Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/102/03. The contractual start date was in February 2013. The draft report began editorial review in September 2016 and was accepted for publication in May 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Allan House is a member of the Health Technology Assessment (HTA) Efficient Study Design Board and of a Programme Grants for Applied Research subpanel. Amy Russell was an associate member of the Health Services and Delivery Research Commissioning Board (Commissioned and Researcher-Led). Claire Hulme is a member of the HTA Commissioning Board. Amanda Farrin is a member of the HTA Themed Call panel.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by House et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Type 2 diabetes
The prevalence of type 2 diabetes, which varies markedly by ethnicity and social deprivation, has increased sharply in recent years, in line with increasing obesity in the general population. Current population prevalence from published figures is between 4% and 5%. 1
Diabetes in people with learning disabilities has been estimated to be more common than it is in the general population,2–5 with a cited prevalence of 9–11%. 6,7 People with learning disabilities also have higher rates of hospital admissions resulting from outpatient-treatable diabetes-related conditions. 8 There are a number of possible explanations for high rates of poorly controlled type 2 diabetes in adults with a learning disability: high prevalence of obesity9–11 and poor dietary habits, prescription medications that increase risk and poor self-management skills. 12 Overall, people with learning disabilities have poorer health outcomes and shorter life expectancy than the average population. 13,14
Learning disability
A learning or intellectual disability is defined as a significant impairment in a person’s intellectual functioning that has been present since childhood. It is less straightforward to define and identify than diabetes, especially at the milder end of the spectrum. It can be defined statistically based on test scores, which typically show a negatively skewed distribution, and in those terms it is often said that 2% of the general population will have some degree of learning disability. 15 However, the picture becomes more complex when an element of functional impairment in real-world activities is built into the definition. Part of the problem is that any functional deficit may not be entirely attributable to intellectual impairment but to (for example) emotional or social problems or missed schooling. Conversely, an adult with intellectual impairment may not come to the attention of statutory or non-statutory agencies if he or she is functioning independently or is well supported by family or some other informal carer. The functional approach to definition is now widespread and accounts for a shift in practice so that in routine discourse learning disability (referring to an intellectual impairment) and learning difficulty (referring to a functional state) are sometimes used interchangeably. An additional confusion is created by the alternative practice of using the term ‘learning difficulty’ to refer to specific educational deficits, such as dyslexia, that are not associated with a more global intellectual disability.
There is a move in academic writing towards the term ‘intellectual disability’ and much of the newer literature uses this term. However, the term learning disability is still used by the great majority of people with such impairments, by their families and other supporters and by third-sector organisations – it is easier to understand and to relate to personal experience. Another problem arises from use of the term learning disability to refer to specific deficits, such as dyslexia, even when it is not associated with more general intellectual impairment or functional deficit. This is not the way the term learning disability is used in this study. For the purposes of this monograph we use the term ‘learning disability’ to encompass all types of intellectual deficit that lead to problems with self-management, except when an alternative term is used in the naming of an agency, service provider or policy of relevance.
Case finding
Case finding for service planning and for research in diabetes is greatly facilitated in the UK by the fact that general practitioners (GPs) are required to maintain a register of all patients with diabetes and are remunerated through the Quality Outcomes Framework (QOF)16 for undertaking various health assessments on an annual cycle.
General practices are also required to maintain a register of their patients with a learning disability and, as a result of increasing evidence of the health inequalities experienced by people with a learning disability, the Disability Rights Commission recommended the introduction of annual health checks17 for people on those registers. In 2009, a Direct Enhanced Service was introduced for adults (aged 18 years or over) with a learning disability and complex needs to be offered an annual health check via their general practice. 18 The Direct Enhanced Service encouraged the verification of general practice-based learning disability registers and local authority involvement in this process, as a means of facilitating the delivery of the health checks.
However, general practice learning disability registers are not uniformly completed throughout the UK. Most registers feature only those people in receipt of health or social services and, as a consequence, most people on the registers have a moderate, severe or profound learning disability. 19 A result is that learning disability registers typically include only 0.5% of the adult population, or about one in four of those with a learning disability. 20 This is an unfortunate state of affairs because it is apparent that adults with a learning disability have high rates of physical illness, and a recent report highlighted their poor levels of health care. 13,21 Take-up of the Direct Enhanced Service has also been variable even for those on the registers. For example in 2013/14, 42% of eligible adults with a learning disability received a health check in Leeds, 44% in Wakefield and 67% in Bradford. 22
Supported self-management
Supported self-help or self-management with health problems is now reasonably well established in that the principles are clear in terms of its core elements, although the intensity with which it is delivered, and its content, have varied considerably between studies. 23–25
In relation to intensity, the main variation is in amount of contact with the support/therapist, which ranges from regular face-to-face meetings to limited telephone contact. In supported self-management (SSM), the usual pattern is that a professional (or trained peer) acts as a ‘therapist’ to help and encourage the nominated patient in using the self-management materials.
With regard to content, all programmes contain an educational and an instructional component, the variation residing mainly in the degree of use of formal techniques for supporting change in behaviour and the degree to which they are theory based. Typical elements include:26
helping people to understand the short, medium and longer-term consequences of health-related behaviour
helping people to feel positive about the benefits of changing their behaviour
building the person’s confidence in their ability to make and sustain changes
recognising how social contexts and relationships may affect a person’s behaviour
helping plan changes in terms of easy steps over time
identifying and planning for situations that might undermine the changes people are trying to make (including planning explicit ‘if–then’ coping strategies to prevent relapse)
encouraging people to make a personal commitment to adopt health-enhancing behaviours by setting (and recording) achievable goals in particular contexts, over a specified time
helping people to use self-regulation techniques (such as self-monitoring, progress review, relapse management and goal revision) to encourage learning from experience
encouraging people to engage the support of others to help them to achieve their behaviour-change goals.
© NICE 2007. PH6 Behaviour Change: General Approaches. Available from www.nice.org.uk/guidance/ph6. All rights reserved. Subject to Notice of Rights NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication
The existing relevant self-management material in learning disability is largely educational and didactic with little or nothing that facilitates self-management, for example advice about self-monitoring. There is also little on the interaction between the person with diabetes and others supporting their care. Many adults with a learning disability do not live entirely independently even when they can be defined as living in the community, that is, not living in a hospital setting. Family members and other informal or formal carers often provide support in the form of help with shopping, cooking, monitoring and prompting about medication and so on. Arrangements here are diverse:27,28 some adults with a learning disability have never left the parental home, some live with a sibling or another relative, some live alone or in shared accommodation with non-resident support or peer support from those with whom they share, and some are married or cohabiting with somebody who may or may not have a learning disability. As many of the positive and negative influences on good diabetes management reside in the immediate social network,29–33 self-management needs to be negotiated not just with the person with diabetes but with their supporter, and flexibility will be needed in negotiating and implementing an intervention.
Research participation for people with a learning disability
The NHS Health Research Agency states that for consent to be considered both legal and ethical it must be given by someone who has been adequately informed; by a person with mental capacity; voluntarily and with no undue influence; and in circumstances in which there is a fair choice. 34 As people with a learning disability have, by definition, a significantly reduced ability to understand new or complex information,35 obtaining informed consent for participation in a feasibility trial provides an ethical challenge to researchers. In addition, many people with a learning disability have communication difficulties and may not always be able to express their understanding (or lack of it) to researchers.
In our study, consent to participation was necessary because the intervention was to change the process of self-management. To assist, advice is available in the form of guidance on implementing the Mental Capacity Act 2005. 36 The Department of Health provides guidance on assessing capacity and obtaining consent for both clinical care and research. 37 The UK Research Governance Framework38 requires that, when necessary, reasonable adjustments must be made to provide accessible research materials. For example, when a person has a learning disability, research information should use ‘easy-read’ or pictorial formats.
In relation to clinical trials, it seems that people with learning disabilities have problems in the same areas as do others:39 understanding randomisation, recognising that they may receive treatment as usual (TAU) and knowing that consent can be withdrawn. The approach to providing information about participation to be involved in a trial therefore needs to be flexible and participant centred, and go beyond standard information sheets – even ‘easy-read’ versions. It will involve finding a form of communication that matches the person’s needs and may well involve a supporter who is familiar with helping that person to make decisions. 40,41
In summary, there is evidence that adults with a learning disability have poor physical health, including high levels of obesity and associated type 2 diabetes. Usual first-line treatment in this situation would be SSM, but there are questions about the suitability of such an approach in the target population: about whether or not reasonable adjustments be made to the self-management intervention, whether or not the resulting intervention can be implemented effectively and, if so, whether or not it leads to useful change. In addition, there is a question about value for money, given the paucity of evidence for cost-effectiveness of behaviour change interventions generally and the likely additional complexity of delivering one in the current context. For these reasons, a pragmatic Phase III randomised controlled trial (RCT) of such an intervention seems appropriate, but there are feasibility questions to be addressed before a trial could be embarked on.
The present study was designed and commissioned to answer those feasibility questions. This report presents the study in the form of three main activities aimed at:
-
establishing the feasibility of identifying and characterising a sample from the target population
-
developing and field testing materials for use in a subsequent RCT
-
conducting a feasibility RCT.
Chapter 2 Methods for case finding and characterising the sample
The starting point in preparing for a RCT in this context has to be if it is possible to identify, consent and recruit a sample from the target population and, if it is, do the potential participants have health-care needs that are likely to be modifiable through SSM? It is not inconceivable, for example, that those who are most easily identified and are able to give consent are also those most able to manage42 their own care and to elicit support in doing so from others in their social network.
Aims and objectives
The aims, in line with the commissioning brief, were to:
-
develop and evaluate a case-finding method to identify participants who have a mild to moderate learning disability and type 2 diabetes who were not taking insulin and who might be suitable for SSM
-
develop procedures for determining, and recording, capacity and obtaining consent
-
recruit a sample meeting the inclusion criteria for the planned feasibility RCT, describe their personal and clinical characteristics and determine their willingness to be considered for participation in the RCT.
The proposed objectives were:
-
the establishment of a practical case-finding method to identify the eligible population without undue cost
-
a robust estimate of the number of people who met the eligibility criteria and the numbers who were willing to consider change in their diabetes management and to participate in the RCT
-
a characterisation of the study population in terms of health, diabetes control, resource use including medication costs, living circumstances and role of supporters in diabetes management.
Design
A cross-sectional observational study.
Setting
We chose three research sites in West Yorkshire, centred on the cities of Leeds, Bradford and Wakefield, to take part in the study. This maximised generalisability of the findings and provided a good test of feasibility of recruitment across a large population. The cities are in the lowest one-third in the UK in relation to deprivation. Bradford has an unusually large population of people of Pakistani heritage (approximately 25%), whereas Leeds and Wakefield are more typical of the UK, with about 15% of the population describing themselves as non-white British. Service configurations for diabetes varied across the three cities. In Leeds, most type 2 diabetes cases were managed exclusively in primary care, with referral to secondary care only for specific problems. There was a tiered system for diabetes care in Bradford, with almost half of general practices managing insulin transfer and several participating in collaborative care of complex cases. In Wakefield, primary care diabetes services were supported by local commissioning arrangements, in which specialist teams tailor their work to individual need. There was also a wide range of services for people with a learning disability in NHS community learning disability services, in social care and in third-sector organisations across these three cities.
Participants and eligibility criteria
People were eligible if they met the following criteria:
-
aged ≥ 18 years
-
diagnosed with type 2 diabetes, controlled with diet alone or hypoglycaemic agents other than insulin
-
mild to moderate learning disability, identified by GP on clinical assessment and confirmed by researcher on the basis of functional history and performance at interview
-
living in the community (not in a hospital setting) and under the care of a general practice in the geographical area covered by the study or willing to agree to a data-sharing agreement.
People were excluded if they had insufficient mental capacity to consent to participate in the research; capacity was assessed at the first meeting with the researcher. The following exclusion criteria were applied after the initial visit and following information gained from the participant’s GP:
-
intellectual impairment acquired from disease or injury in adult life, defined as aged ≥ 16 years, such as that caused by adult-onset dementia or stroke
-
specific educational deficit, such as dyslexia, or autism without learning disability
-
type 1 diabetes, secondary diabetes (such as that caused by steroids, pancreatitis or endocrine disorders) and rare causes of monogenic diabetes (such as maturity-onset diabetes of the young)
-
referral into the study had been through the third sector or another non-medical professional, and eligibility could not be confirmed at interview or from a medical professional such as the GP.
Presence of a learning disability was initially decided by the referrers, who were provided with guidance on how to make the judgement. The person did not need to have a formal recorded diagnosis of a learning disability (as many individuals with intellectual impairment do not). Mild to moderate learning disability was defined by a researcher-led assessment of functional deficits (in daily activities, educational and social attainment and support needs, day-to-day cognitive functions of memory and knowledge) attributable to primary cognitive impairment or to secondary causes acquired in childhood. Mental capacity was assessed by the researcher, following guidelines from the Mental Capacity Act 2005. 36,43 We decided to include only those with mental capacity to consent themselves, excluding those without capacity even when a supporter might have consented, because we wanted to include only individuals who could participate themselves in self-management.
Ethics issues and approval
Ethics approval was granted for the study by the Yorkshire and Humber Research Ethics Committee (reference number 12/YH/0304).
Informed consent
In developing our informed consent procedures and materials we drew on the NHS Health Research Authority’s guidance on supporting informed participation in research, as well as the toolkit supporting informed consent with those lacking capacity [https://connect.le.ac.uk/alctoolkit (accessed 2 January 2018)], the Department of Health guidance on assessing capacity and obtaining consent both for clinical care and research37 and relevant literature. 44 We approached four other research teams identified from the National Institute for Health Research portfolio as working in the area of learning disability, and they kindly shared the materials developed for their own projects (see Acknowledgements). We also worked with our third-sector partners and learning disability specialists to develop appropriate materials.
All researchers were trained in assessing mental capacity by the learning disability consultant on the team (AS). In line with guidelines in the Mental Capacity Act 2005,36 the presumption was of capacity and a judgement of lack of capacity was made only after all practicable steps to help the participant make a decision had been taken by the researcher in collaboration with the person’s supporter. We were mindful that capacity is not fixed and context free, and so capacity was constantly reassessed during the information-giving and data-collection processes. Our aim was to develop a pragmatic approach to consent that balanced ‘self-determination, respect and safety’ with strategies to promote participation in the research. 44
Participants recruited into the study were provided with information about a system of research advocacy that we established with a third-sector organisation, People in Action [http://peopleinaction.org.uk (accessed 2 January 2018)]. They were provided with a confidential number that they or a supporter could call if they wanted to ask questions about the project, express concerns or withdraw if they did not feel they had the confidence to say so directly to a member of the research team.
We were aware that in some cases the supporter of a person with a learning disability may have a learning disability or other mental capacity issues themselves and so we did not make assumptions about their ability to support self-management. A supporter was defined as the main adult who was self-nominated by themselves, or nominated by the participant or by a professional who knows that person, as providing practical help and support in day-to-day living relevant to their diabetes management.
There was a further ethical challenge in considering how to explain the study to those people who had not previously been identified as having a learning disability. This was addressed by developing information materials in collaboration with third-sector organisations and their customers with a learning disability to ensure that the materials were fit for purpose and would be acceptable to our participants. We did not make reference to ‘learning disability’ or ‘learning difficulty’ anywhere in our participant or supporter materials. After suggesting a number of options to service users via People in Action, on its advice we used the abbreviation ‘OK-Diabetes’ to offer a simple memorable project name for participants, again with the aim in mind of not making reference to learning disability in our materials. In each interview, the interviewer discussed with the participant why they had been referred and that the study included people with learning disabilities. It was discussed whether or not a participant minded being in the study and if they felt they had a learning disability.
Safety
We developed a safeguarding policy for the project in consultation with the Safeguarding Adult Partnership Board in Leeds. This set out when researchers should break the confidentiality of the participant and who they should report concerns to depending on the level of perceived risk. The researcher’s obligation to report safeguarding concerns was discussed with the participant. Any non-urgent concerns that did not need an immediate response were reported back to the chief investigator and lead co-investigator for discussion and advice. We also developed a safety protocol for researcher lone working in which researchers were required to call a nominated member of the team prior to going into the interview, and once again straight afterwards.
Screening and case-finding methods
For the reasons described earlier (see Chapter 1, Case finding), we decided that case finding could not simply entail cross-checking people on the learning disability registers with QOF diabetes registers. We developed a multistrand approach to identification of potential participants involving collaboration with both NHS and non-NHS third-sector organisations.
Informing general practitioners and recruiting by direct referral
We wrote to all general practices at the three study sites using a covering letter from their Clinical Commissioning Group (CCG) lead for either diabetes or learning disability. The covering letters were sent to practice managers and/or diabetes lead GPs, gave CCG endorsement to the study and urged practices to take part. Letters were followed up by telephone calls from a researcher. Practices were supplied with project information, examples of the easy-read information that would be sent to participants, a step-by-step guide to recruiting, a recruitment log to record methods of identification, a referral form with Freepost envelope for successful referrals and an easy-read template letter to send to participants who were unable to be reached by telephone. In our original proposal we intended to develop a checklist for health professionals to help them identify potential participants, particularly individuals who may have milder levels of intellectual disability and not be on the learning disability register. We developed a step-by-step recruitment guide for health-care providers (see Appendices 1 and 2), which, in addition to giving information on cross-checking learning disability registers with diabetes registers, informed practitioners how to run the Read Code search against electronic patient records. Our referral forms included a checklist based on one that was developed by the Royal College of Nursing to identify individuals with a learning disability. However, initial returns of the log, despite numerous missing data, suggested that practices were only running the computer-based searches or were finding them the most productive way of identifying people, suggesting that either this checklist was unhelpful or the majority of GPs were unwilling to try and identify a person as having a learning disability who was not already on the register. For this reason the study did not further develop the ‘simple checklist’ as expected, and instead it focused on register and Read Code searches, as these proved to be the most popular method of identification with clinicians.
Practices were offered the opportunity to meet the researcher to discuss the project, as many had concerns about referring a ‘vulnerable’ patient group. The researcher would attend the practice, explain the project and demonstrate the participant information materials to ensure that practice staff knew the study had taken communication issues into consideration. Over the course of the project, practices that did not respond were written to three times and received at least two follow-up telephone calls. Practices that took part in the study were paid service support costs by the Primary Care Research Network.
A researcher attended CCG meetings, giving talks on the project to practice managers and distributing project information. Time for Audit, Research, Governance, Education and Training (TARGET) training was also attended by a researcher who spoke with practice staff and distributed project information. ‘Research-ready’ practices (through what was then the Primary Care Research Network) were recruited through its regular research network meetings. The Commissioning Support Unit supported the project, circulating project information at its events, in newsletters and to its contacts. The researcher attended Continuing Professional Development training relevant to the project and presented to the clinicians there.
Case finding via searches of general practice databases
We developed Read Code searches to help general practices identify potential participants. Read Codes are clinical codes applied to patient records that can create searchable tags of information about a patient but are not limited to diagnosis codes. They can be about a patient’s identity (ethnicity for example), their social circumstances, living and care arrangements or about their symptoms. There are many Read Codes, and codes are not used routinely or consistently across practices. Some codes will automatically include a person on a list or register, such as the diabetes QOF register, or trigger a required response, for example depression symptom codes can trigger an automated reminder to screen for depression; however, not all codes are linked to registers or actions.
Different computer systems use different codes. Bradford and Wakefield use SystmOne (The Phoenix Partnership, TPP, Leeds, UK), whereas the CCGs in Leeds use a combination of SystmOne, EMIS Web (EMIS Health, Leeds, UK) and EMIS LV (EMIS Health, Leeds, UK). The research team met with system specialists and learning disability experts to design a search that could be run on any practice system to create a list of patients whose Read Codes indicated they might have a learning disability or had accessed learning disability services. It would then be up to a member of their direct care team to review these results. The search included diagnostic codes for learning disability and chromosomal or other physical abnormalities, service access codes (e.g. referred to community learning disability team) and functional ability codes (e.g. cannot read). Other codes were discussed but rejected for generating too many results, for example adding the code for ‘unable to perform personal care activity’ added over 16,000 people to the results for all of Bradford.
Two practices agreed to test the search and review the results. Both practices knew their patients with a learning disability very well. They agreed that the search had identified some of the patients known to the practice as having a learning disability but having no formal diagnosis. However, it was agreed there were too many false positives and several codes were removed that had not generated any new eligible patients. Once refined, the Read Code search of codes indicating potential learning disability was then linked to the records of patients who had type 2 diabetes but were not on insulin to further reduce the number of results and ensure eligibility. This search was linked to two other searches and uploaded to practice systems. Practices were able to search their:
-
learning disability register crossed with their QOF diabetes register (excluding type 1 diabetes and people on insulin)
-
whole list for patients with a Read Code that indicated learning disability and type 2 diabetes (excluding insulin)
-
whole list for patients with a Read Code that indicated learning disability.
A member of clinical staff was asked to review the results provided by searches 1 and 2 and recruit all potentially eligible patients. Search 3 was provided to allow practices to review their learning disability registers if they wanted to do so. Searches were embedded in the clinical systems of each practice so they could be directly accessed through their clinical reporting files. In consultation with GPs, it was agreed that if the patient lists generated by the Read code search were too long, clinical staff would not be expected to review them. In practice, the simplicity of this arrangement proved popular as it took only four ‘clicks’ to run all of our searches. Members of the research team gave support on the use of the searches if required.
Secondary care services
Project coapplicants (DN, RA and AS) were located in secondary care settings. They referred patients to the study and recruited colleagues to refer. One site assigned a clinical studies nurse for recruitment. We approached all secondary specialist learning disability services and diabetes services in the three cities directly by letter, indicating our inclusion and exclusion criteria. We asked them to review their case load for files flagged with a learning disability and to keep the study in mind as they saw new patients. A researcher attended the Comprehensive Local Research Networks Diabetes Research Network meetings and encouraged network members to refer to the study. A researcher visited all learning disability community teams and at these meetings a key person was identified who would be the liaison between the research team and the community teams. The learning disability leads for the hospitals were involved in the project recruitment as a key figure to support recruitment in secondary care. The research team also contacted retinal screening services and specialist dentistry services to identify potentially eligible patients.
Third-sector organisations
A significant amount of support for people with a learning disability is provided by non-statutory organisations, for example in relation to advocacy, housing and family issues, health, leisure and employment. In association with partner organisations, People in Action and Tenfold [www.tenfold.org.uk (accessed 2 January 2018)], we identified third-sector services that may be able to support the identification of potential participants in their client base. We worked closely with the learning disability partnership boards in each area to endorse our project to these services and local authority services. We recruited through a variety of services, including housing organisations, care/support organisations, Citizens Advice Bureaux, day centres, advocacy services, religious groups and community volunteering projects. A variety of recruitment methods was used including becoming familiar faces at learning disability events (e.g. in Learning Disability Week), day centres and learning disability organisation networks.
Recruitment procedure
The referrer obtained and documented consent from potential participants to be contacted by the research team. Participants were given a tear-off sheet from the back of the referral form to remind them that they had agreed to contact. A letter and patient information sheet were then sent to the potential participants by the team (and supporter if identified) giving easy-read information about the study (see Appendix 3). This was followed up by a telephone call giving the potential participant (and/or supporter) an opportunity to discuss the study further and obtain verbal consent for a face-to-face interview with the study researcher. Most face-to-face interviews were conducted in the home of the participant.
Following interview, after capacity, consent (see Appendix 4) and a provisional assessment of eligibility were established, participants were registered using a secure, automated 24-hour telephone registration service based at the Clinical Trials Research Unit (CTRU) at the University of Leeds. Following registration, researchers contacted the participant’s GP to inform them of registration (even if they were not the original referrers) and determine final eligibility via their medical notes.
Data collection
There were four main data collection points: (1) referral, (2) initial telephone contact with the referred participant or their supporter, (3) the face-to-face interview with the participant (and supporter if identified) and (4) the participant’s medical notes accessed by their GP. Table 1 summarises the data collected in this part of the study, and the source of the data. Most of the data used to characterise the consenting sample were collected during the face-to-face interview.
| Assessment (including who is involved) | Source of data | |||
|---|---|---|---|---|
| Referral | Telephone | Interview | Medical notes | |
| Screening | ||||
| Demographic data (including age, sex, ethnicity) | ✗ | |||
| Preferred method of contact | ✗ | |||
| Eligibility and consent | ||||
| Eligibility (assessed by referrer and study researcher and confirmed by clinician), including reason for exclusion | ✗ | ✗ | ✗ | ✗ |
| Documented consent to contact | ✗ | |||
| Verbal consent to interview | ✗ | |||
| Mental capacity to consent | ✗ | |||
| Written consent, including access to GP notes | ✗ | |||
| Documented preference to further research contact | ✗ | |||
| Baseline data | ||||
| Presence and role of a supporter | ✗ | ✗ | ||
| Demographic data (including first language, living arrangements, employment status, use of mobile phone and internet) | ✗ | |||
| Self-reported health assessment [including diet, physical activity, mood, smoking, alcohol consumption, comorbidities, self-care activities (e.g. foot check), general and dental health, engagement with health and dental services] | ✗ | |||
| Prescribed diabetes regime (medication) | ✗ | ✗ | ||
| Current physical health state from QOF measures (BP, BMI, HbA1c level, QRISK®2 score) | ✗ | |||
| Service usage (visits to GP, practice nurse, diabetes clinics, home visits, ophthalmologist, podiatrist, dietitian, nephrologist, inpatient stays, attendance at A&E and ‘other’) | ✗ | |||
| Preferences for assistance with diabetes | ✗ | |||
Measures and research materials
All information and consent materials were provided in easy-read format (text supported by pictures) to both participants and supporters. The materials were developed in collaboration with our third-sector partners and produced by easy on the i, an information design service within the learning disability service of the Leeds York Partnership NHS Foundation Trust [www.easyonthei.nhs.uk (accessed 2 January 2018)]. This group includes people with a learning disability. The information resources and the interview schedule were reviewed by employees of CHANGE [www.changepeople.org (accessed 2 January 2018)] who had a learning disability and diabetes. CHANGE is a human rights organisation and service provider that runs an accessible information service.
Baseline researcher interview
The interview (see Appendix 5) used a standardised format to (1) check understanding of the study using an easy-read booklet; (2) assess mental capacity to be involved in the study; (3) obtain written (or verbal when necessary) consent; (4) obtain consent to review medical records for routine clinical measures; (5) establish diabetes management, including diet, physical activity, treatment, self-care awareness and engagement with health services; (6) record mood, feelings about diet, activity levels and having diabetes; (7) identify the role of supporters in the participant’s diabetes management; (8) elicit preferences for further assistance with diabetes management and consent to re-contact for further research; and (9) record a nominated supporter to be involved in further contacts, as applicable.
The interview guide was developed with, and reviewed by, staff from People in Action, a third-sector service provider for people with a learning disability. Measures that required long-term recall (defined as > 1 week) were found to be impractical. The full interview guide was piloted by two employees of CHANGE who had a learning disability and diabetes and was altered based on their feedback.
With one exception, we did not identify standardised existing research measures for mood, diet, attitudes to diabetes or knowledge about diabetes that were appropriate for people with a learning disability in this research setting. 45 Most of the standardised measures that we identified did not have sufficiently robust psychometric properties to overcome doubts about their utility. Even self-report questionnaires with 12–15 items were considered too taxing by our expert advisers and when they depended upon making ranking judgements, such as on a 4-point severity scale, they were beyond the intellectual ability of our participants. Instead, we used such standardised measures as the basis for deriving simple and important questions to ask in the interview. Respondent burden was also a major consideration in our choice of measures and influenced by the cognitive abilities of our sample. A standardised measure that might have been feasible but that takes substantial effort to complete could not be justified unless it met the core needs of the project. For example, although there are standardised depression measures used in learning disabled populations,46 a questionnaire such as the Glasgow Depression Scale,47 even if it could be completed, takes 10–15 minutes with a supporter and would produce a result only indirectly related to our core objectives.
However, because of their particular importance in this study, we did attempt to capture data on diet using the Rapid Eating Assessment for Participants (REAP) – Shortened Version. 45 Although this measure is not designed for people with a learning disability, we supplemented the questions with visual aids, for example, pictures of common foods and a retrospective food diary for the previous week to prompt recall.
The interview employed mainly closed questions (see Appendix 5). When possible, questions were phrased in an explorative conversational form to promote understanding. The interview process was supported by visual aids in the form of laminated picture cards that illustrated the topics the interview would cover and images related to questions about, for example, family, employment, diabetes care, diet, activity and mood.
Evaluating the research process
At the end of each interview the researcher asked the participant how they found the questions on a scale of easy to hard. They were also asked to assess the acceptability of the length of the interview. The interviews were conducted with all registered participants and transcribed.
After each interview, the researcher audio-recorded their own observations using a predefined topic guide. This covered reflections on the consent process and assessing mental capacity for involvement, how well participants understood or were able to respond to questions independently or with support, whether or not the study materials worked well and the researcher’s ability to complete the EuroQol-5 Dimensions (EQ-5D), mood questions and health economics service use questions with the participant and observations about home life and relationship with supporter if present. Journals were initially transcribed to support discussions about eligibility associated with mental capacity; however, they were also used in the analysis phase to supplement the qualitative analysis.
Medical information
A medical information form sent to GPs after the baseline interview included questions to confirm eligibility, obtain recent diabetes-related test results [including glycated haemoglobin (HbA1c) level], comorbidities (as defined by presence on QOF registers), current diabetes medication and health service usage. The information relating to the last two items was collected on forms designed for the project as part of assessing the feasibility of an economic evaluation in a definitive RCT (see Chapter 3).
Outcomes
-
A robust estimate of eligibility, including the pattern and prevalence of uncertainty during eligibility assessments, as measured by: ‘not sure’ boxes ticked for eligibility criteria on the referrals booklet, and on the confirmation of eligibility at registration.
-
Evaluation of a simple case-finding method, according to:
-
the number and rates of referral, including duplicates, consent to research contact, researcher interview, capacity, registration and eligibility
-
methods of identification for the referred and eligible participants
-
contacts – (1) nature of initial contact, (2) number of contacts (by method), (3) who contacts were with, (4) reasons for terminating contact and (5) timelines, including duration of visits.
-
Communication problems for the referred and eligible participants.
-
Identification of a supporter.
-
Patient preferences for time and method of researchers contact.
-
-
Characteristics of the population, according to:
-
demographics – age, sex and ethnicity
-
patient-reported measures obtained through researcher interview including living arrangements, employment status, supporter details, diabetes medication, diabetes care, food, physical activity, general health, mood, feelings about current weight and having diabetes, satisfaction with diet and levels of exercise
-
use of health care and costs associated with health care
-
medical results, including candidate outcome measures [HbA1c level, body mass index (BMI), blood pressure (BP)], other measures of diabetes control and cardiovascular health, presence on QOF registers and resource use including prescribed diabetes medication.
-
Statistical methods
Sample size
Across the three sites we expected an approximate total of ≈1400 people in the target population – assuming a 80% population of adults, 6% diabetes prevalence (in whom 20% use insulin) and 2% learning disability prevalence. We therefore aimed to achieve 50% GP involvement across Leeds, Bradford and Wakefield, equating to approximately 117 practices, and we assumed an eligibility rate of 50% to identify approximately 350 people meeting our eligibility criteria. A sample size of 350 people would allow a conservative estimation of the proportion eligible for the RCT of the target population to a reasonable degree of accuracy [to within 5.2% with a two-sided 95% confidence interval (CI)]. It was this original target that is cited in our funding application and in the original study protocol.
Following a Health Technology Assessment (HTA) programme review of recruitment in June 2014, our original target of 350 was lowered to a minimum of 120 following acknowledgement of a lower than expected recruitment rate. The lower recruitment rate was a result of underestimating the use of insulin in type 2 diabetes, less than expected young-onset type 2 diabetes and a stronger than expected bias of learning disability registers to more severe cases. A higher than expected potential eligibility and acceptance rate for the feasibility RCT meant that we were confident with the new recruitment target. Case-finding recruitment was also extended in order to continue in parallel with RCT recruitment.
Patient populations
Referred population
The referred population consisted of all unique potential participants referred for entry into the case-finding part of the study, including those referred but not registered, and excludes duplicate referrals made for the same person through different sources. ‘All referrals’ refers to every separate referral from any source, including such duplicates.
Eligible population
Participants considered eligible at case finding were all those registered who met the inclusion criteria and none of the exclusion criteria. Participants found to be ineligible after registration (from GP medical notes review) or without documented evidence of informed consent were excluded from this population. When a completed medical review notes form was not received from a GP to confirm eligibility, participants were included if eligibility was confirmed by a researcher and if they were originally referred into the study via a medical professional.
Analysis methods
As this was a case-finding study for a feasibility study, outcomes were not subjected to formal statistical testing and so no hypotheses have been proposed.
For case-finding and eligibility outcomes, percentages were calculated using the total number of participants or forms expected in the relevant population as the denominator (including all participants with missing data for that variable), whereas for outcomes characterising the population, percentages were calculated using the total number of participants with available data as the denominator. Therefore, participants with missing data for each variable were excluded from the denominator and the number of participants excluded is presented alongside each summary. Analyses were carried out using statistical analysis software SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
To provide a robust estimate of eligibility
Analysis presents the flow of participants through the study within a Consolidated Standards of Reporting Trials (CONSORT) framework, including reasons for exclusion at each stage (referral, interview and consent to be approached for further research). A 95% CI for the proportion of eligible patients out of all referrals was calculated.
To evaluate a simple case-finding method to identify participants
Analysis generated descriptive statistics for patients agreeing to researcher contact, patients for whom a researcher visit was conducted, registered patients and the eligible population, when appropriate.
To characterise the population
Analysis generated descriptive statistics for participant demographics, participant-reported measures and measures of diabetes control including resource use for the eligible population, with the exception of demographics also summarised for the referred population. Measures of diabetes control were also presented categorically according to abnormal ranges on standard criteria. 48–51
Exploratory post hoc descriptive subgroup comparisons of diabetes control were made for BMI and HbA1c level by the patient’s response to a number of questions, including feelings around mood, diabetes, eating, weight and exercise, whether or not there was a supporter and whether or not they were taking medications.
Qualitative analysis
The analysis approach for the participant interviews and researcher journals is reported in Qualitative data analysis in the methods for the feasibility RCT, to avoid duplication.
Chapter 3 Development of resources for use in the feasibility randomised controlled trial
Alongside the case-finding study, we undertook work to develop and field-test materials related to the intervention and to its evaluation in the feasibility RCT.
Objectives
-
Identify existing information resources on diabetes self-management suitable for people in our target group to (1) act as TAU and (2) inform the intervention development.
-
Develop a standardised, but flexible, intervention supported by written materials to aid SSM of type 2 diabetes.
-
Field test the intervention, and assess it for acceptability and feasibility of delivery.
-
Develop a simple measure of adherence to the intervention.
-
Determine the most suitable measures for measuring costs (intervention costs and resource use) and the best approach to measuring quality of life in this population.
Developing intervention materials
Early in the study, we established a third-sector working group to collaborate on the development of the intervention materials. The Leeds & York Partnerships NHS Foundation Trust information design service, easy on the i, provided expertise in the development and evaluation of the materials. Our other third-sector partners, People in Action and Tenfold, and their customers with a learning disability, also provided input to the evaluation of the materials.
Development of the treatment as usual materials
Given the variability in care pathways across the three cities, we decided to ensure a basic level of diabetes care and knowledge in all participants. It was agreed this would be done by supplying a standard leaflet to all trial participants. The research team decided to review existing information resources to evaluate if any could be adopted as their standard leaflet.
Members of the third-sector working group, project team members and easy on the i reviewed 23 existing resources developed for use in the UK and aimed at people with a learning disability who have type 2 diabetes (see Appendix 6). We aimed to evaluate these resources in terms of their accessibility and the degree to which they covered the main elements of diabetes self-care, as identified in current guidelines:52
-
understanding what diabetes is
-
understanding the need for healthy diet and regular meal times
-
the importance of doing exercise
-
the importance of following treatment/medication regime
-
how to access services and important check-ups, such as retinal screening and foot care
-
what to do when not feeling well, for example in terms of following treatment
-
managing acute complications, that is, hypoglycaemia and hyperglycaemia.
Each resource was evaluated for comprehensive of coverage of these themes, the quality and appropriateness of images, clarity of text, ease of understanding (e.g. inclusion of medical terms) and relevance to our study population. No stand-alone resource met all these criteria to a high level. However, a leaflet developed over 10 years ago by Diabetes UK and a Leeds-based organisation, CHANGE, was identified as the best single resource in terms of the above criteria. This leaflet was no longer available in electronic format, was outdated in terms of its presentation (black and white line drawings) and some content no longer reflected current health advice guidelines. The team therefore approached Diabetes UK and CHANGE to collaborate on the design of a new resource.
CHANGE, working under the guidance of people with a learning disability, provided the graphic design expertise for the resource and reviews by people with a learning disability at each iteration. The new guide was informed by current best practice in developing accessible information provided by our expert advisers and from a review of the research and national guidelines in this area. Ramzi Ajjan, consultant endocrinologist with special interest in diabetes, reviewed the medical content. Christine Harris-Moores, specialist learning disability dietitian, reviewed the dietary advice. Diabetes UK ensured that the content met its current advice guidelines for type 2 diabetes.
Development of the supported self-management intervention
Supported self-management, which was specified in the commissioning brief as the intervention to be tested, is an existing approach to chronic disease management in which the underlying theory and the basic principles of implementation are already established. Development work in the current project did not, therefore, need to start from the early phases of intervention development as recommended by, for example, the UK’s Medical Research Council53 – elaborating theory and undertaking early-phase proof of concept and efficacy studies. Instead, we planned our development work in five phases:
-
Clarifying the principles of SSM – as summarised in review articles and didactic pieces, and as identifiable from the protocols and final reports of individual studies of self-management in diabetes.
-
Identifying the reported barriers to effective self-management of type 2 diabetes in adults with a learning disability – from published literature.
-
Reviewing existing materials that aim to support self-management of diabetes for people with a learning disability for examples of good practice – in consultation with service users.
-
Synthesising the outputs from the first three phases – to decide on those elements of SSM that are most relevant to the needs of our target population (will respond to likely barriers), and most likely to be acceptable and useful (match the identified good practices). This phase involved a series of problem-structuring and consensus meetings in the research team, checking interim outputs against guidelines on reasonable adjustments and by consultation with experts and service users.
-
Implementation and field testing of early versions – modifying the intervention materials in the light of feedback from research nurses.
At each stage there were regular consultation meetings involving members of the research team with people with a learning disability and their representatives in easy on the i, People in Action and Tenfold, as well as clinical experts in learning disability.
Phases I and II
We realised early in the project that there was no relevant RCT in the area of diabetes self-management in learning disability and, therefore, we did not undertake a formal systematic review of effectiveness studies. Instead, we aimed to use the published literature to identify (1) the principles of self-management that we would hope to embody in our own intervention and (2) those influences on self-management potential in our population that should inform the form or content of a SSM programme.
To help identify published information relevant to our intervention, an information scientist (JW) undertook initial searches in four main areas:
-
SSM in chronic disease
-
self-care in diabetes, including barriers to effective self-care
-
diabetes and a learning disability – a broad search to identify factors that might be specific to the target population
-
descriptions of specific interventions aimed at improving diabetes control in adults with a learning disability.
The search strategies are provided in Appendix 7.
We also reviewed two relevant National Institute for Health and Care Excellence (NICE) guidelines,26,48 two Cochrane Reviews,54,55 a guideline on SSM published by Diabetes UK52 and outlines of national standards in diabetes management from the USA56 and the UK. 57
On the basis of these searches we identified and reviewed 707 titles and abstracts on the topics of self-management of chronic disease, including diabetes, and 350 titles and abstracts on the topic of diabetes in adults with a learning disability.
Titles and abstracts were reviewed by two of the applicants (AH and GL) and full versions of relevant papers were obtained. We categorised retrieved papers as follows:
-
individual self-management programmes or interventions (n = 22)66–87
-
research protocols describing individual self-management interventions (n = 8)88–95
-
observational studies reporting influences on self-management – barriers and enablers (n = 31). 29–33,96–122
Initially, we extracted data from the papers that described the form or content of self-management. We developed an initial framework derived from reviewing the two NICE guidelines,26,48 two Cochrane Reviews54,55 and the guideline on SSM published by Diabetes UK. 52 We then developed this framework by reference to six individual studies in the reports67,74,81,123–125 for which we found reasonably comprehensive descriptions of the intervention. The remaining studies were reviewed against this framework to identify any missing themes, using a modified approach based upon best-fit framework analysis. 126
Next, we reviewed papers describing barriers to effective self-management, influences on interventions or outcomes that were specific to adults with a learning disability. In a series of review meetings we organised the identified influences into a descriptive framework.
Finally, we combined the two frameworks in a narrative synthesis of these literatures to identify the general principles to be adhered to in implementing a pragmatic and sustainable programme of SSM, and the form and content of the specific intervention for this project.
Phase III
For existing self-care resources, a scoping exercise was conducted in which examples of good practice in interventions around health in people with a learning disability were sought from services in the UK. Sources included charities, for example Diabetes UK, local NHS trusts and patient groups, NHS Choices [www.nhs.uk/pages/home.aspx (accessed 2 January 2018)] and Easyhealth [www.easyhealth.org.uk (accessed 2 January 2018)]. The process here was different from that used to develop the TAU intervention described in Development of the treatment as usual materials. In that case we were seeking to identify the best-available resource to adopt in toto; in the present exercise we were seeking specific instances of desirable practice – individual images, approaches to explaining the principles of self-management, or user-friendly resources such as diary sheets.
We identified 18 examples of resources developed in the UK to support self-management of diabetes for people with a learning disability.
Resources were reviewed by a panel consisting of members of the research team and staff and service users from easy on the i. They were asked what they liked and disliked about each resource, and to note implications for the intervention in development. Results were recorded on a structured proforma.
Phase IV
Using the general approach of problem structuring and priority setting,127 preliminary versions of the SSM package – including not only format and content but also tailoring (for easy reading, visibility for those with poor acuity and so on) – were discussed initially in the research team. Finally, we considered guidance on reasonable adjustments to health care designed to ensure access for people with a disability, to check that we were meeting obligations in that respect.
When necessary, a further round of more focused (purposive) reviewing of literature was used to clarify which were the key principles for this population. We generated two lists (Box 1 and Table 2) that we used to help frame final decisions.
-
Food: buying, preparing, eating.
-
Weight control or weight loss.
-
Physical activity or exercise.
-
Looking after your body: foot care, dental care.
-
Healthy living: alcohol, smoking.
-
Taking tablets.
-
Visiting professionals: dental care, medical care, eye checks.
-
Maintaining emotional well-being.
-
Education: about diabetes and what it is, what self-management involves.
-
Problem-solving.
-
Goal-setting, planning.
-
Monitoring and feedback, for example blood glucose levels, weight, dietary intake, tablet take.
-
Skills development: foot care, self-monitoring of blood glucose, preparing food, use of IT.
-
Effective use of other people and resources, for example company when going swimming/walking.
-
Managing emotions and building confidence.
-
Written materials.
-
Charts: fridge door charts, plan your plate, diaries.
-
DVD.
-
Web-based programmes: static or interactive/moderated.
-
Telephone or short message service (SMS) contact: prompts or interactive.
-
IT: beeping fridges, watches, tablet boxes, smart phones, etc.
-
Groups, for example nurse-led, third-sector, exercise group, group education.
-
Professional contact: nurse, diabetes educator, GP.
-
Peer support: informal; trained peer support; family; couples work.
-
Literacy and other intellectual attainment.
-
Sensory impairments.
-
Language difficulties: non-English, comprehension or speech problems.
-
Autistic characteristics.
-
Self-nominated goals or problems.
-
Professionally identified priorities.
-
Living arrangements.
-
Supporter’s priorities.
| Example of | |
|---|---|
| Disability (barrier to good health care) | Adjustment (enabler of good health care) |
| Cognitive disabilities | |
| Memory problems | Prompts, support for appointments |
| Literacy or reading skills deficit | Accessible materials |
| Vision or hearing impairment | Visual aids |
| Speech problems | Time, trained staff |
| Understanding: health risks, necessary actions | Accessible information |
| Personal | |
| History of lack of dignity/respect in services | Staff training |
| Threat to safety including bullying | Safeguarding protocols |
| Lack of personal pleasure/relax and recreation activities | |
| Autonomy/choice | |
| Questionable mental capacity | Staff training in capacity assessment and inclusive practice |
| Practical (instrumental) barriers | |
| Transport | Funding, safe provision |
| Mobility | May need occupational/physiotherapy assessment |
| Finance | Personal budget |
| Treatment burden: timing, side effects | Support with adherence, modified regime |
| Social barriers | |
| Lack of social support/networks | Identify, train and support carers, advocacy, third sector |
| Mental health | |
| Autistic traits | Pacing of change |
| Challenging behaviour | Pacing of change, staff training |
| Low self-confidence | |
| Distress and mental disorder | |
| Other health needs | |
| Epilepsy | Safe environment |
| Physical symptoms or restrictions | |
| Difficulty negotiating health care | |
| Talking with professionals | Staff training and supervision |
| Communicating needs | Learning disability register, health action plan |
| Overcoming stigma | Advocacy |
Principles for priority setting were that:
-
the intervention should respond to known barriers to self-management reported by people with a (learning) disability, including practical problems such as transport, likely attrition from dropout when multiple attendances are expected and ability to accommodate the presence of a supporter
-
the format of the intervention should be likely to encourage self-maintained change beyond an early supported element; in our target population this particularly meant that the intervention should involve supporters who were involved with any aspect of lifestyle (shopping, food choice, physical activity, medication monitoring and so on) relevant to diabetes
-
the intervention should be designed to be readily integrated into usual health-care provision in the NHS to ensure sustainability.
Based on all the advice we received, we wanted to give particular salience to:
-
practical aspects of self-care, such as buying and preparing food, diet change to aim for weight loss and increasing physical activity
-
use of simple (accessible) written materials and charts
-
supportive contact both with a professional and with an informal supporter, if one could be identified
-
use of practical goal-setting, planning to meet goals and self-monitoring.
By contrast, we decided it would be less helpful to focus on:
-
a substantial component of healthy living interventions aimed at alcohol and smoking, because they were not major problems in our group
-
education beyond the basic information in the TAU booklet
-
information technology (IT)-based interventions, such as web, digital versatile disc (DVD), mobile phone, etc., as these were not readily accessible by our participants
-
group-based interventions, as attendance is typically poor and individualisation is harder.
Phase V
We decided that professional support would be provided by diabetes nurses with experience in primary care rather than learning disability nurses: very few of the target population would be in contact with (or taken on by) specialist learning disability services. In routine NHS practice, where 20–25% of patients would be using insulin, diabetes nurses would have more relevant experience; for our population (not all of whom would have been told they had, or would self-describe as having, a learning disability) the diabetes background would be more acceptable. We developed a training plan for the research nurses delivering the intervention covering the underlying principles of mental capacity and of self-management, the individualised elements specific to learning disability, trouble-shooting and dealing with problems (such as behavioural disturbance), and the details of the programme and the materials provided with it. Explicit links were made between the practicalities of the intervention and its rationale in self-management principles (Table 3).
| Principle | Relevant intervention materials (see Appendix 12) |
|---|---|
| Helping people to understand the short-, medium- and longer-term consequences of health-related behaviour | How to do it |
| Looking after my diabetes | |
| Helping people to feel positive about the benefits of changing their behaviour | I am going to . . . |
| Building the person’s confidence in their ability to make and sustain changes | I am going to . . . |
| Recognising how social contexts and relationships may affect a person’s behaviour | My life |
| Who, what, where? | |
| Supporter charts and card | |
| Helping plan changes in terms of easy steps over time | Weekly plan/change plan |
| Identifying and planning for situations that might undermine the changes people are trying to make (including planning explicit ‘if–then’ coping strategies to prevent relapse) | I am going to . . . |
| Encouraging people to make a personal commitment to adopt health-enhancing behaviours by setting (and recording) achievable goals in particular contexts, over a specified time | I am going to . . . |
| Helping people to use self-regulation techniques (such as self-monitoring, progress review, relapse management and goal revision) to encourage learning from experience | My rewards plan |
| Encouraging people to engage the support of others to help them to maintain their behaviour-change goals | Supporter pack and flash cards |
The training programme was delivered by two of the researchers (AH and GL) over three sessions of face-to-face contact with the nurses. An additional session on mental capacity assessment was delivered by Alison Stansfield to the nurses and all research interviewers.
Supervised use of the intervention with three initial cases in the RCT was also arranged. In each case the whole intervention was delivered over a maximum of four visits and the nurses met together with Allan House and Gary Latchford after each visit, to discuss any challenges with implementation. On the basis of this experience, early versions of the intervention were modified in format to make them easier for use by the nurses. In particular, the forms for keeping notes on each contact that were prepared for early versions were found to be overstructured by the nurses and intrusive for use in the field. Once they had familiarised themselves with the principles of self-management and the nature of each contact, the nurses preferred to make free-form notes during contact and then check afterwards that they had recorded all the necessary information and completed the essential standardised forms (case report forms) for the trial.
Both nurses and participants involved in the field-testing reported finding the materials easy to use and the nature of the intervention easy to understand.
The final intervention had four standardised components with associated materials. How they were delivered depended on participant and supporter characteristics and preferences.
-
Establishing the participant’s daily routines and lifestyle: this included current diet and activity routines, participation in daytime social activities or work, shopping and food preparation, current self-reported health and self-management. The main aim of this component was to identify the real social and personal influences in the life of the person with diabetes that would limit their ability to self-manage or that might be mobilised as a resource in supporting self-management.
-
Identifying all supporters and helpers and their roles: a key supporter and other helpers were identified when possible at case finding. Key supporters and other helpers were given written information about the project and if they agreed to support a goal set by the participant, then they were given a written reminder of their role. The main aim was to identify people who might be a useful resource in supporting self-management and to ensure that any changes were embedded in the social network for longer-term maintenance of change.
-
Setting realistic goals for change: the main aim was to avoid prescribing change in the way of good dietary practice or other lifestyle change instead but to support goals suggested by the person with diabetes that were specific, simple and achievable, given the person’s current routines and social support, and consonant with their willingness to make change. The main aim was to encourage engagement in a population usually thought of as having little agency and to introduce the idea of selectable elements in a repertoire of self-management options.
-
Monitoring progress against agreed upon goals: we devised a simple system that did not depend on high levels of functional literacy, using tear-off calendar sheets on which participants noted goal attainment in a yes/no format. The main aim was to encourage active participation in an activity that is a core feature of self-management.
We prepared materials to accompany these activities (see Appendices 8–12).
-
For the nurses: templates for a weekly timetable, a chart to record friends and family and other helpers, charts to be completed in collaboration with the person with diabetes – my life, my likes and do not likes, looking after my diabetes.
-
For the person with diabetes: an OK-Diabetes board to place in a prominent position at home with a visible record of goals including pictorial prompts, for example snack swaps, a written action plan in multiple formats and tear-off slips to record daily actions.
-
For supporters and helpers: an information sheet explaining the study and a card summarising what their role was in helping to support the person with diabetes in meeting their chosen goals.
The research nurse worked through the elements of SSM with the participant, explaining how to use materials and suggesting initial actions and activities. Further contact was negotiated with the person with diabetes. We expected that a total of three or four meetings of 30–60 minutes over 6–8 weeks would be provided, followed by telephone support and advice.
We took steps to ensure consistency in use of the SSM: (1) training and supervision sessions with research nurses, (2) annotation of the intervention materials by research nurses and (3) ensuring nurses had other experience and training in diabetes or learning disability care before the RCT.
Developing an adherence measure
We conducted a review of the literature to determine the methods of adherence measurement reported to date for similar interventions (self-care, self-management) in the same population (learning disability), to see if there were helpful techniques we could adopt.
Search strategy
We ran literature searches on the standard bibliographic databases in July 2013, and updated the searches in July 2015. Full search strategies for each database searched can be found in Appendix 7.
Papers were included if they:
-
described primary research studies
-
involved participants who were adults with a learning disability
-
described a standardised self-management or self-care intervention
-
described an intervention aimed at weight loss or improving self-management of diabetes.
We defined self-management as involving at least (1) some definition of actions relevant to improving the participants’ condition (overweight or diabetes), (2) setting and recording of specific goals or targets related to those actions and (3) monitoring of progress in achieving those goals. The person with a learning disability had to be an active agent in this process – albeit with the help of a supporter at times – so that they were not just a passive recipient of a programme designed and delivered by a third party.
Developing an organising framework
In a series of research team meetings we developed an initial framework for organising the data, based upon our reading of the wider adherence literature as well as the literature identified in the present search. We distinguished steps taken to:
-
ensure the intervention was delivered correctly in form, content and quality
-
measure for research that these approaches to ensuring quality of delivery were employed
-
measure actual provider adherence
-
measure participant adherence.
The first two steps fit with existing literature describing ‘fidelity’ – the degree to which provider delivery is in line with the intended form and content. The last two steps fit with the existing definition of ‘adherence’.
To ensure a measure of adherence was applied to all elements of the intervention and not just a selected few, our initial framework had four categories to describe an intervention:
-
The content of the intervention: topics or components covered, such as (for diabetes self-management) shopping for and preparing food, planning physical activity, taking tablets and avoiding unhealthy behaviours such as smoking or drinking too much alcohol.
-
The techniques employed in the intervention: how it is delivered, for example through education, training in goal-setting, use of self-monitoring and feedback techniques.
-
The platform or format by which it is delivered: for example, written materials, group sessions, self-completion charts, web-based resources or text messaging.
-
The degree of individualisation of the intervention: this could mean use of inclusion and exclusion criteria to define the sample from the target population to whom the intervention is delivered or modification of elements of the intervention to suit the needs of individuals within that sample, for example those with visual impairments.
We used this framework for organising information about adherence, and from each paper we also included detail relating to the type of study participants, type of intervention(s), content of the intervention, how the intervention was delivered, how both provider and participant adherence were measured, collected and scored, and how quality and competence were ensured.
Data synthesis
Our search identified 28 relevant studies, which we used to check and refine our framework. 128–155 As there were so few data available regarding treatment adherence in this population and because they were so heterogeneous, there was no possibility of data pooling. We thus undertook a narrative synthesis, organised according to the framework described in Developing an organising framework, to explore the approaches to measuring adherence in the included studies. A first step was to develop a checklist against which to judge the comprehensiveness of the adherence measure, as in Table 4.
| Elements | Considerations for | ||||
|---|---|---|---|---|---|
| Describing, monitoring and reporting each element | Adherence measurement | ||||
| General intervention details | What type of intervention (e.g. therapy vs. self-management)? | Where is it provided? | Who provides it (and do they need training, supervision)? | Who is it delivered to (population, supporter)? | Measurement of provider credentials, training, supervision, feedback and competence. Consider all those in receipt |
| Content of the intervention | For example, activity classes, learning how to choose the right foods or stopping smoking | Evidence that content was delivered as intended | |||
| How the content is delivered | Technique (e.g. formal education, goal-setting) and method (e.g. individual/group sessions, provision of written materials) | ||||
| Receipt and use of the intervention | For example receipt of materials, attendance at sessions, carrying out self-management tasks or changing diet | Evidence of receipt (e.g. log of attendance, receipt of participant diaries) | |||
Developing an adherence measure
For each component of the intervention (e.g. a list of supporters, identified goals, actions taken to meet goals) a record could be generated either by the nurse or by the person with diabetes. We identified which of these records we could collect routinely for each participant, using the framework as a check to ensure that all aspects of adherence were being recorded. Each of these records was to be captured on a case report form during the trial.
To measure adherence we also developed a scoring system based upon completion rates, which would allow us to estimate the proportion of completed tasks during the SSM intervention, including an overall judgement about whether or not the whole process had been completed at least once. This final score was captured on a summary form to enable collation of these adherence items and recording the adherence score.
The relevant case report forms are in Appendix 13.
Developing the materials for economic evaluation
Service usage information
One of the aims of the case-finding study was to identify health and social care use (and thus cost drivers) for inclusion in a questionnaire in which the data collected would be used in an economic evaluation of a later intervention. A second aim was to identify suitable outcome measures for use in such an economic evaluation.
A literature review informed the development of a questionnaire delivered as part of the request for health and social care usage from participants’ GP surgeries (see Appendix 14). The literature included health and social care resources used:
-
within self-management interventions by people with type 2 diabetes and a learning disability, and the methods by which those data were collected
-
by people with type 2 diabetes within self-management interventions
-
by people with a learning disability and the methods by which those data were collected.
The literature review also aimed to identify the outcome measures used in any cost-effectiveness analyses. Search strategies used four concepts: learning disability, self-care interventions, type 2 diabetes and cost studies. Three separate searches were run in EMBASE, MEDLINE and The Cochrane Library using combinations of three of the four concepts in each. The searches were not limited by language or date of publication. A ‘cost studies’ search filter was used to limit studies to those including some cost data.
Inclusion criteria:
-
cost-effectiveness studies for healthy lifestyle interventions
-
cost-effectiveness studies for diabetes self-management
-
studies with costs or outcomes for people with learning disabilities.
Exclusion criteria:
-
studies reported in abstract form only
-
studies relating to self-monitoring
-
diabetes drug studies.
Searches undertaken between May 2013 and February 2014 identified 1189 unique references. No papers were identified that included cost-effectiveness analysis of self-management interventions in people with diabetes and learning disabilities. Titles and abstracts were reviewed by two people and data extracted using a bespoke data extraction template. Given the nature of the data extracted, no quality assurance assessment of the papers identified was undertaken.
Eight studies156–163 that detailed cost-effectiveness analysis of self-management interventions in people with diabetes met the inclusion criteria. The selected studies were lifestyle modification programmes and telephone interventions (both automated and non-automated). Intervention cost data were collected using a variety of methods, including interviews with health workers, standardised templates for staff and participant questionnaires. Staff costs were the greatest drivers of cost in relation to intervention implementation and were calculated using official wage statistics or personnel records. Administrative costs, such as telephone charges, printing charges and translation costs, were added to the expense of the interventions. Participant out-of-pocket costs were collected in two158,162 of the included studies, using staff-completed templates and from participant-completed questionnaires. Outcomes measured clinical marker data such as HbA1c levels, BMI and risk of cardiovascular disease. Five studies156,160–163 also used quality-adjusted life-years (QALYs); two162,163 calculated QALYs using participant-reported EQ-5D.
Six studies164–169 that detailed costs or costs and outcomes of interventions for people with learning disabilities met the inclusion criteria. Resource-use data were collected in all studies: four of these164,165,168,169 used the Client Service Repository Inventory (CSRI), which is a research instrument that provides retrospective information on service use, and three studies165,168,169 used a proxy informant who was familiar with the person with a learning disability. Resource use (for which GP visits, nurse visits, hospital inpatient and outpatient visits were the most frequently utilised, with chiropodist, psychiatrist and dentist also having high utilisation rates), staff (salaries and training) and accommodation were the main drivers of cost in relation to the cost-of-illness studies;165,167 medications, transportation and carer time were also included. Health outcomes were measured in four of the studies using different methods: QALYs derived from EQ-5D,164 social interaction activity patterns166,168 and an aggression scale plus a Quality Of Life Questionnaire. 169
Overall, the diabetes studies showed a variety of data collection methods, with staff time for intervention delivery as the greatest driver of costs. In the studies including people with learning disabilities, hospital-based care, GP, nurse care and accommodation were the main drivers of costs.
Based on the information retrieved from the literature review and with input from experts on the study team, resource-use questionnaires were developed for completion by GP surgeries, using a modified version of the CSRI (for form 10 see Appendix 4, and for form 2 see Appendix 5). The forms were field-tested with people with a learning disability working with our third-sector collaborators, to ensure that they included all of the main items of relevance. In self-management interventions, patients’ costs are likely to be more important than when considered in more conventional interventions; therefore, the forms identified patient resource use from the perspective of the health and social care sector and from a wider societal perspective. The service usage form was field tested and assessed for content, clarity and acceptability (assessed by proportion of questionnaires returned and missing items).
The measure of outcome within the studies varied. However, the EQ-5D and the Health Utilities Index have been shown to be superior compared with other preference-based measures of health for this population. 170 None of the studies identified in review had used the Health Utilities Index and, thus, based on the frequent use of EQ-5D in the included diabetes literature, the EQ-5D was chosen to be assessed for feasibility as an outcome measure for any subsequent RCT.
Health economics analysis
Completeness of the data collected in the questionnaires was recorded using descriptive statistics, detailing the number and percentage of questionnaires returned and the number and percentage of missing items within the returned questionnaires.
Unit costs for health service resources were obtained from national sources such as the Personal Social Services Reference Unit (PSSRU),171 the British National Formulary (BNF)172 and the NHS reference cost database. 173 If national unit costs were unavailable, the finance departments of trusts participating in the study were asked to provide local cost data. The mean of these costs was used as the unit cost estimate in the analysis. This included the costs of health and social care (service provision and use of other health and social care services) and took into account the productivity costs (time away from work) and out-of-pocket expenditures incurred by the patients (e.g. travel expenses, over-the-counter medicines and supplements and additional costs/savings of any dietary changes). Burden on the supporter was also considered (e.g. productivity costs and out-of-pocket expenses).
Chapter 4 Methods for the feasibility trial
The final element of the originally commissioned research was to undertake a feasibility RCT, with recruitment being based upon the case-finding methods already established and materials for the trial being developed as outlined in Chapter 3.
At one stage during the initial case-finding observational study it seemed plausible that we could recruit substantially greater numbers than were needed for the feasibility RCT, and we were offered additional funding to undertake a larger feasibility trial incorporating a proof of concept assessment for efficacy with a study population size of 150. We modified the protocol accordingly. However, we were unable to achieve the necessary increased sample size and reverted to our original design and objectives. To avoid confusion, we have detailed these various changes in Appendix 17, reporting in this monograph and in the published protocol paper174 the methods and results for the originally planned feasibility RCT.
Aims and objectives
The aim was to undertake a feasibility RCT to address the following objectives:
-
To estimate the recruitment, retention and follow-up rates for a definitive (Phase III) trial.
-
To assess the acceptability, and feasibility, of implementation of the self-management intervention – by measuring adherence, dropouts and negative outcomes such as distress and agitation.
-
To test the feasibility of using a standardised adherence measure to assess delivery and use of self-management techniques.
-
To provide a detailed description of what treatment is delivered to each arm.
-
To assess data collection and the feasibility of collecting a range of physiological, psychological, behavioural and cost-effectiveness outcome measures and of maintaining the blind for subjective outcomes.
-
To measure the variability in the candidate primary outcomes (HbA1c levels and BMI) and main secondary outcomes (in particular EQ-5D score).
-
To use qualitative methods to explore the challenges to the validity of data collection for participant questionnaires (EQ-5D and mood).
-
To use qualitative methods to explore the positive and negative experiences of research involvement and participation in the intervention.
-
To develop a checklist of potential negative outcomes and a related process for their collection.
At the end of the feasibility RCT, the proposed outcomes were:
-
data to inform a decision about the feasibility of a definitive trial, on the basis of expected recruitment, retention or adherence rates
-
estimates of parameters needed to estimate the sample size of a definitive trial
-
refined materials and processes for a definitive trial.
Design
The design of the study was an individually randomised controlled parallel-group feasibility trial of manualised SSM and TAU versus TAU in three sites based around the cities of Leeds, Bradford and Wakefield, involving 80 participants (and, when possible, their supporters), recruited during case finding and consenting to take part in the RCT.
Eligible, consenting participants and their supporters (if available and willing) were randomised on a 1 : 1 basis and followed up for 6 months post randomisation. Participants, supporters, referrers and care providers could not be blinded to treatment allocation. Steps were made, however, to blind all aspects of outcome assessment carried out by study researchers and nurses. To avoid unblinding the researcher, the nurse delivering SSM passed data about the intervention (including adherence) directly to the CTRU.
Data were collected from medical notes by the researchers responsible for outcome assessment and by researcher interview.
We prespecified that if any of the following criteria were met, the results would be incompatible with feasibility of a full Phase III trial:
-
recruitment of ≤ 20 participants by end of 6 months of recruitment in the RCT
-
active or passive withdrawal from researcher follow-up of ≥ 40% of recruited participants
-
non-attendance of ≥ 50% participants in the SSM sessions.
Otherwise, we intended to use the data collected (variability of outcomes and recruitment rates, and information regarding appropriate data collection methods and therapeutic delivery) to optimise the protocol for a subsequent definitive multicentre trial. The ease of collection of candidate primary outcomes, the data quality of these outcomes and their clinical importance were used to choose a primary outcome for the next trial.
Eligibility
Participants from the case-finding study were eligible for the feasibility RCT if they also met all of the following additional criteria:
-
were deemed by researchers after repeat assessment to have the mental capacity to consent to participation in a research trial
-
provided written or verbal (when necessary) informed consent
-
willing to undergo a blood test and measurements to establish HbA1c levels and BMI or, if not, had up-to-date routine values of HbA1c and BMI (ideally within 6 weeks of randomisation)
-
had suboptimal diabetes control defined as a HbA1c level of > 6.5% (equivalent to 48 mmol/mol) OR a BMI of > 25 kg/m2 OR self-reported physical activity below national guidelines.
Participants were excluded if they met any of the following criteria:
-
referred for insulin or put on insulin between identification and randomisation
-
likely to require insulin in the next 3 months
-
not living in the community (i.e. living in hospital) at randomisation
-
declining further assistance with diabetes self-management
-
a member of the same household had already been randomised into the RCT.
From our observations during the case-finding stage of the project we considered that there would be too great a risk of between-group contamination if we randomised more than one occupant of a household, as individuals in the same household would invariably know of each other’s health state and have shared eating arrangements. We considered using cluster randomisation by household to avoid losing potential participants, but this method would have inflated the sample size to an extent that meant there was no benefit.
Supporters were eligible if they met the operational definition of ‘a key person in providing regular practical support in diabetes self-management, who is in contact with the person with diabetes at least weekly’ and who was able and willing to give informed consent. Although it was recognised that there may be several people involved with relevant aspects of a participant’s life (diet, physical activity, clinic attendance and so on), we identified only one main named supporter with whom to conduct the baseline assessment. Other people who were involved could be included as ‘other helpers’ in the self-management plans if desired by the participant but they were not defined as a key supporter.
We did not use absence of a supporter as an exclusion criterion for reasons of equity and because we wanted the intervention to mimic, as far as possible, routine NHS practice in which people would not be denied a supportive intervention because of their living arrangements.
Recruitment and randomisation
Potential participants were approached if they had indicated at case finding that they were interested in further involvement in research, wished to consider changes to how they managed their diabetes and gave consent to be approached by a researcher (Figure 1).
FIGURE 1.
Flow diagram of RCT. RUSAE, related and unexpected serious event.

During a face-to-face interview with a researcher and (when applicable) the research nurse, the researcher (1) further confirmed eligibility (excluding suboptimal diabetes control when blood results were not yet available), (2) sought consent to participate in the RCT, (3) collected baseline data and, as appropriate, (4) sought consent for a nurse to visit and take blood samples and other physical measures prior to randomisation (see Appendices 18 and 19).
If participants declined blood-taking, when possible we tried to ensure that they were recruited in accordance with the date of their next QOF assessment so that we could use those blood results.
Once eligibility was confirmed, following researcher and nurse baseline assessments, receipt of blood results and informed consent, participants were randomised on a 1 : 1 basis, using a secure, automated 24-hour telephone randomisation service based at the University of Leeds to ensure allocation concealment, to receive SSM plus TAU or TAU. A computer-generated minimisation algorithm incorporating a random element (80%) was used, accounting for the following factors:
-
site (Leeds, Bradford or Wakefield)
-
supporter (none, not living with supporter or living with supporter)
-
HbA1c level (< 6.5%, 6.5–8.5% or > 8.5%)
-
BMI (≤ 25 kg/m2 or > 25 kg/m2)
-
physical activity (below or above national guidelines).
The research nurse who had undertaken the baseline visit, but not the researcher, was informed of the outcome of randomisation. The nurse contacted the participants and supporters, and research advocates as appropriate, in the intervention and control arms to explain the randomisation outcome and to make an appointment with those participants allocated to SSM. If no baseline nurse visit had taken place (with baseline blood results obtained via the GP instead), the nurse allocated to inform the participant of their allocation was chosen at random.
Participant information and consent
After discussions with learning disability specialist nurses, we decided that verbal contact would be prioritised when clinicians contacted potential participants, as experience had shown that this was the most acceptable method for the learning disability population. We created an easy-read letter to send out to those participants who could not be contacted by telephone.
Based on our experiences at case finding, we changed the consent process from ticking ‘Yes’ in response to a series of statements to circling ‘Yes’ or ‘No’ after each statement. We wanted to be more inclusive and reduce errors, as many participants in the case-finding study had crossed boxes that contained negative statements such as ‘I know the research team will not tell anyone my name’. We had originally been requested to have tick boxes for participants and initial boxes for supporters but we decided that this unbalanced the participant–supporter relationship and did not acknowledge that supporters also might have a learning disability. The RCT consent form was therefore the same for participants and supporters. The option to obtain verbal consent was also included, as some participants did not want to write or could not grip a pen.
A further addition to the RCT documents was an overview of randomisation provided to potential participants. We searched the literature and current trial documents for ways to explain randomisation and concluded that a visual aid was appropriate to help people understand the process. Researchers found this document useful when taking consent (see Appendix 20).
If present and willing, a supporter was also consented into the RCT. However, at interview we often found that a participant would be accompanied by a supporter who was not the person who supported them with their diabetes management. We altered data collection forms to record the first name and relationship to the participant of their main supporter if not present at interview and arranged to subsequently contact the main supporter.
Supported self-management
The SSM was as described in Chapter 3.
Treatment as usual
All trial participants should have been in receipt of standard treatment for their diabetes. A number of staff and services are involved in providing this care and uncomplicated type 2 diabetes is usually managed through primary care. To ensure all participants had a standardised minimum level of self-care support, we provided an information booklet about type 2 diabetes, which was made accessible for people with a learning disability. This booklet was developed for the study. It was given to participants in both arms of the study (see Appendix 21).
For the TAU group, the nurses posted the leaflet to the participant. For the intervention group, nurses gave it to the participant on their first intervention visit. We did not otherwise attempt to influence the content of standard care.
Data collection
Assessments were undertaken at the following time points, with a guidance window of plus or minus 2 weeks at follow-up, and baseline interviews and measures of no more than 6 weeks before randomisation (Table 5):
-
medical notes review/check (before randomisation)
-
pre-baseline telephone call (before randomisation)
-
baseline researcher interview (before randomisation)
-
research nurse visit to take physical measures (when necessary, before randomisation)
-
6-month researcher follow-up interview and qualitative interview
-
6-month research nurse/medical follow-up.
Weight was measured at nurse visit using research quality digital scales (FVI016 Marsden digital portable adult scales, Marsden Group, Rotherham, UK), BP was measured with a OMRON M7 BP (OMRON Healthcare Europe B.V., LR Hoofddorp, Nigeria) device and waist-to-hip ratio was measured according to a standard protocol in which the nurses were trained.
| Assessment | Time point | ||||||
|---|---|---|---|---|---|---|---|
| Medical notes review/check | Pre-baseline telephone call | Baseline research interview | Baseline research nurse visit | 6-month medical/research nurse follow-up | 6-month follow-up research interview | GP medical notes follow-up | |
| Eligibility and consent | |||||||
| Presence and role of a supporter and/or research advocate | ✗ | ✗ | |||||
| Mental capacity to consent to RCT | ✗ | ||||||
| Eligibility for RCT | ✗ | ✗ | ✗ | ||||
| Consent for RCT | ✗ | ||||||
| Follow-up data | |||||||
| Negative outcomes | ✗ | ✗ | |||||
| Related and unexpected serious adverse events | ✗ | ||||||
| Hospital attendances | ✗ | ✗ | |||||
| Current physical health state (e.g. HbA1c level, BP, BMI, weight, cholesterol levels, high-density lipoprotein/low-density lipoprotein triglycerides concentrations, urea and electrolytes, waist-to-hip ratio) | ✗ | ✗ | ✗ | ||||
| Thyroid function, height | ✗ | ||||||
| QRISK®2 score, retinal screening, diabetes medication, serum creatinine concentrations, microalbuminuria | ✗ | ||||||
| Details of treatment received | ✗ | ||||||
| Adherence to the intervention | ✗ | ||||||
| Prescribed diabetes regime (diet, exercise) | ✗ | ||||||
| Resource use: service and hospital usage | ✗ | ||||||
| Questionnaires (completed at researcher visit) | |||||||
| Health economics questionnaire to cover health and social care costs, participant and supporter expenses and productivity costs | ✗ | ✗ | |||||
| Participant mood (via the PHQ-2) | ✗ | ✗ | |||||
| Health-related quality of life (via the EQ-5D)175 | ✗ | ✗ | |||||
Nurse visits
Initially, to increase efficiency, we aimed to determine whether or not we could obtain certain outcomes (HbA1c levels, BMI) from GP records of diabetes clinical QOF reviews. However, as a result of our experience during case finding, it became clear that such an approach was not feasible because we could not obtain information in a timely and comprehensive way, and we therefore obtained research outcomes ourselves unless participants refused to have measures taken by the research nurses but agreed to us reviewing their medical notes.
Participants were, therefore, asked to undergo a blood test with the research nurse to establish physical measures at baseline and at follow-up. All participants were offered anaesthetic cream for the blood test. Only if a participant declined the physical measures or a blood test did the nurse then attempt to obtain the results from GP records. Whenever possible, the nurse undertaking the follow-up visit was blind to treatment allocation and was one of two nurses who had not seen the participant at baseline, been told their participant’s allocation or delivered the SSM.
Researcher baseline and follow-up questionnaire
For participant- and supporter-completed data, we collected information on supporter details, exercise, service use, medications, employment, accommodation, food consumed, EQ-5D and mood. For the supporter, we collected information on supporter details, service use, employment and EQ-5D. Each question of the EQ-5D was also printed on a separate A4 laminated sheet as an interview aid. We made two changes to the measures we used on the basis of the case-finding study. First, we dropped the REAP because our participants were not able to provide the information necessary to complete it. Second, we decided to use a standardised measure of mood [Patient Health Questionnaire-2 (PHQ-2)],177 as our participants clearly recognised and understood the questions we asked about distress and indicated that it was an important topic to them.
We also recorded participant-reported resource use questions for the questionnaire, based on findings from the literature review and expert input from the study team. The breadth of these questionnaires was wider than the GP-completed questionnaires (reported in Review of general practitioner medical notes) and took a societal perspective.
The interviewers recorded their perceptions of how easy they thought the questions were for the participant to answer.
The feasibility of collecting information on out-of-pocket expenses of supporters relating to travel for participants’ health-care appointments was assessed. Given the expected close involvement of supporters in the self-management process and potential impact on their health-related quality of life, the feasibility of collecting EQ-5D data for supporters or informal carers was also assessed (paid or formal carers/supporters were excluded).
Review of general practitioner medical notes
A brief health and social care questionnaire was again sent to general practices to complete using participants’ medical records, giving details of participants‘ service and hospital usage for the period 4 months post randomisation. The content remained consistent with that requested in the case-finding study with the addition of two new questions about service use on the advice of experts in the team, a diabetes educational course [Diabetes Education and Self Management for Ongoing and Newly Diagnosed (DESMOND) programme] (www.desmond-project.org.uk/) and a chronic illness course (X-PERT; X-PERT Health, Hebden Bridge, UK). In fact, although both are referral options in the regions where participants were located,67,178 no participant had attended either course.
Most recent test results for QRISK®2 score, serum creatinine level, microalbuminuria concentration and diabetes medications were also requested, as were the dates of the participant’s most recent retinal screen and foot examination.
Other costs
We collected information on the resources associated with development and delivery of the intervention. These were based on data such as administrative and researcher records, as well as a detailed description of the development process including number of sessions, staff involved, nurse training and travel, and cost of materials.
Qualitative data collection
Three types of data were collected: participant and support interview data, researcher journals and nurse journals.
Participant and support interviews
At the RCT follow-up after the heath economic data collection, the researcher asked a series of structured questions about the participant’s life during their involvement in the trial. Researchers were given prompts to act as examples for participants and to encourage more detailed answers. The research team assessed the feasibility of collecting self-reported negative outcomes, significant life changes, changes to mood, hospital admissions and falls through self-reporting.
They also asked questions about research participation and solicited feedback on the participant’s perspective on the trial process, for example initial recruitment, researcher visits, nurse visits and intervention experiences.
When designing the interview schedule, the team consulted with people who work with people with learning disabilities. We were told to try to limit the amount of time spent interviewing, as participants struggle to concentrate for long periods of time. Attempts were made to do this but certain data needed to be collected: in the case-finding studies interviews took 1 hour, on average, and in the RCT they took an average of 50 minutes, including introductions and the consent process. The interview schedule was piloted with two project workers from an advocacy charity who had learning disabilities and diabetes who were not participating in the trial. Adjustments were made based upon their feedback.
Participants (and supporters when present) in both the SSM and TAU arms were further interviewed by the researcher at follow-up to obtain qualitative data. A topic guide was used to ask about major life events since the baseline interview, their experiences of being in the research project, any lifestyle changes (diet or exercise) and their views on the TAU booklet. For participants in the SSM arm (and supporters when present), the topic guide also included negative experiences of the self-management intervention to help identify elements of the intervention that were popular and easy to adhere to (facilitators of change) and those that were difficult, unpopular or insufficiently supported (barriers to change).
Researcher journals
A journal was made by the researcher after each RCT follow-up interview, based upon a topic guide (see Appendix 22). These journals reflected upon the research process, ability of participants to recall information, ability of the participants to understand and communicate answers to the researcher’s questions and the involvement of the supporter (if present). Researchers journaled their observations via digital audio-recorders using a predefined topic guide after the baseline visit and the follow-up visit. All the digital recordings were transcribed.
Nurse journals
The study nurses also made journals based upon a topic guide after every baseline and every intervention session (see Appendix 23). All journals were audio-recorded and transcribed verbatim and the recordings were downloaded directly to a secure university server for transcribing. To prevent researchers from becoming unblinded, none of the research nurse journals was read or downloaded into NVivo version 7 (QSR International, Warrington, UK) by the research team before follow-up was completed.
Outcome measures
As noted in the methods for the observational study, a standardised measure that takes substantial effort to complete could not be justified unless it met the core needs of the project. As the feasibility RCT aimed to inform the design and choice of primary outcome for a definitive trial, we concentrated on the outcomes listed below that relate to feasibility of recruitment and retention, intervention delivery and outcome collection. We decided not to use physical activity as an outcome measure, as self-report is too unreliable as a change measure and objective measures (such as accelerometers) would not be justified for such a secondary outcome in a definitive trial.
Recruitment and retention outcomes
-
The number and proportion of initial referrals found eligible for the case-finding study, screened for eligibility for the RCT, found to be eligible, consented and randomised to the RCT.
-
The demographics of (1) participants referred, registered and found eligible at case finding and (2) participants found eligible, consented to and randomised for the RCT.
-
The proportion of randomised participants who have a supporter.
-
The reasons for non-participation (participant and supporter).
-
The method of identification for randomised participants and supporters.
-
The proportion of randomised RCT participants with all the required baseline and follow-up assessments completed, number of withdrawals from follow-up data collection, reasons for withdrawal and number of losses to follow-up.
-
The length of time taken to conduct trial procedures including time from first contact to randomisation, follow-up completion and receipt of medical records.
Intervention delivery outcomes
-
The proportion of participants randomised to SSM who attended at least one session of SSM with the research nurse and who completed all required sessions, the number of dropouts from SSM and the reasons for dropouts.
-
The agreed method of measuring participant, supporter and research nurse adherence to SSM, including uptake and adherence rates and assessment of the feasibility of using a standardised measure: the measure we developed took the form of a checklist used to record which elements of the self-management intervention had been completed, as identified by an independent researcher from the nurses’ case records and from the follow-up research visit (see Appendix 13).
-
The detailed description of what treatment was delivered and received in each arm, including a comparison of TAU across arms from medical records and an assessment of the feasibility of collecting data on TAU pathways.
-
The assessment of the feasibility of collecting data on adherence to SSM and the standard leaflet.
-
The preliminary assessment of the acceptability of the intervention, including negative outcomes, hospital attendances and relevant unexpected severe adverse events.
Outcome data collection
Feasibility outcomes
-
The assessment of the feasibility of blinding researchers to treatment allocation.
-
The proportion of participants who refuse physical measures with available and timely QOF data at baseline and (if necessary) at their follow-up assessments.
-
The completion rates for other data collected, including assessment of the feasibility of collecting health economics data (e.g. participant EQ-5D, NHS and supporter costs or medication use).
-
The missing item-level data on self-reported questionnaires.
Statistical outcomes
-
The distribution and variability of candidate primary and secondary outcomes at 4–6 months post randomisation (e.g. HbA1c levels, BP, BMI, waist-to-hip ratio, EQ-5D, vascular/microvascular risk markers, marker of microvascular disease or participant mood), and assessment for the potential for outcomes to show an effect in a definitive trial, that is, their sensitivity to change.
-
The proportion of participants classed as abnormal on standard criteria for medical markers.
Qualitative outcomes
-
The positive and negative experiences of implementing self-management.
-
The perceptions of the standard leaflet and experience of being in the TAU arm.
Statistical methods
Sample size
To address the feasibility objectives, we planned to recruit 80 participants in total, randomised equally between intervention and control arms, in order to obtain follow-up data on at least 30 participants per arm, as recommended by Lancaster et al. 179 This assumed loss to follow-up would be no greater than 25% at 6 months. As this study was designed to assess the feasibility of conducting a definitive trial, a formal power calculation was not appropriate because effectiveness was not being evaluated. Part of the rationale was to gather data to inform the design of a definitive trial, so estimates of non-adherence and loss to follow-up rates in this patient group were intended to inform power calculations for the definitive trial.
Statistical analysis
Populations
Participants considered eligible for the feasibility RCT consisted of all randomised consenting participants fulfilling all of the inclusion criteria and none of the exclusion criteria. The intention-to-treat population consisted of all randomised participants, regardless of their adherence to the study protocol or eligibility, and participants were grouped according to the treatment to which they were randomised. The screening population consisted of all participants in the case-finding eligible population who agreed to be contacted in the future.
Analyses
All data analyses and summaries were performed using SAS version 9.4. As this was a feasibility study, data were summarised using descriptive summary statistics and estimation (with 95% CIs) only, with no formal statistical testing carried out on any of the outcomes.
The analysis summarised outcomes, presenting descriptive statistics, according to the intention-to-treat population unless otherwise stated. Percentages were calculated using the total number of participants with available data as the denominator, and the number of participants excluded is presented alongside each summary.
The feasibility, and success, of the recruitment strategy was evaluated by summarising the screening, eligibility, consent and randomisation processes, and included the numbers of participants involved at each stage and reasons for non-participation. A recruitment flow diagram depicted the course of participants throughout the recruitment process from case-finding referral through to randomisation in the feasibility RCT, and a recruitment graph presents monthly and cumulative recruitment (see Figures 2 and 3).
In addition to the outcomes listed, the following were also summarised overall and by trial arm: (1) safety, including related unexpected serious adverse events, unplanned hospital admissions, deaths and negative life events; (2) responses to the researcher structured follow-up interview; and (3) additional participant information and living arrangements.
Statistical outcomes
To inform the sample size calculation for the definitive trial, we report summary statistics (including variability), and corresponding 95% CIs overall and for each treatment arm for candidate primary and secondary outcomes at baseline and follow-up (e.g. HbA1c levels, BP, BMI, waist-to-hip ratio, EQ-5D, vascular/microvascular risk markers, marker of microvascular disease or participant mood). Histograms and box plots present the distribution of outcomes. Outcomes reported outside the windows around randomisation and follow-up were included in the analysis. Measures of diabetes control were also presented categorically according to abnormal ranges on standard criteria, as in the case-finding study, with additional microvascular markers (kidney function) categorised as follows:
-
Microalbuminuria level: for men ≤ 3.5 mg and > 3.5 mg and for women ≤ 2.5 mg and > 2.5 mg, indicating early signs or presence of kidney disease.
-
Estimated glomerular filtration rate (eGFR): ≥ 90 ml/minute/1.73 m2, normal function; 60–89 ml/minute/1.73 m2, stage 2, mildly reduced function; 30–59 ml/minute/1.73 m2, stage 3 moderately reduced function (an important cut-off point for clinical care); and < 30 ml/minute/1.73 m2, stages 4 and 5 (would not expect this value for people living in the community).
The risk of metabolic complications according to waist circumference and waist-to-hip ratio was categorised as follows:
-
Waist circumference: not at increased risk, men ≤ 94 cm and women ≤ 80 cm; increased risk, men 94–102 cm and women 80–88 cm; and substantially increased risk, men > 102 cm and women > 88 cm. 180
-
Waist-to-hip ratio: not at increased risk, men < 0.90 and women < 0.85; and substantially increased risk, men ≥ 0.90 and women ≥ 0.85.
Sensitivity of change for candidate primary outcomes was assessed based on within-person change in HbA1c levels and BMI between baseline and follow-up. Summary statistics, effect sizes and histograms depicting the distribution change are presented separately by trial arm.
Exploratory cost-effectiveness analysis
An exploratory cost-effectiveness analysis was undertaken. Given that this is a feasibility study, the purpose of the analysis was not to assess cost-effectiveness but rather to explore the uncertainty around the results through sensitivity analysis and a cost-effectiveness acceptability curve (CEAC). Unit costs were assigned to the data in order to estimate the average cost of health and social care service use and diabetes medications over a 12-month period for the sample. Unit costs were obtained from national sources, including the PSSRU’s Unit Costs of Health and Social Care 2014,171 NHS reference cost database173 and the BNF. 172 The cost analysis was based on complete cases only (i.e. forms that had no missing or unknown data).
Descriptive statistics of costs and EQ-5D scores were calculated by trial arm. Similar to the statistical analysis methods, as this was a case-finding study for a feasibility study, costs and EQ-5D scores were not subjected to formal statistical testing.
The exploratory cost-effectiveness analysis was undertaken from the perspective of the NHS and personal social services and was carried out using health-care resource use collected from general practices and the EQ-5D responses from participants at baseline and follow-up. The evaluation adheres to the methods guidance produced by NICE. 181 The trial analysis was a cost–utility analysis comparing usual care (TAU) with SSM plus usual care (SSM) over the 4- to 6-month trial duration. No discounting was necessary given the time period of data collection (< 1 year). QALYs were derived using participant EQ-5D questionnaire responses, and cost-effectiveness is assessed as the incremental cost per incremental QALY. A price year of 2014 was used in the analysis.
Incremental cost-effectiveness ratios (ICERs) were calculated. 182 An ICER represents the additional cost per QALY gained for each intervention compared with the next best alternative (i.e. SSM vs. TAU) and is calculated as follows:
As a guideline rule, we used the NICE implicit willingness-to-pay threshold of £20,000–30,000 per QALY to determine cost-effectiveness. The intervention is judged to be cost-effective using the lower limit of the NICE acceptance threshold of £20,000 per incremental QALY (λ = £20,000) as the decision rule for the analysis.
Sensitivity analyses
Non-parametric bootstrapping was used to determine the level of sampling uncertainty around the ICER. The bootstrap approach is a non-parametric method that considers the original sample as though it was the population and draws multiple random samples from the original sample by generating 10,000 estimates of incremental costs and benefits from the trial results. The results are presented using cost-effectiveness scatterplots to illustrate the uncertainty surrounding the cost-effectiveness estimates. On the cost-effectiveness plane (which plots incremental QALYs against incremental costs), a result is considered cost-effective if it falls on or below the given cost-effectiveness threshold. The CEAC is derived by calculating the proportion of bootstrapped estimates that are cost-effective across a range of willingness-to-pay thresholds, to show the probability that SSM plus TAU is cost-effective across different threshold values. 183,184
In line with NICE-recommended practice, any cost-effectiveness analysis will require QALYs and utility weights for each health state observed in a trial population. A 2001 HTA report,170 which considered general health status measures for cognitively impaired populations, found the EQ-5D to be superior compared with other preference-based measures of health. We therefore explored the feasibility of use of the EQ-5D for participants and for supporters or informal carers (not including ‘paid’ supporters) and of obtaining details of resources used from GPs.
Qualitative data analysis
The transcripts of researcher interviews were coded verbatim and data were managed within NVivo 10. Data were analysed using a framework approach,185–187 which is an analytic approach that supports the interpretation of qualitative data by following a clear and documented process. It was developed for applied policy-relevant health research, for cases in which the research objectives are already set and answers to specific questions are required. 188 The process involves familiarisation with the data, identifying a framework based upon the aims and objectives, indexing by applying the framework to the data, charting the findings into key themes and summarising and mapping to further synthesise the data. 185 Louise Bryant led the analysis and with Jacqueline Birtwistle created each of the frameworks for the three types of data. Two researchers, Jacqueline Birtwistle and Neda Mahmaodi, indexed a portion each and both indexed the same sample of six transcripts to the framework. Jacqueline Birtwistle and Louise Bryant conducted charting and producing summarised mapping of the data. Amy Russell reviewed all coding and synthesised the summaries in consultation with Louise Bryant. The three types of data were used to lend differing perspectives on the research questions and areas of disagreement, as well as agreement, were reflected upon.
Summary narratives for each chart within each area of interest, for example intervention, standard leaflet and experiences of being in a trial, were created. These, together with the analysis of the nurse and researcher journals, enabled a qualitative interpretation of the participant and nurse experience.
Patient and public involvement
The team continued to work closely with our third-sector partners and the local learning disability information design service during the development of the RCT.
Easy on the i worked with the research team to create accessible information and consent materials for the trial. They also helped create some of the documents used in the intervention with their service user panel. Easy on the i worked closely with us in the decision-making process for the standard leaflet. This was designed by another local learning disability information design organisation called CHANGE. Research materials were also reviewed by our partner, People in Action, a third-sector organisation for people with a learning disability and their families based in Leeds. Each consenting participant was offered contact with a research advocate provided by People in Action.
The role of the research advocate was to ensure that the participant (and their supporter if relevant) had access to someone independent of the project who could provide advice or support with decisions, for example about continuing with or leaving the trial. At the RCT baseline interview, participants were given a contact telephone number for the advocacy service and asked if they would like the researcher to initiate an initial contact from a research advocate. The chief executive of People in Action continued to contribute to the project management group meetings in an advisory capacity.
Chapter 5 Results for case finding and description of the sample
The case-finding and descriptive study results presented here involve findings on:
-
referral, recruitment and eligibility rates
-
an evaluation of our case-finding methods
-
characterisation of the eligible population according to (1) demographics, (2) participant-reported measures of health and health care, (3) health from medical records including measures of diabetes control and (4) health care and other costs.
Referrals, recruitment and eligibility
In total, 365 referrals for 325 individuals were made over 22 months between June 2013 and March 2015, with 172 participants (53%) registered. Figure 2 presents a flow diagram depicting the study conduct and the number of participants involved in each stage of the referral and recruitment process. Figure 3 shows the number of referrals, researcher visits and registrations of participants to the study on a monthly and cumulative basis.
FIGURE 2.
Assessments at baseline and follow-up in the observational study. LD, learning disability. Reproduced with permission from House et al. 176 Randomized controlled feasibility trial of supported self-management in adults with Type 2 diabetes mellitus and an intellectual disability: OK Diabetes, Diabetic Medicine, John Wiley & Sons. © 2018 The Authors. Diabetic Medicine published by John Wiley & Sons Ltd on behalf of Diabetes UK; and Bryant et al. 189 Characterizing adults with Type 2 diabetes mellitus and intellectual disability: outcomes of a case-finding study, Diabetic Medicine, John Wiley & Sons. © 2017 The Authors Diabetic Medicine published by John Wiley & Sons Ltd on behalf of Diabetes UK.
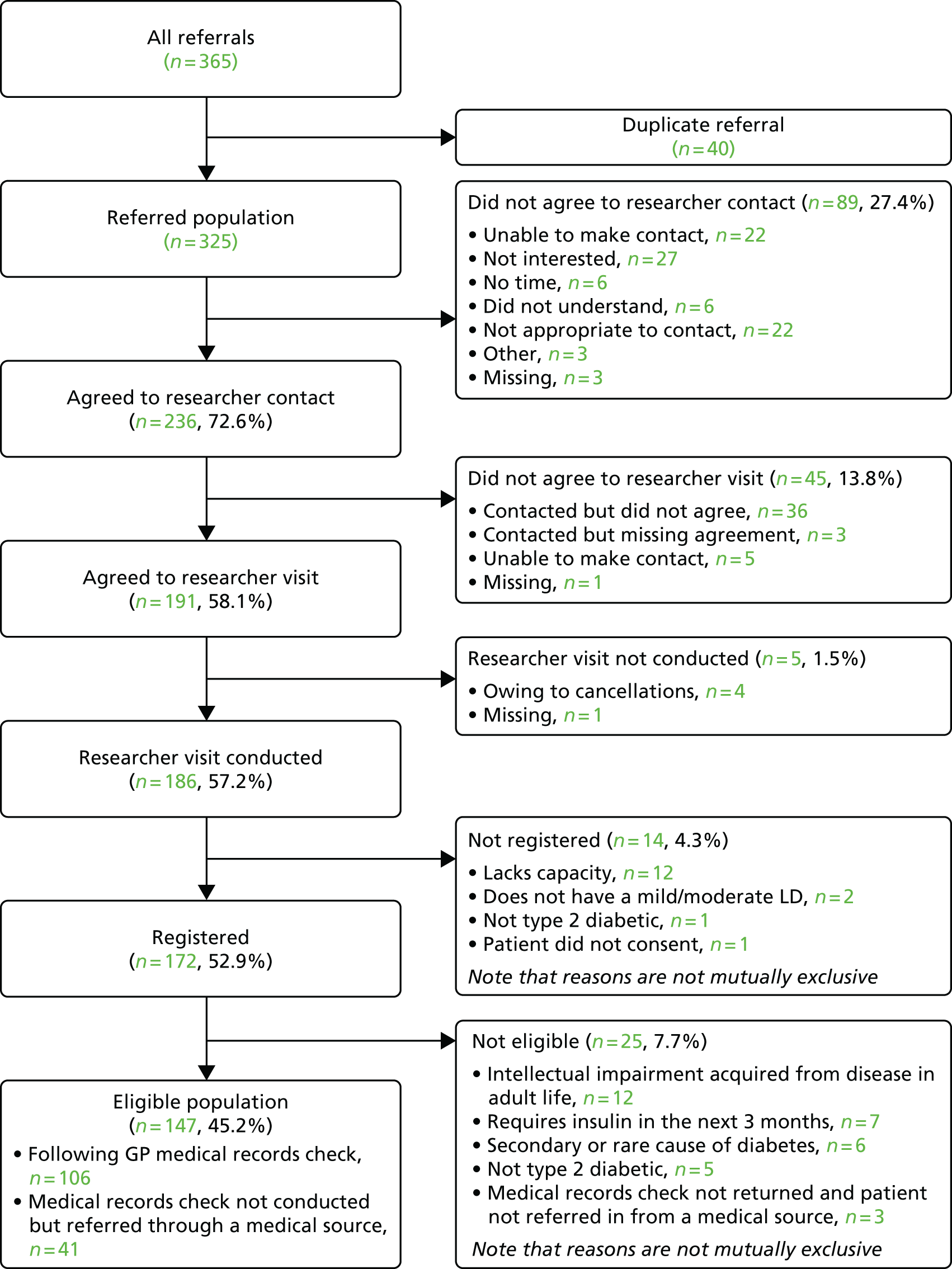
FIGURE 3.
Number of referrals, researcher visits, and registrations per month.
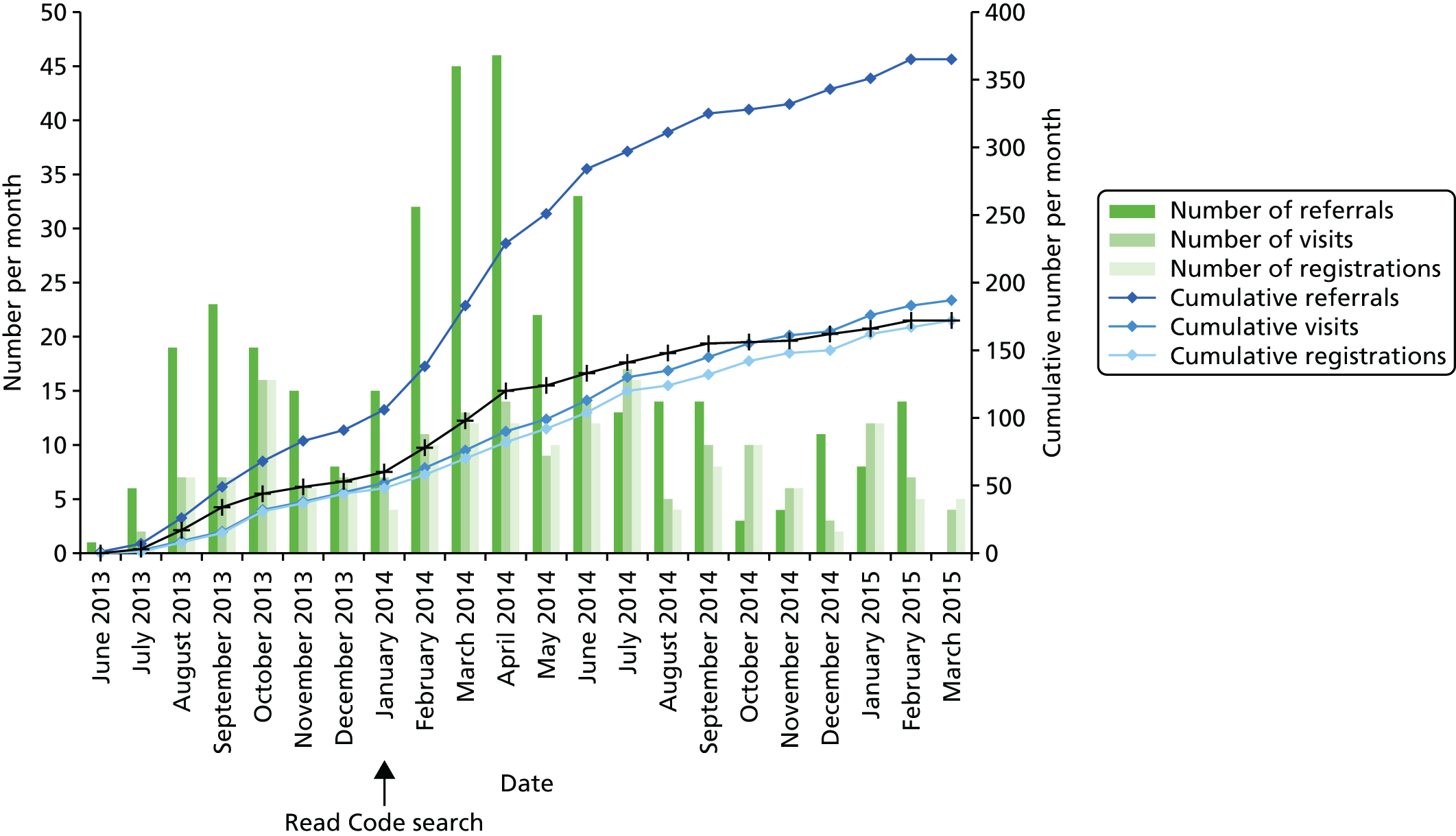
Of the 325 individuals referred, 147 (45%, 95% CI 40% to 51%) were found to be eligible (Table 6) and not meeting the inclusion criteria (or meeting the exclusion criteria) accounted for only 39 (22%) of the ineligible referrals. Of these, the main reason for ineligibility was lack of capacity. Only a few people were found at research assessment to be ineligible as a result of the use of insulin, as most insulin users were excluded by GPs as part of their initial referral process. The main reason that referred individuals were not included in the study was that they, or their supporters, actively chose not to participate in the research once they were approached (n = 69, 39%). The precise reasons for this are unknown, although we noted that recruitment carried out by GP staff was less successful than that carried out by the research nurses using referral lists. It may have been that GP staff were less able or less confident than the nurses in discussing the project with potential participants or their supporters. It is also likely that choosing not to participate was as a result of some supporters feeling that the person with a learning disability was unable to take on additional activities, and perhaps thinking that they would struggle to take part. People with a learning disability may also have been (understandably) wary of letting a stranger into their homes.
| Eligibility | City, n (%) | Total, n (%) | ||
|---|---|---|---|---|
| Leeds | Bradford | Wakefield | ||
| Eligible population | ||||
| Yes | 64 (48.5) | 47 (39.8) | 36 (48.0) | 147 (45.2)a |
| No | 68 (51.5) | 71 (60.2) | 39 (52.0) | 178 (54.8) |
| Total | 132 (100.0) | 118 (100.0) | 75 (100.0) | 325 (100) |
| Ineligible as a result of | ||||
| Research process (see below) | 52 (76.5) | 56 (78.9) | 31 (79.5) | 139 (78.1) |
| Eligibility/exclusion criteria | 16 (23.5) | 15 (21.1) | 8 (20.5) | 39 (21.9) |
| Total | 68 (100.0) | 71 (100.0) | 39 (100.0) | 178 (100) |
| Further reason not eligible (not mutually exclusive) | ||||
| As a result of research process | ||||
| Did not agree to researcher contact | 27 (39.7) | 41 (57.7) | 21 (53.8) | 89 (50.0) |
| Did not agree to see the researcher | 24 (35.3) | 14 (19.7) | 7 (17.9) | 45 (25.3) |
| Could not conduct researcher visit | 1 (1.5) | 1 (1.4) | 3 (7.7) | 5 (2.8) |
| As a result of eligibility criteria | ||||
| Does not have a mild/moderate learning disability | 0 (0.0) | 1 (1.4) | 1 (2.6) | 2 (1.1) |
| Lacks capacity | 4 (5.9) | 4 (5.6) | 2 (5.1) | 10 (5.6) |
| Participant did not consent | 1 (1.5) | 0 (0.0) | 0 (0.0) | 1 (0.6) |
| Learning problems acquired from disease in adult life | 3 (4.4) | 7 (9.9) | 2 (5.1) | 12 (6.7) |
| Not type 2 diabetic | 1 (1.5) | 2 (2.8) | 3 (7.7) | 6 (3.4) |
| Secondary or rare cause of diabetes | 2 (2.9) | 3 (4.2) | 1 (2.6) | 6 (3.4) |
| On insulin or will require it in next 3 months | 4 (5.9) | 1 (1.4) | 2 (5.1) | 7 (3.9) |
| Medical records check not returned and participant not referred from a medical source | 3 (4.4) | 0 (0.0) | 0 (0.0) | 3 (1.7) |
| Total not eligible | 68 (100.0) | 71 (100.0) | 39 (100.0) | 178 (100) |
Rates of choosing not to participate were higher in the potential participants from non-white ethnic minority backgrounds (although the numbers in each group are small; Table 7). We recognised this problem early on in our recruitment and started a more targeted approach, for example through community associations for the South Asian community. We also translated our materials into Urdu and a dialect-speaking researcher visited places of worship to talk about the research. One explanation for this lower participation rate may be cultural reasons associated with the protection of people with a learning disability. It could also be because of non-familiarity with terms such as learning disability in some groups, particularly in South Asian communities.
| Agreement | Ethnicity, n (%) | Missing, n (%) | Total, n (%) | ||||
|---|---|---|---|---|---|---|---|
| White | Mixed | Asian | Black | Other ethnic group | |||
| Agreed to researcher contact | |||||||
| Yes | 191 (76.7) | 4 (66.7) | 29 (64.4) | 1 (50.0) | 1 (100) | 10 (45.5) | 236 (72.6) |
| No | 58 (23.3) | 2 (33.3) | 16 (35.6) | 1 (50.0) | 0 (0.0) | 12 (54.5) | 89 (27.4) |
| Total | 249 (100) | 6 (100) | 45 (100) | 2 (100) | 1 (100) | 22 (100) | 325 (100) |
| Agreed to researcher interview | |||||||
| Yes | 161 (84.3) | 3 (75.0) | 21 (72.4) | 1 (100) | 1 (100) | 4 (40.0) | 191 (80.9) |
| No | 30 (15.7) | 1 (25.0) | 8 (27.6) | 0 (0.0) | 0 (0.0) | 6 (60.0) | 45 (19.1) |
| Total | 191 (100) | 4 (100) | 29 (100) | 1 (100) | 1 (100) | 10 (100) | 236 (100) |
| Researcher visit conducted | |||||||
| Yes | 157 (97.5) | 3 (100.0) | 20 (95.2) | 1 (100) | 1 (100) | 4 (100.0) | 186 (97.4) |
| No | 4 (2.5) | 0 (0.0) | 1 (4.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (2.6) |
| Total | 161 (100) | 3 (100) | 21 (100) | 1 (100) | 1 (100) | 4 (100) | 191 (100) |
It is not known how our experience compares with other research in this population. It is perhaps not too surprising, however, that communication through multiple gate-keepers combined with the sensitivities around working with vulnerable adults, especially those in South Asian communities, leads to these types of recruitment difficulties. To improve this situation in a definite RCT, we would recommend using research nurses to contact referred individuals directly and considering employing a research nurse who is able to speak South Asian languages.
Of the original referrals, 249 out of 325 (77%) described themselves as white. Of those with whom a research interview was eventually conducted, 157 out of 186 (84%) described themselves as white (see Table 7), with most of the rest of those participants interviewed (n = 21, 11%) describing themselves as Asian.
Evaluation of the case-finding method to identify participants
In this section we report our findings about who made referrals to the study, how potential participants were identified and the process of contacting potential participants.
Identification
To evaluate our case-finding methods, the role of referrer and method of contact are presented in Table 8 for all referrals and the referred and eligible populations. The most frequent method of identification was through searching a GP learning disability or diabetes register, leading to 119 (33%) referrals overall, whereas searching the e-health record system using Read Codes led to 78 (21%) referrals. The largest proportion of referrals was made by primary care staff, who provided 202 (55%) referrals. The first 50 practices recruited were asked to complete a log that detailed each stage of identification (see Appendix 1) and to record how many potentially eligible people were found by this method and how many were later found to be ineligible. Only 20 logs were returned, and many were incomplete or had duplicated data in multiple fields. It was agreed that it was not possible to include these data in the analysis because of the high numbers of missing data.
| Method of identification | Population, n (%) | ||
|---|---|---|---|
| All referrals (N = 365) | Referred population (N = 325) | Total eligible (N = 147) | |
| Searched GP learning disability/diabetes register | 119 (32.6) | 116 (35.7) | 47 (32.0) |
| Searched whole e-health record system | 78 (21.4) | 65 (20.0) | 29 (19.7) |
| Considered people who I see | 54 (14.8) | 50 (15.4) | 30 (20.4) |
| Reviewed referrals and/or case files | 36 (9.9) | 24 (7.4) | 6 (4.1) |
| Participant heard about the study and asked | 24 (6.6) | 23 (7.1) | 12 (8.2) |
| Consulted with colleagues | 20 (5.5) | 15 (4.6) | 8 (5.4) |
| Remembered the study when I saw them | 17 (4.7) | 15 (4.6) | 7 (4.8) |
| Other | 4 (1.1) | 4 (1.2) | 1 (0.7) |
| Missing | 13 (3.6) | 13 (4.0) | 7 (4.8) |
| Role of referrer | |||
| General practice staff (GP, nurse, practice manager/administrator) | 202 (55.3) | 187 (57.5) | 82 (55.8) |
| Researcher/research nurse/research administrator/CSO | 46 (12.6) | 39 (12.0) | 20 (13.6) |
| Third sector | 38 (10.4) | 36 (11.1) | 20 (13.6) |
| Community learning disability team | 31 (8.5) | 23 (7.1) | 13 (8.8) |
| Local authority | 11 (3.0) | 10 (3.1) | 2 (1.4) |
| Consultant | 3 (0.8) | 3 (0.9) | 1 (0.7) |
| Community diabetes team member | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| Other | 18 (4.9) | 15 (4.6) | 3 (2.0) |
| Missing | 15 (4.1) | 12 (3.7) | 6 (4.1) |
| Method of contact | |||
| Over the telephone | 211 (57.8) | 189 (58.2) | 82 (55.8) |
| In person | 111 (30.4) | 103 (31.7) | 60 (40.8) |
| Unable to make contact – letter sent | 12 (3.3) | 11 (3.4) | 0 (0.0) |
| Unable to make contact – letter not sent | 13 (3.6) | 12 (3.7) | 0 (0.0) |
| Missing | 18 (4.9) | 10 (3.1) | 5 (3.4) |
Once identified, the referrer contacted participants to request consent for researcher contact, with 111 out of 365 (30%) of all referrals and 60 out of 147 (41%) eligible participants being contacted in person, which suggests a slightly increased consent rate when patients were approached in person.
Table 9 provides further information on the method of identification.
| How identified? | Source of referral, n (%) | Total (N = 365) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| General practice staff (N = 202) | Consultant (N = 3) | Community learning disability team (N = 31) | Third sector (N = 38) | Local authority (N = 11) | Researcher/research nurse/research administrator/CSO (N = 46) | Other (N = 19) | Missing (N = 15) | ||
| Remembered the study when I saw them | 14 (6.9) | 1 (33.3) | 1 (3.2) | 1 (2.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 17 (4.7) |
| Considered people who I see | 15 (7.4) | 1 (33.3) | 8 (26) | 17 (45) | 9 (81.8) | 0 (0.0) | 1 (5.3) | 3 (20.0) | 54 (14.8) |
| Consulted with colleagues | 5 (2.5) | 0 (0.0) | 10 (32) | 2 (5.3) | 0 (0.0) | 3 (6.5) | 0 (0.0) | 0 (0.0) | 20 (5.5) |
| Reviewed referrals and/or case files | 0 (0.0) | 1 (33.3) | 8 (26) | 9 (23.7) | 0 (0.0) | 17 (37.0) | 0 (0.0) | 1 (6.7) | 36 (9.9) |
| Searched a learning disability/diabetes register | 101 (50) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (15.2) | 9 (47) | 2 (13.3) | 119 (33) |
| Searched an electronic database | 61 (30.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (10.9) | 6 (32) | 6 (40.0) | 78 (21.4) |
| Person heard about the study and asked | 0 (0.0) | 0 (0.0) | 1 (3.2) | 8 (21) | 0 (0.0) | 11 (23.9) | 3 (16) | 1 (6.7) | 24 (6.6) |
| Other | 1 (0.5) | 0 (0.0) | 1 (3.2) | 0 (0.0) | 2 (18.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (1.1) |
| Missing | 5 (2.5) | 0 (0.0) | 2 (6.5) | 1 (2.6) | 0 (0.0) | 3 (6.5) | 0 (0.0) | 2 (13.3) | 13 (3.6) |
Table 10 presents the QOF registers (excluding diabetes) that eligible participants were found to be on from their GP medical record check. Of the 103 participants with a GP medical record check and complete data relating to QOF registration, 101 (98%) were on at least one non-diabetes QOF register. Of these 101 participants, the most frequent registers were (1) the learning disability register for 91 (90%) participants (despite most of these not being originally referred following a search of that register), (2) the cardiovascular illnesses register for 37 (37%) participants, (3) the obesity register for 33 (33%) participants and (4) the mental health register for 17 (17%) participants.
| Register | Population,a n (%) |
|---|---|
| On a QOF register | |
| Yes | 101 (68.7) |
| No | 2 (1.3) |
| Missing | 44 (30) |
| Which QOF register/s (not mutually exclusive, n = 101) | |
| Learning disability | 91 (90.1) |
| Cardiovascular illnesses | 37 (36.6) |
| Obesity | 33 (32.7) |
| Mental health | 17 (16.8) |
| Hypothyroidism | 12 (11.9) |
| Depression | 11 (10.9) |
| Chronic kidney disease | 9 (8.9) |
| Epilepsy | 9 (8.9) |
| Asthma | 8 (7.9) |
| Chronic obstructive pulmonary disease | 5 (5.0) |
| Cancer | 4 (4.0) |
| Stroke | 2 (2.0) |
| Otherb | 10 (9.9) |
Recruitment and resources
The number of contact attempts made by the researcher in order to arrange a face-to-face researcher visit for the 236 participants who agreed to researcher contact are presented in Table 11.
| Contact attempts | Type of contact | All contacts (N = 236) | ||||
|---|---|---|---|---|---|---|
| Telephone (N = 236) | E-mail (N = 236) | Text (N = 236) | Letter (N = 236) | Missing (N = 236) | ||
| n | 235 | 235 | 235 | 235 | 235 | 235 |
| n missing | 1 | 1 | 1 | 1 | 1 | 1 |
| Overall number of contacts reported | 713 | 37 | 6 | 16 | 43 | 815 |
| Median number of contacts | 2.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 |
| Range of contacts | 0–23 | 0–3 | 0–2 | 0–2 | 0–4 | 1a–27 |
| At least one contact made, n (%) | ||||||
| Yes | 225 (95) | 28 (11.9) | 5 (2.1) | 14 (5.9) | 29 (12.3) | 235 (99) |
| No | 10 (4.2) | 207 (87) | 230 (97) | 221 (94) | 206 (87) | 0 (0.0) |
| Missing | 1 (0.4) | 1 (0.4) | 1 (0.4) | 1 (0.4) | 1 (0.4) | 1 (0.4) |
A total of 815 contact attempts were reported overall, with a mean of 3.5 contacts and a median of two contacts per participant and ranging from a single contact to 27 contacts. The most frequent method used by the researchers to contact participants was the telephone, accounting for 713 contact attempts. The median time from first to last contact was 3 days (range 1–437 days) in participants agreeing to the researcher visit and 16 days (range 1–349 days) in participants who did not agree to a researcher visit. Once participants had agreed to a researcher visit, the majority (183/186, 98%) required only a single visit to introduce the study, obtain consent and conduct the researcher interview. Three participants required a second visit to complete this interview. The average duration of each researcher visit was 1 hour; however, visits ranged from 15 minutes to just over 3 hours.
Table 12 summarises the involvement of a supporter throughout the recruitment process: referrer contact; provision of contact details to the researcher by the referrer; researcher contact; presence during researcher visits; and informed consent to the study. Of all 325 referred participants, a supporter was contacted by the referrer to obtain consent for researcher contact for 124 (38%) participants. This was either as the sole contact or alongside contact with the participant. The family members of a further 44 (14%) participants were contacted. For the majority of the 236 participants who agreed to researcher contact, the participant’s details, as opposed to the supporter’s, were provided to the researcher by the referrer (n = 143, 61%). Of the 186 participants receiving a researcher visit, a supporter was present in at least one visit for 128 (69%) participants, and of the 172 consenting registered participants, 99 (58%) also had a consenting supporter.
| Nature of involvement | Total, n (%) |
|---|---|
| Who was contacted by the referrer | |
| Participant | 125 (38.5) |
| Participant’s supporter | 86 (26.5) |
| Family member | 34 (10.5) |
| Participant and participant’s supporter | 33 (10.2) |
| Participant and family member | 8 (2.5) |
| Participant‘s supporter and family member | 2 (0.6) |
| Participant, participant‘s supporter and family member | 3 (0.9) |
| Verbal contact not made | 23 (7.1) |
| Missing | 11 (3.4) |
| Total referred population | 325 (100) |
| Whose details were provided to the research team (N = 236) | |
| Participant | 143 (60.6) |
| Supporter | 22 (9.3) |
| Family member | 22 (9.3) |
| Missing | 49 (20.8) |
| Supporter contacted by the researcher (N = 236) | |
| Yes | |
| Supporter contacted | 159 (67.4) |
| No | |
| Participant only contacted | 57 (24.2) |
| Contact made but missing with who | 13 (5.5) |
| No contact possible | 6 (2.5) |
| Missing | 1 (0.4) |
| Supporter present in at least one researcher visit? (N = 186) | |
| Yes | 128 (68.8) |
| No | 58 (31.2) |
| Supporter informed consent | |
| Yes | 99 (57.6) |
| No | 18 (10.5) |
| N/A | 55 (32.0) |
| Total registered | 172 (100) |
Establishing eligibility
Uncertainty during eligibility assessments was a common cause of extra work in recruitment. Uncertainty as a result of ambiguity or missing information in the referrals booklet was common, in 46 (13%) of all 365 referrals, with uncertainty on each criterion as follows: if the participant (1) had a mild or moderate learning disability in 22 (6%) referrals; (2) was taking insulin in 18 (5%) referrals; (3) had type 2 diabetes in six (2%) referrals; and (4) was aged ≥ 18 years in two (0.5%) referrals.
Furthermore, the researcher was uncertain whether or not the participant had capacity to take part in the study at the researcher visit for 3 out of 186 (2%) participants. At registration (172 participants), there was uncertainty about whether or not eight participants (5%) had type 2 diabetes and whether or not 11 participants (6%) required insulin.
Table 13 outlines communication problems for referred participants identified by the referrer prior to passing on contact details to the researcher. Of the referred population, 36 (11%) were reported to have communication problems, of which 13 (36%) were as a result of deafness and 12 (33%) were because of a speech difficulty. This information was missing for 70 (22%) of the referred participants when the participant had not agreed to researcher contact.
| Communication | Population, n (%) | |
|---|---|---|
| Referred (N = 325) | Eligible (N = 147) | |
| Communication problems | ||
| Yes | 36 (11.1) | 15 (10.2) |
| No | 219 (67.4) | 129 (87.8) |
| Missing | 70 (21.5) | 3 (2.0) |
| Type of communication problema | ||
| Behavioural or emotional difficulties | 5 (13.9) | 0 (0.0) |
| Cannot use the telephone | 5 (13.9) | 2 (13.3) |
| Deafness | 13 (36.1) | 8 (53.3) |
| Needs a translator or signer | 2 (5.6) | 2 (13.3) |
| Other | 1 (2.8) | 0 (0.0) |
| Perceptual/vision difficulties | 4 (11.1) | 0 (0.0) |
| Speech difficulties | 12 (33.3) | 5 (33.3) |
| Total | 36 (100) | 15 (100) |
Of the 236 participants agreeing to contact by the researcher, 106 (45%) stated a preference to be contacted via their home telephone, 50 (21%) via their mobile phone, three (1%) via e-mail and 30 (13%) did not mind. Seventy-nine (54%) eligible participants reported having a mobile phone, with only 16 out of 79 (20%) having a smartphone. Only 33 (22%) eligible participants said that they used the internet, 18 of whom said they accessed the internet with a helper.
Characteristics of the eligible population
Demographics
Participant demographics across the referred and eligible populations were similar (Table 14). The mean age of eligible participants was 54.4 years [standard deviation (SD) 12.8 years], 50% were male and 85% were white.
| Characteristic | Population | |
|---|---|---|
| Referred (N = 325) | Eligible (N = 147) | |
| Age at referral (years) | ||
| n | 303 | 147 |
| n missing | 22 | 0 |
| Mean (SD) | 53.5 (13.81) | 54.4 (12.82) |
| Median (range) | 54.0 (18, 93) | 56.0 (19, 83) |
| Sex, n (%) | ||
| Male | 174 (55.9) | 74 (50.3) |
| Female | 137 (44.1) | 73 (49.7) |
| Missing | 14 | 0 |
| Ethnicity, n (%) | ||
| White | 249 (82.2) | 125 (85.0) |
| Mixed | 6 (2.0) | 3 (2.0) |
| Asian | 45 (14.9) | 17 (11.6) |
| Black | 2 (0.7) | 1 (0.7) |
| Other ethnic group | 1 (0.3) | 1 (0.7) |
| Missing | 22 | 0 |
Participant-reported details during researcher interview
During the researcher interviews, participants were asked a series of questions relating to their living and supporter arrangements, medication, diabetes self-care activities, shopping, cooking, eating habits and diet, use of services for diabetes, comorbidities, satisfaction with lifestyle, desire for change and help looking after their diabetes, difficulties carrying out self-care activities and about their well-being, mood and feelings about having diabetes.
Out of the 147 eligible participants, 103 (71%) reported that they did not live on their own. The most frequent relationship of persons living with the participant were others in a shared house for 41 (43%) participants and immediate family for 37 (39%) participants (Table 15).
| Living arrangements | Population,a n (%) |
|---|---|
| Participant lives with anyone | |
| Yes | 103 (71.0) |
| No | 42 (29.0) |
| Missing | 2 |
| Relationship of persons living with participant (N = 103)b,c | |
| Immediate family | 37 (38.5) |
| Extended family | 4 (4.2) |
| Partner/husband/wife | 9 (9.4) |
| Friend | 3 (3.1) |
| Paid supporter | 6 (6.3) |
| Shared house | 41 (42.7) |
| Other | 3 (3.1) |
| Missing | 7 |
| Type of household | |
| Independent | 55 (37.4) |
| Family home with family present | 23 (15.6) |
| Shared home with housemates and regular support | 6 (4.1) |
| Shared home with staff 24 hours/7 days | 35 (23.8) |
| Supported home with staff present or on call 24 hours/7 days | 26 (17.7) |
| Other | 2 (1.4) |
The majority of participants (n = 130, 88%) reported having a supporter (Table 16), someone in their life who helps them with their diabetes and/or shopping and cooking, with 108 participants (74%) having someone who helped with both diabetes and shopping and cooking. Of the 130 participants with a supporter, 71 (56%) lived with this person, most commonly with paid staff on site.
| Supporter details | Population,a n (%) |
|---|---|
| Participant has someone in their life who helps them with their diabetes and/or shopping and cooking | |
| Yes | 130 (88.4) |
| Both with their diabetes and shopping and cooking | 108 (73.5) |
| Diabetes only | 5 (3.4) |
| Shopping/cooking only | 17 (11.6) |
| No | 17 (11.6) |
| Participant lives with person who helps them (N = 130) | |
| Yes | 71 (55.9) |
| No | 56 (44.1) |
| Missing | 3 |
| Relationship of supporter (not mutually exclusive, N = 130) | |
| Extended family | 5 (4.0) |
| Friend | 3 (2.4) |
| Grown-up child of person | 5 (4.0) |
| Immediate family | 38 (30.2) |
| Paid supporter | 69 (54.8) |
| Partner/husband/wife | 10 (7.9) |
| Missing | 4 |
We wanted to know about participants’ understanding of their diabetes and its implications and we found that most participants had ‘found out’ about their diagnosis more than 2 years ago (n = 111, 79%). Twenty-five participants (18%) found out between 6 months and 2 years ago, and only five (3%) had been diagnosed in the previous 6 months.
Table 17 provides participants self-report of medication versus reports from medical records.
| Medication details | Population,a n (%) |
|---|---|
| Self-report | |
| Participant takes tablets for their diabetes | |
| Yes | 118 (80.3) |
| No | 29 (19.7) |
| Participant uses injections for their diabetes | |
| Yes | 2 (1.7) |
| No | 119 (98.3) |
| Missing | 26 |
| GP medical records | |
| Participant taking medication for their diabetes | |
| Yes | 47 (69.1) |
| No | 21 (30.9) |
| Missing | 79 |
| Which medications (not mutually exclusive, N = 47) | |
| Biguanide (metformin) | 41 (87.2) |
| Sulphonylurea | 22 (46.8) |
| Gliptin (DPP-4 inhibitors) | 6 (12.8) |
| Thiazolidinedione (glitazone) | 5 (10.6) |
| SGLT-2 inhibitors | 2 (4.3) |
| Metformin combinations (metformin and sitagliptin) | 1 (2.1) |
| Statins | 2 (4.3) |
| Antihypertensive agent | 3 (6.4) |
| Other | 1 (2.1) |
| Monotherapy or polytherapy (N = 47) | |
| Polytherapy | 22 (46.8) |
| Monotherapy | 25 (53.2) |
Self-reported diabetes medication use is presented in Table 18. Most participants were helped to remember to take their medication in some way and did not collect their own prescriptions. Of the 118 participants taking tablets for their diabetes, 35 (30%) reported that they missed their medications at ‘less than once a week’ or more frequently.
| Medication details | Population,a n (%) |
|---|---|
| Prescribed frequency of dosage | |
| Once a day | 20 (17.9) |
| Twice a day | 52 (46.4) |
| 3 times a day | 29 (25.9) |
| Once a week | 1 (0.9) |
| Other | 10 (8.9) |
| Missing | 6 |
| Routine for remembering tablets (not mutually exclusive) | |
| Prompted by supporter | 56 (48.7) |
| Have poster/sign to jog memory | 1 (0.9) |
| Set an alarm | 3 (2.6) |
| Receive a reminder off someone else | 2 (1.7) |
| Linked to another activity | 31 (27.0) |
| Dosset box/blister pack | 31 (27.0) |
| Other | 20 (17.4) |
| Missing | 3 |
| Way medication obtained | |
| Prescription delivered | 73 (62.9) |
| Someone else collects prescription | 15 (12.9) |
| I collect | 28 (24.1) |
| Missing | 2 |
When questioned about self-care for diabetes, only a minority were able to report awareness of specific activities (Table 19). For example, only 35 (24%) of eligible participants said that they had been told in the past that they needed to check their blood sugar, but as self-monitoring of blood sugar is no longer recommended for type 2 diabetes, lack of monitoring is not inherently problematic. However, all type 2 diabetes patients will be advised to check their feet, but only 51 out of 145 (35%) or participants said that they knew to check their feet and only 74 out of 154 (51%) knew to have their teeth and gums checked.
| Self-care activity | Population,a n (%) |
|---|---|
| Have you been told to check your blood sugar? (N = 147) | 35 (23.8) |
| If yes, how often do you check? (N = 35) | |
| Everyday | 10 (28.6) |
| Some days | 19 (54.3) |
| Never | 4 (11.4) |
| Don’t know | 2 (5.7) |
| Have you been told to check your feet? (N = 145) | 51 (35.2) |
| If yes, how often do you check? (N = 51) | |
| Everyday | 17 (33.3) |
| Some days | 24 (47.1) |
| Never | 7 (13.7) |
| Don’t know | 3 (5.9) |
| Have you been told to have your teeth and gums checked? (N = 145) | 74 (51.0) |
| Do you go to the dentist? (N = 146) | 89 (61.0) |
Tables 20 and 21 provide details of participants’ shopping, cooking, and eating habits. Most participants ate food cooked at home rather than out or ‘takeaways’ and 95% said they had access to a kitchen. Most felt well enough to shop for food or cook. One-third of participants said that they read nutritional information on food when shopping but only just over half of these people said they understood the information (16% of the total sample).
| Experience of shopping | Population,a n (%) |
|---|---|
| Read nutritional information | |
| Yes | 45 (31.3) |
| No | 89 (61.8) |
| Don’t know | 10 (6.9) |
| Missing | 3 |
| If yes, understand nutritional information (n = 45) | |
| Yes | 24 (54.5) |
| No | 18 (40.9) |
| Don’t know | 2 (4.5) |
| Missing | 1 |
| Do you or your family shop and cook rather than eat out/takeaway? | |
| Yes | 138 (93.9) |
| No | 9 (6.1) |
| Do you usually feel well enough to shop or cook? | |
| Yes | 118 (83.1) |
| No | 24 (16.9) |
| Missing | 5 |
| Diet | Self-assessment, n (%) | ||||
|---|---|---|---|---|---|
| Usually/often | Sometimes | Rarely/never | N/A | Missing | |
| Skip breakfast | 16 (11.0) | 34 (23.4) | 95 (65.5) | 2 | |
| Too many takeaways | 36 (24.8) | 46 (31.7) | 63 (43.4) | 2 | |
| Too little wholegrain | 56 (40.9) | 49 (35.8) | 32 (23.4) | 10 | |
| Too little fruit | 58 (40.0) | 49 (33.8) | 38 (26.2) | 2 | |
| Too few vegetables | 43 (30.1) | 61 (42.7) | 39 (27.3) | 4 | |
| Too little dairy | 19 (13.0) | 43 (29.5) | 84 (57.5) | 1 | |
| Too much meat | 34 (23.4) | 57 (39.3) | 54 (37.2) | 2 | |
| Too much processed meat | 45 (30.8) | 59 (40.4) | 34 (23.3) | 8 (5.5) | 1 |
| Too much fried food | 37 (25.5) | 49 (33.8) | 59 (40.7) | 2 | |
| Too many crisps/nuts | 54 (37.2) | 45 (31.0) | 46 (31.7) | 2 | |
| Too much butter/oil | 58 (40.3) | 65 (45.1) | 21 (14.6) | 3 | |
| Too many sweets | 34 (23.3) | 61 (41.8) | 51 (34.9) | 1 | |
| Too many fizzy drinks (soda) | 23 (15.9) | 26 (17.9) | 96 (66.2) | 2 | |
| Too much diet soda | 38 (26.4) | 39 (27.1) | 67 (46.5) | 3 | |
| Too much alcohol | 4 (2.9) | 13 (9.3) | 123 (87.9) | 7 | |
Most participants were willing to acknowledge problems with their diet. Table 21 shows that over one-third of participants self-reported that they ‘usually or often’ ate too many fats (butter/oil) or high-fat snacks and too little wholegrain and fruit. Ten participants were unable to respond to the item about ‘wholegrain’ consumption, even when given examples, such as brown bread or high-fibre cereals. This suggests a lack of understanding about the existence of wholegrain foods and/or their contribution to a healthy diet. Excessive alcohol consumption was not commonly reported in this sample.
In relation to the use of services for diabetes (Table 22), most participants reported that they saw primary care staff rather than secondary care staff. The majority of participants reported no difficulty attending appointments for their diabetes and 102 (70%) participants said that they had someone who accompanied them to appointments. A health passport scheme was active in each of the areas but rarely used.
| Service use | Population,a n (%) |
|---|---|
| Who do you see about your diabetes (not mutually exclusive) | |
| Doctor’s surgery nurse | 135 (91.8) |
| Retinal screening | 117 (79.6) |
| Doctor’s surgery doctor | 92 (62.6) |
| Podiatrist | 72 (49.0) |
| Hospital doctor/consultant | 22 (15.0) |
| Community learning disability team | 19 (12.9) |
| Hospital nurse | 15 (10.2) |
| Dietitian | 10 (6.8) |
| Do you have difficulty attending appointments? | |
| Yes | 38 (25.9) |
| No | 107 (72.8) |
| Don’t know | 2 (1.4) |
| If yes why? (not mutually exclusive, N = 38) | |
| Behavioural/emotional | 11 (37.9) |
| Forgot | 6 (20.7) |
| Transport | 6 (20.7) |
| Too busy | 5 (17.2) |
| Trouble getting appointments at GPs | 5 (17.2) |
| Practical difficulties | 3 (10.3) |
| Timings | 2 (6.9) |
| Financial | 2 (6.9) |
| Location | 1 (3.4) |
| Other | 3 (10.3) |
| Missing | 9 |
| Do you have anything that tells you about your diabetes that you take with you to appointments? | |
| Yes | 21 (14.4) |
| No | 120 (82.2) |
| Don’t know | 5 (3.4) |
| Missing | 1 |
In addition to diabetes, over three-quarters of participants reported conditions that made them ‘poorly’ and for which most were taking medication (Table 23). The most frequent self-reported comorbidities were mental health problems and depression (56/114, 49%) and cardiovascular illness (46/114, 40%).
| Self-reported comorbidities | Population,a n (%) |
|---|---|
| Other than diabetes, is there anything else that makes you poorly? | |
| Yes | 114 (79.2) |
| No | 25 (17.4) |
| Don’t know | 5 (3.5) |
| Missing | 3 |
| If yes what? (not mutually exclusive, N = 114) | |
| Mental health problems/depression | 56 (49.1) |
| Cardiovascular illness | 46 (40.4) |
| Cholesterol | 18 (15.8) |
| Asthma | 17 (14.9) |
| Epilepsy | 17 (14.9) |
| Musculoskeletal problem | 17 (14.9) |
| Partially sighted | 8 (7.0) |
| Hypothyroidism | 7 (6.1) |
| Chronic obstructive pulmonary disease | 4 (3.5) |
| Stroke | 3 (2.6) |
| Chronic kidney disease | 1 (0.9) |
| Other | 36 (31.6) |
| If yes, do you take medication for that? (N = 114) | |
| Yes | 105 (92.1) |
| No | 8 (7.0) |
| Don’t know | 1 (0.9) |
Participants were further asked about their ‘lifestyle’ – diet, exercise, weight and mobility – how this ‘makes them feel’ and whether or not they ‘wanted help’ in these aspects of their life (Table 24). Most participants said that they did some exercise. Of the 126 participants reporting that they exercised, 38 (31%) said that they exercised every day, 64 (53%) said that they exercised every week and 18 (15%) said that they exercised some weeks. The most common activity was walking (n = 117, 93%), although some people attended exercise classes (n = 13, 10%) or went to the gym (n = 10, 8%). Twelve (10%) said that they went swimming, 8 (6%) said that they cycled and 31 (25%) said that they did other forms of activity. Around 40% of participants said that they had mobility problems. About one-third of respondents said that they did not do enough exercise. Over half of participants said that they were unhappy with their weight and 123 out of 145 participants (85%) said that they would like help looking after their diabetes.
| Satisfaction with general health | Population,a n (%) |
|---|---|
| Do you do any exercise? | |
| Yes | 126 (85.7) |
| No | 21 (14.3) |
| If yes, do you think you do enough exercise? | |
| Yes | 83 (68.0) |
| No | 39 (32.0) |
| Missing | 25 |
| How hard do you find doing exercise? | |
| Hard | 48 (38.7) |
| OK | 34 (27.4) |
| Easy | 42 (33.9) |
| Missing | 23 |
| Do you have any problems walking? | |
| No | 86 (58.9) |
| Some | 56 (38.4) |
| Wheelchair user | 4 (2.7) |
| Missing | 1 |
| How do you feel about what you eat? | |
| Generally happy/wants to keep eating the same | 93 (66.0) |
| Generally unhappy/wants to change what they eat | 48 (34.0) |
| Missing | 6 |
| How do you feel about your weight? | |
| Generally happy/wants to stay the same weight | 62 (43.4) |
| Generally unhappy/wants to change weight | 81 (56.6) |
| Missing | 4 |
Table 25 presents participants’ feelings about the perceived difficulty of carrying out self-care activities. Those activities most frequently perceived as being ‘hard’ were doing exercise and healthy eating. Taking medication and attending medical appointments were considered ‘easy’ more frequently, often because the participant had help doing these tasks.
| Difficulty level | Self-care activity, n (%) | |||||
|---|---|---|---|---|---|---|
| Checking blood sugar | Checking feet | Diabetic appointment | Doing exercise | Healthy eating | Taking medication | |
| Hard | 10 (6.9) | 14 (9.6) | 19 (13.0) | 66 (45.2) | 42 (29.0) | 24 (16.3) |
| Easy | 25 (17.4) | 35 (24.0) | 92 (63.0) | 48 (32.9) | 66 (45.5) | 84 (57.1) |
| Neutral | 2 (1.4) | 7 (4.8) | 26 (17.8) | 27 (18.5) | 32 (22.1) | 21 (14.3) |
| Don’t know | 3 (2.1) | 9 (6.2) | 7 (4.8) | 4 (2.7) | 5 (3.4) | 1 (0.7) |
| N/A | 104 (72.2) | 81 (55.5) | 2 (1.4) | 1 (0.7) | 0 (0.0) | 17 (11.6) |
| Missing | 3 | 1 | 1 | 1 | 2 | 0 |
Participants were asked about their well-being, their mood and how they felt about having diabetes. It can be seen from Table 26 that 46 out of 147 (31%) participants reported that they felt poorly either sometimes or most of the time, 51 out of 141 (36%) were either unhappy or worried about their diabetes, 83 out of 146 (57%) felt miserable and sad at least some of the time and 16 out of 146 (11%) reported not enjoying the things they used to.
| Self-reported well-being, mood and feelings | Population,a n (%) |
|---|---|
| How well do you feel most of the time? | |
| Well and healthy all of the time | 32 (21.8) |
| Well and healthy most of the time | 69 (46.9) |
| Poorly sometimes | 33 (22.4) |
| Poorly most of the time | 13 (8.8) |
| Overall how do you feel about having diabetes? | |
| Generally fine | 53 (37.6) |
| Not bothered | 27 (19.1) |
| Generally unhappy | 26 (18.4) |
| Worried | 25 (17.7) |
| Don’t know | 10 (7.1) |
| Missing | 6 |
| Do you feel miserable and sad? | |
| Yes, definitely | 18 (12.3) |
| Yes, sometimes | 65 (44.5) |
| No, not much | 27 (18.5) |
| No, not at all | 36 (24.7) |
| Missing | 1 |
| Do you still enjoy the things you used to? | |
| Yes, definitely | 92 (63.0) |
| Yes, sometimes | 38 (26.0) |
| No, not much | 11 (7.5) |
| No, not at all | 5 (3.4) |
| Missing | 1 |
Finally, participants were asked how they found the questions asked during the researcher visit. Of 145 participants who responded, over half of participants, 79 (54%), said that they had found the questions easy, 44 (30%) found them OK and 18 (12%) found them hard.
Physical measures obtained from general practitioner records
It was difficult to obtain medical information from GPs to allow decisions about eligibility. We followed each request with two reminders when we had no reply and further prompts if the practice had agreed to provide information but did not do so.
As we wanted to know if the available results were recent enough for us to use them to check against inclusion criteria in a subsequent trial, we also checked the timing of the last result obtained from the GP in relation to registration. As shown in Table 27, a substantial proportion of available results were dated many weeks or months before the date of registration, insufficiently recent for the purposes of a RCT.
| Weeks between assessment and registration | Assessment | ||||
|---|---|---|---|---|---|
| Diabetes review | HbA1c level | BP | BMI | Thyroid function | |
| n | 100 | 102 | 101 | 102 | 93 |
| n missing data | 72 | 70 | 71 | 70 | 79 |
| Mean (SD) | –11.6 (20.9) | –10.7 (19.0) | –8.3 (19.6) | –11.7 (24.0) | –39.0 (81.8) |
| Median | –7.6 | –7.4 | –5.4 | –6.4 | –22.4 |
| Range | –79 to 50 | –79 to 42 | –72 to 50 | –98 to 50 | –698 to 28 |
Complete non-response to requests for medical information on eligible participants was also high, for 45 (31%) participants. Clinical characteristics of the eligible participants, as provided by the GPs of participants from their medical records, are shown in Figures 4 and 5 and Table 28. Tables 29 and 30 show other measures of health state and diabetes control.
FIGURE 4.
Distribution of HbA1c level (mmol/mol).
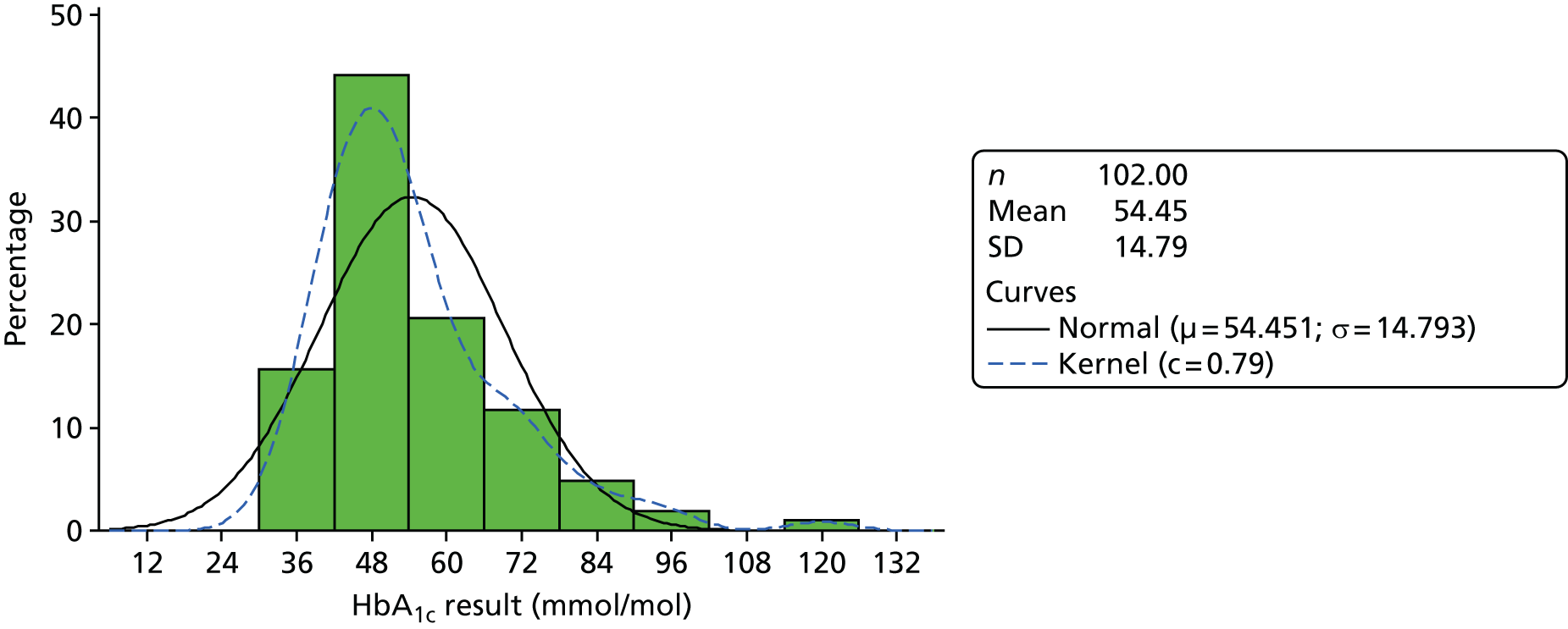
FIGURE 5.
Distribution of BMI.
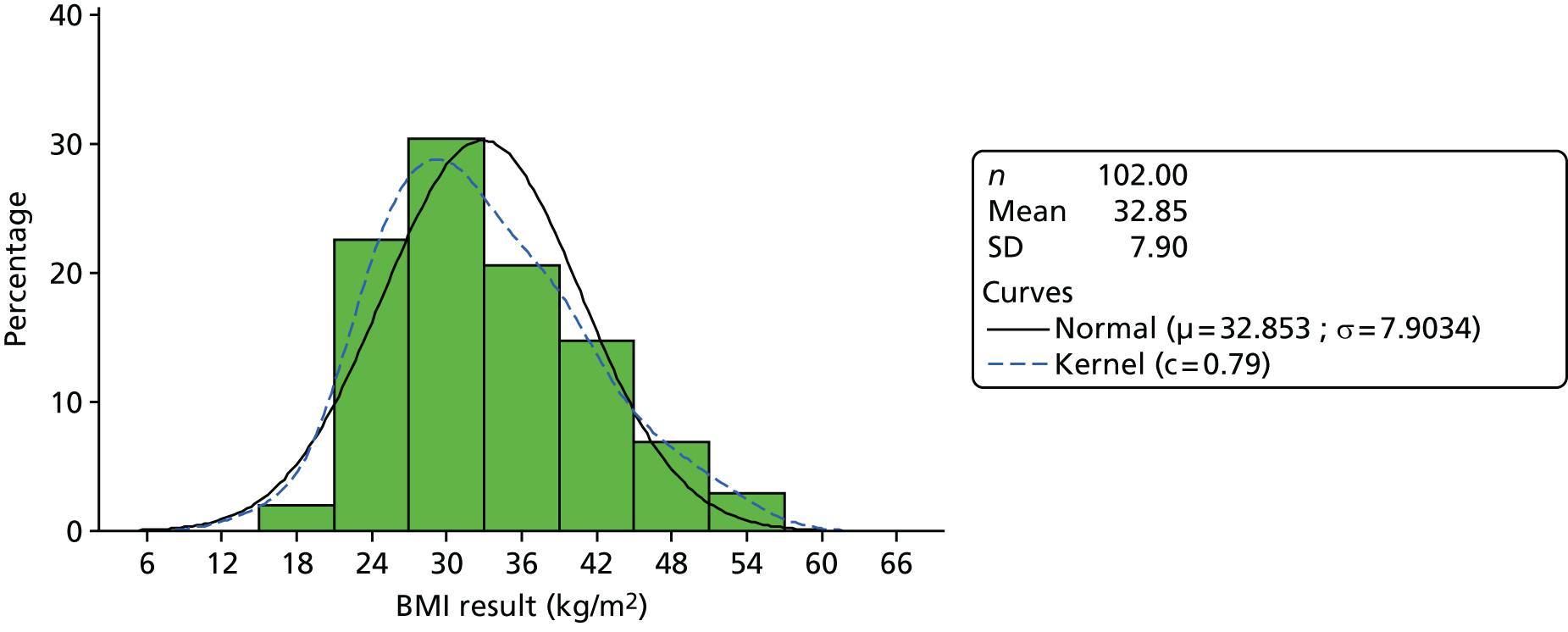
| Measure | |||||
|---|---|---|---|---|---|
| HbA1c | BMI (kg/m2) | BP (mmHg) | |||
| mmol/mol | % | Systolic | Diastolic | ||
| n | 102 | 102 | 102 | 101 | 101 |
| n missing | 45 | 45 | 45 | 46 | 46 |
| Mean (SD) | 54.5 (14.8) | 7.1 (1.4) | 32.9 (7.9) | 124.7 (13.8) | 74.3 (8.4) |
| Median | 51.0 | 6.8 | 31.5 | 122.0 | 74.0 |
| Range | 31–120 | 5.0–13.1 | 16–54 | 96–180 | 55–90 |
| Measure | |||||
|---|---|---|---|---|---|
| TSH level (mIU/l) | QRISK®2 (score %) | Total cholesterol level (mmol/l) | Triglyceride concentration (mmol/l) | Urinary albumin-to-creatinine ratio (mg/mmol) | |
| n | 93 | 72 | 100 | 67 | 93 |
| n missing | 54 | 75 | 47 | 80 | 54 |
| Mean (SD) | 2.3 (2.3) | 19.2 (11.7) | 4.2 (1.1) | 2.2 (1.3) | 5.2 (10.3) |
| Median | 1.7 | 15.9 | 4.1 | 1.9 | 1.4 |
| Range | 0.02–15.80 | 2.8–47.2 | 2.0–7.0 | 0.4–7.0 | 0.1–59.3 |
| Measure | Population,a n (%) |
|---|---|
| HbA1c level (%) (N = 102) | |
| < 6.5 | 36 (35.3) |
| 6.5 to < 7.5 | 38 (37.3) |
| ≥ 7.5 | 28 (27.5) |
| BMI (kg/m2) (N = 102) | |
| < 18.5 (underweight) | 2 (2.0) |
| 18.5–24.9 (normal weight) | 11 (10.8) |
| 25–29.9 (pre-obesity; overweight) | 23 (22.5) |
| 30–34.9 (obese class I) | 27 (26.5) |
| 35–39.9 (obese class II) | 18 (17.6) |
| ≥ 40 (obese class III) | 21 (20.6) |
| Systolic BP (mmHg/kPa) (N = 101) | |
| < 140 (18.7) | 87 (86.1) |
| ≥ 140 (18.7) | 14 (13.9) |
| Diastolic BP (mmHg/kPa) (N = 101) | |
| < 80 (10.7) | 67 (66.3) |
| ≥ 80 (10.7) | 34 (33.7) |
| Total cholesterol level (mmol/l) (N = 100) | |
| < 4 | 43 (43.0) |
| ≥ 4 | 57 (57.0) |
| Triglyceride level (mmol/l) (N = 67) | |
| < 4.5 | 64 (95.5) |
| 4.5–9.9 | 3 (4.5) |
| QRISK®2 score (%) (N = 72) | |
| < 10 | 17 (23.6) |
| 10 to < 20 | 25 (34.7) |
| ≥ 20 | 30 (41.7) |
| Microalbuminuria level (N = 93) | |
| Yes (men > 2.5 mg/mmol; women > 3.5 mg/mmol) | 30 (32.3) |
| No (men ≤ 2.5 mg/mmol; women ≤ 3.5 mg/mmol) | 63 (67.7) |
Participants had a mean HbA1c level of 7.1% (54.5 mmol/mol) with a median level of 6.8% (51 mmol/mol). Approximately one-third, 36 (35%), of participants had a HbA1C level of < 6.5% (48 mmol/mol) and 28 (28%) had HbA1C values that were ≥ 7.5% (58 mmol/mol). BMI ranged from 16 to 54 kg/m2, with a mean of 32 kg/m2 and a median of 31.5 kg/m2. Almost two-thirds of participants, 66 out of 102 (65%), were obese (BMI of > 30 kg/m2) and 21% (21 participants) had a BMI of ≥ 40 kg/m2. There was a positive correlation (Pearson correlation coefficient 0.265; p = 0.0074) between HbA1c level and BMI based on 101 participants with both a BMI and a HbA1c result.
Blood pressure was generally within normal limits. However, 14 out of 101 (14%) participants had a systolic BP of ≥ 140 mmHg, and 34 out of 101 (34%) participants had a diastolic BP of ≥ 80 mmHg.
Total cholesterol level ranged from 2.0 to 7.0 mmol/l, with a mean of 4.2 mmol/l. In 57 out of 100 (57%) participants, their total cholesterol level was ≥ 4 mmol/l. Triglyceride concentration ranged from 0.4 to 7.0 mmol/l, with a mean concentration of 2.2 mmol/l and a median of 1.9 mmol/l. In just 3 out of 67 (4%) participants, their triglyceride concentration was ≥ 4 mmol/l.
Subgroup comparisons for diabetes control
We conducted simple post hoc exploratory subgroup summaries to generate hypotheses for relationships between HbA1c level and BMI and other variables we expected may be associated with adequacy of diabetes management in our population (Table 31). HbA1c levels were slightly lower in those who named a supporter who was involved with their diabetes management, but BMI was more similar in the two groups.
| Factors associated with HbA1c levels and BMI | Measurement | |||||
|---|---|---|---|---|---|---|
| HbA1c level (%) | BMI (kg/m2) | |||||
| Mean (SD) | Median (range) | n missing | Mean (SD) | Median (range) | n missing | |
| Presence of named supporter | ||||||
| Yes (n = 130) | 7.1 (1.37) | 6.8 (5.0–13.1) | 40 | 32.9 (7.98) | 32.0 (16.0–54.0) | 40 |
| No (n = 17) | 7.4 (1.20) | 7.4 (5.9–9.6) | 5 | 32.6 (7.66) | 30.0 (24.0–47.0) | 5 |
| Total (n = 147) | 7.1 (1.35) | 6.8 (5.0–13.1) | 45 | 32.9 (7.90) | 31.5 (16.0–54.0) | 45 |
| Mood: ‘Do you feel miserable or sad?’ | ||||||
| Yes (n = 83) | 7.1 (1.21) | 6.9 (5.3–10.8) | 21 | 34.4 (7.94) | 35.0 (16.0–52.0) | 21 |
| No (n = 63) | 7.1 (1.58) | 6.8 (5.0–13.1) | 24 | 30.4 (7.38) | 30.0 (16.0–54.0) | 24 |
| Missing (n = 1) | 6.6 (.) | 6.6 (6.6–6.6) | 0 | 38.0 | 38.0 (38.0–38.0) | 0 |
| Total (n = 147) | 7.1 (1.35) | 6.8 (5.0–13.1) | 45 | 32.9 (7.90) | 31.5 (16.0–54.0) | 45 |
| Feelings about diabetes: ‘How do you feel about having diabetes?’ | ||||||
| Generally fine/not bothered (n = 80) | 7.3 (1.56) | 7.0 (5.0–13.1) | 29 | 31.2 (8.09) | 30.0 (16.0–54.0) | 29 |
| Generally unhappy/worried (n = 51) | 6.9 (1.04) | 6.6 (5.3–10.2) | 13 | 34.5 (7.70) | 34.0 (23.0–51.0) | 13 |
| Don’t know (n = 10) | 7.1 (1.29) | 6.8 (5.7–9.4) | 3 | 32.4 (4.28) | 33.0 (27.0–37.0) | 3 |
| Missing (n = 6) | 7.0 (1.33) | 6.7 (5.7–9.5) | 0 | 37.2 (8.70) | 39.0 (24.0–46.0) | 0 |
| Total (n = 147) | 7.1 (1.35) | 6.8 (5.0–13.1) | 45 | 32.9 (7.90) | 31.5 (16.0–54.0) | 45 |
| Feelings about eating habits: ‘How do you feel about what you eat?’ | ||||||
| Generally happy/wants to eat the same (n = 93) | 7.2 (1.39) | 6.9 (5.6–13.1) | 30 | 30.4 (7.20) | 30.0 (16.0–54.0) | 30 |
| Generally unhappy/wants to change (n = 48) | 6.9 (1.18) | 6.6 (5.0–10.0) | 14 | 36.6 (7.15) | 36.0 (25.0–51.0) | 15 |
| Missing (n = 6) | 8.0 (1.87) | 7.5 (6.2–10.8) | 1 | 38.0 (9.70) | 40.0 (25.0–52.0) | 0 |
| Total (n = 147) | 7.1 (1.35) | 6.8 (5.0–13.1) | 45 | 32.9 (7.90) | 31.5 (16.0–54.0) | 45 |
| Feelings about weight: ‘How do you feel about your weight?’ | ||||||
| Generally happy/wants to stay the same (n = 62) | 6.8 (1.07) | 6.6 (5.4–10.8) | 19 | 29.5 (6.69) | 28.5 (16.0–54.0) | 20 |
| Generally unhappy/wants to change (n = 81) | 7.2 (1.41) | 6.9 (5.0–13.1) | 26 | 34.8 (7.77) | 34.5 (16.0–51.0) | 25 |
| Missing (n = 4) | 9.1 (1.87) | 9.4 (6.6–10.8) | 0 | 40.0 (9.63) | 39.5 (29.0–52.0) | 0 |
| Total (n = 147) | 7.1 (1.35) | 6.8 (5.0–13.1) | 45 | 32.9 (7.90) | 31.5 (16.0–54.0) | 45 |
| Desire for help with diabetes: ‘Would you like help looking after your diabetes?’ | ||||||
| Yes (n = 123) | 7.1 (1.27) | 6.8 (5.0–10.8) | 34 | 33.3 (8.18) | 32.0 (16.0–54.0) | 34 |
| No (n = 16) | 7.4 (2.30) | 6.6 (5.7–13.1) | 7 | 30.0 (5.66) | 30.0 (23.0–41.0) | 7 |
| Don’t know (n = 6) | 6.9 (0.45) | 6.9 (6.5–7.2) | 4 | 29.0 (1.41) | 29.0 (28.0–30.0) | 4 |
| Missing (n = 2) | 6.6 (0.19) | 6.6 (6.5–6.7) | 0 | 29.5 (4.95) | 29.5 (26.0–33.0) | 0 |
| Total (n = 147) | 7.1 (1.35) | 6.8 (5.0–13.1) | 45 | 32.9 (7.90) | 31.5 (16.0–54.0) | 45 |
| Patient on monotherapy or polytherapy for their diabetes | ||||||
| Not on medication | 6.3 (0.49) | 6.3 (5.3–7.1) | 0 | 30.9 (8.15) | 30.0 (16.0–47.0) | 0 |
| Monotherapy (n = 25) | 6.9 (1.69) | 6.5 (5.0–13.1) | 1 | 32.1 (8.08) | 30.0 (23.0–54.0) | 1 |
| Polytherapy (n = 22) | 7.9 (1.14) | 7.6 (6.2–10.0) | 0 | 34.0 (7.90) | 30.5 (23.0–49.0) | 0 |
| Missing (n = 79) | 7.3 (1.32) | 6.9 (5.7–10.8) | 44 | 33.8 (7.70) | 34.0 (16.0–52.0) | 44 |
| Total (n = 147) | 7.1 (1.35) | 6.8 (5.0–13.1) | 45 | 32.9 (7.90) | 31.5 (16.0–54.0) | 45 |
By contrast, there was a suggestion of an association between BMI, but not HbA1c level, and participants’ mood and self-reported feelings about a number of aspects of their health and lifestyle, with higher BMIs in participants reporting feeling miserable or sad generally, feeling unhappy or worried about diabetes, feeling unhappy about or wanting to change what I eat and feeling unhappy about or wanting to change weight.
Nearly all participants said that they wanted help looking after their diabetes, with those saying ‘no’ having a slightly lower BMI than those saying ‘yes’. Both HbA1c level and BMI were higher in those on polytherapy than in those on monotherapy.
For comparison, the local Commissioning Support Unit provided us with aggregate data for the three cities in our study, based on returns in the QOF scheme (Table 32).
| Measure | Population | |||
|---|---|---|---|---|
| On learning disability register (n = 448) | Not on learning disability register (n = 73,771) | |||
| Not on insulin (n = 348) | On insulin (n = 100) | Not on insulin (n = 53,560) | On insulin (n = 20,211) | |
| Mean HbA1c (mmol/mol), (%) | 55.2 (7.2) | 59.5 (7.6) | 54.6 (7.1) | 67.1 (8.3) |
| Mean BMIa (kg/m2) | 32.0 | 32.4 | 30.7 | 31.6 |
These data indicated several points of interest. First, only 0.6% of the total population was on both the learning disability register and the diabetes register. Second, glycaemic control was similar for those who were and were not on the learning disability register. Third, those on insulin and who were on the learning disability register tended to have better glycaemic control than those on insulin who were not on the learning disability register. Finally, those on the learning disability register tended to be rather heavier than those not (one BMI point is equivalent to 2–3 kg in this population).
Health economics data collection and missing data
Between June 2013 and January 2015, a resource-use questionnaire (form 10, see Appendix 15) for each consented participant was posted to the general practice. The questionnaires asked about the services the participant had used over the previous 12 months. For those eligible participants from whom we received form 10, page 2 was available for 101 out of 147 (69%) participants and page 3 was available for 69 out of 147 (47%) participants.
The data collection process for these forms proved time intensive and difficult. If the form had not been returned after 6 weeks, then the researcher would telephone the general practice to encourage completion; in some instances this required over nine attempts to contact. Other methods of contact proved less rewarding, with e-mails being rarely replied to. When a form had been completed, despite enclosing a prepaid envelope, most practices wanted to use a fax machine to send data to the researcher. This method of data transfer was unreliable and the researcher was left trying to contact the practice manager again (via reception) each time the sending failed.
The identification of services used was challenging for those completing the questionnaire. Often, service use could be accurately counted only by opening every document in a participant’s file to check for referral letters. There were cases when staff at practices refused to complete sections because they were taking too long and they had not realised they were committing to such a long task. Those practice staff who did not want to complete the form were offered the option of a research team member visiting the practice to support them in completing the form; this was accepted on three occasions.
Resource use
General practitioner and practice nurse visits were the most frequent resources used. Almost 93% of the 121 participants visited the GP at least once in a 12-month period and 90% of participants visited the practice nurse. One in three (35%) participants had been to see a podiatrist and over one-quarter (27%) had been to see an ophthalmologist. Over 21% of participants attended the accident and emergency department (A&E) in the preceding 12-month period and 12% had at least one inpatient stay. Other services were much less frequently used (Table 33).
| Service use | Completed form (N = 121), n (%) | Unknown, n (%) | Missing, n (%) | |
|---|---|---|---|---|
| Yes | No | |||
| GP | 112 (92.6) | 7 (5.7) | 2 (1.7) | 0 (0.0) |
| Practice nurse | 109 (90.08) | 10 (8.26) | 1 (0.83) | 1 (0.83) |
| District nurse | 14 (11.57) | 83 (68.6) | 20 (16.53) | 4 (3.31) |
| Diabetic clinic at the hospital | 9 (7.44) | 97 (80.17) | 10 (8.26) | 5 (4.13) |
| Ophthalmologist | 33 (27.27) | 67 (55.37) | 16 (13.22) | 5 (4.13) |
| Podiatrist | 42 (34.71) | 61 (50.41) | 15 (12.40) | 3 (2.48) |
| Dietitian | 12 (9.92) | 86 (71.07) | 20 (16.53) | 3 (2.48) |
| Nephrologist | 3 (2.48) | 99 (81.82) | 13 (10.74) | 6 (4.96) |
| Inpatient stays | 14 (11.57) | 91 (75.21) | 11 (9.09) | 5 (4.13) |
| A&E | 26 (21.49) | 75 (61.98) | 15 (12.40) | 5 (4.13) |
Sixty-one of the questionnaires included medication information, and the details are given in Table 34.
| Diabetes drug type | Taking medication (n = 61) | % taking medication |
|---|---|---|
| Metformin | 48 | 79 |
| Metformin SR (e.g. Glucophage SR, Merck Serono Ltd, Darmstadt, Germany) | 8 | 13 |
| Gliclazide (e.g. Diamicron, Servier Laboratories Ltd, Wexham, UK) | 20 | 33 |
| Glimepiride (e.g. Amaryl, Zentiva, Prague, Czech Republic) | 12 | 20 |
| Sitagliptin (e.g. Januvia, Merck Sharp & Dohme Ltd, Hoddesdon, UK) | 7 | 11 |
| Vildagliptin (e.g. Galvus, Novartis Pharmaceuticals UK Ltd, Camberley, UK) | 1 | 2 |
| Pioglitazone (e.g. Actos, Takeda UK Ltd, High Wycombe, UK) | 6 | 10 |
| Dapagliflozin (e.g. Forxiga, AstraZeneca UK Ltd, Cambridge, UK) | 2 | 3 |
| Metformin in combination with sitagliptin (e.g. Janumet, Merck Sharp & Dohme Ltd, Hoddesdon, UK) | 1 | 2 |
Discussion
Case finding and recruitment
The case-finding study resulted in 365 referrals in 325 patients, with some overlap in referrals from different sources, and identified 147 (95% CI 39.8% to 50.6%) eligible and consenting participants, of whom 132 agreed to be contacted in the future about further research in the study.
Following recruitment, medical record information was requested from GPs to confirm eligibility and attain participants’ most recent routine test results and details of QOF registers, service usage and diabetes medications. This proved to be a major barrier to the study because of difficulties in obtaining this information from GPs. To ensure registered participants were not unnecessarily excluded from the eligible population and allow these participants to proceed through to consideration for the RCT, participants who were referred by their GP, nurse or other medical professional (41 eligible participants, 28%) were confirmed as eligible based on information from the researcher visit.
Participants were largely identified through learning disability or diabetes registers and by primary care staff, but third-sector organisations and community learning disability teams also identified patients. Subsequent researcher contact to arrange face-to face visits to obtain consent was resource intensive with numerous contact attempts, in some cases over a prolonged period of time, with a mean number of 3.5 contacts taking place over a range of 1–437 days. Once they were contacted and had agreed to a face-to-face visit, the majority of participants required only a single visit, with a mean duration of 1 hour and ranging from 15 minutes to 3 hours.
Despite the effort put into recruiting from sources other than GPs, and despite the fact that many of our referrals did not come as a consequence of searching GP learning disability registers, the overwhelming majority of eligible participants were included on a learning disability register. In other words, we did not recruit substantial numbers of participants from the hidden majority of unregistered people with a milder learning disability. It is unclear why, but we suspect the answer is that because this group do not have a diagnosis and do not self-identify as having a learning disability; they are simply unrecognised in any formal process of recruitment, even if their difficulties may be recognised informally when direct contact occurs for whatever reason.
Characterising the sample
The most important finding in relation to living arrangements is that for over half of participants who said they had somebody who supported them, the supporter was a paid member of staff. This matters because, in reality, such support is rarely stable, with a different staff member accompanying participants at health-care and research visits, depending on availability.
Although most participants understood that they had diabetes and knew that they took medication for it, the understanding of dietary and lifestyle management was patchy, with few participants able to interpret nutritional information or knowing what health checks they needed to have. Many respondents had other physical health problems and mental health problems; the latter were especially common. Many respondents said that they felt unhappy, both in general and specifically, because of their diabetes. They reported dissatisfaction with weight and diet and with physical inactivity that they found hard to change. Many agreed that they wanted more help with diabetes and agreed to further research contact.
It proved a challenge to collect routine health data from GP records and, from the absence of recent results in many cases, it is clear that routine data could not be relied upon for outcome measurement in the subsequent feasibility RCT. Data that were available showed reasonable glycaemic control in the sample, and were in line with data for the overall population in the same CCGs provided for us by the Commissioning Support Unit. By comparison, we found high levels of obesity.
The analysis of the results from the GP-collected resource use highlighted the challenge of data collection in primary care. Complete data were obtained for only 33% of eligible participants (48/147). Although 70% of the forms were returned, and the completed forms had few missing items (< 5% missing), there was a high level of service use recorded as unknown. Unsurprisingly, the services provided directly by the GP surgeries had fewer missing or unknown data than those provided by other providers (< 2% and > 12%, respectively). Completion of the resource-use questionnaires was time-consuming and problematic in respect to the intensity of follow-up reminders required to achieve the return rate (including researchers helping with completion in the GP surgeries). Potential reasons for the time-consuming nature of the forms and the missing data were the 12-month time period over which resources were requested and the inability or unwillingness of general practices to complete the time-consuming task.
Implications
What are the implications of these findings for feasibility of a definitive trial? We have shown that it is possible to identify and consent eligible participants from the target population into research. They have important unmet health needs related to type 2 diabetes, especially related to being overweight and inactivity. They have needs for improved self-management in the way of poor knowledge about self-care needs, and many of them express dissatisfaction with their current health and voice a desire to change. We identified two particular challenges. First, despite our best efforts, we identified very few people who were not already known to their GP and other services and were not already on a learning disability register. The hidden majority of people with milder disability remained hidden to us, and yet they may be the population that could benefit most from support in self-management. Second, we were unable to use routinely available primary care data either to confirm eligibility for a RCT or to obtain the comprehensive outcomes necessary for a trial.
Chapter 6 Results: randomised controlled feasibility trial
The randomised controlled feasibility trial results presented here are:
-
recruitment and retention including withdrawals, follow-up, supporter consent and participant demographics
-
intervention delivery, adherence and fidelity
-
participant and supporter feedback
-
success of our outcome data collection
-
statistics on outcomes for variability and estimates of physical measures and mood
-
safety including hospital visits and major life events.
Recruitment and retention
Of the 132 initial eligible participants from the case-finding study who agreed to further research contact, 127 (96%) were contacted to determine if they would be willing to meet with the study researcher again to introduce the trial, obtain consent and establish eligibility for the RCT (Figure 6). Over three-quarters (98/127) of participants received a visit from the researcher, and the majority (92/98) consented to take part in the trial. Following a nurse visit to confirm eligibility (based on HbA1c levels, BMI and level of physical activity) and obtain baseline physical measures, or the acquisition of in-date GP medical records, 82 out of 92 (89%) of these participants went on to be randomised to the trial, with 41 (50%) randomised to receive the SSM intervention and 41 (50%) randomised to receive TAU. Figure 7 presents monthly and cumulative recruitment to the RCT from September 2014 to April 2015.
FIGURE 6.
Randomised controlled trial CONSORT flow diagram. Reproduced with permission from House et al. 176 Randomized controlled feasibility trial of supported self-management in adults with Type 2 diabetes mellitus and an intellectual disability: OK Diabetes, Diabetic Medicine, John Wiley & Sons. © 2018 The Authors. Diabetic Medicine published by John Wiley & Sons Ltd on behalf of Diabetes UK.

FIGURE 7.
Randomised controlled trial recruitment chart.
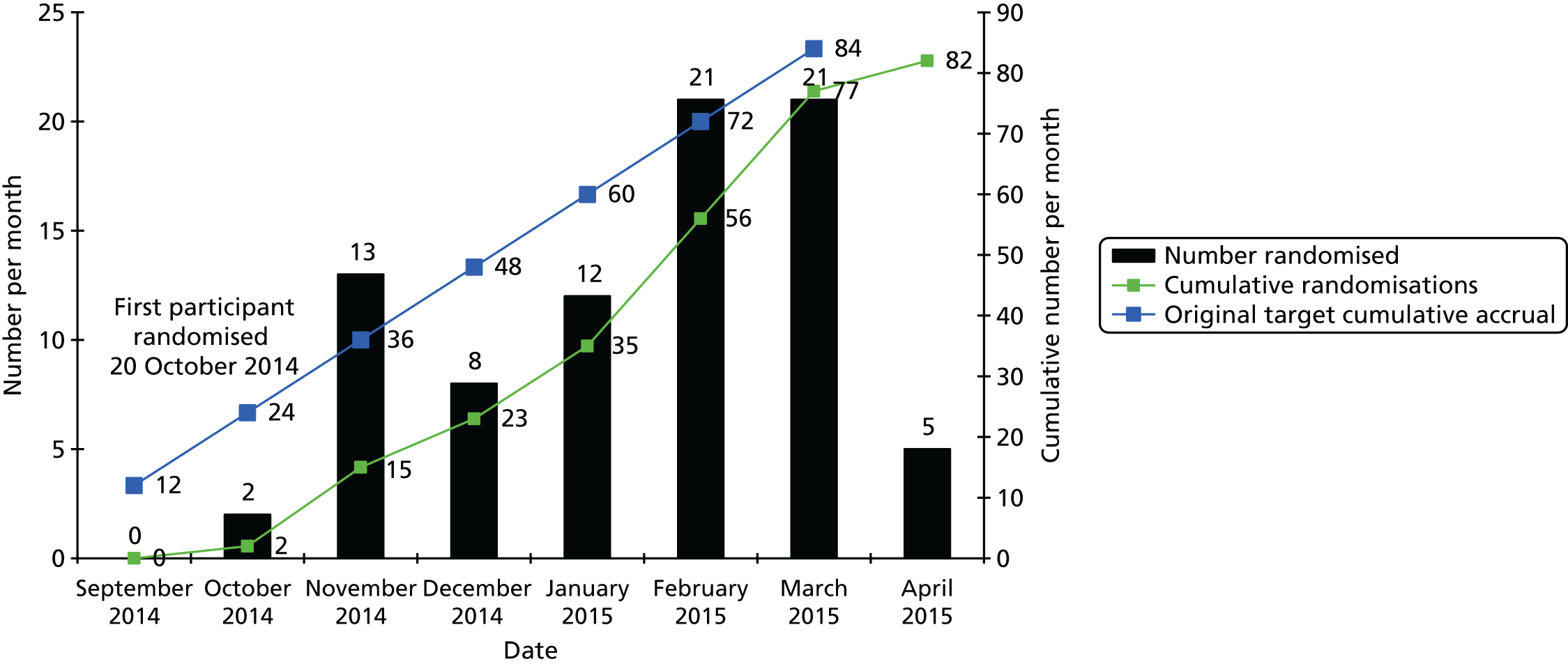
Stratification factors used during randomisation were well balanced across the treatment arms (Table 35; see also Figure 7).
| Stratification factor | Treatment arm, n (%) | Total (N = 82), n (%) | |
|---|---|---|---|
| SSM plus TAU (N = 41) | TAU (N = 41) | ||
| Area | |||
| Leeds | 18 (43.9) | 18 (43.9) | 36 (43.9) |
| Bradford | 12 (29.3) | 12 (29.3) | 24 (29.3) |
| Wakefield | 11 (26.8) | 11 (26.8) | 22 (26.8) |
| HbA1c level | |||
| ≤ 6.5% (≤ 48 mmol/mol) | 15 (36.6) | 16 (39.0) | 31 (37.8) |
| > 6.5% to 8.5% (> 48 to 69 mmol/mol) | 20 (48.8) | 20 (48.8) | 40 (48.8) |
| > 8.5% (> 69 mmol/mol) | 6 (14.6) | 5 (12.2) | 11 (13.4) |
| BMI (kg/m2) | |||
| ≤ 25 | 1 (2.4) | 2 (4.9) | 3 (3.7) |
| > 25 | 40 (97.6) | 39 (95.1) | 79 (96.3) |
| Physical activity | |||
| At or above national guidelines | 1 (2.4) | 0 (0.0) | 1 (1.2) |
| Below national guidelines | 40 (97.6) | 41 (100.0) | 81 (98.8) |
| Supporter available | |||
| No supporter | 9 (22.0) | 11 (26.8) | 20 (24.4) |
| Person does not live with supportera | 23 (56.1) | 22 (53.7) | 45 (54.9) |
| Person lives with supporter | 9 (22.0) | 8 (19.5) | 17 (20.7) |
Participant demographics, including age at initial referral and at RCT randomisation, sex and ethnicity, also appear to be well balanced across trial arms and largely comparable with those of the case-finding population (Table 36).
| Demographic | Case finding | Feasibility RCT randomised participants | |||
|---|---|---|---|---|---|
| Referred population (N = 325) | Eligible population (N = 147) | Treatment arm | Total (N = 82) | ||
| SSM plus TAU (N = 41) | TAU (N = 41) | ||||
| Age (years) at referral/registration (n = 22 missing) | |||||
| Mean (SD) | 53.5 (13.81) | 54.4 (12.82) | 54.8 (10.83) | 56.2 (12.46) | 55.5 (11.62) |
| Median (range) | 54.0 (18–93) | 56.0 (19–83) | 56.0 (29–76) | 57.0 (19–79) | 56.5 (19–79) |
| Age (years) at randomisation | |||||
| Mean (SD) | 55.6 (10.75) | 57.3 (12.26) | 56.4 (11.49) | ||
| Median (range) | 57.0 (30–77) | 58.0 (20–79) | 58.0 (20–79) | ||
| Sex (n = 14 missing), n (%) | |||||
| Male | 174 (55.9) | 74 (50.3) | 20 (48.8) | 20 (48.8) | 40 (48.8) |
| Female | 137 (44.1) | 73 (49.7) | 21 (51.2) | 21 (51.2) | 42 (51.2) |
| Ethnicity (n = 22 missing), n (%) | |||||
| White | 249 (82.2) | 125 (85.0) | 36 (87.8) | 39 (95.1) | 75 (91.5) |
| Mixed | 6 (2.0) | 3 (2.0) | 2 (4.9) | 0 (0.0) | 2 (2.4) |
| Asian | 45 (14.9) | 17 (11.6) | 3 (7.3) | 2 (4.9) | 5 (6.1) |
| Black | 2 (0.7) | 1 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other ethnic group | 1 (0.3) | 1 (0.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Table 37 presents the details of consenting supporters for the 40 (49%) participants with a consenting supporter, with supporter consent observed in 24 (59%) participants in TAU compared with 16 (39%) participants in SSM plus TAU. For the majority of the 40 participants with a consenting supporter, it was a paid supporter who consented to participate (n = 28, 70%) and the supporter did not live with the participant (n = 31, 78%).
| Details of consenting supporter | Treatment arm | Total (N = 82) | |
|---|---|---|---|
| SSM plus TAU (N = 41) | TAU (N = 41) | ||
| Supporter consented to be part of the trial, n (%) | |||
| Yes | 16 (39.0) | 24 (58.5) | 40 (48.8) |
| No | 16 (39.0) | 6 (14.6) | 22 (26.8) |
| No supporter | 9 (22.0) | 11 (26.8) | 20 (24.4) |
| Reason supporter not consented (of those not consented), n (%) | |||
| Not contacted for the trial (not available/present during interview) | 11 (68.8) | 3 (50.0) | 14 (63.6) |
| Not willing to consent | 1 (6.3) | 1 (16.7) | 2 (9.1) |
| Paid supporter: not felt to meet required definitiona | 2 (12.5) | 1 (16.7) | 3 (13.6) |
| Supporter not contactable | 2 (12.5) | 1 (16.7) | 3 (13.6) |
| Supporter age (years) at randomisation (of consented) | |||
| Mean (SD) | 50.7 (14.89) | 49.7 (13.48) | 50.1 (13.86) |
| Median (range) | 57.0 (21–68) | 52.0 (26–77) | 53.0 (21–77) |
| Missing | 1 | 1 | 2 |
| Supporter sex (of consented), n (%) | |||
| Male | 5 (31.3) | 10 (41.7) | 15 (37.5) |
| Female | 11 (68.8) | 14 (58.3) | 25 (62.5) |
| Supporter relationship (of consented), n (%) | |||
| Paid supporter | 11 (68.8) | 17 (70.8) | 28 (70.0) |
| Parent of person | 2 (12.5) | 3 (12.5) | 5 (12.5) |
| Partner/husband/wife | 1 (6.3) | 3 (12.5) | 4 (10.0) |
| Brother of person | 0 (0.0) | 1 (4.2) | 1 (2.5) |
| Grown-up child of person | 1 (6.3) | 0 (0.0) | 1 (2.5) |
| Other family member | 1 (6.3) | 0 (0.0) | 1 (2.5) |
| Live with supporter (of consented), n (%) | |||
| Yes | 3 (18.8) | 6 (25.0) | 9 (22.5) |
| No | 13 (81.3) | 18 (75.0) | 31 (77.5) |
Table 38 gives further details of the participant’s supporter and living arrangements for the 82 randomised participants. We present results at baseline and follow-up to indicate the degree to which these arrangements remained stable over the duration of the study. At baseline, 58 (71%) participants reported that there was someone who helped or supported them with their diabetes in day-to-day life; however, this dropped to 43 out of 77 (56%) participants at follow-up. At baseline, 12 out of 55 (22%) participants with a supporter reported that their main supporter lived with them; however, at follow-up, 13 out of 41 (34%) participants reported living with the supporter. A slightly higher proportion of participants reported having someone who helped them with shopping or cooking, 69 out of 82 (84%) participants reporting such a person at baseline and 61 out of 77 (79%) at follow-up. Again, this person was most frequently a paid supporter.
| Participant details and living arrangements | Time point, n (%) | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | |||||
| Treatment arm | Total (N = 82) | Treatment arm | Total (N = 82) | |||
| SSM plus TAU (N = 41) | TAU (N = 41) | SSM plus TAU (N = 41) | TAU (N = 41) | |||
| Someone who helps/supports you with your diabetes in day-to-day life?a | ||||||
| Yes | 26 (63.4) | 32 (78.0) | 58 (70.7) | 23 (62.2) | 20 (50.0) | 43 (55.8) |
| No | 14 (34.1) | 9 (22.0) | 23 (28.0) | 13 (35.1) | 17 (42.5) | 30 (39.0) |
| Don’t know | 1 (2.4) | 0 (0.0) | 1 (1.2) | 1 (2.7) | 3 (7.5) | 4 (5.2) |
| Missing | 0 | 0 | 0 | 4 | 1 | 5 |
| If yes, who is the main person? | ||||||
| Paid supporter | 16 (61.5) | 24 (77.4) | 40 (70.2) | 10 (50.0) | 14 (77.8) | 24 (63.2) |
| Immediate family | 7 (26.9) | 5 (16.1) | 12 (21.1) | 4 (20.0) | 3 (16.7) | 7 (18.4) |
| Partner/husband/wife | 1 (3.8) | 1 (3.2) | 2 (3.5) | 4 (20.0) | 1 (5.6) | 5 (13.2) |
| Grown-up child of person | 1 (3.8) | 1 (3.2) | 2 (3.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other family member | 1 (3.8) | 0 (0.0) | 1 (1.8) | 1 (5.0) | 0 (0.0) | 1 (2.6) |
| Friend | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.0) | 0 (0.0) | 1 (2.6) |
| Missing | 0 | 1 | 1 | 3 | 2 | 5 |
| If yes, has the supporter changed since the baseline visit? | ||||||
| Yes | 2 (9.1) | 3 (15.8) | 5 (12.2) | |||
| No | 20 (90.9) | 16 (84.2) | 36 (87.8) | |||
| Missing | 1 | 1 | 2 | |||
| If yes, do they live with you? | ||||||
| Yes | 6 (23.1) | 6 (20.7) | 12 (21.8) | 9 (45.0) | 4 (22.2) | 13 (34.2) |
| No | 20 (76.9) | 23 (79.3) | 43 (78.2) | 11 (55.0) | 14 (77.8) | 25 (65.8) |
| Missing | 0 | 3 | 3 | 3 | 2 | 5 |
| Is there anyone who helps you with shopping and cooking? | ||||||
| Yes | 33 (80.5) | 36 (87.8) | 69 (84.1) | 29 (78.4) | 32 (80.0) | 61 (79.2) |
| No | 8 (19.5) | 5 (12.2) | 13 (15.9) | 8 (21.6) | 8 (20.0) | 16 (20.8) |
| Missing | 0 | 0 | 0 | 4 | 1 | 5 |
| If yes, who is the main person? | ||||||
| Paid supporter | 20 (60.6) | 22 (64.7) | 42 (62.7) | 16 (55.2) | 20 (62.5) | 36 (59.0) |
| Immediate family | 7 (21.2) | 8 (23.5) | 15 (22.4) | 6 (20.7) | 9 (28.1) | 15 (24.6) |
| Partner/husband/wife | 2 (6.1) | 1 (2.9) | 3 (4.5) | 3 (10.3) | 2 (6.3) | 5 (8.2) |
| Other family member | 3 (9.1) | 0 (0.0) | 3 (4.5) | 2 (6.9) | 0 (0.0) | 2 (3.3) |
| Grown-up child of person | 1 (3.0) | 2 (5.9) | 3 (4.5) | 1 (3.4) | 0 (0.0) | 1 (1.6) |
| Friend | 0 (0.0) | 1 (2.9) | 1 (1.5) | 1 (3.4) | 0 (0.0) | 1 (1.6) |
| Other relationship | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.1) | 1 (1.6) |
| Missing | 0 | 2 | 2 | 0 | 0 | 0 |
| If yes, has the supporter changed since the baseline visit? | ||||||
| Yes | 2 (7.4) | 4 (14.3) | 6 (10.9) | |||
| No | 25 (92.6) | 24 (85.7) | 49 (89.1) | |||
| Missing | 2 | 4 | 6 | |||
| If yes, do they live with you? | ||||||
| Yes | 10 (32.3) | 7 (23.3) | 17 (27.9) | 9 (32.1) | 7 (23.3) | 16 (27.6) |
| No | 21 (67.7) | 23 (76.7) | 44 (72.1) | 19 (67.9) | 23 (76.7) | 42 (72.4) |
| Missing | 2 | 6 | 8 | 1 | 2 | 3 |
At baseline, 49 out of 82 (60%) participants reported having regular contact with someone else who had diabetes: 23 (56%) SSM participants and 26 (63%) TAU participants. However, although the overall proportion remained similar at follow-up with 41 out of 77 (53%) participants reporting this, there was a marginal shift across the arms with 25 out of 37 (68%) SSM participants and 16 out of 40 (40%) TAU participants reporting regular contact with someone with diabetes.
Finally, similar rates of participants reported regularly attending a day centre, club, organisation, evening event, voluntary work or volunteering: 49 out of 82 (60%) participants at baseline and 43 out of 76 (57%) participants at follow-up. Self-reported levels of exercise were low with 53 out of 77 (69%) participants reporting doing any exercise, and the number of participants actually doing exercise at or above national guidelines was only 4 out of 66 (6%) at baseline.
Before randomisation, participants’ supporters were also invited to consent to take part in data collection for the feasibility trial.
Following randomisation, withdrawals were made for six (7%) participants: four (10%) randomised to receive SSM and two (5%) randomised to receive TAU (Table 39). All six participants withdrew from nurse follow-up, five also withdrew from researcher follow-up and four from further clinical data collection (from GP records). Participants in the SSM arm withdrew, on average, 1.4 months (range 0.4–2.5 months) after randomisation (while in contact to schedule or deliver the intervention), whereas TAU participants withdrew later, following contact to arrange the follow-up visits, at an average of 5.5 months post randomisation.
| Nature of withdrawal | Treatment arm | Total (N = 82) | |
|---|---|---|---|
| SSM plus TAU (N = 41) | TAU (N = 41) | ||
| Withdrawal, n (%) | |||
| From nurse follow-up | 0 (0.0) | 1 (2.4) | 1 (1.2) |
| From researcher and nurse follow-up | 1 (2.4) | 0 (0.0) | 1 (1.2) |
| From researcher and nurse follow-up and further clinical data collection | 3 (7.3) | 1 (2.4) | 4 (4.9) |
| No withdrawal/s | 37 (90.2) | 39 (95.1) | 76 (92.7) |
| Timing (months) of withdrawal with respect to randomisation | |||
| Mean (SD) | 1.4 (0.88) | 5.5 (0.33) | 2.8 (2.22) |
| Median (range) | 1.4 (0.4–2.5) | 5.5 (5.3–5.7) | 2.1 (0.4–5.7) |
Overall conduct and success of participants’ baseline and follow-up researcher and nurse visits and the collection of physical measures (height, weight, BMI, BP and blood test) are presented in Table 40. All randomised participants received a researcher visit at baseline and the follow-up researcher visit was conducted for the 77 (94%) participants without a withdrawal. A baseline nurse visit was conducted for 76 (93%) participants and was not required for five participants as in-date physical measures were obtained from participant’s GP. It was also possible to obtain GP records for the further participant who declined the baseline nurse visit. At follow-up, a nurse visit was conducted for 75 (92%) participants, with no visit for the six participants who had withdrawn, and GP blood test results were obtained for one further participant without nurse follow-up.
| Assessment | Time point, n (%) | |||
|---|---|---|---|---|
| Baseline, total (N = 82) | Follow-up | |||
| Treatment arm | Total (N = 82) | |||
| SSM plus TAU (N = 41) | TAU (N = 41) | |||
| Researcher visit conducted | ||||
| Yes | 82 (100.0) | 37 (90.2) | 40 (97.6) | 77 (93.9) |
| No (person withdrew from researcher follow-up) | 0 (0.0) | 4 (9.8) | 1 (2.4) | 5 (6.1) |
| Nurse visit conducted | ||||
| Yes | 76 (92.7) | 36 (87.8) | 39 (95.1) | 75 (91.5) |
| No | 6 (7.3) | 5 (12.2) | 2 (4.9) | 7 (8.5) |
| Reason nurse visit not conducted (of those without a nurse visit) | ||||
| GP blood test results obtained | 5 (83.3) | 1 (20.0) | 0 (0.0) | 1 (14.3) |
| Person declined blood test (GP results obtained) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Person withdrew from nurse follow-up | 0 (0.0) | 4 (80.0) | 2 (100)a | 6 (85.7) |
Tables 41 and 42 present further details of the researcher and nurse visits and the resources required to conduct the visits, for participants for whom visits were conducted.
| Visit details | Time point | |||
|---|---|---|---|---|
| Baseline, total (N = 82) | Follow-up | |||
| Treatment arm | Total (N = 77) | |||
| SSM plus TAU (N = 37) | TAU (N = 40) | |||
| Number of researcher contact attempts | ||||
| Mean (SD) | 2.6 (2.16) | 1.0 (0.00) | 1.1 (0.22) | 1.0 (0.16) |
| Median (range) | 2.0 (1–12) | 1.0 (1–1) | 1.0 (1–2) | 1.0 (1–2) |
| Missing | 0 | 0 | 0 | 0 |
| Timing of researcher visit | From first contact (days) | From randomisation (months) | ||
| Mean (SD) | 15.9 (16.99) | 4.4 (0.69) | 4.4 (0.62) | 4.4 (0.65) |
| Median (range) | 11.5 (1–112) | 4.2 (3.3–6.0) | 4.2 (3.0–6.2) | 4.2 (3.0–6.2) |
| Missing | 0 | 0 | 0 | 0 |
| Present during interview (not mutually exclusive), n (%) | ||||
| Only the person in the trial | 36 (43.9) | 12 (32.4) | 16 (40.0) | 28 (36.4) |
| Consented supporter | 33 (40.2) | 8 (21.6) | 9 (22.5) | 17 (22.1) |
| Family member or friend | 12 (14.6) | 9 (24.3) | 3 (7.5) | 12 (15.6) |
| Paid supporter | 9 (11.0) | 10 (27.0) | 11 (27.5) | 21 (27.3) |
| Other person | 2 (2.4) | 0 (0.0) | 3 (7.5) | 3 (3.9) |
| Consenting supporter interview conducted, n (%) | ||||
| Yes | 40 (48.8) | 12 (32.4) | 15 (37.5) | 27 (35.1) |
| No | 0 (0.0) | 4 (10.8) | 8 (20.0) | 12 (15.6)b |
| No consenting supporter | 42 (51.2) | 21 (56.8) | 17 (42.5) | 38 (49.4) |
| Visit details | Time point | |||
|---|---|---|---|---|
| Baseline, total (N = 82) | Follow-up | |||
| Treatment arm | Total (N = 75) | |||
| SSM plus TAU (N = 36) | TAU (N = 39) | |||
| Number of nurse contact attempts | ||||
| Mean (SD) | 1.8 (1.22) | 1.9 (1.71) | 2.4 (2.30) | 2.2 (2.04) |
| Median (range) | 1.0 (1–6) | 1.0 (1–10) | 1.0 (1–9) | 1.0 (1–10) |
| Missing | 1 | 0 | 0 | 0 |
| Timing of nurse visit | From first contact (days) | From randomisation (months) | ||
| Mean (SD) | 7.8 (7.71) | 5.1 (0.81) | 5.0 (0.87) | 5.1 (0.84) |
| Median (range) | 6.0 (0–38) | 5.0 (4–7) | 4.8 (4–8) | 4.8 (4–8) |
| Missing | 1 | 0 | 0 | 0 |
| Number of nurse visits required, n (%) | ||||
| 1 | 74 (97.4) | 34 (94.4) | 36 (92.3) | 70 (93.3) |
| 2 | 2 (2.6) | 2 (5.6) | 3 (7.7) | 5 (6.7) |
| Overall duration of nurse visit(s) (minutes) | ||||
| Mean (SD) | 33.6 (22.37) | 27.8 (11.98) | 25.4 (13.37) | 26.5 (12.69) |
| Median (range) | 30.0 (15–180) | 25.0 (15–61) | 21.0 (5–70) | 24.0 (5–70) |
| Missing | 0 | 0 | 0 | 0 |
| Present during interview (not mutually exclusive), n (%) | ||||
| Supporter | 27 (35.5) | 12 (33.3) | 8 (20.5) | 20 (26.7) |
| Other person | 29 (38.2) | 10 (27.8) | 16 (41.0) | 26 (34.7) |
| Only the person in the trial | 25 (32.9) | 14 (38.9) | 18 (46.2) | 32 (42.7) |
A median of two contacts was required to arrange the baseline researcher visit and one contact to arrange the follow-on baseline nurse visit, with the number of contacts required at follow-up decreasing to one for the researcher visit and staying the same for the nurse visit. Researcher follow-up took place a mean of 4.4 months post randomisation (range 3–6.2 months), whereas, as expected, nurse visits took place slightly later at a mean of 4.8 months post randomisation (range 4–8 months).
The timing of visits is presented graphically in Figures 8 and 9.
FIGURE 8.
Time between randomisation and researcher visits.
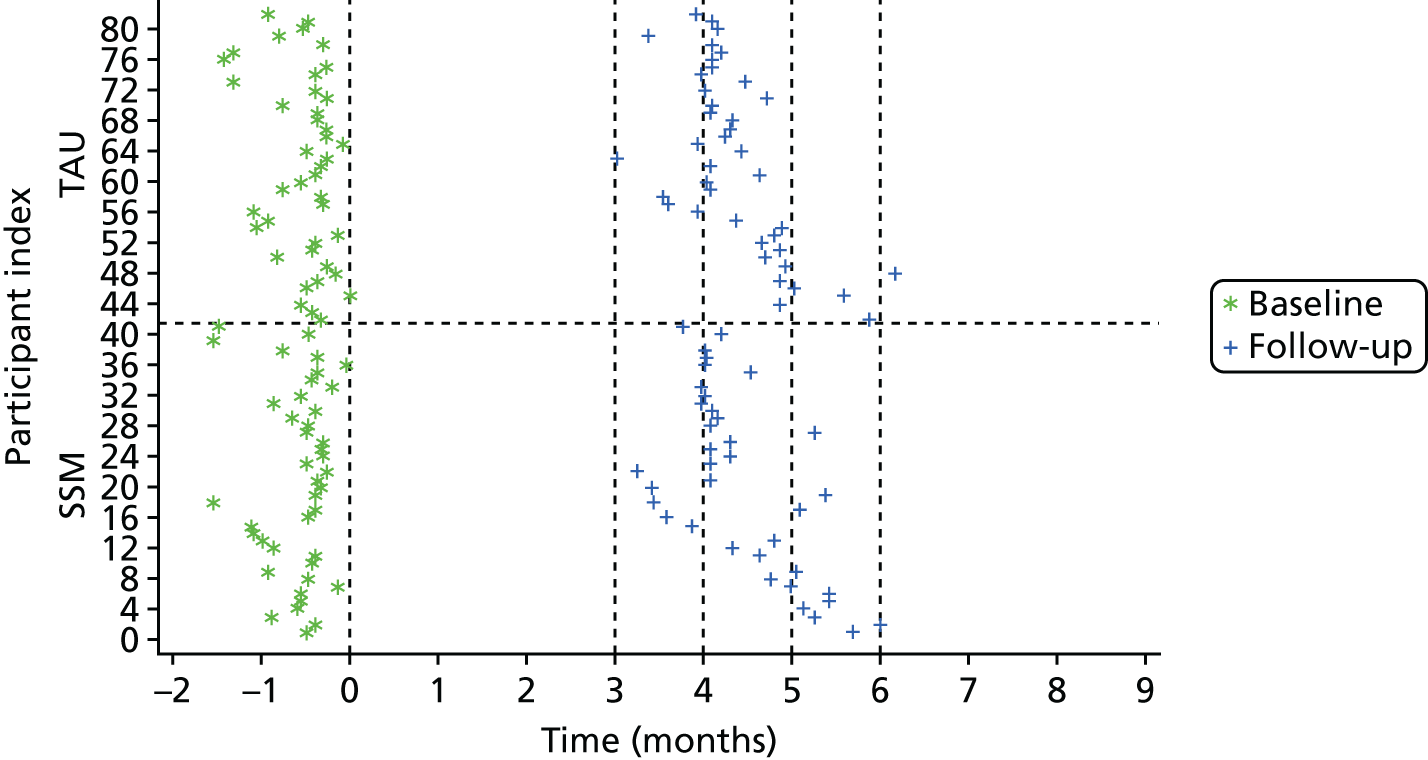
FIGURE 9.
Time between randomisation and nurse visits.

Intervention delivery
Supported self-management
Forty-one (50%) participants were allocated to receive SSM during randomisation. The nurse who was allocated to deliver the intervention saw the participant at baseline, and if no nurse visit had been conducted then the nurse was allocated at random. All participants were seen or contacted to schedule sessions by their allocated nurse.
Thirty-five (85%) participants attended all required sessions, which ranged from two to four sessions, with over three-quarters of all participants (78%) attending at least three sessions (Table 43). Four (10%) of the 41 participants allocated to receive the intervention did not attend any sessions and two (5%) attended only one session and so did not attend all required sessions. Thirty (73%) participants had another person present with them during at least one of their sessions, usually the consenting supporter.
| Attendance | SSM (N = 41), n (%) |
|---|---|
| Attendance summarya | |
| Completed all required sessions (2–4) | 35 (85.4) |
| Did not attend any sessions | 4 (9.8) |
| Did not attend all required sessions (1) | 2 (4.9) |
| Participant referred for a personal budget assessment during SSM? | |
| Yes | 2 (4.9) |
| No | 35 (85.4) |
| N/A: did not attend any sessions | 4 (9.8) |
| Was the standard leaflet given to the participant during the first session? | |
| Yes | 35 (85.4) |
| No | 2 (4.9) |
| N/A: did not attend any sessions | 4 (9.8) |
The standard leaflet was not provided to the four participants who attended no sessions, and it was also not provided to a further two participants who attended only their first SSM session.
For the 37 participants attending at least one session, the first session took place a mean of 28 days post randomisation (range 13–40 days), and the intervention delivery period averaged 49 days from the first to last session (range 0–104 days). Table 44 presents the timing of researcher follow-up visit in relation to randomisation and the number of SSM sessions received; no participant was still receiving SSM by the time of the researcher follow-up visit (Figure 10).
| Timing | SSM (n = 37) |
|---|---|
| Time (days) between randomisation and first attended therapy session | |
| Mean (SD) | 27.9 (19.69) |
| Median (range) | 20.0 (6–91) |
| Time (days) between randomisation and last attended therapy session | |
| Mean (SD) | 77.1 (24.88) |
| Median (range) | 71.0 (15–128) |
| Time (days) between first and last attended therapy sessions | |
| Mean (SD) | 49.2 (19.53) |
| Median (range) | 49.0 (0–104) |
FIGURE 10.
Needle plot of SSM session attendance and timing of research follow-up. Timing of researcher follow-up (points) in relation to duration and end of self-management sessions (bars).
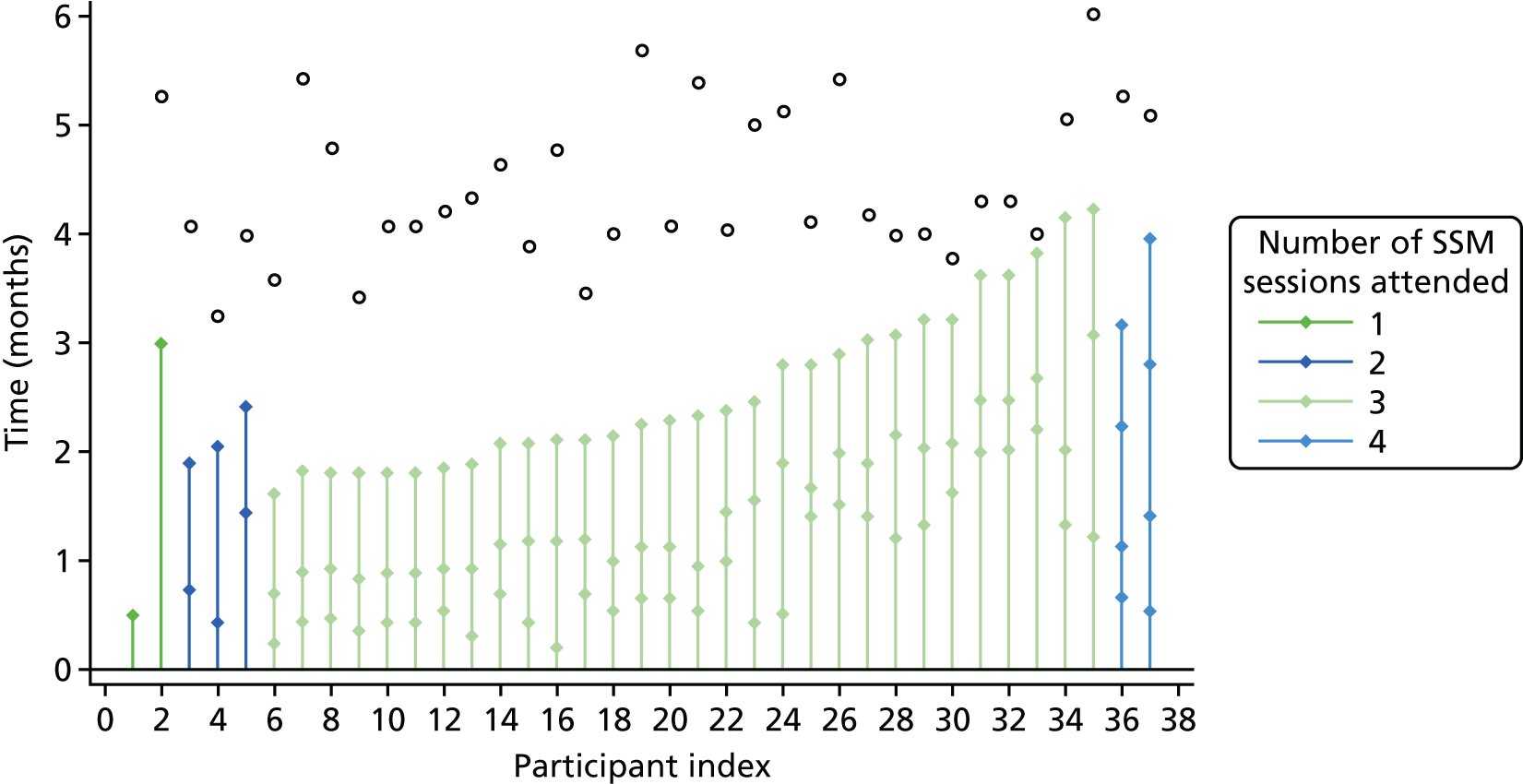
A summary of engagement was reported by the nurse who delivered the intervention (Table 45): 23 out of 40 (58%) participants were deemed to be very engaged with the sessions and 12 out of 40 (30%) were deemed to be very engaged with the materials, 15 out of 41 (37%) were reported to have a very engaged supporter (consenting or non-consenting) and 18 out of 41 (44%) had a further or different person (other supporter, partner or family member) who was engaged in the intervention implementation.
| Engagement | SSM (N = 41), n (%) |
|---|---|
| How engaged was the person with the sessions? | |
| Very engaged | 23 (57.5) |
| Quite engaged | 9 (22.5) |
| Not engaged: attention was not focused | 4 (10.0) |
| N/A: patient did not attend any sessions | 4 (10.0) |
| Missing | 1 |
| How engaged was the person with the materials? | |
| Very engaged | 12 (30.0) |
| Quite engaged | 11 (27.5) |
| Not engaged: hardly used materials | 13 (32.5) |
| N/A: patient did not attend any sessions | 4 (10.0) |
| Missing | 1 |
| How engaged was the supporter with the sessions? | |
| Very engaged | 15 (36.6) |
| Quite engaged | 6 (14.6) |
| Not engaged: attention was not focused | 1 (2.4) |
| Supporter did not attend at all | 8 (19.5) |
| N/A: patient did not attend any sessions/no supporter | 11 (26.8) |
| Was there anyone else who was engaged in the intervention implementation? | |
| Yes | 18 (43.9) |
| Noa | 19 (46.3) |
| N/A: patient did not attend any sessions | 4 (9.8) |
A total of 106 sessions were conducted across the 37 participants attending at least one session, with a typical total intervention time of 2 hours (Table 46). Sessions lasted a mean of 45 minutes (range 13–95 minutes), they largely took place in the participant’s home (92%) and the focus included getting started, setting goals, mapping supporter and checking progress.
| Session details | All SSM sessions (N = 106) |
|---|---|
| Length of session (minutes) | |
| n | 106 |
| Mean (SD) | 44.9 (21.26) |
| Median (range) | 45.0 (13–95) |
| Total length of sessions per participant (minutes) | |
| n | 37 |
| Mean (SD) | 128.6 (44.55) |
| Median (range) | 116.0 (58–258) |
| Location of sessions,a n (%) | |
| Person’s home | 97 (91.5) |
| Day centre | 1 (0.9) |
| Other | 8 (7.5) |
| Consenting supporter or any other person present, n (%) | |
| Yes | 75 (70.8) |
| No | 31 (29.2) |
| Main focus of session? (Not mutually exclusive, per session, N = 106), n (%) | |
| Getting started | 39 (36.8) |
| Setting goals | 38 (35.8) |
| Mapping supporter | 50 (47.2) |
| Checking progress | 38 (35.8) |
| Main focus of session, across all sessions? (Not mutually exclusive, per participant, N = 37), n (%) | |
| Getting started | 37 (100.0) |
| Setting goals | 36 (97.3) |
| Mapping supporter | 37 (100.0) |
| Checking progress | 35 (94.6) |
Finally, Table 47 presents a summary of telephone contact between sessions, excluding contacts for administrative or scheduling reasons.
| Details of telephone contact | SSM (N = 41), n (%) |
|---|---|
| Did the nurse have any telephone contact with the participant | |
| Yesa | 8 (19.5) |
| No | 33 (80.5) |
| Number of contacts per participant | |
| 0 | 33 (80.5) |
| 1 | 4 (9.8) |
| 2 | 3 (7.3) |
| 5 | 1 (2.4) |
| Contact initiated by (N = 15)b | |
| Study nurse | 12 (80.0) |
| Supporter | 1 (6.7) |
| Other | 2 (13.3) |
| Reason for contact (N = 15) | |
| Research process | 6 (40.0) |
| Intervention advice/follow-up from intervention | 4 (26.7) |
| Other clinical reason/calls to health-care professional | 4 (26.7) |
| General diabetes advice | 1 (6.7) |
| Further action required following contact (N = 15) | |
| Yes | 9 (60.0) |
| No | 6 (40.0) |
Adherence and fidelity
Independent review of adherence to, and fidelity of, the intervention took place for all self-management sessions attended by the 37 attending participants.
We assessed which components of the intervention were covered in the first, second, third and fourth sessions, as well as any session overall.
All components of the intervention concerned with assessing day-to-day living arrangements and diabetes management were covered in all participants’ first session, and at least some of these components were revisited during the second session for 10 out of 35 (29%) participants attending at least two sessions.
Goal-setting plans and ‘supporter flashcard(s)’ were covered or provided during first session for just over one-third of participants, were covered for the majority during the second session and in the third session covered for just two participants each.
The most frequent goals identified were to increase physical activity (n = 17, 46%), to eat more fruit (n = 15, 41%), to make snack swaps for food and drink (food, n = 14, 38%; drink, n = 6, 16%) and ‘other dietary improvements’ (e.g. reducing sugar and calorie intake in tea, squash and hot chocolate: n = 10, 27%) (Table 48).
| Identified goals/plans (per participant, not mutually exclusive) | SSM (N = 37), n (%) |
|---|---|
| More physical activity | 18 (48.6) |
| More fruit | 15 (40.5) |
| Snack swaps (food) | 14 (37.8) |
| Other dietary improvementsa | 10 (27.0) |
| Snack swaps (fizzy drinks) | 6 (16.2) |
| More vegetables | 2 (5.4) |
| Tablet taking | 1 (2.7) |
| Retinal screening | 1 (2.7) |
| No goals identified | 2 (5.4) |
A week-to-view calendar recording main fixed activities, attendance at clubs or day centres and meal times.
My lifeA simple chart recording ‘things I like to do’, ‘who I spend time with’, ‘things I don’t like to do’ and ‘my friends and helpers’.
Looking after my diabetesA simple chart recording main activities: ‘cooking and eating’, ‘keeping active’, ‘going to appointments’ and ‘looking after my health’.
I am going to . . .A goal-setting chart recording: ‘what do I want to change?’, ‘how will I make changes?’, ‘where do I want to do things differently?’ and ‘when do I want to change?’
I am going to planA more detailed plan linked to a specific goal – ‘things I can try (to achieve goal)’, ‘who will help me’, ‘what will make it easier and what will make it harder’ – leading to a checklist of planned steps.
Supporter flashcardA simple card given to a key supporter, identified from a ‘my supporters and helpers’ list, that informed them of the goal set and the part the supporter could play in helping the participant to achieve the goal.
Calendar sheetsSmall tear-off sheet for monitoring progress, headed ‘I am going to . . .’ and containing a ‘Did I do it today yes/no?’ check.
In self-monitoring, 16 (43%) returned no calendar sheets, 5 (14%) returned only unmarked calendar sheets, and 16 (43%) returned sheets in which all or some were marked.
Box 2 lists the SSM materials collected to derive an adherence score for each participant. Table 49 presents the details of adherence scoring for each component contributing to the score, with a possible range of scores from zero to eight depending on the number of completed steps or components (see Appendix 13 for details of the intervention materials used in the scoring). Fidelity scores ranged from three to eight, with the majority of participants scoring six or above (n = 32, 87%). There was evidence that the whole process, all steps, were completed at least once for all but two participants.
| Item recorded | SSM (N = 37), n (%) |
|---|---|
| 1. Completed ‘My week timetable and my life chart’ | 37 (100.0) |
| 2. Completed ‘Looking after my diabetes chart’ | 37 (100.0) |
| 3. Completed ‘I am going to chart’ and a ‘What, where, when chart’ | 34 (91.9) |
| 4. Has an ‘I am going to’ box and/or an ‘I am going to plan’ | 36 (97.3) |
| 5. Completed supporters and helpers checklist | 37 (100.0) |
| 6. A flashcard was provided for identified supporter and relevant helpers | 25 (67.6) |
| 7. Calendar sheets: only unmarked calendar sheets returned (one point) | 5 (13.5) |
| 8. Calendar sheets: marked and unmarked, or only marked calendar sheets returned (two points) | 16 (43.2) |
| Adherence score, based on the number of completed steps above (1–8) | |
| 3 | 1 (2.7) |
| 5 | 4 (10.8) |
| 6 | 13 (35.1) |
| 7 | 10 (27.0) |
| 8 | 9 (24.3) |
| The whole process was completed at least once | 35 (94.6) |
Outcome data collection
Unless otherwise stated, the results presented here relate to the intention-to-treat population.
Unblinding
The follow-up nurse was unblinded for a total of 34 (42%) trial participants: 20 (48%) in the SSM arm and 14 (34%) in the TAU arm (Table 50). Most unblinding occurred because the nurse who was allocated at randomisation (who told the participant their treatment allocation and went on to deliver the intervention for allocated participants) also undertook the follow-up visit because of staffing availability. This was the reason for unblinding for 12 out of 20 (60%) participants in the SSM arm and 12 out of 14 (86%) participants in the TAU arm. Other reasons for unblinding included seeing the intervention materials in the SSM arm and also being informed by the participant, supporter or researcher. All unblinding in the TAU arm occurred prior to the follow-up visit, whereas in the SSM arm unblinding occurred during the assessment for six (30%) participants and after the assessment for two (10%) of the 20 unblinded participants.
| Nurse unblinding details | Treatment arm, n (%) | Total (N = 82), n (%) | |
|---|---|---|---|
| SSM plus TAU (N = 41) | TAU (N = 41) | ||
| Nurse unblinded before or during follow-up? | |||
| Yes | 20 (48.8) | 14 (34.1) | 34 (41.5) |
| No | 16 (39.0) | 25 (61.0) | 41 (50.0) |
| No follow-up visit | 5 (12.2) | 2 (4.9) | 7 (8.5) |
| How did unblinding occur? (of those unblinded) | |||
| Delivered intervention/nurse allocated randomisation (assigned follow-up nurse unavailable) | 12 (60.0) | 12 (85.7) | 24 (70.6) |
| Saw intervention materials | 5 (25.0) | 0 (0.0) | 5 (14.7) |
| Informed by participant | 2 (10.0) | 0 (0.0) | 2 (5.9) |
| Informed by supporter | 1 (5.0) | 0 (0.0) | 1 (2.9) |
| Informed by researcher | 0 (0.0) | 1 (7.1) | 1 (2.9) |
| Other | 0 (0.0) | 1 (7.1) | 1 (2.9) |
| When unblinded (of those unblinded) | |||
| Before the assessment | 12 (60.0) | 14 (100.0) | 26 (76.5) |
| During the assessment | 6 (30.0) | 0 (0.0) | 6 (17.6) |
| After the assessment | 2 (10.0) | 0 (0.0) | 2 (5.9) |
| If not unblinded, treatment guess (of those not unblinded) | |||
| SSM | 9 (56.3) | 3 (12.0) | 12 (29.3) |
| TAU | 7 (43.8) | 22 (88.0) | 29 (70.7) |
| Certainty of guess (0: not at all–10 completely sure) | |||
| n | 16 | 25 | |
| Mean (SD) | 5.6 (2.19) | 7.0 (1.49) | 6.5 (1.91) |
| Median (range) | 6.0 (2–8) | 7.0 (3–9) | 7.0 (2–9) |
Researchers were unblinded for a total of 16 (20%) participants (Table 51), for which unblinding occurred before the follow-up assessment. Almost all unblinding occurred in the SSM arm with the researcher unblinded for 15 (31%) participants, and only one participant in the TAU arm. For two participants, one in each arm, an alternative blinded researcher was instead able to conduct the follow-up visit. Most researcher unblinding occurred as the researcher was informed by the participant, for 9 (56%) of the 16 participants.
| Researcher unblinding details | Treatment arm, n (%) | Total (N = 82), n (%) | |
|---|---|---|---|
| SSM plus TAU (N = 41) | TAU (N = 41) | ||
| Researcher unblinded before or during follow-up? | |||
| Yes | 15 (36.6) | 1 (2.4) | 16 (19.5) |
| No | 22 (53.7) | 39 (95.1) | 61 (74.4) |
| No follow-up visit | 4 (9.8) | 1 (2.4) | 5 (6.1) |
| How did unblinding occur? (of those unblinded) | |||
| Informed by participant | 8 (53.3) | 1 (100.0) | 9 (56.3) |
| Informed by advocacy group | 2 (13.3) | 0 (0.0) | 2 (12.5) |
| Saw intervention materials | 2 (13.3) | 0 (0.0) | 2 (12.5) |
| Informed by carer | 1 (6.7) | 0 (0.0) | 1 (6.3) |
| Informed by study nurse | 1 (6.7) | 0 (0.0) | 1 (6.3) |
| Missing | 1 (6.7) | 0 (0.0) | 1 (6.3) |
| When unblinded (of those unblinded) | |||
| Before assessment | 15 (100.0) | 1 (100.0) | 16 (100.0) |
Physical measures
Table 52 summarises the success of attaining the physical measures, height, weight, BMI, waist and hip measurements and BP, and conducting a blood test during the baseline and follow-up nurse visits. For the majority it was possible to collect all measures where a visit went ahead. Most problematic was the blood test, which was not obtained for 5 (6%) of 76 participants at baseline and 4 (5%) of 75 participants at follow-up. However, overall, there was a low participant refusal rate for the blood test (just three refused) and the main problem was poor veins in both arm and hand.
| Collection of physical measures | Baseline, total (N = 82), n (%) | Follow-up, n (%) | ||
|---|---|---|---|---|
| Treatment arm | Total (N = 82) | |||
| SSM plus TAU (N = 41) | TAU (N = 41) | |||
| Nurse visit conducted | ||||
| No (no measures obtained) | 6 (7.3) | 5 (12.2) | 2 (4.9) | 7 (8.5) |
| Yes | 76 (92.7) | 36 (87.8) | 39 (95.1) | 75 (91.5) |
| Measures not obtained during nurse visit | ||||
| Height | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Weight (too heavy for scales) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Waist measurement (measuring tape too small) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hip measurement | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| BMI | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| BP | 2 (2.4) | 0 (0.0) | 2 (4.9) | 2 (2.4) |
| Blood test | 5 (6.1) | 0 (0.0) | 4 (9.8) | 4 (4.9) |
| Reason no BP | ||||
| Cuff too small/unable to get reading | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Person refused | 0 (0.0) | 0 (0.0) | 2 (100.0) | 2 (100.0) |
| Reason no blood test | ||||
| Done at GP | 2 (40.0) | 0 (0.0) | 1 (25.0) | 1 (25.0) |
| Unable to take blood (obtained from GP instead) | 3 (60.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Person refused (obtained from GP instead, n = 2) | 0 (0.0) | 0 (0.0) | 3 (75.0) | 3 (75.0) |
Table 53 presents the number of participants for whom results from GP medical records were required, either because the results could not be obtained during the nurse visit or because there was no nurse visit. GP medical records were required for 14 participants at baseline and 13 at follow-up (the latter had, however, withdrawn from further clinical data collection by follow-up): they were obtained for 11 out of 14 (79%) and 5 out of 13 (39%) participants, respectively. A number of records received did not, however, fall within the ideal 6-week time frame around randomisation or the follow-up researcher visit.
| GP medical records | Baseline, total (N = 82) | Follow-up | ||
|---|---|---|---|---|
| Treatment arm | Total (N = 82) | |||
| SSM plus TAU (N = 41) | TAU (N = 41) | |||
| Number of participants requiring GP medical records | 14 | 5 | 8 | 13 |
| Medical records obtained from the GP, n (%) | ||||
| Yes | 11 (78.6) | 1 (20.0) | 4 (50.0) | 5 (38.5) |
| No | 3 (21.4) | 4 (80.0) | 4 (50.0) | 8 (61.5) |
| Number missing BMI from the nurse | 7 | 5 | 2 | 7 |
| In-date (± 6 weeks) BMI obtained from GP, n (%) | ||||
| Yes | 4 (57.1) | 0 (0.0) | 1 (50.0) | 1 (14.3) |
| No: provided but not in date | 2 (28.6) | 1 (20.0) | 0 (0.0) | 1 (14.3) |
| No: not provided at all | 1 (14.3) | 4 (80.0) | 1 (50.0) | 5 (71.4) |
| Number missing BP from the nurse | 8 | 5 | 4 | 9 |
| In-date (± 6 weeks) BP obtained from GP, n (%) | ||||
| Yes | 2 (25.0) | 0 (0.0) | 1 (25.0) | 1 (11.1) |
| No: provided but not in date | 3 (37.5) | 1 (20.0) | 0 (0.0) | 1 (11.1)e |
| No: not provided at all | 3 (37.5) | 4 (80.0) | 3 (75.0) | 7 (77.8) |
| Number missing blood test results from the nurse | 11 | 5 | 6 | 11 |
| In-date (± 6 weeks) HbA1C level obtained from GP, n (%) | ||||
| Yes | 7 (63.6) | 0 (0.0) | 3 (50.0) | 3 (27.3) |
| No: provided but not in date | 4 (36.4) | 1 (20.0) | 1 (16.7) | 2 (18.2) |
| No: not provided at all | 0 (0.0) | 4 (80.0) | 2 (33.3) | 6 (54.5) |
General practices were also contacted to obtain follow-up medical record information for all participants, including a number of physical measures (QRISK®2 score, serum creatinine level, microalbuminuria concentration, dates of last retinal screening and foot examination), information about patient’s diabetes medications and health-care resource use (Table 54).
| Completion of GP follow-up medical records | Treatment arm, n (%) | Total (N = 82), n (%) | |
|---|---|---|---|
| SSM plus TAU (N = 41) | TAU (N = 41) | ||
| Follow-up medical records information form completed? | |||
| Yes | 35 (85.4) | 36 (87.8) | 71 (86.6) |
| No | 6 (14.6) | 5 (12.2) | 11 (13.4) |
| With respect to randomisation, timing (months) of completion of medical records information | |||
| Mean (SD) | 4.9 (0.95) | 5.2 (0.92) | 5.1 (0.94) |
| Median (range) | 4.5 (4.1–7.7) | 5.3 (4.1–8.1) | 4.7 (4.1–8.1) |
The timing of assessments obtained via the nurse only (height, hip and waist measurements and weight) can be seen in Figure 3. Figure 11 presents the timing and source of assessments of the outcome measures: BMI, BP and HbA1c levels.
FIGURE 11.
Time between randomisation and outcomes. (a) BMI; (b) BP; (c) HbA1c level; and (d) most recent blood test.

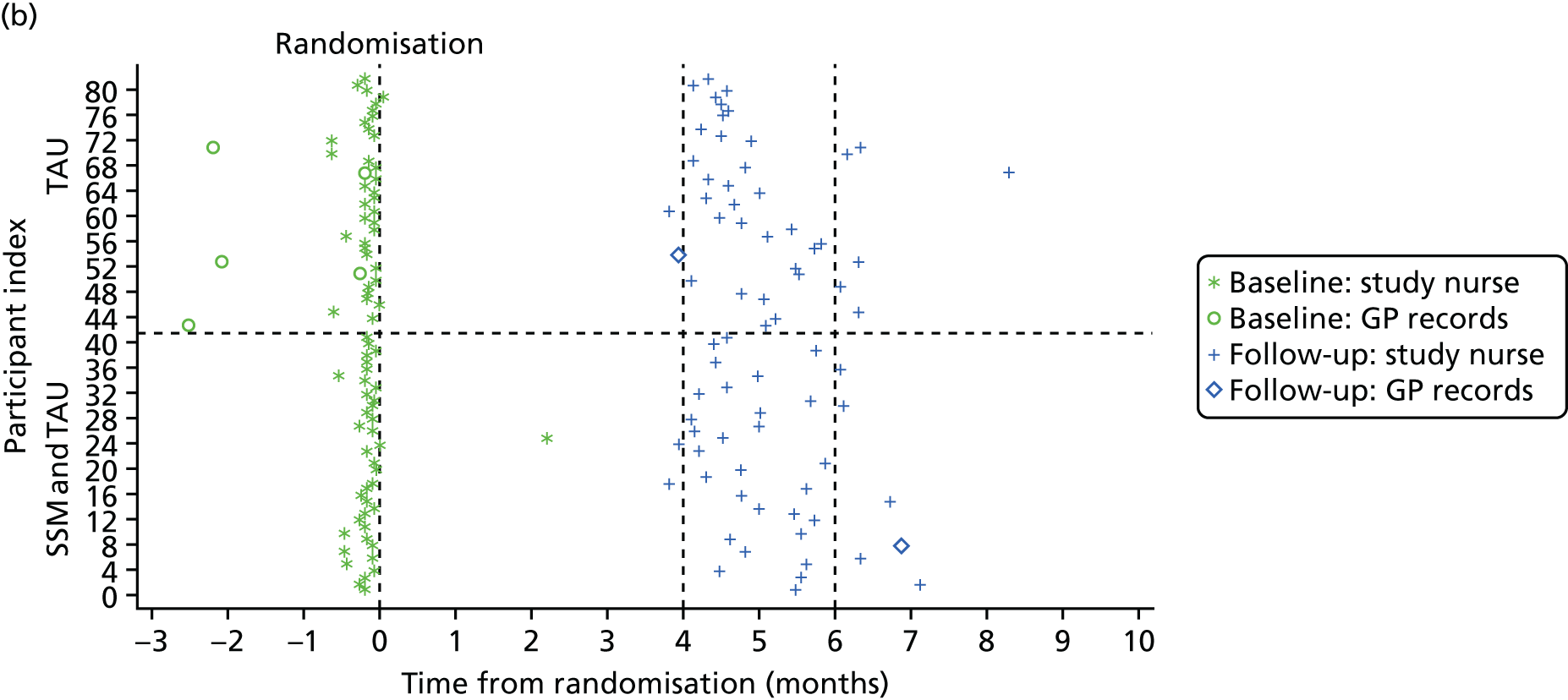

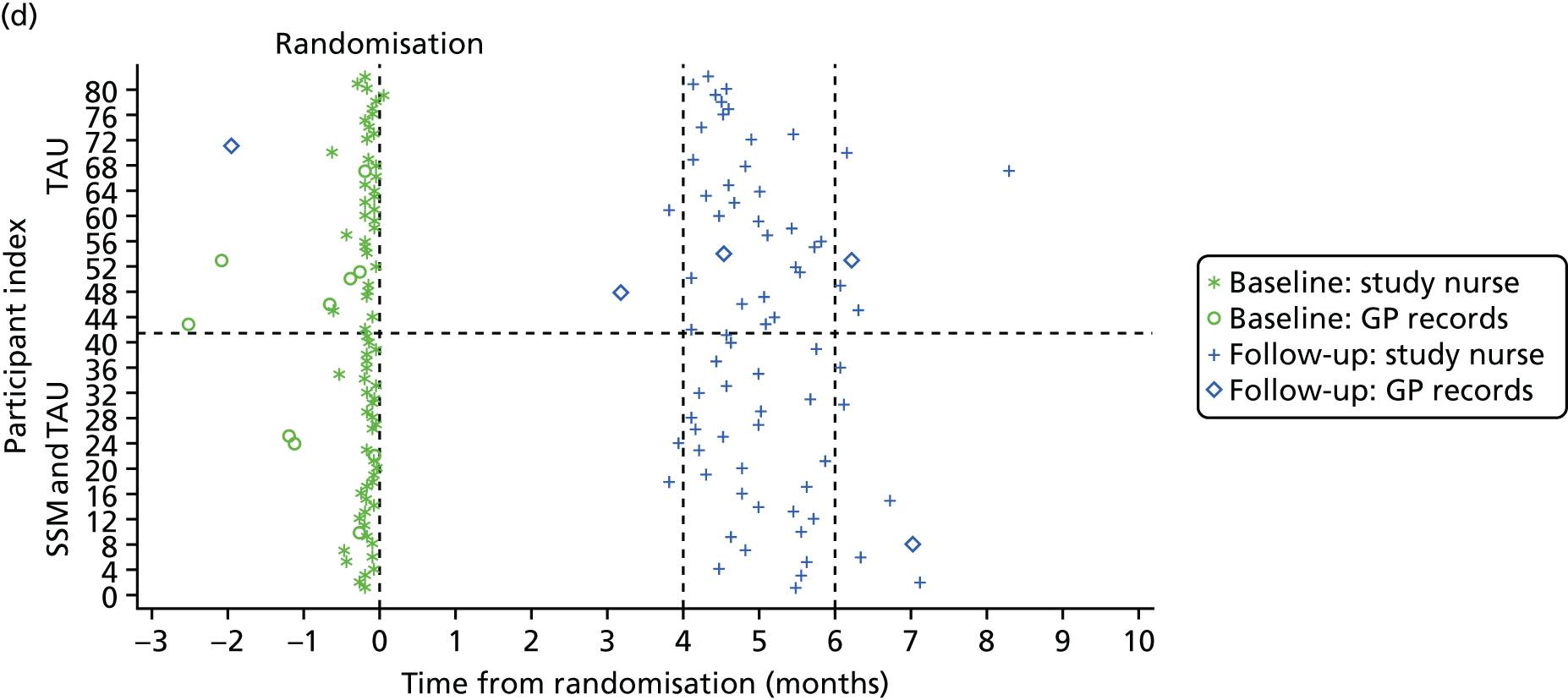
Statistical outcomes
Clinical characteristics of participants at baseline and follow-up are summarised and presented for candidate primary outcomes HbA1c level, BMI (Tables 55 and 56) and BP (Table 57) and other secondary outcomes including waist and hip measurement (Table 58), lipids (Table 59), renal function (Table 60), other outcomes (Table 61, QRISK®2 score, microalbuminuria concentration, medication) and mood (Table 62). The distribution of candidate outcome measures at baseline and follow-up are presented in Figure 12, and the distribution of change for HbA1c level and BMI is presented in Figures 13 and 14, respectively, to inform assessment of their sensitivity to change.
| HbA1c distribution | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | |||||
| Treatment arm | Total (N = 82) | Treatment arm | Total (N = 82) | |||
| SSM plus TAU (N = 41) | TAU (N = 41) | SSM plus TAU (N = 41) | TAU (N = 41) | |||
| HbA1c level (mmol/mol) | ||||||
| Mean (SD) | 57.0 (15.06) | 55.3 (18.03) | 56.1 (16.53) | 54.0 (13.52) | 54.6 (19.54) | 54.3 (16.73) |
| 95% CI | 52.24 to 61.76 | 49.60 to 60.98 | 52.51 to 59.78 | 49.52 to 58.53 | 48.18 to 61.03 | 50.47 to 58.17 |
| Median (range) | 52.0 (37–96) | 49.0 (33–121) | 51.0 (33–121) | 53.0 (31–104) | 49.0 (31–130) | 50.0 (31–130) |
| Missing | 0 | 0 | 0 | 4 | 3 | 7 |
| HbA1c level (% derived) | ||||||
| Mean (SD) | 7.4 (1.38) | 7.2 (1.65) | 7.3 (1.51) | 7.1 (1.24) | 7.1 (1.79) | 7.1 (1.53) |
| 95% CI | 6.93 to 7.80 | 6.69 to 7.73 | 6.95 to 7.62 | 6.68 to 7.51 | 6.56 to 7.73 | 6.77 to 7.47 |
| Median (range) | 6.9 (6–11) | 6.6 (5–13) | 6.8 (5–13) | 7.0 (5–12) | 6.6 (5–14) | 6.7 (5–14) |
| Missing | 0 | 0 | 0 | 4 | 3 | 7 |
| HbA1c level categorised, n (%) | ||||||
| < 6.5% (48 mmol/mol) | 15 (36.6) | 15 (36.6) | 30 (36.6) | 12 (32.4) | 18 (47.4) | 30 (40.0) |
| 6.5 to < 7.5% (48 to < 58 mmol/mol) | 10 (24.4) | 13 (31.7) | 23 (28.0) | 15 (40.5) | 9 (23.7) | 24 (32.0) |
| ≥ 7.5% (58 mmol/mol) | 16 (39.0) | 13 (31.7) | 29 (35.4) | 10 (27.0) | 11 (28.9) | 21 (28.0) |
| Missing | 0 | 0 | 0 | 4 | 3 | 7 |
| BMI distribution | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | |||||
| Treatment arm | Total (N = 82) | Treatment arm | Total (N = 82) | |||
| SSM plus TAU (N = 41) | TAU (N = 41) | SSM plus TAU (N = 41) | TAU (N = 41) | |||
| BMI result | ||||||
| Mean (SD) | 33.8 (6.94) | 34.3 (8.23) | 34.0 (7.58) | 34.2 (8.67) | 34.1 (8.46) | 34.1 (8.51) |
| 95% CI | 31.54 to 35.98 | 31.73 to 36.93 | 32.37 to 35.72 | 31.28 to 37.06 | 31.41 to 36.82 | 32.21 to 36.07 |
| Median (range) | 32.6 (21.7–50.9) | 32.4 (19.3–58.6) | 32.4 (19.3–58.6) | 32.0 (20.8–57.8) | 32.7 (18.6–58.2) | 32.5 (18.6–58.2) |
| Missing | 1 | 0 | 1 | 4 | 1 | 5 |
| BMI (kg/m2), n (%) | ||||||
| 18.5–24.9 (normal) | 1 (2.5) | 1 (2.4) | 2 (2.5) | 3 (8.1) | 1 (2.5) | 4 (5.2) |
| 25–29.9 (overweight) | 11 (27.5) | 14 (34.1) | 25 (30.9) | 10 (27.0) | 14 (35.0) | 24 (31.2) |
| 30–34.9 (obese class I) | 14 (35.0) | 9 (22.0) | 23 (28.4) | 9 (24.3) | 10 (25.0) | 19 (24.7) |
| 35–39.9 (obese class II) | 7 (17.5) | 9 (22.0) | 16 (19.8) | 7 (18.9) | 6 (15.0) | 13 (16.9) |
| ≥ 40 (obese class III) | 7 (17.5) | 8 (19.5) | 15 (18.5) | 8 (21.6) | 9 (22.5) | 17 (22.1) |
| Missing | 1 | 0 | 1 | 4 | 1 | 5 |
| Weight (kg) | ||||||
| Mean (SD) | 89.6 (20.54) | 90.5 (25.14) | 90.0 (22.71) | 91.4 (25.91) | 87.2 (23.74) | 89.2 (24.72) |
| 95% CI | 82.99 to 96.30 | 81.94 to 98.96 | 84.81 to 95.26 | 82.61 to 100.1 | 79.49 to 94.88 | 83.51 to 94.89 |
| Median (range) | 87.1 (60.0–158.5) | 87.0 (55.0–150.2) | 87.0 (55.0–158.5) | 89.2 (49.7–173.0) | 81.5 (53.0–154.7) | 85.0 (49.7–173.0) |
| Missing | 2 | 5 | 7 | 5 | 2 | 7 |
| BP distribution | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | |||||
| Treatment arm | Total (N = 82) | Treatment arm | Total (N = 82) | |||
| SSM plus TAU (N = 41) | TAU (N = 41) | SSM plus TAU (N = 41) | TAU (N = 41) | |||
| Systolic BP (mmHg) | ||||||
| Mean (SD) | 127.8 (16.07) | 125.7 (16.47) | 126.7 (16.20) | 119.4 (18.06) | 122.6 (16.65) | 121.1 (17.32) |
| 95% CI | 122.6 to 133.0 | 120.5 to 131.0 | 123.1 to 130.4 | 113.4 to 125.5 | 117.2 to 128.1 | 117.1 to 125.0 |
| Median (range) | 128.0 (92–153) | 123.0 (91–167) | 125.0 (91–167) | 116.0 (78–180) | 121.0 (96–161) | 117.0 (78–180) |
| Missing | 2 | 1 | 3 | 4 | 3 | 7 |
| Systolic BP (mmHg), n (%) | ||||||
| < 140 | 29 (74.4) | 33 (82.5) | 62 (78.5) | 33 (89.2) | 30 (78.9) | 63 (84.0) |
| ≥ 140 | 10 (25.6) | 7 (17.5) | 17 (21.5) | 4 (10.8) | 8 (21.1) | 12 (16.0) |
| Missing | 2 | 1 | 3 | 4 | 3 | 7 |
| Diastolic BP (mmHg), n (%) | ||||||
| Mean (SD) | 78.7 (10.93) | 77.7 (11.56) | 78.2 (11.19) | 76.3 (9.89) | 74.6 (10.50) | 75.4 (10.17) |
| 95% CI | 75.15 to 82.23 | 73.98 to 81.37 | 75.67 to 80.68 | 72.97 to 79.57 | 71.18 to 78.08 | 73.10 to 77.78 |
| Median (range) | 79.0 (54–97) | 76.0 (49–104) | 78.0 (49–104) | 75.0 (52–97) | 74.5 (48–94) | 75.0 (48–97) |
| Missing | 2 | 1 | 3 | 4 | 3 | 7 |
| Diastolic BP (mmHg), n (%) | ||||||
| < 80 | 20 (51.3) | 27 (67.5) | 47 (59.5) | 24 (64.9) | 24 (63.2) | 48 (64.0) |
| ≥ 80 | 19 (48.7) | 13 (32.5) | 32 (40.5) | 13 (35.1) | 14 (36.8) | 27 (36.0) |
| Missing | 2 | 1 | 3 | 4 | 3 | 7 |
| Waist and hip measurement distribution | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | |||||
| Treatment arm | Total (N = 82) | Treatment arm | Total (N = 82) | |||
| SSM plus TAU (N = 41) | TAU (N = 41) | SSM plus TAU (N = 41) | TAU (N = 41) | |||
| Waist measurement (cm) | ||||||
| Mean (SD) | 112.5 (17.74) | 109.2 (16.58) | 110.9 (17.17) | 113.0 (18.97) | 109.2 (15.91) | 111.0 (17.43) |
| 95% CI | 106.8 to 118.1 | 103.5 to 114.9 | 107.0 to 114.9 | 106.6 to 119.4 | 104.0 to 114.3 | 107.0 to 115.0 |
| Median (range) | 111.0 (86–165) | 105.0 (73–161) | 109.0 (73–165) | 110.0 (85–162) | 106.0 (79–158) | 108.0 (79–162) |
| Missing | 1 | 6 | 7 | 5 | 2 | 7 |
| Waist circumference and risk of metabolic complications,a n (%) | ||||||
| Not at increased risk | 4 (10.0) | 3 (8.6) | 7 (9.3) | 3 (8.3) | 3 (7.7) | 6 (8.0) |
| Increased risk | 3 (7.5) | 3 (8.6) | 6 (8.0) | 3 (8.3) | 4 (10.3) | 7 (9.3) |
| Substantially increased risk | 33 (82.5) | 29 (82.9) | 62 (82.7) | 30 (83.3) | 32 (82.1) | 62 (82.7) |
| Missing | 1 | 6 | 7 | 5 | 2 | 7 |
| Waist-to-hip ratio | ||||||
| Mean (SD) | 0.93 (0.11) | 0.92 (0.21) | 0.93 (0.16) | 0.96 (0.07) | 0.93 (0.07) | 0.94 (0.07) |
| 95% CI | 0.90 to 0.97 | 0.85 to 0.99 | 0.89 to 0.96 | 0.94 to 0.98 | 0.91 to 0.95 | 0.93 to 0.96 |
| Median (range) | 0.95 (0.36–1.09) | 0.94 (0.00–1.62) | 0.94 (0.00–1.62) | 0.96 (0.83–1.12) | 0.92 (0.74–1.06) | 0.93 (0.74–1.12) |
| Missing | 1 | 6 | 7 | 5 | 2 | 7 |
| Waist-to-hip ratio and risk of metabolic complications,b n (%) | ||||||
| Not at increased risk | 7 (17.5) | 8 (22.9) | 15 (20.0) | 3 (8.3) | 5 (12.8) | 8 (10.7) |
| Substantially increased risk | 33 (82.5) | 27 (77.1) | 60 (80.0) | 33 (91.7) | 34 (87.2) | 67 (89.3) |
| Missing | 1 | 6 | 7 | 5 | 2 | 7 |
| Lipid level | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | |||||
| Treatment arm | Total (N = 82) | Treatment arm | Total (N = 82) | |||
| SSM plus TAU (N = 41) | TAU (N = 41) | SSM plus TAU (N = 41) | TAU (N = 41) | |||
| Total cholesterol concentration (mmol/l) | ||||||
| Mean (SD) | 4.0 (0.85) | 4.2 (1.06) | 4.1 (0.96) | 3.8 (0.90) | 4.1 (1.07) | 4.0 (0.99) |
| 95% CI | 3.76 to 4.33 | 3.84 to 4.53 | 3.90 to 4.34 | 3.52 to 4.13 | 3.77 to 4.48 | 3.75 to 4.21 |
| Median (range) | 4.0 (2.3–5.6) | 4.0 (2.6–6.5) | 4.0 (2.3–6.5) | 3.8 (2.3–5.9) | 4.0 (2.7–6.9) | 3.9 (2.3–6.9) |
| Missing | 5 | 2 | 7 | 5 | 4 | 9 |
| Total cholesterol concentration (mmol/l), n (%) | ||||||
| < 4 | 17 (47.2) | 19 (48.7) | 36 (48.0) | 21 (58.3) | 18 (48.6) | 39 (53.4) |
| ≥ 4 | 19 (52.8) | 20 (51.3) | 39 (52.0) | 15 (41.7) | 19 (51.4) | 34 (46.6) |
| Missing | 5 | 2 | 7 | 5 | 4 | 9 |
| Triglycerides concentration (mmol/l) | ||||||
| Mean (SD) | 2.0 (1.15) | 2.2 (1.36) | 2.1 (1.25) | 1.9 (1.04) | 2.0 (1.12) | 2.0 (1.07) |
| 95% CI | 1.65 to 2.44 | 1.68 to 2.62 | 1.80 to 2.40 | 1.58 to 2.29 | 1.60 to 2.37 | 1.71 to 2.22 |
| Median (range) | 1.9 (0.7–5.4) | 1.8 (0.4–6.7) | 1.8 (0.4–6.7) | 1.8 (0.6–5.9) | 1.8 (0.5–6.4) | 1.8 (0.5–6.4) |
| Missing | 6 | 6 | 12 | 6 | 6 | 12 |
| Triglycerides concentration (mmol/l), n (%) | ||||||
| < 4.5 | 33 (94.3) | 33 (94.3) | 66 (94.3) | 34 (97.1) | 34 (97.1) | 68 (97.1) |
| 4.5–9.9 | 2 (5.7) | 2 (5.7) | 4 (5.7) | 1 (2.9) | 1 (2.9) | 2 (2.9) |
| Missing | 6 | 6 | 12 | 6 | 6 | 12 |
| Renal function | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | |||||
| Treatment arm | Total (N = 82) | Treatment arm | Total (N = 82) | |||
| SSM plus TAU (N = 41) | TAU (N = 41) | SSM plus TAU (N = 41) | TAU (N = 41) | |||
| eGFR (ml/minute/1.73 m2) | ||||||
| Missing | 9 | 5 | 14 | 6 | 5 | 11 |
| n known only to be > 90 | 17 | 23 | 40 | 22 | 23 | 45 |
| Mean (SD) | 67.3 (18.84) | 66.5 (16.47) | 66.9 (17.46) | 68.4 (17.56) | 69.1 (17.78) | 68.7 (17.32) |
| 95% CI | 56.83 to 77.70 | 56.59 to 76.49 | 60.16 to 73.70 | 57.77 to 79.00 | 58.33 to 79.82 | 61.74 to 75.73 |
| Median (range) | 72.0 (36–90) | 67.0 (33–89) | 68.0 (33–90) | 71.0 (40–88) | 70.0 (28–89) | 70.5 (28–89) |
| eGFR (ml/minute/1.73 m2), n (%) | ||||||
| ≥ 90 (normal kidney function) | 20 (62.5) | 23 (63.9) | 43 (63.2) | 22 (62.9) | 23 (63.9) | 45 (63.4) |
| 60–89 (mildly reduced kidney function) | 6 (18.8) | 10 (27.8) | 16 (23.5) | 8 (22.9) | 10 (27.8) | 18 (25.4) |
| 30–59 (moderately reduced kidney function) | 6 (18.8) | 3 (8.3) | 9 (13.2) | 5 (14.3) | 2 (5.6) | 7 (9.9) |
| < 30 (severely reduced kidney function) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.8) | 1 (1.4) |
| Missing | 9 | 5 | 14 | 6 | 5 | 11 |
| Urea concentration (mmol/l) | ||||||
| Missing | 9 | 3 | 12 | 8 | 6 | 14 |
| Mean (SD) | 6.0 (1.85) | 6.2 (1.81) | 6.1 (1.81) | 6.1 (1.66) | 6.0 (1.73) | 6.1 (1.68) |
| 95% CI | 5.33 to 6.66 | 5.59 to 6.78 | 5.66 to 6.53 | 5.53 to 6.71 | 5.40 to 6.58 | 5.65 to 6.46 |
| Median (range) | 6.1 (1.2–9.5) | 5.9 (2.7–9.9) | 6.1 (1.2–9.9) | 5.8 (3.4–9.4) | 5.9 (2.0–9.8) | 5.9 (2.0–9.8) |
| Creatinine concentration (µmol/l) | ||||||
| Missing | 8 | 3 | 11 | 4 | 0 | 4 |
| Mean (SD) | 73.7 (24.49) | 66.8 (24.02) | 70.0 (24.32) | 70.1 (24.89) | 67.7 (26.64) | 68.8 (25.68) |
| 95% CI | 65.01 to 82.38 | 58.89 to 74.68 | 64.24 to 75.76 | 61.78 to 78.38 | 59.30 to 76.11 | 63.04 to 74.62 |
| Median (range) | 71.0 (39–131) | 62.0 (39–170) | 66.0 (39–170) | 67.0 (14–140) | 63.0 (40–204) | 64.5 (14–204) |
| Outcomes | Treatment arm, n (%) | Total (N = 82), n (%) | |
|---|---|---|---|
| SSM plus TAU (N = 41) | TAU (N = 41) | ||
| QRISK®2 score (%) | |||
| < 10 | 8 (25.0) | 4 (13.3) | 12 (19.4) |
| 10 to < 20 | 12 (37.5) | 15 (50.0) | 27 (43.5) |
| ≥ 20 | 12 (37.5) | 11 (36.7) | 23 (37.1) |
| Missing | 9 | 11 | 20 |
| Microalbuminuria level (mg) | |||
| Missing | 20 | 19 | 39 |
| Mean (SD) | 26.9 (26.81) | 13.8 (18.90) | 20.2 (23.77) |
| 95% CI | 14.70 to 39.1 | 5.39 to 22.15 | 12.87 to 27.5 |
| Median (range) | 17.0 (1–83) | 5.5 (1–65) | 8.0 (1–83) |
| Microalbuminuria level (mg) | |||
| Yes (men > 2.5 mg/mmol and women > 3.5 mg/mmol) | 16 (76.2) | 17 (77.3) | 33 (76.7) |
| No (men ≤ 2.5 mg/mmol and women ≤ 3.5 mg/mmol) | 5 (23.8) | 5 (22.7) | 10 (23.3) |
| Missing | 20 | 19 | 39 |
| Participant taking medication for their diabetes | |||
| Yes | 29 (82.9) | 28 (77.8) | 57 (80.3) |
| No | 6 (17.1) | 8 (22.2) | 14 (19.7) |
| Missing | 6 | 5 | 11 |
| Which medications (if taking medications, not mutually exclusive) | |||
| Biguanide (metformin) | 25 (86.2) | 23 (82.1) | 48 (84.2) |
| Sulphonylurea | 9 (31.0) | 16 (57.1) | 25 (43.9) |
| Gliptin (DPP-4 inhibitors) | 2 (6.9) | 5 (17.9) | 7 (12.3) |
| Thiazolidinedione (glitazone) | 3 (10.3) | 5 (17.9) | 8 (14.0) |
| SGLT-2 inhibitors | 1 (3.4) | 0 (0.0) | 1 (1.8) |
| GLP-1 analogues | 2 (6.9) | 1 (3.6) | 3 (5.3) |
| Statins | 2 (6.9) | 1 (3.6) | 3 (5.3) |
| Antihypertensive agent | 1 (3.4) | 0 (0.0) | 1 (1.8) |
| Missing | 0 (0.0) | 1 (3.6) | 1 (1.8) |
| Monotherapy or polytherapy (if taking medications) | |||
| Monotherapy (single agent) | 19 (65.5) | 11 (39.3) | 30 (52.6) |
| Polytherapy (more than one agent) | 10 (34.5) | 17 (60.7) | 27 (47.4) |
| Medication changed in last 6 months (self-reported) | |||
| Yes | 3 (8.3) | 4 (10.3) | 7 (9.3) |
| No | 33 (91.7) | 35 (89.7) | 68 (90.7) |
| Missing | 5 | 2 | 7 |
| If yes, how has this changed? | |||
| Frequency | |||
| Decreased | 1 (33.3) | 1 (25.0) | 2 (28.6) |
| Increased | 1 (33.3) | 1 (25.0) | 2 (28.6) |
| Medication type changed | |||
| Added injectable | 1 (33.3) | 0 (0.0) | 1 (14.3) |
| Additional tablet added | 0 (0.0) | 1 (25.0) | 1 (14.3) |
| Unknown how | 0 (0.0) | 1 (25.0) | 1 (14.3) |
| Participant mood | Time point | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | |||||
| Treatment arm | Total (N = 82) | Treatment arm | Total (N = 82) | |||
| SSM plus TAU (N = 41) | TAU (N = 41) | SSM plus TAU (N = 41) | TAU (N = 41) | |||
| Little interest or pleasure doing things, n (%) | ||||||
| Not at all | 27 (65.9) | 28 (68.3) | 55 (67.1) | 22 (59.5) | 33 (86.8) | 55 (73.3) |
| Several days | 9 (22.0) | 9 (22.0) | 18 (22.0) | 10 (27.0) | 2 (5.3) | 12 (16.0) |
| More than half the days | 3 (7.3) | 3 (7.3) | 6 (7.3) | 1 (2.7) | 0 (0.0) | 1 (1.3) |
| Nearly every day | 2 (4.9) | 1 (2.4) | 3 (3.7) | 4 (10.8) | 3 (7.9) | 7 (9.3) |
| Missing | 0 | 0 | 0 | 4 | 3 | 7 |
| Feel down, depressed, n (%) | ||||||
| Not at all | 11 (32.4) | 15 (41.7) | 26 (37.1) | 8 (26.7) | 18 (60.0) | 26 (43.3) |
| Several days | 15 (44.1) | 15 (41.7) | 30 (42.9) | 7 (23.3) | 6 (20.0) | 13 (21.7) |
| More than half the days | 3 (8.8) | 3 (8.3) | 6 (8.6) | 5 (16.7) | 2 (6.7) | 7 (11.7) |
| Nearly every day | 5 (14.7) | 3 (8.3) | 8 (11.4) | 10 (33.3) | 4 (13.3) | 14 (23.3) |
| Missing | 7 | 5 | 12 | 11 | 11 | 22 |
| PHQ-2 score | ||||||
| Missing | 7 | 5 | 12 | 11 | 12 | 23 |
| Mean score (SD) | 1.7 (1.66) | 1.3 (1.51) | 1.5 (1.59) | 2.4 (1.87) | 1.1 (1.80) | 1.7 (1.93) |
| Median score (range) | 1.0 (0, 6) | 1.0 (0, 6) | 1.0 (0, 6) | 2.5 (0, 6) | 0.0 (0, 6) | 1.0 (0, 6) |
| PHQ-2 score according to cut-off point for major depression, n (%) | ||||||
| < 3 units | 24 (70.6) | 29 (80.6) | 53 (75.7) | 15 (50.0) | 23 (79.3) | 38 (64.4) |
| ≥ 3 units | 10 (29.4) | 7 (19.4) | 17 (24.3) | 15 (50.0) | 6 (20.7) | 21 (35.6) |
| Missing | 7 | 5 | 12 | 11 | 12 | 23 |
| Difficult answering PHQ-2, n (%) | ||||||
| No difficulty | 24 (60.0) | 23 (56.1) | 47 (58.0) | 20 (54.1) | 19 (50.0) | 39 (52.0) |
| Some difficulty | 15 (37.5) | 17 (41.5) | 32 (39.5) | 16 (43.2) | 13 (34.2) | 29 (38.7) |
| Extreme difficulty | 1 (2.5) | 1 (2.4) | 2 (2.5) | 1 (2.7) | 6 (15.8) | 7 (9.3) |
| Missing | 1 | 0 | 1 | 4 | 3 | 7 |
FIGURE 12.
Distribution of candidate primary outcome measures at baseline and follow-up by arm in RCT. (a) HbA1c level (mmol/mol); (b) BMI (kg/m2); (c) systolic BP (mmHg); and (d) diastolic BP (mmHg).
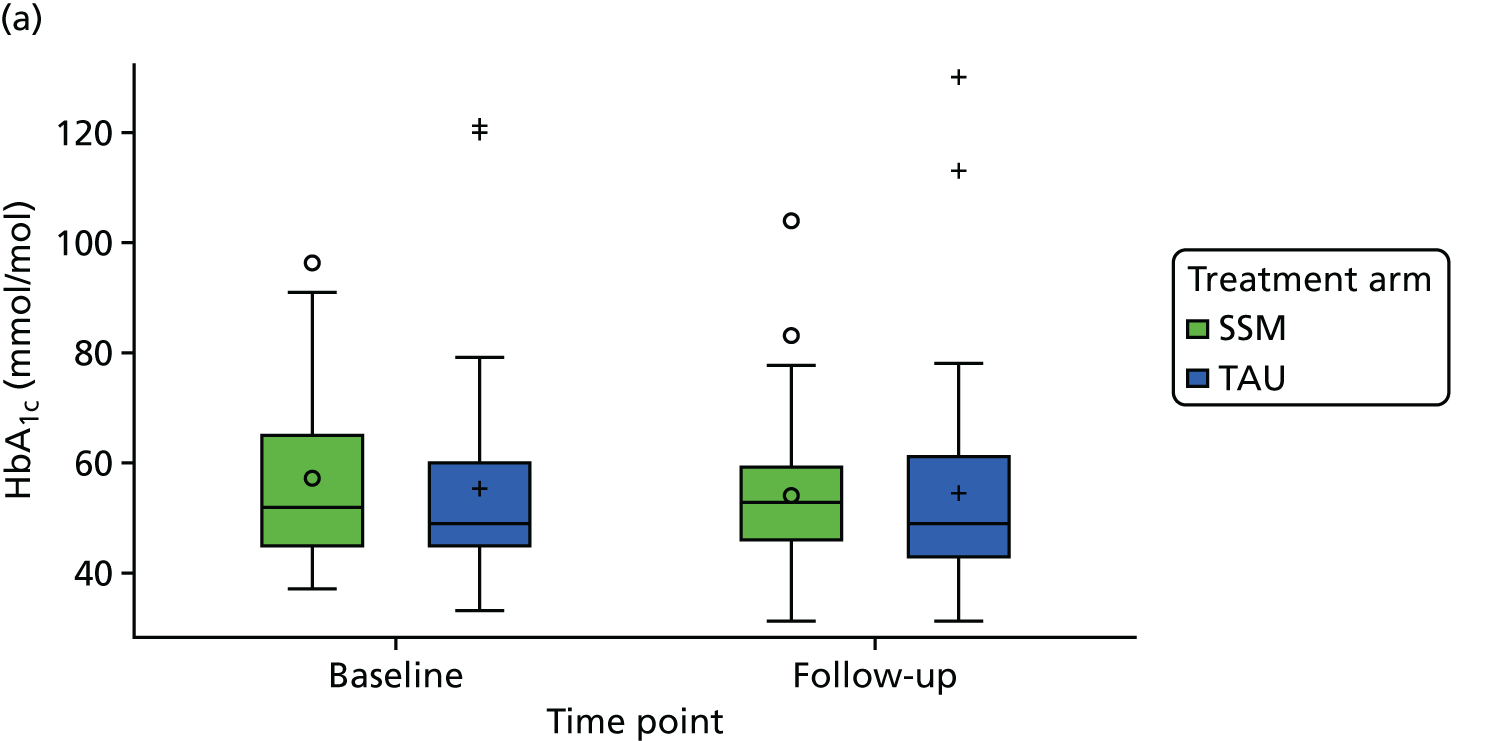
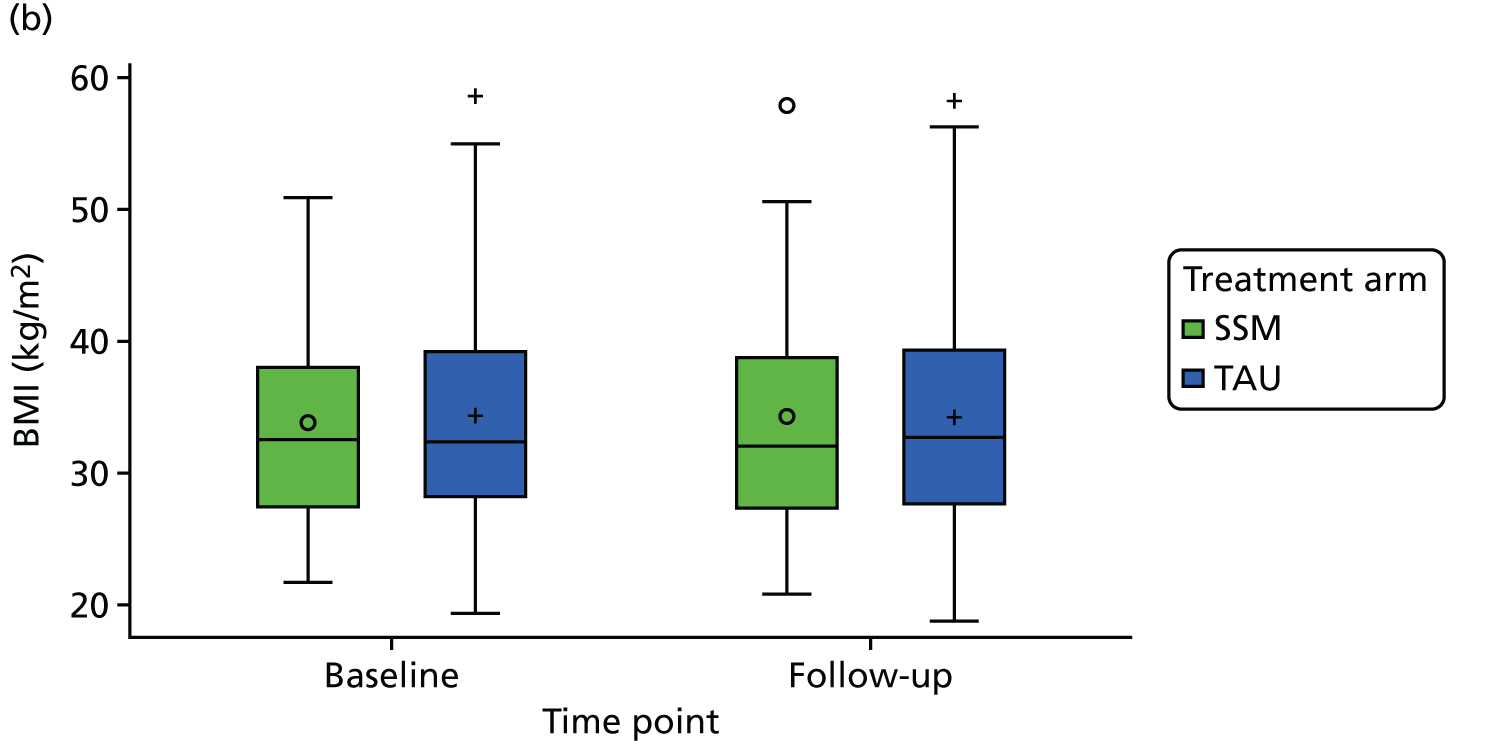

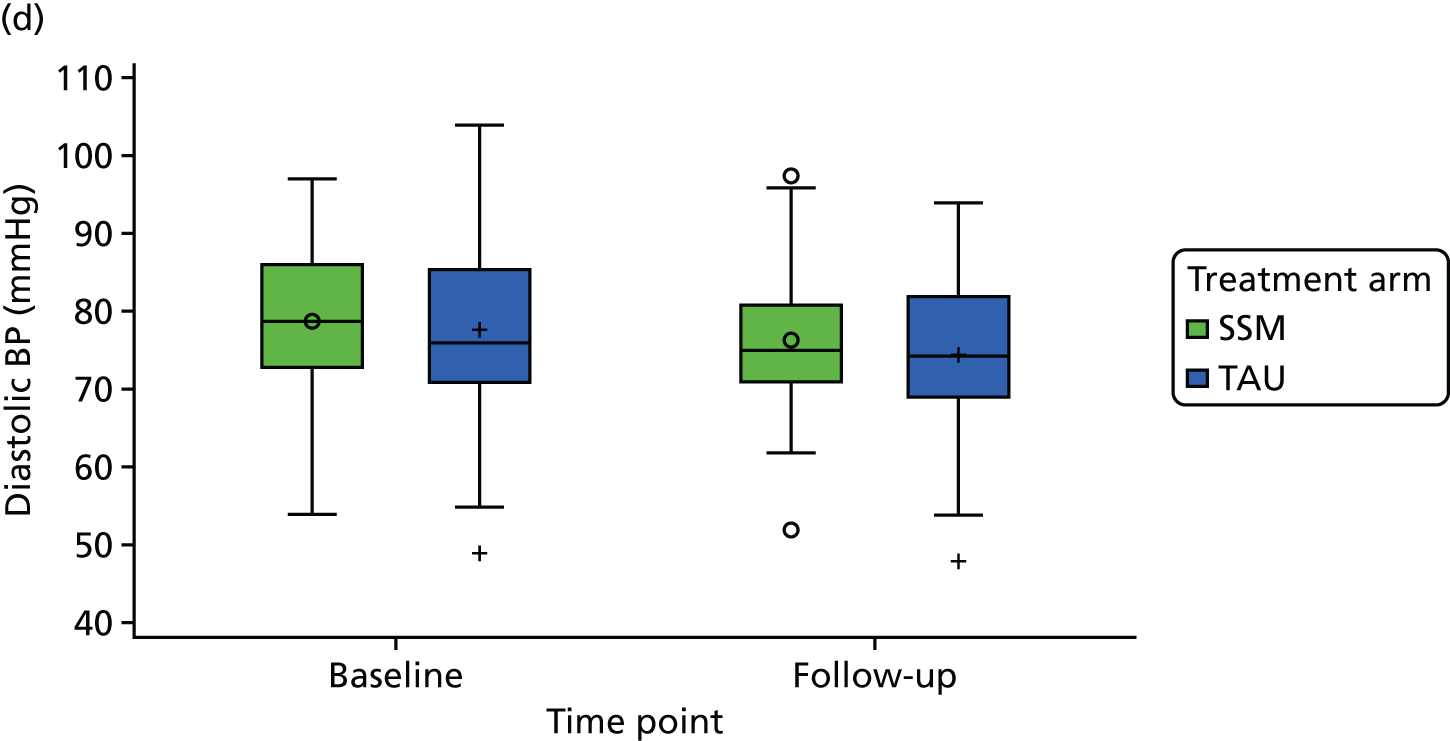
FIGURE 13.
Distribution of change in HbA1c level by allocation. (a) SSM; and (b) TAU. Max., maximum; min., minimum. Reproduced with permission from House et al. 176 Randomized controlled feasibility trial of supported self-management in adults with Type 2 diabetes mellitus and an intellectual disability: OK Diabetes, Diabetic Medicine, John Wiley & Sons. © 2018 The Authors. Diabetic Medicine published by John Wiley & Sons Ltd on behalf of Diabetes UK.

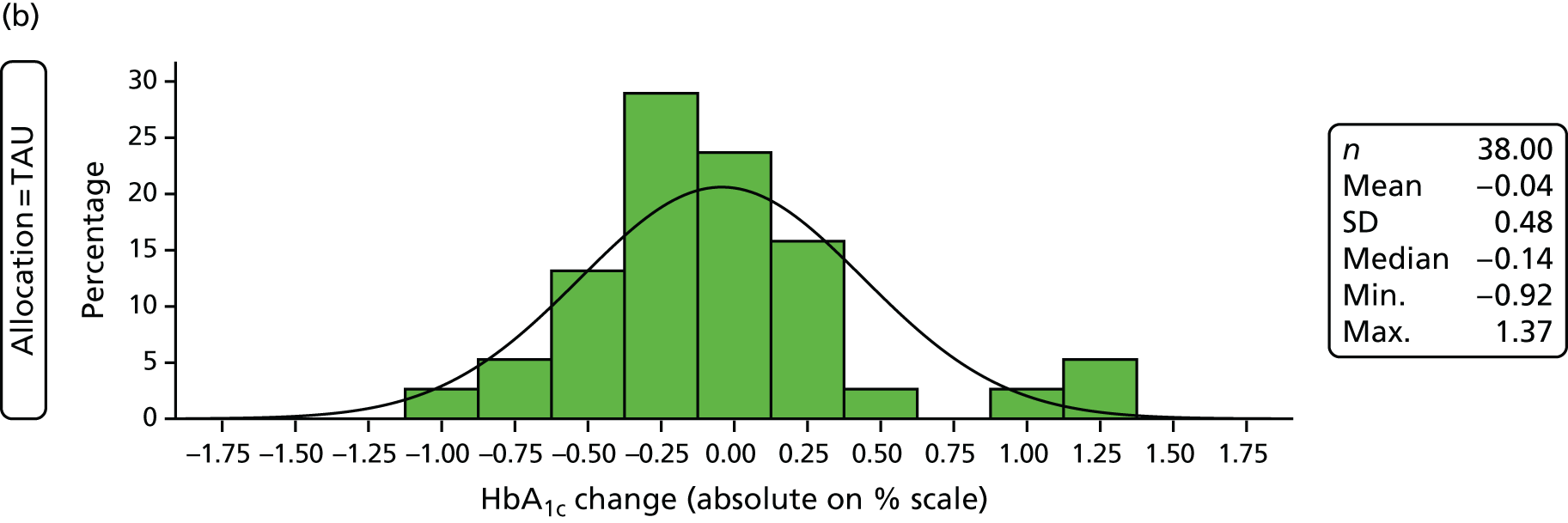
FIGURE 14.
Distribution of change in BMI by allocation. (a) SSM; and (b) TAU. Max., maximum; min., minimum. Reproduced with permission from House et al. 176 Randomized controlled feasibility trial of supported self-management in adults with Type 2 diabetes mellitus and an intellectual disability: OK Diabetes, Diabetic Medicine, John Wiley & Sons. © 2018 The Authors. Diabetic Medicine published by John Wiley & Sons Ltd on behalf of Diabetes UK.
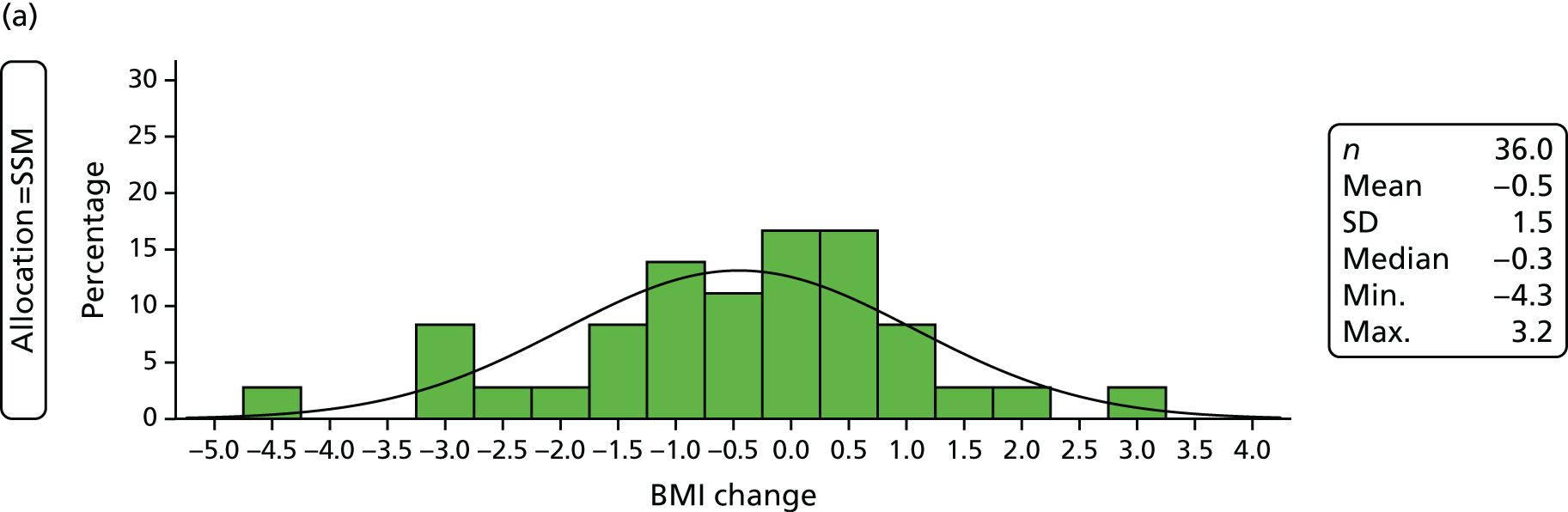
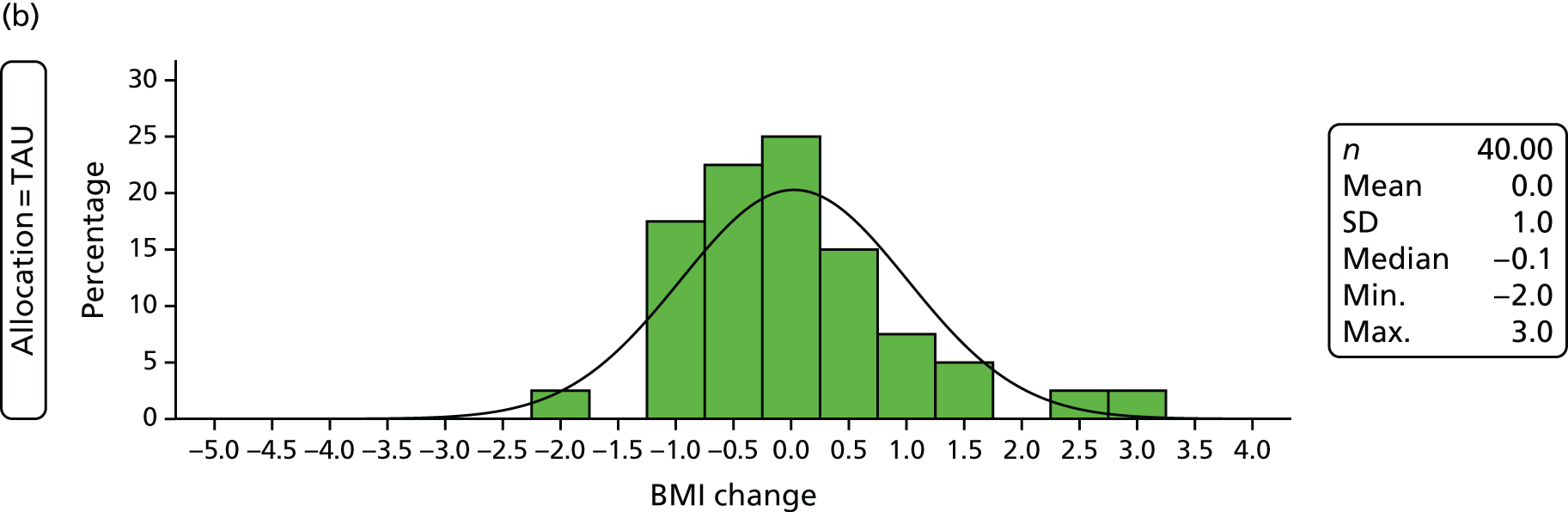
Candidate primary outcomes
Participants had a mean HbA1c level of 56.1 mmol/mol (SD 16.5 mmol/mol) at baseline and 54.3 mmol/mol (SD 16.7 mmol/mol) at follow-up (see Table 56). Compared with NICE guidance, HbA1c levels at follow-up were within the recommended range indicating good diabetes control (< 48 mmol/mol, 6.5%) for 30 out of 75 (40%) participants; however, 21 out of 75 (82%) participants were found to have poor diabetes control (≥ 58 mmol/mol, 7.5%).
Obesity was a problem for our sample. Participants had a mean BMI of 34.0 kg/m2 (SD 7.6 kg/m2) at baseline and 34.1 kg/m2 (SD 8.5 kg/m2) at follow-up, and 49 out of 77 (64%) participants were classed as obese (BMI of ≥ 30 kg/m2) at follow-up (see Table 57).
Candidate primary outcomes; sensitivity to change
Although between-group differences are not presented for our outcomes, a comparison of the mean outcome in each treatment group at follow-up provides an indication of the difference. However, it is not appropriate to rely on mean differences without adjusting for baseline values, especially in the presence of missing data at follow-up. In order to assess the sensitivity to change for candidate primary outcomes, we therefore observed the distribution of change in HbA1c level and BMI between baseline and follow-up (see Figures 13 and 14) and estimated the effect size in the SSM participants.
In the SSM trial arm there were 37 participants with HbA1c level measured at both baseline and follow-up. A mean reduction of 0.17% (SD 0.57%) was observed at follow-up, relating to an effect size of 0.3 (0.17/0.57). The comparative mean reduction in the control arm was 0.04% and the effect size was 0.08 (0.04/0.48).
For the 36 SSM participants with BMI measurements available at baseline and follow-up, there was a mean reduction in BMI of 0.5 kg/m2 (SD 1.5 kg/m2), relating to an effect size of 0.33 (0.5/1.5). The comparative mean change in the control arm was zero and the effect size was zero (0/1).
Secondary outcomes
Mean systolic BP was 126.7 mmHg (SD 16.2 mmHg) at baseline, reducing to 121.1 mmHg (SD 17.3 mmHg) at follow-up, and the mean diastolic BP was 78.2 mmHg (SD 11.2 mmHg) at baseline, reducing to 75.4 mmHg (SD 10.2 mmHg) at follow-up (see Table 57). About half of our sample had BP that was above the recommended levels at baseline and one-third of the sample had this at follow-up.
Similar outcomes were observed between trial arms at baseline and follow-up and across time points; follow-up outcomes for the total sample are referenced below unless otherwise indicated.
Waist and hip measurements suggested that 69 out of 75 (92%) participants were at increased or substantially increased risk of metabolic complications based on their waist circumference and 67 out of 75 (89%) were at substantially increased risk according to their waist-to-hip ratio (see Table 58).
Further outcomes that were collected at follow-up, via participants GP records, include the participant’s most recent QRISK®2 score, serum creatinine level, microalbuminuria level and medications for diabetes (see Table 62). A total of 23 out of 62 (37%) participants were reported to have a QRISK®2 score, for cardiovascular disease risk, of ≥ 20% and 33 out of 43 (77%) of participants had a microalbuminuria (mg) level that was within the elevated range, dependent on sex. Furthermore, 57 out of 71 (80%) participants were reported to be on a medication for their diabetes, of which 27 out of 57 (47%) were on multiple agents. The most frequently reported medications were biguanide (metformin) for 48 out of 57 (84%) participants who were on a medication and sulphonylurea for 25 out of 57 (44%) participants who were on a medication.
Scores of participant mood at baseline and follow-up, as captured by the PHQ-2 during the researcher visit, ranged from the minimum score of 0 units to the maximum score (i.e. most depressed) of 6 units across both time points and arms, with mean scores of 1.5 units at baseline and 1.7 units at follow-up and a median score of 1 unit for both time points (see Table 62). At baseline 17 out of 70 (24%) participants scored above the cut-off score (≥ 3 units) for possible major depression and, despite missing data, this was higher at follow-up, with major depression indicated in 21 out of 59 (36%) participants.
Participants expressed difficulty in answering the two PHQ-2 questions, with just under 50% having some or extreme difficulty at baseline and follow-up. Table 63 further summarises participant’s scores according to difficulty; the results do not suggest that those who found the questions most difficult were especially likely to rate above threshold.
| Difficulty answering the PHQ-2 | Time point, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | |||||||
| PHQ-2 score | Missing (N = 12) | Total (N = 82) | PHQ-2 score | Missing (N = 23) | Total (N = 82) | |||
| < 3 units (N = 53) | < 3 units (N = 17) | < 3 units (N = 38) | < 3 units (N = 21) | |||||
| No difficulty | 32 (61.5) | 12 (70.6) | 3 (25.0) | 47 (58.0) | 22 (61.1) | 7 (33.3) | 10 (55.6) | 39 (52.0) |
| Some difficulty | 19 (36.5) | 4 (23.5) | 9 (75.0) | 32 (39.5) | 11 (30.6) | 13 (61.9) | 5 (27.8) | 29 (38.7) |
| Extreme difficulty | 1 (1.9) | 1 (5.9) | 0 (0.0) | 2 (2.5) | 3 (8.3) | 1 (4.8) | 3 (16.7) | 7 (9.3) |
| Missing | 1 | 0 | 0 | 1 | 2 | 0 | 5 | 7 |
Safety
One death was reported during researcher contact for recruitment to the feasibility RCT and no randomised participants were reported to have died during the follow-up period.
Hospital attendances were reported via the researcher and study nurse (Table 64), through GP follow-up (Table 65) and also from the participant (Table 66).
| Unplanned hospital attendances | Treatment arm | Total (N = 82) | |
|---|---|---|---|
| SSM plus TAU (N = 41) | TAU (N = 41) | ||
| Number of participants with one or more admission, n (%) | 4 (9.8) | 6 (14.6) | 10 (12.2) |
| Number of admissions reported | 4 | 7 | 11 |
| Number of admissions per participant (all participants) | |||
| Mean (SD) | 0.1 (0.30) | 0.2 (0.44) | 0.1 (0.38) |
| Median (range) | 0.0 (0–1) | 0.0 (0–2) | 0.0 (0–2) |
| Missing | 0 | 0 | 0 |
| Reason for presentation (all attendances, not mutually exclusive), n (%) | |||
| Infection | 1 (25.0) | 3 (42.9) | 4 (36.4) |
| Fall | 1 (25.0) | 1 (14.3) | 2 (18.2) |
| Diabetes related/hypoglycaemia (as well as infection) | 0 (0.0) | 2 (28.6) | 2 (18.2) |
| Other | 2 (50.0) | 3 (42.9) | 5 (45.5) |
| Treatment required (all attendances), n (%) | |||
| Yes | 3 (100.0) | 7 (100.0) | 10 (100.0) |
| Missing | 1 | 0 | 1 |
| Outcome of admission (all attendances), n (%) | |||
| Recovered | 1 (25.0) | 4 (57.1) | 5 (45.5) |
| Recovered with residual effects | 3 (75.0) | 2 (28.6) | 5 (45.5) |
| Condition still present and unchanged | 0 (0.0) | 1 (14.3) | 1 (9.1) |
| Length of stay (all attendances, days) | |||
| Mean (SD) | 12.0 (0.00) | 7.4 (10.41) | 8.0 (9.77) |
| Median (range) | 12.0 (12–12) | 1.0 (0–24) | 2.5 (0–24) |
| Missing | 3 | 0 | 3 |
| Hospital attendance | Treatment arm | Total (N = 82) | |
|---|---|---|---|
| SSM plus TAU (N = 41) | TAU (N = 41) | ||
| Participant attended hospital on one or more occasions, n (%) | |||
| Yes | 7 (21.9) | 5 (14.7) | 12 (18.2) |
| No | 25 (78.1) | 29 (85.3) | 54 (81.8) |
| Missing | 9 | 7 | 16 |
| Participant attended hospital for diabetes-related physical illness, n (%) | |||
| No | 28 (100.0) | 33 (100.0) | 61 (100.0) |
| Missing | 13 | 8 | 21 |
| Participant attended hospital for non-diabetes-related physical illness, n (%) | |||
| Yesa | 2 (7.4) | 4 (12.1) | 6 (10.0) |
| No | 25 (92.6) | 29 (87.9) | 54 (90.0) |
| Missing | 14 | 8 | 22 |
| Participant attended hospital for mental illness, n (%) | |||
| Yesb | 1 (3.6) | 0 (0.0) | 1 (1.6) |
| No | 27 (96.4) | 33 (100) | 60 (98.4) |
| Missing | 13 | 8 | 21 |
| Event | Treatment arm | Total (N = 82) | |
|---|---|---|---|
| SSM plus TAU (N = 41) | TAU (N = 41) | ||
| Major life events? n (%) | |||
| Yes | 18 (48.6) | 19 (47.5) | 37 (48.1) |
| No | 19 (51.4) | 21 (52.5) | 40 (51.9) |
| Missinga | 4 | 1 | 5 |
| More upset than usual, n (%) | |||
| Yes | 16 (43.2) | 13 (34.2) | 29 (38.7) |
| No | 21 (56.8) | 25 (65.8) | 46 (61.3) |
| Missinga | 4 | 3 | 7 |
| Had to go to hospital because of your diabetes, n (%) | |||
| Yes | 1 (2.7) | 4 (10.0) | 5 (6.5) |
| No | 36 (97.3) | 36 (90.0) | 72 (93.5) |
| Missinga | 4 | 1 | 5 |
| Falls during activity, n (%) | |||
| Yes | 9 (24.3) | 7 (17.5) | 16 (20.8) |
| No | 28 (75.7) | 33 (82.5) | 61 (79.2) |
| Missinga | 4 | 1 | 5 |
Unplanned hospital attendances were reported by the researcher or study nurse for 10 (12%) participants overall with a total of 11 attendances: four attendances in four (10%) participants in the SSM arm, and seven attendances in six (15%) participants in the TAU arm. When the information could be elicited by the researcher or nurse, no attendances were suspected to be related to feasibility trial procedures. The outcome of the admission in five (45%) attendances was recovery or recovery with residual effects and in one (9%) attendance the condition remained present and unchanged. The length of hospital stay ranged from 0 (day case admission) to 24 days, with a mean of 8 days and a median 2.5 days.
At least one hospital attendance (A&E or admission) was reported by the participant’s GP for 12 out of 66 (18%) participants; 7 out of 32 (22%) in the SSM arm and 5 out of 34 (15%) in the TAU arm. There were notable missing data overall on whether or not a hospital attendance had occurred at all and whether or not the attendance was a result of a diabetes-related physical illness, non-diabetes physical illness or mental health. Nonetheless, no attendances were reported to be attributable to a diabetes-related physical illness; 6 out of 60 (10%) participants had attendances as a result of non-diabetes physical illness and one participant with a previous psychiatric history had an attendance because of mental illness.
During interviews with the researcher, 37 out of 77 (48%) participants reported a major life event (i.e. family bereavement, change in supporter) to have occurred since the baseline visit, 29 out of 75 (39%) reported that they felt more upset than usual, 5 out of 77 (6%) reported going to hospital because of their diabetes and 16 out of 77 (21%) reported having at last one fall during activity (see Table 66).
There was no report from any researcher or GP expressing concern that any of these hospital contacts were attributable to changes brought about either by research participation or by exposure to the SSM intervention. Participant qualitative interviews confirmed these opinions that there was no untoward outcome associated with the intervention. It was clear that participants were able to discuss recent events associated with distress or a change in their health status but did not include contact with researchers as a stressor.
The participant advocacy service did not give us details of individuals but informed us that they had received fewer than six contacts and all were requests about changing appointment times. Nobody called the service to express concerns about the project or to seek advice about withdrawal.
On two occasions, nurses were sufficiently concerned about the mental state of participants to discuss the problem (with AH) and, subsequently, to contact the GP. Both participants were well known to the GP and were in contact with mental health services and the GP did not regard recent changes as either very different from previously or as attributable to the RCT.
Self-reported outcomes
Additional information pertaining to participants’ views on changes in exercise, weight and eating, and drinking habits was collected during the researcher follow-up visit and is presented in Table 67. In total, 32 out of 77 (42%) participants reported that they had changed the amount of exercise they did; however, for eight (25%) of these participants this was a result of a decrease in the amount of exercise. For most, the change in exercise was a result of walking more or less. Overall, 35 out of 77 (45%) participants reported a change in their weight since baseline, with 26 out of 34 (76%) reporting to have lost weight, although 8 out of 34 (24%) participants felt that they had gained weight. Finally, approximately half of the participants, 40 out of 77 (52%), reported changes in what they eat and drink, with the larger proportion of these reporting they eat less, in 24 out of 38 (63%) participants; however, nine (24%) participants reported eating more.
| Self-reported outcomes | Treatment arm | Total (N = 82) | |
|---|---|---|---|
| SSM plus TAU (N = 41) | TAU (N = 41) | ||
| Have you changed the amount of exercise you do?a n (%) | |||
| Yes | 18 (48.6) | 14 (35.0) | 32 (41.6) |
| No | 17 (45.9) | 24 (60.0) | 41 (53.2) |
| Do not know | 2 (5.4) | 2 (5.0) | 4 (5.2) |
| Missing | 4 | 1 | 5 |
| If yes, how has this changed? n (%) | |||
| More exercise | 13 (72.2) | 11 (78.6) | 24 (75.0) |
| Less exercise | 5 (27.8) | 3 (21.4) | 8 (25.0) |
| If yes, what have you changed? | |||
| Walking more | 11 (73.3) | 9 (69.2) | 20 (71.4) |
| Walking less | 3 (20.0) | 3 (23.1) | 6 (21.4) |
| Attending activity classes | 1 (6.7) | 1 (7.7) | 2 (7.1) |
| Missing | 3 | 1 | 4 |
| Has your weight changed?a n (%) | |||
| Yes | 17 (45.9) | 18 (45.0) | 35 (45.5) |
| No | 12 (32.4) | 11 (27.5) | 23 (29.9) |
| Do not know | 8 (21.6) | 11 (27.5) | 19 (24.7) |
| Missing | 4 | 1 | 5 |
| If yes, how has this changed? n (%) | |||
| Lost weight | 13 (81.3) | 13 (72.2) | 26 (76.5) |
| Gained weight | 3 (18.8) | 5 (27.8) | 8 (23.5) |
| Missing | 1 | 0 | 1 |
| Have you changed what you eat or drink?a n (%) | |||
| Yes | 20 (54.1) | 20 (50.0) | 40 (51.9) |
| No | 15 (40.5) | 20 (50.0) | 35 (45.5) |
| Do not know | 2 (5.4) | 0 (0.0) | 2 (2.6) |
| Missing | 4 | 1 | 5 |
| If yes, what have you changed? n (%) | |||
| Eat more | 2 (11.1) | 7 (35.0) | 9 (23.7) |
| Eat less | 13 (72.2) | 11 (55.0) | 24 (63.2) |
| Drink less | 2 (11.1) | 1 (5.0) | 3 (7.9) |
| Drink more | 1 (5.6) | 1 (5.0) | 2 (5.3) |
| Missing | 2 | 0 | 2 |
Health economics: participant questionnaires and interviews
Participants’ interviews were undertaken between September 2014 and September 2015. At baseline, 82 participants were interviewed and 77 were interviewed for a second time at follow-up. The baseline and follow-up questions for participants (see Appendix 19) included items about their accommodation, employment and support; their health service use over the previous 4 weeks, including visits to the hospital and changes to their medications; and diet and physical activities.
Missing data were minimal. However, the interviewers’ recorded perceptions of the ease of completion gives a more in-depth understanding of the challenges for participants in responding to the questions.
Accommodation and employment
For the question about participants’ accommodation, there was only one missing observation across the sample, but the interviewer’s notes recorded that this participant found it difficult to categorise their residence:
Could tell researcher he lived in a flat and staff are always present. Wasn’t able to agree with researcher’s choice, most probably because he didn’t understand the term.
Suggested he lived in sheltered housing when went through options. Then when discussing further we decided it was shared house.
Similar difficulties were experienced with questions about participants’ current employment status. Although there were no missing data for this question, the interviewers recorded 31 participants (37.8%) having difficulty categorising their employment status at baseline:
Participant didn’t answer after the options were put to her. Supporter explained she had a LD [learning disability] and this is why she hasn’t worked. Participant said she didn’t have a disability.
Mix up between volunteering and social life (re: going to a bowling centre and helping out at the bowling centre). Turned out it’s socialising.
Generally, supporters did help to clarify some answers; however, one intervened too quickly at baseline and three intervened at follow-up:
Hard to tell if participant would have eventually answered because supporter jumped in.
Health and social care use
Participants were asked to recall what health and social care services they had used in the past 4 weeks. Very few missing data were reported at baseline and follow-up, and there were only a small number of ‘do not know’ responses (Tables 68 and 69). The interviewers did, however, report a number of respondents having difficulty recalling their health and social care use. The recall time posed particular problems:
Used ‘month’ as time frame and not 4 weeks, since didn’t seem to understand that as much.
Couldn’t remember whether in the last 4 weeks, supporter helped.
The time frame i.e. 4 weeks appeared to confound the participant, rather than the individual questions.
Considered these carefully but it appeared to be a guess at whether visits were in the last 4 weeks – so trouble with perception.
| Service (n = 82) | Response, n | Number of occassions | |||||
|---|---|---|---|---|---|---|---|
| Yes | No | Do not know | Missing | Mean (SD) | Minimum | Maximum | |
| Saw GP at the surgery | 40 | 40 | 2 | 0 | 1.35 (0.580) | 1 | 3 |
| Saw GP at home | 1 | 79 | 2 | 0 | 3.00 (0.00) | 3 | 3 |
| Saw a nurse | 4 | 75 | 3 | 0 | 2.25 (0.957) | 1 | 3 |
| Telephoned a nurse for advice | 32 | 48 | 2 | 0 | 1.55 (0.961) | 1 | 4 |
| Got a repeat prescription | 1 | 79 | 2 | 0 | 1.00 (0.00) | 1 | 1 |
| Got meals on wheels | 51 | 25 | 6 | 0 | 1.32 (0.875) | 1 | 4 |
| Home help came around | 0 | 80 | 2 | 0 | 0.00 (0.00) | 0 | 0 |
| Saw social worker | 14 | 67 | 1 | 0 | 7.45 (8.501) | 1 | 28 |
| Been to A&E | 4 | 77 | 0 | 1 | 1.00 (0.00) | 1 | 1 |
| Stayed in hospital overnight | 2 | 79 | 0 | 1 | 1.5 (0.707) | 1 | 2 |
| Outpatient | 31 | 50 | 1 | 0 | 1.452 (0.850) | 1 | 4 |
| Service (n = 77) | Response, n | Number of occassions | |||||
|---|---|---|---|---|---|---|---|
| Yes | No | Do not know | Missing | Mean (SD) | Minimum | Maximum | |
| Saw GP at the surgery | 26 | 47 | 4 | 0 | 1.44 (0.917) | 1 | 5 |
| Saw GP at home | 3 | 74 | 0 | 0 | 2 (1.414) | 1 | 3 |
| Saw a nurse | 13 | 64 | 0 | 0 | 1.92 (1.441) | 1 | 5 |
| Telephoned a nurse for advice | 23 | 50 | 3 | 1 | 1.36 (1.093) | 1 | 6 |
| Got a repeat prescription | 1 | 73 | 1 | 2 | 3.00 (0.00) | 3 | 3 |
| Got meals on wheels | 73 | 4 | 0 | 0 | 1.00 (0.00) | 1 | 1 |
| Home help came around | 0 | 77 | 0 | 0 | 0.00 (0.00) | 0 | 0 |
| Saw social worker | 14 | 61 | 1 | 1 | 8.75 (11.787) | 1 | 28 |
| Been to A&E | 4 | 72 | 0 | 1 | 2.00 (2.00) | 1 | 5 |
| Stayed in hospital overnight | 3 | 73 | 0 | 1 | 4.00 (5.196) | 1 | 10 |
| Outpatient | 16 | 60 | 1 | 0 | 1.40 (0.910) | 1 | 4 |
Questions relating to changes in medication were also challenging:
Participant thought medication had been increased, supporter checked and said it had not been changed.
Participant seemed to get stuck on describing that the format in which medication arrived has changed. Supporter intervened and confirmed that medication is the same.
Diet and exercise
Participants found the question about what they had eaten the previous day challenging (65% had difficulties with this question at baseline) with difficulties in recall and possibly defensiveness:
Couldn’t remember most meals and got a bit agitated.
Supporter reminded participant of biscuits they had as a treat.
Not sure if participant was embarrassed to disclose food intake or could not remember.
Participant was hesitant to answer because he knew supporter would tell him off.
Questions about exercise and activities were better understood (only 24% had difficulties with the activities question and 27% had difficulties with the exercise question). For those who had difficulty, terminology was a particular problem:
Unsure as to what classed as exercise. Mentioned walking a lot but couldn’t quantify how often, how long for, when last time.
Outcomes
The EQ-5D was incomplete for two participants at baseline and one at follow-up (Table 70). Despite few missing data, interviewers reported that around one-third of participants found the EQ-5D difficult to complete; specifically, many did not understand the language used or, in some cases, what they were being asked to do:
Didn’t understand the word moderate.
Didn’t understand if some problems meant you struggled or that you needed help. Wanted clarification.
| Time point | Participants, n | EQ-5D score | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Minimum | Lower quartile | Median | Upper quartile | Maximum | ||
| Baseline | ||||||||
| All | 80 | 0.6668 | 0.2875 | 0.239 | 0.603 | 0.725 | 0.848 | 1 |
| SSM | 40 | 0.6407 | 0.2866 | 0.181 | 0.551 | 0.701 | 0.832 | 1 |
| TAU | 40 | 0.6929 | 0.2896 | 0.239 | 0.675 | 0.727 | 0.849 | 1 |
| Follow-up | ||||||||
| All | 76 | 0.6641 | 0.3458 | 0.239 | 0.431 | 0.788 | 1 | 1 |
| SSM | 37 | 0.6699 | 0.3225 | 0.041 | 0.552 | 0.743 | 1 | 1 |
| TAU | 39 | 0.6586 | 0.3706 | 0.239 | 0.309 | 0.814 | 1 | 1 |
The supporter provided explanations of the questions for some participants and, in some cases, answered questions on the respondent’s behalf and persuaded respondents to change answers to match their own perceptions of the respondent’s health state. In the case of the respondent’s health state it was clear that often the supporter did not agree with the level chosen by the participant:
Supporter questioned whether he was in pain today as patient talked about past experience.
. . . Support worker changed his mind.
The supporter seemed to suggest the participant’s health was worse than the participant judged.
Would have managed without supporters input.
Supporter answered some questions because participant struggled to. Although unsure if he had been given more time, he might have been able to.
After adjusting for baseline differences in age, sex and baseline EQ-5D, QALYs were calculated for each treatment arm. Table 71 provides these figures. The average total number of QALYs gained over the 6 months was marginally higher in the SSM arm than in the TAU arm. The difference between SSM plus TAU and TAU of 0.0067 QALYs is the equivalent of 2.45 days (0.0067 × 365 days).
| Treatment arm | Responses, n | QALYs | |||
|---|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | ||
| SSM | 36 | 0.3376 | 0.0064 | 0.3285 | 0.3542 |
| TAU | 38 | 0.3309 | 0.0078 | 0.3167 | 0.3545 |
Supporter questionnaire
In total, 12 unpaid supporters completed the supporter interviews at baseline and 10 completed the interviews at follow-up. The interviewers’ notes suggest that, overall, there was very little difficulty in answering questions about their out-of-pocket expenses and their employment status. Just one unpaid supporter at baseline had some difficulty answering the question about their employment status; no reason was given by the researcher. Two had some difficulty recalling if they had accompanied the participant to their health-care visits.
One unpaid supporter was recorded as having some difficulty answering EQ-5D at baseline; the researcher read the questions for them as they were not confident in reading it themselves.
General practice-reported resource use
In order to calculate the mean cost of care for the participants over a 4-month period, we assigned unit costs to each service use (Table 72). Data were not available for the length of stay in hospital and, therefore, each recorded inpatient stay was assumed to be a short stay. The cost analysis was based on complete cases only (i.e. forms which had no missing or unknown data), and this reduced our sample from 70 (85% of randomised participants) to 55 (67% of randomised participants) (Tables 73 and 74).
| Variable | Complete cases, n | Mean number of visits (SD) | Cost (£) | ||
|---|---|---|---|---|---|
| Mean (SD) | Minimum | Maximum cost | |||
| GP | 55 | 3.13 (4.46) | 143.85 (205.16) | 0 | 1058 |
| Practice nurse | 55 | 1.18 (1.47) | 16.18 (20.08) | 0 | 95.83 |
| District nurse | 55 | 0.05 (0.30) | 1.40 (7.70) | 0 | 51.48 |
| Diabetic clinic at the hospital | 55 | 0.04 (0.19) | 3.82 (19.84) | 0 | 105 |
| Ophthalmologist | 55 | 0.13 (0.39) | 9.04 (27.51) | 0 | 142 |
| Podiatrist | 55 | 0.36 (0.62) | 11.64 (19.83) | 0 | 64 |
| Dietitian | 55 | 0.05 (0.23) | 2.02 (8.48) | 0 | 37 |
| Nephrologist | 55 | 0.00 (0.00) | 0.00 (0.00) | 0 | 0 |
| Diabetes educational course | 55 | 0.00 (0.00) | 0.00 (0.00) | 0 | 0 |
| Chronic illness course | 55 | 0.04 (0.19) | 2.36 (12.17) | 0 | 65 |
| Inpatient stays | 55 | 0.13 (0.43) | 77.76 (264.36) | 0 | 1222 |
| A&E | 55 | 0.16 (0.42) | 29.45 (75.61) | 0 | 360 |
| Total | 55 | 5.27 (5.03) | 321.89 (396.52) | 0 | 1947 |
| Resource item | Face-to-face (assumed) cost (£) | Sources |
|---|---|---|
| GP surgery visit/contact | 46.00 | Curtis (2014),171 p. 194, including direct care staff costs with qualification; per-patient contact lasting 11.7 minutes |
| Nurse (general practice) | 13.69 | Curtis (2014),171 p. 192, £53 per hour, 15.5-minute visits |
| District nurse (face to face) | 25.74 | Curtis (2014),171 p. 187, £66 per hour, per hour of patient-related work including qualification, assume home visit of 23.4 minutes |
| Diabetic clinic at the hospital | 105.00 | National Schedule of Reference Costs Year 2014–2015: NHS Trusts PCT Combined,173 307 diabetic medicine: non-consultant-led outpatient attendances |
| Ophthalmologist | 71.00 | National Schedule of Reference Costs Year 2014–2015: NHS Trusts PCT Combined,173 460: medical ophthalmology: consultant-led outpatient attendances |
| Podiatrist | 32.00 | Curtis (2014),171 p. 182, £32 per hour |
| Dietitian | 37.00 | Curtis (2014),171 p. 238, £37 per hour, hospital dietitian |
| Nephrologist | 147.00 | National Schedule of Reference Costs Year 2014–2015: NHS Trusts PCT Combined,173 WF01A: non-admitted face-to-face attendance, follow-up; service code 361: nephrology; national average unit cost: consultant-led outpatient attendance |
| Hospital inpatient stay | 611.00 | Curtis (2014),171 p. 111, national average non-elective short stay, assume all are short stays |
| Hospital outpatient clinic | 109.00 | Curtis (2014),171 p. 111, weighted average of all outpatient procedures |
| Hospital A&E | 180.00 | NHS Reference Costs 2013–14,190 accident and emergency: service code 180: total unit cost |
| Treatment arm | Responses, n | Mean number of visits (SD) | Cost (£) | ||
|---|---|---|---|---|---|
| Mean (SD) | Minimum | Maximum | |||
| GP | |||||
| SSM | 26 | 3.15 (4.40) | 145.08 (202.27) | 0.00 | 782.00 |
| TAU | 29 | 3.10 (4.59) | 142.76 (211.28) | 0.00 | 1058.00 |
| Practice nurse | |||||
| SSM | 26 | 1.35 (1.35) | 18.43 (18.55) | 0.00 | 54.76 |
| TAU | 29 | 1.03 (1.57) | 14.16 (21.49) | 0.00 | 95.83 |
| District nurse | |||||
| SSM | 26 | 0.00 (0.00) | 0.00 (0.00) | 0.00 | 0.00 |
| TAU | 29 | 0.10 (0.41) | 2.66 (10.53) | 0.00 | 51.48 |
| Diabetic clinic at the hospital | |||||
| SSM | 26 | 0.04 (0.20) | 4.04 (20.59) | 0.00 | 105.00 |
| TAU | 29 | 0.03 (0.19) | 3.62 (19.50) | 0.00 | 105.00 |
| Ophthalmologist | |||||
| SSM | 26 | 0.15 (0.46) | 10.92 (32.95) | 0.00 | 142.00 |
| TAU | 29 | 0.10 (0.31) | 7.34 (22.01) | 0.00 | 71.00 |
| Podiatrist | |||||
| SSM | 26 | 0.31 (0.62) | 9.85 (19.77) | 0.00 | 64.00 |
| TAU | 29 | 0.41 (0.63) | 13.24 (20.09) | 0.00 | 64.00 |
| Dietitian | |||||
| SSM plus TAU | 26 | 0.08 (0.27) | 2.85 (10.05) | 0.00 | 37.00 |
| TAU | 29 | 0.03 (0.19) | 1.28 (6.87) | 0.00 | 37.00 |
| Nephrologist | |||||
| SSM | 26 | 0.00 (0.00) | 0.00 (0.00) | 0.00 | 0.00 |
| TAU | 29 | 0.00 (0.00) | 0.00 (0.00) | 0.00 | 0.00 |
| Inpatient stays | |||||
| SSM | 26 | 0.15 (0.46) | 94.00 (283.56) | 0.00 | 1222.00 |
| TAU | 29 | 0.10 (0.41) | 63.21 (250.05) | 0.00 | 1222.00 |
| A&E | |||||
| SSM | 26 | 0.23 (0.51) | 41.54 (92.59) | 0.00 | 360.00 |
| TAU | 29 | 0.10 (0.31) | 18.62 (55.79) | 0.00 | 180.00 |
| Total | |||||
| SSM | 26 | 5.46 (5.23) | 326.51 (398.78) | 0.00 | 1494.00 |
| TAU | 29 | 5.10 (4.94) | 266.89 (351.55) | 32.00 | 1947.00 |
Examining the resource use by treatment arm, there are no important cost differences between the groups (see Table 74).
Table 75 details the recommended dose and costs of diabetes medications prescribed to the participants and Table 76 shows the medication use of the 55 participants with complete information.
| Drug name | Recommended dose as per the BNF and clinician advicea | Price as per the BNF | Cost (£) for 4 months |
|---|---|---|---|
| Metformin | 2000 mga per day | 500 mg, net price of 28-tablet pack = £1.58 | 27.46 |
| Metformin SR (e.g. Glucophage SR, Merck Serono Ltd) | 2000 mga per day | 1 g, 28-tablet pack = £4.26 | 37.02 |
| Gliclazide (e.g. Diamicron, Servier Laboratories Ltd) | 160 mg once a day | 80 mg, net price of 60-tablet pack = £4.38 | 17.76 |
| Glimepiride (e.g. Amaryl, Zentiva) | 4 mg daily | 4 mg, 30-tablet pack = £14.24 | 57.75 |
| Sitagliptin (e.g. Januvia, Merck Sharp & Dohme Ltd) | 100 mg once daily | 100 mg, 28-tablet pack = £33.26 | 144.52 |
| Pioglitazone (e.g. Actos, Takeda UK Ltd) | 30 mg daily | 30 mg, 28-tablet pack = £35.89 | 155.95 |
| Dapagliflozin (e.g. Forxiga, AstraZeneca UK Ltd) | 10 mg daily | 10 mg, 28-tablet pack = £36.59 | 158.99 |
| Exenatide (e.g. Byetta, AstraZeneca UK Ltd) | 10 µg twice daily | 10 µg/dose prefilled pen (60 doses) = £68.24 | 276.75 |
| Exenatide ER (e.g. Bydureon, AstraZeneca UK Ltd) | 2 mg once weekly | 2-mg pen = £18.34 | 318.77 |
| Liraglutide (e.g. Victoza, Novo Nordisk Ltd) | 1.2 mg once daily | 3 × 3-ml prefilled pens = £117.72. Each pen = 15 doses of 1.2 mg | 318.28 |
| Treatment arm | Observed (SSM, n = 26; TAU, n = 29) | % taking medication |
|---|---|---|
| Metformin | ||
| SSM | 14 | 53.85 |
| TAU | 15 | 51.72 |
| Metformin SR (e.g. Glucophage SR, Merck Serono Ltd) | ||
| SSM | 3 | 11.54 |
| TAU | 4 | 13.79 |
| Gliclazide (e.g. Diamicron, Servier Laboratories Ltd) | ||
| SSM | 5 | 19.23 |
| TAU | 7 | 24.13 |
| Glimepiride (e.g. Amaryl, Zentiva) | ||
| SSM | 2 | 7.69 |
| TAU | 5 | 17.24 |
| Sitagliptin (e.g. Januvia, Merck Sharp & Dohme Ltd) | ||
| SSM | 0 | 0.00 |
| TAU | 4 | 13.79 |
| Pioglitazone (e.g. Actos, Takeda UK Ltd) | ||
| SSM | 1 | 3.85 |
| TAU | 5 | 17.24 |
| Dapagliflozin (e.g. Forxiga, AstraZeneca UK Ltd) | ||
| SSM | 1 | 3.85 |
| TAU | 0 | 0.00 |
| Exenatide (e.g. Byetta, AstraZeneca UK Ltd) | ||
| SSM | 1 | 3.85 |
| TAU | 0 | 0.00 |
| Exenatide ER (e.g. Bydureon, AstraZeneca UK Ltd) | ||
| SSM | 1 | 3.85 |
| TAU | 0 | 0.00 |
| Liraglutide (e.g. Victoza, Novo Nordisk Ltd) | ||
| SSM | 1 | 3.85 |
| TAU | 0 | 0.00 |
Intervention costs
Intervention costs were calculated for the SSM arm (Table 77).
| Resource type | Unit cost | Observed | Mean visits | Time (minutes) | Cost (£) |
|---|---|---|---|---|---|
| Sessions with practice nurse | £53 per hour of face-to-face contacta | 37 | 2.6 | 45 | 103.35 |
| Telephone calls | £41 per hour | 8 | 1.875 | 1.5 | 1.92 |
| Total | 105.27 |
Development costs (Table 78) were estimated but not included in the cost-effectiveness analysis.
| Item | Cost (£) |
|---|---|
| Patient boards and information sheet development (easy on the i) | 1200.00 |
| Printing of patient boards | 2808.00 |
| Two × nursea training (2 hours) | 212.00 |
| Additional printing cost | 125.60 |
| Travel | 1852.00 |
| Total | 6197.60 |
Total costs for trial participants are shown in Table 79.
| Treatment arm | Mean cost (£) (SD) |
|---|---|
| Resource use | |
| SSM | 490.05 (598.18) |
| TAU | 400.34 (527.32) |
| Diabetes medications | |
| SSM | 34.41 (9.14) |
| TAU | 42.00 (19.68) |
| Intervention cost | |
| SSM | 105.27 (–) |
| TAU | – |
| Total | |
| SSM | 620.46 (592.03) |
| TAU | 433.65 (525.27) |
Post hoc exploratory cost-effectiveness
Results from a complete-case analysis, based on 55 participants with complete cost and EQ-5D data, yielded a deterministic ICER of £25,244 per QALY. Table 80 shows the deterministic ICER calculated by comparing costs with QALYs.
| Treatment arm | Mean cost (£) | Mean QALY | ICER |
|---|---|---|---|
| SSM | 620.46 | 0.3540 | 25,244.59 |
| TAU | 433.65 | 0.3467 | |
| Incremental | 186.81 | 0.0074 |
Sensitivity analysis
Bootstrapped uncertainty analysis produced a simulation ICER of £43,229 = mean costs (672.47 – 441.88/mean QALYs 0.3402 – 0.3349) (Figure 15).
FIGURE 15.
Cost-effectiveness plane showing the incremental cost and QALYs for SSM compared with TAU.
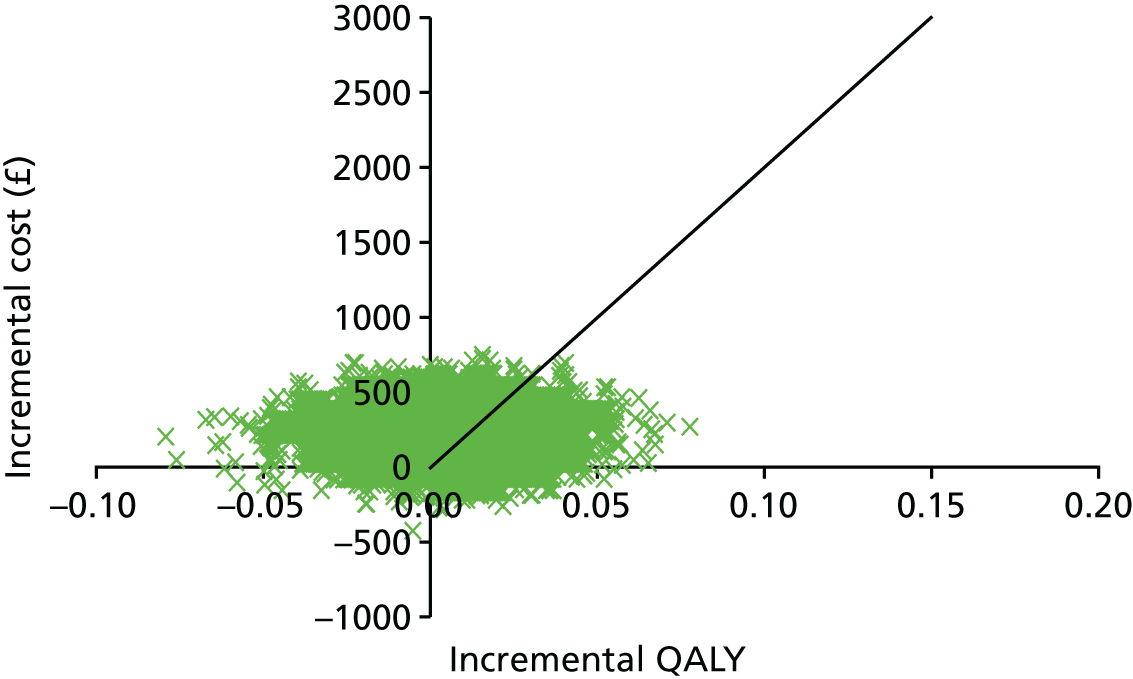
Figure 16 shows the CEAC, which reflects the probability of cost-effectiveness across a range of willingness-to-pay (WTP) values. Bootstrapped uncertainty analysis suggested that SSM plus TAU had only a 37.3% probability of being cost-effective at the £20,000 threshold; this probability rises to 45.3% if the threshold was £30,000.
FIGURE 16.
Cost-effectiveness acceptability curve SSM.
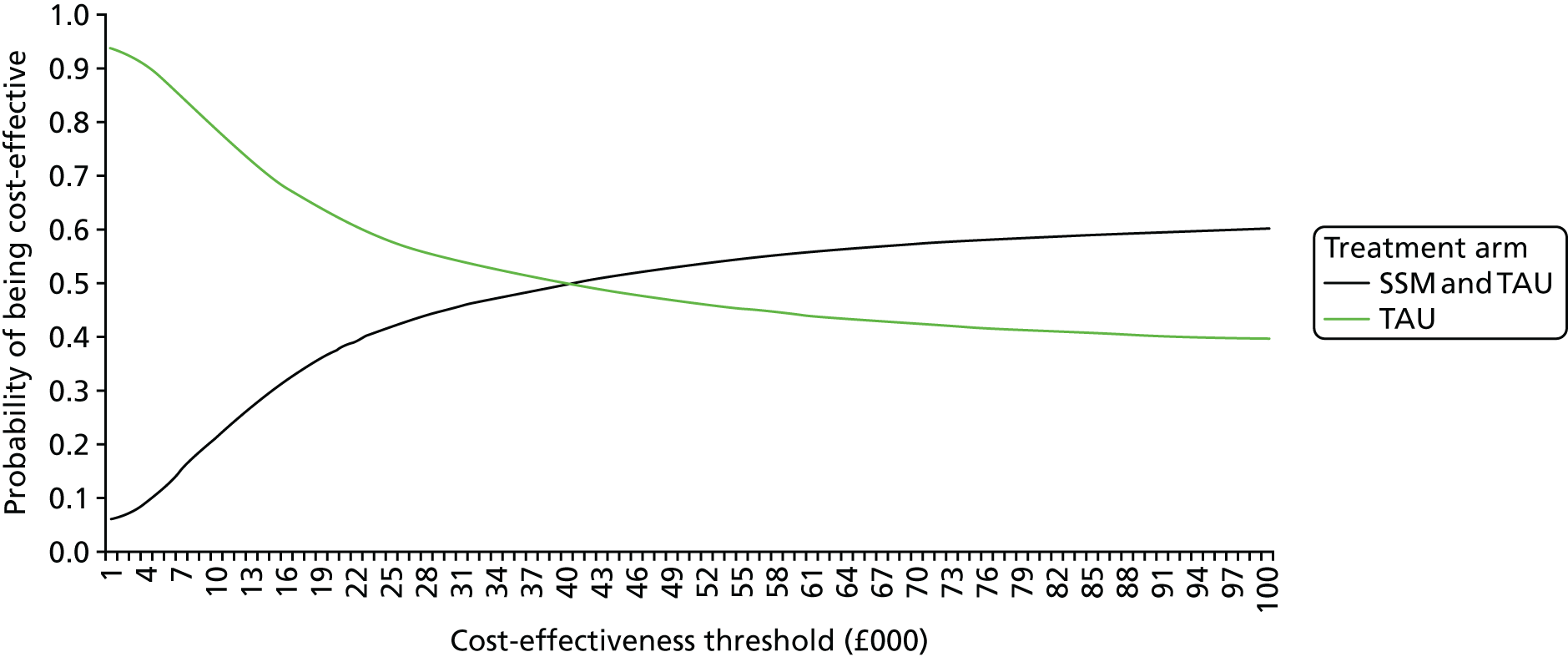
Discussion
The aims of the feasibility trial were to inform the design and feasibility for a definitive Phase III trial, and three criteria were prespecified to indicate unacceptable levels of recruitment, retention and intervention attendance that would prohibit us from proceeding to a full Phase III trial. Acceptable outcomes were achieved for each of these criteria: we recruited 82 participants between September 2014 and April 2015, more than the prespecified 20 required and more than the target of 80. There were active withdrawals for six (7%) participants and overall active or passive withdrawal from researcher or nurse follow-up of seven (9%) participants, which was less than the 40% maximum target prespecified. In addition, there was non-attendance in the manualised intervention sessions for four (10%) participants allocated to SSM, again less than the 50% maximum target prespecified.
Supporters were asked for consent to be part of the trial for 40 out of 82 (49%) participants, the majority of whom were a paid supporter (28/40, 70%) or were not living with the participant (31/40, 78%).
The characteristics of randomised participants were similar to those within the original eligible population from the case-finding study.
Intervention delivery: adherence and fidelity
The majority of participants allocated to receive SSM completed all required sessions (35/41, 85%), attending between two and four sessions each, lasting a mean of 45 minutes, over a mean of a 2.5-month period. Independent review of adherence and fidelity resulted in fidelity scores ranging from three to eight out of a possible eight (with eight being the best fidelity score). For 32 out of 37 (86%) participants who attended at least one session, the fidelity score was less than six and there was evidence that all steps within the intervention were completed at least once for all but two of the participants.
There was a high level of nurse unblinding – for 34 (42%) participants across the arms during outcome data collection at follow-up – largely as a result of difficulties in ensuring that the follow-up nurse was different from the nurse who delivered the intervention or who visited the participants at baseline and passed on their allocation. Researcher unblinding was high in the SSM arm, occurring for 15 (37%) participants (largely because of the researcher being informed by the participant) but it was low in the TAU arm, with unblinding occurring for just one participant.
Outcome measures
During the nurse visits to collect physical measures, only three (4%) participants refused the blood test and seven (9%) did not have a nurse visit at all at follow-up. There was some difficulty again in obtaining missing data from GPs, with medical records successfully obtained from the GP for just 5 out of 13 (38%) participants with missing physical measures at follow-up.
The candidate primary outcomes of mean HbA1c level and BMI were similar in each arm: a HbA1c level of 54.3 mmol/mol (SD 16.7 mmol/mol); and a BMI of 34.1 kg/m2 (SD 8.5 kg/m2), with 49 out of 77 (64%) classed as obese. For the two outcomes, effect sizes in the intervention arm of 0.33 and 0.30 suggest that both HbA1c and BMI may be sufficiently sensitive to change in this population.
Safety
High levels of depression, as judged by the PHQ-2, were indicated in almost one-quarter of all participants at baseline, and this was even higher, being reported by 36% of participants at follow-up; however, almost 50% reported difficulty in answering the PHQ-2.
There was difficulty in eliciting the occurrence and details of unplanned hospital attendances as opposed to routine appointments from participant and supporters, with 10 (12%) participants reporting an unplanned attendance and 12 (15%) also reporting routine day-case attendances. Hospital attendances were also requested from the GP at follow-up; however, data were not provided for 16 (20%) participants and the reason for attendance was also not well completed.
There was no evidence that either mental health problems or hospital attendance were attributable to the intervention.
Economic evaluation
Based on evidence from the literature and expert advice from the research team we developed three questionnaires: two relating to participants’ health and social care use and one for the supporters’ out-of-pocket expenses. We assessed two methods of data collection: GP surgery-completed (covering participants’ health and social care) and interview-administered (covering a wider cost perspective and health-related quality of life using the EQ-5D) questionnaires. In addition, we assessed interview-administered supporter questionnaires to collect out-of-pocket expenses and health-related quality of life, again using the EQ-5D.
The GP-completed questions had a good return rate and content was relevant with service use in line with previous studies. 162–165,168,169 The intensity of reminders and GP visits to assist completion in case finding was not replicated in the feasibility RCT and, in fact, the return rate increased in the second phase of the study. It is not possible to say whether this was because the surgeries became more adept at completion over time or whether it was because the questionnaires were asking about a shorter time span in the RCT. The 12 months’ recall in the initial case-finding study appeared time-consuming and the intensity of reminders and follow-up from the researchers is unlikely to be able to be replicated in a full RCT. Although the findings suggest that 4–6 months may be more feasible, we did not assess repeated completion, that is, whether or not return rates fall over time with more frequent completion for the shorter follow-up rather than 12 months.
In relation to participant-reported service use, participants had difficulty with the concept of time and recalling events. There was also a suggestion of acquiescence, mainly seen in the questions relating to diet. Overall, the accuracy of the data collected using this method may be questionable and would require further exploration. In particular, it would be useful to directly compare the two records of health and social care use completed by the GP surgery and the participants. Although use of electronic data records, such as Hospital Episode Statistics, and primary care databases, such as SystmOne, can provide access to these data and are an alternative to manual collection, the wider societal cost data (such as out-of-pocket expenses and productivity costs) will need to be collected manually.
There were almost no missing EQ-5D data reported. This was perhaps because it was completed by the interviewer. Despite high completion, participants still experienced difficulty answering, with 60% being considered to have some level of difficulty with the EQ-5D questions and needing assistance from the researcher or supporter. The main difficulty experienced was with wording and understanding terms within the questions. Explanations of terms helped. Supporters intervened often, sometimes correcting participant’s answers, even if the participant appeared to have no difficulty with the question. Participants, on occasion, disagreed with supporters, placing a different value on their own health.
Results from the cost-effectiveness analysis was exploratory and illustrates the post hoc uncertainty in the estimates that could potentially be associated with challenges in completing the EQ-5D. Within the scatterplot on the cost-effectiveness plane (see Figure 15) there was a wide spread of points on the horizontal axis, indicating uncertainty in the magnitude and extent of the benefit. Although there was less uncertainty evident in the costs, the CEAC (see Figure 16) shows that the decision uncertainty surrounding adoption is 62.7% at a cost-effectiveness threshold of £20,000 and 54.7% at a threshold of £30,000.
Chapter 7 Involvement in and experiences of the intervention and of research participation: results from a mixed-methods study
Background and aims
In this chapter we present our findings on the participants’ involvement in, and experience of, the research process and intervention from four types of data: (1) researcher-completed structured and semistructured interviews with participant and supporter; (2) transcripts of researcher journals completed after interviews; (3) ease-of-use questionnaires completed by researchers after interviews; and (4) journals completed by the nurses who delivered the intervention after each intervention delivery session.
These data were collected to:
-
assess the feasibility of delivering the SSM intervention in a definitive trial (and potentially in the NHS)
-
explore possible explanations for varying success of the intervention
-
evaluate the data collection measures and methods in this population.
These aims are explored through participant, researcher and nurse perspectives. Participant and supporter perspectives will combine questionnaire and interview data, nurse data are based on nurse journals and researcher perspectives will combine data from journals and ease-of-use questionnaire data.
Results
Participant perspectives
The intervention materials
Researcher-completed questions explored the participant’s perspective on the intervention. Of the 37 participants who had attended at least one session, 32 (89%) remembered being given the ‘OK Diabetes space’ – also referred to as ‘the board’. The majority of people who remembered the board (24/31, 77%) liked it. The researcher asked those who remembered the board if they used the board when the nurse was not there. Nearly half (15/31, 48%) reported they used it at first but stopped and 10 out of 31 (32%) stating that they were still using it.
When evaluated qualitatively in interview, the information sheets (e.g. snack swap, etc.) were, when remembered, evaluated as easy to understand and helpful; respondents said they made them eat more fruit and vegetables or that they were already following the suggestions in the leaflet.
When asked about changes to the intervention, the majority of changes suggested were to make the boards easier to hang and to improve the presentation and durability of the tear-off slips and box. The slips were popular but it was felt they should have more pictures about goals.
Goal-setting
Collaborative goal-setting was a key part of the intervention, yet in researcher interviews participants often struggled to remember setting goals or were not clear about who set them, even when their boards contain evidence of goal-setting. After researcher probing, 26 out of 37 (81%) eventually recalled goal-setting. The majority of goals set (22/26 or 85%) were diet goals. Further details of goal-setting and the intervention are given in Table 81.
| Participant question | SSM participants who remember goal-setting (N = 26), n (%) |
|---|---|
| How easy did you find making the changes set in your goals? | |
| Easy | 14 (53.8) |
| OK | 8 (30.8) |
| Difficult | 4 (15.4) |
| If yes, did a supporter help you with the goal(s)? | |
| Yes | 16 (64.0) |
| No | 5 (20.0) |
| No supporter | 4 (16.0) |
| Missing | 1 |
| Did the nurse give you any leaflets (e.g. snack swap, how to eat fruit)?a | |
| Yes | 13 (40.6) |
| No | 13 (40.6) |
| Do not know | 6 (18.8) |
| Did not remember being part of SSM | 4 (11.1) |
| Missing: no follow-up interview | 1 |
| Did the nurse tell you about any activities/groups/clubs?a | |
| Yes | 8 (25.0) |
| No | 15 (46.9) |
| Do not know | 9 (28.1) |
| Did not remember being part of SSM | 4 (11.1) |
| Missing: no follow-up interview | 1 |
Goal adherence
In interviews with the researcher, the majority of participants could not identify the different aspects of the intervention (goal-setting, adherence, leaflets or folders), making it difficult to evaluate them individually.
Participants in the intervention were asked by researchers about adherence at the follow-up interview. They mostly reported that they found goal adherence easy and had attempted one or both goals. Some were not aware they were working to goals, but descriptions of their behaviour indicated that they were.
Anxiety was a theme that emerged in several interviews as a barrier to goal adherence. Fear of falls, harassment or violence were all mentioned. Those participants who had mobility problems often reported being too fearful to walk alone in case they had a fall and multiple participants talked about falls in wet and icy weather, especially as the intervention was delivered during the winter. Other participants spoke about previous experiences of abuse or harassment by certain people in their local area or previous experience of violence that had made them too fearful to go out alone.
Those who talked about activities had often yet to make any changes. This could be because, logistically, change is slower in an environment where you need to get support to make a change, get a budget for it and secure supporter time, if wanting to be accompanied. It could also be that the person was not going to start these activities but felt that the researcher preferred to hear that they planned to.
Treatment as usual
At follow-up, half of participants remembered the ‘What to do when you have type 2 diabetes’ booklet. Of the participants who remembered it, approximately half said they had read it with their supporter (current or previous) or a family member. Participants answered positively to closed questions about whether or not they trusted the information, if it felt relevant to them, if they learned anything new and if they liked or did not like the booklet. There was some concern in researcher journals that the probing for these questions was leading and participants may have answered to please the researcher.
Most participants liked the booklet, finding it easy to understand or, when explored further in qualitative work, they used neutral phrases such as ‘it was alright’. Most participants said they learned something and it helped, and others were neutral, for example ‘helped a bit, learned a bit’. Some gave examples of how it helped, for example cooking with less oil, reducing chocolate and eating more vegetables and fewer biscuits. Some reported that they did not learn anything, as they knew enough about diabetes already. Researchers asked every participant what changes we could make to the booklet to make it better, but no ideas were given. Table 82 provides further detail.
| Participant question | Treatment arm, n (%) | Total (N = 82), n (%) | |
|---|---|---|---|
| SSM plus TAU (N = 41) | TAU (N = 41) | ||
| Did you get a copy of the ‘What to do when you have type 2 diabetes’ booklet? | |||
| Yes | 22 (59.5) | 21 (52.5) | 43 (55.8) |
| No | 13 (35.1) | 16 (40.0) | 29 (37.7) |
| Do not know | 2 (5.4) | 3 (7.5) | 5 (6.5) |
| Missing | 4 | 1 | 5 |
| If yes, do you still have it? | |||
| Yes | 15 (71.4) | 12 (57.1) | 27 (64.3) |
| No | 4 (19.0) | 1 (4.8) | 5 (11.9) |
| Do not know | 2 (9.5) | 8 (38.1) | 10 (23.8) |
| Missing | 1 | 0 | 1 |
| If yes, did you read it/look through it? | |||
| Yes | 20 (95.2) | 17 (81.0) | 37 (88.1) |
| No | 1 (4.8) | 4 (19.0) | 5 (11.9) |
| Missing | 1 | 0 | 1 |
| If yes, did you read it/look through it with your supporter? | |||
| Yes | 10 (45.5) | 8 (38.1) | 18 (41.9) |
| No | 8 (36.4) | 9 (42.9) | 17 (39.5) |
| Do not know | 1 (4.5) | 0 (0.0) | 1 (2.3) |
| No supporter | 3 (13.6) | 4 (19.0) | 7 (16.3) |
| If yes, did anyone else read it? | |||
| Yes | 7 (35.0) | 7 (36.8) | 14 (35.9) |
| No | 13 (65.0) | 10 (52.6) | 23 (59.0) |
| Do not know | 0 (0.0) | 2 (10.5) | 2 (5.1) |
| Missing | 2 | 2 | 4 |
| If yes, did you like the booklet? | |||
| Yes | 18 (90.0) | 17 (94.4) | 35 (92.1) |
| No | 1 (5.0) | 0 (0.0) | 1 (2.6) |
| Do not know | 1 (5.0) | 1 (5.6) | 2 (5.3) |
| Missing | 2 | 3 | 5 |
| If yes, did you learn anything new about diabetes type 2 from the booklet? | |||
| Yes | 11 (52.4) | 8 (47.1) | 19 (50.0) |
| No | 7 (33.3) | 4 (23.5) | 11 (28.9) |
| Do not know | 3 (14.3) | 5 (29.4) | 8 (21.1) |
| Missing | 1 | 4 | 5 |
| If yes, did the booklet help you look after your diabetes better? | |||
| Yes | 18 (85.7) | 14 (82.4) | 32 (84.2) |
| No | 1 (4.8) | 1 (5.9) | 2 (5.3) |
| Do not know | 2 (9.5) | 2 (11.8) | 4 (10.5) |
| Missing | 1 | 4 | 5 |
| If yes, do you have any ideas about how the booklet could be better? | |||
| Yes | 1 (4.8) | 1 (6.3) | 2 (5.4) |
| No | 18 (85.7) | 11 (68.8) | 29 (78.4) |
| Do not know | 2 (9.5) | 4 (25.0) | 6 (16.2) |
| Missing | 1 | 5 | 6 |
| If yes, did you trust the information in the booklet? | |||
| Yes | 20 (95.2) | 16 (94.1) | 36 (94.7) |
| Do not know | 1 (4.8) | 1 (5.9) | 2 (5.3) |
| Missing | 1 | 4 | 5 |
Self-reporting potential negative outcomes
The questions in this section of the researcher-completed questionnaire were designed to assess the feasibility of collecting information on significant life changes that might affect intervention adherence or health outcomes. All participants were asked by a researcher to recall any ‘big events’ in the last 6 months (also rephrased as ‘since [researcher name] last saw you’ to help give a time frame). Participants were given a list of prompts to be used if they struggled with the concept of ‘big events’ or with recall. These included moving house, illness of person or supporter, death of someone close or a change of supporter. The prompts had been agreed by the research team and the co-applicants, including learning disability specialists.
Participants appeared to perceive these types of events as part of their daily life rather than adverse life events. Interviewers would commonly remind the participants that something they had talked about previously during the visit would be an example of a big life event. Participants demonstrated difficulty in remembering and explaining their health symptoms. When specifically asked about mental health – feeling upset, sleep problems, crying, feeling angry – interviewers needed to probe as participants found it difficult to describe emotions and feelings. Some supporters answered on behalf of the participant and some assisted in probing the participant to answer for themselves. Closed questions on adverse events gained few answers, it was only when the researcher probed further that they got useful information.
‘Big events’ was an abstract concept that always needed defining and exemplifying. For example, after saying that nothing had changed, one participant was encouraged by a supporter to report a death in the family and another reported their own period of illness. A house move was the only ‘big event’ that was independently recalled. Supporters often helped with memory-jogging if they knew the participant’s circumstances, especially when the change was a new key worker. Participants sometimes reported ‘big events’ from a long time ago, for example emergency hospital attendance, and only with probing was this found to be outside the time frame.
The language used to ask about ‘big events’ and to discuss time was often insufficient to elicit recall. It was difficult for researchers to tell when a person could not comprehend time periods, for example the past 6 months, and when they had nothing to report. Multiple probing questions had to be asked:
Since I last saw you has anything happened to you that has been a big event, any big, any sort of big news or anything big that’s happened in your life?
No, don’t think so, no.
Because you mentioned that your parents had died earlier and we couldn’t quite work out how long ago that was, was that this year?
Yeah.
That was this year?
Yeah, yeah.
So both of your parents died this year?
Yeah . . .
And has anything else happened in your life that has been big? Have you been really ill or anything like that?
No, no, no, don’t think so no.
So you’ve not been really ill since I came to see you?
I got engaged to [name], I had a party up at [place], engagement party.
Participants were then asked about change of behaviour and mood, again with prompts to be clear about what we meant. Change was also difficult to comprehend and many reported worse emotional circumstances now than 6 months ago. This is possibly because they were feeling the negative emotions acutely now and could not accurately evaluate the difference between their experiences now and 6 months ago. Some participants had difficulty answering change questions, as these questions require the participant to make a comparison between the present time and 6 months ago:
OK, so in the last 6 months have you been upset more than normal?
No.
No, OK. So would you say that because you were, because you took an overdose that you were feeling more upset? [Supporter and participant had reported participant taking an overdose 2 weeks previously.]
Yeah.
OK. And you said that you panic sometimes don’t you?
Yeah.
Has that stayed the same, has it always been that?
Stayed the same.
Have you been crying more or getting . . .?
I’ve been crying every day.
Interviewer and supporters using probing questions was useful in eliciting information about changes in diet and exercise behaviour. Weight was a more difficult concept to grasp and the question had to be rephrased to suggest that clothes might be feeling tighter or looser or that stomachs might be getting bigger or smaller.
Interviewers tried various methods to explain the concept of quantity and time, and they used terms such as ‘change’, ‘increased’/‘decreased’, ‘more’/‘less’, ‘past’/‘now’. For this group, once a change has been implemented into daily life it may no longer be perceived as a change. One technique that researchers used was to elicit past and present behaviour, find out what had been going on for them and put that to the participant as a change (e.g. ‘so you used to eat that much but now you eat this much?’, ‘did you lose weight because of . . . ?’). This was helpful, but it may have resulted in leading the participant.
The research process
The language of ‘research’ was not familiar to some people. Many people saw each individual element (researcher visit, nurse assessment) as separate and were happy to take part in each part but did not necessarily see them as a linked process that made a research project. The majority said that they knew that we wanted to talk about diabetes but were not clear it was ‘research’:
Now did you know that while you’ve been doing this you’ve been taking part in research?
No I haven’t no.
Do you know what research is?
Yeah I do, it’s to do with health.
This emphasises the concept adopted by this project, that capacity and consent are an ongoing process and must be checked regularly. 191 Each participant will have had research explained to them in case finding when consent was taken and again at baseline when consent was again given, they will also have had reminders about why the researcher was there at every visit.
Individuals who knew they had agreed to take part in research made general statements about being happy to take part (in response to a closed question) and some added that their motivation was to help others:
If it helps other people’s diabetes. If you’re talking to me and I’m talk [sic] to you, and it helps other people with diabetes learn about diabetes then it helps.
Positive feedback on being part of the process included the helpfulness of pictures in the information, being able to read it themselves, knowing they could contact us, enjoying talking to someone about diabetes, enjoying nurse visits, the research benefiting others and that it was interesting and enabled them to find out more about diabetes. There were only a few negative comments: questions were too personal, questions were too difficult, visits were too long and we visited too many times. Changes suggested by participants included wanting more involvement, more nurse visits and more pictures in the literature. Some talked about feeling uncertainty about the process and wanting more information on what it would involve.
Supporter perspectives
Goal-setting and adherence
In interview with researchers, supporters commented on goal-setting and generally agreed with the goals using words such as ‘realistic’ and ‘achievable’. Overall, they reported that it was easy to help the participant to stick to their goals. A lack of communication between supporters was identified when some (non-regular) supporters said they had been supporting healthy eating as a goal but did not know that the intervention was the reason for this. Reported barriers included limited time, checking that participant has done what they say they have, cold weather, family interference and reminding the participant without them perceiving it as nagging and becoming frustrated.
Materials
Most supporter feedback about the intervention was about the board and completing the slips. A small minority felt that the board was not necessary to record goals, but most said its presence motivated participants to achieve their goals. Problems reported were about untruthful slip completion and the unwieldy size of the board.
The information booklet
Over half of supporters questioned remembered the booklet and said that they had read it. All who remembered it trusted the information, but only one-third said that they learned new things. Examples of new learning included helping to change food and exercise behaviours. Despite not always learning new information, 70% of supporters felt that they could use the booklet to benefit the person they supported. Examples of the booklets use were to read it with a participant and to access it for a quick example.
Feedback for change focused on changing the pictures, which did not always fully represent the message they intended to convey, and explaining words such as insulin.
The research process
Few supporters commented on research participation but those who did were overwhelmingly positive about the experience and happy with the involvement of the person they supported, often seeing it as ‘good for them [the participant]’. Some supporters commented that being given enough warning of a researcher or nurse visit was helpful because they could work with the participant to reduce anxiety by preparing them for the visit. During recruitment, researchers found that supporters would often say that they felt the person they support could not take part as they could not understand our questions. By follow-up, only two supporters still had concerns about the understanding of their participant and these were concerns that the participant had not answered research questions accurately so the data obtained would be inaccurate. Visual aids were praised for supporting understanding.
Nurse perspectives
The intervention
The intervention involved several nurse visits in which the following could be covered: social context mapping, goal-setting, the use of a board with tear-off slips to record adherence, the use of information leaflets if appropriate (see Appendices 8–12) and the use of a local activities folder (if appropriate).
The acceptability of the intervention
The nurses thought that most participants benefited from involvement in the project and considered it a successful intervention. This evaluation is in relation to participant engagement and participation in the intervention and not simply adherence to goals. Of the 10 people who lost ≥ 3 kg in weight, nine were in the intervention arm, and so the nurse’s overall perception may be focusing on these key successful individuals.
Nurses reported that participants often initially seemed nervous or showed visible signs of anxiety. Some people who appeared uncommunicative were perceived by the nurses as nervous. For some, nervousness was associated with the physical measures taken at baseline and follow-up, including blood samples and BP.
Most participants were perceived by the nurses to be engaging well with the intervention. The primary problems with engagement in the intervention were participants wanting to keep things as they are, wanting to leave to go to another activity, not wanting to disrupt their normal routine, growing fatigued or appearing to drift off, often letting the supporter do the talking:
She’s quite engaged and then 30 minutes in she began to get bamboozled by everything, I think it was a bit much.
The nurses believed the later factors could be attributed to the supporter taking over, the amount of information covered or the length of visits. Mental health problems were identified in a significant proportion of participants and the nurses reflected that anxiety and depression had a significant effect on an individual’s ability to engage with the intervention process.
Goal-setting
The nurse journals reveal a conflict between the nurses wanting to achieve goal-setting in collaboration with the participant and wanting to grant the participant autonomy to set their own goals when often the participant had indicated they were not open to change:
The participant didn’t come up with the goals herself, it was me who had to prompt her and we talked about a few things and she was very adamant about things that she won’t do so it’s quite difficult, but it was ideas from me but she was happy with them.
The nurses often made suggestions based upon their observations or supporter feedback and worked to find an acceptable goal for the participant. Goals were mainly themed around diet and exercise; however, some were about increasing hobbies and friendship circles, medication adherence, health appointments and daily household activity.
Problems that the nurses reported during goal-setting included participant anxiety, the belief that nothing needed to change, participants being set in own ways and routines, the difficulty of deciding on something achievable, necessary supporter involvement (when supporters were not available) and over-enthusiasm resulting in being over-ambitious or setting too many goals.
Techniques used to set goals were using visual aids to demonstrate amount of sugar in drinks, suggesting very small achievable changes to prevent resistance, discussing barriers together and planning how to overcome these, focusing on increasing or swapping a behaviour rather than reducing and, primarily, getting participants to suggest changes that they would like to make.
Goal adherence
The nurse journals paint a complex picture of the influences on goal adherence. Participant mobility, mood and supporter involvement appear to be the key factors that influenced success. Nurses recorded instances when engaged supporters could make the difference between a forgotten goal and a goal incorporated into daily routine. They also reflected on times when supporters were barriers to success.
The two most common examples of barriers to adherence involving supporters were (1) supporters feeling that they were doing the right thing by encouraging the person to sit while they cooked and cleaned for them, even when the participant’s goal was to be more active, and (2) limited paid supporter time; for example, if a supporter was paid for 2 hours to clean and help the person shower, then accompanying that person for a walk meant that one of the other tasks could not happen. Equally, a person with limited mobility who was taken shopping was often taken in a taxi and pushed round in a wheelchair to speed up the process because of limited time, even when their goal had been to walk more, and examples that were given had included walking in the supermarket or from the bus stop. The most successful goals involved a regular routine activity that had been replaced with some walking, for example not getting a taxi or bus. Organised activities, such as swimming, were limited by supporter availability and transport logistics.
Supporters also acted as facilitators of adherence, commonly sharing the goals with all support staff and regularly reminding the participant that they had agreed to the goal, especially because supporters were often involved in shopping and meal preparation:
Came to the house thinking . . . it wasn’t going to work. I walked in and was greeted by his support worker and it sounds like it’s been a resounding success. There’s pictures, someone has done a laminated thing near the kettle which was suggested to say he was going to have one sugar instead of two and above the fruit bowl for the second goal is saying that he is going to have one red apple a day, absolutely amazed.
Materials
From the nurses’ perspective, elements of the intervention had varying success. They felt that most people were happy to have the board and to use it but that it caused distress in those people who were anxious and used their home, and control over the space, to feel safe. The tear-off sheets were more popular as they were more interactive.
For some people, the nurses employed a more gradual introduction to the resources; however, for most participants the board was introduced according to the intervention plan. Some told the nurses that they wanted to keep using the board and slips, and they said that they would continue to use the board without the slips as a visual aid when the study finished.
The nurses had a folder of information on activities in their local area to support people who wanted an activity-based goal. These activity suggestions were only made if it came up in discussion during goal-setting. It appears that often the activities that people chose were those that they already had in mind rather than the ones that the nurse suggested.
Researcher
The feasibility of collecting outcomes and the acceptability and performance of data collection forms and processes
From analysis of the researcher journals, researchers reported that participants often displayed research fatigue in the interviews. They could also appear confused and sometimes harassed as if they thought they were being asked more and more questions because the researcher did not believe them or was prying. In such cases participants became defensive, edgy and began to close down discussion. Participants said that they were happy to carry on but their answers became shorter as time progressed. In some cases, when the researcher began to ask the supporter questions, the participant appeared to think the researcher was ‘done’ with them and would leave the room or lose interest. RCT follow-up interviews were structured to try to avoid the participant ‘switching off’ during supporter questions. Researchers felt a lesson should be learned about the length of interviews and repeat questioning on one topic with this population.
At the end of each interview researchers completed ease-of-completion ratings for the standard measures at baseline and follow-up. The researchers rated the level of difficulty that the participant had with the question and if the supporter (if present) helped them answer. At feasibility RCT baseline, the two depression screening questions (PHQ-2) were reported as causing ‘no difficulty’ to 47 participants, some difficulty to 32 participants and extreme difficulty to two participants (total n = 81). In the group who had no difficulty, researchers noted that three people were helped by a supporter, 23 were not and the remainder did not have a supporter present. In the group who had some difficulty, 13 people were helped by a supporter, 10 people had a supporter but they did not help and the remaining nine did not have a supporter. Researchers rephrased the questions for eight people in this group and noted that question 1 was the most problematic to five people and that only one person was specifically struggling with question 2. For the two people who had extreme difficulty with the question, one supporter helped the participant and one supporter did not. Both questions were found to be equally problematic to both people in this group.
Supporter involvement in interviews and intervention
Researcher journals reflected on the input of the supporters present at interview. From the sample analysed, three participants had a person there who did not want to be labelled a ‘supporter’ and saw themselves as a chaperone. Of the eight participants with active supporters, four were quite passive and helpful and four answered questions for the person, sometimes over them. The variety in supporter responses suggests that a supporter can be a barrier and a facilitator, very much depending on their personality type.
The research process
Researchers asked a series of questions about research participation towards the end of the interview. They asked closed questions and additional comments were recorded. Very few data were produced from this method. Researcher journals indicate that some participants were tired and growing bored by this stage.
The questions were purely verbal with no visual aid to elicit conversation. In review, the team agreed that this was not the best method to evaluate the research participation of individuals. It had proved necessary, as many data that were collected at baseline needed collection again at follow-up, and it was important to get these answers first for trial outcomes.
Discussion
The intervention was perceived to be feasible to deliver and acceptable to most participants despite it representing a change in routine. The greatest influence on success was seen to be the supporters. The supporters influenced both interviews and results; they could be barriers or enablers depending on the individual supporter. If engaged in the process, people with live-in supporters benefited from regular reminders about the goals. In the case of paid supporters, they often believed that the intervention was a good idea but they had limited time to support an individual. Change of routine could be facilitated with good support and once something new was introduced into a person’s routine it tended to stick; the ‘change’ became the norm and it was not recognised by the participant as a goal but just as part of their routine.
Most participants reported positive feelings about the intervention but it appears that some enjoyed the time with the nurse and having a visitor without really engaging in change to their routine. Exercise-based goals appeared to be the least observed, while changes to diet appeared to be more easily integrated. Exercise goals often required more action to implement than dietary change – having one less sugar in tea takes little time or effort, whereas going swimming requires transport, special clothing and potentially supporter time. Cost was a factor for several participants, which was not surprising in a group for whom so many are not working or only working part-time.
Reporting change or comparisons or measuring time was difficult for participants who struggled with concepts of time. In the ease-of-use reporting for the EQ-5D, certain terminology was noted to confuse people or examples could be taken too literally. Questions needed to be succinct with clear language; those used in the PHQ-2 could confuse people who thought having a ‘little interest’ in things was a positive thing, rather than the more negative having ‘little interest’ in anything.
Self-reporting negative experiences required a more qualitative approach to prompt for significant life events and to explore the participant’s life. It was not possible to assess with simple yes or no questions or to ascertain whether or not negative events related to trial participation without further questioning.
The standard leaflet was a trusted source of information for most and the majority found it useful.
The terminology for research was confusing and unfamiliar to most participants, who had only a little awareness that they were in something called a research project, even though they were happy to take part in each stage of the process and their supporters were happy with their participation.
Chapter 8 General discussion and conclusions
The main findings
We found that it is possible to identify eligible participants in the target population, recruit them into the research and retain them in follow-up. However, case ascertainment was resource intensive – not only did referrers require repeated prompting but each participant was recruited as a result of a multistep process requiring repeated attempts at contact in primary care to seek consent for a research contact, and then multiple attempts at contact by the research team. One explanation was undoubtedly the lack of recording on GP electronic health records or case registers of people who were not formally diagnosed with a learning disability and were not in receipt of services or benefits. Referrals came from diverse sources and were initially identified by referrers in diverse ways. Sole reliance on searching of general practice learning disability registers would not have allowed us to achieve the levels of recruitment that we did. A second practical challenge arose because of the low use of mobile phones in this population; for even those who said they owned a mobile phone (54%), our practical experience was that many were not easily contactable by this route. As a result, although we gained the participation of 60% of general practices covering the research population, it took 18 months in the first case-finding round to find, visit, consent and recruit 147 eligible participants, not all of whom were then interested in participation in the RCT.
On the other hand, once we had made contact with potential participants, retention in research was much easier. At case finding, nearly two-thirds of those referred agreed to contact from a researcher and nearly half of all referrals were eventually registered. Ninety per cent of eligible participants seen agreed to contact for further research, and we recruited our sample for the RCT entirely from this group. Retention in the feasibility trial was excellent, with 85% completing all intervention sessions and candidate primary outcomes being obtained in more than 90%. Furthermore, concerns that participants would not allow physical measures, including blood samples, to be taken by nurses were unfounded. This proved a time-efficient method in comparison with seeking medical record information from a participant’s GP, even though it required the nurses who were conducting home visits to take the blood samples. This was a reflection of the number of attempts at contact and the low levels of information returned from GPs, despite a large amount of researcher time spent contacting practices.
More disappointing was the participation of key supporters. Only a minority of participants in the feasibility RCT had regular, engaged involvement from a consistent supporter. A familiar scenario was the participant living in a shared house with other disabled adults and paid staff providing support; supporters then changed day-by-day according to shifts or the use of temporary staff. This is unfortunate because our experience, shared by other researchers with whom we have discussed this issue, is that involved formal and informal supporters can make a key difference.
Research participants had high levels of comorbidity, with 80% having self-reported illnesses other than diabetes – the most common being mental health problems, cardiovascular disease, asthma, musculoskeletal problems, high cholesterol and epilepsy – and nearly all being on a general practice QOF register in addition to one for diabetes. As expected, weight and obesity were a major problem, and were coupled with low levels of self-reported physical activity. Given these findings, glycaemic control was not as poor as we had expected, comparing favourably, for example, with data from QOF screening in the general population in West Yorkshire. Many of our participants reported dissatisfaction with their diet and their weight, and we suspect that this dissatisfaction, which would have been a more tangible fact to most than a HbA1c level, was an important part of the expressed desire for help in changing, as it is in the wider learning disability population. 192
Comparison with existing data
It is now recognised that the physical health of adults with a learning disability is worse than is that of the general population. Adults with a learning disability are more likely to suffer from epilepsy and other disorders that are associated with the cause of their intellectual deficits, for example congenital heart disease and early-onset Alzheimer’s disease in people with Down syndrome. Such adults are also more at risk of acquired disorders such as cardiovascular disease and diabetes,193,194 which account, along with difficulties with accessing health care, for their higher mortality and reduced life expectancy. 13,195
In relation specifically to diabetes, there is surprisingly little robust evidence, because of problems with case ascertainment, for those with a mild or moderate learning disability who are not recorded as such on general practice registers. It is, however, a reasonable assumption that type 2 diabetes is more common in those with a learning disability than in the general population. 4,5
We were not expecting to find that glycaemic control (as measured by HbA1c) was reasonable in many of our participants in comparison with our local CCG general population figures. The exclusion of people being treated with insulin (which was 27% of the local general population and 20% of those on learning disability registers at the time of our study) will have been one explanation. Our interpretation is that glycaemic control is more amenable to the influence of supporters, who can monitor use of hypoglycaemic agents. Diet is less susceptible to such influence and obesity is associated, as it is in the general population, with lower socioeconomic status. Physical inactivity associated with mobility problems is a particular concern. The third factor of likely relevance is the high rate of mental health problems in the population,196 with the associated use of psychotropic medications known to be a risk both for obesity and for onset of diabetes.
We have been unable to find a description of a self-management intervention that is relevant to our target population. We wanted to ensure that our intervention was both consonant with the principles of SSM and easily incorporated into routine NHS care pathways. Thus, the form and content of our intervention was in line with the general principles of SSM, with an emphasis on realistic goal-setting, identifying likely resources and barriers likely to influence success in reaching those goals and regular self-monitoring of goal attainment. The nature of the ‘supported’ element in such interventions is very variable. We chose to use face-to-face contact because of the participants: few had easy telephone or other IT access and the nature of communication in learning disability makes direct conversation more useful. We employed diabetes research nurses for this purpose. In routine care, each general practice would only have two or three members of the target population and, therefore, practice nurses would not develop the necessary expertise. Learning disability nurses did not seem appropriate because we expected that many of our participants would not self-identify as having a learning disability. We did not want to recruit and train a separate support worker because it would not be a model that worked in routine NHS practice.
Strengths and limitations
Recruitment in primary care and recruitment of adults with a mild or moderate learning disability are both challenges and it is a strength of our study that we were able to recruit and retain the numbers needed for the feasibility RCT. We did not meet our original target for recruitment in case finding but, as a compensation, more of our participants than we had expected agreed to participate in the RCT and thus our original target was met.
We believe there are two main reasons for our success in this respect. The first was the development of an innovative search strategy that was simple to implement in general practice using techniques with principles familiar to practitioners – database and Read Code-based searches. The second was strong engagement with local third-sector organisations that helped us make contacts outside general practice.
We have demonstrated that it is possible to establish procedures for recruitment into trials for adults with a learning disability who have mental capacity by training research staff and by working with a professional team to prepare accessible materials both for information and consent and to support the intervention.
A mixed-method approach for evaluation and the inclusion of qualitative data collection allowed for a further depth of understanding regarding the feasibility of the study.
There were a number of limitations to the study. The exclusion of people taking insulin was part of the commissioning brief but in retrospect it led to the omission of the substantial proportion of the diabetic population who had the worst glycaemic control and, therefore, who had the most to gain by an intervention. We did not include people without mental capacity to consent, arguing that the lack of capacity in that context would be a good indicator of lack of capacity to undertake self-management. However, when a supporter was actively involved and willing, it could have been possible to involve them in implementing some of the intervention, a sort of self-management by proxy. Although we were aware of the need to involve supporters it proved difficult to identify somebody who remained consistently involved in participant’s self-management. There were high levels of unblinding of researchers at follow-up; this would be less of a problem in a larger study in which more researchers could be employed but unintended disclosure by participants is unavoidable.
There were two other uncertainties about our intervention. One is the number of face-to-face sessions involved. Recent reviews been inconsistent on whether or not number of sessions or hours of personal contact is an important determinant of success in changing risks for diabetes onset or cardiovascular disease,197–201 but the most effective programmes seem to have more than three contact sessions. This is a dilemma that is yet to be resolved in debates, for example about national diabetes prevention strategies, because interventions based on minimal patient contact are largely ineffective; however, more intensive contact is unfeasible at the scale required by the population burden and is associated with poor rates of take-up. 121,202,203
The second characteristic of our intervention was its emphasis on diabetes self-management in a broadly defined sense rather than specifically on weight reduction through a calorie-deficit diet, given the centrality of obesity to type 2 diabetes and its very high prevalence in our population. There is a challenge here, too, in that effective weight management programmes require a specific focus,204 whereas the most effective diabetes programmes are said to be multicomponent.
The impact of patient and public involvement, the challenges of patient and public involvement and the lessons learned
Service-user and third-sector involvement were imperative to the project, in helping to develop and pilot research materials (information and consent) and the intervention. People with a learning disability were involved through organisations such as People in Action, easy on the i and CHANGE. The team responded to all comments and feedback, incorporating it into materials and processes whenever possible. The use of a third-sector advocate allowed participants to have an independent source of advice but this was not well utilised and required further explanation. The booklet we produced to support TAU was developed with input from service users, CHANGE and Diabetes UK, and it is now Diabetes UK’s main resource for adults with a learning disability and type 2 diabetes.
Conclusions
Implications for health care
There were two limitations in SSM of type 2 diabetes: the low levels of face-to-face contact time that it provides and the lack of sufficient focus on obesity specifically rather than lifestyle in general. Approaches aimed at achieving and maintaining clinically important weight loss are likely to be more useful in a population for which glycaemic control is not the main issue. To achieve this, health-care programmes, such as those that should be linked to the Learning Disability Health Checks programme, will need to be designed to ensure ease of participation of key supporters.
Implications for research
Research is needed into:
-
Effective ways of identifying the hidden majority of adults with milder learning disabilities, who are in need of support in using health care to which reasonable adjustments have been made, to improve their inclusion both in routine care and in research. Involvement of adults with a learning disability in research starts with case ascertainment. Current approaches do not make it easy to identify those with a mild or moderate learning disability (who are most likely to have capacity to consent) who are not on learning disability registers. Only a few of our participants were not on a learning disability register; however, most referrals to the study came from sources other than by direct searches of those registers.
-
How those without an involved supporter, either because they have no supporter or because their supporter cannot or does not wish to be involved, can participate in research.
-
The effectiveness of obesity treatments in this population, particularly estimating the longer-term outcomes that are important for health benefit. For those who had a BMI measured at baseline and follow-up, a small effect size was observed for BMI reduction in the intervention arm, suggesting that the measure is sensitive to change and that the intervention may yield modest benefits.
-
Improving ways of assessing quality of life in adults with a learning disability, who did not always understand the (unmodifiable) wording of questions in EQ-5D.
The overarching research question: feasibility of a Phase III trial of supported self-management in the target population
We have provided a clear answer to the commissioned question in a number of ways.
-
We were able to develop participant information and consent materials and use them successfully to recruit research participants who had mental capacity to consent.
-
Many of the consenting participants had health needs that would benefit from assistance with improving self-management. Obesity and inactivity were more of a problem than poor glycaemic control; this probably reflected the ability of involved supporters to influence outcomes that depended upon medical intervention rather than lifestyle change.
-
Recruitment was resource intensive but yielded a sample willing to participate in research and who expressed an interest in changing their diet, activity levels and diabetes self-management.
-
It therefore proved possible to recruit and retain people with a mild to moderate learning disability in a RCT aimed at improving supported diabetes self-management. However, it required 18 months to recruit the necessary 80 participants from a population of 1.6 million.
-
Adherence to the intervention was high; the main barrier to delivering the intervention was inconsistency in the involvement of supporters.
-
We were not able to obtain candidate primary outcome measures in a complete and timely manner from GP records, but we were able to obtain them at research visits in > 90% of participants.
-
There were high numbers of missing data for secondary outcomes that depended upon responses from GP records, including those necessary to establish costs for an economic evaluation.
-
Quality of life was difficult to assess because of lack of participant understanding of the structured assessment based on EQ-5D.
-
Cost-effectiveness would be difficult to assess because standardised collection of costs, for example with a modified version of CSRI, rely on proxy reporting and levels of missing data are high in a population living in the community, many of whom do not have a stable named supporter who could provide information.
-
We found no evidence that research participation or exposure to the SSM intervention had an adverse effect on participants.
-
Our results suggest that any intervention effect would be likely to be modest. Our HbA1c results for participants in the feasibility RCT were 56.1 mmol/mol (SD 16.5 mmol/mol) and 7.3% (SD 1.5%) at baseline and 54.3 mmol/mol (SD 16.7 mmol/mol) and 7.1% (SD 1.5%) at follow-up. A clinically important reduction in HbA1c level is usually taken to be at least 5.5 mmol/mol (0.5%), which is equivalent to an effect size of 0.33 (based on the SD 16.5). For a definitive trial to have 90% power (alpha < 0.05), a sample size of 194 per arm would be required or about five times the size of this feasibility trial. Given the substantial effort required for case ascertainment and recruitment, we estimate that this would require 18–24 months recruiting from a population of about 9 million (five times the base population for OK-Diabetes).
Acknowledgements
We would like to express our gratitude to the following people:
The participants and their supporters who generously agreed to give their time to this research.
The employees and volunteers in the third sector and local authorities who provided vital input to the study and dissemination, especially easy on the i (John Burley, Dean Milner-Bell and their colleagues), People in Action (Aquila Choudhry), Tenfold (Kath Lindley) and Rooots Ltd.
The Trial Steering Committee, Dr Lucinda Summers, Professor Tony Holland, Professor Gill Lancaster and Kath Lindley, for support and advice throughout the project.
Staff from the former Commissioning Support Unit, including Paul Carder, Satbir Saggu, Gemma Doran and Stella Johnson, for help with recruitment.
Research nurses, Penny Rice, Julie Bailey and Cherry Coupland, for their hard work and expertise.
Clinical colleagues, Dr Dinesh Nagi, Dr Avijit Biswas, Victoria Donnelly, Dr Ezhil Anand, Elizabeth Nyamadzawo, Dr Peter Lindsay, Tania Swaine, Lucy Greenwood, Kim James and Dr Matt Houghton. Numerous clinicians within the Leeds, Bradford and Wakefield CCGs without whom this research would not have been possible.
Dr Neda Mahmoodi, Natalie King [Leeds Institute of Health Sciences (LIHS)], Alison Fergusson and Vicki McLelland (Leeds Institute of Clinical Trials Research) who contributed to the research.
Dr Sam Browning, Norman Campbell and Christine Harris-Moores for their ideas and enthusiasm.
The Learning Disability Partnership Boards in Leeds and Wakefield, with special mention to Marie Gibb.
Christopher Smith (EpAID trial; improving outcomes in adults with epilepsy and intellectual disability), Michaela Poppe and Angela Hassiotis, Michael King and Becca Beeken (Shape Up LD trial; piloting a manualised weight management programme for overweight and obese persons with mild-moderate learning disabilities), Liz Randell (ANDREA-LD trial; community led ANti-psychotic Drug REduction for Adults with Learning Disabilities) and Craig Melville (Walk Well and Take-5) for sharing materials and experience from their own RCTs.
CHANGE, working under the guidance of people with a learning disability, provided the graphic design expertise for the resource and reviews by people with a learning disability at each iteration.
Contributions of authors
Allan House (Professor of Liaison Psychiatry) contributed to design analysis and write-up of the project, was the chief investigator overseeing project implementation and was the corresponding author for the report.
Louise Bryant (Associate Professor in Medical Psychology) contributed to the design, conduct and analysis of the report, with a lead role on research in adults with learning disability. She also deputised for Professor House.
Amy M Russell (Senior Research Fellow) was a project co-ordinator and was responsible for design of materials, recruitment and data collection and analysis of the report, with joint lead role (with Bryant) in the qualitative analysis.
Alexandra Wright-Hughes (Senior Medical Statistician) was responsible for the design and conduct of the statistical analysis and contributed to the interpretation and write-up of the report.
Liz Graham (Senior Trials Manager) was responsible for management of the feasibility RCT and had a lead role in developing the adherence measure.
Rebecca Walwyn (Lecturer in Medical Statistics) contributed to the design, analysis and interpretation of the project.
Judy M Wright (Senior Information Specialist) designed and undertook all literature searches.
Claire Hulme (Professor of Health Economics) was the senior economist and was responsible for the design, conduct and write-up of the economic evaluation.
John L O’Dwyer (Research Fellow in Health Economics) was responsible for the implementation and write-up of the economic evaluation.
Gary Latchford (Consultant Clinical Psychologist) contributed to the design and implementation of the SSM intervention, including the supervision of nurses.
Shaista Meer (Research Fellow) was responsible for recruitment, data collection and the analysis of the qualitative findings.
Jacqueline C Birtwistle (Research Fellow) was responsible for recruitment, data collection and the analysis of the qualitative findings.
Alison Stansfield (Consultant in Learning Disability) has a lead role in advising on involving adults with learning disability and training researchers in practice related to mental capacity.
Ramzi Ajjan (Consultant in Diabetes and Endocrinology) gave advice on all clinical aspects of the treatment and assessment of type 2 diabetes.
Amanda Farrin (Professor of Clinical Trials and Evaluation of Complex Interventions) was a senior trials methodologist and was responsible for overseeing all aspects of the design and analysis of statistical aspects of the project.
Publications
Russell AM, House A, Bryant L, Farrin A, Graham L, Walwyn R. Reducing health inequities in diabetes care: the OK-Diabetes project. Poster presentation. J Appl Res Intellectual Disabil 2015;28:58.
Russell AM, House A, Bryant L, Farrin A, Walwyn R, Wright-Hughes A. Improving the supported self-management of Type 2 diabetes for adults with learning disabilities: the OK: Diabetes project. Poster presentation. Diabet Med 2015;32:142.
Walwyn REA, Russell AM, Bryant LD, Farrin AJ, Wright-Hughes AM, Graham EH, et al. Supported self-management for adults with type 2 diabetes and a learning disability (OK-Diabetes): study protocol for a randomised controlled feasibility trial. Trials 2015;16:342.
Graham E, Wright J, Walwyn R, Russell AM, Bryant L, Farrin A, House A. Measurement of adherence in a randomised controlled trial of a complex intervention: supported self-management for adults with learning disability and Type 2 Diabetes. BMC Med Res Methodol 2016;16:132.
House A, Bryant L, Russell AM, Wright-Hughes A, Graham L, Walwyn JM, et al. Randomized controlled feasibility trial of supported self-management in adults with Type 2 diabetes mellitus and an intellectual disability: OK Diabetes. Diab Med 2018;35:6.
Bryant LD, Russell AM, Walwyn REA, Farrin AJ, Wright-Hughes A, Graham EH, et al. Characterizing adults with Type 2 diabetes mellitus and intellectual disability: outcomes of a case-finding study. Diabet Med 2018;35:352–9.
Data sharing statement
We will make data available to the scientific community with as few restrictions as feasible, while retaining exclusive use until the publication of major outputs. Enquiries should be directed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- González EL, Johansson S, Wallander MA, Rodríguez LA. Trends in the prevalence and incidence of diabetes in the UK: 1996-2005. J Epidemiol Community Health 2009;63:332-6. https://doi.org/10.1136/jech.2008.080382.
- Straetmans JM, van Schrojenstein Lantman-de Valk HM, Schellevis FG, Dinant GJ. Health problems of people with intellectual disabilities: the impact for general practice. Br J Gen Pract 2007;57:64-6.
- Emerson E, Baines S, Allerton L, Welch V. Health Inequalities and People with Learning Disabilities in the UK: 2010. Durham: Improving Health and Lives Learning Disabilities Observatory; 2010.
- McVilly K, McGillivray J, Curtis A, Lehmann J, Morrish L, Speight J. Diabetes in people with an intellectual disability: a systematic review of prevalence, incidence and impact. Diabet Med 2014;31:897-904. https://doi.org/10.1111/dme.12494.
- MacRae S, Brown M, Karatzias T, Taggart L, Truesdale-Kennedy M, Walley R, et al. Diabetes in people with intellectual disabilities: a systematic review of the literature. Res Dev Disabil 2015;47:352-74. https://doi.org/10.1016/j.ridd.2015.10.003.
- Emerson E. Health status and health risks of the ‘hidden majority’ of adults with intellectual disability. Intellect Dev Disabil 2011;49:155-65. https://doi.org/10.1352/1934-9556-49.3.155.
- Glover G, Emerson E, Eccles R. Using Local Data to Monitor the Health Needs of People with Learning Disabilities. Durham: Improving Health and Lives Learning Disabilities Observatory; 2012.
- Balogh R, Lake J, Lin E, Wilton A, Lunsky Y. Disparities in diabetes prevalence and preventable hospitalizations in people with intellectual and developmental disability: a population-based study. Diabet Med 2015;32:235-42. https://doi.org/10.1111/dme.12573.
- Hsieh K, Rimmer JH, Heller T. Obesity and associated factors in adults with intellectual disability. J Intellect Disabil Res 2014;58:851-63. https://doi.org/10.1111/jir.12100.
- Melville C, Hamilton S, Hankey C, Miller S, Boyle S. The prevalence and determinants of obesity in adults with intellectual disabilities. Obes Rev 2007;8:223-30. https://doi.org/10.1111/j.1467-789X.2006.00296.x.
- Taggart L, Coates V, Truesdale-Kennedy M. Management and quality indicators of diabetes mellitus in people with intellectual disabilities. J Intellect Disabil Res 2013;57:1152-63.
- De Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011;10:52-77. https://doi.org/10.1002/j.2051-5545.2011.tb00014.x.
- Heslop P, Blair P, Fleming P, Hoghton M, Marriott A, Russ L. Confidential Inquiry into Premature Deaths of People with Learning Disabilities (CIPOLD). Bristol: Norah Fry Research Centre; 2007.
- Heslop P, Blair P, Fleming P, Hoghton M, Marriott A, Russ L. Confidential Inquiry into Premature Deaths of People with Learning Disabilities (CIPOLD). Bristol: Norah Fry Research Centre; 2013.
- Emerson E, Hatton C, Robertson J, Baines S, Christie A, Glover G. People with Learning Disabilities in England: 2012. Durham: Improving Health and Lives Learning Disabilities Observatory; 2013.
- QOF 201516 Results. Leeds: NHS Digital; 2016.
- Turner S, Robinson C. Health Checks for People with Learning Disabilities: Implications and Actions for Commissioners. Durham: Improving Health and Lives Learning Disabilities Observatory; 2010.
- NHS England . Enhanced Services 2009. www.nhsemployers.org/your-workforce/primary-care-contacts/general-medical-services/enhanced-services/archive-2008-2012/enhanced-services-2008-09 (accessed 14 February 2017).
- Chauhan U, Reeve J, Kontopantelis E, Hinder S, Nelson P, Doran T. Impact of the English Directly Enhanced Service (DES) for Learning Disability. Manchester: University of Manchester; 2012.
- Emerson E, Glover G. The ‘transition cliff’ in the administrative prevalence of learning disabilities in England. Tizard Learning Disability Review 2012;17:139-43. https://doi.org/10.1108/13595471211240988.
- Death by Indifference: 74 Deaths and Counting. A Progress Report 5 Years On. London: MENCAP; 2012.
- Public Health England . National General Practice Profiles 2015. www.fingertips.phe.org.uk/profile/general-practice/data (accessed 14 February 2017).
- Newman S, Steed L, Mulligan K. Self-management interventions for chronic illness. Lancet 2004;364:1523-37. https://doi.org/10.1016/S0140-6736(04)17277-2.
- Heinrich E, Schaper NC, de Vries NK. Self-management interventions for type 2 diabetes: a systematic review. Eur Diab Nursing 2010;7:71-6. https://doi.org/10.1002/edn.160.
- Radhakrishnan K. The efficacy of tailored interventions for self-management outcomes of type 2 diabetes, hypertension or heart disease: a systematic review. J Adv Nurs 2012;68:496-510. https://doi.org/10.1111/j.1365-2648.2011.05860.x.
- Behaviour Change: General Approaches. London: NICE; 2007.
- Braddock D, Emerson E, Felce D, Stancliffe RJ. Living circumstances of children and adults with mental retardation or developmental disabilities in the United States, Canada, England and Wales, and Australia. Ment Retard Dev Disabil Res Rev 2001;7:115-21. https://doi.org/10.1002/mrdd.1016.
- Forrester-Jones R, Carpenter J, Coolen-Schrijner P, Cambridge P, Tate A, Beecham J, et al. The social networks of people with intellectual disability living in the community 12 years after resettlement from long-stay hospitals. J Appl Res Intellect Disabil 2006;19:285-95. https://doi.org/10.1111/j.1468-3148.2006.00263.x.
- Cardol M, Rijken M, van Schrojenstein Lantman-de Valk H. Attitudes and dilemmas of caregivers supporting people with intellectual disabilities who have diabetes. Patient Educ Couns 2012;87:383-8. https://doi.org/10.1016/j.pec.2011.11.010.
- Hale LA, Trip HT, Whitehead L, Conder J. Self-management abilities of diabetes in people with an intellectual disability living in New Zealand. J Policy Pract Intellect Disabil 2011;8:223-30. https://doi.org/10.1111/j.1741-1130.2011.00314.x.
- Rintala T-M, Jaatinen P, Paavilainen E, Åstedt-Kurki P. Interrelation between adult persons with diabetes and their family a systematic review of the literature. J Fam Nurs 2013;19:3-28. https://doi.org/10.1177/1074840712471899.
- Stopford R, Winkley K, Ismail K. Social support and glycemic control in type 2 diabetes: a systematic review of observational studies. Patient Educ Couns 2013;93:549-58. https://doi.org/10.1016/j.pec.2013.08.016.
- Fisher L, Chesla CA, Bartz RJ, Gilliss C, Skaff MA, Sabogal F, et al. The family and type 2 diabetes: a framework for intervention. Diabetes Educ 1998;24:599-607. https://doi.org/10.1177/014572179802400504.
- NHS Health Research Authority . Principles of Consent n.d. www.hra-decisiontools.org.uk/consent/principles-general.html (accessed 30 October 2015).
- Valuing People: A New Strategy for Learning Disability for the 21st Century. London: Department of Health; 2001.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Seeking Consent: Working with People with Learning Disabilities. London: Department of Health; 2001.
- Research Governance Framework for Health and Social Care. London: Department of Health; 2005.
- Robotham D, Hassiotis A. Randomised controlled trials in learning disabilities: a review of participant experiences. Advances in Mental Health and Learning Disabilities 2009;3:42-6. https://doi.org/10.1108/17530180200900008.
- Iacono T. Ethical challenges and complexities of including people with intellectual disability as participants in research. J Intellect Dev Disabil 2006;31:173-9. https://doi.org/10.1080/13668250600876392.
- Iacono T, Murray V. Issues of informed consent in conducting medical research involving people with intellectual disability. J Appl Res Intellect Disabil 2003;16:41-5. https://doi.org/10.1046/j.1468-3148.2003.00141.x.
- Alderson SL, Russell AM, McLintock K, Potrata B, House A, Foy R. Incentivised case finding for depression in patients with chronic heart disease and diabetes in primary care: an ethnographic study. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005146.
- Mental Capacity Act. Code of Practice. London: The Stationery Office; 2005.
- McDonald K, Patka M. ‘There is no black or white’: scientific community views on ethics in intellectual and developmental disability research. J Policy Pract Intellect Disabil 2012;9:206-14. https://doi.org/10.1111/j.1741-1130.2012.00348.x.
- Segal-Isaacson C, Wylie-Rosett J, Gans KM. Validation of a short dietary assessment questionnaire: the Rapid Eating and Activity Assessment for Participants short version (REAP-S). Diabetes Educ 2004;30:774-81. https://doi.org/10.1177/014572170403000512.
- Hermans H, Evenhuis HM. Characteristics of instruments screening for depression in adults with intellectual disabilities: systematic review. Res Dev Disabil 2010;31:1109-20. https://doi.org/10.1016/j.ridd.2010.04.023.
- Cuthill FM, Espie CA, Cooper SA. Development and psychometric properties of the Glasgow Depression Scale for people with a learning disability. Individual and carer supplement versions. Br J Psychiatry 2003;182:347-53. https://doi.org/10.1192/bjp.182.4.347.
- Type 2 Diabetes: Prevention in People at High Risk. London: NICE; 2012.
- Cooper A, O’Flynn N. Guidelines: risk assessment and lipid modification for primary and secondary prevention of cardiovascular disease: summary of NICE guidance. BMJ 2008;336:1246-8. https://doi.org/10.1136/bmj.39554.624086.AD.
- Krause T, Lovibond K, Caulfield M, McCormack T, Williams B. Guideline Development Group . Management of hypertension: summary of NICE guidance. BMJ 2011;343. https://doi.org/10.1136/bmj.d4891.
- Type 2 Diabetes in Adults: Management. London: NICE; 2015.
- Diabetes UK . Improving Supported Self-Management for People With Diabetes 2009. www.diabetes.org.uk/Documents/Reports/Supported_self-management.pdf (accessed 15 February 2017).
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Deakin T, McShane CE, Cade JE, Williams RD. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;2. https://doi.org/10.1002/14651858.CD003417.pub2.
- Duke SA, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2009;1. https://doi.org/10.1002/14651858.CD005268.pub2.
- Funnell MM, Brown TL, Childs BP, Haas LB, Hosey GM, Jensen B, et al. National standards for diabetes self-management education. Diabetes Care 2008;31. https://doi.org/10.2337/dc08-S097.
- National Diabetes Audit – 2013–2014 and 2014–2015: Report 1, Care Processes and Treatment Targets. Leeds: NHS Digital; 2016.
- Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns 2016;99:926-43. https://doi.org/10.1016/j.pec.2015.11.003.
- Clark M. Diabetes self-management education: a review of published studies. Prim Care Diabetes 2008;2:113-20. https://doi.org/10.1016/j.pcd.2008.04.004.
- Gucciardi E, Chan VW-S, Manuel L, Sidani S. A systematic literature review of diabetes self-management education features to improve diabetes education in women of Black African/Caribbean and Hispanic/Latin American ethnicity. Patient Educ Couns 2013;92:235-45. https://doi.org/10.1016/j.pec.2013.03.007.
- Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 2004;363:1589-97. https://doi.org/10.1016/S0140-6736(04)16202-8.
- Jarvis J, Skinner TC, Carey ME, Davies MJ. How can structured self-management patient education improve outcomes in people with type 2 diabetes?. Diabetes Obes Metab 2010;12:12-9. https://doi.org/10.1111/j.1463-1326.2009.01098.x.
- Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care 2002;25:1159-71. https://doi.org/10.2337/diacare.25.7.1159.
- Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. J Diabetes Metab Disord 2013;12. https://doi.org/10.1186/2251-6581-12-14.
- Steinsbekk A, Rygg L, Lisulo M, Rise MB, Fretheim A. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. A systematic review with meta-analysis. BMC Health Serv Res 2012;12. https://doi.org/10.1186/1472-6963-12-213.
- DAFNE Study Group . Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325.
- Deakin TA, Cade JE, Williams R, Greenwood DC. Structured patient education: the diabetes X-PERT Programme makes a difference. Diabet Med 2006;23:944-54. https://doi.org/10.1111/j.1464-5491.2006.01906.x.
- Glasgow RE, Toobert DJ, Hampson SE. Effects of a brief office-based intervention to facilitate diabetes dietary self-management. Diabetes Care 1996;19:835-42. https://doi.org/10.2337/diacare.19.8.835.
- Jansink R, Braspenning J, Keizer E, van der Weijden T, Elwyn G, Grol R. No identifiable Hb1Ac or lifestyle change after a comprehensive diabetes programme including motivational interviewing: a cluster randomised trial. Scand J Prim Health Care 2013;31:119-27. https://doi.org/10.3109/02813432.2013.797178.
- Khunti K, Gray LJ, Skinner T, Carey ME, Realf K, Dallosso H, et al. Effectiveness of a diabetes education and self management programme (DESMOND) for people with newly diagnosed type 2 diabetes mellitus: three year follow-up of a cluster randomised controlled trial in primary care. BMJ 2012;344. https://doi.org/10.1136/bmj.e2333.
- Klug C, Toobert DJ, Fogerty M. Healthy Changes for living with diabetes: an evidence-based community diabetes self-management program. Diabetes Educ 2008;34:1053-61. https://doi.org/10.1177/0145721708325886.
- Lennox N, Edie G, Taylor M, Rey-Conde T, McPhee J. Diabetes, to the point: designing a website about diabetes for adults with intellectual disability and carers. Technol Disabil 2009;21:11-8.
- Lennox NG, Rey-Conde TF, McPhee JH, Taylor M. The development and evaluation of resources about diabetes management for people with intellectual disability and their care providers. J Dev Disabil 2009;15:77-80.
- Liu S, Bi A, Fu D, Fu H, Luo W, Ma X, et al. Effectiveness of using group visit model to support diabetes patient self-management in rural communities of Shanghai: a randomized controlled trial. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-1043.
- Lorig K, Sobel D, Stewart A, Brown BW, Bandura A, Ritter P, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care 1999;37:5-14. https://doi.org/10.1097/00005650-199901000-00003.
- McGowan PT. Self-management education and support in chronic disease management. Prim Care 2012;39:307-25. https://doi.org/10.1016/j.pop.2012.03.005.
- McMahon GT, Fonda SJ, Gomes HE, Alexis G, Conlin PR. A randomized comparison of online- and telephone-based care management with internet training alone in adult patients with poorly controlled type 2 diabetes. Diabetes Technol Ther 2012;14:1060-7. https://doi.org/10.1089/dia.2012.0137.
- Mitchell B, Armour C, Lee M, Song YJ, Stewart K, Peterson G, et al. Diabetes Medication Assistance Service: the pharmacist’s role in supporting patient self-management of type 2 diabetes (T2DM) in Australia. Patient Educ Couns 2011;83:288-94. https://doi.org/10.1016/j.pec.2011.04.027.
- Molsted S, Tribler J, Poulsen PB, Snorgaard O. The effects and costs of a group-based education programme for self-management of patients with type 2 diabetes. A community-based study. Health Educ Res 2012;27:804-13. https://doi.org/10.1093/her/cyr053.
- Müller N, Stengel D, Kloos C, Ristow M, Wolf G, Müller UA. Improvement of HbA(1c) and stable weight loss 2 years after an outpatient treatment and teaching program for patients with type 2 diabetes without insulin therapy based on urine glucose self-monitoring. Int J Gen Med 2012;5:241-7. https://doi.org/10.2147/IJGM.S28505.
- Naik AD, Palmer N, Petersen NJ, Street RL, Rao R, Suarez-Almazor M, et al. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Arch Intern Med 2011;171:453-9. https://doi.org/10.1001/archinternmed.2011.70.
- Song MS, Kim HS. Intensive management program to improve glycosylated hemoglobin levels and adherence to diet in patients with type 2 diabetes. Appl Nurs Res 2009;22:42-7. https://doi.org/10.1016/j.apnr.2007.05.004.
- Thoolen BJ, de Ridder D, Bensing J, Gorter K, Rutten G. Beyond good intentions: the role of proactive coping in achieving sustained behavioural change in the context of diabetes management. Psychol Health 2009;24:237-54. https://doi.org/10.1080/08870440701864504.
- Trento M, Passera P, Borgo E, Tomalino M, Bajardi M, Cavallo F, et al. A 5-year randomized controlled study of learning, problem solving ability, and quality of life modifications in people with type 2 diabetes managed by group care. Diabetes Care 2004;27:670-5. https://doi.org/10.2337/diacare.27.3.670.
- van der Wulp I, de Leeuw JR, Gorter KJ, Rutten GE. Effectiveness of peer-led self-management coaching for patients recently diagnosed with type 2 diabetes mellitus in primary care: a randomized controlled trial. Diabet Med 2012;29:e390-7. https://doi.org/10.1111/j.1464-5491.2012.03629.x.
- Zanetti ML, Otero LM, Peres DS, dos Santos MA, Guimarães FPdM, Freitas MCF. Progress of the patients with diabetes mellitus who were managed with the staged diabetes management framework. Acta Paul Enferm 2007;20:338-44. https://doi.org/10.1590/S0103-21002007000300016.
- Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New Engl J Med 2002;2002:393-40.
- Christie D, Strange V, Allen E, Oliver S, Wong ICK, Smith F, et al. Maximising engagement, motivation and long term change in a structured intensive education programme in diabetes for children, young people and their families: child and adolescent structured competencies approach to diabetes education (CASCADE). BMC Pediatr 2009;9. https://doi.org/10.1186/1471-2431-9-57.
- Debussche X, Collin F, Fianu A, Balcou-Debussche M, Fouet-Rosiers I, Koleck M, et al. Structured self-management education maintained over two years in insufficiently controlled type 2 diabetes patients: the ERMIES randomised trial in Reunion Island. Cardiovasc Diabetol 2012;11. https://doi.org/10.1186/1475-2840-11-91.
- Gray LJ, Khunti K, Williams S, Goldby S, Troughton J, Yates T, et al. Let’s prevent diabetes: study protocol for a cluster randomised controlled trial of an educational intervention in a multi-ethnic UK population with screen detected impaired glucose regulation. Cardiovasc Diabetol 2012;11. https://doi.org/10.1186/1475-2840-11-56.
- Johnson ST, Mundt C, Soprovich A, Wozniak L, Plotnikoff RC, Johnson JA. Healthy eating and active living for diabetes in primary care networks (HEALD-PCN): rationale, design, and evaluation of a pragmatic controlled trial for adults with type 2 diabetes. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-455.
- Lascar N, Kennedy A, Jackson N, Daley A, Dowswell G, Thompson D, et al. Exercise to preserve beta cell function in recent-onset type 1 diabetes mellitus (EXTOD): a study protocol for a pilot randomized controlled trial. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-180.
- Ma J, King AC, Wilson SR, Xiao L, Stafford RS. Evaluation of lifestyle interventions to treat elevated cardiometabolic risk in primary care (E-LITE): a randomized controlled trial. BMC Fam Pract 2009;10. https://doi.org/10.1186/1471-2296-10-71.
- Murray E, Dack C, Barnard M, Farmer A, Li J, Michie S, et al. HeLP-Diabetes: randomised controlled trial protocol. BMC Health Serv Res 2015;15. https://doi.org/10.1186/s12913-015-1246-9.
- Simmons D, Prevost AT, Bunn C, Holman D, Parker RA, Cohn S, et al. Impact of community based peer support in type 2 diabetes: a cluster randomised controlled trial of individual and/or group approaches. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0120277.
- Baker RM. Economic rationality and health and lifestyle choices for people with diabetes. Soc Sci Med 2006;63:2341-53. https://doi.org/10.1016/j.socscimed.2006.06.007.
- Bayliss EA, Steiner JF, Fernald DH, Crane LA, Main DS. Descriptions of barriers to self-care by persons with comorbid chronic diseases. Ann Fam Med 2003;1:15-21. https://doi.org/10.1370/afm.4.
- Beverly EA, Miller CK, Wray LA. Spousal support and food-related behavior change in middle-aged and older adults living with type 2 diabetes. Health Educ Behav 2008;35:707-20.
- Cardol M, Rijken M, van Schrojenstein Lantman-de Valk H. People with mild to moderate intellectual disability talking about their diabetes and how they manage. J Intellect Disabil Res 2012;56:351-60. https://doi.org/10.1111/j.1365-2788.2011.01472.x.
- Cartwright L, Reid M, Hammersley R, Blackburn C, Glover L. Food choice by people with intellectual disabilities at day centres: a qualitative study. J Intellect Disabil 2014;19:103-15. https://doi.org/10.1177/1744629514563423.
- Caton S, Chadwick D, Chapman M, Turnbull S, Mitchell D, Stansfield J. Healthy lifestyles for adults with intellectual disability: knowledge, barriers, and facilitators. J Intellect Dev Disabil 2012;37:248-59. https://doi.org/10.3109/13668250.2012.703645.
- Chlebowy DO, Hood S, LaJoie AS. Facilitators and barriers to self-management of type 2 diabetes among urban African American adults focus group findings. Diabetes Educ 2010;36:897-905. https://doi.org/10.1177/0145721710385579.
- Dagnan D. Psychosocial interventions for people with intellectual disabilities and mental ill-health. Curr Opin Psychiatry 2007;20:456-60. https://doi.org/10.1097/YCO.0b013e3282ab9963.
- Ding EL, Prescott MR, Watson KT, Bui N, Makarechi L, Zoughbie DE. Abstract 009: microclinic social network lifestyle intervention for weight loss and obesity management: a 10-month randomized controlled trial. Circulation 2013;127.
- Dysch C, Chung MC, Fox J. How do people with intellectual disabilities and diabetes experience and perceive their illness?. J Appl Res Intellect Disabil 2012;25:39-4. https://doi.org/10.1111/j.1468-3148.2011.00641.x.
- Gill JM, Mainous AG, Diamond JJ, Lenhard MJ. Impact of provider continuity on quality of care for persons with diabetes mellitus. Ann Fam Med 2003;1:162-70. https://doi.org/10.1370/afm.22.
- Glasgow R, Nelson C, Strycker L, King D. Using RE-AIM metrics to evaluate diabetes self-management support interventions. Am J Prev Med 2006;30:67-73. https://doi.org/10.1016/j.amepre.2005.08.037.
- Glasgow RE, Toobert DJ, Gillette CD. Psychosocial barriers to diabetes self-management and quality of life. Diabetes Spectrum 2001;14:33-41. https://doi.org/10.2337/diaspect.14.1.33.
- Gomersall T, Madill A, Summers LK. Getting one’s thoughts straight: a dialogical analysis of women’s accounts of poorly controlled type 2 diabetes. Psychol Health 2012;27:378-93. https://doi.org/10.1080/08870446.2011.583649.
- Harvey JN, Lawson VL. The importance of health belief models in determining self-care behaviour in diabetes. Diabet Med 2009;26:5-13. https://doi.org/10.1111/j.1464-5491.2008.02628.x.
- Hawkins A, Look R. Levels of engagement and barriers to physical activity in a population of adults with learning disabilities. Br J Learn Disabil 2006;34:220-6. https://doi.org/10.1111/j.1468-3156.2005.00381.x.
- Majeed-Ariss R, Jackson C, Knapp P, Cheater FM. British-Pakistani women’s perspectives of diabetes self-management: the role of identity. J Clin Nurs 2015;24:2571-80. https://doi.org/10.1111/jocn.12865.
- Nagelkerk J, Reick K, Meengs L. Perceived barriers and effective strategies to diabetes self-management. J Adv Nurs 2006;54:151-8. https://doi.org/10.1111/j.1365-2648.2006.03799.x.
- Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Barriers to diabetes management: patient and provider factors. Diabetes Res Clin Pract 2011;93:1-9. https://doi.org/10.1016/j.diabres.2011.02.002.
- Nath C. Literacy and diabetes self-management. Am J Nurs 2007;107:43-9. https://doi.org/10.1097/01.NAJ.0000277829.28043.93.
- Perri MG, Sears SF, Clark JE. Strategies for improving maintenance of weight loss. Toward a continuous care model of obesity management. Diabetes Care 1993;16:200-9. https://doi.org/10.2337/diacare.16.1.200.
- Rouse L, Finlay WM. Repertoires of responsibility for diabetes management by adults with intellectual disabilities and those who support them. Sociol Health Illn 2016;38:1243-57. https://doi.org/10.1111/1467-9566.12454.
- Skovlund SE, Peyrot M. The Diabetes Attitudes, Wishes, and Needs (DAWN) program: a new approach to improving outcomes of diabetes care. Diabetes Spectrum 2005;18:136-42. https://doi.org/10.2337/diaspect.18.3.136.
- Trief P, Sandberg JG, Ploutz-Snyder R, Brittain R, Cibula D, Scales K, et al. Promoting couples collaboration in type 2 diabetes: the diabetes support project pilot data. Fam Syst Health 2011;29. https://doi.org/10.1037/a0024564.
- Whitehead LC, Trip HT, Hale LA, Conder J. Negotiated autonomy in diabetes self-management: the experiences of adults with intellectual disability and their support workers. J Intellect Disabil Res 2016;60:389-97. https://doi.org/10.1111/jir.12257.
- Winkley K, Evwierhoma C, Amiel S, Lempp H, Ismail K, Forbes A. Patient explanations for non-attendance at structured diabetes education sessions for newly diagnosed type 2 diabetes: a qualitative study. Diabet Med 2015;32:120-8. https://doi.org/10.1111/dme.12556.
- Zulman DM, Rosland AM, Choi H, Langa KM, Heisler M. The influence of diabetes psychosocial attributes and self-management practices on change in diabetes status. Patient Educ Couns 2012;87:74-80. https://doi.org/10.1016/j.pec.2011.07.013.
- Funnell MM, Anderson RM. Empowerment and self-management of diabetes. Clinical Diabetes 2004;22:123-7. https://doi.org/10.2337/diaclin.22.3.123.
- Group DPPR . The diabetes prevention program (DPP). Diabetes Care 2002;25:2165-71. https://doi.org/10.2337/diacare.25.12.2165.
- Skinner TC, Carey ME, Cradock S, Daly H, Davies MJ, Doherty Y, et al. Diabetes Education and Self-Management for Ongoing and Newly Diagnosed (DESMOND): process modelling of pilot study. Patient Educ Couns 2006;64:369-77. https://doi.org/10.1016/j.pec.2006.04.007.
- Carroll C, Booth A, Cooper K. A worked example of’ best fit’ framework synthesis: a systematic review of views concerning the taking of some potential chemopreventive agents. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-29.
- Mingers J, Rosenhead J. Problem structuring methods in action. Eur J Oper Res 2004;152:530-54. https://doi.org/10.1016/S0377-2217(03)00056-0.
- Aronow HU, Hahn JE. Stay well and healthy! Pilot study findings from an inhome preventive healthcare programme for persons ageing with intellectual and/or developmental disabilities. J Appl Res Intellect Disabil 2005;18:163-73. https://doi.org/10.1111/j.1468-3148.2005.00245.x.
- Bazzano AT, Zeldin AS, Diab IRS, Garro NM, Allevato NA, Lehrer D, et al. The Healthy Lifestyle Change Program: a pilot of a community-based health promotion intervention for adults with developmental disabilities. Am J Prev Med 2009;37:S201-S8. https://doi.org/10.1016/j.amepre.2009.08.005.
- Bodde AE, Seo D-C, Frey GC, Lohrmann DK, Van Puymbroeck M. Developing a physical activity education curriculum for adults with intellectual disabilities. Health Promot Pract 2012;13:116-23. https://doi.org/10.1177/1524839910381698.
- Bodde AE, Seo D-C, Frey GC, Van Puymbroeck M, Lohrmann DK. The effect of a designed health education intervention on physical activity knowledge and participation of adults with intellectual disabilities. Am J Health Promot 2012;26:313-16. https://doi.org/10.4278/ajhp.100408-ARB-112.
- Bradley S. Tackling obesity in people with learning disability. Learn Disabil Pract 2005;8:10-4. https://doi.org/10.7748/ldp2005.09.8.7.10.c1637.
- Chapman MJ, Craven MJ, Chadwick DD. Fighting fit? An evaluation of health practitioner input to improve healthy living and reduce obesity for adults with learning disabilities. J Intellect Disabil 2005;9:131-44. https://doi.org/10.1177/1744629505053926.
- Elinder LS, Bergstrom H, Hagberg J, Wihlman U, Hagstromer M. Promoting a healthy diet and physical activity in adults with intellectual disabilities living in community residences: design and evaluation of a cluster-randomized intervention. BMC Public Health 2010;10. https://doi.org/10.1186/1471-2458-10-761.
- Ewing G, McDermott S, Thomas-Koger M, Whitner W, Pierce K. Evaluation of a cardiovascular health program for participants with mental retardation and normal learners. Health Educ Behav 2004;31:77-8. https://doi.org/10.1177/1090198103259162.
- Fisher E. Behavioral weight reduction program for mentally retarded adult females. Percept Mot Skills 1986;62:359-62. https://doi.org/10.2466/pms.1986.62.2.359.
- Fox RA, Rosenberg R, Rotatori AF. Parent involvement in a treatment program for obese retarded adults. J Behav Ther Exp Psychiatry 1985;16:45-8. https://doi.org/10.1016/0005-7916(85)90029-1.
- Hahn JE, Aronow HU. A pilot of a gerontological advanced practice nurse preventive intervention. J Appl Res Intellect Disabil 2005;18:131-42. https://doi.org/10.1111/j.1468-3148.2005.00242.x.
- Heller T, Hsieh K, Rimmer JH. Attitudinal and psychosocial outcomes of a fitness and health education program on adults with down syndrome. Am J Ment Retard 2004;109:175-85. https://doi.org/10.1352/0895-8017(2004)109<175:AAPOOA>2.0.CO;2.
- Humphries K, Traci MA, Seekins T. Nutrition education and support program for community-dwelling adults with intellectual disabilities. Intellect Dev Disabil 2008;46:335-45. https://doi.org/10.1352/2008.46:335-345.
- Jones MC, Walley RM, Leech A, Paterson M, Common S, Metcalf C. Using goal attainment scaling to evaluate a needs-led exercise programme for people with severe and profound intellectual disabilities. J Intellect Disabil 2006;10:317-35. https://doi.org/10.1177/1744629506070051.
- Lunsky Y, Straiko A, Armstrong S. Women be healthy: evaluation of a women’s health curriculum for women with intellectual disabilities. J Appl Res Intellect Disabil 2003;16:247-53. https://doi.org/10.1046/j.1468-3148.2003.00160.x.
- Mann J, Zhou H, McDermott S, Poston MB, MacLean J, William E. Healthy behavior change of adults with mental retardation: attendance in a health promotion program. Am J Ment Retard 2006;111:62-73. https://doi.org/10.1352/0895-8017(2006)111[62:HBCOAW]2.0.CO;2.
- McCarran MS, Andrasik F. Behavioral weight-loss for multiply-handicapped adults: assessing caretaker involvement and measures of behavior change. Addict Behav 1990;15:13-20. https://doi.org/10.1016/0306-4603(90)90003-G.
- McDermott S, Whitner W, Thomas-Koger M, Mann JR, Clarkson J, Barnes TL, et al. An efficacy trial of ‘Steps to Your Health’, a health promotion programme for adults with intellectual disability. Health Educ J 2012;71:278-90. https://doi.org/10.1177/0017896912441240.
- Marshall D, McConkey R, Moore G. Obesity in people with intellectual disabilities: the impact of nurse-led health screenings and health promotion activities. J Adv Nurs 2003;41:147-53. https://doi.org/10.1046/j.1365-2648.2003.02522.x.
- Mate-Kole CC, Danquah SA, Twum M, Danquah AO. Outcomes of a nonaversive behavior intervention in intellectually impaired individuals using goal attainment scaling. Nurs Res 1999;48:220-5. https://doi.org/10.1097/00006199-199907000-00005.
- Pett M, Clark L, Eldredge A, Cardell B, Jordan K, Chambless C, et al. Effecting healthy lifestyle changes in overweight and obese young adults with intellectual disability. Am J Intellect Dev Disabil 2013;118:224-43. https://doi.org/10.1352/1944-7558-118.3.224.
- Poynor L. Steps to fitness: a health and well-being pilot project: with rates of obesity in the UK reaching epidemic proportions, programmes designed to tackle the problem have been criticised for bypassing certain population groups. But a project designed to promote exercise and healthy living among people with learning disabilities in Surrey has achieved many of its objectives. Lisa Poynor explains. Learning Disability Practice 2008;11:10-5. https://doi.org/10.7748/ldp2008.04.11.3.10.c6475.
- Rotatori AF, Fox R. The effectiveness of a behavioral weight reduction program for moderately retarded adolescents. Behavior Therapy 1980;11:410-16. https://doi.org/10.1016/S0005-7894(80)80057-8.
- Rotatori AF, Fox R, Switzky H. A parent-teacher administered weight reduction program for obese Down’s Syndrome adolescents. J Behav Ther Exp Psychiatry 1979;10:339-41. https://doi.org/10.1016/0005-7916(79)90013-2.
- Rotatori AF, Fox R, Switzky H. Multicomponent behavioral program for achieving weight loss in adult mentally retarded persons. Ment Retard 1980;18:31-3.
- Rotatori AF, Zinkgraf S, Matson J, Fox R, Sexton D, Wade P. The effect of two weight reduction maintenance strategies for moderately/mildly retarded adults. J Obesity Weight Reg 1986;5:18-22.
- Sailer AB, Miltenberger RG, Johnson B, Zetocha K, Egemo-Helm K, Hegstad H. Evaluation of a weight loss treatment program for individuals with mild mental retardation. Child Fam Behav Ther 2006;28:15-28. https://doi.org/10.1300/J019v28n02_02.
- Wilhite B, Biren G, Spencer L. Fitness Intervention for Adults with Developmental Disabilities and their Caregivers. Ther Recreation J 2012;46.
- Brown HS, Wilson KJ, Pagan JA, Arcari CM, Martinez M, Smith K, et al. Cost-effectiveness analysis of a community health worker intervention for low-income Hispanic adults with diabetes. Prev Chronic Dis 2012;9. https://doi.org/10.5888/pcd9.120074.
- Schechter CB, Cohen HW, Shmukler C, Walker EA. Intervention costs and cost-effectiveness of a successful telephonic intervention to promote diabetes control. Diabetes Care 2012;35:2156-60. https://doi.org/10.2337/dc12-0048.
- Ritzwoller D, Sukhanova A, Glasgow R, La S, King L, Gaglio B, et al. Intervention costs and cost-effectiveness for a multiple-risk-factor diabetes self-management trial for Latinas: economic analysis of Viva Bien! Provisional abstract. Transl Behav Med 2011;1:427-35. https://doi.org/10.1007/s13142-011-0037-z.
- Kaplan MR, Atkins KC, Wilson KD. The cost-utility of diet and exercise interventions in non-insulin-dependent diabetes mellitus. Structured abstract. Health Promot Int 1988;2:331-40. https://doi.org/10.1093/heapro/2.4.331.
- Handley MA, Shumway M, Schillinger D. Cost-effectiveness of automated telephone self-management support with nurse care management among patients with diabetes. Ann Fam Med 2008;6:512-18. https://doi.org/10.1370/afm.889.
- Brownson CA, Hoerger TJ, Fisher EB, Kilpatrick KE. Cost-effectiveness of diabetes self-management programs in community primary care settings. Diabetes Educ 2009;35:761-9. https://doi.org/10.1177/0145721709340931.
- Bower P, Kennedy A, Reeves D, Rogers A, Blakeman T, Chew-Graham C, et al. A cluster randomised controlled trial of the clinical and cost-effectiveness of a ‘whole systems’ model of self-management support for the management of long- term conditions in primary care: trial protocol. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-7.
- Riddell MA, Renwick C, Wolfe R, Colgan S, Dunbar J, Hagger V, et al. Cluster randomized controlled trial of a peer support program for people with diabetes: study protocol for the Australasian Peers for Progress study. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-843.
- Beeken RJ, Spanos D, Fovargue S, Hunter R, Omar R, Hassiotis A, et al. Piloting a manualised weight management programme (Shape Up-LD) for overweight and obese persons with mild-moderate learning disabilities: study protocol for a pilot randomised controlled trial. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-71.
- Strydom A, Romeo R, Perez-Achiaga N, Livingston G, King M, Knapp M, et al. Service use and cost of mental disorder in older adults with intellectual disability. Br J Psychiatry 2010;196:133-8. https://doi.org/10.1192/bjp.bp.108.060939.
- Knobbe CA, Carey SP, Rhodes L, Horner RH. Benefit-cost analysis of community residential versus institutional services for adults with severe mental retardation and challenging behaviors. Am J Ment Retard 1995;99:533-41.
- Polder JJ, Meerding WJ, Bonneux L, van der Maas PJ. Healthcare costs of intellectual disability in the Netherlands: a cost-of-illness perspective. J Intellect Disabil Res 2002;46:168-78. https://doi.org/10.1046/j.1365-2788.2002.00384.x.
- Robertson J, Emerson E, Hatton C, Elliott J, McIntosh B, Swift P, et al. Longitudinal analysis of the impact and cost of person-centered planning for people with intellectual disabilities in England. Am J Ment Retard 2006;111:400-16. https://doi.org/10.1352/0895-8017(2006)111[400:LAOTIA]2.0.CO;2.
- Tyrer P, Oliver-Africano P, Romeo R, Knapp M, Dickens S, Bouras N, et al. Neuroleptics in the treatment of aggressive challenging behaviour for people with intellectual disabilities: a randomised controlled trial (NACHBID). Health Technol Assess 2009;13. https://doi.org/10.3310/hta13210.
- Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J. General health status measures for people with cognitive impairment: learning disability and acquired brain injury. Health Technol Assess 2001;5. https://doi.org/10.3310/hta5060.
- Curtis L. Unit Costs of Health and Social Care 2014. Kent: Personal Social Services Research Unit; 2015.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2013.
- National Schedule of Reference Costs Year 2014–2015: NHS Trusts PCT Combined. London: Department of Health; 2015.
- Walwyn RE, Russell AM, Bryant LD, Farrin AJ, Wright-Hughes AM, Graham EH, et al. Supported self-management for adults with type 2 diabetes and a learning disability (OK-Diabetes): study protocol for a randomised controlled feasibility trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-0832-9.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- House A, Bryant L, Russell AM, Wright-Hughes A, Graham L, Walwyn R, et al. Randomized controlled feasibility trial of supported self-management in adults with Type 2 diabetes mellitus and an intellectual disability: OK Diabetes. Diabet Med 2018;35:776-88. https://doi.org/10.1111/dme.13626.
- Löwe B, Kroenke K, Gräfe K. Detecting and monitoring depression with a two-item questionnaire (PHQ-2). J Psychosom Res 2005;58:163-71. https://doi.org/10.1016/j.jpsychores.2004.09.006.
- Gillett M, Dallosso HM, Dixon S, Brennan A, Carey ME, Campbell MJ, et al. Delivering the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cost effectiveness analysis. BMJ 2010;341. https://doi.org/10.1136/bmj.c4093.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. https://doi.org/10.1111/j.2002.384.doc.x.
- Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: a consensus on type 2 diabetes prevention. Diabet Med 2007;24:451-63. https://doi.org/10.1111/j.1464-5491.2007.02157.x.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Drummond MF, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; 2005.
- Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves – facts, fallacies and frequently asked questions. Health Econ 2004;13:405-15. https://doi.org/10.1002/hec.903.
- van Hout BA, Al MJ, Gordon GS, Rutten FF. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ 1994;3:309-19. https://doi.org/10.1002/hec.4730030505.
- Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ 2000;320:114-16. https://doi.org/10.1136/bmj.320.7227.114.
- Smith J, Firth J. Qualitative data analysis: the framework approach. Nurse Res 2011;18:52-6. https://doi.org/10.7748/nr2011.01.18.2.52.c8284.
- Ritchie J, Spencer L, Huberman M, Miles MB. The Qualitative Researcher’s Companion. Thousand Oaks, CA: Sage Publications; 2002.
- Ritchie J, Spencer L, Bryman A, Burgess R. Analyzing Qualitative Data. London: Routledge; 1994.
- Bryant LD, Russell AM, Walwyn REA, Farrin AJ, Wright-Hughes A, Graham EH, et al. Characterizing adults with Type 2 diabetes mellitus and intellectual disability: outcomes of a case-finding study. Diabet Med 2018;35:352-9. https://doi.org/10.1111/dme.13510.
- NHS Reference Costs 2013–14. London: Department of Health; 2014.
- Cameron L, Murphy J. Obtaining consent to participate in research: the issues involved in including people with a range of learning and communication disabilities. Br J Learn Disabil 2007;35:113-20. https://doi.org/10.1111/j.1468-3156.2006.00404.x.
- Jones N, Melville CA, Harris L, Bleazard L, Hankey CR. A qualitative study exploring why adults with intellectual disabilities and obesity want to lose weight and views of their carers. BMC Obes 2015;2. https://doi.org/10.1186/s40608-015-0080-2.
- Emerson E, Hatton C, Baines S, Robertson J. The physical health of British adults with intellectual disability: cross sectional study. Int J Equity Health 2016;15. https://doi.org/10.1186/s12939-016-0296-x.
- Emerson E, Llewellyn G, Hatton C, Hindmarsh G, Robertson J, Man WY, et al. The health of parents with and without intellectual impairment in the UK. J Intellect Disabil Res 2015;59:1142-54. https://doi.org/10.1111/jir.12218.
- Heslop P, Blair PS, Fleming P, Hoghton M, Marriott A, Russ L. The Confidential Inquiry into premature deaths of people with intellectual disabilities in the UK: a population-based study. Lancet 2014;383:889-95. https://doi.org/10.1016/S0140-6736(13)62026-7.
- Cooper SA, Smiley E, Morrison J, Williamson A, Allan L. An epidemiological investigation of affective disorders with a population-based cohort of 1023 adults with intellectual disabilities. Psychol Med 2007;37:873-82. https://doi.org/10.1017/S0033291707009968.
- Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL. Combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med 2015;163:437-51. https://doi.org/10.7326/M15-0452.
- Hobbs N, Godfrey A, Lara J, Errington L, Meyer TD, Rochester L, et al. Are behavioral interventions effective in increasing physical activity at 12 to 36 months in adults aged 55 to 70 years? A systematic review and meta-analysis. BMC Med 2013;11. https://doi.org/10.1186/1741-7015-11-75.
- Johns DJ, Hartmann-Boyce J, Jebb SA, Aveyard P. Group BWMR . Diet or exercise interventions vs combined behavioral weight management programs: a systematic review and meta-analysis of direct comparisons. J Acad Nutri Diet 2014;114:1557-68. https://doi.org/10.1016/j.jand.2014.07.005.
- Lin JS, O’connor E, Evans CV, Senger CA, Rowland MG, Groom HC. Behavioral counseling to promote a healthy lifestyle in persons with cardiovascular risk factors: a systematic review for the US preventive services task force. Ann Intern Med 2014;161:568-78. https://doi.org/10.7326/M14-0130.
- Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. JAMA 2014;312:1779-91. https://doi.org/10.1001/jama.2014.14173.
- Cardona-Morrell M, Rychetnik L, Morrell SL, Espinel PT, Bauman A. Reduction of diabetes risk in routine clinical practice: are physical activity and nutrition interventions feasible and are the outcomes from reference trials replicable? A systematic review and meta-analysis. BMC Public Health 2010;10. https://doi.org/10.1186/1471-2458-10-653.
- Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care 2014;37:922-33. https://doi.org/10.2337/dc13-2195.
- Spanos D, Melville CA, Hankey CR. Weight management interventions in adults with intellectual disabilities and obesity: a systematic review of the evidence. Nutr J 2013;12. https://doi.org/10.1186/1475-2891-12-132.
- Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol 2005;23:7199-206. https://doi.org/10.1200/JCO.2005.01.149.
- ADVANCE Collaborative Group . ADVANCE – Action in Diabetes and Vascular Disease: patient recruitment and characteristics of the study population at baseline. Diabet Med 2005;22:882-8. https://doi.org/10.1111/j.1464-5491.2005.01596.x.
- Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545-59. https://doi.org/10.1056/NEJMoa0802743.
Appendix 1 Step-by-step identification guide
Appendix 2 Simple checklist from referral form
Appendix 3 Case-finding study patient information booklet
Appendix 4 Case-finding study consent form
Appendix 5 Case-finding study researcher researcher administered baseline booklet
Appendix 6 List of resources
| Title of resource | Source/author |
|---|---|
| Getting on with Diabetes: Beyond Words book | Sheila Hollins and Rachel Besser and Libby Dowling |
| My Living Well with Diabetes folder (aimed at people whose first language is not English and who does not have a learning disability) | NHS Bradford and Airedale Trust |
| What to Do When You Have Type 2 Diabetes | Diabetes UK and CHANGE |
| Understanding Diabetes: Easy-Read Guide and Compact Disc | Bradford Talking Media |
| Understanding Diabetes: I Have Diabetes | Walsall Council |
| Understanding Diabetes: Visiting the Nurse | |
| Understanding Diabetes: Words and Meanings | |
| My Health Action Plan for Diabetes | |
| Diabetes | Shropshire County NHS Primary Care Trust |
| Diabetes Type 2 | Easyhealth |
| Getting Started If You Have Diabetes | Bristol Central Community Learning Disability Team |
| My Plate Planner | New York City Health |
| Type 2 and Coronary Heart Disease Risk Assessment | Isle of Wight NHS Healthcare Trust |
| Diabetes (Type 2): A Guide for People with Learning Disability and Carers | Reading Borough Council and Berkshire West NHS Primary Care Trust |
| Diabetes Care. Taking Care of Your Eyes | Cheshire and Wirral Partnership NHS Trust |
| SeeAbility diabetic eye screening | NHS Screening Programmes |
| Diabetes and Your Feet/What is Diabetes?/Diabetes & Healthy Eating/My Diabetes Daily Reminder/Diabetes Care Schedule/Diabetes Pills: What You Need to Know/Exercise & Diabetes | Learning about Diabetes |
| Diabetes | Foundation for People with Learning Disability |
| You Have Diabetes | Derbyshire County NHS |
| Steven’s story | Leeds and York Partnership NHS Trust |
| Living Well with Diabetes: A Book about Diabetes | North Tyneside Primary Care Trust and Northumbria Healthcare NHS Foundation Trust |
| Diabetes 15 Healthcare Essentials | Diabetes UK |
| Advice on diabetes | Widgit symbols |
| Type 2 Diabetes: Pictorial Information about Type 2 Diabetes for People with a Learning Disability | Health and Social Care Public Health Agency and Northern Health and Social Care Trust |
| Healthy Eating Sheet/Good Exercise Routine (for People with Down’s Syndrome) | Down’s Syndrome Association (UK) |
| Health Eating – Tips for Parents and Carers/Getting Active – Tips for Parents and Carers | |
| Step by Step – For Health Fitness and Fun | NHS Ayrshire & Arran |
Appendix 7 Literature searches for intervention and adherence
Scoping searches revealed a very small research literature on self-care interventions for people with diabetes and learning difficulties. Literature searches were therefore developed to identify relevant studies from larger research areas. Citation searches targeted known self-care projects.
Searches:
-
self-care interventions for adults with diabetes
-
self-care interventions for adults with learning difficulties (and their carers)
-
adherence to self-care interventions for diabetes control or weight loss in people with learning difficulties
-
citation searches of five key papers describing self-care interventions.
Literature search for studies of self-care interventions for diabetes
We searched for studies describing self-care interventions for diabetes to provide an overview of this literature and identify core studies that would be relevant to our project. In light of the volume of diabetes research and the difficulty in searching precisely for ‘self-care’ interventions we designed two targeted literature searches.
-
Search 1a identified systematic reviews of diabetes and self-care interventions.
-
Search 1b identified all study types, but used a focused strategy to identify reports likely to have substantial relevant ‘self-care intervention’ content.
The MEDLINE search below illustrates the two search strategies. Translated versions of these searches were developed and run in the Cumulative Index to Nursing and Allied Health Literature (CINAHL), EMBASE, MEDLINE and PsycINFO.
Search1a: systematic reviews of diabetes and self-care interventions
MEDLINE (via Ovid)
Date range searched: 1946 to week 4 August 2013.
Search strategy
-
exp Diabetes Mellitus/ (315,941)
-
diabet*.tw. (388,226)
-
niddm.tw. (6747)
-
mody.tw. (831)
-
or/1-4 [Diabetes] (440,597)
-
exp diet/ (193,464)
-
nutrition therapy/ (876)
-
food habits/ (20,699)
-
dietetics/ (5113)
-
nutritional sciences/ (6553)
-
diet therapy.fs. (38,787)
-
((diet* or eat or eating) adj2 (behav* or habit* or therap* or treatment* or intervention*)).tw. (35,676)
-
(weight adj2 (loss* or control or gain* or reduc*)).tw. (107,235)
-
exp weight loss/ (28,469)
-
exp Obesity/dh, dt, pc, th [Diet Therapy, Drug Therapy, Prevention & Control, Therapy] (35,150)
-
exp exercise/ (111,033)
-
exp exercise therapy/ (29,744)
-
(physical adj2 (exercis* or activit*)).tw. (63,376)
-
exercise*.ti. (77,740)
-
medication adherence/ (7051)
-
patient compliance/ (48,041)
-
(adher* or nonadher* or non-adher*).tw. (115,842)
-
(compliance or noncompliance or non-compliance).tw. (78,237)
-
or/6-23 [Diet or Exercise or Adherence] (751,483)
-
self care/ or blood glucose self-monitoring/ or self administration/ or self medication/ (40,793)
-
(self adj3 (help or care* or manage* or administer* or monitor* or medicat* or treat* or inject*)).tw. (59,402)
-
exp Motivation/ (128,593)
-
Patient Education as Topic/ (70,775)
-
*health promotion/mt (9371)
-
exp computer-assisted instruction/ (9418)
-
exp teaching materials/ (95,949)
-
((patient or patients) adj5 (information or leaflet* or education* or educate* or book* or pamphlet* or resource* or dvd* or cd or cds or “cd rom*” or multimedia or web* or internet*)).tw. (82,812)
-
(education adj2 (program* or session*)).tw. (21,085)
-
((skill or skills) adj2 teach*).tw. (2304)
-
Reminder Systems/ (2362)
-
(reminder* or prompt* or cue).tw. (106,427)
-
Problem Solving/ (21,253)
-
(goal* adj3 (set or setting or attain* or achiev* or motivat*)).tw. (20,888)
-
or/25-38 [Self care or associated interventions] (589,918)
-
exp Patient Care Management/ (531,179)
-
5 and 24 and 39 and 40 [Diabetes AND Diet/exercise/Adherence AND self care AND patient mgt] (1275)
-
exp animals/ not (exp animals/ and exp humans/) (4,027,003)
-
(adolescent/ or exp child/ or exp infant/) not ((adolescent/ or exp child/ or exp infant/) and exp adult/) (1,512,502)
-
japan*.lg. (398,760)
-
or/42-44 [exclusions] (5,868,877)
-
41 not 45 (1169)
-
limit 46 to “reviews (best balance of sensitivity and specificity)” (232)
Search 1b: diabetes and self-care interventions – core studies
MEDLINE (via Ovid)
Date range searched: 1946 to 4 week August 2013.
Search strategy
-
exp *Diabetes Mellitus/ (250,043)
-
diabet*.ti. (222,237)
-
or/1-2 [Diabetes] (265,273)
-
exp *diet/ (82,798)
-
*nutrition therapy/ (590)
-
*food habits/ (10,233)
-
*dietetics/ (4036)
-
*nutritional sciences/ (5182)
-
diet therapy.fs. (38,787)
-
((diet* or eat or eating) adj2 (behav* or habit* or therap* or treatment* or intervention*)).ti. (7223)
-
(weight adj2 (loss* or control or gain* or reduc*)).ti. (16,240)
-
exp *weight loss/ (12,859)
-
exp *Obesity/dh, dt, pc, th [Diet Therapy, Drug Therapy, Prevention & Control, Therapy] (21,650)
-
exp *exercise/ (70,762)
-
exp *exercise therapy/ (19,603)
-
(physical adj2 (exercis* or activit*)).ti. (20,849)
-
exercise*.ti. (77,740)
-
*medication adherence/ (4157)
-
*patient compliance/ (19,282)
-
(adher* or nonadher* or non-adher*).ti. (21,260)
-
(compliance or noncompliance or non-compliance).ti. (14,666)
-
or/4-21 [Diet or Exercise or Adherence] (336,482)
-
*self care/ or *blood glucose self-monitoring/ or *self administration/ or *self medication/ (17,827)
-
(self adj3 (help or care* or manage* or administer* or monitor* or medicat* or treat* or inject*)).ti. (12,104)
-
23 or 24 [self care] (23,250)
-
exp Patient Care Management/ (531,179)
-
3 and 22 and 25 and 26 (72)
-
exp animals/ not (exp animals/ and exp humans/) (4,027,003)
-
2 (adolescent/ or exp child/ or exp infant/) not ((adolescent/ or exp child/ or exp infant/) and exp adult/) (1,512,502)
-
japan*.lg. (398,760)
-
or/28-30 [exclusions] (5,868,877)
-
27 not 31 (62)
Literature search for self-care interventions for people with learning difficulties (and their carers)
We searched for core studies describing self-care interventions for people with learning difficulties (and their carers) that also included health behaviour and patient understanding.
The MEDLINE search below illustrates the strategy used. Translated versions were developed and run in CINAHL, The Cochrane Library, EMBASE, Health Management Information Consortium, MEDLINE and PsycINFO.
MEDLINE (via Ovid)
Date range searched: 1946 to week 2 March 2013.
Search strategy
-
learning disorder/ (12,013)
-
Mentally Disabled Persons/ (2113)
-
Intellectual Disability/ (45,430)
-
((Learning or intellectual*) adj3 (disab* or disorder* or disturbance* or impair* or difficulty or difficulties)).tw. (20,324)
-
(development* adj1 (disab* or disorder* or disturbance* or difficulty or difficulties)).tw. (9239)
-
((Subnormal* or below normal) adj3 (intellect* or mental* or learning or IQ)).tw. (548)
-
(Mental* adj3 (disab* or impair* or deficien* or retard* or handicap*)).tw. (36,310)
-
or/1-7 [Learning Disabilities] (92,107)
-
self care/ or blood glucose self-monitoring/ or self administration/ or self medication/ (36853)
-
Patient Education as Topic/ (66,504)
-
“education of mentally retarded”/ (5483)
-
(self adj3 (care* or manage* or administer* or monitor* or medicat* or treat* or inject*)).tw. (49,010)
-
((skill or skills) adj2 teach*).tw. (2124)
-
((patient or patients) adj3 (information or leaflet* or education* or educate* or book* or pamphlet* or resource* or dvd* or CD* or multimedia or web*)).tw. (62,294)
-
((carer* or caregiver*) adj3 (information or leaflet* or education* or educate* or book* or pamphlet* or resource* or dvd* or CD* or multimedia or web*)).tw. (1487)
-
(education adj2 (program* or session*)).tw. (19,444)
-
exp Program evaluation/ or exp computer-Assisted Instruction/ or exp Teaching materials/ (148,194)
-
*health promotion/mt (7935)
-
or/9-18 [Self Care or Patient Education Methods] (346,459)
-
health behavior/ or patient compliance/ or medication adherence/ or exp self-examination/ or treatment refusal/ (88,519)
-
exp Life Style/ (58,961)
-
(health* adj2 (behav* or lifestyle*)).tw. (23,864)
-
exp Diet/ or Nutrition Therapy/ (179,881)
-
diet therapy.fs. (36,213)
-
Food Habits/ (18,838)
-
nutritional sciences/ or dietetics/ (10,774)
-
((Diet* or eat or eating) adj2 (behav* or habit*)).tw. (15,408)
-
(weight adj2 (loss* or control or gain* or reduc*)).tw. (96,778)
-
exp Weight Loss/ (25,384)
-
exp Obesity/ (125,479)
-
exp Exercise/ (100,222)
-
exp Exercise Therapy/ (26,894)
-
(physical adj2 (exercise* or activit*)).tw. (55,029)
-
exercise*.ti. (72,804)
-
or/20-34 [Diet, Exercise & Healthy behaviour] (687,397)
-
Health Knowledge, Attitudes, Practice/ (65075)
-
health literacy/ or patient medication knowledge/ (1117)
-
“Education of Intellectually Disabled”/ (5483)
-
(Learning adj1 (disab* or disorder* or disturbance* or impair* or difficulty or difficulties) adj7 (understand* or comprehend* or know of knowledge)).tw. (91)
-
((patient or patients or client or clients) adj4 (understand* or comprehend*)).tw. (10,563)
-
((patient or patients or client or clients) adj2 (know or knowledge)).tw. (4799)
-
or/36-41 (84,733)
-
8 and 19 and 35 and 42 (90)
Literature search for studies of adherence, self-care, learning difficulties and diabetes control or weight-loss interventions
We searched for core studies describing adherence to self-care diabetes interventions or self-care weight loss interventions for people with learning difficulties.
The MEDLINE search below illustrates the strategy used. Translated versions were developed and run in CINAHL, EMBASE and PsycINFO.
MEDLINE (via Ovid)
Date range searched: 1946 to week 1 July 2015.
Search strategy
-
learning disorder/ (12,972)
-
Mentally Disabled Persons/ (2251)
-
Cognition Disorders/ (54,539)
-
Intellectual Disability/ (48,467)
-
((Development* or Learning or intellectual*) adj3 (disab* or disorder* or disturbance* or impair* or difficulty or difficulties)).tw. (50,199)
-
((Subnormal* or below normal) adj3 (intellect* or mental* or learning or IQ)).tw. (584)
-
(Mental* adj3 (disab* or impair* or deficien* or retard* or handicap*)).tw. (38,478)
-
(cogniti* adj5 (disab* or disorder* or disturbance* or impair* or difficulty or difficulties)).tw. (53,021)
-
or/1-8 [Learning Disabilities] (196,692)
-
exp Diabetes Mellitus, Type 2/ (93,609)
-
Diabetes Mellitus/ (95,037)
-
(diabet* adj3 (ii or “type 2” or two)).tw. (83,896)
-
(diabet* adj3 (“non insulin dependent” or stable or “adult onset” or “maturity onset” or “ketosis resistant” or “slow onset”)).tw. (12,796)
-
niddm.tw. (6735)
-
mody.tw. (862)
-
or/10-15 [Diabetes Type 2] (208,839)
-
exp diet/ (207,219)
-
nutrition therapy/ (1011)
-
food habits/ (22,770)
-
dietetics/ (5281)
-
nutritional sciences/ (6783)
-
diet therapy.fs. (41,403)
-
((diet* or eat or eating) adj2 (behav* or habit* or therap* or treatment* or intervention*)).tw. (38,346)
-
[or/17-30 [Diet or Exercise]] (0)
-
[or/33-46 [Self Care Interventions]] (0)
-
[or/48-70 [Intervention Studies]] (0)
-
[or/84-87] (0)
-
learning disorder/ (12,972)
-
Mentally Disabled Persons/ (2251)
-
Cognition Disorders/ (54,539)
-
Intellectual Disability/ (48,467)
-
((Development* or Learning or intellectual*) adj3 (disab* or disorder* or disturbance* or impair* or difficulty or difficulties)).tw. (50,199)
-
((Subnormal* or below normal) adj3 (intellect* or mental* or learning or IQ)).tw. (584)
-
(Mental* adj3 (disab* or impair* or deficien* or retard* or handicap*)).tw. (38,478)
-
(cogniti* adj5 (disab* or disorder* or disturbance* or impair* or difficulty or difficulties)).tw. (53,021)
-
or/28-35 [Learning Disabilities] (196,692)
-
exp Diabetes Mellitus, Type 2/ (93,609)
-
Diabetes Mellitus/ (95,037)
-
(diabet* adj3 (ii or “type 2” or two)).tw. (83,896)
-
(diabet* adj3 (“non insulin dependent” or stable or “adult onset” or “maturity onset” or “ketosis resistant” or “slow onset”)).tw. (12,796)
-
niddm.tw. (6735)
-
mody.tw. (862)
-
or/37-42 [Diabetes Type 2] (208,839)
-
exp diet/ (207,219)
-
nutrition therapy/ (1011)
-
food habits/ (22,770)
-
dietetics/ (5281)
-
nutritional sciences/ (6783)
-
diet therapy.fs. (41,403)
-
((diet* or eat or eating) adj2 (behav* or habit* or therap* or treatment* or intervention*)).tw. (38,346)
-
(weight adj2 (loss* or control or gain* or reduc*)).tw. (113,342)
-
exp obesity/ (153,138)
-
exp weight loss/ (30,778)
-
exp exercise/ (128,302)
-
exp exercise therapy/ (32,963)
-
(physical adj2 (exercis* or activit*)).tw. (68,935)
-
exercise*.ti. (81,555)
-
or/44-57 [Diet or Exercise] (670,444)
-
43 or 58 [Diabetes or Diet or Exercise] (833,366)
-
self care/ or blood glucose self-monitoring/ or self administration/ or self medication/ (42,897)
-
(self adj3 (help or care* or manage* or administer* or monitor* or medicat* or treat* or inject*)).tw. (62,976)
-
(goal* adj3 set*).tw. (4581)
-
exp Motivation/ (134,568)
-
Patient Education as Topic/ (72,978)
-
*health promotion/mt (10,199)
-
exp computer-assisted instruction/ (9932)
-
exp teaching materials/ (99,158)
-
((patient or patients) adj5 (information or leaflet* or education* or educate* or book* or pamphlet* or resource* or dvd* or cd or cds or “cd rom*” or multimedia or web* or internet*)).tw. (88,842)
-
(education adj2 (program* or session*)).tw. (22,029)
-
((skill or skills) adj2 teach*).tw. (2431)
-
self-help devices/ (3676)
-
((“self help” or “self care” or “self manage*”) adj5 (device* or tool* or technolog*)).tw. (531)
-
(assistive adj2 technolog*).tw. (957)
-
or/60-73 [Self Care Interventions] (484,452)
-
clinical trial/ or clinical trial, phase i/ or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or controlled clinical trial/ or multicenter study/ or randomized controlled trial/ (819,898)
-
exp Clinical Trials as Topic/ (297,339)
-
Evaluation studies/ (207,012)
-
Validation studies/ (73,500)
-
research design/ or cross-over studies/ or double-blind method/ or matched-pair analysis/ or random allocation/ or “reproducibility of results”/ or sample size/ or exp “sensitivity and specificity”/ or single-blind method/ or Early Termination of Clinical Trials/ (956,355)
-
(pre post or pre test or post test or non ramdomi?ed or quasi experiment).tw. (11,136)
-
Feasibility studies/ (47,606)
-
Intervention studies/ (7958)
-
Pilot projects/ (89,042)
-
exp program evaluation/ (59,216)
-
placebo*.tw. (160,364)
-
(random* adj3 (study or studies or trial or trials)).tw. (268,390)
-
(random* adj3 (allocation or assign* or allocate*)).tw. (96,507)
-
(study adj (pilot or feasibility or evaluation or validation)).tw. (801)
-
(studies adj (pilot or feasibility or evaluation or validation)).tw. (212)
-
((blind or mask*) adj2 (singl* or doubl* or trebl* or tripl*)).tw. (121,904)
-
(control adj group*).tw. (287,783)
-
(“outcome study” or “outcome studies” or quasiexperimental or “quasi experimental” or quasi-experimental or “pseudo experimental”).tw. (11,545)
-
case reports/ (1,759,292)
-
(crossover* or “cross over*” or cross-over*).tw. (59,207)
-
((trial or trials) adj2 (clinical or controlled or cluster or factorial)).tw. (333,156)
-
case stud*.tw. (52,289)
-
intervention.tw. (336,369)
-
or/75-97 [Intervention Studies] (4,346,949)
-
medication adherence/ (9523)
-
patient compliance/ (49,291)
-
(adher* or nonadher* or non-adher*).tw. (121,440)
-
(compliance or noncompliance or non-compliance).tw. (81,763)
-
99 or 100 or 101 or 102 [Adherence search] (226,017)
-
98 or 103 [Intervention or Adherence studies] (4,502,963)
-
36 and 59 and 74 and 104 (230)
-
“Patient Acceptance of Health Care”/ or Treatment Refusal/ or Patient Participation/ or exp Patient Satisfaction/ or Patient Dropouts/ (130,985)
-
exp Attitude to Health/ (314,603)
-
106 or 107 [extra compliance terms] (319,579)
-
104 or 108 [Intervention or Adherence or compliance studies] (4,681,354)
-
36 and 59 and 74 and 109 (256)
-
exp animals/ not (exp animals/ and exp humans/) (4,078,149)
-
(adolescent/ or exp child/ or exp infant/) not ((adolescent/ or exp child/ or exp infant/) and exp adult/) (1,581,504)
-
dementia.tw. (66,389)
-
exp dementia/ (128,598)
-
or/111-114 (5,795,650)
-
105 not 115 [original strategy] (161)
-
110 not 115 [additional compliance terms added] (182)
Citation searches of five key papers describing self-care interventions
Search for papers citing the following article. Search results were limited to records including the word ‘diabetes’ (Web of Science):
-
Newman S, Steed L, Mulligan K. Self-management interventions for chronic illness. Lancet 2004;364:1523–37.
Searches for citations of the following articles and the references listed in their bibliographies (Web of Science, Google Scholar):
-
DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325:746.
-
Davies MJ, Skinner TC, Cradock S, Doherty Y, Oliver L, Khunti K. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336:491–95.
-
Deakin TA, Cade JE, Williams R, Greenwood DC. Structured patient education: the Diabetes X-PERT Programme makes a difference. Diabetic Medicine 2006;23:944–54.
-
Naik AD, Palmer N, Petersen NJ, Street RL Jr, Rao R, Suarez-Almazor M, Haidet P. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Arch Intern Med 2011;171:453.
Appendix 8 Intervention materials
Appendix 9 How-to sheet: snack swaps
Appendix 10 How-to sheet: eat more fruit
Appendix 11 How-to sheet: be more active
Appendix 12 How-to sheet: eat more vegetables
Appendix 13 Adherence to intervention checklist
Appendix 14 Literature searches for health economics
In May 2013 we searched the following databases: EMBASE Classic and EMBASE (1947 to 2 May 2013), MEDLINE (via Ovid) 1946 to week 4 April 2013, MEDLINE In-Process & Other Non-Indexed Citations (via Ovid) 3 May 2013, NHS Economic Evaluations Database, The Cochrane Library (Wiley Online Library), Cost-effectiveness Analysis Registry and EconPapers (Research Papers in Economics, RePEC). Final update searches were completed in October 2013.
Search strategies were developed iteratively from April to October 2013 based on five concepts: learning disabilities, self-care interventions, type 2 diabetes, cost studies and economic models.
Four separate searches were run in each database using alternative combinations of three of the five concepts in each one. The searches were designed to identify studies that included costs or economic models for:
-
people with learning disabilities and diabetes
-
self-care interventions for people with learning disabilities
-
self-care interventions for people with diabetes
-
diabetes and self and care and model.
The searches were not limited by language. The search identifying cost studies of self-care interventions for people with diabetes published was limited by the publication date 2011 to present. No other search had publication date limits. The LIHS Information Specialist ‘cost studies’ and ‘economic models’ search filters were used to identify studies that include some cost data or an economic model.
The electronic searches identified 1495 references, which were reduced to 1189 once duplicates were removed.
Search 1: literature search for studies involving people with learning disabilities and diabetes
EconPapers (http://econpapers.repec.org/paper/)
Date range searched: inception to 30 October 2013.
Diabetes and learning and disability*
EMBASE Classic and EMBASE
Date range searched: 1947 to 29 October 2013.
Search strategy
-
exp learning disorder/ (27,339)
-
intellectual impairment/ (9052)
-
cognitive defect/ (91,973)
-
mental deficiency/ (68,124)
-
((Development* or Learning or intellectual*) adj3 (disab* or disorder* or disturbance* or impair* or difficulty or difficulties)).tw. (65,311)
-
((Subnormal* or below normal) adj3 (intellect* or mental* or learning or IQ)).tw. (1021)
-
(Mental* adj3 (disab* or impair* or deficien* or retard* or handicap*)).tw. (56,765)
-
(cogniti* adj3 (disab* or disorder* or disturbance* or impair* or difficulty or difficulties)).tw. (62,128)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (271,572)
-
health economics/ (33,378)
-
exp economic evaluation/ (206,596)
-
exp health care cost/ (198,664)
-
pharmacoeconomics/ or “drug cost”/ or drug utilization/ or “utilization review”/ (144,564)
-
socioeconomics/ and economics/ (14,776)
-
*socioeconomics/ (17,925)
-
*fee/ (6384)
-
*“cost”/ (12,941)
-
cost*.ti. (102,621)
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or evaluat* or analy* or study or studies or consequenc* or compar* or efficienc* or variable or unit or estimate* or variable* or unit)).ab. (135,415)
-
(price or prices or pricing).tw. (32,512)
-
(economic* or pharmacoeconomic* or pharmaco-economic*).tw. (204,513)
-
budget*.tw. (24,149)
-
(value adj1 (money or monetary)).tw. (447)
-
(financ* adj2 (cost* or data or “health care”)).tw. (7208)
-
financ*.tw. and economics/ (12,600)
-
(expenditure* not energy).tw. (24,214)
-
quality adjusted life year/ (11,415)
-
(eq-5d or eq5d or euroquol*).tw. (4765)
-
quality adjusted life.tw. (8202)
-
(qaly or qalys or qald or qale or qtime).tw. (7909)
-
disability adjusted life.tw. (1454)
-
(daly or dalys).tw. (1520)
-
(SF6D or sf 6d or short form 6d or shortform6d).tw. (623)
-
health* year* equivalent*.tw. (42)
-
(hye or hyes).tw. (85)
-
health utilit*.tw. (1487)
-
(hui1 or hui2 or hui3).tw. (349)
-
disutil*.tw. (336)
-
standard gamble*.tw. (763)
-
(time trade off or time tradeoff).tw. (1173)
-
(hqol or h qol or hr qol).tw. (715)
-
(pqol or qls).tw. (376)
-
or/10-42 (768,575)
-
exp animals/ not (exp animals/ and exp humans/) (4,726,495)
-
exp nonhuman/ not (exp nonhuman/ and exp human/) (3,333,799)
-
exp experimental animal/ (488,258)
-
exp veterinary medicine/ (33,074)
-
animal experiment/ (1,729,886)
-
((energy or oxygen* or metaboli*) adj3 (expenditure* or cost*)).tw. (27,522)
-
or/44-49 (6,189,732)
-
43 not 50 (719,336)
-
*diabetes mellitus/ (178,039)
-
limit 52 to yr=“1900 - 1980” (48,629)
-
*non insulin dependent diabetes mellitus/ (81,724)
-
(diabet* adj3 (ii or “type 2” or two)).tw. (114,777)
-
(diabet* adj3 (“non insulin dependent” or stable or “adult onset” or “maturity onset” or “ketosis resistant” or “slow onset”)).tw. (15,837)
-
(niddm or mody).tw. (9030)
-
or/53-57 (192,025)
-
9 and 51 and 58 (40)
MEDLINE (via Ovid)
Date range searched: 1946 to week 3 October 2013.
Search strategy
-
exp Diabetes Mellitus, Type 2/ (86,901)
-
Diabetes Mellitus/ (91,304)
-
(diabet* adj3 (ii or “type 2” or two)).tw. (78,151)
-
(diabet* adj3 (“non insulin dependent” or stable or “adult onset” or “maturity onset” or “ketosis resistant” or “slow onset”)).tw. (12,714)
-
niddm.tw. (6757)
-
mody.tw. (833)
-
or/1-6 [Diabetes-2] (197,176)
-
learning disorder/ (12,696)
-
Mentally Disabled Persons/ (2245)
-
Cognition Disorders/ (51,623)
-
Intellectual Disability/ (46,835)
-
((Development* or Learning or intellectual*) adj3 (disab* or disorder* or disturbance* or impair* or difficulty or difficulties)).tw. (47,085)
-
((Subnormal* or below normal) adj3 (intellect* or mental* or learning or IQ)).tw. (554)
-
(Mental* adj3 (disab* or impair* or deficien* or retard* or handicap*)).tw. (38,310)
-
(cogniti* adj5 (disab* or disorder* or disturbance* or impair* or difficulty or difficulties)).tw. (48,265)
-
or/8-15 [Learning Disabilities] (186,563)
-
Economics/ (27,116)
-
exp Economics, Dental/ (3950)
-
exp Economics, Nursing/ (3880)
-
exp Economics, Medical/ (13,511)
-
exp Economics, pharmaceutical/ (2607)
-
exp Economics, Hospital/ (19,414)
-
exp “Costs and Cost Analysis”/ (182,668)
-
exp “Fees and Charges”/ (27,050)
-
exp budgets/ (12,003)
-
exp “Value of Life”/ec [Economics] (243)
-
budget*.tw. (17,212)
-
cost*.ti. (77,598)
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or evaluat* or analy* or study or studies or consequenc* or compar* or efficienc* or variable or unit or estimate* or variable* or unit)).ab. (97,878)
-
(economic* or pharmacoeconomic* or pharmaco-economic*).tw. (144,648)
-
(price or prices or pricing).tw. (22,254)
-
(financ* adj2 (cost* or data or “health care”)).tw. (5646)
-
(fee or fees).tw. (11,622)
-
(value adj1 (money or monetary)).tw. (330)
-
quality-adjusted life years/ (7230)
-
(eq-5d or eq5d or euroquol*).tw. (2793)
-
exp models, economic/ (10314)
-
markov chains/ (10,634)
-
quality adjusted life.tw. (6065)
-
(qaly or qalys or qald or qale or qtime).tw. (5000)
-
disability adjusted life.tw. (1190)
-
(daly or dalys).tw. (1140)
-
health* year* equivalent*.tw. (37)
-
(hye or hyes).tw. (53)
-
(hui1 or hui2 or hui3).tw. (282)
-
disutil*.tw. (211)
-
standard gamble*.tw. (666)
-
(time trade off or time tradeoff).tw. (936)
-
(hqol or h qol or hrqol or hr qol).tw. (7389)
-
(pqol or qls).tw. (245)
-
(sf6d or sf 6d or short form 6d or shortform 6d or sf sixd or sf six d).tw. (408)
-
exp animals/ not (exp animals/ and exp humans/) (4,051,824)
-
exp Veterinary Medicine/ (20,736)
-
exp Animal Experimentation/ (6411)
-
((energy or oxygen* or metaboli*) adj3 (expenditure* or cost*)).tw. (22,022)
-
or/52-55 (4,083,859)
-
or/17-51 (473,985)
-
57 not 56 [Cost studies] (444,824)
-
58 and 7 and 16 (31)
Search 2: literature search for self-care interventions for people with learning disabilities
EconPapers (http://econpapers.repec.org/paper/)
Date range searched: inception to 30 October 2013.
Search strategy
Diabetes and self and care and (learn or mental or psychology or psychological or cognitive or cognition).
EMBASE Classic and EMBASE
Date range searched: 1947 to 29 October 2013.
Search strategy
-
exp learning disorder/ (27,339)
-
intellectual impairment/ (9052)
-
*cognitive defect/ (32,966)
-
mental deficiency/ (68,124)
-
((Learning or intellectual*) adj3 (disab* or disorder* or disturbance* or impair* or difficulty or difficulties)).tw. (31,418)
-
(development* adj1 (disab* or disorder* or disturbance* or difficulty or difficulties)).tw. (14,389)
-
((Subnormal* or below normal) adj3 (intellect* or mental* or learning or IQ)).tw. (1021)
-
(Mental* adj3 (disab* or impair* or deficien* or retard* or handicap*)).tw. (56,765)
-
or/1-8 [Learning Disability] (173,679)
-
self care/ or self help/ or self medication/ (48,240)
-
self monitoring/ (3645)
-
(self adj3 (care* or manage* or administer* or monitor* or medicat* or treat* or inject*)).tw. (73,393)
-
drug self administration/ (7675)
-
((skill or skills) adj2 teach*).tw. (3025)
-
((patient or patients) adj5 (information or leaflet* or education* or educate* or book* or pamphlet* or resource* or dvd* or CD* or multimedia or web* or internet or “cd-rom”)).tw. (161,628)
-
((carer* or caregiver*) adj3 (information or leaflet* or education* or educate* or book* or pamphlet* or resource* or dvd* or CD* or multimedia or web*)).tw. (2455)
-
(education adj2 (program* or session*)).tw. (27,835)
-
*health promotion/ (30,240)
-
*patient education/ (24,654)
-
exp daily life activity/ (55,298)
-
exp independence/ or exp Functional Independence Measure/ or independen*.ti. (78,837)
-
exp assistive technology/ or (assistive adj2 technology).tw. or (enabl* adj4 technology).tw. (4501)
-
((increas* or improve* or better) adj5 (independ* or “self-relian*” or “self-care” or “self confiden*”)).tw. (31,309)
-
exp motivation/ (69,853)
-
or/10-24 [Self Care or Patient Education Methods] (549,847)
-
health economics/ (33,378)
-
exp economic evaluation/ (206,596)
-
exp health care cost/ (198,664)
-
pharmacoeconomics/ or “drug cost”/ or drug utilization/ or “utilization review”/ (144,564)
-
socioeconomics/ and economics/ (14,776)
-
*socioeconomics/ (17,925)
-
*fee/ (6384)
-
*“cost”/ (12,941)
-
cost*.ti. (102,621)
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or evaluat* or analy* or study or studies or consequenc* or compar* or efficienc* or variable or unit or estimate* or variable* or unit)).ab. (135,415)
-
(price or prices or pricing).tw. (32,512)
-
(economic* or pharmacoeconomic* or pharmaco-economic*).tw. (204,513)
-
budget*.tw. (24,149)
-
(value adj1 (money or monetary)).tw. (447)
-
(financ* adj2 (cost* or data or “health care”)).tw. (7208)
-
financ*.tw. and economics/ (12,600)
-
(expenditure* not energy).tw. (24,214)
-
quality adjusted life year/ (11,415)
-
(eq-5d or eq5d or euroquol*).tw. (4765)
-
quality adjusted life.tw. (8202)
-
(qaly or qalys or qald or qale or qtime).tw. (7909)
-
disability adjusted life.tw. (1454)
-
(daly or dalys).tw. (1520)
-
(SF6D or sf 6d or short form 6d or shortform6d).tw. (623)
-
health* year* equivalent*.tw. (42)
-
(hye or hyes).tw. (85)
-
health utilit*.tw. (1487)
-
(hui1 or hui2 or hui3).tw. (349)
-
disutil*.tw. (336)
-
standard gamble*.tw. (763)
-
(time trade off or time tradeoff).tw. (1173)
-
(hqol or h qol or hr qol).tw. (715)
-
(pqol or qls).tw. (376)
-
or/26-58 (768,575)
-
exp animals/ not (exp animals/ and exp humans/) (4,726,495)
-
exp nonhuman/ not (exp nonhuman/ and exp human/) (3,333,799)
-
exp experimental animal/ (488,258)
-
exp veterinary medicine/ (33,074)
-
animal experiment/ (1,729,886)
-
((energy or oxygen* or metaboli*) adj3 (expenditure* or cost*)).tw. (27,522)
-
or/60-65 (6,189,732)
-
59 not 66 (719,336)
-
9 and 25 and 67 (392)
MEDLINE (via Ovid)
Date range searched: 1946 to week 3 October 2013.
Search strategy
-
learning disorder/ (12,696)
-
Mentally Disabled Persons/ (2245)
-
Intellectual Disability/ (46,835)
-
((Learning or intellectual*) adj3 (disab* or disorder* or disturbance* or impair* or difficulty or difficulties)).tw. (22,734)
-
(development* adj1 (disab* or disorder* or disturbance* or difficulty or difficulties)).tw. (10,242)
-
((Subnormal* or below normal) adj3 (intellect* or mental* or learning or IQ)).tw. (554)
-
(Mental* adj3 (disab* or impair* or deficien* or retard* or handicap*)).tw. (38,310)
-
or/1-7 [Learning Disabilities] (98,050)
-
self care/ or blood glucose self-monitoring/ or self administration/ or self medication/ (41,064)
-
Patient Education as Topic/ (71,159)
-
caregivers/ (21,389)
-
“education of mentally retarded”/ (5603)
-
(self adj3 (care* or manage* or administer* or monitor* or medicat* or treat* or inject*)).tw. (55,760)
-
((skill or skills) adj2 teach*).tw. (2324)
-
((patient or patients) adj5 (information or leaflet* or education* or educate* or book* or pamphlet* or resource* or dvd* or CD* or multimedia or web* or internet or “cd rom”)).tw. (109,203)
-
((carer* or caregiver*) adj3 (information or leaflet* or education* or educate* or book* or pamphlet* or resource* or dvd* or CD* or multimedia or web*)).tw. (1710)
-
(education adj2 (program* or session*)).tw. (21,227)
-
exp Program evaluation/ or exp computer-Assisted Instruction/ or exp Teaching materials/ (159,218)
-
*health promotion/mt (9471)
-
“Activities of daily living”/ (50,778)
-
independen*.tw. (718,313)
-
Self-Help Devices/ (3429)
-
(assistive adj2 technology).tw. (718)
-
(enabl* adj4 technology).tw. (2244)
-
((increas* or improve* or better) adj5 (independ* or “self-relian*” or “self-care” or “self confiden*”)).tw. (23,624)
-
(feeding adj2 skill*).tw. (178)
-
or/9-26 [Self Care SEARCH 2] (1,170,195)
-
Economics/ (27,116)
-
exp Economics, Dental/ (3950)
-
exp Economics, Nursing/ (3880)
-
exp Economics, Medical/ (13,511)
-
exp Economics, pharmaceutical/ (2607)
-
exp Economics, Hospital/ (19,414)
-
exp “Costs and Cost Analysis”/ (182,668)
-
exp “Fees and Charges”/ (27,050)
-
exp budgets/ (12,003)
-
exp “Value of Life”/ec [Economics] (243)
-
budget*.tw. (17,212)
-
cost*.ti. (77,598)
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or evaluat* or analy* or study or studies or consequenc* or compar* or efficienc* or variable or unit or estimate* or variable* or unit)).ab. (97,878)
-
(economic* or pharmacoeconomic* or pharmaco-economic*).tw. (144,648)
-
(price or prices or pricing).tw. (22,254)
-
(financ* adj2 (cost* or data or “health care”)).tw. (5646)
-
(fee or fees).tw. (11,622)
-
(value adj1 (money or monetary)).tw. (330)
-
quality-adjusted life years/ (7230)
-
(eq-5d or eq5d or euroquol*).tw. (2793)
-
exp models, economic/ (10314)
-
markov chains/ (10,634)
-
quality adjusted life.tw. (6065)
-
(qaly or qalys or qald or qale or qtime).tw. (5000)
-
disability adjusted life.tw. (1190)
-
(daly or dalys).tw. (1140)
-
health* year* equivalent*.tw. (37)
-
(hye or hyes).tw. (53)
-
(hui1 or hui2 or hui3).tw. (282)
-
disutil*.tw. (211)
-
standard gamble*.tw. (666)
-
(time trade off or time tradeoff).tw. (936)
-
(hqol or h qol or hrqol or hr qol).tw. (7389)
-
(pqol or qls).tw. (245)
-
(sf6d or sf 6d or short form 6d or shortform 6d or sf sixd or sf six d).tw. (408)
-
exp animals/ not (exp animals/ and exp humans/) (4,051,824)
-
exp Veterinary Medicine/ (20,736)
-
exp Animal Experimentation/ (6411)
-
((energy or oxygen* or metaboli*) adj3 (expenditure* or cost*)).tw. (22,022)
-
or/63-66 (4,083,859)
-
or/28-62 (473,985)
-
68 not 67 (444,824)
-
69 and 8 and 27 (470)
Search 3: literature search for self-care interventions for people with diabetes
EconPapers (http://econpapers.repec.org/paper/)
Date range searched: inception to 30 October 2013.
Search strategy
Diabetes and self and care and mental.
EMBASE Classic and EMBASE
Date range searched: 1947 to 29 October 2013.
Search strategy
-
*diabetes mellitus/ (178,039)
-
limit 1 to yr=“1900 - 1980” (48,629)
-
*non insulin dependent diabetes mellitus/ (81,724)
-
(diabet* adj3 (ii or “type 2” or two)).tw. (114,777)
-
(diabet* adj3 (“non insulin dependent” or stable or “adult onset” or “maturity onset” or “ketosis resistant” or “slow onset”)).tw. (15,837)
-
(niddm or mody).tw. (9030)
-
or/2-6 (192,025)
-
(self adj3 (help or care* or manage* or administer* or monitor* or medicat* or treat* or inject*)).tw. (79,016)
-
(support* adj5 (care or caregiv* or carer*)).tw. (37,333)
-
((shared or sharing or share) adj5 (care or caregiv* or carer*)).tw. (4415)
-
(patient adj5 (information or leaflet* or education* or educate*)).tw. (46,148)
-
((skill or skills) adj5 (train* or teach*)).tw. (18,862)
-
self care/ or self help/ or self medication/ (48,240)
-
drug self administration/ (7675)
-
self monitoring/ (3645)
-
patient education/ (86,159)
-
caregiver/ or caregiver burden/ or caregiver support/ (39,442)
-
or/8-17 (302,670)
-
exp economic evaluation/ (206,596)
-
*“cost”/ (12,941)
-
cost*.ti. (102,621)
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or evaluat* or analy* or study or studies or consequenc* or compar* or efficienc* or variable or unit or estimate* or variable* or unit)).ab. (135,415)
-
quality adjusted life year/ (11,415)
-
(eq-5d or eq5d or euroquol*).tw. (4765)
-
quality adjusted life.tw. (8202)
-
(qaly or qalys or qald or qale or qtime).tw. (7909)
-
exp animals/ not (exp animals/ and exp humans/) (4,726,495)
-
exp nonhuman/ not (exp nonhuman/ and exp human/) (3,333,799)
-
exp experimental animal/ (488,258)
-
exp veterinary medicine/ (33,074)
-
animal experiment/ (1,729,886)
-
((energy or oxygen* or metaboli*) adj3 (expenditure* or cost*)).tw. (27,522)
-
or/27-32 (6,189,732)
-
or/19-26 (331,577)
-
34 not 33 [focussed Health Econ search] (310,047)
-
7 and 18 and 35 (478)
-
limit 36 to yr=“2011 -Current” (141)
MEDLINE (via Ovid)
Date range searched: 1946 to week 3 October 2013.
Search strategy
-
exp Diabetes Mellitus, Type 2/ (86,901)
-
Diabetes Mellitus/ (91,304)
-
(diabet* adj3 (ii or “type 2” or two)).tw. (78,151)
-
(diabet* adj3 (“non insulin dependent” or stable or “adult onset” or “maturity onset” or “ketosis resistant” or “slow onset”)).tw. (12,714)
-
niddm.tw. (6757)
-
mody.tw. (833)
-
or/1-6 (197,176)
-
self care/ or blood glucose self-monitoring/ or self administration/ or self medication/ (41,064)
-
Patient Education as Topic/ (71,159)
-
“education of mentally retarded”/ (5603)
-
Caregivers/ (21,389)
-
(self adj3 (help or care* or manage* or administer* or monitor* or medicat* or treat* or inject*)).tw. (59,885)
-
(support* adj5 (care or caregiv* or carer*)).tw. (26,911)
-
((shared or sharing or share) adj5 (care or caregiv* or carer*)).tw. (3286)
-
(patient adj5 (information or leaflet* or education* or educate*)).tw. (32,301)
-
((skill or skills) adj5 (train* or teach*)).tw. (13,772)
-
or/8-16 [Self Care SEARCH 1] (228,863)
-
exp “Costs and Cost Analysis”/ (182,668)
-
cost*.ti. (77,598)
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or evaluat* or analy* or study or studies or consequenc* or compar* or efficienc* or variable or unit or estimate* or variable* or unit)).ab. (97,878)
-
quality-adjusted life years/ (7230)
-
(eq-5d or eq5d or euroquol*).tw. (2793)
-
exp models, economic/ (10,314)
-
markov chains/ (10,634)
-
quality adjusted life.tw. (6065)
-
(qaly or qalys or qald or qale or qtime).tw. (5000)
-
exp animals/ not (exp animals/ and exp humans/) (4,051,824)
-
exp Veterinary Medicine/ (20,736)
-
exp Animal Experimentation/ (6411)
-
((energy or oxygen* or metaboli*) adj3 (expenditure* or cost*)).tw. (22,022)
-
or/27-30 (4,083,859)
-
or/18-26 (275,483)
-
32 not 31 [Focussed cost studies filter] (261,737)
-
7 and 17 and 33 (714)
-
limit 34 to yr=“2011 -Current” (160)
Search 4: literature search for economic models of diabetes and self-care
EconPapers (http://econpapers.repec.org/paper/)
Date range searched: inception to 30 October 2013.
Search strategy
Diabetes and self and care and model.
EMBASE Classic and EMBASE
Date range searched: 1947 to 29 October 2013.
Search strategy
-
*diabetes mellitus/ (178,000)
-
limit 1 to yr=“1900 - 1980” (48,629)
-
*non insulin dependent diabetes mellitus/ (81,684)
-
(diabet* adj3 (ii or “type 2” or two)).tw. (114,709)
-
(diabet* adj3 (“non insulin dependent” or stable or “adult onset” or “maturity onset” or “ketosis resistant” or “slow onset”)).tw. (15,837)
-
(niddm or mody).tw. (9030)
-
or/2-6 (191,951)
-
(self adj3 (help or care* or manage* or administer* or monitor* or medicat* or treat* or inject*)).tw. (78,984)
-
(support* adj5 (care or caregiv* or carer*)).tw. (37,322)
-
((shared or sharing or share) adj5 (care or caregiv* or carer*)).tw. (4411)
-
(patient adj5 (information or leaflet* or education* or educate*)).tw. (46,132)
-
((skill or skills) adj5 (train* or teach*)).tw. (18,861)
-
self care/ or self help/ or self medication/ (48,222)
-
drug self administration/ (7669)
-
self monitoring/ (3642)
-
patient education/ (86,142)
-
caregiver/ or caregiver burden/ or caregiver support/ (39,426)
-
or/8-17 (302,569)
-
simulation/ or computer simulation/ (160,204)
-
decision making/ (139,713)
-
19 and 20 (1665)
-
*statistical model/ (15,362)
-
decision support system/ (12,791)
-
hidden markov model/ (1154)
-
(econom* adj2 model*).tw. (3630)
-
((simulat* adj3 model*) and decision*).tw. (1611)
-
microsimulat*.tw. (554)
-
(patient level adj8 simulat*).tw. (80)
-
(discrete event* adj5 simulat*).tw. (561)
-
(discrete event* adj8 model*).tw. (450)
-
(decision* adj5 model*).tw. (11,201)
-
(model* adj5 markov*).tw. (10,213)
-
(decision adj2 tree*).tw. (5460)
-
or/21-33 (56,266)
-
7 and 18 and 34 (72)
MEDLINE (via Ovid)
Date range searched: 1946 to week 3 October 2013.
Search strategy
-
exp Diabetes Mellitus, Type 2/ (86,901)
-
Diabetes Mellitus/ (91,304)
-
(diabet* adj3 (ii or “type 2” or two)).tw. (78,151)
-
(diabet* adj3 (“non insulin dependent” or stable or “adult onset” or “maturity onset” or “ketosis resistant” or “slow onset”)).tw. (12,714)
-
niddm.tw. (6757)
-
mody.tw. (833)
-
or/1-6 [Diabetes-2] (197,176)
-
models, economic/ or models, econometric/ (10,314)
-
markov chain/ (10,634)
-
Decision Trees/ (8976)
-
decision support techniques/ (12,182)
-
microsimulat*.tw. (426)
-
(simulat* adj3 model*).tw. and decision*.mp. (1270)
-
(discrete event* adj5 simulat*).tw. (380)
-
(discrete event* adj8 model*).tw. (302)
-
(decision* adj5 model*).tw. (8452)
-
(model* adj5 markov*).tw. (8102)
-
self care/ or blood glucose self-monitoring/ or self administration/ or self medication/ (41,064)
-
Patient Education as Topic/ (71,159)
-
“education of mentally retarded”/ (5603)
-
Caregivers/ (21,389)
-
(self adj3 (help or care* or manage* or administer* or monitor* or medicat* or treat* or inject*)).tw. (59,885)
-
(support* adj5 (care or caregiv* or carer*)).tw. (26,911)
-
((shared or sharing or share) adj5 (care or caregiv* or carer*)).tw. (3286)
-
(patient adj5 (information or leaflet* or education* or educate*)).tw. (32,301)
-
((skill or skills) adj5 (train* or teach*)).tw. (13,772)
-
or/18-26 [Self Care SEARCH 1] (228,863)
-
or/8-17 [econ models] (47,754)
-
7 and 27 and 28 (98)
Additional searches
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid)
Search 1 – same as MEDLINE.
Search 2 – same as MEDLINE.
Search 3 – same as MEDLINE.
Search 4 – same as MEDLINE.
NHS Economic Evaluation Database, Issue 3 of 4, (The Cochrane Library, Wiley Online Library)
Accessed July 2013.
Search strategy
#1 MeSH descriptor: [Diabetes Mellitus] this term only
#2 * from 1966 to 1983
#3 #1 and #2
#4 MeSH descriptor: [Diabetes Mellitus, Type 2] this term only
#5 (diabet* near/3 (ii or “type 2” or two)):ti,ab,kw OR (diabet* near/3 (“non insulin dependent” or stable or “adult onset” or “maturity onset” or “ketosis resistant” or “slow onset”)):ti,ab,kw OR niddm or mody:au
#6 #3 or #4 or #5
#7 MeSH descriptor: [Self Care] explode all trees
#8 MeSH descriptor: [Patient Education as Topic] this term only
#9 MeSH descriptor: [Education of Intellectually Disabled] this term only
#10 MeSH descriptor: [Caregivers] this term only
#11 (self near/3 (help or care* or manage* or administer* or monitor* or medicat* or treat* or inject*)):ti,ab,kw OR (support* near/5 (care or caregiv* or carer*)):ti,ab,kw OR ((shared or sharing or share) near/5 (care or caregiv* or carer*)):ti,ab,kw OR (patient near/5 (information or leaflet* or education* or educate*)):ti,ab,kw OR ((skill or skills) near/5 (train* or teach*)):ti,ab,kw
#12 #7 or #8 or #9 or #10 or #11
#13 #6 and #12
TUFTS Cost-Effectiveness Analysis Registry (https://research.tufts-nemc.org/cear4/SearchingtheCEARegistry/SearchtheCEARegistry.aspx)
Search dates: 7 May 2013, 30 October 2013.
Search strategy
Diabetes AND (disability OR self care) 0 hits.
Appendix 15 Case-finding study health economics resource-use questionnaire and medical records request form
Appendix 16 Randomised controlled trial health economics resource use questionnaire and medical records information request form
Appendix 17 Protocol amendments
NHS ethics permission was given on 9 July 2012 (reference number 12/YH/0304). Subsequently, substantial amendments were made to the participant information sheets, recruitment and referral materials and a substantial amendment was made to the protocol and processes for the feasibility RCT following a monitoring visit with the funders. This final amendment to the process put into place plans to evaluate the proof of concept; however, recruitment did not proceed and therefore the study continued to be an assessment of the feasibility of a Phase III trial as originally intended.
Recruitment materials
We created a poster for GPs to display in their waiting rooms that invited participants to request more information about the study.
Feasibility randomised controlled trial process
Our original protocol outlined the initial stages of our project, the observational case-finding study. It detailed the feasibility RCT on the understanding that certain elements would have detail added to them when we had collected observational case-finding data.
The recruitment target for the observational case-finding study was reduced from 350 to a minimum of 120 after a monitoring visit with the funders to explore slow recruitment but high levels of agreement to further contact for the feasibility RCT (see Assessment of proof of concept). Recruitment to the observational case-finding study was extended to continue alongside recruitment to the feasibility RCT. The most significant change was that we realised that relying purely on data from general practice was feasible, as gaining information from practices was proving extremely time-consuming. We had initially thought that participants would not let our nurses take a blood sample but, in conversation, many participants expressed a preference for a nurse visit at home to take a blood sample rather than visiting their GP. We altered our process so our experienced research nurses could take blood samples as well as physical measures in a participant’s home or location of their choosing.
Furthermore, a plan to move from the feasibility RCT to a definitive Phase III trial was put in place with details of the Trial Steering Committee contribution and an interim analysis. It was agreed that if recruitment to the feasibility RCT was slow, the Trial Steering Committee could meet to decide to change the ratio of allocation to the two arms of the trial from 1 : 1. This proved to not be necessary.
The eligibility for the feasibility RCT was clarified to define what poor diabetes management was (i.e. HbA1c level of > 6.5%, BMI of > 25 kg/m2 or self-reported physical activity below national guideline levels). Data collection was widened to include the collection of cholesterol, waist-to-hip ratio, participant mood, and urea and electrolytes.
We introduced the standard leaflet to ensure a basic level of diabetes care and knowledge in both arms of the trial. We also confirmed details of our intervention for the committee. A new participant information booklet was created for the feasibility RCT and trialled. A further change was the introduction of a research advocate, who is a person who is not on the research team and can supply impartial information and advice to participants. An easy-read sheet was provided to explain the role of the research advocate to participants and their supporters (see Patient and public involvement).
Assessment of proof of concept
Following a HTA programme review of recruitment in June 2014, our original feasibility target of 80 was increased to 150 participants. This was because, although there was a lower expected recruitment rate in case finding, initial estimates suggested a higher potential eligibility and acceptance rate for the further contact for the feasibility study. The intention of increasing the feasibility RCT sample size was to allow the study to also address proof of concept in relation to potential efficacy of the intervention. Should feasibility and proof of concept be demonstrated, it was planned for the trial to proceed seamlessly into a full Phase III evaluation.
Sample size
To address the proof of concept objective, we planned to use methods developed for Phase II screening trials in oncology205 in which preliminary and non-definitive randomised treatment comparisons are made, carefully adjusting the false-positive (α) and false-negative (β) error rates so the target treatment effect is appropriate but the sample size remains restricted. We assumed that a reduction of HbA1c level by 5 mmol/mol or more at 6 months would be clinically important. This change from our initial application, in which we quoted 10 mmol/mol or 1% as being a desirable change, was based on clinical advice from Dr Ramzi Ajjan and is equivalent to an effect size of 0.33 based upon data from the Action in Diabetes and Vascular Disease (ADVANCE) trial206 and preliminary data from our observational case-finding study. Allowing for 25% loss to follow-up (refusal of blood tests or researcher visit), 150 patients would adequately power (82%) us to detect a reduction in the primary outcome of HbA1c of 0.5% for the intervention compared with control, assuming a common SD of 1.5% [as per the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial207] using a one-sided t-test with a significance level of 20% to test for this degree of superiority. If the decision was made to continue to a full trial, this cohort would then form an internal pilot for a definitive Phase III RCT.
Outcomes
The extension to 150 participants aimed to assess proof of concept in relation to potential efficacy of the intervention, according to the following outcomes:
-
change in HbA1c level at 6 months post randomisation (primary)
-
change in BP, BMI or total cholesterol at 6 months post randomisation (secondary).
Statistical analysis
To assess proof of concept, it was planned that 80% one-sided CIs would be presented for the change in HbA1C level, BP, BMI and total cholesterol from baseline to 6 months’ follow-up.
Interim analyses
No interim analyses were initially planned, however, following the request from the funders to consider extending the feasibility RCT to assess proof of concept, the following interim analyses were planned:
-
Decision point 1 (1 March 2015) – whether or not to proceed beyond our original feasibility target to recruit 150 participants. Proceed if:
-
we have recruited as expected n ≥ 70, with acceptance rate indicating we can recruit to target from the remaining case-finding participants
-
the willingness to take up the intervention makes it plausible that there could be an effect, that is there was > 75% adherence to therapy appointments.
If we proceeded to recruit 150 participants, then we planned to follow the whole sample up for 12 months. Should the definitive Phase III trial proceed, this would allow all participants in this earlier phase to also be included in the main trial.
-
Decision point 2 (1 December 2015) – whether or not to start set-up for a definitive Phase III trial, based on interim analyses of the 6-month outcome data for the first 75–80 participants and reviewed by the Data Monitoring and Ethics Committee.
-
Decision point 3 (1 June 2016) – whether or not to proceed to the definitive Phase III trial based on interim analyses of the 6-month outcome data for the full 150 participants and reviewed by the Data and Ethics Committee.
Outcome of interim analysis
The first interim analysis took place using data as of 5 March 2015.
At this time, 168 patients had been registered to the case-finding observational study, of which 59 (35.1%) had been recruited and randomised into Phase II; 68 (40.5%) had not and would not be recruited (not eligible, did not agree, could not be contacted); 11 (6.5%) were in the process of being recruited; and a further 30 (17.9%) had the potential to be screened for recruitment, leaving a shortfall of at least 50 participants to meet the extended sample size of 150 participants.
Of the 59 participants recruited to the feasibility RCT, 30 had been randomised to receive SSM, of which 18 (60%) were known to have attended at least one treatment session with the nurse and two were known to have withdrawn from sessions. Of participants randomised to the intervention more than 1 month ago, 18/21 (85.7%) had attended at least one session.
As a result of this interim analysis, the Trial Steering Committee agreed that sufficient case-finding participants remained to recruit the original sample of 80 patients, but not to extend the feasibility trial to recruit 150 participants or to proceed to further decision points. It was agreed that participants could be followed up between 4 and 6 months following randomisation.
Appendix 18 Randomised controlled trial information booklet (includes consent form)
Appendix 19 Randomised controlled trial researcher administered baseline booklet
Appendix 20 Visual representation of randomisation
Appendix 22 Post-randomised controlled trial follow-up interview researcher journal guide
For researcher: topic guide to journal after randomised controlled trial follow-up
-
Could the person remember their previous interview?
-
Do you think they knew they were in a research project?
-
Did they identify any big life changes and did you know about any others that they did not identify?
-
Did the supporter sound like they’d been engaged in:
-
The research process?
-
The intervention?
-
Using the TAU leaflet?
-
Reflections on:
-
person’s experience of recruitment and nurse visits (blood samples and/or intervention)
-
person’s experience of receiving TAU OR person’s experience of seeing the nurse for intervention.
If they were in the intervention:
-
Where was the board?
-
Could they locate it?
-
What about the folder?
-
Were there tear-off sheets?
-
How had they used them?
Appendix 23 Guidance for journalling for nurses in randomised controlled trials
For nurses: topic guide to journal after randomised controlled trial follow-up
What enables people do self-management?
Think of some of the people you saw who did make changes, what helped them make those changes?
-
What helps people stick to changes they have made?
-
If people made changes but did not stick with them, what happened?
What stops people doing self-management?
Think of some of the people you saw who did not make changes, what was it that stopped them?
Was it only them or were other people involved?
Was there anything that could have been changed that you think would have made them make the changes?
What have you learnt about what influences a person’s ability to self-manage?
What has a big impact on whether they do or do not manage it?
Appendix 24 Case-finding study researcher post interview journal guide
Post interview journal
Points to reflect on/make observations about:
Consent process
Any problems with the consent process?
For person?
Or supporter?
Any questions about information sheet or consent form?
Any comments on referral process?
Capacity
To what extent did the participant understand what diabetes is?
To what extent did the participant understand what research is?
General
To what extent did the person understand the questions?
Were there any that were found to be very hard/confusing?
Were there any communication problems?
Were there any indications the person was not telling the truth?
Were there any indications the person was answering to please the researcher? What form did this take?
Any questions refused?
Any question answered too precisely (e.g. I NEVER EVER eat cake)?
Were there any problems with the research process, e.g. took too long, interrupted, nowhere to put down materials?
Materials
Of the visual material what worked well if any?
What did the person have trouble with if any?
Did you require a weekly food planner to answer diet questions?
Did you require a weekly exercise chart to answer exercise questions?
Did they know what blood sugar testing/foot checks/other measure were?
Supporter
Were there any problems defining who the diabetes supporter was?
Did the supporter answer any questions? Which ones?
What was the interaction between the person and the supporter?
Did you get a sense of how engaged or resistant supporter was to things like diet and exercise change?
Did the supporter tell you anything about the person you think is relevant?
Home life
Any observations about the type of accommodation?
Where the interview took place?
Observations about participant
What is this person like?
Should anything be noted about their mobility/build/mood/clothing?
As above for supporter?
Glossary
- Clinical Commissioning Group
- A clinically led statutory NHS body responsible for the planning and commissioning of health-care services for its local area.
- Comprehensive Local Research Networks
- An organisation providing the infrastructure to support high-quality clinical research in the NHS.
- Direct Enhanced Service
- A scheme that health service commissioners in the NHS are required to establish, which is linked to national priorities and agreements.
- EMIS LV
- A commercially available electronic health-care record system for primary care, superseded by EMIS Web.
- EMIS Web
- A commercially available electronic health-care record system for primary care, supersedes EMIS LV.
- EuroQol-5 Dimensions
- A standardised instrument for measuring generic health status.
- HbA1c
- A term meaning glycated haemoglobin, which is used as a measure of blood glucose levels over the last 2–3 months.
- Quality Outcomes Framework
- An annual reward and incentive programme detailing general practice achievement results.
- Read Code
- A letter- and number-coded thesaurus of clinical terms that has been used in the NHS since 1985.
- SystmOne
- A commercially available electronic integrated health-care record system.
- Third sector
- An organisation that is neither public sector nor private sector. This includes voluntary and community organisations and social enterprises.
- Time for Audit, Research, Governance, Education and Training
- A system of protected teaching time for general practitioners and practice nurses working in the NHS.
List of abbreviations
- A&E
- accident and emergency department
- BMI
- body mass index
- BNF
- British National Formulary
- BP
- blood pressure
- CCG
- Clinical Commissioning Group
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CONSORT
- Consolidated Standards of Reporting Trials
- CSRI
- Client Service Repository Inventory
- CTRU
- Clinical Trials Research Unit
- DVD
- digital versatile disc
- eGFR
- estimated glomerular filtration rate
- EQ-5D
- EuroQol-5 Dimensions
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IT
- information technology
- LIHS
- Leeds Institute of Health Sciences
- NICE
- National Institute for Health and Care Excellence
- PHQ-2
- Patient Health Questionnaire-2
- PSSRU
- Personal Social Services Reference Unit
- QALY
- quality-adjusted life-year
- QOF
- Quality Outcomes Framework
- RCT
- randomised controlled trial
- REAP
- Rapid Eating Assessment for Participants
- SD
- standard deviation
- SSM
- supported self-management
- TAU
- treatment as usual