Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/57/24. The contractual start date was in September 2012. The draft report began editorial review in June 2017 and was accepted for publication in December 2017. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Alan A Montgomery reports membership of the National Institute for Health Research (NIHR) Health Technology Assessment Clinical Evaluation and Trials Board. Catherine Sackley and Roshan das Nair report membership of the NIHR Health Services and Delivery Research Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by das Nair et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Parts of this chapter have been reproduced with permission from das Nair et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Background
Traumatic brain injuries (TBIs) are defined as an alteration in brain function, or other evidence of brain pathology, caused by an external force. 2 In 2013–14, the total number of people who were admitted for a TBI in the UK was 162,544 (254 per 100,000 population). 3 As well as TBIs sustained by civilians in daily life, TBIs among military personnel have also been a major contributor to mortality and morbidity. 4,5 The epidemiological variations seen in TBIs observed in the armed forces are the result of different approaches to screening, definitions of TBI and methods of reporting. 6
The costs of morbidity from TBIs are incurred by the health-care system and those outside it (in terms of loss of productivity as a result of short-term sick leave and early retirement), as well as through non-medical costs (e.g. transformation of the house or work environment). In addition, informal care by family or friends also adds to the costs of care for affected individuals. For TBI, the direct medical costs and indirect costs were estimated at US$60B in the USA in 2000. 7 The full costs of dealing with memory problems caused by TBI in the UK are not known. Care costs escalate when interventions are provided on an inpatient basis, but Salazar et al. 8 have demonstrated that the benefits of inpatient and home cognitive rehabilitation programmes for TBI, in terms of return to duty (for military personnel) or employment, were similar.
Impairment of memory is one of the most common cognitive deficits reported by people with TBIs, affecting 40–60% of patients. 9,10 These memory problems are not only persistent, but also debilitating and difficult to treat. 11 Memory deficits may also affect the extent to which patients engage with other interventions and rehabilitation. The safety of such patients can also be compromised, making them vulnerable citizens in the home (e.g. forgetting to turn the stove off), community (e.g. forgetting road rules) and work (e.g. forgetting important documents) settings. Memory problems consequently have a devastating effect on the psychological well-being of the individuals and others around them. 12
Cognitive rehabilitation is a structured set of therapeutic activities designed to retrain an individual’s cognitive functions. Memory rehabilitation is a domain-specific type of cognitive rehabilitation that focuses on improving memory and helping people deal with the consequences of memory problems. In the UK, memory rehabilitation is offered by some services as a means to help people cope with their cognitive problems.
Research evidence
Individual studies
Some randomised controlled trials (RCTs) have demonstrated the effectiveness of cognitive rehabilitation following brain injuries. These have mainly focused on attention, executive functions and visual neglect, but memory rehabilitation specifically has not been sufficiently researched. 13 Most evidence for memory rehabilitation comes from single case experimental design studies and controlled clinical trials, with the few RCTs and quasi-RCTs in this area offering some support for the effectiveness of intervention.
Wilson et al. 14 examined a paging system used as an external memory aid. This enabled participants to achieve more memory-related goals than when NeuroPage was not available. Doornhein and de Haan15 reported that patients who received a memory training programme performed significantly better than those in a pseudo-treatment control group on trained memory tasks, but no differences were observed in subjective ratings of everyday memory functions. Kaschel et al. 16 reported that imagery mnemonics significantly improved delayed recall of verbal material and reduced observer-rated reports of memory failures. However, systematic reviews of memory rehabilitation have not found evidence to support or refute the effectiveness of such programmes. 17,18 This lack of evidence is partly because of a lack of well-designed trials, which has led one of the largest meta-analyses to conclude that ‘the results for memory rehabilitation are mixed and weak’ (p. 33). 13 The authors of this review suggested that ‘researchers need to reduce reliance on single-subject and single group designs’ (p. 34) and recommended more RCT evidence, a view supported by others. 19 At a symposium on disorders of memory, Wilson20 called for ‘better evaluation of memory rehabilitation programmes’ (p. e4–5). This is a conclusion that more recent systematic reviews of memory rehabilitation following TBI,18 stroke21 and multiple sclerosis22 also reached. This conclusion has been attributed mainly to the dearth of high-quality RCTs, but may also reflect the lack of common outcomes measures that are responsive to the effects of memory rehabilitation.
In a small-scale RCT (n = 72) to evaluate a group memory rehabilitation programme,23 patients with memory problems were randomly allocated to one of three group treatment programmes: compensation strategy training, restitution or a self-help attention placebo control. Although the results showed no statistically significant differences in outcomes, they indicated that the interventions seemed worthy of further evaluation. This was supported by the qualitatively analysed participant feedback interviews,24 which suggested improvements in knowledge and skills with regard to memory aid use, among other improvements. This small trial provided feasibility and pilot data for the current trial.
Literature reviews
A narrative review25 found cognitive rehabilitation to be beneficial for treating cognitive deficits following brain damage. Cernich et al. 26 reviewed evidence for cognitive rehabilitation in TBI and recommended that, although RCTs had demonstrated the utility of specific rehabilitation approaches to attention retraining and retraining of executive functioning skills, further research was needed on rehabilitation techniques in other domains of cognition (such as memory). They also suggested that training in the use of supportive devices to improve an individual’s daily activities was central to their ability to function independently.
Systematic reviews, such as that by Cicerone et al.,27 published in 2000, concluded that there was strong evidence of the effectiveness of treatments for memory problems after a TBI. The updated review,25 published in 2005, continued to endorse this view. These reviews included both RCTs and single case studies. However, Rohling et al. ’s13 more stringent meta-analytic re-examination of both reviews by Cicerone et al. 25,27 included 115 studies of cognitive rehabilitation trials and found mixed, or at best, only weak, support for memory treatment. It is worth noting that, of all of the included studies (of TBI and stroke), only 30 (26.1%) were classified as being in ‘class I’ (i.e. well-designed, prospective RCTs) and only 14 specifically focused on memory. Also of note was that these studies were small, with an average of 16.9 and 18.5 participants in the treatment and control arms, respectively.
Bergquist et al. 28 found that people with TBI not only were willing to use the internet to receive cognitive rehabilitation, but also were satisfied with the treatment. Encouraged by results from imaging studies that have shown neuroplasticity, Spreij et al. 29 conducted a systematic review that offered novel insights into remediation-oriented approaches for the rehabilitation of memory deficits following acquired brain injuries (ABIs). They classified the 15 studies that they included in their review as falling within the rubric of (1) virtual reality (VR) training, (2) computer-based cognitive retraining (CBCR) and (3) non-invasive brain stimulation (NBS). They concluded that CBCR was the most promising of these interventions, with all seven of the CBCR studies they included showing positive effects. However, closer inspection of these studies (and the other VR and NBS studies) showed that they were not methodologically robust (some were not RCTs) and the outcomes included were mainly impairment-level measures. Furthermore, most of these studies included mixed diagnosis samples and, therefore, it was not possible to extract what the specific effects of these interventions would be on people with a TBI. More robust mixed-methods RCTs are therefore still required to evaluate the effectiveness of these newer forms of cognitive rehabilitation for people with TBIs.
Another review30 that focused specifically on computerised cognitive training (CCT) in ABI and on outcomes classified within the framework of the International Classification of Functioning, Disability and Health (ICF)31 included 96 primary studies that evaluated CCT. The authors noted that only 15% of these studies represented ‘level 1′ evidence (i.e. good-quality RCTs). Interestingly, the authors also reported that, although the population with TBI was the most studied population, only two of the 31 TBI studies were considered to offer ‘level 1′ evidence. Overall, their findings suggest that CCT has limited positive impacts on outcomes that relate to activity or participation, although only 43% of the studies included an outcome measure that assessed activity or participation.
Clinical guidelines
There are recommendations for the provision of cognitive rehabilitation for people with ABIs. Older national and international guidelines for TBI rehabilitation, such as the Brain Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine’s published practice guidelines for rehabilitation after TBI and stroke,32 the European Federation of Neurological Societies Task Force on Cognitive Rehabilitation’s guidelines for stroke and TBI33,34 and the national clinical guidelines for rehabilitation following ABIs from the Royal College of Physicians and the British Society of Rehabilitation Medicine,35 found limited high-quality evidence supporting some forms of cognitive rehabilitation, specifically, treatments for memory problems after TBI. This was mainly because of a lack of RCTs, with most evaluations being uncontrolled trials or single case experimental designs.
The national clinical guidelines for rehabilitation following ABIs from the Royal College of Physicians and the British Society of Rehabilitation Medicine35 recommend that patients with persistent cognitive deficits following ABI should be offered cognitive rehabilitation, which may include compensatory strategies, including the use of memory aids, to help manage memory problems in daily life and support independence. They further state that ‘trial-and-error’ learning should be avoided. Again, the level of recommendation is low because of the low quality of the evidence. Therefore, it is perhaps unsurprising that most previous recommendations have been qualified by the need for more research.
The national clinical guidelines for brain injury rehabilitation in adults from the Scottish Intercollegiate Guidelines Network (SIGN)36 recommend training in the use of compensatory strategies for people with memory problems following TBI, focusing on improving the management of memory problems in daily life rather than underlying memory impairment. Recommendations differ depending on the severity of memory problems, with both internal and external strategies advised for those with mild to moderate impairment, whereas the focus for those with severe memory impairment should be on improving functional abilities through external aids.
However, this is a ‘grade D’ recommendation, based on level 3 (non-analytical studies) and level 4 (expert opinion) evidence and on extrapolating level 2 evidence (well-conducted case–control or cohort studies). This SIGN guideline forms the basis of the National Institute for Health and Care Excellence (NICE) guidance on head injury. 37
More recently, recommendations for the management of memory problems following TBI by an international team of researchers and clinicians38 concluded that there is ‘good evidence for the integration of internal and external compensatory memory strategies that are implemented using instructional procedures for rehabilitation for memory impairments’ but that the ‘evidence for the efficacy of restorative strategies currently remains weak’ (p. 369). However, this conclusion was arrived at on the basis of few RCTs, many of which had a small sample size and a large number of outcomes, did not report power analyses and did not consider the longer-term effects of the intervention.
On the basis of the foregoing discussion, and in response to a commissioned call by the National Institute for Health Research Health Technology Assessment (HTA) programme,39 we designed the ReMemBrIn trial to address the concerns raised by authors of individual studies, systematic reviews and clinical guidelines. The trial, funded by the HTA programme, was designed to assess the effectiveness of a group memory rehabilitation programme, on the basis of recent research suggestions from researchers and clinicians,19 our own pilot study23 and current clinical guidelines40,41 and clinical practice in the UK. Furthermore, in line with the Better Value in the NHS report,42 we designed this project to deliver value for money through innovative changes to current clinical practice leading to improved patient outcomes.
Rationale
Currently, patients with TBI experiencing memory problems do not routinely receive cognitive rehabilitation after the outpatient rehabilitation phase, even though their abilities and needs may change once they are discharged from clinical services. This is in part because of the current lack of evidence for the clinical effectiveness and cost-effectiveness of memory rehabilitation and because of resource limitations. This study sought to address these issues.
Research question
What is the clinical effectiveness and cost-effectiveness of memory rehabilitation for people with memory problems following TBI?
Objectives
Primary objective
The primary objective was to determine whether attending a group-based memory rehabilitation programme (the intervention) was associated with improved subjective reports of the management of memory problems in daily life, as measured using the Everyday Memory Questionnaire – patient version (EMQ-p),43 compared with a usual-care control.
Secondary objectives
The secondary objectives were to assess:
-
whether the intervention was associated with improvements in participants’:
-
objectively assessed memory abilities
-
ability to achieve individually set goals
-
health-related quality of life (HRQoL)
-
cognitive, emotional, and social well-being.
-
-
the cost-effectiveness of the intervention.
Chapter 2 Methods
Parts of this chapter have been reproduced with permission from das Nair et al. 1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Design
The ReMemBrIn study was a multicentre, two-arm, parallel-group randomised controlled superiority trial of a group-based memory rehabilitation programme, provided in addition to usual care, compared with usual care alone. Participants were randomised in clusters of between four and six. Clusters were allocated to memory rehabilitation or usual care in a ratio of 1 : 1.
An economic analysis was conducted to determine the costs and cost-effectiveness of group memory rehabilitation compared with usual care (see Chapter 4). In addition, a nested qualitative substudy sought to explore participants’ experiences of the group memory rehabilitation and usual care (see Chapter 5).
Study setting and participants
Sites
The trial was conducted in nine sites in the UK (see Appendix 1). Each site was a NHS trust providing rehabilitation services for people with TBI.
We initially intended to recruit from four sites, but we activated new sites owing to old ones shutting down because of their participant pools being exhausted. The original four sites were opened to recruitment between February and April 2013. Because of staff turnover and staff recruitment difficulties, two of these sites were subsequently closed to recruitment and were replaced by two new sites that opened in March and November 2014, respectively. To address delays in recruitment, three additional sites were also opened between March and June 2015.
Identification of participants
Participants were identified through NHS services at the participating sites. This included searching hospital databases and departmental records for people with head injuries from rehabilitation medicine, neurosurgery and clinical psychology and neuropsychology departments. In-clinic recruitment also took place from rehabilitation consultant-led clinics and outpatient TBI rehabilitation clinics. Participants were also identified from similar sources at other NHS trusts acting as participant identification centres (PICs). In addition, posters were displayed in clinic areas in the hospitals. Participants were also identified by self-referral as a result of publicity by local and national charities and patient groups (e.g. head injury charities) and advertising to the general public through the study website, on various support group websites and newsletters and through features on television and radio programmes. In order to include military personnel, participants were sought from a military rehabilitation centre and a NHS surgical centre treating personnel from the armed forces.
Clinical teams sent an invitation letter to individuals who were identified as potential participants. This letter included a participant information sheet, a consent form, a contact details slip and a prepaid reply envelope. If potential participants were interested in taking part, they were asked to complete the contact slip and return it in the envelope directly to the assistant psychologist (AP) at their nearest site.
Informed consent
Written consent was obtained by the AP and participants were given a copy of the consent form for their records. Participant information sheets and consent forms were based on those developed for the pilot study,23 and these had been checked for clarity and readability by a service user representative. Potential participants had the opportunity to read about the study and discuss it with other clinical staff members, family and friends and the research team before they decided whether or not to take part. They had a minimum of 24 hours to do this. Potential participants also had the opportunity to read the participant information sheet and consent forms with the AP at their first assessment.
Eligibility criteria
Eligible participants were those who:
-
Were admitted to hospital with a TBI sustained > 3 months prior to recruitment.
-
Had memory problems, defined as a score or ≥ 24 on the EMQ-p and/or a score below the 25th percentile on the Rivermead Behavioural Memory Test – third edition (RBMT-3),44 as assessed at the initial screening assessment. A score of ≥ 24 on the EMQ-p is two standard deviations (SDs) below the mean for healthy participants (Professor Nadina B Lincoln, University of Nottingham, 2017, personal communication). The 25th percentile cut-off for the RBMT-3 indicates below average objectively assessed memory ability. 44
-
Were aged 18–69 years. The upper age limit was applied in order not to include those with age-related memory problems.
-
Were able to travel to one of the study sites and attend group sessions. Participants had to live within the geographical area covered by the sites and be able to travel to sites. We offered travel expenses to all participants who requested it. Participants also needed to be willing to receive treatment in a group if allocated to the intervention.
-
Gave informed consent.
Potential participants were excluded if they:
-
Were unable to engage in group treatment if allocated. This was assessed by the clinicians at the recruitment sites, with reasons for exclusion including severe aural sensory problems. Those who had behavioural problems that would interfere with group treatment were not considered.
-
Were participating in other psychological intervention studies, assessed by self-report.
-
Had impairment of language that would make them unable to take part in the rehabilitation group activities, defined as a score of < 17 on the Sheffield Screening Test for Acquired Language Disorders (SST)45 completed at the initial screening assessment. In accordance with the test manual, participants who scored < 17 on the SST were considered to have impairment of language that would limit their ability to complete the intervention.
Study procedures
We expected participants to be involved in the study for approximately 13 months from the initial screening assessment to the final follow-up visit 12 months from randomisation. The data collected at each time point are shown in Appendix 2 and are detailed below. Data were collected through a combination of self-report questionnaires completed by participants and their relatives/friends and face-to-face assessments with participants, completed by a research assistant (RA) during study visits. Visits took place at participants’ homes whenever possible. However, if there were concerns about the suitability of the home environment or if a participant preferred, assessments were conducted at NHS sites or community venues.
Initial screening visit
At the initial screening visit, the AP first explained the study and made clear that the initial assessments were required to check that the participant met the inclusion criteria, to obtain demographic and clinical data and to conduct baseline assessments for those who were eligible. The AP responded to queries and obtained informed consent prior to conducting the following initial assessments, questionnaire completion and demographic data collection:
-
EMQ-p:43 this is a subjective, patient-centred outcome measure with good ecological and face validity46,47 and has been previously used in cognitive rehabilitation studies. 23,48 The EMQ-p comprises 28 items asking about the frequency of memory failures in everyday life over the past month. Each item is rated on a five-point Likert scale (from ‘once or less in the last month/never’ to ‘once or more a day’). Total scores range from 0 to 112, with higher scores indicating more frequent memory problems.
-
RBMT-3:44 this is a standardised objective measure of memory, with adequate psychometric properties. This was chosen as an objective measure that closely reflected daily life memory ability. It has also been used as an outcome measure in other studies of memory rehabilitation. 16,23,49–52 A General Memory Index (GMI) score was derived to provide an assessment of overall memory abilities. GMI values range between 52 and 174 and are standardised on a representative sample from the UK44 to have a mean of 100 (SD 15). Lower scores indicate more significant memory impairment.
-
National Adult Reading Test (NART):53 the premorbid level of intellectual functioning, required to interpret RBMT-3 scores, was estimated using the NART.
-
SST:45 this was used to assess eligibility for the trial on the basis of language ability.
-
General Health Questionnaire 30-item version (GHQ-30):54 this is a 30-item questionnaire that was designed to detect psychological distress in the general population. It assesses participants’ mood over the past few weeks compared with their usual mood. The GHQ-30 was chosen as it is suitable for postal administration and is easy to complete. The General Health Questionnaire (GHQ) (12-, 28- or 30-item versions) has also been shown to be responsive to the effects of psychological interventions in people with neurological conditions48,55–57 and has been used in previous TBI and rehabilitation studies. 23 Likert scoring was used for the GHQ-30 for the clinical outcome, with scores ranging from 0 to 90 and higher scores indicating more psychological distress. The alternative GHQ scoring methodology (0–0–1–1) was applied for the health economic evaluation.
In addition, we collected demographic information from participants at the screening assessment. This included gender, date of birth, ethnicity, date of TBI (self-reported by participants), duration of the initial hospital stay for the TBI, current employment status, living arrangements, military status and highest educational achievement.
The following clinical information on participants’ brain injury was collected from medical notes when these could be accessed at the recruiting site:
-
severity of injury assessed by the Glasgow Coma Scale (GCS)58 score closest to admission and the worst total score
-
date of TBI (verified from medical notes)
-
type of brain injury (open or closed)
-
other neurological conditions.
Scores from the EMQ-p, RBMT-3 and SST completed at the initial screening visit were used to confirm eligibility for the trial. Patients who did not meet the eligibility criteria following the initial screening visit were notified by letter to thank them for their interest in the study and a brief report of their test results was provided if requested. Those who met the inclusion criteria were invited to continue in the trial, if they were happy to do so, and proceeded to the second assessment visit.
Participants continuing in the trial were asked to nominate a relative/friend who knew about their memory problems in daily life, although this was not a mandatory requirement of trial participation. A questionnaire booklet for the relative/friend was sent to eligible participants following the initial screening visit. Participants were asked to pass this on to their relative/friend and return completed questionnaires at the second assessment visit.
Second assessment visit
The following questionnaires were completed at the second visit, conducted 2 weeks (±1 week) after the initial screening visit:
-
EuroQol-5 Dimensions, five-level version (EQ-5D-5L):59 this is a validated, generalised health profile questionnaire used to assess HRQoL. EQ-5D-5L scores are used to derive utilities, which can be used to calculate quality-adjusted life-years (QALYs).
-
Service use questionnaire (SUQ): we used a bespoke self-report SUQ to assess NHS health-care utilisation. The data collected included use of community-based services, such as contacts with general practitioners (GPs), practice nurses, other community-based professionals and community-based social care services and medication. Use of hospital services [including outpatient appointments, accident and emergency department attendance, day-care services and hospitalisation] was also captured. We asked participants to report services used for their memory problems and for other reasons separately. The period covered by the questionnaire was the previous 3 months. This was considered long enough for people to have received a range of services but not so long that they would have forgotten what they had received. The SUQ was adapted from a questionnaire used in previous studies. 60,61
In addition, the participants’ nominated relative/friend completed the following questionnaire prior to the visit, which was collected by the AP:
-
Everyday Memory Questionnaire – relative version (EMQ-r):43 this is a parallel version of the EMQ-p that offers an independent rating by a relative/friend of the memory problems that a person experiences. The EMQ-r was included to identify any effect of treatment on daily life problems as observed by another person, which might not have been detected by the participants themselves. EMQ-r scores range from 0 to 112, with higher scores indicating more frequent memory problems.
Participants were also asked to set the short- and long-term goals that they would like to achieve by the end of the study. With the assistance of the AP, each participant set between one and five personal short- and long-term goals. The AP also checked participants’ availability to attend for treatment in the event that they were assigned to the memory rehabilitation group, in order to form clusters of participants for randomisation.
Randomisation
Eligible patients were randomised following screening and baseline data collection.
Formation of clusters
Clusters of four to six participants were formed by the AP at each site prior to randomisation. This cluster size was selected as we considered this the optimal number of participants for the memory rehabilitation group sessions based on our previous experience of delivering the intervention. Furthermore, if the cluster sizes were larger participants may have needed to wait longer after the baseline assessments for a group to be formed. Clusters were based on participants’ availability to attend for treatment at the same time and same venue, should they be allocated to the memory rehabilitation arm. In the period while waiting for group allocation, the AP remained in regular contact with the participants to inform them when it was likely that there would be sufficient participants to form a group and to maintain their interest in the trial.
Participants who were awaiting randomisation at the time that their site closed to recruitment were sent a letter informing them that the AP had not been able to recruit enough people to create a group at a time and place that was convenient for them and that their participation in the trial was therefore at an end.
Randomisation
Participants were randomised in clusters of four to six to the memory rehabilitation arm or a usual-care control using a 1 : 1 ratio. The randomisation was based on a computer-generated pseudo-random code using random permuted blocks of randomly varying size, created by the Nottingham Clinical Trials Unit (NCTU) in accordance with its standard operating procedure (SOP) and held on a secure server. The randomisation was stratified by study site. Access to the sequence was limited to the NCTU information technology manager. The AP at the site accessed the allocation for each cluster by means of a remote, internet-based randomisation system developed and maintained by the NCTU. The sequence of treatment allocations was concealed from the study statistician until all participants were assigned and recruitment and data collection and all other study-related assessments were complete.
Intervention
Participants were randomised to group memory rehabilitation in addition to usual care or usual care alone.
Usual care
All participants received their usual clinical care during the trial. Based on our knowledge of the recruiting sites, we expected that the majority of participants would no longer be receiving any formal rehabilitation but that they may be attending employment rehabilitation services or self-help groups or receiving support from specialist charities, such as Headway. Any additional interventions that people received were noted in the SUQ completed at the follow-up assessments.
Memory rehabilitation
The intervention was offered in groups of between four and six participants. Each group was led by an AP trained to deliver the intervention. Sessions were held at NHS sites or community venues. Participants were offered 10 group memory rehabilitation sessions, lasting approximately 1.5 hours each, which were planned to take place once a week for 10 weeks. However, sessions could be rearranged if necessary (e.g. because of staff or participant absence). The sessions followed a treatment manual. 18 The intervention included restitution strategies to retrain memory functions, including attention retraining (such as letter cancellation), and strategies to improve encoding and retrieval (such as deep-level processing). Compensation strategies were taught, including mnemonics (such as chunking, use of first letter cues, rhymes), use of external devices (such as diaries, mobile phones and calendars) and ways of coping with memory problems. The importance of ‘errorless learning’ (not making errors while learning new material and, therefore, preventing learning the errors)62 was also taught. The emphasis was on identifying the most appropriate strategies to help individuals overcome their memory problems and on providing participants with a range of memory techniques that they could adapt and use depending on their needs. This intervention provided an opportunity for revision of strategies taught during inpatient rehabilitation and discussion of their application in a community setting.
Treatment fidelity
The fidelity of the group rehabilitation programme was assured in a number of ways:
-
Manualised treatment. The group memory rehabilitation programme followed a manual (see Report Supplementary Material 1) that was developed and tested in the pilot study. A detailed description of the manual has been published. 18,23 The manual was accompanied by facilitator notes to guide delivery of the sessions (see Report Supplementary Material 2).
-
Training and supervision. Staff delivering the intervention (APs) were psychology graduates with clinical experience. A clinical psychologist provided study-specific training on conducting baseline assessments and the delivery of the intervention. In addition, monthly teleconferences between all APs, a clinical psychologist and NCTU staff provided an opportunity for peer group supervision. Furthermore, additional monthly one-to-one supervision with a clinical psychologist allowed for discussion of specific challenges relating to treatment or assessment. To ensure continuity and consistency, when staff changes occurred, old staff completed a ‘handover’ document for new staff, who were trained by the same trainers.
-
Fidelity assessment. Formal fidelity assessment of the group memory rehabilitation was undertaken through analysis of video recordings of treatment sessions. Intervention sessions were video recorded by APs facilitating the groups. APs were asked to video record all treatment groups, unless it was the first group run by the AP or participants had not given consent to be recorded. Practices for video recording drew on guidance on minimising the intrusiveness of the recording. 63,64
Sessions were selected for analysis in order to include sessions from the start, middle and end of the 10-week course and from each site. As far as possible, only recordings that covered a complete session were analysed. A coding schedule was developed based on the components of treatment described in the manual, listing possible activities for both the APs and participants (see Appendix 3).
A distinction was made between non-rehabilitation activities (e.g. social chat, information about sessions, preparing tasks or materials) and rehabilitation activities (e.g. discussing educational material, recap of previous session). An independent researcher coded the videos using a time sampling procedure. Observations were made on the minute, every minute, throughout the video recording. For each observation, the activities of the AP and participants were given the appropriate activity code. A sample of coding was checked by another observer and discrepancies were resolved by discussion. Data from coding sheets were entered into the SPSS statistics programme for analysis (version 21; IBM Corporation, Armonk, NY, USA).
Requirements for usual care were not specified and so no measures of fidelity were applied.
Blinding
Blinding of participants and the APs delivering the intervention to treatment allocation was not possible. RAs collecting outcome data at the 6- and 12-month follow-up visits were blind to treatment allocation. RAs were not involved in the delivery of the intervention. To prevent unblinding, at the start of each follow-up visit, the RAs reminded participants of the importance of the RAs remaining blind to treatment allocation and asked that participants did not discuss any aspects of their involvement in the study. At the beginning of each follow-up visit, the RAs recorded whether they had been unblinded prior to the visit and recorded their opinion of each participant’s treatment allocation using the following categories: definitely control, probably control, probably intervention or definitely intervention. At the end of each visit, the RAs recorded whether they had been unblinded during the visit and again recorded their opinion of each participant’s treatment allocation.
Follow-up
Outcomes were assessed at 6 and 12 months after randomisation to assess the immediate and longer-term effects of the intervention. The primary time point of interest was 6 months after randomisation. This time point was chosen to allow time to complete the 10 group sessions, while still allowing for one group session to be rescheduled if it had to be cancelled through illness or for other unforeseen circumstances. The 12-month assessment was carried out to determine whether or not any treatment gains had been maintained over time.
All reasonable attempts were made to contact any participant lost to follow-up during the course of the study in order to complete assessments. Participants were contacted by telephone in the first instance when follow-up visits were due. If telephone contact could not be made, a letter was sent to the participant’s last known address, so that the participant could contact the outcome assessor to arrange the appointment or provide updated contact details.
The following assessments were completed at the follow-up visits:
-
RBMT-3.
-
Assessment of individual goal attainment: each participant’s individual goals were evaluated in terms of the degree to which each goal had been met on a four-point Likert scale: ‘not met at all’, ‘met a little’, ‘mostly met’ and ‘fully met’. The participant and researcher discussed the extent to which goals were met and jointly determined the goal attainment. The average goal attainment score was used as the secondary outcome (with attainment coded as 0 for ‘not met at all’ and 3 for ‘fully met’). Goal attainment scaling (GAS) has been used in memory rehabilitation studies and has been recommended as an outcome measure of choice for cognitive rehabilitation. 65
In addition, a questionnaire pack was posted to participants before their 6- and 12-month appointments. Participants were asked to complete this questionnaire pack at home and return it by post to the trial co-ordinating centre in the prepaid envelope provided as soon as possible. If questionnaires had not been returned by the time of the follow-up visit they were collected by the RA at the follow-up visit.
The questionnaire pack included the following questionnaires for completion by participants:
-
EMQ-p.
-
GHQ-30.
-
EQ-5D-5L.
-
SUQ.
-
European Brain Injury Questionnaire – patient version (EBIQ-p):66,67 this contains 63 items that assess the subjective experience of cognitive, emotional and social difficulties experienced by people with brain injury; there are an additional three items that ask about the impact of the participant’s brain injury on their relative/friend. Each item is rated on a three-point Likert scale, ‘not at all’, ‘a little’ or ‘a lot’, depending on how much each has been experienced over the past month. The EBIQ-p is a clinically reliable measure that is used to determine the subjective well-being of people with brain injury and to assess change in subjective concerns over time. 67,68 It is used in rehabilitation centres as an outcome measure. 68 We used the modified subscales proposed by Bateman et al. 69 In this model, EBIQ-p scores range between 1 and 3 on each of seven subscales, with higher scores indicating greater difficulties. The seven subscales are:
-
somatic (seven items)
-
cognitive (12 items)
-
impulsivity (10 items)
-
depression (five items)
-
social interaction (five items)
-
fatigue (eight items)
-
communication (four items).
-
In addition, the following questionnaires, for completion by the participants’ nominated relative/friend, were included in the questionnaire pack sent to participants. Participants were asked to pass these on to their relative/friend for completion:
-
EMQ-r.
-
European Brain Injury Questionnaire – relative version (EBIQ-r). 67 This is a parallel version of the EBIQ-p, completed by the participant’s relative/friend to assess the cognitive, emotional and social difficulties experienced by people with brain injury.
Originally, all questionnaires were intended to be returned by post only; however, the procedure was changed after a HTA programme monitoring visit in December 2014 so that follow-up questionnaires were collected at the follow-up visit by the outcome assessor if these had not been posted back. This was in response to a poor return rate because of the previous reliance on postal returns.
The returned questionnaire packs were checked for completeness and participants were telephoned if items were missing or we needed clarification about their responses (e.g. unclear marking on questionnaires). Participants were also telephoned if their questionnaire packs were not received before the follow-up visit.
Qualitative feedback interviews
A sample of participants was invited to take part in qualitative feedback interviews, conducted within 2 months of the 6-month follow-up appointment. The interviews were intended to provide feedback on the participants’ experience of being involved in the trial, their experience of usual care and, for those in the intervention groups, their experience of receiving group memory rehabilitation. The qualitative analysis is described in detail in Chapter 5.
End of the study
Participants left the study when they had completed the 12-month follow-up. The end of the study was defined as the time of the last participant’s 12-month follow-up appointment, although questionnaires were accepted after completion of the final visit to allow for any delays in return.
Premature discontinuation from the intervention or withdrawal from follow-up was reported and reasons for withdrawal (if given) were documented. If a participant discontinued treatment but agreed to remain in the trial, outcome data collection continued in accordance with the protocol1 [the protocol is also available at www.journalslibrary.nihr.ac.uk/programmes/hta/105724/#/ (accessed 1 August 2018)]. Participants were informed at the start of the study that data collected up to the point of withdrawal would be retained and used in the final analysis. We did not replace participants who withdrew.
Outcome measures
All measures were selected on the basis of their clinical utility, relevant psychometric properties and ease of use for participants. Furthermore, the measures reflect the three levels of the ICF31 domains – impairment, activity limitations and participation restrictions – thereby embracing the aims and spirit of cognitive rehabilitation. 70,71
Primary outcome
-
Frequency of memory failures in daily life assessed using the EMQ-p at 6 months’ follow-up.
Secondary outcomes
-
Objective measure of memory problems assessed by the RBMT-3.
-
Mood assessed with the GHQ-30.
-
Individual goal attainment.
-
Subjective experience of brain injury assessed with the EBIQ-p.
-
Subjective report of frequency of memory problems in daily life in the longer term assessed using the EMQ-p.
-
Subjective report of the importance of memory problems in daily life. To assess this, we added to the EMQ-p a measure of how important each item was. The rationale was that, even if some items were forgotten less frequently than others, these may be more significant if participants viewed these items as being more important than other items. The 28 EMQ-p items were therefore also rated for importance on a five-point Likert scale (from ‘not at all important’ to ‘very important’). Importance scores ranged from 0 to 112, with higher scores indicating more important memory problems.
Relative/friend-completed outcomes
-
Relative/friend report of the frequency of participants’ memory problems in daily life assessed using the EMQ-r.
-
Relative/friend report of the experience of brain injury assessed using the EBIQ-r.
Health economic outcomes
-
Quality of life assessed using the EQ-5D-5L.
-
Service use assessed using the bespoke SUQ.
Research governance
The study was conducted in accordance with the recommendations for clinicians involved in research on human subjects adopted by the 18th World Medical Assembly (Helsinki, 1964)72 and later revisions, the NHS Research Governance Framework for Health and Social Care (second edition)73 and the principles of the International Conference of Harmonisation Good Clinical Practice guidelines. 74
Trial registration
The study was prospectively registered as ISRCTN65792154 on 17 October 2012.
Ethics
The National Research Ethics Service – East Midlands (Nottingham 1) gave ethics approval for the study for NHS participants (reference 12/EM/0324) and the Ministry of Defence Research Ethics Committee gave approval for recruiting military participants (reference 374/PPE/12).
Site initiation and training
Prior to the commencement of the study, members of the central research team (chief investigator and/or co-chief investigator and NCTU staff) met with study collaborators from each site to discuss implementation and training issues to ensure that all staff members were familiar with all aspects of the study. New staff were trained before starting work on the trial. A clinical psychologist and the NCTU staff provided study-specific training on the trial documentation and database.
Protocol deviations
A protocol deviation was defined as an unanticipated or unintentional divergence or departure from the expected conduct of the study inconsistent with the protocol, consent document or other study procedures. Protocol deviations were recorded on the electronic case report form by APs and RAs. Protocol violations were defined as deviations that affected eligibility or outcome measures, as assessed by the Trial Management Group (TMG).
Oversight
We convened a number of oversight groups to monitor study progress and conduct throughout the trial. The general roles and responsibilities of these groups were outlined in the protocol, with specific charters also developed for the independent Trial Steering Committee (TSC) and Data Monitoring Committee (DMC).
Trial Management Group
The TMG comprised the co-chief investigators and members of the NCTU responsible for the running of the trial, who met regularly throughout the trial. This group was responsible for the day-to-day running of the trial.
Trial Steering Committee
The TSC was responsible for overseeing the conduct of the study. The TSC had an independent chairperson and five independent members. Independent members were rehabilitation professionals and patient/carer representatives who were not otherwise involved in the trial. Members of the study team, including the chief investigator, co-chief investigator, service user co-applicant and trial manager, were also part of the TSC. The TSC advised on recruitment strategies, monitored the progress of recruitment and checked adherence to the study protocol. Observers from the funder and the sponsor were invited to TSC meetings.
Data Monitoring Committee
The DMC was an independent group, the members had no other involvement with the study. Members of this committee included two rehabilitation professionals and an experienced study statistician. The role of the DMC was to safeguard the interests of trial participants, with particular reference to the safety of the intervention, monitor the overall progress and conduct of the trial and assist and advise the investigators to protect the validity and credibility of the trial.
The TSC and the DMC met independently of each other, with the DMC providing reports to the TSC.
Safety monitoring
The risks of the study were assessed during protocol development. The assessment of memory may have made participants aware of memory problems that they did not know that they had. As a result, the main risk associated with this trial was considered to be distress caused by this realisation. However, such distress was considered unlikely and any distress caused was deemed likely to be mild. Overall, therefore, the risk of the trial was assessed as negligible. As a result, data on adverse events or serious adverse events were not collected in this study. The independent DMC instead was provided with a report detailing hospital and GP visits (either related to TBI or otherwise), recorded from participant-reported SUQs for all participants, and any deaths. This was agreed by the sponsor, the Research Ethics Committee, the DMC and the TSC.
‘Notable events’ occurring during both assessments and treatment were recorded throughout the trial. Notable events were those that were assessed by the APs or RAs as being out of the ordinary, such as problems arising during group sessions or issues that might pose a risk to participants or researchers. These were reviewed by the study team on an ongoing basis and were reported to the DMC/TSC during routine meetings.
Patient and public involvement
During protocol development, service user and carer representatives with experience of TBI and/or rehabilitation in NHS services advised on recruitment and dissemination options and contributed to the development of the intervention manual and the lay summary of the project. One service user representative had experience of NHS rehabilitation services following his head injury and participated in our pilot study, so was able to provided first-hand experience of the intervention. He told us what he and the peers in his group enjoyed and found useful and what they did not find useful. This information enabled us to make some changes to the manual and content of the intervention. We also recruited a carer representative who had caring responsibilities for a person with TBI. The service user co-applicant and a carer helped us advertise the study by being part of a video about our study and by taking part in a radio and television interview about brain injury and our study. The service user co-applicant was involved in project management decisions, project approval through the Integrated Research Application System and recruitment and consent (by contributing to the development of participant information sheets).
A service user and carer and representatives from relevant charities (e.g. Headway and Combat Stress) were members of the TSC and DMC. Their involvement in these committees enabled us to check with them when any amendments to the protocol were required.
We developed participant and public newsletters to keep participants and the public informed about the progress of our study. These were sent to participants, clinicians and local head injury charities so that they could be cascaded to interested members of the public. We sent the final plain English summary of our findings to our carer representative to assess its readability and we made changes where these were required. All service user involvement was resourced appropriately.
Payments to participants
Participants were not paid to take part in the trial but reasonable travel expenses for attendance at trial assessments and intervention sessions were reimbursed.
Statistical methods
Sample size
The sample size calculation was based on the primary outcome measure (EMQ-p) at 6 months post randomisation. The main study aim was to detect a minimum clinically relevant difference in mean EMQ-p score of 12 points between the memory rehabilitation arm and the usual-care arm. In the absence of any agreed and published minimum clinical relevant differences on the EMQ-p, we deemed a 12-point difference on this measure to be a clinically significant change, based on our pilot data18 and clinical interviews. A common SD of 21.9 from the pilot study gave us an effect size of 0.55. For the sample size calculation, a two-sided type 1 error of 0.05 and power of 90% and a fixed-effects model at the level of the four original planned sites were used, with 10% of the total variation due to between-site variation. The participants were cluster randomised and a cluster size of six was used for the sample size calculation, with an intracluster correlation coefficient (ICC) of 0.1. Using the Optimal Design software (version 3.01; William T. Grant Foundation, New York, NY, USA) with these parameters, the calculation gave 10 clusters (five clusters for each allocation) per site (40 in total). Data from the pilot study, and taking account of the fact that the control arm received only usual care, suggested a possible dropout rate of 20%. Therefore, we needed 26 clusters for each allocation (52 in total), which amounted to 312 participants randomised in total.
Statistical analysis
Statistical analyses are detailed in the statistical analysis plan (SAP) [URL: www.journalslibrary.nihr.ac.uk/programmes/hta/105724/#/ (accessed 17 August 2018)], which was finalised prior to database lock and release of the treatment allocation codes for analysis. All analyses were carried out using Stata®/SE 13.1 (StataCorp LP, College Station, TX, USA).
Preliminary analysis
We used descriptive statistics of demographic and clinical measures to examine balance between the two arms. The internal consistency of the EMQ-p, EMQ-r and GHQ-30 was also evaluated using Cronbach’s alpha.
Analysis populations
The main approach used in the analysis was to analyse participants as randomised regardless of the number of memory rehabilitation sessions attended [intention to treat (ITT)], for all primary and secondary outcomes.
Data used at each time point were as follows:
-
the outcomes at 6 months were questionnaires/visits completed within 9 months of randomisation (i.e. within 275 nights of randomisation)
-
the outcomes at 12 months were questionnaires/visits completed within 15 months of randomisation (i.e. within 456 nights of randomisation).
Outcomes completed outside these time periods were not used, other than in a sensitivity analysis for the primary outcome. The main analyses were based on participants with available data, with no imputation for participants with missing outcomes.
Descriptive analyses
We described the adherence to the intervention by tabulating the attendance at each session and summarising the number of sessions that each participant attended. The reasons for non-attendance at sessions were also described and summarised.
The numbers of participants returning the questionnaire booklet and completing the 6- and 12-month follow-up visits were summarised in the two arms along with the number of days between randomisation and completion. The pattern of missing outcome data was explored, overall and in the two arms, and baseline characteristics were compared between participants with and without primary outcome data.
Missing data in questionnaires
We imputed missing items in questionnaires using the mean of the completed items if < 10% of the items in the questionnaire were not completed. Scores were therefore calculable when ≥ 25 of the 28 items were completed on the EMQ-p and EMQ-r, ≥ 27 of the 30 items were completed on the GHQ-30, ≥ 11 of the 12 items were completed on the EBIQ-p and EBIQ-r cognitive subscale and ≥ 9 of the 10 items were completed on the EBIQ-p and EBIQ-r impulsivity subscale. Scores for all other EBIQ-p and EBIQ-r subscales were calculable only if all items in the subscale were completed. If > 10% of items were missed, outcomes were treated as missing.
If scores from the questionnaires remained missing at baseline after the process outlined above (or other baseline information was missing), in order to be able to include all participants in the regression analysis of the outcome score, we imputed these baseline data using the mean score at each site. These simple imputation methods are superior to more complicated imputation methods when baseline variables are included in an adjusted analysis to improve the precision of the treatment effect. 75 Note that this imputation was carried out only for the regression analyses and not for summarising the baseline scores.
Primary outcome
For the primary analysis we estimated the difference in mean EMQ-p score between the two arms at the 6-month follow-up using a multilevel linear model, with baseline EMQ-p score and site as covariates. Although participants were randomly allocated in clusters, individuals in the usual-care arm had no contact with each other and outcomes in this arm were therefore assumed to be independent. However, participants in the intervention arm attended group memory rehabilitation sessions, which needed to be accounted for in the analysis. We therefore used a fully heteroscedastic model, as suggested by Roberts and Roberts,76 for the analysis of trials comparing group-based treatments with individual-based treatment as usual, when, as is the case here, there is adjustment for individual-level covariates. This model estimates group-level residual variance in the intervention arm and also permits individual-level residual variance to differ between the intervention arm and the control arm. 76,77 Assumptions made in the multilevel linear model were checked using diagnostic plots. The ICC in the intervention arm was estimated using the estimates of the group-level residual variance and individual residual variance in the intervention arm. 77
Sensitivity analyses for the primary outcome
We conducted the following sensitivity analyses:
-
Including all 6-month questionnaires. We repeated the analysis described above including participants whose 6-month questionnaires were returned after the 9-month post-randomisation window.
-
Additional adjustment for baseline variables with an observed imbalance. We included baseline variables with an observed imbalance (based on comparison of summary statistics only, not statistical testing) as additional covariates in the multilevel model for the 6-month EMQ-p score.
-
Multiple imputation of missing primary outcome data. We performed multiple imputation using chained equations (MICE) separately for each arm, under the assumption that missing data were missing at random. 78 Variables included in the imputation model were site, age and gender, baseline variables identified as predictive of dropout (by examination only), prognostic baseline variables (EMQ-p, RBMT-3 GMI and GHQ-30), RBMT-3 GMI at the 6- and 12-month visit and 12-month EMQ-p score. In addition, for the intervention arm, the number of intervention sessions attended was included. Forty data sets were imputed and the results of the analyses on the imputed data sets were combined using Rubin’s rules. 78
-
Estimation of the complier average causal effect. We used instrumental variable regression to estimate the effect of the intervention for participants who would comply with the allocated treatment whichever arm they were randomised to. 79,80 Participants in the intervention arm were classified as adherent if they attended at least four memory rehabilitation sessions. The instrumental variable regression model included baseline EMQ-p score and recruiting site and used a clustered sandwich estimator to estimate the variance to allow for correlation between randomisation clusters (vce cluster option in Stata). We estimated the complier average causal effect using both the observed data and the multiply imputed data.
We performed a prespecified exploratory subgroup analysis for the primary outcome according to memory impairment at baseline (using the RBMT-3 GMI score, an objective measure of memory) by including an interaction term in the model for the primary analysis. The RBMT-3 GMI score at baseline was categorised into three groups on the basis of classifications provided by the test publisher:81 significant memory impairment (scores of ≤ 69), borderline/moderate memory impairment (scores of 70–84) and average and above average range (scores of ≥ 85).
During the trial, a Rasch analysis of the EMQ-p was performed using an independent data set of patients with TBI (Rachel Johnson, Roshan das Nair and Nadina B Lincoln, University of Nottingham, 2017, personal communication). We performed an exploratory analysis using this Rasch conversion of the EMQ-p scores and compared scores between the two arms using the multilevel model described above.
After the planned analyses were conducted, at two meetings with collaborators and investigators, time since TBI was raised as a potentially important factor with regard to whether or not patients could benefit from the intervention. We therefore conducted a post hoc subgroup analysis of time since TBI, using the methods described above.
Secondary outcomes at 6 and 12 months
We analysed the secondary outcomes using the multilevel model described for the primary outcome. Estimates of the intervention effect are presented as difference in means with 95% confidence intervals (CIs). For the goal attainment outcome, the number of goals set was additionally included as a covariate. Estimates of the ICC in the memory rehabilitation arm for each outcome, calculated from the multilevel models, are provided in Appendix 4.
Of the seven subscales of the EBIQ-p (and EBIQ-r), the cognitive, depression, communication and difficulties in social interaction subscales were used in a formal comparison between arms, as the content of the group memory intervention was most likely to have an impact on these subscales. The other subscales (impulsivity, somatic and fatigue) were summarised using descriptive statistics only.
Sensitivity analyses for goal attainment secondary outcomes
Participants set at least one short- and one long-term goal but could set up to five. An interaction term between the number of goals set (one or more than one) and treatment arm was included in the model for the goal attainment score to explore whether there was evidence of any differential effect of the intervention according to the number of goals set at baseline. We hypothesised that it could be harder for participants who set more than one goal to meet all of their goals than for participants who set, and who therefore focused on, one individual goal.
Goals set at the start of the trial should have been SMART (specific, measurable, assignable, realistic and time-related) goals so that they could be assessed at the 6- and 12-month follow-up visits as being met or not being met. During the trial it became apparent that not all goals set by the APs at baseline were measurable. As a sensitivity analysis, each goal was classified as SMART or not by one of the trial APs and a sample was independently checked by the chief investigator. We then repeated the analysis for goal attainment including only SMART goals.
Chapter 3 Results
Recruitment
Recruitment commenced in February 2013 and continued until December 2015 when the recruitment target was met (see Appendix 5). The original planned recruitment period was extended by 8 months because recruitment rates were lower than expected. This was in part because of staff turnover and delays in recruiting new staff, resulting in a number of sites being inactive during the recruitment period.
Between February 2013 and December 2015, we screened 4023 people and consented 466. Of the 3557 people screened but not consented, the main reason was not replying to the letter of invitation (n = 1710, 43%); 1129 (28%) people were not eligible for the trial and 718 (18%) were not enrolled for other reasons (Figure 1). Further details are provided in Appendix 6.
FIGURE 1.
Participant flow chart. Reproduced from das Nair et al. 82 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
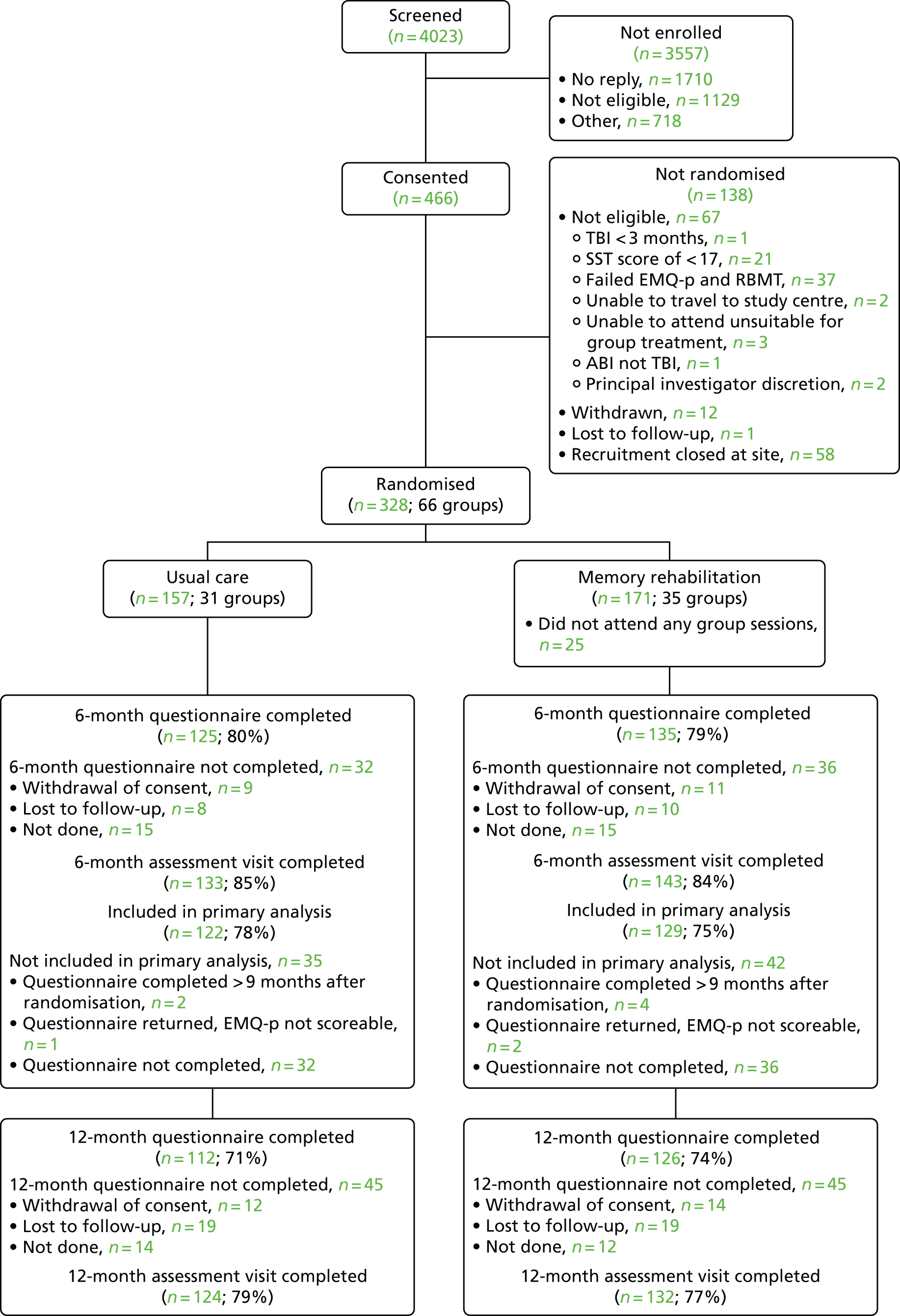
Of the 466 people who gave consent, 328 (70%) were randomised. Non-randomisation after consent was the result of non-eligibility, recruitment being closed at the site (because of the site closing either during the trial or at the end of recruitment in December 2015) and participants withdrawing consent or no longer being contactable (see Figure 1).
Of the 328 participants randomised, 157 (48%) were randomised to usual care and 171 (52%) to memory rehabilitation in addition to usual care (see Figure 1). The mean size of the cluster randomised was five. The randomisation target of 312 was exceeded because of the requirement to randomise clusters of participants who could attend the intervention sessions at the same time, if allocated to the intervention, at the five sites remaining open at the end of recruitment.
Participants were randomised in clusters of four to six and the randomisation was stratified by site. The number of clusters randomised to each arm within each site was well balanced (see Appendix 7). More participants were randomised to the memory rehabilitation arm.
Participants waited a median of 18 days between the second assessment and randomisation (see Appendix 7). However, a small number of participants (n = 23) waited for ≥ 6 months to be randomised; this was because they had to wait both for other participants who could attend the intervention sessions at the same time and for the AP to be available within the site to deliver the intervention.
Baseline data
The mean age of participants was 45 years (SD 12 years), 239 (73%) were men and almost all (96%) were white (Table 1). We randomised 31 participants (9%) who were serving or who had served in the military, including participants from the Territorial Army and reservists (see Table 1). There was a wide variation in the time since the TBI at randomisation, from 3 months to almost 49 years. The median time since TBI was just over 4 years (see Table 1).
| Characteristic | Trial arm | Total (N = 328) | |
|---|---|---|---|
| Usual care (N = 157) | Memory rehabilitation (N = 171) | ||
| Age (years) | |||
| Mean (SD) | 45.1 (12.5) | 45.8 (11.5) | 45.4 (12) |
| Median (25th, 75th centile) | 45 (36, 55) | 47 (38, 54) | 46 (36, 54) |
| Min., max. | 19, 69 | 20, 68 | 19, 69 |
| Gender, n (%) | |||
| Men | 116 (74) | 123 (72) | 239 (73) |
| Women | 41 (26) | 48 (28) | 89 (27) |
| Ethnicity, n (%) | |||
| White | 147 (94) | 167 (98) | 314 (96) |
| Black | 6 (4) | 2 (1) | 8 (2) |
| Mixed race | 3 (2) | 1 (1) | 4 (1) |
| Other | 1 (1) | 1 (1) | 2 (1) |
| Residential status, n (%) | |||
| Lives alone | 44 (28) | 43 (25) | 87 (27) |
| Lives with others | 106 (68) | 120 (70) | 226 (69) |
| Living with informal carer | 2 (1) | 1 (1) | 3 (1) |
| Living with formal carer | 2 (1) | 0 | 2 (1) |
| Living in care home | 3 (2) | 7 (4) | 10 (3) |
| Highest educational attainment, n (%) | |||
| Below GCSE | 26 (17) | 29 (17) | 55 (17) |
| GCSE | 54 (34) | 49 (29) | 103 (31) |
| A level | 42 (27) | 34 (20) | 76 (23) |
| Degree | 24 (15) | 41 (24) | 65 (20) |
| Higher degree | 10 (6) | 17 (10) | 27 (8) |
| Not known | 1 (1) | 1 (1) | 2 (1) |
| Employment status at screening (not mutually exclusive), n (%) | |||
| Not employed | 80 (51) | 85 (50) | 165 (50) |
| Employed full time | 25 (16) | 38 (22) | 63 (19) |
| In education full time | 2 (1) | 1 (1) | 3 (1) |
| Voluntary full time | 1 (1) | – | 1 (< 0.5) |
| Retired | 17 (11) | 15 (9) | 32 (10) |
| Employed part time | 25 (16) | 19 (11) | 44 (13) |
| Voluntary part time | 9 (6) | 17 (10) | 26 (8) |
| Current military service,a n (%) | |||
| Military | 4 (3) | 0 | 4 (1) |
| TA/reservist | 0 | 2 (1) | 2 (1) |
| Non-military | 153 (97) | 169 (99) | 322 (98) |
| Previous military service, n (%) | |||
| Military | 14 (9) | 11 (6) | 25 (8) |
| TA/reservist | 2 (1) | 4 (2) | 6 (2) |
| Non-military | 141 (90) | 156 (91) | 297 (91) |
| TBI during service | 3 (2) | 1 (1) | 4 (1) |
| Time since TBI (months)b | |||
| Mean (SD) | 99 (114.8) | 102.6 (113.4) | 100.9 (113.9) |
| Median (25th, 75th centile) | 46 (23, 116) | 58 (24, 148) | 52 (24, 129.5) |
| Min., max. | 4, 520 | 3, 587 | 3, 587 |
| Length of initial hospital stay for TBI (days)c | |||
| Mean (SD) | 81.8 (108.6) | 86.5 (143.5) | 84.2 (127.7) |
| Median (25th, 75th centile) | 35 (7, 120) | 35.5 (10, 93.5) | 35 (9, 116) |
| Min., max.d | 0, 468 | 0, 999 | 0, 999 |
| n | 148 | 160 | 308 |
| Length of hospital stay unknown, n (%) | 9 (6) | 11 (6) | 20 (6) |
Characteristics assessed at baseline were well balanced, although a greater proportion of participants in the memory rehabilitation arm than in the usual-care arm had a degree or higher degree and the median time since TBI was slightly longer in the memory rehabilitation arm (approximately 4 years in the usual-care arm and approximately 5 years in the memory rehabilitation arm).
Data from the memory, mood and quality-of-life assessments completed prior to randomisation are shown in Table 2. Scores were well balanced between arms; however, when the RBMT-3 GMI was categorised into levels of memory impairment a smaller percentage of participants in the memory rehabilitation arm than in the usual-care arm were classified as having a significant impairment (39% in the usual-care arm vs. 29% in the memory rehabilitation arm; see Table 2).
| Assessment | Trial arm | Total (N = 328) | |
|---|---|---|---|
| Usual care (N = 157) | Memory rehabilitation (N = 171) | ||
| EMQ-p – frequency of problems | |||
| Mean (SD) | 50.1 (24.6) | 47.4 (21) | 48.7 (22.8) |
| Median (25th, 75th centile) | 50 (33, 65.2) | 47.7 (30, 63) | 48 (32, 64) |
| Min., max. | 0, 105 | 5, 102 | 0, 105 |
| na | 156 | 171 | 327 |
| EMQ-p – importance of problems | |||
| Mean (SD) | 70.6 (22.4) | 65.7 (23.5) | 68 (23) |
| Median (25th, 75th centile) | 72 (56, 87) | 69 (51, 83) | 70.5 (54, 84) |
| Min., max. | 2, 112 | 0, 112 | 0, 112 |
| n | 152 | 170 | 322 |
| RBMT-3 GMI | |||
| Mean (SD) | 76.3 (14.5) | 77.7 (13.6) | 77 (14) |
| Median (25th, 75th centile) | 75 (65, 85) | 77 (67, 85) | 76 (66.5, 85) |
| Min., max. | 53, 114 | 53, 127 | 53, 127 |
| n | 157 | 171 | 328 |
| Level of memory impairment based on the RBMT-3, n (%) | |||
| Significant memory impairment (score of ≤ 69) | 61 (39) | 50 (29) | 111 (34) |
| Borderline/moderate memory impairment (score 70–84) | 54 (34) | 77 (45) | 131 (40) |
| Average or above average (score of ≥ 85) | 42 (27) | 44 (26) | 86 (26) |
| GHQ-30 | |||
| Mean (SD) | 35.3 (16.3) | 36.1 (15.4) | 35.8 (15.8) |
| Median (25th, 75th centile) | 33 (21, 47) | 34 (25, 45) | 33 (24, 45.3) |
| Min., max. | 6, 90 | 6, 84 | 6, 90 |
| n | 154 | 170 | 324 |
| Estimated premorbid IQ from the NART | |||
| Mean (SD) | 106.5 (10) | 108.1 (10.2) | 107.4 (10.1) |
| Median (25th, 75th centile) | 103 (99, 116) | 109.5 (100, 117) | 105 (100, 117) |
| Min., max. | 86, 126 | 87, 128 | 86, 128 |
| n | 155 | 170 | 325 |
| SST | |||
| Mean (SD) | 19.3 (0.9) | 19.4 (0.9) | 19.4 (0.9) |
| Median (25th, 75th centile) | 20 (19, 20) | 20 (19, 20) | 20 (19, 20) |
| Min., max. | 17, 20 | 17, 20 | 17, 20 |
| n | 157 | 171 | 328 |
| EMQ-r – frequency of problems | |||
| Mean (SD) | 46.4 (24.4) | 42 (28.4) | 44.1 (26.6) |
| Median (25th, 75th centile) | 46.7 (27, 65) | 35.3 (18.7, 64) | 38.5 (22, 64.5) |
| Min., max. | 0, 107 | 0, 108 | 0, 108 |
| n | 95 | 105 | 200 |
| EMQ-r – importance of problems | |||
| Mean (SD) | 71.5 (24.5) | 71.8 (21) | 71.7 (22.7) |
| Median (25th, 75th centile) | 76 (64, 87) | 72 (60, 88) | 75 (60, 87) |
| Min., max. | 0, 112 | 4, 112 | 0, 112 |
| n | 90 | 101 | 191 |
The internal consistency of the EMQ-p and GHQ-30 at baseline using Cronbach’s alpha was 0.93 for the EMQ-p and 0.95 for the GHQ-30.
Relative/friend participation in the trial
In total, 210 relatives or friends of participants agreed to take part in the trial by returning a questionnaire at baseline or follow-up (64% in each arm). The scores from the EMQ-r were similar in the two arms at baseline (see Table 2). The internal consistency of the EMQ-r using Cronbach’s alpha was 0.96.
Group memory rehabilitation sessions
Attendance at group sessions
Participants attended a mean of 6.3 sessions (SD 3.5 sessions), with 131 (77%) participants attending four or more sessions (Table 3). There were several reasons that participants did not attend sessions; these are shown in Table 3.
| Summary | Memory rehabilitation (N = 171) |
|---|---|
| Number of sessions attended | |
| Mean (SD) | 6.3 (3.5) |
| Median (25th, 75th centile) | 8 (4, 9) |
| Min., max. | 0, 10 |
| 0–2, n (%) | 36 (21) |
| 3–7, n (%) | 44 (26) |
| 8–10, n (%) | 91 (53) |
| At least four, n (%) | 131 (77) |
| Total number of sessions missed | 627 |
| Reason for missing sessions – number of sessions (number of participants)a | |
| Did not want to continue | 122 (16) |
| Withdrew from study | 52 (7) |
| Lost to follow-up (unable to contact) | 70 (7) |
| Forgot to attend | 11 (10) |
| Unwell | 83 (40) |
| Holiday | 59 (36) |
| Work/family commitments | 114 (34) |
| No reason given | 94 (23) |
| Otherb | 22 (16) |
Attendance at sessions decreased over time. Some groups were well attended throughout. In one group, the final four sessions were not attended by any participants (see Appendix 9).
In total, 17 APs delivered group sessions during the trial. The number of sessions that each AP ran ranged between 1 and 47, with a median of 20 (25th, 75th centile = 10, 28).
Analysis of treatment fidelity
The number of video recordings retrieved from each site are shown in Appendix 10 (see Table 35). No videos were retrieved from three sites. Sites 2 and 4 recruited only one intervention group each and, therefore, the sessions were not recorded as it was the first group conducted by the APs at those sites. At site 7, two intervention groups were recruited, one of which was the first for the AP; for the second group the recordings were not available for analysis. At two sites (sites 5 and 6) there were very few recordings retrieved as the APs did not understand that all sessions needed to be recorded. For 25 sessions the recording stopped partway through the session because of technical problems (e.g. the recorder battery being completely discharged). Overall, there were some recordings from six of the nine sites.
A selection of all recordings of complete sessions was analysed; seven recordings of complete sessions were not included because sufficient recordings from the site or the session had already been included in the analysis. In addition, as there were no complete recordings of session 1, two recordings that were almost complete were analysed for session 1. A summary of the video recordings analysed is provided in Appendix 10 (see Table 36). Between two and five recordings of each session were analysed. A total of 31 sessions were included in the fidelity analysis, approximately 9% of the 350 memory rehabilitation sessions delivered during the trial.
The frequency and percentage of each activity code were calculated for each session; these are provided in Table 4 for APs and Table 5 for participants.
| Category | Session | Total | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Non-rehabilitation | ||||||||||||||||||||||
| Introductions | 1 | 1.8 | 0 | 0 | 0 | 0 | 1 | 0.7 | 1 | 1.0 | 0 | 0 | 1 | 1.6 | 0 | 0 | 1 | 0.7 | 0 | 0 | 5 | 0.4 |
| Social chat | 3 | 5.5 | 2 | 1.3 | 2 | 4.5 | 2 | 1.4 | 4 | 3.9 | 3 | 2.1 | 0 | 0 | 9 | 4.2 | 8 | 5.8 | 5 | 4.1 | 38 | 3.2 |
| Preparing materials, tasks | 2 | 3.6 | 5 | 3.3 | 0 | 0 | 1 | 0.7 | 0 | 0 | 1 | 0.7 | 0 | 0 | 2 | 0.9 | 4 | 2.9 | 8 | 6.5 | 23 | 2.0 |
| Information about sessions, venue, group | 20 | 36.4 | 3 | 2.0 | 2 | 4.5 | 4 | 2.9 | 6 | 5.8 | 2 | 1.4 | 1 | 1.6 | 4 | 1.9 | 1 | 0.7 | 13 | 10.6 | 56 | 4.8 |
| Hospital visit discussion | 1 | 1.8 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.2 |
| Describing emotions and coping strategies | 1 | 1.8 | 3 | 2.0 | 0 | 0 | 1 | 0.7 | 0 | 0 | 0 | 0 | 1 | 1.6 | 0 | 0 | 1 | 0.7 | 1 | 0.8 | 8 | 0.7 |
| Rehabilitation skills | ||||||||||||||||||||||
| Facilitating discussion (non-specific prompts) | 5 | 9.1 | 3 | 2.0 | 0 | 0 | 13 | 9.4 | 0 | 0 | 5 | 3.4 | 0 | 0 | 4 | 1.9 | 4 | 2.9 | 7 | 5.7 | 41 | 3.5 |
| Providing feedback not directly related to manual | 1 | 1.8 | 5 | 3.3 | 0 | 0 | 3 | 2.2 | 5 | 4.9 | 0 | 0 | 0 | 0 | 5 | 2.3 | 5 | 3.6 | 4 | 3.3 | 28 | 2.4 |
| Providing encouragement/reassurance | 4 | 7.3 | 6 | 3.9 | 0 | 0 | 6 | 4.3 | 4 | 3.9 | 4 | 2.7 | 1 | 1.6 | 6 | 2.8 | 3 | 2.2 | 3 | 2.4 | 37 | 3.2 |
| Summarising | 0 | 0 | 1 | 0.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 | 1 | 0.7 | 0 | 0 | 3 | 0.3 |
| Paraphrasing | 1 | 1.8 | 8 | 5.2 | 0 | 0 | 4 | 2.9 | 2 | 1.9 | 2 | 1.4 | 1 | 1.6 | 3 | 1.4 | 1 | 0.7 | 0 | 0 | 22 | 1.9 |
| Rehabilitation activities | ||||||||||||||||||||||
| Presenting/discussing educational material | 13 | 23.6 | 81 | 52.9 | 21 | 47.7 | 41 | 29.7 | 24 | 23.3 | 48 | 32.9 | 12 | 19.7 | 57 | 26.8 | 31 | 22.6 | 41 | 33.3 | 369 | 31.5 |
| Presenting/discussing strategies | 1 | 1.8 | 2 | 1.3 | 18 | 40.9 | 53 | 38.4 | 49 | 47.6 | 75 | 51.4 | 44 | 72.1 | 102 | 47.9 | 60 | 43.8 | 16 | 13.0 | 420 | 35.8 |
| Providing general information on memory not related to manual | 2 | 3.6 | 25 | 16.3 | 1 | 2.3 | 2 | 1.4 | 2 | 1.9 | 2 | 1.4 | 0 | 0 | 3 | 1.4 | 2 | 1.5 | 3 | 2.4 | 42 | 3.6 |
| Recap of previous session | 0 | 0 | 9 | 5.9 | 0 | 0 | 7 | 5.1 | 5 | 4.9 | 4 | 2.7 | 0 | 0 | 17 | 8.0 | 15 | 10.9 | 22 | 17.9 | 79 | 6.7 |
| Total | 55 | 100 | 153 | 100 | 44 | 100 | 138 | 100 | 103 | 100 | 146 | 100 | 61 | 100 | 213 | 100 | 137 | 100 | 123 | 100 | 1173 | 100 |
| Category | Session | Total | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Non-rehabilitation | ||||||||||||||||||||||
| Introductions | 5 | 6.2 | 0 | 0 | 0 | 0 | 3 | 2.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 | 0 | 0 | 9 | 0.8 |
| Social chat | 14 | 17.3 | 6 | 8.7 | 5 | 21.7 | 13 | 8.8 | 22 | 14.8 | 10 | 8.1 | 2 | 5.9 | 40 | 22.7 | 46 | 26.1 | 27 | 13.8 | 185 | 15.8 |
| Preparing materials, tasks | 2 | 2.5 | 2 | 2.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.6 | 0 | 0 | 1 | 0.5 | 6 | 0.5 |
| Information about sessions, venue, group | 3 | 3.7 | 0 | 0 | 1 | 4.3 | 3 | 2.0 | 1 | 0.7 | 2 | 1.6 | 0 | 0 | 1 | 0.6 | 2 | 1.1 | 5 | 2.6 | 18 | 1.5 |
| Hospital visit discussion | 8 | 9.9 | 0 | 0 | 0 | 0 | 2 | 1.4 | 1 | 0.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0.9 |
| Describing emotions and coping strategies | 13 | 16.0 | 10 | 14.5 | 0 | 0 | 6 | 4.1 | 2 | 1.3 | 0 | 0 | 1 | 2.9 | 4 | 2.3 | 10 | 5.7 | 28 | 14.4 | 74 | 6.3 |
| Rehabilitation activities | ||||||||||||||||||||||
| Discussing/filling in educational material | 13 | 16.0 | 17 | 24.6 | 11 | 47.8 | 63 | 42.9 | 51 | 34.2 | 32 | 26.0 | 19 | 55.9 | 12 | 6.8 | 11 | 6.3 | 53 | 27.2 | 282 | 24.0 |
| Discussing strategies | 6 | 7.4 | 8 | 11.6 | 5 | 21.7 | 28 | 19.0 | 51 | 34.2 | 62 | 50.4 | 6 | 17.6 | 76 | 43.2 | 95 | 54.0 | 45 | 23.1 | 382 | 32.6 |
| Asking for information | 0 | 0 | 6 | 8.7 | 1 | 4.3 | 4 | 2.7 | 2 | 1.3 | 0 | 0 | 2 | 5.9 | 3 | 1.7 | 0 | 0 | 3 | 1.5 | 21 | 1.8 |
| Feedback on home activities | 0 | 0 | 5 | 7.2 | 0 | 0 | 4 | 2.7 | 13 | 8.7 | 4 | 3.3 | 3 | 8.8 | 14 | 8.0 | 6 | 3.4 | 20 | 10.3 | 69 | 5.9 |
| Describing problems related to memory | 17 | 21.0 | 15 | 21.7 | 0 | 0 | 21 | 14.3 | 6 | 4.0 | 13 | 10.6 | 1 | 2.9 | 25 | 14.2 | 5 | 2.8 | 13 | 6.7 | 116 | 9.9 |
| Total | 81 | 100 | 69 | 100 | 23 | 100 | 147 | 100 | 149 | 100 | 123 | 100 | 34 | 100 | 176 | 100 | 176 | 100 | 195 | 100 | 1173 | 100 |
The main non-rehabilitation activity of APs was providing information about sessions, which occurred in just under 5% of AP observations. The main rehabilitation activities of APs were discussing the educational material (31.5%) and discussing memory strategies (35.8%). The main rehabilitation skills of APs were facilitating discussion and providing encouragement. Overall, APs spent 88.9% of the time on rehabilitation skills and activities. The pattern across time showed that the initial session included more non-rehabilitation activities (50.9%), whereas all subsequent sessions included more rehabilitation activities (78.0–95.9%).
For participants, the main non-rehabilitation activity was social chat, which occurred in about 16% of observations. The main rehabilitation activities were discussing the educational material (24.0%) and discussing memory strategies (32.6%). Overall, participants spent 74.2% of the sessions on rehabilitation activities. The pattern of activities across time showed that the initial session included more non-rehabilitation activities (55.6%), whereas all subsequent sessions included more rehabilitation activities (62.1–91.1%).
Overall, the results indicate that the APs followed the guidelines in the manual by providing and discussing rehabilitation strategies and this was mirrored by participants, who also discussed the educational materials and strategies. Most sessions included summarising and paraphrasing by the AP and the provision of general information on memory not related to the manual. Most sessions included descriptions of problems related to memory.
To examine the consistency between sites, we compared the distribution of observations at each site. The results are shown in Appendix 10 (see Tables 37 and 38).
The distribution of AP activities was similar across the sites. The AP at site 5 spent more time organising sessions, for example discussing the venue and the time of the groups (20.9%), and more time facilitating discussion (16.4%) than the APs at the other sites. Most variation between sites occurred in the time spent presenting and discussing strategies, which varied from 16.4% (site 5) to 65.6% (site 9). Providing a recap of the previous session was more frequent at site 3 (17.2%) than at the other sites.
From the participants’ perspective, those at site 5 spent more time discussing information about the sessions (4.4%) and hospital visits (11.1%). Participant rehabilitation activities were similar between the sites. Site 3 participants spent the most time discussing the educational materials (35.6%) and participants at sites 5 and 9 spent the highest proportion of time discussing strategies (42.5% and 41.8%, respectively). Site 6 participants spent the highest proportion of time describing problems related to memory (20.9%).
Overall, the results of the fidelity analysis indicate that the components of therapy described in the manual were delivered to participants. Each session included the essential components of therapy. The distribution of time was as expected, with session 1 being introductory and providing an opportunity for participants to get to know each other and later sessions focusing on the rehabilitation content. This suggests that the outcomes reflect the effect of the intervention, as described in the manual.
Follow-up
Follow-up assessments were completed between October 2013 and December 2016. At the 6-month follow-up, 260 (79%) participants returned the questionnaire booklet and 276 (84%) participants completed the assessment visit (Table 6). Questionnaire booklet return and visit completion were similar in the two arms.
| Summary | Follow-up time point | |||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||
| Usual care (N = 157) | Memory rehabilitation (N = 171) | Total (N = 328) | Usual care (N = 157) | Memory rehabilitation (N = 171) | Total (N = 328) | |
| Face-to-face visit, n (%) | ||||||
| Attended | 133 (85) | 143 (84) | 276 (84) | 124 (79) | 132 (77) | 256 (78) |
| Not donea | 7 (4) | 7 (4) | 14 (4) | 2 (1) | 6 (4) | 8 (2) |
| Discontinued | 17 (11) | 21 (12) | 38 (12) | 31 (20) | 33 (19) | 64 (20) |
| Lost to follow-up | 8 | 10 | 18 | 19 | 19 | 38 |
| Death | 0 | 0 | 0 | 0 | 0 | 0 |
| Withdrawal of consent | 9 | 11 | 20 | 12 | 14 | 26 |
| Days to visit from randomisation | ||||||
| Median (25th, 75th centile) | 183 (180, 189) | 184 (182, 189) | 184 (180, 189) | 364 (361, 368) | 366 (362, 370) | 364 (361, 370) |
| Min., max. | 169, 251 | 153, 291 | 153, 291 | 351, 415 | 330, 440 | 330, 440 |
| Visit completed within 3 months of due date, n (%) | 133 (85) | 142 (83) | 275 (84) | 124 (79) | 132 (77) | 256 (78) |
| Participant questionnaire booklet | ||||||
| Returned | 125 (80) | 135 (79) | 260 (79) | 112 (71) | 126 (74) | 238 (73) |
| Not donea | 15 (10) | 15 (9) | 30 (9) | 14 (9) | 12 (7) | 26 (8) |
| Discontinued | 17 (11) | 21 (12) | 38 (12) | 31 (20) | 33 (19) | 64 (20) |
| Lost to follow-up | 8 | 10 | 18 | 19 | 19 | 38 |
| Withdrawal of consent | 9 | 11 | 20 | 12 | 14 | 26 |
| Days to completion from randomisation | ||||||
| Median (25th, 75th centile) | 174 (167, 188) | 174 (166, 185) | 174 (166, 186) | 356.5 (349.5, 364) | 357 (349, 366) | 357 (349, 364) |
| Min., max. | 159, 278 | 159, 286 | 159, 286 | 340, 611 | 316, 438 | 316, 611 |
| Questionnaire completed within 3 months of due date | 123 (78) | 131 (77) | 254 (77) | 111 (71) | 126 (74) | 237 (72) |
At the 12-month follow-up, 238 (73%) of the participants returned the questionnaire booklet and 256 (78%) completed the assessment visit; completion rates were again similar in the two arms (see Table 6).
Most visits (n = 275, 84% at 6 months; n = 256, 78% at 12 months) and questionnaires (n = 254, 77% at 6 months; n = 237, 72% at 12 months) were completed within 3 months of the scheduled time point.
A total of 64 (20%) participants did not complete any of the 12-month follow-up, either because they withdrew consent (8%) or they were lost to follow-up (12%). Numbers lost to follow-up and withdrawing consent were similar in each arm. Twenty-six participants (8%) withdrew from the trial: 12 in the usual-care arm and 14 in the memory rehabilitation arm (see Table 6). The main reasons for withdrawal were health problems, lack of time or personal or family issues. Three participants withdrew from the usual-care arm because of not being randomised to group memory rehabilitation. Four participants from the memory rehabilitation arm withdrew because they did not find the sessions as they had expected or because they felt that the sessions were having a negative impact on their mood.
Of the 328 participants randomised, some follow-up data (e.g. questionnaire or visit at the 6- or 12-month follow-up) were collected for 290 (88%) participants.
Inclusion in primary analysis of the primary outcome
In total, 122 (78%) participants in the usual-care arm and 129 (75%) in the memory rehabilitation arm were included in the primary analysis of the EMQ-p at the 6-month follow-up. Three participants in the usual-care arm and six in the memory rehabilitation arm who returned questionnaires were not included, because of either completing the questionnaire > 9 months from randomisation or not completing enough items on the EMQ-p for it to be scored (see Figure 1).
Participants with no primary outcome data tended to have a slightly lower level of educational attainment and slightly more memory problems at baseline based on both patient and relative/friend report. There were no important differences in other baseline characteristics (see Appendix 11).
Unblinding at follow-up visits
Research assistants reported being unblinded more often in the memory rehabilitation arm than in the usual-care arm, both prior to and during the visits (see Appendix 12). The percentage of participants assessed correctly as ‘definitely’ in their allocated arm was higher in the memory rehabilitation arm than the usual-care arm. The most frequent assessment of allocation at each time point was ‘probably control’.
The Kappa statistic was used to assess the agreement between a participant’s actual treatment allocation and the RA’s opinion of treatment allocation (collapsing ‘probably’ and ‘definitely’ into one category). Kappa values of ≤ 0.20 are considered to indicate no or poor agreement, 0.21–0.40 indicate fair agreement, 0.41–0.6 indicate moderate agreement, 0.61–0.8 indicate substantial agreement and ≥ 0.81 indicate near perfect agreement. Kappa values were fair before goal assessment and moderate after goal assessment (see Appendix 12).
Relative/friend questionnaire follow-up
Of the 210 relatives/friends who agreed to participate in the trial, the questionnaire booklet was returned by 144 (69%) at the 6-month follow-up and 131 (62%) at the 12-month follow-up (see Appendix 13). A slightly higher percentage of relatives/friends in the usual-care arm returned the questionnaire booklet at the 6-month follow-up; however, the percentage returning the booklet at the 12-month follow-up was similar in the two arms.
Primary outcome (EMQ-p) at the 6-month follow-up
Primary analysis
The mean EMQ-p score was lower in both arms at the 6-month follow-up than at baseline; however, there was no clinically important difference between the two arms (Table 7).
| Trial arm | Time point, mean (SD) score | Adjusted difference in mean scores (95% CI) | p-value | |
|---|---|---|---|---|
| Baseline | 6-month follow-up | |||
| Usual care (n = 122) | 48.9 (23.9) | 44.1 (24.6) | ||
| Memory rehabilitation (n = 129) | 45.9 (21.0) | 38.8 (26.1) | –2.1 (–6.7 to 2.5) | 0.37 |
The mean number of participants in each cluster with EMQ-P data at follow-up was 3.9 in the usual-care arm and 3.7 in the memory rehabilitation arm. Diagnostic plots were used to check the assumptions in the model, with no strong evidence that these were not met. The estimated ICC for the participant EMQ-p was 0.05 in the memory rehabilitation arm.
Sensitivity analyses for the primary outcome
The estimates of the difference in EMQ-p scores between the two arms at the 6-month follow-up, after additional adjustment for baseline variables, multiple imputation (assuming that missing outcomes were missing at random) and the complier average causal effect estimate, were slightly greater than in the primary analysis (Table 8 and see Appendix 14, Figure 15). However, the lower limit of the 95% CI for all of these sensitivity analyses was greater than –12, the minimum clinically relevant difference specified in the sample size calculation.
| Analysis type | n | Adjusted difference in mean scores (95% CI) |
|---|---|---|
| Additional adjustment for educational attainment and time since TBI (log-transformed) because of slight imbalance at baseline | 251 | –2.8 (–7.2 to 1.7) |
| Including participants completing the 6 month questionnaire booklet > 9 months after randomisation | 257 | –2.0 (–6.5 to 2.5) |
| After multiple imputation of missing outcome dataa | 328 | –2.4 (–7.1 to 2.3) |
| Complier average causal effect using observed data at 6 months | 251 | –2.6 (–7.9 to 2.7) |
| Complier average causal effect at 6 months after multiple imputation of missing outcome data | 328 | –3.2 (–9.3 to 2.9) |
We conducted a further sensitivity analysis to explore the robustness of the results if missing EMQ-p data at the 6-month follow-up were not missing at random. Under the extreme assumption of scores in the usual-care arm being 12 points worse than as imputed under the missing at random assumption, the lower limit of the 95% CI was –9.8.
The analysis after Rasch conversion of the EMQ-p scores gave similar results to using the standard EMQ-p total scores (see Appendix 14).
Subgroup analysis for the primary outcome
We conducted a prespecified subgroup analysis based on the baseline level of memory impairment, as assessed on the RBMT-3. The difference in mean EMQ-p score for those with borderline/moderate memory impairment favoured the memory rehabilitation arm but there was no evidence of a difference in the effect of the group memory rehabilitation sessions across the subgroups (p-value for interaction = 0.12) (see Table 9 and Appendix 15).
| Subgroup | Time point, mean (SD) score | Adjusted difference in mean scores (95% CI) | Adjusted interaction effect (95% CI) | |
|---|---|---|---|---|
| Baseline | 6-month follow-up | |||
| RBMT-3 GMI score of ≥ 85 (average and above average range) | ||||
| Usual care (n = 34) | 43.4 (15.0) | 36.0 (20.5) | ||
| Memory rehabilitation (n = 35) | 42.7 (16.9) | 34.4 (21.9) | –0.1 (–8.3 to 8.1) | |
| RBMT-3 GMI score of 70–84 (borderline/moderate memory impairment) | ||||
| Usual care (n = 43) | 45.7 (25.0) | 43.9 (25.6) | ||
| Memory rehabilitation (n = 59) | 43.5 (20.8) | 34.0 (23.9) | –7.1 (–13.9 to –0.3) | –7.0 (–17.5 to 3.4) |
| RBMT-3 GMI score of ≤ 69 (significant memory impairment) | ||||
| Usual care (n = 45) | 56.3 (26.9) | 50.4 (25.1) | ||
| Memory rehabilitation (n = 35) | 53.2 (23.7) | 51.3 (29.8) | 3.3 (–4.4 to 11.0) | 3.4 (–7.7 to 14.6) |
The results of the post hoc subgroup analysis conducted on time since TBI are shown in Appendix 16. There was no evidence of a difference in the intervention effect on the basis of time since TBI based on this analysis (p-value for interaction effect = 0.48).
Secondary outcomes
EMQ-p at the 12-month follow-up
There was no clinically important difference in EMQ-p scores between the two arms at the 12-month follow-up (Table 10). The EMQ-p importance scores at the 6- and 12-month follow-ups are reported in Appendix 17. The importance scale was originally added to be able to investigate the effect of memory rehabilitation on memory problems weighted for their importance. However, analysis of data from a subsequent independent study83 showed no effect of weighting items by their importance and, therefore, this was not included in the main analyses.
| Outcome | Time point, mean (SD) score | Adjusted difference in mean scores (95% CI) | |
|---|---|---|---|
| Baseline | Follow-up | ||
| EMQ-p – frequency of problems | |||
| 12-month follow-up | |||
| Usual care (n = 107) | 47.5 (24.6) | 43.0 (26.7) | |
| Memory rehabilitation (n = 124) | 46.7 (20.4) | 38.0 (25.0) | –4.8 (–9.6 to 0.0) |
| GMI scores from the RBMT-3 | |||
| 6-month follow-upa | |||
| Usual care (n = 133) | 77.1 (14.5) | 79.1 (15) | |
| Memory rehabilitation (n = 141) | 78.9 (13.7) | 82.7 (14) | 2.5 (0.1 to 4.8) |
| 12-month follow-upb | |||
| Usual care (n = 124) | 76.2 (14.0) | 84.0 (18.4) | |
| Memory rehabilitation (n = 131) | 79.5 (12.8) | 87.2 (15.7) | 0.5 (–2.6 to 3.6) |
| GHQ-30 | |||
| 6-month follow-up | |||
| Usual care (n = 110) | 33.9 (15.7) | 34.1 (16.8) | |
| Memory rehabilitation (n = 124) | 36.2 (15.4) | 33.6 (16.3) | –1.6 (–5.3 to 2.1) |
| 12-month follow-up | |||
| Usual care (n = 103) | 33.4 (15.8) | 32.5 (18.8) | |
| Memory rehabilitation (n = 119) | 35.7 (15.3) | 33.1 (18.5) | –0.2 (–4.5 to 4.1) |
| EMQ-r – frequency of problems | |||
| 6-month follow-up | |||
| Usual care (n = 66) | 43.2 (23.1) | 40.9 (25.9) | |
| Memory rehabilitation (n = 68) | 39.4 (26.3) | 31.8 (24.5) | –4.2 (–10.1 to 1.7) |
| 12-month follow-up | |||
| Usual care (n = 57) | 42.9 (23.5) | 37.6 (26.6) | |
| Memory rehabilitation (n = 67) | 40.0 (26.7) | 32.2 (26.2) | –5.3 (–12.0 to 1.4) |
Objectively assessed memory ability
The GMI scores from the RBMT-3 were slightly higher at the 6-month follow-up in the memory rehabilitation arm than in the usual-care arm. However, by the 12-month follow-up there was no evidence of a difference between the arms (see Table 10).
Goal attainment
Participants set on average 2.5 (SD 1.2) short-term goals and 2.4 (SD 1.2) long-term goals at the second assessment. These included goals such as better recall of names and dates, remembering forthcoming tasks or appointments and improved memory of past events. We were unable to set goals for three participants (two randomised to the usual-care arm and one randomised to the memory rehabilitation arm); two participants did not wish to set any goals and one participant was not able to as they were preoccupied with other non-memory issues.
For short- and long-term goals, the goal attainment scores favoured the memory rehabilitation arm at both the 6-month follow-up and the 12-month follow-up (Table 11). There was no evidence of a difference in the effect of the intervention on the basis of the number of goals set (p-values for the interaction effect between treatment arm and one goal set or more than one goal set: 0.67 and 0.59 for short-term goals and 0.07 and 0.67 for long-term goals at 6 and 12 months, respectively).
| Outcome | Follow up time point | |||||
|---|---|---|---|---|---|---|
| 6 monthsa | 12 monthsb | |||||
| n | Mean (SD) score | Adjusted difference in mean scores (95% CI) | n | Mean (SD) score | Adjusted difference in mean scores (95% CI) | |
| Goal attainmentc | ||||||
| Short-term goal attainment average score | ||||||
| Usual care | 131 | 1.2 (1.0) | 123 | 1.5 (1.1) | ||
| Memory rehabilitation | 141 | 1.8 (1.0) | 0.6 (0.3 to 0.9) | 131 | 1.8 (0.9) | 0.3 (0.0 to 0.5) |
| Long term goal attainment average score | ||||||
| Usual care | 131 | 1.0 (0.9) | 123 | 1.3 (1.0) | ||
| Memory rehabilitation | 141 | 1.5 (1.0) | 0.5 (0.2 to 0.7) | 131 | 1.6 (1.0) | 0.4 (0.1 to 0.6) |
| EBIQ-p subscale scoresd | ||||||
| Cognitive subscale | ||||||
| Usual care | 109 | 1.97 (0.43) | 99 | 1.94 (0.47) | ||
| Memory rehabilitation | 121 | 1.89 (0.45) | –0.05 (–0.17 to 0.06) | 117 | 1.88 (0.46) | –0.05 (–0.17 to 0.08) |
| Depression subscale | ||||||
| Usual care | 109 | 1.68 (0.62) | 97 | 1.63 (0.63) | ||
| Memory rehabilitation | 118 | 1.76 (0.62) | 0.06 (–0.10 to 0.23) | 118 | 1.77 (0.64) | 0.16 (–0.01 to 0.34) |
| Communication subscale | ||||||
| Usual care | 110 | 1.86 (0.53) | 99 | 1.90 (0.57) | ||
| Memory rehabilitation | 120 | 1.92 (0.57) | 0.06 (–0.10 to 0.21) | 115 | 1.85 (0.57) | –0.05 (–0.21 to 0.11) |
| Difficulties with social interaction subscale | ||||||
| Usual care | 110 | 1.71 (0.48) | 97 | 1.71 (0.45) | ||
| Memory rehabilitation | 120 | 1.82 (0.50) | 0.09 (–0.04 to 0.22) | 118 | 1.77 (0.48) | 0.05 (–0.08 to 0.18) |
| Impulsivity subscale | ||||||
| Usual care | 108 | 1.7 (0.50) | 97 | 1.64 (0.48) | ||
| Memory rehabilitation | 121 | 1.8 (0.51) | NAe | 118 | 1.76 (0.50) | NAe |
| Somatic subscale | ||||||
| Usual care | 110 | 1.94 (0.52) | 96 | 1.91 (0.51) | ||
| Memory rehabilitation | 120 | 1.95 (0.52) | NAe | 115 | 1.89 (0.50) | NAe |
| Fatigue subscale | ||||||
| Usual care | 107 | 2.01 (0.47) | 99 | 1.99 (0.55) | ||
| Memory rehabilitation | 120 | 2.00 (0.50) | NAe | 117 | 1.97 (0.51) | NAe |
Of the 819 short-term goals set, 692 (84%) were classified as SMART. Of the 775 long-term goals set, 679 (88%) were classified as SMART. Thirty participants (9%) set no SMART short-term goals and 35 participants (11%) set no SMART long-term goals. The results from the analysis of goal attainment including only SMART goals were similar to the results from the analysis including all goals (see Appendix 18).
Mood
The GHQ-30 scores were similar in the two arms at both time points (see Table 10).
Cognitive, emotional and social well-being
Scores from all subscales of the EBIQ-p were similar in the two arms at both the 6-month follow-up and the 12-month follow-up (see Table 11).
Relative/friend secondary outcomes
The differences between the two arms at follow-up based on the relative/friend assessment (Table 12) were consistent with the results of the participant-completed questionnaires (see Table 10), indicating that there was no clinically important difference between the two arms on the EMQ-r, even though this outcome was completed for only a subgroup of participants. The EMQ-r importance scores at the 6- and 12-month follow-ups are reported in Appendix 19.
| EBIQ-r subscale | Follow up time point | |||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||
| n | Mean (SD) score | Adjusted difference in mean scores (95% CI) | n | Mean (SD) score | Adjusted difference in mean scores (95% CI) | |
| Cognitive subscale | ||||||
| Usual care | 72 | 1.98 (0.50) | 60 | 1.88 (0.52) | ||
| Memory rehabilitation | 69 | 1.89 (0.52) | –0.06 (–0.23 to 0.12) | 68 | 1.91 (0.53) | 0.00 (–0.18 to 0.19) |
| Depression subscale | ||||||
| Usual care | 67 | 1.67 (0.65) | 59 | 1.64 (0.61) | ||
| Memory rehabilitation | 68 | 1.71 (0.59) | 0.10 (–0.11 to 0.31) | 69 | 1.77 (0.57) | 0.13 (–0.08 to 0.34) |
| Communication subscale | ||||||
| Usual care | 71 | 1.76 (0.65) | 59 | 1.72 (0.59) | ||
| Memory rehabilitation | 70 | 1.75 (0.58) | 0.00 (–0.20 to 0.21) | 69 | 1.80 (0.59) | 0.04 (–0.17 to 0.26) |
| Difficulties with social interaction subscale | ||||||
| Usual care | 71 | 1.95 (0.57) | 59 | 1.85 (0.52) | ||
| Memory rehabilitation | 67 | 1.97 (0.51) | 0.05 (–0.13 to 0.23) | 63 | 1.97 (0.56) | 0.11 (–0.08 to 0.30) |
| Impulsivity subscale | ||||||
| Usual care | 72 | 1.92 (0.61) | 60 | 1.83 (0.56) | ||
| Memory rehabilitation | 69 | 1.93 (0.55) | NAa | 68 | 1.97 (0.59) | NAa |
| Somatic subscale | ||||||
| Usual care | 69 | 1.95 (0.50) | 56 | 1.79 (0.47) | ||
| Memory rehabilitation | 68 | 1.97 (0.51) | NAa | 66 | 1.92 (0.54) | NAa |
| Fatigue subscale | ||||||
| Usual care | 70 | 2.01 (0.50) | 59 | 1.92 (0.51) | ||
| Memory rehabilitation | 68 | 1.97 (0.54) | NAa | 66 | 2.02 (0.55) | NAa |
Notable events
There were 11 notable events recorded during the trial: two occurred during contact with potential participants before consent, four occurred during memory rehabilitation groups and five were reported during follow-up visits.
The events reported during memory rehabilitation groups included issues with group dynamics, such as disagreements between participants, which were addressed within the group sessions. The events reported during follow-up included inappropriate behaviour exhibited by some participants towards the RA.
Protocol deviations
All reported protocol deviations were reviewed by the TMG prior to data lock. None was considered to constitute a violation of the protocol.
Chapter 4 Health economics
Introduction and aim
This chapter reports the methods and results of the economic evaluation conducted alongside the trial. The aim was to assess the cost-effectiveness of the group-based memory rehabilitation programme (the intervention) in addition to usual care compared with usual care alone. In line with NICE guidelines,84 a UK NHS and personal social services perspective was taken.
Methods
We took two approaches to the economic evaluation:
-
a within-trial evaluation to assess the cost-effectiveness of the group-based memory rehabilitation programme compared with usual care based on the time horizon of the trial (12 months)
-
a decision-analytic model to estimate the cost-effectiveness of the group-based memory rehabilitation programme compared with usual care over longer time periods.
A summary of the health economic analyses was set out in the trial protocol, with an agreed analysis plan in place prior to data lock and commencement of the analysis.
Within-trial economic evaluation
The primary economic analysis was a cost–utility analysis (incremental cost per QALY gained) at 12 months based on area under the curve (AUC) analysis. To present a comparable time point of interest with the clinical analyses, we undertook additional analyses of the 6-month follow-up data. Secondary cost-effectiveness analyses were undertaken to assess the incremental cost per point improvement based on the primary clinical outcome (EMQ-p) and assessment of mood (GHQ-30) at the 6-month follow-up in order to reflect the primary time point of interest, with additional consideration at the 12-month follow-up to reflect a comparison of costs over potential maintenance of treatment gains over time. As the within-trial evaluation did not exceed 12 months, costs and outcomes were not discounted.
Resource use and costs
Resource use and associated costs were calculated across the following broad categories:
-
the implementation and delivery of the group-based memory rehabilitation programme
-
health and personal social care resource use.
All resource use data were valued in Great British pounds using published unit costs85–87 at 2016 prices. If a 2016 unit cost was not available, a unit cost from a previous version (e.g. an earlier edition of the Unit Costs of Health and Social Care) was used and inflated to 2016 costs using the Bank of England inflation calculator. 88
Costs associated with the implementation and delivery of the group-based memory rehabilitation programme
We estimated the cost of implementation and delivery of the intervention during the trial using information from the trial team and data obtained within the trial (e.g. number of participants). The key resources and associated costs of delivering the intervention focused on the opportunity costs of:
-
AP training to deliver the intervention
-
delivery of the intervention by APs to trial participants.
We assumed that, in standard clinical practice, the intervention would be delivered in a NHS setting, thus the costs of travel and room hire and other capital costs were not included. Resources and associated costs related to research and the management and administration of the trial were excluded. Costs of supplying course booklets, stationery and refreshments were included. No provision for supervision was included in the intervention cost. It was instead assumed that the costs and resources related to supervision were part of standard clinical practice for the delivery of any intervention by APs.
The skill mix, time and grade of staff involved in delivering the intervention were obtained from information collated by the trial team. These costs were aggregated to give a total cost, given the number of groups to which participants were allocated and the number of sessions offered.
Individual-level resource use and associated costs within the group memory rehabilitation programme
We assessed individual-level resource use using the SUQ, as previously described in Chapter 2. Resource use was initially summated across each time point for each individual into discrete categories (primary care, medication use within primary care, secondary care).
For primary care costs, we used published unit costs based on average contact times. 85 We obtained NHS net prices for medications from the British National Formulary (BNF). 86 When the dosage and prescription length were recorded by the participant, the cost of the medication was calculated. When the dosage or prescription length was either not recorded by the participant or unclear, the cost of the standard adult dosage as per the BNF was used. We calculated the cost of secondary care attendances by applying unit costs from national published sources87 to the usage reported on the questionnaires. When possible for inpatient admissions, the type of procedure and length of stay were matched and the national average unit cost used. When no length of stay was stated, the lowest national average unit cost was used, based on the lowest case-mix classification (CC) score (i.e. 0–1). As only a small number of participants did not report any length of stay, this approach was preferred to a weighted cost approach, which would have substantially overestimated the cost of service use contacts. When an unspecified or ambiguous visit to a hospital was indicated, but no length of stay was stated, an average general surgery cost was used to ensure that these contacts were appropriately captured.
Although the SUQ allowed participants to differentiate between resource use as a consequence of memory problems and resource use for other reasons (e.g. other comorbidities), this information was not obtained for medication use. We decided, in consultation with the trial team, that it would be difficult for participants to classify resource use on the basis of whether it was for memory problems or for other problems. Therefore, the decision was made to use total resource use (i.e. service use because of memory problems and for other reasons) within the base-case analysis. Appropriate sensitivity analysis was undertaken to assess the impact of including resource use attributed to memory problems only.
Resource use and costs were summated to give an overall cost for the memory rehabilitation arm (including intervention costs) and usual-care arm over the trial period (12 months), with an interim assessment at the 6-month follow-up point also reported to reflect the time points of interest. The mean total cost and difference in mean total cost (with 95% CIs) per participant between the memory rehabilitation arm and the usual-care arm were compared using the two-tailed Student’s t-test, following the ITT principle.
A description of unit costs associated with service resource and medication use is presented in Appendices 20 and 21, respectively.
Health utilities and quality-adjusted life-years
Health-related quality-of-life data were collated using the EQ-5D-5L questionnaire, as described in Chapter 2. The reported EQ-5D-5L values were used to derive utilities based on the UK social tariff. 89 Individual-level utility scores across each assessment point were summated for the memory rehabilitation and usual-care arms, with QALYs for each patient calculated based on the utility scores at different points using the AUC approach. We assumed linear interpolation between the measurement points.
Summary statistics across each time point were used to describe and compare QALYs between the two arms from baseline to 12 months for the primary analysis, with additional analysis to derive QALYs from baseline to the 6-month follow-up. The impact of a baseline imbalance in utilities was examined, with adjusted analyses on similar covariates (baseline score, treatment arm and site) undertaken, commensurate with the statistical analysis methods of the clinical outcomes. Mean utilities and QALYs gained per participant were evaluated between the memory rehabilitation arm and the usual-care arm using the difference-in-difference approach and compared using the Student’s t-test.
Clinical outcomes
The primary outcome data (EMQ-p) and GHQ-30 scores were analysed with the difference in scores (points) calculated (as described in Chapter 2) at the 6-month follow-up (as per the primary clinical analysis) and at 12 months (to assess whether an improvement was maintained over time). The analysis of the EMQ-p is commensurate with the clinical analysis, with a difference-in-difference approach adopted to determine the incremental effect of the intervention compared with usual care. For the analysis of the GHQ-30, the GHQ-30 (0–0–1–1) scoring methodology was applied. This differs from the clinical analysis, which adopted the Likert (0–1–2–3) scoring methodology. Higher scores on the EMQ-p are indicative of more frequent memory problems and higher scores on the GHQ-30 are indicative of greater psychological distress. Therefore, ‘improvement’ over time would be characterised by a decreasing score.
Missing data
The problems concerning missing data are particularly relevant to health economic analysis as missing items relating to health-care service usage may undervalue the total costs, whereas missing outcome data may be correlated with effects, as those individuals without information may be systematically different from those for whom all information is observed. 90 A complete case analysis would therefore result in meaningful data being excluded. 90 We therefore adopted appropriate techniques to provide a comprehensive investigation of the impact of missing data on our estimations of cost-effectiveness.
For the questionnaires used within the economic analysis (i.e. EQ-5D-5L, EMQ-p and GHQ-30) we followed the same rules for missing data as applied to the analysis of clinical outcomes (see Chapter 2). To ensure that comparable consideration was given to missing data in relation to the SUQ, we devised similar rules with the trial team.
For both costs and outcomes, two imputation methods were used for the health economic analysis. Simple imputation using the site-specific treatment arm mean and MICE were undertaken on costs and outcomes for both the 6-month follow-up and the 12-month follow-up to impute missing items. Mean imputation provides a single estimate for each missing item. However, it is unable to account for the uncertainty inherent in missing data, resulting in a smaller standard error (SE), and can result in biased estimates because of covariance and correlations being decreased in magnitude compared with alternative methods. The multiple imputation method used was similar to the imputation of the primary outcome data performed, that is, chained equations were undertaken separately for each allocated arm under the assumption that data were missing at random, using similar predictor variables (with baseline EQ-5D-5L score used) with similar data sets included and results of imputed sets combined following Rubin’s rules. 91 Because of the typically skewed distribution of cost data, predictive mean matching was used. Missing outcome data were imputed using truncated regression to restrict imputed scores to the range of valid scores for each outcome.
For the base-case cost-effectiveness analyses, we used the multiply imputed data to construct incremental cost-effectiveness ratios (ICERs). The non-imputed and mean imputed data are presented to fully reflect the impact on the base-case ICERs when different scenarios for missing data are considered.
Incremental-cost-effectiveness analysis
As summarised in the description of the within-trial evaluation, a series of cost-effectiveness analyses were undertaken, with a cost–utility analysis undertaken as the primary analysis. This comparative analysis of incremental costs and effects can be summarised in terms of an ICER.
The ICER can be represented as:
where C1 and E1 are the costs and effects of the memory rehabilitation arm, respectively; C0 and E0 are the costs and effects of the usual-care arm, respectively; and ΔC and ΔE are the incremental costs and effects of the intervention compared with usual care.
The ICER is reported to determine the cost-effectiveness of the intervention compared with competing alternatives and to aid decision-making. Although NICE84 reports a base cost-effectiveness threshold of £20,000 per QALY, cost-effectiveness is a spectrum rather than a dichotomy, with the maximum threshold increasing dependent on circumstances. The reported ICERs from our analysis are presented to assist the decision-making process and are not an absolute statement on whether the intervention can be deemed cost-effective.
We also calculated net monetary benefits (NMBs) based on the following equation:
The NMB represents the value of the intervention in monetary terms given a willingness-to-pay (WTP) threshold, λ. 91 When the incremental NMB, calculated as the difference in NMB between the intervention and usual care, is positive, the intervention is identified as cost-effective at the given threshold, relative to usual care. 92
For the cost-effectiveness analyses (i.e. incremental cost per point improvement in EMQ-p and GHQ-30 scores), no accepted WTP threshold could be identified. However, as the WTP threshold is based on important end points of relevance and importance to the assessment of the effect of memory problems, decision-makers and clinicians can judge the results against different thresholds as part of a full examination of cost-effectiveness.
Sensitivity analysis
A series of one-way sensitivity analyses were undertaken to assess the impact of parameter and methodological uncertainty on the estimates of cost-effectiveness. For primary and secondary outcomes, we assessed the impact on the base-case ICER of altering key parameters (i.e. costs, QALYs and outcomes scores) based on lower and upper 95% CIs for incremental costs and outcomes.
We also assessed the extent of uncertainty based on imputation method, as documented earlier. Scenario analysis was conducted to ascertain the potential impact of changes to input parameters (as measured by the EMQ-p and GHQ-30) on the base-case ICER. Sensitivity analyses of the intervention cost were conducted on the available data case analysis.
Bootstrapping was undertaken to address the joint uncertainty and impact on the ICER; 1000 simulations were undertaken using random sampling of the distributions of costs and outcomes, presented on a cost-effectiveness plane. The cost-effectiveness plane is presented as a scatterplot of the point estimates obtained as a result of the 1000 runs depicted in four quadrants (see Appendix 22), which can be summarised as follows:
-
North-west (upper-left) quadrant – the intervention is dominated by usual care. The intervention is more costly and less effective than usual care.
-
North-east (upper-right) quadrant – further evaluation required. The intervention is more costly and more effective than usual care. The ICER is computed to assess whether the net incremental health gain is worth the incremental cost.
-
South-west (lower-left) quadrant – further evaluation required. The intervention is less costly and less effective than usual care. The ICER is computed to assess whether the cost saving is worth the net incremental health loss.
-
South-east (lower-right) quadrant – the intervention is dominant compared with usual care and unambiguously preferred to usual care. The intervention is less costly and more effective than usual care.
For consistency with the cost-effectiveness plane for QALYs, the incremental effect for the EMQ-p and GHQ-30 was reversed (i.e. multiplied by –1) such that a positive value was representative of an improvement in everyday memory or mood. Cost-effectiveness acceptability curves (CEACs) were produced to present the probability of the intervention being considered cost-effective at alternative WTP thresholds. For the cost per QALY CEAC, the WTP threshold based on NICE guidance84 was used. No similar WTP threshold exists for the clinical outcomes.
Long-term cost-effectiveness
We constructed a decision-analytic model to extrapolate the findings from the trial to estimate the cost-effectiveness of the group-based memory rehabilitation programme compared with usual care beyond the trial horizon of 12 months. A cost–utility analysis was undertaken. We based the modelling exercise on extrapolating results from the within-trial analysis to longer-term cost per QALY estimates, supplemented with data sources from the literature and, when necessary, clinical opinion from the trial team.
Modelling approach
We developed a Markov model given that TBIs are considered to have a disease and treatment pathway consistent with chronic health conditions (e.g. with evolving progression of the condition over time). The decision-analytic Markov model was developed using Microsoft Excel® 2013 with coding in Visual Basic for Applications (Microsoft Corporation, Redmond, WA, USA). The Markov model simulated costs and QALYs over a minimum 5-year time horizon using four mutually exclusive health states (low, moderate, high and death). As the time horizon exceeded 12 months we discounted all future costs and QALYs at the UK Treasury discount rate of 3.5% per year, as recommended by NICE. 84
The cost and utility data reported at the 12-month follow-up within the trial were used alongside external data such as UK life tables and standard mortality ratios to estimate longer-term costs and QALYs. Time horizons of 5 and 10 years were chosen for the analysis although the model was constructed to undertake a lifetime horizon should suitable data be available in the future. Although this was an amendment from the original protocol, in which a lifetime horizon was proposed, we chose this approach (1) based on the availability of plausible estimates of longer-term effects from external literature sources and (2) because, when necessary, appropriate clinical assumptions could be made by the trial team.
We categorised psychological distress into three mutually exclusive states of no/mild distress, moderate distress and severe distress, pro-rating the categories defined by Rai et al. 93 for the GHQ-12 to the GHQ-30 as follows:
-
GHQ-30 scores of < 5.5 were classified as no/mild distress
-
GHQ-30 scores between 5.5 and 17.5 were classified as moderate distress
-
GHQ-30 scores of ≥ 17.5 were classified as severe distress.
Participants could also transition to a fourth state of death, which is an absorbing state with participants unable to transition out of this state.
Model structure
The basic outline of the Markov model used to evaluate the longer-term cost-effectiveness of the intervention is illustrated in Appendix 23. For the memory rehabilitation and usual-care arms, separate Markov processes with different input parameters were obtained. The same structure, depicted in Appendix 23, would, however, apply.
We assumed that the sample adhered to the trial protocol. For each 6-month cycle, individuals were categorised into one of three mood states based on their GHQ-30 score or into the absorbing death state. We assumed that psychological distress in individuals with TBI can either worsen or improve, that is, the GHQ-30 score could either increase or decrease from the value observed at the previous time point. Moreover, transitions from the high mood state to the low mood state (or vice versa) were permitted in addition to sequential transitions, that is, high to moderate to low. Aside from death, we assumed that there were no intervention-related adverse events that impacted on an individual’s state of psychological distress.
Model inputs
The base-case analysis was estimated using a cohort of 10,000 patients. The population was consistent with the trial, that is, men represented 72% of the model cohort and the mean age at entry was 45 years. Base-case transition probabilities were defined by those observed in the trial between baseline and 6 months’ follow-up and between 6 months’ and 12 months’ follow-up. For intervals beyond the 12-month within-trial horizon, we assumed that the 6-month to 12-month transition probabilities would persist at intervals beyond 12 months. This assumption was made because of a lack of published data beyond the 12-month follow-up.
To account for missing items observed in the trial, mean imputed data were used, despite the limitations highlighted earlier, to provide a single transition for each individual and prevent scenarios in which an individual occupied multiple states at a given time point. This provided a single set of transition probabilities at each time point. Sensitivity analysis was conducted to determine how changes in transition probabilities influenced the ICER.
Details of the model parameters used are presented in Appendix 24. A summary of the base-case model inputs and one-way sensitivity analyses is presented in Table 13.
| Parameter | Lower | Base case | Upper |
|---|---|---|---|
| Age (years) | 30 | 45 | 65 |
| Discount rate (%) | 1.5 | 3.5 | 5.0 |
| Intervention cost (£) | 116.90 | 167 | 217.10 |
| Cost per cycle in GHQ-30 state | –30% | Various (state dependent) | + 30% |
| Utility per cycle in GHQ-30 state | –30% | Various (state dependent) | + 30% |
| Transition probability to lower GHQ-30 state | –20% | Various (state dependent) | + 20% |
| Transition probability to higher GHQ-30 states | –20% | Various (state dependent) | + 20% |
Clinical/epidemiological inputs
No deaths were reported in the trial. We therefore assumed that patients would not be at an increased risk of death from the intervention. Additionally, the model did not include any provision for the occurrence of serious adverse events, as these were not assessed within the trial. In the model, all-cause age-related mortality rates were obtained from the Office for National Statistics (ONS),94 based on mid-year population estimates for 2013–15. Considering that individuals with a TBI are recognised to have a higher all-cause mortality rate and shorter life expectancy than the general population,93,95 we adjusted the general population mortality using the TBI standardised mortality ratio (SMR) presented by Brooks et al. 96
Costs
All costs were based on the within-trial costs reported (see Appendix 25). Costs associated with each health state defined by the model included the costs relating to delivery of the intervention and any health-care service usage recorded by participants over the preceding 6-month interval. For cycles beyond the in-trial 12-month horizon, costs were assumed to be the same as for the 6- to 12-month cycle. No provision was made in the model for any top-up sessions relating to the intervention.
Utilities
The model applied different utility values to each of the non-dead health states, differentiated by trial arm. In accordance with the manner in which costs were handled, all utilities were based on those observed in the within-trial analysis reported. For cycles beyond the within-trial 12-month horizon, utilities were assumed to be the same as for the 6- to 12-month cycle.
Incremental cost-effectiveness ratio
Incremental cost-effectiveness ratios were calculated as in the within-trial analysis, based on a 5- and 10-year time horizon. As this was a cost–utility analysis, we applied the same approach as in the assessment of the base-case results against the WTP threshold set out by NICE. 84
Sensitivity analysis
Similar sensitivity analyses were conducted as outlined for the within-trial evaluation.
We conducted a series of one-way sensitivity analyses by manipulating the values of the main parameters in the model (i.e. costs, QALYs and transition probabilities) and assessing the subsequent impact on the ICER. Table 13 presents a list of the one-way sensitivity analyses conducted.
In general, the upper and lower values of the input parameters were arbitrarily chosen, but were considered appropriate given the uncertainty inherent in the parameter values. The lower discount rate of 1.5% is consistent with NICE guidelines. 84 Costs and QALYs were discounted at the same rate under all scenarios. Sensitivity analyses on transition probabilities also affect the probability that an individual remains within their current state; however, they do not affect the probability of transitioning to the death state.
To account for the simultaneous uncertainty regarding the input parameters, a probabilistic sensitivity analysis was conducted. Cost-effectiveness planes and CEACs illustrating the probability that the intervention is cost-effective across a range of threshold values were produced.
Results
Intervention cost
The resource use involved in delivering the intervention and associated costs are summarised in Table 14, with a detailed account of each component presented in Appendix 25. The total cost of memory rehabilitation per participant was £167.
| Resource | Total (£) |
|---|---|
| Training costs (nine sites) | 1818 |
| Administration costs (35 groups) | 875 |
| Session costs (350 sessions) | 24,500 |
| Participant costs (171 participants) | 1402 |
| Total cost | 28,595 |
| Cost per participant (171 participants) | 167 |
The first resource-intensive component was AP training in the memory rehabilitation programme. One clinical psychologist (based on NHS Agenda for Change grade 8a) delivered the training across all sites. We did not include any travel costs as training would occur on site in standard practice. In addition, the opportunity cost of each of the APs attending the training was included, with costs estimated using published unit costs. 85 The training involved a one-off session. Ongoing monitoring/supervision of the APs during the trial (one-to-one supervision between the AP and the clinical psychologist) was considered part of standard clinical practice. Sensitivity analysis was conducted to determine the impact of the addition of supervision on the intervention cost and the resulting ICERs.
The second component was the delivery of the intervention by the APs across the trial sites. This was based on the 171 participants randomised to the memory rehabilitation arm, each of whom was allocated to one of 35 groups of four to six individuals across the nine sites involved in the trial. Each group that received the intervention was invited to attend 10 sessions, giving a total of 350 sessions. We made the assumption that the costs of delivery would be the same for each session, regardless of attendance, for example even if participants withdrew or did not attend. We assumed that the delivery of one 90-minute session required 2 hours of time from the AP, inclusive of session preparation. Administrative support was also included for 1 hour per group in total for the 10 sessions delivered.
Resource use and costs
The resource use and associated costs for available cases is summarised in Appendix 26 with summary of costs presented in Table 15. We observed that visits to primary care services and use of inpatient services are the primary cost drivers for both the memory rehabilitation and usual-care arms. The number of participants with inpatient appointments for either arm at each time point is small (< 10%), yet costs attributed to these contacts are high with a maximum of £9444. In contrast, a much higher proportion (> 65%) report costs associated with visits to primary care or medication; however, these costs are typically much lower yet more frequent. The costs per patient associated with resource use in the memory rehabilitation and usual-care arms across the time points are summarised in Table 15.
| Group | Cost (£), mean (SD) | 95% CI (£) | Difference in mean costs (£) (95% CI)a | p-value |
|---|---|---|---|---|
| Baseline | ||||
| Usual care (n = 155) | 453.325 (849.822) | 318.480 to 588.171 | 45.990 (–171.671 to 263.652) | 0.697 |
| Memory rehabilitation (n = 170) | 499.315 (1112.967) | 330.805 to 667.826 | ||
| 6 months | ||||
| Usual care (n = 111) | 587.123 (1543.587) | 297.903 to 876.344 | –255.336 (–362.273 to –148.398) | < 0.001*** |
| Memory rehabilitation (n = 120) | 328.078 (687.450) | 203.816 to 452.339 | ||
| 12 months | ||||
| Usual care (n = 101) | 355.034 (824.026) | 195.565 to 514.504 | 18.353 (–24.782 to 61.488) | 0.403 |
| Memory rehabilitation (n = 121) | 372.092 (824.026) | 211.335 to 532.848 | ||
| Total service resource usage costs up to 12 months (excluding intervention cost) | ||||
| Usual care (n = 93) | 1184.905 (1877.947) | 811.303 to 1584.820 | 83.624 (–302.345 to 469.593) | 0.670 |
| Memory rehabilitation (n = 103) | 1281.685 (2001.812) | 890.452 to 1672.919 | ||
| Total costs up to 12 months (including intervention cost) | ||||
| Usual care (n = 87) | 1184.905 (1877.947) | 782.903 to 1586.908 | 250.624 (–135.345 to 636.593) | 0.202 |
| Memory rehabilitation (n = 102) | 1449.329 (2001.812) | 1054.196 to 1844.461 | ||
| Total costs up to 6 months (including intervention cost) | ||||
| Usual care (n = 110) | 1002.020 (1537.326) | 610.984 to 1393.056 | 30.113 (–340.619 to 400.845) | 0.873 |
| Memory rehabilitation (n = 119) | 1026.477 (2052.678) | 746.240 to 1306.714 | ||
The total mean cost per participant was £1449 (SE £199.19) and £1185 (SE £202.22) for the memory rehabilitation and usual-care arms, respectively. The aggregate service use cost was £84 (95% CI £302.35 to £469.59) higher for the memory rehabilitation arm than for the usual-care arm. Including the cost of the intervention, the memory rehabilitation arm had a higher total cost than the usual-care arm of £251 (95% CI –£135.35 to £636.59) at 12 months and £30 (95% CI –£340.62 to £400.85) at 6 months.
Impact of missing data on cost estimations
Service use questionnaire data were obtained for 98.8% of participants at baseline (see Appendix 27). However, at the 6- and 12-month follow-ups, questionnaires were missing for approximately 20% and 29% of participants, in each arm, respectively, with more missing data in the usual-care arm (36%) than in the memory rehabilitation arm (29%) at the 12-month follow-up. The planned approach to missing data was subsequently adopted.
Although numerical differences between the trial arms were seen depending on the imputation method used, there was no statistically significant impact on the total costs, with the memory rehabilitation arm showing a higher total mean cost per participant at the 12-month follow-up. With the inherent issues associated with skewed costs, imputed costs were subsequently bootstrapped to derive means and 95% CIs to be used in the base case (Table 16).
| Group | Mean (SE) | 95% CI | Difference (SE) | p-value of difference |
|---|---|---|---|---|
| Baseline | ||||
| Usual care (n = 157) | 456.152 (70.206) | 317.402 to 594.902 | 44.447 (111.880) | 0.691 |
| Memory rehabilitation (n = 171) | 500.599 (85.388) | 332.019 to 669.178 | ||
| 6 months | ||||
| Usual care (n = 157) | 576.071 (126.547) | 325.267 to 826.875 | –247.060 (138.067) | 0.075 |
| Memory rehabilitation (n = 171) | 329.011 (63.367) | 203.293 to 454.729 | ||
| 12 months | ||||
| Usual care (n = 157) | 391.396 (86.106) | 220.008 to 562.784 | 8.718 (116.615) | 0.941 |
| Memory rehabilitation (n = 171) | 400.115 (80.789) | 239.918 to 560.311 | ||
| Total service resource usage costs up to 12 months (excluding intervention cost) | ||||
| Usual care (n = 157) | 1423.619 (197.761) | 1031.972 to 1815.265 | –193.894 (249.157) | 0.437 |
| Memory rehabilitation (n = 171) | 1229.724 (154.178) | 924.911 to 1534.538 | ||
| Total costs up to 12 months (including intervention cost) | ||||
| Usual care (n = 157) | 1423.619 (197.761) | 1031.972 to 1815.265 | –26.894 (249.157) | 0.914 |
| Memory rehabilitation (n = 171) | 1396.724 (154.178) | 1091.910 to 1701.538 | ||
| Total costs up to 6 months (including intervention cost) | ||||
| Usual care (n = 157) | 1032.222 (165.900) | 703.941 to 1360.504 | –35.613 (201.227) | 0.860 |
| Memory rehabilitation (n = 171) | 996.610 (119.018) | 761.459 to 1231.760 | ||
Outcomes
EuroQol-5 Dimensions five-level version utilities
The EQ-5D-5L utility scores and QALY increments, based on AUC analysis, across the trial assessment points, based on the available cases for the memory rehabilitation and usual-care arms, are presented in Table 17.
| Time point | Trial arm | Adjusted differencea (95% CI) | |||
|---|---|---|---|---|---|
| Usual care | Memory rehabilitation | ||||
| EQ-5D-5L score, mean (SD) | Change over time (95% CI) | EQ-5D-5L score, mean (SD) | Change over time (95% CI) | ||
| Baseline | 0.581 (0.275) (n = 157) | 0.637 (0.266) (n = 171) | |||
| 6 months | 0.581 (0.299) (n = 119) | –0.020 (–0.063 to 0.024) | 0.642 (0.269) (n = 128) | –0.007 (–0.047 to 0.033) | 0.060 (0.015 to 0.105) |
| 12 months | 0.625 (0.253) (n = 123) | –0.001 (–0.043 to 0.041) | 0.644 (0.264) (n = 106) | –0.010 (–0.047 to 0.027) | 0.019 (–0.026 to 0.063) |
| QALY gain at 6 months (95% CI) | –0.005 (–0.016 to 0.006) | –0.002 (–0.012 to 0.008) | 0.003 (–0.002 to 0.008) | ||
| QALY gain at 12 months (95% CI) | 0.000 (–0.011 to 0.010) | –0.002 (–0.012 to 0.007) | –0.004 (–0.016 to 0.007) | ||
At 6 months, small QALY losses were observed for both arms; the incremental QALY gain of 0.003 in the memory rehabilitation arm compared with the usual-care arm was not significant at the 5% level (p = 0.154). At 12 months, small QALY loses were observed, with a small incremental QALY gain of 0.004 in the usual-care arm compared with the memory rehabilitation arm, which was not significant at the 5% level (p = 0.743). The incremental QALY gains calculated from the mean imputed and multiply imputed data are analogous to those calculated from the available cases presented above, with a small gain for the memory rehabilitation arm at 6 months and a small gain for the usual-care arm at 12 months.
Impact of missing data
There was 100% completion of the EQ-5D-5L questionnaire at baseline, with the number of missing data increasing at 6 months and 12 months across both arms. No clear pattern of missing data was evident. Most participants who missed one follow-up assessment had another follow-up assessment available. A descriptive profile of EQ-5D-5L questionnaire completion is presented in Appendix 28.
When the EQ-5D-5L utility and subsequent QALY gains were examined using the imputation methods, there were no statistically significant differences in within-group utility scores or between-group QALY gains at either the 6-month follow-up or the 12-month follow-up. Table 18 presents the results based on multiple imputation; the results of the mean imputation are presented in Appendix 29.
| Time point | Trial arm | |||
|---|---|---|---|---|
| Usual care (n = 157) | Memory rehabilitation (n = 171) | |||
| EQ-5D-5L score, mean | Change over time (95% CI) | EQ-5D-5L score, mean | Change over time (95% CI) | |
| Baseline | 0.581 | 0.637 | ||
| 6 months | 0.553 | –0.028 (–0.071 to 0.015) | 0.625 | –0.012 (–0.050 to 0.027) |
| 12 months | 0.589 | 0.008 (–0.034 to 0.051) | 0.624 | –0.013 (–0.050 to 0.023) |
| QALY gain at 6 months (95% CI) | –0.007 (–0.018 to 0.004) | –0.003 (–0.013 to 0.007) | ||
| QALY gain at 12 months (95% CI) | 0.004 (–0.017 to 0.025) | –0.007 (–0.025 to 0.012) | ||
Similar to the results presented in Table 17, there was a small QALY loss in both arms at 6 months’ follow-up (memory rehabilitation: –0.003, 95% CI –0.013 to 0.007; usual care: –0.007, 95% CI –0.0018 to 0.004). At the 12-month follow-up, this translated into a small QALY gain in the usual care arm (0.004, 95% CI –0.017 to 0.025) compared with a small QALY loss in the memory rehabilitation arm (–0.007, 95% CI –0.025 to 0.012). These differences did not reach statistical significance, with p-values of 0.536 and 0.442 at 6 months and 12 months, respectively.
Clinical outcomes
The EMQ-p scores used for the health economic analyses are presented in Tables 7 and 10 and the per-point improvements at the 6- and 12-month follow-ups for the GHQ-30 are presented in Table 19. There was an improvement in everyday memory at both the 6-month follow-up and the 12-month follow-up compared with baseline for both arms of the trial, but the difference between arms was not statistically significant at the 5% level.
| Time point | Trial arm | Adjusted differencea (95% CI) | |||
|---|---|---|---|---|---|
| Usual care | Memory rehabilitation | ||||
| GHQ-30 score, mean (SD) | Change over time (95% CI) | GHQ-30 score, mean (SD) | Change over time (95% CI) | ||
| Baseline (n = 157) | 9.6 (8.1) | 9.7 (8.0) | |||
| 6 months (n = 110) | 9.1 (8.4) | 0.0 (–1.3 to 1.4) | 8.6 (8.1) | –1.2 (–2.7 to 0.2) | –1.2 (–2.1 to –0.3) |
| 12 months (n = 103) | 8.2 (8.1) | –0.4 (–2.1 to 1.3) | 8.4 (8.8) | –1.1 (–2.7 to 0.5) | –0.7 (–1.7 to 0.4) |
Although there was a significant difference between arms in GHQ-30 scores at 6 months, with the memory rehabilitation arm having a greater reduction in mood score, there was no significant difference in EMQ-p scores at either follow-up or in GHQ-30 scores at 12 months. The finding of a significant difference at 6 months in the GHQ-30 score differs from the findings of the statistical analysis of clinical outcomes. This is because of the use of GHQ-30 scoring in the health economic analysis, rather than the Likert scoring used in the analysis of clinical outcomes.
Impact of missing data
There were missing data within the questionnaires at each of the follow-up assessments. When the imputation methods were examined, numerical differences were seen but these did not demonstrate any statistically significant differences between the trial arms. The results using the multiply imputed data are presented in Table 20 and mean imputations for the EMQ-p and GHQ-30 are reported in Appendices 30 and 31, respectively.
| Time point | Trial arm | Difference, p-value | |||
|---|---|---|---|---|---|
| Usual care (n = 157) | Memory rehabilitation (n = 171) | ||||
| Mean (SD) score | Change over time (95% CI) | Mean (SD) score | Change over time (95% CI) | ||
| EMQ-p | |||||
| Baseline | 50.075 (1.953) | 47.363 (1.606) | |||
| 6 months | 45.622 (2.165) | –4.453 (–7.756 to –1.149) | 40.589 (2.061) | –6.774 (–9.732 to –3.817) | 0.297 |
| 12 months | 44.370 (2.248) | –5.705 (–9.118 to –2.292) | 40.248 (2.112) | –7.115 (–10.442 to –3.788) | 0.560 |
| GHQ-30 | |||||
| Baseline | 9.601 (0.646) | 9.670 (0.613) | |||
| 6 months | 9.901 (0.771) | 0.300 (–1.127 to 1.727) | 8.972 (0.683) | –0.698 (–2.065 to 0.669) | 0.320 |
| 12 months | 10.143 (0.944) | 0.542 (–1.324 to 2.409) | 9.474 (0.771) | –0.195 (–1.763 to 1.372) | 0.551 |
Reductions in the multiply imputed EMQ-p score were observed for both the memory rehabilitation arm and the usual-care arm at 6 and 12 months’ follow-up. These results are commensurate with those presented in the analysis of clinical outcomes. The difference was not statistically significant at either 6 months’ (p = 0.297) or 12 months’ (p = 0.560) follow-up.
For the GHQ-30 score, although a reduction was observed in the memory rehabilitation arm at both 6 and 12 months’ follow-up relative to baseline, an increase was observed relative to baseline in the usual-care arm at both time points. This indicates an improvement in mood for the memory rehabilitation arm compared with a worsening in mood for the usual-care arm. There was a worsening in mood for both the memory rehabilitation arm and the usual-care arm between the 6-month follow-up and the 12-month follow-up. Despite using the GHQ (0–0–1–1) scoring rather than the Likert (0–1–2–3) scoring, these results are broadly comparable to the clinical outcomes, with no statistically significant difference in GHQ score within or between arms at either the 6-month follow-up or the 12-month follow-up.
Incremental cost-effectiveness analyses
The results of the cost–utility analysis are presented in Table 21. This presents the ICERs calculated using the available cases and multiple imputation approaches.
| Imputation method | Incremental | ICER (£) | NMBa (£) | NMBb (£) | |
|---|---|---|---|---|---|
| Cost (£) | Effect | ||||
| 12 months | |||||
| Multiply imputed | –26.895 | –0.011 | 2445 (SW quadrant, further investigation required) | –193.10 | –303.10 |
| Available cases | 250.62 | –0.004 | –62,656 (NW quadrant, usual care dominant) | –330.62 | –370.62 |
| Mean imputed | –26.197 | –0.023 | 1139 (SW quadrant, further investigation required) | –443.80 | –663.80 |
| 6 months | |||||
| Multiply imputed | –35.612 | 0.004 | –8903 (SE quadrant, intervention dominant) | 115.61 | 155.61 |
| Available cases | 30.11 | 0.003 | 10,038 (NE quadrant, further investigation) required | 29.89 | 59.89 |
| Mean imputed | –38.146 | 0.001 | –38,146 (SE quadrant, intervention dominant) | 58.15 | 68.15 |
The base-case analysis (incremental cost per QALY gained at 12 months, based on multiple imputation) showed the intervention to be slightly less effective but less costly than usual care, with a reported ICER of £2445. At 6 months the intervention was found to dominate usual care, with slightly lower costs and slightly higher QALYs, but these were not statistically significant. Uncertainty was seen in these findings as the results changed at 12 and 6 months, depending on the imputation method used.
Incremental cost per point improvement in EMQ-p and GHQ-30 scores at 6 and 12 months
Table 22 presents the ICERs for the EMQ-p and GHQ-30, respectively. As higher scores in the EMQ-p represent more frequent forgetting in daily life, and higher scores in the GHQ-30 indicate more psychological distress, negative incremental effects will be observed where the intervention is more effective than usual care. To calculate appropriate ICERs consistent with those for the EQ-5D-5L presented above, the denominator of the equation presented earlier is multiplied by –1.
| Imputation method | Incremental | ICER (£) | |
|---|---|---|---|
| Cost (£) | Effect | ||
| 12 months | |||
| EMQ-p | |||
| Multiply imputed | –26.895 | –1.4 | –19.07 (SE quadrant, intervention dominant) |
| Available cases | 250.62 | –4.8 | 52.21 (NE quadrant, further investigation required) |
| Mean imputed | –26.197 | –2.5 | –10.58 (SE quadrant, intervention dominant) |
| GHQ-30 | |||
| Multiply imputed | –26.895 | –0.7 | –36.49 (SE quadrant, intervention dominant) |
| Available cases | 250.62 | –0.2 | 1253.10 (NE quadrant, further investigation required) |
| Mean imputed | –26.197 | 0.4 | 70.23 (SW quadrant, further investigation required) |
| 6 months | |||
| EMQ-p | |||
| Multiply imputed | –35.61 | –2.3 | –15.34 (SE quadrant, intervention dominant) |
| Available cases | 30.11 | –2.1 | 14.34 (NE quadrant, further investigation required) |
| Mean imputed | –38.146 | –1.4 | –27.36 (SE quadrant, intervention dominant) |
| GHQ-30 | |||
| Multiply imputed | –35.61 | –1.0 | –35.68 (SE quadrant, intervention dominant) |
| Available cases | 30.11 | –1.6 | 18.82 (NE quadrant, further investigation required) |
| Mean imputed | –38.146 | –0.2 | –205.09 (SE quadrant, intervention dominant) |
For both the EMQ-p and the GHQ-30, in the primary analysis based on multiply imputed data at 12 and 6 months, the intervention dominated usual care because of non-statistically significant lower costs and improved EMQ-p and GHQ-30 scores. Again, uncertainty arises, with the results varying based on the imputation method used.
Sensitivity analysis
Sensitivity analysis using the upper and lower 95% CI bounds for the primary cost per QALY gained analysis is presented in Appendix 32. For two of the four scenarios, the intervention was dominated by usual care (higher costs and smaller effects for the intervention), namely when the upper 95% CI for both costs and outcomes was used and when the upper bound for costs was compared with the lower bound for QALYs.
In summary, the results suggest that the findings are not robust to changes in these parameters.
A further sensitivity analysis (based on available cases) was conducted on the intervention cost by including the costs of AP supervision by a clinical psychologist. Based on the recommendations for good practice presented by the British Psychological Society,97 we assumed that a minimum of three one-to-one supervision sessions of 1 hour were required at each site for the 10 weekly sessions. For a band 8a clinical psychologist at £66 per hour and a mid-band 5 AP at £35 per hour,85 a total of £2727 is incurred in addition to the total cost of rehabilitation presented in Table 14. The effect on the total cost and the ICER is presented in Table 23.
| Group | Cost (£), mean (SD) | 95% CI (£) | Difference in mean costs (£) (95% CI)a | p-value |
|---|---|---|---|---|
| Total costs up to 12 months (including intervention cost) | ||||
| Usual care (n = 88) | 1184.905 (857.282) | 782.903 to 1586.908 | 275.539 (–70.275 to 678.959) | 0.111 |
| Memory rehabilitation (n = 102) | 1460.444 (1678.229) | 1130.808 to 1790.079 | ||
| Total costs up to 6 months (including intervention cost) | ||||
| Usual care (n = 110) | 1002.020 (1190.572) | 610.984 to 1393.056 | 56.313 (–314.273 to 426.898) | 0.765 |
| Memory rehabilitation (n = 119) | 1040.160 (1634.991) | 743.358 to 1336.962 | ||
Taking into account these changes to the intervention cost, the intervention was found to be dominated by usual care at 12 months; at 6 months the ICER was £18,771 per QALY gained (Table 24).
| Imputation method | Incremental | ICER (£) | NMBa (£) | NMBb (£) | |
|---|---|---|---|---|---|
| Cost (£) | Effect | ||||
| 12 months | |||||
| Available cases | 275.54 | –0.004 | –68,885 (NW quadrant, usual care dominant) | –355.54 | –395.54 |
| 6 months | |||||
| Available cases | 56.313 | 0.003 | 18,771 (NE quadrant, further investigation required) | 3.687 | 33.687 |
The results of the bootstrapped replications are presented as a cost-effectiveness plane and corresponding CEAC for the primary cost–utility analysis (cost per QALY). As the multiply imputed base case for the EMQ-p and GHQ-30 at both 6 months and 12 months, presented in Table 22, indicates dominance of the intervention (lower cost and higher effects than usual care), cost-effectiveness planes and CEACs are not presented for the secondary cost-effectiveness analyses. The cost-effectiveness plane for cost per QALY gained is presented as a scatterplot of the point estimates obtained from the 5000 runs, depicted as quadrants (north-east, south-east, south-west and north-west), as illustrated in Appendix 22. The CEAC graph shows the probability of the intervention being considered cost-effective at different monetary thresholds.
Figures 2 and 3 present the cost-effectiveness plane and CEAC for the incremental cost per QALY gained at 12 months, respectively.
FIGURE 2.
Cost-effectiveness plane for the bootstrapped incremental cost per QALY gained at 12 months. Reproduced from das Nair et al. 82 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
FIGURE 3.
Cost-effectiveness acceptability curve for the bootstrapped incremental cost per QALY gained at 12 months.
Figure 2 characterises the uncertainty in the cost-effectiveness analysis, with point estimates distributed across all four quadrants. The largest proportion of estimates is located in the south-west quadrant, consistent with the intervention being less costly and less effective than usual care. The associated CEAC in Figure 3 shows that, at a threshold value of ≤ £30,000 per QALY gained, the probability that the intervention is cost-effective is 24.5%. At a threshold of £20,000 per QALY gained, the probability of cost-effectiveness is 29.0%. The cost-effectiveness plane and corresponding CEAC for the additional analysis at 6 months are presented in Figures 4 and 5, respectively.
FIGURE 4.
Cost-effectiveness plane for the bootstrapped incremental cost per QALY gained at 6 months.

FIGURE 5.
Cost-effectiveness acceptability curve for the bootstrapped incremental cost per QALY gained at 6 months.
In accordance with the 12-month results presented in Figure 2, at 6 months the cost-effectiveness plane presented in Figure 4 further demonstrates the uncertainty inherent in the cost-effectiveness results, with all four quadrants populated. In contrast to the 12-month analysis, the largest proportion of estimates is located in the south-east quadrant, consistent with the intervention being less costly and more effective than usual care; the intervention dominates usual care. The incremental effect is, however, small. The associated CEAC presented in Figure 5 illustrates that, at a WTP threshold of £20,000 per QALY gained, the intervention has a 68.1% probability of being cost-effective. At a higher WTP threshold of £30,000 per QALY gained, the intervention has a 70.6% probability of being cost-effective.
It should, however, be noted that bootstrapped replications of the cost per point improvement ICERs for the EMQ-p and GHQ-30 based on mean imputed data produced cost-effectiveness planes that were somewhat analogous to those presented in Figures 2 and 3 for the cost–utility analysis. Estimates are spread across all four quadrants, further demonstrating the uncertainty observed within the results. For both secondary outcomes at 12 months and 6 months, the largest proportion of point estimates is located in the north-east and south-east quadrants, associated with the intervention being more effective than usual care.
In Table 22 the base-case cost-effectiveness analyses of the EMQ-p and GHQ based on multiply imputed data found that the intervention dominated usual care at both 6 months and 12 months. The resulting cost-effectiveness planes for these outcomes show the largest proportion of bootstrapped estimates in the south-east quadrant, consistent with the intervention being less costly and more effective than usual care. The probability of intervention dominance based on the proportion of bootstrapped estimates in the south-east quadrant of the cost-effectiveness plane was 43.9% and 50.1% for the EMQ-p and 43.5% and 50.8% for the GHQ-30 at 6 and 12 months’ follow-up, respectively.
For both secondary health economic outcomes, the probability of dominance at 6 months is larger than that at 12 months, consistent with the larger effect observed for the shorter time horizon. Similar levels of dominance are, however, observed for the EMQ-p and GHQ.
Results of the model-based analysis to estimate longer-term cost-effectiveness
For a longer-term perspective, the cost-effectiveness estimates were extrapolated over horizons of 5 and 10 years using the Markov model presented in Appendix 23. The deterministic cost-effectiveness results obtained from the model for the 5- and 10-year horizons are presented in Appendices 33 and 34, respectively. The base-case analysis was carried out using a cohort of 10,000 patients aged 45 years, corresponding to the average age of participants in the trial. The base-case results are presented in Table 25.
| Horizon | Trial arm | Incremental | ICER (£) | ||||
|---|---|---|---|---|---|---|---|
| Memory rehabilitation | Usual care | ||||||
| Cost (£) | QALYs | Cost (£) | QALYs | Cost (£) | QALYs | ||
| 5 years | 3599.08 | 2.952 | 3404.27 | 2.651 | 194.81 | 0.301 | 646.36 |
| 10 years | 6451.25 | 5.340 | 5976.51 | 4.806 | 474.73 | 0.535 | 887.76 |
The results indicate that, for a 5-year horizon, the intervention is cost-effective, with an incremental cost of £194.81 and incremental QALYs of 0.301, resulting in an ICER of £646.36 per QALY. For the 10-year horizon, the intervention is again shown to result in higher costs and more QALYs, with an ICER of £887.76. These results suggest that, for longer horizons, the intervention is cost-effective.
One-way sensitivity analyses were conducted on the base-case results presented in Table 25 using a variety of parameter inputs, including age at which individuals enter the model, the discount rate applied to costs and outcomes, the intervention cost, costs and outcomes related to each health state and transition probabilities. A summary of the results is presented in Appendices 33 and 34.
In general, the ICER results for both the 5-year horizon and the 10-year horizon appear robust to one-way sensitivity analysis changes in the input parameters, with positive incremental costs and QALYs. However, by reducing the cost associated with one cycle in each health state by 30%, for both the 5-year and the 10-year horizons, the intervention becomes less costly than usual care, with incremental QALYs remaining positive. This represents a shift from the base case, in which the intervention was more effective and more costly than usual care, generating a positive ICER (north-east quadrant of the cost-effectiveness plane), to a scenario in which the intervention is dominant, being less costly but more effective than usual care (south-east quadrant of the cost-effectiveness plane).
The one-way sensitivity analyses presented above suggest that the base-case scenario is generally insensitive to a range of model inputs. The results of the probabilistic sensitivity analyses, which consider uncertainty regarding a variety of input parameters simultaneously, are illustrated in cost-effectiveness planes and corresponding CEACs in Figures 6–9.
FIGURE 6.
Probabilistic cost-effectiveness plane: 5-year horizon.
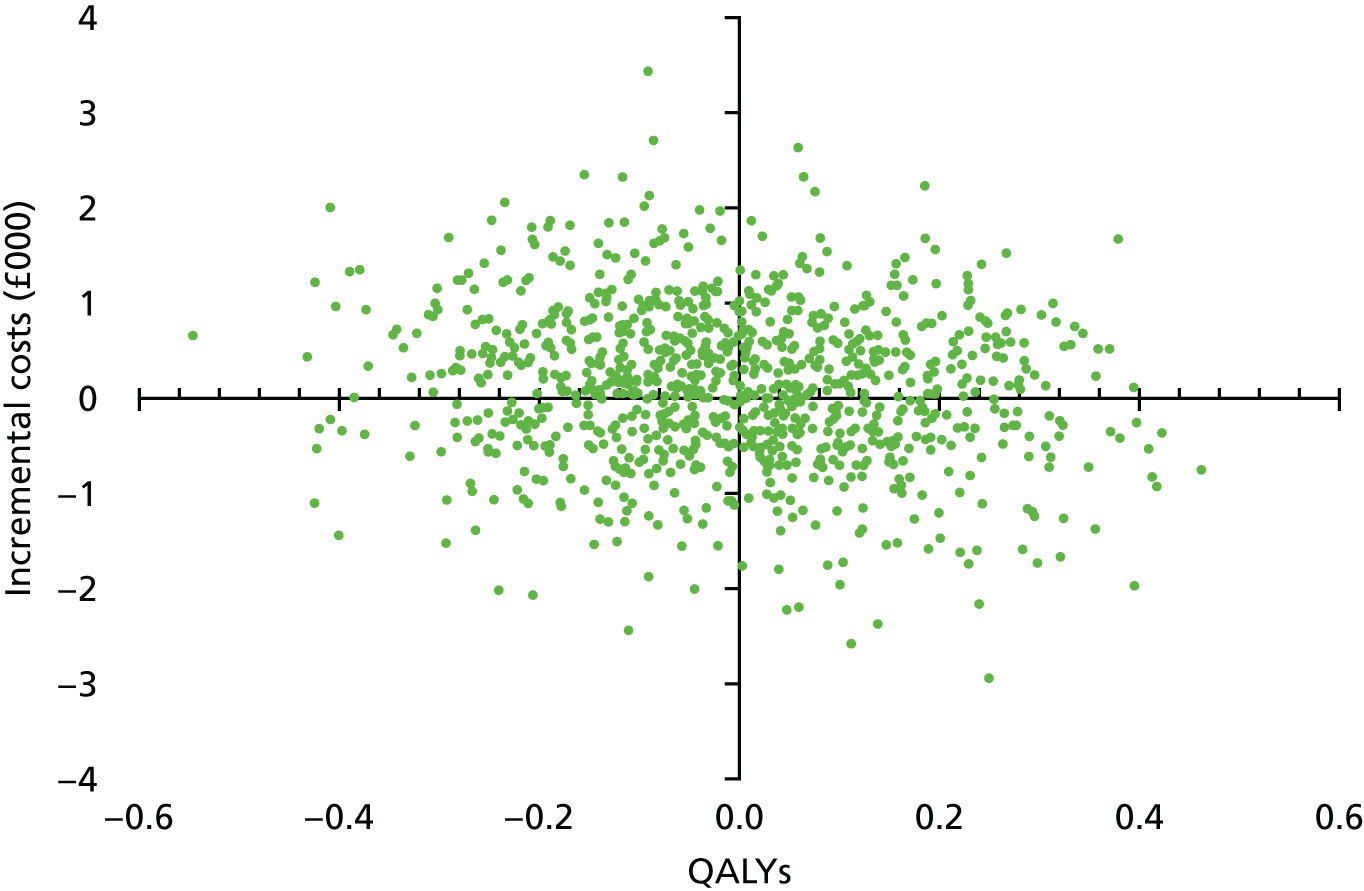
FIGURE 7.
Probabilistic CEAC: 5-year horizon.

FIGURE 8.
Probabilistic cost-effectiveness plane: 10-year horizon.
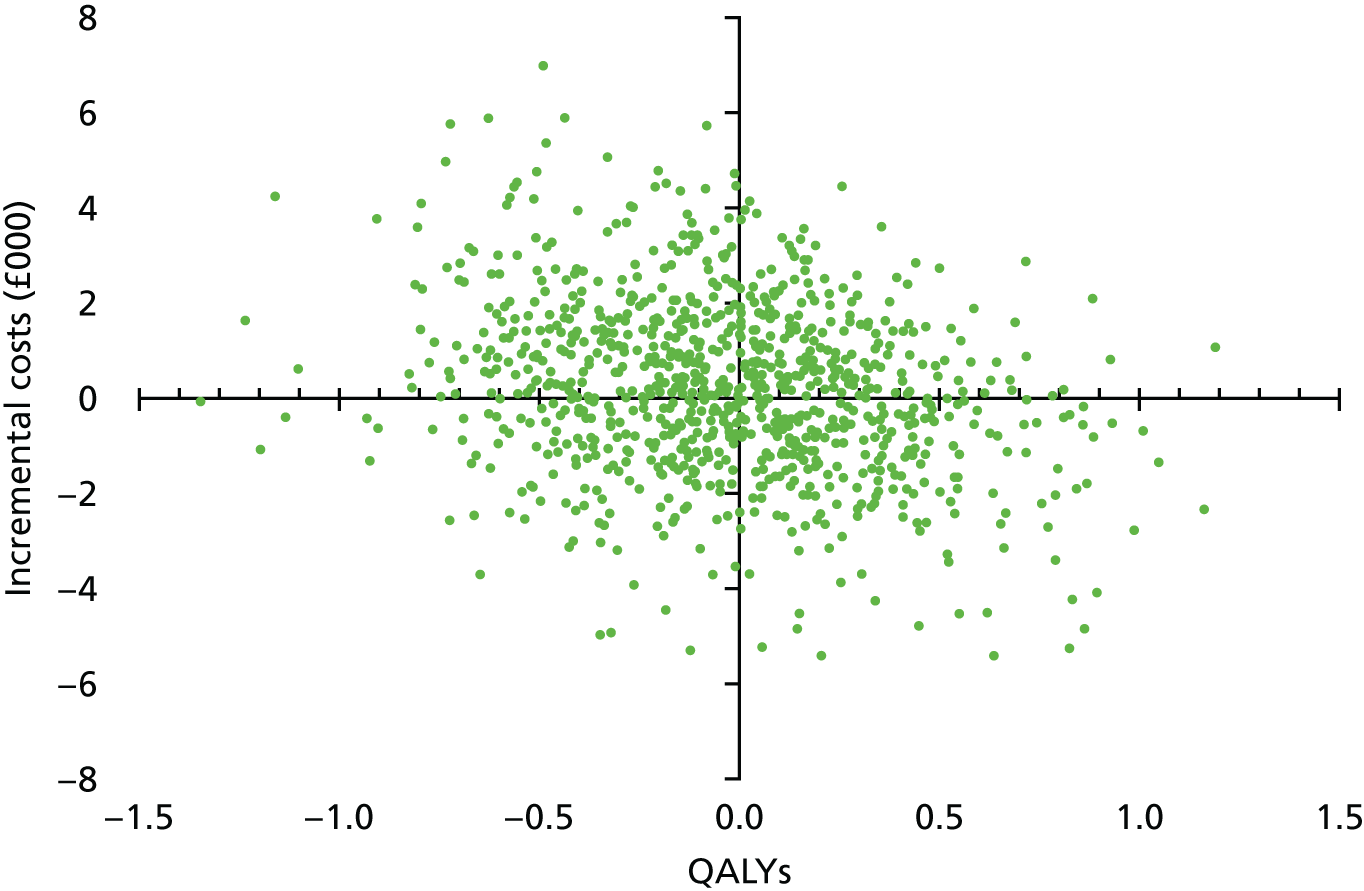
FIGURE 9.
Probabilistic CEAC: 10-year horizon.
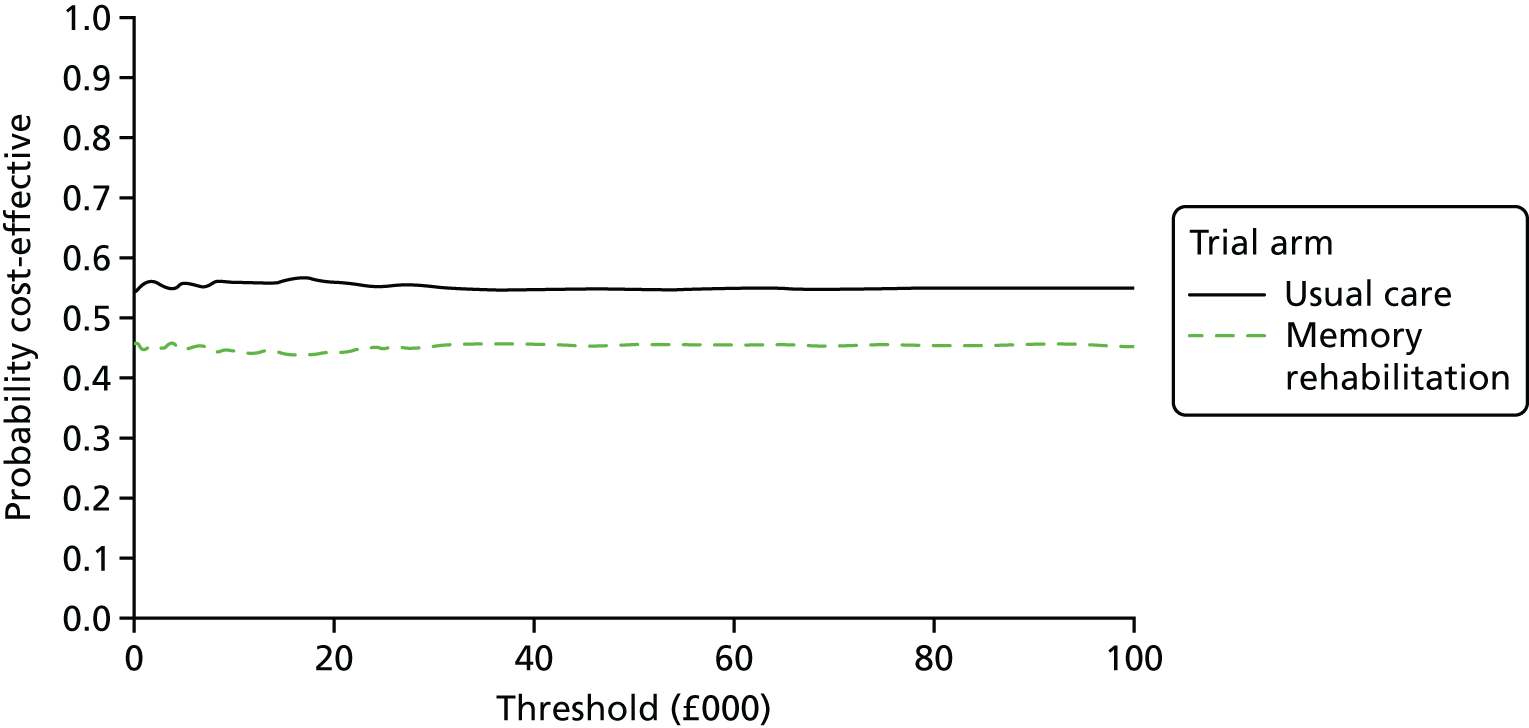
Consistent with the cost-effectiveness planes for the within-trial analysis (incremental cost per QALY gain at the 12-month and 6-month follow-ups), the 5-year and 10-year horizons illustrate substantial uncertainty, with all four quadrants populated (see Figures 6 and 8). The CEACs presented in Figures 7 and 9 indicate that the probability that the intervention is cost-effective is around 45% for both the 5-year horizon and the 10-year horizon, irrespective of the threshold value, λ.
Discussion
Overall, the findings from the health economic evaluation show that there was ambiguity in the findings, with results not remaining robust when different imputation approaches and time points were taken into account and when the impact of variation in costs and outcomes was examined, given the small (statistically non-significant) differences between treatment arms and wide CIs seen.
The cost-effectiveness planes for all outcomes and time horizons in the analyses showed dispersion of point estimates of incremental costs and effects across all four quadrants and illustrate the uncertainty in our findings.
It is important to consider our results not just from the point of view of the technical assessment of cost-effectiveness. Memory rehabilitation was estimated to cost £167 per participant (based on the multiply imputed data); this resulted in small cost differences between treatment arms. We also based our comparator on usual care and, given the pragmatic nature of the trial, this could have reflected different models of care within the trial arm and associated resources (and costs). However, the interpretation of small effect sizes is also important in the health economic analysis; the numerically small QALY gains seen are not statistically significant and are unlikely to be considered clinically meaningful. The analysis of our findings based on a ‘per point improvement’ basis should also be considered fully in light of the fact that no statistically significant or clinical meaningful differences were seen, as reported in Chapter 3.
There are limitations to our health economic analyses. The methods used to collect resource use data within this population should be carefully considered, for example, whether recall bias was a potential issue (albeit this applies to both arms). The SUQ used and data derived were not sufficiently clear to enable us to fully examine resource use specifically attributed to memory problems and thus we took all resource use and costs into account. Although this may have overestimated costs, this applied to both arms. We undertook a thorough investigation of our data, given the scenario that, in the non-imputed analysis, costs were higher for the memory rehabilitation arm at baseline and 6–12 months. Although there was evidence of a reduction in service usage costs between baseline and 6 months for the memory rehabilitation arm, after including the cost of the intervention, the difference in costs between the memory rehabilitation arm and the usual-care arm was not statistically significant.
One potential limitation of our analysis of service usage data is that we considered only a health and personal social services perspective; because of the nature of rehabilitation, there could be potential downstream effects on the patient, carer and family and wider impacts, such as on employment, that have not been evaluated by this study. Instead, the health economic analysis used the EQ-5D-5L as its primary outcome measure. This provided a more ‘general’ assessment of patient outcomes and could (in theory) capture broader aspects of HRQoL rather than a single dimension of mood or function, as captured in the other trial outcomes. However, the trade-off is that the use of such a generic measure can often result in a lack of precision in the estimation of health effects.
We fully considered whether or not it was feasible and plausible to undertake a model-based analysis to explore the longer-term cost-effectiveness of the intervention, which had been set out in the protocol and health economics analysis plan. In light of the trial findings, we chose to do so to ensure that we conducted a comprehensive analysis as originally set out. The results of the longer-term cost-effectiveness, based on our model-based evaluation, should be interpreted with caution, although they do give a useful indicator of expected benefits. We created a de novo model (as far as the data and external evidence allowed us) using the results of a robustly conducted trial alongside reasonable, albeit basic, assumptions to examine the longer-term cost-effectiveness. This may provide a basis for future health economic analyses.
We argue that the decision to amend the original protocol and not undertake analyses using a lifetime horizon but instead base analyses on mid-term horizons (5 and 10 years) was a sensible compromise. Sufficient evidence is not available to precisely determine the costs and outcomes associated with longer-term horizons. Therefore, extrapolating the trial results in the model to a lifetime horizon would likely lead to misleading or erroneous conclusions.
One potential area for consideration is how future analyses could capture some of the ‘wider’ benefits of such interventions, given that the qualitative findings in Chapter 5 indicate the potential for benefit across broader outcomes. This raises potential questions including further examination of patient preferences for interventions to support memory rehabilitation and the inclusion of other outcomes of interest to fully consider the impact of group memory rehabilitation in terms of value for money.
The trial has raised important questions with regard to how future trials should be developed in this area. Similar questions should also be considered in the development of any future health economic evaluations. Although the conclusions from our health economic analyses are appropriately cautious as they must be made ‘on the evidence’ as presented, the need for high-quality methodically driven but ‘real world’-led economic evaluations must be part of any future agenda in deriving the evidence on whether to invest in this area of patient care.
Chapter 5 Qualitative feedback interviews
Rationale
Mixed-methods designs are increasingly being used in evidence-based practice research. 98,99 Qualitative research can explore and explain the real-world complexities involved in participating in a trial or being a recipient of health-care interventions. This nested qualitative study provided a separate evaluation of memory rehabilitation, which served as an adjunct to the primary trial. We believed that insights from these qualitative data and the analysis would serve to inform developments of the intervention programme in the future and to generate user-oriented proposals about areas for further investigations.
Aims
Although there is an overlap in terms of the broad aims of the trial and this study, the specific focus of this study was an experiential and phenomenological one, with a view to provide a nuanced account of participant experiences. The aims of this substudy were therefore linked to the main aim of the trial (i.e. to evaluate the effectiveness of memory rehabilitation in TBI), but specifically, we wanted to explore participant experiences of:
-
being involved in the trial
-
receiving group memory rehabilitation
-
using strategies to cope with memory impairment
-
usual care.
Methods
We conducted one-to-one feedback interviews with participants enrolled in the trial within 2 months of completion of the 6-month follow-up assessment.
Sampling
Participants were purposefully selected from a subgroup of trial participants who had originally consented to being invited for interview. We used a maximum variation sampling strategy, a purposive sampling technique used to identify and select participants who have important shared experiences that cut across different subgroups based on demographic, clinical or other variables (see Palinkas et al. 100 for a review of purposive sampling techniques). Maximum variation sampling was used to achieve multiple perspectives that demonstrate real-world complexities. 101 The selection strategy was designed to include participants with varying demographic features (age, gender, ethnicity) and levels of memory impairment (based on the EMQ-p conducted at baseline) and from different sites. Once the first few participants had been interviewed, we then selected others who were different from the first interviewees (in terms of demographics and memory impairment). This iterative process continued until we had recruited the required number of participants.
Sample size
In determining the sample size for the feedback interviews, we followed Onwuegbuzie and Leech’s102 recommendation of identifying a corpus of studies that used the same design and in which data saturation was reached and examining the sample sizes in these studies. Data saturation (the point at which further data collection is unlikely to lead to new insights) has been described as an ‘elastic’ concept and the desirable degree of saturation depends, in part, on the nature and breadth of the research question. 103 Indeed, the concept of saturation is a contested one and there is no one-size-fits-all method to reach data saturation. 104,105
Our meta-synthesis of published qualitative studies of group-based memory rehabilitation106 revealed sample sizes of 10–38 participants. We therefore opted to recruit 32 participants, aiming for 16 from the usual-care arm and 16 from the memory rehabilitation arm. We believed that data saturation would be achieved with this number of interviews. Furthermore, this would allow us to sample four participants each from the memory rehabilitation and usual-care arm from four sites. However, as we increased the number of sites as the trial progressed, we altered the sampling frame to recruit at least two participants from each site where possible.
Procedure
Consent to be interviewed was checked with the participants selected for this study and, when participants were willing to participate, an interview was arranged. To reduce social desirability response bias, a researcher who was not involved with the participants’ assessment or treatment conducted the interviews. This researcher was not blind to treatment allocation, as she needed to follow a semistructured interview schedule (see Appendix 35), which included general questions for all participants and specific questions for those who had received the intervention and those who were in the usual-care arm.
The interviews took place face to face in participants’ homes. At the interview, the researcher reiterated the purpose of the interview and informed participants that the interview was being audio recorded on a dictaphone. During the interview, participants were invited to discuss their thoughts as freely as possible, although the researcher used the interview schedule to guide the interview agenda and to create some comparability across the interviews. 107 Participants were assured confidentiality and had the option to pause or stop the interview or skip questions if they chose to. The audio recordings were transcribed verbatim by a professional transcription service.
Analysis
The transcripts were analysed using framework analysis. 108 Framework analysis is a specific type of thematic analysis109 that is atheoretical and allows researchers to collapse large data sets of qualitative data along broad areas of research interest determined a priori. It is more deductive and structured than other forms of thematic analysis and usually yields a matrix of cases and codes, with data placed in ‘cells’ that contain chunks of the interview data transcripts. Through a constant comparison method (both within and across cases), the data are then reduced to the key themes. Themes can be broken down to subthemes and further subsidiary themes. This method is useful in multidisciplinary research teams with more than one researcher analysing the data. 109
Two researchers (SC and HC) read and reread the interview transcripts a number of times to familiarise themselves with the data. Each separately conducted a line-by-line coding of half of the transcripts before swapping over to check the codes. Disagreements were resolved by discussion with another researcher (RdN). Thereafter, we created a ‘master map’ based on the aims of this substudy, which served as the framework to map the data onto. Mind maps for each transcript based on its coding, which was discussed with the third researcher (RdN), were then created. Use of mind maps is not common in framework analysis, but we included this step because mind maps allow us to see how the various themes, subthemes and codes connect with each other, thereby suggesting possible relations between them. We then populated the framework matrix and four themes (and subthemes) were extracted from the condensed data.
In Results, we discuss each theme and subtheme. For each theme and subtheme we provide relevant quotes from the participant feedback interviews, with quotes attributed to participants (demarcated by a unique participant identification number, gender, age and treatment allocation). We describe our results using quantity measures such as ‘all participants’, ‘most’, ‘half’, ‘almost half’ and ‘a few’, with this order representing decreasing values.
Quality considerations
To ensure the quality of the study, we adopted the criteria of Mays and Pope110 by providing a clear exposition of the methods of data collection, sampling and analysis; our reflexive position; attending to negative cases; the context; and ‘fair dealing’ with respect to providing different perspectives so as not to privilege one group over another.
We approached this substudy from a critical realist epistemological perspective. Critical realism, where language is assumed to be used to construct our social realities but that these constructions are limited to that which is grounded in the material world,111 functions as a ‘general methodological framework for research but is not associated with any particular set of methods’. 112 This perspective helps researchers explain social events or issues and suggest ways to address these. 112
Results
Thirty-two participants (10 women and 22 men) were interviewed. Interviews were conducted in two tranches and lasted between 13 and 69 minutes. Participants were aged between 24 and 68 years at randomisation and all were white. The time since TBI ranged from approximately 9 months to > 34 years. We recruited participants from eight of the nine sites. We did not recruit from one site because the site was closed to recruitment before participants reached the 6-month follow-up. Details of the sites that we recruited from and the composition of the participants in terms of whether they were memory rehabilitation or usual-care participants are shown in Appendix 36.
Feedback on the trial
The first theme relates to how participants felt about taking part in the trial. Participants described their motivation for taking part, experiences of specific trial procedures, experiences of the assessment procedures, preferences for the group format and reactions to the allocation. Participants also provided their overall impressions of the trial (Figure 10).
FIGURE 10.
Thematic map of the ‘feedback on trial’ theme.

Motivation for taking part
Most participants appeared to be driven to take part in the study by altruism. Many spoke of wanting to help others:
I’m just helping you guys out so that, so that what you can get can help somebody else out, you know, in the future.
01003, male, 58 years, usual care
So to go onto this [study] was good because I thought if this gets other people out of a situation that I was in then that’s why I wanted to do it.
06049, male, 68 years, usual care
Participants also thought that by taking part in the study they would receive some information about their memory problems and some help to deal with these problems:
It was a matter of floundering around thereafter [after discharge from hospital], er that’s why when this [study] came up we jumped at the opportunity to take part.
04005, male, 56 years, intervention
I wanted to get as much information as I could really about how it [brain injury] affects me and how I can deal with it. So that was my idea of going along with it [the study] in the first place.
05042, male, 51 years, intervention
Trial procedures
Participants had positive feedback regarding the organisation of the trial. In addition, the qualitative study was praised for providing an opportunity to raise more attention about TBI and memory rehabilitation, including the need for increased funding for such services:
From the practical viewpoint I thought the study was done well. You’re kept informed people, errm, kept appointments, etc. it was all organised, kept to there’s no issues there.
04010, male, 55 years, usual care
I’m very pleased that I’ve been part of this feedback . . . I think it [trial] is a very good thing to do I think more emphasis, more pressure on the powers that be to allocate more funding to this [TBI memory rehabilitation] because it’s very important and it does not receive anywhere near the amount of attention that it deserves.
07012, male, 37 years, usual care
The trial also helped usual-care arm participants understand that they were not alone with their TBI, despite not attending the memory rehabilitation groups as part of the intervention:
It’s [taking part in the trial] opened my eyes an awful lot, of how things are, and how I’ve got to accept my life is now and it’s not just me, there’s god knows how many out there are in the same position.
01005, male, 43 years, usual care
It [taking part in the trial] was good, it, it was informative, it helped me understand I’m not the only one with the problems.
01017, female, 30 years, usual care
Experience of assessments
Overall, participants were positive about the assessments that they undertook as part of the trial. The fact that they were conducted at home or at other convenient locations (e.g. local hospital) was highlighted as ideal in terms of travel arrangements and as a way of allowing participants to remain in their comfort zone. Additionally, participants found the assessments helpful with regard to highlighting their specific memory impairments and strengths:
The assessments have been done; it was either at [names location] when we were first looking at enrolling in the course, not exactly far to travel and then all the assessments and discussions afterwards have been here at the home – so that’s ideal for us.
03004, female, 39 years, intervention
Well my psychologist comes here [home] . . . I prefer it, it’s awful, it’s terrible . . . but this is my comfort zone . . . I feel better in my own home . . . I feel safe. I don’t know why, it’s weird isn’t it.
09018, female, 51 years, usual care
It was helpful that they [researchers] got back to me and showed me where the deficits were, with where I was lacking sort of thing.
03022, male, 25 years, usual care
However, one participant reported that she did not like the fact that the assessments highlighted her specific memory weaknesses:
I’m not sure if it [assessment] wasn’t useful but I didn’t really like the faces when she [researcher] showed me the faces [from an assessment] . . . which is probably because I needed more help with that, do you know what I mean? . . . But I knew that was a weakness and I don’t like weakness.
08007, female, 41 years, intervention
Although most participants were positive about the assessments, some felt that they were challenging and not necessarily reflective of real-life memory concerns. Additionally, one participant did not understand the need for repeated assessments throughout the trial:
I found it [the assessments] taxing in a way . . . some of the tests were OK, others because of problems with my memory and that was a bit taxing, do you know what I mean, but overall it was good to do, if that makes sense, yeah.
09018, female, 51 years, usual care
It’s [assessments] either focused on stuff I’m not very good at anyway, so like remembering names and faces, you’ve got so many faces and they all look similar and they all have like are relatively the same . . . they’re people I don’t really know or care about, I’m not going to remember them.
08002, male, 31 years usual care
You start thinking, why are you doing this [the assessment] . . . and then I had a reassessment and we had to do it all again which I thought was quite, well we’ve been here before haven’t we, do we need to do it again kind of thing.
03005, female, 56 years, intervention
Preference for rehabilitation format
The majority of participants stated that they would rather experience memory rehabilitation as part of a group than on a one-to-one basis, with the reason often being the benefit of sharing ideas with other participants who have first-hand knowledge of having a TBI.
In a group it’s much better cause then you can get the impact of someone else and they can give you an idea or vice versa so, erm but individual, no disrespect, but it’s like, yeah well I’m telling you all these things and you’ll try tell me, but they [group members] know what it’s like, but the actual person [AP] doesn’t.
01010, female, 24 years, intervention
It would probably be helpful to have a group-based thing . . . because one-on-one you don’t necessarily know what you, what you currently want help with . . . if you’re in a group you kind of hear other people’s struggles and you kind of come out and go ‘yeah, I have some problems with that’.
08002, male, 31 years, usual care
However, a few participants would have preferred one-to-one memory rehabilitation. They felt that one-to-one sessions would allow their relevant concerns to be addressed and enable them to discuss personal issues, as well as allowing them to concentrate more easily than in group situations:
One-to-one probably because I have had, I’ve been to other group sessions where I’ve found it’s not necessarily been very relevant which might sound a bit stupid . . . but I’ve definitely found, from my experiences, one-to-one sort of treatment and rehabs have been more useful. Because you can talk about your personal issues.
04008, female, 25 years, usual care
I would probably prefer one-to-one . . . I tell you why because in [rehabilitation centre] sometimes I cannot concentrate because there’s so much rambling, because there’s people with different levels of head injury, right, and some of them really ramble on and on and on and on and arrgghh! So sometimes I have to sit right at the back.
09018, female, 51 years, usual care
A few participants were unsure of their preference because they could see the benefits of both one-to-one and group rehabilitation formats.
I don’t know. I think you could cover the same things more tailored to individuals faster [in] one-to-one, but then obviously, you know, other patients . . . have got contributions to make that the person that’s running it [groups] don’t have, as well, and . . . it is quite nice to see other people with the same issues.
07002, female, 29 years, intervention
I think given my character I’m a very vivacious person I probably would have been good in a group but as you might have seen today I can go off on a tangent and I’m probably better with someone focusing on me . . . I don’t think a group would have been the wrong thing but better might have been I should try both.
07012, male, 37 years, usual care
Reaction to ‘usual-care’ allocation
Half of the participants randomised to the usual-care group stated that they had no problem with their allocation. This was often because of an understanding of the need for a control arm, although one participant expressed relief at not having to fit the weekly memory rehabilitation group sessions into his busy working schedule:
Which I don’t have a problem with [being in a control group] because I do understand the concept of how you need to have a baseline and I’d said that I was quite happy to participate in the study and, yes you’ve got to have willing baseline otherwise a study’s meaningless.
04010, male, 55 years, usual care
Kind of a bit relieved . . . a bit of me was worrying, thinking ‘how am I going to squeeze that [rehabilitation sessions] in with everything else?’
08002, male, 31 years, usual care
However, a few participants were disappointed with being allocated to the usual-care arm because they had hoped to receive the intervention, which they believed would improve their memory:
It would have been nice to have been in the other [i.e. treatment] group . . . Just to see what you were doing and if it worked and made a difference.
04008, female, 25 years, usual care
I was a little disappointed actually I was hoping that I might erm, experience something new that would help my memory.
04009, male, 46 years, usual care
A few participants misunderstood the randomisation process and believed that it was their performance on the baseline assessments that decided their allocation:
I didn’t really mind which, which group I’d, I‘d end up in er cause obviously we went through the various er questions and, that determine over time which group you went into.
03014, male, 27 years, usual care
It [the study test] was to see whether I’d be allocated into the, a group to go on to other tests or another different group, which obviously, which wouldn’t go on to the tests.
03022, male, 25 years, usual care
Overall impressions of the trial
When asked for their overall impressions of the trial, a few participants mentioned the personal characteristics of the trial staff:
All the assistants that have been involved in the study with me have been you know very easy going and explained everything to me.
03014, male, 37 years, usual care
I would like to say that erm all the people I’ve met and talked to have been very happy and very polite and very pleasant.
04009, male, 46 years, usual care
Overall, participants expressed satisfaction with taking part in the trial, but when asked specifically for suggestions for improvement they recommended that usual-care arm participants be sent the intervention manual at the end of the trial, a crossover study design (so that everyone received the intervention), a wider variety of assessments, more frequent assessments, more detailed feedback on assessments and a written timescale of key study dates (e.g. assessments):
Maybe the number maybe there could be more assessments, that would have been more helpful and give me a better idea between one and the next.
07012, male, 37 years, usual care
Experience of the rehabilitation group
The second theme related to how participants randomised to receive the intervention experienced the memory rehabilitation groups. Participants described the timing of the intervention in relation to their TBI, the format of the group, the content of the intervention manual, their experience of the group facilitator and the perceived effects of the group (Figure 11).
FIGURE 11.
Thematic map of the ‘experience of group’ theme.

Timing of the intervention
Participants randomised to the memory rehabilitation group stated that they would have preferred to receive the memory rehabilitation sooner after their TBI, but ‘not too soon’ because of difficulty processing information immediately after their injury:
Yes, earlier would have been good . . . But not right at the beginning, because right at the beginning, it would have just gone in one ear and out the other, effectively.
07002, female, 29 years, intervention
Personally, from my point of view, it [memory rehabilitation] would have helped me if it was like 6–12 months after the accident rather than 4 years later.
03004, female, 39 years, intervention
Although participants in the usual-care arm were not asked specifically about the timing of memory rehabilitation, one usual-care arm participant’s feedback is valuable here. This participant felt that there was a need for memory rehabilitation at the optimal time after TBI. She described a lack of engagement with a neurorehabilitation centre in the year after her TBI:
They [clinical staff] were quite keen to do different courses and different things with me but at the time I was very reluctant, I just wasn’t in the right frame of mind I think, but had it been further down the line, because I was still quite unwell and not myself after the accident and I just wasn’t interested.
04008, female, 25 years, usual care
The extended length of time between participants’ TBI and the intervention meant that some participants found it challenging to adopt the new strategies being taught in the memory rehabilitation group:
So trying to learn, it’s like with a head injury and that you, you’re learning stuff like, it’s like you’re learning from new kind of thing, but trying to learn new strategies like, oh what was the one she [AP] said, like a dictaphone for instance, see that wouldn’t work for me, so I wouldn’t have tried it.
01010, female, 24 years, intervention
I’d muddled through and found strategies that worked for me and then to be thrown into a group, that’s the wrong term to use but to attend that group, and I’d be thrown 101 other strategies it’s like, hang on a minute I’m doing the, I’m using these strategies and it works for me and I can forget all those strategies and look at applying new ones and that takes effort and it takes energy and it takes thinking whereas had I not been going into a routine and using some of my own strategies I might have been a bit more susceptible to taking some of those on board.
03004, female, 39 years, intervention
This was linked to participants having already developed their own strategies. Almost half of the participants in the memory rehabilitation arm already knew some of the strategies taught in the memory rehabilitation group either because they had worked them out themselves or because they had attended other rehabilitation sessions:
Er yeah the, these strategies er, the strategies er I used most, I found at least half of them I use anyway.
01012, male, 59 years, intervention
I was doing quite a lot of it anyway from my own work but there were some extra tips that I picked up.
08007, female, 41 years, intervention
Format
The majority of participants described benefits gained from the group format of the memory rehabilitation. This included a feeling of not being alone with their TBI, sharing experiences with people in a similar situation, conversing with people who understand (as opposed to a lack of understanding outside the group), social interaction and sharing ideas or strategies:
We both [participant and other group member] found that talking to other people who’d had brain injury made you feel more normal, made you feel like it wasn’t just you.
05053, male, 32 years, intervention
the good thing about it [group] was the problems that the other people at the group were having which were the same as mine with the memory and everything, very, very similar.
06045, male, 56 years, intervention
I liked meeting others who’ve had brain injury and how they go about their day-to-day life and conversing really erm on how . . . other people you know in the, in the work, in their day-to-day life do not always understand you, maybe you’re a bit slower or a bit, you know.
03001, female, 54 years, intervention
The benefit of the group was to erm [pause] to realise that er, [pause] that, that I could get out and about and, and interact with these people socially.
01012, male, 59 years, intervention
It was really good to see us like, not only me giving ‘em ideas but them, them throwing ideas back at me, maybe that they haven’t tried yet.
01010, female, 24 years, intervention
Participants also commented on the composition of the group in relation to group attendance, the benefits of seeing other people with TBI looking well, feeling like a ‘fraud’ because of self-comparison of ability with the ability of those with more severe disabilities in the group, and conflicts within the group:
The other thing as I say, sadly er a, a couple of the guys erm didn’t turn up, I mean one was working . . . I just thought it was a shame that erm people didn’t turn up.
04005, male, 56 years, intervention
The hopes and dreams of these people [participants] have been er have been shattered you know, where, and er because of their circumstances but still they were, still they were happy within themselves and that to me was, that was important.
01012, male, 59 years, intervention
When you listen to the other people [participants] and especially two of the other people and you think oh my god it happened so long ago and they’re still really struggling. And it was really, it used to upset me, I felt like a fraud.
03005, female, 56 years, intervention
I’d had enough of it and I just said ‘will you shut up, you’re putting me off’ [to another group member] and it was like, even though everyone else was thinking it, I was the only one that had to say it but I thought no, it’s no use for me, you’re spoiling it [attending the group] for me, because I even thought of not going because the thought of seeing her [participant] again I thought oh, here we go.
05042, male, 51 years, intervention
Most participants were satisfied with the format of the memory rehabilitation group. However, the following suggestions were made when participants were specifically asked for ways that we could improve the intervention: more group sessions, longer sessions so that the information could be taught at a slower pace, the use of social events before and after the intervention, and a follow-up group to check the maintenance of memory strategy use:
Apart from being able to erm [pause] do the course for a longer period of time to be able to actually utilise things, [pause] but what I, what I want and what is really achievable, I don’t know if it’s feasible.
01015, male, 33 years, intervention
Instead of having I don’t know how many hours in a session I, I thought that if you could get an afternoon session, now maybe you classify it as too long, to go through things, I’d say a little bit slower.
04005, male, 56 years, intervention
I think the group was such a nice group I think we should be a good thing to organise like at the end of it was sit down and have a night out or go to the pub or something.
04002, male, 24 years, intervention
Well whether it was between the sessions or, erm if the sessions continues say, if they continued like, you know say you meet once every 3 months or once every, whatever and you, you met up with those people and you, and you know perhaps the lady [AP] or, or somebody from the memory place said you know have you, how’ve you been getting on with your er, your diaries, have you got all your diaries, have you been filling them out regularly.
03003, male, 55 years, intervention
Content of the manual
Almost half of the participants provided positive feedback on the content of the manual used in the memory rehabilitation groups. This included an appreciation of the group tasks, the belief that the structure of the sessions was good and stating that the language used in the manual was clear:
You have activities toward the end of each week, that’s good, because sometimes it is the practical experience of doing something that actually kind of consolidates the learning of it.
09008, male, 33 years, intervention
But I think the way it was set out was good, the way the leaflets – well, like a booklet explaining everything, which you followed where they explained what they were trying to do.
05042, male, 51 years, intervention
So the material was clear and concise and targeted so I don’t think er, I don’t think anything could be [improved].
01012, male, 59 years, intervention
However, one participant felt that the manual was written in a way that was difficult to understand:
I think there are elements of it which are written in PhD language.
09008, male, 33 years, intervention
In terms of negative feedback, a few participants stated that each session contained too much information. Additionally, one participant reported that the content was repetitive and another expressed a strong dislike for a particular relaxation exercise:
If I had to be really picky it was just that some of the sessions were very intense and there was a lot of information in that.
03004, female, 39 years, intervention
I think some of the stuff was repetitive. Because I’d never come across these sort of strategies they were using for improving memory, I found it a bit confusing, Some sort of overlapped with each other. I’m trying to think, what am I trying to do with it? It’s just like the last bit or something else.
05042, male, 51 years, intervention
There were certain points where it was – there was one page, I remember exactly what page it was, it really, it just pressed my button of ‘I don’t like this!’ It was page, I think it was 74, and it talked about relaxing . . . and I remember thinking I’m not going to relax. I’m not being told to relax. What made me totally not relax was being told to relax.
09008, male, 33 years, intervention
Group facilitator
Half of the participants spoke positively about the facilitators (APs) who led the memory rehabilitation groups. In particular, participants praised the facilitators’ knowledge, skill and positive personality traits:
[Facilitator] was very good and she listened too, we’d get into our own little debates and you know sort of, you sit there for a minute and she was very good at explaining things and she’d go over things at the beginning of the next session.
03005, female, 56 years, intervention
I thought [facilitator] was she was a nice person. I mean she was very, she was like, she obviously, you could tell she obviously cared by what she was doing it wasn’t just pay check. So that was nice.
04002, male, 24 years, intervention
However, one participant felt that the group facilitator treated him differently from the other group members. This was particularly in relation to how the facilitator was perceived to spend more time with, and give more attention to, some individuals than others:
He [other group member] could see there was a – I don’t want to say ‘favouritism’, but it was like – it was like he would get . . . 60% of attention, if you like. And the other one would get 30% and I’d get 10% [laughing]. It wasn’t equal.
09006, male, 53 years, intervention
Effects of the group
All participants reported positive effects of attending the memory rehabilitation group sessions. Besides the general positive comments [‘I found it all helpful’ (05053, male, 32 years, intervention)], participants commented on learning new strategies, the reinforcement of existing strategies, increased external strategy use, improvement in memory, increased understanding of how memory works, improvement in mood, increased confidence, reduced frustration/increased acceptance and contact with group members outside the group:
But now you guys have given me more techniques like how to use them, my phone, my laptop, my iPad, so erm and erm I’m erm my card in my wallet, just check and they’re all there and I texted me my card in my wallet.
04002, male, 24 years, intervention
I’m going to say reiterated, er what, what we’d found that we were doing the right things.
04005, male, 56 years, intervention
Memory wise, well I write a hell of a lot more down now so, I don’t forget half of much on that sense of things.
01015, male, 33 years, intervention
My wife says it [memory] has [improved], yes it has [pause and sighs] . . . I think it’s improved.
04005, male, 56 years, intervention
I suppose I’m more understanding, I’ve got more understanding of how brain injuries affect you, especially your memory, day-to-day things.
05042, male, 51 years, intervention
It’s given me confidence for a start to be able to travel outside of me little bubble, before it came to that point I never could quite get on a bus.
01015, male, 33 years, intervention
I mean it’s got better since I’ve been going to the group especially, has got better I’m not getting, I’m not getting as frustrated when people move things.
01010, female, 24 years, intervention
One of the girls there [name], I mean, we met a couple of times, yeah for coffee and it was nice, really nice.
03001, female, 54 years, intervention
However, two participants reported that the groups had a negative impact on their mood. One participant found it very upsetting to hear the other group members talk about their struggles with their TBI (see quote from participant 03005 under the format of the group subtheme), the other occasionally worried about her use of strategies:
It’s just err a reminder to be me to try and be, as you know, as more on the ball or more, more, but that gives me, that aches, how, how can I put it, that gives me a headaches when I worry or con-concern or not so concern, when I am thinking about that too much.
03001, female, 54 years, intervention
Strategy use
Participants from the memory rehabilitation group spoke about the strategies that they were taught in the group sessions and which ones they found helpful or unhelpful. This is covered in some of the quotations related to the effects of the group in the previous theme. This theme therefore relates to the participants’ use of strategies to improve their memory that were learnt outside the group. Participants described strategies that they had learnt and were using prior to their TBI, strategies that they generated themselves, strategies that were offered by other sources and the significance of their strategy use (Figure 12).
FIGURE 12.
Thematic map of the ‘strategy use’ theme.
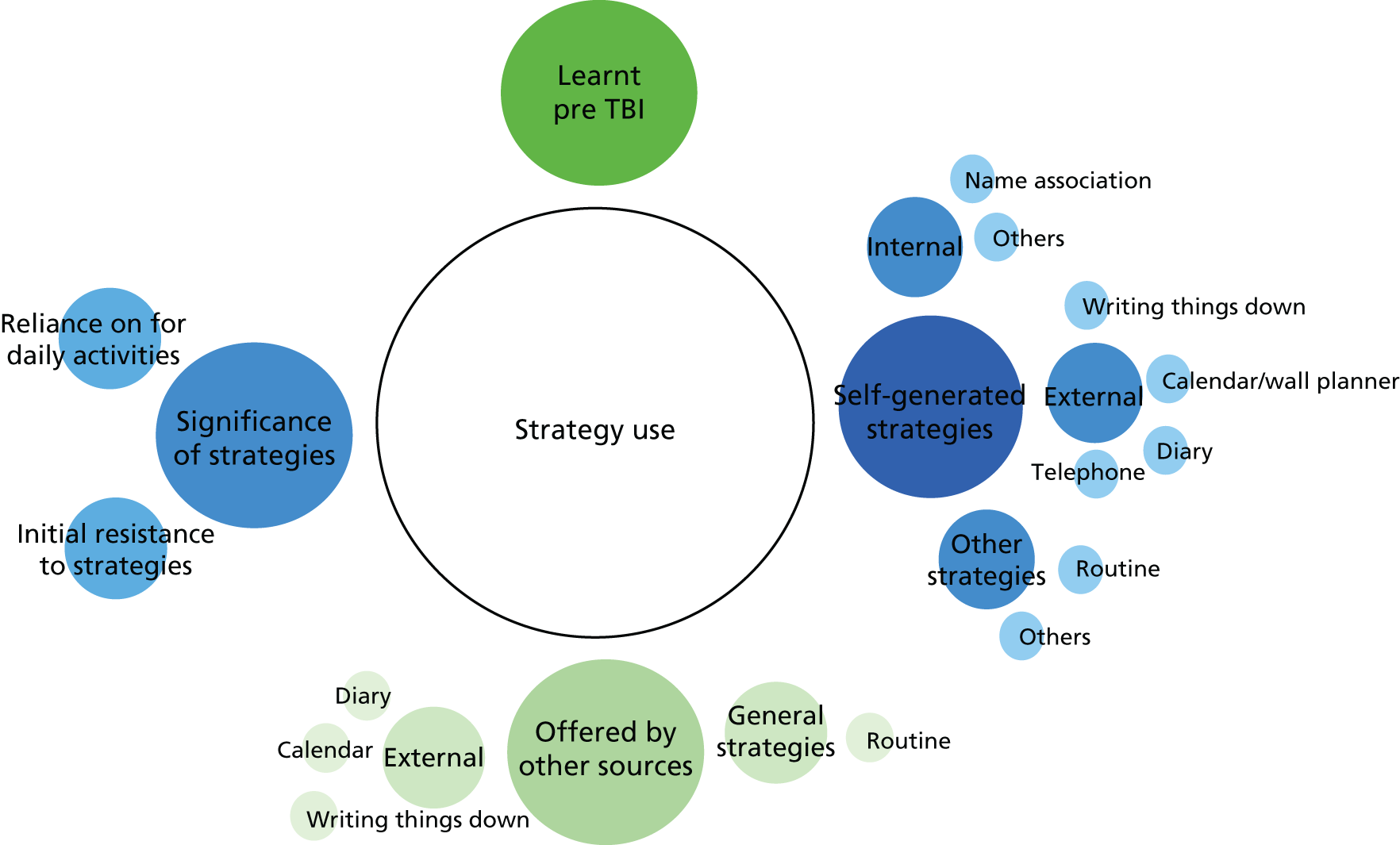
Learnt pre traumatic brain injury
A minority of participants reported developing memory rehabilitation strategies before their TBI as part of their employment:
Several years before the accident I’d done some, I’d learnt some memory techniques for use in work when, when I was a consultant, erm and for use around presentations so I’ve learnt like pegging and erm you know sort of weaving in a story.
03015, male, 36 years, usual care
Well a lot of the strategies I had . . . er a lot of the strategies [at the group] was stuff that er we used in the police . . . for er evidence gathering, erm investigations, etc., erm so a lot of the strategies I already knew but not with this context, if that makes sense.
04005, male, 56 years intervention
Self-generated strategies
Linked to the preceding subtheme, almost half of the participants had generated their own memory rehabilitation strategies. For some participants these were internal strategies, including name association and attempting to remember specific aspects of an event:
I mean I try to do association, so name association, erm, I’m, I’m terrible, I mean [name] I’ve got, but only because you’re, you’re here on your own, erm if there was two or three of you I probably wouldn’t get who was who.
04009, male, 46 years, usual care
I try and make something memorable happen . . . even if it’s talking to a new person and talking to them and erm, in that situation I think oh yeah I remember him, he had the bright yellow jacket on, I spoke to him, that was a day, it was a Thursday.
01017, female, 30 years, usual care
The majority of self-generated strategies were external memory aids. Writing things down was the most frequently used self-generated strategy, followed by inputting notes into a mobile phone. The use of a calendar, wall planner or a diary was the least frequently used strategy:
You find your own way of doing things and getting around and doing stuff, which for me is writing a lot of things down.
03004, female, 39 years, intervention
So I kind of always have my phone with me, so I’ve got lots of notes and things in there, written down.
07002, female, 29 years, intervention
Well, the main thing really is mainly with this, a calendar . . . Everything’s on there.
05042, male, 51 years, intervention
Yeah over the years I’ve found having a, a diary to hand, always, I can always keep track of my main events during the day.
03014, male, 37 years, usual care
Almost one-quarter of participants reported the use of other self-generated strategies. This was predominantly relying on routines, although one participant used word substitution and another used relaxation techniques:
I try and think, ok I’ll do things at certain times and put things, I try and put things in places which I usually forget to do but I try and look in certain places first and see if I’ve put it there.
04008, female, 28 years, usual care
If it happens in a sentence or something like that I can kind of er slide my way around it, the word that I can’t remember, so that people know what I’m talking about.
01003, male, 58 years, usual care
Almost one-quarter of participants specifically stated that they had had to generate their own strategies because of a lack of memory rehabilitation or support from other sources. For one participant this was cause for considerable distress:
I just give up and work it out myself. ’Cause I guess, in a way, I’m lucky in that I do – I’m, you know, clever enough or whatever to have that own resources to try and do that. So, yeah – so I know that I looked up things online and I got things from memory charities and stuff like booklets myself, to try to help myself . . . it would be better for them [doctors] to be giving me information, rather than not even telling me to go away, look it up and for me to have done something like this [intervention] group or – you know, just anything rather than just the feeling of, like – you know, as if it’s then your fault because it’s not been acknowledged that it’s actually an issue. [Crying]
07002, female, 29 years, intervention
I’ve done a lot of things just myself . . . I had to do a lot of recovery on my own.
01017, female, 30 years, usual care
This lack of memory rehabilitation or support is described further under the fourth theme, ‘usual care’.
Offered by other sources
Just under half of the participants had received advice or support on keeping a routine from other sources:
I have a routine, that’s another thing that I learnt in either the TBI team there and that or maybe is it [name of occupational therapist].
01010, female, 24 years, intervention
We [ABI team] did do some strategy stuff, sort of around making, er you know sort of substituting routine for memory if you like . . . making sure you always put things in the, in the same place and, and that sort of thing.
03015, male, 36 years, usual care
In terms of external memory strategies offered by other sources, writing things down was the most frequently used strategy, followed by the use of a calendar or diary:
That [inpatient rehabilitation] was one of the places that we, you know started to . . . say that it would be a good idea if you are not sure . . . where you want to go, what you want to wear or what you want to do, you know, write it down.
03001, female, 54 years, intervention
I put them all up there, like phone numbers and stuff like that on the calendar. I’ve got one next to me when I sit down if I’m in there. I write down on me calendar, like, ‘got to see me home Friday at 1 o’clock’, or whatever [suggested by the Brain Injury Trust].
05067, male, 53 years, usual care
She [from the TBI team] told me that it was the right thing to do, get a diary and that it would help.
01005, male, 43 years, usual care
Significance of strategies
A few participants felt an initial resistance to adopting memory strategies after their TBI. However, these participants did persevere with the strategies and were then able to experience the benefits:
I do remember my opinion at the time was I’ve never had a diary, don’t need a diary . . . I gave in and got one and now it’s got to the point, that, yeah again she was right, I was wrong cause I live by it.
01005, male, 43 years, usual care
I find them quite tedious and patronising and really you know, saying ‘Ohhh’, you know like sort of getting a pad out or whatever and writing things down and you know . . . But it does help.
03022, male, 25 years, usual care
Almost one-third of participants relied heavily on their strategies (particularly external memory strategies) in their daily activities:
I had to have an alarm to pick my children up from school, because otherwise I would forget.
07002, female, 29 years, intervention
I use, erm like my memory as my diary and my phone, this is like my lifeline, without them I’m you know, I’m just not, I, I’m useless.
01010, female, 24 years, intervention
Usual care
The fourth theme related to the participants’ experience of rehabilitation external to the trial. Participants described a lack of memory rehabilitation, the types of memory rehabilitation that they did access and rehabilitation offered for non-memory issues (Figure 13).
FIGURE 13.
Thematic map of the ‘usual-care’ theme.
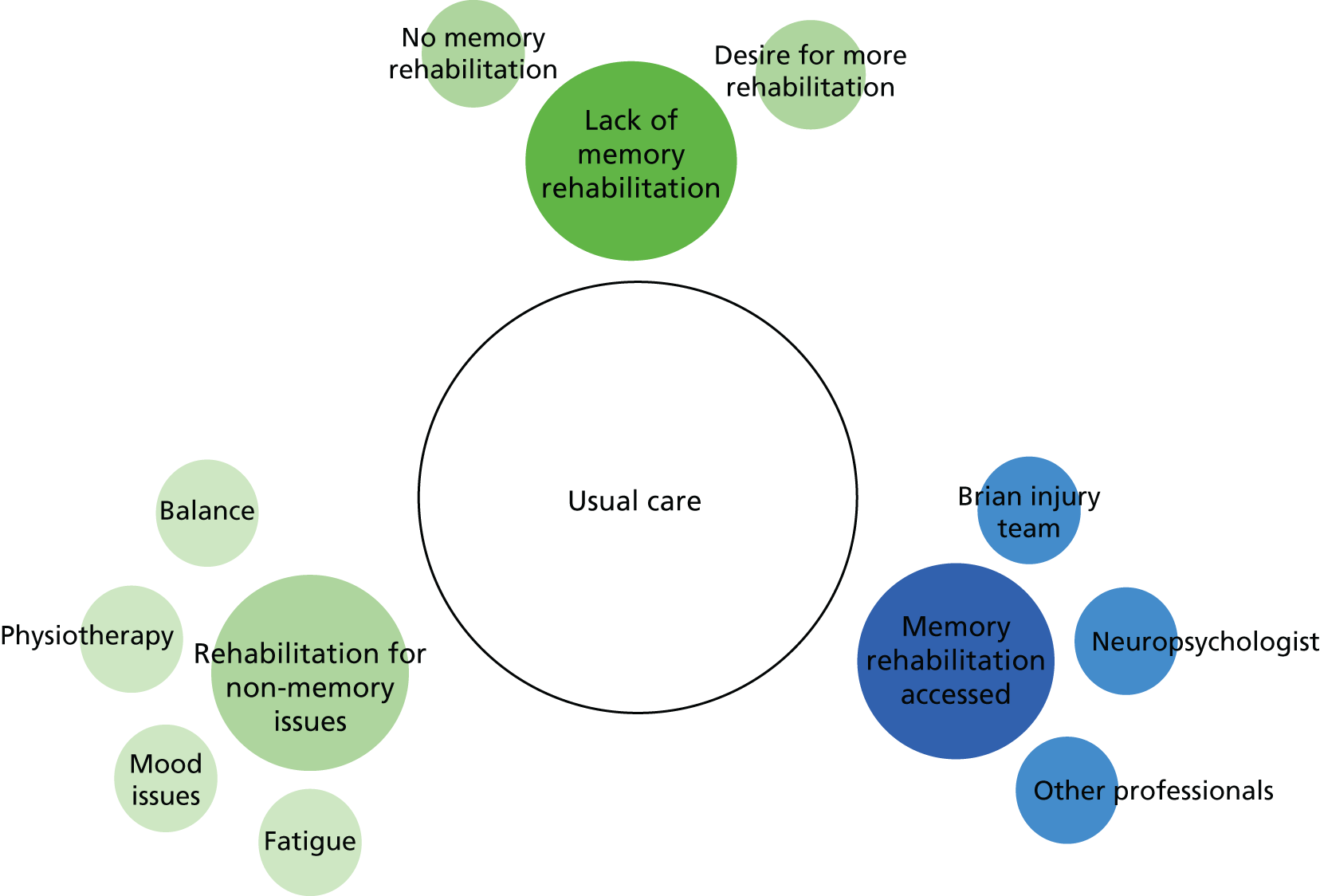
Lack of memory rehabilitation
The level of memory rehabilitation received by the participants following their TBI varied greatly, with the majority reporting that they had received no memory rehabilitation at all external to the trial:
I do think that there is a massive gap in between there’s a massive gap I think for me I mean this is my personal experience after the accident hospital you’re discharged goodbye, good luck.
07012, male, 37 years, usual care
He [group member] got the care package, if you like, from day 1 . . . mine has been a DIY job . . . learning how to cope and – with not understanding other people – you know, people think you’re taking the mickey by forgetting to do that task or doing – you know, etcetera.
09006, male, 53 years, intervention
One participant felt that she did not receive memory rehabilitation because of performing adequately on memory tests shortly after her TBI, whereas, for her, her score represented a deterioration compared with before her injury:
So what I then found really difficult was they’re like, ‘Oh, well, you’re middle of the range on these tests. You’re fine’. And I’m like, ‘Yes, but I wouldn’t have been middle of the range so, actually, I’m not fine’ . . . And I think that I wasn’t able to articulate that at the time, because I wasn’t able to explain my thoughts properly then. So I think I just kind of got dismissed . . . I felt a huge amount of frustration and powerlessness, because I didn’t get any help, because I wasn’t able to explain that I needed it.
07002, female, 29 years, intervention
Perhaps not surprisingly, many participants stated that they wanted more support and rehabilitation to help with their memory:
It would be nice if there was access to errm memory techniques or a programme.
04010, male, 55 years, usual care
I would like more support, or – and certainly ideas and what I could do, not necessarily, well and probably also techniques of helping myself, but also stuff I could do to improve it [memory] as opposed to just working around, because I don’t want to just take notes all the time.
08002, male, 31 years, usual care
Memory rehabilitation received outside the trial
Almost half of the participants discussed the types of memory rehabilitation that they had accessed through sources other than the trial. The most common sources accessed were brain injury teams and neuropsychologists, with other sources including inpatient rehabilitation teams, charities, work, private companies and social workers:
The way I see it is what I’ve had with rehab and the TBI team and everything else they’ve, they’ve just been amazing.
01010, female, 24 years, intervention
Obviously I’ve gone through a few things with [neuropsychologist], and you know . . . sort of how, you know how I can help with writing things down and certain strategies.
03022, male, 25 years, usual care
Well when I was in hospital, when I was like an inpatient I think I did have erm I did have some memory but wasn’t very common.
04002, male, 24 years, intervention
That’s the Brain Injury Trust did that to me [suggested strategies], yeah.
05067, male, 53 years, usual care
I’ve had like coaching, like kind of business kind of coaching and various different things to try and put strategies in place so I, I’ll try anything.
03015, male, 36 years, usual care
I saw the rehab assistant from . . . July 2015 to December then got discharged officially in January this year, so the length of time that [private rehabilitation company] had been around for is a kind of, sort of, story of what . . . like cognitive rehab and all daily independent living.
09008, male, 33 years, intervention
Yeah, yeah, yeah, the social worker from the brain injury unit, I think she’s from [place], she was brilliant, she was good, she was very, very good . . . and she, you know, obviously because she works with the brain injury department, they knew a lot of the pitfalls of having this accident and a lot of the problems that you will have in the future, so she sort of pre-empted and tried to prevent a lot of them as well, which did work.
06045, male, 56 years, intervention
Other (non-memory) rehabilitation received
As could be expected, participants experienced a range of other health issues comorbid to their TBI for which they also attended rehabilitation. The most common ‘other’ rehabilitation was for mood issues and a few spoke about fatigue management groups and courses, physiotherapy and balance rehabilitation:
Well now they [Brain Injury Trust] come round and just check me mood swings because I used to get in bad moods and that like, but I’m not scared of nothing . . . they come round and check me pills and they put me on these anti-depressant pills even though I don’t feel depressed. But then they put me dose up and it has calmed me down a bit.
05067, male, 53 years, usual care
I’ve been to [place name, fatigue management group] and they showed me what happens because that’s why I sleep all the time.
05067, male, 53 years, usual care
But I got seen by the physios a bit longer than, than I should have done. I got seen by the intermediate community team longer than I should have been and they tried to have more of a smooth transition, that’s why they looked after me a bit longer, they wanted to just hand over as they see it, me being ready for life.
08002, male, 31 years, usual care
After my head injury yes, because I couldn’t balance, I lost my sense of balance . . . So through [neurological rehabilitation unit] . . . erm I was under the care of a couple of people there erm there was actually one who I can speak really highly of, [staff member], she put in place coping mechanisms.
04010, male, 55 years, usual care
Discussion
One of the challenges of an interview study requiring people with memory problems to recall specific events that happened over the 6 months previously is that some people tend to forget some details. Indeed, this was the case with five participants, who could not remember the study. Four participants who had received the intervention also had limited memory of the groups, but did recall that they were taught to use some ‘strategies’ to cope with their memory problems and that some of these strategies were useful. Five participants could not recall whether or not they had received any memory rehabilitation outside the trial. However, the majority of the interviewees provided detailed and rich accounts of their experiences of being involved in the trial and also their experiences before the trial. Therefore, the results of this study should be interpreted in light of the difficulties that some participants faced.
Some participants were motivated to participate in the trial by altruism, feeling that their participation, even if it did not help them, would help others in the future. The other major reason for participation was because participants needed help with their memory problems. Participants were clear that there was a lack of provision of memory rehabilitation as part of their usual care. Some participants received rehabilitation for other non-memory issues (for problems with mood, fatigue and balance), with only a few receiving help for their memory problems from their TBI team, neuropsychologists and other allied health professionals. This support was often felt to be insufficient.
Although most participants reported that they preferred the group intervention to one-to-one interventions, there were some who were ambivalent and some who would have preferred one-to-one sessions. The reasons for this are that participants felt that there would have been greater focus on them as individuals and their problems in individual sessions and that intervention material that was not seen as ‘relevant’ to them would be left out. Although the majority felt that the groups were beneficial, there are aspects related to the composition of the groups, timing of the groups, the materials used in the groups and the groups’ facilitators that need to be carefully considered when delivering group interventions.
Diversity in a group can be a strength but also a problem for some. Having people with different levels of severity of TBI was frustrating for those with milder levels of memory problems; one participant even felt like a ‘fraud’ because their memory problems were not as severe as those of some of the other group members. Participants also felt that their preference for a group format was based on their own personality (e.g. being ‘vivacious’). Most, however, felt that being with others with a similar condition made them feel ‘not alone’ and that learning from peers was a beneficial aspect of the intervention. Although some participants felt good about seeing others who appeared to be coping well after their TBI, others felt bad about seeing people, even years after their TBI, not coping.
Another aspect of the delivery of the group related to the time since participants’ TBI. Participants described an ‘optimal’ time (not too early but not too late) after TBI when they thought that they would have benefited the most from memory rehabilitation. Although some participants felt that they already had strategies in place, because they had been living with their TBI for some time before the trial, we had hoped that the memory rehabilitation group sessions would enable them to fine tune the use of these strategies. However, because of the length of time since their TBI, some participants felt that incorporating new strategies with their old ones was a challenge. When participants had strategies in place, these were often self-generated, having been learnt and used successfully before the TBI. However, some participants were taught these strategies (e.g. keeping a routine, using external memory aids) by rehabilitation professionals after their TBI. Most attested to the positive benefits of using such strategies.
All participants reported benefits from having attended the intervention groups. The aspects that they felt had improved as a result of the intervention have also been reported in previous studies. 24,106,113 Improvements related to learning new strategies, reinforcing the use of existing strategies and increased use of external strategies, all related to improved memory. Furthermore, participants also felt that they understood how memory worked. Concomitant improvements in confidence and acceptance and reduced frustrations (with themselves) were also reported. Although most participants reported improved mood as a result of attending the group sessions, a few felt that being in a group with severely memory-impaired people affected them adversely.
For some participants the group served an important social function. Participants enjoyed meeting others ‘like them’, who ‘understood them’ (because of their shared condition). In fact, some participants wanted to extend the relatively formal group meeting to more informal social outings. Some participants did not want the groups to end. The social aspect of being part of a group of people with TBI helped participants to not feel isolated. Indeed, people with TBI often report that their social networks have markedly declined following their injury114 and the groups may serve to establish new networks.
Most participants found the manual useful. They thought that the content was useful and it was easy to understand. There were a few, however, who thought that the language was too academic and that too much information was covered in too short a time. However, others thought that there was too much repetition. We had structured the manual to provide a summary at the end of each session and, in addition, at the beginning of the next session there was a reminder of what had been covered in the previous session. Some participants found this helpful, but this is perhaps what others felt was repetition. This again may point towards the challenges of running a group with participants of very different cognitive abilities.
The group facilitators played a key role in the success of the groups. Most participants found that the facilitators were knowledgeable and competent to manage the groups. Others spoke about the importance of the facilitators’ listening skills and making participants feel understood. A few participants did have interpersonal issues with their facilitator. This may have been because of the facilitators’ inability to deal effectively with a problem within the group or may have arisen because of the participants’ interpersonal communication style,115 difficulties with personal relationships116 and neurobehavioural problems (such as impulsive behaviour and reduced frustration tolerance), which are documented psychosocial sequelae of TBI. 117 Further training and support for facilitators may help in this regard.
Chapter 6 Discussion
Context
This pragmatic trial was designed and conducted in response to a commissioned call for ‘proposals concerning people needing physical or psychological rehabilitation following trauma in a military or civilian context’ from the HTA programme. 39 This call recognised the need for further research to develop services that support those who live with the disabilities that are sequelae of trauma, which includes TBI. Cognitive problems, especially memory problems, are common among those with TBIs and negatively affect people’s personal, social and professional lives. Indeed, cognitive impairments are the leading cause of TBI-related disability and affect approximately 43% of those with moderate to severe injuries. 118 The intervention in the ReMemBrIn trial included memory retraining and both internal and external strategies (thereby making it a comprehensive memory rehabilitation intervention) and the trial addressed most of the limitations identified in previous memory rehabilitation trials in that it was an adequately powered pragmatic observer-blinded Phase III RCT that assessed both clinical effectiveness and cost-effectiveness and which included outcomes at an activity and participation level.
Summary of findings
Clinical effectiveness
Our results indicate that there was no benefit of this memory rehabilitation programme for this group of people with TBI, with no clinically important difference on the EMQ-p (the primary outcome) between the two arms at the 6-month follow-up. Although the difference in mean EMQ-p scores between the arms for those with borderline/moderate memory impairment favoured the memory rehabilitation arm, there was no statistical evidence of any overall subgroup effect. There were also no important differences between the arms in terms of memory ability, mood, quality of life, and cognitive, emotional and social well-being at the 6- or 12-month follow-up. Goal attainment scores, however, favoured the memory rehabilitation arm at both 6 and 12 months’ follow-up. No safety concerns were raised and no deaths were reported.
Cost-effectiveness
There was ambiguity in our cost-effectiveness findings, which was driven by methodological uncertainty (based on the impact of different imputation methods) and parameter uncertainty (based on different time points, outcomes and costs). Although some of the findings suggest that memory rehabilitation could be seen as cost-effective, with lower costs and lower effects observed for the cost per QALY gained at 12 months, this was based on small, imprecise differences in costs and outcomes. When we take into account all of the uncertainty in our results, memory rehabilitation is unlikely to be considered cost-effective. The results of the cost-effectiveness analyses based on the two clinical outcomes (EMQ-p and GHQ-30) showed consistent results, with the intervention seen as dominant for the base-case multiply imputed data (i.e. lower cost, greater effect). Again, these results are based on small, non-significant differences in costs and effects and uncertainty in our findings was seen when these results were further examined. It should be noted that any technical finding of cost-effectiveness does not overwrite that there was no clinical benefit of memory rehabilitation for this group of participants.
Qualitative analysis
Despite having memory problems, most people could recall their experience of being involved in the trial, and those who had received the intervention could recall what they felt about the group sessions. Most participants’ experiences of being involved in the trial were positive. Most participants preferred a group format to one-to-one memory rehabilitation, but this finding may have been the result of a selection bias (i.e. people agreed to take part knowing that they may receive a group intervention). Many participants reported the benefits of being in a group in terms of their improved use of strategies and improved levels of confidence, acceptance and mood. For many, the group also served a social function. The composition of the group (in terms of the severity of people’s disabilities) affected participants’ experiences of the sessions. There appeared to be an ‘optimal’ time after injury when people felt that they would have benefited most from the sessions, which was not too early but not too late either. Participants found the intervention manual helpful. The success of the group also depended on the knowledge and skills of the group facilitator.
Interpretation
There are several reasons why this intervention may not have been effective overall. The following factors may have had an impact on the overall outcome of the trial.
Time since traumatic brain injury
One consideration is that a high proportion of participants were recruited relatively late after TBI, with a median time since injury of just over 4 years. Hoofien et al. 119 found that, even a decade post injury, people with TBI had cognitive problems and needed professional assistance to maintain a reasonable quality of life. Therefore, we expected rehabilitation at this late stage to be useful because people’s lifestyle and expectations change after discharge from rehabilitation and new demands may require the use of different strategies. In addition, Tsaousides and Gordon120 suggested that cognitive rehabilitation is effective at any time post injury.
It may be that people had already learnt many of the strategies that were taught in the group sessions or had established ways of coping that suited them and they were unlikely to adopt new methods of coping. Our qualitative study provided some support for this assumption (see Timing of the intervention in Chapter 5). Indeed, other studies121,122 have found that there are various factors related to the use of strategies (particularly external memory aids). From their survey of people with ABIs, Evans et al. 121 observed that many people were using memory aids, with many more aids being used after the ABI than premorbidly. They also found no relationship between rehabilitation and the level of memory aid use. Interestingly, they also found that the longer the time since the injury, the fewer memory aids were used. This again calls into question the impact of time since injury on strategy use and benefit finding from the rehabilitation programme.
However, our results suggest that additional rehabilitation at this late stage conferred no benefits over usual care alone. Post hoc subgroup analysis in relation to time since injury (categorised into ≤ 2 years, 2–10 years and > 10 years) found no differences in outcomes. Therefore, this is an aspect that needs to be investigated further in future studies.
Severity of memory impairment
As this was a pragmatic trial, we recruited people with a wide range of severity of memory problems, which reflects the range of patients seen in clinical practice. Some reported memory problems in daily life but were within the average range or above average on the RBMT-3. Although this may be an impairment relative to their premorbid level, it could also reflect mood problems. It is well recognised that there is a strong relationship between reported memory problems in daily life and mood problems in people with neurological conditions. 123–126 Therefore, some of those recruited may have reported memory problems associated with low mood and, therefore, would be unlikely to benefit from memory rehabilitation. However, despite some previous studies demonstrating better mood in those who had cognitive rehabilitation than in those who did not,127,128 and previous meta-syntheses of qualitative studies demonstrating this,106,113 our quantitative data suggested no significant differences between the memory rehabilitation arm and the usual-care arm at the 6- or 12-month follow-up in relation to mood. Interestingly, our qualitative study (see Effects of the group in Chapter 5) found mixed results, with some people finding benefits in relation to mood as a consequence of attending the group sessions, but others finding the groups distressing. Furthermore, there were some participants with very severe problems who may have been too impaired to cope with the demands of the intervention. This is supported by the finding that, although we found no benefit overall, those with a moderate level of impairment on the RBMT-3 showed the greatest benefit from rehabilitation. This is also consistent with recommendations38 that suggest teaching memory rehabilitation strategies based on the level of severity of memory impairment.
Format of memory rehabilitation delivery
The intervention was provided on a group basis because we felt that this was more likely to be cost-effective and resource efficient than individual sessions and, based on previous research,24,106 we also felt that participants may benefit from interacting with others in a similar situation. The latter point was supported by the qualitative feedback in which the group aspect was seen to be useful by most participants (see Preference for rehabilitation format and Format in Chapter 5). Furthermore, Cicerone et al. ,25 on the basis of their systematic review, recommended that ‘Group-based interventions may be considered for remediation of memory deficits after TBI’ as a ‘Practice Option’ (defined as evidence based on class II or III studies). However, groups do not suit all (again, evident from our qualitative findings) and selecting only those who prefer group to individual treatment may have enhanced any benefits. Perhaps providing a few individual sessions before participants are allocated to a group or alongside group sessions may enable therapists to better prepare patients for group sessions (e.g. helping them to deal with issues such as being with others with severe disabilities) and improve attendance at groups. 129
The treatment was structured to be delivered as weekly sessions for 10 weeks. Perhaps greater spacing between sessions would have allowed participants to practise their between-session tasks and to become more familiar with one strategy before learning about another. In addition, the provision of top-up sessions over a longer period of time may have helped participants retain information that had been learnt. Indeed, our qualitative study documented the perceived need for longer sessions and top-up sessions (see Format in Chapter 5). However, additional sessions may incur additional costs.
Attendance at and dose of memory rehabilitation
Given that our sample had memory problems, attendance at the memory rehabilitation groups was good, with 77% of participants attending four or more sessions. We assumed, based on our previous studies,23,24 that, given the modular structure of the programme, people would find some benefit from attending at least four sessions. This is also consistent with our clinical practice. Furthermore, in this study, the reasons given for non-attendance were also mainly the reasons that are encountered in clinical practice, such as clashing appointments, rather than because participants withdrew or did not want to continue with the group sessions. In the Rohling et al. 13 meta-analysis, the mean treatment duration was 13.3 weeks (SD 14.2 weeks), which is similar to the 10 weekly sessions in our trial.
However, one challenge that clinicians and researchers face is determining the optimum ‘dose’ of the intervention. Very few rehabilitation studies have investigated in detail the optimum dose and format of memory rehabilitation. Even the large systematic reviews13,25 have not been able to consider the dose–response effect in memory rehabilitation. One reason for this is that primary studies do not always provide sufficient details of interventions. 130 We expect that reporting guidelines such as the Template for Intervention Description and Replication (TIDieR) checklist and guide131 and other more specific guidelines for reporting group memory rehabilitation programmes130 will improve the reporting of these interventions. Therefore, it seems that more Phase II studies are needed to optimise the recruitment of suitable participants and to provide the optimum format of the intervention before progressing to a Phase III trial. Indeed, Cicerone et al. 25 stated that ‘future research should move beyond the simple question of whether cognitive rehabilitation is effective, and examine the therapy factors and patient characteristics that optimise the clinical outcomes of cognitive rehabilitation’. Thus, attendance rates may have affected outcomes.
Choice of outcomes
We included a variety of outcomes relating to memory (subjective and objective, patient and relative reports), mood, cognitive, social and emotional well-being and goal attainment, as well as health economic outcomes.
There is debate among researchers and clinicians working in neuropsychological rehabilitation about the most appropriate outcome measures. 132 When the trial was designed, we felt that a subjective report should be the primary end point because this assesses the effect of memory problems on everyday life and provides a patient-centred outcome rather than the views of health-care professionals. This is also suitable for independent completion and return by post, which helped to ensure blind assessment of the outcome. Other studies have considered goal attainment as a primary outcome. 65,133 Although we consider this a valid and useful measure, we felt that it would be more suitable as a secondary outcome measure because the goals set are strongly influenced by the expectation of the treatment and the goals are likely to change over the course of the year when follow-up assessments are conducted.
Although GAS, in which goals are set and assessed in collaboration between patients and clinicians, is considered very much a part of rehabilitation in clinical practice, its application as an outcome measure in rehabilitation studies poses certain challenges. 134 Indeed, Bovend’Eerdt et al. 135 documented the challenges of using GAS as a blinded outcome measure by an independent assessor (who has not been part of a patient’s clinical team). In a previous crossover trial136 that used goal attainment as a primary outcome, the randomisation protocol had to be modified to accommodate patient-relevant goals that would fit with the timing of the intervention. For instance, participants originally allocated to the intervention phase were moved to the control phase during school holidays if the goal was to remind them to pick up their children from school. This therefore potentially biased the study.
When we compare the findings from the quantitative and qualitative parts of this study, we find a discrepancy, with the qualitative study finding benefits of memory rehabilitation. Rohling et al. 13 noted that ‘it is not uncommon for patients and providers to report improvements in the real-world task of compensating for memory impairment, while the psychometric measures show little or no change’. Goal attainment may be one way to accurately capture individual patient targets in a systematic and quantitative way, without having to resort to general preformatted questions that may not be applicable to all. The use of GAS may also help bridge the gap between what we find in qualitative studies and what we find in quantitative studies of memory rehabilitation, because it has the ability to identify what matters most to patients. For instance, in our trial the qualitative findings suggested that participants found improvements in levels of confidence and acceptance, aspects of improvement that may not have been identified in standardised questionnaires. Indeed, in our trial, participants’ individually set goals were better met in the memory rehabilitation arm than in the usual-care arm at both 6 and 12 months’ follow-up. Therefore, if some of the concerns raised about the use of GAS can be allayed, GAS may be a useful outcome to consider in future trials.
Strengths and limitations
The findings from this trial should be viewed in light of its strengths and limitations. The key limitations are discussed in the following sections.
Limitations
Heterogeneity
As a pragmatic trial, our inclusion criteria were necessarily broad, which replicates how memory rehabilitation is delivered in clinical practice and contributes to the generalisability of the findings. However, this also meant that the sample was heterogeneous, but our trial was not designed to detect differences in the effect of the group memory rehabilitation (i.e. subgroup effects) according to baseline factors. Therefore, we intend to further analyse the data in this respect; the findings will provide us with some indications of which groups of people may benefit most from memory rehabilitation, and this would need to be tested out in future trials.
Generalisability
It was necessary to screen a large number of potential participants to recruit the required sample size, and a large proportion of those screened were not enrolled. This is largely because of the methods of recruitment used, which relied on postal invitations, which are known to suffer from poor response rates;137 indeed, non-response to the postal invitations accounted for 43% of people screened but not consented in the current trial. By comparison, only 28% of people were excluded on the grounds of eligibility, lending support to the representativeness of the current sample.
As a pragmatic trial, to reduce selection bias, we used multiple recruitment sources and strategies, including self-referrals. Unfortunately, this meant that we did not have demographic data on all those who were approached and invited or those who chose to self-refer as a result of the study publicity material. Furthermore, we were unable to collect this information at the recruitment stage because those interested had not given consent for us to collect these data for research purposes. Therefore, we cannot be certain whether or not there were differences between the characteristics of those who wanted to take part in the study and the characteristics of those who did not.
Research suggests that white, middle-class, highly educated men tend to be over-represented in health-care research in most Western countries. 138 Indeed, in our trial 96% of our sample identified as white. Surprisingly, the literature is sparse regarding racial and ethnic variations in the epidemiology of TBI in the UK. One older study,139 however, found substantial differences based on ethnic groups in relation to seasonal variations and differences in length of hospital stay following TBI. In relation to gender, typically men are over-represented by 3 : 1 in all subgroups of TBI. 140 Men formed more than two-thirds of our sample.
Comparability with other trials
Rather than reviewing individual studies, we have opted to compare our findings with those of published reviews. More men than women sustain brain injuries, with ratios estimated to be between 2 : 1 and 3 : 1. 141 This is consistent with our findings, with 73% of participants being male. In the Rohling et al. 13 systematic review of cognitive rehabilitation following ABI, the authors identified eight studies that considered rehabilitation of memory difficulties in TBI. In this review, the mean age of TBI participants was 29.1 years (SD 15.9 years), whereas our participants were older, with a mean age of 45.4 years (SD 12 years). The mean treatment duration was 13.3 weeks (SD 14.2 weeks), which is similar to our treatment duration of 10 weekly sessions.
The Cicerone et al. 25 review discusses the various treatment strategies covered in memory rehabilitation in ABI. Studies that they included in the review used internal memory aids (e.g. visual imagery techniques), memory retraining and external memory aids (e.g. diaries, pagers and other compensatory strategies). This suggests that the content of our intervention programme was comprehensive, elements of which have been investigated in other trials. Most of these trials suggested that participants benefited from using these strategies.
Fidelity of the intervention
It was a strength of the study that we were able to assess the fidelity of the intervention. The video analysis suggested that the therapists were delivering the intervention as planned and in accordance with the manual. However, the main limitation of the video analysis is that recordings were incomplete and did not include all sites. Some sessions were only partially recorded; recordings stopped partway through a session, probably because of the camera battery running low, or did not continue after the break, possibly because of the AP forgetting to turn the camera on again. Some recordings were lost and not transferred to the main site. This limited the recordings that could be included in the analysis and meant that not all sessions were observed at each site. Therefore, we cannot be certain that all sessions at all sites were delivered consistently in accordance with the manual. However, based on feedback obtained during our monthly peer and supervisor meetings with the APs, we do not expect that the unrecorded sessions were markedly different from the recorded ones.
Lack of an ‘active’ control arm
One of the challenges of conducting complex intervention trials, particularly in rehabilitation contexts, is having an ‘active’ control arm. In our pilot trial,23 we included an attention placebo self-help group as the control arm. In this group, participants met to discuss their health and memory problems and were taught relaxation exercises. The group facilitator did not initiate any memory talk. Our fidelity analysis of these sessions compared with the treatment sessions142 demonstrated that the self-help group underwent very little discussion around ‘memory’ and significantly less than in our treatment groups. However, our qualitative research24 suggested that even people in this self-help group were reporting some benefits of attending the sessions. Therefore, in the ReMemBrIn trial, we opted to have a treatment-as-usual control arm. However, based on the qualitative data (see Chapter 5, Usual care), it would appear that most people did not get much by way of usual care. This is a challenge that has been recognised in evaluating complex intervention and rehabilitation trials. 143
Strengths
Despite the limitations reported in the previous section, we believe that this study has several strengths and has avoided many of the methodological weaknesses identified in previous trials of memory rehabilitation in TBI.
Methodological quality
The ReMemBrIn trial was a RCT. We took care to ensure allocation concealment and blinding of outcome assessors. Our SAP was agreed in advance of the data-lock stage. We have reported the trial in line with the Consolidated Standards of Reporting Trials (CONSORT) guidelines144 and the content, format and delivery of the intervention in line with the TIDieR131 and other relevant guidelines. 130 This should enable other researchers who wish to replicate this study to deliver the intervention as we have.
Sample size
Most previous studies of memory rehabilitation have been small and underpowered. This is an issue within the wider field of cognitive rehabilitation studies. The systematic review of Rohling et al. 13 identified RCTs of TBI with sample sizes ranging from 4 to 22. The ReMemBrIn trial was powered to detect a minimum clinically significant difference in mean EMQ-p score of 12 between the memory rehabilitation arm and the usual-care arm, taking into account between-site variations and clustering as a result of the intervention being delivered in a group. The target sample size of 312 was exceeded, with a total of 328 randomised participants, making this, to our knowledge, the largest trial of its kind to date.
Standardised manualised treatment
We were able standardise the treatment by having a manual and facilitator notes for facilitators at each site and a workbook for all participants in the memory rehabilitation arm. Facilitator training happened centrally, and monthly peer supervision and one-to-one supervision with one supervisor for all APs who delivered the intervention enabled sharing of experiences, which we hoped would encourage APs to adhere to the manual and facilitator notes. In the training programme and through supervision, we also taught the APs how they could adhere to the manual but also adapt the contents of each session to the needs of individuals within the group and the group as a whole.
Assessment of treatment fidelity
Our videos of the rehabilitation sessions from the various sites were coded and rated by two independent raters, which enabled us to determine the consistency of delivery of the intervention. There was some variation between sites, but part of this may have been because of variations in the sessions available for analysis between sites. Site 5 recordings were mainly from early sessions whereas recordings from sites 3, 8 and 9 were mainly from later sessions. This may account for the high proportion of time spent discussing the venue and organisational issues in the recordings from site 5 rather than discussing strategies. This site also included more facilitation of discussion, probably because this was needed more in the early sessions than in the later sessions.
Time sampling, in which behaviours are observed and recorded for a specific period of time,145 has been used mainly for ward-based observations of behavioural problems but we have found it useful to adapt this method to assess the ‘content fidelity’ of group-based rehabilitation interventions delivered in a research context with participants with neurological conditions. 142,146
Content fidelity has been defined as ‘the extent to which each intervention component was effectively delivered to the participants in terms of required content’. 147 The recording of activities indicated that appropriate content was covered. ‘Process fidelity’ was evident from the therapist skills observed, such as facilitating discussion, summarising and paraphrasing. However, process fidelity also involves the interpersonal skills of the therapist147 and there was no attempt to rate the quality of the interactions.
The fidelity to the intervention manual and consistency between therapists could have been improved if we had analysed the first group conducted by all APs and provided them with detailed feedback. This could then have been carried out routinely to ensure that there was no ‘drift’ in terms of intervention delivery for the rest of the trial. This of course has resource implications; therefore, in this trial, we analysed the video data only at the end of the trial.
In the delivery of complex interventions, we cannot be certain what the key ingredients are that would contribute to a positive outcome. In this study, we decided on the coding frame based on the activities and skills described in the manual. However, there may be other important aspects of the group sessions, such as group cohesiveness and therapist experience, which were not monitored.
Follow-up completion rates
Although 84% of the sample completed the assessment visit at the 6-month follow-up, only 75% and 78% of the participant questionnaires from the memory rehabilitation and usual-care arms, respectively, could be included in the primary analysis. This is slightly below our 80% response rate target; however, sensitivity analyses using multiple imputation to impute missing outcome data gave very similar results to the analysis using the available data.
Activity- and participation-level outcomes
Outcomes related to activity and participation levels of the ICF framework. 31 Frontera et al. ,143 in their summary of the Rehabilitation Research at the National Institutes of Health: Moving the Field Forward conference, stated that ‘In rehabilitation research, outcomes are often complex, occur across the domains of the International Classification of Function, and include patient-reported as well as performance-based and instrumented outcomes’. Many of the previous studies used impairment-level measures to assess the outcome of memory rehabilitation. Although impairment-level measures are useful in understanding the potential mechanisms that underlie changes effected by rehabilitation, as outcomes, they may not generalise to meaningful functional outcomes for patients. 148 Many of these impairment-level measures are ‘objective’ measures (i.e. based on cognitive tests). However, studies have also shown discrepancies between perceived and objective cognitive functioning,149,150 with subjective ratings being influenced by mood and self-efficacy. We acknowledge that the EMQ-p would have been influenced by these factors, but felt that it was the appropriate primary outcome because we expected participants who received the intervention to report a reduction in forgetting and improvements in coping with memory problems, which is in keeping with the spirit of cognitive rehabilitation. 70 Furthermore, we also included an objective measure of memory and relatives’ assessment of participants’ memory failures as secondary outcomes. Goal attainment was also a secondary outcome.
Another strength related to outcomes is that we assessed participants at both 6 and 12 months’ follow-up; therefore, in this study we were able to evaluate the longevity of the treatment effect, if any. Most previous studies have assessed outcomes only proximal to the end of the intervention.
Economic analysis
Past studies have considered only the clinical effectiveness of memory rehabilitation. To our knowledge, this is the first trial to have determined the cost of delivering memory rehabilitation and the cost-effectiveness of memory rehabilitation.
Lessons learnt
Throughout the trial we experienced some delays. These largely occurred as a result of unforeseen circumstances beyond our control. At one site, the AP was not allowed access to the patient database after there was disagreement between the trust’s Caldicott Guardian and its research and development (R&D) department (after all approvals were granted) in relation to data access. This resulted in us having to raise this with the head of R&D at that trust and required us to recruit a new AP at the trust (instead of a secondment from the sponsoring trust). One site changed its organisational structure from being a NHS trust to being a social enterprise without a R&D department. We therefore had to subcontract a research consortium to act as that site’s R&D approvers. At one site, there was a delay in recruiting the AP because of an impasse between the comprehensive local research network and the NHS trust regarding finances. This took 5 months to resolve. In these instances, a considerable amount of time was spent in escalating the issue to the trust’s R&D directors and chief executives, with a view to resolving the issues.
In addition to these problems, we did have one recurrent problem: keeping APs in post. We chose to appoint APs to deliver the intervention because they had the qualifications and skills to deliver low-intensity manualised interventions under the supervision of a clinical psychologist. They often deliver such interventions in the NHS and are seen as a safe, cost-efficient way of providing psychological therapies for some circumscribed problems.
Assistant psychologists are graduates who work in NHS trusts, typically for 1 year, before they move on to work in another area (seeing a different patient group) or they are successful in obtaining a place on a doctorate in clinical psychology programme. Therefore, by appointing the best and most experienced APs, we could retain them only for a year, needing to readvertise and recruit to each post a number of times at each site. To mitigate delays in recruitment, we developed and shared to the principal investigators at all of the sites’ draft advertisements, job descriptions and person specifications for the job. We also kept a log of when APs’ contracts were coming to an end and worked closely with the APs to ensure that they would give us sufficient notice to allow us to recruit someone to their post, preferably before they left. Knowing the patterns of when APs were scheduled to leave the team to start on their doctoral course (if successful in obtaining a place) allowed us to factor this in to our recruitment projections, with a slowing down of participant recruitment around this time. This therefore provided us with a more realistic monthly recruitment target.
These delays caused an overall delay in participant recruitment. However, when we did have staff available in active sites, participant recruitment was not a problem (see Figure 14 and Appendix 5).
We attempted to recruit military personnel into the trial. We obtained the necessary research ethics approval from the Ministry of Defence, had a TSC member who had experience of working with military charities and recruited a PIC to identify potential participants. Despite various attempts to identify potential participants with this PIC and relevant military charities, we did not succeed in recruiting many participants from the military. Future studies may benefit from having a military co-applicant within the research team and may need to have confirmed support from military command.
Another delay occurred between assessing and randomising participants. Participants waited a median of 18 days between the second assessment and randomisation. This is inevitable in studies in which the intervention is delivered in a group format. We needed to gather a sufficient number of participants who were eligible to take part in the study and could attend at a particular time and place. We did learn, however, that targeting a specific geographical area (e.g. postcodes within a small area) by sending our recruitment mailouts in small batches as opposed to one large mailout to a large area enabled us to form clusters faster, as participants were able to travel to the venue where the groups were held. Interestingly, the delay did not deter people from wanting to participate. However, in conditions in which people’s cognitive function can fluctuate or deteriorate, such as in multiple sclerosis, this may mean that their baseline assessments may not reflect their cognitive ability at the start of the intervention.
We experienced problems in collecting postal outcome questionnaires from our participants, as their memory problems resulted in them forgetting to complete the questionnaires or send them back to us. This resulted in a lower than expected initial response rate for the outcome questionnaires. However, we were able to identify this problem quite early on and institute a recovery plan. This plan necessitated the outcome assessor checking with participants at the time of their 6-month outcome visit whether or not they had completed the questionnaire. If they had not, a new questionnaire pack was provided for them to complete and for the outcome assessor to return to the NCTU. This change to our procedure resulted in an increase in our response rate for the 6-month questionnaire, from 73% before we instituted this plan to 81.5% after.
We trust that the lessons that we have learnt serve to inform future researchers when they develop their research protocols.
Chapter 7 Conclusions
Implications for practice
This trial has not shown any benefit of this group memory rehabilitation for people with TBI late after their injury. However, people continue to report memory problems later after injury and the qualitative feedback from the participants who received the intervention was positive. Clinicians therefore need to identify what interventions may be useful at this late stage after TBI.
Recommendations for research
The results of this study highlight the need for more adequately powered studies of memory rehabilitation in patients with TBI. Despite extensive development work and early studies suggesting that the intervention was potentially effective, the findings suggest that our group-based memory rehabilitation conferred no benefit over usual care in the NHS.
-
There are a range of other memory rehabilitation strategies, such as computer games, mnemonics training and visual imagery, and these all need full-scale evaluation. Interventions can be delivered in individual or group formats and can be delivered face to face or on a computer. Future research may consider the usefulness of online and internet-based memory rehabilitation. The effect of the format of delivery on outcomes needs to be established. In addition, the question of what works best for whom requires further consideration of participant selection to ensure that people receive the treatment that is most appropriate to their needs. All of these issues require more RCTs of high methodological quality.
-
We used a usual-care control arm to determine whether the memory rehabilitation programme conferred any benefits over and above what was provided in the NHS. However, usual care is difficult to document and varies between sites. Future studies would benefit from developing appropriate attention control interventions to assess the specific effects of the intervention. Although we have previously used an attention control for memory rehabilitation,23 this proved difficult to deliver as a plausible control activity of equivalent duration and intensity. Utilising other support services, such a Headway groups, as a control intervention may enable better identification of the essential components of treatment.
-
Future research will need to consider more small-scale efficacy studies to establish appropriate selection criteria for group memory rehabilitation programmes, so that interventions can be tailored to those who can benefit. Research will also need to offer more information on usual care for people with memory problems following TBI so that group memory rehabilitation can be evaluated in those who have not already been taught the strategies covered in the group programme.
Acknowledgements
We thank all those who took part in the trial and clinical staff at the participating sites and PICs for their support.
We also thank the staff working on behalf of the trial sponsor, Nottingham University Hospitals NHS Trust, who have supported the trial: Maria Koufali, Brian Thompson, Kate Horton, Amy Farmer, Joanne Thornhill, Hannah Driver and Lisa Edwards.
Parts of this report have been reproduced from the ReMemBrIn trial protocol. 1 The protocol has been published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.
The ReMemBrIn Trial Collaborative Group
Co-applicants
Nicola Brain (Consultant Physician in Rehabilitation Medicine) contributed to the development of the grant application and trial protocol.
Anthony Pink (Service User Representative) contributed to the development of the grant application and trial protocol and sat on the TSC.
Trial and data management
Margo Childs (Senior Trial Manager from 2013 to 2015), Kirsty Sprange (Senior Trial Manager from 2015), Sandip Stapleton (Trial Manager until 2014), Dawn Coleby (Trial Manager from 2014 to 2015), Amy Evans (Trial Manager from 2015 to 2016), Florence Day (Trial Manager from 2016), Jo Hobbs (Trial Co-ordinator from 2015), Pat Morris (Trial Administrator until 2016), Natalie Wakefield (Trial Administrator from 2016), Lucinda Murphy (Senior Data Manager until 2014), Brian Barnes (Data Co-ordinator), Natasha Bryant (Data Administrator), Daniel Simpkins (IT Manager), Essam Eliwa (IT Programmer until 2014), Keith Whitaker (IT Programmer) and Alexandra Erven (Research Facilitator).
Statistical support
Graham Warren (until 2013), Alan Montgomery (from 2013) and Lucy Bradshaw (from 2013).
Health economics
Ceri Phillips, Deb Fitzsimmons, Shaun Harris and Ioan Humphreys.
Trial Steering Committee (independent members)
Jonathan Evans (chairperson) (Professor of Applied Neuropsychology), Derick Wade (Consultant in Neurological Rehabilitation), Charlotte Leask (Headway Representative), Declan McNicholl (Consultant Clinical Psychologist/Neuropsychologist), Janice Mackenzie (Consultant Clinical Neuropsychologist), Dawn Flynn (Carer Representative) and Morgan O'Connell (Consultant Psychiatrist until 2014).
Data Monitoring Committee (independent members)
Andrew Bateman (chairperson) (Clinical Manager, Director of Research), Diane Playford (Professor of Rehabilitation) and Amanda Farrin (Professor of Clinical Trials and Evaluation of Complex Interventions).
Sites
Cheshire and Wirral Partnership NHS Foundation Trust: Gavin Newby (Principal Investigator), Joanne Crossley (AP), Emma Marsh (AP) and Heather Condon (AP).
Nottingham University Hospitals NHS Trust: Patrick Vesey (Principal Investigator), Emma Sinclair (AP), Kristy-Jane Martin (AP) and Lucy Morris (AP).
Birmingham Community Healthcare NHS Trust: Theresa Powell (Principal Investigator) and Tanya Burton (AP).
Central Surrey Health: Annette Schwartz (Principal Investigator) and Lucy Ratcliffe (AP).
The Walton Centre NHS Foundation Trust: Catherine McMahon (Principal Investigator), Perry Moore (Co-principal Investigator), Katy Hughes (AP), Alexandra Whitelock (AP), Alexandra Cunliffe (AP) and John Wilson (RA).
Sheffield Health and Social Care NHS Foundation Trust: Alistair Atherton (Principal Investigator), Cormac Duffy (AP) and Serena Vanzan (AP).
St George's Healthcare NHS Trust: Laura Madeley (Principal Investigator), Jessica Budgett (AP).
North Bristol NHS Trust: Simon Gerhand (Principal Investigator) and Stephanie Pegnall (AP).
South Tees Hospitals NHS Foundation Trust: Stephen Evans (Principal Investigator), Natasha Anderson (AP) and Jenna Moffitt (Co-Investigator).
Participant identification centres
Derbyshire Community Health Services NHS Foundation Trust: Susan Reed.
Derby Teaching Hospitals NHS Foundation Trust: Nigel Schofield.
University Hospitals of Leicester NHS Trust: Graham Lowings.
County Durham and Darlington NHS Foundation Trust: Janet Walker.
Outcome assessors
Sara Clarke, Hannah Carpenter, Holly Chappell, Luke Squires and Rachel Harnell.
Qualitative interviewers
Kerry Burvil (née Dyer) and Hannah Carpenter.
Contributions of authors
Roshan das Nair (Professor of Clinical Psychology and Neuropsychology) was the Chief Investigator and co-authored the final report.
Lucy E Bradshaw (Medical Statistician, NCTU) analysed the clinical effectiveness data and prepared the results for publication.
Hannah Carpenter (Research Associate) jointly conducted the qualitative analysis and contributed to preparation of the qualitative results for publication.
Sara Clarke (Research Assistant) jointly conducted the qualitative and video analyses and contributed to preparation of the qualitative and video results for publication.
Florence Day (Clinical Trial Manager, NCTU) was the Trial Manager from July 2016 and contributed to preparation of the final report.
Avril Drummond (Professor of Healthcare Research, Director of Research and Occupational Therapist) contributed to the development of the grant application and trial protocol and contributed important intellectual content to the report.
Deborah Fitzsimmons (Professor of Health Outcome Research) oversaw the health economics analysis and contributed important intellectual content to the report.
Shaun Harris (Research Assistant, Swansea Centre for Health Economics) analysed the health economics data and contributed important intellectual content to the report.
Alan A Montgomery (Professor of Medical Statistics and Clinical Trials) oversaw the clinical effectiveness analysis and contributed important intellectual content to the report.
Gavin Newby (Clinical Neuropsychologist) contributed to the development of the grant application and trial protocol and contributed important intellectual content to the report.
Catherine Sackley (Professor of Rehabilitation) contributed to the development of the grant application and trial protocol and contributed important intellectual content to the report.
Nadina B Lincoln (Professor of Clinical Psychology) was the Co-chief Investigator and co-authored the final report.
Publications
das Nair R, Lincoln NB, Fitzsimmons D, Brain N, Montgomery AA, Bradshaw L, et al. Rehabilitation of memory following brain injury (ReMemBrIn): study protocol for a randomised controlled trial. Trials 2015;16:6.
das Nair R, Bradshaw LE, Day FEC, Drummond A, Fitzsimmons D, Harris S, et al. Clinical and cost-effectiveness of memory rehabilitation following traumatic brain injury: a pragmatic cluster randomised controlled trial [published online ahead of print April 12 2019]. Clin Rehabil 2019.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- das Nair R, Lincoln NB, Ftizsimmons D, Brain N, Montgomery A, Bradshaw L, et al. Rehabilitation of memory following brain injury (ReMemBrIn): study protocol for a randomised controlled trial. Trials 2015;16. https://doi.org/10.1186/1745-6215-16-6.
- Menon DK, Schwab K, Wright DW, Maas AI. Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health . Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil 2010;91:1637-40. https://doi.org/10.1016/j.apmr.2010.05.017.
- Headway . Acquired Brain Injury: The Numbers Behind the Hidden Disability 2015. www.headway.org.uk/media/2883/acquired-brain-injury-the-numbers-behind-the-hidden-disability.pdf (accessed 18 April 2017).
- Schneiderman AI, Braver ER, Kang HK. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol 2008;167:1446-52. https://doi.org/10.1093/aje/kwn068.
- Wojcik BE, Stein CR, Bagg K, Humphrey RJ, Orosco J. Traumatic brain injury hospitalizations of US army soldiers deployed to Afghanistan and Iraq. Am J Prev Med 2010;38:S108-16. https://doi.org/10.1016/j.amepre.2009.10.006.
- Risdall JE, Menon DK. Traumatic brain injury. Philos Trans R Soc Lond B Biol Sci 2011;366:241-50. https://doi.org/10.1098/rstb.2010.0230.
- Finkelstein EA, Corso PS, Miller TR. Incidence and Economic Burden of Injuries in the United States. New York: Oxford University Press; 2006.
- Salazar AM, Warden DL, Schwab K, Spector J, Braverman S, Walter J, et al. Cognitive rehabilitation for traumatic brain injury: a randomized trial. Defense and Veterans Head Injury Program (DVHIP) Study Group. JAMA 2000;283:3075-81. https://doi.org/10.1001/jama.283.23.3075.
- Goldstein FC, Levin HS. Cognitive outcome after mild and moderate traumatic brain injury in older adults. J Clin Exp Neuropsychol 2001;23:739-53. https://doi.org/10.1076/jcen.23.6.739.1028.
- Richardson JTE. Clinical and Neuropsychological Aspects of Closed Head Injury. London: Taylor & Francis; 2000.
- Williamson DJG, Scott JG, Adams RL, Adams RL, Parsons OA, Culbertson JL, et al. Neuropsychology for Clinical Practice: Etiology, Assessment and Treatment of Common Neurological Disorders. Washington, DC: American Psychological Association; 1996.
- Skeel RL, Edwards S, Johnstone B, Stonnington HH. Rehabilitation of Neuropsychological Disorders: A Practical Guide for Rehabilitation Professionals. New York, NY: Taylor & Francis; 2001.
- Rohling ML, Faust ME, Beverly B, Demakis G. Effectiveness of cognitive rehabilitation following acquired brain injury: a meta-analytic re-examination of Cicerone et al.’s (2000, 2005) systematic reviews. Neuropsychology 2009;23:20-39. https://doi.org/10.1037/a0013659.
- Wilson BA, Emslie H, Quirk K, Evans J, Watson P. A randomized control trial to evaluate a paging system for people with traumatic brain injury. Brain Inj 2005;19:891-4. https://doi.org/10.1080/02699050400002363.
- Doornhein K, de Haan EHF. Cognitive training for memory deficits in stroke patients. Neuropsychol Rehabil 1998;8:393-400. https://doi.org/10.1080/713755579.
- Kaschel R, Della Sala S, Cantagallo A, Fahlbock A, Laaksonen R, Kazen M. Imagery mnemonics for the rehabilitation of memory: a randomised group controlled trial. Neuropsychol Rehabil 2002;12:127-53. https://doi.org/10.1080/09602010143000211.
- Majid MJ, Lincoln NB, Weyman NI. Cognitive rehabilitation for memory deficits following stroke. Cochrane Database Syst Rev 2002;2.
- das Nair R. Effectiveness of Memory Rehabilitation Following Brain Damage 2008.
- Ptak R, der Linden MV, Schnider A. Cognitive rehabilitation of episodic memory disorders: from theory to practice. Front Hum Neurosci 2010;4. https://doi.org/10.3389/fnhum.2010.00057.
- Wilson BA. Rehabilitation of memory disorders. J Neurol Neurosurg Psychiatry 2010;81:e4-5. https://doi.org/10.1136/jnnp.2010.217554.9.
- das Nair R, Cogger H, Worthington E, Lincoln NB. Cognitive rehabilitation for memory deficits after stroke. Cochrane Database Syst Rev 2016;9. https://doi.org/10.1002/14651858.CD002293.pub3.
- das Nair R, Martin KJ, Lincoln NB. Memory rehabilitation for people with multiple sclerosis. Cochrane Database Syst Rev 2016;3. https://doi.org/10.1002/14651858.CD008754.pub3.
- das Nair R, Lincoln NB. Evaluation of rehabilitation of memory in neurological disabilities (ReMiND): a randomized controlled trial. Clin Rehabil 2012;26:894-903. https://doi.org/10.1177/0269215511435424.
- das Nair R, Lincoln NB. The effectiveness of memory rehabilitation following neurological disabilities: a qualitative inquiry of patient perspectives. Neuropsychol Rehabil 2013;23:528-45. https://doi.org/10.1080/09602011.2013.792290.
- Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Arch Phys Med Rehabil 2005;86:1681-92. https://doi.org/10.1016/j.apmr.2005.03.024.
- Cernich AN, Kurtz SM, Mordecai KL, Ryan PB. Cognitive rehabilitation in traumatic brain injury. Curr Treat Options Neurol 2010;12:412-23. https://doi.org/10.1007/s11940-010-0085-6.
- Cicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, et al. Evidence-based cognitive rehabilitation: recommendations for clinical practice. Arch Phys Med Rehabil 2000;81:1596-615. https://doi.org/10.1053/apmr.2000.19240.
- Bergquist TF, Thompson K, Gehl C, Munoz Pineda J. Satisfaction ratings after receiving internet-based cognitive rehabilitation in persons with memory impairments after severe acquired brain injury. Telemed J E Health 2010;16:417-23. https://doi.org/10.1089/tmj.2009.0118.
- Spreij LA, Visser-Meily JM, van Heugten CM, Nijboer TC. Novel insights into the rehabilitation of memory post acquired brain injury: a systematic review. Front Hum Neurosci 2014;8. https://doi.org/10.3389/fnhum.2014.00993.
- Sigmundsdottir L, Longley WA, Tate RL. Computerised cognitive training in acquired brain injury: a systematic review of outcomes using the International Classification of Functioning (ICF). Neuropsychol Rehabil 2016;26:673-741. https://doi.org/10.1080/09602011.2016.1140657.
- International Classification of Functioning, Disability and Health. Geneva: World Health Organization; 2001.
- Harley JP, Allen C, Braciszewski TL, Cicerone KD, Dahlberg C, Evans S, et al. Guidelines for cognitive rehabilitation. NeuroRehabilitation 1992;2:62-7.
- Cappa SF, Benke T, Clarke S, Rossi B, Stemmer B, van Heugten CM. European Federation of Neurological Societies . EFNS guidelines on cognitive rehabilitation: report of an EFNS task force. Eur J Neurol 2003;10:11-23. https://doi.org/10.1046/j.1468-1331.2003.00537.x.
- Cappa SF, Benke T, Clarke S, Rossi B, Stemmer B, van Heugten CM. Task Force on Cognitive Rehabilitation . EFNS guidelines on cognitive rehabilitation: report of an EFNS task force. Eur J Neurol 2005;12:665-80. https://doi.org/10.1111/j.1468-1331.2005.01330.x.
- Rehabilitation Following Acquired Brain Injury: National Clinical Guidelines. London: RCP and BSRM; 2003.
- Scottish Intercollegiate Guidelines Network . Brain Injury Rehabilitation in Adults 2013. www.sign.ac.uk/assets/sign130.pdf (accessed 18 April 2017).
- National Institute for Health and Care Excellence . Quality Statement 7: Community Rehabilitation Services for People (Aged 16 and Over) With Traumatic Brain Injury 2014. www.nice.org.uk/guidance/qs74/chapter/Quality-statement-7-Community-rehabilitation-services-for-people-aged-16-and-over-with-traumatic-brain-injury (accessed 18 April 2017).
- Velikonja D, Tate R, Ponsford J, McIntyre A, Janzen S, Bayley M. INCOG Expert Panel . INCOG recommendations for management of cognition following traumatic brain injury, part V: memory. J Head Trauma Rehabil 2014;29:369-86. https://doi.org/10.1097/HTR.0000000000000069.
- Department of Health . New Trauma Research 2010. http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/MediaCentre/Pressreleasesarchive/DH_114389 (accessed 18 April 2017).
- Bayley MT, Tate R, Douglas JM, Turkstra LS, Ponsford J, Stergiou-Kita M, et al. INCOG guidelines for cognitive rehabilitation following traumatic brain injury: methods and overview. J Head Trauma Rehabil 2014;29:290-306. https://doi.org/10.1097/HTR.0000000000000070.
- Clinical Neuropsychology and Rehabilitation Services for Adults with Acquired Brain Injury. Leicester: British Psychological Society; 2005.
- Alderwick H, Robertson R, Appleby J, Dunn P, Maguire D. Better Value in the NHS: The Role of Changes in Clinical Practice. London: King’s Fund; 2015.
- Sunderland A, Harris JE, Baddeley AD. Do laboratory tests predict everyday memory – a neuropsychological study. J Verbal Learn Verbal Behav 1983;22:341-57. https://doi.org/10.1016/S0022-5371(83)90229-3.
- Wilson BA, Greenfield E, Clare L, Baddeley A, Cockburn J, Watson P, et al. Rivermead Behavioural Memory Test – Third Edition (RBMT-3). London: Pearson; 2008.
- Syder D, Body R, Parker M. Sheffield Screening Test for Acquired Language Disorder. Windsor: NFER-Nelson; 1993.
- Cornish IM. Factor structure of the everyday memory questionnaire. Br J Psychol 2000;91:427-38. https://doi.org/10.1348/000712600161916.
- Royle J, Lincoln NB. The Everyday Memory Questionnaire-revised: development of a 13-item scale. Disabil Rehabil 2008;30:114-21. https://doi.org/10.1080/09638280701223876.
- Carr SE, das Nair R, Schwartz AF, Lincoln NB. Group memory rehabilitation for people with multiple sclerosis: a feasibility randomized controlled trial. Clin Rehabil 2014;28:552-61. https://doi.org/10.1177/0269215513512336.
- Chiaravalloti ND, Wylie G, Leavitt V, Deluca J. Increased cerebral activation after behavioral treatment for memory deficits in MS. J Neurol 2012;259:1337-46. https://doi.org/10.1007/s00415-011-6353-x.
- Tam SF, Man WK. Evaluating computer-assisted memory retraining programmes for people with post-head injury amnesia. Brain Inj 2004;18:461-70. https://doi.org/10.1080/02699050310001646099.
- Dou ZL, Man DW, Ou HN, Zheng JL, Tam SF. Computerized errorless learning-based memory rehabilitation for Chinese patients with brain injury: a preliminary quasi-experimental clinical design study. Brain Inj 2006;20:219-25. https://doi.org/10.1080/02699050500488215.
- Björkdahl A, Akerlund E, Svensson S, Esbjörnsson E. A randomized study of computerized working memory training and effects on functioning in everyday life for patients with brain injury. Brain Inj 2013;27:1658-65. https://doi.org/10.3109/02699052.2013.830196.
- Nelson HE, Willison J. National Adult Reading Test. Chiswick: NFER-Nelson; 1991.
- Goldberg RJ, Williams PA. User’s Guide to the General Health Questionnaire. Windsor: NFER-Nelson; 1988.
- Watkins CL, Auton MF, Deans CF, Dickinson HA, Jack CI, Lightbody CE, et al. Motivational interviewing early after acute stroke: a randomized, controlled trial. Stroke 2007;38:1004-9. https://doi.org/10.1161/01.STR.0000258114.28006.d7.
- Moss-Morris R, Dennison L, Landau S, Yardley L, Silber E, Chalder T. A randomized controlled trial of cognitive behavioral therapy (CBT) for adjusting to multiple sclerosis (the saMS trial): does CBT work and for whom does it work?. J Consult Clin Psychol 2013;81:251-62. https://doi.org/10.1037/a0029132.
- Lincoln NB, Yuill F, Holmes J, Drummond AE, Constantinescu CS, Armstrong S, et al. Evaluation of an adjustment group for people with multiple sclerosis and low mood: a randomized controlled trial. Mult Scler 2011;17:1250-7. https://doi.org/10.1177/1352458511408753.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2:81-4. https://doi.org/10.1016/S0140-6736(74)91639-0.
- EuroQol Group . EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990;3:199-208.
- Humphreys I, Drummond AE, Phillips C, Lincoln NB. Cost-effectiveness of an adjustment group for people with multiple sclerosis and low mood: a randomized trial. Clin Rehabil 2013;27:963-71. https://doi.org/10.1177/0269215513488608.
- Humphreys I, Thomas S, Phillips C, Lincoln N. Cost analysis of the Communication and Low Mood (CALM) randomised trial of behavioural therapy for stroke patients with aphasia. Clin Rehabil 2015;29:30-41. https://doi.org/10.1177/0269215514537656.
- Wilson BA, Baddeley A, Evans J, Shiel A. Errorless learning in the rehabilitation of memory-impaired people. Neuropsychol Rehabil 1994;4:307-26. https://doi.org/10.1080/09602019408401463.
- Jordan B, Henderson A. Interaction Analysis: Foundations and Practice 1995. http://lrs.ed.uiuc.edu/students/c-merkel/document4.HTM (accessed 18 April 2011).
- Heath C, Silverman D. Qualitative Methods. London: Sage; 1997.
- Bouwens SF, van Heugten CM, Verhey FR. The practical use of goal attainment scaling for people with acquired brain injury who receive cognitive rehabilitation. Clin Rehabil 2009;23:310-20. https://doi.org/10.1177/0269215508101744.
- Teasdale TW. EBIQ Questionnaire n.d. http://teasdale.psy.ku.dk/EBIQ.pdf (accessed 18 April 2017).
- Teasdale TW, Christensen AL, Willmes K, Deloche G, Braga L, Stachowiak F, et al. Subjective experience in brain-injured patients and their close relatives: a European Brain Injury Questionnaire study. Brain Inj 1997;11:543-63. https://doi.org/10.1080/026990597123250.
- Sopena S, Dewar BK, Nannery R, Teasdale TW, Wilson BA. The European Brain Injury Questionnaire (EBIQ) as a reliable outcome measure for use with people with brain injury. Brain Inj 2007;21:1063-8. https://doi.org/10.1080/02699050701630342.
- Bateman A, Teasdale TW, Willmes K. Assessing construct validity of the self-rating version of the European Brain Injury Questionnaire (EBIQ) using Rasch analysis. Neuropsychol Rehabil 2009;19:941-54. https://doi.org/10.1080/09602010903021170.
- Wilson BA. Towards a comprehensive model of cognitive rehabilitation. Neuropsychol Rehabil 2002;12:97-110. https://doi.org/10.1080/09602010244000020.
- Wade D, Halligan P, Wade D. The Effectiveness of Rehabilitation for Cognitive Deficits. Oxford: Oxford University Press; 2005.
- World Medical Association (WMA) . WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects n.d. www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed 5 July 2018).
- Department of Heath . Research Governance Framework for Health and Social Care Second Edition, 2005 n.d. www.gov.uk/government/uploads/system/uploads/attachment_data/file/139565/dh_4122427.pdf (accessed 5 July 2018).
- European Medicines Agency . ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice n.d. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4_2016_1109.pdf (accessed 1 August 2018).
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005;24:993-1007. https://doi.org/10.1002/sim.1981.
- Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clin Trials 2005;2:152-62. https://doi.org/10.1191/1740774505cn076oa.
- Baldwin SA, Bauer DJ, Stice E, Rohde P. Evaluating models for partially clustered designs. Psychol Methods 2011;16:149-65. https://doi.org/10.1037/a0023464.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- White IR. Uses and limitations of randomization-based efficacy estimators. Stat Methods Med Res 2005;14:327-47. https://doi.org/10.1191/0962280205sm406oa.
- Shrier I, Steele RJ, Verhagen E, Herbert R, Riddell CA, Kaufman JS. Beyond intention to treat: what is the right question?. Clin Trials 2014;11:28-37. https://doi.org/10.1177/1740774513504151.
- Pearson Assessment . Rivermead Behavioural Memory Test – Third Edition (RBMT-3) – Frequently Asked Questions n.d. www.pearsonclinical.co.uk/Psychology/AdultCognitionNeuropsychologyandLanguage/AdultMemory/RivermeadBehaviouralMemoryTestThirdEditionRBMT3/ForThisProduct/FrequentlyAskedQuestions.aspx (accessed 9 June 2017).
- das Nair R, Bradshaw LE, Day FEC, Drummond A, Fitzsimmons D, Harris S, et al. Clinical and cost-effectiveness of memory rehabilitation following traumatic brain injury: a pragmatic cluster randomised controlled trial [published online April 12 2019]. Clin Rehabil 2019.
- Mackenzie K. Assessing the Saliency of Everyday Memory Failures: The Modified Everyday Memory Questionnaire 2014.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2016. Canterbury: Personal Social Services Research Unit, University of Kent; 2016.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- Department of Health . NHS Reference Costs 2015 to 2016 n.d. www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed 18 April 2017).
- Bank of England . Inflation Calculator n.d. www.bankofengland.co.uk/education/Pages/resources/inflationtools/calculator/default.aspx (accessed 21 April 2017).
- EuroQol . EQ-5D n.d. www.euroqol.org/ (accessed 8 May 2017).
- York Health Economics Consortium . Net Monetary Benefit 2016. www.yhec.co.uk/glossary/net-monetary-benefit/ (accessed 18 April 2017).
- Briggs AH. Handling uncertainty in cost-effectiveness models. PharmacoEconomics 2000;17:479-500. https://doi.org/10.2165/00019053-200017050-00006.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley; 1987.
- Rai D, Kosidou K, Lundberg M, Araya R, Lewis G, Magnusson C. Psychological distress and risk of long-term disability: population-based longitudinal study. J Epidemiol Community Health 2012;66:586-92. https://doi.org/10.1136/jech.2010.119644.
- Office for National Statistics . National Life Tables: England and Wales 2016. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesenglandandwalesreferencetables (accessed 2 November 2016).
- Ventura T, Harrison-Felix C, Carlson N, Diguiseppi C, Gabella B, Brown A, et al. Mortality after discharge from acute care hospitalization with traumatic brain injury: a population-based study. Arch Phys Med Rehabil 2010;91:20-9. https://doi.org/10.1016/j.apmr.2009.08.151.
- Brooks JC, Shavelle RM, Strauss DJ, Hammond FM, Harrison-Felix CL. Long-term survival after traumatic brain injury part II: life expectancy. Arch Phys Med Rehabil 2015;96:1000-5. https://doi.org/10.1016/j.apmr.2015.02.002.
- DCP Policy on Clinical Supervision. Leicester: British Psychological Society; 2014.
- Johnson RB, Onwuegbuzie AJ. Mixed methods research: a research paradigm whose time has come. Educ Res 2004;33:14-26. https://doi.org/10.3102/0013189X033007014.
- O’Cathain A, Murphy E, Nicholl J. Why, and how, mixed methods research is undertaken in health services research in England: a mixed methods study. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-85.
- Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health 2015;42:533-44. https://doi.org/10.1007/s10488-013-0528-y.
- Creswell JW. Educational Research: Planning, Conducting, and Evaluating Quantitative and Qualitative Research. Upper Saddle River, NJ: Pearson Education; 2002.
- Onwuegbuzie AJ, Leech NL. A call for qualitative power analyses. Qual Quant 2007;41:105-21. https://doi.org/10.1007/s11135-005-1098-1.
- Mason M. Sample size and saturation in PhD studies using qualitative interviews. Forum Qual Soc Res 2010;11.
- Guest G, Bunce A, Johnson L. How many interviews are enough?. Field Methods 2006;18:59-82. https://doi.org/10.1177/1525822X05279903.
- Fusch PI, Ness LR. Are we there yet? Data saturation in qualitative research. Qual Rep 2015;20:1408-16.
- das Nair R, Martin KJ, Sinclair EJ. A meta-synthesis of qualitative research on perceptions of people with long-term neurological conditions about group-based memory rehabilitation. Neuropsychol Rehabil 2015;25:479-502. https://doi.org/10.1080/09602011.2014.971820.
- Willig C. Introducing Qualitative Research in Psychology: Adventures in Theory and Method. Maidenhead: McGraw Hill/Open University Press; 2008.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage; 2003.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Mays N, Pope C. Qualitative research in health care. Assessing quality in qualitative research. BMJ 2000;320:50-2. https://doi.org/10.1136/bmj.320.7226.50.
- Archer M, Bhaskar R, Collier A, Lawson T, Norrie A. Critical Realism: Essential Readings. London: Routledge; 2013.
- Fletcher AJ. Applying critical realism in qualitative research: methodology meets method. Int J Social Res Methodol 2017;20:181-94. https://doi.org/10.1080/13645579.2016.1144401.
- Klein OA, Drummond A, Mhizha-Murira JR, Mansford L, das Nair R. Effectiveness of cognitive rehabilitation for people with multiple sclerosis: a meta-synthesis of patient perspectives [published online ahead of print 1 May 2017]. Neuropsychol Rehabil 2017. https://doi.org/10.1080/09602011.2017.1309323.
- Finset A, Dyrnes S, Krogstad JM, Berstad J. Self-reported social networks and interpersonal support 2 years after severe traumatic brain injury. Brain Inj 1995;9:141-50. https://doi.org/10.3109/02699059509008187.
- O’Flaherty CA, Douglas JM. Living with cognitive-communicative difficulties following traumatic brain injury: using a model of interpersonal communication to characterize the subjective experience. Aphasiology 1997;11:889-911. https://doi.org/10.1080/02687039708250463.
- Ponsford JL, Downing MG, Olver J, Ponsford M, Acher R, Carty M, et al. Longitudinal follow-up of patients with traumatic brain injury: outcome at two, five, and ten years post-injury. J Neurotrauma 2014;31:64-77. https://doi.org/10.1089/neu.2013.2997.
- Ashman TA, Gordon WA, Cantor JB, Hibbard MR. Neurobehavioral consequences of traumatic brain injury. Mt Sinai J Med 2006;73:999-1005.
- Rabinowitz AR, Levin HS. Cognitive sequelae of traumatic brain injury. Psychiatr Clin North Am 2014;37:1-11. https://doi.org/10.1016/j.psc.2013.11.004.
- Hoofien D, Gilboa A, Vakil E, Donovick PJ. Traumatic brain injury (TBI) 10–20 years later: a comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Inj 2001;15:189-20. https://doi.org/10.1080/026990501300005659.
- Tsaousides T, Gordon WA. Cognitive rehabilitation following traumatic brain injury: assessment to treatment. Mt Sinai J Med 2009;76:173-81. https://doi.org/10.1002/msj.20099.
- Evans JJ, Wilson BA, Needham P, Brentnall S. Who makes good use of memory aids? Results of a survey of people with acquired brain injury. J Int Neuropsychol Soc 2003;9:925-35. https://doi.org/10.1017/S1355617703960127.
- Wilson BA, Watson PC. A practical framework for understanding compensatory behaviour in people with organic memory impairment. Memory 1996;4:465-86. https://doi.org/10.1080/741940776.
- Marino SE, Meador KJ, Loring DW, Okun MS, Fernandez HH, Fessler AJ, et al. Subjective perception of cognition is related to mood and not performance. Epilepsy Behav 2009;14:459-64. https://doi.org/10.1016/j.yebeh.2008.12.007.
- Elixhauser A, Leidy NK, Meador K, Means E, Willian MK. The relationship between memory performance, perceived cognitive function, and mood in patients with epilepsy. Epilepsy Res 1999;37:13-24. https://doi.org/10.1016/S0920-1211(99)00036-4.
- Slavin MJ, Brodaty H, Kochan NA, Crawford JD, Trollor JN, Draper B, et al. Prevalence and predictors of ‘subjective cognitive complaints’ in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry 2010;18:701-10. https://doi.org/10.1097/JGP.0b013e3181df49fb.
- Yates JA, Clare L, Woods RT, MRC CFAS. Subjective memory complaints, mood and MCI: a follow-up study. Aging Ment Health 2017;21:313-21. https://doi.org/10.1080/13607863.2015.1081150.
- Kurz A, Pohl C, Ramsenthaler M, Sorg C. Cognitive rehabilitation in patients with mild cognitive impairment. Int J Geriatr Psychiatry 2009;24:163-8. https://doi.org/10.1002/gps.2086.
- Bergquist T, Gehl C, Mandrekar J, Lepore S, Hanna S, Osten A, et al. The effect of internet-based cognitive rehabilitation in persons with memory impairments after severe traumatic brain injury. Brain Inj 2009;23:790-9. https://doi.org/10.1080/02699050903196688.
- das Nair R, Kontou E, Smale K, Barker A, Lincoln NB. Comparing individual and group intervention for psychological adjustment in people with multiple sclerosis: a feasibility randomised controlled trial. Clin Rehabil 2016;30:1156-64. https://doi.org/10.1177/0269215515616446.
- Martin KJ, Sinclair EJ, das Nair R. Descriptions of memory rehabilitation group interventions for neurological conditions: a systematic review. Clin Rehabil 2016;30:705-13. https://doi.org/10.1177/0269215515595273.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Lincoln NB, das Nair R, Stuss DT, Winocur G, Robertson IH. Cognitive Rehabilitation. New York: Cambridge University Press; 2008.
- Rockwood K, Joyce B, Stolee P. Use of goal attainment scaling in measuring clinically important change in cognitive rehabilitation patients. J Clin Epidemiol 1997;50:581-8. https://doi.org/10.1016/S0895-4356(97)00014-0.
- Turner-Stokes L. Goal attainment scaling and its relationship with standardized outcome measures: a commentary. J Rehabil Med 2011;43:70-2. https://doi.org/10.2340/16501977-0656.
- Bovend’Eerdt TJ, Dawes H, Izadi H, Wade DT. Agreement between two different scoring procedures for goal attainment scaling is low. J Rehabil Med 2011;43:46-9. https://doi.org/10.2340/16501977-0624.
- Wilson BA, Emslie HC, Quirk K, Evans JJ. Reducing everyday memory and planning problems by means of a paging system: a randomised control crossover study. J Neurol Neurosurg Psychiatry 2001;70:477-82. https://doi.org/10.1136/jnnp.70.4.477.
- Brown AW, Moessner AM, Mandrekar J, Diehl NN, Leibson CL, Malec JF. A survey of very-long-term outcomes after traumatic brain injury among members of a population-based incident cohort. J Neurotrauma 2011;28:167-76. https://doi.org/10.1089/neu.2010.1400.
- National Institutes of Health (NIH) Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research. Maryland, MD: National Institutes of Health; 1994.
- Moles DR, Gilthorpe MS, Wilson RC, Bedi R. Variations in admission to hospital for head injury and assault to the head. Part 2: ethnic group. Br J Oral Maxillofac Surg 1999;37:301-8. https://doi.org/10.1054/bjom.1998.0040.
- BMJ Best Practice . Assessment of Traumatic Brain Injury, Acute 2016. http://bestpractice.bmj.com/best-practice/monograph/515.html (accessed 9 June 2017).
- Kennedy MR, Turkstra L. Group intervention studies in the cognitive rehabilitation of individuals with traumatic brain injury: challenges faced by researchers. Neuropsychol Rev 2006;16:151-9. https://doi.org/10.1007/s11065-006-9012-8.
- O’Brien MC, das Nair R, Lincoln NB. A comparison of the content of memory rehabilitation groups for patients with neurological disabilities. Neuropsychol Rehabil 2013;23:321-32. https://doi.org/10.1080/09602011.2012.753920.
- Frontera WR, Bean JF, Damiano D, Ehrlich-Jones L, Fried-Oken M, Jette A, et al. Rehabilitation research at the National Institutes of Health: moving the field forward (Executive Summary). Arch Phys Med Rehabil 2017;98:795-803. https://doi.org/10.1016/j.apmr.2017.02.001.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials 2010;340.
- Ponsford J, Sloan S, Snow P. Traumatic Brain Injury: Rehabilitation for Everyday Adaptive Living. Hove: Psychology Press; 2013.
- Smale KJ, Carr SE, Schwartz AF, das Nair R, Lincoln NB. An evaluation of treatment integrity in a randomised controlled trial of memory rehabilitation for people with multiple sclerosis. Clin Rehabil 2015;29:493-9. https://doi.org/10.1177/0269215514548733.
- Dumas JE, Lynch AM, Laughlin JE, Phillips Smith E, Prinz RJ. Promoting intervention fidelity. Conceptual issues, methods, and preliminary results from the EARLY ALLIANCE prevention trial. Am J Prev Med 2001;20:38-47. https://doi.org/10.1016/S0749-3797(00)00272-5.
- Hajek VE, Gagnon S, Ruderman JE. Cognitive and functional assessments of stroke patients: an analysis of their relation. Arch Phys Med Rehabil 1997;78:1331-7. https://doi.org/10.1016/S0003-9993(97)90306-3.
- Middleton LS, Denney DR, Lynch SG, Parmenter B. The relationship between perceived and objective cognitive functioning in multiple sclerosis. Arch Clin Neuropsychol 2006;21:487-94. https://doi.org/10.1016/j.acn.2006.06.008.
- Strober LB, Binder A, Nikelshpur OM, Chiaravalloti N, DeLuca J. The Perceived Deficits Questionnaire: perception, deficit, or distress?. Int J MS Care 2016;18:183-90. https://doi.org/10.7224/1537-2073.2015-028.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
Appendix 1 Participating sites
| Lead NHS trust | Date opened to recruitment | Number of months open |
|---|---|---|
| Cheshire and Wirral Partnership NHS Foundation Trust | 6 February 2013 | 23 |
| Nottingham University Hospitals NHS Trust | 7 February 2013 | 31 |
| Birmingham Community Healthcare NHS Trust | 18 April 2013 | 5 |
| Central Surrey Health | 30 April 2013 | 7 |
| The Walton Centre NHS Foundation Trust | 12 March 2014 | 21 |
| Sheffield Health and Social Care NHS Foundation Trust | 3 November 2014 | 13 |
| St George’s Healthcare NHS Trust | 18 March 2015 | 9 |
| North Bristol NHS Trust | 1 May 2015 | 7 |
| South Tees Hospitals NHS Foundation Trust | 12 June 2015 | 6 |
Appendix 2 Plan of study procedures and data collection
| Assessments | Time point | |||
|---|---|---|---|---|
| Initial screening assessment | Second assessment visit | 6-month follow-up | 12-month follow-up | |
| Initial eligibility screening | ✓a | |||
| Informed consent | ✓a | |||
| Demographic information | ✓a | |||
| EMQ-p | ✓a | ✓b | ✓b | |
| RBMT-3 | ✓a | ✓a | ✓a | |
| SST | ✓a | |||
| NART | ✓a | |||
| GHQ-30 | ✓a | ✓b | ✓b | |
| Setting of short- and long-term goals | ✓a | |||
| EQ-5D-5L | ✓a | ✓b | ✓b | |
| SUQ | ✓a | ✓b | ✓b | |
| EMQ-r | ✓b | ✓b | ✓b | |
| Check availability for treatment group | ✓a | |||
| EBIQ-p | ✓b | ✓b | ||
| EBIQ-r | ✓b | ✓b | ||
| RA opinion on participants’ treatment group before and after assessment of goals | ✓a | ✓a | ||
| Assessment of individual goal attainment | ✓a | ✓a | ||
| Qualitative feedback interviews with a sample of participants | ✓c | |||
| Clinical data collected from medical notes where available | ✓ | |||
Appendix 3 Treatment fidelity coding schedule
| Activites | |||||||
|---|---|---|---|---|---|---|---|
| Non-rehabilitation | Rehabilitation | ||||||
| Activities | Code | AP skills | Code | AP activities | Code | Participant | Code |
| Introductions | T1, P1 | Facilitating discussion (non-specific prompts) | T7 | Presenting/discussing educational material | T10 | Discussing/filling in educational material | P10 |
| Social chat | T2, P2 | Providing feedback not directly related to content of manual | T8 | Presenting/discussing strategies | T11 | Discussing strategies | P11 |
| Preparing materials, tasks, etc. | T3, P3 | Providing encouragement/reassurance | T9 | Providing general information related to memory that is not covered in educational material | T15 | Asking for information | P12 |
| Information about sessions, venue, group, etc. | T4, P4 | Summarising | T16 | Recap of previous session | T18 | Feedback on home activities | P13 |
| Hospital visit discussion | T5, P5 | Paraphrasing | T17 | Describing problems related to memory | P14 | ||
| Describing emotions and coping strategies | T6, P6 | ||||||
Appendix 4 Estimates of the intracluster correlation coefficient in the memory rehabilitation arm for each outcome calculated from the multilevel models
| Outcome | Time point | |
|---|---|---|
| 6 months | 12 months | |
| EMQ-p – frequency of problems | 0.05 | 0.00 |
| EMQ-p – importance of problems | 0.00 | 0.00 |
| GHQ-30 | 0.07 | 0.00 |
| RBMT-3 (GMI) | 0.00 | 0.00 |
| Short-term goal achievement average score | 0.13 | 0.00 |
| Long-term goal achievement average score | 0.06 | 0.00 |
| EBIQ-p – cognitive | 0.00 | 0.00 |
| EBIQ-p – depression | 0.00 | 0.00 |
| EBIQ-p – communication | 0.09 | 0.03 |
| EBIQ-p – difficulties in social interactions | 0.00 | 0.00 |
| EMQ-r – frequency of problems | 0.00 | 0.00 |
| EMQ-r – importance of problems | 0.00 | 0.01 |
| EMQ-r – cognitive | 0.08 | 0.00 |
| EMQ-r – depression | 0.00 | 0.00 |
| EMQ-r – communication | 0.04 | 0.09 |
| EMQ-r – difficulties in social interactions | 0.00 | 0.00 |
Appendix 5 Cumulative recruitment against target
FIGURE 14.
Cumulative recruitment: actual and predicted.

Appendix 6 Further details of patients who did not enrol in the trial
| Reason | Number of participants | Percentage of those screened |
|---|---|---|
| Unsuitable for group | 236 | 6 |
| Involved in other study | 1 | 0 |
| Impairment of language | 26 | 1 |
| TBI < 3 months previously | 8 | 0 |
| Aged < 18 years | 33 | 1 |
| Aged > 69 years | 622 | 15 |
| Unable to travel | 196 | 5 |
| Unable to consent | 7 | 0 |
| Total | 1129 | 28 |
| Other reasons for non-enrolment | Number of participants | Percentage of those screened |
|---|---|---|
| Deceased | 119 | 3 |
| Not TBI | 167 | 4 |
| No memory difficulties | 105 | 3 |
| Declined | 75 | 2 |
| Unable to contact | 66 | 2 |
| English-language difficulties | 36 | 1 |
| Working or no time | 24 | 1 |
| Out of area | 23 | 1 |
| Other illness | 16 | 0 |
| Not accepted/did not engage | 12 | 0 |
| Cause of brain injury unknown | 10 | 0 |
| Did not attend | 9 | 0 |
| Clinician’s advice | 9 | 0 |
| Other rehabilitation | 7 | 0 |
| Recruitment closed at site | 5 | 0 |
| Student, so unable to attend | 2 | 0 |
| Not known | 33 | 1 |
| Total | 718 | 18 |
Appendix 7 Randomisation by trial arm and site
| Summary | Trial arm, n | Total, n | |
|---|---|---|---|
| Usual care | Memory rehabilitation | ||
| Number of clusters randomised | |||
| Site 1 | 8 | 8 | 16 |
| Site 2 | 0 | 1 | 1 |
| Site 3 | 4 | 6 | 10 |
| Site 4 | 2 | 1 | 3 |
| Site 5 | 7 | 7 | 14 |
| Site 6 | 6 | 6 | 12 |
| Site 7 | 1 | 2 | 3 |
| Site 8 | 1 | 2 | 3 |
| Site 9 | 2 | 2 | 4 |
| Number of participants randomised | |||
| Site 1 | 38 | 39 | 77 |
| Site 2 | 0 | 5 | 5 |
| Site 3 | 19 | 27 | 46 |
| Site 4 | 10 | 4 | 14 |
| Site 5 | 36 | 33 | 69 |
| Site 6 | 33 | 33 | 66 |
| Site 7 | 5 | 8 | 13 |
| Site 8 | 4 | 11 | 15 |
| Site 9 | 12 | 11 | 23 |
| Size of cluster randomised | |||
| 4 | 10 | 12 | 22 |
| 5 | 9 | 15 | 24 |
| 6 | 12 | 8 | 20 |
| Days between initial screening and randomisation | |||
| Median (25th, 75th centile) | 39 (20, 110) | 29 (21, 62) | 33 (21, 78.5) |
| Min., max. | 6, 455 | 5, 317 | 5, 455 |
| Days between second assessment and randomisation | |||
| Median (25th, 75th centile) | 22 (7, 90) | 15 (8, 42) | 18 (8, 54.5) |
| Min., max. | 0, 444 | 0, 282 | 0, 444 |
Appendix 8 Other clinical data collected at baseline
| Variable | Trial arm | Total (N = 328) | |
|---|---|---|---|
| Usual care (N = 157) | Memory rehabilitation (N = 171) | ||
| Clinical notes available, n (%) | 58 (37) | 42 (25) | 100 (30) |
| Clinical notes not available, n (%) | 99 (63) | 129 (75) | 228 (70) |
| Type of head injury, n (%) | |||
| Open | 5 (9) | 4 (10) | 9 (9) |
| Closed | 39 (67) | 31 (74) | 70 (70) |
| Unknown | 14 (24) | 7 (17) | 21 (21) |
| Severity of the head injury (GCSa) | |||
| Closest to admission | |||
| Median (25th, 75th centile) | 10 (6, 14) | 12 (6, 15) | 11.5 (6, 14) |
| Min., max. | 3, 15 | 3, 15 | 3, 15 |
| n | 25 | 21 | 46 |
| Unknown, n (%) | 33 (57) | 21 (50) | 54 (54) |
| Worst total score | |||
| Median (25th, 75th centile) | 8.5 (4, 14) | 9 (4, 14) | 8.5 (4, 14) |
| Min., max. | 3, 15 | 3, 15 | 3, 15 |
| n | 22 | 18 | 40 |
| Unknown, n (%) | 36 (62) | 24 (57) | 60 (60) |
| Other neurological conditions,b n (%) | |||
| None | 35 (60) | 31 (74) | 66 (66) |
| Stroke | 0 | 2 (5) | 2 (2) |
| Subarachnoid haemorrhage | 3 (5) | 5 (12) | 8 (8) |
| Epilepsy | 8 (14) | 4 (10) | 12 (12) |
| Multiple sclerosis | 0 | 0 | 0 |
| Parkinson’s disease | 0 | 0 | 0 |
| Otherc | 4 (7) | 0 | 4 (4) |
| Unknown | 8 (14) | 1 (2) | 9 (9) |
Appendix 9 Attendance at group memory rehabilitation
| Session | Attendance | Duration (minutes) | Size of group in attendance | |||
|---|---|---|---|---|---|---|
| n | % | Median | Min., max. | Median | Min., max. | |
| 1 | 124 | 73 | 90 | 60, 110 | 4 | 1, 6 |
| 2 | 124 | 73 | 90 | 60, 110 | 4 | 1, 6 |
| 3 | 114 | 67 | 90 | 75, 185 | 3 | 1, 6 |
| 4 | 108 | 63 | 90 | 70, 110 | 3 | 1, 5 |
| 5 | 108 | 63 | 90 | 60, 100 | 3 | 1, 5 |
| 6 | 101 | 59 | 90 | 60, 95 | 3 | 1, 5 |
| 7 | 103 | 60 | 90 | 60, 105 | 3 | 0, 6 |
| 8 | 106 | 62 | 90 | 60, 110 | 3 | 0, 6 |
| 9 | 94 | 55 | 90 | 50, 110 | 3 | 0, 5 |
| 10 | 101 | 59 | 90 | 60, 120 | 3 | 0, 5 |
Appendix 10 Additional details of the treatment fidelity analysis
| Site | Group | Videos | |
|---|---|---|---|
| Available | Included in analysis | ||
| 1 | 003 | Sessions 4–10 | Sessions 4–6 and 8–10 |
| 011 | Sessions 5 and 7–9 | Sessions 8 and 9 | |
| 012 | Sessions 9 and 10 | Sessions 9 and 10 | |
| 020 | Sessions 1, 2, 4, 6 and 7 | Sessions 1, 2, 4 and 6 | |
| 023 | Sessions 2–5 and 8 | Sessions 2 and 5 | |
| 024 | Sessions 2–4 | Sessions 3 and 4 | |
| 031 | Sessions 1, 3–5 and 8–10 | ||
| 036 | Sessions 1 and 3–8 | ||
| 2 | 008 | First group | |
| 3 | 001 | First group | |
| 017 | Session 4, 5, 9 and 10 | Sessions 9 and 10 | |
| 018 | Sessions 8 and 9 | Session 8 | |
| 021 | Sessions 3 and 8 | ||
| 029 | No recordings | ||
| 032 | No recordings | ||
| 4 | 004 | First group | |
| 5 | 016 | Sessions 1–5 | Sessions 1 and 4 |
| 028 | No recordings | ||
| 041 | No recordings | ||
| 042 | No recordings | ||
| 044 | No recordings | ||
| 061 | No recordings | ||
| 066 | No recordings | ||
| 6 | 038 | First group | |
| 039 | No recordings | ||
| 045 | Session 6 | Session 6 | |
| 047 | Sessions 2 and 9 | Session 2 | |
| 050 | No recordings | ||
| 055 | No recordings | ||
| 7 | 048 | First group | |
| 062 | Camera missing | ||
| 8 | 053 | First group | |
| 057 | Sessions 8–10 | Sessions 8 and 10 | |
| 9 | 056 | First group | |
| 058 | Sessions 5–9 | Sessions 5–8 | |
| Site | Session | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| 1 | 1 | 2 | 1 | 3 | 2 | 2 | 0 | 2 | 3 | 2 | 18 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 |
| 5 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 6 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 |
| 9 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 4 |
| Total | 2 | 3 | 1 | 4 | 3 | 4 | 1 | 5 | 4 | 4 | 31 |
| Category | Site | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 6 | 8 | 9 | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Non-rehabilitation activities | ||||||||||||||
| Introductions | 3 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1.1 | 5 | 0.4 |
| Social chat | 24 | 3.7 | 4 | 3.4 | 3 | 4.5 | 1 | 1.3 | 2 | 3.0 | 4 | 2.2 | 38 | 3.2 |
| Preparing materials, tasks, etc. | 17 | 2.6 | 2 | 1.7 | 1 | 1.5 | 0 | 0 | 3 | 4.5 | 0 | 0 | 23 | 2.0 |
| Information about sessions, venue, group, etc. | 32 | 4.9 | 4 | 3.4 | 14 | 20.9 | 1 | 1.3 | 1 | 1.5 | 4 | 2.2 | 56 | 4.8 |
| Hospital visit discussion | 0 | 0 | 0 | 0 | 1 | 1.5 | 0 | 0 | 0 | 0 | 1 | 0.5 | 2 | 0.2 |
| Describing emotions and coping strategies | 6 | 0.9 | 1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 | 8 | 0.7 |
| Rehabilitation skills | ||||||||||||||
| Facilitating discussion (non-specific prompts) | 18 | 2.7 | 1 | 0.9 | 11 | 16.4 | 2 | 2.5 | 6 | 9.0 | 3 | 1.6 | 41 | 3.5 |
| Providing feedback not directly related to manual | 19 | 2.9 | 0 | 0 | 2 | 3.0 | 2 | 2.5 | 5 | 7.5 | 0 | 0 | 28 | 2.4 |
| Providing encouragement/reassurance | 24 | 3.7 | 1 | 0.9 | 3 | 4.5 | 3 | 3.8 | 0 | 0 | 6 | 3.2 | 37 | 3.2 |
| Summarising | 3 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0.3 |
| Paraphrasing | 13 | 2.0 | 1 | 0.9 | 1 | 1.5 | 6 | 7.5 | 0 | 0 | 1 | 0.5 | 22 | 1.9 |
| Rehabilitation activities | ||||||||||||||
| Presenting/discussing educational material | 200 | 30.4 | 40 | 34.5 | 19 | 28.4 | 43 | 53.8 | 28 | 41.8 | 39 | 21.0 | 369 | 31.5 |
| Presenting/discussing strategies | 216 | 32.9 | 42 | 36.2 | 11 | 16.4 | 11 | 13.8 | 18 | 26.9 | 122 | 65.6 | 420 | 35.8 |
| Providing general information on memory not related to manual | 35 | 5.36 | 0 | 0 | 1 | 1.5 | 3 | 3.8 | 1 | 1.5 | 2 | 1.1 | 42 | 3.6 |
| Recap of previous session | 47 | 7.2 | 20 | 17.2 | 0 | 0 | 8 | 10.0 | 3 | 4.5 | 1 | 0.5 | 79 | 6.7 |
| Total | 657 | 100 | 116 | 100 | 67 | 100 | 80 | 100 | 67 | 100 | 186 | 100 | 1173 | 100 |
| Category | Site | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 6 | 8 | 9 | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Non-rehabilitation | ||||||||||||||
| Introductions | 7 | 1.1 | 0 | 0 | 2 | 2.2 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 0.8 |
| Social chat | 109 | 16.4 | 25 | 17.1 | 9 | 10.0 | 2 | 4.4 | 14 | 20.0 | 26 | 16.5 | 185 | 15.8 |
| Preparing materials, tasks, etc. | 6 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0.5 |
| Information about sessions, venue, group, etc. | 11 | 1.7 | 1 | 0.7 | 4 | 4.4 | 0 | 0 | 1 | 1.4 | 1 | 0.6 | 18 | 1.5 |
| Hospital visit discussion | 1 | 0.2 | 0 | 0 | 10 | 11.1 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0.9 |
| Describing emotions and coping strategies | 50 | 7.5 | 9 | 6.2 | 3 | 3.3 | 1 | 2.2 | 10 | 14.3 | 1 | 0.6 | 74 | 6.3 |
| Rehabilitation | ||||||||||||||
| Discussing/filling in educational material | 147 | 22.1 | 31 | 21.2 | 32 | 35.6 | 8 | 17.8 | 16 | 22.9 | 48 | 30.4 | 282 | 24.0 |
| Discussing strategies | 216 | 32.5 | 62 | 42.5 | 15 | 16.7 | 12 | 26.7 | 11 | 15.7 | 66 | 41.8 | 382 | 32.6 |
| Asking for information | 10 | 1.5 | 4 | 2.7 | 0 | 0 | 5 | 11.1 | 0 | 0 | 2 | 1.3 | 21 | 1.8 |
| Feedback on home activities | 32 | 4.8 | 9 | 6.2 | 0 | 0 | 4 | 8.9 | 13 | 18.6 | 11 | 7.0 | 69 | 5.9 |
| Describing problems related to memory | 75 | 11.3 | 5 | 3.4 | 15 | 16.7 | 13 | 28.9 | 5 | 7.1 | 3 | 1.9 | 116 | 9.9 |
| Total | 664 | 100 | 146 | 100 | 90 | 100 | 45 | 100 | 70 | 100 | 158 | 100 | 1173 | 100 |
Appendix 11 Baseline characteristics and assessments completed according to primary outcome completion and allocated trial arm
| Characteristic/assessment | Trial arm | |||
|---|---|---|---|---|
| Usual care | Memory rehabilitation | |||
| No primary outcomea (N = 35) | Primary outcome (N = 122) | No primary outcomea (N = 42) | Primary outcome (N = 129) | |
| Age (years) | ||||
| Mean (SD) | 45.4 (11.1) | 45 (12.9) | 44.1 (11.6) | 46.3 (11.5) |
| Median (25th, 75th centile) | 46 (38, 54) | 44.5 (35, 55) | 45 (35, 53) | 48 (39, 54) |
| Min., max. | 25, 63 | 19, 69 | 20, 65 | 21, 68 |
| Gender, n (%) | ||||
| Men | 28 (80) | 88 (72) | 30 (71) | 93 (72) |
| Women | 7 (20) | 34 (28) | 12 (29) | 36 (28) |
| Ethnicity, n (%) | ||||
| White | 30 (86) | 117 (96) | 42 (100) | 125 (97) |
| Black | 4 (11) | 2 (2) | 0 | 2 (2) |
| Mixed ethnicity | 0 | 3 (2) | 0 | 1 (1) |
| Other | 1 (3) | 0 | 0 | 1 (1) |
| Residential status, n (%) | ||||
| Lives alone | 8 (23) | 36 (30) | 11 (26) | 32 (25) |
| Lives with others | 25 (71) | 81 (66) | 28 (67) | 92 (71) |
| Living with informal care | 1 (3) | 1 (1) | 0 | 1 (1) |
| Living with formal care | 0 | 2 (2) | 0 | 0 |
| Living in care home | 1 (3) | 2 (2) | 3 (7) | 4 (3) |
| Highest educational attainment, n (%) | ||||
| Below GCSE | 7 (20) | 19 (16) | 10 (24) | 19 (15) |
| GCSE | 17 (49) | 37 (30) | 16 (38) | 33 (26) |
| A Level | 4 (11) | 38 (31) | 8 (19) | 26 (20) |
| Degree | 6 (17) | 18 (15) | 5 (12) | 36 (28) |
| Higher degree | 0 | 10 (8) | 3 (7) | 14 (11) |
| Not known | 1 (3) | 0 | 0 | 1 (1) |
| Current military service, n (%) | ||||
| Military | 3 (9) | 1 (1) | 0 | 0 |
| TA/reservist | 0 | 0 | 0 | 2 (2) |
| Non-military | 32 (91) | 121 (99) | 42 (100) | 127 (98) |
| Previous military service, n (%) | ||||
| Military | 4 (11) | 10 (8) | 3 (7) | 8 (6) |
| TA/reservist | 1 (3) | 1 (1) | 2 (5) | 2 (2) |
| Non-military | 30 (86) | 111 (91) | 37 (88) | 119 (92) |
| TBI during service, n (%) | 2 (6) | 1 (1) | 0 | 1 (1) |
| Time since TBI (months) | ||||
| Mean (SD) | 103.9 (139.3) | 97.6 (107.4) | 102.4 (106.5) | 102.7 (116) |
| Median (25th, 75th centile) | 44 (21, 101) | 49 (24, 119) | 68 (24, 151) | 55 (27, 139) |
| Min., max. | 4, 491 | 4, 520 | 4, 410 | 3, 587 |
| Length of initial hospital stay for TBI (days) | ||||
| Mean (SD) | 61.2 (95.5) | 87.7 (111.7) | 97.4 (200.5) | 83 (120.5) |
| Median (25th, 75th centile) | 21 (7, 63) | 36 (7, 122) | 35 (9, 84) | 38 (11, 120) |
| Min., max. | 1, 468 | 0, 465 | 0, 999 | 0, 999 |
| n | 33 | 115 | 39 | 121 |
| Length of hospital stay unknown, n | 2 | 7 | 3 | 8 |
| EMQ-p score – frequency of problems | ||||
| Mean (SD) | 54.1 (26.5) | 48.9 (23.9) | 51.9 (20.5) | 45.9 (21) |
| Median (25th, 75th centile) | 53 (33, 75.4) | 48 (32, 64) | 56 (43, 68) | 44 (30, 60) |
| Min., max. | 12, 105 | 0, 105 | 8, 93 | 5, 102 |
| n | 35 | 121 | 42 | 129 |
| EMQ-p score – importance of problems | ||||
| Mean (SD) | 76.7 (18.3) | 68.8 (23.2) | 66.7 (19.8) | 65.4 (24.6) |
| Median (25th, 75th centile) | 80.9 (67, 89) | 70.5 (54, 85) | 69.5 (51, 81.9) | 69 (51.5, 84) |
| Min., max. | 27, 108 | 2, 112 | 5, 101 | 0, 112 |
| n | 34 | 118 | 42 | 128 |
| RBMT-3 GMI score | ||||
| Mean (SD) | 73.5 (13.8) | 77.2 (14.6) | 74 (12) | 78.8 (13.9) |
| Median (25th, 75th centile) | 71 (63, 84) | 76 (66, 86) | 74.5 (65, 83) | 78 (68, 85) |
| Min., max. | 53, 106 | 53, 114 | 53, 98 | 56, 127 |
| n | 35 | 122 | 42 | 129 |
| GHQ-30 score | ||||
| Mean (SD) | 39.4 (17.7) | 34.2 (15.8) | 34.7 (14.5) | 36.6 (15.7) |
| Median (25th, 75th centile) | 36.5 (27, 52) | 32.5 (21, 45) | 32.5 (24, 39) | 34.5 (25, 45.3) |
| Min., max. | 10, 90 | 6, 82 | 6, 70 | 10, 84 |
| n | 34 | 120 | 42 | 128 |
| Estimated premorbid IQ (NART) | ||||
| Mean (SD) | 105.4 (9.5) | 106.9 (10.1) | 106 (10.2) | 108.8 (10.1) |
| Median (25th, 75th centile) | 102 (100, 116) | 103 (99, 117) | 103 (100, 114) | 112 (100, 117) |
| Min., max. | 89, 121 | 86, 126 | 88, 124 | 87, 128 |
| n | 33 | 122 | 42 | 128 |
| SST score | ||||
| Mean (SD) | 19.4 (0.9) | 19.3 (0.9) | 19.1 (1.0) | 19.5 (0.8) |
| Median (25th, 75th centile) | 20 (19, 20) | 20 (19, 20) | 19 (19, 20) | 20 (19, 20) |
| Min., max. | 17, 20 | 17, 20 | 17, 20 | 17, 20 |
| n | 35 | 122 | 42 | 129 |
| Level of memory impairment based on RBMT-3 score, n (%) | ||||
| Significant memory impairment (≤ 69) | 16 (46) | 45 (37) | 15 (36) | 35 (27) |
| Borderline/moderate memory impairment (70–84) | 11 (31) | 43 (35) | 18 (43) | 59 (46) |
| Average range or above average range (≥ 85) | 8 (23) | 34 (28) | 9 (21) | 35 (27) |
| Relative/friend agreed to participate in trial, n (%) | 17 (49) | 83 (68) | 27 (64) | 83 (64) |
| EMQ-r score – frequency of problems | ||||
| Mean (SD) | 53 (28) | 45.2 (23.7) | 56.8 (29.7) | 37.4 (26.6) |
| Median (25th, 75th centile) | 55.5 (24, 77.6) | 41 (27, 58.2) | 62 (30, 83) | 32.3 (17, 58.5) |
| Min., max. | 10, 98 | 0, 107 | 6.2, 108 | 0, 104 |
| n | 14 | 81 | 25 | 80 |
| EMQ-r score – importance of problems | ||||
| Mean (SD) | 78.3 (14.6) | 70.2 (25.9) | 74.5 (19.5) | 70.8 (21.6) |
| Median (25th, 75th centile) | 80.8 (67.4, 88) | 76 (58, 87) | 71 (63, 88.4) | 72.6 (60, 87) |
| Min., max. | 52.7, 104 | 0, 112 | 33, 112 | 4, 112 |
| n | 15 | 75 | 27 | 74 |
Appendix 12 Unblinding of outcome assessors
| Variable | Follow-up time point | |||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||
| Usual care (N = 133), n (%) | Memory rehabilitation (N = 143), n (%) | Kappa statistic | Usual care (N = 124), n (%) | Memory rehabilitation (N = 132), n (%) | Kappa statistic | |
| Unblinded prior to the visit | 9 (7) | 35 (24) | 9 (7) | 28 (21) | ||
| Unblinded during the visit | 16 (12) | 52 (36) | 7 (6) | 36 (27) | ||
| Unblinded prior to and/or during the visit | 21 (16) | 64 (45) | 13 (10) | 42 (32) | ||
| Opinion of treatment allocation prior to goal assessment | 0.24 | 0.26 | ||||
| Definitely control | 9 (7) | 0 | 7 (6) | 1 (1) | ||
| Probably control | 109 (82) | 92 (64) | 111 (90) | 90 (68) | ||
| Probably intervention | 15 (11) | 19 (13) | 4 (3) | 14 (11) | ||
| Definitely intervention | 0 | 32 (22) | 2 (2) | 27 (20) | ||
| Opinion of treatment allocation after goal assessment | 0.42 | 0.46 | ||||
| Definitely control | 21 (16) | 2 (1) | 9 (7) | 2 (2) | ||
| Probably control | 97 (73) | 64 (45) | 108 (87) | 61 (46) | ||
| Probably intervention | 14 (11) | 17 (12) | 6 (5) | 28 (21) | ||
| Definitely intervention | 1 (1) | 60 (42) | 1 (1) | 41 (31) | ||
Appendix 13 Relative/friend questionnaire booklet return
| Variable | Follow-up time point | |||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | |||||
| Usual care (N = 100), n (%) | Memory rehabilitation (N = 110), n (%) | Total (N = 210), n (%) | Usual care (N = 100), n (%) | Memory rehabilitation (N = 110), n (%) | Total (N = 210), n (%) | |
| Questionnaire bookleta | ||||||
| Returned | 73 (73) | 71 (65) | 144 (68) | 61 (61) | 70 (64) | 131 (62) |
| Not returned | 21 (21) | 27 (25) | 48 (22) | 24 (24) | 22 (20) | 46 (22) |
| Participant discontinued | 6 (6) | 12 (11) | 18 (8) | 15 (15) | 18 (16) | 33 (16) |
| Days to completion from randomisation | ||||||
| Median (25th, 75th centile) | 171 (167, 179) | 174 (168, 184) | 173 (167, 181) | 352 (349, 360) | 358 (353, 365) | 355 (350, 363) |
| Min., max. | 161, 277 | 156, 268 | 156, 277 | 341, 433 | 345, 420 | 341, 433 |
| Questionnaire completed within 3 months of due dateb | 72 (72) | 71 (65) | 143 (68) | 61 (61) | 70 (64) | 131 (62) |
Appendix 14 Sensitivity analyses for the primary outcome
FIGURE 15.
Sensitivity analysis for the difference in mean EMQ-p score at the 6-month follow-up.
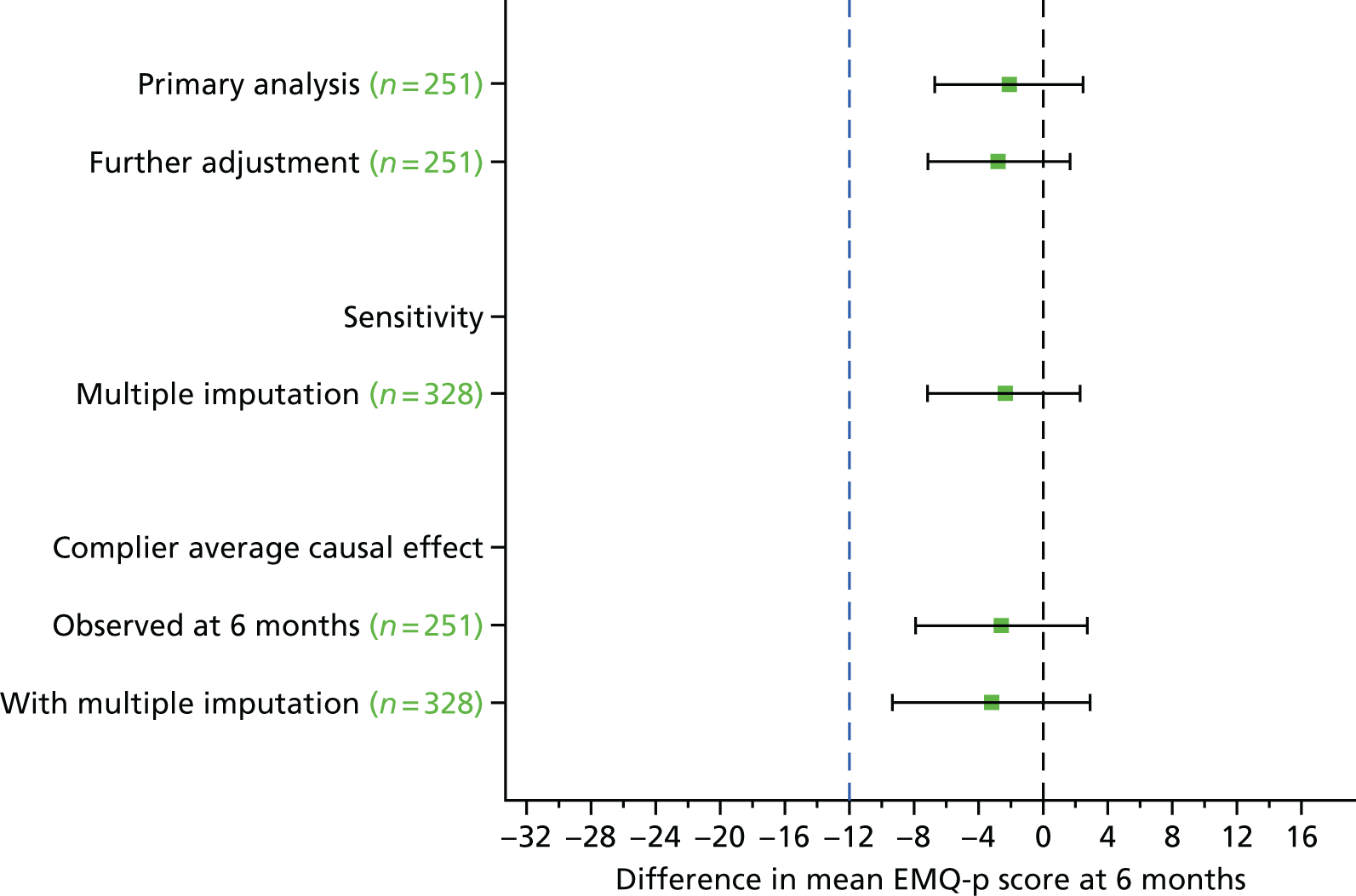
| Trial arm | Time point, mean (SD) | Adjusted difference in means (95% CI) | |
|---|---|---|---|
| Baseline | 6 months | ||
| Usual care (n = 122) | 21.7 (8.1) | 20.0 (8.3) | |
| Memory rehabilitation (n = 129) | 21.3 (6.8) | 17.7 (9.5) | –1.2 (–3.0 to 0.5) |
Appendix 15 Subgroup analyses conducted on baseline memory impairment
FIGURE 16.
Difference in mean EMQ-p scores at the 6-month follow-up on the basis of impairment at baseline.

Appendix 16 Subgroup analyses conducted on time since traumatic brain injury
Note: this subgroup analysis was not prespecified in the SAP.
The categories for time since TBI were discussed at a trial management meeting prior to analysis. The categories initially proposed were quartiles, that is, ≤ 2 years, 2–4 years, 4–10 years and > 10 years. However, it was felt that there was a minimal clinical difference between those at 2 years post injury and those at 4 years post injury. Three categories were therefore agreed: ≤ 2 years, > 2 years to 10 years and > 10 years.
| Subgroup | Time point, mean (SD) | Adjusted difference in means (95% CI) | Adjusted interaction effect (95% CI) | |
|---|---|---|---|---|
| Baseline | 6 months | |||
| ≤ 2 years since TBI | ||||
| Usual care (n = 31) | 50.0 (22.5) | 43.1 (28.0) | ||
| Memory rehabilitation (n = 30) | 43.4 (20.4) | 34.3 (25.8) | –2.1 (–10.9 to 6.7) | |
| > 2 years to 10 years since TBI | ||||
| Usual care (n = 61) | 46.6 (23.9) | 42.7 (24.1) | ||
| Memory rehabilitation (n = 58) | 41.6 (20.5) | 34.5 (24.0) | –4.9 (–11.3 to 1.6) | –2.8 (–13.5 to 7.9) |
| > 10 years since TBI | ||||
| Usual care (n = 30) | 52.6 (25.7) | 47.8 (22.1) | ||
| Memory rehabilitation (n = 41) | 53.8 (20.5) | 48.1 (27.3) | 1.5 (–6.7 to 9.7) | 3.6 (–8.3 to 15.5) |
Appendix 17 The EMQ-p importance scores at the 6- and 12-month follow-ups
| Time point and trial arm | Score, mean (SD) | Adjusted difference in means (95% CI) | |
|---|---|---|---|
| Baseline | Follow-up | ||
| 6-month follow-up | |||
| Usual care (n = 119) | 69.5 (23.0) | 73.9 (24.3) | |
| Memory rehabilitation (n = 124) | 65.5 (24.9) | 68.7 (25.8) | –4.0 (–9.8 to 1.8) |
| 12-month follow-up | |||
| Usual care (n = 106) | 69.9 (23.9) | 73.6 (25.2) | |
| Memory rehabilitation (n = 123) | 66.9 (23.2) | 67.9 (24.5) | –4.3 (–10.3 to 1.7) |
Appendix 18 Analysis of goal attainment for SMART goals
| Summary | 6 months | 12 months | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | Adjusted difference in means (95% CI) | n | Mean (SD) | Adjusted difference in means (95% CI) | |
| Short-term goal attainment average score | ||||||
| Usual care | 126 | 1.2 (1.1) | 119 | 1.6 (1.1) | ||
| Memory rehabilitation | 126 | 1.8 (1.0) | 0.6 (0.3 to 0.9) | 117 | 1.9 (1.0) | 0.2 (0.0 to 0.5) |
| Long-term goal attainment average score | ||||||
| Usual care | 123 | 1.0 (0.9) | 117 | 1.3 (1.0) | ||
| Memory rehabilitation | 121 | 1.5 (1.0) | 0.6 (0.3 to 0.8) | 114 | 1.7 (1.0) | 0.4 (0.1 to 0.7) |
Appendix 19 The EMQ-r importance scores at the 6- and 12-month follow-ups
| Time point and trial arm | Score, mean (SD) | Adjusted difference in means (95% CI) | |
|---|---|---|---|
| Baseline | Follow-up | ||
| 6-month follow-up | |||
| Usual care (n = 64) | 68.2 (27.8) | 71.8 (25.2) | |
| Memory rehabilitation (n = 69) | 69.4 (22.0) | 68.7 (25.3) | –4.8 (–11.2 to 1.6) |
| 12-month follow-up | |||
| Usual care (n = 57) | 69.5 (27.6) | 65.3 (28.2) | |
| Memory rehabilitation (n = 64) | 69.7 (21.4) | 64.5 (26.4) | –0.6 (–8.1 to 6.9) |
Appendix 20 Unit costs associated with service resource use
| Resource input | Unit cost (£) | Source | Notes |
|---|---|---|---|
| Community-based health care | |||
| GP surgery visit (per surgery consultation lasting 9.22 minutes) | 36 | PSSRU 2016 (p. 145)85 | |
| GP telephone consultation (per telephone consultation lasting on average 4 minutes) | 14.60 | PSSRU 2016 (p. 147)85 | |
| GP home visit (per out-of-surgery visit lasting 23.4 minutes) | 120 | PSSRU 2013 (p. 191)85 (inflated to 2016 prices using Bank of England inflation calculator) | |
| Practice nurse surgery consultation | 43 | PSSRU 2016 (p. 143)85 | |
| Surgery nurse telephone consultation (per telephone consultation lasting on average 6.56 minutes) | 7.90 | PSSRU 2016 (p. 147)85 | |
| Specialist nurse (Agenda for Change band 7) | 52 | PSSRU 2016 (p. 142)85 | |
| Community pharmacist | 42 | PSSRU 2016 (p. 201)85 | This cost is based on ‘Cost per working hour’ from the PSSRU. It was assumed that this would cover the cost of prescribing as well as any possible consultations |
| Community physiotherapist | 32 | PSSRU 2016 (p. 200)85 | |
| Community occupational therapist | 32 | PSSRU 2016 (p. 200)85 | |
| Community speech and language therapist | 32 | PSSRU 2016 (p. 200)85 | |
| Podiatrist | 32 | PSSRU 2016 (p. 200)85 | |
| Dietitian | 32 | PSSRU 2016 (p. 200)85 | |
| Clinical psychologist (per hour of client contact) | 74 | PSSRU 2016 (p. 201)85 | |
| Assistant clinical psychologist (per hour of client contact) | 32 | PSSRU 2016 (p. 201)85 | |
| Psychiatrist | 74 | PSSRU 2016 (p. 201) | |
| Elective surgery as inpatient | |||
| Muscular, Balance, Cranial or Peripheral Nerve Disorders, Epilepsy or Head Injury, with CC Score 15+ | 7605 | NHS Reference Costs 2015 to 2016 (AA26C)87 | |
| Muscular, Balance, Cranial or Peripheral Nerve Disorders, Epilepsy or Head Injury, with CC Score 6–8 | 2932 | NHS Reference Costs 2015 to 2016 (AA26F)87 | |
| Headache, Migraine or Cerebrospinal Fluid Leak, with CC Score 0–6 | 1672 | NHS Reference Costs 2015 to 2016 (AA31E)87 | |
| Minor, Cataract or Lens Procedures | 919 | NHS Reference Costs 2015 to 2016 (BZ33Z)87 | |
| Major, Mouth or Throat Procedures, 19 years and over, with CC Score 2+ | 2951 | NHS Reference Costs 2015 to 2016 (CA83A)87 | |
| Complex Maxillofacial Procedures with CC Score 0 | 5507 | NHS Reference Costs 2015 to 2016 (CA91B)87 | |
| Reduction or Fixation, of Jaw | 2854 | NHS Reference Costs 2015 to 2016 (CA96Z)87 | |
| Pulmonary, Pleural or Other Tuberculosis, without Interventions, with CC Score 0–2 | 1923 | NHS Reference Costs 2015 to 2016 (DZ14J)87 | |
| Unspecified Chest Pain with CC Score 11+ | 1345 | NHS Reference Costs 2015 to 2016 (EB12A)87 | |
| Major General Abdominal Procedures, 1 year and under, with CC Score 0–1 | 5090 | NHS Reference Costs 2015 to 2016 (FZ12U)87 | |
| Low Back Pain without Interventions, with CC Score 0–2 | 1510 | NHS Reference Costs 2015 to 2016 (HC32K)87 | |
| Foot Fracture with Single Intervention, with CC Score 0–1 | 5544 | NHS Reference Costs 2015 to 2016 (HE31C)87 | |
| Intermediate Knee Procedures for Non-Trauma, 19 years and over, with CC Score 0–1 | 2647 | NHS Reference Costs 2015 to 2016 (HN24C)87 | |
| Non-Malignant Prostate Disorders without Interventions, with CC Score 6 + | 1926 | NHS Reference Costs 2015 to 2016 (LB28E)87 | |
| Major Open, Scrotum, Testis or Vas Deferens Procedures, with CC Score 0–1 | 2638 | NHS Reference Costs 2015 to 2016 (LB52B)87 | |
| Labour without Specified Delivery | 1110 | NHS Reference Costs 2015 to 2016 (NZ25Z)87 | |
| Allergy or Adverse Allergic Reaction | 1288 | NHS Reference Costs 2015 to 2016 (WH05Z)87 | |
| Unspecified Oedema with CC Score 2+ | 2360 | NHS Reference Costs 2015 to 2016 (WH10A) | |
| Percutaneous Single Drainage of Abdominal Abscess, with CC Score 2–4 | 5226 | NHS Reference Costs 2015 to 2016 (YF04B)87 | |
| Open Arteriovenous Fistula, Graft or Shunt Procedures | 2451 | NHS Reference Costs 2015 to 2016 (YQ42Z)87 | |
| Non-elective surgery as an inpatient | |||
| Muscular, Balance, Cranial or Peripheral Nerve Disorders, Epilepsy or Head Injury, with CC Score 15+ | 6687 | NHS Reference Costs 2015 to 2016 (AA26C)87 | The SUQ does not distinguish between elective and non-elective surgery; therefore, assumptions were made based on the description given by the participant, the assumed severity given the description and the length of stay provided. All three of these considerations were used to estimate whether surgery was elective or non-elective |
| Liver Failure Disorders with Multiple Interventions | 6199 | NHS Reference Costs 2015 to 2016 (GC01C)87 | |
| Very Major Knee Procedures for Non-Trauma with CC Score 0–1 | 6692 | NHS Reference Costs 2015 to 2016 (HN22E)87 | |
| Allergy or Adverse Allergic Reaction (non-elective long stay) | 1298 | NHS Reference Costs 2015 to 2016 (WH05Z)87 | |
| Unspecified Pain with CC Score 0 | 1537 | NHS Reference Costs 2015 to 2016 (WH08B)87 | |
| Tendency to Fall, Senility or Other Conditions Affecting Cognitive Functions, without Interventions, with CC Score 0–1 | 1847 | NHS Reference Costs 2015 to 2016 (WH09G)87 | |
| Fever of Unknown Origin without Interventions, with CC Score 0–3 | 1576 | NHS Reference Costs 2015 to 2016 (WJ07D)87 | |
| Allergy or Adverse Allergic Reaction (non-elective short stay) | 375 | NHS Reference Costs 2015 to 2016 (WH05Z)87 | |
| Outpatient | |||
| General surgery | 130 | NHS Reference Costs 2015 to 2016 87 | Because of ambiguous descriptions of outpatient visits (e.g. ‘gone to hospital’), this figure was used to capture items not able to be individually costed |
| Medications | |||
| Prescribed medication | Various | BNF86 | Various costs. See Appendix 21 for full list of medication |
Appendix 21 Unit costs associated with medication use
| Drug | Dose (per oral tablet unless other stated) | Cost (£) |
|---|---|---|
| Aladronic acid | 10 mg | 0.96 |
| Allopurinal (AAH Pharmaceuticals Ltd) | 100 mg | 1.12 |
| Amitriptyline (AAH Pharmaceuticals Ltd) | 10 mg | 0.96 |
| Amiodarone (AHH Pharmaceuticals Ltd) | 100 mg | 1.29 |
| Amlodipine (AAH Pharmaceuticals Ltd) | 10 mg | 0.91 |
| Amoxicillin (AAH Pharmaceuticals Ltd) | 250 mg | 1.30 |
| Mesalazine (Asacol®, MR Allergan Ltd) | 400 mg | 26.72 |
| Ascorbic acid | 200 mg | 18.59 |
| Aspirin (AAH Pharmaceuticals Ltd) | 75 mg | 0.81 |
| Salbutamol Airomer 100 ml (100 mg per 1 dose) (Autoinhalver®, Teva UK Ltd) | 200 doses | 7.87 |
| Atenolol (AA Pharmaceuticals) | 50 mg | 0.87 |
| Atorvastatin (AAH Pharmaceuticals Ltd) | 10 mg | 1.57 |
| Avistatin 10 mg | 1.57 | |
| Azathioprine (AAH Pharmaceuticals Ltd) | 25 mg | 3.24 |
| Baclofen (AAH Pharmaceuticals Ltd) | 10 mg | 1.92 |
| Beclometasone 50 mg/dose (AAH Pharmaceuticals Ltd) | 200 doses | 5.36 |
| Bendroflumethiazide (AAH Pharmaceuticals Ltd) | 2.5 mg | 0.80 |
| Bezafibrate (AAH Pharmaceuticals Ltd) | 200 mg | 3.25 |
| Bisoprolol (Sandoz Ltd) | 1.25 mg | 0.91 |
| Buprenorphine (Actavis UK Ltd) | 440 mcg | 1.60 |
| Buscopan (Sanofi) | 10 mg | 3.00 |
| Buprenorphine (Butrans® 5 mcg/hour transdermal patches, Napp Pharmaceuticals Ltd) | 1 patch | 17.60 |
| Beclometasone 50 mcg/dose (Dipropionate Nasobec Aqueous® 50 mcg/dose nasal spray, Teva UK Ltd) | 2.49 | |
| Clobetasone Buyrate (Eumovate® 0.05% ointment, GlaxoSmithKline UK Ltd) | 500 mcg per 1 g | 1.86 |
| Calcium carbonate (Adcal-D3®, Kyowa Kirin Ltd) | 1500 mg | 3.65 |
| Cabergoline (AAH Pharmaceuticals Ltd) | 500 mcg | 34.93 |
| Carbamazepine (Tegretol®, Novartis Pharmaceuticals UK Ltd) | 110 mg | 2.51 |
| Co-amilofruse (AAH Pharmaceuticals Ltd) | 2.5 mg/20 mg | 5.57 |
| Co-codamol (Galen Ltd) | 15 mg/500 mg | 6.74 |
| Codeine (AAH Pharmaceuticals Ltd) | 15 mg | 1.44 |
| Colecalciferol (AAH Pharmaceuticals Ltd) | 800 units | 3.60 |
| Colesevelam (Sanofi) | 625 mg | 96.10 |
| Colestyramine (Oral powder sachets, Teva UK Ltd) | 4 g | 31.85 |
| Desogestrel (Cerelle® Consillient Health Ltd) | 75 mcg | 3.17 |
| Cyanocobalamin (AAH Pharmaceuticals Ltd) | 50 mcg | 8.99 |
| Cyclizine (AMCo) | 50 mg | 10.85 |
| Cardicor (Medreich PLC) | 1.25 mg | 2.35 |
| Cerazette® (Merck Sharp and Dohme Ltd) | 75 mcg | 9.55 |
| Certraline (Accord Healthcare Ltd) | 50 mg | 1.83 |
| Cetirizine hydrochloride (Teval UK Ltd) | 10 mg | 1.87 |
| Chlorhexidine [Gluconate Mouthwash (mint) 0.2%, Numark Ltd] | 2 mg per 1 ml | 1.26 |
| Cholecalciferol (AAH Pharmaceuticals Ltd) | 800 unit | £3.60 |
| Cinchocaine 0.5%/hydrocortisone 0.5% (Ultraproct Ointment®, Meadow Laboratories Ltd) | 5 mg per 1 g | 10.34 |
| Cinnarizine (AAH Phamaecuticals Ltd) | 15 mg | 5.59 |
| Citalopram (AAH Pharmaceuticals Ltd) | 10 mg | 1.21 |
| Clomipramine (AAH Pharmaceuticals Ltd) | 10 mg | 1.55 |
| Clonazepam | N/A | 6.94 |
| Clonidine (Sandoz Ltd) | 25 mcg | 10.08 |
| Clopidogrel (Macleods Pharma UK Ltd) | 75 mg | 1.82 |
| Digoxin (Actavis UK Ltd) | 62.5 mcg | 2.44 |
| Dihydrocodeine (AAH Pharmaceuticals Ltd) | 30 mg | 1.43 |
| Diltiazem (Modified-release, AAH Pharmaceuticals Ltd) | 60 mg | 37.91 |
| Dioxepan | 10 mg/2 ml | 3.77 |
| Disulfiram (AAH Pharmaceuticals Ltd) | 200 mg | 31.00 |
| Ducusate (DulcoEase®, Sanofi) | 100 mg | 2.09 |
| Doxazosin (Actavis UK) | 4 mg | 0.90 |
| Duloxetine (Aspire Pharma Ltd) | 60 mg | 27.72 |
| Desmopressin (AAH Pharmaceuticals Ltd) | 200 mcg | 8.76 |
| Tolterodine (Detrusitol® Pfizer Ltd) | 2 mg | 30.56 |
| Diazepam (Teva UK Ltd) | 20 mg | 1.02 |
| Diclofenac (AAH Pharmaceuticals Ltd) | 25 mg | 1.25 |
| Digoxin (Actavis UK Ltd) | 62.5 mcg | 2.44 |
| Enalapril (AAH Pharmaceuticals Ltd) | 10 mg | 1.15 |
| Erythromycin (Phoenix Healthcare Distribution Ltd) | 250 mg | 5.61 |
| Ezetimibe (AMCo) | 10 mg | 26.31 |
| Felodipine (Modified Release, DE Pharmaceuticals) | 5 mg | 4.21 |
| Felodipine (Modified Release, DE Pharmaceuticals) | 2.5 mg | 6.31 |
| Ferrous fumarate (Galfer, Thornton & Ross Ltd) | 305 mg | 2.30 |
| Ferrous sulfate (AAH Pharmaceuticals Ltd) | 200 mg | 1.06 |
| Fexofenadine hydrochloride (AAH Pharmaceuticals Ltd) | 180 mg | 3.65 |
| Finasteride (AAH Pharmaceuticals Ltd) | 5 mg | 1.73 |
| Flucloxacillin Sodium (AAH Pharmaceuticals Ltd) | 550 mg | 2.46 |
| Fluoxetine (Wockhardt UK Ltd) | 20 mg | 1.11 |
| Fluticasone (Accuhaler®) | 50 mcg | 6.38 |
| Folic acid (Intrapharm Laboratories Ltd) | 5 mg | 1.02 |
| Frusemide (AAH Pharmaceuticals Ltd) | 20 mg | 0.83 |
| Gabapentin (Accord Healthcare Ltd) | 600 mg | 4.36 |
| Gliclazide (Almus Pharmaceuticals Ltd) | 80 mg | 2.06 |
| Glucosamine and chondroitin (combined) | N/A | 18.40 |
| Hayfever Tablets | 10 mg | 2.91 |
| Hormone replacement therapy | N/A | 6.52 |
| Hydrocortisone (AMCo) | 10 mg | 78.50 |
| Hydroxychloroquine (Creo Pharma Ltd) | 200 mg | 5.31 |
| Ibuprofen (Wockhardt UK Ltd) | 200 mg | 0.20 |
| Imipramine (AAH pharmaceuticals Ltd) | 10 mg | 1.32 |
| Inhaler | N/A | 18.00 |
| Insulin for sub-cutaneous injection | 100 unit per 1 ml | 30.68 |
| Irbesartan (Macleods Pharma UK Ltd) | 150 mg | 2.72 |
| Levetiracetam (Keppra®, UCB Pharma Ltd) | 250 mg | 28.01 |
| Lacidipine [Alliance Healthcare (Distribution) Ltd] | 2 mg | 2.95 |
| Lactulose oral solution (300 ml) (AAH Pharmaceuticals Ltd) | 680 mg per 1 ml | 1.95 |
| Lamictal (lamotrigine) (GlaxoSmithKline) | 100 mg | 69.04 |
| Lamotrigine (Accord Healthcare Ltd) | 25 mg | 2.21 |
| Lansoprazole (AAH Pharmaceuticals Ltd) | 30 mg | 1.10 |
| Lantus® | N/A | 1.10 |
| Levetiracetam (Aurobino Pharma Ltd) | 250 mg | 7.24 |
| Levothyroxine (AAH Pharmaceuticals Ltd) | 25 mcg | 2.02 |
| Lisinopril (Accord Healthcare Ltd) | 20 mg | 0.89 |
| Loperamide (Norimode®, Tillomed Laboratories Ltd) | 2 mg | 2.15 |
| Loratadine | 10 mg | 1.06 |
| Losartan potassium (Mylan) | 25 mg | 1.15 |
| Lymecyline capsule (AAH Pharmaceuticals Ltd) | 408 mg | 8.58 |
| Lactulose oral solution (300 ml) (AAH Pharmaceuticals Ltd) | 680 mg per 1 ml | 1.95 |
| Lamictal (lamotrigine) (GlaxoSmithKline) | 100 mg | 69.04 |
| Lamotrigine (Accord Healthcare Ltd) | 25 mg | 2.21 |
| Lansoprazole (AAH Pharmaceuticals Ltd) | 30 mg | 1.10 |
| Lantus® | N/A | 1.10 |
| Levetiracetam (Aurobino Pharma Ltd) | 250 mg | 7.24 |
| Levothyroxine (AAH Pharmaceuticals Ltd) | 25 mcg | 2.02 |
| Lisinopril (Accord Healthcare Ltd) | 20 mg | 0.89 |
| Loperamide (Norimode®, Tillomed Laboratories Ltd) | 2 mg | 2.15 |
| Loratadine | 10 mg | 1.06 |
| Mebeverine (Alliance Health Care Distribution Ltd) | 135 mg | 4.22 |
| Metformin modified release (Almus Pharmaceuticals Ltd) | 500 mg | 2.66 |
| Methotrexate (Sandoz Ltd) | 2.5 mg | 2.40 |
| Metronidazole | N/A | 15.99 |
| Mirtazapine (AAH Pharmaceuticals Ltd) | 15 mg | 2.03 |
| Montelukast (Actavis UK Ltd) | 10 mg | 2.41 |
| Mebeverine (Alliance Health Care Distribution Ltd) | 135 mg | 4.22 |
| Metformin modified release (Almus Pharmaceuticals Ltd) | 500 mg | 2.66 |
| Methotrexate (Sandoz Ltd) | 2.5 mg | 2.40 |
| Metronidazole | N/A | 15.99 |
| Mirtazapine (AAH Pharmaceuticals Ltd) | 15 mg | 2.03 |
| Montelukast (Actavis UK Ltd) | 10 mg | 2.41 |
| Mebeverine (Alliance Health Care Distribution Ltd) | 135 mg | 4.22 |
| Mesalazine (Pentesa® Suppositories Ferring Pharmaceuticals Ltd) | 1 g | 36.89 |
| Nortriptyline (Focus Pharmaceuticals Ltd) | 10 mg | 12.06 |
| Naproxen (Actavis UK Ltd) | 250 mg | 1.54 |
| Neproxin (AAH Pharmaceuticals) | 500 mg | 1.12 |
| Nicorandil [Alliance Healthcare (Distribution) Ltd] | 20 mg | 3.66 |
| Nicotine | N/A | 7.54 |
| Nifedipine | N/A | 1.74 |
| Nitrazepam | 5 mg | 1.74 |
| Neproxin (AAH Pharmaceuticals) | 500 mg | 1.12 |
| Nicorandil [Alliance Healthcare (Distribution) Ltd] | 20 mg | 3.66 |
| Nitrofurantion | 100 mg | 3.53 |
| Olanzapine | 2.5 mg | 1.13 |
| Omeprazole gastro-resistant (Tillomed Laboratories Ltd) | 20 mg | 13.80 |
| Oxcarbazepine (AAH Pharmaceuticals Ltd) | 150 mg | 9.29 |
| Oxybutynin (AAH Pharmaceuticals Ltd) | 5 mg | 2.06 |
| Oxycodone hydrochloride 5 mg (Ethypharm UK Ltd) | 11.43 | |
| OxyNorm (Hydrochloride Oxeltra® modified release, Wockhardt UK Ltd) | 10 mg | 22.86 |
| Oxytetracycline (Cresent Pharma Ltd) | 250 mg | 1.14 |
| Pantoprazole (Sandoz Ltd) | 40 mg | 1.18 |
| Paracetamol | 500 mg | 0.19 |
| Paroxetine (AAH Pharmaceuticals Ltd) | 20 mg | 2.70 |
| Phenytoin sodium (AAH Pharmaceuticals Ltd) | 100 mg | 15.98 |
| Phenytoin sodium | 100 mg | 57.38 |
| Pizotifen (Soverign Medical Ltd) | 1.5 mg | 7.22 |
| Pramipexole | 180 mcg | 2.25 |
| Pravastatin (Mylan) | 10 mg | 1.85 |
| Prednisolone steroid (AAH Pharmaceuticals Ltd) | 2.5 mg | 1.24 |
| Prednisolone [Strides Shausn (UK) Ltd] | 1 mg | 1.07 |
| Pregabalin (Lyrica®, Pfizer Ltd) | 20 mg | 64.40 |
| Prochlorperazine (Alliance Healthcare Ltd) | 5 mg | 1.12 |
| Propranolol Bedranol (Almus Pharmaceuticals Ltd) | 80 mg | 3.22 |
| Propiverine Hydrochloride (Detrunorm®, AMCo) | 15 mg | 18.00 |
| Propranolol (AAH Pharmaceuticals Ltd) | 10 mg | 1.45 |
| Quetiapine | N/A | 1.44 |
| Quinine sulphate (AAH Pharmaceuticals) | 200 mg | 2.17 |
| Ranitidine (Accord Healthcare Ltd) | 150 mg | 1.37 |
| Ramipril [Alliance Healthcare (Distrbution) Ltd] | 1.25 mg | 1.07 |
| Risperidone (AAH Pharmceuticals Ltd) | 4 mg | 1.76 |
| Rivaroxaban (Xarelto®, Bayer Plc) | 10 mg | 58.80 |
| Rosuvastatin Tablets (AAH Pharmaceuticals) | 10 mg | 18.03 |
| Salbutamol (Inhaler 100 mcg/dose, AAH Pharmaceuticals Ltd) | 200 doses | 1.91 |
| Senna (Senokot® Forum Health Products) | 7.5 mg/5 ml | 3.99 |
| Fluticasone with Salmeterol (Seretide 100® Accuhaler 100 mcg per 1 dose, GlaxoSmithKline UK Ltd) | 60 doses | 18.00 |
| Sertraline (Accord Healthcare Ltd) | 50 mg | 1.83 |
| Simvastatin (Brown and Burk UK Ltd) | 20 mg | 2.02 |
| Sitagliptin with Metformin (Janumet® 50 mg/1000 mg Merck Sharp & Dohme Ltd) | 1 g/50 mg | 33.26 |
| Sodium valporate Gastro-resistant (Alliance Healthcare Distribution Ltd) | 200 mg | 4.75 |
| Sodium Valporate (Epilim®, Sanofi) | 200 mg | 7.70 |
| Solifenacin (Vesicare®, Astellas Pharma Ltd) | 5 mg | 27.62 |
| Co-codamol (Solpadol®, Sanofi) | 30 mg/500 mg | 2.20 |
| Statins | N/A | 0.84 |
| Sublingual tablets (Buprenorphine with Naloxone Suboxone®, Indivicor UK Ltd) | 8 mg/2 mg | 76.19 |
| Sumatriptan (Mylan) | 50 mg | 1.61 |
| Symbicort Inhaler & Ventolin | 100 mcg/6 mcg/dose | 33.00 |
| Tamsulosin 400 mcg (Contiflo XL, Ranbaxy UK Ltd) | 400 mcg | 4.47 |
| Tegretol Carbamazepine (Novartis Pharmaceuticals UK Ltd) | 400 mg | 5.02 |
| Temazepam | N/A | 10.70 |
| Thiamine (Kent Pharmaceuticals Ltd) | 100 mg | 10.13 |
| Levothyroxine (Teva UK Ltd) | 25 mcg | 2.02 |
| Temazepam (AAH Pharmaceuticals) | 10 mg | 10.70 |
| Topiramate | 100 mg | 2.37 |
| Tovias (Fesoterodine fumarate) | 8 mg | 25.78 |
| Tramadol | 50 mg | 1.20 |
| Tranexamic Acid | 500 mg | 6.73 |
| Trazodone (Focus Pharmaceuticals Ltd) | 150 mg | 30.34 |
| Ticagrelor (Brilique, AstraZeneca) | 60 mg | 54.60 |
| Trihexyphenidyl | 2 mg | 8.27 |
| Trileptal (Oxcarbazepine, Novartis Pharmaceuticals UK Ltd) | 150 mg | 12.24 |
| Tylex (Co-codamol, UCB Pharma Ltd) | 30 mg/500 mg | 9.06 |
| Venlafaxine (Venaxx XL, AMCo) | 75 mg | 10.44 |
| Venlafaxine hydrochloride (Venlalic XL) | 75 mg | 2.35 |
| Ventolin | 100 mcg/dose | 1.50 |
| Sildenafil (Viagra, Pfizer Ltd) | 25 mg | 16.59 |
| Vitamin B | N/A | 1.95 |
| Vitamin D | N/A | 8.99 |
| Valproic Acid (Depakote® Imported United States) | 125 mg | 17.08 |
| Vitamins with Minerals and Trace elements (Ketovite® Tablets Essential Pharmaceuticals Ltd) | N/A | 9.21 |
| Warfarin | 1 mg | 0.31 |
| Zinc sulfate monohydrate (Effervescent Tablets Sugar Free) | 125 mg | 4.32 |
| Zolmitriptan | 5 mg | 1.22 |
| Zolpidem | 7.5 mg | 1.19 |
| Zopiclone | 7.5 mg | 1.17 |
Appendix 22 Illustration of the cost-effectiveness plane
FIGURE 17.
Illustration of the cost-effectiveness plane.
Appendix 23 Markov model
FIGURE 18.
Markov model structure.
Appendix 24 Model parameters
From Brooks et al. ,96 for the low and moderate categories of GHQ-30 scores, the SMR was defined by the ‘walks well’ category whereas the ‘some walking’ SMR was used for those with high GHQ-30 scores. As detailed by Briggs et al. ,151 to transform probabilities between periods of differential length, the derivation of instantaneous rates from which the appropriate probabilities can be calculated is required. To convert the yearly SMR adjusted mortality probability presented by Brooks et al. ,96 for a cycle length of 6 months, the following formulae were used, where pn and rn are the n-month probability and rate of death, respectively:
Using these formulae, the 12-month mortality probability was transformed to the monthly death rate, which was subsequently transformed back to a 6-month probability. The adjusted 6-month mortality rates are presented in Table 49.
| Age (years) | Unadjusted mortality rate (1 year) | Mortality rates | |||
|---|---|---|---|---|---|
| Low/moderate | High | ||||
| SMR – ‘walks well’ | Adjusted mortality rate (6 months) | SMR – ‘some walking’ | Adjusted mortality rate (6 months) | ||
| 20 | 0.000442 | 3.10 | 0.00058 | 3.80 | 0.00071 |
| 30 | 0.000464 | 3.10 | 0.00092 | 3.80 | 0.00113 |
| 40 | 0.000455 | 2.90 | 0.00189 | 4.50 | 0.00294 |
| 50 | 0.000530 | 2.90 | 0.00414 | 4.50 | 0.00643 |
| 60 | 0.000527 | 1.40 | 0.00500 | 2.70 | 0.00966 |
| 70 | 0.000557 | 1.40 | 0.01215 | 2.70 | 0.02357 |
| 80 | 0.000594 | 0.70 | 0.01808 | 1.50 | 0.03916 |
| 90 | 0.000608 | 0.70 | 0.05733 | 1.50 | 0.12745 |
| 100 | 0.000597 | 0.70 | 0.13712 | 1.50 | 0.32723 |
Given the adjusted mortality rates presented in Table 49, Table 50 defines the baseline transition probabilities for an individual aged 45 years.
| GHQ-30 category | Trial arm | Distribution | Source | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Memory rehabililation | Usual care | |||||||||
| Low | Moderate | High | Death | Low | Moderate | High | Death | |||
| Transition probability: 0–6 months | ||||||||||
| Low GHQ-30 | 0.715 | 0.188 | 0.094 | 0.003 | 0.716 | 0.239 | 0.043 | 0.002 | Beta | Trial data, Brooks et al.,96 ONS94 |
| Moderate GHQ-30 | 0.347 | 0.499 | 0.152 | 0.003 | 0.288 | 0.466 | 0.244 | 0.002 | Beta | Trial data, Brooks et al.,96 ONS94 |
| High GHQ-30 | 0.159 | 0.358 | 0.478 | 0.004 | 0.199 | 0.299 | 0.498 | 0.004 | Beta | Trial data, Brooks et al.,96 ONS94 |
| Death | 0.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 0.000 | 1.000 | Beta | Trial data, Brooks et al.,96 ONS94 |
| Transition probability: 6–12 months | ||||||||||
| Low GHQ-30 | 0.802 | 0.117 | 0.078 | 0.003 | 0.696 | 0.162 | 0.139 | 0.002 | Beta | Trial data, Brooks et al.,96 ONS94 |
| Moderate GHQ-30 | 0.332 | 0.526 | 0.138 | 0.003 | 0.374 | 0.561 | 0.062 | 0.002 | Beta | Trial data, Brooks et al.,96 ONS94 |
| High GHQ-30 | 0.111 | 0.387 | 0.498 | 0.005 | 0.124 | 0.373 | 0.498 | 0.004 | Beta | Trial data, Brooks et al.,96 ONS94 |
| Death | 0.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 0.000 | 1.000 | Beta | Trial data, Brooks et al.,96 ONS94 |
For the three non-dead health states, the mean costs and utilities and associated SEs are presented in Table 51.
| GHQ-30 category | Trial arm | Distribution | Source | |||
|---|---|---|---|---|---|---|
| Memory rehabilitation | Usual care | |||||
| Mean cost (£) | SE (£) | Mean cost (£) | SE (£) | |||
| 0–6 months | ||||||
| Low GHQ-30 | 242.88 | 54.23 | 304.00 | 64.33 | Gamma | Trial data |
| Moderate GHQ-30 | 452.06 | 188.14 | 471.43 | 126.69 | Gamma | Trial data |
| High GHQ-30 | 353.17 | 204.01 | 715.58 | 171.38 | Gamma | Trial data |
| 6–12 months | ||||||
| Low GHQ-30 | 242.88 | 76.26 | 204.14 | 37.95 | Gamma | Trial data |
| Moderate GHQ-30 | 452.06 | 65.36 | 820.75 | 200.75 | Gamma | Trial data |
| High GHQ-30 | 353.17 | 108.56 | 547.90 | 237.74 | Gamma | Trial data |
| 12+ months | ||||||
| Low GHQ-30 | 343.36 | 104.82 | 211.91 | 49.54 | Gamma | Trial data |
| Moderate GHQ-30 | 315.47 | 51.41 | 442.50 | 66.37 | Gamma | Trial data |
| High GHQ-30 | 694.19 | 286.69 | 561.34 | 324.25 | Gamma | Trial data |
| Mean utility | SE | Mean utility | SE | Distribution | Source | |
| 0–6 months | ||||||
| Low GHQ-30 | 0.710 | 0.029 | 0.653 | 0.037 | Beta | Trial data |
| Moderate GHQ-30 | 0.660 | 0.029 | 0.569 | 0.031 | Beta | Trial data |
| High GHQ-30 | 0.443 | 0.047 | 0.461 | 0.046 | Beta | Trial data |
| 6–12 months | ||||||
| Low GHQ-30 | 0.742 | 0.025 | 0.672 | 0.032 | Beta | Trial data |
| Moderate GHQ-30 | 0.626 | 0.017 | 0.559 | 0.027 | Beta | Trial data |
| High GHQ-30 | 0.428 | 0.076 | 0.410 | 0.059 | Beta | Trial data |
| 12+ months | ||||||
| Low GHQ-30 | 0.724 | 0.028 | 0.699 | 0.027 | Beta | Trial data |
| Moderate GHQ-30 | 0.635 | 0.016 | 0.595 | 0.024 | Beta | Trial data |
| High GHQ-30 | 0.442 | 0.063 | 0.530 | 0.056 | Beta | Trial data |
Appendix 25 Costs associated with training and delivery of the intervention
| Costs | Resource | Unit cost (£) | Resource usage | Cost (£) | Total cost (£) | Unit cost source/description |
|---|---|---|---|---|---|---|
| Training costs | Training psychologist band 8a | 66 | 2 hours | 132 | PSSRU 2016,85 bands 5–8 (p. 185) | |
| Training AP (mid-band 5) | 35 | 2 hours | 70 | PSSRU 2016,85 band 5 (p. 185) | ||
| Cost per site | 202 | |||||
| One-off training cost (subtotal) | 1818 | Based on nine sites | ||||
| Delivery costs | ||||||
| Per-group variable costs | Administration staff (band 3) | 25 | 1 hour per group | 25 | PSSRU 2016,85 band 3 (pp. 188 and 199) | |
| Cost per group | 25 | |||||
| Total administration cost for groups (subtotal) | 875 | Based on 35 groups | ||||
| Per-session variable costs | AP (mid-band 5) | 35 | 2 hours per session | 70 | PSSRU 2016,85 band 5 (p. 185) | |
| Total per session | 70 | |||||
| Total cost of sessions (subtotal) | 24,500 | Based on 350 sessions | ||||
| Per-participant variable costs | Cost per manual | 2.20 | One manual per participant | 2.20 | As advised by trial team | |
| Refreshments | 0.50 | £0.50 per participant per session | 5.00 | As advised by trial team | ||
| Stationery costs (e.g. pens, miscellaneous) | 1.00 | Estimated total £1 per participant for all sessions | 1.00 | As advised by trial team | ||
| Total per participant | 8.20 | |||||
| Per-participant costs (subtotal) | 1402 | Based on 171 participants | ||||
| Total cost of intervention | Overall cost of rehabilitation intervention | 28,595 | ||||
| Cost of rehabilitation per participant | 167 | |||||
Appendix 26 Resource utilisation and service usage costs (available cases)
| Resource utilisation | Trial arm | n | Sum | Mean | SD | Difference in means (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Number of GP consultations (linked to memory problems) per patient | Usual care | 130 | 19 | 0.12 | 0.44 | 0.056 (–0.024 to 0.137) | 0.168 |
| Memory rehabilitation | 137 | 11 | 0.06 | 0.29 | |||
| Number of GP consultations (for other reasons) per patient | Usual care | 151 | 283 | 1.80 | 2.05 | 0.311 (–0.168 to 0.790) | 0.202 |
| Memory rehabilitation | 157 | 255 | 1.49 | 2.33 | |||
| Number of practice nurse consultations (linked to memory problems) per patient | Usual care | 129 | 5 | 0.03 | 0.29 | 0.008 (–0.056 to 0.073) | 0.797 |
| Memory rehabilitation | 134 | 4 | 0.02 | 0.31 | |||
| Number of practice nurse consultations (for other reasons) per patient | Usual care | 137 | 68 | 0.43 | 1.01 | –0.456 (–0.954 to 0.042) | 0.073 |
| Memory rehabilitation | 147 | 152 | 0.89 | 3.02 | |||
| Number of consultations with other health-care professionals (linked to memory problems) per patient | Usual care | 131 | 66 | 0.42 | 2.22 | 0.046 (–0.346 to 0.434) | 0.817 |
| Memory rehabilitation | 139 | 64 | 0.37 | 1.31 | |||
| Number of consultations with other health-care professionals (for other reasons) per patient | Usual care | 144 | 349 | 2.22 | 5.23 | 0.0241 (–1.035 to 1.083) | 0.964 |
| Memory rehabilitation | 156 | 376 | 2.20 | 4.52 | |||
| Number of times an inpatient because of memory problems | Usual care | 157 | 0 | 0 | 0 | – | – |
| Memory rehabilitation | 171 | 0 | 0 | 0 | |||
| Number of days an inpatient because of memory problems | Usual care | 157 | 0 | 0 | 0 | – | – |
| Memory rehabilitation | 171 | 0 | 0 | 0 | |||
| Number of times an inpatient for other reasons | Usual care | 134 | 11 | 0.07 | 0.28 | –0.006 (–0.070 to 0.058) | 0.855 |
| Memory rehabilitation | 139 | 13 | 0.08 | 0.31 | |||
| Number of days an inpatient for other reasons | Usual care | 24 | 33 | 0.21 | 0.99 | –0.094 (–0.447 to 0.259) | 0.601 |
| Memory rehabilitation | 27 | 52 | 0.30 | 2.04 | |||
| Number of GP home consultations (linked to memory problems) per patient | Usual care | 157 | 0 | 0 | 0 | – | – |
| Memory rehabilitation | 171 | 0 | 0 | 0 | |||
| Number of GP home consultations (for other reasons) per patient | Usual care | 129 | 5 | 0.03 | 0.21 | –0.003 (–0.063 to 0.057) | 0.915 |
| Memory rehabilitation | 136 | 6 | 0.04 | 0.32 | |||
| Number of practice nurse home consultations (linked to memory problems) per patient | Usual care | 128 | 0 | 0.00 | 0.00 | –0.006 (–0.018 to 0.006) | 0.339 |
| Memory rehabilitation | 134 | 1 | 0.01 | 0.08 | |||
| Number of practice nurse home consultations (for other reasons) per patient | Usual care | 130 | 73 | 0.46 | 3.35 | 0.436 (–0.070 to 0.941) | 0.091 |
| Memory rehabilitation | 137 | 5 | 0.03 | 0.23 | |||
| Number of home consultations with other health-care professionals (linked to memory problems) per patient | Usual care | 128 | 26 | 0.17 | 0.72 | –0.829 (–2.059 to 0.402) | 0.186 |
| Memory rehabilitation | 136 | 170 | 0.99 | 7.81 | |||
| Number of home consultations with other health-care professionals (for other reasons) per patient | Usual care | 132 | 169 | 1.08 | 3.61 | –0.198 (–1.072 to 0.675) | 0.655 |
| Memory rehabilitation | 143 | 218 | 1.27 | 4.35 |
| Service usage | Mean (SE) (£) | 95% CI (£) | Difference in means (95% CI) (£)a | p-value |
|---|---|---|---|---|
| Primary care visits | ||||
| Usual care (n = 155) | 206.06 (28.20) | 150.346 to 261.780 | –3.54 (–73.53 to 66.44) | 0.921 |
| Memory rehabilitation (n = 170) | 202.52 (22.19) | 158.71 to 246.33 | ||
| Primary care home visits | ||||
| Usual care (n = 155) | 74.172 (17.55) | 39.494 to 108.850 | –2.84 (–53.05 to 47.37) | 0.911 |
| Memory rehabilitation (n = 170) | 71.33 (18.38) | 35.05 to 107.60 | ||
| Inpatient | ||||
| Usual care (n = 155) | 156.85 (56.10) | 46.023 to 267.668 | 54.90 (–134.78 to 244.59) | 0.570 |
| Memory rehabilitation (n = 170) | 211.75 (76.54) | 60.64 to 362.85 | ||
| Medication | ||||
| Usual care (n = 155) | 16.24 (2.04) | 12.21 to 20.28 | –2.52 (–8.13 to 3.08) | 0.376 |
| Memory rehabilitation (n = 170) | 13.72 (1.98) | 9.81 to 17.63 | ||
| Resource utilisation | Trial arm | n | Sum | Mean | SD | Difference in means (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Number of GP consultations (linked to memory problems) per patient at 6 months | Usual care | 84 | 44 | 0.28 | 0.75 | 0.076 (–0.089 to 0.240) | 0.368 |
| Memory rehabilitation | 102 | 35 | 0.20 | 0.77 | |||
| Number of GP consultations (for other reasons) per patient at 6 months | Usual care | 85 | 132 | 0.84 | 1.55 | 0.133 (–0.159 to 0.426) | 0.371 |
| Memory rehabilitation | 101 | 121 | 0.71 | 1.12 | |||
| Number of practice nurse consultations (linked to memory problems) per patient at 6 months | Usual care | 83 | 19 | 0.12 | 0.57 | 0.033 (–0.076 to 0.143) | 0.549 |
| Memory rehabilitation | 97 | 15 | 0.09 | 0.43 | |||
| Number of practice nurse consultations (for other reasons) per patient at 6 months | Usual care | 74 | 47 | 0.30 | 1.06 | –0.011 (–0.249 to 0.228) | 0.931 |
| Memory rehabilitation | 88 | 53 | 0.31 | 1.13 | |||
| Number of consultations with other health-care professionals (linked to memory problems) per patient at 6 months | Usual care | 86 | 83 | 0.53 | 1.76 | 0.218 (–0.099 to 0.537) | 0.177 |
| Memory rehabilitation | 101 | 53 | 0.31 | 1.13 | |||
| Number of consultations with other health-care professionals (for other reasons) per patient at 6 months | Usual care | 79 | 84 | 0.54 | 1.72 | –0.395 (–0.857 to 0.067) | 0.094 |
| Memory rehabilitation | 87 | 159 | 0.93 | 2.44 | |||
| Number of times an inpatient because of memory problems at 6 months | Usual care | 80 | 4 | 0.03 | 0.19 | 0.008 (–0.032 to 0.048) | 0.694 |
| Memory rehabilitation | 97 | 3 | 0.02 | 0.17 | |||
| Number of days an inpatient because of memory problems at 6 months | Usual care | 33 | 9.5 | 0.61 | 0.64 | 0.043 (–0.058 to 0.143) | 0.401 |
| Memory rehabilitation | 32 | 3 | 0.18 | 0.17 | |||
| Number of times an inpatient for other reasons at 6 months | Usual care | 77 | 14 | 0.09 | 0.41 | 0.025 (–0.050 to 0.100) | 0.516 |
| Memory rehabilitation | 85 | 11 | 0.06 | 0.27 | |||
| Number of days an inpatient for other reasons at 6 months | Usual care | 13 | 27 | 0.17 | 1.06 | –0.027 (–0.251 to 0.197) | 0.814 |
| Memory rehabilitation | 12 | 34 | 0.20 | 1.00 | |||
| Number of GP home consultations (linked to memory problems) per patient at 6 months | Usual care | 81 | 7 | 0.04 | 0.36 | 0.009 (–0.065 to 0.084) | 0.803 |
| Memory rehabilitation | 104 | 6 | 0.04 | 0.32 | |||
| Number of GP home consultations (for other reasons) per patient at 6 months | Usual care | 75 | 11 | 0.07 | 0.54 | 0.017 (–0.079 to 0.114) | 0.724 |
| Memory rehabilitation | 86 | 9 | 0.05 | 0.33 | |||
| Number of practice nurse home consultations (linked to memory problems) per patient at 6 months | Usual care | 83 | 3 | 0.02 | 0.24 | 0.019 (–0.017 to 0.055) | 0.297 |
| Memory rehabilitation | 102 | 0 | 0.00 | 0.00 | |||
| Number of practice nurse home consultations (for other reasons) per patient at 6 months | Usual care | 76 | 99 | 0.63 | 5.57 | 0.607 (–0.231 to 1.445) | 0.155 |
| Memory rehabilitation | 87 | 4 | 0.02 | 0.19 | |||
| Number of home consultations with other health-care professionals (linked to memory problems) per patient at 6 months | Usual care | 84 | 52 | 0.33 | 1.61 | 0.214 (–0.042 to 0.471) | 0.102 |
| Memory rehabilitation | 100 | 20 | 0.12 | 0.55 | |||
| Number of home consultations with other health-care professionals (for other reasons) per patient at 6 months | Usual care | 74 | 43 | 0.27 | 1.65 | –0.036 (–0.365 to 0.292) | 0.829 |
| Memory rehabilitation | 84 | 53 | 0.31 | 1.37 |
| Service usage | Mean (SE) (£) | 95% CI (£) | Difference in means (95% CI) (£)a | p-value |
|---|---|---|---|---|
| Primary care visits | ||||
| Usual care (n = 112) | 127.88 (17.30) | (93.61 to 162.16) | –23.83 (–64.05 to 16.39) | 0.244 |
| Memory rehabilitation (n = 121) | 104.05 (11.38) | (81.52 to 126.58) | ||
| Primary care home visits | ||||
| Usual care (n = 112) | 85.27 (29.26) | (27.28 to 143.26) | –47.20 (–106.70 to 12.30) | 0.119 |
| Memory rehabilitation (n = 121) | 38.07 (10.51) | (17.26 to 58.89) | ||
| Inpatient | ||||
| Usual care (n = 112) | 356.47 (130.23) | (98.41 to 279.26) | –189.00 (–461.57 to 83.57) | 0.173 |
| Memory rehabilitation (n = 121) | 167.46 (56.47) | (55.67 to 279.26) | ||
| Medication | ||||
| Usual care (n = 112) | 13.76 (2.21) | (9.37 to 18.15) | 3.41 (–3.82 to 10.63) | 0.353 |
| Memory rehabilitation (n = 121) | 17.17 (2.87) | (11.48 to 22.85) | ||
| Resource utilisation | Trial arm | n | Sum | Mean | SD | Difference in means (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Number of GP consultations (linked to memory problems) per patient at 12 months | Usual care | 75 | 39 | 0.25 | 1.01 | 0.044 (–0.147 to 0.235) | 0.652 |
| Memory rehabilitation | 101 | 35 | 0.20 | 0.73 | |||
| Number of GP consultations (for other reasons) per patient at 12 months | Usual care | 88 | 105 | 0.67 | 1.23 | –0.138 (–0.424 to 0.147) | 0.341 |
| Memory rehabilitation | 101 | 138 | 0.81 | 1.38 | |||
| Number of practice nurse consultations (linked to memory problems) per patient at 12 months | Usual care | 81 | 12 | 0.08 | 0.40 | 0.018 (–0.066 to 0.102) | 0.674 |
| Memory rehabilitation | 98 | 10 | 0.06 | 0.37 | |||
| Number of practice nurse consultations (for other reasons) per patient at 12 months | Usual care | 82 | 66 | 0.42 | 1.86 | –0.012 (–0.440 to 0.415) | 0.955 |
| Memory rehabilitation | 97 | 74 | 0.43 | 2.06 | |||
| Number of consultations with other health-care professionals (linked to memory problems) per patient at 12 months | Usual care | 83 | 58 | 0.37 | 1.40 | 0.019 (–0.281 to 0.318) | 0.903 |
| Memory rehabilitation | 95 | 60 | 0.35 | 1.36 | |||
| Number of consultations with other health-care professionals (for other reasons) per patient at 12 months | Usual care | 77 | 92 | 0.59 | 1.82 | –0.209 (–0.664 to 0.246) | 0.366 |
| Memory rehabilitation | 91 | 136 | 0.80 | 2.32 | |||
| Number of times an inpatient because of memory problems at 12 months | Usual care | 79 | 5 | 0.03 | 0.21 | –0.003 (–0.055 to 0.049) | 0.902 |
| Memory rehabilitation | 99 | 6 | 0.04 | 0.26 | |||
| Number of days an inpatient because of memory problems at 12 months | Usual care | 33 | 11 | 0.70 | 0.61 | –0.012 (–0.203 to 0.180) | 0.904 |
| Memory rehabilitation | 32 | 14 | 0.08 | 1.07 | |||
| Number of times an inpatient for other reasons at 12 months | Usual care | 70 | 4 | 0.03 | 0.16 | –0.027 (–0.076 to 0.022) | 0.275 |
| Memory rehabilitation | 87 | 9 | 0.05 | 0.27 | |||
| Number of days an inpatient for other reasons at 12 months | Usual care | 12 | 7 | 0.04 | 0.29 | –0.037 (–0.138 to 0.063) | 0.466 |
| Memory rehabilitation | 14 | 14 | 0.08 | 0.58 | |||
| Number of GP home consultations (linked to memory problems) per patient at 12 months | Usual care | 74 | 3 | 0.02 | 0.24 | 0.019 (–0.017 to 0.055) | 0.297 |
| Memory rehabilitation | 102 | 0 | 0.00 | 0.00 | |||
| Number of GP home consultations (for other reasons) per patient at 12 months | Usual care | 73 | 11 | 0.07 | 0.43 | –0.000 (–0.088 to 0.089) | 0.998 |
| Memory rehabilitation | 96 | 12 | 0.07 | 0.38 | |||
| Number of practice nurse home consultations (linked to memory problems) per patient at 12 months | Usual care | 77 | 4 | 0.03 | 0.32 | –0.507 (–1.600 to 0.587) | 0.363 |
| Memory rehabilitation | 103 | 91 | 0.53 | 6.96 | |||
| Number of practice nurse home consultations (for other reasons) per patient at 12 months | Usual care | 71 | 44 | 0.28 | 2.91 | 0.269 (–0.170 to 0.707) | 0.229 |
| Memory rehabilitation | 94 | 2 | 0.01 | 0.11 | |||
| Number of home consultations with other health-care professionals (linked to memory problems) per patient at 12 months | Usual care | 73 | 24 | 0.15 | 1.25 | –0.017 (–0.278 to 0.245) | 0.900 |
| Memory rehabilitation | 99 | 29 | 0.17 | 1.16 | |||
| Number of home consultations with other health-care professionals (for other reasons) per patient at 12 months | Usual care | 71 | 46 | 0.29 | 2.06 | 0.024 (–0.343 to 0.392) | 0.898 |
| Memory rehabilitation | 93 | 46 | 0.27 | 1.26 |
| Service usage | Mean (SE) (£) | 95% CI (£) | Difference in means (95% CI) (£)a | p-value |
|---|---|---|---|---|
| Primary care visits | ||||
| Usual care (n = 105) | 127.87 (19.06) | (90.08 to 165.67) | –0.31 (–53.10 to 52.49) | 0.991 |
| Memory rehabilitation (n = 122) | 127.56 (18.67) | (90.60 to 164.53) | ||
| Primary care home visits | ||||
| Usual care (n = 105) | 49.30 (19.83) | (9.98 to 88.62) | 7.65 (–70.70 to 86.00) | 0.848 |
| Memory rehabilitation (n = 122) | 56.95 (32.70) | (–7.78 to 121.67) | ||
| Inpatient | ||||
| Usual care (n = 105) | 162.77 (72.56) | (18.89 to 306.66) | 6.46 (–196.61 to 209.53) | 0.950 |
| Memory rehabilitation (n = 122) | 169.23 (72.38) | (25.93 to 312.54) | ||
| Medication | ||||
| Usual care (n = 105) | 15.09 (2.94) | (9.26 to 20.91) | 3.26 (–5.56 to 12.08) | 0.467 |
| Memory rehabilitation (n = 122) | 18.35 (3.29) | (11.83 to 24.87) | ||
Appendix 27 Service use questionnaire completion
| SUQ completeness | Time point, n (%) | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | ||||
| Usual care (N = 157) | Memory rehabilitation (N = 171) | Usual care (N = 157) | Memory rehabilitation (N = 171) | Usual care (N = 157) | Memory rehabilitation (N = 171) | |
| Complete | 154 (98.09) | 170 (99.42) | 111 (70.70) | 120 (70.18) | 101 (64.33) | 121 (70.76) |
| Missing (assumed zero) | 2 (1.27) | 1 (0.64) | 1 (0.64) | 2 (1.17) | 0 (0.00) | 1 (0.58) |
| Missing | 1 (0.64) | 0 (0.00) | 45 (28.66) | 49 (28.65) | 56 (35.67) | 49 (28.65) |
Appendix 28 EuroQol-5 Dimensions, five-level version, missing data
| EQ-5D-5L 6-month questionnaire | EQ-5D-5L 12-month questionnaire, n (%) | |||||
|---|---|---|---|---|---|---|
| Usual care (N = 157) | Memory rehabilitation (N = 171) | |||||
| Complete | Missing | Total | Complete | Missing | Total | |
| Complete | 99 (63.1) | 20 (12.7) | 119 (75.8) | 110 (64.3) | 18 (10.5) | 128 (74.9) |
| Missing | 7 (4.5) | 31 (19.7) | 38 (24.2) | 13 (7.6) | 30 (17.5) | 43 (25.1) |
| Total | 106 (67.5) | 51 (32.5) | 157 (100.0) | 123 (71.9) | 48 (28.1) | 171 (100.0) |
Appendix 29 EuroQol-5 Dimensions, five-level version, utilities and quality-adjusted life-years gained over time (mean imputed data)
| Time point | Trial arm | Difference in means (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
| Usual care (n = 157) | Memory rehabilitation (n = 171) | |||||
| Mean EQ-5D-5L score | Change over time (95% CI) | Mean EQ-5D-5L score | Change over time (95% CI) | |||
| Baseline | 0.581 | 0.637 | ||||
| 6 months | 0.581 | 0.000 (–0.041 to 0.040) | 0.639 | 0.002 (–0.036 to 0.039) | 0.002 (–0.052 to 0.057) | 0.935 |
| 12 months | 0.628 | 0.047 (0.004 to 0.090) | 0.644 | 0.007 (–0.027 to 0.041) | –0.040 (–0.093 to 0.014) | 0.148 |
| QALY gain at 6 months (95% CI) | 0.000 (–0.020 to 0.020) | 0.001 (–0.018 to 0.020) | 0.001 (–0.026 to 0.028) | 0.935 | ||
| QALY gain at 12 months (95% CI) | 0.023 (0.002 to 0.045) | 0.004 (–0.013 to 0.020) | –0.020 (–0.047 to 0.007) | 0.148 | ||
Appendix 30 Change in EMQ-p score over time (mean imputed data)
| Time point | Trial arm | Difference in means (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
| Usual care (n = 157) | Memory rehabilitation (n = 171) | |||||
| Mean EMQ-p score | Change over time (95% CI) | Mean EMQ-p score | Change over time (95% CI) | |||
| Baseline | 50.075 (1.953) | 47.363 (1.606) | ||||
| 6 months | 43.679 (1.749) | –6.397 (–9.681 to –3.112) | 39.572 (1.800) | –7.791 (–10.678 to –4.903) | –1.394 (5.735 to 2.946) | 0.528 |
| 12 months | 43.268 (1.774) | –6.807 (–10.119 to –3.495) | 38.323 (1.707) | –9.049 (–12.081 to –6.015) | –2.242 (–6.707 to 2.224) | 0.324 |
Appendix 31 Change in GHQ-30 score over time (mean imputed data)
| Time point | Trial arm | Difference in means (95% CI) | p-value | |||
|---|---|---|---|---|---|---|
| Usual care (n = 157) | Memory rehabilitation (n = 171) | |||||
| Mean GHQ-30 score | Change over time (95% CI) | Mean GHQ-30 score | Change over time (95% CI) | |||
| Baseline | 9.601 | 9.670 | ||||
| 6 months | 8.811 | –0.790 (–2.003 to 0.422) | 8.693 | –0.976 (–2.177 to 0.224) | –0.186 (–1.889 to 1.517) | 0.830 |
| 12 months | 8.089 | –1.512 (–2.867 to –0.158) | 8.492 | –1.179 (–2.494 to 0.135) | 0.373 (–1.549 to 2.215) | 0.728 |
Appendix 32 One-way sensitivity analyses of cost-effectiveness (multiply imputed data)
| Cost (£) | |||
|---|---|---|---|
| Time point, analysis | Incremental cost (£) (memory rehabilitation – usual care) | Incremental effect (memory rehabilitation – usual care) | ICER (£) |
| 6 months | |||
| Base case | –35.612 | 0.004 | –8903 (intervention dominant) |
| Upper 95% bound net cost | 57.519 | 0.005 | 11,504 (north-east quadrant) |
| Upper 95% bound QALYs | |||
| Lower 95% bound net cost | –128.744 | 0.003 | –42,915 (intervention dominant) |
| Lower 95% bound QALYs | |||
| Upper 95% bound net cost | 57.519 | 0.003 | 57.519 (north-east quadrant) |
| Lower 95% bound QALYs | |||
| Lower 95% bound net cost | –128.744 | 0.005 | –25,749 (intervention dominant) |
| Upper 95% bound QALYs | |||
| 12 months | |||
| Base case | –26.895 | –0.011 | 2445 (south-west quadrant) |
| Upper 95% bound net cost | 59.94 | –0.008 | –7493 (usual care dominant) |
| Upper 95% bound QALYs | |||
| Lower 95% bound net cost | –280.73 | –0.013 | 21,595 (south-west quadrant) |
| Lower 95% bound QALYs | |||
| Upper 95% bound net cost | 59.94 | –0.013 | –4611 (usual care dominant) |
| Lower 95% bound QALYs | |||
| Lower 95% bound net cost | –280.73 | –0.008 | 35,091 (south-west quadrant) |
| Upper 95% bound QALYs | |||
| Cost (£) | |||
|---|---|---|---|
| Time point, analysis | Incremental cost (£) (memory rehabilitation – usual care) | Incremental effect (memory rehabilitation – usual care) | ICER (£) |
| 6 months | |||
| Base case | –35.612 | –2.322 | –15.34 (intervention dominant) |
| Upper 95% bound net cost | 57.519 | –2.667 | 21.56 (north-east quadrant) |
| Upper 95% bound EMQ-p score | |||
| Lower 95% bound net cost | –128.744 | –1.976 | –65.16 (intervention dominant) |
| Lower 95% bound EMQ-p score | |||
| Upper 95% bound net cost | 57.519 | –1.976 | 29.11 (north-east quadrant) |
| Lower 95% bound EMQ-p score | |||
| Lower 95% bound net cost | –128.744 | –2.667 | –48.26 (intervention dominant) |
| Upper 95% bound EMQ-p score | |||
| 12 months | |||
| Base case | –26.895 | –1.410 | –19.07 (intervention dominant) |
| Upper 95% bound net cost | 59.94 | –1.496 | 40.07 (north-east quadrant) |
| Upper 95% bound EMQ-p score | |||
| Lower 95% bound net cost | –280.73 | –1.325 | –211.93 (intervention dominant) |
| Lower 95% bound EMQ-p score | |||
| Upper 95% bound net cost | 59.94 | –1.325 | 45.25 (north-east quadrant) |
| Lower 95% bound EMQ-p score | |||
| Lower 95% bound net cost | –280.73 | –1.496 | 187.68 (intervention dominant) |
| Upper 95% bound EMQ-p score | |||
| Cost (£) | |||
|---|---|---|---|
| Time point, analysis | Incremental cost (£) (memory rehabilitation – usual care) | Incremental effect (memory rehabilitation – usual care) | ICER (£) |
| 6 months | |||
| Base case | –35.612 | –0.998 | –35.70 (intervention dominant) |
| Upper 95% bound net cost | 57.519 | –1.058 | 54.37 (north-east quadrant) |
| Upper 95% bound GHQ-30 score | |||
| Lower 95% bound net cost | –128.744 | –0.937 | –137.35 (intervention dominant) |
| Lower 95% bound GHQ-30 score | |||
| Upper 95% bound net cost | 57.519 | –0.937 | 61.36 (north-east quadrant) |
| Lower 95% bound GHQ-30 score | |||
| Lower 95% bound net cost | –128.744 | –1.058 | –121.71 (intervention dominant) |
| Upper 95% bound GHQ-30 score | |||
| 12 months | |||
| Base case | –26.895 | –0.737 | –36.49 (intervention dominant) |
| Upper 95% bound net cost | 59.94 | –0.439 | 136.54 (north-east quadrant) |
| Upper 95% bound GHQ-30 score | |||
| Lower 95% bound net cost | –280.73 | –1.037 | –270.71 (intervention dominant) |
| Lower 95% bound GHQ-30 score | |||
| Upper 95% bound net cost | 59.94 | –1.037 | 57.80 (north-east quadrant) |
| Lower 95% bound GHQ-30 score | |||
| Lower 95% bound net cost | –280.73 | –0.439 | –639.48 (intervention dominant) |
| Upper 95% bound GHQ-30 score | |||
Appendix 33 One-way sensitivity analysis of longer-term cost-effectiveness: 5-year horizon
| Time point, analysis | Trial arm | Incremental | ICER (£) | ||||
|---|---|---|---|---|---|---|---|
| Memory rehabilitation | Usual care | ||||||
| Cost (£) | QALY | Cost (£) | QALY | Cost (£) | QALY | ||
| Base-case analysis | 3599.08 | 2.952 | 3404.27 | 2.651 | 194.81 | 0.301 | 646.36 |
| One-way sensitivity analysis | |||||||
| Age 30 years | 3644.67 | 2.990 | 3442.43 | 2.681 | 202.24 | 0.309 | 655.35 |
| Age 65 years | 3422.51 | 2.848 | 3280.97 | 2.552 | 141.54 | 0.296 | 477.62 |
| Discount rate 1.5% | 3770.16 | 3.096 | 3561.15 | 2.780 | 209.01 | 0.316 | 661.17 |
| Discount rate 5.0% | 3479.91 | 2.852 | 3294.90 | 2.561 | 185.01 | 0.291 | 635.50 |
| +30% intervention cost | 3649.18 | 2.952 | 3404.27 | 2.651 | 244.91 | 0.301 | 812.59 |
| –30% intervention cost | 3548.98 | 2.952 | 3404.27 | 2.651 | 144.71 | 0.301 | 480.13 |
| +30% cost per cycle in GHQ state | 4628.70 | 2.952 | 3404.27 | 2.651 | 1224.43 | 0.301 | 4062.58 |
| –30% cost per cycle in GHQ state | 2569.45 | 2.952 | 3404.27 | 2.651 | –834.82 | 0.301 | –2769.87 |
| +30% utility per cycle in GHQ state | 3599.08 | 3.838 | 3404.27 | 3.446 | 194.81 | 0.392 | 497.20 |
| –30% utility per cycle in GHQ state | 3599.08 | 2.067 | 3404.27 | 1.856 | 194.81 | 0.211 | 923.37 |
| +20% transition probability higher GHQ state | 3674.46 | 2.904 | 3526.14 | 2.612 | 148.31 | 0.292 | 507.06 |
| +20% transition probability lower GHQ state | 3526.03 | 3.001 | 3277.91 | 2.690 | 248.12 | 0.311 | 797.73 |
| –20% transition probability higher GHQ state | 3521.44 | 3.004 | 3263.71 | 2.695 | 257.73 | 0.309 | 834.92 |
| –20% transition probability lower GHQ state | 3697.16 | 2.891 | 3553.60 | 2.603 | 143.56 | 0.288 | 498.47 |
Appendix 34 One-way sensitivity analysis of longer-term cost-effectiveness: 10-year horizon
| Time point, analysis | Trial arm | Incremental | ICER (£) | ||||
|---|---|---|---|---|---|---|---|
| Memory rehabilitation | Usual care | ||||||
| Cost (£) | QALY | Cost (£) | QALY | Cost (£) | QALY | ||
| Base-case analysis | 6451.25 | 5.340 | 5976.51 | 4.806 | 474.73 | 0.535 | 887.76 |
| One-way sensitivity analysis | |||||||
| Age 30 years | 6627.24 | 5.486 | 6120.75 | 4.924 | 506.49 | 0.562 | 900.57 |
| Age 65 years | 5905.55 | 4.931 | 5503.20 | 4.417 | 402.35 | 0.514 | 782.30 |
| Discount rate 1.5% | 7066.71 | 5.856 | 6534.38 | 5.271 | 532.33 | 0.586 | 908.93 |
| Discount rate 5.0% | 6044.64 | 4.999 | 5607.79 | 4.498 | 436.86 | 0.501 | 871.83 |
| +30% intervention cost | 6501.35 | 5.340 | 5,976.51 | 4.806 | 524.83 | 0.535 | 981.44 |
| –30% intervention cost | 6401.15 | 5.340 | 5,976.51 | 4.806 | 424.63 | 0.535 | 794.07 |
| +30% cost per cycle in GHQ state | 8336.52 | 5.340 | 5976.51 | 4.806 | 2,360.01 | 0.535 | 4413.23 |
| –30% cost per cycle in GHQ state | 4565.97 | 5.340 | 5976.51 | 4.806 | –1410.54 | 0.535 | –2637.72 |
| +30% utility per cycle in GHQ state | 6451.25 | 6.942 | 5976.51 | 6.247 | 474.73 | 0.695 | 682.89 |
| –30% utility per cycle in GHQ state | 6451.25 | 3.738 | 5976.51 | 3.364 | 474.73 | 0.374 | 1268.22 |
| +20% transition probability higher GHQ state | 6596.52 | 5.248 | 6196.88 | 4.732 | 399.64 | 0.516 | 774.87 |
| +20% transition probability lower GHQ state | 6318.55 | 5.430 | 5753.57 | 4.878 | 564.99 | 0.552 | 1023.36 |
| –20% transition probability higher GHQ state | 6301.55 | 5.442 | 5717.64 | 4.891 | 583.91 | 0.551 | 1059.79 |
| –20% transition probability lower GHQ state | 6635.93 | 5.224 | 6246.03 | 4.716 | 389.90 | 0.509 | 766.74 |
Appendix 35 Semistructured interview schedule
Appendix 36 Interview recruitment
| Study site | Trial arm, n | Total, n | |
|---|---|---|---|
| Usual care | Memory rehabilitation | ||
| 1 | 4 | 3 | 7 |
| 2 | 0 | 0 | 0 |
| 3 | 3 | 4 | 7 |
| 4 | 4 | 2 | 6 |
| 5 | 1 | 2 | 3 |
| 6 | 1 | 1 | 2 |
| 7 | 1 | 1 | 2 |
| 8 | 1 | 1 | 2 |
| 9 | 1 | 2 | 3 |
| Total | 16 | 16 | 32 |
List of abbreviations
- ABI
- acquired brain injury
- AP
- assistant psychologist
- AUC
- area under the curve
- BNF
- British National Formulary
- CBCR
- computer-based cognitive retraining
- CC
- case-mix classification
- CCT
- computerised cognitive training
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- DMC
- Data Monitoring Committee
- EBIQ
- European Brain Injury Questionnaire
- EBIQ-p
- European Brain Injury Questionnaire – patient version
- EBIQ-r
- European Brain Injury Questionnaire – relative version
- EMQ-p
- Everyday Memory Questionnaire – patient version
- EMQ-r
- Everyday Memory Questionnaire – relative version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GAS
- goal attainment scaling
- GCS
- Glasgow Coma Scale
- GHQ
- General Health Questionnaire
- GHQ-30
- General Health Questionnaire 30-item version
- GMI
- General Memory Index
- GP
- general practitioner
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ICF
- International Classification of Functioning, Disability and Health
- ITT
- intention to treat
- MICE
- multiple imputation using chained equations
- NART
- National Adult Reading Test
- NBS
- non-invasive brain stimulation
- NCTU
- Nottingham Clinical Trials Unit
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- ONS
- Office for National Statistics
- PIC
- participant identification centre
- QALY
- quality-adjusted life-year
- R&D
- research and development
- RA
- research assistant
- RBMT-3
- Rivermead Behavioural Memory Test – third edition
- RCT
- randomised controlled trial
- SAP
- statistical analysis plan
- SD
- standard deviation
- SE
- standard error
- SIGN
- Scottish Intercollegiate Guidelines Network
- SMART
- specific, measurable, assignable, realistic and time related
- SMR
- standardised mortality ratio
- SOP
- standard operating procedure
- SST
- Sheffield Screening Test for Acquired Language Disorders
- SUQ
- service use questionnaire
- TBI
- traumatic brain injury
- TIDieR
- Template for Intervention Description and Replication
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VR
- virtual reality
- WTP
- willingness to pay
