards for patients who remained on treatment. This approach is consistent with the assumption made in the previous York model.
FIGURE 12.
Illustration of HAQ-DI score change for patients responding to a biologic treatment in the UCB Pharma model.
In both models the change in PASI score is derived from the distribution of PASI responses. The approaches followed by each company are consistent with the approach and assumptions of the York model.
The two submissions also account for the correlation between PASI 75 and PsARC using a similar method to the York model. However, both companies source data on the correlation coefficients from their own trial data as opposed to the data used in the York model.
Both submissions also incorporate an adjustment to HAQ-DI and PASI scores in order to account for possible ‘placebo’ or ‘expectation’ effects in order to generalise the treatment effects from the RCTs to routine practice. The methods of adjustment follow the same approach as the York model, by reducing the change in HAQ-DI score for biologics by the weighted average of change in HAQ-DI score for PsARC responders and non-responders across the standard of care (SoC) arm. A similar approach is followed for PASI. Consequently, SoC patients were not assumed to experience any HAQ-DI or PASI score improvement in the models.
The Novartis model assumes that, when a treatment is withdrawn, patients rebound to their baseline HAQ-DI score (i.e. rebound equal to gain) and that their HAQ-DI score continues to deteriorate in line with the natural history of HAQ-DI (i.e. a constant monthly rate of HAQ-DI deterioration). In contrast, the UCB Pharma submission assumes that the HAQ-DI trajectory of patients switching to a subsequent treatment initially rebounds to a higher (i.e. worse) HAQ-DI value than the original baseline.
The two submissions include a sex-specific multiplier effect for PsA mortality. The Novartis submission applied the RRs reported in Wong et al. 12 (1.65 and 1.59 for men and women, respectively) to life tables from the general population. The impact of these multiplier effects was assessed by removing the effects in a scenario analysis. In the UCB Pharma submission, a standardised mortality ratio of 1.36 was applied for males and females. 14 This represents an updated analysis of the cohort from Wong et al. 12
Intervention and comparators
According to the BSR guidelines, biologic treatments should be considered for patients with active PsA who have inadequately responded to two previous conventional disease-modifying antirheumatic drugs. 127 However, in accordance with the NICE scope112 and the licences for SEC and CZP, the two submissions have addressed three different subpopulations, including the one prior non-biologic DMARD population. The three subpopulations specified in the NICE scope are:
-
subpopulation 1 (biologic naive, one prior DMARD): people who have received one prior non-biologic DMARD
-
subpopulation 2 (biologic naive, two or more prior DMARDs): people whose disease has not responded adequately to at least two prior non-biologic DMARDs
-
subpopulation 3 (biologic experienced or contraindicated): people whose disease has not responded adequately to non-biologic DMARDs and not adequately responded to biological therapies (including ETN, ADA, INF and GOL), or for whom biologic therapies are contraindicated.
There are two areas where the CSs appear to deviate from the specified NICE scope. 112 First, subpopulation 2 is subsequently defined by UCB Pharma as all biologic-naive people. Hence, subpopulation 2 is presented by UCB Pharma as an expansion of subpopulation 1 (i.e. representing one or more prior DMARDs). In contrast, the Novartis submission specifies subpopulation 2 in accordance with the NICE scope (i.e. inadequate response to at least two DMARDs). Second, both companies focus on the biologic-experienced population for subpopulation 3. Hence, neither company separately considers people in whom biologic therapies (including ETN, ADA, INF and GOL) are contraindicated.
The interventions and comparators in both submissions are specified separately for each of the three subpopulations. Conceptually there are important differences between the submissions in terms of the scope of the models and the approaches used to model the interventions and comparators:
-
The UCB Pharma model has been developed to assess the cost-effectiveness of the interventions in the context of a treatment pathway and, hence, explicitly considers subsequent treatment lines by modelling separate sequences. The length and composition of the sequences differ across each of the three subpopulations.
-
The base-case model from Novartis for each subpopulation focuses on each specific point in the pathway (i.e. the point that a decision to initiate a new intervention would be made for each subpopulation) and does not attempt to formally model the sequences of subsequent treatments. Instead, the impact of further treatment and associated sequences is explored as part of a separate scenario and is presented as an exploratory analysis. Novartis justifies this approach given the limitations in the data available to model sequencing of treatments and the lack of formal guidelines concerning the order in which biologics should be used sequentially.
The interventions and comparators in each subpopulation are summarised in Figure 13 (UCB Pharma) and Figure 14 (Novartis). The figures illustrate the different approaches employed by the companies and the focus on the entire pathway (sequences and different lines) in the UCB Pharma submission compared with the approach used by Novartis in its base case.
FIGURE 13.
Interventions and comparators according to subpopulations (UCB Pharma). (a) Subpopulation 1 (biologic naive, one prior DMARD); (b) subpopulation 2 (all biologic naive, one or more prior DMARDs); and (c) subpopulation 3 (biologic experienced).
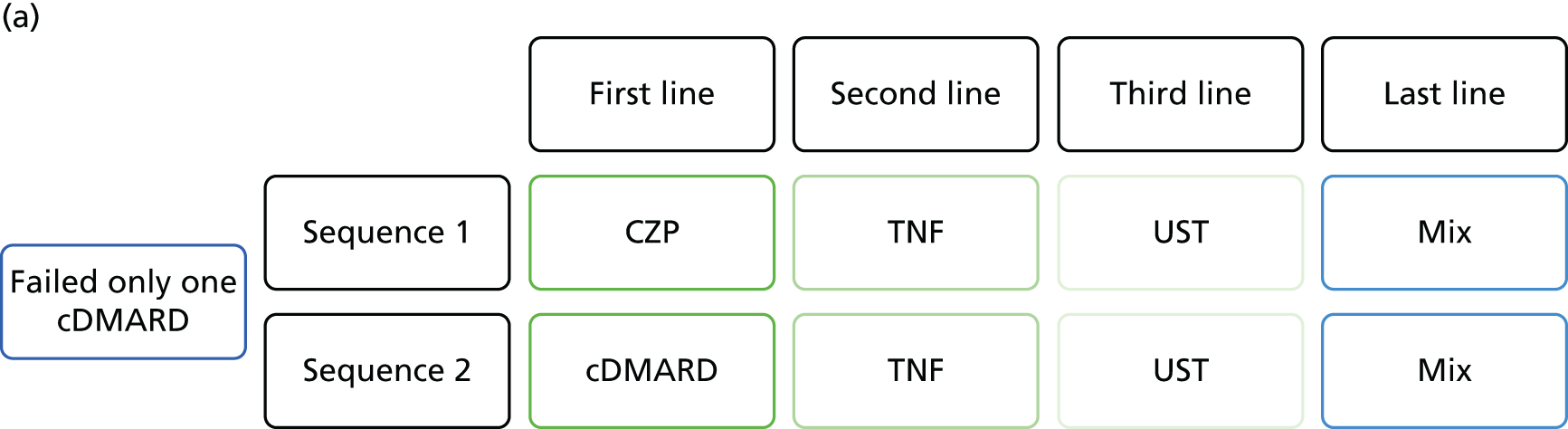
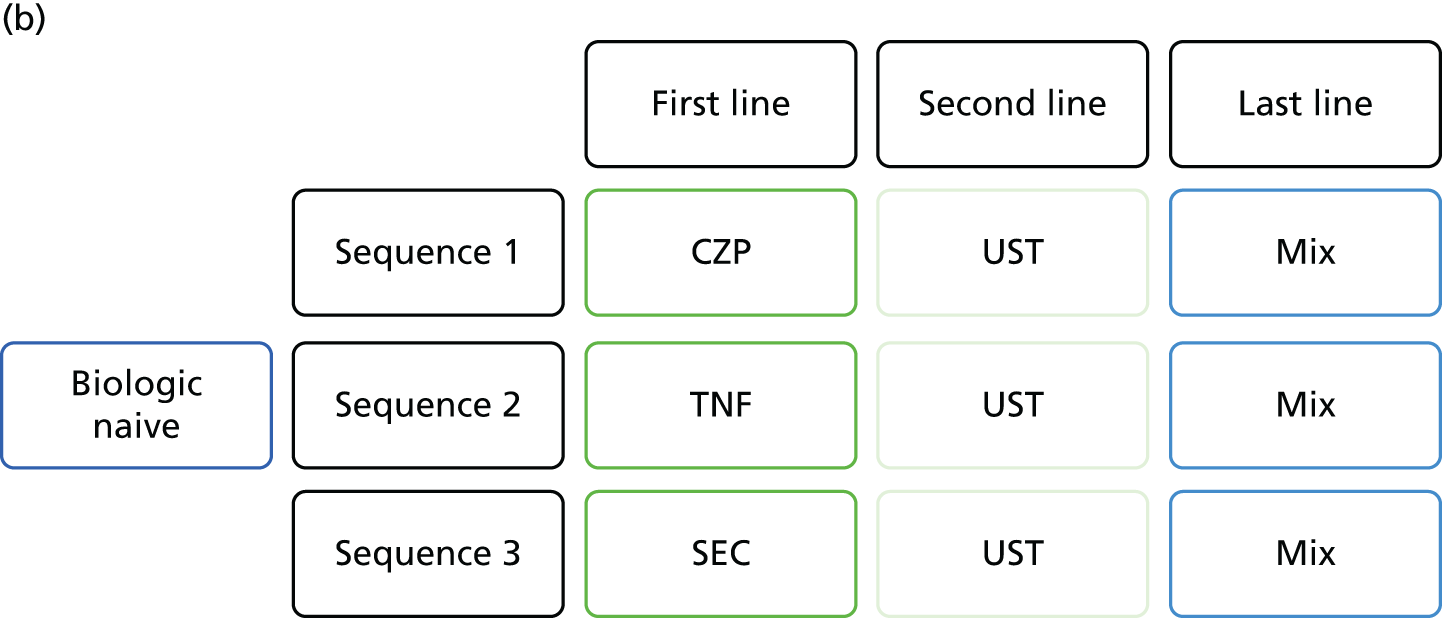
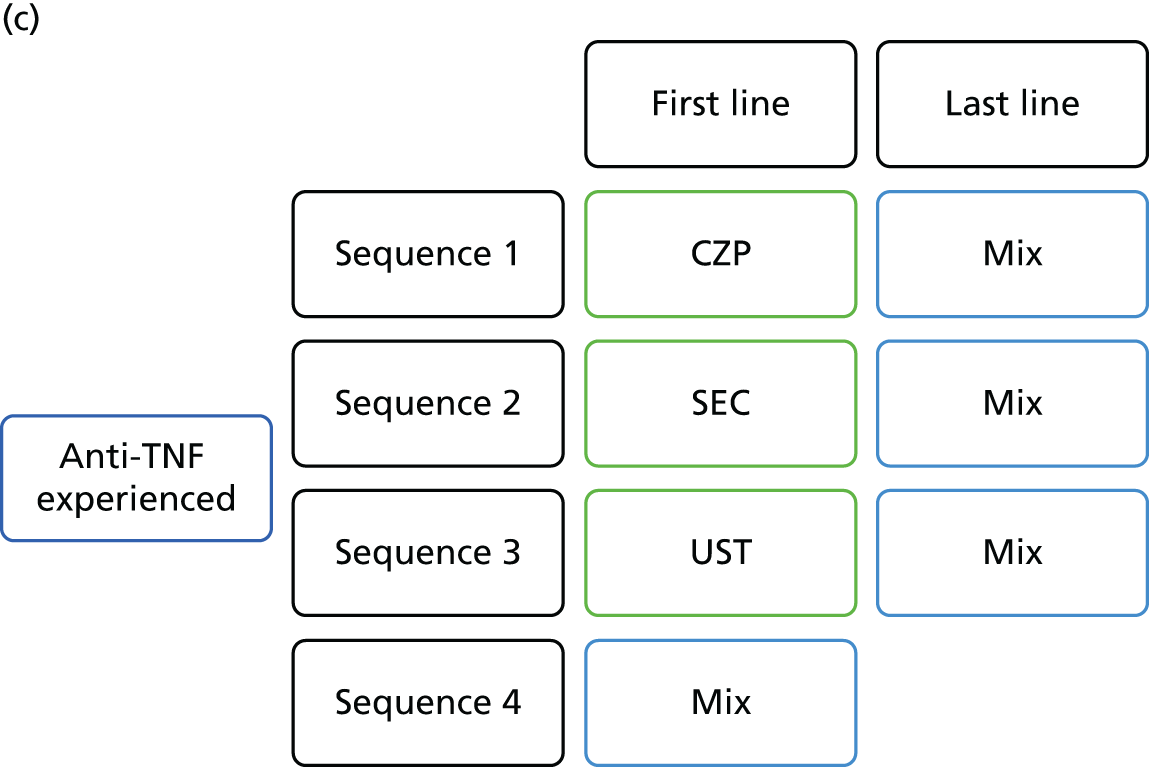
FIGURE 14.
Interventions and comparators according to subpopulations (Novartis).
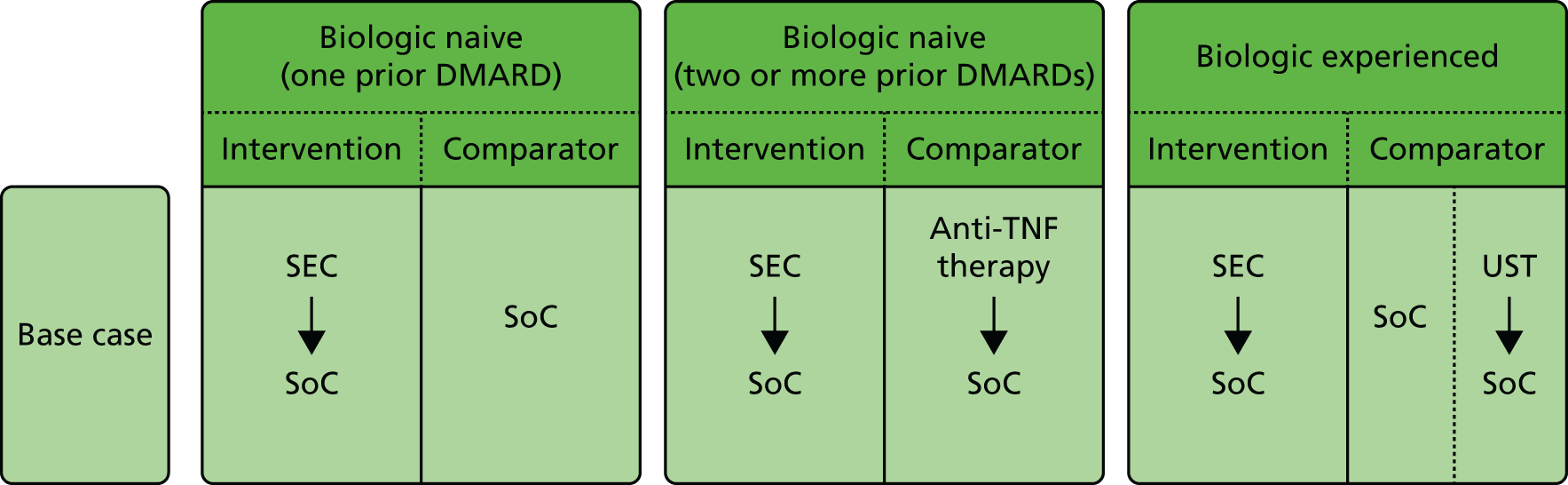
Subpopulation 1: biologic naive (one prior DMARD)
In the UCB Pharma model, two sequences are compared in subpopulation 1:
-
sequence 1: first line (CZP) → second line (TNF) → third line (UST) → last line (mix)
-
sequence 2: first line (cDMARD) → second line (TNF) → third line (UST) → last line (mix).
The sequences differ in terms of the first-line therapy (CZP or cDMARDs) and the subsequent lines of therapies (up to three further lines) in both sequences are assumed to be identical. Primary and secondary failures to first-line therapy are assumed to move onto a second-line treatment comprising a mixture of four TNF-α inhibitors (ETN, INF, ADA and GOL). The mixture of the four TNF-α inhibitors is modelled assuming an equal market share (25%) and costs and outcomes are estimated as the weighted sum. Following failure of the mixture of TNFs, patients are assumed to move onto UST as a third-line treatment before moving onto the last line (mix). The last line (mix) is defined as a mixture of cDMARDs (base case: MTX = 58.8%, leflunomide = 1.5%, sulfasalazine, 2.9% MTX sodium) and palliation (34.6%).
The UCB Pharma submission states that, although SEC is also a relevant comparator in this subpopulation (i.e. a third sequence starting with SEC), the lack of published clinical evidence specifically on the one prior DMARD subpopulation precluded SEC from being formally included.
In the Novartis model, the intervention assessed in subpopulation 1 is 150 mg of SEC and the comparator is SoC (defined as 100% use of MTX, dose 25 mg per week). Similarly, the lack of published clinical evidence specifically on the one prior DMARD subpopulation precluded CZP from being formally included in the Novartis submission. Following primary or secondary treatment failure of SEC, patients are assumed to move to SoC (MTX) without further biologic treatment.
Although 300 mg of SEC is the licensed dose for biologic-experienced patients with concomitant moderate–severe psoriasis, Novartis stated three reasons why the 300-mg dose was included for biologic-naive patients (subpopulations 1 and 2):
-
the use of 300 mg of SEC for moderate–severe psoriasis is already recommended based on a separate appraisal in this indication
-
no comparator data for biologic-naive PsA patients with concomitant moderate–severe psoriasis were reported to be available
-
the subgroup of biologic-naive patients with concomitant moderate–severe psoriasis in the FUTURE 2 trial48 was too small to appropriately inform model inputs.
Subpopulation 2: biologic naive (one or more prior DMARDs, UCB Pharma; two or more prior DMARDs, Novartis)
In the UCB Pharma model, three main sequences are compared in subpopulation 2:
-
sequence 1: first line (CZP) → second line (UST) → last line (mix)
-
sequence 2: first line (TNF) → second line (UST) → last line (mix)
-
sequence 3: first line (SEC) → second line (UST) → last line (mix).
The sequences start with CZP, other TNF-α inhibitors (ETN, INF, ADA and GOL) or SEC. In contrast to subpopulation 1, the four other TNF-α inhibitors are evaluated as alternative first-line treatments. Hence, sequence 2 actually comprises four separate sequences with ETN, INF, ADA or GOL specified as the first-line treatment. The six sequences assessed in subpopulation 2 are thus:
-
sequence 1: first line (CZP) → second line (UST) → last line (mix)
-
sequence 2: first line (ETN) → second line (UST) → last line (mix)
-
sequence 3: first line (INF) → second line (UST) → last line (mix)
-
sequence 4: first line (ADA) → second line (UST) → last line (mix)
-
sequence 5: first line (GOL) → second line (UST) → last line (mix)
-
sequence 6: first line (SEC) → second line (UST) → last line (mix).
Primary and secondary failures to first-line treatment are assumed to subsequently move onto UST before moving onto ‘mix’ (similarly defined as in subpopulation 1 as a mixture of cDMARDs and palliation).
The UCB Pharma model does not separately model the 150- and 300-mg doses of SEC for subpopulation 2. Instead, a single SEC sequence is modelled based on a weighted approach according to prevalence of moderate–severe plaque psoriasis in subpopulation 2 and assuming that 53.7% of patients would have a PASI score of > 10 units at baseline. The proportion used as the basis for weighting is referenced to an academic-in-confidence study and no further details are reported. The weighting is discussed only in the context of costing and, hence, it is unclear whether or not the efficacy estimates for SEC were similarly weighted.
In the Novartis model, the treatment assessed in subpopulation 2 is 150 mg of SEC and five TNF-α inhibitors (CZP, ETN, INF, ADA and GOL). Primary and secondary failures are assumed to subsequently move onto SoC without biologic therapy (100% use of MTX, dose 25 mg per week).
The Novartis submission also considers a separate scenario (exploratory analysis) for subpopulation 2 in which it is assumed that patients can move onto a mixed biologic therapy, prior to moving to SoC. The mixed biologic treatment therapy comprises a mix of all biologics other than that received at first line. This mixed strategy is assigned a weighted average efficacy, costs and AE incidence rates. The weights assumed are not formally specified, but appear to be based on a similar approach to that taken by UCB Pharma (i.e. assuming each has the same market share). Two scenarios were considered in which either the same first-line efficacy is assumed for the mixed biologic therapy or a 20% decline in efficacy for HAQ-DI, PsARC and PASI response while on second-line therapy.
Available biosimilars for ETN and INF are also included in the two submissions as part of separate scenario analyses.
Subpopulation 3: biologic experienced
In the UCB Pharma model, four sequences are compared in subpopulation 3:
-
sequence 1: first line (CZP) → last line (mix)
-
sequence 2: first line (300 mg of SEC) → last line (mix)
-
sequence 3: first line (UST) → last line (mix)
-
sequence 4: first line (mix).
In common with the other subpopulations, the sequences for subpopulation 3 differ in terms of the first-line therapy (CZP, 300 mg of SEC, UST or mix), and the subsequent line of therapy (mix – comprising a mixture of cDMARDs and palliative care) is assumed to be identical. The SEC sequence is modelled based on the 300-mg dose in accordance with the licensed dose for biologic-experienced patients.
In the Novartis model, the intervention assessed in subpopulation 3 is 300 mg of SEC, and UST and SoC are included as separate comparators. The Novartis submission does not discuss why CZP is not included as a separate comparator for subpopulation 3. Following primary or secondary treatment failure of SEC or UST, patients are assumed to move to SoC without further biologic treatment (i.e. MTX).
Patient characteristics
The UCB Pharma submission uses the RAPID-PsA trial47 and specifies different baseline characteristics for the three subpopulations. In the Novartis submission, baseline characteristics were reported to be similar across subgroups in the FUTURE 2 trial48 and, hence, the same values were assigned to all patient characteristics apart from PASI score.
Tables 66 and 67 report the values applied in the two company models. The subpopulations are broadly similar in terms of age and weight; however, there are some differences in terms of baseline HAQ-DI and PASI scores assumed across the separate models. The UCB Pharma submission applies an increasing baseline mean HAQ-DI score across subpopulations 1–3, which contrasts with the same HAQ-DI score applied across the three subpopulations in the Novartis submission. There appears to be more variation in the baseline PASI scores between the submissions, with mean PASI scores assumed to be > 10 units and ≤ 10 units, respectively, in the UCB Pharma and Novartis submissions for each of the subpopulations.
| Feature | Subpopulation | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Age (years), mean | Confidential information has been removed | 47 | 49 |
| % female | Confidential information has been removed | 55.6 | 53.8 |
| Weight (kg), mean (SD) | Confidential information has been removed | 84 (18) | 87 (20) |
| HAQ-DI score (units), mean | Confidential information has been removed | 1.29 | 1.37 |
| PASI score (units), mean | Confidential information has been removed | 11.58 | 12.04 |
| Feature | Subpopulation | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Age (years), mean | 47.96 | 47.96 | 47.96 |
| % female | 51.6 | 51.6 | 51.6 |
| Weight (kg), mean (SD) | 87.11 (19.66) | 87.11 (19.66) | 87.11 (19.66) |
| HAQ-DI score (units), mean | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| PASI score (units), mean | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
The differences in the mean PASI scores appear to be an important source of variation between the two submissions. By assuming a mean PASI score of > 10 units, the UCB Pharma base-case results relate to an ‘average’ PsA patient with concomitant moderate–severe psoriasis (i.e. ≥ 3% of BSA affected and a PASI score of > 10 units). In contrast, the Novartis base-case results relate to an ‘average’ PsA patient with concomitant mild–moderate psoriasis (≥ 3% of BSA affected and a PASI score of ≤ 10 units). These differences are likely to have an impact on subsequent costs and outcomes, most importantly in terms of the appropriate dosing and costs assumed for SEC (i.e. 150 or 300 mg depending on the presence and severity of concomitant psoriasis) in the naive subpopulations (i.e. subpopulations 1 and 2).
The UCB Pharma submission presents separate deterministic sensitivity analyses based on different PASI scores. These sensitivity analyses were presented for two alternative baseline PASI scores (0 and 12.5 units). These sensitivity analyses essentially reflect separate subgroups without concomitant psoriasis (mean PASI score = 0 units), and a subgroup with concomitant moderate–severe psoriasis (mean PASI score = 12.5 units). The Novartis model does not present separate subgroup results or sensitivity analyses in relation to the baseline PASI score.
Given that PASI is directly observable and because the severity of concomitant psoriasis means that different SEC dosages are appropriate for the separate subgroups (i.e. 150 mg of SEC for naive patients without concomitant psoriasis or with concomitant mild–moderate psoriasis and 300 mg of SEC for experienced patients and for naive patients with concomitant moderate–severe psoriasis), it would appear more appropriate for both companies to have more explicitly modelled the three specific subgroups within each of the subpopulations as opposed to assuming a single ‘average’ PsA patient or cohort. These three subgroups are:
-
PsA without concomitant psoriasis
-
PsA with concomitant mild–moderate psoriasis (≥ 3% of BSA and a PASI score of ≤ 10 units)
-
PsA with concomitant moderate–severe psoriasis (≥ 3% of BSA and a PASI score of > 10 units).
Withdrawal from treatment and the natural history of psoriatic arthritis
Following treatment failure and withdrawal, the Novartis submission assumes that patients’ HAQ-DI and PASI scores will revert to the original baseline values, which is consistent with the ‘rebound equal to gain’ approach previously applied in the York model. In contrast, the UCB Pharma submission assumes that the HAQ-DI score trajectory of patients switching to a subsequent treatment initially rebounds to a higher (i.e. worse) HAQ-DI value than the original baseline. The value assumed for rebound is equal to the baseline value plus the HAQ-DI score change for the previous treatment’s PsARC non-responders. Furthermore, when switching from the second to the third line of treatment, this rebound increases further, representing the baseline plus the previous two treatments’ change in HAQ-DI score for non-PsARC responders. For example, in a treatment sequence addressing subpopulation 1, the baseline HAQ-DI score is assumed to be (confidential information has been removed); on switching to the second line of treatment, this initially increases to (confidential information has been removed), and increases further to (confidential information has been removed) and (confidential information has been removed). The UCB Pharma submission does not include any discussion or justification for this approach.
The natural progression of PsA (i.e. in the absence of biologic treatments), in terms of increasing the HAQ-DI score, is reflected in both models using the approach adopted in the York model. The two models assume that the HAQ-DI score linearly increases over time by 0.018 units every 3 months until it reaches the maximum, 3 units. This increasing HAQ-DI score is applied in conventional treatment arms of both models and to patients who subsequently move on to conventional (i.e. non-biologic) treatment.
Both the UCB Pharma and Novartis models consider the possibility that patients who initially respond to treatment may subsequently withdraw from treatment in the longer-term model. Based on safety and tolerability data from the FUTURE 1 and 2 trials (see Chapter 4),46,48 the Novartis submission derived the discontinuation rates for patients receiving 150 and 300 mg of SEC. This was (confidential information has been removed) and (confidential information has been removed) for the first year and (confidential information has been removed) and (confidential information has been removed) for subsequent years (applied until the end of the model). These values were used for all comparators in the base case and alternative values were examined in sensitivity analysis, in which withdrawal rate values were derived from different trials (Table 68 shows these values).
| Time point | Treatment, annual discontinuation rate (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 150 mg of SEC | 300 mg of SEC | CZP | ETN | ADA | INF | GOL | UST | |
| Year 1 | Confidential information has been removed | Confidential information has been removed | 15.1 | 15.6 | 15.0 | 13.9 | 29.5 | 26.1 |
| Year 2+ | Confidential information has been removed | Confidential information has been removed | 15.1 | 15.6 | 15.0 | 8.5 | 29.5 | 11.3 |
The UCB Pharma model assumes an annual discontinuation rate of 16.5% for all biologic treatments. This figure is consistent with the assumption and data used to inform the York model. A further assumption was also included in the UCB Pharma model such that if a patient continued on a therapy for at least 48 months there would be no risk of longer-term withdrawal beyond this time point. This assumption was justified as a result of the lack of data reporting long-term withdrawal rates.
Sources and synthesis of effectiveness
The main clinical outcomes included in the company models were PsARC and PASI (50, 75 and 90) response, and HAQ-DI score changes conditional on PsARC response. The sources and assumptions of the effectiveness evidence used in the base case of each of the economic models are summarised in detail in Appendix 6. A brief overview is provided below and is specifically focused on the relationship between the meta-analyses undertaken by each company and the specific inputs and assumptions applied to each subpopulation within the economic models.
Subpopulation 1: biologic naive (one prior DMARD)
For the biologic-naive (one prior DMARD) subpopulation, both companies used the results from post hoc subgroup analyses of the naive subgroup (one prior DMARD) from either the RAPID-PsA (UCB Pharma)47 or FUTURE 2 (Novartis)48 trials to inform PsARC and PASI responses and conditional HAQ-DI scores.
Subpopulation 2: biologic naive (one or more prior DMARDs, UCB Pharma; two or more prior DMARDs, Novartis)
The PsARC and PASI responses were derived directly from the estimates of the separate NMAs undertaken by each company. The patients used for subpopulation for each NMA differed. The UCB Pharma estimates were derived from a NMA based on trials (or relevant subgroups) of biologic-naive patients only. In the absence of subgroup data for SEC for biologic-naive patients, UCB Pharma included a separate assumption that the effectiveness of SEC (confidential information has been removed). In contrast, Novartis used the results from its NMA based on the overall population (i.e. including both naive and experienced patients for some trials) results for all treatments.
A variety of different sources and assumptions were used to inform HAQ-DI change scores, including results from the NMA, external published estimates and assumptions.
Subpopulation 3: biologic experienced
There were important differences in the approaches and assumptions used by each company for subpopulation 3. The UCB Pharma model included PsARC and PASI response estimates for CZP and SoC directly from a subgroup of biologic-experienced patients from the RAPID-PsA trial47 and then applied separate assumptions for 300 mg of SEC and UST. In contrast, Novartis assumed a common reduction in the efficacy of biologic-experienced patients based on a comparison between biologic-naive and biologic-experienced subgroups in the FUTURE 2 trial. The efficacy reductions were subsequently applied to the all-population NMA. The following reductions were applied:
-
PsARC reduced by (confidential information has been removed)%
-
PASI 50–74 reduced by (confidential information has been removed)%
-
PASI 75–89 reduced by (confidential information has been removed)%
-
PASI 90–99 reduced by (confidential information has been removed)%.
For HAQ-DI change scores, the UCB Pharma model derived data for CZP and SoC directly from the biologic-experienced subgroup of the RAPID-PsA trial47 and used separate assumptions for UST and 300 mg of SEC. Novartis assumed the same change scores as applied to subpopulation 2.
Sources of utility data
The two manufacturers’ submissions present separate utility algorithms derived from patient data in the FUTURE248 (Novartis) and RAPID-PsA47 (UCB Pharma) trials. These algorithms are estimated to determine the independent contribution of HAQ-DI and PASI scores to health utilities.
Table 69 shows the parameters used in each submission, alongside the values used in the York model. The Novartis algorithm, in addition to HAQ-DI and PASI, also includes age, sex and the baseline utility as explanatory variables, together with the response status for anti-TNF treatment. This implies that a different algorithm was defined according to PsARC response status. The algorithm also accounts for the decline in utility over time by including age as a covariate. Both submissions also used the algorithm adopted by the York model within a separate scenario analysis. The UCB Pharma and York algorithms are broadly consistent; however, the Novartis algorithm predicts a much smaller coefficient for HAQ-DI score (–0.172 units as opposed to –0.298 units in the York algorithm and –0.258 units in the UCB Pharma model). This implies a much smaller utility decrement for a unit increase in HAQ-DI score.
| Parameter | Submission | York model (SE) | |
|---|---|---|---|
| Novartis: FUTURE 248 (SE) | UCB Pharma: RAPID-PsA47 (SE) | ||
| Intercept | Confidential information has been removed | Confidential information has been removed | 0.897 (0.006) |
| HAQ-DI score | Confidential information has been removed | Confidential information has been removed | –0.298 (0.006) |
| PASI total score | Confidential information has been removed | Confidential information has been removed | –0.004 (0.0003) |
| EQ-5D coefficient | Confidential information has been removed | N/A | N/A |
| Anti-TNF therapy status (anti-TNF naive was used as the reference for anti-TNF therapy status) | |||
| Inadequate responder | Confidential information has been removed | N/A | N/A |
| Sex (female was used as the reference) | |||
| Male | Confidential information has been removed | N/A | N/A |
| Age (years) | Confidential information has been removed | N/A | N/A |
Summary of resource utilisation and costs data
In both models, resource use and costs were categorised in terms of drug acquisition, administration and monitoring and associated health state costs (i.e. according to HAQ-DI and PASI scores). In both models, it was assumed that DMARDs were used concomitantly with all biologic treatments (58% using MTX in the UCB Pharma model and 100% using MTX in the Novartis model). AE costs were included only in the Novartis model.
Drug acquisition costs
Both models estimated the acquisition costs for CZP based on the Patient Access Scheme currently under approval. There were differences in the approaches and costs used by the companies for SEC. In the Novartis model, the acquisition costs for 150 and 300 mg of SEC were based on the Patient Access Scheme for SEC. The Novartis model also evaluated only the 300-mg dose for the biologic-experienced subpopulation and the 150-mg dose for subpopulations 1 and 2 for reasons previously outlined. In the UCB Pharma model, the acquisition costs for SEC were based on the list prices and a weighted cost was estimated for subpopulations 1 and 2 based on the 150- and 300-mg doses, based on the proportion of patients assumed to have concomitant moderate–severe psoriasis.
Both companies used national list prices [British National Formulary (BNF)139 and an online and print prescribing database for health professionals (MIMS)] for other comparators and incorporated existing Patient Access Schemes for UST and GOL. In addition, both companies used a similar approach to estimating acquisition costs for INF by assuming a normal distribution of weights to determine the required number of vials based on patient-level data in the FUTURE 248 [mean 87.11 kg, standard deviation (SD) 19.66 kg] and RAPID-PsA47 (mean 84.34 kg, SD 18.77 kg) trials. The drug acquisition costs for biosimilars in both submissions were sourced from MIMS (in 2016) and were approximately 90% of the price of the originator product.
Drug administration and monitoring costs
In terms of drug administration costs, the Novartis model assumed a half-day inpatient visit for each infusion for INF (£326.46). For all other (subcutaneously administered) biologics, resource use associated with administration was based on a single 30-minute session with a specialist community nurse in the first 3-month period in order to train patients in self-administration (£37.50). No administration costs were assumed for MTX.
In contrast, the UCB Pharma model assumed a cost of £159 for each infusion for INF based on the cost of delivering a simple parenteral chemotherapy (first attendance). For all other (subcutaneously administered) biologics and MTX, the UCB Pharma model assumed a cost of £43 based on the cost of a 1-hour nurse visit at a GP practice.
Although the two submissions included the same laboratory tests for monitoring PsA patients, there were differences in the costs that are applied for these. In the UCB Pharma submission, monitoring costs were defined as laboratory tests and estimated at £117.60 for the first 3 months and £21 for the subsequent 3 months. The monitoring costs for biologics applied in the Novartis model were lower, at £79 for the first 3 months and £4.20 for the subsequent 3 months.
Adverse events
Only the Novartis submission included the resource costs of AEs. These comprised the costs of TB reactivation (£3054) and other serious infections (£1527), based on the approach used for a separate NICE appraisal for ankylosing spondylitis (see TA383104).
Health Assessment Questionnaire-Disability Index and Psoriasis Area and Severity Index costs
In the Novartis submission, HAQ-DI and PASI costs were estimated using the same approach as the York model (uprated to 2016 costs). Table 70 shows the inputs used by Novartis and the previous estimates used in the York model.
| Input | Cost (£) | Unit | |
|---|---|---|---|
| York model | Novartis model | ||
| Intercept | 233 | 255.78 | Per 3 months |
| Cost per HAQ-DI score change | 103 | 113.07 | Per 1-unit change per 3 months |
| Health states | |||
| Uncontrolled psoriasis (PASI < 75 units) | 198 | 217.36 | Per 3 months |
| Controlled psoriasis (PASI ≥ 75 units) | 16 | 17.56 | Per 3 months |
In the UCB Pharma submission, health state costs for HAQ-DI and PASI were derived from a separate study by Poole et al. 138 The Poole et al. 138 study utilised data from a sample of PsA patients from the BSRBR to develop a multivariate model estimating disease severity from parameters routinely available in primary care data. The multivariate model was subsequently applied to routine data from The Health Improvement Network (THIN) to link to treatment and resource costs. These costs include costs of drugs, contacts with a GP and other health-care professionals, tests, hospital outpatient attendances and inpatient admissions. The relationship between disease severity and costs, based on HAQ-DI score, was then estimated using a generalised linear model. Table 71 shows the coefficients from the generalised linear model.
| Coefficient | Mean | SE |
|---|---|---|
| Intercept | 3.537 | 0.010 |
| HAQ-DI coefficient | 2.048 | 0.006 |
| Age coefficient | 0.026 | 0.000 |
| Interaction coefficient, for interaction between HAQ-DI and age | −0.012 | 0.000 |
Annual costs applied in the model were estimated using the following regression:
An adjustment was applied in the UCB Pharma model to avoid double counting prescription costs, which accounted for 38% of the total costs in the Poole et al. 138 study. Hence, HAQ-DI costs were assumed to be 62% of the total costs. The final costs were then uprated to 2015 values.
The UCB Pharma submission stated that, since the costs from Poole et al. 138 included all medical resource use for PsA patients, adding additional PASI-related costs would result in double counting. Consequently, PASI-related costs were not included in the model base case. A sensitivity analysis including PASI-related costs was undertaken based on the method used in the York model, with costs uprated to 2015 values.
Cost-effectiveness results from the company submissions
Subpopulation 1: biologic naive (one prior DMARD)
The base-case (deterministic) results for subpopulation 1 are reported in Tables 72 (UCB Pharma model) and 73 (Novartis model). The UCB Pharma model reports an ICER of £23,666 per QALY based on the comparison of a sequence starting with CZP and a separate sequence starting with cDMARDs. The Novartis model reports an ICER of £12,189 per QALY based on a comparison of 150 mg of SEC versus SoC. Neither company included both CZP and SEC as relevant comparators in this subpopulation and hence direct comparisons of CZP and SEC are not possible in this subpopulation.
| Treatment | Total cost (£) | Total QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| cDMARDs | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | – |
| CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 23,666 |
| Treatment | Total costs (£) | Total QALYs | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| SoC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | – |
| 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 12,189 |
There appear to be large differences in the total costs and QALYs reported for the comparator treatment across the separate models. This may be partly explained by the different model time horizons (50 years in the UCB Pharma model and 40 years in the Novartis model), the inclusion of subsequent lines of biologic therapy and the different sources of cost data for HAQ-DI and PASI. The UCB Pharma submission reports higher incremental costs and QALYs for CZP relative to the comparator treatment than does the Novartis submission for 150 mg of SEC.
Subpopulation 2: biologic naive (one or more prior DMARDs, UCB Pharma; two or more prior DMARDs, Novartis)
The base-case (deterministic) results for subpopulation 2 are reported in Tables 74 (UCB Pharma model) and 75 (Novartis model). The UCB Pharma model reports that CZP dominates all the other treatments, including SEC. In contrast, the Novartis model reports that 150 mg of SEC dominates all the other treatments with the exception of SoC (less costly and less effective than 150 mg of SEC) and INF (more costly and more effective than 150 mg of SEC). The ICER of 150 mg of SEC versus SoC is reported in the Novartis submission to be £10,549 per QALY and the ICER of INF versus 150 mg of SEC is £220,558 per QALY.
| Treatment | Total cost (£) | Total QALYs | Incremental costs vs. next least costly intervention (£) | Incremental QALYs vs. next least costly intervention | ICER vs. next least costly intervention (£) |
|---|---|---|---|---|---|
| CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | – |
| ADA | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Dominated |
| GOL | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Dominated |
| ETN | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Dominated |
| SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Dominated |
| INF | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Dominated |
| Treatment | Total cost (£) | Total QALYs | Incremental costs vs. SoC (£) | Incremental QALYs vs. SoC | ICER vs. SoC (QALYs) (£) | ICER vs. next least costly intervention (£) |
|---|---|---|---|---|---|---|
| SoC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | – | – |
| 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 10,549 | 10,549 |
| CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 28,432 | Dominated by SEC |
| ETN | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 31,280 | Dominated by SEC |
| GOL | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 33,802 | Dominated by SEC |
| ETN | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 32,706 | Dominated by SEC |
| INF | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 53,223 | 220,558 |
As both companies included both CZP and SEC as relevant comparators in this subpopulation, a direct comparison between the submissions is possible for subpopulation 2. Both companies report their own treatment to be the most cost-effective treatment at conventional cost-effectiveness thresholds and both report that their specific treatment dominates the other. The contrasting conclusions could arise from several important differences previously noted, including (1) different NMA approaches (i.e. the use of 24-week data by UCB Pharma in the base case vs. 12- to 16-week data from Novartis); (2) different acquisition costs and dosages assumed for SEC (weighted estimate for SEC based on list price costs of 150 mg of SEC and 300 mg of SEC in the UCB Pharma submission vs. Patient Access Scheme price for 150 mg of SEC assumed in the Novartis submission); (3) inclusion of subsequent lines of biologic therapy in the UCB Pharma submission; and (4) different sources of cost data for HAQ-DI and PASI and different model horizons.
As the UCB Pharma model did not present comparisons against a strategy of no biologic therapy, it is difficult to determine the external validity of the results presented for the comparator treatments. In contrast, the Novartis submission presents both fully incremental ICERs and pairwise ICERs versus SoC. The presentation of the pairwise ICERs versus SoC provides an important basis to consider issues of cross-validation based on the consistency of the findings for the comparator treatments and those reported from the broader comparator review presented earlier in Chapter 5. It is notable that the ICERs reported for the comparator treatments (ADA, ETN, GOL and INF) in the Novartis submission appear higher (i.e. less favourable) than reported in previous studies. Indeed, none of these comparator treatments would appear to be cost-effective versus SoC at conventional cost-effectiveness thresholds. The reason for this difference and implications in terms of external validity is not discussed in the Novartis submission.
Subpopulation 3: biologic experienced
The base-case (deterministic) results for subpopulation 3 are reported in Tables 76 (UCB Pharma model) and 77 (Novartis model). The UCB Pharma model reports that CZP dominates UST and 300 mg of SEC. The least costly and least effective non-dominated treatment in the UCB Pharma model is mix (i.e. a mixture of cDMARDs and palliative care). The ICER of CZP versus mix is reported to be £8894 per QALY. In contrast, the Novartis model reports that 300 mg of SEC extendedly dominates CZP and UST. The ICER of 300 mg of SEC versus SoC is reported to be £27,562 per QALY.
| Treatment | Total cost (£) | Total QALYs | Incremental costs vs. next least costly alternative (£) | Incremental QALYs vs. next least costly intervention | ICER vs. next least costly intervention (£) |
|---|---|---|---|---|---|
| Mix | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | – |
| CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 8894 |
| UST | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Dominated by CZP |
| 300 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Dominated by CZP |
| Treatment | Total cost (£) | Total QALYs | Incremental costs vs. SoC (£) | Incremental QALYs vs. SoC | ICER vs. SoC (QALYs) (£) | ICER vs. next least costly intervention (£) |
|---|---|---|---|---|---|---|
| SoC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | – | – |
| CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 29,538 | Extendedly dominated |
| UST | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 37,228 | Extendedly dominated |
| 300 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | 27,562 | 27,562 |
Similar to the conclusions reported for subpopulation 2, both companies report their own treatment to be the most cost-effective treatment at conventional cost-effectiveness thresholds and both report that their specific treatment either dominates (UCB Pharma model) or extendedly dominates (Novartis model) the other.
The Novartis submission, again, presents both fully incremental ICERs and pairwise ICERs versus SoC for subpopulation 3. Although pairwise comparisons versus the non-biologic comparator (mix) are not presented in the UCB Pharma submission, these can be estimated for UST versus mix from the data reported in its ICER results table. As with subpopulation 2, these provide an opportunity to consider issues of cross-validation in terms of the consistency of findings for one of the comparator treatments (UST) considered in the broader review. The ICER for UST versus SoC is reported to be £37,228 per QALY in the Novartis submission, indicating that UST is not cost-effective compared with SoC at conventional cost-effectiveness thresholds. Again, this appears inconsistent with previous studies reporting the cost-effectiveness of UST in a biologic-experienced population, and the reasons and possible implications in terms of external validity are not discussed in the Novartis submission. One possible explanation is the different approaches used in the Novartis submission for the experienced population (i.e. applying a common reduction in the efficacy rate to all treatments based on a comparison between the biologic-naive and biologic-experienced subgroups based on the FUTURE 2 trial48 data, as opposed to using the actual subgroup data reported for UST). The pairwise comparison for UST versus mix, estimated from the results presented in the UCB Pharma results table, results in an ICER of £28,068 per QALY. This appears reasonably consistent with the ICER reported in TA34035 for UST (£25,393 per QALY).
Relevance of submitted cost-effectiveness evidence for National Institute for Health and Care Excellence decision-making: summary and motivation for de novo model
The CSs are the only studies that directly assess the decision problem in relation to the new interventions [i.e. the positioning of these treatments within the pathway for PsA (biologic-naive and biologic-experienced populations)]. Although the studies, in relation to the broader comparators, are helpful in terms of highlighting similarities and possible differences between the approaches being applied by the separate companies and those previously used for previous TA appraisals, they are not directly relevant to the evaluation of SEC and CZP.
In general, the structure and approaches of both models were similar in many key respects to the York model conducted for TA19933 (ETN, ADA and INF). The main differences were:
-
The timing of the initial response period was assumed to be 24 weeks in the UCB Pharma submission and 3 months (i.e. 12–16 weeks) in both the Novartis submission (with the exception of UST) and the York model. The justification provided by Novartis for assuming 3 months for the initial response period was to ensure consistency with previous NICE appraisals and BSR/British Health Professionals in Rheumatology guidelines and to maximise the data included in the NMA. UCB Pharma justified the 24-week period based on 2011 EULAR guidelines, although results were also reported as part of separate sensitivity analysis assuming a 3-month response period.
-
The definition of response in the Novartis base case (PsARC and PASI) differed from that used in the base-case approaches by both UCB Pharma and the previous York model (PsARC only). The Novartis submission presented a separate sensitivity analysis assuming that response was assessed using just PsARC, and this reported only minor differences from its base case.
-
The UCB Pharma base case focused on sequences and the incorporation of subsequent lines of treatments as opposed to presenting this as a separate exploratory scenario (Novartis and York models).
-
In common with the York model, the Novartis model assumed that the HAQ-DI score gain reported at 3 months was the maximum reduction achieved on treatment and assumed no further change (i.e. increase or decrease) beyond this period for patients while they remained on this treatment. In contrast, the UCB Pharma model employed different assumptions during the initial 9-month treatment (i.e. that the highest rate of change is obtained at 4 weeks, but further improvements in HAQ-DI score are possible during a period of 9 months for a responding patient who remains on treatment). After 9 months, the UCB Pharma model assumed no further change beyond this period for patients while they remained on this treatment.
-
Assumptions related to the rebound effect on HAQ-DI score following treatment withdrawal. The UCB Pharma submission assumes that a patient’s HAQ-DI score rebounds to a worse position than the original baseline value when they switch to the next treatment. Both the Novartis and York models assume that a patient’s HAQ-DI score rebounds to its original baseline value.
-
The Novartis and UCB Pharma submissions include additional subpopulations (subpopulations 1 and 3), based on the broader scope for the appraisal of SEC and CZP compared with the scope of TA199. 33
-
The Novartis submission estimates costs associated with HAQ-DI and PASI based on the same sources and assumptions previously used in the York model. In contrast, the UCB Pharma submission based costs on a separate study by Poole et al. 138 and justified this on the basis that the use of a PsA population was more appropriate than deriving costs based on a RA population and employing separate assumptions for PASI costs.
-
Although UCB Pharma assumed the same annual withdrawal rate as the York model (16.5% per annum), the UCB Pharma submission applied this only to the first 4 years of a treatment. Thereafter it was assumed that no patient would withdraw. This assumption was justified by UCB Pharma based on the lack of longer-term evidence reported for withdrawal. Novartis utilised withdrawal data from its trial population (FUTURE 2 trial48) and applied a (confidential information has been removed) per annum rate for the first year and (confidential information has been removed) for subsequent years.
-
By assuming a mean PASI score of > 10 units, the UCB Pharma base-case results relate to an ‘average’ PsA patient with concomitant moderate–severe psoriasis (i.e. ≥ 3% of BSA affected and a PASI score of > 10 units). In contrast, the Novartis base-case results relate to an ‘average’ PsA patient with concomitant mild–moderate psoriasis (≥ 3% of BSA affected and a PASI score of ≤ 10 units), similar to the base case in the York model. Both the UCB Pharma and York model also presented separate sensitivity analyses based on different PASI scores, which reflected subgroups of PsA patients without concomitant psoriasis and with concomitant moderate–severe psoriasis. Separate sensitivity and scenario analyses were not presented in the Novartis submission.
-
The time horizon was assumed to be 40 years in the Novartis and York models and 50 years in the UCB Pharma model.
As highlighted in Results, drawing robust conclusions from the results reported from the separate companies is challenging given the differences noted in the approaches and data sources employed. Comparisons in subpopulation 1 are not possible as neither company included the other treatment in their comparisons. The difficulty of comparing results across subpopulations 2 and 3 are further hampered by the different assumptions made concerning the dosage of SEC included and in both subpopulations 2 and 3 based on the use of list prices for SEC in the UCB Pharma submission and Patient Access Scheme prices in the Novartis submission.
Assessments of cross-validity were possible for subpopulations 2 and 3 based on the Novartis results presented for comparator treatment and those reported in previous studies. The results from the Novartis model did not appear consistent with the cost-effectiveness reported for the comparator treatment assessed in previous NICE TAs (see TA199,33 TA220133 and TA34035). A discussion of possible reasons for this difference was not provided in the Novartis submission. An assessment of cross-validity was possible only in terms of subpopulation 3 for the UCB Pharma submission. Here the reported ICER appeared reasonably consistent for the main comparator treatment (UST) and the ICER reported in the previous NICE TA (TA34035).
Given the different approaches and assumptions employed by the companies, there remains considerable uncertainty regarding the cost-effectiveness of both SEC and CZP in each of the subpopulations and potential implications for the NHS. These differences make it challenging to draw robust conclusions from the current submissions, particularly given the contradictory findings reported for several of the subpopulations in terms of the relative cost-effectiveness of SEC and CZP. Furthermore, neither company incorporated the full range of interventions and comparators as stated in the NICE scope112 across all three subpopulations. The following chapter describes the development of a de novo model that attempts to address several areas of remaining uncertainty and to apply a consistent basis for evaluating the cost-effectiveness of the full range of interventions and comparators as stated in the NICE scope112 across all three subpopulations.
Chapter 6 Independent economic assessment
Introduction
The review of published models, and the CSs, show that the underlying structure used to model the cost-effectiveness of treatments for PsA has remained largely unaltered since the previous York model for TA199. 33 Despite the similarity observed across studies in terms of the model structure, important differences were identified in terms of associated assumptions and data sources. None of these can be considered unequivocally superior to the others; however, there are a number of issues with each of the currently available models (see Chapter 5, Relevance of submitted cost-effectiveness evidence for National Institute for Health and Care Excellence decision-making: summary and motivation for de novo model).
In terms of the previous York model, this does not consider all of the subpopulations defined in the NICE scope112 for this assessment. Currently available guidance, issued by NICE on the use of biologics in PsA,140 recommends that patients try two cDMARDs over a 6-month period before they can be considered for biologic treatment in accordance with current BSR guidelines. However, as defined in the NICE scope for this appraisal, three subpopulations need to be considered:
-
subpopulation 1 (biologic naive, one prior DMARD)
-
subpopulation 2 (biologic naive, two or more prior DMARDs)
-
subpopulation 3 (biologic experienced or contraindicated).
The two CSs consider these three subpopulations in their economic models; however, neither includes the full range of relevant treatments for all of the subpopulations and neither specifically considers patients contraindicated to existing biologic treatments.
In modelling the cost-effectiveness of available treatments, it is also important to consider the possibility that patients may switch to another active treatment, following primary failure (non-response) or secondary withdrawal (initial response with later withdrawal due to AE or loss of efficacy). Therefore, a key objective of the de novo model is to assess the cost-effectiveness of SEC and CZP for PsA within possible sequences of available treatments.
Methods
Overview
A decision-analytic model was developed to estimate the cost-effectiveness of SEC and CZP compared with other relevant comparators, including ETN, INF, ADA, GOL, UST and BSC for the treatment of adult PsA. BSC is defined as a mix of cDMARDs and usual care (see Choice of intervention and comparators). A different set of comparators are defined according to each subpopulation of interest (see Patient characteristics).
The cost-effectiveness model takes the form of a Markov cohort model with 3-monthly cycles, developed using R programing language (see Appendix 7 for the full model code). A lifetime horizon (40 years) is assumed. A half-cycle correction was not applied as the cycle length is 3 months, which is relatively short and, therefore, half-cycle correction is unlikely to be required. 119
Although the model shares a number of important characteristics with the previous York model, several significant changes have also been implemented. These include:
-
The base-case model attempts to replicate ‘real-world’ clinical practice, in terms of incorporating subsequent biologic treatments following a primary lack of response or secondary failure. Ignoring these subsequent treatment lines and/or assuming patients move directly onto BSC following failure of an initial biologic treatment, could result in overly optimistic estimates of cost-effectiveness of new (and more effective) interventions. This may arise because the consequences of treatment failure are likely to be overstated compared with real-world clinical practice, as additional treatment options remain which are more cost-effective than BSC alone. Although exploratory scenarios were considered in the previous York model in relation to treatment sequences, the formal inclusion of further lines of treatment within the base model necessitated significant amendments to the previous R code.
-
The model now includes the three subpopulations specified in the NICE scope112 for this appraisal.
-
Rather than presenting a single base case reflecting an ‘average’ PsA patient, heterogeneity in terms of baseline PASI score is now formally addressed by presenting results for three distinct subgroups within each subpopulation: (1) PsA without concomitant psoriasis; (2) PsA with concomitant mild–moderate psoriasis (≥ 3% of BSA and a PASI score of ≤ 10 units); and (3) PsA with concomitant moderate–severe psoriasis (≥ 3% of BSA and a PASI score of > 10 units). Differences in baseline PASI score were previously considered in the previous York model as part of a sensitivity analysis. However, as the decision problem differs across the specific subgroups as a result of the different licensed dosages of SEC, it was considered more appropriate to model these subgroups separately.
Outcomes are expressed using QALYs. 141 The QALY provides a summary measure combining estimates of the remaining length of life (life-years) and its associated quality. QALYs are derived from health-related utilities by multiplying a utility value (quality of life) by the time spent with this utility (length of life). Utility values are generated from the main clinical outcomes of the disease, HAQ-DI reflecting the arthritis component and PASI representing the psoriasis element (see Sources of utility data). These clinical scores (HAQ-DI = 0–3 units and PASI = 0–72 units) represent the health states of the model and are also associated with health-care resource use and costs (see Health state costs).
The parameters of the model were obtained from published literature, data reported in the CSs and the results of the evidence synthesis in Chapter 4. The model adopts a NHS and Personal Social Services perspective. A price year of 2016 is assumed and a 3.5% annual discount rate is applied to costs and QALYs. 125 Probabilistic sensitivity analysis (PSA) was conducted as is reported separately from the deterministic results.
Model structure and assumptions
Figure 15 illustrates the model structure. The structure remains largely unchanged since the previous York model (see Figure 8). However, in the updated York model, patients who withdraw from an initial treatment during cycle 1 because of a lack of response or as a result of AEs (or later cycles for patients who initially respond) are assumed to be eligible to receive further treatments prior to moving to BSC. The subsequent treatment lines are defined separately for each of the three subpopulations (see Choice of intervention and comparators).
FIGURE 15.
Overview of the model structure.

Patients enter the model and receive one of the treatments or BSC, relevant to each particular subgroup. Patients remain on treatment for 3 months (13 weeks), after which, if they respond, defined using PsARC, they continue on the treatment; otherwise they move to BSC or another biologic treatment, if the sequence allows.
The PsARC response data reported in the clinical trials (see Chapter 4) dichotomise patients into two groups: responders and non-responders (as a result of lack of efficacy or AEs). In accordance with current BSR guidelines (and to ensure consistency with previous NICE TAs), only PsARC response is used to determine continuation on treatment. PsARC responders/non-responders are further stratified according to PASI response status, to provide a more granular assessment of utilities and costs. PsARC and PASI responses are assumed to be correlated. For consistency, the same correlation coefficient (0.4) applied in the previous York model is assumed. This value is also assumed to apply across all subpopulations, subgroups and individual treatments.
The PASI changes observed in the clinical trials are categorised according to the proportion of patients who achieve at least 50%, 75% and 90% improvement in their baseline PASI score (PASI 50, PASI 75 and PASI 90, respectively). The calculation of the expected improvement in PASI score for PASI 75 responders and non-responders is equivalent to the approach used in the previous York model. 33 That is, the new model also assumes that patients who achieve a PASI 75 response will gain at least a 75% improvement in psoriasis compared with baseline PASI score, with some achieving a 90% improvement. Similarly, patients who do not achieve a PASI 75 response may achieve PASI 50.
Functional capability, in terms of the arthritis component of the disease, is measured using the HAQ-DI. A relationship between PsARC response and HAQ-DI score is explicitly considered in the current model. The change in baseline HAQ-DI score is assumed to be conditional on PsARC response status. To ensure that the treatment effect is reproducible in the clinical practice, an adjustment for the placebo or expectation effect is applied within the new model. This adjustment follows the same methods employed in the previous York model.
An individual’s HAQ-DI and PASI score determine health state costs (in addition to treatment-related costs) and QALYs; hence, the model tracks these clinical scores over time. The new model employs ‘tunnel’ states142 to reflect how long patients stay in a particular health state (HAQ-DI and PASI scores) and when they move (switch to another treatment) (see Choice of intervention and comparators). The ability to build multidimensional arrays, facilitated through the use of R, enables this functionality and the inclusion of subsequent lines of treatments, either after the initial response period or during the longer-term period. 143
After the treatment response period, responders are subject to an ongoing risk of withdrawal from treatment as a result of lack of efficacy or the occurrence of AEs (modelled together as an overall risk of withdrawal). HAQ-DI and PASI scores again change according to the second-line treatment received and associated response status. It is assumed that PsARC responders continuing on treatment after the initial 3-month response period maintain their improvement in HAQ-DI and PASI scores until subsequent withdrawal (i.e. no progression in HAQ-DI and PASI scores). Once patients withdraw from treatment to BSC, or to another biologic treatment, their HAQ-DI and PASI scores rebound to their baseline values (see Withdrawal from treatment and the natural history of psoriatic arthritis).
A summary of data inputs used in the model is given in Table 78. These are described in detail in the relevant sections that follow. The effectiveness data utilised in the model are shown separately in Table 79 in Sources of effectiveness data. The variable names in both tables follow those used in the R code, reported in Appendix 7.
| Description | Variable name | Mean | SE | Source/appendix |
|---|---|---|---|---|
| Sex: male = 1, female = 0 | Male | 1 | ||
| Baseline HAQ-DI score | HAQ-DI0 | 1.22 | Mean of RCTs (see Chapter 4) | |
| Baseline age | Age | 47 | Mean of RCTs (see Chapter 4) | |
| Model time horizon cycles | num_cycles | 160 | Clinical opinion | |
| Cycle length, year | Cl | 0.25 | ||
| Discount rate (per year) | r | 0.035 | UK treasury144 | |
| Utility function intercept | h0 | 0.897 | 0.006 | Rodgers et al., 201133 |
| Change in utility for a 1-unit change in HAQ-DI score | h1 | –0.298 | 0.006 | |
| Change in utility for a 1-unit change in PASI score | h2 | –0.004 | 0.0003 | |
| Interaction term HAQ-DI PASI | h3 | 0 | 10 × E–5 | |
| Change in HAQ-DI score while on treatment per 3-month period | HAQ-DI1.d | 0 | Rodgers et al., 201133 | |
| Change in HAQ-DI score while not on treatment per 3-month period | HAQ-DI1.w | 0.018 | 0.007 | Rodgers et al., 201133 |
| Rebound in HAQ-DI score on withdrawal (compared with HAQ-DI score at baseline) (zero means ‘rebound equal to initial gain’) | loss.w | 0 | Assumption | |
| Intercept of regression of log-mortality vs. age in men | ln.R.g.m | –10.25 | 0.046 | Gompertz parameters parameterising life table data for England and Wales145 |
| Intercept of regression of log-mortality vs. age in women | ln.R.g.f | –11.10 | 0.046 | |
| Change in log-mortality with additional year of age in men aged > 40 years | a.g.m | 0.094 | 0.0006 | |
| Change in log-mortality with additional year of age in women aged > 40 years | a.g.f | 0.101 | 0.0006 | |
| Standardised mortality ratio for PsA vs. general population | SMRmen SMRwomen | 1.36 | Ali et al., 200714 | |
| Log-withdrawal rate from biologics per year | ln.long.yr | –1.823 | 0.2044 | Rodgers et al., 201133 |
| Correlation between PASI 75 and PsARC | rho.new | 0.4 | 0.1 | ADEPT55 |
Patient characteristics
As discussed in Overview, the NICE scope112 for this appraisal specified three specific subpopulations of interest, reflecting the various stages of the treatment pathway for adult PsA. These three subpopulations are subsequently referred to as:
-
subpopulation 1: biologic naive, one previous cDMARD
-
subpopulation 2: biologic naive, two or more previous cDMARDs
-
subpopulation 3: biologic experienced.
Within subpopulation 3, the availability of evidence relating to CZP, necessitates the specification of a further scenario analysis to address the subgroup of patients who have previously responded to biologic treatment (primary responders), but who have subsequently withdrawn as a result of loss of efficacy or the occurrence of an AE.
In addition, in the NICE scope112 for this appraisal, a further population (subpopulation 4) contraindicated to TNF-α inhibitors (including ETN, ADA, INF and GOL) was also considered for SEC. CZP was not considered within the contraindicated population on the basis that, in patients in whom other TNF-α inhibitors are contraindicated, CZP (a new TNF-α inhibitor) would probably also be contraindicated.
In the updated York model, separate versions of the model are specified, representing each of the three main subpopulations. In the base case of each of these models, the baseline age is assumed to be 47 years and mean baseline HAQ-DI score is 1.22 units. These values represent the average baseline characteristics from the included trials (see Chapter 4). Baseline weight is required for administration of INF; however, not all trials report these values. Here the weight distribution reported in the RAPID-PsA trial47 is used (see Treatment costs).
As discussed in Chapter 5, it is also important to consider the impact of differences in baseline characteristics, in terms of HAQ-DI and, particularly, PASI scores, and the impact that these differences have on cost-effectiveness and the choice of optimal treatment. This is a particular issue in terms of the severity of concomitant psoriasis, as 300 mg of SEC, as opposed to the standard dose of 150 mg of SEC, is approved in patients with more severe psoriasis. To explore the impact of severity of the psoriasis component of the disease on cost-effectiveness, separate analyses are presented according to three concomitant psoriasis subgroups. Clinical opinion suggests that about 50% of patients who receive biologic treatment have mild or minimal concomitant psoriasis (< 3% of BSA or a PASI score of < 2.5 units), 25% have mild–moderate concomitant psoriasis (a baseline PASI score between 2.5 and 10 units) and 25% have moderate–severe concomitant psoriasis (a PASI score of > 10 units). 128 These definitions have been used as the basis for the three concomitant psoriasis subgroups formally considered here:
-
no concomitant psoriasis, with a baseline PASI score of 0 units
-
mild–moderate concomitant psoriasis, with a baseline PASI score of 7.3 units (the same value used in the previous York model)
-
moderate–severe concomitant psoriasis, with a baseline PASI score of 12.5 units (the same value used as part of a separate sensitivity analysis presented in the previous York model).
In the absence of effectiveness data reported for these subgroups, an assumption is made that treatments are similarly effective (in relative terms) for each subgroup within the separate subpopulations. Hence, the differences in cost-effectiveness for these subgroups are driven entirely by the different baseline PASI scores and the subsequent impact on costs and outcomes of these differences.
Baseline HAQ-DI scores are assumed the same across the separate subpopulations and PASI subgroups. Differences in baseline HAQ-DI scores were considered in a separate sensitivity analysis based on estimates reported in the UCB Pharma submission.
Choice of intervention and comparators
-
In subpopulation 1, only SEC, CZP and BSC are included in accordance with the NICE scope. 112 Based on the licence of SEC, 150 mg of SEC is included for the no-concomitant PASI and mild–moderate PASI subgroups in the naive populations and 300 mg of SEC for the severe psoriasis subgroup.
-
In subpopulation 2, SEC, CZP and other TNF-α inhibitors (ETN, INF, ADA and GOL) are considered to be relevant treatment alternatives in accordance with the NICE scope. BSC includes cDMARDs according to the placebo response rates as observed in the trials and costs according to HAQ-DI and PASI health states (see Health state costs). Again, in accordance with the licence of SEC, 150 mg of SEC is evaluated for the no-concomitant PASI and mild–moderate PASI subgroups in the naive populations and 300 mg of SEC for the severe psoriasis subgroup.
-
In subpopulation 3, 300 mg of SEC, CZP, UST and BSC (as defined above) are regarded as relevant treatment alternatives in accordance with the NICE scope. 112 As previously stated, as the data available for CZP inform only a subgroup of subpopulation 3, a separate analysis is conducted for CZP compared with BSC (see Patient characteristics).
-
In the additional contraindicated subpopulation (subpopulation 4), SEC, UST and BSC (as defined above) are regarded as relevant treatment alternatives. An assumption is made for this subpopulation that patients in whom TNF-α inhibitors are contraindicated are biologic naive and hence the effectiveness data are derived from this population. In reality, it is recognised that contraindications (e.g. infection, TB activation) may arise after a TNF-α inhibitor has been tried. However, for simplicity this analysis assumes patients are biologic naive. Hence, in accordance with the licence of SEC, 150 mg of SEC is evaluated for the no-concomitant PASI and mild–moderate PASI subgroups in the naive populations and 300 mg of SEC for the severe psoriasis subgroup.
In accordance with the NICE scope112 for this appraisal, APR was not included as a comparator in any of the subpopulations, as at the time this report was completed it had not been approved for use in adult PsA by NICE.
A key element of updating the previous York economic model is the formal incorporation of subsequent lines of therapy assumed within the base case. Specifically, the updated model allows for patients to move (switch) to a second treatment rather than to BSC as a result of primary non-response or secondary failure of treatment. The model also allows third- and fourth-line treatments. This functionality is enabled in the R by including tunnel states to track the HAQ-DI and PASI scores of patients who switch therapy. Tunnel states are generated for every cycle in the model (160 cycles). Further tunnel states are generated within this structure where patients can switch to a third and fourth treatment. This significantly increases the size of the Markov structure compared with the previous York model.
The length of the treatment sequence depends on the subpopulation: subpopulation 1 (biologic naive, one previous cDMARD) is eligible to receive three lines of treatment before moving to BSC; subpopulation 2 (biologic naive, two or more previous cDMARDs) is eligible to receive two lines of treatment before moving to BSC; and subpopulation 3 (biologic experienced) is eligible to receive one treatment before moving to BSC. Subpopulation 4 is assumed to be equivalent to subpopulation 3 in terms of sequencing, the only difference being the use of 150 mg of SEC as opposed to 300 mg of SEC.
The sequences of treatments are shown in Figures 16–18 for the main subpopulations 1, 2 and 3, respectively. Only the biologic-naive populations are eligible to receive further active treatments once they have failed on their initial treatment.
FIGURE 16.
Treatment sequences in subpopulation 1.

FIGURE 17.
Treatment sequences in subpopulation 2.
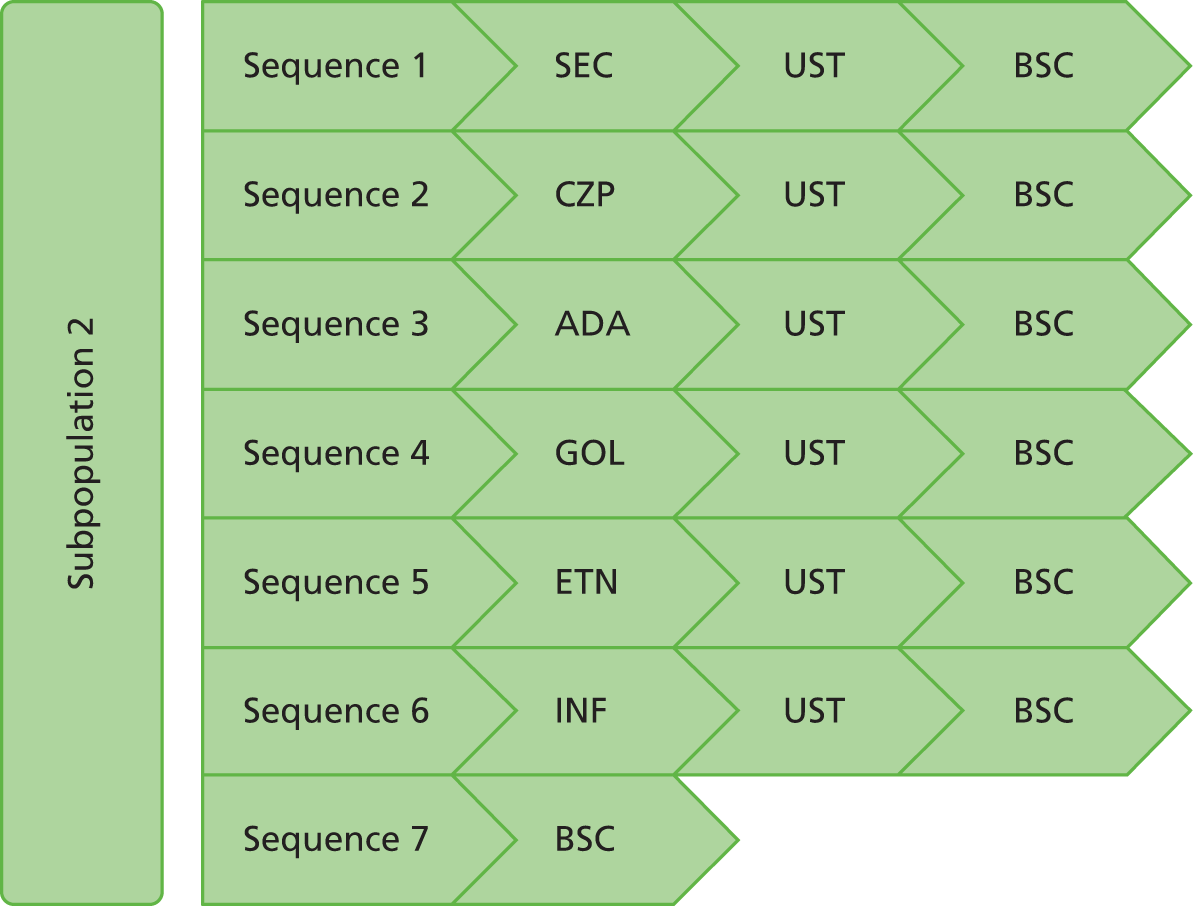
FIGURE 18.
Treatment sequences in subpopulation 3.

-
In subpopulation 1, patients may be eligible to receive further biologics. ETN is assumed to be the next biologic treatment as part of the overall sequence, on the basis that is it the lowest cost currently approved biologic and because it was consistently reported to be more cost-effective than other TNFs in previously published studies. 33 Following failure of ETN, patients are assumed to receive UST before moving onto BSC.
-
In subpopulation 2, patients are assumed to subsequently receive UST (approved in the biologic-experienced population) before moving onto BSC.
-
Patients in subpopulation 3 are assumed to move to BSC after failure of 300 mg of SEC, UST or CZP (secondary failures only).
Etanercept and INF are available as the originator products or biosimilars. The originator product of ETN is ENBREL and the biosimilar version is Benepali (SB4, Biogen Idec Ltd, Maidenhead, UK). The originator product of INF is REMICADE® (Janssen Pharmaceuticals/ Merck Sharp & Dohme) and the biosimilar versions are Inflectra® (Hospira UK Ltd, Maidenhead, UK), Remsima (Napp Pharmaceuticals Ltd, Cambridge, UK) and SB2 (Samsung Bioepis Co. Ltd, Seoul, Korea). In each of the base-case scenarios, the list prices for the originator products of ETN and INF are assumed. A separate analysis is presented using the prices of the biosimilar products (see Appendix 8). The biosimilar analysis is restricted to subpopulation 2. In this separate analysis the biosimilar versions are assumed to be equivalent to the originator products in terms of effectiveness.
Withdrawal from treatment and the natural history of psoriatic arthritis
As the psoriasis element of PsA is not progressive, it is assumed that PASI score does not increase over time for patients receiving BSC. The arthritis element of PsA is assumed to be progressive, consistent with the clinical evidence (see Chapter 4). Therefore, for patients not receiving biologic therapies, the HAQ-DI score is assumed to worsen over time, reflecting the decrease in functional capability as the arthritis component of the disease progresses. In the absence of a more appropriate alternative identified in the review of long-term open-label data (see Chapter 3, Open-label extension studies) and registry data (see Chapter 3, Review of anti-tumour necrosis factor patient registry studies), the rate determined in the previous York model, derived from the NOAR, was utilised in the updated York model. This rate of 0.018 units per 3-month cycle is assumed to be constant over time. Figure 12 shows the trajectory of HAQ-DI scores over time, for patients receiving BSC alone.
For PsARC responders, there is a risk of withdrawal following the first cycle of the model (3 months). This risk is due to AEs and loss of efficacy. Based on the previous York model, this probability is estimated from a meta-analysis of registry data from several countries to be –1.823 (SE 0.2044) on the log-scale, or exp(–1.823 + 0.5 × 0.20442) = 0.165 per year. This probability of withdrawal (0.165 per year) is assumed to be independent of HAQ-DI and PASI score in the model, relevant for all comparators and is constant over time. Alternative scenarios were specified according to those reported in the CSs (see Scenario analyses).
Following withdrawal, the ‘rebound’ of HAQ-DI and PASI scores is assumed to be equivalent to the gain. This assumption is consistent with the previous York model (see Figure 12). The rebound effect is assumed to happen immediately following withdrawal.
Sources of effectiveness data
The effectiveness data applied in the economic model are derived from the NMA, reported separately in Chapter 5. Three outcomes were included in the NMA to inform the economic model: (1) PsARC response, (2) change in HAQ-DI score conditional on PsARC response and (3) PASI 50, PASI 75 and PASI 90 responses.
The NMA implemented separate models for the pooling of treatment effects and placebo responses. A number of alternative models were implemented to explore the possibility of placebo response determining the effectiveness of alternative treatments, and also whether or not there was similarity between treatment effects for treatments of the same class. These are discussed in detail in Chapter 5. The following sections specify the approaches used in the economic model for each of the three outcomes.
Psoriatic Arthritis Response Criteria response
Chapter 5 details the data available for PsARC response, for each of the comparators. The NMA implemented seven alternative models for PsARC response in the naive populations (see Table 41). Owing to data limitations, these could be specified only for all biologic-naive patients (i.e. not separately for subpopulations 1 and 2). Of these seven models, two were considered to be the preferred models on the basis of model fit, goodness-of-fit statistics and clinical plausibility. These are:
-
Model A1: no baseline adjustment. Assumes that the treatments are independent (fixed effect) and, therefore, utilises the baseline and treatment effects as observed in the trial.
-
Model D2: a metaregression on baseline risk (placebo response). Treatments within a class have similar (exchangeable) effectiveness and depend on the effect of the placebo arm. Shrunken estimates are reported to account for the differences between treatments. The Genovese et al. 56 and Mease et al. 53 trials are included.
Results for the two preferred PsARC models, in the naive population, are presented in Table 43. These show the median probabilities and ORs.
For the biologic-experienced population (subpopulation 3), it was not possible to conduct a metaregression because of data limitations; therefore, only independent analysis estimates are available for this subpopulation (model A1). As discussed in Patient characteristics, the data from the RAPID-PsA trial (CZP)47 were not included in the analysis. Results for the biologic-experienced population are presented in Table 45. These show the median probabilities and ORs.
Health Assessment Questionnaire-Disability Index changes conditional on Psoriatic Arthritis Response Criteria response
Given that HAQ-DI scores are modelled conditional on PsARC response, modelling an interaction effect between baseline and treatment effect was deemed to be less relevant, and a metaregression model was not implemented on HAQ-DI (see Chapter 4, Health Assessment Questionnaire-Disability Index conditional on Psoriatic Arthritis Response Criteria response/non-response). Instead, three models are implemented in the biologic-naive populations (see Chapter 4, Health Assessment Questionnaire-Disability Index changes conditional on Psoriatic Arthritis Response Criteria response/non-response, Subpopulation: biologic naive), two of which model a class effect for treatments. Again, as a result of data limitations, these could be specified only for all biologic-naive patients. Of these three models, two were considered to be the preferred models on the basis of model fit, goodness-of-fit statistics and clinical plausibility. These are:
-
Model E1: no baseline adjustment. Assumes that the treatments are independent (fixed effect) and, therefore, utilises the baseline and treatment effects as observed in the trial.
-
Model E2: no baseline adjustment. A class effect is applied comprising three groups: anti-TNFs, ILs and APR. Treatments are similar within class (exchangeable) and there is a fixed effect across studies.
The results for the two preferred HAQ-DI change models, in the naive population, are presented in Table 47. These show the absolute median changes (with a more negative number representing a larger HAQ-DI score improvement).
For the biologic-experienced population (subpopulation 3), it was not possible to determine a class effect; therefore, only independent analysis estimates are available for this subpopulation (model E1). As discussed in Chapter 4, Health Assessment Questionnaire-Disability Index conditional on Psoriatic Arthritis Response Criteria response/non-response, the data from the RAPID-PsA trial (CZP)47 were not included in the analysis. Results for the biologic-experienced population are presented in Table 49. These show the absolute median/mean HAQ-DI score changes.
Psoriasis Area and Severity Index 50, 75 and 90 responses
Chapter 5 details the data available for PASI response, for each of the comparators. The NMA utilised a framework of analysis that evaluated the probability of PASI responses in different categories of PASI thresholds (50/75/90) within a single model. For the economic model this was used to determine the probabilities of achieving PASI 50, PASI 75 and PASI 90.
The NMA implemented three alternative models for PASI response in the naive populations (see Table 51). Owing to data limitations, these could be specified only for all biologic-naive patients. Of these three models, two were considered to be the preferred models on the basis of model fit, goodness-of-fit statistics and clinical plausibility. These are:
-
Model F1: no baseline adjustment. Assumes that treatments are independent and fixed effect on cut-off points/thresholds.
-
Model G2: common interaction term with baseline effect. Assumes that treatments are independent, but treatment effects are adjusted with the trial-specific baseline effects assuming a common interaction term.
The results for the two preferred PASI response models, in the naive population, are presented in Table 53. These show the median probabilities for PASI 50, PASI 75 and PASI 90.
For the biologic-experienced population (subpopulation 3), it was not possible to determine a class effect; therefore, only independent analysis estimates are available for this subpopulation (model F1). As discussed in Chapter 4, Psoriasis Area and Severity Index Psoriasis Area and Severity Index response, the data from the RAPID-PsA trial (CZP)47 were not included in the analysis. Results for the biologic-experienced population are presented in Table 55. These show the median/mean probabilities and ORs.
Combinations of evidence synthesis estimates utilised in the economic model
As discussed in the sections above, results are available for two alternative evidence synthesis models, for each of the three outcomes (PsARC response, change in HAQ-DI score conditional on PsARC response and PASI 50, PASI 75 and PASI 90 responses). The economic model utilises two combinations of these results for PsARC response, HAQ-DI score conditional on PsARC response and PASI response. These are:
-
independent analysis: PsARC response (model A1), HAQ-DI conditional on PsARC response (model E1) and PASI response (model F1)
-
metaregression: PsARC response (model D2), HAQ-DI conditional on PsARC response (model E2) and PASI response (model G2).
Table 79 presents the effectiveness data used in the updated York model. The clinical effectiveness results reported in Chapter 4 are, on the whole, reported as medians. The economic model instead utilises the means from the NMA. The means represent the most appropriate values for the economic model in order to inform a decision regarding the expected cost-effectiveness of competing treatments.
| Parameter | Name | Value | Source | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo responses for biologic-naive population: treatment effects from the independent analysis | ||||||||
| Probability of a PsARC response | p.psarc.plac2 | 0.3073 | See Chapter 4 | |||||
| Change in HAQ-DI score given a PsARC response | HAQ-DI.resp.plac2 | –0.2629 | ||||||
| Probability of a PASI 50 response | p.pasi.50.plac2 | 0.153 | ||||||
| Probability of a PASI 75 response | p.pasi.75.plac2 | 0.054 | ||||||
| Probability of a PASI 90 response | p.pasi.90.plac2 | 0.015 | ||||||
| Placebo responses for biologic-naive population: treatment effects from the metaregression | ||||||||
| Probability of a PsARC response | p.psarc.plac2 | 0.3073 | See Chapter 4 | |||||
| Change in HAQ-DI score given a PsARC response | HAQ-DI.resp.plac2 | –0.2579 | ||||||
| Probability of a PASI 50 response | p.pasi.50.plac2 | 0.155 | ||||||
| Probability of a PASI 75 response | p.pasi.75.plac2 | 0.055 | ||||||
| Probability of a PASI 90 response | p.pasi.90.plac2 | 0.016 | ||||||
| Placebo responses for biologic-experienced population: treatment effects from the independent analysis | ||||||||
| Probability of a PsARC response | p.psarc.plac3 | 0.268 | See Chapter 4 | |||||
| Change in HAQ-DI score given a PsARC response | HAQ-DI.resp.plac3 | –0.134 | ||||||
| Probability of a PASI 50 response | p.pasi.50.plac3 | 0.103 | ||||||
| Probability of a PASI 75 response | p.pasi.75.plac3 | 0.012 | ||||||
| Probability of a PASI 90 response | p.pasi.90.plac3 | 0.004 | ||||||
| Description | Variable name | Treatment | ||||||
| ETN | INF | ADA | GOL | CZP | 150 mg of SEC | 300 mg of SEC | ||
| Treatments’ input data for biologic-naive population: treatment effects from the independent analysis | ||||||||
| Probability of a PsARC response | psarc2 | 0.77 | 0.8114 | 0.6421 | 0.8168 | 0.5697 | 0.5849 | 0.5870 |
| Change in HAQ-DI score in the first 3 months given no PsARC response | HAQ-DI.noresp2 | –0.20 | –0.1966 | –0.1344 | –0.0634 | –0.0683 | –0.0825 | –0.0535 |
| Change in HAQ-DI score in the first 3 months given a PsARC response | HAQ-DI.resp2 | –0.6407 | –0.66 | –0.4889 | –0.4385 | –0.4284 | –0.3947 | –0.5472 |
| Probability of a PASI 50 response | p.pasi.50_2 | 0.411 | 0.918 | 0.675 | 0.732 | 0.441 | 0.801 | 0.819 |
| Probability of a PASI 75 response | pasi75_2 | 0.209 | 0.789 | 0.448 | 0.514 | 0.231 | 0.603 | 0.627 |
| Probability of a PASI 90 response | p.pasi.90_2 | 0.084 | 0.593 | 0.242 | 0.297 | 0.097 | 0.380 | 0.405 |
| Treatments’ input data for biologic-naive population: treatment effects from the metaregression | ||||||||
| Probability of a PsARC response | psarc2 | 0.74 | 0.74 | 0.60 | 0.71 | 0.71 | 0.73 | 0.73 |
| Change in HAQ-DI score in the first 3 months given no PsARC response | HAQ-DI.noresp2 | –0.15 | –0.15 | –0.13 | –0.11 | –0.12 | –0.09 | –0.08 |
| Change in HAQ-DI score in the first 3 months given a PsARC response | HAQ-DI.resp2 | –0.59 | –0.60 | –0.50 | –0.48 | –0.47 | –0.43 | –0.51 |
| Probability of a PASI 50 response | p.pasi.50_2 | 0.43 | 0.77 | 0.66 | 0.54 | 0.66 | 0.77 | 0.79 |
| Probability of a PASI 75 response | pasi75_2 | 0.24 | 0.57 | 0.43 | 0.32 | 0.44 | 0.57 | 0.60 |
| Probability of a PASI 90 response | p.pasi.90_2 | 0.11 | 0.36 | 0.23 | 0.16 | 0.24 | 0.36 | 0.39 |
| Description | Variable name | Treatment | ||||||
| CZP | 300 mg of SEC | UST | ||||||
| Treatments’ input data for biologic-experienced population: treatment effects from the independent analysis | ||||||||
| Probability of a PsARC response | Psarc3 | Confidential information has been removed | 0.674 | 0.562 | ||||
| Change in HAQ-DI score in the first 3 months given no PsARC response | HAQ-DI.noresp3 | Confidential information has been removed | –0.4295 | 0.0015 | ||||
| Change in HAQ-DI score in the first 3 months given a PsARC response | HAQ-DI.resp3 | Confidential information has been removed | –0.3838 | –0.32 | ||||
| Probability of a PASI 50 response | p.pasi.50_3 | 0.56 | 0.875 | 0.628 | ||||
| Probability of a PASI 75 response | pasi75_3 | 0.41 | 0.598 | 0.279 | ||||
| Probability of a PASI 90 response | p.pasi.90_3 | 0.19 | 0.365 | 0.12 | ||||
Correlation between Psoriatic Arthritis Response Criteria and Psoriasis Area and Severity Index responses
Although treatment continuation is determined by PsARC response, the model needs to consider the proportion of those patients who achieve PASI 75 together with PsARC, as this cohort has a different PASI score, and hence incurs different costs and QALYs. Based on previously published models and the CSs, a positive correlation between the two main responses in the model, PsARC and PASI 75, is included in the base-case model. The correlation coefficient value used in the model is 0.4, taken from the analysis conducted as part of the previous York model.
Table 80 shows the effect of treatment, in terms of PsARC and PASI 75 response probabilities, utilising the results from the evidence synthesis model performing independent analysis. The positive correlation columns account for the correlation between these two outcomes to generate the proportion of patients achieving joint only and joint plus skin improvement together. The no correlation columns assume independence between the two responses (no correlation coefficient applied). The no correlation columns are shown only for illustration here, as these values are not employed in the updated York model. Assuming a positive correlation between PsARC and PASI (the assumption in the updated York model), ETN has the highest probability of a joint only response and INF the lowest probability of a joint only response. For both a joint and skin response, INF has the highest probability and CZP the lowest probability.
| Treatment | Evidence synthesis | Correlation | ||||
|---|---|---|---|---|---|---|
| Positive | No | |||||
| PsARC | PASI 75 | Joints only (PsARC) | Joints and skin (PsARC + PASI) | Joints only (PsARC) | Joints and skin (PSARC + PASI) | |
| ETN | 0.770 | 0.227 | 0.525 | 0.245 | 0.595 | 0.175 |
| INF | 0.811 | 0.785 | 0.110 | 0.701 | 0.175 | 0.637 |
| ADA | 0.642 | 0.449 | 0.259 | 0.384 | 0.354 | 0.288 |
| GOL | 0.817 | 0.514 | 0.320 | 0.497 | 0.397 | 0.420 |
| CZP | 0.570 | 0.236 | 0.351 | 0.218 | 0.435 | 0.134 |
| 150 mg of SEC | 0.585 | 0.600 | 0.138 | 0.447 | 0.234 | 0.351 |
| 300 mg of SEC | 0.587 | 0.623 | 0.126 | 0.461 | 0.221 | 0.366 |
| USTa | 0.486 | 0.319 | 0.238 | 0.248 | 0.331 | 0.155 |
Table 81 also shows the effect of treatment, in terms of PsARC and PASI 75 response probabilities, but instead utilises the evidence synthesis outcomes based on metaregression. There are some differences between the independent probabilities and the metaregression probabilities, reflecting the adjustments made to the relative effectiveness of treatments using class effect shrunken estimates in the metaregression, as opposed to relative treatments effects as observed in the trials in the independent analysis (see Chapter 4). Assuming a positive correlation between PsARC and PASI, again ETN has the highest probability of a joint only response; however, 300 mg of SEC has the lowest probability of a joint only response. For both a joint and skin response, 300 mg of SEC has the highest and ETN has the lowest probability.
| Treatment | The evidence synthesis outcome | Correlation | ||||
|---|---|---|---|---|---|---|
| Positive | No | |||||
| PsARC | PASI 75 | Joints only (PsARC) | Joints and skin (PsARC + PASI) | Joints only (PsARC) | Joints and skin (PsARC + PASI) | |
| ETN | 0.740 | 0.238 | 0.489 | 0.251 | 0.564 | 0.176 |
| INF | 0.740 | 0.573 | 0.229 | 0.511 | 0.316 | 0.424 |
| ADA | 0.594 | 0.430 | 0.241 | 0.353 | 0.338 | 0.256 |
| GOL | 0.706 | 0.323 | 0.393 | 0.313 | 0.478 | 0.228 |
| CZP | 0.710 | 0.436 | 0.310 | 0.399 | 0.400 | 0.309 |
| 150 mg of SEC | 0.728 | 0.575 | 0.222 | 0.507 | 0.309 | 0.419 |
| 300 mg of SEC | 0.730 | 0.600 | 0.205 | 0.525 | 0.292 | 0.438 |
| USTa | 0.589 | 0.401 | 0.256 | 0.333 | 0.353 | 0.237 |
Mortality
All-cause mortality is incorporated by applying a risk of death during each model cycle. The mortality risk is not assumed to be structurally related to response or treatments received. Instead a common excess mortality risk is assumed for all PsA patients compared with general population mortality risks. The general population mortality risk is obtained from life tables for England and Wales and is specified separately for males and females, although the model averages across these as it does not generate results separately for males and females. Similar to the previous York model, a Gompertz function was fitted to life table data (see Table 78). The excess mortality risk associated with PSA is modelled assuming a HR of 1.3614 compared with the general population. This value is based on an updated analysis of the same source used in the previous York model and hence employs a different estimate from the one previously assumed.
Sources of utility data
Health utility is measured as a function of HAQ-DI and PASI. A separate search was undertaken to identify alternative utility algorithms (see Appendix 9). In the absence of finding any published sources reporting alternative algorithms to the one applied in the previous York model, the same algorithm was used. This algorithm is based on a linear function relating the expected utility to HAQ-DI and PASI. The same utility function is applied to all subpopulations, subgroups and treatments.
Figure 19 shows the trajectories of utility according to a patients HAQ-DI score over time, for BSC, remaining on treatment and treatment withdrawal at 5 years.
FIGURE 19.
Utility corresponding to alternative HAQ-DI trajectories.
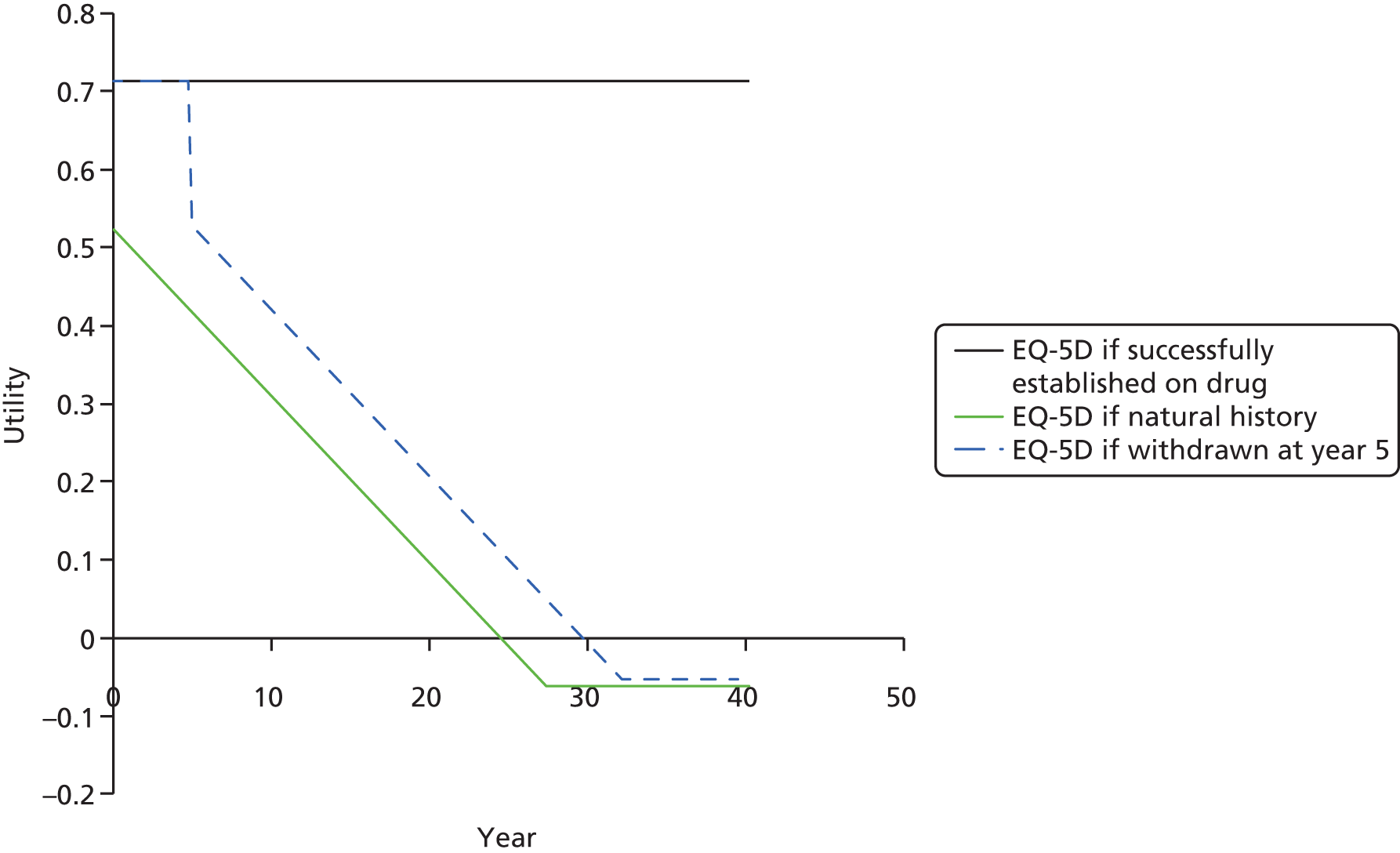
The equation below shows this relationship:
The utility function provided by one of the companies (Novartis) includes coefficients, namely baseline EQ-5D score, which cannot be utilised easily in the current model structure. UCB Pharma used a similar function to the previous York model but with a smaller coefficient for PASI (0.001 rather than 0.004). Given that this algorithm is very similar to the previous York model, separate scenarios, using alternative utility algorithms are not considered.
Sources of resource utilisation and costs data
Costs in the model are determined from the treatment costs (acquisition, administration and monitoring) and changes in health service utilisation driven by disease status (HAQ-DI and PASI scores). The resource use assumptions and costs applied to each of these categories are discussed in the sections below. Further searches were conducted to identify alternative sources of health state costs. The searches and results are described in Appendix 10.
Treatment costs
Table 82 shows the treatment-related costs applied in the updated York model. These costs are based on the list prices for SEC and CZP (biosimilar costs and Patient Access Scheme prices are used in the separate analysis). Costs are presented for the first and subsequent cycles and in terms of annual costs.
| Time period | Treatment cost (£) | |||||||
|---|---|---|---|---|---|---|---|---|
| ETN | INF | ADA | GOL | CZP | 150 mg of SEC | 300 mg of SEC | UST | |
| First cycle | 2541 | 7887 | 2506 | 2498 | 3784 | 4475 | 8741 | 4503 |
| Subsequent cycles | 2336 | 3672 | 2301 | 2293 | 2149 | 1832 | 3661 | 2151 |
| Annual cost | 9549 | 18,902 | 9409 | 9377 | 10,232 | 9972 | 19,722 | 10,957 |
Each of the existing models (published and CSs) presents different resource use assumptions and unit costs, which are used to cost drug treatment, administration and monitoring of patients. Different assumptions have been used regarding the dosing of drugs and resource use for administration and monitoring (see Chapter 5, Summary of resource utilisation and costs data in the York model and Chapter 5, Summary of resource utilisation and costs data). The current York model sought to specify the most appropriate resource use associated with drug acquisition, administration and monitoring patients for each of the treatment options.
The resource use items from the previous York model33 have been updated for ETN, INF and ADA, reflecting evidence from a recent appraisal in ankylosing spondylosis. 104 The assumptions regarding resource use for GOL have been taken from the GOL STA,70 and the assumptions regarding the resource use for UST have been taken from the UST STA. 35 The resource use for SEC and CZP has been derived using the Summary of Product Characteristics, MIMS, clinical advice and BSR guidelines. The treatments’ dosing schedules were obtained from the Summary of Product Characteristics found on the Electronic Medicines Compendium website.
The dose for INF was determined by a patient’s weight, that is, 5 mg for each 1 kg. These weights were derived using the weight distribution reported in the RAPID-PsA trial. 47 All assumptions made regarding resource use have been validated with the clinical expert for this appraisal.
Table 83 summarises the drug acquisition, administration and monitoring costs used in the updated York model. Further details of these costs are given in the sections below.
| Treatment | Cost (£) | |||||||
|---|---|---|---|---|---|---|---|---|
| First cycle (13 weeks) | Subsequent cycles | |||||||
| Acquisition | Administration | Monitoring | Total | Acquisition | Administration | Monitoring | Total | |
| ETN | 2332 | 43 | 166 | 2541 | 2332 | 0 | 4 | 2336 |
| INF | 7147 | 574 | 166 | 7887 | 3395 | 273 | 4 | 3672 |
| ADA | 2297 | 43 | 166 | 2506 | 2297 | 0 | 4 | 2301 |
| GOL | 2289 | 43 | 166 | 2498 | 2289 | 0 | 4 | 2293 |
| CZP | 3575 | 43 | 166 | 3784 | 2145 | 0 | 4 | 2149 |
| 150 mg of SEC | 4266 | 43 | 166 | 4475 | 1828 | 0 | 4 | 1832 |
| 300 mg of SEC | 8532 | 43 | 166 | 8741 | 3656 | 0 | 4 | 3661 |
| UST | 4294 | 43 | 166 | 4503 | 2147 | 0 | 4 | 2151 |
Drug acquisition
Table 84 shows the number of vials assumed for each treatment, during the first cycle (the loading phase) and subsequent cycles. In the loading phase, 400 mg of CZP is given at weeks 0, 2 and 4. Subsequently, 200 mg is given every 2 weeks. Patients receive MTX (7.5 mg) alongside CZP, in accordance with the licence. For patients with mild–moderate psoriasis, the recommended dose of SEC is 150 mg, with initial dosing at weeks 0, 1, 2 and 3, followed by monthly maintenance dosing starting at week 4. For patients with moderate–severe psoriasis, or those who are biologic experienced, the recommended dose is 300 mg, with initial dosing at weeks 0, 1, 2 and 3, followed by monthly maintenance dosing starting at week 4. Each 300-mg dose is given as two subcutaneous injections of 150 mg.
| Treatment | Number of vials | |
|---|---|---|
| First cycle | Subsequent cycles | |
| ETN | 26 | 26 |
| INF | Weight based | Weighted based |
| ADA | 6.5 | 6.5 |
| GOL | 3 | 3 |
| CZP | 10 | 6 |
| 150 mg of SEC | 7 | 3 |
| 300 mg of SEC | 7 | 3 |
| UST | 2 | 1 |
For the other treatments, the following assumptions were made:
-
Six and a half vials of ADA are assumed given in every 3-month cycle. This does not represent vial sharing; instead the total yearly numbers of vials is equally divided by each 3-month (13-week) cycle.
-
Twenty-six vials of ETN are assumed given in the first cycle (two 25-mg prefilled syringes per week), followed by 26 vials for all subsequent cycles.
-
GOL is given as a 50-mg dose once a month. In patients with a body weight of > 100 kg who do not achieve an adequate clinical response after three or four doses, the dose of GOL can be increased to 100 mg once a month. The company (Janssen Pharmaceuticals) provides this double dose at the same price as the 50-mg dose as part of an approved Patient Access Scheme.
-
UST is given as an initial dose of 45 mg, followed by a 45-mg dose 4 weeks later, and then every 12 weeks thereafter. Alternatively, 90 mg may be used in patients with a body weight of > 100 kg. Similarly, the company (Janssen Pharmaceuticals) offers this double dose at an equivalent price as part of an approved Patient Access Scheme.
Infliximab is given at 0, 2 and 6 weeks, then every 8 weeks, with the number of vials administered at each time point determined by the patient’s weight. Baseline weight is taken from the weight distribution reported in the RAPID-PsA trial. 47 Table 85 shows the proportion of patients in each weight category in the RAPID-PsA trial47 and the number of INF vials required.
| Patient’s weight (kg) | Number of vials required | Dose (mg) | Proportion of population |
|---|---|---|---|
| 20 | 1 | 100 | 0.0003 |
| 40 | 2 | 200 | 0.0087 |
| 60 | 3 | 300 | 0.0878 |
| 80 | 4 | 400 | 0.3105 |
| 100 | 5 | 500 | 0.3898 |
| 120 | 6 | 600 | 0.1740 |
| 140 | 7 | 700 | 0.0273 |
| 160 | 8 | 800 | 0.0015 |
The drug acquisition costs used in the current York model are shown in Table 86. The acquisition costs of the drugs represent the list prices in the base-case analysis. The list prices are taken from the BNF139 and MIMS. 134 An analysis utilising non-list prices (biosimilar costs), for some of the comparators, is presented in Appendix 8. Biosimilar costs used are presented in Appendix 8. A separate analysis is also presented using the Patient Access Scheme prices for CZP and SEC as part of a separate and confidential appendix.
| Treatment | Cost (£; 2016) | Source |
|---|---|---|
| INF (100-mg vial): Inflectra/Remsima | 419.62 | MIMS134 |
| ETN (25-mg syringe): ENBREL | 89.50 | MIMS134 |
| ADA (40-mg syringe): Humera | 352.14 | MIMS134 |
| GOL (50-mg syringe; 100-mg syringe): SIMPONI | 762.97; 1525.94 | MIMS134 |
| UST (45-mg syringe; 90-mg syringe) | 2147; 2147 | MIMS134 |
| SEC (150-mg syringe) | 609.39 | MIMS134 |
| CZP (200-mg syringe) | 357.50 | MIMS134 |
| MTX (7.5 mg) | 0.30 | BNF139 |
A separate acquisition cost was not applied to BSC and, therefore, the cost of BSC is assumed to be entirely captured in terms of health state costs. These represent the full HAQ-DI costs (without discounting the prescribing costs) and the uncontrolled psoriasis costs (see Health state costs).
Drug administration
For all treatments, other than INF, an administration cost was applied only on the first cycle, therefore assuming self-administration in the subsequent cycles. This was assigned a cost of a 1-hour nurse visit in a GP practice (£43) (Personal Social Services Research Unit; PSSRU136). INF requires intravenous (i.v.) infusion and, therefore, the administration cost for INF was assumed to represent the cost of delivering simple parenteral chemotherapy at first attendance (£159; reference costs 2015137). These costs are the same as those used in the UCB Pharma model. The administration costs assumed in the updated model are shown in Table 87.
| Method of administration | Cost (£) | |
|---|---|---|
| First cycle | Subsequent cycles | |
| Subcutaneously | 43 | – |
| Intravenously | 159 | 159 |
Initiation and monitoring
A summary of the initiation and monitoring resource use assumptions is reported Table 88. The resource use assumptions for laboratory testing for biologic treatment initiation and monitoring have been sourced from the previous York model and updated using the Hospital and Community Health Service Pay and Prices Index from the PSSRU. 137 These conform to guidelines from the BSR127 for the use of biologics.
| Item | Initiation and monitoring costs (£) | Frequency | ||
|---|---|---|---|---|
| First cycle | Subsequent cycles | First cycle | Subsequent cycles | |
| Full blood count | 6.18 | 1.54 | 2 | 0.5 |
| ESR test | 6.11 | 1.53 | 2 | 0.5 |
| Liver function test | 1.56 | 0.39 | 2 | 0.5 |
| Urea and electrolytes test | 2.86 | 0.72 | 2 | 0.5 |
| Chest radiography | 27.11 | 0.00 | 1 | 0 |
| TB Heaf test | 9.03 | 0.00 | 1 | 0 |
| Antinuclear antibody test | 4.81 | 0.00 | 1 | 0 |
| Double-stranded DNA test | 4.81 | 0.00 | 1 | 0 |
| Specialist visit | 103.53 | 0.00 | 1 | 0 |
| Total | 166.01 | 4.18 | ||
Psoriatic arthritis patients on biologic therapy are assumed to undertake a series of tests at treatment initiation and at 3 months when assessing initial treatment response [i.e. a full blood count, erythrocyte sedimentation rate (ESR) test, liver function test, urea and electrolytes test]. Additional testing is assumed to be conducted once during the initial period (i.e. chest radiography, TB Heaf test, antinuclear antibody test and a double-stranded deoxyribonucleic acid test). Patients on biologics are also assumed to visit a specialist (rheumatologist) twice during the initial 3-month period (at treatment initiation and when assessing a response). The cost of a rheumatologist visit was applied only in the first cycle. The assumption that subsequent visit costs would be encapsulated within health state costs and has been applied in similar appraisals104 and in the company models. The cost of a rheumatology visit was taken from the NHS Reference Costs 2014 to 2015. 137
Health state costs
In order to generate an estimate of the lifetime costs for each of the treatments, estimates of resource use and costs associated with HAQ-DI and PASI are required. As reported in Chapter 5, the previous York model used separate studies and assumptions to estimate HAQ-DI- and PASI-related costs.
A search of the published literature was undertaken to identify alternative published evidence regarding the resource use and costs associated with the management of PsA in the UK (see Appendix 11). The only other published source identified in the search that specifically reported estimates of costs according to HAQ-DI and/or PASI was the study from Poole et al. 138 This study was used in the UCB Pharma submission and was previously described in Chapter 5.
The alternative approaches identified, which could be used to estimate HAQ-DI and PASI costs, represent an important area of remaining uncertainty. One potential advantage of the Poole et al. 138 study is that the estimates according to HAQ-DI score are derived from a sample of PsA patients as opposed to a sample of RA patients. However, Poole et al. 138 noted important differences in the authors’ predictions, with markedly higher costs predicted for equivalent HAQ-DI scores for PsA patients than those previously reported for RA patients. Although the authors of the Poole et al. 138 study stated that this could indicate important differences in the economic burden associated with PsA compared with RA, they also acknowledged that the differences might simply be attributed to differences in methods and/or the requirement to predict HAQ-DI score in the THIN data set using a separate regression model from the BSRBR. A number of further limitations were also noted in Poole et al. ,138 including (1) the predicted HAQ-DI score did not cover the full range (0–3 units) and applying the generalised linear model to predict for the full range could result in substantial errors, particularly for the more severe event of the range; and (2) PASI data were not available in either the BSBR or THIN data. These additional limitations are particularly important in the context of the current model, as HAQ-DI predictions are required across the full range of HAQ-DI scores and that separate PASI subgroups are modelled.
Having identified important differences in the predictions based on the separate sources and noting the potential limitations identified in the Poole et al. 138 study, the final HAQ-DI costs were based on the same function used in the previous York model, with costs uprated to current prices. This assumption also ensures consistency across the separate NICE TAs. Despite some concerns with the Poole et al. 138 study, the fact that it provides the only source of costs specific to PsA makes it potentially relevant for the updated York model. The use of the Poole et al. 138 study is therefore explored as a separate scenario (see Scenario analyses).
The costs according to HAQ-DI scores address only the arthritis component of PsA; therefore, additional costs were required to capture the psoriasis element of the disease. The current York model addresses three subgroups according to psoriasis severity (see Patient characteristics). It was assumed patients without concomitant psoriasis would not incur additional psoriasis-related costs. In the absence of identifying any other relevant UK costing studies to inform PASI estimates for the mild–moderate and moderate–severe PASI subgroups, the same sources as in the previous York model were assumed and the same assumptions were made. Hence, the costs assumed for treating mild–moderate psoriasis in patients who do not use biologics or who do not respond to biologics (PASI 75) were based on NHS unit costs of phototherapy137 and a UK RCT. 146 Similarly, for patients with moderate or severe psoriasis, costs were based on a Dutch RCT adjusted to UK price levels (see Hartman et al. 131). Costs from the previous York model were uprated to the current price year (2016).
The psoriasis-related costs applied to PASI 75 non-responders and for patients not receiving biologics are shown in Table 89 for each of the psoriasis subgroups.
| Cost | Psoriasis subgroups | ||
|---|---|---|---|
| Without psoriasis | Mild to moderate | Moderate to severe | |
| Baseline PASI score | 0.0 | 7.3 | 12.5 |
| Uncontrolled psoriasis | 0.0 | 223 | 638 |
| Controlled psoriasis (PASI 75 response) | 0.0 | 18 | 18 |
Scenario analyses
As described in Patient characteristics, a further subgroup of subpopulation 3 was considered as part of a separate scenario analysis. This separate scenario is presented to reflect that the data reported for CZP in biologic-experienced patients are applicable only to patients who initially responded to the previous biologic therapy (i.e. secondary failure of treatment), and are is not directly comparable to the data for UST and SEC ,which include primary and secondary treatment failures. This separate scenario includes only CZP and BSC. Other subgroups, in terms of extent of psoriasis (measured using PASI), are presented as part of the base-case analysis.
In addition, a number of scenarios are specified to explore the robustness of some of the assumptions made in the model, focusing on key areas where these deviate from assumptions made in the CSs:
-
Applying an alternative cost function from Poole et al. 138
-
Alternative assumptions regarding withdrawals. Two scenarios were specified: (1) the withdrawal rate for SEC is assumed to be 50% of the base-case value from year 2; and (2) all treatments are associated with a withdrawal rate equivalent to 50% of the base-case values from year 5. The first withdrawal scenario is similar to the assumption made in the Novartis model, in which lower withdrawal rates are reported for SEC in the second year of treatment. The second withdrawal scenario was undertaken to assess the robustness of the results to assumptions made regarding the constant rate of withdrawal applied in the model. Given the lack of longer-term data to inform an alternative, time-dependent withdrawal rate, an assumption was made that patients who remained on therapy at 5 years would no longer be at risk of subsequent withdrawals. This is similar to the assumption made in the UCB Pharma model, but not as extreme in that patients are still permitted to withdraw albeit at a reduced rate and from a slightly later time point (5 years as opposed to 4 years).
-
Baseline HAQ-DI score according to subpopulation. Equivalent to the separate baseline HAQ-DI scores assumed in the UCB Pharma model, three separate baseline scores were applied according to the subpopulation: (confidential information has been removed) for subpopulation 1, (confidential information has been removed) for subpopulation 2 and (confidential information has been removed) for subpopulation 3.
Analytic methods
The expected costs and QALYs of the alternative treatment strategies are determined for each subpopulation and PASI subgroup and the relative cost-effectiveness of the strategies is then compared using standard decision rules, estimating ICERs as appropriate. 147 The ICER examines the additional cost that one strategy incurs over another and compares this with the additional benefits. The ICER estimate represents the additional cost required to generate one additional unit of health outcome (QALY). When more than two strategies are being compared, the ICERs are calculated using the following process:
-
The strategies are ranked in terms of mean QALYs (from the least effective to the most effective).
-
If a strategy is more costly and less effective than any previous strategy, then this strategy is said to be dominated and is excluded from the calculation of the ICERs.
-
The ICERs are calculated for each successive alternative, from the least effective to the most effective; if the ICER for a given strategy is higher than that of any more effective strategy, then this strategy is ruled out on the basis of extended dominance.
-
Finally, the ICERs are recalculated, excluding any strategies that are ruled out by principles of dominance or extended dominance.
The resulting ICERs then provide the basis for establishing which strategy appears optimal based on cost-effectiveness considerations, that is, which strategy (or strategies) appears to provide good value for money to the NHS. Guidance from NICE suggests that an incremental cost per additional QALY of around £20,000–30,000 is considered to represent an appropriate threshold to establish value for money to the NHS. 125
In addition to determining which strategy appears optimal based on fully incremental comparisons of all treatments simultaneously, separate pairwise ICERs are presented for each treatment versus BSC alone. These pairwise ICERs are helpful in informing assessments of cross-validity (i.e. providing a comparable basis to compare particular treatments with previously published results). These comparisons may also be informative if strategies are ruled out from the fully incremental calculations based on differences between treatments that are not considered clinically or economically significant. In this situation, comparing the pairwise ICERs for each individual treatment with a common comparator may provide further information to inform subsequent decisions.
The model was run several times, once for the main base-case analysis (for each subpopulation and PASI subgroup) and then for a number of alternative scenarios to consider alternative assumptions related to key aspects of the base-case approach (see Scenario analysis). Given the large number of subpopulation, subgroup and scenario combinations, it has not been possible to conduct PSA, although this functionality is included in the model.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis is used to assess the implications of parameter uncertainty (the imprecision with which input parameters are estimated), in terms of the estimates of cost-effectiveness. The uncertainty in each parameter was represented using a probability distribution and the PSA was carried out using Monte Carlo simulation. The rate of change of the HAQ-DI score while not on treatment was assigned a gamma distribution to ensure that values are strictly positive. All other uncertain parameters were assigned normal distributions using the mean and SE. The treatment effect parameters used in the model, PsARC response, conditional change in HAQ-DI score and PASI responses, utilise the convergence diagnostic and output analysis (CODA) output from the evidence synthesis models (see Sources of effectiveness data).
This analysis reflects the decision uncertainty associated with the optimal treatment. PSA generates distributions (20,000 iterations) of total costs and QALYs, and shows the probability that a treatment is cost-effective at thresholds of £20,000 and £30,000. This was performed for the three subpopulations, defined by the patient’s position in the treatment pathway, and also on the three subgroups of concomitant psoriasis severity.
This analysis utilised the two evidence synthesis outputs: the independent and the metaregression analyses. Given the mathematically intensive operations, represented by 20,000 iterations for each version of the model, the computation time is a major challenge. This may potentially reach 2 months on a desktop machine. Therefore, there was a need to run the probabilistic model on the University of York’s supercomputer. This necessitated some flexibility in the code allowing the model to be run in parallel on hundreds of processors within the supercomputer.
Results
Results of the base-case cost-effectiveness analysis
According to the three main subpopulations (biologic naive, one prior or two or more prior DMARDs; and biologic experienced), results for three separate concomitant psoriasis subgroups (baseline PASI score = 0, 7.5 or 12.5) are presented and discussed in the following sections. For ease of presentation and interpretation, individual ICER tables are presented only for the independent analysis from the evidence synthesis in the main body of the report and summary tables used to compare with the results based on metaregression approach. Individual ICER tables based on the metaregression are also reported separately in Appendix 12.
All results presented in Results are based on the list prices for SEC and CZP and the originator products for INF and ETN. Results are presented for the base-case models, according to subpopulation and psoriasis subgroup, for the scenarios as specified and the PSA. A separate confidential appendix is included which incorporates the confidential Patient Access Scheme prices for CZP and SEC. Scenarios including biosimilar prices are also presented separately in Appendix 8.
Subpopulation 1: biologic naive (one prior DMARD)
The cost-effectiveness results for subpopulation 1 are shown for the three subgroups according to the level of concomitant psoriasis (moderate–severe psoriasis, mild–moderate psoriasis and no concomitant psoriasis) in Tables 90–92.
In the moderate–severe psoriasis subgroup (Table 90), 300 mg of SEC is the most effective strategy (QALYs = 8.52), followed by CZP (QALYS = 8.38) and BSC (QALYs = 5.31). In terms of costs, 300 mg of SEC is also the mostly costly strategy (£179,692) followed by CZP (£159,951) and BSC (£95,965). Based on the fully incremental ICERs, the ICER of CZP compared with BSC is £20,870 per QALY and the ICER of 300 mg of SEC compared with CZP is £134,783 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next-best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 95,965 | 5.312 | – | – | – | – |
| CZP | 159,951 | 8.377 | 63,987 | 3.066 | 20,870 | 20,870 |
| 300 mg of SEC | 179,692 | 8.524 | 19,741 | 0.146 | 134,783 | 26,064 |
The individual pairwise ICERs for CZP and 300 mg of SEC compared with BSC are £20,870 and £26,064 per QALY, respectively.
In the mild–moderate psoriasis group (Table 91), 150 mg of SEC is the most effective strategy (QALYs = 8.69), followed by CZP (QALYs = 8.68) and BSC (QALYs = 5.68). In terms of costs, CZP is now the most costly strategy (£135,946), followed by 150 mg of SEC (£132,500) and BSC (£67,000). Based on the fully incremental ICERs, CZP is dominated by 150 mg of SEC. The ICER of 150 mg of SEC compared with BSC is £21,772 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 67,000 | 5.676 | – | – | – | – |
| CZP | 135,946 | 8.667 | – | – | Dominateda | 23,052 |
| 150 mg of SEC | 132,500 | 8.685 | 65,500 | 3.009 | 21,772 | 21,772 |
The individual pairwise ICERs for CZP and 150 mg of SEC compared with BSC are £23,052 and £21,772 per QALY, respectively.
In the no concomitant psoriasis subgroup (Table 92), CZP is the most effective strategy (QALYs = 9.074), followed by 150 mg of SEC (QALYs = 9.067) and BSC (QALYs = 6.188). In terms of costs, CZP is also the most costly strategy (£122,832), followed by 150 mg of SEC (£120,303) and BSC (£51,436). Based on the fully incremental ICERs, the ICER for 150 mg of SEC compared with BSC is £23,928 per QALY and the ICER of CZP compared with 150 mg of SEC is £346,785 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 51,436 | 6.188 | – | – | – | – |
| 150 mg of SEC | 120,303 | 9.067 | 68,866 | 2.878 | 23,928 | 23,928 |
| CZP | 122,832 | 9.074 | 2529 | 0.007 | 346,785 | 24,744 |
The individual pairwise ICERs for 150 mg of SEC and CZP compared with BSC are £23,928 and £24,774 per QALY, respectively.
There are a number of important differences evident across the separate concomitant psoriasis subgroups for subpopulation 1. Mean costs are higher (and mean QALYs lower) for all treatments depending on the presence and severity of concomitant psoriasis, demonstrating the important contribution of psoriasis to costs and HRQoL, and to subsequent ICER estimates. The difference in mean QALYs between SEC and CZP is greatest in the moderate–severe psoriasis subgroup, with 300 mg of SEC reported to be the most effective strategy. The difference appears largely attributable to the higher average PASI responses (PASI 50, PASI 75 and PASI 90), estimated for 300 mg of SEC compared with CZP from the independent evidence synthesis. The differences in PASI outcomes become less important as the severity of concomitant psoriasis is reduced and the differences are now based on comparisons between 150 mg of SEC and CZP. The difference in QALYs between 150 mg of SEC and CZP is subsequently reduced in the mild–moderate psoriasis subgroup (QALY difference still in favour of 150 mg of SEC), and reduced again in the subgroup with no concomitant psoriasis (QALY difference now in favour of CZP). As the influence of PASI outcomes is reduced, the differences in both the PsARC response rate and the HAQ-DI change scores conditional on PsARC response between the treatments become more important. Although the PsARC response rate was estimated to be marginally higher for 150 mg of SEC than CZP (probability = 0.58 vs. 0.57), marginally higher conditional HAQ-DI changes were then estimated for CZP than 150 mg of SEC (–0.43 vs. –0.39). In the no concomitant psoriasis subgroup, in which differences in PASI response are no longer relevant, the higher conditional HAQ-DI score assumed for CZP appears to offset the higher PsARC response rate for 150 mg of SEC. However, subsequent differences in QALY outcomes appear minor between 150 mg of SEC and CZP (0.007 QALYs in favour of CZP).
In terms of the pairwise ICERs reported versus BSC, the ICERs for CZP vary between £20,870 (moderate–severe psoriasis) and £24,744 (no concomitant psoriasis) per QALY across the psoriasis subgroups. The ICERs for SEC range from £23,052 (mild–moderate psoriasis) to £26,064 per QALY (moderate–severe psoriasis). The ICERs versus BSC for SEC do not follow the same pattern as for CZP (i.e. more favourable ICERs as severity of concomitant psoriasis increases), as a result of the different dosages assumed for SEC and the higher cost of 300 mg of SEC assumed in the moderate–severe psoriasis subgroup.
Table 93 illustrates the differences between the independent analysis and the metaregression evidence synthesis for each of the subgroups in subpopulation 1 (full results are presented in Appendix 12). The pairwise ICERs for each of the treatments compared with BSC are presented along with the optimal (or most cost-effective) treatment strategy determined based on the fully incremental ICER comparisons at thresholds of £20,000 and £30,000 per QALY.
| NMA approach | ICERs vs. BSC (£) | Optimal treatment strategy at a threshold of | |||
|---|---|---|---|---|---|
| CZP | 150 mg of SEC | 300 mg of SEC | £20,000 | £30,000 | |
| Moderate–severe psoriasis | |||||
| Independent analysis | 20,870 | – | 26,064 | BSC | CZP |
| Metaregression | 19,908 | – | 27,033 | CZP | CZP |
| Mild–moderate psoriasis | |||||
| Independent analysis | 23,052 | 21,772 | – | BSC | 150 mg of SEC |
| Metaregression | 22,446 | 21,287 | – | BSC | 150 mg of SEC |
| No concomitant psoriasis | |||||
| Independent analysis | 24,744 | 23,928 | – | BSC | 150 mg of SEC |
| Metaregression | 24,388 | 23,408 | – | BSC | 150 mg of SEC |
In summary, the differences in the pairwise ICERs estimated using the alternative synthesis models have only a minor effect. Furthermore, the optimal treatment remains consistent across the two evidence synthesis approaches using a threshold of £30,000 per QALY. At a threshold of £20,000 the optimal treatment changes in the moderate–severe subgroup. CZP is now the most cost-effective treatment as its ICER compared with BSC now falls below the threshold (£19,908), based on the results of the metaregression.
Subpopulation 2: biologic naive (two or more prior DMARDs)
The cost-effectiveness results for subpopulation 2 are reported according to the level of concomitant psoriasis (moderate–severe psoriasis, mild–moderate psoriasis and no concomitant psoriasis) in Tables 94–96.
As discussed in Choice of intervention and comparators, it is assumed that, after failing the first biologic treatment, patients move (switch) to UST as a second-line treatment before moving to BSC. In the moderate–severe subgroup (Table 94), 300 mg of SEC treatment is compared in this population, as opposed to 150 mg of SEC, as the licence for SEC states that a 300-mg dose is appropriate for patients with severe psoriasis (PASI score of > 10 units). The cost-effectiveness results for this subgroup show that 300 mg of SEC is dominated by other comparators (ADA, GOL and ETN), as it incurs higher costs and results in fewer QALYs. CZP is extendedly dominated (by a linear combination of ADA and BSC). Of the remaining non-dominated alternatives, the ICER of ADA versus BSC is £20,074 per QALY, the ICER of GOL versus ADA is £20,976 per QALY, the ICER of ETN versus GOL is £21,215 per QALY and the ICER of INF is £131,716 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 95,965 | 5.312 | – | – | – | – |
| CZP | 137,240 | 7.226 | – | – | Extendedly dominateda | 21,564 |
| 300 mg of SEC | 157,086 | 7.379 | – | – | Dominateda | 29,569 |
| ADA | 138,109 | 7.411 | 42,144 | 2.100 | 20,074 | 20,074 |
| GOL | 142,850 | 7.637 | 4741 | 0.226 | 20,976 | 20,161 |
| ETN | 144,585 | 7.719 | 1735 | 0.082 | 21,215 | 20,197 |
| INF | 167,126 | 7.890 | 22,541 | 0.171 | 131,716 | 27,599 |
The individual pairwise ICERs for CZP and 300 mg of SEC compared with BSC are £21,564 and £29,569 per QALY, respectively.
Table 95 shows the results for the mild–moderate psoriasis subgroup. In this subgroup CZP is the least effective biologic treatment, generating 7.537 QALYs, whereas INF generates the highest QALYs (8.161). Fully incremental analysis shows that CZP is dominated by 150 mg of SEC, GOL is dominated by ETN, and ADA is extendedly dominated (linear combination of 150 mg of SEC and ETN). Of the remaining non-dominated alternatives, the ICER of 150 mg of SEC versus BSC is £22,032 per QALY, the ICER of ETN versus 150 mg of SEC is £23,256 per QALY and the ICER of INF versus ETN is £193,063 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 67,000 | 5.676 | – | – | – | – |
| CZP | 111,856 | 7.537 | – | – | Dominateda | 24,103 |
| 150 mg of SEC | 108,508 | 7.560 | 41,508 | 1.884 | 22,032 | 22,032 |
| ADA | 114,039 | 7.708 | – | – | Extendedly dominateda | 23,149 |
| GOL | 119,624 | 7.923 | – | – | Dominateda | 23,419 |
| ETN | 119,326 | 8.025 | 10,818 | 0.465 | 23,256 | 22,274 |
| INF | 145,569 | 8.161 | 26,243 | 0.136 | 193,063 | 31,616 |
The individual pairwise ICERs for CZP and 150 mg of SEC compared with BSC are £24,103 and £22,032 per QALY, respectively.
For the no concomitant psoriasis subgroup (PASI score = 0) (Table 96), INF maintains its position as the most effective treatment (8.543 QALYs), whereas 150 mg of SEC is now the least effective option. As expected in this subgroup, the ICERs versus BSC increase compared with the mild–moderate and severe psoriasis subgroups, as a result of the benefits being driven entirely by HAQ-DI as opposed to a combination of HAQ-DI and PASI. The incremental cost-effectiveness analysis shows that GOL is dominated by ETN and 150 mg of SEC, and CZP and ADA are extendedly dominated. Of the non-dominated alternatives, the ICER of ETN versus BSC is £23,833 per QALY and the ICER of INF versus ETN is £324,502 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 51,436 | 6.188 | – | – | – | – |
| 150 mg of SEC | 95,632 | 7.972 | – | – | Extendedly dominateda | 24,773 |
| CZP | 98,060 | 7.974 | – | – | Extendedly dominateda | 26,105 |
| ADA | 100,893 | 8.125 | – | – | Extendedly dominateda | 25,532 |
| GOL | 106,895 | 8.325 | – | – | Dominateda | 25,951 |
| ETN | 105,592 | 8.456 | 54,156 | 2.268 | 23,883 | 23,883 |
| INF | 133,664 | 8.543 | 28,071 | 0.087 | 324,502 | 34,930 |
The individual pairwise ICERs for CZP and 150 mg of SEC compared with BSC are £26,105 and £24,773 per QALY, respectively.
Table 97 summarises the differences between the independent analysis and the metaregression evidence synthesis for each of the separate psoriasis subgroups within subpopulation 2 (full results are available in Appendix 12). The pairwise ICERs for each of the treatments compared with BSC are presented along with the optimal (or most cost-effective) treatment at thresholds of £20,000 and £30,000 per QALY, using the full incremental results. Although there are only minimal differences in the pairwise ICERs, in this subpopulation the optimal treatment alters across the two evidence synthesis approaches. Both approaches accord in terms of the optimal strategy at a threshold of £20,000 for the mild–moderate and no concomitant psoriasis subgroups. In the moderate–severe subgroup, the ICER for CZP (compared with BSC – its next best) falls below £20,000; therefore, at this threshold it represents the optimal treatment. Using the metaregression estimates, CZP, as opposed to ETN, represents the most cost-effective option at a threshold value of £30,000 per QALY in the moderate–severe psoriasis group. The optimal treatment switches from ETN to 150 mg of SEC in the mild–moderate and non-concomitant psoriasis subgroups. These differences are driven by the increased relative effectiveness of CZP and 150 mg of SEC in the metaregression approach (see Chapter 4).
| NMA approach | ICERs vs. BSC (£) | Optimal treatment strategy at a threshold of | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CZP | 150 mg of SEC | 300 mg of SEC | ADA | GOL | ETN | INF | £20,000 | £30,000 | |
| Moderate–severe psoriasis | |||||||||
| Independent analysis | 21,564 | – | 29,569 | 20,074 | 20,074 | 20,197 | 27,599 | BSC | ETN |
| Metaregression | 19,923 | – | 30,456 | 20,092 | 20,767 | 20,552 | 29,138 | CZP | CZP |
| Mild–moderate psoriasis | |||||||||
| Independent analysis | 24,103 | 22,032 | – | 23,149 | 23,419 | 22,274 | 31,616 | BSC | ETN |
| Metaregression | 22,939 | 21,177 | – | 23,130 | 23,408 | 22,750 | 32,703 | BSC | 150 mg of SEC |
| No concomitant psoriasis | |||||||||
| Independent analysis | 26,105 | 24,773 | – | 25,532 | 25,951 | 23,883 | 34,930 | BSC | ETN |
| Metaregression | 25,275 | 23,768 | – | 25,485 | 25,475 | 24,460 | 35,689 | BSC | 150 mg of SEC |
Subpopulation 3: biologic experienced
Tables 98–100 present the results for subpopulation 3 for the moderate–severe, mild–moderate and no concomitant psoriasis subgroups, respectively. Only an independent analysis is available for this subpopulation, because of the smaller number of data available (see Sources of effectiveness data). In this subpopulation, 300 mg of SEC is considered as a relevant comparator, alongside UST and BSC. The clinical trial data for UST and 300 mg of SEC come from a mix of biologic-experienced patients: those who have not responded to biologic treatment (primary non-responders) and those who have responded but subsequently failed the treatment (secondary failures). CZP is not included in this model as only patients who had a primary response to a biologic treatment (secondary failures) were included in the RAPID-PsA trial. 47 Primary non-responders were explicitly excluded from this trial and, therefore, the population represents a separate subgroup of the overall biologic-experienced subpopulation (those that have previously had a response). The results for CZP are presented separately in Results of subgroup analysis: biologic-experienced secondary failures.
Table 98 shows the results of the moderate–severe psoriasis subgroup. The most effective and expensive treatment is 300 mg of SEC, generating greater QALYs than UST (6.632 vs. 6.334 QALYs) and incurring higher costs (£143,534 vs. £118,127). In the fully incremental analysis, the ICER of UST versus BSC is £21,684 per QALY and the ICER of 300 mg of SEC is £85,013 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 95,965 | 5.312 | – | – | – | – |
| UST | 118,127 | 6.334 | 22,162 | 1.022 | 21,684 | 21,685 |
| 300 mg of SEC | 143,534 | 6.632 | 25,407 | 0.299 | 85,013 | 36,013 |
The individual pairwise ICER for 300 mg of SEC compared with BSC is £36,013.
Table 99 shows the results of the mild–moderate psoriasis subgroup. In this subgroup, 300 mg of SEC is the most effective and expensive treatment, generating more QALYs than UST (6.945 vs. 6.666) and incurring higher costs (£118,564 vs. £91,246). In the fully incremental analysis, the ICER of UST versus BSC is £24,510 per QALY and the ICER of 300 mg of SEC versus UST is £97,713 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 67,000 | 5.676 | – | – | – | – |
| UST | 91,246 | 6.666 | 24,246 | 0.989 | 24,510 | 24,510 |
| 300 mg of SEC | 118,564 | 6.945 | 27,318 | 0.280 | 97,713 | 40,639 |
The individual pairwise ICER for 300 mg of SEC compared with BSC is £40,639.
Table 100 shows the results of non-evaluable psoriasis subgroup The most effective and expensive treatment is 300 mg of SEC, generating more QALYs than UST (7.384 vs. 7.132 QALYs) and incurring higher costs (£104,973 vs. £76,712). In the fully incremental analysis, the ICER of UST versus BSC is £26,797 per QALY and the ICER of 300 mg of SEC versus UST is £111,927 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 51,436 | 6.188 | – | – | – | – |
| UST | 76,712 | 7.132 | 25,275 | 0.943 | 26,797 | 26,797 |
| 300 mg of SEC | 104,973 | 7.384 | 28,261 | 0.252 | 111,927 | 44,774 |
The individual pairwise ICER for 300 mg of SEC compared with BSC is £44,774.
Subpopulation 4: TNF-α inhibitors contraindicated
As described in Patient characteristics, a separate scenario is required for patients in whom existing TNF-α inhibitors (INF, ETN, ADA and GOL) are contraindicated. These patients are likely to be a combination of biologic-naive and biologic-experienced patients who have experienced a significant AE. SEC, UST and BSC were included as comparators. CZP was not included as it was assumed that other TNF-α inhibitors, including CZP, would also be contraindicated in these patients. As described in Sources of effectiveness data, in the absence of effectiveness data specific to these patients, the analysis was undertaken using the naive populations from the SEC and UST trials. Only an independent analysis is available for this subpopulation, because of the smaller number of data available (see Sources of effectiveness data).
Table 101 shows the results of the moderate–severe psoriasis subgroup. The most effective and expensive treatment is 300 mg of SEC, generating more QALYs than UST (6.530 vs. 6.274 QALYs) and incurring higher costs (£137,936 vs. £115,216). In the fully incremental analysis, the ICER of UST versus BSC is £19,969 per QALY and the ICER of 300 mg of SEC versus UST is £89,302 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 95,965 | 5.312 | – | – | – | – |
| UST | 115,216 | 6.276 | 19,252 | 0.964 | 19,969 | 19,969 |
| 300 mg of SEC | 137,936 | 6.530 | 22,720 | 0.254 | 89,302 | 34,445 |
The individual pairwise ICER for 300 mg of SEC compared with BSC is £34,445.
Table 102 shows the results of the mild–moderate psoriasis subgroup. The most effective treatment is 150 mg of SEC, generating more QALYs than UST (6.739 vs. 6.613 QALYs). It incurs lower costs than UST (£87,559 vs. £88,280). In the fully incremental analysis, UST is dominated by 150 mg of SEC. The ICER of 150 mg of SEC versus BSC is £19,349 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 67,000 | 5.676 | – | – | – | – |
| UST | 88,280 | 6.613 | Dominateda | – | – | 22,708 |
| 150 mg of SEC | 87,559 | 6.739 | 20,558 | 1.063 | 19,349 | 19,349 |
Table 103 shows the results of the no concomitant psoriasis subgroup. The most effective and expensive treatment is 150 mg of SEC, generating more QALYs than UST (7.190 vs. 7.088 QALYs) and incurring higher costs (£73,798 vs. £73,717). In the fully incremental analysis, UST is extendedly dominated by 150 mg of SEC. The ICER of 150 mg of SEC compared with BSC is £22,334 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 51,436 | 6.188 | – | – | – | – |
| UST | 73,717 | 7.088 | – | – | Extendedly dominateda | 24,781 |
| 150 mg of SEC | 73,798 | 7.190 | 22,362 | 1.001 | 22,334 | 22,334 |
Results of the scenario analyses
As discussed in Scenario analyses, a number of scenario analyses were conducted to explore the impact of various model assumptions. These scenarios were conducted for the three main subpopulations and were intended to accord with assumptions and data employed in the CSs. These scenarios therefore aid comparison across the models (see Scenario analyses).
Details of the scenarios are given in Scenario analyses. First, baseline HAQ-DI score is specified according to the subpopulation of interest. Second, the costs assigned according to HAQ-DI score were taken from Poole et al. 138 as opposed to Kobelt et al. 129 Third, two alternative withdrawal scenarios were specified. The results of these alternative scenarios are summarised in Tables 104–106 for each of the three main subpopulations. The pairwise ICERs for each of the treatments compared with BSC are presented along with the optimal (or most cost-effective) treatment at thresholds of £20,000 and £30,000 per QALY, using the fully incremental ICERs. List prices are used in all of these scenarios. Independent analyses from the evidence synthesis are also employed throughout. The HAQ-DI costs and withdrawal scenarios are specified for only subpopulations 2 and 3. The full results for these scenarios are presented in Appendix 13.
Table 104 illustrates the differences between the base case and the alternative scenarios for each of the concomitant psoriasis subgroups in subpopulation 1. The optimal treatment is consistent across the two scenarios, base case and using a subpopulation-specific baseline HAQ-DI score. In the moderate–severe subgroup, the optimal treatment is BSC at a threshold of £20,000 and CZP at a threshold of £30,000. In the mild–moderate and no concomitant subgroups, the optimal treatment is BSC at a threshold of £20,000 and 150 mg of SEC at a threshold of £30,000. The lower ICERs for SEC in these two subgroups are driven by the lower acquisition costs of the 150-mg dose than of the 300-mg dose used in the moderate–severe subgroup.
| Scenario | ICERs vs. BSC (£) | Optimal treatment strategy at a threshold of | |||
|---|---|---|---|---|---|
| CZP | 150 mg of SEC | 300 mg of SEC | £20,000 | £30,000 | |
| Moderate–severe psoriasis | |||||
| Base case | 20,870 | – | 26,064 | BSC | CZP |
| Baseline HAQ-DI by subpopulation | 20,709 | – | 25,873 | BSC | CZP |
| Mild–moderate psoriasis | |||||
| Base case | 23,052 | 21,772 | – | BSC | 150 mg of SEC |
| Baseline HAQ-DI by subpopulation | 22,874 | 21,604 | – | BSC | 150 mg of SEC |
| No concomitant psoriasis | |||||
| Base case | 24,744 | 23,928 | – | BSC | 150 mg of SEC |
| Baseline HAQ-DI by subpopulation | 24,543 | 23,732 | – | BSC | 150 mg of SEC |
Table 105 illustrates the differences between the base case and the alternative scenarios for each of the subgroups in subpopulation 2. Aside from the use of the HAQ-DI costs reported by Poole et al. ,138 the optimal treatment is consistent across all scenarios, BSC at a threshold of £20,000 and ETN at a threshold of £30,000. Using the Poole et al. 138 costs significantly reduces the ICERs for all treatments relative to BSC, as it estimates a much higher cost for BSC. As a result, ETN, as opposed to BSC, is considered to be the most cost-effective treatment at a threshold of £20,000. At a threshold of £30,000, ETN remains the optimal treatment despite the reduced ICERs for all the treatments.
| Scenario | ICERs vs. BSC (£) | Optimal treatment strategy at a threshold of | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CZP | 150 mg of SEC | 300 mg of SEC | ADA | GOL | ETN | INF | £20,000 | £30,000 | |
| Moderate–severe psoriasis | |||||||||
| Base case | 21,564 | – | 29,569 | 20,074 | 20,074 | 20,197 | 27,599 | BSC | ETN |
| Baseline HAQ-DI by subpopulation | 21,809 | – | 29,877 | 20,295 | 20,384 | 20,409 | 27,866 | BSC | ETN |
| Poole et al.138 HAQ-DI costs | 3115 | – | 13,500 | 3069 | 3244 | 2842 | 13,036 | ETN | ETN |
| Withdrawal scenario 1 | 21,560 | – | 30,461 | 20,074 | 20,161 | 20,197 | 27,599 | BSC | ETN |
| Withdrawal scenario 2 | 21,791 | – | 29,562 | 20,406 | 20,545 | 20,555 | 27,750 | BSC | ETN |
| Mild–moderate psoriasis | |||||||||
| Base case | 24,103 | 22,032 | – | 23,149 | 23,419 | 22,274 | 31,616 | BSC | ETN |
| Baseline HAQ-DI by subpopulation | 24,395 | 22,294 | – | 23,418 | 23,687 | 22,514 | 31,938 | BSC | ETN |
| Poole et al.138 HAQ-DI costs | 3205 | 1698 | – | 3171 | 3358 | 2913 | 13,526 | ETN | ETN |
| Withdrawal scenario 1 | 24,107 | 21,291 | – | 23,153 | 23,418 | 22,274 | 31,616 | BSC | ETN |
| Withdrawal scenario 2 | 24,459 | 22,267 | – | 23,623 | 23,946 | 22,734 | 31,911 | BSC | ETN |
| No concomitant psoriasis | |||||||||
| Base case | 26,105 | 24,773 | – | 25,532 | 25,951 | 23,883 | 34,930 | BSC | ETN |
| Baseline HAQ-DI by subpopulation | 26,444 | 25,096 | – | 25,851 | 26,267 | 24,150 | 35,311 | BSC | ETN |
| Poole et al.138 HAQ-DI costs | 3341 | 1794 | – | 3328 | 3531 | 3018 | 14,279 | ETN | ETN |
| Withdrawal scenario 1 | 26,117 | 24,219 | – | 25,542 | 25,951 | 23,883 | 34,930 | BSC | ETN |
| Withdrawal scenario 2 | 26,570 | 25,138 | – | 26,129 | 26,604 | 24,427 | 35,352 | BSC | ETN |
Table 106 illustrates the differences between the base case and the alternative scenarios for each of the subgroups in subpopulation 3. Like subpopulation 2, aside from the use of the Poole et al. 138 costs, the optimal treatment is consistent across all scenarios: BSC at a threshold of £20,000 and UST at a threshold of £30,000. Using the Poole et al. 138 costs significantly reduces the ICERs for all treatments relative to BSC, as it estimates a much higher cost for BSC (see Appendix 13, Alternative Health Assessment Questionnaire-Disability Index costs from Poole et al. ). As a result, UST, as opposed to BSC, is considered to be the most cost-effective treatment at a threshold of £20,000. At a threshold of £30,000, UST remains the optimal treatment, despite the reduced ICERs across all treatments.
| Scenario | ICERs vs. BSC (£) | Optimal treatment strategy at a threshold of | ||
|---|---|---|---|---|
| UST | 300 mg of SEC | £20,000 | £30,000 | |
| Moderate–severe psoriasis | ||||
| Base case | 21,685 | 36,013 | BSC | UST |
| Baseline HAQ-DI by subpopulation | 22,309 | 26,926 | BSC | UST |
| Poole et al.138 HAQ-DI costs | 2778 | 20,154 | UST | UST |
| Withdrawal scenario 1 | 21,685 | 35,876 | BSC | UST |
| Withdrawal scenario 2 | 21,829 | 36,276 | BSC | UST |
| Mild–moderate psoriasis | ||||
| Base case | 24,510 | 40,639 | BSC | UST |
| Baseline HAQ-DI by subpopulation | 25,239 | 41,721 | BSC | UST |
| Poole et al.138 HAQ-DI costs | 2870 | 20,981 | UST | UST |
| Withdrawal scenario 1 | 24,510 | 40,749 | BSC | UST |
| Withdrawal scenario 2 | 24,763 | 41,081 | BSC | UST |
| No concomitant psoriasis | ||||
| Base case | 26,797 | 111,927 | BSC | UST |
| Baseline HAQ-DI by subpopulation | 27,638 | 46,057 | BSC | UST |
| Poole et al.138 HAQ-DI costs | 3010 | 22,264 | UST | UST |
| Withdrawal scenario 1 | 26,797 | 45,105 | BSC | UST |
| Withdrawal scenario 2 | 27,142 | 45,389 | BSC | UST |
Results of subgroup analysis: biologic-experienced secondary failures
As discussed in Subpopulation 3: biologic experienced, the RAPID-PsA trial47 includes only experienced patients who had a primary response to a biologic treatment (secondary failures), representing a specific subgroup of the overall biologic-experienced subpopulation. In the absence of data for other comparators for this subgroup, the comparison is restricted to CZP and BSC. The results for this subgroup of biologic-experienced patients are presented in Tables 107–109.
| Treatment | Cost (£) | QALY | Incremental cost (£) | Incremental QALY | ICER vs. BSC (£) |
|---|---|---|---|---|---|
| BSC | 95,965 | 5.312 | – | – | – |
| CZP | 121,314 | 6.841 | 25,349 | 1.530 | 16,573 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. BSC (£) |
|---|---|---|---|---|---|
| BSC | 67,000 | 5.676 | – | – | – |
| CZP | 95,470 | 7.166 | 28,470 | 1.490 | 19,113 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. BSC (£) |
|---|---|---|---|---|---|
| BSC | 51,436 | 6.188 | – | – | – |
| CZP | 81,447 | 7.622 | 30,011 | 1.433 | 20,937 |
In the biologic-experienced subgroup including only secondary failures, the ICERs of CZP versus BSC are £16,573, £19,113 and £20,973 for moderate–severe, mild–moderate and no concomitant psoriasis patients, respectively.
Results from the probabilistic sensitivity analysis
Results for the three main subpopulations (biologic naive with one prior DMARD, biologic naive with two or more prior DMARDs and biologic experienced), and for three separate concomitant psoriasis subgroups (baseline PASI score = 0, 7.5 or 12.5), are presented and discussed in the following sections. For ease of presentation and interpretation, only tables for the independent analysis from the evidence synthesis are presented in the main body of the report, and summary tables are used to compare with the results based on metaregression approach.
All results presented in Results are based on the list prices for SEC and CZP and the originator products for INF and ETN. A separate confidential appendix is included which incorporates the Patient Access Scheme prices for CZP and SEC.
In each of the 15 versions of the model, the expected model outputs are not equal to the output evaluated at the expected values of the parameters of the model [deterministic analysis (DA)], showing that the model is non-linear.
Subpopulation 1: biologic naive (one prior DMARD)
The probabilistic cost-effectiveness results for subpopulation 1 are shown for the three subgroups according to the level of concomitant psoriasis (moderate–severe psoriasis, mild–moderate psoriasis and no concomitant psoriasis) in Tables 110–112.
Table 110 shows that the means from the PSA imply the same optimal treatment (CZP) as the DA. The probability that CZP is cost-effective at a threshold of £20,000 is 0.39. At a threshold of £30,000 this increases to 0.53. Using the metaregression results increases the likelihood of CZP being cost-effective to 0.46 at a £20,000 threshold and to 0.63 at a £30,000 threshold.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | Pairwise ICER vs. BSC (£) | Probability of being cost-effective at a threshold of | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | |||||||
| BSC | 95,849 | 5.363 | – | – | – | – | 0.51 | 0.20 |
| CZP | 160,096 | 8.363 | 64,247 | 3.000 | 21,417 | 21,417 | 0.39 | 0.53 |
| 300 mg of SEC | 179,594 | 8.661 | 19,498 | 0.298 | 65,416 | 25,394 | 0.10 | 0.26 |
In the mild–moderate psoriasis group (Table 111), again the cost-effectiveness results from the means of the PSA are similar to the results obtained from the DA; 150 mg of SEC represents the optimal treatment at a threshold between £20,000 and £30,000. This is highly uncertain; the probability that CZP is cost-effective at threshold of £20,000 is 0.17. At a threshold of £30,000 this increases to 0.30. Using the metaregression results again produces similar results.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | Pairwise ICER vs. BSC (£) | Probability of being cost-effective at a threshold of | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | |||||||
| BSC | 66,885 | 5.727 | – | – | – | – | 0.46 | 0.20 |
| CZP | 135,999 | 8.653 | 69,114 | 2.926 | Dominated | 23,621 | 0.17 | 0.30 |
| 150 mg of SEC | 132,284 | 8.822 | –3714 | 0.168 | 21,136 | 21,136 | 0.37 | 0.50 |
In the no concomitant psoriasis subgroup (Table 112), the probabilistic results again imply the same optimal treatment (150 mg of SEC). The probability that 150 mg of SEC is cost-effective at a threshold of £20,000 is 0.28. This increases to 0.45 at a threshold of £30,000. Using metaregression analysis gives very similar results.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | Pairwise ICER vs. BSC (£) | Probability of being cost-effective at a threshold of | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | |||||||
| BSC | 51,321 | 6.239 | – | – | – | – | 0.59 | 0.26 |
| CZP | 122,839 | 9.061 | 71,518 | 2.822 | Dominated | 25,342 | 0.13 | 0.29 |
| 150 mg of SEC | 120,028 | 9.204 | –2810 | 0.142 | 23,177 | 23,177 | 0.28 | 0.45 |
Table 113 illustrates the differences between the independent analysis and the metaregression evidence synthesis for each of the subgroups in subpopulation 1 using the means from the PSA. The pairwise ICERs for each of the treatments compared with BSC are presented along with the optimal (or most cost-effective) treatment strategy determined based on the fully incremental ICER comparisons at thresholds of £20,000 and £30,000 per QALY.
| NMA approach | ICERs vs. BSC (£) | Optimal treatment strategy at a threshold of | |||
|---|---|---|---|---|---|
| CZP | 150 mg of SEC | 300 mg of SEC | £20,000 | £30,000 | |
| Moderate–severe psoriasis | |||||
| Independent analysis | 21,417 | – | 25,394 | BSC | CZP |
| Metaregression | 20,621 | – | 26,766 | BSC | CZP |
| Mild–moderate psoriasis | |||||
| Independent analysis | 23,621 | 21,136 | – | BSC | 150 mg of SEC |
| Metaregression | 23,280 | 20,993 | – | BSC | 150 mg of SEC |
| No concomitant psoriasis | |||||
| Independent analysis | 25,342 | 23,177 | – | BSC | 150 mg of SEC |
| Metaregression | 25,334 | 23,090 | – | BSC | 150 mg of SEC |
In summary, the differences in the pairwise ICERs estimated using the alternative synthesis models have only a minor effect. Furthermore, the optimal treatment remains consistent across the two evidence synthesis approaches using a threshold of £30,000 per QALY. At a threshold of £20,000 the optimal treatment is BSC, unlike the DA results. The ICER for CZP compared with BSC now is beyond the threshold (£20,621) based on the results of the metaregression.
Subpopulation 2: biologic naive (two or more prior DMARDs)
The means from the PSA for subpopulation 2 are reported according to the level of concomitant psoriasis (moderate–severe psoriasis, mild–moderate psoriasis and no concomitant psoriasis) in Tables 114–116.
In the moderate–severe subgroup (Table 114), the PSA results imply a different optimal treatment from the DA results; it switches from ETN to GOL. This is driven by the skewed nature of the PASI 75 data. Figure 20 shows that the PASI 75 data for ETN have the widest variation, with the mean having greater value than the median, indicating that the data are rightly skewed. PASI 75 response plays a more important role in this subgroup than in those with the mild–moderate or no concomitant psoriasis.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | Pairwise ICER vs. BSC (£) | Probability of being cost-effective at a threshold of | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | |||||||
| BSC | 95,849 | 5.363 | – | – | – | – | 0.26 | 0.10 |
| CZP | 137,306 | 7.255 | 41,457 | 1.893 | Extendedly dominated | 21,906 | 0.13 | 0.11 |
| ADA | 138,117 | 7.494 | 811 | 0.239 | Extendedly dominated | 19,831 | 0.16 | 0.16 |
| 300 mg of SEC | 156,926 | 7.531 | 18,809 | 0.036 | Dominated | 28,176 | 0.03 | 0.07 |
| GOL | 142,645 | 7.753 | –14,281 | 0.223 | 19,577 | 19,577 | 0.20 | 0.23 |
| ETN | 144,518 | 7.800 | 1873 | 0.047 | 39,854 | 19,968 | 0.21 | 0.26 |
| INF | 166,776 | 8.075 | 22,257 | 0.275 | 81,064 | 26,153 | 0.01 | 0.08 |
FIGURE 20.
Range of values and distributions for PASI 75 response for the treatments.

There is a high degree of uncertainty around the choice of optimal treatment (GOL); the probability that GOL is cost-effective is 0.20 at a threshold of £20,000 and 0.23 at a threshold of £30,000. Using the metaregression estimates reduces the difference between the QALYs for GOL and ETN, making ETN within the threshold of £30,000 at £25,886 per QALY compared with GOL. Again, this decision is highly uncertain; probability of being cost-effective is 0.20 and 0.25 at a threshold of £20,000 and £30,000, respectively.
Table 115 shows the results for the mild–moderate psoriasis subgroup. In this subgroup, the optimal treatment (ETN) is consistent for the PSA and DA results. The probability that ETN is cost-effective is 0.13 at a threshold of £20,000 and 0.22 at a threshold of £30,000. Using the metaregression estimates increases the decision uncertainty associated with ETN and makes 150 mg of SEC the optimal treatment within a threshold of £30,000 and with a probability of being cost-effective of 0.21.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | Pairwise ICER vs. BSC (£) | Probability of being cost-effective at a threshold of | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | |||||||
| BSC | 66,885 | 5.727 | – | – | – | – | 0.28 | 0.13 |
| CZP | 111,852 | 7.567 | 44,967 | 1.839 | Dominated | 24,446 | 0.14 | 0.12 |
| 150 mg of SEC | 108,252 | 7.712 | –3600 | 0.145 | 20,844 | 20,844 | 0.20 | 0.18 |
| ADA | 113,980 | 7.791 | 5728 | 0.079 | Extendedly dominated | 22,819 | 0.11 | 0.13 |
| GOL | 119,349 | 8.040 | 5369 | 0.248 | Dominated | 22,691 | 0.13 | 0.18 |
| ETN | 119,168 | 8.107 | –181 | 0.068 | 27,619 | 21,969 | 0.13 | 0.22 |
| INF | 145,152 | 8.346 | 25,985 | 0.238 | 108,986 | 29,893 | 0.00 | 0.05 |
For the no concomitant psoriasis subgroup (PASI score = 0) (Table 116), the choice of optimal treatment (ETN) is consistent across the PSA and DA results. The probability that ETN is cost-effective is highly uncertain, with a probability of 0.12 at a threshold of £20,000 and of 0.22 at a threshold of £30,000. Using metaregression switches the optimal treatment (see Table 8). The uncertainty associated with the optimal treatment (150 mg of SEC) is somewhat less uncertain, the probability being 0.19.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | Pairwise ICER vs. BSC (£) | Probability of being cost-effective at a threshold of | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | |||||||
| BSC | 51,321 | 6.239 | – | – | – | – | 0.33 | 0.16 |
| CZP | 98,022 | 8.004 | 46,701 | 1.765 | Dominated | 26,461 | 0.14 | 0.13 |
| 150 mg of SEC | 95,329 | 8.123 | –2693 | 0.119 | 23,356 | 23,356 | 0.19 | 0.17 |
| ADA | 100,800 | 8.208 | 5471 | 0.085 | Extendedly dominated | 25,129 | 0.10 | 0.13 |
| GOL | 106,585 | 8.441 | 5785 | 0.233 | Dominated | 25,095 | 0.11 | 0.16 |
| ETN | 105,389 | 8.538 | –1196 | 0.097 | 24,248 | 23,517 | 0.12 | 0.22 |
| INF | 133,214 | 8.726 | 27,826 | 0.188 | 148,259 | 32,932 | 0.00 | 0.03 |
Table 117 summarises the differences between the independent analysis and the metaregression evidence synthesis for each of the separate psoriasis subgroups within subpopulation 2. Although there are only minimal differences in the pairwise ICERs in this subpopulation, the optimal treatment alters across the two evidence synthesis approaches. In the moderate–severe subgroup, it switches from ETN to GOL because of the skewness of the PASI 75 data for ETN. In the mild–moderate and no concomitant subgroups, the optimal treatment switches from ETN to 150 mg of SEC. These differences are driven by the increased relative effectiveness of 150 mg of SEC in the metaregression approach.
| NMA approach | ICERs vs. BSC (£) | Optimal treatment strategy at a threshold of | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CZP | 150 mg of SEC | 300 mg of SEC | ADA | GOL | ETN | INF | £20,000 | £30,000 | |
| Moderate–severe psoriasis | |||||||||
| Independent analysis | 21,906 | – | 28,176 | 19,831 | 19,577 | 19,968 | 26,153 | BSC | GOL |
| Metaregression | 20,256 | – | 29,289 | 19,812 | 20,038 | 20,285 | 27,411 | BSC | ETN |
| Mild–moderate psoriasis | |||||||||
| Independent analysis | 24,446 | 20,844 | – | 22,819 | 22,691 | 21,969 | 29,893 | BSC | ETN |
| Metaregression | 23,279 | 20,262 | – | 22,752 | 22,543 | 22,406 | 30,690 | BSC | 150 mg of SEC |
| No concomitant psoriasis | |||||||||
| Independent analysis | 26,461 | 23,356 | – | 25,129 | 25,095 | 23,517 | 32,932 | BSC | ETN |
| Metaregression | 25,630 | 22,675 | – | 25,023 | 24,484 | 24,052 | 33,391 | BSC | 150 mg of SEC |
Subpopulation 3: biologic experienced
Tables 118–120 present the results for subpopulation 3 for the moderate–severe, mild–moderate and no concomitant psoriasis subgroups. Similar to the DA results, in the moderate–severe subgroup, UST is the optimal treatment at thresholds of £20,000 and £30,000. The probability of UST being cost-effective at a threshold of £20,000 is 0.48. This increases to 0.50 using a threshold of £30,000.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | Pairwise ICER vs. BSC (£) | Probability of being cost-effective at a threshold of | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | |||||||
| BSC | 95,849 | 5.363 | – | – | – | – | 0.44 | 0.34 |
| UST | 117,666 | 6.605 | 21,817 | 1.242 | 17,571 | 17,571 | 0.48 | 0.50 |
| 300 mg of SEC | 143,629 | 6.636 | 25,964 | 0.032 | 818,886 | 37,524 | 0.09 | 0.16 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | Pairwise ICER vs. BSC (£) | Probability of being cost-effective at a threshold of | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | |||||||
| BSC | 66,885 | 5.727 | – | – | – | – | 0.47 | 0.36 |
| UST | 90,719 | 6.935 | 23,835 | 1.208 | 19,731 | 19,731 | 0.45 | 0.49 |
| 300 mg of SEC | 118,576 | 6.950 | 27,857 | 0.014 | 1,961,907 | 42,295 | 0.07 | 0.14 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | Pairwise ICER vs. BSC (£) | Probability of being cost-effective at a threshold of | |
|---|---|---|---|---|---|---|---|---|
| £20,000 | £30,000 | |||||||
| BSC | 51,321 | 6.239 | – | – | – | – | 0.50 | 0.38 |
| 300 mg of SEC | 104,944 | 7.389 | 53,624 | 1.150 | Dominated | 46,617 | 0.07 | 0.13 |
| UST | 76,152 | 7.400 | –28,792 | 0.010 | 21,394 | 21,394 | 0.43 | 0.49 |
Table 119 shows the results for the mild–moderate psoriasis subgroup. The optimal treatment remains UST, with the probability that it is cost-effective at a threshold of £20,000 being 0.45. This increases to 0.49 at a threshold of £30,000.
Table 120 shows the results of non-evaluable psoriasis subgroup. In this subgroup, again, the choice of optimal treatment (UST) is consistent across the PSA and DA. The probability that UST is cost-effective at a threshold of £20,000 is 0.43 and at a threshold of £30,000 is 0.49.
Summary of the model results
The current York model specifies three main subpopulations according to the position in the pathway of treatment:
-
subpopulation 1: biologic naive, one previous cDMARD
-
subpopulation 2: biologic naive, two or more previous cDMARDs
-
subpopulation 3: biologic experienced.
For subpopulation 3, CZP was excluded on the basis that data were available for only a subset of biologic-experienced patients (see Patient characteristics and Choice of intervention and comparators). A separate scenario was conducted for secondary failures as a result of the availability of data for CZP. This scenario includes only CZP versus BSC.
Three subgroups are also specified within each of the three subpopulations. These subgroups refer to the severity of concomitant psoriasis:
-
no concomitant psoriasis
-
mild–moderate concomitant psoriasis
-
moderate–severe concomitant psoriasis.
A fourth subpopulation is also specified, which defines a population in which TNF-α inhibitors are contraindicated (subpopulation 4). A number of scenarios are specified to explore the robustness of some of the assumptions made in the model: rate of withdrawals beyond the first cycle and source of costs relating to HAQ-DI. In addition, separate analyses were conducted using biosimilar prices for ETN and INF and Patient Access Scheme prices for CZP and SEC.
Base-case results
Under base-case assumptions and using the independent analysis from the evidence synthesis, the results for each of the three subpopulations can be summarised as:
-
For subpopulation 1:
-
CZP is likely to be the optimal treatment in the moderate–severe psoriasis group (ICER = £20,870 compared with BSC). The individual pairwise ICER for 300 mg of SEC compared with BSC is £26,064 per QALY.
-
In the mild–moderate psoriasis group, CZP is dominated by 150 mg of SEC, which has an ICER of £21,772 compared with BSC. The individual pairwise ICER for CZP compared with BSC is £23,052 per QALY.
-
In the no concomitant psoriasis subgroup, CZP is no longer dominated by 150 mg of SEC; however, its ICER is substantial compared with 150 mg of SEC (£346,785). The ICER for 150 mg of SEC increases to £23,928, compared with BSC. The individual pairwise ICER for CZP compared with BSC is £24,774 per QALY.
-
-
For subpopulation 2:
-
ETN is likely to be the optimal treatment in the moderate–severe subgroup, with an ICER of £21,210 compared with GOL. The individual pairwise ICERs for CZP and 300 mg of SEC compared with BSC are £21,564 and £29,569 per QALY, respectively.
-
For the mild–moderate psoriasis subgroup, again ETN appears to be the optimal treatment, with an ICER of £23,256 compared with 150 mg of SEC. The individual pairwise ICERs for CZP and 150 mg of SEC compared with BSC are £24,103 and £22,032 per QALY, respectively.
-
For the no concomitant psoriasis subgroup, the ICERs increase for all treatments. ETN is likely to be the optimal treatment in this subgroup, with an ICER of £23,883 compared with BSC. The individual pairwise ICERs for CZP and 150 mg of SEC compared with BSC are £24,103 and £22,032 per QALY, respectively.
-
-
For subpopulation 3:
-
UST is likely to be the optimal treatment for the moderate–severe psoriasis subgroup, with an ICER of £21,684 compared with BSC. The individual pairwise ICER for 300 mg of SEC compared with BSC is £36,013 per QALY.
-
In the mild–moderate psoriasis subgroup, the ICER for UST compared with BSC increases to £24,510. The individual pairwise ICER for 300 mg of SEC compared with BSC is £40,639 per QALY.
-
In the non-evaluable psoriasis subgroup, UST is likely to be the optimal treatment, at thresholds below £30,000, with an ICER of £26,797 compared with BSC. The individual pairwise ICER for 300 mg of SEC compared with BSC is £44,774 per QALY.
-
For subpopulations 1 and 2, separate effectiveness results are also available utilising a metaregression approach. The differences between the independent analysis and the metaregression can be summarised as:
-
In subpopulation 1 the use of the metaregression evidence has a minimal impact on the pairwise ICERs; however, at a threshold of £20,000 the optimal treatment changes in the moderate–severe subgroup. CZP is now likely to be the most cost-effective treatment, as its ICER, compared with BSC, falls below the threshold (£19,908).
-
In subpopulation 2, again, there are only minimal differences in the pairwise ICERs; however, the optimal treatment is not consistent across the two evidence synthesis approaches. Both approaches accord in terms of the optimal strategy at a threshold of £20,000 for the mild–moderate and no concomitant subgroups. In the moderate–severe subgroup, the ICER for CZP (compared with BSC – its next best) falls below £20,000, therefore at this threshold it represents the optimal treatment. Using the metaregression estimates, CZP, as opposed to ETN, represents the most cost-effective optimal treatment at a threshold value of £30,000 per QALY in the moderate–severe psoriasis group. In addition, the optimal treatment switches from ETN to 150 mg of SEC in the mild–moderate and no concomitant psoriasis subgroups.
In the contraindicated subgroup (subpopulation 4):
-
UST appears to the most cost-effective treatment in moderate–severe psoriasis patients, with an ICER of £19,969 compared with BSC. The individual pairwise ICER for 300 mg of SEC compared with BSC is £34,445 per QALY.
-
In mild–moderate psoriasis patients, UST is dominated by 150 mg of SEC. Compared with BSC, 150 mg of SEC has an ICER of £19,349.
-
In the no concomitant psoriasis patients, UST is extendedly dominated by 150 mg of SEC. Compared with BSC, 150 mg of SEC has an ICER of £22,334.
In the biologic-experienced subgroup, including only secondary failures, CZP seems to be the cost-effective treatment compared with BSC, with ICERs of £16,573, £19,113 and £20,973 for moderate–severe, mild–moderate and no concomitant psoriasis patients, respectively.
Results using biosimilar prices
When using biosimilar prices for ETN and INF in subpopulation 2, the ICERs for ETN compared with BSC and for INF compared with ETN decrease. The ICER for ETN compared with its next best alternative (BSC) in the moderate–severe subgroup falls below the threshold of £20,000; therefore, at this threshold, using the biosimilar prices for ETN, the optimal treatments switches from BSC to ETN. For the mild–moderate and no concomitant psoriasis subgroups the optimal treatments remains unchanged.
Scenario results
A number of scenarios were specified to explore the sensitivity of results to some of the assumptions made in the model. Alternative scenarios were specified for the three main subpopulations, although withdrawal scenarios and the use of Poole et al. 138 costs were conducted only for subpopulations 2 and 3. List prices and originator products (ETN and INF) are used in all of these scenarios. Independent analyses from the evidence synthesis are also employed throughout. The results can be summarised as:
-
In subpopulation 1, the optimal treatment is consistent across the two scenarios, base case and using a subpopulation-specific baseline HAQ-DI score.
-
In subpopulation 2, aside from the use of the Poole et al. 138 HAQ-DI costs, the optimal treatment is consistent across all scenarios. Using the Poole et al. 138 costs significantly reduces the ICERs for all treatments relative to BSC, as it estimates a much higher cost for BSC. As a result, ETN, as opposed to BSC, is identified to be the most cost-effective treatment at a threshold of £20,000 per QALY. At a threshold of £30,000 per QALY, ETN remains the optimal treatment despite the reduced ICERs for all the treatments.
-
In subpopulation 3, aside from the use of the Poole et al. 138 costs, the optimal treatment is consistent across all scenarios. Using the Poole et al. 138 costs significantly reduces the ICERs for all treatments relative to BSC, as it estimates a much higher cost for BSC. As a result, UST, as opposed to BSC, is considered to be the most cost-effective treatment at a threshold of £20,000 per QALY. At a threshold of £30,000 per QALY, UST remains the optimal treatment despite the reduced ICERs across all treatments.
Probabilistic sensitivity analysis
-
In all subpopulations and subgroups according to level of psoriasis, the PSA demonstrates considerable decision uncertainty regarding the optimal treatment, at both £20,000 and £30,000 thresholds.
-
The ICERs are broadly consistent between the deterministic and the means of the PSA. Although there are only small differences in the ICER, the optimal treatment does change in a few instances:
-
in subpopulation 1, at a threshold of £20,000 the optimal treatment is BSC, unlike the deterministic results, where either CZP or SEC are optimal
-
in subpopulation 2 the optimal treatment changes in the moderate–severe subgroup, from ETN in the deterministic results to GOL in the means of the PSA.
-
External validation of results
Comparison of updated York model results with company model results
In the absence of a list price analysis from either of the companies, it is not possible to make direct comparisons between the updated York model results and those from the Novartis and UCB Pharma submissions. In general, the structure and approaches of both company models were similar in many key respects to the updated York model and models developed as part of previous appraisals. However, as highlighted in Chapter 5, further challenges arise when trying to make comparisons between the results of the updated York model, similar to those we faced when trying to make comparisons between the CSs, given the differences identified in the approaches and data sources employed. On this basis we consider that direct comparisons between the ICER results would not be sufficiently meaningful.
The main advantage of the York model is that it facilitates a more consistent basis for evaluating CZP and SEC by ensuring comparability in methods and inputs (including prices). In addition, the York model attempts to include all relevant treatments within each subpopulation and more explicitly considers issues around the appropriate dosing for SEC by undertaking separate subgroup analyses based on the presence and severity of concomitant psoriasis.
Comparison of updated York model results with published models’ results
It is possible to compare some of the results of the updated York model with those from previously published models, namely the three models developed as part of previous appraisals in this area (TA199,33 TA220133 and TA34035), and a published update of the previous York model by Cawson et al. 36 (see Table 3). This comparison is somewhat restricted by the more limited scope in previously published models. In TA199,33 TA220133 and Cawson et al. ,36 only subpopulation 2 was considered. TA34035 also included an analysis for subpopulations 3 and 4 together [experienced and contraindicated (termed ineligible)]. All previously published models looked at the extent of concomitant psoriasis; however, this was included only as limited scenario analyses and full results are only available for the average severity of psoriasis: mild–moderate. It is also noted that none of the previously published models included the comparators CZP or SEC.
In terms of the results for subpopulation 2, the ICERs for ETN versus the next best treatment are broadly consistent across the updated York model and the four published models (£16,426 in Cawson et al. 36 to £23,256 in the updated York model, mild–moderate psoriasis subgroup). For subpopulation 3, TA34035 included a separate analysis of a biologic-experienced/contraindicated population for UST. In this analysis, the ICER for UST compared with BSC was £25,393. This result is very similar to those from subpopulation 3 of the updated York model results, in which the ICER for UST compared with BSC, in the mild–moderate psoriasis subgroup, is £24,510. In the contraindicated subgroup (subpopulation 4 of the York model), in mild–moderate psoriasis patients, the ICER for UST compared with BSC is again broadly consistent at £22,708. In the full incremental analysis for this subpopulation, however, UST is dominated by 150 mg of SEC and 150 mg of SEC has an ICER of £19,349 compared with BSC.
Discussion of the York model
The previous York model has been updated for this appraisal. This includes an update of the evidence used to populate the model and a number of updates to the model structure and assumptions. Specifically, the updated York model differs from the previous York in several respects:
-
The model now incorporates subsequent biologic treatments following primary lack of response or secondary failure.
-
The model now includes the three subpopulations specified in the NICE scope112 for this appraisal.
-
Rather than presenting a single base case reflecting an ‘average’ PsA patient, heterogeneity in terms of baseline PASI score is now formally addressed by presenting results for three distinct subgroups within each subpopulation.
In addition, the updated York model includes the comparators CZP and SEC and considers the cost-effectiveness of these treatments in each of the subpopulations. The updated York model also considers several key uncertainties: the acquisition cost of SEC and CZP (list or Patient Access Scheme prices); the products for ETN and INF (originator or biosimilar); the source algorithm used to link progression in HAQ-DI score to costs; and assumptions regarding the longer-term rate of withdrawal for primary responders.
The model utilises all currently available evidence to generate estimates of clinical effectiveness using NMA. Alternative models are specified for the NMA, and a more limited set of models is chosen on the basis of model fit, goodness-of-fit statistics and clinical plausibility. These alternative models (independent analysis and metaregression) are each used in the economic model and the sensitivity of model results to these alternative evidence synthesis models assessed.
Using list prices, SEC and CZP are likely to be considered cost-effective only in subpopulation 1 (biologic naive, one prior DMARD). In subpopulation 2, ETN is likely to be the optimal treatment across all psoriasis subgroups and, in subpopulation 3, UST is likely to be the optimal treatment across all psoriasis subgroups. The cost-effectiveness results are, however, sensitive to a number of assumptions made in the model, namely the choice of NMA model used to determine clinical effectiveness and the algorithm used to link HAQ-DI score to health state costs.
The updated York model also has a number of limitations, which have largely been imposed by a lack of available data to inform aspects of the model. First, subpopulation 1 includes only the comparators CZP, SEC and BSC, as per the NICE scope. 112 It is recognised, however, that there may be other comparators relevant for this subpopulation. In particular, patients who have received only one prior DMARD may be eligible to receive a second DMARD. It was not possible within the scope of this appraisal to assess the evidence for DMARDs and, therefore, include this as a formal comparator in this subpopulation. The extremely low cost of DMARDs (7.5 mg of MTX is £0.30) makes it likely that these would be considered cost-effective in this population. In addition, the licences for the other biologic treatments (ETN, INF, ADA and GOL) do not preclude their use in the one-DMARD population and, therefore, these could be considered to be relevant comparators in subpopulation 1. Indeed, this subpopulation appears to not have been considered in previously published models, largely because the scope of these models has closely followed existing BSR guidelines and criteria for commencing biologic treatments (i.e. that the PsA has not responded to adequate trials of at least two standard DMARDs, administered either individually or in combination), as opposed to reflecting important differences in the licences of existing biologic treatments and those for SEC and CZP.
Second, the clinical effectiveness evidence synthesised in the NMA does not differentiate between subpopulations 1 and 2 as a result of the limited data availability. This means that it was possible to differentiate these two populations only on the basis of the comparators included and the subsequent treatments received following primary failure or secondary withdrawal. Related to this, the subpopulation 1 analysis makes the assumption that ETN is the next treatment received, following failure of 150 mg of SEC or CZP. It is likely that other treatments could be used as second line in this population. Owing to the large number of possible treatment sequences for subpopulation 1, it was not feasible as part of this appraisal to determine the optimal sequence for all potential treatments. Modelling multiple lines of biologic treatments would also require evidence on any degradation effect for subsequent lines. Such evidence is sparse in PsA and that which exists does not consider the full set of biologic treatments considered in this appraisal.
Finally, it has not been possible to update a number of the assumptions in the York model, specifically the rate of withdrawal for primary responders, the progression in HAQ-DI score for those receiving treatment, and the progression of HAQ-DI score for those remaining on treatment. These assumptions rely on non-experimental data and, unfortunately, within the time constraints of this appraisal, it was not possible to gain access to registry data to update these assumptions, although attempts to do so were made.
Given these uncertainties and possible limitations, and the lack of direct head-to-head evidence for the alternative treatments, the results from the fully incremental cost-effectiveness analyses should be carefully considered alongside the separate pairwise comparisons presented against BSC. The significant efficacy of all biologic treatments was evident in the important QALY differences reported compared with BSC alone. In contrast, differences between the alternative biologic therapies were much less significant and, in some instances, may not be clinically meaningful. Hence, there remains considerable uncertainty in relation to defining an optimal treatment or pathway of care. The PSA also demonstrates considerable decision uncertainty regarding the optimal treatment, at both £20,000 and £30,000 thresholds.
Chapter 7 Assessment of factors relevant to the NHS and other parties
The potential extra cost to the NHS of providing SEC and CZP to adult patients with PsA is unclear, as the prevalence of UK PsA patients in subpopulation 1 is somewhat uncertain.
Chapter 8 Discussion
Statement of principal findings
The systematic review of the efficacy of SEC, CZP and relevant comparator therapies in patients with PsA identified an evidence base of generally high-quality randomised trials. The results of the pivotal randomised trials of SEC (FUTURE 2 trial48) and CZP (RAPID-PsA trial47) demonstrated their short-term efficacy for treating PsA. When considering the whole-trial populations, both SEC and CZP were associated with statistically significant improvements in all key clinical outcomes. At 3 months, patients taking SEC were around six times more likely to be ACR 50 responders – an important clinical outcome to patients – than patients taking placebo. Patients taking CZP were around three times more likely to be ACR 50 responders than placebo patients. Clinically important improvements in activities of daily living (assessed using the HAQ-DI) were also evident for both therapies, particularly in patients who were PsARC responders. In addition, both SEC and CZP significantly improved measures of HRQoL and the resolution of enthesitis and dactylitis.
However, when the populations from these two trials were split into subgroups based on previous biologic experience, results for the biologic-experienced subgroups became difficult to interpret. This was as a result of both the low numbers of placebo patients (and placebo events) and the differences in placebo response rates across subgroups; it was therefore not possible to make robust conclusions about the relative efficacy of SEC and CZP across these subgroups.
Subgroup results from PsA patients recruited to trials of patients with quite severe psoriasis suggested SEC may be particularly efficacious in treating the psoriasis symptoms of PsA.
The results from open-label trial extension studies that radiographically assessed joint damage indicated that, after 2 years of treatment, CZP effectively reduced disease progression, with benefits being similar to those observed in the open-label studies for the other biologics. For SEC, fewer result details were available at 2 years, although results also indicated effective reduction in radiographic disease progression. Meaningful treatment comparisons of longer-term data for other outcomes were difficult to undertake because of the variation in both time points assessed and in methodological approaches used for data analyses (confidential information has been removed).
The trials identified to inform a comparison of SEC and CZP with other biologics were performed across a 15-year period and variation in placebo response was evident for some important outcomes, with larger placebo response rates seen in the more recent trials. Furthermore, there was important heterogeneity across trials with regard to patients’ previous use of a biologic therapy: subgroups of biologic-experienced patients were recruited only in more recent trials. Our NMAs were therefore performed on the biologic-naive and biologic-experienced subgroups separately, and included models which adjusted for, and explored, the different rates of placebo response across trials.
The NMA results – both adjusted and unadjusted – demonstrated that, in biologic-naive patients, SEC and CZP were more effective than placebo in terms of achieving PsARC and ACR responses. There was though some uncertainty regarding the relative effectiveness of SEC and CZP when compared with each other and with all other biologics: they had fairly similar effectiveness when compared with the other anti-TNFs, although they were possibly slightly more effective than UST. However, both SEC and CZP appeared to be more effective than APR. In terms of psoriasis outcomes in biologic-naive patients, treatment with SEC and INF resulted in the best PASI results when compared with other therapies, although the differences for most comparisons were not statistically significant.
The median HAQ-DI score change, conditional on a PsARC response, was highest with INF and ETN, followed by 300 mg of SEC, but 150 mg of SEC and CZP were worse than all treatments except for APR.
Only three trials recruited biologic-experienced patients: one each of SEC, CZP and UST. Unfortunately. data from the CZP trial had to be excluded from the NMAs because this trial included a more restricted biologic-experienced population, which was not comparable to the biologic-experienced populations in the other two trials. The NMA results showed that the probabilities of PsARC and ACR responses with SEC and UST were quite similar, as was the change in HAQ-DI score in PsARC responders. Patient numbers were particularly limited for the biologic-experienced PASI analyses, as they were based on a subgroup (prior use of a biologic) of a subgroup (psoriasis on ≥ 3% of BSA), so estimates from the NMA were highly uncertain. However, the results suggested that the probabilities of achieving PASI responses were higher for SEC than for UST.
Results from studies of patient registries that recorded biologic use suggested that, although patients benefit from a second or further anti-TNFs, the expected benefit from anti-TNFs diminishes after switching, with a reduced chance of response and reduced drug survival. The paucity of observational data on the natural history of PsA meant that it was difficult to produce accurate estimates of yearly disease progression rates in patients not taking anti-TNFs.
Results from three systematic reviews of AEs suggested that CZP was associated with statistically significantly more SAEs and serious infections than placebo. SEC was not included in these systematic reviews of AEs, probably as a result of the limited availability of data at the time. Although the safety data for SEC appear promising, the fairly small number of trials for which data are currently available means that there is still some uncertainty regarding its safety.
Strengths and limitations of the assessment
Strengths
The systematic review was performed using transparent, reproducible and robust methods. Our comprehensive searches therefore sought to identify all relevant published and unpublished trials, which minimised the possibility of publication or language biases affecting the review results. The possibility of reviewer errors and biases affecting this assessment was minimised by performing review processes in duplicate. A thorough evaluation of the risk of bias in each randomised trial was performed. We conducted many NMAs to investigate the relative efficacy of all the comparator agents. Additionally, and in order to improve the methodological similarity of the trial data included in our analyses, we successfully obtained previously unpublished data relating to two key trials (for which manufacturer submission data were not available).
A further key strength of our review was the breadth of its scope: in addition to randomised trials we included other types of study, such as non-randomised trial extension studies, registry studies of patients taking anti-TNFs, systematic reviews and other large studies of adverse effects of anti-TNFs and studies of the natural history of PsA. Our review was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
The updated York model confers several advantages over current published cost-effectiveness studies, namely the inclusion of the three subpopulations according to the position in the pathway of treatment, the explicit consideration of the severity of concomitant psoriasis and the modelling of subsequent treatments following primary non-response or secondary failure. Like the company models, the updated York model includes the comparators CZP and SEC. In addition, it considers the cost-effectiveness of these treatments in each of the subpopulations and more explicitly considers issues around the appropriate dosing for SEC by undertaking separate subgroup analyses based on the presence and severity of concomitant psoriasis.
The updated York model also considers several key uncertainties: the acquisition cost of SEC and CZP (list or Patient Access Scheme prices); the products for ETN and INF (branded or biosimilars); the source algorithm used to link progression in HAQ-DI score to costs; and assumptions regarding the longer-term rate of withdrawal for primary responders.
The model utilises all currently available evidence to generate estimates of clinical effectiveness using a NMA. Alternative models are specified for the NMA, and a more limited set of models is chosen on the basis of model fit, goodness-of-fit statistics and clinical plausibility. These alternative models (independent analysis and metaregression) are each used in the economic model and the sensitivity of model results to these alternative evidence synthesis models assessed. The York model facilitates a more consistent basis for evaluating CZP and SEC by ensuring comparability in methods and inputs.
Limitations
Data from randomised, fully blinded populations were available only for up to around 3 or 4 months for most of the trials included in our review (after which patients could cross over to active treatments); much of the RCT evidence was therefore quite short term in nature. Some of the earlier trials were also limited by small sample sizes (increasing the possibility of results being attributable to chance, rather than being attributable to treatment). The variation in placebo responses over time was also a limitation of the available data, although we sought to address this in our NMAs (using metaregression adjustments). Although we also evaluated long-term results from studies that were not RCTs, data from such studies may have been affected by biases or confounding and often either key method details were absent from publications or methods were found to be suboptimal. Much less reliability and certainty could therefore be ascribed to the results obtained from these other studies.
As discussed previously, the updated York model does have a number of limitations, which have largely been imposed by a lack of available data to inform aspects of the model.
Of particular note is the fact that subpopulation 1 includes only the comparators CZP, SEC and BSC, as per the NICE scope. 112 It is recognised, however, that there may be other comparators relevant for this subpopulation. In particular, patients who have received only one prior DMARD may be eligible to receive a second DMARD. It was not possible within the scope of this appraisal to assess the evidence for DMARDs and, therefore, include this as a formal comparator in this subpopulation. In addition, the licences for the other biologic treatments (ETN, INF, ADA and GOL) do not appear to preclude their use in the one-DMARD population and, therefore, these could be considered to be relevant comparators in subpopulation 1. Indeed, this subpopulation appears to not have been considered in previous models, largely because the scope of these models has closely followed existing BSR guidelines and criteria for commencing biologic treatments (i.e. that the PsA has not responded to adequate trials of at least two standard DMARDs, administered either individually or in combination), as opposed to reflecting important differences in the licences of existing biologic treatments and those for SEC and CZP.
Uncertainties
-
The magnitude of SEC and CZP treatment effects in biologic-experienced patients is uncertain because the trial subgroup sample sizes were small, and the subgroup in the CZP trial was not appropriately representative of the biologic-experienced population that would be seen in clinical practice.
-
The limitations and variations in the design and reporting of long-term studies means that there is uncertainty whether or not there are differences in efficacy and safety between the different therapies in the long term.
-
The long-term impact of SEC and CZP (and other anti-TNFs) on other important outcomes, such as cardiovascular disease and mortality, is uncertain.
The cost-effectiveness results are potentially sensitive to a number of assumptions made in the model, namely the choice of NMA model used to determine clinical effectiveness and the algorithm used to link HAQ-DI score to health state costs. Given these uncertainties and the lack of direct head-to-head evidence for the alternative treatments, the results from the fully incremental cost-effectiveness analyses should also be considered alongside the separate pairwise comparisons presented against BSC. The significant efficacy of all biologic treatments was evident in the important QALY differences reported, compared with BSC alone. In contrast, differences between the alternative biologic therapies were much less significant and in some instances may not be clinically meaningful. Hence, there remains considerable uncertainty in relation to defining an optimal treatment or pathway of care. Indeed, the PSA demonstrates considerable decision uncertainty regarding the optimal treatment, at both £20,000 and £30,000 thresholds.
Chapter 9 Conclusions
Although the NMAs were based on data from high-quality randomised trials, heterogeneity across trials meant that the analyses had to be performed in biologic-naive and biologic-experienced subpopulations separately, and also needed to include models which adjusted for the different rates of placebo response evident across trials. The NMA results for the biologic-naive subpopulation indicated that, although SEC and CZP were effective across all outcomes after 3 months’ therapy, their relative effectiveness compared with ETN, ADA, GOL and INF and with each other was uncertain (the rankings of treatment varied with outcome and analysis). However, both agents did seem consistently more effective than APR. The results also indicated that SEC and INF were the most effective in terms of treating psoriasis (PASI response). Only SEC and UST could be included in the analyses of the biologic-experienced subpopulation. The results showed that, across all outcomes analysed, both SEC and UST were significantly more effective than placebo. Most of the results suggested that SEC may be better than UST. However, the patient numbers in this subpopulation were quite low; the results were therefore uncertain (with wide overlapping CrIs).
The results from open-label trial extension studies which radiographically assessed joint damage suggest that both CZP and SEC effectively reduce disease progression. Published systematic reviews of AEs have suggested CZP is associated with statistically significantly more SAEs and serious infections than placebo. Although the safety data for SEC appear promising, the fairly small number of trials for which data are currently available means that there is still some uncertainty regarding its safety.
Economic modelling found that these new biologics can be considered a cost-effective use of NHS resources when compared with the other therapies currently recommended by NICE for treating PsA. Which treatment is most cost-effective depends on which previous treatments a patient has tried and not responded to, the severity of the psoriasis symptoms, and the price of the treatment. Some of the study’s results were somewhat limited because not enough relevant clinical trial data were available.
Implications for service provision
-
The clinical evidence indicates that SEC and CZP are only two of a number of effective treatments for the treatment of active PsA.
-
For patients with PsA and significant psoriasis, SEC may be one of the more effective biologic treatments.
-
The limited long-term evidence suggests some beneficial impact of radiographic disease progression.
Suggested research priorities
-
Adequately powered randomised trials are needed to inform the clinical effectiveness of biologics in biologic-experienced populations.
-
Future trials should consider using newer composite disease outcome measures which have recently been developed for PsA, such as the Composite Psoriatic arthritis Disease Activity Index, the PsA disease activity score, the Disease Activity index for PSoriatic Arthritis (DAPSA) and minimal disease activity.
-
Further research is required to better elucidate the impact of biologics on radiographic disease progression and HAQ-DI score in the long term. This requires the use of real-world data.
-
With the continuing introduction of new biologic drugs and continued collection of data through biologic registries, further analysis of the data to investigate patterns of drug switching and the long-term effectiveness and safety of biologics is warranted. Radiographic outcomes should be evaluated given the significance of radiographic damage as a measure of disease progression and treatment effects.
-
Although randomised head-to-head trials – which directly compare different biologics – would yield very useful results, their design and recruitment strategies may require very careful thought. Different biologics are administered at different rates and time points; therefore, to achieve adequate blinding, patients would need both their randomised treatment injections and placebo injections corresponding to the comparator biologic regimen (i.e. patients would receive many more injections than would be needed if they took a biologic outside a trial). When considering this, together with the known benefits of biologics, and the likely large trial population that would be needed to detect efficacy differences between different biologics, consideration of trial recruitment and compliance issues should be key when conducting pilot studies. INF is delivered intravenously so would be even more difficult to study in a head-to-head blinded trial.
-
Larger-scale, longer-term studies are required to determine the HRQoL impact of response to treatment and changes in functional capacity (measured using HAQ-DI) and psoriasis (measured using PASI). These should include the full range of PsA severities.
-
Larger-scale studies are required to determine the cost implications of response to treatment and changes in functional capacity (measured using HAQ-DI) and psoriasis (measured using PASI). These should be undertaken in a PsA population and include the full range of PsA severities.
Acknowledgements
This study, carried out under YODA project #2016-0897, used data obtained from the YODA project, which has an agreement with Janssen Research & Development, LLC. The interpretation and reporting of research using this data are solely the responsibility of the authors and does not necessarily represent the official views of the YODA project or Janssen Research & Development, LLC.
We would like to thank Dr Sofia Dias, Research Fellow, School of Social & Community Medicine, University of Bristol, for advice on the evidence synthesis models.
Contributions of authors
Mark Corbett, Research Fellow and Systematic Reviewer, contributed to the protocol, study selection, data extraction, validity assessments and synthesis of the included studies. He also contributed to the interpretation of the results and the writing of the report.
Fadi Chehadah, Research Fellow and Health Economist, contributed to the protocol, the development of the economic model, the review of economic analyses, the interpretation of the results and the writing of the report.
Mousumi Biswas, Research Fellow in Health Economics, contributed to developing the synthesis models and undertook the evidence synthesis. She also contributed to the interpretation of the results and the writing of the report.
Thirimon Moe-Byrne, Research Fellow and Systematic Reviewer, contributed to the protocol, study selection, data extraction, and validity assessment of the included studies and the writing of the report.
Stephen Palmer, Professor of Health Economics, contributed to the protocol development and to all aspects of the cost-effectiveness work including the writing of the report.
Marta Soares, Senior Research Fellow in Health Economics and Statistician, contributed to developing the synthesis models and the writing of the report.
Matthew Walton, Research Training Fellow in Systematic Reviews, performed and wrote the sections of the report relating to the reviews of patient registry studies and natural history studies.
Melissa Harden, Information Specialist, contributed to the protocol development, developed the search strategies, conducted a range of searches to locate studies, and wrote the sections of the report relating to the literature searches.
Pauline Ho, Consultant Rheumatologist, provided expert clinical advice, contributed to the protocol and interpretation of the results and commented on drafts of the report.
Nerys Woolacott, Reader in Health Technology Assessment, contributed to the protocol, study selection and synthesis of the included studies. She also contributed to the interpretation of the results and the writing of the report, and took overall responsibility for the clinical effectiveness section.
Laura Bojke, Senior Research Fellow in Health Economics, had overall responsibility for the cost-effectiveness sections. She contributed to the development of the protocol, the economic model and the economic analyses. She also contributed to the interpretation of the results and the writing of the report.
Data sharing statement
Requests for access to data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Reveille JD. Spondyloarthritis 2013. www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Spondyloarthritis (accessed 28 July 2016).
- Arthritis Foundation . What Is Psoriatic Arthritis? n.d. www.arthritis.org/about-arthritis/types/psoriatic-arthritis/what-is-psoriatic-arthritis.php (accessed 7 July 2016).
- Emery P, Ash Z. Psoriatic Arthritis 2013. www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Psoriatic-Arthritis (accessed 28 July 2016).
- Shiel WC. Psoriatic Arthritis 2015. www.medicinenet.com/psoriatic_arthritis/article.htm (accessed 25 November 2015).
- Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA) – an analysis of 220 patients. Q J Med 1987;62:127-41.
- Torre Alonso JC, Rodriguez Perez A, Arribas Castrillo JM, Ballina Garcia J, Riestra Noriega JL, Lopez Larrea C. Psoriatic arthritis (PA): a clinical, immunological and radiological study of 180 patients. Br J Rheumatol 1991;30:245-50. https://doi.org/10.1093/rheumatology/30.4.245.
- Galadari H, Fuchs B, Lebwohl M. Newly available treatments for psoriatic arthritis and their impact on skin psoriasis. Int J Dermatol 2003;42:231-7. https://doi.org/10.1046/j.1365-4362.2003.01449.x.
- Ruderman EM. Evaluation and management of psoriatic arthritis: the role of biologic therapy. J Am Acad Dermatol 2003;49:125-32. https://doi.org/10.1016/S0190-9622(03)01145-9.
- Michelsen B, Fiane R, Diamantopoulos AP, Soldal DM, Hansen IJ, Sokka T, et al. A comparison of disease burden in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0123582.
- Kavanaugh A, Mease PJ, Purcaru O, van der Heijde D. High economic burden of moderate to severe psoriatic arthritis on paid work and household productivity: baseline results from the RAPID-PsA study (poster SAT0275). Ann Rheum Dis 2013;72. https://doi.org/10.1136/annrheumdis-2013-eular.2000.
- Mease P, Goffe BS. Diagnosis and treatment of psoriatic arthritis. J Am Acad Dermatol 2005;52:1-19. https://doi.org/10.1016/j.jaad.2004.06.013.
- Wong K, Gladman DD, Husted J, Long JA, Farewell VT. Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum 1997;40:1868-72. https://doi.org/10.1002/1529-0131(199710)40:10<1868::AID-ART21>3.0.CO;2-W.
- Gladman DD, Farewell VT, Wong K, Husted J. Mortality studies in psoriatic arthritis: results from a single outpatient center. II. Prognostic indicators for death. Arthritis Rheum 1998;41:1103-10. https://doi.org/10.1002/1529-0131(199806)41:6<1103::AID-ART18>3.0.CO;2-N.
- Ali Y, Tom BD, Schentag CT, Farewell VT, Gladman DD. Improved survival in psoriatic arthritis with calendar time. Arthritis Rheum 2007;56:2708-14. https://doi.org/10.1002/art.22800.
- Helliwell PS, Taylor WJ. Classification and diagnostic criteria for psoriatic arthritis. Ann Rheum Dis 2005;64:ii3-8. https://doi.org/10.1136/ard.2004.032318.
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973;3:55-78. https://doi.org/10.1016/0049-0172(73)90035-8.
- Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665-73. https://doi.org/10.1002/art.21972.
- Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64:ii14-7. https://doi.org/10.1136/ard.2004.032482.
- Salisbury NHS Foundation Trust . Referral Pathway for Psoriatic Arthritis n.d. www.icid.salisbury.nhs.uk/ClinicalManagement/Rheumatology/Pages/PsA.aspx (accessed 7 July 2016).
- Bowcock AM. Understanding the pathogenesis of psoriasis, psoriatic arthritis, and autoimmunity via a fusion of molecular genetics and immunology. Immunol Res 2005;32:45-56. https://doi.org/10.1385/IR:32:1-3:045.
- Leung YY, Tam LS, Kun EW, Li EK. Psoriatic arthritis as a distinct disease entity. J Postgrad Med 2007;53:63-71. https://doi.org/10.4103/0022-3859.30334.
- Ritchlin CT, Qureshi AA, de Vlam K, Pitzalis C, Helliwell PS, Mease PJ, et al. Biomarkers in psoriasis and psoriatic arthritis: GRAPPA 2008. J Rheumatol 2010;37:462-7. https://doi.org/10.3899/jrheum.090957.
- GRAPPA . Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) 2016. www.grappanetwork.org/ (accessed 12 July 2016).
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727-35. https://doi.org/10.1002/art.1780380602.
- Mease PJ, Antoni CE, Gladman DD, Taylor WJ. Psoriatic arthritis assessment tools in clinical trials. Ann Rheum Dis 2005;64:ii49-54. https://doi.org/10.1136/ard.2004.034165.
- Wong PC, Leung YY, Li EK, Tam LS. Measuring disease activity in psoriatic arthritis. Int J Rheumatol 2012;2012. https://doi.org/10.1155/2012/839425.
- Chang CA, Gottlieb AB, Lizzul PF. Management of Psoriatic Arthritis from the View of the Dermatologist: Assessment of PsA 2011. www.medscape.org/viewarticle/749147_2 (accessed 6 July 2016).
- Kavanaugh A, Cassell S. The assessment of disease activity and outcomes in psoriatic arthritis. Clin Exp Rheumatol 2005;23:142-7.
- Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499-510. https://doi.org/10.1136/annrheumdis-2015-208337.
- Mease PJ. Psoriatic arthritis: update on pathophysiology, assessment and management. Ann Rheum Dis 2011;70:i77-84. https://doi.org/10.1136/ard.2010.140582.
- Coates LC, Tillett W, Chandler D, Helliwell PS, Korendowych E, Kyle S, et al. The 2012 BSR and BHPR guideline for the treatment of psoriatic arthritis with biologics. Rheumatology 2013;52:1754-7. https://doi.org/10.1093/rheumatology/ket187.
- Haberhauer G, Strehblow C, Fasching P. Observational study of switching anti-TNF agents in ankylosing spondylitis and psoriatic arthritis versus rheumatoid arthritis. Wien Med Wochenschr 2010;160:220-4. https://doi.org/10.1007/s10354-010-0795-0.
- Rodgers M, Epstein D, Bojke L, Yang H, Craig D, Fonseca T, et al. Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis: a systematic review and economic evaluation. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15100.
- Yang H, Craig D, Epstein D, Bojke L, Light K, Bruce IN, et al. Golimumab for the treatment of psoriatic arthritis: a NICE single technology appraisal. PharmacoEconomics 2012;30:257-70. https://doi.org/10.2165/11595920-000000000-00000.
- O’Connor J, Rice S, Smith A, Rodgers M, Lopez RR, Craig D, et al. The clinical and cost effectiveness of ustekinumab for the treatment of psoriatic arthritis: a critique of the evidence. PharmacoEconomics 2016;34. https://doi.org/10.1007/s40273-015-0350-3.
- Cawson MR, Mitchell SA, Knight C, Wildey H, Spurden D, Bird A, et al. Systematic review, network meta-analysis and economic evaluation of biological therapy for the management of active psoriatic arthritis. BMC Musculoskelet Disord 2014;15. https://doi.org/10.1186/1471-2474-15-26.
- Ungprasert P, Thongprayoon C, Davis JM. Indirect comparisons of the efficacy of biological agents in patients with psoriatic arthritis with an inadequate response to traditional disease-modifying anti-rheumatic drugs or to non-steroidal anti-inflammatory drugs: a meta-analysis. Semin Arthritis Rheum 2016;45:428-38. https://doi.org/10.1016/j.semarthrit.2015.09.004.
- Migliore A, Bizzi E, Broccoli S, Lagana B. Indirect comparison of etanercept, infliximab, and adalimumab for psoriatic arthritis: mixed treatment comparison using placebo as common comparator. Clin Rheumatol 2012;31:133-7. https://doi.org/10.1007/s10067-011-1790-6.
- Ramiro S, Smolen JS, Landewe R, van der Heijde D, Dougados M, Emery P, et al. Pharmacological treatment of psoriatic arthritis: a systematic literature review for the 2015 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2016;75:490-8. https://doi.org/10.1136/annrheumdis-2015-208466.
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Res Synth Methods 2014;5:79-85. https://doi.org/10.1002/jrsm.1090.
- Maneiro JR, Souto A, Salgado E, Mera A, Gomez-Reino JJ. Predictors of response to TNF antagonists in patients with ankylosing spondylitis and psoriatic arthritis: systematic review and meta-analysis. RMD Open 2015;1. https://doi.org/10.1136/rmdopen-2014-000017.
- Sterry W, Ortonne JP, Kirkham B, Brocq O, Robertson D, Pedersen RD, et al. Comparison of two etanercept regimens for treatment of psoriasis and psoriatic arthritis: PRESTA randomised double blind multicentre trial. BMJ 2010;340. https://doi.org/10.1136/bmj.c147.
- Schett G, Wollenhaupt J, Papp K, Joos R, Rodrigues JF, Vessey AR, et al. Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2012;64:3156-67. https://doi.org/10.1002/art.34627.
- Torii H, Nakagawa H. Japanese Infliximab Study investigators . Infliximab monotherapy in Japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci 2010;59:40-9. https://doi.org/10.1016/j.jdermsci.2010.04.014.
- Baranauskaite A, Raffayová H, Kungurov NV, Kubanova A, Venalis A, Helmle L, et al. Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: the RESPOND study. Ann Rheum Dis 2012;71:541-8. https://doi.org/10.1136/ard.2011.152223.
- Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med 2015;373:1329-39. https://doi.org/10.1056/NEJMoa1412679.
- Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 2014;73:48-55. https://doi.org/10.1136/annrheumdis-2013-203696.
- McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1137-46. https://doi.org/10.1016/S0140-6736(15)61134-5.
- Gottlieb AB, Langley RG, Philipp S, Sigurgeirsson B, Blauvelt A, Martin R, et al. Secukinumab improves physical function in subjects with plaque psoriasis and psoriatic arthritis: results from two randomized, phase 3 trials. J Drugs Dermatol 2015;14:821-33.
- Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 2009;60:976-86. https://doi.org/10.1002/art.24403.
- Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 2005;52:1227-36. https://doi.org/10.1002/art.20967.
- Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005;64:1150-7. https://doi.org/10.1136/ard.2004.032268.
- Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 2000;356:385-90. https://doi.org/10.1016/S0140-6736(00)02530-7.
- Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004;50:2264-72. https://doi.org/10.1002/art.20335.
- Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279-89. https://doi.org/10.1002/art.21306.
- Genovese MC, Mease PJ, Thomson GT, Kivitz AJ, Perdok RJ, Weinberg MA, et al. Safety and efficacy of adalimumab in treatment of patients with psoriatic arthritis who had failed disease modifying antirheumatic drug therapy. J Rheumatol 2007;34:1040-50.
- Mease PJ, van der Heijde D, Ritchlin CT, Cuchacovich R, Shuler CL, Lee CH, et al. A randomized, double-blind, active-and placebo-controlled phase 3 study of efficacy and safety of ixekizumab, adalimumab, and placebo therapy in patients naive to biologic disease modifying anti-rheumatic drugs with active psoriatic arthritis. Arthritis Rheumatol 2015;67.
- McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet 2013;382:780-9. https://doi.org/10.1016/S0140-6736(13)60594-2.
- Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014;73:990-9. https://doi.org/10.1136/annrheumdis-2013-204655.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, Adebajo AO, Wollenhaupt J, Gladman DD, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis 2014;73:1020-6. https://doi.org/10.1136/annrheumdis-2013-205056.
- National Institute for Health and Care Excellence (NICE) . Psoriatic Arthritis (Active) – Apremilast (Post DMARDs) [ID682] 2015. www.nice.org.uk/guidance/TA372/documents/psoriatic-arthritis-active-apremilast-post-dmards-id682-committee-papers-2 (accessed 19 May 2016).
- Gottlieb AB, Thaci D, Blauvelt A, Milutinovic M, Mpofu S. Secukinumab improves skin symptoms and physical functioning compared with ustekinumab in patients with moderate to severe psoriasis with concomitant psoriatic arthritis: subanalysis of a randomized, double blind, parallel-group, active comparator-controlled phase 3b trial. Arthritis Rheumatol 2015;67.
- Thaçi D, Blauvelt A, Reich K, Tsai TF, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol 2015;73:400-9. https://doi.org/10.1016/j.jaad.2015.05.013.
- Atteno M, Peluso R, Costa L, Padula S, Iervolino S, Caso F, et al. Comparison of effectiveness and safety of infliximab, etanercept, and adalimumab in psoriatic arthritis patients who experienced an inadequate response to previous disease-modifying antirheumatic drugs. Clin Rheumatol 2010;29:399-403. https://doi.org/10.1007/s10067-009-1340-7.
- Corbett M, Sideris E, Palmer S, Harden M, Woolacott N, Bojke L. Evidence Review Group’s Report: Apremilast for Treating Active Psoriatic Arthritis. Southampton: National Institute for Health Research; 2015.
- Craig D, O’Connor J, Rodgers M, Rodriguez-Lopez R, Smith A, Woolacott N. Evidence Review Group’s Report: Ustekinumab for Treating Active and Progressive Psoriatic Arthritis. Southampton: National Institute for Health Research; 2013.
- Gottlieb AB, Mease PJ, Cuchacovich RS, Shuler CL, Lin CY, Burge RT, et al. Ixekizumab improves physical function, quality of life, and work productivity in biologic disease-modifying antirheumatic drug-naive patients with active psoriatic arthritis. Arthritis Rheumatol 2015;67.
- Gottlieb AB, Sigurgeirsson B, Blauvelt A, Mpfofu S, Martin R, Papavassilis C. Secukinumab shows substantial improvement in both psoriasis symptoms and physical functioning in moderate-to-severe plaque psoriasis patients with psoriatic arthritis: a subanalysis of a phase 3, multicenter, double-blind, placebo-controlled study. Arthritis Rheum 2013;65:S136-7.
- Assessment Report: Otezla. London: European Medicines Agency; 2014.
- Yang H, Epstein D, Bojke L, Craig D, Light K, Bruce I, et al. Evidence Review Group’s Report: Golimumab for the Treatment of Psoriatic Arthritis. Southampton: National Institute for Health Research; 2010.
- Novartis Pharmaceuticals . 24 Week Efficacy and 3-Year Safety and Efficacy of Secukinumab in Active Psoriatic Arthritis 2013. https://ClinicalTrials.gov/show/NCT01989468 (accessed 7 December 2015).
- Novartis Pharmaceuticals . Efficacy at 24 Weeks With Long Term Safety, Tolerability and Efficacy up to 5 Years of Secukinumab in Patients of Active Psoriatic Arthritis 2012. https://ClinicalTrials.gov/show/NCT01752634 (accessed 7 December 2015).
- Novartis Pharmaceuticals . Study to Demonstrate the Efficacy (Including Inhibition of Structural Damage), Safety and Tolerability up to 2 Years of Secukinumab in Active Psoriatic Arthritis 2015. https://ClinicalTrials.gov/show/NCT02404350 (accessed 7 December 2016).
- Mease P, Deodhar A, Fleischmann R, Wollenhaupt J, Gladman D, Leszczyński P, et al. Effect of certolizumab pegol over 96 weeks in patients with psoriatic arthritis with and without prior antitumour necrosis factor exposure. RMD Open 2015;1. https://doi.org/10.1136/rmdopen-2015-000119.
- Kavanaugh A, McInnes IB, Mease P, Krueger GG, Gladman D, van der Heijde D, et al. Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study). Ann Rheum Dis 2014;73:1689-94. https://doi.org/10.1136/annrheumdis-2013-204902.
- Krueger GG. Effects of golimumab on the dermatologic manifestations of psoriatic arthritis: 5-year results from the long-term extension of the randomized, placebo-controlled, GO-REVEAL study. J Am Acad Dermatol 2013;68.
- Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Continued inhibition of radiographic progression in patients with psoriatic arthritis following 2 years of treatment with etanercept. J Rheumatol 2006;33:712-21.
- Mease PJ, Ory P, Sharp JT, Ritchlin CT, Van den Bosch F, Wellborne F, et al. Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT). Ann Rheum Dis 2009;68:702-9. https://doi.org/10.1136/ard.2008.092767.
- Antoni CE, Kavanaugh A, van der Heijde D, Beutler A, Keenan G, Zhou B, et al. Two-year efficacy and safety of infliximab treatment in patients with active psoriatic arthritis: findings of the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT). J Rheumatol 2008;35:869-76.
- Kavanaugh A, Puig L, Gottlieb AB, Ritchlin C, Li S, Wang Y, et al. Maintenance of clinical efficacy and radiographic benefit through 2 years of ustekinumab therapy in patients with active psoriatic arthritis: results from a randomized, placebo-controlled phase III trial. Arthritis Care Res 2015;67:1739-49. https://doi.org/10.1002/acr.22645.
- Kavanaugh A, Puig L, Gottlieb A, Ritchlin C, Li S, Wang Y, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 2-year results from a phase 3, multicenter, double-blind, placebo-controlled study. Ann Rheum Dis 2014;73:737-8. https://doi.org/10.1136/annrheumdis-2014-eular.2283.
- Bird P, Adebajo A, Gladman D, Kavanaugh A, Mease P, Gomez-Reino J, et al. Long-term (104-week) efficacy and safety profile of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis: results from a phase III, randomized, controlled trial and open-label extension (PALACE 1). Intern Med J 2015;45:39-40.
- Saad AA, Ashcroft DM, Watson KD, Hyrich KL, Noyce PR, Symmons DPM, et al. Persistence with anti-tumour necrosis factor therapies in patients with psoriatic arthritis: observational study from the British Society of Rheumatology Biologics Register. Arthritis Res Ther 2009;11. https://doi.org/10.1186/ar2670.
- Fagerli K, Watson K, Packham J, Symmons D, Hyrich K. Predicting successful long-term treatment with tumour necrosis factor-alpha inhibitors in patients with psoriatic arthritis. Arthritis Rheumatol 2014;66:S679-S80.
- Kristensen LE, Gulfe A, Saxne T, Geborek P. Efficacy and tolerability of anti-tumour necrosis factor therapy in psoriatic arthritis patients: results from the South Swedish Arthritis Treatment Group register. Ann Rheum Dis 2008;67:364-9. https://doi.org/10.1136/ard.2007.073544.
- Simard JF, Arkema EV, Sundström A, Geborek P, Saxne T, Baecklund E, et al. Ten years with biologics: to whom do data on effectiveness and safety apply?. Rheumatology 2011;50:204-13. https://doi.org/10.1093/rheumatology/keq326.
- Mease PJ, Collier DH, Saunders KC, Li G, Kremer JM, Greenberg JD. Comparative effectiveness of biologic monotherapy versus combination therapy for patients with psoriatic arthritis: results from the Corrona registry. RMD Open 2015;1. https://doi.org/10.1136/rmdopen-2015-000181.
- Glintborg B, Østergaard M, Dreyer L, Krogh NS, Tarp U, Hansen MS, et al. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor α therapy: results from the nationwide Danish DANBIO registry. Arthritis Rheum 2011;63:382-90. https://doi.org/10.1002/art.30117.
- Chen JS, Makovey J, Lassere M, Buchbinder R, March LM. Comparative effectiveness of anti-tumor necrosis factor drugs on health-related quality of life among patients with inflammatory arthritis. Arthritis Care Res 2014;66:464-72. https://doi.org/10.1002/acr.22151.
- Fagerli KM, Lie E, van der Heijde D, Heiberg MS, Lexberg AS, Rødevand E, et al. The role of methotrexate co-medication in TNF-inhibitor treatment in patients with psoriatic arthritis: results from 440 patients included in the NOR-DMARD study. Ann Rheum Dis 2014;73:132-7. https://doi.org/10.1136/annrheumdis-2012-202347.
- Carmona L, Gomez-Reino J. BIOBADASER group . Survival of TNF antagonists in spondyloarthritis is better than in rheumatoid arthritis. Data from the Spanish registry BIOBADASER. Arthritis Res Ther 2006;8. https://doi.org/10.1186/ar1941.
- Glintborg B, Gudbjornsson B, Krogh NS, Omerovic E, Manilo N, Holland-Fischer M, et al. Impact of different infliximab dose regimens on treatment response and drug survival in 462 patients with psoriatic arthritis: results from the nationwide registries DANBIO and ICEBIO. Rheumatology 2014;53:2100-9. https://doi.org/10.1093/rheumatology/keu252.
- Iannone F, Lopriore S, Bucci R, Scioscia C, Anelli MG, Notarnicola A, et al. Two-year survival rates of anti-TNF-α therapy in psoriatic arthritis (PsA) patients with either polyarticular or oligoarticular PsA. Scand J Rheumatol 2015;44:192-9. https://doi.org/10.3109/03009742.2014.962081.
- Glintborg B, Ostergaard M, Krogh NS, Andersen MD, Tarp U, Loft AG, et al. Clinical response, drug survival, and predictors thereof among 548 patients with psoriatic arthritis who switched tumor necrosis factor inhibitor therapy: results from the Danish nationwide DANBIO registry. Arthritis Rheum 2013;65:1213-23. https://doi.org/10.1002/art.37876.
- Fagerli KM, Lie E, van der Heijde D, Heiberg MS, Kalstad S, Rødevand E, et al. Switching between TNF inhibitors in psoriatic arthritis: data from the NOR-DMARD study. Ann Rheum Dis 2013;72:1840-4. https://doi.org/10.1136/annrheumdis-2012-203018.
- Eder L, Thavaneswaran A, Chandran V, Gladman DD. Tumour necrosis factor α blockers are more effective than methotrexate in the inhibition of radiographic joint damage progression among patients with psoriatic arthritis. Ann Rheum Dis 2014;73:1007-11. https://doi.org/10.1136/annrheumdis-2012-202959.
- Saad AA, Ashcroft DM, Watson KD, Symmons DPM, Noyce PR, Hyrich KL, et al. Improvements in quality of life and functional status in patients with psoriatic arthritis receiving anti-tumor necrosis factor therapies. Arthritis Care Res 2010;62:345-53. https://doi.org/10.1002/acr.20104.
- Husted JA, Tom BD, Farewell VT, Schentag CT, Gladman DD. Description and prediction of physical functional disability in psoriatic arthritis: a longitudinal analysis using a Markov model approach. Arthritis Rheum 2005;53:404-9. https://doi.org/10.1002/art.21177.
- Kane D, Stafford L, Bresnihan B, Fitzgerald O. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology 2003;42:1460-8. https://doi.org/10.1093/rheumatology/keg384.
- Morgan C, Lunt M, Bunn D, Scott DG, Symmons DP. Five-year outcome of a primary-care-based inception cohort of patients with inflammatory polyarthritis plus psoriasis. Rheumatology 2007;46:1819-23. https://doi.org/10.1093/rheumatology/kem270.
- Mease PJ, McInnes IB, Gottlieb AB, Widmer A, Pricop L, Mpofu S. Secukinumab safety and tolerability in patients with active psoriatic arthritis and psoriasis: results from a pooled safety analysis. Arthritis Rheumatol 2015;67.
- Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev 2011;2. https://doi.org/10.1002/14651858.CD008794.pub2.
- Askling J, Fahrbach K, Nordstrom B, Ross S, Schmid CH, Symmons D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf 2011;20:119-30. https://doi.org/10.1002/pds.2046.
- Corbett M, Soares M, Jhuti G, Rice S, Spackman E, Sideris E, et al. Tumour necrosis factor-alpha inhibitors for ankylosing spondylitis and non-radiographic axial spondyloarthritis: a systematic review and economic evaluation. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20090.
- Tarp S, Tarp U, Andersen LS, Lorenzen T, Lindegaard HM, Stoltenberg M, et al. Serious adverse events associated with using biological agents to treat rheumatic diseases: network meta-analysis from a national guideline panel. Arthritis Rheum 2013;65:S997-8.
- Capogrosso-Sansone A, Mantarro S, Blandizzi C, Montagnani S, Ruggiero E, Saporiti A, et al. Update of certolizumab pegol safety profile: a systematic review and meta-analysis. Drug Saf 2014;37:844-5.
- Girolomoni G, Altomare G, Ayala F, Berardesca E, Calzavara-Pinton P, Chimenti S, et al. Safety of anti-TNFα agents in the treatment of psoriasis and psoriatic arthritis. Immunopharmacol Immunotoxicol 2012;34:548-60. https://doi.org/10.3109/08923973.2011.653646.
- Dixon WG, Hyrich KL, Watson KD, Lunt M. The influence of anti-TNF therapy upon the incidence and severity of serious lower respiratory tract infections in patients with rheumatoid arthritis: results from the BSR biologics register (BSRBR). Rheumatology 2008;47.
- Mariette X, Tubach F, Bagheri H, Bardet M, Berthelot JM, Gaudin P, et al. Lymphoma in patients treated with anti-TNF: results of the 3-year prospective French RATIO registry. Ann Rheum Dis 2010;69:400-8. https://doi.org/10.1136/ard.2009.117762.
- Zisman D, Bitterman H, Shalom G, Feldhamer I, Comanesther D, Batat E, et al. Psoriatic arthritis treatment and the risk of herpes zoster. Ann Rheum Dis 2016;75:131-5. https://doi.org/10.1136/annrheumdis-2013-205148.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 1: Introduction to Evidence Synthesis for Decision Making 2011. http://scharr.dept.shef.ac.uk/nicedsu/technical-support-documents/evidence-synthesis-tsd-series/.
- Multiple Technology Appraisal. Certolizumab Pegol and Secukinumab for Treating Active Psoriatic Arthritis Following Inadequate Response to Disease Modifying Antirheumatic Drugs [ID579]. Final Scope. London: NICE; 2015.
- Julious S, Wong SJ. How biased are indirect comparisons, particularly when comparisons are made over time in controlled trials?. Drug Inf J 2008;42:625-33. https://doi.org/10.1177/009286150804200610.
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU Technical Support Document 3: Heterogeneity: Subgroups, Meta-Regression, Bias and Bias-Adjustment 2011. http://scharr.dept.shef.ac.uk/nicedsu/technical-support-documents/evidence-synthesis-tsd-series/ (accessed December 2016).
- Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit (with discussion). J R Stat Soc Series B Stat Methodol 2002;64:583-639. https://doi.org/10.1111/1467-9868.00353.
- Mease PJ, Woolley JM, Bitman B, Wang BC, Globe DR, Singh A. Minimally important difference of Health Assessment Questionnaire in psoriatic arthritis: relating thresholds of improvement in functional ability to patient-rated importance and satisfaction. J Rheumatol 2011;38:2461-5. https://doi.org/10.3899/jrheum.110546.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials 2011. http://scharr.dept.shef.ac.uk/nicedsu/technical-support-documents/evidence-synthesis-tsd-series/ (accessed December 2016).
- Yang H, Epstein D, Bojke L, Craig D, Light K, Bruce I, et al. Golimumab for the treatment of psoriatic arthritis. Health Technol Assess 2011;15. https://doi.org/10.3310/hta15suppl1/10.
- Drummond M, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Codreanu C, Mogosanu C, Joita M, Purcaru O. Cost-effectiveness of certolizumab pegol in the treatment of active rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis in Romania. Value Health 2014;17. https://doi.org/10.1016/j.jval.2014.08.2608.
- Tzanetakos C, Vassilopoulos D, Kourlaba G, Christou P, Maniadakis N. Cost–utility analysis of certolizumab pegol for the treatment of active psoriatic arthritis in Greece. Value Health 2015;18:A646-7. https://doi.org/10.1016/j.jval.2015.09.2319.
- Bojke L, Epstein D, Craig D, Rodgers M, Woolacott N, Yang H, et al. Modelling the cost-effectiveness of biologic treatments for psoriatic arthritis. Rheumatology 2011;50:39-47. https://doi.org/10.1093/rheumatology/ker245.
- Einarson TR, Bereza BG, Bobro I, Efremova E, Lelli F. Economic analysis of ustekinumab for psoriatic arthritis in Russia. Value Health 2015;18. https://doi.org/10.1016/j.jval.2015.09.2325.
- Wang X, Bansback N, Anis A, Joshi AD, Rao S, Wolff M, et al. Economic evaluation model of biologic therapies for moderate to severe psoriatic arthritis in Germany. Value Health 2012;15. https://doi.org/10.1016/j.jval.2012.08.1392.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Madan J, Ades T, Barton P, Bojke L, Choy E, Helliwell P, et al. Consensus decision models for biologics in rheumatoid and psoriatic arthritis: recommendations of a multidisciplinary working party. Rheumatol Ther 2015;2:113-25. https://doi.org/10.1007/s40744-015-0020-0.
- Kyle S, Chandler D, Griffiths CE, Helliwell P, Lewis J, McInnes I, et al. Guideline for anti-TNF-alpha therapy in psoriatic arthritis. Rheumatology 2005;44:390-7. https://doi.org/10.1093/rheumatology/keh514.
- Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler DA, et al. British Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009. Br J Dermatol 2009;161:987-1019. https://doi.org/10.1111/j.1365-2133.2009.09505.x.
- Kobelt G, Jönsson L, Lindgren P, Young A, Eberhardt K. Modeling the progression of rheumatoid arthritis: a two-country model to estimate costs and consequences of rheumatoid arthritis. Arthritis Rheum 2002;46:2310-19. https://doi.org/10.1002/art.10471.
- Bansback NJ, Ara R, Barkham N, Brennan A, Fraser AD, Conway P, et al. Estimating the cost and health status consequences of treatment with TNF antagonists in patients with psoriatic arthritis. Rheumatology 2006;45:1029-38. https://doi.org/10.1093/rheumatology/kel147.
- Hartman M, Prins M, Swinkels OQ, Severens JL, De Boo T, Van Der Wilt GJ, et al. Cost-effectiveness analysis of a psoriasis care instruction programme with dithranol compared with UVB phototherapy and inpatient dithranol treatment. Br J Dermatol 2002;147:538-44. https://doi.org/10.1046/j.1365-2133.2002.04920.x.
- Etanercept, Infliximab and Adalimumab for the Treatment of Psoriatic Arthritis. London: NICE; 2010.
- Cummins E, Asseburg C, Prasad M, Buchanan J, Punekar YS. Cost effectiveness of golimumab for the treatment of active psoriatic arthritis. Eur J Health Econ 2012;13:801-9. https://doi.org/10.1007/s10198-011-0335-x.
- Monthly Index of Medical Specialities (MIMS). London: Haymarket Media Group Ltd; 2016.
- British National Formulary 2015. London: BMJ Group and Pharmaceutical Press; 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- NHS Reference Costs 2014 to 2015. London: DH; 2015.
- Poole CD, Lebmeier M, Ara R, Rafia R, Currie CJ. Estimation of health care costs as a function of disease severity in people with psoriatic arthritis in the UK. Rheumatology 2010;49:1949-56. https://doi.org/10.1093/rheumatology/keq182.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2016.
- Etanercept, Infliximab and Adalimumab for the Treatment of Psoriatic Arthritis [TA199]. London: NICE; 2010.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. Centre for Health Economics Discussion Paper 138. Centre for Health Economics, University of York: York; 1995.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Asaria M, Walker S, Palmer S, Gale CP, Shah AD, Abrams KR, et al. Using electronic health records to predict costs and outcomes in stable coronary artery disease. Heart 2016;102:755-62. https://doi.org/10.1136/heartjnl-2015-308850.
- The Green Book: Appraisal and Evaluation in Central Government. London: HM Treasury; 2013.
- National Life Tables, UK: 2013–2015. Newport: Office for National Statistics; 2015.
- Poyner TF, Wall ARJ, Adnitt PI, Menday AP. Economic impact of psoriasis treatment on the patient and on the National Health Service. J Dermatol Treatment 1999;10:25-9.
- Karlsson G, Johannesson M. The decision rules of cost-effectiveness analysis. PharmacoEconomics 1996;9:113-20. https://doi.org/10.2165/00019053-199609020-00003.
- Lefebvre C, Manheimer E, Glanville J, Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011). The Cochrane Collaboration; 2011.
- Lefebvre C, Eisinga A, McDonald S, Paul N. Enhancing access to reports of clinical trials published world-wide – the contribution of EMBASE records to the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library. Emerg Themes Epidemiol 2008;5. https://doi.org/10.1186/1742-7622-5-13.
- Centre for Reviews and Dissemination . Search Strategies for DARE 2015. www.crd.york.ac.uk/crdweb/searchstrategies.asp (accessed 15 December 2015).
- Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis – results of two phase 3 trials. N Engl J Med 2014;371:326-38. https://doi.org/10.1056/NEJMoa1314258.
- Adams R, Walsh C, Veale D, Bresnihan B, FitzGerald O, Barry M. Understanding the relationship between the EQ-5D, SF-6D, HAQ and disease activity in inflammatory arthritis. PharmacoEconomics 2010;28:477-87. https://doi.org/10.2165/11533010-000000000-00000.
- Adams R, Craig BM, Walsh CD, Veale DJ, Bresnihan B, FitzGerald O, et al. The impact of a revised EQ-5D population scoring on preference-based utility scores in an inflammatory arthritis cohort. Value Health 2011;14:921-7. https://doi.org/10.1016/j.jval.2011.03.002.
- Brodszky V, Péntek M, Bálint PV, Géher P, Hajdu O, Hodinka L, et al. Comparison of the Psoriatic Arthritis Quality of Life (PsAQoL) questionnaire, the functional status (HAQ) and utility (EQ-5D) measures in psoriatic arthritis: results from a cross-sectional survey. Scand J Rheumatol 2010;39:303-9. https://doi.org/10.3109/03009740903468982.
- Gratacós J, Daudén E, Gómez-Reino J, Moreno JC, Casado MÁ, Rodríguez-Valverde V. Health-related quality of life in psoriatic arthritis patients in Spain. Reumatol Clin 2014;10:25-31. https://doi.org/10.1016/j.reuma.2013.05.006.
- Leung YY, Png ME, Wee HL, Thumboo J. Comparison of EuroQol-5D and short form-6D utility scores in multiethnic Asian patients with psoriatic arthritis: a cross-sectional study. J Rheumatol 2013;40:859-65. https://doi.org/10.3899/jrheum.120782.
- Picchianti-Diamanti A, Germano V, Ferlito C, Migliore A, D’Amelio R, Lagana B. Health-related quality of life and disability in patients with rheumatoid, early rheumatoid and early psoriatic arthritis treated with etanercept. Qual Life Res 2010;19:821-6. https://doi.org/10.1007/s11136-010-9651-3.
- Stolfa J. Golimumab in the PsA treatment. Rheumatologia 2010;24:31-7.
Appendix 1 Database search strategies
MEDLINE
Via Ovid: http://ovidsp.ovid.com/
Date range searched: 1946 to November week 3 2015.
Date searched: 1 December 2015.
Records retrieved: 712.
The Cochrane highly sensitive search strategy for identifying randomised trials in Ovid MEDLINE: sensitivity maximising version was used to limit retrieval to clinical trials (lines 25–35). 148
The search was updated on 28 April 2016 and retrieved 749 records.
Search strategy
-
Arthritis, Psoriatic/ (4144)
-
(psoria$ adj2 (arthrit$ or arthropath$)).ti,ab. (6043)
-
1 or 2 (6887)
-
(Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7).af. (763)
-
3 and 4 (53)
-
(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6).af. (88)
-
3 and 6 (18)
-
(golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5).af. (431)
-
(2010$ or 2011$ or 2012$ or 2013$ or 2014$ or 2015$).ed. (4,809,341)
-
3 and 8 and 9 (89)
-
(apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9).af. (92)
-
(2014$ or 2015$).ed. (1,668,230)
-
3 and 11 and 12 (22)
-
(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0).af. (536)
-
(2012$ or 2013$ or 2014$ or 2015$).ed. (3,233,078)
-
3 and 14 and 15 (86)
-
(inflectra or remsima or CT-P13).af. (17)
-
3 and 17 (1)
-
(etanercept or enbrel or 185243-69-0).af. (5831)
-
(infliximab or remicade or 170277-31-3).af. (9674)
-
(adalimumab or humira or D2E7 or (D2 adj E7) or 331731-18-1).af. (4205)
-
19 or 20 or 21 (14,458)
-
(2009$ or 2010$ or 2011$ or 2012$ or 2013$ or 2014$ or 2015$).ed. (5,535,938)
-
3 and 22 and 23 (650)
-
randomized controlled trial.pt. (417,039)
-
controlled clinical trial.pt. (92,231)
-
randomized.ab. (308,924)
-
placebo.ab. (159,456)
-
drug therapy.fs. (1,860,741)
-
randomly.ab. (218,795)
-
trial.ab. (321,356)
-
groups.ab. (1,376,975)
-
or/25-32 (3,513,844)
-
exp animals/ not humans/ (4,152,952)
-
33 not 34 (2,995,700)
-
5 or 7 or 10 or 13 or 16 or 18 or 24 (765)
-
35 and 36 (712)
Key
/ = indexing term [medical subject heading (MeSH) heading].
exp = exploded indexing term (MeSH heading).
$ = truncation.
ti,ab = terms in either title or abstract fields.
af = terms in any field.
ed = entry date – date added to the database.
pt = publication type.
fs = floating subheading.
adj = terms next to each other (order specified).
adj2 = terms within two words of each other (any order).
MEDLINE In-Process & Other Non-Indexed Citations
Via Ovid: http://ovidsp.ovid.com/
Date range searched: 30 November 2015.
Date searched: on 1 December 2015.
Records retrieved: 157.
The search was updated on 28 April 2016 and retrieved 168 records.
Search strategy
-
Arthritis, Psoriatic/ (0)
-
(psoria$ adj2 (arthrit$ or arthropath$)).ti,ab. (655)
-
1 or 2 (655)
-
(Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7).af. (126)
-
3 and 4 (16)
-
(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6).af. (45)
-
3 and 6 (10)
-
(golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5).af. (97)
-
3 and 8 (13)
-
(apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9).af. (45)
-
3 and 10 (25)
-
(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0).af. (148)
-
3 and 12 (36)
-
(inflectra or remsima or CT-P13).af. (19)
-
3 and 14 (0)
-
(etanercept or enbrel or 185243-69-0).af. (542)
-
(infliximab or remicade or 170277-31-3).af. (994)
-
(adalimumab or humira or D2E7 or (D2 adj E7) or 331731-18-1).af. (631)
-
16 or 17 or 18 (1560)
-
3 and 19 (97)
-
5 or 7 or 9 or 11 or 13 or 15 or 20 (157)
Key
/ = indexing term (MeSH heading).
$ = truncation.
ti,ab = terms in either title or abstract fields.
af = terms in any field.
adj = terms next to each other (order specified).
adj2 = terms within two words of each other (any order).
Cochrane Central Register of Controlled Trials
Via Wiley Online Library: http://onlinelibrary.wiley.com/
Issue 11 of 12, November 2015.
Date searched: 1 December 2015.
Records retrieved: 225.
The strategy below was used to search CENTRAL and CDSR.
The search was updated on 28 April 2016 and retrieved 249 records from CENTRAL.
Search strategy
#1 MeSH descriptor: [Arthritis, Psoriatic] this term only (199)
#2 (psoria* near/2 (arthrit* or arthropath*)):ti,ab,kw (560)
#3 #1 or #2 (560)
#4 (Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7):ti,ab,kw (191)
#5 #3 and #4 (24)
#6 (secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6):ti,ab,kw (124)
#7 #3 and #6 (28)
#8 (golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5):ti,ab,kw Publication Year from 2010 to 2015 (210)
#9 #3 and #8 (40)
#10 (apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9):ti,ab,kw Publication Year from 2014 to 2015 (35)
#11 #3 and #10 (21)
#12 (ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0):ti,ab,kw Publication Year from 2012 to 2015 (102)
#13 #3 and #12 (39)
#14 (inflectra or remsima or CT-P13):ti,ab,kw (15)
#15 #3 and #14 (4)
#16 (etanercept or enbrel or 185243-69-0):ti,ab,kw Publication Year from 2009 to 2015 (577)
#17 (infliximab or remicade or 170277-31-3):ti,ab,kw Publication Year from 2009 to 2015 (655)
#18 (adalimumab or humira or D2E7 or (D2 next E7) or 331731-18-1):ti,ab,kw Publication Year from 2009 to 2015 (722)
#19 #16 or #17 or #18 (1551)
#20 #3 and #19 (116)
#21 #5 or #7 or #9 or #11 or #13 or #15 or #20 (250)
#22 #5 or #7 or #9 or #11 or #13 or #15 or #20 in Cochrane Reviews (Reviews and Protocols) and Trials (228)
Note that 228 results at line #22 include Cochrane Reviews or Protocols as well as trials from CENTRAL.
Key
MeSH descriptor = indexing term (MeSH heading).
* = truncation.
ti,ab,kw = terms in either title or abstract or keyword fields.
near/2 = terms within two words of each other (any order).
next = terms are next to each other.
Cochrane Database of Systematic Reviews
Via Wiley Online Library: http://onlinelibrary.wiley.com/
Issue 12 of 12, December 2015.
Date searched: 1 December 2015.
Records retrieved: three.
See above under CENTRAL for search strategy used.
The search was updated on 28 April 2016 and retrieved three records from CDSR.
Database of Abstracts of Reviews of Effects
Via: www.crd.york.ac.uk/CRDWeb/
Date range searched: inception to 31 March 2015.
Date searched: 1 December 2015.
Records retrieved: 13.
The strategy below was used to search DARE and NHS EED.
As DARE and NHS EED were no longer receiving new records after 31 March 2015 these searches were not updated.
Search strategy
| 1 | MeSH DESCRIPTOR Arthritis, Psoriatic | 55 |
| 2 | ((psoria* NEAR2 (arthrit* or arthropath*))) | 88 |
| 3 | (((arthrit* or arthropath*) NEAR2 psoria*)) | 68 |
| 4 | (Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7) | 33 |
| 5 | (secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6) | 7 |
| 6 | (golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5) where LPD from 1 January 2010 to 31 March 2015 | 31 |
| 7 | (apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9) where LPD from 1 January 2014 to 31 March 2015 | 1 |
| 8 | (ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0) where LPD from 1 January 2012 to 31 March 2015 | 22 |
| 9 | (inflectra or remsima or CT-P13) | 5 |
| 10 | (etanercept or enbrel or 185243-69-0) where LPD from 1 January 2009 to 31 March 2015 | 137 |
| 11 | (infliximab or remicade or 170277-31-3) where LPD from 1 January 2009 to 31 March 2015 | 204 |
| 12 | (adalimumab or humira or D2E7 or D2-E7 or 331731-18-1) where LPD from 1 January 2009 to 31 March 2015 | 152 |
| 13 | #1 OR #2 OR #3 | 92 |
| 14 | #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 | 321 |
| 15 | #13 AND #14 | 39 |
| 16 | (#13 AND #14) in DARE | 13 |
| 17 | (#13 AND #14) in NHS EED | 8 |
| 18 | (#13 AND #14) in HTA | 18 |
Key
-
MeSH DESCRIPTOR = indexing term (MeSH heading).
-
* = truncation.
-
NEAR2 = terms within two words of each other (order specified).
EMBASE
Via Ovid: http://ovidsp.ovid.com/
Date range searched: 1974 to 2015 November 30.
Date searched: 1 December 2015.
Records retrieved: 639.
A search strategy developed by Lefebvre et al. to limit retrieval of studies to RCTs was used (see lines 38–52). 149
The search was updated on 28 April 2016 and retrieved 744 records.
Search strategy
-
psoriatic arthritis/ (13,050)
-
(psoria$ adj2 (arthrit$ or arthropath$)).ti,ab. (11,246)
-
1 or 2 (15,353)
-
certolizumab pegol/ (3506)
-
(Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7).af. (4212)
-
4 or 5 (4212)
-
3 and 6 (548)
-
secukinumab/ (601)
-
(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6).af. (679)
-
8 or 9 (679)
-
3 and 10 (199)
-
golimumab/ (2969)
-
(golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5).af. (3054)
-
12 or 13 (3054)
-
(2010$ or 2011$ or 2012$ or 2013$ or 2014$ or 2015$).em. (8,021,136)
-
3 and 14 and 15 (708)
-
apremilast/ (456)
-
(apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9).af. (490)
-
17 or 18 (490)
-
(2014$ or 2015$).em. (3,442,925)
-
3 and 19 and 20 (170)
-
ustekinumab/ (2445)
-
(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0).af. (2559)
-
22 or 23 (2559)
-
(2012$ or 2013$ or 2014$ or 2015$).em. (6,165,443)
-
3 and 24 and 25 (565)
-
(inflectra or remsima or CT-P13).af. (123)
-
3 and 27 (21)
-
etanercept/ (21,668)
-
(etanercept or enbrel or 185243-69-0).af. (22,500)
-
infliximab/ (33,968)
-
(infliximab or remicade or 170277-31-3).af. (34,643)
-
adalimumab/ (18,932)
-
(adalimumab or humira or D2E7 or (D2 adj E7) or 331731-18-1).af. (19,317)
-
or/29-34 (47,513)
-
(2009$ or 2010$ or 2011$ or 2012$ or 2013$ or 2014$ or 2015$).em. (9,378,944)
-
3 and 35 and 36 (3116)
-
random$.ti,ab. (1,044,993)
-
factorial$.ti,ab. (26,816)
-
crossover$.ti,ab. (55,631)
-
cross-over$.ti,ab. (24,911)
-
placebo$.ti,ab. (230,032)
-
(doubl$ adj blind$).ti,ab. (163,599)
-
(singl$ adj blind$).ti,ab. (16,962)
-
assign$.ti,ab. (278,181)
-
allocat$.ti,ab. (100,141)
-
volunteer$.ti,ab. (201,600)
-
Crossover Procedure/ (45,294)
-
double blind procedure/ (127,551)
-
Randomized Controlled Trial/ (392,436)
-
single blind procedure/ (21,379)
-
or/38-51 (1,651,603)
-
7 or 11 or 16 or 21 or 26 or 28 or 37 (3624)
-
52 and 53 (639)
-
animal/ (1,708,125)
-
exp animal experiment/ (1,900,985)
-
nonhuman/ (4,661,466)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (5,213,728)
-
or/55-58 (7,584,705)
-
exp human/ (16,613,065)
-
human experiment/ (345,688)
-
60 or 61 (16,614,514)
-
59 not (59 and 62) (5,821,013)
-
54 not 63 (639)
Key
/ = indexing term (Emtree heading).
exp = exploded indexing term (Emtree heading).
$ = truncation.
ti,ab = terms in either title or abstract fields.
af = all fields.
pt = publication type.
sh = subject heading field.
adj2 = terms within two words of each other (any order).
em = entry week – date added to the database.
Health Technology Assessment database
Via: www.crd.york.ac.uk/CRDWeb/
Date range searched: inception to 31 March 2015.
Date searched: 1 December 2015.
Records retrieved: 18.
The search was updated on 28 April 2016 and retrieved 20 records.
Search strategy
| 1 | MeSH DESCRIPTOR Arthritis, Psoriatic | 55 |
| 2 | ((psoria* NEAR2 (arthrit* or arthropath*))) | 88 |
| 3 | (((arthrit* or arthropath*) NEAR2 psoria*)) | 68 |
| 4 | (Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7) | 33 |
| 5 | (secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6) | 7 |
| 6 | (golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5) where LPD from 1 January 2010 to 1 December 2015 | 31 |
| 7 | (apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9) where LPD from 1 January 2014 to 1 December 2015 | 4 |
| 8 | (ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0) where LPD from 1 January 2012 to 1 December 2015 | 28 |
| 9 | (inflectra or remsima or CT-P13) | 5 |
| 10 | (etanercept or enbrel or 185243-69-0) where LPD from 1 January 2009 to 1 December 2015 | 176 |
| 11 | (infliximab or remicade or 170277-31-3) where LPD from 1 January 2009 to 1 December 2015 | 267 |
| 12 | (adalimumab or humira or D2E7 or D2-E7 or 331731-18-1) where LPD from 1 January 2009 to 1 December 2015 | 204 |
| 13 | #1 OR #2 OR #3 | 92 |
| 14 | #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 | 403 |
| 15 | #13 AND #14 | 46 |
| 16 | (#13 AND #14) in HTA | 18 |
Key
-
MeSH DESCRIPTOR = indexing term (MeSH heading).
-
* = truncation.
-
NEAR2 = terms within two words of each other (order specified).
PubMed
Via: www.ncbi.nlm.nih.gov/pubmed/
Date searched: 1 December 2015.
Records retrieved: 779.
The Cochrane highly sensitive search strategy for identifying randomised trials in PubMed sensitivity maximising version was used to limit retrieval to clinical trials. 148
The search was updated on 28 April 2016 and retrieved 844 records.
Search strategy
Search ((((((((((((‘Arthritis, Psoriatic’[Mesh:noexp]) OR (psoria*[Title/Abstract] AND arthrit*[Title/Abstract]) OR (psoria*[Title/Abstract] AND arthropath*[Title/Abstract]))) AND ((Certolizumab OR Cimzia OR CZP OR CDP870 OR CDP-870 OR 428863-50-7)))) OR ((((‘Arthritis, Psoriatic’[Mesh:noexp]) OR (psoria*[Title/Abstract] AND arthrit*[Title/Abstract]) OR (psoria*[Title/Abstract] AND arthropath*[Title/Abstract]))) AND ((secukinumab OR Cosentyx OR AIN457 OR AIN-457 OR 1229022-83-6)))) OR ((((‘Arthritis, Psoriatic’[Mesh:noexp]) OR (psoria*[Title/Abstract] AND arthrit*[Title/Abstract]) OR (psoria*[Title/Abstract] AND arthropath*[Title/Abstract]))) AND (((golimumab OR simponi OR CNTO148 OR CNTO-148 OR 476181-74-5)) AND (‘2010/01/01’[Date - Entrez] : ‘3000’[Date - Entrez])))) OR ((((‘Arthritis, Psoriatic’[Mesh:noexp]) OR (psoria*[Title/Abstract] AND arthrit*[Title/Abstract]) OR (psoria*[Title/Abstract] AND arthropath*[Title/Abstract]))) AND (((apremilast OR otezla OR otezia OR CC10004 OR CC-10004 OR 608141-41-9)) AND (‘2014/01/01’[Date - Entrez] : ‘3000’[Date - Entrez])))) OR ((((‘Arthritis, Psoriatic’[Mesh:noexp]) OR (psoria*[Title/Abstract] AND arthrit*[Title/Abstract]) OR (psoria*[Title/Abstract] AND arthropath*[Title/Abstract]))) AND (((ustekinumab OR stelara OR CNTO1275 OR CNTO-1275 OR 815610-63-0)) AND (‘2012/01/01’[Date - Entrez] : ‘3000’[Date - Entrez])))) OR ((((‘Arthritis, Psoriatic’[Mesh:noexp]) OR (psoria*[Title/Abstract] AND arthrit*[Title/Abstract]) OR (psoria*[Title/Abstract] AND arthropath*[Title/Abstract]))) AND ((inflectra OR remsima OR CT-P13)))) OR ((((‘Arthritis, Psoriatic’[Mesh:noexp]) OR (psoria*[Title/Abstract] AND arthrit*[Title/Abstract]) OR (psoria*[Title/Abstract] AND arthropath*[Title/Abstract]))) AND (((((((etanercept OR enbrel OR 185243-69-0))) AND (‘2009/01/01’[Date - Entrez] : ‘3000’[Date - Entrez]))) OR ((((infliximab OR remicade OR 170277-31-3))) AND (‘2009/01/01’[Date - Entrez] : ‘3000’[Date - Entrez]))) OR ((((adalimumab OR humira OR D2E7 OR D2-E7 OR 331731-18-1))) AND (‘2009/01/01’[Date - Entrez] : ‘3000’[Date - Entrez])))))) AND ((((((((((randomized controlled trial[Publication Type]) OR controlled clinical trial[Publication Type]) OR randomized[Title/Abstract]) OR placebo[Title/Abstract]) OR drug therapy[sh]) OR randomly[Title/Abstract]) OR trial[Title/Abstract]) OR groups[Title/Abstract])) NOT (animals[mh] NOT humans[mh]))
Key
[Mesh] = exploded indexing term (MeSH heading).
[mh] = exploded indexing term (MeSH heading).
[Mesh:NoExp] = indexing term (MeSH heading) not exploded.
* = truncation.
[Title/Abstract]) = terms in either title or abstract fields.
[Publication Type] = terms in the publication type field.
[Date - Entrez] = date added to the database.
[sh] = subheading.
Science Citation Index
Via Web of Science, Thomson Reuters: http://thomsonreuters.com/thomson-reuters-web-of-science/
Date range searched: 1900 to 28 November 2015.
Date searched: 1 December 2015.
Records retrieved: 712.
Strategy below was used to search SCI and the CPCI-S. As both databases were searched together the records retrieved refer to results from both databases.
The search was updated on 28 April 2016 and retrieved 796 records from both databases.
Search strategy
| # 27 | 712 | #26 AND #25 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 26 | 1284 | #18 OR #13 OR #11 OR #9 OR #7 OR #5 OR #3 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 25 | 5,529,680 | #23 NOT #24 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 24 | 3,812,114 | TS=(animal or animals or dog or dogs or hamster* or mice or mouse or rat or rats or bovine or sheep or guinea*) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 23 | 6,341,875 | #22 OR #21 OR #20 OR #19 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 22 | 5,414,453 | TS=(placebo* or random* or control* or prospectiv* or volunteer*) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 21 | 486,891 | TS=(clinic* SAME trial*) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 20 | 227,219 | TS=((singl* or doubl* or trebl* or tripl*) SAME (blind* or mask*)) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 19 | 1,143,892 | TS=((study or studies) SAME design*) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 18 | 973 | #17 AND #1 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 17 | 13,195 | #16 OR #15 OR #14 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=2009-2015 | ||
| # 16 | 4497 | TS=(adalimumab or humira or D2E7 or (D2 NEAR/1 E7) or 331731-18-1) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=2009-2015 | ||
| # 15 | 8564 | TS=(infliximab or remicade or 170277-31-3) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=2009-2015 | ||
| # 14 | 4505 | TS=(etanercept or enbrel or 185243-69-0) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=2009-2015 | ||
| # 13 | 4 | #12 AND #1 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 12 | 48 | TS=(inflectra or remsima or CT-P13) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 11 | 151 | #10 AND #1 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 10 | 632 | TS=(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=2012-2015 | ||
| # 9 | 61 | #8 AND #1 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 8 | 126 | TS=(apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=2014-2015 | ||
| # 7 | 137 | #6 AND #1 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 6 | 594 | TS=(golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=2010-2015 | ||
| # 5 | 54 | #4 AND #1 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 4 | 257 | TS=(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 3 | 101 | #2 AND #1 |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 2 | 1386 | TS=(Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years | ||
| # 1 | 9294 | TS=(psoria* NEAR/2 (arthrit* or arthropath*)) |
| Indexes=SCI-EXPANDED, CPCI-S Timespan=All years |
Key
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields.
* = truncation.
‘ ‘ = phrase search.
NEAR/2 = terms within two words of each other (any order).
SAME = terms within the same sentence.
Ongoing, unpublished or grey literature search strategies
ClinicalTrials.gov
Date searched: 7 December 2015.
Records retrieved: 99.
The searches were updated on 28 April 2016 and retrieved 110 records.
Search strategy
-
Six studies found for: ((psoriatic arthritis OR psoriatic arthropathy) AND (Certolizumab OR Cimzia OR CZP OR CDP870 OR CDP-870 OR 428863-50-7))
-
Eleven studies found for: ((psoriatic arthritis OR psoriatic arthropathy) AND (secukinumab OR Cosentyx OR AIN457 OR AIN-457 OR 1229022-83-6))
-
Thirteen studies found for: (psoriatic arthritis OR psoriatic arthropathy) AND (golimumab OR simponi OR CNTO148 OR CNTO-148 OR 476181-74-5) | received from 1 January 2010 to 7 December 2015
-
Two studies found for: (psoriatic arthritis OR psoriatic arthropathy) AND (apremilast OR otezla OR otezia OR CC10004 OR CC-10004 OR 608141-41-9) | received from 1 January 2014 to 7 December 2015
-
Three studies found for: (psoriatic arthritis OR psoriatic arthropathy) AND (ustekinumab OR stelara OR CNTO1275 OR CNTO-1275 OR 815610-63-0) | received from 1 January 2012 to 7 December 2015
-
Two studies found for: (psoriatic arthritis OR psoriatic arthropathy) AND (inflectra OR remsima OR CT-P13)
-
Eighteen studies found for: (psoriatic arthritis OR psoriatic arthropathy) AND (etanercept OR enbrel OR 185243-69-0) | received from 1 January 2009 to 7 December 2015
-
Eleven studies found for: (psoriatic arthritis OR psoriatic arthropathy) AND (infliximab OR remicade OR 170277-31-3) | received from 1 January 2009 to 7 December 2015
-
Thirty-three studies found for: (psoriatic arthritis OR psoriatic arthropathy) AND (adalimumab OR humira OR D2E7 OR D2-E7 OR 331731-18-1) | received from 1 January 2009 to 7 December 2015
Conference Proceedings Citation Index: Science
Via Web of Science, Thomson Reuters: http://thomsonreuters.com/thomson-reuters-web-of-science/
Date range searched: 1990 to 28 November 2015.
Date searched: 1 December 2015.
Records retrieved: 712.
See above under SCI for search strategy used. As both databases were searched together the records retrieved refers to results from both databases.
The search was updated on 28 April 2016 and retrieved 796 records from both databases.
EU Clinical Trials Register
www.clinicaltrialsregister.eu/ctr-search/search
Date searched: 7 December 2015.
Records retrieved: 29.
The searches were updated on 28 April 2016 and retrieved two new records.
Search strategy
-
Thirteen result(s) found for: (psoriatic arthritis OR psoriatic arthropathy) AND (Certolizumab OR Cimzia OR CZP OR CDP870 OR CDP-870 OR 428863-50-7 OR secukinumab OR Cosentyx OR AIN457 OR AIN-457 OR 1229022-83-6 OR inflectra OR remsima OR CT-P13)
-
Four result(s) found for: (psoriatic arthritis OR psoriatic arthropathy) AND (golimumab OR simponi OR CNTO148 OR CNTO-148 OR 476181-74-5) date limit 01/01/2010-07/12/2015
-
No result(s) found for: (psoriatic arthritis OR psoriatic arthropathy) AND (apremilast OR otezla OR otezia OR CC10004 OR CC-10004 OR 608141-41-9) date limits – 01/01/2014-07/12/2015
-
Three result(s) found for: (psoriatic arthritis OR psoriatic arthropathy) AND (ustekinumab OR stelara OR CNTO1275 OR CNTO-1275 OR 815610-63-0) date limits 01/01/2012-07/12/2015
-
Nine result(s) found for: (psoriatic arthritis OR psoriatic arthropathy) AND (etanercept OR enbrel OR 185243-69-0 OR infliximab OR remicade OR 170277-31-3 OR adalimumab OR humira OR D2E7 OR D2-E7 OR 331731-18-1) date limits 01/01/2009-07/12/2015
PROSPERO
Date searched: 4 December 2015.
Records retrieved: 25.
Search: psoriatic arthritis in all fields.
The search was updated on 28 April 2016 and retrieved nine new records.
World Health Organization’s International Clinical Trials Registry Platform
Date searched: 7 December 2015.
Records retrieved: 113.
The searches were updated on 28 April 2016 and retrieved five new records.
Search strategy
-
Condition: (psoriatic arthritis OR psoriatic arthropathy) AND Intervention: (Certolizumab OR Cimzia OR CZP OR CDP870 OR CDP-870 OR 428863-50-7 OR secukinumab OR Cosentyx OR AIN457 OR AIN-457 OR 1229022-83-6 OR inflectra OR remsima OR CT-P13)
Twenty-nine trials found.
-
Condition: (psoriatic arthritis OR psoriatic arthropathy) AND Intervention: (golimumab OR simponi OR CNTO148 OR CNTO-148 OR 476181-74-5) limits 1 January 2010 to 7 December 2015
Sixteen trials found.
-
Condition: (psoriatic arthritis OR psoriatic arthropathy) AND Intervention: (apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9) limits 1 January 2014 to 7 December 2015
No records found.
-
Condition: (psoriatic arthritis OR psoriatic arthropathy) AND Intervention: (ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0) limits 1 January 2012 to 7 December 2015
Two trials found.
-
Condition: (psoriatic arthritis OR psoriatic arthropathy) AND Intervention: (etanercept OR enbrel OR 185243-69-0 OR infliximab OR remicade OR 170277-31-3 OR adalimumab OR humira OR D2E7 OR D2-E7 OR 331731-18-1) limits 1 January 2009 to 7 December 2015
Eighty-six trials found.
Extra searches for systematic reviews
As DARE ceased at the end of March 2015, searches for systematic reviews were carried out on MEDLINE and EMBASE to ensure that any relevant systematic reviews were identified.
EMBASE
Via Ovid: http://ovidsp.ovid.com/
Date range searched: 1974 to 30 November 2015.
Date searched: 1 December 2015.
Records retrieved: 82.
The following strategy includes a search strategy designed to locate reviews for DARE in Ovid EMBASE (see lines 35–129). 150
The search was updated on 28 April 2016 and retrieved 139 records.
Search strategy
-
psoriatic arthritis/ (13,050)
-
(psoria$ adj2 (arthrit$ or arthropath$)).ti,ab. (11,246)
-
1 or 2 (15,353)
-
certolizumab pegol/ (3506)
-
(Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7).af. (4212)
-
4 or 5 (4212)
-
3 and 6 (548)
-
secukinumab/ (601)
-
(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6).af. (679)
-
8 or 9 (679)
-
3 and 10 (199)
-
golimumab/ (2969)
-
(golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5).af. (3054)
-
12 or 13 (3054)
-
3 and 14 (806)
-
apremilast/ (456)
-
(apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9).af. (490)
-
16 or 17 (490)
-
3 and 18 (231)
-
ustekinumab/ (2445)
-
(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0).af. (2559)
-
20 or 21 (2559)
-
3 and 22 (754)
-
(inflectra or remsima or CT-P13).af. (123)
-
3 and 24 (21)
-
etanercept/ (21,668)
-
(etanercept or enbrel or 185243-69-0).af. (22,500)
-
infliximab/ (33,968)
-
(infliximab or remicade or 170277-31-3).af. (34,643)
-
adalimumab/ (18,932)
-
(adalimumab or humira or D2E7 or (D2 adj E7) or 331731-18-1).af. (19,317)
-
or/26-31 (47,513)
-
3 and 32 (4302)
-
7 or 11 or 15 or 19 or 23 or 25 or 33 (4863)
-
systematic$ review$.ti,ab. (95,091)
-
systematic$ literature review$.ti,ab. (6884)
-
‘systematic review’/ (98,895)
-
‘systematic review (topic)’/ (13,418)
-
meta analysis/ (102,483)
-
‘meta analysis (topic)’/ (23,719)
-
meta-analytic$.ti,ab. (5089)
-
meta-analysis.ti,ab. (92,607)
-
metanalysis.ti,ab. (351)
-
metaanalysis.ti,ab. (4420)
-
meta analysis.ti,ab. (92,607)
-
meta-synthesis.ti,ab. (333)
-
metasynthesis.ti,ab. (173)
-
meta synthesis.ti,ab. (333)
-
meta-regression.ti,ab. (4113)
-
metaregression.ti,ab. (569)
-
meta regression.ti,ab. (4113)
-
(synthes$ adj3 literature).ti,ab. (2047)
-
(synthes$ adj3 evidence).ti,ab. (5649)
-
(synthes$ adj2 qualitative).ti,ab. (939)
-
integrative review.ti,ab. (1084)
-
data synthesis.ti,ab. (10,020)
-
(research synthesis or narrative synthesis).ti,ab. (1100)
-
(systematic study or systematic studies).ti,ab. (9606)
-
(systematic comparison$ or systematic overview$).ti,ab. (2447)
-
(systematic adj2 search$).ti,ab. (14,698)
-
systematic$ literature research$.ti,ab. (172)
-
(review adj3 scientific literature).ti,ab. (1182)
-
(literature review adj2 side effect$).ti,ab. (11)
-
(literature review adj2 adverse effect$).ti,ab. (2)
-
(literature review adj2 adverse event$).ti,ab. (9)
-
(evidence-based adj2 review).ti,ab. (2599)
-
comprehensive review.ti,ab. (9891)
-
critical review.ti,ab. (13,722)
-
critical analysis.ti,ab. (6783)
-
quantitative review.ti,ab. (596)
-
structured review.ti,ab. (712)
-
realist review.ti,ab. (93)
-
realist synthesis.ti,ab. (61)
-
(pooled adj2 analysis).ti,ab. (10,726)
-
(pooled data adj6 (studies or trials)).ti,ab. (1727)
-
(medline and (inclusion adj3 criteria)).ti,ab. (13,602)
-
(search adj (strateg$ or term$)).ti,ab. (23,159)
-
or/35-77 (313,391)
-
medline.ab. (82,933)
-
pubmed.ab. (59,842)
-
cochrane.ab. (49,544)
-
embase.ab. (49,331)
-
cinahl.ab. (14,619)
-
psyc?lit.ab. (963)
-
psyc?info.ab. (11,667)
-
lilacs.ab. (4162)
-
(literature adj3 search$).ab. (41,110)
-
(database$ adj3 search$).ab. (38,127)
-
(bibliographic adj3 search$).ab. (1761)
-
(electronic adj3 search$).ab. (13,296)
-
(electronic adj3 database$).ab. (18,556)
-
(computeri?ed adj3 search$).ab. (3348)
-
(internet adj3 search$).ab. (2745)
-
included studies.ab. (12,116)
-
(inclusion adj3 studies).ab. (10,022)
-
inclusion criteria.ab. (73,458)
-
selection criteria.ab. (23,235)
-
predefined criteria.ab. (1684)
-
predetermined criteria.ab. (980)
-
(assess$ adj3 (quality or validity)).ab. (62,963)
-
(select$ adj3 (study or studies)).ab. (56,413)
-
(data adj3 extract$).ab. (46,092)
-
extracted data.ab. (9890)
-
(data adj2 abstracted).ab. (5666)
-
(data adj3 abstraction).ab. (1428)
-
published intervention$.ab. (148)
-
((study or studies) adj2 evaluat$).ab. (168,567)
-
(intervention$ adj2 evaluat$).ab. (9530)
-
confidence interval$.ab. (302,095)
-
heterogeneity.ab. (130,769)
-
pooled.ab. (71,894)
-
pooling.ab. (10,965)
-
odds ratio$.ab. (209,779)
-
(Jadad or coding).ab. (151,963)
-
evidence-based.ti,ab. (89,257)
-
or/79-115 (1,249,442)
-
review.pt. (2,121,803)
-
116 and 117 (155,285)
-
review.ti. (354,800)
-
116 and 119 (79,064)
-
(review$ adj10 (papers or trials or trial data or studies or evidence or intervention$ or evaluation$ or outcome$ or findings)).ti,ab. (349,461)
-
(retriev$ adj10 (papers or trials or studies or evidence or intervention$ or evaluation$ or outcome$ or findings)).ti,ab. (17,449)
-
78 or 118 or 120 or 121 or 122 (648,468)
-
letter.pt. (918,884)
-
editorial.pt. (497,918)
-
124 or 125 (1,416,802)
-
123 not 126 (636,540)
-
(animal/ or nonhuman/) not exp human/ (4,935,282)
-
127 not 128 (611,316)
-
34 and 129 (558)
-
2015$.em. (1,962,120)
-
130 and 131 (82)
Key
/ = indexing term (Emtree heading).
exp = exploded indexing term (Emtree heading).
$ = truncation.
? = optional wildcard – one or no characters.
ti,ab = terms in either title or abstract fields.
af = all fields.
pt = publication type.
sh = subject heading field.
adj2 = terms within two words of each other (any order).
em = entry week – date added to the database.
MEDLINE
Via Ovid: http://ovidsp.ovid.com/
Date range searched: 1946 to November week 3 2015.
Date searched: 1 December 2015.
Records retrieved: nine.
The following strategy includes a search strategy designed to locate reviews for DARE in Ovid MEDLINE (see lines 22–98). 150
The search was updated on 28 April 2016 and retrieved 25 records.
Search strategy
-
Arthritis, Psoriatic/ (4144)
-
(psoria$ adj2 (arthrit$ or arthropath$)).ti,ab. (6043)
-
1 or 2 (6887)
-
(Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7).af. (763)
-
3 and 4 (53)
-
(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6).af. (88)
-
3 and 6 (18)
-
(golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5).af. (431)
-
3 and 8 (104)
-
(apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9).af. (92)
-
3 and 10 (29)
-
(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0).af. (536)
-
3 and 12 (114)
-
(inflectra or remsima or CT-P13).af. (17)
-
3 and 14 (1)
-
(etanercept or enbrel or 185243-69-0).af. (5831)
-
(infliximab or remicade or 170277-31-3).af. (9674)
-
(adalimumab or humira or D2E7 or (D2 adj E7) or 331731-18-1).af. (4205)
-
16 or 17 or 18 (14,458)
-
3 and 19 (1129)
-
5 or 7 or 9 or 11 or 13 or 15 or 20 (1267)
-
systematic$ review$.ti,ab. (62,767)
-
meta-analysis as topic/ (15,063)
-
meta-analytic$.ti,ab. (3875)
-
meta-analysis.ti,ab,pt. (80,432)
-
metanalysis.ti,ab. (130)
-
metaanalysis.ti,ab. (1122)
-
meta analysis.ti,ab. (61,044)
-
meta-synthesis.ti,ab. (245)
-
metasynthesis.ti,ab. (143)
-
meta synthesis.ti,ab. (245)
-
meta-regression.ti,ab. (2799)
-
metaregression.ti,ab. (315)
-
meta regression.ti,ab. (2799)
-
(synthes$ adj3 literature).ti,ab. (1446)
-
(synthes$ adj3 evidence).ti,ab. (4369)
-
integrative review.ti,ab. (943)
-
data synthesis.ti,ab. (7556)
-
(research synthesis or narrative synthesis).ti,ab. (821)
-
(systematic study or systematic studies).ti,ab. (6891)
-
(systematic comparison$ or systematic overview$).ti,ab. (1891)
-
evidence based review.ti,ab. (1253)
-
comprehensive review.ti,ab. (6999)
-
critical review.ti,ab. (10,688)
-
quantitative review.ti,ab. (474)
-
structured review.ti,ab. (490)
-
realist review.ti,ab. (58)
-
realist synthesis.ti,ab. (44)
-
or/22-48 (164,741)
-
review.pt. (2,034,742)
-
medline.ab. (60,574)
-
pubmed.ab. (36,054)
-
cochrane.ab. (34,003)
-
embase.ab. (33,609)
-
cinahl.ab. (11,111)
-
psyc?lit.ab. (871)
-
psyc?info.ab. (7994)
-
(literature adj3 search$).ab. (27,401)
-
(database$ adj3 search$).ab. (26,195)
-
(bibliographic adj3 search$).ab. (1303)
-
(electronic adj3 search$).ab. (9505)
-
(electronic adj3 database$).ab. (11,568)
-
(computeri?ed adj3 search$).ab. (2654)
-
(internet adj3 search$).ab. (1771)
-
included studies.ab. (7960)
-
(inclusion adj3 studies).ab. (7019)
-
inclusion criteria.ab. (37,933)
-
selection criteria.ab. (21,191)
-
predefined criteria.ab. (1159)
-
predetermined criteria.ab. (756)
-
(assess$ adj3 (quality or validity)).ab. (42,982)
-
(select$ adj3 (study or studies)).ab. (39,117)
-
(data adj3 extract$).ab. (31,055)
-
extracted data.ab. (7660)
-
(data adj2 abstracted).ab. (3467)
-
(data adj3 abstraction).ab. (878)
-
published intervention$.ab. (108)
-
((study or studies) adj2 evaluat$).ab. (110,270)
-
(intervention$ adj2 evaluat$).ab. (6324)
-
confidence interval$.ab. (243,474)
-
heterogeneity.ab. (97,658)
-
pooled.ab. (48,633)
-
pooling.ab. (7960)
-
odds ratio$.ab. (161,734)
-
(Jadad or coding).ab. (123,582)
-
or/51-85 (846,853)
-
50 and 86 (138,063)
-
review.ti. (262,483)
-
88 and 86 (51,780)
-
(review$ adj4 (papers or trials or studies or evidence or intervention$ or evaluation$)).ti,ab. (105,599)
-
49 or 87 or 89 or 90 (305,581)
-
letter.pt. (928,972)
-
editorial.pt. (379,192)
-
comment.pt. (631,763)
-
92 or 93 or 94 (1,437,876)
-
91 not 95 (297,485)
-
exp animals/ not humans/ (4,152,952)
-
96 not 97 (287,212)
-
21 and 98 (96)
-
2015$.ed. (777,364)
-
99 and 100 (9)
Key
/ = indexing term (MeSH heading).
exp = exploded indexing term (MeSH heading).
$ = truncation.
? = optional wildcard – one or no characters.
ti,a. = terms in either title or abstract fields.
af = terms in any field.
ed = entry date – date added to the database.
pt = publication type.
adj = terms next to each other (order specified).
Appendix 2 Inclusion and exclusion criteria of the included studies
| Study and drug | Inclusion criteria | Exclusion criteria |
|---|---|---|
| FUTURE 2;48 SEC |
|
|
| ERASURE;49,151 SEC |
|
|
| FIXTURE;49,151 SEC |
|
|
| CLEAR;62,63 SEC |
|
|
| aSPIRIT-P1;57,67 ADA |
|
|
| RAPID-PsA;47 CZP |
|
|
| PALACE 1, 2, 3;60,61,65 APR |
|
|
| PSUMMIT 2;59,66 UST |
|
|
| PSUMMIT 1;66 UST |
|
|
| Atteno et al., 2010;64 ETN vs. ADA vs. INF |
|
|
| GO-REVEAL;50 GOL |
|
|
| Genovese et al., 2007;56 ADA |
|
|
| ADEPT;55 ADA |
|
|
| IMPACT;51 INF | Diagnosed PsA for ≥ 6 months
|
|
| IMPACT 2;52 INF |
|
|
| Mease et al. 2004;54 ETN |
|
|
| Mease et al., 2000;53 ETN |
|
|
Appendix 3 Detailed evidence synthesis
Detailed evidence synthesis framework
The evidence synthesis was undertaken using WinBUGS (version 1.4.3). WinBUGS is a Bayesian analysis software tool that, through the use of Markov chain Monte Carlo, calculates posterior distributions for the parameters of interest given likelihood functions derived from data and prior probabilities (uninformative priors were used throughout). There were few individual studies on each treatment; therefore, fixed-effect models were used across studies in all analyses. Parameter estimates for all functional parameters were reported from the models. These differ by outcome, and further details are presented in the subsections that follow. Treatment effects were expressed in relation to placebo. Owing to the sparse evidence imposing a high level of uncertainty over estimates of functional parameters, point estimates are medians throughout. Some models assumed exchangeability across treatments within a class. Within such models we reported the estimates for each treatment (called shrunken estimates), rather than the class medians, allowing us to represent any residual differences across treatments.
All PsARC response, and HAQ-DI conditional on PsARC response, models were run for 20,000 iterations after a burn-in of 30,000 on two chains. All PASI response and ACR response models were run for 20,000 iterations after a burn-in of 50,000 on two chains. The level of credibility used was 95% (i.e. 95% CrIs). The DIC statistic, convergence and autocorrelation were all assessed and informed model selection. Thinning was considered where autocorrelation was high. Model fit statistics are reported in the form of DIC and residual deviance.
Data used for the ustekinumab (PSUMMIT) trials
The marketing authorisation for UST differs from that of the other biologics in terms of how long treatment should be continued before clinicians should consider stopping treatment. Although the recommendation for UST is for doctors to consider stopping treatment if there is no response after 28 weeks, for the other biologics the stopping time frames range between 12 and 16 weeks. However, the PSUMMIT trials58,59,66 had an early escape crossover design at week 16, just like several other trials included in the NMA (including the FUTURE 248 and RAPID-PsA trials47). Using the post-early escape 24-week data from the PSUMMIT trials but pre-early escape data from the other trials would introduce methodological heterogeneity across treatments, which could potentially have implications on results. With this in mind we obtained 12-week data for the PSUMMIT trials via the YODA project (see Chapter 3, Methods for reviewing clinical effectiveness). Although biologic-naive and biologic-experienced subgroup data were extracted for several relevant outcomes from the PSUMMIT clinical study reports, these subgroup data were not available for PsARC at 12 weeks for PSUMMIT 2,59,66 although they were available for the full population.
The data from YODA showed that results for the PsARC and HAQ-DI outcomes were very similar at 12 and 24 weeks in both PSUMMIT trials (Table 121). 58,59,66 Conversely, the 12- and 24-week results appear different for the PASI outcomes, particularly at the higher thresholds. A similar pattern of results (when comparing 12 and 24 weeks) can be seen in the RAPID-PsA trial,47 but is less evident in the SEC FUTURE 2 trial48 (see Table 121). Some differences across treatments may be attributable to variations in analysis approaches used with respect to non-responder imputations in early escapers. It was also noted that in ADEPT,55 which was placebo controlled and blinded up to 24 weeks without early escape, there was around a 10% increase in PASI 75 and PASI 90 response rates going from 12 to 24 weeks.
| Trial and arm | Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PsARC | HAQ-DI (units)a | PASI 50 | PASI 75 | PASI 90 | ||||||
| 12 weeks | 24 weeks | 12 weeks | 24 weeks | 12 weeks | 24 weeks | 12 weeks | 24 weeks | 12 weeks | 24 weeks | |
| FUTURE 2;48 150 mg of SEC | 69 | Confidential information has been removed | NR | –0.48 | 83 | NR | 53 | 43 | 33 | 33 |
| FUTURE 2;48 300 mg of SEC | 72 | Confidential information has been removed | NR | –0.56 | 83 | NR | 59 | 63 | 39 | 49 |
| RAPID-PsA;47 CZP 200 mg | 73 | 78 | –0.45 | –0.52 | 69 | 74 | 47 | 62 | 22 | 47 |
| RAPID-PsA;47 CZP 400 mg | 66 | 77 | –0.39 | –0.43 | 63 | 72 | 47 | 61 | 20 | 36 |
| PSUMMIT 1;58,66 45 mg of UST | 59 | 56 | –0.28 | –0.31 | 61 | 78 | 39 | 57 | 19 | 41 |
| PSUMMIT 2;59,66 45 mg of UST | 52 | 55 | –0.21 | –0.21 | 64 | 68 | 39 | 51 | 20 | 30 |
Based on these observations, and to allow our analyses to include subgroup data from both PSUMMIT trials,58,59,66 we used the 24-week PSUMMIT trial data for the analyses of PsARC and HAQ-DI, on the assumption that they fairly reflected the 12-week results. For the analyses of PASI and ACR outcomes (where the 12- and 24-week results differed), we used the 12-week data.
For completeness, and to allow comparison with the placebo groups and the 24-week data, the PsARC and HAQ-DI 12-week data for the PSUMMIT trial full populations are as follows: PSUMMIT 1,58,66 PsARC responders 121 of 205 for 45 mg of UST and 75 of 206 for placebo; HAQ-DI mean change from baseline –0.28 units (SD 0.487 units) for 45 mg of UST and –0.1 units (SD 0.384 units) for placebo; PSUMMIT 2,59,66 PsARC responders 54 of 103 for 45 mg of UST and 33 of 104 for placebo; HAQ-DI mean change from baseline –0.21 units (SD 0.472 units) for 45 mg of UST and –0.07 units (SD 0.398 units) for placebo.
Psoriatic Arthritis Response Criteria response
Detailed methods for the biologic-naive subpopulation
Each trial reported the number of events (PsARC responses) in the placebo and the number of events under treatments (rit), where i represents a trial (i = 1, . . . , 14) and t represents a treatment (t = 1, . . . , 10). Across all models, it was assumed that that rit are binomially distributed, with probability parameter pit representing the probability of an event (PsARC response) in treatment arm t of trial i. As the parameters of interest, pit, are probabilities and, therefore, can take only values between 0 and 1, we modelled these on the logit scale (log-odds). We implemented separate models for the pooling of treatment effects and of placebo responses.
Treatment effect models
The treatment effect model assumed the baseline and treatment effects to be additive on the logit scale Logit(pit) = µi + δt. This means that log-ORs were pooled across trials. In the treatment effect models, the baselines were considered trial specific (unconstrained). We implemented a set of alternative models in what concerns the specification of treatment effects. We first explored a model with independent treatment effects across treatments. We then explored the possibility of placebo response determining the effectiveness of alternative treatments (with treatment effects still assumed independent). We also explored whether or not there was similarity between treatment effects for treatments of the same class.
Exploring placebo response as a treatment effect modifier
The trial-specific data show that higher placebo rates are associated with lower relative effectiveness estimates. Our investigations regarding trial designs and patient characteristics did not identify a clear reason for such differences, although placebo response rates appear to have increased over time. We investigated the effect of placebo response as a treatment effect modifier. It should be noted that the source of any relationship between placebo response and treatment effect is unclear and the reader should interpret the results carefully and with caution.
Figure 21 shows the relationships between trial-specific observed placebo responses and ORs on log-odds scale in the biologic-naive population. Considering placebo response as a treatment effect modifier in the independent treatment-effects analysis, only multiple studies of the same treatment (two or more studies) can inform the placebo effect. Hence, treatments from the single trials (i.e. CZP, SEC and GOL) do not contribute to the interaction in the independent treatment-effects analysis. In Figure 21, the solid lines within the plot reflect the relationship between the trials of the same treatments. Those with a steeper slope will indicate a stronger effect modification of placebo response (i.e. stronger association between placebo response and treatment effects). The highest effects are seen between trials of ADA and ETN – lines in green in Figure 21. Among the trials on ETN, the Mease et al. trial53 has the smallest number of participants and the response rates in placebo and treatment arms are very different from other trials. Similarly, the smallest trial of ADA (i.e. Genovese et al. 56) reports a similar proportion of PRs but very different response to treatment compared with the ADEPT55 of ADA. Therefore, the Mease et al. 53 and Genovese et al. 56 trials could contribute most (and possibly unreasonably so) to the estimation of interaction term (beta). It should be noted that the effect of placebo is consistently negative across all trials (i.e. higher placebo rates are associated with lower relative effectiveness estimates in the trial evidence). Exclusion of both Mease et al. 53 and Genovese et al. 56 will probably result in a much less pronounced placebo effect but it will still be negative.
FIGURE 21.
The PsARC response in the biologic-naive subpopulation: plot of trial-specific observed log-odds of placebo responses and ORs on log-scale (all 13 trials).

Given the issue of heterogeneity in terms of unexplained differences in placebo response rates across the trials, analyses were undertaken, including a metaregression adjusting for placebo response. We used the baseline risk in each trial for the adjustment, taking into account the error in the estimation of baseline risk and its correlation to the ORs. 114 Sensitivity analyses excluding both the Mease et al. 53 and Genovese et al. 56 trials were performed. Note that the effect of excluding these studies will be more pronounced if independent treatment effects are considered, rather than class effects. In the treatment effects as class analysis, all treatments assume to have equal or similar treatment effects; therefore, all studies within the class will contribute to the interaction term (compare dashed lines in Figures 21 and 22, in which all biologics as a class was assumed). The metaregression model includes an interaction term between the treatment effect (log-OR) and the trial-level estimate of placebo log-odds of response. By including such an interaction term, analyses will assume that the relative effectiveness of each of the treatments is not constant, but is associated with the response rate in the placebo arm. Treatment effects are no longer independent of the placebo response, but will be predicted for a particular value for the response rate in the placebo arm – usually the mean across the trials. The ranking of treatments is expected to differ from that estimated in the primary analyses (without the metaregression being imposed). This is because if, for example, the metaregression shows that trials with higher placebo response rates are associated with lower treatment effects, then treatments such as SEC that have been trialled only under a high placebo response will be predicted to have shown higher effectiveness in a different trial with a placebo response equal to the mean observed across trials.
Exploring treatment effects as class
In the context of an adjusted model for placebo response, we explored the possibility of there being class effects. Three different class groupings were considered: all treatments as a single class; all biologics as a class with APR separate; and, to reflect the pharmacology, anti-TNFs grouped, ILs grouped and APR separate. In addition, we explored two within-class assumptions: assuming treatments within a class to have equal effectiveness and, alternatively, that treatments within a class have similar (exchangeable) effectiveness (described by a normal distribution with an estimated mean and variance). Fixed effects across studies were assumed for all models. We have not considered models assuming exchangeability between classes.
Summary of all treatment effect models explored
All models implemented for evidence synthesis of PsARC response are presented in Table 122. Detailed coding of the models is presented in Table 123.
| Sets of analysis | Between-studies assumption | Treatment | Metaregression | Class |
|---|---|---|---|---|
| A1 | FE | Independent | No baseline adjustment | No class effect |
| B1 | FE | Independent | Common interaction term with log-odds of response in placebo arm | No class effect |
| C1 | FE | Equal | class | Common interaction term with log-odds of response in placebo arm | Independent class effect: class = {all treatments} |
| C2 | FE | Equal | class, remaining treatments independenta | Independent class effect: class = APR independent {all remaining biologics} | |
| C3 | FE | Equal | class, remaining treatments independenta | Independent class effect: class = {anti-TNFs, ILs}; APR independent | |
| D1 | FE | Exchangeable | class, remaining treatments independenta | Common interaction term with log-odds of response in placebo arm | Independent class effect: class = APR independent {all other biologics} |
| D2 | FE | Exchangeable | class, remaining treatments independenta | Independent class effect: class = {anti-TNFs, ILs}; APR independent |
| Model A1 | Model B1 |
|---|---|
| Likelihoodrit~Binomial(pit,nit)ModelLogit(pit)=µi+δtPriorsδt~dnorm(0,0.000001),µi~dnorm(0,0.000001) | Likelihoodrit~Binomial(pit,nit)ModelLogit(pit)=µi+δt+β(µi−µ¯)Priorsδt~dnorm(0,0.000001),µi~dnorm(0,0.000001),β~dnorm(0,0.000001) |
Assumptions:
|
Assumptions:
|
| Models C1, C2 and C3 | Models D1 and D2 |
| Likelihoodrit~Binomial(pit,nit)ModelLogit(pit)=µi+δt+β(µi−µ¯)δt=δcPriorsδc~dnorm(0,0.000001)µi~dnorm(0,0.000001)β~dnorm(0,0.000001) | Likelihoodrit~Binomial(pit,nit)ModelLogit(pit)=µi+δt+β(µi−µ¯)δt~dnorm(Classc,1/γ2)PriorsClassc~dnorm(0,0.000001)γ~dunif(0,10)µi~dnorm(0,0.000001)β~dnorm(0,0.000001) |
| C1: class = {all biologics} | D1: APR independent; class = {all other biologics} |
| C2: APR independent; class = {all other biologics} | D2: class = {anti-TNFs, ILs}; APR independent |
| C3: class = {anti-TNFs, ILs}; APR independent | |
Assumptions:
|
Assumptions:
|
| Pooling of placebo effects | |
| Likelihood:rplaci~dbin(pplaci,ni)ModelLogit(pplaci)=PDiPDi~dnorm(Mean,1/σ2)Priors:Mean~dnorm(0,0.000001)σ~duni(0,10) | |
In summary, this model assumes:
|
|
As stated earlier, sensitivity analyses around the adjustment for placebo response were performed: sets of analyses (models A1, B1, C1, C2, C3, D1 and D2) were conducted for PsARC response excluding the Mease et al. 53 and Genovese et al. 56 trials.
Placebo response synthesis model
To estimate baseline effect, the number of events in the placebo arm reported within each trial (ri1) was assumed to be binomially distributed and the log-odds for placebo was pooled across trials. A random effect was assumed between studies. The trial-specific effects for placebo PDi were estimated from a common distribution PDi ∼ dnorm(mean,1/σ2). The random effect was defined using a mean and variance parameters (mean and σ, respectively). Mean was assigned a non-informative normal prior distribution and σ was assigned a uniform prior. Results of the analysis are presented in Detailed results for the biologic-naive subpopulation.
Detailed results for the biologic-naive subpopulation
Summary results of Psoriatic Arthritis Response Criteria response
Tables 124 and 125 show summary results of PsARC response including and excluding the Genovese et al. 56 and Mease et al. 53 studies.
| Metaregression | No | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||
| Treatments | Ind | Ind | = | class | = | class | = | class | ∼ | classa | ∼ | classa | ||||||||
| Class | No | No | {All} | {APR, other} | {ILs, TNFs, APR} | {APR, other} | {ILs, TNFs, APR} | ||||||||
| Log-odds placebo | A1 | r b | B1 | r b | C1 | r b | C2 | r b | C3 | r b | D1 | r b | D2 | r b | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 300 mg of SEC | –0.16 | 1.178 | 5 | 2.110 | 1 | 1.844 | 3 | 1.833 | 3 | ||||||
| 150 mg of SEC | –0.16 | 1.175 | 6 | 2.104 | 2 | 1.285 | 2 | 1.839 | 4 | 1.822 | 4 | ||||
| UST | –0.51 | 0.758 | 9 | 1.187 | 7 | 1.197 | 8 | 1.174 | 8 | ||||||
| CZP | –0.28 | 1.094 | 7 | 1.837 | 5 | 1.278 | 1 | 1.565 | 1 | 1.722 | 5 | 1.716 | 5 | ||
| GOL | –1.32 | 2.339 | 1 | 1.619 | 6 | 1.692 | 6 | 1.712 | 6 | ||||||
| ADA | –1.02 | 1.401 | 4 | 1.081 | 8 | 1.648 | 1 | 1.201 | 7 | 1.201 | 7 | ||||
| INF | –1.15 | 2.296 | 2 | 1.870 | 4 | 1.853 | 2 | 1.875 | 1 | ||||||
| ETN | –0.99 | 2.043 | 3 | 1.917 | 3 | 1.856 | 1 | 1.872 | 2 | ||||||
| APR | –0.85 | 0.813 | 8 | 0.765 | 9 | 0.756 | 2 | 0.779 | 3 | 0.769 | 9 | 0.771 | 9 | ||
| Beta (mean) | – | –1.471 | –0.498 | –1.692 | –1.061 | –1.264 | –1.225 | ||||||||
| Residual deviancec | 29.9 | 27.2 | 59.2 | 46.8 | 47.5 | 27.8 | 27.9 | ||||||||
| DIC | 193.1 | 190.5 | 148.0 | 203.8 | 199.1 | 190.0 | 190.3 | ||||||||
| Metaregression | No | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||
| Treatments | Ind | Ind | = | class | = | class | = | class | ∼ | classa | ∼ | classa | ||||||||
| Class | No | No | {All} | {APR, other} | {IL, TNF, APR} | {APR, other} | {ILs, TNFs, APR} | ||||||||
| Log-odds placebo | A1 | r b | B1 | r b | C1 | r b | C2 | r b | C3 | r b | D1 | r b | D2 | r b | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 300 mg of SEC | –0.16 | 1.176 | 5 | 1.928 | 2 | 1.775 | 2 | 1.682 | 4 | ||||||
| 150 mg of SEC | –0.16 | 1.169 | 6 | 1.914 | 3 | 1.259 | 2 | 1.766 | 3 | 1.674 | 5 | ||||
| UST | –0.51 | 0.757 | 9 | 1.099 | 8 | 1.179 | 8 | 1.127 | 8 | ||||||
| CZP | –0.28 | 1.092 | 7 | 1.686 | 6 | 1.665 | 6 | 1.640 | 6 | ||||||
| GOL | –1.32 | 2.341 | 1 | 1.761 | 5 | 1.294 | 1 | 1.577 | 1 | 1.680 | 1 | 1.729 | 4 | 1.778 | 2 |
| ADA | –1.05 | 1.526 | 4 | 1.251 | 7 | 1.344 | 7 | 1.377 | 7 | ||||||
| INF | –1.15 | 2.301 | 2 | 1.953 | 1 | 1.864 | 1 | 1.897 | 1 | ||||||
| ETN | –0.80 | 1.784 | 3 | 1.781 | 4 | 1.725 | 5 | 1.748 | 3 | ||||||
| APR | –0.85 | 0.814 | 8 | 0.772 | 9 | 0.761 | 2 | 0.781 | 3 | 0.773 | 9 | 0.777 | 9 | ||
| Beta (mean) | – | –1.149 | –1.680 | –1.481 | –0.903 | –1.131 | –1.018 | ||||||||
| Residual deviancec | 23.6 | 22.6 | 52.2 | 38.2 | 36.3 | 22.3 | 22.8 | ||||||||
| DIC | 169.8 | 168.7 | 147.9 | 177.8 | 176.0 | 167.0 | 167.7 | ||||||||
Detailed results of Psoriatic Arthritis Response Criteria response
Results of the baseline effects (placebo)
The mean baseline effect is estimated to be –0.81 (Table 126).
Results of treatment effects models
More detailed results of models A1, B1, C1, C2, C3, D1 and D2 are presented next.
Results including Genovese et al. and Mease et al. studies
The results of the models A1, B1, C1, C2, C3, D1 and D2, including the Genovese et al. 56 and Mease et al. 53 studies, are presented in Tables 127–133.
Results of analysis assuming treatments are independent including all studies (Table 127).
| Treatment | OR | Treatment effects (log-odds) | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| 300 mg of SEC | 3.499 | 3.246 | 1.559 to 6.886 | 1.181 | 1.178 | 0.444 to 1.930 |
| 150 mg of SEC | 3.503 | 3.239 | 1.540 to 6.955 | 1.179 | 1.175 | 0.432 to 1.939 |
| UST | 2.172 | 2.134 | 1.489 to 3.070 | 0.759 | 0.758 | 0.398 to 1.122 |
| CZP | 3.082 | 2.985 | 1.880 to 4.813 | 1.096 | 1.094 | 0.631 to 1.571 |
| GOL | 10.890 | 10.370 | 5.865 to 18.980 | 2.343 | 2.339 | 1.769 to 2.943 |
| ADA | 4.159 | 4.059 | 2.703 to 6.212 | 1.403 | 1.401 | 0.994 to 1.827 |
| INF | 10.330 | 9.931 | 5.914 to 17.060 | 2.299 | 2.296 | 1.777 to 2.837 |
| ETN | 8.063 | 7.712 | 4.529 to 13.580 | 2.047 | 2.043 | 1.510 to 2.609 |
| APR | 2.276 | 2.255 | 1.733 to 2.941 | 0.813 | 0.813 | 0.550 to 1.079 |
| Residual deviancea | 29.86 | |||||
| DIC | 193.148 | |||||
Metaregression results including all studies
Results of analysis assuming treatments are independent (Table 128).
| Treatment | OR | Treatment effects (log-odds) | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| 300 mg of SEC | 10.560 | 8.251 | 3.244 to 26.790 | 2.142 | 2.110 | 1.177 to 3.288 |
| 150 mg of SEC | 10.410 | 8.196 | 3.174 to 26.980 | 2.135 | 2.104 | 1.155 to 3.295 |
| UST | 3.441 | 3.276 | 2.117 to 5.752 | 1.201 | 1.187 | 0.750 to 1.750 |
| CZP | 7.024 | 6.277 | 3.166 to 14.980 | 1.861 | 1.837 | 1.153 to 2.707 |
| GOL | 5.360 | 5.049 | 2.000 to 10.400 | 1.593 | 1.619 | 0.693 to 2.342 |
| ADA | 2.989 | 2.947 | 1.745 to 4.404 | 1.067 | 1.081 | 0.557 to 1.483 |
| INF | 6.702 | 6.488 | 3.345 to 11.120 | 1.856 | 1.870 | 1.207 to 2.408 |
| ETN | 7.018 | 6.804 | 4.026 to 11.250 | 1.914 | 1.917 | 1.393 to 2.420 |
| APR | 2.160 | 2.150 | 1.684 to 2.691 | 0.763 | 0.765 | 0.521 to 0.990 |
| Beta | –1.471 | –1.459 | –2.769 to –0.216 | |||
| Residual deviancea | 27.17 | |||||
| DIC | 190.495 | |||||
Results of analyses assuming treatments as class (Tables 129–133).
| Treatment/parameter | OR | Treatment effects (log-odds) | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| Biologics as class | 3.612 | 3.589 | 2.730 to 4.648 | 1.275 | 1.278 | 1.004 to 1.537 |
| Beta | –0.498 | 0.523 | –3.711 to 2.483 | |||
| Residual deviancea | 59.24 | |||||
| DIC | 147.961 | |||||
| Treatment/parameter | OR | Treatment effects (log-odds) | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| Biologics as class (excluding APR) | 4.805 | 4.782 | 4.099 to 5.657 | 1.566 | 1.565 | 1.411 to 1.733 |
| APR | 2.142 | 2.130 | 1.676 to 2.670 | 0.755 | 0.756 | 0.516 to 0.982 |
| Beta | –1.692 | –1.666 | –2.406 to –1.122 | |||
| Residual deviancea | 46.83 | |||||
| DIC | 203.806 | |||||
| Treatment/parameter | OR | Treatment effects (log-odds) | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| ILs as class | 3.755 | 3.616 | 1.880 to 6.573 | 1.273 | 1.285 | 0.631 to 1.883 |
| Anti-TNFs as class | 5.238 | 5.195 | 4.036 to 6.710 | 1.648 | 1.648 | 1.395 to 1.904 |
| APR | 2.194 | 2.179 | 1.726 to 2.751 | 0.779 | 0.779 | 0.546 to 1.012 |
| Beta | –1.061 | –1.025 | –1.864 to –0.462 | |||
| Residual deviancea | 47.54 | |||||
| DIC | 199.129 | |||||
| Treatment | OR | Predicted mean distribution | Shrunken or independent treatment effects (log-odds) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| 300 mg of SEC | 5.331 | 5.206 | 3.675 to 7.737 | 1.657 | 1.647 | 0.653 to 2.714 | 1.859 | 1.844 | 1.343 to 2.456 |
| 150 mg of SEC | 1.853 | 1.839 | 1.332 to 2.451 | ||||||
| UST | 1.202 | 1.197 | 0.885 to 1.538 | ||||||
| CZP | 1.731 | 1.722 | 1.342 to 2.165 | ||||||
| GOL | 1.689 | 1.692 | 1.233 to 2.122 | ||||||
| ADA | 1.197 | 1.201 | 0.861 to 1.509 | ||||||
| INF | 1.854 | 1.853 | 1.462 to 2.254 | ||||||
| ETN | 1.859 | 1.856 | 1.481 to 2.258 | ||||||
| APR | 2.166 | 2.157 | 1.765 to 2.609 | 0.768 | 0.769 | 0.568 to 0.959 | |||
| γa | 0.437 | 0.398 | 0.187 to 0.924 | ||||||
| Beta | –1.264 | –1.261 | –1.917 to –0.633 | ||||||
| Residual devianceb | 27.76 | ||||||||
| DIC | 189.961 | ||||||||
| Treatment | OR | Predicted mean distribution | Shrunken or independent treatment effects (log-odds) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| 300 mg of SEC | 5.521 | 4.982 | 2.326 to 11.920 | 1.618 | 1.597 | 0.315 to 3.016 | 1.841 | 1.833 | 1.146 to 2.588 |
| 150 mg of SEC | 1.832 | 1.822 | 1.133 to 2.588 | ||||||
| UST | 1.180 | 1.174 | 0.809 to 1.580 | ||||||
| CZP | 5.546 | 5.340 | 3.112 to 9.147 | 1.671 | 1.673 | 0.424 to 2.891 | 1.722 | 1.716 | 1.278 to 2.209 |
| GOL | 1.707 | 1.712 | 1.173 to 2.204 | ||||||
| ADA | 1.199 | 1.201 | 0.834 to 1.548 | ||||||
| INF | 1.874 | 1.875 | 1.430 to 2.306 | ||||||
| ETN | 1.874 | 1.872 | 1.476 to 2.287 | ||||||
| APR | 2.172 | 2.162 | 1.763 to 2.638 | 0.770 | 0.771 | 0.567 to 0.970 | |||
| γa | 0.491 | 0.437 | 0.193 to 1.107 | ||||||
| Beta | –1.225 | –1.227 | –2.039 to –0.393 | ||||||
| Residual devianceb | 27.92 | ||||||||
| DIC | 190.342 | ||||||||
Results excluding Genovese et al. and Mease et al. studies
The results of the models A1, B1, C1, C2, C3, D1 and D2, excluding the Genovese et al. 56 and Mease et al. 53 studies, are presented in Tables 134–140.
The results of analysis assuming treatments are independent excluding the Genovese et al. 56 and Mease et al. 53 studies (Table 134).
| Treatment | OR | Treatment effects (log-odds) | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| 300 mg of SEC | 3.492 | 3.240 | 1.554 to 6.920 | 1.178 | 1.176 | 0.441 to 1.934 |
| 150 mg of SEC | 3.486 | 3.218 | 1.543 to 6.982 | 1.174 | 1.169 | 0.434 to 1.943 |
| UST | 2.168 | 2.131 | 1.486 to 3.062 | 0.757 | 0.757 | 0.396 to 1.119 |
| CZP | 3.076 | 2.980 | 1.861 to 4.820 | 1.094 | 1.092 | 0.621 to 1.573 |
| GOL | 10.910 | 10.390 | 5.869 to 18.920 | 2.345 | 2.341 | 1.770 to 2.940 |
| ADA | 4.746 | 4.602 | 2.856 to 7.491 | 1.527 | 1.526 | 1.049 to 2.014 |
| INF | 10.380 | 9.983 | 5.954 to 17.210 | 2.303 | 2.301 | 1.784 to 2.845 |
| ETN | 6.269 | 5.956 | 3.264 to 11.070 | 1.787 | 1.784 | 1.183 to 2.404 |
| APR | 2.278 | 2.257 | 1.739 to 2.931 | 0.814 | 0.814 | 0.553 to 1.075 |
| Residual deviancea | 23.63 | |||||
| DIC | 169.761 | |||||
Metaregressions results excluding Genovese et al. and Mease et al. studies
Results of analysis assuming treatments are independent (Table 135).
| Treatments | OR | Treatment effects (log-odds) | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| 300 mg of SEC | 9.534 | 6.872 | 2.132 to 23.890 | 1.932 | 1.928 | 0.757 to 3.174 |
| 150 mg of SEC | 9.980 | 6.779 | 2.091 to 23.470 | 1.925 | 1.914 | 0.738 to 3.156 |
| UST | 3.178 | 3.001 | 1.748 to 5.410 | 1.103 | 1.099 | 0.558 to 1.688 |
| CZP | 6.248 | 5.400 | 2.241 to 13.770 | 1.695 | 1.686 | 0.807 to 2.622 |
| GOL | 8.068 | 5.818 | 2.233 to 14.620 | 1.757 | 1.761 | 0.803 to 2.682 |
| ADA | 3.647 | 3.494 | 1.940 to 5.920 | 1.245 | 1.251 | 0.663 to 1.778 |
| INF | 7.572 | 7.049 | 3.629 to 13.280 | 1.952 | 1.953 | 1.289 to 2.587 |
| ETN | 6.218 | 5.936 | 3.477 to 10.280 | 1.783 | 1.781 | 1.246 to 2.330 |
| APR | 2.181 | 2.165 | 1.707 to 2.729 | 0.772 | 0.772 | 0.535 to 1.004 |
| Beta | –1.149 | –1.151 | –2.727 to 0.406 | |||
| Residual deviancea | 22.601 | |||||
| DIC | 168.708 | |||||
Results of analyses assuming treatments as class (Tables 136–140).
| Treatment/parameter | OR | Treatment effects (log-odds) | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| Biologics as class | 3.679 | 3.649 | 2.749 to 4.794 | 1.293 | 1.294 | 1.011 to 1.567 |
| Beta | –1.680 | –2.560 | –4.050 to 2.094 | |||
| Residual deviancea | 52.16 | |||||
| DIC | 147.920 | |||||
| Treatment/parameter | OR | Treatment effects (log-odds) | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| Biologics as class (excluding APR) | 4.867 | 4.843 | 4.192 to 5.682 | 1.580 | 1.577 | 1.433 to 1.737 |
| APR | 2.151 | 2.141 | 1.730 to 2.622 | 0.760 | 0.761 | 0.548 to 0.964 |
| Beta | –1.481 | –1.455 | –2.122 to –0.996 | |||
| Residual deviancea | 38.16 | |||||
| DIC | 177.825 | |||||
| Treatment/parameter | OR | Treatment effects (log-odds) | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| ILs as class | 3.559 | 3.520 | 2.289 to 5.069 | 1.250 | 1.259 | 0.828 to 1.623 |
| Anti-TNFs as class | 5.392 | 5.363 | 4.500 to 6.460 | 1.681 | 1.680 | 1.504 to 1.866 |
| APR | 2.195 | 2.183 | 1.796 to 2.652 | 0.781 | 0.781 | 0.586 to 0.976 |
| Beta | –0.903 | –0.906 | –1.725 to –0.087 | |||
| Residual deviancea | 36.30 | |||||
| DIC | 175.979 | |||||
| Treatment | OR | Predicted mean distribution | Shrunken or independent treatment effects (log-odds) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| 300 mg of SEC | 5.214 | 5.115 | 3.706 to 7.350 | 1.637 | 1.633 | 0.713 to 2.563 | 1.787 | 1.775 | 1.296 to 2.335 |
| 150 mg of SEC | 1.778 | 1.766 | 1.273 to 2.338 | ||||||
| UST | 1.180 | 1.179 | 0.857 to 1.507 | ||||||
| CZP | 1.668 | 1.665 | 1.283 to 2.067 | ||||||
| GOL | 1.733 | 1.729 | 1.329 to 2.157 | ||||||
| ADA | 1.341 | 1.344 | 0.991 to 1.669 | ||||||
| INF | 1.869 | 1.864 | 1.499 to 2.264 | ||||||
| ETN | 1.731 | 1.725 | 1.355 to 2.141 | ||||||
| APR | 2.177 | 2.167 | 1.791 to 2.621 | 0.773 | 0.773 | 0.583 to 0.964 | |||
| γa | 0.385 | 0.350 | 0.148 to 0.824 | ||||||
| Beta | –1.131 | –1.128 | –1.750 to –0.528 | ||||||
| Residual devianceb | 22.34 | ||||||||
| DIC | 167.044 | ||||||||
| Treatment | OR | Predicted mean distribution | Shrunken or independent treatment effects (log-odds) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| 300 mg of SEC | 4.805 | 4.428 | 2.225 to 9.507 | 1.503 | 1.478 | 0.383 to 2.754 | 1.688 | 1.682 | 1.012 to 2.390 |
| 150 mg of SEC | 1.679 | 1.674 | 0.998 to 2.399 | ||||||
| UST | 1.127 | 1.127 | 0.756 to 1.498 | ||||||
| CZP | 5.566 | 5.408 | 3.436 to 8.554 | 1.695 | 1.689 | 0.670 to 2.750 | 1.640 | 1.640 | 1.218 to 2.064 |
| GOL | 1.781 | 1.778 | 1.314 to 2.258 | ||||||
| ADA | 1.376 | 1.377 | 0.985 to 1.757 | ||||||
| INF | 1.904 | 1.897 | 1.512 to 2.329 | ||||||
| ETN | 1.752 | 1.748 | 1.359 to 2.165 | ||||||
| APR | 2.187 | 2.176 | 1.796 to 2.642 | 0.778 | 0.777 | 0.586 to 0.972 | |||
| γa | 0.407 | 0.362 | 0.123 to 0.968 | ||||||
| Beta | –1.018 | –1.019 | –1.781 to –0.245 | ||||||
| Residual devianceb | 22.77 | ||||||||
| DIC | 167.708 | ||||||||
Preferred models
The unadjusted model A1 fits the data as well as any of the other models and generates results that reflect the observed results. The placebo response-adjusted model B1 fits well compared with the unadjusted model A1 (smaller DIC and residual deviance), but not significantly so as the difference in DIC is < 5 points. Considering the placebo-adjusted models, it must be borne in mind that without any clear rationale for the placebo effect, the results must be interpreted with caution. The results (rankings) generated by model B1 are very different from the observed trial results.
Regarding possible class effects, the analyses found that an assumption of equal class effect for the treatments does not produce a better-fitting model (models C1, C2, C3) than assuming independent treatment effects (models A1, B1) or similar treatment effects (models D1, D2). There was little difference in goodness-of-fit statistics (DIC and residual deviance) between models D1 and D2, and we consider the exchangeable class effect model (D2) which utilised two classes (ILs and anti-TNFs) with APR separate to be the most clinically plausible. The results (rankings) generated by models D1 and D2 are same, but are very different from the observed trial results.
Comparing treatment effects in models A1, B1 and D2, the treatment effects are very different from each other. INF and 300 mg of SEC appeared to be the most effective in models D2 and B1, respectively, but GOL is the most effective in model A1. UST appeared to be least effective in model A1, whereas APR appeared to be least effective in models B1 and D2.
In the sensitivity analyses on Genovese et al. 56 and Mease et al. ,53 excluding those two studies from the analysis affects the treatment effects, resulting in changes of the treatment effects ranking. Despite the results of the adjusted model (B1) being sensitive to the exclusion of Mease et al. 53 and Genovese et al. 56 (with rankings changing), there are two reasons why this analysis has not been adopted as the main one. First, exclusion of these studies may appear to be selective, and second it is less relevant in the context of our preferred model that assumes a class effect (compare D2 with and without Mease et al. 53 and Genovese et al. 56). Therefore, these two trials were not excluded from our preferred analysis.
Hence, we consider models A1 and D2 including Genovese et al. 56 and Mease et al. 53 to be our preferred models.
Comparison of the network meta-analysis of Psoriatic Arthritis Response Criteria responses in the company submissions (Novartis and UCB Pharma), a previous multiple technology appraisal (Rodgers et al.) and the current Assessment Group
Each of the two CSs combined evidence using Bayesian evidence synthesis methods to estimate probability of PsARC responses to inform the economic model. UCB Pharma and the AG included analysis of subpopulations in the main NMA and analysed both subpopulations (biologic naive and experienced) separately, whereas Novartis considered overall population as the main NMA, and the analysis included a more complete set of treatments and trials. The AG refers to the subgroup NMA conducted by Novartis (i.e. biologic naive) in this comparison. A brief comparison of the methods used and key model assumptions by the AG, CS and previous MTA is presented in Tables 141 and 142.
| Domains compared | Rodgers et al., 201133 | CS | CS | |
|---|---|---|---|---|
| Novartis | UCB Pharma | AG | ||
| Model | Binomial logit model | Binomial logit model | Binomial logit model | Binomial logit model |
| Results reported | Probability of PsARC response for each treatment | RRs of each treatment compared with SEC; and probability of PsARC response for each treatmenta | ORs reported for the biologic-naive subpopulation, but results were not reported for the biologic-experienced subpopulation | ORs and probability of PsARC response for each treatment |
| Time point | At 12 weeks (data from the 12-week or closest time point after 12 weeks – normally 14 or 16 weeks) | At 12 weeks (data from the 12-week or closest time point after 12 weeks – normally 14 or 16 weeks) | Primary analysis at 24 weeks (by treatments), sensitivity analysis was conducted at 12 weeks including data on 12 weeks or closest time point after 12 weeksb | At 12 weeks (data from the 12-week or closest time point after 12 weeks – normally 14 or 16 weeks); UST outcomes at 24 weeks were included and assumed equivalent to outcomes at 12 weeks |
| Comments | – | Modelled probabilities are presented graphically | – | – |
| Data regarding subpopulation of biologic naive | ||||
| Studies used in the analysis | ADEPT;55 Genovese et al.;56 IMPACT;51 IMPACT 2;52 and Mease et al.53,54 | ADEPT;55 Genovese et al.;56 FUTURE 2;48 GO-REVEAL;50 IMPACT 2;52 and Mease et al.54 | ADEPT;55 Genovese et al.;56 GO-REVEAL;50 IMPACT;51 IMPACT 2;52 Mease et al.;53,54 and RAPID-PsA47 (12- to 16-week analysis) | ADEPT;55 FUTURE 2;48 Genovese et al.;56 GO-REVEAL;50 IMPACT;51 IMPACT 2;52 Mease et al.;53,54 PALACE 1;60 PALACE 2;61 PALACE 3;65 PSUMMIT 1;58 PSUMMIT 2;59,66 and RAPID-PsA47 |
| Drugs evaluated | 40 mg of ADA; 5 mg/kg of INF; and 25 mg of ETN | 40 mg of ADA; 25 mg of ETN; 50 and 100 mg of GOL; 5 mg/kg of INF; and 150 and 300 mg of SEC | 40 mg of ADA; 400 mg of CZP; 25 mg of ETN; 50 mg of GOL; and 5 mg/kg of INF | 40 mg of ADA; 30 mg of APR; 400 mg of CZP; 25 mg of ETN; 50 mg of GOL; 5 mg/kg of INF; 150 and 300 mg of SEC; and 45 mg of UST |
| Data regarding subpopulation of biologic experienced | ||||
| Studies used in the analysis | – | – | Not clear | FUTURE 2;48 and PSUMMIT 259,66 |
| Drugs evaluated | – | – | Not clear | 300 mg of SEC; and 45 mg of UST |
| Domains compared | Rodgers et al., 201133 | CS | AG | |
|---|---|---|---|---|
| Novartis | UCB Pharma | |||
| Model | Binomial logit model | Binomial logit model | Binomial logit model | Binomial logit model |
| Fixed or random effects between studies | Random effects on studies | Random effects on studies | Fixed effects on studies (for both biologic-naive and biologic-experienced subpopulation) | Fixed effects on studies (for both biologic-naive and biologic-experienced subpopulation) |
| Baselines | Common-effect model was used to estimate baseline | Common-effect model was used to estimate baseline | Common-effect model was used to estimate baseline | Common-effect model was used to estimate baseline |
| Treatment effects | Treatments were assumed to be independent of each other | Treatments were assumed to be independent of each other | For the biologic-naive subpopulation the treatment effects are exchangeable within classes (anti-TNFs = ADA, IFX, ETN, GOL). For the biologic-experienced subpopulation the treatments were assumed to be independent of each other | For the biologic-naive subpopulation:
|
| Model adjusted for placebo response | Unadjusted | Unadjusted | Adjusted for biologic-naive subpopulation, but unadjusted forbiologic-experienced subpopulation | Independent treatment effects models were unadjusted, but analysis assuming exchangeable class effects model was adjusted for the placebo response |
| Interaction term (beta) | – | – | Common interaction term in adjusted model | Common interaction term in adjusted model |
A key difference between the NMAs presented concerns the trials included in each analysis. Only the AG NMA for the biologic-naive subgroup includes all comparators and all trials. The UCB Pharma analysis for the biologic-naive subgroup includes all treatments but misses only some APR trials. The Novartis NMA does not include CZP or APR for the biologic-naive subgroup analysis and does not include all trials for the other treatments. The Rodgers et al. 33 analysis was limited to the treatments available at that time.
The evidence synthesis is not clear in UCB Pharma’s main submission for the biologic-experienced subgroup, and results for this subgroup were not reported. Novartis did not conduct a NMA for the biologic-experienced subgroup. Therefore, it was not plausible to compare the AG’s NMA with the CS for the biologic-experienced subgroup.
Another key difference relates to the primary time point analysed: most NMAs used 12 weeks, but the UCB Pharma analysis used 24 weeks as its primary time point, although it did include a 12-week sensitivity analysis.
All analyses considered a binomial logit model (both companies, previous MTA and AG). Both the AG and UCB Pharma consider fixed effect on studies, whereas Novartis considers random effects. Both the AG and UCB Pharma consider baseline risk adjustment to reflect effects of differences in trial-specific placebo response on treatment effects in the biologic-naive population whereas Novartis did not consider such adjustment for subgroup analysis.
Another key difference relates to the PsARC responses data included in the analysis. An inconsistency was identified by the AG in the Novartis submission in PsARC response data for SEC and revised PsARC response data were provided late in the assessment. Therefore, it is plausible that Novartis NMA used the incorrect data for the analysis. Additionally, the AG’s extracted PsARC response data from some studies do not match with the Novartis data, particularly for Mease et al. 54 trial and two ADA trials (ADEPT,55 Genovese et al. 56). The plausible explanation for the difference is that the AG consistently used ITT denominators rather than the ‘modified ITT’ approach which was sometimes used by the CS (whereby only patients who have received at least one dose of their randomised treatment are considered).
The results of the AG NMA are compared with those of the other NMAs in Tables 143 and 144. Table 143 shows the probabilities of PsARC response for the biologic-naive subgroup, estimated by the different models – Rodgers et al. ,33 Novartis and AG (the UCB Pharma results are presented only as ORs) – and Table 144 compares the ORs from the AG NMA with those from the UCB Pharma analysis. The results of the AG unadjusted NMA are mostly consistent with the previous MTA as well as the Novartis results, except for the SEC. The differences are largely because Novartis included a different PsARC response data set. The estimated probabilities in the AG’s analysis are more precise than Novartis’ results. Given the differences in model assumptions and included studies, the ranking of the treatment effects is similar between UCB Pharma and the AG’s adjusted NMA (see Table 144).
| Treatment | Rodgers et al. (2011),33 mean (95% CrI) | Novartis, mean | AG, median (95% CrI) | |
|---|---|---|---|---|
| Unadjusted, independent treatment | Adjusted for placebo response, class effects assumed | |||
| Placebo | 0.25 (0.18 to 0.32) | Confidential information has been removed | 0.31 (0.26 to 0.36) | 0.31 (0.26 to 0.36) |
| 300 mg of SEC | NC | Confidential information has been removed | 0.59 (0.40 to 0.76) | 0.73 (0.57 to 0.86) |
| 150 mg of SEC | NC | Confidential information has been removed | 0.59 (0.40 to 0.76) | 0.73 (0.57 to 0.86) |
| UST | NC | NC | 0.49 (0.38 to 0.60) | 0.59 (0.48 to 0.70) |
| CZP | NC | NC | 0.57 (0.44 to 0.69) | 0.71 (0.60 to 0.81) |
| 50 mg of GOL | NC | Confidential information has been removed | 0.82 (0.71 to 0.90) | 0.71 (0.58 to 0.81) |
| ADA | 0.59 (0.44 to 0.71) | Confidential information has been removed | 0.64 (0.53 to 0.75) | 0.60 (0.49 to 0.69) |
| INF | 0.80 (0.67 to 0.89) | Confidential information has been removed | 0.81 (0.71 to 0.89) | 0.74 (0.63 to 0.83) |
| ETN | 0.71 (0.57 to 0.83) | Confidential information has been removed | 0.77 (0.65 to 0.86) | 0.74 (0.64 to 0.82) |
| APR | NC | NC | 0.50 (0.41 to 0.59) | 0.49 (0.41 to 0.57) |
| Treatment | UCB Pharma, mean (95% CrI) | AG, mean (95% CrI) | |
|---|---|---|---|
| Unadjusted, independent treatment | Adjusted for placebo response, class effects assumed | ||
| 300 mg of SEC | NC | 3.25 (1.56 to 6.89) | 6.25 (3.15 to 13.31) |
| SEC 150 | NC | 3.24 (1.54 to 6.96) | 6.18 (3.10 to 13.30) |
| UST | NC | 2.13 (1.49 to 3.07) | 3.24 (2.25 to 4.86) |
| CZP | Confidential information has been removed | 2.99 (1.88 to 4.81) | 5.56 (3.59 to 9.11) |
| GOL | Confidential information has been removed | 10.37 (5.87 to 18.98) | 5.54 (3.23 to 9.06) |
| ADA | Confidential information has been removed | 4.06 (2.70 to 6.21) | 3.33 (2.30 to 4.70) |
| INF | Confidential information has been removed | 9.93 (5.91 to 17.06) | 6.52 (4.18 to 10.04) |
| ETN | Confidential information has been removed | 7.71 (4.53 to 13.58) | 6.50 (4.38 to 9.85) |
| APR | NC | 2.26 (1.73 to 2.94) | 2.16 (1.76 to 2.64) |
WinBUG codes of preferred model
HAQ-DI score changes conditional on PsARC response/non-response
Detailed methods for the biologic-naive subpopulation
We consider three models to estimate the HAQ-DI score changes conditional on PsARC response. Model E1 considers that treatments are independent and considers fixed effects across studies. Models E2 and E3 apply a class effects on three groups: anti-TNFs, ILs and APR. This class effect reflects the best-fitting class effect model for PsARC (see Detailed results for the biologic-naive subpopulation). Model E2 assumes that the treatments are similar within class (exchangeable) and fixed effect across studies, and model E3 considers that the treatments are equal within class and fixed effect across studies. A detailed description of the model and underlying assumptions are presented in Table 145.
| Model E1 | Model E2 | Model E3 |
|---|---|---|
| LikelihoodHAQPNRi~dnorm(µPNRi,1/varPNRi)HAQPRi~dnorm(µPRi,1/varPRi)HAQTNRij~dnorm(µTNRij,1/varTNRij)HAQTRij~dnorm(µTRij,1/varTRij)ModelµPNRi=baselineiµPRi=µPNRi+δ.diffPRµTNRij=µPNRi+δ.diffTNRjµTRij=µPNRi+δ.diffTRjPriorsbaselinei~dnorm(0,0.000001)δ.diffPR~dnorm(0,0.000001)δ.diffTNRj~dnorm(0,0.000001)δ.diffTRj~dnorm(0,0.000001) | LikelihoodHAQPNRi~dnorm(µPNRi,1/varPNRi)HAQPRi~dnorm(µPRi,1/varPRi)HAQTNRij~dnorm(µTNRij,1/varTNRij)HAQTRij~dnorm(µTRij,1/varTRij)ModelµPNRi=baselineiµPRi=µPNRi+δ.diffPRµTNRij=µPNRi+δ.diffTNRjµTRij=µPNRi+δ.diffTRjδ.diffTNRj~dnorm(δ.diffTNR.C,1/γTNR2)δ.diffTRj~dnorm(δ.diffTR.C,1/γTR2)Priorsbaselinei~dnorm(0,0.000001)δ.diffPR~dnorm(0,0.000001)δ.diffTNR.C~dnorm(0,0.000001)δ.diffTR.C~dnorm(0,0.000001)γTNR~dunif(0,10)γTNR~dunif(0,10) | LikelihoodHAQPNRi~dnorm(µPNRi,1/varPNRi)HAQPRi~dnorm(µPRi,1/varPRi)HAQTNRij~dnorm(µTNRij,1/varTNRij)HAQTRij~dnorm(µTRij,1/varTRij)ModelµPNRi=baselineiµPRi=µPNRi+δ.diffPRµTNRij=µPNRi+δ.diffTNRjδ.diffTNRj=δ.diffTNR.CµTRij=µPNRi+δ.diffTRjδ.diffTRj=δ.diffTR.CPriorsbaselinei~dnorm(0,0.000001)δ.diffPR~dnorm(0,0.000001)δ.diffTNR.C~dnorm(0,0.000001)δ.diffTR.C~dnorm(0,0.000001) |
Assumptions:
|
Assumptions:
|
Assumptions:
|
The model defines TR as treatment responder, TNR as treatment non-responder, PR as placebo responder and PNR as placebo non-responder; I represents the trial and j the alternative treatments. The observed quantities (i.e. HAQ-DI score changes in PRs and PNRs, and in TRs and TNRs) have a normal distribution for the likelihood.
Changes in HAQ-DI scores in all groups are assumed relative to changes in HAQ-DI in PNRs – µPNRi. This parameter was left unconstrained (allowed to differ between trials), and non-informative normal prior distributions were assigned (baselinei). The relative effects of placebo on those who respond in the placebo arm (δ.diffPR) were assumed to be additive to µPNRi and were pooled across trials. The relative effects of treatments on those who do not respond (δ.diffTNRj) and on those who respond (δ.diffTRj) are additive to µPNRi, and were assumed to be treatment specific. In pooling these parameters, we assumed fixed effects across studies. Within a fixed-effects model, parameters δ.diffPR, δ.diffTNRj, and δ.diffTRj were assigned non-informative normal prior distributions.
Detailed results for the biologic-naive subpopulation
Summary results of Health Assessment Questionnaire-Disability Index changes conditional on Psoriatic Arthritis Response Criteria response
The summary results from three models are presented in Table 146 as absolute changes in HAQ-DI scores in relation to baseline.
| Treatments | Independent treatment | Exchangeable | class | Equal | class | |||||||||
| Studies | FE | FE | FE | |||||||||
| E1 | E2a | E3 | PsARC response vs. non-response | |||||||||
| PsARC response | PsARC non-response | PsARC response | PsARC non-response | PsARC response | PsARC non-response | E1 | rb | E2a | rb | E3 | rb | |
| Placebo | –0.26 | –0.26 | –0.25 | –0.26 | 10 | –0.26 | 10 | –0.25 | 4 | |||
| 150 mg of SEC | –0.39 | –0.08 | –0.44 | –0.09 | –0.31 | 8 | –0.35 | 8 | ||||
| 300 mg of SEC | –0.55 | –0.05 | –0.51 | –0.08 | –0.47 | –0.08 | –0.49 | 1 | –0.43 | 3 | –0.39 | 1 |
| UST | –0.49 | –0.10 | –0.48 | –0.09 | –0.39 | 4 | –0.39 | 4 | ||||
| CZP | –0.43 | –0.07 | –0.47 | –0.12 | –0.36 | 6 | –0.35 | 7 | ||||
| GOL | –0.44 | –0.06 | –0.49 | –0.11 | –0.52 | –0.13 | –0.38 | 5 | –0.37 | 5 | –0.39 | 1 |
| ADA | –0.49 | –0.13 | –0.50 | –0.13 | –0.36 | 7 | –0.37 | 6 | ||||
| INF | –0.66 | –0.20 | –0.60 | –0.14 | –0.46 | 2 | –0.46 | 1 | ||||
| ETN | –0.64 | –0.20 | –0.59 | –0.14 | –0.44 | 3 | –0.45 | 2 | ||||
| APR | –0.36 | –0.09 | –0.36 | –0.09 | –0.36 | –0.09 | –0.27 | 9 | –0.27 | 9 | –0.27 | 3 |
| DIC | –126.0 | –133.0 | –131.4 | |||||||||
Detailed results of Health Assessment Questionnaire-Disability Index changes conditional on Psoriatic Arthritis Response Criteria response
The results of HAQ-DI score changes conditional on PsARC response or non-response are presented in Tables 147–149.
| Treatment | HAQ-DI score changes in PsARC response in relation to PNR | HAQ-DI score changes in PsARC non-response in relation to PNR | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| Placebo/baseline effect | –0.263 | –0.263 | –0.301 to –0.224 | |||
| 150 mg of SEC | –0.394 | –0.395 | –0.553 to –0.236 | –0.083 | –0.083 | –0.389 to 0.220 |
| 300 mg of SEC | –0.547 | –0.547 | –0.722 to –0.369 | –0.053 | –0.053 | –0.288 to 0.182 |
| UST | –0.488 | –0.488 | –0.597 to –0.379 | –0.098 | –0.097 | –0.208 to 0.012 |
| CZP | –0.429 | –0.429 | –0.530 to –0.326 | –0.069 | –0.069 | –0.194 to 0.057 |
| GOL | –0.439 | –0.439 | –0.585 to –0.293 | –0.063 | –0.064 | –0.182 to 0.055 |
| ADA | –0.489 | –0.489 | –0.583 to –0.395 | –0.135 | –0.134 | –0.237 to –0.032 |
| INF | –0.660 | –0.660 | –0.771 to –0.548 | –0.196 | –0.196 | –0.311 to –0.083 |
| ETN | –0.640 | –0.640 | –0.767 to –0.515 | –0.200 | –0.200 | –0.348 to –0.054 |
| APR | –0.362 | –0.362 | –0.432 to –0.291 | –0.089 | –0.089 | –0.157 to –0.022 |
| DIC | –125.96 | |||||
| Treatment | HAQ-DI score changes in PsARC response in relation to PNR | HAQ-DI score changes in PsARC non-response in relation to PNR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predicted mean | Shrunken/independent estimates | Predicted mean | Shrunken/independent estimates | |||||||||
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| Placebo | –0.258 | –0.258 | –0.296 to –0.220 | |||||||||
| 150 mg of SEC | –0.432 | –0.435 | –0.557 to –0.294 | –0.085 | –0.085 | –0.228 to 0.057 | ||||||
| 300 mg of SEC | –0.475 | –0.474 | –0.751 to –0.203 | –0.512 | –0.509 | –0.658 to –0.378 | –0.083 | –0.083 | –0.253 to 0.086 | –0.077 | –0.078 | –0.205 to 0.062 |
| UST | –0.481 | –0.480 | –0.580 to –0.383 | –0.088 | –0.087 | –0.186 to 0.009 | ||||||
| CZP | –0.468 | –0.470 | –0.558 to –0.370 | –0.116 | –0.118 | –0.196 to –0.021 | ||||||
| GOL | –0.482 | –0.486 | –0.594 to –0.354 | –0.110 | –0.114 | –0.188 to –0.013 | ||||||
| ADA | –0.530 | –0.529 | –0.784 to –0.279 | –0.499 | –0.500 | –0.581 to –0.414 | –0.130 | –0.130 | –0.274 to 0.012 | –0.133 | –0.132 | –0.209 to –0.058 |
| INF | –0.605 | –0.603 | –0.716 to –0.502 | –0.147 | –0.144 | –0.240 to –0.071 | ||||||
| ETN | –0.593 | –0.591 | –0.717 to –0.486 | –0.147 | –0.143 | –0.255 to –0.063 | ||||||
| APR | –0.361 | –0.361 | –0.430 to –0.289 | –0.088 | –0.088 | –0.155 to –0.020 | ||||||
| DIC | –133.03 | |||||||||||
| Treatment | HAQ-DI score changes in PsARC response in relation to PNR | HAQ-DI score changes in PsARC non-response in relation to PNR | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 95% CrI | Mean | Median | 95% CrI | |
| Placebo/baseline effect | –0.254 | –0.254 | –0.291 to –0.217 | |||
| ILs as class | –0.473 | –0.473 | –0.554 to –0.393 | –0.083 | –0.083 | –0.176 to 0.013 |
| Anti-TNFs as class | –0.524 | –0.524 | –0.575 to –0.474 | –0.131 | –0.131 | –0.185 to –0.077 |
| APR | –0.359 | –0.359 | –0.430 to –0.290 | –0.087 | –0.087 | –0.155 to –0.018 |
| DIC | –131.37 | |||||
Preferred models
The model fit statistics (DIC) indicate that neither class effect model (E2 or E3) is a better fit for the data than the unadjusted, independent treatments model (E1). The fit of both of the class effect models was similar, but the one that allowed exchangeability within classes (E2) was considered to be the most clinically plausible. For the purposes of the economic model in Chapter 6, models E1 and E2 were the preferred models.
Comparison of the network meta-analysis of Health Assessment Questionnaire-Disability Index score changes conditional on Psoriatic Arthritis Response Criteria response/non-response in the company submissions (Novartis and UCB Pharma), a previous multiple technology appraisal (Rodgers et al.) and the current Assessment Group
The previous MTA by Rodgers et al. 33 and the current AG assessment conducted a NMA for HAQ-DI score changes conditional on PsARC response/non-response outcome using Bayesian methods. Novartis did not conduct a meta-analysis for this outcome. UCB Pharma conducted meta-analysis for HAQ-DI score change in PsARC responders and non-responders with data extracted from Rodgers et al. 33 The HTA report assumed an additive effect for the effect of treatment in TRs versus that for PRs (UCB Pharma’s submission, p. 133). Although results of the analysis were presented in the economic section of UCB Pharma’s submission, detailed information about evidence synthesis was not provided. Hence, it is difficult to compare UCB Pharma’s submission with the AG evidence synthesis. The key assumptions for the NMA are presented in Table 150.
| Domains compared | Rodgers et al., 201133 | UCB Pharma | AGa |
|---|---|---|---|
| Key assumptions for model |
|
|
|
| Time points | HAQ-DI at 12 weeks conditional on PsARC response at 12 weeks | At 24 weeks | HAQ-DI at 12 weeks conditional on PsARC response at 12 weeks |
| Results reported | Changes in HAQ-DI given PsARC response/non-response to treatment | Changes in HAQ-DI given PsARC response/non-response to treatmentb | Changes in HAQ-DI given PsARC response/non-response to treatment |
| Data regarding subpopulation of biologic naive | |||
| Studies used in the analysis | ADEPT;55 Genovese et al.;56 IMPACT;51 IMPACT 2;52 and Mease et al.53,54 | ADEPT;55 FUTURE 2;48 GO-REVEAL;50 IMPACT 2;52 SPIRIT-P1;57,67 Mease et al.;54 and RAPID-PsA47 (24 weeks) | ADEPT;55 FUTURE 2;48 Genovese et al.;56 GO-REVEAL;50 IMPACT;51 IMPACT 2;52 Mease et al.;54 PALACE 1;60 PALACE 2;61 PALACE 3;65 PSUMMIT 1;58 PSUMMIT 2;59,66 and RAPID-PsA47 |
| Drugs evaluated | 40 mg of ADA; 5 mg/kg INF; and 25 mg of ETN | 40 mg of ADA; CZP; 25 mg of ETN; 50 mg of GOL; 5 mg/kg of INF; and SEC | 40 mg of ADA; 30 mg of APR; CZP; 25 mg of ETN; 50 mg of GOL; 5 mg/kg of INF; 150 and 300 mg of SEC; and 45 mg of UST |
| Data regarding subpopulation of biologic experienced | |||
| Studies used in the analysis | – | FUTURE 2;48 PSUMMIT 2;59,66 and RAPID-PsA47 (24 weeks) | FUTURE 2;48 and PSUMMIT 259,66 |
| Drugs evaluated | – | CZP; SEC; and 45 mg of UST | 300 mg of SEC; and 45 mg of UST |
As mentioned before, the NMA of UCB Pharma is difficult to compare with the AG’s NMA; therefore, only the NMA of Rodgers et al. 33 was compared with the AG’s NMA.
A key difference between the NMAs presented is the trials included in each analysis. The AG’s NMA includes nine active treatments and 13 trials, whereas the Rodgers et al. 33 analysis was limited to the treatments available at that time. Another key difference between Rodgers et al. 33 and AG’s analyses was the assumption of the effects on studies. Rodgers et al. 33 assumed random effect on studies, whereas the AG considered fixed effect on studies. Despite differences in model assumption, the results of the current assessment are fairly similar to those of Rodgers et al. 33 (Table 151).
| Treatment | Rodgers et al. (2011),33 mean (95% CrI) | AG (independent treatments), median (95% CrI) |
|---|---|---|
| HAQ-DI score changes conditional on PsARC response | ||
| Placebo | –0.244 (–0.337 to –0.151) | –0.263 (–0.301 to –0.224) |
| 150 mg of SEC | NC | –0.395 (–0.553 to –0.236) |
| 300 mg of SEC | NC | –0.547 (–0.722 to –0.369) |
| CZP | NC | –0.429 (–0.530 to –0.326) |
| UST | NC | –0.488 (–0.597 to –0.379) |
| GOL | NC | –0.439 (–0.585 to –0.293) |
| ADA | –0.477 (–0.596 to –0.351) | –0.489 (–0.583 to –0.395) |
| INF | –0.657 (–0.793 to –0.523) | –0.660 (–0.771 to –0.548) |
| ETN | –0.630 (–0.805 to –0.455) | –0.640 (–0.767 to –0.515) |
| APR | NC | –0.362 (–0.432 to –0.291) |
| HAQ-DI score changes conditional on PsARC non-response | ||
| 150 mg of SEC | NC | –0.083 (–0.389 to 0.220) |
| 300 mg of SEC | NC | –0.053 (–0.288 to 0.182) |
| CZP | NC | –0.069 (–0.194 to 0.057) |
| UST | NC | –0.097 (–0.208 to 0.012) |
| GOL | NC | –0.064 (–0.182 to 0.055) |
| ADA | –0.130 (–0.188 to 0.065) | –0.134 (–0.237 to –0.032) |
| INF | –0.194 (–0.333 to –0.057) | –0.196 (–0.311 to –0.083) |
| ETN | –0.190 (–0.381 to 0.000) | –0.200 (–0.348 to –0.054) |
| APR | NC | –0.089 (–0.157 to –0.022) |
WinBUG codes of preferred model
Psoriasis Area and Severity Index response
Detailed methods for the biologic-naive subpopulation
Treatment effect models
The NMA for PASI utilised a framework of analysis that evaluated the probability of PASI responses in different categories of PASI thresholds (50/75/90) within a single model: the single model included all categories of PASI and generated a single effect estimate for each treatment and also probabilities of achieving PASI 50, PASI 75 and PASI 90. Specifically, the model considered a multinomial likelihood and a probit link for ordered categorical data. 117
In brief, trials report rikj, the number of patients in arm k of trial i belonging to different, mutually exclusive categories j = 1, 2, 3, where these categories represent the different thresholds of PASI score (e.g. 50%, 75%, or 90% improvement). The responses for each arm k of trial i in category j follows a multinomial distribution as:
which has been parameterised as a series of conditional binomial distributions, with parameters of interest the probabilities, pikj, that a patient in arm k (k = 1, 2, 3) of trial i (i = 1, . . . ; see Table 153) belongs to category j (j = 1, 2, 3). We use the probit link function, the inverse of the normal cumulative distribution function Φ, to define the pikj as a function of a set of threshold values, zj. The threshold values (estimated within the model) are such that the probability that the standard normal (probit score) will take a value ≤ z1 will reflect the probability of obtaining a PASI response of < 50%, that is, 1 – PASI 50. The probability that the standard normal will take a value ≤ z2 will reflect the probability of obtaining a PASI response of < 75%, that is, 1 – PASI 75, and, analogously, evaluating Φ at z3 will approximate 1 – PASI 95. Placebo and treatments are assumed to shift the mean of the distribution. This means that the pooled effect of taking the experimental treatment instead of the control is to change the probit score (or z-score) of the control arm, by di,1 SDs. Therefore, the model is written as pikj=Φ(µi+zj+δi,1kI{k≠1}). The terms zj as the differences on the standard normal scale between the response to category j and the response to category j-1 in all the arms of trial i.
We assumed that the baselines, µi,were trial specific (unconstrained) and were given non-informative prior. A non-informative prior was assigned to the treatment effects parameter (δt). A uniform prior was assigned to the parameter zj.
Analogously to the analyses on PsARC, alternative assumptions were tested in two analyses. The first assumed independent treatment effects and did not include any metaregression for placebo effects (model F1). As the number of trials to inform each treatment effect was small, a fixed-effect model was used. In a second analysis, we explored the impact on treatment effects of adjusting for placebo responses [i.e. baseline effects (metaregression model)]. As can be seen from Chapter 4, Data, there are large differences between trials for PASI responses in placebo arms, ranging between 0% and 27% (0% in IMPACT51 and 27% in the RAPID-PsA47 trial). IMPACT51 had very small sample size and reported 0% response in placebo arm and 100% response in treatment arm, which leads to very extreme values for placebo adjustment. Therefore, IMPACT51 could not be included in the metaregression analysis. Unlike the analysis for PsARC, for PASI we did not assume a class effect as the evidence from individual trials does not support such an assumption. Table 152 presents the key assumptions for the models implemented for PASI response and detailed coding of the models is presented in Table 153.
| Sets of analyses | Between-studies assumption | Treatment | Metaregression | Thresholds (i.e. cut-off points) | Baseline effect for metaregression |
|---|---|---|---|---|---|
| F1 | FE | Independent | No baseline adjustment | FE | – |
| G1 | FE | Independent | No baseline adjustment | FE | – |
| G2 | FE | Independent | Common interaction term with baseline effect | FE | Adjusted with trial-specific baseline effects |
| Models F1 and G1 | Model G2 |
|---|---|
| Likelihoodrikj~Binomial(pikj,nikj)Modelqikj=1−(pikCi,j+1/pikCi,j)θikj=µi+δti,k−δti,1+zjpikCij=1−ADikjADikj=ϕ(θik,j−1)Priorsδt~dnorm(0,0.000001)µi~dnorm(0,0.000001)zj~dunif(0,5) | Likelihoodrikj~Binomial(pikjnikj)Modelqikj=1−(pikCi,j+1/pikCi,j)θikj=µi+δti,k−δti,1+zj+β(µi−µ¯)pikCij=1−ADikjADikj=ϕ(θik,j−1)Priorsδt~dnorm(0,0.01)µi~dnorm(0,0.01)β~dnorm(0,0.01)zj~dunif(0,5) |
Assumptions:
|
Assumptions:
|
Model F1 considers that treatments are independent of each other and fixed effect on cut-off points/thresholds. Model G1 considers the same assumption as model F1, but IMPACT was excluded from the analysis. Model G2 assumes treatments are independent of each other, but treatment effects are adjusted with the trial-specific baseline effects assuming a common interaction term (beta).
The preferred model was used to evaluate estimated probability of achieving PASI 50, PASI 75, PASI 90 responses on treatment t, using Tjt = 1 – Φ(A + δt + zj), where A is the pooled baseline effect described below.
We adopted the WinBUG code presented in the decision support unit technical support document 2117 for the analysis although we identified that the model was not specifying the z-score correctly in the linear predictor specification when the first category of the response data (in this case PASI 50) was missing. A correction was made to incorporate the correct specification for the z-score in the linear predictor specification.
Baseline effect
The baseline effect, A, was estimated as A=Σµi1NS, where µi1 is the baseline effects, where i is the studies and 1 = placebo; NS is the number of studies (in this case NS = 13).
Detailed results for the biologic-naive subpopulation
Summary results of Psoriasis Area and Severity Index response
Table 154 presents the results of the treatment effects for PASI responses estimated from the three models with measures of goodness of fit. There were no issues with convergence.
| Metaregression | No | No | Yes | |||
| Treatments | Ind | Ind | Ind | |||
| Cut-off points | FE | FE | FE | |||
| F1 | r a | G1 | r a | G2 | r a | |
|---|---|---|---|---|---|---|
| Placebo | 1.024 | – | 0.983 | – | 1.015 | – |
| 300 mg of SEC | –1.936 | 2 | –1.932 | 2 | –1.864 | 1 |
| 150 mg of SEC | –1.870 | 3 | –1.865 | 3 | –1.798 | 2 |
| CZP | –0.875 | 7 | –0.873 | 7 | –1.424 | 4 |
| UST | –1.134 | 6 | –1.131 | 6 | –1.342 | 6 |
| GOL | –1.645 | 4 | –1.635 | 4 | –1.141 | 7 |
| ADA | –1.477 | 5 | –1.476 | 5 | –1.422 | 5 |
| INF | –2.412 | 1 | –2.276 | 1 | –1.798 | 2 |
| ETN | –0.798 | 8 | –0.797 | 8 | –0.849 | 8 |
| APR | –0.749 | 9 | –0.748 | 9 | –0.815 | 9 |
| Beta | – | – | –1.310 | |||
| Residual deviance | 76.6b | 62.5c | 58.4c | |||
| DIC | 318.9 | 297.2 | 293.7 | |||
Detailed results of Psoriasis Area and Severity Index response
More detailed results of the models F1, G1 and G2 are presented in Tables 155–157.
| Treatment/parameter | Treatment effects | ||
|---|---|---|---|
| Mean | Median | 97% CrI | |
| Baseline effect | 1.025 | 1.024 | 0.903 to 1.149 |
| 300 mg of SEC | –1.941 | –1.936 | –2.628 to –1.280 |
| 150 mg of SEC | –1.877 | –1.870 | –2.540 to –1.238 |
| CZP | –0.877 | –0.875 | –1.239 to –0.523 |
| UST | –1.135 | –1.134 | –1.407 to –0.868 |
| GOL | –1.647 | –1.645 | –2.100 to –1.212 |
| ADA | –1.480 | –1.477 | –1.831 to –1.142 |
| INF | –2.414 | –2.412 | –2.841 to –2.006 |
| ETN | –0.801 | –0.798 | –1.639 to 0.025 |
| APR | –0.750 | –0.749 | –0.987 to –0.513 |
| z1, PASI 50 | – | – | – |
| z2, PASI 75 | 0.586 | 0.585 | 0.523 to 0.651 |
| z3, PASI 90 | 1.153 | 1.153 | 1.059 to 1.251 |
| Residual deviancea | 76.6 | ||
| DIC | 318.948 | ||
| Treatment/parameter | Treatment effects | ||
|---|---|---|---|
| Mean | Median | 97% CrI | |
| Baseline effect | 0.984 | 0.983 | 0.867 to 1.103 |
| 300 mg of SEC | –1.935 | –1.932 | –2.612 to –1.287 |
| 150 mg of SEC | –1.869 | –1.865 | –2.528 to –1.236 |
| CZP | –0.874 | –0.873 | –1.237 to –0.519 |
| UST | –1.131 | –1.131 | –1.402 to –0.863 |
| GOL | –1.641 | –1.635 | –2.097 to –1.212 |
| ADA | –1.478 | –1.476 | –1.834 to –1.136 |
| INF | –2.280 | –2.276 | –2.730 to –1.847 |
| ETN | –0.800 | –0.797 | –1.645 to 0.021 |
| APR | –0.748 | –0.748 | –0.983 to –0.510 |
| z1, PASI 50 | – | – | – |
| z2, PASI 75 | 0.578 | 0.577 | 0.516 to 0.642 |
| z3, PASI 90 | 1.136 | 1.136 | 1.043 to 1.235 |
| Residual deviancea | 62.54 | ||
| DIC | 297.153 | ||
| Treatment/parameter | Treatment effects | ||
|---|---|---|---|
| Mean | Median | 97% CrI | |
| Baseline effect | 1.016 | 1.015 | 0.888 to 1.153 |
| 300 mg of SEC | –1.860 | –1.864 | –2.330 to –1.363 |
| 150 mg of SEC | –1.793 | –1.798 | –2.231 to –1.316 |
| CZP | –1.433 | –1.424 | –1.888 to –1.040 |
| UST | –1.346 | –1.342 | –1.596 to –1.121 |
| GOL | –1.127 | –1.141 | –1.499 to –0.667 |
| ADA | –1.421 | –1.422 | –1.668 to –1.167 |
| INF | –1.788 | –1.798 | –2.173 to –1.313 |
| ETN | –0.846 | –0.849 | –1.478 to –0.198 |
| APR | –0.816 | –0.815 | –0.999 to –0.640 |
| Beta | –1.310 | –1.297 | –2.164 to –0.495 |
| z1, PASI 50 | – | – | – |
| z2, PASI 75 | 0.582 | 0.582 | 0.520 to 0.647 |
| z3, PASI 90 | 1.141 | 1.141 | 1.044 to 1.238 |
| Residual deviancea | 58.44 | ||
| DIC | 293.702 | ||
Preferred models
The results of models G1 and F1 are similar except for a small effect on the estimate of effect for INF; therefore, model F1 is the preferred unadjusted model as it does not exclude a trial. In model G2, DIC and residual deviance are lower than model G1, indicating that the model fits well with the existing data and the data support the assumption of adjustment with baseline effects. Therefore, we considered models F1 and G2 to be our preferred models.
Comparison of evidence synthesis of Psoriasis Area and Severity Index responses in the company submissions (Novartis and UCB Pharma), a previous multiple technology appraisal (Rodgers et al.) and the current Assessment Group
Both the Novartis and the UCB Pharma submissions combined PASI response evidence using Bayesian evidence synthesis methods. Each of the two CSs estimated probability of achieving PASI responses in three categories (50/75/90) to inform the economic model. A brief comparison of the methods used with key model assumptions, by the AG, CS and previous MTA are presented in Tables 158 and 159.
| Domains compared | Rodgers et al., 201133 | CS | AG | |
|---|---|---|---|---|
| Novartis | UCB Pharma | |||
| Model | Conditional multinomial probit model | Conditional multinomial probit model | Conditional multinomial probit model | Conditional multinomial probit model |
| Results reported | Probability of PASI response in three categories: 50, 75 and 90 | Probability of PASI response in three categories: 50, 75 and 90 | Probability of PASI response in three categories: 50, 75 and 90 | Probability of PASI response in three categories: 50, 75 and 90 |
| Time points | At 12 weeks (data from the 12-week or closest time point after 12 weeks – normally 14 or 16 weeks) | At 12 weeks (data from the 12-week or closest time point after 12 weeks – normally 14 or 16 weeks) | Primary analysis at 24 weeks (by treatments), sensitivity analysis was conducted at 12 weeks including data on 12 weeks or closest time point after 12 weeksa | At 12 weeks (data from the 12-week or closest time point after 12 weeks – normally 14 or 16 weeks) |
| Comments | Modelled probabilities are presented graphically | |||
| Data regarding subpopulation of biologic naive | ||||
| Studies used in the analysis | ADEPT;55 IMPACT;51 IMPACT 2;52 and Mease et al.53,54 | ADEPT;55 FUTURE 2;48 GO-REVEAL;50 and IMPACT 252 | ADEPT;55 GO-REVEAL;50 IMPACT;51 IMPACT 2;52 SPIRIT-P1;57,67 Mease et al.;53 and RAPID-PsA47 (12–16 weeks analysis) | ADEPT;55 FUTURE 2;48 GO-REVEAL;50 IMPACT;51 IMPACT 2;52 Mease et al.;53 PALACE 1;60 PALACE 2;61 PALACE 3;65 PSUMMIT 1;58 PSUMMIT 2;59,66 RAPID-PsA;47 and SPIRIT-P157,67 |
| Drugs evaluated | 40 mg of ADA; 5 mg/kg of INF; and 25 mg of ETN | 40 mg of ADA; 50 mg of GOL and 100 mg; 5 mg/kg of INF; and 150 and 300 mg of SEC | 40 mg of ADA; 400 mg of CZP; 25 mg of ETN; 50 mg of GOL; and 5 mg/kg of INF | 40 mg of ADA; 30 mg of APR; 400 mg of CZP; 25 mg of ETN; 50 mg of GOL; 5 mg/kg of INF; 150 and 300 mg of SEC; and 45 mg of UST |
| Data regarding subpopulation of biologic experienced | ||||
| Studies used in the analysis | – | – | FUTURE 2;48 PSUMMIT 2;59,66 and RAPID-PsA47 (24-week analysis) | FUTURE 2;48 and PSUMMIT 259,66 |
| Drugs evaluated | – | – | CZP; 300 mg of SEC; and 45 mg of UST | 300 mg of SEC; and 45 mg of UST |
| Domains compared | Rodgers et al., 201133 | CS | AG | |
|---|---|---|---|---|
| Novartis | UCB Pharma | |||
| Model | Conditional multinomial probit model | Conditional multinomial probit model | Conditional multinomial probit model | Conditional multinomial probit model |
| Fixed or random effects between studies | Fixed effects on studies | Random effects on studies for biologic-naive subpopulation analysis | Random effects on studies for biologic-naive subpopulation analysis and fixed effects for biologic-experienced subpopulation analysis | Fixed effects on studies (for both biologic-naive and biologic-experienced subpopulation) |
| Baselines | Common-effect model was used to estimate baseline | Common-effect model was used to estimate baseline | Common-effect model was used to estimate baseline | Common-effect model was used to estimate baseline |
| Treatment effects | Treatments were assumed to be independent of each other | Treatments were assumed to be independent of each other | Treatments were assumed to be independent of each other | Treatments were assumed to be independent of each other |
| Model adjusted for the placebo response | Unadjusted | Unadjusted | Unadjusted | Considered both unadjusted and adjusted model for biologic-naive subpopulation; considered unadjusted model for biologic-experienced subpopulation |
| Interaction term (beta) | – | – | – | Common interaction term for adjusted model |
| Probit/logit score thresholds | Thresholds were assumed to be fixed across trials | Thresholds were assumed to be fixed across trials | Thresholds were assumed to be fixed across trials | Thresholds were assumed to be fixed across trials |
As mentioned before, UCB Pharma and the AG included subpopulations in the main NMA and analysed both subpopulations (biologic naive and experienced) separately, whereas Novartis considered overall population as the main NMA, and the analysis included a more complete set of treatments and trials. This comparison refers to the Novartis’ NMA of subgroup (i.e. biologic naive).
A key difference between the NMAs presented concerns the trials included in each analysis. Only the AG’s NMA for the biologic-naive subgroup includes all comparators and all trials. The Rodgers et al. 33 analysis was limited to the treatments available at that time. The UCB Pharma analysis for the biologic-naive subgroup includes all treatments, but misses only some APR trials. The Novartis NMA for the biologic-naive subgroup does not include CZP or APR and does not include all trials for the other treatments. The AG considered to exclude the RAPID-PsA trial47 in the NMA for the biologic-experienced subgroup, whereas UCB Pharma included the trial data in the analysis. Novartis did not conduct a NMA for this outcome for the biologic-experienced subgroup.
Another key difference between the models was the assumption of effects on studies. The AG and Rodgers et al. 33 consider fixed effects on studies, whereas UCB Pharma and Novartis consider random effect on studies for the biologic-naive subgroup and fixed effect on studies for the biologic-experienced subgroup analysis. Another difference was the primary time point used. The AG, a previous MTA and Novartis conducted analyses at the 12-week time point, whereas UCB Pharma conducted primary analysis at 24 weeks and a sensitivity analysis considering the 12-week time point.
Table 160 shows the NMA results for (probabilities of) PASI response for the biologic-naive subpopulation estimated by the four NMAs. Across all the analyses, INF has the highest effectiveness following SEC among the treatment evaluated. The estimated probabilities in the AG’s analysis are more precise than either of the CSs.
| Treatment | Probability of PASI responses in the biologic-naive subpopulation at 12 weeks (12–16 weeks) | ||||
|---|---|---|---|---|---|
| Rodgers et al. (2011),33 mean (95% CrI) | CS | AG, median (95% CI) | |||
| Novartis, mean | UCB Pharma, mean (95% CI) | Unadjusted | Adjusted | ||
| Placebo | |||||
| PASI 50 | 0.131 (0.09 to 0.18) | Confidential information has been removed | Confidential information has been removed | 0.15 (0.13 to 0.18) | 0.16 (0.12 to 0.19) |
| PASI 75 | 0.045 (0.03 to 0.07) | Confidential information has been removed | Confidential information has been removed | 0.05 (0.04 to 0.07) | 0.06 (0.04 to 0.07) |
| PASI 90 | 0.017 (0.01 to 0.03) | Confidential information has been removed | Confidential information has been removed | 0.02 (0.01 to 0.02) | 0.02 (0.01 to 0.02) |
| 300 mg of SEC | |||||
| PASI 50 | NC | Confidential information has been removed | NC | 0.82 (0.61 to 0.94) | 0.80 (0.62 to 0.91) |
| PASI 75 | Confidential information has been removed | 0.63 (0.38 to 0.84) | 0.60 (0.40 to 0.78) | ||
| PASI 90 | Confidential information has been removed | 0.41 (0.19 to 0.67) | 0.38 (0.21 to 0.58) | ||
| 150 mg of SEC | |||||
| PASI 50 | NC | Confidential information has been removed | NC | 0.80 (0.59 to 0.93) | 0.78 (0.60 to 0.90) |
| PASI 75 | Confidential information has been removed | 0.60 (0.36 to 0.82) | 0.58 (0.38 to 0.75) | ||
| PASI 90 | Confidential information has been removed | 0.38 (0.18 to 0.63) | 0.36 (0.19 to 0.54) | ||
| CZP | |||||
| PASI 50 | NC | NC | Confidential information has been removed | 0.44 (0.31 to 0.59) | 0.66 (0.50 to 0.82) |
| PASI 75 | Confidential information has been removed | 0.23 (0.14 to 0.36) | 0.43 (0.29 to 0.63) | ||
| PASI 90 | Confidential information has been removed | 0.10 (0.05 to 0.18) | 0.23 (0.13 to 0.41) | ||
| UST | |||||
| PASI 50 | NC | NC | NC | 0.54 (0.44 to 0.65) | 0.63 (0.52 to 0.74) |
| PASI 75 | 0.32 (0.23 to 0.42) | 0.40 (0.30 to 0.52) | |||
| PASI 90 | 0.15 (0.09 to 0.22) | 0.21 (0.14 to 0.31) | |||
| 50 mg of GOL | |||||
| PASI 50 | NC | Confidential information has been removed | Confidential information has been removed | 0.73 (0.58 to 0.86) | 0.55 (0.36 to 0.70) |
| PASI 75 | Confidential information has been removed | Confidential information has been removed | 0.51 (0.35 to 0.68) | 0.32 (0.17 to 0.48) | |
| PASI 90 | Confidential information has been removed | Confidential information has been removed | 0.30 (0.17 to 0.47) | 0.15 (0.07 to 0.27) | |
| ADA | |||||
| PASI 50 | 0.738 (0.55 to 0.88) | Confidential information has been removed | Confidential information has been removed | 0.68 (0.55 to 0.78) | 0.66 (0.54 to 0.76) |
| PASI 75 | 0.477 (0.28 to 0.69) | Confidential information has been removed | Confidential information has been removed | 0.45 (0.32 to 0.58) | 0.43 (0.32 to 0.55) |
| PASI 90 | 0.257 (0.12 to 0.45) | Confidential information has been removed | Confidential information has been removed | 0.24 (0.15 to 0.36) | 0.23 (0.15 to 0.33) |
| INF | |||||
| PASI 50 | 0.913 (0.82 to 0.97) | Confidential information has been removed | Confidential information has been removed | 0.92 (0.84 to 0.96) | 0.78 (0.61 to 0.88) |
| PASI 75 | 0.769 (0.59 to 0.90) | Confidential information has been removed | Confidential information has been removed | 0.79 (0.67 to 0.88) | 0.58 (0.39 to 0.73) |
| PASI 90 | 0.557 (0.35 to 0.77) | Confidential information has been removed | Confidential information has been removed | 0.59 (0.44 to 0.73) | 0.36 (0.20 to 0.52) |
| ETN | |||||
| PASI 50 | 0.403 (0.24 to 0.59) | NC | Confidential information has been removed | 0.41 (0.15 to 0.72) | 0.43 (0.20 to 0.69) |
| PASI 75 | 0.177 (0.09 to 0.31) | Confidential information has been removed | 0.21 (0.05 to 0.50) | 0.23 (0.08 to 0.47) | |
| PASI 90 | 0.074 (0.03 to 0.15) | Confidential information has been removed | 0.08 (0.01 to 0.29) | 0.10 (0.02 to 0.26) | |
| APR | |||||
| PASI 50 | NC | NC | NC | 0.39 (0.31 to 0.49) | 0.42 (0.33 to 0.52) |
| PASI 75 | 0.20 (0.14 to 0.27) | 0.22 (0.16 to 0.30) | |||
| PASI 90 | 0.08 (0.05 to 0.12) | 0.09 (0.06 to 0.14) | |||
Given the differences in model assumptions and the included studies, the results are slightly different for GOL, ADA and ETN. Between the previous and current assessment, differences in the ADA estimates are the result of additional data on ADA from the SPIRIT-P1. 57,67 In the Novartis submission, the estimated probabilities are much lower for 50 mg of GOL. The differences are plausible as the AG and Novartis used different sets of data and model assumptions. In the UCB Pharma submission, the estimated probabilities for ETN are much lower than obtained in previous and current assessments. The difference is largely because UCB Pharma used different PASI 50 response data in the analysis.
Rodgers et al. 33 and Novartis did not include an analysis for the treatment-experienced subgroup. Table 161 presents the PASI results from the AG and UCB Pharma NMAs for the biologic-experienced subpopulation. However, the results are not comparable between the AG and UCB Pharma analyses as probabilities were estimated at two different time points (12 weeks and 24 weeks), and it is evident that the PASI response differs between these two time points.
| Treatment | Probability of PASI responses in the biologic-experienced subpopulation, mean (95% CrI) | |
|---|---|---|
| UCB Pharma, at 24 weeks | AG, at 12 weeks (12–16 weeks) | |
| Placebo | ||
| PASI 50 | Confidential information has been removed | 0.088 (0.01 to 0.28) |
| PASI 75 | Confidential information has been removed | 0.012 (0.00 to 0.06) |
| PASI 90 | Confidential information has been removed | 0.002 (0.00 to 0.02) |
| 300 mg of SEC | ||
| PASI 50 | Confidential information has been removed | 0.875 (0.46 to 1.00) |
| PASI 75 | Confidential information has been removed | 0.598 (0.23 to 0.89) |
| PASI 90 | Confidential information has been removed | 0.365 (0.08 to 0.75) |
| UST | ||
| PASI 50 | Confidential information has been removed | 0.628 (0.29 to 0.89) |
| PASI 75 | Confidential information has been removed | 0.279 (0.07 to 0.61) |
| PASI 90 | Confidential information has been removed | 0.120 (0.01 to 0.42) |
| CZP | ||
| PASI 50 | Confidential information has been removed | NC |
| PASI 75 | Confidential information has been removed | NC |
| PASI 90 | Confidential information has been removed | NC |
WinBUG codes of preferred model
American College of Rheumatology response
Detailed methods for the biologic-naive subpopulation
The NMA for ACR utilised a similar framework of analysis to that used to estimate probability of PASI responses. In brief, the model considered a multinomial likelihood and a probit link for ordered categorical data. 117
Analogously to the analyses on PsARC, sets of alternative assumptions were tested. We explored the effect of differences in trial-specific placebo responses on treatment effect undertaking a metaregression. In the context of an adjusted model for placebo response, we explored the possibility of there being class effects. Three different class groupings were considered: all treatments as a single class; all biologics as a class with APR separate; and, to reflect the pharmacology, anti-TNFs grouped, ILs grouped and APR separate. Additionally, we explored two within-class assumptions: assuming treatments within a class to have equal effectiveness and, alternatively, assuming that those treatments within a class have similar (exchangeable) effectiveness. Fixed effects across studies were assumed for all models. We have not considered models assuming exchangeability between classes.
Summary of all treatment effect models explored
All models implemented for evidence synthesis of ACR response are presented in Table 162. Detailed coding of the models is presented in Table 163.
| Sets of analysis | Between studies assumption | Treatment | Metaregression | Class |
|---|---|---|---|---|
| H1 | FE | Independent | No baseline adjustment | No class effect |
| I1 | FE | Independent | Common interaction term with baseline effect | No class effect |
| J1 | FE | Equal | class | Common interaction term with baseline effect | Independent class effect: class = {all treatments} |
| J2 | FE | Equal | class, remaining treatments independenta | Independent class effect: class = APR independent {all remaining biologics} | |
| J3 | FE | Equal | class, remaining treatments independenta | Independent class effect: class = {anti-TNFs, ILs}; APR independent | |
| K1 | FE | Exchangeable | class, remaining treatments independenta | Common interaction term with baseline effect | Independent class effect: class = APR independent {all other biologics} |
| K2 | FE | Exchangeable | class, remaining treatments independenta | Independent class effect: class = {anti-TNFs, ILs}; APR independent |
| Model H1 | Model I1 |
|---|---|
| Likelihoodrikj~Binomial(pikj,nikj)Modelqikj=1−(pikCi,j+1/pikCi,j)θikj=µi+δti,k−δti,1+zjpikCij=1−ADikjADikj=ϕ(θik,j−1)Priors(anti−TNF−naive−analysis)δt~dnorm(0,0.000001)µi~dnorm(0,0.000001)zj~dunif(0,5)Priors(anti−TNF−experiensed−analysis)δt~dnorm(0,0.01)µi~dnorm(0,0.01)zj~dunif(0,5) | Likelihoodrikj~Binomial(pikjnikj)Modelqikj=1−(pikCi,j+1/pikCi,j)θikj=µi+δti,k−δti,1+zj+β(µi−µ¯)pikCij=1−ADikjADikj=ϕ(θik,j−1)Priorsδt~dnorm(0,0.01)µi~dnorm(0,0.01)β~dnorm(0,0.01)zj~dunif(0,5) |
Assumptions:
|
Assumptions:
|
| Models J1, J2 and J3 | Models K1 and K2 |
| Likelihoodrikj~Binomial(pikjnikj)Modelqikj=1−(pikCi,j+1/pikCi,j)θikj=µi+δti,k−δti,1+zj+β(µi−µ¯)pikCij=1−ADikjADikj=ϕ(θik,j−1)δt~δcPriorsδc~dnorm(0,0.01)µi~dnorm(0,0.01)β~dnorm(0,0.01)zj~dunif(0,5) | Likelihoodrikj~Binomial(pikjnikj)Modelqikj=1−(pikCi,j+1/pikCi,j)θikj=µi+δti,k−δti,1+zj+β(µi−µ¯)pikCij=1−ADikjADikj=ϕ(θik,j−1)δt~dnorm(Classc,1/γ2)PriorsClassc~dnorm(0,0.01)µi~dnorm(0,0.01)β~dnorm(0,0.01)zj~dunif(0,5)γ~dunif(0,10) |
| J1: class = {all biologics} | K1: APR independent; class = {all other biologics} |
| J2: APR independent; class = {all other biologics} | K2: class = {anti-TNFs, ILs}; APR independent |
| J3: class = {anti-TNFs, ILs}; APR independent | |
Assumptions:
|
Assumptions:
|
Model H1 considers that the effectiveness of each treatment is independent of the effectiveness of other treatments. Model I1 considers the relative effectiveness of the alternative treatments to be independent of the effectiveness of other treatments, but that the effectiveness of all treatments depends on the response in the placebo arm. Model J1 considers the treatments to be equal in terms of their effectiveness, but dependent on the effect of the placebo arm. Models J2 and J3 consider the treatments to be equal in terms of their effectiveness within class, but dependent on the effect of the placebo arm. Models K1 and K2 assume the treatments to have a similar, but not equal, effectiveness and to be dependent on the effect of the placebo arm; this model introduces more flexibility than those that assume treatment effects to be equal (models J2 and J3), but does not fully assume treatments to differ as in model H1. It does imply that there are differences between the effectiveness of treatments that we may not be able to explain but that we should consider. These may be a result of differences between the treatments themselves or because of differences in the design of the trials used to evaluate each treatment.
Detailed results for the biologic-naive subpopulation
Summary results of American College of Rheumatology response
Table 164 presents the results of the treatment effects for ACR responses estimated from the seven models with measures of goodness of fit. There were no issues with convergence.
| Metaregression | No | Yes | Yes | Yes | Yes | Yes | Yes | |||||||
| Treatments | Ind | Ind | = | class {all} | = | class (APR, other) | = | class (ILs, TNFs, APR) | ∼ | classa (APR, other) | ∼ | classa (ILs, TNFs, APR) | |||||||
| Cut-off points | FE | FE | FE | FE | FE | FE | FE | |||||||
| H1 | r b | I1 | r b | J1 | r b | J2 | r b | J3 | r b | K1 | r b | K2 | r b | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | 0.952 | 0.961 | 0.882 | 0.966 | 0.966 | 0.963 | 0.961 | |||||||
| 300 mg of SEC | –0.914 | 6 | –1.397 | 2 | –1.274 | 2 | –1.236 | 3 | ||||||
| 150 mg of SEC | –0.932 | 5 | –1.415 | 1 | –1.094 | 1 | –1.095 | 1 | –1.283 | 1 | –1.246 | 2 | ||
| UST | –0.570 | 8 | –0.722 | 8 | –0.750 | 8 | –0.732 | 8 | ||||||
| CZP | –0.811 | 7 | –1.265 | 3 | –0.830 | 1 | –1.193 | 5 | –1.176 | 5 | ||||
| GOL | –1.429 | 2 | –0.918 | 7 | –1.010 | 7 | –1.040 | 7 | ||||||
| ADA | –1.072 | 4 | –1.126 | 6 | –0.609 | 2 | –1.121 | 6 | –1.124 | 6 | ||||
| INF | –1.617 | 1 | –1.212 | 5 | –1.246 | 3 | –1.269 | 1 | ||||||
| ETN | –1.362 | 3 | –1.214 | 4 | –1.215 | 4 | –1.228 | 4 | ||||||
| APR | –0.509 | 9 | –0.592 | 9 | –0.610 | 2 | –0.014 | 3 | –0.581 | 9 | –0.576 | 9 | ||
| Beta (mean) | –1.276 | 1.327 | –1.627 | –1.621 | –1.099 | –1.018 | ||||||||
| Residual deviancec | 120.0 | 119.1 | 156.1 | 148.3 | 148.3 | 120.0 | 120.4 | |||||||
| DIC | 482.22 | 480.94 | 511.66 | 503.43 | 503.37 | 480.90 | 481.10 | |||||||
Detailed results of American College of Rheumatology response
More detailed results of the models H1, I1, J1, J2, J3, K1 and K2 are presented in Tables 165–171.
| Treatment/parameter | Treatment effects | ||
|---|---|---|---|
| Mean | Median | 97% CrI | |
| Baseline effect | 0.952 | 0.952 | 0.874 to 1.031 |
| 300 mg of SEC | –0.915 | –0.914 | –1.319 to –0.512 |
| 150 mg of SEC | –0.932 | –0.932 | –1.347 to –0.525 |
| UST | –0.570 | –0.570 | –0.797 to –0.349 |
| CZP | –0.811 | –0.811 | –1.090 to –0.530 |
| GOL | –1.431 | –1.429 | –1.810 to –1.068 |
| ADA | –1.072 | –1.072 | –1.274 to –0.870 |
| INF | –1.619 | –1.617 | –1.943 to –1.306 |
| ETN | –1.364 | –1.362 | –1.688 to –1.050 |
| APR | –0.509 | –0.509 | –0.672 to –0.346 |
| z1, ACR 20 | – | – | – |
| z2, ACR 50 | 0.661 | 0.661 | 0.615 to 0.709 |
| z3, ACR 70 | 1.284 | 1.283 | 1.213 to 1.356 |
| Residual deviancea | 120.00 | ||
| DIC | 482.22 | ||
| Treatment/parameter | Treatment effects | ||
|---|---|---|---|
| Mean | Median | 97% CrI | |
| Baseline effect | 0.962 | 0.961 | 0.880 to 1.046 |
| 300 mg of SEC | –1.402 | –1.397 | –1.890 to –0.939 |
| 150 mg of SEC | –1.421 | –1.415 | –1.920 to –0.953 |
| UST | –0.725 | –0.722 | –0.939 to –0.526 |
| CZP | –1.268 | –1.265 | –1.666 to –0.874 |
| GOL | –0.910 | –0.918 | –1.362 to –0.433 |
| ADA | –1.127 | –1.126 | –1.290 to –0.973 |
| INF | –1.207 | –1.212 | –1.578 to –0.812 |
| ETN | –1.209 | –1.214 | –1.455 to –0.931 |
| APR | –0.594 | –0.592 | –0.738 to –0.459 |
| Beta | –1.276 | –1.297 | –2.164 to –0.274 |
| z1, ACR 20 | – | – | – |
| z2, ACR 50 | 0.661 | 0.661 | 0.615 to 0.709 |
| z3, ACR 70 | 1.283 | 1.282 | 1.212 to 1.356 |
| Residual deviancea | 119.10 | ||
| DIC | 480.94 | ||
| Treatment/parameter | Treatment effects | ||
|---|---|---|---|
| Mean | Median | 97% CrI | |
| Baseline effect | 0.882 | 0.882 | 0.812 to 0.953 |
| All biologics as a class | –0.825 | –0.830 | –0.992 to –0.624 |
| Beta | 1.327 | 1.236 | 0.399 to 2.792 |
| z1, ACR 20 | – | – | – |
| z2, ACR 50 | 0.656 | 0.655 | 0.610 to 0.702 |
| z3, ACR 70 | 1.272 | 1.272 | 1.201 to 1.345 |
| Residual deviancea | 156.1 | ||
| DIC | 511.66 | ||
| Treatment/parameter | Treatment effects | ||
|---|---|---|---|
| Mean | Median | 97% CrI | |
| Baseline effect | 0.967 | 0.966 | 0.886 to 1.051 |
| All biologics (except APR) | –1.095 | –1.094 | –1.190 to –1.005 |
| APR | –0.614 | –0.610 | –0.773 to –0.474 |
| Beta | –1.627 | –1.627 | –2.365 to –0.926 |
| z1, ACR 20 | – | – | – |
| z2, ACR 50 | 0.657 | 0.656 | 0.611 to 0.704 |
| z3, ACR 70 | 1.272 | 1.271 | 1.201 to 1.345 |
| Residual deviancea | 148.30 | ||
| DIC | 503.43 | ||
| Treatment/parameter | Treatment effects | ||
|---|---|---|---|
| Mean | Median | 97% CrI | |
| Baseline effect | 0.967 | 0.966 | 0.886 to 1.049 |
| ILs as class | –1.095 | –1.095 | –1.189 to –1.005 |
| Anti-TNFs as class | –0.612 | –0.609 | –0.767 to –0.474 |
| APR | 0.021 | –0.014 | –19.450 to 19.720 |
| Beta | –1.621 | –1.619 | –2.349 to –0.918 |
| z1, ACR 20 | |||
| z2, ACR 50 | 0.657 | 0.656 | 0.611 to 0.704 |
| z3, ACR 70 | 1.272 | 1.271 | 1.201 to 1.344 |
| Residual deviancea | 148.30 | ||
| DIC | |||
| Treatment/parameter | Predicted mean distribution | Shrunken or independent estimates | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 97% CrI | Mean | Median | 97% CrI | |
| Baseline effect | – | – | – | 0.963 | 0.963 | 0.880 to 1.049 |
| 300 mg of SEC | –1.137 | –1.135 | –1.750 to –0.534 | –1.278 | –1.274 | –1.582 to –0.994 |
| 150 mg of SEC | –1.287 | –1.283 | –1.597 to –0.998 | |||
| UST | –0.750 | –0.750 | –0.919 to –0.582 | |||
| CZP | –1.195 | –1.193 | –1.437 to –0.961 | |||
| GOL | –1.007 | –1.010 | –1.264 to –0.733 | |||
| ADA | –1.122 | –1.121 | –1.257 to –0.990 | |||
| INF | –1.244 | –1.246 | –1.479 to –1.005 | |||
| ETN | –1.214 | –1.215 | –1.410 to –1.013 | |||
| APR | – | – | – | –0.581 | –0.581 | –0.700 to –0.465 |
| Beta | – | – | – | –1.099 | –1.103 | –1.646 to –0.534 |
| γa | – | – | – | 0.264 | 0.240 | 0.123 to 0.547 |
| z1, ACR 20 | – | – | – | – | – | – |
| z2, ACR 50 | – | – | – | 0.660 | 0.660 | 0.614 to 0.709 |
| z3, ACR 70 | – | – | – | 1.280 | 1.280 | 1.209 to 1.354 |
| Residual devianceb | 120.00 | |||||
| DIC | 480.90 | |||||
| Treatment/parameter | Predicted mean distribution | Shrunken or independent estimates | ||||
|---|---|---|---|---|---|---|
| Mean | Median | 97% CrI | Mean | Median | 97% CrI | |
| Baseline effect | – | – | – | 0.961 | 0.961 | 0.878 to 1.046 |
| 300 mg of SEC | –1.069 | –1.054 | –1.869 to –0.345 | –1.234 | –1.236 | –1.609 to –0.845 |
| 150 mg of SEC | –1.243 | –1.246 | –1.628 to –0.854 | |||
| UST | –0.733 | –0.732 | –0.913 to –0.552 | |||
| CZP | –1.167 | –1.170 | –1.862 to –0.464 | –1.178 | –1.176 | –1.443 to –0.924 |
| GOL | –1.038 | –1.040 | –1.350 to –0.718 | |||
| ADA | –1.123 | –1.124 | –1.259 to –0.988 | |||
| INF | –1.268 | –1.269 | –1.530 to –1.003 | |||
| ETN | –1.228 | –1.228 | –1.432 to –1.021 | |||
| APR | – | – | – | –0.576 | –0.576 | –0.700 to –0.453 |
| Beta | –1.018 | –1.028 | –1.671 to –0.334 | |||
| γa | 0.280 | 0.248 | 0.107 to 0.643 | |||
| z1, ACR 20 | – | – | – | – | – | – |
| z2, ACR 50 | 0.661 | 0.660 | 0.615 to 0.708 | |||
| z3, ACR 70 | 1.281 | 1.281 | 1.210 to 1.354 | |||
| Residual devianceb | 120.40 | |||||
| DIC | ||||||
Preferred models
The unadjusted model H1 fits the data as well as any of the other models and generates results that reflect the observed results. Considering the placebo-adjusted models, model I1 generated results (rankings) which do not reflect well the observed trial results; and it must be borne in mind that, without any clear rationale for the placebo effect, the results must be interpreted with caution. Using an assumption of equal class effect for the treatments does not produce a better-fitting model (models J1, J2 and J3) than assuming independent treatment effects (models H1 and I1) or similar (exchangeable) treatment effects (models K1 and K2). In addition, there was little difference in goodness-of-fit statistics (DIC and residual deviance) between models K1 and K2, and we consider the exchangeable class effect model, which utilised two classes (ILs and anti-TNFs) with APR separate, to be the most clinically plausible. Hence, our preferred models are models H1 and K2.
Comparison of evidence synthesis of American College of Rheumatology responses in the company submissions, a previous multiple technology appraisal and the Assessment Group
Both the Novartis and the UCB Pharma submissions combined ACR outcome evidence using Bayesian evidence synthesis methods. Both submissions estimated probability of achieving ACR responses in three categories (20/50/70) and conducted binary analysis of the ACR categories separately to inform clinical effectiveness. However, the AG and a previous MTA estimated probability of achieving ACR responses in three categories (20/50/70) to inform clinical effectiveness. Therefore, the comparison between the CSs and AG is limited to the estimation of probability of achieving ACR responses in three categories (20/50/70). A brief comparison of the methods used with key model assumptions, by the AG, CSs and a previous MTA, is presented in Tables 172 and 173.
| Domains compared | Rodgers et al., 201133 | CS | AG | |
|---|---|---|---|---|
| Novartis | UCB Pharma | |||
| Model | Conditional multinomial probit model | Conditional multinomial probit model | Conditional multinomial probit model | Conditional multinomial probit model |
| Results reported | Probability of ACR response in three categories 20/50/70 | Probability of ACR response in three categories 20/50/70 | Probability of ACR response in three categories 20/50/70 for biologic-experienced subpopulation, but did not present probabilities for the biologic-naive subpopulation | Probability of ACR response in three categories 20/50/70 |
| Time points | At 12 weeks (data from the 12-week or closest time point after 12 weeks – normally 14 or 16 weeks) | At 12 weeks (data from the 12-week or closest time point after 12 weeks – normally 14 or 16 weeks) | Primary analysis at 24 weeks (by treatments), sensitivity analysis was conducted at 12 weeks including data on 12 weeks or closest time point after 12 weeksa | At 12 weeks (data from the 12-week or closest time point after 12 weeks – normally 14 or 16 weeks) |
| Comments | Modelled probabilities are presented graphically | |||
| Data regarding biologic-naive subpopulation | ||||
| Studies used in the analysis | ADEPT;55 Genovese et al.;56 IMPACT;51 IMPACT 2;52 and Mease et al.53,54 | ADEPT;55 FUTURE 2;48 Genovese et al.;56 GO-REVEAL;50 IMPACT 2;52 Mease et al.;54 PALACE 1;60 PSUMMIT 1;58 PSUMMIT 2;59,66 and RAPID-PsA47 | ADEPT;55 Genovese et al.;56 GO-REVEAL;50 IMPACT;51 IMPACT 2;52 SPIRIT-P1;57,67 PALACE 1;60 PALACE 3;65 PSUMMIT 1;58 Mease et al.;53,54 and RAPID-PsA47 (12–16 weeks analysis) | ADEPT;55 FUTURE 2;48 Genovese et al.;56 GO-REVEAL;50 IMPACT 2;52 Mease et al.;54 PALACE 1;60 PALACE 2;61 PALACE 3;65 PSUMMIT 1;58 PSUMMIT 2;59,66 RAPID-PsA;47 and SPIRIT-P157,67 |
| Drugs evaluated | 40 mg of ADA; 5 mg/kg of INF; and 25 mg of ETN | 40 mg of ADA; 20 and 30 mg of APR; 200 and 400 mg of CZP; 25 mg of ETN; 50 and 100 mg of GOL; 5 mg/kg of INF; 150 and 300 mg of SEC; 45 and 90 mg of UST | 40 mg of ADA; 20 mg of APR and 30 mg; 400 mg of CZP; 25 mg of ETN; 50 mg of GOL; and 5 mg/kg of INF | 40 mg of ADA; 30 mg of APR; 400 mg of CZP; 25 mg of ETN; 50 mg of GOL; 5 mg/kg of INF; 150 and 300 mg of SEC; and 45 mg of UST |
| Data regarding biologic-experienced subpopulation | ||||
| Studies used in the analysis | NC | FUTURE 2;48 PALACE 1;60 PSUMMIT 2;59,66 and RAPID-PsA47 | bFUTURE 1;46 FUTURE 2;48 PSUMMIT 2;59,66 and RAPID-PsA47 (24 weeks’ analysis) | FUTURE 2;48 and PSUMMIT 259, |
| Drugs evaluated | NC | 20 and 30 mg of APR; 200 and 400 mg of CZP; 150 and 300 mg of SEC; and, 45 and 90 mg of UST | CZP; 300 mg of SEC; and 45 mg of UST | 300 mg of SEC; and 45 mg of UST |
| Domains compared | Rodgers et al., 201133 | CS | AG | |
|---|---|---|---|---|
| Novartis | UCB Pharma | |||
| Model | Conditional multinomial probit model | Conditional multinomial probit model | Conditional multinomial probit model | Conditional multinomial probit model |
| Fixed or random effects between studies | Fixed effects on studies | Random effect on studies for biologic-naive subgroup analysis; and fixed effects on studies for biologic-experienced subgroup analysis | Random effect on studies for biologic-naive subpopulation analysis and fixed effect for biologic-experienced subpopulation analysis | Fixed effects on studies (for both subpopulation analyses) |
| Baselines | Common-effect model was used to estimate baseline | Common-effect model was used to estimate baseline | Common-effect model was used to estimate baseline | Common-effect model was used to estimate baseline |
| Treatment effects | Treatments were assumed to be independent of each other | Treatments were assumed to be independent of each other | Treatments were assumed to be independent of each other | For the biologic-naive subpopulation:
|
| Model adjusted for the placebo response | Unadjusted | Unadjusted | Unadjusted | Independent treatment effects model was unadjusted, but analysis assuming exchangeable class effects model was adjusted for the placebo response |
| Interaction term (beta) | – | – | – | Common interaction term for adjusted model |
| Probit/logit score thresholds | Thresholds were assumed to be fixed across trials | Thresholds were assumed to be fixed across trials | Thresholds were assumed to be fixed across trials | Thresholds were assumed to be fixed across trials |
Like other outcomes, a key difference between the ACR NMAs presented concerned the trials included in each analysis. The AG’s NMA for the biologic-naive subgroup includes all comparators and all trials. Rodgers et al. ’s33 analysis was limited to the treatments available at that time. The UCB Pharma analysis for the biologic-naive subgroup includes all treatments, but misses one APR trial. The Novartis NMA for the biologic-naive subgroup included a more complete set of treatments and trials for this outcome. Both submissions included the RAPID-PsA trial47 in the biologic-experienced subgroup analysis, whereas the AG excluded the RAPID-PsA trial47 from the analysis. It should be noted that this comparison refers to Novartis’ NMAs of subgroups. As mentioned before, the Novartis submission presented a NMA for all patients (treatment naive and experienced combined).
A key difference between models was the assumption of effects on studies. The AG and Rodgers et al. 33 consider fixed effects on studies, whereas UCB Pharma and Novartis consider random effect on studies for the biologic-naive subgroup and fixed effect on studies for the biologic-experienced subgroup analysis. Like other outcomes, another key difference relates to the primary time point used. The AG, the previous MTA and Novartis conducted analyses at the 12-week time point, whereas UCB Pharma conducted primary analysis at 24 weeks and sensitivity analysis considering a 12-week time point.
Table 174 shows the three NMA results for (probabilities of) ACR response for the biologic-naive subpopulation. In comparison with the Novartis analysis and the AG unadjusted analysis, the estimated probabilities in the three categories are lower for INF, but higher for ADA. The differences are largely because Novartis included a different data set. UCB Pharma chose binary analysis of ACR 20 and ACR 50 over probability of achieving ACR responses in three categories (20/50/70) to be the preferred analysis, and did not present the results of probability of ACR responses for the biologic-naive subgroup. Therefore, it was not plausible to compare the AG’s results for the biologic-naive population with UCB Pharma results.
| Treatment | Probability of ACR responses in the biologic-naive subpopulation at 12 weeks (12–16 weeks) | |||
|---|---|---|---|---|
| Rodgers et al. (2011),33 mean (95% CrI) | Novartis, mean | AG, median (95% CrI) | ||
| Unadjusted | Adjusted | |||
| Placebo | ||||
| ACR 20 | 0.14 (0.11 to 0.17) | Confidential information has been removed | 0.17 (0.15 to 0.19) | 0.17 (0.15 to 0.19) |
| ACR 50 | 0.05 (0.04 to 0.07) | Confidential information has been removed | 0.05 (0.04 to 0.06) | 0.05 (0.04 to 0.06) |
| ACR 70 | 0.01 (0.01 to 0.03) | Confidential information has been removed | 0.01 (0.01 to 0.02) | 0.01 (0.01 to 0.02) |
| 300 mg of SEC | ||||
| ACR 20 | NC | Confidential information has been removed | 0.49 (0.33 to 0.64) | 0.61 (0.46 to 0.75) |
| ACR 50 | Confidential information has been removed | 0.24 (0.14 to 0.38) | 0.35 (0.22 to 0.50) | |
| ACR 70 | Confidential information has been removed | 0.09 (0.04 to 0.18) | 0.16 (0.08 to 0.27) | |
| 150 mg of SEC | ||||
| ACR 20 | NC | Confidential information has been removed | 0.49 (0.34 to 0.65) | 0.61 (0.46 to 0.75) |
| ACR 50 | Confidential information has been removed | 0.25 (0.14 to 0.39) | 0.35 (0.22 to 0.51) | |
| ACR 70 | Confidential information has been removed | 0.10 (0.04 to 0.19) | 0.16 (0.08 to 0.27) | |
| 45 mg of UST | ||||
| ACR 20 | NC | Confidential information has been removed | 0.35 (0.27 to 0.44) | 0.41 (0.34 to 0.49) |
| ACR 50 | Confidential information has been removed | 0.15 (0.10 to 0.21) | 0.19 (0.14 to 0.25) | |
| ACR 70 | Confidential information has been removed | 0.05 (0.03 to 0.08) | 0.07 (0.04 to 0.10) | |
| CZP | ||||
| ACR 20 | NC | Confidential information has been removed | 0.44 (0.34 to 0.55) | 0.58 (0.49 to 0.69) |
| ACR 50 | Confidential information has been removed | 0.21 (0.14 to 0.30) | 0.33 (0.24 to 0.43) | |
| ACR 70 | Confidential information has been removed | 0.08 (0.04 to 0.13) | 0.14 (0.09 to 0.22) | |
| 50 mg of GOL | ||||
| ACR 20 | NC | Confidential information has been removed | 0.68 (0.55 to 0.80) | 0.53 (0.40 to 0.66) |
| ACR 50 | Confidential information has been removed | 0.43 (0.30 to 0.57) | 0.28 (0.18 to 0.40) | |
| ACR 70 | Confidential information has been removed | 0.21 (0.12 to 0.33) | 0.11 (0.06 to 0.19) | |
| ADA | ||||
| ACR 20 | 0.56 (0.43 to 0.69) | Confidential information has been removed | 0.55 (0.47 to 0.62) | 0.56 (0.50 to 0.63) |
| ACR 50 | 0.31 (0.21 to 0.44) | Confidential information has been removed | 0.29 (0.23 to 0.36) | 0.31 (0.26 to 0.37) |
| ACR 70 | 0.13 (0.08 to 0.21) | Confidential information has been removed | 0.12 (0.09 to 0.17) | 0.13 (0.10 to 0.17) |
| INF | ||||
| ACR 20 | 0.68 (0.53 to 0.81) | Confidential information has been removed | 0.75 (0.65 to 0.83) | 0.62 (0.51 to 0.72) |
| ACR 50 | 0.43 (0.29 to 0.59) | Confidential information has been removed | 0.50 (0.39 to 0.62) | 0.36 (0.26 to 0.47) |
| ACR 70 | 0.20 (0.11 to 0.33) | Confidential information has been removed | 0.27 (0.18 to 0.38) | 0.17 (0.10 to 0.24) |
| ETN | ||||
| ACR 20 | 0.61 (0.46 to 0.75) | Confidential information has been removed | 0.66 (0.55 to 0.76) | 0.61 (0.51 to 0.69) |
| ACR 50 | 0.36 (0.23 to 0.52) | Confidential information has been removed | 0.40 (0.29 to 0.52) | 0.35 (0.27 to 0.43) |
| ACR 70 | 0.16 (0.09 to 0.26) | Confidential information has been removed | 0.19 (0.12 to 0.29) | 0.16 (0.11 to 0.21) |
| 30 mg of APR | ||||
| ACR 20 | NC | Confidential information has been removed | 0.33 (0.27 to 0.39) | 0.35 (0.30 to 0.41) |
| ACR 50 | Confidential information has been removed | 0.13 (0.10 to 0.17) | 0.15 (0.12 to 0.19) | |
| ACR 70 | Confidential information has been removed | 0.04 (0.03 to 0.06) | 0.05 (0.03 to 0.07) | |
While comparing results of the biologic-experienced subgroup, the results are not comparable between the AG and UCB Pharma, as probabilities were estimated at two different time points (12 weeks and 24 weeks). There are differences in the Novartis and AG estimates, largely because Novartis included a different data set (Table 175).
| Treatment | Probability of ACR responses in the biologic-experienced subpopulation | ||
|---|---|---|---|
| CS | AG at 12 weeks (12–16 weeks), median (95% CrI) | ||
| Novartis, mean | UCB Pharma, at 24 weeks, mean (95% CrI) | ||
| Placebo | |||
| ACR 20 | Confidential information has been removed | Confidential information has been removed | 0.14 (0.08 to 0.22) |
| ACR 50 | Confidential information has been removed | Confidential information has been removed | 0.03 (0.01 to 0.06) |
| ACR 70 | Confidential information has been removed | Confidential information has been removed | 0.01 (0.00 to 0.02) |
| 300 mg of SEC | |||
| ACR 20 | Confidential information has been removed | Confidential information has been removed | 0.36 (0.19 to 0.57) |
| ACR 50 | Confidential information has been removed | Confidential information has been removed | 0.11 (0.04 to 0.25) |
| ACR 70 | Confidential information has been removed | Confidential information has been removed | 0.03 (0.01 to 0.11) |
| 45 mg of UST | |||
| ACR 20 | Confidential information has been removed | Confidential information has been removed | 0.42 (0.26 to 0.59) |
| ACR 50 | Confidential information has been removed | Confidential information has been removed | 0.14 (0.06 to 0.27) |
| ACR 70 | Confidential information has been removed | Confidential information has been removed | 0.05 (0.01 to 0.12) |
| CZP | |||
| ACR 20 | Confidential information has been removed | Confidential information has been removed | NC |
| ACR 50 | Confidential information has been removed | Confidential information has been removed | NC |
| ACR 70 | Confidential information has been removed | Confidential information has been removed | NC |
WinBUG codes of preferred model
Appendix 4 Search strategy for cost-effectiveness studies
MEDLINE and MEDLINE In-Process & Other Non-Indexed Citations
The search strategy was developed in MEDLINE (via Ovid) by an information specialist, with input from the project team. The strategy included terms for PsA combined, using the Boolean operator AND, with terms for the eight drugs. No language or geographical limits were applied. A search strategy to limit retrieval to economic evaluations was used, where available. The search strategy was adapted for use in the other resources searched.
The following databases were searched: MEDLINE; MEDLINE In-Process & Other Non-Indexed Citations; CENTRAL; Conference Proceedings Citation Index – Science; EconLit; EMBASE; NHS EED; PubMed; and the SCI.
The results from the searches were imported into an EndNote library (x7, Thomson Reuters, CA, USA) and de-duplicated. After de-duplication in EndNote, a total of 722 records were available for screening.
Via Ovid: http://ovidsp.ovid.com/
Date range searched: 1946 to present.
Date searched: 15 February 2016.
Records retrieved: 73.
Search strategy
-
Arthritis, Psoriatic/ (4255)
-
(psoria$ adj2 (arthrit$ or arthropath$)).ti,ab. (6719)
-
1 or 2 (7560)
-
Certolizumab Pegol/ (329)
-
(Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7).af. (873)
-
4 or 5 (873)
-
3 and 6 (69)
-
(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6).af. (144)
-
3 and 8 (33)
-
(golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5).af. (530)
-
(2010$ or 2011$ or 2012$ or 2013$ or 2014$ or 2015$ or 2016$).ed. (5,936,425)
-
3 and 10 and 11 (93)
-
(apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9).af. (142)
-
(2014$ or 2015$ or 2016$).ed. (2,019,613)
-
3 and 13 and 14 (29)
-
Ustekinumab/ (386)
-
(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0).af. (684)
-
16 or 17 (684)
-
(2012$ or 2013$ or 2014$ or 2015$ or 2016$).ed. (3,931,892)
-
3 and 18 and 19 (97)
-
(inflectra or remsima or CT-P13).af. (45)
-
3 and 21 (2)
-
Etanercept/ (4522)
-
(etanercept or enbrel or 185243-69-0).af. (6317)
-
Infliximab/ (7584)
-
(infliximab or remicade or 170277-31-3).af. (10,459)
-
Adalimumab/ (3151)
-
(adalimumab or humira or D2E7 or (D2 adj E7) or 331731-18-1).af. (4791)
-
or/23-28 (15,794)
-
(2009$ or 2010$ or 2011$ or 2012$ or 2013$ or 2014$ or 2015$ or 2016$).ed. (6,691,099)
-
3 and 29 and 30 (686)
-
7 or 9 or 12 or 15 or 20 or 22 or 31 (846)
-
economics/ (26,633)
-
exp ‘costs and cost analysis’/ (193,882)
-
economics, dental/ (1876)
-
exp ‘economics, hospital’/ (21,057)
-
economics, medical/ (8845)
-
economics, nursing/ (3933)
-
economics, pharmaceutical/ (2601)
-
(economic$ or cost$ or price or prices or pricing or pharmacoeconomic$).ti,ab. (563,319)
-
(expenditure$ not energy).ti,ab. (20,845)
-
value for money.ti,ab. (1132)
-
budget$.ti,ab. (21,354)
-
or/33-43 (695,859)
-
((energy or oxygen) adj cost).ti,ab. (3171)
-
(metabolic adj cost).ti,ab. (962)
-
((energy or oxygen) adj expenditure).ti,ab. (18,791)
-
or/45-47 (22,130)
-
44 not 48 (690,811)
-
letter.pt. (901,537)
-
editorial.pt. (393,586)
-
historical article.pt. (326,263)
-
or/50-52 (1,605,365)
-
49 not 53 (659,853)
-
exp animals/ not humans/ (4,184,674)
-
54 not 55 (613,314)
-
32 and 56 (73)
Key
/ = indexing term (MeSH heading).
exp = exploded indexing term (MeSH heading).
$ = truncation.
ti,ab = terms in either title or abstract fields.
af = terms in any field.
ed = entry date – date added to database.
pt = publication type.
adj = terms next to each other (order specified).
adj2 = terms within two words of each other (any order).
Cochrane Central Register of Controlled Trials
Via Wiley Online Library: http://onlinelibrary.wiley.com/
Issue 1 of 12, January 2016.
Date searched: 16 February 2016.
Records retrieved: 240.
Search strategy
#1 MeSH descriptor: [Arthritis, Psoriatic] this term only (224)
#2 (psoria* near/2 (arthrit* or arthropath*)):ti,ab,kw (582)
#3 #1 or #2 (582)
#4 MeSH descriptor: [Certolizumab Pegol] this term only (57)
#5 (Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7):ti,ab,kw (211)
#6 #4 or #5 (211)
#7 #3 and #6 (29)
#8 (secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6):ti,ab,kw (140)
#9 #3 and #8 (30)
#10 (golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5):ti,ab,kw Publication Year from 2010 to 2016 (227)
#11 #3 and #10 Publication Year from 2010 to 2016 (43)
#12 (apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9):ti,ab,kw Publication Year from 2014 to 2016 (48)
#13 #3 and #12 Publication Year from 2014 to 2016 (24)
#14 MeSH descriptor: [Ustekinumab] this term only (48)
#15 (ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0):ti,ab,kw Publication Year from 2012 to 2016 (111)
#16 #14 or #15 Publication Year from 2012 to 2016 (111)
#17 #3 and #16 Publication Year from 2012 to 2016 (41)
#18 (inflectra or remsima or CT-P13):ti,ab,kw (16)
#19 #3 and #18 (4)
#20 MeSH descriptor: [Etanercept] this term only (381)
#21 (etanercept or enbrel or 185243-69-0):ti,ab,kw Publication Year from 2009 to 2016 (638)
#22 MeSH descriptor: [Infliximab] this term only (431)
#23 (infliximab or remicade or 170277-31-3):ti,ab,kw Publication Year from 2009 to 2016 (718)
#24 MeSH descriptor: [Adalimumab] this term only (236)
#25 (adalimumab or humira or D2E7 or (D2 next E7) or 331731-18-1):ti,ab,kw Publication Year from 2009 to 2016 (775)
#26 #20 or #21 or #22 or #23 or #24 or #25 Publication Year from 2009 to 2016 (1685)
#27 #3 and #26 Publication Year from 2009 to 2016 (123)
#28 #7 or #9 or #11 or #13 or #17 or #19 or #27 (265)
#29 #7 or #9 or #11 or #13 or #17 or #19 or #27 in Trials (240)
Key
MeSH descriptor = indexing term (MeSH heading).
* = truncation.
ti,ab,kw = terms in either title or abstract or keyword fields.
near/2 = terms within two words of each other (any order).
next = terms are next to each other.
Conference Proceedings Citation Index – Science
Via Web of Science, Thomson Reuters: http://thomsonreuters.com/thomson-reuters-web-of-science/
Date range searched: 1990 to 12 February 2016.
Date searched: 15 February 2016.
Records retrieved: four.
Search strategy
| # 22 | 4 | #21 OR #19 |
| Indexes=CPCI-S Timespan=All years | ||
| # 21 | 3 | #20 not #16 |
| Indexes=CPCI-S Timespan=2009-2016 | ||
| # 20 | 3 | #15 AND #14 AND #3 |
| Indexes=CPCI-S Timespan=2009-2016 | ||
| # 19 | 1 | #18 not #16 |
| Indexes=CPCI-S Timespan=All years | ||
| # 18 | 1 | #17 AND #15 AND #3 |
| Indexes=CPCI-S Timespan=All years | ||
| # 17 | 868 | #9 OR #8 OR #7 OR #6 OR #5 OR #4 |
| Indexes=CPCI-S Timespan=All years | ||
| # 16 | 305,948 | TS=(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep or guinea*) |
| Indexes=CPCI-S Timespan=All years | ||
| # 15 | 389,653 | TS=(economic* or cost* or price or prices or pricing or pharmacoeconomic*) |
| Indexes=CPCI-S Timespan=All years | ||
| # 14 | 1811 | #13 |
| Indexes=CPCI-S Timespan=2009-2016 | ||
| # 13 | 4801 | #12 OR #11 OR #10 |
| Indexes=CPCI-S Timespan=All years | ||
| # 12 | 1317 | TS=(adalimumab or humira or D2E7 or D2-E7 or 331731-18-1) |
| Indexes=CPCI-S Timespan=All years | ||
| # 11 | 2706 | TS=(infliximab or remicade or 170277-31-3) |
| Indexes=CPCI-S Timespan=All years | ||
| # 10 | 1338 | TS=(etanercept or enbrel or 185243-69-0) |
| Indexes=CPCI-S Timespan=All years | ||
| # 9 | 7 | TS=(inflectra or remsima or CT-P13) |
| Indexes=CPCI-S Timespan=All years | ||
| # 8 | 177 | TS=(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0) |
| Indexes=CPCI-S Timespan=All years | ||
| # 7 | 69 | TS=(apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9) |
| Indexes=CPCI-S Timespan=All years | ||
| # 6 | 176 | TS=(golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5) |
| Indexes=CPCI-S Timespan=All years | ||
| # 5 | 76 | TS=(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6) |
| Indexes=CPCI-S Timespan=All years | ||
| # 4 | 367 | TS=(Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7) |
| Indexes=CPCI-S Timespan=All years | ||
| # 3 | 1638 | #2 OR #1 |
| Indexes=CPCI-S Timespan=All years | ||
| # 2 | 30 | TS=(psoria* same arthropath*) |
| Indexes=CPCI-S Timespan=All years | ||
| # 1 | 1625 | TS=(psoria* same arthrit*) |
| Indexes=CPCI-S Timespan=All years |
Key
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields.
* = truncation.
‘ ‘ = phrase search.
EconLit
Via Ovid: http://ovidsp.ovid.com/
Date range searched: 1886 to January 2016.
Date searched: 15 February 2016.
Records retrieved: one.
Search strategy
-
(psoria$ adj2 (arthrit$ or arthropath$)).ti,ab. (4)
-
(Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7).af. (0)
-
(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6).af. (0)
-
(golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5).af. (1)
-
(apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9).af. (0)
-
(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0).af. (0)
-
(inflectra or remsima or CT-P13).af. (1)
-
(etanercept or enbrel or 185243-69-0).af. (9)
-
(infliximab or remicade or 170277-31-3).af. (11)
-
(adalimumab or humira or D2E7 or (D2 adj E7) or 331731-18-1).af. (4)
-
2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 (16)
-
1 and 11 (1)
Key
$ = truncation.
ti,ab = terms in either title or abstract fields.
af = all fields.
adj2 = terms within two words of each other (any order).
EMBASE
Via Ovid: http://ovidsp.ovid.com/
Date range searched: 1974 to 2016 February 12.
Date searched: 15 February 2016.
Records retrieved: 429.
Search strategy
-
psoriatic arthritis/ (13,665)
-
(psoria$ adj2 (arthrit$ or arthropath$)).ti,ab. (11,842)
-
1 or 2 (16,004)
-
certolizumab pegol/ (3636)
-
(Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7).af. (4412)
-
4 or 5 (4412)
-
3 and 6 (593)
-
secukinumab/ (674)
-
(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6).af. (752)
-
8 or 9 (752)
-
3 and 10 (236)
-
golimumab/ (3205)
-
(golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5).af. (3296)
-
12 or 13 (3296)
-
(2010$ or 2011$ or 2012$ or 2013$ or 2014$ or 2015$ or 2016$).em. (7,964,340)
-
3 and 14 and 15 (734)
-
apremilast/ (493)
-
(apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9).af. (529)
-
17 or 18 (529)
-
(2014$ or 2015$ or 2016$).em. (3,487,544)
-
3 and 19 and 20 (180)
-
ustekinumab/ (2546)
-
(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0).af. (2662)
-
22 or 23 (2662)
-
(2012$ or 2013$ or 2014$ or 2015$ or 2016$).em. (6,135,553)
-
3 and 24 and 25 (579)
-
(inflectra or remsima or CT-P13).af. (137)
-
3 and 27 (20)
-
etanercept/ (22,267)
-
(etanercept or enbrel or 185243-69-0).af. (23,098)
-
infliximab/ (34,699)
-
(infliximab or remicade or 170277-31-3).af. (35,399)
-
adalimumab/ (19,622)
-
(adalimumab or humira or D2E7 or (D2 adj E7) or 331731-18-1).af. (20,032)
-
or/29-34 (48,727)
-
(2009$ or 2010$ or 2011$ or 2012$ or 2013$ or 2014$ or 2015$ or 2016$).em. (9,322,795)
-
3 and 35 and 36 (3158)
-
7 or 11 or 16 or 21 or 26 or 28 or 37 (3754)
-
Health Economics/ (35,095)
-
exp Economic Evaluation/ (238,057)
-
exp Health Care Cost/ (228,961)
-
pharmacoeconomics/ (6245)
-
39 or 40 or 41 or 42 (427,297)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (717,152)
-
(expenditure$ not energy).ti,ab. (27,886)
-
(value adj2 money).ti,ab. (1653)
-
budget$.ti,ab. (27,874)
-
44 or 45 or 46 or 47 (744,311)
-
43 or 48 (940,487)
-
letter.pt. (924,109)
-
editorial.pt. (499,866)
-
note.pt. (628,173)
-
50 or 51 or 52 (2,052,148)
-
49 not 53 (858,063)
-
(metabolic adj cost).ti,ab. (1050)
-
((energy or oxygen) adj cost).ti,ab. (3462)
-
((energy or oxygen) adj expenditure).ti,ab. (23,424)
-
55 or 56 or 57 (27,048)
-
54 not 58 (852,398)
-
animal/ (1,703,995)
-
exp animal experiment/ (1,909,383)
-
nonhuman/ (4,685,261)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (5,233,856)
-
60 or 61 or 62 or 63 (7,617,710)
-
exp human/ (16,737,281)
-
human experiment/ (347,954)
-
65 or 66 (16,738,727)
-
64 not (64 and 67) (5,838,485)
-
59 not 68 (781,570)
-
38 and 69 (429)
Key
/ = indexing term (Emtree heading).
exp = exploded indexing term (Emtree heading).
$ = truncation.
ti,ab = terms in either title or abstract fields.
af = all fields.
pt = publication type.
sh = subject heading field.
adj2 = terms within two words of each other (any order).
em = entry week – date added to the database.
NHS Economic Evaluations Database
URL: www.crd.york.ac.uk/CRDWeb/
Date range searched: inception to 31 March 2015.
Date searched: 16 February 2016.
Records retrieved: 14.
Search strategy
| 1 | (MeSH DESCRIPTOR Arthritis, Psoriatic) in NHS EED | 11 |
| 2 | (((psoria* NEAR2 (arthrit* or arthropath*)))) in NHS EED | 17 |
| 3 | (((arthrit* or arthropath*) NEAR2 psoria*)) in NHS EED | 12 |
| 4 | MeSH DESCRIPTOR Certolizumab Pegol in NHS EED | 2 |
| 5 | ((Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7)) in NHS EED | 3 |
| 6 | ((secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6)) in NHS EED | 0 |
| 7 | ((golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5)) in NHS EED where lpd from 1 January 2010 to 31 March 2015 | 2 |
| 8 | ((apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9) ) in NHS EED where lpd from 1 January 2014 to 31 March 2015 | 0 |
| 9 | MeSH DESCRIPTOR Ustekinumab in NHS EED | 7 |
| 10 | ((ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0) ) in NHS EED where lpd from 1 January 2012 to 31 March 2015 | 9 |
| 11 | ((inflectra or remsima or CT-P13)) in NHS EED | 0 |
| 12 | MeSH DESCRIPTOR Etanercept in NHS EED | 52 |
| 13 | MeSH DESCRIPTOR Infliximab in NHS EED | 75 |
| 14 | MeSH DESCRIPTOR Adalimumab in NHS EED | 47 |
| 15 | ((etanercept or enbrel or 185243-69-0)) in NHS EED where lpd from 1 January 2009 to 31 March 2015 | 61 |
| 16 | ((infliximab or remicade or 170277-31-3)) in NHS EED where lpd from 1 January 2009 to 31 March 2015 | 85 |
| 17 | ((adalimumab or humira or D2E7 or D2-E7 or 331731-18-1) ) in NHS EED where lpd from 1 January 2009 to 31 March 2015 | 64 |
| 18 | #1 OR #2 OR #3 | 17 |
| 19 | #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 | 135 |
| 20 | #18 AND #19 | 14 |
Key
MeSH DESCRIPTOR = indexing term (MeSH heading).
* = truncation.
NEAR2 = terms within two words of each other (order specified).
PubMed
URL: www.ncbi.nlm.nih.gov/pubmed/
Date searched: 16 February 2016.
Records retrieved: 58.
Search strategy
((economic evaluation*[TIAB] OR economic analy*[TIAB] OR cost analy*[TIAB] OR cost effectiveness[TIAB] OR cost benefit*[TIAB] OR cost utilit*[TIAB]) OR (‘Costs and Cost Analysis’[Mesh])) AND ((((‘Arthritis, Psoriatic’[Mesh:noexp]) OR (psoria*[Title/Abstract] AND arthrit*[Title/Abstract]) OR (psoria*[Title/Abstract] AND arthropath*[Title/Abstract]))) AND ((‘Certolizumab Pegol’[Mesh:noexp]) OR (Certolizumab OR Cimzia OR CZP OR CDP870 OR CDP-870 OR 428863-50-7) OR (secukinumab OR Cosentyx OR AIN457 OR AIN-457 OR 1229022-83-6) OR ((golimumab OR simponi OR CNTO148 OR CNTO-148 OR 476181-74-5) AND ‘2010/01/01’[Date - Entrez] : ‘3000’[Date - Entrez]) OR ((apremilast OR otezla OR otezia OR CC10004 OR CC-10004 OR 608141-41-9) AND (‘2014/01/01’[Date - Entrez] : ‘3000’[Date - Entrez])) OR (‘Ustekinumab’[Mesh:noexp]) OR ((ustekinumab OR stelara OR CNTO1275 OR CNTO-1275 OR 815610-63-0) AND (‘2012/01/01’[Date - Entrez] : ‘3000’[Date - Entrez])) OR (inflectra OR remsima OR CT-P13) OR (‘Etanercept’[Mesh:noexp]) OR ((etanercept OR enbrel OR 185243-69-0) AND (‘2009/01/01’[Date - Entrez] : ‘3000’[Date - Entrez])) OR (‘Infliximab’[Mesh:noexp]) OR ((infliximab OR remicade OR 170277-31-3) AND (‘2009/01/01’[Date - Entrez] : ‘3000’[Date - Entrez])) OR (‘Adalimumab’[Mesh:noexp]) OR ((adalimumab OR humira OR D2E7 OR D2-E7 OR 331731-18-1) AND (‘2009/01/01’[Date - Entrez] : ‘3000’[Date - Entrez]))))
Key
[Mesh] = exploded indexing term (MeSH heading).
[Mesh:noexp] = indexing term (MeSH heading) not exploded.
* = truncation.
[Title/Abstract]) = terms in either title or abstract fields.
[Date - Entrez] = date added to the database.
Science Citation Index
Via Web of Science, Thomson Reuters: http://thomsonreuters.com/thomson-reuters-web-of-science/
Date range searched: 1900 to 12 February 2016.
Date searched: 15 February 2016.
Records retrieved: 111.
Search strategy
| # 23 | 111 | #22 OR #19 |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 22 | 95 | #21 |
| Indexes=SCI-EXPANDED Timespan=2009-2016 | ||
| # 21 | 143 | #20 not #16 |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 20 | 149 | #15 AND #14 AND #3 |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 19 | 38 | #18 not #16 |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 18 | 39 | #17 AND #15 AND #3 |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 17 | 3,371 | #9 OR #8 OR #7 OR #6 OR #5 OR #4 |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 16 | 3,889,643 | TS=(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep or guinea*) |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 15 | 1,036,604 | TS=(economic* or cost* or price or prices or pricing or pharmacoeconomic*) |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 14 | 23,253 | #13 |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 13 | 23,253 | #12 OR #11 OR #10 |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 12 | 6,187 | TS=(adalimumab or humira or D2E7 or D2-E7 or 331731-18-1) |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 11 | 15,582 | TS=(infliximab or remicade or 170277-31-3) |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 10 | 8,277 | TS=(etanercept or enbrel or 185243-69-0) |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 9 | 56 | TS=(inflectra or remsima or CT-P13) |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 8 | 962 | TS=(ustekinumab or stelara or CNTO1275 or CNTO-1275 or 815610-63-0) |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 7 | 240 | TS=(apremilast or otezla or otezia or CC10004 or CC-10004 or 608141-41-9) |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 6 | 709 | TS=(golimumab or simponi or CNTO148 or CNTO-148 or 476181-74-5) |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 5 | 275 | TS=(secukinumab or Cosentyx or AIN457 or AIN-457 or 1229022-83-6) |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 4 | 1,407 | TS=(Certolizumab or Cimzia or CZP or CDP870 or CDP-870 or 428863-50-7) |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 3 | 11,992 | #2 OR #1 |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 2 | 659 | TS=(psoria* same arthropath*) |
| Indexes=SCI-EXPANDED Timespan=All years | ||
| # 1 | 11,744 | TS=(psoria* same arthrit*) |
| Indexes=SCI-EXPANDED Timespan=All years |
Key
TS = topic tag; searches terms in title, abstract, author keywords and keywords plus fields.
* = truncation.
SAME = terms within the same sentence.
Appendix 5 Quality assessment checklists for company-submitted models
Checklist for the Novartis model
| Study question | Grade | Comments |
|---|---|---|
|
✓ | |
|
✗ | In the one prior DMARD population and the anti-TNF experienced population |
|
✓ | |
| Selection of alternatives | ||
|
✗ | In the one prior DMARD population, other anti-TNFs can be applicable |
|
✓ | |
|
✓ | |
| Form of evaluation | ||
|
✓ | |
|
N/A | |
| Effectiveness data | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | WinBUGS code presented |
| Costs | ||
|
✗ | Severe psoriasis costs are not accounted |
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
| Benefit measurement and valuation | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Decision modelling | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Discounting | ||
|
✓ | |
|
✓ | |
| Allowance for uncertainty | ||
| Stochastic analysis of patient-level data | ||
|
N/A | |
|
N/A | |
|
N/A | |
| Stochastic analysis of decision models | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
| Deterministic analysis | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Presentation of results | ||
|
✓ | |
|
✓ | |
|
✓ | |
Checklist for the UCB Pharma model
| Study question | Grade | Comments |
|---|---|---|
|
✓ | |
|
✗ | In the one prior DMARD population |
|
✓ | |
| Selection of alternatives | ||
|
✓ | |
|
✗ | It was not clear how the SEC was modelled as the cost refers to a mix of the two strengths of SEC, 150 mg and 300 mg |
|
✓ | |
| Form of evaluation | ||
|
✓ | |
|
N/A | |
| Effectiveness data | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | WinBUGS code presented |
| Costs | ||
|
✗ | Severe psoriasis costs are not accounted |
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
| Benefit measurement and valuation | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Decision modelling | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Discounting | ||
|
✓ | |
|
✓ | |
| Allowance for uncertainty | ||
| Stochastic analysis of patient-level data | ||
|
N/A | |
|
N/A | |
|
N/A | |
| Stochastic analysis of decision models | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
| Deterministic analysis | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Presentation of results | ||
|
✗ | Reporting the incremental results was not performed properly |
|
✓ | |
|
✓ | |
Appendix 6 Clinical effectiveness inputs applied in the company models
Subpopulation 1: biologic naive, one prior DMARD
| Treatment | PsARC | PASI 50 | PASI 75 | PASI 90 | Source |
|---|---|---|---|---|---|
| CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | RAPID-PsA47 trial subgroup (one prior DMARD) |
| cDMARD | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | RAPID-PsA47 trial subgroup (one prior DMARD) |
| Treatment | PsARC | PASI 50 | PASI 75 | PASI 90 | Source |
|---|---|---|---|---|---|
| 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | FUTURE 248 trial subgroup (one prior DMARD) |
| SoC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | FUTURE 248 trial subgroup (one prior DMARD) |
| Treatment | PsARC responders | PsARC non-responders | Source |
|---|---|---|---|
| CZP | Confidential information has been removed | Confidential information has been removed | RAPID-PsA47 trial subgroup (one prior DMARD) |
| cDMARD | Confidential information has been removed | Confidential information has been removed | RAPID-PsA47 trial subgroup (one prior DMARD) |
| Treatment | PsARC responders | PsARC non-responders | Source |
|---|---|---|---|
| 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | FUTURE 248 subgroup (one prior DMARD) |
| SoC | Confidential information has been removed | Confidential information has been removed | FUTURE 248 subgroup (one prior DMARD) |
Subpopulation 2: biologic naive (one or more prior DMARDs, UCB Pharma; two or more prior DMARDs, Novartis)
| Treatment | PsARC | PASI 50 | PASI 75 | PASI 90 | Source |
|---|---|---|---|---|---|
| CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NMA, naive population |
| 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| ETN | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NMA, naive population |
| INF | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NMA, naive population |
| ADA | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NMA, naive population |
| GOL | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NMA, naive population |
| Treatment | PsARC | PASI 50 | PASI 75 | PASI 90 | Source |
|---|---|---|---|---|---|
| 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NMA, overall population |
| CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NMA, overall population |
| ETN | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NMA, overall population |
| INF | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NMA, overall population |
| ADA | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NMA, overall population |
| GOL | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NMA, overall population |
| SoC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | NMA, overall population |
| Treatment | PsARC responders | PsARC non-responders | Source |
|---|---|---|---|
| CZP | Confidential information has been removed | Confidential information has been removed | NMA, naive population |
| 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| ETN | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| INF | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| ADA | Confidential information has been removed | Confidential information has been removed | NMA, naive population |
| GOL | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed |
| Treatment | PsARC responders | PsARC non-responders | Source |
|---|---|---|---|
| 150 mg of SEC | Confidential information has been removed | Confidential information has been removed | FUTURE 2 trial48 |
| CZP | –0.558 | –0.15 | Assumption: average TNF effect |
| ETN | –0.64 | –0.2 | Cawson et al., 201436 |
| INF | –0.66 | –0.2 | Cawson et al., 201436 |
| ADA | –0.49 | –0.14 | Cawson et al., 201436 |
| GOL | –0.44 | –0.06 | Cawson et al., 201436 |
| SoC | Confidential information has been removed | Confidential information has been removed | FUTURE 2 trial48 |
Subpopulation 3: biologic experienced
| Treatment | PsARC | PASI 50 | PASI 75 | PASI 90 | Source |
|---|---|---|---|---|---|
| CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | RAPID-PsA trial,47 experienced subgroup |
| 300 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Assumption |
| UST | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Assumption |
| Mix/SoC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | RAPID-PsA trial,47 experienced subgroup |
| Treatment | PsARC | PASI 50 | PASI 75 | PASI 90 | Source |
|---|---|---|---|---|---|
| 300 mg of SEC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Common efficacy reduction from the FUTURE 2 trial48 |
| CZP | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Common efficacy reduction from the FUTURE 2 trial48 |
| UST | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Common efficacy reduction from the FUTURE 2 trial48 |
| SoC | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Confidential information has been removed | Common efficacy reduction from the FUTURE 2 trial48 |
| Treatment | PsARC responders | PsARC non-responders | Source |
|---|---|---|---|
| CZP | Confidential information has been removed | Confidential information has been removed | RAPID-PsA trial47 |
| 300 mg of SEC | Confidential information has been removed | Confidential information has been removed | Assumption |
| UST | Confidential information has been removed | Confidential information has been removed | Assumption |
| Mix/SoC | Confidential information has been removed | Confidential information has been removed | RAPID-PsA trial47 |
| Treatment | PsARC responders | PsARC non-responders | Source |
|---|---|---|---|
| 300 mg of SEC | Confidential information has been removed | Confidential information has been removed | Assumption |
| CZP | Confidential information has been removed | Confidential information has been removed | Assumption |
| UST | Confidential information has been removed | Confidential information has been removed | Assumption |
| SoC | Confidential information has been removed | Confidential information has been removed | Assumption |
Appendix 7 R code for the updated York model
Confidential information has been removed.
Appendix 8 Cost-effectiveness results using infliximab and etanercept biosimilar prices, subpopulation 2
In a separate scenario analysis, biosimilar prices,134 as opposed to list prices for ETN and INF, were used in subpopulation 2 (see Chapter 6, Choice of intervention and comparators). This reduces the acquisition cost for ETN from £2332 to £2139 in the first cycle and subsequent cycles. For INF, the acquisition cost falls from £7147 to £6432 in the first cycle and from £3395 to £3056 in subsequent cycles. The results for the three subgroups according to concomitant psoriasis are shown below (see Tables 188–190).
Table 188 shows the results for the mild–moderate psoriasis subgroup. In this subgroup, CZP is the least effective biologic treatment, generating 7.226 QALYs, whereas INF generates the highest numbers of QALYs (7.890). Fully incremental analysis shows that 300 mg of SEC is dominated by ADA, GOL and ETN, GOL is dominated by ETN, and CZP and ADA are extendedly dominated. Of the remaining non-dominated alternatives, the ICER of ETN versus BSC is £18,906 per QALY and the ICER of INF versus ETN is £114,044 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 95,965 | 5.312 | – | – | – | – |
| CZP | 137,240 | 7.226 | Extendedly dominated | – | – | 21,560 |
| 300 mg of SEC | 157,086 | 7.379 | Dominated | – | – | 29,564 |
| ADA | 138,109 | 7.411 | Extendedly dominated | – | – | 20,074 |
| GOL | 142,850 | 7.637 | Dominated | – | – | 20,161 |
| ETN_Sim | 141,477 | 7.719 | 45,512 | 2.407 | 18,906 | 18,906 |
| INF_Sim | 160,993 | 7.890 | 19,517 | 0.171 | 114,044 | 25,220 |
The individual pairwise ICERs for CZP and 300 mg of SEC compared with BSC are £21,560 and £29,564 per QALY, respectively.
Table 189 shows the results for the mild–moderate psoriasis subgroup. In this subgroup, CZP is the least effective biologic treatment, generating 7.537 QALYs, whereas INF generates the highest number of QALYs (8.161). Performing fully incremental analysis shows that CZP is dominated by 150 mg of SEC, GOL is dominated by ETN, and 150 mg of SEC and ADA are extendedly dominated. Of the remaining non-dominated alternatives, the ICER of ETN versus BSC is £20,951 per QALY and the ICER of INF versus ETN is £170,815 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 67,000 | 5.676 | – | – | – | – |
| CZP | 111,856 | 7.537 | Dominated | – | – | 24,107 |
| 150 mg of SEC | 108,508 | 7.560 | Extendedly dominated | – | – | 22,032 |
| ADA | 114,039 | 7.708 | Extendedly dominated | – | – | 23,153 |
| GOL | 119,624 | 7.923 | Dominated | – | – | 23,418 |
| ETN_Sim | 116,218 | 8.025 | 49,217 | 2.349 | 20,951 | 20,951 |
| INF_Sim | 139,436 | 8.161 | 23,218 | 0.136 | 170,815 | 29,148 |
The individual pairwise ICERs for CZP and 150 mg of SEC compared with BSC are £24,107 and £22,032 per QALY, respectively.
For the no concomitant psoriasis subgroup (PASI score = 0) (Table 190), INF maintains its position as the most effective treatment (8.543 QALYs), whereas 150 mg of SEC is now the least effective treatment (7.972 QALYs). As expected in this subgroup, the ICERs versus BSC increase compared with the mild–moderate and severe psoriasis subgroups as a result of benefits being driven entirely by HAQ-DI benefits as opposed to HAQ-DI and PASI. The incremental cost-effectiveness analysis shows that GOL is dominated by ETN. CZP, 150 mg of SEC and ADA are extendedly dominated. Of the non-dominated alternatives, the ICER of ETN versus BSC is £22,512 per QALY and the ICER of INF versus ETN is £289,542 per QALY.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 51,436 | 6.188 | – | – | – | – |
| 150 mg of SEC | 95,632 | 7.972 | Extendedly dominated | – | – | 24,782 |
| CZP | 98,060 | 7.974 | Extendedly dominated | – | – | 26,117 |
| ADA | 100,893 | 8.125 | Extendedly dominated | – | – | 25,542 |
| GOL | 106,895 | 8.325 | Dominated | – | – | 25,951 |
| ETN_Sim | 102,484 | 8.456 | 51,047 | 2.268 | 22,512 | 22,512 |
| INF_Sim | 127,531 | 8.543 | 25,047 | 0.087 | 289,542 | 32,325 |
The individual pairwise ICERs for CZP and 150 mg of SEC compared with BSC are £26,117 and £24,782 per QALY, respectively.
Appendix 9 Estimating health-related quality of life for the updated York model
In order to generate an estimate of the lifetime QALYs for each of the treatments, the disease-specific measures, HAQ-DI and PASI, at each cycle of the model, must be mapped onto the utilities scores associated with particular HAQ-DI and PASI combinations. This assumes that HAQ-DI and PASI capture all of the relevant information regarding a PsA patient’s quality of life. In the previous York model,33 this relationship was estimated from analyses provided by the company (Wyeth), it carried out ordinary least squares regressions of EQ-5D utility versus HAQ-DI, PASI and an interaction term HAQ-DI × PASI, in participants in key RCTs. The utility function is given below with standard errors in parentheses:
The interaction between HAQ-DI and PASI did not reach statistical significance at the 5% level and was therefore excluded from the regression model. Table 191 presents the results of Wyeth linear regressions of utility versus HAQ-DI, PASI and HAQ-DI × PASI.
| Wyeth | Coefficients | Variance–covariance matrices | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | z | p > z | Intercept | HAQ-DI | PASI | HAQ-DI × PASI | |
| Intercept | 0.895 | 0.007 | 128.652 | 0.000 | 0.000048430 | |||
| HAQ-DI | –0.295 | 0.008 | –37.157 | 0.000 | –0.000030080 | 0.000062880 | ||
| PASI | –0.004 | 0.000 | –9.039 | 0.000 | –0.000001640 | 0.000000947 | 0.000000207 | |
| HAQ-DI × PASI | 0.000 | 0.000 | –0.669 | 0.504 | 0.000001311 | –0.000002207 | –0.000000136 | 0.000000183 |
The Psoriasis Randomized Etanercept study in Subjects with psoriaTic Arthritis (PRESTA) trial42 was used to determine this algorithm. The PRESTA trial is a 24-week clinical study comparing two forms of ETN and includes 752 patients with PsA. The study was originally designed to detect any differences in treatment efficacy for skin manifestations of psoriasis, but these patients also had diagnosed (by a rheumatologist) PsA.
Comparison of the Wyeth algorithm with that from other companies, in the previous York model, showed that the results were similar in all data sets. This indicates that the relationship between HAQ-DI, PASI and utility is stable across independent clinical trials, and gives some assurance about the generalisability to the wider PsA population.
We performed a systematic search to identify any subsequent papers which include mapping functions from HAQ-DI and PASI to utilities (post December 2009). This was not restricted to utilities measured using the EQ-5D. The search strategy can be seen in Appendix 10. This identified 2573 potentially relevant records after deduplication. After initial screening, 40 of these records were actually related to PsA and contained information on (preference-rated) quality of life. Of these, only 11 suggested the use of a mapping function to link a preference-based measure of quality of life, such as the EQ-5D or the SF-36, to disease-specific measures, including the HAQ-DI and PASI. Five of these were available only as conference abstracts. The remaining six papers were screened for inclusion (see Table 192 for a summary of these studies). In conclusion, none of the papers offers a mapping function that will allow the disease-specific measures, HAQ-DI and PASI to be mapped onto a utility score. The existing York utility algorithm is therefore used in the current version of the economic model.
| Publication (first author and year of publication) | Population | Measures included | Mapping function made explicit in paper? | Relevant for economic model? |
|---|---|---|---|---|
| Adams et al., 2010152 | Patients with RA and PsA (n = 504) | HAQ-DI, SF-6D, EQ-5D, EULAR and DAS | Yes, presented separately for EQ-5D and SF-6D | Does not include the PASI |
| Adams et al., 2011153 | Patients with RA and PsA (n = 504) | HAQ-DI, SF-36, EQ-5D (revised) and EQ-5D (original) | Yes, presented separately for EQ-5D and SF-6D | Does not include the PASI |
| Brodszky et al., 2010154 | Patients with PsA (n = 183) | Hungarian versions of HAQ-DI, EQ-5D, PsAQoL, DAS28, VAS, PASI and BASDI | No, looked at correlations between measures individually but no mapping | No |
| Gratacós et al., 2014155 | Patients with PsA (n = 287) | PASI, HAQ-DI, number of swollen and tender joints, SF-36 and EQ-5D | Yes, multivariate analysis conducted | Does not include the HAQ-DI in the EQ-5D model; instead includes the number of swollen and tender joints and the PASI. EQ-5D not included in the HAQ-DI model |
| Leung et al., 2013156 | Patients with PsA (n = 86) | EQ-5D and SF-6D | Not undertaken. Does not include the HAQ-DI or PASI | No |
| Picchianti-Diamanti et al., 2010157 | Patients with RA and PsA (n = 80) | HAQ-DI, SF-36 and DAS | Not undertaken. Reports scores separately | No |
Appendix 10 Search strategy for utility studies
Database
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations.
Ovid MEDLINE(R).
Date range searched: 1946 to present.
Search strategy
-
(sf36 or sf 36).ti,ab. (15,462)
-
(eq5d or eq 5d or euroqol or euro qol).ti,ab. (5427)
-
(short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).ti,ab. (7310)
-
(hrql or hrqol or h qol or hql or hqol).ti,ab. (12,181)
-
(hye or hyes or health$ year$ equivalent$ or health utilit$).ti,ab. (1375)
-
health related quality of life.ti,ab. (26,941)
-
rosser.ti,ab. (74)
-
(standard gamble$ or time trade off or time tradeoff or ‘tto’ or willingness to pay).ti,ab. (4653)
-
(utilities or utility or daly or dalys or disability adjusted life).ti,ab. (140,271)
-
‘Quality of Life’/ (132,981)
-
(quality of life or life quality).ti,ab. (178,851)
-
health status indicators/ (20,944)
-
quality adjusted life year/ (8035)
-
(qaly$ or quality adjusted).ti,ab. (9209)
-
(qwb$ or hui or hui1 or hui2 or hui3 or qwi).ti,ab. (1249)
-
(quality of wellbeing or quality of well being).ti,ab. (360)
-
preference based.ti,ab. (841)
-
(dermatology life quality index or health status).ti,ab. (42,673)
-
(state$ adj2 (value or values or valuing or valued)).ti,ab. (2630)
-
(dlqi or hspv).ti,ab. (688)
-
general health questionnaire.ti,ab. (3748)
-
nottingham health profile.ti,ab. (1019)
-
patient generated index.ti,ab. (44)
-
sickness impact profile.ti,ab. (1019)
-
(ghq or nhp or pgi or sip or uksip or wtp).ti,ab. (10,048)
-
or/1-25 (425,323)
-
(PSAQoL or psoriatic arthritis quality of life or PsA quality of life).ti,ab. (14)
-
(PASI or psoriasis area severity index).ti,ab. (1737)
-
(PsARC or Psoriatic Arthritis Response Criteria).ti,ab. (44)
-
(HAQ-DI or Health Assessment Questionnaire).ti,ab. (3581)
-
or/27-30 (5285)
-
Arthritis, Psoriatic/ (4270)
-
(psoria$ adj2 (arthrit$ or arthropath$)).ti,ab. (6737)
-
32 or 33 (7581)
-
26 and 34 (655)
-
31 and 34 (424)
-
35 or 36 (918)
-
(letter or editorial or comment).pt. (1,456,654)
-
37 not 38 (902)
-
exp animals/ not humans/ (4,189,142)
-
39 not 40 (899)
-
limit 41 to yr=‘2009 -Current’ (595)
Appendix 11 Identifying additional psoriatic arthritis health state costs
Methods
This is a very broad literature and an exhaustive review was beyond the time constraints of this appraisal. Instead, a rapid review was undertaken of the following sources, since the previous MTA (December 2009):
-
evidence presented to previous NICE appraisals of PsA treatments
-
the CSs to the current appraisal
-
citation searches using Rodgers et al. 33
Relevant cost data for the economic model must satisfy the following criteria:
-
The data should be specific to patients with PsA.
-
The data must show a causal relationship from the HAQ-DI and PASI to subsequent health service utilisation and costs.
-
The data should report mean costs conditional on the HAQ-DI and PASI and measures of sampling uncertainty.
-
The data should measure costs not charges or prices.
-
Preferably data would be taken from the UK; where this is not possible, it is important to assess whether or not studies from other countries are likely to be generalisable to the UK, particularly countries with mixed public/private financing such as the USA.
-
The data should measure all direct health-care costs in the hospital, outpatient and community; productivity losses should be reported separately (the base-case model excludes productivity losses in accordance with the NICE reference case).
-
The data should estimate the costs of medications separately from those of other health services; the economic model includes these costs separately from the effect of HAQ-DI/PASI on costs.
-
The data should state the price year, the currency and other data to allow adjustment to the UK in 2016.
Results
An additional relevant reference was found from the recent STA for APR in PsA. In this, the company identified a paper by Poole et al. 138 The citation searches for Rodgers et al. 33 did not identify any further published studies. One conference abstract was identified;158 however, the costs relating to PsA patients have not been published and contact with the author did not receive a response. The GOL and UST STAs both used the Rodgers et al. ’s33 algorithms for costs. The advantages and disadvantages of the previous York HAQ-DI costs and Poole et al. ’s138 costs are discussed in Chapter 6, Health state costs.
Appendix 12 Metaregression results
Results utilising the metaregression estimates for effectiveness parameters are presented in Tables 193–198 for each of the subpopulations and subgroups.
Subpopulation 1
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 95,965 | 5.312 | – | – | – | – |
| CZP | 161,347 | 8.596 | 65,382 | 3.284 | 19,908 | 19,908 |
| 300 mg of SEC | 186,956 | 8.677 | 25,609 | 0.082 | 313,571 | 27,033 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 67,000 | 5.676 | – | – | – | – |
| 150 mg of SEC | 134,957 | 8.869 | 67,956 | 3.192 | 21,287 | 21,287 |
| CZP | 138,698 | 8.870 | 3741 | 0.002 | 2,010,048 | 22,446 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 51,436 | 6.188 | – | – | – | – |
| 150 mg of SEC | 122,938 | 9.243 | 71,502 | 3.055 | 23,408 | 23,408 |
| CZP | 126,253 | 9.256 | 3315 | 0.013 | 252,218 | 24,388 |
Subpopulation 2
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 95,965 | 5.312 | – | – | – | – |
| ADA | 136,766 | 7.342 | – | – | Extendedly dominated | 20,092 |
| GOL | 141,113 | 7.486 | – | – | Dominated | Confidential information has been removed |
| CZP | 139,489 | 7.496 | 43,524 | 2.185 | 19,923 | 19,923 |
| 300 mg of SEC | 165,222 | 7.586 | – | – | Dominated | Confidential information has been removed |
| ETN | 143,538 | 7.626 | 4049 | 0.130 | 31,090 | 20,552 |
| INF | 165,132 | 7.685 | 21,594 | 0.059 | 366,216 | 29,138 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 67,000 | 5.676 | – | – | – | – |
| ADA | 112,468 | 7.642 | – | – | Dominated | 23,130 |
| GOL | 116,438 | 7.788 | – | – | Dominated | Confidential information has been removed |
| CZP | 115,516 | 7.791 | – | – | Dominated | Confidential information has been removed |
| 150 mg of SEC | 111,894 | 7.796 | 44,894 | 2.120 | 21,177 | 21,177 |
| ETN | 118,339 | 7.933 | 6445 | 0.137 | 47,137 | 22,750 |
| INF | 142,056 | 7.971 | 23,717 | 0.038 | 616,950 | 32,703 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 51,436 | 6.188 | – | – | – | – |
| ADA | 99,209 | 8.063 | – | – | Extendedly dominated | 25,485 |
| 150 mg of SEC | 99,225 | 8.199 | 47,789 | 2.011 | 23,768 | 23,768 |
| CZP | 102,418 | 8.205 | – | – | Extendedly dominated | Confidential information has been removed |
| GOL | 102,993 | 8.212 | – | – | Extendedly dominated | Confidential information has been removed |
| ETN | 104,635 | 8.363 | 5410 | 0.164 | 32,926 | 24,460 |
| INF | 129,401 | 8.373 | 24,766 | 0.010 | 2,571,503 | 35,689 |
Appendix 13 Results from alternative scenarios
Baseline Health Assessment Questionnaire-Disability Index according to subpopulation
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 95,460 | 5.540 | – | – | – | – |
| CZP | 159,431 | 8.629 | 63,971 | 3.089 | 20,709 | 20,709 |
| 300 mg of SEC | 179,172 | 8.775 | 19,741 | 0.146 | 134,880 | 25,873 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 66,495 | 5.904 | – | – | – | – |
| CZP | 135,426 | 8.917 | Dominated | – | – | 22,874 |
| 150 mg of SEC | 131,980 | 8.935 | 65,485 | 3.031 | 21,604 | 21,604 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 50,931 | 6.414 | – | – | – | – |
| 150 mg of SEC | 119,783 | 9.315 | 68,852 | 2.901 | 23,732 | 23,732 |
| CZP | 122,312 | 9.322 | 2529 | 0.007 | 351,603 | 24,543 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 96,544 | 5.049 | – | – | – | – |
| CZP | 137,839 | 6.942 | Extendedly dominated | – | – | 21,809 |
| 300 mg of SEC | 157,685 | 7.095 | Dominated | – | – | 29,877 |
| ADA | 138,709 | 7.127 | 42,165 | 2.078 | 20,295 | 20,295 |
| GOL | 143,451 | 7.350 | Extendedly dominated | – | – | 20,384 |
| ETN | 145,186 | 7.432 | 6477 | 0.306 | 21,183 | 20,409 |
| INF | 167,727 | 7.603 | 22,541 | 0.171 | 131,805 | 27,866 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 67,580 | 5.416 | – | – | – | – |
| CZP | 112,455 | 7.255 | Dominated | – | – | 24,395 |
| 150 mg of SEC | 109,107 | 7.278 | 41,527 | 1.863 | 22,294 | 22,294 |
| ADA | 114,639 | 7.425 | Extendedly dominated | – | – | 23,418 |
| GOL | 120,225 | 7.638 | Extendedly dominated | – | – | 23,687 |
| ETN | 119,927 | 7.741 | 10,820 | 0.462 | 23,400 | 22,514 |
| INF | 146,170 | 7.876 | 26,243 | 0.136 | 193,511 | 31,938 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 52,016 | 5.930 | – | – | – | – |
| 150 mg of SEC | 96,231 | 7.692 | Extendedly dominated | – | – | 25,096 |
| CZP | 98,659 | 7.694 | Extendedly dominated | – | – | 26,444 |
| ADA | 101,493 | 7.844 | Extendedly dominated | – | – | 25,851 |
| GOL | 107,496 | 8.042 | Dominated | – | – | 26,267 |
| ETN | 106,193 | 8.173 | 54,178 | 2.243 | 24,150 | 24,150 |
| INF | 134,265 | 8.259 | 28,072 | 0.086 | 326,736 | 35,311 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 97,192 | 4.756 | – | – | – | – |
| UST | 119,384 | 5.750 | 22,192 | 0.995 | 22,309 | 22,309 |
| 300 mg of SEC | 144,796 | 6.045 | 25,412 | 0.294 | 86,320 | 36,926 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 68,228 | 5.124 | – | – | – | – |
| UST | 92,503 | 6.086 | 24,276 | 0.962 | 25,239 | 25,239 |
| 300 mg of SEC | 119,826 | 6.361 | 27,323 | 0.275 | 99,385 | 41,721 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 52,664 | 5.641 | – | – | – | – |
| UST | 77,968 | 6.556 | 25,305 | 0.916 | 27,638 | 27,638 |
| 300 mg of SEC | 106,235 | 6.804 | 28,267 | 0.248 | 114,170 | 46,057 |
Alternative Health Assessment Questionnaire-Disability Index costs from Poole et al.
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 137,167 | 5.312 | – | – | – | – |
| CZP | 143,130 | 7.226 | Extendedly dominated | – | – | 3115 |
| 300 mg of SEC | 165,077 | 7.379 | Dominated | – | – | 13,500 |
| ADA | 143,610 | 7.411 | Extendedly dominated | – | – | 3069 |
| GOL | 144,712 | 7.637 | Dominated | – | – | 3244 |
| ETN | 144,009 | 7.719 | 6843 | 2.407 | 2842 | 2842 |
| INF | 170,780 | 7.890 | 26,771 | 0.171 | 156,435 | 13,036 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 137,167 | 5.676 | – | – | – | – |
| CZP | 143,130 | 7.537 | Dominated | – | – | 3205 |
| 150 mg of SEC | 140,366 | 7.560 | 3199 | 1.884 | 1698 | 1698 |
| ADA | 143,610 | 7.708 | Extendedly dominated | – | – | 3171 |
| GOL | 144,712 | 7.923 | Dominated | – | – | 3358 |
| ETN | 144,009 | 8.025 | 3643 | 0.465 | 7832 | 2913 |
| INF | 170,780 | 8.161 | 26,771 | 0.136 | 196,949 | 13,526 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 137,167 | 6.188 | – | – | – | – |
| 150 mg of SEC | 140,366 | 7.972 | 3199 | 1.783 | 1794 | 1794 |
| CZP | 143,130 | 7.974 | Extendedly dominated | – | – | 3341 |
| ADA | 143,610 | 8.125 | Extendedly dominated | – | – | 3328 |
| GOL | 144,712 | 8.325 | Dominated | – | – | 3531 |
| ETN | 144,009 | 8.456 | 6843 | 2.268 | 3018 | 3018 |
| INF | 170,780 | 8.543 | 26,771 | 0.087 | 309,469 | 14,279 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 137,167 | 5.312 | – | – | – | – |
| UST | 140,006 | 6.334 | 2840 | 1.022 | 2778 | 2778 |
| 300 mg of SEC | 163,788 | 6.632 | 23,781 | 0.299 | 79,576 | 20,154 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 137,167 | 5.676 | – | – | – | – |
| UST | 140,006 | 6.666 | 2840 | 0.989 | 2870 | 2870 |
| 300 mg of SEC | 163,788 | 6.945 | 23,781 | 0.280 | 85,064 | 20,981 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 137,167 | 6.188 | – | – | – | – |
| UST | 140,006 | 7.132 | 2840 | 0.943 | 3010 | 3010 |
| 300 mg of SEC | 163,788 | 7.384 | 23,781 | 0.252 | 94,184 | 22,264 |
Withdrawal scenario 1
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 95,965 | 5.312 | – | – | – | – |
| CZP | 137,240 | 7.226 | Extendedly dominated | – | – | 21,560 |
| ADA | 138,109 | 7.411 | 42,144 | 2.100 | 20,074 | 20,074 |
| GOL | 142,850 | 7.637 | 4741 | 0.226 | 20,976 | 20,161 |
| ETN | 144,585 | 7.719 | 1735 | 0.082 | 21,215 | 20,197 |
| 300 mg of SEC | 172,821 | 7.835 | Dominated | – | – | 30,461 |
| INF | 167,126 | 7.890 | 22,541 | 0.171 | 131,716 | 27,599 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 67,000 | 5.676 | – | – | – | – |
| CZP | 111,856 | 7.537 | Extendedly dominated | – | – | 24,107 |
| ADA | 114,039 | 7.708 | Extendedly dominated | – | – | 23,153 |
| GOL | 119,624 | 7.923 | Dominated | – | – | 23,418 |
| 150 mg of SEC | 115,157 | 7.938 | 48,157 | 2.262 | 21,291 | 21,291 |
| ETN | 119,326 | 8.025 | 4169 | 0.087 | 47,734 | 22,274 |
| INF | 145,569 | 8.161 | 26,243 | 0.136 | 193,063 | 31,616 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 51,436 | 6.188 | – | – | – | – |
| CZP | 98,060 | 7.974 | Extendedly dominated | – | – | 26,117 |
| ADA | 100,893 | 8.125 | Extendedly dominated | – | – | 25,542 |
| 150 mg of SEC | 103,136 | 8.323 | Extendedly dominated | – | – | 24,219 |
| GOL | 106,895 | 8.325 | Dominated | – | – | 25,951 |
| ETN | 105,592 | 8.456 | 54,156 | 2.268 | 23,883 | 23,883 |
| INF | 133,664 | 8.543 | 28,071 | 0.087 | 324,502 | 34,930 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 95,965 | 5.312 | – | – | – | – |
| UST | 118,127 | 6.334 | 22,162 | 1.022 | 21,685 | 21,685 |
| 300 mg of SEC | 164,019 | 7.208 | 45,892 | 0.875 | 52,454 | 35,876 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 67,000 | 5.676 | – | – | – | – |
| UST | 91,246 | 6.666 | 24,246 | 0.989 | 24,510 | 24,510 |
| 300 mg of SEC | 141,128 | 7.495 | 49,881 | 0.830 | 60,105 | 40,749 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 51,436 | 6.188 | – | – | – | – |
| UST | 76,712 | 7.132 | 25,275 | 0.943 | 26,797 | 26,797 |
| 300 mg of SEC | 128,564 | 7.898 | 51,852 | 0.767 | 67,626 | 45,105 |
Withdrawal scenario 2
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 95,965 | 5.312 | – | – | – | – |
| CZP | 145,291 | 7.575 | Extendedly dominated | – | – | 21,791 |
| 300 mg of SEC | 168,369 | 7.761 | Dominated | – | – | 29,562 |
| ADA | 146,695 | 7.798 | Extendedly dominated | – | – | 20,406 |
| GOL | 152,626 | 8.069 | 56,661 | 2.758 | 20,545 | 20,545 |
| ETN | 154,686 | 8.168 | 2060 | 0.099 | 20,827 | 20,555 |
| INF | 180,980 | 8.375 | 26,294 | 0.207 | 127,152 | 27,750 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 67,000 | 5.676 | – | – | – | – |
| CZP | 120,762 | 7.874 | Dominated | – | – | 24,459 |
| 150 mg of SEC | 116,558 | 7.902 | 49,558 | 2.226 | 22,267 | 22,267 |
| ADA | 123,771 | 8.080 | Extendedly dominated | – | – | 23,623 |
| GOL | 130,746 | 8.338 | Dominated | – | – | 23,946 |
| ETN | 130,329 | 8.462 | 13,771 | 0.560 | 24,593 | 22,734 |
| INF | 161,129 | 8.626 | 30,800 | 0.164 | 187,663 | 31,911 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 51,436 | 6.188 | – | – | – | – |
| 150 mg of SEC | 104,305 | 8.292 | Extendedly dominated | – | – | 25,138 |
| CZP | 107,389 | 8.294 | Extendedly dominated | – | – | 26,570 |
| ADA | 111,192 | 8.475 | Extendedly dominated | – | – | 26,129 |
| GOL | 118,682 | 8.716 | Dominated | – | – | 26,604 |
| ETN | 117,041 | 8.874 | 65,605 | 2.686 | 24,427 | 24,427 |
| INF | 150,067 | 8.978 | 33,026 | 0.104 | 316,876 | 35,352 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 95,965 | 5.312 | – | – | – | – |
| UST | 122,062 | 6.507 | 26,098 | 1.196 | 21,829 | 21,829 |
| 300 mg of SEC | 152,067 | 6.858 | 30,004 | 0.351 | 85,485 | 36,276 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 67,000 | 5.676 | – | – | – | – |
| UST | 95,632 | 6.833 | 28,631 | 1.156 | 24,763 | 24,763 |
| 300 mg of SEC | 127,960 | 7.160 | 32,328 | 0.328 | 98,657 | 41,081 |
| Treatment | Cost (£) | QALYs | Incremental cost (£) | Incremental QALYs | ICER vs. next best option (£) | ICER vs. BSC (£) |
|---|---|---|---|---|---|---|
| BSC | 51,436 | 6.188 | – | – | – | – |
| UST | 81,319 | 7.289 | 29,883 | 1.101 | 27,142 | 27,142 |
| 300 mg of SEC | 114,795 | 7.584 | 33,476 | 0.295 | 113,494 | 45,389 |
Appendix 14 Quality assessment checklists for published cost-effectiveness models
Checklist for the Rodgers et al.33 model
| Study question | Grade | Comments |
|---|---|---|
|
✓ | |
|
✓ | |
|
✓ | |
| Selection of alternatives | ||
|
✗ | UST, GOL, SEC and CZP not included |
|
✓ | |
|
✓ | |
| Form of evaluation | ||
|
✓ | |
|
N/A | |
| Effectiveness data | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | WinBUGS code presented |
| Costs | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
| Benefit measurement and valuation | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Decision modelling | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Discounting | ||
|
✓ | |
|
✓ | |
| Allowance for uncertainty | ||
| Stochastic analysis of patient-level data | ||
|
N/A | |
|
N/A | |
|
N/A | |
| Stochastic analysis of decision models | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
| Deterministic analysis | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Presentation of results | ||
|
✓ | |
|
✓ | |
|
✓ | |
Checklist for the golimumab model70
| Study question | Grade | Comments |
|---|---|---|
|
✓ | |
|
✓ | |
|
✓ | |
| Selection of alternatives | ||
|
✗ | Biologics compared with palliative care, which is defined as DMARDs |
| Comparators UST, SEC and CZP not included | ||
|
✗ | Does not describe what the series of DMARDs are |
|
✓ | |
| Form of evaluation | ||
|
✓ | |
|
N/A | |
| Effectiveness data | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | WinBUGS code presented |
| Costs | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
|
N/A | |
|
✓ | |
| Benefit measurement and valuation | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Decision modelling | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Discounting | ||
|
✓ | |
|
✓ | |
| Allowance for uncertainty | ||
| Stochastic analysis of patient-level data | ||
|
N/A | |
|
N/A | |
|
N/A | |
| Stochastic analysis of decision models | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
| Deterministic analysis | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Presentation of results | ||
|
✗ | Calculated incorrectly |
|
✗ | |
|
✓ | |
Checklist for the ustekinumab model66
| Study question | Grade | Comments |
|---|---|---|
|
✓ | |
|
✓ | |
|
✓ | |
| Selection of alternatives | ||
|
✗ | Conventional management was not specifically defined, but reflects treatment with non-biologics. SEC and CZP not included |
|
✗ | Does not describe what the series of DMARDs are |
|
✓ | |
| Form of evaluation | ||
|
✓ | |
|
N/A | |
| Effectiveness data | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | WinBUGS code presented |
| Costs | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
|
N/A | |
|
✓ | |
| Benefit measurement and valuation | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Decision modelling | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Discounting | ||
|
✓ | |
|
✓ | |
| Allowance for uncertainty | ||
| Stochastic analysis of patient-level data | ||
|
N/A | |
|
N/A | |
|
N/A | |
| Stochastic analysis of decision models | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
| Deterministic analysis | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Presentation of results | ||
|
✓ | |
|
✓ | |
|
✓ | |
Checklist for the Cawson et al.36 model
| Study question | Grade | Comments |
|---|---|---|
|
✓ | |
|
✓ | |
|
✓ | |
| Selection of alternatives | ||
|
✗ | Conventional management was not specifically defined, but reflects treatment with non-biologics. SEC and CZP not included |
|
✗ | Does not describe what the series of DMARDs are |
|
✓ | |
| Form of evaluation | ||
|
✓ | |
|
N/A | |
| Effectiveness data | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | WinBUGS code presented |
| Costs | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
|
N/A | |
|
✓ | |
| Benefit measurement and valuation | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Decision modelling | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Discounting | ||
|
✓ | |
|
✓ | |
| Allowance for uncertainty | ||
| Stochastic analysis of patient-level data | ||
|
N/A | |
|
N/A | |
|
N/A | |
| Stochastic analysis of decision models | ||
|
✓ | |
|
✓ | |
|
✓ | |
|
✓ | |
| Deterministic analysis | ||
|
✓ | |
|
✓ | |
|
✓ | |
| Presentation of results | ||
|
✓ | |
|
✓ | |
|
✓ | |
Glossary
- Adverse effect
- An abnormal or harmful effect, such as death, a physical symptom or visible illness, caused by, and attributable to, exposure to a chemical (e.g. a drug). An effect may be classed as adverse if it causes functional or anatomical damage or irreversible change in the homeostasis of the organism, or if it increases the susceptibility of the organism to another chemical or biological stress.
- American College of Rheumatology improvement criteria
- Measures of the improvement in disease severity based on threshold percentage improvements of 20%, 50% or 70%. To meet the criteria, a reduction in the tender joint count and swollen joint count and an improvement in at least three out of the five additional measures (patient and physician global health assessment, pain, disability and levels of an acute-phase reactant) are required.
- Anti-tumour necrosis factor/biologic experienced
- Previously treated with a biologic therapy.
- Anti-tumour necrosis factor/biologic naive
- Not previously treated with a biologic therapy.
- Apremilast
- An orally administered small-molecule drug that inhibits an enzyme involved in tumour necrosis factor production. Apremilast (Otezla®, Celgene Corporation, Summit, NJ, USA) is not a biologic therapy.
- Arthritis
- A disorder involving inflammation of the joint(s), but which is often taken to include all joint disorders. Joints can be permanently damaged through the disease process of arthritis.
- Articular
- Of or relating to joints.
- Between-study variance
- A measure of statistical heterogeneity that depends on the scale of the outcome measured. It represents the variation in reported study effects over and above the variation expected given the within-study variation.
- Biological therapy (biologic)
- Any pharmaceutical product derived from biological sources. Biologics used in the treatment of psoriatic arthritis treatment are generally monoclonal antibodies that bind to, and inactivate, immune cell-signalling molecules (e.g. tumour necrosis factor and interleukins), thereby dampening the inflammatory response.
- Biosimilar
- An imitation biological medical product (such as an anti-tumour necrosis factor) usually marketed by a manufacturer other than the manufacturer of the original biological product once a patent has expired. It should be similar to the original licensed product in terms of safety and efficacy.
- C-reactive protein
- A protein found in the blood, the concentration of which is a raised by inflammation, for example in rheumatoid arthritis, and the level of which is used as a measure of disease activity.
- Ciclosporin
- A medication originally developed to prevent the immune system from rejecting transplanted organs but which has also proved helpful in treating psoriasis.
- Confidence interval
- The typical (‘classical’ or ‘frequentist’) definition is the range within which the ‘true’ value (e.g. size of effect of an intervention) would be expected to lie if sampling could be repeated a large number of times (e.g. 95% or 99%).
- Cost–benefit analysis
- An economic analysis that converts the effects or consequences of interventions into the same monetary terms as the costs and compares them using a measure of net benefit or a cost–benefit ratio.
- Cost-effectiveness analysis
- An economic analysis that expresses the effects or consequences of interventions on a single dimension. This would normally be expressed in ‘natural’ units (e.g. cases cured, life-years gained). The difference between interventions in terms of costs and effects is typically expressed as an incremental cost-effectiveness ratio (e.g. the incremental cost per life-year gained).
- Cost–utility analysis
- The same as a cost-effectiveness analysis, but the effects or consequences of interventions are expressed in generic units of health gain, usually quality-adjusted life-years.
- Credible interval
- In Bayesian statistics, a posterior probability interval estimation that incorporates problem-specific contextual information from the prior distribution. It is used for a purpose similar to that of a confidence interval in frequentist statistics.
- Crohn’s disease
- An inflammatory condition of the digestive tract; rheumatic diseases are often associated with it and ulcerative colitis is related to it.
- Dactylitis
- Inflammation of an entire digit caused by simultaneous joint and tendon inflammation.
- Deviance information criterion
- A model fit statistic and used for Bayesian model comparison. The model with the smallest deviance information criterion is estimated to be the model that would best predict a replicate data set that has the same structure as that currently observed.
- Disease-modifying antirheumatic drugs
- Drugs capable of modifying the progression of rheumatic disease. The term is, however, applied to what are now considered to be traditional (or conventional) disease-modifying drugs, in particular sulfasalazine, methotrexate and ciclosporin, as well as azathioprine, cyclophosphamide, antimalarials, penicillamine and gold. The newer agent leflunomide is also a disease-modifying antirheumatic drug. Biologics are not generally referred to as disease-modifying antirheumatic drugs, although occasionally biological disease-modifying antirheumatic drugs may be used.
- Dominated
- A term, used in this report in the cost-effectiveness sections, that describes a treatment associated with higher costs and a lower number of quality-adjusted life-years than another treatment.
- Enthesitis
- Inflammation of the region where tendons and ligaments attach to the bone (enthesis).
- Erythrocyte sedimentation rate
- One of the tests designed to measure the degree of inflammation.
- EuroQol-5 Dimensions questionnaire
- A standardised instrument for measuring generic health-related quality of life, used in the computation of the number of quality-adjusted life-years gained.
- Extendedly dominated
- A term, used in this report in the cost-effectiveness sections, to describe a strategy in which the incremental cost-effectiveness ratio is higher than that of the next most effective strategy. Therefore, an extendedly dominated strategy produces additional gains in effectiveness at incremental costs higher than those of the next most effective strategy.
- Fixed-effect model
- A statistical model that stipulates that the units under analysis (e.g. people in a trial or study in a meta-analysis) are the ones of interest, and thus constitute the entire population of units. Only within-study variation is taken to influence the uncertainty of results (as reflected in the confidence interval) of a meta-analysis using a fixed-effect model.
- Health Assessment Questionnaire-Disability Index
- A self-administered questionnaire measuring an individual’s physical disability and pain. It scores ability to perform various activities between 0 (without any difficulty) and 3 (unable to do). It is reported as an average of all activity scores.
- Heterogeneity
- In systematic reviews, the variability or differences between studies in the estimates of effects. A distinction is sometimes made between ‘statistical heterogeneity’ (differences in the reported effects), ‘methodological heterogeneity’ (differences in study design) and ‘clinical heterogeneity’ (differences between studies in key characteristics of the participants, interventions or outcome measures).
- Intention-to-treat analysis
- An analysis in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not.
- Leeds Dactylitis Index
- A measure of swelling between digital joints. A dactylometer is used to measure the circumference of an affected digit, and the contralateral unaffected digit, and the ratio of the circumferences is calculated. If both hands are affected, a standard reference is used to calculate the ratio. A difference in circumference of ≥ 10% defines a finger with dactylitis. The tenderness of each digit is also taken into account to generate a score for each. If multiple digits are affected, the scores for each are added together.
- Leeds Enthesitis Index
- A measure of tenderness over six tendon attachment sites (enthuses). It also includes an assessment for soft tissue swelling. It is scored from 0 to 6.
- Methotrexate
- One of the oldest chemotherapy drugs used in the treatment of cancer and autoimmune diseases, such as rheumatoid and psoriatic arthritis.
- Modified total Sharp score
- One of several radiological assessments used to measure joint damage in psoriatic arthritis. This method grades all joints of the hand separately for erosions and joint space narrowing for 64 and 52 joints (out of a maximum score of 149), respectively, with higher scores representing greater damage. The total Sharp score is modified to include other joints in the assessment.
- Monoclonal antibody
- An antibody produced using a single clone of cells with affinity for one particular antigen.
- Network meta-analysis
- (Synonyms: mixed treatment comparison, indirect treatment comparison.) Used when there is insufficient direct evidence linking two interventions, this is a type of meta-analysis comparing three or more different treatments using both direct comparison within randomised controlled trials and indirect comparison between trials based on a common comparator (such as placebo).
- Non-steroidal anti-inflammatory drug
- Any of a large range of drugs in the aspirin family, prescribed for different kinds of arthritis, that reduces inflammation and controls pain, swelling and stiffness.
- Placebo
- An inactive substance or procedure administered to a patient, usually to compare its effects with those of a real drug or other intervention, but sometimes for the psychological benefit to the patient through a belief that she/he is receiving treatment.
- Plaque psoriasis
- The most common form of psoriasis, also known as psoriasis vulgaris, characterised by red, raised lesions covered by silvery scales. About 80% of patients with psoriasis have this type.
- Psoriasis
- A chronic skin disease characterised by inflammation and scaling. Scaling occurs when cells in the outer layer of skin are produced faster than normal and build up on the skin’s surface. It is thought to be caused by a disorder of the immune system.
- Psoriasis Area and Severity Index
- A measure of the extent of skin affected and of the redness, scaliness and thickness of psoriatic plaques. Response is presented as PASI 50, PASI 75 or PASI 90, the number being the percentage reduction in Psoriasis Area and Severity Index score from baseline.
- Psoriatic arthritis
- A disease characterised by stiffness, pain and swelling in the joints, especially of the hands and feet. It affects about 30% of people with psoriasis. Early diagnosis and treatment can help inhibit the progression of joint deterioration.
- Psoriatic Arthritis Response Criteria response
- An improvement of at least 30% in the tender or swollen joint count as well as a 1-point improvement on a 5-point scale of the patient’s and/or physician’s assessment. The National Institute for Health and Care Excellence defines a response as an improvement in two or more of the four assessment criteria (with no worsening of any of these four measures).
- Quality-adjusted life-year
- An index of health gain according to which survival duration is weighted or adjusted by the patient’s quality of life during the survival period. It has the advantage of incorporating changes in both quantity (mortality) and quality (morbidity) of life.
- Quality of life
- A concept incorporating all the factors that might have an impact on an individual’s life, including factors such as the absence of disease or infirmity as well as other factors that might affect that individual’s physical, mental and social well-being.
- Random-effects model
- A statistical model sometimes used in meta-analysis in which both within-study sampling error (variance) and between-studies variation are included in the assessment of the uncertainty (confidence interval) of the results of a meta-analysis.
- Randomised controlled trial
- (Synonym: randomised clinical trial.) An experiment in which investigators randomly allocate eligible people to intervention groups to receive or not receive one or more interventions that are being compared.
- Relative risk
- (Synonym: risk ratio.) The ratio of risk in the intervention group to the risk in the control group. The risk (proportion, probability or rate) is the ratio of people with an event in a group to the total number in the group. A relative risk of 1 indicates no difference between comparison groups. In the case of undesirable outcomes, a relative risk of < 1 indicates that the intervention was effective in reducing the risk of that outcome.
- Remission
- A lessening or abatement of the symptoms of a disease.
- Residual deviance
- An analysis used for model comparison and goodness of fit. It is equal to the deviance for a given model minus the deviance for a saturated model. A saturated model is one in which all of the predictions from the model are equal to the observed data values. Total residual deviance should approximate the number of data points for a good fit.
- Rheumatoid arthritis
- A chronic autoimmune disease characterised by pain, stiffness, inflammation, swelling, and, sometimes, destruction of joints.
- Sensitivity analysis
- An analysis used to determine how sensitive the results of a study or systematic review are to changes in how it was done. It is used to assess how robust the results are to uncertain decisions or assumptions about the data and the methods that were used.
- Short Form questionnaire-36 items
- A patient-reported survey of general health status.
- Statistical significance
- An estimate of the probability of an association (effect) as large as or larger than what is observed in a study occurring by chance, usually expressed as a p-value.
- Subpopulation 1
- Patients who are biologic naive but have tried one previous conventional disease-modifying antirheumatic drug.
- Subpopulation 2
- Patients who are biologic naive but have tried two or more previous conventional disease-modifying antirheumatic drugs.
- Subpopulation 3
- Patients who are biologic experienced.
- Tender joint count and swollen joint count
- Assessment of the condition of 28 joints important to functional status. Used in the calculation of several composite disease activity scores such as Disease Activity Score 28.
- Tumour necrosis factor alpha
- A cell signalling molecule (cytokine) involved in the inflammatory response pathway, known to be fundamental to the pathological processes causing psoriasis and psoriatic arthritis. Plays a key role in onset and persistence of joint and skin inflammation.
List of abbreviations
- ACR
- American College of Rheumatology
- ACR 20
- 20% improvement in the American College of Rheumatology criteria
- ACR 50
- 50% improvement in the American College of Rheumatology criteria
- ACR 70
- 70% improvement in the American College of Rheumatology criteria
- ADA
- adalimumab
- ADEPT
- ADalimumab Effectiveness in Psoriatic arthritis Trial
- AE
- adverse event
- AG
- Assessment Group
- APR
- apremilast
- BNF
- British National Formulary
- BSA
- body surface area
- BSC
- best supportive care
- BSR
- British Society for Rheumatology
- BSRBR
- British Society for Rheumatology Biologics Register
- CASPAR
- Classification Criteria for Psoriatic Arthritis
- cDMARD
- conventional disease-modifying antirheumatic drug
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CLEAR
- Efficacy of Secukinumab Compared to Ustekinumab in Patients with Plaque-type Psoriasis
- CPCI-S
- Conference Proceedings Citation Index – Science
- CrI
- credible interval
- CRP
- C-reactive protein
- CS
- company submission
- CZP
- certolizumab pegol
- DA
- deterministic analysis
- DANBIO
- Danish Database for Biological Therapies
- DARE
- Database of Abstracts of Reviews of Effects
- DIC
- deviance information criterion
- DMARD
- disease-modifying antirheumatic drug
- EQ-5D
- EuroQol-5 Dimensions
- ERASURE
- Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis
- ERG
- Evidence Review Group
- ESR
- erythrocyte sedimentation rate
- ETN
- etanercept
- EULAR
- European League Against Rheumatism
- FIXTURE
- Full Year Investigative Examination of Secukinumab vs. Etanercept Using Two Dosing Regimens to Determine Efficacy in Psoriasis
- FUTURE
- Efficacy at 24 Weeks and Long Term Safety, Tolerability and Efficacy up to 2 Years of Secukinumab (AIN457) in Patients With Active Psoriatic Arthritis
- GO-REVEAL
- Golimumab – A Randomized Evaluation of Safety and Efficacy in Subjects with Psoriatic Arthritis Using a Human Anti-TNF Monoclonal Antibody
- GOL
- golimumab
- GP
- general practitioner
- HAQ-DI
- Health Assessment Questionnaire-Disability Index
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- i.v.
- intravenous
- ICER
- incremental cost-effectiveness ratio
- IL
- interleukin
- IMPACT
- Infliximab Multinational Psoriatic Arthritis Controlled Trial
- INF
- infliximab
- ITT
- intention to treat
- LDI
- Leeds Dactylitis Index
- LEI
- Leeds Enthesitis Index
- LOCF
- last observation carried forward
- MeSH
- medical subject heading
- MIMS
- online and print prescribing database for health professionals
- MTA
- multiple technology appraisal
- mTSS
- modified total Sharp score
- MTX
- methotrexate
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NOAR
- Norfolk Arthritis Register
- NOR-DMARD
- Norwegian Antirheumatic Drug Register
- NSAID
- non-steroidal anti-inflammatory drug
- OR
- odds ratio
- PALACE
- Psoriatic Arthritis Long-term Assessment of Clinical Efficacy
- PASI
- Psoriasis Area and Severity Index
- PASI 50
- 50% reduction in the Psoriasis Area and Severity Index
- PASI 75
- 75% reduction in the Psoriasis Area and Severity Index
- PASI 90
- 90% reduction in the Psoriasis Area and Severity Index
- PNR
- placebo non-responder
- PR
- placebo responder
- PRESTA
- Psoriasis Randomized Etanercept study in Subjects with psoriaTic Arthritis
- PsA
- psoriatic arthritis
- PSA
- probabilistic sensitivity analysis
- PsARC
- Psoriatic Arthritis Response Criteria
- PSSRU
- Personal Social Services Research Unit
- PSUMMIT
- Study of the Safety and Effectiveness of Ustekinumab in Patients With Psoriatic Arthritis
- QALY
- quality-adjusted life-year
- RA
- rheumatoid arthritis
- RAPID-PsA
- Certolizumab Pegol in Subjects With Adult Onset Active and Progressive Psoriatic Arthritis
- RCT
- randomised controlled trial
- RR
- relative risk
- SAE
- serious adverse event
- SCI
- Science Citation Index
- SD
- standard deviation
- SE
- standard error
- SEC
- secukinumab
- SF-36
- Short Form questionnaire-36 items
- SJC
- swollen joint count
- SoC
- standard of care
- SPIRIT-P1
- Study of Ixekizumab in Participants With Active Psoriatic Arthritis
- STA
- single technology appraisal
- TA
- technology appraisal
- TB
- tuberculosis
- THIN
- The Health Improvement Network
- TJC
- tender joint count
- TNF
- tumour necrosis factor
- TNF-α
- tumour necrosis factor alpha
- TNR
- treatment non-responder
- TR
- treatment responder
- UST
- ustekinumab
- VAS
- visual analogue scale
- YODA
- Yale University Open Data Access
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed confidential. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of confidential data removed and replaced by the statement ‘confidential information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.