Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/104/40. The contractual start date was in September 2012. The draft report began editorial review in September 2018 and was accepted for publication in December 2018. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Gus Gazzard, David Garway-Heath, Rachael Hunter, Gareth Ambler, Catey Bunce, Richard Wormald, Keith Barton, Gary Rubin and Marta Buszewicz have received a grant from the National Institute for Health Research (NIHR) for the submitted work. Gus Gazzard reports grants from Lumenis (Borehamwood, UK) during the conduct of the study; grants from Ellex Medical Lasers (Adelaide, SA, Australia), Ivantis, Inc. (Irvine, CA, USA) and Thea Pharmaceuticals (Keele, UK) outside the submitted work; and personal fees from Allergan (Dublin, Ireland), Alcon (Fort Worth, TX, USA), Glaukos Corporation (San Clemente, CA, USA), Santen Pharmaceutical Co., Ltd (Osaka, Japan) and Thea Pharmaceuticals outside the submitted work. David Garway-Heath reports personal fees from Aerie Pharmaceuticals, Inc. (Durham, NC, USA), Alcon, Allergan, Bausch + Lomb (Rochester, NY, USA), Quark Pharmaceuticals, Inc. (Ness Ziona, Israel), Quethera Limited and Roche (Basel, Switzerland); grants from the Alcon Research Institute; and grants and personal fees from Pfizer (New York, NY, USA) and Santen Pharmaceutical Co., Ltd, outside the submitted work. In addition, David Garway-Heath was a member of the Health Technology Assessment (HTA) Clinical Trials Board from 2014 to 2017. Keith Barton received a grant from NIHR for the Treatment of Advanced Glaucoma Study during the conduct of the study. In addition, Keith Barton reports grants from Johnson & Johnson Vision (Santa Ana, CA, USA), New World Medical (Rancho Cucamonga, CA, USA), Alcon, Merck & Co. (Kenilworth, NJ, USA), Allergan and Refocus Group (Dallas, TX, USA); that he has had other financial relationships with Alcon, Merck & Co., Allergan, Refocus, AqueSys Inc. (Taipei, Taiwan), Ivantis, Carl Zeiss Meditec AG (Jena, Germany), Kowa Europe GmbH (Düsseldorf, Germany), Santen Pharmaceutical Co., Ltd, Transcend Medical (Scottsboro, AL, USA), Glaukos (San Clemente, CA, USA), Amakem NV (Diepenbeek, Belgium), Thea Pharmaceuticals, Alimera Sciences (Alpharette, GA, USA), Pfizer, Advanced Ophthalmic Implants Pte Ltd (Singapore), Vision Futures (UK) Ltd (London, UK), London Claremont Clinic Ltd (London, UK) and Vision Medical Events Ltd (London, UK), outside the submitted work; and that he has a patent with Ophthalmic Implants (PTE) Ltd. Stephen Morris was a member of NIHR Health Services and Delivery Research (HSDR) Research Funding Board (2014–19), the NIHR HSDR Commissioned Board (2014–16), the NIHR HSDR Evidence Synthesis Sub Board (2016), the NIHR HTA Clinical Evaluation and Trials Board (2007–10), the NIHR HTA Commissioning Board (2009–13), the NIHR Public Health Research Funding Board (2011–17) and the NIHR Programme Grants for Applied Research expert subpanel (2017–present). Marta Buszewicz was a member of the HTA Mental Health Panel from January to May 2018. In September 2018 this panel was amalgamated into the HTA Prioritisation Committee C (mental health, women and children’s health), of which Marta Buszewicz was also a member. Marta Buszewicz has also been a member of the NIHR Research for Patient Benefit, London, funding panel since May 2017.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Gazzard et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background
Primary open-angle glaucoma and ocular hypertension
Primary open-angle glaucoma (OAG) is a common, irreversible optic neuropathy affecting the vision of predominantly older adults. It is strongly associated with elevated intraocular pressure (IOP), but may also occur with IOP in the normal range. Glaucoma results in progressive visual field (VF) loss and is a leading cause of blindness worldwide. In the UK, glaucoma affects over half a million individuals, with over one-quarter of a million aged > 65 years. 1 It is a leading cause of visual morbidity, accounting for 12% of blind registrations. 2 Glaucoma is a significant cause of falls, road traffic accidents3 and loss of independence in the elderly (even in the case of mild asymptomatic disease),3,4 and can significantly reduce the quality of life (QoL) of these patients. 5–8
Ocular hypertension (OHT) is a state of raised IOP without optic nerve damage, which can progress to OAG in some patients. 9,10 Those with a higher IOP, lower central corneal thickness (CCT) and a family history of OAG are more at risk of developing glaucoma. 11 IOP is the only modifiable risk factor for the development of OAG, the reduction of which is proven to slow down the progression of OAG or the conversion of OHT to OAG. 10,12–14 National Institute for Health and Care Excellence (NICE) guidelines recommend that patients with OHT at high risk of developing OAG should be offered treatment. 15
Current first-line treatment options
Medical therapy (eyedrops) is widely used and is currently the most commonly used first-line treatment for mild to moderate disease,16 leading to approximately 1.2 million prescriptions per month in the UK. 17 Medical treatment is usually lifelong; patients may need to instil multiple eyedrops, which can become expensive, while also experiencing side effects that limit the acceptability of the treatment and impair their QoL. 18–21
Although the effectiveness of IOP-lowering eyedrops is irrefutable, they come with a number of aesthetic, sight- and potentially life-threatening side effects: pain on instillation, conjunctival hyperaemia, elongation and darkening of eyelashes, iris colour changes, periocular skin pigmentation, allergic reactions, accelerated cataract formation, cystoid macular oedema, anterior uveitis and reactivation of herpes simplex keratitis,19,20 in addition to serious respiratory (e.g. airway obstruction) and cardiovascular side effects in some, falls and increased mortality. 22,23 These adverse effects influence the acceptability of eyedrops to patients, as well as patients’ concordance with medical treatment plans and their QoL. Indeed, reported non-compliance rates for medical treatment range from 24% to 80%, depending on definition. 24–26 Approximately 22% of initial treatment regimens need adjustment27 and up to 50% of patients discontinue their medication within 6 months of the initial consultation. 24 Although patients with diagnosed glaucoma are more likely than those with suspected glaucoma to adhere to therapy,24 side effects are likely to have a direct adverse effect on patients diagnosed with OHT, in whom proper IOP control can reduce the incidence of OAG. 28 Medical management of OAG and OHT requires regular monitoring and modification of therapy by ophthalmologists, as well as multiple hospital visits for patients. 29
Long-term use of topical IOP-lowering medication induces significant subclinical conjunctival inflammation and conjunctival fibroblast activation by medications or preservatives,30–32 and has been shown to have a negative impact on the success rates of subsequent surgical intervention33 (long-term eyedrop use is a known powerful risk factor for later surgical failure). 31,34
Laser trabeculoplasty (an alternative treatment method) has been inconsistently performed in the UK; NICE has identified a lack of evidence governing its use. 17 The procedure involves a single, painless outpatient application of laser to the trabecular meshwork using a contact lens. It is easy to deliver by clinicians competent in gonioscopy and has a wide safety margin. Selective laser trabeculoplasty (SLT) uses bursts of nanosecond pulses with a larger spot (400 microns) and lowers IOP by increasing the aqueous humour outflow through the trabecular meshwork by causing increased macrophage activity and trabecular tissue remodelling. 35,36
The IOP-lowering effect of SLT is comparable with that of prostaglandin analogues,37,38 the current first-line medical treatment recommended by NICE. SLT can delay and, in some cases, prevent the need for eyedrops. 38 The effects are not permanent; however, SLT does not prejudice the effectiveness of later medical or surgical treatments. Because minimal trabecular meshwork damage is caused, SLT has also been shown to have good efficacy on repeat treatment. 39–41
The use of SLT for lowering IOP has been inconsistent and has in the past often been reserved as a last resort before surgery, possibly because of concerns arising from older forms of laser trabeculoplasty. Use of SLT as a first-line treatment has the potential to offer patients an eyedrop-free window of several years, remove the concerns about concordance with medication and reduce both hospital visits and side effects compared with medical therapy.
Glaucoma filtration surgery is usually reserved for those who continue to lose vision despite other treatments. It has a significant failure rate and may cause permanent ocular discomfort and, rarely, chronic pain. 42,43 A study comparing medical management (eyedrops) with surgery (trabeculectomy) as a first-line treatment for advanced OAG is currently under way. 44
Economic burden of treatment to the NHS
The treatment of OAG and OHT can incur significant costs to both the patient and the NHS. Direct treatment costs in the UK were estimated at an equivalent of US$1337 per patient per year in 1999;45 up to 61% of these costs were for IOP-lowering medication. 46 In 2012, > 8 million glaucoma treatment-related items were dispensed in the community alone, costing > £105M, with increases in the number of items dispensed and their cost reported annually. 47 Both direct costs and indirect costs are higher for more severe disease,48 suggesting that effective IOP control early in the course of the disease is likely to reduce later costs, as well as improve vision-dependent health-related quality of life (HRQoL).
Extensive economic modelling of SLT has taken place in various health-care systems worldwide. In the USA, the 5-year cumulative costs per patient were lower for SLT than for eyedrops and surgery, whereas an Australian study found that every AU$1 spent on laser treatment resulted in a saving of AU$2.50 compared with initial medical therapy, with projections of increasing cost savings over time. 49,50 The time threshold at which bilateral SLT would become less costly than bilateral use of topical medication has also been modelled. 51 It was found that SLT became less costly than most brand-name medications within 1 year and less costly than generic latanoprost and generic timolol eyedrops after 13 and 40 months, respectively. This is supported by a projected 6-year cost comparison of primary SLT with primary medical therapy in OAG treatment in a Canadian health-care model;46 if primary SLT had to be repeated between 2 and 3 years, use of primary SLT over mono-, bi-, and tri-drug therapy produced a 6-year cumulative cost saving of CA$580.52, CA$2042.54 and CA$3366.65 per patient, respectively. Similar findings have also been published for the management of both mild and moderate glaucoma. 52
Although the limited existing data are very difficult to apply to the UK population, the above data would suggest annual savings to the NHS of £2.4M in direct treatment costs for new OAG patients alone from a Laser-1st (initial SLT followed by routine medical treatment) paradigm. This rises to £16.8M per year if a conservative 20% of new OHT/OAG referrals require treatment. Australian data give far higher predictions: were SLT to be extended to previously diagnosed patients, as is common practice in the USA, cost savings would be up to 20 times higher. Indirect cost savings (e.g. reduced visual loss) are, of course, greater still.
Use of selective laser trabeculoplasty as a first-line treatment for open-angle glaucoma/ocular hypertension
Initial treatment with SLT potentially offers an ‘eyedrop-free window’ of several years, removes concerns about concordance and possibly reduces the need for multiple eyedrops, even years later. Even when insufficient as a sole therapy, SLT may reduce the intensity of subsequent medical treatment and possibly the need for later surgery. A single outpatient treatment is likely to be more acceptable to patients than daily self-administration of eyedrops, securing 100% concordance from those attending for treatment and resulting in fewer hospital visits and fewer side effects than eyedrop therapy alone.
Although SLT is an existing technology, proven to lower IOP, neither HRQoL nor cost-effectiveness has been compared with outcomes in patients who received eyedrops as a first-line treatment. A Laser-1st pathway allows an eyedrop-free period and, possibly, lower intensity of treatment. This is likely to be associated with greater HRQoL, improved patient acceptability and better treatment compliance, with fewer patient visits resulting from treatment changes and fewer adverse events (AEs), at a much lower cost than treatment with eyedrops. 46,50,53
Uptake of SLT by surgeons in the UK has so far been limited because of past experiences with older laser technology. SLT is delivered in an outpatient setting using topical anaesthesia and is quick and pain free. It is simple and safe to deliver and has a wide safety margin and good repeatability. Widespread uptake of SLT has the potential to substantially improve HRQoL for many patients and produce substantial cost savings to the NHS (lower medication costs, reduced side effects, fewer hospital visits, lower surgery rates and indirect savings from care costs for fewer visually impaired patients).
Rationale for research
Research recommendations by NICE17 and a Cochrane systematic review54 have identified the need for robust randomised controlled trials investigating the efficacy and cost-effectiveness of SLT as a first-line treatment for OAG and OHT.
Aims and objectives
Hypothesis
Our hypothesis is that, in patients with newly diagnosed OHT or OAG, primary treatment with SLT (Laser-1st) leads to a better HRQoL than primary treatment with IOP-lowering eyedrops (Medicine-1st), and that this is associated with reduced costs, better clinical outcomes and an improved tolerability of treatment.
Primary objective
To determine if, in a pragmatic study that mirrors the realities of clinical decision-making, a Laser-1st pathway delivers a better HRQoL at 3 years than a Medicine-1st (routine medical treatment only) pathway, in the management of patients with OAG and OHT.
Secondary objectives
To determine whether or not a Laser-1st treatment pathway:
-
costs less than the conventional treatment pathway of Medicine-1st
-
achieves the desired level of IOP with less intensive treatment over the course of the study
-
leads to equivalent levels of visual function after 3 years
-
is better tolerated by patients.
Chapter 2 Methods
Trial design
The Laser in Glaucoma and Ocular Hypertension (LiGHT) trial was designed to evaluate the difference in HRQoL, cost and clinical efficacy between two first-line treatment arms for OAG and OHT. The LiGHT trial is a multicentre, randomised clinical trial, unmasked to treatment allocation, with two treatment arms: initial SLT followed by routine medical treatment (Laser-1st) and routine medical treatment only (Medicine-1st). 55,56
Eligible patients were randomised in a 1 : 1 ratio to receive either SLT (Laser-1st) or medical therapy (Medicine-1st) as the first-line treatment for OAG or OHT. All measurements influencing treatment escalation decisions [VF, Heidelberg retinal tomography (HRT) and IOP] were made by masked observers. Patients were monitored for 3 years and monitoring intervals were guided by a defined protocol to avoid bias in clinical decision-making. A clinical decision algorithm, attempting to capture the complexities of clinical practice, defined triggers for escalation. This was a pragmatic trial aiming to mirror the ‘real-world’ patient experience of treatment as closely as possible and seeking to capture the full effects of laser treatment.
Ethics approval and research governance
The study adhered to the tenets of the Declaration of Helsinki. Ethics approval was granted by the City Road and Hampstead Research and Ethics Committee (former Moorfields and Whittington Research Ethics Committee then East London and The City Research Ethics Committee 1, reference 12/LO/0940) on 20 June 2012. The LiGHT trial is registered as ISRCTN32038223 [the full protocol can be accessed at URL: www.moorfields.nhs.uk/sites/default/files/LiGHT%20Trial%20Protocol%203.0%20-%2020-5-2015_3.pdf (accessed 3 May 2019)].
Patient population
The LiGHT trial aimed to recruit patients with newly diagnosed OAG or OHT in one or both eyes from six collaborating specialist glaucoma clinics at large ophthalmic centres in the UK (see Appendix 1).
Inclusion criteria
Parts of this text have been reproduced from Gazzard et al. 58 © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (http://creativecommons.org/licenses/by/4.0/).
Patients were required to have newly diagnosed OAG or OHT in one or both eyes, which needed treatment. Definitions of OAG and OHT, as well as criteria for initiating treatment, are shown in Appendix 2. The following criteria were also specified:56
-
A decision to treat had been made by a glaucoma specialist consultant ophthalmologist.
-
Patients were aged > 18 years and were able to provide informed consent.
-
Patients were able to complete QoL, disease-specific symptom and cost questionnaires in English (physical help with completion and assistance with reading was permitted, as long as an interpreter was not required).
-
It was possible to perform a VF test in the study eye(s) with < 15% false positives.
Exclusion criteria
Parts of this text have been reproduced from Gazzard et al. 58 © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (http://creativecommons.org/licenses/by/4.0/).
Patients were not considered for the study if there was:56
-
advanced glaucoma in the potentially eligible eye as determined by Early Manifest Glaucoma Trial (EMGT I)59 criteria (77 VF loss mean deviation (MD) worse than –12 dB in the better eye or –15 dB in the worse eye)
-
secondary glaucoma (e.g. pigment dispersion syndrome, rubeosis, trauma, etc.) or any angle closure
-
any contraindication to SLT (e.g. unable to sit at the laser-mounted slit-lamp, past history of or active uveitis, neovascular glaucoma, inadequate visualisation of trabecular meshwork)
-
an inability to use topical medical therapy because of, for example, physical infirmity and a lack of carers able to administer daily eyedrops
-
a previous treatment for OAG or OHT
-
congenital or early childhood glaucoma
-
a visually significant cataract in symptomatic patients who want to undergo cataract surgery
-
any current, active treatment for another ophthalmic condition in the hospital eye service (this applied to both eyes, even if one was not in the trial, as the fellow eye might affect the patient’s visit frequency)
-
any history of retinal ischaemia, macular oedema or diabetic retinopathy
-
age-related macular degeneration with neovascularisation in either eye or geographic atrophy
-
visual acuity (VA) worse than 6/36 in a study eye; non-progressive VA loss better than 6/36 owing to any comorbidity was permitted provided that it did not affect the response to treatment or later surgical choices and that it was not under active follow-up (e.g. an old, isolated retinal scar no longer under review or amblyopia)
-
any previous intraocular surgery, except uncomplicated phacoemulsification, at least 1 year before recruitment (this applied to both eyes, even if one was not in the trial, as it could affect the required treatment intensity and visit frequency for any glaucoma in the fellow eye)
-
pregnancy at the time of recruitment or intention to become pregnant within the duration of the trial
-
medical unsuitability for completion of the trial (e.g. suffering from a terminal illness or too unwell to be able to attend hospital clinic visits)
-
recent involvement in another interventional research study (within 3 months) of any topic.
Recruitment
Internal pilot study
We conducted a 9-month internal pilot at Moorfields Eye Hospital (MEH) (the central trial site and largest recruiting site). This ensured that recruitment rates were adequate and that all procedures were in place, before roll-out to other sites. Data collected included number of eligible patients approached, proportion entering the trial and recruitment rates.
Recruitment strategy and identification of participants
Patients attending the hospital eye service for the first treatment of OAG/OHT were assessed for eligibility before treatment and, if eligible, were informed of the study by the local trial co-ordinator (along with written information). To maximise potential coverage of all eligible patients, a trial staff member was available daily to attend clinics and counsel potential subjects. Local trial staff screened all new referrals (by referral letter or electronic patient record) and identified those possibly eligible, with reminders for the clinic staff. Regular education of clinical staff and clinic-wide information posters for staff and patients raised awareness of the study and reminded clinicians of the opportunity for recruitment. 56
Recruitment process and informed consent
Eligible patients were approached and introduced to the aims, methods, anticipated benefits and potential hazards of the study, and were eventually invited to participate by a member of the LiGHT team. Introducing the patients to the study and inviting them to participate was done either by face-to-face discussions with the trial team members or by the use of audiovisual material (video); the video conveyed the same information as the face-to-face discussions with the trial team members, but was delivered by the chief investigator (video content/script is shown in Appendix 3). The use of the video in the recruitment process maximised the time efficiency of the recruiters, as often more than one patient had to be approached simultaneously.
After the invitation to participate, ample time was given to the patients to consider participation. Written informed consent was obtained on a separate day, usually the day of the baseline assessment (see Baseline assessment), by either the good clinical practice (GCP)-trained local trial ophthalmologist or the local trial optometrist who had been delegated this duty by the chief investigator/principal investigator (PI) on the delegation log. Consent was obtained with the support of extensive clearly written information (in English) that had been reviewed and approved by our patient-led lay advisory group (LAG). Patients who had difficulty in giving informed consent did not form part of this study. A copy of the signed informed consent was given to the participant and the original signed form was retained at the study site.
If new safety information resulted in significant changes in the risk/benefit assessment, the consent form was reviewed and updated if necessary. All patients, including those already being treated, were given any new information, a copy of the revised form and reconsented to continue in the study.
Baseline assessment
At the baseline assessment, and after informed consent was provided, participants underwent VA testing, slit-lamp examination, automated VF testing [Humphrey Field Analyser Mark II (Carl Zeiss Meditec, Dublin, CA, USA) and the Swedish Interactive Threshold Algorithm standard 24-2 programme], HRT optic disc imaging, IOP measurement, gonioscopy, CCT measurement and assessment of the optic discs, maculae and fundi. The patients also completed the following baseline questionnaires: EuroQol-5 Dimensions, five-level version (EQ-5D-5L),60 Glaucoma Utility Index (GUI),61 Glaucoma Symptom Scale (GSS),62 Glaucoma Quality of Life-15 (GQL-15; a visual function, rather than quality-of-life measure)8 and a modified version of the Client Service Receipt Inventory (CSRI) questionnaire. 63
Randomisation and masking
Following the completion of all baseline assessments, eligible patients were randomised to one of two treatment groups: SLT (Laser-1st) or topical medical therapy (Medicine-1st). Randomisation was undertaken online on the same day by the clinical staff who obtained informed consent, using a web-based randomisation service (Sealed Envelope, London, UK) and achieving full allocation concealment. Stratified randomisation with random block sizes was used to randomise in a 1 : 1 ratio at the level of the patient, with the stratification factors of diagnosis (OHT/OAG) and treatment centre. Following randomisation, the details of the treatment and specific arrangements and instructions were communicated to the patients by a member of the trial team. Owing to the pragmatic design of this trial, the patients and clinicians were unmasked to the treatment arm; however, all clinical measurements (IOP, VF, HRT) were carried out by masked observers and treatment decisions were masked by the use of a computerised evidence-based decision support algorithm. 56
Treatment arm allocation
Laser-1st pathway
Selective laser trabeculoplasty was delivered to 360° of the trabecular meshwork with one 360° retreatment used as the first escalation of treatment, if required. To ensure quality control of SLT delivery and to minimise variation between surgeons, standardisation was achieved by a stringent protocol defining laser settings and technique, including the range of acceptable powers (see Appendix 4). All treating clinicians were given training before recruitment and had at least one laser treatment directly observed by the chief investigator. After two SLT treatments, if further treatment escalation was required, the Laser-1st pathway patients embarked on medical treatment and followed the Medicine-1st algorithms. Significant complications of laser treatment, if they occurred (e.g. corneal oedema, intraocular haemorrhage, severe uveitis, IOP spike > 15 mmHg, peripheral anterior synechiae), meant that a second treatment with SLT was contraindicated. Other new medical conditions (such as a new history of uveitis or rubeosis) also precluded repeat SLT. 56
Medicine-1st pathway
Medical treatment of glaucoma involves several distinct steps that require standardisation: choice of drugs, number of agents permitted and rules for switching between or adding drugs. International best practice guidelines advocate changing medication if the target is not reached, with the addition or switching of medication (based on the magnitude of initial response). 64–66 Surgery was offered once maximum treatment intensity was reached; this varied between patients, but required definition to minimise inter-surgeon variation (see Maximum medical treatment).
Choice of agent
No mainstream medications were prohibited, but medication classes for first-, second- or third-line treatment were defined as per NICE1 and European Glaucoma Society guidance:67
-
first line: prostaglandin analogue
-
second line: beta-blocker (once in the morning or in a prostaglandin analogue combination)
-
third or fourth line: topical carbonic anhydrase inhibitor or alpha-adrenoceptor agonist.
Systemic carbonic anhydrase inhibitors were permitted only as a temporising measure while awaiting surgery and did not influence treatment escalation. Cholinergic agonists were not accepted as topical medications for OAG.
Treatment changes
Treatment was escalated under the following circumstances:
-
Strong evidence of progression (see Defining progression) irrespective of IOP.
-
IOP above the target IOP (see Adding/switching medication) by > 4 mmHg68 at a single visit (irrespective of evidence for progression).
-
IOP above target by < 4 mmHg plus less strong evidence for progression (see Defining progression). If the IOP was above target by less than the threshold with no evidence for progression, then the target IOP was re-evaluated.
Adding/switching medication
The incremental escalation of the treatment protocol defined stepwise increases in treatment. Patients’ medications were switched if the pre- and post-treatment IOP difference was no greater than the measurement error. If there was a greater reduction but the eye was still not at target, then the next medication was added. The progression of glaucomatous optic neuropathy (GON) when at target IOP, also triggered a stepwise increase in treatment and a lowering of the target. 56
Maximum medical treatment
Maximum medical treatment (MMT) is the most intensive combination of eyedrops a given individual can reasonably, reliably and safely use. The MMT varies between patients depending on their comorbidities, side effects and patient-specific concordance factors. Although there is variation in the attitudes of surgeons to polypharmacy, it is widely accepted that additional medications result in a lower percentage reduction in IOP. Evidence shows there are profound reductions in compliance with complex dosage schedules. NICE guidance15 recommends offering surgery after only two drugs have failed to control IOP. In the LiGHT trial, treatment with multiple different medications was limited and MMT was defined in terms of the maximum number of drugs (three) and dosages per day (five drops). The MMT was often less owing to drug intolerance, contraindications and patient factors. 56
Disease stratification and initiation of treatment
The NICE-recommended thresholds were used for defining disease (OAG or OHT) for entry into the study, as well as in initiating treatment (see Appendix 2). 15 The patients’ clinical evaluation and test outcomes were then entered into the clinical decision algorithm and a disease category and stage were determined. The algorithm used severity criteria from the Canadian target IOP workshop,69 with central-field loss severity criteria defined according to Mills et al. 70 (Table 1). Severity stratification determined the follow-up frequency.
| Severity | Definition for treatment target IOP | ||||
|---|---|---|---|---|---|
| Optic nerve | VF MD | Central (10°) scotoma on VF | |||
| OHT | Healthy | Any | No GON-related VF loss | ||
| Mild OAG | GON | + | > –6 dB | + | None |
| Moderate OAG | GON | + | < –6 dB and > –12 dB | or | At least one central 5º point < 15 dB but none < 0 dB and only one hemifield with a central point < 15 dB |
| Severe OAG | GON | + | < –12 dB | or | Any central 5º point with sensitivity < 0 dB. Both hemifields contain point(s) < 15 dB within 5º of fixation |
Computerised decision algorithm
The follow-up and treatment escalation protocols were enabled by custom-written clinical decision support software (DSS), which permitted real-time decision-making based on the analysis of multiple clinical measures, including HRT optic disc analysis, VF assessment and IOP measurements. Predefined objective indicators of either disc or field deterioration [change in mean neuroretinal rim area, as determined by HRT, or VF glaucoma progression analysis (GPA)], or IOP above target all triggered earlier follow-up and/or increased treatment intensity.
Setting individual target intraocular pressure
Once the decision to treat was made, a treatment target IOP (target) was set. The target was eye specific and was objectively defined and adjusted by the computerised decision algorithm to avoid bias from unmasked treating clinicians. The lowest permitted target was 8 mmHg for OAG and 18 mmHg for OHT. Although CCT has an effect on IOP measurement and risk of progression, the true magnitude of this interaction is unknown because of complex non-linear interactions between CCT, ‘true’ IOP and corneal material properties; CCT was therefore not used in the algorithm for setting a target IOP. 56 Myopia and family history were also not included in this algorithm, as data on the effect size of these risk factors on progression rates are weak. 71 The target IOP was either an absolute reduction to below a specified level or a percentage reduction from baseline, whichever was lower. The process of setting the IOP target is illustrated in Figure 1. Greater reductions were required for greater disease severity as defined by Canadian glaucoma study criteria. 73
FIGURE 1.
Process for target IOP setting. a, Disease stratification according to Mills et al. 70 POAG, primary open-angle glaucoma. Adapted by permission from BMJ Publishing Group Limited. The Laser in Glaucoma and Ocular Hypertension (LiGHT) trial. A multicentre randomised controlled trial: baseline patient characteristics, Konstantakopoulou E, Gazzard G, Vickerstaff V, Jiang Y, Nathwani N, Hunter R, Ambler G, Bunce C, volume 102, pp. 599–603, 2018. 72

Failure to meet target intraocular pressure and target intraocular pressure re-evaluation
Parts of this text have been reproduced from Gazzard et al. 58 © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (http://creativecommons.org/licenses/by/4.0/).
Diurnal fluctuation and measurement error both lead to variation in measured IOP. Kotecha et al. 68 have shown that inter-visit variation may nonetheless be as much as ± 4 mmHg. 68 To prevent an inappropriate escalation to a more intensive treatment, it is therefore important to repeat measurements that deviate only slightly from target. Criteria for failure to meet, and to reassess, target IOP follow those of the Canadian glaucoma study,74 taking into account that inter-visit variation in IOP measurement may be as much as ± 4 mmHg:
-
If IOP in an eye was ≥ 2 mmHg but < 4 mmHg above target on two consecutive visits and showed possible or definite progression, then the treatment was intensified and the target remained unchanged.
-
If IOP in an eye was ≥ 2 mmHg and < 4 mmHg above target on two consecutive visits and showed no progression (with a minimum of three post-baseline follow-up VF tests required to confirm progression, as per EMGT I),59 then the target was adjusted upwards. In this case the target IOP was revised to the mean of the previous three visits, during which progression did not occur. If VF testing had been carried out at fewer than three follow-up visits, additional visits were required to confirm stability before the target was relaxed.
-
If IOP in an eye was ≥ 4 mmHg from target at any visit, then the eye was considered to have failed to reach target and treatment intensity was increased to the next level (unless already at the maximum), irrespective of any progression, unless the clinician identified poor concordance with treatment. In such cases the target remained unchanged. In the presence of poor concordance and in the absence of progression, additional measures to improve concordance before escalation of treatment were permitted, as in usual clinical practice.
-
If the IOP of an eye on MMT was ≥ 2 mmHg from target and showed definite progression, then glaucoma drainage surgery was offered to the patient.
-
If the IOP of an eye on MMT was ≥ 2 mmHg from target and showed possible progression, then the follow-up frequency was increased until progression was either confirmed or ruled out.
-
If the IOP of an eye on MMT was ≥ 2 mmHg from target but below maximum IOP (maximum IOP is that above which surgery may be offered even without progression: OHT, 35 mmHg; mild glaucoma, 24 mmHg; moderate and severe glaucoma, 21 mmHg), and showed no progression (with at least three follow-up VFs), then the target was adjusted (revised to the mean of the previous three visits) with an increase in follow-up frequency. If VF testing had been carried out at fewer than three follow-up visits, additional visits were required to confirm stability.
-
A patient with an eye with IOP above the maximum IOP may have been offered surgery without progression at the discretion of the treating surgeon.
-
If there was progression and the IOP was at target, then the target IOP was reduced by 20% (according to the Canadian glaucoma study protocol),74 with a lower limit of 8 mmHg, and treatment intensified accordingly.
Failure to meet target can be a result of poor concordance as well as a lack of drug efficacy. As is normal practice, compliance was discussed and patients were counselled at each visit, using validated ‘ask–tell–ask’ techniques. 75–77 Patients were given standard written information from the International Glaucoma Association, face-to-face instruction in eyedrop administration and the offer of further nurse-led support.
Where poor concordance was thought to be the contributing factor, education with written information and repeated face-to-face instruction in eyedrop administration was given. If the decision was made to educate rather than escalate a patient who was not at target, then the reason for an algorithm over-ride was recorded (non-concordance) and the patient recalled after 8 weeks for a repeat IOP check visit. 56
Treatment escalation
To minimise bias for escalating treatment, standardised criteria for any additional intervention were used, in accordance a protocol following international guidelines by the European Glaucoma Society,64 American Academy of Ophthalmology Preferred Practice Pattern65 and the South East Asia Glaucoma Interest Group. 66 Treatment is escalated under the following circumstances:56
-
Strong evidence of progression irrespective of IOP (see Defining progression).
-
IOP above target by > 4 mmHg at a single visit (irrespective of evidence of progression).
-
IOP above target by < 4 mmHg and less strong evidence of progression (see Defining progression). If the IOP is above target by < 4 mmHg with no evidence of progression, then the treatment target IOP is re-evaluated (see Failure to meet target intraocular pressure and target intraocular pressure re-evaluation).
The process for escalating treatment is shown in Figures 2 and 3.
FIGURE 2.
Process for escalating treatment in OHT. a, On two consecutive visits; b, as per protocol; and c, until progression confirmed/refuted. VF progression required three follow-up VF assessments. Maximal IOP, IOP above which surgery was offered even without progression or 35 mmHg for OHT. Adapted by permission from BMJ Publishing Group Limited. The Laser in Glaucoma and Ocular Hypertension (LiGHT) trial. A multicentre randomised controlled trial: baseline patient characteristics, Konstantakopoulou E, Gazzard G, Vickerstaff V, Jiang Y, Nathwani N, Hunter R, Ambler G, Bunce C, volume 102, pp. 599–603, 2018. 72
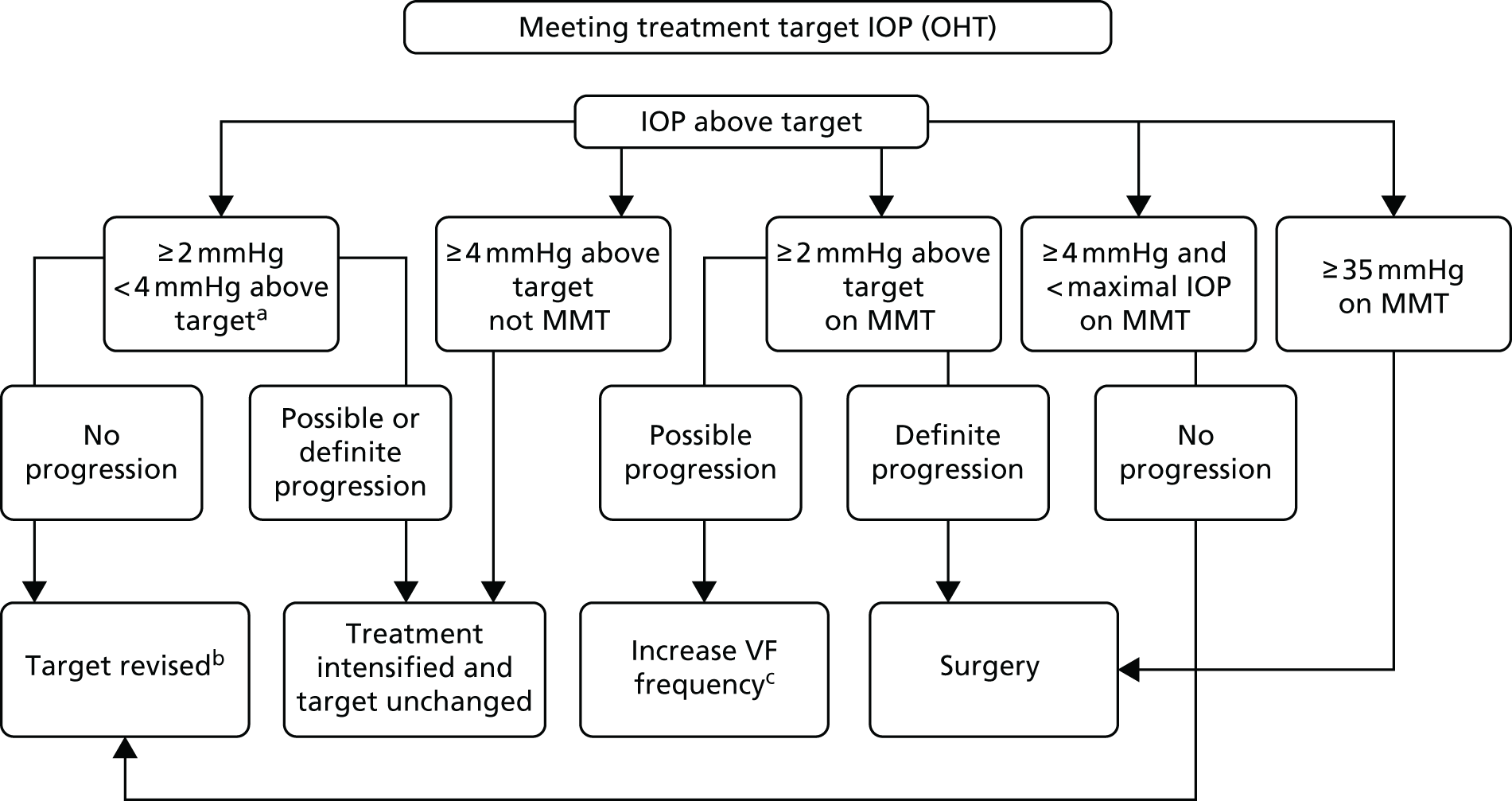
FIGURE 3.
Process for escalating treatment in OAG. a, On two consecutive visits; b, as per protocol; and c, until progression confirmed/refuted. VF progression required three follow-up VF assessments. Maximal IOP, IOP above which surgery was offered even without progression or 35 mmHg for OHT. Adapted by permission from BMJ Publishing Group Limited. The Laser in Glaucoma and Ocular Hypertension (LiGHT) trial. A multicentre randomised controlled trial: baseline patient characteristics, Konstantakopoulou E, Gazzard G, Vickerstaff V, Jiang Y, Nathwani N, Hunter R, Ambler G, Bunce C, volume 102, pp. 599–603, 2018. 72

More stringent criteria than those used for laser or medical treatment were applied before being referred for surgery. This reflected the greater risk to a patient’s vision from surgical complications. Strong evidence of progression and/or failure to meet target was usually required in all but the most severe disease. However, extreme elevations of IOP could be an indication for surgery without progression, with lower thresholds in more damaged eyes. Any patient in whom IOP was at or above the maximum was reviewed (in person or remotely) by the PI, who decided whether or not surgery was indicated. In accordance with the principle of patient-centred care, the decision to operate was always a collaboration between clinician and patient. When an IOP-lowering surgical intervention was indicated, cataract surgery was permitted (in the presence of cataract, i.e. not clear lens extraction) when this was the consultant’s usual practice.
Defining progression
Visual field progression
Worsening of VF loss was defined as ‘likely’ or ‘possible’ in the absence of any identifiable retinal or neurological cause. The ‘minimum data set’ to determine VF progression was two reliable baseline VF measurements followed by three follow-up VF tests. ‘Likely VF progression’ was defined as ≥ 3 points on the Humphrey Visual Field (HVF) GPA software (Carl Zeiss Meditec, Dublin, CA, USA) at p < 0.05 for change on three consecutive occasions. ‘Possible VF progression’ was ≥ 3 points on Humphrey Visual Field GPA software at p < 0.05 for change on two consecutive occasions. VF series were independently assessed for progression using the automated algorithm software at each visit. Any treatment escalation triggered by worsening VF loss had to be agreed by a senior clinician after excluding retinal or neurological causes. 56
Optic disc progression
Chauhan et al. 78 showed that sequential HRT three-disc assessment performed as well as, or better than, ‘experts’ judging monoscopic photos. Simultaneous stereoscopic disc photography has been considered a gold standard, but it is rarely available. Worsening of disc damage was defined as a rate of neuroretinal rim loss exceeding 1% of baseline rim area per year on a minimum of five repeat HRT images. This slope value was selected as approximately double that of age-related rim area loss and gave a similar specificity to VF trend analyses. 79
Open-angle glaucoma progression
Progression of glaucoma is defined as:
-
Strong evidence: GPA ‘likely progression’ and/or HRT rim area > 1% per year (p < 0.001).
-
Less strong evidence: GPA ‘possible progression’ and/or HRT rim area > 1% per year (p < 0.01).
Algorithm over-ride
In the following cases the algorithm was over-ridden by the treating consultant if:
-
Poor concordance was thought to be the contributing factor to failure to meet IOP target and was followed by patient education and a recall 8 weeks after for an IOP check.
-
It was felt that it was in the patient’s best interest to over-ride the algorithm’s decision to either revise the target IOP (upwards or downwards) or to escalate treatment.
The reason for the over-ride was recorded.
Follow-up procedure
Follow-up intervals were set at entry to the study, based on disease severity and lifetime risk of loss of vision, according to NICE guidance,15 and subsequently adjusted on the basis of IOP control, disease progression or adverse reactions. Disease stability, along with all available data, was taken into consideration, but testing for progression did not independently determine follow-up intervals. The routine schedule of appointments for patients who remained at or below the target IOP, without progression or treatment change, and who had no adverse reactions requiring earlier assessment, is shown in Table 2. Additional VF tests were permissible at any visit if clinically necessary to confirm possible progression. Variation in follow-up intervals was permitted to accommodate the clinician’s judgement and/or patient choice. 56
| Disease severity category | First visit | Routine follow-up intervals in months | ||||||
|---|---|---|---|---|---|---|---|---|
| Second visita | Third visit | Fourth visit | Fifth visit | Sixth visit | Seventh visit | Eighth visit | ||
| OHT | Randomisation and treatment | 2 | 4 | 6 | 12 | 12 | 12 | 12 |
| Mild OAG | 2 | 4 | 6 | 6 | 12 | 12 | 12 | |
| Moderate OAG | 2 | 4 | 6 | 6 | 6 | 6 | 6 | |
| Severe OAG | 1–2 | 4 | 6 | 6 | 6 | 6 | 6 | |
Participants in the Laser-1st arm were reviewed 2 and 8 weeks after SLT application. After the 8-week review in the Laser-1st group and for all treatment changes in the Medicine-1st arm, patients were reviewed at 2 months, following which their treatment was changed (with consequent early assessment of response to second treatment) or they entered a disease severity-tailored routine follow-up schedule. Follow-up of patients with severe OAG was at the discretion of the consultant ophthalmologist. If an eye showed ‘possible progression’, then the follow-up frequency was increased to every 3–4 months until progression was confirmed or ruled out with additional VF testing or HRT. No further tests were conducted at additional visits for IOP check alone. All contacts with medical professionals and optometrists were captured for cost data. Information on contact with health-care providers was collected via the CSRI, a validated method of collecting health-care cost data. 63
Follow-up intervals were planned within the ranges specified by NICE guidance15 and were independently determined on the basis of IOP control or adverse reactions, to minimise bias. The main driver for follow-up frequency was treatment in pursuit of control. Disease stability was considered using all available data, but testing for progression did not independently determine follow-up. Patients who required medication changes or additional laser treatment and patients who suffered AEs or showed progression of glaucoma were seen sooner and reverted to schedule when stable. The worse or more unstable of each patient’s two eyes determined the follow-up interval, whereas treatment was individualised to the needs of each eye.
Follow-up clinical assessments
The schedule of assessments (all assessments were part of routine care) is shown in Table 3. After the full baseline assessment, all patients underwent VF testing and HRT to assess progression at each follow-up visit. The EuroQol-5 Dimensions (EQ-5D-5L) and other HRQoL questionnaires were assessed at baseline and 6-monthly thereafter, with additional questionnaires as outlined in Questionnaires.
| Investigation | Time of follow-upa | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | First checka | Third visit (6 months) | First year | 18 months | Second year | Patient specificb | Third year | |
| Clinical examination (including disc and IOP) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Dilated fundus examination | Yes | – | Yes | Yes | Yes | Yes | Yes | Yes |
| Gonioscopy | Yes | – | – | – | – | – | – | Yes |
| VF test | Yes | – | Yes | Yes | Yes | Yes | Yes | Yes |
| Optic nerve imaging (HRT) | Yes | – | Yes | Yes | Yes | Yes | Yes | Yes |
| EQ-5D-5L | Yes | – | Yes | Yes | Yes | Yes | – | Yes |
| GUI | Yes | – | Yes | Yes | Yes | Yes | – | Yes |
| GSS | Yes | – | Yes | Yes | Yes | Yes | – | Yes |
| CSRIc | Yes | – | Yes | Yes | Yes | Yes | – | Yes |
Questionnaires
The content of the questionnaires was determined by the use of a number of validated, widely accepted existing questionnaires as follows:
-
EQ-5D-5L
-
GUI
-
GSS
-
GQL-15.
Additionally, a modified CSRI was used and two questions regarding concordance. The content of the questionnaires used can be found as supplementary material [see URL: www.journalslibrary.nihr.ac.uk/programmes/hta/0910440/#/documentation (accessed 23 April 2019)]; a sample of each questionnaire completed is presented in Appendix 5. The patient and public involvement group reviewed the final questionnaire for layout and clarity to ensure ease of completion.
Questionnaire delivery and follow-up
The baseline questionnaires were self-completed by participants in a private room, at the time of enrolment, after informed consent had been given but before randomisation. Participants were required to have sufficient English knowledge that translation was not required [practical assistance with the layout (e.g. some questionnaires were printed double-sided, some questions had conditional formatting depending on the patients’ response) and completion of the form were permitted].
Subsequent questionnaires were sent out by post for self-completion at 6-monthly intervals; up to two written reminders followed by one telephone follow-up were implemented in the case of non-response. In the event of a telephone follow-up, if the patient was willing, only the primary outcome measure was collected. Aiming to incentivise LiGHT participants to return the vital final questionnaire, a high street voucher worth £5.00 was sent by post along with the final set of questionnaires to each participant. The central site at MEH managed all questionnaires across all collaborating sites.
Follow-up has been extended beyond the primary study to look additionally at HRQoL outcomes at 6 years; questionnaires are will be posted to participants every 6 months for the duration of the extended period.
Adverse events and serious adverse events
An AE was defined as an unfavourable medical occurrence in a patient that was not necessarily caused by the treatment. GCP guidelines67 were used to determine if AEs should be classified serious [serious adverse events (SAEs)]. AEs and SAEs were reported in accordance with standard operating procedures (SOPs) and GCP guidelines, to achieve standardisation across sites and between treatment arms, with an annual safety report to the Research and Ethics Committee.
Primary outcome measure
The primary outcome measure was HRQoL in patients with OAG or OHT treated with SLT first, compared with HRQoL in patients treated with topical medication first, measured using EQ-5D-5L utility scores at 3 years.
Secondary outcome measures
The secondary outcomes were as follows:56
-
Treatment pathway health-care resource use, cost and cost-effectiveness. Health-care resource use was ascertained from the record of treatment episodes and additional health-care contacts using a modified CSRI. 63 The cost components included the cost of SLT, number of visits, number and type of medications and glaucoma surgeries, and clinical tests.
-
Glaucoma-specific treatment-related QoL was measured using the GUI, from which quality-adjusted life-years (QALYs) can also be derived.
-
Patient-reported disease and treatment-related symptoms using the GSS.
-
Patient-reported visual function using the GQL-15.
-
Objective measurements of pathway effectiveness for IOP-lowering and visual function preservation (e.g. treatment intensity and time taken to achieve target IOP, the number of target IOP revisions, proportion of patients achieving target after each year of treatment, number of patients with confirmed disease deterioration and rates of ocular surgery).
-
Objective safety measures for each pathway.
-
Concordance assessed by two questions shown to predict the probability of non-concordance. 80
Reproduced with permission from The Laser in Glaucoma and Ocular Hypertension (LiGHT) trial. A multicentre randomised controlled trial: baseline patient characteristics, Konstantakopoulou E, Gazzard G, Vickerstaff V, Jiang Y, Nathwani N, Hunter R, Ambler G, Bunce C, volume 102, pp. 599–603, 2018 with permission from BMJ Publishing Group Ltd. 72
Data collection and management
To standardise data collection and management, researchers were trained to follow specific SOPs for each stage of data handling. Identical electronic and hard-copy case report forms (CRFs) were designed according to a standard CRF template. A web-based database for the Priment Clinical Trials Unit, managed by the company ‘SealedEnvelope’, was used for database entry with direct data entry at the time of patient visit. This included extensive internal consistency and range checking, with a hard-copy backup CRF in case of information technology (IT) failure. Records were identifiable only by unique, confidential trial identification number with no patient-identifiable information included. All data were contemporaneously entered directly into the web-based database CRF.
Data from patient completed questionnaires received by post were scanned on receipt for e-copy back-up and entered onto the database within 1 week of receipt by the trial data management officer. Questionnaire data were from validated, standardised tools (EQ-5D-5L, GUI, GQL-15, GSS and CSRI) (see Table 3). The central site at MEH managed the inputting of data from all questionnaires across all collaborating sites.
Statistical analysis plan
The statistical analysis plan has been published previously. 81 All patients were analysed in the treatment arm to which they were randomised. All analyses were performed in Stata® version 14 (StataCorp LP, College Station, TX, USA).
Sample size
The sample size for the study was 718 participants. This number of participants was required to detect a difference of 0.05 in EQ-5D-5L between the two arms at 36 months using a two-sample t-test at the 5% significance level, with 90% power, assuming a common standard deviation (SD) of 0.1982 and a 15% loss to follow-up. 81
Baseline
The baseline characteristics of each arm were summarised as means and SDs for continuous, symmetric variables, medians and interquartile ranges (IQRs) for continuous, skewed variables and frequencies and percentages for categorical variables. These summaries were based only on observed data. No significance testing was performed.
Primary outcome
The primary outcome measure was HRQoL measured using the EQ-5D-5L at 36 months. The EQ-5D-5L questionnaire was analysed using a linear regression model, with an adjustment for the randomisation factors (severity and centre), baseline IOP, the baseline value of EQ-5D-5L and whether one or two eyes were affected at baseline.
For the primary outcome, the unit of analysis was the patient. If both of a patient’s eyes were included in the study, we used the worse eye at baseline for severity and baseline IOP covariates. The worse eye was defined using the MD at baseline, with the worse eye having the most negative MD.
The primary analysis used outcome data measured at 36 months. If these were missing, we imputed these missing data using the outcome measured at 30 months.
Secondary outcomes
The secondary outcomes were analysed using regression methods appropriate for the type of outcome. These models were also adjusted using the covariates mentioned above. The results from all secondary analyses are presented as estimates with confidence intervals (CIs).
Exploratory analyses
We used mixed-effect models, using all patient outcome data over the 36 months, to investigate how the primary and secondary outcomes changed over time. Such models allow the analysis of repeated outcome measurements data (recorded every 6 months), as well as taking into account the correlation between measurements from the same patient. Standard regression models assume independence between observations, which typically means that separate models are required for each time point. The mixed-effect models allowed modelling all time points (baseline and 6, 12, 18, 24, 30 and 36 months) in a single model, by explicitly modelling both the within- and the between-patient variability. 83
By using interaction terms between randomisation arm and time, we investigated differences between the groups over time.
We also used a similar mixed-effect model using all patient data over the 36 months, to evaluate the treatment effects at 36 months by using the exact times that the questionnaires were completed. Finally, using all the patient data over the 36 months, we used a mixed-effects model to explore the average treatment effect over the 36 months.
Analysis of missing data
Potential bias as a result of missing data was investigated by descriptively comparing the baseline characteristics of the trial participants with complete follow-up measurements with those who had incomplete follow-up or no outcome data.
Analysis of homogeneity
To explore the homogeneity (or otherwise) of the intervention effect on the primary outcome, we examined the treatment effect across the following: age (as a continuous measure); severity of glaucoma (using the two groups OHT/OAG used during randomisation process); baseline IOP (as a continuous measure); and sex. The results from these analyses should be treated as exploratory and hypothesis generating, as the trial was not powered for these analyses.
Sensitivity analyses
First, we ran sensitivity analyses that adjusted for variables associated with missingness. We performed logistic regression analyses (with missing ‘yes’ or ‘no’ as the outcome), to identify predictors of missing data. When predictors associated with both missing data and outcomes were found, we refitted the primary analysis model, adjusting for these predictors of missingness.
Second, to take into account any missing data, we used a multiple imputation approach. The imputation model included the outcome of interest, sociodemographic variables and any other variables potentially related to missingness and HRQoL. The imputations were performed separately by treatment arm.
Economic evaluation
The aim of the economic evaluation was to calculate the mean incremental cost per QALY of Laser-1st compared with Medicine-1st. Health and social care costs and QALYs were calculated for the within-trial period (36 months). The outputs were:
-
mean total patient-level QALYs by trial arm
-
mean cost per patient of laser treatment in the Laser-1st arm
-
mean cost per patient of eyedrop treatment for glaucoma by trial arm
-
mean cost per patient of surgery by trial arm
-
mean total health-care cost per patient over 3 years by trial arm
-
mean increment cost per QALY of Laser-1st compared with Medicine-1st and 95% CIs
-
cost-effectiveness planes
-
cost-effectiveness acceptability curves (CEACs).
Quality-adjusted life-years
Mean patient-level QALYs by trial arm were calculated as the area under the curve using patient-level responses to EQ-5D-5L at each follow-up time point84 and the formula by Devlin et al. 85 Patients who died were imputed as zero from the date of death until the end of the trial. We assumed a straight line from the last follow-up time point until death. As the EQ-5D-5L is the primary outcome for the trial, mean patient responses at each follow-up time point are reported as part of the repeated-measures analysis. The mean incremental difference in QALYs was calculated using ordinary least squares regression and included covariates for randomisation arm, baseline EQ-5D-5L values, randomisation factors (severity and centre), baseline IOP and number of eyes affected at baseline.
Quality-adjusted life-years were discounted from 12 months to 3 years at an annual rate of 3.5%. 86 Ninety-five per cent CIs were calculated using bootstrapping, bias corrected and 5000 replications, given that we assume that the data are not normally distributed. Although there was a high rate of return for the EQ-5D-5L at 36 months (91%), data were missing for each time point, which meant that only 73% of patients had complete data across all time points for calculation of QALYs. Multiple imputation using chained equations was used to impute the data for 35 data sets, including age, highest education attainment, employment and diabetic status, included as variables identified as being predictive of missingness.
Cost of selective laser trabeculoplasty
The cost of SLT was calculated using bottom-up microcosting based on data collected from sites. Sites reported the cost of the machine maintenance costs, how sessions were run (dedicated sessions for SLT or as part of a routine session), the grade and number of staff for each session and the number of patients treated per session and per year. Staff wages and overheads were taken from the Personal Social Services Resource Unit (PSSRU). 87 The cost per patient of using the machine was based on an annuitised formula,88 accounting for the number of patients seen in a ‘typical’ site per year and assuming a laser lifetime of 10 years. The number of SLTs per patient was reported.
Cost of drops for open-angle glaucoma and ocular hypertension
We report the mean cost of eyedrops by trial arm over 3 years. Information on eyedrops prescription, including drug name, dose, number of eyes, number of drops per eye and frequency, was collected as part of trial monitoring processes. Each prescription was costed using the British National Formulary (2018) to calculate the cost per bottle. 89 This was divided by the number of drops per bottle to calculate the cost per day. To calculate the number of days per medication, it was assumed that patients would take the medication from the day of prescription until the next medication change. The mean total cost per patient was then the cost per day of the prescribed eyedrops multiplied by the number of days the medication was prescribed for.
Total ophthalmology-related costs
In addition to eyedrops and laser, information was collected from the patient files on ocular surgery and planned and unplanned specialist ophthalmologist visits. These included a 2-week IOP check as part of the trial process; however, this check would not occur if the service was rolled out and hence this IOP check was removed from the primary analysis. Descriptive statistics for ophthalmology resource use are reported in Chapter 3, Ocular-related costs. Ocular surgery and ophthalmologist outpatient appointments were costed using the NHS Reference Costs 2016–17. 90 We report the mean cost per patient at 3 years for each type of ophthalmology cost, as well as total costs discounted at an annual rate of 3.5%86 by trial arm. Ninety-five per cent bias-corrected CIs were calculated using bootstrapping and 5000 replications. Given that data were taken from patient files, it was not possible to identify missing data (it was assumed that if patients did not have an appointment or surgery reported, this was because none occurred). As a result, the intention to treat (ITT) was based on all the patients, assuming that the appointment data collected are correct.
Other health-care costs
Health-care resource use, including optician contacts, community health-care contacts and acute health-care contacts, was collected from a modified version of the CSRI91 at baseline and at 6, 12, 18, 24, 30 and 36 months, asking about eye-related and non-eye-related resource use in the past 6 months. Information on inpatient stays and day cases was checked against SAE data. SAEs not reported in the CSRI were included in the total inpatient cost. Resource use was costed using unit costs from PSSRU87 except for optometrist visits,92 heart bypass surgery90 and cancer deaths (Table 4). 93 Mean costs by trial arm at each time point were by ocular- and non-ocular-related costs over 3 years.
| Resource use | Unit cost (£) (per contact) | Source |
|---|---|---|
| Trabeculectomy | 1436 | NHS Reference Costs 2016–17 90 |
| Ophthalmology appointments | 91 | NHS Reference Costs 2016–17 90 |
| Optometrist visit | 52 | Violato et al.92 |
| Planned inpatient stay | 3903 | Curtis87 |
| Unplanned inpatient stay: short durationa | 628 | Curtis87 |
| Unplanned inpatient stay: long durationb | 2953 | Curtis87 |
| A&E attendance: admitted | 221 | NHS Reference Costs 2016–17 90 |
| A&E attendance: not admitted | 128 | NHS Reference Costs 2016–17 90 |
| Outpatient attendance | 137 | Curtis87 |
| GP contact: in practice | 31 | Curtis87 |
| GP contact: telephone | 24 | Curtis87 |
| GP contact: at home | 80 | Curtis87 |
| GP practice nurse | 36 | Curtis87 |
| Social care | 59 | Curtis87 |
| Home care | 26 | Curtis87 |
| Other community contacts | 57 | NHS Reference Costs 2016–17 90 |
| Cancer death | 6129 | Georghiou and Bardsley93 |
Total health and social care costs
The cost components included in the analysis were the cost of SLT, OAG medication and other health-care costs. We report the mean cost per patient in addition to an adjusted cost, adjusting for baseline service use using regression analysis. Mean costs were based on a complete-case analysis, with only optician and CSRI resource use excluding inpatient stays missing (an analysis imputing for missing CSRI data using chained equations has been included). The mean incremental difference in costs is calculated using ordinary least squares regression and includes covariates for randomisation arm, baseline EQ-5D-5L values, randomisation factors (severity and centre), baseline IOP and number of eyes affected at baseline. We used bias-corrected bootstrapping to calculate 95% CIs, given that we assumed that the data are not normally distributed. All costs are reported in 2016/17 Great British pounds.
Incremental cost-effectiveness ratio
The incremental cost-effectiveness ratio (ICER) was defined as the mean incremental cost of Laser-1st compared with Medicine-1st and divided by the mean incremental QALYs of laser treatment compared with eyedrops. The mean incremental differences were adjusted for baseline values, randomisation factors (severity and centre), baseline IOP and number of eyes affected at baseline. To account for the correlation between costs and QALYs, seemingly unrelated regression was used to calculate the numerator and denominator of the ICER. ICERs are reported for total costs, as defined in Total health and social care costs, and ophthalmology only costs, as defined in Total ophthalmology-related costs. Costs and QALYs from 12 months until 36 months are discounted at an annual rate of 3.5%. 86 The final results for total costs and QALYs are based on data imputed using chained equations for QALYs and CSRI, and using the missing at random methodology described in Leurent et al. 57 for calculating CEACs using bootstrapping and multiple imputation for 200 draws of each of the 35 imputed data sets for 7000 replications in total.
Cost-effectiveness acceptability curve
A CEAC is reported using the bootstrap imputed data (200 draws of each of the 35 imputed data sets for 7000 replications in total), for a range of values of willingness to pay for a QALY. We report the probability that Laser-1st is cost-effective compared with Medicine-1st at a willingness to pay for a QALY of £20,000 and £30,000 for (1) total costs and (2) ophthalmology only costs.
Secondary analyses
The following secondary analyses were conducted:
-
For the primary analysis SLT was costed using microcosting. Some assumptions of the microcosting, for example the number of patients per site per year, or how sessions are run, may have an impact on the total cost. As a result, we planned to examine the impact of modifying the assumptions on the total cost of SLT per patient and hence the ICER. The cost of SLT as estimated from NHS reference costs90 was used in the analysis.
-
In the primary analysis we removed the cost of the 2-week IOP check, given that this was unlikely to occur in practice. One could hypothesise that patients obtained some minor benefit from this check and hence its costs could be included in the analysis. A secondary analysis including the 2-week IOP check has been included.
-
QALYs were calculated using utility scores generated from the GUI61 and the same methodology for calculating QALYs as above. The results were combined with the costs, as above, to report the mean incremental cost per QALY of Laser-1st compared with Medicine-1st, using the GUI.
Patient and public involvement: lay advisory group
Glaucoma patients and relatives from the Cochrane Eyes and Vision Group Consumer Panel formed our independent LAG. Consultation on trial design, choice of outcome measures, recruitment and treatment acceptability took place by e-mail and through online discussions via the Facebook social networking site (Facebook, Inc., Menlo Park, CA, USA; Group: ‘Public Eye – LiGHT Trial Discussion Forum’). All of the suggestions made have been incorporated (e.g. requests to monitor all symptoms in detail, a safety concern about ‘rapid loss of pressure control’ after SLT and more explanation of the relationship between eyedrops and surgical failure). An ‘expert patient’ with treated glaucoma reviewed and commented on the study protocol as a service user member for the Trial Steering Committee (TSC) and another service user representative from the International Glaucoma Association was invited to join. The LAG contributed to the development of tailored information leaflets and consent forms with further consultation with service user groups and via the Friends of Moorfields charity. A survey of 100 new patients attending MEH to assess the acceptability of an invitation to participate in such a trial, before the commencement of the trial, had a 70% positive response.
Patients diagnosed and treated for glaucoma also provided input to a questionnaire sent in 2015 (see Appendix 6), allowing us to design an extension for the main LiGHT trial; the questionnaire looked into the views of these treated patients on current treatment options and their willingness to switch from eyedrops to laser.
As required by the NHS, in line with INVOLVE national guidelines and in accordance with UK Clinical Research Collaboration policy, the results are being communicated to patients, for example via NHS Choices and patient advocate groups (e.g. International Glaucoma Association), and the findings have been published in open access media. 58
Study oversight and management
Study co-ordination in London
The Trial Management Team was composed of the chief investigator, central trial manager (CTM), central trial optometrist (CTO), central research optometrist (CRO), lead trial statistician and trial statistician, members of the University College London Priment Clinical Trials Unit, trial data officers, co-applicants and trial optometrists. The team met monthly on average to ensure the smooth running of the trial and troubleshooting. The duties of the CTM were to support the organisation of the study [investigator meetings, TSC and Data Management Committee (DMC) meetings, training, etc.] and have a study management role, including monitoring data collection according to established milestones, maintaining trial records, co-ordinating data management between local sites and the central clinical trial unit, facilitating user involvement in the project through LAG meetings and working alongside the CTO and facilitating the recruitment and follow-up of study participants.
Local organisation in centres
The chief investigator (consultant ophthalmologist) was the local PI at the central site (MEH), who co-ordinated the local ethics approval and sat on the TSC. The local study co-ordinator administrated the follow-up and recall of patients, liaising with the Trial Management Team. The local trial clinicians were an ophthalmologist, a fellow or an optometrist who were responsible for the recruitment, treatment and follow-up of trial participants. They had regular conference calls with the Trial Management Team for the duration of the study. The local trial clinicians were directly accountable to the local PIs. There were regular conference calls to all local clinicians and PIs to troubleshoot local issues. The chief investigator closely supervised the CTM, CTO and CRO with regular meetings.
Trial Steering Committee
The TSC was composed, in accordance with GCP, of an ophthalmologist as the independent TSC chairperson, a chief investigator, an independent clinician with relevant expertise, a sponsor representative, a Central and East London Comprehensive Local Research Network representative, an independent health economist, an independent statistician and two patient representatives. The trial manager, chief investigator, lead trial statistician and trial statistician were invited to report as required. The TSC met at least 6-monthly and minutes were taken.
Data and safety monitoring
Data and safety monitoring by the University College London Priment Clinical Trials Unit involved regular reports from the CTM, including recruitment and drop-out rates, adherence to SOPs, number failing to meet target or progressing, and AEs. The chief investigator maintained day-to-day responsibility for the trial with the CTM to ensure that the trial was conducted, recorded and reported in accordance with the protocol, GCP94 guidelines and SOPs.
The DMC was composed of the following individuals in accordance with GCP guidelines: (1) a DMC chairperson, (2) an independent trial statistician and (3) two additional glaucoma or ophthalmic trials specialists. The DMC met annually (or more often if appropriate), timed to report to the TSC. During recruitment, interim reports were supplied to the DMC, together with any analyses it requested.
The above committees followed SOPs set by MEH and the University College London Priment Clinical Trials Unit, and complied with guidelines issued by the National Institute for Health Research (NIHR) Health and Technology Assessment panel for clinical trials.
Data monitoring
We completed double data entry for the EQ-5D-5L for all completed questionnaires for all time points. The second data entry was completed by a different individual to the person doing the first entry. The first data entry was then matched with the second data entry and any discrepancies were checked and resolved by referring back to the hard-copy questionnaire.
The trial research team performed checks on 100% of the clinical baseline and eligibility data. Monitoring activity across sites was carried out at scheduled intervals and was adapted to the demonstration of errors by the collaborating sites (see Appendix 7). Protocol deviations and violations were recorded throughout the study and appropriate action was taken to prevent similar events from taking place in the future.
Protocol amendments
A series of minor amendments have taken place after the commencement of the trial and were submitted to the funder, as well as gaining ethics approval. Below is a list of the major protocol amendments:
-
addition of audio-visual material to assist with recruitment of patients
-
collection of blood, tears and saliva samples
-
addition of the ocular response analyser (Reichert Ophthalmic Instruments, Inc., Buffalo, NY, USA) to the assessments
-
extension of the trial to 6 years
-
ocular surface disease questionnaire (extension only).
Chapter 3 Results
The main results of the study have been published in Gazzard et al. 58 © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 licence (http://creativecommons.org/licenses/by/4.0/).
Recruitment
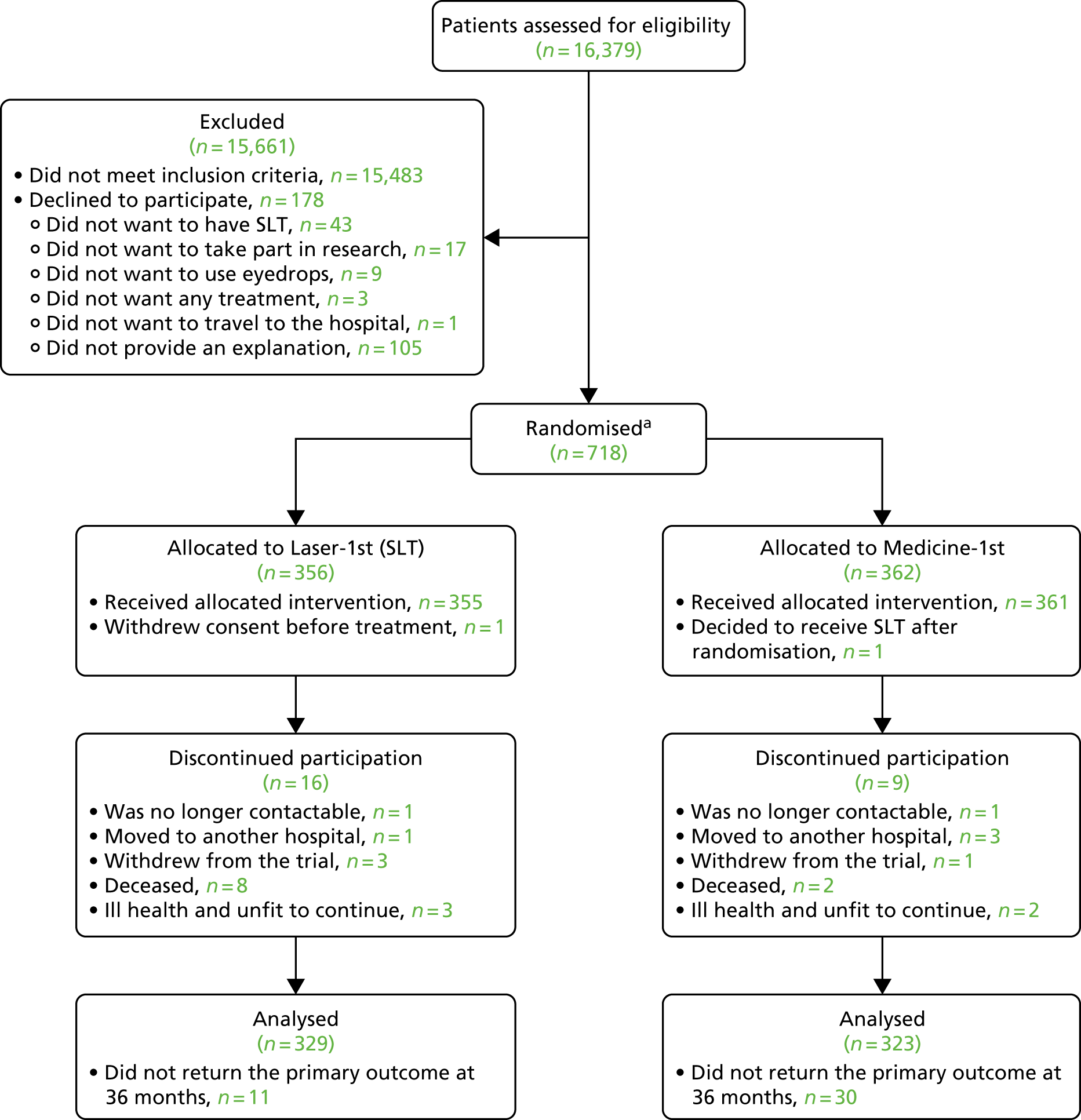
A total of 16,379 patients were assessed for eligibility; 15,483 were excluded as they did not meet the inclusion criteria. Of the 896 patients who were eligible across the six participating NHS centres, a total of 718 (1235 eyes) were recruited for the study (80.1% participation rate). A recruitment chart for the total recruitment period can be found in Appendix 8. A total of 178 eligible patients declined to participate. Of the patients who declined to participate, 43 did not want to have SLT, 17 did not want to take part in research, nine did not want to use eyedrops, three did not want to receive any treatment, one did not want to travel to the hospital and 105 did not provide an explanation.
Participant flow
A total of 718 patients (1235 eyes) were randomised: 356 patients (613 eyes) were allocated to SLT (Laser-1st pathway) and 362 patients (622 eyes) to medical treatment (Medicine-1st pathway) (Figure 4). Two patients were randomised twice owing to failure, as a result of which the initial randomisation was not visible. Subsequently, a second randomisation was carried out; one of these patients had initially been randomised to medication (non-visible randomisation), but was subsequently randomised to, and received, SLT. The second patient was initially randomised to SLT (non-visible randomisation), but was later randomised to, and received, medication. Four patients who did not meet the eligibility criteria were randomised in error and were subsequently removed from the study (see Appendix 9).
FIGURE 4.
The LiGHT trial Consolidated Standards of Reporting Trials (CONSORT) flow diagram. a, Two patients were randomised twice owing to IT failure, as a result of which the initial randomisation was not visible, and subsequently a second randomisation was carried out. Reproduced from Gazzard et al. 58 © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (http://creativecommons.org/licenses/by/4.0/).
Participant baseline characteristics
Reproduced with permission from The Laser in Glaucoma and Ocular Hypertension (LiGHT) trial. A multicentre randomised controlled trial: baseline patient characteristics, Konstantakopoulou E, Gazzard G, Vickerstaff V, Jiang Y, Nathwani N, Hunter R, Ambler G, Bunce C, volume 102, pp. 599–603, 2018 with permission from BMJ Publishing Group Ltd. 72
Participant baseline characteristics are shown in Table 5. Of the 718 patients recruited, approximately 70% were based in London: 52% were recruited by MEH and almost 15% from Guy’s and St Thomas’ Hospital. The average age of the patients was 63.1 years (± 11.8 years), with more male patients recruited than female (55.3% male vs. 44.7% female). In total, 70% of all participants were white, and black was the second largest ethnic group (20%). Thirty per cent of patients reported a family history of glaucoma affecting at least one first-degree relative. Systemic hypertension (defined as the use of prescribed antihypertensive medication) was recorded in 35% of the patients. Use of systemic antihypertensive medication was recorded in these patients; of those, 5% were using beta-blockers, 16% were using calcium channel blockers and 14% were using angiotensin-converting enzyme inhibitors; 27% of all patients were on statins. In total, 11% of the patients were smokers at the time of recruitment. Approximately 30% of the LiGHT patients had a degree or equivalent qualification, 13% had achieved higher education, 12% had achieved Advanced Level or equivalent and 45% did not pursue an education beyond 16 years of age.
| Characteristic | Total (N = 718), n (%) | Medicine-1st (N = 362), n (%) | Laser-1st (N = 356), n (%) |
|---|---|---|---|
| Centre | |||
| MEH | 374 (52.1) | 187 (51.7) | 187 (52.5) |
| Hinchingbrooke Hospital | 82 (11.4) | 41 (11.3) | 41 (11.5) |
| Guy’s and St Thomas’ Hospital | 106 (14.8) | 55 (15.2) | 51 (14.3) |
| Queen’s University Belfast | 30 (4.2) | 15 (4.1) | 15 (4.2) |
| Norfolk and Norwich University Hospital | 89 (12.4) | 46 (12.7) | 43 (12.1) |
| York Hospital | 37 (5.2) | 18 (5.0) | 19 (5.3) |
| Age (years), mean (SD) | 63.1 (11.8) | 62.7 (11.6) | 63.4 (12.0) |
| Sex, n (%) | |||
| Male | 397 (55.3) | 197 (54.4) | 200 (56.2) |
| Female | 321 (44.7) | 165 (45.6) | 156 (43.8) |
| Ethnicity, n (%)a | |||
| Asian | 51 (7.1) | 28 (7.7) | 23 (6.5) |
| Black | 146 (20.3) | 69 (19.1) | 77 (21.6) |
| White | 501 (69.8) | 258 (71.3) | 243 (68.3) |
| Other | 20 (2.8) | 7 (1.9) | 13 (3.7) |
| Diagnosis, n (%) | |||
| OAG | 555 (77.3) | 282 (77.9) | 273 (76.7) |
| OHT | 163 (22.7) | 80 (22.1) | 83 (23.3) |
| General health conditions, n (%) | |||
| Asthma | 93 (13.0) | 45 (12.4) | 48 (13.5) |
| Hypertension | 251 (35.0) | 119 (32.9) | 132 (37.1) |
| Diabetes | 82 (11.4) | 40 (11.1) | 42 (11.8) |
| Angina | 21 (2.9) | 11 (3.0) | 10 (2.8) |
| Cardiac arrhythmia | 37 (5.2) | 20 (5.5) | 17 (4.8) |
| Medication, n (%) | |||
| Statins | 196 (27.3) | 92 (25.4) | 104 (29.2) |
| Systemic beta-blockers | 34 (4.7) | 12 (3.3) | 22 (6.2) |
| Calcium channel blocker | 116 (16.2) | 60 (16.6) | 56 (15.7) |
| ACE inhibitors | 100 (13.9) | 43 (11.9) | 57 (16.0) |
| Corticosteroids | 42 (5.9) | 20 (5.5) | 22 (6.2) |
| Family ocular history of glaucoma,b n (%) | 214 (30.0) | 107 (29.6) | 107 (30.1) |
| Highest education achievement, n (%) | |||
| Degree or equivalent | 216 (30.1) | 106 (29.3) | 110 (30.9) |
| Higher education | 94 (13.1) | 39 (10.8) | 55 (15.5) |
| A level or equivalent | 88 (12.3) | 49 (13.5) | 39 (11.0) |
| GCSE | 155 (21.6) | 84 (23.2) | 71 (19.9) |
| Other qualifications | 59 (8.2) | 30 (8.3) | 29 (8.2) |
| No qualification | 106 (14.8) | 54 (14.9) | 52 (14.6) |
A total of 301 patients (41.9%) had bilateral OAG, 161 patients (22.4%) had unilateral OAG (fellow eye healthy), 93 patients (13.0%) had OAG in one eye and OHT in the other eye, 124 patients (17.3%) had bilateral OHT and 39 patients (5.4%) had unilateral OHT (fellow eye healthy). A total of 555 patients (77.2%) were classified as having OAG (if at least one eye was affected by OAG) and 163 patients (22.7%) were classified as having OHT; both eyes were eligible for the trial in 517 patients (72.0%), only the right eye was eligible in 96 patients (13.4%), and only the left eye was eligible in 105 patients (14.6%); 55% of the better eyes were right eyes. 72
The baseline patient characteristics were similar between the two groups in terms of age, sex distribution, ethnicity, general health and family history of glaucoma (see Table 5). There were small differences in the medication and education of the patients. The eye characteristics were also similar between the two treatment arms, with VA, VF MD, HRT optic disc rim area, IOP and CCT comparable between the two treatment arms (Table 6).
| Characteristic | N | All eyes (N = 1235) | Medicine-1st (N = 622) | Laser-1st (N = 613) |
|---|---|---|---|---|
| Diagnosis, n (%) | ||||
| OHT | 380 (30.8) | 185 (29.7) | 195 (31.8) | |
| Mild OAG | 636 (51.5) | 325 (52.3) | 311 (50.7) | |
| Moderate OAG | 144 (11.7) | 77 (12.4) | 67 (10.9) | |
| Severe OAG | 75 (6.1) | 35 (5.6) | 40 (6.5) | |
| Refractive error (spherical D), mean (SD) | 1225 | –0.23 (3.0) | –0.2 (2.7) | –0.3 (3.2) |
| VA, mean (SD) | 1235 | 0.1 (0.12) | 0.1 (0.1) | 0.1 (0.2) |
| VF MD (dB), mean (SD) | 1233 | –3.0 (3.45) | –3.0 (3.6) | –3.0 (3.4) |
| HRT rim area, mean (SD) | 1128 | 1.2 (0.4) | 1.1 (0.4) | 1.2 (0.4) |
| IOP, mean (SD) | 1233 | 24.5 (5.1) | 24.4 (5.0) | 24.5 (5.2) |
| CCT (µm), mean (SD) | 1229 | 551.1 (37.2) | 551.6 (36.3) | 550.7 (38.1) |
| PXF, n (%) | 1233 | 17 (1.4) | 12 (1.9) | 5 (0.8) |
| Pseudophakia, n (%) | 1233 | 72 (5.8) | 33 (5.3) | 39 (6.4) |
The baseline scores for QoL (EQ-5D-5L, GSS, GUI and GQL-15) are shown in Table 7. The two treatment arms had similar average EQ-5D-5L scores (Medicine-1st 0.92 ± 0.13, Laser-1st 0.91 ± 0.13; higher scores indicate better HRQoL), GUI scores (Medicine-1st 0.89 ± 0.11, Laser-1st 0.89 ± 0.12; higher scores indicate better HRQoL) and GQL-15 scores (Medicine-1st 18.7 ± 5.6, Laser-1st 18.9 ± 6.6; higher scores indicate worse HRQoL) at baseline. The Medicine-1st arm showed slightly higher average GSS scores at baseline than the Laser-1st arm (Medicine-1st 83.3 ± 16.6, Laser-1st 81.4 ± 17.2; higher scores indicate better HRQoL).
| Measure | Overall (N = 717), mean (SD) | Medicine-1st (N = 362), mean (SD) | Laser-1st (N = 355), mean (SD) |
|---|---|---|---|
| EQ-5D-5L index | 0.91 (0.13) | 0.92 (0.13) | 0.91 (0.13) |
| GUIa | 0.89 (0.12) | 0.89 (0.11) | 0.89 (0.12) |
| GSSb | 82.4 (16.9) | 83.3 (16.6) | 81.4 (17.2) |
| Subscales | |||
| Symptom | 80.2 (19.7) | 81.2 (19.4) | 79.1 (20.1) |
| Function | 85.6 (17.6) | 86.4 (17.3) | 84.8 (17.8) |
| GQL-15a | 18.8 (6.1) | 18.7 (5.6) | 18.9 (6.6) |
| Subscales | |||
| Central | 2.5 (1.0) | 2.5 (1.0) | 2.5 (1.0) |
| Peripheral | 8.5 (3.1) | 8.4 (2.9) | 8.5 (3.4) |
| Dark | 7.9 (2.9) | 7.9 (2.8) | 7.9 (3.0) |
| Outdoor | 1.1 (0.4) | 1.1 (0.4) | 1.1 (0.4) |
Primary outcome return rates
A total of 652 patients returned the primary outcome at the trial’s end point at 36 months (overall return rate was 91%: 92% for the SLT arm and 89% for the Medicine-1st arm), and were included in the ITT analysis (with imputation used for missingness). An additional 21 patients supplied 30-month data, which was used to impute their missing 36-month data, such that 673 patients were included in the primary ITT analysis.
Losses to follow-up
A total of 16 patients in the Laser-1st arm and nine patients in the Medicine-1st arm discontinued participation (see Figure 4). In total, two patients were lost to follow-up and were no longer contactable, four patients moved to a different hospital, four patients withdrew from the trial, five patients could not continue participation owing to ill health and 10 patients died.
Quality of life
Primary outcome: EQ-5D-5L
At 36 months, the Laser-1st arm had an average EQ-5D-5L score of 0.90 (SD 0.16), compared with 0.89 (SD 0.18) in the Medicine-1st arm, suggesting little difference between the two treatment arms [adjusted mean difference (Laser-1st – Medicine-1st) 0.01, 95% CI –0.01 to 0.03; p = 0.23] (Table 8 and Figure 5). The results were confirmed in sensitivity analyses (see Appendix 10). Taking into account the outcome data from all time points across 36 months, the two treatment arms had similar EQ-5D-5L scores at 36 months [adjusted mean difference 0.02 (95% CI –0.00 to 0.03) and 0.01 (95% CI –0.01 to 0.02), when using exact times of questionnaire returns).
| Measure | Medicine-1st | Laser-1st | Adjusted mean differencea | 95% CI | p-value | ||
|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||||
| Primary analysis at 36 months | |||||||
| EQ-5D-5L | 336 | 0.89 (0.18) | 337 | 0.90 (0.16) | 0.01 | –0.01 to 0.03 | 0.230 |
| GUI | 299 | 0.89 (0.13) | 303 | 0.89 (0.13) | 0.01 | –0.01 to 0.03 | |
| GSS | 281 | 83.3 (17.3) | 294 | 83.1 (17.7) | 1.6 | –0.8 to 4.0 | |
| GQL-15 | 297 | 19.8 (7.8) | 304 | 19.8 (7.2) | –0.4 | –1.3 to 0.6 | |
| QALY | 263 | 2.70 (0.42) | 261 | 2.74 (0.37) | 0.025 | 0.02 to 0.07 | 0.289 |
| QALY (discounted) | 263 | 2.62 (0.41) | 261 | 2.65 (0.36) | 0.024 | –0.02 to 0.07 | 0.286 |
| Repeated measures analysis across 36 months | |||||||
| EQ-5D-5L | |||||||
| Baseline | 362 | 0.92 (0.13) | 355 | 0.91 (0.13) | |||
| 6 months | 332 | 0.90 (0.15) | 330 | 0.91 (0.13) | 0.01 | –0.01 to 0.03 | |
| 12 months | 327 | 0.91 (0.14) | 327 | 0.91 (0.14) | 0.01 | –0.01 to 0.02 | |
| 18 months | 329 | 0.90 (0.16) | 325 | 0.90 (0.16) | 0.00 | –0.02 to 0.02 | |
| 24 months | 326 | 0.91 (0.14) | 326 | 0.91 (0.14) | –0.00 | –0.02 to 0.02 | |
| 30 months | 320 | 0.90 (0.15) | 317 | 0.90 (0.15) | 0.00 | –0.01 to 0.02 | |
| 36 months | 323 | 0.89 (0.18) | 329 | 0.90 (0.16) | 0.02 | –0.00 to 0.03 | |
| GUI | |||||||
| Baseline | 361 | 0.89 (0.11) | 355 | 0.89 (0.12) | |||
| 6 months | 330 | 0.90 (0.11) | 329 | 0.91 (0.10) | 0.01 | –0.00 to 0.03 | |
| 12 months | 315 | 0.89 (0.12) | 320 | 0.91 (0.11) | 0.01 | –0.00 to 0.03 | |
| 18 months | 305 | 0.89 (0.12) | 303 | 0.90 (0.13) | 0.01 | –0.01 to 0.02 | |
| 24 months | 298 | 0.89 (0.12) | 305 | 0.90 (0.11) | 0.02 | 0.00 to 0.03 | |
| 30 months | 299 | 0.88 (0.12) | 291 | 0.89 (0.12) | 0.02 | 0.00 to 0.03 | |
| 36 months | 300 | 0.89 (0.13) | 303 | 0.89 (0.13) | 0.01 | –0.01 to 0.02 | |
| GSS | |||||||
| Baseline | 357 | 83.3 (16.6) | 353 | 81.4 (17.2) | |||
| 6 months | 321 | 83.0 (16.3) | 320 | 85.6 (14.9) | 4.0 | 2.0 to 6.0 | |
| 12 months | 310 | 83.0 (17.6) | 309 | 85.2 (15.4) | 2.9 | 0.8 to 4.9 | |
| 18 months | 295 | 83.1 (16.8) | 294 | 84.6 (15.8) | 2.8 | 0.7 to 4.8 | |
| 24 months | 287 | 83.3 (16.4) | 290 | 83.3 (16.3) | 1.4 | –0.7 to 3.5 | |
| 30 months | 288 | 81.3 (17.6) | 276 | 84.1 (16.7) | 3.5 | 1.5 to 5.6 | |
| 36 months | 282 | 83.3 (17.3) | 296 | 83.1 (17.7) | 2.2 | 0.1 to 4.2 | |
| GQL-15 | |||||||
| Baseline | 361 | 18.7 (5.6) | 355 | 18.9 (6.6) | |||
| 6 months | 323 | 18.8 (5.6) | 324 | 18.3 (5.4) | –0.8 | –1.6 to 0.0 | |
| 12 months | 314 | 19.2 (7.2) | 318 | 18.8 (6.6) | –0.5 | –1.4 to 0.3 | |
| 18 months | 302 | 19.1 (6.4) | 298 | 18.9 (6.5) | –0.6 | –1.4 to 0.2 | |
| 24 months | 289 | 19.5 (7.3) | 298 | 19.2 (6.7) | –0.5 | –1.3 to 0.4 | |
| 30 months | 293 | 19.9 (7.1) | 287 | 19.6 (7.9) | –0.3 | –1.1 to 0.5 | |
| 36 months | 298 | 19.8 (7.8) | 304 | 19.8 (7.2) | –0.4 | –1.2 to 0.4 | |
FIGURE 5.
Mean (a) EQ-5D-5L, (b) GUI, (c) GSS and (d) GQL-15 scores at each time point, across 36 months. Time point ‘0’ refers to pre-treatment. a, Higher scores on EQ-5D-5L, GUI and GSS indicate better HRQoL; b, higher scores on GQL-15 indicate worse HRQoL. Adapted by permission from BMJ Publishing Group Limited. The Laser in Glaucoma and Ocular Hypertension (LiGHT) trial. A multicentre randomised controlled trial: baseline patient characteristics, Konstantakopoulou E, Gazzard G, Vickerstaff V, Jiang Y, Nathwani N, Hunter R, Ambler G, Bunce C, volume 102, pp. 599–603, 2018. 72
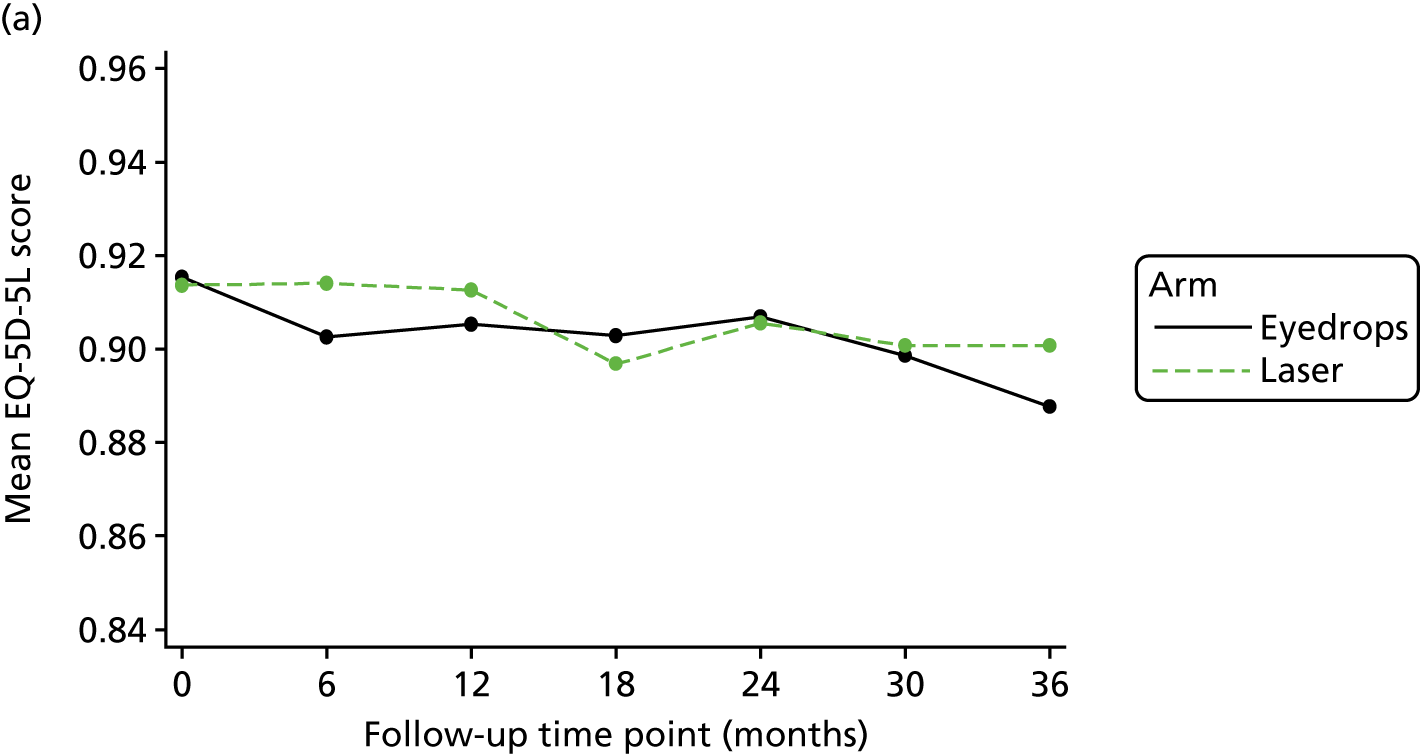
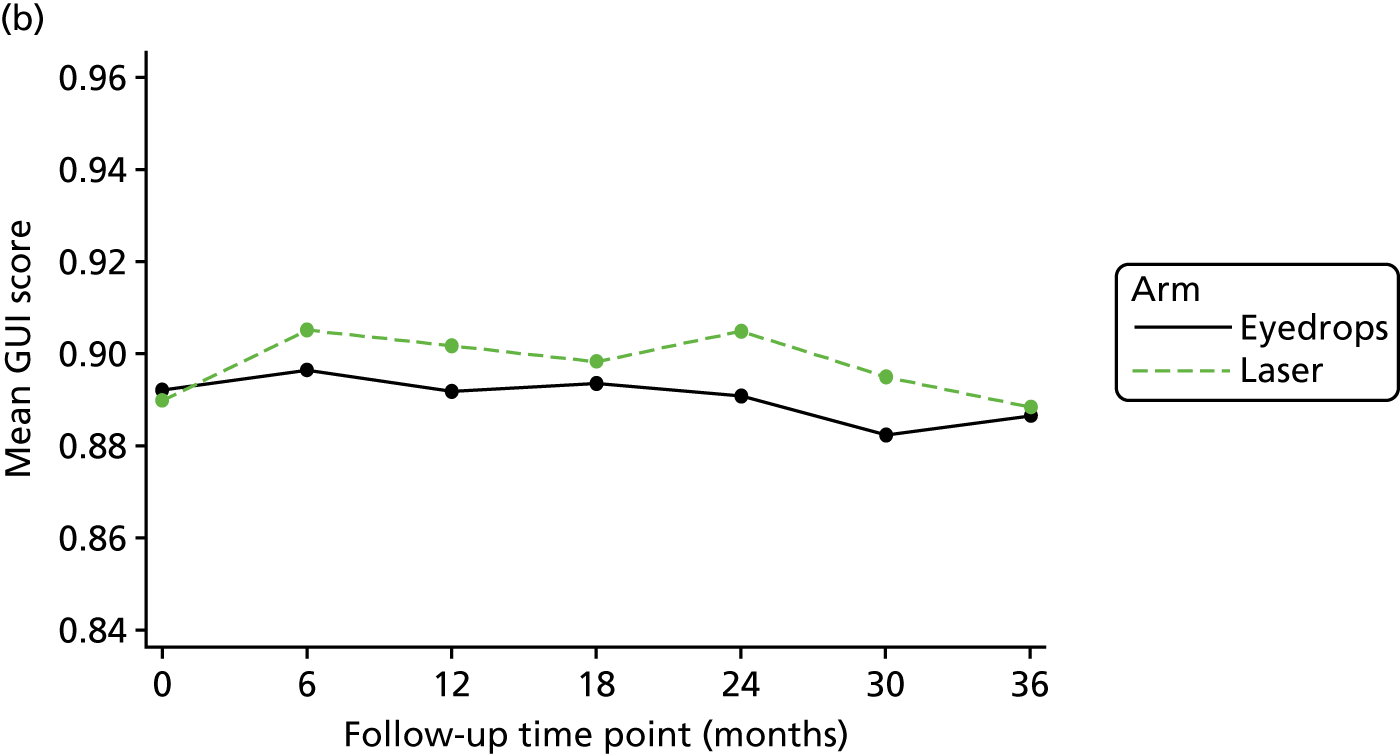

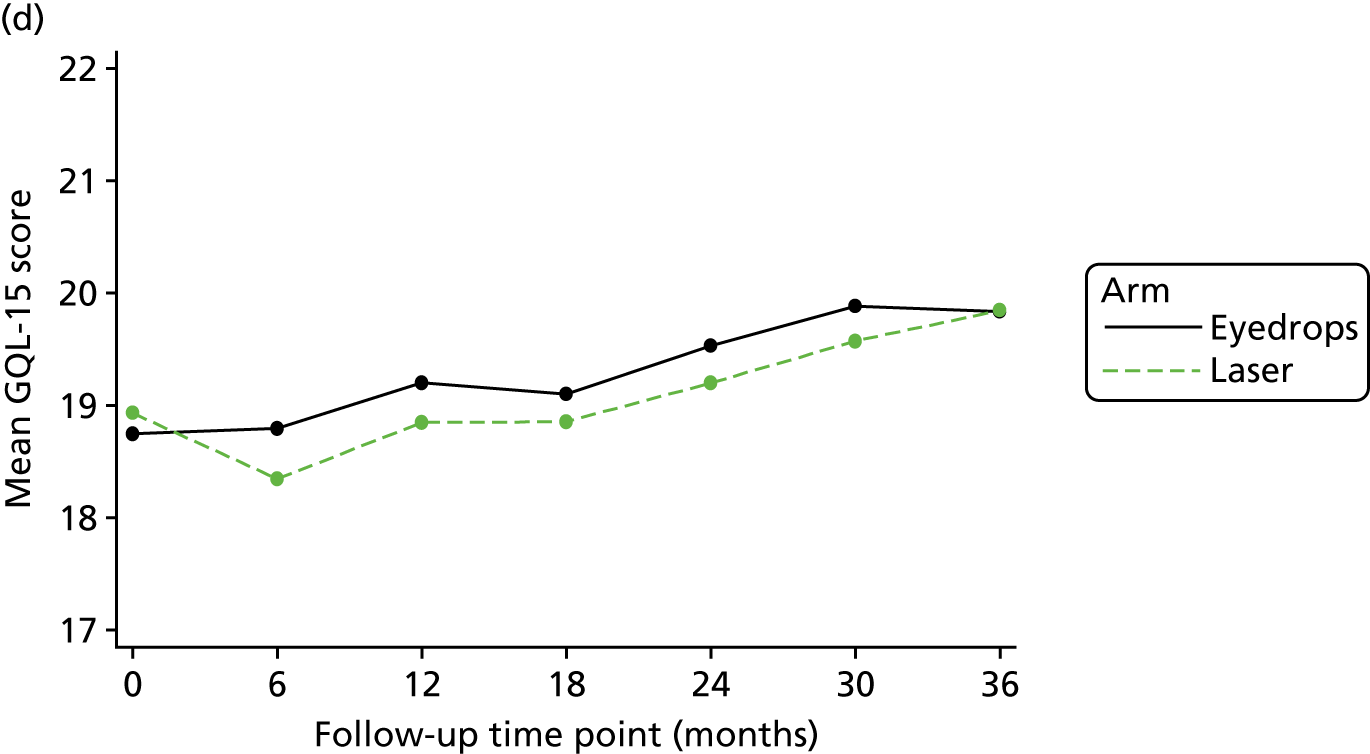
Secondary outcomes: Glaucoma Utility Index, Glaucoma Symptom Scale and Glaucoma Quality of Life-15
The average score on the GUI was 0.89 (SD 0.13) in the Laser-1st arm, compared with 0.89 (SD 0.13) in the Medicine-1st arm (adjusted mean difference 0.007, 95% CI –0.010 to 0.025) (see Table 8 and Figure 5). The mean GSS score at 36 months was 83.3 (SD 17.3) in the Laser-1st arm, compared with 83.1 (SD 17.7) in the Medicine-1st arm (adjusted mean difference 1.595, 95% CI –0.797 to 3.988). Mean GQL-15 scores at 36 months were similar (19.8 in the Laser-1st arm and 19.8 in the Medicine-1st arm, adjusted mean difference –0.368, 95% CI –0.605 to 1.341). Secondary HRQoL outcomes (GUI, GSS and GQL-15) generally suggested slightly better HRQoL in the Laser-1st cohort (see Table 8). Repeated-measures analysis showed worse GSS scores in the Medicine-1st arm at five out of six time points over 36 months.
Pathway clinical effectiveness
At 12 months, 606 eyes (98.9%) were available for analysis in the Medicine-1st arm and 608 eyes (97.8%) in the Laser-1st arm. At 24 months, 564 eyes (92.0%) were available for analysis in the Medicine-1st arm and 576 eyes (92.6%) in the Laser-1st arm. At 36 months, 536 eyes (87.7%) of 314 patients in the Laser-1st arm and 536 eyes (86.2%) of 312 patients in the Medicine-1st arm were available for analysis of clinical outcomes.
Visual function
Measures of visual function at 36 months are shown in Table 9 for both treatment arms. VA at 36 months was comparable between the two treatment arms [0.08 (SD 0.17) log of the minimum angle of resolution (logMAR) for Medicine-1st compared with 0.07 (0.18) for Laser-1st]. In both treatment arms, patients with moderate and severe OAG showed worse VA than those with OHT and mild OAG [logMAR for the Medicine-1st and Laser-1st arms, respectively: severe OAG, 0.16 (SD 0.23) and 0.15 (SD 0.18); moderate OAG, 0.12 (SD 0.16) and 0.11 (SD 0.24); mild OAG, 0.06 (SD 0.15) and 0.08 (SD 0.17); and OHT, 0.08 (SD 0.19) and 0.02 (SD 0.15)]. VF MD was also comparable between the two treatment arms at 36 months [–3.21 (SD 3.76) dB for Medicine-1st compared with –3.19 (SD 3.92) dB for Laser-1st]; VF MD values among those with OHT, as well as those with mild, moderate or severe OAG, were similar in the two treatment arms. VF pattern SD was similar between the two treatment arms at 36 months [3.98 (SD 3.29) dB for Medicine-1st, compared with 3.91 (SD 3.23) dB for Laser-1st]. IOP was reduced from baseline levels for both groups and showed comparable measures at 36 months across all severity categories [16.3 mmHg (SD 3.9) for Medicine-1st, compared with 16.6 mmHg (SD 3.6) for Laser-1st] (see Table 9).
| Medicine-1st, mean (SD) | Laser-1st, mean (SD) | |
|---|---|---|
| VA (logMAR) at 36 months | 0.08 (0.17) | 0.07 (0.18) |
| OHT | 0.08 (0.19) | 0.02 (0.15) |
| Mild OAG | 0.06 (0.15) | 0.08 (0.17) |
| Moderate OAG | 0.12 (0.16) | 0.11 (0.24) |
| Severe OAG | 0.16 (0.23) | 0.15 (0.18) |
| VF MD at 36 months | –3.21 (3.76) | –3.19 (3.92) |
| OHT | –0.94 (1.92) | –1.05 (1.98) |
| Mild OAG | –2.14 (1.95) | –1.99 (1.93) |
| Moderate OAG | –7.21 (1.92) | –7.96 (2.04) |
| Severe OAG | –10.50 (5.01) | –10.24 (4.93) |
| VF pattern SD at 36 months | 3.98 (3.29) | 3.91 (3.23) |
| OHT | 2.00 (1.19) | 2.11 (1.31) |
| Mild OAG | 3.01 (1.94) | 2.84 (1.63) |
| Moderate OAG | 7.56 (2.89) | 8.40 (3.03) |
| Severe OAG | 10.41 (2.77) | 9.63 (2.58) |
| IOP at 36 months | 16.29 (3.87) | 16.63 (3.62) |
| OHT | 18.7 (3.73) | 18.2 (3.73) |
| Mild OAG | 15.7 (3.45) | 16.4 (3.17) |
| Moderate OAG | 14.7 (3.49) | 14.4 (3.07) |
| Severe OAG | 15.5 (4.17) | 15.5 (4.16) |
Achieving target intraocular pressure
A total of 91% of patients treated with Laser-1st achieved target IOP at the first planned visit, compared with 89.6% of those treated with Medicine-1st (Table 10). Over 36 months, target IOP was achieved at 93.0% of visits in the Laser-1st arm, compared with 91.3% of visits in the Medicine-1st arm.
| Medicine-1st | Laser-1st | |
|---|---|---|
| Eyes achieving target IOP at first planned visit (%)a | 89.6 | 91.0 |
| Proportion of visits at target IOP over 36 months (%) | 91.3 | 93.0 |
| Eyes at target IOP at 12 months, % (n) | 96.2 (583) | 94.7 (576) |
| OHT | 97.6 (166) | 95.3 (183) |
| Mild OAG | 96.4 (320) | 95.6 (301) |
| Moderate OAG | 96.5 (55) | 90.7 (49) |
| Severe OAG | 91.3 (42) | 91.5 (43) |
| Eyes at target IOP at 24 months, % (n) | 94.1 (531) | 96.0 (553) |
| OHT | 92.8 (142) | 98.3 (171) |
| Mild OAG | 94.5 (294) | 95.9 (281) |
| Moderate OAG | 95.3 (61) | 95.7 (66) |
| Severe OAG | 89.5 (34) | 87.5 (35) |
| Eyes at target IOP at 36 months, % (n) | 93.1 (499) | 95.0 (509) |
| OHT | 92.0 (127) | 95.6 (151) |
| Mild OAG | 94.6 (261) | 96.3 (259) |
| Moderate OAG | 94.5 (69) | 96.5 (55) |
| Severe OAG | 85.7 (42) | 84.6 (44) |
| IOP fluctuation over 36 months, mean (SD) | 2.5 (1.4) | 2.3 (1.0) |
At 12 months, 94.7% of eyes (n = 576) in the Laser-1st arm met or were below the target IOP, compared with 96.2% of the eyes in the Medicine-1st arm (see Table 10). In the first 12 months after treatment, the proportion of eyes that were consistently at target IOP among patients with OHT or mild or moderate OAG was higher in the Medicine-1st arm, but, among those with severe OAG, the proportion of eyes achieving target IOP was similar in both arms (91.3% in the Medicine-1st arm vs. 91.5% in the Laser-1st arm). This trend was reversed at the end of the second year of the trial, when 96.0% of eyes in the Laser-1st arm (n = 553) were at target IOP, compared with 94.1% of eyes the Medicine-1st arm (n = 531). Similarly, more eyes with severe OAG, treated with Medicine-1st, were at target IOP compared with those treated with Laser-1st (89.5% vs. 87.5%, respectively). At 36 months, a total of 95% of eyes (n = 509) were at target IOP in the Laser-1st pathway, compared with 93.1% of eyes (n = 499) in the Medicine-1st pathway. The proportion of eyes at target IOP among patients with OHT or mild or moderate OAG was higher in the Laser-1st arm than in the Medicine-1st arm, but, among those with severe OAG, a higher proportion of eyes treated with Medicine-1st were at target IOP (85.7% in the Medicine-1st arm vs. 84.6% in the Laser-1st arm). IOP appears to have fluctuated marginally more in the Medicine-1st arm than in the Laser-1st arm (2.5 mmHg vs. 2.3 mmHg).
Treatment intensity to achieve target intraocular pressure
In the first year after treatment, 85.9% of eyes (n = 522) treated with Laser-1st were at target IOP without the use of IOP-lowering eyedrops (Table 11). This reduced to 81.6% (n = 470) after the second year and to 78.2% (n = 419) at the end of the trial, at 36 months. In comparison, in the Medicine-1st arm, 82.2% of eyes (n = 498) were being treated with a single medication at 12 months, falling to 71.5% of eyes (n = 403) at 24 months and 64.6% of eyes (n = 346) at 36 months. The benefit of not using any eyedrops to control IOP was lost for 103 eyes in the Laser-1st arm, whereas 152 eyes in the Medicine-1st arm lost the benefit of a single drop per eye and had to add a second medication to control their IOP.
| Medicine-1st, n (%) | Laser-1st, n (%) | |
|---|---|---|
| Number of SLT treatments per eye at 12 monthsa | 4 | 701 |
| One SLT treatment | 4 | 521(85.3) |
| Two SLT treatments | 0 | 90 (14.7) |
| Three SLT treatmentsb | 0 | 0 |
| Number of medications per eye at target IOP at 12 monthsb | ||
| No medication | 6 (1.0) | 522 (85.9) |
| One medication | 498 (82.2) | 49 (8.1) |
| Two medications | 67 (11.1) | 4 (0.7) |
| Three medications | 11 (1.8) | 1 (0.1) |
| Four medications | 1 (0.2) | 0 (0.0) |
| Number of SLT treatments per eye at 24 monthsa | 4 | 733 |
| One SLT treatment | 4 | 489 (80) |
| Two SLT treatments | 0 | 122 (20) |
| Three SLT treatmentsb | 0 | 0 |
| Number of medications per eye at target IOP at 24 monthsb | ||
| No medication | 14 (2.5) | 470 (81.6) |
| One medication | 403 (71.5) | 73 (12.7) |
| Two medications | 94 (16.7) | 8 (1.4) |
| Three medications | 18 (3.2) | 2 (0.3) |
| Four medications | 2 (0.4) | 0 (0.0) |
| Number of SLT treatments per eye at 36 monthsa | 6a | 770 |
| One SLT treatment | 6 | 453 (74.0) |
| Two SLT treatments | 0 | 157 (26.0) |
| Three SLT treatmentsb | 0 | 1 (0.2) |
| Number of medications per eye at target IOP at 36 monthsb | ||
| No medication | 16 (3.0) | 419 (78.2) |
| One medication | 346 (64.6) | 64 (12.0) |
| Two medications | 99 (18.5) | 21 (3.9) |
| Three medications | 35 (6.5) | 4 (0.8) |
| Four medications | 3 (0.6) | 1 (0.2) |
At the end of the first year, a total of 701 SLT procedures had taken place, with 521 eyes having had a single SLT and 90 eyes having had two SLTs (see Table 11). During the second year, a further 32 SLTs were performed for on eyes that had been treated with SLT during the first 12 months. During the third year of the trial, 35 eyes underwent a second SLT and one eye underwent a third SLT (protocol deviation; see Appendix 9).
At the end of the trial, at 36 months, target IOP was achieved without IOP medication in 78.2% of the eyes (n = 419) treated with Laser-1st (see Table 11); of these, 76.6% (n = 321) had required only one SLT application. Of the Laser-1st patients, 74.2% (n = 233, 95% CI 69.3% to 78.6%) were eyedrop free at 36 months. A total of 64.6% (n = 346) of the eyes treated with Medicine-1st were being treated with a single medication at 36 months.
Control of disease
More treatment escalations took place in the Medicine-1st arm (n = 348) than in the Laser-1st arm (n = 299). Thirty-six eyes in the Medicine-1st arm showed algorithm-confirmed disease deterioration (three eyes converted from OHT to OAG and in 33 eyes OAG progressed), compared with 23 eyes in the Laser-1st arm (two eyes showed OHT conversion to OAG and in 21 eyes OAG worsened) (Table 12).
| Medicine-1st | Laser-1st | |
|---|---|---|
| Treatment escalations over 36 months (n)a | 348 | 299 |
| Disease progression during the trial, % (n) | 5.8 (36) | 3.8 (23) |
| From OHT to OAG (n)b | 3 | 2 |
| OAG progression (n) | 33 | 21 |
| Algorithm defined VF progression | 27 | 18 |
| Algorithm defined optic disc progression | 3 | 2 |
| Algorithm-defined VF and disc progression | 3 | 1 |
| IOP target revisions | 38 (38 eyes, 33 patients) | 41 (38 eyes, 37 patients) |
| Upwards IOP revisions over 36 months (n) | 22 | 26 |
| OHT | 4 | 5 |
| Mild OAG | 8 | 14 |
| Moderate OAG | 7 | 1 |
| Severe OAG | 3 | 6 |
| Downwards IOP revisions over 36 months (n) | 16 | 15 |
| OHT | 1 | 2 |
| Mild OAG | 5 | 5 |
| Moderate OAG | 6 | 5 |
| Severe OAG | 4 | 3 |
| Glaucoma surgeries (n) | ||
| Trabeculectomy | 11 | 0 |
| Trabeculectomy revision | 7 (5 eyes) | 0 |
Over the 36-month duration of the trial, target IOP was revised in 38 eyes, in 33 patients, in the Medicine-1st arm (total 38 IOP revisions) and in 38 eyes, in 37 patients, in the Laser-1st arm (total 41 IOP revisions) (see Table 12). IOP was revised downwards in 31 eyes (16 in the Medicine-1st arm and 15 in the Laser-1st arm) because of objective signs of disease deterioration/progression despite the IOP target being met. The vast majority of the downward revisions of target IOP (28 out of 31) were for eyes with OAG. Additionally, in 48 cases (22 in the Medicine-1st arm and 26 in the Laser-1st arm), the IOP target was revised upwards, despite the initial IOP target not having been met repeatedly, because there was no evidence of disease deterioration/progression. There were proportionally more upwards target IOP revisions in eyes with mild OAG (8 out of 22 in the Medicine-1st arm and 14 out of 26 in the Laser-1st arm). Eleven eyes (1.8%) required IOP-lowering surgery (trabeculectomy) in the Medicine-1st arm, compared with none in the Laser-1st arm.
Safety profile
Adverse events
A total of 1196 AEs were reported for the Medicine-1st arm, compared with 900 in the Laser-1st arm, although the number of patients reporting at least one AE was balanced (260 patients in the Medicine-1st arm vs. 261 in the Laser-1st arm) (Table 13). On average, four AEs were reported per patient treated with Medicine-1st compared with three AEs per patient treated with Laser-1st.
| Summary | Medicine-1st | Laser-1st | Total | |||
|---|---|---|---|---|---|---|
| Total number of AEs | 1196 | 900 | 2096 | |||
| Number of patients reporting at least one AE | 260 | 261 | 521 | |||
| Number of AEs reported per person, mediana (IQR) | 4 (2–8) | 3 (1–5) | 3 (2–7) | |||
| Events, n | Patients, n (%) | Events, n | Patients, n (%) | Events, n | Patients, n (%) | |
| Systemic AEs/symptoms | 298 | 115 (31.8) | 236 | 98 (27.6) | 534 | 213 (29.7) |
| Pulmonary problems | 23 | 14 (3.9) | 24 | 12 (3.4) | 47 | 26 (3.6) |
| Cardiac events | 6 | 5 (1.4) | 8 | 5 (1.4) | 14 | 10 (1.4) |
| Heart block | 1 | 1 (0.3) | 0 | 0 (0) | 1 | 1 (0.0) |
| Cardiac arrhythmia | 5 | 4 (1.1) | 8 | 5 (1.4) | 13 | 9 (1.3) |
| Drug-related events | 148 | 52 (14.4) | 87 | 23 (6.5) | 235 | 75 (10.5) |
| Impotence | 10 | 3 (0.8) | 7 | 4 (1.1) | 17 | 7 (1.0) |
| Depression | 18 | 9 (2.5) | 14 | 4 (1.1) | 32 | 13 (1.8) |
| Somnolence/tiredness | 60 | 31 (8.6) | 34 | 17 (4.8) | 94 | 48 (6.7) |
| Nightmares | 21 | 11 (3.0) | 15 | 4 (1.1) | 36 | 15 (2.1) |
| Generalised skin rash | 18 | 11 (3.0) | 13 | 8 (2.3) | 31 | 19 (2.6) |
| Taste disturbance | 21 | 18 (5.0) | 4 | 3 (0.8) | 25 | 21 (2.9) |
| Otherb | 121 | 82 (22.7) | 117 | 78 (22.0) | 238 | 160 (22.3) |
| Ophthalmic AEs | 809 | 241 (66.6) | 492 | 188 (53.0) | 1388 | 429 (59.8) |
| Aesthetic eyedrop side effects | 117 | 56 (15.5) | 12 | 7 (2.0) | 129 | 63 (8.8) |
| Change in iris colour | 6 | 4 (1.1) | 1 | 1 (0.3) | 7 | 5 (0.7) |
| Periocular pigmentation | 24 | 16 (4.4) | 4 | 4 (1.1) | 28 | 20 (2.8) |
| Excessive lash growth | 87 | 48 (13.3) | 7 | 5 (1.4) | 94 | 53 (7.4) |
| Ophthalmic allergic reactions | 33 | 17 (4.7) | 18 | 13 (3.7) | 51 | 30 (4.2) |
| Periocular skin rash | 16 | 10 (2.8) | 5 | 5 (1.4) | 21 | 15 (2.1) |
| Allergy | 17 | 11 (3.0) | 13 | 8 (2.3) | 30 | 19 (2.6) |
| Uveitis | 1 | 1 (0.3) | 2 | 2 (0.6) | 3 | 3 (0.4) |
| Reactivation of herpes | 1 | 1 (0.3) | 1 | 1 (0.3) | 2 | 2 (0.3) |
| Otherc | 744 | 118 (32.6) | 459 | 117 (33.0) | 1041 | 235 (32.8) |
| Laser-related AEs | 2 | 1 (0.3) | 172 | 122 (34.4) | 180 | 123 (17.2) |
| IOP spike post SLTd | 0 | 0 | 6 | 6 (1.7) | 6 | 6 (0.8) |
| Inflammation | 0 | 0 (0) | 1 | 1 (0.3) | 1 | 1 (0.1) |
| Discomfort | 1 | 1 (0.3) | 92 | 82 (23.1) | 93 | 83 (11.6) |
| Blurred vision | 0 | 0 (0) | 23 | 21 (5.9) | 23 | 21 (2.9) |
| Change in refraction | 0 | 0 (0) | 5 | 4 (1.1) | 5 | 4 (0.6) |
| Otherc | 1 | 1 (0.3) | 51 | 47 (13.2) | 52 | 48 (6.7) |
Overall, systemic AEs were similar between the two treatment arms (see Table 13): 26 cardiac AEs in the Medicine-1st arm vs. 28 in the Laser-1st arm and six pulmonary systemic AEs in the Medicine-1st arm vs. eight in the Laser-1st arm. Eyedrop-related systemic AEs (impotence, depression, somnolence/tiredness, taste disturbance, skin rash and nightmares) were reported more often and by more patients in the Medicine-1st arm [148 events reported by 52 patients (14.4%)] than in the Laser-1st arm [87 events reported by 23 patients (6.5%)].
There were more ophthalmic eyedrop-related AEs reported by patients in the Medicine-1st arm (150 aesthetic side effects and topical allergic reactions reported by 73 patients) than reported by patients in the Laser-1st arm (30 events reported by 20 patients). There were a total of 80 treatment changes (not escalations) attributable to eyedrop side effects or intolerances during the course of the trial; 69 changes to treatment were applied to 59 eyes in 41 patients (11.3% of patients) treated with Medicine-1st and 11 changes to treatment were applied to seven eyes in four patients (1.1% of patients) treated with Laser-1st.
Transient discomfort, blurred vision, photophobia and hyperaemia after the SLT treatment were reported by 34.4% (n = 122) of the patients in the Laser-1st arm and were of a transient nature. AEs (including variations in the number of laser shots, visualisation of angle, breaks taken and discomfort) were reported for 14 patients during the SLT procedure.
There were no sight-threatening complications of SLT (see Table 13). Cases of reactivation of herpes simplex keratitis (one in each treatment arm) and uveitis (two in the Laser-1st arm and one in the Medicine-1st arm) were comparable in the two treatment arms. In only six eyes in six patients was a post-SLT IOP rise noted (> 5 mmHg), identified on the day of laser treatment. Only one of these eyes required treatment. There were no peripheral anterior synechiae.
Serious adverse events
Overall, serious adverse events were balanced between the two treatment arms (Table 14); there were 97 events in the Medicine-1st arm, reported by 69 patients, and 107 events in the Laser-1st arm, reported by 64 patients. The most common ocular SAEs were vascular occlusions, retinal detachments, choroidal neovascularisation and angle closure. In terms of systemic SAEs, pulmonary problems requiring hospitalisations were balanced between the Medicine-1st and the Laser-1st arms (three and two, respectively), as were cardiac events (seven and nine, respectively). There were few and balanced cerebrovascular accidents (one in the Medicine-1st arm and with two in the Laser-1st arm). There were more cancer diagnoses (n = 15) and deaths (n = 8) in the Laser-1st arm than in the Medicine-1st arm (nine and two events, respectively).
| Medicine-1st | Laser-1st | Total | ||||
|---|---|---|---|---|---|---|
| Total number of events | 97 | 107 | 204 | |||
| Total number of patients reporting | 69 | 64 | 133 | |||
| Events, n | n (%) | Events, n | n (%) | Events, n | n (%) | |
| Ocular | 9 | 6 (1.7) | 10 | 8 (2.2) | 17 | 14 (1.9) |
| CRVO/BRVO | 1 | 1 | 1 | 1 | 2 | 2 |
| Retinal detachment | 1 | 1 | 3 | 2 | 4 | 3 |
| Anterior chamber surgery | 1 | 1 | 0 | 0 | 1 | 1 |
| Posterior segment surgery | 1 | 1 | 0 | 0 | 1 | 1 |
| Corneal ulcer | 1 | 1 | 0 | 0 | 1 | 1 |
| CNV | 2 | 2 | 3 | 3 | 5 | 5 |
| Angle closure requiring intervention | 2 | 1 | 2 | 1 | 4 | 2 |
| Post-traumatic uveitis | 0 | 0 | 1 | 1 | 1 | 1 |
| Pulmonary problemsa | 3 | 3 (0.8) | 2 | 2 (0.5) | 5 | 5 (0.7) |
| Cerebrovascular accidents | 1 | 1 (0.3) | 2 | 2 (0.5) | 3 | 3 (0.4) |
| Cardiac eventsa | 7 | 7 (1.9) | 9 | 8 (2.2) | 16 | 15 (2.1) |
| Cancer | 9 | 8 (2.2) | 15 | 13 (3.6) | 24 | 21 (2.9) |
| Death | 2 | 2 (0.5) | 8 | 8 (2.2) | 10 | 10 (1.4) |
| Other systemic | 66 | 50 (15.3) | 61 | 43 (12.1) | 127 | 93 (13) |
Ocular comorbidities and cataract
Ocular comorbidities developing during the course of the trial are shown in Table 15. Overall, these were balanced between the two arms and not related to the treatment. Twenty-five cataract extractions were carried out in 17 patients treated in the Medicine-1st arm and 13 cataract extractions were carried out in 11 patients treated in the Laser-1st arm.
| Medicine-1st (n) | Laser-1st (n) | Total (N) | ||||
|---|---|---|---|---|---|---|
| Events | Patients reporting | Events | Patients reporting | Events | Patients reporting | |
| Ocular comorbidities | 12 | 16 | 28 | 22 | ||
| Central retinal artery occlusion | 3 | 2 | 1 | 1 | 4 | 3 |
| Branch retinal artery occlusion | 1 | 1 | 2 | 1 | 3 | 2 |
| Diabetic retinopathy | 0 | 0 | 1 | 1 | 1 | 1 |
| Diabetic macular oedema | 0 | 0 | 3 | 2 | 3 | 2 |
| Retinal detachment/tear | 1 | 1 | 3 | 2 | 4 | 3 |
| Anterior chamber surgery | 1 | 1 | 0 | 0 | 1 | 1 |
| Posterior segment surgerya | 1 | 1 | 0 | 0 | 1 | 1 |
| Corneal ulcer | 1 | 1 | 0 | 0 | 1 | 1 |
| CNV | 2 | 2 | 3 | 3 | 5 | 5 |
| Angle closure requiring interventionb | 2 | 1 | 2 | 1 | 4 | 2 |
| Post-traumatic uveitis | 0 | 0 | 1 | 1 | 1 | 1 |
| Cataract surgeries | 25 | 17 | 13 | 11 | 38 | 28 |
Concordance/compliance
At baseline, concordance with treatment was lower among patients who were to be treated with Medicine-1st than among those allocated to treatment with Laser-1st (the proportion taking their eyedrops correctly was 75% vs. 92.5%, respectively). By the end of the trial, at 36 months, self-reported concordance had improved and was similar between the two treatment arms (99% of eyedrops used correctly) (Tables 16 and 17).
| Follow-up time point (months) | |||||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 | 12 | 18 | 24 | 30 | 36 | |
| What percentage of your eyedrops do you think you took correctly (in past month)? | |||||||
| Total responses (n) | 10 | 41 | 49 | 61 | 62 | 65 | 77 |
| Median (IQR) | 92.5 (75–100) | 99 (90–100) | 98 (90–75) | 99 (80–100) | 99 (85–100) | 99 (90–100) | 99 (90–100) |
| I’m the sort of person who follows doctors’ orders exactly | |||||||
| Total responses (n) | 351 | 324 | 311 | 299 | 300 | 286 | 299 |
| Strongly agree, n (%) | 266 (75.8) | 245 (75.6) | 226 (72.7) | 212 (70.9) | 209 (69.7) | 203 (71.0) | 210 (70.2) |
| Somewhat agree, n (%) | 77 (21.9) | 70 (21.6) | 74 (23.8) | 73 (24.4) | 80 (26.7) | 74 (25.9) | 79 (26.4) |
| Neither agree nor disagree, n (%) | 7 (2.0) | 5 (1.5) | 8 (2.6) | 10 (3.3) | 6 (2.0) | 4 (1.4) | 7 (2.3) |
| Somewhat disagree, n (%) | 0 (0) | 1 (0.3) | 3 (1) | 2 (0.7) | 4 (1.3) | 5 (1.8) | 2 (0.7) |
| Strongly disagree, n (%) | 1 (0.3) | 3 (0.9) | 0 (0) | 2 (0.7) | 1 (0.3) | 0 (0) | 1 (0.3) |
| Follow-up time point (months) | |||||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 | 12 | 18 | 24 | 30 | 36 | |
| What percentage of your eyedrops do you think you took correctly (in past month)? | |||||||
| Total responses (n) | 11 | 319 | 298 | 289 | 290 | 284 | 286 |
| Median (IQR) | 75 (25–100) | 99 (90–100) | 99 (90–100) | 99 (95–100) | 99 (90–100) | 99 (93–100) | 99 (90–100) |
| I’m the sort of person who follows doctors’ orders exactly | |||||||
| n | 354 | 324 | 305 | 302 | 290 | 293 | 294 |
| Strongly agree, n (%) | 249 (70.3) | 247 (76.2) | 226 (74.1) | 221 (73.2) | 211 (72.8) | 219 (74.7) | 211 (71.8) |
| Somewhat agree, n (%) | 82 (23.2) | 73 (22.5) | 73 (23.9) | 73 (24.2) | 75 (25.9) | 68 (23.2) | 78 (26.5) |
| Neither agree nor disagree, n (%) | 16 (4.5) | 1 (0.3) | 5 (1.6) | 5 (1.7) | 2 (0.7) | 4 (1.4) | 3 (1) |
| Somewhat disagree, n (%) | 5 (1.4) | 2 (0.6) | 1 (0.3) | 2 (0.7) | 1 (0.3) | 2 (0.7) | 1 (0.3) |
| Strongly disagree, n (%) | 2 (0.6) | 1 (0.3) | 0 (0) | 1 (0.3) | 1 (0.3) | 0 (0) | 1 (0.3) |
Cost-effectiveness
Throughout the 36 months of the trial, patients treated with Medicine-1st made 2907 ophthalmology outpatient visits and patients treated with Laser-1st made 3441 visits. The latter includes visits at 2 weeks after the SLT treatment, which served as a safety check. None of these visits revealed a pathology that changed the course of management. A detailed table summarising all of the medical contacts is shown in Appendix 12 (see Table 28).
Quality-adjusted life-years
Descriptive statistics for the EQ-5D-5L are reported in Table 8. In the complete-case analysis, the Laser-1st arm had a mean of 2.63 adjusted and discounted QALYs across 3 years (n = 261, 95% CI 2.60 to 2.66), with 2.61 QALYs in the Medicine-1st arm (n = 263, 95% CI 2.57 to 2.64) with an adjusted difference of 0.025 (95% CI –0.020 to 0.070; p = 0.277). In the multiple imputation analysis, there was an adjusted difference of 0.014 [standard error (SE) 0.220, 95% CI –0.029 to 0.057; p = 0.526].
Cost of selective laser trabeculoplasty
There were a range of different models for delivering SLT across the different sites. Although all sites had a dedicated laser session, this was usually attended by a mixture of patients, some receiving other types of laser treatment. The procedure was performed by ophthalmologists of a range of grades, covering registrar through to consultant. Supporting staff may have been a health-care assistant or a lower-grade nurse. Sessions tended to last 4 hours, with sites treating between five and eight patients at each session. Depending on the number of sessions, sites may treat between 350 and 200 patients a year with the laser (the laser can be used for procedures other than just SLT).
At a cost of £38,995 for the machine, and an annual maintenance cost of £6395, the cost per patient for the machine, annuitising for a 10-year lifespan, is £32 per patient if one assumes that each site sees 300 patients per year (Lumenis, 2018, personal communication). Alternatively, the per patient cost is £55 if one assumes that each site sees 200 patients per year. If it is assumed that the procedure is carried out by a consultant, takes 30 minutes and there is a mixed model of care between nurses and health-care assistants (half and half), the total staff cost is £64 per patient . If the procedure takes 45 minutes, it is £97 per patient, using the same mix of staff (overheads and oncosts are included in the salary costs).
As a result, the total cost of a SLT is likely to be between £96 and £151 depending on the assumptions made. We have used the upper estimate of £151 as the cost per patient for a SLT to use the more conservative estimate. Descriptive statistics for SLTs are reported in Table 11. The average total cost per patient for SLT is reported in Table 18.
| Cost component | Medicine-1st (n = 362), mean (SD) | Laser-1st (n = 356), mean (SD) | Difference, mean (95% CI) |
|---|---|---|---|
| SLT | 3 (22) | 208 (82) | 205 (196 to 213) |
| Eyedrops | 526 (202) | 61 (144) | –465 (–491 to –440) |
| Ocular surgery | 242 (709) | 109 (386) | –134 (–218 to –50) |
| Preoperative assessment | 17 (50) | 8 (32) | –9 (–15 to –3) |
| Postoperative assessment | 1 (14) | 0.3 (5) | –0.5 (–2 to 1) |
| IOP checksa | 170 (290) | 34 (111) | –135 (–168 to –103) |
| Scheduled checks | 446 (144) | 535 (150) | 90 (68 to 111) |
| Unscheduled checks | 26 (86) | 21 (57) | –5 (–16 to 5) |
| 3-year check | 63 (42) | 67 (40) | 4 (–2 to 10) |
| Total | 1495 (1083) | 1044 (608) | –451 (–580 to –322) |
Ocular-related costs
Descriptive statistics for eyedrops, surgery and IOP appointments are reported in Table 11. Total ophthalmology costs collected from patient files are reported in Tables 11 and 18. Patients randomised to Laser-1st had significantly higher costs for SLT and scheduled ophthalmology checks (excluding the 2-week IOP check). Patients randomised to Medicine-1st had significantly higher costs for eyedrops, ocular surgery (including preoperative assessment) and IOP checks. The ophthalmology-related costs in the Medicine-1st arm were £451 (95% CI –£580 to –£322) higher in the unadjusted analysis and £447 (95% CI –£573 to –£322) higher in the adjusted analysis with bootstrapped bias-corrected CIs. There were no significant differences between the two groups in community eye-related costs collected using the CSRI (Table 19).
| Cost component | Medicine-1st, mean (SD) | Laser 1st, mean (SD) | Difference, mean (95% CI) | ||
|---|---|---|---|---|---|
| Baseline (n = 354) | 3 years (n = 223) | Baseline (n = 348) | 3 years (n = 217) | ||
| Optometrist | 49 (37) | 125 (95) | 47 (39) | 139 (111) | 14 (–7 to 35) |
| Community costsa (eye related) | 7 (16) | 23 (43) | 7 (16) | 19 (50) | –4 (–12 to 5) |
| Total (£) | 56 (43) | 133 (109) | 54 (45) | 141 (130) | 8 (–14 to 30) |
Other health-care resource use
There were no significant differences between the two arms in health-care costs collected using the CSRI (Table 20), with a difference of –£319 (95% –£757 to £118) for the adjusted analysis with 95% bias-corrected bootstrapped CIs. If missing data are imputed using chained equations, then the adjusted discounted difference is £36 (95% CI –£366 to £437).
| Cost component | Medicine-1st, mean (SD) | Laser-1st, mean (SD) | Difference, mean (95% CI) | ||
|---|---|---|---|---|---|
| Baseline (n = 354) | 3 years (n = 224) | Baseline (n = 348) | 3 years (n = 231) | ||
| General practitionera | 47 (65) | 138 (171) | 48 (79) | 133 (133) | –5 (–36 to 26) |
| Social care | 4 (40) | 27 (109) | 2 (15) | 26 (134) | –1 (–25 to 22) |
| A&E attendances | 9 (40) | 60 (163) | 13 (51) | 51 (147) | –9 (–39 to 22) |
| Acute outpatientb | 115 (182) | 525 (683) | 97 (172) | 436 (706) | –89 (–227 to 49) |
| Day cases | 256 (610) | 1184 (2071) | 230 (602) | 920 (1577) | –264 (–619 to 90) |
| Total (£) | 425 (712) | 1776 (2538) | 386 (719) | 1389 (2100) | –387 (–815 to 41) |
Inpatient costs have been calculated separately, given that information on inpatient stays could be supplemented with SAE data. The mean inpatient cost over 3 years (discounted) for patients randomised to Medicine-1st is £799 (SD £2592), with a mean cost of £1095 (SD £3252) for patients randomised to Laser-1st and an adjusted difference of £336 (95% CI –£97 to £770), with CIs calculated from bias-corrected bootstrap.
Total health and social care costs
Total costs include the cost of SLTs, eyedrops, eye-related costs and non-eye-related health and social care costs. Including all costs with no imputation, the total adjusted cost for patients randomised to Medicine-1st over 3 years, discounted, is £3993 (SE £215, 95% CI £3571 to £4414) and for Laser-1st it is £3890 (SE £245, 95% CI £3409 to £4371), with a difference of –£103 (SE £325, 95% CI –£739 to £534; p = 0.752). Including imputed missing community data, the difference in costs is –£105 (SE £348, 95% CI –£788 to £579; p = 0.764).
Incremental cost-effectiveness ratio and cost-effectiveness acceptability curve
For ophthalmology and total costs, Laser-1st dominates Medicine-1st in that it results in more QALYs for a lower cost. For ophthalmology-only costs, the results of the multiple imputation (QALYs imputed only) and bootstrap, accounting for correlation between costs and QALYs using seemingly unrelated regression, are that Laser-1st results in an average cost saving of –£458 per patient, with 95% of iterations falling between –£585 and –£345, and 0.014 additional QALYs, with 95% of bootstrap replications falling between –0.018 and 0.046. If non-eye-related costs are also included, the average cost saving of Laser-1st is –£126 per patient, with 95% of bootstrap replications falling between –£796 and £487.
The CEAC is presented in Figure 6. If ophthalmology-only costs are included, at a £20,000 and £30,000 willingness to pay for a QALY, there is a 97% and 93% probability, respectively, that Laser-1st is cost-effective compared with Medicine-1st over 3 years, discounted and adjusted. For all health-care-related costs, including non-eye-related costs, at both a £20,000 and a £30,000 willingness to pay for a QALY, there is a 68% chance that Laser 1st is cost-effective compared with Medicine-1st, discounted and adjusted, over 3 years.
FIGURE 6.
Cost-effectiveness acceptability curve of Laser-1st compared with Medicine-1st based on bootstrapped, imputed, discounted and adjusted data: ophthalmology costs and all health-care costs. Reproduced from Gazzard et al. 58 © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license (http://creativecommons.org/licenses/by/4.0/).
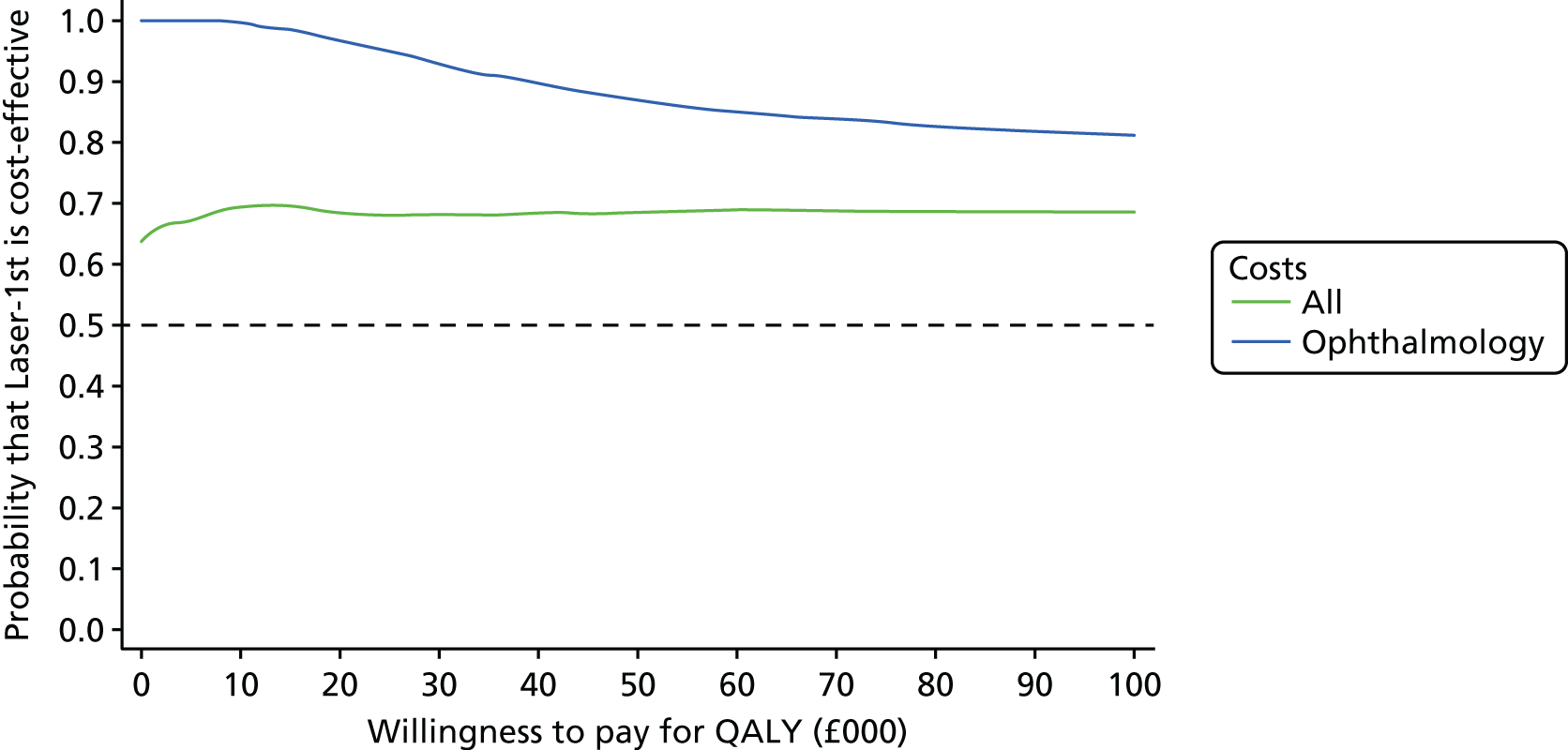
Sensitivity analysis
-
If the SLT cost was at the lower end of the microcosting estimate, Laser-1st would result in a £176 cost saving compared with eyedrops for all health-care costs. The cost of a SLT based on NHS reference costs90 is £188. If this value is used, Laser-1st results in £89 in cost savings if all health-care costs are included.
-
The average cost of the 2-week check following SLT in the Laser-1st arm was £128 and £20 in the Medicine-1st arm, with the 2-week check costing an additional £108 for patients randomised to Laser-1st. If the 2-week check is included in the analysis, Laser-1st results in £18 of cost savings if all health-care costs are included.
-
In the complete-case analysis, the Laser-1st arm results in a mean of 2.63 adjusted and discounted QALYs over 3 years (n = 261, 95% CI 2.60 to 2.65), with 2.61 QALYs in the Medicine-1st arm (n = 263, 95% CI 2.57 to 2.62) and an adjusted difference of 0.032 (95% CI –0.003 to 0.068; p = 0.075). Laser-1st dominates Medicine-1st, with lower costs and more QALYs.
Protocol deviations and violations
A list of protocol deviations and violations is shown in Appendix 9.
Chapter 4 Health economic decision model
Introduction
We collected costs and monitored QoL over the 3-year time horizon of the trial. However, it is possible that further costs and benefits will accrue beyond the time horizon of the trial.
Aim
The aim of the economic evaluation was to calculate the mean incremental cost per QALY of Laser-1st compared with Medicine-1st for the lifetime of the patient. Health service costs and QALYs were calculated for the lifetime of patients, drawing on data from the trial and the literature where appropriate.
Methods
Design
In the lifetime model, cost-effectiveness was calculated in terms of the incremental cost per QALY of Laser-1st compared with Medicine-1st. The model was developed and populated based on available evidence, including the data collected during the trial. Based on previously identified models73 and expert clinical input, the proposed design is a Markov state-transition model that allows movement between glaucoma states. Values for the model have predominantly been taken from data collected from the trial. A systematic search of the literature was also conducted to ratify the evidence from the trial with that in the wider literature. Estimates for mortality have been derived from national data sets. 75
The model has cycles of 6 months’ duration and calculates expected costs and outcomes for a hypothetical cohort of patients with the same age, sex, ethnicity and deprivation composition as patients enrolled in the LiGHT trial. The number of cycles was determined by the number of years between the first cycle and when all patients in the model had died. Costs and QALYs have been discounted at 3.5% per year, in line with NICE guidelines. 76 The health states in the model were OHT, mild glaucoma, moderate glaucoma, severe glaucoma and death. Health states were defined with associated costs and utility values.
Transition probabilities were obtained from the LiGHT trial findings for the first 3 years of the model and a combination of published studies and LiGHT trial findings for the remaining years of the analysis. Given the duration of follow-up in the trial, health status utility and annual costs associated with each Markov state were based on within-trial data; mean utilities and costs for each state were calculated based on the patient-level data in the 3-year follow-up period in the study. These values were utilised in the long-run model. The within-trial values were also compared and supplemented with data from published studies (see Traverso et al. 77), if appropriate. We undertook deterministic (one-way, two-way, multiway) analyses and a probabilistic sensitivity analysis (PSA), the latter assuming appropriate distributions and parameter values. 79 The values from the PSA were used to construct a CEAC, which shows the probability that Laser-1st is cost-effective compared with Medicine-1st over the full lifetime of patients for a range of values of the NHS’s willingness to pay for an additional QALY.
The costs of the treatment pathway were also estimated using a Markov model that estimated the cost of eyedrops, surgery and SLT and the costs for patients who were ‘drops free’. This model was run in conjunction with the aforementioned model and the results were combined.
Markov model structure
The model structure is presented in Figure 1. Generally speaking, OAG is a non-reversible condition, which is reflected in the model structure, in which it was not possible for patients to return to a less severe glaucoma state at the end of each cycle. However, in reality, it is expected that a number of patients will break this assumption as a result of fluctuation within patients, measurement error and other procedures (e.g. cataract surgery), rather than true improvement. Only two patients in the trial were at a better state at 36 months than at baseline (both in the medication arm). These patients were removed from the analysis, assuming measurement error.
Patients in both arms progressed through health states until the entire cohort entered the ‘death’ state. The rate of progression was calculated based on patients severity at the 36-month follow-up compared with baseline. Cycle-specific transition probability was calculated using the method and formula set out in Briggs et al. 79 of r = –[ln(1 – P)]/t and transition probability = 1 – exp(–rt), where r is the rate, P is the probability of an event and t is time.
The model was developed in Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA).
Costing of the treatment pathway
The second and third Markov models were developed to capture treatment costs, including the costs of eyedrops, surgery and SLT. The model structures for each pathway are shown in Figures 7 and 8. The costs estimated in the model were combined with the health state costs in the disease state model (Figure 9).
FIGURE 7.
Medicine-1st treatment pathway.
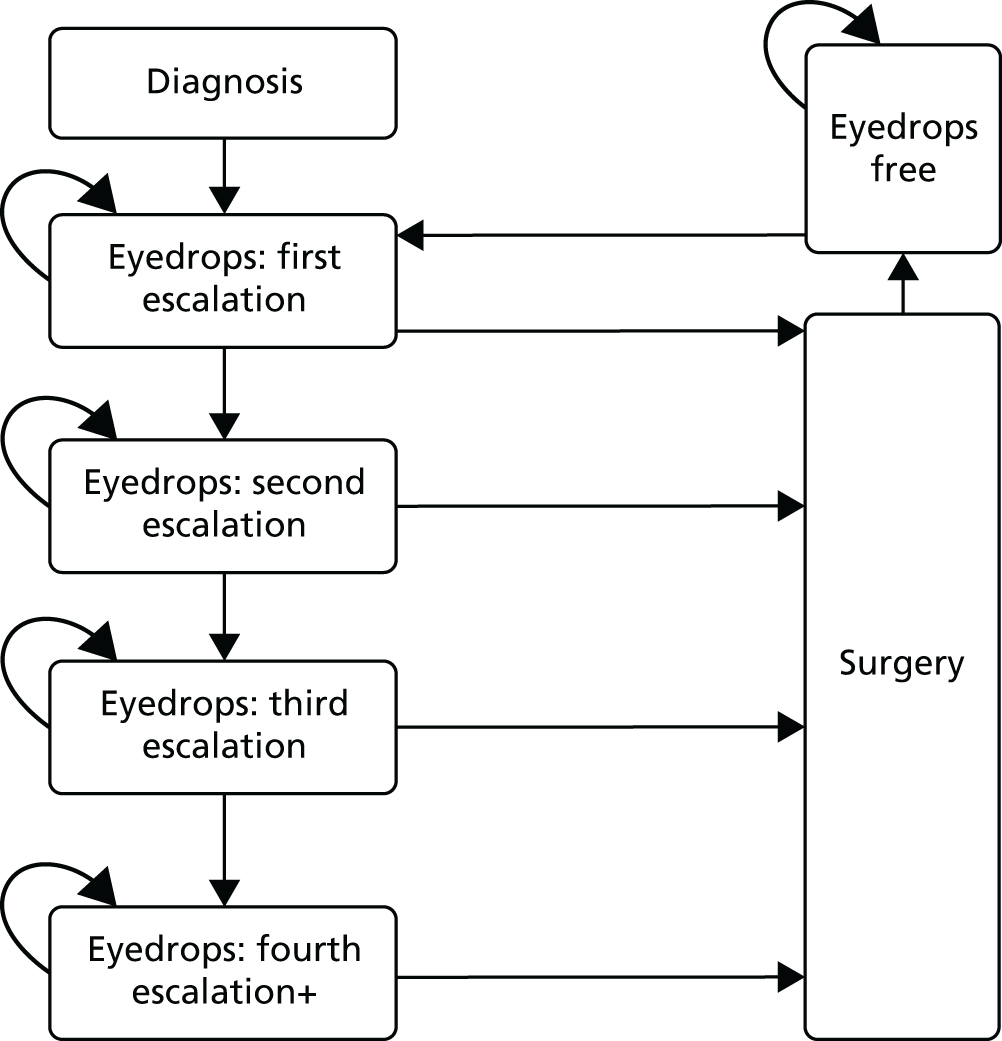
FIGURE 8.
Laser-1st treatment pathway.

FIGURE 9.
Markov model structure.
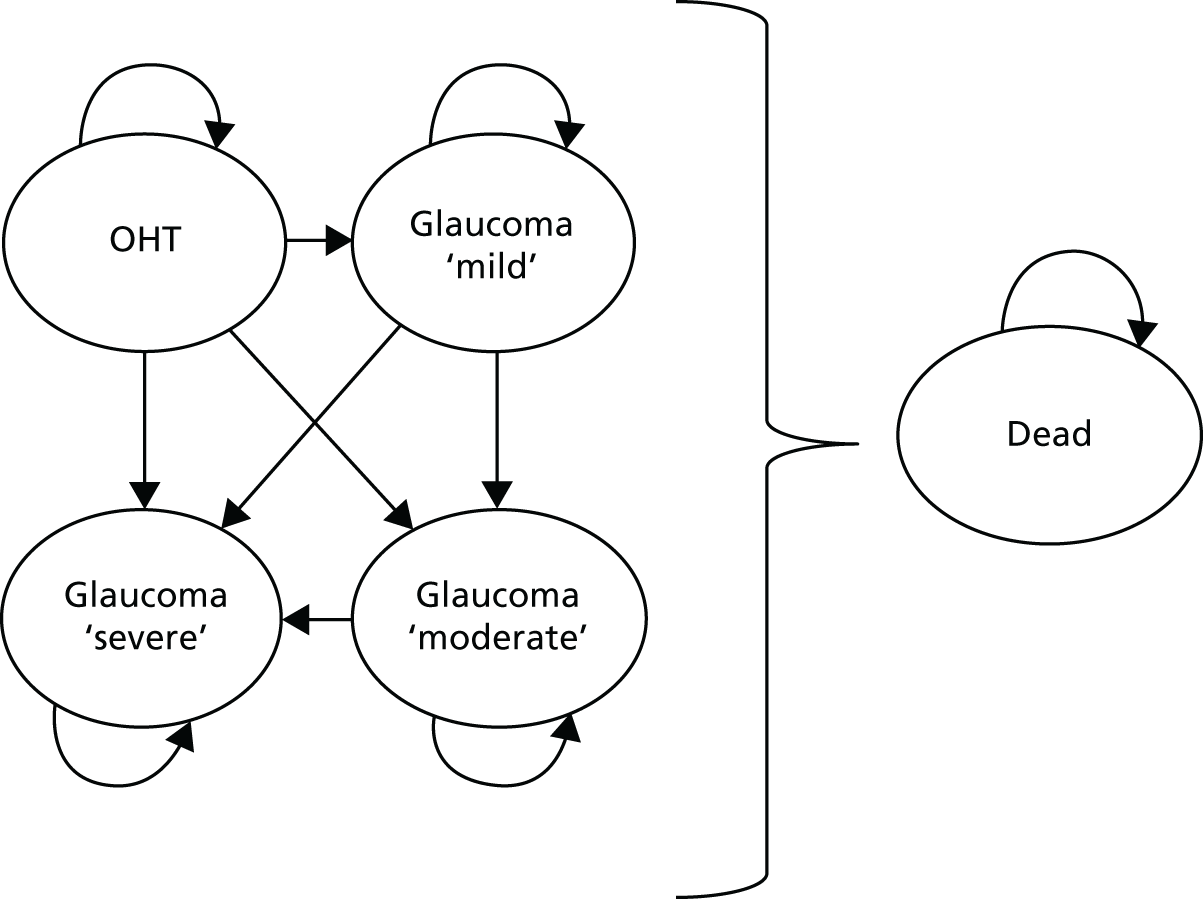
The cost of eyedrops depended on whether the patient was receiving first, second, third or fourth escalation treatment. The model allowed for patients to remain in each eyedrops subcategory after each cycle or progress to the next treatment escalation. Patients could stay in the surgery state for only one cycle, following which they moved into the eyedrops-free window. Patients could also remain in the eyedrops-free state for multiple cycles.
The Laser-1st pathway included a state for patients who received SLT and allowed a number of patients to undergo a second laser treatment, although this transition could be made only once, in line with the trial protocol.
Eyedrops treatment
The cost of eyedrops was calculated as the average for patients who received their first, second, third and fourth escalation of treatment by eyedrops. The fourth escalation group was inclusive of subsequent lines of medical therapy.
Time-to-eyedrops analysis
The proportion of people moving into each of the eyedrops escalations was based on the number of changes made to patients’ eyedrops medication over the 3 years of the trial by trial arm. The same methodology for calculating cycle-specific transition probabilities, as described in Markov model structure, was used.
Time-to-surgery analysis
We conducted a survival analysis with Weibull distribution to calculate the probability of surgery in each cycle by trial arm. The Weibull model was calculated and applied in the model using the methodology in Briggs et al. ,79 to extrapolate beyond the time horizon of the trial.
Literature search
A literature search was undertaken to identify previous economic evaluations with modelling components in OHT and glaucoma, and epidemiological studies of utility scores, costs or disease progression in OHT and glaucoma. We searched the following databases: York Centre for Reviews and Dissemination database, cost-effectiveness registry, The Cochrane Library and MEDLINE. Search terms relating to costs, utility tariffs, QALYs and glaucoma were used.
Population
Patients with a diagnosis of OAG or OHT with a decision to treat made by a consultant glaucoma specialist. The age, sex, ethnicity and socioeconomic deprivation of patients was assumed to be the same as the patients enrolled in the LiGHT trial.
Intervention and comparator
Intervention
Initial treatment with SLT: Laser-1st.
Comparator
Current standard initial treatment with topical medication alone: Medicine-1st.
Perspective
Costs were from the perspectives of NHS England and Personal and Social Services (PSS).
Time horizon
The time horizon for the model was the lifetime (maximum 30 years) for a hypothetical cohort of patients distributed across age bands in line with the trial population. All costs and QALYs were discounted by 3.5%, in line with NICE guidance. 76
Cycle length
The cycle length in the model was 6 months. The cycle length specifies the time interval at which the cohort can change health state.
Health states
Health states in the model were used to allocate costs and consequences as a result of various events. Events within a state were assumed to happen at the start of the 6-month cycle and, hence, costs and consequences for the period were calculated from the first time that the patient entered a state. There were five health states in the model as follows:
-
OHT
-
glaucoma ‘mild’
-
glaucoma ‘moderate’
-
glaucoma ‘severe’
-
death.
The health states in the model are displayed in Figure 1. The mild, moderate and severe glaucoma health states and OHT were defined in accordance with the structured protocols applied (see Table 1). The definitions were based on the Canadian IOP guidelines. 69 The definition of health states from any costs and QALYs assigned from the literature was carefully compared with the health state definitions used in the trial for suitability. In particular, the ‘severe’ state as defined in the trial was likely to be less severe than in other trials because of the nature of the recruitment selection process, as a result of which patients with more severe disease were not considered suitable for the study.
Death was an absorbing state in the model, meaning that any individual can move into this state from any other state within the model. Once a patient entered the death state in the model, no costs or utilities were applied.
Costs
Individual costs included in the model were as follows:
-
Cost of SLT: the mean cost per patient of SLT in the Laser-1st arm was £151 per SLT based on the figure reported in the trial-based health economic evaluation (see Cost-effectiveness analysis).
-
Cost of trabeculectomy: the cost of trabeculectomy was taken from NHS reference costs 2016/1790 and as a day-case cost.
-
Cost of eyedrops: the cost of eyedrops was calculated using trial data and the average cost per patient at each eyedrop change.
-
Health state costs: health-care costs were calculated as the average 36 months’ health and social costs for patients with OHT or one of the three OAG health states at 36 months and by trial arm. They included all costs, excluding SLT, eyedrops and surgery (trabeculectomy). They included the cost of ophthalmology appointments, including IOP checks.
Quality-adjusted life-years
Quality-adjusted life-years for each health state were calculated based on patients’ OHT and OAG severity at 35 months and the mean GUI61 for those patients by trial arm. Utility values collected using the EQ-5D-5L were applied in the sensitivity analysis.
Cost-effectiveness analysis
We calculated the total costs and QALYs for patients with OAG and OHT treated by Laser-1st compared with Medicine-1st.
The ICER was calculated as the ratio of the difference in total cost for a patient over a lifetime for the intervention and the comparator, and the difference in total QALYs for a patient over a lifetime for the treatment and the comparator:79
All future benefits (QALYs) and costs were discounted in line with NICE guidance76 to capture time preferences for costs and benefits.
Sensitivity analysis
We undertook deterministic (one-way, two-way and multiway) analyses and a PSA, the latter assuming appropriate distributions and parameter values. 79 The values from the PSA were used to construct a CEAC, which showed a probability that Laser 1st is cost-effective compared with Medicine-1st over the full lifetime of patients, for a range of values of the NHS’s willingness to pay for an additional QALY.
Probabilistic sensitivity analysis and cost-effectiveness acceptability curve
We conducted a full PSA to generate a CEAC and calculate the probability that a given option was cost-effective compared with a range of prestated comparators based on 1000 iterations of the model. All values in the model were assigned appropriate distributions and parameter values. 79
Deterministic
We varied input variables, either individually or in combination, whereas other variables are held at their baseline value.
Results
Inputs for model
The inputs for the model are reported in Table 21. There was a significant reduction in the rate of surgery in the Laser-1st arm compared with the Medicine-1st arm (hazard ratio 0.156, 95% CI 0.046 to 0.527).
| Variable | Mean | SE | Distribution | Source |
|---|---|---|---|---|
| Age at time 0 | 63 | |||
| Patients OHT at time 0 (%) | 30 | |||
| Patients mild at time 0 (%) | 49 | |||
| Patients moderate at time 0 (%) | 15 | |||
| Patients severe at time 0 (%) | 6 | |||
| Transition probabilities variables (1 year) | ||||
| Laser-1st | ||||
| OHT to mild | 0.07 | 0.04 | Beta | |
| OHT to moderate | 0.04 | 0.04 | Beta | |
| OHT to severe | 0.02 | 0.02 | Beta | |
| Mild to moderate | 0.08 | 0.04 | Beta | |
| Mild to severe | 0.04 | 0.03 | Beta | |
| Moderate to severe | 0.22 | 0.12 | Beta | |
| Second SLT | 0.10 | 0.03 | Beta | |
| Medicine free to first-line medicine | 0.07 | 0.02 | Beta | |
| First- to second-line medicine | 0.17 | 0.06 | Beta | |
| Second- to third-line medicine | 0.05 | 0.04 | Beta | |
| Third- to fourth-line medicine | 0.01 | 0.02 | Beta | |
| Medicine-1st | ||||
| 1-year probability of OHT to mild | 0.10 | 0.05 | Beta | |
| 1-year probability of OHT to moderate | 0.03 | 0.03 | Beta | |
| 1-year probability of OHT to severe | 0.01 | 0.02 | Beta | |
| 1-year probability of mild to moderate | 0.07 | 0.04 | Beta | |
| 1-year probability of mild to severe | 0.06 | 0.03 | Beta | |
| 1-year probability of moderate to severe | 0.17 | 0.13 | Beta | |
| First- to second-line medicine | 0.15 | 0.03 | Beta | |
| Second- to third-line medicine | 0.07 | 0.02 | Beta | |
| Third- to fourth-line medicine | 0.03 | 0.02 | Beta | |
| Medicine free after surgery to first-line medicine | 0.15 | 0.03 | Beta | |
| Time to surgery (3-month rates) | ||||
| Laser-1st | –1.86 | 0.62 | LogNormal | |
| Constant | –10.88 | 1.61 | LogNormal | |
| Gamma | 2.85 | 0.57 | LogNormal | |
| Resource cost (per year) | ||||
| Laser-1st OHT | £1087 | 824 | Gamma | |
| Laser-1st mild | £798 | 217 | Gamma | |
| Laser-1st moderate | £1162 | 598 | Gamma | |
| Laser-1st severe | £1109 | 637 | Gamma | |
| Medicine-1st OHT | £772 | 283 | Gamma | |
| Medicine-1st mild | £874 | 197 | Gamma | |
| Medicine-1st moderate | £1072 | 422 | Gamma | |
| Medicine-1st severe | £1500 | 744 | Gamma | |
| Cost of medicine first line | £152 | 50 | Gamma | |
| Cost of medicine second line | £159 | 70 | Gamma | |
| Cost of medicine third line | £179 | 74 | Gamma | |
| Cost of medicine fourth line | £180 | 74 | Gamma | |
| Surgery | £1454 | 299 | Gamma | NHS Reference Costs 2016–17 90 |
| Utility of Markov states per cycle | ||||
| Laser-1st OHT | 0.9034 | 0.02 | Beta | |
| Laser-1st mild | 0.905 | 0.013 | Beta | |
| Laser-1st moderate | 0.8622 | 0.021 | Beta | |
| Laser-1st severe | 0.88 | 0.023 | Beta | |
| Medicine-1st OHT | 0.8934 | 0.022 | Beta | |
| Medicine-1st mild | 0.9085 | 0.011 | Beta | |
| Medicine-1st moderate | 0.8762 | 0.02497 | Beta | |
| Medicine-1st severe | 0.8455 | 0.0206 | Beta | |
Literature search
A total of 1506 papers were identified as part of the literature search. Thirty-four papers45,46,48,50,52–54,73,95–120 were identified as being relevant to the model, and included information on transition probabilities, utilities and costs. Overall, data from the trial fit better with the model than data that could be extracted from the 34 papers identified to be relevant. The best utility scores were those from the trial because we were able to derive trial-arm specific utilities and from the GUI. The values were similar to values published elsewhere in the literature; for example, Hernández et al. 103 reported utilities of 0.8015, 0.8015, 0.7471 and 0.7133 for OHT and mild, moderate and severe glaucoma, respectively. Stein et al. 95 reported values of 0.92, 0.89 and 0.86 for mild, moderate and severe glaucoma, respectively. The values reported in Hernández et al. 103 were used in a sensitivity analysis for the model.
Cost-effectiveness analysis
The average lifetime cost for Laser-1st based on 1000 runs of the PSA was £17,541 per patient, with an average cost per patient of £20,435 for the Medicine-1st arm and a difference of –£2894. Laser-1st resulted in an average QALY of 12.5 over the lifetime time horizon, compared with 12.3 for Medicine-1st (difference of 0.2 QALYs). Laser-1st dominated Medicine-1st in that it was cost saving and resulted in additional QALYs.
There was a 90% probability that Laser-1st is cost-effective compared with medication at a willingness to pay £20,000 for a QALY (Figure 10).
FIGURE 10.
Cost-effectiveness acceptability curve: probability that Laser-1st is cost-effective for a range of values of willingness to pay for a QALY.
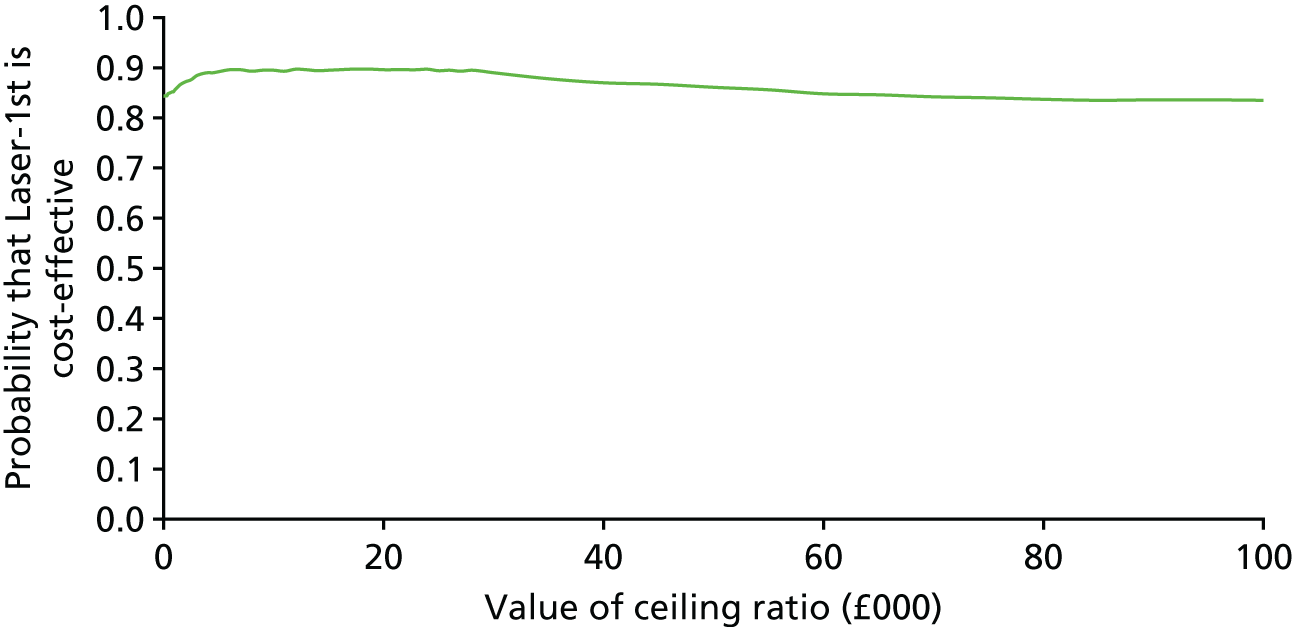
Sensitivity analysis
If the utility health state values were substituted for those from Hernández et al. ,103 there was no difference in QALYs between the two groups (an average of 10.6 QALYs per patient over the lifetime time horizon).
Conclusion
When costs, outcomes and surgery rates were projected to a lifetime time horizon, there was a 90% probability that Laser-1st is cost-effective. This is similar to the findings of trial-based analysis, strengthening the finding that Laser-1st is cost saving compared with Medicine-1st.
Chapter 5 Discussion and conclusions
Summary of findings
This multicentre randomised controlled trial compared initial treatment of OAG or OHT using SLT followed by medication, if required, with the use of IOP-lowering medication alone. Patient HRQoL, clinical efficacy and cost-effectiveness were investigated in six NHS settings in the UK. The study demonstrates that initial SLT is cost-effective, with better clinical outcomes and a trend towards better HRQoL compared with the prescription of IOP-lowering eyedrops from the outset.
Quality of life
The Laser-1st and Medicine-1st treatment arms showed comparable EQ-5D-5L scores at the trial’s end point, at 36 months. The trial’s protocol, whereby each eye was treated to an eye-specific IOP target, led to minimal disease-related differences, such as visual function outcomes. Indeed, VA, VF MD and VF pattern SD were comparable between the two treatment arms at 36 months (see Table 9), and this was reflected in similar EQ-5D-5L scores between the two pathways (see Table 8). The above average baseline HRQoL,82,121–123 weak sensitivity of the EQ-5D-5L to detect glaucoma-specific effects on HRQoL82,123,124 and relatively short duration of this trial, compared with the time for disease progression, may have contributed to the lack of superiority of the EQ-5D-5L in the Laser-1st approach. Recent data on patient self-reported outcome measures (including EQ-5D-5L) now confirm that these may not be sensitive enough to function as primary end points in clinical trials. 125
Glaucoma-specific instruments (e.g. GUI and GQL-15) are better at capturing differences in glaucoma severity than the effect of treatment side effects on patients’ HRQoL. Indeed, the QoL of patients with glaucoma has been related to the extent of VF loss when using the GQL-15. 8,121,126,127 The GUI attributes less weight to local side effects and provides generic health outcome measures and measures of glaucoma severity. 61 The lack of a significant difference in the GUI and GQL-15 in this study was, therefore, somewhat expected; each eye was treated to target and stringent controls over disease progression minimised any substantial differences in disease severity.
The GSS evaluates a visual and an ocular comfort-related domain. The six non-visual ophthalmic symptoms are formed around an ocular comfort domain (burning/smarting/stinging, tearing, dryness, itching, soreness/tiredness and a feeling of something in the eye), and are related to treatment side effects and their measures. The patients were asked to rate their difficulties around blurry/dim vision, hard to see in daylight, hard to see in dark places and haloes around lights, in relation to the four visual ophthalmic symptoms. The GSS has been shown to correlate well with traditional measures of visual function (such as VA and VF),62 which in this study’s end point were comparable between the two treatment arms. Repeated-measures analysis showed worse GSS scores for the Medicine-1st arm at five out of six time points over the course of the 36 months of the trial. Better GSS scores for the Laser-1st arm may represent differences that arise from eyedrop use (64.6% of the patients in the Medication-1st arm were using only one drop per day) (see Table 11), but potentially reflect differences in baseline scores between the two treatment arms (83.3 for the Medicine-1st arm and 81.4 for the Laser 1st arm) (see Table 7).
Clinical efficacy
The treatments were equally effective in lowering IOP and reaching the target IOP for the first time, with 89.6% of the Medicine-1st eyes reaching target at the first planned follow-up visit, compared with 91.0% of the Laser-1st eyes (see Table 10). By 3 years, however, 95% of the Laser-1st eyes were at target IOP, compared with 93.1% of the eyes treated with Medicine-1st. Although Medicine-1st provided more eyes at target IOP at 12 months (96.2% for Medicine-1st compared with 94.7% for Laser-1st) (see Table 10), Laser-1st achieved more eyes at target IOP over the second and the third years of treatment. Interestingly, the percentage of eyes with severe OAG that were at target IOP after the second and third years of treatment was higher in the Medicine-1st arm than in the Laser-1st arm (89.5% compared with 87.5%, respectively, at 24 months and 85.7% compared with 84.6%, respectively at 36 months); the proportions of severe OAG eyes achieving IOP target at 12 months were comparable (91.3% and 91.5%, respectively). A possible explanation may be the limit to which SLT may reduce IOP; severe OAG is likely to have low IOP targets, which may be easier to achieve with more than one medication. A number of studies have reported that SLT reduces IOP by 15–32%,128–134 and another study found a reduction of up to 29.4% at 6 months, up to 30% at 12 months, up to 27.8% at 2 years and up to 25.1% at 3 years. 135
Overall, IOP was at target for 93% of the Laser-1st visits, compared with 91% of the Medicine-1st visits, over the 36-month duration of the trial. IOP control with topical medication (eyedrops) may rely on patient concordance with treatment; indeed, one report94 found that IOP-lowering eyedrops were available to patients for only 69% of the time, whereas concordance has been reported to range between 76% and 86%. More discouraging figures have been reported for patients on multiple eyedrops. 136,137 In this trial, however, self-reported concordance was very high, with patients’ concordance reportedly improving during the 36 months of treatment (see Tables 16 and 17). SLT has also been proposed to provide better diurnal IOP stability, because of its continuous effect on the trabecular meshwork, in contrast to the episodic administration of medication. 138–141 This trial showed a comparable IOP fluctuation between Medicine-1st and Laser-1st (2.5 mmHg and 2.3 mmHg, respectively).
By 36 months, 78.2% (95% CI 74.7% to 81.4%) of the eyes treated with Laser-1st were at target without the need for any topical IOP-lowering medication (eyedrops). In comparison, in 64.6% of eyes treated with Medicine-1st only a single eyedrop was necessary to control IOP. Primary SLT gave eyedrop-free IOP control for at least 36 months to 74.2% of patients (95% CI 69.3% to 78.6%), substantially higher than reported in previous studies that used less stringent success criteria and which used SLT exclusively as the primary treatment. 37,142–144 Prior treatment and more severe disease have been suggested to reduce the magnitude of IOP lowering with SLT,37,38,142–144 possibly explaining the results of this trial in treatment-naive patients. Pre-trial activities with glaucoma patients (LAG) identified eyedrop-free disease control as the most desired outcome, with 90% of a patient focus group feeling that even unilateral eyedrop freedom is beneficial. Concerns about eyedrop use, particularly associated with challenges from cognitive and physical impairment, were rated as a priority by patients in the James Lind Alliance survey of sight loss research questions. 145
By 36 months, rates of disease deterioration were higher in the Medicine-1st arm than in the Laser-1st arm [5.8% (36 eyes) vs. 3.8% (23 eyes), respectively], despite the treat-to-target design, tailoring treatment intensity to disease severity and treatment response. The vast majority of disease progression happened in eyes with OAG (33 out of 36 eyes in the Medicine-1st arm and 21 out of 23 eyes in the Laser-1st arm) (see Table 12). Additionally, there were more treatment escalations over 36 months in the Medicine-1st arm (348, compared with 299 in the Laser-1st arm).
Upwards (22 in the Medicine-1st arm and 26 in the Laser-1st arm) and downwards (16 in the Medicine-1st arm and 15 in the Laser-1st arm) target IOP revisions were overall balanced between the two treatment arms. By 36 months, 11 eyes in the Medicine-1st arm, but none in the Laser-1st arm, had required IOP-lowering surgery.
Safety
This trial demonstrates a greater safety of SLT than previously reported, with low rates of SLT-related AEs. 143 Rates of systemic AEs were balanced between the two treatment arms, indicating a safe treatment pathway for both Medicine-1st and Laser-1st (i.e. no excess cardiac or pulmonary events in the Medicine-1st arm and no systemic AEs as a result of SLT) (see Tables 13 and 14). Differences in the rates of cancer diagnoses and deaths between the two treatment arms (see Table 14) are attributable to chance, with no medical link between the treatments that were administered and the events that took place.
Eyedrop-related systemic and ophthalmic AEs were reported by more patients and more often in the Medicine-1st arm (see Table 13). Ophthalmic AEs led to a change in treatment for 11.3% of the patients treated with Medicine-1st, when IOP was otherwise well controlled.
Selective laser trabeculoplasty resulted in at least one transient AE in 34.4% of the patients treated with Laser-1st. Out of 776 SLT procedures, one patient developed an IOP spike requiring treatment, compared with reported rates of up to 28.8%, possibly because treatment was administered at an earlier stage of disease in this trial. 143 The IOP check conventionally done 2 weeks after SLT did not change management for any of the patients and consequently appears unnecessary.
The ocular comorbidities developed during the trial spanned a range of conditions commonly seen in patients of the age group of this cohort (retinal vascular occlusions, diabetic retinopathy, retinal tears and detachments, macular degeneration, etc.) (see Table 15). The rate of cataract surgery was lower in the Laser-1st arm (13 eyes in the Laser-1st arm, compared with 25 eyes in the Medicine-1st arm) (see Table 15), supporting existing evidence that IOP-lowering eyedrops are associated with a greater incidence of nuclear cataract and earlier need for surgical removal. 12,59,146–148
Economic evaluation
The Laser-1st approach resulted in a significant reduction in the cost of surgery and IOP-lowering medication, with an overall cost saving to the NHS of £451 per patient in specialist ophthalmology costs. The trial-based economic evaluation found that there is a 97% probability that SLT is a cost-effective treatment for OAG and OHT at a £20,000 willingness to pay for a QALY, from an ophthalmology cost perspective. Resource use information was collected from patient files and trial monitoring data and hence is likely to be complete, with limited bias as a result of loss to follow-up or missing data. Including non-eye-related health-care costs alongside ophthalmology costs, the average cost per patient for Laser-1st remained less than that for Medicine-1st, but the differences between the two groups were not significant, with the wide CIs resulting in a 68% probability that Laser-1st is cost-effective compared with Medicine-1st. Non-ocular health-care cost data were, however, based on self-reported health-care resource use and may be unreliable or incomplete. Expensive systemic AEs unrelated to OHT or OAG, such as cancer, may have also skewed the cost results. In the health economic decision model, in which costs and utilities are projected for the lifetime of patients, there is a 90% probability that Laser-1st is cost-effective compared with Medicine-1st at a willingness to pay £20,000 for a QALY health-care cost perspective, with an average cost saving per patient to the NHS of £2894.
Previous economic assessments have attempted to estimate the relative costs of SLT using only economic modelling or estimates of the treatment costs, instead of a direct cost assessment plus modelling. 50,51,53 Compared with mono or multiple drug therapy, and allowing for repetition of SLT within 2–3 years, cost savings have been predicted at 6 years for a Canadian health-care system. 46 The present study, conducted in a NHS setting following pragmatic clinical approaches for the treatment of OAG/OHT, indicates that SLT is cost-effective over a 3-year period and is likely to remain cost-effective over the lifetime of the patients. These findings have important implications for patients and health-care systems. Patients are concerned about the use of IOP-lowering eyedrops,145 and widespread uptake of the Laser-1st strategy would lead to an eyedrop-free interval of at least 36 months for three-quarters of patients, while also providing savings for the NHS. If necessary, additional SLT may further increase the duration of this eyedrop-free window. Patients with OAG have an average life expectancy from diagnosis of 9–13 years,149–151 and so an eyedrop-free period of ≥ 3 years may significant increase the quality of their remaining life. The requirement for intense medical or surgical regimes may be deferred or completely averted by SLT and potentially with improved surgical success rates and still lower cost. 31,33,34
The health economic decision model has some limitations. First, the mortality rate used is not glaucoma specific. Rather, the same all-cause mortality rate was applied across all health states. This is a reasonable assumption given that studies have not found a difference in all-cause mortality rates between patients with and without glaucoma. 149 There is, however, an increased risk of cardiovascular disease in older patients (aged > 75 years), which was not incorporated into the model. This is unlikely to have made a significant impact on the results of the model, given the small difference in OAG progression between the two arms.
The lifetime economic model originally set out to include variables from other studies in the literature, including costs, utilities and disease progression. As noted in other studies that have modelled the progression of OAG, there is heterogeneity in how OHT and OAG are defined, making it difficult to incorporate such values into glaucoma models. 73 Burr et al. 73 conducted a systematic review of studies for glaucoma progression and identified 1-year progression probabilities for mild to moderate glaucoma, moderate to severe glaucoma and severe glaucoma to visually impaired. The progression values for the two health states that overlap with our model for Medicine-1st patients are different from those observed in the LiGHT trial; higher for mild to moderate progression (0.2 in Burr et al. 73 vs. 0.07 in the LiGHT trial) and lower for moderate to severe progression (0.07 in Burr et al. 73 vs. 0.17 in the LiGHT trial). This difference is likely to be a result of differences in the populations used to determine transitions probabilities, as the values for Burr et al. 73 are from observational studies of people at different stages of OAG, whereas the LiGHT trial recruited patients newly diagnosed with OHT or OAG. It may also be as a result of differences in how the various stages are defined, with Burr et al. 73 restricting the definition to mean definition score only, omitting an OHT health state and including an additional visual impairment state. Costs were also more suitably obtained from the trial or NHS reference costs90 given that most studies report costs for medication for OHT and OAG health states, or the costs for eyedrops progression, ophthalmology appointments and trabeculectomy (glaucoma surgery) only. 77 Our model is novel, in that we were able to project medication and trabeculectomy costs over the lifetime of patients while modelling the health-care costs associated with OHT and OAG health states separately, using LiGHT trial data. As discussed, our utility values for health states are similar to those quoted in other studies;73,95,103 modifying these has limited impact on the results, particularly as using published data means that we are unable to use utilities specific to trial arms.
Strengths and limitations
This study mirrored pragmatic clinical practice by tailoring treatment to the patient. Individual IOP targets were based on pre-treatment IOP and disease severity, and adapted both to treatment response and to progression of the disease. Consequently, the concluding findings on disease progression, achievement of target IOP and cost are highly relevant to normal clinical practice. The ‘treat-to-target’ design with computerised decision-supported treatment interventions and follow-up intervals captured the complexity of real-life clinical decision-making, but also allowed objective and unbiased decisions based on clinical observations.
This trial was unmasked. An unmasked design was essential to capture any treatment effects on patients’ perception, which is clinical reality. As an important benefit of SLT is an eyedrop-free treatment window; a study design requiring a treatment arm with placebo eyedrops would have prevented assessment of benefits attributable to IOP control without the use of medication. The impact of treatment on subsequent medication-taking behaviours and concordance was also of interest and will be fully revealed in the extension of this trial. Similarly, treating clinicians had to be unmasked to be able to choose appropriate treatment escalations.
The EQ-5D-5L questionnaire, a generic tool eliciting utility values in multiple settings, may not have been the most sensitive tool to investigate HRQoL in the two treatment arms for a 3-year trial, but is a requirement for a NICE cost–utility analysis. OAG can be asymptomatic, even at levels sufficient to make driving unsafe. 152 Although potentially associated with significant visual impairment over longer periods, only small changes in vision occurred over the 36 months duration of the trial, and this finding may be related to the lack of a difference in the primary outcome at 36 months.
Chapter 6 Conclusions
In summary, this trial shows that treating newly diagnosed OAG and OHT patients with SLT can safely provide predominantly eyedrop-free IOP control over a minimum of 3 years, with less intense treatment, fewer AEs and a reduced need for ocular surgery at a lower cost per QALY than standard medical therapy alone, with a similar effect on patients’ generic HRQoL.
Implications for health care
The results of this randomised controlled trial are widely generalisable, as patients with OHT and both low- and high-pressure OAG were included, from a range of backgrounds and ethnic origins. This trial focused on newly diagnosed, treatment-naive patients with OHT and/or mild or moderate OAG; the results should, therefore, be interpreted with caution in relation to the efficacy of SLT in advanced OAG stages, or in eyes previously treated for high IOP. There are important implications for resource-poor health-care settings, where access to medication is a major barrier to glaucoma treatment. Adequate eyedrop-free IOP control for years after is a promising treatment paradigm for regions of Africa where glaucoma prevalence is high. Longer follow-up, already under way, will permit us to answer further questions regarding the effect of prior SLT on later medication-taking behaviour, treatment intensity and longitudinal HRQoL.
The data support a change in practice; however, clinicians need to consider the perceived necessity of monitoring visits by the patient, in the absence of daily medication, as has been suggested by in the past for other chronic illnesses. 153 Primary SLT is a safe, clinically efficient and cost-effective alternative to eyedrops that can be offered as a first-line treatment to patients with OAG or OHT, in need of IOP reduction.
Recommendations for research
This study addresses the initial 36-month period after initiation of treatment for OAG and/or OHT. OAG and OHT are chronic conditions and treatment is almost always lifelong. Longitudinal research into the clinical efficacy of SLT as a first-line treatment could specify the long-term differences (if any) of disease progression, treatment intensity and ocular surgery rates between a Laser-1st and a Medicine-1st pathway, as well as any effect on subsequent medication-taking behaviours. Longitudinal HRQoL in OAG and OHT will also help clinicians understand the impact medical treatment has on patients over a longer period of time, where more intense medical treatment might become necessary.
Acknowledgements
We are thankful to all the patients who participated in this trial. Our particular recognitions and remembrances are for Dominic Carrington (MEH research technician), who sadly and suddenly passed away shortly before the completion of the study.
Members of the Trial Steering Committee
Independent chairperson: Professor John Sparrow, Honorary Professor, University of Bristol.
Chief investigator: Mr Gus Gazzard, Consultant Ophthalmic Surgeon, MEH.
Trial manager: Dr Amanda Davis, MEH.
Independent clinician with relevant expertise: Professor James Morgan, Professor of Ophthalmology, Cardiff University.
Independent statistician: Dr Marta (Garcia-Finana) Van der Hoek, Reader in Biostatistics, University of Liverpool.
Lead statistician: Dr Gareth Ambler, Senior lecturer in Medical Statistics, University College London.
Patient and public involvement representative: Ms Shelia Page, expert patient.
Independent members of the Data Monitoring Committee
Independent statistician: Professor Chris Rogers, Professor of Medical Statistics and Clinical Trials, University of Bristol.
Independent clinician: Mr John Salmon, Consultant Ophthalmologist, Oxford Eye Hospital.
Independent triallist: Professor Caroline Free, Professor of Primary Care and Epidemiology, London School of Hygiene and Tropical Medicine.
Project management group
Mr Gus Gazzard was the chief investigator of the trial; Dr Amanda Davis was the CTM of the trial (with temporary covers from Ms Ayse Barnes and Ms Danielle Reid); Mr Neil Nathwani was the trial’s CTO; Dr Evgenia Konstantakopoulou was the trial’s CRO; Dr Gareth Ambler was the lead trial statistician; Ms Victoria Vickerstaff was the trial statistician; and Ms Taniya Chowdhury was the trial data officer.
Staff facilitating recruitment and data collection
Moorfields Eye Hospital: Ms Emily Dowse, Ms Karine Girard-Claudon, Ms Seetal Savania-Dholakia and Ms Gurveen Panesar (optometrists); Mr Emerson Tingco, Ms Kanom Bibi, Mr Charles Amoah, Mr Dominic Carrington, Mr Mohamed Illiyas and Mr Yoganand Jeetun (technicians); and Ms Taniya Chowdhury, Ms Varshini Parayoganathan and Ms Nana Fatimah Ogunfemi (data officers).
Guy’s and St’ Thomas’ Hospital: Mr Sheng Lim (local PI); Mr Arij Daas, Ms Panayiota Founti and Mr Pouya Alaghband (research fellows); and Ms Priya Morjaria, Mr Andrew Ho, Ms Jenny Lee, Mr Kishan Patel, Mr Sandip Patel and Ms Seema Rajoria (optometrists).
Royal Victoria Hospital Belfast: Ms Sarah Wilson (local PI); Ms Karen Gillvray and Ms Angela Knox (research fellows); and Ms Tricia Douglas, Ms Katie Graham and Ms Deirdre Burns (optometrists).
Hinchingbrooke Hospital: Professor Rupert Bourne (local PI); and Ms Jane Kean (optometrists).
York Hospital: Mr Timothy Manners (local PI); and Ms Archana Airody (research fellow).
Norfolk and Norwich University Hospital: Mr David Broadway (local PI); and Mr Nuwan Niyadurupola (research fellow).
Contributions of authors
Gus Gazzard (Chief Investigator, Consultant Ophthalmic Surgeon) led the initial conception and design of the trial and writing of the protocol, acquired funding and ethics approval, is the chief investigator, had complete involvement and oversight of the trial, provided clinical expertise and was a major contributor to writing the manuscript.
Evgenia Konstantakopoulou (Research Optometrist) was involved in the day-to-day running of the trial, data collection and data interpretation, wrote the manuscript together with Gus Gazzard and was responsible for the production of the final report.
David Garway-Heath (Professor of Ophthalmology) co-designed the trial and the trial protocol, provided expert advice on clinical and methodological aspects and was involved in the drafting and critical revision of the manuscript.
Anurag Garg (Research Fellow) was involved in the acquisition of the data, data analysis and interpretation, contributed to the writing of Chapter 3 and critically reviewed the manuscript.
Victoria Vickerstaff (Senior Research Fellow) was involved in the design of the statistical analysis plan and the writing of the relevant paper, the data analysis, wrote parts of Chapter 2, provided guidance on the writing of Chapter 3 and critically reviewed the manuscript.
Rachael Hunter (Associate Professor Health Economics) contributed to the design of the outcome measures and data collection as well as the health economics analysis, wrote Chapter 4 and parts of Chapter 3.
Gareth Ambler (Associate Professor) was involved in the design of the statistical analysis plan and the writing of the relevant paper, contributed to the design of the outcome measures, the data to be collected and the data analysis, and was involved in the critical revision of the manuscript.
Catey Bunce (Reader Medical Statistics) contributed to the design of the outcome measures and the data to be collected, provided expert advice on statistical and methodological aspects of the trial and was involved in the critical revision of the manuscript.
Richard Wormald (Consultant Ophthalmologist) was involved in the critical revision of the study design and protocol, provided expert advice on clinical and methodological aspects of the trial and was involved in the critical revision of the manuscript.
Neil Nathwani (Research Optometrist) was involved in the design of the trial, data collection, data interpretation and critical revision of the manuscript. He was responsible for the clinical running of the trial in the central site.
Keith Barton (Consultant Ophthalmologist) was involved in the drafting of the protocol and critical revision of the study design and manuscript, and provided clinical expertise.
Gary Rubin (Professor of Ophthalmology) was involved in the drafting of the study design, provided expert advice on clinical and methodological aspects of the trial, and was involved in the critical revision of the manuscript.
Stephen Morris (Professor Health Economics) contributed to the design of the outcome measures and the data to be collected, and the critical revision of the manuscript.
Marta Buszewicz (Associate Professor in Primary Care) contributed to the original trial design, provided methodological guidance and was involved in the critical revision of the manuscript.
Publications
Vickerstaff V, Ambler G, Bunce C, Xing W, Gazzard G, LiGHT Trial Study Group. Statistical analysis plan for the Laser-1st versus Drops-1st for Glaucoma and Ocular Hypertension Trial (LiGHT): a multi-centre randomised controlled trial. Trials 2015;16:517. https://doi.org/10.1186/s13063-015-1047-9
Gazzard G, Konstantakopoulou E, Garway-Heath D, Barton K, Wormald R, Morris S, et al. Laser in Glaucoma and Ocular Hypertension (LiGHT) trial. A multicentre, randomised controlled trial: design and methodology. Br J Ophthalmol 2018;102:593–8. https://doi.org/10.1136/bjophthalmol-2017-310877
Konstantakopoulou E, Gazzard G, Vickerstaff V, Jiang Y, Nathwani N, Hunter R, et al. The laser in glaucoma and ocular hypertension (LiGHT) trial. A multicentre randomised controlled trial: baseline patient characteristics. Br J Ophthalmol 2018;102:599–603. https://doi.org/10.1136/bjophthalmol-2017-310870
Gazzard G, Konstantakopoulou E, Garway-Heath D, Garg A, Vickerstaff V, Hunter R, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet 2019;393:1505–16.
Data-sharing statement
All available data can be obtained from the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data are vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives. More about the background to this citation can be found here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Baasanhu J, Johnson GJ, Burendei G, Minassian DC. Prevalence and causes of blindness and visual impairment in Mongolia: a survey of populations aged 40 years and older. Bull World Health Organ 1994;72:771-6.
- Bunce C, Wormald R. Leading causes of certification for blindness and partial sight in England & Wales. BMC Public Health 2006;6. https://doi.org/10.1186/1471-2458-6-58.
- Haymes SA, Leblanc RP, Nicolela MT, Chiasson LA, Chauhan BC. Risk of falls and motor vehicle collisions in glaucoma. Invest Ophthalmol Vis Sci 2007;48:1149-55. https://doi.org/10.1167/iovs.06-0886.
- Haymes SA, LeBlanc RP, Nicolela MT, Chiasson LA, Chauhan BC. Glaucoma and on-road driving performance. Invest Ophthalmol Vis Sci 2008;49:3035-41. https://doi.org/10.1167/iovs.07-1609.
- Parrish RK, Gedde SJ, Scott IU, Feuer WJ, Schiffman JC, Mangione CM, et al. Visual function and quality of life among patients with glaucoma. Arch Ophthalmol 1997;115:1447-55. https://doi.org/10.1001/archopht.1997.01100160617016.
- Nelson P, Aspinall P, O’Brien C. Patients’ perception of visual impairment in glaucoma: a pilot study. Br J Ophthalmol 1999;83:546-52. https://doi.org/10.1136/bjo.83.5.546.
- Gutierrez P, Wilson MR, Johnson C, Gordon M, Cioffi GA, Ritch R, et al. Influence of glaucomatous visual field loss on health-related quality of life. Arch Ophthalmol 1997;115:777-84. https://doi.org/10.1001/archopht.1997.01100150779014.
- Nelson P, Aspinall P, Papasouliotis O, Worton B, O’Brien C. Quality of life in glaucoma and its relationship with visual function. J Glaucoma 2003;12:139-50. https://doi.org/10.1097/00061198-200304000-00009.
- Higginbotham EJ, Gordon MO, Beiser JA, Drake MV, Bennett GR, Wilson MR, et al. The Ocular Hypertension Treatment Study: topical medication delays or prevents primary open-angle glaucoma in African American individuals. Arch Ophthalmol 2004;122:813-20. https://doi.org/10.1001/archopht.122.6.813.
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:701-13. https://doi.org/10.1001/archopht.120.6.701.
- Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol 1999;117:573-83. https://doi.org/10.1001/archopht.117.5.573.
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Early Manifest Glaucoma Trial Group . Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002;120:1268-79. https://doi.org/10.1001/archopht.120.10.1268.
- Garway-Heath DF, Crabb DP, Bunce C, Lascaratos G, Amalfitano F, Anand N, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet 2015;385:1295-304. https://doi.org/10.1016/S0140-6736(14)62111-5.
- Ederer F, Gaasterland DA, Dally LG, Kim J, VanVeldhuisen PC, Blackwell B, et al. The Advanced Glaucoma Intervention Study (AGIS): 13. Comparison of treatment outcomes within race: 10-year results. Ophthalmology 2004;111:651-64. https://doi.org/10.1016/j.ophtha.2003.09.025.
- NICE: Guidance on Glaucoma: Diagnosis and Management of Chronic Open Angle Glaucoma and Ocular Hypertension. London: NICE; 2010.
- Royal College of Ophthalmologists . Focus in Ophthalmology – Glaucoma Management n.d. www.mrcophth.com/focus1/Glaucoma%20Management.htm (accessed 10 July 2015).
- Review of Clinical Guideline (CG85) – Glaucoma: Diagnosis and Management of Chronic Open Angle Glaucoma and Ocular Hypertension: Costing Report. London: NICE; 2009.
- Kirwan JF, Nightingale JA, Bunce C, Wormald R. Beta blockers for glaucoma and excess risk of airways obstruction: population based cohort study. BMJ 2002;325:1396-7. https://doi.org/10.1136/bmj.325.7377.1396.
- Fukuchi T, Wakai K, Suda K, Nakatsue T, Sawada H, Hara H, et al. Incidence, severity and factors related to drug-induced keratoepitheliopathy with glaucoma medications. Clin Ophthalmol 2010;4:203-9. https://doi.org/10.2147/OPTH.S9716.
- Alm A, Grierson I, Shields MB. Side effects associated with prostaglandin analog therapy. Surv Ophthalmol 2008;53:S93-105. https://doi.org/10.1016/j.survophthal.2008.08.004.
- Balkrishnan R, Bond JB, Byerly WG, Camacho FT, Anderson RT. Medication-related predictors of health-related quality of life in glaucoma patients enrolled in a Medicare health maintenance organization. Am J Geriatr Pharmacother 2003;1:75-81. https://doi.org/10.1016/S1543-5946(03)90003-1.
- Sharkawi E, Franks W. Glaucoma and mortality. Ophthalmology 2008;115:213-14. https://doi.org/10.1016/j.ophtha.2007.03.088.
- Roughead EE, Kalisch LM, Pratt NL, Killer G, Barnard A, Gilbert AL. Managing glaucoma in those with co-morbidity: not as easy as it seems. Ophthalmic Epidemiol 2012;19:74-82. https://doi.org/10.3109/09286586.2011.638743.
- Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol 2005;140:598-606. https://doi.org/10.1016/j.ajo.2005.04.051.
- Tsai JC. Medication adherence in glaucoma: approaches for optimizing patient compliance. Curr Opin Ophthalmol 2006;17:190-5. https://doi.org/10.1097/01.icu.0000193078.47616.aa.
- Tsai JC. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology 2009;116:30-6. https://doi.org/10.1016/j.ophtha.2009.06.024.
- Rahman MQ, Montgomery DM, Lazaridou MN. Surveillance of glaucoma medical therapy in a Glasgow teaching hospital: 26 years’ experience. Br J Ophthalmol 2009;93:1572-5. https://doi.org/10.1136/bjo.2008.151522.
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol 2002;120:714-20. https://doi.org/10.1001/archopht.120.6.714.
- Rahman MQ, Abeysinghe SS, Kelly S, Roskell NS, Shannon PR, Abdlseaed AA, et al. Persistence of glaucoma medical therapy in the Glasgow Glaucoma Database. Br J Ophthalmol 2011;95:966-70. https://doi.org/10.1136/bjo.2010.188607.
- Broadway D, Grierson I, Hitchings R. Adverse effects of topical antiglaucomatous medications on the conjunctiva. Br J Ophthalmol 1993;77:590-6. https://doi.org/10.1136/bjo.77.9.590.
- Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. I. The conjunctival cell profile. Arch Ophthalmol 1994;112:1437-45. https://doi.org/10.1001/archopht.1994.01090230051020.
- Broadway D, Hitchings R, Grierson I. Topical antiglaucomatous therapy: adverse effects on the conjunctiva and implications for filtration surgery. J Glaucoma 1995;4. https://doi.org/10.1097/00061198-199504000-00012.
- Broadway DC, Chang LP. Trabeculectomy, risk factors for failure and the preoperative state of the conjunctiva. J Glaucoma 2001;10:237-49. https://doi.org/10.1097/00061198-200106000-00017.
- Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol 1994;112:1446-54. https://doi.org/10.1001/archopht.1994.01090230060021.
- Alvarado JA, Katz LJ, Trivedi S, Shifera AS. Monocyte modulation of aqueous outflow and recruitment to the trabecular meshwork following selective laser trabeculoplasty. Arch Ophthalmol 2010;128:731-7. https://doi.org/10.1001/archophthalmol.2010.85.
- Kagan DB, Gorfinkel NS, Hutnik CM. Mechanisms of selective laser trabeculoplasty: a review. Clin Experiment Ophthalmol 2014;42:675-81. https://doi.org/10.1111/ceo.12281.
- McIlraith I, Strasfeld M, Colev G, Hutnik CM. Selective laser trabeculoplasty as initial and adjunctive treatment for open-angle glaucoma. J Glaucoma 2006;15:124-30. https://doi.org/10.1097/00061198-200604000-00009.
- Nagar M, Ogunyomade A, O’Brart DP, Howes F, Marshall J. A randomised, prospective study comparing selective laser trabeculoplasty with latanoprost for the control of intraocular pressure in ocular hypertension and open angle glaucoma. Br J Ophthalmol 2005;89:1413-17. https://doi.org/10.1136/bjo.2004.052795.
- Khouri AS, Lari HB, Berezina TL, Maltzman B, Fechtner RD. Long term efficacy of repeat selective laser trabeculoplasty. J Ophthalmic Vis Res 2014;9:444-8. https://doi.org/10.4103/2008-322X.150814.
- Khouri AS, Lin J, Berezina TL, Maltzman B, Fechtner RD. Repeat selective laser trabeculoplasty can be effective in eyes with initial modest response. Middle East Afr J Ophthalmol 2014;21:205-9. https://doi.org/10.4103/0974-9233.134668.
- Avery N, Ang GS, Nicholas S, Wells A. Repeatability of primary selective laser trabeculoplasty in patients with primary open-angle glaucoma. Int Ophthalmol 2013;33:501-6. https://doi.org/10.1007/s10792-013-9729-3.
- Barton K. Bleb dysesthesia. J Glaucoma 2003;12:281-4. https://doi.org/10.1097/00061198-200306000-00018.
- Budenz DL, Hoffman K, Zacchei A. Glaucoma filtering bleb dysesthesia. Am J Ophthalmol 2001;131:626-30. https://doi.org/10.1016/S0002-9394(00)00901-6.
- King AJ, Fernie G, Azuara-Blanco A, Burr JM, Garway-Heath T, Sparrow JM, et al. Treatment of Advanced Glaucoma Study: a multicentre randomised controlled trial comparing primary medical treatment with primary trabeculectomy for people with newly diagnosed advanced glaucoma-study protocol. Br J Ophthalmol 2018;102:922-8. https://doi.org/10.1136/bjophthalmol-2017-310902.
- Kobelt G, Jönsson L. Modeling cost of treatment with new topical treatments for glaucoma. Results from France and the United Kingdom. Int J Technol Assess Health Care 1999;15:207-19. https://doi.org/10.1017/S0266462399015299.
- Lee R, Hutnik CM. Projected cost comparison of selective laser trabeculoplasty versus glaucoma medication in the Ontario Health Insurance Plan. Can J Ophthalmol 2006;41:449-56. https://doi.org/10.1016/S0008-4182(06)80006-2.
- Slade J. Eye Health Data Summary – A Review of Published Data in England 2014.
- Lee PP, Walt JG, Doyle JJ, Kotak SV, Evans SJ, Budenz DL, et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol 2006;124:12-9. https://doi.org/10.1001/archopht.124.1.12.
- Taylor HR. Glaucoma: where to now?. Ophthalmology 2009;116:821-2. https://doi.org/10.1016/j.ophtha.2009.01.042.
- Dirani M, Crowston JG, Taylor PS, Moore PT, Rogers S, Pezzullo ML, et al. Economic impact of primary open-angle glaucoma in Australia. Clin Experiment Ophthalmol 2011;39:623-32. https://doi.org/10.1111/j.1442-9071.2011.02530.x.
- Seider MI, Keenan JD, Han Y. Cost of selective laser trabeculoplasty vs topical medications for glaucoma. Arch Ophthalmol 2012;130:529-30. https://doi.org/10.1001/archophthalmol.2012.355.
- Guedes RA, Guedes VM, Gomes CE, Chaoubah A. Maximizing cost-effectiveness by adjusting treatment strategy according to glaucoma severity. Medicine 2016;95. https://doi.org/10.1097/MD.0000000000005745.
- Cantor LB, Katz LJ, Cheng JW, Chen E, Tong KB, Peabody JW. Economic evaluation of medication, laser trabeculoplasty and filtering surgeries in treating patients with glaucoma in the US. Curr Med Res Opin 2008;24:2905-18. https://doi.org/10.1185/03007990802379996.
- Rolim de Moura C, Paranhos A, Wormald R. Laser trabeculoplasty for open angle glaucoma. Cochrane Database Syst Rev 2007;4. https://doi.org/10.1002/14651858.CD003919.pub2.
- Gazzard G, Konstantakopoulou E, Garway-Heath D, Barton K, Wormald R, Morris S, et al. Laser in Glaucoma and Ocular Hypertension (LiGHT) Trial. A multicentre, randomised controlled trial: design and methodology. Br J Ophthalmol 2018;102:593-8. https://doi.org/10.1136/bjophthalmol-2017-310877.
- National Institute for Health Research . Health-Related Quality of Life in Two Treatment Pathways for Newly Diagnosed Open Angle Glaucoma and Ocular Hypertension: An Unmasked, Multi-Centre, Randomised Controlled Trial of Initial Selective Laser Trabeculoplasty Versus Conventional Medical Therapy n.d. www.journalslibrary.nihr.ac.uk/programmes/hta/0910440/#/ (accessed 7 May 2019).
- Leurent B, Gomes M, Faria R, Morris S, Grieve R, Carpenter JR. Sensitivity analysis for not-at-random missing data in trial-based cost-effectiveness analysis: a tutorial. PharmacoEconomics 2018;36:889-901. https://doi.org/10.1007/s40273-018-0650-5.
- Gazzard G, Konstantakopoulou E, Garway-Heath D, Garg A, Vickerstaff V, Hunter R, et al. Selective laser trabeculoplasty vs drops for the treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. Lancet 2019;393:1505-16. https://doi.org/10.1016/S0140-6736(18)32213-X.
- Leske MC, Heijl A, Hyman L, Bengtsson B. Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology 1999;106:2144-53. https://doi.org/10.1016/S0161-6420(99)90497-9.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Burr JM, Kilonzo M, Vale L, Ryan M. Developing a preference-based Glaucoma Utility Index using a discrete choice experiment. Optom Vis Sci 2007;84:797-808. https://doi.org/10.1097/OPX.0b013e3181339f30.
- Lee BL, Gutierrez P, Gordon M, Wilson MR, Cioffi GA, Ritch R, et al. The Glaucoma Symptom Scale. A brief index of glaucoma-specific symptoms. Arch Ophthalmol 1998;116:861-6. https://doi.org/10.1001/archopht.116.7.861.
- Patel A, Rendu A, Moran P, Leese M, Mann A, Knapp M. A comparison of two methods of collecting economic data in primary care. Fam Pract 2005;22:323-7. https://doi.org/10.1093/fampra/cmi027.
- European Glaucoma Society . Terminology and Guidelines for Glaucoma 3rd Edn 2008. www.eugs.org/eng/guidelines.asp (accessed 12 May 2015).
- Primary Open-Angle Glaucoma: Preferred Practice Pattern. San Francisco, CA: American Academy of Ophthalmology; 2005.
- South East Asia Gluacoma Interest Group: Asia Pacific Glaucoma Guidelines. Hong Kong: SEAGIG; 2003.
- Good Clinical Practice. London: MHRA; 2010.
- Kotecha A, Crabb DP, Spratt A, Garway-Heath DF. The relationship between diurnal variations in intraocular pressure measurements and central corneal thickness and corneal hysteresis. Invest Ophthalmol Vis Sci 2009;50:4229-36. https://doi.org/10.1167/iovs.08-2955.
- Damji KF, Behki R, Wang L. Target IOP Workshop participants . Canadian perspectives in glaucoma management: setting target intraocular pressure range. Can J Ophthalmol 2003;38:189-97. https://doi.org/10.1016/S0008-4182(03)80060-1.
- Mills RP, Budenz DL, Lee PP, Noecker RJ, Walt JG, Siegartel LR, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Ophthalmol 2006;141:24-30. https://doi.org/10.1016/j.ajo.2005.07.044.
- Lee JR, Kim S, Lee JY, Back S, Lee KS, Kook MS. Is myopic optic disc appearance a risk factor for rapid progression in medically treated glaucomatous eyes with confirmed visual field progression?. J Glaucoma 2016;25:330-7. https://doi.org/10.1097/ijg.0000000000000218.
- Konstantakopoulou E, Gazzard G, Vickerstaff V, Jiang Y, Nathwani N, Hunter R, et al. The laser in glaucoma and ocular hypertension (LiGHT) trial. A multicentre randomised controlled trial: baseline patient characteristics. Br J Ophthalmol 2018;102:599-603. https://doi.org/10.1136/bjophthalmol-2017-310870.
- Burr JM, Mowatt G, Hernández R, Siddiqui MA, Cook J, Lourenco T, et al. The clinical effectiveness and cost-effectiveness of screening for open-angle glaucoma: a systematic review and economic evaluation. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11410.
- Canadian Glaucoma Study Group . Canadian Glaucoma Study: 1. Study design, baseline characteristics, and preliminary analyses. Can J Ophthalmol 2006;41:566-75. https://doi.org/i06-057.
- Death Registrations Summary Tables – England and Wales. Newport: Office for National Statistics; 2016.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Traverso CE, Walt JG, Kelly SP, Hommer AH, Bron AM, Denis P, et al. Direct costs of glaucoma and severity of the disease: a multinational long term study of resource utilisation in Europe. Br J Ophthalmol 2005;89:1245-9. https://doi.org/10.1136/bjo.2005.067355.
- Chauhan BC, Hutchison DM, Artes PH, Caprioli J, Jonas JB, LeBlanc RP, et al. Optic disc progression in glaucoma: comparison of confocal scanning laser tomography to optic disc photographs in a prospective study. Invest Ophthalmol Vis Sci 2009;50:1682-91. https://doi.org/10.1167/iovs.08-2457.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Chang DS, Friedman DS, Frazier T, Plyler R, Boland MV. Development and validation of a predictive model for nonadherence with once-daily glaucoma medications. Ophthalmology 2013;120:1396-402. https://doi.org/10.1016/j.ophtha.2013.01.002.
- Vickerstaff V, Ambler G, King M, Nazareth I, Omar RZ. Are multiple primary outcomes analysed appropriately in randomised controlled trials? A review. Contemp Clin Trials 2015;45:8-12. https://doi.org/S1551-7144(15)30053-7.
- Aspinall PA, Johnson ZK, Azuara-Blanco A, Montarzino A, Brice R, Vickers A. Evaluation of quality of life and priorities of patients with glaucoma. Invest Ophthalmol Vis Sci 2008;49:1907-15. https://doi.org/10.1167/iovs.07-0559.
- Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med 1997;16:2349-80. https://doi.org/10.1002/(SICI)1097-0258(19971030)16:20<2349::AID-SIM667>3.0.CO;2-E.
- Hunter RM, Baio G, Butt T, Morris S, Round J, Freemantle N. An educational review of the statistical issues in analysing utility data for cost-utility analysis. PharmacoEconomics 2015;33:355-66. https://doi.org/10.1007/s40273-014-0247-6.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Guides to the Methods of Technology Appraisal. London: NICE; 2013.
- Curtis L. Unit Costs of Health and Social Care 2017. Canterbury: PSSRU, University of Kent; 2017.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes: Fourth Edition. Oxford: Oxford University Press; 2015.
- British National Formulary (Online). London: BMJ Group and Pharmaceutical Press; n.d.
- National Schedule of Reference Costs 2016–17. London: DHSC; 2018.
- Beecham JK, Knapp MRJ, Thornicroft G, Brewin C, Wing JK. Measuring Mental Health Needs. London: Edward Gaskell Publishers; 1992.
- Violato M, Dakin H, Chakravarthy U, Reeves BC, Peto T, Hogg RE, et al. Cost-effectiveness of community versus hospital eye service follow-up for patients with quiescent treated age-related macular degeneration alongside the ECHoES randomised trial. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011121.
- Georghiou T, Bardsley M. Exploring the Cost of Care at the End of Life. London: Nuffield Trust; 2014.
- Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol 2008;53:S57-68. https://doi.org/10.1016/j.survophthal.2008.08.002.
- Stein JD, Kim DD, Peck WW, Giannetti SM, Hutton DW. Cost-effectiveness of medications compared with laser trabeculoplasty in patients with newly diagnosed open-angle glaucoma. Arch Ophthalmol 2012;130:497-505. https://doi.org/10.1001/archophthalmol.2011.2727.
- Rahman MQ, Beard SM, Discombe R, Sharma R, Montgomery DM. Direct healthcare costs of glaucoma treatment. Br J Ophthalmol 2013;97:720-4. https://doi.org/10.1136/bjophthalmol-2012-302525.
- Holmstrom S, Buchholz P, Walt J, Wickstrom J, Aagren M. The cost-effectiveness of bimatoprost, latanoprost and timolol in treatment of primary open angle glaucoma in five European countries. Curr Med Res Opin 2006;22:897-905. https://doi.org/10.1185/030079906X104687.
- Crane GJ, Kymes SM, Hiller JE, Casson R, Martin A, Karnon JD. Accounting for costs, QALYs, and capacity constraints: using discrete-event simulation to evaluate alternative service delivery and organizational scenarios for hospital-based glaucoma services. Med Decis Making 2013;33:986-97. https://doi.org/10.1177/0272989X13478195.
- Li EY, Tham CC, Chi SC, Lam DS. Cost-effectiveness of treating normal tension glaucoma. Invest Ophthalmol Vis Sci 2013;54:3394-9. https://doi.org/10.1167/iovs.12-11549.
- Stewart WC, Stewart JA, Mychaskiw MA. Cost-effectiveness of latanoprost and timolol maleate for the treatment of glaucoma in Scandinavia and the United Kingdom, using a decision-analytic health economic model. Eye 2009;23:132-40. https://doi.org/10.1038/sj.eye.6702964.
- van Gestel A, Webers CA, Severens JL, Beckers HJ, Jansonius NM, Hendrikse, et al. The long-term outcomes of four alternative treatment strategies for primary open-angle glaucoma. Acta Ophthalmol 2012;90:20-31. https://doi.org/10.1111/j.1755-3768.2011.02318.x.
- Boodhna T, Crabb DP. More frequent, more costly? Health economic modelling aspects of monitoring glaucoma patients in England. BMC Health Serv Res 2016;16. https://doi.org/10.1186/s12913-016-1849-9.
- Hernandez R, Burr JM, Vale L, Azuara-Blanco A, Cook JA, Banister K, et al. Monitoring ocular hypertension, how much and how often? A cost-effectiveness perspective. Br J Ophthalmol 2016;100:1263-8. https://doi.org/10.1136/bjophthalmol-2015-306757.
- Kaplan RI, De Moraes CG, Cioffi GA, Al-Aswad LA, Blumberg DM. Comparative cost-effectiveness of the baerveldt implant, trabeculectomy with mitomycin, and medical treatment. JAMA Ophthalmol 2015;133:560-7. https://doi.org/10.1001/jamaophthalmol.2015.44.
- Thomas S, Hodge W, Malvankar-Mehta M. The cost-effectiveness analysis of teleglaucoma screening device. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0137913.
- Xu X, Ma YY, Zou HD. Cost–utility analysis of cataract surgery in advanced glaucoma patients. J Glaucoma 2016;25:e657-62. https://doi.org/10.1097/IJG.0000000000000313.
- Blumberg DM, Vaswani R, Nong E, Al-Aswad L, Cioffi GA. A comparative effectiveness analysis of visual field outcomes after projected glaucoma screening using SD-OCT in African American communities. Invest Ophthalmol Vis Sci 2014;55:3491-500. https://doi.org/10.1167/iovs.14-14014.
- Orme M, Collins S, Loftus J. Long-term medical management of primary open-angle glaucoma and ocular hypertension in the UK: optimizing cost-effectiveness and clinic resources by minimizing therapy switches. J Glaucoma 2012;21:433-49. https://doi.org/10.1097/IJG.0b013e31821dac2a.
- Rein DB, Wittenborn JS, Lee PP, Wirth KE, Sorensen SW, Hoerger TJ, et al. The cost-effectiveness of routine office-based identification and subsequent medical treatment of primary open-angle glaucoma in the United States. Ophthalmology 2009;116:823-32. https://doi.org/10.1016/j.ophtha.2008.12.056.
- Stewart WC, Leech J, Sharpe ED, Kulze J, Ellyn J, Day DG. An economic analysis of switching to latanoprost from a beta-blocker or adding brimonidine or latanoprost to a beta-blocker in open-angle glaucoma or ocular hypertension. Am J Manag Care 2002;8:S240-8.
- Vaahtoranta-Lehtonen H, Tuulonen A, Aronen P, Sintonen H, Suoranta L, Kovanen N, et al. Cost effectiveness and cost utility of an organized screening programme for glaucoma. Acta Ophthalmol Scand 2007;85:508-18. https://doi.org/10.1111/j.1755-3768.2007.00947.x.
- Burr J, Hernandez R, Ramsay C, Prior M, Campbell S, Azuara-Blanco A, et al. Is it worthwhile to conduct a randomized controlled trial of glaucoma screening in the United Kingdom?. J Health Serv Res Policy 2014;19:42-51. https://doi.org/10.1177/1355819613499748.
- van Gestel A, Schouten JS, Beckers HJ, Severens JL, Hendrikse F, Webers CA. The long term effectiveness and cost-effectiveness of initiating treatment for ocular hypertension. Acta Ophthalmol 2014;92:513-23. https://doi.org/10.1111/aos.12328.
- Burr J, Azuara-Blanco A, Avenell A, Tuulonen A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst Rev 2012;9. https://doi.org/10.1002/14651858.CD004399.pub3.
- Burr JM, Botello-Pinzon P, Takwoingi Y, Hernandez R, Vazquez-Montes M, Elders A, et al. Surveillance for ocular hypertension: an evidence synthesis and economic evaluation. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16290.
- Hernandez RA, Burr JM, Vale LD. Economic evaluation of screening for open-angle glaucoma. Int J Technol Assess Health Care 2008;24:203-11. https://doi.org/10.1017/S0266462308080288.
- Kymes SM, Plotzke MR, Kass MA, Boland MV, Gordon MO. Effect of patient's life expectancy on the cost-effectiveness of treatment for ocular hypertension. Arch Ophthalmol 2010;128:613-18. https://doi.org/10.1001/archophthalmol.2010.83.
- Kymes SM, Plotzke MR, Li JZ, Nichol MB, Wu J, Fain J. The increased cost of medical services for people diagnosed with primary open-angle glaucoma: a decision analytic approach. Am J Ophthalmol 2010;150:74-81. https://doi.org/10.1016/j.ajo.2010.01.037.
- Kymes SM, Kass MA, Anderson DR, Miller JP, Gordon MO. Management of ocular hypertension: a cost-effectiveness approach from the Ocular Hypertension Treatment Study. Am J Ophthalmol 2006;141:997-1008. https://doi.org/10.1016/j.ajo.2006.01.019.
- Peeters A, Schouten JS, Webers CA, Prins MH, Hendrikse F, Severens JL. Cost-effectiveness of early detection and treatment of ocular hypertension and primary open-angle glaucoma by the ophthalmologist. Eye 2008;22:354-62. https://doi.org/10.1038/sj.eye.6702637.
- van Gestel A, Webers CA, Beckers HJ, van Dongen MC, Severens JL, Hendrikse F, et al. The relationship between visual field loss in glaucoma and health-related quality-of-life. Eye 2010;24:1759-69. https://doi.org/10.1038/eye.2010.133.
- Szende A, Janssen B, Cabases J. Self-Reported Population Health: An International Perspective Based on EQ-5D. London: SpringerOpen; 2014.
- Kobelt G, Jonsson B, Bergström A, Chen E, Lindén C, Alm A. Cost-effectiveness analysis in glaucoma: what drives utility? Results from a pilot study in Sweden. Acta Ophthalmol Scand 2006;84:363-71. https://doi.org/10.1111/j.1600-0420.2005.00621.x.
- Tosh J, Brazier J, Evans P, Longworth L. A review of generic preference-based measures of health-related quality of life in visual disorders. Value Health 2012;15:118-27. https://doi.org/10.1016/j.jval.2011.08.002.
- Jones L, Garway-Heath DF, Azuara-Blanco A, Crabb DP. United Kingdom Glaucoma Treatment Study Investigators . Are patient self-reported outcome measures sensitive enough to be used as end points in clinical trials?: evidence from the United Kingdom Glaucoma Treatment Study. Ophthalmology 2019;126:682-9.
- Medeiros FA, Gracitelli CP, Boer ER, Weinreb RN, Zangwill LM, Rosen PN. Longitudinal changes in quality of life and rates of progressive visual field loss in glaucoma patients. Ophthalmology 2015;122:293-301. https://doi.org/10.1016/j.ophtha.2014.08.014.
- Goldberg I, Clement CI, Chiang TH, Walt JG, Lee LJ, Graham S, et al. Assessing quality of life in patients with glaucoma using the Glaucoma Quality of Life-15 (GQL-15) questionnaire. J Glaucoma 2009;18:6-12. https://doi.org/10.1097/IJG.0b013e3181752c83.
- Gracner T. Intraocular pressure response of capsular glaucoma and primary open-angle glaucoma to selective Nd:YAG laser trabeculoplasty: a prospective, comparative clinical trial. Eur J Ophthalmol 2002;12:287-92. https://doi.org/10.1177/112067210201200406.
- Shazly TA, Latina MA. Intraocular pressure response to selective laser trabeculoplasty in the first treated eye vs the fellow eye. Arch Ophthalmol 2011;129:699-702. https://doi.org/10.1001/archophthalmol.2011.108.
- Shazly TA, Latina MA, Dagianis JJ, Chitturi S. Effect of prior cataract surgery on the long-term outcome of selective laser trabeculoplasty. Clin Ophthalmol 2011;5:377-80. https://doi.org/10.2147/OPTH.S17237.
- Gracner T, Falez M, Gracner B, Pahor D. Long-term follow-up of selective laser trabeculoplasty in primary open-angle glaucoma. Klin Monbl Augenheilkd 2006;223:743-7. https://doi.org/10.1055/s-2006-926725.
- Melamed S, Ben Simon GJ, Levkovitch-Verbin H. Selective laser trabeculoplasty as primary treatment for open-angle glaucoma: a prospective, nonrandomized pilot study. Arch Ophthalmol 2003;121:957-60. https://doi.org/10.1001/archopht.121.7.957.
- Koucheki B, Hashemi H. Selective laser trabeculoplasty in the treatment of open-angle glaucoma. J Glaucoma 2012;21:65-70. https://doi.org/10.1097/IJG.0b013e3182027596.
- Lai JS, Chua JK, Tham CC, Lam DS. Five-year follow up of selective laser trabeculoplasty in Chinese eyes. Clin Experiment Ophthalmol 2004;32:368-72. https://doi.org/10.1111/j.1442-9071.2004.00839.x.
- Leahy KE, White AJ. Selective laser trabeculoplasty: current perspectives. Clin Ophthalmol 2015;9:833-41. https://doi.org/10.2147/OPTH.S53490.
- Robin AL, Covert D. Does adjunctive glaucoma therapy affect adherence to the initial primary therapy?. Ophthalmology 2005;112:863-8. https://doi.org/10.1016/j.ophtha.2004.12.026.
- Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol 2007;144:533-40. https://doi.org/10.1016/j.ajo.2007.06.012.
- Prasad N, Murthy S, Dagianis JJ, Latina MA. A comparison of the intervisit intraocular pressure fluctuation after 180 and 360 degrees of selective laser trabeculoplasty (SLT) as a primary therapy in primary open-angle glaucoma and ocular hypertension. J Glaucoma 2009;18:157-60. https://doi.org/10.1097/IJG.0b013e3181752c97.
- Elsås T, Junk H, Johnsen H. Diurnal intraocular pressure after successful primary laser trabeculoplasty. Am J Ophthalmol 1991;112:67-9. https://doi.org/10.1016/S0002-9394(14)76215-4.
- Greenidge KC, Spaeth GL, Fiol-Silva Z. Effect of argon laser trabeculoplasty on the glaucomatous diurnal curve. Ophthalmology 1983;90:800-4. https://doi.org/10.1016/S0161-6420(83)34479-1.
- Tojo N, Oka M, Miyakoshi A, Ozaki H, Hayashi A. Comparison of fluctuations of intraocular pressure before and after selective laser trabeculoplasty in normal-tension glaucoma patients. J Glaucoma 2014;23:e138-43. https://doi.org/10.1097/IJG.0000000000000026.
- Nagar M, Luhishi E, Shah N. Intraocular pressure control and fluctuation: the effect of treatment with selective laser trabeculoplasty. Br J Ophthalmol 2009;93:497-501. https://doi.org/10.1136/bjo.2008.148510.
- Wong MO, Lee JW, Choy BN, Chan JC, Lai JS. Systematic review and meta-analysis on the efficacy of selective laser trabeculoplasty in open-angle glaucoma. Surv Ophthalmol 2015;60:36-50. https://doi.org/10.1016/j.survophthal.2014.06.006.
- Li X, Wang W, Zhang X. Meta-analysis of selective laser trabeculoplasty versus topical medication in the treatment of open-angle glaucoma. BMC Ophthalmol 2015;15. https://doi.org/10.1186/s12886-015-0091-2.
- Priorities for Glaucoma Research. Southampton: James Lind Alliance; 2015.
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Early Manifest Glaucoma Trial Group . Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 2003;121:48-56. https://doi.org/10.1001/archopht.121.1.48.
- Wu SY, Nemesure B, Hennis A, Schachat AP, Hyman L, Leske MC. Barbados Eye Studies Group . Open-angle glaucoma and mortality: the Barbados Eye Studies. Arch Ophthalmol 2008;126:365-70. https://doi.org/10.1001/archophthalmol.2007.77.
- Stewart WC, Stewart JA, Nasser QJ, Nassar QJ, Mychaskiw MA. Cost-effectiveness of treating ocular hypertension. Ophthalmology 2008;115:94-8. https://doi.org/10.1016/j.ophtha.2007.01.040.
- Lee AJ, Wang JJ, Kifley A, Mitchell P. Open-angle glaucoma and cardiovascular mortality: the Blue Mountains Eye Study. Ophthalmology 2006;113:1069-76. https://doi.org/10.1016/j.ophtha.2006.02.062.
- Quigley HA, Tielsch JM, Katz J, Sommer A. Rate of progression in open-angle glaucoma estimated from cross-sectional prevalence of visual field damage. Am J Ophthalmol 1996;122:355-63. https://doi.org/10.1016/S0002-9394(14)72062-8.
- Saunders LJ, Russell RA, Kirwan JF, McNaught AI, Crabb DP. Examining visual field loss in patients in glaucoma clinics during their predicted remaining lifetime. Invest Ophthalmol Vis Sci 2014;55:102-9. https://doi.org/10.1167/iovs.13-13006.
- Saunders LJ, Russell RA, Crabb DP. Practical landmarks for visual field disability in glaucoma. Br J Ophthalmol 2012;96:1185-9. https://doi.org/10.1136/bjophthalmol-2012-301827.
- Patterson BL, Charlton P, Richard S. Non-attendance in chronic disease clinics: a matter of non-compliance?. J Nurs Healthc Chronic Illn 2010;2:63-74. https://doi.org/10.1111/j.1752-9824.2010.01048.x.
Appendix 1 Recruiting sites
| Participating centre | Local PI | Screening start date | Screening end date |
|---|---|---|---|
| MEH, City Road Campus | Mr Gus Gazzard | October 2012 | October 2014 |
| MEH at St George’s Hospital | Mr Gus Gazzard | October 2012 | October 2014 |
| MEH at Northwick Park Hospital |
Mr Nicholas Strouthidis Mr Hari Jayaram |
October 2012 | October 2014 |
| Royal Victoria Hospital, Belfast | Ms Sarah Wilson | November 2013 | October 2014 |
| Guy’s and St Thomas’ Hospital | Mr Sheng Lim | July 2013 | October 2014 |
| Hinchingbrooke Hospital | Professor Rupert Bourne | July 2013 | October 2014 |
| Norfolk and Norwich University Hospitals NHS Foundation Trust | Mr David Broadway | July 2013 | October 2014 |
| York Hospital |
Mr Timothy Manners Ms Joanna Liput |
November 2013 | October 2014 |
Appendix 2 Definitions of open-angle glaucoma, ocular hypertension and treatment requirements
We have used the NICE recommended thresholds for initiating treatment,1 with stringent diagnostic definitions of disease (OAG or OHT) for entry into the study.
Diagnosis of open-angle glaucoma and treatment requirements
Primary OAG is defined as an open drainage angle (no iridotrabecular contact on non-indentation gonioscopy in primary position, trabecular meshwork visible over 360°), with no secondary causes (such as trauma):
-
and reproducible glaucomatous VF defects as tested by the Swedish Interactive Threshold Algorithm on the Humphrey Visual Field Analyser (i.e. reproducible defect, in at least, of two or more contiguous points with a p-value < 0.01 loss or greater, or three or more contiguous points with a p-value < 0.05 loss or greater, or abnormal Glaucoma Hemifield Test)
-
or GON with localised absence of the neuroretinal rim, or cup disc ratio of ≥ 0.7, or asymmetry of cup disc ratio of ≥ 0.2 in similar-sized eyes/optic discs
-
and deemed to require treatment in the opinion of the treating (fellowship-trained) glaucoma specialist.
Subjects with pseudo-exfoliation are eligible (as for EMGT I82).
Subjects with GON and IOP in the normal range are therefore eligible. ‘Phasing’ (diurnal IOP pressure measurements) will be performed at the discretion of the treating clinician, and, if performed, the maximum IOP recorded will be used as that day’s measurement.
Diagnosis of ocular hypertension and treatment requirements
Ocular hypertension with IOP > 21 mmHg and requiring treatment as per NICE guidelines. 1 NICE OHT guidelines treat four categories of OHT on the basis of CCT and age (the rest are monitored for 3–5 years).
Appendix 3 Video script
This video has been designed to inform you about a research study that is ongoing at Moorfields Eye Hospital. The video will introduce you to glaucoma and ocular hypertension, the various treatment options that are available and will eventually invite you to take part in a research study investigating the quality of your life after treatment. We would be grateful if you could spend 5–10 minutes watching this video.
Glaucoma is a disease of the optic nerve, which connects the eye to the brain. Glaucoma slowly progresses over a period of years; at the early stages people may not notice anything abnormal, but in advanced disease people may notice loss of vision. At the early stages, glaucoma can be treated with eyedrops or a laser treatment, which aim to control the condition and minimise future damage. Early diagnosis is important because any damage cannot be reversed. If glaucoma is left untreated, it can cause visual impairment. Glaucoma may be caused by raised eye pressure, but sometimes glaucoma develops despite a normal pressure inside the eyes, owing to a poorer blood supply or a weaker optic nerve.
Ocular hypertension is a condition where the pressure of the eyes is above normal limits, without, however, this causing any damage to the optic nerve. Some people have higher pressures than others. It has been shown that ocular hypertension puts people at a higher risk for developing glaucoma. Some people with ocular hypertension may, however, never develop glaucoma.
The pressure of your eyes is important, as your eyes function properly under a certain amount of pressure. If this pressure increases the optic nerve can be damaged. The amount of damage may depend on how high the pressure is, how long it lasts, and whether there is a poor blood supply or other weaknesses of the optic nerve. By lowering the pressure damage is slowed down.
At the moment, in the NHS, nearly all patients who have glaucoma or ocular hypertension are treated by eyedrops. Once started, eyedrops usually have to be continued for life. Not all patients like using eyedrops daily, however, and these patients might be suitable to a gentle laser therapy called selective laser trabeculoplasty. This laser treatment is not experimental; it is used commonly in the UK and for a number of years in the United States and its efficacy has been proven. At the moment the laser treatment is not offered as a first-line treatment in the NHS.
This study is designed to investigate the quality of life in patients treated first time either with eyedrops or with laser and is being funded by the National Institute for Health Research. The study will use questionnaires that the patients will have to fill in every 6 months. A secondary aim is to assess the cost of these treatments to the NHS.
Because we don’t know which treatment will prove preferable for the patients’ quality of life or which treatment is more cost-effective for the NHS, patients in this study will be assigned randomly to one of the two treatments and the two groups will then be compared. This type of study is called a randomised controlled trial, where half the patients will be randomly assigned by a computer to eyedrops and half will be assigned to laser treatment. If we assigned you to a group we might show preference to a specific treatment and if you were to choose, you might be biased towards one treatment.
Participation in this study is entirely voluntary. If you decide not to take part this will have no effect on the quality of care you receive at Moorfields Eye Hospital. If you decide to take part you will only be asked to attend the clinic one extra time compared with the usual clinic, but we can reimburse your travel expenses. The reason we will ask you to come one extra time is to give you time to think about the study and allow you to ask us any questions you might have.
If you decide to take part in this study you will be monitored by our specially trained optometrist and you will do the exact same tests you would do in a normal clinic. This is because the study is a real-life study, investigating your quality of life. This study will last in total 5 years, but each patient will be monitored for 6 years after the treatment is started. For this period of time you will be asked to attend the clinic between five and seven times. If you do not take part in the study you will be asked to attend the clinic six to seven times. After the end of the study you will continued to be monitored by the glaucoma clinics at Moorfields Eye Hospital as usual.
If you decide to take part you will be asked to fill in a questionnaire about your health and about your eyes. You will then be assigned to having drops or laser and you will be seen a few weeks later to assess if the treatment is working, just as we would if you were not in the study. Twice a year we will send you a questionnaire by post, which after filling in you can return to us in a pre-paid envelope that we will also send.
As every treatment, the treatments in this study might have some side effects. Eyedrops are used for approximately 30 years and can have mild or more severe side effects. Drops can cause mild discomfort or redness of the eyes, which usually settle soon, but in some cases they might make asthma worse. We will make sure we ask you all the necessary questions about your health before prescribing any drops. Some types of eyedrops should not be given to pregnant women. Any woman who is pregnant or who is planning to become pregnant should therefore not take part in this study. If a woman taking part in the study becomes pregnant she should let the research team know immediately. If the drops are not lowering your eye pressure enough you will be offered different or additional eyedrops.
The laser has been used successfully for 10–20 years. It is not a surgery and it is safe, easy and painless to deliver. In some people it might cause a small discomfort for a few days, which can be treated with anti-inflammatory eyedrops. Very rarely the laser might cause the eyes to be blurry for a few weeks or it may cause the pressure of the eyes to increase. If this happens we will give you drops to use for a few days. Laser treatment is effective in 80–90% of patients and its effect might wear off after a few years. If this happens the laser can be repeated once more. If for some reason we still need to reduce your eye pressure we will give you drops.
This study has no direct risks or benefits to you, as it is designed to mimic normal clinical practise. This means that you will be doing exactly what you would be doing in a normal glaucoma clinic and nothing additional to that. If during the study you decide you don’t want to take part any more you can withdraw without providing a reason and without this affecting your care at Moorfields Eye Hospital. If you choose to withdraw you will then be monitored by a normal clinic. If you decide to take part you will be assigned an ID and all the information will be kept strictly confidential.
Two of the patients who have already taken part in our study have kindly agreed to explain the reasons they decided to participate and their experience so far.
Having explained why Moorfields Eye Hospital is conducting this study I hope you will be willing to consider taking part. My colleagues will now give you an information sheet about this study and will be able to answer any further questions you might have in relation to the study.
Thank you very much for your time.
Appendix 4 Selective laser trabeculoplasty delivery protocol
Training was given to all treating surgeons before recruitment and at least one laser treatment was observed by the chief investigator. The treating surgeon was the local PI or a fellowship-trained glaucoma specialist who was eligible to apply for a UK consultant surgeon post or for inclusion on the UK General Medical Council specialist register and who had performed at least 25 previous SLT treatments.
Selective laser trabeculoplasty was delivered to 360° of the trabecular meshwork, with one 360° retreatment as the first escalation of treatment if required. The model of SLT laser used was not restricted, and the wavelength and spot size were the same. To minimise variation between surgeons, standardisation was achieved by a stringent protocol defining laser settings and technique.
Pretreatment with iopidine (0.5% or 1%) at least 15 minutes before treatments was mandatory, unless contraindicated for medical reasons, in which case alternative medications such as oral acetazolamide [Apraclonidine (Novartis Pharmaceuticals UK Ltd)] could be used. If no prophylaxis against IOP spikes was used, close post-treatment monitoring of IOP for 2 hours was necessary.
One hundred non-overlapping shots (25 per quadrant) of a preset 3 nanoseconds duration and a preset 400 μm spot size were used, with the laser energy varied from 0.3 mJ to 1.4 mJ by the clinician using any laser gonioscopy lens (as long as the appropriate magnification was observed, e.g. ‘Latina’ was acceptable but ‘Magnaview’ was not). The desired end point was the production of a few fine ‘champagne bubbles’; larger gas bubbles and trabecular meshwork blanching were not acceptable, and if seen the operator should have titrated the power downwards in 0.1-mJ increments. Pigmented trabecular meshwork will have required lower energy (from 0.3 mJ to 1.2 mJ) than non-pigmented and it was advisable to start treatments at 0.4 mJ. The Goldmann applanation tonometry IOP was measured 1 hour after treatment.
After treatment with SLT, patients were not asked to use anti-inflammatory eyedrops routinely; however, they were provided with a bottle of topical non-steroidal anti-inflammatory eyedrops for use only if they were in significant discomfort, despite simple oral analgesia such as paracetamol. [This is now standard practice in most units worldwide (Mark Latina, Tufts University School of Medicine, MA, USA, personal communication).] Topical steroids were not to be permitted post laser. Demonstrations of correct eyedrop technique were given at baseline and whenever needed thereafter.
Any rise in IOP of > 10 mmHg or that puts the patient at risk of visual loss was treated at the discretion of the treating ophthalmologist with an earlier recheck of IOP (e.g. at 2 hours, 1 day or 1 week) and/or a short-term course of topical or systemic aqueous suppressants as necessary. An IOP rise necessitating medical treatment or an extra visit alone would constitute an AE and be independently logged as such.
The first post-SLT follow-up was at 2 weeks for an IOP check and assessment of potential side effects. No reintervention/treatment escalation decisions for non-response were made at this point; a further follow-up 6 weeks later was scheduled to allow time for the full effects of laser to occur. Patients at target 8 weeks after SLT were subsequently reviewed as per the interval determined by the severity category. Patients not at target after a single SLT received another treatment of 360° (100 spots) at the same energy settings, with re-evaluation after 2 weeks. After retreatment, a 6-week follow-up was given whether at target or not, unless, in the opinion of the treating clinician, a dangerously high IOP posed a significant risk to vision, in which case earlier follow-up was allowed to avoid an unsafe delay in medical therapy.
It is possible that an IOP rise following SLT could be severe enough to prevent a safe repeat of SLT should the target not have been met in future, particularly with more severe glaucoma. In this case, treatment escalation with eyedrops rather than repeating the SLT was permitted. This required an algorithm over-ride and thus was automatically logged and monitored. This may have occurred as an immediate (but transient) or persistent post-SLT pressure rise. Any immediate IOP rise > 40 mmHg despite pre-treatment iopidine or any rise of > 5 mmHg that persisted 8 weeks after laser would usually prevent further SLT treatment. After two SLT treatments, participants in the Laser-1st pathway embarked on medical treatment and followed the Medicine-1st algorithms. If the participant subsequently underwent drainage surgery which failed in the course of the trial, the step-wise medical intervention algorithm was initiated again and further SLT was not permitted.
Appendix 5 Questionnaire: sample patient response
Appendix 6 Patient and public involvement survey sent to patients
Appendix 7 Frequency of data monitoring across sites
| Site | Patients (n) | Monitored (n) | Monitored (%) | Notes |
|---|---|---|---|---|
| Guy’s and St Thomas’ Hospital | 106 | 106 | 100.0 | January 2015–February 2016 |
| Guy’s and St Thomas’ Hospital (repeat) | 106 | 30 | 28.3 | April 2016–October 2017 |
| Hinchingbrooke Hospital | 82 | 38 | 46.3 | |
| York Hospital | 37 | 19 | 51.4 | |
| Royal Victoria Hospital, Belfast | 30 | 11 | 36.7 | |
| Norfolk and Norwich University Hospitals NHS Foundation Trust | 89 | 40 | 44.9 |
Appendix 8 Recruitment
| Date | Monthly target | Cumulative overall target | Actual recruitment | Cumulative actual recruitment |
|---|---|---|---|---|
| October 2012 | 10 | 10 | 7 | 7 |
| November 2012 | 17 | 27 | 24 | 31 |
| December 2012 | 17 | 44 | 10 | 41 |
| January 2013 | 17 | 61 | 19 | 60 |
| February 2013 | 17 | 78 | 13 | 73 |
| March 2013 | 17 | 95 | 11 | 84 |
| April 2013 | 17 | 112 | 12 | 96 |
| May 2013 | 17 | 129 | 16 | 112 |
| June 2013 | 17 | 146 | 7 | 119 |
| July 2013 | 26 | 172 | 33 | 151 |
| August 2013 | 39 | 211 | 22 | 173 |
| September 2013 | 39 | 250 | 27 | 200 |
| October 2013 | 39 | 289 | 39 | 239 |
| November 2013 | 39 | 328 | 39 | 278 |
| December 2013 | 39 | 367 | 40 | 318 |
| January 2014 | 39 | 406 | 47 | 365 |
| February 2014 | 39 | 445 | 36 | 401 |
| March 2014 | 39 | 484 | 45 | 446 |
| April 2014 | 39 | 523 | 47 | 493 |
| May 2014 | 39 | 562 | 23 | 516 |
| June 2014 | 39 | 601 | 37 | 553 |
| July 2014 | 39 | 640 | 53 | 606 |
| August 2014 | 39 | 679 | 26 | 632 |
| September 2014 | 39 | 718 | 38 | 670 |
| October 2014 | 39 | 718 | 49 | 719 |
Appendix 9 Protocol deviations and violations
| Patient ID | Site | Date of event | Violation/deviation | Preventative action |
|---|---|---|---|---|
| MEH-070 | MEH | 27 February 2013 | Violation: ineligible patient randomised | Any patient who is judged to have angles < 10°, or where there is a conflict of diagnosis, should be checked by the chief investigator/PI prior to randomisation |
|
YOR-246 YOR-265 YOR-286 YOR-287 YOR-319 |
York | 8 January 2014 | Deviation: health-care assistants have been performing SITA fast visual fields instead of SITA standard | Research manager has sent an e-mail to all centres (PIs and co-ordinators) to ensure that patients are tested using SITA standard and not SITA fast. VF are now being done by the research team in York rather than health-care assistants |
| HIN-340 | Hinchingbrooke | 15 January 2014 | Deviation: after the patient was randomised they decided to withdraw as they wanted to have only one of the two treatments | E-mailed the site explaining that patients who are adamant about receiving a particular treatment should not be consented to participate as this is a randomised trial. Site is happy to continue with this guidance. Mr Bourne confirmed that the patient did not express a preference to a treatment prior to randomisation, but once randomised they had made a preference. Patient has been withdrawn and will have their preferred treatment on the NHS |
| GST-472 | Guy’s and St’ Thomas’ | 14 April 2014 | Violation: ineligible patient randomised | VF MD more than the eligibility criteria |
| YOR-265 | York | 17 December 2013; 14 January 2014 |
Deviation: patient previously randomised to SLT. Patient did not attend 2-week IOP check and York hospital rescheduled the appointment. At this appointment, the algorithm was updated which should not have been Violation: HRT test was not done and data not entered on to the algorithm on the day of the appointment. Data were also entered for the wrong follow-up visit as a result of data being entered at the IOP check visit when this should not have been entered. The patient was then prescribed eyedrops as an increase in treatment as a clinical decision over-riding the algorithm |
Provided intense training for all team members on 28 January 2014 |
| BEL-613 | Belfast | 8 August 2104 | Deviation: patient was recruited and randomised to laser. As the patient’s IOP increased, Ms Wilson decided to start eyedrops and not go ahead with the laser | |
| GST-486 | Guy’s and St’ Thomas’ | 28 April 2014 | Violations: ineligible patient randomised owing to VF MD criteria | |
|
GST-512 GST-156 GST-261 GST-541 GST-531 GST-552 GST-585 GST-598 GST-596 GST-430 GST-570 GST-544 GST-561 GST-571 GST-530 GST-358 GST-239 |
Guy’s and St’ Thomas’ | 20 November 14 | Violations: appointment schedule had not been followed | Has been noted and corrected. Centre retrained on protocol |
| NOR-349 | Norfolk and Norwich | 20 January 2014 | Violation: patient not eligible owing to PDS | |
| GST-178 | Guy’s and St’ Thomas’ | 11 January 2016 | Deviation: third SLT done in the left eye | |
| GST | Guy’s and St’ Thomas’ | Violation: Mr Lim had advised all patients to take Acular (ketorolac) for 3/7 t.i.d. following SLT. However, the protocol and SOP for SLT states ‘Acular as required only’ for everyone is indeed at variance with the agreed protocol | Acular for everyone is indeed at variance with the agreed protocol, but consistency with what has already been done within a unit is important. It was proposed that repeat lasers get the same as what Mr Lim has already done |
Appendix 10 Results of sensitivity analyses
| Approach | Adjusted mean differencea (Laser-1st – Medicine-1st) | 95% CI |
|---|---|---|
| 1. Using exact dates | 0.007 | –0.009 to 0.022 |
| 2. Adjusted for variables associated with missingness | 0.011 | –0.008 to 0.030 |
| 3. Multiple imputation | 0.016 | –0.004 to 0.035 |
| 4. Mapping algorithm | 0.019 | –0.005 to 0.042 |
| 5. Data recorded within 3 months of 36 months | 0.012 | –0.007 to 0.031 |
| 6. Robust SEs | 0.012 | –0.007 to 0.031 |
| 7. Beta regression | 0.002 | –0.010 to 0.013 |
Appendix 11 Details of ophthalmic- and laser-related adverse events
| Medicine-1st | Laser-1st | Total | ||||
|---|---|---|---|---|---|---|
| n of events | n (%) of patients | n of events | n (%) of patients | n of events | n (%) of patients | |
| Other ophthalmic-related AEs | 744 | 118 (32.6) | 459 | 117 (33.0) | 1041 | 235 (32.8) |
| Conjunctival injection | 109 | 61 (16.9) | 33 | 25 (7.0) | 142 | 86 (12.0) |
| Ocular irritation, discomfort or dry eye | 239 | 125 (34.5) | 147 | 97 (27.3) | 386 | 222 (31.0) |
| Itching | 103 | 51 (14.1) | 73 | 44 (12.4) | 176 | 95 (13.2) |
| Stinging on instillation | 89 | 53 (14.6) | 18 | 11 (3.1) | 107 | 64 (8.9) |
| Optic disc haemorrhage | 4 | 4 (1.1) | 8 | 7 (2.0) | 12 | 11 (1.5) |
| Macular haemorrhage | 0 | 0 (0) | 0 | 0 (0) | 0 | 0 (0) |
| Subconjunctival haemorrhage | 9 | 8 (2.2) | 2 | 2 (0.6) | 11 | 10 (1.4) |
| Cataract | 14 | 13 (3.6) | 19 | 17 (4.8) | 33 | 30 (4.2) |
| Blurred vision | 19 | 18 (5.0) | 12 | 12 (3.4) | 31 | 30 (4.2) |
| Change in vision | 16 | 14 (3.9) | 9 | 9 (2.5) | 25 | 23 (3.2) |
| Floater(s) | 5 | 5 (1.4) | 11 | 8 (2.3) | 16 | 13 (1.8) |
| Flashes | 4 | 4 (1.1) | 8 | 7 (2.0) | 12 | 11 (1.5) |
| Conjunctivitis | 8 | 8 (2.2) | 6 | 5 (1.4) | 14 | 13 (1.8) |
| Watery eye | 8 | 7 (1.9) | 13 | 11 (3.1) | 21 | 18 (2.5) |
| Glare | 4 | 4 (1.1) | 6 | 5 (1.4) | 10 | 9 (1.3) |
| Pain/sore eye | 10 | 10 (2.8) | 8 | 8 (2.3) | 18 | 18 (2.5) |
| Blepharitis | 6 | 6 (1.7) | 0 | 0 (0) | 6 | 6 (0.8) |
| Swollen eye(s) | 3 | 3 (0.8) | 1 | 1 (0.3) | 4 | 4 (0.6) |
| Photophobia | 4 | 4 (1.1) | 4 | 3 (0.8) | 8 | 7 (1.0) |
| CRVO | 2 | 1 (0.3) | 0 | 0 (0) | 2 | 1 (0.1) |
| BRVO | 1 | 1 (0.3) | 2 | 1 (0.3) | 3 | 2 (0.3) |
| Diabetic retinopathy | 0 | 0 (0) | 1 | 1 (0.3) | 1 | 1 (0.1) |
| Diabetic macular oedema | 0 | 0 (0) | 3 | 2 (0.6) | 3 | 2 (0.3) |
| Other laser-related AEs | 1 | 1 (0.3) | 51 | 47 (13.2) | 52 | 48 (6.7) |
| Photophobia | 0 | 0 (0) | 21 | 20 (5.6) | 21 | 20 (2.8) |
| Hyperaemia | 0 | 0 (0) | 3 | 3 (0.8) | 3 | 3 (0.4) |
| Discomfort | 0 | 6 | 6 | |||
| Breaks taken during procedure | 0 | 0 | 0 | |||
| Fewer laser shots | 0 | 3 | 3 | |||
| Visualisation of angle/other angle issues | 0 | 5 | 5 | |||
Appendix 12 Medical contacts
| Baseline | 6 months | 12 months | 18 months | 24 months | 30 months | 36 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medicine-1st (n = 362) | Laser-1st (n = 356) | Medicine-1st (n = 326) | Laser-1st (n = 329) | Medicine-1st (n = 317) | Laser-1st (n = 317) | Medicine-1st (n = 302) | Laser-1st (n = 302) | Medicine-1st | Laser-1st | Medicine-1st | Laser-1st | Medicine-1st | Laser-1st | |
| Eye related, mean (SD) | ||||||||||||||
| Optician | 0.898 (0.691) | 0.874 (0.729) | 0.336 (0.600) | 0.464 (0.789) | 0.364 (0.569) | 0.422 (0.660) | 0.397 (0.611) | 0.412 (0.714) | 0.409 (0.546) | 0.456 (0.597) | 0.394 (0.637) | 0.399 (0.631) | 0.497 (0.718) | 0.492 (0.721) |
| General practitionera | 0.192 (0.477) | 0.186 (0.493) | 0.095 (0.408) | 0.116 (0.630) | 0.073 (0.363) | 0.050 (0.314) | 0.05 (0.273) | 0.053 (0.412) | 0.112 (0.493) | 0.076 (0.387) | 0.081 (0.378) | 0.1 (0.539) | 0.125 (0.729) | 0.122 (0.631) |
| General practitioner nurse | 0.045 (0.363) | 0.074 (0.371) | 0.074 (0.378) | 0.068 (0.317) | 0.064 (0.304) | 0.065 (0.517) | 0.086 (0.384) | 0.034 (0.246) | 0.090 (0.407) | 0.026 (0.229) | 0.121 (0.817) | 0.053 (0.254) | 0.063 (0.319) | 0.072 (0.339) |
| Social worker | 0.003 (0.053) | 0.003 (0.053) | 0.003 (0.055) | 0.003 (0.055) | 0.003 (0.056) | 0 | 0 | 0 | 0.003 (0.058) | 0.003 (0.057) | 0 | 0 | 0 | 0.003 (0.058) |
| Home care worker | 0.003 (0.053) | 0.008 (0.092) | 0.006 (0.078) | 0 | 0 | 0 | 0.003 (0.058) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other community services | 0 | 0 | 0.003 (0.056) | 0.003 (0.056) | 0 | 0 | 0.007 (0.083) | 0.010 (0.175) | 0 | 0 | 0 | 0 | 0 | 0.007 (0.081) |
| Non-eye related, mean (SD) | ||||||||||||||
| General practitionera | 1.346 (1.909) | 1.427 (2.446) | 1.356 (2.039) | 1.405 (2.097) | 1.070 (1.550) | 1.196 (2.081) | 1.174 (1.711) | 1.296 (2.004) | 1.150 (2.122) | 1.079 (1.814) | 1.182 (2.060) | 1.073 (1.725) | 0.979 (1.664) | 1.132 (1.924) |
| General practitioner nurse | 0.415 (0.999) | 0.394 (1.046) | 0.439 (1.023) | 0.480 (1.096) | 0.316 (0.666) | 0.549 (1.371) | 0.424 (0.840) | 0.529 (1.211) | 0.406 (1.374) | 0.365 (0.811) | 0.435 (1.003) | 0.356 (0.827) | 0.378 (0.786) | 0.493 (0.991) |
| Social worker | 0.006 (0.075) | 0.011 (0.106) | 0.009 (0.096) | 0.003 (0.055) | 0.003 (0.056) | 0.010 (0.126) | 0 | 0.003 (0.058) | 0.017 (0.174) | 0.026 (0.354) | 0.007 (0.083) | 0.003 (0.059) | 0.024 (0.266) | 0.003 (0.058) |
| Home care worker | 0 | 0.008 (0.092) | 0.003 (0.056) | 0.006 (0.078) | 0.044 (0.733) | 0.010 (0.126) | 0.068 (1.16) | 0 | 0.010 (0.175) | 0 | 0.007 (0.083) | 0 | 0.003 (0.058) | 0 |
| Other community services | 0.073 (0.705) | 0.025 (0.231) | 0.157 (1.269) | 0.142 (1.093) | 0.039 (0.312) | 0.089 (1.168) | 0.084 (1.024) | 0.127 (1.113) | 0.032 (0.243) | 0.010 (0.101) | 0.101 (0.860) | 0.042 (0.311) | 0.048 (0.421) | 0.154 (1.473) |
| Acute hospital servicesb | ||||||||||||||
| Outpatient, mean (SD) | 0.838 (1.331) | 0.707 (1.259) | 0.808 (1.826) | 0.911 (1.948) | 0.551 (1.350) | 0.543 (1.230) | 0.620 (1.489) | 0.568 (1.283) | 0.562 (1.193) | 0.685 (1.602) | 0.553 (1.431) | 0.582 (1.491) | 0.642 (1.378) | 0.5 (1.092) |
| A&E attendance, mean (SD) | 0.062 (0.252) | 0.085 (0.343) | 0.049 (0.256) | 0.085 (0.447) | 0.061 (0.299) | 0.098 (0.472) | 0.077 (0.313) | 0.06 (0.370) | 0.048 (0.244) | 0.093 (0.641) | 0.075 (0.381) | 0.070 (0.294) | 0.083 (0.416) | 0.067 (0.288) |
| Day case, mean (SD) | 0.352 (0.839) | 0.316 (0.828) | 0.297 (1.091) | 0.287 (0.826) | 0.244 (0.690) | 0.220 (0.688) | 0.306 (0.950) | 0.194 (0.584) | 0.199 (0.771) | 0.276 (0.837) | 0.264 (0.890) | 0.345 (1.275) | 0.315 (1.022) | 0.248 (0.759) |
| Planned inpatient admission, n (%) | 11 (3) | 6 (2) | 6 (2) | 8 (2) | 5 (2) | 8 (3) | 10 (3) | 10 (3) | 2 (1) | 8 (3) | 6 (2) | 8 (3) | 5 (2) | 5 (2) |
| LOS planned inpatient admissions, mean (SD)c | 1.091 (0.302) | 1 (0) | 1.333 (0.516) | 1.125 (0.354) | 1 (0) | 1.125 (0.354) | 1.1 (0.316) | 1.1 (0.316) | 1 (0) | 1.125 (0.354) | 1.667 (0.516) | 1.5 (1.414) | 1.4 (0.548) | 1 (0) |
| Emergency inpatient admission, n (%) | 5 (1) | 3 (1) | 5 (2) | 9 (3) | 8 (3) | 10 (3) | 9 (3) | 8 (3) | 3 (1) | 5 (2) | 8 (3) | 11 (4) | 10 (3) | 6 (2) |
| LOS emergency inpatient admissions, mean (SD)c | 1.2 (0.447) | 1 (0) | 1 (0) | 1.889 (2.315) | 1.25 (0.463) | 1 (0) | 1 (0) | 1.125 (0.354) | 1 (0) | 1 (0) | 1 (0) | 1.545 (0.688) | 1.7 (1.337) | 1.5 (1.224) |
List of abbreviations
- AE
- adverse event
- CCT
- central corneal thickness
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CRF
- case report form
- CRO
- central research optometrist
- CSRI
- Client Service Receipt Inventory
- CTM
- central trial manager
- CTO
- central trial optometrist
- DMC
- Data Management Committee
- DSS
- decision support software
- EMGT I
- Early Manifest Glaucoma Trial
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GCP
- good clinical practice
- GON
- glaucomatous optic neuropathy
- GPA
- glaucoma progression analysis
- GQL-15
- Glaucoma Quality of Life-15
- GSS
- Glaucoma Symptom Scale
- GUI
- Glaucoma Utility Index
- HRQoL
- health-related quality of life
- HRT
- Heidelberg retinal tomography
- ICER
- incremental cost-effectiveness ratio
- IOP
- intraocular pressure
- IQR
- interquartile range
- IT
- information technology
- ITT
- intention to treat
- LAG
- lay advisory group
- Laser-1st
- initial selective laser trabeculoplasty followed by routine medical treatment
- LiGHT
- Laser in Glaucoma and Ocular Hypertension
- logMAR
- log of the minimum angle of resolution
- MD
- mean deviation
- Medicine-1st
- routine medical treatment only
- MEH
- Moorfields Eye Hospital
- MMT
- maximum medical treatment
- NICE
- National Institute For Health and Care Excellence
- NIHR
- National Institute for Health Research
- OAG
- open-angle glaucoma
- OHT
- ocular hypertension
- PI
- principal investigator
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Resource Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SLT
- selective laser trabeculoplasty
- SOP
- standard operating procedure
- TSC
- Trial Steering Committee
- VA
- visual acuity
- VF
- visual field
