Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/80/04. The contractual start date was in April 2012. The draft report began editorial review in April 2017 and was accepted for publication in September 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Sarah E Lamb was on the Health Technology Assessment (HTA) Additional Capacity Funding Board, HTA End of Life Care and Add-on Studies Board, HTA Prioritisation Group Board and the HTA Trauma Board.
Disclaimer
This report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Lamb et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Definition and prevalence of dementia
Dementia is a syndrome characterised by acquired, progressive deterioration in memory, general cognitive function, self-care and personality. Probable dementia according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)1 criteria is defined by:
-
memory impairment with cognitive disturbance in at least one of the following domains – aphasia (language impairment), apraxia (motor impairment), agnosia (impairment of object recognition) or executive functioning (planning, sequencing, abstracting)
-
functional decline – increasing impairment in functional ability (social, occupational, personal/self-care) related to cognitive deficits.
Dementia affects older people to a much greater extent than younger people. As a rule of thumb, the prevalence of dementia in developed countries doubles in successive 5-year age groups within the age range of 65–99 years, from under 1% for people aged 65–69 years, to about 35% in people aged 95–99 years. 2 Prevalence is similar in men and women. 2 About 60% of cases of dementia in developed countries are caused by Alzheimer’s disease, and about 20% by vascular dementia,3 while mixed Alzheimer’s/vascular dementia and dementia with Lewy bodies are the other common causes.
Recent reports have suggested that the prevalence of dementia is reducing across Western Europe4 and in the USA. 5 The hypothesised reasons for this reduction are an increase in educational achievements in successive birth cohorts and other medical, social and behavioural factors that are still under investigation. However, current prevalence figures indicate that in the UK > 800,000 people have dementia, with an annual cost to the economy of £23B. 6 Although more recent estimates of dementia prevalence have revised this figure down to 670,000 people,7 this is still a very substantial number of people.
Reducing the burden of dementia is a priority for the UK government. In February 2015, the Prime Minister reiterated the 2012 dementia statement and set a challenge for the UK to become the best country in the world for dementia care and research. 8
Evidence for the effect of exercise on cognition
There is currently no cure for dementia, only interventions to reduce risk and alleviate symptoms. The protective effects of moderate levels of physical activity on the progression of subjective memory impairment and mild cognitive impairment (MCI) to dementia have been observed consistently in several large-scale epidemiological studies. 9–11 Studies of Alzheimer’s disease in transgenic mice and dogs suggest several potential mechanisms by which exercise may prevent the progression of dementia. Brain-derived neurotrophic factor is stimulated by exercise in mice with Alzheimer’s disease, leading to increased concentrations in many areas of the brain including the hippocampus, which is thought to have a key role in mediating some of the effects of dementia. 12 Other effects of exercise observed in murine dementia models include improved synaptic function,13 attenuated mitochondrial reactive oxygen species production,14 delayed loss of myelinated fibres in the brain15 and a reduction of damaging beta amyloid oligomers. 16 Epidemiological studies of the human brain have shown positive associations between physical activity and the volume of the hippocampus and other areas of the central nervous system sensitive to pathological change in dementia,17 all of which are targets that are considered important for treatment. 18
These studies provide evidential support for the theoretical model of increasing physical activity and exercise in people with dementia with the aim of alleviating cognitive symptoms. At the time of finalising the protocol, the then most recent Cochrane review of exercise programmes for people with dementia (final search date October 2013)19 included 17 studies, nine of which assessed the effect of exercise on cognition. No clear conclusions could be drawn regarding the effect of exercise on cognition in people with dementia because of unexplained heterogeneity in the data, and the review was unable to make recommendations as to the type and dose of exercise (i.e. intensity, frequency and duration).
The Cochrane review19 ended its searches in October 2013 and we ran an update to this review with searches ending in September 2016. We identified an additional 14 studies investigating dementia and MCI patients (reported in Chapter 6) and two other systematic reviews20,21 of patient trials have been reported during the trial. One of these systematic reviews concluded that exercise was an effective intervention20 and the other that it was not. 21
Current management of dementia in the UK
The treatments for dementia recommended by the National Institute for Health and Care Excellence (NICE)22 are cholinesterase inhibitors (donepezil, galantamine and rivastigmine), but only for mild to moderate Alzheimer’s disease, not for vascular dementia. Memantine is supported by NICE for limited use in moderate to severe dementia or in patients who are unable to tolerate cholinesterase inhibitors. 22 Many people with mild to moderate dementia (MMD) and their families require additional services, mainly to mitigate functional loss, such as carer training, home carers, day care, respite admissions, sitting services and carer support services.
Potential role of exercise in the management of dementia
Physical exercise is a candidate non-pharmacological treatment for dementia and there is a considerable amount of literature given to expanding underlying mechanistic hypotheses and rationale.
Although much of the current evidence informing the choice of type of exercise to improve cognition is derived from animal studies, and studies of healthy humans or people with MCI, it is a widely held belief that exercise has the potential to be an important intervention in the management of people with dementia. In addition to any possible effects on cognition, exercise should improve physical fitness and functioning, and the selection of the right type of exercise stimulus could reduce fall risk, improve mobility and reduce cardiovascular risk factors, just as in people who are not cognitively impaired. The main challenge is to design a programme that people with dementia can engage with, and adhere to in the longer term, which is of sufficient intensity and frequency to achieve the desired effect.
To date there are no recommendations as to which behaviour change techniques (BCTs) are the most effective for people with dementia. There are, however, recommendations regarding generic BCTs to increase physical activity adherence in adults23 and for older people without dementia. 24,25 The key active components include self-regulatory BCTs (e.g. goal-setting, self-monitoring), feedback and reviewing of previously set goals.
The Dementia and Physical Activity trial
The aim was to establish whether or not exercise is effective in slowing or improving cognitive decline in community-dwelling adults with MMD.
Research objectives
To undertake a definitive randomised controlled trial (RCT) to estimate the effects of an exercise or physical activity intervention that is feasible for delivery within the current constraints of NHS delivery.
Our objectives were to:
-
refine an existing intervention for delivery to community-dwelling populations of people with dementia, including a systematic review to inform intervention development
-
pilot critical procedures in the intervention and trial
-
complete a definitive, individually RCT to estimate the effectiveness of exercise in addition to usual care on cognitive impairment (primary outcome), function and quality of life in people with mild or moderate dementia, and on carer burden in carers
-
complete a parallel cost study and conduct an economic analysis from a health-care and societal perspective
-
investigate intervention effects in predefined subgroups of gender and dementia severity
-
undertake a qualitative study into the experiences of participants and carers taking part in the intervention.
Overview of report
The report is structured in seven chapters. We present the methods, results and a brief discussion of each of the main components of the study within each chapter. The results of the systematic review, which we have undertaken as a continual process throughout the trial, are presented in Chapter 6. We finish with an overarching discussion and conclusion.
Chapter 2 Intervention development and description
Parts of this report are based on Brown et al. ,26 in which the intervention description has been published. © 2015 Chartered Society of Physiotherapy. Published by Elsevier Inc. All rights reserved. Reproduced with permission.
Introduction
The development of the intervention followed a pathway and method we established in earlier National Institute for Health Research (NIHR) Health Technology Assessment (HTA)-funded studies27–30 and in accordance with the Medical Research Council recommendations for the development of complex interventions. 31 Exercise interventions in older people are underpinned by a well-established physiological and clinical evidence base and guidelines; however, these do not necessarily include people with cognitive impairment. 32 We examined existing relevant reviews and undertook a systematic review specifically to identify RCTs that examined the effect of exercise upon cognitive function in people with MCI or dementia, as summarised in Chapter 6. In addition, we considered the observational and animal study evidence base specific to exercise and cognitive impairment. Finally, patient, carer and clinician expertise was used to draw the intervention package together into a programme. The refinement, acceptability and feasibility of trial procedures and the exercise intervention were tested in pre pilot and pilot studies between June 2011 and July 2012.
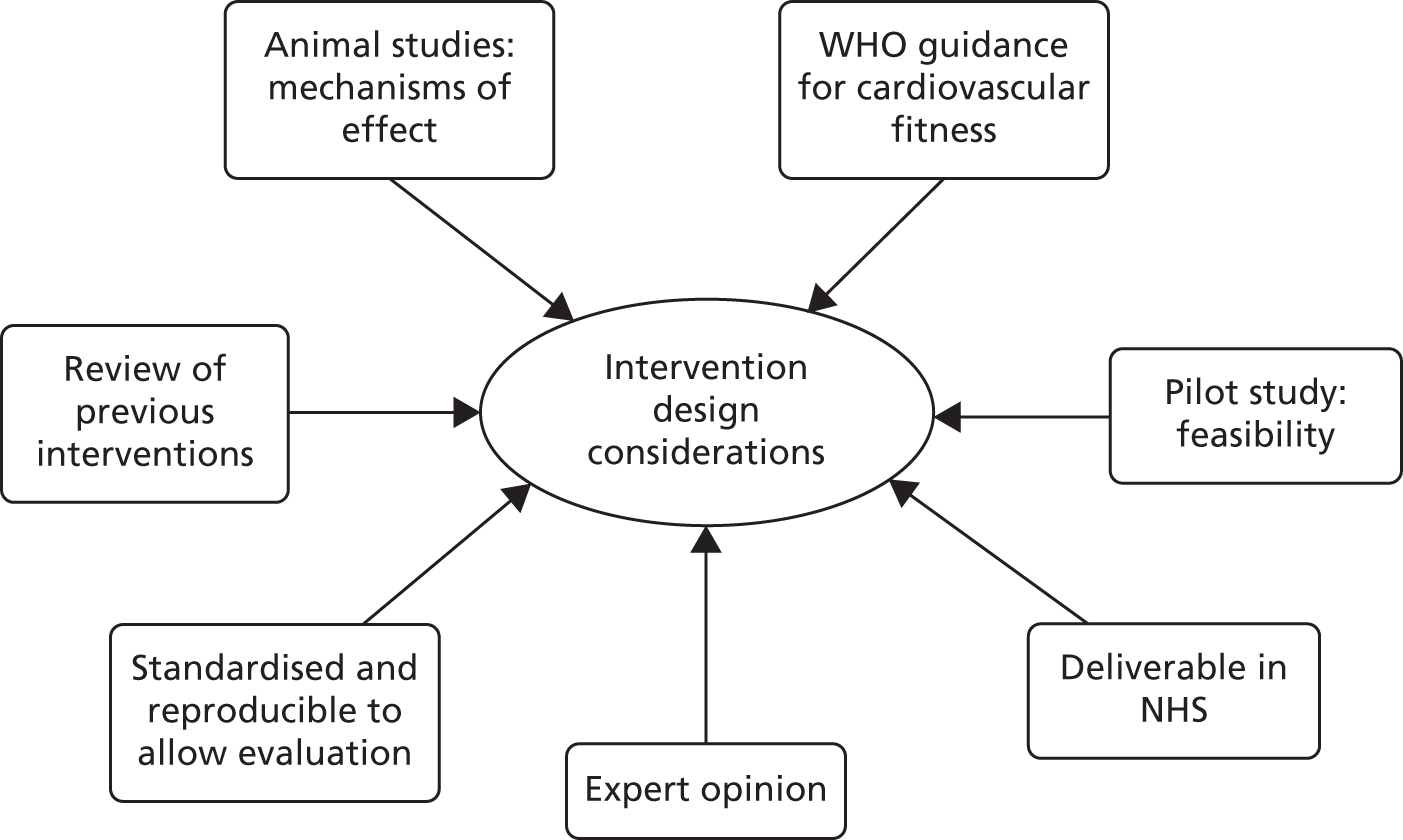
Figure 1 demonstrates the various information sources and considerations that were combined to formulate the final Dementia and Physical Activity (DAPA) intervention.
FIGURE 1.
Intervention design considerations. WHO, World Health Organization. © 2015 Chartered Society of Physiotherapy. Published by Elsevier Inc. All rights reserved. Reproduced with permission.
Rationale and development of the intervention
Evidence review
We defined exercise as ‘a subcategory of leisure time physical activity in which planned, structured and repetitive bodily movements are performed to improve or maintain one or more components of physical fitness’. 33 Physical activity was taken to mean any activity (including exercise itself) that could mimic the physiological effects of exercise, for example gardening or housework. From our systematic review of trials and review of systematic reviews, we developed an evidence-based rationale for the intervention content.
The most recent Cochrane review of exercise programmes for people with dementia19 included 17 studies, nine of which assessed the effect of exercise on cognition. No clear conclusions could be drawn regarding the effect of exercise on cognition in people with dementia because of unexplained heterogeneity in the data. The review was unable to make recommendations as to the type and dose of exercise (i.e. intensity, frequency and duration).
Exercise type
To inform our decision around the types of exercise to deliver, we examined existing exercise models and evidence for mechanisms of action. There is evidence that aerobic exercise increases vascularisation of the brain and triggers the release of neurotrophic substances, which support cognitive functions. 34 Studies in healthy older adults report that increased aerobic fitness is associated with better preservation of grey matter volume and larger hippocampus volume. The hippocampus contributes to spatial memory and consolidation of short-term memory. 35 In dementia patients, the hippocampus is atrophied. Similarly, resistance training alters levels of circulating substances (insulin-like growth factor 1, homocysteine)36 in a manner that may positively affect cognitive function. Supporting evidence is summarised in Table 1. Hence, it was decided to include both aerobic and resistance training elements in the exercise programme.
| Evidence | References | Implications | Element of the DAPA intervention |
|---|---|---|---|
| Aerobic exercise leads to increased brain vascularisation in animal model | Lista and Sorrentino34 | Increased oxygenation to the brain enhances cognitive performance | Inclusion of aerobic exercise |
| Aerobic exercise leads to release of brain-derived neurotrophic factor in animal model | Lista and Sorrentino34 | Formation of new neurones and new synapses in the brain | Inclusion of aerobic exercise |
| Amount of cardiovascular exercise required for general health and fitness | Garber et al.32 | The American College of Sports Medicine recommends 150 minutes per week at moderate intensity for adults | Dosage of aerobic activity |
| Resistance training at 50% 1RM leads to decreased homocysteine levels | Vincent et al.37 | High levels of homocysteine linked to increased risk of cognitive impairment | Inclusion of resistance exercise |
| Resistance training leads to increased levels of insulin-like growth factor 1 | Suetta et al.36 | Low levels of insulin-like growth factor 1 linked to reduced cognitive performance | Inclusion of resistance exercise |
| Feasibility of people with dementia using exercise bicycles | Yu and Swartwood38 | Exercise bicycles can be used by people with dementia | Use of exercise bicycles for aerobic exercise |
| Prevalence of dyspraxia in people with dementia | Zoltan39 | Dyspraxia is not uncommon in people with dementia | Use of simple, functional movements, such as sit-to-stand |
| Behavioural interventions, for example goal-setting, feedback on physical activity | Conn et al.40 | These strategies increase physical activity in healthy adults | Use of behavioural interventions |
| Peer support in maintaining physical activity | Cress et al.41 | Peer support is associated with exercise adherence in older adults | Use of group exercise, involvement of carers |
| Social component of physical activity | NICE42 | The social component of activity is important to older adults, so group exercise is recommended | Use of group exercise |
| Written goal-setting and planning, recording physical exercise | Cress et al.41 | Written contracts assist exercise adherence in older adults | Use of written goals and exercise plans, use of exercise calendar |
| Use of exercise logs | NICE42 | Written exercise logs recommended for older people | Use of exercise calendar |
| Performance feedback | Cress et al.41 | Provision of accurate feedback and positive reinforcement assists exercise adherence in older adults | Use of 6MWT review, verbal feedback during classes |
| Feasibility of the DAPA intervention | DAPA pilot | Intervention practicable in this population | Entire intervention |
Dose of exercise
There is a robust evidence base around the quantity of exercise needed to elicit health benefits in healthy older people, but these are not specific to cognitive outcomes. 32 Our systematic review (see Chapter 6) found that protocols for exercise interventions ranged from 60 to 210 minutes of exercise per week, with an average of 122 minutes per week. Although there is no direct evidence to inform dosing for improvement in cognitive performance, there is a link between cardiovascular status and dementia. We used the guidance, produced by the World Health Organization (WHO), for the quantity and quality of exercise required for cardiorespiratory and musculoskeletal good health for adults and older adults. 43 This WHO guidance recommends at least 150 minutes per week of moderate-intensity cardiorespiratory exercise. The same guidance recommends resistance exercises for the major muscle groups two or three times a week for musculoskeletal good health. Hence, we selected the target intensity as, at least, moderate to moderate to hard level for both exercise forms for an overall total of 150 minutes per week.
To gauge an individual’s fitness and exertion levels (mild, moderate, hard) we used the 6-minute walk test (6MWT). This assessment is relatively simple to implement, and an adapted version has been shown to be reliable in people with Alzheimer’s disease. 44 It requires less time to complete than the Incremental Shuttle Walk Test45 and, unlike the cycle ergometry test,46 requires no special equipment. We selected the Borg Scale of Perceived Exertion, adapted for use by people with dementia,47 as the primary method of assessing exercise intensity during sessions, coupled with soft indicators such as sweating, skin colour and breathing rate. Although heart rate could have been, and was occasionally planned to be, used as an indicator, these measures are complicated in situations in which, as we expected, there was a higher prevalence of beta-blockers and drugs/devices/conditions influencing heart rate.
We selected cycling as the core aerobic exercise38 because the cycling action is a simple, intuitive one and the risk of falls is minimised.
For resistance exercise, the gold standard method for identifying an initial resistance load is the one-repetition maximum using an isokinetic dynamometer. 48 However, exposing untrained individuals to resistance that is higher than 80% of the one-repetition maximum increases the likelihood of an adverse event (AE) and is not recommended for older people. 49 Given the probable frailty of the participants, we selected a validated method to prescribe the resistance by estimating the maximum number of lifts that could be made with good form for a given weight. 49 As dyspraxia is common among people with dementia,39 the exercises chosen were simple, functional movements aimed at enhancing participation and were selected to engage the major muscle groups, in accordance with the guidance from the WHO. 32
Duration of exercise intervention
Determination of the total duration of an intervention was challenging. Our systematic review (see Chapter 6) identified intervention duration ranges from 1.5 to 12 months, with an average duration of 5 months. Our funders requested that the duration of the intervention was 4 months in order to maximise the chance of it being found to be cost-effective, which is documented in Appendix 1.
To maximise the chance of the intervention being cost-effective, we used group supervision (with each participant working to their individual prescription) for 4 months, and included BCTs plus suggestions for continued activities to encourage continued physical activity.
Exercise intervention adherence
Behavioural modification is an important component of the intervention, to make adherence to the exercise intervention more likely. Simply providing information about the health benefits of exercise is not effective in increasing older adults’ participation in exercise. 41 The BCT taxonomy of Michie et al. 50 provides standardised definitions of 93 techniques used in behaviour change interventions and promotes clarity in the reporting of intervention content. During our intervention development there was no specific guidance for which BCTs to use for people with dementia. We adapted guidance for healthy older people32 and the NHS guidance for behaviour change,51 and examined BCT evidence for healthy older adults. A review identified self-efficacy as the most consistent predictor of initiation and maintenance of physical activity in adults aged > 50 years. 52 The BCTs of ‘goal-setting’ and ‘activity contracts’ are associated with increased physical activity levels and improved self-efficacy in adults. 53 The NHS guidance for behaviour change details how to implement these BCTs into an intervention. Group exercise, compared with individual home-based exercise, has been shown to improve exercise adherence in older people. 54 Substantial parts of the DAPA behavioural intervention depended on telephone contact, and there is precedence for this. A study of older women undertaking a 26-week group-based exercise programme found that those who were given a telephone-assisted coping plan, via a single telephone call, had better adherence to the programme than those who were given only written advice. 55 Staff encouraged a fun atmosphere in the classes, as enjoyment has been shown to improve exercise adherence in adults attending exercise groups. 56 Encouragement from spouses and carers has been recognised to strengthen self-efficacy and improve adherence. 57 Carers were welcome to come along to the sessions. They had no formal role during the class and did not participate as either coaches or individuals during the session. A break-out area was organised where carers could chat among themselves. When they wished to be, carers were involved in identifying activities that the participants were likely to find enjoyable and in encouraging compliance with home exercise.
Expert opinion
A key part of the intervention development process was the advice received from clinicians and other experts, including patient groups. The intervention development team gained opinions from specialist physiotherapy teams and visited exercise or physical activity group programmes that were being run for the target population. The team members also talked to people with MMD and their carers via local Alzheimer’s Society activities, as well as within the pre-pilot and pilot studies.
Guidelines
The intervention design was supported by information from a number of guidelines, principally the American College of Sports Medicine’s guidelines for exercise testing and prescription,32 NICE’s dementia guidelines58 and the WHO’s Global Recommendations on Physical Activity for Health. 43
The pilot studies
We ran two pilot studies. The first tested the feasibility of using the OPERA trial intervention59 (a randomised trial of an exercise intervention for older people in residential and nursing accommodation based on a music approach) in the DAPA trial target population. It was found that even the highest level of the OPERA exercise activities were not able to create an adequate exercise challenge, as the DAPA trial population were younger and fitter than the OPERA population. As a result, the intervention development team created an innovative and specially targeted exercise programme.
The acceptability and feasibility of the new intervention (including the behaviour change support strategies) was then tested in a second pilot study (n = 6 people with dementia).
Informal feedback interviews were conducted with the participants, carers and physiotherapists involved. No qualitative analysis was conducted on these data, but the data were used in real time to inform intervention development. Participants found the DAPA intervention to be acceptable, feasible and enjoyable. The exercise activities were able to create an adequate exercise challenge for the DAPA trial target group (using heart rate monitoring). This pilot study also highlighted that some participants needed more assistance and supervision than others and that the involvement of carers facilitated attendance and adherence in terms of timings, transport and emotional support, and so this also was accounted for in the programme design. Participants identified concerns that they held before starting the groups, which were used to inform our recruitment strategy information documents. Outcome measures and some aspects of the recruitment processes were also tested during the pilot phases.
The Dementia and Physical Activity intervention
A summary of the final DAPA intervention is provided in Figure 2. The exercise intervention was divided into two parts: a supervised part lasting 4 months and a supported, unsupervised component lasting an additional 8 months. The supervised part comprised a pre-exercise assessment, twice-weekly exercise sessions of approximately 1 hour’s duration (including 50 minutes of exercise at the target intensity) for 4 months with a target of at least 50 minutes of unsupervised activity at moderate intensity, to achieve a total of 150 minutes per week. The exercises sessions were a combined aerobic and resistance training schedule at moderate to hard intensity, with supervision in groups of up to eight participants.
FIGURE 2.
The DAPA intervention. © 2015 Chartered Society of Physiotherapy. Published by Elsevier Inc. All rights reserved. Reproduced with permission.

Individual pre-exercise assessment
The pre-exercise assessment assessed the participant’s general health, current fitness and activity levels. The participant and/or carer was asked about cardiovascular, respiratory, neurological, musculoskeletal and psychiatric conditions, as well as diabetes mellitus and recent acute illnesses (for details please see Appendix 2). Current and previous physical activity levels, along with any physical, perceptual or sensory limitations, for example limited range of shoulder movement, were noted and considered in the individual tailoring of exercises. Carers were encouraged to attend when possible to provide additional information to the therapist, for example on medications, comorbidities and exercise preferences.
The main element of the pre-exercise assessment was to set the initial intensity of exercise for the sessions. A 6MWT was completed using two marker cones set up 15 m apart, and the participant was instructed to walk between and around the cones for 6 minutes, with standardised directions and phrases of encouragement. 44 The distance walked in 6 minutes was noted. The participant also wore a heart rate monitor, and the resting and average heart rates were recorded. This allowed calculation of the initial target aerobic intensity according to Luxton. 60 For resistance exercises, the maximum weight that could be lifted in good form over 20 repetitions was estimated.
Exercise sessions
Exercise sessions required a venue with sufficient accessible space and numbers of fixed cycles, weighted jackets and dumb-bells. The sessions required a physiotherapist and an exercise assistant, unless the number of participants were substantially fewer than the target of eight. The exercise assistant was usually an exercise instructor or had worked in health care, for example as a physiotherapy assistant.
Warm-up
A simple warm-up comprising movements of the neck, shoulders, trunk, hips and knees, together with marching on the spot was performed, lasting about 5 minutes.
Aerobic
The target intensity for aerobic training was 25 minutes in each session, with at least 15 minutes at moderate to hard intensity. Intensity was individually tailored to each participant’s baseline fitness, recognising that a low load may be a moderate challenge to some but a low challenge to others. The period of cycling was built over the sessions. For all sessions, the first 5 minutes of the aerobic exercise was spent cycling at low intensity. At the outset of the programme all participants were started at low intensity. The aerobic challenge was progressed by increasing the total duration spent cycling, up to 25 minutes, as well as the duration spent exercising at moderate and hard intensity over the ensuing sessions.
Resistance
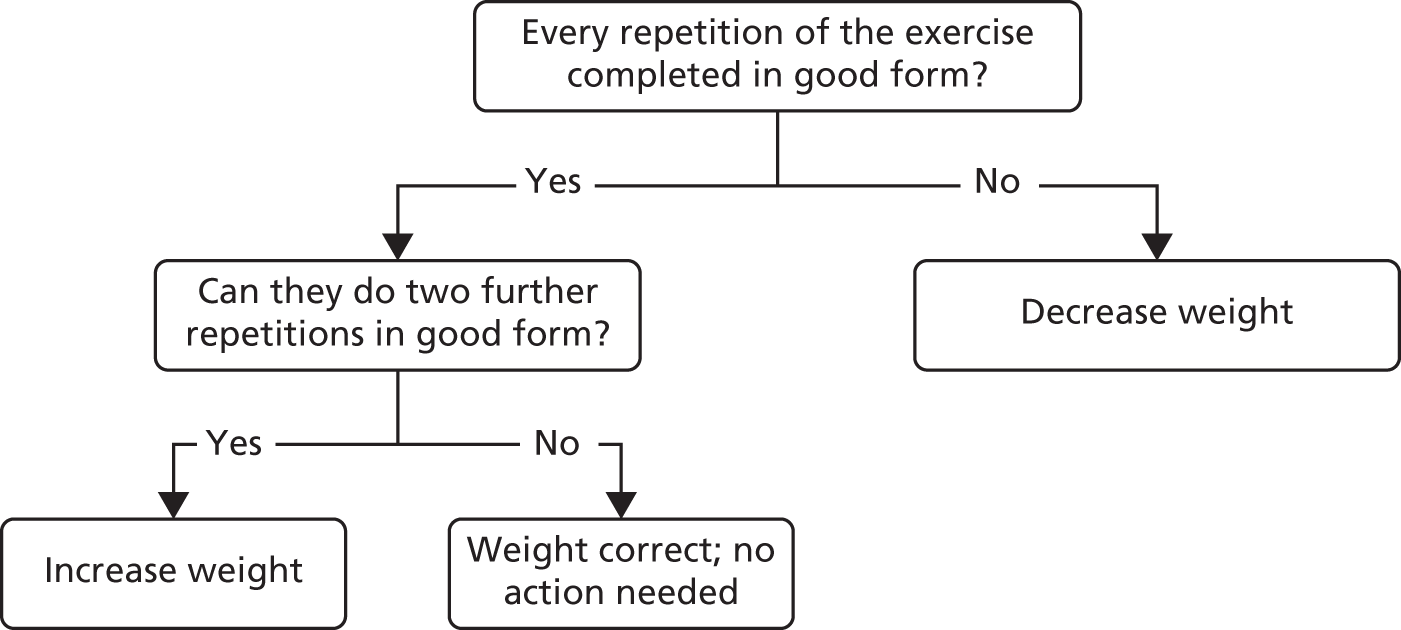
Progression was individually tailored using recognised methods. 61 The resistance exercises always included sit-to-stand, using weighted belts and jackets, and biceps curls, using dumb-bells as resistance, and were set according to principles shown in Figure 3. Suppliers are detailed in Appendix 3.
FIGURE 3.
Calibrating load for resistance exercises. © 2015 Chartered Society of Physiotherapy. Published by Elsevier Inc. All rights reserved. Reproduced with permission.
Role of exercise assistant and physiotherapist
The physiotherapists were responsible for the examination and assessment of the participants, setting the prescription and reviewing and progressing the exercise prescription. The physiotherapists supervised the exercise assistants during the sessions. The role of the exercise assistants was to assist in welcoming carers and participants to the class, to monitor and encourage people during the class and to provide assistance when required. The physiotherapists undertook the telephone follow-up calls.
Good form = correct postural alignment maintained, contraction is controlled, full range of movement is used, normal breathing is maintained.
Ratamess et al. 61
The initial resistance load was determined on the basis that the lift should be at least moderately hard and on clinical observations, with the ability to lift 20 repetitions in ‘good’ form being the principal guide. Table 2 gives typical ranges of initial resistance for the sit-to-stand exercise. The weight lifted was increased, with participants performing 15 repetitions 3 weeks into classes and 10 repetitions at a higher weight again at 7 weeks. The weight was subsequently increased if the participant could perform two additional repetitions with good form, or decreased if the participant could not perform the required number of repetitions. A further 1–3 exercises (depending on time available) were selected from shoulder forward raise, shoulder lateral raise and shoulder press, again using dumb-bells as resistance. The resistance training element of the exercise class lasted approximately 25 minutes. The participants were congratulated and presented with a certificate at their last exercise class, to reinforce continuation with unsupervised exercise.
| Sit-to-stand form category | Gender, starting resistance (kg) | |
|---|---|---|
| Men | Women | |
| Excellent: no use of hands, compensations or momentum, good control throughout the movement | 8–12 | 6–8 |
| Fair: use of hands, compensations or momentum but able to perform movement without these when prompted, with reasonable control throughout the movement | 4–7 | 3–5 |
| Poor: use of hands, compensations or momentum in order to perform movement, poor control observed | 0–3 | 0–2 |
Unsupervised activity
During the supervised part of the intervention, the participant was encouraged to undertake an additional 50 minutes of activity per week at a moderate intensity at home. The physiotherapist helped the participant to find an enjoyable or, at least, an acceptable activity, to maximise the likelihood of adherence, and to signpost the participant to local exercise facilities or groups. After the 4 months of exercise classes, all of the 150 minutes of activity per week at moderate intensity was carried out unsupervised.
Behaviour change techniques
Goal-setting
During the supervised exercise intervention, a system of goal-setting was used that focused on the goals and activities that were important to the participants. The first goal-setting took place between weeks 3 and 5 of the supervised exercise sessions and focused on an action plan with specific physical activities, which were to be undertaken at agreed locations and times. The second goal-setting took place between weeks 13 and 16 and focused on a plan for the transition from the supervised to the unsupervised exercise period. This focused on how, and where, to perform the unsupervised exercise and included an exercise opportunities booklet detailing local facilities and opportunities.
Review behavioural goals (objective and subjective)
Between weeks 17 and 22, the 6MWT was used to review participants’ progress and to act as a prompt to revise goals and identify barriers or facilitators. An exercise calendar was given to participants and carers to encourage self-monitoring of behaviour, as a visual prompt to remind the participant to undertake physical activity and to assist the physiotherapist to monitor unsupervised exercise. The face-to-face meeting with the participant and (if possible) carer was to review the targets from the second target-setting discussion and amend them if necessary, together with praise and problem-solving assistance.
Behavioural contract
The action plan (goal-setting) was signed by the participant and physiotherapist to reinforce the commitment to carry it out.
Identification of barriers or facilitators and solutions
Telephone contact was made with participants who did not attend exercise sessions to identify and problem-solve any barriers to class attendance and encourage the habit of regular attendance. During the unsupervised part of the intervention, three telephone calls and one face-to-face meeting took place. The telephone calls were made approximately 2–3 weeks, 14–16 weeks and 22–23 weeks after the supervised exercise sessions had finished, and were intended to provide praise and encouragement and assist in solving any difficulties encountered in exercising independently.
Dementia-specific considerations
People with dementia commonly have difficulties with communication,62 as well as poor memory. Using aids to memory and communication (e.g. name badges for staff, participants and carers), observation of facial expression and body language, and the use of alternative wording to aid understanding were included as part of the conduct of the exercise classes. Background noise and distractions were minimised by having a separate room (not a public gym) for the sessions. Demonstration and instruction on how to perform the exercises were provided by the physiotherapist or exercise assistant. For participants with dyspraxia, copying a movement (mirroring) was sometimes easier than following verbal instructions, and hands-on guidance was appropriate for some individuals. 63 In some cases, a modified (but safe) version of the movement was allowed, if the participant was unable to perform the movement correctly.
Quality control assessments
The intervention sites were visited by trial research physiotherapists (Nicola Atherton, Deborah Brown, Katie Spanjers, Lousia Stonehewer and Janet Lowe) to conduct quality control (QC) assessments. The first QC assessment at each site was conducted during the first few weeks of delivering the interventions. The aim of these visits was to ensure that the intervention was being delivered in a standardised manner and that the physiotherapist and exercise assistant could demonstrate competency in all aspects of the intervention. The QC assessor also provided, as needed, clinical supervision and support to the intervention staff. Items assessed included:
-
correct adherence to the exercise procedures focusing on ensuring effective delivery of an adequate exercise dose
-
correct completion of all paperwork, especially the structured treatment forms that record the exercise intensity and duration (enabling accurate calculation of the exercise dose delivered)
-
evidence of the use of a person-centred care approach with appropriate support given to participants
-
communication between the staff regarding participants’ progress and needs
-
completion and return of AE documentation (when required).
This visit also allowed the provision of support and advice to staff to enhance their clinical competencies and assist with the smooth running of the exercise group.
A second QC visit was made after some of the formal behavioural support activities (such as goal-setting) had commenced. When possible, these activities were observed; when this was not possible, assessment was made through inspection of the documentation and a discussion with the physiotherapist who carried out the activity, with corrective guidance being given as needed. The QC assessor also assessed the continued correct adherence to the exercise procedures, with an emphasis on the use of progressions and provision of adequate exercise challenges, and the correct completion of clinical records. The QC forms are available in Appendix 4. At the end of each of these visits, feedback was provided to the staff delivering the intervention and further visits arranged if problems were found in the QC assessment or if the staff needed further support. A QC form was completed and signed by both the assessor and the physiotherapist (provided that the latter agreed with its findings).
Results of the quality control visits
There were 26 physiotherapists and 17 exercise assistants delivering the DAPA intervention across the DAPA trial sites. Of the physiotherapists, in the first QC assessment, 23 were rated as ‘satisfactory’, two were rated as ‘minor concerns’ and one had missing forms. Those who were rated as ‘minor concerns’ were followed up within 2 weeks of the initial assessment and both then reached a ‘satisfactory’ rating. Of the exercise assistants, 13 achieved a rating of ‘satisfactory’, three were rated as ‘minor concerns’ and one had missing forms. In the follow-up assessment, all three exercise assistants who had been rated as ‘minor concerns’, then reached the ‘satisfactory’ rating.
In the second QC assessment, all physiotherapists were rated as ‘satisfactory’, apart from three who had a missing assessment, and all exercise assistants were rated as ‘satisfactory’, apart from one who was rated as ‘minor concerns’ and one who had missing forms (unable to complete the QC visit). The exercise assistant who was rated as ‘minor concerns’ was rated as the same at their follow-up assessment.
The third and fourth QC assessments were performed only on physiotherapists, as they carried the predominant load and responsibility for the intervention. At the third QC assessment, all physiotherapists were rated as ‘satisfactory’ and one had missing forms.
At the fourth QC assessment, 10 physiotherapists were rated as ‘minor concerns’. In the follow-up assessment, it was not possible to reassess 4 out of these 10 physiotherapists and the remaining therapists were reclassified as a rating of ‘satisfactory.’
In all cases where forms were missing, the QC visit could not be completed within the scheduled time period, before the next QC visit was due.
Manuals are available at www.octru.ox.ac.uk/trials/trials-completed/dapa (accessed 17 April 2018).
Patient and public involvement
The study benefited from broad-ranging patient and public involvement. At the outset of the study people who had experience of caring with people with dementia were identified and asked to join the Trial Steering Committee and were independent voting members of the committee. We made broad informal consultation with carers and people with dementia as we undertook the pilot work for the study both for the design of the intervention and for research design. We engaged in public meetings on dementia and research to gain input into the study. At the end of the study, we presented the results to members of the public, including an invitation for all participants to attend the meeting along with their carers. We had good attendance and feedback about the study, which we were able to incorporate into our final report.
Chapter 3 Randomised controlled trial: methods, results and brief discussion
The protocol for the RCT was published in Atherton et al. 64
Aim
The primary aim was to estimate the effect of the exercise intervention on cognitive and functional decline in community-dwelling adults with MMD.
Randomised controlled trial design
The design was a multicentred, randomised parallel-group trial comparing a 4-month, supervised, moderate- to hard-intensity exercise training regime with follow-on behavioural support for long-term physical activity change with usual care.
Methods
Participant recruitment and setting
We recruited adults (aged ≥ 18 years) with a diagnosis of any type of dementia and their carers (when available). The dementia needed to be mild to moderate at the time of recruitment. Participants were recruited from 15 regions in the UK.
Approach and initial information
Participants were recruited from four sources:
-
NHS secondary care memory clinics and services: authorised NHS staff identified potential participants using electronic or case record searches to screen for eligibility. The lists were reviewed by a clinician involved in the clinical care of the participant and checked for patients who should be excluded. Potential participants and their carers were approached by their clinician or authorised NHS staff member either face to face at a clinic or by letter.
-
General practice registers of people with dementia: general practices searched their registers of patients to identify people with a diagnostic code for dementia. In most practices, this was done by a search of databases using Read Codes or Quality Outcomes Framework codes. General practitioners approved the final list of names and sent invitation letters to potential participants.
-
Research network participant-interested databases [e.g. the Dementia and Neurodegenerative Diseases Research Network (DeNDRoN); Join Dementia Research]: the UK Department of Health and Social Care has funded a number of networks and projects to enable people with dementia to register their interest in participating in research and to enable a rapid and simple approach. Participants were identified by a nursing or clinical staff of DeNDRoN from these databases. Participants deemed as meeting the inclusion criteria were contacted by DeNDRoN staff by telephone to explain the study and, if potentially eligible, sent an invitation letter.
-
Other dementia resources (e.g. Alzheimer’s cafes): we approached community-based dementia resources directly to determine if they were willing to approach potential participants. When service providers agreed and approached potential participants, a researcher from the team then visited the centres and provided further explanation about the study to potential participants and their carers. When participants or carers were interested, the researcher assessed their potential eligibility and contacted their health-care provider to confirm eligibility.
All procedures were consistent with relevant legislation to ensure data confidentiality. All invitations were accompanied by written information about what was involved in the study, supplemented by a verbal explanation when the opportunity arose. In all instances, the participants indicated their potential willingness to participate by sending a reply slip to either the local co-ordinating centres or the research office at the University of Warwick Clinical Trials Unit (WCTU) (depending on the local set-up). Once a participant confirmed their willingness to participate, they were contacted by a member of the recruitment team (a registered nurse or allied health professional with appropriate research training) to further assess eligibility and explain the trial. In all instances, participants received the written and verbal information and were given a minimum of 48 hours to decide whether or not they wished to join the trial. Potential participants were visited in their own homes to record consent in writing and to confirm eligibility [Standardised Mini Mental State Examination (sMMSE) score and functional abilities]. A baseline assessment was carried out and the participant was registered and randomised.
Eligibility criteria
Participants were required to:
-
have probable dementia according to the DSM-IV criteria1
-
have probable MMD (a score of > 10 on the sMMSE)65
-
be able to participate in a structured exercise programme determined by:
-
being able to sit in a chair and walk 10 feet without human assistance
-
having no unstable medical conditions, for example unstable angina, or acute or terminal illness
-
-
live in the community, alone or with a friend, relative or carer, or in sheltered accommodation.
Consent
People with MMD may lack the necessary mental capacity to provide fully informed consent. All potential participants were assessed for their capacity to consent by the registered research nurses or physiotherapists conducting the baseline visit. All nurses and physiotherapists were trained specifically in assessing and taking consent in trials recruiting people with dementia using the principles of the Mental Capacity Act 2005. 66 Training was provided by the NIHR Clinical Research Network or DeNDRoN and/or the lead research physiotherapist responsible for recruitment. When potential participants were assessed as having capacity, informed consent was obtained from the individual. If potential participants were assessed as lacking capacity, agreement to participate was still sought from these individuals. In this situation, additional advice would be sought from the primary carer or personal consultee on whether or not the person who lacked capacity should take part in the project and what their past and present wishes and feelings would have been about taking part. If participants were unable to give informed consent, and for whom no personal consultee was available, a nominated consultee (e.g. health-care professional) who was well-placed and prepared to act on behalf of the potential participant was sought. When a potential participant lacked capacity to provide informed consent, and a nominated consultee could not be found, that person was not recruited. Agreement for continued participation was checked at each follow-up visit. Consent was sought from carers, for the data on carers, after consent for participation had been secured from the person with dementia.
Risks and benefits
All participants had access to usual care, and thus no treatment was withheld from trial participants. There is some limited evidence of a very small increased risk of injury as a result of becoming more physically active. In our physical activity programme, however, health-care professionals tailored progressive exercises to individual need/ability and delivered the programme to small groups of participants in a supervised and safe context.
Intervention
The usual-care arm
All participants had, or were receiving, usual care consistent with the recommendation of NICE’s clinical guidance,67 comprising diagnosis, information provision and limited social support. We recognised that the usual-care interventions vary across the country and, hence, we stratified the randomisation by region to ensure these effects were randomly distributed. There was no limit on co-interventions during the trial; medications and other treatments could be initiated, continued or discontinued at the discretion of the clinical team responsible for the care of the participants. We collected data on treatments provided to both arms of the trial at baseline and during the follow-up period, and described these in the study reports. Exercise is not currently part of recommended usual care for people with dementia, but each study participant (in both the control and intervention groups) was given one of two information sheets produced by the Department of Health and Social Care, appropriate to their age group. The information sheets recommend physical activity levels for adults (aged 19–64 years)68 and older adults (aged ≥ 65 years). 69
The Dementia and Physical Activity (intervention) arm
The intervention arm is fully described in Chapter 2.
Assessment data
Baseline data on the participants (persons with dementia)
Potential participants were visited at home by a registered research nurse or physiotherapist. Informed consent was obtained (see Consent), then eligibility was checked, including carrying out the sMMSE. Once the participant was confirmed as being eligible, descriptive data were collected, including age, gender, marital status, ethnicity, educational attainment and employment status.
The next stage was to collect the data for the primary and secondary outcomes, as given in Table 3. The type of dementia (vascular, Alzheimer’s or mixed) was ascertained after the baseline visit by reference to the participant’s medical notes.
| Domain | Measure | Description | Completed by |
|---|---|---|---|
| Primary | |||
| Cognition | ADAS-Cog70 | Takes 30–40 minutes to complete. Includes 11 tasks targeting three domains (memory, language and praxis). Scores range from 0 to 70 points, with higher scores indicating greater cognitive impairment. A 4-point difference is considered clinically important79 | Participant |
| Secondary | |||
| Function | BADLS71 | Takes 15 minutes to complete. Includes 20 daily activities. Scores range from 0 to 60 points, with higher scores indicating greater impairment | Carer (rating participant) |
| HRQoL | EQ-5D-3L72 | Takes a few minutes to complete. Includes health state classification system with five dimensions and a VAS thermometer. Scores on the classification system range from 0 to 25, with higher scores indicating better quality of life. Scores on the VAS range from 0 to 100, with 100 equating to the best health state. These two scores can be combined into an index value 0.0–1.0, the higher value indicates better quality of life |
Participant (rating self) Carer (rating self) Carer (rating participant)a |
| Dementia quality of life |
QoL-AD73 QoL-AD proxy |
Takes 10–15 minutes to complete. Includes 13 items. Scores range from 13 to 52 points, with higher scores indicating less impairment |
Participant (rating self) Carer (rating participant)a |
| Behavioural symptoms | NPI74 | Takes 10 minutes to complete. Includes 12 behavioural domains. Scores range from 0 to 144 points, with higher scores indicating greater impairment | Carer (rating participant) |
| Carer burden | ZBI75 | Takes 5 minutes to complete. Includes 22 items regarding direct stress to carers. Scores range from 0 to 88 points, with higher scores indicating greater stress | Carer (rating self) |
| Health- and social-care usage to inform the health economics analysis | CSRI76 | Administered by trained assessor. Takes 20 minutes to complete. Includes 29 items covering five domains regarding information about use of health- and social-care services, other economic impacts (such as time off work because of illness) and sociodemographic information. Used to inform health economic study | Carer with participant (rating participant) |
The primary outcome was the global cognition score of the Alzheimer’s Disease Assessment Scale – Cognitive Subscale (ADAS-Cog). 70 The funder’s brief was clear that the primary purpose of the trial and of the exercise programme should be to determine whether or not exercise can modify cognitive functioning. Cognitive deficits are central to dementia and widely understood as the most important treatment target. 77 The ADAS-Cog was collected directly from the participant, takes about 30–40 minutes to administer and has established sensitivity to change. It is widely considered the gold standard primary outcome in treatment trials for dementia, with relatively well-established treatment effect sizes. 78 We also collected the maze and number of cancellation optional items of the ADAS-Cog as additional items to be reported separately.
Initially, the primary outcome measure was to be the sMMSE, which, although widely used in clinical evaluation, is a very global, relatively insensitive measure of cognitive function. The ADAS-Cog is acknowledged to be more sensitive in detecting cognitive change in individuals with MMD than a variety of other measures, including the sMMSE. 79 The use of a valid but more sensitive primary outcome measure (the ADAS-Cog) made it possible to reduce the sample size of the study.
Secondary outcomes were chosen to reflect the broad impact of dementia on function, behaviour and quality of life. When possible, we chose instruments that were dementia specific, well validated and not excessively burdensome. Secondary outcomes are also detailed in Table 3. In all instances we used the published guidance about who the primary respondent for the questionnaire should be. We used the Bristol Activities of Daily Living Scale (BADLS). 71 This carer-rated instrument of participant ability is dementia specific, sensitive to change and widely used in clinical trials. We also collected data using the Quality of Life in Alzheimer’s Disease (QoL-AD) scale. 73 This is a 13-item dementia-specific scale that can be completed by a carer or participant; both were collected for the DAPA trial but the participant response was considered the primary data source. We also collected the EuroQol-5 Dimensions, three-level version (EQ-5D-3L),72 a 5-dimension generic (i.e. not dementia-specific) measure of health-related quality of life (HRQoL). The EQ-5D-3L was reported by both the participant and carer, with the participant being the primary data source, and it allows a calculation of health utilities for application in economic evaluations.
We used the Neuropsychiatric Inventory (NPI),74 which includes important predictors of carer breakdown such as depression and agitation. We collected cost data using the Client Services Receipt Inventory (CSRI). 76 This detailed questionnaire is designed to be used with a carer and covers all social, health care, medication use and out-of-pocket expenses. Initially, mood was to be a secondary outcome, as measured by the Cornell Scale for Depression in Dementia. 80 This was removed prior to the collection of any data as mood is covered by the NPI and it would have added unnecessarily to participant burden during data collection.
Carer
We recorded carer age, gender, ethnicity, details about the relationship they had with the person with dementia and how much care they provided. Carers were asked to complete the Zarit Burden Interview (ZBI)75 and the EQ-5D-3L72 to assess their own HRQoL. These outcomes are all among those recommended by a consensus recommendation of outcome scales for non-drug interventional studies in dementia. 81
Outcome assessment training and quality control
All staff involved in recruitment and baseline assessments received a 1-day face-to-face training session, supplemented by a detailed operational recruitment manual. The ADAS-Cog is a measure that needs initial training, shadowing and practise over time to become proficient. Recruitment staff had differing experience in using this measure; therefore, training was adapted to accommodate this. Those who had a working knowledge of the measure attended a data collection training day that included rating a video, discussion around individual items and performing an ADAS-Cog simulation with a trainer to assess practical competency. Based on competency, it was decided whether or not the rater required further shadowing and training. Inexperienced raters had an initial introduction to the measure, shadowed experienced raters and practised until they were ready to undertake the training day. Raters were also required to pass a competency test. This day included training for the sMMSE screening tool and other outcome measures.
The QC included the observation of at least one baseline home visit per researcher to ensure recruitment and data collection processes were followed correctly. All questionnaire data were sent to the WCTU, where it was checked on receipt for discrepancies and errors. Feedback was provided to the research nurses and therapists by e-mail to improve standards. Further QC visits were used to check source data collection and completion of paperwork as necessary.
Process evaluation
A range of process evaluation measures was undertaken. Some were trial process evaluation markers, including time from randomisation to first assessment and first group attendance. In addition, we collected detailed information about baseline prescription of exercise, assessment variables including comorbidity and baseline 6MWT, session attendance and the dose of exercise delivered in each session. Dose was collected on the amount of weight lifted and total number of repetitions in each session. For the cycling activity, we recorded the amount of time at low-intensity and moderate- to high-intensity exercise. We reassessed 6MWT distance at 6 weeks after the beginning of the group intervention. Data were summarised for each participant for each session and we report the weight lifted during a session as the product of the number of repetitions and the amount of weight lifted for each exercise divided by the number of participants who attended that session number.
Follow-up
Follow-up data collection was also conducted face to face and, in most instances, we were able to maintain continuity in the personnel who collected the data across all time points. These interviews were also carried out in the participants’ homes. Data for the primary and secondary outcomes were collected in the same way as at baseline, together with some additional data. The carer was asked ‘How much benefit have you gained from being in the DAPA trial?’ and ‘How much has the dementia of the person you care for changed in the past 6 months?’. The CSRI was usually completed by the carer for the participant at these time points, but a person with no carer could give as much information as they were able.
Randomisation and masking
The unit of randomisation was the individual patient. Randomisation was stratified by region and dementia severity (sMMSE score of ≥ 20 for mild and < 20 for moderate). Participants were randomised to:
-
usual care
-
usual care plus exercise programme (intervention).
Randomisation was 2 : 1 in favour of the intervention group, to allow exercise groups to be assembled in a shorter period of time and minimise participant withdrawal between randomisation and the exercise classes commencing.
The random allocation sequence was generated by an independent statistician, using a computerised random number generator, and implemented by a central telephone registration and randomisation service at the WCTU.
Research clinicians registered participants after obtaining consent, confirming eligibility and undertaking the baseline assessment. Once registered, the allocation was generated and, after the researcher had left the participant’s home, the WCTU trial team informed the participant of their allocation and made arrangements for treatment referral. Neither the intervention providers nor participants could be masked to treatment allocation. If a research clinician became unmasked, then follow-up assessments were conducted by different research workers, and all study personnel who were involved with data entry, follow-up assessments, and management were masked until the final analysis was complete.
Post-randomisation withdrawals
Participants were able to withdraw from the trial intervention and/or the trial at any time without prejudice. Participants who withdrew from the intervention were followed up, whenever possible, and data were collected as per the protocol until the end of the trial, unless they specifically withdrew from follow-up.
Participants who became unable to participate in the exercise intervention were withdrawn from the intervention by the physiotherapist but followed up at 6 and 12 months, unless they indicated that they did not wish this. Carers were able to withdraw from the trial without this affecting the inclusion of the participant with dementia.
Documentation was completed on withdrawal to confirm the date and reason for withdrawal (if available).
Data management
All data were managed within the framework of the Data Protection Act 199882 and the standard operating procedures of WCTU. Data were entered onto a bespoke application and stored on a secure WCTU server with daily, weekly and monthly back-ups. All case report forms and accompanying papers (excluding consent forms) were stored in a lockable cabinet at WCTU in individual, numbered participant files. The files were kept in numerical order and had restricted access. Consent forms were stored separately in a different cabinet to the case report forms, as they contained identifiable information. Intervention forms were anonymised and stored in a lockable cabinet. All data were checked for completeness and validity prior to data entry. Researchers were requested to check data for completeness prior to returning the case report forms to the trial team at WCTU. A further check was made by an appropriate member of the trial team and queries were clarified by the completing researcher prior to data entry. Data were not checked and entered by the same trial personnel.
A detailed data management plan was written that provided full details of the management of the data, including formalising the tracking and collection of data from centres and participants, and guidance on telephone contact with participants and carers.
Data analyses
Sample size
A sample size of 360 participants would provide 80% power to detect a minimum clinical between-group difference of 2.45 points [baseline standard deviation (SD) of 7.8 points based on 66 participants] on the ADAS-Cog at 12 months with a 5% level of significance and 2 : 1 randomisation in favour of the intervention. This equated to a standardised effect size of 0.31. An overall difference of 2–2.5 change points on the ADAS-Cog is considered to be a worthwhile target. 83 To account for therapist effects, we inflated the sample size using a design effect of 1.04 (intracluster correlation = 0.01) assuming that there are five participants per group (recognising that it may not be possible to achieve and retain eight recruits to each group), which gave a sample size of 375. The sample size was further inflated to account for 20% loss to follow-up, of which 10% was estimated would be attributable to death. Thus, a final minimum sample size of 468 participants was required, with 312 participants to be randomly allocated to the intervention arm and 156 participants to the control arm.
Note that the sample size initially calculated was 728 participants based on the sMMSE score as the primary outcome. The use of ADAS-Cog score rather than sMMSE score as the primary outcome allowed the sample size to be reduced to 468. This change in sample size occurred before we started the trial and was approved as a formal protocol amendment.
Primary analyses
Data were summarised and reported in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines for RCTs,84 and we used intention-to-treat (ITT) analyses as the primary analysis.
For the primary analyses, multilevel models were used with a random effect for region to estimate the treatment effects and 95% confidence intervals (CIs). The clustering effects of therapist and group were assessed by measuring the intracluster correlation. Therapist and/or group effects were not included in the multilevel model if the clustering effects were found to be negligible. The models were adjusted for important covariates (age, gender, region, sMMSE score and baseline ADAS-Cog score). Owing to the nature of the population, certain items on the ADAS-Cog were missing and, hence, the missingness was classed as being not random. Thus, for the primary analyses, participants with missing ADAS-Cog outcome at each time point in the observed data had their item-level responses reviewed on an individual basis. If any items were missing as a result of the participant being either cognitively unable, too distressed or refusing to answer, then we estimated the treatment effects using multiple imputation (MI) methods. This approach was taken as we are aware that the missing item-level responses for the ADAS-Cog are non-ignorable. Therefore, doing a complete-case analysis (as done so in the first sensitivity analysis) could give highly biased results. However, baseline variables, such as baseline ADAS-Cog score and sMMSE score, are quite likely to be good predictors of missing items. We therefore applied MI methods, which is considered a plausible approach to address non-ignorable missing data, to impute missing item-level data, enabling us to compute and analyse the ADAS-Cog scores. 85
Sensitivity analyses
In addition to the primary analysis, three sensitivity analysis data sets were used to carry out sensitivity analyses. For the first sensitivity analysis, the treatment effect was estimated using the observed data with missing values present in the ADAS-Cog. This sensitivity analysis data set consisted of participants who provided complete primary outcome data. For the second sensitivity analysis, participants with missing ADAS-Cog outcome in the observed data had the worst score assigned at the item level, provided the item was missing as a result of the participant being either cognitively unable, too distressed or refusing to answer. For the third sensitivity analysis, an item response theory (IRT) approach was used. Between the estimation of the sample size and the finalisation of the statistical analysis plan, the literature had moved to suggest that the ADAS-Cog does not measure a single patient trait (cognitive impairment) but rather it measures cognitive impairment in multiple cognitive domains. 86 Therefore, IRT was used to assess treatment effects in each of the cognitive domains, namely language, memory and praxis86 (see Appendix 5).
Secondary analyses
For secondary analyses, we estimated treatment effects over the 12-month time period using longitudinal models adjusting for the same variables used in the primary analysis.
Subgroup analyses
Prespecified subgroup analyses looking at severity of cognitive impairment (sMMSE score of ≥ 20 for mild and < 20 for moderate), type of dementia (Alzheimer’s vs. other), physical performance (no problems walking vs. some problems/confined to bed, taken from the EQ-5D-3L) and gender (female vs. male) were conducted using formal tests of interaction. 87
Complier-average causal effect analysis
We measured compliance with the intervention by the number of sessions attended. This information was collected by the therapist providing the treatment. Complier-average causal effect (CACE) analysis was used to assess the effect of compliance with the intervention on the primary outcome. 88
Data set access
The final data set was accessible to all study members after data lock. The chief investigator assumed overall responsibility for the data report and had full access to the trial data set. There were no contractual agreements that limited access for investigators.
Serious adverse event and adverse event reporting
An AE was defined as any untoward medical occurrence in a participant that did not necessarily have a causal relationship with this treatment. These were most likely to be identified by the physiotherapist during the exercise sessions, from information at the sign-in, or after completion of the exercise sessions during support telephone calls or the face-to-face meeting.
As each participant had a pre-exercise assessment done, this provided information on comorbidities. The trial population included many participants aged > 70 years old and, therefore, they had many of the common chronic diseases of older age, for example osteoarthritis. It was expected that participants would experience some uncomfortable effects of participation in the intervention, for example muscle or joint soreness in response to exercise. Provided that these followed an expected pattern (e.g. as for delayed-onset muscle soreness), needed simple modifications to the exercise activity (e.g. changes to the bicycle seat height) or were non-serious exacerbations of existing medical conditions, they were not considered as AEs.
A serious adverse event (SAE) was an AE that fulfilled one or more of the following criteria:
-
resulted in death
-
was immediately life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
required medical intervention to prevent one of the above.
The SAEs to be reported were defined as those that occurred within 2 hours of completing the exercise sessions or follow-on physical activities. SAEs were reported to the Trial Co-ordinating Centre within 24 hours of the physiotherapist becoming aware of them. The Trial Co-ordinating Centre was responsible for reporting AEs to the sponsor and ethics committee within required timelines.
The relationship of SAEs to trial treatment was assessed by the chief investigator and this was recorded on each SAE form. All SAEs were recorded in the trial database, when appropriate, reported to and reviewed by the Data Monitoring and Ethics Committee (DMEC) throughout the trial, and were followed up to resolution.
Monitoring and approval
Trial Steering Committee
A Trial Steering Committee was responsible for monitoring and supervising the progress of the trial towards its interim and overall milestones.
Data Monitoring and Ethics Committee
The DMEC was independent of the trial and monitored the ethical, safety and data integrity aspects of the trial.
Formal approvals
The original ethics approval for this project was granted on 19 January 2012. A substantial amendment for the pre-pilot study was granted on 31 May 2012. Another amendment regarding randomisation was granted on 17 July 2012. The third amendment for a change to the primary outcome measure to ADAS-Cog was also granted on 17 July 2012. Amendments 4–6 were changes to the intervention materials based on the results of the pre-pilot study, and these were granted on 17 January 2013. Amendment 7, regarding sample size, was granted on 7 July 2014; and the final amendment, to add more elements to the qualitative study into the project, was granted on 19 January 2015.
Results
Participant flow
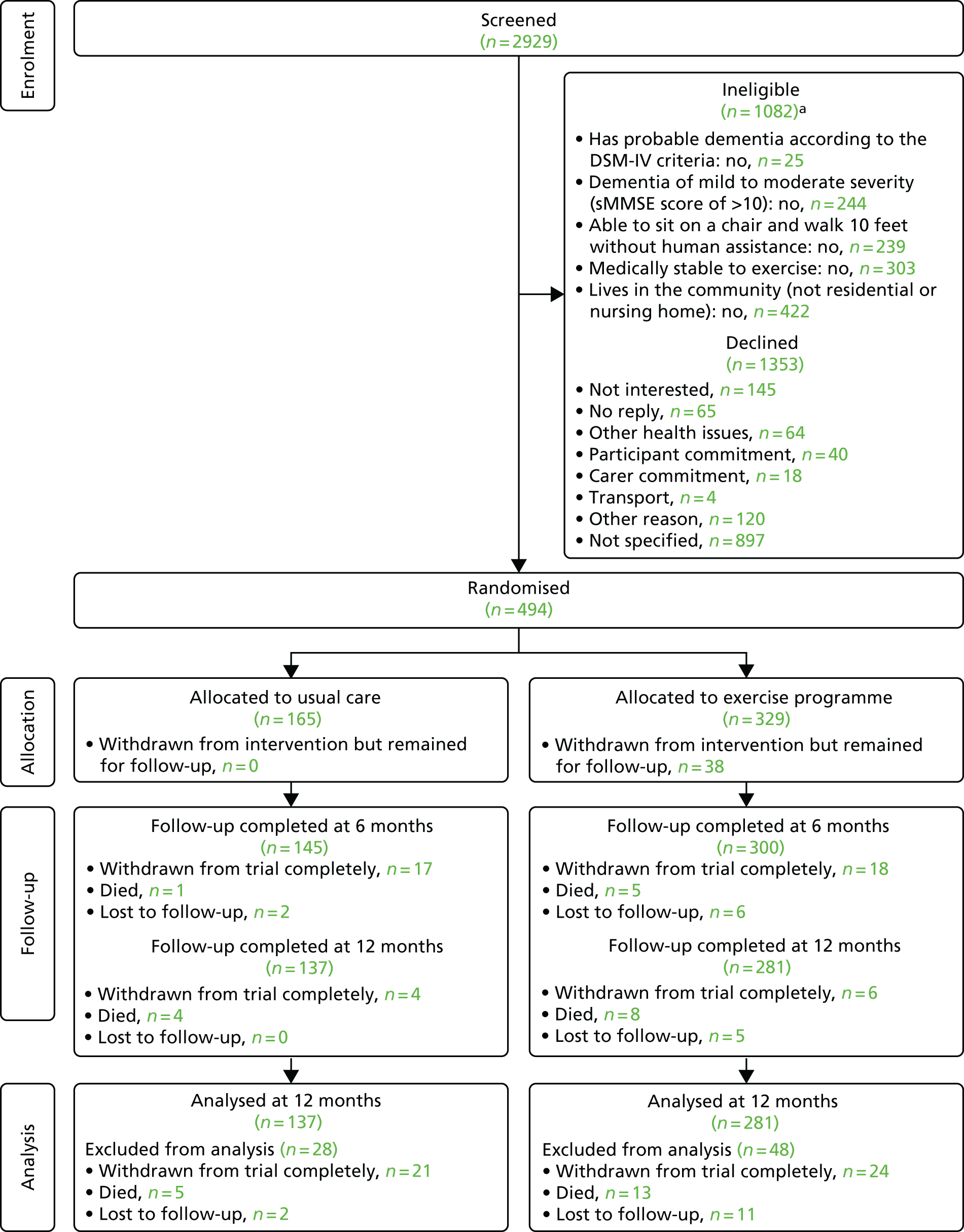
The CONSORT flow diagram (Figure 4) describes the overall flow of participants through the study and Table 53, Appendix 6, describes the flow summarised by each region.
FIGURE 4.
The CONSORT flow diagram for the DAPA trial. a, Participants have more than one reason for ineligibility.
Recruitment
Screening
Recruitment occurred between 1 February 2013 and 24 June 2015 across 15 different regions. A total of 2929 potential participants were identified through the screening process, of whom 1082 were ineligible. The remaining 63% (1847/2929) of people were approached to participate in the trial through various organisations within each region (see Appendix 6, Table 54) using different approach methods (Table 4). Around 41% (750/1847) of these people were sourced through secondary care organisations and the main approach method was by letter (50%) and telephone (25%). Of the 1847 people approached, 73.3% (1353/1847) declined and 25.4% (461/1847) were eligible but were unwilling or unable to participate.
| Approach method | Region (n) | Total (n) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Berkshire | Black Country | Coventry | Devon and Exeter | Gloucestershire & Herefordshire | Greater Manchester West | Leicester | North East London | Northampton | Nuneaton | Oxford | Rugby | Solent | South Warwickshire | Worcester | ||
| Alzheimer’s Cafe | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5 |
| Clinic | 0 | 67 | 34 | 0 | 1 | 18 | 69 | 19 | 0 | 14 | 10 | 15 | 18 | 1 | 30 | 277 |
| Letter | 2 | 92 | 187 | 13 | 15 | 0 | 0 | 0 | 1 | 131 | 80 | 74 | 0 | 107 | 216 | 918 |
| Telephone | 37 | 0 | 0 | 3 | 0 | 9 | 16 | 22 | 215 | 0 | 165 | 0 | 0 | 0 | 0 | 467 |
| Other | 6 | 12 | 30 | 6 | 6 | 5 | 16 | 0 | 0 | 0 | 24 | 8 | 1 | 8 | 0 | 122 |
| Unknown | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 32 | 0 | 58 |
| Total | 45 | 171 | 252 | 24 | 22 | 36 | 101 | 41 | 217 | 146 | 279 | 99 | 19 | 148 | 247 | 1847 |
Recruitment
Of the 2929 participants screened, 17% (494/2929) of the participants were deemed eligible and consented to trial participation (Table 5). The majority of participants were able to provide informed consent (376/484, 76.1%), some required a personal consultee (117/494, 23.7%), and one required a nominated consultee (1/494, 0.2%). The personal consultee was the carer in most situations.
| Method of consent | Treatment, n (%) | Total (N = 494), n (%) | |
|---|---|---|---|
| Usual care (N = 165) | Exercise programme (N = 329) | ||
| Participant | 126 (76.4) | 250 (76.0) | 376 (76.1) |
| Personal consultee | 38 (23.0) | 79 (24.0) | 117 (23.7) |
| Nominated consultee | 1 (0.6) | 0 | 1 (0.2) |
| Missing | 0 | 0 | 0 |
Although the original target sample size was set as 468 participants, by the time this target was reached a further 26 participants had been recruited and randomised because of the nature of recruiting participants to fill exercise groups. The final recruitment total was, therefore, 494 participants. There were no participants randomised in error. The proportion of participants in each arm across all regions is listed in Table 6 and across the randomisation strata is given in Table 55, Appendix 6.
| Region | Treatment, n (%) | Total (N = 494), n (%) | |
|---|---|---|---|
| Usual care (N = 165) | Exercise programme (N = 329) | ||
| Berkshire | 13 (31.7) | 28 (68.3) | 41 (8.3) |
| Black Country | 15 (33.3) | 30 (66.7) | 45 (9.1) |
| Coventry | 18 (33.3) | 36 (66.7) | 54 (10.9) |
| Devon and Exeter | 4 (40.0) | 6 (60.0) | 10 (2.0) |
| Gloucestershire & Herefordshire | 6 (33.3) | 12 (66.7) | 18 (3.6) |
| Greater Manchester West | 6 (33.3) | 12 (66.7) | 18 (3.6) |
| Leicester | 5 (29.4) | 12 (70.6) | 17 (3.5) |
| North East London | 6 (33.3) | 12 (66.7) | 18 (3.6) |
| Northampton | 21 (33.9) | 41 (66.1) | 62 (12.6) |
| Nuneaton | 8 (34.8) | 15 (65.2) | 23 (4.7) |
| Oxford | 25 (33.3) | 50 (66.7) | 75 (15.2) |
| Rugby | 8 (33.3) | 16 (66.7) | 24 (4.9) |
| Solent | 4 (40.0) | 6 (60.0) | 10 (2.0) |
| South Warwickshire | 12 (33.3) | 24 (66.7) | 36 (7.3) |
| Worcester | 14 (32.6) | 29 (67.4) | 43 (8.7) |
Participant baseline data
Participant baseline characteristics
The dementia diagnosis of participants at the baseline assessment has been summarised in Table 7, with the most common diagnoses being dementia in Alzheimer’s disease with late onset at 35.6% (176/494), atypical or mixed type at 20.9% (103/494) and unspecified at 15.4% (76/494). The baseline demographic characteristics and outcome measures of the randomised participants are summarised by treatment group in Table 8. Overall, the demographic characteristics were well matched across treatment groups, with the majority of participants being white males with an average age of 77.4 years (SD 7.9 years), who were married and living with their wife/husband/partner.
| Dementia diagnosis | Treatment, n (%) | Total (N = 494), n (%) | |
|---|---|---|---|
| Usual care (N = 165) | Exercise programme (N = 329) | ||
| Dementia in Alzheimer’s disease | |||
| With early onset | 9 (5.5) | 25 (7.6) | 34 (6.9) |
| With late onset | 56 (33.9) | 120 (36.5) | 176 (35.6) |
| Atypical or mixed type | 33 (20.0) | 70 (21.3) | 103 (20.9) |
| Unspecified | 29 (17.6) | 47 (14.3) | 76 (15.4) |
| Multi-infarct dementia | 3 (1.8) | 7 (2.1) | 10 (2.0) |
| Mixed cortical and subcortical vascular dementia | 2 (1.2) | 0 | 2 (0.4) |
| Vascular dementia, unspecified | 17 (10.3) | 27 (8.2) | 44 (8.9) |
| Dementia in | |||
| Pick’s disease | 1 (0.6) | 3 (0.9) | 4 (0.8) |
| Parkinson’s disease | 5 (3.0) | 13 (3.9) | 18 (3.6) |
| Other specified diseases classified elsewhere | 2 (1.2) | 3 (0.9) | 5 (1.0) |
| Unspecified dementia | 8 (4.9) | 14 (4.3) | 22 (4.5) |
| Characteristic | Randomised sample | Sample providing primary outcome | ||
|---|---|---|---|---|
| Usual care (N = 165) | Exercise programme (N = 329) | Usual care (N = 137) | Exercise programme (N = 278) | |
| Age (years), mean (SD) | 78.4 (7.6) | 76.9 (7.9) | 78.1 (7.7) | 76.9 (7.7) |
| Gender (male), n (%) | 106 (64.2) | 195 (59.3) | 86 (62.8) | 166 (59.7) |
| Living arrangements, n (%) | ||||
| Live alone | 35 (21.2) | 62 (18.8) | 29 (21.2) | 46 (16.5) |
| Live with relatives | 5 (3.0) | 18 (5.5) | 4 (2.9) | 15 (5.4) |
| Live with wife/husband/partner | 125 (75.8) | 248 (75.4) | 104 (75.9) | 216 (77.7) |
| Living with friends | 0 | 1 (0.3) | 0 | 1 (0.4) |
| Ethnicity, n (%) | ||||
| White | 157 (95.2) | 321 (97.6) | 130 (94.9) | 274 (98.6) |
| Other | 8 (4.8) | 8 (2.4) | 7 (5.1) | 4 (1.4) |
| Highest level of education, n (%) | ||||
| Degree/degree equivalent (including higher degree)/NVQ4/NVQ5 | 24 (14.5) | 57 (17.3) | 20 (14.6) | 51 (18.3) |
| Higher education below degree | 16 (9.7) | 28 (8.5) | 11 (8.0) | 23 (8.3) |
| NVQ3/GCE A-level equivalent | 10 (6.1) | 14 (4.3) | 9 (6.6) | 12 (4.3) |
| NVQ2/GCE O-level/GCSE-level equivalent/school certificate | 29 (17.6) | 60 (18.2) | 25 (18.2) | 50 (18.0) |
| Other vocational/work-related qualifications | 35 (21.2) | 68 (20.7) | 29 (21.2) | 57 (20.5) |
| No qualification | 49 (29.7) | 99 (30.1) | 41 (29.9) | 84 (30.2) |
| Total number of medications, mean (SD) | 5.5 (3.1) | 5.7 (3.7) | 5.6 (3.2) | 5.5 (3.5) |
| Dementia medications, n (%) | ||||
| Donepezil | 84/155 (54.2) | 166/318 (52.2) | 70/129 (54.3) | 148/270 (54.8) |
| Rivastigmine | 0 | 6/318 (1.9) | 0 | 3/270 (1.1) |
| Galantamine | 1/155 (0.6) | 6/318 (1.9) | 0 | 0 |
| Memantine | 8/155 (5.2) | 10/318 (3.1) | 8/129 (6.2) | 4/270 (1.5) |
| ADAS-Cog score, mean (SD) | 21.8 (7.7) | 21.4 (9.6) | 21.4 (7.8) | 21.2 (9.5) |
| Language subscale score, mean (SD) | 2.7 (3.0) | 3.0 (3.6) | 2.7 (3.0) | 2.9 (3.6) |
| Memory subscale score, mean (SD) | 17.4 (4.8) | 16.7 (6.2) | 17.1 (4.9) | 16.6 (6.1) |
| Praxis subscale score, mean (SD) | 1.7 (1.5) | 1.7 (1.7) | 1.6 (1.5) | 1.7 (1.6) |
| sMMSE score, mean (SD) | 21.6 (4.6) | 22.0 (4.7) | 22.1 (4.6) | 22.1 (4.6) |
| EQ-5D-3L score (self-reported), mean (SD) | 0.85 (0.18) | 0.82 (0.20) | 0.86 (0.16) | 0.84 (0.19) |
| EQ-5D-3L score (proxy-reported), mean (SD) | 0.70 (0.24) | 0.68 (0.24) | 0.72 (0.22) | 0.69 (0.24) |
| QoL-AD score (self-reported), mean (SD) | 39.3 (5.2) | 38.7 (5.6) | 39.4 (5.0) | 39.1 (5.4) |
| NPI score (proxy-reported), mean (SD) | 13.3 (13.2) | 12.8 (15.0) | 12.7 (12.2) | 12.7 (15.1) |
| BADLS score (proxy-report), mean (SD) | 11.4 (8.5) | 12.0 (8.4) | 10.9 (8.1) | 11.6 (8.1) |
| Fallen in the last 6 months (yes), n (%) | 56 (36.4) | 90 (29.5) | 41 (31.8) | 70 (27.1) |
| Number of falls in last 6 months, mean (SD) | 2.8 (4.9) | 2.7 (3.3) | 3.1 (5.5) | 2.8 (3.7) |
| Broken bones in last 6 months (yes), n (%) | 5 (3.3) | 9 (3.0) | 2 (1.6) | 9 (3.5) |
| Carer age (years), mean (SD) | 70.2 (10.5) | 69.1 (11.4) | 70.1 (10.4) | 69.8 (10.7) |
| Carer gender (male), n (%) | 29 (18.8) | 87 (28.5) | 25 (19.4) | 74 (28.7) |
| Carer relationship, n (%) | ||||
| Spouse | 117 (76.0) | 239 (78.4) | 98 (76.0) | 209 (81.0) |
| Son/daughter (in law) | 32 (20.8) | 55 (18.0) | 27 (20.9) | 42 (16.3) |
| Other | 4 (2.6) | 11 (3.6) | 3 (2.3) | 7 (2.7) |
| Frequency of caring, n (%) | ||||
| Daily | 120 (77.9) | 254 (83.3) | 100 (77.5) | 217 (84.1) |
| 4–6 times a week | 7 (4.6) | 14 (4.6) | 5 (3.9) | 9 (3.5) |
| 1–3 times a week | 16 (10.4) | 24 (7.9) | 15 (11.6) | 21 (8.1) |
| Less than once a month | 9 (5.8) | 8 (2.6) | 7 (5.4) | 7 (2.7) |
| ZBI score, mean (SD) | 29.0 (15.7) | 30.6 (15.4) | 28.5 (15.7) | 30.2 (15.0) |
| Carer EQ-5D-3L score, mean (SD) | 0.82 (0.23) | 0.79 (0.21) | 0.81 (0.23) | 0.79 (0.21) |
Participant baseline medications
Medication use was similar in both treatment groups; the overall mean number of medications being taken was 5.7 (SD 3.5) and donepezil was the most used medication (50.6%) (see Table 8).
Participant baseline outcome measures
A summary of the baseline outcome measures is given in Table 8. We report only the primary data sources, as data were sufficiently complete that we did not have to rely on alternative sources (i.e. proxy). All outcome measures were similar across treatment groups at baseline. The overall mean imputed ADAS-Cog score (primary outcome) was 21.5 (SD 9.0) out of a possible score of 70, for which a higher score indicates greater cognitive impairment. The three subscales of the primary outcome, namely language, memory and praxis, had mean scores of 2.9 (SD 3.4), 16.9 (5.8) and 1.7 (SD 1.6), respectively.
Participant baseline outcome measures
A summary of the baseline outcome measures is given in Table 8. We report only the primary data sources as data were sufficiently complete that we did not have to rely on alternative sources (i.e. proxy). All outcome measures were similar across treatment groups at baseline. The overall mean imputed ADAS-Cog score (primary outcome) was 21.5 (SD 9.0) out of a possible score of 70, for which a higher score indicates greater cognitive impairment. The three subscales of the primary outcome, namely language, memory and praxis, had mean scores of 2.9 (SD 3.4), 16.9 (5.8) and 1.7 (SD 1.6), respectively.
Comparing the baseline self-reported and proxy-reported outcome measures
Self-reported and proxy-reported data were available for most of the participants at baseline (see Table 9). In total, self-reported data were provided by 98.4% (486/494) of the participants for the EQ-5D-3L, 98.0% (484/494) for the EQ-5D-3L visual analogue scale (VAS) and 85.8% (424/494) for the QoL-AD. The only reason that most participants with self-reported outcomes did not have proxy data was if their carer did not give consent to participate and provide data. The self-reported and proxy-outcome measures were compared for all participants who had both sets of data available at baseline (see Table 10). There is evidence of a significant difference between the self-reported and proxy-reported outcome measures when the participants reported a significantly higher quality of life. Therefore, participants rated their quality of life significantly more highly than their carers did.
| Type of respondent | Treatment arm, n (%) | Total (N = 494), n (%) | |
|---|---|---|---|
| Usual care (N = 165) | Exercise programme (N = 329) | ||
| EQ-5D-3L score | |||
| No self-reported data or proxy-reported data | 3 (1.8) | 0 | 3 (0.6) |
| Self-reported data onlya | 10 (6.1) | 25 (7.6) | 35 (7.1) |
| Proxy-reported data only | 3 (1.8) | 2 (0.6) | 5 (1.0) |
| Both self-reported data and proxy-reported data | 149 (90.3) | 302 (91.8) | 451 (91.3) |
| EQ-5D-3L VAS score | |||
| No self-reported data or proxy-reported data | 1 (0.6) | 0 | 1 (0.2) |
| Self-reported data onlya | 10 (6.1) | 25 (7.6) | 35 (7.1) |
| Proxy-reported data only | 3 (1.8) | 6 (1.8) | 9 (1.8) |
| Both self-reported data and proxy-reported data | 151 (91.5) | 298 (90.6) | 449 (90.9) |
| QoL-AD score | |||
| No self-reported data or proxy-reported data | 10 (6.1) | 23 (7.0) | 33 (6.7) |
| Self-reported data onlya | 31 (18.8) | 44 (13.4) | 75 (15.2) |
| Proxy-reported data only | 15 (9.1) | 22 (6.7) | 37 (7.5) |
| Both self-reported data and proxy-reported data | 109 (66.1) | 240 (73.9) | 349 (70.6) |
| Outcome | Data | Unadjusted estimate (95% CI) | p-value | |
|---|---|---|---|---|
| Self reported | Proxy reported | |||
| EQ-5D-3L score | ||||
| n | 451 | 451 | 0.14 (0.12 to 0.16) | < 0001a |
| Mean (SD) | 0.83 (0.19) | 0.69 (0.24) | ||
| Range | –0.07 to 1 | –0.35 to 1 | ||
| EQ-5D-3L VAS score | ||||
| n | 449 | 449 | 11.1 (9.1 to 13.0) | < 0.001 |
| Mean (SD) | 79.0 (18.0) | 68.0 (18.9) | ||
| Range | 20 to 100 | 5 to 100 | ||
| QoL-AD score | ||||
| n | 349 | 349 | 5.9 (5.2 to 6.6) | < 0.001 |
| Mean (SD) | 38.9 (5.4) | 32.9 (6.0) | ||
| Range | 21 to 52 | 14 to 51 | ||
Falls and fracture
In total, 31.8% (146/459) of the participants had a fall in the last 6 months at baseline. There was no evidence of a difference in the mean number of falls between the two treatment groups with an overall mean of 2.7 falls (SD 4.0 falls) in the last 6 months. About 3% (14/459) of all participants had broken bones as a result of falling in the last 6 months. There was no evidence of a difference in the mean number of broken bones between the two groups with an overall mean of 1.3 (SD 0.6) broken bones in the last 6 months (see Table 8).
Carer baseline data
Carer baseline characteristics
Of the 494 participants attending the baseline assessment, 96.2% (475/494) had a carer. Of those with a carer, 96.6% (459/475) of the carers consented to participate in the study. The carer baseline characteristics and outcome measures have been summarised, by treatment group, in Table 11. There is a higher number of female carers in the usual-care arm (81.2%) than in the exercise arm (71.5%). Apart from this, the carer demographic characteristics and outcome measures are well matched across treatment groups, with the majority of carers being white females with an average age of 69.5 years (SD 11.1 years) who are providing care on a daily basis. Most of the carers (99.1%) knew the participant before they had dementia and the reason for this was that they were either their spouse (77.6%) or their son/daughter (in-law) (18.9%). Around 91% of the carers had knowledge of the participant’s night-time behaviour.
| Characteristic/outcome | Treatment arm, n (%) | Total (N = 459), n (%) | |
|---|---|---|---|
| Usual care (N = 154) | Exercise programme (N = 305) | ||
| Age (years) | |||
| Mean (SD) | 70.2 (10.5) | 69.1 (11.4) | 69.5 (11.1) |
| Range | 36.5 to 91.3 | 33.1 to 95.3 | 33.1 to 95.3 |
| Gender (male) | 29 (18.8) | 87 (28.5) | 116 (25.3) |
| Knew participant before they had dementia (yes) | 152 (98.7) | 303 (99.3) | 455 (99.1) |
| Carer role | |||
| Only caregiver | 112 (72.7) | 236 (77.4) | 348 (75.8) |
| Main carer but share caring responsibilities | 26 (16.9) | 56 (18.3) | 82 (17.9) |
| Share caring responsibilities with others | 9 (5.8) | 9 (2.9) | 18 (3.9) |
| Share caring responsibilities but someone else is the main carer | 4 (2.6) | 2 (0.7) | 6 (1.3) |
| Other | 1 (0.7) | 0 | 1 (0.2) |
| Missing | 2 (1.3) | 2 (0.7) | 4 (0.9) |
| Relationship to participant | |||
| Spouse | 117 (76.0) | 239 (78.4) | 356 (77.6) |
| Son/daughter (in-law) | 32 (20.8) | 55 (18.0) | 87 (18.9) |
| Other relative | 1 (0.6) | 5 (1.6) | 6 (1.3) |
| Friend, neighbour, acquaintance | 0 | 3 (1.0) | 3 (0.7) |
| Paid carer | 1 (0.6) | 0 | 1 (0.2) |
| Other | 2 (1.4) | 3 (1.0) | 5 (1.1) |
| Missing | 1 (0.6) | 0 | 1 (0.2) |
| How often you provide care to the participant | |||
| Daily | 120 (77.9) | 254 (83.3) | 374 (81.5) |
| 4–6 times a week | 7 (4.6) | 14 (4.6) | 21 (4.6) |
| 1–3 times a week | 16 (10.4) | 24 (7.9) | 40 (8.7) |
| Less than once a month | 9 (5.8) | 8 (2.6) | 17 (3.7) |
| Missing | 2 (1.3) | 5 (1.6) | 7 (1.5) |
| Do you have knowledge of the participant’s night-time behaviour | |||
| Yes | 140 (90.9) | 279 (91.5) | 419 (91.3) |
| Missing | 0 | 1 (0.3) | 1 (0.2) |
| Ethnicity | |||
| White | 147 (95.5) | 301 (98.7) | 448 (97.6) |
| Other | 6 (3.9) | 4 (1.3) | 10 (2.2) |
| Missing | 1 (0.6) | 0 | 1 (0.2) |
| EQ-5D-3L utility score | |||
| Mean (SD) | 0.82 (0.23) | 0.79 (0.21) | 0.80 (0.22) |
| Range | –0.18 to 1 | –0.02 to 1 | –0.18 to 1 |
| Missing | 1 | 1 | 2 |
| EQ-5D-3L VAS score | |||
| Mean (SD) | 77.7 (18.4) | 77.3 (17.7) | 77.5 (17.9) |
| Range | 3 to 100 | 20 to 100 | 3 to 100 |
| Missing | 1 | 1 | 2 |
| ZBI score | |||
| Mean (SD) | 29.0 (15.7) | 30.6 (15.4) | 30.1 (15.5) |
| Range | 0 to 76 | 2 to 77 | 0 to 77 |
| Missing | 8 | 5 | 13 |
Carer role and relationship
For most participants in both arms their spouse was the only caregiver: 66.2% (102/154) in the usual-care arm and 69.8% (213/305) in the exercise arm. Many of the remaining participants had either their spouse or son or daughter (in-law) as their main carer.
Carer baseline outcome measures
A summary of the carer baseline outcomes measures is given in Table 11. All outcome measures were similar across treatment groups at baseline. Carers had an overall mean EQ-5D-3L utility score of 0.8 (SD 0.22) and mean EQ-5D-3L VAS score of 77.5 (SD 17.9) with mild to moderate carer burden reflected by a mean Zarit Burden Interview (ZBI) score of 30.1 (SD 15.5).
Participant follow-up
During the study 90% (445/494) and 85% (418/494) of the participants were followed up at 6 months and 12 months (see Figure 4), respectively. In both arms, all follow-up was completed within the intended time frames. The mean time from randomisation to the 6-month follow-up time point was 6.2 months (SD 0.50 months) and to the 12-month follow-up time point was 12.3 months (SD 0.56 months).
Withdrawals and loss to follow-up
At 12 months, the combined withdrawal and loss to follow-up rate was 12 out of 165 (13.9%) for the usual-care arm, and 35 out of 329 (10.6%) for the exercise arm (see Table 9), which was not statistically significantly different. The number of losses between these two categories was different, as shown in Table 12. In total, 9.1% (45/494) of the participants withdrew from the trial completely during follow-up, with 12.7% (21/165) of these requests being in the usual-care arm compared to 7.3% (24/329) in the exercise arm, which was significantly different (p < 0.001). A complete withdrawal request from the participant also meant that their carer also completely withdrew. Loss to follow-up at 12 months was 2 out of 165 patients in usual care (1.2%) and 11 out of 329 patients (3.3%) in the exercise arm.
| Type of withdrawal | Treatment arm, n (%) | Total (N = 494), n (%) | |
|---|---|---|---|
| Usual care (N = 165) | Exercise programme (N = 329) | ||
| Has ceased trial treatment or procedure but remains on follow-up | 0 | 38 (11.6)a | 38 (7.7) |
| Has withdrawn from the trial completely and will not be followed up | 21 (12.7) | 24 (7.3) | 45 (9.1) |
| Participant has died | 5 (3.0) | 13 (4.0) | 18 (3.6) |
| Physiotherapist has recommended withdrawal from treatment | 0 | 5 (1.5)b | 5 (1.0) |
| Lost to follow-up | 2 (1.2) | 11 (3.3) | 13 (2.6) |
Table 13 presents the time from randomisation to complete withdrawal by treatment arm. There was no evidence of a significant difference between the two arms in the time from randomisation to complete withdrawal from the trial (p = 0.724).
| Characteristic/outcome | Treatment arm | Unadjusted estimate (95% CI) | p-value | |
|---|---|---|---|---|
| Usual care (N = 165) | Exercise programme (N = 329) | |||
| Time (days) from randomisation to withdrawal from trial | ||||
| n | 21 | 24 | –12.6 (–84.4 to 59.1) | 0.724 |
| Mean (SD) | 183.9 (103.4) | 196.5 (131.3) | ||
| Range | 1–363 | 2–517 | ||
| Time (days) from randomisation to death | ||||
| n | 5 | 13 | 37.5 (–59.1 to 134.1) | 0.423 |
| Mean (SD) | 288.4 (76.5) | 250.9 (89.7) | ||
| Range | 167–346 | 89–372 | ||
In the exercise arm, 13.1% (43/329) of the participants had ceased only the trial treatment procedure (i.e. still remained on follow-up), of which 11.6% (38/329) were by request of the participant and 1.5% (5/329) were recommended by the physiotherapist (see Table 12). Of these, 10 participants later withdrew completely from the trial.
Deaths
The number of participants who died by the end of the study was similar in both arms with 3.6% (18/494) deaths in total, as shown in Table 12.
Table 13 presents the time from randomisation to death by treatment arm. There was no evidence of a significant difference between the two trial arms in the time from randomisation to death (p = 0.423).
Comparison of retained participants to those lost to follow-up
Tables 56 and 57, Appendix 6, present the baseline characteristics of participants not completing (died, withdrew and lost to follow-up) and completing follow-up at 6 months and 12 months, respectively. At both time points, people who completed follow-up were, on average, younger than those who died but were similar in age to those who withdrew or were lost to follow-up. There is evidence of a significant association between marital status and whether or not a participant completed follow-up, with those who are separated or divorced being less likely to complete follow-up. Moreover, participants completing follow-up had significantly better HRQoL at 6 months (measured by the EQ-5D-3L) and at 12 months (measured by the EQ-5D-3L and QoL-AD) than those not completing follow-up. At 12 months, participants completing follow-up had significantly better cognition, as measured by the sMMSE score, than the non-completers. In addition, both the ADAS-Cog (imputed) and the ADAS-Cog (raw) scores suggest that, on average, participants who died had poorer cognition than those who completed follow-up at both time points.
Protocol violations
Research protocol violations
A summary of the protocol violations has been presented by treatment arm in Table 14. There were no participants who were found to be ineligible once randomised.
| Category | Treatment arm, n (%) | Total (N = 494), n (%) | |
|---|---|---|---|
| Usual care (N = 165) | Exercise programme (N = 329) | ||
| Randomised but ineligible | 0 | 0 | 0 |
| Withdrawals (received opposite treatment to allocation)a | 0 | 41 (12.5) | 41 (8.3) |
| Non-adherence to treatment (≤ 75% compliance) | 0 | 106 (32.2) | 106 (21.5) |
| Incomplete follow-upb | 19 (11.5) | 26 (7.9) | 45 (9.1) |
Clinical protocol violations
Of the 329 participants who were allocated to the exercise arm, 12.5% (41/329) withdrew from the intervention alone, having completed only ≤ 75% of their scheduled sessions. In the exercise arm, 35% (115/329) of the participants were non-compliers, that is, they attended ≤ 75% of their scheduled sessions. In total, 9.1% (45/494) of the participants did not complete follow-up at any of the time points.
Outcomes and analyses
Numbers analysed
All 494 randomised participants completed their baseline assessments (usual-care arm, n = 165; exercise arm, n = 329). A reduced number of 445 participants provided 6-month follow-up data (usual-care arm, n = 145; exercise arm, n = 300) and a total of 418 participants provided 12-month follow-up data (usual-care arm, n = 137; exercise arm, n = 281).
Table 15 summarises the completeness of the ADAS-Cog, both overall and at the item level, at each time point. The ADAS-Cog had missing items for 5.7% (28/494) of the participants at baseline, 6.7% (30/445) at 6 months and 12.9% (54/418) at 12 months. The two items that were mostly missing were the word recognition and remember test items.
| Time point, pattern number | ADAS-Cog item | Number of items Missing | Frequency of patients with pattern | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Word recall | Commands | Constructional praxis | Naming | Ideational praxis | Orientation | Word recognition | Remember test | Comprehension of spoken language | Word finding | Language | |||
| Baseline | |||||||||||||
| 1 | + | + | + | + | + | + | + | + | + | + | + | 0 | 466 |
| 2 | + | + | + | + | + | + | + | + | + | 2 | 17 | ||
| 3 | + | + | + | + | + | + | + | + | + | + | 1 | 7 | |
| 4 | + | + | + | + | + | + | + | + | + | + | 1 | 1 | |
| 5 | + | + | + | + | + | + | + | + | + | + | 1 | 1 | |
| 6 | + | + | + | + | + | + | + | + | 3 | 1 | |||
| 7 | + | + | + | + | + | + | + | + | 3 | 1 | |||
| 6 months | |||||||||||||
| 1 | + | + | + | + | + | + | + | + | + | + | + | 0 | 415 |
| 2 | + | + | + | + | + | + | + | + | + | 2 | 11 | ||
| 3 | 11 | 8 | |||||||||||
| 4 | + | + | + | + | + | + | + | + | + | + | 1 | 2 | |
| 5 | + | + | + | + | + | + | + | + | 3 | 2 | |||
| 6 | + | + | + | + | + | + | + | + | + | + | 1 | 1 | |
| 7 | + | + | + | + | + | + | + | + | + | + | 1 | 1 | |
| 8 | + | + | + | + | + | + | + | + | + | 2 | 1 | ||
| 9 | + | + | + | + | + | + | + | + | + | 2 | 1 | ||
| 10 | + | + | + | + | + | + | + | + | 3 | 1 | |||
| 11 | + | + | + | + | + | + | + | + | 3 | 1 | |||
| 12 | + | + | + | + | + | + | + | 4 | 1 | ||||
| 12 months | |||||||||||||
| 1 | + | + | + | + | + | + | + | + | + | + | + | 0 | 364 |
| 2 | + | + | + | + | + | + | + | + | + | 2 | 20 | ||
| 3 | 11 | 10 | |||||||||||
| 4 | + | + | + | + | + | + | + | + | + | 3 | 5 | ||
| 5 | + | + | + | + | + | + | + | + | + | + | 1 | 4 | |
| 6 | + | + | + | + | + | + | + | + | + | + | 1 | 2 | |
| 7 | + | + | + | + | + | + | + | + | 3 | 2 | |||
| 8 | + | + | + | + | + | + | + | + | + | + | 1 | 1 | |
| 9 | + | + | + | + | + | + | + | + | + | + | 1 | 1 | |
| 10 | + | + | + | + | + | + | + | + | 3 | 1 | |||
| 11 | + | + | + | + | + | + | + | + | 3 | 1 | |||
| 12 | + | + | + | + | + | + | + | 4 | 1 | ||||
| 13 | + | + | + | + | + | + | + | 4 | 1 | ||||
| 14 | + | + | + | + | + | + | 5 | 1 | |||||
| 15 | + | + | + | + | + | + | 5 | 1 | |||||
| 16 | + | + | + | + | + | + | 5 | 1 | |||||
| 17 | + | + | + | + | 7 | 1 | |||||||
| 18 | + | + | + | + | 7 | 1 | |||||||
Primary outcome: Alzheimer’s Disease Assessment Scale – Cognitive Subscale (imputed at the item level)
On average, cognition declined over time (Tables 16 and 17 and Figures 5 and 6). There was evidence of a statistically significant difference at 12 months, which suggests that participants in the usual-care arm have a lower ADAS-Cog score (better cognition) of 1.4 (95% CI –2.62 to –0.17) than participants in the exercise arm (Table 17 and Figure 6).
| Outcome | Treatment arm | Adjusted estimate (95% CI)a | p-value | |
|---|---|---|---|---|
| Usual care | Exercise programme | |||
| ADAS-Cog (imputed) | ||||
| n | 145 | 298 | ||
| Mean (SD) | 22.4 (9.4) | 22.9 (11.6) | –0.6 (–1.58 to 0.39) | 0.237 |
| Outcome | Treatment arm | Adjusted estimate (95% CI)a | p-value | |
|---|---|---|---|---|
| Usual care | Exercise programme | |||
| ADAS-Cog (imputed) | ||||
| n | 137 | 278 | ||
| Mean (SD) | 23.8 (10.4) | 25.2 (12.3) | –1.4 (–2.62 to –0.17) | 0.026 |
FIGURE 5.
Plot of the unadjusted ADAS-Cog score (imputed) over time by treatment arm.
FIGURE 6.
Plot of adjusted treatment estimates (mean difference and 95% CI) for the ADAS-Cog score at 6 and 12 months.

Secondary outcomes
Secondary outcomes collected at 6 months and 12 months have been presented in Tables 18 and 19, respectively.
| Outcome | Treatment arm | Adjusted estimate (95% CI)a | p-value | |
|---|---|---|---|---|
| Usual care | Exercise programme | |||
| Language subscale score (imputed) | ||||
| n | 145 | 299 | ||
| Mean (SD) | 3.2 (3.7) | 3.5 (4.4) | 0.01 (–0.42 to 0.45) | 0.959 |
| Memory subscale score (imputed) | ||||
| n | 145 | 298 | ||
| Mean (SD) | 17.3 (5.6) | 17.3 (6.9) | –0.5 (–1.18 to 0.27) | 0.218 |
| Praxis subscale score (imputed) | ||||
| n | 145 | 299 | ||
| Mean (SD) | 1.9 (1.8) | 2.1 (2.0) | –0.03 (–0.27 to 0.21) | 0.811 |
| EQ-5D-3L score (self-reported) | ||||
| n | 139 | 292 | ||
| Mean (SD) | 0.83 (0.21) | 0.80 (0.21) | 0.02 (–0.01 to 0.06) | 0.240 |
| EQ-5D-3L VAS score (self-reported) | ||||
| n | 138 | 288 | ||
| Mean (SD) | 78.7 (18.8) | 75.4 (20.6) | –0.1 (–3.62 to 3.36) | 0.942 |
| QoL-AD score (self-reported) | ||||
| n | 124 | 263 | ||
| Mean (SD) | 39.0 (5.9) | 38.9 (6.1) | –0.1 (–0.98 to 0.84) | 0.879 |
| NPI score (proxy-reported) | ||||
| n | 110 | 234 | ||
| Mean (SD) | 14.8 (15.6) | 15.2 (16.1) | –0.5 (–3.08 to 2.05) | 0.695 |
| BADLS score (proxy-reported) | ||||
| n | 129 | 271 | ||
| Mean (SD) | 14.6 (10.4) | 14.6 (9.5) | 0.8 (–0.31 to 1.96) | 0.153 |
| Outcome | Treatment arm | Adjusted estimate (95% CI)a | p-value | |
|---|---|---|---|---|
| Usual care | Exercise programme | |||
| Language subscale (imputed) | ||||
| n | 137 | 280 | ||
| Mean (SD) | 3.8 (4.7) | 4.4 (5.2) | –0.2 (–0.75 to 0.44) | 0.611 |
| Memory subscale (imputed) | ||||
| n | 137 | 279 | ||
| Mean (SD) | 18.1 (5.6) | 18.5 (6.7) | –0.8 (–1.56 to 0.02) | 0.056 |
| Praxis subscale (imputed) | ||||
| n | 137 | 281 | ||
| Median (range) | 1 (0–9) | 2 (0–9) | – | 0.084b |
| EQ-5D-3L score (self-reported) | ||||
| n | 131 | 261 | ||
| Mean (SD) | 0.82 (0.25) | 0.81 (0.22) | –0.002 (–0.04 to 0.04) | 0.928 |
| EQ-5D-3L VAS score (self-reported) | ||||
| n | 124 | 261 | ||
| Mean (SD) | 78.3 (19.4) | 75.5 (19.3) | 1.4 (–2.38 to 5.23) | 0.464 |
| QoL-AD score (self-reported) | ||||
| n | 119 | 237 | ||
| Mean (SD) | 39.1 (5.7) | 38.4 (5.8) | 0.7 (–0.21 to 1.65) | 0.127 |
| NPI score (proxy-reported) | ||||
| n | 105 | 215 | ||
| Mean (SD) | 13.5 (13.1) | 16.2 (15.9) | –2.1 (–4.83 to 0.65) | 0.135 |
| BADLS score (proxy-reported) | ||||
| n | 124 | 251 | ||
| Mean (SD) | 15.9 (9.7) | 17.0 (10.2) | –0.6 (–2.05 to 0.78) | 0.380 |
Alzheimer’s Disease Assessment Scale – Cognitive Subscale (imputed) subscale scores
The ADAS-Cog subscale scores of language, memory and praxis at 6 months and 12 months have been presented in Tables 18 and 19, respectively. No statistical differences were found in the subscale scores between the two treatment groups at any of the time points.
Health-related quality of life (EuroQol-5 Dimensions, three-level version and Quality of Life in Alzheimer’s Disease)
There was no evidence of a difference between the two treatment groups for the HRQoL measures, that is, the EQ-5D-3L (utility) score, EQ-5D-3L VAS score and QoL-AD score at 6 months and 12 months (see Tables 18 and 19).
Behavioural symptoms (Neuropsychiatric Inventory)
There was no evidence of a difference between the two treatment groups at 6 months’ follow-up and at 12 months’ follow-up (see Tables 18 and 19).
Function (Bristol Activities of Daily Living Scale)
There was no evidence of a difference between the two treatment groups at 6 months’ follow-up and at 12 months’ follow-up (see Tables 18 and 19).
Carer outcomes
A summary of the carer-reported outcomes collected at 6 months and 12 months has been presented in Table 20.
| Time point, outcome | Treatment arm | Adjusted estimate (95% CI)a | p-value | |
|---|---|---|---|---|
| Usual care | Exercise programme | |||
| 6 months | ||||
| EQ-5D-3L score | ||||
| n | 132 | 277 | ||
| Mean (SD) | 0.77 (0.24) | 0.76 (0.23) | –0.004 (–0.04 to 0.03) | 0.839 |
| EQ-5D-3L VAS score | ||||
| n | 136 | 277 | ||
| Mean (SD) | 72.4 (20.7) | 73.4 (19.7) | –1.4 (–4.65 to 1.76) | 0.376 |
| ZBI score | ||||
| n | 122 | 273 | ||
| Mean (SD) | 32.9 (17.1) | 33.9 (16.0) | 0.06 (–1.96 to 2.08) | 0.955 |
| How much benefit have you gained from being involved in the DAPA trial? | ||||
| Substantial benefit, n (%) | 10 (7.3) | 79 (28.1) | < 0.001b | |
| Moderate benefit, n (%) | 44 (32.4) | 126 (44.8) | ||
| No benefit, n (%) | 79 (58.1) | 69 (24.5) | ||
| Moderate harm, n (%) | 0 | 1 (0.4) | ||
| Substantial harm, n (%) | 0 | 1 (0.4) | ||
| Missing, n (%) | 3 (2.2) | 5 (1.8) | ||
| 12 months | ||||
| EQ-5D-3L score | ||||
| n | 129 | 261 | ||
| Mean (SD) | 0.78 (0.23) | 0.76 (0.24) | –0.002 (–0.04 to 0.04) | 0.936 |
| EQ-5D-3L VAS score | ||||
| n | 129 | 261 | ||
| Mean (SD) | 75.1 (18.7) | 74.5 (18.6) | 0.2 (–2.87 to 3.27) | 0.897 |
| ZBI score | ||||
| n | 125 | 256 | ||
| Mean (SD) | 32.7 (16.6) | 34.5 (16.1) | –0.5 (–2.78 to 1.72) | 0.644 |
| How much benefit have you gained from being involved in the DAPA trial? | ||||
| Substantial benefit, n (%) | 13 (10.0) | 54 (20.5) | 0.001b | |
| Moderate benefit, n (%) | 56 (43.1) | 133 (50.6) | ||
| No benefit, n (%) | 60 (46.1) | 72 (27.4) | ||
| Moderate harm, n (%) | 0 | 0 | ||
| Substantial harm, n (%) | 0 | 1 (0.4) | ||
| Missing, n (%) | 1 (0.8) | 3 (1.1) | ||
Carer health-related quality of life (EuroQol-5 Dimensions, three-level version)
There was no evidence of a difference between the two treatment groups for the carer’s HRQoL measures, namely the EQ-5D-3L (utility) score and EQ-5D-3L VAS score, at 6 months and 12 months (see Table 20).
Carer burden (Zarit Burden Interview)
Carer burden was assessed using the ZBI, which was completed by the carer. No evidence of a difference in the carer’s mean ZBI score was observed between the two treatment groups at 6 months’ and 12 months’ follow-up (see Table 20).
Carer benefit from the study
Carer benefit was measured using a five-level Likert scale, that is, substantial benefit to substantial harm. At 6 months, most carers (around 73%) in the exercise group felt that they had gained moderate or substantial benefit, whereas the majority of carers in the usual-care arm (58.1%) felt that they had gained no benefit (see Table 20). Similarly, at 12 months, most carers (around 71%) in the exercise group felt that they had gained moderate or substantial benefit, whereas the majority of carers in the usual-care arm felt that they had gained no benefit (46.1%) or gained moderate benefit (43.1%).
Participant dementia change
Participant dementia change was reported by their carer at 6 and 12 months’ follow-up and was measured using a five-level Likert scale, that is, much improved to much worsened. There was no evidence of a difference between the two treatment groups at both time points. At 6 months, around 23% of carers reported no change in the participant and 52% felt that the participant had slightly worsened. At 12 months, 18% of carers reported no change in the participant, 55% felt that the participant had slightly worsened and 17% felt that the participant had much worsened.
Participant falls and fractures
Table 21 summarises the falls and fractures of the person being cared for, as reported by the carer, at 6 and 12 months. There was no evidence of a significant difference in the reported number of falls and the number of broken bones at 6 and 12 months’ follow-up sustained by the participant. Moreover, there was no evidence of a significant difference in the number of falls over the entire follow-up period.
| Time point, mobility outcome | Treatment arm | Adjusted estimate (95% CI) | p-value | |
|---|---|---|---|---|
| Usual care | Exercise programme | |||
| 6 months | ||||
| In the last 6 months, have they had any falls including a slip or trip, following which they have come to rest on the ground, floor, or lower level? | ||||
| Yes, n (%) | 45 (33.1) | 93 (33.1) | ||
| Missing, n (%) | 0 | 3 (1.1) | OR 0.9 (0.61 to 1.48) | 0.817 |
| If yes, how many times have they fallen within the last 6 months? | ||||
| n | 44 | 93 | ||
| Mean (SD) | 4.1 (7.4) | 3.6 (6.2) | IRRa 1.2 (0.81 to 1.74) | 0.383 |
| Median (range) | 2 (1–48) | 2 (1–50) | ||
| Missing | 1 | 0 | ||
| Have they had any broken bones in the last 6 months as a result of falling? | ||||
| Yes, n (%) | 1 (0.7) | 4 (1.4) | ||
| Missing, n (%) | 0 | 4 (1.4) | OR 0.5 (0.05 to 4.61) | 0.533 |
| If yes, how many broken bones have they had within the last 6 months as a result of falling? | ||||
| n | 1 | 4 | ||
| Mean (SD) | 1 | 1 (0) | IRRa 1 (0.11 to 8.95) | 1.00 |
| Median (range) | 1 (1–1) | 1 (1–1) | ||
| 12 months | ||||
| In the last 6 months, have they had any falls including a slip or trip, following which they have come to rest on the ground, floor, or lower level? | ||||
| Yes, n (%) | 38 (29.2) | 89 (33.8) | ||
| Missing, n (%) | 2 (1.5) | 2 (0.8) | OR 0.8 (0.49 to 1.24) | 0.285 |
| If yes, how many times have they fallen within the last 6 months? | ||||
| n | 37 | 88 | ||
| Mean (SD) | 6.4 (13.5) | 3.7 (6.2) | IRRa 1.1 (0.75 to 1.56) | 0.690 |
| Median (range) | 2 (1–70) | 2 (1–50) | ||
| Missing | 1 | 1 | ||
| Have they had any broken bones in the last 6 months as a result of falling? | ||||
| Yes, n (%) | 4 (3.1) | 7 (2.7) | ||
| Missing, n (%) | 2 (1.5) | 3 (1.1) | OR 1.2 (0.33 to 4.33) | 0.779 |
| If yes, how many broken bones have they had within the last 6 months as a result of falling? | ||||
| n | 4 | 7 | ||
| Mean (SD) | 1 (0) | 1.3 (0.5) | IRRa 0.8 (0.24 to 2.53) | 0.676 |
| Median (range) | 1 (1–1) | 1 (1–2) | ||
| Entire follow-up period (6 and 12 months) | ||||
| If yes, how many times have they fallen within the last 6 months? | ||||
| n | 139 | 284 | ||
| Mean (SD) | 3.0 (11.1) | 2.3 (6.1) | IRRa 1.1 (0.84 to 1.33) | 0.655 |
| Median (range) | 0 (0–96) | 0 (0–53) | ||
Secondary analyses
Sensitivity analyses
Three sensitivity analyses were conducted to see how the treatment effect estimate differs to the primary analysis if the ADAS-Cog (primary outcome) was computed or analysed differently. The first sensitivity analysis estimated the treatment effect using the ADAS-Cog score that was computed using the observed data, that is, only the data provided by those participants who were well enough to complete. Table 22 presents the results of this sensitivity analysis. There was evidence of a statistically significant difference at 12 months, with an estimated between-group difference of –1.7 (95% CI –2.97 to –0.39) in favour of the usual-care arm. These results are similar to the primary analysis results, with the between-group difference here being slightly larger at 12 months (Figure 7).
| Outcome | Treatment arm | Adjusted estimate (95% CI)a | p-value | |
|---|---|---|---|---|
| Usual care | Exercise programme | |||
| ADAS-Cog at baseline | ||||
| n | 152 | 314 | ||
| Mean (SD) | 21.4 (7.4) | 20.6 (8.9) | ||
| Missing | 13 | 15 | ||
| ADAS-Cog at 6 months | ||||
| n | 135 | 280 | ||
| Mean (SD) | 21.4 (8.5) | 21.7 (10.3) | –0.7 (–1.72 to 0.35) | 0.196 |
| Missing | 10 | 20 | ||
| ADAS-Cog at 12 months | ||||
| n | 119 | 245 | ||
| Mean (SD) | 22.4 (9.7) | 22.9 (10.6) | –1.7 (–2.97 to –0.39) | 0.011 |
| Missing | 18 | 36 | ||
FIGURE 7.
Plot of adjusted ADAS-Cog score (imputed) estimates at 12 months for the primary analysis and sensitivity analyses.
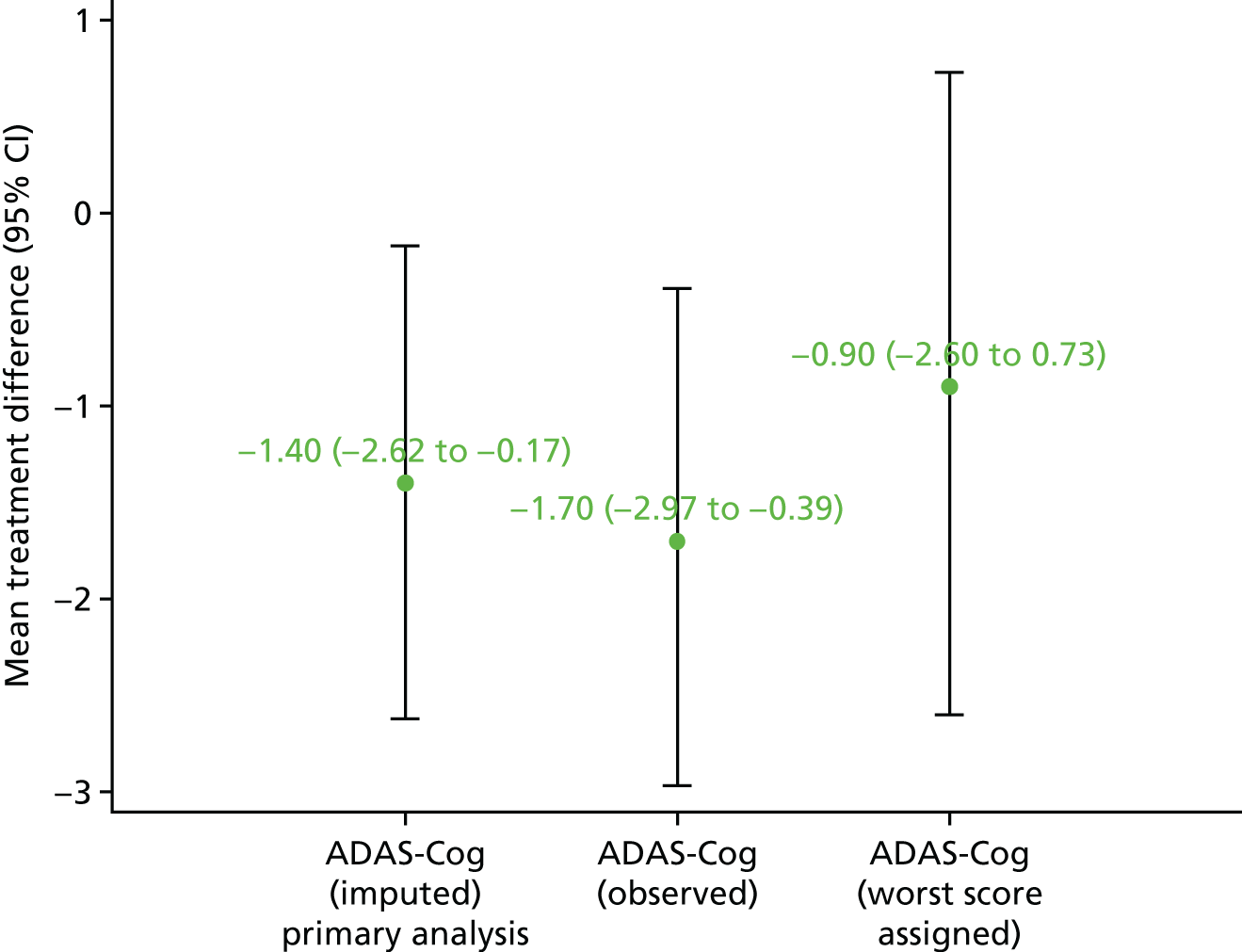
The second sensitivity analysis estimated the treatment effect using the ADAS-Cog score that was computed using the observed data, for which the worst score was assigned at the item level if the participant was too distressed or cognitively unable to answer the item. Table 23 presents the results of this sensitivity analysis. There was no evidence of a difference between the two groups at 6 months (estimated between-group difference 0.4, 95% CI –0.83 to 1.66) and 12 months (estimated between-group difference –0.9, 95% CI –2.60 to 0.73) at 12 months. Here, the estimated between-group difference at 12 months was smaller than that estimated by the primary analysis (see Figure 7).
| Outcome | Treatment arm | Adjusted estimate (95% CI)a | p-value | |
|---|---|---|---|---|
| Usual care | Exercise programme | |||
| ADAS-Cog at baseline | ||||
| n | 163 | 329 | – | – |
| Mean (SD) | 22.0 (8.1) | 21.7 (10.1) | ||
| Missing | 2 | 0 | ||
| ADAS-Cog at 6 months | ||||
| n | 145 | 298 | ||
| Mean (SD) | 23.8 (12.8) | 23.3 (12.5) | 0.4 (–0.83 to 1.66) | 0.515 |
| Missing | 0 | 2 | ||
| ADAS-Cog at 12 months | ||||
| n | 137 | 278 | ||
| Mean (SD) | 25.5 (13.7) | 26.6 (14.8) | –0.9 (–2.60 to 0.73) | 0.271 |
| Missing | 0 | 3 | ||
The third sensitivity analysis used IRT to analyse the ADAS-Cog computed using the observed data. Moreover, the analysis looked at three particular traits measured by the ADAS-Cog: language, memory and praxis. The baseline model converged within 0.001 tolerance after 305 Metropolis–Hastings Robbins–Monro iterations. The model fit was adequate, with a root-mean-square error of approximation of 0.047 (95% CI 0.043 to 0.051). Furthermore, individual items fit the model well, with all chi-squared statistics being insignificant. The memory and language traits had a correlation of 0.78, the memory and praxis traits 0.76 and the language and praxis traits 0.72. A summary of the abilities at each time point is presented in Table 58, Appendix 6. Tables 60–62, Appendix 6, present univariate analyses of the between-group differences for each of the traits at baseline and at 6 months and 12 months, respectively. The respondent ability scores at each time point for each latent trait have also been presented as a box plot in Figure 22, Appendix 6. No significant between-group differences were observed in the univariate analyses. The adjusted analyses results for the language, memory and praxis scores at 12 months are presented in Tables 61–63, Appendix 6, respectively. There was no evidence of a significant difference between the two groups for any of the three traits at 12 months.
Complier-average causal effect
A CACE analysis was conducted to estimate the treatment effect at each time point, having adjusted for non-compliance. Participants in the exercise arm who attended > 75% of their scheduled sessions were defined as compliers of treatment. The baseline outcome measures for the usual-care arm and for compliers and non-compliers in the treatment arm have been summarised in Table 24.
| Outcome | Usual care | Compliance status | Mean difference (95% CI)a | p-valueb | |
|---|---|---|---|---|---|
| Complier | Non-complier | ||||
| Age (years) | |||||
| n | 165 | 214 | 115 | ||
| Mean (SD) | 78.4 (7.6) | 76.4 (7.8) | 77.7 (8.1) | 1.2 (–0.57 to 3.03) | 0.178 |
| Gender (male), n (%) | 106 (64.2) | 144 (67.3) | 51 (44.4) | – | < 0.001 |
| Living arrangements | |||||
| Live alone, n (%) | 35 (21.2) | 32 (14.9) | 30 (26.1) | – | 0.030 |
| Live with relatives, n (%) | 5 (3.0) | 13 (6.1) | 5 (4.3) | ||
| Live with wife/husband/partner, n (%) | 125 (75.8) | 169 (79.0) | 79 (68.7) | ||
| Living with friends, n (%) | 0 | 0 | 1 (0.9) | ||
| ADAS-Cog (imputed) | |||||
| n | 163 | 214 | 115 | ||
| Mean (SD) | 21.8 (7.7) | 21.9 (9.7) | 20.5 (9.4) | –1.4 (–3.55 to 0.81) | 0.217 |
| ADAS-Cog (raw score) | |||||
| n | 152 | 201 | 113 | ||
| Mean (SD) | 21.4 (7.4) | 20.9 (8.8) | 20.2 (9.2) | –0.6 (–2.70 to 1.44) | 0.550 |
| Language subscale (imputed) | |||||
| n | 165 | 214 | 115 | ||
| Mean (SD) | 2.7 (3.0) | 3.2 (3.6) | 2.7 (3.6) | –0.5 (–1.29 to 0.33) | 0.241 |
| Memory subscale (imputed) | |||||
| n | 163 | 214 | 115 | ||
| Mean (SD) | 17.4 (4.8) | 17.0 (6.3) | 16.1 (5.9) | –0.8 (–2.26 to 0.56) | 0.239 |
| Praxis subscale (imputed) | |||||
| n | 165 | 214 | 115 | ||
| Mean (SD) | 1.7 (1.5) | 1.8 (1.7) | 1.7 (1.6) | –0.04 (–0.42 to 0.33) | 0.823 |
| Language subscale (raw score) | |||||
| n | 161 | 211 | 114 | ||
| Mean (SD) | 2.7 (3.0) | 3.1 (3.4) | 2.6 (3.6) | –0.4 (–1.24 to 0.35) | 0.275 |
| Memory subscale (raw score) | |||||
| n | 156 | 205 | 113 | ||
| Mean (SD) | 17.1 (4.7) | 16.7 (6.3) | 16.0 (5.9) | –0.7 (–2.08 to 0.75) | 0.356 |
| Praxis subscale (raw score) | |||||
| n | 165 | 213 | 115 | ||
| Mean (SD) | 1.7 (1.5) | 1.8 (1.7) | 1.7 (1.6) | –0.04 (–0.42 to 0.34) | 0.840 |
| sMMSE score | |||||
| n | 165 | 214 | 115 | ||
| Mean (SD) | 21.6 (4.6) | 22.0 (4.8) | 21.9 (4.5) | –0.1 (–1.15 to 0.98) | 0.874 |
| EQ-5D-3L score (self-reported) | |||||
| n | 159 | 212 | 115 | ||
| Mean (SD) | 0.85 (0.18) | 0.83 (0.19) | 0.79 (0.22) | –0.05 (–0.10 to –0.004) | 0.033 |
| EQ-5D-3L VAS score (self-reported) | |||||
| n | 161 | 210 | 113 | ||
| Mean (SD) | 81.8 (17.7) | 77.7 (18.2) | 74.9 (19.0) | –2.9 (–7.13 to 1.35) | 0.181 |
| QoL-AD score (self-reported) | |||||
| n | 140 | 186 | 98 | ||
| Mean (SD) | 39.3 (5.2) | 38.9 (5.7) | 38.2 (5.5) | –0.7 (–2.12 to 0.63) | 0.287 |
| NPI score (proxy-reported) | |||||
| n | 119 | 164 | 76 | ||
| Mean (SD) | 13.3 (13.2) | 12.5 (14.3) | 13.7 (16.6) | 1.3 (–2.82 to 5.38) | 0.540 |
| BADLS score (proxy-reported) | |||||
| n | 143 | 188 | 99 | ||
| Mean (SD) | 11.4 (8.5) | 11.7 (8.2) | 12.8 (8.6) | 1.1 (–0.91 to 3.18) | 0.276 |
Statistically significant differences in the baseline characteristics were found when comparing compliers and non-compliers. The compliers were mostly male, lived with their wife or partner and had better quality of life, as measured by the EQ-5D-3L. The ITT and CACE analysis estimates have been presented in Table 25. Similar to the ITT results, the CACE results found no difference in treatment effect at 6 months but found a statistically significant treatment effect of –2.0 (95% CI –3.87 to –0.22) at 12 months in the primary outcome (imputed ADAS-Cog), having adjusted for non-compliance. The CACE analysis estimates of treatment effect at 6 months and 12 months were larger than the ITT estimates.
| Time point | ITT covariate model | Standardised effect at 12 monthsb | CACE covariate model | Standardised effect at 12 monthsb | ||
|---|---|---|---|---|---|---|
| Mean compliance difference (95% CI)a | p-value | Mean compliance difference (95% CI)c | p-value | |||
| ADAS-Cog (imputed) score | ||||||
| 6 months | –0.6 (–1.58 to 0.39) | 0.237 | – | –0.8 (–2.22 to 0.65) | 0.282 | – |
| 12 months | –1.4 (–2.62 to –0.17) | 0.026 | –0.16 | –2.0 (–3.87 to –0.22) | 0.028 | –0.23 |
Subgroup analyses
Subgroup analyses were conducted on gender, sMMSE score, EQ-5D-3L mobility score and type of dementia using the 12-month ADAS-Cog (imputed) score as the outcome measure. No statistically significant subgroup effects were found (Table 26).
| Subgroups | Treatment arm | Effect estimate (95% CI) | Interaction effect (95% CI) | Interaction p-value | |||
|---|---|---|---|---|---|---|---|
| Usual care | Exercise programme | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| Gender | |||||||
| Male | 86 | 23.9 (11.4) | 166 | 23.9 (11.8) | –1.2 (–2.78 to 0.46) | –0.6 (–3.17 to 1.88) | 0.616 |
| Female | 51 | 23.7 (8.5) | 112 | 27.3 (12.9) | –1.8 (–3.60 to 0.08) | ||
| sMMSE score | |||||||
| < 20 | 36 | 34.3 (10.9) | 84 | 37.7 (10.8) | –2.8 (–5.32 to –0.27) | 1.8 (–0.98 to 4.50) | 0.207 |
| ≥ 20 | 101 | 20.1 (7.2) | 194 | 19.8 (8.4) | –0.9 (–2.32 to 0.46) | ||
| EQ-5D-3L mobility score | |||||||
| No problems walking | 103 | 24.5 (10.5) | 204 | 26.6 (12.8) | –1.3 (–2.65 to 0.10) | 0.00005 (–2.86 to 2.86) | 1.000 |
| Some problems/confined to bed | 33 | 21.7 (10.0) | 74 | 21.4 (10.0) | –1.6 (–4.21 to 1.00) | ||
| Type of dementia | |||||||
| Alzheimer’s | 108 | 23.7 (10.0) | 227 | 25.6 (12.4) | –1.1 (–2.41 to 0.29) | 1.3 (–1.81 to 4.35) | 0.417 |
| Other (mixed, vascular, other types) | 29 | 24.5 (11.8) | 51 | 23.5 (11.9) | –2.7 (–5.58 to 0.16) | ||
Longitudinal analysis
A longitudinal analysis was conducted (n = 494). The analysis adjusted for age, gender, sMMSE score and region. No statistically significant between group difference was found [–0.3 (95% CI –1.69 to 1.05)].
Adverse events and serious adverse events
During the entire study period, 31 AEs were reported and four SAEs were reported, all of which were from the exercise programme arm (Table 27). Around 74% of the AEs and 50% of the SAEs occurred during the session or within 2 hours of the session. The remaining AEs and SAEs occurred during follow-on physical activities. An assessment of all AEs and SAEs has been summarised in Table 28. All SAEs were assessed by the chief investigator. Of the four reported SAEs, none was expected and unrelated, two were expected and related, one was unexpected and unrelated, and one was unexpected and related (see Appendix 7 for SAE details).
| AE and SAE summaries | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| Usual care | Exercise programme | ||
| AEs | |||
| Number of AEs reported | 0 | 31 (100.0) | 31 (100.0) |
| When did AE occur | |||
| Occurred during session | 0 | 15 (48.4) | 15 (48.4) |
| Within 2 hours of session | 0 | 8 (25.8) | 8 (25.8) |
| During follow-on physical activities | 0 | 8 (25.8) | 8 (25.8) |
| SAEs | |||
| Number of SAEs reported | 0 | 4 (100.0) | 4 (100.0) |
| When did SAE occur | |||
| Occurred during session | 0 | 1 (25.0) | 1 (25.0) |
| Within 2 hours of session | 0 | 1 (25.0) | 1 (25.0) |
| During follow-on physical activities | 0 | 2 (50.0) | 2 (50.0) |
| Reason AE deemed serious | |||
| Participant died | 0 | 0 | 0 |
| Participant is in life-threatening condition | 0 | 0 | 0 |
| Participant required hospitalisation or prolongation of existing hospitalisation | 0 | 2 (50.0) | 2 (50.0) |
| Participant left with persistent or significant disability or incapacity | 0 | 0 | 0 |
| Participant required medical intervention to prevent one of the above | 0 | 2 (50.0) | 2 (50.0) |
| Assessment of AEs and SAEs | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| Usual care | Exercise programme | ||
| AE related to trial procedure (clinician/researcher) | |||
| Definitely | 0 | 8 (25.8) | 8 (25.8) |
| Probably | 0 | 9 (29.0) | 9 (29.0) |
| Possibly | 0 | 8 (25.8) | 8 (25.8) |
| Unlikely | 0 | 5 (16.2) | 5 (16.2) |
| Unrelated | 0 | 1 (3.2) | 1 (3.2) |
| SAE related to trial procedure (clinician/researcher) | |||
| Definitely | 0 | 0 | 0 |
| Probably | 0 | 0 | 0 |
| Possibly | 0 | 3 (75.0) | 3 (75.0) |
| Unlikely | 0 | 1 (25.0) | 1 (25.0) |
| Unrelated | 0 | 0 | 0 |
| SAE related to trial procedure (clinician/researcher) | |||
| Expected | 0 | 2 (50.0) | 2 (50.0) |
| Unexpected | 0 | 2 (50.0) | 2 (50.0) |
| Chief investigator assessment of SAE | |||
| Expected and unrelated | 0 | 0 | 0 |
| Expected and related | 0 | 2 (50.0) | 2 (50.0) |
| Unexpected and unrelated | 0 | 1 (25.0) | 1 (25.0) |
| Unexpected and related | 0 | 1 (25.0) | 1 (25.0) |
Process evaluation
Process measurements
The time to pre-assessment and first session remained within the protocol-defined boundaries (Table 29).
| Summary statistic | Time (days) | ||
|---|---|---|---|
| From randomisation to pre-assessment | From randomisation to session 1 | From pre-assessment to session 1 | |
| n | 317 | 319 | 315 |
| Mean (SD) | 18.3 (12.2) | 24.3 (13.4) | 5.9 (4.1) |
| Range | 1–90 | 1–101 | 0–27 |
| Missing | 12 | 10 | 14 |
The delivery of the exercise intervention has been summarised in Table 30. The average number of sessions attended by each participant was 21 (SD 8.7).
| Region | Number of physiotherapists involved in delivery | Number of exercise instructors involved in delivery | Number (%) of participants attending pre-assessment | Mean (SD) number of sessions attended |
|---|---|---|---|---|
| Berkshire | 5 | 3 | 27 (96.4) | 18.4 (10.0) |
| Black Country | 7 | 3 | 26 (86.7) | 17.2 (11.4) |
| Coventry | 12 | 5 | 35 (97.2) | 21.1 (8.2) |
| Devon and Exeter | 3 | 2 | 6 (100.0) | 24.2 (5.0) |
| Gloucestershire and Herefordshire | 5 | 3 | 12 (100.0) | 25.1 (2.7) |
| Greater Manchester West | 2 | 1 | 12 (100.0) | 18.7 (8.9) |
| Leicester | 7 | 0 | 10 (83.3) | 20.2 (11.3) |
| North East London | 5 | 2 | 11 (91.7) | 17 (10.1) |
| Northampton | 6 | 3 | 40 (97.6) | 23.2 (7.6) |
| Nuneaton | 12 | 5 | 15 (100.0) | 23.1 (8.0) |
| Oxford | 9 | 7 | 50 (100.0) | 22.6 (7.1) |
| Rugby | 12 | 5 | 15 (93.8) | 17.3 (10.0) |
| Solent | 2 | 2 | 6 (100.0) | 17.2 (10.6) |
| South Warwickshire | 12 | 5 | 24 (100.0) | 24.4 (5.0) |
| Worcester | 7 | 6 | 28 (96.6) | 20.8 (8.8) |
| Total | 106 | 52 | 317 (96.4) | 21.0 (8.7) |
Table 31 details the compliance rates of participants in the intervention group. In total, 65% of the participants in the intervention group demonstrated > 75% compliance with the exercises and 79.9% achieved > 50% compliance.
| Compliance range (%) | Number (%) of participants (n = 329) |
|---|---|
| ≤ 25 | 44 (13.4) |
| > 25 and ≤ 50 | 22 (6.7) |
| > 50 and ≤ 75 | 49 (14.9) |
| > 75 and ≤ 100 | 214 (65.0) |
Participant’s individual fitness was measured with the 6MWT. The reasons for non-completion and the pre- and post-intervention data for the 6MWT are presented in Table 32.
| Test components | Time point of assessment | |
|---|---|---|
| Pre exercise (n = 317)a | Post exercise (n = 233)b | |
| Walking aid used, n (%) | ||
| Yes | 58 (18.3) | 30 (12.9) |
| Missing | 4 (1.3) | 2 (0.8) |
| Was test completed, n (%) | ||
| Yes | 310 (97.8) | 229 (98.3) |
| Missing | 2 (0.6) | 1 (0.4) |
| If test completed, was it completed in the standardised manner, n (%) | ||
| Yes | 262 (82.7) | 193 (82.8) |
| Missing | 10 (3.1) | 14 (6.0) |
| Test not completed because participant became anxious, n (%) | ||
| Yes | 0 | 1 (0.4) |
| Missing | 0 | 0 |
| Test not completed because of signs of overexertion, n (%) | ||
| Yes | 1 (0.3) | 1 (0.4) |
| Missing | 0 | 0 |
| Test not completed because participant became unsteady, n (%) | ||
| Yes | 0 | 0 |
| Missing | 0 | 0 |
| Test not completed because participant declined to continue, n (%) | ||
| Yes | 1 (0.3) | 1 (0.4) |
| Missing | 0 | 0 |
| Test not completed because of pain, n (%) | ||
| Yes | 2 (0.6) | 0 |
| Missing | 0 | 0 |
| Test not completed because test disrupted, n (%) | ||
| Yes | 0 | 1 (0.4) |
| Missing | 0 | 0 |
| Metabolic equivalents | ||
| Mean (SD) | 2.6 (0.6) | 2.7 (0.6) |
| Range | 1.1 to 5.6 | 1.2 to 5.5 |
| Missing | 3 | 2 |
| % heart rate reserve | ||
| Mean (SD) | 32.4 (21.2) | 36.7 (24.0) |
| Range | –82 to 158 | 4 to 94 |
| Missing | 16 | 204 |
| Heart rate walking speed index | ||
| Mean (SD) | 21.0 (18.5) | 18.5 (12.5) |
| Range | 0 to 222.9 | 1.1 to 115.9 |
| Missing | 15 | 24 |
The demographics, dose received and physical activity of the participants in the intervention arm have been summarised in Table 33 by compliance status. By taking part in the intervention, participants were able to lift more weight for the sit-to-stand exercise, indicating an improvement in strength (see Table 33 and Figure 8). On average, across all sessions, participants fully achieved their target number of repetitions and sets for the sit-to-stand exercise as shown in Figure 9. Regarding the aerobic component (cycling) of the intervention, participants were able to achieve a large proportion of their set target at each session both at moderate and hard intensity, as shown in Figure 10. We modelled the association between the dose achieved and cognition for both the aerobic and resistance components of the intervention; however, no evidence of an association was found.
| Participants | Compliance status | All (n = 329) | |
|---|---|---|---|
| Compliers (n = 214) | Non-compliers (n = 115) | ||
| Demographics | |||
| Age (years), mean (SD) | 76.4 (7.8) | 77.7 (8.1) | 76.9 (7.9) |
| Gender (male), n (%) | 144 (67.3) | 51 (44.4) | 195 (59.3) |
| ADAS-Cog (imputed) score, mean (SD) | 21.9 (9.7) | 20.5 (9.4) | 21.4 (9.6) |
| ADAS-Cog (raw score), mean (SD) | 20.9 (8.8) | 20.2 (9.2) | 20.6 (8.9) |
| Language subscale (imputed), mean (SD) | 3.2 (3.6) | 2.7 (3.6) | 3.0 (3.6) |
| Memory subscale (imputed), mean (SD) | 17.0 (6.3) | 16.1 (5.9) | 16.7 (6.2) |
| Praxis subscale (imputed), mean (SD) | 1.8 (1.7) | 1.7 (1.6) | 1.7 (1.7) |
| sMMSE score, mean (SD) | 22.0 (4.8) | 21.9 (4.5) | 22.0 (4.7) |
| EQ-5D-3L score (self-reported), mean (SD) | 0.83 (0.19) | 0.79 (0.22) | 0.82 (0.20) |
| QoL-AD score (self-reported), mean (SD) | 38.9 (5.7) | 38.2 (5.5) | 38.7 (5.6) |
| NPI score (proxy-reported), mean (SD) | 12.5 (14.3) | 13.7 (16.6) | 12.8 (15.0) |
| BADLS score (proxy-reported), mean (SD) | 11.7 (8.2) | 12.8 (8.6) | 12.0 (8.4) |
| Medical conditions,a n (%) | |||
| Heart or circulatory | 102 (47.7) | 52 (50.5) | 154 (48.6) |
| GTN spray | 18 (8.4) | 13 (12.6) | 31 (9.8) |
| Lung disease | 22 (10.3) | 18 (17.5) | 40 (12.6) |
| Inhaler | 22 (10.3) | 14 (13.6) | 36 (11.4) |
| Diabetes | 38 (17.8) | 21 (20.4) | 59 (18.6) |
| Neurological condition | 42 (19.6) | 15 (14.6) | 57 (18.0) |
| Limiting joint or muscle pain | 117 (54.7) | 60 (58.3) | 177 (55.8) |
| Broken bone in last 6 months | 14 (6.5) | 8 (7.8) | 22 (6.9) |
| Mental illness | 65 (30.4) | 45 (43.7) | 110 (34.7) |
| Number of medical conditions | |||
| No conditions | 27 (12.6) | 12 (11.7) | 39 (12.3) |
| One condition | 56 (26.2) | 21 (20.4) | 77 (24.3) |
| Two conditions | 57 (26.6) | 23 (22.3) | 80 (25.2) |
| ≥ Three conditions | 74 (34.6) | 47 (45.6) | 121 (38.2) |
| Dose receivedb | |||
| Number of sessions attended | |||
| Mean (SD) | 26.2 (2.1) | 11.2 (8.0) | 21.0 (8.7) |
| Range | 22–30 | 0–22 | 0–30 |
| Sit to stand | |||
| Repetitions,c mean (SD) | 432.8 (115.5) | 229.2 (117.8) | 370.7 (149.3) |
| Start weight (kg), mean (SD) | 4.3 (4.1) | 3.8 (3.4) | 4.2 (3.9) |
| Finish weight (kg), mean (SD) | 8.7 (9.1) | 6.4 (5.4) | 8.0 (8.2) |
| Difference between start and finish weight (kg), mean difference (95% CI) | 4.4 (3.2 to 5.6) | 2.6 (1.7 to 3.5) | 3.9 (3.0 to 4.8) |
| Total weight lifted (kg),d median (IQR) | 3460.8 (1857.3–5537.1) | 1307.5 (276–2284) | 2569.8 (1231.4– 4672) |
| Arm exercises | |||
| Repetitions,c mean (SD) | 1031.4 (225.5) | 556.1 (277.3) | 886.5 (326.5) |
| Total weight lifted,d median (IQR) | 2469.4 (1626.3– 3444.2) | 1001.9 (463–1574) | 1933.5 (1105–2905) |
| Cycling (total number of minutes) | |||
| Low intensity, mean (SD) | 210.5 (70.7) | 131.5 (72.6) | 186.6 (79.9) |
| Medium intensity, mean (SD) | 287.9 (90.8) | 121.5 (94.7) | 237.5 (119.6) |
| High intensity, mean (SD) | 66.2 (58.3) | 14.6 (27.7) | 50.6 (56.2) |
| First session (total number of minutes) | |||
| Target, mean (SD) | 14.5 (2.1) | 14.2 (2.8) | 14.4 (2.3) |
| Actual, mean (SD) | 13.8 (3.3) | 13.1 (4.3) | 13.6 (3.7) |
| Target, moderate or high intensity, mean (SD) | 0 | 0.4 (2.1) | 0.1 (1.2) |
| Actual, moderate or high intensity, mean (SD) | 0.4 (1.7) | 0.7 (2.2) | 0.5 (1.8) |
| Last session (total number of minutes) | |||
| Target, mean (SD) | 21.3 (7.1) | 20.8 (5.5) | 21.1 (6.7) |
| Actual, mean (SD) | 19.9 (8.0) | 19.4 (7.0) | 19.8 (7.7) |
| Target, moderate or high, mean (SD) | 16.0 (6.8) | 12.9 (8.2) | 15.0 (7.3) |
| Actual, moderate or high, mean (SD) | 14.7 (7.5) | 10.6 (8.5) | 13.5 (8.0) |
| Fitness tests | |||
| 6MWT | |||
| Baseline 6MWT distance (m), mean (SD) | 340.0 (114.0) | 315.4 (108.7) | 332.1 (112.7) |
| 6-week 6MWT walk distance (m), mean (SD) | 363.0 (118.1) | 355.8 (101.6) | 361.8 (115.3) |
| Difference in 6MWT distance, mean difference from 0 to 6 weeks (95% CI) | 19.6 (12.5 to 26.7) | 10.7 (–6.3 to 27.8) | 18.1 (11.6 to 24.6) |
| Self-reported physical activitye | |||
| First telephone call, n (%) | |||
| No physical activity | 19 (9.0) | 7 (11.9) | 26 (9.6) |
| Some physical activity | 186 (88.2) | 47 (79.7) | 233 (86.3) |
| Second telephone call, n (%) | |||
| No physical activity | 12 (5.9) | 8 (14.8) | 20 (7.8) |
| Some physical activity | 179 (88.6) | 42 (77.8) | 221 (86.3) |
| Third telephone call, n (%) | |||
| No physical activity | 20 (9.9) | 8 (15.1) | 28 (10.9) |
| Some physical activity | 177 (87.2) | 40 (75.5) | 217 (84.8) |
| First telephone call (minutes per week) | |||
| n | 205 | 54 | 259 |
| Median (IQR) | 150 (60–250) | 115 (60–230) | 140 (60–250) |
| Second telephone call (minutes per week) | |||
| n | 191 | 50 | 241 |
| Median (IQR) | 150 (80–270) | 97.5 (35–150) | 150 (70–250) |
| Third telephone call (minutes per week) | |||
| n | 197 | 48 | 245 |
| Median (IQR) | 145 (70–275) | 117.5 (30–220) | 140 (60–270) |
FIGURE 8.
Plot of the median (interquartile range) weight lifted (kg) over time by compliance status for the sit-to-stand exercise. (a) Complier and (b) non-complier. The total number of participants attending each session is presented above each interquartile range bar. IQR, interquartile range.
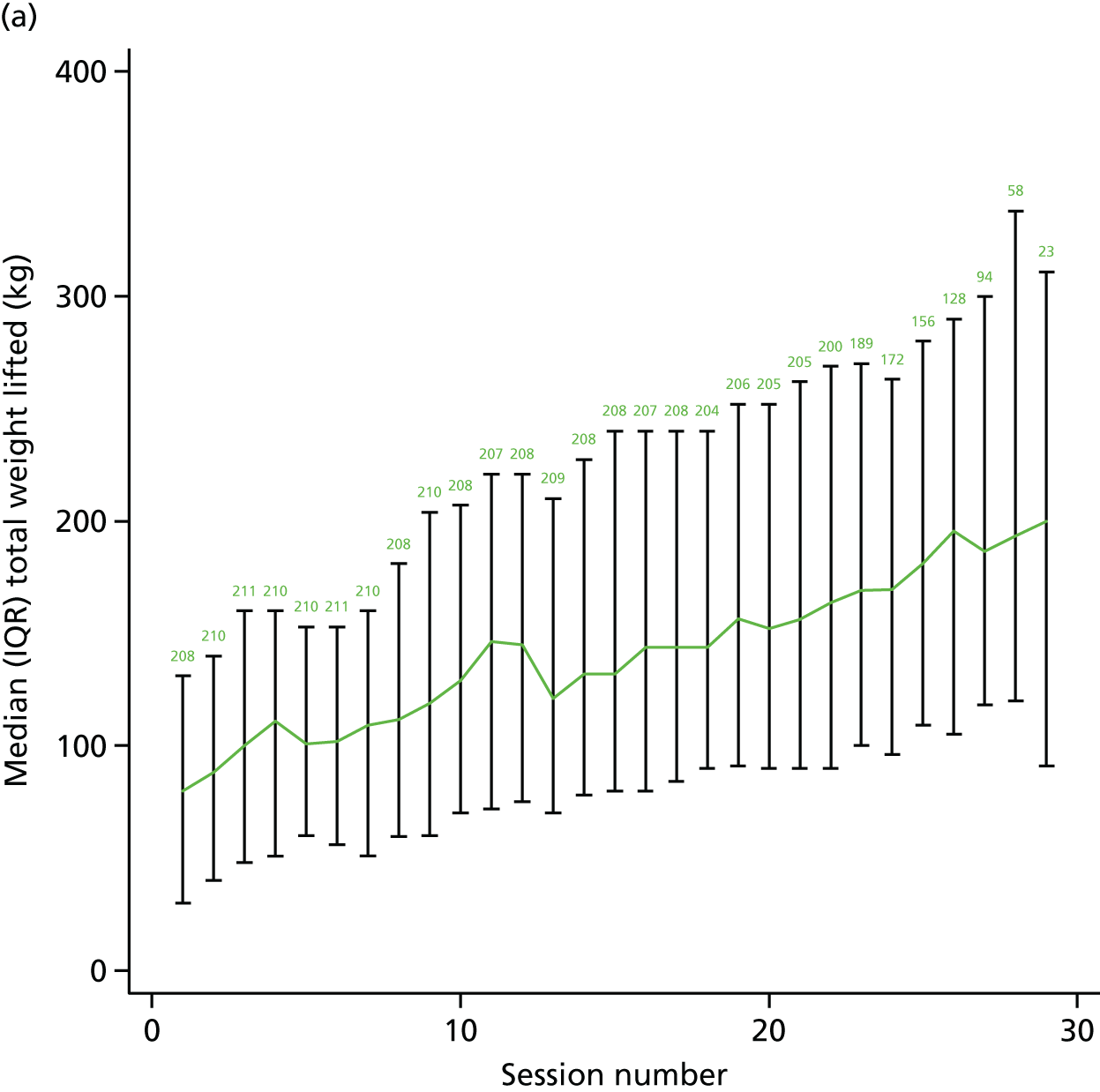
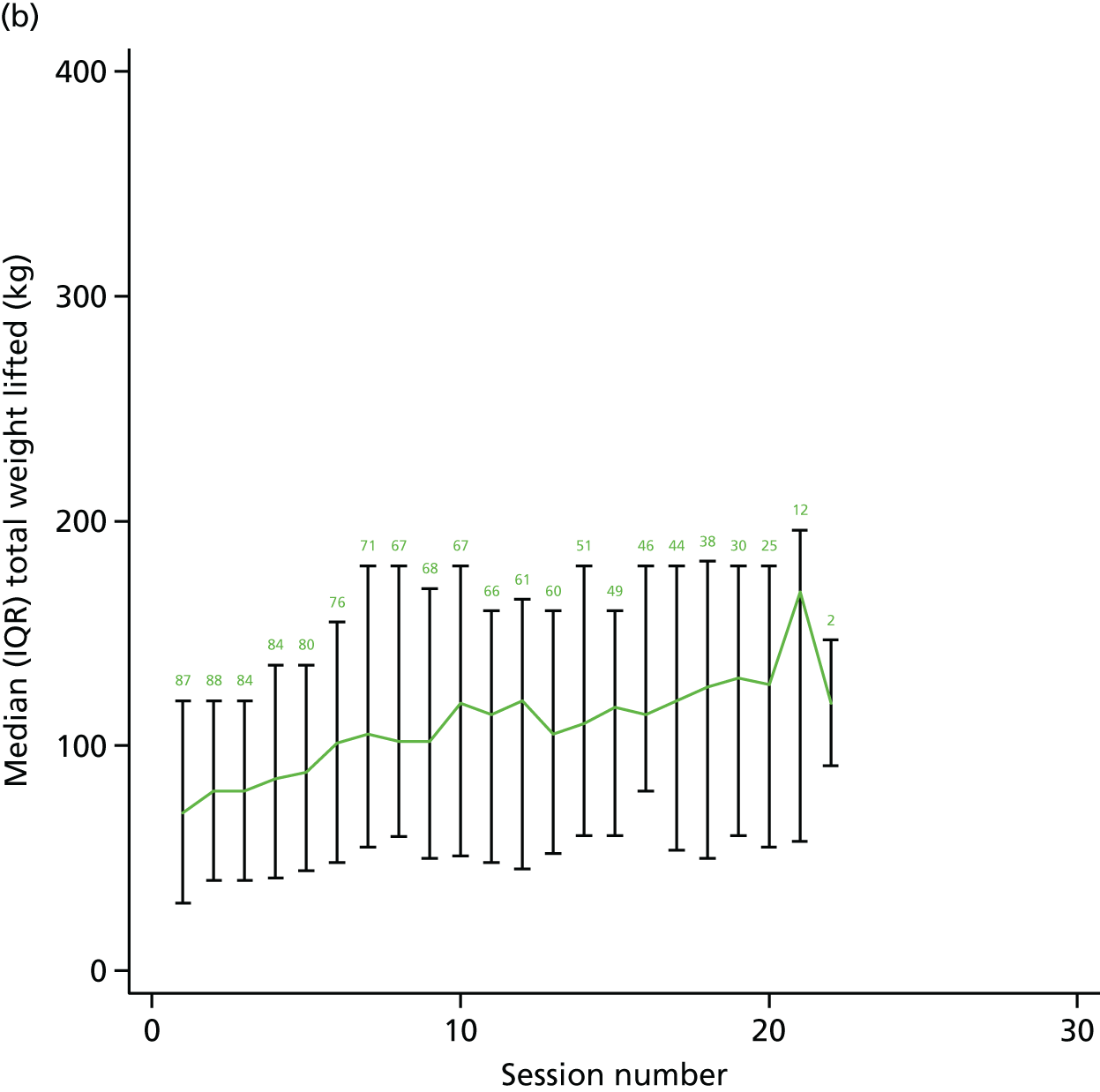
FIGURE 9.
Mean % target repetitions and sets achieved by the intervention group for the sit-to-stand exercise.

FIGURE 10.
Mean % target achieved by the intervention group at moderate and hard intensity.

The intervention was intended to increase participants’ fitness levels through increasing exercise intensity over the intervention period. From all of the outcomes generated by the 6MWT, the distance travelled has been proven as a reliable estimate of fitness. 60 There is evidence of a statistically significant increase in the distance walked post assessment compared with pre-assessment (paired t-test, p < 0.001), suggesting that the intervention was successful in increasing participants’ fitness levels.
Discussion
The clinical effectiveness evaluation of the exercise on global cognition demonstrated some statistical evidence of a difference in favour of usual care. Moreover, the intervention had no impact on behavioural symptoms, HRQoL, daily functioning, falls or carer burden.
The results of this clinical effectiveness analysis differs to results from many smaller single-centre studies. 19 However, the quality of this study is a key discerning factor, which is a major strength. Compared with other studies, this study has a larger representative sample size with high intervention compliance and follow-up rates. Moreover, we used prospective registration, robust allocation concealment, independent computer-based randomisation and masked outcome assessment. Hence, we are confident that the clinical effectiveness analysis results provide a reliable estimate of the effects of moderate- to high-intensity exercise in this population and setting. Although there was some difference in how participants withdrew from the trial, with more participants from the usual-care arm providing notification of formal withdrawal as opposed to not responding to a request for follow-up, the overall levels of participant retention were the same. The comparability of the baseline characteristics of people who were analysed at 12 months was good.
There are some limitations to this study. First, we were faced with the challenge of finding an appropriate way of dealing with the item-level missingness in the primary outcome measure (ADAS-Cog). After much discussion with the DMEC and Trial Steering Committee, it was agreed that MI at the item level was the most sensible approach to take, for which items were only imputed if it was known that the participant was too unwell to complete it. The sensitivity analyses demonstrated that, regardless of the method used for handling missingness, the conclusions drawn were still the same, hence giving us confidence in the findings from the primary analysis. A second limitation was that the prespecified subgroup analyses were underpowered, although these were still substantially larger than any other previous studies. However, the direction of the effect estimates supported our conclusions. Finally, as we collected physical parameters from the intervention arm only, we cannot definitively conclude that the strength and fitness improvements of participants is caused by intervention, although it seems unlikely that there are other explanations.
In conclusion, moderate- to high-intensity exercise training does not reduce cognitive decline in people with MMD in a community-dwelling setting and may result in worsening of cognition.
Chapter 4 Qualitative study
Objectives
To conduct a qualitative study in parallel to the supervised component of the exercise intervention, the aim of which was to provide insight into participants’ and carers’ attitudes and experiences of taking part in the experimental intervention and physiotherapists’ experiences of, and attitudes to, delivering it.
Research questions
-
For participants and their carers, what are the experiences of, and attitudes to, taking part in the experimental intervention?
-
For physiotherapists, what are the experiences of, and attitudes to, delivering the experimental intervention?
Methods
Sampling and recruitment
Sampling of participants and their carers was consecutive and participants were drawn from five intervention delivery sites. Sites were selected to reflect a range of settings and reflexive observations were carried out at each site. Once observations of the sites were completed, we invited participants and carers to take part in an interview. Participants had already consented to be approached to take part in the qualitative study as part of the consent process for the RCT. Ten participants and 10 carers were approached in person, asked if they would like to learn more about taking part in an interview and given a participant information leaflet. At the next observation, they were approached again and asked if, having read the information, they would like to agree to an interview. Once an interview date was agreed, participants were asked to sign an interview consent form. Participants were advised that they could terminate the interview at any time and that it would not affect their participation in the intervention.
As we began to reach data saturation, we checked that the sample broadly reflected the population of the intervention arm of the trial in relation to gender, ethnicity and social class. All of the physiotherapists delivering the intervention at the included sites were approached for interview and offered a participant information leaflet and an informed consent form.
Data collection
Observations
A qualified and experienced qualitative researcher (Samantha Lyle) observed classes at three time points across the duration of the 4-month-long supervised aspect of the intervention. At the start of the observations, the researcher was introduced to participants by the physiotherapist who was delivering the class. Observations consisted of watching participants and carers and the interactions of the physiotherapists with them as they arrived to the classes, and watching and taking handwritten notes of the delivery and end of the classes. The researcher took notes while visible to those present. She also took time to engage briefly with participants in order to hear about their particular experience on that day. Observations notes were typed up and formed part of the data set. No one made any objections to being observed.
Interviews
A semistructured interview guide was developed by the research team to gather data from the participants, carers and physiotherapists to answer the research questions (see Appendix 8). This was tested and refined during the second pilot study. The interviews were carried out by a researcher who was trained and experienced in qualitative research methodologies (Samantha Lyle).
Participants and carers were offered the opportunity to be interviewed separately or together. When both expressed indifference, the researcher suggested that the interviews be conducted separately starting with the participant, thereby allowing participants to engage in the research as autonomous research subjects89,90 and allowing carers the opportunity to speak freely.
Physiotherapists were offered the choice of being interviewed at their workplace or at home via telephone. The interviews were audio-recorded and transcribed verbatim.
Interviewing participants with cognitive impairment
There are a number of obstacles to interviewing people with cognitive impairment: short-term memory problems, difficulties in abstract thinking and expressive language, a lack of insight and awareness of their diagnosis, and damage to their sense of self related to their experiences of diagnosis and symptoms of dementia. 89,91–97 We sought to overcome these obstacles by having the interviewer adopt an attitude that assumes that the person with dementia has something valuable to say, listen carefully and accept the interviewee as they are and with an openness to understanding them. The researcher took time to meet interviewees before the formal interview in order to build rapport and gauge expressive skills. During the interviews, the researcher was comfortable with long pauses and the expression of strong emotions, avoided the use of complex concepts, adjusted language to align with participants’ language and used direct interview styles when expressive skills were very limited. 89,94
Data analysis
Our data comprised:
-
notes taken during observations of exercise classes
-
interviews with trial participants
-
interviews with carers of trial participants
-
interviews with physiotherapists delivering the classes.
The data were analysed as a single data set. Identifying information was anonymised to ensure interviewees’ confidentiality was maintained. Interview transcripts and field notes were uploaded into NVivo version 10 (QSR International, Warrington, UK), a computer program for qualitative data, to assist with data management and analysis.
Analysis was thematic and broadly followed the principles of grounded theory. 98 A thematic analysis involves identifying, describing, analysing, interpreting and reporting repeated themes and categories in the data that relate to the research questions and represents some level of patterned response or meaning across a data set. 99 We compared data relating to an individual participant from the participant themselves, their carer and their physiotherapist. The data were initially organised into broad descriptive codes such as ‘experiences of the intervention’. The data within these broad codes were reread and subcodes were identified such as ‘positive experiences of participants’, negative experiences of participants’ and ‘ambivalent experiences of participants’. During the coding process, we compared data between participants, between carers and between physiotherapists to sharpen our understanding and refine our subcodes. Transcripts were analysed by the researcher who carried out the interviews (Samantha Lyle). A second researcher (Frances Griffiths) independently analysed approximately 20% of the transcripts. 99 The two researchers met to discuss and reflect on the coding and analysis as it proceeded. Discussion of the analytic process and findings were shared within the wider research team, including the chief investigator (Sarah E Lamb), for their reflections, which further informed subcode refinement. Disagreements regarding the identification, description, analysis or interpretation of data were resolved through discussion and further analysis.
Results
Data collected and the characteristics of sites and interviewees
In total, five sites were observed four times, for approximately 1.5 hours each, between November 2013 and March 2015. Settings for the delivery of the intervention included pleasant leisure centres with cafes and soft seating for carers to use, large warehouse-style gyms situated on industrial estates with very loud heating systems and rather run-down local authority amenities with no facilities for carers to use. They were located in a range of urban, suburban and rural settings.
Eight participants took part in an interview and two declined to do so. Six carers agreed to take part in an interview and two declined. Six participants chose to be interviewed on their own and two chose to have their carer present (one was a spouse who had her interview on her own and the other was a friend). Five carers chose to be interviewed on their own and one to be interviewed with the participant. All participant interviews took place in their homes and three carer interviews took place in quiet rooms while classes were being delivered. Interviews lasted between 30 minutes and 2 hours.
All five physiotherapists delivering the intervention at the included sites agreed to an interview (one via the telephone).
The participants interviewed were slightly younger than the overall study sample. They were all white British, with five men and three women. Their cognitive function indicated only mild dementia for all but one participant who had moderate dementia. Their mean cognitive function score is slightly better than that of the main study sample. The sample of carers interviewed (n = 6) comprised two men and four women, all were white British and their current level of burden ranged from 8 (low burden) to 64 (high burden). Table 34 describes the interviewees.
| Participant ID number | Interview | Characteristic | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Gender | Ethnicity | sMMSE score out of 30a | Carer ID number | Interview | Relationship to participant | Gender | Ethnicity | ZBI score out of 80b | ||
| P4 | Solo | 67 | Female | White British | 21 | N/A | Not interviewed | Husband | Male | White British | 30 |
| P5 | Solo | 71 | Male | White British | 19 | N/A | Not interviewed | Wife | Female | White British | 38 |
| P6 | Solo | 81 | Female | White British | 24 | C1 | Solo | Son | Male | White British | 16 |
| P7 | Solo | 76 | Male | White British | 28 | C3 | Solo | Wife | Female | White British | 20 |
| P8 | Joint | 71 | Male | White British | 24 | C9 | Solo | Wife | Female | White British | 64 |
| P10 | Solo | 87 | Male | White British | 26 | C11 | Solo | Wife | Female | White British | 19 |
| P12 | Joint | 77 | Male | White British | 24 | C13 | Joint | Friend | Female | White British | 8 |
| P16 | Solo | 73 | Female | White British | 21 | C17 | Solo | Wife | Male | White British | 24 |
Participant and carer experiences of taking part in the intervention
In this section, we first describe positive experiences that the participants and carers had of the intervention and then the more burdensome aspects of their experiences.
Participants enjoy being with other people
All participants had something positive to say about their experiences. Reasons they gave for enjoying the classes were the company, that it was fun and that there was humour and dialogue:
I think the company of everybody you know. I mean we all had a sit down afterwards and we had a good laugh while we’re doing it.
Patient 12 (male, sMMSE score of 24)
The intervention protocol states that ‘fun, pleasure and enjoyment are recognised as possible motivators to exercise participation for older people’. Those delivering the intervention were observed in all but one class to encourage chit-chat, teasing, joking and laughing within the group, particularly when trying to motivate participants:
[Exercise assistant] chats to all the participants, mentions the World Cup, asks how people’s grandchildren are while they are getting ready, makes small jokes, generally ‘joshing’ with participants. There is a fun mood in the room, people are chatting and joking, during and in-between the resistance work.
Observation field notes, 10 July 2014
In some classes, this type of banter was initiated by participants. When discussing a class in which there was a lot of banter, one participant who was asked what they enjoyed about the class said:
It’s just . . . it’s actually um, being with other people and like-minded people.
Patient 16 (female, sMMSE score of 21)
One group did not engage in banter but participants 6 and 7 expressed enjoyment in attending the class:
I like going to the classes, I’m always [laughs] ready to go . . . I enjoy doing it all.
Patient 6 (female, sMMSE score of 24)
I like . . . well I like it all actually, I very much appreciate having it.
Patient 7 (male, sMMSE score of 28)
For one participant, sharing the experience with other people with dementia was an important part of the enjoyment:
I think it’s just been a lovely time, and you see like-minded people around you and it’s lovely to see them, you know, how they’re enjoying it . . . with Alzheimer’s. Yeah, with Alzheimer’s . . . it’s just nice to be there for them as well as me, I can understand you know with the Alzheimer’s, you can have a chat with them.
Patient 16 (female, sMMSE score of 21)
Participant 16 was the only participant to explicitly say that she enjoyed the classes because she was around other people living with dementia. However, others talked about gaining pleasure from seeing others do well in the class. Participant 5 had been a competitive road cyclist and was still very active at the time he attended the classes. He talked about how other class members had progressed:
Two ladies, they didn’t think much about weights and they were sort of ‘mmmm we don’t do weights’ and the person in charge, she got me started and the ladies said ‘I’ll do it with little weights then’ and they started with little weights and they weren’t too sure about it but after a few weeks they were saying I think I could get to a heavier weight and they could see me going up on my weights so they thought we’ll try it.
Patient 5 (male, sMMSE score of 19)
Participant 7 spoke also gained pleasure in seeing others in class do well:
It’s nice to see the ladies and particularly one or two of the frailer ladies who have picked up.
Patient 7 (male, sMMSE score of 28)
Participants gain pleasure from exercising
One of the ladies who participant 5 described as frail, described enjoying the exercise:
I feel a bit tired but I feel as if I’ve done the exercises, but yes I’m glad I do them.
Patient 6 (female, sMMSE score of 24)
Participant 10 described his enjoyment:
I’m enjoying it. I’m very tired when I come out because I do 25 minutes on the bike, about 2 miles on the bike.
Patient 10 (male, sMMSE score of 26)
Although all participants reported some positive experiences, some also found the classes burdensome. We will return to this after considering carer perspectives on the experiences of participants.
Carers’ perspectives on the experiences of participants of the intervention
All carers spoke positively about the experiences of participants of taking part in the trial and did not perceive the trial to be burdensome. They were confident that the person they cared for enjoyed the classes. They talked about the enjoyment of being with other people, of the exercise itself and of having something to do regularly:
[He] really, really does enjoy it, yes. I think it’s the men all getting together and they just all of them get on with the exercises. It is really good.
Carer 11 (wife, ZBI score of 19)
Well he’s absolutely loving it. He is really enjoying the programme; he doesn’t want it to end. I think he’s benefiting from it . . . being with other people . . . the exercise itself but also doing something specific twice a week.
Carer 3 (wife, ZBI score of 20)
Carer 1 talked about his mother’s sense of achievement:
I think it’s been excellent . . . she’s getting a sense of achievement . . . she tells me how long she’s ridden on the bike.
Carer 1 (son, ZBI score of 16)
Some carers talked in positive terms about how tired the classes made participants:
He’s enjoyed it and it’s done him good even . . . especially the last month, because they’ve pushed him and pushed him, he’s been absolutely nearly incoherent he’s been that tired and luckily fallen asleep straight away . . . I think it’s good.
Carer 9 (wife, ZBI score of 64)
Carers’ own experiences of the intervention
Most carers were very happy to have been randomised to the intervention arm of the trial:
Oh I was over the moon yeah . . . it just sort of gave focus to a couple of days of the week.
Carer 1 (son, ZBI score of 16)
All carers said they appreciated the opportunity to be with other carers in a similar position:
I’ve actually appreciated the opportunity to talk about it [being a carer/dementia] with you know, outside of just friends, that’s been quite good for me to be able to talk about it and . . . it has been nice to talk to other carers.
Carer 3 (wife, ZBI score of 20)
When the participants were attending the classes, carers had free time:
Sometimes I will nip up to [town] and do a bit of shopping and come back. He doesn’t mind.
Carer 11 (wife, ZBI score of 19)
I’ve got a couple of hours that I can catch up on a bit of ironing, I can have a quick vac round and when [participant 8] comes back I can sit with him.
Carer 9 (wife, ZBI score of 64)
This break from the burden of caring for someone with dementia was very welcome. Even when carers stayed at the class venue, taking part in the trial was a welcome change.
The intervention as burden for participant and carer
Although all participants and carers talked about positive experiences, some also talked about the intervention in terms of being a burden. We also know from trial physiotherapists that some carers found it very difficult to get participants to attend and so they withdrew from the intervention and were not interviewed. Three participants who were interviewed talked about what they found difficult about the classes.
Participant 4 talked about, and was seen to enjoy her time at, the class, particularly the social interaction, but she also found some of the exercise ‘painful’ and ‘a bit of a chore’:
I find I’m getting very tired in an afternoon, I don’t like the bikes. They are very, very uncomfortable.
Patient 4 (female, sMMSE score of 21)
However, she saw the classes as beneficial:
I’d do it again, I’ve done it, I’m coming up towards the end of it now and I think there’s got to be some reason and some benefit for me. The exercise in itself is beneficial.
Patient 4 (female, sMMSE score of 21)
The therapeutic alliance between participant and physiotherapist
Therapeutic alliance is a term used to describe the fit between therapists and patients; a good or positive alliance potentially contributes to patient well-being and successful rehabilitation,101 although this is contested. 102 A good therapeutic alliance must be continually reproduced between the patient and the therapist. 103 Trying to maintain a good therapeutic alliance with participants who have dementia is challenging.
One physiotherapist seems to have overestimated what participant 5 could achieve, while perhaps underestimating his understanding of what was possible:
She loaded the whole waistcoat up with lead, the fronts, the middle, the lot, everything and she sat me on this chair and then she said right stand up so I went to get up and she said no, no put your feet there, now get up and I said you can’t do that . . . I knew what I was doing and how to do it and I said I can’t move. She said you’re not going until you do it once so I got up and I put my knee out, I thought this is it, I’m going to be punished like this.
Patient 5 (male, sMMSE score of 19)
Participant 5 reported his experience to the trial team and was offered a different class in which he enjoyed the exercise and the company of the other members of the class:
In contrast, participant 8 talked about how too much surveillance contributed to an incident that he found stigmatising. This participant was observed to have a lot of energy and was described to the researcher as having a big personality and that he could sometimes be disruptive. He was positive about the physiotherapist and exercise assistant but he felt that their surveillance of him undermined his sense of autonomy:
They are frightened to death that you’re going to do something and injure yourself . . . if I pick up a dumb-bell and start doing oh my . . . [they say], ‘just a minute, just a minute’ . . . and I think well I’ve just had a bit of a [telling off] for doing something on my own and there’s these other people they’re doing the same. I don’t feel as though I’ve got anything at the moment. My son calls them ‘window lickers’ and ‘smell of wee tours’ when they all get on the bus . . . it’s only a little thing, it uh . . . when I say uh, ‘oh I’ll be back in a minute’, ‘no, no hang on a minute, where are you going?’ I said ‘I’m going to the toilet’ and then I’ve got [exercise assistant] standing outside the lavatory door!
Patient 8 (male, sMMSE score of 24)
The wife of participant 8 joined the interview saying that those leading the class had behaved reasonably in the situation and that the participant lacked insight (the gym was in a warehouse with the toilets near the exit and no one at the exit to see people leave). However, participant 8 had sufficient insight to express that he felt was being treated like people who his son call ‘gagas’ and ‘window lickers’.
Participants getting to the classes
Of those participants interviewed, three were unaccompanied to the classes at least some of the time. Participants 5 and 8 arrived by taxi (provided by the trial) and participant 7 drove himself, with his wife not accompanying him at least some of the time. Of the carers who had to accompany participants to the class, none of them spoke of the difficulty in doing so. Carer 1 was still in work but he lived locally to his mother and was able to work his around his job, and his brother was also on hand should he be unable to take her to the odd class. He attributed the ease of getting her to classes to her willingness to attend:
When I pulled into her drive I could see her figure behind the door waiting, so she was just ready for me.
Carer 1 (son, ZBI score of 16)
Do participants and carers think that the classes ‘worked’?
Some participants spontaneously offered their view on whether or not they thought the classes ‘worked’ and others were asked directly. With the exception of one participant and one carer, no one reported that they thought the classes had made an impact on their dementia. But, with the exception of one carer, everyone interviewed thought that there had been a positive impact on participants physically and that the class had generally done them some good.
Improving physical health and functioning
Having dementia makes the already difficult act of self-assessment even more difficult, as recent past experiences are lost to memory. Participant 6, in her early 80s and described as frail by other participants, carers and the physiotherapist leading her class, struggled to follow the question of whether or not the classes had worked. When asked if she thought the classes had any impact on her physical health, she said it had:
I never thought there was anything wrong with my physical health I must say, but I do feel that I’m getting along a bit quicker and everything and, I think it could have been my younger son said ‘you’re moving a lot faster’ or something but I’ve always moved fast.
Patient 6 (female, sMMSE score of 24)
Participant 12 and his friend, carer 13, agreed that his ability to walk unaided had improved and the participant seemed to feel better about himself:
I think these classes were very, very good, before I went to them I struggled with my walking . . . I had to have walking sticks and everything. Now I’m walking normally. I don’t like to feel that I’m helpless, I don’t like to feel like . . . a disabled person. People look at you and say ‘oh look at that poor old gentleman’ . . . I don’t want to be old.
Patient 12 (male, sMMSE score of 24)
One carer talked about how the classes had enabled her to change what she felt that she could expect of her husband with dementia and what he could expect of himself. The carer was reassured by his improved physical functioning, as this lessened her worry about whether or not she would be able to manage to continue caring for him:
He was walking really slowly and I used to say to him ‘oh come on move, you can walk better than this’. Well this has made him realise ‘I can do this’, you know and it’s made him much more active and doing things now and . . . because he was getting up like this . . . [demonstrates slow, painful stand up] . . . And I said come on, get up, you can do it and of course in class he has to do this you know. And so when he starts it at home I say you can do it in class you can do it at home. The longer he can stay physically fit the better it will be for me because I’m not going to be able to help him, especially with my neck problem and back problem . . . So it’s a win–win situation as far as I’m concerned. If it hasn’t helped him with his memory well we both understood that, it’s not a waste of time at all.
Carer 3 (wife, ZBI score of 20)
Little or no change on cognitive function
Almost all carers and participants did not think the intervention had made a difference to the participant’s cognitive function. When asked directly about this, participant 12 thought that the classes had helped his memory ‘a little bit’ (participant 12, sMMSE score of 24) but his carer (carer, sMMSE score of 13) disagreed, shaking her head for the researcher. Carer 17 thought that the exercise may have improved participant 16’s memory, influenced by the assessment of the psychiatric nurse:
The psychiatric nurse . . . came 5 weeks ago just before the start of DAPA and she came again last week, and her view is that his memory has definitely improved. Subjective but . . . and I think that it has improved. I can’t tell how much but he seems brighter and seems to be able to remember things that probably were going to be difficult, if not impossible, before.
Carer 17 (wife, ZBI score of 24)
He went on to explain that his wife’s mood had changed to being more positive so the effect of the exercise may have lifted depression symptoms:
One of the effects that the Alzheimer’s was . . . she would clamp on very negative things and to repeat those quite regularly. She would have difficulty in dropping things that had happened in the past but she seems more able to do that now and more able to look at the positive side of things and enjoy things . . . it’s something that is happening, and the only thing that’s different is the exercise class.
Carer 17 (wife, ZBI score of 24)
Those participants who were not sure if there had been any change in their memory were nevertheless positive about their improved physical functioning:
I don’t know whether it’s done my dementia any good but I know now that yes I can lift those heavy weights.
Patient 4 (female, sMMSE score of 21)
Participant 7 talked about physical improvement and then said:
I don’t know whether . . . I’ve been surprised with one or two things I have remembered . . . But no . . . there are other things that still elude you.
Patient 7 (male, sMMSE score of 28)
Of a participant with Parkinson’s disease, carer 9 said about the intervention classes:
It’s good to exercise because his muscles and his tendons will get . . . [tight] . . . I don’t know about mental ability, from my point of view I don’t think they’ve made an awful lot of difference.
Carer 9 (wife, ZBI score of 64)
No change
One carer and the participant did not think that the classes had made any physical difference, explaining that he was fairly active before the classes:
Always just pottering about in the garden or doing something, you know he’s not one to be sitting down all the time.
Carer 11 (wife, ZBI score of 19)
Physiotherapists’ experiences of delivering the intervention
We now consider the experience of the physiotherapists, how they delivered the intervention while negotiating the participant’s dementia symptoms and how they negotiated the label of dementia and its associated stigma.
Dementia symptoms and delivering an exercise intervention
Physiotherapists faced the challenge of engaging participants with dyspraxia, ataxia, memory problems and low mood in exercise. Strategies used by the physiotherapists for dyspraxia included getting the participants to copy or mirror their actions, giving them assistance or verbal feedback and not expecting the participant to perform the movement perfectly. Some participants would copy or mirror their actions all the time, not just for the exercises. Talking about a participant who seemed to be in a world of his own most of the time, the physiotherapist said:
I always noticed that he followed, so if you went forward he went forward, if you sat down he sat, if you stood he stood, which was tricky if you were dealing with someone else . . . Sometimes even though I didn’t want to sit at that moment I’ll sit because I knew that he will sit. And then before he gets to stand, because he’s not as fast as me, I’ll probably quickly walk up to him and whisper in his ear, ‘sit down, I just need to pop over there’, then he will sit.
Physiotherapist 21
Participants found it hard to learn to get on the bikes, even though the physiotherapists would break down a task into its component parts:
I’ve had to physically guide them through the movement. It’s taken them longer to actually get the feel of what we’re asking them to do, but not to the extent that they haven’t fitted in with the class. Getting on and off a bike is something that is not as straightforward as you might think, I mean you can demonstrate it, you can talk them through it and you could actually physically get them to do it, but then they get off. When they get back on again 2 seconds later they really haven’t got it, they don’t know physically what they’re doing.
Physiotherapist 1
The physiotherapists learned to allow for the different abilities of participants to process instructions:
Some participants present quite well but then you start to learn actually that perhaps they’re not fully understanding all that you’re telling them.
Physiotherapist 15
Physiotherapists would give very explicit instructions and avoided talking to participants while exercising so as not to distract them:
[At the end of an exercise] two of the ladies in my class would . . . sit there and hold their weights so you explicitly have to tell them to put them down. Now I don’t know whether that’s a dementia thing or it’s just a politeness thing. It’s interesting to see, and I think the processing thing is a big one . . . it’s not stopping anybody, you have to be aware because they may be one down in their repetition. You can’t talk to them while they’re cycling because if they stop for 5 minutes then that’s 5 minutes of their exercising gone.
Physiotherapist 14
Memory problems posed a number of challenges. If participants did not have a carer living with them, remembering to come to the classes could be an issue. However, one physiotherapist describes this problem as being no different from other classes:
I will make sure they’ve got things written down and that if you’re worried you’ll call them and remind them in that sense, but then I think I would do that anyway even if they weren’t classed as dementia.
Physiotherapist 14
Repetition, talking about the same thing, within the same class or from class to class, was common:
There were some difficulties with their memory, often people will tell me the same thing a lot.
Physiotherapist 21
Although physiotherapists acknowledged that repetition would be difficult for a partner or family member, all five agreed that it was a symptom that they ignored by pretending that every time was the first time the participant has said something. This, it was felt, was the kindest way to react to this:
There’s no reason for them to know that they’ve repeated themselves because it’s embarrassing for someone potentially if they don’t think that their dementia is that bad and, they, people can get quite upset potentially or agitated from knowing. If somebody says have I asked you that already and I would be like yes but don’t worry.
Physiotherapist 14
Another physiotherapist took a similar approach:
Every opportunity he got he went back to [the same] conversation and talked about things he had said before, you just learn to listen and take it in as new information every single time.
Physiotherapist 21
The physiotherapists suggested that ignoring the fact that some participants repeated themselves and, therefore, shielding them from social embarrassment, was a normal part of their role. Participants dealt with the repetition of other participants in a similar way:
[Other participant] is a little bit ahead of me [in dementia progression] because she kept saying every week I made these, these trousers, I made these myself. I just said, every time I said, brilliant that is, you know . . . I wouldn’t have dared to say to her yes we’ve heard it six times now, no I just kept saying that’s marvellous, I don’t know how you do it.
Patient 5 (male, sMMSE score of 19)
Some participants were prone to get lost if they went away from the group:
Quite a few participants we’ve had to accompany, even just to the toilet, just so they don’t get lost.
Physiotherapist 15
At one venue, a participant did not make it into the venue:
The second time she was brought there and she didn’t come into the building, [I spent the] entire time out looking for her and I couldn’t find her. It turns out, she got into a taxi and went home . . . and then she had no recollection of having left the house.
Physiotherapist 21
Some participants were difficult to engage owing to their low mood:
There was a chap in one of the groups who . . . I think he was probably a very introspective man even before he got dementia, but he, um, basically he would go through phases of just extreme brooding on negative events and it was quite difficult to communicate with him at times like that . . . he would do the exercises but . . . someone who is in that sort of psychological state probably needs a lot more supervising.
Physiotherapist 1
When asked if the participant’s low mood interfered with delivery of the intervention, the physiotherapist said it made no difference to the physical exercises but did make a difference to the atmosphere in the group:
It was like a black cloud over the group . . . the behavioural aspects of the intervention, the self-efficacy and the positive feelings around exercise the we’re trying to instil . . . that would have been affected.
Physiotherapist 1
The physiotherapists reported that participants mostly tried to do their best, although needed some reassurance:
You have to reassure people that they’re not actually doing themselves any harm but most people are pretty keen.
Physiotherapist 1
The physiotherapists gave lots of positive feedback to encourage the participants:
I can see them getting fitter and that’s part of how we motivate them, so we’ll say, this week you’re lifting more weights, you know your technique is much better and you’re on the bike for longer, you’re working much harder.
Physiotherapist 21
Navigating the stigma of dementia
During observation and interviews, physiotherapists revealed their awareness of the sensitivities and stigma associated with dementia. The word dementia was not often used, with phrases such as ‘memory problems’ being used instead. The following example is from a physiotherapist describing how she explained to a participant why they were at the class:
You’re here because we’re hoping that the class will help your, because I never want to say condition to a patient just in case they don’t understand but like thinking and memory ability . . . it’s difficult, dementia’s like a stigmatised word without a doubt. Then she said ‘oh yes, I remember, they think I’m doolally’ and I said ‘well it’s not that, it’s maybe that your memory isn’t as good as it was but, um, hopefully the class might help but I can’t promise you’.
Physiotherapist 14
It can be argued that the patient used an even more stigmatising word ‘doolally’ about herself. The other participants seemed to think so, as the following, from the other physiotherapist delivering the same class, suggests:
I don’t usually raise it [dementia] unless they do . . . but in all the groups that I’ve been with they’ve all spoken about it in one form or another, amongst themselves and with us, so it’s not like been taboo. A lady this week said to the group ‘oh, I’m doolally’ and one of the other participants went up to her and said ‘I’ve been diagnosed with Alzheimer’s’, and we were just . . . we were just all sort of pitching in you know this doesn’t mean that you’re doolally. They all suddenly came together as a group, it was really quite nice, and started talking about it.
Physiotherapist 15
Most of the physiotherapists decided whether or not to use the word dementia on a case-by-case basis:
That was a concern for me because I couldn’t really tell where each individual was as far as, you know, their diagnosis was concerned. So if this is someone who hasn’t accepted it, I don’t want to be trying to shove it down their throat, and if this is someone who has, how do I know? So I sort of let them initiate the conversations [until] I had a definite idea of where the individual stood with, you know, dementia in general.
Physiotherapist 21
This same physiotherapist was also concerned about not reinforcing other stigmatising terms:
I find it difficult when you have people who say things like ‘oh yeah I’m a bit cuckoo aren’t I?’ or ‘yeah my memory’s shit’, things like that. Now as much as you get that dementia is a terrible disease I cannot affirm that statement . . . I think I shied away from such conversations and said something like, well let’s hope this helps you know, just to put a very positive vague cloak over it.
Physiotherapist 21
Our data suggest that this was tricky social and emotional terrain for the physiotherapists and that they engaged in emotional work104,105 in order to negotiate it. The physiotherapists talked about how they tried to remember that the participants are more than their symptoms or diagnosis:
I don’t think you have to have this dementia head on, you’re trying to understand them as an individual. That’s no different with somebody with dementia. But I suppose being aware you might need to make some allowances and some adaptations with possibly needing a little bit more time, but again I think that would be the same working with the very, very old age groups.
Physiotherapist 18
Discussion
Participants and carers were positive about the exercise classes, with only a few having negative things to say about them. Participants and carers also enjoyed being with other people having similar experiences and for carers the classes were a welcome change or break from their caring responsibility. Nearly all participants gained in terms of physical fitness but very few experienced a change in cognitive function. However, participants and carers remained positive about the classes. Helping participants maintain their physical function contributed to participants living well with dementia. In lieu of a cure, being able to live well with dementia is increasingly being advocated as a humane and practical response to the disease106–110 along with the concept of ‘active ageing’. 111
Dementia symptoms posed challenges for the physiotherapists trying to enable participants to undertake the physical exercises. However, they developed strategies for dealing with these. What was trickier was negotiating the stigma of the label dementia. Despite increasing public awareness, dementia is still a stigmatised and stigmatising condition. 91,93,95,96,112–122 Our analysis suggests that the physiotherapists were aware of the sensitivities and stigma surrounding the condition and took a cautious approach, allowing the participants to lead them.
Strengths and limitations of the study
The analysis and interpretation of the interview data were not influenced by the results of the trial, as it was completed 6 months before the trial outcome was available. We captured perspectives from all three groups of stakeholders involved in the trial. Although the number of participants who were interviewed, eight participants and seven carers, is relatively small even for a qualitative study, interviews were long and participants were engaged with and observed over several hours from five sites. Participants were able to contribute their views despite their impaired cognitive function. However, these interviews were challenging because of the idiosyncratic way in which participants responded to questions. Very often participants would go off on long tangents. Gauging whether or not participants understood the question, or if their responses were excessively compliant, was sometimes difficult during the interview, especially when participants’ answers were short. However, some answers were extensive and nuanced. There were no participants who struggled to recall the classes, but many participants found it difficult to assess changes to their physical and mental health. Although interviewing participants was not straightforward, this research enabled participants to give voice to their own experiences. 94,116,118,123
Our sample did not include anyone from an ethnic minority. We had no interviewees who lived without a carer. Participants and carers for whom the burden of dementia was becoming too much may not have agreed to interview. We failed to interview participants who did not commence the intervention.
Given that the participants and carers knew the purpose of the exercise class when asked directly whether or not it had worked, we would expect responses to be influenced by what might be considered socially desirable – the idea that the intervention had made a difference or at least the desire to tell the trial team it had. A few participants tried to suggest there had been change but, apart from the participant whose depression symptoms improved, there was consensus that it had not worked – there was no improvement in cognitive function.
Conclusion
Told from the perspectives of participants, carers and physiotherapists, we have learned that:
-
participants did not experience an improvement in cognitive function, except one who seemed to experience improvement in depression symptoms accompanied by some improvement in memory
-
all participants, with the exception of one, experienced a worthwhile improvement in physical function
-
participants enjoyed being with other people at the classes
-
carers appreciated the classes as it gave them a break from their caring responsibilities, they met other people in a similar situation and they saw the person they were caring for enjoying the classes and improving in physical fitness
-
the physiotherapists faced challenges helping participants with dementia symptoms undertake the exercises but solved them pragmatically; it was difficult for them to negotiate the stigma of the label dementia and they approached this case by case, being sensitive to how the participant talked about their condition.
Attending physical exercise classes contributed to the ability of participants and carers to live well with dementia but they did not experience improvement in cognitive function.
Chapter 5 Economic evaluation
Overview of economic evaluation
A prospective economic evaluation was conducted alongside the RCT with the objective of estimating the cost-effectiveness of a 4-month supervised moderate- to high-intensity exercise training regime and ongoing supported physical activity programme (exercise) compared with usual care. The economic evaluation took the form of a cost–utility analysis, expressed in terms of incremental cost per quality-adjusted life-year (QALY) gained. The primary analysis is based on a NHS and personal social services (PSS) perspective, as recommended by NICE, and excludes broader societal costs (e.g. by families or informal carers) associated with the exercise programme. 124 A sensitivity analysis was conducted to recalculate cost-effectiveness from a societal perspective.
Measurement of resource use and costs
The incremental costs associated with the exercise programme were determined through a comprehensive strategy that encompassed two strands of research: the estimation of (1) costs associated with the delivery of the intervention and (2) broader health and personal social service resource inputs and costs.
Costing of the exercise (intervention) programme
A specific focus of the economic evaluation was the assessment of the cost of delivering the exercise programme, including the costs of training of accredited health-care professionals and the cost of delivering group sessions, participant monitoring activities and any follow-up/management. This involved asking physiotherapists and exercise assistants in each site to prospectively complete, in detail, weekly activity logs reporting the number of hours spent delivering each exercise session, including preparation time, programme delivery time, indirect administrative activities, telephone contacts, as well as intervention-related training and supervision activities costs. The type of travel (e.g. car, taxi), distance travelled and time spent travelling by each physiotherapist and exercise assistant as a result of intervention-related activities were reported on a weekly basis. Additional expenditures associated with the exercise equipment, treatment manual and related paperwork were also recorded. The costs of venue hire were estimated separately for each site. When venue hire costs were not available or when venues were made available for use free of charge by providers, the mean venue hire cost from the remaining sites was applied. The total costs of delivering the exercise programme in terms of the average (mean) cost per session per attending participant for each group within each site were estimated (at the group level). For the baseline analysis, estimates of average cost per session per attending participant excluded practitioner travel costs, as it was assumed that delivery of the exercise programme in routine NHS settings would not result in additional travel costs by physiotherapists and exercise assistants. However, the effects of this assumption were tested in the sensitivity analyses.
Collection of broader resource-use data
Data were collected on broader health and personal social service and broader societal resource inputs, between randomisation and 12 months post randomisation, that were deemed relevant. Trial participants were required to complete resource-use questionnaires via face-to-face interviews (researcher administered) at baseline and at 6 and 12 months post randomisation using a modified version of the CSRI (version 1.0),125 that was modified based on the experiences of the economic evaluation that was conducted alongside the Donepezil and Memantine for Moderate-to-Severe Alzheimer's Disease (DOMINO-AD) trial. 126 The data collected from participants at each assessment point covered their use of sheltered housing or care home accommodation, hospital care and day care services, community-based health care, community-based social care, medicines, aids and equipment. Details of travel costs (borne by trial participants or their family members or friends) owing to the trial participants’ health status or contacts (visits) with health or social services over the relevant time horizons were also collected. Medication use was categorised by drug name (or active constituent), mode of administration, dose frequency and duration.
Valuation of resource use
Resource inputs were valued using a combination of primary research and data collated from secondary national tariff sets, using standard accounting methods. Staff time (indirect or direct) for the delivery of the exercise programme was determined from hourly unit costs for each Agenda for Change band. 127 Unit cost estimates for staffing inputs were inclusive of staff salaries, qualification costs, employer’s on-costs and associated revenue and capital overheads. Costs relating to travel (for each mile) for practitioners delivering the exercise intervention were determined from the Automobile Association (AA) for travel by car,128 and estimates published in the Department for Transport’s Public Service Vehicle Survey: Bus Statistics for travel by public transport. 129 Inpatient admissions during the study (overall and by type) were determined from NHS reference cost trust schedules. 130 Other hospital-based care costs were valued by applying unit costs from national tariffs. 131 NHS medication prices (per mg) were obtained from the NHS Digital’s drug costs. 132 Participant-level costs for medication use were estimated based on reported doses and frequencies, when available, or otherwise based on an assumed daily dose. Gender-specific median earnings data were applied to occupational classifications derived from self-reported work status information to determine the costs of time taken off work by trial participants (or by their family members or carers). In addition, data reported by the participants as part of the follow-up resource-use questionnaires were used to determine other family-borne costs. The NHS Hospital and Community Health Services Pay and Prices Index was used to inflate or deflate costs when necessary to 2014–15 prices (GBP). 127 No discounting of costs was applied because the cost-effectiveness of the exercise programme was determined over a 1-year time horizon (Table 35).
| Resource item | Unit cost (£) | Unit of analysis | Source |
|---|---|---|---|
| Intervention arm | |||
| Physiotherapist grade | |||
| 5 | 36.00 | Per hour | Unit Costs of Health and Social Care 2014,134 p. 179 |
| 6 | 44.00 | Per hour | Unit Costs of Health and Social Care 2014,134 p. 179 |
| 7 | 52.00 | Per hour | Unit Costs of Health and Social Care 2014,134 p. 179 |
| 8 | 62.00 | Per hour | Unit Costs of Health and Social Care 2014,134 p. 179 |
| Exercise assistant grade | |||
| 3 | 25.00 | Per hour | Unit Costs of Health and Social Care 2014,134 p. 179 |
| 4 | 28.00 | Per hour | Unit Costs of Health and Social Care 2014,134 p. 179 |
| 5 | 36.00 | Per hour | Unit Costs of Health and Social Care 2014,134 p. 179 |
| 6 | 45.00 | Per hour | Unit Costs of Health and Social Care 2014,134 p. 179 |
| 7 | 54.00 | Per hour | Unit Costs of Health and Social Care 2014,134 p. 179 |
| Venue hire | Range: 23.00–80.00 | Per session | From venue list price |
| Equipmenta | Range 0.44–335.70 | Fixed | NHS Supply Chain. NHS Supply Chain 2015. URL: https://my.supplychain.nhs.uk/catalogue (accessed 7 June 2016) |
| Patient accommodation | |||
| Extra care housing | 455.00 | Per week | Unit Costs of Health and Social Care 2015,127 p. 41 |
| Care home with nursing care | 1110.00 | Per week | Unit Costs of Health and Social Care 2015,127 p. 39 |
| Care home with personal care | 1134.00 | Per week | Unit Costs of Health and Social Care 2015,127 p. 39 |
| Acute psychiatric ward | 252.90 | Per day | Unit Costs of Health and Social Care 2010,133 p. 70 |
| Rehabilitation ward | 636.00 | Per bed-day | Unit Costs of Health and Social Care 2015,127 p. 1104 |
| Rehabilitation ward | 17,358.10 | Per year | Unit Costs of Health and Social Care 2014,134 p. 119 |
| General medical ward | 400.00 | Per day | Department of Health and Social Care communication, data set request, 2015. URL: https://data.gov.uk/data-request/nhs-hospital-stay (accessed 1 October 2016) |
| Hospital services | |||
| Continuing care respite | |||
| Inpatient | 635.60 | Per week | Scottish Government. Respite Care, Scotland 2014, page 3. URL: www.gov.scot/Resource/0046/00461672.pdf (accessed 7 June 2016) |
| 743.60 | Per 4 days | NHS Reference Costs 2014–2015,130 p. 6 | |
| Other hospital inpatient ward | 403.60 | Per day | Department of Health and Social Care communication, data set request, 2014–15. URL: https://data.gov.uk/data-request/nhs-hospital-stay (accessed 1 October 2016) |
| Outpatient services | 112.00 | Per visit | Unit Costs of Health and Social Care 2015,127 p. 107 |
| Accident and emergency department | 133.20 | Per visit | NHS Reference Costs 2014–2015 130 |
| Day hospital | 113.00 | Per visit/day | Unit Costs of Health and Social Care 2014,134 p. 115 |
| Day care services | |||
| LA social services | |||
| Day care | 59.50 | Per visit | Unit Costs of Health and Social Care 2014 134 |
| 13.10 | Per hour | Unit Costs of Health and Social Care 2014 134 | |
| 45.40 | Per 3.5 hours | Unit Costs of Health and Social Care 2014 134 | |
| Voluntary and private | |||
| Day care | 41.40 | Per visit | Unit Costs of Health and Social Care 2014 134 |
| 10.20 | Per hour | Unit Costs of Health and Social Care 2014 134 | |
| 35.30 | Per 3.5 hours | Unit Costs of Health and Social Care 2014 134 | |
| NHS (not hospital) | |||
| Day care† | 36.00 | Per hour | Unit Costs of Health and Social Care 2015,127 section 9, p. 164, section 1.6; scientific and professional staff, assumed to be band 5 |
| Lunch club† | 12.00 | Per day | Age UK. Milton Keynes Age UK; 2016. URL: www.ageuk.org.uk/miltonkeynes/activities-and-events1/lunch-clubs/ (accessed 7 June 2016) |
| Social clubb,† | 12.30 | Per year | Age UK. Nuneaton 050 Friendship Centre; 2016. URL: www.ageuk.org.uk/about-us/local-services-search/groups/nuneaton-050-friendship-centre-england (accessed 7 June 2016) |
| General health community services | |||
| Geriatrician | |||
| Office | 134.20 | Per contact | Unit Costs of Health and Social Care 2015,127 p. 199 |
| GP | |||
| Office | 44.40 | Per 11-minute contact | General practitioner – Unit Costs of Health and Social Care 2015,127 p. 177, 10.8b |
| Home | 90.40 | Per home visit | General practitioner – Unit Costs of Health and Social Care 2015,127 p. 177, 10.8b |
| Practice nurse | |||
| Office | 43.40 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 174, 10.6 |
| Home | 56.50 | Per contact | Unit Costs of Health and Social Care 2015,127 p. 174, 10.6 |
| District nurse | |||
| Office | 50.40 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 169, 10.1 |
| Home | 67.60 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 169, 10.1 |
| Health visitor | |||
| Office | 50.40 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 171, 10.3 |
| Home | 76.70 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 171, 10.3 |
| Incontinence nurse | |||
| Office | 50.40 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 172, 10.4 |
| Home | 75.70 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 172, 10.4 |
| Occupational therapist | |||
| Office – hospital | 38.30 | Per hour | Unit Costs of Health and Social Care 2014,134 p. 218, 13.2 |
| Office – community | 36.30 | Per hour | Unit Costs of Health and Social Care 2014,134 p. 180, 9.2 |
| Home† | 48.30 | Per hour | Unit Costs of Health and Social Care 2010,133 p. 152, 9.1 |
| Physiotherapist | |||
| Office – hospital | 38.30 | Per hour | Unit Costs of Health and Social Care (2014–2015), p. 217, 13.1 |
| Office – community | 36.30 | Per hour | Unit Costs of Health and Social Care 2014,134 p. 179, 9.2 |
| Home† | 44.00 | Per hour | Unit Costs of Health and Social Care 2010,133 p. 151, 9.1 |
| Alternative medicine therapist | |||
| Office† | 31.50 | Per contact | The Role of Complementary and Alternative Medicine in the NHS. An Investigation into the Potential Contribution of Mainstream Complementary Therapies to Healthcare in the UK,135 figure 7. URL: www.getwelluk.com/uploadedFiles/Publications/SmallwoodReport.pdf (accessed 7 June 2016) |
| Home† | 31.50 | Per contact | The Role of Complementary and Alternative Medicine in the NHS. An Investigation into the Potential Contribution of Mainstream Complementary Therapies to Healthcare in the UK,135 figure 7 |
| Mental health service | |||
| CPN/CMHN | |||
| Office | 42.40 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 199, 12.1 |
| Home | 74.70 | Per contact | Unit Costs of Health and Social Care 2015,127 p. 188 |
| Community psychiatrist | |||
| Office | 77.80 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 18 |
| Psychologist | |||
| Office | 61.50 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 183, 9–5 |
| Home | 139.20 | Per contact | Unit Costs of Health and Social Care 2015,127 p. 183, 9–5 |
| Social care services | |||
| Care manager | |||
| Office | 39.30 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 211 |
| Home | 39.30 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 211 |
| Social worker | |||
| Office | 68.60 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 205 |
| Home | 93.80 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 205 |
| Home care worker | |||
| Office | 19.20 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 192, 11.6 |
| Home | 24.20 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 192, 11.6 |
| Care support worker | |||
| Office | 24.20 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 223, 13.7 |
| Home | 24.20 | Per hour | Unit Costs of Health and Social Care 2015,127 p. 223, 13.7 |
| Chiropodist | |||
| Office | 32.30 | Per contact | Unit Costs of Health and Social Care 2015,127 p. 182, 9.4 |
| Home | 42.40 | Per contact | Unit Costs of Health and Social Care 2015,127 p. 182, 9.4 |
| Sitting scheme | |||
| Home† | 10.10 | Per hour | Northallerton & District Voluntary Service Association,136 2016. URL: www.ndvsa.co.uk/index.php/carers-respite-sitting-scheme (accessed 7 June 2016) |
| Meals on wheels | |||
| Home | 46.40 | Per week | Unit Costs of Health and Social Care 2015,127 p. 118 |
| Laundry service | |||
| Home† | 9.00 | Per hour | Laing.137 URL: www.laingbuisson.co.uk/portals/1/media_packs/Fact_Sheets/Fair_Price_ThrdEd_2008.pdf (accessed 7 June 2016) |
| Self-help group | |||
| Office – health service | 36.30 | Per contact | Unit Costs of Health and Social Care 2015,127 p. 118 |
| Office – local authority† | 5.00 | Per week | Shropshire Community Directory, 2016. URL: http://search3.openobjects.com/kb5/shropshire/cd/view.page?record%20=%20796AeasoHso (accessed 7 June 2016) |
| Office – voluntary† | 5.00 | Per week | Assumed to be the same as local authority |
| Office – private† | 5.00 | Per week | Not identifiable – assumed to be same as local authority |
| Office – health service† | 5.00 | Per contact | Assumed to be the same as for local authority support self-help group |
| Private health services | |||
| GP | |||
| Office –private | 70.00 | 15-minute contact | Bupa,138 2015. URL: www.bupa.co.uk/health/bupa-on-demand/gp-services (accessed 1 October 2016) |
| Nurse | 65.10 | Per contact | Assume 50% above NHS unit cost Unit Costs of Health and Social Care 2015,127 p. 174 |
| Physiotherapist | |||
| Office | 55.00 | Per contact/session | Nuffield Health,139 2016. URL: www.nuffieldhealth.com/physiotherapy/faqs (accessed 1 October 2016) |
| Home† | 65.00 | Per contact/session | London Home Visit Physiotherapy,140 2015. URL: www.londonhomevisitphysiotherapy.com/about-us/fees/ (accessed 1 October 2016) |
| Community psychiatrist | 150.00 | Per hour | Psychiatry UK, 2016.141 URL: www.psychiatry-uk.com/fees/ (accessed 1 October 2016) |
| Equipment and adaptations | |||
| Various aidsc | Range: 4.80–930.00 | Per item | NHS Supply Chain. NHS Supply Chain 2015. URL: https://my.supplychain.nhs.uk/catalogue/ (accessed 7 June 2016) |
| Medications | |||
| Various medications | Range: 0.02–207.00 | Per dose | NHS Digital,132 2015 |
| Other | |||
| Various itemsd | Range: 13.10–134.20 | Per visit/contact | Provider sources (e.g. website) |
| Time taken off work | |||
| National average | 530.00 | Per 37.5-hour week | Office for National Statistics,142 2015. URL: www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2015provisionalresults (accessed 1 October 2016) |
| Parking | Range: 1.00–3.00 | Per hour | Participant reported |
| Other | 34.00–88.00 | Per contact | Participant reported |
Calculation of utilities and quality-adjusted life-years
The economic evaluation estimated QALY profiles for trial participants, based on participant and carer reports of preference-based HRQoL outcomes. The HRQoL of trial participants was assessed using the EQ-5D-3L,143 measured at baseline and at 6 and 12 months post randomisation, as a secondary outcome of the trial. The EQ-5D-3L consists of two principal measurement components. The first is a descriptive system, which defines HRQoL across five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Responses in each dimension are categorised into three ordinal levels, namely (1) no problems, (2) some or moderate problems and (3) severe or extreme problems. For the purposes of the economic evaluation, the UK time trade-off tariff was applied to each set of responses to generate an EQ-5D-3L utility score (preference weight) for each trial participant. 143 Resulting utility scores range from –0.59 to 1.0, with 0 representing death and 1.0 representing full health; values below 0 are indicative of health states worse than death. The second measurement component of the EQ-5D-3L consists of a 20-cm vertical VAS ranging from 100 (best imaginable health state) to 0 (worst imaginable health state), which provided an indication of the respondent’s assessment of the trial participant’s health status on the day of the survey. QALYs were calculated as the area under the baseline-adjusted utility curve, and were calculated using linear interpolation between baseline and between 6 and 12 months post-randomisation utility scores. No discounting of QALYs was applied because cost-effectiveness was determined over a 1-year time horizon.
Missing data
Multiple imputation (MI) using the method of chained equations was used for the base-case analysis to impute missing data. This avoids potential biases associated with complete-case analysis and is consistent with good practice guidance. MI was used at the aggregate level (i.e. on missing total costs or QALYs). When data are missing at random, MI provides unbiased estimates of treatment effect. The missing at random assumption was explored in the data, using logistic regression for missingness of total costs and QALYs for each time point (separately) as a function of baseline variables. A model was used to generate multiple imputed data sets for treatment groups, in which missing values were estimated conditional on available covariates, which included baseline costs, baseline utilities, age, gender and baseline sMMSE score (< 20; ≥ 20). With MI, a complete data set is generated, reflecting the distributions and correlations between variables in the observed data. Mean matching, using predictive methods, was used to improve estimates of imputed values as normality could not be assumed. Each imputed data set was analysed independently using model-based approaches; the estimates obtained were pooled to generate mean and variance estimates of costs and QALYs using Rubin’s rule in order to capture within and between variances for imputed samples. Information loss from finite imputation sampling was minimised using 20 data sets, resulting in minimal loss of efficiency (< 0.5%) when compared with infinite sampling. As the fraction of information missing was reasonably low, n = 20 imputation sets were considered adequate. 144 Imputed and observed values were compared to establish that imputation did not introduce bias into subsequent estimation.
Analyses of resource use, costs and outcome data
Resource-use items were summarised by treatment group and assessment point; differences between groups were analysed using two-sample t-tests for continuous variables. Mean [standard error (SE)] values of each resource use and cost type were estimated by trial allocation group for each time period. Costs were estimated from a NHS and PSS perspective for the baseline analysis. This was also repeated from a broader societal perspective for a separate sensitivity analysis. Differences between trial groups in terms of costs, along with their respective CIs, were estimated and reported. Non-parametric bootstrap estimates using 10,000 replications124 were also calculated for differences along with their respective CIs. For the EQ-5D-3L dimension, the proportion of participants with suboptimal levels of function (moderate problems or severe or extreme problems) at each assessment point were compared between trial groups using the chi-squared (χ2) test. EQ-5D-3L utility score differences at each follow-up point between the trial groups were tested using two-sample t-tests for unequal variance.
Regression-based methods, namely, seemingly unrelated regression, were used to estimate mean incremental changes in costs and QALYs and these accounted for the correlation between costs and outcomes within the data while adjusting for covariates, including baseline costs and utility scores to adjust for potential baseline imbalances. Non-parametric bootstrap methods were used to generate the joint distributions of costs and outcomes to populate the cost-effectiveness plane. Bootstrapping (specifically bias-corrected non-parametric bootstrapping) is a resampling method which jointly resamples costs and outcomes from the observed data while maintaining the sample correlation structure. From each bootstrap sample (10,000 samples in our analyses), differences in costs and QALYs were estimated. Mean estimates are reported with their 95% CIs.
Cost-effectiveness analyses
The incremental cost-effectiveness ratio (ICER) was estimated as the difference between the trial comparators in mean total costs divided by the difference in mean total QALYs. Value for money was determined by comparing the ICER with a cost-effectiveness threshold value; typically, the NICE cost-effectiveness threshold for British studies ranges between £20,000 and £30,000 per QALY. In addition, a £15,000 cost-effectiveness threshold was used to reflect recent evidence around the declining value of the cost-effectiveness threshold. 145 This represents society’s willingness to pay for an additional QALY; lower ICER values than the threshold could be considered cost-effective for use in the NHS. Base-case assumptions were explored using a range of supportive sensitivity analyses.
The incremental net monetary benefit (INMB) of switching to the exercise programme was also reported as a recalculation of the ICER at a range of cost-effectiveness thresholds. The INMB succinctly describes the resource gain (or loss) when investing in a new intervention when resources can be used elsewhere at the same threshold. INMB estimates were used to generate cost-effectiveness acceptability curves. The cost-effectiveness acceptability curve illustrates the likelihood that interventions are cost-effective as the cost-effectiveness threshold varies.
All statistical analyses and cost-effectiveness modelling were conducted in SAS® software version 9.4 on a Windows platform. SAS and all other SAS Institute Inc., product or service names are registered trademarks or trademarks of SAS Institute Inc., in the USA and other countries. The symbol ® indicates USA registration.
Sensitivity and subgroup analyses
Several sensitivity analyses were undertaken to assess the impact on the base-case economic evaluation. The following sensitivity analyses were undertaken in line with the trial’s prespecified health economics analysis plan (version 4, 5 May 2015):
-
restricting the analyses to complete cases (i.e. those with complete cost and outcome data throughout the trial time horizon)
-
adopting a wider societal perspective that included costs incurred by all sectors of the economy and by families and informal carers
-
recalculating the trial participants’ QALY profiles using the carer-reported EQ-5D-3L scores
-
recalculating the average cost per exercise session per attending participant by taking into account practitioner travel costs
-
varying the cohort size for the exercise programme to the lowest number of participants attending across all groups (n = 3)
-
varying the cohort size for the exercise programme to the highest number of participants attending across all groups (n = 10)
-
setting the venue hire costs to zero on the assumption that delivery of the exercise programme in routine NHS settings rather than community venues may be associated with zero opportunity costs.
Prespecified subgroup analyses were also conducted for the main cost-effectiveness results to explore heterogeneity in the trial population. These were conducted by:
-
gender (male, female)
-
baseline sMMSE score (< 20, ≥ 20).
Results
Study population
A total of 494 participants were randomised into the trial: 329 to the exercise programme (experimental arm) and to usual care (control arm). Complete baseline information was available for 488 participants (exercise arm, n = 326; control arm, n = 162). Consequently, the baseline study population for the bulk of the health economic analyses is 488 participants. Between 91% and 99% of all health resource-use data were complete at baseline for the exercise group; for the usual-care group, this ranged between 91% and 98% (Table 36). Similarly, these values ranged between 84% and 91% and 78% to 86% at 6 months; and between 77% and 85% and 78% and 82% at 12 months, for exercise and usual care, respectively. A complete QALY profile was available for 435 (88%) participants based on the patient-reported EQ-5D-3L (84% for the carer-reported EQ-5D-3L).
| Time point | Treatment arm, n (%) | |||||
|---|---|---|---|---|---|---|
| Exercise programme (N = 329) | Usual care (N = 165) | |||||
| Completeda | Missingb | Unavailablec | Completed | Missing | Unavailable | |
| Q2 patient accommodation | ||||||
| Baseline | 326 (99.09) | 0 (0.00) | 3 (0.91) | 162 (98.18) | 0 (0.00) | 3 (1.82) |
| 6 months | 298 (90.58) | 1 (0.30) | 30 (9.12) | 142 (86.06) | 1 (0.61) | 22 (13.33) |
| 12 months | 280 (85.11) | 2 (0.61) | 47 (14.29) | 136 (82.42) | 0 (0.00) | 29 (17.58) |
| Q5a hospital services | ||||||
| Baseline | 327 (99.09) | 1 (0.30) | 2 (0.61) | 162 (98.18) | 0 (0.00) | 3 (1.82) |
| 6 months | 298 (90.58) | 1 (0.30) | 30 (9.12) | 141 (85.45) | 2 (1.21) | 22 (13.33) |
| 12 months | 280 (85.11) | 2 (0.61) | 47 (14.29) | 135 (81.82) | 1 (0.61) | 29 (17.58) |
| Q5c day services | ||||||
| Baseline | 325 (98.78) | 2 (0.61) | 2 (0.61) | 162 (98.18) | 0 (0.00) | 3 (1.82) |
| 6 months | 298 (90.58) | 1 (0.30) | 30 (9.12) | 141 (85.45) | 2 (1.21) | 22 (13.33) |
| 12 months | 280 (85.11) | 2 (0.61) | 47 (14.29) | 135 (81.82) | 1 (0.61) | 29 (17.58) |
| Q5e general community health services | ||||||
| Baseline | 326 (99.09) | 1 (0.30) | 2 (0.61) | 162 (98.18) | 0 (0.00) | 3 (1.82) |
| 6 months | 297 (90.27) | 2 (0.61) | 30 (9.12) | 141 (85.45) | 2 (1.21) | 22 (13.33) |
| 12 months | 280 (85.11) | 2 (0.61) | 47 (14.29) | 135 (81.82) | 1 (0.61) | 29 (17.58) |
| Q5g community mental health services | ||||||
| Baseline | 326 (99.09) | 1 (0.30) | 2 (0.61) | 162 (98.18) | 0 (0.00) | 3 (1.82) |
| 6 months | 298 (90.58) | 1 (0.30) | 30 (9.12) | 141 (85.45) | 2 (1.21) | 22 (13.33) |
| 12 months | 280 (85.11) | 2 (0.61) | 47 (14.29) | 135 (81.82) | 1 (0.61) | 29 (17.58) |
| Q5i social care services | ||||||
| Baseline | 326 (99.09) | 1 (0.30) | 2 (0.61) | 161 (97.58) | 1 (0.61) | 3 (1.82) |
| 6 months | 298 (90.58) | 1 (0.30) | 30 (9.12) | 141 (85.45) | 2 (1.21) | 22 (13.33) |
| 12 months | 280 (85.11) | 2 (0.61) | 47 (14.29) | 135 (81.82) | 1 (0.61) | 29 (17.58) |
| Q6a equipment, adaptations | ||||||
| Baseline | 325 (98.78) | 1 (0.30) | 3 (0.91) | 162 (98.18) | 0 (0.00) | 3 (1.82) |
| 6 months | 298 (90.58) | 1 (0.30) | 30 (9.12) | 141 (85.45) | 2 (1.21) | 22 (13.33) |
| 12 months | 280 (85.11) | 2 (0.61) | 47 (14.29) | 136 (82.42) | 0 (0.00) | 29 (17.58) |
| Q7a medications | ||||||
| Baseline | 326 (99.09) | 0 (0.00) | 3 (0.91) | 162 (98.18) | 0 (0.00) | 3 (1.82) |
| 6 months | 297 (90.27) | 2 (0.61) | 30 (9.12) | 141 (85.45) | 2 (1.21) | 22 (13.33) |
| 12 months | 280 (85.11) | 2 (0.61) | 47 (14.29) | 135 (81.82) | 1 (0.61) | 29 (17.58) |
| Q8 patient/carer travel | ||||||
| Baseline | 326 (99.09) | 0 (0.00) | 3 (0.91) | 162 (98.18) | 0 (0.00) | 3 (1.82) |
| 6 months | 297 (90.27) | 2 (0.61) | 30 (9.12) | 141 (85.45) | 2 (1.21) | 22 (13.33) |
| 12 months | 280 (85.11) | 2 (0.61) | 47 (14.29) | 135 (81.82) | 1 (0.61) | 29 (17.58) |
| Q9d given up or cut down on work | ||||||
| Baseline | 303 (92.10) | 23 (6.99) | 3 (0.91) | 153 (92.73) | 9 (5.45) | 3 (1.82) |
| 6 months | 278 (84.50) | 21 (6.38) | 30 (9.12) | 131 (79.39) | 12 (7.27) | 22 (13.33) |
| 12 months | 256 (77.81) | 26 (7.90) | 47 (14.29) | 128 (77.58) | 8 (4.85) | 29 (17.58) |
| Q10c time off work | ||||||
| Baseline | 299 (90.88) | 27 (8.21) | 3 (0.91) | 150 (90.91) | 12 (7.27) | 3 (1.82) |
| 6 months | 277 (84.19) | 22 (6.69) | 30 (9.12) | 129 (78.18) | 14 (8.48) | 22 (13.33) |
| 12 months | 255 (77.51) | 27 (8.21) | 47 (14.29) | 128 (77.58) | 8 (4.85) | 29 (17.58) |
| EQ-5D-3L index (patient) | ||||||
| Baseline | 321 (97.56) | 6 (1.82) | 2 (0.61) | 159 (96.34) | 2 (1.21) | 4 (2.42) |
| 6 months | 288 (87.54) | 11 (3.34) | 30 (9.12) | 137 (83.03) | 7 (4.24) | 21 (12.73) |
| 12 months | 255 (77.51) | 26 (7.90) | 48 (14.59) | 124 (75.15) | 12 (7.27) | 29 (17.58) |
| EQ-5D-3L index (carer) | ||||||
| Baseline | 302 (91.79) | 2 (0.61) | 25 (7.59) | 153 (92.73) | 1 (0.61) | 11 (6.67) |
| 6 months | 276 (83.89) | 4 (1.22) | 49 (14.89) | 132 (80.00) | 4 (2.42) | 29 (17.58) |
| 12 months | 261 (79.33) | 2 (0.61) | 66 (20.06) | 129 (78.18) | 0 (0.00) | 36 (21.82) |
Resource use and costs
Cost of intervention
Estimates of the total costs of delivering the exercise programme are provided in Tables 37 and 38 for each group within each study site. The cost components are aggregated into four headings, namely (1) staff costs, inclusive of training activities, planning, direct delivery, administrative activities, meetings with professionals, telephone calls and supervision activities associated with group delivery, (2) travel costs, based on distances travelled by practitioners by mode of transport, (3) venue hire costs and (4) equipment and other costs for each site, including cost of belts, stopwatches, timers, cones, lap counters, compact discs, stationery (e.g. pens, erasers) and trial manuals, associated with group delivery. Total intervention costs are also presented within each group within each site. These varied between £4443.90 (Worcester, cohort 53) and £11,342 (Wolverhampton, cohort 50).
| Site | Cohorta | Costs (£) | |||||
|---|---|---|---|---|---|---|---|
| Staff | Travelb | Venue | Equipmentc | Totald | Total (including travel) | ||
| Gloucestershire & Herefordshire | 1 | 5640.00 | 2915.20 | 1194.80 | 109.60 | 6944.40 | 9859.60 |
| Gloucestershire & Herefordshire | 2 | 5640.00 | 1027.90 | 1194.80 | 96.10 | 6930.90 | 7958.80 |
| Abingdon | 3 | 4702.40 | 2152.90 | 2400.00 | 112.30 | 7214.70 | 9367.60 |
| Amersham | 4 | 5085.00 | 3657.60 | 713.00 | 142.20 | 5940.20 | 9597.80 |
| Amersham | 5 | 5997.80 | 3476.60 | 782.00 | 124.20 | 6904.00 | 10,380.60 |
| Amersham | 6 | 7971.60 | 5559.50 | 667.00 | 121.60 | 8760.20 | 14,319.70 |
| Atrium | 7 | 6144.60 | 621.20 | 1450.00 | 84.10 | 7678.70 | 8299.90 |
| Atrium | 8 | 7403.80 | 970.10 | 2500.00 | 195.50 | 10,099.30 | 11,069.40 |
| Atrium | 9 | 6273.60 | 938.80 | 1500.00 | 72.30 | 7845.90 | 8784.70 |
| Atrium | 10 | 6656.80 | 2030.40 | 1600.00 | 132.20 | 8389.00 | 10,419.40 |
| Atrium | 11 | 6947.80 | 907.60 | 1450.00 | 96.10 | 8493.90 | 9401.50 |
| Atrium | 12 | 6087.60 | 907.60 | 1600.00 | 118.10 | 7805.70 | 8713.30 |
| Aylesbury | 13 | 4523.30 | 1927.10 | 775.00 | 114.90 | 5413.20 | 7340.30 |
| Banbury | 14 | 6473.50 | 3547.40 | 1710.00 | 133.40 | 8316.90 | 11,864.30 |
| Bromsgrove | 15 | 5594.80 | 2937.50 | 1740.00 | 84.10 | 7418.90 | 10,356.40 |
| Bromsgrove | 16 | 7223.80 | 2462.60 | 1740.00 | 83.30 | 9047.10 | 11,509.70 |
| Daventry | 17 | 8358.30 | 3231.80 | 1929.70 | 133.40 | 10,421.40 | 13,653.20 |
| Exeter | 18 | 5307.80 | 5716.20 | 1194.80 | 109.60 | 6612.20 | 12,328.40 |
| High Wycombe | 19 | 4047.50 | 1941.80 | 2320.00 | 109.60 | 6477.10 | 8418.90 |
| Jubilee | 20 | 6111.00 | 1743.90 | 1720.00 | 108.80 | 7939.80 | 9683.70 |
| Jubilee | 21 | 5594.30 | 909.80 | 1200.00 | 59.10 | 6853.40 | 7763.20 |
| Jubilee | 22 | 5521.00 | 909.80 | 1160.00 | 121.60 | 6802.60 | 7712.40 |
| Kenilworth | 23 | 6824.30 | 1546.60 | 1550.00 | 114.90 | 8489.20 | 10,035.80 |
| Kettering | 24 | 5742.90 | 3310.90 | 1359.60 | 120.20 | 7222.80 | 10,533.60 |
| Loughborough | 25 | 6664.60 | 4238.00 | 2310.00 | 134.70 | 9109.40 | 13,347.30 |
| Maidenhead | 26 | 4087.80 | 1428.20 | 1682.00 | 96.90 | 5866.70 | 7294.90 |
| Maidenhead | 27 | 6207.50 | 2839.50 | 1682.00 | 121.60 | 8011.10 | 10,850.60 |
| Melton Mowbray | 28 | 5535.30 | 3368.40 | 1162.90 | 71.40 | 6769.60 | 10,138.00 |
| North East London | 29 | 7011.00 | 7490.00 | 1277.20 | 114.90 | 8403.10 | 15,893.10 |
| North East London | 30 | 6362.00 | 892.50 | 1318.40 | 75.80 | 7756.20 | 8648.70 |
| Newbury | 31 | 5682.10 | 1982.70 | 1240.00 | 101.30 | 7023.40 | 9006.10 |
| Northampton | 32 | 6839.00 | 4002.20 | 1750.00 | 171.70 | 8760.70 | 12,762.90 |
| Northampton | 33 | 6439.20 | 2797.20 | 1450.00 | 96.10 | 7985.30 | 10,782.50 |
| Northampton | 34 | 6244.10 | 3915.50 | 1700.00 | 139.20 | 8083.30 | 11,998.80 |
| Oxford Brookes | 35 | 5345.60 | 4506.90 | 2030.00 | 84.10 | 7459.70 | 11,966.60 |
| Reading | 36 | 7768.10 | 3049.40 | 1740.00 | 84.10 | 9592.20 | 12,641.60 |
| Salford | 37 | 6931.00 | 3412.30 | 1194.80 | 109.60 | 8235.40 | 11,647.70 |
| Salford | 38 | 5711.20 | 2514.60 | 1194.80 | 96.10 | 7002.10 | 9516.70 |
| Sandwell | 39 | 5474.70 | 2379.50 | 1120.00 | 119.30 | 6714.00 | 9093.50 |
| Sandwell | 40 | 6354.80 | 3020.40 | 1320.00 | 106.60 | 7781.40 | 10,801.80 |
| Sandwell | 41 | 6823.10 | 2073.30 | 1160.00 | 83.30 | 8066.40 | 10,139.70 |
| Solent | 42 | 6138.30 | 3405.90 | 1606.80 | 136.00 | 7881.10 | 11,287.00 |
| St Cross | 43 | 6365.20 | 3733.00 | 925.00 | 195.90 | 7486.10 | 11,219.10 |
| Wellingborough | 44 | 5374.30 | 5269.50 | 1194.80 | 84.10 | 6653.20 | 11,922.70 |
| Wildmoor Spa | 45 | 7492.60 | 1722.10 | 1277.20 | 142.20 | 8912.00 | 10,634.10 |
| Wildmoor Spa | 46 | 7912.90 | 525.50 | 1277.20 | 101.40 | 9291.50 | 9817.00 |
| Wildmoor Spa | 47 | 4647.90 | 2548.00 | 1194.80 | 83.30 | 5926.00 | 8474.00 |
| Wokingham | 48 | 4163.50 | 3056.30 | 1194.80 | 96.90 | 5455.20 | 8511.50 |
| Wolston | 49 | 4983.80 | 1595.50 | 1194.80 | 122.40 | 6301.00 | 7896.50 |
| Wolverhampton | 50 | 9659.40 | 3460.50 | 1565.60 | 116.70 | 11341.70 | 14,802.20 |
| Wolverhampton | 51 | 5852.80 | 3036.20 | 1236.00 | 85.50 | 7174.30 | 10,210.50 |
| Worcester | 52 | 5400.00 | 4211.80 | 700.00 | 119.30 | 6219.30 | 10,431.10 |
| Worcester | 53 | 3635.60 | 3784.60 | 725.00 | 83.30 | 4443.90 | 8228.50 |
| Worcester | 54 | 6331.50 | 3793.80 | 850.00 | 139.20 | 7320.70 | 11,114.50 |
| Venue | Cohort | Participants per cohort | Total number of sessions delivered | Mean number of sessions attended | Mean cost (£) per session/participanta | Mean cost (£) per session/participant including practitioner travel costs | Sensitivity analyses, mean cost (£) per session/participant | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Participants per cohort | Participants per cohort, including practitioner travel costs | |||||||||
| n = 3 | n = 10 | n = 3 | n = 10 | |||||||
| Gloucestershire & Hereford | 1 | 6 | 29 | 23.50 | 49.30 | 69.90 | 98.50 | 29.60 | 139.90 | 42.00 |
| Gloucestershire & Hereford | 2 | 6 | 29 | 26.70 | 43.30 | 49.70 | 86.60 | 26.00 | 99.50 | 29.80 |
| Abingdon | 3 | 6 | 30 | 23.80 | 50.50 | 65.50 | 100.90 | 30.30 | 131.00 | 39.30 |
| Amersham | 4 | 8 | 29 | 26.00 | 28.60 | 46.10 | 76.20 | 22.80 | 123.00 | 36.90 |
| Amersham | 5 | 7 | 29 | 25.60 | 38.60 | 58.00 | 90.00 | 27.00 | 135.30 | 40.60 |
| Amersham | 6 | 8 | 29 | 24.10 | 45.40 | 74.20 | 121.00 | 36.30 | 197.90 | 59.40 |
| Atrium | 7 | 4 | 29 | 23.80 | 80.80 | 87.40 | 107.80 | 32.30 | 116.50 | 34.90 |
| Atrium | 8 | 8 | 31 | 24.80 | 51.00 | 55.90 | 136.00 | 40.80 | 149.10 | 44.70 |
| Atrium | 9 | 4 | 30 | 18.30 | 107.50 | 120.30 | 143.30 | 43.00 | 160.50 | 48.10 |
| Atrium | 10 | 8 | 29 | 21.30 | 49.30 | 61.30 | 131.60 | 39.50 | 163.40 | 49.00 |
| Atrium | 11 | 6 | 29 | 15.30 | 92.30 | 102.20 | 184.60 | 55.40 | 204.40 | 61.30 |
| Atrium | 12 | 7 | 29 | 24.30 | 45.90 | 51.30 | 107.10 | 32.10 | 119.60 | 35.90 |
| Aylesbury | 13 | 6 | 29 | 25.70 | 35.20 | 47.70 | 70.30 | 21.10 | 95.30 | 28.60 |
| Banbury | 14 | 6 | 29 | 21.80 | 63.50 | 90.60 | 127.00 | 38.10 | 181.10 | 54.30 |
| Bromsgrove | 15 | 4 | 29 | 27.50 | 67.40 | 94.10 | 89.90 | 27.00 | 125.50 | 37.70 |
| Bromsgrove | 16 | 5 | 29 | 26.00 | 69.60 | 88.50 | 116.00 | 34.80 | 147.60 | 44.30 |
| Daventry | 17 | 6 | 29 | 26.00 | 66.80 | 87.50 | 133.60 | 40.10 | 175.00 | 52.50 |
| Exeter | 18 | 6 | 29 | 24.20 | 45.60 | 85.00 | 91.20 | 27.40 | 170.00 | 51.00 |
| High Wycombe | 19 | 6 | 29 | 22.50 | 48.00 | 62.40 | 96.00 | 28.80 | 124.70 | 37.40 |
| Jubilee | 20 | 4 | 29 | 23.50 | 84.50 | 103.00 | 112.60 | 33.80 | 137.40 | 41.20 |
| Jubilee | 21 | 3 | 29 | 22.70 | 100.80 | 114.20 | 100.80 | 30.20 | 114.20 | 34.20 |
| Jubilee | 22 | 8 | 29 | 24.10 | 35.20 | 40.00 | 94.00 | 28.20 | 106.60 | 32.00 |
| Kenilworth | 23 | 6 | 29 | 26.20 | 54.10 | 63.90 | 108.10 | 32.40 | 127.80 | 38.40 |
| Kettering | 24 | 6 | 31 | 25.30 | 47.50 | 69.30 | 95.00 | 28.50 | 138.60 | 41.60 |
| Loughborough | 25 | 7 | 29 | 26.40 | 49.20 | 72.10 | 114.90 | 34.50 | 168.30 | 50.50 |
| Maidenhead | 26 | 5 | 29 | 27.00 | 43.50 | 54.00 | 72.40 | 21.70 | 90.10 | 27.00 |
| Maidenhead | 27 | 8 | 29 | 21.60 | 46.30 | 62.70 | 123.50 | 37.00 | 167.30 | 50.20 |
| Melton Mowbray | 28 | 3 | 29 | 25.70 | 87.90 | 131.70 | 87.90 | 26.40 | 131.70 | 39.50 |
| North East London | 29 | 6 | 29 | 20.70 | 67.80 | 128.20 | 135.50 | 40.70 | 256.30 | 76.90 |
| North East London | 30 | 4 | 28 | 20.00 | 97.00 | 108.10 | 129.30 | 38.80 | 144.10 | 43.20 |
| Newbury | 31 | 5 | 29 | 21.40 | 65.60 | 84.20 | 109.40 | 32.80 | 140.30 | 42.10 |
| Northampton | 32 | 9 | 29 | 24.90 | 39.10 | 57.00 | 117.30 | 35.20 | 170.90 | 51.30 |
| Northampton | 33 | 6 | 29 | 25.30 | 52.50 | 70.90 | 105.10 | 31.50 | 141.90 | 42.60 |
| Northampton | 34 | 8 | 29 | 24.60 | 41.00 | 60.90 | 109.40 | 32.80 | 162.40 | 48.70 |
| Oxford Brookes | 35 | 4 | 29 | 18.30 | 102.20 | 163.90 | 136.20 | 40.90 | 218.60 | 65.60 |
| Reading | 36 | 4 | 29 | 23.50 | 102.00 | 134.50 | 136.10 | 40.80 | 179.30 | 53.80 |
| Salford | 37 | 6 | 29 | 22.70 | 60.60 | 85.60 | 121.10 | 36.30 | 171.30 | 51.40 |
| Salford | 38 | 6 | 29 | 15.20 | 76.90 | 104.60 | 153.90 | 46.20 | 209.20 | 62.70 |
| Sandwell | 39 | 7 | 28 | 21.70 | 44.20 | 59.80 | 103.10 | 30.90 | 139.60 | 41.90 |
| Sandwell | 40 | 6 | 31 | 22.00 | 59.00 | 81.80 | 117.90 | 35.40 | 163.70 | 49.10 |
| Sandwell | 41 | 5 | 29 | 24.60 | 65.60 | 82.40 | 109.30 | 32.80 | 137.40 | 41.20 |
| Solent | 42 | 6 | 29 | 17.30 | 75.80 | 108.50 | 151.60 | 45.50 | 217.10 | 65.10 |
| St Cross | 43 | 10 | 31 | 21.70 | 34.50 | 51.70 | 115.00 | 34.50 | 172.30 | 51.70 |
| Wellingborough | 44 | 4 | 29 | 27.30 | 61.00 | 109.40 | 81.40 | 24.40 | 145.80 | 43.80 |
| Wildmoor Spa | 45 | 8 | 29 | 25.90 | 43.10 | 51.40 | 114.80 | 34.40 | 137.00 | 41.10 |
| Wildmoor Spa | 46 | 6 | 29 | 20.50 | 75.50 | 79.80 | 151.10 | 45.30 | 159.60 | 47.90 |
| Wildmoor Spa | 47 | 5 | 29 | 19.80 | 59.90 | 85.60 | 99.80 | 29.90 | 142.70 | 42.80 |
| Wokingham | 48 | 5 | 29 | 20.80 | 52.50 | 81.80 | 87.40 | 26.20 | 136.40 | 40.90 |
| Wolston | 49 | 7 | 29 | 22.40 | 40.10 | 50.30 | 93.60 | 28.10 | 117.40 | 35.20 |
| Wolverhampton | 50 | 5 | 32 | 21.80 | 104.10 | 135.80 | 173.40 | 52.00 | 226.30 | 67.90 |
| Wolverhampton | 51 | 5 | 29 | 26.40 | 54.40 | 77.40 | 90.60 | 27.20 | 128.90 | 38.70 |
| Worcester | 52 | 7 | 28 | 22.60 | 39.40 | 66.00 | 91.80 | 27.60 | 154.00 | 46.20 |
| Worcester | 53 | 5 | 29 | 17.40 | 51.10 | 94.60 | 85.10 | 25.50 | 157.60 | 47.30 |
| Worcester | 54 | 8 | 29 | 24.50 | 37.40 | 56.70 | 99.60 | 29.90 | 151.20 | 45.40 |
Group- and site-specific estimates of average cost per exercise session per attending participant were estimated using the total cost data in Table 37 and data on group size and mean session attendance reported in Table 38. These average costs varied from £28.60 (Amersham, cohort 4) to £107.50 (Atrium, cohort 9). Table 38 also reports group- and site-specific estimates of average cost per exercise session per attending participant following sensitivity analyses; varying the number of exercise group participants from three (lowest observed) to 10 (highest observed) per cohort. As expected, increases in values for both the session attendance variable and the group size variable had the tendency to decrease the average cost per exercise session per participant.
Broader resource use
Table 39 shows resource-use values for participants with complete data by trial allocation, resource-use category and study period. The resource-use values are presented for subcategories of resource use, including accommodation, hospital services, day care services, general community health services, community mental health services, social care services, equipment (adaptation and repairs), medication use, participant travel and other resource items. Societal resource items included privately provided community health and mental health services, and time taken off work.
| Resource category (unit) | Treatment arm | |||
|---|---|---|---|---|
| Exercise programme | Usual care | |||
| Mean (SE) | na (%) | Mean (SE) | na (%) | |
| Patient accommodation (number of nights) | N = 326 | N = 162 | ||
| Care home providing nursing care | 0.0 (0.02) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| Care home providing personal care | 0.0 (0.00) | 0 (0.0) | 0.1 (0.13) | 1 (0.6) |
| Dual-registered home (providing both personal and nursing care) | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| General medical ward | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Rehabilitation ward | 0.0 (0.00) | 0 (0.0) | 0.3 (0.26) | 1 (0.6) |
| Acute psychiatric ward | 0.0 (0.03) | 3 (0.9) | 0.1 (0.06) | 3 (1.9) |
| Hospital services | N = 327 | N = 162 | ||
| General medical ward (days) | 0.2 (0.06) | 12 (3.7) | 0.1 (0.06) | 4 (2.5) |
| Continuing care/respite in-patient ward (days) | 0.0 (0.04) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| Rehabilitation ward (days) | 0.0 (0.00) | 0 (0.0) | 0.0 (0.01) | 1 (0.6) |
| Acute psychiatric ward (days) | 0.1 (0.03) | 5 (1.5) | 0.0 (0.02) | 3 (1.9) |
| Other hospital in-patient ward (days) | 0.6 (0.06) | 103 (31.5) | 0.7 (0.13) | 54 (33.3) |
| Out-patient services (appointments) | 0.0 (0.01) | 15 (4.6) | 0.1 (0.02) | 8 (4.9) |
| Accident and emergency (appointments) | 0.1 (0.04) | 9 (2.8) | 0.0 (0.01) | 2 (1.2) |
| Day hospital (days) | 0.0 (0.01) | 3 (0.9) | 0.0 (0.01) | 1 (0.6) |
| Other | 0.0 (0.02) | 9 (2.8) | 0.0 (0.02) | 5 (3.1) |
| Day care services | N = 325 | N = 162 | ||
| Local authority social service (half days) | 0.1 (0.04) | 6 (1.8) | 0.1 (0.05) | 6 (3.7) |
| Voluntary/private (half days) | 0.1 (0.04) | 19 (5.8) | 0.5 (0.33) | 8 (4.9) |
| NHS (not hospital) (half days) | 0.1 (0.04) | 9 (2.8) | 0.0 (0.02) | 2 (1.2) |
| Unit (accommodation) (half days) | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Lunch club (visits) | 0.1 (0.02) | 10 (3.1) | 0.0 (0.01) | 1 (0.6) |
| Social club (visits) | 0.0 (0.01) | 9 (2.8) | 0.1 (0.08) | 6 (3.7) |
| Other | 0.0 (0.01) | 12 (3.7) | 0.2 (0.15) | 6 (3.7) |
| General community health services (number of visits) | N = 326 | N = 162 | ||
| Geriatrician | ||||
| Office visit | 0.0 (0.01) | 5 (1.5) | 0.0 (0.01) | 2 (1.2) |
| General practitioner | ||||
| Office visit | 1.1 (0.08) | 187 (57.4) | 1.2 (0.13) | 94 (58.0) |
| Home visit | 0.1 (0.03) | 8 (2.5) | 0.0 (0.02) | 6 (3.7) |
| Practice nurse (GP clinic) | ||||
| Office visit | 0.7 (0.13) | 91 (27.9) | 0.6 (0.10) | 47 (29.0) |
| Home visit | 0.0 (0.00) | 2 (0.6) | 0.0 (0.01) | 2 (1.2) |
| District nurse | ||||
| Office visit | 0.0 (0.02) | 2 (0.6) | 0.0 (0.01) | 2 (1.2) |
| Home visit | 0.0 (0.02) | 6 (1.8) | 0.0 (0.01) | 2 (1.2) |
| Health visitor | ||||
| Home visit | 0.0 (0.04) | 2 (0.6) | 0.0 (0.00) | 0 (0.0) |
| Incontinence nurse | ||||
| Office visit | 0.0 (0.01) | 4 (1.2) | 0.0 (0.02) | 2 (1.2) |
| Home visit | 0.0 (0.00) | 1 (0.3) | 0.0 (0.01) | 1 (0.6) |
| Occupational therapist | ||||
| Office visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Home visit | 0.0 (0.02) | 5 (1.5) | 0.0 (0.02) | 3 (1.9) |
| Physiotherapist | ||||
| Office visit | 0.1 (0.03) | 8 (2.5) | 0.0 (0.04) | 2 (1.2) |
| Home visit | 0.0 (0.01) | 3 (0.9) | 0.1 (0.07) | 3 (1.9) |
| Alternative medicine/therapist | ||||
| Office visit | 0.0 (0.01) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| Home visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Other | ||||
| Office visit | 0.1 (0.03) | 20 (6.1) | 0.1 (0.05) | 11 (6.8) |
| Home visit | 0.0 (0.01) | 3 (0.9) | 0.0 (0.02) | 3 (1.9) |
| Exercise class/physical activity | 0.0 (0.00) | 2 (0.6) | 0.0 (0.04) | 2 (1.2) |
| Optician | 0.1 (0.04) | 9 (2.8) | 0.1 (0.07) | 3 (1.9) |
| Dentist | 0.1 (0.02) | 10 (3.1) | 0.1 (0.07) | 3 (1.9) |
| Community mental health services (number of visits) | N = 326 | N = 162 | ||
| CPN/CMHN | ||||
| Office visit | 0.1 (0.02) | 33 (10.1) | 0.1 (0.02) | 11 (6.8) |
| Home visit | 0.2 (0.10) | 34 (10.4) | 0.1 (0.07) | 12 (7.4) |
| Community psychiatrist | ||||
| Office visit | 0.3 (0.03) | 75 (23.0) | 0.3 (0.05) | 41 (25.3) |
| Home visit | 0.0 (0.01) | 9 (2.8) | 0.0 (0.02) | 5 (3.1) |
| Psychologist | ||||
| Office visit | 0.0 (0.01) | 7 (2.1) | 0.0 (0.00) | 0 (0.0) |
| Home visit | 0.0 (0.00) | 2 (0.6) | 0.0 (0.02) | 2 (1.2) |
| Other | ||||
| Office visit | 0.1 (0.02) | 9 (2.8) | 0.2 (0.15) | 2 (1.2) |
| Home visit | 0.1 (0.10) | 5 (1.5) | 0.0 (0.04) | 2 (1.2) |
| Social care services (number of visits) | N = 326 | N = 161 | ||
| Care manager | ||||
| Home | 0.0 (0.00) | 2 (0.6) | 0.0 (0.00) | 0 (0.0) |
| Social worker | ||||
| Office | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Home | 0.0 (0.02) | 9 (2.8) | 0.0 (0.01) | 5 (3.1) |
| Home care worker | ||||
| Home | 0.7 (0.41) | 7 (2.1) | 2.6 (2.44) | 2 (1.2) |
| Carer worker | ||||
| Office | 0.0 (0.00) | 1 (0.3) | 0.0 (0.01) | 1 (0.6) |
| Carer worker – home | 0.1 (0.06) | 5 (1.5) | 0.0 (0.01) | 1 (0.6) |
| Chiropodist | ||||
| Office | 0.1 (0.02) | 17 (5.2) | 0.0 (0.02) | 4 (2.5) |
| Home | 0.0 (0.02) | 6 (1.8) | 0.1 (0.03) | 5 (3.1) |
| Sitting scheme | ||||
| Home | 0.1 (0.08) | 2 (0.6) | 0.0 (0.00) | 0 (0.0) |
| Meals on wheels | ||||
| Home | 0.5 (0.34) | 2 (0.6) | 0.0 (0.00) | 0 (0.0) |
| Self-help group | ||||
| Office | 0.1 (0.05) | 7 (2.1) | 0.1 (0.03) | 5 (3.1) |
| Home | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Self-help group carer | ||||
| Office | 0.0 (0.01) | 3 (0.9) | 0.1 (0.05) | 3 (1.9) |
| Home | 0.0 (0.00) | 0 (0.0) | 0.0 (0.01) | 1 (0.6) |
| Other | ||||
| Office | 0.1 (0.06) | 8 (2.5) | 0.1 (0.05) | 4 (2.5) |
| Home | 0.0 (0.02) | 5 (1.5) | 0.0 (0.02) | 3 (1.9) |
| Equipment, adaptations/repairs, n (%) | N = 325 | N = 162 | ||
| Health service | 0.03 (0.01) | 11 (3.4) | 0.06 (0.02) | 10 (6.2) |
| Local authority | 0.0 (0.00) | 0 (0.0) | 0.01 (0.01) | 1 (0.6) |
| Voluntary organisation | 0.0 (0.00) | 1 (0.3) | 0.01 (0.01) | 2 (1.2) |
| Self-financed | 0.04 (0.01) | 14 (4.3) | 0.04 (0.01) | 6 (3.7) |
| Private organisation | 0.05 (0.01) | 19 (5.8) | 0.11 (0.02) | 16 (9.9) |
| Medications, n (%) | N = 325 | N = 162 | ||
| Number (%) of participants with: | ||||
| One medication | 0.0 (0.01) | 113 (34.7) | 0.31 (0.04) | 51 (31.5) |
| Two medications | 0.2 (0.02) | 59 (18.1) | 0.12 (0.03) | 20 (12.3) |
| Three medications | 0.1 (0.02) | 39 (12.0) | 0.19 (0.03) | 31 (19.1) |
| More than three medications | 0.0 (0.01) | 113 (34.7) | 0.36 (0.04) | 58 (35.8) |
| Participant travel, n (%) | N = 326 | |||
| Hospital service visit | ||||
| Bus | 0.1 (0.01) | 16 (4.9) | 0.05 (0.02) | 8 (4.9) |
| Car | 0.3 (0.02) | 92 (28.2) | 0.25 (0.03) | 40 (24.8) |
| Taxi | 0.01 (0.01) | 6 (1.8) | 0.03 (0.01) | 5 (3.1) |
| Train | 0.0 (0.01) | 2 (0.6) | 0.0 (0.00) | 0 (0.0) |
| Walk/cycle | 0.0 (0.01) | 3 (0.9) | 0.0 (0.00) | 0 (0.0) |
| Ambulance | 0.01 (0.01) | 6 (1.8) | 0.01 (0.01) | 1 (0.6) |
| Voluntary | 0.0 (0.01) | 2 (0.6) | 0.01 (0.01) | 2 (1.2) |
| Hospital day visit | ||||
| Bus | 0.0 (0.01) | 4 (1.2) | 0.01 (0.01) | 2 (1.2) |
| Car | 0.0 (0.01) | 23 (7.1) | 0.07 (0.02) | 12 (7.4) |
| Taxi | 0.0 (0.00) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| Walk/cycle | 0.0 (0.01) | 3 (0.9) | 0.01 (0.01) | 2 (1.2) |
| Voluntary | 0.0 (0.01) | 4 (1.2) | 0.02 (0.01) | 3 (1.9) |
| General health community visit | ||||
| Bus | 0.1 (0.01) | 19 (5.8) | 0.07 (0.02) | 12 (7.4) |
| Car | 0.1 (0.01) | 205 (62.9) | 0.56 (0.04) | 90 (55.9) |
| Taxi | 0.0 (0.00) | 8 (2.5) | 0.04 (0.01) | 6 (3.7) |
| Walk/cycle | 0.2 (0.02) | 52 (16.0) | 0.2 (0.03) | 32 (19.8) |
| Ambulance | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Voluntary | 0.0 (0.00) | 2 (0.6) | 0.0 (0.00) | 0 (0.0) |
| Mental health community visit | ||||
| Bus | 0.0 (0.01) | 13 (4.0) | 0.05 (0.02) | 8 (4.9) |
| Car | 0.0 (0.01) | 123 (37.7) | 0.35 (0.04) | 56 (34.7) |
| Taxi | 0.0 (0.01) | 5 (1.5) | 0.01 (0.01) | 1 (0.6) |
| Walk/cycle | 0.0 (0.00) | 3 (0.9) | 0.01 (0.01) | 2 (1.2) |
| Ambulance | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Voluntary | 0.0 (0.00) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| Social care visit | ||||
| Bus | 0.0 (0.00) | 4 (1.2) | 0.01 (0.01) | 2 (1.2) |
| Car | 0.1 (0.01) | 26 (8.0) | 0.09 (0.02) | 14 (8.6) |
| Taxi | 0.0 (0.00) | 2 (0.6) | 0.01 (0.01) | 2 (1.2) |
| Walk/cycle | 0.0 (0.01) | 4 (1.2) | 0.01 (0.01) | 2 (1.2) |
| Ambulance | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Voluntary | 0.0 (0.00) | 2 (0.6) | 0.01 (0.01) | 2 (1.2) |
| Privately provided general community health servicesb | N = 326 | N = 162 | ||
| 0.1 (0.03) | 10 (3.0) | 0.0 (0.03) | 2 (1.2) | |
| Privately provided mental health services | N = 326 | N = 162 | ||
| 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) | |
| Time off work | N = 303 | N = 153 | ||
| Hours | 0.7 (0.20) | 15 (4.9) | 0.6 (0.29) | 5 (3.2) |
| Days | 0.1 (0.04) | 4 (1.3) | 0.1 (0.03) | 6 (3.9) |
Notably, among participants with complete resource-use data, the most frequent health resource inputs were GP visits, hospital stays, practice nurse visits and community psychiatrist contacts (see Table 39). About 57% versus 58%, 69% versus 66% and 66% versus 64% of participants had at least one visit to the GP at baseline, 6 months post randomisation and 12 months post randomisation for exercise versus usual care, respectively. The mean (SE) number of GP contacts per participant over 12 months was 1.8 (0.14) in the exercise arm compared with 1.7 (0.27) in the usual-care arm (see Table 39).
Community mental health care use, primarily through psychiatric support, fell over time in both groups relative to baseline, and by 12 months it was 4% lower in the exercise group (10% vs. 14%; p = 0.2269). No noticeable differences in terms of health-care resource use were observed post baseline for hospital stays, practice nurse visits and community psychiatrist contacts. Resource-use frequencies in other categories were low; hence, meaningful comparisons could not be easily made. There were no marked differences between the trial arms in terms of the proportion of participants who incurred travel costs or lost earnings as a result of their health state or their contacts with health- and social-care professionals. Resource-use values at the individual participant level were combined with unit costs for each resource item (Tables 40 and 41) to estimate economic costs for each resource category.
| Resource category (unit) | Treatment arm | |||
|---|---|---|---|---|
| Exercise programme | Usual care | |||
| Mean (SE) | na (%) | Mean (SE) | na (%) | |
| Patient accommodation (number of nights) | N = 298 | N = 142 | ||
| Care home providing nursing care | 0.0 (0.04) | 1 (0.3) | 0.1 (0.10) | 1 (0.7) |
| Care home providing personal care | 0.0 (0.00) | 0 (0.0) | 0.0 (0.05) | 1 (0.7) |
| Dual-registered home (providing both personal and nursing care) | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| General medical ward | 0.0 (0.00) | 0 (0.0) | 0.1 (0.10) | 1 (0.7) |
| Rehabilitation ward | 0.0 (0.03) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| Acute psychiatric ward | 0.2 (0.08) | 10 (3.4) | 0.2 (0.15) | 2 (1.4) |
| Hospital services | N = 298 | N = 141 | ||
| General medical ward (days) | 0.5 (0.14) | 22 (7.4) | 0.3 (0.16) | 6 (4.3) |
| Continuing care/respite inpatient ward (days) | 0.1 (0.10) | 2 (0.7) | 0.0 (0.00) | 0 (0.0) |
| Rehabilitation ward (days) | 0.7 (0.66) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| Acute psychiatric ward (days) | 0.3 (0.14) | 11 (3.7) | 0.2 (0.14) | 6 (4.3) |
| Other hospital in-patient ward (days) | 1.2 (0.18) | 120 (40.3) | 1.2 (0.20) | 59 (41.8) |
| Outpatient services (appointments) | 0.1 (0.02) | 30 (10.1) | 0.1 (0.02) | 10 (7.1) |
| Accident and emergency (appointments) | 0.1 (0.03) | 19 (6.4) | 0.1 (0.03) | 8 (5.7) |
| Day hospital (days) | 0.0 (0.01) | 6 (2.0) | 0.0 (0.01) | 2 (1.4) |
| Other | 0.2 (0.06) | 13 (4.4) | 0.1 (0.03) | 6 (4.3) |
| Day care services | N = 298 | N = 141 | ||
| Local authority social service (half-days) | 0.0 (0.02) | 6 (2.0) | 0.2 (0.07) | 8 (5.7) |
| Voluntary/private (half-days) | 0.2 (0.04) | 27 (9.1) | 0.1 (0.04) | 6 (4.3) |
| NHS (not hospital) (half-days) | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Unit (accommodation) (half-days) | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Lunch club (visits) | 0.0 (0.02) | 8 (2.7) | 0.0 (0.02) | 5 (3.5) |
| Social club (visits) | 0.0 (0.01) | 11 (3.7) | 0.2 (0.17) | 5 (3.5) |
| Other | 0.1 (0.02) | 10 (3.4) | 0.1 (0.06) | 10 (7.1) |
| General community health services (number of visits) | N = 297 | N = 141 | ||
| Geriatrician | ||||
| Office visit | 0.0 (0.00) | 1 (0.3) | 0.0 (0.01) | 1 (0.7) |
| General practitioner | ||||
| Office visit | 2.0 (0.15) | 205 (69.0) | 1.9 (0.24) | 93 (66.0) |
| Home visit | 0.1 (0.02) | 16 (5.4) | 0.0 (0.02) | 4 (2.8) |
| Practice nurse (GP clinic) | ||||
| Office visit | 1.3 (0.25) | 130 (43.8) | 1.2 (0.27) | 61 (43.3) |
| Home visit | 0.0 (0.01) | 3 (1.0) | 0.0 (0.04) | 2 (1.4) |
| District nurse | ||||
| Office visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.01) | 1 (0.7) |
| Home visit | 0.2 (0.17) | 9 (3.0) | 0.0 (0.01) | 2 (1.4) |
| Health visitor | ||||
| Home visit | 0.0 (0.00) | 2 (0.7) | 0.0 (0.00) | 0 (0.0) |
| Incontinence nurse | ||||
| Office visit | 0.0 (0.01) | 5 (1.7) | 0.0 (0.02) | 2 (1.4) |
| Home visit | 0.0 (0.01) | 3 (1.0) | 0.0 (0.01) | 1 (0.7) |
| Occupational therapist | ||||
| Office visit | 0.0 (0.01) | 2 (0.7) | 0.1 (0.07) | 1 (0.7) |
| Home visit | 0.0 (0.01) | 6 (2.0) | 0.1 (0.04) | 8 (5.7) |
| Physiotherapist | ||||
| Office visit | 0.1 (0.06) | 12 (4.0) | 0.0 (0.02) | 4 (2.8) |
| Home visit | 0.1 (0.04) | 6 (2.0) | 0.0 (0.02) | 2 (1.4) |
| Alternative medicine/therapist | ||||
| Office visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Home visit | 0.1 (0.05) | 2 (0.7) | 0.0 (0.00) | 0 (0.0) |
| Other | ||||
| Office visit | 0.1 (0.04) | 21 (7.1) | 0.3 (0.12) | 13 (9.2) |
| Exercise class/physical activity | 0.0 (0.01) | 2 (0.7) | 0.0 (0.01) | 1 (0.7) |
| Optician | 0.2 (0.17) | 9 (3.0) | 0.0 (0.02) | 3 (2.1) |
| Dentist | 0.1 (0.06) | 12 (4.0) | 0.0 (0.02) | 4 (2.8) |
| Community mental health services (number of visits) | N = 298 | N = 141 | ||
| CPN/CMHN | ||||
| Office visit | 0.1 (0.02) | 29 (9.7) | 0.1 (0.03) | 15 (10.6) |
| Home visit | 0.3 (0.17) | 30 (10.1) | 0.3 (0.11) | 11 (7.8) |
| Community psychiatrist | ||||
| Office visit | 0.2 (0.03) | 56 (18.8) | 0.2 (0.04) | 29 (20.6) |
| Home visit | 0.0 (0.01) | 6 (2.0) | 0.0 (0.01) | 4 (2.8) |
| Psychologist | ||||
| Office visit | 0.0 (0.02) | 3 (1.0) | 0.2 (0.11) | 2 (1.4) |
| Home visit | 0.0 (0.01) | 2 (0.7) | 0.1 (0.06) | 2 (1.4) |
| Other | ||||
| Office visit | 0.1 (0.05) | 12 (4.0) | 0.5 (0.35) | 5 (3.5) |
| Home visit | 0.3 (0.25) | 9 (3.0) | 0.0 (0.03) | 4 (2.8) |
| Social care services (number of visits) | N = 298 | N = 141 | ||
| Care manager | ||||
| Home | 0.0 (0.01) | 3 (1.0) | 0.0 (0.01) | 1 (0.7) |
| Social worker | ||||
| Office | 0.0 (0.00) | 0 (0.0) | 0.0 (0.01) | 1 (0.7) |
| Home | 0.1 (0.02) | 16 (5.4) | 0.1 (0.05) | 7 (5.0) |
| Home care worker | ||||
| Home | 1.9 (1.32) | 3 (1.0) | 0.3 (0.34) | 1 (0.7) |
| Carer worker | ||||
| Office | 0.0 (0.01) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| Home | 1.5 (0.72) | 11 (3.7) | 0.7 (0.74) | 2 (1.4) |
| Chiropodist | ||||
| Office | 0.1 (0.03) | 13 (4.4) | 0.2 (0.07) | 12 (8.5) |
| Home | 0.0 (0.02) | 7 (2.3) | 0.0 (0.03) | 2 (1.4) |
| Sitting scheme | ||||
| Home | 0.2 (0.18) | 2 (0.7) | 0.0 (0.02) | 1 (0.7) |
| Meals on Wheels | ||||
| Home | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Self-help group | ||||
| Office | 0.1 (0.06) | 6 (2.0) | 0.5 (0.26) | 5 (3.5) |
| Home | 0.0 (0.00) | 0 (0.0) | 0.0 (0.04) | 2 (1.4) |
| Self-help group carer | ||||
| Office | 0.0 (0.02) | 2 (0.7) | 0.0 (0.00) | 0 (0.0) |
| Home | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Other | ||||
| Office | 0.2 (0.11) | 6 (2.0) | 0.0 (0.02) | 3 (2.1) |
| Home | 0.0 (0.02) | 4 (1.3) | 0.0 (0.01) | 2 (1.4) |
| Equipment, adaptations/repairs, n (%) | N = 298 | N = 141 | ||
| Health service | 0.04 (0.01) | 13 (4.4) | 0.06 (0.02) | 9 (6.3) |
| Local authority | 0.01 (0.01) | 3 (1.0) | 0.01 (0.01) | 1 (0.7) |
| Voluntary organisation | 0.0 (0.00) | 1 (0.3) | 0.01 (0.01) | 1 (0.7) |
| Self-financed | 0.04 (0.01) | 13 (4.4) | 0.06 (0.02) | 9 (6.3) |
| Private organisation | 0.13 (0.02) | 38 (12.8) | 0.13 (0.03) | 18 (12.7) |
| Medications, n (%) | N = 297 | N = 141 | ||
| Number (%) of participants with | ||||
| One medication | 0.14 (0.02) | 41 (13.8) | 0.17 (0.03) | 24 (17.0) |
| Two medications | 0.21 (0.02) | 62 (20.9) | 0.15 (0.03) | 21 (14.9) |
| Three medications | 0.13 (0.02) | 39 (13.1) | 0.11 (0.03) | 16 (11.3) |
| More than three medications | 0.05 (0.01) | 157 (52.8) | 0.57 (0.04) | 81 (57.4) |
| Participant travel (societal), n (%) | N = 298 | N = 141 | ||
| Hospital service visit | ||||
| Bus | 0.07 (0.02) | 22 (7.4) | 0.07 (0.02) | 10 (7.0) |
| Car | 0.03 (0.01) | 105 (35.2) | 0.39 (0.04) | 55 (39.0) |
| Taxi | 0.02 (0.01) | 7 (2.3) | 0.06 (0.02) | 8 (5.6) |
| Train | 0.0 (0.00) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| Walk/cycle | 0.01 (0.00) | 2 (0.7) | 0.0 (0.00) | 0 (0.0) |
| Ambulance | 0.05 (0.01) | 16 (5.4) | 0.02 (0.01) | 3 (2.1) |
| Voluntary | 0.01 (0.01) | 3 (1.0) | 0.01 (0.01) | 1 (0.7) |
| Hospital day visit | ||||
| Bus | 0.0 (0.00) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| Car | 0.03 (0.01) | 8 (2.7) | 0.03 (0.01) | 4 (2.8) |
| Taxi | 0.0 (0.00) | 1 (0.3) | 0.01 (0.01) | 1 (0.7) |
| Walk/cycle | 0.0 (0.00) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| Voluntary | 0.01 (0.00) | 2 (0.7) | 0.01 (0.01) | 2 (1.4) |
| General health community visit | ||||
| Bus | 0.07 (0.02) | 22 (7.4) | 0.04 (0.02) | 6 (4.2) |
| Car | 0.06 (0.01) | 177 (59.4) | 0.58 (0.04) | 82 (58.1) |
| Taxi | 0.02 (0.01) | 7 (2.3) | 0.05 (0.02) | 7 (4.9) |
| Walk/cycle | 0.17 (0.02) | 50 (16.8) | 0.18 (0.03) | 25 (17.8) |
| Ambulance | 0.0 (0.00) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| Voluntary | 0.0 (0.00) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| Mental health community visit | ||||
| Bus | 0.04 (0.01) | 13 (4.4) | 0.04 (0.02) | 6 (4.2) |
| Car | 0.03 (0.01) | 106 (35.6) | 0.37 (0.04) | 52 (36.9) |
| Taxi | 0.02 (0.01) | 5 (1.7) | 0.01 (0.01) | 1 (0.7) |
| Walk/cycle | 0.01 (0.01) | 4 (1.3) | 0.01 (0.01) | 2 (1.4) |
| Ambulance | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Voluntary | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Social care visit | ||||
| Bus | 0.02 (0.01) | 5 (1.7) | 0.01 (0.01) | 2 (1.4) |
| Car | 0.07 (0.01) | 20 (6.7) | 0.12 (0.03) | 17 (12.0) |
| Taxi | 0.01 (0.01) | 3 (1.0) | 0.02 (0.01) | 3 (2.1) |
| Walk/cycle | 0.01 (0.01) | 3 (1.0) | 0.01 (0.01) | 1 (0.7) |
| Ambulance | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Voluntary | 0.01 (0.00) | 2 (0.7) | 0.01 (0.01) | 1 (0.7) |
| Privately provided general community health servicesb | N = 297 | N = 141 | ||
| General practitioner | ||||
| Office visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Practice nurse (GP clinic) | ||||
| Office visit | 0.0 (0.00) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| District nurse | ||||
| Home visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Physiotherapist | ||||
| Office visit | 0.0 (0.01) | 1 (0.3) | 0.0 (0.01) | 1 (0.7) |
| Home visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Alternative medicine/therapist | ||||
| Office visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Other | ||||
| Office visit | 0.0 (0.01) | 4 (1.3) | 0.4 (0.21) | 5 (3.5) |
| Home visit | 0.0 (0.02) | 2 (0.7) | 0.0 (0.00) | 0 (0.0) |
| Privately provided mental health services | N = 298 | N = 141 | ||
| Community psychiatrist | ||||
| Office visit | 0.0 (0.00) | 1 (0.3) | 0.0 (0.01) | 1 (0.7) |
| Other | ||||
| Home visit | 0.1 (0.14) | 1 (0.3) | 0.0 (0.00) | 0 (0.0) |
| Time off work (societal) | N = 278 | N = 131 | ||
| Hours | 0.6 (0.16) | 17 (5.7) | 0.3 (0.17) | 3 (2.1) |
| Days | 0.4 (0.15) | 14 (4.7) | 0.3 (0.11) | 9 (6.3) |
| Resource category (unit) | Treatment arm | |||
|---|---|---|---|---|
| Exercise programme | Usual care | |||
| Mean (SE) | na (%) | Mean (SE) | na (%) | |
| Patient accommodation (number of nights) | N = 280 | N = 136 | ||
| Care home providing nursing care | 0.3 (0.22) | 2 (0.7) | 0.2 (0.22) | 1 (0.7) |
| Care home providing personal care | 0.5 (0.24) | 4 (1.4) | 0.1 (0.05) | 2 (1.5) |
| Dual-registered home (providing both personal and nursing care) | 0.2 (0.13) | 3 (1.1) | 0.0 (0.00) | 0 (0.0) |
| General medical ward | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Rehabilitation ward | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Acute psychiatric ward | 1.0 (0.39) | 15 (5.4) | 0.9 (0.70) | 2 (1.5) |
| Hospital services | N = 280 | N = 135 | ||
| General medical ward (days) | 1.1 (0.40) | 28 (10.0) | 1.4 (0.74) | 13 (9.6) |
| Continuing care/respite inpatient ward (days) | 0.0 (0.03) | 1 (0.4) | 0.0 (0.01) | 1 (0.7) |
| Rehabilitation ward (days) | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Acute psychiatric ward (days) | 0.1 (0.11) | 3 (1.1) | 0.0 (0.00) | 0 (0.0) |
| Other hospital inpatient ward (days) | 1.0 (0.13) | 102 (36.4) | 1.1 (0.18) | 53 (39.3) |
| Outpatient services (appointments) | 0.1 (0.03) | 29 (10.4) | 0.1 (0.02) | 9 (6.7) |
| Accident and emergency (appointments) | 0.1 (0.05) | 13 (4.6) | 0.1 (0.02) | 8 (5.9) |
| Day hospital (days) | 0.0 (0.01) | 3 (1.1) | 0.0 (0.01) | 3 (2.2) |
| Other | 0.3 (0.17) | 8 (2.9) | 0.0 (0.01) | 2 (1.5) |
| Day care service | N = 280 | N = 135 | ||
| Local authority social service (half-days) | 0.0 (0.02) | 8 (2.9) | 0.1 (0.05) | 8 (5.9) |
| Voluntary/private (half-days) | 0.2 (0.04) | 35 (12.5) | 0.2 (0.13) | 10 (7.4) |
| NHS (not hospital) (half-days) | 0.0 (0.00) | 0 (0.0) | 0.1 (0.04) | 3 (2.2) |
| Unit (accommodation) (half-days) | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Lunch club (visits) | 0.1 (0.02) | 9 (3.2) | 0.0 (0.01) | 4 (3.0) |
| Social club (visits) | 0.1 (0.06) | 9 (3.2) | 0.1 (0.06) | 3 (2.2) |
| Other | 0.2 (0.08) | 24 (8.6) | 0.1 (0.06) | 7 (5.2) |
| General community health services (number of visits) | N = 280 | N = 135 | ||
| Geriatrician | ||||
| Office visit | 0.0 (0.00) | 1 (0.4) | 0.0 (0.01) | 1 (0.7) |
| General practitioner | ||||
| Office visit | 1.8 (0.14) | 184 (65.7) | 1.7 (0.27) | 86 (63.7) |
| Home visit | 0.1 (0.03) | 19 (6.8) | 0.2 (0.10) | 13 (9.6) |
| Practice nurse (GP clinic) | ||||
| Office visit | 1.0 (0.24) | 109 (38.9) | 0.8 (0.13) | 53 (39.3) |
| Home visit | 0.1 (0.05) | 5 (1.8) | 0.2 (0.19) | 2 (1.5) |
| District nurse | ||||
| Office visit | 0.1 (0.04) | 5 (1.8) | 0.0 (0.00) | 0 (0.0) |
| Home visit | 0.3 (0.18) | 10 (3.6) | 0.1 (0.09) | 2 (1.5) |
| Health visitor | ||||
| Home visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Incontinence nurse | ||||
| Office visit | 0.0 (0.01) | 7 (2.5) | 0.0 (0.01) | 2 (1.5) |
| Home visit | 0.0 (0.01) | 7 (2.5) | 0.1 (0.03) | 5 (3.7) |
| Occupational therapist | ||||
| Office visit | 0.0 (0.01) | 1 (0.4) | 0.0 (0.00) | 0 (0.0) |
| Home visit | 0.1 (0.02) | 11 (3.9) | 0.1 (0.04) | 2 (1.5) |
| Physiotherapist | ||||
| Office visit | 0.1 (0.04) | 8 (2.9) | 0.2 (0.09) | 7 (5.2) |
| Home visit | 0.1 (0.04) | 7 (2.5) | 0.1 (0.06) | 3 (2.2) |
| Alternative medicine/therapist | ||||
| Office visit | 0.0 (0.00) | 1 (0.4) | 0.0 (0.00) | 0 (0.0) |
| Home visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Other | ||||
| Exercise class/physical activity | 0.3 (0.22) | 2 (0.7) | 0.0 (0.01) | 1 (0.7) |
| Optician | 0.1 (0.05) | 12 (4.3) | 0.0 (0.01) | 4 (3.0) |
| Dentist | 0.2 (0.04) | 14 (5.0) | 0.1 (0.03) | 5 (3.7) |
| Office visit | 0.2 (0.04) | 26 (9.3) | 0.1 (0.05) | 6 (4.4) |
| Home visit | 0.5 (0.40) | 12 (4.3) | 0.5 (0.44) | 2 (1.5) |
| Community mental health services (number of visits) | N = 280 | N = 136 | ||
| CPN/CMHN | ||||
| Office visit | 0.1 (0.02) | 27 (9.6) | 0.1 (0.03) | 18 (13.3) |
| Home visit | 0.5 (0.29) | 29 (10.4) | 0.1 (0.06) | 7 (5.2) |
| Community psychiatrist | ||||
| Office visit | 0.1 (0.02) | 28 (10.0) | 0.2 (0.04) | 19 (14.1) |
| Home visit | 0.1 (0.02) | 9 (3.2) | 0.0 (0.01) | 2 (1.5) |
| Psychologist | ||||
| Office visit | 0.0 (0.00) | 0 (0.0) | 0.1 (0.10) | 2 (1.5) |
| Home visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Other | ||||
| Office visit | 0.1 (0.06) | 6 (2.1) | 0.2 (0.15) | 2 (1.5) |
| Home visit | 0.0 (0.01) | 3 (1.1) | 0.1 (0.11) | 3 (2.2) |
| Social care services (number of visits) | N = 280 | N = 135 | ||
| Care manager | ||||
| Home | 0.0 (0.01) | 4 (1.4) | 0.0 (0.01) | 2 (1.5) |
| Social worker | ||||
| Office | 0.0 (0.00) | 0 (0.0) | 0.0 (0.01) | 1 (0.7) |
| Home | 0.1 (0.04) | 19 (6.8) | 0.2 (0.05) | 14 (10.4) |
| Home care worker | ||||
| Home | 3.0 (1.69) | 9 (3.2) | 8.7 (4.91) | 7 (5.2) |
| Carer worker | ||||
| Office | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Home | 2.4 (1.29) | 8 (2.9) | 1.3 (1.24) | 2 (1.5) |
| Chiropodist | ||||
| Office | 0.1 (0.04) | 14 (5.0) | 0.2 (0.06) | 13 (9.6) |
| Home | 0.0 (0.02) | 2 (0.7) | 0.3 (0.22) | 2 (1.5) |
| Sitting scheme | ||||
| Home | 0.1 (0.11) | 3 (1.1) | 0.4 (0.36) | 1 (0.7) |
| Meals on wheels | ||||
| Home | 0.1 (0.09) | 1 (0.4) | 1.2 (1.24) | 1 (0.7) |
| Self-help group | ||||
| Office | 0.1 (0.07) | 1 (0.4) | 0.0 (0.04) | 1 (0.7) |
| Home | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Self-help group carer | ||||
| Office | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Home | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Other | ||||
| Office | 0.1 (0.04) | 5 (1.8) | 0.3 (0.23) | 2 (1.5) |
| Home | 0.8 (0.67) | 3 (1.1) | 0.2 (0.19) | 2 (1.5) |
| Equipment, adaptations/repairs, n (%) | N = 280 | N = 136 | ||
| Self-financed | 0.08 (0.02) | 21 (7.5) | 0.11 (0.03) | 15 (11.0) |
| Health service | 0.08 (0.02) | 22 (7.9) | 0.22 (0.04) | 30 (22.1) |
| Local authority | 0.02 (0.01) | 6 (2.1) | 0.07 (0.02) | 9 (6.6) |
| Voluntary organisation | 0.0 (0.00) | 0 (0.00) | 0.03 (0.01) | 4 (2.9) |
| Private organisation | 0.17 (0.02) | 48 (17.1) | 0.2 (0.03) | 27 (19.9) |
| Medications, n (%) | N = 280 | N = 135 | ||
| Number (%) of participants with | ||||
| One medication | 0.12 (0.02) | 33 (11.8) | 0.13 (0.03) | 17 (12.6) |
| Two medications | 0.35 (0.03) | 98 (35.0) | 0.4 (0.04) | 54 (40.0) |
| Three medications | 0.15 (0.02) | 41 (14.6) | 0.13 (0.03) | 17 (12.6) |
| More than three medications | 0.04 (0.01) | 108 (38.6) | 0.35 (0.04) | 47 (34.8) |
| Participant travel (societal), n (%) | N = 280 | N = 135 | ||
| Hospital service visit | ||||
| Bus | 0.05 (0.01) | 13 (4.6) | 0.07 (0.02) | 9 (6.7) |
| Car | 0.04 (0.01) | 104 (37.1) | 0.4 (0.04) | 54 (40.0) |
| Taxi | 0.01 (0.01) | 4 (1.4) | 0.04 (0.02) | 6 (4.4) |
| Train | 0.0 (0.00) | 1 (0.4) | 0.0 (0.00) | 0 (0.00) |
| Walk/cycle | 0.01 (0.01) | 2 (0.7) | 0.02 (0.01) | 3 (2.2) |
| Ambulance | 0.09 (0.02) | 24 (8.6) | 0.04 (0.02) | 6 (4.4) |
| Voluntary | 0.0 (0.00) | 1 (0.4) | 0.0 (0.00) | 0 (0.00) |
| Hospital day visit | ||||
| Bus | 0.03 (0.01) | 9 (3.2) | 0.01 (0.01) | 2 (1.5) |
| Car | 0.08 (0.02) | 23 (8.2) | 0.1 (0.03) | 13 (9.6) |
| Taxi | 0.0 (0.00) | 1 (0.4) | 0.0 (0.00) | 0 (0.00) |
| Walk/cycle | 0.0 (0.00) | 0.0 (0.00) | 0.0 (0.00) | 0 (0.00) |
| Voluntary | 0.01 (0.01) | 2 (0.7) | 0.0 (0.00) | 0 (0.00) |
| General health community visit | ||||
| Bus | 0.06 (0.01) | 17 (6.1) | 0.05 (0.02) | 7 (5.1) |
| Car | 0.06 (0.01) | 164 (58.6) | 0.6 (0.04) | 81 (59.6) |
| Taxi | 0.02 (0.01) | 5 (1.8) | 0.03 (0.01) | 4 (2.9) |
| Walk/cycle | 0.16 (0.02) | 44 (15.7) | 0.16 (0.03) | 22 (16.2) |
| Ambulance | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Voluntary | 0.0 (0.00) | 1 (0.4) | 0.0 (0.00) | 0 (0.00) |
| Mental health community visit | ||||
| Bus | 0.04 (0.01) | 12 (4.3) | 0.04 (0.02) | 6 (4.4) |
| Car | 0.34 (0.03) | 94 (33.6) | 0.35 (0.04) | 48 (35.3) |
| Taxi | 0.01 (0.01) | 4 (1.4) | 0.01 (0.01) | 1 (0.7) |
| Walk/cycle | 0.01 (0.01) | 4 (1.4) | 0.01 (0.01) | 2 (1.5) |
| Ambulance | 0.0 (0.00) | 1 (0.4) | 0.0 (0.00) | 0 (0.00) |
| Voluntary | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.00) |
| Social care visit | ||||
| Bus | 0.01 (0.01) | 3 (1.1) | 0.01 (0.01) | 1 (0.7) |
| Car | 0.08 (0.02) | 21 (7.5) | 0.1 (0.03) | 13 (9.6) |
| Taxi | 0.01 (0.01) | 2 (0.7) | 0.02 (0.01) | 3 (2.2) |
| Walk/cycle | 0.01 (0.01) | 4 (1.4) | 0.01 (0.01) | 1 (0.7) |
| Ambulance | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.00) |
| Voluntary | 0.0 (0.00) | 1 (0.4) | 0.01 (0.01) | 1 (0.7) |
| Privately provided general community health servicesb | N = 280 | N = 135 | ||
| General practitioner | ||||
| Office visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.01) | 1 (0.7) |
| Practice nurse (GP clinic) | ||||
| Office visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| District nurse | ||||
| Home visit | 0.0 (0.02) | 1 (0.4) | 0.0 (0.00) | 0 (0.0) |
| Physiotherapist | ||||
| Office visit | 0.0 (0.02) | 1 (0.4) | 0.0 (0.04) | 1 (0.7) |
| Home visit | 0.0 (0.02) | 1 (0.4) | 0.0 (0.01) | 1 (0.7) |
| Alternative medicine/therapist | ||||
| Office visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.01) | 1 (0.7) |
| Other | ||||
| Office visit | 0.1 (0.09) | 5 (1.8) | 0.0 (0.02) | 3 (2.2) |
| Home visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.04) | 1 (0.7) |
| Privately provided mental health (community) | N = 280 | N = 136 | ||
| Community psychiatrist | ||||
| Office visit | 0.0 (0.00) | 1 (0.4) | 0.0 (0.00) | 0 (0.0) |
| Home visit | 0.0 (0.00) | 0 (0.0) | 0.0 (0.00) | 0 (0.0) |
| Time off work (societal) | N = 255 | N = 128 | ||
| Hours | 0.4 (0.14) | 8 (3.1) | 0.4 (0.20) | 4 (3.1) |
| Days | 0.3 (0.11) | 9 (3.5) | 0.1 (0.03) | 5 (3.9) |
Economic costs
Economic costs for participants with complete data are presented in Tables 42–45 by trial group, study period and cost category. With the exception of the cost of the exercise intervention, there were no statistically significant differences between the trial groups in any cost category, at any time point. The mean cost of the exercise programme in participants with complete resource-use data over the entire follow-up period was £1269 (SE £30) (see Table 45). Over the entire follow-up period, and for participants with complete data, the mean total NHS and personal social service costs, inclusive of the cost of the intervention, were £5945 (SE £492) in the intervention arm compared with £4597 (SE £444) in the control arm, generating a mean cost difference of £1347 (bootstrap 95% CI £8 to £2136; p = 0.00426). Over the entire follow-up period, and for participants with complete data, the mean total societal costs, inclusive of the cost of the intervention, were £6063 (SE £494) in the intervention arm compared with £4761 (SE £447) in the control arm, generating a mean cost difference of £1301 (bootstrap 95% CI £3 to £2096; p = 0.00479) (see Table 45).
| Cost category by period | Treatment arm, mean cost (£) (SE) | Mean cost (£) difference | p-valuea | Bootstrap 95% CIb | |
|---|---|---|---|---|---|
| Exercise programme (n = 326) | Usual care (n = 162) | ||||
| NHS/PSS costs | |||||
| Patient accommodation | 2.90 (2.89) | 201.90 (179.92) | –199.00 | 0.2704 | –597.00 to 3.30 |
| Hospital services | 315.50 (33.96) | 303.30 (52.94) | 12.20 | 0.8459 | –131.00 to 107.30 |
| Day care services | 8.60 (2.63) | 15.90 (5.44) | –7.30 | 0.2302 | –19.90 to 3.60 |
| General community health services | 101.40 (7.53) | 93.10 (9.86) | 8.30 | 0.5048 | –17.60 to 33.30 |
| Community mental health services | 60.90 (7.53) | 53.30 (6.28) | 7.60 | 0.4365 | –6.70 to 28.90 |
| Social care services | 105.90 (26.67) | 163.50 (69.20) | –57.60 | 0.4381 | –196.80 to 59.00 |
| Equipment, adaptations/repairs | 1.00 (0.74) | 0.80 (0.43) | 0.23 | 0.7811 | –0.80 to 2.20 |
| Patient travelc | 2.00 (0.21) | 2.10 (0.34) | –0.10 | 0.7861 | –0.90 to 0.76 |
| Concomitant/prescription medications | 57.3 (8.83) | 38.30 (7.39) | 18.90 | 0.1000 | –1.30 to 41.60 |
| Other | 25.1 (10.93) | 41.00 (30.18) | –15.90 | 0.6312 | –83.00 to 38.90 |
| Total (NHS/PSS) | 680.62 (48.23) | 913.10 (201.56) | –232.50 | 0.2748 | –648.10 to 26.10 |
| Broader societal costs | |||||
| Privately provided general community health services | 2.90 (1.61) | 2.40 (1.80) | 0.55 | 0.8175 | –5.20 to 5.00 |
| Privately provided mental health services | 10.30 (7.29) | 6.00 (3.07) | 4.30 | 0.5856 | –8.40 to 17.00 |
| Patient equipment | 2.20 (1.35) | 1.20 (0.94) | 1.00 | 0.5116 | –2.50 to 3.90 |
| Patient traveld | 0.70 (0.20) | 0.90 (0.33) | –0.20 | 0.5920 | –1.00 to 0.48 |
| Time off work | |||||
| Hours | 9.30 (2.87) | 8.20 (4.06) | 1.000 | 0.8320 | –10.30 to 9.50 |
| Days | 5.50 (4.01) | 7.10 (3.17) | –1.50 | 0.7559 | –10.30 to 9.80 |
| Total broader societal | 30.90 (5.37) | 25.80 (5.52) | –5.10 | 0.9956 | –17.40 to 8.30 |
| Total (societal) | 711.50 (48.90) | 938.90 (202.05) | –227.40 | 0.2374 | –670.90 to 6.70 |
| Cost category by period | Treatment arm, mean cost (£) (SE) | Mean cost (£) difference | p-valuea | Bootstrap 95% CIb | |
|---|---|---|---|---|---|
| Exercise programme (n = 298) | Usual care (n = 142) | ||||
| NHS/PSS costs | |||||
| Patient accommodation | 37.60 (24.11) | 43.00 (27.59) | –5.39 | 0.8831 | –69.10 to 50.70 |
| Hospital services | 590.10 (75.69) | 676.70 (103.80) | –86.6 | 0.5006 | –299.40 to 140.60 |
| Day care services | 9.80 (1.96) | 12.20 (3.18) | –2.40 | 0.5111 | –8.50 to 4.80 |
| General community health services | 138.60 (15.60) | 136.00 (13.07) | 2.60 | 0.8945 | –28.10 to 32.00 |
| Community mental health services | 60.30 (4.48) | 69.80 (11.66) | –9.50 | 0.4421 | –34.10 to 13.40 |
| Social care services | 224.50 (68.74) | 246.90 (80.22) | –22.40 | 0.8332 | –249.40 to 162.50 |
| Equipment, adaptations/repairs | 0.80 (0.30) | 2.60 (2.13) | –1.80 | 0.4067 | –6.20 to 0.920 |
| Patient travelc | 2.70 (0.32) | 2.80 (0.35) | –0.10 | 0.8264 | –0.88 to 0.79 |
| Concomitant/prescription medications | 331.80 (33.73) | 343.90 (69.95) | –12.10 | 0.8778 | –157.60 to 122.10 |
| Other | 36.70 (14.13) | 40.00 (21.58) | –3.30 | 0.9096 | –59.00 to 41.40 |
| Total (NHS/PSS) | 1432.90 (119.87) | 1573.00 (150.01) | –140.10 | 0.4890 | –569.70 to 71.10 |
| Broader societal costs | |||||
| Privately provided general community health services | 4.50 (2.18) | 1.90 (1.31) | 2.50 | 0.3079 | –2.10 to 7.20 |
| Privately provided mental health services | 8.00 (2.68) | 19.10 (15.39) | –11.00 | 0.4877 | –53.40 to 9.60 |
| Patient equipment | 2.00 (1.24) | 1.30 (1.06) | 0.69 | 0.6712 | –2.50 to 3.70 |
| Patient traveld | 1.10 (0.31) | 1.20 (0.32) | –0.0097 | 0.8278 | –1.40 to 0.98 |
| Time off work | |||||
| Hours | 8.80 (2.68) | 4.10 (2.34) | 4.70 | 0.1820 | –1.30 to 12.10 |
| Days | 39.60 (15.98) | 27.60 (11.48) | 12.00 | 0.5412 | –33.20 to 49.70 |
| Total broader societal | 64.00 (16.91) | 55.10 (12.55) | 8.90 | 0.7463 | –39.20 to 33.40 |
| Total (societal) | 1496.90 (123.61) | 1628.10 (151.16) | –131.20 | 0.5198 | –566.80 to 85.10 |
| Cost category by period | Treatment arm, mean cost (£) (SE) | Mean cost (£) difference | p-valuea | Bootstrap 95% CIb | |
|---|---|---|---|---|---|
| Exercise programme (n = 280) | Usual care (n = 142) | ||||
| NHS/PSS costs | |||||
| Patient accommodation | 150.00 (55.71) | 11.40 (8.20) | 138.60 | 0.0143 | 37.80 to 257.90 |
| Hospital services | 1429.20 (454.93) | 1150.30 (290.21) | 278.90 | 0.6043 | –636.30 to 1392.50 |
| Day care services | 24.10 (4.28) | 37.00 (8.56) | –12.80 | 0.1827 | –32.00 to 7.24 |
| General community health services | 227.70 (28.008) | 211.60 (23.14) | 16.20 | 0.6576 | –58.30 to 80.40 |
| Community mental health services | 103.30 (26.32) | 80.40 (14.87) | 22.90 | 0.4411 | –31.80 to 75.00 |
| Social care services | 422.50 (92.75) | 513.00 (152.95) | –90.50 | 0.6128 | –373.30 to 230.00 |
| Equipment, adaptations/repairs | 1.10 (0.45) | 11.90 (10.24) | –10.80 | 0.2939 | –31.40 to 0.87 |
| Patient travelc | 3.00 (0.33) | 4.20 (0.92) | –1.20 | 0.2328 | –3.40 to 0.16 |
| Concomitant/prescription medications | 714.40 (66.48) | 723.50 (118.67) | –9.10 | 0.9428 | –307.00 to 255.70 |
| Other | 167.80 (30.85) | 281.00 (211.91) | –113.30 | 0.6197 | –493.30 to 152.00 |
| Total (NHS/PSS) | 3243.10 (479.93) | 3024.30 (389.91) | 219.30 | 0.7644 | –1170.20 to 919.70 |
| Broader societal costs | |||||
| Privately provided general community health services | 4.60 (2.26) | 1.50 (1.09) | 3.20 | 0.2031 | –1.62 to 7.40 |
| Privately provided mental health services | 11.60 (4.16) | 84.40 (72.15) | –72.85 | 0.3238 | –187.05 to 7.80 |
| Patient equipment | 6.50 (3.70) | 6.10 (4.59) | 0.420 | 0.9489 | –15.10 to 13.40 |
| Patient traveld | 1.00 (0.31) | 2.20 (0.90) | –1.20 | 0.2066 | –3.50 to 0.10 |
| Time off work | |||||
| Hours | 5.00 (1.94) | 6.20 (3.05) | –1.20 | 0.7345 | –9.60 to 4.80 |
| Days | 25.60 (11.21) | 8.20 (4.11) | 17.40 | 0.1443 | –3.70 to 38.40 |
| Total broader societal | 54.30 (13.85) | 108.60 (19.94) | –54.30 | 0.0256e | –108.20 to –27.10 |
| Total (societal) | 3297.40 (481.22) | 3132.90 (398.75) | 165.30 | 0.8222 | –1240.10 to 871.90 |
| Cost category by period | Treatment arm, mean cost (£) (SE) | Mean cost (£) difference | p-valuea | Bootstrap 95% CIb | |
|---|---|---|---|---|---|
| Exercise programme (n = 280) | Usual care (n = 136) | ||||
| NHS/PSS costs | |||||
| Patient accommodation | 187.60 (58.02) | 54.40 (30.16) | 133.20 | 0.0513 | –6.90 to 210.80 |
| Hospital services | 2019.30 (466.80) | 1827.00 (320.04) | 192.30 | 0.7342 | –1001.90 to 858.10 |
| Day care services | 33.90 (4.82) | 49.20 (10.25) | –15.30 | 0.1685 | –36.90 to 1.12 |
| General community health services | 366.30 (38.25) | 347.60 (27.88) | 18.70 | 0.6438 | –62.30 to 64.90 |
| Community mental health services | 163.60 (27.08) | 150.20 (23.81) | 13.40 | 0.7108 | –62.20 to 56.30 |
| Social care services | 647.00 (123.90) | 759.90 (190.09) | –112.90 | 0.6190 | –565.30 to 169.30 |
| Equipment, adaptations/repairs | 1.90 (0.62) | 14.50 (10.23) | –12.60 | 0.2092 | –30.60 to 1.74 |
| Patient travelc | 5.70 (0.58) | 7.00 (1.01) | –1.30 | 0.2651 | –3.60 to 0.16 |
| Concomitant/prescription medications | 1046.20 (78.66) | 1067.40 (161.72) | –21.20 | 0.9081 | –372.30 to 209.40 |
| Other | 204.50 (37.86) | 32.00 (184.50) | –116.50 | 0.5366 | –466.40 to 140.40 |
| Total (NHS/PSS) | 4676.20 (507.66) | 4597.30 (444.35) | 78.70 | 0.9066 | –1336.70 to 880.30 |
| Broader societal costs | |||||
| Privately provided general community health services | 9.10 (4.32) | 3.4 (2.33) | 5.70 | 0.2431 | –4.80 to 11.40 |
| Privately provided mental health services | 19.60 (5.35) | 103.5 (73.61) | –83.90 | 0.2562 | –216.50 to 21.10 |
| Patient equipment | 8.50 (4.12) | 7.4 (4.77) | 1.10 | 0.8617 | –11.80 to 8.80 |
| Patient traveld | 2.10 (0.57) | 3.4 (0.97) | –1.30 | 0.2656 | –3.60 to 0.16 |
| Time off work | |||||
| Hours | 13.80 (3.92) | 10.3 (4.24) | 3.50 | 0.5417 | –8.30 to 10.40 |
| Days | 65.20 (19.19) | 35.8 (12.46) | 29.40 | 0.2001 | –19.10 to 56.30 |
| Total broader societal | 118.30 (20.55) | 163.7 (20.01) | –45.40 | 0.0594 | –104.10 to 1.40 |
| Total (societal) | 4794.30 (510.66) | 4761.00 (447.24) | 33.30 | 0.9609 | –1390.50 to 838.80 |
| Intervention costs | 1268.70 (29.56) | ||||
| Total PSS/NHS including intervention costs | 5944.90 (491.75) | 4597.30 (444.35) | 1347.40 | 0.0426 | 8.20 to 2135.70 |
| Total societal including intervention costs | 6063.00 (494.08) | 4761.10 (447.24) | 1301.90 | 0.0479 | 2.80 to 2095.50 |
Health-related quality-of-life outcomes
There were no statistically significant differences in suboptimal levels of function in HRQoL for either participant- or carer-reported dimensions of the EQ-5D-3L, between the exercise and usual-care groups, at each of the 6- and 12-month follow-up time points (Tables 46 and 47). For complete cases, the mean (SE) patient-reported QALY estimate was 0.787 (SE 0.012) versus 0.826 (SE 0.019) for exercise versus control (Table 48); this was 0.758 (SE 0.014) versus 0.782 (SE 0.020) based on the carer-reported EQ-5D-3L, respectively. There were no statistically significant differences in the overall EQ-5D-3L utility scores or EQ-5D-3L VAS scores between the exercise and usual-care groups at each of the follow-up time points. There were no statistically significant differences in either participant- or carer-reported QALY estimates (see Table 48).
| Time point | EQ-5D-3L item (level), n (%) | EQ-5D-3L VAS, mean (SE) | EQ-5D-3L utility, mean (SE) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mobility | Self care | Usual activities | Pain/discomfort | Anxiety/depression | |||||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |||
| Baseline (N = 494) | |||||||||||||||||
| Exercise (n = 329) | 234 (71) | 95 (29) | 0 | 283 (86) | 44 (13) | 1 (0) | 240 (73) | 82 (25) | 6 (2) | 209 (64) | 110 (33) | 8 (2) | 230 (70) | 96 (29) | 2 (1) | 76.74 (1.03) | 0.819 (0.011) |
| Control (n = 165) | 121 (73) | 42 (25) | 0 | 142 (86) | 21 (13) | 0 | 123 (75) | 35 (21) | 3 (2) | 110 (67) | 51 (31) | 2 (1) | 120 (73) | 41 (25) | 0 | 81.83 (1.39) | 0.849 (0.014) |
| p-valuea | 0.0035 | 0.1043 | |||||||||||||||
| 6 months (N = 445) | |||||||||||||||||
| Exercise (n = 300) | 202 (67) | 93 (31) | 0 | 251 (84) | 39 (13) | 3 (1) | 202 (67) | 79 (26) | 12 (4) | 183 (61) | 106 (35) | 5 (2) | 201 (67) | 88 (29) | 5 (2) | 75.42 (1.21) | 0.800 (0.013) |
| Control (n = 145) | 101 (70) | 39 (27) | 0 | 116 (80) | 23 (16) | 1 (1) | 108 (74) | 31 (21) | 1 (1) | 99 (68) | 37 (26) | 4 (3) | 103 (71) | 34 (23) | 2 (1) | 78.67 (1.60) | 0.833 (0.018) |
| p-valuea | 0.1067 | 0.1480 | |||||||||||||||
| 12 months (N = 418) | |||||||||||||||||
| Exercise (n = 281) | 184 (65) | 86 (31) | 0 | 223 (79) | 39 (14) | 7 (2) | 184 (65) | 72 (26) | 9 (3) | 171 (61) | 93 (33) | 5 (2) | 183 (65) | 79 (28) | 3 (1) | 75.46 (1.19) | 0.805 (0.014) |
| Control (n = 137) | 92 (67) | 39 (28) | 1 (1) | 113 (82) | 14 (10) | 4 (3) | 97 (71) | 30 (22) | 4 (3) | 88 (64) | 40 (29) | 4 (3) | 100 (73) | 29 (21) | 3 (2) | 78.27 (1.74) | 0.816 (0.022) |
| p-valuea | 0.1829 | 0.6815 | |||||||||||||||
| Time Point | EQ-5D-3L item (level), n (%) | EQ-5D-3L VAS, mean (SE) | EQ-5D-3L utility, mean (SE) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mobility | Self care | Usual activities | Pain/discomfort | Anxiety/depression | |||||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |||
| Baseline (N = 459) | |||||||||||||||||
| Exercise (n = 305) | 229 (75) | 76 (25) | 0 | 292 (96) | 13 (4) | 0 | 228 (75) | 74 (24) | 3 (1) | 155 (51) | 139 (46) | 11 (4) | 165 (54) | 135 (44) | 4 (1) | 77.35 (1.02) | 0.794 (0.012) |
| Control (n = 154) | 120 (78) | 34 (22) | 0 | 151 (98) | 3 (2) | 0 | 121 (79) | 32 (21) | 1 (1) | 96 (62) | 50 (32) | 8 (5) | 93 (60) | 57 (37) | 3 (2) | 77.67 (1.49) | 0.819 (0.019) |
| p-valuea | 0.8601 | 0.2558 | |||||||||||||||
| 6 months (N = 417) | |||||||||||||||||
| Exercise (n = 281) | 187 (67) | 91 (32) | 0 | 269 (96) | 9 (3) | 0 | 195 (69) | 81 (29) | 2 (1) | 133 (47) | 131 (47) | 14 (5) | 133 (47) | 135 (48) | 9 (3) | 73.37 (1.18) | 0.760 (0.014) |
| Control (n = 136) | 101 (74) | 33 (24) | 0 | 129 (95) | 6 (4) | 0 | 96 (71) | 35 (26) | 3 (2) | 70 (51) | 56 (41) | 9 (7) | 66 (49) | 66 (49) | 2 (1) | 72.38 (1.77) | 0.774 (0.021) |
| p-valuea | 0.6417 | 0.5731 | |||||||||||||||
| 12 months (N = 393) | |||||||||||||||||
| Exercise (n = 263) | 177 (67) | 84 (32) | 0 | 250 (95) | 11 (4) | 0 | 183 (70) | 76 (29) | 2 (1) | 128 (49) | 122 (46) | 11 (4) | 136 (52) | 112 (43) | 13 (5) | 74.52 (1.15) | 0.765 (0.015) |
| Control (n = 130) | 99 (76) | 30 (23) | 0 | 128 (98) | 1 (1) | 0 | 88 (68) | 39 (30) | 2 (2) | 67 (52) | 54 (42) | 8 (6) | 68 (52) | 58 (45) | 3 (2) | 75.09 (1.64) | 0.779 (0.020) |
| p-valuea | 0.7742 | 0.5795 | |||||||||||||||
| Treatment arm | QALY (EQ-5D-3L) | |||
|---|---|---|---|---|
| Participant | Carer | |||
| n | Mean (SE) | n | Mean (SE) | |
| Exercise | 294 | 0.787 (0.012) | 279 | 0.758 (0.014) |
| Usual care | 141 | 0.826 (0.019) | 137 | 0.782 (0.020) |
| Mean differencea | –0.039 | –0.024 | ||
| p-value | 0.090 | 0.330 | ||
| 95% CI | –0.083 to 0.0061 | –0.073 to 0.0324 | ||
Cost-effectiveness results
Baseline analysis
The incremental cost-effectiveness of the exercise programme is shown in Table 49 for the participants with costs and QALY data subject to MI. When a NHS/PSS perspective was adopted (i.e. that adopted for the baseline analysis) and health outcomes were measured in terms of QALYs, the mean (SE) total cost was £5580 (SE £436) in the exercise group, compared with £3917 (SE £620) in the usual-care group, generating a mean incremental cost of £1663. The mean incremental cost-effectiveness of the exercise intervention was estimated at –£74,227 per QALY, that is, on average, the intervention was associated with a higher net cost and a lower net effect and was dominated in health economic terms.
| Type of analysis | Incremental cost (95% CI) | Incremental QALYs (95% CI) | ICERa (95% CI) | Probability of cost-effectiveness | INMB | ||||
|---|---|---|---|---|---|---|---|---|---|
| pb | pc | pd | INMBa,b | INMBa,c | INMBa,d | ||||
| Base case (NHS/PSS perspective) | |||||||||
| Imputed attributable costs and QALYs, covariate- and baseline-adjusted EQ-5D-3L utility score | 1663 (120 to 3207) | –0.0220 (–0.0621 to 0.0181) | Dominated | 0.0011 | 0.0012 | 0.0014 | –2158 (–3455 to –969) | –2306 (–3678 to –1041) | –2601 (–4128 to –1176) |
| Sensitivity analyses | |||||||||
| 1. Complete cases attributable costs and QALYs, and baseline-adjusted EQ-5D-3L utility score | 1549 (458 to 2764) | –0.0254 (–0.0592 to 0.0084) | Dominated | 0.0044 | 0.0044 | 0.0050 | –1943 (–3238 to –756) | –2071 (–3420 to –828) | –2325 (–3823 to –922) |
| 2. Imputed societal attributable costs and QALYs, covariate- and baseline-adjusted EQ-5D-3L utility score | 1574 (6 to 3123) | –0.0220 (–0.0621 to 0.0181) | Dominated | 0.0079 | 0.0079 | 0.0068 | –1710 (–2896 to –503) | –2233 (–3789 to –777) | –2412 (–3936 to –972) |
| 3. Imputed attributable costs and QALYs, covariate- and baseline-adjusted carer-reported EQ-5D-3L utility score | 1663 (120 to 3207) | –0.00665 (–0.0453 to 0.0320) | Dominated | 0.0026 | 0.0027 | 0.0044 | –1867 (–3094 to –757) | –1917 (–3182 to –757) | –2017 (–3380 to –738) |
| 4. Imputed attributable costs and QALYs, covariate- and baseline-adjusted EQ-5D-3L utility score, including practitioner travel costs | 1971 (959 to 3122) | –0.0220 (–0.0621 to 0.0181) | Dominated | 0 | 0.0010 | 0.0025 | –2264 (–3439 to –1124) | –2379 (–3625 to –1178) | –2610 (–4034 to –1216) |
| 5. Imputed attributable costs and QALYs, covariate- and baseline-adjusted EQ-5D-3L utility score assuming cohort size (n = 3) | 2773 (2458 to 2954) | –0.0220 (–0.0621 to 0.0181) | Dominated | 0 | 0 | 0.0001 | –3055 (–3327 to –2790) | –3172 (–3454 to –2891) | –3406 (–3723 to –3085) |
| 6. Imputed attributable costs and QALYs, covariate- and baseline-adjusted EQ-5D-3L utility score assuming cohort size (n = 10) | 983 (669 to 1165) | –0.0220 (–0.0621 to 0.0181) | Dominated | 0.0495 | 0.0486 | 0.0511 | –1265 (–1538 to –1000) | –1382 (–1663 to –1102) | –1616 (–1931 to –1294) |
| 7. Imputed attributable costs and QALYs, covariate- and baseline-adjusted EQ-5D-3L utility score, excluding venue hire costs | 1203 (–61 to 2240) | –0.0220 (–0.0621 to 0.0181) | Dominated | 0.0250 | 0.0260 | 0.050 | –1417 (–2698 to –219) | –1543 (–2892 to –286) | –1796 (–3297 to –364) |
| Subgroup analyses (gender and sMMSE score) | |||||||||
| Imputed attributable costs and QALYs, covariate- and baseline-adjusted EQ-5D-3L utility score | |||||||||
| Male | 1383 (23 to 3068) | –0.0263 (–0.049 to 0.027) | Dominated | 0.0461 | 0.0486 | 0.0608 | –1631 (–3346 to –33) | –1688 (–3469 to –12) | –1802 (–3784 to –105) |
| Female | 1511 (–74 to 3126) | –0.0215 (–0.087 to 0.0127) | Dominated | 0.0012 | 0.0016 | 0.0030 | –2140 (–3744 to –568) | –2239 (–4028 to –499) | –2440 (–4632 to –322) |
| Baseline sMMSE score of < 20 | 1206 (804 to 1385) | –0.00204 (–0.0135 to 0.00935) | Dominated | 0.0318 | 0.0326 | 0.0375 | –1128 (–1470 to –779) | –1139 (–1519 to –753) | –1161 (–1624 to –696) |
| Baseline sMMSE score of ≥ 20 | 1951 (1585 to 2331) | –0.0334 (–0.0415 to 0.0253) | Dominated | 0.0090 | 0.0011 | 0.0021 | –2453 (–2856 to –2066) | –2621 (–3042 to –2216) | –2955 (–3415 to –2505) |
The associated mean INMB at cost-effectiveness thresholds of £15,000, £20,000 and £30,000 per QALY were –£2158, –£2306 and –£2601, respectively (see Table 49). The base-case mean INMB was < 0, suggesting that the exercise group would result in an average NHS/PSS loss of about £2158 (INMB –£2158, 95% CI –£3455 to –£969). The cost-effectiveness plane (Figure 11) shows that the vast majority of the ICER values lie in the north-west quadrant. These result in a probability of cost-effectiveness close to zero (Figure 12), that is, if decision-makers are willing to pay between £15,000 and £30,000 for an additional QALY, the probability that the exercise intervention is cost-effective is very low < 1% (see Table 49).
FIGURE 11.
Cost-effectiveness plane for exercise programme: incremental cost (£) vs. incremental QALY: base case.

FIGURE 12.
Cost-effectiveness acceptability curve: base case.
Sensitivity analyses
Several sensitivity analyses were undertaken to assess the impact of uncertainty surrounding key parameters or methodological features on the cost-effectiveness results. The probability that the exercise intervention is cost-effective remained relatively static (< 1%) for the majority of the sensitivity analyses (complete cases, societal costs, carer-reported EQ-5D-3L score, inclusion of practitioner travel costs and changes in the number of participants per cohort to the lowest number observed). When venue hire costs were excluded and when the number of participants per cohort was set at the highest number observed across all groups, the probability that the exercise intervention is cost-effective increased but remained at < 5%. All sensitivity analyses show the average INMB is unlikely to be positive, as all upper limits of the 95% CIs remain below zero (see Table 49 and Figures 13–15).
FIGURE 13.
Forest plot of sensitivity and subgroup analyses (impact on INMB): cost-effectiveness threshold of £30,000/QALY.
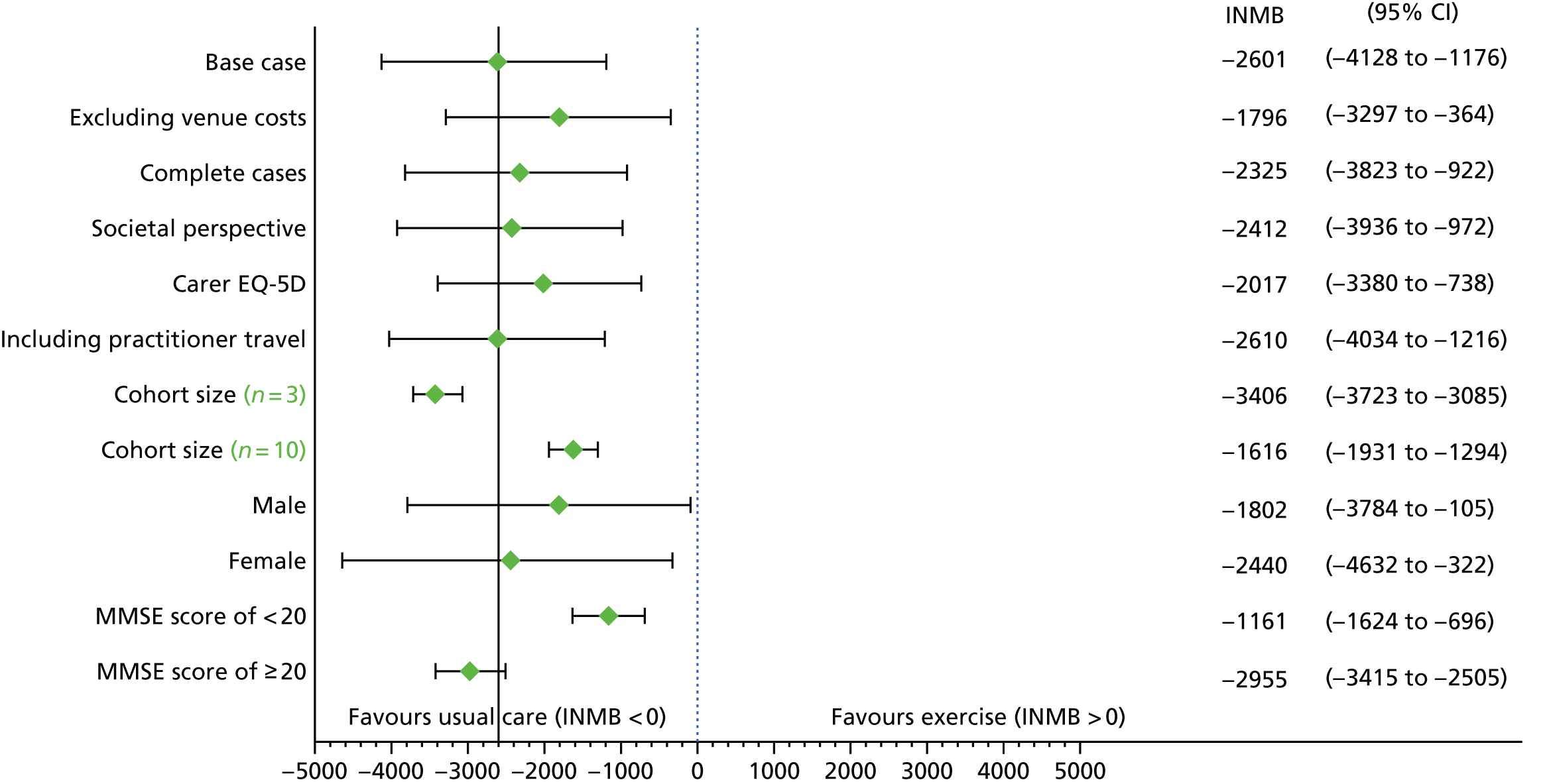
FIGURE 14.
Forest plot of sensitivity and subgroup analyses (impact on INMB): cost-effectiveness threshold of £20,000/QALY.

FIGURE 15.
Forest plot of sensitivity and subgroup analyses (impact on INMB): cost-effectiveness threshold of £15,000/QALY.
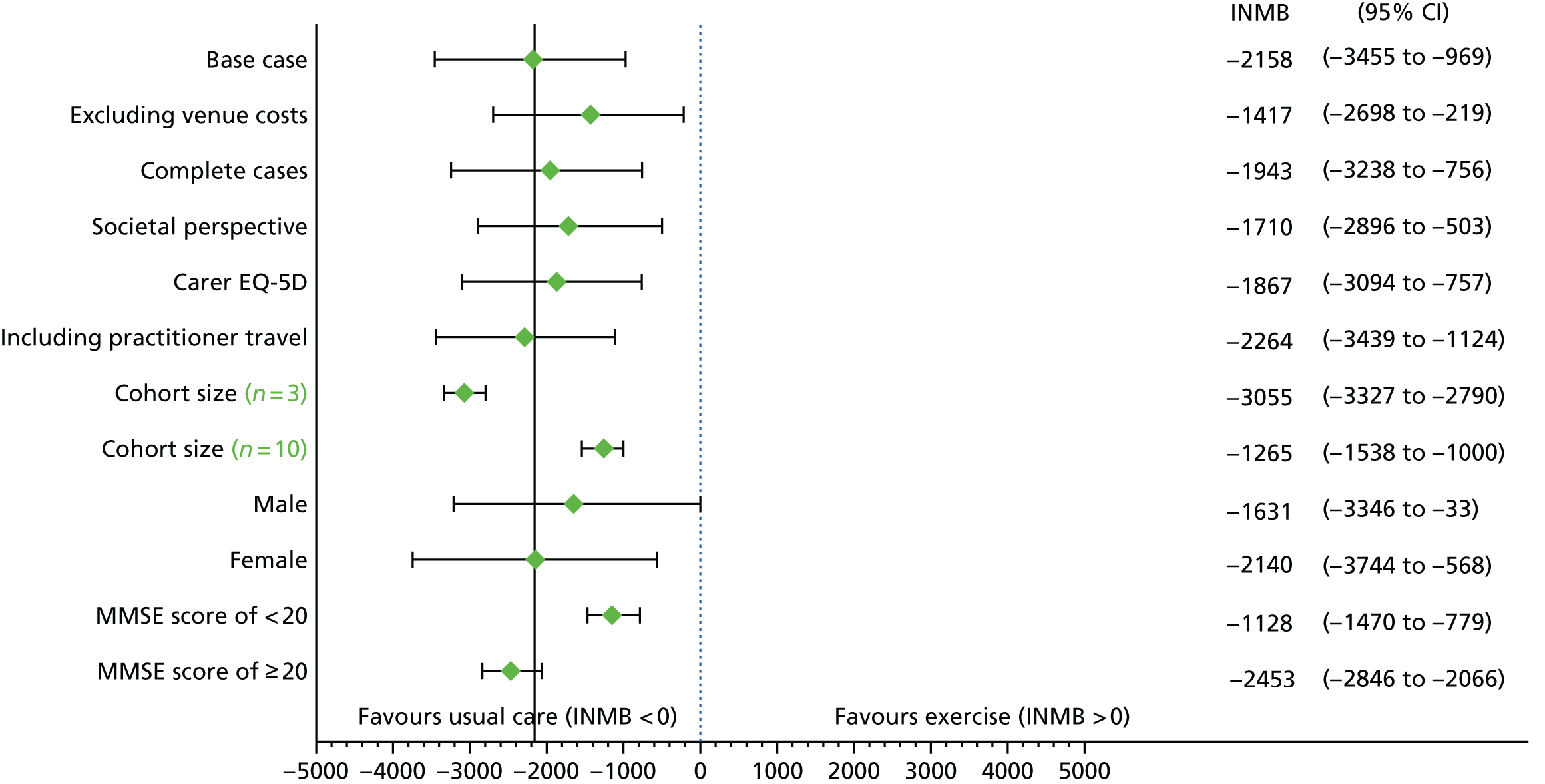
Subgroup analyses
Four subgroups were subjected to cost-effectiveness analyses to explore the heterogeneity in cost-effectiveness results: males, females, baseline sMMSE score of < 20 and baseline sMMSE score of ≥ 20 (see Table 49 and Figures 13–15). Both subgroup analyses were based on the patient-reported EQ-5D-3L score using MI adjusted for covariates. There was no evidence that gender or the baseline sMMSE score has a significant effect on the cost-effectiveness of the exercise programme.
Discussion
This trial-based economic evaluation revealed that the exercise programme is not cost-effective compared with usual care for adults with MMD in a community-dwelling setting. The INMB estimate was negative, a finding that remained robust to several sensitivity and subgroup analyses. The clear-cut result of the trial-based economic evaluation precluded the need for extrapolation of cost-effectiveness over a longer-time horizon than observed within the trial. This would have required the development of a de novo decision-analytic model according to accepted guidelines for good practice in decision-analytic modelling and the general principles outlined in the NICE ‘reference case’. 124,146
The main reason for lack of cost-effectiveness appears to stem from a combination of higher cost in the experimental group with no evidence of effectiveness based on EQ-5D-3L-derived QALYs, although, from a NHS and PSS perspective, a number of costs were, on average, slightly lower (although not statistically significant) within the exercise group.
There is little evidence for cost-effectiveness of exercise in dementia patients reported in literature. This cost-effectiveness analysis using patient-level data is based on the largest RCT of its kind reported to date. The results from these analyses are consistent with a recent cost-effectiveness analysis of exercise as a therapy for behavioural and psychological symptoms of dementia. 147 In that smaller (n = 131) RCT [EVIDEM-E; (Evidence Based Interventions in Dementia Exercise Therapy)] the exercise therapy was not cost-effective based on the incremental cost per QALY metric. In the DAPA study, we confirm this conclusion. Results from other much smaller (n = 40) trials148 suggest that community-based exercise programmes confer cognitive and physical benefits with the potential to show cost-effectiveness but this has not been substantiated.
The main strengths of this analysis are that the trial was prospectively designed for a cost-effectiveness analysis using individual-level data. Costs and outcomes were carefully considered in the design of this trial with the purpose of reaching a robust conclusion with respect to cost-effectiveness in a large sample of individuals. There were, however, several limitations to this cost-effectiveness analysis. In the absence of any effect in either the primary clinical outcome or the EQ-5D-3L-based QALYs, usual care was dominant in health economic terms. Unless the expected costs are lower for the exercise group, usual care would almost always dominate.
Second, QALYs were based on utility measurement at just two time points post randomisation. Although the trial did not yield benefits, the assumption of linearity of HRQoL between data collection points is uncertain and more uncertain when missing data are present. Third, despite the longitudinal nature of the study, resource use was retrospectively recalled by trial participants, which is likely to result in recall bias. Fourth, similar pilot or Phase II trials may have been useful in identifying the critical costs that drive cost-effectiveness. Instead, data for a broad spectrum of cost categories were collected that, on average, had little impact on the ICER. Many costs items did not occur (see Tables 40 and 41) and a reduced form of the CSRI in this setting may be advisable, with a focus on the largest and more relevant costs. In addition, the CSRI could be improved in several places, as it leads to many categories of ‘other’ costs that are very difficult and time-consuming to cost. Many of these costs had little impact on the results. Sensitivity analyses, for example, showed the cohort size to be the most influential factor on the INMB and not some of the cost components that were incorporated into the analysis.
Finally, the 95% CIs surrounding the incremental QALYs do not exclude the possibility of a small QALY benefit because the 95% CIs contain the value zero (see Table 49). However, the upper limit of these intervals from the sensitivity analyses never exceeds 0.03 (carer-reported EQ-5D-3L), in which case for an observed base-case incremental cost of £1683, the ICER would be very unlikely to be < £55,000 per QALY – rendering it not cost-effective at NICE thresholds in the most optimistic case.
In conclusion, the data collected in the DAPA trial strongly support a hypothesis that exercise therapy in addition to usual care, when compared with usual care alone, is more expensive and less effective.
Chapter 6 Systematic review
Effects of exercise in adults with dementia: a systematic review and meta-analysis of randomised controlled trials
We initiated the first version of this systematic review at the application stage to NIHR. We have updated the review at key stages. First it was updated during the intervention development stage (see Chapter 2) and then again as the results of the trial were finalised.
Aim
The aim was to undertake a systematic, comprehensive and replicable search for RCTs of exercise interventions in dementia, in which data on cognitive impairment were reported, and to assess the quality of evidence. We prespecified the reporting of global and specific domains of cognition and subgroup analyses defined by diagnosis (Alzheimer’s disease or any dementia), the setting from which participants were recruited (community or residential), intervention duration (< 4 or ≥ 4 months), total weekly exercise duration (< 150 or ≥ 150 minutes), exercise intensity (moderate or high) and intervention type (aerobic, resistance or mixed). We also explored length of follow-up as a potential source of heterogeneity in the review.
Methods
Search methods
Studies were identified using electronic searches of PubMed, Allied and Complementary Medicine Database (AMED), MEDLINE In-Process & Other Non-Indexed Citations (via OVID SP), EMBASE (via OVID SP), PsycINFO (via ProQuest), Applied Social Sciences Index and Abstracts (ASSIA), Latin American and Caribbean Health Sciences Literature (LILACS) and Cumulative Index to Nursing and Allied Health Literature(CINAHL; via EBSCOhost), as well as ALOIS (Cochrane Dementia and Cognitive Improvement Group), a specialist register of dementia studies. The original search was from inception to March 2014 and was updated to 13 September 2016. In addition, to identify any undetected studies, forward and backward citation tracking of systematic reviews and included studies was conducted and study authors, experts and research groups were contacted when necessary. An example of the search strategy used for the MEDLINE search is given in Appendix 9.
Inclusion criteria
Design
Inclusion was restricted to RCTs or studies from which RCT evidence could be extracted.
Participants
We included studies of people with probable dementia, diagnosed by a clinician and/or using recognised assessment measures. Studies were not restricted based on severity of dementia. Details on dementia severity, as indicated by either a recognised cognitive impairment scale or author assessment, were extracted. Studies must have sampled from community settings or residential care and not hospitals or psychiatric institutions. Two studies with more general populations (thus including participants without cognitive impairment) were included, as data were made available on the subgroup of eligible participants consistent with a clinical diagnosis of dementia. 149
Intervention
Included studies delivered interventions that prescribe an exercise programme, for which exercise is defined as any planned or structured movement of the body performed systematically for the purpose of gaining physical fitness. 150 Studies of interventions that provided general advice about exercise without prescribing an exercise programme were excluded. We included physical activity within our definition of exercise if the activity was prescribed and structured for the purpose of gaining fitness, for example prescribed walking programmes. We defined and categorised exercise types using the Taxonomy of Fall Prevention Interventions, which includes descriptors for the types and dose parameters for exercise and physical activity interventions used in older populations. 151 This included gait/balance/functional training (D100), strength/resistance training (D101), flexibility training (D102) and three-dimensional training, such as tai chi (D103), physical activity (D104) and endurance activity (D105).
Studies of multicomponent interventions were included if exercise was a core component. When studies had two or more exercise intervention arms, data from the exercise arm delivering the highest exercise dose (frequency, intensity and/or duration) were used. We defined low- and high-dose exercise using WHO’s guidance,106 which combines frequency and duration of an exercise session into low frequency (< 150 minutes per week) and high frequency ≥ 150 minutes per week) and intensity into low (3–6 metabolic equivalents) and high intensity (> 6 metabolic equivalents). We categorised intervention duration into short (< 4 months) and long duration (≥ 4 months), recognising that it takes a minimum of 6–8 weeks for physiological conditioning of the muscular and cardiovascular systems to occur, and between 8 and 16 weeks for improvements in physical function to emerge. 152
Control
Any study using a control group in which exercise was not a component was eligible for inclusion (including pharmacological, exercise advice or no active treatment). The control groups were classified as attention control (non-exercise activity/social contact of the same duration and frequency as the exposure to exercise in the exercise arm), active control (non-exercise-based activity and/or social contact for a duration or frequency less than that provided to participants in the exercise arm) or treatment as usual. When studies had two or more control arms, data from the attention control arm were used.
Outcomes
Studies were only included if they measured global and/or specific cognitive function outcomes. Specific cognitive functions were attention, memory, language, praxis and executive function, provided these were tested with recognised methods. If a study used two measurements that tested the same cognitive function, then we included the outcome measure with stronger psychometric validity based on the literature and expert opinion. In the case of outcome measures having similar psychometric properties, we used the measure that was most commonly used across our included studies.
Data collection
Search results were merged, duplicates were removed and studies reviewed for eligibility. Each reviewer’s decisions were quality checked by another author (Bethan Copsey, Susanne Finnegan and Deborah Brown). Disagreements were discussed with a fourth author for a final decision (Beth Fordham). All authors are either experienced in the dementia and exercise research field or experienced systematic reviewers. The eligible studies were retrieved for full-text review and assessed using the same process used for the original search results.
Data were extracted on study setting, inclusion/exclusion criteria, participant characteristics, intervention and control treatments and outcomes. If data were not available and could not be calculated from the reported details, we contacted the primary author for clarification. The quality of studies was assessed using the Cochrane risk-of-bias assessment. 153 All data extraction was performed by two independent reviewers and disagreements were resolved with Beth Fordham. For papers not published in English, data extraction and quality assessment (risk of bias) were performed by a native speaker.
We also include within the meta-analysis the results of the DAPA trial to place the results of the study into the context of the broader literature. Assessments of DAPA study quality were performed by researchers independent of the trial team (Beth Fordham and Bethan Copsey).
We defined studies as being rated as having a low risk of bias if they reported the use of random sequence generation and allocation concealment, if an additional two criteria were assessed as having a low risk of bias and if no criteria were assessed as having a high risk of bias. Studies were categorised as having a high risk of bias if they did not report the use of random sequence generation or allocation concealment or if two other criteria were rated as having a high risk of bias. Studies were rated as having unclear risk of bias if either random sequence generation, allocation concealment or three other criteria had not been reported sufficiently well to be able to be assessed or if one criterion, other than random sequence generation or allocation concealment, was rated as having a high risk of bias.
Data analysis
The primary analysis pooled data from the earliest follow-up time point after completion of the intervention. When study reporting did not include the required summary data, these were, when possible, calculated from the reported data. The most common example was calculation of the SD from the reported SE. For cluster RCTs, when clustering was not taken into account during the analysis, the effective sample size was calculated using the number of clusters. 85,154
Data synthesis
Meta-analyses were conducted using random-effects models and the results were synthesised using the inverse variance method in RevMan version 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). 85
For characteristics of study populations (e.g. age), summary statistics were presented using the median and range of the population average for each study. For trial characteristics (e.g. follow-up time period), summary statistics were presented using the median and range across studies. For intervention characteristics (e.g. intervention duration), summary statistics were presented using the mean and range across studies.
Treatment effects were summarised as the standardised mean differences (as outcome measures differed between studies). 85 Effect sizes were interpreted as follows: small, 0.2; moderate, 0.5; and large, 0.8. 155 A positive effect size indicated a treatment effect in favour of exercise.
Heterogeneity was assessed using the chi-squared and I2 statistics. 156 The interpretation of I2 values were as follows: 0–40%, might not be important; 30–60%, moderate heterogeneity; 50–90%, substantial heterogeneity; and 75–100%, considerable heterogeneity. 85 When at least 10 studies were included in a meta-analysis, publication bias was assessed using visual assessment of funnel plots and Egger’s test. 157
Subgroup analysis
When there were at least two studies in each subgroup, subgroup analysis was undertaken on the following factors: diagnosis [Alzheimer’s disease or any dementia (Alzheimer’s disease and non-Alzheimer’s disease)], setting (community or residential), length of follow-up (< 20 or ≥ 20 weeks), intervention duration (< 4 or ≥ 4 months), weekly exercise dose (< 150 or ≥ 150 minutes) and intervention type (aerobic, resistance, three-dimensional or mixed). Pooled effects were calculated within each subgroup and a chi-squared test was performed to test for interactions.
Sensitivity analysis
Sensitivity analyses were conducted in order to assess the robustness of the summary effect estimates for global cognition. First, inclusion was restricted to studies with low overall risk of bias. Second, inclusion was restricted to studies with an attention control arm only. Third, study results were included from the follow-up time point furthest from the end of the intervention. Sensitivity analyses were undertaken when two or more studies met the inclusion criterion.
Results
Database searches identified 2436 articles and additional searching within systematic reviews identified another two articles. After removing duplicates, 1796 abstracts were screened and, from these, 193 full-text articles were assessed for eligibility. One study was published in Spanish158 and all others were published in English. The flow of studies is presented in Figure 16.
FIGURE 16.
Flow of studies.
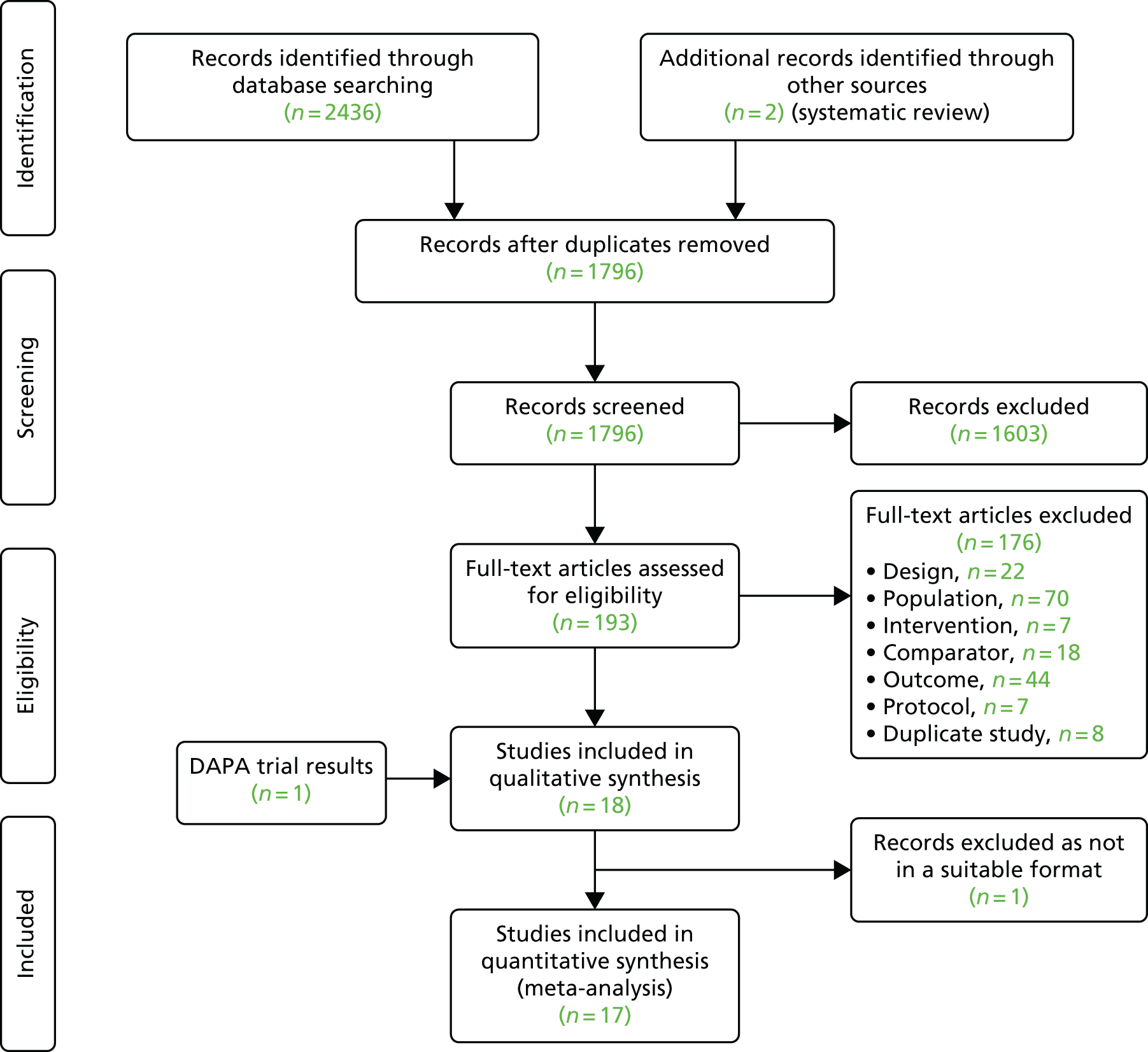
Summary of included studies
Characteristics of included studies are shown in Table 50. We included 19 studies, the majority of these (13/19, 68%) sampled participants who had MMD, as most studies restricted eligibility to participants with a sMMSE score of 10–26. 158–163,165,167,169,170,172,173,DAPA RCT Eleven studies included participants with Alzheimer’s disease only148,158,159,164–166,168,169,171–173 and eight included various types of dementia, including Alzheimer’s Disease, or did not report the disease type. 149,160–163,167,170,DAPA RCT Participants were either recruited from community settings (9/19, 58%)148,158,164,165,167–169,173,DAPA RCT or nursing homes. Three studies (3/19, 16%)164,165,172 had an upper-age restriction within their eligibility criteria, and for these studies the average age of participants ranged from 70 to 85 years. For studies with no upper age restriction, the average age of participants within each study sample ranged from 71 to 87 years (median 78 years). The study with the median proportion of females had a 65% proportion in favour of women (n = 23/35) and the range in proportions of women was 31–81%. The cognitive outcome measures used in each study and those that were included in our analysis are presented in Table 51.
| Study | Arms | n | Disease type | Baseline cognition | Control | Intervention | Intervention characteristicsa |
|---|---|---|---|---|---|---|---|
| Aguiar et al.159 | 2 | 40 | Alzheimer’s disease | Mild, moderate (sMMSE score of > 12) | Usual care: rivastigmine transdermal patch delivering daily dose | Mixed: rivastigmine patch, stretching, walking or resistance, and balance |
Type: D100, D101, D102, D105 Frequency: 80 minutes per week Duration: 6 months |
| Arcoverde et al.160 | 2 | 20 | Various | Mild, moderate (sMMSE score of ≥ 15) | Usual care: continuation of previous medical management | Aerobic: treadmill-based exercise |
Type: D102, D105 Frequency: 150 minutes per week Duration: 6 months |
| Bossers et al.161 | 3 | 123 | Various | Mild, moderate (sMMSE score of 9–23) | Attention: social intervention | Mixed:
|
Type: D101, D105 Frequency: 70 minutes per week Duration: 3 months |
| Cheng et al.162 | 3 | 117 | Not reported | Mild, moderate (sMMSE score of 10–24) | Attention: C1: handicraftsb C2: Mahjong | Three-dimensional only: yang-style tai chi |
Type: D103 Frequency: 50–100 minutes per week Duration: 2.5 months |
| DAPA RCT | 2 | 494 | Various | Mild, moderate (sMMSE score of > 10) | Usual care | Mixed: cycling, sit-to-stand, weighted belted and jackets, bicep curls, dumb-bells |
Type: D101, D105 Frequency: 150 minutes per week Duration: 4 months supervised, 8 months unsupervised |
| Eggermont et al.163 | 2 | 97 | Not reported | Mild, moderate (sMMSE score of 10–24) | Attention: social intervention | Aerobic: indoor walking at self-selected speed |
Type: D100, D104 Frequency: 280 minutes per week Duration: 6 months |
| Hoffmann et al.164 | 2 | 200 | Alzheimer’s disease | Mild (sMMSE score of > 19) | Usual care: treatment within memory clinic (medical or other) | Aerobic: ergometer static bicycle, cross-trainer and treadmill |
Type: D105 Frequency: 135 minutes per week Duration: 4 months |
| Holthoff et al.165 | 2 | 30 | Alzheimer’s disease | Mild, moderate (CDR of 1–2) | Active: monthly clinical visits and counselling to increase physical activity | Mixed: seated pedalling action and alternated passive, assisted and resisted lower limb movement |
Type: D101, D105 Frequency: 120 minutes per week Duration: 2.25 months |
| Kemoun et al.166 | 2 | 38 | Alzheimer’s disease | Mild, moderate, severe (sMMSE score of < 23) | Usual care: no additional input | Aerobic: articular mobilisation and muscle stimulation then walking, static cycling, balance/endurance activity |
Type: D100, D103 D105 Frequency: 105 minutes per week Duration: 3 months |
| López et al.158 | 2 | 60 | Alzheimer’s disease | Mild, moderate (sMMSE score of 10–24) | Usual care: telephone follow-up | Resistance: warm up/stretching/flexibility, walking, balance exercises and moderate cardiovascular resistance work (aerobic) with word recall during exercise |
Type: D100, D101 D102 Frequency: 180 minutes per week Duration: 3 months |
| Miu et al.167 | 2 | 85 | Various | Mild, moderate (sMMSE score of 10–26) | Usual care: usual care included drug treatment | Aerobic: treadmill, bicycle and arm ergometer |
Type: D102, D105 Frequency: 90 minutes per week Duration: 3 months |
| Pitkälä et al.168 | 3 | 210 | Alzheimer’s disease | NR (clinical diagnosis) | Active: usual care and oral and written advice about nutrition and exercise |
|
Type: D100, D101, D105 Frequency: 150 minutes per week Duration: 1.5 months |
| Steinberg et al.169 | 2 | 27 | Alzheimer’s disease | Mild, moderate (sMMSE score of ≥ 10) | Active: a home safety assessment | Aerobic: daily exercise programme with caregivers |
Type: D104 Frequency: 90 minutes per week Duration: 12 months |
| Stevens and Killeen170 | 3 | 75 | Not reported | Mild, moderate (sMMSE score of 10–22) |
Attention: social intervention Usual care: no intervention |
Aerobic: group-based aerobic exercise programme |
Type: D104 Frequency: 90 minutes per week Duration: 3 months |
| Underwood et al.149 | 2 | 247c | Various | Mild, moderate, severe | Active: usual care plus depression awareness training for care home staff | Mixed: progressive aerobic and resistance training activities |
Type: D101, D105 Frequency: 180 minutes per week Duration: 3.75 months |
| Venturelli et al.171 | 2 | 24 | Alzheimer’s disease | Moderate, severe (sMMSE score of 5–15) | Usual care: leisure activities | Aerobic: walking programme with caregivers |
Type: D105 Frequency: 120 minutes per week Duration: 6 months |
| Venturelli et al.172 | 4 | 80 | Alzheimer’s disease | Moderate (sMMSE score of 10–15) |
C1 attention: cognitive training (reality orientation) C2 usual care |
Aerobic: walking programme with caregiver |
Type: D105 Frequency: 300 minutes per week Duration: 3 months |
| Vreugdenhill et al.148 | 2 | 40 | Alzheimer’s disease | NR (clinical diagnosis) | Active: telephone calls to check on well-being | Mixed: daily home exercise programme with carers |
Type: D100, D101 D105 Frequency: 60 minutes per week Duration: 12 months |
| Yang et al.173 | 2 | 50 | Alzheimer’s disease | Mild, moderate (sMMSE score of 10–24) | Active: health education intervention | Aerobic: cycling training at moderate intensity (70% MHR) |
Type: D105 Frequency: 120 minutes per week Duration: 3 months |
| Study | Outcome measure | |||||
|---|---|---|---|---|---|---|
| Global cognition | Language | Memory | Attention | Executive function | Praxis | |
| Aguiar et al.159 | sMMSEa | |||||
| Arcoverde et al.160 | sMMSE, CAMCOGa | Verbal fluencya | WMS-R digit span forward,a RAVLT | TMT-Aa | Stroop testa | Clock drawing testa |
| Bossers et al.161 | sMMSEa | Verbal fluencya | WMS-R digit span forward,a RBMT | TMT-Aa | Stroop testa | Picture testa |
| Cheng et al.162 | sMMSE, CDR-SOBa | WMS-R digit span forward,a RAVLT | ||||
| DAPA trial | sMMSE, ADAS-Coga | ADAS-Cog language subscale | ADAS-Cog memory subscale | ADAS-Cog praxis subscale | ||
| Eggermont et al.163 | Category fluency, letter fluency | |||||
| Hoffmann et al.164 | sMMSEa | Category fluency,a letter fluency | ADAS-Cog VMT delayed,a ADAS-Cog VMT immediate | Stroop test, symbol digit modalities testa | ||
| Holthoff et al.165 | sMMSEa | CERAD,a PVF (F-A-S) | ||||
| Kemoun et al.166 | ERFCa | |||||
| López et al.158 | sMMSEa | List learning delayed recall,a Rey complex figure test | TMT-A,a TMT-B | |||
| Miu et al.167 | sMMSE, ADAS-Coga | |||||
| Pitkälä et al.168 | FIM-Coga | Category fluencyb | Clock drawing testb | |||
| Steinberg et al.169 | BNT, Hopkins VLT | |||||
| Stevens and Killeen170 | Clock drawing test | |||||
| Underwood et al.149 | sMMSEa | |||||
| Venturelli et al.171 | sMMSEa | |||||
| Venturelli et al.172 | sMMSEa | |||||
| Vreugdenhill et al.148 | sMMSE, ADAS-Coga | |||||
| Yang et al.173 | sMMSEa | |||||
The majority of the interventions (10/19, 53%) were aerobic exercise alone. 160,163,164,166,167,169–173 Seven studies used a mixed-resistance and aerobic exercise. 148,149,159,161,165,168,DAPA RCT One study used resistance exercise only158 and one study used three-dimensional exercises (tai chi and yoga only). 162 The interventions varied in the mode of delivery. Some used fixed equipment (mainly in community settings), including treadmills and static bicycles (n = 7/19, 37%),168,164–167,173,DAPA RCT and others used functional training activities, including walking (n = 11/19, 58%). 148,158,159,161,163,166,168,169,171,172,DAPA RCT The mode of delivery was not associated with disease type. Across the 19 included studies, the average number of minutes of exercise per week was 142, ranging from 60 to 300 minutes. Seven of the included studies149,158,160,163,168,172,DAPA RCT equalled or surpassed the 150 minutes per week recommended by WHO’s guidelines. 43 The interventions continued for an average of 4.5 months, ranging from 1.5 to 12 months, and the majority were supervised. There was a paucity in reporting of exercise intensity, only 2 out of 20 studies explicitly described the intensity. 158,173 Both of these studies used moderate-intensity exercise.
Nine studies used a usual-care control arm158–160,164,166,167,170,171,DAPA RCT and the most common control group treatment was educational initiatives (4/19). 149,168,172,173 Five studies (n = 5/19, 26%)161,162,168,170,172 had three or more treatment arms. For inclusion in the meta-analysis, it was necessary to select one of two eligible control treatments for two studies (n = 2/19, 11%)170,172 and to select one of two eligible intervention treatments for two studies (n = 2/19, 11%). 161,168
Study quality
The sample sizes of the included RCTs are presented as a histogram in Figure 17. The sample sizes ranged from 20 to 494 participants (median 75 participants). All studies assessed the effect of exercise immediately following the intervention period. The median time from randomisation to first follow-up after the interventions was 4 months from baseline (range 1.5–6 months). Eight studies reported further time points, the longest of which was 12 months after randomisation (8 months after completion of the intervention).
FIGURE 17.
Histogram of sample sizes.
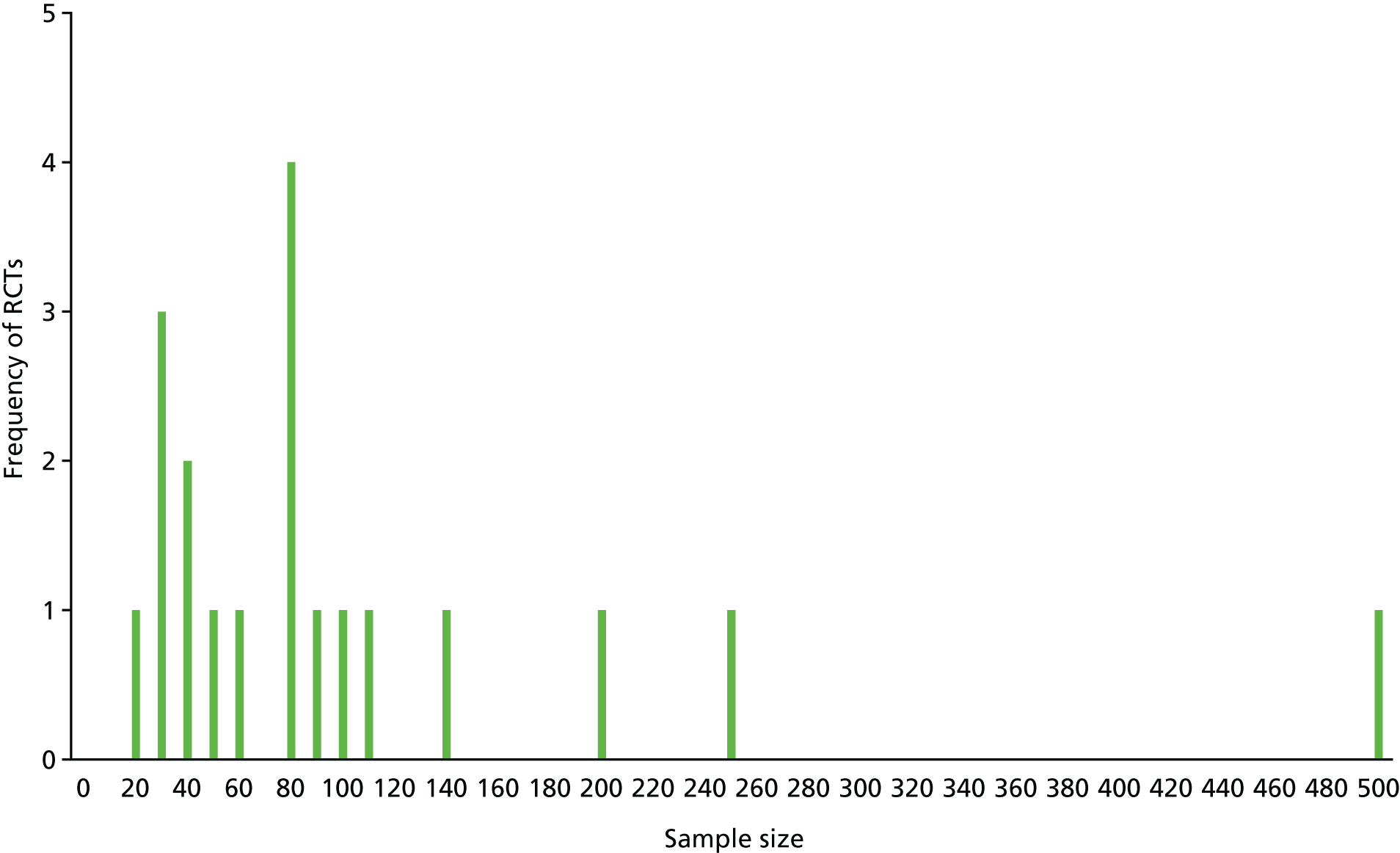
The risk-of-bias ratings are given in Figure 18. The overall risk-of-bias scores indicated that the majority of studies had a rating of unclear risk of bias (n = 15/19, 79%), three had a rating of low risk of bias and two had a rating of high risk of bias. Most studies were judged as ‘unclear’ overall, as they did not report the use of adequate random sequence generation or allocation concealment.
FIGURE 18.
Risk-of-bias table. Low risk of bias is represented by + (dark green), unclear risk of bias is represented by ? (green) and high risk of bias is represented by – (light green).

Effects on global cognition
The global cognitive outcomes analysis included 16 studies (n = 1420 participants). A pooled analysis reported a small–medium effect size of 0.36 (95% CI 0.12 to 0.61) in favour of exercise, with evidence of substantial heterogeneity (I2 = 76%). Additional analyses to explore heterogeneity found that using data generated from the final follow-up assessments generated similar effects and levels of heterogeneity, as did restricting studies to those using an attention control comparison. The pooled effect size in the strata of studies that were assessed as having a low risk of bias was null (standardised mean difference 0.001, 95% CI –0.18 to 0.21, I2 = 13%) (Table 52).
| Analysis description | Studies (n) | Participants (n) | Effect estimate [standardised mean difference (95% CI)] | I2 (%) |
|---|---|---|---|---|
| Primary analysis | 16 | 1420 | 0.36 (0.12 to 0.61) | 76 |
| Secondary analysis (longest follow-up) | 16 | 1358 | 0.37 (0.12 to 0.63) | 78 |
| Subgroup analysis | ||||
| Condition | p < 0.01 | |||
| Alzheimer’s disease only | 10 | 622 | 0.62 (0.22 to 1.02) | 80 |
| Alzheimer’s disease and non-Alzheimer’s disease | 6 | 798 | –0.01 (–0.15 to 0.14) | 0 |
| Setting | p = 0.85 | |||
| Residential | 6 | 342 | 0.41 (–0.07 to 0.90) | 77 |
| Community | 10 | 1078 | 0.36 (0.06 to 0.66) | 78 |
| Follow-up period (weeks) | p = 0.26 | |||
| < 20 | 11 | 758 | 0.26 (0.02 to 0.50) | 58 |
| ≥ 20 | 5 | 662 | 0.67 (0.00 to 1.33) | 90 |
| Intervention duration (months) | p = 0.65 | |||
| < 4 | 10 | 672 | 0.33 (0.02 to 0.64) | 73 |
| ≥ 4 | 6 | 748 | 0.46 (–0.00 to 0.92) | 82 |
| Intervention dose (minutes) | p = 0.12 | |||
| < 150 | 10 | 1049 | 0.20 (–0.03 to 0.44) | 63 |
| ≥ 150 | 6 | 371 | 0.71 (0.11 to 1.30) | 84 |
| Intervention type | p = 0.17 | |||
| Aerobic only | 8 | 541 | 0.48 (0.04 to 0.92) | 81 |
| Mixed | 6 | 745 | 0.14 (–0.07 to 0.36) | 33 |
| Sensitivity analysis | ||||
| Attention control | 2 | 146 | 0.05 (–0.28 to 0.37) | 0 |
| Low risk of bias | 2 | 569 | 0.01 (–0.18 to 0.21) | 13 |
Subgroup analysis on cognition: by exercise and disease type
Subgroup analysis found that the effects of exercise on global cognition differed significantly by disease type (Figure 19). There was a large, statistically significant effect on global cognition in favour of exercise (standardised mean difference 0.62, 95% CI 0.22 to 1.02) in studies that sampled participants with Alzheimer’s disease only. However, the level of heterogeneity was substantial (I2 = 80%) and this effect was not evident when limited to trials with low risk of bias (I2 = 13% in studies rated as having a low risk of bias). We found no effect on global cognition in undifferentiated dementia populations.
FIGURE 19.
Forest plot: global cognition by type of dementia.

Using data from patients with Alzheimer’s disease only within the DAPA trial, the overall effect size was reduced but still remained statistically significant (Figure 20).
FIGURE 20.
Forest plot: global cognition – Alzheimer’s disease only.
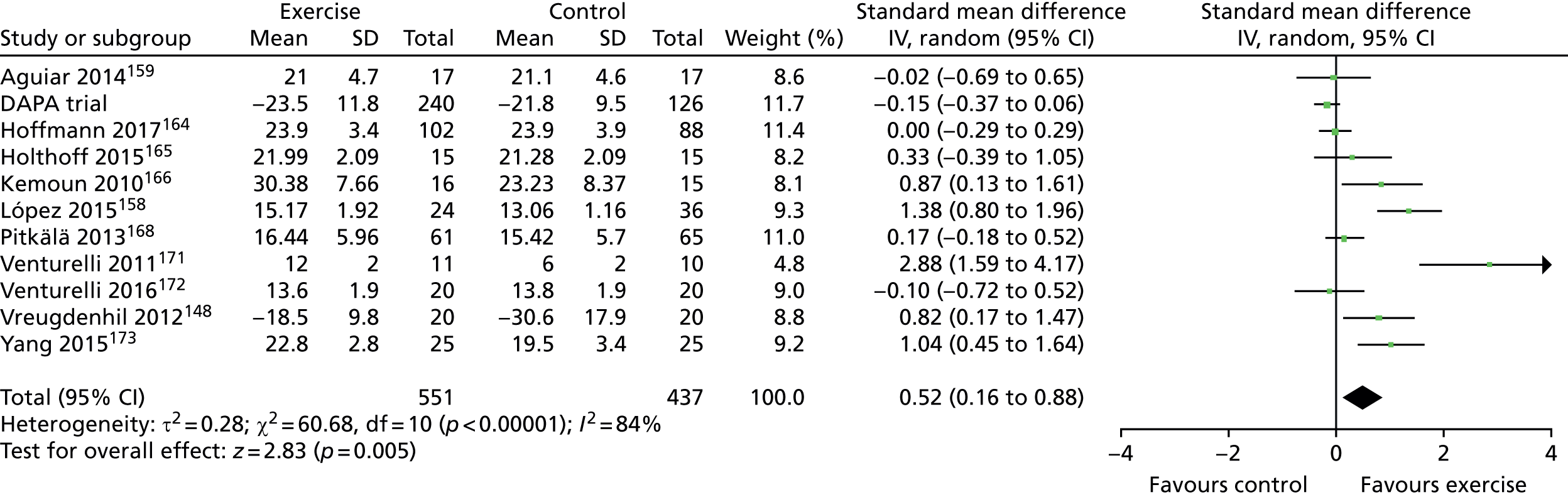
Subgroup analysis indicated that effects on global cognition were similar for aerobic exercise, resistance or a combination of both (Figure 21). Similarly, there were no statistically significant differences in the effects of exercise on cognition by setting, exercise duration or exercise dose. We could not conduct an analysis by intensity, as few studies reported this information.
FIGURE 21.
Forest plot: global cognition by type of exercise.

Effects on specific cognitive domains
The majority of studies reported global cognition scores only (n = 13/28, 46%). Some studies reported outcomes in specific cognitive domains only (n = 4/28, 14%) and some reported a global outcome and other independent measures of specific cognitive domains (n = 11/28, 39%). There was no evidence of clinically worthwhile benefits or statistically significant effects for attention, memory, language, executive function or praxis (see Table 53). There was lower heterogeneity in the effects of specific cognitive outcomes (I2 < 50%) than for the measures of global cognition. For studies that reported both global and specific cognitive measures there was a lack of concordance in the estimates of effect. Studies that measured both global cognition and attention or memory found larger heterogeneity in the global measure and substantially less in the specific measures (Figure 22).
FIGURE 22.
Forest plot: global and specific outcomes reported within same trials.

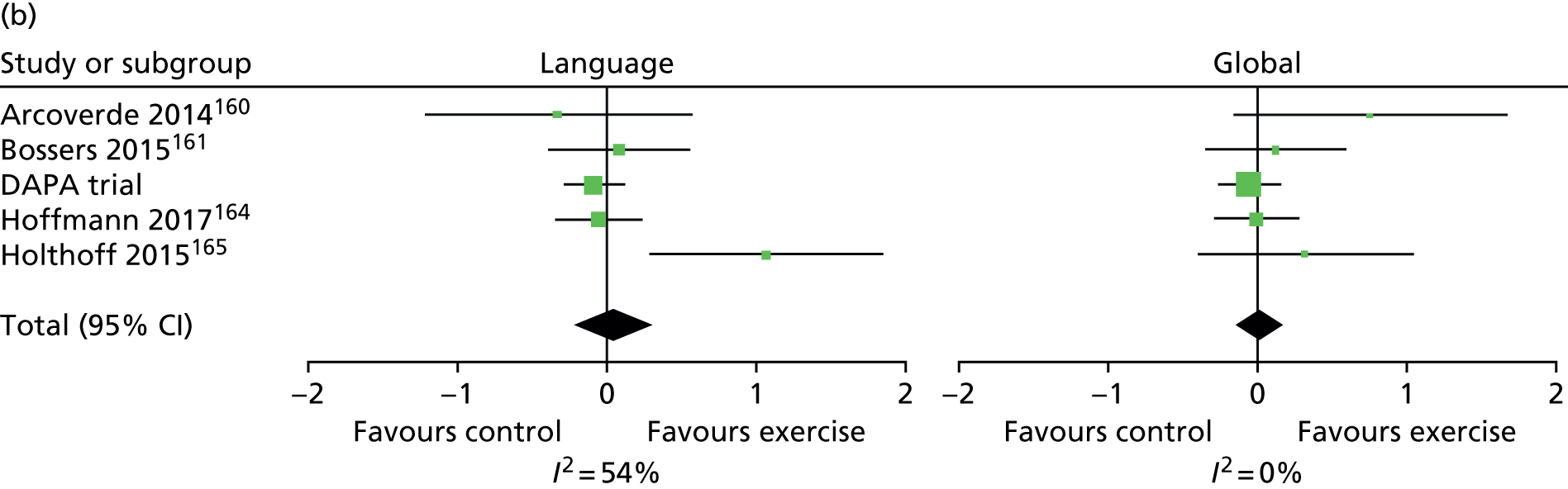
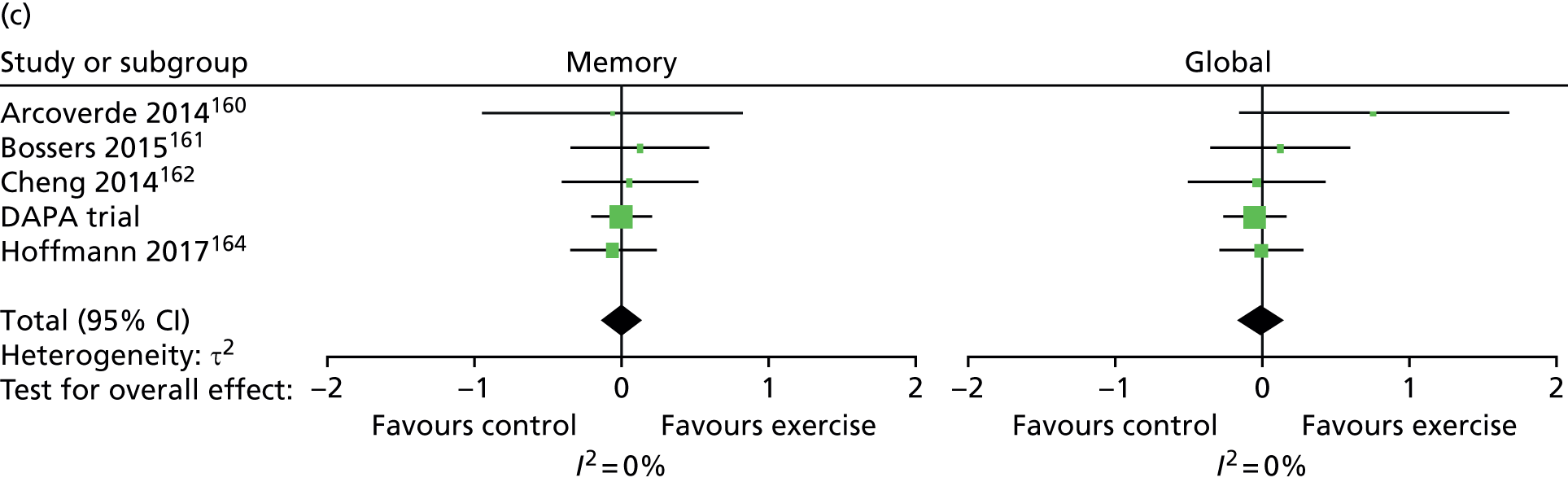


Summary
The literature presents a confusing picture with very few well-conducted trials. When well-conducted trials are available, these point to a negligible effect of exercise on cognition in people with dementia regardless of the aetiology of the disease. The DAPA trial has been important in confirming these emerging trends and is the largest study to date.
Although quite large in number, nearly half of the trials are single centre and extremely small in their sample size. The quality of these studies is highly questionable, with most failing to report even the most basic elements of recognised good practice in clinical trials. The inadequacies of simple randomisation, even when the sample size is large, have been highlighted in a recent simulation study. 174 Failure of investigators to properly understand key concepts in reporting studies has also been reported. 175 All of the studies in the review are open label and protection against a range of biases associated with masked assessment are inadequately addressed in all but a few trials.
A recent study has found temporal differences in global and specific cognitive decline within older adults and recommends a focus upon specific rather than global physical and cognitive functioning. 176 Our exploration of a range of specific cognitive functions suggested no effect in memory, praxis, language or attention.
Chapter 7 Discussion
An overview of the study findings and key messages
The discussion focuses on the interpretation of the findings, the internal and external validity of the trial and associated analysis, the implications for clinical practice in the NHS and further research.
The DAPA trial was a definitive, pragmatic, rigorous, well-conducted trial to estimate the effect of a moderate- to high-intensity exercise intervention on cognitive and functional decline in community-dwelling adults with MMD. The trial included a large sample who have demonstrated good intervention compliance and follow-up rates over a moderate to long follow-up time period (12 months). Our primary ITT analysis reports a small, statistically significant, negative effect upon cognitive impairment. There were no significant effects upon any of the secondary outcomes regarding function and quality of life for people with MMD. Neither was there an effect upon caregiver burden nor quality of life. In summary, the exercise-based intervention did not change outcomes for participants or carers and may have made cognition slightly worse.
A secondary aim included updating and expanding a systematic review into this topic area. Upon completion of our review, we were surprised that even the most recent publications examining exercise interventions for people with dementia were rated as having unclear or high risk of bias. Our review’s primary analysis suggested that there is a small to moderate positive effect upon cognitive impairment within this population. However, this assumption is based on a less than rigorous evidence base.
The key message that emerged from the quantitative and qualitative results is that exercise may worsen cognitive decline and does not change functional outcomes, quality of life or carer burden. However, structured exercise sessions do seem to bring enjoyment and respite to people living with this incredibly challenging condition and also to their carers.
External validity and generalisability of the findings
The baseline descriptive analyses demonstrated that we have recruited a representative sample of community-dwelling adults with MMD to satisfy our sample size estimate calculation, allowing us to perform our prespecified statistical analyses with confidence on a sample that is largely representative of the UK dementia population. We recruited rather more sites than the seven sites originally specified in the protocol because of the need to spread the cost and resource implication of the intervention across more health sites, and because of the challenges of recruitment.
The intervention and control groups were well balanced at baseline. The baseline and demographic data of our recruited participants reflect the patterns observed in the general dementia population. Our sample included slightly higher Alzheimer’s disease diagnoses (78.8%) than that reported globally (62%). 3,177 Consequently, our sample had a smaller proportion of people with vascular types (acute, multi-infarct, mixed, other and unspecified) (11.3%) than reported globally (20%) or other aetiologies (Pick’s disease, Parkinson’s disease, other specified and unspecified) than reported globally (20%).
Dementia prevalence is reported to be equal between genders. 2 Our sample had a ratio of roughly 60 : 40 (male to female), which seems appropriate, especially when considering the demographics collected across the studies included in our systematic review, in which the percentage of females in the study populations ranged from 31% to 81%. The CACE analyses indicated that men were more likely to comply with the intervention than women. The age of our participants spanned across early- to late-onset dementia (≥ 65 years old); however, the mean (77.4 years) and median (78.3 years) both rested at late-onset dementia. When compared with the included studies from our systematic review, when there was no age restriction, the age of participants was reported to range from 68 to 87 years, with a median of 77 years. Our sample appears to be representative.
The majority of our participants were married (73.7%); however, the next largest group were those who were widowed (17.4%). When combining those who were widowed, divorced or single (i.e. without a long-term partner), this equated to 23.6%. The majority of participants were living with their partner (75.5%) and nearly 20% of the participants lived alone. This is slightly lower than the observation that, prior to 2002, one-third of people with dementia live on their own in the UK, as reviewed in Miranda-Castillo et al. 178 The qualitative chapter findings (see Chapter 4) exemplified the importance of considering practical considerations such as travelling to and from structured exercise sessions. If a person is single and living alone, they may lack the motivation and practical logistics to attend an exercise session. When examining the demographic data from those who withdrew from the study, there was no pattern to suggest that people who were without a long-term partner and/or who were living alone were more likely to drop out or be lost to follow-up. However, the CACE analyses suggest that participants were more likely to comply with the intervention if they were not living alone.
Our sample seems to mirror the ethnic proportions reported in the second report of the Cognitive Functioning and Ageing Studies for dementia patients in the UK. 7 The Cognitive Functioning and Ageing Studies report identified that, in 2011, 3.7% of people with dementia in the UK were from black, Asian and minority ethnic groups and our sample included 3.2% of participants from these groups.
Our population sample has a mean age of leaving school of 16 years, with only 16.4% having a degree (or equivalent) level of education and the majority having no qualification (30%). This is similar to the Office for National Statistics census data,179 which show that people > 65 years in UK are more likely to have no qualifications (52.9%) than any other age group. However, the age range from 16 to 64 years shows that 30% of people have a degree, or equivalent, level of education.
Our inclusion criteria stipulated that participants must have probable dementia, classified by clinician (according to DSM-IV), which was mild to moderate in severity (sMMSE score of > 10). The systematic review (see Chapter 6) found that the majority of included studies (13/28) classified the severity of their study population as mild to moderate (sMMSE score of 10–26). The trial sample’s sMMSE scores range from 11 to 30 (out of a possible maximum score of 30), with the higher scores indicating less impairment. This range crosses the NICE guideline boundaries of mild dementia into MCI (a score of 26 on sMMSE). 67 The mean sMMSE score was 21.9 (SD 4.6), which is classified as mild dementia severity. This is echoed with the baseline ADAS-Cog scores, which find that there is a mean score of 20.9, with a large range from 5.3 to 52 (out of a possible 70), with a higher score indicating more impairment. These scores are indicative rather than confirmatory, and expert clinical opinion explains that many people with early-stage dementia are likely to have mild impairment scores yet still have the disease. The quantitative description demonstrates that our participants have a broad range in their level of cognitive impairment. Variation in dementia symptom was witnessed in the qualitative chapter (see Chapter 4), in which one participant clearly explained that he did not identify with the other people in his exercise classes and that their symptoms were more severe than his.
The sample demonstrates the expected dementia population trend of a 4-point ADAS-Cog deterioration over the 12-month79 study period, which demonstrates that the ADAS-Cog measurement is detecting normal deterioration but that it has still not detected a change in progression as a result of the intervention.
We examined the subdomains of the ADAS-Cog composite score. The sample mean scores present substantial memory impairment but the mean scores for language and praxis functions do not seem to indicate, as yet, a great affect. The ability to understand commands and communicate (language) and synthesise and sequence motor movements (praxis) remains relatively intact in our sample and these seem to be functions that could be necessary to enable people to participate in an intervention such as exercise.
The outcome measures for the quality of life of the participants were completed by the participants and, when possible, their carers (proxy). The ratings of the carers were significantly lower than the participants’ own ratings, indicating that the carers perceive the participants to have a worse quality of life than the participants believe themselves to have. This lack of patient–carer agreement on quality-of-life outcomes is well established in dementia populations180 and in other chronic conditions. 181 Carer’s ratings of the quality of life of people with dementia is likely to be influenced by the type of carer relationship (spouse or child), patient’s mood status and the participant’s activities of daily living. 180 The majority of our carers were spouses, who tend to give better QoL scores for their spouse than a child would for their parent. We were able to follow the validated scoring guidelines of all measures and, when appropriate, obtained good completion rates from the person with dementia. The conclusions of our study would not have been altered had we chosen to use proxy responses for variables such as the EQ-5D-3L. Falls were a secondary measure and we did not use a falls calendar, which is the gold standard method of collecting falls data,182 because of the already substantial commitment that people with dementia and their carers were making to the study. Any under or overestimation of falls that resulted from the carer recall should be randomly distributed across the sample.
In summary, our sample appears to be largely representative of the general dementia population in terms of demographics. The sample also reflects those from previously published exercise intervention trials in this target group.
Our findings are based on a sample that is representative of the population likely to engage with exercise as an intervention for dementia. In comparison to previous trials, we recruited a substantially larger sample size, used a sensitive measure of cognitive impairment and maintained high levels of follow-up. We used prospective registration and robust allocation concealment, independent computer-generated randomisation and masked outcome assessment.
Participant follow-up
For such a large and relatively long-term (12-months) trial, we achieved a good follow-up rate. The majority of participants who withdrew from the intervention and control arms did so between the point of randomisation and the 6-month follow-up. When examining the demographic data of those who remained in the trial, those who withdrew, those who were lost to follow-up and those who died during the trial (at 6 and 12 months’ follow-up), there are no concerning trends. The characteristics of the sample providing data were well matched.
When examining baseline cognitive impairment and QoL scores, those who died during the trial were, as to be expected, older and had greater impairment and lower QoL ratings than those who did not die. When examining those who withdrew or were lost to follow-up, they had similar cognitive impairment scores (sMMSE and ADAS-Cog) to those who remained in the trial but their QoL ratings (EQ-5D-3L and QoL-AD scores) indicated poorer quality of life.
The individual variation in participant ability and the challenges that this poses to the therapists leading the sessions and to exercise prescription was reflected in the qualitative interviews. Some participants felt that the therapists were overly cautious and underestimated their physical abilities, whereas some other participants felt the exercises were uncomfortably hard in some situations. A key finding from the qualitative data was the importance of maintaining a positive working therapeutic relationship and open communication. Without this, motivation deteriorates, expectations and capabilities can be misunderstood and compliance will deteriorate. Overall, the participants, their carers and the physiotherapists found this to be a rewarding programme in many different ways. When asked about whether or not the intervention had an effect, the response of participants in the qualitative study suggested that it was not improving their cognitive functioning but that it was improving physical fitness/strength and emotional well-being.
Some of the benefits of the intervention were attributed to enjoying the sociality of performing exercises in a group. The data show that the mean number of participants per group was six people and only one group had as few as three people per group.
About half of the cohort was able to maintain physical activity after cessation of the intervention.
Participant compliance
Compliance with exercise was good and 65% of participants achieved between 75% and 100% compliance to the intervention. When we examined the CACE analysis, we see that the people who were not compliant were more likely to be women and people living alone. Men seemed to really appreciate the physical challenge of using the bikes and carers were very important in helping to get people to the classes. We did not find any other statistically significant differences between the usual-care group, the intervention compliers and the intervention non-compliers on any descriptive or outcome measurements.
Critique of the methods
The trial was designed to test an intervention that could be delivered in routine practice. The groups were feasible and the number of AEs was small. From a physiological perspective, the protocol improved physical fitness parameters and walking speed within a range that would be considered worthwhile. 53,61,62 The sample was diverse in baseline fitness, as selection was based predominantly on cognition. The exercise protocol was able to account for higher levels of fitness but this was challenging. Our prespecified subgroup analyses suggest that there is no hidden effect within the sample, for example related to baseline mobility, cognition or broad classification of underlying disease. The choice of exercises was also driven by the underlying hypothesis of targeting cognitive impairment. At the time of designing the intervention, most evidence/theory supported aerobic and strength conditioning as the prime pathways to target. We did not include cognitive training or psychomotor training (for example training reaction time) to ensure that the effects of the intervention could be attributed to aerobic and strength training disease pathways. There was no evidence to suggest that balance exercise could modify cognition at the time of developing the intervention and, therefore, it was not included. Although we did not randomise the behavioural elements underpinning the programme and cannot make robust conclusions about the effects of these elements, the good session compliance suggests that the behavioural support was adequate, at least during the first 4 months of the programme.
We monitored the dose delivered through session records. In contrast to similar high-intensity interventions that improve muscle function in older people without dementia,63 we started with a higher initial strength challenge and then progressed participants at a similar rate. The strength dose was higher than that achieved in residential care settings, in which a similar intervention was found to be ineffective in changing cognitive and BADLS status but improved balance. 183,184 Hence, it seems likely that we have delivered sufficient dose. Greater compliance with session attendance was associated with further reduction in cognition and even higher doses may incur greater harm.
The results of this trial disagree with those of many small single-centre studies. Study quality is likely to play a role, with many previous studies having uncertain allocation concealment and very poor levels of masking. There are some limitations to our work. We collected physical parameters only in the exercise arm and, hence, cannot conclude definitively that the intervention improves physical fitness. Subgroup analyses may be underpowered, as the proportion of people in the various strata was not distributed optimally,57,183,184 but the direction of effect estimates support our conclusions. In the absence of definitive guidance and rationale, we used a mixed exercise programme, and it may be that longer periods of a single training modality may be effective. However, the feasibility of getting participants to sustain a single type of activity through an entire session is improbable. We did not include an attention control as our intention was for a pragmatic trial. Participants and carers were not masked to allocation and this is an unavoidable limitation.
On a positive note, the qualitative study suggested that in the small sample interviewed, people were able to exercise safely and they appeared to enjoy the experience and it improved confidence in movement and self-efficacy. We found no evidence of improved mood or quality of life, suggesting that any perceived benefits might be either positive reporting or transitory.
The changes in physical fitness did not translate into improvements in transfers, mobility or functional activities. To achieve these outcomes may require greater motor relearning than we included in our programme. Falls were not reduced and this may be because we did not specifically target balance within the intervention. Carers may also be reluctant to encourage people to re-establish functional activities through fear of their relative falling or because of well-established caring roles. Although the quality of life of carers was not different from the general population, the carer burden was high. Two sessions per week may have provided some respite, but these are likely to be insignificant in comparison with other behavioural challenges.
We updated and expanded a systematic review in this topic area as part of the project. The quality of the reviewed studies was very low and the literature was dominated by small single-centre studies. A review published during the trial suggested that the effect of exercise on cognition may be specific to Alzheimer’s disease. 20 We were unable to support this finding within the cohort of DAPA trial patients with Alzheimer’s disease only, which is surprising given the size of the overall effect reported across pooled studies. Addition of the DAPA trial estimates to the systematic review reduced the estimated effect in people with Alzheimer’s disease but did not eradicate it. There remain a number of concerns with the synthesis of the literature for this indication and intervention. There are multiple small studies of low quality. The chance of overestimating effects or conducting a biased experiment are high in these situations. The level of heterogeneity reported in global measures of cognition was also well above accepted levels. When we examined effects in specific areas of cognitive impairment, heterogeneity was much lower and effects were null. There is some preliminary evidence that supports different dementia types (Alzheimer’s disease or vascular dementia) influencing different specific cognitive functions (language or executive function) to varying degrees. 185
Another important finding was that this high-intensity exercise intervention was well tolerated by participants. Participants’ carers and the physiotherapists delivering the intervention felt it was a suitable structured activity for people with dementia. Despite the intervention group sample displaying a high level of comorbid conditions, including nearly 50% living with a heart/circulatory condition and over 50% living with joint or muscle pain, there were remarkably few AEs.
It was a disappointing finding that there were no changes to carers’ quality of life, considering the enjoyment and benefits reported in the qualitative study. However, on reflection of the bigger picture, we can see that the burden on carers is unrelenting. Carers may have found these two sessions per week to be a respite but they then have to cope with a range of challenges throughout the day and night. It is also worth considering that the quality-of-life measurements were taken at 6 and 12 months post intervention. So although they may have enjoyed the intervention when it was running, the continued deterioration of their loved one (an average of 4 ADAS-Cog points per year79) will undoubtedly compound their burden and the deterioration of their quality of life. It could be argued that a longer period of exercise classes may have had a better effect, but there was no signal of this within a time period that is consistent with physiological change in muscles and the cardiovascular system. Longer-term provision will be more costly and, hence, there is a need to demonstrate even greater benefit to achieve effects at the current levels of willingness to pay. There is also the possibility that longer periods of moderate- to high-intensity exercise may incur greater detriment to cognitive function.
Cost-effectiveness
The data collected in the DAPA trial strongly support a hypothesis that exercise therapy in addition to usual care, when compared with usual care alone, is not cost-effective.
Future research questions
This was a well-conducted and large trial with good compliance with a moderate- to high-intensity dose of exercise. Follow-up rates were good and the sample was representative of those likely to participate. The logistical difficulties and cost of running sessions was substantial. Motor relearning and ability to maximise functional gains from physical fitness are a suggested focus for future research.
Acknowledgements
We would like to acknowledge and thank all the therapists involved in delivering the trial intervention.
Trial management group
Professor Sarah E Lamb (Chief Investigator); Dr Bart Sheehan (Co-applicant); Dr Dipesh Mistry (Trial Statistician); Dr Ranjit Lall (Co-applicant, Statistician); Kamran Khan and Iftekar Khan (Health Economists); Professor Stavros Petrou (Co-applicant; lead Health Economist); Dr Sukhdeep Dosanjh and Sharisse Alleyne (Trial Co-ordinators); Susie Hennings (Senior Project Manager); Vivien Nichols and Susanne Finnegan (Recruitment leads); Nicky Atherton and Debbie Brown (Intervention leads); and Helen Collins (DeNDRoN advisor).
Trial steering committee
Professor Brian Lawlor (chairperson), Angela Clayton Turner (lay member; patient and public involvement), Dr Jenny Freeman (independent member), Professor Sarah E Lamb (non-independent member), Professor Paul McCrone (independent member) and Dr Bart Sheehan (non-independent member).
Data Monitoring and Ethics Committee
Professor Roy Jones (chairperson), Professor Julian Hughes and Professor Patrick Phillips.
Statistician
Dr Dipesh Mistry.
Trial team
University of Warwick
N Atherton, S Bridgewater, E Eyre, S Finnegan, L Hall, L Hill, P Hall, H Johnson, G Kaur, L Langdon, J Lowe, S Mathews, J Millichap, J Nussbaum, I On-kar, C Ritchie, V Russell, G Scott, S Shore, A Slowther, K Spanjers, L Stonehewer, M Thorogood, J Todd, A Ullah, H Waters, L Woods, E Withers and P Zeh.
University of Oxford
A Bond, D Brown, C Byrne, R McShane, H Richmond, N Thomas and J Thompson.
Research sites teams
Oxford Health NHS Foundation Trust
C Dransfield, F Le Frenais, C Hall and O Rye.
Berkshire Healthcare NHS Foundation Trust
R Carson, M Clarke, H Eaton, H Ellis, A Farrand, S Gardner, C Harducas, L Rigby and J Wilson.
Black Country Partnership NHS Foundation Trust
L Hill, L Johnson, L Lord, L Johnson, T Qassam, S Sadier, A Shipman, L South, J Statham, J Tomkins and D Weaver.
Coventry and Warwickshire Partnership Trust
B Coope, D Craddock, A Johal, J Lee, J Lindsay, J Tucker and R Vanderputt.
Royal Devon & Exeter NHS Foundation Trust
V Cross, G Glithens-Mather, L Martin, C O’Reilly, E Rogers and R Sheridan.
Greater Manchester West NHS Foundation Trust
K Birtwell, J Brooke, A Davis, C Hinze, S Hussain, A Kennedy, H Mistry, R Noble, R Norton, E Oughton, V Sherwin and P Tinker.
Leicester Partnership NHS Trust
D Glancey, H Karrin and M Marudkar.
Northamptonshire Healthcare NHS Foundation Trust
G Borley, T Crisp, P Koranteng, A Lovesy and S Vogel.
North East London NHS Foundation Trust
B Browne, L Colbourn, A Feast, E Hanratty, R Legerd, R Niland-Smith, Theresa Sullivan, Tony Sullivan, A Streater and H St Roas.
Solent NHS Trust
M Anderton, R Blake, K Brown, S Marriott, S Simpson and A Thornhill.
2gether NHS Foundation Trust (Gloucestershire & Herefordshire)
L Colbourn, F Dawe, T Kuruvilla, L Moore, R Niland-Smith, M Phillips, G Riley and A Uthup.
Contribution of authors
Sarah E Lamb (Professor of Rehabilitation, Chief Investigator) was responsible for study conception and design, and the writing and reviewing of the report.
Dipesh Mistry (Research Fellow, Trial Statistician) was a member of TMG, and was responsible for the statistical analysis of the trial and for the writing and reviewing of the report.
Sharisse Alleyne (Trial Co-ordinator) was a member of the TMG, and was responsible for the writing and reviewing of the report.
Nicky Atherton (Research Fellow, Intervention Lead) was a former member of the TMG, and was responsible for the writing and reviewing of the report.
Deborah Brown (Research Fellow, Intervention Team Lead) was a member of the TMG, and was responsible for the writing and reviewing of the report.
Bethan Copsey (Medical Statistician) was responsible for the DAPA trial systematic review, and was responsible for the writing and reviewing of the report.
Sukhdeep Dosanjh (Trial Co-ordinator) was a former member of the TMG, and was responsible for the writing and reviewing of the report.
Susanne Finnegan (Research Physiotherapist) was responsible for the organisation of the intervention and intervention quality assurance in the later stages of the project. In addition, she was responsible for data extraction and contributed to the systematic review.
Beth Fordham (Research Fellow) was responsible for the writing and reviewing of the report.
Frances Griffiths (Professor of Medicine in Society, Co-applicant) was a member of the TMG, and was responsible for protocol development, the qualitative analysis of the trial, and for the writing and reviewing of the report.
Susie Hennings (Senior Project Manager) was a member of the TMG, and was responsible for the writing and reviewing of the report.
Iftekhar Khan (Research Fellow, Health Economist) was a member of the TMG, responsible for the economic analysis of the trial, and was responsible for the writing and reviewing of the report.
Kamran Khan (Research Associate, Health Economist) was a former member of the TMG, responsible for the economic analysis of the trial, and was also responsible for the writing and reviewing of the report.
Ranjit Lall (Principal Research Fellow, Co-applicant) developed the protocol, was a member of the TMG and is responsible for the statistical analysis of the trial, and was responsible for the writing and reviewing of the report.
Samantha Lyle (Research Fellow) was responsible for the qualitative analysis of the trial and was involved in the writing and reviewing of the report.
Vivien Nichols (Research Associate, Recruitment Lead) was a member of the TMG and was involved in the writing and reviewing of the report.
Stavros Petrou (Professor of Health Economics, Co-applicant) was involved in protocol development, was a member of the TMG, was responsible for the economic analysis of the trial and was involved in the writing and reviewing of the report.
Peter Zeh (Recruitment Network Facilitator) was a member of the TMG and was involved in the writing and reviewing of the report.
Bart Sheehan (Consultant in Psychological Medicine) was involved in the study design, in clinical responsibility and in the writing and reviewing of the report.
Publications
Lamb SE, Sheehan B, Atherton N, Nichols V, Collins H, Mistry D, et al. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ 2018;361:K1675. https://doi.org/10.1136/bmj.k1675
Brown D, Spanjers K, Atherton N, Lowe J, Stonehewer L, Bridle C, et al. Development of an exercise intervention to improve cognition in people with mild to moderate dementia: Dementia And Physical Activity (DAPA Trial). Physiotherapy 2015;101:126–34.
Data sharing statement
Requests for data sharing for secondary research purposes can be addressed to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994.
- Rocca WA, Hofman A, Brayne C, Breteler MMB, Clarke M, Copeland JRM, et al. Frequency and distribution of Alzheimer’s disease in Europe: a collaborative study of 1980–90 prevalence findings. Ann Neurol 1991;30:381-90. https://doi.org/10.1002/ana.410300310.
- Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimer’s and vascular types. Biomed Res Int 2014;2014. https://doi.org/10.1155/2014/908915.
- Wu Y-T, Fratiglioni L, Matthews FE, Lobo A, Breteler MMB, Skoog I, et al. Dementia in western Europe: epidemiological evidence and implications for policy making. Lancet Neurol 2015;15:116-24. https://doi.org/10.1016/S1474-4422(15)00092-7.
- Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, et al. A comparison of the Prevalence of Dementia in the United States in 2000 and 2012. JAMA Intern Med 2017:51-8. https://doi.org/10.1001/jamainternmed.2016.6807.
- Luengo-Fernandez R, Leal J, Gray Ai. Dementia 2010: The Economic Burden of Dementia and Associated Research Funding in the United Kingdom. Cambridge: Alzheimer’s Research Trust; 2010.
- Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 2013;382:1405-12. https://doi.org/10.1016/S0140-6736(13)61570-6.
- The Prime Minister’s Challenge on Dementia 2020. London: Department of Health and Social Care; 2015.
- Geda YE, Roberts RO, Knopman DS, Christianson TJH, Pankratz VS, Ivnik RJ, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol 2010;67. https://doi.org/10.1001/archneurol.2009.297.
- Etgen T, Sander D, Huntgeburth U, Poppert H, Förstl H, Bickel H. Physical activity and incident cognitive impairment in elderly persons: the INVADE study. Arch Intern Med 2010;170:186-93. https://doi.org/10.1001/archinternmed.2009.498.
- Erickson KI, Kramer AF. Aerobic exercise effects on cognitive and neural plasticity in older adults. Br J Sports Med 2009;43:22-4. https://doi.org/10.1136/bjsm.2008.0052498.
- Xiong JY, Li SC, Sun YX, Zhang XS, Dong ZZ, Zhong P, et al. Long-term treadmill exercise improves spatial memory of male APPswe/PS1dE9 mice by regulation of BDNF expression and microglia activation. Biol Sport 2015;32:295-300. https://doi.org/10.5604/20831862.1163692.
- Revilla S, Suñol C, García-Mesa Y, Giménez-Llort L, Sanfeliu C, Cristòfol R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology 2014;81:55-63. https://doi.org/10.1016/j.neuropharm.2014.001.0037.
- Bo H, Kang W, Jiang N, Wang X, Zhang Y, Ji LL. Exercise-induced neuroprotection of hippocampus in APP/PS1 transgenic mice via upregulation of mitochondrial 8-oxoguanine DNA glycosylase. Oxid Med Cell Longev 2014;2014. https://doi.org/10.1155/2014/834502.
- Chao F, Zhang L, Luo Y, Xiao Q, Lv F, He Q, et al. Running exercise reduces myelinated fiber loss in the dentate gyrus of the hippocampus in app/ps1 transgenic mice. Curr Alzheimer Res 2015;12:377-83. https://doi.org/10.2174/1567205012666150325183011.
- Moore KM, Girens RE, Larson SK, Jones MR, Restivo JL, Holtzman DM, et al. A spectrum of exercise training reduces soluble Abeta in a dose-dependent manner in a mouse model of Alzheimer’s disease. Neurobiol Dis 2016;85:218-24. https://doi.org/10.1016/j.nbd.2015.11.004.
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 2009;19:1030-9. https://doi.org/10.1002/hipo.20547.
- Deslandes A, Moraes H, Ferreira C, Veiga H, Silveira H, Mouta R, et al. Exercise and mental health: many reasons to move. Neuropsychobiology 2009;59:191-8. https://doi.org/10.1159/000223730.
- Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. Exercise programs for people with dementia. Cochrane Database of Syst Rev 2015;4. https://doi.org/10.1002/14651858.CD006489.pub4.
- Groot C, Hooghiemstra AM, Raijmakers PG, van Berckel BN, Scheltens P, Scherder EJ, et al. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res Rev 2016;25:13-2. https://doi.org/10.1016/j.arr.2015.11.0005.
- Öhman H, Savikko N, Strandberg TE, Pitkälä KH. Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: a systematic review. Dement Geriatr Cogn Disord 2014;38:347-65. https://doi.org/10.1159/000365388.
- Alzheimer’s Disease – Donepezil, Galantamine, Rivastigmine and Memantine. London: National Institute for Health and Care Excellence; 2011.
- Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol 2009;28. https://doi.org/10.1037/a0016136.
- Conn VS, Valentine JC, Cooper HM. Interventions to increase physical activity among aging adults: a meta-analysis. Ann Behav Med 2002;24:190-20. https://doi.org/10.1207/S15324796ABM2403_04.
- Heath GW, Parra DC, Sarmiento OL, Andersen LB, Owen N, Goenka S, et al. Evidence-based intervention in physical activity: lessons from around the world. Lancet 2012;380:272-81. https://doi.org/10.1016/S0140-6736(12)60816-2.
- Brown D, Spanjers K, Atherton N, Lowe J, Stonehewer L, Bridle C, et al. Development of an exercise intervention to improve cognition in people with mild to moderate dementia: Dementia And Physical Activity (DAPA) Trial, registration ISRCTN32612072. Physiotherapy 2015;101:126-34. https://doi.org/10.1016/j.physio.2015.01.002.
- Hansen Z, Daykin A, Lamb SE. A cognitive-behavioural programme for the management of low back pain in primary care: a description and justification of the intervention used in the Back Skills Training Trial (BeST; ISRCTN 54717854). Physiotherapy 2010;96:87-94. https://doi.org/10.1016/j.physio.2009.009.0008.
- Lamb SE, Lall R, Hansen Z, Castelnuovo E, Withers EJ, Nichols V, et al. A multicentred randomised controlled trial of a primary care-based cognitive behavioural programme for low back pain. The Back Skills Training (BeST) trial. Health Technol Assess 2010;14. https://doi.org/10.3310/hta14410.
- Lamb SE, Williams MA, Williamson EM, Gates S, Withers EJ, Mt-Isa S, et al. Managing Injuries of the Neck Trial (MINT): a randomised controlled trial of treatments for whiplash injuries. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16490.
- Williamson E, Williams M, Hansen Z, Joseph S, Lamb SE. Development and delivery of a physiotherapy intervention for the early management of whiplash injuries: the Managing Injuries of Neck Trial (MINT) Intervention. Physiotherapy 2009;95:15-23. https://doi.org/10.1016/j.physio.2008.11.0001.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand: quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sport Exer 2011;43:1334-59. https://doi.org/10.1249/MSS.0b013e318213fefb.
- Hardman AE, Stensel DJ. Physical Activity and Health: The Evidence Explained. London: Routledge; 2009.
- Lista I, Sorrentino G. Biological mechanisms of physical activity in preventing cognitive decline. Cell Mol Neurobiol 2010;30:493-50. https://doi.org/10.1007/s10571-009-9488-x.
- Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc 2011;86:876-84. https://doi.org/10.4065/mcp.2011.00252.
- Suetta C, Clemmensen C, Andersen JL, Magnusson SP, Schjerling P, Kjaer M. Coordinated increase in skeletal muscle fiber area and expression of IGF-I with resistance exercise in elderly post-operative patients. Growth Horm IGF Res 2010;20:134-40. https://doi.org/10.1016/j.ghir.2009.11.005.
- Vincent KR, Braith RW, Bottiglieri T, Vincent HK, Lowenthal DT. Homocysteine and lipoprotein levels following resistance training in older adults. Prev Cardiol 2003;6:197-203. https://doi.org/10.1111/j.1520-037X.2003.01723.x.
- Yu F, Swartwood RM. Feasibility and perception of the impact from aerobic exercise in older adults with Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2012;27:397-405. https://doi.org/10.1177/1533317512453492.
- Zoltan B. Vision, Perception, and Cognition: A Manual for the Evaluation and Treatment of the Neurologically Impaired Adult. Thorofare, NJ: Slack Inc.; 2007.
- Conn VS, Hafdahl AR, Mehr DR. Interventions to increase physical activity among healthy adults: meta-analysis of outcomes. Am J Public Health 2011;101:751-8. https://doi.org/10.2105/AJPH.2010.194381.
- Cress ME, Buchner DM, Prohaska T, Rimmer J, Brown M, Macera C, et al. Physical activity programs and behavior counseling in older adult populations. Med Sci Sport Exer 2004;36:1997-2003. https://doi.org/10.1249/01.MSS.0000145451.08166.97.
- Dementia: Supporting People with Dementia and their Carers in Health and Social Care. London: National Institute for Health and Care Excellence; 2006.
- Global Recommendations on Physical Activity for Health. Geneva: WHO; 2010.
- Ries JD, Echternach JL, Nof L, Gagnon Blodgett M. Test-retest reliability and minimal detectable change scores for the timed ‘up & go’ test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys Ther 2009;89:569-79. https://doi.org/10.2522/ptj.20080258.
- Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992;47:1019-24. https://doi.org/10.1136/thx.47.12.1019.
- Roca J, Whipp BJ, Agusti AGN, Anderson SD, Casaburi R, Cotes JE, et al. Clinical exercise testing with reference to lung diseases: indications, standardization and interpretation strategies. Eur Respir J 1997;10:2662-89. https://doi.org/10.1183/09031936.97.10112662.
- Yu F. Developing an Aerobic Exercise Training Program for Older Adults With Alzheimer’s Disease n.d. www.alz.org/mnnd/documents/breakout_session_303-yu.pdf (accessed 17 October 2011).
- Drouin JM, Valovich-McLeod TC, Shultz SJ, Gansneder BM, Perrin DH. Reliability and validity of the Biodex system 3 pro isokinetic dynamometer velocity, torque and position measurements. Eur J Appl Physiol 2004;91:22-9. https://doi.org/10.1007/s00421-003-0933-0.
- Wood TM, Maddalozzo GF, Harter RA. Accuracy of seven equations for Predicting 1-RM performance of apparently healthy, sedentary older adults. Meas Physl Ed Exer Sci 2002;6:67-94. https://doi.org/10.1207/S15327841MPEE0602_1.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. https://doi.org/10.1007/s12160-013-9486-6.
- Michie S, Rumsey N, Fussell A, Hardeman W, Johnston M, Newman S, et al. Improving Health: Changing Behaviour, NHS Health Trainer Handbook. London: Department of Health and Social Care; 2008.
- van Stralen MM, De Vries H, Mudde AN, Bolman C, Lechner L. Determinants of initiation and maintenance of physical activity among older adults: a literature review. Health Psychol Rev 2009;3:147-20. https://doi.org/10.1080/17437190903229462.
- Williams SL, French DP. What are the most effective intervention techniques for changing physical activity self-efficacy and physical activity behaviour – and are they the same?. Health Educ Res 2011;26:308-22. https://doi.org/10.1093/her/cyr005.
- Cyarto EV, Brown WJ, Marshall AL. Retention, adherence and compliance: important considerations for home- and group-based resistance training programs for older adults. J Sci Med Sport 2006;9:402-12. https://doi.org/10.1016/j.jsams.2006.06.020.
- Evers A, Klusmann V, Ziegelmann JP, Schwarzer R, Heuser I. Long-term adherence to a physical activity intervention: the role of telephone-assisted vs. self-administered coping plans and strategy use. Psychol Health 2012;27:784-97. https://doi.org/10.1080/08870446.2011.582114.
- Hagberg LA, Lindahl B, Nyberg L, Hellénius ML. Importance of enjoyment when promoting physical exercise. Scand J Med Sci Sports 2009;19:740-7. https://doi.org/10.1111/j.1600-0838.2008.000844.x.
- Resnick B, D’Adamo C. Factors associated with exercise among older adults in a continuing care retirement community. Rehabil Nurs 2011;36:47-53. https://doi.org/10.1002/j.2048-7940.2011.tb00065.x.
- Dementia: The NICE–SCIE Guideline on Supporting People with Dementia and their Carers in Health and Social Care 2007. Leicester: The British Psychological Society; 2007.
- Underwood M, Lamb S, Eldridge S, Sheehan B, Slowther A. Exercise for depression in care home residents: a randomised controlled trial with cost-effectiveness analysis (OPERA). Health Technol Asses 2013;17.
- Luxton N, Alison JA, Wu J, Mackey MG. Relationship between field walking tests and incremental cycle ergometry in COPD. Respirology 2008;13:856-62. https://doi.org/10.1111/j.1440–1843.2008.001355.x.
- Ratamess NA, Alvar BA, Evetoch TE, Housh TJ, Ben Kibler W, Kraemer WJ, et al. Progression models in resistance training for healthy adults. Med Sci Sport Exer 2009;41:687-708. https://doi.org/10.1249/MSS.0b013e3181915670.
- Harris J, Marshall M. Perspectives on Rehabilitation and Dementia. London: Jessica Kingsley; 2005.
- Beck C, Heacock P, Rapp CG, Mercer SO. Assisting cognitively impaired elders with activities of daily living. Am J Alzheimers Dis 1993;8:11-20. https://doi.org/10.1177/153331759300800602.
- Atherton N, Bridle C, Brown D, Collins H, Dosanjh S, Griffiths F, et al. Dementia and Physical Activity (DAPA) - an exercise intervention to improve cognition in people with mild to moderate dementia: study protocol for a randomized controlled trial. Trials 2016;17. https://doi.org/10.1186/s13063-016-1288-2.
- Vertesi A, Lever JA, Molloy DW, Sanderson B, Tuttle I, Pokoradi L, et al. Standardized Mini-Mental State Examination. Use and interpretation. Can Fam Physician 2001;47:2018-23.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Dementia: Supporting People with Dementia and their Carers in Health and Social Care. London: National Institute for Health and Care Excellence; 2006.
- Physical Activity Guidelines for Adults (19–64 years). London: Department of Health and Social Care; 2011.
- Physical Activity Guidelines for Older Adults (65+ years). London: Department of Health and Social Care; 2011.
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry 1984;141:1356-64. https://doi.org/10.1176/ajp.141.11.1356.
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age Ageing 1996;25:113-20. https://doi.org/10.1093/ageing/25.2.113.
- EuroQuol Group . EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer’s disease: patient and caregiver reports. J Ment Health Aging 1999;5:21-32.
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308-14. https://doi.org/10.1212/WNL.44.12.2308.
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649-55. https://doi.org/10.1093/geront/20.6.649.
- Beecham J, Knapp M. Costing Psychiatric Interventions: DIRUM 1992. www.dirum.org/instruments/details/44 (accessed accessed 17 April 2018).
- Webster L, Groskreutz D, Grinbergs-Saull A, Howard R, O’Brien JT, Mountain G, et al. Development of a core outcome set for disease modification trials in mild to moderate dementia: a systematic review, patient and public consultation and consensus recommendations. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21260.
- Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ 2005;331:321-7. https://doi.org/10.1136/bmj.331.7512.321.
- Rockwood K, Fay S, Gorman M, Carver D, Graham JE. The clinical meaningfulness of ADAS-Cog changes in Alzheimer’s disease patients treated with donepezil in an open-label trial. BMC Neurol 2007;7. https://doi.org/10.1186/1471-2377-7-26.
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biol Psychiatry 1988;23:271-84. https://doi.org/10.1016/0006-3223(88)90038-8.
- Moniz-Cook E, Vernooij-Dassen M, Woods R, Verhey F, Chattat R, Vugt M, et al. A European consensus on outcome measures for psychosocial intervention research in dementia care. Aging Ment Health 2008;12:14-29. https://doi.org/10.1080/13607860801919850.
- Data Protection Act 1998. London: The Stationery Office; 1998.
- Tak EC, van Uffelen JG, Paw MJ, van Mechelen W, Hopman-Rock M. Adherence to exercise programs and determinants of maintenance in older adults with mild cognitive impairment. J Aging Phys Act 2012;20:32-46. https://doi.org/10.1123/japa.20.1.32.
- Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c332.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.00 [updated March 2011]. London: The Cochrane Collaboration; 2011.
- Verma N, Beretvas SN, Pascual B, Masdeu JC, Markey MK. New scoring methodology improves the sensitivity of the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) in clinical trials. Alzheimers Res Ther 2015;7. https://doi.org/10.1186/s13195-015-0151-0.
- Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess 2001;5. https://doi.org/10.3310/hta5330.
- Dunn G, Melnick EL, Everitt BS. Encyclopedia of Quantitative Risk Analysis and Assessment. Chichester: John Wiley & Sons, Ltd; 2014.
- Lloyd V, Gatherer A, Kalsy S. Conducting qualitative interview research with people with expressive language difficulties. Qual Health Res 2006;16:1386-404. https://doi.org/10.1177/1049732306293846.
- Bartlett R, O’Connor D. From personhood to citizenship: broadening the lens for dementia practice and research. J Aging Stud 2007;21:107-18. https://doi.org/10.1016/j.jaging.2006.09.002.
- Caddell LS, Clare L. I’m still the same person: the impact of early-stage dementia on identity. Dementia 2011;10:379-98. https://doi.org/10.1177/1471301211408255.
- Dröes RM. Insight in coping with dementia: listening to the voice of those who suffer from it. Aging Ment Health 2007;11:115-18. https://doi.org/10.1080/13607860601154658.
- Kitwood T, Bredin K. Towards a theory of dementia care: personhood and well-being. Ageing Soc 1992;12:269-87. https://doi.org/10.1017/S0144686X0000502X.
- Moore TF, Hollett J. Giving voice to persons living with dementia: the researcher’s opportunities and challenges. Nurs Sci Q 2003;16:163-7. https://doi.org/10.1177/0894318403251793251793.
- Österholm JH, Samuelsson S. Orally positioning persons with dementia in assessment meetings. Ageing Soc 2013;35:367-88. https://doi.org/10.1017/S0144686X13000755.
- Pesonen HM, Remes AM, Isola A. Ethical aspects of researching subjective experiences in early-stage dementia. Nurs Ethics 2011;18:651-61. https://doi.org/10.1177/0969733011408046.
- Proctor G. Listening to older women with dementia: relationships, voices and power. Disabil Soc 2001;16:361-76. https://doi.org/10.1080/09687590120045932.
- Charmaz K. Constructing Grounded Theory. London: SAGE Publications; 2014.
- Bryman A. Social Research Methods. Oxford: Oxford University Press; 2001.
- Glotzbecker MP, Bono CM, Wood KB, Harris MB. Thromboembolic disease in spinal surgery: a systematic review. Spine 2009;34:291-303. https://doi.org/10.1097/BRS.00b013e318195601d.
- Ferreira PH, Ferreira ML, Maher CG, Refshauge KM, Latimer J, Adams RD. The therapeutic alliance between clinicians and patients predicts outcome in chronic low back pain. Phys Ther 2013;93:470-8. https://doi.org/10.2522/ptj.20120137.
- Kumlin IW, Kroksmark T. The first encounter. Physiotherapists’ conceptions of establishing therapeutic relationships. Scand J Caring Sci 1992;6:37-44. https://doi.org/10.1111/j.1471-6712.1992.tb00121.x.
- Hall AM, Ferreira PH, Maher CG, Latimer J, Ferreira ML. The influence of the therapist-patient relationship on treatment outcome in physical rehabilitation: a systematic review. Phys Ther 2010;90:1099-110. https://doi.org/10.2522/ptj.20090245.
- Mann S. A health-care model of emotional labour: an evaluation of the literature and development of a model. J Health Organ Manag 2005;19:304-17. https://doi.org/10.1108/14777260510615369.
- Mann S, Cowburn J. Emotional labour and stress within mental health nursing. J Psychiatr Ment Health Nurs 2005;12:154-62. https://doi.org/10.1111/j.1365-2850.2004.00807.x.
- Dementia: A Public Health Priority. Geneva: WHO; 2012.
- MacRae H. Self and other: the importance of social interaction and social relationships in shaping the experience of early-stage Alzheimer’s disease. J Aging Stud 2011;25:445-56. https://doi.org/10.1016/j.jaging.2011.06.001.
- Wolverson EL, Clarke C, Moniz-Cook E. Remaining hopeful in early-stage dementia: a qualitative study. Aging Ment Health 2010;14:450-60. https://doi.org/10.1080/13607860903483110.
- Vikström S, Josephsson S, Stigsdotter-Neely A, Nygård L. Engagement in activities: experiences of persons with dementia and their caregiving spouses. Dementia 2008;7:251-70. https://doi.org/10.1177/1471301208091164.
- Macrae H. ‘Making the best you can of it’: living with early-stage Alzheimer’s disease. Sociol Health Illn 2008;30:396-412. https://doi.org/10.1111/j.1467-9566.2007.001056.x.
- Lamb S. Permanent personhood or meaningful decline? Toward a critical anthropology of successful aging. J Aging Stud 2014;29:41-52. https://doi.org/10.1016/j.jaging.2013.12.0006.
- Burgener SC, Buckwalter K, Perkhounkova Y, Liu MF. The effects of perceived stigma on quality of life outcomes in persons with early-stage dementia: longitudinal findings: part 2. Dementia 2013.
- Werner P, Mittelman MS, Goldstein D, Heinik J. Family stigma and caregiver burden in Alzheimer’s disease. Gerontologist 2012;52:89-97. https://doi.org/10.1093/geront/gnr117.
- Beard RL. Advocating voice: organisational, historical and social milieux of the Alzheimer’s disease movement. Sociol Heath Ill 2004;26:797-819. https://doi.org/10.1111/j.0141-9889.2004.00419.x.
- Kitwood T. The experience of dementia. Aging Ment Health 1997;1:13-22. https://doi.org/10.1080/13607869757344.
- Kitwood T. Towards a theory of dementia care: the interpersonal process. Ageing Soc 1993;13:51-67. https://doi.org/10.1017/S0144686X00000647.
- Weaks D, Wilkinson H, McLeod J. Daring to tell: the importance of telling others about a diagnosis of dementia. Ageing Soc 2015;35:765-84. https://doi.org/10.1017/S0144686X13001074.
- O’Sullivan G, Hocking C, Spence D. Dementia: the need for attitudinal change. Dementia 2014;13:483-97. https://doi.org/10.1177/1471301213478241.
- Kindell J, Sage K, Wilkinson R, Keady J. Living with semantic dementia: a case study of one family’s experience. Qual Health Res 2014;24:401-11. https://doi.org/10.1177/1049732314521900.
- Brittain K, Corner L, Robinson L, Bond J. Ageing in place and technologies of place: the lived experience of people with dementia in changing social, physical and technological environments. Sociol Health Ill 2010;32:272-87. https://doi.org/10.1111/j.1467-9566.2009.01203.x.
- Dobbs D, Eckert JK, Rubinstein B, Keimig L, Clark L, Frankowski AC, et al. An ethnographic study of stigma and ageism in residential care or assisted living. Gerontologist 2008;48:517-26. https://doi.org/10.1093/geront/48.4.517.
- Sweeting H, Gilhooly M. Dementia and the phenomenon of social death. Sociol Health Ill 1997;19:93-117. https://doi.org/10.1111/j.1467-9566.1997.tb00017.x.
- Carmody J, Traynor V, Marchetti E. Barriers to qualitative dementia research: the elephant in the room. Qual Health Res 2015;25:1013-19. https://doi.org/10.1177/1049732314554099.
- Guide to the Methods of Technology Appraisal 2013. London: National Institute for Health and Care Excellence; 2013.
- Beecham J, Knapp M, Thornicroft G, Brewin J, Wing. Measuring Mental Health. London: Gaskell; 1992.
- Knapp M, King D, Romeo R, Adams J, Baldwin A, Ballard C, et al. Cost-effectiveness of donepezil and memantine in moderate to severe Alzheimer’s disease (the DOMINO-AD trial). Int J Geriatr Psychiatry 2017;32:1205-16. https://doi.org/10.1002/gps.4583.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015. Canterbury: PSSRU, University of Kent; 2015.
- Motoring Costs 2014. London: Automobile Association; 2014.
- Public Service Vehicle Survey: Bus Statistics. London: Department for Transport; 2015.
- NHS Reference Costs 2014–2015. London: Department of Health and Social Care; 2014.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: PSSRU, University of Kent; 2013.
- Prescription Cost Analysis, England – 2015. Leeds: NHS Digital; 2015.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; 2010.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: PSSRU, University of Kent; 2014.
- Smallwood C. The Role of Complementary and Alternative Medicine in the NHS. An Investigation into the Potential Contribution of Mainstream Complementary Therapies to Healthcare in the UK. London: Get Well UK; 2005.
- Northallerton & District Voluntary Service Association . Carers Respite Sitting Scheme n.d. www.ndvsa.co.uk/index.php/carers-respite-sitting-scheme (accessed accessed on 17 April 2018).
- Liang W. Calculating a Fair Price for Care: A Toolkit for Residential and Nursing Care Costs. York: Joseph Rowntree Foundation; 2008.
- Bupa . Private GP Services 2015. www.bupa.co.uk/health/bupa-on-demand/gp-services (accessed accessed on 17 April 2018).
- Nuffield Health . Physiotherapy FAQs – Help &Amp; Advice 2016. www.nuffieldhealth.com/physiotherapy/faqs (accessed accessed on 17 April 2018).
- London Home Visit Physiotherapy . Fees 2015. www.londonhomevisitphysiotherapy.com/about-us/fees (accessed accessed on 17 April 2018).
- Psychiatry UK . Our Fees 2015. www.psychiatry-uk.com/fees (accessed accessed on 17 April 2018).
- Office for National Statistics . Annual Survey of Hours and Earnings: 2015 Provisional Results 2015. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2015provisionalresults (accessed accessed on 17 April 2018).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci 2007;8:206-13. https://doi.org/10.1007/s11121-007-0070-9.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the estimation of the National Institute for Health and Care Excellence cost-effectiveness threshold. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19140.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8. https://doi.org/10.3310/hta8360.
- D’Amico F, Rehill A, Knapp M, Lowery D, Cerga-Pashoja A, Griffin M, et al. Cost-effectiveness of exercise as a therapy for behavioural and psychological symptoms of dementia within the EVIDEM-E randomised controlled trial. Int J Geriatr Psych 2016;31:656-65. https://doi.org/10.1002/gps.4376.
- Vreugdenhil A, Cannell J, Davies A, Razay G. A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: a randomized controlled trial. Scand J Caring Sci 2012;26:12-9. https://doi.org/10.1111/j.1471-6712.2011.00895.x.
- Underwood M, Lamb SE, Eldridge S, Sheehan B, Slowther AM, Spencer A, et al. Exercise for depression in elderly residents of care homes: a cluster-randomised controlled trial. Lancet 2013;382:41-9. https://doi.org/10.1016/S0140-6736(13)60649-2.
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985;100:126-31.
- Lamb SE, Becker C, Gillespie LD, Smith JL, Finnegan S, Potter R, et al. Reporting of complex interventions in clinical trials: development of a taxonomy to classify and describe fall-prevention interventions. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-125.
- Byrne C, Faure C, Keene DJ, Lamb SE. Ageing, muscle power and physical function: a systematic review and implications for pragmatic training interventions. Sports Med 2016;46:1311-32. https://doi.org/10.1007/s40279-016-0489-x.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. https://doi.org/10.1136/bmj.d5928.
- Rao JN, Scott AJ. A simple method for the analysis of clustered binary data. Biometrics 1992;48:577-85. https://doi.org/10.2307/2532311.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. https://doi.org/10.1002/sim.1186.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. https://doi.org/10.1136/bmj.315.7109.629.
- López N, Véliz A, Soto-Añari M, Ollari J, Chesta S, Allegri R. Efectos de un programa combinado de actividad física y entrenamiento cognitivo en pacientes chilenos con Alzheimer leve. Neurolog Argentina 2015;7:131-9. https://doi.org/10.1016/j.neuarg.2015.04.001.
- Aguiar P, Monteiro L, Feres A, Gomes I, Melo A. Rivastigmine transdermal patch and physical exercises for alzheimer’s disease: a randomized clinical trial. Curr Alzheimer Res 2014;11:532-7. https://doi.org/10.2174/1567205011666140618102224.
- Arcoverde C, Deslandes A, Moraes H, Almeida C, de Araujo NB, Vasques PE, et al. Treadmill training as an augmentation treatment for Alzheimer’s disease: a pilot randomized controlled study. Arq Neuro-Psiquiat 2014;72:190-6. https://doi.org/10.1590/0004-282X20130231.
- Bossers WJR, van der Woude LHV, Boersma F, Hortobagyi T, Scherder EJA, van Heuvelen MJG. A 9-week aerobic and strength training program improves cognitive and motor function in patients with dementia: a randomized, controlled trial. Am J Geriat Psychiat 2015;23:1106-16. https://doi.org/10.1016/j.jagp.2014.12.191.
- Cheng ST, Chow PK, Song YQ, Yu EC, Chan AC, Lee TM, et al. Mental and physical activities delay cognitive decline in older persons with dementia. Am J Geriatr Psychiatry 2014;22:63-74. https://doi.org/10.1016/j.jagp.2013.001.0060.
- Eggermont LH, Swaab DF, Hol EM, Scherder EJ. Walking the line: a randomised trial on the effects of a short term walking programme on cognition in dementia. J Neurol Neurosurg Psychiatr 2009;80:802-4. https://doi.org/10.1136/jnnp.2008.158444.
- Hoffmann VS, Baccarani M, Hasford J, Castagnetti F, Di Raimondo F, Casado LF, et al. Treatment and outcome of 2904 CML patients from the EUTOS population-based registry. Leukemia 2017;31:593-601. https://doi.org/10.1038/leu.2016.246.
- Holthoff VA, Marschner K, Scharf M, Steding J, Meyer S, Koch R, et al. Effects of physical activity training in patients with Alzheimer’s dementia: results of a pilot RCT study. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.00121478.
- Kemoun G, Thibaud M, Roumagne N, Carette P, Albinet C, Toussaint L, et al. Effects of a physical training programme on cognitive function and walking efficiency in elderly persons with dementia. Dement Geriatr Cogn 2010;29:109-14. https://doi.org/10.1159/000272435.
- Miu D, Szeto S, Mak Y. A randomised controlled trial on the effect of exercise on physical, cognitive and affective function in dementia subjects. Asian J Gerontol Geriatr 2008;3:8-16.
- Pitkälä KH, Pöysti MM, Laakkonen ML, Tilvis RS, Savikko N, Kautiainen H, et al. Effects of the Finnish Alzheimer disease exercise trial (FINALEX): a randomized controlled trial. JAMA Intern Med 2013;173:894-901. https://doi.org/10.1001/jamainternmed.2013.359.
- Steinberg M, Leoutsakos JM, Podewils LJ, Lyketsos CG. Evaluation of a home-based exercise program in the treatment of Alzheimer’s disease: the Maximizing Independence in Dementia (MIND) study. Int J Geriatr Psychiatry 2009;24:680-5. https://doi.org/10.1002/gps.2175.
- Stevens J, Killeen M. A randomised controlled trial testing the impact of exercise on cognitive symptoms and disability of residents with dementia. Contemp Nurse 2006;21:32-40. https://doi.org/10.5555/conu.2006.21.1.32.
- Venturelli M, Scarsini R, Schena F. Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am J Alzheimers Dis Other Demen 2011;26:381-8. https://doi.org/10.1177/1533317511418956.
- Venturelli M, Sollima A, Ce E, Limonta E, Bisconti AV, Brasioli A, et al. Effectiveness of exercise- and cognitive-based treatments on salivary cortisol levels and sundowning syndrome symptoms in patients with Alzheimer’s disease. J Alzheimers Dis 2016;53:1631-40. https://doi.org/10.3233/JAD-160392.
- Yang SY, Shan CL, Qing H, Wang W, Zhu Y, Yin MM, et al. The effects of aerobic exercise on cognitive function of Alzheimer’s disease patients. CNS Neurol Disord Drug Targets 2015;14:1292-7. https://doi.org/10.2174/1871527315666151111123319.
- Nguyen TL, Collins GS, Lamy A, Devereaux PJ, Daurès JP, Landais P, et al. Simple randomization did not protect against bias in smaller trials. J Clin Epidemiol 2017;84:105-13. https://doi.org/10.1016/j.jclinepi.2017.02.010.
- Hopewell S, Boutron I, Altman DG, Barbour G, Moher D, Montori V, et al. Impact of a web-based tool (WebCONSORT) to improve the reporting of randomised trials: results of a randomised controlled trial. BMC Med 2016;14. https://doi.org/10.1186/s12916-016-0736-x.
- Stijntjes M, Aartsen MJ, Taekema DG, Gussekloo J, Huisman M, Meskers CG, et al. Temporal relationship between cognitive and physical performance in middle-aged to oldest old people. J Gerontol A Biol Sci Med Sci 2017;72:662-8.
- Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Cornas-Herrera A, et al. Dementia UK: Update. London: Alzheimer’s Society; 2014.
- Miranda-Castillo C, Woods B, Orrell M. People with dementia living alone: what are their needs and what kind of support are they receiving?. Int Psychogeriatr 2010;22:607-17. https://doi.org/10.1017/S104161021000013X.
- 2011 Census Analysis, Local Area Analysis of Qualifications Across England and Wales Release. Newport: Office for National Statistics; 2014.
- Orgeta V, Edwards RT, Hounsome B, Orrell M, Woods B. The use of the EQ-5D as a measure of health-related quality of life in people with dementia and their carers. Qual Life Res 2015;24:315-24. https://doi.org/10.1007/s11136-014-0770-0.
- Rand S, Caiels J. Using Proxies to Assess Quality of Life: A Review of the Issues and Challenges. Canterbury: Quality and Outcomes of Person-Centred Care Policy Research Unit; 2015.
- Lamb SE, Jorstad-Stein EC, Hauer K, Becker C. Prevention of Falls Network Europe and Outcomes Consensus Group . Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc 2005;53:1618-22. https://doi.org/10.1111/j.1532-5415.2005.53455.x.
- Toots A, Littbrand H, Lindelöf N, Wiklund R, Holmberg H, Nordström P, et al. Effects of a high-intensity functional exercise program on dependence in activities of daily living and balance in older adults with dementia. J Am Geriatr Soc 2016;64:55-64. https://doi.org/10.1111/jgs.13880.
- Littbrand H, Rosendahl E, Lindelof N, Lundin-Olsson L, Gustafson Y, Nyberg L. A high-intensity functional weight-bearing exercise program for older people dependent in activities of daily living and living in residential care facilities: evaluation of the applicability with focus on cognitive function. Phys Ther 2006;86:489-98.
- Looi JC, Sachdev PS. Differentiation of vascular dementia from AD on neuropsychological tests. Neurology 1999;53:670-8. https://doi.org/10.1212/WNL.53.4.670.
- Verma N, Markey MK. Item Response Analysis of Alzheimer’s Disease Assessment Scale n.d.
- Cai L. High-dimensional exploratory item factor analysis by a Metropolis–Hastings Robbins–Monro algorithm. Psychometrika 2010;75:33-57. https://doi.org/10.1007/s11336-009-9136-x.
Appendix 1 Health Technology Assessment correspondence regarding 4-month restriction on the intervention design
Appendix 2 Pre-exercise assessment form
Appendix 3 Suppliers of exercise equipment
| Standard materials list for intervention | Manufacturer | Manufacturer’s location |
|---|---|---|
| Marker cones | The Safety Supply Company | Wembley, UK |
| Lap counter | Silver-Line Tools Ltd | Yeovil, UK |
| Heart rate monitor | Polar Electro (UK) Ltd | Warwick, UK |
| 15-m tape measure | Silver-Line Tools Ltd | Yeovil, UK |
| Chair cushions | Fitness-Mad [The Mad Group (HQ) Ltd] | Evesham, UK |
| Fully weighted belts (mixed sizes) | Rehabus | Lerum, Sverige |
| Fully loaded 20-lb weighted vests | All Pro Exercise Products Inc. | Longboat Key, FL, USA |
| Handweights | Fitness-Mad [The Mad Group (HQ) Ltd] | Evesham, UK |
| Stopwatch | Scientific Laboratory Supplies Ltd | Hessle, UK |
| Grey crates (× 8) | The Hill Company | West Thurrock, UK |
| Flatbed trolley (× 2) | Key (Family member of Manutan) | Verwood, UK |
| Gel seat cover | Selle Royal | Pozzoleone, Italy |
| Portable bike | 3D Innovations LLC | Greeley, CO, USA |
| Recliner bike | Powerhouse Fitness | Glasgow, UK |
| Upright bike | Powerhouse Fitness | Glasgow, UK |
Appendix 4 Quality control forms for sites
Appendix 5 Item response theory analysis
We made an analysis of the DAPA trial data using IRT analysis. We assumed that cognitive impairment is a continuous score, such that higher scores on the ADAS-Cog denote higher levels of impairment. In the original IRT analysis of the ADAS-Cog, Verma and Markey186 found that cognitive impairment is composed of three subtraits: memory, language and praxis. This model is consistent with clinical opinion.
To create the ADAS-Cog-IRT, the ADAS-Cog items were first assessed for use in an IRT analysis. This consisted of examining item-level frequency tables for all data collected and checking for response categories that were sparsely used. Dichotomous items with low response rates were dealt with as follows:
-
commands – ceiling and fist items were combined to create ‘easy’ commands owing to their low incorrect response rate
-
construction – circle and rectangle were combined to create ‘easy’ construction
-
naming objects:
-
flower, bed, whistle and pencil were combined into ‘high frequency’
-
rattle, mask, scissors and comb were combined into ‘medium frequency’
-
-
naming fingers – the thumb item was removed owing to the low number of incorrect responses
-
orientation – the name item was removed owing to the low number of incorrect responses.
For polytomous items, categories with low response frequencies were merged together, and for the word recall trials, the mean score was used (rounded to the nearest integer). Patients with any incomplete responses to the remaining ADAS-Cog items were then removed from further analyses.
In line with the methods of Verma and Markey,186 the two-parameter model was fitted to all dichotomous items apart from orientation – season; naming objects – funnel; and constructional praxis – cube, which were fitted with the three-parameter model. The two-parameter models consist of parameters of characteristic (slope) and difficulty (intercept). The characteristic of each item represents the sensitivity of the item to detect different abilities, with lager characteristics (greater slopes) being able to detect more subtle differences in abilities. The item difficulty (intercept or location) represents the point where half of the population affirm the item, and is the point at which the item slope is steepest. For the three-parameter model, the third parameter represents ‘guessing’, or the fact that respondents of lower ability may randomly guess which answer is correct, rather than having the ability to pass the item. For the DAPA study, it represents items for which persons with low levels of cognitive impairment find the item difficult, even though their impairment is low enough that they should not have affirmed the item.
The graded response model was used for all polytomous items. The graded response model fits an item response curve (probability of affirming the item) to each possible item response separately. The responses are assumed ordered, but not at fixed intervals. This captures the fact that for each item, the interval of cognitive impairment between ‘mild’ and ‘moderate’ is not necessarily the same as the difference in cognitive impairment between ‘moderate’ and ‘moderately severe’.
Rather than fitting a novel model, the structure reported by Verma and Markey186 was used, as this model had been tested on real and simulated clinical trial data and was found to have good clinical meaning. Hence, the baseline data were then used to obtain the parameter estimates of the following item structure:
-
memory trait – word recall, orientation items and word recognition
-
language trait – naming objects and fingers items, spoken language, language comprehension, word finding and remembering test instruction
-
praxis trait – commands items, construction items and ideational praxis items.
The model was estimated using the mirt package in R (The R Foundation for Statistical Computing, Vienna, Austria) using a Metropolis–Hastings Robbins–Monro algorithm. 187 Similarly to factor analysis, an oblimin factor rotation was applied to allow each factor trait to correlate freely with each other.
This estimated model was then used to calculate the respondent’s ability for each latent trait at each ADAS-Cog response. Baseline data were used to estimate the model, as there would be no influence from the trial interventions on the data, and the balance between the groups was ensured by randomisation. No patient-level data were added to the model.
Hence, the ADAS-Cog-IRT at a single follow-up point consists of the estimated ability for each of the three traits separately. As the model allowed correlations between traits, total scores were not constructed. Scaling factors were not applied; consequently, all trait abilities were generated using IRT standard parameters, that is, the distribution of abilities for each latent trait is centred on a mean of 0 and a SD of 1.
Appendix 6 Supplementary analyses
| Phase and data | Region, n (%) | Total, n (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Berkshire | Black Country | Coventry | Devon and Exeter | Greater Manchester West | Gloucestershire and Herefordshire | Leicester | Northampton | North East London | Nuneaton | Oxford | Rugby | Solent | South Warwickshire | Worcester | ||
| From entry into the trial up to pre randomisation | ||||||||||||||||
| Total number of patients approached | 46 | 180 | 589 | 26 | 44 | 27 | 148 | 274 | 57 | 382 | 381 | 174 | 21 | 172 | 408 | 2929 |
| Excluded patients not meeting the eligibility criteriaa | 1 (2) | 9 (5) | 337 (57) | 2 (8) | 8 (18) | 5 (19) | 47 (32) | 57 (21) | 16 (28) | 236 (62) | 102 (27) | 75 (43) | 2 (10) | 24 (14) | 161 (39) | 1082 (37) |
| Randomisation | ||||||||||||||||
| Patients satisfying the entry inclusion criteriaa | 41 (89) | 45 (25) | 54 (9) | 10 (38) | 18 (41) | 18 (67) | 17 (11) | 62 (23) | 18 (32) | 23 (6) | 75 (20) | 24 (14) | 10 (48) | 36 (21) | 43 (11) | 494 (17) |
| Patients satisfying the entry inclusion criteriaa | 4 (9) | 126 (70) | 198 (34) | 14 (54) | 18 (41) | 4 (15) | 84 (57) | 155 (57) | 23 (40) | 123 (32) | 204 (54) | 75 (43) | 9 (43) | 112 (65) | 204 (50) | 1353 (46) |
| Patients randomised but ineligible | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Follow-upb | ||||||||||||||||
| No follow-up data at any time points | 4 (10) | 5 (11) | 6 (11) | 1 (10) | 0 | 1 (6) | 4 (24) | 7 (11) | 3 (17) | 1 (4) | 6 (8) | 2 (8) | 2 (20) | 2 (6) | 1 (2) | 45 (9) |
| Follow-up data available for 6 months only | 6 (15) | 3 (7) | 5 (9) | 0 | 1 (6) | 1 (6) | 1 (6) | 6 (10) | 1 (6) | 1 (4) | 3 (4) | 0 | 1 (10) | 2 (6) | 0 | 31 (6) |
| Follow-up data available for 12 months only | 0 | 0 | 1 (2) | 0 | 0 | 0 | 0 | 1 (2) | 0 | 0 | 0 | 0 | 0 | 0 | 2 (5) | 4 (1) |
| Follow-up data available for both 6 and 12 months | 31 (76) | 37 (82) | 42 (78) | 9 (90) | 17 (94) | 16 (89) | 12 (71) | 48 (77) | 14 (78) | 21 (91) | 66 (88) | 22 (92) | 7 (70) | 32 (89) | 40 (93) | 414 (84) |
| Diedb | ||||||||||||||||
| Patient dies post randomisation up to 6 months’ follow-up | 1 (2) | 0 | 2 (4) | 0 | 0 | 0 | 0 | 1 (2) | 0 | 0 | 1 (1) | 0 | 0 | 1 (3) | 0 | 6 (1) |
| Patient died during 6 to 12 months’ follow-up | 2 (5) | 0 | 3 (6) | 0 | 0 | 1 (6) | 1 (6) | 2 (3) | 0 | 0 | 1 (1) | 0 | 0 | 2 (6) | 0 | 12 (2) |
| Withdrawalsb | ||||||||||||||||
| Patient withdrew post randomisation up to 6 months’ follow-up | 3 (7) | 4 (9) | 4 (7) | 1 (10) | 0 | 1 (6) | 4 (24) | 5 (8) | 2 (11) | 1 (4) | 5 (7) | 2 (8) | 2 (20) | 1 (3) | 0 | 35 (7) |
| Patient withdrew during 6 to 12 months’ follow-up | 4 (10) | 3 (7) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (3) | 0 | 1 (10) | 0 | 0 | 10 (2) |
| Non-responders (follow-up)b | ||||||||||||||||
| Non-response to 6-month follow-up | 0 | 1 (2) | 1 (2) | 0 | 0 | 0 | 0 | 2 (3) | 1 (6) | 0 | 0 | 0 | 0 | 0 | 3 (7) | 8 (2) |
| Non-response to 6 to 12 months’ follow-up | 0 | 1 (2) | 2 (4) | 0 | 1 (6) | 0 | 0 | 5 (8) | 2 (11) | 1 (4) | 0 | 0 | 0 | 0 | 1 (2) | 13 (3) |
| Data source | Region | Total, n (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Berkshire | Black Country | Coventry | Devon and Exeter | Gloucestershire and Herefordshire | Greater Manchester West | Leicester | North East London | Northampton | Nuneaton | Oxford | Rugby | Solent | South Warwickshire | Worcester | ||
| Primary care | 0 | 67 | 115 | 0 | 2 | 0 | 17 | 0 | 2 | 71 | 0 | 7 | 0 | 57 | 217 | 555 |
| RiL | 34 | 0 | 0 | 0 | 0 | 1 | 16 | 0 | 215 | 0 | 207 | 0 | 0 | 0 | 0 | 473 |
| Secondary care | 6 | 92 | 111 | 21 | 20 | 29 | 68 | 40 | 0 | 75 | 71 | 88 | 19 | 80 | 30 | 750 |
| Other | 5 | 12 | 26 | 3 | 0 | 6 | 0 | 1 | 0 | 0 | 1 | 4 | 0 | 11 | 0 | 69 |
| Total | 45 | 171 | 252 | 24 | 22 | 36 | 101 | 41 | 217 | 146 | 279 | 99 | 19 | 148 | 247 | 1847 |
| Region | sMMSE score | |||
|---|---|---|---|---|
| Treatment arm, < 20 | Treatment arm, ≥ 20 | |||
| Usual care | Exercise programme | Usual care | Exercise programme | |
| Berkshire | 4 | 5 | 9 | 23 |
| Black Country | 7 | 12 | 8 | 18 |
| Coventry | 7 | 13 | 11 | 23 |
| Devon and Exeter | 1 | 3 | 3 | 3 |
| Gloucestershire and Herefordshire | 1 | 2 | 5 | 10 |
| Greater Manchester West | 2 | 5 | 4 | 7 |
| Leicester | 0 | 2 | 5 | 10 |
| North East London | 2 | 2 | 4 | 10 |
| Northampton | 7 | 8 | 14 | 33 |
| Nuneaton | 3 | 8 | 5 | 7 |
| Oxford | 6 | 17 | 19 | 33 |
| Rugby | 3 | 6 | 5 | 10 |
| Solent | 0 | 1 | 4 | 5 |
| South Warwickshire | 5 | 10 | 7 | 14 |
| Worcester | 3 | 6 | 11 | 23 |
| Total | 51 | 100 | 114 | 229 |
| Characteristic/outcome | Died (N = 6) | Withdrew (N = 35) | Lost to follow-up (N = 8) | Completed follow-up (N = 445) | p-value for difference between completers and non-completersa |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 80.1 (4.3) | 77.8 (8.7) | 78.1 (6.6) | 77.3 (7.9) | 0.464 |
| Range | 74.1 to 87.4 | 51.1 to 92.2 | 65.9 to 88.6 | 50.4 to 94.6 | |
| Gender (male), n (%) | 4 (66.7) | 24 (68.6) | 4 (50.0) | 269 (60.5) | 0.508 |
| Marital status, n (%) | |||||
| Single | 0 | 1 (2.9) | 1 (12.5) | 7 (1.6) | 0.013 |
| Married | 3 (50.0) | 25 (71.4) | 4 (50.0) | 332 (74.6) | |
| Separated | 0 | 2 (5.7) | 1 (12.5) | 4 (0.9) | |
| Divorced | 2 (33.3) | 2 (5.7) | 0 | 11 (2.5) | |
| Widowed | 1 (16.7) | 4 (11.4) | 2 (25.0) | 79 (17.7) | |
| Cohabiting | 0 | 1 (2.9) | 0 | 11 (2.5) | |
| Missing | 0 | 0 | 0 | 1 (0.2) | |
| Living arrangements, n (%) | |||||
| Live alone | 3 (50.0) | 8 (22.9) | 4 (50.0) | 82 (18.4) | 0.195 |
| Live with relatives | 0 | 1 (2.9) | 0 | 22 (5.0) | |
| Live with wife/husband/partner | 3 (50.0) | 26 (74.2) | 4 (50.0) | 340 (76.4) | |
| Living with friends | 0 | 0 | 0 | 1 (0.2) | |
| Ethnicity, n (%) | |||||
| White | 5 (83.3) | 33 (94.2) | 8 (100.0) | 432 (97.1) | 0.05 |
| Other | 1 (16.7) | 2 (5.8) | 0 | 13 (2.9) | |
| Age (years) left full-time education | |||||
| Mean (SD) | 16.8 (2.4) | 16.1 (1.8) | 15.6 (0.7) | 16.2 (2.7) | 0.919 |
| Range | 14 to 21 | 14 to 20 | 15 to 17 | 7 to 31 | |
| Missing | 0 | 0 | 0 | 8 | |
| Highest level of education, n (%) | |||||
| Degree/degree equivalent (including higher degree)/NVQ4/NVQ5 | 1 (16.7) | 5 (14.3) | 1 (12.5) | 74 (16.6) | 0.884 |
| Higher education below degree | 1 (16.7) | 5 (14.3) | 0 | 38 (8.5) | |
| NVQ3/GCE A-level equivalent | 0 | 1 (2.9) | 1 (12.5) | 22 (4.9) | |
| NVQ2/GCE O-level/GCSE-level equivalent/school certificate | 1 (16.7) | 8 (22.8) | 1 (12.5) | 79 (17.8) | |
| Other vocational/work-related qualifications | 0 | 5 (14.3) | 3 (37.5) | 95 (21.4) | |
| No qualification | 3 (49.9) | 11 (31.4) | 2 (25.0) | 132 (29.7) | |
| Missing | 0 | 0 | 0 | 5 (1.1) | |
| ADAS-Cog (imputed) | |||||
| Mean (SD) | 27.4 (12.5) | 22.7 (8.0) | 21.5 (6.5) | 21.4 (9.1) | 0.209 |
| Range | 12 to 41.7 | 8.7 to 43.4 | 15 to 35.7 | 5.3 to 52.7 | |
| Missing | 0 | 0 | 0 | 2 | |
| ADAS-Cog (raw score) | |||||
| Mean (SD) | 27.4 (12.5) | 21.6 (6.8) | 21.5 (6.5) | 20.7 (8.5) | 0.219 |
| Range | 12 to 41.7 | 8.7 to 33.3 | 15 to 35.7 | 5.3 to 52 | |
| Missing | 0 | 2 | 0 | 26 | |
| sMMSE score | |||||
| Mean (SD) | 18.2 (5.2) | 21.1 (4.3) | 22.6 (3.7) | 21.9 (4.7) | 0.157 |
| Range | 12 to 25 | 13 to 27 | 15 to 26 | 11 to 30 | |
| EQ-5D-3L score (self-reported) | |||||
| Mean (SD) | 0.76 (0.42) | 0.75 (0.19) | 0.67 (0.39) | 0.84 (0.18) | < 0.001 |
| Range | –0.02 to 1 | 0.19 to 1 | –0.02 to 1 | –0.07 to 1 | |
| Missing | 0 | 2 | 0 | 6 | |
| EQ-5D-3L VAS score (self-reported) | |||||
| Mean (SD) | 79.3 (27.0) | 74.3 (22.8) | 71 (20.3) | 78.9 (17.8) | 0.105 |
| Range | 30 to 100 | 20 to 100 | 49 to 100 | 20 to 100 | |
| Missing | 0 | 1 | 0 | 9 | |
| QoL-AD score (self-reported) | |||||
| Mean (SD) | 43 (4.6) | 37.0 (6.0) | 38.4 (5.5) | 39.0 (5.4) | 0.267 |
| Range | 36 to 49 | 27 to 48 | 30 to 47 | 21 to 52 | |
| Missing | 1 | 5 | 1 | 63 | |
| Characteristic/outcome | Died (N = 18) | Withdrew (N = 45) | Lost to follow-up (N = 13) | Completed follow-up (N = 418) | p-value for difference between completers and non-completersa |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 81.0 (5.3) | 77.6 (8.4) | 77.0 (7.9) | 77.2 (7.9) | 0.260 |
| Range | 70.0 to 88.8 | 51.1 to 92.2 | 63.5 to 88.6 | 50.4 to 94.6 | |
| Gender (male), n (%) | 13 (72.2) | 27 (60.0) | 7 (53.8) | 254 (60.8) | 0.860 |
| Marital status, n (%) | |||||
| Single | 0 | 1 (2.2) | 2 (15.4) | 6 (1.4) | 0.008 |
| Married | 14 (77.9) | 32 (71.1) | 3 (23.1) | 315 (75.3) | |
| Separated | 0 | 2 (4.4) | 1 (7.7) | 4 (1.0) | |
| Divorced | 2 (11.1) | 3 (6.7) | 1 (7.7) | 9 (2.2) | |
| Widowed | 1 (5.5) | 6 (13.4) | 5 (38.4) | 74 (17.7) | |
| Cohabiting | 1 (5.5) | 1 (2.2) | 1 (7.7) | 9 (2.2) | |
| Missing | 0 | 0 | 0 | 1 (0.2) | |
| Living arrangements, n (%) | |||||
| Live alone | 3 (16.7) | 11 (24.4) | 7 (53.8) | 76 (18.2) | 0.244 |
| Live with relatives | 0 | 2 (4.4) | 2 (15.4) | 19 (4.6) | |
| Live with wife/husband/partner | 15 (83.3) | 32 (71.2) | 4 (30.8) | 322 (77.0) | |
| Living with friends | 0 | 0 | 0 | 1 (0.2) | |
| Ethnicity, n (%) | |||||
| White | 15 (83.3) | 43 (95.6) | 13 (100.0) | 407 (97.4) | 0.022 |
| Other | 3 (16.7) | 2 (4.4) | 0 | 11 (2.6) | |
| Age left full-time education (years) | |||||
| Mean (SD) | 17.4 (3.0) | 15.9 (1.7) | 15.5 (1.0) | 16.2 (2.7) | 0.996 |
| Range | 14 to 25 | 14 to 20 | 14 to 18 | 7 to 31 | |
| Missing | 1 | 0 | 0 | 7 | |
| Highest level of education, n (%) | |||||
| Degree/degree equivalent (including higher degree)/NVQ4/NVQ5 | 3 (16.6) | 5 (11.1) | 0 | 73 (17.5) | 0.566 |
| Higher education below degree | 5 (27.7) | 5 (11.1) | 0 | 34 (8.1) | |
| NVQ3/GCE A-level equivalent | 1 (5.6) | 1 (2.2) | 1 (7.7) | 21 (5.0) | |
| NVQ2/GCE O-level/GCSE-level equivalent/school certificate | 1 (5.6) | 11 (24.4) | 2 (15.4) | 75 (17.9) | |
| Other vocational/work-related qualifications | 1 (5.6) | 9 (20.0) | 6 (46.1) | 87 (20.8) | |
| No qualification | 6 (33.3) | 13 (29.0) | 4 (30.8) | 125 (29.9) | |
| Missing | 1 (5.6) | 1 (2.2) | 0 | 3 (0.8) | |
| ADAS-Cog (imputed) | |||||
| Mean (SD) | 26.1 (11.2) | 22.0 (8.6) | 21 (9.1) | 21.3 (8.9) | |
| Range | 12 to 52.7 | 5.3 to 44.7 | 8.7 to 42.7 | 5.7 to 52 | |
| Missing | 0 | 0 | 0 | 2 | |
| ADAS-Cog (raw score) | |||||
| Mean (SD) | 23.5 (8.5) | 21.3 (7.7) | 21 (9.1) | 20.8 (8.5) | 0.372 |
| Range | 12 to 41.7 | 5.3 to 44.7 | 8.7 to 42.7 | 5.7 to 52 | |
| Missing | 2 | 4 | 0 | 22 | |
| sMMSE score | |||||
| Mean (SD) | 19.1 (5.2) | 21.2 (4.7) | 21.5 (4.2) | 22.1 (4.6) | 0.023 |
| Range | 11 to 29 | 13 to 29 | 13 to 28 | 11 to 30 | |
| EQ-5D-3L score (self-reported) | |||||
| Mean (SD) | 0.71 (0.35) | 0.77 (0.18) | 0.65 (0.26) | 0.85 (0.18) | < 0.001 |
| Range | –0.07 to 1 | 0.19 to 1 | –0.02 to 1 | –0.07 to 1 | |
| Missing | 2 | 2 | 0 | 4 | |
| EQ-5D-3L VAS score (self-reported) | |||||
| Mean (SD) | 73.6 (24.2) | 74.9 (22.5) | 69.8 (20.1) | 79.3 (17.5) | 0.104 |
| Range | 30 to 100 | 20 to 100 | 36 to 95 | 20 to 100 | |
| Missing | 2 | 1 | 0 | 7 | |
| QoL-AD score (self-reported) | |||||
| Mean (SD) | 37.2 (7.3) | 36.7 (6.1) | 37.5 (5.2) | 39.2 (5.3) | 0.003 |
| Range | 23 to 49 | 21 to 48 | 26 to 43 | 25 to 52 | |
| Missing | 5 | 7 | 3 | 55 | |
| Trait | Time point | n | Mean | SD | Minimum | Q1 | Median | Q3 | Maximum |
|---|---|---|---|---|---|---|---|---|---|
| Memory | Baseline | 451 | 0 | 0.91 | –2.08 | –0.71 | –0.03 | 0.67 | 2.68 |
| 6 months | 403 | 0.06 | 0.88 | –2.61 | –0.62 | 0.02 | 0.69 | 3.31 | |
| 12 months | 386 | 0.02 | 0.82 | –2.23 | –0.52 | 0.01 | 0.64 | 2.39 | |
| Language | Baseline | 451 | 0 | 0.89 | –1.94 | –0.68 | –0.1 | 0.68 | 2.77 |
| 6 months | 403 | 0.06 | 0.93 | –2.29 | –0.65 | –0.06 | 0.8 | 3.6 | |
| 12 months | 386 | 0.02 | 0.82 | –1.99 | –0.59 | –0.01 | 0.69 | 2.76 | |
| Praxis | Baseline | 451 | 0 | 0.87 | –1.73 | –0.69 | –0.1 | 0.65 | 2.93 |
| 6 months | 403 | 0 | 0.85 | –2.15 | –0.64 | –0.1 | 0.62 | 2.99 | |
| 12 months | 386 | –0.02 | 0.81 | –1.85 | –0.64 | –0.11 | 0.52 | 2.59 |
| Trait | Arm | n | Mean | SD | p-value |
|---|---|---|---|---|---|
| Memory | 1 | 301 | 0 | 0.89 | 0.932 |
| 2 | 150 | 0 | 0.94 | ||
| Language | 1 | 301 | –0.01 | 0.88 | 0.753 |
| 2 | 150 | 0.02 | 0.92 | ||
| Praxis | 1 | 301 | –0.02 | 0.85 | 0.499 |
| 2 | 150 | 0.04 | 0.91 |
| Trait | Arm | n | Mean | SD | p-value |
|---|---|---|---|---|---|
| Memory | 1 | 276 | 0.1 | 0.89 | 0.205 |
| 2 | 127 | –0.02 | 0.85 | ||
| Language | 1 | 276 | 0.1 | 0.94 | 0.228 |
| 2 | 127 | –0.02 | 0.93 | ||
| Praxis | 1 | 276 | 0.04 | 0.88 | 0.097 |
| 2 | 127 | –0.1 | 0.78 |
| Trait | Arm | n | Mean | SD | p-value |
|---|---|---|---|---|---|
| Memory | 1 | 261 | 0.01 | 0.83 | 0.763 |
| 2 | 125 | 0.04 | 0.82 | ||
| Language | 1 | 261 | 0.02 | 0.81 | 0.826 |
| 2 | 125 | 0.04 | 0.86 | ||
| Praxis | 1 | 261 | 0 | 0.8 | 0.554 |
| 2 | 125 | –0.06 | 0.83 |
FIGURE 23.
Box plots of respondent ability scores at each follow-up point for each latent trait. (a) Memory; (b) language; and (c) praxis.
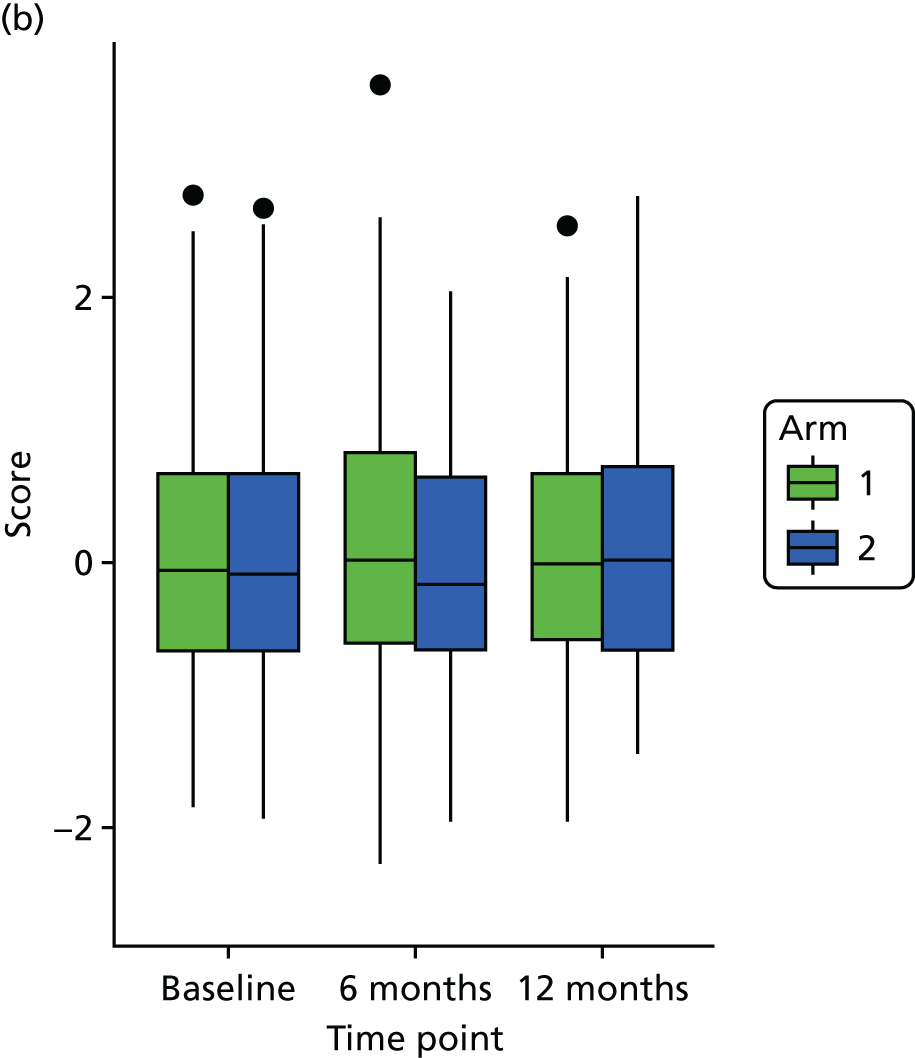
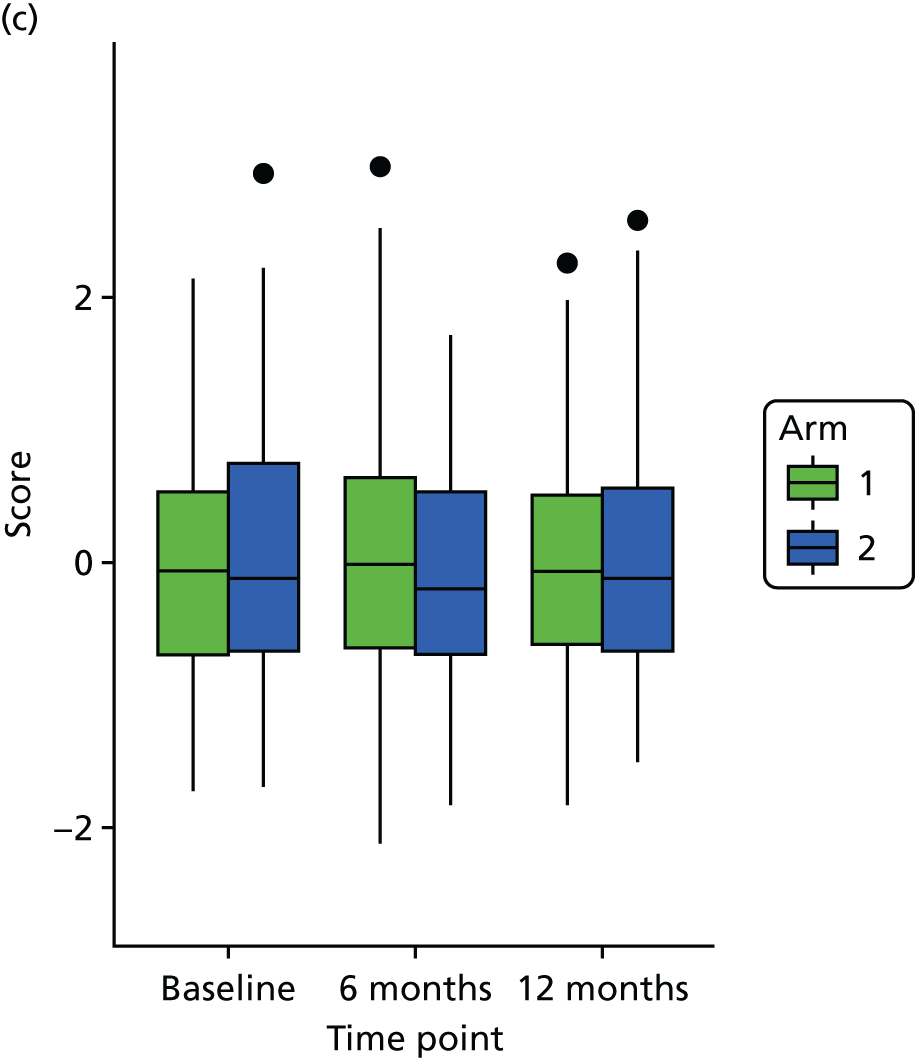
| Variables | Estimate | 95% CI | p-value |
|---|---|---|---|
| Baseline score | 0.000 | –0.09 to 0.10 | 0.98 |
| Arm: arm 2 | 0.002 | –0.16 to 0.18 | 0.801 |
| sMMSE score: score of ≥ 20 | 0.16 | –0.02 to 0.35 | 0.082 |
| Variables | Estimate | 95% CI | p-value |
|---|---|---|---|
| Baseline score | 0.00 | –0.09 to 0.09 | 0.998 |
| Arm: arm 2 | 0.004 | –0.15 to 0.22 | 0.678 |
| sMMSE score: score of ≥ 20 | 0.23 | 0.05 to 0.42 | 0.015 |
| Variables | Estimate | 95% CI | p-value |
|---|---|---|---|
| Baseline score | –0.05 | –0.22 to 0.14 | 0.607 |
| Arm: arm 2 | 0.05 | –0.05 to 0.15 | 0.286 |
| sMMSE score: score of ≥ 20 | –0.06 | –0.24 to 0.12 | 0.512 |
| Baseline score | 0.09 | –0.08 to 0.28 | 0.346 |
Appendix 7 Serious adverse event details
| SAE number | Event details | Description |
|---|---|---|
| 1 | Participant was participating in ‘Walks for Health’ as part of follow-on physical activity after cessation of exercise classes. Fell, suffered lacerations to face, may need surgery. Admitted to hospital same day | Fall |
| 2 | Participant reported gradual worsening of hip pain over 2 weeks – initially did not feel the class was the cause and happy to continue. Pain got worse so he saw an osteopath privately who recommended he cease all physical exercise. Two weeks later wife reported he is still in a lot of pain and will not be returning to the class, as he is awaiting urgent MRI scan | Pain |
| 3 | Last exercise session therefore shorter session, only two PRT exercises completed. Sitting awaiting refreshments, spoke to participant who reported start of chest pain and has taken GTN spray. Sat with patient – pain not resolving with GTN. Asked centre staff to assist. Monitored pain for few minutes then noted participant’s colour changing. HR reduced to high 48s (normal 60s) and closing eyes. Was able to respond to me but intermittent. Asked centre staff to call for help – patient lowered to floor. Resuscitate officer arrived in minutes – defibrillator applied but not required. Taken to A&E for monitoring. Plan to admit | Hospital admission |
| 4 | Participant on walk, fell, required hospitals admission for facial injury. Diagnosed with urinary tract infection, discharged home after approximately 1 month. Has returned to previous level of functional ability, but walking is slightly more unsteady | Hospital admission |
Appendix 8 Semistructured interview guides for participants, carers and physiotherapists
Appendix 9 Systematic review search strategy
Search strategy for update (inception to September 2016)
MEDLINE In-Process & Other Non-Indexed Citations (PubMed)
Search strategy
-
dementia [MeSH]
-
alzheimer*
-
lewy bod*
-
fronto-temporal
-
picks
-
korsako*
-
binswanger
-
‘primary progressive aphasia’
-
‘kluver bucy’
-
‘cognition disorders’
-
cognitive impair*
-
memory impair*
-
OR/1-12
-
aged [MeSH]
-
old*
-
elder*
-
‘middle aged’
-
‘frail elderly’
-
OR/14-18
-
exercise [MeSH]
-
physical activit*
-
‘resistance exercise’
-
‘strength training’
-
‘weight training’
-
‘anaerobic exercise’
-
‘aerobic exercise’
-
run*
-
swim*
-
walk*
-
danc*
-
cycling
-
yoga
-
‘tai ji’
-
OR/20-33
-
randomised controlled trial [pt]
-
controlled clinical trial [pt]
-
randomised [tiab]
-
placebo [tiab]
-
randomly [tiab]
-
trial [tiab]
-
groups [tiab]
-
OR/35-41
-
13. AND 19. AND 34. AND 42.
List of abbreviations
- 6MWT
- 6-minute walk test
- ADAS-Cog
- Alzheimer’s Disease Assessment Scale – Cognitive Subscale
- AE
- adverse event
- BADLS
- Bristol Activities of Daily Living Scale
- BCT
- behaviour change technique
- CACE
- complier-average causal effect
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CSRI
- Client Services Receipt Inventory
- DAPA
- Dementia and Physical Activity
- DeNDRoN
- Dementia and Neurodegenerative Research Network
- DMEC
- Data Monitoring and Ethics Committee
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- INMB
- incremental net monetary benefit
- IRT
- item response theory
- ITT
- intention to treat
- MCI
- mild cognitive impairment
- MI
- multiple imputation
- MMD
- mild to moderate dementia
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPI
- Neuropsychiatric Inventory
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- QC
- quality control
- QoL-AD
- Quality of Life in Alzheimer’s Disease scale
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- sMMSE
- Standardised Mini Mental State Examination
- VAS
- visual analogue scale
- WCTU
- Warwick Clinical Trials Unit
- WHO
- World Health Organization
- ZBI
- Zarit Burden Interview
