Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/153/01. The contractual start date was in May 2014. The draft report began editorial review in November 2017 and was accepted for publication in April 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Matthew J Ridd is a member of the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) General Board. Nick A Francis was a member of the HTA Antimicrobial Resistance Themed Call Board during the study. Amanda Roberts is a member of the HTA General Board. As patient and public involvement co-applicant, Amanda Roberts also received an attendance allowance and travel expenses from the University of Southampton in order to attend meetings. Emma Thomas-Jones reports grants from the NIHR HTA programme during the conduct of the study. Hywel C Williams is Director of the NIHR HTA programme. Paul Little is a member of the NIHR Journals Library Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2018. This work was produced by Santer et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2018 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific background
Eczema is very common, affecting > 20% of children at some point during their first 5 years of life. 1 Eczema has a significant impact on quality of life (QoL)2 and can cause distress to affected children and their families because of sleep disturbance, itching and scratching. 3 The term ‘atopic eczema’ (synonymous with ‘atopic dermatitis’) is widely used to denote a clinical phenotype, rather than those who are truly atopic as defined by the presence of immunoglobulin E-specific antibodies to common environmental allergens. In this study, we use the term ‘eczema’ throughout to refer to the ‘atopic eczema’ clinical phenotype, in accordance with the recommended nomenclature of the World Allergy Organization. 4,5
Skin complaints are the second most common reason for general practitioner (GP) consultations in children aged < 5 years. 6 Health and societal costs of eczema care are difficult to estimate as they vary widely by population under study, but eczema is thought to cause a similar economic burden to that for asthma. 7,8
Emollients for the treatment of childhood eczema
Guidelines suggest that emollients form the mainstay of treatment for eczema and should be used regularly by all patients alongside other treatments, such as topical corticosteroids (TCSs), when necessary to treat flare-ups. 9 Emollients are thought to act by providing a protective layer over the skin, decreasing moisture loss and occluding against irritants.
There are three methods of application of emollients: (1) leave-on (directly applied) emollients, where emollients are applied to the skin and left to soak in, (2) soap substitutes, where emollients are used instead of soap or other washing products, and (3) bath emollients (or bath additives), which are oil and/or emulsifiers designed to disperse in the bath. All three approaches are often used together (referred to as ‘complete emollient therapy’). 5,9 In this report, the term ‘bath additives’ rather than ‘bath emollients’ is used to emphasise the differences between the three methods of application (i.e. these three methods differ in their proposed actions and evidence relating to their effectiveness should also be considered separately).
Although there is widespread clinical consensus on the need for leave-on emollients and soap substitutes, there is less agreement regarding the additional benefits of emollient bath additives. 10–13
Bath additives for the treatment of childhood eczema
A previous systematic review has revealed no convincing evidence for the use of emollient bath additives in the treatment of eczema;10,11 available data consist of case series and very small trials. One study14 in which parents were asked to soak one of their child’s arms for 15 minutes a day for 2 weeks in a basin containing water with bath additive found that clinical assessment by blinded observer was worse for the arm that was soaked daily than for the unsoaked arm in eight of the nine children, suggesting the possibility that bath additives may be harmful. No trials of emollient bath additives have been published since 200715 and trial registries reveal no ongoing studies.
In addition to concerns about cost-effectiveness, potential harms from using bath additives include skin irritation and greasier bath surfaces, which can increase the risk of slips and accidents13 (listed in the Summary of Product Characteristics of leading brands). There is also a concern that people who use bath additives in place of leave-on emollients are receiving substandard emollient therapy. 12
The effectiveness of adding bleach bath additives has also not been demonstrated in a small randomised trial, although 5 out of 18 participants in the bleach bath group experienced mild burning/stinging or dry skin. 16 Two small randomised studies17,18 compared ‘bath emollient’ with ‘bath emollient plus antiseptic’ on a range of outcomes, but there were no significant differences between groups, including colony counts of Staphylococcus aureus. 19 For this reason, we chose to exclude bath additives that incorporate an antiseptic, because of the absence of benefit and the possible increased risk of skin irritation. 5,20
In 2007, the Drug and Therapeutics Bulletin noted that the NHS spends > £16M per year on bath additives (at an average cost of £6.29 per item), representing 38% of the total cost of treatments prescribed for preschool children with eczema and matching the proportion spent on emollients for application directly to the skin. 9
Despite the absence of evidence and the possibility of potential harms, bath additives are widely prescribed at a cost of > £23M per year to the NHS in England. 21 Currently, prescribing advice varies widely. An analysis of 216 formularies from Clinical Commissioning Groups in England and Local Health Boards in Wales22 showed that 68% recommended the use of bath additives, 15% allowed their use but did not encourage it, 13% did not mention bath additives and 5% did not recommend their use.
Pragmatic trial design
Pragmatic clinical trials aim to test the effectiveness of an intervention in a real-life setting in order to recruit a study population that is as similar as possible to the population on which the intervention is meant to be used. Whereas an explanatory clinical trial aims to answer the question ‘Can this intervention work under ideal conditions?’, a pragmatic approach seeks to answer the question ‘Does this intervention work under usual conditions?’. 23,24 Features of pragmatic trials include the use of clinically important outcomes and common participant-reported outcomes, long-term follow-up and encouragement of participants to adhere to the intervention only to the extent that would be anticipated in usual care.
Although relatively few pragmatic trials have been carried out in dermatology,25 we felt that a definitive pragmatic clinical trial, including outcomes of relevance to participants and including long-term follow-up, was the most appropriate design to address the question of the effectiveness of bath additives in addition to standard eczema care in everyday care. 5
Blinding
We chose an ‘open-label’ design as it would not be possible to create a convincing placebo for bath additives, which make the bath feel ‘greasy’, and many families of children with eczema will already have experience of using them. We wished to design a trial with a clinical outcome relevant to participants (as below). Ideally, we would also have included an objective assessment of eczema severity carried out by a blinded assessor. However, this would have increased burden for participants as additional face-to-face assessments would have been required, particularly as the relapsing and remitting nature of eczema means that follow-up assessment at a single time point is problematic. As our primary outcome was participant reported and, therefore, unblinded, incurring substantial additional costs for an objective secondary outcome did not seem warranted.
Participant-reported outcome measure
We wished to design a trial with a clinical outcome relevant to participants. In eczema, the appearance of the skin does not always closely reflect symptoms causing a major impact on the child and family, such as sleep disturbance and itch. 26 It was therefore particularly important to design a trial with a validated participant-reported primary outcome. 5
We chose the Patient Oriented Eczema Measure (POEM)27,28 as our participant-reported primary outcome measure. POEM comprises seven questions about eczema symptoms over the previous week that are summed to give a score from 0 (no eczema) to 28 (worst possible eczema). POEM is a patient-reported outcome that can be used by proxy (carer report), demonstrates good validity, repeatability and responsiveness to change29 and is recommended by the National Institute for Health and Care Excellence (NICE)9 and the international Harmonising Outcome Measures for Eczema (HOME) initiative. 30,31
Capturing meaningful outcomes for people with eczema is complicated by the relapsing and remitting nature of the condition. Therefore, gathering information regularly over time is essential to understand disease burden32 and to assess the impact of interventions. We therefore chose to collect POEM scores on a weekly basis from participants, as has been used successfully in other eczema trials. 33 An article reporting on the acceptability and practicality of weekly POEM completion is in preparation. 34
Development of research priority
This trial was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme following a commissioned call advertised in February 2012. The research topic was suggested via the NIHR HTA website topic suggestion form and was approved in December 2011.
The James Lind Alliance Priority Setting Partnership (PSP) for Eczema published its top 10 priority topics in 2012. 35 Even though this call was not advertised directly as a result of the PSP, it does address some of the issues that patients, carers and clinicians highlighted, including priorities around bathing/washing and also around the best ways to use emollients.
Objectives
The objectives were to determine the clinical effectiveness and cost-effectiveness of adding bath emollient to the standard management of eczema in children, which includes regular application of leave-on emollients with use of TCSs as required.
The trial was registered before commencing on 13 December 2013 and the protocol was published online in August 2015. 5
Chapter 2 Methods
Trial design
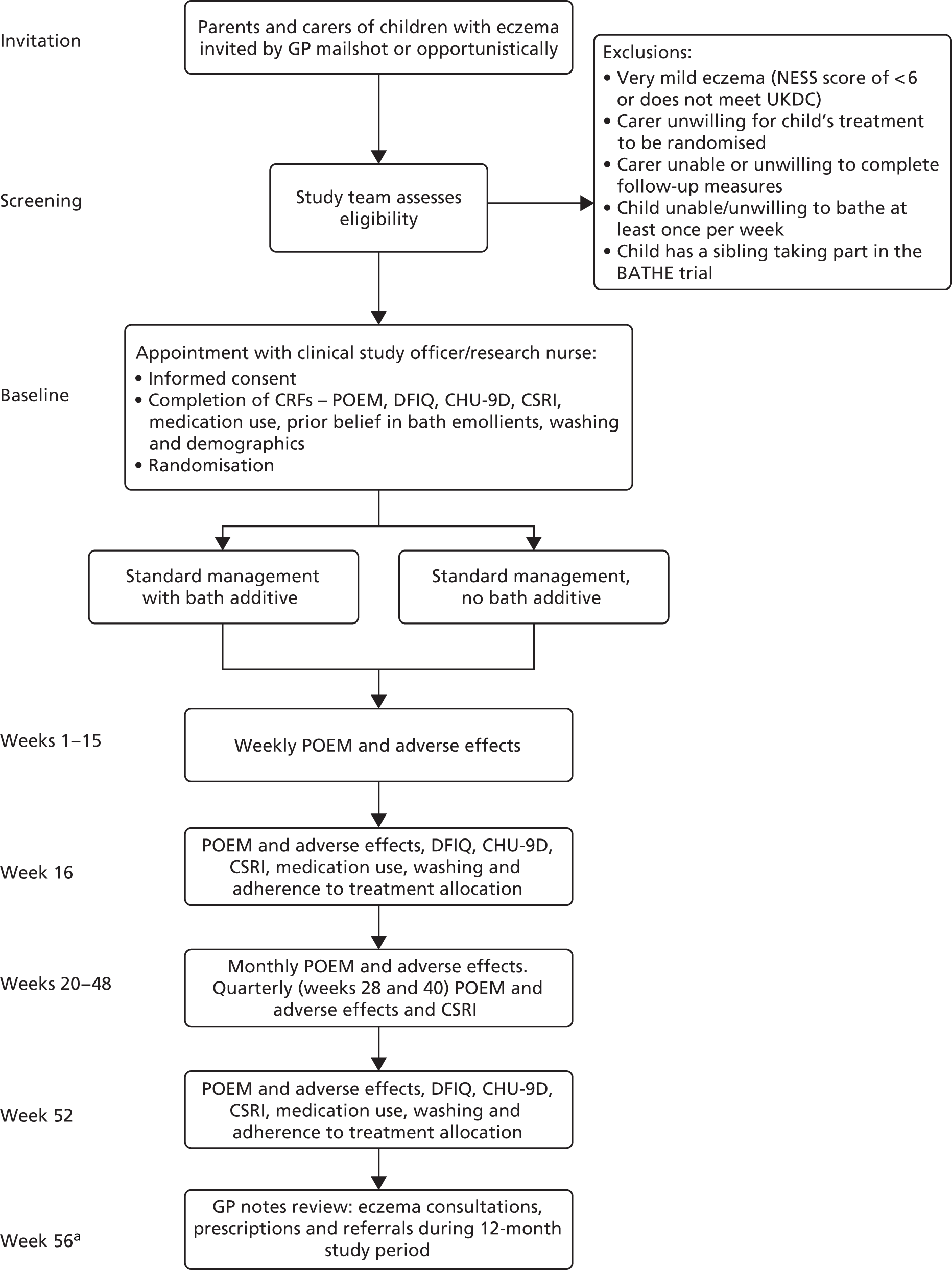
The Bath Additives for the Treatment of Eczema in cHildren (BATHE) trial was a pragmatic, randomised, open-label, multicentre, superiority trial with two parallel groups and a primary outcome of long-term control as measured by POEM weekly scores over 16 weeks. Children were randomised in a 1 : 1 ratio to either standard eczema care plus bath additives or standard eczema care only for 12 months (Figure 1).
FIGURE 1.
Participant flow through study. a, At least 4 weeks allowed for clinical letters to be received at surgery and scanned into patient record. CHU-9D, Child Health Utility-9 Dimensions; CRF, case report form; CSRI, client service receipt inventory; DFIQ, Dermatitis Family Impact Questionnaire; NESS, Nottingham Eczema Severity Score; UKDC, UK Diagnostic Criteria for Atopic Dermatitis.
Changes to trial protocol
As recruitment was ahead of target, and following discussion with the Trial Steering Committee, in October 2015 the decision was taken to increase the target sample size from 423 to 491 participants, to allow an analysis by treatment adherence in addition to an intention-to-treat (ITT) analysis. Assuming that 80% of participants were strictly adherent to their allocated treatment, recruitment of an additional 68 participants would be required to retain 90% power for a per-protocol analysis.
In addition, although not requiring an amendment to the trial protocol, concerns were raised that the participant information sheet was overly formal and text heavy and that this might be contributing to a lower than expected response rate. A colourful summary information leaflet was therefore designed, to be added to the patient invitation pack (see Appendix 1). The additional leaflet was included in mailshots from 12 June 2015 (63% of the invitations), but there is no evidence that its inclusion affected either the number of responses or the proportion of positive replies.
Participants
Participant identification
Participants were recruited exclusively through GP surgeries in Wales, the west of England and southern England. All recruiting sites were members of their local clinical research networks (CRNs) [NIHR CRN Wessex, NIHR CRN West of England and National Institute for Social Care and Health Research (NISCHR) in Wales] and were reimbursed for their time via the Service Support Costs scheme [Attributing the costs of health and social care Research and Development (AcoRD). URL: www.nihr.ac.uk/funding-and-support/study-support-service/early-contact-and-engagement/acord/ (accessed 10 September 2018)]. A total of 96 GP surgeries took part in the trial.
Postal invitation
Sites were provided with the instructions to conduct a search of their electronic records for potentially eligible children. The inclusion criteria were child aged between 12 months and 12 years, with a recorded diagnosis of eczema (Read Codes: eczema not otherwise specified, atopic eczema/dermatitis, infantile eczema) and who had obtained one or more prescriptions for drugs acting on the skin36 within the past 12 months. The lists that were produced were then screened for further exclusions by the children’s GPs (e.g. recent bereavement, child protection issues). When finalised, staff at the surgery merged the list of names and addresses with the trial invitation materials using a secure online mailing service. Trial staff were aware of how many invitation letters were being sent from each site, but did not have access to the details of the mailing list.
The trial invitation pack consisted of a covering letter printed on the surgery’s letterhead paper, an information sheet, a reply slip incorporating an eczema screening questionnaire (see Appendix 2) and a prepaid reply envelope. Children’s names were used within the text of the letter and invitation letters were addressed ‘to the parent/guardian’ of the child. Patient identification numbers were assigned to each invitee when the mail merge was performed and were printed on the reply slip to permit anonymous replies. The participant identification number format (six digits consisting of centre number, site number and patient number, in the format X-XX-XXX) also enabled trial staff to monitor response rates at the surgery level.
Opportunistic invitation
Sites were also provided with invitation packs to hand out opportunistically to parents/carers of children with eczema. Although it was hoped that all eligible children would have received an invitation through the post, packs were provided in anticipation of new eczema diagnoses and families newly registered with participating surgeries. The trial team was not generally notified when an invitation pack had been handed out by surgery staff, and the overall number of opportunistic recruitment packs distributed is not known; however, 35 (2.4%) of the responses received were returned from opportunistic invitations, of which 34 were from children who were willing to take part, 19 (54%) of whom went on to participate in the trial.
All documents in the mailing pack were supplied in both English and Welsh to patients of those surgeries where Welsh is spoken, except for the screening questionnaire. This questionnaire was supplied only in English as neither of the measures it incorporates [UK Diagnostic Criteria for Atopic Dermatitis (UKDC) and Nottingham Eczema Severity Score (NESS)] have been validated in the Welsh language. Materials that were included in the patient invitation pack can be found in Appendices 1–4.
Reponses
Parents/carers could respond to the invitation letter by either returning the reply slip in the prepaid envelope or by entering the same data into a secure online questionnaire hosted by the University of Southampton (URL: www.iSurvey.soton.ac.uk; accessed 3 August 2018).
Eligibility
Children were eligible to participate if:
-
they were aged between 12 months and 12 years
-
they fulfilled UKDC (see following paragraphs)
-
they scored mild to severe eczema severity over the past 12 months on the NESS (i.e. a score of > 5, excluding very mild eczema – see following paragraphs)
-
they bathed at least once a week
-
their parent/carer (or themselves) was willing for randomisation to either bath additive or no bath additive
-
they had no sibling(s) participating in the trial
-
they were not taking part in other clinical trials.
To avoid inviting ineligible children to baseline screening appointments, an initial assessment of eligibility was determined using a screening form, completed by the parent/carer, that combined the UKDC and the NESS, as below.
The UKDC are a refinement of the Hanifin and Rajka37 clinical criteria for diagnosing eczema and are the most extensively validated way of diagnosing eczema; they have been widely used in epidemiological and clinical studies. 38 The UKDC questionnaire consists of a single inclusion/exclusion question (‘in the last year, has your child had an itchy skin condition?’) followed by a further five questions about the clinical course of the disease. Atopic dermatitis, or eczema, is indicated by a score of three or more positive responses from these five questions.
For the purposes of the BATHE trial, the requirement for visible flexural dermatitis was approximated by assessing the NESS body diagram for marks indicating the presence of eczema in the wrist, ankle, elbow and facial areas (see Appendix 2). In addition, the question relating to child or family history of atopy, being age adapted and involving some explanation, was not included on the screening questionnaire form. This question was asked by telephone or e-mail only if an additional point was required to reach eligibility (i.e. the child scored ≥ 5 on the NESS and ≥ 2 on the UKDC).
The NESS assesses eczema severity over the previous 12 months and comprises three questions: duration of eczema symptoms over past 12 months, frequency of sleep disturbance and current extent of eczema as represented by parental marking of a diagram39 (see Appendix 2).
The NESS severity classifications are mild (score of 3–8), moderate (9–11) and severe (12–15). 39 However, the majority of children in primary care fall into the ‘mild’ category and a score of ≥ 9 would exclude 82% of children with eczema in primary care, whereas a cut-off point of ≥ 6 would exclude only 40% of children with ‘very mild’ eczema. Children with a score of ≤ 5 were excluded, as floor effects would make it unlikely that changes in eczema severity could be detected.
The screening forms were returned in a prepaid envelope to the trial centre in Southampton, where responses were entered into a secure database [Microsoft Access® (Microsoft Corporation, Redmond, WA, USA)]. The database calculated eligibility for each child using an algorithm (see Appendix 5). To be eligible to take part in the BATHE trial, children were required to meet the UKDC and to score ≥ 5 on the NESS. Those who reached eligibility on the NESS but scored only 2 on the UKDC were contacted by the clinical studies officer/research nurse (CSO/RN) in order to ask the additional question about atopy.
When screening forms did not meet eligibility criteria, parents/carers were sent a letter of thanks and explanation, together with a booklet explaining the best way to wash children with eczema. Children’s details were not collected until the recruitment appointment and, therefore, children who were not recruited into the BATHE trial remained anonymous.
Recruitment
All contact with families regarding recruitment was conducted by a clinical studies officer or research nurse. In the Southampton and Bristol centres, the clinical studies officers were study-employed staff, whereas, in the Cardiff centre, recruitment was carried out by clinical studies officers or research nurses from the NISCHR, who may work across several research projects concurrently. The majority of trial staff were trained together at a whole-day workshop prior to the start of the recruitment period; however, the large number of staff in Wales meant that some new staff required handover training from trained research nurses.
Parents/carers of eligible children who expressed a willingness to take part in the trial were contacted by their local CSO/RN. After re-establishing eligibility, a recruitment appointment was made. The majority of appointments were held at the child’s GP surgery, with a small number held at the child’s home when this option was preferred by the family. The child did not need to attend the appointment, but whenever they were present efforts were made to include them in the discussion. Two summary child information leaflets were prepared to facilitate their understanding and assent forms were available for older children (see Appendices 6 and 7).
The mean number of days between completion of the screening form and the recruitment appointment was 24, and 370 (77%) participants were recruited within 30 days of the screening form completion date. When the time elapsed between completion of the screening form and the recruitment appointment was ≥ 30 days, the CSO/RN rechecked eligibility by asking parents/carers to confirm the time-sensitive questions from the NESS (questions five to seven of the screening form). Eligibility was recalculated by the CSO/RN and the data held in the trial management database were updated.
Baseline data were entered directly into the online software by the parent/carer wherever possible. This enabled the CSO/RN to familiarise the participants with logging in and using the software, but occasionally it added considerable time to the appointment. The recruitment process routinely took between 40 and 60 minutes and, therefore, parents/carers were reimbursed for their time and expenses with a £10 gift voucher.
Engagement
Following the baseline appointment, there were no other face-to-face meetings and so efforts were made to help participants to remain engaged with the trial throughout the full 12 months. A study logo of a rubber duck was adopted and used in many different ways: participating children (and their siblings) were invited to colour in and name a duck, which was then uploaded to a gallery on the study website. The logo was also used to identify small gifts, which were provided at the baseline appointment [a bath duck with study logo, Post-it® (3M, Bracknell, UK) notes and a novelty eraser] and birthday and Christmas cards and quarterly newsletters were sent about study progress. The newsletters were also uploaded to the study website, where participants were able to access key trial information and get in touch with the study team if required (URL: www.southampton.ac.uk/bathe; accessed 3 August 2018). No incentives were given to participants; however, a thank you card and a £10 voucher were sent to parents/carers shortly before each of the 16- and 52-week questionnaires were due in recognition of the time spent completing them, and all participants were eligible for inclusion in a prize draw for a tablet computer at the end of the study.
Interventions
Standard care with bath additive
In addition to the child’s usual skincare regimen, parents/carers were asked to use one of the three most commonly used bath additives [Balneum® (Almirall Ltd, Middlesex, UK), Aveeno® (Johnson & Johnson Ltd, Maidenhead, UK) or Oilatum® (Stiefel Skin Science Solutions, a GlaxoSmithKline company, Middlesex, UK)] at least once per week, in accordance with the manufacturer’s instructions. Having discussed any carer preferences, the CSO/RN asked staff at the surgery to set up a repeat prescription on the child’s medical record. In the event of an adverse reaction to the bath additive, parents/carers were free to change the prescription to another of the recommended products at any point during the trial. Bath additives other than the three recommended products are also available and parents/carers were free to choose any bath additive they wished, provided that it did not contain any additional active ingredients, such as antipruritic or antimicrobial agents. A full list of acceptable bath additives was provided to the surgery in the study site file and was available on the study website (see Appendix 8). Both groups also received further advice (as below).
Standard care without bath additive
Parents/carers were requested to avoid using any emollient product that had been designed to be poured into the bath for the 12 months of their participation. It was reiterated that it was not known whether or not bath additives provided any benefit to children with eczema and that we did not believe that their child would experience any worsening of their eczema as a result of participating in this trial. Parents/carers in the no bath additive group were advised that they should treat their child’s eczema exactly as they normally would, using leave-on emollients regularly and TCSs to treat flare-ups, and consulting health professionals as they normally would. As many participants had family members who also had eczema, and in accordance with the pragmatic nature of the trial, no specific instructions were given to parents/carers with regard to bath additives that might already be present in the home; instead, the main focus was on ensuring that parents/carers were in equipoise with and engaged with the aims of the research.
Advice given to both groups
We aimed for no difference in soap use between groups and both groups were therefore given the same advice about how to wash. Both groups were advised to use a leave-on emollient as a soap substitute. When parents/carers were keen to use existing wash products, they were advised that these could be used for direct application to skin but should not be added to the bath water, as this could potentially have the same effect as a bath additive. All participants were provided with a copy of the BATHE trial Study Washing Leaflet that was based on best practice guidelines developed by the Nottingham Support Group for Carers of Children with Eczema (see Appendix 9). 40
All parents/carers were reminded that they were free to use any other medications as they normally would and to visit their doctor or dermatologist as usual, if required. The standard operating procedure (SOP) that was used by recruiters during the baseline appointment is available in Appendix 10.
Data collection
Parents/carers were able to choose to complete the trial questionnaires either online or by post following discussion at the recruitment appointment, although online questionnaire completion was strongly encouraged. Online questionnaires became available on the seventh day following recruitment and every 7 days thereafter. Notifications were automatically sent by e-mail when the questionnaire went ‘live’ and reminders were sent after 48 hours if it had not been completed. Participants could also opt to receive the notifications and reminders by automated text. Both e-mails and text messages contained a hyperlink to the online questionnaire and participants were required to log in using their e-mail address and password in order to enter data. Of the 9784 times that parents/carers logged in to the website, 5963 logins (61%) were from mobile devices. A further 2909 logins (30%) were from computers or laptops, whereas 912 logins (9%) were from tablets. 34
The 16-week questionnaire, and subsequent monthly questionnaires, remained available to complete online for 28 days. However, in order to encourage timely completion of the primary and secondary outcomes, a reminder letter and paper copy of the 16- and 52-week questionnaires were posted out if the online questionnaire had not been completed within 7 days. A total of 80 reminders were posted at week 16 and 107 reminders were posted at week 52.
Weekly paper questionnaires were printed in booklets of four. The first booklet, covering weeks 1–4, was marked with the day of the week on which the questionnaires should be completed and then handed to the parent at recruitment. Subsequent questionnaires were posted to participants shortly before they were due, together with a prepaid envelope. The data from the paper questionnaires were entered into a secure database and were merged with the data collected online prior to analysis.
Data management
The online data collection software was built using LifeGuide software (University of Southampton, Southampton, UK) and validated by Southampton Clinical Trials Unit. The clinical data were separated from the personally identifiable information and both data sets were stored on secure servers.
In the trial office, data from paper screening forms and questionnaires were entered into a password-protected Microsoft Access database. The clinical data were again stored separately from personally identifiable information in two data sets on a secure server. Paper forms were separated from each other and stored in locked filing cabinets in the trial office.
The data sets were merged and stripped of any identifying data prior to analysis.
Outcomes
Primary outcome
The primary outcome was eczema severity as measured by the POEM completed weekly for 16 weeks. The POEM is a patient-reported outcome that scores symptoms over the previous week. It consists of seven questions that can be completed by the child’s parent/carer and provides a severity score on a scale from 0 to 28. The published minimal clinically important difference of the POEM is 3.0. 41,42 The POEM was the only patient-reported outcome measure for eczema to demonstrate validity and repeatability in a systematic review by Schmitt et al. 29 and it was adopted as the preferred patient-reported outcome measure by the HOME initiative in 2015. 30,43 Our primary outcome measure is based on repeated measures of POEM data collected weekly over 16 weeks because these reflect the impact of this relapsing and remitting chronic condition better than comparing outcomes at a single follow-up point. Because of the burden of weekly data collection on participants, weekly data collection was limited to the first 16 weeks of the trial.
Secondary outcomes
-
The number of eczema exacerbations resulting in a primary health-care consultation over 1 year, assessed by a review of participants’ primary care records. An exacerbation for this trial is defined as a consultation where there is mention of eczema and a topical steroid or topical calcineurin inhibitor (TCI) has been prescribed. The notes review form was based on those used in other recent eczema trials and recorded the number of consultations, prescriptions and referrals over the 12 months’ trial participation (see Appendix 11). Notes reviews were carried out by members of the trial team at not less than 13 months after the recruitment date in order to allow time for clinic letters to be received and scanned into the patients’ notes.
-
Eczema severity over 1 year as measured by POEM every 4 weeks, from 16 weeks to 12 months.
-
Disease-specific QoL at baseline, 16 weeks and 1 year, measured by the Dermatitis Family Impact Questionnaire (DFIQ). 44 The DFIQ is an internationally recognised validated instrument that measures the impact of a child’s eczema on the family’s QoL. The questionnaire consists of 10 questions and the total score ranges from 0 (no impact on family life) to 30 (maximum impact on family life).
-
Generic QoL as measured by the Child Health Utility-9 Dimensions (CHU-9D) at baseline, 16 weeks and 1 year. CHU-9D45 is a paediatric QoL measure developed in children aged 7–11 years. It captures issues pertinent to childhood eczema, such as sleep disturbance and the child’s mood, and is therefore more suitable for measuring the QoL in families affected by eczema than the EuroQol-5 Dimensions (EQ-5D). There are no suitable utility measures validated for very young children aged 1–4 years; however, personal communication (Dr Katherine Stevens, University of Sheffield, 2014) suggested guidance for using the CHU-9D in very young children. In addition, the CHU-9D performed well in children aged 1–4 years in the Supporting Parents and carers of Children with Eczema (SpaCE) trial and its use in younger age groups is currently being trialled elsewhere. 46,47
-
Type (strength) and quantity of topical steroid/calcineurin inhibitors prescribed during trial participation, measured by GP notes review.
Sample size
The sample size was calculated for repeated measures analysis of variance (ANOVA) in weekly POEM scores over the 16-week observation period. Using weekly data from a similar population to that in the Softened Water Eczema (SWET) trial,48 we aimed to detect a mean difference of 2.0 [standard deviation (SD) 7.0] between the intervention and control groups. The published minimal clinically important difference for POEM is 3.0,41,42 but we wished to detect a difference of 2.0 because of the expectation of low baseline POEM scores in a population recruited entirely through primary care, and because we wished to be able to detect this small difference as the intervention is relatively inexpensive and even small effect sizes may be cost-effective. An alpha of 0.05 and a power of 90% with a correlation between repeated measures of 0.70 gives a sample size of 338 participants. Allowing for 20% loss to follow-up gave a total sample size of 423 participants.
We aimed to report a per-protocol analysis in addition to a primary ITT analysis, and early data suggested that approximately 80% of participants in both groups were strictly adherent to treatment allocation. We were concerned that if only 80% of participants were adherent to treatment allocation then we would have usable data for only 270 participants. To get back up to 90% power for this group, we submitted an ethics amendment requesting recruitment of an additional 68 participants, giving a revised target recruitment of 491 participants.
Randomisation
Randomisation was performed using LifeGuide49 software hosted by the University of Southampton and validated by Southampton Clinical Trials Unit. At the time of trial setup, LifeGuide was unable to easily perform block randomisation and the additional programming time would have resulted in delays to the trial. Simple randomisation was therefore used, stratified by centre. Although this can result in imbalances, it was felt that with strata of > 100 participants each, the overall balance between groups would be preserved.
A backup randomisation system was established for occasions when internet access to LifeGuide was not available or when the parent had opted to complete the trial questionnaires on paper. A second set of random treatment allocations was programmed into a Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) spreadsheet by the trial statistician. The spreadsheet similarly allocated treatments on a 1 : 1 basis, stratified by recruiting centre, and the treatment allocation was not revealed until the participant had been recruited. A total of 30 randomisations were conducted using this offline method.
Allocation concealment mechanism
Both the online and the offline randomisation procedures were conducted immediately at the end of the recruitment appointment, following completion of the baseline questionnaire. It was therefore impossible for the treatment allocation to be known prior to study entry. Once randomisation was complete, however, the participant, CSO/RN and the participant’s clinical team were all aware of which group the child had been allocated to.
Implementation
Once recruitment and randomisation was complete, the lead GP at the site was notified of the child’s enrolment into the study and their allocated treatment group was noted on a form completed by the recruiting CSO/RN. The form requested that repeat prescriptions be set up for children allocated to the bath additive group and it also recorded the parent/carer’s preferred bath additive, if any. Practices were recommended to add a Read Code to the child’s electronic record to indicate that they were enrolled in a clinical trial and/or to add an electronic alert or other notification to remind clinicians of their treatment allocation. This, however, was not enforced and the focus remained on ensuring that parents/carers were fully engaged with the aims and requirements of the study.
Blinding
Given the nature of bath emollient, which adds a greasy film to water, it was impossible to manufacture a convincing placebo treatment or to blind the participants to their treatment allocation. Participants were therefore fully aware of the treatment regime they were being asked to adhere to and report on. In order to support families throughout their 12-month participation, the trial team were also not blinded to the treatment allocated.
The trial statisticians carrying out the analysis were blind to allocation group and the statistical analyses were independently verified.
Statistical methods
The primary analysis for the total POEM score was performed using a mixed multilevel mixed model (MMLM) framework with observations over time from weeks 1–16 (level 1) nested within participants (level 2) nested within centres (level 3). Unadjusted results are reported, as well as results adjusting for baseline POEM, recruitment region as covariates and any significant confounders. Confounders were defined as variables associated with both the exposure and the outcome that significantly contribute to the multivariable model (defined as a p-value of < 0.05 or by modifying the effect estimate by > 10%). The following variables were identified a priori as possible confounders: child age, child gender, carer age, carer gender, carer education, prior belief in bath emollients, type of emollient used, other medication used and other items used when washing (e.g. soap/shampoo/soap substitute).
The model used all of the observed data and made the assumption that missing POEM scores are missing at random. The model included a random effect for centre (random intercept) and patient (random intercept and slope on time) to allow for between-patient and between-centre differences at baseline and between-patient differences in the rate of change over time (if a treatment/time interaction was significant), and fixed effects for baseline covariates. An unstructured covariance matrix was used.
The assumptions of the normality of the residuals from the fixed part of the model and the normality of the random effects at the cluster level were checked.
For the analysis of secondary outcomes, repeated measures analysis in line with that used for the primary outcome was used for the monthly POEM up to 1 year. 5 For other secondary outcomes, linear regression was used for continuous outcomes if the assumptions were met; otherwise, non-parametric analyses were used. Logistic regression was used for dichotomous outcomes and a suitable count model, as determined by goodness of fit measures, was used for count data. All analyses controlled for stratification variables and potential confounders. Preplanned sensitivity analysis and exploratory subgroup analyses were carried out as set out in the statistical analysis plan.
To test the sensitivity of the results to missing data, a chained equations multiple imputation model was used to impute the missing values. This analysis used 100 imputations and included all the outcomes and covariates included in the adjusted analysis of the primary outcome.
For all models, participants were analysed in the group to which they were randomised, regardless of their adherence to that allocation (ITT analysis). The only exception to this was the per-protocol analysis, for which participants were analysed on the basis of their reported use of bath additives. The reported use of bath additives was collected at 16 weeks and 52 weeks in both groups using the categorical response options ‘every time’, ‘more than half the time’, ‘less than half the time’ and ‘never’. This allows two possible definitions for adherence to the intervention.
Adherence definition one
Defined as the bath additive group using emollient bath additives ‘more than half the time’ or ‘every time’ and the no bath additive group using emollient bath additives ‘less than half the time’ or ‘never’.
Adherence definition two
These figures include only participants who reported using emollient bath additives ‘every time’ or ‘never’ (i.e. excluding participants who report using emollient both additives ‘more than half the time’ and ‘less than half the time’).
We report the effect sizes for the primary outcome based on both definitions of adherence. These can then be compared with the effect size in the ‘as randomised’ ITT population.
Chapter 3 Results
Participant recruitment
Invitation packs were sent to the parents/carers of 12,504 children and 1451 responses were received. A total of 1343 responses were returned by post; 832 respondents (62%) were willing to be contacted and 431 (32%) went on to participate in the trial. A total of 108 responses were received electronically; 88 respondents (81%) were willing to take part and 52 (48%) went on to be recruited. A total of 35 (2.4%) of the responses received were returned from opportunistic invitations; 34 respondents were willing to take part and 19 (54%) went on to participate in the trial.
A total of 237 parents/carers declined participation and returned a blank screening form. A further 104 answered ‘No’ to the first question of the UKDC (‘In the last year, has your child had an itchy skin condition?’) and did not fill in any other information about their child’s condition. A total of 188 parents/carers indicated that they were unwilling or unable to take part but did complete the screening questionnaire. Table 1 summarises the information received about these children’s eczema, in comparison with children who went on to take part.
| Respondent characteristic | Responder | |
|---|---|---|
| Declined participation (N = 185) | Recruited (N = 482) | |
| Age group, n (%) | ||
| ≤ 18 months | 9 (5) | 29 (6) |
| > 18 months but < 4 years | 59 (32) | 162 (34) |
| ≥ 4 years | 117 (63) | 291 (60) |
| Eligibility, n (%) | (N = 186) | (N = 482) |
| Eligible | 87 (47) | 482 (100) |
| Query eligible | 19 (10) | – |
| Not eligible | 80 (43) | – |
| Eczema severity (eligible responders), mean (SD) | (N = 87) | (N = 482) |
| NESS (0–15) | 9.14 (2.31) | 9.52 (2.33) |
| UKDC score (0–4) | 3.36 (0.48) | 3.25 (0.61) |
| Belief in bath additives (1–9)a | (N = 183) | (N = 475) |
| Mean (SD) | 5.6 (2.3) | 4.6 (2.0) |
| Do not know | 34 (18%) | 97 (20%) |
| Bath additive use in past month, n (%) | (N = 180) | (N = 481) |
| Never | 69 (38) | 126 (26) |
| Less than half the time | 36 (20) | 109 (23) |
| More than half the time | 23 (13) | 96 (20) |
| Every time | 52 (29) | 150 (31) |
There were 920 replies expressing a willingness to be contacted and these replies also included a completed screening questionnaire. Of these, 229 did not meet the clinical criteria required to enter the trial. Overall, 662 children met the clinical eligibility criteria and were approached regarding participation, of whom 483 entered the trial and 179 were not recruited for the reasons shown in Figure 2.
Figure 2 shows the flow of participants through the screening and recruitment process.
FIGURE 2.
Recruitment flow chart. a, Unwilling to be randomised/change current treatment, n = 58; eczema no longer a problem, n = 31; child mostly showers, n = 16; child refused, n = 2; other/not specified reason, n = 29. b, Did not meet UKDC, n = 65; NESS score of < 6, n = 76; and did not meet either criterion, n = 88.
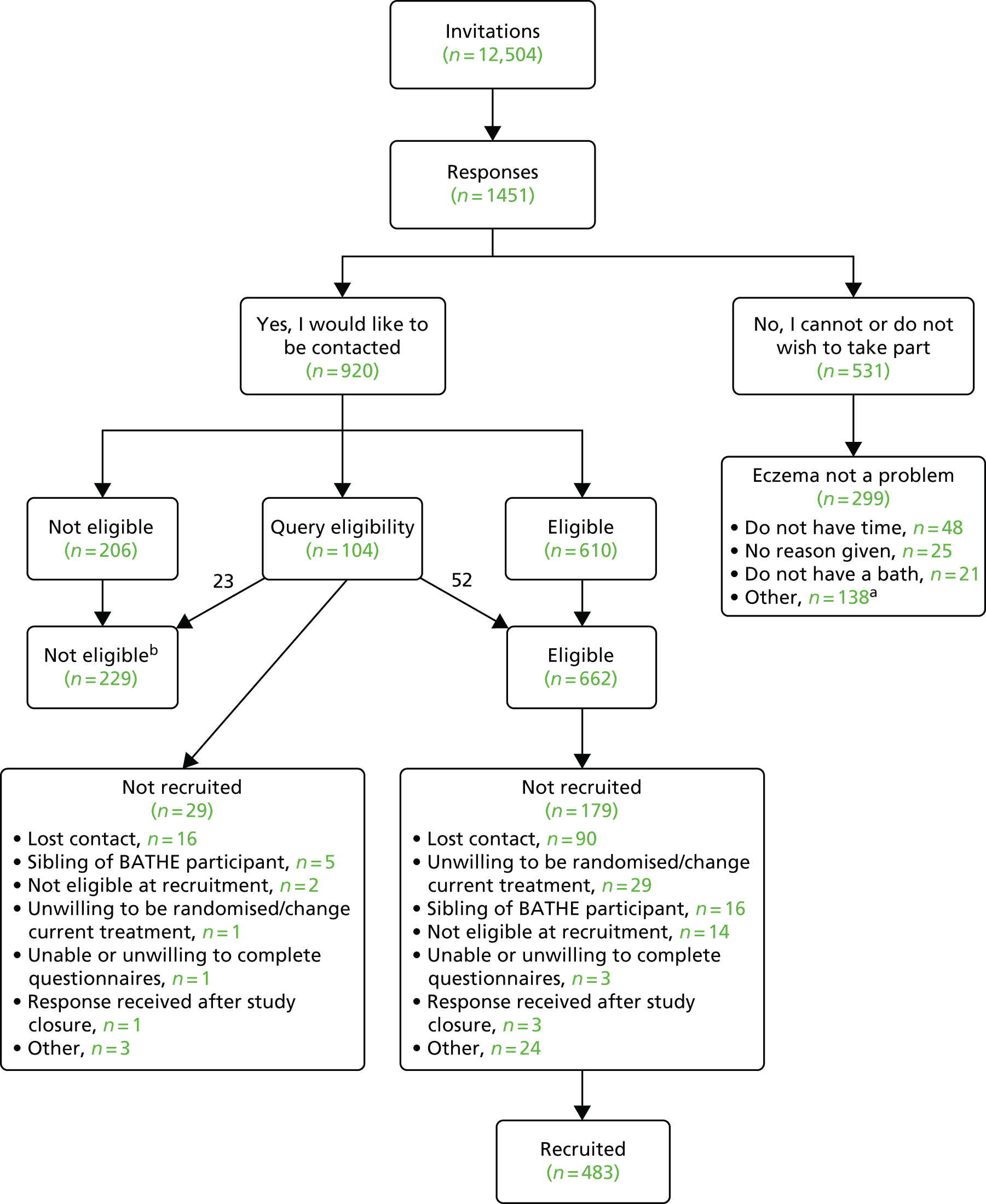
Figure 3 shows the number of participants who withdrew or who were lost to follow-up during the study period. Four participants formally withdrew during the 16-week primary outcome period, three from the Cardiff centre and one from the Bristol centre; all withdrawals were from the bath additive group. Permission was obtained from three parents to use the data already collected, and one requested that all data collected be removed. A total of 42 participants were lost to follow-up by the time of the 16-week primary outcome (22 in the bath additive group and 20 in the no bath additive group) and a further four 16-week questionnaires were not received from participants who remained enrolled.
FIGURE 3.
The BATHE participant flow chart. a, Reported eczema had not been a problem for some time, although screening form clearly indicated eligibility. b, One parent wished to bathe two children together but felt that bath additive was worsening the symptoms of the child not enrolled in the study; one was finding improvement with new creams and, despite reassurance, did not wish this to affect the study results; one child was withdrawn by their GP after experiencing a rash. CRF, case report form.

A total of 429 (89.6%) participants opted to complete their weekly questionnaire online. Of the remaining 51 participants, 27 (5.6%) requested the paper option at recruitment and 24 (5.0%) were switched from online to paper format after discussion with the study team. Reasons for this were primarily related to technical issues, as some parents/carers became discouraged after having problems logging in to the online database. Login problems were occasionally compounded by a failure to understand the automated nature of the system and the inability to complete POEMs retrospectively. This was particularly problematic for a small number of participants who experienced delays in obtaining their prescribed bath emollients and, therefore, did not ‘start’ participation in week 1. Although attempts were made to telephone all families during the first week, as a matter of courtesy and in order to address any problems, some parents could not be reached and it is not possible to determine how many participants were lost to follow-up because of technical or logistical problems.
The majority of trial participants needed no prompting to complete the trial and only 25% of the trial participants were contacted by telephone or e-mail during the 16-week primary outcome period because of failure to complete the questionnaires.
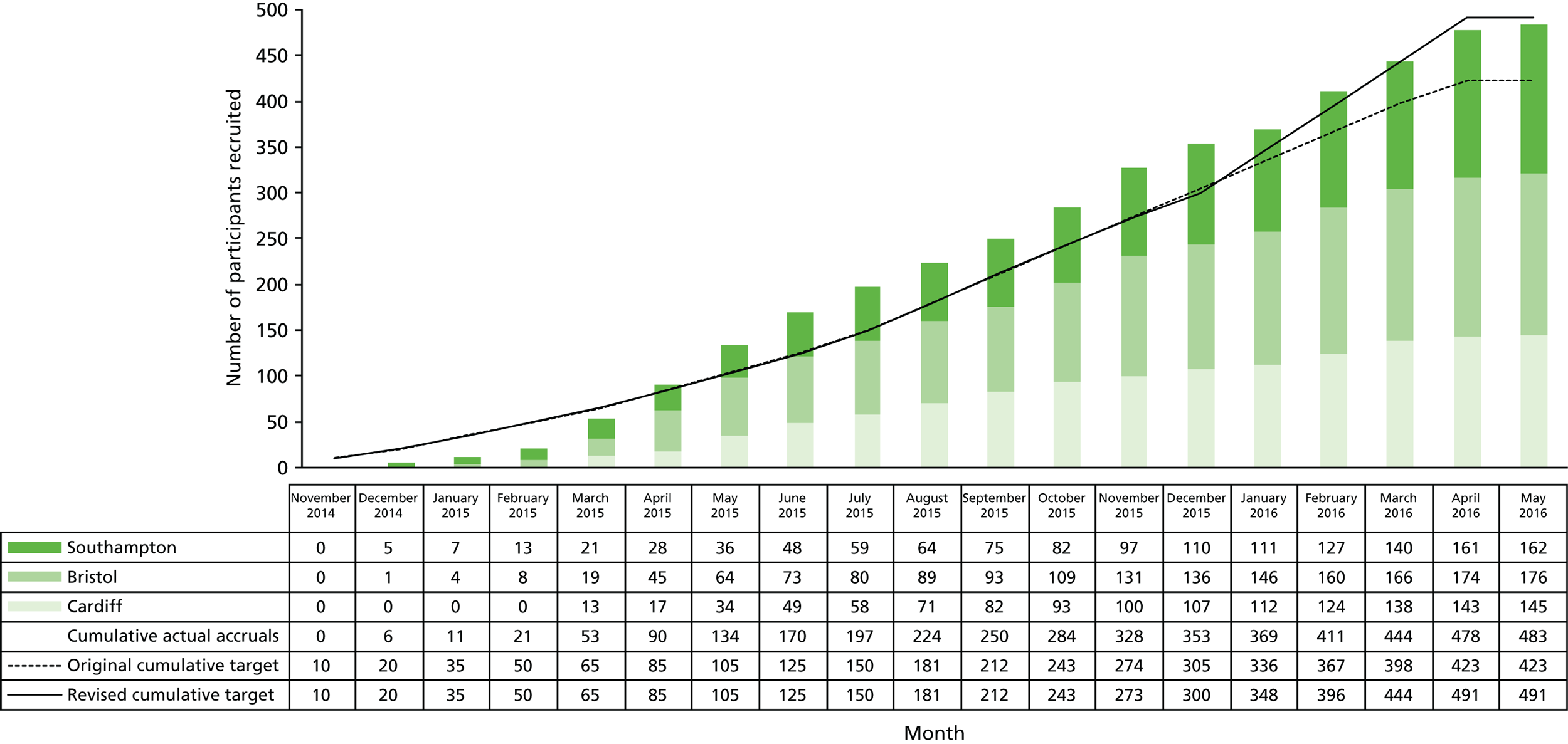
Recruitment dates
Recruitment took place from November 2014 to May 2016. The original target of 423 participants was reached in March 2016 and permission was obtained to continue recruiting participants up to an increased target of 491 participants as discussed in Chapter 2, Changes to trial protocol. Recruitment was stopped at the end of May 2016 as planned, with a total of 483 participants enrolled (Figure 4). The 52-week follow-up questionnaires and notes reviews of the last recruited participants were completed in June 2017.
FIGURE 4.
Recruitment by centre.
Baseline data
Table 2 shows that, although there were more participants allocated to the bath additive group than the no bath additive group (see Chapter 5, Discussion), the key characteristics were well balanced at baseline.
| Participant characteristics | Treatment group | Total (N = 482) | |
|---|---|---|---|
| Bath additive (N = 264) | No bath additive (N = 218) | ||
| Child age (years), mean (SD) | 5.4 (2.9) | 5.2 (2.9) | 5.3 (2.9) |
| Child gender, n (%) | |||
| Male | 138 (52.3) | 100 (45.9) | 238 (49.4) |
| Female | 126 (47.7) | 118 (54.1) | 244 (50.6) |
| Carer age (years), mean (SD) | 36.5 (6.5) | 35.9 (6.7) | 36.2 (6.5) |
| Carer gender, n (%) | |||
| Male | 11/258 (4.3) | 12/212 (5.7) | 23/470 (4.9) |
| Female | 247/258 (95.7) | 200/212 (94.3) | 447/470 (95.1) |
| Ethnicity, n (%) | |||
| White | 228/257 (86.0) | 176/215 (81.9) | 397/472 (84.1) |
| Black | 6/257 (1.9) | 9/215 (4.2) | 15/472 (3.2) |
| Asian | 15/257 (5.8) | 16/215 (7.4) | 31/472 (6.6) |
| Mixed race | 10/257 (3.9) | 9/215 (4.2) | 19/472 (4.0) |
| Chinese | 2/257 (0.8) | 3/215 (1.4) | 5/472 (1.1) |
| Other | 3/257 (1.2) | 2/215 (0.9) | 5/472 (1.1) |
| Highest qualification, n (%) | |||
| Not answered | 6/257 (2.3) | 3/213 (1.4) | 9/470 (1.9) |
| Degree or equivalent | 106/257 (41.3) | 90/213 (42.3) | 197/470 (41.7) |
| Diploma or equivalent | 56/257 (21.8) | 37/213 (17.4) | 95/470 (19.8) |
| A level | 25/257 (9.7) | 24/213 (11.3) | 49/470 (10.4) |
| GSCE/O level | 50/257 (19.5) | 38/213 (17.8) | 88/470 (18.7) |
| Other | 12/257 (4.7) | 16/213 (7.5) | 29/470 (6.0) |
| None | 2/257 (0.8) | 5/213 (2.4) | 7/470 (1.5) |
| Cost of living, n (%) | |||
| Not answered | 7/257 (2.7) | 3/213 (1.4) | 10/470 (2.1) |
| Finding it a strain | 11/257 (4.3) | 3/213 (1.4) | 14/470 (3.0) |
| Have to be careful | 105/257 (40.9) | 82/213 (38.5) | 187/470 (39.8) |
| Able to manage | 99/257 (38.5) | 90/213 (42.3) | 189/470 (40.2) |
| Quite comfortable | 35/257 (13.6) | 35/213 (16.4) | 70/470 (14.9) |
| Prior belief in bath additives (1–9)a | 5.1 (2.2) | 4.8 (2.3) | 5.0 (2.3) |
| POEM scores, mean (SD) | 9.5 (5.7) | 10.1 (5.8) | 9.8 (5.8) |
| Mild (0–7), n (%) | 114 (43.2) | 73 (33.5) | 187 (38.8) |
| Moderate (8–16), n (%) | 119 (45.1) | 114 (52.3) | 233 (48.3) |
| Severe (17–28), n (%) | 31 (11.7) | 31 (14.2) | 62 (12.9) |
| DFIQ score, median (IQR) | 2 (1–6) | 3 (1–7) | 3 (1–7) |
| NESS score, mean (SD) | 9.5 (2.3) | 9.5 (2.3) | 9.5 (2.3) |
| CHU-9D score (utility values), mean (SD) | 0.90 (0.1) | 0.90 (0.1) | 0.90 (0.1) |
Numbers analysed
The questionnaire response rate was high, with 76.7% of participants completing questionnaires for > 80% of the time points for the primary outcome (12 out of 16 weekly questionnaires to 16 weeks). There were no marked differences in completeness of the data by randomisation group (see Table 3 and Figure 5).
| Measure of completion | Treatment group | Total (N = 482) | |
|---|---|---|---|
| Bath additive (N = 264) | No bath additive (N = 218) | ||
| Number of weekly questionnaires completed during the 16-week primary outcome period, mean (SD) | 13.1 (4.5) | 12.7 (4.7) | 12.9 (4.6) |
| > 12 questionnaires completed, n (%) | 209 (79.1) | 161 (73.9) | 370 (76.7) |
| Weekly questionnaire return, n (%) | |||
| Week 1 | 224 (84.8) | 191 (87.6) | 415 (86.1) |
| Week 2 | 222 (84.1) | 183 (83.9) | 405 (84.0) |
| Week 3 | 223 (84.5) | 176 (80.7) | 399 (82.8) |
| Week 4 | 219 (83.0) | 182 (83.5) | 401 (83.2) |
| Week 5 | 216 (81.8) | 175 (80.3) | 391 (81.1) |
| Week 6 | 224 (84.8) | 175 (80.3) | 399 (82.8) |
| Week 7 | 209 (79.1) | 173 (79.4) | 382 (79.2) |
| Week 8 | 216 (81.8) | 172 (78.9) | 388 (80.5) |
| Week 9 | 221 (83.7) | 163 (74.8) | 384 (79.7) |
| Week 10 | 220 (83.3) | 168 (77.1) | 388 (80.5) |
| Week 11 | 203 (76.8) | 161 (73.9) | 364 (75.5) |
| Week 12 | 204 (77.2) | 165 (75.7) | 369 (76.6) |
| Week 13 | 203 (76.9) | 165 (75.7) | 368 (76.3) |
| Week 14 | 203 (76.9) | 167 (76.6) | 370 (76.8) |
| Week 15 | 207 (78.4) | 173 (79.4) | 380 (78.8) |
| Week 16 | 236 (89.4) | 194 (89.0) | 430 (89.2) |
| 52-week questionnaire return, n (%) | 209 (79.2) | 178 (81.6) | 387 (80.3) |
| Initial method of questionnaire completion, n (%) | |||
| Online | 235 (89.0) | 196 (89.9) | 431 (89.4) |
| By post | 15 (5.7) | 12 (5.5) | 27 (5.6) |
| Switched method of completion during study, n (%) | 14 (5.3) | 10 (4.6) | 24 (5.0) |
FIGURE 5.
Questionnaire completion during the 16-week primary outcome period.

Outcomes
Primary outcomes
All 461 participants who had completed at least one POEM following baseline were included in this analysis. The results in Table 4 indicate no statistically significant difference in weekly POEM scores between the two groups over the 16-week period. Of the prespecified potential confounders (child age, child gender, ethnic group, carer age, carer gender, carer education, prior belief in bath emollients, type of emollient used, other medication used, other items used when washing, such as soap/shampoo/soap substitute), only ethnic group, steroid use and soap substitute use were statistically significant and, therefore, retained in the models. After controlling for baseline severity and these confounders, and allowing for the clustering of patients within centres and responses within patients over time, the POEM score in the no bath additive group was 0.41 points higher than in the bath additive group (95% CI –0.27 to 1.10). Unadjusted POEM scores showed < 1 point difference between groups. These differences are not considered to be clinically meaningful, given the published minimal clinically important difference for POEM of 3.0. 41,42
| Time period | Treatment group, mean (SD) | |
|---|---|---|
| Bath additive | No bath additive | |
| Baseline | 9.5 (5.7) | 10.1 (5.8) |
| Week 1 | 8.3 (5.6) | 9.1 (5.9) |
| Week 2 | 7.8 (5.5) | 8.2 (5.9) |
| Week 3 | 7.4 (5.3) | 8.5 (5.9) |
| Week 4 | 7.6 (6.0) | 8.6 (6.1) |
| Week 5 | 7.6 (5.9) | 8.3 (6.0) |
| Week 6 | 7.8 (6.3) | 8.4 (5.7) |
| Week 7 | 7.5 (6.1) | 8.8 (6.1) |
| Week 8 | 7.3 (6.2) | 8.2 (5.8) |
| Week 9 | 7.2 (5.9) | 8.1 (5.8) |
| Week 10 | 7.3 (5.8) | 8.5 (5.8) |
| Week 11 | 7.6 (6.1) | 8.2 (6.0) |
| Week 12 | 7.7 (6.2) | 7.9 (5.9) |
| Week 13 | 7.1 (5.9) | 8.3 (6.2) |
| Week 14 | 7.2 (6.3) | 7.9 (6.3) |
| Week 15 | 7.0 (6.3) | 8.4 (6.5) |
| Week 16 | 7.1 (6.1) | 8.2 (6.3) |
There was no statistically significant interaction between treatment and time (interaction term 0.04, 95% CI –0.02 to 0.11; p = 0.204).
Patient Oriented Eczema Measure weekly for 16 weeks
There is some fluctuation in between-group differences in POEM score over the 16-week period, with some results statistically significant and others not. However, all the between-group differences are < 2 points, suggesting that even those that are statistically significant are not likely to be clinically meaningful as they are below the POEM minimal clinically important difference of 3.0 (Tables 4 and 5 and Figure 6). Moreover, it is likely that some significant results may be found because of multiple testing (type I error).
| POEM scores | Treatment group, mean (SD) | Univariate difference in mean POEM (95% CI) | Adjusted difference in mean POEMa (95% CI) | |
|---|---|---|---|---|
| Bath additive | No bath additive | |||
| Primary outcome: 16-week repeated measures | – | – | – | – |
| Over the 16-week primary outcome period (repeated measures) | 7.5 (6.0) | 8.4 (6.0) | 0.90 (–0.03 to 1.83) | 0.41 (–0.27 to 1.10) |
FIGURE 6.
Mean POEM scores over the 16-week primary outcome period, by group.

Imputed analysis
As a sensitivity analysis, primary outcome was examined using imputed values for all missing weekly data using an individual chained equations multiple imputation model. Table 6 shows that this produced an adjusted difference in mean POEM score between groups of 0.43 (95% CI –0.26 to 1.12). This is a very similar result to our primary analysis.
| Adherence to allocated treatment | Treatment group, mean (SD) | Univariate difference in mean POEM (95% CI) | Adjusted difference in mean POEMa (95% CI) | |
|---|---|---|---|---|
| Bath additive | No bath additive | |||
| Primary outcome: 16 week repeated measures | – | – | – | – |
| Over the 16 week primary outcome period (repeated measures) | 7.4 (6.0) | 8.5 (6.0) | 0.96 (0.05 to 1.87) | 0.43 (–0.26 to 1.12) |
Adherence to allocated treatment (per-protocol analysis)
Parent/carer report of adherence to treatment allocation group at 16 weeks recorded 92.7% of participants in the bath additive group using bath additive ‘every time’ (73.8%) or ‘more than half the time’ (18.9%). Similarly, 92.1% of those in the no bath additive group said that they used bath additives either ‘never’ (87.4%) or ‘less than half the time’ (4.7%) (Table 7).
| Adherence to allocated treatment | Treatment group, n (%) | |
|---|---|---|
| Bath additive (N = 233) | No bath additive (N = 191) | |
| Use of bath additives | ||
| Every time | 172 (73.8) | 14 (7.3) |
| More than half the time | 44 (18.9) | 1 (0.5) |
| Less than half the time | 15 (6.4) | 9 (4.7) |
| Never | 2 (0.9) | 167 (87.4) |
| Number of baths per week | (N = 221) | (N = 176) |
| 1–2 | 70 (31.7) | 54 (30.7) |
| 3–4 | 74 (33.5) | 56 (31.8) |
| 5–6 | 45 (20.4) | 39 (22.2) |
| ≥ 7, n (%) | 32 (14.5) | 27 (15.3) |
We considered two possible definitions of adherence and on neither basis was there a statistically significant difference between the groups (Table 8).
| Adherence to allocated treatment | Difference in mean POEM (95% CI) | |
|---|---|---|
| Univariate | Adjusted | |
| 16-week repeated measures model | ||
| ‘More than half’ or ‘every time’ compared with ‘less than half the time’ or ‘never’ | 0.35 (–0.58 to 1.28) | 0.32 (–0.37 to 1.02) |
| ‘Every time’ compared with ‘never’ | 0.23 (–0.79 to 1.24) | 0.38 (–0.39 to 1.15) |
Participants were also asked about adherence at 52 weeks. Table 9 shows that adherence remained high.
| Exacerbations | Treatment group, n (%) | |
|---|---|---|
| Bath additive (N = 203) | No bath additive (N = 176) | |
| Use of bath additives | ||
| Every time | 118 (58.1) | 9 (5.1) |
| More than half the time | 55 (27.1) | 4 (2.3) |
| Less than half the time | 20 (9.9) | 18 (10.2) |
| Never | 10 (4.9) | 145 (82.4) |
| Number of baths per week | ||
| 1–2 | 69 (36.5) | 57 (35.6) |
| 3–4 | 65 (34.4) | 50 (31.3) |
| 5–6 | 28 (14.8) | 29 (18.1) |
| ≥ 7 | 27 (14.3) | 24 (15.0) |
Secondary outcomes
Exacerbations
In total, we had exacerbations data for 470 (97.5%) children. Of these, 257 (54.7%) had at least one exacerbation as defined in the protocol (i.e. GP notes review recorded consultations in which there was mention of eczema and topical steroid or TCI was advised or prescribed).
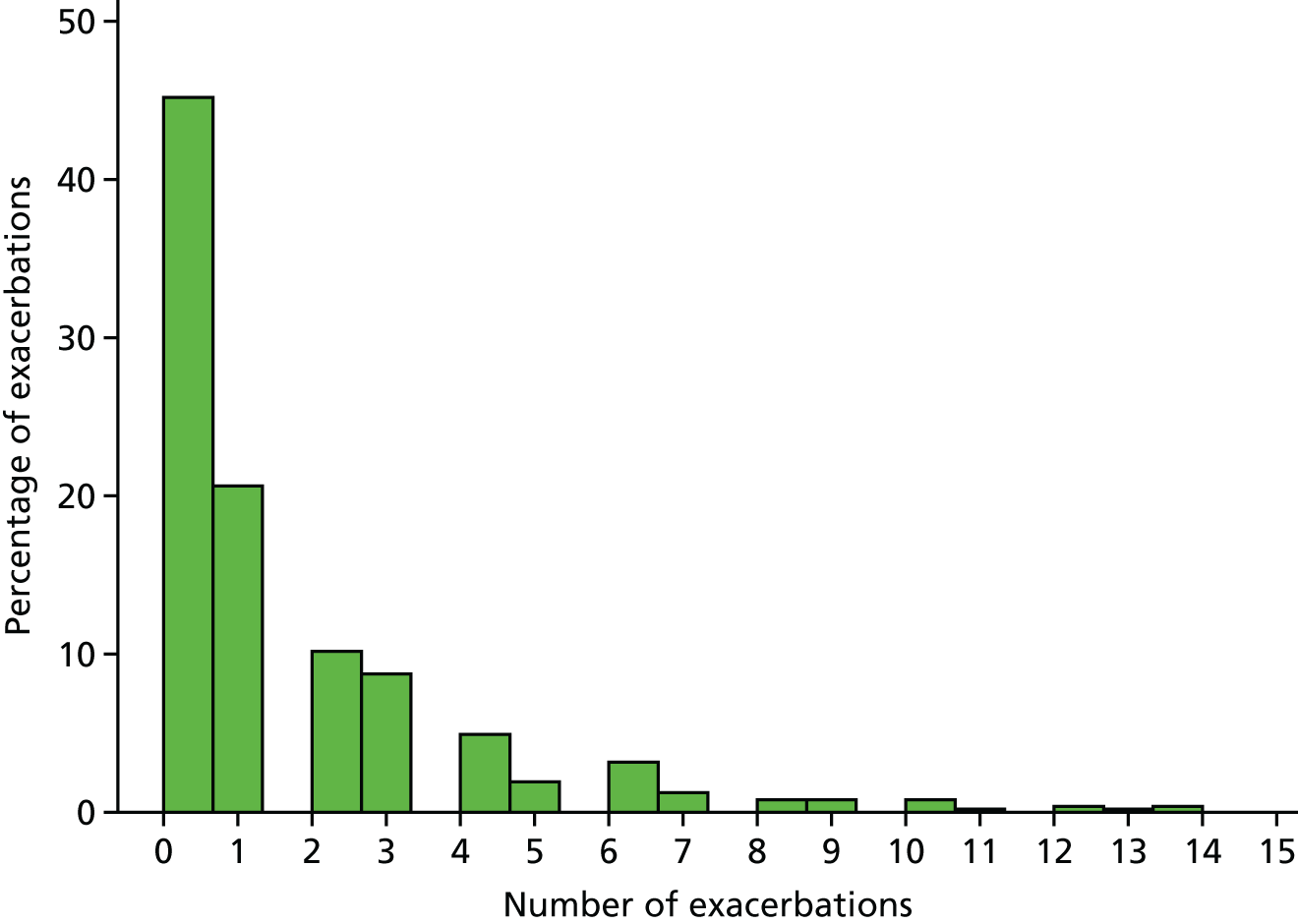
The distributions of the number of exacerbations is skewed and follows an approximately negative binomial distribution (Figure 7). As shown in Table 10, on average, the number of exacerbations was similar between groups. The unadjusted results indicated slightly more exacerbations in the no bath additive group, but in the adjusted model there was no significant difference in the number of exacerbations between groups.
FIGURE 7.
Distribution of exacerbations.
| Time point | Treatment group | RR exacerbations (95% CI) | ||
|---|---|---|---|---|
| Bath additive | No bath additive | Univariate | Adjusted | |
| Median number of exacerbations (IQR) | 1 (0–2) | 1 (0–3) | 1.33 (1.02 to 1.75) | 1.24 (0.96 to 1.60) |
Eczema severity over 1 year (from baseline to 52 weeks): monthly Patient Orientated Eczema Measure scores
The difference between groups in monthly POEM scores from baseline to 52 weeks was explored and tended to be non-significant (Table 11 and Figure 8).
| POEM scores | Treatment group, mean (SD) | |
|---|---|---|
| Bath additive | No bath additive | |
| Baseline | 9.5 (5.7) | 10.1 (5.8) |
| Week 4 | 7.6 (6.0) | 8.6 (6.1) |
| Week 8 | 7.3 (6.2) | 8.2 (5.8) |
| Week 12 | 7.7 (6.2) | 7.9 (5.9) |
| Week 16 | 7.1 (6.1) | 8.2 (6.3) |
| Week 20 | 6.9 (6.2) | 8.7 (6.5) |
| Week 24 | 7.3 (6.5) | 8.3 (6.7) |
| Week 28 | 7.4 (6.4) | 8.8 (6.5) |
| Week 32 | 7.8 (6.7) | 8.7 (7.0) |
| Week 36 | 7.2 (6.8) | 8.8 (6.8) |
| Week 40 | 7.3 (6.4) | 8.8 (6.7) |
| Week 44 | 6.9 (6.3) | 8.2 (6.4) |
| Week 48 | 7.4 (6.6) | 8.6 (6.9) |
| Week 52 | 7.1 (6.2) | 8.0 (6.4) |
FIGURE 8.
Patient Oriented Eczema Measure scores during the 52-week secondary outcome period, by group.
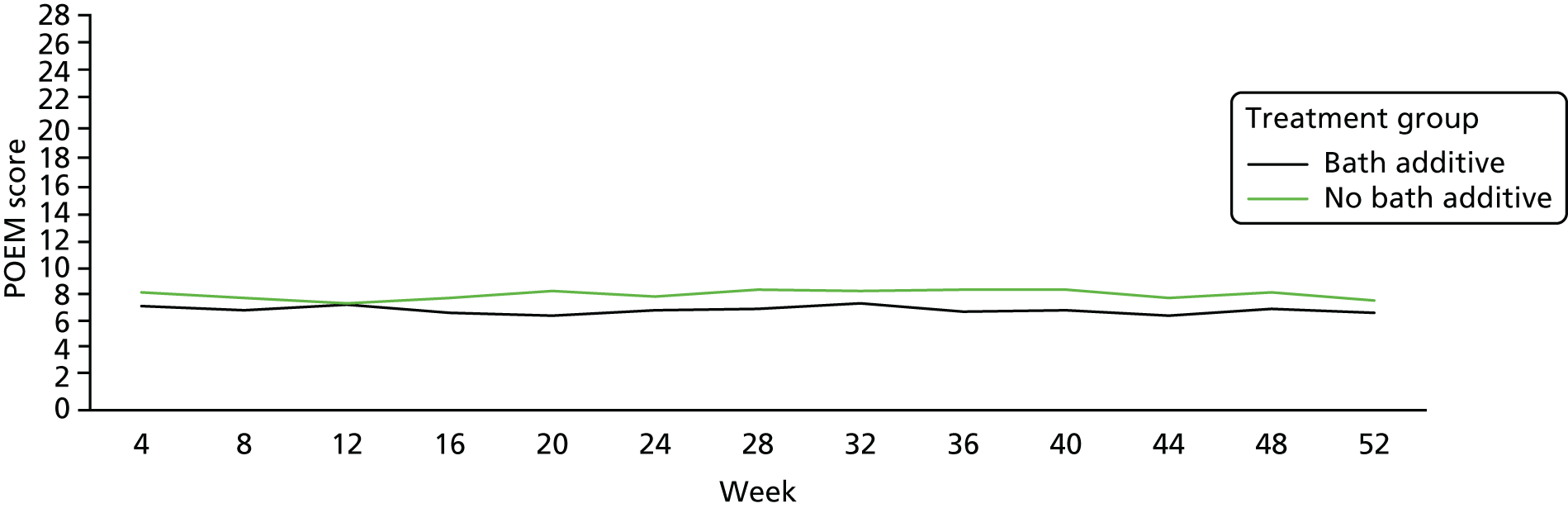
There was no statistically significant difference between the two groups at 52 weeks, based on monthly POEM scores over the period, and the CIs were well below 2 points (Table 12).
| Time point | Treatment group, mean (SD) | Univariate difference in mean POEM (95% CI) | Adjusted difference in mean POEM (95% CI) | |
|---|---|---|---|---|
| Bath additive | No bath additive | |||
| Secondary outcome: monthly repeated measures | ||||
| Over the 52-week period (repeated measures) | 7.3 (6.3) | 8.4 (6.4) | 0.99 (0.03 to 1.96) | 0.75 (–0.05 to 1.55) |
Dermatitis Family Impact Questionnaire
The distribution of the DFIQ was very skewed, with almost two-thirds of participants (62.9%) scoring ≤ 4 out of 27 on the scale. Therefore, a non-parametric approach has been used. The quantile regression compares the median values rather than the means. There was no statistically significant difference in the DFIQ at either 16 or 52 weeks (Table 13).
| Prescriptions | Treatment group | Univariate difference in median DFIQ (95% CI) | Adjusted difference in median DFIQ (95% CI) | |
|---|---|---|---|---|
| Bath additive (n = 230) | No bath additive (n = 186) | |||
| Median DFIQ at baseline (IQR) | 2 (1–6) | 3 (1–7) | – | – |
| Median DFIQ at 16 weeks (IQR) | 2 (0–5) | 3 (1–7) | 1.00 (0.09 to 1.91) | 0.29 (–0.57 to 1.14) |
| Median DFIQ at 52 weeks (IQR) | 2 (0–5) | 2 (0–6) | 0.00 (–0.93 to 0.93) | –0.29 (–1.36 to 0.79) |
Health-related quality of life
See Chapter 4, Health economic evaluation, for a discussion of the CHU-9D.
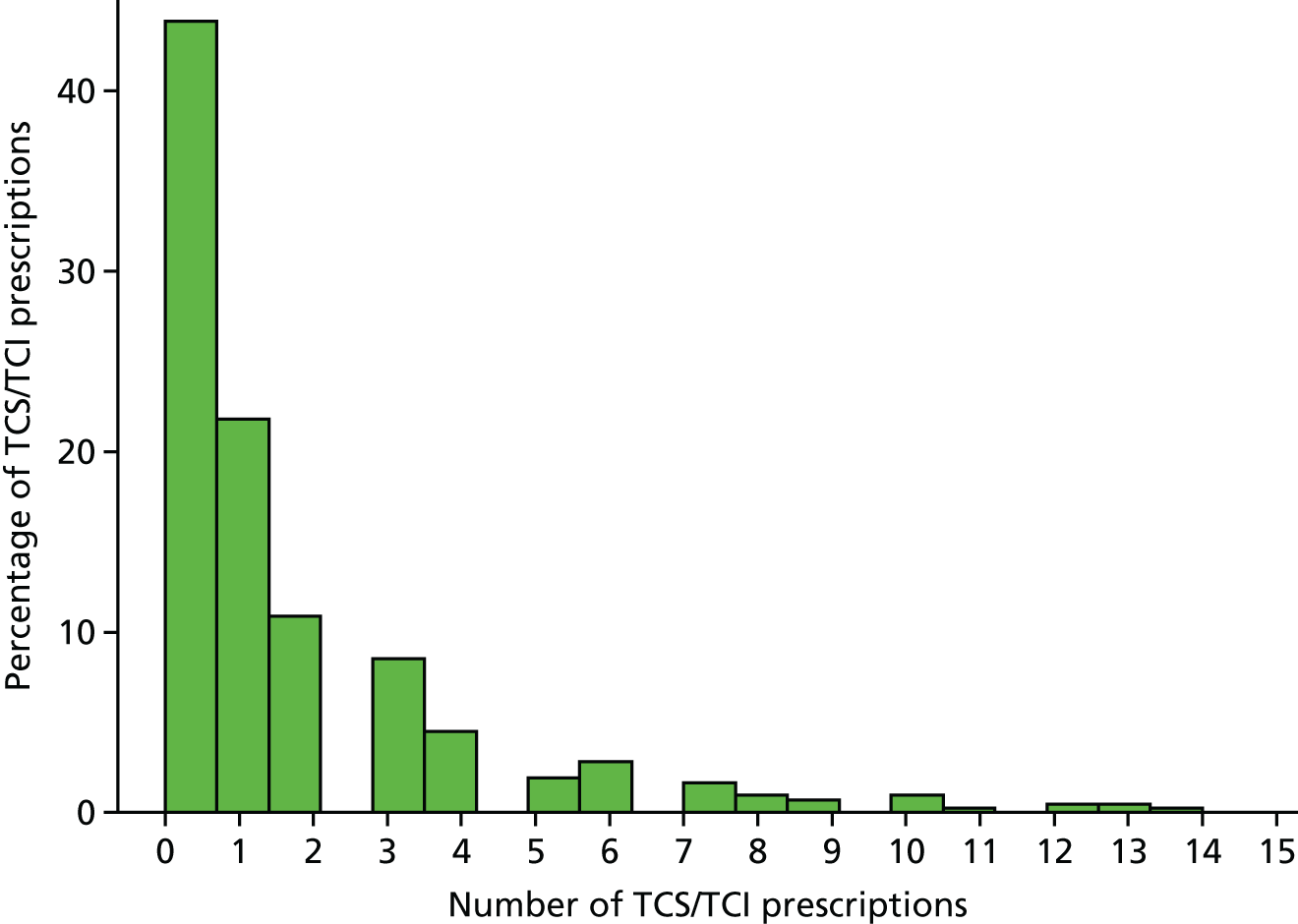
Use of topical corticosteroids and topical calcineurin inhibitors
There were a total of 671 prescriptions for TCSs and 32 prescriptions for TCIs (Table 14). As shown in Figure 9, the distribution is skewed, with 44% children receiving no TCS or TCI prescription and 85% receiving fewer than five prescriptions over the 52-week study period.
| Number of prescriptions | Treatment group | |
|---|---|---|
| Bath additive (n = 258) | No bath additive (n = 164) | |
| Total number of TCS/TCI prescriptions | 325 | 346 |
| Median number of TCS/TCI prescriptions | 0 (0–2) | 1 (0–3) |
FIGURE 9.
Prescriptions for TCSs and TCIs.
Subgroup analysis
All of these analyses are intended to be hypothesis generating rather than hypothesis testing as the trial was not powered to explore the effect in subgroups and there is a risk of type I error, in which a statistically significant result is found simply because the data have been tested multiple times rather than because a genuine difference exists between the groups. However, there seems to be weak evidence in favour of an interaction with age. We cannot exclude the possibility of a small effect of bath additives among children aged < 5 years, as the no bath additive group had a POEM score 1.3 points higher than the group with bath additives. The upper limit of the 95% CI was 2.3, still below the now widely accepted POEM minimal clinically important difference of 3 points but reaching what we said would be considered clinically meaningful in this trial (i.e. 2 points).
A significant interaction effect was also seen in the frequency of bathing as reported at 16 weeks. There was no statistically significant difference in those children who bathed 1–4 times per week; however, in those children who bathed ≥ 5 times per week, the POEM score was 2.3 points higher (95% CI 0.63 to 3.91) in the no bath additive group. The upper CI reached 3.9, suggesting that there may be a clinically meaningful benefit to bath additives in this group, but this is a small group, with only 77 in the bath additive group and 66 in the no bath additive group (Table 15).
| Primary outcome: 16-week repeated measures | N (%) | Treatment group, mean (SD) | Interaction term (95% CI) | Adjusted difference in mean POEMa (95% CI) | |
|---|---|---|---|---|---|
| Bath additive | No bath additive | ||||
| Age (years) | |||||
| < 5 | 256 (53.1) | 6.99 (5.67) | 9.09 (6.01) | –1.43 (–2.02 to –0.15) | 1.29 (0.33 to 2.25) |
| ≥ 5 | 226 (46.9) | 7.97 (6.24) | 7.52 (5.92) | –0.29 (–1.21 to 0.63) | |
| Baseline severity | |||||
| Clear/mild (0–7) | 187 (38.8) | 4.78 (4.26) | 5.22 (4.58) | –0.05 (–1.14 to 1.05) | –0.07 (–1.08 to 0.95) |
| Moderate (8–16) | 233 (48.3) | 8.14 (5.54) | 9.18 (5.46) | 0.65 (–0.45 to 1.74) | |
| Severe/very severe | 62 (12.9) | 14.63 (6.16) | 13.03 (6.92) | –1.16 (–3.62 to 1.32) | |
| Use of leave-on emollient | |||||
| 0–4 days per week | 138 (28.6) | 7.64 (6.68) | 6.43 (5.42) | –0.02 (–2.05 to 2.01) | 0.26 (–1.34 to 1.86) |
| 5–7 days per week | 344 (71.4) | 8.61 (5.74) | 7.93 (6.14) | 0.69 (–0.39 to 1.76) | |
| TCS use | |||||
| Any | 241 (50.7) | 8.40 (6.19) | 9.35 (6.21) | 0.52 (–1.35 to 2.40) | 1.22 (–0.18 to 2.63) |
| None | 234 (49.3) | 6.63 (5.64) | 7.39 (5.66) | 0.58 (–0.64 to 1.81) | |
| Frequency of bathing at 16 weeks | |||||
| 1–4 times per week | 255 (64.1) | 7.93 (5.94) | 8.00 (5.82) | 2.14 (0.21 to 4.07) | –0.26 (–1.38 to 0.87) |
| ≥ 5 times per week | 143 (35.9) | 6.30 (5.70) | 8.75 (6.12) | 2.27 (0.63 to 3.91) | |
| Prior belief in bath additive | |||||
| 1–3 low belief | 106 (29.4) | 7.93 (6.10) | 9.27 (6.25) | 0.85 (–0.52 to 2.21) | 1.17 (–0.78 to 3.13) |
| 4–6 moderate belief | 158 (43.8) | 8.37 (6.06) | 8.68 (6.02) | –0.16 (–1.77 to 1.45) | |
| 7–9 high belief | 97 (26.9) | 5.70 (5.06) | 7.09 (6.05) | 1.80 (0.04 to 3.56) | |
| Use of soap substitute at 16 weeks | |||||
| Any | 89 (20.8) | 8.09 (6.10) | 9.31 (5.88) | 1.30 (–0.97 to 3.57) | 1.72 (–0.44 to 3.88) |
| None | 340 (79.3) | 7.17 (5.82) | 7.99 (5.87) | 0.36 (–0.63 to 1.35) | |
Adverse events
No serious adverse events (SAEs) were reported during the trial period; however, one SAE, which occurred in the bath additive group and was unrelated to the trial intervention, was detected during the notes review process.
Over the first 16 weeks, 34.5% in the bath additive group and 35.4% in the no bath additive group reported at least one adverse event on weekly questionnaires. There was no statistically significant difference between the groups [odds ratio (OR) 1.40, 95% CI 0.79 to 2.47] (Table 16).
| Adverse events | Treatment group, n (%) | |
|---|---|---|
| Bath additive | No bath additive | |
| 16 weeks | ||
| Slips | 44 (17.5) | 52 (24.8) |
| Stinging | 4 (1.6) | 4 (1.9) |
| Redness | 35 (13.9) | 48 (23.0) |
| Refuses a bath | 21 (8.3) | 25 (12.0) |
| 52 weeks | ||
| Slips | 56 (22.2) | 63 (30.1) |
| Stinging | 7 (2.8) | 4 (1.9) |
| Redness | 44 (17.5) | 61 (29.2) |
| Refuses a bath | 30 (11.9) | 31 (14.8) |
Over the full 52-week study period, 40.2% of the bath additive group and 41.3% of the no bath additive group reported at least one AE on questionnaires. As at 52 weeks, there was no statistically significant difference between the groups (OR 1.36, 95% CI 0.80 to 2.33).
Chapter 4 Health economic evaluation
This chapter presents the economic analysis of the relative resource use, costs, clinical effectiveness and cost-effectiveness outcomes of emollient bath additives when used in addition to standard management versus standard management without bath additives for childhood eczema.
Introduction
Eczema is a skin condition that is very common in children. The economic implications of eczema in children are described in the introduction (see Chapter 1) of this report and are well documented in the literature. 33 Recommendations by the NICE guideline9 on childhood eczema suggest the use of a ‘complete emollient therapy’ that includes bath emollient (bath additives) in addition to emollient cream and soap substitutes. However, the guideline also highlights the uncertainty from limited evidence on the benefit of including bath additives in this combination of treatments. In addition to the clinical question that this uncertainty generates, there is also an economic question to be addressed. This is even more important in the current economic climate in which NHS resources are extremely limited. Given that some estimates have suggested that bath additives account for more than one-third of the total costs treating eczema in childhood,9,13 the relevance of the economic question becomes crucially important.
Economic evaluations alongside clinical trials (EEACT) provide timely information with high internal validity. 50 When conducting EEACT, the quality of the economic information derived depends on the features of the trial’s design. However, it is generally acknowledged that pragmatic effectiveness trials are the best vehicle for economic studies. 50 For the economic analysis, the pragmatic nature of the trial means that the external validity of the economic results is increased by avoiding protocol-driven biases, such as artificial resource use.
Economic evaluations alongside clinical trials involve the comparative analysis of alternative interventions in terms of their costs and benefits. 51,52 Methodological guidelines for EEACT differ in their recommendations for the most appropriate perspective that should be adopted. As a minimum, it is recommended that analysts adopt a health system perspective for analysis. For England and Wales, this is currently considered to include the NHS and personal social services. 53
The BATHE trial was a multicentre, pragmatic, non-blinded, randomised controlled trial with two parallel arms. The study population for the BATHE trial is described in Chapter 3, Results. Ideally, the economic evaluation would be factored into sample size calculations using standard methods, based on asymptotic normality, or by simulation. 54,55 However, it is common for the sample size of the trial to be based on the primary clinical outcome alone. As a consequence, sample size restrictions necessitate a focus on estimation rather than hypothesis testing for our economic evaluation. 56 The sample size in the BATHE trial was calculated for repeated measures ANOVA in weekly POEM scores over the 16-week observation period. In our protocol and the health economics analysis plan (HEAP) we stated our intention to conduct a cost-effectiveness analysis using the primary outcome, POEM, a cost–utility analysis using utility values obtained from the paediatric CHU-9D and to also report cost per exacerbation avoided. However, the economic results in this study reveal that none of these outcomes showed a clinically important difference. Therefore, our approach was to proceed by reporting our findings and ultimately presenting our economic evaluation in the form of cost–consequences analysis,57 as per our HEAP. Following this, the economic evaluation was conducted from the NHS and Personal Social Services perspective; however, family-borne costs (FbCs), in the form of additional analyses, were also incorporated and the combined results are also reported in this chapter.
Methods
The range of cost and effectiveness outcomes used are described in this chapter. The economic evaluation used resource use, cost and effectiveness outcomes data collected for all of the participants enrolled in the BATHE trial as described in Chapter 3, Results. The time points of the evaluation were the 16- and 52-week follow-up periods used for the trial. The maximum 52-week follow-up period of the trial means that discounting of costs and outcomes was not relevant and was not conducted. The intervention was conducted in primary care settings; however, the setting for the economic evaluation covers both primary and secondary care resource use.
The analyses were carried out using the ITT approach, and individual patient data were estimated for each participant. All economic analyses were performed using Stata® version 14 (StataCorp LP, College Station, TX, USA) and Microsoft Excel.
Resource use and valuation, health-related quality of life and data collection tools
Resource use
The categories of resource use included in this study were determined by the perspective of the analysis (NHS). For each participant, health-care resource utilisation was measured using two data sources.
First, a modified version of the Client Service Receipt Inventory (CSRI) questionnaire58 adapted for the BATHE trial was used to collect resources reported by parents/carers. The CSRIs were completed at baseline asking parents about resource use over the 3-month period prior to randomisation, at 16 weeks (the trial primary end point) and after that in 3-month intervals up to 52 weeks, as this is considered to be the most appropriate recall length for reporting. 59 The CSRI asked about resource use stemming from the child’s eczema and, in addition to providing information on primary and secondary care resource use, it also allowed reporting of resources associated with salient clinical events, such as days lost from school for children and from work for parents because of their child’s eczema as well as FbCs.
Second, GP electronic patient records were examined in order to record resource use on study case report forms called GP notes review (NR) throughout this report. The selection process for each resource variable was undertaken by assessors and the principal investigator (MS) identifying the ‘eczema-related’ items as opposed to ‘other’ resource use. The NR forms used in this study were designed to capture the frequency and intensity of care provided to each child, based on GP practice record and any complications experienced. The NR forms recorded GP and other primary care consultations, referrals to secondary care, as well as prescriptions and medications during the trial period for each participant.
We have used the triangulation of resource use data to validate our data by cross-verifying the same information. The data were used not in a complementary manner, integrating results, but for verification and better understanding of the data. Resource use, recorded using both approaches, is reported separately in the form of mean (SD) and 95% CIs, taking into account the skewness of the data.
Valuation
The valuation of resources used within the trial period involves quantifying each resource item used by multiplying it by the perspective unit cost of each item. The products estimated were summarised to calculate the individual cost for each participant. The principal costs are those associated with the use of bath additives and the primary and secondary health-care contacts and medications attributed to eczema. The comprehensive profile of resources captured for each participant was valued using national tariffs and expressed in Great British pounds, 2016 prices. The primary care resource use items were valued using the Personal Social Services Research Unit Unit Costs of Health and Social Care 2016. 60 The resource use items from the secondary care data were mainly valued using the NHS Reference Costs for 2015 to 2016. 61 When necessary, the unit costs were adjusted for inflation using the hospital and community health services index. 61
Costs
Once resources were identified and valued, individual patient costs were estimated, mean estimates by group were calculated and differences in mean costs (ΔC) and mean effects (ΔE) between the groups were calculated. Arithmetic means were used to estimate differences between groups. Independent sample t-tests were used to test for differences in costs and effects observed between the groups; all statistical tests were two-tailed. However, cost data often do not conform to the assumptions for standard statistical tests for comparing differences in arithmetic means. They are usually right skewed and truncated at zero because of the small number of participants with high resource use and those participants who incur no costs. The assessment of uncertainty for each measure was estimated and reported in the form of SDs and CIs for point estimates using regression models. 50
There were five main cost categories created from the data collection tools: (1) primary care resource use, (2) secondary care, including hospital admissions and accident and emergency (A&E), (3) medications for eczema, (4) prescriptions for eczema and (5) FbCs. Days lost from school for children and days lost from work because of their child’s eczema for parents are also reported.
Effectiveness outcomes
Three alternative outcomes were identified as relevant and of interest to policy-makers, providers, funders of care and patients. These were:
Health-related quality of life
Eczema has been shown to have a detrimental effect on children’s QoL. 2,3 The paediatric QoL measure CHU-9D was used to collect data at baseline and at 16- and 52-week follow-up. In contrast to adult measures for HRQoL, for which there is widespread consensus in support of using the EQ-5D questionnaire, there is no consensus regarding the paediatric HRQoL measures. However CHU-9D, which is a relatively recently developed measure, has gained ground within the paediatric research community. 46 Responses obtained using the CHU-9D questionnaire were used to estimate utility values for each participant, reporting quality-adjusted life-years (QALYs) gained.
Analysis
Our analysis follows a prespecified HEAP. The purpose of the economic analysis was to estimate costs, cost and effectiveness outcome differences associated with the treatment, the variability of differences and whether or not the differences occurred by chance. Our economic results reported here are expressed in terms of net and incremental costs and effectiveness outcomes.
Regression models were used to estimate net and incremental cost and effectiveness outcomes and to adjust for confounders where appropriate. We used MMLMs controlling for baseline POEM and allowing for clustering of patients within centres, thereby following the same process undertaken for the statistical analysis for consistency of results.
Correlation and baseline covariates for costs and quality-adjusted life-years
We conducted correlation analysis to assess variables for inclusion as confounders in the analyses of cost and QALY outcomes. This allowed us to identify covariates for the outcomes of interest performing adjustment and including these variables in our analysis. Therefore, in addition to reporting unadjusted and adjusted results for baseline covariates, the effects of age and severity of eczema are also assessed and reported in the form of exploratory analysis.
Missing data
Extracts of health-care contact records were available from all trial sites; these were cross-checked against the CSRI questionnaires completed by parents/carers, ensuring that any conflicts or omissions were detected and corrected. The results of the economic evaluation were restricted to the observed data, making the assumption that any missing observations were missing at random. For the CSRI and the CHU-9D, this includes cases in which the parents failed to complete the questionnaires and for the NR data include cases of participants who left the participating GP surgery during the trial period and for whom the date of departure was unknown. However, the CSRI questionnaires were completed irrespective of any change in GP surgery occurring during the trial period. Therefore, the missing observations among the two data sources were compared and considered missing at random only when the observed data supported this assumption.
Addressing uncertainty
The sampling uncertainty of the economic outcomes were reported by estimating and reporting SDs for within-group estimates of costs and outcomes and CIs for between-group differences and comparison. To address potential threats arising from unrepresentative recruiting centres, we used hypothesis testing of homogeneous results across centres. The primary outcome (POEM) allowed us also to report costs by severity levels. 28 To avoid the danger of spurious subgroup effects and the probability of finding a difference due solely to random variation, we report these results in the form of exploratory MMLM analysis.
Results
The sample population for the economic analysis was 482 participants in total, the same as the statistical analysis. Missing values are indicated in each section below. In our sample, 264 participants were included in the bath additives group and 218 participants in the no bath additives group. The clinical and sociodemographic characteristics of the BATHE participants included in our economic study were well balanced between groups (see Table 2). The sections below report resource use and cost estimates from both data sources (i.e. CSRI and GP NR), and Table 17 presents the unit costs used for the valuation of the resources used.
| Resource or service | Unit cost (£)a | Source |
|---|---|---|
| GP | 36.00 | Unit Costs of Health and Social Care 2016 60 |
| GP telephone call | 14.60 | Unit Costs of Health and Social Care 2016 60 |
| Practice nurse | 13.22 | Unit Costs of Health and Social Care 2016 60 |
| Walk-in centre, out of hours | 43.10 | Unit Costs of Health and Social Care 2016 60 |
| Allergy clinic | 168.67 | NHS Reference Costs 2015 to 2016 61 |
| Child health | 70.00 | NHS Reference Costs 2015 to 2016 61 |
| Paediatric respiratory medicine | 218.38 | NHS Reference Costs 2015 to 2016 61 |
| Paediatric dermatology | 135.41 | NHS Reference Costs 2015 to 2016 61 |
| Dermatology nurse (specialist nurse) | 86.00 | Unit Costs of Health and Social Care 2016 60 |
| Paediatrics | 194.36 | NHS Reference Costs 2015 to 2016 61 |
| Nutrition and dietetics | 71.17 | NHS Reference Costs 2015 to 2016 61 |
| Out of hours | 138.01 | Unit Costs of Health and Social Care 2016 60 |
| Eye unit | 96.34 | NHS Reference Costs 2015 to 2016 61 |
| Health visitor | 53.00 | NHS Reference Costs 2015 to 2016 61 |
| Other outpatients | 135.00 | Unit Costs of Health and Social Care 2016 60 |
| Phototherapy unit | 85.61 | NHS Reference Costs 2015 to 2016 61 |
| Paediatric clinical immunology | 235.69 | NHS Reference Costs 2015 to 2016 61 |
| A&E | 98.00 | Unit Costs of Health and Social Care 2016 60 |
| A&E by ambulance | 146.86 | NHS Reference Costs 2015 to 2016 61 |
| Hospital admissions (dermatology) | 987.50 | NHS Reference Costs 2015 to 2016 61 |
| Non-specified hospital admissions | 236.00 | NHS Reference Costs 2015 to 2016 61 |
| Clinical genetics | 439.45 | NHS Reference Costs 2015 to 2016 61 |
| Microbiology | 7.63 | NHS Reference Costs 2015 to 2016 61 |
| Phlebotomy | 3.37 | NHS Reference Costs 2015 to 2016 61 |
| Hospital pharmacist | 72.00 | Unit Costs of Health and Social Care 2016 60 |
| Chinese herbal specialist consultation | 35.00 | Private appointment (web) |
| NHS 111, cost per call | 8.41 | Unit Costs of Health and Social Care 2016 60 |
| Prescription cost | 8.00 | Unit Costs of Health and Social Care 2016 60 |
| Bath additives (costed by item) | – | BNF 68 (2016)36 |
| Various medications (costed by item) | – | BNF 68 (2016)36 |
Resource use, costs and intervention costs
Resource use and costs source: Client Service Receipt Inventory
Resource use
Table 18 presents the eczema-related primary and secondary care consultations at baseline and at 16- and 52-week follow-ups as reported by parents completing the CSRI questionnaires. The baseline number of consultations showed no significant difference between the two groups. The total mean number of consultations was 0.89 (SD 1.4) and 0.95 (SD 1.6) for the bath additives and no bath additives groups, respectively, with a difference of –0.06 (95% CI –0.32 to 0.21). This difference indicated that there was no need to adjust for baseline resource use differences.
| Resource or service | Treatment group, mean (SD) | Difference, mean (95% CI) | |
|---|---|---|---|
| Bath additives | No bath additives | ||
| Baseline | n = 264 | n = 218 | |
| GP | 0.70 (1.1) | 0.75 (1.2) | –0.05 (–0.25 to 0.16) |
| Practice nurse | 0.06 (0.4) | 0.05 (0.3) | 0.02 (–0.05 to 0.09) |
| Paediatric dermatologist | 0.05 (0.2) | 0.04 (0.2) | 0.00 (–0.04 to 0.05) |
| Dermatology nurse | 0.01 (0.1) | 0.02 (0.2) | –0.01 (–0.04 to 0.01) |
| Paediatrics | 0.02 (0.2) | 0.01 (0.1) | 0.01 (–0.02 to 0.04) |
| Allergy clinic | 0.01 (0.1) | 0.01 (0.1) | –0.01 (–0.02 to 0.01) |
| Other outpatients | 0.00 (0.0) | 0.00 (0.1) | 0.00 (–0.01 to 0.00) |
| Out of hours | 0.01 (0.1) | 0.03 (0.2) | –0.02 (–0.06 to 0.01) |
| A&E | 0.00 (0.0) | 0.01 (0.2) | –0.01 (–0.03 to 0.00) |
| Dermatology admissions | 0.00 (0.0) | 0.00 (0.0) | 0.00 (0.00 to 0.00) |
| Other | 0.03 (0.3) | 0.02 (0.1) | 0.02 (–0.02 to 0.05) |
| Total number of consultations | 0.89 (1.4) | 0.95 (1.6) | –0.06 (–0.32 to 0.21) |
| 16 weeks | n = 236 | n = 194 | |
| GP | 0.40 (0.9) | 0.68 (1.3) | –0.29 (–0.50 to –0.08) |
| Practice nurse | 0.03 (0.2) | 0.01 (0.2) | 0.01 (–0.02 to 0.05) |
| Paediatric dermatologist | 0.05 (0.3) | 0.07 (0.4) | –0.02 (–0.08 to 0.05) |
| Dermatology nurse | 0.02 (0.1) | 0.01 (0.1) | 0.00 (–0.02 to 0.03) |
| Paediatrics | 0.01 (0.1) | 0.01 (0.1) | 0.00 (–0.02 to 0.02) |
| Allergy clinic | 0.01 (0.1) | 0.04 (0.3) | –0.04 (–0.07 to 0.00) |
| Other outpatients | 0.00 (0.0) | 0.00 (0.0) | 0.00 (0.00 to 0.00) |
| Out of hours | 0.01 (0.1) | 0.03 (0.2) | –0.02 (–0.05 to 0.01) |
| A&E | 0.01 (0.1) | 0.00 (0.0) | 0.01 (–0.01 to 0.02) |
| Dermatology admissions | 0.00 (0.0) | 0.00 (0.0) | 0.00 (0.00 to 0.00) |
| Other | 0.02 (0.1) | 0.03 (0.2) | –0.01 (–0.05 to 0.03) |
| Total number of consultations | 0.53 (1.2) | 0.88 (1.7) | –0.35 (–0.62 to –0.08) |
| 52 weeks | n = 203 | n = 184 | |
| GP | 1.19 (2.0) | 1.67 (2.4) | –0.48 (–0.92 to –0.05) |
| Practice nurse | 0.10 (0.6) | 0.04 (0.3) | 0.05 (–0.03 to 0.14) |
| Paediatric dermatologist | 0.18 (0.8) | 0.14 (0.8) | 0.03 (–0.13 to 0.20) |
| Dermatology nurse | 0.08 (0.7) | 0.06 (0.3) | 0.03 (–0.08 to 0.13) |
| Paediatrics | 0.02 (0.2) | 0.05 (0.3) | –0.03 (–0.08 to 0.02) |
| Allergy clinic | 0.03 (0.3) | 0.10 (0.6) | –0.07 (–0.15 to 0.02) |
| Other outpatients | 0.00 (0.1) | 0.02 (0.1) | –0.01 (–0.03 to 0.01) |
| Out of hours | 0.06 (0.4) | 0.06 (0.3) | 0.00 (–0.07 to 0.08) |
| A&E | 0.01 (0.1) | 0.00 (0.0) | 0.01 (–0.01 to 0.03) |
| Dermatology admissions | 0.00 (0.0) | 0.01 (0.1) | –0.01 (–0.02 to 0.00) |
| Other | 0.05 (0.3) | 0.18 (1.1) | –0.13 (–0.29 to 0.03) |
| Total number of consultations | 1.73 (3.1) | 2.32 (3.7) | –0.59 (–1.27 to 0.08) |
The mean number of primary and secondary care consultations at 16 weeks was 0.53 (SD 1.2) for the bath additives group and 0.88 (SD 1.7) for the no bath additives group, with a statistically significant difference of –0.35 (95% CI –0.62 to –0.08), indicating that fewer consultations were reported within the bath additives group. However, this statistically significant difference was not retained in the 52-week results (see Table 18).
Costs
Following valuation (see Table 17) of the resources used, Table 19 presents the costs estimated from the parent-reported CSRI, for baseline and 16- and 52-week follow-ups. The mean estimates for the bath additives group and the no bath additives group at 16 weeks were £26.52 (SD £74.4) and £47.42 (SD £116.6), respectively, showing a statistically significant difference of –£20.89 (95% CI –£39.13 to –£2.65), indicating a decrease in the bath additives group in the costs of primary and secondary care consultations. As with the resource use data, the statistical significance of the difference was not retained in the 52-week costs of primary and secondary care consultations, which showed a difference of –£28.38 (95% CI –£80.06 to –£23.30).
| Resource or service | Treatment group, mean (SD) | Difference, mean (95% CI) | |
|---|---|---|---|
| Bath additives | No bath additives | ||
| Baseline | n = 264 | n = 218 | |
| GP | 25.23 (39.2) | 26.92 (43.8) | –1.69 (–9.12 to 5.74) |
| Practice nurse | 0.85 (5.4) | 0.61 (4.4) | 0.24 (–0.65 to 1.14) |
| Paediatric dermatologist | 6.16 (32.8) | 5.59 (30.0) | 0.56 (–5.11 to 6.24) |
| Dermatology nurse | 0.98 (11.8) | 1.97 (12.9) | –1.00 (–3.21 to 1.22) |
| Paediatrics | 4.42 (41.3) | 1.78 (18.6) | 2.63 (–3.30 to 8.57) |
| Allergy clinic | 1.28 (14.7) | 2.32 (19.7) | –1.04 (–4.12 to 2.03) |
| Other outpatients | 0.00 (0.0) | 0.62 (9.1) | –0.62 (–1.72 to 0.49) |
| Out of hours | 1.02 (16.6) | 4.33 (30.1) | –3.31 (–7.57 to 0.94) |
| A&E | 0.00 (0.0) | 1.35 (14.8) | –1.35 (–3.14 to 0.44) |
| Dermatology admissions | 0.00 (0.0) | 0.00 (0.0) | 0.00 (0.0) |
| Other | 1.18 (15.4) | 1.53 (12.0) | –0.35 (–2.86 to 2.16) |
| Total costs of consultations | 41.11 (86.5) | 47.02 (100.1) | –5.92 (–22.61 to 10.78) |
| 16 weeks | n = 236 | n = 194 | |
| GP | 14.44 (33.1) | 24.74 (46.4) | –10.30 (–17.85 to –2.75) |
| Practice nurse | 0.37 (2.5) | 0.18 (2.5) | 0.19 (–0.29 to 0.67) |
| Paediatric dermatologist | 6.89 (41.9) | 9.31 (51.1) | –2.42 (–11.24 to 6.40) |
| Dermatology nurse | 1.46 (12.9) | 1.18 (11.6) | 0.28 (–2.07 to 2.62) |
| Paediatrics | 1.10 (16.9) | 1.34 (18.6) | –0.24 (–3.60 to 3.13) |
| Allergy clinic | 0.95 (14.6) | 6.96 (45.3) | –6.00 (–12.15 to 0.14) |
| Other outpatients | 0.00 (0.0) | 0.00 (0.0) | 0.00 (0.0) |
| Out of hours | 0.76 (11.7) | 3.71 (31.5) | –2.95 (–7.31 to 1.41) |
| A&E | 0.55 (8.5) | 0.00 (0.0) | 0.55 (–0.65 to 1.75) |
| Dermatology admissions | 0.00 (0.0) | 0.00 (0.0) | 0.00 (0.0) |
| Other | 0.00 (0.0) | 0.00 (0.0) | 0.00 (0.0) |
| Total costs of consultations | 26.52 (74.4) | 47.42 (116.6) | –20.89 (–39.13 to –2.65) |
| 52 weeks | n = 203 | n = 184 | |
| GP | 43.03 (70.9) | 60.33 (85.8) | –17.29 (–32.97 to –1.62) |
| Practice nurse | 1.28 (7.3) | 0.55 (3.4) | 0.73 (–0.43 to 1.89) |
| Paediatric dermatologist | 26.68 (117.2) | 19.38 (108.3) | 7.30 (–15.32 to 29.92) |
| Dermatology nurse | 7.20 (60.6) | 4.99 (24.6) | 2.22 (–7.20 to 11.63) |
| Paediatrics | 4.15 (47.4) | 9.86 (56.2) | –5.71 (–16.07 to 4.65) |
| Allergy clinic | 5.26 (44.3) | 16.50 (95.3) | –11.24 (–25.87 to 3.40) |
| Other outpatients | 0.67 (9.5) | 2.20 (17.1) | –1.54 (–4.27 to 1.20) |
| Out of hours | 8.20 (54.9) | 7.58 (43.6) | 0.62 (–9.35 to 10.59) |
| A&E | 1.13 (11.4) | 0.00 (0.0) | 1.13 (–0.53 to 2.78) |
| Dermatology admissions | 0.00 (0.0) | 5.37 (72.8) | –5.37 (–15.41 to 4.68) |
| Other | 0.85 (9.7) | 0.08 (1.1) | 0.77 (–0.64 to 2.18) |
| Total costs of consultations | 98.45 (235.1) | 126.83 (281.5) | –28.38 (–80.06 to 23.30) |
Resource use and costs source: general practitioner notes review
Resource use
Table 20 presents resource use data for eczema, recorded from the electronic GP notes review for the 52-week follow-up. As for the CSRI estimates, these estimates are resources used for eczema-related consultations. In the 52-week follow-up, participants in the bath additives group had a mean of 1.01 (SD 1.8) consultations and the no bath additives group had a mean of 1.43 (SD 2.8) consultations. The difference between the two groups, however, as seen in the data from the CSRI for the same period, was not statistically significant: –0.42 (95% CI –0.83 to 0.00) (see Table 20). The annual mean number of prescriptions related to eczema were 5.47 (SD 8.4) for the bath additives group and 6.40 (SD 8.6) for the no bath additives group, with a non-significant difference of –0.93 (95% CI –2.47 to 0.61). As expected, the number of prescriptions for bath additives showed a statistically significant difference of 3.55 (95% CI 2.99 to 4.10).
| Resource or service | Treatment group, mean (SD) | Difference, mean (95% CI) | |
|---|---|---|---|
| Bath additives (n = 261) | No bath additives (n = 214) | ||
| 52 weeks | |||
| GP | 0.54 (1.0) | 0.67 (1.2) | –0.13 (–0.33 to 0.07) |
| GP telephone call | 0.10 (0.4) | 0.13 (0.5) | –0.03 (–0.11 to 0.06) |
| Practice nurse | 0.11 (0.4) | 0.26 (0.9) | –0.15 (–0.27 to –0.03) |
| Walk-in centre/out of hours | 0.04 (0.2) | 0.08 (0.5) | –0.04 (–0.11 to 0.03) |
| Total primary care consultations | 0.79 (1.4) | 1.14 (2.0) | –0.35 (–0.66 to –0.05) |
| Allergy clinic | 0.01 (0.1) | 0.02 (0.1) | –0.01 (–0.03 to 0.01) |
| Child health | 0.00 (0.0) | 0.01 (0.2) | –0.01 (0.04 to 0.01) |
| Respiratory–asthma clinic | 0.00 (0.1) | 0.00 (0.0) | 0.00 (0.00 to 0.01) |
| Paediatric dermatologist | 0.15 (0.6) | 0.13 (0.7) | 0.02 (–0.10 to 0.14) |
| Dermatology nurse (specialist nurse) | 0.00 (0.1) | 0.02 (0.2) | –0.01 (–0.04 to 0.01) |
| Paediatrician | 0.02 (0.2) | 0.03 (0.2) | –0.01 (–0.04 to 0.03) |
| Dietitian | 0.01 (0.1) | 0.01 (0.1) | 0.00 (–0.02 to 0.02) |
| Out of hours | 0.00 (0.1) | 0.02 (0.1) | –0.01 (–0.03 to 0.00) |
| Eye unit | 0.00 (0.0) | 0.00 (0.1) | 0.00 (–0.01 to 0.00) |
| Health visitor | 0.00 (0.0) | 0.00 (0.1) | 0.00 (–0.01 to 0.00) |
| Other outpatients | 0.00 (0.0) | 0.00 (0.1) | 0.00 (–0.01 to 0.00) |
| Phototherapy | 0.00 (0.1) | 0.00 (0.0) | 0.00 (0.00 to 0.01) |
| Paediatric clinical immunology | 0.00 (0.1) | 0.00 (0.0) | 0.00 (0.00 to 0.01) |
| A&E | 0.00 (0.0) | 0.01 (0.2) | –0.01 (–0.03 to 0.00) |
| A&E by ambulance | 0.00 (0.0) | 0.00 (0.1) | 0.00 (–0.01 to 0.00) |
| Emergency | 0.00 (0.0) | 0.01 (0.1) | –0.01 (–0.03 to 0.01) |
| Dermatology admissions | 0.01 (0.1) | 0.00 (0.1) | 0.00 (–0.01 to 0.02) |
| Hospital nights (> 1) | 0.00 (0.1) | 0.00 (0.1) | 0.00 (–0.01 to 0.01) |
| Total secondary care consultations | 0.22 (0.8) | 0.29 (1.2) | –0.07 (–0.25 to 0.12) |
| Total number of consultations | 1.01 (1.8) | 1.43 (2.8) | –0.42 (–0.83 to 0.00) |
| Prescriptions related to eczema | 5.47 (8.4) | 6.40 (8.6) | –0.93 (–2.47 to 0.61) |
| Bath additives prescriptions | 3.96 (3.9) | 0.41 (1.6) | 3.55 (2.99 to 4.10) |
The primary and secondary care number of consultations for health issues other than eczema (see Table 20) showed very similar results, with a non-statistically significant difference of –0.69 (95% CI –1.59 to 0.22) between the two groups during the 52-week trial follow-up.
Costs
Following valuation of the resources used, Table 21 presents the costs at the 52-week follow-up. Similar results to CSRI data were obtained using the NR data, with the total costs of consultations estimated as £54.63 (SD £133.20) and £73.00 (SD £210.20) for the bath additives group and the no bath additives group, respectively, with a non-statistically significant difference of –£18.37 (–£49.57 to –£12.84). The difference between the two groups in cost of prescriptions for eczema was –£7.41 (–£19.72 to £4.91), whereas the difference in cost of medications for eczema was –£6.36 (–£15.91 to £3.19); none reached statistical significance. The results presented in Tables 18–25 have not been adjusted for potential baseline covariates.
| Resource or service | Treatment group, mean cost (£) (SD) | Difference, mean (95% CI) | |
|---|---|---|---|
| Bath additives (n = 261) | No bath additives (n = 214) | ||
| 52 weeks | |||
| GP | 17.70 (34.5) | 22.05 (38.6) | –4.35 (–10.95 to 2.25) |
| GP telephone call | 1.07 (4.2) | 1.35 (5.5) | –0.28 (–1.16 to 0.59) |
| Practice nurse | 1.47 (5.3) | 3.46 (11.9) | –1.99 (–3.60 to –0.37) |
| Walk-in centre/out of hours | 1.65 (8.3) | 3.42 (22.8) | –1.77 (–4.76 to 1.22) |
| Total primary care consultations | 21.89 (38.6) | 30.29 (54.7) | –8.40 (–16.84 to 0.05) |
| Allergy clinic | 1.94 (18.0) | 2.36 (19.9) | –0.43 (–3.85 to 2.99) |
| Child health | 0.00 (0.0) | 1.31 (19.1) | –1.31 (–3.64 to 1.02) |
| Respiratory–asthma clinic | 0.84 (13.5) | 1.02 (14.9) | –0.18 (–2.75 to 2.38) |
| Paediatric dermatologist | 20.49 (85.1) | 19.29 (107.7) | 1.20 (–16.18 to 18.58) |
| Dermatology nurse (specialist nurse) | 0.99 (11.9) | 2.41 (16.5) | –1.42 (–3.99 to 1.14) |
| Paediatrician | 4.37 (28.6) | 6.59 (50.5) | –2.23 (–9.47 to 5.02) |
| Dietitian | 0.55 (8.8) | 0.67 (6.9) | –0.12 (–1.57 to 1.33) |
| Out-of-hours emergency | 0.53 (8.5) | 3.87 (26.5) | –3.34 (–6.76 to 0.08) |
| Eye unit | 0.00 (0.0) | 0.45 (6.6) | –0.45 (–1.25 to 0.35) |
| Health visitor | 0.00 (0.0) | 0.25 (3.6) | –0.25 (–0.69 to 0.19) |
| Other outpatients | 0.00 (0.0) | 0.63 (9.2) | –0.63 (–1.75 to 0.49) |
| Phototherapy | 0.33 (5.3) | 0.00 (0.0) | 0.33 (–0.38 to 1.04) |
| Paediatric clinical immunology | 0.90 (14.6) | 0.00 (0.0) | 0.90 (–1.06 to 2.86) |
| A&E | 0.00 (0.0) | 2.06 (22.4) | –2.06 (–4.78 to 0.67) |
| A&E by ambulance | 0.00 (0.0) | 0.69 (10.0) | –0.69 (–1.91 to 0.53) |
| Dermatology admissions | 1.81 (20.6) | 1.10 (16.1) | 0.71 (–2.69 to 4.10) |
| Hospital nights (> 1) | 0.00 (0.1) | 0.00 (0.1) | 0.00 (–0.01 to 0.01) |
| Total secondary care consultations | 32.74 (114.8) | 42.71 (179.2) | –9.97 (–36.67 to 16.73) |
| Total costs of consultations | 54.63 (133.2) | 73.00 (210.2) | –18.37 (–49.57 to 12.84) |
| Prescriptions (any eczema, no bath additives) | 43.76 (66.9) | 51.17 (69.1) | –7.41 (–19.72 to 4.91) |
| Bath additives prescriptions | 31.66 (31.1) | 3.29 (12.8) | 28.37 (23.91 to 32.84) |
| Medications related to eczema | 30.28 (49.4) | 36.64 (56.3) | –6.36 (–15.91 to 3.19) |
| Bath additives | 20.22 (19.5) | 2.03 (7.6) | 18.19 (15.41 to 20.97) |
| Intervention costs | 51.88 (50.2) | 5.32 (20.4) | 46.56 (39.37 to 53.75) |
| Health system costs | 180.50 (237.0) | 166.12 (293.0) | 14.38 (–33.45 to 62.21) |
| FbCs | 90.93 (276.6) | 142.30 (390.1) | –51.37 (–118.49 to 15.74) |
| Total costs (health system and family-borne) | 281.78 (426.0) | 306.23 (513.9) | –24.45 (–119.06 to 70.17) |
Intervention costs
The intervention costs were estimated from the NR data and are presented in Table 22, for the 52-week follow-up. The intervention cost includes the actual prescription costs in addition to the cost of the bath additives. The total mean intervention cost for the bath additives group was £51.88 (SD £50.20). As stated in Resource use, a small number of participants in the no bath additives group received prescriptions for bath emollients and the mean cost of these was estimated to be £5.32 (SD £20.40). The cost of bath additives during the 52-week follow-up for the bath additives group was £20.22 (SD £19.50) and the cost of prescriptions for bath additives was £31.66 (SD £31.10).
| Resource or service | Treatment group, mean cost (£) (SD) | Difference, mean (95% CI) | |
|---|---|---|---|
| Bath additives (n = 261) | No bath additives (n = 214) | ||
| 52 weeks | |||
| GP | 1.48 (2.0) | 1.86 (2.8) | –0.38 (–0.81 to 0.06) |
| GP telephone call | 0.58 (1.2) | 0.68 (1.5) | –0.10 (–0.35 to 0.14) |
| Practice nurse | 0.82 (1.2) | 0.77 (1.3) | 0.05 (–0.18 to 0.27) |
| Walk-in centre, out of hours | 0.30 (0.6) | 0.43 (1.0) | –0.13 (–0.27 to 0.01) |
| Allergy clinic | 0.00 (0.1) | 0.01 (0.2) | –0.01 (–0.03 to 0.01) |
| Child health | 0.00 (0.1) | 0.00 (0.0) | 0.00 (0.00 to 0.01) |
| Asthma clinic | 0.00 (0.1) | 0.00 (0.0) | 0.00 (0.00 to 0.01) |
| Paediatric dermatologist | 0.01 (0.1) | 0.01 (0.1) | 0.00 (–0.02 to 0.01) |
| Paediatrician | 0.07 (0.4) | 0.08 (0.4) | –0.01 (–0.09 to 0.06) |
| Dietitian | 0.00 (0.0) | 0.00 (0.1) | 0.00 (–0.01 to 0.00) |
| Out-of-hours emergency | 0.06 (0.2) | 0.12 (0.6) | –0.06 (–0.14 to 0.01) |
| Eye casualty | 0.00 (0.1) | 0.00 (0.1) | 0.00 (–0.01 to 0.01) |
| Paediatric clinical immunology | 0.00 (0.1) | 0.00 (0.0) | 0.00 (0.00 to 0.01) |
| A&E | 0.06 (0.3) | 0.07 (0.3) | –0.01 (–0.07 to 0.05) |
| A&E by ambulance | 0.00 (0.0) | 0.00 (0.1) | 0.00 (–0.01 to 0.00) |
| Emergency | 0.00 (0.0) | 0.01 (0.1) | –0.01 (–0.02 to 0.00) |
| Dermatology admissions | 0.04 (0.2) | 3.74 (4.8) | 0.00 (–0.04 to 0.03) |
| Paediatric admissions | 0.00 (0.1) | 0.00 (0.1) | 0.00 (–0.01 to 0.01) |
| Hospital nights (> 1) | 0.06 (0.3) | 0.00 (0.0) | –0.01 (–0.07 to 0.05) |
| Other outpatients | 0.71 (1.3) | 0.68 (1.2) | 0.03 (–0.19 to 0.25) |
| Total number of non-eczema consultations | 4.14 (4.0) | 4.75 (5.5) | –0.61 (–1.47 to 0.25) |
Family-borne costs
Table 23 presents estimates of the FbCs as reported by parents within the CSRI questionnaire. The FbCs were estimated at baseline and at 16-week and 52-week follow-up, but none of the differences assessed at each time period reached statistical significance. Overall, parents within the no bath additives group annually spent £51.37 (95% CI –£118.49 to £15.74) more on household items for their child’s eczema than parents within the bath additives group (see Table 23), but this difference was not statistically significant.
| Household itemsa | Treatment group, mean cost (£) (SD) | Difference, mean (95% CI) | |
|---|---|---|---|
| Bath additives (n = 264) | No bath additives (n = 218) | ||
| Baseline | |||
| Clothes | 7.09 (63.3) | 4.45 (15.5) | 2.64 (–5.99 to 11.27) |
| Food | 4.13 (16.9) | 5.39 (19.4) | –1.26 (–4.51 to 1.99) |
| Over-the-counter products | 7.15 (15.7) | 9.02 (19.9) | –1.87 (–5.06 to 1.31) |
| Laundry | 7.64 (20.5) | 8.47 (18.2) | –0.83 (–4.33 to 2.68) |
| Equipment | 1.92 (12.8) | 0.83 (12.2) | 1.09 (–1.15 to 3.34) |
| Travel costs | 0.78 (6.6) | 0.66 (3.3) | 0.12 (–0.84 to 1.08) |
| Complementary medicine | 2.56 (37.0) | 0.47 (3.4) | 2.10 (–2.84 to 7.04) |
| Other | 2.27 (36.9) | 0.07 (0.8) | 2.20 (–2.72 to 7.12) |
| Total family-borne extra costs | 33.54 (105.6) | 29.35 (49.5) | 4.19 (–11.09 to 19.47) |
| 16 weeks | n = 236 | n = 194 | |
| Clothes | 3.42 (11.5) | 4.38 (16.7) | –0.96 (–3.64 to 1.72) |
| Food | 2.60 (10.1) | 4.19 (20.4) | –1.59 (–4.56 to 1.39) |
| Over-the-counter products | 6.23 (14.5) | 7.35 (17.2) | –1.12 (–4.13 to 1.89) |
| Laundry | 4.98 (11.7) | 8.01 (19.0) | –3.03 (–5.97 to –0.09) |
| Equipment | 0.71 (5.6) | 0.86 (5.7) | –0.15 (–1.23 to 0.92) |
| Travel costs | 0.85 (8.9) | 0.49 (2.9) | 0.35 (–0.96 to 1.67) |
| Complementary medicine | 6.32 (95.5) | 0.93 (6.5) | 5.39 (–8.11 to 18.89) |
| Other | 0.05 (0.6) | 0.23 (2.2) | –0.18 (–0.46 to 0.11) |
| Total family-borne extra costs | 25.15 (112.6) | 26.44 (52.7) | –1.29 (–18.55 to 15.97) |
| 52 weeks | n = 203 | n = 184 | |
| Clothes | 11.58 (29.8) | 22.15 (99.1) | –10.57 (–24.90 to 3.76) |
| Food | 12.86 (45.6) | 26.57 (112.5) | –13.72 (–30.59 to 3.15) |
| Laundry | 20.11 (45.3) | 28.70 (73.3) | –15.86 (–35.98 to 4.27) |
| Over-the-counter products | 23.23 (42.3) | 39.09 (138.9) | –8.59 (–20.64 to 3.46) |
| Equipment | 2.46 (12.8) | 6.99 (47.5) | –4.53 (–11.33 to 2.28) |
| Travel costs | 2.87 (21.7) | 4.10 (20.8) | –1.24 (–5.49 to 3.02) |
| Complementary medicine | 8.44 (110.4) | 7.93 (48.7) | 0.51 (–16.84 to 17.86) |
| Other | 9.24 (98.4) | 6.76 (53.0) | 2.48 (–13.55 to 18.51) |
| Total family-borne extra costs | 90.93 (276.6) | 142.30 (390.1) | –51.37 (–118.49 to 15.74) |
Figure 10 highlights the skewed distribution of FbCs, showing that this difference is to a great extent driven by outliers. When these outliers were normalised by assigning mean values to them, the mean difference of the FbCs was reduced to –£40.28 (–£78.78 to –£1.78). Figure 11 shows the normalised data, highlighting the similarities between the two groups.
Days lost from school for children and days lost from work for parents because of eczema
Table 24 shows days lost from school or nursery for children and days lost from work for parents because of their child’s eczema. Overall, there is no statistically significant difference between the two groups at any time point, including baseline.
| Days lost | Treatment group | Difference, mean (95% CI) | |||
|---|---|---|---|---|---|
| Bath additives | No bath additives | ||||
| n | Mean (SD) | n | Mean (SD) | ||
| From school/nursery | |||||
| Baseline | 264 | 0.36 (1.6) | 218 | 0.19 (0.9) | 0.17 (–0.07 to 0.40) |
| Week 16 | 236 | 0.25 (0.9) | 194 | 0.17 (0.9) | 0.07 (–0.10 to 0.25) |
| Week 52 | 210 | 0.67 (2.0) | 189 | 0.51 (2.2) | 0.16 (–0.25 to 0.58) |
| From work (parents) | |||||
| Baseline to week 16 | 264 | 0.51 (3.0) | 218 | 0.43 (1.7) | 0.07 (–0.38 to 0.52) |
| Week 16 to week 52 | 236 | 0.17 (0.8) | 194 | 0.14 (0.7) | 0.03 (–0.12 to 0.18) |
| 52-week period | 210 | 0.71 (2.3) | 189 | 0.47 (1.7) | 0.24 (–0.16 to 0.64) |
Health-related quality of life, health profiles, utility scores and quality-adjusted life-years
Table 25 summarises the mean utility values generated using the data from the preference-based QoL measure CHU-9D at baseline and at 16 weeks and 52 weeks. There was no difference between the two groups at baseline and minimal differences in opposite directions at 16 weeks and 52 weeks. The 52-week mean QALY per participant was 0.90 (SD 0.1) for the bath additives group and 0.91 (SD 0.1) for the no bath additives group. Similarly, there was no significant difference detected at the 16-week follow-up. Therefore, in line with primary outcome POEM, both the utility values and the QALYs are extremely similar in both groups, indicating no effect of bath additives on HRQoL.
| QoL outcomes | Treatment group | Difference, mean (95% CI) | |||
|---|---|---|---|---|---|
| Bath additives | No bath additives | ||||
| n | Mean (SD) | n | Mean (SD) | ||
| Utility values (CHU-9D) | |||||
| Baseline | 264 | 0.90 (0.1) | 218 | 0.90 (0.1) | 0.00 (–0.02 to 0.02) |
| Week 16 | 211 | 0.91 (0.1) | 173 | 0.89 (0.1) | 0.01 (–0.01 to 0.03) |
| Week 52 | 177 | 0.90 (0.1) | 150 | 0.91 (0.1) | –0.01 (–0.03 to 0.01) |
| QALYs | |||||
| Baseline to week 16 | 211 | 0.30 (0.0) | 173 | 0.30 (0.0) | 0.00 (0.00 to 0.01) |
| Week 16 to week 52 | 174 | 0.61 (0.1) | 147 | 0.60 (0.1) | 0.00 (–0.01 to 0.01) |
| 52-week period | 174 | 0.91 (0.1) | 147 | 0.90 (0.1) | 0.00 (–0.01 to 0.02) |
Analysis
Table 26 presents differences between groups for costs and QALYs: (1) controlling for centre and (2) adjusted for baseline severity (POEM) and allowing for the clustering of patients within centre. Both the unadjusted (Table 25 and see Tables 20 and 22) the adjusted results (Table 26) present no statistically significant differences between the two groups. This was the case using both data sources for costs. The cost difference between the data sources reported here could be attributable to parents’ different perspective classifying resources used as opposed to GP records (eczema related vs. other health issues).
| Outcomes | n | Difference, mean (95% CI) | |
|---|---|---|---|
| 1a | 2b | ||
| 16 weeks | |||
| HRQoL | |||
| QALYs | 384 | 0.00 (0.00 to 0.01) | 0.00 (0.00 to 0.00) |
| Costs (£) | |||
| HsC – CSRI | 430 | –22.57 (–40.66 to –4.47) | –20.80 (–38.64 to –2.95) |
| FbC – CSRI | 430 | –1.53 (–18.74 to 15.69) | 0.15 (–16.83 to 17.14) |
| 52 weeks | |||
| HRQoL | |||
| QALYs | 321 | 0.00 (–0.01 to 0.02) | 0.00 (–0.02 to 0.02) |
| Costs (£) | |||
| HsC – CSRI | 387 | –33.50 (–84.48 to 17.49) | –28.85 (–78.58 to 20.88) |
| FbC – CSRI | 387 | –52.36 (–118.99 to 14.27) | –47.56 (–113.19 to 18.07) |
| Total health system costs – NR (£) | 474 | 11.87 (–35.62 to 59.35) | 19.18 (–26.33 to 64.70) |
Table 27 presents a summary of the key findings of the economic evaluation.
| Key findings at 52-week follow-up | Treatment group | Difference, mean (95% CI) | |||
|---|---|---|---|---|---|
| Bath additives | No bath additives | ||||
| n | Mean (SD) | n | Mean (SD) | ||
| Full sample | |||||
| HsC (£)a | 260 | 180.50 (237.0) | 214 | 166.12 (293.0) | 14.38 (–33.45 to 62.21) |
| QALYs | 174 | 0.91 (0.1) | 147 | 0.90 (0.1) | 0.00 (–0.01 to 0.02) |
| Children < 5 years old | |||||
| HsC (£)a | 132 | 190.58 (247.0) | 118 | 188.55 (306.0) | 2.03 (–66.94 to 71.0) |
| QALYs | 88 | 0.91 (0.1) | 73 | 0.90 (0.1) | 0.01 (–0.01 to 0.03) |
| Children > 5 years old | |||||
| HsC (£)a | 128 | 170.11 (226.6) | 96 | 138.55 (275.2) | 31.56 (–34.58 to 97.69) |
| QALYs | 86 | 0.90 (0.1) | 74 | 0.90 (0.1) | 0.00 (–0.03 to 0.02) |
For the purpose of the analysis that follows, the NR data source acted as the reference data source. Table 27 presents the key findings for the full sample. However, because of uncertainties in measuring QoL for very young children, we have also presented our results according to whether participants were younger or older than 5 years. This was done in order to avoid any measurement issues affecting the very young group diluting the results of the older children group. Although potential limitations measuring QoL for very young children still remain, our results (QALYs) show that there were no different conclusions to draw.
Our sample was not powered to detect subgroup differences; however, in the form of the exploratory analysis that we report, Table 28 shows that the most important factors determining costs and QALYs in children with eczema are the severity of their symptoms and their age, with younger children incurring higher costs. In our 52-week results, severity of symptoms was associated with increased costs up to £203 per year for the most severe cases. Similarly, severity of symptoms was associated with reduced QALYs from 0.09 for the most severe symptoms to 0.04 for the less severe symptoms. The results from our exploratory analysis should be interpreted with caution, but we consider them indicative.
| Explanatory variable | Cost, adjusted mean (95% CI); p-value | 52-week QALYs; adjusted mean (95% CI); p-value (n = 321) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 16 weeks (n = 430) | 52 weeks (n = 474) | ||||||||
| Bath additives group | –18.11 (–36.12 to –0.09); 0.049 | 25.16 (–21.16 to 71.48); 0.287 | 0.00 (–0.02 to 0.01); 0.878 | ||||||
| Effect of other explanatory variables | |||||||||
| Severity of eczemaa | |||||||||
| Mild | – | – | – | – | – | – | – | – | – |
| Moderate | 14.03 (–5.23 to 33.29); 0.153 | 72.70 (23.09 to 122.31); 0.004 | –0.04 (–0.06 to –0.02); 0.000 | ||||||
| Severe | 36.19 (7.42 to 64.96); 0.014 | 202.56 (128.49 to 276.62); 0.000 | –0.09 (–0.11 to –0.06); 0.000 | ||||||
| Age category | |||||||||
| < 5 years old | 24.02 (–41.90 to –6.15); 0.008 | 41.63 (–87.72 to 4.46); 0.077 | 0.00 (–0.02 to 0.02); 0.901 | ||||||
| Constant | 40.11 (30.97 to 97.29); 0.021 | 51.90 (8.87 to 178.19); 0.245 | 0.94 (0.91 to 0.97); 0.000 | ||||||
Implications of the results
We aimed to assess whether or not bath additives, when used in addition to standard management versus standard management alone, could provide a cost-effective treatment option. The economic study shows that in children with eczema, the use of bath additives does not provide any additional economic or otherwise benefit. Therefore, bath additives cannot be considered value for money for the NHS. Given the amount spent annually on bath additives, this has important implications for the NHS and decision-makers alike.
Strengths and weaknesses
A strength of this economic study is the use of alternative data sources estimating the health system costs. This allowed us to present a comprehensive resource use profile for children with eczema. We also consider the pragmatic nature of this trial, which reinforces the external validity of our results, to be a strength.
The broad spectrum of the age of the children included in our trial is a limitation when assessing QoL, especially as there are no suitable measures to assess the QoL of very young children. Although we cannot eliminate this limitation, we have reported our results for the full sample alongside the two different groups (< 5 years/> 5 years) to avoid diluting the conclusions drawn by presenting only the full sample, which includes the very young age group and introduces additional uncertainty due to measurement limitations.
Conclusions
Given the finding of no clinical effectiveness of the intervention in this study, it could be argued that a full cost-effectiveness analysis has nothing to offer in addition to what is already presented in this chapter. We have, however, presented our results at length adopting a cost–consequences analysis format, in line with our HEAP. During this trial, we collected comprehensive data for both the costs and QoL, and we believe that these data present a valuable source of information for future studies and decision-making.
Comparing the alternative data sources, the difference between primary and secondary care consultations reported by parents (CSRI) and recorded within the GP electronic records (NR) perhaps shows the different perceptions classifying consultations as being for eczema or not, and this raises important questions for future economic studies. By comparing the two data sources, we also validated our assumption that missing data were missing at random.
Given that emollient bath additives account for more than one-third of the total costs of treating eczema in childhood,9,13 we believe that the relevance of our economic results is essential.
Chapter 5 Discussion
Main findings
This trial found no evidence of any clinical benefit or cost-effectiveness of adding bath additive to the standard management of eczema in children aged between 12 months and 12 years. After controlling for baseline variables, the weekly POEM score over 16 weeks in the no bath additive group was 0.41 points higher than in the bath additives group (95% CI –0.27 to 1.10) on a scale of 0 (clear) to 28 (most severe). The narrow 95% CIs suggest that a clinically important treatment effect is unlikely to have been missed and is well within the meaningful difference of 2.0 between groups that we aimed to detect.
Although not powered to look at subgroups, prespecified exploratory subgroup analyses explored features that could plausibly modify the effectiveness of bath additives including age, baseline severity, use of leave-on emollient, use of TCSs/TCIs, use of soap substitute, frequency of bathing and prior belief in bath emollient. Although most of these showed no significant differences between groups, we could not exclude the possibility that younger children or children bathing ≥ 5 times per week might experience very modest benefit [1.29 (95% CI 0.33 to 2.25) and 2.27 (95% CI 0.63 to 3.91), respectively]. Although the published minimal clinically important difference for POEM is now widely regarded as being 3.0,41,42 at the trial design stage we based our sample size calculation on a difference of 2.0, given that this could be regarded as meaningful for an inexpensive intervention in a population with mild eczema.
Primary analyses were carried out on an ITT basis. We carried out a per-protocol analysis in addition to this in order to explore the effect of adherence to randomised treatment allocation. This allowed an estimate of effectiveness for children whose carers reported that they had regularly used bath additives rather than the effectiveness of being allocated to the bath additive group; this also showed no statistically significant difference between groups.
Relevance to existing literature
Previous systematic reviews have noted the sparse randomised trial evidence for bath additives and have been unable to draw conclusions regarding their effectiveness. 9 Current prescribing guidelines vary, but a recent analysis of 216 formularies in England and Wales showed that 68% recommend their use. 22 This is the largest trial to date exploring the effectiveness of these widely used products.
In a large case series (post-marketing surveillance) of 3566 people,62 most of whom had eczema and were aged ≤ 5 years, the tolerability of the bath additive was stated to be ‘good’ or ‘very good’ for 96.8% of participants. However, few data were presented on other treatments used and the absence of an experimental design meant that the effectiveness of the treatment cannot be inferred.
Although the addition of antiseptics to emollients is used by some for the prevention of infected eczema, there is no robust evidence for this. 63 There are some reports that irritant reactions are more frequent in individuals treated with bath additives with added antiseptics. 20 The trial reported here supports the finding that most bath additives are well tolerated but did not explore the effectiveness or tolerability of bath additives containing antiseptics/antimicrobials or antipruritics.
Strengths
This was an adequately powered randomised controlled trial, with high follow-up rates and good adherence to randomised trial allocations.
As an unblinded trial with a participant-reported measure as the primary outcome, there was a risk of bias in favour of the trial intervention, which is widely prescribed in childhood eczema in the UK. However, a convincing placebo is not possible for emollient bath additives and we wished to design a trial with a clinical outcome relevant to participants. As bath additives are a relatively inexpensive and widely used intervention, we felt that the risk of expectation of benefit leading to systematic bias in outcome reporting was less problematic. Having made this design decision, the finding of no significant difference between groups gives additional reassurance that we are very unlikely to be missing a meaningful benefit from the use of bath additives in childhood eczema.
Limitations
Adherence to allocation group
This trial aimed to test only the effectiveness of adding bath additives to bath water and aimed for no difference in soap use between groups. Standard advice for both groups was to use a leave-on emollient as a soap substitute. When parents/carers were keen to use an existing wash product, we advised that direct application to skin constituted use of the product as a soap substitute and this was compatible with their child entering the trial. However, if they wished to add an emollient or other bath additive product to bath water then they were not eligible to take part.
Self-report and NR data both suggest that most people adhered to this treatment allocation, but there are limitations in the strength of both of these data sources as participants may have potentially misreported. In addition, for NR data, receiving a prescription does not equate to use of a product and not receiving a prescription does not necessarily mean not using the product, given that bath additives are available to purchase over the counter. However, parents/carers were encouraged to report difficulties with allocation openly and we have no reason to believe that there was misreporting. Furthermore, to use bath additives regularly requires obtaining repeat prescriptions, which most participants in the bath additive group did. Purchasing bath additives over the counter is relatively unusual for children as all NHS prescriptions for under-16-year-olds are free in the UK.
Imbalance in group sizes at baseline
We used simple randomisation in this trial, stratified by centre. Simple randomisation preserves allocation concealment, which is often better than stratified randomisation and is less subject to technical errors. 64 Although achieving balance on key covariates may seem appealing, studies have shown that it adds little in terms of statistical efficiency to the approach taken in this study of adjusting in the analysis. 65,66
Although simple randomisation can result in imbalances in the numbers recruited to each group, in a large trial, such as BATHE, the overall balance between groups should be preserved. The baseline characteristics showed that, although there were slightly more participants allocated to the bath additive group than the no bath additive group, the key characteristics were well balanced.
Participant-reported outcome measure: specific limitations
Given the young age group, the primary outcome (POEM) was recorded by proxy by parents/carers, although they were encouraged to involve children where possible. POEM is well validated for use by patient or by proxy (carer report). 29 POEM is recommended by NICE9 and the international HOME initiative. 30
Generalisability
Participants were recruited through primary care in southern England, the west of England and in Wales. As > 90% of eczema is managed in primary care in the UK, these findings would be applicable to most children with eczema. The response rate to letters of invitation from practices, although in keeping with similar studies,67 was relatively low, suggesting that participating families may have been particularly motivated to be involved in research. However, of those who replied that they did not wish to participate, by far the most common reason for this was that their child’s eczema was no longer a problem. It seems likely that many who did not respond would have not returned the reply slip for this reason.
Emollient bath additives are popular eczema treatments with some parents/carers and some prescribers in countries where baths, as opposed to showers, are the norm, although there is international lack of consensus about their role (Professor Masutaka Furue, Kyushu University, Japan, 13 October 2017; Dr Roberto Takaoka, University of São Paulo, Brazil, 12 October 2017; and Professor Eric Simpson, Oregon Health and Science University, USA, 12 October 2017; personal communication).
Chapter 6 Conclusions
Implications for health care
This trial provides useful information for parents/carers who are seeking to manage their child’s eczema. Findings from this trial indicate that parents/carers can be advised that adding bath additive to bath water is unlikely to provide additional benefit over standard therapy in childhood eczema. Although we cannot completely exclude the possibility that children aged < 5 years who bath frequently might benefit, this is unlikely to be a clinically meaningful benefit.
Prescribers and policy-makers have already started to look at limiting the use of emollient bath additives in some Clinical Commissioning Group (CCG) areas as a potential cost saving, although an analysis of CCGs (England) and Local Health Boards (Wales) in 2016–17 showed that 68% still recommended the use of bath additives. 22 The findings of this trial would support the suggestion that adding emollients to bath water has no benefit, or minimal benefit at best, and prescribing budgets and parental energies may be best spent on more effective treatments. However, emollient products may be used for more than one purpose and some emollients marketed as bath additives are used as soap substitutes. Our study has not addressed the question of whether or not ‘bath additives’ are effective as soap substitutes, but it has found that advising parents/carers that pouring bath additives into bath water is not effective.
Anecdotal and qualitative evidence (Santer et al. 68 and Dr Miriam Santer, University of Southampton, 2015, personal communication) suggests that some families use emollient bath additives as an alternative to leave-on emollients as a response to child resistance. As there is good evidence for the effectiveness of leave-on emollients,69 our findings suggest that families may be turning to an ineffective treatment (bath additives) because of barriers experienced in adhering to an effective eczema treatment (leave-on emollients). Barriers to regular application of topical treatments, such as leave-on emollients, include the time-consuming nature of such treatments and child resistance. 68 Some strategies to overcome barriers, such as involving the child in treatment, allowing them to choose an emollient that they like, distracting the child during treatment or using rewards, are likely to be more helpful than others, such as physically restraining the child or reducing the frequency of applications. 68 Promoting positive strategies to facilitate regular topical treatment use seems more likely to be helpful than using emollient bath additives as an alternative to leave-on emollients.
It is essential to highlight to prescribers and policy-makers that, although this trial found that pouring emollient bath additives into bath water was not beneficial, the role of emollients as soap substitutes or leave-on emollients has not been explored. Both soap substitutes and leave-on emollients have a much stronger a priori rationale for effectiveness than emollient bath additives and greater clinical consensus around their effectiveness, even though there are relatively few large trials in this area. 69 It is therefore important that policy-makers do not conflate the findings of this study, which relates to a specific method of delivering emollients to the skin via bath water, with the use of emollients as leave-on preparations or as soap substitutes as recommended by the NICE guideline on eczema in children. 9
Recommendations for research
Some participants in this trial were using emollient bath additives as soap substitutes and further research is needed to explore what the best soap substitute is for use in eczema.
Several questions around emollients and washing in eczema that were highlighted in the James Lind Alliance PSP34 remain outstanding, in particular the shared priority from patients and health-care professionals, ‘which emollient is the most effective and safe in treating eczema?’. This is currently being addressed by the NIHR HTA-funded Best Emollient for Eczema (BEE) study (15/130/07). The priority from patients and carers, namely ‘what is the best way for people with eczema to wash: frequency of washing, water temperatures, bath versus shower?’ remains unanswered. The priority from health-care professionals, namely ‘how effective are interventions to reduce skin infections in the management of eczema?’, is also related to the question of bathing and also remains unanswered.
Chapter 7 Patient and public involvement
In line with other NIHR research, patient and public involvement (PPI) was seen as a key element of the research from the outset. However, the PPI focus was secondary to the primary question of the effectiveness of bath additives and the Guidance for Reporting Involvement of Patients and the Public 2 short form checklist70 are therefore used for reporting PPI activities rather than the Guidance for Reporting Involvement of Patients and the Public long form (see Table 29). 71
| Section and topic | Item | Reported in section |
|---|---|---|
| Aim | The aim of PPI in this study was to ensure that all aspects of trial design were acceptable to patients and parents/carers and to maximise dissemination of findings | See Topic prioritisation |
| The study question had been prioritised by the NIHR HTA programme prior to funding | ||
| Methods | Amanda Roberts, an experienced PPI co-applicant, was involved in all stages of trial design, management and interpretation | See details in Trial design and Trial conduct |
| We consulted the CEBD Patient Panel at the study design stage and during recruitment and will feed back findings to this group | ||
| We carried out an online survey through social media at the study design stage, including > 200 parents/carers | ||
| We invited additional patient representatives (and paid travel expenses) to the end-of-study meeting for presentation and discussion of study findings. This included representatives of national patient advocacy groups (NSGCCE, NES, Eczema Outreach Scotland) | ||
| Study results | We maintained good relationships with the PPI co-applicant and wider PPI links. Questions remain from some in the wider eczema community about why this trial was prioritised. All involved in the trial are keen that the findings should not be used to limit choice for people with eczema and their families around emollient prescriptions | See End-of-study meeting |
| Discussion and conclusions | We maintained good rates of recruitment and very good rates of retention in the trial and believe that this is related to the focus on keeping trial procedures as easy as possible for participating families, assisted by advice from PPI | See Discussion and conclusions |
| Reflections/critical perspective | The study went well but the dissemination and reception of findings will be crucial to its impact and we are at the very early stages of this process. However, the experience of our PPI co-applicant and other PPI input means that we hope to be in a strong position for anticipating and mitigating against potential negative impacts from the dissemination of study findings | – |
Topic prioritisation
This trial was funded by the NIHR HTA programme as the result of a commissioned call. The research topic had been suggested through the NIHR HTA website topic suggestion form. The NIHR HTA programme has PPI embedded in its prioritisation processes and Boards.
The James Lind Alliance PSP for Eczema34 published its top 10 priority topics in 2012. Even though this call was not advertised directly as a result of the PSP, it addresses issues that patients, carers and clinicians highlighted as an outcome of the PSP, including priorities around bathing/washing and also around the best ways to use emollients.
Patient and public involvement co-applicant
Amanda Roberts has eczema, has two (grown-up) children with eczema and is an experienced PPI representative on a number of bodies, including the Centre for Evidence-Based Dermatology (CEBD) Patient Panel. Amanda is in contact with many carers of children with eczema through running the Nottingham Support Group for Carers of Children with Eczema (NSGCCE).
Trial design
The trial design benefited from having an experienced PPI representative as a member of our research team. Amanda Roberts contributed at all stages of trial design, including joining trial development telephone conferences, being copied in on all correspondence and drafts. Furthermore, between outline and full grant application, we carried out a workshop with members of the CEBD Patient Panel and carried out an online survey, including questions about information that parents/carers would like to receive in the trial information sheets and willingness to take part in the proposed trial. These provided useful feedback, although participants in both the survey and the workshop were typically caring for children with moderate or severe eczema, so it was noted at the time that, as the majority of study participants would have mild eczema, there was a possibility that their carers might hold different views.
We found that 33% (67 out of 203) of survey respondents would not be happy to participate if allocated to the ‘usual-care’ group and 9.3% (19 out of 204) would not wish to participate if allocated to the bath additive group. Two workshop members also had reservations about randomisation, one holding a strong view that one particular bath additive helped her child’s eczema and another who felt that bath additives did not help her child’s eczema.
These concerns highlighted the need for careful development of participant information leaflets, in consultation with patient/parent support groups, to communicate clinical equipoise about the benefits of bath additives. These concerns also confirmed the need for collection of ‘prior belief’ in bath additive prior to recruitment, as well as to ask invited participants if they would share reasons for declining to participate, so that we could seek to measure to what extent these concerns influenced recruitment.
Focus group members and survey respondents were also concerned about what would happen if their child’s eczema deteriorated while they were in the study. Robust discussion and SOPs around consent processes were developed to ensure that carers were prepared to be randomised to either group. However, it was also made clear to parents/carers that they would receive all other treatment as usual, remain free to consult their usual clinical team as needed and could change bath additive if necessary. When these measures were insufficient, they could, of course, withdraw from the study.
We found that the duration of the study was perceived as a barrier by some completing the survey, supporting our decision to use weekly measures to 16 weeks as our primary outcome rather than 12 months. Furthermore, we found that a small proportion were not using baths, regardless of age, so we included bath use of at least once a week as an eligibility criterion.
Trial conduct
Amanda Roberts attended the team training day in 2014 and gave a presentation on patient/parent/carer perspectives to all trial staff. She attended further face-to-face meetings on trial conduct in 2016 and 2017.
Study materials: Amanda Roberts and members of the CEBD Patient Panel were involved in reviewing and revising the patient information sheet, child information sheet, consent form (see Appendix 12), assent form (see Appendix 13) and invitation letter.
Newsletters: Amanda Roberts was involved in providing content for quarterly newsletters to participants’ families and reviewing the newsletters prior to them being circulated.
After the first few months of recruitment, we found that we were getting relatively low response rates. We therefore discussed recruitment at a CEBD PPI meeting in March 2015. Attendees responded that the patient information sheet was too dense and unfriendly. Using this feedback, a short and colourful summary leaflet was designed and included in the invitation pack from July 2015, but we were unable to detect any change in the response rate as a result of the additional information.
The independent Trial Steering Committee, appointed by the NIHR HTA programme, included independent PPI representative Rosemary Humphries.
End-of-study meeting for presentation and discussion on interpretation of findings
We invited additional patient representatives to the end-of-study discussion of findings, including representatives of national patient advocacy groups (NSGCCE, National Eczema Society, Eczema Outreach Scotland). Attendees included Amanda Roberts (PPI co-applicant), another member of the CEBD Patient Panel (a mother of children with severe eczema), the Chief Executive and Director of Services of the National Eczema Society and the PPI member of the BATHE Trial Steering Committee.
Patient representatives raised concerns that the trial results might be used to justify removing bath emollients from prescribing formularies and that some families rely on bath emollients as soap substitutes. Others acknowledged that understanding the likely effectiveness of these products was potentially useful for parents trying to work out how best to ‘live with eczema’. The study team highlighted that the trial design was more likely to result in a false-positive result than a false-negative result, as it was an open trial with a participant-reported primary outcome measure (and was thus open to expectation bias). All felt that it was better for prescribers and commissioners to make prescribing decisions based on high-quality evidence, although they would not wish to see the use of emollient soap substitutes compromised through misinterpretation of trial findings.
The context of these concerns is that CCG formularies are increasingly restricting the availability of emollients in general, and bath additives in particular, and the National Eczema Society reports that patients are having problems accessing preferred products. Others were also concerned that there is an underlying drive to remove all emollients from the prescribing guidelines, which would leave patients and carers in a difficult position and potentially lead to more flare-ups and greater service use. It was agreed that we need to be clear in all communications that emollients should still be available and that this study suggests only that pouring them into the bath does not offer benefit. All agreed that using the term ‘bath additives’ rather than ‘bath emollients’ might avert some confusion.
Discussion and conclusions
Amanda Roberts was an integral member of the team from the first decision to respond to the call and was an enthusiastic, reliable and inspiring member of the team in terms of maintaining the focus of the study on providing answers for parents/carers on one of the key questions in eczema care. Her input was invaluable.
The following quotation illustrates her perspective on her involvement:
My take on what I contributed to the trial was this: everyone on the team was enthusiastic about bearing in mind the needs of the patients and carers – but they had other hats. I reminded people in telephone conferences things – like producing the newsletters etc. – that had been perhaps lost sight of in all the business of the trial.
Input from the CEBD Patient Panel was useful at a number of points, but the other aspect of PPI input that was particularly valuable was obtaining feedback on results from a wide range of stakeholders, particularly PPI, at the end-of-study meeting for discussion of interpretation of findings. This prepared and briefed us for report writing, identifying early on how to communicate messages about trial findings and preparing wider materials for dissemination with trial participants, parents/carers of children with eczema and clinical and commissioning communities.
Acknowledgements
We are very grateful to all the families who took part in the BATHE trial and for their enthusiasm and commitment to finding out the best ways to treat childhood eczema. We would also like to thank all the staff at the participating practices without whom research in the NHS would be impossible. Thanks also go to the staff of the local CRNs for facilitating the recruitment of practices and the delivery of this research.
We would like to thank the LifeGuide team, particularly Mary Steele and Lucy Yardley, Jo Musgrove at Southampton CTU, iSolutions IT support at the University of Southampton and the Sponsor at the University of Southampton.
We would like to thank the NIHR HTA programme for funding this trial and for ongoing support and advice.
Contributions of authors
Miriam Santer (Associate Professor in Primary Care Research) contributed to the conception and design of the study, trial oversight (as chief investigator) and interpretation of the data and drafted and critically reviewed the report.
Kate Rumsby (Clinical Trial Manager) contributed to the design of the study, managed the trial, contributed to interpretation of the data and drafted and critically reviewed the report.
Matthew J Ridd (Consultant Senior Lecturer in Primary Health Care) contributed to the conception and design of the study, was responsible for oversight of the trial at the Bristol centre (as principal investigator), contributed to interpretation of the data and critically reviewed the report.
Nick A Francis (Senior Clinical Research Fellow) contributed to the design of the study, was responsible for oversight of the trial at the Cardiff centre (as principal investigator), contributed to interpretation of the data and critically reviewed the report.
Beth Stuart (Senior Research Fellow and Medical Statistician) contributed to the conception and design of the study, was responsible for the statistical analysis plan, carried out the statistical analyses, contributed to interpretation of the data and drafted and critically reviewed the report.
Maria Chorozoglou (Senior Research Fellow and Health Economist) contributed to the design of the study, carried out the health economics analyses, contributed to interpretation of the data and drafted and critically reviewed the report.
Amanda Roberts (Patient Representative) contributed to the conception and design of the study and interpretation of the data and critically reviewed the report.
Lyn Liddiard (Clinical Studies Officer) contributed to the management of the study and to the collection of data and critically reviewed the report.
Claire Nollett (Clinical Studies Officer) contributed to the management of the study and to the collection of data and critically reviewed the report.
Julie Hooper (Trial Administrator) contributed to the management of the study and to the collection of data and critically reviewed the report.
Martina Prude (Clinical Studies Officer) contributed to the management of the study and to the collection of data and critically reviewed the report.
Wendy Wood (Research Adviser in NIHR Research Design Service and former Senior Trials Manager Southampton CTU) contributed to the conception and design of the study, was responsible for oversight of study setup and critically reviewed the report.
Emma Thomas-Jones (Senior Trial Manager) contributed to the design of the study, contributed to interpretation of the data and drafted and critically reviewed the report.
Taeko Becque (Research Fellow and Medical Statistician) carried out statistical analyses and critically reviewed the report.
Kim S Thomas (Professor of Applied Dermatology Research and Co-Director of the Centre of Evidence-Based Dermatology) contributed to the conception and design of the study and contributed to interpretation of the data, and drafted and critically reviewed the report.
Hywel C Williams (Professor of Dermato-Epidemiology and Co-Director of the Centre of Evidence-Based Dermatology) contributed to the conception and design of the study, contributed to interpretation of the data and drafted and critically reviewed the report.
Paul Little (Professor of Primary Care Research) contributed to the conception and design of the study, contributed to interpretation of the data and drafted and critically reviewed the report.
Publications
Santer M, Rumsby K, Ridd MJ, Francis NA, Stuart B, Chorozoglou M, et al. Bath additives for the treatment of childhood eczema (BATHE): protocol for multicentre parallel group randomised trial. BMJ Open 2015;5:e009575. https://doi.org/10.1136/bmjopen-2015-009575
Santer M, Ridd MJ, Francis NA, Stuart B, Rumsby K, Chorozoglou M, et al. Emollient bath additives for the treatment of childhood eczema (BATHE): multicentre pragmatic parallel group randomised controlled trial of clinical and cost effectiveness. BMJ 2018;361:k1332.
Stuart B, Rumsby K, Santer M, Ridd M, Francis N, Chorozoglou M, et al. Feasibility of weekly participant-reported data collection in a pragmatic randomised controlled trial in primary care: experiences from the BATHE trial (Bath Additives for the Treatment of cHildhood Eczema). Trials 2018; in press.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups . Is eczema really on the increase worldwide?. J Allergy Clin Immunol 2008;121:947-54.e15. https://doi.org/10.1016/j.jaci.2007.11.004.
- Karimkhani C, Dellavalle RP, Coffeng LE, Flohr C, Hay RJ, Langan SM, et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatol 2017;153:406-12. https://doi.org/10.1001/jamadermatol.2016.5538.
- Chamlin SL, Frieden IJ, Williams ML, Chren MM. Effects of atopic dermatitis on young American children and their families. Pediatrics 2004;114:607-11. https://doi.org/10.1542/peds.2004-0374.
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004;113:832-6. https://doi.org/10.1016/j.jaci.2003.12.591.
- Santer M, Rumsby K, Ridd MJ, Francis NA, Stuart B, Chorozoglou M, et al. Bath additives for the treatment of childhood eczema (BATHE): protocol for multicentre parallel group randomised trial. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-009575.
- McCormick A, Fleming D, Charlton J. Morbidity Statistics from General Practice: Fourth National Study 1991–1992. London: The Stationery Office; 1995.
- Herd RM, Tidman MJ, Prescott RJ, Hunter JAA. The cost of atopic dermatitis. Br J Dermatol 1996;135:20-3. https://doi.org/10.1111/j.1365-2133.1996.tb03601.x.
- Verboom P, Hakkaart-Van L, Sturkenboom M, De Zeeuw R, Menke H, Rutten F. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol 2002;147:716-24. https://doi.org/10.1046/j.1365-2133.2002.04964.x.
- Atopic Eczema in Under 12s: Diagnosis and Management. London: National Institute for Health and Care Excellence; 2007.
- Nankervis H, Thomas KS, Delamere FM, Barbarot S, Rogers NK, Williams HC. Scoping systematic review of treatments for eczema. Programme Grants Appl Res 2016;4.
- Nankervis H, Thomas KS, Delamere FM, Barbarot S, Smith S, Rogers NK, et al. What is the evidence base for atopic eczema treatments? A summary of published randomized controlled trials. Br J Dermatol 2017;176:910-27. https://doi.org/10.1111/bjd.14999.
- Tarr A, Iheanacho I. Should we use bath emollients for atopic eczema?. BMJ 2009;339. https://doi.org/10.1136/bmj.b4273.
- Anonymous . Bath emollients for atopic eczema: why use them?. Drug Ther Bull 2007;45:73-5. https://doi.org/10.1136/dtb.2007.09.0015.
- White M, Batten T, Ormerod A. Adverse effects of a daily bathing routine on children with atopic dermatitis. J Dermatol Treat 1994;5:21-3. https://doi.org/10.3109/09546639409081841.
- Global Resource for Eczema Trials (GREAT). Nottingham: University of Nottingham, Centre of Evidence Based Dermatology; n.d.
- Wong SM, Ng TG, Baba R. Efficacy and safety of sodium hypochlorite (bleach) baths in patients with moderate to severe atopic dermatitis in Malaysia. J Dermatol 2013;40:874-80. https://doi.org/10.1111/1346-8138.12265.
- Holland KT, Bojar RA, Cunliffe WJ, Lever R, Levy J. The Bacteriology of Eczema (Round Table series). London: Royal Society of Medicine Press Limited; 1995.
- Harper J, Lever R, Levy J. The Bacteriology of Eczema (Round Table Series). London: Royal Society of Medicine Press Limited; 1995.
- Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess 2000;4.
- Ling TC, Highet AS. Irritant reactions to an antiseptic bath emollient. J Dermatolog Treat 2000;11:263-7. https://doi.org/10.1080/09546630050517216.
- Cost Effective Prescribing of Emollients. London: NHS PrescQIPP; 2015.
- Chan JQP, Ridd M. A Comparison of Emollient Prescribing Guidelines for Atopic Eczema and Other Dry Skin Conditions across Clinical Commissioning Groups in England n.d.
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. CMAJ 2009;180:E47-57. https://doi.org/10.1503/cmaj.090523.
- Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350. https://doi.org/10.1136/bmj.h2147.
- Williams HC, Burden-Teh E, Nunn AJ. What is a pragmatic clinical trial?. J Investig Dermatol 2015;135:1-3. https://doi.org/10.1038/jid.2015.13.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol 2004;150:284-90. https://doi.org/10.1111/j.1365-2133.2004.05776.x.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004;140:1513-19. https://doi.org/10.1001/archderm.140.12.1513.
- Charman CR, Venn AJ, Ravenscroft JC, Williams HC. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol 2013;169:1326-32. https://doi.org/10.1111/bjd.12590.
- Schmitt J, Langan S, Deckert S, Svensson A, von Kobyletzki L, Thomas K, et al. Harmonising Outcome Measures for Atopic Dermatitis (HOME) Initiative . Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. J Allergy Clin Immunol 2013;132:1337-47. https://doi.org/10.1016/j.jaci.2013.07.008.
- Spuls PI, Gerbens LAA, Simpson E, Apfelbacher CJ, Chalmers JR, Thomas KS, et al. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol 2017;176:979-84. https://doi.org/10.1111/bjd.15179.
- Harmonising Outcome Measures for Eczema (HOME). Nottingham: University of Nottingham, Centre of Evidence Based Dermatology; n.d.
- Schmitt J, Langan S, Stamm T, Williams HC. Harmonizing Outcome Measurements in Eczema (HOME) Delphi panel . Core outcome domains for controlled trials and clinical recordkeeping in eczema: international multiperspective Delphi consensus process. J Invest Dermatol 2011;131:623-30. https://doi.org/10.1038/jid.2010.303.
- Thomas KS, Bradshaw LE, Sach TH, Batchelor JM, Lawton S, Harrison EF, et al. Silk garments plus standard care compared with standard care for treating eczema in children: A randomised, controlled, observer-blind, pragmatic trial (CLOTHES Trial). PLOS Med 2017;14. https://doi.org/10.1371/journal.pmed.1002280.
- Stuart B, Rumsby K, Santer M, Ridd M, Francis N, Chorozoglou M, et al. Feasibility of weekly participant-reported data collection in a pragmatic randomised controlled trial in primary care: experiences from the BATHE trial (Bath Additives for the Treatment of cHildhood Eczema). Trials 2018.
- Batchelor JM, Ridd MJ, Clarke T, Ahmed A, Cox M, Crowe S, et al. The Eczema Priority Setting Partnership: a collaboration between patients, carers, clinicians and researchers to identify and prioritize important research questions for the treatment of eczema. Br J Dermatol 2013;168:577-82. https://doi.org/10.1111/bjd.12040.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2015.
- Williams HC, Burney PG, Hay RJ, Archer CB, Shipley MJ, Hunter JJ, et al. The UK Working Party’s Diagnostic Criteria for Atopic Dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol 1994;131:383-96. https://doi.org/10.1111/j.1365-2133.1994.tb08530.x.
- Vakharia PP, Chopra R, Silverberg JI. Systematic review of diagnostic criteria used in atopic dermatitis randomized controlled trials. Am J Clin Dermatol 2018;19:15-22. https://doi.org/10.1007/s40257-017-0299-4.
- Emerson RM, Charman CR, Williams HC. The Nottingham Eczema Severity Score: preliminary refinement of the Rajka and Langeland grading. Br J Dermatol 2000;142:288-97. https://doi.org/10.1046/j.1365-2133.2000.03300.x.
- Nottingham Support Group for Carers of Children with Eczema . Welcome to the Nottingham Support Group for Carers of Children With Eczema (NSGCCE) n.d. www.nottinghameczema.org.uk/ (accessed 20 February 2014).
- Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012;67:99-106. https://doi.org/10.1111/j.1398-9995.2011.02719.x.
- Gaunt DM, Metcalfe C, Ridd M. The Patient-Oriented Eczema Measure in young children: responsiveness and minimal clinically important difference. Allergy 2016;71:1620-5. https://doi.org/10.1111/all.12942.
- Chalmers JR, Simpson E, Apfelbacher CJ, Thomas KS, von Kobyletzki L, Schmitt J, et al. Report from the fourth international consensus meeting to harmonize core outcome measures for atopic eczema/dermatitis clinical trials (HOME initiative). Br J Dermatol 2016;175:69-7. https://doi.org/10.1111/bjd.14773.
- Lawson V, Lewis-Jones MS, Finlay AY, Reid P, Owens RG. The family impact of childhood atopic dermatitis: the Dermatitis Family Impact Questionnaire. Br J Dermatol 1998;138:107-13. https://doi.org/10.1046/j.1365-2133.1998.02034.x.
- Stevens K. Assessing the performance of a new generic measure of health-related quality of life for children and refining it for use in health state valuation. Appl Health Econ Health Policy 2011;9:157-69. https://doi.org/10.2165/11587350-000000000-00000.
- Canaway AG, Frew EJ. Measuring preference-based quality of life in children aged 6-7 years: a comparison of the performance of the CHU-9D and EQ-5D-Y – the WAVES pilot study. Qual Life Res 2013;22:173-83. https://doi.org/10.1007/s11136-012-0119-5.
- A Brief Overview of the Child Health Utility 9D (CHU9D). University of Sheffield: School of Health and Related Research; n.d.
- Thomas KS, Dean T, O’Leary C, Sach TH, Koller K, Frost A, et al. SWET Trial Team . A randomised controlled trial of ion-exchange water softeners for the treatment of eczema in children. PLOS Med 2011;8. https://doi.org/10.1371/journal.pmed.1000395.
- About LifeGuide. Southamptom: University of Southampton; n.d.
- Ramsey S, Wilke R, Briggs A, Brown R, Buxton M, Chawla A, et al. Best Practices For Economic Analysis Alongside Clinical Trials: An ISPOR RCT–CEA Task Force Report. Lawrenceville, NJ: International Society for Pharmacoeconomics and Outcomes Research (ISPOR); 2004.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; 2015.
- Rudmik L, Drummond M. Health economic evaluation: important principles and methodology. Laryngoscope 2013;123:1341-7. https://doi.org/10.1002/lary.23943.
- Guide to the Methods of Technology Appraisal 2013. London: National Institute for Health and Care Excellence; n.d.
- Briggs A, Tambour M. The design and analysis of stochastic cost-effectiveness studies for the evaluation of health care interventions. Drug Inf J 2001;35:1455-68. https://doi.org/10.1177/009286150103500441.
- Laska EM, Meisner M, Siegel C. Power and sample size in cost-effectiveness analysis. Med Decis Making 1999;19:339-43. https://doi.org/10.1177/0272989X9901900312.
- Gardner MJ, Altman DG. Confidence intervals rather than P values: estimation rather than hypothesis testing. Br Med J 1986;292:746-50. https://doi.org/10.1136/bmj.292.6522.746.
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. New York, NY: Oxford University Press; 2010.
- Beecham J, Knapp M, Thornicroft G, Brewin CR, Wing J. Measuring Mental Health Needs. London: Gaskell/Royal College of Psychiatrists; 2001.
- Beecham J. CSRI – Background Information and Manual. Canterbury: Personal Social Sciences Research Unit; n.d.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- NHS Reference Costs 2015 to 2016. London: Department of Health and Social Care; 2016.
- Bettzuege-Pfaff BI, Melzer A. Treating dry skin and pruritus with a bath oil containing soya oil and lauromacrogols. Curr Med Res Opin 2005;21:1735-9. https://doi.org/10.1185/030079905X62963.
- Bath-Hextall FJ, Birnie AJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema: an updated Cochrane review. Br J Dermatol 2010;163:12-26. https://doi.org/10.1111/j.1365-2133.2010.09743.x.
- Hewitt CE, Torgerson DJ. Is restricted randomisation necessary?. BMJ 2006;332:1506-8. https://doi.org/10.1136/bmj.332.7556.1506.
- Grizzle JE. A note on stratifying versus complete random assignment in clinical trials. Control Clin Trials 1982;3:365-8. https://doi.org/10.1016/0197-2456(82)90026-5.
- Rosenberger WF, Lachin JM. Randomization in Clinical Trials: Theory and Practice. New York, NY: John Wiley & Sons Inc.; 2002.
- Ridd MJ, Garfield K, Gaunt DM, Hollinghurst S, Redmond NM, Powell K, et al. Choice of Moisturiser for Eczema Treatment (COMET): feasibility study of a randomised controlled parallel group trial in children recruited from primary care. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-012021.
- Santer M, Burgess H, Yardley L, Ersser SJ, Lewis-Jones S, Muller I, et al. Managing childhood eczema: qualitative study exploring carers’ experiences of barriers and facilitators to treatment adherence. J Adv Nurs 2013;69:2493-501. https://doi.org/10.1111/jan.12133.
- van Zuuren EJ, Fedorowicz Z, Arents BWM. Emollients and moisturizers for eczema: abridged Cochrane systematic review including GRADE assessments. Br J Dermatol 2017;177:1256-71. https://doi.org/10.1111/bjd.15602.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
- Staniszewska S, Brett J, Mockford C, Barber R. The GRIPP checklist: strengthening the quality of patient and public involvement reporting in research. Int J Technol Assess Health Care 2011;27:391-9. https://doi.org/10.1017/S0266462311000481.
Appendix 1 Summary patient information leaflet (from 12 July 2015)
The graphics in this PDF have been reproduced with permission from Fotolia (© Fotolia 2018) [New York City, NY, USA; URL: https://en.fotolia.com (accessed August 2018)].
Appendix 2 Screening form/reply slip
Appendix 3 Patient invitation letter
Appendix 4 Patient information sheet
Appendix 5 Algorithm for calculating eligibility using Microsoft Access
Appendix 6 Information leaflet for older children (aged ≥ 6 years)
The graphics in this PDF have been reproduced with permission from Fotolia (© Fotolia 2018) [New York City, NY, USA; URL: https://en.fotolia.com (accessed August 2018)].
Appendix 7 Information leaflet for younger children (aged ≤ 5 years)
The graphics in this PDF have been reproduced with permission from Fotolia (© Fotolia 2018) [New York City, NY, USA; URL: https://en.fotolia.com (accessed August 2018)].
Appendix 8 List of bath additives permitted for use in Bath Additives for the Treatment of Eczema in cHildren
Appendix 10 Baseline appointment standard operating procedure
Appendix 11 Notes review form
Appendix 12 Consent form
Appendix 13 Assent form
Appendix 14 Calculating eligibility for clinical studies officer/research nurses standard operating procedure
Appendix 15 Additional information for future sample size calculations
Glossary
- Bath additive/bath emollient
- Emollients to be added to bath water. Not to be confused with leave-on emollients (applied directly to the skin) or soap substitute emollients (used instead of soap).
- CFH Docmail Ltd
- A company that provides secure mass mailing at reduced cost. Compliant with the latest international data security standards, Docmail has been used extensively within the NHS for many years.
- Clinical studies officer/research nurse
- Staff employed by the academic/health-care institutions that are hosting the study to recruit participants. Research nurses also have clinical qualifications.
- Confidence interval
- In statistics, a range around a measurement that estimates its precision.
- Good clinical practice for clinical trials
- An international system of accreditation whereby the rights, safety and well-being of research participants are protected and research data are of the highest quality.
- Health Technology Assessment programme
- A National Institute for Health Research funding stream that supports research of immediate benefit to patients, clinicians and policy-makers.
- LifeGuide
- Customisable online software to support health interventions, established by the Department of Psychology at the University of Southampton.
- National Institute for Health Research
- A division of the Department of Health and Social Care that supports health-care research in the UK.
- National Institute for Social Care and Health Research
- Now Health and Care Research Wales, this organisation of the Welsh Government supports health-care research and development in Wales.
- No bath additives
- Standard eczema care without bath additives (control group).
- Serious adverse event
- In human drug trials, any untoward medical occurrence that results in death, is life-threatening, requires inpatient hospitalisation or causes prolongation of existing hospitalisation. A serious adverse event is not necessarily related to the product under investigation.
- Standard deviation
- A statistical measurement that quantifies the dispersion of measurements around the mean.
List of abbreviations
- A&E
- accident and emergency
- ANOVA
- analysis of variance
- BATHE
- Bath Additives for the Treatment of Eczema in cHildren
- CCG
- Clinical Commissioning Group
- CEBD
- Centre for Evidence-Based Dermatology
- CHU-9D
- Child Health Utility-9 Dimensions
- CI
- confidence interval
- CRN
- Clinical Research Network
- CSO/RN
- clinical studies officer/research nurse
- CSRI
- Client Service Receipt Inventory
- DFIQ
- Dermatitis Family Impact Questionnaire
- EEACT
- economic evaluations alongside clinical trials
- EQ-5D
- EuroQol-5 Dimensions
- FbC
- family-borne cost
- GP
- general practitioner
- HEAP
- health economics analysis plan
- HOME
- Harmonising Outcome Measures for Eczema
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ITT
- intention to treat
- MMLM
- mixed multilevel model
- NESS
- Nottingham Eczema Severity Score
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NISCHR
- National Institute for Social Care and Health Research
- NR
- notes review
- NSGCCE
- Nottingham Support Group for Carers of Children with Eczema
- OR
- odds ratio
- POEM
- Patient Oriented Eczema Measure
- PPI
- patient and public involvement
- PSP
- Priority Setting Partnership
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- SAE
- serious adverse event
- SD
- standard deviation
- SOP
- standard operating procedure
- TCI
- topical calcineurin inhibitor
- TCS
- topical corticosteroid
- UKDC
- UK Diagnostic Criteria for Atopic Dermatitis


