Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/103/01. The contractual start date was in February 2013. The draft report began editorial review in July 2017 and was accepted for publication in November 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Peter Tyrer is a member of the Health Technology Assessment (HTA) Commissioning Board. Joseph G Reilly has received project funding from the Drug Safety Research Unit as part of an unrestricted grant provided by Merck Pharmaceuticals. Alan Montgomery is part of the HTA Clinical Evaluation and Trials Board. Institutions for all authors have received funding from the National Institute for Health Research for other studies.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Crawford et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
The importance of borderline personality disorder
Borderline personality disorder (BPD) is a severe mental health condition that is characterised by affective instability, recurrent suicidal behaviour and impaired interpersonal functioning. 1 It is estimated that between 0.5% and 2% of people have BPD. 2 The levels of BPD among people in contact with mental health services are far higher; as many as one-fifth of people who are admitted to inpatient mental health units in the UK have this diagnosis. 3
People with BPD are more likely to experience other mental health problems such as anxiety, depression and substance misuse. Of those who attend emergency medical services following deliberate self-harm, 1 in 10 have BPD,4 and the rate of completed suicide among people with this condition is 50 times higher than in the general population. 5 People with this condition have poor social functioning; many are socially isolated and most are unemployed or on long-term sick leave. 6 People with BPD are also more likely to experience poor physical health7 and mortality due to cardiovascular disease and other physical health problems is higher. 8 The reasons for this are unclear. Although it is possible that the high levels of emotional distress that people with BPD experience are associated with more somatic symptoms,9 it seems likely that higher levels of smoking and substance misuse are important. 10 People with BPD may neglect themselves, and problems in maintaining interpersonal relationships may make it more difficult for them to obtain the physical health care they need when unwell. 10
Treatment of borderline personality disorder
Concerns have been expressed about the quality of services for people with BPD. 11 Many people who have this diagnosis report that they are dissatisfied with the treatment they receive,12,13 and mental health practitioners often find it difficult to work with people with this condition. 14
Although psychological treatments, such as dialectical behaviour therapy and mentalisation-based therapy, have been shown to improve the mental health of people with BPD,15 most people with this disorder do not have access to specialist psychological treatment services. Among those who do, many do not engage with psychological treatment, and as many as half of those who do engage drop out before the treatment has been completed. 16 People with the most severe problems are less likely to engage successfully in psychological treatments than those with milder forms of the disorder. 16,17
No drug treatments are licensed for the treatment of BPD. Despite this, people with this condition are often prescribed large amounts of psychotropic medication. 18 Antidepressants are widely used, despite evidence that they do not improve the mental health or social functioning of people with BPD. 19 The results of randomised trials of antipsychotic medications are equivocal. Although some studies have shown short-term reductions in symptoms of anger and hostility, the longer-term effects of these drugs are not known. 19
The role of mood stabilisers
Affective instability and higher than expected levels of comorbidity with bipolar disorder among people with BPD have led to considerable interest in the role that mood stabilisers may play in improving the mental health of people with this disorder. 20 Investigation of the role of mood stabilisers was highlighted as a priority for future research in the National Institute for Health and Care Excellence (NICE) guidelines on the recognition and management of BPD. 11 Research into the effects of established mood stabilisers, such as lithium and carbamazepine, has been limited because of their toxicity in overdose, which is a not infrequent occurrence among people with this condition. 21 Another concern about the use of mood stabilisers in people with BPD is the increased incidence of birth defects among children born to women taking these drugs. 22 Most people with BPD who are in contact with mental health services are women of child-bearing age. Many women with BPD report impulsive behaviour, including unplanned and unprotected sex. Data from women who take these drugs for epilepsy have shown that levels of major congenital malformations are higher among those taking valproate than among those taking lamotrigine. 23 Concerns have also been raised about long-term cognitive impairment among children born to women taking valproate. 24
Lamotrigine is a mood stabiliser with antiepileptic and analgesic properties. 25,26 The mechanism of action of lamotrigine in patients with bipolar affective disorder is poorly understood but may relate to enhancing the action of the inhibitory neurotransmitters, including gamma-aminobutyric acid. 27
Evidence in support of the use of lamotrigine for people with BPD comes from three open-label studies and two placebo-controlled trials. 19 The two randomised controlled trials of lamotrigine for people with BPD have reported positive findings. The first trial, by Tritt et al. ,28 involved 24 women who were recruited mainly from advertisements placed in primary care practices. In comparison with those women taking the placebo, women taking up to 200 mg of lamotrigine were found to have lower levels of anger 8 weeks later. The second trial, by Reich et al. ,29 recruited 28 men and women through websites and television and radio advertisements. Those participants who were randomised to receive up to 225 mg of lamotrigine were subsequently found to have lower levels of affective instability and impulsiveness [assessed using the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD)] 12 weeks later. A key limitation of both studies is that the degree of severity may have been lower than that seen among people with BPD who are treated in secondary care mental health services. Data on levels of global functioning at the time of the baseline assessment in the trial by Reich et al. 29 show that mean levels of impaired social function were in the ‘moderate’ range. Both studies focused on short-term effects of lamotrigine and neither examined the costs or cost-effectiveness of this intervention.
Lamotrigine is associated with a range of side effects, which include a skin rash that is reported in up to 10% of people taking the drug and a more severe cutaneous reaction, Stevens–Johnson syndrome, which is estimated to occur in < 0.1% of people taking this drug. 30 The incidence of this problem is reduced by gradual dose escalation and care with interacting agents, such as valproate and oral contraceptives. However, serious events are rare, and the drug is widely used in the UK for the treatment of people with bipolar disorder; therefore, psychiatrists are familiar with its dose titration requirements and the need for vigilance regarding severe cutaneous adverse reactions.
The LABILE study
The Lamotrigine And Borderline personality disorder: Investigating Long-term Effectiveness (LABILE) trial was designed to compare the clinical effectiveness and cost-effectiveness of lamotrigine plus usual care with an inactive placebo plus usual care over a 1-year period. The main aim of the study was to test whether or not prescribing lamotrigine in addition to usual treatment reduces symptoms of this condition, improves social functioning and quality of life, reduces the incidence of suicidal behaviour, reduces the level of alcohol and substance misuse and lowers the amount of antipsychotic and other psychotropic medication that people are prescribed. The trial also examined the cost, cost-effectiveness and cost–utility of adding lamotrigine to usual care for adults with BPD.
Chapter 2 Methods
Design
The LABILE trial was a multicentre, two-arm, parallel-group, double-blind, placebo-controlled, individually randomised trial of lamotrigine versus placebo with 12-, 24- and 52-week follow-up assessments. The trial included an integrated clinical and economic evaluation.
Study setting
Study participants were recruited from secondary care mental health services in England including inpatient units, outpatient clinics and community mental health teams. There were six recruitment centres altogether in London (Central and North West London NHS Foundation Trust, Oxleas NHS Foundation Trust, West London Mental Health NHS Trust), the East Midlands (Nottinghamshire Healthcare NHS Foundation Trust, Derbyshire Healthcare NHS Foundation Trust) and the north-east of England (Tees, Esk and Wear Valleys NHS Foundation Trust).
Participants
To be eligible to take part in the study, potential participants had to be aged ≥ 18 years, meet the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) diagnostic criteria for BPD and be willing and able to provide written informed consent to take part in the study. Potential participants were excluded if they:
-
had a coexisting diagnosis of bipolar affective disorder (type I and II) or psychotic disorder (schizophrenia, schizoaffective disorder or mood disorder with psychotic features)
-
were already being prescribed a mood stabiliser (lithium, carbamazepine or valproate) or had had one within the past 4 weeks
-
had a known medical history of liver or kidney impairment
-
had cognitive or language difficulties that prevented them from providing informed consent.
In addition to this, women were excluded from the study if they were pregnant, planning a pregnancy or of child-bearing age and not using adequate contraception.
Interventions
Those who were allocated to the active arm of the trial were prescribed encapsulated generic lamotrigine, titrated according to the established British National Formulary protocol30 but with the titration occurring at standardised 14-day intervals. The dose was altered for participants who were taking the combined oral contraceptive pill, which affects the metabolism of lamotrigine. For all participants, the starting dose was 25 mg per day and this was increased to 50 mg after 2 weeks, 100 mg after 4 weeks and 200 mg per day after 6 weeks. The dose was maintained at 200 mg unless the participant was taking the combined oral contraceptive pill, in which case it was further increased to 300 mg after 8 weeks and 400 mg per day after 10 weeks. However, the same dose could be prescribed again for an additional 2 weeks during titration and a lower maintenance dose utilised throughout participation when this was clinically indicated, such as when tolerability or emergent side effects were a concern.
Those who were allocated to the placebo arm of the trial were given capsules identical in appearance to those containing active lamotrigine, but backfilled with lactose monohydrate. This was prescribed in the same regime as that used in the active arm of the trial.
Trial medication was issued to patients fortnightly to cover the dose titration period, with a 17-day supply provided in case the next supply was delayed for any reason, such as a participant not attending a scheduled meeting. Once the maintenance dose was reached, further trial medication was provided either fortnightly or 4-weekly, as decided by the prescriber based on an assessment of risk of intentional overdose. In any instance when a participant had, intentionally or unintentionally, stopped taking trial medication for a period of ≥ 5 consecutive days, they were returned to a prescribed dosage of 25 mg daily and re-titrated gradually to their maintenance dose.
Usual care
Usual care in the trial comprised contact with primary care and secondary care health services, including access to psychological treatment services and inpatient admission if required. No restrictions were imposed on the use of other treatments, except that those who remained in the trial were not to be prescribed lamotrigine (aside from trial medication) or any other mood stabiliser (lithium, carbamazepine or sodium valproate).
Assessments
Assessment of eligibility and for determining randomisation strata
We assessed eligibility using the items on BPD from the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II). 31,32 We planned to use data from other sections of the SCID-II to establish the severity of the participant’s personality disorder33 but, following feedback from researchers and service users, we replaced this with the self-completed International Personality Disorder Examination (IPDE) screening questionnaire (DSM-IV version) to reduce the amount of time that it took to complete the baseline assessment. 34 We used the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I)35 to assess whether or not potential participants had bipolar affective disorder (type I or II) and excluded those who did. Hypomanic symptoms were assessed using the Hypomanic Checklist-32 items (HCL-32),36 a relatively short screening questionnaire that can distinguish those with bipolar disorder from those with unipolar depression.
Primary outcome
The primary outcome was symptoms of BPD measured using the ZAN-BPD37 52 weeks after randomisation. The ZAN-BPD is a widely used measure of the symptoms and behavioural problems experienced by people with BPD. The scale includes four subscores for the domains of affective disturbance, cognitive disturbance, impulsivity and disturbed relationships, which characterise the signs and symptoms of BPD. The ZAN-BPD has been used in previous studies of pharmacological and psychological treatments for people with BPD. 29,38–40 It is reliable (intraclass correlation coefficients for inter-rater reliability = 0.96 and test–retest reliability = 0.93), has high convergent validity with structured clinical ratings of symptoms of BPD and is sensitive to change. 37
The lead researcher on the study received personal training on the use of the ZAN-BPD from Professor Mary Zanarini (who developed the scale). This researcher then trained the other researchers, initially by using vignettes and then by discussing participants whom they had assessed. In order to test the reliability of the assessment of the primary outcome among researchers, we arranged for 27 participants to be simultaneously rated by two separate researchers and we calculated the extent to which total scores on the scale were correlated.
Secondary outcomes
The following secondary outcomes were assessed:
-
Scores on the ZAN-BPD in the 52 weeks after randomisation using repeated measures analysis of data collected at 12, 24 and 52 weeks’ follow-up.
-
Total score on the 21-item Beck Depression Inventory (BDI)41 at 12, 24 and 52 weeks. The BDI has been widely used as a self-completed questionnaire, provides a valid assessment of the severity of depressive symptoms and can be completed in less than 10 minutes. 42
-
Incidence and severity of suicidal behaviour and self-harm using the Acts of Deliberate Self-Harm Inventory43 at 12, 24 and 52 weeks. This structured interview collects detailed information about the number and severity of episodes of self-harm and suicidal acts, and has been used successfully in other trials of treatments for people with BPD. 44
-
Social functioning using the Social Functioning Questionnaire (SFQ) at 12, 24 and 52 weeks. This questionnaire is an eight-item self-report scale that asks people about problems across a range of settings that people with BPD often experience. 45
-
Health-related quality of life, using the EuroQoL-5 Dimensions, three-level version (EQ-5D-3L),46 at 12, 24 and 52 weeks. The EQ-5D-3L provides a brief and reliable measure of health-related quality of life, which is responsive to change in people with BPD. 47
-
Side effects, using a pro forma designed to cover the possible effects listed in the British National Formulary entry for lamotrigine,30 at 12, 24, and 52 weeks (see Appendix 1).
-
Use of alcohol and other drugs at 52 weeks after randomisation, using the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST). 48 This short questionnaire provides a reliable and valid screening test for problem substance use. 49
-
Use of concomitant psychotropic medication, defined as the proportion of people taking psychotropic medication and the proportion of people taking antipsychotics, at 52 weeks after randomisation.
-
Total cost of health and social services. We collected data on use of resource using the Adult Service Use Schedule (AD-SUS), adapted for use in this trial based on previous research involving people with personality disorders,50 at 12, 24 and 52 weeks (see Appendix 2). This questionnaire collects detailed data on use of all hospital and community health and social care services. At baseline, we used the AD-SUS to record service use over the previous 12 weeks and at the trial follow-up time points we used the AD-SUS to record service use since the previous assessment; thus, the entire study period was covered.
Adherence
We assessed adherence to study medication at the 12-, 24- and 52-week assessments using the Morisky Medication four-item Adherence Scale. 51 This is a four-item questionnaire that provides a valid estimate of adherence with psychotropic medication. 52 The total score ranges from 0 to 4, with higher scores indicating higher adherence. In addition to this, researchers asked participants about their use of trial medication when each prescription was renewed and any intentional or unintentional treatment breaks were recorded.
Blinding
All patients, carers and referring psychiatrists were blinded to treatment assignment until the participant had left the trial or until 52 weeks post randomisation (whichever was the longer). Blinding of investigators, researchers, the trial manager and the trial statistician was maintained until all data were entered, the database was locked and initial analyses of trial data were complete. The exception to this was for participants whose referring psychiatrist was also the principal investigator, in which case the allocation for that particular participant was revealed following the final assessment.
Site pharmacies were unblinded to trial arm allocation and were provided with a list of the randomisation codes and corresponding trial arm allocation for that site. The trial medication was produced with tear-off labels that identified it as being lamotrigine or placebo in a coded format, so that pharmacy staff could dispense the appropriate medication for a participant. Pharmacy procedures required that the tear-off label was removed during dispensing and added to trial documents for accountability. The need to maintain the blinding of researchers and other individuals at the site was made clear to those delegated to work on the trial within the pharmacy.
Unblinding at the end of the follow-up period
At 52 weeks after a participant was randomised into the study, regardless of whether or not they withdrew from the study early or completed the participation period in full, a letter was sent to the referring prescriber informing them of the participant’s allocation status. When a participant had completed the participation period in full, this allowed the prescriber time to make arrangements for the participant to continue on lamotrigine if appropriate and desired. On completion of the 52-week follow-up assessment, the participant was advised to contact their prescriber to discuss their trial arm allocation and their future treatment.
An individual, who had no other role in the trial, was unblinded for the purpose of informing the referring clinician of the trial arm of the allocation of participants, as part of routine unblinding.
Emergency unblinding
In anticipation of an emergency, such as an overdose of trial medication, Emergency Scientific and Medical Services (ESMS) Global Ltd was contracted to provide a 24-hour emergency unblinding telephone service. All requests for unblinding were recorded.
Study logistics
Recruitment
Potential participants were initially approached about the trial by any health-care professional who was involved in their care, providing that the consultant psychiatrist for the team had agreed in principle to patients under their care taking part in the study.
If a psychiatrist or other health-care professional had a patient under their care who they believed met the eligibility criteria, they then introduced the patient to the trial and provided them with an information sheet.
When the patient provided verbal agreement to discuss their eligibility and possible enrolment into the trial with a member of the research team, a screening number was assigned and contact details passed on to the research team to discuss consent.
Potential participants were given a minimum of 24 hours from receiving the information sheet to consider the information and the opportunity to question the investigator, their general practitioner (GP) or other independent parties regarding participation in the trial.
Screening and baseline
If written informed consent was given and documented, then the referring clinician completed a document to confirm their medical opinion of the participant’s eligibility and a researcher completed the screening assessment (Table 1) with the participant to assess eligibility. If the participant fulfilled all the eligibility criteria, then the baseline assessment was also completed and they were randomised into the trial. Following randomisation, the participant’s GP and consultant were informed of their enrolment into the trial.
| Assessments | Time point | ||||
|---|---|---|---|---|---|
| Screening | Baseline | Follow-up | |||
| 12-week | 24-week | 52-week | |||
| SCID-IIa | ✗ | – | – | – | – |
| SCID-Ib | ✗ | – | – | – | – |
| IPDE screening questionnaire (DSM-IV version) | ✗ | – | – | – | – |
| HCL-32 | ✗ | – | – | – | – |
| ASSIST | – | ✗ | – | – | ✗ |
| Four-item Morisky Medication Adherence Scale | – | – | ✗ | ✗ | ✗ |
| ZAN-BPD | – | ✗ | ✗ | ✗ | ✗ |
| BDI | – | ✗ | ✗ | ✗ | ✗ |
| Acts of Deliberate Self-harm Inventory | – | ✗ | ✗ | ✗ | ✗ |
| SFQ | – | ✗ | ✗ | ✗ | ✗ |
| EQ-5D-3L | – | ✗ | ✗ | ✗ | ✗ |
| Side effects | – | ✗ | ✗ | ✗ | ✗ |
| Modified Adult Service User Schedule | – | ✗ | ✗ | ✗ | ✗ |
Assignment of interventions
Study participants were randomly allocated to the intervention (lamotrigine) or comparator (placebo) arm of the trial by an automated randomisation service operated by Nottingham Clinical Trials Unit. The randomisation sequence was generated using permuted stacked blocks, with block size randomly assigned to 4 or 6. Allocation was 1 : 1, stratified by recruitment site, severity of personality disorder and extent of bipolarity. We used data from the IPDE screening questionnaire to establish whether participants met criteria for probable cluster A or cluster C personality disorders (‘complex personality disorder’) or whether they met only probable criteria for borderline and other cluster B personality disorders (‘simple personality disorder’) according to the criteria developed by Tyrer and Johnson. 33 We used the extent of bipolarity, measured as total score on the HCL-32, to examine the extent of bipolarity (low, a score of 0–13, or high, a score of ≥ 14). 54
Follow-up
Prior to providing a new supply of trial medication, the participant was contacted to elicit details of any adverse events (AEs) that occurred, to determine if there had been any intentional or unintentional breaks in their taking of the trial mediation and to ascertain whether or not they wished to continue with the trial.
Participants received an assessment at 12, 24 and 52 weeks. The timing and sequence of all assessments are summarised in Table 1.
Data management
Data were entered onto a secure web-based database. Access was restricted by user identifiers and passwords (encrypted using a one-way encryption method). Study data will be archived securely and then safely destroyed after 15 years.
Sample size
We based the sample size calculation for the study on our primary hypothesis: for people with BPD who are in contact with mental health services, the addition of lamotrigine to usual treatment will reduce symptoms of their disorder at 52 weeks’ follow-up, according to the total score on the ZAN-BPD.
The ZAN-BPD has been used to examine the clinical effectiveness of a range of psychological and pharmacological treatments for people with BPD. In a randomised trial of a modified form of group-based cognitive behavioural therapy, Blum et al. 40 found that there were improvements in mental health and reduced use of emergency medical services among those who were randomised to problem-solving therapy. These improvements were associated with a difference of 3.6 [standard deviation (SD) 6.9] in total ZAN-BPD score. The ZAN-BPD rating scale was also used to examine the clinical effectiveness of lamotrigine for people with BPD in a randomised trial conducted by Reich et al. 29 In this small trial (n = 28), a non-statistically significant difference of 5.6 (SD 6.75) in total score on the ZAN-BPD was found at 12 weeks. Seventeen (61%) people in the trial completed all 12 weeks of the study and the levels of adherence to trial medications in those that completed the study were judged to be high.
Anticipating that levels of adherence to trial medications would be lower in the LABILE trial than in the study by Reich et al. ,29 we powered the study on the basis of a smaller difference in ZAN-BPD score of 3.0 (SD 6.75). The sample size was calculated using Stata® version 13.1 (StataCorp LP, College Station, TX, USA).
A total of 214 participants (107 receiving lamotrigine and 107 receiving placebo) would need to be randomised to have 90% power to detect a minimal clinically relevant difference of 3.0 (SD 6.75) in total score on the ZAN-BPD at 52 weeks, using a 0.05 level of statistical significance. To take account of 15% loss to follow-up at 52 weeks, the sample size was increased to 252. However, this was further revised to 266 during the course of the trial to account for a greater loss to follow-up of 25%.
Statistical analyses
The analysis and reporting of the trial was conducted in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines. 55 A detailed statistical analysis plan was developed and agreed with the Independent Data Monitoring and Ethics Committee, and this was finalised prior to the completion of data collection, the database lock and the unblinding of the study. Continuous variables were summarised in terms of the mean, SD, median, lower and upper quartiles, minimum, maximum and number of observations. Categorical variables were summarised in terms of frequency counts and percentages. All data were analysed using Stata, version 13.1.
Preliminary analyses
Descriptive statistics of demographic and clinical measures were used to examine the balance between the randomised arms at baseline.
Primary analysis
The primary analysis was performed according to the intention-to-treat principle on the available case set, without imputation of missing data. The analysis was adjusted by site, baseline ZAN-BPD score, severity of personality disorder (simple or complex) and the extent of bipolarity (score of ≥ 14 or < 14).
Secondary analyses
For secondary analyses of ZAN-BPD scores at 12 and 24 weeks, randomised groups were compared using a mixed model for repeated outcome measures, adjusted by the same stratification variables used for the primary analysis. We investigated whether any treatment effects were sustained or emerged later by including an interaction term between treatment and time in the model. In the absence of a time effect, the effectiveness parameter was the average difference in mean ZAN-BPD score over the 52-week period along with 95% confidence interval (CI) and exact p-value.
Sensitivity analyses
Sensitivity analyses of the primary outcome were conducted to:
-
further adjust for any variable with marked imbalance at baseline
-
investigate the impact of missing data, using multiple imputation.
Complier-average causal effect analyses
We investigated the effect of treatment adherence using complier-average causal effect (CACE) estimation methods. Intention-to-treat analysis does not represent treatment effect under non-compliance of treatment; therefore, we used CACE analysis to explore whether or not the treatment effect was directly affected by the level of compliance. The level of compliance was examined using both dichotomous and continuous measures: (1) dichotomous – whether or not the participant had taken medication at a dose of ≥ 100 mg without interruption during the 52 weeks prior to the final follow-up interview; and (2) continuous – the percentage of weeks that the patient took the medication at a dose of ≥ 100 mg during the 52-week treatment period.
Analyses of secondary outcomes
The secondary outcomes were BDI score, incidence of deliberate self-harm, SFQ score, alcohol and any other substance use, and antipsychotic medication use. The secondary outcomes were analysed in a similar manner to the primary analysis. A generalised linear model was used for continuous outcomes and logistic regression model for binary outcomes.
Safety reporting
For safety data, including AEs, serious adverse events (SAEs) and suspected unexpected serious adverse reactions, we presented basic summary statistics, that is, the number of AEs or side effects of different categories and the number and proportion of participants who reported at least one AE or SAE within each treatment arm.
Health economics analysis
The primary economic evaluation took a NHS/personal social services perspective, including only costs incurred to health and social care services, following guidance from NICE. 56 Although previous studies in people with BPD have found that health and social care are the key cost drivers in this patient group,44 it is also clear that BPD can have an impact on not only an individual’s ability to work but also their absence from work as a result of sickness. 57 Therefore, productivity losses were included in a sensitivity analysis.
Calculation of costs
Costs for the economic evaluation were calculated in three stages: identification, measurement and valuation. The first stage of identification ensures that all relevant resources are included in the evaluation; these are the resources that are particularly relevant for people with BPD and which were identified from published studies,44,58 meetings with clinicians and discussions with our patient representatives. Resource use was collected in the following areas:
-
lamotrigine – drug costs and time with dispensing clinician
-
hospital services – inpatient admissions (including admissions for physical and mental health problems), outpatient/day case appointments (for physical and mental health problems), accident and emergency attendances
-
community services – GP (in person, on the telephone and at home), practice nurse, mental health care co-ordinator/key worker, psychiatrist, psychologist, community psychiatric nurse, social worker, counsellor/therapist, NHS walk-in clinic, advice service (e.g. Citizens Advice), complementary therapist
-
medication.
During data collection there was also an opportunity for respondents to report any other relevant service use, including group therapy, day centre, dietitian, drug and alcohol services, eating disorder services, physiotherapist or podiatrist.
Data on the use of all identified services were collected using a range of methods. Information on the dispensing and dosage of lamotrigine was taken from clinician records. Other data were obtained from participants using a modified version of the AD-SUS, adapted for use in people with BPD on the basis of previous research in this area. 58 The use of community services was collected in interview, in which the study participant was asked to recall which, from a list of community services, they had contacted over the previous period. In addition, researchers used the AD-SUS to collect information on the participant’s occupational status (e.g. employed, unemployed, student, retired) and the number of hours and days taken off work as a result of ill-health. The AD-SUS was administered at baseline and at 12, 24 and 52 weeks’ follow-up. At baseline, the participant was asked to recall the services that they had used over the previous 12 weeks and at the trial follow-up time points, the participant was asked, using the AD-SUS, about the services that they had used since the previous assessment, so that the entire follow-up period was covered.
The total cost of the resources used by each study participant was calculated by applying a unit cost to each item of resource use. All unit costs were for the financial year 2015/16. The cost of lamotrigine was taken from the British National Formulary,59 using a generic cost from a standard NHS supplier. The cost of time with the dispensing clinician was taken from the Unit Costs of Health and Social Care. 60 It was assumed that the clinician was a psychiatrist, who had a 10-minute consultation with the participant during the titration period, followed by 10 minutes every 4 weeks thereafter. All other unit costs were sourced from standard sources and are detailed in Appendix 3. Productivity losses were calculated on the basis of days missed from work using information on gross annual pay collected in the AD-SUS. The total cost for each participant was the sum of all their costs. Discounting was not necessary, as costs were collected over a 1-year period only.
Calculation of quality-adjusted life-years
The EQ-5D-3L responses were converted to utility scores using findings from a sample of representative UK adults. 61 These utility scores were then used to calculate quality-adjusted life-years (QALYs) using the area under the curve approach, in which changes in utility scores were assumed to follow a linear path. 62 No discounting of QALYs was necessary. 62
Data analysis
For the main analysis, complete-case analysis was used in which participants with missing data were excluded. Multiple imputation of missing cases was carried out in a sensitivity analysis. A single missing item from an otherwise complete data set was imputed using mean imputation, so that the participant could be included in the complete-case analysis.
The average use of different types of services by randomised group over 52 weeks’ follow-up was tabulated and reported descriptively as the mean number of contacts and the percentage of each group using that service at least once. No statistical comparisons between service uses were completed in order to avoid problems with multiple testing and to keep the focus of the evaluation on costs and cost-effectiveness.
The total average cost between randomised groups over 52 weeks’ follow-up was compared using generalised linear regression models with the following covariates: stratification variables (study centre, severity of personality disorder and extent of bipolarity) and baseline costs. The validity of the results were confirmed by examining the CIs from bias-corrected, non-parametric bootstrapping. 63 The use of parametric tests is recommended for cost data, despite its skewed distribution, because it allows for inferences to be made on the arithmetic mean, which is the most meaningful summary statistic for cost. 64
Cost-effectiveness analysis
The cost-effectiveness analysis allowed costs and outcomes to be considered together in a decision-making context. The primary cost-effectiveness analysis used QALYs derived from the EQ-5D-3L; a secondary cost-effectiveness analysis used the ZAN-BPD measure. Cost-effectiveness was first assessed through the calculation of incremental cost-effectiveness ratios (ICERs), which are a summary statistic of the difference in mean cost between randomised groups divided by the difference in mean effect. 65 An ICER is calculated from the means of the randomised group, so there remains statistical uncertainty as to the accuracy of the ICER as a summary statistic. Therefore, 5000 resamples (bootstrapping) from the cost and outcomes data were used to generate a new distribution of mean costs and outcomes. 66 These distributions were then plotted onto a cost-effectiveness plane for interpretation. 65 Replications that fall in the south-west quadrant of the plane suggest that lamotrigine is less costly and less effective than the placebo, replications that fall in the south-east quadrant suggest that lamotrigine is less costly and more effective than the placebo, replications in the north-west quadrant suggest that lamotrigine is more costly and less effective than the placebo and replications in the north-east quadrant suggest that lamotrigine is more costly and more effective than the placebo.
Next, the bootstrapped replications were used to calculate the probability that lamotrigine was the ‘optimal’ choice, depending on the maximum value (willingness to pay, λ) that a decision-maker might be willing to pay for an improvement in outcome. The willingness-to-pay value varied between likely minimum and maximum values, and the results plotted on a graph result in a cost-effectiveness acceptability curve. 67 All cost-effectiveness analyses were adjusted for baseline stratification variables by site, baseline ZAN-BPD score, severity of personality disorder (simple or complex) and score on the HCL-32 (i.e. a score of ≥ 14 or < 14), baseline costs and baseline EQ-5D-3L tariff.
Sensitivity analysis
A number of sensitivity analyses were carried out to test the robustness of the analysis to key assumptions:
-
varying the economic perspective to include productivity losses
-
examining the impact of missing data through multiple imputation of missing cases.
Service user involvement
Plans for the study were presented at a research seminar at the British and Irish Group for the Study of Personality Disorder, which included service user representatives. These discussions, together with feedback from Fenella Lemonsky (an expert by experience and a co-applicant on the study), helped us to decide which mood stabiliser we should examine. We also used feedback from service users and results of a Delphi study of users of personality disorder services to help us decide. 58
Fenella Lemonsky remained an active member of the project management group throughout the study. Additional input from people with lived experience of using services was provided by Sally Strange and Jennie Parker. Service users reviewed materials for publicising the study and commented on a draft version of the patient information sheet.
Service users also contributed to the communication of study findings; Sally Strange and Jennie Parker helped us interpret study findings and develop our recommendations for services and for future research. Jennie Parker also commented on a draft of the lay summary of the study findings, which we have distributed to study participants, and Fenella Lemonsky presented the results of the study at the annual conference of the British and Irish Group for the Study Personality Disorders in 2017.
An independent service user, Jenny Trite, was an active member of the Trial Steering Group throughout the course of the study.
Ethics approval and governance
The trial was approved by the London-Central Research Ethics Committee (reference number 2/LO/1514). In accordance with the current revision of the Declaration of Helsinki68 (amended October 2000, with additional footnotes added in 2002 and 2004), a participant had the right to stop trial treatment and to withdraw from the trial at any time and for any reason, without prejudice to his or her future medical care by the physician or at the institution, and was not obliged to give his or her reasons for doing so. The investigator could also withdraw a participant from trial treatment at any time in the interest of the participant’s health and well-being or for administrative reasons. Trial follow-up continued after treatment was withdrawn, unless the participant withdrew consent.
All potential participants were provided with written and verbal information about the study before being asked to provide written informed consent to participate in the study.
Progress of the study was overseen by a Trial Steering Committee and a Data Monitoring and Ethics Committee.
Changes to trial design
Change to design between funding proposal and trial commencement
-
Following concerns about the length of time it would take to assess eligibility, we modified our initial plan, which was to assess all aspects of personality disorder using the SCID-II semistructured interview (which can take up to 90 minutes to complete), and instead used only the section of the SCID-II that establishes whether or not a person meets diagnostic criteria for BPD. As we wanted to obtain information about whether or not a person had simple or complex personality disorder, we also used the self-complete IPDE screening questionnaire. This questionnaire takes less than 15 minutes to complete. 69
-
We added the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) questionnaire to the 52-week follow-up assessment to enable us to assess whether or not offering study participants lamotrigine had any impact on their use of alcohol and illicit drugs. 48
-
Initial plans to measure participants’ weight at 24 and 52 weeks were removed from the assessment schedule because of problems identifying scales in many of the locations where follow-up assessments were due to be conducted.
Change to design after trial commencement
An additional recruitment site was opened several months into the recruitment period (Derbyshire Healthcare NHS Foundation Trust).
Loss to follow-up during trial participation was expected to be 15%, but an interim assessment of the retention rate showed that it was approximately 20%. Therefore, the target sample size was increased to 266.
Chapter 3 Results
Between July 2013 and October 2015, 413 participants were referred to the study, of whom 296 (71.7%) were screened. Among the potential participants who were screened, 276 (93.2%) met the inclusion criteria and were randomised. Of the 276 participants randomised, 139 were allocated to the placebo plus usual care arm and 137 were allocated to the lamotrigine plus usual care arm. The CONSORT flow diagram for the LABILE trial is presented in Figure 1. Follow-up interviews took place between October 2013 and October 2016. The rates of follow-up were similar between treatment arms; overall, 71% of participants attended the 52-week follow-up. There were no instances in which researchers were unblinded to the participant’s allocation status prior to completion of collection of 52-week outcome data. Scores on the ZAN-BPD from pairs of researchers who separately rated 27 participants were highly correlated (intraclass correlation coefficient 0.98, 95% CI 0.95 to 0.99).
FIGURE 1.
The CONSORT flow diagram for the study.

Baseline characteristics of randomised participants
The demographic and clinical data of each trial arm are summarised in Table 2. The results of the outcome assessments at baseline are presented for each arm in Table 3. In terms of baseline comparability, the lamotrigine and placebo groups were well matched.
| Characteristic | Treatment group | |
|---|---|---|
| Lamotrigine (N = 137) | Placebo (N = 139) | |
| Age (years) at randomisation | ||
| Mean (SD) | 36.0 (11) | 36.2 (11) |
| Sex, n (%) | ||
| Male | 34 (25) | 34 (24) |
| Female | 103 (75) | 105 (76) |
| Ethnicity, n (%) | ||
| White | 123 (90) | 123 (90) |
| Black | 7 (5) | 4 (3) |
| Asian | 1 (1) | 2 (1) |
| Mixed | 6 (4) | 8 (6) |
| Missing | 0 | 2 (1) |
| Employment status, n (%) | ||
| Employed | 34 (25) | 26 (19) |
| Unemployed | 95 (69) | 105 (76) |
| Student | 4 (3) | 1 (1) |
| Retired | 2 (1) | 2 (1) |
| Missing | 2 (1) | 5 (4) |
| Total score HCL-32 | ||
| Mean (SD) | 21.2 (5.5) | 22.5 (5.2) |
| n | 72 | 75 |
| Severity of personality disorder, n (%) | ||
| Simple | 0 | 2 (1) |
| Complex | 137 (100) | 137 (99) |
| Outcome | Treatment group | |
|---|---|---|
| Lamotrigine (N = 137) | Placebo (N = 139) | |
| ZAN-BPD | ||
| Mean score (SD) | 16.6 (5.8) | 17.4 (6.2) |
| n | 135a | 138a |
| ASSIST,b n (%) | ||
| Alcohol | 53 (39) | 54 (39) |
| Cannabis | 35 (26) | 27 (19) |
| Cocaine | 11 (8) | 15 (11) |
| Amphetamine-type stimulants | 10 (7) | 9 (6) |
| Inhalants | 2 (1) | 2 (1) |
| Sedatives or sleeping pills | 14 (10) | 16 (12) |
| Hallucinogens | 1 (1) | 4 (3) |
| Opiates | 7 (5) | 9 (6) |
| Other | 6 (4) | 2 (1) |
| BDI | ||
| Mean score (SD) | 39.8 (11.7) | 38.4 (10.2) |
| n | 135 | 138 |
| SFQ | ||
| Mean score (SD) | 15.0 (4.1) | 14.9 (4.5) |
| n | 135 | 137 |
| EQ-5D-3L health state | ||
| Mean score (SD) | 42.6 (24) | 43.8 (20.9) |
| n | 135 | 137 |
| Total number of side effects, median (IQR) | ||
| Mild | 4 (2–7) | 4 (1–7) |
| Moderate | 3 (2–6) | 3 (1–6) |
| Severe | 1 (0–3) | 1 (0–3) |
| Deliberate self-harm,c n (%) | ||
| No | 39 (28) | 51 (37) |
| Yes | 96 (70) | 87 (63) |
| Unknown | 2 (2) | 1 (< 0.5) |
The ZAN-BPD is a measure of symptoms and behavioural problems and the total score ranges from 0 to 36; a lower score indicates a better outcome. The BDI measures the severity of depression symptoms and the score ranges from 0 to 63; a lower score indicates a better outcome. The SFQ score measures social functioning problems and the score ranges from 0 to 24; a lower score indicates a better outcome.
Adherence to protocol and trial medication
Details of the number of participants who completed each of the follow-up assessments are presented in Table 4, broken down by study arm. The median time between randomisation and the completion of these assessments is also presented. In total, 195 (71%) participants completed the 52-week follow-up.
| Time point | Treatment group | |
|---|---|---|
| Lamotrigine (N = 137) | Placebo (N = 139) | |
| 12 weeks | ||
| Number of participants who completed the assessment, n (%) | 111 (81) | 104 (74) |
| Average time in days from randomisation, mean (SD) | 13.8 (2.6) | 13.7 (1.9) |
| 24 weeks | ||
| Number of participants who completed the assessment, n (%) | 98 (72) | 98 (71) |
| Average time in days from randomisation, mean (SD) | 26 (3.0) | 26 (2.5) |
| 52 weeks | ||
| Number of participants who completed the assessment, n (%) | 97 (71) | 98 (71) |
| Average time in days from randomisation, mean (SD) | 51.8 (3.8) | 51.6 (2.4) |
Table 5 summarises the key parameters of trial medication adherence and prescribing over the course of participation for participants in each arm. In total, 93 (34%) participants completed the trial medication per protocol, and similar proportions were shown in both arms. Although 191 (69%) study participants were taking the trial medication 12 weeks after randomisation, only 107 (39%) were taking it at the end of the 1-year follow-up period. Self-rated adherence, assessed using the total score on the four-item Morisky Medication Adherence Scale at 12, 24 and 52 weeks’ follow-up (Table 6), also showed similar levels of compliance with trial medication.
| Outcome | Treatment group | |
|---|---|---|
| Lamotrigine (N = 137) | Placebo (N = 139) | |
| Was the IMP received as per protocol? [n (%)]a | ||
| No | 93 (68) | 90 (65) |
| Yes | 44 (32) | 49 (35) |
| Percentage of the 52-week period that the participant was taking ≥ 100 mg | ||
| Mean (SD) | 60 (35) | 66 (35) |
| Range | 2–94 | 2–98 |
| Number of weeks that the participant received IMP | ||
| Median (IQR) | 32 (9–52) | 46 (7–52) |
| Minimum, maximum | 0, 52 | 0, 52 |
| Number (%) of participants taking IMP | ||
| At 12 weeks | 95 (69) | 95 (68) |
| At 52 weeks | 49 (36) | 58 (42) |
| Dose (mg) of IMP taken | ||
| At 12 weeks | ||
| Median (IQR) | 200 (200–200) | 200 (200–200) |
| Range | 25–400 | 25–400 |
| At 52 weeks | ||
| Median (IQR) | 200 (200–200) | 200 (200–200) |
| Range | 100–400 | 200–400 |
| Time point | Treatment group | |
|---|---|---|
| Lamotrigine (N = 137) | Placebo (N = 139) | |
| 12 weeks | ||
| Median score (IQR) | 3 (2–4) | 3 (2–4) |
| n | 109 | 99 |
| 24 weeks | ||
| Median score (IQR) | 3 (2–4) | 3 (2–4) |
| n | 89 | 91 |
| 52 weeks | ||
| Median score (IQR) | 3 (2–4) | 3 (2–4) |
| n | 88 | 82 |
Primary outcome
The total score on the ZAN-BPD decreased for study participants as a whole between baseline and 12 weeks and then remained fairly stable throughout the remainder of the follow-up. This pattern was seen in both arms. No difference was seen in adjusted mean total ZAN-BPD score at 52 weeks (Table 7).
| Time point | Treatment group | Adjusted differencea (95% CI); p-value | |
|---|---|---|---|
| Lamotrigine (N = 137) | Placebo (N = 139) | ||
| Baseline | |||
| Mean score (SD) | 16.6 (5.8) | 17.4 (6.2) | – |
| n | 135 | 138 | |
| 12 weeks | |||
| Mean score (SD) | 11.5 (5.7) | 11.5 (7.1) | – |
| n | 111 | 104 | |
| 24 weeks | |||
| Mean score (SD) | 11.9 (6.1) | 11.9 (7.0) | – |
| n | 98 | 98 | |
| 52 weeks | |||
| Mean (SD) | 11.3 (6.6) | 11.5 (7.7) | 0.1 (–1.8 to 2.0); p = 0.91 |
| n | 97 | 98 | |
The lack of treatment effect was supported by the results of the sensitivity analyses (Table 8), which included the following:
-
using repeated measures analysis to include ZAN-BPD scores at all visits
-
adjusting baseline variable with imbalance (no formal statistical testing for differences)
-
multiple imputation of missing data using chained equations
-
CACE analysis to investigate the impact of compliance level on treatment effect.
| Treatment group | Adjusted difference in meansa | 95% CI | Analysis scenario |
|---|---|---|---|
| Lamotrigine | 0.0 | –1.25 to 1.26 | Repeated measureb |
| Placebo | – | – | – |
| Lamotrigine | 0.0 | –1.90 to 1.90 | Further adjustment of baseline datac |
| Placebo | – | – | – |
| Lamotrigine | –0.1 | –1.90 to 1.80 | Multiple imputation of missing datad |
| Placebo | – | – | – |
| Lamotrigine | 0.3 | –3.70 to 4.30 | Using dichotomous treatment adherence indicatore |
| Placebo | – | – | – |
| Lamotrigine | 0.0 | 0.02 to 0.03 | Using continuous treatment adherence indicatore |
| Placebo | – | – | – |
Secondary outcomes
Comparison of secondary outcomes at 52 weeks between those in the two treatment arms of the trial revealed no statistically significant differences (Table 9). The proportion of people who were prescribed psychotropic medication in the year following randomisation was 93% of those in the lamotrigine arm and 95% of those in the placebo arm of the trial, of whom 16% in the lamotrigine arm and 17% in the placebo arm of the trial were prescribed antipsychotic medication. We also found no evidence of clinically important differences in secondary outcomes at 12 or 26 weeks (see Appendix 4). No differences were seen on any of the subscores of the ZAN-BPD at 52 weeks. The adjusted mean difference for those prescribed lamotrigine was –0.1 (95% CI –0.9 to 0.7) on the affective disturbance subscore, 0.1 (95% CI –0.4 to 0.7) for cognitive disturbance, –0.1 (95% CI –0.6 to 0.5) for impulsivity and 0.0 (95% CI –0.5 to 0.5) for disturbed relationships compared with those in the placebo arm of the trial (see Appendix 4, Table 22).
| Outcome measure, treatment group | Time point | Adjusted differencea (95% CI) | p-value | |
|---|---|---|---|---|
| Baseline | 52-week follow-up | |||
| Depression score (as measured by the BDI) (N = 180), mean score (SD) | ||||
| Lamotrigine | 39.8 (11.7) | 28.8 (16.1) | –0.2 (–4.5 to 4.1) | 0.937 |
| Placebo | 38.4 (10.2) | 28.7 (15.5) | – | |
| Deliberate self-harmb (N = 179), n (%) | ||||
| Lamotrigine | 96 (70) | 45 (46) | 1.25 (0.68 to 2.28) | 0.464 |
| Placebo | 87 (63) | 38 (39) | – | |
| Social functioning (as measured by the SFQ) (N = 179), mean score (SD) | ||||
| Lamotrigine | 15 (4.1) | 12.4 (4.3) | 0 (–1.2 to 1.2) | 0.987 |
| Placebo | 14.9 (4.5) | 12.3 (4.9) | – | |
| Alcohol use (as measured by ASSIST) (N = 178), n (%) | ||||
| Lamotrigine | 53 (39) | 28 (31) | 1.4 (0.7 to 2.7) | 0.354 |
| Placebo | 54 (39) | 22 (25) | – | |
| Other substance misuse (as measured by ASSIST) (N = 178), n (%) | ||||
| Lamotrigine | 54 (39) | 27 (30) | 1.2 (0.6 to 2.3) | 0.598 |
| Placebo | 47 (34) | 23 (26) | – | |
Safety
Tables 10 and 11 show that there was an excess of AEs in the placebo group but the incidence of those classified as serious did not differ across treatment arms. There were three deaths in the trial, all of which occurred in the placebo arm. No suspected unexpected serious adverse reactions were recorded.
| AE | Treatment group | |
|---|---|---|
| Lamotrigine (N = 137) | Placebo (N = 139) | |
| Total number of AEs | 246 | 285 |
| Total number of participants with at least one AE, n (%) | 77 (56) | 93 (67) |
| Total number of AEs by system organ class | ||
| Blood and lymphatic system disorders | 2 | 3 |
| Cardiac disorders | 0 | 1 |
| Endocrine disorders | 0 | 1 |
| Eye disorders | 1 | 6 |
| Gastrointestinal disorders | 38 | 55 |
| General disorders and administration site conditions | 14 | 14 |
| Hepatobiliary disorders | 1 | 0 |
| Immune system disorders | 1 | 1 |
| Infections and infestations | 23 | 38 |
| Injury, poisoning and procedural complications | 17 | 39 |
| Investigations | 7 | 3 |
| Metabolism and nutrition disorders | 2 | 1 |
| Musculoskeletal and connective tissue disorders | 8 | 7 |
| Nervous system disorders | 32 | 31 |
| Pregnancy, puerperium and perinatal conditions | 3 | 2 |
| Psychiatric disorders | 37 | 40 |
| Renal and urinary disorders | 1 | 0 |
| Reproductive system and breast disorders | 3 | 1 |
| Respiratory, thoracic and mediastinal disorders | 16 | 9 |
| Skin and subcutaneous tissue disorders | 35 | 31 |
| Social circumstances | 1 | 1 |
| Surgical and medical procedures | 4 | 1 |
| SAE | Treatment group | |
|---|---|---|
| Lamotrigine (N = 137) | Placebo (N = 139) | |
| Total number of SAEs | 36 | 48 |
| Total number of participants with at least one SAE, n (%) | 26 (19) | 32 (23) |
| Total number of SAEs by system organ class | ||
| Eye disorders | 0 | 1 |
| Gastrointestinal disorders | 1 | 3 |
| General disorders and administration site conditions | 2 | 1 |
| Immune system disorders | 1 | 0 |
| Infections and infestations | 0 | 1 |
| Injury, poisoning and procedural complications | 6 | 19 |
| Metabolism and nutrition disorders | 1 | 0 |
| Musculoskeletal and connective tissue disorders | 1 | 0 |
| Nervous system disorders | 1 | 0 |
| Pregnancy, puerperium and perinatal conditions | 3 | 2 |
| Psychiatric disorders | 16 | 20 |
| Renal and urinary disorders | 1 | 0 |
| Respiratory, thoracic and mediastinal disorders | 1 | 0 |
| Social circumstances | 1 | 0 |
| Surgical and medical procedures | 1 | 1 |
Using a pro forma to enquire about the presence of specific known side effects of lamotrigine revealed no difference between the groups at any assessment time points (Table 12).
| Time point, side effect level | Treatment group, median number of side effects (IQR) | |
|---|---|---|
| Lamotrigine (n = 137) | Placebo (n = 139) | |
| At baseline | ||
| Mild | 4 (2–7) | 4 (1–7) |
| Moderate | 3 (2–6) | 3 (1–6) |
| Severe | 1 (0–3) | 1 (0–3) |
| At 12 weeks | ||
| Mild | 9 (5–13) | 7.5 (4–14) |
| Moderate | 6 (3–9) | 6 (4–9) |
| Severe | 2 (0–5) | 1 (0–4) |
| At 24 weeks | ||
| Mild | 11.5 (7.5–18) | 13 (6–20) |
| Moderate | 9 (5.5–12.5) | 8.5 (4–14.5) |
| Severe | 2.5 (0–7.5) | 2 (0–6) |
| At 52 weeks | ||
| Mild | 17 (11–24.5) | 16 (8–25) |
| Moderate | 12 (6–19) | 11 (4.5–17) |
| Severe | 3 (0–10) | 3 (1–8.5) |
Chapter 4 Economic evaluation
The availability of service-use data at each follow-up period is summarised in Table 13, which shows that full service-use information was available for 61% of participants in the lamotrigine group and 57% of participants in the placebo group.
| Time point | Treatment group, n (%) | |
|---|---|---|
| Lamotrigine | Placebo | |
| Baseline | 135 (100) | 137 (100) |
| 12 weeks | 110 (80) | 104 (77) |
| 24 weeks | 98 (72) | 97 (72) |
| 52 weeks | 91 (66) | 88 (65) |
| All periods | 83 (61) | 77 (57) |
Service use
All resources used by study participants over the 52-week follow-up are summarised in Table 14. There are some noticeable differences in the use of hospital services over the follow-up period; participants in the lamotrigine group had an average of 12 nights of inpatient care compared with six nights of inpatient care in the placebo group. The SDs and ranges reported alongside the means in the table suggest that this difference in mean costs was because of a small number of participants who had long stays in hospital. Outpatient appointments and accident and emergency attendances were similar between groups.
| Service | Treatment group | |||||
|---|---|---|---|---|---|---|
| Lamotrigine (n = 83) | Placebo (n = 77) | |||||
| Mean (SD)a | Range | %b | Mean (SD)a | Range | %b | |
| Inpatient nights | 12.66 (34.59) | 0–239 | 46 | 6.37 (17.95) | 0–127 | 35 |
| Outpatient contacts | 4.47 (7.26) | 0–39 | 67 | 5.36 (9.69) | 0–66 | 77 |
| A&E contacts | 3.86 (8.47) | 0–69 | 70 | 3.52 (5.90) | 0–28 | 61 |
| General practice | 20.43 (24.13) | 0–157 | 98 | 17.16 (16.57) | 1–83 | 100 |
| Health care | 28.90 (23.19) | 0–85 | 95 | 21.23 (25.60) | 0–167 | 95 |
| Mental health services | 12.65 (15.13) | 0–84 | 96 | 13.29 (19.56) | 0–148 | 96 |
| Social care | 13.88 (49.36) | 0–429 | 73 | 12.25 (36.37) | 0–291 | 70 |
| Complementary services | 1.02 (9.33) | 0–85 | 1 | 0.08 (0.68) | 0–6 | 1 |
| Any medication | – | – | 93 | – | – | 95 |
| Any antipsychotic medication | – | – | 16 | – | – | 17 |
In general, the use of community health services was similar in both groups. Between 98% and 100% of participants saw their GP at least once over the period of follow-up, and, on average, the number of times a participant saw their GP was between 17 and 20. Use of other health-care services (practice nurse, walk-in clinic, dietitian, physiotherapist, podiatrist) and community mental health services (psychiatrist, psychologist, care co-ordinator/key worker, psychiatric nurse, counsellor/therapist, group therapy, day centre, drug and alcohol services, eating disorder services) was equally high.
Cost
At baseline, on average, costs were £5618 in the lamotrigine group and £3555 in the placebo group. The adjusted difference of £2376 was not statistically significant (95% CI –£108.13 to £4860.37; p = 0.061). The mean average cost differences between the groups are detailed in Table 15. The average drug and prescribing cost of lamotrigine was £242.69. The difference in hospital costs between the lamotrigine and placebo groups (£7294.09 vs. £4711.22) reflects the different average duration of inpatient stays. Other medication costs were also higher in the lamotrigine group (£674.10 vs. £302.64), resulting in higher average total costs in the lamotrigine group (£12,244.32) than in the placebo group (£8495.41), although this difference in cost was not statistically significant (95% CI –£1886.61 to £3169.83; p = 0.617).
| Cost item | Treatment group, mean cost (SD) | Differencea | 95% CIa | p-valuea | |
|---|---|---|---|---|---|
| Lamotrigine (n = 83) | Placebo (n = 77) | ||||
| Intervention | 242.69 (95.99) | 0.00 (0.00) | 244.78 | 222.71 to 266.84 | |
| Hospital | 7294.09 (15,894.87) | 4711.22 (10,057.64) | 416.76 | –1798.50 to 2632.01 | |
| Community | 4033.44 (3599.40) | 3481.54 (3090.88) | 158.94 | –437.91 to 755.79 | |
| Medication | 674.10 (2457.12) | 302.64 (971.04) | 23.73 | –302.89 to 350.35 | |
| Total | 12,244.32 (17,442.80) | 8495.41 (11,349.10) | 641.61 | –1886.61 to 3169.83 | 0.617 |
Outcomes
The EQ-5D-3L scores at baseline and all follow-up points are detailed in Table 16. There were very few between-group differences in EQ-5D-3L scores and the resulting QALYs. The QALYs in the lamotrigine group were 0.287 and in the placebo group were 0.299, not significantly different (95% CI –0.057 to 0.034; p = 0.612).
| Time point | Treatment group | |||
|---|---|---|---|---|
| Lamotrigine | Placebo | |||
| n | Mean score (SD) | n | Mean score (SD) | |
| Baseline | 135 | 0.424 (0.336) | 137 | 0.446 (0.343) |
| 12 weeks | 109 | 0.488 (0.365) | 104 | 0.509 (0.344) |
| 24 weeks | 93 | 0.473 (0.366) | 91 | 0.525 (0.341) |
| 52 weeks | 86 | 0.47 (0.355) | 80 | 0.516 (0.353) |
| All-period QALYa | 83 | 0.287 (0.023) | 77 | 0.299 (0.292) |
Cost-effectiveness analysis
The ICER for the QALY outcome using adjusted mean differences is not reported. As it was negative, the lamotrigine treatment was dominated by the placebo. The ICER for ZAN-BPD using adjusted mean differences was £641.61/0.22 = £2916 per unit change in ZAN-BPD score.
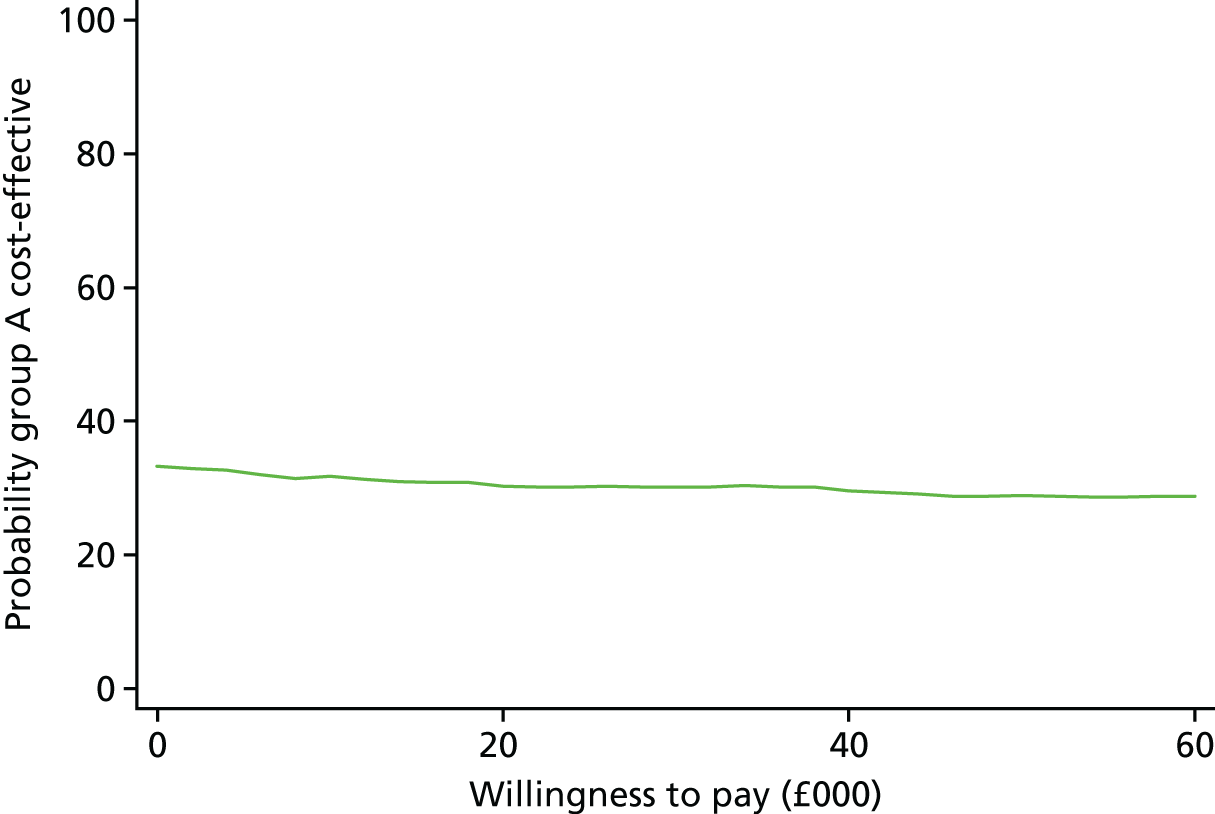
The uncertainty around the ICER for QALYs is shown in Figure 2. The bootstrapped replications are present in all four quadrants of the plane: 44% appear in the less effective, more costly, quadrant; 23% in the more costly, more effective, quadrant; 21% in the less effective, less costly, quadrant; and 12% in the less costly, more effective, quadrant. The green line denotes the willingness-to-pay value of £20,000 per QALY. As there are only relatively few replications below this line, it suggests that lamotrigine treatment is not cost-effective in terms of QALYs. The cost-effectiveness acceptability curve in Figure 3 gives a clear representation of the cost-effectiveness plane. There are no willingness-to-pay values at which the probability of lamotrigine treatment being cost-effective is > 35%.
FIGURE 2.
Scatterplot on a cost-effectiveness plane of differences in costs vs. differences in QALYs.

FIGURE 3.
Cost-effectiveness acceptability curve showing the probability that lamotrigine treatment is cost-effective compared with placebo at different values that a decision-maker might be willing to pay for increases in QALYs.
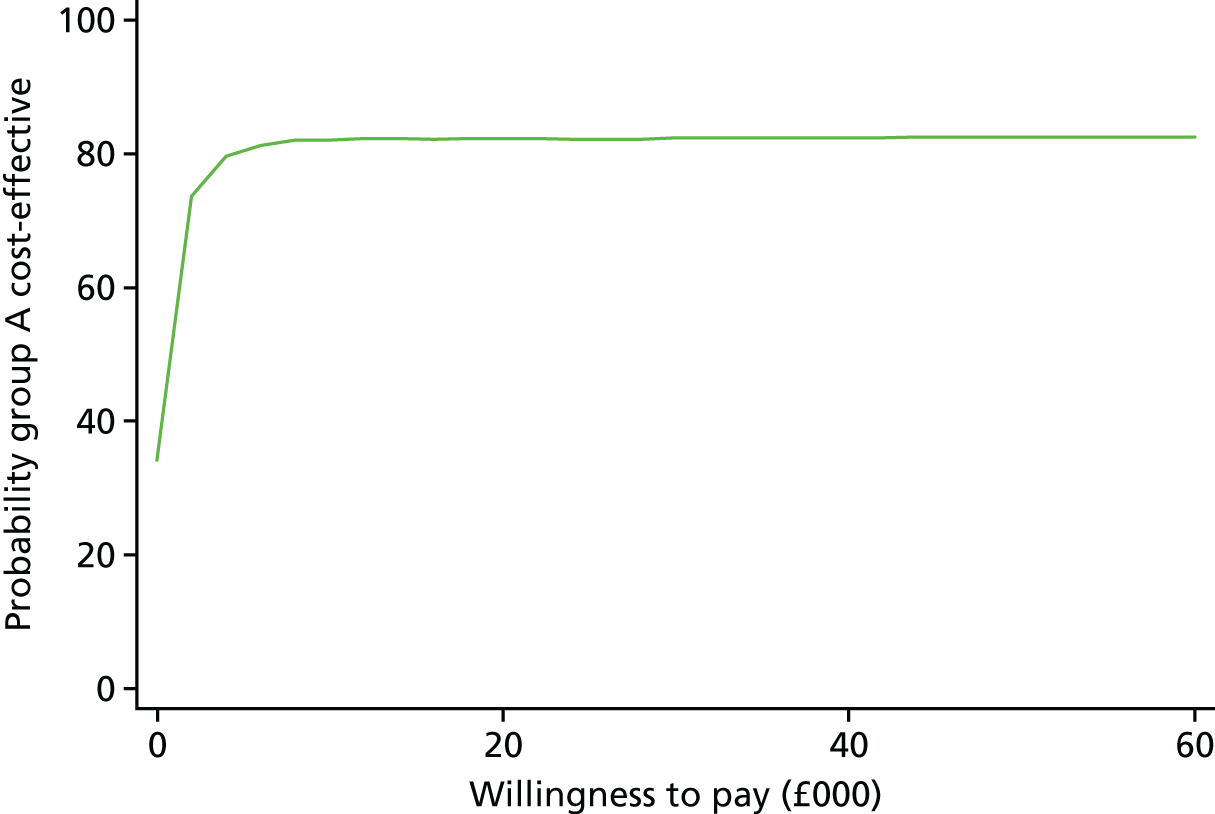
The bootstrapped replications for the ZAN-BPD outcomes are shown in Figure 4. Here, the replications are mainly to the right of the y-axis, suggesting that outcomes were slightly better in the lamotrigine treatment group; however, the lack of a difference in cost between the groups is reflected in 54% of replications being in the more costly, more effective, quadrant. The resulting cost-effectiveness acceptability curve in Figure 5 can only be indicative, as we do not know the willingness-to-pay values for a unit change in ZAN-BPD score. The curve suggests that there is a probability of > 60% that lamotrigine treatment is cost-effective, but only when willingness-to-pay values for a unit change in ZAN-BPD score are greater than £1000.
FIGURE 4.
Scatterplot on a cost-effectiveness plane of differences in costs vs. differences in ZAN-BPD.
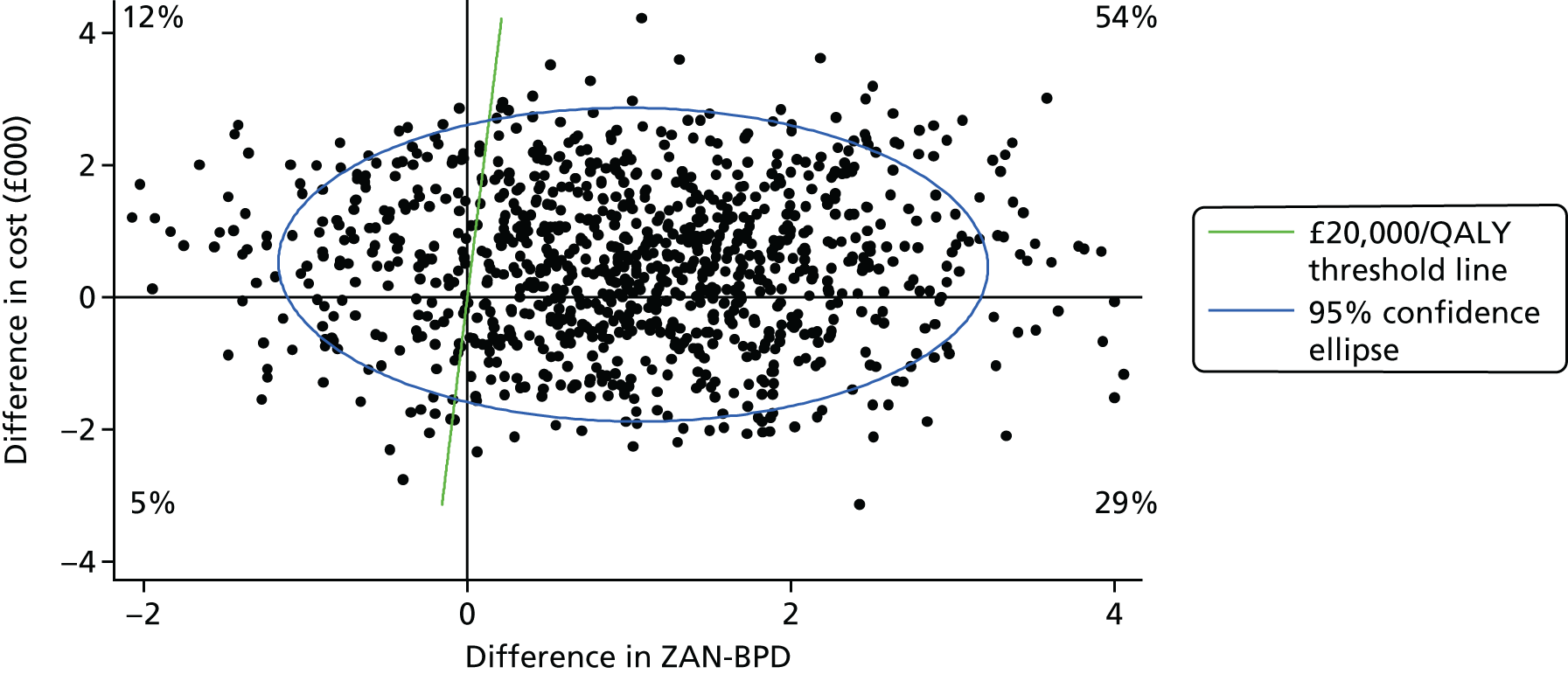
FIGURE 5.
Cost-effectiveness acceptability curve showing the probability that lamotrigine treatment is cost-effective compared with placebo at different values that a decision-maker might be willing to pay for decreases in ZAN-BPD scores.
Sensitivity analysis
The sensitivity analyses are presented in Table 17 and show that varying the economic perspective to include productivity losses and examining the impact of missing data through multiple imputation of missing cases have no impact on the finding that lamotrigine treatment is not cost-saving and thus it is unlikely to be cost-effective compared with placebo.
| Type of analysis | Treatment group, mean cost (SD) | Cost differencea | 95% CIa | p-valuea | |
|---|---|---|---|---|---|
| Lamotrigine | Placebo | ||||
| Main analysis (n = 160) | 12,244.32 (17,442.80) | 8495.41 (11,349.10) | 641.61 | –1886.61 to 3169.83 | 0.617 |
| Total cost including productivity losses (n = 160) | 12,378.70 (17,410.19) | 8634.52 (11,299.95) | 661.31 | –1877.68 to 3200.30 | 0.608 |
| Multiple imputation of missing cases | 12,655.64 (1822.07) | 7209.06 (1283.66) | 1773.42 | –76.94 to 3623.77 | 0.06 |
Chapter 5 Discussion
Data from this randomised trial of adding lamotrigine treatment to usual care for people receiving secondary care mental health services for BPD show that the effect of this intervention was no different from that of offering an inert placebo in addition to usual care. Follow-up data collected from 195 (70.7%) out of 276 participants at 12 months showed no difference in score on the ZAN-BPD (adjusted mean difference 0.1, 95% CI –1.8 to 2.0). When differences in the primary outcome were compared over the course of the 12-month follow-up period, among the 234 participants who completed at least one follow-up assessment, no difference was observed in the adjusted ZAN-BPD score (0.0, 95% CI –1.9 to 1.9). Although costs associated with prescribing lamotrigine were small compared with those of inpatient and community care, we did not find evidence that offering patients with BPD lamotrigine was a cost-effective use of resources, given the large number of resources needed to be invested for very small changes in the ZAN-PD score outcome.
Levels of adherence to trial medication were low, with only one-third (n = 93, 33.7%) of study participants taking trial mediation throughout the 1-year follow-up period, as specified in the study protocol. Levels of adherence were higher during the first 12 weeks of the study, at which point two-thirds of participants were taking trial medication (n = 190, 68.8%). However, differences between the treatment arms in the severity of symptoms of BPD were not found at the 12-week assessment, despite medication compliance being higher during this period. In a secondary analysis using CACE methods, we found no evidence that greater adherence to medication was associated with any benefit to patients in the lamotrigine treatment arm of the trial.
In addition to there being no difference in the scores on ZAN-BPD between the treatment arms, treatment with lamotrigine was no better than placebo in terms of improvement on any other measures of mental health. Levels of depression, likelihood of self-harming or suicidal behaviour and likelihood of problem drug or alcohol use were all comparable across the groups at follow-up. Social functioning was also equivalent across groups. There were no deaths in the lamotrigine arm of the trial over the 12-month follow-up period, but three among those in the placebo arm of the trial. Quality of life, assessed using the EQ-5D-3L, was poor in both groups, a finding which is consistent with other studies in people with BPD. 47
The number of AEs reported was higher in the placebo arm than in the lamotrigine treatment arm, and more participants in the placebo arm than in the lamotrigine treatment arm had at least one AE (67% vs. 56%). Serious AEs were comparable across treatment arms. A pro forma to elicit information about the known side effects of lamotrigine at each assessment showed that these were comparable across the treatment arm. Taken together, these data suggest that lamotrigine was well tolerated in participants in the active arm of the trial.
Strengths and weaknesses of the study
The LABILE trial is the first ever UK-based study of a medical treatment for people with BPD. Data were collected from participants receiving secondary care from NHS mental health services in six large mental health trusts in the north, south and centre of England. The study was designed to maximise internal validity. This included using independent remote randomisation to avoid unmasking researchers and adhering to an analysis plan that was finalised and shared with the Independent Data Monitoring and Ethics Committee and Trial Steering Group prior to the start of data analysis. Members of the research team drafted their conclusions and recommendations while still masked to treatments received by the two trial arms.
We used a validated measure of severity of BPD that is acceptable to patients and sensitive to change. 37,40 All researchers were trained to use the measure, and inter-rater reliability between researchers was high.
One of the main strengths of the LABILE trial is that participants were followed up over a 12-month period. BPD is a long-term condition, but previous drug trials have not conducted double-blind assessments beyond 12 weeks.
We recruited 11% more participants than we originally planned and the study was sufficiently powered to detect a minimum clinically significant difference in the severity of symptoms of BPD. We over-recruited, as it became clear that we were not going to achieve our ambitious target of following up 85% of participants to 12 months after randomisation. The 71% rate of follow-up that we achieved was very similar to that achieved in a UK study of problem-solving therapy for people with personality disorder71 and may represent a more realistic target for studies that aim to follow up people with personality disorder in community-based studies. A planned secondary analysis using multiple imputation to account for missing data found no difference between the study arms.
In this pragmatic trial, we attempted to replicate clinical practice in the NHS. However, one area in which we were unable to do this was in the means by which participants obtained their medication. Most participants typically had medication delivered to them in person or by post, rather than collecting medication from a local pharmacy as required by this study. This meant that participants had more regular contact with staff than they would have done in normal clinical practice (once every 2 weeks during titration and once a month for the majority of participants once the recommended dose was achieved). Although levels of adherence to medication were low, we believe that the additional contact that participants had with study researchers meant that the level of adherence may have been higher in the trial than would be seen in routine clinical practice.
Comparison with results of previous trials
In contrast to the results of the LABILE study, the two previous randomised trials of lamotrigine treatment for people with BPD both reported positive effects. 28,29 Both trials were smaller, had a larger number of exclusion criteria and followed up participants for a shorter period of time. There are a number of other differences between the LABILE study and these other trials, which are summarised in Table 18.
| Characteristics of study and sample | Trial | ||
|---|---|---|---|
| LABILE studya | aReich et al.29 | aTritt et al.28 | |
| Total sample size | 276 | 27 | 24 |
| Source of study participants | Clinical referral from inpatient and community secondary care mental health services | Members of the public who responded to websites and adverts on television and radio | Advertisements placed in primary care clinics |
| Exclusion criteria |
|
|
|
| Randomisation | Independent | Prearranged random number sequence | In-house |
| Age (years), mean | 36 | 32 | 29 |
| Female (%) | 75 | 89 | 100 |
| Employed (%) | 22 | Not stated | 100 |
| Mean ZAN-BPD score | 17.0 | 18.8 | Not assessed |
| Previous mental health hospitalisation | 34.4% in the last 6 months | 51.9% lifetime | 19% lifetime |
| Mean dose | 200 mg | 93.3 mg | 200 mg |
| Adherence (% taking trial medication) | 66.7 | 80 | Not stated |
| Funding | Public | Industry | No funding declared |
In their trial of 24 women with BPD recruited from advertisements in primary care clinics, Tritt et al. 28 reported statistically significant reductions in four out of five scales of the State–Trait Anger Expression Inventory at 8 weeks. Reich et al. 29 randomised 28 men and women with BPD who were recruited from websites and advertisements on local television and radio stations. Although a statistically significant difference was not seen in total score on the ZAN-BPD at 12 weeks, the team reported differences in two out of the four subscales of this measure (affective lability and impulsivity). 29
The primary outcome of the LABILE study was assessed at 1 year, but in exploring the reasons for differences in outcomes between these trials, we have focused on data from the 12-week follow-up assessment for comparison. We believe that a number of factors may have resulted in the differences in the results between the LABILE study and the two previous randomised trials. First, we cannot rule out the possibility that apparent differences between groups in the two earlier trials result from chance. Randomisation does not guarantee that treatment arms are balanced in small-scale trials and it is possible that differences in study outcomes resulted from confounding.
The LABILE study was designed to generate evidence that can be used by secondary care mental health services. We therefore limited our exclusion criteria and recruited people who had high levels of contact with mental health services and major impairments in social functioning. This approach meant that we were able to recruit people with the type of complex and severe problems that people with BPD who use NHS secondary care mental health services generally have. A commonly used marker of severity is whether or not people have other coexisting personality problems in addition to BPD. 33 It is of note that all but one of the people who took part in the LABILE trial met criteria for other personality disorders and had the types of complex personality-related problems that people in contact with NHS secondary services generally have. In contrast, the two previous trials of lamotrigine treatment for people with BPD excluded people who were misusing alcohol or other drugs, were actively suicidal or were receiving inpatient treatment. Therefore, there are important differences in the sample that we recruited compared with those in the two previous trials. For instance, 73% of participants in the trial conducted by Tritt et al. 28 were employed, compared with only 22% in the LABILE trial, and none of those recruited by Tritt et al. had a recent history of deliberate self-harm, compared with two-thirds of participants in the LABILE trial. It is possible that lamotrigine treatment reduces the symptoms of BPD among people who have less complex mental health problems, higher level of social functioning and lower levels of substance misuse than those we recruited to the LABILE trial.
Previous research has shown that people with more complex and severe personality problems are less likely to adhere to treatment. 17 It is possible that participants in the LABILE study were less adherent to trial medication than those in the two previous trials. Tritt et al. 28 did not report data on adherence. Reich et al. 29 did not provide detailed information about adherence but did note that 3 (20%) out of the 15 participants in the active arm of the trial were no longer taking medication at 12 weeks, compared with 86 (31%) of 276 participants in the LABILE trial who were no longer taking lamotrigine at 12 weeks. Although we cannot rule out the possibility that differences in levels of adherence are responsible for differences in outcomes between these trials, the results of the CACE analysis do not suggest that higher levels of adherence to lamotrigine result in greater improvements in mental health.
It is possible that other aspects of the design of previous trials contributed to their positive effects. For instance, in the LABILE trial we had a rigorous process for maintaining blinding through the use of a computerised system, managed by an independent team that allocated study participants. In contrast, Tritt et al. 28 used an internal group to oversee treatment allocation. Insufficient information is provided in the paper by Reich et al. 29 to establish the process they used to randomise participants.
The LABILE trial is the only one of the three studies to be publicly funded. The trial by Reich et al. 29 was funded by a manufacturer of Lamictal® (a proprietary form of lamotrigine; GlaxoSmithKline UK Ltd, Brentford, UK) and the trial by Tritt et al. 28 was self-funded.
Finally, we are not aware of any unpublished trials of lamotrigine for people with BPD, but we cannot rule out the possibility that publication bias helps to explain the absence of previous negative trials of this treatment approach.
Implications for clinical practice
People with BPD experience high levels of emotional distress at times of crisis and may behave in an erratic or impulsive way that puts their health at risk. Emotional distress, suicidal behaviour or thoughts of suicide may lead people into contact with mental health services at such times. Interpersonal problems and negative experiences of previous contact with services often make it difficult for people with BPD to trust health-care professionals or feel reassured by their attempts to provide support and advice. In such circumstances, the prescription of medication can provide a clear signal to the patient that they are being taken seriously and health-care staff may also feel reassured that they are ‘doing something’ under difficult circumstances. Data from a national audit of prescribing practice suggest that one-quarter of people who are in contact with mental health services are being prescribed a mood stabiliser. 72 Some patients report that prescribing medication during a crisis can help bring about short-term reductions in emotional distress. The results of the LABILE study support the clinical impression that prescribing lamotrigine can lead to improvements in mental health but suggest that this benefit is no greater than would be found if a placebo were to be prescribed.
Current NICE guidelines73 for the treatment of BPD recommend that future research should be conducted to examine the impact of mood stabilisers on people with the condition, but they conclude that there was insufficient evidence to recommend any drug treatment at the time of publication. The results of the LABILE trial provide evidence to support current NICE recommendations and emphasise the need to use alternative approaches to help people with BPD cope with crises and take steps to improve their mental health. The two best-established treatments for BPD are dialectical behaviour therapy and mentalisation-based treatment. 74 More recently, evidence has begun to emerge that Systems Training for Emotional Predictability and Problem Solving (STEPPS),40 schema-focused therapy75 and day therapeutic community treatment76 can also improve the health of people with BPD. Clinicians who are working with people in secondary care mental health services should be aware of how they can help support people through mental health crises and how to access evidence-based psychological treatments when these are available.
In the LABILE trial, we took great care not to recruit women who were pregnant, wanting to become pregnant or having regular unprotected sex. Despite the assurances that potential recruits gave us, six participants subsequently became pregnant during the course of the trial. Although lamotrigine treatment has been shown to be relatively safe in pregnancy, this is not true of all mood stabilisers – notably sodium valproate. 23 In January 2015, the Medicines and Healthcare products Regulatory Agency issued a warning advising clinicians to avoid prescribing sodium valproate to women of child-bearing age when possible. 77 Despite this, a recent national audit showed that 11% of women with BPD who are in contact with secondary care mental health services are currently being prescribed this drug. 72 The data from the LABILE study showing the high level of unplanned pregnancies among women with BPD emphasise the importance of avoiding the use of unlicensed medications, such as sodium valproate, that are potentially teratogenic.
Future research
Existing evidence-based psychological treatments for BPD are lengthy, intensive and expensive. Previous research has demonstrated that, when compared with treatment as usual, these interventions are associated with reduced levels of deliberate self-harm and contact with health-care services. 74 However, studies that compare the effects of these interventions with high-quality control treatments delivered in a consistent manner show less, if any, additional benefit. 78,79 Reductions in self-harm and contact with health-care services raise the possibility that specialist psychological treatment for BPD provides an effective use of available resources, but very few data on the costs and cost-effectiveness of these treatments exist. Further research is needed to examine the costs and cost-effectiveness of specialist psychological treatment for people with BPD compared with high-quality structured general psychiatric management. Such research is needed to help policy-makers make better decisions about investing in specialist treatment services, which many patients are currently unable to access in the NHS.
Even were such services to become more widely available, a substantial minority of people with BPD would be unwilling or unable to use these group-based treatments. There is a pressing need for new research to examine alternative psychological approaches to helping people with BPD cope better with emotional crises and improve their mental health. Computer-assisted self-help interventions and training and support for friends and family of people with BPD are all areas worthy of further exploration. 80
An area of particular concern is how best to help people with severe BPD who are treated in inpatient mental health units. Following a number of open-label studies of clozapine treatment for people with BPD,81,82 the use of this atypical antipsychotic drug is becoming more widespread. Evidence from cohort studies suggests that this type of medication may help reduce impulsive self-harming behaviour and reduce the need for inpatient treatment. 82 However, this drug is also associated with potential harms, including blood dyscrasia,82 which have the potential to be fatal if not properly monitored and treated. The large number of potential benefits and harms of clozapine for the treatment of inpatients with severe BPD provides a strong case for a placebo-controlled randomised trial of this drug. Given the high rate of drop-out in this and other Phase III trials of community-based interventions for people with BPD, consideration should be given using primary outcomes that do not require direct patient contact.
Conclusions
Based on the results of this trial, we have not shown any benefits of prescribing lamotrigine for people with BPD. These results provide further support for current NICE guidelines, which state that medication should not be used specifically for BPD and consideration should be given to referring people for specialist psychological treatment when these services are available.
Acknowledgements
We thank all those who took part in the study and clinical staff at Central and North West London NHS Foundation Trust, Derbyshire Healthcare NHS Foundation Trust, Nottinghamshire Healthcare NHS Foundation Trust, Oxleas NHS Foundation Trust, Tees, Esk and Wear Valleys NHS Foundation Trust and West London Mental Health NHS Trust for their help with completing the study, especially Ms Lauren Bryant, Ms Adele Mason, Mr John Lawton, Dr Ben Lucas, Dr Paul Mallett, Dr Tom McLaren, Dr Ayodeji Morah, Dr Bal Powar, Dr Paola Rossin, Dr Zahir Shah, Dr Vineet Singh and Ms Danielle Wilson.
We thank the Clinical Research Network for publicising the study and supporting the recruitment and follow-up of study participants. We would especially like to thank Pat Daly, Anthony Davis, Tara Harvey, Bianca Hinds-Walters, Natalie Marking, Antoinette McNulty, Lorraine O’Connell, Sandra Simpson, Lisa Thompson and Audrey Williamson for supporting study recruitment and helping with participant follow-up. We also thank members of the trial co-ordinating team at Imperial College London, including Claudia Adele, Amy Claringbold, Zoe Cotton, Kavi Gakhal, Ana Arbeloa del Moral, Lesley O’Connell and Dilveer Sually, for overseeing the administration of the trial.
We are grateful to all members of the Trial Steering Committee [Maxine Patel (chairperson 2013–15), Paul Moran (chairperson 2015–16), Delia Bishara, Oliver Dale, Sandra O’Sullivan and Jenny Trite], the Data Monitoring and Ethics Committees [Nicol Ferrier (chairperson), Tony Johnson and Steve Pearce] for their support and guidance. We are grateful to Sally Strange and Jennie Parker for their input to the project as experts by experience, to Sally for advising on study logistics and to both Sally and Jennie for helping us interpret and communicate study findings.
Contributions of authors
Mike J Crawford (Professor of Mental Health, Centre for Psychiatry, Imperial College London) was the chief investigator of the study, and contributed to the design of the study and the study protocol and the preparation of this manuscript.
Rahil Sanatinia (Research Associate, Centre for Psychiatry, Imperial College London) provided clinical leadership, supported recruitment of study participants and contributed to the preparation of this manuscript.
Barbara Barrett (Reader, King’s College London) developed plans for the economic evaluation, contributed to the design of the study and the study protocol and preparation of this manuscript.
Gillian Cunningham (Research Associate, Tees, Esk and Wear Valleys NHS Foundation Trust) recruited and followed up study participants and contributed to the preparation of this manuscript.
Oliver Dale (Consultant Psychiatrist, West London Mental Health NHS Trust) was a principal investigator and contributed to the conduct of the study and the preparation of this manuscript.
Poushali Ganguli (Research Associate, King’s College London) contributed to the management, analysis and interpretation of economic data and to the preparation of this manuscript.
Geoff Lawrence-Smith (Consultant Psychiatrist, Oxleas NHS Foundation Trust) was a principal investigator, and contributed to the conduct of the study and the preparation of this manuscript.
Verity C Leeson (Clinical Trials Manager, Centre for Psychiatry, Imperial College London) co-ordinated the trial, contributed to the development of the study protocol and preparation of this manuscript.
Fenella Lemonsky (Expert by Experience, Centre for Psychiatry, Imperial College London) oversaw service user involvement in the study and contributed to the design of the study protocol and preparation of this manuscript.
Georgia Lykomitrou-Matthews (Research Associate, University of Nottingham) recruited and followed up study participants and contributed to the writing of this manuscript.
Alan Montgomery (Professor, University of Nottingham) contributed to the development of the analysis plan for the study and the study protocol and the preparation of this manuscript.
Richard Morriss (Professor, University of Nottingham) was a principal investigator, and contributed to the design and conduct of the study and the preparation of this manuscript.
Jasna Munjiza (Consultant Psychiatrist, Central and North West London NHS Foundation Trust) contributed to the design of the study and the study protocol and preparation of this manuscript.
Carol Paton (Chief Pharmacist, Oxleas NHS Foundation Trust) was the lead pharmacist on the project, and contributed to the design of the study and the study protocol and preparation of this manuscript.
Iwona Skorodzien (Research Associate, University of Nottingham) recruited and followed up study participants and contributed to the preparation of this manuscript.
Vineet Singh (Consultant Psychiatrist, Derbyshire Healthcare NHS Foundation Trust) was a principal investigator, and contributed to the conduct of the study and the preparation of this manuscript.
Wei Tan (Statistician, University of Nottingham) contributed to the development of analysis plan for the study and the study protocol and the preparation of this manuscript.
Peter Tyrer (Professor, Centre for Psychiatry, Imperial College London) contributed to the design of the study and the study protocol and preparation of this manuscript.
Joseph G Reilly (Professor, University of Durham and Tees, Esk and Wear Valleys NHS Foundation Trust) was a principal investigator, and contributed to the design and conduct of the study and the preparation of this manuscript.
All authors read and approved the final manuscript.
Publications
Crawford MJ, McLaren T, Reilly J. Role of mood stabilisers in treatment of borderline personality disorder. BMJ 2014;349:g5378.
Crawford MJ, Sanatinia R, Barrett B, Byford S, Cunningham G, Gakhal K, et al. Lamotrigine versus inert placebo in the treatment of borderline personality disorder: study protocol for a randomized controlled trial. Trials 2015;16:308.
Crawford MJ, Sanatinia R, Barrett B, Cunningham G, Dale O, Ganguli P, Lawrence-Smith G, et al. The clinical effectiveness and cost-effectiveness of lamotrigine in borderline personality disorder: a randomized placebo-controlled trial [published online ahead of print April 6 2018]. Am J Psychiatry 2018. https://doi.org/10.1176/appi.ajp.2018.17091006
Data sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994.
- Coid J, Yang M, Tyrer P, Roberts A, Ullrich S. Prevalence and correlates of personality disorder in Great Britain. Br J Psychiatry 2006;188:423-31. https://doi.org/10.1192/bjp.188.5.423.
- Hayward M, Slade M, Moran PA. Personality disorders and unmet needs among psychiatric inpatients. Psychiatr Serv 2006;57:538-43. https://doi.org/10.1176/ps.2006.57.4.538.
- Haw C, Hawton K, Houston K, Townsend E. Psychiatric and personality disorders in deliberate self-harm patients. Br J Psychiatry 2001;178:48-54. https://doi.org/10.1192/bjp.178.1.48.
- Lieb K, Zanarini MC, Schmahl C, Linehan MM, Bohus M. Borderline personality disorder. Lancet 2004;364:453-61. https://doi.org/10.1016/S0140-6736(04)16770-6.
- Skodol AE, Gunderson JG, McGlashan TH, Dyck IR, Stout RL, Bender DS, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry 2002;159:276-83. https://doi.org/10.1176/appi.ajp.159.2.276.
- El-Gabalawy R, Katz LY, Sareen J. Comorbidity and associated severity of borderline personality disorder and physical health conditions in a nationally representative sample. Psychosom Med 2010;72:641-7. https://doi.org/10.1097/PSY.0b013e3181e10c7b.
- Fok ML, Hayes RD, Chang CK, Stewart R, Callard FJ, Moran P. Life expectancy at birth and all-cause mortality among people with personality disorder. J Psychosom Res 2012;73:104-7. https://doi.org/10.1016/j.jpsychores.2012.05.001.
- Sanatinia R, Wang D, Tyrer P, Tyrer H, Crawford M, Cooper S, et al. Impact of personality status on the outcomes and cost of cognitive-behavioural therapy for health anxiety. Br J Psychiatry 2016;209:244-50. https://doi.org/10.1192/bjp.bp.115.173526.
- Sanatinia R, Middleton SM, Lin T, Dale O, Crawford MJ. Quality of physical health care among patients with personality disorder. Personal Ment Health 2015;9:319-29. https://doi.org/10.1002/pmh.1303.
- Borderline Personality Disorder: Treatment and Management. London: NICE; 2009.
- Ramon S, Castillo H, Morant N. Experiencing personality disorder: a participative research. Int J Soc Psychiatry 2001;47:1-15. https://doi.org/10.1177/002076400104700401.
- Haigh R. Services for People with Personality Disorder: The Thoughts of Service Users. London: Department of Health and Social Care; 2002.
- Gallop R, Lancee WJ, Garfinkel P. How nursing staff respond to the label ‘borderline personality disorder’. Hosp Community Psychiatry 1989;40:815-19. https://doi.org/10.1176/ps.40.8.815.
- Binks CA, Fenton M, McCarthy L, Lee T, Adams CE, Duggan C. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev 2006;1. https://doi.org/10.1002/14651858.CD005652.
- McMurran M, Huband N, Overton E. Non-completion of personality disorder treatments: a systematic review of correlates, consequences, and interventions. Clin Psychol Rev 2010;30:277-87. https://doi.org/10.1016/j.cpr.2009.12.002.
- Crawford MJ, Price K, Gordon F, Josson M, Taylor B, Bateman A, et al. Engagement and retention in specialist services for people with personality disorder. Acta Psychiatr Scand 2009;119:304-11. https://doi.org/10.1111/j.1600-0447.2008.01306.x.
- Crawford MJ, Kakad S, Rendel C, Mansour NA, Crugel M, Liu KW, et al. Medication prescribed to people with personality disorder: the influence of patient factors and treatment setting. Acta Psychiatr Scand 2011;124:396-402. https://doi.org/10.1111/j.1600-0447.2011.01728.x.
- Lieb K, Völlm B, Rücker G, Timmer A, Stoffers JM. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. Br J Psychiatry 2010;196:4-12. https://doi.org/10.1192/bjp.bp.108.062984.
- Crawford MJ, MacLaren T, Reilly JG. Are mood stabilisers helpful in treatment of borderline personality disorder?. BMJ 2014;349. https://doi.org/10.1136/bmj.g5378.
- Sukumaran S, Herbert J, Tracey J, Delanty N. Safety of newer generation anti-epileptic drugs in non-accidental overdose: an Irish population study. Seizure 2005;14:151-6. https://doi.org/10.1016/j.seizure.2004.02.005.
- Tomson T, Battino D, French J, Harden C, Holmes L, Morrow J, et al. Antiepileptic drug exposure and major congenital malformations: the role of pregnancy registries. Epilepsy Behav 2007;11:277-82. https://doi.org/10.1016/j.yebeh.2007.08.015.
- Harden CL. Antiepileptic drug teratogenesis: what are the risks for congenital malformations and adverse cognitive outcomes?. Int Rev Neurobiol 2008;83:205-13. https://doi.org/10.1016/S0074-7742(08)00011-1.
- Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 2009;360:1597-605. https://doi.org/10.1056/NEJMoa0803531.
- Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry 2009;194:4-9. https://doi.org/10.1192/bjp.bp.107.048504.
- Geddes JR, Gardiner A, Rendell J, Voysey M, Tunbridge E, Hinds C, et al. Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (CEQUEL): a 2 × 2 factorial randomised trial. Lancet Psychiatry 2016;3:31-9. https://doi.org/10.1016/S2215-0366(15)00450-2.
- Ketter TA, Manji HK, Post RM. Potential mechanisms of action of lamotrigine in the treatment of bipolar disorders. J Clin Psychopharmacol 2003;23:484-95. https://doi.org/10.1097/01.jcp.0000088915.02635.e8.
- Tritt K, Nickel C, Lahmann C, Leiberich PK, Rother WK, Loew TH, et al. Lamotrigine treatment of aggression in female borderline-patients: a randomized, double-blind, placebo-controlled study. J Psychopharmacol 2005;19:287-91. https://doi.org/10.1177/0269881105051540.
- Reich DB, Zanarini MC, Bieri KA. A preliminary study of lamotrigine in the treatment of affective instability in borderline personality disorder. Int Clin Psychopharmacol 2009;24:270-5. https://doi.org/10.1097/YIC.0b013e32832d6c2f.
- British National Formulary 74. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2017.
- First MB, Spitzer RL, Gibbon M, Williams JBW, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II), Version 2.0. New York, NY: American Psychiatric Publishing, Inc.; 1994.
- Maffei C, Fossati A, Agostoni I, Barraco A, Bagnato M, Deborah D, et al. Interrater reliability and internal consistency of the structured clinical interview for DSM-IV axis II personality disorders (SCID-II), version 2.0. J Pers Disord 1997;11:279-84. https://doi.org/10.1521/pedi.1997.11.3.279.
- Tyrer P, Johnson T. Establishing the severity of personality disorder. Am J Psychiatry 1996;153:1593-7. https://doi.org/10.1176/ajp.153.12.1593.
- Loranger A. International Personality Disorder Examination (IPDE) Manual. White Plains, NY: Cornell Medical Center; 1995.
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. New York, NY: New York Biometrics Research, New York State Psychiatric Institute; 2002.
- Gamma A, Angst J, Azorin JM, Bowden CL, Perugi G, Vieta E, et al. Transcultural validity of the Hypomania Checklist-32 (HCL-32) in patients with major depressive episodes. Bipolar Disord 2013;15:701-12. https://doi.org/10.1111/bdi.12101.
- Zanarini MC, Vujanovic AA, Parachini EA, Boulanger JL, Frankenburg FR, Hennen J. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. J Pers Disord 2003;17:233-42. https://doi.org/10.1521/pedi.17.3.233.22147.
- Blum N, Pfohl B, John DS, Monahan P, Black DW. STEPPS: a cognitive-behavioral systems-based group treatment for outpatients with borderline personality disorder – a preliminary report. Compr Psychiatry 2002;43:301-10. https://doi.org/10.1053/comp.2002.33497.
- Zanarini MC, Schulz SC, Detke HC, Tanaka Y, Zhao F, Lin D, et al. A dose comparison of olanzapine for the treatment of borderline personality disorder: a 12-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2011;72:1353-62. https://doi.org/10.4088/JCP.08m04138yel.
- Blum N, St John D, Pfohl B, Stuart S, McCormick B, Allen J, et al. Systems Training for Emotional Predictability and Problem Solving (STEPPS) for outpatients with borderline personality disorder: a randomized controlled trial and 1-year follow-up. Am J Psychiatry 2008;165:468-78. https://doi.org/10.1176/appi.ajp.2007.07071079.
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561-71. https://doi.org/10.1001/archpsyc.1961.01710120031004.
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years later. Clin Psychol Rev 1988;8:77-100. https://doi.org/10.1016/0272-7358(88)90050-5.
- Davidson KM. Cognitive Therapy for Personality Disorders: A Guide for Clinicians. Hove: Routledge; 2007.
- Palmer S, Davidson K, Tyrer P, Gumley A, Tata P, Norrie J, et al. The cost-effectiveness of cognitive behavior therapy for borderline personality disorder: results from the BOSCOT trial. J Pers Disord 2006;20:466-81. https://doi.org/10.1521/pedi.2006.20.5.466.
- Tyrer P, Nur U, Crawford M, Karlsen S, McLean C, Rao B, et al. The Social Functioning Questionnaire: a rapid and robust measure of perceived functioning. Int J Soc Psychiatry 2005;51:265-75. https://doi.org/10.1177/0020764005057391.
- Kind P, Spilker B. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia, PA: Lippincott-Raven; 1996.
- van Asselt AD, Dirksen CD, Arntz A, Giesen-Bloo JH, Severens JL. The EQ-5D: a useful quality of life measure in borderline personality disorder?. Eur Psychiatry 2009;24:79-85. https://doi.org/10.1016/j.eurpsy.2008.11.001.
- WHO ASSIST Working Group . The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction 2002;97:1183-94. https://doi.org/10.1046/j.1360-0443.2002.00185.x.
- Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev 2005;24:217-26. https://doi.org/10.1080/09595230500170266.
- Borschmann R, Barrett B, Hellier JM, Byford S, Henderson C, Rose D, et al. Joint crisis plans for people with borderline personality disorder: feasibility and outcomes in a randomised controlled trial. Br J Psychiatry 2013;202:357-64. https://doi.org/10.1192/bjp.bp.112.117762.
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67-74. https://doi.org/10.1097/00005650-198601000-00007.
- George CF, Peveler RC, Heiliger S, Thompson C. Compliance with tricyclic antidepressants: the value of four different methods of assessment. Br J Clin Pharmacol 2000;50:166-71. https://doi.org/10.1046/j.1365-2125.2000.00244.x.
- Crawford MJ, Sanatinia R, Barrett B, Byford S, Cunningham G, Gakhal K, et al. Lamotrigine versus inert placebo in the treatment of borderline personality disorder: study protocol for a randomized controlled trial and economic evaluation. Trials 2015;16. https://doi.org/10.1186/s13063-015-0823-x.
- Angst J, Adolfsson R, Benazzi F, Gamma A, Hantouche E, Meyer TD, et al. The HCL-32: towards a self-assessment tool for hypomanic symptoms in outpatients. J Affect Disord 2005;88:217-33. https://doi.org/10.1016/j.jad.2005.05.011.
- Moher D, Hopewell S, Schulz K, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:698-702. https://doi.org/10.1136/bmj.c869.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- van Asselt AD, Dirksen CD, Arntz A, Severens JL. The cost of borderline personality disorder: societal cost of illness in BPD-patients. Eur Psychiatry 2007;22:354-61. https://doi.org/10.1016/j.eurpsy.2007.04.001.
- Ranger M, Tyrer P, Miloseska K, Fourie H, Khaleel I, North B, et al. Cost-effectiveness of nidotherapy for comorbid personality disorder and severe mental illness: randomized controlled trial. Epidemiol Psichiatr Soc 2009;18:128-36.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2015.
- Curtis L, Burns A. Unit Costs of Health & Social Care 2015. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQoL: Results from a UK General Population Survey. York: Centre for Health Economics, University of York; 1995.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993.
- Thompson SG, Barber JA. How should cost data in pragmatic randomised controlled trials be analysed?. BMJ 2000;320:1197-2000. https://doi.org/10.1136/bmj.320.7243.1197.
- van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ 1994;3:309-19. https://doi.org/10.1002/hec.4730030505.
- Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219-36. https://doi.org/10.1002/1097-0258(20001215)19:23<3219::AID-SIM623>3.0.CO;2-P.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Loranger A, Sartorius N, Andreoli A, Berger P, Buchiem P, Channabasavanna S. The International Personality Disorder Examination. The World Health Organization/Alcohol, Drug Abuse and Mental Health Administration international pilot study of personality disorders. Arch Gen Psychiatr 1994;51:215-24. https://doi.org/10.1001/archpsyc.1994.03950030051005.
- MedDRA . The Medical Dictionary for Regulatory Activities n.d. www.meddra.org (accessed 19 January 2018).
- McMurran M, Crawford MJ, Reilly J, Delport J, McCrone P, Whitham D, et al. Psychoeducation with problem-solving (PEPS) therapy for adults with personality disorder: a pragmatic randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of a manualised intervention to improve social functioning. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20520.
- Paton C, Crawford MJ, Bhatti SF, Patel MX, Barnes TR. The use of psychotropic medication in patients with emotionally unstable personality disorder under the care of UK mental health services. J Clin Psychiatry 2015;76:e512-8. https://doi.org/10.4088/JCP.14m09228.
- Borderline Personality Disorder: The NICE Guideline on Treatment and Management. London: The British Psychological Society and The Royal College of Psychiatrists; 2009.
- Stoffers JM, Völlm BA, Rücker G, Timmer A, Huband N, Lieb K. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst Rev 2012;8. https://doi.org/10.1002/14651858.CD005652.pub2.
- Giesen-Bloo J, van Dyck R, Spinhoven P, van Tilburg W, Dirksen C, van Asselt T, et al. Outpatient psychotherapy for borderline personality disorder: randomized trial of schema-focused therapy vs. transference-focused psychotherapy. Arch Gen Psychiatry 2006;63:649-58. https://doi.org/10.1001/archpsyc.63.6.649.
- Pearce S, Scott L, Attwood G, Saunders K, Dean M, deRidder R, et al. A randomized controlled trial of democratic therapeutic community treatment for personality disorder. Br J Psychiatry 2017;210:149-55. https://doi.org/10.1192/bjp.bp.116.184366.
- Medicines and Healthcare Products Regulatory Agency . Medicines related to valproate: risk of abnormal pregnancy outcomes. Drug Safety Update 2015;8.
- McMain SF, Links PS, Gnam WH, Guimond T, Cardish RJ, Korman L, et al. A randomized trial of dialectical behavior therapy versus general psychiatric management for borderline personality disorder. Am J Psychiatry 2009;166:1365-74. https://doi.org/10.1176/appi.ajp.2009.09010039.
- Bateman A, Fonagy P. Randomized controlled trial of outpatient mentalization-based treatment versus structured clinical management for borderline personality disorder. Am J Psychiatry 2009;166:1355-64. https://doi.org/10.1176/appi.ajp.2009.09040539.
- van’t Hof E, Cuijpers P, Stein DJ. Self-help and Internet-guided interventions in depression and anxiety disorders: a systematic review of meta-analyses. CNS Spectr 2009;14:34-40. https://doi.org/10.1017/S1092852900027279.
- Parker GF. Clozapine and borderline personality disorder. Psychiatr Serv 2002;53:348-9. https://doi.org/10.1176/appi.ps.53.3.348.
- Frogley C, Anagnostakis K, Mitchell S, Mason F, Taylor D, Dickens G, et al. A case series of clozapine for borderline personality disorder. Ann Clin Psychiatry 2013;25:125-34.
- NHS Reference Costs 2015. London: Department of Health and Social Care; 2016.
Appendix 1 Pro forma for recording possible side effects of trial medication
Appendix 2 Modified version of the Adult Service Use Schedule as used in this trial
Reproduced with permission from Dr Barbara Barrett (2018, King’s College London, personal communication).
Appendix 3 Unit costs and sources for economic evaluation
| Cost item | Unit cost (£) | Source |
|---|---|---|
| Inpatient stay, per night | 538–587 | NHS Reference Costs 2015 83 |
| Outpatient, per appointment | 42–298 | NHS Reference Costs 2015 83 |
| Accident and emergency, per attendance | 114–192 | NHS Reference Costs 2015 83 |
| Ambulance, per call | 96 | NHS Reference Costs 2015 83 |
| GP contact, per minute of contact | 2.82–3.60 | Curtis and Burns60 |
| Practice nurse, per minute of contact | 0.72 | Curtis and Burns60 |
| Community mental health services, per minute of contact | 0.68–4.35 | Curtis and Burns60 |
| Community health services, per minute of contact | 0.50–2.53 | Curtis and Burns60 |
Appendix 4 Secondary outcomes at secondary time points
| Time point | Treatment group | |
|---|---|---|
| Lamotrigine (N = 137) | Placebo (N = 139) | |
| Baseline | ||
| Mean score (SD) | 39.8 (11.7) | 38.4 (10.2) |
| Median score (IQR) | 41 (31–49) | 39 (30–46) |
| n | 135 | 138 |
| 12 weeks | ||
| Mean score (SD) | 29.9 (15.7) | 29 (14.5) |
| Median score (IQR) | 30 (19–42) | 31 (17–40) |
| n | 110 | 104 |
| 24 weeks | ||
| Mean score (SD) | 30.7 (15.1) | 29.9 (15.1) |
| Median score (IQR) | 32 (20–42) | 31 (17–42) |
| n | 98 | 97 |
| 52 weeks | ||
| Mean score (SD) | 28.8 (16.1) | 28.7 (15.5) |
| Median score (IQR) | 29 (14–42) | 29 (16–41) |
| n | 92 | 88 |
| Time point | Treatment group, n (%) | |
|---|---|---|
| Lamotrigine (N = 137) | Placebo (N = 139) | |
| Baseline | ||
| No | 39 (28) | 51 (37) |
| Yes | 96 (70) | 87 (63) |
| Unknown | 2 (2) | 1 (< 0.5) |
| 12 weeks | ||
| No | 59 (54) | 62 (60) |
| Yes | 51 (46) | 41 (40) |
| Unknown | 1 (< 0.5) | 1 (< 0.5) |
| 24 weeks | ||
| No | 56 (57) | 60 (62) |
| Yes | 42 (43) | 37 (38) |
| Unknown | 0 | 1 (< 0.5) |
| 52 weeks | ||
| No | 46 (47) | 50 (51) |
| Yes | 45 (46) | 38 (39) |
| Unknown | 6 (7) | 10 (10) |
| Time point | Treatment group | |
|---|---|---|
| Lamotrigine (N = 137) | Placebo (N = 139) | |
| Baseline | ||
| Mean score (SD) | 15 (4.1) | 14.9 (4.5) |
| Median score (IQR) | 15 (12–18) | 15 (12–18) |
| n | 135 | 137 |
| 12 weeks | ||
| Mean score (SD) | 12.6 (4.5) | 12.6 (5.2) |
| Median score (IQR) | 12 (9–16) | 12 (9–17) |
| n | 110 | 104 |
| 24 weeks | ||
| Mean score (SD) | 11.8 (4.3) | 12.2 (4.9) |
| Median score (IQR) | 12 (9–14) | 12 (9–15) |
| n | 98 | 97 |
| 52 weeks | ||
| Mean score (SD) | 12.4 (4.3) | 12.3 (4.9) |
| Median score (IQR) | 13 (10–15) | 12 (8–15) |
| n | 91 | 88 |
| Subscore | Time point | Adjusted difference in means (95% CI)a | p-value | |
|---|---|---|---|---|
| Baseline | 52 weeks’ follow-up | |||
| Affective disturbance | ||||
| Lamotrigine | 6.9 (2.2) | 4.7 (2.8) | –0.1 (–0.9 to 0.7) | 0.850 |
| Placebo | 7.2 (2.4) | 4.9 (3.1) | – | |
| Cognitive disturbance | ||||
| Lamotrigine | 4 (2) | 2.7 (2.2) | 0.1 (–0.4 to 0.7) | 0.615 |
| Placebo | 4.3 (2) | 2.6 (2.1) | – | |
| Impulsivity | ||||
| Lamotrigine | 3 (2.1) | 2.1 (2) | –0.1 (–0.6 to 0.5) | 0.824 |
| Placebo | 3 (1.8) | 2.1 (2.1) | – | |
| Disturbed relationship | ||||
| Lamotrigine | 2.7 (1.9) | 1.7 (1.8) | 0 (–0.5 to 0.5) | 0.982 |
| Placebo | 2.9 (2) | 1.8 (2) | – | |
List of abbreviations
- AD-SUS
- Adult Service Use Schedule
- AE
- adverse event
- ASSIST
- Alcohol, Smoking and Substance Involvement Screening Test
- BDI
- Beck Depression Inventory
- BPD
- borderline personality disorder
- CACE
- complier-average causal effect
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- EQ-5D-3L
- EuroQoL-5 Dimensions, three-level version
- GP
- general practitioner
- HCL-32
- Hypomanic Checklist-32 items
- ICER
- incremental cost-effectiveness ratio
- IPDE
- International Personality Disorder Examination
- LABILE
- Lamotrigine And Borderline personality disorder: Investigating Long-term Effectiveness
- NICE
- National Institute for Health and Care Excellence
- QALY
- quality-adjusted life-year
- SAE
- serious adverse event
- SCID-I
- Structured Clinical Interview for DSM-IV Axis I Disorders
- SCID-II
- Structured Clinical Interview for DSM-IV Axis II Personality Disorders
- SD
- standard deviation
- SFQ
- Social Functioning Questionnaire
- ZAN-BPD
- Zanarini Rating Scale for Borderline Personality Disorder
