Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/188/05. The contractual start date was in September 2014. The draft report began editorial review in September 2016 and was accepted for publication in June 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Amanda Farrin is a member of the Health Technology Assessment (HTA) Themed Call Panel. David Meads is a member of the Elective and Emergency Specialist Care National Institute for Health Research HTA panel.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Bennett et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2017 Queen’s Printer and Controller of HMSO
Chapter 1 Supporting self-management of analgesia and related treatments at the end of life
Summary of Health Technology Assessment brief
In October 2012, the Health Technology Assessment (HTA) published a commissioning brief entitled ‘Self-management of pain relief, nausea and constipation for patients approaching the end of life’. Applicants were asked to address the research question of whether or not a patient support tool could improve the self-management of medication for pain, nausea and constipation in patients approaching the end of life. This call was based on the recognition that:
Enhanced patient-family health decision making can improve the overall quality of end of life care. As life-limiting illnesses progress, the number of disease related symptoms typically increases. Medication regimens can be complex and pain, nausea and constipation are among the common symptoms that often fluctuate and may be appropriate for self-management by patients and their family carers. Patient decision aids have been shown to be effective in facilitating informed decision making and it may be that a self-management aid could help patients and their families to manage their medication regimens to improve pain, nausea and constipation symptom control. A feasibility study is needed to develop a support aid and to assess its acceptability.
This report contains the research conducted in response to this brief (see Appendix 1 for full HTA brief).
Summary of current evidence and policy context
Approximately 160,000 people die from cancer each year in the UK, a number that is expected to rise to 193,000 by 2030. 1 Evidence suggests that 45–56% of patients with advanced cancer (72,000–89,600 each year in the UK), experience pain of moderate to severe intensity before they die. 2,3 Detailed information on pain in patients approaching the end of life with non-cancer diseases is less widely available.
Since 1986, the focus of pain treatment for patients approaching the end of life has been the use of strong opioids based on the World Health Organization’s ‘analgesic ladder’. 4 Initial studies suggested that this approach could control pain in around 73% of cancer patients. 5,6 Despite widespread availability of strong opioids in the UK, at least 32% of patients with cancer are undertreated for their pain. 3,7
Patients at the end of life report that their preferred place of care and death is home. 8 The National Survey of Bereaved People (VOICES) has evaluated the perceptions of the care given to recently deceased persons (not just those with cancer) since 2011. 9 In 2015, only 18% reported that pain was controlled ‘completely, all the time’ at home, compared with 38% in hospital and 63% in hospice. Not surprisingly, uncontrolled pain is the most frequent reason for community-based cancer patients to contact out-of-hours primary care services. 10
Although there is evidence that improved pain management for patients with advanced disease is associated with involvement of palliative care, the evidence base is not consistent in reflecting significant methodological heterogeneity. 11 Little is known about the service constituents that are responsible for improved pain management.
In 2008, the Department of Health published a strategy for end-of-life care as it recognised that many people did not have what could be described as a ‘good death’: being treated as an individual with dignity and respect, being without pain and other symptoms, being in familiar surroundings and being in the company of close family and/or friends. 12 A National Institute for Health and Care Excellence (NICE) quality standard was subsequently issued in 2011 to define and support high-quality end-of-life care, specifically including pain management. 13
In 2016, the British Medical Association interviewed 269 members of the public and 237 doctors regarding the provision of end-of-life care by the NHS. 14 For both the public and doctors, pain was the most feared aspect of dying, echoing the findings of the national VOICES survey and underlining the importance of good pain control at the end of life.
A succession of key reports have emphasised the urgent need to improve end-of-life care services in the NHS because of unacceptable variation in access to and experience of care. 15 The Leadership Alliance for the Care of Dying People’s One Chance to Get it Right – Improving People’s Experience of Care in the Last Few Days and Hours of Life16 recognised that pain and symptom control should be among the five key priorities of care in the NHS. In 2015, NHS England led Ambitions for Palliative and End of Life Care: A National Framework for Local Action 2015–2020,17 which described access to care and maximising comfort as two of its six ambitions for improving services. The Parliamentary Health Committee on End of Life Care reported in 2015 that round-the-clock access to community nurses and specialist outreach palliative care for pain relief are some of the actions that could facilitate a shift in quality of care. 18
The Palliative and End of Life Care Priority Setting Partnership, led by the James Lind Alliance, undertook a survey of 700 patients and carers and a similar number of professionals involved in end-of-life care, regarding research priorities. 19 Of the 83 shortlisted areas, one-third of the top 10 research priorities related to symptom management, with pain specifically mentioned. In response to this, the National Institute for Health Research (NIHR) undertook a themed review of palliative care research that it funds, summarised in the NIHR report Better Endings. 20
Overall, NIHR research has identified persistent inequalities and variations in care, with poorly co-ordinated services and limited access to specialist palliative care. In addition, place of death may not be the most important aspect of care for many; managing pain and other symptoms and the quality of care are key for patients and their family, whatever the setting. These reports,12–15 all published in the last 2 years, serve to highlight that although good-quality end-of-life care can be defined, currently within the NHS patients experience poor pain control at home, the public remain understandably fearful of a painful death and there is unacceptable variation in access to good care in the NHS. Providing better support to enable patients to self-manage with more confidence is likely to be an important mechanism in improving outcomes for patients with pain from advanced disease. 21 Therefore, this research proposal was timely and important in supporting NHS priorities and informing ways to improve the experiences of dying patients and those that survive them.
Background rationale
One important influence on the quality of pain management for patients at home concerns the information and understanding that patients have regarding their pain and their analgesic medication. Misunderstandings by patients regarding opioids inhibit good pain control22 and we have found that this is particularly true for older patients. 23 Our own research has also shown that patients and their carers face daily dilemmas on the best way to balance pain relief with the adverse effects of analgesia and the consequent impact of both on daily activities. 24,25 Attitudes and knowledge of health-care professionals (HCPs) towards opioids is likely to influence the quality of information provided to patients26 and the increasing complexity of opioid choices in end-of-life care may further reduce the confidence of non-specialist practitioners. 27
However, addressing the concerns of patients leads to improvements in pain control,28 and this process relies on specific contexts that support behavioural change in patients, carers and professionals. 29–31 Although education and self-management support are largely seen as nursing tasks rather than medical tasks,30 our research suggests that pharmacists can make important contributions too. 32,33
Despite there being a good understanding of patient and carer concerns regarding opioid analgesia and related side effects, much less is known about the optimal means of addressing these concerns,34 which is why they have been highlighted by NICE guidance. 35 Simple information in the form of leaflets or video may help,36 but may be insufficient to make a tangible impact on patients’ perceptions of confidence to self-manage.
The research recommendation 4.1 from recent NICE guidance on the use of opioids in palliative care calls for clinically effective and cost-effective methods of addressing patient and carer concerns about strong opioids, including anticipating and managing adverse effects. 35,37 Moreover, the NICE guidance indicates that as well as constipation and nausea, drowsiness is one of the most common side effects of pain medication and one that bothers patients most. All three side effects need to be addressed for optimal pain management and we therefore extended the scope of the HTA brief to incorporate drowsiness in the intervention.
Feasibility study aims and objectives
We aimed to develop an intervention that enables patients approaching the end of life and their carers to more confidently manage medications for pain (specifically strong opioids), nausea, constipation and drowsiness at home. We designed this project with a patient-centred approach at the heart of our development plan, nested within a theoretically informed behaviour change framework. The expected benefits of the intervention for patients were improvements in symptom relief, feeling empowered with increased knowledge and skills to recognise worsening symptoms or adverse effects, being able to self-initiate therapeutic adjustments and knowing how and when to access help from the health-care system.
We defined our intervention as a set of materials and coaching procedures that deliver knowledge, facilitate the generation of specific action plans and enhance the user’s skills to monitor and reflect on their actions. We judged that our intervention would be optimally delivered by clinical nurse specialists (CNSs) who work within specialist palliative care teams. We thought that patients would be most likely to benefit from the intervention if they were adults (aged > 18 years), approaching the end of life, suffering from significant pain and being cared for in their own home, being treated with, or due to start treatment with, opioids for pain, and experiencing (or anticipating) adverse effects of these medications. We were particularly keen to embed the principles of experience-based co-design into the development, modelling and testing of our prototype intervention, and to evaluate this within a theoretical framework for characterising and designing behaviour change interventions. 38,39
Our objectives were divided into three distinct phases, which is in line with the Medical Research Council framework on developing and evaluating complex interventions. 40 We also planned to use the explanatory models of normalisation process theory to evaluate factors that will support implementation. 41 This would offer a clear path for implementation into the wider NHS should the effectiveness of the intervention be established in a future definitive randomised controlled trial (RCT).
Phase I: development objectives
-
Establish a patient and public involvement (PPI) panel.
-
Establish the content of a prototype intervention and a manualisation strategy that includes a protocol to standardise (1) the training of HCPs and (2) the delivery of the intervention by HCPs to patients and carers.
-
Understand self-management needs and capabilities of patients and carers related to strong opioid medication.
-
Define usual care.
Phase II: modelling objectives
-
Refine the prototype intervention and manualisation strategy.
Phase III: feasibility assessment objectives
-
Assess acceptability and up-take of the intervention in a mixed-methods observational study involving patients, informal carers and HCPs from four palliative care services.
-
Assess the feasibility of obtaining outcome data for a larger trial.
Patient and public involvement
We have had sustained PPI throughout all stages of the Self-Management of Analgesia and Related Treatments at the end of life (SMART) study. PPI has been an integral part of all our study processes from inception and development of the research ideas, development of the funding application, through to delivering the research project and interpreting the study findings. We have engaged with PPI in a number of ways. First, through a PPI coapplicant (JG), we benefited from expert PPI input to help prioritise the research question and ensure that the delivery of the intervention is undertaken in a way that is meaningful and relevant to patients approaching the end of life and their carers. Second, in the first phase of the project we established a dedicated PPI panel that informed and helped refine the content and delivery strategy of the SMART intervention as well as review patient study materials (i.e. information sheet, consent form, patient questionnaire). Last, we recruited an independent PPI representative to be part of the Steering Committee, which has oversight and responsibility for the project, and gave the study team insight and direction throughout the design, delivery and completion of the project.
Success criteria
Ultimately, we aimed to establish the acceptability and uptake of our prototype self-management support toolkit (SMST) and determine the feasibility of evaluating this intervention within a larger trial. In order to judge whether or not we had achieved our aims, we agreed our success criteria beforehand to be as follows.
Phase I
-
Establishment of a PPI panel and assessment of members’ support and training requirements.
-
Development of a usual-care protocol based on literature review and clinical practice observations.
-
Development of prototype intervention materials, manualisation strategy and usual care protocol.
Phase II
-
Establish members of focus groups.
-
Development of refined intervention materials and manualisation strategy.
Phase III
-
Sampling strategy: recruit three patients per month at each site within 4 months.
-
Feasibility of data collection: key clinical and health economic measures, and health-care resource measures, have sufficient complete data to estimate primary study end points.
-
Trial experience: patients and carers reporting acceptable and sustained use of intervention materials.
Chapter 2 Development of the SMART intervention
Phase I: overview of initial contextual work
This chapter describes the intervention development process. First, it summarises the literature scoped to inform the development of the intervention. Second, it recounts the exploratory activities undertaken with specialist palliative care health professionals, patients and carers, to derive a concept of self-management of analgesia (opioids) and related treatments (for nausea, constipation and drowsiness) at the end of life.
Through this development process it was possible to generate a preliminary model of self-management that was then tested further through interviews and focus groups with patients, carers and HCPs. This chapter will also describe how this model was then used to specify and inform the components, including content and form, of the SMART intervention. This process aligned with the theoretical modelling phase of the Medical Research Council framework for complex interventions. 42,43
Initially, contextual work was performed to frame self-management within the field of palliative pain management. Much is written about self-management, but the focus is predominantly on long-term condition management. 44 However, a survey of 90 cancer patients living at home receiving community-based palliative care identified three key factors associated with successful pain management. 45 The first factor is maintaining a sense of control over managing medicines by modifying the schedule of taking medicines around daily routines and planned activities. The second factor is negotiating a balance (trade-off) between symptom control and the impact of medicines’ side effects on cognitive and physical functioning. Furthermore, Hansen et al. 46 identified that 40% of end-of-life patients regularly do not use analgesia despite reporting moderate to severe pain and indicating an awareness that regular medication was the most effective self-management behaviour for controlling pain. These authors concluded that patients were making important trade-offs between pain relief and the side effects of medications and this helped maintain a sense of control. The third factor associated with successful pain management identified by Bennett et al. 45 is a broad base of support. Community palliative care nurses and community pharmacists were seen as key HCPs supplemented by support from a carer (family member or friend). The authors concluded that a community-based intervention that is flexible and responsive to patients’ needs, involving carers and community-based HCPs, is most likely to be successful.
Information provision has been identified as a fundamental component of interventions in advanced disease. 37,47 Tailored information provision equips patients and carers with the necessary skills and confidence to drive behaviour change. 39,48 However, on its own it is insufficient to drive significant improvements in patient-reported outcomes, such as pain and quality of life. Contextual factors associated with successful pain management are the development of a trusting relationship with health-care providers, having dedicated time to focus on medicines management and confidence in exerting self-control over a daily analgesic routine. 47,49 Identifying patient and carer needs was recognised by NICE as the basis for delivering tailored support in its guidance on improving supportive and palliative care for adults with cancer. 37 Therefore, in addition to information provision, regular assessment and review of patient needs is a key component in supporting self-management of pain medication in this context. 37,47
In order to understand the context more fully, six semistructured interviews were initially undertaken with patients and carers recruited from a palliative care outpatient service. Interviews focused on identifying the need to self-manage medicines at the end of life and exploring perceived barriers to and facilitators of managing medicines at home. This led to the delineation of five themes:
-
communication and understanding
-
addressing fears and concerns
-
information requirements
-
carer-specific needs
-
making trade-offs.
These themes were used to shape early thinking about the dimensions of the intervention, which were further refined in later phases, as described below.
Evidence synthesis
Scoping of the literature
Scoping of the literature was undertaken following the preliminary work described above. The objective of this exercise was to examine:
-
the content and form of previous interventions effective in improving pain management by patients (see Appendix 2)
-
key systematic reviews of what can be done to support self-management across a range of long-term conditions of relevance to this particular application at the end of life
-
key literature and reviews that identify factors that enable HCPs to support patient self-management
-
existing public guidance based on NICE clinical guideline 140 – Opioids in Palliative Care (Box 1)
-
description and potential components of self-management at the end of life (Box 2).
-
NICE (2012) information for managing pain with the strong opioids in people with advanced progressive disease – information for the public. 37
-
All Wales Medicines Strategy Group: Opioids in Palliative Care – Patient Information Manual. 50
Managing pain with strong opioids: some treatments may not be suitable depending on your circumstances, definition of palliative care and its purpose of alleviating pain and discomfort to improve quality of life.
Information about taking strong opioids: if you are offered strong opioids, HCPs should explain when and why strong opioids are used to treat pain; how effective they are likely to be at relieving your pain; about taking strong opioids for background pain and breakthrough pain, including how, when and how often to take them; how long pain relief should last, possible side effects and signs to watch out for that might mean there is too much of the medication in your system; and how to store strong opioids safely.
Discussing your concerns: if you are worried about addiction and side effects, your HCP should reassure you that addiction is very unlikely and that you will be monitored for side effects. Strong opioids can be offered at different stages and doing so does not necessarily mean that you are close to the end of your life.
Starting treatment with opioids: different forms, short acting vs. slow release, no standard dose.
If you have trouble swallowing: if pain is stable you should be offered a patch.
Reviewing pain control: need for regular reviews especially at the beginning.
Continuing treatment: sustained-release form.
Treating breakthrough pain: immediate-release form, advice from specialist if uncontrolled.
Managing side effectsConstipation: definition and statement that it affects nearly everyone who takes strong opioids. You should be offered laxatives and a description of how laxatives work; they can take time to work so take them as advised and your HCP may change the type of opioid if your constipation is severe.
Nausea: you may experience feeling sick when starting strong opioids or when the dose is increased, but this is likely to last only a short time. If it persists, you should be offered anti-sickness medication.
Drowsiness: you may experience mild drowsiness or problems with concentration when starting strong opioids or the dose is increased, but this is likely to last only a short time. Your HCP should warn you that having problems might affect your ability to carry out some tasks, such as driving. If you have severe or long-lasting problems and your pain is under control, your HCP may discuss the possibility of reducing the dose of opioid with you. If your pain is not controlled, then your HCP may consider changing the opioid and if the problems are not relieved by these changes then your HCP may seek specialist advice.
Questions to ask a HCP: tell me more about strong opioids for pain relief? Can you tell me about the side effects associated with taking strong opioids? Will I become addicted to strong opioids? How long will it take for this medication to work? What do I do if I am still in pain after taking strong opioids? What are my options for taking some other type of pain relief? Can you give me some written material about strong opioid treatment?
Sources of more information: CancerHelp UK, Macmillan Cancer Support, British Pain Society and NHS Choices.
The above authors provided a definition (via concept analysis) of self-management support within the context of palliative care:
. . . Assessing, planning, and implementing appropriate care to support the patient to be given the means to master or deal with their illness or its effects.
The authors proposed eight potential professional roles to support self-management. These roles were undefined but labelled as advocate, educator, facilitator, problem-solver, communicator, goal-setter, monitor and reporter.
References: Schumacher et al.52,53These authors demonstrated that, for oncology patients (and their carers) in the USA, the practical experiences of day-to-day management of pain medications could be both challenging and onerous. Navigating the systems with complex webs of people and rules was a lengthy and tedious challenge causing frustration, effort and added anxiety. These issues revolved around getting prescriptions, obtaining medicines, understanding, organising, storing, scheduling, remembering and taking.
UnderstandingOnce patients and carers obtained medicines they were immediately faced with understanding the medicines they had brought home. Lack of understanding led to uncontrolled pain. Many areas of confusion were evident; keeping the purpose and names of medications straight was one. Medication names were ‘not in English’ (i.e. plain English); many referred to medicines by appearance, and the use of abbreviations was seen as confusing, as were drugs with similar names. Lack of understanding about maximum daily dose limits was common (including not knowing that opioids do not have a dose ceiling). Understanding the meaning of dosing intervals was an issue for some (e.g. every 3 days). Utilising the wide variety of information sources was challenging and information printed on packs and inserts was too small for some to read.
OrganisingOrganising presented a host of issues because of the sheer number and various forms of medicines prescribed for regular use, p.r.n. (as required) or both. Participants used a wide range of highly individual strategies: elaborate daily rituals involving, for example, zip lock bags; differentiation of medicines by colour; lining up of medicine bottles with magnifying glass; taking out pills and putting them into a glass. Lack of organisational systems presented safety risks such as medicines sitting out in glasses:
Participants used their home environments for pain medication management in highly individualized ways. Countertops, drawers, tables, windowsills, cabinets, boxes, bags, dishes, alarm clocks, whiteboards, computers, and mobile devices were all used. Individuals and pets living in the home were taken into account. Visitors were a consideration, especially visiting grandchildren.
Schumacher et al. p. 78753
Storing
This refers to putting medicines safely away. Hiding medicines from patients was a strategy used when carers feared that the patient would get confused and take too much. Storing medication generally involved hiding them and locking them up.
SchedulingThis refers to working out the best time to take medicines in relation to daily lifestyles. Some used pain as a cue to take next regular dose earlier than scheduled, rather than take rescue medicines; this fitted with the mindset of taking medicines only when pain is present.
RememberingRemembering use of dosette boxes helped but this was not failsafe. All affected by fatigue, drowsiness and confusion.
TakingThis was straightforward for most, but some experienced challenges (e.g. erroneously cutting sustained-release pills).
Theoretical underpinning of the intervention
The literature-scoping work informed the selection of theory focused on self-efficacy and behaviour change to best suit the developing intervention. Self-efficacy is a key component of Bandura’s social cognitive theory. 54,55 The theory of social cognition states that knowledge acquisition can be directly related to observing others within the context of interactions and experiences. According to this theory, self-efficacy is the belief in an individual’s capabilities to organise and carry out courses of action to manage situations. Bandura states that behavioural techniques can be used to target the four sources of self-efficacy: mastery experience, role modelling, verbal persuasion and the regulation of physiological and affective states. Michie et al. 39 undertook a systematic search and consultation with behaviour change experts to identify frameworks of behaviour change interventions. These were then evaluated for comprehensiveness, coherence and a clear link to a model of behaviour. A new framework was subsequently developed to fully meet all these criteria: the behaviour change wheel. The wheel characterises behaviour change interventions around nine intervention functions aimed at addressing deficits in one or more of three essential conditions for behaviour change: Capability, Opportunity and Motivation (termed the COM-B system). The behaviour change wheel identifies three main target constructs (sources of behaviour), which are capability (physical and psychological), motivation (automatic and reflective) and opportunity (social and physical). Around these target constructs are nine intervention functions (education, persuasion, incentivisation, coercion, training, enablement, modelling, environmental restructuring and restrictions), which represent ways to address deficits in one or more of the target conditions.
Key learning from studies conducted by the team
The intervention development was also informed by key learning from two studies undertaken by members of the research team. These were Cancer Carer Medicines Management (CCMM),56 funded by Dimbleby Marie Curie Cancer Care, and Improving the Management of Pain from Advanced Cancer in the Community (IMPACCT), a programme grant funded by the NIHR (RP-PG-0610-10114).
The SMART intervention drew on carer needs for medicines management at the end of life identified from the CCMM study (see Appendix 3), including needs for information about pain medicines and side effects, changing beliefs about opioids and providing skills and opportunities for self-evaluation. The results of the CCMM study also showed that a conversation-driven intervention, delivered by nurses in routine practice and supported with a toolkit of resources, was acceptable to carers and to nurses and was compatible with their existing practice. CCMM showed some evidence of benefit in influencing carers’ knowledge, beliefs and behaviours related to management of pain medicines. Principles of the CCMM conversational process (assessment, education and review), as well as elements of the toolkit (information leaflets, medication chart, pain diary and contact details for local and national services), informed the development of the SMART intervention.
Learning from the IMPACCT study was derived from a review of the optimal components of educational interventions for advanced cancer pain, which was carried out as part of the study (see Appendix 4). In this meta-review,57 the authors used Michie et al. ’s39 behaviour change wheel as theoretical underpinning. Mapping findings from six reviews and two papers led to identification of five out of the nine behaviour change wheel intervention functions. These were considered essential for successful interventions:
-
education, for example providing written information about pain management, including analgesic and non-pharmacological approaches
-
training, for example providing instruction, demonstration and coaching of new skills (techniques for managing daily drug regimes, relaxation techniques)
-
enablement and persuasion, for example overcoming cognitive and emotional barriers to pain management through addressing concerns about tolerance or addiction
-
environmental restructuring and resources, for example incorporating the delivery of education for self-management into the usual care provided by specific health professionals, such as specialist nurses, primary care practice nurses and community pharmacists
-
modelling, for example patients talking to other patients about their successful use of various pain management strategies.
Phase II: refining and detailing the intervention
This section describes the work undertaken to refine and detail the intervention. In particular it describes a series of interviews and focus groups conducted with patients, carers and HCPs. These data sources, together with work described in the previous section, were used to derive the content and form of the intervention for SMART.
Aim
The aim of this phase of the study was to refine and detail an intervention for self-management of analgesia (opioids) and related treatments (for nausea, constipation and drowsiness) at the end of life.
Objectives
The literature scoping informed the objectives and these were to:
-
explore views regarding the nature and components of supported self-management regarding analgesia and related treatments at the end of life, and test our preliminary model of self-management in this context
-
introduce participants to, and ask for their views on, aspects of self-management in this context – in particular our selected definition of supported self-management in palliative care (Johnston et al. 51) and practical difficulties regarding supply and medicines taking encountered by patients (Schumacher et al. 52,53) (see Table 2).
For HCPs, the objectives were to:
-
reveal the self-management promoting activities and behaviours already used by specialist palliative care HCPs and gain views on the professional supportive self-management roles proposed by Johnston et al. 51
-
rehearse previous examples of interventions to test possible delivery modes and how these could link to existing patterns of practice.
For patients and carers, the objectives were also to:
-
understand what supported self-management at the end of life was for them
-
explore patient and carer needs using the framework of Schumacher et al. ’s52,53 commonly encountered practical difficulties regarding supply and the taking of medicines
-
gain views regarding potential options for the content, form and delivery of the intervention.
Approach to data collection
Focus groups and interviews were held in two geographical regions: Hampshire and Yorkshire. They were conducted with patients, their carers and specialist palliative care health professionals (including service managers and commissioners).
Inclusion criteria
Patients were included if they:
-
were aged ≥ 25 years and considered to be in the last year of life
-
were experiencing pain
-
were being treated with, or starting, opioid analgesia
-
were experiencing, or anticipating, adverse effects of nausea, constipation and drowsiness
-
were living at home
-
were being cared for by specialist community-based palliative care services in Hampshire or Yorkshire
-
had capacity to consent.
Carers were included if:
-
they were the primary carer of a patient meeting the above inclusion criteria and
-
the patient gave consent to their involvement.
Health-care professionals were included if they were:
-
CNSs and doctors who were part of specialist palliative care teams or
-
service providers or managers of specialist palliative care services or
-
local commissioners of palliative care services.
Sampling strategy and recruitment
To access a range of individuals (HCPs, patients and carers), we aimed to recruit 35 participants via various strategies across four hospices and two acute NHS trusts. The sampling and recruitment strategy is detailed in Table 1.
| Hampshire | ||
|---|---|---|
| HCPsa | ||
| Acute trust 1 + hospice 1: eight hospital-based CNSs, six consultants and one specialist registrar were invited to participate. Of these, two hospital-based CNSs attended the focus group. 18 community palliative care CNSs were invited to participate, five attended the focus group that occurred at this hospice | Hospice 2: staff across the hospice were invited to attend. Three attendees participated in the focus group at this hospice and a further three travelled to attend the focus group at the other hospice | Acute trust 2: one lead nurse/commissioner was invited to take part in an interview and this took place |
| Patients and carersb | ||
| Acute trust: 17 patients were referred via a hospital-based palliative care team, all except four were approached by the researcher (seven did not meet the eligibility criteria). Two patients were recruited and interviewed | Hospice 1: 39 patients and carers attending day hospice sessions were informed about the study. Five patients and two carers (who met the eligibility criteria) approached the researcher after these sessions and four patients and two carers were recruited (n = 6) | Hospice 2: six patients were referred by day hospice staff as meeting the study’s eligibility criteria. All were spoken to by the researcher and five patients were recruited, with two carers (n = 7) |
| Yorkshire | ||
| Focus groupsc | ||
| HCPs: an entire community palliative care CNS team based at a hospice were invited to take part in a focus group and all attended on the day (n = 4). One palliative care consultant was invited to take part in an interview and this took place | Patients and carers: 10 eligible individuals were approached via a hospice outpatient clinic. Of the 10, four attended the focus group, four had agreed to participate but failed to attend, and two declined | |
Focus group and interview guides
Topic/interview guides (as well as supporting slide/card packs) were developed to meet the study objectives (see Appendices 5–8). A number of tools were used to manage the interview and focus group discussions:
-
a definition of supported self-management in the context of palliative care (Johnston et al. 51)
-
eight proposed professional roles to support self-management in the context of palliative care (Johnston et al. 48)
-
the practical difficulties around supply and medicine taking encountered by patients and carers in the work of Schumacher et al. 52,53
-
the content and form of previous interventions to improve pain management by patients. 58–61
The interviews and focus groups were conducted by the study’s researchers. The focus groups were conducted with a co-facilitator another researcher with expertise in the field, present to aid moderation.
Data analysis
The audio files from the interviews and focus groups were professionally transcribed. They were listened to by the researchers alongside the transcripts to check for complete accuracy. Both study researchers familiarised themselves with the data by reading and rereading the transcripts and identifying key issues, concepts and themes. Initial coding took the form of indexing on the transcripts, and each research fellow summarised the key themes from the data related to the sites in their regional area. The themes were discussed for comparative purposes. Natasha Campling then coded the entire data set for all issues, aspects and themes that were relevant to supported self-management in this field. Coding was performed in NVivo (version 11; QSR International, Warrington, UK) utilising framework analysis. 62
The development process
The process of intervention development is illustrated in Figure 1. The contextual work and literature scoping informed a preliminary model of supported SMART. This model, a definition of self-management,51 practical difficulties related to supply and medicine-taking52,53 and the content and form of previous interventions58–61 were used as tools within the focus groups and interviews to gain participants' views. The result was development of a concept of self-management, to inform the required components (including the content and form) of the intervention: a four-step educational approach and toolkit, plus a training package to enable nurses to deliver the intervention within a feasibility study.
FIGURE 1.
Intervention development process.

Findings from the intervention development interviews and focus groups
The sample
The sample was composed of 38 participants recruited via the two geographical regions of Hampshire and Yorkshire (Table 2). The demographics for the HCPs and patients and carers are presented in Tables 3 and 4.
| Patient and carer sample (n = 19) | HCP sample (n = 19) |
|---|---|
| Yorkshire | |
| 1 focus group, n = 4 patients | 1 focus group, n = 4 CNSs |
| 1 face-to-face interview, n = 1 consultant | |
| Hampshire | |
| 11 interviews, n = 11 patients, n = 4 carers | 2 focus groups: n = 10, 9 CNSs + 1 specialist registrar; n = 3, 2 inpatient unit nurses, 1 lecturer/practitioner |
| 1 telephone interview, n = 1 lead nurse/commissioner | |
| Overall total | |
| 38 participants | |
| Demographic characteristic | HCPs, n (N = 19) |
|---|---|
| Sex | |
| Female | 18 |
| Male | 1 |
| Professional background | |
| Nursing | 17 |
| Medicine | 2 |
| Main working environment | |
| Hospice inpatient | 4 |
| Hospice education | 1 |
| Community | 10 |
| Hospital | 2 |
| Community and day hospice | 1 |
| Hospital, hospice and community | 1 |
| Length of time in current post | |
| Years, mean (range) | 7 (0.5–24) |
| Length of time in palliative care specialism | |
| Years, mean (range) | 13 (1–27) |
| Demographic characteristic | Patients, n (N = 15) | Carers, n (N = 4) |
|---|---|---|
| Sex | ||
| Female | 7 | 4 |
| Male | 8 | 0 |
| Age | ||
| Years, mean (range) | 66 (47–84) | 69 (52–80) |
| Cancer site | ||
| Bile duct | 1 | |
| Breast | 1 | |
| Colon | 1 | |
| Lung | 4 | |
| Lung (pleural mesothelioma) | 1 | |
| Oesophagus | 1 | |
| Pancreas | 1 | |
| Prostate | 3 | |
| Skin (melanoma) | 1 | |
| Uterus | 1 | |
| Educational level | ||
| Degree level or above | 4 | 2 |
| Below degree level | 6 | 2 |
| No qualifications | 5 | |
Concept of supported self-management in analgesia and related treatments at the end of life
The development process, informed by the varied sources of learning outlined in Evidence synthesis, enabled the generation of a preliminary model of supported self-management in analgesia and related treatments at the end of life. The model was tested, within this specific context, through the interviews and focus groups with patients, carers and HCPs. The model included the self-management definition, professional roles outlined by Johnston et al. 51 and the practical difficulties regarding medicines access, supply and taking outlined by Schumacher et al. 52,53
This process revealed new components of supported self-management within the end-of-life context. It displayed an ever-changing process enacted on a continuum of behaviours dependent on the responsibility taken by the patient, carer and specialist nurse. This was a complex web of behaviours, varying day by day, if not hour by hour, within this context. With continual disease progression, there were frequent changes in symptoms and side effects from both medication and palliative treatments, with behaviours profoundly affected. This context was complicated by the surrounding ‘swirl’ of what individuals and their families were already striving to deal with, the wider context of psychological distress and high levels of carer strain. Individuals in this context could be struggling to cope with a palliative care diagnosis and there was anxiety and clinical depression of both patients and/or carers. Consequently, the capabilities of the patient and carer fluctuated greatly, influencing supportive self-management roles and the required behaviours of the specialist nurse.
The concept is presented here as it is key to understanding the development and form of the intervention. The key components of the concept, the issues of responsibility and the supported self-management roles of the patient, carer and CNS are outlined. Figure 2 is a diagrammatic representation of the concept.
FIGURE 2.
A concept of supported SMART.
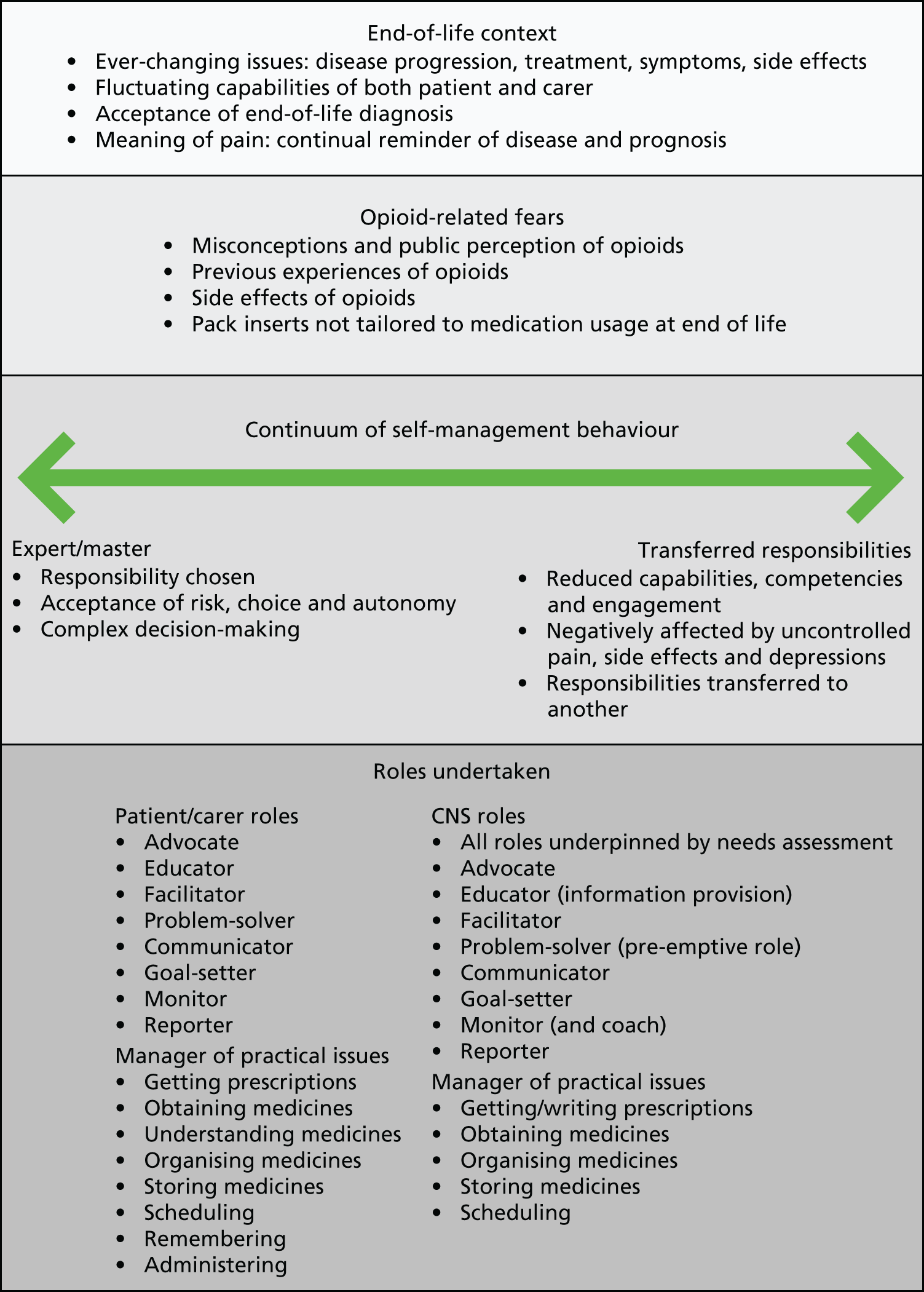
Patient roles
Some study patients participated in managing their medicines almost entirely themselves; however, this was the experience of a small minority. Those who had nurse specialist input and/or a carer who played a role in supporting self-management enacted their own roles differently as a result, leading ultimately to a change in their resulting behaviours. This was the experience of the majority of the patient sample. The roles undertaken by patient participants are mapped to the roles to support self-management proposed by Johnston et al. 51 in Table 5.
| Johnston et al.51 roles to support self-management | Intervention development interview and focus group participants | ||
|---|---|---|---|
| Patients | Carers | CNSs | |
| Advocate | For themselves (e.g. requesting alternative opioids/forms if side effects are not acceptable) | Total advocacy role when needed | Right type and route of drug |
| Educator | Of carer if required, anticipation of future changes (i.e. planning for worsening condition) | Of patient and CNS when needed | Refining knowledge for individuals, providing instruction |
| Facilitator | Of relationships/access to medicines (e.g. GP, HCPs and carer, carer and community pharmacist) | Manager of the practical supply and medicine-taking issues when needed | Preparing for transitions, ‘home feels like you have not got someone to speak to just around the corner’ |
| Problem-solver | Access to medicines and navigating the supply system, side effects management and off-setting doses | Pre-emptive (e.g. regarding stock management or suggesting need for breakthrough analgesia) | Best drug and side effect profile for individual, sorting out when supplies get in a muddle, pre-emptive problem-solving (‘always have a plan B’) |
| Communicator | Of relevant information to all – family and HCPs | ‘Is it helping?’ Encouraging discussion with patient | Picking style of communication for individual, knowing the family and patient (mediator as well as communicator) |
| Goal-setter | Self-planning, planning with a GP or joint planning with CNS | Often in relation to getting out and about (e.g. getting out of the house for a coffee, going to a favourite place) | Proposing options and allowing the individual to decide what they would prefer and putting a plan together |
| Monitor | Writing down of breakthrough doses and noting effectiveness | Diary recording | How much information has been understood? Monitoring involvement of patient in decisions and reviewing effectiveness of medicines |
| Reporter | Of relevant symptom experiences and side effects | Evaluation of the effectiveness of the medications | To wider palliative care team and GPs |
The patients often played an advocacy role on their own behalf, for example requesting alternative analgesics/opioids where they found the side effects unacceptable and they were unable to manage them. They also educated their carer, if they had one, regarding their medicines so that if their condition changed or they had a bad day then they could rely on them to safely administer their medications. This often took the form of listing their medications, creating a simple timetable of what they took and when and keeping this in a location within their home that could be easily referred to. This aided their communicator role whereby they transferred relevant information regarding their medicines, their effectiveness and their experience of side effects to HCPs [particularly general practitioners (GPs) and CNSs]. Those patients who were under the care of a community palliative care CNS often set joint plans/goals with their CNS, whereas others not under the care of a CNS made their own plans and goals and/or negotiated these with their GP (e.g. coming off a neuropathic agent because of unacceptable side effects).
In addition, the patients facilitated relationships with their HCPs and carers so as to aid access to their medicines. Patients worked at relationships with those who were key to managing their medicines and supporting their self-management: CNSs and GPs, as well as community pharmacists. They often found that knowing their pharmacist aided the supply and stocking of their medicines, resulting in them obtaining their medicines quickly and without delays in the system. At times, pharmacists put in repeat prescription requests for patients because of these relationships, meaning that the patient then just had to arrange to collect the medications from the pharmacy or could use pharmacy delivery services, when available.
The role of facilitating/managing the practical issues related to supply and medicine-taking was frequently an onerous one for patients. They had to get prescriptions, obtain the medicines, understand them once they had been dispensed, organise the medicines at home to keep track of them, store them, schedule them around their routine, remember to take them and, finally, actually administer them.
The patients played a problem-solving role navigating the difficulties in the medicines supply system. They also problem-solved the side effects of their opioids, making decisions to appropriately balance the benefits of pain control with a manageable level of side effects. This was an individual balance (e.g. titrating laxatives on a daily basis to offset the common side effect of constipation). Others for whom nausea was an issue titrated antiemetics on a daily basis. The side effect of drowsiness led some to delay doses or take smaller doses to minimise this. Furthermore, at times a small minority of expert self-managers made complex decisions regarding the dose of opioid to take when it was prescribed within a range.
The patients monitored their symptoms, side effects and the effectiveness of their medicines, often keeping their own records of this, particularly in relation to the administration of ‘as required’ doses for breakthrough pain. This was facilitated by the input of community palliative care CNSs or GPs, who prompted patients to consider how much were taking, when they were taking it and how they found it (H2HCPfocusgroup). As a result, the patients were often in a position to accurately report their relevant symptom and side effect experiences, and changes, to their HCPs.
Carer roles
The supportive self-management roles of the carer fluctuated in relation to changes in the competencies and engagement of the individual patient. However, a few patients had always handed over responsibility for medicines management to their carer: ‘she just always did it . . . I tend to be . . . not worried enough about it you know. I basically need looking after that’s the truth of the matter’ (H1Pt004). The roles undertaken by carer participants are mapped to the roles to support self-management proposed by Johnston et al. 51 in Table 5.
Carers often took on an all-encompassing advocacy role for the patient, particularly when difficulties arose with challenging side effects or poorly controlled pain. Advocacy took the form of working or facilitating the supply system in relation to managing all the practical issues of getting prescriptions, obtaining the medicines, understanding the medicines, organising the medicines in the home environment to keep stock of them, storing them safely, scheduling them around the patient’s routine, remembering (i.e. reminding the individual to take the medicines) and actually administering the medicines if required.
Facilitating and advocating on behalf of the patient in relation to obtaining the medicines was complex, onerous and a hugely time-consuming process for many carers. One patient outlined his difficulties (lengthy delays) in obtaining his fentanyl patches through a non-palliative care specialist pharmacy. This left his wife needing to make in-person visits to speak to the pharmacist on his behalf, only for her to be equally frustrated and leave the pharmacy without the patches, in tears, because she could not answer the question ‘Who’s prescribed these?’:
I’m not sure if it’s the chemist . . . when I rang through and said ‘Here look, what about these patches?’ and the woman said ‘What are they?’ And then I said to her – and she said ‘Yes, well we have got them down on the list, but I don’t know where they are.’ So in actual fact, on like that again . . .
If you lived alone, somebody very elderly . . .
Yes, you need someone to work the system.
Yes! Yes!
As with the various roles of the patient and CNS, the carers’ roles were complexly interwoven. The facilitator role for carers, as in the following example, was one of monitoring pain and the effectiveness of medicines via a pain diary. In turn, this facilitated the administration of analgesia by the carer to the patient, creating the end result of confidence and control over the situation:
. . . I would take an example actually it was quite difficult, as a facilitator it’s not quite between health-care professionals and patient, it was actually between patient and carer. And it goes back to what we were saying earlier on about carers being reluctant to give it [morphine] or patients being reluctant to take it. And it’s where a diary was very useful ‘cos it actually empowered the carer to feel that she was doing something useful to help with her spouse’s pain but also that she felt more in control that she wasn’t giving it more often than she should do or she was writing that it had an effect or didn’t have an effect and where they were going with it. And she was far happier to give it if she was documenting things than just on his say so that ‘I’m in pain’, and he was saying that ‘I’m in pain and she won’t give it to me’ . . . She admitted she wasn’t giving it because she was frightened she was going to be giving too much and how would she know when to stop and the diary actually facilitated being able to give [it], it was confidence . . .
H1HCPfocusgroup
Carers often played a monitoring role highlighting and watching for condition, symptom and side effect changes. Indeed, their monitoring was often astute because of the acuity of observation by someone who knew the patient best. As a result, carers could play an educator role of both the patient and CNS, highlighting these changes as required. This linked closely with their communicator and reporting role, as carers often aided the monitoring of the effectiveness of medicines by asking the patient simple questions such as ‘Is it helping? Does that help?’:
. . . And every so often, I say to you don’t I? ‘How are you on the laxatives?’ And it seems ridiculous doesn’t it, because . . . that’s the best [thing] that’s happened, is you’ve managed to get it [opioids vs. constipation] at a level which is not a problem for you haven’t you . . . ?
Carer-H2Pt004
Consequently, the carers encouraged discussion with the patient and could report this information to HCPs, the CNS and GP as required.
The carers often took a lead in establishing small goals for the patient that they knew were of importance to the individual. With effective medicines and side effect management, goals frequently set were in relation to getting out of the house and continuing to visit favourite places for the individual.
The role of problem-solver was arguably the greatest of the roles played by the carer. In the words of one carer, ‘I try and stop problems happening’ (Carer-H2Pt004). As with the CNS problem-solving role, this was in the main pre-emptive carer role, resolving potential problems. This was particularly the case in terms of asking the individual about their pain, so as to be able to administer ‘as required’ analgesia. Carers also pre-emptively stock managed, requesting medicines before they ran out, and chased GP practices for prescriptions and pharmacies if medications had not been dispensed as requested (e.g. in a different form or were missing).
Clinical nurse specialist roles
In order to evaluate which roles were required, and at what point, the nurses assessed the competencies not only of the patient but also of the carer. The roles undertaken by the CNSs are mapped to the roles to support self-management proposed by Johnston et al. 51 in Table 5. It was recognised that nurses’ provision of supportive self-management roles would fluctuate in relation to patient and carer needs and that at times the roles would be challenging:
. . . I think all of these [roles] will probably peak in difficulty, at times depending on the situation. As a professional, there could be a nightmare sometimes, in a person’s home advocating for that patient, if . . . you have a family who have distinct feelings that are opposing the patient, that’s really . . . difficult. The monitoring, there will be times when that, even on the inpatient unit, that’s got to be a challenge at times, depending on the complexity of the patient, and the capacity of the staff, and staffing levels . . .
H2HCPfocusgroup
Within this challenging context, the nurses emphasised the importance of ensuring that the individual patient had the right drug via the right route. For them this was a clear role of advocacy:
I met a lady with head and neck cancer that was really compromising her mouth and she was just starting on opiates, thought patch that’s going to be the best way to go . . . Spoke to the GP who said fine, then they changed their minds and went back to the tablets because someone in the practice had gone on a palliative care course and was told that MST [Morphine Sulfate Tablets modified release] it’s cheap and cheerful start everyone on that. So then the relative rings up we’ve just gone to collect the patches and it’s tablets so I’m like ‘Oh god’ ring up again, ‘there is a reason why we said patches, I know they’re expensive but she can’t open her mouth’. So like ridiculous!
It’s about being that patient’s advocate isn’t it . . .
The supportive communicator role was vital to the nurses and they emphasised the complexity of communicating well to ‘the agenda of the patient’ using language that would be understood, while highlighting ‘what they need to know, because they might not be interested in all the things that you want to say’ (H2HCPfocusgroup):
. . . I think you have to really pick your style of communication with each individual, this is what X was saying about knowing your family, knowing your patient, ‘cos sometimes you are as much a mediator as communicator. We can sometimes have a relative that just simply doesn’t believe in morphine . . . they will withhold it from them . . . And then others where they will perhaps give a little too much, then you have to sort of be kind in how you say these things, because they want to make it better . . . Yes, so communication is quite hard; you have to get that right, don’t you . . . ?
H2HCPfocusgroup
All the supportive roles of the CNS interlinked and overlapped, particularly that of communicator and educator. This role of educator was viewed as one of providing ‘instruction and information regarding medicines’ (H2HCPfocusgroup). The ever-increasing role of the internet as a source of information for patients and their families was also recognised so that the supportive role of the nurses was often seen as one of helping to ‘refine’ this knowledge for individuals. The need to provide education for carers specifically was viewed as important, but it was argued that these supportive needs may not be met in practice:
. . . You get carers who the knowledge gap is so huge for them, they want to help they want to know what to do and we need to be filling that knowledge gap for them appropriately . . . I think for the carer what they want is the right information and we don’t currently meet that need I don’t think. We try . . .
H1HCPfocusgroup
In order to meet the informational needs of patients and their carers (within their educational role) the nurses recognised that a number of issues needed to be identified and then addressed. In summary, they were:
-
the starting point, working out how the individual best learns and then tailoring the information to this; verbal information reinforced by written information (+ technological alternatives if possible) at the right pace, via stepwise provision
-
identifying the types of pain and which medications are best suited to the types of pain for that individual
-
outlining each medicine, what it is, what it’s for and how to take it
-
explaining the requirement to adjust medications on an ongoing basis and establishing this as baseline understanding; highlighting that there are always alternatives if pain is uncontrolled or side effects are viewed as intolerable
-
informing on the side effects, the benefits versus the burdens and the likelihood of the individual experiencing them
-
outlining the need for laxatives and working out the balance between opioid dosage and laxatives required for the individual
-
discussing and revealing the individual’s fears, challenging and correcting opioid-related preconceptions
-
explaining the lack of dosing ceilings for opioids and being clear regarding the relative lack of required dosing intervals for ‘as required’ doses for breakthrough pain
-
highlighting the importance of monitoring the effectiveness of the medications (especially in relation to the pain experience); the need to record breakthrough doses so that regular opioid doses can be increased/altered if required
-
signposting the individual and carer to contacts for concerns/questions, outlining the most suitable contacts for specific situations that the individual may encounter.
Within their problem-solving role, the nurses sought to work out the best drugs and dosages with the most tolerable side effect profiles for the individual, recognising that this required fine tuning over time, often in conflict with the end-of-life context. The nurses also assisted the patients/carers with problem-solving in relation to the practical (supply and administration related) issues of getting prescriptions, obtaining the medicines, organising the medicines at home, storing the medicines safely and scheduling the medicines around their daily routines. For example:
. . . Getting prescriptions . . . we spend a lot of our time trying to sort that out, and you can understand how patients really struggle with [it]. I mean one chap . . . it has taken so many phone calls and so much of my time . . . a youngish intelligent chap and he has just really struggled with that. I think the other issue is sometimes they get 28 tablets and then you change them, then that knocks their whole sort of repeat prescription out of balance . . . When you’re wanting dosette boxes as well they’re really difficult, they’ve already started, then you’re adding something in and changing them, to add bits in, that’s really problematic . . .
H1HCPfocusgroup
This could be a time-consuming role for nurses as medication supplies got ‘out of sync’ for patients with any prescription alteration. For example, increases in dosage meant that supplies lasted for shorter periods and ran out in advance of supplies of other medications.
The problem-solving role was often implemented in a pre-emptive way. This was referred to as ‘mind-reading’ or being ‘a problem-solver in advance’, which necessitated always having ‘a plan B’ (such as knowing who to contact or consideration and education of the individual patient in relation to potential crisis episodes, e.g. chest inflections or bowel obstruction):
. . . You’re anticipating, you’re pre-empting what might happen to be able to talk it through with that patient and to that carer to be able to give them you know a toolkit of who to ring, when to ring and why they might ring. How to deal with the uncertainties of do I ring now, do I ring later, but the security of knowing that there is somebody to ring . . .
H1HCPfocusgroup
The professional participants stressed the imperativeness of this pre-emptive problem-solving role and the fact that it mirrored the wider requirements of end-of-life care in general:
. . . The notion of the discussion about symptom control and analgesia and medicine things, the pre-emptive nature of it links in with the much bigger picture, doesn’t it, I think of palliative and end of life care now. The fact that all of it is about pre-empting and pre-planning, advance care planning, you know the Gold Standard Framework; getting people on a register and pre-empting their kind of deterioration, all of those things. It mirrors in a more distilled way the bigger picture of things . . .
H2HCPfocusgroup
Indeed, part of the pre-emptive problem-solving role within the context of end of life was the requirement to prepare individuals and their carers for transition not only in terms of deterioration of condition, but also in terms of transition between care settings, frequently between inpatient units/hospices and home. This could also be seen as integral to the role of facilitator, whereby the nurses prepared the patients and their carers for these transitions by enabling them to use inpatient stays ‘like a pit-stop’ where they could initiate, develop or refine their information and knowledge of medication management. For example:
. . . So part of it before they go home is about talking through what our rationale has been for their medicines and what type of pain we’re looking at to take for certain things. What we’ll have on the discharge sheet that goes with them, there’s like a medication chart of what their drugs are and why they take them, when they take them but you can be a little bit more distinct can’t we on the type of pains so they’ve got some slight signpost, as to what to take, where when and how . . .
H1HCPfocusgroup
Another professional role was that of goal-setting. This was often about proposing different options to the patient in relation to their medicines management, allowing the individual to decide between the proposed courses of action and then putting a joint plan together based on the individual’s preferences. These plans were then relayed and discussed with the wider palliative care team and GPs as needed (to support medication changes) under the role of reporter. Furthermore, the CNS role of monitor intermeshed in practice with the role of goal-setter (involvement in decision-making and shared responsibility where possible). The nurses continually monitored ‘how much the patient has understood’:
. . . Whatever you do, if you are setting goals if you are solving a problem, or if you are educating, whatever you are doing you have to check that . . . the message has arrived . . .
H2HCPfocusgroup
. . . In terms of monitoring as well I think it’s about involving the patient in those decisions isn’t it so you know having given them some education actually when you’re reviewing things you know saying to them so are you happy then that we’re still on the same dose for now, you know they’ve got that involvement in that haven’t they, it’s like an agreed shared sort of responsibility . . .
H1HCPfocusgroup
The monitoring role was seen as an imperative professional responsibility, particularly when starting individuals on new medications. The nurses also emphasised the value of face-to-face monitoring in the context of end of life. In the words of one:
. . . You can see people’s responses, you can work with them at their timing to answer questions. I mean one gentleman I went into, I talked to him about his medication, and reading his bottles, and actually I discovered he couldn’t read. And it was something as basic as that, making sure that . . . I then put symbols on there that he felt represented like his water tablet, I put a droplet of water on a little label on his bottle. So I think it’s a blended approach really, you know, just to phone them up, say ‘How are you doing?’ and if you sense that this is not going . . . The things you are listening to aren’t representative of somebody managing, then you actually go back and reassess them face to face; there is nothing quite like eyeballing a patient . . . !
HCPW001
The continuum of self-management behaviours
The patient, carer and CNS roles outlined above were enacted on a far-reaching continuum. This continuum of behaviours ranged from, at one end, expertise and mastery, with the individual taking full responsibility for complex decision-making, accepting the associated risks, to, at the other end, transfer of responsibility to another (the carer and/or CNS) because of patients’ and carers’ reduced capabilities and engagement in self-management behaviours, sometimes negatively affected by uncontrolled pain, the side effects of opioids (particularly drowsiness), clinical depression and memory loss:
I’ve seen both sorts . . . Obviously this is reflecting a distinct change in the type of patient we are seeing as well. I would say the younger ones coming along are becoming masters; they are gaining information from the internet, using every resource they have; they are thinking outside of pure medication as being an option for their pain control, and yes, I have seen . . . I can think now in my mind’s eye of a ‘master’. But prior to when I began in Hospice care, everybody needed to be told, and there remains a little group of people, usually in the older age groups that still need that and actually feel quite burdened by being expected to choose, and make decisions.
Very much so. There is a shift in people’s tendency. Before it was ‘You decide, you are the expert’, now it is ‘Wait a moment, I am the expert of my body and my health, so I . . . give me the knowledge, give me the information so that I can make an informed decision’.
Is it something that you negotiate with patients, whether you expect them to self-manage and master all these things? Or is it something that is sort of tacitly understood?
. . . You can tell, straight away when you start talking to them about what they understand and what they want from us; that comes across very quickly, which group they are going to fall into . . .
All study participants highlighted the individual-level variation in the range of self-management behaviours enacted:
. . . You’ll get some who don’t want anything to do with their medicines and you sort it out and then people that want to know everything will want to do their own as much as they can . . .
H1HCPfocusgroup
Those patients who, when discussing the role they played in managing their medicines, reported feeling in control, often referred to how relatively ‘lucky’ they were in terms of being able to ‘think about it and work it out’. These individuals at one end of the continuum had accepted full responsibility for their role and were in their eyes ‘doing it all’ themselves, but with backup strategies in place and knowledge of whom to contact if anything changed.
The HCPs often spoke about those individuals at polar ends of the continuum but there was also wide variation in behaviours and choices made by those individuals who were not at either end of the continuum. Indeed, behaviours and choices were never static, but ever changing. In addition, there was wide variation within the group of individuals assessed by their HCPs as ‘self-managing’ of their analgesia and related treatments. For example, one professional discussed a patient who was managing his medications, but without the adjustments that she ideally would have recommended:
. . . A man that’s really angry and frustrated, he’s young but he was diagnosed late. He’s had lots of frustrations with chemo, and things like that. So he’s quite resistant to changes, and that’s fine, so we’ve just left him [medication wise] as he is; he’s not managed quite properly, not adequately in our eyes, but he is doing what he wants to do at the moment, so he’s doing it . . .
H4HCPfocusgroup
Where does the responsibility lie?
The competencies and engagement of the individual, and their acceptance of responsibility, affected their enactment of self-management behaviours and, thus, the roles required of the carer and CNS. The CNSs recognised the importance of assessing the individual’s ability to understand, their capabilities and their potential engagement (what the individual was currently doing and what they would like to do):
. . . Asking them to go to through their medicines, some people haven’t got a clue, and other people don’t even need to get the boxes or list out and they can tell you absolutely everything they’ve had, and they’ve got lists and the diary, and it all written down. And other people haven’t got a clue.
As well as trying to establish what level of understanding they’ve got, you are using that information about how much they have engaged with their medicines and things to try and determine the scope for them to self-manage . . .
For the nurses, it was about recognising that individuals make their own analgesia-related choices within their home environments and that their autonomy to do so should be respected:
. . . I always say that it’s their pain and it’s up to them how they choose to manage it we can give them the medications or the tools but you know ultimately if they’re happy to live with a certain level of pain, they don’t want to use their medications, that’s their choice we’re there just to give them and advise them how to use things but ultimately it’s up to them . . .
H1HCPfocusgroup
Indeed, some patients made deliberate decisions to withhold or reduce doses of opioids to offset the side effects that they were experiencing. These decisions were about balancing pain control against the things that they wished to achieve:
. . . So if I’ve got pain, sometimes I won’t have that [oxycodone immediate-release formulation; OxyNorm, NAPP Pharmaceuticals], and I choose not to, because I don’t want to get tired and sleepy as I want to drive, or I want to do something, so I’ll manage it in a different way, not necessarily by taking drugs . . .
H3Ptfocusgroup
The context of end of life
The overarching context of end of life had a profound influence on the supportive self-management behaviours of the patient, carer and CNS. Within this context, there were ever-changing issues related to continual disease progression and subsequent changes in symptoms and side effects from both medication and palliative treatments. As a result, the behaviours were ever changing.
This context was further complicated at the end of life by the surrounding swirl of what individuals and their families were already striving to deal with (the wider context), and of psychological distress and anxiety as well as high levels of carer strain. Thus, individuals in this context may be struggling to cope with a palliative care diagnosis and there may be anxiety and potential clinical depression of both patients and/or carers. As a result, the capabilities of both the patient and carer fluctuated greatly, influencing the supportive self-management roles and the required behaviours of the CNS in particular.
Opioid-related fears
The data demonstrated that patients’ and carers’ behaviours in relation to opioid management were strongly affected by misconceptions and common public perceptions of these medicines. It was commonplace for patients and their carers to hold fears or assumptions regarding opioids:
-
fear that the individual taking them will become addicted to these medicines, ‘you hear of so many people get[ting] addicted to certain things’ (H1Pt004)
-
assumption that there is a ceiling dose for opioids as with other medicines
-
fear of overdosing, even by taking just one extra dose
-
fear that these medicines are ‘killers’ because of press accounts of abuse of opioids (H2HCPfocusgroup)
-
assumption that the individual will develop a tolerance to the opioids and the pain will therefore not be controlled as a result
-
fear that death of the individual is imminent if they are started on opioids [i.e. ‘I’m dying’ (H1HCPfocusgroup)]
-
fear that if the individual takes these medicines now then there is ‘nothing later’ for them to take in the future ‘so I’ll avoid it if I can’ (H1HCPfocusgroup).
These fears and assumptions affected the self-management behaviours of patients. This was clearly articulated by one focus group participant who required a hospice admission as a result:
. . . My self-management wasn’t terribly good, that’s why I ended up in here [the hospice] in the first place. But it wasn’t to do with side effects, because I don’t actually suffer particularly bad side effects. It’s more to do with the psychological attitude that I was taking morphine, and that I didn’t want to over-take and the doctors kept saying to me ‘No we are giving you such small doses you can self-medicate, you can up this to 5 mls, etc.’. So it was getting my head around that, because I’ve spent a lifetime of being the sort of person that just carries on; never go to the doctors, that’s why I ended up here! You know, I’ve never been one to take a lot of painkillers, and now I’m sitting here, I’ve got lower back pain, and I could do with some morphine! But I just thought ‘Oooh, I’ll just wait till after this study [focus group]’, that’s the sort of person I am. So it’s been sort of, just ‘cos I’m strong, a Yorkshire woman that doesn’t like to admit failure or weakness, I think that’s been the main issue with me . . . At the time I was taking . . . morphine, and it was for pain relief. And I had the slow release, and that was OK, every 12 hours. And then if I needed to have the little extra, you know, I was the same as the other ladies, ‘Oh I don’t think I’ll be taking any of that!’ you know. Until somebody did once say to me one day ‘You know you are on a very low dose you know’, and I thought ‘Ooooh! How dare you!’ I thought I was a hard drug taker!
H3Ptfocusgroup
The HCP also highlighted that carer-held fears could be projected onto the patient, negatively affecting self-management behaviours:
. . . sometimes the fears of the relatives project on to the patient. The patient might start out all right, and then the minute they say to their nearest and dearest, or the next door neighbour ‘I’m on this now’ and then they just get all these horror stories, and then you’ve got that to kind of circumvent as well. So you know, it’s never black and white . . .
H4HCPfocusgroup
Arguably, the most common fears related to opioid usage were not the ones referred to above, but were related to the side effects of the medicines themselves:
. . . I think probably the greatest fears are not so much the addiction but sedation or constipation and again it’s a reluctance, ‘I won’t take it unless I need to ‘cos I don’t want those effects’ . . .
H1HCPfocusgroup
Patients referred to being reluctant to take opioids for fear of both constipation and drowsiness. The fear of constipation and the subsequent difficulties in balancing doses of laxatives with opioid intake was particularly troublesome for some. A number of the sample had accounts of faecal impaction requiring admissions; as a result, the fear of constipation was profound:
My main concern is that if I get some pain, I take extra morphine. I’m on a patch at the moment, so if I change the dose of the morphine, I have to change the dose that I take of the laxative. And of course, the first time ‘oh yeah, OK, let’s bang it up by another one of the sachets’. And of course I was then for the next 2 days on the loo! So ‘oh let’s cut it down’, by which time ‘Oh god I haven’t been to the loo now for 2 days!’
H2Pt004
I’ve never before seen people so frightened of constipation, as he has been.
Carer
The SMART intervention
The development process and resulting conceptual work described above generated the SMART intervention that comprised both a four-step educational approach and a SMST. The intervention was designed to be delivered via a feasibility study to patients by community-based palliative care CNSs. The approach to delivery involved nesting the intervention in a clinical encounter (nurse–patient consultation) and was enacted through a conversational process.
The four-step educational approach
The four-step educational approach was maintained from the study protocol, with data confirming that such an approach was an appropriate way to proceed. Our contextual work had identified that needs assessment, information provision and regular review were important components of a supported self-management intervention that would potentially fit well into nurses’ usual practice processes. Goal-setting (with associated action plans) was proposed following expert educational psychologist input (SM) as the mechanism by which information provision could lead to behaviour change. In addition, a meta-review of self-management interventions for long-term conditions identified that action plans for deteriorating conditions were a key component of successful interventions. 21 The approach was designed to sit alongside the everyday practice of specialist nurses, with four cyclical steps to occur at each nurse–patient visit.
Step 1: needs assessment
A detailed assessment of patients’ needs is part of usual specialist practice and was intended to take place at all SMART study nurse visits between the CNS and patient. The CNSs were to identify specific concerns and needs related to self-managing opioid medicines at home, such as:
-
fears and concerns related to taking opioids and their side effects
-
self-management capabilities and issues preventing supported self-management of opioids at home (e.g. getting prescriptions and obtaining medicines or dealing with breakthrough pain)
-
relevant contact information for further advice and information.
Step 2: information provision
Verbal information reinforced by the provision of SMST resources. Following needs assessment, the CNSs were to discuss any issues raised and use the SMST to provide tailored, staged and relevant educational materials via the study resources (outlined below).
Step 3: goal-setting
Development of a self-management focused action plan. The third step aimed to guide patients towards developing self-management focused goals at each visit. CNSs were to help patients identify goals that were achievable through a set of actions that could be reviewed and modified at each visit.
Step 4: review and coaching
Review of the action plan and provision of support. The intent was for the trial CNSs to review patients’ self-management capabilities and progress at each visit and provide coaching and support to develop, maintain and improve self-management behaviour. This was planned to involve the reviewing of self-management goals and the evaluation of action plans. Nurses were also to focus on identifying barriers for individuals to meet self-management goals or steps in their action plans, suitable supported self-management strategies for the trial patient and/or carer and problem-solving techniques. Follow-up and reinforcement of information and self-management strategies were planned to reflect usual patterns of and forms of contact and review (i.e. face to face and via telephone).
The self-management support toolkit
The evidence from the data was mapped to components of the intervention (both the educational approach and proposed SMST resources), resulting in the framework outlined in Table 6. The framework also linked the intervention components to the target participant understanding and behaviour in relation to self-management, including the behaviour source,39 the target supportive self-management roles of the specialist nurse,51 the self-efficacy techniques to be used by the nurse54,55 and the intervention function,39 and served to ensure a theoretically modelled intervention.
| Target participant understanding/behaviour | Target professional self-management rolea | Self-efficacy techniqueb | Intervention component | Evidence from the data | Target construct (behaviour source)c | Intervention functiond |
|---|---|---|---|---|---|---|
| Willingness to self-manage opioids and side effects | 1, 2, 3 | Verbal persuasion | Recruitment and consent process pre-intervention delivery; educational approach | Majority see it as part of their responsibility:you’ve got to look after yourself quite a bit, and then know who to turn to if you can’t find the answer | Capability; motivation | Education; persuasion |
| Shared understanding of side effects | 2, 5 | Mastery experience | Educational approach; factsheet | Importance of acknowledgement. Need for ‘normalisation’ to reduce fear. For patients, opioid drowsiness not always perceived as diminishing. Prescription of laxatives but ‘they never said you will need it’. Lack of focus on opioid nausea and drowsiness by HCPs | Motivation | Education |
| Self-titration to negate side effects (drowsiness and constipation) | 2, 4, 5, 6 | Verbal persuasion; mastery experience; role modelling | Educational approach; factsheet | Unmet need. Patients learn this through trial and error, accounts of ‘crisis’ situations with impaction, underdosing to control drowsiness | Capability | Training; incentivisation; enablement |
| Misconceptions and unnecessary fears regarding opioids | 2, 5 | Verbal persuasion; mastery experience; emotional regulation | Educational approach; factsheet | Issues: holding back on medications (e.g. stock piling for a rainy day); ceiling amount and as needed 4-hourly dose; ‘I’m imminently dying’; addiction; tolerance | Motivation | Education; persuasion; enablement |
| Safe drug administration: control of what taking, why and when (+ safe storage and not removing medicines from boxes) | 2, 3, 5, 7, 8 | Verbal persuasion; mastery experience; role modelling | Information chart (what, when, rationale, appearance); dosette box if required; podcast – patient | Some unmet needs here: some ‘muddled’ patients not always aware of drug names or purpose; using pet names for drugs, etc.; confusion over millilitres vs. milligrams; literacy level; need for removal of ‘old’ drugs; information chart would be helpful for carers too | Capability | Education; training; modelling; enablement |
| Recognition of pain patterns | 2, 4, 5, 7 | Verbal persuasion; mastery experience | Educational approach; factsheet; pain diary | Importance in order to control pain | Capability; motivation | Education; persuasion; incentivisation; training |
| Monitoring the effectiveness of the medicines | 2, 3, 4, 5, 6, 7, 8 | Verbal persuasion; mastery experience; role modelling | Educational approach; factsheet; pain and side effect diary; podcast – HCPs | Importance for HCPs to be able to goal-set and manage symptoms effectively, shared responsibility with patient | Capability; motivation | Education; persuasion; incentivisation; training; modelling; enablement |
| Control over practical issues: checking stock, ordering, collecting, etc. | 1, 2, 3, 4, 5 | Verbal persuasion; mastery experience; role modelling | Factsheet; podcast – patient | Practical issues can be onerous, sapping energy unnecessarily (physical journeys, lack of syncing of supplies, etc.) | Capability; motivation; opportunity – physical | Education; incentivisation; modelling; enablement |
| Checking of dispensed medicines | 2, 7, 8 | Verbal persuasion; mastery experience | Educational approach factsheet | Not always right: supplies may be missing or not in correct form. Use community pharmacist as resource | Capability; motivation | Education; persuasion; training |
| Back up plans: knowledge of who to contact, when, plus out of hours | 1, 2, 3, 4 | Verbal persuasion; mastery experience | Educational approach; factsheet | Key message in patient/carer data – unmet needs | Motivation | Education; enablement |
| Understanding of initial verbal information giving reinforced by additional resources | 2, 3, 5 | Verbal persuasion; mastery experience; role modelling | Factsheets; podcasts; list of good websites, signpost where to go | Patients and/or carers ‘bombarded’ with information. Recognition that where information is given in it is not always taken in and that it needs to be reinforced by other resources | Capability; motivation | Education; training; modelling; enablement |
As a result of this framework, the SMST resources were developed to address the evidence from the data. The resources comprised eight factsheets, two podcasts, a pain diary, medication charts and goal-setting sheets. The factsheets were drafted to encompass all the core themes and issues outlined by the patients, carers and palliative care HCPs. They went through iterative cycles in the development process. The study PPI group was asked to review the content of all the factsheets, as were specialist HCPs from two different palliative care teams who were not going to be involved in the feasibility study (in order to prevent potential issues of bias and/or contamination). The final stage of development was review of the factsheets by an expert in health literacy (HB). The presentation and structure of the facts were recast with reference to the evidence on how people make sense of illness and decisions about treatment. 63,64 Factsheet content did not change, but readability was improved (assessed using Simple Measure of Gobbledygook readability formula65) by shortening sentences and eliminating some longer words (except drug names). The text was subdivided in all the factsheets into small titled sections to facilitate review by patients, with spaces provided for patients and carers to write notes or questions. These changes improved the factsheets’ health literacy and utility to support patients’ reasoning about treatment in the context of their experience of illness and lifestyle.
The factsheets
-
Managing pain with opioid medicines.
-
Contacts for advice and further information.
-
Getting prescriptions and obtaining medicines.
-
Organising opioid medicines.
-
Fitting pain control into my daily routine.
-
Checking opioid medicines are managing pain.
-
Common concerns when taking opioid medicines.
-
Keeping on top of side effects.
The eight factsheets are reproduced in Appendix 9. Each factsheet was designed to be used as a standalone educational resource or in combination to provide a set of educational materials relevant to the trial patient. Only factsheet 2 (contacts for advice and further information) was a core factsheet resource that the CNSs were asked to deliver to the respective trial patient on their first SMART visit.
The podcast films
The data demonstrated that individuals value resources being available in various forms to meet individual need. Therefore, two audio-visual podcasts were developed as an alternative medium:
-
‘The Practical Issues of Managing Medicines’ – when a patient described the practical methods (self-management strategies) they used to monitor their medication stock levels, order new medicines and organise them at home.
-
‘Monitoring the Effectiveness of Medicines’ – two experienced palliative care specialists discussed why monitoring the effectiveness of medicines is valuable in relation to self-management and why specialists may ask individuals to do this.
Structured guides (see Appendices 10 and 11) were written from the data to guide filming and the films were edited to produce two short 5- to 6-minute podcasts. This length of the podcast files was guided by the data, which suggested that patients and carers would watch short films and could benefit from them. Furthermore, it is known from existing resources (such as www.healthtalk.org/) that there is value that patients place in hearing authentic voices recounting their experiences and strategies in dealing with health-related issues and problems. Even when strategies are not ones that individual patients are likely to use themselves, it can help them to consider alternative strategies that might work for them. The podcasts were designed to be delivered to patients on a digital versatile disc (DVD) or memory stick (for computer use).
The pain diary and medication chart
In order to develop versions of a pain diary and medication chart to suit this specific context, existing medication charts and pain diaries used at the sites were requested and reviewed. In addition, a selection were sampled from online sources (e.g. professional-focused medication administration records) and patient-focused booklets (e.g. those produced by Macmillan Cancer Support). Two resource charts resulted in:
-
a pain diary to record and track pain which could be used by the patient (and their CNS) to monitor the effects of opioid medication and any side effects (see Appendix 12)
-
a medication chart which could be used to help patients organise, remember and take their medicines (see Appendix 13).
The goal-setting sheets
The final SMST resources, self-management-focused goal-setting sheets, were developed in consultation with an expert educational psychologist (SM). They were entitled ‘Things I would like to achieve over the next week’ (see Appendix 14) and were the second of two core resources of the SMST (the first being the ‘Contacts for advice and further information’ factsheet). The sheets were designed so that a trial patient could set one or two key goals at each visit and then with the help of their CNS set an action plan with small, practical steps to meet the goals. At the bottom of each sheet was space for the patient to review with their CNS the progress, or otherwise, that had been made in meeting the goals so as to inform development of the action plan where needed.
Training development
A training package was developed prior to the feasibility study to enable the study nurses to deliver the intervention. The training package was developed from the following:
-
A philosophical standpoint and definition of self-management at the end of life derived from our developing conceptual analysis (Evidence synthesis and Phase II: refining and detailing the intervention) and drawn from the work of Johnston et al. 51
-
To date, there has been a lack of specification in the literature about how self-management focused conversations regarding analgesia and related treatments at end of life should be enacted – it is not known what practitioners do and need to do to help support patients to self-manage. To illustrate the nature of a conversation in this context, a conversation was modelled using ethno-drama from a real-life case exemplar, by nurse educators for Masters students attending a module on cancer, palliative and end of life care.
-
Literature focused on the self-management of long-term conditions, such as systematic reviews and practice guidelines (e.g. Canadian practice guidelines on self-management in chronic care66), which emphasises the importance of action-planning was also used to inform the training development, as were the delivery strategies of similar educational interventions by specialist nurses as part of complex intervention studies. 67
-
An awareness that those delivering the intervention would be both self-selecting and palliative care nurses specialists with pre-existing expertise and skills.
The research team built on the four-step educational approach of needs assessment, information giving, goal-setting and review and coaching to develop a training approach that modelled the four steps within a therapeutic conversational process between the specialist nurse and patient. The educational approach and conversational process were underpinned by recognition of the importance of:
-
good communication skills, identifying concerns, clarifying and exploring
-
not imposing solutions to professionally identified problems and being open to all patient-identified concerns
-
agreeing realistic goals and action plans and reviewing these at each visit – noting detail on whether or not the action plan was followed and the patient’s ability to carry out the plan.
A bespoke training package resulted, comprising a workshop session, combined with additional resources for the study research fellows to deploy with the nurses over the course of the trial to reinforce nurses’ engagement in the study and support their delivery of the intervention. Further details regarding the training package are given in Chapter 3, SMART intervention.
Conclusion
The work presented in this chapter was undertaken to define the concept of supported self-management at the end of life and to develop the theoretical underpinning for the content of the intervention. This work provided a basis on which to develop and refine the content of the intervention through interviews and focus groups with patients, carers and HCPs. The findings from these activities resulted in the development of a theoretically informed behaviour change intervention to enable study nurses to deliver the intervention. We had planned to embed the principles of experience-based co-design within this phase of the work, and to a large extent this was achieved. However, the constraints on working with a population of very sick people meant that we used an iterative process and, where possible, sought views and involvement in a number of different ways. In summary, the development process resulted in the SMART intervention comprising a four-step educational approach and SMST, both of which are summarised in Box 3. The intervention was to be delivered to the individual patient by their community-based palliative care CNS using a conversational process, within the context of a clinical consultation. Mechanisms of impact through which the SMART intervention is hypothesised to lead to a medicines self-management behaviour change are summarised in a logic model (Table 7).
Assess beliefs, behaviour and knowledge related to pain and pain medicines.
(2) Information provisionProvide specific information (discuss content of appropriate factsheets + podcasts).
Provide ‘contacts for advice’ factsheet at first visit.
(3) Goal-settingCollaboratively set goals based on the patient’s needs (complete ‘things I would like to achieve . . .’ at each visit).
Complete ‘what I will do to help get me there’ to enable the goals to be met (small practical steps).
(4) Review and coachingPlan follow-up (face-to-face visits and telephone calls).
Identify barriers, strategies, problem-solving techniques and support.
The SMST Factsheets-
Managing pain with opioid medicines.
-
Common concerns when taking opioid medicines.
-
Keeping on top of side effects.
-
Checking opioid medicines are managing pain.
-
Getting prescriptions and obtaining medicines.
-
Organising opioid medicines.
-
Fitting pain control into my daily routine.
-
Contacts for advice and further information. a
-
Pain diary.
-
Medication chart.
-
Monitoring the effectiveness of medicines.
-
The practical issues of managing medicines.
-
Goal-setting sheets. a
Core SMST resources that every patient received.
| Problem | Opportunity | Intervention description | Implementation | Mechanisms of impact | Outcomes of individual elements of the intervention | Outcomes of the overall intervention |
|---|---|---|---|---|---|---|
| Enhanced supported decision-making can improve overall quality of end-of-life care. People living with advanced disease often experience fluctuating symptoms that require complex medication regimens. Supporting self-management could help patients and their family to manage their medications to improve pain and related symptoms | Development of a SMST that will be delivered by nurses, designed to help patients improve their knowledge, skills and confidence to manage medicines for pain, constipation, nausea and drowsiness |
Four-step supported self-management process Step 1, needs assessment – identify current medicines management behaviours, beliefs, knowledge of pain medicines Step 2, information provision – tailored information specific to identified concerns which will positively alter behaviour, address beliefs Step 3, setting self-management goals – realistic and achievable plan that responds to concerns and has potential to improve pain management Step 4, regular review – set a date to review the action plan and provide additional information and coaching |
Nurse delivery: the four-step educational approach will be delivered by specialist palliative care nurses working with community-based patients over a minimum of three sessions. The first session should be within 1 week of baseline data collection. Study nurses will assess self-management needs, provide tailored information and plan pain management goals (steps 1–3). Over the 6-week follow-up study, nurses will provide at least two additional follow-up meetings to review self-management needs, provide additional educational resources and review success with goals (step 4) Nurse training to deliver the intervention: study nurses will attend a half-day training workshop that will cover concepts and key features of self-management; the components of the intervention and the conversational process; who will use the educational resources with patients; worked-examples and practice using resources with patients; ongoing support (peer-to-peer support and support from research fellows) Resources to support nurse training: nurses will also receive the following resources to support the training: a diagrammatic representation of the four-step SMART intervention; the definition of self-management in palliative care and related professional roles; self-management conversational prompts to use with patients; an audio-recording of a modelled self-management conversation; summary sheet for making action plans with patients Ongoing support for duration of trial: to support the study nurses throughout the trial, the research fellows will meet with them regularly (approximately once a week) to support them to provide the intervention to their patients |
|
Clearly identified needs and improved patient and carer engagement in self-management via increased knowledge of obtaining and managing medicines and how to access support. Clearly identified action plans to support goal achievement. Patients empowered with increased knowledge and skills to recognise worsening symptoms, be able to self-initiate therapeutic adjustments and know how and when to access help from their local health-care system | Reduced pain intensity and reduced interference from pain in daily activities. Reduced need for out-of-hours support. Improved quality of life and self-efficacy |
Chapter 3 A feasibility study assessing the SMART intervention and trial processes
Introduction
This chapter focuses on Phase III of the SMART study: the feasibility study. As outlined in Chapter 2, The SMART intervention, the SMART intervention is designed to support self-management of analgesia and related treatments for patients approaching the end of life. It was developed with HCPs, patients and carers to distil best practice, and reviewed by our dedicated PPI panel.
Aim and objectives
The aim of the feasibility study was to assess both the feasibility of undertaking a definitive RCT and the acceptability of the SMART intervention. This aim was achieved by collecting data on the following specific objectives:
-
patient, carer and nurse eligibility, recruitment and follow-up rates
-
fidelity of the SMART intervention delivery (see Trial outcomes for definition)
-
patient, carer and nurse acceptability of the SMART intervention
-
contamination of non-study nurses (i.e. the feasibility of blinding non-study nurses working within study recruitment sites)
-
completion rates, variability and suitability of patient-reported outcomes
-
the extent of carer involvement.
The data from this feasibility study will be used to inform the design, intervention delivery strategy, sample size, outcome measures and operational aspects of a definitive trial aimed at establishing the effectiveness of the SMART intervention.
Design and setting
We conducted a multicentre mixed-methods single-arm pre–post observational feasibility study of the SMART intervention in patients living at home with advanced pain recruited from four community palliative care services.
The SMART intervention is an evidence-based supported self-management educational intervention delivered by CNSs in partnership with patients (and carers when appropriate) living at home with pain from advanced disease. The aim of the SMART intervention is to improve pain management and quality of life by enabling patients and carers to better self-manage analgesia (specifically strong opioids) and related treatments. The feasibility study was conducted in four community palliative care services: two in Yorkshire and the Humber and two in Hampshire. Within each community palliative care service, between two and four community-based CNSs were trained in the delivery of the SMART intervention (referred to as study nurses hereafter), which was a total of 12 overall. In addition, two research fellows co-ordinated trial recruitment and follow-up. Data were collected at baseline and at the 2-, 4- and 6-week follow-up time points.
Patient and public involvement
Specific engagement for this feasibility study took place via regular study meetings and individual correspondence with PPI panel members. PPI panel members have been closely involved in the design and delivery of this feasibility study. PPI representatives were involved with:
-
reviewing the content and format of the SMART intervention materials and the nurse delivery strategy
-
reviewing and feeding back to the study team on the draft patient study materials, including the self-reported outcome measures included in the patient questionnaire, which led to the removal of one questionnaire [Beliefs about Medicine Questionnaire(3)] as panel members felt the wording to be inappropriate for the end-of-life context.
Ethics approval and research governance
Research ethics approval was sought in September 2015 from North West – Lancaster Research Ethics Committee (REC). Provisional ethics approval was given on 10 October 2015 with a request for further clarification on seven minor points (no changes to the protocol were requested). Favourable ethics opinion was confirmed on 27 October 2015 (REC reference number 15/NW/0797). Following favourable ethics opinion from the REC, management permission (research and development approval) was obtained from University Hospital Southampton NHS Foundation Trust’s research and development department, as well as local site approvals.
Amendments to protocol
There was one substantial amendment, which outlined three changes to the protocol. This was approved on 23 November 2015 and the changes to protocol are summarised below.
-
Changes to patient-reported outcome measures: replacement of the Self-Management Ability Scale68 with the Self-Efficacy for Managing Chronic Disease Scale (SES)69 and removal of the Beliefs about Medicines Questionnaire,70 because it was felt to be too burdensome for participants to complete.
-
Change to assessing fidelity of delivering the SMART intervention: study nurses would be asked to audio-record the nurse–patient consultations when the SMART intervention was used.
-
Change to wording of ‘weekly’ to ‘regular’ review of patients’ progress with goal-setting review so that the protocol reflects study nurses’ usual practice.
See Appendix 15 for communication with the REC.
Participants
Participants were adults with advanced disease living at home (with or without a carer) and prescribed opioid medication and experiencing (or anticipating) side effects from these medications.
Eligibility criteria
Patients were eligible for participation if they:
-
were aged ≥ 18 years
-
had been prescribed strong opioid analgesia
-
were living at home
-
were being cared for by specialist community palliative care services
-
were considered by the clinical team likely to survive beyond 6 weeks of follow-up
-
had the capacity to provide informed consent.
Patients were ineligible for participation if they:
-
had insufficient literacy or proficiency in English to contribute to the data collection that was required for the research.
Carers were eligible to take part in an end-of-study interview if:
-
they were the primary carer of a patient meeting the above inclusion criteria
-
the patient whom they cared for had consented to their involvement.
Recruitment procedures
Sites
Study nurses and patient participants (and carers, when appropriate) were identified from community palliative care services at four hospices: two in Hampshire (site codes HANTS1 and HANTS2) and two in Yorkshire and the Humber (site codes YKHB1 and YKHB2). Identifying patients from community palliative care services offered the most efficient access to patients approaching the end of life who were living at home. Hospital-based palliative care services were used as recruitment sites for the focus groups and interviews described in the previous chapter. However, patients recruited from hospital-based palliative care services were either inpatients or in transition between inpatient and community health-care services and, therefore, not living in their own homes. Therefore, it was decided not to recruit from hospital-based palliative care services for the feasibility study as the focus was to identify patients who were managing pain in their own homes.
Nurse recruitment: identification and consent
At each recruitment site, between two and four community-based palliative care CNSs were identified by contacting service team leaders; 12 CNSs (hereafter referred to as study nurses) were identified from the four recruitment sites. An invitation was sent to all CNSs in the four community teams to attend a brief presentation by the SMART study research team. Following this, CNSs interested in the study were invited to attend a half-day workshop at which they received further information and training on the trial procedures and delivery of the intervention.
All study nurses who were trained to use the SMART intervention were also invited to take part in a one-off face-to-face interview at the end of the trial. Study nurses were given a recruitment pack (see Appendix 16) by a researcher, who explained the purpose of the qualitative interview. Study nurses were asked to provide written informed consent to take part in an interview with the research no less than 24 hours after receiving a recruitment pack and having had sufficient opportunity to ask any questions about their participation.
Non-study nurses working with research active sites were identified by contacting community service-lead CNSs. An e-mail was sent to lead CNSs with a link to an online survey and a request to circulate among all palliative care CNSs within their teams.
Participant recruitment: eligibility, approach and informed consent
Potentially eligible patients were identified by screening all new referrals and existing patients on study nurses’ caseloads against the eligibility criteria. Screening caseloads was done by a clinical research nurse (CRN) or researchers with the study nurses. This activity was recorded on a screening log (see Appendix 17), kept at each site. Before eligible patients were approached, their participation was discussed among the study nurse, researchers and wider clinical team to consider patients’ capacity to participate.
Eligible patients were first approached face to face by their CNS, who gave a verbal explanation of the study and provided them with a recruitment pack, consisting of an invitation letter, a patient information sheet and a consent form (see Appendix 17). At this point, patients not interested in participating were thanked for their time and asked if they would say briefly why they refused participation and, if willing, whether or not they thought the SMART intervention was acceptable in principle.
To identify carer participants, patients who were interested in the study were asked if they had a main informal carer (family member or friend) who may also be interested in participating in an interview with a researcher. If a main informal carer was identified, they were provided with a recruitment pack for carers (see Appendix 17).
Patients (and carers) interested in knowing more about participating in the SMART study were asked to provide their contact details and told that a researcher would contact them to discuss the study in more detail and answer any questions that they may have about participation. At this point, all subsequent recruitment and consent activities were completed by a researcher or CRN.
Patients were then contacted by telephone or face to face by a researcher to discuss the SMART study and answer any questions they had about participation. At this point, patients who refused participation were thanked for their time and asked if they would say briefly why they did not wish to participate.
Following provision of the recruitment pack, patients and their carers were given at least 24 hours to consider their participation and were encouraged to discuss the study with their family/friends and other HCPs.
Patients (and carers) willing to participate were asked to provide full written informed consent, following which they were recruited into the trial, and hereafter are referred to as participants.
This study nurse-led approach to identification and introduction and researcher/CRN-led approach to recruitment was the most efficient way to identifying eligible interested patients while keeping the burden of recruitment to a minimum for the study nurses and separate from their clinical practice.
Recruitment schedule
We estimated that within a 4-month recruitment period approximately 450 new patients would be referred to the four recruitment sites included in our study. We assumed that two-thirds of patients would have pain (n = 300), of which 50% would be eligible (n = 150). Based on these assumptions, we conservatively estimated that 20% of eligible patients would agree to participant (n = 30), which gave a predicted recruitment rate of eight consented participants per month across all sites for 4 months.
The SMART intervention
Training of the clinical nurse specialists
A bespoke training package was designed (outlined in Chapter 2, Training development), comprising a half-day workshop session. Following the training session, additional resource packs were developed for the study researchers to deploy with the nurses over the course of the trial to reinforce their engagement in the study and support their delivery of the intervention.
All 12 study nurses attended an initial SMART training workshop. The workshops were run in Hampshire and Yorkshire, with nurses from the two hospices in each region attending the same workshop. Sessions were facilitated by an expert Nursing and Midwifery Council-registered nurse educator who leads postgraduate palliative and end-of-life care education provision. In attendance, and acting as co-facilitators, were two study researchers. Sessions were experiential in approach, requiring reflection and demonstration of practice, and were run using the SMART training plan (Table 8) and supported with resources developed by the research fellows for the study nurses (see Appendix 3).
| Time | Activity | Facilitator prompts |
|---|---|---|
| 13.00–13.10 |
Thanks Housekeeping Overview of plan, setting scene of intervention and approach to training (ask questions as go along, repeat things as needed, centred on participants) Introduction to each other and the things that they have been thinking about in terms of getting ready to provide the intervention |
Facilitator to make a mental note of the things participants have been thinking about as these will be good examples later of the importance to assess beliefs, behaviours and knowledge |
| 13.10–13.20 | Warm-up exercise: the nature of self-management and conversations | Ask participants to select one or two cards from a range of possibilities – cards are pictorial, depicting wide variety of different scenes and patterns. Ask participants to choose the card(s) that bring to mind the features of self-management present in the clinical example they have been thinking about in preparation for the session:We asked you to think of an example from your practice that brought to mind what self-management means in your practice. Keeping these features in your mind, choose a card or couple of cards that best depict these featuresOnce everyone, including facilitators, have a card(s), ask participants to talk in pairs about their cards and the features of self-management that they have in mind. Allow 5 minutes per person |
| 13.20–13.30 | Identify key features of self-management | Feedback to group: explain rules of feedback, each person asked to feedback. Feedback not commented on, time for discussion afterwards, once everyone has fed back. Ask each person to hold up their card(s) and explain the features of self-management that the card brings to mind. Once everyone has fed back, the facilitator summarises features that have been raised and integrates this with the features of self-management emphasised within the intervention. Facilitator emphasises that the intervention is designed to enhance pain management through enhanced self-management of pain medications |
| 13.30–13.45 | Introduce focus of conversational process: identify and build on what the participants intrinsically know about conversations | Ask the participants to go back into their pairs and think back to the conversation that they had about the cards, when they were explaining reasons for choosing the selected cards. Ask the participants to describe the process of the conversation that they had together: How did they start? How did the conversation develop? How did it become focused? How did it finish? Facilitator lays out a conversational process on the wall or floor or table using A4 sheets of paper in different colours for each phase of conversation (four phases as above). The phases of conversation are labelled on the coloured paper. Ask the participants to ‘walk through’ the conversation they had together – this is best done by physically walking through (walking along the process on the floor/along the wall). Facilitator draws out what happened at each phase and makes a note on a Post-it® note (3M, Cynthiana, KY, USA) of what happened and agrees with whole group where this Post-it note belongs. When all pairs have completed this process, facilitator summaries the process of conversation in terms of what the purpose is of each phase and frames this in relation to the intervention (e.g. so this phase of the conversation is about identifying a focus, a title for the rest of the conversation, this phase is about transmitting understanding the focus and clarifying meaning, this phase of the conversation draws the points together and shapes the next thing to happen – in this case what you will feed back to group, in clinical practice what actions you will take). Facilitator then links this conversational process with the SMART intervention – we are going to use a similar conversational process in this intervention, so one of the key points is that you already know the process we are going to follow, the key thing is that this conversation needs to carry the things that will make the intervention different from an everyday conversation |
| 13.45–14.00 | Outline conversational phases and components | Facilitator walks participants through intervention conversational process emphasising the four different components. Reinforce this process by providing a diagram of the process. Go through process illustrated within the diagram, giving examples of how the process would flow in clinical practice when discussing pain medicines with a patient |
| 14.00–14.15 | Introduce next part of the session | Next hour focused on the interventional process, going to put what we know about self-management and conversational process into practice in relation to supporting self-management of pain medicines. Facilitator models intervention process with colleague – brief example |
| 14.15–14.30 | Break | During break facilitator outlines an example on board which is going to be used for the rehearsals |
| 14.30 | Welcome everyone back | Facilitator summarises what has happened so far and plan for this part of the session – to work closely through the process as a group, using the case outlined on board, using forum theatre technique |
| 14.30–15.00 | Participant-worked example | Intervention-worked example – participants guiding facilitator in the interventional process |
| 15.00–15.10 |
Introduce next section – resources Overview of resources available for use within the intervention and principle of these being a toolkit |
Short presentation of resources available and how these are intended to be used |
| 15.10–15.25 | Practice introducing and using resources | Participants to work in pairs and practise how they would introduce a resource to a patient and how they would shape this part of the conversation to encourage self-management. Pairs to make a plan about what they need to do to get to know the resources – ask them to write an action plan that follows what is needed in a self-management plan – realistic, time orientated, outcome orientated, etc. Then ask them to add an action around the whole intervention – what they need to do to be able to provide intervention |
| 15.25 | Introduce last section of day – ongoing support | Group discussion – facilitator introduces purpose of this last section – how to support each other and how the researchers can support them to provide the intervention. The process for eliciting this information will follow the intervention thus allowing participants to model and reinforce the process and give them additional vicarious experience of the intervention. Facilitator asks participants to work in pairs, taking 10 minutes each to work through process of peer reflective support – each taking role of peer supporter and peer discussant. Use intervention process to shape reflective conversation:
|
| 15.45 |
Bring things together Ask participants to summarise by explaining the intervention in their own words Finish round by asking researchers to summarise the intervention in their own words Close, thank participants, summarise plan for follow-up and resources available |
During the workshop session, it became apparent that the nurses wanted further detail on the research process, such as eligibility criteria, screening, approach and consent, before they felt able to give their attention to intervention delivery itself. As a result, these processes had to be outlined by the co-facilitators. This took time during the workshops, leaving no time to model the conversational process for and with the nurses, or the goal-setting, in particular the process of effective action-planning once goals are set. To respond to this, additional resource packs were created for the study nurses following the workshops. It was the intention that these resources would be given to the nurses via weekly opportunities to reflect on delivery of the intervention through face-to-face visits with the study researchers.
Additional resource packs comprised (1) a 30-minute nurse–patient modelled self-management-focused conversation between two skilled nurse educators (one of whom was the workshop session facilitator), which was supplied as an mp3 file on a memory sticks with an expectation that nurses would listen to the ‘model’ conversation; (2) self-management conversation prompts (see Appendix 18), outlining potential questions mapped to the four steps of the educational approach; and (3) a ‘making action plans’ document (see Appendix 19), outlining specific actions needed to help a patient meet their self-management focused goal(s). The use of these resources enabled the researchers to provide ongoing coaching and support to the study nurses during the trial and additionally helped to promote ongoing engagement of the nurses.
In order to help formalise and aid standardisation of the approach to ongoing nurse engagement, an aide memoire for the researchers to use with the study nurses at site visits was developed, which prompted questions related to the nurses’ experience of each step of the educational approach (see Appendix 20). A related framework to standardise the documentation of these discussions via researcher field notes was also developed (see Appendix 21).
Interventions details and schedule of delivery
Study nurses were asked to begin using the intervention with participants within 7 days of consent and baseline data collection. As described in Chapter 2, The SMART intervention, the SMART intervention comprised both a four-step educational approach and a SMST. In brief, the four-step educational approach was designed to reflect the everyday practice of specialist nurses and included an assessment of participants’ needs, provision of information, goal-setting and review and coaching of self-management progress. The SMST comprised eight factsheets, two podcast films, a pain diary, a medication chart and goal-setting sheets.
It was intended that the intervention would be delivered by study nurses each time they visited their participants (and a carer when appropriate) over the 6-week study period (each visit was referred to as a ‘SMART visit’). Study nurses were asked to visit participants a minimum of three times during this 6-week period (i.e. at least once a fortnight). During each visit, study nurses were required to use a conversational approach to go through the four steps of the educational approach and provide the resources from the SMST as required. As a minimum, study nurses were required to provide the factsheet ‘Contacts and further information’ and the goal-setting sheets as core resources at their first SMART intervention visit.
Assessment and data collection
Screening data collection
Screening data were collected by a researcher or CRNs on all the patients on the study nurse’s caseload. Data were collected on sex, age, referral status (existing patient or new referral) and eligibility.
Baseline data collection
The researcher or CRN completed the baseline case report form (CRF), which captured data on participants’ medical history (e.g. type of advanced disease, date of diagnosis), reason for referral to palliative care, palliative treatments received (within the past month) and current medications for pain, nausea and constipation. The baseline CRF also asked participants whether or not, in a future study, they would take part if they were randomised to receive either the intervention or standard care as usual (yes/no response). This question was included to inform the acceptability of randomisation processes in a future definitive trial.
Participants were also asked to complete an outcome measure pack comprising five validated self-reported outcome measures on pain, self-efficacy, common symptoms, quality of life and satisfaction with medicines information. These outcome measures are described in Participant self-reported outcome measures.
Follow-up schedule
Participants were followed up for 6 weeks from the date of baseline assessment. Six-week follow-up was chosen because the risk of short survival in this population meant that demonstrating early and sustained improvements in self-management within a few weeks would be particularly important in this context. During the 6-week follow-up period, participants were contacted by a researcher at three time points post baseline:
-
week 2 (day 14)
-
week 4 (day 28)
-
week 6 (day 42).
The exact timing of follow-up visits was flexible to fit around participants’ medical and other appointments, although efforts were made to keep follow-up visits within ± 2 days of the scheduled appointment date. Follow-up visits were usually conducted face to face between the participant, their carer and a researcher. Participants were offered a telephone follow-up if this was more convenient.
At each follow-up visit the researcher completed a follow-up CRF and asked the participant to complete the outcome measure pack (as described above). The follow-up CRF captured data on any of the following activities since the last follow-up (or baseline) visit:
-
which factsheets had been given to the participant
-
whether or not any self-management goals had been set (or reviewed)
-
whether or not the participant and/or carer had watched either of the video podcasts.
Participant and carer interview
As part of the final follow-up visit (week 6) participants (and carers, when appropriate) were asked to take part in an audio-recorded face-to-face semistructured interview together with a study researcher. Participants and carers were interviewed together. The topic guide for these interviews focused on two broad areas. First, participants and their carers were asked what they thought about taking part in the trial and how they were managing their medicines. These questions were intended to explore participants’ and carers’ acceptability of trial procedures, what they thought of the intervention itself and how it had been delivered to them by their CNS. These questions were designed to understand the extent to which participants and their carers were aware of the formalised educational process, the acceptability of the SMST resources (i.e. what they liked and did not like about it), their continued use of the intervention and whether or not their participation had any effect on their confidence with managing medicines. Interview guides are reproduced in Appendix 22.
Study nurse interview
In addition to participant and carer interviews, all study nurses were invited to take part in an audio-recorded semistructured interview after follow-up had closed for all participants at their site. These interviews covered a range of topics designed to capture their experiences of participating in the research process (including the training workshop), as well as their views on the acceptability of the intervention and whether or not they felt that they could integrate it into their clinical practice. The topic guides for the study nurse interviews are reproduced in Appendix 22.
Final data collection
At each site, when the last participant had completed 6 weeks of follow-up (or had withdrawn from the trial) a final data collection CRF was completed to capture the following data on all patients at that site:
-
date of death or date last known to be alive
-
place of death and preferred place of death (if known)
-
health-care resource use over the 6-week follow-up period (these data are reported in Chapter 4 as they relate to the health economic evaluation of the overall intervention)
-
current medication prescribed for pain, nausea and constipation.
Adverse events
The intervention was evaluated within a patient population with advanced disease and who are approaching the end of life. Thus, it was expected that episodes of acute illness or infection, new medical problems and deterioration of existing medical problems would occur and could result in prolonged hospitalisation, hospital readmission, significant or permanent disability or incapacity, or death. In recognition of this, events fulfilling the definition of an adverse event or serious adverse event were not reported in this study unless the event resulted from administration of any research procedure.
Non-study nurse data collection: assessing contamination
To inform the design of a future definitive trial, it was considered appropriate to evaluate whether or not within individual sites the practice of non-study nurses was influenced as a consequence of working in a team where their colleagues were using the SMART intervention. To assess contamination, an online survey was sent to all non-study nurses working in the community palliative care teams at the four recruitment sites. The survey captured data on:
-
demographics (age, sex, grade/band)
-
duration of palliative care experience
-
whether or not they were an independent prescriber
-
whether or not they were aware of the SMART study and what it was about
-
whether they had discussed the SMART study with colleagues who were using the intervention
-
if they were aware of the study or had discussed it with a colleague, whether or not this had influenced their own practice supporting medicines management with their patients.
A copy of the online survey questions to non-study CNSs is reproduced in Appendix 23.
Participant self-reported outcome measures
Pain was measured using the short-form Brief Pain Inventory (BPI). 71 Using a 0–10 numerical rating scale (anchored 0, ‘no pain’, and 10, ‘pain as bad as you can imagine’), the BPI allows respondents to rate their worst, least and average pain intensity during the past 24 hours, as well as present pain. The responses to the four pain intensity items are reported separately. Using a 0–10 numerical rating scale (anchored 0, ‘does not interfere’, and 10, ‘completely interferes’), respondents are also asked to rate the extent to which pain interferes with general activity, mood, walking ability, normal work, relations with other people, sleep and enjoyment of life.
Self-efficacy was measured using the SES (adapted for palliative care). 69 This scale contains six items that assess an individual’s confidence with managing symptoms of illness and was adapted for a palliative care context. Each item is measured on a 1–10 numerical rating scale, anchored 1, ‘not confident at all’, and 10, ‘totally confident’.
Symptom burden was measured using the Edmonton Symptom Assessment Scale (ESAS). 72 The ESAS is a 10-item tool designed to assess common symptoms in palliative care patients. Each item is measured using a 0–10 numerical rating scale anchored 0, ‘no (symptom)’, and 10, ‘worst (symptom)’. The ESAS was originally developed in cancer patients but has been extensively used at end of life. The final item, ‘other problems’, was modified to represent drowsiness.
Health-related quality of life was measured using the EuroQol-5 Dimensions, five-level version (EQ-5D-5L),73 which is a standardised, generic measure of health-related quality of life. It provides a single index value for describing and valuing health status calculated from a simple descriptive profile consisting of the following five dimensions: usual activities, self-care, mobility, pain/discomfort and anxiety/depression. The EQ-5D-5L index is reported not in this chapter, but in Chapter 4, as it relates to the health economic evaluation of the overall SMART intervention. The EQ-5D-5L also provides an overall measure of health-related quality of life by asking respondents to rate their present health state from 0, ‘worst health you can imagine’, to 100, ‘best health you can imagine’, which is reported in this chapter.
Satisfaction with medicines information was measured using the Satisfaction with Information about Medicines Scale (SIMS),74 which consists of 17 items about the types of information required to facilitate safe self-management. For each item, respondents are asked to rate the amount of information they have received using the following response scale: too much, about right, too little, none received or none needed.
Trial outcomes
For consistency, reporting of the feasibility study outcomes is presented in line with the trial aims (see Aim and objectives): to assess the feasibility of conducting the trial procedures and the acceptability and fidelity of the SMART intervention to participants, carers and study nurses.
Feasibility of conducting trial procedures
Process outcomes providing measures of the feasibility of study procedures included eligibility rates, recruitment rates and follow-up rates at 2, 4 and 6 weeks; the fidelity of delivery (i.e. number of SMART intervention visits delivered per participant); completion and acceptability of participant self-reported outcome measures; completion of patient health-care records to collect outcomes; estimates of variability in patient-reported outcome measures; the extent of contamination of non-study nurses working in research-active teams; and level of carer involvement based on the number of carers willing to take part in a face-to-face interview.
The feasibility of conducting the study procedures was also assessed through semistructured face-to-face interviews with participants, carers and study nurses. Interview guides were developed (see Appendix 22) to explore the feasibility of the trial processes.
Choosing a primary outcome measure for a definitive trial
The primary aim of a definitive trial would be to observe a reduction in average pain intensity (measured using the BPI average pain intensity item) and is therefore a candidate primary outcome for a future definitive trial. However, the complexity of symptom control within an end-of-life population may mean that the average pain intensity scores do not improve over the course of the study period despite the participant having received some benefit from using the intervention with their study nurse. It is recognised that changes in pain interference (measured as a composite score of the seven interference items on the BPI) and self-efficacy (measured on the SES), may be more responsive outcomes. As outlined in the logic model presented in Chapter 2 (see Table 7), the SMART intervention is aimed at improving medicines self-management behaviours; therefore, it is possible that participants may have experienced improvements in self-efficacy and interference from pain (mediated via information provision, goal-setting and regular review and couching) without any direct improvement on pain severity score. Indeed, stability in pain severity score over the study period may indicate improvements in overall medicine self-management in the context of declining health. Therefore, the BPI pain intensity, pain interference measure and the SES self-efficacy score were assessed as candidate primary outcomes for a definitive trial.
Fidelity of intervention delivery
Intervention delivery ‘as planned’ was defined as:
-
initiation within 7 days of baseline
-
a minimum of three SMART study nurse visits over the 6-week follow-up period
-
assessment of participants’ self-management needs/requirements (step 1)
-
tailored information resource provision from the SMST (step 2)
-
self-management goal-setting and action-planning (step 3)
-
regular review and coaching of self-management goals (step 4).
To assess whether or not the SMART intervention was delivered as planned, nurses were asked to record the details of each SMART visit on a standardised CRF. This form captured information on:
-
self-management needs identified and discussed
-
intervention factsheets provided to participants
-
self-management goals set or reviewed
-
changes made to participants’ analgesic medication and who made the change
-
details of any additional contact with participants between SMART visits.
The number of CRFs completed per participant was used as a proxy indicator of the number of SMART study nurse visits each participant has received. When study nurse CRFs were missing, participants’ clinical records were searched for evidence of a SMART visit having taken placed. To capture evidence of goal-setting, the goal-setting sheets were printed on carbon copy paper: the top copy was kept by the participants in their SMST folder, one copy was kept by the study nurses in the participant’s notes and one copy was collected by the researchers (either from the study nurse or from the participant at each follow-up visit) as evidence of goal-setting having taken place. When goal-setting sheets were missing, participants’ clinical records were searched for evidence of goal-setting.
Fidelity of the intervention delivery was also assessed by the researchers. At each follow-up visit the researchers completed a CRF capturing data on which SMST resources had been received by participants since the previous follow-up, whether or not the participants had set or reviewed their self-management goals, whether or not they had watched the video podcasts (if yes, which ones) and whether or not they were still using the SMART intervention.
In addition to the above quantitative assessment of intervention delivery, fidelity was also assessed during interviews with participants (and carers) after they completed 6 weeks of follow-up and with the study nurses at the end of the trial. Interviews included questions focusing on the extent to which the intervention had been delivered as intended as well as any barriers to or facilitators of delivering the intervention or using the intervention.
Finally, in order to develop an ‘intervention delivery fidelity checklist’ to be used in a future national multicentre definitive trial, study nurses were asked if they would be willing to audio-record their patient consultations when they used the SMART intervention. It was intended that these audio transcripts would be judged against a checklist of key elements required for intervention delivery.
Acceptability of the SMART intervention
Acceptability of the SMART intervention was assessed during recruitment by asking all patients who received an information pack whether or not they thought the intervention was acceptable in principle (yes/no response).
During the study period participants’ and carers’ acceptability of the intervention was assessed quantitatively by evaluating the number of participants agreeing to use the intervention compared with the number indicating that they were still using the intervention at each follow-up. Participant and carer engagement with the SMST was assessed at each follow-up visit by the question ‘Are you still using the SMART toolkit?’.
The end-of-study interviews with participants, carers and study nurses (described above) also assessed acceptability and usage of the SMART intervention. Interview guides (see Appendix 22) were developed to explore participants’, carers’ and study nurses’ experience of using the intervention.
Sample size
A practical approach was taken to recruit an adequate sample size to evaluate the feasibility of conducting a definitive trial. Browne75 suggests that when previous data sets are not available to estimate the sample size required to achieve a planned power to conduct the necessary quantitative evaluations, a pilot sample of 30 participants per arm is sufficient to estimate the population standard deviation (SD). A sample size of 30 patients would allow the 95% confidence interval (CI) around the proportion of patients with at least a 30% reduction in average pain intensity on the BPI to be calculated within 0.164 degrees of precision, assuming a 30% response rate (participants with ≥ 30% reduction, 0.3 ± 0.164).
Analysis plan
The overarching approach to data analyses (for both qualitative and quantitative outcomes) was informed by the recommendations of Moore et al. 76 regarding process evaluation of complex interventions. The logic model presented in Chapter 2, Conclusion, establishes the causal assumptions underpinning the mechanisms of action of the SMART intervention. The qualitative and quantitative analyses described here focus on developing this understanding by evaluating the data in terms of its mechanisms of actions and the consequences that will trigger behaviour change.
Qualitative data: analysis of interview data
The audio files from the interviews were professionally transcribed. They were listened to alongside the transcripts by the researchers (NC and MM) to check for complete accuracy and ensure data familiarity. The data were coded utilising framework analysis62 by indexing transcripts for all issues relevant to the feasibility of conducting the trial processes (i.e. deliverability), acceptability and usage of the intervention, perceived benefits and any potential disadvantages of both the research design and the intervention.
The interview data were analysed within a framework designed for the study based on the recommendations of Moore et al. 76 regarding process evaluation of complex interventions. The analysis framework, and the ultimate higher level of analysis, focuses on the mechanisms of action, the participant, carer or study nurse responses to the research design or intervention, the mediating factors and the consequences.
The analyses of qualitative findings are presented in full in Appendix 24. These analyses focus on the feasibility and deliverability of the trial processes, acceptability and usage of the intervention, perceived benefits and possible disadvantages of both the research design and the intervention itself, including the four-step educational approach and the SMST.
Quantitative data
Unless otherwise stated, all percentages were calculated using the total number of participants (or forms completed) within the relevant population, which was the denominator (i.e. excluding all participants with missing data for that variable). All percentages, means, medians, interquartile ranges (IQRs), ranges, SDs, standard errors and 95% CIs will be rounded to one decimal place (or two significant figures for numbers < 0.1). To account for the variation in the amount of intervention received by participants, all calculations and analyses were performed on an intention-to-treat basis (i.e. based on all consented participants). This pragmatic approach to include all consented participants was taken as it more closely reflects the real-life situation in which the amount of palliative care support received by patients varies. All calculations and analyses were carried out using Stata® version 12 (StataCorp LP, College Station, TX, USA).
Assessing feasibility of conducting trial procedures
Recruitment and retention
The feasibility of the recruitment strategy was evaluated by summarising the screening, eligibility, approach and consent processes and included the numbers of participants involved at each stage and reasons for non-participation. A recruitment flow diagram depicts the course of participants throughout the screening and recruitment process. A recruitment graph presents monthly and cumulative recruitment figures. These data were summarised overall and by recruitment site.
Demographic characteristics for consented participants are presented overall and by research site. The number/proportion of participants with a carer and consenting carer was summarised, as were carer reasons for non-participation and the details of the consenting carer. Participant retention during follow-up, including the number of participants withdrawing or who were lost to follow-up (hereafter referred to as dropouts), together with the timing and reason for dropouts, is presented overall and by recruitment site.
Acceptability of randomisation
Participants’ acceptance of being randomised to either standard care or SMART intervention was assessed by summarising the number/proportion of participants indicating that they would agree to be randomised.
Participant self-reported outcome data
Completion rates of all participant responses to the questionnaire packs as well as missing item-level data were summarised at each time point. In this section of the analysis, missing data were classed as a category in their own right, and all percentages were calculated using the total number of participants or forms expected in the relevant population as the denominator (i.e. including participants with missing data for that variable). As outlined in our initial grant application, we had hoped to explore the impact of potential confounders, such as disease state, age, sex, level of support and recruitment site, on the potential for participants to benefit from the intervention. However, owing to the small number of participants taking part, statistical analysis was not conducted to explore potential confounders.
Summary statistics and corresponding 95% CIs are reported for participant self-reported outcomes at each time point and mean differences were calculated between baseline and 6-week follow-up time point. The number and proportion of participants with a meaningful reduction in average pain intensity and pain interference were summarised as recommended by Dworkin et al. :77 a decrease in BPI pain intensity of ≥ 2.0 points or ≥ 30%; and a decrease in BPI pain interference of ≥ 1 point at each follow-up time point compared with baseline.
To evaluate the performance of candidate primary outcomes (described in Trial outcomes) for a definitive trial, statistical and contextual factors were taken into consideration. These included the proportion of missing data, any evident floor or ceiling effects, precision (variability) of the outcome measures based on 95% CIs (around mean responses at each time point, mean difference score between baseline and 6-week follow-up and the SD of responses) and responsiveness to change based on the observed effect size and the distribution of change by 6 weeks.
Survival following study entry
To understand how close to the end of life participants were, the number of days between study entry and date of death were calculated for participants with a known date of death (i.e. who died during the follow-up period or after follow-up but before the final data collection). For participants known to be alive at the end of the study, the median (range) number of days between baseline and the date the patients were last known to be alive was summarised.
Derivation of participant self-reported outcomes
For the four pain intensity items on the short-form BPI71 (pain at its ‘worst,’ ‘least,’ ‘average,’ in past 24 hours and pain ‘now’), the mean item response was calculated with scores ranging from 0 to 10, with higher scores indicating greater pain intensity. A pain interference score was obtained by calculating the mean of the responses to the seven pain interference items (where four or more of the seven items were completed). Scores ranged from 0 to 10, with higher scores indicating greater interference from pain.
For the SES,69 a summary scale score was obtained by calculating the mean of the six items (where four or more items were completed). Scores ranged from 0 to 10, with higher scores indicating higher self-efficacy.
For the ESAS,72 a summary score was obtained by calculating the mean of the nine symptom items (pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, well-being and shortness of breath), which were summed at each time point to give the mean scale score representing the extent of symptom burden. Scores ranged from 0 to 10, with higher scores indicating increased symptom burden.
A measure of health-related quality of life was derived by summing the present health state item on the EQ-5D-5L73 at each time point. The item is scored from 0 to 100 and then divided by 100. Item scores are reported between 0 and 1, with higher scores indicating better health status.
Responses to the 17 items on the SIMS74 were summed (responses too much, too little and none received are scored 0; responses about right and none needed are scored 1), to give a total satisfaction score at each time point. Scores range from 0 to 17, with higher scores indicating higher satisfaction with information received about medicines.
Fidelity and acceptability of the SMART intervention
Intervention delivery and fidelity
The uptake and retention rate of the intervention were evaluated by summarising the number of SMART visits each patient had from a study nurse during the 6-week study period. These data were summarised as the mean number of SMART visits received by all participants. The analysis was evaluated within the context of total screening and eligibility rates, as well as the number of participating CNSs, to provide an indication of overall capacity to deliver the intervention per protocol. The average length of time for each intervention SMART visit was also summarised, together with the number (and proportion) of participants receiving at least three SMART visits.
Adherence to the SMST delivery strategy for the education resources (the factsheets, the pain diary, the medication chart, podcast films and goal-setting sheets) was assessed by summarising which resources were present in participants’ SMST folders at each follow-up visit. The number/proportion of patients indicating that they had watched the podcast films at each follow-up visit was also summarised.
Acceptability
Acceptability of the SMART intervention was assessed, primarily summarising data from the qualitative semistructured interviews with participants, carers and study nurses. Quantitative evaluation of participants’ acceptability was achieved by first summarising responses to the ‘acceptable in principle’ question, and then by comparing the SMART intervention uptake rate with the number of participants reporting that they are still using the intervention at 6 weeks follow-up. For all participants, reported use of the factsheets and DVD podcasts, as reported at the follow-up visits with the study researcher, will be summarised.
Data accuracy
To evaluate the accuracy of data input, a random check of 20% of the data entered into the trial database was carried out prior to data cleaning and analysis. This process identified that data entry accuracy was very high: > 99% across all the questionnaire and study CRFs.
Missing data
Attempts were made to retrieve missing data via a thorough data cleaning process, including a 10% check of all data entered by hand. Every effort was made to obtain complete dates for all key data. Completion rates of all participants’ responses to the outcome measures packs as well as missing item-level data were summarised at each time point. Within this section of the analysis, missing data were classed as a category in their own right and all percentages were calculated using the total number of participants or forms expected in the relevant population as the denominator (i.e. including participants with missing data for that variable).
Participant recruitment, retention and characteristics
Participant flow
Figure 3 presents the Consolidated Standards of Reporting Trials (CONSORT) flow diagram of participant recruitment from screening through consent and intervention delivery to follow-up completion. In total, 417 patients were screened against the eligibility criteria (Figure 4), of whom 103 (24%) were eligible. Of the eligible patients, 37 (36%) were approached, of whom 22 (59%) were interested and 19 (51%) consented and were recruited and are hereafter referred to as participants. Of the 19 consented participants, 15 (79%) completed 6 weeks of follow-up. A total of 17 participants (89%) received the intervention.
FIGURE 3.
The CONSORT flow diagram of recruitment. a, Calculated as the proportion of consented participants.

FIGURE 4.
Weekly screening rates by referral status.

Over the 4 months of the trial, the recruitment rate was 4.75 consented participants per month. Figure 5 presents the weekly recruitment figures for each site (bars) and the weekly cumulative accrual rate against target accrual rate (dotted line). The median (range) days between approach and consent was 7 (4–39) days.
FIGURE 5.
Weekly recruitment figures by site.

Recruitment sites
HANTS1 and HANTS2 were the two community palliative care services in Hampshire; YKHB1 and YKHB2 were the two community palliative care services in Yorkshire and the Humber. Stacked columns in Figure 5 show the weekly recruitment rate by site. The dots and lines show target cumulative accrual rate (black line) and the actual cumulative accrual rate (blue line).
Eligibility criteria
Table 9 summarises the screening activity undertaken across all sites and presents basic demographics for patients screened. Based on previous studies undertaken by the research team, we had assumed that 66% of screened patients would be ineligible for participation; however, 314 out of the 417 patients (75%) screened were ineligible. The primary reason was not having been prescribed strong opioid analgesia (Table 10). Our screening procedure did not stipulate rescreening of ineligible patients, which may have identified patients who had subsequently been prescribed opioid analgesia. The researcher field notes taken during the screening process identified that many patients were ineligible because they were not prescribed strong opioids but were prescribed weak opioids for pain.
| Variable | Recruitment site, n (%) | ||||
|---|---|---|---|---|---|
| All | HANTS1 | HANTS2 | YKHB1 | YKHB2 | |
| Screened | 417 (100) | 148 (35.5) | 57 (13.7) | 148 (35.5) | 64 (15.4) |
| Sex | |||||
| Male | 118 (45.3) | 60 (40.8) | 28 (50) | 71 (48) | 29 (45.3) |
| Female | 227 (54.7) | 87 (59.2) | 28 (50) | 77 (52) | 35 (54.7) |
| Age (years) | 73 (22–97) | 72 (37–97) | 76 (35–95) | 74 (39–96) | 69 (22–92) |
| Referral status | |||||
| New | 202 (48.4) | 64 (43.2) | 33 (57.9) | 95 (64.2) | 10 (15.6) |
| Existing | 215 (51.6) | 84 (56.8) | 24 (42.1) | 53 (35.8) | 54 (84.4) |
| Eligible | |||||
| Yes | 103 (25.4) | 24 (16.9) | 18 (35.1) | 39 (26.4) | 22 (33.4) |
| No | 314 (74.6) | 124 (83.1) | 39 (64.9) | 109 (73.6) | 42 (65.6) |
| Approacheda | |||||
| Yes | 37 (36) | 9 (36) | 10 (55.6) | 13 (33) | 5 (22.7) |
| No | 66 (64) | 15 (64) | 8 (44.4) | 26 (67) | 17 (77.3) |
| Interestedb | |||||
| Yes | 22 (60) | 7 | 4 | 7 | 4 |
| No | 15 (40) | 2 | 6 | 6 | 1 |
| Consentedc | |||||
| Yes | 19 (86.4) | 6 | 3 | 6 | 4 |
| No | 3 (13.6) | 1 | 1 | 1 | 0 |
| Reason for screening failures | n (%) |
|---|---|
| Not prescribed strong opioid | 168 (53.5) |
| < 6 weeks survival | 61 (19.4) |
| Patient lacks capacity to consent | 29 (9.2) |
| Patient not living at home | 19 (6.1) |
| Discharge | 18 (5.7) |
| Strong opioids for breathlessness | 11 (3.5) |
| Other | 8 (2.5) |
| All | 314 (100) |
The interviews with study nurses revealed that the eligibility criteria were initially seen as acceptable by being relatively broad. However, the study nurses were surprised by the lack of individuals who met the eligibility criteria. Moreover, those who did meet the eligibility criteria often had complex end-of-life needs and would have been ‘on their reserves to do it [very fatigued and only just able to participate]’ (H2CNS002). One study nurse responded that ‘It’s interesting that . . . there’s quite a lot of patients that aren’t even on opioids’ (H1CNS002). Another study nurse indicated that ‘I was surprised in a way that I didn’t have any patients that would be applicable for the study . . . maybe, that shows how complex the patients are that come to us, and how quickly people who may possibly have been suitable deteriorated’ (H1CNS004).
A mediating factor in the low eligibility rate was the number of study nurses involved in the study. Across the four sites there were 37 full-time equivalent CNSs, of whom 12 (33%) volunteered to take part in the SMART study. Consequently, the pool of patients available to screen was smaller than expected and those who were ‘eligible’ often had very complex needs that were not captured by the broad eligibility criteria.
Patient referral rate
We had assumed that approximately 450 new patients would be referred to the four community palliative care services during the recruitment period (a rate of 112 per month). As we recruited only 12 nurses to take part in the study, the actual number of new referrals from these 12 study nurses over 4 months of recruitment was 202 (a rate of 50 per month).
Screening and initial approach processes
The initial screen for eligible patients included screening all existing patients on the study nurses’ caseloads. Subsequent caseload screening was of new referrals (see Figure 4). Consequently, 54% (n = 227) of all patients were screened within the first 3 weeks of recruitment, resulting in 70% (n = 72) of all eligible patients being identified within the same 3-week period. Given that study nurses’ appointments with patients were usually weekly, fortnightly or ‘as required’, they had to prioritise which patients they approached first based on the next appointment date. This meant that many eligible patients identified in the first 3 weeks of the trial were not approached (reasons described below), or by the time the study nurses were able to see them had become ineligible because of declining health (i.e. they were not anticipated to survive more than 6 weeks).
Just over one-third of eligible patients were approached and invited to participate in SMART (see Figure 3). Reasons for the low approach rate were largely missing because of low CRF completion rates (71% missing; Table 11); however, end-of-study interviews and researcher field notes provided more explanatory evidence. Study nurses indicated during interviews that approaching eligible patients was not a problem in itself (i.e. patients were not intrinsically opposed to finding out about the research). However, the often complicated and rapidly fluctuating circumstances of the end-of-life context meant that it was often not appropriate to approach eligible participants about participating. In addition, some patients had already been approached about participating in other drug trials and study nurses felt that they ‘couldn’t burden them with something else at that time’ (H1CNS002). Many eligible patients, as a result of their complex situation, were frequently admitted to alternative care settings (hospital, hospice or nursing home) for symptom control or respite care; therefore, study nurses found it challenging to find a relatively stable period during which to approach them about participating.
| Reason patients were not approached | n (%) |
|---|---|
| Died | 7 (10.6) |
| Discharged | 3 (4.5) |
| Inpatient | 4 (6) |
| Too unwell | 2 (3) |
| Other | 3 (4.5) |
| Missinga | 47 (71) |
| All | 66 (100) |
Another factor affecting the screening and approach processes was the contact time between study nurses and researchers or CRNs to undertake screening activity, which was not consistent across all sites due to pressure on study nurses’ time and the size of their caseloads. The number of patients screened per CNS varied from 17 to 68 (median 36). Maintaining weekly appointments with each CNS to screen new referrals and review recruitment of eligible patients was challenging and, although the screening process was seen as deliverable and acceptable by the study nurses, they were very aware that they (and sometimes the CRNs) were not available when they had said they would be for screening appointments. One that responded: ‘It was only tricky because of time’ (H1CNS002). Existing pressures on study nurses and their often high caseloads meant that screening appointments frequently needed to be rearranged/reattempted, with the result that screening was missed on some weeks. One study nurse stated she found the screening onerous and two said they would have preferred to screen with just the researcher (rather than screening with two people, e.g. the CRN and research fellow). Consequently, screening was undertaken regularly, but not always on a weekly basis.
Participant recruitment
Despite the low approach rate, the proportion of patients interested in participating after reading the information sheet was higher than expected (60%; see Figure 3). The majority of those who refused to participate did so because they felt that they were too unwell at the time of being asked. Participant interviews revealed two primary motivations for taking part:
-
It was an opportunity to give something back and to help others.
-
It was a way for them (and their carer) to learn something new that might help them to manage their pain.
Of the 37 patients who were given an information sheet, 19 (51.4%) consented to participate (hereafter referred to as participants), of whom nine (47%) also had a carer who consented.
Nurse recruitment
Across the four sites, 12 CNSs were trained to deliver the SMART intervention with their patients. The demographic details of the 12 study nurses are summarised in Table 12. Eleven of the 12 nurses completed an interview with a researcher at the end of the trial about their experiences of being part of the trial and delivering the intervention.
| Variable | Nurses, n (%) | |
|---|---|---|
| Study (N = 12) | Non-study (N = 15) | |
| Site | ||
| HANST1 | 4 (36.4) | 4 (26.7) |
| HANTS2 | 2 (18.2) | 3 (20) |
| YKHB1 | 3 (18.2) | 4 (26.7) |
| YKHB2 | 3 (27.3) | 4 (26.7) |
| Age | ||
| Median years (range) | 53 (49–54) | 51 (43–62) |
| Sex | ||
| Female | 12 (100) | 15 (100) |
| Band | ||
| 6 | 2 (16.7) | 4 (26.7) |
| 7 | 10 (83.3) | 9 (60) |
| 8 | 0 (–) | 2 (13) |
| Time working in palliative care | ||
| Median years (IQR) | 8.2 (1–16.7) | 10.5 (3.75–15.5) |
| Independent prescriber | ||
| Yes | 6 (54.6) | 4 (26.7) |
| No | 5 (45.4) | 11 (73.3) |
| Take part in an interview | ||
| Yes | 11 (92) | |
| No | 1 (8) | |
Three study nurses (25%, two in Hampshire and one in Yorkshire and Humber) recruited no participants. Three study nurses (25%) recruited one participant each, three (25%) recruited two participants each, two (17%) recruited three participants each and one (8%) recruited four participants.
Study nurses were asked whether or not they would be willing to audio-record their consultations with participants when using the SMART intervention to enable the research team to develop an intervention delivery checklist to assess fidelity. This was generally met with apprehension by the study nurses, although the majority agreed to do it. To allow study nurses time to get used to using the intervention it was decided that they would start recording SMART intervention consultations with their second participant. However, the completion rate for this part of the study was poor, primarily because half of the study nurses recruited one participant or none. In the end, only two nurses (in Hampshire) audio-recorded their SMART intervention consultations; however, this provided insufficient data to test an intervention delivery checklist. For a future definitive trial, alternative methods should be considered.
Baseline participant characteristics
Participants’ baseline demographic factors and clinical characteristics were broadly similar across the four sites (Table 13). Participants recruited from the two Hampshire sites had slightly lower (worse) Australia-modified Karnofsky Performance Scale score than the two northern hospices. There was a range of disease types, with the most common being breast cancer (26%), followed by liver or pancreatic cancer (16%). One patient had a non-cancer diagnosis (liver cirrhosis). Although the study was open to patients with any type of advanced disease, we found that most of those on strong opioids, and, therefore, eligible for the study, were patients with cancer.
| Variable | Recruitment site, n (%) | ||||
|---|---|---|---|---|---|
| All | HANTS1 | HANTS2 | YKHB1 | YKHB2 | |
| Participants recruited | 19 (100) | 6 (31.6) | 3 (15.8) | 6 (31.6) | 4 (21) |
| Age (years) | 66 (48–88) | 68 (63–88) | 68 (51–73) | 60 (48–82) | 59 (50–69) |
| Sex | |||||
| Male | 8 (42.1) | 2 | 1 | 2 | 3 |
| Female | 11 (57.9) | 4 | 2 | 4 | 1 |
| Karnofsky score | |||||
| Entry | 60 (50–70) | 55 (50–60) | 50 (50–70) | 60 (60–70) | 70 (60–70) |
| Completion | 60 (40–60) | 60 (50–60) | 50 (0–50)a | 60 (40–70) | 65 (30–70) |
| Primary disease | |||||
| Breast cancer | 5 (26.3) | 1 | 2 | 4 | 1 |
| Lung cancer | 4 (21) | 3 | 1 | 1 | 1 |
| Bowel cancer | 1 (5.3) | 1 | 1 | 1 | |
| Gynaecological cancer | 1 (5.3) | 1 | 1 | ||
| Pancreas/liver cancer | 3 (15.8) | ||||
| Liver cirrhosis | 1 (5.3) | ||||
| Bone cancer | 1 (5.3) | ||||
| Head/neck cancer | 1 (5.3) | ||||
| Metastatic sarcoma | 1 (5.3) | ||||
| Unknown primary | 1 (5.3) | ||||
| Time to referralb | |||||
| Days | 211 (17–414) | 17 (9–570) | 40 (12–292) | 414 (211–505) | 172 (19–330) |
| Weeks | 30 (2–59) | 2 (1–81) | 6 (2–42) | 59 (30–72) | 25 (3–48) |
| Reason for referral | |||||
| Pain only | 8 (42.1) | ||||
| Psychological support only | 1 (5.2) | ||||
| Pain + psychological support | 6 (31.5) | ||||
| Other symptoms | 4 (21) | ||||
| Pall treatments | |||||
| Yes | 10 (52.6) | 0 | 2 | 4 | 4 |
| No | 9 (47.4) | 6 | 1 | 2 | 0 |
| Treatment types | |||||
| None | 9 (47.4) | 6 | 1 | 2 | 1 |
| Chemotherapy | 6 (31.6) | 0 | 2 | 3 | 2 |
| Chemotherapy + radiotherapy | 3 (15.8) | 1 | 1 | ||
| Ascites drainage | 1 (5.3) | ||||
| Carer recruitedc | |||||
| Yes | 9 (47.5) | 4 | 2 | 1 | 2 |
| No | 10 (52.6) | 2 | 1 | 5 | 2 |
| Completed 6-week follow-up | |||||
| Yes | 15 (79) | 5 | 2 | 5 | 3 |
| No | 4 (21) | 1 | 1 | 1 | 1 |
There were differences in the median time from diagnosis of advanced disease to referral to palliative care services between the Hampshire and Yorkshire recruitment sites: the median (IQR) time between diagnosis and referral in Hampshire was 4 (IQR 2–61) weeks compared with 38.5 (IQR 9–59) weeks in Yorkshire and the Humber. The end-of-study interview data with the study nurses from both Hampshire sites emphasised that late presentation at diagnosis (i.e. more advanced disease) meant that many patients referred to palliative care services were close to the end of life and subsequently had limited exposure to specialist palliative care services.
Across all four sites, the most common reasons for referral to palliative care services were for pain control and psychological support. Just over half of participants (n = 10) were undergoing palliative treatment at the time of enrolment. Six patients were receiving palliative chemotherapy and three were receiving palliative chemotherapy plus radiotherapy.
At baseline, all participants were prescribed at least one strong opioid for pain relief (Table 14). Two participants (10%) had one strong opioid prescription, 15 participants (79%) had two strong opioid prescriptions (for background and breakthrough pain), one participant (5%) had three strong opioid prescriptions [two background (tablets + patch) and one breakthrough] and one participant (5%) had five strong opioid prescriptions. Of the 15 participants who received two strong opioid prescriptions, three (16%) were also prescribed a weak opioid. Co prescribing rates of laxatives, antiemetics, neuropathic analgesics and non-opioid analgesics were 74% (n = 14), 79% (n = 15), 68% (n = 13) and 74% (n = 14), respectively.
| Variable | All, n (%) (N = 19) |
|---|---|
| Strong opioid | |
| None | 0 |
| 1–3 | 18 (94.7) |
| ≥ 4 | 1 (5.3) |
| Weak opioid | |
| None | 16 (84.2) |
| 1–3 | 3 (15.8) |
| ≥ 4 | 0 |
| Non-opioid analgesic | |
| None | 5 (26.3) |
| 1–3 | 14 (73.7) |
| ≥ 4 | 0 |
| Neuropathic analgesic | |
| None | 6 (31.6) |
| 1–3 | 13 (68.4) |
| ≥ 4 | 0 |
| Laxative | |
| None | 5 (26.3) |
| 1–3 | 14 (73.7) |
| ≥ 4 | 0 |
| Antiemetic | |
| None | 4 (21) |
| 1–3 | 14 (73.7) |
| ≥ 4 | 1 (5.3) |
Randomisation acceptability
During the baseline visit, participants’ general willingness and acceptability of randomisation was assessed by asking the question, ‘If the study had been designed so that those taking part would be randomly selected to receive either the intervention or standard care would you have taken part?’.
A total of 17 out of 19 participants (89%) responded to this question by saying that they would. One participant did not understand the concept of randomisation and, therefore, preferred not to comment either way. One participant–carer dyad indicated anxiety about the relinquishing of control associated with randomisation and that maintaining control of ‘critical decisions in palliative care’ was important to them and this was the reason why they would not have agreed to randomisation.
Researcher follow-up
All follow-up visits were conducted by one of two researchers, face to face at participants’ homes. Overall, participant retention was high: of the 19 participants who consented, 15 (79%) completed 6 weeks of follow-up (see Figure 3). One participant completed all follow-up visits with a researcher but did not receive the intervention because of complex pain management issues that prevented the study nurse from devoting time within the consultation to initiating the use of the SMART intervention. Therefore, of the 19 consented participants, 14 (74%) received the intervention and completed 6 weeks of follow-up. However, an intention-to-treat analysis approach was taken to account for the variation in the amount of SMART intervention received by participants; therefore, all consented participants were included in the analysis. As such, the denominator at baseline was 19 and at all follow-up time points was 15.
Withdrawals
The end-of-life context meant that many patients experienced uncontrolled symptoms (not just pain) and infections during the course of the 6-week study period. Despite this, relatively few participants were lost to follow-up. There were four dropouts, all associated with a rapid unexpected decline in health:
-
One participant died 1 week after consenting, having received no SMART study nurse visits (only baseline data obtained).
-
One participant died between baseline and the 2-week follow-up, having received two SMART study nurse visits (only baseline data obtained).
-
One participant withdrew from researcher follow-up visits because of uncontrolled pain in week 2 (prior to week 2 follow-up), but continued to use the goal-setting element of the SMART intervention with the study nurse on four more occasions (baseline data obtained + evidence of four goal-setting sheets from the study nurse).
-
One participant withdrew between baseline and the 2-week follow-up because of declining health and was subsequently moved to a nursing home where they were no longer managing their medicines; this participant received one SMART study nurse visit (only baseline data obtained).
In order to capture accurately why, when and from what participants withdraw, a future trial should explicitly record what parts of the trial participants are withdrawing from (i.e. the research elements or the intervention elements).
Acceptability of study length and frequency of follow-up visits
The 6-week study period was universally seen as ‘about right’ or ‘just right’ by the participants and their carers: ‘I think it’s just about right actually, you probably need that length of time to get any results’ (H2Pt019) and ‘It seems to have gone quick’ (H1Pt001-C). The study nurses also appeared to agree on the acceptability and deliverability of a 6-week study period.
In terms of the deliverability of follow-up appointments with researchers, Figure 6 shows that in the majority of cases it was feasible to conduct three follow-up visits within a tight time frame of 2 days either side of the scheduled fortnightly follow-ups. Nevertheless, it was not always possible to conduct follow-up visits within this tight time frame (see Figure 6). This is not surprising given the complex nature of participants’ homes.
FIGURE 6.
Time elapsed between baseline and researcher follow-up visits.
Interview data revealed that participants and carers all stated that the frequency of researcher visits (fortnightly) was acceptable, and some looked forward to these visits. Participants responded, ‘It’s been very nice you coming in’ (H2Pt019) and ‘It’s been lovely. I enjoy you coming . . . you’re so easy to talk to, you ask the right questions’ (H1Pt009). These responses indicate that people approaching the end of life valued taking part in a research study and that they saw the researcher as external to their care team and were able to develop a positive relationship with them over the 6-week duration of the study.
Participant and carer interviews
Thirteen participants completed an interview with a researcher (Table 15). Although nine carers consented at baseline to participate in an interview, only seven were available to complete an interview with a researcher.
| Variable | n (%) |
|---|---|
| Number of participant interviews completed | 13 (68.4) |
| Previous (or current) occupationa | |
| Manual worker | 5 (38) |
| Health-care worker | 2 (15) |
| Professional/managerial | 4 (31) |
| Academia | 2 (15) |
| Qualificationsa | |
| None | 4 (31) |
| Below university degree | 6 (46) |
| University degree or higher | 3 (23) |
| Number of carers presenta | 7 (54) |
| Carer occupationb | |
| Manual worker | 2 (29) |
| Health-care worker | 2 (29) |
| Professional/managerial | 3 (42) |
| Carer qualificationb | |
| None | 1 (14) |
| Below university degree | 5 (71) |
| University degree or higher | 1 (14) |
Survival following study entry
At the close of the study, three participants were known to have died. Two participants died during the follow-up period at 21 days and 14 days following baseline assessment, respectively. One patient died after the follow-up period, 71 days following baseline assessment. Two participants withdrew from the study follow-up data collection for reasons of declining health; the number of days between study entry and withdrawal was 14 days in one case and 27 days in the other. These data are summarised in Figure 7.
FIGURE 7.
Survival (days) for each participant.

Fidelity and acceptability of the SMART intervention
Intervention delivery
To what extent was study nurse training provided as planned?
During the interviews, study nurses were asked to comment on the training workshop (described in SMART intervention). Responses to the experiential, reflective style of the training workshops were mixed; they varied widely from generally or overtly positive through to neutral and negative responses. The reflective nature of the session appealed to some, whereas others found it challenging and preferred alternative approaches (e.g. along the lines of advanced communications training, accompanied by a video of a modelled conversation and then subsequent group discussion). However, critical reflection on existing practice is necessary to stimulate change, which is fundamental to the intervention delivery. 78,79 Nevertheless, there was a general view that the sessions covered what the nurses needed to know in anticipation of their study involvement.
There was a mixed reception to the underpinning self-management ethos of the sessions. During the workshops, the Johnston et al. 51 definition of self-management support in palliative nursing was explored, as well as the related eight professional roles from advocate to reporter (see Appendix 25). Some of the study nurses appeared to readily understand the supported self-management ethos, whereas others were more challenged by it. The self-management ethos of the study and the training sessions challenged the professional behaviours and identity of some of the specialist palliative care nurses, whose therapeutic role is often measured by their effectiveness to ameliorate pain. The nurse interviews also revealed that it was harder for some nurses to adapt their practice from imposing their views (i.e. telling patients how to use opioids) to collaborative discussion. Such discussion focused on the individual patient using and developing their own self-management strategies as result of the information provided by the nurse.
There was feedback that the four-step educational approach was viewed as akin to usual specialist practice, but some nurses felt that the workshop sessions made the four steps seem more complicated than it actually was. The nurses generally felt that the four-step approach mirrored normal practice and, consequently, was not viewed as being distinctive or novel. The study nurses reported that they valued the training materials supplied during and after the training workshop (see Appendices 18 and 19, and Figure 8). In particular, the nurses liked the figure illustrating the self-management conversation prompts mapped to the four steps of the educational approach (see Figure 8). Despite this, there was variation in the extent to which nursers referred to the training resources over the course of the study, with the majority of use being at the start of the trial.
FIGURE 8.
Diagram of the SMART four-step educational process.

There was insufficient opportunity during the training workshop for the study nurses to become familiar with the research process, which led to some anxiety during the workshop. This had an impact on time available for role modelling a conversation in practice. Therefore, a future definitive trial should consider providing an opportunity to receive information and ask questions about the research process prior to any training.
Based on our finding of a mixed reception to the self-management ethos, it appears important that nurses process the intervention and rehearse how they deliver self-management-focused conversations that fit their own practice. There are likely to be different timelines regarding adoption of such a focus, and nurses vary in how much they need to practise this before it becomes embedded. Therefore, some form of further training and support for those involved in delivering the intervention is desirable and could be combined with efforts to assure intervention fidelity.
The busy and time-pressured reality of clinical practice for these specialist nurses, who often managed large caseloads, meant that the delivery of ongoing training and support during the course of the trial was often problematic. These issues were captured in the researchers’ field notes and included difficulty making appointments to meet the study nurses. Visits usually had to be made at the start of their working day. However, not all the study nurses visited their office before going out to visit patients, and none wanted to make appointments at the end of the day. Furthermore, visits to the study nurses were complicated by nurses covering weekend working and, therefore, having days off in the week. Once appointments were made, the study nurses were not always subsequently available (because of extended/unexpected patient visits, over-running meetings, their own illness, etc.). Nonetheless, when they were available, they were usually open to discussing their experiences of delivering the intervention (utilising the four-step educational approach), but time devoted to this was always pressured or limited. Over the course of trial, the study nurses were usually visited by the study researchers once a fortnight depending on their availability, which was less often than the weekly frequency originally intended.
To what extent was the intervention delivered by study nurses as planned?
Table 16 summarises the pattern of the intervention delivery for each participant. Overall, there were 52 SMART study nurse visits across all participants, evidenced by completion of study nurse CRFs. When study nurse CRFs were missing, evidence of a SMART intervention visit having taken place was gathered from participants’ clinical records, the presence of a goal-setting sheet having been completed (with date) or from researchers’ field notes. The final column in Table 16 groups the participants based on whether they received the intervention as planned (group A), partially (groups B and C) or not at all (groups D and E).
| ID – site | Start 7 days? | Number of SMART visits | Average visit time (minutes) | New resources at each follow-upa | Number of factsheets received | Number of goal-setting sheets received | Podcast films watched? | Intervention delivered as plannedb | Complete all follow-up visits? | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 2 | Week 4 | Week 6 | |||||||||
| 1 – YKHB2 | Yes | 3 | 62 | 1, 5, 7, 10, 11 | 6, 11 | 2, 3, 4, 8, 9, 11 | 10 | 3 | No | A | Yes |
| 2 – YKHB2 | Yes | 3 | 60 | 1, 7, 8, 11 | 4, 9, 10, 11 | 2, 3, 5, 6, 11 | 10 | 3 | Yes | A | Yes |
| 3 – HANTS1 | Yes | 4 | 47 | 2, 3, 7, 11 | 10, 11 | 11 | 4 | 3 | No | A | Yes |
| 4 – HANTS1 | Yes | 5 | 44 | 2, 3, 8, 11 | 5, 11 | 11 | 4 | 5 | No | A | Yes |
| 5 – HANTS1 | Yes | 4 | 71 | 1, 2, 4, 8, 10, 11 | 3, 7, 11 | 11 | 7 | 4 | No | A | Yes |
| 6 – HANTS1 | Yes | 3 | 47 | 1, 2, 11 | 7, 8, 9, 11 | 11 | 5 | 3 | No | A | Yes |
| 7 – HANTS1 | Yes | 4 | 55 | 1, 2, 10, 11 | 8, 11 | 11 | 4 | 3 | No | A | Yes |
| 8 – HANTS2 | Yes | 3 | 62 | 2, 3, 5, 11 | 11 | 9, 10, 11 | 3 | 3 | Yes | A | Yes |
| 9 – HANTS2 | Yes | 4 | 52 | 2, 7, 9, 10, 11 | 11 | 11 | 4 | 4 | Yes | A | Yes |
| 10 – YKHB1 | Yes | 2 | 60 | 11 | 11 | 11 | 0 | 2 | No | A | Yes |
| 11 – YKHB2 | Yes | 3 | 45 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 | 11 | 11 | 10 | 3 | No | B | Yes |
| 12 – YKHB1 | Yes | 3 | 60 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 | 11 | 11 | 10 | 3 | Yes | B | Yes |
| 13 – YKHB1 | No | 3 | 60 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 | 11 | 11 | 10 | 3 | No | B | Yes |
| 14 – YKHB1c | Yes | 4 | 22 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 | – | – | 10 | 4 | No | B | No |
| 15 – YKHB1 | No | 1 | 60 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 | – | – | 10 | 1 | Yes | C | Yes |
| 16 – YKHB2d | Yes | 2 | 70 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 | – | – | 10 | 1 | No | C | No |
| 17 – HANTS2e | Yes | 1 | 95 | 7, 8, 10, 11 | – | – | 3 | 1 | No | C | No |
| 18 – YKHB1f | No | 0 | 0 | – | – | – | 0 | 0 | – | D | Yes |
| 19 – HANTS1g | No | 0 | 0 | – | – | – | 0 | 0 | – | D | No |
Of the 19 participants who consented, 17 (89%) had a first SMART study nurse visit and started using the intervention (see Table 16). From this point, 10 participants (53%) received the intervention as planned (group A). One participant in group A decided not to have the factsheets after reading through them, but did engage with the goal-setting. As the information provision was designed to be tailored to the participants’ needs, this still met the criteria for this element of the intervention. Four participants (21%, group B) received the minimum number of SMART study nurse visits, goal-setting, and review and coaching, but the information provision was not staged as they received all the factsheet on their first SMART study nurse visit. This does not necessarily represent inappropriate delivery of the intervention resources; however, it was not clear whether all the factsheets were delivered on the first visit at the participant’s request (which would satisfy the criteria for tailored provision of information) or whether the study nurses handed them all over in one go. One participant in group B [identification (ID) 14 – YKHB1; see Table 16] withdrew from researcher follow-ups because of uncontrolled pain but continued to engage with the goal-setting and reviewing (steps 3 and 4 of the intervention). Three participants (16%, group C) did receive the intervention materials and the goal-setting, but did not receive the minimum number of SMART study nurse visits. However, in group C one participant died during the first 2 weeks of the study and one participant was lost to follow-up because of rapidly declining health (see Table 16). Finally, two participants (10.5%, group D) did not receive any elements of the intervention: one participant died within a week of giving consent and the other did not receive the intervention (because of complex pain management issues that prevented the study nurse from starting the SMART intervention), but did complete all researcher follow-up visits.
The timing of study nurse visits is summarised in Figure 9, which shows that participants generally received a SMART study nurse visit once a week or once a fortnight. The average duration of SMART study nurse visits was 57.2 minutes (SD 15.1 minutes), but this covers the whole consultation and not just time spent using the SMART intervention. Interview data with study nurses revealed that they were able to successfully deliver the SMART intervention during their visits and the pattern of delivery was acceptable as it matched their normal visiting pattern. However, in relation to the acceptability of the length of the SMART study nurse visits, the nurses were very conscious of the extra time required for study visits (approximately 30 minutes for the first visit and 15 minutes for each further visit) and the impact this had on their workload. One study nurse responded:
We [one study nurse and another study nurse] talked about timekeeping a lot, because in my first couple of SMART study appointments they were really long, partly because the lady I had, it was quite difficult to keep to time with them anyway.
H2CNS001
FIGURE 9.
Timing of SMART study nurse visits.

The study nurses managed to successfully accommodate this additional workload without changes to their usual care patterns, except for one (part-time) study nurse who asked a colleague to follow up some patients on her caseload by telephone on a single day because of a SMART study visit. The extra time required for SMART study nurse visits led the study nurses at the individual sites to discuss this impact with one another.
To what extent were each of the intervention elements implemented as planned?
Overall, the deliverability of the four-step educational approach was initially seen by the study nurses as challenging and required practice. However, they perceived the delivery of the four steps in a therapeutic conversational style, which enabled it to flow naturally and recognised them as being inherently part of what specialist nurse practice looks like. Study nurses responded:
The four-step process sort of reflects the nursing process really doesn’t it? You know that’s what you do, or what you should be doing. But I think having it in your head more concretely and having things that you do at each of those steps just makes it more real.
H4CNS003
I followed this process . . . I found that kind of reflected pretty much what we do with that sort of pain assessment and their usage of medication assessment that followed quite well anyway.
H4CNS001
Fully embedding the concept of supported self-management in end-of-life care took time and practice for the study nurses. The reflective patient-led approach to the educational process challenged their desire to go in and immediately ‘come up with solutions’ (H2CNS002). In addition, all the study nurses were experienced palliative care clinicians and the standardised approach to the four steps challenged their own working style which they had evolved. Consequently, the shift in thinking required for delivery of the self-management ethos within the educational approach was accommodated more successfully by some of the study nurses than by others. For example, one said, ‘I need to just take a little step back and let the patient tell me what they want to do a little bit more’ (H2CNS002).
The deliverability of the individual elements of the four-step educational approach was mixed. The quality/completeness of evidence for each step varied owing to poor completion of the study nurse CRFs, which were intended to document what had been delivered by the study nurses at each SMART visit (Table 17). Undertaking the needs assessment (step 1) was universally recognised by the study nurses as part of their usual practice and did not present as a challenging aspect of the intervention. Table 17 shows that, based on the study nurse CRFs and the presence of staged information provision and goal-setting (which were predicated on having undertaken a needs assessment), all of the 17 participants who started using the intervention received a needs assessment.
| ID – sitea | Step | |||
|---|---|---|---|---|
| 1: need assessmentb | 2: staged information provisionc | 3: goal-settingd | 4: review and coachinge | |
| 1 – YKHB2 | Yes | Yes | Yes | Yes |
| 2 – YKHB2 | Yes | Yes | Yes | Yes |
| 3 – HANTS1 | Yes | Yes | Yes | Yes |
| 4 – HANTS1 | Yes | Yes | Yes | Yes |
| 5 – HANTS1 | Yes | Yes | Yes | Yes |
| 6 – HANTS1 | Yes | Yes | Yes | Yes |
| 7 – HANTS1 | Yes | Yes | Yes | Yes |
| 8 – HANTS2 | Yes | Yes | Yes | Yes |
| 9 – HANTS2 | Yes | Yes | Yes | Yes |
| 10 – YKHB1 | Yesf | Yes | Yes | Yes |
| 11 – YKHB2 | Yes | Unclear | Yes | Yes |
| 12 – YKHB1 | Yesf | Unclear | Yes | Yes |
| 13 – YKHB1 | Yesf | Unclear | Yes | Yes |
| 14 – YKHB1 | Yes | Unclear | Yes | Yes |
| 15 – YKHB1 | Yesf | No | Yes | No |
| 16 – YKHB2 | Yes | No | Yes | Yes |
| 17 – HANTS2 | Yes | No | Yes | No |
| 18 – YKHB1 | No | No | No | No |
| 19 – HANTS1 | No | No | No | No |
Overall, step 2 (giving information supported by educational resources in a staged and tailored approach) was adhered to for the majority of participants. A total of 10 out of 19 participants (52.6%) received the educational resources as intended (see Table 16, group A), whereas four participants received all the factsheets resources at the first SMART visit. It is unclear whether or not this latter method of information provision was at the participant’s request; however, one study nurse responded that ‘I just handed everything over’ (H4CNS001). This type of delivery may have negatively influenced the acceptability of the factsheets for these participants. The following argument for giving all the factsheets on the first SMART visit was put forward by one study nurse:
When somebody’s newly started on an opioid . . . you can’t kind of pre-guess what they’re going to need. And to me the whole point of it is that you are giving them the tools to be able to self-manage . . . they should have all of them [the factsheets] at the beginning so that they’ve got the information there.
H4CNS001
Table 16 shows that all of the 17 participants who started using the SMART intervention received a goal-setting sheet on their first SMART visit (step 3, resource 11). Subsequently, of the 14 participants who received the intervention and completed a 6-week follow-up visit (see Table 16, IDs 1–14), only one did not receive continued goal-setting and regular review and coaching (step 4). The study nurses universally perceived the goal-setting and regular review process as acceptable and deliverable. They identified the goal-setting as a core component of the intervention and perceived value in it because it formalised and evidenced their specialist practice. It also facilitated review and coaching as the previous goals because they were there to ‘reflect back on’ (H4CNS003). Consequently, goal-setting often became the mechanism by which participants were helped to focus on doing things for themselves (i.e. implementing self-management strategies). Participants and carers also recognised the value of the goal-setting process as focusing on their needs and motivating behaviour change:
That’s been helpful . . . I think it has made me a bit more explicit about setting goals and saying to [CNS name] ‘I’d like to do this, can you help me do this?’
H3Pt002
If you set a goal, even if you don’t reach it, I still think it’s a good thing to do.
H4Pt013
Participants’ perceived disadvantages of goal-setting were related to having different expectations of the process, ‘sometimes your perception of what they are going to write is just completely different to what they come out with’ (H2CNS001). Some participants struggled to think what their goals would be in the context of clinical depression or a degree of memory loss.
In addition to the goal-setting, the only other core factsheet resource was ‘contacts and further information’ (resource 2), which the CNSs were asked to deliver to the trial patient on their first SMART visit. Thirteen (68%) participants received this factsheet on their first SMART study nurse visit (see Table 16). By the 6-week follow-up time point, a further two participants had received this core factsheet.
Overall deliverability of the intervention
A total of 14 out of 19 (74%) participants received the intervention as intended or partially as intended (see Table 16, groups A and B). Evidence of the four steps of the intervention having been completed was present for the majority of participants who started using the intervention (see Table 17). The end-of-life context provided a complex set of circumstances within which study nurses had to deliver the intervention. Consequently, not all participants were able to fully engage with all elements of the intervention; however, overall the four-step educational approach appears to have been adhered to by the study nurses.
Acceptability of the intervention
Acceptability of the self-management support toolkit components
Overall, the participants found the factsheet resources to be easy to read, clear and not too long. In the case of the few participants who received factsheets and did not read them or just scanned them, the resources were usually read by their carer and were perceived to be of benefit to them. The factsheet ‘Contacts and further information’ was often poorly delivered (i.e. not completed by nurses), but participants saw it as highly relevant and acceptable: ‘all the numbers you need are there, it’s a brilliant idea’ (H1Pt015-C) and ‘it’s just reassurance you know, an easy reference, just in case’ (H2Pt007).
Other than the core resources, factsheets that were commonly delivered on the first visit were ‘Managing pain with opioids’ (n = 11) and ‘Common concerns’ (n = 10) (see Table 16). Both were considered by study nurses to be essential resources as they helped to address concerns and expectations, ‘I think that perhaps all patients should have that information [common concerns factsheet] as a standard’ (H1CNS001). By the end of 6-week follow-up, 10 participants had received all 10 factsheet resources (see Table 16).
The medication chart (resource 10) was delivered to 14 participants (see Table 16); however, the frequent non-completion of the medication chart by the study nurses meant that its use and benefit varied. Some nurses provided and completed a simplified one-page medication chart, whereas others helped participants to produce their own versions (either paper based or using a spreadsheet). A number of participants indicated that the medication charts helped them to plan activities away from the home by preparing the necessary medicines to take with them – something that they previously would not have engaged in. Similarly, the medication chart also facilitated carer involvement as it allowed for pre-emptive planning for deterioration of the participant (i.e. carers were able to familiarise themselves with the medication chart and respond appropriately if the participant was unable to).
The pain diary (resource 9) was successfully delivered to 11 participants (see Table 16) who viewed it as acceptable, with the majority using it regularly. Participants and carers responded during the end-of-study interview that the pain diary formalised and recorded information about the timing, intensity of pain and outcome of analgesia comprehensively. Participants also responded that it helped with managing breakthrough opioid medication because it enabled them to monitor pain intensity rating throughout the day. The study nurses universally saw the pain diary as a helpful tool to monitor pain and evaluate the effectiveness of analgesic medication and non-drug pain relief strategies (e.g. distraction or hot bath). Overall, participants reported that the pain diaries helped them to keep track of the effectiveness of their medicines by recording when a pain episode occurred, what action was taken and the response. This had the effect of relieving the pressure and anxiety of having to remember these details. In addition, it made participants, carers and study nurses aware of the pattern of pain events throughout the day and stimulated conversations around adjustments to medicines and pain management in general.
The podcast films were watched by five participants who perceived them to be acceptable and:
. . . reassuring . . . I think the information was very useful, because it did home in on the fact that you’re in control . . . that came across very clear.
H2Pt019
Nevertheless, it was noted that the podcast films (as with some of the factsheet resources) would have been of greater use earlier on in participant’s experience of managing pain with opioid medicines. For example:
I think that would be useful if I was at the start of the process, but now, with all the things that were said in the DVD, I kind of already knew, especially the chap who was managing his prostate cancer, I’ve been through the same process myself.
H3Pt002
Importantly, participants responded that they valued the authenticity of the subjects in the films (one patient and two specialist palliative care nurses), particularly as they were sharing their experience and self-management strategies. Only one participant who received the podcast films did not watch them, which was in the context of untreated depression. Study nurses responded during the end-of-study interviews that they thought the podcast films were helpful for participants and their carers, and having an alternative to paper-based information suited some people well. However, overall the podcast films were not offered by the study nurses to all participants. This may have been an issue of practicality; for example, one study nurse said that she forgot about them because they were separate from the main file with the factsheet resources, whereas another noted that she did not carry the DVD/memory sticks with them when she visited participants.
Finally, the goal-setting sheets were universally seen by participants, carers and study nurses as acceptable. The study nurses responded during the end-of-study interviews that the goal-setting sheets formalised and evidenced their specialists practice as well as facilitating the review and coaching of previously made goals:
I could actually say to you now, with the patients, I’m actually at this point with them . . . we’ve set these goals and I’m off today to reflect on those and identify any other issue.
H4CNS003
Participants also responded positively to the goal-setting, indicating that the process was manageable and helped them to be more explicit about the things they wanted to achieve. It also helped stimulate participants’ thinking around performing the tasks necessary for self-managing medicines such as:
. . . right this is what we’ve got to do now, and get this sorted . . . it’s made us more aware to help things along.
H1Pt015
Completion rate for the goal-setting sheets was high: of the 52 SMART study nurse visits that occurred across all participants, there was evidence of goal-setting (or review of goal-setting) at 44 (85%). In the majority of cases, the goal-setting sheets were delivered and completed well, with appropriate patient-focused goals set and action plans made to achieve the goals. One participant who withdrew from researcher follow-ups (due to uncontrolled pain) continued to use the goal-setting sheets.
Overall, the patients, carers and CNSs always perceived some benefit to the SMST. The goal-setting was the most frequently valued element, but often other elements were also liked (e.g. the pain diary). The factsheets often reinforced information provided by the CNSs or that the patients already knew and they stimulated patients and carers to ask further questions. If the factsheets were not read by the patient, then they were often valued by their carer. The CNSs particularly valued the common concerns factsheet. The podcast films were valued by those who had been provided with them.
Overall, there was a range of participant responses to the SMST as a whole from the overtly positive, ‘we’ll treasure that’ (H1Pt001), through to more general, ‘it has helped me, definitely’ (H3Pt041); a minority of patients did not fully engage with it. Generally, the study nurses viewed the SMST as of value particularly as a resource to support verbal information provision and to refer back to when reviewing self-management progress. For example:
It’s just a solid piece of evidence, rather than us just trying to explain things to patients and sort of jot things down for them, they’ve actually got information that we can leave with them that they can use . . . I’d like to be able to use these tools with other people that come onto my caseload. I think they are very useful.
H4CNS003
To have all of this to give them, kind of backs up what we say, rather than it’s just you talking to the patient, and then the minute you’ve left the house they’ve got nothing then to hold on to.
H4CNS001
However, given the complex end-of-life circumstances for some participants, not all were able to engage with the intervention or benefited from it directly themselves. For example:
Unfortunately things have gone from bad to worse with him [study patient] deteriorating and [name of carer] not being very well, I think it was just perhaps a bit too much.
H1CNS001
Acceptability of the four-step educational approach
The deliverability of the four-step approach was seen as acceptable to the study nurses, who did not perceive that they needed any additional skills to deliver it; it was viewed as a normal part of the specialist role. Still, study nurses perceived value in the explicit nature of the four-step educational approach as it formalised and gave structure to supporting participants and their carers to self-manage medicines within the complexity of the end-of-life context with frequent deterioration of health and depression.
All participants stated that they derived a benefit from the intervention, but the extent to whch the participants were aware of, or acknowledged, the four-step approach varied. The acceptability of the delivery of the intervention and resulting benefit were increased for the participants and their carers because of the almost universal value that they placed on contact with their study nurse (particularly when face to face and in their own homes).
The second part of the four-step approach was provision of information tailored to patients’ needs. Frequently, delivery of the SMST resources (i.e. the factsheets) by the study nurses was less than ideal, which, as a result, had an impact on the acceptability and benefit of the resources, particularly when they were not discussed or talked through, or when all the factsheets were given all together as a large file. There was also difficulty in providing the intervention at the most appropriate time for participants (and their carers) while they were well enough to engage in it, given the unpredictability of end-of-life context.
Overall, the four-step educational approach helped to stimulate suggestions for self-management strategies and then enabled patients, carers and study nurses to determine which strategies worked for the individual. In addition, the intervention as a whole (see Box 3) stimulated appropriate questioning by the patient or carer to the CNS; for example, ‘it’s been easy to ask questions’ (H1Pt001). Participants who were already effective self-managers prior to the trial felt that they would have used the materials more if they had received them earlier (at their first contact with their CNS). For example:
I’ve been doing this for over a year. And a lot of the things in here I knew. And frankly, I’m a very organised person, so I’ve got all the diaries, and I’ve got all the prescriptions, and they’re all online. Whereas it takes a while to get to that stage, to figure out what you’re meant to be doing, how you’re meant to be doing it.
H3Pt002
Completion of study nurse case report forms
Study nurses were asked to complete a brief CRF after each SMART visit, documenting what had been delivered. Completion rates were high; study nurses’ CRFs were completed in 43 out of 52 SMART intervention visits that were delivered across the whole feasibility study (83% completion rate). For SMART study nurse visits for which study nurse CRFs were missing, information on the date of visit was gathered from participant clinical records and evidence of the four-step educational process and the use of the SMST tool resources (including whether or not goal-setting sheets had been completed) was gathered by researchers at follow-up visits. For four participants [all from the same site in Yorkshire and the Humber (YKHB1)] no study nurse CRFs were completed.
Participant self-reported outcomes
Exploratory analysis of participant self-reported outcomes
Descriptive statistics (mean and 95% CIs) of the participant self-reported outcome measures at each time point and at 6 weeks compared with baseline (difference) are presented in Table 18 and summarised overall by time point. Owing to the small number of participants taking part, further outcome summaries by potential confounders such as disease state, age, sex, level of support and recruitment site, were not undertaken.
| Participant-reported outcome | Study time point | Differencea | |||
|---|---|---|---|---|---|
| Baseline | Week 2 | Week 4 | Week 6 | ||
| BPI (scale 0–10) | |||||
| Average pain | 4.3 (3.1 to 5.6) | 3.3 (2 to 4.6) | 4.1 (2.4 to 5.7) | 3.5 (2.3 to 4.8) | –0.2 (–1.5 to 1.1) |
| Pain interference | 4.3 (3.1 to 5.5) | 3.5 (1.8 to 5.2) | 2.7 (2.4 to 5) | 2.5 (1.4 to 3.6) | –1.6 (–2.8 to –0.4) |
| Worst pain | 6.1 (4.5 to 7.6) | 5.3 (3.4 to 7.2) | 5.9 (3.9 to 7.8) | 5.4 (3.6 to 7.2) | –0.1 (–1.5 to 1.4) |
| Least pain | 2.8 (1.4 to 4.2) | 2 (0.7 to 3.3) | 2.7 (1.2 to 4.2) | 2.5 (1.1 to 3.8) | 0.1 (–1.1 to 1.3) |
| Present pain | 2.9 (1.8 to 4.1) | 2.7 (1.5 to 3.8) | 3.5 (1.8 to 5.1) | 3.7 (1.9 to 5.5) | 0.8 (–0.8 to 2.4) |
| SES (scale 0–10) | |||||
| Total score | 7.1 (6.3 to 7.9) | 7 (6.2 to 7.8) | 7.5 (6.5 to 8.5) | 7.7 (6.7 to 8.6) | 0.7 (0.3 to 1.1) |
| ESAS (scale 0–10) | |||||
| Total score | 2.3 (1.7 to 2.9) | 2.6 (1.6 to 3.6) | 2.9 (2.3 to 3.5) | 2.7 (1.9 to 3.5) | 0.1 (–0.5 to 0.7) |
| EQ-5D (scale 0–1) | |||||
| Health status | 0.52 (0.4 to 0.63) | 0.56 (0.44 to 0.68) | 0.52 (0.4 to 0.63) | 0.58 (0.44 to 0.7) | 0.05 (–0.11 to 0.21) |
| SIMS (scale 0–17) | |||||
| Total score | 11.7 (9.7 to 13.8) | 13.8 (11.4 to 16.2) | 13.4 (11.4 to 15.4) | 13.7 (7.5 to 20) | 1.7 (–4.5 to 7.8) |
This study was not powered to detect any changes in outcome measure score, and there was no change in average pain scores; however, there was a slight reduction in interference from pain (–1.6, 95% CI –2.8 to –0.4) and a modest increase (0.7, 95% CI 0.3 to 1.2) in self-efficacy scores (see Table 18). There was no overall change in the intensity of common end-of-life symptoms (ESAS), health-related quality of life [EuroQol-5 Dimensions (EQ-5D)] or satisfaction with information about medicines (SIMS).
Figures 10–12 present histograms of the change in average pain, pain interference and self-efficacy scores, respectively. These histograms show that, for the majority of participants, average pain intensity worsened over the study period (i.e. scores of > 0), whereas there was greater stability or improvement across the board on BPI pain interference (i.e. scores of ≥ 0) and, largely, there were improvements for all participants on the self-efficacy scale (i.e. scores of > 0). Table 19 presents the variability (SD) with 95% CI for candidate primary outcome measures, along with the estimated effect size for the change in average pain, pain intensity and self-efficacy at 6 weeks compared with baseline.
FIGURE 10.
Histogram of change in BPI average pain intensity scores. N.B. change scores calculated week 6 score minus baseline score.

FIGURE 11.
Histogram of change in BPI pain interference score. N.B. change scores calculated week 6 score minus baseline score.
FIGURE 12.
Histogram of change in SES score. N.B. change scores calculated week 6 score minus baseline score; > 0 indicates improvement.
| Time point | Candidate primary outcome variable, SD (95% CI) | ||
|---|---|---|---|
| BPI average pain | BPI pain interference | SES | |
| Follow-up week 2 | 2.6 (1.9 to 3.8) | 2.5 (1.9 to 3.7) | 1.6 (1.2 to 2.4) |
| Follow-up week 4 | 2.4 (1.7 to 3.7) | 3.1 (2.2 to 4.8) | 1.4 (1.0 to 2.2) |
| Follow-up week 6 | 3.0 (2.2 to 4.7) | 2.4 (1.7 to 3.8) | 1.8 (1.3 to 2.9) |
The number, proportion and 95% CI around the proportion of participants with clinically meaningful reduction in average pain and pain interference are summarised in Table 20. These data show that at follow-up weeks 2 and 6 there were more responders based on pain interference than average pain intensity.
| Time point | Responders | |||||
|---|---|---|---|---|---|---|
| BPI average pain | BPI pain interference | |||||
| Number of responders | % | 95% CI | Number of responders | % | 95% CI | |
| Follow-up week 2 | 4 | 26.7 | 7.8 to 55.1 | 6 | 40.0 | 16.3 to 67.7 |
| Follow-up week 4 | 3 | 20.0 | 4.3 to 48.1 | 3 | 20.0 | 4.3 to 48.1 |
| Follow-up week 6 | 3 | 20.0 | 4.3 to 48.1 | 7 | 46.7 | 4.3 to 48.1 |
Participant acceptability of self-reported outcomes
Generally, participants’ experience of completing the self-reported outcome measures was acceptable; however, there were some limitations related to the wording of some questions given the end-of-life context (e.g. ‘normal work’ and ‘enjoyment of life’ on the interference subscale of the BPI). A small number of participants criticised the ‘duplicity’ (H4Pt001) of some questions, given the combination of five different measures. Overall, there was dislike for the SIMS as many participants experienced difficulty remembering specific information that they had received about their medicines over the preceding 2 weeks.
Overall, completion rates for the questionnaire packs were high: all questionnaires were completed by participants with a researcher at each time point. The proportion of missing data at individual item level was very low (Table 21) and did not prevent any summary scores from being calculated at any time point. The proportion of missing data was < 3% for all participant self-reported outcome measures at all time points, except for BPI at the week 4 follow-up (3.9%). The most commonly missing item on the BPI was the final item about the extent to which pain interferes with enjoyment of life (missing in three cases from the same participant); a number of participants responded that they felt that this question was inappropriate for people approaching the end of life. Similarly, for the EQ-5D, one response to the final item asking respondents to rate their overall health from best to worst was missing at all time points (from the same participant in each case). The field notes kept by the researchers identified a general lack of acceptability of this question by participants. One participant responded during the end-of-study interview, ‘it’s a stupid question to ask people in palliative care’ (H1Pt011-C), when asked specifically about this response item. The SIMS was least liked by the participants; nevertheless the proportion of missing data was extremely low.
| Study time point | Outcome measure | Number of items overall (all participants) | Number of (%) missing items |
|---|---|---|---|
| Baseline (n = 19) | BPIa | 228 | 2 (0.9) |
| SESb | 114 | 0 (–) | |
| ESASc | 190 | 3 (1.6) | |
| EQ-5Db | 114 | 2 (1.8) | |
| SIMSd | 323 | 0 (–) | |
| Week 2 follow-up (n = 15) | BPIa | 180 | 4 (2.2) |
| SESb | 90 | 1 (1.1) | |
| ESASc | 150 | 2 (1.3) | |
| EQ-5Db | 90 | 1 (1.1) | |
| SIMSd | 255 | 1 (0.8) | |
| Week 4 follow-up (n = 15) | BPIa | 180 | 7 (3.9) |
| SESb | 90 | 0 (–) | |
| ESASc | 150 | 4 (2.7) | |
| EQ-5Db | 90 | 1 (1.1) | |
| SIMSd | 255 | 0 (–) | |
| Week 6 follow-up (n = 15) | BPIa | 180 | 5 (2.8) |
| SESb | 90 | 0 (–) | |
| ESASc | 150 | 2 (1.3) | |
| EQ-5Db | 90 | 1 (1.1) | |
| SIMSd | 255 | 0 (–) |
Primary outcome measure for a definitive trial
The level of missing data for the BPI average pain, BPI pain interference and SES was negligible (see Table 21). There were no ceiling or floor effects for the BPI average pain (maximum score = 10, reported by n = 0; minimum score = 0, reported by n = 1). For BPI pain interference, no ceiling effects were found (maximum score = 10, reported by n = 0); however, marginal floor effects were observed (minimum score = 0, reported by n = 4). No floor or ceiling effects were found on the SES (maximum score = 10, reported by n = 0; minimum score = 0, reported by n = 0). There was greater stability or improvement by 6-week follow-up across the board on BPI pain interference (i.e. scores of ≤ 0) compared with BPI average pain intensity and, largely, there were improvements for all participants on the self-efficacy scale. Taking account of the variability in participants’ change in scores from baseline to 6 weeks (based on the upper limit of the 95% CI for the SD), a large effect size of 0.65 was observed on the SES and 0.46 on the BPI pain intensity scale, whereas a negligible effect size of 0.05 was observed on the BPI average pain scale (Table 22).
| Outcome | n | Difference at 6 weeks compared with baseline | |||
|---|---|---|---|---|---|
| Mean difference (95% CI) | SD (95% CI) | Effect size (mean/SD) | Effect size (mean/SD upper limit) | ||
| BPI average pain | 15 | –0.2 (–1.5 to 1.1) | 2.4 (1.8 to 3.8) | 0.2/2.4 = 0.082 | 0.2/3.8 = 0.05 |
| BPI interference | 15 | –1.6 (–2.8 to –0.4) | 2.2 (1.6 to 3.5) | 1.6/2.2 = 0.73 | 1.6/3.5 = 0.46 |
| SES | 15 | 0.7 (0.3 to 1.1) | 0.7 (0.5 to 1.1) | 0.74/0.72 = 1.03 | 0.74/1.14 = 0.65 |
The results suggest that the SES and BPI pain interference scale are more responsive to change and should be considered for the primary outcome for a definitive trial. Estimates of mean scores, variability and effect sizes are provided in Tables 18, 19 and 22 to inform future sample size calculations.
Assessing contamination of non-study nurses
The survey assessing contamination of non-study nurses was sent to all non-study CNSs working at the four recruitment sites (n = 37). The demographics and responses are summarised in Table 12. The overall response rate was 41% (n = 15) and, like the study nurses, non-study nurses were all female, of a similar age and had worked in specialist palliative care services for a similar length of time. However, fewer non-study nurses were independent prescribers.
The responses to the non-study nurse survey are summarised in Table 23. A general awareness of the presence of the SMART study was high among non-study nurses. Of the 15 respondents, only one (7%) was unaware of the SMART study and, of the remaining 14 responders, nine (64%) were aware of what the SMART study was about in a general sense. One non-study nurse responded: ‘not aware of what it involves or spoken to my colleagues about it. Just from the title that it is a study about patients having more control in managing their medication’ (NSN004).
| Non-study survey questions | n (%) |
|---|---|
| Aware of SMART study? | |
| Yes | 14 (94) |
| No | 1 (6) |
| Aware of what the SMART study is about? | |
| Yes | 9 (60) |
| No | 6 (40) |
| Discuss SMART study with a study nurse? | |
| Yes | 1 (6) |
| No | 14 (94) |
| Influenced or changed practice? | |
| Yes | 0 (–) |
| No | 15 (100) |
| Have you seen a SMART participant? | |
| Yes | 2 (13) |
| No | 13 (87) |
In terms of direct communication about the study, only one respondent (7%) had discussed the SMART study with one of the study nurses. Concerning contamination of non-study nurse usual practice, all respondents indicated that their own practice was not influenced or changed as a consequence of working in a team in which their colleagues were using the SMART intervention. Two respondents indicated that they had each seen one SMART participant when covering for a study nurse: one during a hospital admission and one in an outpatient clinic.
Adverse events
There were no serious adverse events attributed to the trial intervention or trial processes. Two participants were admitted as inpatients (one to a hospice and one to a hospital) for symptom control.
Conclusions
In conclusion, the feasibility study of the SMART intervention was a single-arm trial in which 19 participants were recruited over 4 months from four community palliative care services via 12 palliative care CNSs who were trained in the delivery of the intervention. In total, 17 participants commenced the SMART intervention with a trained study nurse and 15 were followed up at 2, 4 and 6 weeks following baseline assessment. Through this study we have demonstrated that our research process, study nurse training schedule and intervention delivery strategy are feasible and acceptable within a sample of community-based individuals approaching the end of life, their carers and palliative care CNSs. A total of 74% of participants received the intervention as intended (or partially as intended), with flexibility of delivery necessary to allow for the complex circumstances of managing symptoms at the end of life. The follow-up rate was 79% at 6 weeks, higher than follow-up rates observed in previous trials of similar community-based populations.
Although the analysis of participant self-reported outcome was exploratory, the data overall tended to favour improvements in pain interference and self-efficacy over improvements in pain intensity. The results of the feasibility study were used to determine which outcome should be considered as the primary outcome for a future definitive RCT and to estimate the sample size required for such a trial. The next chapter demonstrates the feasibility of conducting a health economic evaluation of the SMART intervention.
Chapter 4 The SMART health economics: feasibility of economic evaluation and preliminary cost-effectiveness
Introduction
Before investment in new health-care interventions can be made, convincing evidence of the value for money of those interventions must be provided. The SMART feasibility study included resources for health economic research to be conducted that would help determine both the feasibility of an economic evaluation in this patient group and setting and whether or not there is the potential for the SMART intervention to be cost-effective. 80
Aims and objectives
The overall aim of the health economic research was to establish the feasibility of an economic evaluation of SMART and preliminary estimates of cost-effectiveness. Specific objectives were to:
-
determine acceptability and completeness of resource use and utility measures in this setting
-
establish the cost of the SMART intervention
-
develop a decision-analytic model that would permit the generation of cost-effectiveness estimates
-
employ the model to test effectiveness scenarios
-
employ the model to estimate the value of further research.
Feasibility
The SMART feasibility study was used to test the acceptability of the data collection forms. Acceptability is evidenced by the level of missing data. The completeness of the data collected in the outcome measures pack was recorded using descriptive statistics, detailing the number and percentage of questionnaires returned and the number and percentage of missing items within the returned questionnaires.
Resource use
Data collection forms to identify the health and social care services that individuals used were completed by researchers using clinical records. The form, which was adapted from one used in the IMPACCT study, is included in Appendix 3.
Quality of life (utility)
In order to calculate quality-adjusted life-years (QALYs), it is necessary to collect data on health state utility. The EQ-5D is NICE’s preferred measure of health state. The feasibility of using the new EQ-5D-5L was explored. 81 The EQ-5D-5L formed part of the interview-administered questionnaire and was scored using a newly developed UK tariff. 82
The small sample size and absence of a control group meant that an economic evaluation based on patient-level analysis of the data was not possible. Thus, estimates of cost-effectiveness were based on a decision-analytic modelling (DAM) approach.
Cost-effectiveness and decision-analytic model
We conducted a preliminary economic evaluation of the SMART intervention plus standard care compared with standard care alone following the NICE reference case. 83 Outcomes were expressed as QALYs and costs calculated from the perspective of the NHS and Personal Social Services (PSS). A DAM developed for the NIHR-funded IMPACCT project was adapted for use here. In the absence of trial data, the DAM allows us to estimate the cost-effectiveness of the SMART intervention and explore, through sensitivity and scenario analyses, the levels of effectiveness and costs that would yield acceptable value for money metrics for the intervention. The DAM is a simplified representation of the patient pathway describing the major health and cost events that occur over a relevant time period.
Model structure, cycle length and time horizon
The SMART DAM (Figure 13) was adapted from the IMPACCT DAM and is a Markov model with weekly cycles that runs for a time horizon of 52 weeks. The model structure, time horizon, cycle length and parameters were developed using the findings from a model literature review and expert and patient opinion. The model was developed in line with current best-practice standards. 84,85
FIGURE 13.
Diagram of the SMART DAM.

The DAM comprises health states based on level of pain severity (no/mild pain, moderate pain, severe pain) and death (see Figure 13). Within each of the pain health states, the occurrence of side effects (i.e. constipation, drowsiness and nausea) was permitted.
In the DAM, a cohort of hypothetical patients (mean age 72.4 years) who have advanced cancer and pain move (or transit) through the health states in accordance with specified transition probabilities. Each health state has a mean cost and utility value associated with it so that the cohort members accrue QALYs and costs as the 52 weeks pass. Patients can move between pain states and between pain states and death. Side effects are not represented as separate health states.
Model parameters
The model parameter values are described in Tables 19 and 21. They were derived from a number of sources, including the IMPACCT patient survey data, data from the literature, analysis of palliative care patient survival data and analysis of a previous advanced cancer trial. 86
Cost and utility parameters
The model uses resource use and utility data collected from participants during the NIHR-funded IMPACCT study. Between August 2013 and June 2014, 248 patients were recruited to the study and completed a survey. Community-based patients with pain from advanced cancer who were aged ≥ 18 years were eligible for the study. Patients with advanced cancer were defined as those patients with metastatic cancer (histological, cytological or radiological evidence) and/or those receiving anticancer therapy with palliative intent. Patients with pain were defined as those receiving analgesic treatment for cancer symptom-related and/or therapy-related pain. Patients had to be able to complete the questionnaires and provide informed consent to participate. Thirteen palliative care services across England recruited patients to the study. The participants completed a resource use questionnaire capturing health-care use, pain rating scales, the EuroQol-5 Dimensions, three-level version, and additional utility measures: the European Organisation for Research and Treatment of Cancer 8 Domains (EORTC-8D)87 and ICEpop CAPability measure for Adults (ICECAP-A). 88
The resource use questionnaire asked participants to recall use of primary and community care (e.g. GP visits and nurse contact) and secondary or hospital care (e.g. visits to accident and emergency and hospice stays) in the previous 4 weeks. Unit costs were assigned to the data in order to estimate the average cost of health and social care service use for the sample. Unit costs were obtained from national sources including the Personal Social Services Research Unit (PSSRU) Unit Costs of Health and Social Care 2015,89 NHS Reference Costs 2014–201580 and the British National Formulary. 90
Mean cost and utility estimates (with variance) were estimated for each of the model pain health states (Table 24). Individuals were classified into pain health state using a 0–10 pain severity rating scale, where:
-
0–4 = no/mild pain
-
5–6 = moderate pain
-
7–10 = severe pain.
| Parameter | Mean | SD | Source |
|---|---|---|---|
| No/mild pain | 0.526 | 0.282 | IMPACCT patient survey91 |
| Moderate pain | 0.423 | 0.296 | IMPACCT patient survey91 |
| Severe pain | 0.149 | 0.321 | IMPACCT patient survey91 |
| Decrement for nausea | –0.084 | 0.028 | COUGAR II trial data86 |
| Decrement for constipation | –0.052 | 0.027 | COUGAR II trial data86 |
| Decrement for drowsiness | –0.222 | 0.022 | COUGAR II trial data86 |
The impact of side effects (nausea, constipation and drowsiness) were estimated using a previous trial data set that included EQ-5D, cost and side effect data. COUGAR II86 was a trial of chemotherapy compared with active symptom control for those with refractory oesophagogastric adenocarcinoma. 92 These data were thought appropriate as the participants were at the end of life and had similar characteristics to those in the survival estimation and IMPACCT survey samples. 91 A regression model was run predicting, in turn, EQ-5D and costs, and using side effect identifiers based on European Organisation for Research and Treatment of Cancer – Quality of Life Questionnaire C30 (EORTC-QLQ C30) questions as predictors. The beta coefficients on each of the predictors denote the utility decrement or cost impact associated with each side effect. These were applied in an additive way in the model.
The development and implementation of the new tool were costed following consultation with the SMART study researchers. In addition to the participant-completed data, the resources associated with development and delivery of the SMART intervention were recorded based on routine data, such as administrative records and participant records, as well as a detailed description of the intervention costs. The costs of material development, printing and nurse training are included in Table 25.
| Parameter | Mean (£) | SD (£) | Source |
|---|---|---|---|
| No/mild pain | 531.49 | 1021.82 | IMPACCT patient survey91 |
| Moderate pain | 720.55 | 1038.83 | IMPACCT patient survey91 |
| Severe pain | 1089.12 | 1455.65 | IMPACCT patient survey91 |
| Increment for nausea | 93.93 | 47.32 | COUGAR II trial data86 |
| Increment for constipation | 33.23 | 46.60 | COUGAR II trial data86 |
| Increment for drowsiness | 81.04 | 36.97 | COUGAR II trial data86 |
| Cost for SMART intervention | 320.25 | – | SMART – see Table 33 |
Survival parameter
Although it was assumed that neither of the interventions compared in this evaluation influences mortality, it was necessary still to estimate background survival in this population. The survival of the hypothetical model cohort was estimated using a parametric regression, which was fitted to data gathered in another IMPACCT workstream. The data (n = 4638, of whom 84% of patients had a cancer diagnosis and 16% a non-cancer diagnosis) were retrospectively collected on all patient referrals to specialist palliative care services in the city of Leeds, West Yorkshire, over a 2-year period (2012–14) and contained variables on date of referral to palliative care, age, sex and date of death. The characteristics of the sample are described in Table 26. There was no censoring of death and a Kaplan–Meier (K–M) curve was estimated for the time between referral and death. Negative numbers were assumed to be errors and, where they occurred, resulted in the cases being dropped.
| Parameter | n (%) (N = 4638) | Mean | SD | Range |
|---|---|---|---|---|
| Female | 2372 (51) | – | – | – |
| Age (years) | 4638 (100) | 72.44 | 13.54 | 17–108 |
| Survival (days) | 4638 (100) | 80.77 | 117.81 | 0–933 |
A number of models were applied to the data, including exponential, Weibull and Gompertz. Visual comparison of the modelled survival curve with the K–M curve and the Akaike information criterion were used to judge model quality. Weibull had the lowest Akaike information criterion (16,891.5 vs. 17,760.15 for exponential and 16,891.5 vs. 17,138.03 for Gompertz functions) and had good fit with the observed K–M curve. Age and sex covariates were tested in the models but only age was found to be significant. The results of the Weibull regression are shown in Table 27 and Figure 14. As the gamma factor was significant, the use of the Weibull model is justified as this indicates a non-constant (and declining) hazard function. The same risk estimates from this analysis were applied to all health states and the Markov model was relaxed to allow these risk estimates to vary over time. The survival model estimates were permitted to vary in the probabilistic sensitivity analysis following Cholesky decomposition for correlated regression parameters. During the 52-week model time horizon, 97% of the cohort were expected to have died. Survival was assumed to be unrelated to pain, and thus the mortality rate was the same for all pain health states.
| Parameter | Coefficient | SE | z | p > z | 95% CI | Hazard ratio |
|---|---|---|---|---|---|---|
| Gamma (_ln/p) | –0.306 | 0.011 | –27.220 | 0.000 | –0.328 to –0.284 | 0.737 |
| Constant | –2.019 | 0.083 | –24.330 | 0.000 | –2.182 to –1.857 | 0.133 |
| Age | 0.005 | 0.001 | 4.640 | 0.000 | 0.003 to 0.007 | 1.005 |
| p | 0.737 | 0.008 | 0.721 to 0.753 | |||
| 1/p | 1.358 | 0.015 | 1.328 to 1.388 |
FIGURE 14.
Weibull estimated survival (weeks after referral to palliative care).
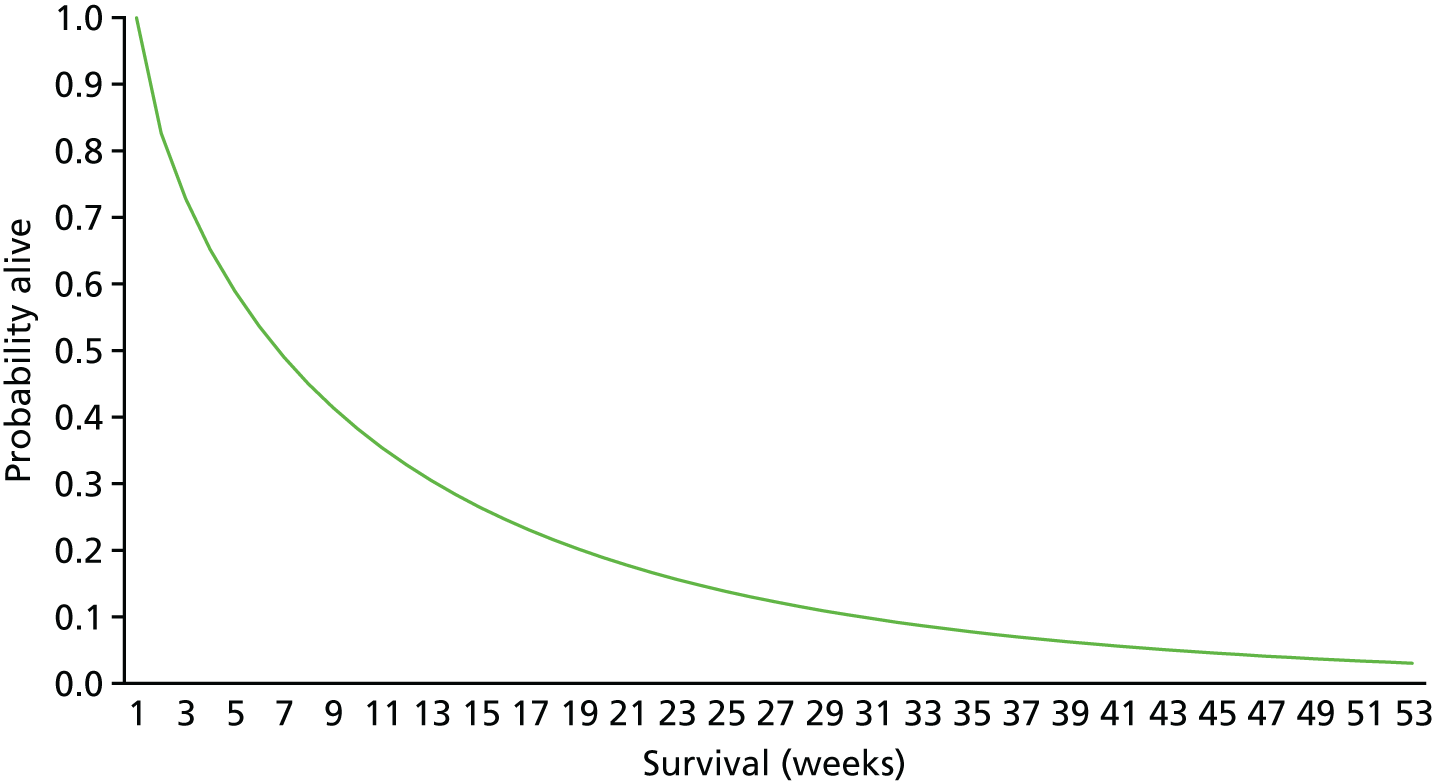
Transition probability parameters
Table 28 summarises the transition probabilities used in the model. The starting proportions of those in each pain health state and of those with each of the three side effects were taken from the IMPACCT patient survey, but allowed to vary in sensitivity analyses. Data from the feasibility study could not reliably inform on the effectiveness of SMART as the study was not powered to do so. In the absence of effectiveness data on the impact of the SMART intervention on pain and side effect management, we relied on expert opinion on the likely effectiveness of the intervention (Professor Michael Bennett, University of Leeds) and on a review of the literature. Given the uncertainty over the intervention effectiveness, we explored scenarios in the model which assumed, for example, that the SMART intervention led to an overall reduction in the proportion of people with side effects of 5% and a weekly reduction in pain status (from moderate and severe to no/mild) of 1% per week. The baseline pain and side effect levels and pain progression were assumed equivalent to those estimated in the standard care arm. To help inform model parameter estimation, literature searches were conducted in June and July 2016 across the databases MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, EMBASE and The Cochrane Library to identify economic models of behavioural and educational self-management interventions for patients to manage pain and side effects of medications at the end of life.
| Parameter | Mean (£) | SD (£) | Source |
|---|---|---|---|
| Background survival | See Table 26 | N/A | Palliative care referral data |
| Starting proportions | |||
| No/mild pain | 0.439 | N/A | IMPACCT patient survey91 |
| Moderate pain | 0.305 | N/A | IMPACCT patient survey91 |
| Severe pain | 0.256 | N/A | IMPACCT patient survey91 |
| Nausea | 0.212 | N/A | IMPACCT patient survey91 |
| Constipation | 0.331 | N/A | |
| Drowsiness | 0.773 | N/A | |
| Pain progression | See Table 26 | N/A | COUGAR II trial data86 |
| Effectiveness | |||
| Standard care: pain | 0 | No change in pain progression – assumption | |
| Standard care: side effects | 0 | No change in side effects – assumption | |
| SMART intervention: pain | –0.001 | Weekly transitions: moderate to no pain and severe to moderate – assumption | |
| SMART intervention: side effects | –0.05 | Assumption | |
The change in pain status in standard care was modelled using the COUGAR II trial data set. 86 In COUGAR II, patients completed the EQ-5D measure at 3- and 6-weekly follow-ups during the trial, meaning that a substantial number of longitudinal data were available for this group. The EQ-5D pain and discomfort item response options were considered to be equivalent to the health states in the model defined by the numeric pain scale categories. Thus, the EQ-5D responses ‘I have no pain or discomfort’, ‘I have moderate pain or discomfort’ and ‘I have extreme pain or discomfort’ were assumed roughly equivalent to the pain rating categories of 0–4, 5–6 and 7–10, respectively. Hence, by observing changes in EQ-5D pain item responses over time, we were able to estimate the change in pain status over time at the end of life.
The EQ-5D pain item response was predicted in a multinomial regression with study week number and survival included as covariates. Thus, the coefficient on the week covariate indicates the likelihood of change in pain item response as time progresses, after controlling for survival. The results of the regression are shown in Table 29 and indicate an increase in pain level over time, albeit a small one. Marginal effects were used to estimate the transition probabilities for pain progression using the multinomial results. These results inform only on the change in proportions over time (and not all the possible transitions between health states), and the COUGAR data were insufficient to inform on all possible pain health state transitions. Therefore, we assumed that transitions occurred only from the ‘no pain’ group to moderate and severe pain groups. This background pain progression at the end of life was assumed to be the same in both arms, but the scenario analyses allowed for improvements in health status in the SMART intervention arm.
| Model parameter | ||||||
|---|---|---|---|---|---|---|
| n | 639 | |||||
| Log-ratio χ2(4) | 30.99 | |||||
| Probability > χ2 | 0.0000 | |||||
| Pseudo-R2 | 0.0299 | |||||
| Log-likelihood | –502.644 | |||||
| Pain parameter | Coefficient | SE | z-value | p-value | Lower CI | Upper CI |
| No pain (base) | ||||||
| Moderate pain | ||||||
| Week | 0.0270 | 0.0092 | 2.9500 | 0.0030 | 0.0091 | 0.0450 |
| Survival | –0.0031 | 0.0006 | –4.9600 | 0.0000 | –0.0043 | –0.0019 |
| Constant | 1.2037 | 0.1617 | 7.4500 | 0.0000 | 0.8868 | 1.5205 |
| Extreme pain | ||||||
| Week | 0.0524 | 0.0170 | 3.0800 | 0.0020 | 0.0191 | 0.0858 |
| Survival | –0.0029 | 0.0015 | –2.0200 | 0.0440 | –0.0058 | –0.0001 |
| Constant | –1.6192 | 0.3482 | –4.6500 | 0.0000 | –2.3017 | –0.9368 |
Cost-effectiveness analysis
The economic evaluation follows the NICE reference case and hence a cost–utility analysis was conducted with a cost per incremental QALY presented from UK NHS and PSS perspectives. The costs are reported in 2015 prices and patient health is measured in terms of QALYs. Cost-effectiveness was assessed using the incremental cost-effectiveness ratio (ICER) and net monetary benefit (NMB) values, and a range of sensitivity analyses were conducted to explore the impact of key model assumptions and parameter uncertainty on the results.
The ICER is calculated by dividing the difference in mean costs between two arms by the difference in mean QALYs between the two arms:
where CSMART and ESMART are the expected cost and effectiveness of the intervention (i.e. SMART) and CUC and EUC are the expected cost and effectiveness of the usual care arm. The incremental cost and effect of the SMART arm compared with usual care arm are represented by ΔC and ΔE, respectively. The NICE willingness to pay per incremental QALY threshold [(λ) = £20,000] was used to define cost-effectiveness. ICERs < £20,000 are usually indicative of cost-effectiveness. It was assumed that the NICE end-of-life criteria were not met as these require an intervention to deliver an average increase in survival of 3 months over usual care.
We account for parameter uncertainty in non-linear models by assigning probability distributions to each of the input parameters and randomly drawing from these probabilities over the 10,000 Monte Carlo simulations. This probabilistic sensitivity analysis allows the calculation of 10,000 ICERs and informs on the level of uncertainty in the model. The probabilistic sensitivity analysis results were plotted on the cost-effectiveness plane and NMB estimates used to generate the cost-effectiveness acceptability curve (CEAC). 92 The CEAC illustrates the probability that each intervention would be cost-effective given a range of willingness-to-pay thresholds per incremental QALY.
The NMB was derived thus:
Discounting was not required as all costs and benefits were experienced within 1 year. A half-cycle correction was applied to account for the likelihood that model health state transitions occur half-way through the model cycles. All analyses were conducted in Stata software (version 14) and Excel® (2013; Microsoft Corporation, Redmond, WA, USA).
Feasibility results
Resource use
Full resource use data for 6 weeks were collected for 15 of the 19 participants (78.9%). Four participants (21.1%) were lost to follow-up and, therefore, their patient records were not accessed. It was of note that use of health-care services outside the GP practice, for example by the community team, was often not recorded. Further investigation of how these services may be captured is required. Despite these challenges, the researcher-completed outcome measure packs were completed.
There were 164 recorded instances of health-care service use for 15 participants over the 6-week study period. As seen in Table 30, community nurse and palliative care CNSs were the services most frequently accessed.
| Service | Outpatient/day hospice | Home | Inpatient admission | Telephone | Total |
|---|---|---|---|---|---|
| GP | 3 | 15 | 0 | 6 | 24 |
| CNS palliative care | 5 | 21 | 7 | 16 | 49 |
| Doctor palliative care | 3 | 2 | 0 | 4 | 9 |
| Community nurse | 20 | 29 | 0 | 8 | 57 |
| Secondary care | 7 | 0 | 3 | 0 | 10 |
| Nurse other | 6 | 3 | 0 | 6 | 15 |
| Total | 44 | 70 | 10 | 40 | 164 |
In order to calculate mean cost of care for each participant over the 6-week period, we assigned unit costs to each service use (see Appendix 26 for unit costs). Cost data were completed for 15 participants. As seen in Table 31, with unit costs applied to service use it can be observed that the main drivers of cost are GP, CNS and district nurse home visits.
| Service | Type | n | Number of uses | Mean cost (£) | SD (£) | Minimum (£) | Maximum (£) |
|---|---|---|---|---|---|---|---|
| GP | Outpatient/day hospice | 1 | 3 | 132.00 | 0.00 | 132.00 | 132.00 |
| Home | 8 | 15 | 168.75 | 89.19 | 90.00 | 360.00 | |
| Telephone | 4 | 6 | 40.50 | 15.59 | 27.00 | 54.00 | |
| CNS palliative care | Outpatient/day hospice | 3 | 5 | 62.10 | 21.51 | 37.26 | 74.52 |
| Home | 8 | 21 | 97.81 | 59.54 | 37.26 | 223.56 | |
| Inpatient admission | 2 | 7 | 130.41 | 131.73 | 37.26 | 223.56 | |
| Telephone | 8 | 16 | 33.06 | 17.67 | 16.53 | 66.12 | |
| Doctor palliative care | Outpatient/day hospice | 1 | 3 | 501.00 | 0.00 | 501.00 | 501.00 |
| Home | 2 | 2 | 167.00 | 0.00 | 167.00 | 167.00 | |
| Community nurse | Outpatient/day hospice | 4 | 20 | 186.30 | 80.49 | 74.52 | 260.82 |
| Home | 6 | 29 | 180.09 | 211.32 | 37.26 | 596.16 | |
| Telephone | 5 | 8 | 26.45 | 9.05 | 16.53 | 33.06 | |
| Secondary care | Outpatient/day hospice | 7 | 7 | 167.00 | 0.00 | 167.00 | 167.00 |
| Inpatient admission | 1 | 3 | 501.00 | 0.00 | 501.00 | 501.00 | |
| Total cost (£) | 536.07 | 513.04 | 90.00 | 1998.77 |
The questionnaire also included space to record prescribed medications. These data were collected for the final 2 weeks for 15 patients and included only prescribed medications and not over-the-counter medications and treatments the patient may have paid for themselves. These data have not been included in the descriptive analysis.
Quality of life
In respect of assessment of quality of life, of the 19 participants in the study, 15 had complete information for calculation of EQ-5D-5L. The four participants who were lost to follow-up had missing data for this part of the questionnaire. Mean change from baseline to week 6 was 0.1079. Table 32 provides a summary of the EQ-5D-5L.
| Time point | Observed | Missing | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Baseline | 19 | 0 | 0.564 | 0.227 | 0.122 | 0.927 |
| Week 2 (follow-up 1) | 15 | 4 | 0.679 | 0.196 | 0.193 | 0.942 |
| Week 4 (follow-up 2) | 15 | 4 | 0.603 | 0.206 | 0.184 | 0.942 |
| Week 6 (follow-up 3) | 15 | 4 | 0.672 | 0.223 | 0.108 | 0.942 |
Intervention costs
Intervention costs were calculated and included for SMART. The total intervention cost is estimated at £320.25 per patient (Table 33). Development costs were also estimated; this included material development (printing SMST resources), training sessions for nurses and researchers and time to deliver intervention (Table 34).
| Resource type | Unit cost (£) | Mean number of visits | Time (minutes) | Cost (£) |
|---|---|---|---|---|
| Sessions with CNSa | 91 (per hour of face-to-face contact) | 3.25 | 55 | 271.10 |
| SMART toolkit contents (see Appendix 9 for more detail) | 43.87 | N/A | N/A | 43.87 |
| DVD pressing | 3.60 | N/A | N/A | 3.60 |
| Memory sticks | 1.68 | N/A | N/A | 1.68 |
| Total | 320.25 |
| Itema | Total cost (£) |
|---|---|
| Intervention toolkit folders | 2293.55 |
| SMART DVDs | 949.44 |
| Seven × nurseb training | 3276.00 |
| Trainer expenses | 2220.00 |
| Researcher preparation and delivery time | 354.60 |
| Total | 9093.59 |
Cost-effectiveness results
Results for the base-case cost-effectiveness analysis of SMART compared with usual care are presented in the Table 35. Based on the model assumptions regarding pain progression and SMART intervention impact, it can be seen that SMART is more effective and less costly than usual care. SMART can be said to dominate usual care, indicating that there are cost savings to be made from the introduction of SMART.
| Strategy | Total cost (£) | Total QALY | Incremental cost (£) | Incremental QALY | ICER | NMB (£) | Net health benefit |
|---|---|---|---|---|---|---|---|
| SMART | 8921 | 0.0473 | –175 | 0.009 | SMART dominates | 5 | 0.0025 |
| Usual care | 9096 | 0.0387 |
The results of the one-way sensitivity analyses conducted for SMART compared with usual care are given in Table 36. Assuming that there was a 2.5% reduction in side effects and a 0.005% reduction in pain led to a drop in QALY gain of 0.0041. This meant that costs for SMART were now higher than for usual care, but with an additional health benefit of 0.0045 QALYs per patient. This results in an ICER of £11,977 per additional QALY, which still indicates cost-effectiveness. Sensitivity analyses were conducted using different utility values, the EORTC-QLQ C30 and the ICECAP-A quality-of-life measures. The results of both of these analyses were similar, with SMART once again dominating standard care alone.
| Sensitivity analysis | Strategy | Total cost (£) | Total QALY | Incremental cost (£) | Incremental QALY s | ICER (£) |
|---|---|---|---|---|---|---|
| Reducing effectiveness by 50% | SMART | 9151 | 0.0432 | 55 | 0.0045 | 11,977 |
| Usual care | 9096 | 0.0387 | ||||
| Using ICECAP-A utility values | SMART | 8921 | 0.0847 | –175 | 0.0055 | SMART dominates |
| Usual care | 9096 | 0.0792 | ||||
| Using EORTC utility values | SMART | 8921 | 0.1000 | –175 | 0.0054 | SMART dominates |
| Usual care | 9096 | 0.0946 | ||||
| Increasing SMART intervention costs by 100% | SMART | 9241 | 0.0473 | 145 | 0.0086 | 16,778 |
| Usual care | 9096 | 0.0387 | ||||
| Assuming no extra visit costs for SMART | SMART | 8650 | 0.0473 | –446 | 0.0086 | SMART dominates |
| Usual care | 9096 | 0.0387 | ||||
| Halving the costs of the cancer for each severity group | SMART | 5073 | 0.0473 | 16 | 0.0086 | 1830 |
| Usual care | 5057 | 0.0387 | ||||
| Scenario where side effect costs and utility decrement are halved | SMART | 8468 | 0.0670 | –119 | 0.0068 | SMART dominates |
| Usual care | 8587 | 0.0602 |
With costs of SMART increased 100%, it can now be seen that SMART becomes a more expensive intervention. However, the ICER of £16,778 remains below the NICE cost per QALY threshold of £20,000. One assumption that could be made is that nurse visits to the patient do not increase because of SMART. This extra visit cost was removed, leading to an intervention cost of £49.15. This decrease in incremental cost served to make SMART more cost-effective. With the costs of cancer for each pain severity group halved, SMART becomes a more expensive intervention; however, again, with an ICER of £1830, this remains below the NICE cost per QALY threshold.
The uncertainty around the model results can be seen in Figure 15, the cost-effectiveness plane. The results vary widely, with ICERs scattered across the cost-effectiveness plane. As the spread of ICER cloud is greater vertically than horizontally, there appears to be greater uncertainty in the costs than in the QALYs.
FIGURE 15.
Cost-effectiveness plane.
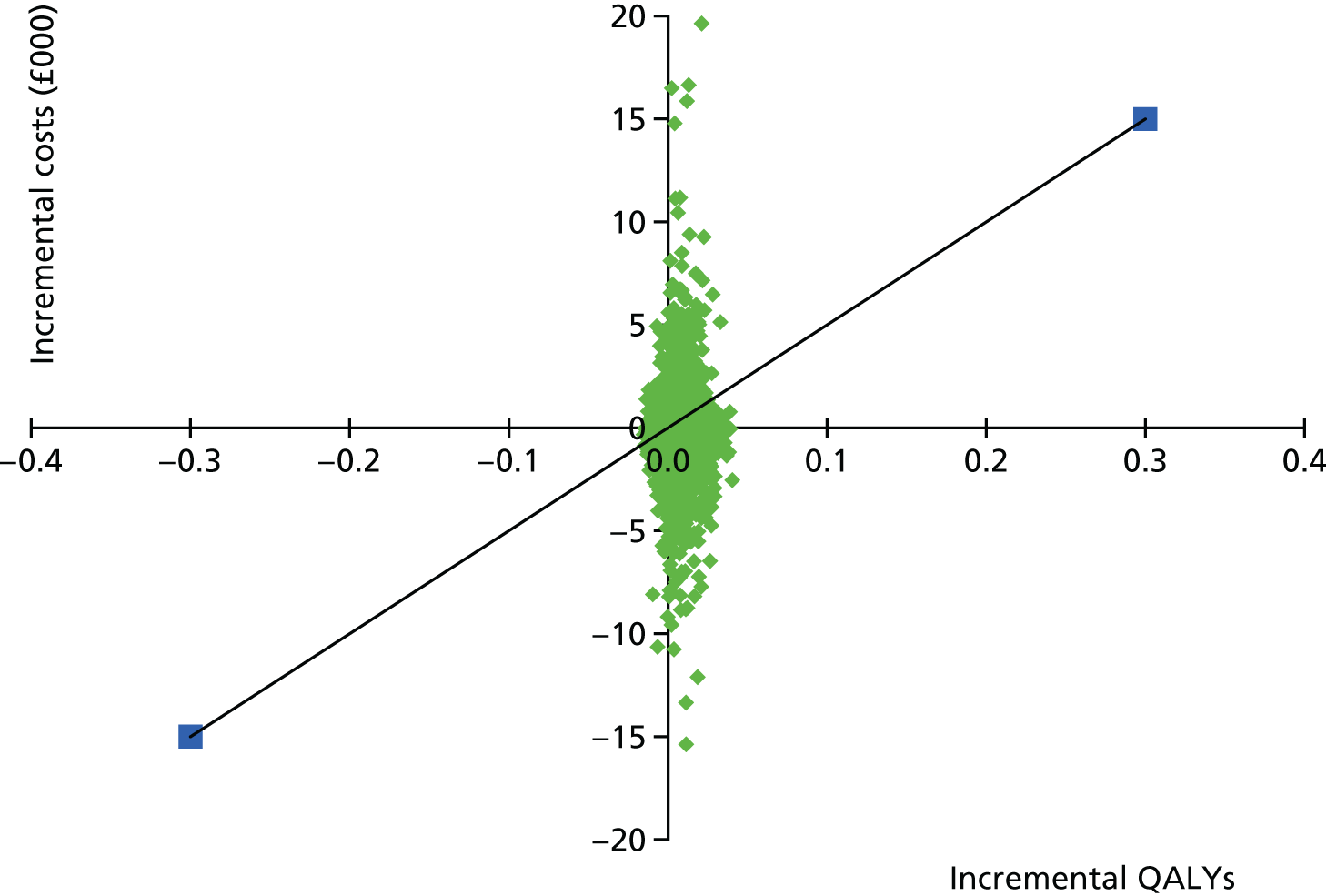
The uncertainty around the cost-effectiveness is further represented in the CEAC in Figure 16. The CEAC shows the likelihood that SMART will be acceptable to a decision-maker, given a particular threshold. SMART has a 68.9% probability of being cost-effective at a £20,000 per QALY threshold, increasing to 78.3% at a £50,000 per QALY threshold.
FIGURE 16.
Cost-effectiveness acceptability curve: SMART vs. usual care. WTP, willingness to pay.

Feasibility of conducting a cost-effectiveness evaluation of SMART
An economic evaluation was conducted to assess the feasibility of estimating cost-effectiveness of SMART compared with usual care in patients at the end of life who receive opioids. The feasibility of a trial-based evaluation was also explored. The evaluation consisted of an economic decision-analytic model, in which cost-effectiveness was assessed for the remaining survival time of patients from a NHS and PSS perspective over 1 year.
The costs of developing and implementing the SMART intervention are relatively modest and, in this analysis, these costs are recovered in savings brought about by improved management of pain and opioid side effects. Given the assumptions made relating to effectiveness, the SMART intervention led to cost savings and yielded incremental QALYs in our base case and many of the deterministic sensitivity analyses. These QALY gains are small, although this is to be expected as this population has a limited survival time in which to benefit. In general, the results are robust to one-way parameter changes and SMART appears to be cost-effective compared with standard care alone.
The probabilistic sensitivity analysis highlights moderate uncertainty in the model as the SMART intervention has a 69% chance of being cost-effective. Given the uncertainty around the cost-effectiveness and data highlighted above, and as effectiveness parameters were based on plausible scenarios, the results should be treated with caution. However, the results indicate that a low-cost intervention such as SMART could be cost-effective in this population even if the impact on pain and side effect management were modest and suggests that further research is warranted.
A full economic evaluation of patient-level data from a RCT is required to allow confidence in decision-making. The feasibility aspects of this study suggest that this should be possible. Although missing data on the utility measures were minimal, greater effort may be required in the collection of cost data and access to centrally held health-care use records may be optimal.
Chapter 5 Discussion and conclusions
We have shown that the evaluation of a supportive self-management intervention for patients requiring analgesia, and who are approaching the end life, is feasible.
Summary of specific feasibility study outcomes
Developed a construct of supported self-management in palliative care in relation to analgesic medicines
This was derived through a synthesis of our existing research and literature reviews of patient needs, behaviour change theory, existing interventions and ways to optimise the context in which HCPs can provide support. The development of the supportive self-management concept included key contextual factors in end-of-life care pain management, patients’ concerns about analgesia and the roles and responsibilities of patients, carers and professionals that we represented on a continuum in relation to self-management behaviours.
Developed and refined an intervention consisting of a self-management toolkit and a four-step education approach
We explored and refined our concept of supported self-management with 11 patients, eight carers and 19 HCPs though interviews and focus groups, informing the development of our intervention. From these data, we developed a four-step education approach that consisted of a needs assessment, including capacity for self-management, provision of information, goal-setting and review and coaching. We supported this approach with a self-management toolkit that comprised information factsheets, a pain chart and medication diary, goal-setting sheets and two 5- to 6-minute podcasts. We mapped data from our literature review and interviews against target behaviours and techniques to enhance self-efficacy to ensure that the toolkit and education approach would address the needs of patients, would be based on sound theoretical principles of enhancing self-management and would therefore be more likely to be effective in practice.
Developed and delivered a brief training programme for clinical nurse specialists in palliative care
The intervention was designed to be delivered to patients by community-based CNSs in palliative care. We engaged an expert nurse educator to design and deliver the training, which modelled the four steps within a clinical encounter and was based on a therapeutic conversational process between the specialist nurse and patient. This training included reflection, experiential learning and the development of a modelled self-management-focused conversation between two nurse educators.
Conducted a feasibility study of the intervention to inform the design of a future randomised controlled trial
This tested the recruitment and follow-up rates, fidelity of treatment delivery and suitability of outcome measures. We trained 12 CNSs from four UK hospices in the delivery of the intervention. During the 4-month study period, we identified 103 eligible patients from 417 who were screened. The most common reasons for ineligibility were patients not treated with a strong opioid (53%) and expected survival of < 6 weeks (19%). Of the 103 eligible patients, 37 (36%) were approached, of whom 19 (51% of those approached) agreed to participate and 15 completed the 6-week follow-up period. We found that 13 out of these 15 patients received all components of the intervention. Most patients (13/15) received a minimum of three visits during the 6-week study period. Rates of missing data for our outcome measures were very low. Although we observed no changes in our measures of pain intensity, we did observe improvements in our measures of interference from pain and in enhancing self-efficacy, which our evidence synthesis and interview data highlighted as being more important outcomes to patients than pain intensity alone.
Acceptability of intervention
Through qualitative interviews with patients and CNSs who participated in our feasibility study, we were able to understand the acceptability of the intervention and potential challenges within a large RCT. Patients and carers perceived that they all derived some benefit to them from the SMST, but the degree and nature of this benefit was variable and dependent on individual circumstances and preferences. The goal-setting sheets were the most frequently valued element; however, there were often other elements that were liked (e.g. the pain diary). The value of the factsheets to patients and carers appeared to be in reinforcing information that they had already been provided with by their CNS. Nurses reported that, although some patients found the concept of supported self-management more difficult to grasp, in general they felt that the educational approach was in keeping with their usual practice. All of them valued the training and materials. The busy and time-pressured reality of clinical practice for these specialist nurses, who often managed large caseloads, meant that the delivery of the intervention per protocol during the course of the trial varied. Patients perceived that the intervention was most effective when nurses delivered the factsheets according to need, completed contact information sheets, reviewed patients’ diaries and set goals. We assessed the potential for contamination of nurses not involved in the study by those nurses who were. We found that, although there was a general awareness of the study, there was no evidence that practice had changed or that the intervention was used by nurses who were not involved in the study.
Health economic analysis
We estimated cost-effectiveness of the SMART intervention within this feasibility study using a DAM approach because of the small number of patients and the lack of a control arm. We demonstrated that it was feasible to collect information to inform a full economic evaluation within a definitive RCT. The cost–utility analysis suggested that the SMART intervention appears to be cost-effective compared with standard care alone and could lead to cost savings. The SMART intervention yielded QALY gains and cost savings in our base case and many of the deterministic sensitivity analyses.
Success criteria and key learning points
Ultimately, we aimed to establish the acceptability and uptake of the SMART intervention and determine the feasibility of evaluating this intervention within a definitive trial. In order to judge whether or not we had achieved our aims, we agreed our success criteria beforehand (see Chapter 1, Success criteria). Here we review the extent to which we have met these criteria.
Phase I
Establishment of patient and public involvement panel
This was achieved within 3 months of starting the SMART project. The PPI panel consisted of carers and bereaved carers of patients who had received palliative care in the community. PPI panel members were invited to quarterly PPI meetings as well as the biannual investigators meeting and Study Steering Group meetings. PPI panel members were involved in reviewing our initial concept of self-management and the components of our prototype SMART intervention prior to the modelling focus groups. A PPI panel member reviewed participant study materials (e.g. information sheets) and contributed to interpreting the results of the feasibility trial during the investigator meetings.
Development of a concept of usual care based on literature review and clinical practice observations
This was achieved by reviewing the contextual policy literature on delivering end-of-life care in the community (see Chapter 1) and during interviews with CNSs, GPs and consultant palliative care clinicians working in hospice and community palliative care services (see Chapter 2).
Development components of a prototype SMART intervention and delivery strategy
This was achieved throughout the first phase of the SMART project, during which the literature was reviewed on supported self management of chronic diseases and end-of-life care. The literature scoping work informed the theory-driven development of a prototype SMART intervention based on the theories of self-efficacy and behaviour change that were best suited to the developing intervention. 39,54
Phase II
Establish members of focus groups
Recruitment of patients, carers and HCPs to the Phase II focus groups was achieved through community palliative care services. The Phase II methodology was amended to allow patients to take part in a interview format if they were to unwell or preferred not to attend a focus group but still wished to participate.
Development of refined intervention materials and delivery strategy
This was achieved through an iterative process involving patients, carers, HCPs, the research team, expert advice from the study co-applicants, the PPI panel and the Study Steering Group.
Phase III
Recruit three participants per month per each site within 4-month recruitment period
The observed recruitment rate was 1.2 participants per month per site for 4 months. Our initial recruitment strategy was an estimate based on the caseloads of all CNSs working within community services at the four sites. We recruited approximately one-third of all full-time equivalent CNSs working within the recruitment sites. The observed new referral rate was approximately what we had expected (4.2 patients per month per site vs. 3 patients per month per site, respectively); however, we overestimated the proportion of patients that we expected would be prescribed strong opioids for pain, which led to a screening failure rate of 53.5%. Therefore, to increase the recruitment rate to three participants per month per site, a future definitive trial should consider recruiting all full-time equivalent CNSs within community palliative care services at each of the sites and increase the range of the intervention (and consequently the eligibility criteria) to include patients on weak opioids.
Feasibility of data collection
The overall level of missing data for the participant self-reported outcomes and the measures of health-care resource use was very low. These findings indicate that the deliverability of data collection methodology was feasible through face-to-face researcher visits. Data from our previous research (IMPACCT) indicate that follow-up data collection rates would be far worse if data were not collected face to face. Participant acceptability and preferences of the self-reported outcomes were assessed by evaluating the level of missing data for each outcome measure and the post-study interviews with participants.
Estimating primary study end point
Owing to the low level of missing data, we were able to evaluate the variability in the participant self-reported outcome measures across the follow-up time points. We identified that the BPI pain interference scale and SES are the measures most likely to be sensitive to change over the defined follow-up period. This fits with the mechanisms of impact of the intervention outlined in the logic model presented in Chapter 2, which identified that the SMART intervention would lead to improvements in participants’ self-confidence (self-efficacy) in managing analgesic medication and consequently reduce the impact of pain on activities of daily living within the context of declining health. The self-efficacy scale was most acceptable to participants (some participants did not like the final question on the BPI interference scale) and demonstrated the largest potential effect size.
Participant, carer and study nurse acceptability of the study experience
Participants and their carers universally indicate that their overall experience of the study was positive and they had sustained use of the intervention resources over the 6-week follow-up time point. The study nurses also indicated that the study process was acceptable; however, ongoing training and support throughout the trial period were necessary to improve the fidelity of delivery of the intervention and a future definitive trial should consider implementing a sustained programme of ‘top-up’ training sessions for study nurses.
Limitation of feasibility and considerations for a future definitive randomised control trial
To inform the design of a future definitive RCT of the SMART intervention, we have identified the key learning points and highlighted opportunities to address them.
Recruitment
Limitation
The feasibility study was limited by the lower than anticipated recruitment rate.
Solution
Extend the range of eligible participants to include people prescribed weak opioids. Research field notes identified that many patients who were screened as ineligible because they were not prescribed strong opioids were in fact prescribed weak opioids for pain. Recruited patients already taking strong opioids reported that they would have benefited from the intervention before or at the point of commencing strong opioids.
Intervention content
Limitation
The content of the intervention was focused on strong opioids only.
Solution
Modify the content of the SMST resources and CNS training manual to include information about weak opioids. This process should include further stakeholder input (i.e. patients, carers and HCPs) to ensure that the content of the SMST resources remains relevant and acceptable to individuals prescribed weak opioids.
Intervention delivery
Limitation
The fidelity of intervention delivery was limited by the need for ongoing training and support throughout the trial period.
Solution
Modify training and support for CNSs to ensure that the research process is more clearly articulated and that the concepts of self-management and means to support this by nurses are better understood to reduce variations in the fidelity of intervention delivery.
Trial support
Limitation
Sites where CRN support was not secured had lower recruitment rates.
Solution
Securing CRN support at each site to assist researchers with screening, recruitment, follow-up visits and final data collection, and to assist study nurses with completion of CRFs relevant to the delivery of the intervention.
Trial processes
Limitation
The process for recording withdrawal was not sufficiently detailed.
Solution
Clear reporting of withdrawal procedures with levels of withdrawal to capture patients who withdraw from researcher follow-up (because of the burden of continuing with these visits), but continue to use the intervention with the study nurse.
Outcomes and outcome measures
Limitation
The number of follow-up visits was considered burdensome for some participants.
Solution
Reduce the number of follow-up visits from three to two (weeks 3 and 6 following baseline). Data from participant self-reported outcome measures indicated little change in the variables between weeks 2 and 4. Based on the acceptability to participants and large potential effect size, we recommend that the primary outcome measure for a further definitive trial would be the SES. Consider using interference from pain as a second primary outcomes rather than pain intensity.
Trial design
Limitation
The feasibility trial did not have a control arm or assess the feasibility of randomisation processes.
Solution
Contamination of non-study nurses was low at participating sites; therefore, a parallel design could be considered for this reason. However, this design would be feasible only if patients allocated to the control arm were seen by untrained nurses and those allocated to intervention arm were seen by trained nurses. Current services are delivered by nurses with geographically based caseloads and so their eligible patients could be allocated to either arm. A cluster design with nurse as the cluster would lead to the most efficient trial design, while accounting for nurses’ geographically aligned caseloads. If nurses within a service are willing to be randomly allocated to either intervention or the control, then all eligible patients within a service could still be considered for recruitment, maximising accrual rate while increasing the number of clusters of participants. In addition, having intervention and non-intervention nurses within each site further minimises between-site differences. Therefore, we recommend a cluster trial design at the level of the nurse for a future definitive trial, as we have shown that there is little contamination of nurses within a site and it allows participants to be seen by their geographically aligned nurse without having to cluster at the site level. Furthermore, we recommend that a future definitive trial would have an internal pilot study to evaluate the feasibility of randomisation processes and generate pilot data required to inform the sample size for a definitive trial.
Conclusion
We have shown that the evaluation of a supportive self-management intervention for patients requiring analgesia, and who are approaching the end life, is feasible. Our success criteria were largely met and, for those that were not, we have identified clear means to succeed within a future trial through a detailed process evaluation of our feasibility study. The key considerations in the design of future definitive trial have been identified, which we believe is now feasible to undertake.
Acknowledgements
The authors wish to acknowledge the funding for this study from the NIHR HTA programme, as well as support from the research managers at the NIHR Evaluation, Trials and Studies Coordinating Centre. We would like to thank the clinical staff at the palliative care services in Hampshire and Yorkshire involved in Phases I and II of the SMART project for participating in interviews and focus groups as well as reviewing draft versions of the intervention resources. We express particular thanks to the 12 study nurses who volunteered to take part in the feasibility study and were trained to deliver the SMART intervention to their patients and who were instrumental in recruiting patients and arranging study appointments. We thank also the non-clinical staff at the four recruitment sites for their input into the administration of the feasibility study and facilitating communication between the research team, the study nurses and patients.
We gratefully acknowledge and thank the patients and their carers who contributed to the SMART study either by participating in the interviews and focus groups in the first two phases or by participating in the feasibility trial. The information and findings gathered from patients and their carers over the course of the SMART study was invaluable in shaping the content and form of the intervention.
We also thank the members for the PPI panel for contributing to the development of the SMART study, for review of patient materials and of the intervention resources and for interpretation of the study findings. We express particular thanks to Jean Gallagher, who provided expert PPI input at all stages of the study from the inception of the SMART study funding application through to interpreting the findings of the feasibility study and drafting of the final report.
We acknowledge Professor Sue Duke from the University of Southampton for her support in designing and delivering the study nurse training workshops that were part of the feasibility trail in Phase III. Thanks also to the nurses who responded to the ‘non-study nurses survey’, which helped the research team to evaluate the level of contamination of non-study nurses working within research active sites.
Contributions of authors
Professor Michael I Bennett, St Gemma’s Professor of Palliative Medicine, led the research as chief investigator. He led the design of the overall study, co-ordinated the funding proposal application, had overall supervision of the research, facilitated the Project Advisory Group meeting, reviewed the content of the SMART intervention and study materials and wrote the final report.
Dr Matthew R Mulvey, Research Fellow, University of Leeds, contributed to the design of the overall study and wrote the funding proposal application, project managed and co-ordinated the day-to-day running of the research, facilitated the PPI panel meetings, completed the literature scoping in Phase I, facilitated the focus groups and interviews for Phases I and II, developed the content and format of the SMART intervention resources and study materials, led the NHS REC approval applications, co-ordinated the site site-up, patient recruitment and data collection for the feasibility study sites in Yorkshire and the Humber, facilitated the study nurse training workshops, performed the quantitative analysis of the feasibility study data, interpreted in the finings from the feasibility study and led the writing of the final report.
Dr Natasha Campling, Research Fellow, co-ordinated the delivery of the research at the Hampshire sites, facilitated the focus groups and interviews for Phase II, performed the qualitative analyses of data from Phases I and II, developed the framework of supported self-management within palliative care services, developed the content and format of the SMART intervention resources, developed a bespoke training package and learning resources for the study nurses, led the development and production of the podcast films, co-ordinated the site set-up, patient recruitment and data collection of the feasibility study in Hampshire, performed the qualitative analyses of the data of the feasibility study, interpreted in the finings from the feasibility study and wrote the final report.
Professor Sue Latter, Professor of Nursing, contributed to the design of the overall study and writing of the funding proposal application, supervised the research in Hampshire, reviewed the content of the SMART intervention and study materials and contributed to the writing of the final report.
Professor Alison Richardson, Clinical Professor in Cancer Nursing and End-of-Life Care, contributed to the design of the overall study and writing of the funding proposal application, supervised the research in Hampshire, reviewed the content of the SMART intervention and study materials and contributed to the writing of the final report.
Professor Hilary Bekker, Professor of Medical Decision-Making, contributed to the design of the overall study and the writing of the funding proposal application and helped to develop the content of the SMART intervention resources by providing expertise in health literacy.
Professor Alison Blenkinsopp, Professor of Pharmacy Practice, contributed to the design of the overall study and interpretation of study findings.
Mr Paul Carder, Research Manager, contributed to the design of the overall study and interpretation of study findings.
Professor Jose Closs, Professor of Nursing Research, contributed to the design of the overall study and interpretation of study findings.
Professor Amanda Farrin, Professor of Clinical Trials and Evaluation of Complex Interventions, contributed to the design of the overall study and interpretation of study findings with a focus on the requirements for a future definitive trial.
Dr Kate Flemming, Senior Lecturer, contributed to the design of the overall study, interpretation of study findings and provided expert advice on the literature reviewing activities in Phases I and II.
Ms Jean Gallagher, PPI Co-applicant, contributed to the design of the overall study, reviewed SMART intervention resources and study materials (including participant information sheets and questionnaires) and helped to interpret the results of the feasibility trial.
Dr David Meads, Lecturer in Health Economics, helped design the overall study and led the health economic feasibility evaluation and modelling.
Professor Stephen Morley, Professor of Clinical Psychology, contributed to the design of the overall study, development of the framework of supported self-management and interpretation of the study findings.
Mr John O’Dwyer, Research Fellow in Health Economics, conducted the health economic feasibility evaluation and modelling.
Ms Alexandra Wright-Hughes, Senior Medical Statistician, contributed to the design of the overall study, helped to develop the protocol and analysis plan for the feasibility study and contributed to the quantitative analysis and interpretation of the feasibility study data and writing of the final report.
Ms Suzanne Hartley, Head of Trial Management, contributed to the content and development of the feasibility study protocol and development of the study materials.
Publication
Campling N, Richardson A, Mulvey M, Bennett M, Johnston B, Latter S. Self-management support at the end of life: patients’, carers’ and professionals’ perspectives on managing medicines. Int J Nurs Stud 2017;76:45–54.
Data sharing statement
Data collected as part of the SMART study are held securely at the University of Leeds. The research team will consider applications to share data provided sufficient governance procedures and approvals are in place. Enquires should be made to the corresponding author Professor Michael Bennett (m.i.bennett@leeds.ac.uk).
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Secondary Cancer Mortality Statistics. 2015.
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437-49. https://doi.org/10.1093/annonc/mdm056.
- Breivik H, Cherny N, Collett B, de Conno F, Filbet M, Foubert AJ, et al. Cancer-related pain: a pan-European survey of prevalence, treatment, and patient attitudes. Ann Oncol 2009;20:1420-33. https://doi.org/10.1093/annonc/mdp001.
- World Health Organization . Cancer Pain Relief Secondary Cancer Pain Relief 1996. http://whqlibdoc.who.int/publications/9241544821.pdf (accessed 1 July 2016).
- Zech DF, Grond S, Lynch J, Hertel D, Lehmann KA. Validation of World Health Organization guidelines for cancer pain relief: a 10-year prospective study. Pain 1995;63:65-76. https://doi.org/10.1016/0304-3959(95)00017-M.
- Bennett MI, McQuay HJ, Kelso E, Moore A. Systematic Reviews in Pain Research: Methodology Refined. Seattle, WA: IASP Press; 2008.
- Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E, Cavuto S, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol 2014;32:4149-54. https://doi.org/10.1200/JCO.2014.56.0383.
- Gomes B, Higginson IJ, Calanzani N, Cohen J, Deliens L, Daveson BA, et al. Preferences for place of death if faced with advanced cancer: a population survey in England, Flanders, Germany, Italy, the Netherlands, Portugal and Spain. Ann Oncol 2012;23:2006-15. https://doi.org/10.1093/annonc/mdr602.
- Office for National Statistics . National Survey of Bereaved People (VOICES) 2015. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthcaresystem/bulletins/nationalsurveyofbereavedpeoplevoices/2015-07-09 (accessed 1 July 2016).
- Adam R, Wassell P, Murchie P. Why do patients with cancer access out-of-hours primary care? A retrospective study. Br J Gen Pract 2014;64:e99-104. https://doi.org/10.3399/bjgp14X677158.
- Davis MP, Temel JS, Balboni T, Glare PA. Review of the trials which examine early integration of outpatient and home palliative care for patients with serious illnesses. Ann Palliat Med 2015;4:99-121.
- End of Life Care Strategy – Promoting High Quality Care For All Adults at the End of Life. London: Department of Health; 2008.
- End of Life Care for Adults. London: NICE; 2011.
- End-of-Life Care and Physician-Assisted Dying. London: BMA; 2016.
- Care Quality Commission . Identifying Variation in End of Life Care Commissioning 2014. www.cqc.org.uk/content/identifying-variation-end-life-care-commissioning (accessed 1 July 2016).
- One Chance to Get it Right – Improving People’s Experience of Care in the Last Few Days and Hours of Life. London: LACDP; 2014.
- Ambitions for Palliative and End of Life Care: A National Framework for Local Action 2015–2020. London: NPELCP; 2015.
- End of Life Care Report 2014–2015. London: House of Commons; 2015.
- Putting Patients, Carers and Clinicians at the Heart of Palliative and End of Life Care Research. London: PeolcPSP; 2015.
- Better Endings Right Care, Right Place, Right Time. London: NIHR; 2015.
- Taylor SJC, Pinnock H, Epiphaniou E, Pearce G, Parke HL, Schwappach A, et al. A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions: PRISMS – Practical systematic Review of Self-Management Support for long-term conditions. Health Serv Deliv Res 2014;2.
- Gunnarsdottir S, Donovan HS, Serlin RC, Voge C, Ward S. Patient-related barriers to pain management: the Barriers Questionnaire II (BQ-II). Pain 2002;99:385-96. https://doi.org/10.1016/S0304-3959(02)00243-9.
- Closs SJ, Chatwin J, Bennett MI. Cancer pain management at home (II): does age influence attitudes towards pain and analgesia?. Suppor Care Cancer 2009;17:781-6. https://doi.org/10.1007/s00520-008-0548-4.
- Manzano A, Ziegler L, Bennett M. Exploring interference from analgesia in patients with cancer pain: a longitudinal qualitative study. J Clin Nurs 2014;23:1877-88. https://doi.org/10.1111/jocn.12447.
- Hacket J, Godfrey M, Bennett MI. Patient and caregiver perspectives on managing pain in advanced cancer: a qualitative longitudinal study. Palliat Med 2016;30:711-19. https://doi.org/10.1177/0269216316628407.
- Gardiner C, Gott M, Ingleton C, Hughes P, Winslow M, Bennett MI. Attitudes of health care professionals to opioid prescribing in end-of-life care: a qualitative focus group study. J Pain Symptom Manage 2012;44:206-14. https://doi.org/10.1016/j.jpainsymman.2011.09.008.
- Gao W, Gulliford M, Bennett MI, Murtagh FE, Higginson IJ. Managing cancer pain at the end of life with multiple strong opioids: a population-based retrospective cohort study in primary care. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0079266.
- Bennett MI, Bagnall AM, José Closs S. How effective are patient-based educational interventions in the management of cancer pain? Systematic review and meta-analysis. Pain 2009;143:192-9. https://doi.org/10.1016/j.pain.2009.01.016.
- Bennett MI, Flemming K, Closs SJ. Education in cancer pain management. Curr Opin Support Palliat Care 2011;5:20-4. https://doi.org/10.1097/SPC.0b013e328342c607.
- Flemming K, Closs SJ, Foy R, Bennett MI. Education in advanced disease. J Pain Symptom Manage 2012;43:885-901. https://doi.org/10.1016/j.jpainsymman.2011.05.023.
- Latter S, Hopkinson JB, Lowson E, Hughes JA, Hughes J, Duke S, et al. Supporting carers to manage pain medication in cancer patients at the end of life: a feasibility trial [published online ahead of print June 1 2017]. Palliat Med 2017. http://journals.sagepub.com/doi/10.1177/0269216317715197.
- Bennett MI, Bagnall AM, Raine G, Closs SJ, Blenkinsopp A, Dickman A, et al. Educational interventions by pharmacists to patients with chronic pain: systematic review and meta-analysis. Clin J Pain 2011;27:623-30. https://doi.org/10.1097/AJP.0b013e31821b6be4.
- Savage I, Blenkinsopp A, Closs SJ, Bennett MI. ‘Like doing a jigsaw with half the parts missing’: community pharmacists and the management of cancer pain in the community. Int J Pharm Pract 2013;21:151-60. https://doi.org/10.1111/j.2042–7174.2012.00245.x.
- Latter S, Hopkinson JB, Richardson A, Hughes JA, Lowson E, Edwards D. How can we help family carers manage pain medicines for patients with advanced cancer? A systematic review of intervention studies. BMJ Support Palliat Care 2016;6:263-75. https://doi.org/10.1136/bmjspcare-2015-000958.
- Bennett MI, Graham J, Schmidt-Hansen M, Prettyjohns M, Arnold S. Guideline Development Group . Prescribing strong opioids for pain in adult palliative care: summary of NICE guidance. BMJ 2012;344. https://doi.org/10.1136/bmj.e2806.
- Capewell C, Gregory W, Closs S, Bennett M. Brief DVD-based educational intervention for patients with cancer pain: feasibility study. Palliat Med 2010;24:616-22. https://doi.org/10.1177/0269216310371704.
- NICE CG140: Palliative Care for Adults: Strong Opioids for Pain Relief. London: NICE; 2012.
- Bate P, Robert G. Experience-based design: from redesigning the system around the patient to co-designing services with the patient. Qual Saf Health Care 2006;15:307-10. https://doi.org/10.1136/qshc.2005.016527.
- Michie S, van Stralen MM, West R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
- A Framework for Development and Evaluation of RCTs for Complex Interventions to Improve Health. London: Medical Research Council and Public Health Research Board; 2000.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. https://doi.org/10.1136/bmj.321.7262.694.
- A Practical Guide to Self-Management Support – Key Components for Successful Implementation. London: The Health Foundation; 2015.
- Bennett MI, Closs SJ, Chatwin J. Cancer pain management at home (I): do older patients experience less effective management than younger patients?. Support Care Cancer 2009;17:787-92. https://doi.org/10.1007/s00520-008-0549-3.
- Hansen L, Leo MC, Chang MF, Zucker BL, Sasaki A. Pain and self-care behaviours in adult patients with end-stage liver disease: a longitudinal description. J Palliat Care 2014;30:32-40.
- Koller A, Miaskowski C, De Geest S, Opitz O, Spichiger E. A systematic evaluation of content, structure, and efficacy of interventions to improve patients’ self-management of cancer pain. J Pain Symptom Manage 2012;44:264-84. https://doi.org/10.1016/j.jpainsymman.2011.08.015.
- Michie S, Abraham C, Eccles MP, Francis JJ, Hardeman W, Johnston M. Strengthening evaluation and implementation by specifying components of behaviour change interventions: a study protocol. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-10.
- Sand AM, Harris J, Rosland JH. Living with advanced cancer and short life expectancy: patients’ experiences with managing medication. J Palliat Care 2009;25:85-91.
- Opioids in Palliative Care – Patient Information Manual. Cardiff: Palliative Care Cymru Implementation Board, Velindre NHS Trust, Cardiff & Vale University Health; 2012.
- Johnston B, Rogerson L, Macijauskiene J, Blaževičiene A, Cholewka P. An exploration of self-management support in the context of palliative nursing: a modified concept analysis. BMC Nurs 2014;13. https://doi.org/10.1186/1472-6955-13-21.
- Schumacher KL, Plano Clark VL, West CM, Dodd MJ, Rabow MW, . Pain medication management processes used by oncology outpatients and family caregivers part I: health systems contexts. J Pain Symptom Manage 2014;48:770-83. https://doi.org/10.1016/j.jpainsymman.2013.12.242.
- Schumacher KL, Plano Clark VL, West CM, Dodd MJ, Rabow MW, . Pain medication management processes used by oncology outpatients and family caregivers part II: home and lifestyle contexts. J Pain Symptom Manage 2014;48:784-96. https://doi.org/10.1016/j.jpainsymman.2013.12.247.
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191-215. https://doi.org/10.1037/0033-295X.84.2.191.
- Bandura A. The Exercise of Control. New York, NY: Freeman; 1997.
- Latter S, Hopkinson J, Lowson E, Duke S, Anstey S, Bennett MI, et al. Study protocol for a feasibility trial of Cancer Carer Medicines Management (CCMM): an educational intervention for carer management of pain medication in cancer patients at end of life. Work Pap Health Sci 2014;1:1-7.
- Flemming K, Closs J, Hughes N, Bennett M. Using qualitative research to overcome the shortcomings of systematic reviews when designing of a self-management intervention for advanced cancer pain. Int J Qual Methods 2016;15:1-11. https://doi.org/10.1177/1609406916670656.
- West CM, Dodd MJ, Paul SM, Schumacher K, Tripathy D, Koo P, et al. The PRO-SELF(c): Pain Control Program – an effective approach for cancer pain management. Oncol Nurs Forum 2003;30:65-73. https://doi.org/10.1188/03.ONF.65-73.
- Fahey KF, Rao SM, Douglas MK, Thomas ML, Elliott JE, Miaskowski C. Nurse coaching to explore and modify patient attitudinal barriers interfering with effective cancer pain management. Oncol Nurs Forum 2008;35:233-40. https://doi.org/10.1188/08.ONF.233-240.
- Ward S, Donovan H, Gunnarsdottir S, Serlin RC, Shapiro GR, Hughes S. A randomized trial of a representational intervention to decrease cancer pain (RIDcancerPain). Health Psychol 2008;27:59-67. https://doi.org/10.1037/0278-6133.27.1.59.
- Cagle JG, Zimmerman S, Cohen LW, Porter LS, Hanson LC, Reed D. EMPOWER: an intervention to address barriers to pain management in hospice. J Pain Symptom Manage 2015;49:1-12. https://doi.org/10.1016/j.jpainsymman.2014.05.007.
- Richie J, Spencer L, Burgess BA. Analysing Qualitative Data. London: Routledge; 1994.
- Bekker HG, Thornton J, Airey CM, Connelly JB, Hewison J, Robinson MB, et al. Informed decision making: an annotated bibliography and systematic review. Health Technol Assess 1999;3.
- Bekker HL. Patient Educ Couns 2010;78:357-64. https://doi.org/10.1016/j.pec.2010.01.002.
- Readability – How to Produce Clear Written Materials for a Range of Readers. Leicester: NIACE; 2009.
- Strategies to Support Self-Management in Chronic Conditions: Collaboration with Clients. Ontario, ON: RAINO; 2010.
- Latter S, Sibley A, Skinner TC, Cradock S, Zinken KM, Lussier MT, et al. The impact of an intervention for nurse prescribers on consultations to promote patient medicine-taking in diabetes: a mixed methods study. Int J Nurs Stud 2010;47:1126-38. https://doi.org/10.1016/j.ijnurstu.2010.02.004.
- Cramm JM, Strating MM, de Vreede PL, Steverink N, Nieboer AP. Validation of the self-management ability scale (SMAS) and development and validation of a shorter scale (SMAS-S) among older patients shortly after hospitalisation. Health Qual Life Outcomes 2012;10. https://doi.org/10.1186/1477-7525-10-9.
- Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract 2001;4:256-62.
- Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999;14:1-24. https://doi.org/10.1080/08870449908407311.
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap 1994;23:129-38.
- Watanabe SM, Nekolaichuk C, Beaumont C, Johnson L, Myers J, Strasser F. A multicenter study comparing two numerical versions of the Edmonton Symptom Assessment System in palliative care patients. J Pain Symptom Manage 2011;41:456-68. https://doi.org/10.1016/j.jpainsymman.2010.04.020.
- EuroQol, Group . EuroQol – a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Horne R, Hankins M, Jenkins R. The Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and research. Qual Health Care 2001;10:135-40. https://doi.org/10.1136/qhc.0100135.
- Browne RH. On the use of a pilot sample for sample size determination. Stat Med 1995;14:1933-40. https://doi.org/10.1002/sim.4780141709.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105-21. https://doi.org/10.1016/j.jpain.2007.09.005.
- Rolfe G, Jasper M, Freshwater D. Critical Reflection in Practice: Generating Knowledge for Care. Basingstoke: Palgrave Macmillan; 2010.
- Duke S, Bulman C, Schutz S. Reflective Practice in Nursing. Chichester: Wiley-Blackwell; 2013.
- Department of Health . NHS Reference Costs 2014–2015 2015. www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015 (accessed 1 July 2016).
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Devlin N, Shah K, Feng Y, Mulhern B, Van Hout B. Valuing Health-Related Quality of Life: An EQ-5D-5L Value Set for England. London: OHE Working Paper; 2016.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Caro JJ, Briggs AH, Siebert U, Kuntz KM. ISPOR-SMDM Modeling Good Research Practices Task Force . Modeling good research practices – overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force – 1. Value Health 2012;15:796-803. https://doi.org/10.1016/j.jval.2012.06.012.
- Edlin R, McCabe C, Hulme C, Hall P, Wright J. Cost Effectiveness Modelling for Technology Assessment. London: Springer; 2015.
- Meads DM, Marshall A, Hulme CT, Dunn JA, Ford HE. The cost effectiveness of docetaxel and active symptom control versus active symptom control alone for refractory oesophagogastric adenocarcinoma: economic analysis of the COUGAR-02 Trial. PharmacoEconomics 2016;34:33-42. https://doi.org/10.1007/s40273-015-0324-5.
- Rowen D, Brazier J, Young T, Gaugris S, Craig BM, King MT, et al. Deriving a preference-based measure for cancer using the EORTC QLQ-C30. Value Health 2011;14:721-31. https://doi.org/10.1016/j.jval.2011.01.004.
- Al-Janabi H, Flynn TN, Coast J. Development of a self-report measure of capability wellbeing for adults: the ICECAP-A. Qual Life Res 2012;21:167-76. https://doi.org/10.1007/s11136-011-9927–2.
- Curtis LA. Unit Costs of Health and Social Care 2015. Canterbury: Personal Social Services Research Unit; 2016.
- British National Formulary (Online). London: BMJ Group and Pharmaceutical Press; 2014.
- Meads DM, O’Dwyer JL, Hulme CT, Chintakayala P, Vinall-Collier K, Bennett MI. Patient preferences for pain management in advanced cancer: results from a discrete choice experiment [published online ahead of print March 31 2017]. Patient 2017. https://doi.org/10.1007/s40271-017-0236-x.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- Lau DT, Berman R, Halpern L, Pickard AS, Schrauf R, Witt W. Exploring factors that influence informal caregiving in medication management for home hospice patients. J Palliat Med 2010;13:1085-90. https://doi.org/10.1089/jpm.2010.0082.
- Kimberlin C, Brushwood D, Allen W, Radson E, Wilson D. Cancer patient and caregiver experiences: communication and pain management issues. J Pain Symptom Manage 2004;28:566-78. https://doi.org/10.1016/j.jpainsymman.2004.03.005.
- Mehta A, Cohen SR, Carnevale FA, Ezer H, Ducharme F. Family caregivers of palliative cancer patients at home: the puzzle of pain management. J Palliat Care 2010;26:184-93.
- Mehta A, Cohen SR, Carnevale FA, Ezer H, Ducharme F. Strategizing a game plan: family caregivers of palliative patients engaged in the process of pain management. Cancer Nurs 2010;33:461-9. https://doi.org/10.1097/NCC.0b013e3181de72cc.
- Mehta A, Cohen SR, Ezer H, Carnevale FA, Ducharme F. Striving to respond to palliative care patients’ pain at home: a puzzle for family caregivers. Oncol Nurs Forum 2011;38:E37-45. https://doi.org/10.1188/11.ONF.E37-E45.
- Schumacher KL, Koresawa S, West C, Hawkins C, Johnson C, Wais E, et al. Putting cancer pain management regimens into practice at home. J Pain Symptom Manage 2002;23:369-82. https://doi.org/10.1016/S0885-3924(02)00385-8.
- Hopkinson JB, Richardson A. A mixed-methods qualitative research study to develop a complex intervention for weight loss and anorexia in advanced cancer: the family approach to weight and eating. Palliat Med 2015;29:164-76. https://doi.org/10.1177/0269216314556924.
Appendix 1 National Institute for Health Research Health Technology Assessment programme commissioning brief
Appendix 2 The content and form of previous interventions to improve pain management
West CM, Dodd MJ, Paul SM, Schumacher K, Tripathy D, Koo P, et al. The PRO-SELF(c): Pain Control Program – an effective approach for cancer pain management. Oncol Nurs Forum 2003;30:65–73. 58
| PRO-SELF: a pain control programme for cancer pain management | |
|---|---|
| Time period | Intervention |
| Week 1 |
Nurses meet patients and family caregivers in their homes Conduct an in-depth assessment session, identifying areas of knowledge deficit Review answers to questions on the knowledge and attitude questionnaire Review the PRO-SELF: pain control booklet and use the teaching guide to enhance information in the booklet Review baseline pain scores and pain pattern Review pain medicines and drug administration schedule Educate patients regarding optimal administration of pain medications, set up medicines in the dosette box Instruct patients on how to complete a daily pain management diary Review side effects checklist and discuss prevention and management of side effects Educate patients on how to discuss with their HCPs the need for change in the pain management plan if appropriate Review how to contact nurses for pain management questions |
| Week 2 |
Nurses telephone patients and family caregivers Review pain scores and medication use during the previous week Reinforce teaching about use of analgesics, side effects management and concerns about addiction if needed Determine if patients had to see HCPs for pain management or if analgesic prescription changed during the previous week Answer questions about pain management |
| Week 3 | Home visit: same as week 2 |
| Week 4 | Telephone call: same as week 2 |
| Week 5 | Telephone call: same as week 2 |
| Week 6 | Home visit: same as week 2 |
Fahey KF, Rao SM, Douglas MK, Thomas ML, Elliott JE, Miaskowski C. Nurse coaching to explore and modify patient attitudinal barriers interfering with effective cancer pain management. Oncol Nurs Forum 2008;35:233–40. 59
Coaching intervention: nurse teaching to explore and address patient attitudinal barriers interfering with effective cancer pain management.
Four telephone calls over 6 weeks:
-
greeting – initiate call by listening and outlining plan for the session
-
current issue – consider and explore attitudinal barriers; promote patient’s recognition of attitudinal barriers interfering with adequate pain management
-
problem – help patient describe and consider the nature and extent of problem that is interfering with better pain management
-
problem impact – explore specifics about how the problem (and related beliefs and behaviours) is affecting the patient
-
short-term goals – encourage identification of short-term goals and promote exploration of specific behaviours that might help the patient reach goals
-
strategies – list strategies and options for overcoming the barriers
-
tasks – select tasks that will support the removal of attitudinal barriers, enhance self-confidence and improve pain management
-
summary – summarise the discussion and allow for questions.
Ward S, Donovan H, Gunnarsdottir S, Serlin RC, Shapiro GR, Hughes S. A randomized trial of a representational intervention to decrease cancer pain (RIDcancerPain). Health Psychol 2008;27:59–67. 60
RIDcancer PAIN +: an intervention to decrease cancer pain.
Seven elements:
-
Patients asked to describe their beliefs about their pain in terms of cause, timeline, consequences and control (assessment interview). The intervener listened carefully for mention of barriers to pain management such as fears of addiction or concerns about side effects.
-
Gaps, confusions and misconceptions about reporting pain and using analgesics were identified and discussed.
-
Creating conditions for change – patients discussed the losses that result from the misconceptions.
-
Intervener provided information to fill the gaps and replace confusions that had been identified. An educational message had been prepared for each of the common attitudinal barriers (fatalism about cancer pain management, exaggerated fear of addiction, worry about developing tolerance, concern about side effects, fear of being a complainer and worry about masking changes in disease status). The messages were developed from evidence in the literature and had been used previously.
-
Summary and discussion of the benefits of adopting this new information.
-
The patient created a plan for changing the way he/she managed pain.
The first six elements were covered in a single session that lasts from 20 to 80 minutes, depending on the number of misconceptions that were identified.
-
Evaluation of coping plans – took place during follow-up telephone calls that occurred 2 and 4 weeks after the first session. The intervener reviewed the plan with the patient to work out if the patient was meeting their goals, to determine which coping strategies had been most useful and to revise the plan. These follow-up calls lasted approximately 5–10 minutes.
Cagle JG, Zimmerman S, Cohen LW, Porter LS, Hanson LC, Reed D. EMPOWER: an intervention to address barriers to pain management in hospice. J Pain Symptom Manage 2015;49:1–12. 61
EMPOWER: an intervention to address barriers to pain management in hospice patients.
Components:
-
Staff training
-
an overview of barriers to pain management in hospice
-
an in-depth discussion of common barriers and suggestions for addressing patient and family fears and misconceptions
-
instructions on use of the EMPOWER screen
-
strategies to improve patient caregiver medication management
-
presentation of case examples
-
distribution of written material outlining the components of the intervention.
-
-
The EMPOWER screen
-
Hospice nurses were instructed to screen family caregivers and, if possible, the patient, using the EMPOWER screen during admission. The screen consisted of eight yes/no questions to identify common concerns related to pain and pain management. The concerns included addiction, side effects, pain as a sign of weakness, being perceived as drug seeking, being a bother, building a tolerance, taking/giving too much and that the medicines would not work.
-
-
Tailored education using the EMPOWER brochure
-
If any of the eight barriers to pain management were identified, hospice staff gave the patient/family member the EMPOWER brochure, which included evidence-based statements, and reviewed its content. The statements were written to address common fears and misinformed beliefs while aiding communication between patients, caregivers and hospice providers. For example, to address concerns about tolerance the statements say –
-
– ‘It is normal for your body to adjust to the pain medication. Your dose can be increased if necessary so the medication keeps working’
-
– ‘Using medication now will not prevent it from working in the future’.
-
-
When no barriers were identified during the screen, families still received the brochure, but there was no discussion of its content.
-
-
Follow-up
-
Staff were instructed to document patient and family concerns in the medical chart and discuss identified concerns during team meetings.
-
Appendix 3 Summary of learning Cancer Carer Medicines Management
A Phase I/II feasibility trial of Cancer Carer Medicines Management: an educational intervention for carer management of pain medication in cancer patients at end of life (research data available to the team)
The Phase I systematic review and Phase II patient and carer interviews
Beliefs
-
Evidence of beliefs that initially hindered, reduced or delayed pain control.
-
Fears of addiction/dependency (not being able to come off it if started).
-
Fears of having to rely on morphine.
-
-
Patients self-managing to avoid ‘crises’ (i.e. pre-empting emergencies and seeking to avoid hospital admissions).
-
Fear of side effects (e.g. reluctance to increase baseline pain medicines for fear of drowsiness); therefore, use of pro re nata medication instead.
-
Concern about out-of-hours care and treatment.
-
Common wish to take the minimum medication possible – often underpinned by rationale ‘so that they can have more later’.
Skills
-
Carer often acts as the person who reminds the patient to take medicines, helping when patients become confused or forgetful, ensuring that medicines taken at appropriate times.
-
Development of routines to suit home life (e.g. to prepare medicines in evening for next day or week).
Recurrent contextual issues
-
Lack of in-depth conversations with patient and carer regarding pain medications across control group.
-
Problems with 111 service in a crisis.
Value of intervention
-
Most found some aspects of it helpful.
-
Carers commented on the value of CCMM resources in the toolkit, particularly for information, reassurance and supporting problem-solving.
-
Some positive changes in medicines management (e.g. increased acceptance of the need for opiates), knowledge being reinforced or enhanced, and behavioural change (e.g. responding more readily to patients’ requests for pain relief and improved systems in place for giving and recording medicines).
Nurse data
-
Only some aspects of the intervention were perceived as distinct from current practice.
-
The toolkit (e.g. the information about opioids was seen as a new and useful resource).
-
The structured conversational process was considered to be similar to nurses’ routine practice.
-
Value of having written materials about opioids and introducing more systematic techniques for managing pain medication.
-
Positive experiences of training helped nurse engagement.
-
Nurses’ accounts emphasised the diversity of patient and carer circumstances, experiences and needs. The adaptability of the intervention, the extent to which nurses could individualise its delivery was perceived as crucial to its usefulness.
-
Nurses did not fully exploit the adaptability of the toolkit. Although some nurses introduced the toolkit resources selectively, there was a tendency to use the toolkit as a package that they gave to carers in its entirety with the expectation that they would decide for themselves which tools would be useful.
-
The focus on pain was seen by some nurses as limiting its usefulness. They argued that carers typically managed multiple medications for a range of symptoms at end of life so broadening the intervention to accommodate that would increase its applicability and acceptability to carers. Nurses were critical of some written resources, which they felt should be comprehensive (all medicines for cancer) rather than pain specific (e.g. the medicines chart).
-
Most argued that introducing the intervention earlier in the course of a patient’s illness would be easier, more appropriate and of greater benefit. They gave examples of carers who were unable to engage with the intervention because they were overwhelmed and distressed.
-
The nurses reported that the intervention had facilitated communication and relationship building.
-
Most found value in the intervention and identified advantages in offering carers written information about analgesics and simple formats for documenting pain and medication. Some thought the intervention had influenced their practice: they would be more likely in future to include carers in discussions and encourage them to keep records.
Carer need literature
Lau et al.93
-
Interviews with informal caregivers (n = 23) and hospice providers (n = 22).
-
Caregivers’ life experience and self-confidence facilitated medication management.
-
Caregivers’ negative emotional states, cognitive and physical impairments, low literacy, other competing responsibilities, as well as patients’ negative emotional states and complex medication needs were limiting factors.
Kimberlin et al.94
-
Focus groups and interviews with cancer patients (n = 22) and family caregivers (n = 16).
-
Seven themes emerged suggesting improvements that are needed in the communication process. These included (1) improving the process of information exchange, (2) increasing active participation of patient and caregiver in the care process, (3) improving provider relationship-building skills, (4) overcoming time barriers, (5) addressing fears regarding use of pain management medications, (6) fostering appropriate involvement of family and caregivers in the communication process and (7) improving co-ordination of care among providers.
Mehta et al.95
-
Grounded theory study of family caregivers (n = 24).
-
Derived an explanatory model of how family caregivers manage the pain of cancer patients at home involving four main processes: ‘drawing on past experiences’; ‘strategizing a game plan’ (accepting responsibility for pain management, establishing relationships with patients and health-care team and seeking information on pain and pain management); ‘striving to respond to pain’ (including implementing strategies for pain relief, determining the characteristics of pain and verifying the degree to which pain relief strategies are successful); and ‘gauging the best fit’ (a decision-making process that links all the processes, recognising parameters and limitations, and then joins the pieces together).
Mehta et al.96
-
Grounded theory study of family caregivers (n = 24).
-
Family caregivers are not always well prepared and require appropriate support to ensure optimal pain control.
-
Understanding that family caregivers are continuously engaged in specific processes as they prepare for and implement pain management strategies can help HCPs tailor their interventions.
Mehta et al.97
-
Grounded theory study of family caregivers (n = 24).
-
Caregivers assessed different types of pain and, therefore, were experimenting with different types of interventions. Not all family caregivers were able to distinguish between the different pains afflicting patients and, consequently, were not selecting the most appropriate interventions. This often led to poorly managed pain and frustrated family caregivers.
Schumacher et al.98
-
Transcribed interactions between intervention nurses and patients (n = 52) and their family caregivers (n = 33).
-
Describes the difficulties with pain management that patients and family caregivers bring to a nurse’s attention during a teaching and coaching intervention. Found patients had difficulty in seven areas when they attempted to put a pain management regimen into practice, namely (1) obtaining the prescribed medication(s), (2) accessing information, (3) tailoring prescribed regimens to meet individual needs, (4) managing side effects, (5) cognitively processing information, (6) managing new or unusual pain and (7) managing multiple symptoms simultaneously. The findings suggest that the provision of information about cancer pain management to patients and their family caregivers is not sufficient to improve pain control in the home care setting.
Appendix 4 Summary of learning IMPACCT
Meta-review57 (of six reviews and two papers) describing the optimal components for an educational intervention for advanced cancer pain using Michie et al. ’s39 behaviour change wheel as theoretical underpinning.
Information on pain management
-
Providing education to patients approaching the end of life to self-manage their pain is known to reduce pain.
-
Include name and type of medication, routes of administration, around the clock/as needed, schedule and dosing.
-
Patients with cancer pain should routinely be provided with patient-based education to improve knowledge on managing pain and analgesia.
-
Provide consistent screening for misunderstandings about pain and analgesia prior to commencing analgesic therapy. Address these aspects through clear advice and information.
-
Written or audio-visual material supporting the advice should be given to the patient to take away.
Cognitive barriers to pain management
Includes concerns about tolerance, addiction, fatalism, religious fatalism, being a good patient, side effects of medication are inevitable and unmanageable, masking signs of disease progression, distracting clinicians from treating the disease, harming the immune system, injections and respiratory depression. Patients and carers view pain as a referent for disease status (i.e. worsening pain = worsening disease). Morphine use has particularly strong symbolism of addiction and tolerance and its introduction into a patient’s life is seen/interpreted as a signal of impending death.
Information on how to implement self-management strategies
-
It is not known which aspects of self-management education interventions are most effective (content, timing, frequency, mode of delivery).
-
Information and advice should be so that a lay person could improve his/her knowledge of pain management.
-
Behavioural instructions on how to perform the desired behaviour means skill building via instructions, so that a lay person could actually perform desired pain management.
-
Skills-based interventions showed greater effectiveness compared with education approaches in reducing pain severity (not statistically significant, but considered a promising finding). Skills-based interventions defined as ‘changing patients’ dysfunctional beliefs about pain and promote the use of specific skills to manage it (e.g. distraction, relaxation). Specific components varied, targeting all three attributes of knowledge, skills, attitudes of cancer pain and its management.
-
Specialist nurses and pharmacists might be the most appropriate HCPs to deliver pain management advice.
Contextual factors that need to be addressed in an intervention to support self-management of cancer pain
-
Patient/carer level (intrinsic): psychological and physical capability to engage with an intervention; reflective processes around planning and managing cancer pain.
-
HCP level (extrinsic): appropriate education/training of the HCP to deliver education (the intervention) to patients; the HCP requires protected time to deliver education (the intervention) to patients (written material and face-to-face educational session of not less than 15 minutes); multidisciplinary involvement.
Themes from qualitative studies
Control, knowledge, meaning of morphine, adherence, impact of pain and trust. Qualitative research evidences indicates that patients constantly make a trade-off between the impact of pain against the impact from analgesia on physical and cognitive function.
Intervention components are those activities that should be included in an educational intervention in order to change the behaviour of individuals. The authors identified five out of the nine behaviour change wheel intervention functions and suggest that these should be included in any educational intervention promoting self-management of advanced cancer pain:
-
education, for example providing written information about pain management, including analgesic and non-pharmacological approaches
-
training, for example providing instruction, demonstration and coaching of new skills (techniques for managing daily drug regimes, relaxation techniques)
-
enablement and persuasion, for example overcoming cognitive and emotional barriers to pain management through addressing concerns about tolerance or addiction
-
environmental restructuring and resources, for example incorporating the delivery of education for self-management into the usual care provided by specific health professionals, such as specialist nurses, primary care practice nurses and community pharmacists
-
modelling, for example patients talking to other patients about their successful use of various pain management strategies.
Appendix 5 Focus group topic guide/interview guide: patients/carers
Appendix 6 Card pack used in the patient/carer interviews and focus group
Appendix 7 Focus group topic guide/interview guide: health-care professionals
Appendix 8 Card pack used in the health-care professional interviews and focus groups
Appendix 9 SMART self-management support toolkit factsheets
Appendix 10 Topic guide for structured patient podcast
Appendix 11 Topic guide for structured health-care professional podcast
Appendix 12 Pain diary
Appendix 13 Medication chart
Appendix 14 Goal-setting sheets
Appendix 15 Communication with the Research Ethics Committee
The Research Ethics Committee’s amendment notification and amendment approval letter
Appendix 16 Recruitment packs: patients, carers and study nurses
Appendix 17 Feasibility study case report forms
Appendix 18 Nurse self-management conversation prompts
Supportive self-management in palliative care
. . . assessing, planning and implementing appropriate care to enable the patient to live until they die and supporting the patient to be given the means to master or deal with their illness themselves.
Johnston et al. 51
Conversational process
| Phase of self-management conversation | Approach/role (Johnston et al.51) | Skills | Example |
|---|---|---|---|
| Orientation to self-management |
|
Emotion work: take account of what is important to the person, their identity, to their previous experience and how they are making sense of the current situation66 | |
| Focus on maintaining normality (Johnston et al.51) | |||
| Suspend concerns about time the conversation will take | |||
Assess: purpose to identify
|
|
Start with open question focused on intention of conversation | Can you tell me how you have been getting on with managing your pain medicines? |
| Recognise and validate the feeling expressed by the patient | It sounds as though this has been really toughIt sounds as though you have been working hard to try and get on top of your pain | ||
| Use probing questions to find out in more detail about how the person is managing their pain medicines – what they are doing, how they are understanding analgesics and the beliefs influencing this understanding | So one of the challenges you are facing is . . . can you tell me a little more about . . .Are there other concerns that are influencing how you are managing your medications? | ||
| Check for other concerns and repeat process of gathering and reframing | Can I just check whether you have any concerns about taking medications for your pain; for example, many people worry that they will get addicted to the medicines or that if they take them now they will not work so well if the pain gets worse, or some people have worries that relate to their own or a family member’s experiences of taking pain medicines in the past | ||
| If not already mentioned, check whether or not person has any concerns raised by beliefs about pain medications (e.g. typical fears about opioids such as dependence and tolerance, meaning of being on opioids, etc.) and previous experience of pain medications (such as family members who have taken opioids) | |||
| Ask patient to identify most pressing concern(s) | So to summarise, the key things influencing your ability to manage your pain are (list a, b, c, etc.)Which of these is most important for us to address today? | ||
| Agree a plan for what to discuss today – negotiate to add a topic to the patient’s list if you feel is it significant to improving their pain medicine management | OK let’s talk about that a bit more XIf you need to add a topic: ‘Once we’ve done that I would like to talk a bit more about Y because I think this will also help. Would that be OK?’ | ||
Inform: purpose
|
|
Restate the issue to be discussed | So the issue you have identified as being most challenging is . . . |
| Normalise the issue | This is something that many people have concerns about | ||
| Introduce the educational resource relevant to this issue | I think this resource will be helpful, let me go through it with you/tell you about it . . . | ||
| Reinforce the information: key points | This factsheet/podcast explains that it is important to a, b, c | ||
| Check understanding/response | How does this sound to you? Does this information help your concerns? | ||
| Address any remaining concerns (may need to reassess concerns and provide other information) | |||
| If appropriate suggest ways of monitoring pain (pain diary and pain medication chart) | |||
Setting self-management goals: purpose
|
|
Begin to explore some possibilities for positively influencing pain management | Having gone through this information, do you think there are some things that you can try which will help you to manage your medicines/pain differently? |
| Check out the things suggested | Which of these ideas will be realistic to try this week?Are there things that will make this difficult for you to do? | ||
| Refine the suggested plan | So just to recap, you are going to (state what the patient is going to do), because this will help your pain by (state how this behaviour is going to help manage medicines or control pain). If you do this you are hoping that (state expectation of action) by (state time frame) | ||
| Record suggested plan | Let’s just make a note of what you are hoping to achieve in the next week, so that we can review how things have gone when we next meet | ||
| Agreeing self-management goals |
|
Remind patient of resources available to support agreed plan | Don’t forget that there are some other things available to support you with your plan. I’m going to leave the fact sheet with you so that you and your family can read and refer to it, in your own time. There are other sources of reliable information available on the internet so I’ll leave you a list of where to look. Also, if for any reason the plan we have discussed is not possible or your pain increases then we would want to know. (Then go through who to telephone/contact etc.) |
| Regular review |
|
Make a plan to review | So the last thing is to make a date and time for us to review the plan that you have made and your pain management . . . |
Appendix 19 Making action plans
Making action plans with individuals
One of the most important self-management skills is goal-setting. Goals often need to be broken into smaller, more achievable steps or tasks. Once a goal has been set, it needs to be decided how exactly it can be achieved, by making an action plan.
An action plan should be time limited (i.e. 1 or 2 weeks) and be related to a goal that the individual really wants to achieve. The individual should be able to achieve the action plan and the plan should be very specific, specifying what, how much, when and how often.
Parts of a personal action plan
-
Something the individual wants to do.
-
Reasonable/achievable (something that the individual could expect to be able to achieve within the week).
-
Action/behaviour specific.
-
Answer the questions: what, how much, when, how often.
Consider
-
The specific steps needed to achieve the goal (include what, when, how, where and how often).
-
The things that could make it difficult to achieve the goal.
-
The plan for overcoming these challenges.
-
Supports and resources needed to achieve the goal.
Appendix 20 Aide memoire for researchers to use with study nurses
Researcher aide memoire
Supporting the nurses in their use of the four-step educational approach:
Needs assessment
-
What is your experience of asking patients how they are managing their pain medicines?
-
How are you finding using the four-step conversational process?
Information provision
-
Thinking about your experience of providing the intervention, are there parts of it that feel easier/more appropriate to do than others?
-
Could we do anything additional to support you in delivering the process? Are there any extra resources/materials that might be of help to you?
Goal-setting
-
Thinking about the process of goal-setting with study patients, what are the kinds of things patients are prioritising? Do you feel that this prioritisation process is helpful in supporting self-management?
-
Are you managing to use the goal-setting sheet at each visit? How are you finding this? What sort of action plans are you developing with the patients (are they practical steps, action orientated, time specified, barrier focused with strategies for overcoming these, etc.)?
Review and coaching
-
I’ll be here next week; can we talk over these issues again to see if there are any changes or additional things that would help you?
Appendix 21 Framework for researcher field notes
Ongoing supportive visits
Visits with CNSs – reinforcing the workshop session.
Needs assessment
-
Response to how she/he is finding asking patients about their needs (beliefs, behaviours and knowledge), resulting discussion, any researcher recommendations.
Information provision
-
Response to how she/he is finding providing information, issues raised and researcher’s response, when appropriate.
Goal-setting
-
Response to how she/he is finding using the goal-setting sheet, resulting discussion and recommendations.
Coaching
-
Response to how she is finding the reviewing with the study patient(s), resulting discussion and coaching of CNS by researcher.
Delivery and discussion of further training resources:
-
For example, Johnston et al. ’s51 self-management definition, self-management conversational prompts, audio file of modelled self-management conversation, action-planning sheet.
Response of the CNS to the above process and trial delivery.
Any confounding factors?
-
Lack of CNS availability/time?
-
Lack of receptiveness? Is there an apparent reason for this?
Researcher reflexivity
-
Should I have handled anything differently?
-
What lessons can be learnt for the other CNSs or other patients in terms of delivery of the intervention by the respective CNS?
Appendix 22 Feasibility study interview guide
Appendix 23 Non-study clinical nurse specialist survey
Appendix 24 Analyses of the feasibility study qualitative findings
Thirteen patients and seven carers participated in 13 interviews at the end of their involvement in the feasibility trial (Table 37). They were asked about their last main occupation and their highest educational achievements (Table 38).
| Research design and processes | The intervention |
|---|---|
Deliverability, acceptability, perceived benefits and disadvantages of:
|
Deliverability of four-step approach Data sources: CNS interviews and researcher field notes |
|
Deliverability, acceptability, perceived benefits and disadvantages of:Data source: patient/carer interviews Deliverability, acceptability, perceived benefits and disadvantages of:Data sources: patient/carer interviews and researcher field notes |
Deliverability of SMST Data sources: CNS interviews and researcher field notes |
|
Deliverability of:Data source: study mapping Excel spreadsheets Deliverability of:Data source: study mapping Excel spreadsheets |
Acceptability of four-step approach Data sources: CNS interviews and researcher field notes |
Deliverability, acceptability, perceived benefits and disadvantages of:
|
Acceptability of SMST Data source: CNS interviews |
|
Perceived benefits of four-step approach Data source: CNS interviews |
|
|
Perceived benefits of SMST Data sources: CNS interviews and patient/carer interviews |
|
|
Perceived disadvantages of four-step approach Data source: CNS interviews |
|
|
Perceived disadvantages of SMST Data source: CNS interviews and patient/carer interviews |
| Demographic data: patient and carer Phase III interview sample | |||
|---|---|---|---|
| Number of interviews |
n = 13 Thirteen patients, seven carers participated in dyad interviews with patient Remaining six patients recruited to feasibility study – one admitted to hospice post trial, other five withdrawal or loss to follow-up of the patient during course of trial |
||
| Last main (or current) occupation | |||
| Patients | Car mechanic, care worker, coach trip business, education welfare officer, electrical engineer, fitters mate, nurse, professor, publisher, secretary, senior lecturer, shop manager, vehicle inspector | ||
| Carers | Anglican minster, architect, brick layer, care worker, nursery school worker, secretary, security worker | ||
| Number of qualifications | Professional, vocational or other work-related qualifications below degree level | Professional, vocational or other work-related qualifications at degree level or higher | |
| Patients | 4 | 6 | 3 |
| Carers | 1 | 5 | 1 |
All but one (n = 11) of the 12 study nurses participated in an interview at the end of the feasibility trial (Table 39).
| Demographic data: CNS Phase III interview sample | |
|---|---|
| Number of interviews | n = 11 |
| Location | n = 5 Yorkshire based (out of 6 study nurses); n = 6 Hampshire based (out of 6 study nurses) |
The Phase III qualitative data results have been presented in tabular form for clarity and brevity, within an analysis framework designed for the study. The initial focus was on the findings regarding deliverability, acceptability, perceived benefits and possible disadvantages of both the research design and the intervention itself. 99 In line with the recommendations of Moore et al. 76 regarding process evaluation of complex interventions, the final column of the analysis framework, and the ultimate higher level of analysis, focuses on the mechanisms of action – the participant responses to the research design or intervention, the mediating factors and the consequences (Tables 40 and 41).
| Feasibility of research design and processes | Findings from the data: deliverability, acceptability, perceived benefits, disadvantages | Mechanism of action: participant responses to the research design and processes, mediating factors, consequences |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Feasibility of intervention delivery | Findings from the data: deliverability, acceptability, perceived benefits, disadvantages | Mechanism of action: participant responses to and interactions with the intervention, mediating factors, consequences |
|---|---|---|
The four-step educational approach:
|
|
|
| The SMST: all the factsheets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Appendix 25 Definition of self-management and professional roles
Johnston et al. ’s definition of supported self-management in palliative care:51
Assessing, planning, and implementing appropriate care to support the patient to be given the means to master or deal with their illness or its effects. Supported self-management in advanced disease, by nurses, can, empower people to acknowledge the impact of their condition on their life, and enable them, where possible, to face the range of challenges they may have, and identify areas where they need further support, help or care. Therefore, for individuals it’s about being provided with the means to master or deal with problems rather than relinquish them to others.
Johnston et al. 51
Johnston et al. ’s related professional roles:51
Self-Management Support: Professional Roles
Advocate – To support self-management and the right of palliative patients to receive appropriate medicines to meet their symptom control needs
Educator – To provide instruction regarding medicines to allow patients to self-manage
Facilitator – To promote relationships between healthcare professionals and patient/carer to enable effective access to and use of medicines
Problem Solver – To use expertise (underpinned by robust needs assessment) to work out whether current medicines and dosages are appropriate, or whether they should be altered
Communicator – To facilitate communication between individuals e.g. encouraging a patient to discuss their pain with their carer
Goal Setter – To identify specific goals that the patient wishes to achieve, and the methods to achieve the goals. This is motivational and enhances self-management performance
Monitor – To observe and constantly re-assess self-management of medicines over time. This requires evaluation of an individual’s capacity to self-manage vs. their willingness to engage and compliance
Reporter – To gather information and report it e.g. at multidisciplinary team meetings
Johnston et al. 51
These extracts are reproduced from Johnston et al. 51 © Johnston et al. ; licensee BioMed Central Ltd. 2014. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.
Appendix 26 The SMART intervention costs
| SMART toolkit contents: unit title | Quantity | Pages | Cost (£) | Unit cost (£) |
|---|---|---|---|---|
| Goals folder | 50 | 4 | 164.79 | 3.30 |
| NCR sets | 300 | 4 | 120.26 | 0.40 |
| Folder (with insert and spine) | 50 | 1 | 435.70 | 8.71 |
| Checking opioid medicines are managing pain | 50 | 4 | 116.64 | 2.33 |
| Pain diary | 50 | 4 | 116.64 | 2.33 |
| Common concerns | 50 | 4 | 116.64 | 2.33 |
| Contacts and further information | 50 | 4 | 116.64 | 2.33 |
| Keeping on top of side effects | 50 | 4 | 116.64 | 2.33 |
| Organising opioid medicines | 50 | 4 | 116.64 | 2.33 |
| Fitting pain control into my daily routine | 50 | 8 | 218.24 | 4.36 |
| Getting prescriptions and obtaining medicines | 50 | 8 | 218.24 | 4.36 |
| Managing pain with opioid medicines | 50 | 8 | 218.24 | 4.36 |
| Medicine chart | 50 | 8 | 218.24 | 4.36 |
| Total SMART folders | 2293.55 | 43.83 |
List of abbreviations
- BPI
- Brief Pain Inventory
- CCMM
- Cancer Carer Medicines Management
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CNS
- clinical nurse specialist
- CRF
- case report form
- CRN
- clinical research nurse
- DAM
- decision-analytic modelling
- DVD
- digital versatile disc
- EORTC-QLQ C30
- European Organisation for Research and Treatment of Cancer – Quality of Life Questionnaire C30
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ESAS
- Edmonton Symptom Assessment Scale
- GP
- general practitioner
- HCP
- health-care professional
- HTA
- Health Technology Assessment
- ICECAP-A
- ICEpop CAPability measure for Adults
- ICER
- incremental cost-effectiveness ratio
- ID
- identification
- IMPACCT
- Improving the Management of Pain from Advanced Cancer in the Community
- IQR
- interquartile range
- K–M
- Kaplan–Meier
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- PPI
- patient and public involvement
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SD
- standard deviation
- SES
- Self-Efficacy for Managing Chronic Disease Scale
- SIMS
- Satisfaction with Information about Medicines Scale
- SMART
- Self-Management of Analgesia and Related Treatments at the end of life
- SMST
- self-management support toolkit
- VOICES
- National Survey of Bereaved People