Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/78/02. The contractual start date was in June 2015. The draft report began editorial review in January 2019 and was accepted for publication in April 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Louise Robinson reports grants from the National Institute for Health Research (NIHR) Professor award and a NIHR Senior Investigator award outside the submitted work. Lynn Rochester reports grants from the Medical Research Council, the European Union, the Engineering and Physical Sciences Research Council, the Wellcome Trust (London, UK), Parkinson’s UK (London, UK), NIHR [Programme Development Grant, Physical Activity Interventions to Improve Outcomes for people with Rare Neurological Conditions (PARC), co-applicant (2018–20); and Health Technology Assessment, PDSAFE: a randomised controlled trial of the effectiveness of PDSAFE to prevent falls among people with Parkinson’s disease, co-applicant (2013–17)] and the Stroke Association (London, UK) outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2019. This work was produced by Allan et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2019 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Background and context
Recent estimates suggest that there are 850,000 people living with dementia in the UK, of whom 70% live in the community. 1 People with dementia (PWD) living in their own homes experience almost 10 times more incident falls than other older people and their falls are more likely to result in injury. 2 When injuries are sustained, PWD are less likely than cognitively intact older people to recover well. 3
Evidence shows that falls are a common reason for hospital admission in PWD4 and that most admissions for PWD with an injury are due to a fall. 5 Despite this, current UK guidelines for treatment of older people following a fall do not specifically address the needs of PWD;6 the new dementia guidelines7 recommend that falls services address the specific needs of PWD, but provide few details on how this can be achieved. The World Health Organization8 report on falls prevention in older people refers to cognitive impairment only as a risk factor for falls. There is little evidence regarding the care pathways currently experienced by PWD presenting with a fall-related injury.
For older people without dementia, there is good evidence that a multifactorial intervention by a specialist falls service will prevent further falls. 9–12 Such interventions are usually tailored to the individual and are directed at known risk factors for falls. However, their effectiveness for PWD is unclear. 13 It is possible that the lack of demonstrated efficacy is because risk factors for falls may differ in PWD or be more frequent or specific to dementia, for example wandering,14 behavioural disturbance,15 Parkinsonism,16,17 severity of cognitive impairment,16 functional impairment18 and use of neuroleptic drugs. 19,20 Nevertheless, and despite the lack of evidence, PWD are often referred directly to the local falls service. Such services are not usually tailored to meet the needs of PWD. It is possible that the referral may achieve other benefits for the PWD, such as medication review, treatment of other comorbidities or provision of aids to support activities of daily living (ADL), but it is not known if a falls service is the best setting for addressing these goals. Indeed, it is not known what goals are of most importance to PWD who fall.
In designing any kind of intervention to address the problem of fall-related injuries in PWD, it is vital that the intervention addresses outcomes of importance to PWD themselves, their informal carers (i.e. unpaid family members of friends who support the PWD, hereafter referred to as carers) and their care professionals. We accessed the Core Outcome Measures in Effectiveness Trials (COMET) database21 and found no consensus regarding suitable outcomes for fall-related injury, although there were two publications regarding interventions of relevance in this situation: the Prevention of Falls Network Europe (ProFaNE) consensus on a common outcome data set for fall injury prevention trials22 (domains include falls, injuries, psychological consequences of falling, health-related quality of life and physical activity) and the European Consensus on outcome measures for psychosocial intervention research in dementia care23 (domains include patient mood, quality of life, ADL, behaviour, carer mood and carer burden).
There is a range of ways in which improved management of fall-related injuries might reduce adverse sequelae for PWD and their carers. First, any fall in older people, whether injurious or not, is known to frequently result in fear of falling and psychological morbidity, which may lead the person to restrict their mobility, resulting in deconditioning and a cycle of further loss of mobility and frailty. 24 A successful intervention may reduce psychological morbidity and improve well-being. 25 Second, if physical recovery from the injury itself is poor, further restriction of mobility may happen and independence in ADL may decline. These restrictions may result in reduced social participation, increased burden for informal carers and increased need for formal care. Such problems lead to reduced well-being and quality of life for PWD, and substantial costs to both health and social care systems. A successful intervention may support the maintenance of physical ability and independence or reduce the degree of physical decline and loss of independence. We are not aware of any clinical trials that have specifically tried to address the management of all fall-related injuries in PWD.
Therefore, although PWD who sustain fall-related injuries currently receive a range of health interventions, a single model of care for this specific situation has not previously been described to our knowledge. Given all of the aspects of care relevant to the situation as described previously, it is apparent that a new model of care would take the form of a complex intervention. Given the frequency of falls in PWD, it is clear that this is an important area for research, although the potential demand for such an intervention is not known. There is also no current consensus on the best outcomes with which to measure the impact of such an intervention or its cost-effectiveness. This report describes the process of developing and testing the feasibility of a new intervention to help this patient group.
Research objectives
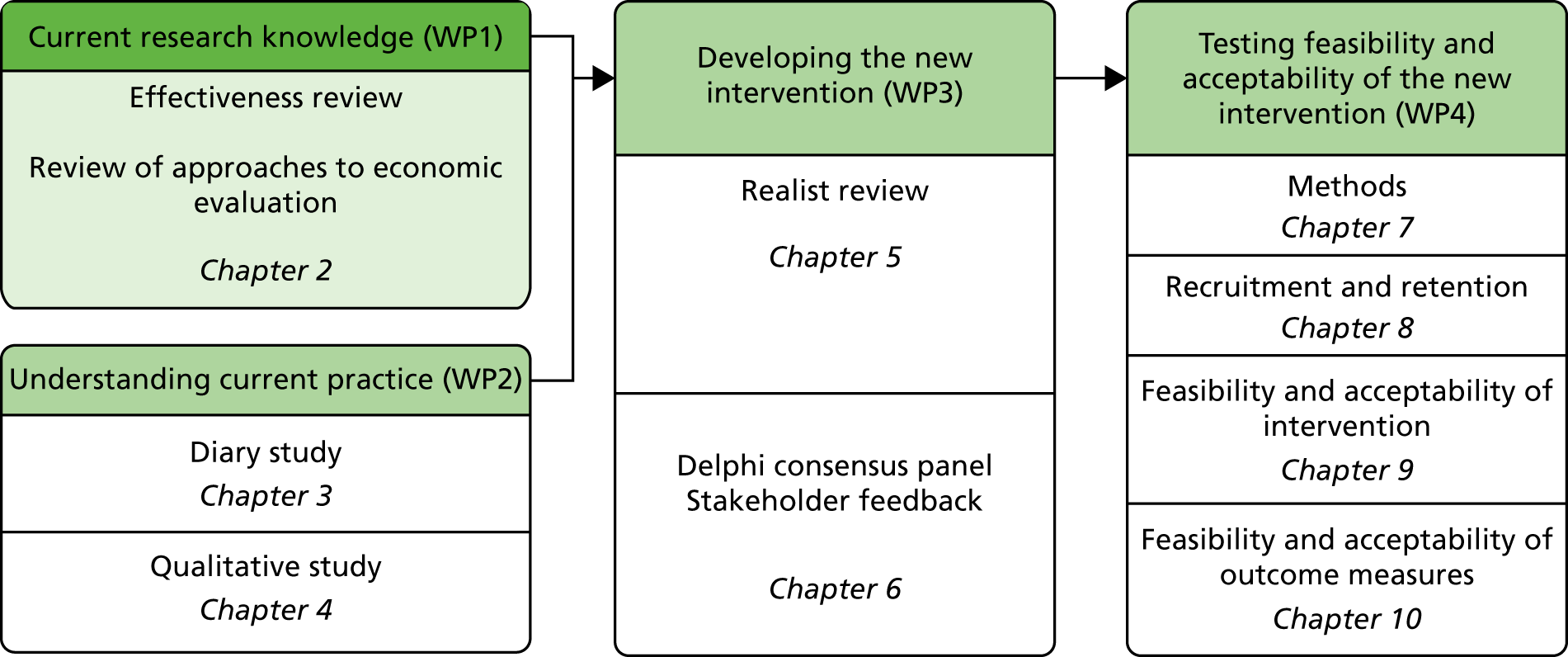
The overall aim of this study was to assess, through a series of work packages (WPs), whether or not it is possible to design a complex intervention to improve the outcome of fall-related injuries in PWD living in their own homes (Figure 1). During the course of the study, the objective was broadened to include people with a fall necessitating health-care attention and not just those with injurious falls.
FIGURE 1.
Overview of study and report structure.
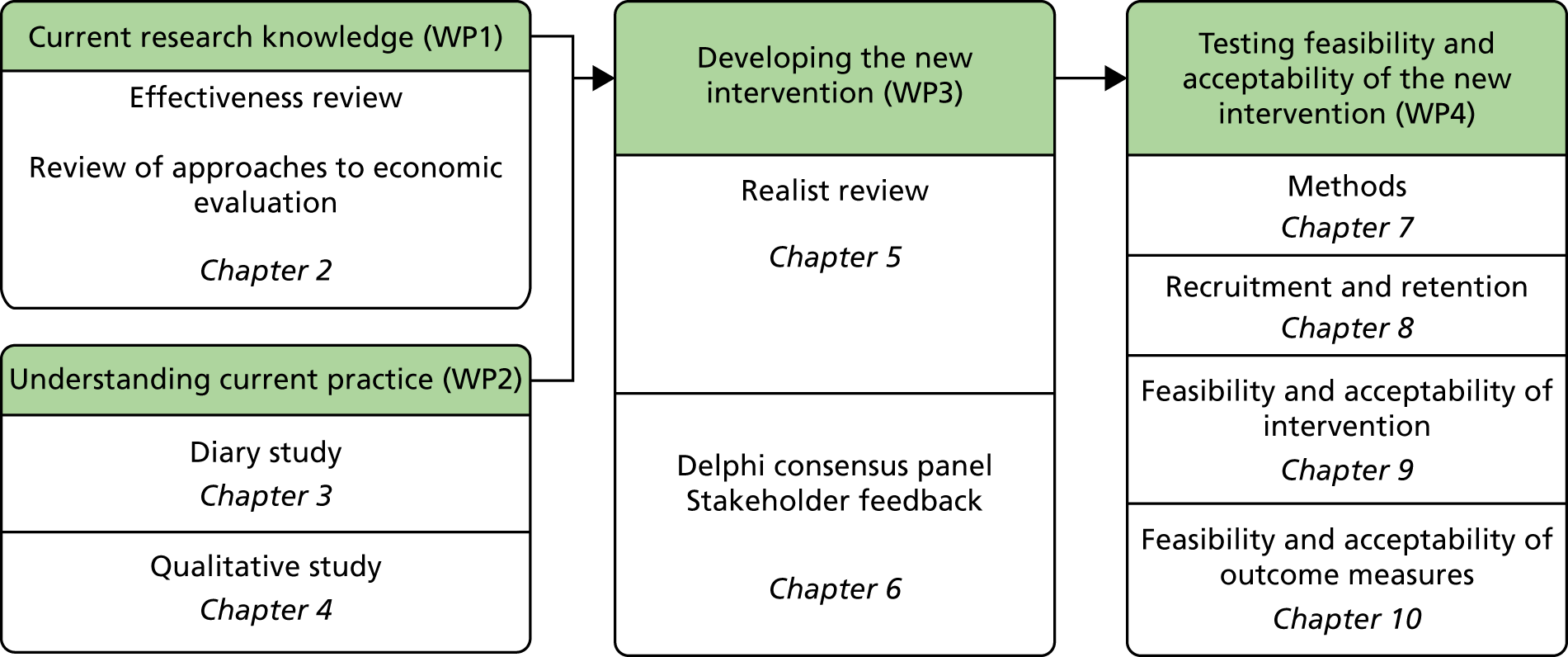
Primary objectives
Work package 1: current research knowledge
-
To conduct a systematic literature review to synthesise the current evidence regarding the management of fall-related injuries in PWD.
-
To investigate what evidence is currently available regarding the clinical effectiveness and cost-effectiveness of interventions aimed at improving the outcome of fall-related injuries in dementia.
Work package 2: understanding current practice
-
To quantify PWD presenting to health services with a fall-related injury in three UK sites.
-
To understand current care pathways (‘usual care’) experienced and the services used by a subgroup of PWD who completed a falls diary for 12 weeks following a fall.
-
To identify care needs and ideas for intervention, and prioritise the outcomes that are important to participants and their carers.
Work package 3: developing the new intervention
-
To develop an intervention to improve outcomes for PWD following a fall, drawing on the findings of WP1 and WP2.
-
To describe the outcome measures to evaluate the clinical effectiveness and cost-effectiveness of the intervention.
-
To validate the proposed intervention through qualitative work with stakeholders, including some participants from WP2.
Work package 4: testing the feasibility and acceptability of the new intervention
-
To conduct a non-randomised feasibility study to deliver the new intervention to 10 PWD in each of the three sites.
Secondary objectives
-
To use the data collected in WP1 and WP2 to develop data-collection tools for use in the evaluation of a new intervention.
-
To assess the factors influencing the acceptability and implementation of the intervention and to determine whether or not to progress to a full-scale randomised controlled trial (RCT).
Chapter 2 Reviews of effectiveness and approaches to economic evaluation
Parts of this chapter are adapted from Robalino et al. 26 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
There is no consensus on how best to manage PWD who have had a fall. As part of this study, two reviews were conducted. The first focused on the effectiveness of different interventions targeted at PWD who have experienced a fall. The aim of this review was to help inform the development of the intervention to be piloted in WP4 (see Chapter 7). In addition to assessing the intervention’s effectiveness and safety, it was important to evaluate whether or not it would represent value for money. The second review, therefore, synthesised existing evidence on economic evaluations of falls prevention interventions in PWD. It was not stipulated in the economic review that the population had to have previously experienced a fall as the methods for evaluating a falls prevention intervention would be the same among those with and without a previous fall. The aim of this review was to identify the most appropriate methods and outcomes for an economic evaluation of the intervention to be developed for PWD following a fall.
Effectiveness review
The aim of this systematic review was to synthesise all existing research evidence evaluating the effectiveness of interventions intended to improve the physical and psychological well-being of PWD who had experienced a fall. The full review has been published separately. 26
Methods
The protocol for this review was registered with PROSPERO (reference CRD42016029565). 27 The review was informed by Cochrane methods28 and described in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines. 29
Selection of eligible studies
Eligible studies were randomised or quasi-experimental trials that recruited PWD living in the community who had experienced an injurious or non-injurious fall and received any type of intervention aiming to improve the fall outcomes of PWD. Comparator groups in the trials had to be receiving usual care. Eligible primary outcomes were measures of performance-oriented assessment of mobility (e.g. the Tinetti score30) and measures of performance in ADL (e.g. Barthel Index31). Secondary outcomes of interest were length of hospital stay, place of discharge post intervention, recurrent fall or injury and readmission to hospital.
We excluded trials that (1) recruited only cognitively intact patients or a mix of patients where results for PWD were not reported separately, (2) recruited exclusively from care homes or (3) were not published in the English language.
An experienced information specialist searched eight bibliographic databases and two trials registries for reports of eligible studies from database inception to November 2015 and updated the MEDLINE search in January 2018. The search contained the following facets: [dementia] AND [falls or fall-related injuries] AND [interventions or RCT filter where available]. For each facet, thesaurus headings and keyword synonyms were combined in accordance with good practice in systematic review literature searching, and translated as appropriate between databases. Reference lists of included studies and relevant systematic reviews were searched for further eligible studies, and all results were collated in an EndNote (Clarivate Analytics, Philadelphia, PA, USA) library. Two reviewers independently screened titles and abstracts in EndNote, and then they screened the full texts of the resulting potentially eligible studies. Discrepancies were resolved by discussion and reference to a third reviewer. One reviewer extracted data to a bespoke data extraction form in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) and a second reviewer checked it. Data extracted included details of the study population [e.g. Mini Mental State Examination (MMSE) score32], setting (e.g. ward), the intervention (e.g. care team and services used), the comparator and outcomes (e.g. mobility and length of hospital stay) measured at baseline and follow-up. We e-mailed authors to request data missing from eligible studies.
Critical appraisal and synthesis
Two reviewers independently used the Cochrane risk-of-bias tool33 to critically appraise each included study outcome, and discrepancies were resolved by discussion and referral to a third reviewer. The Cochrane tool facilitates a judgement of low, unclear or high risk of each of selection, performance, detection, attrition and reporting bias.
We planned to carry out a meta-analysis, but few of the included studies measured the same outcome, and, even when they did, different outcome measures were used, thereby precluding a valid statistical analysis. Consequently, we carried out a narrative synthesis, broadly categorising studies by intervention and presenting detailed results by outcome.
Results
Selection of eligible studies
The initial search returned 1071 studies after deduplication (Figure 2). Of these, 991 were excluded by screening titles and abstracts, and the full texts of 80 were assessed for eligibility. Of these, 69 were excluded because they were not RCTs or quasi-experimental studies, they did not include PWD or the PWD did not reside in the community. A total of 11 studies remained for narrative synthesis, but four had missing data that could not be obtained. Seven studies were included in the narrative synthesis.
FIGURE 2.
Effectiveness review PRISMA flow diagram of study inclusion and exclusion. Adapted from Robalino et al. 26 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original.

Characteristics of included studies
Six RCTs and one quasi-experimental study were included in this review. 34–40 In the quasi-experimental study, patients were recruited in two phases. In the first phase, all consecutive patients from two sites were recruited to the control group; in the second phase, all were recruited to the intervention group. The trials recruited mostly patients with hip fractures in hospitals and emergency departments (EDs) in high-income countries. All studies included both cognitively intact patients and patients diagnosed with dementia, except one that recruited patients with at least mild dementia (MMSE score of < 24 points). 34
Five studies evaluated multidisciplinary in-hospital post-surgical geriatric assessment, which varied in terms of the type of ward (e.g. geriatric vs. orthopaedic), mix of multidisciplinary staff and components of the intervention. 35,37,40 All studies in this group included a core team of a geriatrician, nurse, occupational therapist (OT) and physiotherapist, plus other staff, such as social workers or dietitians, as required. The interventions included different combinations of components, for example early-discharge planning, post-discharge home visits and weekly team meetings.
One study evaluated multifactorial assessment and intervention in patients presenting at an ED post fall, utilised a multidisciplinary team (MDT) similar to that used in the in-hospital geriatric assessment and followed up with risk assessments in patients’ homes. 34 Patients were then offered a variety of interventions based on the risk assessments; these interventions included home-based exercise, home hazard modification, medication review and optical correction by an optician.
The final study provided an annual dose of intravenous zoledronic acid to participants in an attempt to reduce recurrent falls and further fractures by improving bone health. 36
Critical appraisal of studies
The included studies were mostly at low risk of selection bias, and many were at high risk of performance and detection biases due to difficulties in blinding participants and/or personnel to interventions and outcomes (a common scenario with complex interventions). The risk of bias for attrition and reporting was less well reported.
Outcomes
Three RCTs34,37,38 and one quasi-experimental study40 reported different measures of mobility following the intervention, of which three studies reported limited improvement or retention of mobility in the intervention group compared with the control group. Those studies utilising multidisciplinary in-hospital postsurgical geriatric assessment reported short-term improvements in gait, but long-term improvements were either not reported or proved statistically insignificant. 37,38,40 The studies used different mobility scales that exhibited relatively little overlap in the components measured.
Three studies reported recurrent falls post intervention,34,36,37 of which one37 reported a reduction in inpatient falls in the treatment group (4%) compared with the control group (31%) (p = 0.006), although there was no difference in the rate of new fractures. A second study34 reported no difference in the number of patients with falls, the median number of falls or the median number of weeks before the second fall. The final study36 found no difference in falls for PWD, but reported a reduction in recurrent fractures at 6 months in the cognitively impaired patients.
Three studies reported on post-intervention ADL,37–39 utilising four tools that had limited overlap, with only two common items (feeding and transferring). The results were not consistent between studies.
Three studies measured length of hospital stay using multidisciplinary in-hospital post-surgical geriatric assessment, but with varying components. 35,38,39 Two studies35,39 showed a decreased length of stay for those with mild or moderate (but not severe) dementia, and the other study38 reported a significantly higher median length of stay in the intervention group.
All of the studies utilising multidisciplinary in-hospital post-surgical geriatric assessment and intervention reported on the place of discharge. 35,37–40 Three reported that PWD were more likely to return to independent living following the intervention,35,39,40 and the other two described no difference between the intervention and control groups. 37,38
Two studies reported no evidence of impact on readmission rates to hospital. 34,38
Discussion
The effectiveness of interventions to improve outcomes for PWD who fall was highly heterogeneous in terms of all interventions compared, the outcomes considered and the patient populations considered. Three of these studies used multidisciplinary in-hospital post-surgical geriatric assessment, which showed improvements in some outcomes within their treatment groups, regardless of mental status. 41–43 Overall, the risk of bias in the studies was mixed and their results conflicted even when similar interventions were utilised.
Four eligible studies provided no useable data for this review;41–44 we contacted the authors to clarify reported results or request subgroup data where they were reported to be available, but received no response.
The term ‘Comprehensive Geriatric Assessment’ (CGA) was not used consistently with respect to the staff delivering the intervention, frequency of MDT meetings, discharge planning, post-discharge in-home follow-up, falls assessment and prevention, or medication management. Current evidence suggests that CGA is likely to benefit older people hospitalised with acute conditions owing to these services generally providing a multidimensional, multidisciplinary approach that includes the identification of medical, social and functional needs, as well as the development of an integrated and co-ordinated care plan to address those needs. 45,46 The question of whether or not there is a need for adaptation of CGA for PWD has not been addressed.
Generally, the earlier a patient is mobilised, the better the outcome with regard to reduced length of stay and discharge to independent living. Patients with mild and moderate dementia showed better outcomes than those with more severe dementia.
Strengths and limitations
This review used robust methods including prespecified inclusion and exclusion criteria, a comprehensive search and duplicate screening, data extraction and critical appraisal procedures. However, we were unable to include all of the relevant studies in the synthesis; despite efforts to contact authors of four studies, we were unable to obtain their data grouped according to dementia status.
The searches were carried out to inform the panel meeting in WP3 and updated in January 2018 for publication of the effectiveness review. As they have not been updated again for this report, the findings should be interpreted as informing only that WP and not for current clinical decision-making.
Conclusions
We found gaps in the evidence base. Most of the study populations presented with hip fracture in hospital so interventions may not be applicable to soft tissue injuries or other types of fracture, and these studies provided no guidance about managing fall-related injuries in primary care. Most of the studies were not aimed at PWD, and subgroup analysis was used to report the effects of interventions targeted at the general older population on PWD.
Review of approaches to economic evaluation
The aim of this review was to understand the current cost-effectiveness evidence base in the area to inform the design of a potential future economic evaluation of the DIFRID (Developing an Intervention for Fall related Injuries in Dementia) intervention should it proceed to a definitive trial. The review identified economic evaluations of fall prevention interventions in PWD to make recommendations about:
-
how best to capture the resources used to provide the intervention and any changes in subsequent use of services
-
appropriate outcomes that would (1) capture the benefits of the intervention, (2) be appropriate for use with PWD and (3) provide information for an economic evaluation relevant to policy-makers.
Drawing on the findings of the review, we developed and piloted data collection tools to collect information on health-care resource use in WP2 (see Chapter 3) and WP4 (see Chapter 10).
Methods
Searches were formed of two facets and were based on an amended version of the NHS Economic Evaluation Database search filter where necessary. 47 The facets were (1) dementia and (2) falls or fall-related injuries. Only studies including full economic evaluations were eligible (i.e. studies that compared two or more interventions in terms of both their costs and their outcomes). 48 The search was extended to incorporate patients with cognitive impairment as many approaches might be equally applicable to this patient group.
Electronic database searches were conducted in August 2016. The following databases were searched: MEDLINE, EMBASE and NHS Economic Evaluation Database. An example of the search strategy used in MEDLINE is provided in Appendix 1. Citations of potentially relevant studies were also checked for additional eligible studies, as were citations in any previously conducted literature reviews relevant to the topic that were identified. Protocols of ongoing studies were also included if they provided information on the planned economic evaluation.
Selection of eligible studies
The following inclusion criteria were applied:
-
reported in the English language
-
reports of full economic evaluations – cost–benefit, cost–utility, cost-minimisation and cost-effectiveness analysis
-
patients had any diagnosis of cognitive impairment
-
the intervention was falls prevention
-
the comparator was usual care or no intervention
-
the economic outcomes were costs, falls, quality-adjusted life-years (QALYs), incremental cost-effectiveness ratio (ICER) (e.g. incremental cost per QALY gained or incremental cost per fall prevented).
We adopted the same exclusion criteria as were used in the effectiveness review with the exception that care home studies were included. An additional criterion for the cost-effectiveness review was that studies were excluded if they did not incorporate falls into the economic evaluation.
Critical appraisal and synthesis
Titles and abstracts of all studies identified by the search were assessed by two reviewers using EndNote. Full texts of potentially eligible studies were then obtained. Discrepancies were resolved by discussion and a third reviewer when needed. Data were extracted by one reviewer using a prespecified data extraction form. Data collected included details of the study population (e.g. PWD), the intervention (e.g. rehabilitation classes), the comparator and outcomes (costs and falls or QALYs). The range of interventions, populations and outcomes reported in the included studies was described. The quality of included studies was assessed against a commonly used checklist for reporting economic analyses. 49
Similarly to the effectiveness review, risk of bias was assessed using the Cochrane risk-of-bias tool. 33
Results
Selection of eligible studies
The initial search returned 1252 reports. Eleven papers were excluded after deduplication and 124 were excluded as they were not in English. A further report was identified from an ineligible report that was a literature review concerning fall interventions. 50 Overall, six reports were deemed potentially relevant and the full papers were obtained.
Four reports were excluded after the full texts were reviewed. One paper was a critical review of another paper that was selected for full review. 51 One paper estimated the cost-effectiveness of falls prevention of a range of interventions using the results of a systematic review to populate a Markov model. 50 The population included in that review were people aged ≥ 65 years, but their exclusion criteria included ‘special populations (e.g. stroke or osteoporosis)’; therefore, we cannot assume that people with cognitive impairment were included in the model. 50 Two of the excluded papers, one protocol and one economic evaluation based on a RCT, evaluated exercise-based interventions in PWD in nursing homes [the LEDEN (Effects of a long-term exercise programme on functional ability in people with dementia living in nursing homes) study52] and patients with Alzheimer’s disease (AD) [FINALEX (FINnish ALzheimer disease EXercise trial)53]. The aims of these studies were to improve functional ability (LEDEN) and to improve physical functioning and mobility (FINALEX). Overall, although both studies collected falls as a secondary outcome, it was not the primary objective of their intervention and neither incorporated number of falls as an outcome measure in their economic evaluations. The LEDEN study protocol outlined the outcome measures for the economic evaluation as costs and changes in functional ability measured using the Alzheimer Disease Cooperative Study ADL-severe. 54 FINALEX estimated the ICER as the cost per dyad (patient with AD and their carer who was a spouse they resided with). 53
Included studies
Two papers met the inclusion criteria, both of which were protocols. 55,56 The i-FOCIS RCT aims to examine whether or not an individually tailored, cognitive impairment-specific approach to the delivery of an exercise and home-hazard-reduction programme can reduce the rate of falls in community-dwelling cognitively impaired older people. 56 The Encouraging Best Practice in Residential Aged Care (EBPRAC) programme aims to improve evidence-based clinical care for residents in aged-care homes and to enable nationally consistent application of this care. 55 Both proposed studies will be undertaken in Australia.
A PRISMA breakdown of study inclusion and exclusion is presented in Figure 3.
FIGURE 3.
Economic evaluation review PRISMA flow diagram of study inclusion and exclusion. CI, cognitive impairment.

Participant and study characteristics
The studies have different target populations. One study targets people aged ≥ 65 years living in the community with cognitive impairment (n = 360)56 and the other targets residents of residential aged-care facilities, including PWD (nine residential aged-care facilities, 670 patients and 650 staff will be invited to participate). 55
Perspective of the studies
The protocols55,56 suggest that the economic evaluations will be conducted from the perspective of (1) the health and community service provider (i-FOCIS) and (2) the societal and residential aged-care facility (EBPRAC).
Resource use data (costs)
The i-FOCIS RCT will capture information on the consumable, reusable and capital resources required to deliver the interventions as part of the trial. Data on health-care resources, including medications, will be captured via self-reported monthly calendars for 12 months. Costs will be collected from routine sources and out-of-pocket expenses for the patients and their carers will be estimated from a previous published study. 57
The EBPRAC programme will determine the resources needed to deliver the intervention by monitoring the resource use during the project implementation for 12 months. These resources will be costed using market values where possible. Fall-related health-care resource use will be collected from two participating sites. There is no information provided on where these costs will be collected from.
Outcome measures
The rate of falls will be the primary outcome for both of these studies, and both economic evaluations will incorporate this into their analysis. The primary economic outcomes are the cost per fall prevented (i-FOCIS56) and the cost per fall (EBPRAC55). The i-FOCIS RCT incorporated additional outcome measures in the economic evaluation: falls requiring medical attention, ED presentation avoided, hospital admission avoided and QALYs estimated using the EuroQol-5 Dimensions, five-level version (EQ-5D-5L).
Economic evaluation
The i-FOCIS RCT will analyse its data as a within-trial cost-effectiveness analysis. Appropriate sensitivity analyses, including deterministic and stochastic analyses, will be used to address any uncertainty in costs, effects and cost-effectiveness. The probability of the intervention being considered cost-effective at current willingness-to-pay thresholds will be presented as cost-effectiveness acceptability curves (CEACs).
The EBPRAC programme will determine whether or not there is a reduction in the cost per fall associated with the intervention by analysing the cost per fall pre and post intervention implementation. Cost per fall will be estimated by modelling the costs collected from two participating sites. Sensitivity analyses will be carried out to address any uncertainty surrounding costs and effects.
Duration of the studies and data-collection time points
The i-FOCIS RCT has a 12-month follow-up, with clinical and quality-of-life outcomes collected at baseline, 6 months and 12 months. The number of falls and health-care resource use are collected using monthly diaries. The EBPRAC programme will be a 2-year study; it also includes a review of the literature, hence it is unclear when the intervention will be implemented. However, although the study team will review falls data every 6 months, it is unclear when other outcome measures will be collected. An economic model will be used to estimate costs and effects 1, 2, 3, 4 and 5 years post intervention implementation.
Quality of the studies
As the papers suitable for inclusion were protocols, there is insufficient detail on the methodology of the economic evaluations for us to evaluate the quality of the proposed analyses using standard criteria. 49 The i-FOCIS RCT is not accounting for any longer-term costs and benefits that may be accrued after the intervention is implemented. If the intervention is effective, this could create potential bias if the follow-up is not sufficiently long for benefits and possible cost savings in subsequent care to offset the cost of the intervention. The i-FOCIS RCT is not considering the potential impact of the intervention on carers, despite their involvement in the home exercise programme by supervising practice sessions and assisting in delivering the sessions at home.
The duration of the EBPRAC programme is unclear, but if costs and outcomes are going to be estimated beyond a 1-year time frame in an economic model then discounting needs to be considered. There was no detail provided on the type of economic evaluation model being undertaken. Therefore, it is unclear if the approach provided will be sufficient to capture costs and benefits in the longer term.
Discussion
Both cost-effectiveness and cost–utility analyses are currently being incorporated into studies evaluating a falls-prevention intervention in people with cognitive impairment. It is likely that the DIFRID intervention, described in Chapter 6, will involve a number of health-care resources and a MDT given the complexity of the health problem. It is recommended that each individual resource needed to deliver the intervention should be identified and costed using routine sources where available. Costs estimated from routine sources are arguably less reliable than those estimate from time-based materials costing; however, they can be a good representation of the estimated cost associated with these resources. Arguably, for a study seeking to inform NHS and social care decision-makers, the perspective of the economic evaluation should be that of the health-care provider (the NHS) and Personal Social Services. The inclusion of direct and indirect costs to the person with dementia and the carer is important to understand the impact of care on these people as this informs judgements about efficiency and fairness (i.e. equity). Such costs for a UK context are best considered as part of a sensitivity analysis.
For the cost-effectiveness analysis, the effectiveness outcome would be reported as a physical unit: the number of falls. 55,56 The inclusion of this outcome measure would enable comparison of the DIFRID intervention with other interventions aimed at reducing the number of falls in PWD. In WP2 (see Chapter 3), the number of falls was self-reported by participants and captured in a falls diary. Although using self-reported data may not be the most reliable source of data collection, it is also being used in the i-FOCIS RCT56 and has been successfully used in other studies. 58
For the cost–utility analysis, the preferred generic utility-based measure of health-related quality of life is the EuroQol-5 Dimensions (EQ-5D). 59 As a generic measure, the EQ-5D facilitates the comparison of interventions across conditions and is recommended by the National Institute for Health and Care Excellence for use in technology appraisals in England. 60 This questionnaire has five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The original version of the EQ-5D, now called the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), has three levels (no problems, some problems and extreme problems) for each question. The tool has been revised and has been expanded to five levels (no problems, slight problems, moderate problems, severe problems and extreme problems) for each question (EQ-5D-5L). 61 The EQ-5D-5L is recommended as part of this economic evaluation as it is arguably more sensitive than the three-level version62 and it is also being used in the i-FOCIS study. 56 Furthermore, the methodological work on improving the tool and providing scoring systems is now concentrated on the EQ-5D-5L. A proxy version of the EQ-5D-5L should also be completed by carers as previous studies have found that PWD are unlikely to report ‘extreme problems’. 63,64 The proxy version, on average, estimates lower quality-of-life values than the self-completed version and is more likely to be sensitive to changes in quality of life. 63–65 The inclusion of both the self-completed version and the proxy version of the EQ-5D means that any uncertainty in the overall cost-effectiveness depending on who completed the EQ-5D can be explored. 64
For the economic evaluation, costs and outcomes should be discounted at recommended rates60 if the follow-up period of the study is beyond a 1-year time horizon. Consideration also has to be given to any uncertainty that arises as part of trial-based economic evaluations. Deterministic sensitivity analysis can be used to address any uncertainty surrounding assumptions made during the analysis. A stochastic sensitivity analysis, using, for example, the bootstrapping technique,66 would be appropriate to explore the impact of the statistical imprecision surrounding estimates of costs, effects and cost-effectiveness. Uncertainty surrounding the cost-effectiveness ratio should be presented on the cost-effectiveness plane67 and as CEACs.
Strengths and limitations
There are a number of limitations of this review. First, although the comprehensive search generated over 1200 hits, only six full-text papers were deemed eligible for further review after the screening process and only two protocol papers were eligible. This indicates that few fall prevention interventions in PWD have included economic evaluations. Second, not including falls recovery in the search terms means that we may have missed some potentially eligible studies that focused on recovery and rehabilitation post fall. Third, the strict inclusion criteria of falls prevention interventions meant that two potentially relevant studies were excluded51,52 and additional sources evaluating interventions for PWD more generally were not identified. The rationale for focusing the review on economic evaluations of falls prevention interventions was to ensure that any recommendations made for a future definitive study are comparable with existing literature in this area. Finally, the risk of bias was not determined for the two eligible studies. This is a potential limitation of our results but in the context of this review it was not a major concern as the focus was to identify the most appropriate economic evaluation methodology.
The searches were carried out to inform the panel meeting in WP3. As they have not been updated for this report, the findings should be interpreted as informing only that WP and not for current clinical decision making.
Conclusions
The inclusion of economic evaluations to determine the efficiency of alternative courses of action is recommended to inform policy-makers in the UK. 60 To conduct an economic evaluation, considerations need to be made to both the costs and the outcomes of these courses of action. Given the low level of evidence from existing studies, future economic evaluations should (1) identify and cost all of the resources required to deliver the intervention and any subsequent health and social care resource use, (2) measure outcomes using both number of falls and QALYs (using the EQ-5D-5L) and (3) undertake sensitivity analyses to address any uncertainty in the analysis.
Chapter 3 Incidence of fall-related injuries and the diary study

Introduction
The reviews reported in Chapter 2 indicate a lack of evidence regarding the effectiveness of fall interventions for PWD and limited attention to the economic evaluation of such interventions. PWD who sustain fall-related injuries may currently receive a range of health interventions, but the current models of care for this specific situation have not previously been described and the potential demand for such an intervention is not known. In order to develop a new complex intervention for this situation, we wished to describe current usual care and assess the demand for a future intervention to ascertain the feasibility of recruitment in WP4. We planned to do this by measuring the incidence of fall-related injuries presenting via three settings: the ED, paramedic attendances and primary care consultations.
In a subgroup of people presenting with fall-related injuries, we piloted a data collection tool in the form of a diary to collect data about falls, help at home and usual care. Information on usual care was obtained by analysing the health-care services used by diary participants after a fall-related injury. We planned that this diary would also be used to refine the design of a data collection tool used in WP4 to meet the requirement to identify subsequent health and social care resource use and capture data on the number of falls (see Chapter 2).
In order to obtain further information about experiences of usual care, some participants in the diary study also took part in a qualitative interview (described in Chapter 4). This chapter describes the incidence of fall-related injuries and the findings of the diary study.
Aim
The aim of the diary study was to determine the feasibility of recruiting PWD through different settings and to pilot the data collection tool prior to the feasibility study in WP4.
Methods
Setting
The study was carried out in three sites (Newcastle upon Tyne, North Tees and Norwich), reflecting a range of NHS practice to allow for generalisability. These sites covered both urban areas and rural areas, included a NHS trust with a long history of innovation in falls services and had dementia diagnosis rates both above and below the national average.
Three potential clinical settings were identified where we anticipated PWD with a fall-related injury would present:
-
Primary care – patients with a known diagnosis of dementia presenting with a fall-related injury to any primary care professional at participating practices in the NHS Clinical Commissioning Groups (CCGs) involved in the study (Newcastle Gateshead CCG, Hartlepool and Stockton CCG and Norwich CCG).
-
The community – paramedics attending calls to a person with possible dementia presenting with a fall-related injury. This applied to calls within the postcodes served by the CCGs mentioned above.
-
Secondary care – patients with possible dementia, resident within the postcodes served by participating CCGs, presenting to the EDs of participating sites.
The study took place in all three settings at each research site.
Inclusion criteria
Participants were required to:
-
Have a known diagnosis of dementia, made prior to entry into the study, by a specialist in dementia care (geriatrician, neurologist or old-age psychiatrist). The potential participant’s general practitioner (GP) was asked to confirm that the potential participant was on the practice’s Quality and Outcomes Framework (QOF) register of PWD, or the GP confirmed that the person’s records contained confirmed Read codes that would result in the QOF register being updated to include this person. Appropriate Read codes (and their equivalent International Classification of Diseases codes) for including a person on the QOF register are given in Appendix 2.
-
Have sustained at least one fall-related injury within the 48 hours prior to their identification as a potential study participant. The fall causing this injury was known as the index fall. A fall was defined as an event whereby a person comes to lie on the ground or another lower level with or without loss of consciousness. 68 Injuries were defined using International Classification of Diseases, Tenth Edition, Clinical Modification, diagnosis codes: ‘Injury, poisoning and certain other consequences of external causes S00-T88’. 69 A fall-related injury was defined as an injury that came about as a direct consequence of the index fall.
-
Be dwelling in the community at the time of the index fall.
-
Have a carer to assist with completion of the diaries (for those in the diary study).
Exclusion criteria
Participants found to be dwelling in residential or nursing care accommodation or to have been a hospital inpatient at the time of the index fall were excluded.
In addition, potential participants were excluded from the diary study if:
-
A diagnosis of dementia could not be confirmed by consultation with the GP or via the secondary care notes within 2 weeks of their being identified as a potential participant.
-
The participant or carer refused to provide consent.
Recruitment
In primary care, patients on the dementia QOF register had a flag applied to their records. If a primary care consultation with these patients took place, the professional was alerted to determine whether or not the consultation was due to a fall-related injury and, if it was, the consultation was added to the screening log. Consent was to be sought from the patient and/or their carer for the research team to contact them with further information about the diary study.
In the community, paramedics attending a person with a fall routinely refer the person to the local integrated falls services via a logistics desk. Basic information about comorbidities is sought by the person receiving the referral at the time of the referral. During the period of recruitment, the teams were asked to include a question about whether or not it was possible that the person may have dementia. This information could be obtained by a direct history of known dementia or confusion from the person or their carer, or, if not available, if the person appeared to be confused in the opinion of the paramedic. All persons with possible dementia who had sustained an injury were added to the screening log. The paramedic was to seek verbal consent for the research team to contact the person with further information about the diary study.
In secondary care, ED staff were asked to consider whether or not patients presenting with fall-related injuries had possible or known dementia and record this in their notes. ED staff were also asked to seek verbal consent from these patients at the time of the consultation for the research team to contact them with further information about the diary study. All cases of fall-related injuries presenting to the ED were screened by a clinical trials assistant (CTA) for evidence of a dementia diagnosis, possible dementia or other evidence of confusion, and all such cases were added to the screening log.
The CTA at each site monitored the screening logs 5 days per week for potential participants. They made a record of any duplicates presenting to the ED via the paramedics and recorded on both logs. The presence or absence of dementia was determined for all cases on the screening log.
For the diary study, CTAs contacted those who had given consent to be contacted by the research team. A participant information sheet (PIS) was sent by post as soon as practicable after the potential participant was detected. The potential participant was contacted by telephone once they received the PIS to answer any questions and determine whether or not they were interested in taking part. However, owing to small numbers of potential participants in the ED being asked for consent to release their details to the research team, this process was changed during the course of the study in accordance with an amendment submitted to Newcastle and North Tyneside 2 Research Ethics Committee. This allowed the CTA to send potential participants a PIS after they had left the department if the clinician had been unable to gain verbal consent for contact by the research team during the ED consultation (usually because of time constraints).
In the absence of published data on usual care provided to PWD following an injurious fall, we used the diary study to capture information on existing care pathways. We anticipated that up to an average of 20 patients per site would need to join the diary study in order identify the full range of usual care pathways provided (1–2 participants per week at each site). Once data saturation was reached, we aimed to continue to record incidence, but participants would not be asked to join the diary study.
Consent
Consent was not obtained for the diagnosis of dementia to be checked before adding the participant to the screening log. Approval was given by the Health Research Authority Confidentiality Advisory Group (reference 16/CAG/0057) for the researcher to obtain the name, age, sex, injury code and NHS number from the ED or paramedic service and use this information to contact the relevant GP to find out if the person was on their dementia QOF register. If the participant did not become a member of the diary study, patient-identifiable information was discarded.
Participants in the diary study were required to give informed consent in accordance with the Declaration of Helsinki. 70 Owing to the nature of dementia, some participants lacked the capacity to give full informed consent. In these cases, the provisions of the Mental Capacity Act (2005) applied. 71 Participants were asked to give consent appropriate to their level of understanding, ranging from written informed consent to account being taken of verbal and non-verbal communication in determining willingness to participate. In those individuals found to be without capacity to give full informed consent, the CTA identified a personal or nominated consultee and sought their advice regarding participation. Any patient appearing distressed by participation or withdrawing consent was excluded from the study without prejudice to clinical care. A favourable opinion was given by the North East – Newcastle and North Tyneside 2 Research Ethics Committee (reference 16/NE/0011).
Baseline assessments
Baseline data were recorded for participants consenting to the diary study. Data included medical history, medication history, dementia subtype and further details of the type and code of injury, location and circumstances of the fall, early treatment, any referral made by the attending professional and involvement of a carer. Cognition was assessed using the Montreal Cognitive Assessment (MoCA). 72
Diary study
The diaries were completed by the PWD, who were assisted by their carer as needed. Each diary collected information over a 4-week period. Participants were asked to complete three diaries in total. The objectives of the diary were to determine the feasibility of collecting information on number of falls (the predicted primary outcome for a future definitive study) and to identify potential patient pathways following a fall. It was agreed to pilot a Health Utilisation Questionnaire (HUQ) within the diary; this collected information to support a health economic analysis from the perspective of the health-care provider and Personal Social Services in a definitive trial. The format of this diary was similar to that of the data-collection tool used in the i-FOCIS trial. 56
For WP2, the diary and the HUQ were combined as one data-collection tool. The HUQ collected information on help at home (from carers or professionals), primary and secondary health-care resource use, social care and out-of-pocket expenses. The recall of the HUQ questions varied from daily to every 4 weeks. The rationale for daily recall for home help was to understand the daily burden on carers and determine whether or not the introduction of the intervention would affect their daily activities. Weekly recall was used to collect information on the most-common health-care resources likely to be used following a fall. Finally, 4-week recall was used for social care and participant expenses to minimise the burden on participants. The diary, including the HUQ, used in WP2 is provided in Appendix 3.
The HUQ was detailed as its purpose was to understand and record the type of health and social care used by PWD and the frequency of any reported use. The diary was piloted and amended prior to WP2 using feedback from patient and public involvement (PPI) representatives from VOICE (www.voice-global.org), a local involvement group. The main changes to the diary before it was administered in WP2 were extending the health-care treatment options provided in particular physiotherapy appointments. The overall aim of the diary study from an economics perspective was to inform the data collection tool to be piloted in WP4.
Analysis
Participant characteristics were analysed using descriptive statistics. Monthly presentation rates of potentially eligible participants were calculated for each setting per dementia case recorded on the QOF registers, giving an estimate of the potential future demand for an effective complex intervention in the NHS. The proportion of potentially eligible participants consenting to initial contact and then to full participation in the diary study was calculated, giving an indication of likely recruitment rates to WP4 and any future clinical trial.
Results
Incidence of fall-related injuries
Data were collected from the ED and paramedic attendances in all three sites. Primary care data were collected from eight general practices in Newcastle and 15 general practices in Norwich. Practices in North Tees declined to take part in the study owing to the burden of extracting the data required.
The total number of people presenting with fall-related injuries recorded across all sites and settings was 257 (Newcastle, 65; North Tees, 40; Norwich, 152), which gives a presentation rate of 42 cases per month. The majority of cases presented in the ED (n = 211), followed by primary care (n = 40), with very few presenting via paramedic attendances alone (n = 6). Table 1 gives the number of falls in each setting in the three sites. In the ED, the monthly presentation rate per dementia case recorded on the CCG QOF registers was 0.0029 (0.0027 cases per month per dementia case in Newcastle, 0.0024 in North Tees and 0.0033 in Norwich). In primary care, the monthly presentation rate per dementia case recorded on the practice QOF registers was 0.0035 (0.0058 cases per month per dementia case in Newcastle and 0.0028 in Norwich).
| Setting | Site, n (%) | Total (all three sites), n (%) | ||
|---|---|---|---|---|
| Newcastle | North Tees | Norwich | ||
| Primary care attendance | 14 (21.5) | 0 (0.0) | 26 (17.1) | 40 (15.6) |
| Paramedic attendance | 4 (6.2) | 0 (0.0) | 2 (1.3) | 6 (2.33) |
| ED attendance | 47 (72.3) | 40 (100.0) | 124 (81.6) | 211 (82.1) |
| Total | 65 (25.3) | 40 (15.6) | 152 (59.1) | 257 (100.0) |
The mean age of the fallers was 85 years [standard deviation (SD) 6.1 years]. Fallers were older in Norwich than in Newcastle [Newcastle, mean 84 years (SD 6.61 years); North Tees, mean 84 years (SD 6.04 years); Norwich, mean 86 years (SD 5.80 years); mean difference –2.07 years; p = 0.022]. Two-thirds of fallers were female (Newcastle, 65%; North Tees, 80%; Norwich, 66%), which did not differ significantly between sites (p = 0.192). Table 2 gives the dementia subtype diagnoses of the fallers. The most common dementia subtype was AD (58%), followed by vascular dementia (VAD) (26%). It took a mean of 10 days from the date of the fall to confirm whether or not patients were on the dementia QOF register, but this was established within 1 week in 81% of cases. Table 3 summarises the types of injuries with which fallers presented. The most common type was soft tissue injury (44%), followed by head, neck and facial injury (37%). Nearly 11% of fallers presented with a fracture.
| Dementia subtype | n (%) |
|---|---|
| AD | 148 (57.6) |
| Vascular dementia | 66 (25.7) |
| Dementia with Lewy bodies | 4 (1.5) |
| Parkinson’s disease dementia | 1 (0.4) |
| Frontotemporal dementia | 1 (0.4) |
| Unspecified dementia | 37 (14.4) |
| Total | 257 (100.0) |
| Injury type | n (%) |
|---|---|
| Soft tissue injury (head, neck and facial injury) | 94 (36.6) |
| Other soft tissue injury | 113 (44.0) |
| Hip fracture | 12 (4.7) |
| Other fracture | 15 (5.8) |
| Amputation injury | 1 (0.4) |
| Unspecified injury | 17 (6.6) |
| Multiple unspecified injuries | 5 (1.9) |
| Total | 257 (100.0) |
Diary study
Recruitment
Thirteen participants were recruited to the diary study: 12 were recruited via the ED and one was recruited via a paramedic. The mean age of the participants was 87 years, and seven participants (54%) were female. Seven participants had AD and six had VAD. The mean MoCA score was 13.6 points, indicating moderate cognitive impairment. One participant withdrew before completing any diaries but initial baseline data were retained.
Early treatment of the participants
One participant already had a falls unit referral in progress and one further participant was offered a falls unit referral but declined. One participant was already receiving physiotherapy, three participants were offered a new physiotherapy referral and one was referred to a rehabilitation unit. One participant was referred to their GP. No referrals were made for the remaining five participants.
Data completeness
The return rate for the first diary was 75% (n = 9), but this reduced to 50% (n = 6) for diaries 2 and 3. One participant went into a care home during the study and completed diary 1. One participant died during the study but had completed diaries 1 and 2 and partially completed diary 3. Carers were contacted to return the remaining outstanding diaries but, despite several telephone reminders, they were never returned.
Number of falls
A total of 11 falls were reported by four participants during the diary study. Two participants reported having one fall each, one participant reported having four falls and one participant reported having five falls. In the falls diaries returned, few data were missing.
Health-care utilisation questionnaire
The level of missing data within the HUQ section of the diaries was relatively low, suggesting that those participants who completed the diary had few problems with completing these questions. The individual response rate to the weekly HUQ questions is provided in Appendix 4. The lowest response rate to an individual question, based on those who responded to the diary, was 50%. However, it should be noted that in later diaries it appeared that some participants only completed the HUQ questions that were relevant to them, suggesting that the frequency of the questions became burdensome. This is supported by the following extract from the qualitative data:
We’re not going to do the diary. There’s no point. There’s no point doing the diary [. . .] What could we say? It’s hard work, every day, writing.
Carer 9b (joint interview with carers 9a and 9b)
Data on nine participants who completed at least one diary were summarised to inform any modifications to the HUQ for WP4. Although these data are not necessarily reflective of all PWD who have had a fall, they give us an indication of the types of resources used by PWD and the frequency of their use. On average, little use of health care was reported during the 12-week study period. The maximum reported health care was for diary 1 in week 2, when six participants reported using at least one health-care service.
Appendix 4 summarises the total resource use per participant in the diary study. On average, participants reported using at least one health-care service for 3 weeks of their 12-week follow-up period. Arguably, the median results presented are more representative of the actual health-care resource use as one participant reported high health-care resource use: 28 day-case visits and 30 nights in hospital over the 12 weeks. Although high, this volume of care is not unusual in clinical trials, in which a small number of participants tend to have very high use of services.
Participant out-of-pocket expenditure
Paid health and social care
At the end of each diary, there was a section on out-of-pocket expenditure, which recorded whether or not participants had paid for any health or social care and, if so, what they had paid for and how much they paid. Over the 12 weeks, three participants reported paying for care; however, only two participants provided information on the cost. One participant reported paying for care in all three diaries and paid a total of £664 for home care and day centre care. The other participant reported paying £35 for a visit to a chiropractor. The participant who did not provide information on how much they paid stated they had paid for spectacles.
Paid other help
Over the 12-week diary period, five participants reported paying for additional help, most commonly a cleaner (four participants). Appendix 5 summarises the type and cost of paid help. The average total cost paid by these five participants over the 12 weeks was £98.
Carer’s Allowance
Two participants reported receiving a Carer’s Allowance: one carer received £90 per week and the other received £50 per week.
Help at home
As part of WP2, it was decided to collect daily information on help at home to gauge the level of assistance reported by PWD. The inclusion of the ‘help at home’ section was to identify what activities carers usually participated in.
The open-text boxes allowed participants to provide detailed information on the help they received. This ranged from help with medications to 24-hour care. The majority of care reported related to daily activities such as making dinner and going to the shops. Assistance could benefit the person with dementia and/or the carer:
Carer for meds [medications] and breakfast. Pick up and drop off for church. Carer for meds and tea.
Data from diary (DS11)
Paramedic came looked him over took him to North Tees Hospital. My three daughters and two granddaughters called after it just happened, made me a cup of tea, went to hospital with me. Then brought me home. Six hours they stayed with us.
Data from diary (DS05)
Opportunity costs for carers
The most frequent activity that carers would be undertaking if they were not assisting the person with dementia was housework (71%), followed by leisure time (57%). Fewer than 30% of carers missed paid work to assist the person with dementia.
Discussion
The incidence of fall-related injuries was much lower than expected. In our previous study, the total incidence of falls in people with mild to moderate dementia was 9118 per 1000 person-years. 2 A secondary analysis of the data in our previous study showed that there were 0.044 injuries per person per month among PWD with AD or VAD. Our findings that only 0.0029 cases per person per month presented to the ED and 0.0035 cases per person per month presented to primary care suggest that fewer than 15% of injuries sustained by PWD are brought to the attention of health-care practitioners. This assumes that our procedures for identifying both fall-related injuries and that a person with a fall-related injury had dementia were robust. In primary care, we are confident that the diagnosis of dementia was robust because the GPs had direct access to QOF registers. In the ED, where carers gave a history of dementia, this is likely to have been accurate. However, a carer may have been absent or the carer may not have been aware of the diagnosis. In the case of diagnosis by paramedics, the very small number of cases suggest that systems for picking up dementia were not robust. This has implications for WP4 and any future trial of an intervention to improve outcomes for PWD with fall-related injuries. If dementia is not identified at the time of presentation, it would be difficult to ensure that a referral to an appropriate intervention is made.
The limited number of diary data collected indicated that most PWD received very little health-care input following the index fall. Although 8 out of the 13 participants were offered an initial referral to a falls unit, physiotherapy or a rehabilitation unit, the diaries of the 12 participants who completed them show very little use of these services over the course of the subsequent 12 weeks. This suggests that people may have received an initial assessment but were deemed not appropriate for further input or were still waiting for the referral to be followed up. Further evidence of this was found in our qualitative study, which is discussed in Chapter 4.
Strengths and limitations
For the diary study, we did not reach our target of 60 participants. We believe that the requirement for health professionals to seek permission from potential participants to share their contact details with the research team contributed to poor recruitment owing to time constraints, particularly in the ED. Although we submitted an amendment to modify recruitment procedures (see Recruitment), approval was not received in time to make a material difference. The modified approach was implemented in most sites in WP4.
With regard to data completion, 75% of participants completed the first diary and 50% completed diaries 2 and 3. Where diaries were returned, the daily recording of falls was successful, and this is supported by the use of a diary in other studies with PWD. 58 The relatively poor completion rates for the HUQ, compared with completion rates for a falls diary alone in previous studies, suggest that the additional questions may have been off-putting or too time consuming for participants. The low rates of reported health-care and social care use suggest that the HUQ could be simplified for the feasibility study (see Chapter 7). A limitation of the HUQ was that a number of unrelated expenses (e.g. spectacles) were reported. Furthermore, some regular expenses were only reported once (e.g. for a cleaner); participants may have thought that it was unnecessary to include these in all diaries.
Conclusions
The incidence and diary studies suggested that recruitment rates to a future trial may be lower than anticipated. However, PWD were found to be receiving very few services, suggesting that there is scope for a new intervention to improve outcomes.
Chapter 4 Current pathways and opportunities for intervention

Parts of this chapter are adapted from Bamford et al. 73 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text. Parts of this chapter are adapted from Wheatley et al. 74 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
The effectiveness review provided limited guidance on the core components of an intervention to improve outcomes for PWD with fall-related injuries and highlighted uncertainty over the most appropriate outcome measures. Although the diary study provided some insight into current service use in the 12-week period following an injurious fall, low recruitment rates meant that we were unlikely to have captured the diversity of care pathways. Qualitative work was conducted with a range of stakeholders to provide additional data.
Aim
The qualitative component of WP2 aimed to develop a better understanding of current pathways and identify opportunities for intervention. Objectives were to:
-
explore the range of services currently available to PWD following an injurious fall
-
identify the needs of PWD and carers following an injurious fall and ascertain the extent to which these were currently met
-
explore ideas for service development or intervention with a range of stakeholders
-
identify outcomes of importance to PWD and their families.
We included health and social care professionals, PWD who had experienced a fall and their carers. We also aimed to identify the care needs of, and outcomes of importance to, PWD and their families.
Methods
Sampling
Participants were drawn from the three sites described in Chapter 3. Professionals were initially identified through interviews with the principal investigator of each site. We then used snowball sampling75 to identify relevant health and social care services in each area. Recruitment continued until data saturation was reached.
Professionals taking part in interviews and focus groups were asked whether or not they and their colleagues would be willing for us to observe routine practice. We selected a diverse range of services across the three sites for observation. All patients due to be seen on the agreed date(s) for observation were eligible to take part.
Patients and carers were recruited for interviews through either observation or the diary study (see Chapter 3). The process of sampling and recruitment is summarised in Figure 4.
FIGURE 4.
Summary of sampling and recruitment processes. Reproduced with permission from Bamford et al. 73 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original.

Recruitment and consent
Professionals who were invited to all parts of the study were provided with a PIS. Prior to data collection, professionals gave either verbal consent (telephone interviews) or written consent (face-to-face interviews, focus groups, observation).
The PWD and carers seen by participating services on dates selected for observation were provided with a brief information sheet by staff (in advance of home visits or on arrival at clinics). Verbal consent was sought for observation. Prior to observation of group interventions (e.g. exercise classes), we obtained verbal consent from all participants.
Some PWD and carers who were observed were invited to take part in an interview. In selecting potential interviewees, the researcher aimed to sample people with a range of injuries, presenting to different services and who lived in the community. Only PWD thought to have capacity to consent to an interview were invited. In addition, participants in the diary study (see Chapter 3) were asked if they were willing for their details to be passed to the qualitative team as part of the initial consent process. We also recruited a small number of participants without cognitive impairment from exercise groups. Regardless of how potential interviewees were identified, a PIS was provided to those who expressed an interest. Formal written consent was sought prior to the interview.
Data collection and analysis
Interviews and focus groups
Topic guides were used to structure interviews and focus groups (see Appendix 6). Those for professionals explored service organisation, the perceived success of current interventions, the views and use of outcome measures, experience of working with PWD, challenges specific to falls in PWD and ideas for intervention. Most interviews with professionals were carried out by telephone. We supplemented professional interviews with five local focus groups held in participants’ places of work.
Interviews with PWD and carers explored their falls history, experience of services, desired outcomes and ideas for intervention. Interviews with exercise group participants without cognitive impairment focused on their views and experiences of the inclusion of PWD in such groups. Interviews with all patients and carers were conducted face to face, in their homes or in another venue of their choice. Participating dyads were interviewed either individually or jointly according to their preference.
Observation
During observation, we considered the interactions, content and if and how interventions were tailored to individuals. Detailed ethnographic field notes were recorded during and after each period of observation,76 which usually lasted for a single shift or clinic session. In field notes, patients were identified only by age and sex; the only information recorded on companions was their relationship to the patient.
Data management and analysis
Interviews and focus groups were audio-recorded. Professional interviews and focus groups were initially summarised onto a structured pro forma. Data-rich audio recordings were transcribed in full. All interviews with patients and carers were transcribed for analysis. Transcripts were checked and anonymised, with participants allocated a unique identifier, prior to analysis.
We adopted a separate thematic approach to each data set (interviews and focus groups with professionals, interviews with patients and carers, observation) to avoid assuming that themes from one data set were necessarily relevant to another. We then mapped areas of consistency and discrepancy across data sets to create a new integrated coding frame. This was then applied to each data set using NVivo 11 (QSR International, Warrington, UK).
Research governance approvals
Newcastle University provided an ethics review for the initial interviews and focus groups with professionals, and any necessary permissions were obtained from research and development departments of participating trusts. Approval for observation and interviews with patients and carers was given by Newcastle and North Tyneside 1 Research Ethics Committee (reference 15/NE/0397), Newcastle and North Tyneside 2 Research Ethics Committee (reference 16/NE/0011) and the Health Research Authority. Additional approvals were received from participating trusts and social services departments as required. For non-statutory agencies, approval was sought from senior managers.
Results
Qualitative findings relating to care pathways for PWD following a fall and the need for staff training have been published73,74 and are summarised below. Quotations are accompanied by a participant identifier, with role and service type given for professionals.
Participants
In total, 53 professionals were interviewed across the three sites and an additional 28 took part in five focus groups. Interviews lasted between 20 and 60 minutes and focus groups lasted between 40 and 65 minutes. Participants included consultants, GPs, nurses, OTs, physiotherapists, paramedics, service managers, support workers and clinical commissioners.
Initial observations focused on services that we anticipated would be difficult to capture through the diary study (Table 4). Although we intended to recruit additional PWD and carers for observation through the diary study, this proved impractical as a result of low recruitment rates and limited use of community services by diary participants (see Chapter 3). In total, 20 professionals were observed delivering care to 85 patients.
| Service type | NHS | Social care | Third sector |
|---|---|---|---|
| First response services | Paramedics |
|
|
| Hospital services |
|
Facilitated discharge team | |
| Other residential services | Specialist rehabilitation unit | Specialist rehabilitation unit | |
| Domiciliary services |
|
Telecare | |
| Community services (including primary care) | Exercise classes |
We approached 17 PWD and 19 carers for interview (21 were identified through observation, 15 were identified through the diary study), of whom four PWD and nine carers consented to and completed interviews. We additionally included four cognitively intact older people from exercise classes.
The findings are presented under three main headings: (1) views on the need for a new intervention for PWD after an injurious fall, (2) views on the content and delivery of a new intervention and (3) issues in assessing outcomes of a new intervention and the outcome measures currently used by participants.
Perceived relevance of a new intervention
In discussing opportunities for intervention, some professionals queried whether or not a specific intervention was required for PWD and the proposed timing of the intervention. Some professionals believed that any service that would benefit PWD would also benefit a broader population of older people and, therefore, thought that a specific intervention was not required:
I can’t think that there’s an intervention that I would specifically want to offer to a demented patient, compared with simply a slightly more frail, elderly person.
Professional 33, GP, primary care (interview)
Others, however, thought that a specific intervention would be useful to address the increased risk of institutionalisation, reduced quality of life and well-being of PWD following an injurious fall.
A number of professionals challenged the study brief (to focus on injurious falls), arguing that an earlier, more proactive intervention was needed, rather than intervening only after an injurious fall:
I think there are a lot of things around prevention. I’ve always said that once a patient’s fallen, it’s too late. They’ve fallen.
Professional 70, paramedic (interview)
Even if a preventative approach was not adopted, other participants suggested that any new intervention should be available to all PWD who fell, regardless of whether or not an injury was sustained.
Proposed content and delivery of a new intervention
Through discussion of the shortcomings of existing services, and examples of good practice, participants identified key components of a new intervention. These components were grouped into three main themes, each of which included subthemes. An overview of the themes, and their implications for a new intervention, is provided in Table 5. We then provide a description of each theme, with illustrative data.
| Theme | Subthemes | Implications for a new intervention |
|---|---|---|
| 1. Supportive service organisation | Clear pathways |
|
| Flexible service delivery |
|
|
| Effective information-sharing | Involve a MDT to improve access to information | |
| Holistic approach | Include holistic assessment and ensure that all factors contributing to falls are addressed | |
| 2. Staff attitudes, knowledge and skills | Attitudes to dementia | Provide staff training on attitudes to dementia |
| Knowledge and understanding of dementia | Provide staff training on dementia | |
| Practical skills for working with PWD | Provide staff training on a range of practical skills including communication, engaging PWD and embedding activities into daily routines | |
| 3. Supporting carers and their role in interventions | Assessing carer capacity and stress |
|
| Training and educating carers |
|
Theme 1: supportive service organisation
Interviews and observation highlighted a number of ways in which the current organisation of services could undermine or enhance the recovery of PWD following a fall. 74
Clear pathways
A recurrent theme in interviews with professionals and carers was the fragmentation of care pathways for PWD following a fall. Because local falls and dementia services were often led by different individuals, knowledge of the full range of services available and how to access them was often patchy.
Drawing on our data, we identified 21 distinct types of service potentially available to PWD following a fall. Not all service types were available in all areas but Figure 5 shows a composite set of services organised by the point in the falls trajectory at which they are typically involved. Initial services (hyperacute and acute phases) primarily focus on injury management, whereas services provided later in the trajectory (post acute and longer term) focus on rehabilitation, risk management and the prevention of future falls. Figure 5 also indicates which agencies typically provide the service and whether it is delivered in the patient’s home or elsewhere.
FIGURE 5.
Overview of services potentially available to PWD with fall-related injuries. Adapted from Wheatley et al. 74 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original.
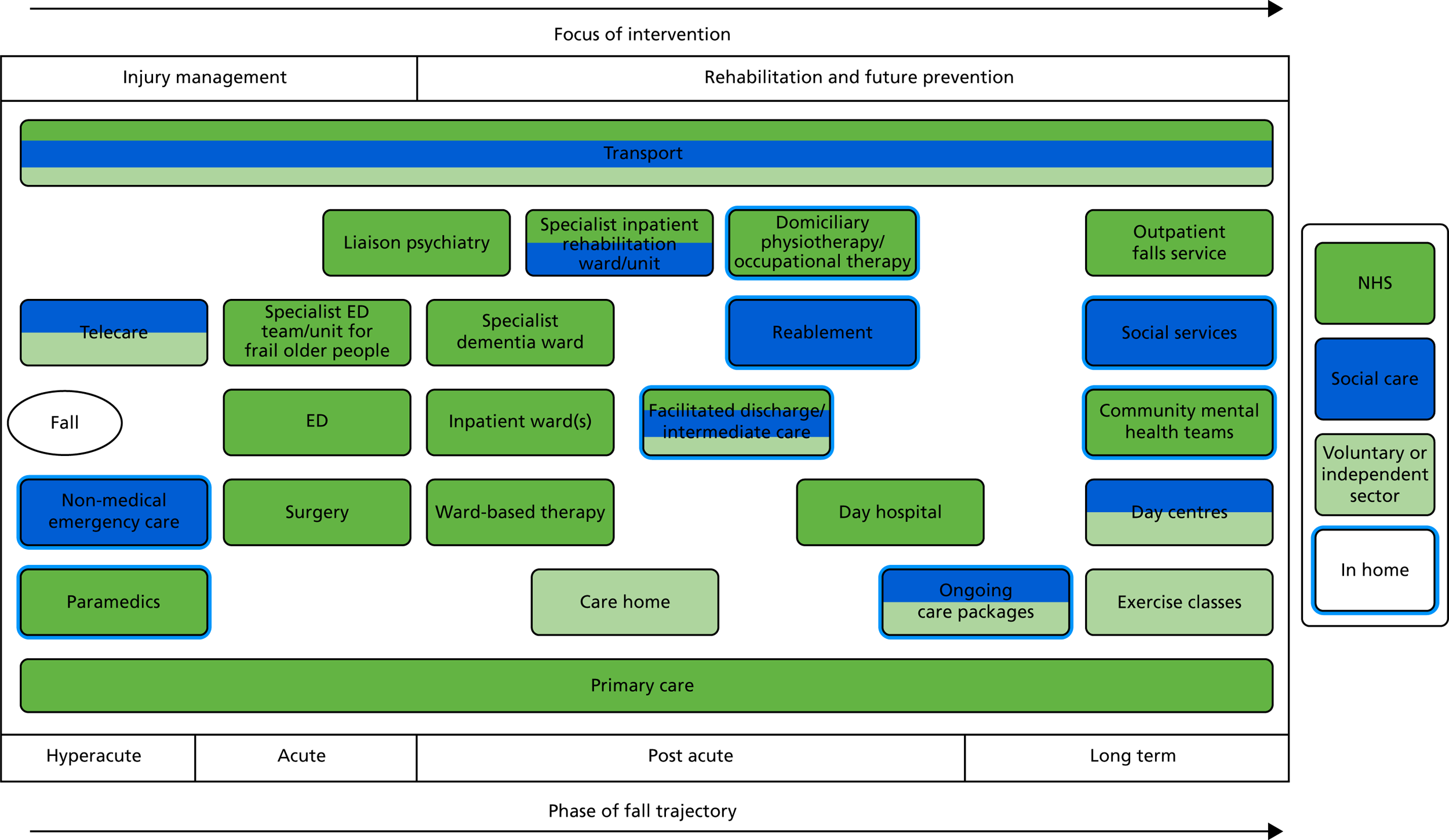
An individual person with dementia with a fall-related injury will access only a limited range of services. Although the pathway might appear linear, it is possible for many of these services to be accessed in parallel and people with recurrent falls may have multiple iterations of different service configurations. Although it is difficult to identify a ‘typical’ pathway, we have provided an example of a possible care pathway for a person with dementia who fell at home and sustained a fractured neck of femur (Figure 6).
FIGURE 6.
Example pathway for a person with dementia with a fractured neck of femur. Adapted from Wheatley et al. 74 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original.

A lack of knowledge of locally available services could result in PWD not receiving appropriate support; this was particularly problematic for paramedics who covered large geographical areas. Professionals emphasised the need for improved integration of existing services, for example through a single point of contact, better signposting, clear eligibility criteria and referral systems:
A standardisation of systems would be helpful. So you know, if that patient has dementia and they fall and they injure themselves, then you can ring a number to get them referred, if it’s out of hours, to a particular team who will come out in a couple of days and check that.
Professional 13, paramedic (interview)
We found one example of successful service integration among first response services. One telecare service was integrated with the ambulance and NHS 111 services, which allowed for flexibility and could reduce response times:
They are only supposed to attend non-injurious falls, so if they get there and there are signs of injury, they call the paramedics (and sometimes the paramedics refer patients to them if there is a non-injurious fall).
Field notes of urgent unplanned needs service visit, 20160728
Key services on the care pathway were thought unsuitable for PWD. The ED environment in particular was identified as problematic for PWD, despite attempts to make some EDs more dementia friendly (e.g. use of volunteers to give non-clinical attention or distraction techniques). Concerns about care provided outside the PWD’ home environment were raised, because this could lead to loss of confidence and connection with their home if admission was prolonged:
When people come in here, the quicker you can get them home the better, because the longer they’re out of their own environment the harder it is, they become very dependent here. We see it quite often where people do go home and it fails, because they’ve been here too long.
Professional 30 (joint interview with Professional 30 and Professional 31, managers, reablement service and specialist dementia unit)
Access to community support could influence length of admission. If equipment or packages of care were not available, PWD sometimes remained in rehabilitation services longer than intended or were transferred to an alternative service. A community matron model, with a dedicated person to help navigate services, was suggested to facilitate prompt discharge. This would be valued by carers, who often lacked knowledge of available services or how to access support:
[The patient’s] granddaughter says that someone said that he should have had a quad stick but that hasn’t been sorted. She doesn’t really know who is supposed to deal with that or where they’re supposed to get it from.
Field notes of domiciliary visit by physiotherapist from reablement service, 20160714
Flexible service delivery
Many professionals highlighted the poor ‘fit’ between the needs of PWD and current service organisation. The emphasis on shifts between health and social care and time-limited interventions disrupted continuity of care. Staff emphasised the need for time to enable PWD to develop rapport and trust:
When they know they’re having problems with their memory, if they know they’re not managing but they’ve got to trust you before they’re going to give you the information that you need as to what they’re struggling with. You’re not going to get that from one half an hour visit. You’ve got to build up the picture over a period of time.
Professional 62, team lead, facilitated discharge service (interview)
Lack of continuity was also challenging for professionals who relied on detailed knowledge of PWD to tailor interventions appropriately. Time-limited interventions were thought not to take account of the need for PWD to have more rehabilitation sessions, over a longer period of time, in order to achieve the same progress as older people who were cognitively intact:
You shouldn’t be able to rush people for multiple reasons. They could be afraid, they could be in pain, they could be constipated, getting down to the root of it, finding out what the triggers are. [. . .] You’ve got secondary things due to vascular changes, from strokes and things. How tired they may be. If somebody gets very tired, you can’t do a long session. You have to do lots of little sessions.
Professional 48, physiotherapist, ward-based therapy (interview)
In the context of a deteriorating condition, ongoing review was recommended by some professionals to avoid the loss of functional gains and address any emerging problems or changes:
You will only maintain your muscle strength and range of movement for a period of time before it starts to decline again if you are not doing those activities.
Professional 10, senior OT, ward-based therapy (interview)
No services providing long-term review or follow-up were identified. Carers would also have welcomed more follow-up, to address changing needs and ensure that existing equipment was still appropriate:
Dad’s got a pressure pad under his chair there and a pressure pad under his bed but he no longer has pressure sores. We’re still using them, but does he actually need them anymore? I don’t know.
Carer 8 (joint interview with Patient 8 and Carer 8)
Effective information-sharing
A need for effective systems for sharing information across service boundaries (e.g. primary and secondary care, health and social care) was identified. Joint information systems remained underdeveloped, with many services not being able to access even basic information (e.g. whether or not a formal diagnosis of dementia had been made). A lack of joint information systems led to unnecessary duplication of effort and frustration for staff:
[Professional 120, physiotherapist] comments that it would make a lot more sense if all the information was consolidated in one place for everyone to have access to. [. . .] She takes the patient’s notes to her office to fill in the referral paperwork for [short-stay rehabilitation unit], which is a 7 page document. She says that she doesn’t know why the referral is so long and complicated, since the [short-stay rehabilitation unit] doesn’t trust their data and will redo all the tests when the patient gets there [. . .] It takes her 45 minutes to complete and fax the referral.
Field notes of facilitated discharge service, 20161018
Multidisciplinary team meetings, including staff with access to different information systems, were identified as a successful way of sharing information.
Carers similarly commented on the lack of ‘joined-up working’ between different services, which could lead to conflicting advice or interventions:
[Home carers] brought a load of occupational therapists in to change some of the equipment over because they thought it wasn’t suitable, even though the hospital had provided what they thought was right. [. . .] although, that [a hoist] is very useful, the stand assist they brought originally meant Dad had to work at it, which exercised his arm muscles and his leg muscles and that’s what I would have preferred to have seen staying here, although, it was more work for us to move around and so forth. It’s easier for the carers to use that [hoist]. It’s harder for them to use the other one.
Carer 8 (joint interview with Patient 8 and Carer 8)
This is one of several examples of carers expressing their uncertainty over equipment and aids. Where there was no explicit discussion of the rationale for changing equipment, or detailed guidance on how to determine when aids were no longer needed, carers were unsure what to do for the best. In the above example, the change of equipment creates concern over whether or not the new equipment was chosen for staff convenience rather than to maximise recovery.
A holistic approach
Holistic assessment was seen as the most appropriate way of identifying and treating factors that could potentially contribute to falls. Opportunities for such assessment, however, seemed limited. Falls clinics and day hospitals routinely adopted a holistic approach, but not all PWD were referred to these services. The extent to which holistic assessment was provided in other settings was unclear. Professionals recommended that assessment of PWD with falls should include visual perception and spatial judgement, footwear and foot care, bone health and hydration. Addressing mood and anxiety was also important to rehabilitation outcomes:
I think in terms of addressing depression as well, people might have a reduced level of activity if they’re depressed. Obviously, that’s not great. If someone’s sitting for a long time, that’s going to affect their mobility. If people aren’t feeling particularly motivated, it does affect what they’re doing.
Professional 71, senior worker (focus group with specialist inpatient rehabilitation workers)
Formal tools were used most frequently to assess cognition and fear of falling. The potential value of detailed assessment in facilitating staff to tailor their approach to meet the needs of an individual person with dementia is illustrated by the following description:
We do specialist assessments in terms of what cognitive level they’re at, so that will tell us how we should be giving instructions to them, what their abilities still are. Even things like how we should be modifying the environment to best meet their needs. You can actually kind of predict what the risk is going to be in relation to falls, based on their personal cognitive level. So it’s using the strengths that they’ve still got to get the best that we can out of it.
Professional 79, OT clinical lead (focus group with mental health professionals)
Less systematic approaches were typically used in assessing other areas. Despite frequent discussion of the difficulties of assessing pain in PWD, only two paramedics described using a formal pain assessment tool (the Abbey Pain Scale77). Given the difficulties in identifying pain in some PWD, some professionals suggested that pain relief should be given routinely because it potentially:
[. . .] doesn’t do any harm and actually usually [we] can find some quite marked effects.
Professional 81, nurse consultant (focus group with mental health professionals)
The observation highlighted several occasions when pain relief was inadequate. In the following example, a person with dementia became irritated during an examination in the ED:
The doctor then helped the patient move onto the bed so that she could examine the knee fully. It was clear that the examination was painful at some points and the patient became quite cross saying ‘She’s the wickedest woman in [place]’ [. . .] The doctor took this in good part but I don’t think she really explained to the patient why she was having to move the leg and cause her pain. The patient had clearly had enough, saying ‘Well, just stop it!’.
Field notes of ED, 20160406
There may have been a number of reasons for the patient becoming un-co-operative at this point: she had not received pain relief for more than 4 hours and was potentially hungry, thirsty and tired, as she had missed lunch and her usual nap.
Theme 2: staff attitudes, knowledge and skills
The second main theme influencing the quality of existing falls services for PWD related to staff attitudes, knowledge and skills. 73 A range of approaches to staff training and development were identified, including basic training for all staff, access to specialist advice through MDT meetings, links with specialist dementia services (e.g. challenging behaviour teams) or (where available) links with the local lead for falls and dementia, and joint visits with experienced colleagues or local specialists.
Attitudes to dementia
Recognising and facilitating the rehabilitation potential of PWD require staff to be willing to invest in working with this patient group. Comments made during interviews and observation of service delivery indicated negative attitudes towards PWD among some staff. The ability of PWD, particularly those with more severe cognitive impairment, to benefit from an intervention following an injurious fall was questioned by some staff who perceived them as ‘not rehabable’ or ‘untrainable’:
And unfortunately the term ‘not rehabable’ is not uncommon, used in relation to patients presenting with cognitive issues, which could be delirium as well as patients with diagnosed or undiagnosed dementia. And is used by all professionals and grades of staff, from consultant geriatricians down.
Professional 79, OT clinical lead (focus group with mental health professionals)
Assumptions that falls were an inevitable consequence of dementia meant that detailed investigation following a fall was not always seen as relevant or necessary, because the problems were simply attributed to dementia:
I think partly because I would be thinking that the dementia or the lack of awareness is partly behind why they’re actually falling and hurting themselves, rather than falling and not falling. I’ve already identified a pathology in that group, i.e. that they’re demented, where the non-demented ones, I’m looking for another pathology.
Professional 33, GP, primary care (interview)
Negative attitudes to dementia were also evident in the extent to which staff were willing to persevere with PWD and their families who appeared reluctant to engage or were not adhering to proposed activities. Although some staff sought to address these issues by building rapport and adapting their approach, others seemed to accept reluctance to engage at face value and simply withdrew the service.
Knowledge and understanding of dementia
Many participating professionals had received no formal training in dementia. Some had attended brief courses or completed online modules, and others had learned through professional experience, from colleagues or through personal experience with dementia:
I’ve had people in the family with dementia, and I’ve done a lot of looking into how to talk to people, how to deal with people [. . .] I do think it has benefited my work, and I know when my grandma was ill a few years ago, I did a lot of research on just little things around how to talk to people, what might be missing from their lives, that sort of thing.
Professional 23, exercise class instructor, non-statutory sector (interview)
Many professionals expressed a desire for further training in working with PWD. Staff working for a mental health trust particularly highlighted the need for professionals to better understand ‘challenging behaviour’ as a barrier to rehabilitation.
Practical skills in working with people with dementia
In addition to greater understanding of dementia and the potential impacts on individual PWD, staff required a range of skills to work effectively with this patient group. These have been described elsewhere73 and are briefly summarised in Table 6. Specific skills identified by participants and through the observation included communication, use of observation, engaging PWD, tailoring interventions to build on existing interests and activities and embedding interventions into daily life.
| Skill | Example |
|---|---|
| Communication | [Professional 111] does tests on the arm that is damaged from the stroke, including range of movement, grip etc. There’s a misunderstanding about pain at this point. She says to [the patient], ‘Let me know if it’s sore’, but I’m not sure he understands. She just kind of says it and then goes straight in, without giving him time to process. It seems clear that he is in pain, to the point where his granddaughter says, ‘Oh, granddad, say if it hurts’. Then [Professional 111] stops and rephrases the thing about the pain, and after that he is quite good about saying where the pain isField notes of domiciliary visit by physiotherapist from reablement service, 20160714 |
| Observation and demonstration | Because a lot of our assessment is skilled observation. I think it’s quite specific to this specialism, because you can’t always rely on the person’s account to be accurate, and we’ve got lots of patients, especially in the early stages, that have got really good verbal skills and they can mask the level of impairment that they’ve actually got. But with an OT assessment they can’t mask functioning. So we will get them to show us everythingProfessional 79, OT clinical lead (focus group with mental health professionals) |
| Engaging PWD | [Professional 51] says ‘I wanted to test you, [Patient 1]’. She gets a large ball from the cupboard, about the size of a beach ball. [Patient 1] becomes very animated, much more engaged than he has been. He says ‘Oh, football.’ [. . .] They stand in the middle of the gym and do some bouncing and catching of the ball, and then throwing and catching. [Professional 51] checks after each activity if he has experienced any dizzinessShe then fetches cones and a plastic football from the cupboard, which [Patient 1] greets enthusiastically ‘That’s the way forward!’Field notes of day hospital physiotherapy session, 20160504 |
| Tailoring interventions | [Patient 1] has new trainers – last time he came, [Professional 51] had recommended that he get new shoes because his old ones had holes in. [. . .] [After the consultation] [Professional 51] mentions the shoes and says that someone other than the patient’s wife had to say it: [Patient 1] loved his old shoes even though they had holes. [Professional 51] wrote down the instruction to get new shoes so that his wife could have something to refer toField notes of day hospital physiotherapy session, 20160504 |
| Embedding interventions | There is quite a bit of evidence now if you incorporate your strength and balance exercises into your routines it can be as effective. For me that is where we need to work on with the dementia patients. If you can put it into their habits so it becomes habitual then you perhaps don’t need to be going to a programme that you have to do specific things at specific timesProfessional 10, senior OT, ward-based therapy (interview) |
Some of the quotations in Table 6 demonstrate the creativity and resourcefulness of staff in trying to engage with PWD and achieve desired outcomes. Without these practical skills, staff could struggle to engage PWD, which could reinforce the perception of some staff that PWD were unsuitable for intervention.
Many professionals recognised that PWD could have difficulty in retaining abstract and unfamiliar exercises. Making interventions understandable and appealing to the individual was therefore seen as essential. Some participants, particularly patients, talked about the importance of the intervention being fun and enjoyable. To enable activities to be tailored in this way, assessment needed to capture information about the person with dementia’s personality, likes and dislikes.
Theme 3: supporting carers and their role in interventions
Carers were recognised as potentially having a key role in facilitating rehabilitation of PWD; however, there were tensions regarding their capacity to support interventions and attitudes to positive risk.
Assessing carer capacity and stress
Because interventions were often time limited, some professionals recommended a cascade model in which relevant exercises or activities would be integrated into regular visits by a support worker or into daily routines by a carer:
I worked with somebody who had really, really bad dementia and who’d had lots of falls, and we thought he wouldn’t work with a therapist because they were scary. He didn’t understand what we were offering, so we taught his daughter all of his exercises and did all the risk assessments and that with her. Then, she did them with Dad and we just reviewed once a week, and he did really, really well.
Professional 08, manager, specialist inpatient rehabilitation (interview)
Although this is a positive example of involving a carer in rehabilitation, carers did not always have the capacity to support interventions in this way. Some staff implicitly assumed that carers would take an active role in any intervention, for example by encouraging activities between sessions and after an intervention was withdrawn. Staff understanding and recognition of carer stress varied. Some staff seemed to lack insight into the realities of caring for a person with dementia and occasionally expressed critical and even hostile views towards carers. For example, some exercise instructors expressed frustration that carers saw their service as an opportunity to have a break rather than attending the class with the person with dementia. Other staff had more insight into the challenges and stress of caring for PWD:
It is a huge demand on families as well, which I think people forget sometimes how stressful it is for the family to have someone at home where things are not working out and where they are worrying about them. They do know that they really don’t want them to go into hospital, but they are really at the end of their tether providing the support. That is something that we see very frequently here.
Professional 06, consultant geriatrician, outpatients falls service (interview)
In addition to stress, the capacity of carers to support interventions was influenced by their own health problems, work commitments and whether or not they lived with or near the person with dementia.
Training and education for carers
A number of professionals highlighted the potential for carers to undermine interventions because of a lack of understanding. Some carers were perceived as ‘overprotective’ and risk averse by both PWD and professionals. Typically, such carers tried to restrict patient mobility or daily activities because they were unfamiliar with the concept of positive risk or the dangers of deconditioning. In this context, professionals described the need for ‘mindset work’ to encourage carers to actively encourage the PWD to get up and move about. One rehabilitation ward actively involved carers in rehabilitation, for example by supervising patients while they were walking. In this setting, staff also trained carers on how to carry out certain tasks, such as transferring a patient from a bed to a commode. In contrast, other services provided little information on how to support a person with dementia following discharge:
When Dad [Patient 8] was released, I was given about a 5-minute demonstration of handling, how to transfer people to sit down onto a stand or onto the bed and moving them around and that was it. They’re released into your care.
Carer 8 (joint interview with Patient 8 and Carer 8)
Professionals suggested that carers would benefit from additional education and awareness raising on the importance of keeping moving, hydration and nutrition in reducing the risk of falls. Carers and professionals also emphasised the value of practical advice on how best to help the PWD get up after a fall.
Outcome measures
Assessing outcomes was seen as challenging in the context of a degenerative condition, particularly where interventions took place over an extended period of time. For example, one exercise programme lasted for 9 months and the high dropout rate for PWD made it difficult to evaluate programme effectiveness. Six services reported using their own judgement as opposed to a formal measure, and three relied on the number of falls to assess outcomes. Fifteen formal outcome measures were identified by participants (see Appendix 7). These focused on functional ability, quality of life, goal-setting, psychological well-being and satisfaction. Functional measures were most commonly used, with the Barthel Index31 and Tinetti Balance Assessment30 being the most common. Although goal-setting was used by five participants, none used specific measures relating to goal-setting. These findings indicate a wide range of outcome measures that could potentially be relevant in evaluating the outcomes of new interventions, although not all may be appropriate for PWD.
Discussion
The findings suggest that improving outcomes after a fall depends on recognising and facilitating the rehabilitation potential of PWD. The integrative analysis of all qualitative data identified three key areas that need to be addressed by the intervention to ensure that this aim is achieved in practice.
The first of these was ensuring that services are organised in the most effective and supportive way for PWD. This included having clear and efficient care pathways for PWD following a fall; evidence from other contexts suggests that this can improve care outcomes. 78,79 Improving links between services, particularly across health and social care boundaries, could also be beneficial, although this remains challenging in the UK. 80 Flexibility in service organisation to allow care to be tailored to the individual needs of PWD was seen as particularly important. This included varying the number, frequency or duration of intervention sessions, as well as tailoring the content of the intervention to individuals. Evidence exists to support such a tailored, person-centred approach to rehabilitation following hip fracture,81 although further research is needed.
The attitudes, knowledge and skills of professionals working with PWD was another key area to be addressed. Developing a positive, rather than fatalistic, attitude towards PWD was also important; again, the presence of negative attitudes was congruent with previous research in this area. 82,83 Few health and social care professionals had received formal dementia training, a finding reported by several previous studies of staff delivering care following hip fracture. 84–86 We identified a need for staff delivering the intervention to have both theoretical and practical understandings of dementia. In particular, practical skills in communication, observation, engaging PWD and tailoring and embedding interventions are required. In addition, staff require creativity and resourcefulness in adapting their approach to suit individual PWD.
The final key area was supporting carers and their role in interventions. This included ensuring that carers had the capacity and willingness to provide any assistance required by the intervention, and finding other solutions if not. Support, training and education for carers was seen as an important factor in improving this capacity.
Strengths and limitations
The strengths of the study were the inclusion of multiple perspectives, in particular being able to observe staff with differing levels of experience and interest in dementia care. The direct observation of service delivery highlighted a range of issues relating to communication skills that were not emphasised during interviews. This is not surprising because considerable work has highlighted the reluctance of older people to criticise services. 87 Staff were not always aware of their own communication styles or need for training.
We experienced a number of recruitment difficulties, with two sites (social services and a mental health trust) being unable to recruit any participants for observation or patients and carers for interview. Coupled with lower than anticipated recruitment via the diary study, this resulted in relatively small numbers of PWD and carers being included. With the observational data, it is important to acknowledge that we observed only a snapshot of existing services. Although some of the sessions we observed suggested a lack of attention to engagement and embedding, we do not know the extent to which such work had previously taken place or was planned for the future.
Conclusions
Most professionals participating in this initial exploratory work felt that it was both possible and desirable to develop an intervention to improve the outcome of fall-related injuries in PWD. A range of suggestions for improving existing services and potential components of a new intervention were identified, although some of these may fall outside the scope of the intervention to be developed, and the full range of ideas were presented to the consensus panel as part of WP3 (see Chapter 6). Additional information for the consensus panel was obtained through a realist review, which built on the qualitative analysis, as described in Chapter 5.
Chapter 5 Realist review and theory generation

Parts of this chapter have been adapted from Wheatley et al. 88 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
Given the small number of articles included in the effectiveness review, we additionally used a realist approach89 to synthesise the current evidence regarding the management of falls in dementia, to build on the qualitative work reported in Chapter 4 and to develop a theory regarding how a new intervention might work. 90,91 The aims of the realist review were to identify the essential components of an intervention aimed at improving care for PWD with fall-related injuries and to hypothesise the mechanisms underpinning successful falls prevention and/or rehabilitation interventions for PWD.
Methods
Realist methodology involves the development of context–mechanism–outcome configurations (CMOcs), which aim to identify what works (mechanism), for whom and in what circumstances (context) to achieve a particular intervention outcome. 89
We used an iterative approach to the review, integrating data from the semistructured interviews and focus groups with professionals (described in Chapter 4) with literature searches to develop and refine our emerging theory to underpin the intervention. This was a pragmatic decision based on the intervention time scales and the availability of qualitative data, as research approvals were not yet in place for interviews and observation with PWD and carers.
The initial phase involved the development of ‘if–then’ statements from the qualitative data by the qualitative team. 92 We then refined the if–then statements, looking for data that could be interpreted as a context, a mechanism or an outcome. Initial CMOcs were presented to a group of clinician co-applicants working on the realist review and further refined using their feedback. This preliminary CMOc framework formed the basis for extracting data from the literature.
Search strategy
Multiple searches were inductively carried out. An initial comprehensive search (in November 2015) focused on dementia, falls and fall-related injuries, and interventions. Subsequent targeted searches (in March 2017) were conducted for particular areas where the research team felt insufficient information had been found. Additional papers were identified through citation chaining of included papers and relevant systematic reviews and through hand-searches. All searches were restricted to the English language. Figure 7 demonstrates the flow of studies in these multiple, iterative searches.
FIGURE 7.
Diagram of the search, screening, selection and extraction process. Reproduced with permission from Wheatley et al. 88 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original.
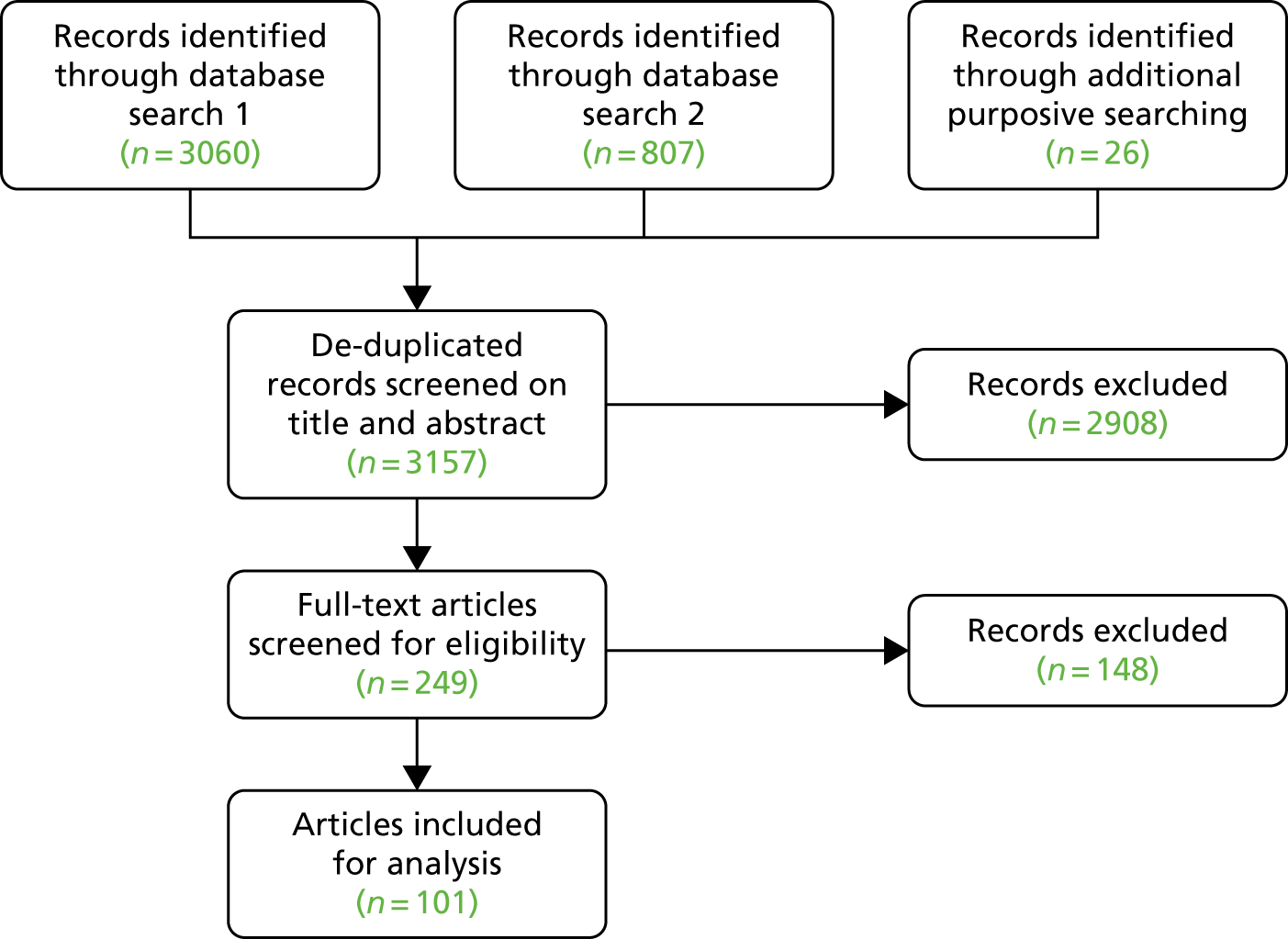
Search 1: comprehensive search
Electronic searches were conducted in MEDLINE, Cochrane Central Register of Controlled Trials, Health Management Information Consortium, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science, Allied and Complementary Medicine Database and Physiotherapy Evidence Database (see Appendix 8 for an example of the search strategy). This search was designed around three distinct concepts: dementia, falls and fall-related injuries, and interventions. This was translated to other databases, making appropriate adjustments for individual thesaurus headings and syntax. Citation chaining was used for included papers and relevant systematic reviews to identify additional papers of interest. Trials registers were searched, but further grey literature searching was not conducted. Results from all databases were imported to EndNote.
Search 2: targeted searches
Targeted searches for CMOcs 1, 2, 4 and 6 (see Table 7) took place in MEDLINE and CINAHL on EBSCOhost (see Appendix 9 for an example targeted search strategy). The major subject heading field was used to identify studies that were focused on dementia or older people and relevant CMOcs.
Data extraction and theory generation
Data were extracted from the included papers using a bespoke online form. This form included questions about the methodology of each study, including information on rigour using the Mixed Methods Appraisal Tool,93 details of any intervention described in a study based on the Template for Intervention Description and Replication (TIDieR) framework94 and space to extract evidence relating to the initial (or new) CMOcs. Data from each paper were extracted by two reviewers independently: one clinician and one non-clinician. The extracted data were presented for discussion at a team meeting and any disagreements between reviewers were resolved. The qualitative team then analysed and summarised the data relating to each CMOc. Following this process, the wording of each CMOc was refined as appropriate. The process was repeated for additional papers identified through targeted searches and citation chaining.
Results
We identified nine CMOcs, which we grouped into three themes (Table 7). 88 Evidence relating to each of these themes is described in the following sections. At the end of this section, we provide a table indicating the intervention components suggested by the CMOcs (see Table 8).
| Themes from realist review | CMOcs | Theme and subtheme from qualitative work |
|---|---|---|
| Ensuring that the circumstances of rehabilitation are optimised for PWD | CMOc1: managing pain | Addressing barriers to participation |
| CMOc2: ensuring a supportive environment | Addressing barriers to participation | |
| CMOc3: recognising and treating comorbidities | Treating factors contributing to falls | |
| Compensating for the reduced ability of PWD to self-manage | CMOc4: tailoring interventions and embedding in everyday life |
Making interventions meaningful and enjoyable Embedding interventions into daily life |
| CMOc5: providing ongoing support | Flexible service delivery | |
| CMOc6: involving carers in intervention delivery | Negotiating carer role in intervention | |
| Equipping the workforce with the necessary skills and information to care for this patient group | CMOc7: developing a detailed understanding of the patient | Addressing barriers to participation |
| CMOc8: upskilling the workforce |
Attitudes to dementia Creativity and resourcefulness Communication skills Ongoing training and support |
|
| CMOc9: improving pathways and referral |
Enhancing pathways Information-sharing |
Ensuring that the circumstances of rehabilitation are optimised for people with dementia
Context–mechanism–outcome configuration 1: managing pain
Studies show associations between pain and poorer mobility and physical functioning in people with cognitive impairment or dementia. 95–97 Pain is also linked with sleep disturbance, leading to impaired cognitive function as well as increases in depression, aggression and agitation in PWD. 98–102 This evidence suggests that PWD who are in pain may benefit less from an intervention owing to difficulties with mobility and reduced compliance stemming from pain and increased behavioural issues.
However, one study of a physician recommendation tool aimed at reducing delirium following hip fracture repair in those aged ≥ 65 years, including PWD, found that adherence to pain management recommendations was lower than adherence for other recommendations, for example those relating to fluid/electrolyte balance or early mobilisation. 103 Round-the-clock paracetamol recommendations were made 40% of the time, with an adherence rate of 32%; this was the lowest of all reported adherence rates. The authors did not provide enough information to elucidate why the adherence rate was so low for pain management, although the study suggests that hospital staff may underestimate the importance of pain management for recovery.
Context–mechanism–outcome configuration 2: ensuring a supportive environment
The environment surrounding a person with dementia can have a significant impact on their well-being, and hospitalisation may result in a deterioration in patients’ health. 104 Moreover, PWD may find it difficult to ask for help with their basic needs, such as hydration, which may lead to these being overlooked by hospital staff. 105 However, several hospital-based studies that aimed to reduce length of stay by facilitating discharge were not effective. 41,106,107 Authors speculated that sparse dementia care facilities in the community contributed to the lack of improvement in length of stay. 106
Although PWD exhibited poor adherence to home-based rehabilitation in one study,34 other studies suggest that home-based rehabilitation for people with cognitive impairment and hip fracture could be feasible for at least some patients. 108–110 Factors affecting adherence to exercise interventions in the home environment include recommendations from health professionals, the perceived value or benefit of the intervention, attitudes towards (structured) exercise, the presence of a tangible measure of adherence (such as an exercise recording sheet) and carer burden and support, as well as external factors such as bad weather or absence from the home. 111
Context–mechanism–outcome configuration 3: recognising and treating comorbidities
Older patients with hip fractures often have associated comorbidities – including thinking, moving and mood disorders – that may go unrecognised and increase mortality risk during and after hospitalisation for the fracture. 105,112,113 The need for holistic assessments to discover and manage falls risk factors emerged as an important theme from the literature. Moreover, identifying patients with dementia in itself can be challenging, with the result that cognitive impairment and delirium may go undiagnosed. 105 Including routine assessment of cognitive function in holistic assessment is, therefore, essential. 112 A structured assessment, such as CGA, was shown to improve outcomes for older people who have fallen;103,113,114 however, an advisory CGA model was not effective. 41 Where no holistic assessments were used, authors reflected that they may have aided patient and carer understanding of the causes of falls and helped to address other health issues that otherwise affected therapy. 115,116
Few papers specifically addressed psychosocial elements of assessment; two addressed depression and mood disorders as comorbidities113,114 and one paper included social isolation. 114
Compensating for the reduced ability of people with dementia to self-manage
Context–mechanism–outcome configuration 4: tailoring interventions and embedding in everyday life
Cognitive impairment might affect the ability of patients to comply with instructions and, consequently, their rehabilitation success. 34,41,103,106,108,113,114,117–119 Individually tailoring exercises to the physical and cognitive abilities of PWD was described as ‘vital’ to successful interventions for this patient group,109 although this required specialised training for staff and carers involved in intervention delivery. 109,120
Difficulty understanding and complying with instructions was not always linked with poor performance: in one study of physical training in dementia, not being able to remember the instructions did not seem to have an impact on the outcomes of interest. 119 Procedural memory may be intact in some PWD, meaning that they could learn skills and procedures crucial to their rehabilitation success. 114 Similarly, making activities and exercises person centred and relevant to the lives and interests of PWD (e.g. by building golf into an intervention for a golf enthusiast) was suggested as a way of overcoming poor adherence. 109 Training carers to function as ‘co-therapists’ at home could also help to overcome limited learning ability in cognitively impaired people. 115
Context–mechanism–outcome configuration 5: providing ongoing support
Evidence suggests that maintenance of progress is important for rehabilitation in all patient groups. In older adults, ‘failure of rehabilitation’, including deterioration, further falls and inability to cope, can be a common cause of hospital readmission, accounting for 24% of readmissions following hip fracture surgery. 116 Despite this, none of the intervention studies reviewed included long-term follow-up care. One hip fracture rehabilitation trial specifically recognised that cognitively impaired patients may have difficulty maintaining their improvements; although follow-ups had been planned to test this, the outcomes of the follow-ups were unclear. 114 A review of falls prevention interventions for older people found that, on average, only half of participants adhered to interventions after 12 months; the authors recommended increased follow-up appointments and implementing guidelines on promoting falls prevention interventions. 121
Context–mechanism–outcome configuration 6: involving carers in intervention delivery
The involvement of family carers is recommended to improve adherence and outcomes of interventions, although there is little evidence as to the mechanism by which this takes place. 113–115,122 However, this relies on an implicit assumption that carers have capacity to assist in intervention delivery. Caring for a person with dementia can be very difficult and stressful for family members, and many report feeling isolated, helpless and overstretched from providing care as well as dealing with their own health problems. 104,115 Thus, an intervention needs to take carers’ other commitments into account and have realistic expectations of carers. 86 This might involve exploring concerns about time requirements and disruption to routines. 123 Further barriers to the involvement of carers in interventions include the difficulty of acknowledging that they need help123 and negative attitudes of other family members. 124
Factors that could facilitate carer involvement in interventions and help ease carer burden include explicitly discussing potential benefits to PWD and carers,111,123 including benefits to carers of increased exercise125 and participation in daily activities. 126 Activating social support networks, engaging secondary carers to increase carer resources, or implementing peer support may also help to increase carer engagement with interventions,127–129 although one study found that carer burden was unrelated to additional helpers. 130 Additional factors include engaging with carers to uncover the causes of falls131 and offering the intervention at the right time,123 although the latter may be difficult to achieve in practice. Finally, it is important to recognise the heterogeneity of carers and to tailor interventions appropriately to them as well as to PWD. 120,132–135
Evidence suggests that carers may have a fatalistic view of falls and feel that little can be done to stop them occurring. 131 Fear of the care recipient falling can lead to behaviours that negatively influence the relationship between the carer and the care recipient. 104
Equipping the workforce with the necessary skills and information to care for this patient group
Context–mechanism–outcome configuration 7: developing a detailed understanding of the patient
A number of studies explicitly recognised that PWD may struggle with giving full and accurate histories. 105,107,116 Strategies such as gathering information from additional sources, such as carers105 or patients’ GPs,107 in addition to patient’s own words, were used. A qualitative study of carers for people with AD found that patients often relied on their carers to remember the facts surrounding their falls rather attempting to remember this information themselves: couples had ‘joint memories’ of a fall, and carers would prompt the patient’s recollection or confirm the patient’s account. 104
A clinician–carer communication tool called TOP 5 engaged clinical staff in a structured process with carers to record tips and strategies to aid personalised care of patients with dementia. 117 Carers reported high satisfaction with the way that clinicians had used the TOP 5 strategies and the majority of both carers and clinicians perceived that patients were less agitated and distressed, although no data were given on the patients’ satisfaction with the care received. The study also reported a decrease in the use of restraints and antipsychotic drugs in managing PWD. Data on fall rates were difficult to interpret owing to limitations in the study design.
Context–mechanism–outcome configuration 8: upskilling the workforce
Several authors recognised the value of providing specialised training of staff to work with older adults and PWD,41,107,116,117,136 although few authors provided detailed information on the content of such training. An ‘education unit-centred model’, which aimed to familiarise non-specialist staff with the specifics of geriatric care, was suggested for transferring expertise between specialists and non-specialists. 41 Training in engaging with carers was required for successful uptake of the TOP 5 intervention. 117 In addition, negative attitudes towards PWD were reported. 104,114,120,133–135
Context–mechanism–outcome configuration 9: improving pathways and referral
No papers were identified that explicitly discussed a lack of knowledge about local care pathways as a barrier to the care of PWD with fall-related injuries. However, there were a number of relevant issues in relation to resourcing, collaborative working and referrals to other services. Collaboration between professionals emerged as an important factor in whether or not patients received effective treatment. 112 Three studies described delegation of work previously undertaken by physicians to nurse practitioners and other health-care providers; these new processes of care were successfully integrated for two interventions,137,138 but another attained low referral rates relating to poor documentation by physicians and poor uptake by patients. 136
Few data were available concerning the processes through which decisions were made about referrals. Some authors speculated that a range of social and contextual factors may influence decisions to refer patients to new interventions and services, including lack of confidence in the service provided, reluctance to share responsibility for patient care or a perception that the patient would not benefit from the service. 136 Another study reported that patients with dementia and the oldest old received less occupational therapy,116 suggesting that preconceptions about patients may also influence decision-making, although this may also reflect patient reluctance to engage with the intervention. 136 The availability of resources may also influence the ability of physicians to discharge patients to other services. 106
Context-mechanism-outcome configurations and supporting evidence
The CMOcs presented in Table 8 were developed using both the qualitative data presented in the previous chapter and the data from the indicated papers.
| CMOc | Context | Mechanism (resource) | Mechanism (reasoning) | Outcome | References |
|---|---|---|---|---|---|
| CMOc1: managing pain | Cognitive impairment may limit the ability of PWD to articulate pain | Staff use non-verbal pain signifiers and/or give blanket pain relief | PWD are not in pain | Capacity to engage with an intervention increases | 95–103 |
| CMOc2: ensuring a supportive environment | Cognitive impairment may limit the ability of PWD to adapt to and cope with new environments | Intervention assessment and delivery takes place in appropriate, accessible and familiar environments | PWD feel comfortable and less distracted | Anxiety and challenging behaviours are reduced | 34,41,104–111 |
| CMOc3: recognising and treating comorbidities | The role of comorbidities may be underestimated in dementia | Holistic biopsychosocial assessment is employed | Staff understand the range of factors contributing to falls and are able to treat comorbidities more effectively | Falls risk may be reduced and recovery enhanced in patients with dementia | 41,103,105,106,112–116 |
| CMOc4: tailoring interventions and embedding in everyday life | Cognitive impairment may limit the ability of PWD to comply with instructions and form habits | Staff tailor the intervention (e.g. exercises) to the circumstances of PWD and embed it in their existing routines | Intervention becomes routine and habitual | More successful rehabilitation can be achieved | 34,41,103,106,108,109,113–115,117–120 |
| CMOc5: providing ongoing support | Cognitive impairment may limit the ability of PWD to self-manage changes in circumstances | Ongoing follow-up is provided | Staff are able to reinforce previous interventions and adapt them to meet changing needs | Improvements in mobility are sustained and new falls risks reduced | 114,116 |
| CMOc6: involving carers in intervention delivery | The burden on carers is high when caring for relatives or friends with dementia who are at risk of falling | Carer support and education is provided | Carer stress is reduced and skills increased | Carers’ capacity to assist with the delivery of interventions increases | 86,104,111,113–115,120,122–135 |
| CMOc7: developing a detailed understanding of the patient | Cognitive impairment may limit the ability of PWD to pass on information | Staff use multiple sources of information including carers and direct observation | Staff gain a better understanding of the individual | Staff are able to provide appropriate, tailored care | 104,105,107,116,117 |
| CMOc8: upskilling the workforce | Current staff knowledge of, and attitudes to, dementia are variable | Increased dementia training is provided | Staff gain skills in and understanding of rehabilitation for PWD | Staff ability and willingness to engage with PWD is enhanced | 41,104,107,114,116,117,120,133–136 |
| CMOc9: improving pathways and referral | Care pathways are often unclear | A centralised, collaborative pathway is developed and disseminated | Staff are better equipped to refer to the most-appropriate services | Service users receive better treatment | 106,112,116,136–138 |
Discussion
The findings of the realist review suggest a number of important components of interventions for fall-related injuries in PWD, as well as potential mechanisms underpinning successful interventions for this patient group. These are represented by the nine CMOcs, which were further grouped into three broad themes: (1) ensuring that the circumstances of rehabilitation are optimised for PWD, (2) compensating for the reduced ability of PWD to self-manage and (3) equipping the workforce with the necessary skills and information to care for this patient group. Drawing on the data relating to each of these themes, we suggest a number of components for inclusion in the final intervention (see Table 8).
Optimising the circumstances of rehabilitation for PWD means ensuring that basic needs, such as appropriate food, water, comfort and pain relief, are met. Given the evidence that suggests lower adherence to recommendations relating to pain relief,103 the intervention needs to consider strategies to engage health-care professionals in managing pain. This might include training for staff on recognising pain and/or ensuring that staff have the necessary permissions to prescribe or administer pain relief. Locating the intervention in a familiar environment such as the home may also facilitate engagement. Home-based rehabilitation may potentially produce good results,108–110 and pre-empting potential problems with advice (e.g. to continue to do exercises while on holiday) could help to increase adherence. If the home is not deemed suitable, specialised rehabilitation units designed for the needs of PWD may be more appropriate than general wards. 108,139 Initial structured, comprehensive assessment emerged as important to ensure that comorbidities were correctly identified and treated. However, the relative lack of emphasis on psychosocial elements of assessment in the studies examined suggests that staff training may be required to stress the importance of holistic assessment and emphasise aspects such as social support networks as part of discharge procedures. Ensuring that MDTs include experts in mental health care as well as physical health could be another potential way to address this issue. Routine assessment of cognitive function may also be necessary to ensure that dementia is correctly identified. 105
Compensating for the reduced ability of PWD to self-manage requires designing rehabilitation strategies around repetition and embedding interventions (e.g. exercises) into existing habits and routines. Indeed, patient memory and understanding may not be necessary. 114 The comprehensive initial assessments recommended in the previous theme (ensuring that the circumstances of rehabilitation are optimised for PWD) should ideally be used to tailor exercises and other treatments to daily life, including flexibility in the timing and duration of the intervention. A staff training component may be required to teach embedding and contextualising techniques. Failure of rehabilitation is a common cause of readmission,116 which the implementation of regular follow-up visits or a longer initial intervention period may help mitigate. Many studies have relied on input from carers to help compensate for the reduced ability to self-manage in PWD,113–115,122 but this may place an additional burden on carers. Therefore, the intervention should involve carers in the decision-making process and include strategies to aid with carer burden and stress, such as support and training for carers. Providing patients and carers with information about falls risks and prevention may promote their self-efficacy, reduce their anxieties and increase their skill in managing falls.
The theme of equipping the workforce with the necessary skills and information to care for PWD draws on elements from across the findings. Although the importance of information gathering for tailoring has already been identified, it may also be valuable to ensure that multiple sources are used when gathering information. Formalising collective memories of PWD and carers104 may help to ensure that care is person centred, for example by using a model similar to the TOP 5 strategy. 107,117 Again, the extra burden placed on carers should be considered. The intervention should provide staff training in dementia, including strategies to manage challenging behaviour. Staff should be challenged on their preconceived attitudes towards older adults and those with dementia to better understand their needs and goals. Training should cover the dementia-specific adaptation to practice described in the previous themes, with a particular focus on communication. In order to create continuity of care, staffing for the intervention should be consistent. Delivering the intervention through multidisciplinary, collaborative teams may also encourage information-sharing. Based on the lack of knowledge of local services identified in the qualitative study, the intervention should also provide education and pathway resources for staff to encourage referral to other services. However, the available evidence106,116,136 suggests that a simple lack of knowledge of services in staff members may not be the only barrier to the use of care pathways. This is a further area in which improving staff attitudes may be of value.
Strengths and limitations
A strength of this review is the integration of literature and qualitative data. A realist approach also allowed us to consider and synthesise a broader range of evidence, which was important in the light of the limited evidence identified in the effectiveness review (see Chapter 2). The review is limited by the lack of published evidence found to support concepts that were clearly articulated in the qualitative data, such as in CMOc5 (providing ongoing support). Further research is needed in these areas. Owing to time limitations, we were not able to conduct additional targeted searches for all CMOcs. The searches were carried out between November 2015 and March 2017 and have not been updated; thus, this chapter reflects the evidence that was presented to the consensus panel to inform the development of the intervention.
Conclusions
Through the use of realist methodology to examine the qualitative data from health and social care professionals as well as the existing literature, we were able to develop theories around what would be required in a successful new intervention for PWD with fall-related injuries. These theories were presented to a panel of experts as part of the Delphi consensus process, which is described in Chapter 6.
Chapter 6 Prioritising, operationalising and validating intervention components
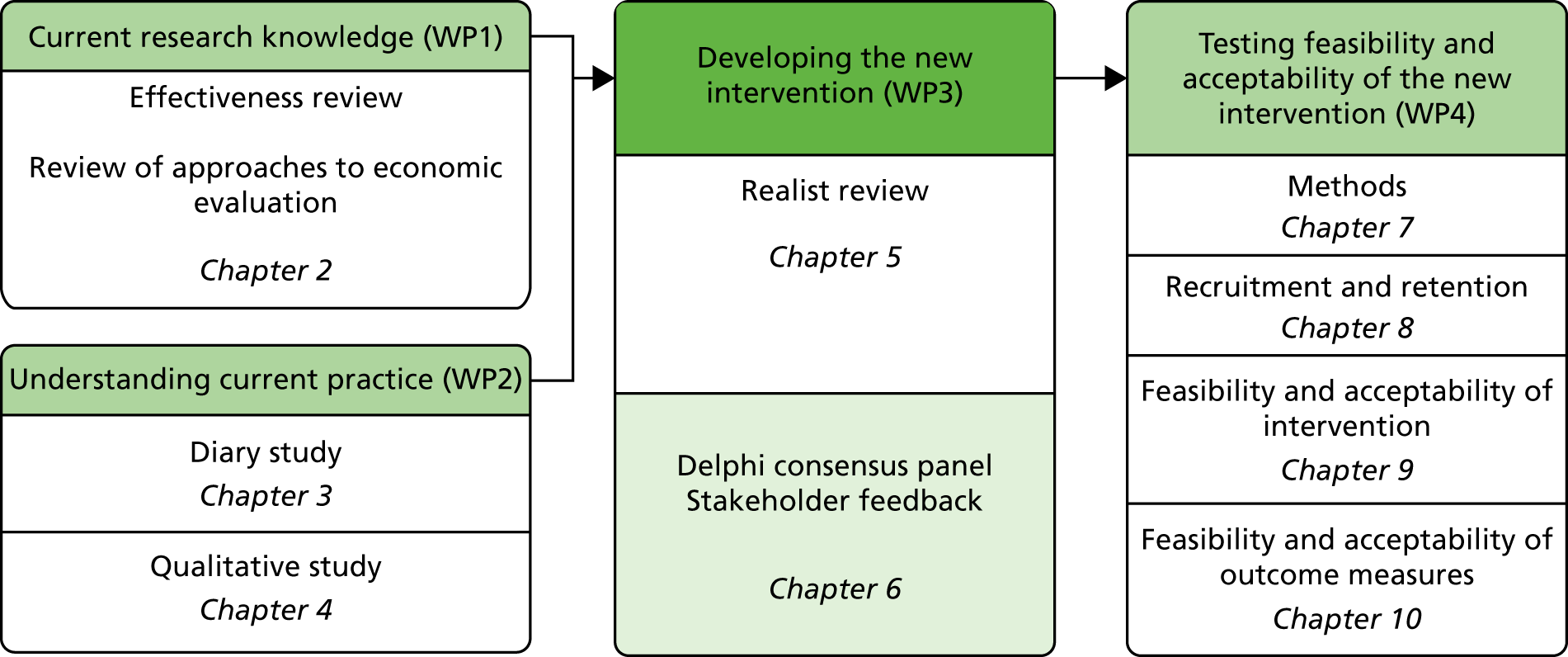
Parts of this chapter have been adapted from Wheatley et al. 88 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
The reviews, diary study and qualitative work reported in previous chapters suggested a range of potential components for a new intervention for fall-related injuries in PWD. The diary study (see Chapter 3) additionally provided insight into the practicalities of recruitment to such an intervention. The objectives of the final stage of intervention development were to:
-
prioritise and operationalise intervention components
-
consider feasible sources for recruitment and appropriate targets
-
agree outcome measures to evaluate the clinical effectiveness and cost-effectiveness of the intervention
-
validate the proposed intervention through qualitative work with stakeholders
-
produce a logic model summarising the rationale underpinning the intervention.
The results of this development process have been published88 and are elaborated below.
Methods
The first three objectives were addressed by convening a consensus panel that participated in two meetings and completed two rounds of a Delphi survey. 140 The proposed intervention was validated through qualitative interviews and focus groups with a range of stakeholders; the logic model was then produced by members of the research team and reviewed by the Trial Oversight Committee (TOC).
Consensus panel
We convened a panel of 24 health and social care professionals with expertise in falls and/or dementia. The expertise represented included geriatric medicine, old-age psychiatry, emergency medicine, physiotherapy, occupational therapy, general nursing, mental health nursing and social work. The panel met twice (in March 2017 and June 2017). Prior to the first meeting, the panel received summaries of the effectiveness review (see Chapter 2), the qualitative work (see Chapter 4) and the realist review (see Chapter 5). At the first meeting, members were split into groups facilitated by a member of the research team to discuss:
-
the feasibility and setting of the proposed feasibility study
-
the content of the intervention
-
outcome measures for the intervention.
Groups reported back to each other and areas of initial agreement and dissent were identified, together with recommendations regarding the content and design of the proposed intervention.
The second meeting followed a similar format, with presentations by the research team followed by group discussions and feedback. The key topics discussed at the second meeting were:
-
the details of the proposed intervention (including the proposed roles of the physiotherapist, OT and geriatrician)
-
the final report on the diary study
-
stakeholder feedback on the proposed intervention.
Small-group discussions were facilitated as at the first meeting and groups fed back key points. All discussions at both meetings were audio-recorded with participants’ consent and transcribed for analysis.
Delphi surveys
In between the two face-to-face meetings, we used a modified Delphi panel approach140–142 to achieve consensus on the intervention. This approach ensured that the design of the new intervention took account of the full range of stakeholders’ views and not just those of the research team or those who were most vocal in the meetings.
A threshold of two-thirds of stakeholders being in agreement was the target chosen to represent consensus for issues refined through iterative rounds. Only the independent moderator (Beth Edgar, non-participant in the survey) was able to access identification details of respondents. We hoped that the anonymity of responses would facilitate free expression of opinion throughout the study.
Members were asked to respond to specific questions regarding feasibility of the setting, staffing and other components of the complex intervention as well as outcome measures for the feasibility study. After each round, the research team summarised comments and presented these together with the proportion agreeing with each question back to the panel. Questions were refined on the basis of the feedback and a second round of the survey was sent to participants. Following the second round, consensus was achieved on the majority of questions, and outstanding areas of disagreement were discussed in the second panel meeting. Consensus statements are given in Appendix 10.
Stakeholder feedback
Between the two consensus panel meetings, stakeholder feedback was sought on the emerging intervention. Participants were identified from those who took part in the earlier qualitative work, together with additional professionals recruited via local contacts and patients and carers from the North East and North Cumbria Clinical Research Network Case Register. We summarised the key components of the intervention and presented them as the basis for discussion. Interviews and focus groups were audio-recorded and transcribed for analysis. A summary of the key findings was presented to the consensus panel at the second meeting.
Collation of results and logic modelling
The results of consensus panel discussion and stakeholder feedback, along with the set of agreed consensus statements, were collated and used to finalise the protocol for the feasibility study and to model the intervention. We developed our model by adapting existing logic model templates. 143,144 The model was developed by the qualitative team and reviewed by the TOC.
Preparation of intervention materials and manual
Resources identified in the intervention-modelling process (i.e. the assessment document and manual) were developed by the research team with reference to the final consensus statements, protocol and logic model. Additional training materials for presentation to intervention staff were derived from the manual.
Results
Consensus results
Feasibility and setting
Panel members critically discussed the inclusion criteria. They expressed views that were similar to those of the stakeholders interviewed in WP2 (see Chapter 4, Recruitment and consent) that including only injurious falls was too restrictive, instead suggesting:
Do we want to redefine that then? By something like it’s a fall which is sufficient to a lower secondary service or telecare or some agency or the GP. You know, it might not be injury directly but it’s been serious enough to require an intervention.
Panel member 22
Other eligibility criteria were also discussed, with some panel members arguing that anyone with a fall in the previous 6 months should be included. This issue was included in the consensus survey to gauge the views of all members.
Content of the intervention
Consensus panel members were also asked to prioritise potential intervention components suggested by the qualitative data and realist review, as well as indicating an opinion on their feasibility (see Appendix 11). At this stage, no suggested components were rated as undesirable. Supporting carers was seen as both essential and feasible by the majority of respondents. A number of participants felt that some suggested components were not feasible within the confines of current resources; in particular, this included introducing proactive maintenance/follow-up, ensuring consistency in staffing for the intervention and tailoring the timings of intervention sessions to fit with participants’ routines and daily rhythms. There was uncertainty around the feasibility of introducing system-level changes and some aspects of engaging PWD. This information informed the development of the consensus survey. Following the surveys, a set of consensus statements was agreed by the panel (see Appendix 10). A response rate of 58% was achieved for the consensus survey in round 1 and 54% was achieved for the consensus survey in round 2.
There was some discussion of the proposed content of the training to be delivered as part of the intervention. The UK Department of Health and Social Care has funded the Dementia Training Standards Framework with the aim of supporting the development and delivery of appropriate and consistent education and training on dementia for the health and care workforce. 145 This consists of three tiers of training: training targeted at all of those working in health and social care settings (tier 1), staff with regular contact with PWD (tier 2) and experts working with PWD (tier 3). The consensus panel agreed in the Delphi rounds that staff delivering the intervention should receive tier 2 training.
Outcome measures for the intervention
There was considerable debate concerning the most appropriate outcome measure(s) for the feasibility study at the second meeting. A range of possible outcome measures were identified in small-group discussions (number of falls; time to first fall; goal achievement, functional abilities and quality of life). However, there was little consensus, and shortcomings were identified for most options. Although the number of falls is commonly used as an outcome measure, some panel members highlighted potential shortcomings of relying on this outcome:
I’m a physio[therapist] by background and what I really don’t like is that when someone comes in after someone’s had a fall and all the intervention is about reducing their falls risk, which means that people are terrified to get out. So they spend more time sitting in a chair and, yes, you reduce their falls risk, but [. . .] they’ve got pressure sores or they now cannot walk independently, because they’ve lost all their muscle mass that they would need to be able to do that. So I think it’s really important that we don’t go down that route of just focusing on the number of falls.
Panel member 23
The difficulties in collecting accurate information led to concerns over relying on other measures, such as time to first fall and goal-setting:
If time to first fall is going to be primary, how do we assess that, is it just a question of repeatedly asking the patient and hoping that they remember?
Panel member 04
I suppose part of it recognising like you say that the goal-setting is difficult sometimes in the later stages in terms of that dialogue sometimes. It’s then to use what would the person previously have chosen to do which is why I think it’s so important to get as much information from a range of resources as well.
Panel member 19
Because we were conducting a feasibility study, we did not need to specify a primary outcome measure. We therefore included most of the outcomes suggested by the panel: number of falls, quality of life, goal attainment and functional abilities (see Chapter 7).
Stakeholder feedback
Data were collected through focus groups with professionals (n = 13 across four focus groups), interviews with professionals (n = 2) and interviews with patients (n = 2) and carers (n = 3). Stakeholder feedback was generally positive and the majority of suggested intervention components were approved. No components were rejected outright, although a number of queries and suggestions were raised. These focused on assessment and MDT composition; intervention content and duration; staff training; and outcome measures (described in the following sections).
Assessment and multidisciplinary team composition
The concept of holistic assessment was welcomed by all stakeholders. Additional areas for assessment suggested by participants included foot assessment, pain assessment (using a formal tool), nutrition [using the Malnutrition Universal Screening Tool (MUST)146], frailty, existing equipment and aids, cognitive impairment and social circumstances. The quality of holistic assessment and ability to translate the results into practice was emphasised:
It is someone that needs to pick up the cues as well, so it needs to be that real holistic assessment. For example, incontinence, you know, you are not going to engage someone in an exercise programme, or encourage them to stabilise their gait, their balance or posture if actually their real problem is they are retaining urine. They are getting overflow, and when they stand up to go they have a real sense of urgency and they are desperate. You can put in every intervention you like. [. . .] But, actually they need specialist intervention around for getting that sorted out. Then, exercise intervention might really work.
Professional 122, pain nurse (focus group with care home practitioners)
The different perspectives and expertise of MDT members, as well as enabling access to notes held by different agencies, could potentially facilitate this process of translation. Additional staff suggested for inclusion in the MDT included a dietitian/nutritionist; Alzheimer’s Society (London, UK) carer support and/or outreach workers; welfare rights and advocacy advisers; a community psychiatric nurse; and a social worker (particularly if the assessment did not include social circumstances).
These additional assessments and staff were discussed at the second consensus meeting. The feasibility of organising MDT meetings was questioned, particularly in rural areas. However, it was suggested that virtual MDT meetings could be possible if concerns over security and encryption could be resolved. Some stakeholders suggested that patients should be reviewed by the MDT at the mid-point and/or towards the end of the intervention. This would ensure that referrals were under way and that services were in place to help with maintaining progress after the completion of the 12-week intervention. In addition, the MDT could act as a resource and suggest alternative approaches if staff were having difficulties in engaging PWD:
You may know early on whether what you’re doing is working. [. . .] If you get to that point and think, this isn’t going well, you can go back to all those people that you’re going to link into and make sure you plug into them.
Professional 31, manager, specialist dementia unit (interview)
Intervention content and duration
The intensity and duration of the intervention proved to be the most contentious aspects, with diverse views being expressed. Community-based professionals, particularly those in rural areas, raised some concerns over the feasibility of delivering this number of sessions both within and outside the context of a trial. The need to tailor the number and duration of individual sessions was also emphasised:
Because you need at least, you know, half of that time even strike up a rapport, for them to remember, possibly, who you are, for you to engage with the carer, and that’s before you’ve even done anything and before you’ve even assessed the person or given them any intervention. That’s every time, because every time is like a new time.
Professional 124, physiotherapist (focus group with community health and social care professionals)
Although a 12-week period was thought to be appropriate by some participants, others questioned whether or not this would be long enough for all referrals to have been acted on and for alternative services to have been put in place to provide ongoing support. Some carers expressed concerns about what would happen at the end of the intervention:
So, that would be my only concern. You’re leaving people, then, in limbo. You’re offering them something that isn’t there anymore. It was there, but oh, that’s not there now.
Carer 12 (interview)
Some PWD and carers felt that the proposed intervention seemed similar to services they had previously received and either would not benefit them or would be more appropriate for other people:
Carer 13 felt that the proposed intervention was very similar to what they had already had – OT has been and rearranged furniture, equipment such as stair lift installed, doctor has done a home visit to check medications, dietitian has been, memory clinic input.
Field notes of interview with Patient 13 and Carer 13
Although the components of the intervention appeared similar to this participant, key differences that were not necessarily apparent to them were the intensity of the intervention, focus on personal goals and staff training in dementia. At the time of the interviews, the intervention had not been finalised and this illustrates the limitations of the timing of the feedback interviews. Factors influencing stakeholder perceptions of the potential value of the intervention included the severity of their dementia or comorbidities such as Parkinson’s disease.
In terms of content, stakeholders emphasised the need to include mental and social stimulation in the programme of meaningful activities. Exploring the barriers (including cultural barriers) to meaningful activity was also suggested because this would inform how best to engage patients. Although professionals generally agreed with developing the intervention around individual goals, some carers and patients had reservations about this approach, highlighting concerns around safety:
I wouldn’t trust my dad to go down the shop. It depends, obviously, on the individual patient. But yes, I think, certainly safety and looking at that sort of thing.
Carer 12 (interview)
I don’t think I could do it. Like, make a cup of tea. I wouldn’t trust myself.
Patient 13 (interview with Patient 13 and Carer 13)
These comments confirm the importance of education on ‘positive risk’, an aspect of the intervention emphasised by professionals in the initial interviews. Another recommended addition to the intervention was training on harm minimisation and how to get up after a fall:
It is teaching them how to get up, different scenarios, what you would use if you were outside in the high street. If you were in the garden, if you are in the high street. If you were squashed between a wardrobe and a bed, how would you do it?
Professional 117, exercise class instructor, non-statutory sector (interview)
Staff training
Professionals supported the inclusion of staff training, in particular the need for training on tailoring interventions, engaging and motivating PWD:
I think what’s jumping out to me is the dependence on the staff training. From a list of interventions none of those are really, hugely, a step away from what we cover. But I know, definitely, still in our organisation staff still need to understand that you can’t deliver the same package to someone with a physical condition as to somebody with some challenges, whatever they are.
Professional 35, dementia and falls co-ordinator (focus group with community health and social care professionals)
Participants stressed the need to allow adequate time, to deliver training in ‘bite-size chunks’ and to supplement training with a summary of short, concise bullet points to aid retention. On-the-job modelling was identified as a valuable approach to staff training.
Outcome measures
In common with the consensus panel, some professionals questioned the use of number of falls as an outcome measure, particularly in relation to the varied needs of PWD and the idea that there might be a ‘ceiling’ for improvement in more advanced dementia:
. . . with some clients we may not be able to reduce the number of falls and the diagnosis of dementia in itself predisposes somebody to a high risk of falls, and also high risk of injury as well. But whether there are any injury-prevention measures we can focus on, because those sorts of things we may be able to protect against. There may be some protective factors, whereas we may not be able to reduce the fall threat.
Professional 124, physiotherapist (focus group with community health and social care professionals)
Logic model of final intervention
A logic model can be helpful in clarifying causal assumptions of interventions,143 and developing such a model is recommended by UK Medical Research Council (MRC) guidance. 147 We identified the resources required for the intervention, activities to be undertaken, anticipated outcomes and longer-term impacts for each of the three key stakeholder groups involved (professionals, PWD and carers) (Figure 8). The process of identifying the pathways through which we anticipated outcomes would be achieved helped to inform data collection for the process evaluation to be conducted as part of the feasibility study.
FIGURE 8.
Logic model of the DIFRID intervention. This figure is adapted from Wheatley et al. 88 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original.
Overview of the DIFRID intervention
The intervention comprised a tailored programme of activities centred on goals identified and agreed with the person with dementia and their carers. The intervention was delivered face to face to individual PWD and carers by trained rehabilitation staff [OTs, physiotherapists and rehabilitation support workers (variously called support workers, therapy assistants or assistant practitioners in different sites, hereafter referred to as ‘support workers’] at participants’ homes. Rehabilitation staff attended a half-day training programme that covered skills needed to work effectively with PWD, study procedures and all components of the intervention including assessment, goal attainment scaling (GAS) and activity-planning. The assessment included questions about participant likes and dislikes and daily routines to allow staff to develop a tailored programme of meaningful activities to achieve desired goals. The initial assessment comprised two sessions: one conducted by an OT, the other by a physiotherapist. Intervention sessions were mostly delivered by support workers, although there was scope for up to four visits from both the OT and the physiotherapist. Up to 22 intervention sessions in total were available over a 12-week period. The intervention is summarised using the TIDieR framework in Table 9. 94
| Item | Description |
|---|---|
| 1. Brief name: provide the name or a phrase that describes the intervention | DIFRID: Developing an Intervention for Fall related Injuries in Dementia |
| 2. Why: describe any rationale, theory, or goal of the elements essential to the intervention | Detailed qualitative work and a realist review have informed the underlying principles of the DIFRID intervention, which are:
|
| 3. What materials: describe any physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery or in training of intervention providers. Provide information on where the materials can be accessed (such as online appendix, URL) |
Training materials, staff manual, assessment and intervention document, patient diary Additional materials (e.g. exercise sheets) may be provided to participants at the discretion of individual therapists Materials can be obtained from the corresponding author |
| 4. Procedures: describe each of the procedures, activities, and/or processes used in the intervention, including any enabling or support activities | Each participant will receive a detailed, holistic assessment in their home including discussion of their likes, dislikes and personal goals, and an assessment of carer need. Following these assessments, each participant will be discussed at a MDT meeting to decide the most appropriate activity programme to achieve the participant’s goals, and make any referrals required. The participant will then receive intervention sessions at home in which their progress will be recorded and their programme of activities adjusted as required. At the mid-point and end of the intervention, goals will be reviewed and any additional needs discussed to arrange ongoing support on conclusion of the intervention |
| 5. Who provided: for each category of intervention provider (such as psychologist, nursing assistant), describe their expertise, background, and any specific training given |
OTs, physiotherapists and support workers Half-day training programme focusing on working effectively with PWD, assessment, goal-setting and developing tailored activity programmes |
| 6. How: describe the modes of delivery (such as face to face or by some other mechanism, such as internet or telephone) of the intervention and whether it was provided individually or in a group | Individual face-to-face sessions |
| 7. Where: describe the type(s) of location(s) where the intervention occurred, including any necessary infrastructure or relevant features | Participants’ homes |
| 8. When and how much: describe the number of times the intervention was delivered and over what period of time including the number of sessions, their schedule, and their duration, intensity, or dose | The intervention was designed to be flexible according to PWD needs. Each person with dementia could have up to four sessions with a physiotherapist, up to four sessions with an OT and up to 14 sessions with a support worker over a period of 12 weeks. Review sessions were scheduled at 6 weeks and 12 weeks |
| 9. Tailoring: if the intervention was planned to be personalised, titrated or adapted, then describe what, why, when, and how | Intervention to focus on goals set by the PWD and carer through a tailored programme of meaningful and enjoyable activities |
| 10. Changes: if the intervention was modified during the course of the study, describe the changes (what, why, when, and how). | No formal modifications were made |
| 11. How well – planned: if intervention adherence or fidelity was assessed, describe how and by whom, and if any strategies were used to maintain or improve fidelity, describe them | Intervention delivery was assessed through review of completed assessment documentation (LMA) and analysis of goals (AS) |
| 12. How well – actual: if intervention adherence or fidelity was assessed, describe the extent to which the intervention was delivered as planned | See Chapter 9, Feasibility and acceptability of the DIFRID intervention |
Discussion
The iterative process of developing the DIFRID intervention involved mixed methods and included a wide range of stakeholders throughout. This process allowed us to integrate practical empirical data from experts and practitioners with evidence from previous studies to create a robust, theoretically informed design for a new intervention.
Furthermore, the consensus panel provided access to a wide range of expertise. This facilitated decision-making where there was no clear direction from earlier WPs (e.g. outcome measures) or where problems had been identified (e.g. recruitment). The content of the Delphi survey was informed by the initial assessment of the desirability and feasibility of the components that emerged from the initial qualitative work and realist review. The Delphi approach allowed us to gauge the extent to which different components of the intervention were supported. 142
Stakeholder feedback on the proposed intervention was generally positive, although some participants found it difficult to comment as the intervention presented was still under development.
The production of CMOcs and the logic model helped us hypothesise about how change would happen and made assumptions explicit. 89 The logic model was also used to inform the process evaluation (see Chapters 8–10).
Strengths and limitations
A strength of the intervention development process was that it was iterative and included a range of stakeholder perspectives. However, the panel did not include PPI representatives or a range of social care professionals. Furthermore, we did not achieve full attendance at the two meetings. It is possible that different priorities would have been expressed had more perspectives been included. The inclusion of a range of professionals, PWD and carers to give feedback on the proposed intervention may have compensated for this. However, the timing of the stakeholder interviews and focus groups meant that the intervention was at a relatively early stage of development and the lack of supporting documentation (e.g. the assessment document) made providing feedback difficult for some participants. In the future, it would be beneficial for stakeholders to have the opportunity to review intervention documents.
Some of the issues raised by stakeholders and the consensus panel (e.g. shifting the focus away from injurious falls or including longer-term follow-up) could not be addressed in the feasibility study because they were outside the scope of the study brief or were unfeasible within the time available.
Conclusions
Using Delphi consensus techniques, we developed a new intervention to help PWD following a fall necessitating health-care attention. The feasibility of this intervention was tested in the next phase of the DIFRID project.
Chapter 7 Methods for a feasibility study of the DIFRID intervention

Parts of this chapter are adapted from Allan et al. 148 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
The process of developing the DIFRID intervention has been described in previous chapters. This chapter focuses on the methods and Chapters 8–10 focus on the key findings of a feasibility study of the intervention. The primary aim of the feasibility study was to determine whether or not to progress to a full-scale RCT of the DIFRID intervention to evaluate its efficacy and cost-effectiveness in preventing falls and improving secondary outcomes for PWD who have experienced a fall necessitating health-care attention. The specific objectives were to explore the feasibility and acceptability of:
-
recruitment and retention
-
implementation of the DIFRID intervention
-
proposed outcome measures.
Mixed methods were used and the full protocol for the study has been published elsewhere. 148 A summary of the quantitative methods is provided in the following section, followed by a description of the embedded process evaluation in Process evaluation. The findings for each objective of the feasibility study are then reported in Chapter 8 (recruitment and retention), Chapter 9 (implementation of the DIFRID intervention) and Chapter 10 (outcome measures).
Quantitative methods: recruitment and consent
This study was designed to be was a single-arm feasibility study of the DIFRID intervention.
Target sample size and eligibility criteria
The sample size for the feasibility study was agreed by the expert consensus panel to be 35 participants and subsequently reduced to 30 by the TOC. This decision reflected the TOC’s expertise as to how many participants would be needed to measure feasibility outcomes, balanced with the time for recruitment available and the likely potential recruitment rates estimated from our observational work in an earlier stage of this research programme. It was anticipated that a total of 30 PWD and 30 carers would provide sufficient data to answer feasibility questions including estimation of potential recruitment rates, intervention adherence and rates of completion of data-collection tools.
Recruitment criteria were similar to those for the diary study described in Chapter 3. However, in the light of the recruitment difficulties experienced in WP2, two key changes were made.
The first change related to the nature of the fall. Although stakeholders and the consensus panel had argued that any falls should be eligible, this was considered too great a deviation from the initial research brief for the project. However, with the agreement of the TOC, we modified the eligibility criteria to include any falls for which health-care attention had been sought; this could include contacts with NHS 111 (a free-to-call single non-emergency-number medical helpline operating in England and Scotland), a district/practice nurse or minor injuries unit, in addition to presentation to any of the services directly involved in recruitment.
The second change concerned the recency of the fall. By including any falls that had occurred in the 1 month prior to a patient’s identification as a potential study participant, we hoped to facilitate recruitment. The remaining inclusion criteria were unchanged. Briefly, participants were required to:
-
have a known diagnosis of dementia (see Chapter 3, Inclusion criteria)
-
have had a fall using the definition of an index fall provided in Chapter 3, Inclusion criteria
-
be dwelling in the community at the time of the index fall and returning to the community at the time of the intervention
-
have a carer available to assist with completion of the diaries
-
have capacity to consent to participation, or have a personal or nominated consultee able to give an opinion on the participation of the person with dementia.
As in the diary study (see Chapter 3, Exclusion criteria), potential participants were excluded if:
-
a diagnosis of dementia could not be confirmed by the primary care team within 2 weeks
-
they were dwelling in residential or nursing care, or were a hospital inpatient at the time of the index fall
-
they refused consent, or lacked capacity and either did not have a personal/nominated consultee or their consultee declined participation
-
they were unable to communicate in English
-
their carer declined participation in the study.
Identification and recruitment of people with dementia
Participants were recruited from three geographical areas in the UK (Newcastle upon Tyne, North Tees, and Norwich). Recruitment settings included those described in WP2 (see Chapter 3): the ED, paramedic attendances and primary care consultations. In the light of lower than anticipated recruitment from these services (see Chapter 3, Discussion), we aimed to recruit from additional services based on feedback from stakeholders and the consensus panel, namely telecare services, supported discharge teams, rehabilitation outreach teams and Admiral Nurses. We also included PWD from two research registers (North East and North Cumbria Clinical Research Network Case Register and Join Dementia Research).
Confirmation of the eligibility of people with dementia
With the exception of potential participants identified through primary care (who were identified via the dementia QOF register), we first confirmed that the participant had a diagnosis of dementia prior to formal recruitment to the study. At first identification in the relevant setting, participants were given (in person) or posted a summary PIS. In community settings, participants were asked to send an opt-in form giving their contact details. In secondary care settings, it was possible to access contact details via patient notes. After they received the summary, all potential participants were contacted by the CTA by telephone. During the initial telephone call from the CTA to discuss participation, the CTA sought verbal consent to contact the general practice to check whether or not the person was on the dementia QOF register.
If the participant was on the dementia QOF register, the CTA sent a full PIS and subsequently contacted the potential participant to confirm eligibility. A home visit was arranged for those who were still interested to obtain consent and undertake a baseline assessment.
Quantitative methods: data collection and follow-up
Baseline assessments and data
Baseline data for the outcome measures were collected during a home visit by a CTA for PWD and carers consenting to the intervention study within 2 weeks of confirmation of eligibility. Outcome measures are shown in Table 10. The EQ-5D-5L was included based on the findings from the economic review (see Chapter 2, Discussion). Unless indicated otherwise, measures were administered by a CTA in the person with dementia’s own home at baseline and 12-week follow-up.
| Measure | Completed by | Time to complete (minutes) | Baseline visit | 12-week follow-up visit |
|---|---|---|---|---|
| MoCA72 | Patient | 10 | ✓ | N/A |
| EQ-5D-5L61 | Patient | 5 | ✓ | ✓ |
| QOL-AD149 | Patient | 5–10 | ✓ | ✓ |
| MFES150 | Patient | 5–15 | ✓ | ✓ |
| GAS151 | Patient and therapist | 20–40 | ✓a | ✓a |
| TUG152 | Patient and therapist | 5 | ✓a | ✓a |
| DAD153 | Informal carer (proxy) | 15 | ✓ | ✓ |
| EQ-5D-5L | Informal carer (proxy) | 5 | ✓ | ✓ |
| QOL-AD | Informal carer (proxy) | 5–10 | ✓ | ✓ |
| HUQ | Patient and informal carer (proxy) | 20 | ✓ | ✓ |
| ZBI154 | Informal carer | 10 | ✓ | ✓ |
Following the baseline assessment, the CTA sent a structured referral form with details of the baseline assessments of the PWD and carers to the intervention team. The intervention team then arranged an initial intervention assessment within 2 weeks.
The therapists recorded the Timed Up and Go Test (TUG)152 at their initial intervention assessment and final intervention visits. As part of the intervention, therapists set individualised goals with participants using GAS. 151 The process of GAS included discussing the suggested goals with the participant and carer and modifying them if required. Further discussion focused on agreeing what success would look like if the goal was achieved. This was to ensure that goals were tailored to the participant, that everyone agreed each goal would be worth striving for, and that everyone had a realistic expectation of what was likely to be achieved. The goals were agreed with the PWD by the therapists at the first therapy session and assigned ‘weights’ for importance and difficulty. GAS is a method of scoring the extent to which these goals are achieved in a way that is standardised for analysis. 151,155 Progress towards goals was measured at 6 weeks and at the final intervention visit, allowing a numerical score to be calculated.
Follow-up assessments
At 12 weeks, the CTA carried out a second visit to repeat most of the outcome measures completed at the baseline assessment (see Table 10). The exception was the MoCA,72 which was not repeated as the intervention was not expected to have an impact on cognition. During this visit, the CTA completed the HUQ with the carer to determine health-care and social care use by the person with dementia in the preceding 12 weeks.
Other outcome measures included the number of falls that were recorded in a diary by the participant supported by the carer (see Appendix 12). The diary also included space to record the activities undertaken each week.
Health Utilisation Questionnaire
The HUQ was refined for the feasibility study by reducing the number of questions and extending the recall for health-care resource use to 12 weeks. The diary included space to record services used each week; this provided an aide-memoire during the interview with the CTA (see Appendix 12). This is consistent with the recall period in other trials with frail participants. 58,156,157 To support this extended recall period, a section was included in the diary at the end of every week for participants to record any health-care information they could reflect on when completing the HUQ.
To further reduce the burden on participants, the HUQ was separated from the falls diary for WP4. In addition, in WP4 the HUQ was completed by the researcher with the participant using their diary as a prompt when responding to the questions. A similar approach was used in a recent study looking at care for young people with complex health needs (cerebral palsy, autism spectrum disorder and diabetes mellitus). 158
Questions relating to out-of-pocket expenses and Carer’s Allowance remained in the HUQ for WP4. However, given that the questionnaire was being completed by a researcher, only information relevant to a fall was collected, thus minimising the inclusion of non-fall-related expenses incurred by PWD (e.g. spectacles) and minimising any uncertainty surrounding the regularity of expenses incurred.
The results of the HUQ piloted in WP4 are presented in Chapter 10.
Quantitative analysis
The main analysis was of feasibility outcomes. We report the numbers of eligible participants seen over the recruitment period, and the resulting rates of recruitment, retention and data completion. The majority of the outcome data are presented in descriptive tables presenting percentages, means and SDs.
Feasibility of recruitment and retention
We aimed to explore the feasibility of different approaches to the identification and recruitment of PWD by describing the:
-
number of PWD identified through community and secondary care, and case register/Join Dementia Research
-
proportion of PWD who gave permission for us to check their medical records to determine eligibility
-
proportion of PWD who met the eligibility criteria
-
proportion of eligible PWD who agreed to participate in the study
-
proportion of eligible carers who agreed to participate in the study
-
proportion of participating PWD and carers who started the intervention
-
proportion of participating PWD and carers who remained in the study until study completion
-
proportion of participating PWD and carers completing each outcome measure at baseline and 12-week follow-up.
Findings relating to recruitment and retention are reported in Chapter 8.
Feasibility and acceptability of intervention delivery
Quantitative analysis of intervention delivery considered:
-
the proportion of staff attending all training and supervision sessions and MDT meetings
-
the number, frequency and duration of training and supervision sessions and MDT meetings
-
time spent with the patient and time spent travelling to appointments
-
the proportion of PWD discussed at MDT meetings and actions taken
-
the proportion of PWD seen by a geriatrician
-
the proportion of PWD reviewed by the MDT at 6 and 12 weeks and actions taken
-
how the assessment documentation was used in practice, for example whether or not all sections were completed
-
the nature of goals set and the alignment of activities with these goals
-
referrals made to other services
-
adherence to agreed activities by PWD.
Findings relating to the feasibility and acceptability of the DIFRID intervention are reported in Chapter 9.
Feasibility and acceptability of outcome measures
We examined the response rates, acceptability and feasibility of outcome measures described in Table 10 that could be used in a definitive trial. Additional data were collected through the process evaluation (see the following section). Findings relating to the outcome measures are reported in Chapter 10.
Process evaluation
The quantitative data were supplemented with qualitative data from the process evaluation, which provided a more nuanced understanding and allowed us to explore if and how the intervention would need to be adapted prior to a RCT.
Recruitment and consent
The initial consent process with PWD and carers included consent for optional participation in the process evaluation. We intended to purposively select a sample of consenting PWD and carers for observation and interview; however, the number of study participants was so small that we approached all PWD and carers who had given consent to be approached for the process evaluation. We aimed to observe the delivery of all components of the intervention in all sites. This enabled us to explore (1) if and how the sessions were tailored to individuals, (2) if and how activities were embedded into usual routines and (3) the role of the carer in the intervention.
We aimed to include a range of professionals in the process evaluation, including those developing and delivering training, staff delivering the intervention, MDT members, professionals receiving referrals as a result of the intervention, CTAs responsible for recruitment and professionals involved in making the initial approach to PWD and carers. All professionals received a PIS. This was followed up by e-mail or telephone to discuss participation and, if appropriate, arrange an interview. Consent was sought from all professionals for interviews. For staff delivering the intervention, participation in observation was seen as an integral part of their role and, therefore, formal written consent was not sought, although verbal consent was obtained.
Data collection
Interviews and focus groups
We conducted semistructured interviews with PWD, carers and professionals involved in training, recruitment and intervention delivery. Interviews explored the feasibility and acceptability of the DIFRID intervention, including the number and content of intervention sessions, the ‘fit’ of the intervention with other services and participants’ perceptions of achieved outcomes. Interviews were structured with the aid of a topic guide informed by normalisation process theory (NPT)159 (see Appendix 6). NPT aims to understand implementation through four key constructs: coherence (the extent to which an intervention ‘makes sense’ and has a clear purpose and objective), cognitive participation (willingness and ability to invest time and energy to make the intervention work), collective action (the resources, skills and organisational support required to make an intervention work) and reflexive monitoring (formal and informal mechanisms for judging whether or not the intervention is worthwhile).
Interviews with PWD and carers were conducted face to face in participants’ homes. Interviews with professionals were carried out by telephone or face to face at Newcastle University, according to preference and availability. One focus group with professionals was conducted where it was practical and feasible to bring staff together. The focus group used the same topic guide as that used for the professional interviews. Interviews with CTAs focused on their perceptions of the feasibility and acceptability of the different approaches to patient identification and the outcome measures.
Observation
We observed intervention training, intervention delivery and MDT meetings. During observation, we paid specific attention to interpersonal aspects, intervention fidelity and the extent to which the intervention was tailored to individuals. Details of observations were recorded in anonymised field notes. Informal discussions were completed following some observation sessions and recorded in field notes.
Qualitative analysis
Interviews and focus groups were audio-recorded and transcribed in full. Transcripts were checked and anonymised by a researcher prior to analysis.
We adopted a thematic approach to analysis. 160 Selected transcripts and field notes were read and discussed by the qualitative team in data workshops and an initial coding frame was developed. Additional data were then reviewed and discussed in further data workshops; this led to the identification of new codes arising from the data, and modification of the coding frame. Once review of new data led to no new insights or themes, the coding frame was finalised. Data were then coded with the aid of NVivo 11. After all transcripts and field notes were coded, the contents of the codes were analysed in depth through the production and discussion of narrative summaries.
The sources of quotations included in the report are anonymised by participant identifiers as described in Chapter 4.
Data collected for the process evaluation
The data set comprised 21 interviews, one focus group, five informal discussions and 14 episodes of observation (Table 11). The intervention sessions observed were delivered by therapists and support workers at various points in the intervention trajectory, including one initial and one final intervention session. Although the lead clinician in each site was asked to keep a record of MDT meetings, this was not returned to the research team. Furthermore, despite the efforts of the qualitative researcher, it proved possible to arrange observation of only one local MDT meeting, although two other teleconferences at the same site were ‘observed’. It was also not possible to interview the geriatricians involved. Limited information, therefore, is available on the frequency, format and content of MDT meetings. Although we had intended to interview professionals to whom referrals had been made, we received details of only one referral during the data collection period and the professional involved did not respond to our request for an interview.
| Participant/observation type | Number of participants |
|---|---|
| Interviews (n = 21) | |
| PWD | 1 |
| Carer | 3 |
| Joint: PWD and carer(s) | 3 |
| Professional | 14 |
| Focus group (n = 1) | |
| Professional | 9 |
| Informal discussions (n = 5) | |
| Professional | 5 |
| Observation (n = 14) | |
| Training sessions | 3 |
| MDT meetings | 3 |
| Intervention sessions | 8 |
Criteria for progression to full trial
Stop/Go criteria were developed for progression to a definitive trial (Table 12).
| Criteria | Go | Stop |
|---|---|---|
| % of eligible participants consenting to the feasibility study | ≥ 60 | ≤ 40 |
| % of participants attending ≥ 60% of the planned intervention sessions | ≥ 80 | ≤ 20 |
| % of participants providing key outcome data at 12 weeks | ≥ 70 | < 50 |
| Intervention has acceptable fidelity? | Yes | No |
| Intervention is acceptable to participants and professionals? | Yes | No |
In addition to the quantitative indicators, we included two indicators based primarily on the qualitative work. The first related to whether or not the intervention could be delivered with fidelity (i.e. the content, frequency, duration and quality of the intervention were delivered as set out in the intervention delivery manual). The second was an indication that the intervention was perceived as acceptable to both participants and professionals.
Intermediate outcomes were defined as amber. Whether or not to progress to a full trial was discussed by the TOC.
Ethics approval
Approval was given by Newcastle and North Tyneside 2 Research Ethics Committee (reference 17/NE/0297) and the Health Research Authority.
Chapter 8 Recruitment and retention

Introduction
This chapter describes recruitment and retention in the feasibility study and presents qualitative feedback on recruitment processes. Seven methodological issues relating to recruitment and retention for feasibility studies have been suggested,161,162 although not all of these were applicable to the present study. In this chapter, we consider the following issues:
-
What factors influenced eligibility and what proportion of those approached were eligible?
-
Was recruitment successful?
-
Did eligible participants consent?
-
Was retention in the study good?
Screening and recruitment of people with dementia
The flow of participants through the study and reasons for non-recruitment are shown in the Consolidated Standards of Reporting Trials (CONSORT) flow diagram (Figure 9).
FIGURE 9.
The CONSORT flow diagram.

A total of 113 people were screened for eligibility, of whom 29 (26%) were eligible. The most common reason for non-eligibility was not having a fall in the previous month (this mainly applied to people who were contacted via research registers). Other reasons included being unable to contact the person, the person being too unwell to participate, the person was moving into a care home, the person had died, the person lived too far away to receive the intervention, no carer was available and the person was not on the dementia QOF register. Some PWD on research registers declined before answering eligibility questions. The setting in which potential participants were screened is given in Table 13.
| Setting | Site, number of potential participants screened (number recruited) | Total, number of potential participants screened (number recruited) | ||
|---|---|---|---|---|
| Newcastle | North Tees | Norwich | ||
| Primary care | 1 (0) | 1 (1) | 0 (0) | 2 (1) |
| Paramedic attendance | 0 (0) | 0 (0) | 2 (1) | 2 (1) |
| ED | 27 (5) | 32 (2) | 2 (0) | 61 (7) |
| Supported discharge team | 0 (0) | 0 (0) | 2 (0) | 2 (0) |
| Community rehabilitation | 2 (1) | 0 (0) | 1 (1) | 3 (2) |
| Research register | 31 (0) | 0 (0) | 11 (0) | 42 (0) |
| Admiral nurse | 1 (1) | N/A | 0 (0) | 1 (1) |
| Total | 62 (7) | 33 (3) | 18 (2) | 113 (12) |
The majority of the potential participants were screened in the ED (54%). The exception was in Norwich, where screening was hampered because local approval processes did not allow the CTA to access patient ED records directly; instead we had to rely on a clinician approaching patients about the study while they were in the ED. This was a permanent decision that could not be resolved. As a result, it is likely that a number of potential participants were missed in the Norwich ED.
We do not have data about the potential participants in primary care or paramedic attendance who were given a summary PIS but did not return the opt-in form. Two potential participants returned forms from primary care and two returned forms from paramedic attendances. In Newcastle, 13 potential primary care participants received an opt-in form, of whom one returned the form and was screened. In North Tees, 29 potential primary care participants received a summary, of whom one returned the opt-in form, but we know that, owing to a misunderstanding, the practice sent the PIS to all PWD who had ever fallen rather than those who had fallen in the previous month. In Norwich, three potential primary care participants received the PIS, none of whom returned the opt-in form. In the East of England Ambulance Service, four summary PISs were given out and two opt-in forms were returned. We contacted the North East Ambulance Service to find out how many summary PISs were given out in Newcastle and North Tees but did not receive a reply. No opt-in forms were received via the paramedics in Newcastle and North Tees, suggesting that forms may not have been given out. Despite initial agreement to participate from telecare services in two sites, long delays with research governance in one site followed by difficulties in re-engaging with the service meant that no patients were ever approached. The second telecare service ultimately decided against participation owing to staff shortages.
Of the 29 PWD who were eligible, 12 people agreed to have their dementia status confirmed and to take part in the study (41% of eligible participants). Of those who declined, the patient declined in nine cases and the carer declined in eight cases. There were no significant differences in baseline characteristics between those who agreed and those who declined to participate in the study [enrolled mean age 81 years (SD 5.58 years), not enrolled mean age 81 years (SD 8.09 years), mean difference –0.37 years; enrolled 75% male, not enrolled 41% male; p = 0.176].
All final assessments of outcome measures were completed by the CTA for all participants (except the PWD who died shortly after recruitment). However, two participants did not complete all planned intervention visits. One participant had a fall and sustained a neck of femur fracture after visit 10 and did not have a final therapy visit. A second participant requested to stop intervention sessions after visit 10 but did agree to a final therapy visit at week 12.
Sample characteristics
Of those who received the intervention (n = 11), seven (63%) were male. The mean age was 80 years (SD 5.8 years). Six (55%) had minimal schooling, three (27%) had additional education and two (18%) had university-level education. Ten (90%) lived in their own home and one (9%) lived in sheltered housing. Seven (64%) lived with a spouse, one (9%) lived with another relative and three (27%) lived alone. For six (55%) participants, their informal carer was their spouse; for two (18%) participants, their informal carer was an adult child; and for three (27%) participants, their informal carer was another relative.
Five (46%) participants had AD, three (27%) had VAD and three (27%) had mixed dementias. Four (36%) participants had capacity and gave their own consent to participate; the remaining seven (64%) lacked capacity and consent was given by a consultee.
Views on recruitment processes
Data on recruitment were obtained through interviews with CTAs and other professionals involved in recruitment (see Chapter 7, Data collected for the process evaluation). Comments on recruitment related to two key areas: the eligibility criteria and feasibility of recruitment processes. No specific feedback was obtained on retention because in this section we rely on data from staff involved in recruitment.
Eligibility criteria
Further modification of the eligibility criteria was recommended by professionals involved in recruitment to ensure a more inclusive approach, in particular by:
-
extending the time period between the index fall and recruitment
-
including all falls and near misses
-
including PWD without a carer.
Although the time period within which the PWD had fallen was extended from 48 hours (in the diary study; see Chapter 3) to 1 month, this was still thought to be too restrictive, and to have contributed to low recruitment rates:
Other people I had to exclude because they fitted all of the other criteria but their fall wasn’t within a month, it was just outside the month. I think that month window of somebody having a fall was a bit too restrictive.
Professional 142, CTA (interview)
A key disadvantage of the requirement to have fallen within the previous month was that some PWD were either still in hospital or were already receiving services and consequently were reluctant to consent to an additional intervention:
I do have a list of the reasons why people declined. I think a lot of it was carers saying, ‘There’s just too much going on. She’s just got out of hospital,’ or, ‘She’s just getting over a fall. She’s got carers suddenly coming in four times a day, now is not the right time.’. So yes, I think there was a lot of people that just felt that they had too much on their plate.
Professional 140, CTA (interview)
Offering the intervention 3 months after a fall would have allowed for recovery and the provision of standard services (which were often time limited; see Chapter 4, Data collection and analysis). However, it is noteworthy that we received only three opt-in forms from PWD registered with the general practice that inadvertently sent study information to all PWD on the dementia register who had (ever) fallen. This suggests that extending the period since the fall had only a small impact on opt-in rates from primary care.
Although we had modified the eligibility criteria to include PWD with a fall for which health-care attention had been sought (rather than an injurious fall as in the diary study; see Chapter 3), participants argued that those experiencing ‘near falls’ should also have been eligible:
There was quite a lot of people that said, ‘Oh well, they had a near fall. They stumbled and they managed to grab onto me’, or, ‘They managed to hold onto the wall.’. One guy had walked into the door frame because he had stumbled and then hit his head off the door frame. That’s not a fall as such because it didn’t meet the ground but it’s a clear balance issue.
Professional 140, CTA (interview)
Within the context of the feasibility study, the inclusion of a carer was essential to provide outcome data; however, some CTAs felt that more PWD could have been recruited without this requirement:
There were people that I [could have] recruited but they were restricted because they didn’t have a carer. So, although the patient themselves was eligible, they weren’t eligible because they didn’t have anyone to fill in the proxy questionnaire.
Professional 142, CTA (interview)
Feasibility of recruitment processes
Recruitment materials, such as the PIS, were generally thought to be fit for purpose and acceptable for PWD. The 8-week recruitment period was considered too short by some, either because of the large volume of patients to screen from research registers combined with limited staff availability or because of communication issues. For example, community staff in one site were unaware that they had approval to start recruitment, which delayed the start of screening. The misunderstanding of one GP surgery over the requirement for the PWD to have fallen within the previous month also suggests that communication and follow-up between the research team, local CTAs and participating services could have been improved. The experience of the CTAs varied considerably between sites. The site initiation visit alone, even with follow-up contacts, was insufficient to ensure that inexperienced CTAs fully understood and enacted study procedures correctly, and briefed local services accurately.
We had added recruitment from the case register to try to enhance recruitment rates; however, this proved time-consuming and yielded few eligible PWD:
We had over 100 matches in terms of the dementia side of things but the actual database or the information that was gathered didn’t record if somebody had actually had a history of falls. I had to contact every single match, or a lot of the matches that were in our area, to actually check whether they’d had a fall, to see if they were eligible or not. A lot of the ones that I had screened at that initial process weren’t eligible.
Professional 142, CTA (interview)
Although the above quotation refers to more than 100 matches, only 42 of these were reported to have been screened. This suggests either inaccuracies in reporting eligibility and screening data or the lack of resources to contact all of those identified via registers to confirm eligibility.
Based on our experience in the diary study (see Chapter 3), we tried to facilitate recruitment from the ED by using embedded CTAs as we had successfully done in WP2; however, this was not possible in one site. This confirmed that relying on ED clinicians to introduce the study was unworkable in practice:
In ED it’s very pressurised. So, it’s a 4-hour target to get them out. You need to give them more time. So, I think more time and more explanation and being able to go back to them and say, ‘You were in ED yesterday would you mind taking part in this?’. Maybe when . . . Having the availability to go and talk to them in their own home a day or two later might increase your pick-up?
Professional 132, consultant, older people’s ED (interview)
Discussion
There were uncertainties about the feasibility of achieving the target of 30 PWD from the outset of the study; nevertheless, we met the progression criterion of recruiting at least 40% of eligible PWD. If we had been able to extend the recruitment period for the study, we may have been able to reach our recruitment target.
The findings have important implications for any potential further implementation of the DIFRID intervention. Research governance processes affected recruitment in two ways: inconsistent research governance requirements (1) undermined the use of a CTA to facilitate recruitment in one ED and (2) were likely to have resulted in under-recruitment from this service. Obtaining approvals for the inclusion of telecare services was challenging: in one site, there were significant delays that eventually led to a loss of interest from the service, and, in another, we experienced considerable difficulties in identifying the department responsible for approving the work. Staff shortages and pressures of work led to one telecare service eventually declining to participate; as already discussed, these factors are also likely to have affected recruitment in the ED where we were unable to use an embedded CTA.
Although these factors were largely outside the control of the research team, the qualitative work highlighted the need for improved systems and communication between the research team and local sites and within local sites to improve co-ordination regarding recruitment.
The failure to recruit any PWD from research registers suggests that this is not a viable approach to recruitment in the light of the resources required to contact large numbers of PWD to check eligibility. Although changing the eligibility criteria to include falls in the previous 3 or 6 months was suggested, this had little impact on recruitment rates in one general practice where the requirement to have fallen in the previous month was inadvertently omitted. A more convincing argument for extending the period since the fall is to enable all acute interventions to have been completed prior to recruitment to the study.
Although there are published data suggesting that falls are common in PWD,2 we experienced recruitment difficulties despite modifying the eligibility criteria and including alternative services and approaches to recruitment. The low recruitment rates in both the diary study (see Chapter 3) and the feasibility study (see Chapter 8) suggest that PWD may not seek health-care attention following a fall. Although they may contact telecare services via an alarm system, we were unsuccessful in our attempts to include such services. We are therefore unable to conclude whether or not telecare services would represent a viable source for recruitment.
Chapter 9 Feasibility and acceptability of the DIFRID intervention

Introduction
In this chapter, we present findings relating to the feasibility and acceptability of the intervention. We focus on three key methodological issues relating to intervention delivery in feasibility research:161,162
-
intervention adherence (both the adherence of front-line staff to the intervention and the extent to which PWD engaged in the intervention and adhered to the planned activities)
-
acceptability of the intervention
-
whether or not it was possible to calculate intervention costs and duration.
Two further issues161 relate to whether or not the logistics of running a multicentre trial were assessed and whether or not all components of the protocol worked together. The extent to which we are able to address these issues is limited owing to the scale of our feasibility study and the nature of the data collected (see Chapter 7).
The findings are presented for each component of the intervention in sequence (i.e. training and supervision; assessment; MDT meetings; referrals; goal-setting and activity-planning; ongoing intervention sessions; reviews and future-planning), followed by a review of the costs of the intervention and the logistics of intervention delivery. We conclude the chapter by drawing together the findings and their implications using the NPT framework. 159
Training and supervision
The aim of the training was to ensure that staff felt equipped and confident to deliver the DIFRID intervention by improving their knowledge and understanding of dementia and discussing the intervention components. The training was developed by the research team and the physiotherapist and OT who were seconded to the team to facilitate intervention development and training. Concerns about the feasibility of delivering a day-long training programme meant that training was condensed into a single half-day session. The areas covered by the training and approximate time allocated to each are shown in Table 14. The intention had been to provide all professionals with the equivalent of tier 2 dementia training;145 however, just under 1 hour was allocated specifically to working with dementia.
| Topic | Time allocation (minutes) |
|---|---|
| Introduction | 5 |
| What is the DIFRID intervention? | 10 |
| Introduction to working with PWD | 50 |
| Patient identification, assessment and referral | 10 |
| Assessment | 20 |
| MDT meetings | 10 |
| GAS | 30 |
| Developing activity plans | 25 |
| Project diary | 5 |
| Intervention sessions | 15 |
| 6- and 12-week reviews | 10 |
| Consent, withdrawal and adverse events | 10 |
| Process evaluation | 10 |
| Final questions and close | 10 |
Attendance at training
All staff responsible for delivering the intervention (physiotherapists, OTs and support workers) were invited to attend the training sessions. All relevant staff attended the training at two sites, but only therapists attended at the third site. This was because of difficulties in identifying which support workers would be involved in delivering the intervention and, therefore, needed to attend the training:
The way it worked, there wasn’t a designated support worker who was going to be able to follow through with all those patients, and it totally depended on who had capacity at the time [. . .] We have got so many support workers, you never know until it actually comes to that day who is going to be able to pick it up. It just happened that the guys here happened to have the capacity at the time, and that’s how they got involved. In terms of the training, it would have been really difficult to identify who.
Professional 141, physiotherapist (focus group)
This subsequently created difficulties for the support workers, who did not feel that they had been fully briefed on the intervention despite having a key role in its delivery, and had implications for the therapists, who had to explain study procedures and documentation (about which they themselves were uncertain) to their colleagues.
Views on training content
Many front-line staff felt positively about the training and found it useful, although some commented that it was similar to their existing approach. Participants valued the section on dementia and commented on the new understandings they had gained through the training:
I think the explanation of the eyesight issues was really good as well, and we’ve used that numerous times now with other patients and for training ourselves. So that was good.
Professional 154, physiotherapist (interview)
The manual supporting the training was well received, with many professionals using it as a reference document. Some professionals shared the information from the training and manual with colleagues not involved in the intervention, confirming the value of these resources:
It’s been really useful sharing that with the rest of our team, actually [. . .] about the dementia, at the beginning of that manual, that information. We shared that with our teams. That’s all really straightforward and telling us exactly what to do. So that was really good.
Professional 153, physiotherapist (interview)
One area in which staff would have welcomed more guidance was the duration of sessions. Although the manual clearly defined the maximum number of sessions to be delivered, there was no information about the duration of individual sessions. Some staff used the opportunity to facilitate outings taking several hours, but others would have welcomed clearer guidance:
I think it was just a little bit more confusing. ‘How long are we supposed to spend? What happens if you do have a long visit but then you want to go the next week to see them and you’ve already done 3 and a half hours this week?’. I think it was just . . . I don’t know how the timing could be changed, sort of thing. Do we just say, ‘Right, you’re going to do 12 visits and it’s however long you want to spend to achieve the goal, whatever’s going to be set’?
Professional 155, therapy assistant (interview)
Supervision
No formal supervision of intervention delivery was established, although staff were invited to contact the research team for advice and had access to their normal supervision arrangements. Despite the research team proactively offering support, only one person contacted the therapists seconded to the research team:
I sent two e-mails to one of the team leaders. I sent one to an OT as well, just asking them if they had any problems and to feel free – if they wanted to discuss anything – to get in touch. They didn’t. I was quite surprised, really, because it wasn’t necessarily their . . . It’s an area of interest to them, but it wasn’t really their expertise or specialist interest that they’re known for. I would’ve thought they would’ve had a few questions.
Professional 144, research team and training (interview)
Although some staff felt that they had addressed all of their queries through use of the manual and MDT discussion, others, on reflection, felt that they would have benefited from more direct supervision. Some staff were unsure who to contact about queries and resolved them through discussions with colleagues:
We didn’t quite understand all the timings. We got ourselves a bit confuddled on that, but we just went with whatever we thought was right, to be honest.
Professional 154, physiotherapist (interview)
Organisation of training
Both therapists seconded to the research team felt that the training session should have been longer, with more practical content. This could have allowed a more interactive approach, for example through the use of scenarios to give participants practical experience and enable them to begin to develop their skills:
That’s always the thing that’s concerned me, maybe that time was just a bit too snappy. [. . .] I think, for people who are going to participate in the programme, they do need more training. Like we said before, about the intervention itself and their attitudes and beliefs towards people with dementia and their expectations of people with dementia. I think, in most cases I would say those professionals’ expectations are lower than they should – particularly in the earlier stages of dementia.
Professional 144, research team and training (interview)
Participants similarly felt that more or longer training sessions would have been useful:
It was good, but I think having a bit more of it might have been nice, having it a bit longer so they could go into things in a bit more detail. The practical element of it was good.
Professional 150, OT (interview)
In the light of concerns over demands on staff time, it was suggested that, in future, the training should be presented as part of continuing professional development to maximise the benefit to participants. For two sites, there were delays of 2 or 3 months between the training and initial referrals; unsurprisingly, staff felt that they had forgotten things they had learned, particularly around the practical aspects of the intervention. Staff recommended minimising the gap between the training and beginning the intervention as well as having clear arrangements for addressing any queries. An alternative strategy would be to provide a follow-up training session once recruitment had started, so that staff had the opportunity to discuss their first PWD with the specialist therapists involved in training.
Assessment
Eleven PWD received two assessments by the therapists. The assessment comprised three main sections: a generic section to be completed by the therapist (either physiotherapist or OT) making the initial visit, a physiotherapy assessment and an occupational therapy assessment. The section on goals and action planning was completed by the therapist who made the second visit. Although it was intended that both assessment visits would take place during week 1, the mean time between assessment visits was 7.3 days (SD 4.47 days) and 54% of participants took longer than 1 week to complete. This was followed by a mean of 9 days (SD 3.03 days) before the first intervention session; thus, the intervention did not start as promptly as anticipated. An overview of the assessment components and completion rates is given in Table 15. Assessment documents were completed in full with the exception of those for osteoporosis risk (36%), TUG score (82%) and lying and standing blood pressure (82%). The initial osteoporosis risk assessment [Fracture Risk Assessment (FRAX)]163 was completed by the CTA as part of the baseline outcome assessment and passed onto the therapists with the referral document. The low level of completion suggests that the scores either were not included in the referral document or were not transferred to the assessment document. A needs list was completed for 9 of the 11 participants (82%) but action planning was completed for all. Because the action planning was derived from the needs list, this suggests that the needs list may have been seen as redundant by some.
| Component | n (%) completed |
|---|---|
| Professional responsible: first therapist | |
| History and circumstances of index fall and any injuries sustained | 11 (100.0) |
| Details of treatment offered so far and services already involved | 11 (100.0) |
| Past medical history and comorbidities | 11 (100.0) |
| Medication | 11 (100.0) |
| Osteoporosis risk | 4 (36.4) |
| Assessment of risk factors for falls | 11 (100.0) |
| Current mobility | 11 (100.0) |
| Current levels of activity, routines and likes and dislikes for activities | 11 (100.0) |
| Is there any challenging behaviour or sleep disturbance? | 11 (100.0) |
| How is the carer coping? | 11 (100.0) |
| How does the carer feel about being involved in and promoting the activities? | 11 (100.0) |
| Professional responsible: physiotherapist | |
| General observations and posture including pain, tone and sensation | 11 (100.0) |
| Lying and standing blood pressure | 9 (81.8) |
| Range of movement | 11 (100.0) |
| Muscle strength | 11 (100.0) |
| TUG | 9 (81.8) |
| Professional responsible: OT | |
| Home environment | 11 (100.0) |
| Self-care/productivity | 11 (100.0) |
| Affect | 11 (100.0) |
| Cognition | 11 (100.0) |
| Awareness of falls risk and impact on ADL | 11 (100.0) |
| Perception/sensory impairments | 11 (100.0) |
| Professional responsible: both | |
| Needs list | 9 (81.8) |
| Professional responsible: second therapist | |
| Action planning | 11 (100.0) |
| Patient and carer goals | 11 (100.0) |
Some professionals reported that the initial assessments worked well and successfully incorporated new components, such as measuring blood pressure or the person with dementia’s ability to multitask, into the assessment:
It was good, yes. It was quite lengthy. I could follow it through fine. I managed to do the blood pressure. So, that’s good. The assessments were all ones that we are used to anyway. Apart from when you have to count and do the activity. We don’t normally count backwards and do the activity. [. . .] I think the length was fine. The patient seemed OK with it.
Professional 130, physiotherapist (interview)
Although some staff conducted additional tests not included in the assessment document for individual PWD where they seemed relevant (e.g. to get an indication of the patient’s stamina), others felt that the assessment was too long and that there was repetition between the physiotherapist and OT assessments and the baseline measures completed by the CTA. It was suggested that the assessment be combined into a single visit with both therapists to reduce burden on the PWD and carers. In teams with generic roles where a single therapist would normally cover all components of the assessment, the division between occupational therapy and physiotherapy assessment was artificial:
By the time I went out I was the third professional going and asking lots of questions. [. . .] I could sense that the wife was feeling a little bit like, ‘I feel like I’ve been asked these questions before.’. On reflection, perhaps if I’d have gone out with the physio[therapist], and we’d done the assessment together, that might have been less onerous for the carer.
Professional 150, OT (interview)
One thing that we found a little bit difficult was that, because we’re so generic here, I would go out and normally do the physio[therapist] and the OT bits, so I was having to hold myself back.
Professional 155, physiotherapist (interview)
Despite the inclusion of questions about carer assessment, capacity to support the intervention and training needs in the assessment documentation, there was little evidence that these areas were considered.
Multidisciplinary team meetings
It was intended that on completion of the assessment each patient would be discussed by a MDT, including all staff directly involved in the assessment or delivery of the intervention sessions (physiotherapist, OT and support workers) and a geriatrician. The aim of the MDT was to discuss the assessment and develop an action plan based on patient and carer goals. Intended outcomes were to agree:
-
the types of activities and interventions that are most appropriate to meet this patient’s goals
-
the referrals required and a named individual responsible for each
-
the key worker for the participant
-
the number of intervention sessions needed for the first 6 weeks and who would deliver them.
It proved difficult to identify a geriatrician to join the MDT in one site; instead, the therapists used an existing contact to discuss medical issues. However, this meant that the intended holistic review of all PWD by a MDT was not possible in this site. Owing to the difficulties in arranging meetings where members worked in different locations, only one face-to-face MDT meeting with a geriatrician was held. Other MDT meetings were conducted by telephone (sometimes without teleconference facilities). Staff with experience of both a face-to-face meeting and a telephone meeting agreed that face-to-face MDT meetings where multiple PWD were discussed were more effective:
I definitely thought face to face was better. [. . .] I think because when you are face to face, everybody could sort of add their little bit, whereas when you were on the telephone, I would have a conversation and then say pass the phone over to [Professional 141] for her little bit of conversation. It wasn’t as joined up, because obviously I couldn’t hear the conversation that [Professional 141] was having, and she could only hear my responses on the bit that I was having.
Professional 137, OT (focus group)
Not all team members involved in intervention delivery participated in MDT meetings; sometimes support workers fed back information to therapists who then met with the geriatrician:
We had our team meet up and then we had the consultant phone in. That was useful because both patients had postural hypotension, on assessment. So that was useful to have their advice on that. We only, really, had one formal MDT because that was the only medical thing we needed to talk about. Otherwise it was more ad hoc. We’ve been e-mailing feedback to each other, after interventions, and talking in the office and stuff like that, when we’re around. I hope that’s OK.
Professional 153, physiotherapist (interview)
This quotation suggests that, at this site, the MDT was seen as only being relevant when there was a ‘medical thing’ to discuss rather than being an integral part of the assessment. Following assessment and MDT meetings, a total of 16 referrals were made, suggesting that participants had significant unmet needs. Two participants were referred to a geriatrician, three were referred to their GP, one was referred to a continence adviser, one was referred to wheelchair services, two were referred to equipment services and six were referred to other services. Only one carer was referred to a carers’ centre.
Goal-setting and activity-planning
By centring the intervention on goals identified by PWD and carers (refined by the MDT if needed) we hoped to maximise engagement and motivation. At the first intervention session, goals were agreed with the participant, the GAS form was completed and the project diary was introduced. Details of the rating of goals at the outset and end of therapy are provided in Chapter 10, because this relates to their potential use as outcome measures.
Overall, 31 goals were recorded; these were reviewed and categorised by one of the therapists involved in developing the intervention. Four goals were excluded as they were either too vague or were signposting or actions rather than goals (e.g. ‘proper medical assessment’). The remaining 27 goals were grouped into four categories: outdoor activities (n = 12), self-care (n = 7), indoor household tasks (n = 5) and indoor leisure activities (n = 3). The scope of the goals varied considerably; for example, one goal was ‘To be able to go into town on the community bus and access coffee shop three times over 12 weeks’, whereas another was ‘To stand for long enough to brush own teeth and hair on a daily basis’. This suggests that goals were tailored to the specific abilities of the PWD involved. Staff sometimes struggled to set goals that followed SMART (Specific, Measurable, Attainable, Realistic and Timely) principles, suggesting that additional training or review of goals may have been useful.
The assessment and intervention documentation included space for up to three goals; this was often interpreted by staff as a requirement for each person with dementia to have exactly three goals. For PWD who identified one or two challenging goals, there was sometimes not sufficient time, energy or motivation to tackle a third goal as well. Where PWD and therapists were struggling to identify goals, there was a tendency in some MDT meetings to add ‘default’ goals that were neither grounded in the assessment nor of particular interest to the PWD, such as making a hot drink. Alternatively, therapists sometimes substituted actions for goals:
They were struggling to come up with a third goal. They have already begun the process of getting assistive device for the toilet so [Professional 130] thought that this could also be a goal.
Field notes from informal discussion with Professional 130, physiotherapist
Despite some of the challenges of goal-setting, it proved successful with some PWD to the extent that some therapists intended to integrate GAS into their normal practice:
I quite enjoyed the goal-setting. [. . .] Using the GAS score was really interesting, and it’s something that we are going to try and include with our own patients as well.
Professional 154, physiotherapist (interview)
Defining expected outcomes as part of GAS provided a series of smaller goals that enabled participants to document progress. For example, for one person with dementia with the goal of being able to ‘exit the property and walk round the driveway to the car’, the following steps were described:
It was walking outside to the patio, which he cracked. It was walking outside to the car, which he did. It was getting in and out of the car, which I did with the OT, with a little bit of equipment. He had a swivel cushion. We talked about a handle, but actually he didn’t need it. It was practising that a few times, which we did. Then he goes out with his family quite a lot in the car now. Then it was walking a little bit further. [. . .] There are lots of little goals that we’ve just kept moving along, moving along, moving along.
Professional 134, support worker (interview)
However, goal-setting was not always as successful, particularly with PWD with more severe impairment who were unable to grasp the purpose of goal-setting or to retain goals. It was suggested that the term ‘goal’ was potentially off-putting to PWD, and that framing this part of the intervention in a different way might have been more successful:
It’s usually older clientele, and they’re just not used to the word goal. So we would be, obviously, using different wording around that. What would they like to get out of it or what were they expecting? All different wording, really, to try and tease out anything, but it’s been really hard to get anything.
Professional 153, physiotherapist (interview)
Even when PWD identified goals, these were not always included. In the following quotation, the person with dementia’s goals seem more cognitive than physical, which may explain why the therapist found these goals difficult to operationalise. In the second quotation, the therapist viewed the goals as unrealistic:
[Patient 17] does not have many goals but he manages quite well currently, for example with his drinks and meals. He didn’t rate much of anything on the Compass of Life, though they used it. He said that his goals are to anticipate things better and to understand whether he’s doing the right thing.
Field notes from informal discussion with Professional 130, physiotherapist
He had very unrealistic goals about playing boules and setting up a boules club in [local area], and going [abroad], returning to his house [abroad]. He would talk a lot about that, a lot, and really, really desperately wanting to get those things set up [. . .] It was very difficult to try and get him to focus on other things, because realistically we were never going to meet any of those goals.
Professional 141, physiotherapist (focus group)
However, some therapists expressed surprise at the progress achieved by some PWD, suggesting that ambitious goals should not be ruled out:
I questioned myself whether she would achieve getting into town on the bus on her own, and she did. That amazed me; that absolutely amazed me.
Professional 154, physiotherapist (interview)
Some staff recognised that some of the difficulties with goal-setting may have stemmed from their own lack of skill or experience; more training or supervision might have helped them to find ways of engaging more successfully with PWD:
I’d probably like more training on the psychological side of motivating people that have the cognitive problems, because it can be quite a barrier. I do think we tend to say, and it might be lack of training, ‘Oh, well, they won’t do it, that’s it then’. Yes, it might be, but I think sometimes training might teach us otherwise, you know?
Professional 147, OT (interview)
A final issue relating to goal-setting concerned the timing; therapists found that some PWD had more ideas about goals once they had developed rapport with staff and had become more familiar with the process:
After the relationship developed, he could then feel comfortable about discussing other things. I think, initially, he found it more difficult to pinpoint anything in particular, because he wasn’t used to being asked to do that.
Professional 133, support worker (interview)
Ongoing intervention sessions
The intervention was tailored to each participant based on the needs identified at the initial assessment(s). On average, participants reported having 12 planned intervention visits over the 12-week follow-up period {mean 12 [SD 5], median 11 [interquartile range (IQR) 9–17] visits}. Four participants had at least one intervention session that was not delivered, despite staff recording travel time or time spent at the visit (three of these participants missed either one or two visits and one participant missed seven visits). Reasons for missed visits included ‘patient not at home’, ‘patient requested visit not take place’, ‘10 min discussion with reablement team’ and ‘no answer at door’. The average number of intervention sessions that took place was 11 [mean 11 (SD 3.9), median 10 (IQR 9–14)]. Therefore, 94% of planned visits took place. The number of sessions potentially available as specified in the manual and the number delivered by different professionals are summarised in Table 16.
| Professional | Number of sessions | ||||
|---|---|---|---|---|---|
| Potentially available | Actually delivered, mean (SD) | Median (IQR) | Minimum | Maximum | |
| Support worker | Up to 16 | 8.73 (4.15) | 8 (7–11) | 0 | 16 |
| Physiotherapist | Up to 3 | 2.45 (1.37) | 2 (1–4) | 1 | 5 |
| OT | Up to 3 | 0.64 (0.67) | 1 (0–1) | 0 | 2 |
The number of visits was, therefore, substantially lower than envisaged, particularly for OTs. This may have been owing to difficulties with the logistics of intervention delivery (see Factors influencing implementation of the intervention) and the fact that two participants discontinued therapy after session 10.
In each intervention session, staff were intended to review the activities the participant had undertaken since the previous visit, check the project diary and discuss any falls (forwarding details of any adverse events to the principal investigator). Documentation of this process was recorded on 77% of occasions. Having reviewed the activities, staff then considered whether or not and how to modify activities in order to progress towards the goals. The activities were to be informed by the participant’s likes and dislikes and the type of activity they were most interested in and, therefore, most likely to follow. The manual and training emphasised the importance of embedding activities into participants’ everyday lives, for example walking to the local shop (if they routinely needed items such as a newspaper or milk) or walking the dog. Documentation of the activity-planning process was available for 81% of occasions.
Observation of intervention sessions indicated varied approaches to activity-planning. We observed some PWD being given exercise sheets and being advised how many of each exercise to do and how frequently. There was little evidence of explicit discussion of how the exercises would help PWD to achieve their goals. Review of therapist notes and patient diaries highlighted the tendency of some therapists to rely heavily on exercises. In some cases, this was despite explicitly noting that the person with dementia would prefer to undertake activities and was not adhering to the exercise programme:
. . . motivated to exercises but would prefer to be doing activity rather than just doing exercises.
Notes of intervention (Patient 22)
Furthermore, over-emphasis on terms such as ‘exercise’ and ‘fitness’ could alienate PWD:
[Professional 129] tells him that she wants to do some exercises with him to improve his fitness and he is quite resistant to this, saying ‘I’m an old man. I don’t need fitness’.
Field notes of intervention session, Patient 19 and Professional 129, physiotherapist
There was also evidence in the notes that some therapists relied heavily on carers to support the intervention between visits, suggesting that ways of embedding activities were not always considered and that carer capacity was not necessarily considered:
I would say they need to have somebody that is able to facilitate it [. . .] I just think if you can’t treat at the level of intensity required for carry over, there is no point in starting what you are then not going to finish. I think if you are giving daily balance exercises and they do them once a week, you are going to make your intervention look ineffective, because it has not been done at the required intensity. Or the other way round. That would be massively increasing the amount of support worker involvement, which would be extremely expensive.
Professional 129, physiotherapist (focus group)
Although some aspects of the intervention were similar to usual practice, the key difference identified by staff was the increased time available. This allowed staff to monitor progress more closely, engage more creatively and design more tailored activity programmes, which was seen as beneficial to PWD:
I think maybe we’d seen him more than we would do normally. Normally we would give them exercises and say to the carer, ‘Can you get him to do these?’. We’d review them a couple of times and then hope that they would carry on doing them. So, it’s more that supervision, that we don’t normally offer.
Professional 130, physiotherapist (interview)
Having the time to do it, I suppose, was good, rather than being limited in my follow-up, which is what it would normally be. I felt that we did a really full job. [. . .] If I had done it in my job role, we’d have probably just had to dash in and dash out. I don’t think it would have achieved the confidence giving that perhaps she needed, and he needed.
Professional 134, support worker (interview)
In addition to enabling staff to provide more hands-on help with activities, extra time also facilitated the development of rapport and gave staff greater insight into the day-to-day lives of PWD:
You built a relationship with that particular patient/client. You actually got to know their world and the people around them as well, which you wouldn’t necessarily do on a 2-week basis. [. . .] But it just made you see their world as well on a longer term, and what it is like for them on a day-to-day basis. That is where we could see definitely from a positive point of view, the intervention is needed out there.
Professional 151, support worker (focus group)
In contrast to the assessment document, which was easy to follow and complete, the paperwork for recording intervention sessions could be difficult to understand and time consuming to use. This was exacerbated by the overlap with the project diary (in which activities were also to be recorded as an aide-memoire for the PWD and carers) and need to maintain the usual clinical notes:
Just with having the notes in the house and having the notes here, and some of the tables and the charts, it wasn’t really clear how to fill them in. I filled them in the best that I could in the way that felt appropriate to me, but whether that was what you are looking for I don’t know. It just felt like a lot of duplicating. We had to do it here and then put it on our system as well, so it was time consuming.
Professional 141, physiotherapist (focus group)
Although the project diaries could have been an effective way for intervention team members to communicate with one another, they were not consistently used as intended. Although the person delivering the intervention was supposed to update the list of activities in the diary at each visit, this was not consistently done. Some teams met informally or communicated via e-mail or telephone to discuss their work with PWD, and others suggested introducing more joint visits:
You know, maybe another review visit at a certain point. We were relying on [support worker] all the time to come back and tell us, ‘Oh, when’s our next visit?’. So it perhaps would’ve been nice to have had another – even a joint visit in the middle somewhere, or at a certain point.
Professional 154, physiotherapist (interview)
Reviews and future-planning
Two formal reviews were scheduled during the intervention: one at the approximate mid-point (6 weeks) and one at the end of the intervention (12 weeks). The purposes of these reviews were to:
-
check if all the referrals from the MDT meeting had been acted on
-
record the GAS scores at these time points
-
discuss the participant’s progress
-
consider plans for progression and ongoing support.
In addition, at the final review, the therapist repeated the TUG and explored PWD’ and carers’ views on the intervention, including whether or not they would like to be referred to any ongoing services to facilitate maintenance and progression [e.g. Staying Steady (Newcastle)/community-based balance groups]. We observed one final review, which suggested that the PWD had enjoyed the intervention and that some suggestions had been embedded:
When asked about what she liked about the intervention, [Patient 25] responded that she enjoyed the ‘chat’ and found the visits ‘uplifting’. [. . .] She said that she was likely to continue going to town on her own. [. . .] As part of the intervention, [Professional 155, support worker] had installed a whiteboard for reminders. [Patient 25] described that she had initially found this difficult to remember and required a lot of prompting from husband, therapists and her daughter. However, this had become more embedded over the course of the study and [Patient 25] and her husband said that they this was something they would also continue using.
Field notes from observation of physiotherapist and support worker intervention session
This extract highlights the work required to successfully embed new ideas; the length of the intervention and support of the person with dementia’s family were key to the successful implementation of the reminder system.
Resources to deliver the intervention
As part of the intervention documentation, staff were asked to record travelling time as well as the length of visits. However, this was not always straightforward; sometimes staff forgot, and visits were often organised sequentially, so it was difficult to separate out the travel associated with one specific patient:
Sometimes, if you had other patients and you were going straight from my patient to there, I forgot to fill in what time I set off and then what time I got back.
Professional 155, physiotherapist (interview)
Table 17 summarises the available information on time spent travelling to and from and delivering the different sessions. One participant was missing information on the time spent at their second assessment and the time spent travelling back from this assessment. Information on time was available for eight participants at their final visit.
| Resource use | n | Time (minutes) | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Minimum | Maximum | ||
| Assessment 1 | |||||
| Travelling time to the assessment | 11 | 26.36 (14.68) | 20 (15–35) | 10 | 60 |
| Travelling time leaving the assessment | 11 | 27.27 (14.55) | 25 (15–40) | 5 | 50 |
| Time at the assessment | 11 | 85.55 (24.09) | 75 (60–101) | 60 | 130 |
| Assessment 2 | |||||
| Travelling time to the assessment | 11 | 24.09 (12.61) | 20 (15–35) | 10 | 45 |
| Travelling time leaving the assessment | 10 | 20.50 (10.12) | 15 (15–30) | 5 | 35 |
| Time at the assessment | 10 | 77.00 (33.60) | 65 (50–110) | 45 | 130 |
| Intervention sessions | |||||
| Travelling time to an intervention visita | 11 | 22.0 (6.7) | 12.7 (17.9–26.2) | 10.3 | 35 |
| Travelling time from an intervention visit | 11 | 21.8 (7.9) | 19.4 (16.1–29.5) | 10.6 | 37.1 |
| Time spent at an intervention visit | 11 | 57.01 (28.4) | 50 (35–82.5) | 30 | 121.7 |
| Final visit | |||||
| Travelling time to the final visitb | 8 | 21.9 (8.4) | 20 (15–25) | 15 | 40 |
| Travelling time leaving the final visit | 8 | 25.6 (14.5) | 22.5 (17.5–25) | 15 | 60 |
| Time at the final visit | 8 | 51.3 (24.5) | 40 (37.5–65) | 30 | 95 |
On average, the travel time and time spent at visits were similar regardless of the type of visit. However, as expected, the most time spent at a visit was to assess the participant and tailor the intervention to their needs, with the least amount of time spent at the final visit.
Logistics of intervention delivery
The lack of dedicated posts to deliver the intervention meant that we relied on existing staff to take on extra hours. Furthermore, this approach limited the extent to which the intervention sessions could be tailored to individual PWD and carers:
[Professional 157, support worker] commented that 9:30 was not really the best time to arrange sessions for the patient but she was constrained by her own schedule – she had to arrange to see study patients on her usual day off and had been scheduled to work half a day, in the morning [. . .] She also mentioned that the hospital had changed her days several times, which meant that she had not been able to consistently offer [Patient 26] a day and time for appointments. This in turn had led to [Patient 26] declining visits on several occasions.
Field notes from observation of support worker observation session
In addition to staff availability, sessions could be disrupted by other commitments of PWD and their carers. Some staff were faced with working outside their usual geographical area. As well as increasing travelling time, staff were not necessarily aware of which support services were available in the area, limiting their ability to signpost PWD and carers to other services.
Although most staff valued the opportunity to deliver a more extensive intervention, some commented that this had in part been due to low recruitment rates and queried whether or not they would have been able to provide such an effective intervention if recruitment had been more successful:
I mean, the amount of work that [Professional 155, support worker] has put in has been brilliant, you know, we couldn’t have done it without all the input that she put in. Had we have had more patients, I don’t know how that would have affected [Professional 155]’s caseload. That would be my query, but just looking at the research, you know, ideal.
Professional 154, physiotherapist (interview)
Delays in reimbursement meant that staff in one team had to deliver the intervention within their normal working hours. This may account for the reduced number of visits and tendency of some staff to revert to usual patterns of working. For example, one person with dementia received only three intervention visits because the therapist felt that the person with dementia’s carer was successfully supporting the exercise programme provided.
Factors influencing implementation of the intervention
Understanding the likelihood of new interventions being successfully embedded into routine practice is a key component of feasibility work. NPT is a well-established theory that has been used in over 100 studies seeking to understand factors influencing implementation. 164 We used the framework of NPT to inform data collection and analysis. NPT considers both the individual work and the collective work required for successful implementation of a new intervention. It focuses on four key areas: (1) coherence – whether or not the new intervention makes sense to stakeholders and is clearly different from current practice, (2) cognitive participation – whether or not stakeholders engage with and invest in the new intervention, (3) collective action – whether or not the new intervention is adequately supported in terms of resources, skills and training, and (4) reflexive monitoring – whether or not stakeholders can determine the impacts of the new intervention and adapt it to suit the local context. 159 An overview of the key factors influencing the implementation of the DIFRID intervention within the feasibility study and recommendations for future implementation is provided in Table 18.
| NPT construct | Key factors influencing implementation | Recommendations for future implementation |
|---|---|---|
| Coherence: making sense of the DIFRID intervention | Although some staff felt that the DIFRID intervention was similar to usual practice, key differences were the extended time available and use of GAS | A more interactive approach to training may help staff to understand more clearly whether or not and how the intervention differs from their usual practice |
| Training was valued and provided new insights into dementia | Consider expanding training to increase practical focus on working with PWD | |
| Uncertainties about intervention delivery arose when face-to-face work with PWD started | Provide ongoing support, either via a ‘top-up’ training session or through intervention supervision sessions | |
| Most stakeholders could see the potential value of the intervention | Build on this through tailoring the intervention to ensure early success | |
| Cognitive participation: engaging with the DIFRID intervention | Staff saw the intervention as a legitimate part of their role and were willing to try new ways of working | |
| Geriatricians had limited engagement in the planned MDT meetings |
Explore barriers to involvement and ways of addressing these Clarify purpose of the MDT |
|
| PWD’ engagement in goal-setting varied |
Emphasise motivational strategies in training and supervision Discourage the use of ‘default’ goals Consider how emerging goals can be incorporated into the intervention |
|
| Few sites had key individuals to drive the intervention forward | Identify mechanisms through which closer relationships can be developed with sites in general and with key individuals | |
| Collective action: enacting the DIFRID intervention | The intervention was successfully integrated into existing work and relationships | Ensure that all staff involved in intervention delivery attend training and supervision |
| Some staff tended to revert to established ways of working by focusing on exercise and relying on carers to ensure adherence | Use supervision to monitor how the intervention is being delivered | |
| Staff would have welcomed more opportunities for practical skill development | Consider expanding training to increase practical focus on working with PWD | |
| Intervention paperwork (especially the project diary) could be confusing and cumbersome | Adapt the paperwork to make tracking activities easier; reinforce the purpose of the project diary | |
| Evidence of continued reliance on contextless exercises | Address through improved training and supervision | |
| Reflexive monitoring: reflecting on and adapting the DIFRID intervention | The lack of external supervision meant that staff did not have the opportunity to refine skills in goal-setting | Provide intervention supervision to review goals and help staff to develop and embed skills |
| Some PWD found it difficult to identify goals at the outset of therapy | Consider ways of adapting and adding goals throughout therapy | |
| Planning ahead for the end of the intervention seemed minimal | Provide additional training at the point when PWD are approaching the end of the intervention | |
| Successful achievement of goals could challenge staff preconceptions about the abilities of PWD to benefit from intervention | Tailor the intervention to ensure early success with the possibility of extending goals |
The NPT analysis highlights some key areas for future development. Difficulties in translating theory into practice, in relation to both the intervention and working with PWD, highlight the need to ensure that training has a practical focus, and to provide additional training or supervision to develop the skills needed for successful implementation. The findings also suggest that more attention is needed to foster investment in and engagement with the intervention. Having strong local leadership is a well-established component of implementation strategies,165 yet was not achieved in all sites. Specifically, building relationships with local geriatricians may be one way of increasing buy-in in the MDTs while also providing local leadership. A key issue was the tendency of staff to revert to established approaches, for example by focusing on exercises; similar problems with introducing new ways of working were encountered in a previous study. 58 Intervention supervision may be a key way of monitoring and addressing this issue and could link to the involvement of geriatricians if they were willing to take on this role. The limited data collected suggest that early outcomes could either challenge preconceptions about dementia (if successful) or confirm them (if staff were unable to engage PWD in goal-setting). Appropriate supervision could provide opportunities to share outcomes, problem-solve as a team and learn from one another.
Discussion
The findings of the feasibility study suggest that the DIFRID intervention is both feasible and acceptable to stakeholders.
Adherence to the initial assessment was relatively good. There were, however, some difficulties in identifying meaningful goals with or for PWD. Difficulties in goal-setting with older people have previously been reported. 166,167 This suggests that further training and review of goals by a specialist member of the research team is needed, particularly in the early stages while skills are still developing. It was clear that the goals achieved sometimes exceeded the expectations of staff; successful work with PWD could, therefore, help to challenge the negative attitudes towards dementia expressed by some professionals (see Chapter 4, Data collection and analysis).
There was evidence of poor implementation of two key aspects of the intervention: (1) MDT meetings and (2) carer assessment, support and training. The difficulties in identifying a geriatrician in one site, and limited opportunities for discussion in other sites, meant that the holistic assessment and collaborative goal-setting envisaged in the intervention was not always realised. Further consideration is needed regarding the recruitment of geriatricians to support MDT meetings, clarification of the purpose of the meetings and documentation of such meetings. Although the intervention was intended to assess and address carer needs alongside those of the PWD, the lack of explicit attention to this in the study paperwork meant that there was little evidence of staff exploring carer needs for support, education or training in any detail.
Given that the feasibility study took place in three sites, we gained some insight into the logistics of running a multicentre trial. Ongoing supervision or training is a key area for development. Concerns about the willingness of staff to commit time and effort to training led to relatively brief training sessions; this seemed insufficient to ensure that staff fully understood study procedures and provided little time for skill development.
The organisation of participating services also highlighted the need for the intervention to be flexible to fit with the local context; for example, in services in which staff have a more generic role, requiring parts of the assessment to be conducted by an OT and a physiotherapist may need further justification and discussion with local services.
Service organisation further suggests that a cluster RCT may be the most appropriate design for a future trial of the intervention. Randomising individual PWD is unlikely to be feasible because it would require individual front-line staff to alter their behaviour for some PWD but not for others. Randomising individual members of staff is also likely to be problematic because support workers typically work across all team members. Furthermore, the finding that some teams found the intervention manual sufficiently useful to share it with colleagues highlights the potential for contamination within teams.
Strengths and limitations
The findings suggest a number of ways of optimising the intervention prior to further testing or evaluation. The limitations relate to the small number of PWD recruited and the limited data obtained on certain aspects of the intervention, in particular MDT meetings and review sessions. Relying on existing staff to deliver the intervention by working additional hours resulted in a lack of flexibility in the timing of sessions, which was at odds with the intention to deliver the intervention in ways that fitted around PWD’ and carers’ routines and preferences.
Conclusions
The study has highlighted the feasibility of delivering a creative, tailored, individual approach to intervention for PWD following a fall. Although the intervention required greater investment of time than in usual practice, many staff valued the opportunity to work more closely with PWD and carers.
Chapter 10 Feasibility and acceptability of outcome measures

Introduction
This chapter summarises the feasibility of the data collection tools used to capture information on the outcomes collected (outlined in Chapter 7) during the feasibility study. We focus on the extent to which the measures proved feasible and acceptable to PWD and their carers, and the CTAs responsible for the administration of these measures. We also consider the outcomes collected by the therapists during the intervention. Two methodological issues relating to intervention delivery in feasibility research are addressed: whether or not outcome assessments were complete and whether or not the outcome measures were those that were most appropriate. 161,162
Clinical trials assistant-administered outcome measures
The CTAs were responsible for collecting outcome data from PWD and carers at baseline and 12-week follow-up. There was some confusion surrounding the timing of the 12-week follow-up assessments: one CTA calculated the 12-week follow-up from the date of the CTA’s baseline assessment, whereas the other two CTAs calculated the date from the therapists’ initial assessment visit. Clearer communication and documentation of this process are required.
Outcome assessment measures were generally completed in full and all questions were generally completed with the exception of Quality of Life in Alzheimer’s Disease (QOL-AD) and EQ-5D-5L. At baseline, all PWD (n = 11) completed the EQ-5D-5L, with 82% (n = 9) of carers completing the proxy version. At 12 weeks, 91% (n = 10) of both PWD and carers completed the appropriate version of the EQ-5D-5L. There was a completed proxy version of the EQ-5D-5L questionnaire for the PWD who had not completed this measure at follow-up. All self-reported and proxy EQ-5D-5L questionnaires that were completed had no missing data for any of the domains. One CTA misunderstood that the proxy questionnaires were to be completed even if the PWD also completed their version of the questionnaire (at both time points). Summary data for the outcome measures are given in Table 19.
| Time point | n | Scores | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Minimum | Maximum | ||
| DAD (%) (maximum score is 100; higher scores are better) | |||||
| Baseline | 11 | 48.5 (28.9) | 60.0 (17.5–70.0) | 5.0 | 92.5 |
| Follow-up | 11 | 45.5 (27.6) | 50.0 (14.7–75.0) | 10.0 | 80.0 |
| MFES (points) (maximum score is 10; higher scores are better) | |||||
| Baseline | 11 | 6.51 (2.40) | 7.14 (5.14–8.00) | 1.93 | 10.0 |
| Follow-up | 11 | 7.40 (3.09) | 8.50 (7.30–9.32) | 0 | 10.0 |
| QOL-AD participant (points) (maximum score is 52; higher scores are better) | |||||
| Baseline | 11 | 33.7 (8.03) | 34.0 (28.5–37.0) | 20 | 51 |
| Follow-up | 10 | 34.4 (7.86) | 35.0 (29.0–37.0) | 23 | 48 |
| QOL-AD proxy (points) (maximum score is 52; higher scores are better) | |||||
| Baseline | 9 | 28.3 (6.32) | 29.0 (24.0–31.0) | 18 | 37 |
| Follow-up | 10 | 28.3 (6.48) | 26.0 (24.0–32.0) | 19 | 39 |
| Zarit Burden Scale (points) (maximum score is 88; lower scores are better) | |||||
| Baseline | 11 | 27.0 (11.9) | 21.0 (19.0–37.0) | 15 | 52 |
| Follow-up | 11 | 29.7 (11.9) | 32.0 (21.0–41.0) | 10 | 46 |
| EQ-5D-5La (utility: maximum score is 1.0; higher scores are better; VAS: maximum score is 100; higher scores are better) | |||||
| Baseline utility score | 11 | 0.67 (0.23) | 0.73 (0.48–0.88) | 0.17 | 0.94 |
| Baseline VAS | 11 | 65.9 (15.5) | 60 (55–80) | 40 | 90 |
| Follow-up utility score | 10 | 0.79 (0.14) | 0.79 (0.71–0.89) | 0.57 | 1.00 |
| Follow-up VAS | 10 | 72.7 (22.3) | 73.5 (50–95) | 40 | 100 |
| EQ-5D-5L proxy | |||||
| Baseline utility score | 9 | 0.58 (0.19) | 0.47 (0.46–0.73) | 0.33 | 0.87 |
| Baseline VAS | 9 | 49.4 (23.1) | 50 (40–70) | 10 | 80 |
| Follow-up utility score | 10 | 0.60 (0.21) | 0.65 (0.55–0.71) | 0.20 | 0.87 |
| Follow-up VAS | 10 | 55.9 (22.3) | 55 (35–75) | 25 | 89 |
Health Utilisation Questionnaire
As described in Chapter 7, the HUQ was completed by the CTA during the 12-week follow-up assessment. The HUQ collected information on any contact the participant had with health-care and social services and any out-of-pocket expenditure. The response rate to the HUQ was very high, with all 11 participants providing information. Each question was also completed well, with only one participant not providing information on the number of ED visits they had over the previous 12 weeks (Table 20). The data are presented as the number of visits reported by participants who responded ‘Yes’ to visiting a health-care provider. For example three participants reported visiting a GP at the general practice and, on average, those participants reported visiting a GP three times over the 12-week follow-up period.
| Area of resource use | Number of participants using the service | Number of visits for those who did use the service | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Minimum | Maximum | ||
| General practice consultations | 3 | 3.00 (2.65) | 2 (1–6) | 1 | 6 |
| Nurse practice consultations | 3 | 1.00 (0.00) | 1 (1–1) | 1 | 1 |
| GP phone consultations | 0 | 0.00 (N/A) | 0 (0–0) | 0 | 0 |
| Nurse phone consultations | 1 | 1.00 (N/A) | 1 (1–1) | 1 | 1 |
| GP home consultations | 1 | 2.00 (N/A) | 2 (2–2) | 2 | 2 |
| Nurse home consultations | 3 | 1.67 (1.15) | 1 (1–3) | 1 | 3 |
| OT consultations | 1 | 1.00 (N/A) | 1 (1–1) | 1 | 1 |
| OT home consultations | 2 | 1.00 (0.00) | 1 (1–1) | 1 | 1 |
| Physiotherapist consultations | 3 | 1.00 (0.00) | 1 (1–1) | 1 | 1 |
| Physiotherapist home consultations | 1 | 1.00 (N/A) | 1 (1–1) | 1 | 1 |
| Outpatient visits | 5 | 2.40 (1.67) | 2 (1–3) | 1 | 5 |
| Emergency ambulance uses | 4 | 1.00 (0.00) | 1 (1–1) | 1 | 1 |
| ED visits | 3 | 1.00 (0.00) | 1 (1–1) | 1 | 1 |
| Day-case visits | 1 | 1.00 (N/A) | 1 (1–1) | 1 | 1 |
| Inpatient nights | 2 | 1.00 (0.00) | 1 (1–1) | 1 | 1 |
| Day hospital (rehabilitation unit) visits | 0 | 0.00 (N/A) | 0 (0–0) | 0 | 0 |
| Rehabilitation classes | 0 | 0.00 (N/A) | 0 (0–0) | 0 | 0 |
| Social worker visits | 0 | 0.00 (N/A) | 0 (0–0) | 0 | 0 |
| Social worker home visits | 3 | 1.00 (0.00) | 1 (1–1) | 1 | 1 |
Five participants reported receiving a Carer’s Allowance, of which four participants provided information on how much, on average, they received each week. The weekly reported amount varied between participants (£112.00, £300.00 and £55.00) with one participant reporting that they received £585.00 annually.
One participant reported purchasing a pressure pad for £105.00 and shower stool for £20.00. Three participants reported paying for other help: (1) £50.00 per week for a ‘Carer once daily’ and £8.00 per week for a ‘falls detection band’, (2) £20.00 per week for unspecified help and (3) £5.50 per morning for exercise classes. Additional information on other health-care visits was provided by three participants and included carers twice daily to aid washing/dressing, three visits to a dentist, one visit to a podiatrist and one visit to an optician. Additional detail on the type of visits reported was provided in the Other Details section. The HUQ is thus a feasible tool to use to capture health-care resources used by PWD.
Therapist-completed outcome measures
As part of their initial and final visits, the lead therapist completed two outcome measures for the participant. One was a physical test of mobility and balance (TUG) and the other was GAS, which involved identifying goals, weighting the importance and difficulty of these goals, defining a range of expected outcomes and rating the person with dementia’s current abilities. The GAS was intended to be completed at the 6-week review in addition to at baseline and the 12-week follow-up.
The TUG was completed for 10 of the PWD at baseline and 9 out of the 11 PWD at follow-up. The GAS had considerable missing data, with complete information at all time points for only three PWD (Table 21).
| Time point | n | Scores | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Minimum | Maximum | ||
| TUG score (seconds) | |||||
| Baseline | 10 | 29.6 (16.5) | 27.5 (17.0–35.0) | 11 | 69 |
| Follow-up | 9 | 26.5 (16.2) | 21.9 (15.0–26.0) | 12 | 65 |
| GAS (points) | |||||
| Baseline | 3 | 35.3 (4.07) | 37.6 (30.6–37.7) | 30.6 | 37.7 |
| Follow-up | 3 | 62.7 (11.0) | 68.6 (59.3–69.0) | 50.0 | 69.4 |
Detailed examination of data relating to the 27 goals identified on the GAS indicated that there were marked problems with completion of ratings of the importance and difficulty of the goals identified (Table 22). Therapists appeared to improve completion of the ratings of current performance in relation to the goal at later time points, but ratings for all three time points were provided for only around three-quarters of the goals identified. Ratings at all three time points were complete for all goals identified for just over half of the PWD (54.5%) (see Table 22).
| Component | n (%) of goals (N = 27) | n (%) of PWD with information for at least one goal (N = 11) | n (%) of PWD with information for all goals (N = 11) |
|---|---|---|---|
| Goal identified | 27 (100) | 11 (100) | 11 (100) |
| Importance rated | 7 (25.9) | 3 (27.3) | 3 (27.3) |
| Difficulty rated | 7 (25.9) | 3 (27.3) | 3 (27.3) |
| Expected outcomes defined | 20 (74.1) | 8 (72.7) | 8 (72.7) |
| Achievement rated at baseline | 21 (77.8) | 9 (81.8) | 8 (72.7) |
| Achievement rated at 6 weeks | 24 (88.9) | 10 (90.1) | 9 (81.8) |
| Achievement rated at 12-week follow-up | 26 (96.3) | 10 (90.1) | 10 (90.1) |
| Achievement rated at all three time points | 19 (70.4) | 8 (72.7) | 6 (54.5) |
Reviewing the ratings of PWD’ abilities at the three time points provides some insight into the potential sensitivity of the use of GAS to changes resulting from the intervention. Ratings were available for at least one goal for eight PWD at all three time points, and only these data are included in the analysis (Table 23). The findings show that performance did not deteriorate for any goals over the period of the study, and that there was no improvement on a single goal. At 6 weeks, the ratings of three goals were unchanged; the performance on the remaining goals had improved (with an average increase of 2 points). At the 12-week follow-up, the most common outcome was for PWD to have maintained progress with their goals, with no change from their score at 6 weeks; however, five PWD made further gains on six goals, all but one by a single point (with one person with dementia gaining 2 points on one of their goals).
| Score | Change in goal attainment (points) | ||
|---|---|---|---|
| Baseline to 6 weeks | 6 to 12 weeks | Baseline to 12 weeks | |
| Score (points) (n) | |||
| 0 (no change) | 3 | 13 | 1 |
| 1 | 4 | 5 | 5 |
| 2 | 5 | 1 | 3 |
| 3 | 3 | 0 | 5 |
| 4 (maximum improvement) | 4 | 0 | 5 |
| Mean (median) change (points) | 2.1 (2) | 0.4 (0) | 2.4 (3) |
Self-completed outcome measure
Of the 11 participants, 10 (91%) completed the falls diaries. In total, 9 out of 10 (90%) had at least one fall. The median number of falls was 2 (IQR 1–6, range 0–23). A single participant had a very large number of falls (23). This very frail participant had very frequent falls before coming into the study and these continued.
Safety reporting
Adverse and serious adverse events were as expected and none were judged to be directly related to the intervention.
There were 12 adverse events reported in six participants:
-
swollen leg; drained and tired; dizziness
-
dizziness
-
facial injury caused by jewellery; buttock pain; elbow and shoulder pain
-
painful legs and hip
-
back pain
-
two instances of back pain; sickness.
There were four serious adverse events reported in four participants:
-
hospital admission with symptoms of stroke
-
hospital admission with fall and left radial head fracture
-
hospital admission with fall and neck of femur fracture
-
hospital admission with fall.
Acceptability of outcome measures
Feedback from PWD, carers and CTAs completing the outcome measures indicated that the most common concern related to the duration of the baseline and follow-up assessments:
I know that we had to do all the paperwork that we did, but I think it could be streamlined a bit, I think there was a bit of repetitiveness in the questions we were using [. . .] for the patients, it was a bit too much when you’re sat in the house. We only had, like, 90 minutes but I couldn’t do the first one in less than 2 hours because he kept getting upset and crying, it was very difficult.
Professional 145, CTA (interview)
I thought that was a bit long and drawn out, 2 hours. It was about 2 hours to start with. I didn’t think it had got to be anywhere near that long, but in the end, that went quickly. We just didn’t know what to expect. I’d got no idea, no idea at all. I just said yes thinking, ‘Well, if it doesn’t work out and he doesn’t like it, we can just stop it anyway.’.
Carer 16a (joint interview with Carer 16a, Carer 16b and Patient 16)
One carer found the question relating to Carer’s Allowance offensive and was not willing to discuss finances; however, these questions appeared acceptable to other participants. Some of the CTAs identified problems with administering the Modified Falls Efficacy Scale (MFES) and QOL-AD to PWD. The wording of the MFES was thought to be complex for PWD, and simpler phrasing was suggested:
For example, the Modified Falls Efficacy Scale, that was hard to explain to the patient that had more advanced dementia. It’s almost like a double negative. So, you’re asking them, ‘How confident are you that you won’t fall doing a certain thing,’ whereas, if you could just say, ‘Are you worried about falling when you get dressed and undressed’, that’s a much more straightforward question.
Professional 140, CTA (interview)
Although the CTAs recognised the importance of asking the questions of standardised scales verbatim, in practice they found that PWD often needed further explanation or clarification, especially when the questions were ambiguous, for example on the QOL-AD scale, which may have also affected the validity of the data:
I think there’s probably better quality-of-life questions [. . .] so physical health, that’s fair enough. Physical health, ‘How would you rate your physical health? Poor, fair, good, excellent.’. That’s pretty straightforward. Energy, that’s pretty straightforward. Mood, that’s pretty straightforward. Then, things like family. What about family? The support from your family? Whether your family live nearby? So, they’d look in and say, ‘Well, what do you mean, family?’. Do you know what I mean? ‘How do you rate your family?’. What? How proud you are of them? It’s a really difficult one to say.
Professional 140, CTA (interview)
The comments highlight the need for training for the CTAs responsible for collecting outcome data to ensure that standardised scales are being approached in the same way.
Relevance of the outcome measures to changes resulting from the intervention
The goals set focused on outdoor activities, indoor household tasks, indoor leisure activities and self-care; it is probable that achieving these goals would have an impact on quality of life as measured by QOL-AD and EQ-5D-5L. The relevance of the MFES for this study is unclear as only two PWD had significant fear of falling. One consequence of dementia is that some PWD may lose insight into their difficulties and may not be aware of their falls risk. Although we anticipated that GAS would provide a tailored and sensitive assessment of changes made during the intervention, there were difficulties in identifying appropriate goals, and the data required for scoring was often incomplete. These changes could be addressed through further training and supervision. Of more concern are the comments made by front-line staff about the timing of the goal-setting, which have implications for using GAS as an outcome measure.
Discussion
We have demonstrated that assessments were completed at all time points for 9 out of 11 PWD and 9 out of 11 carers. Furthermore, examination of completion of individual measures indicated few missing data. Only one person with dementia could not complete the TUG at baseline and follow-up, which is a good completion rate for a balance and mobility measure. Another participant could not complete the TUG at follow-up because of a fractured hip. The feasibility of using the EQ-5D-5L as a means of estimating health state utilities for PWD was demonstrated, even with those PWD who did not have capacity to consent to study participation for themselves. Removing the HUQ from the patient diary and completing it within a face-to-face interview proved successful, in terms of both potentially increasing completion rates for the (now simplified) diary and acceptability to carers. The high completion rate of the HUQ supports its use in a future definitive study. The feasibility of using the Disability Assessment for Dementia (DAD) and Zarit Burden Scales with the carer was also demonstrated with complete data.
The MFES was completed by all PWD, but in the light of the comments on the complexity of the MFES, in future it may be worth considering using the iconographical version of the Falls Efficacy Scale, which includes illustrations of common activities as verbal cues. 168 This has been validated in a number of countries and appears to have good validity and reliability. 147,168
Confusion over the timing of outcome measures and completion of proxy measures highlights the need for additional training for CTAs responsible for data collection. Our findings also suggest that training on outcome measures would ensure a consistent approach and give CTAs the opportunity to share and resolve common difficulties in administration or with question wording.
As described in Chapter 9, staff delivering the intervention required more training and supervision on the use of GAS. Although changes in scores were consistently positive, there were problems with missing data, particularly on the ratings of importance and difficulty, which are required to produce standardised scores. 151 A key advantage of GAS is that it is tailored to the priorities of individual participants; therefore, it may be more sensitive to change than standardised outcome measures.
The number of falls reported in the falls diary highlights the frequency of falling in this population. Together with the data on adverse events and health-care utilisation, the findings indicate that serious falls resulting in hospitalisation appear common (reported by 3 out of our 11 participants). That three such falls occurred during our 12-week intervention highlights the need for careful monitoring of adverse events in any future implementation and also indicates the financial and personal costs of falling for PWD and their carers.
Strengths and limitations
We supplemented data on completion rates of outcome measures with interview data with those responsible for administering or completing the measures. This highlighted that even when measures had good completion rates, the wording was sometimes complex and difficult to explain to PWD. One limitation of this aspect of the study was that the research team had no access to the notes maintained by the therapists (which included the TUG, DAD and GAS) until after the end of the intervention and completion of qualitative data collection. Opportunities for exploring the reasons for poor completion of the GAS were therefore missed.
Conclusions
The findings confirm that many of the measures used in the feasibility study are suitable for use in a future trial of the intervention. These outcomes were selected by the panel in WP3 and were possible to obtain. The importance of additional training for CTAs and staff delivering the intervention was highlighted to ensure a consistent approach and minimise missing data.
Chapter 11 Discussion/conclusions
Summary of the key findings
This report describes a series of mixed-methods approaches to answer the questions ‘Is it possible to develop a complex intervention to improve fall-related injuries in PWD living in their own homes?’, ‘What is the feasibility and acceptability of the intervention?’ and ‘Is it feasible to plan a future RCT to evaluate the efficacy of the DIFRID intervention?’. We showed that it was possible to design an intervention, although a key change was that the intervention should be delivered to all PWD living in their own homes who present with a fall requiring health-care attention and not just those who sustain an injury.
In Chapter 2, we described a systematic review assessing the previous evidence of effectiveness of interventions to improve outcomes for PWD who fall. We found gaps in the evidence base. The studies used different interventions, reported multiple different outcomes and included people with cognitive impairment as well as those diagnosed with dementia. The quality of evidence was mixed and the results across the studies conflicted even when similar interventions were utilised. Most of the study populations presented with hip fracture in hospital so interventions may not be applicable to soft tissue injuries or other types of fracture, and these studies provided no guidance about managing fall-related injuries in primary care. This suggested there was still a need for research into whether or not an effective intervention for fall-related injuries in dementia could be designed and delivered.
In Chapter 2, we also looked at how such an intervention should be evaluated. We concluded that the evaluation of a falls prevention intervention should identify and cost all of the resources required to deliver the intervention and any subsequent health and social care resource use. The outcomes that need to be considered are the number of falls and the QALYs based on responses to the EQ-5D-5L. Sensitivity analyses should be adopted to address any uncertainty.
In Chapter 3, we wished to describe current usual care and assess the demand for a future intervention for PWD who sustain a fall-related injury. We found that the incidence of fall-related injuries coming to attention in the settings of the ED, paramedic attendances and primary care consultations was much lower than expected. However, for those who did present, it was evident that usual care consisted of very little input. This suggested there was scope for improvement in the care received by such PWD. The HUQ was piloted as part of the diary study and refined for WP4 based on the data provided and feedback from the qualitative interviews.
In Chapter 4, we used qualitative methods, including interviews, focus groups and observation, to develop a better understanding of current care pathways and identify opportunities for intervention. The findings suggested that improving outcomes for PWD after a fall depends on recognising and facilitating the rehabilitation potential of PWD. The three key areas that need to be addressed are (1) ensuring that services are organised in the most effective and supportive way for PWD, (2) improving attitudes, knowledge and skills of professionals working with PWD, and (3) supporting carers and their role in the interventions.
In Chapter 5, we used a realist approach to synthesise the current evidence regarding the management of falls in dementia, further develop the key areas identified in Chapter 4 and develop theory regarding how a new intervention might work. We developed nine CMOcs, which were further grouped into three broad themes: (1) ensuring that the circumstances of rehabilitation are optimised for PWD, (2) compensating for the reduced ability of PWD to self-manage and (3) equipping the workforce with the necessary skills and information to care for this patient group.
In Chapter 6, we prioritised, operationalised and validated components of a complex intervention. We did this by convening a consensus panel that participated in two meetings and two Delphi consensus rounds. At this stage, the panel decided that the intervention should be delivered to those who had experienced a fall requiring health-care attention and not just those who had sustained an injury. The intervention that was designed was a multidisciplinary intervention to be carried out in PWD’ own homes over 12 weeks. Up to 22 intervention sessions could be undertaken but this was to be tailored according to the person with dementia’s need. The detailed methods for the intervention to be tested in the feasibility study were described in Chapter 7.
In Chapters 8–10, we described the results of the feasibility study in terms of recruitment and retention (see Chapter 8), acceptability of the intervention (see Chapter 9) and feasibility of outcome measures (see Chapter 10). We were unable to achieve the target of 30 PWD, which is not surprising given the lower than expected incidence of fall-related injuries coming to attention in the ED, paramedic attendances or primary care consultations, as we found in Chapter 3. It is likely that we would have been successful had we been able to extend the recruitment period; however, this was not possible within the funding envelope for the study. Nevertheless, we met the progression criterion of recruiting ≥ 40% of eligible PWD. The study suggested that the DIFRID intervention is both feasible and acceptable to stakeholders. A number of modifications were recommended to address some of the issues arising during the feasibility testing. These mainly centred on the need to expand training in the intervention for the staff delivering it and also clarifying the process of goal-setting. It was suggested that goal-setting could be carried out over a longer period at the start of the intervention to enable PWD to engage more in the goal-setting process. The process of measuring outcomes was largely successful. However, we did identify a need for more training for both CTAs and therapy staff, particularly in the use of the GAS. The costs associated with the intervention and subsequent health-care resource use of PWD were identified and included in the data collection tools piloted in WP4. Overall, the data collection measures were completed well. The data collection tools derived for this study can be used in a future trial evaluating this intervention and we would recommend that the HUQ is recorded by the CTA alongside the other outcome measures. The unit costs associated with each of these resources would need to be identified as part of a pilot trial.
Strengths and limitations
There were a number of strengths and limitations of the study. In Chapter 2, the systematic review followed established review methodologies including comprehensive searching for evidence and independent risk-of-bias assessment. However, the number of studies identified was small and four studies that otherwise met the inclusion criteria for this review could not be included. We were not able to perform a meta-analysis and we found significant gaps in the evidence base, especially for non-hip-fracture injuries. The studies did not show evidence of any particular adaptation of the approach, enhancement of the skills or composition of MDTs given that they were working with a population that was different from that of older people without a cognitive impairment. In addition, most of the interventions were not aimed at patients with known dementia; subgroup analysis was used to report the effects of general interventions on this group. The review of approaches to evaluate cost-effectiveness also found only a small number of studies. Not including falls recovery in the search terms means that we may have missed some potentially eligible studies. The risk of bias was not determined for the two eligible studies. This is a potential limitation of our results but in the context of this review it was not a major concern as the focus was on what sort of economic evaluation methodology to follow.
In Chapter 3, we used careful methods to quantify the number of people presenting with a fall-related injury but the incidence was much lower than expected. We believe that presentations to paramedics may have been particularly underestimated. Unfortunately, in the diary study we did not reach our target of 60 participants. We believe that the requirement for health professionals to seek permission from potential participants to share their contact details with the research team contributed to poor recruitment due to time constraints and so this was modified for WP4. Completion of the diaries was fairly successful and we modified our approach for WP4 in the light of the burden of completing health service use questions. Given the low level of use of health services by participants completing the diary study, we were able to identify a clear gap in current services that could be addressed by a new intervention.
In Chapter 4, the strengths of the study were the inclusion of multiple perspectives, in particular being able to observe staff with differing levels of experience and interest in dementia care. The direct observation of service delivery highlighted a range of issues relating to communication skills that were not emphasised during interviews. However, we experienced a number of recruitment difficulties, resulting in relatively small numbers of PWD and carers being included in both observations and qualitative interviews. Nevertheless, a range of suggestions for improving existing services and potential components of a new intervention were identified, which were presented to the consensus panel as part of WP3.
In Chapter 5, a realist approach allowed us to consider and synthesise a broader range of evidence, which was important in the light of the limited evidence identified in the effectiveness review. We used established methods of realist review. 91 The review was limited by the lack of published evidence found to support concepts that were clearly articulated in the qualitative data, such as in CMOc5 (providing ongoing support). Further research is needed in these areas. Owing to time limitations, we were not able to conduct additional targeted searches for all CMOcs and the review was completed by the time of the consensus panel meeting, so more research may have been published in these areas since we completed our review.
In Chapter 6, we used an intervention development process that was iterative and included a range of stakeholder perspectives. The development of the intervention was strengthened by including a range of professionals with expertise in falls prevention and rehabilitation. However, we did not include PPI representatives as the expected technical level of the presentations involved was not thought to be suitable for PPI involvement. We did not achieve full participation at the two meetings or in the Delphi rounds. The stakeholder interviews took place at an early stage of development of the intervention between the two meetings and it would have been useful to have time for more stakeholder interviews after development of the intervention materials. The development process enabled us to successfully develop the methods described in Chapter 7.
In Chapter 8, we showed that it was possible to recruit participants to receive the intervention, but a limitation was that because of the short time period for recruitment we did not reach our target number of participants. Nevertheless, it is a strength that we met the progression criterion of recruiting ≥ 40% of eligible patients. Retention to completion of the outcome assessments was good, although two participants did not receive the full 12 weeks of intervention sessions. In one case, this was because of a hip fracture; in the other case, this was a result of participant request. Adverse events and serious adverse events were as expected for this type of study in which frail older people can be expected to have a number of events unrelated to the study intervention. The participant who fractured their hip was having frequent falls both before and after receiving the intervention; therefore, it is not possible to be sure that their injury was directly attributable to increased activity from the intervention.
In Chapter 9, we were successful in using a process evaluation to suggest a number of ways of optimising the intervention prior to further testing or evaluation. This study comprehensively addressed the requirements of the MRC guidance on process evaluations. 169 However, a limitation is that we only had a small number of participants and carers. Nevertheless, the ways that we can optimise the intervention will be useful in further evaluations of this intervention.
In Chapter 10, we were successful in demonstrating that the outcome measures we selected could be completed by most participants and their carers. It is a strength that we supplemented data on completion rates of outcome measures with interview data with those responsible for administering or completing the measures. This highlighted that even when measures had good completion rates, the wording was sometimes complex and difficult to explain to PWD. One limitation of this aspect of the study was that the research team had no access to the notes maintained by the therapists (which included the TUG, DAD and GAS) until after the end of the intervention and completion of qualitative data collection. Opportunities for exploring the reasons for poor completion of the GAS were, therefore, missed. The findings confirm that many of the measures used in the feasibility study are suitable for use in a future trial of the intervention. The importance of additional training for CTAs and staff delivering the intervention was highlighted to ensure a consistent approach and to minimise missing data.
Implications for taking the DIFRID intervention forward
Our study used a comprehensive approach to developing a complex intervention for falls in PWD using the MRC framework. We have designed a tailored, individual approach to falls prevention in PWD and our systematic review identified that this approach has not been evaluated in previous studies. We are aware of only two trial protocols for studies that will aim to prevent falls in PWD. 56,170 Both of these protocols are aimed at the primary prevention of falls in PWD. Although the inclusion criteria do allow PWD to have had a fall before entering the study, the majority of participants in these two trials will not have experienced a fall. It is important to note that PWD who have already experienced a fall may have more severe dementia than those who are yet to experience a fall and, therefore, studies specifically in the group who have experienced a fall are needed.
We were able to meet most of the progression criteria for progress to a full trial, although the percentage of eligible participants consenting to a feasibility study was rated as amber. However, the number of people who met the eligibility criteria was lower than we were expecting at the outset of this research and a number of factors will need to be taken into consideration regarding potential recruitment rates for a full trial. We identified an important need for the CTA to have access to the details of the potential participants rather than relying on ED staff to ask if details could be shared with the CTA. In the inclusion criteria, extending the period since the fall is likely to be helpful to enable all acute interventions to have been completed prior to recruitment to the study. This is likely to be particularly useful in identifying participants from primary care. The use of research registers is unlikely to be a helpful way of identifying participants.
We identified a number of modifications to the intervention that would be useful. There were some difficulties in identifying meaningful goals with or for PWD. This suggests that further training and review of goals by a specialist member of the research team is needed, particularly in the early stages while skills are still developing. We also found that further training in working with PWD would be valued by the intervention teams. Further consideration is needed regarding the recruitment of geriatricians to support MDT meetings, clarification of the purpose of the meetings and documentation of such meetings. Although the intervention was intended to assess and address carer needs alongside those of the PWD, it is clear that more attention needs to be given to carer assessment and intervention in the materials for the intervention.
We did not have a control group in the present study and, therefore, we were unable to test procedures of randomisation and whether or not participants would be willing to be randomised. Given the modifications to the intervention that have been suggested, we recommend that it would be useful to further refine the intervention in a pilot trial before proceeding to a full trial. This would enable the procedures for randomisation to be tested as well as allowing further opportunity for refinement of the intervention.
In future work, we will apply for funding for a pilot trial. We propose a cluster randomised design. Recruitment challenges will be addressed by increasing the number of sites and lengthening the recruitment period. We will also ensure that a CTA is able to screen notes directly in each site. The time since the fall allowed in the inclusion criteria will be extended to 6–12 months. Additional training for therapists will be provided and geriatricians will be specifically recruited at each site to participate in the MDT. Additional support will be provided to carers.
Conclusions
The study has highlighted the feasibility of delivering a creative, tailored, individual approach to intervention for PWD following a fall. Although the intervention required greater investment of time than in usual practice, many staff valued the opportunity to work more closely with PWD and carers. We conclude that further research is now needed to refine this intervention through a pilot RCT.
Chapter 12 Patient and public involvement
Aims
The aims of the PPI in the study were to:
-
ensure that the study was relevant to PWD and their carers
-
ensure that the participant-facing materials were understandable and suitable for purpose
-
assist with the dissemination of the study.
Methods
During the development of the study protocol, a focus group was held to discuss the commissioning brief and help the team develop their ideas for the protocol. This was convened by VOICE, an organisation that aims to capture the public’s vast experience, ideas, opinions and expectations of research, innovation and policy developments that affect their lives. After funding was awarded, VOICE advertised for representatives to join the PPI panel for the study. Two volunteers joined the Programme Management Group and one joined the TOC and attended these meetings regularly.
The members of the Programme Management Group met the researchers at regular intervals during the study. They reviewed all participant-facing materials and made suggestions as to how they could be improved. After WPs 1–3, they worked with the research team to produce lay summaries of the reports about each WP. These were placed on the study website (https://research.ncl.ac.uk/difrid/) and disseminated to individuals involved in the study.
Results
The initial focus group supported the importance and relevance of the research topic identified by the commissioning brief and supported the design of the protocol. However, members of the focus group did not make any suggestions that altered the overall design of the study.
The presence of PPI representatives was very helpful in designing participant-facing materials. Changes were made to the designs as a result of their suggestions.
The lay summaries were made more readable as a result of the input of the representatives.
The presence of representatives at Programme Management Group meetings and TOC meetings enabled discussion of the potential impact of the study on participants and ways of encouraging recruitment.
Discussion and conclusions
Involvement of PPI representatives was a positive aspect of the DIFRID study. Without their involvement, the participant-facing materials may not have been so engaging for participants. The team were able to balance the need to collect accurate data with the need not to place an undue burden on participants. Dissemination to members of the public via the study website was assisted by their involvement.
Reflections/critical perspective
Because of the time scale for initial development of the protocol for the funding call, there may have been limited opportunities for the focus group to contribute to the design of the protocol. A series of focus groups may have encouraged further discussion.
Only three people contributed to ongoing PPI once the study was funded and this may have placed some burden on those taking part. A larger group would be useful for future studies.
Acknowledgements
The DIFRID team would like to acknowledge the contribution of the members of the consensus panel: Dr Fiona Shaw (Consultant Geriatrician, Newcastle upon Tyne Hospitals NHS Foundation Trust), Dr John-Paul Taylor (Senior Clinical Lecturer and Honorary Consultant in Old Age Psychiatry, Institute of Neuroscience, Newcastle University and Northumberland, Tyne and Wear NHS Trust), Dr Simon Thacker (Lead Consultant Psychiatrist, Royal Derby Hospital), Ms Louise McCarthy (Lead Research Nurse, Older Peoples’ Services, Norfolk and Suffolk Foundation Trust), Professor Pam Dawson [Associate Dean (Strategic Workforce Planning and Development), Northumbria University], Dr Pat Chung (Senior Lecturer, Occupational Therapy Pathway, Canterbury Christ Church University) and five additional panel members.
We would like to acknowledge the work of our PPI representatives Pam Harris and Helen Tumilty.
We would like to acknowledge the members of the TOC: Professor Rowan Harwood (Chairperson), Professor Emma Reynish, Dr Catherine Bailey and Ms Beryl Downing (PPI representative).
The Clinical Research Networks in the North East and Cumbria and the Eastern region supported the recruitment of participants to the study.
We are also grateful to all of the study participants, to Dr Miriam Boyles and Dr Caroline Shaw for additional qualitative data collection and analysis, to Dr Sue Lord for additional data extraction and to Mrs Beth Edgar for administrative support.
Contributions of authors
Louise M Allan (https://orcid.org/0000-0002-8912-4901) (Professor of Geriatric Medicine) was the principal investigator for the study. She contributed to drafting the report, analysed the quantitative data, contributed to the realist review, developed the manual and developed and delivered the training.
Alison Wheatley (https://orcid.org/0000-0002-6051-0286) (Research Associate) contributed to drafting the report, collected and analysed the qualitative data, contributed to the realist review, developed the logic model, developed the manual and developed and delivered the training.
Amy Smith (https://orcid.org/0000-0003-1398-9623) (Occupational Therapist) was a member of the consensus panel, oversaw the delivery of the intervention, developed the manual and developed and delivered the training.
Elizabeth Flynn (https://orcid.org/0000-0003-0646-4722) (Physiotherapist) was a member of the consensus panel, oversaw the delivery of the intervention, developed the manual and developed and delivered the training.
Tara Homer (https://orcid.org/0000-0002-6664-0671) (Research Associate, Health Economics) contributed to drafting the report, analysed the quantitative data for the health economics and was a member of the consensus panel.
Shannon Robalino (https://orcid.org/0000-0002-6359-1827) (Guest Researcher) contributed to the systematic review.
Fiona R Beyer (https://orcid.org/0000-0002-6396-3467) (Information Specialist) contributed to the systematic review and the drafting of the report.
Christopher Fox (https://orcid.org/0000-0001-9480-5704) (Professor of Old Age Psychiatry) contributed to the realist review, oversaw the study at the Norwich site and was a member of the consensus panel.
Denise Howel (https://orcid.org/0000-0002-0033-548X) (Senior Lecturer, Statistics) advised on design and statistical analysis of WP2 and WP4.
Robert Barber (https://orcid.org/0000-0001-9464-8446) (Honorary Clinical Senior Lecturer, Old Age Psychiatry) contributed to the realist review, was a member of the consensus panel and oversaw the Clinical Research Network staff at the Newcastle site.
Jim Anthony Connolly (https://orcid.org/0000-0002-3819-7772) (Consultant Physician, ED) oversaw the study in the Newcastle ED and was a member of the consensus panel.
Louise Robinson (https://orcid.org/0000-0003-0209-2503) (Professor of Primary Care and Ageing) advised on the primary care aspects of the study.
Steve Wayne Parry (https://orcid.org/0000-0002-8364-9192) (Clinical Senior Lecturer, Geriatric Medicine) contributed to the realist review and was a member of the consensus panel.
Lynn Rochester (https://orcid.org/0000-0001-5774-9272) (Professor of Human Movement Science) contributed to the realist review and was a member of the consensus panel.
Lynne Corner (https://orcid.org/0000-0002-6806-0304) (Director of Engagement) advised on PPI in the study.
Claire Bamford (https://orcid.org/0000-0003-2885-801X) (Senior Research Associate) led the qualitative work, contributed to drafting the report, collected and analysed the qualitative data, contributed to the realist review, developed the logic model and developed the manual.
Publications
Allan LM, Wheatley A, Flynn E, Smith A, Fox C, Howel D, et al. Is it feasible to deliver a complex intervention to improve the outcome of falls in people with dementia? A protocol for the DIFRID feasibility study. Pilot Feasibility Stud 2018;4:1–13.
Bamford C, Wheatley A, Shaw C, Allan LM. Equipping staff with the skills to maximise recovery of people with dementia after an injurious fall. Aging Ment Health 2018;15:1–9.
Robalino S, Nyakang’o SB, Beyer FR, Fox C, Allan LM. Effectiveness of interventions aimed at improving physical and psychological outcomes of fall-related injuries in people with dementia: a narrative systematic review. Syst Rev 2018;7:1–11.
Wheatley A, Bamford C, Shaw C, Boyles M, Fox C, Allan LM. Service organisation for people with dementia after an injurious fall: challenges and opportunities. Age Ageing 2019;48:454–8.
Wheatley A, Bamford C, Shaw C, Flynn E, Smith A, Beyer F, et al. Developing an Intervention for Fall-Related Injuries in Dementia (DIFRID): an integrated, mixed-methods approach. BMC Geriatr 2019;19:57.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Herrera A, et al. Dementia UK: Second Edition – Overview. Alzheimer’s Society; 2014.
- Allan LM, Ballard CG, Rowan EN, Kenny RA. Incidence and prediction of falls in dementia: a prospective study in older people. PLOS ONE 2009;4. https://doi.org/10.1371/journal.pone.0005521.
- Shaw FE. Falls in cognitive impairment and dementia. Clin Geriatr Med 2002;18:159-73. https://doi.org/10.1016/S0749-0690(02)00003-4.
- Voisin T, Sourdet S, Cantet C, Andrieu S, Vellas B. Descriptive analysis of hospitalizations of patients with Alzheimer’s disease: a two-year prospective study of 686 patients from the REAL.FR study. J Nutr Health Aging 2009;13:890-2. https://doi.org/10.1007/s12603-009-0247-y.
- Rowe MA, Fehrenbach N. Injuries sustained by community-dwelling individuals with dementia. Clin Nurs Res 2004;13:98-110. https://doi.org/10.1177/1054773803262520.
- National Institute for Health and Care Excellence (NICE) . Falls in Older People (NICE Quality Standard No. 86) 2017.
- National Institute for Health and Care Excellence (NICE) . Dementia: Assessment, Management and Support for People Living With Dementia and Their Carers 2018. www.nice.org.uk/guidance/ng97 (accessed 27 March 2019).
- World Health Organization (WHO) . WHO Global Report on Falls Prevention in Older Age 2007.
- National Institute for Health and Care Excellence (NICE) . Clinical Practice Guideline for the Assessment and Prevention of Falls in Older People 2004.
- Clevenger CK, Chu TA, Yang Z, Hepburn KW. Clinical care of persons with dementia in the emergency department: a review of the literature and agenda for research. J Am Geriatr Soc 2012;60:1742-8. https://doi.org/10.1111/j.1532-5415.2012.04108.x.
- Smith T, Hameed Y, Cross J, Sahota O, Fox C. Assessment of people with cognitive impairment and hip fracture: a systematic review and meta-analysis. Arch Gerontol Geriatr 2013;57:117-26. https://doi.org/10.1016/j.archger.2013.04.009.
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012;9. https://doi.org/10.1002/14651858.CD007146.pub3.
- Winter H, Watt K, Peel NM. Falls prevention interventions for community-dwelling older persons with cognitive impairment: a systematic review. Int Psychogeriatr 2013;25:215-27. https://doi.org/10.1017/S1041610212001573.
- Buchner DM, Larson EB. Falls and fractures in patients with Alzheimer-type dementia. JAMA 1987;257:1492-5. https://doi.org/10.1001/jama.1987.03390110068028.
- Pellfolk T, Gustafsson T, Gustafson Y, Karlsson S. Risk factors for falls among residents with dementia living in group dwellings. Int Psychogeriatr 2009;21:187-94. https://doi.org/10.1017/S1041610208007837.
- Asada T, Kariya T, Kinoshita T, Asaka A, Morikawa S, Yoshioka M, et al. Predictors of fall-related injuries among community-dwelling elderly people with dementia. Age Ageing 1996;25:22-8. https://doi.org/10.1093/ageing/25.1.22.
- Ballard CG, Shaw F, Lowery K, McKeith I, Kenny R. The prevalence, assessment and associations of falls in dementia with Lewy bodies and Alzheimer’s disease. Dement Geriatr Cogn Disord 1999;10:97-103. https://doi.org/10.1159/000017108.
- Härlein J, Dassen T, Halfens RJ, Heinze C. Fall risk factors in older people with dementia or cognitive impairment: a systematic review. J Adv Nurs 2009;65:922-33. https://doi.org/10.1111/j.1365-2648.2008.04950.x.
- Katz IR, Rupnow M, Kozma C, Schneider L. Risperidone and falls in ambulatory nursing home residents with dementia and psychosis or agitation: secondary analysis of a double-blind, placebo-controlled trial. Am J Geriatr Psychiatry 2004;12:499-508. https://doi.org/10.1176/appi.ajgp.12.5.499.
- Sterke CS, van Beeck EF, van der Velde N, Ziere G, Petrovic M, Looman CW, et al. New insights: dose-response relationship between psychotropic drugs and falls – a study in nursing home residents with dementia. J Clin Pharmacol 2012;52:947-55. https://doi.org/10.1177/0091270011405665.
- COMET Initiative . COMET Initiative Database 2018. www.comet-initiative.org/ (accessed 27 March 2019).
- Lamb SE, Jørstad-Stein EC, Hauer K, Becker C. Prevention of Falls Network Europe and Outcomes Consensus Group . Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc 2005;53:1618-22. https://doi.org/10.1111/j.1532-5415.2005.53455.x.
- Moniz-Cook E, Vernooij-Dassen M, Woods R, Verhey F, Chattat R, De Vugt M, et al. A European consensus on outcome measures for psychosocial intervention research in dementia care. Aging Ment Health 2008;12:14-29. https://doi.org/10.1080/13607860801919850.
- Deshpande N, Metter EJ, Lauretani F, Bandinelli S, Guralnik J, Ferrucci L. Activity restriction induced by fear of falling and objective and subjective measures of physical function: a prospective cohort study. J Am Geriatr Soc 2008;56:615-20. https://doi.org/10.1111/j.1532-5415.2007.01639.x.
- Vaapio SS, Salminen MJ, Ojanlatva A, Kivelä SL. Quality of life as an outcome of fall prevention interventions among the aged: a systematic review. Eur J Public Health 2009;19:7-15. https://doi.org/10.1093/eurpub/ckn099.
- Robalino S, Nyakang’o SB, Beyer FR, Fox C, Allan LM. Effectiveness of interventions aimed at improving physical and psychological outcomes of fall-related injuries in people with dementia: a narrative systematic review. Syst Rev 2018;7. https://doi.org/10.1186/s13643-018-0697-6.
- Beyer F, Robalino S, Fox C, Rochester L, Parry S, Barber R, et al. Effectiveness of interventions aimed at improving physical and psychological outcomes of fall-related injuries in people with dementia. PROSPERO; 2016.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Chichester: The Cochrane Collaboration; 2011.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc 1986;34:119-26. https://doi.org/10.1111/j.1532-5415.1986.tb05480.x.
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61-5. https://doi.org/10.1037/t02366-000.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. https://doi.org/10.1016/0022-3956(75)90026-6.
- Higgins JPT, Altman DG, Sterne JAC, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Chichester: The Cochrane Collaboration; 2011.
- Shaw FE, Bond J, Richardson DA, Dawson P, Steen IN, McKeith IG, et al. Multifactorial intervention after a fall in older people with cognitive impairment and dementia presenting to the accident and emergency department: randomised controlled trial. BMJ 2003;326. https://doi.org/10.1136/bmj.326.7380.73.
- Huusko TM, Karppi P, Avikainen V, Kautiainen H, Sulkava R. Randomised, clinically controlled trial of intensive geriatric rehabilitation in patients with hip fracture: subgroup analysis of patients with dementia. BMJ 2000;321:1107-11. https://doi.org/10.1136/bmj.321.7269.1107.
- Prieto-Alhambra D, Judge A, Arden NK, Cooper C, Lyles KW, Javaid MK. Fracture prevention in patients with cognitive impairment presenting with a hip fracture: secondary analysis of data from the HORIZON Recurrent Fracture Trial. Osteoporos Int 2014;25:77-83. https://doi.org/10.1007/s00198-013-2420-8.
- Stenvall M, Berggren M, Lundström M, Gustafson Y, Olofsson B. A multidisciplinary intervention program improved the outcome after hip fracture for people with dementia: subgroup analyses of a randomized controlled trial. Arch Gerontol Geriatr 2012;54:e284-9. https://doi.org/10.1016/j.archger.2011.08.013.
- Watne LO, Torbergsen AC, Conroy S, Engedal K, Frihagen F, Hjorthaug GA, et al. The effect of a pre- and postoperative orthogeriatric service on cognitive function in patients with hip fracture: randomized controlled trial (Oslo Orthogeriatric Trial). BMC Med 2014;12. https://doi.org/10.1186/1741-7015-12-63.
- Kennie DC, Reid J, Richardson IR, Kiamari AA, Kelt C. Effectiveness of geriatric rehabilitative care after fractures of the proximal femur in elderly women: a randomised clinical trial. BMJ 1988;297:1083-6. https://doi.org/10.1136/bmj.297.6656.1083.
- McGilton KS, Davis AM, Naglie G, Mahomed N, Flannery J, Jaglal S, et al. Evaluation of patient-centered rehabilitation model targeting older persons with a hip fracture, including those with cognitive impairment. BMC Geriatr 2013;13. https://doi.org/10.1186/1471-2318-13-136.
- Deschodt M, Braes T, Broos P, Sermon A, Boonen S, Flamaing J, et al. Effect of an inpatient geriatric consultation team on functional outcome, mortality, institutionalization, and readmission rate in older adults with hip fracture: a controlled trial. J Am Geriatr Soc 2011;59:1299-308. https://doi.org/10.1111/j.1532-5415.2011.03488.x.
- Prestmo A, Saltvedt I, Helbostad JL, Taraldsen K, Thingstad P, Lydersen S, et al. Who benefits from orthogeriatric treatment? Results from the Trondheim hip-fracture trial. BMC Geriatr 2016;16. https://doi.org/10.1186/s12877-016-0218-1.
- Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc 2005;53:1476-82. https://doi.org/10.1111/j.1532-5415.2005.53466.x.
- Huusko TM, Karppi P, Kautiainen H, Suominen H, Avikainen V, Sulkava R. Randomized, double-blind, clinically controlled trial of intranasal calcitonin treatment in patients with hip fracture. Calcif Tissue Int 2002;71:478-84. https://doi.org/10.1007/s00223-001-2111-x.
- Parker SG, McLeod A, McCue P, Phelps K, Bardsley M, Roberts HC, et al. New horizons in comprehensive geriatric assessment. Age Ageing 2017;46:713-21. https://doi.org/10.1093/ageing/afx104.
- Pilotto A, Cella A, Pilotto A, Daragjati J, Veronese N, Musacchio C, et al. Three decades of comprehensive geriatric assessment: evidence coming from different healthcare settings and specific clinical conditions. J Am Med Dir Assoc 2017;18:192-192.e11. https://doi.org/10.1016/j.jamda.2016.11.004.
- Centre for Reviews and Dissemination (CRD) . Welcome to the CRD Database: Search Strategies n.d. www.crd.york.ac.uk/crdweb/searchstrategies.asp (accessed 3 August 2016).
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; 2005.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ: The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. https://doi.org/10.1136/bmj.313.7052.275.
- Medical Advisory Secretariat . The Falls/Fractures Economic Model in Ontario Residents Aged 65 Years and Over (FEMOR). Ont Health Technol Assess Ser 2008;8:1-54.
- Centre for Reviews and Dissemination (CRD) . Effects of the Finnish Alzheimers Disease Exercise Trial (FINALEX): A Randomised Controlled Trial (Structured Abstract) 2015. www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?ID=22013025468 (accessed 22 August 2019).
- de Souto Barreto P, Denormandie P, Lepage B, Armaingaud D, Rapp T, Chauvin P, et al. Effects of a long-term exercise programme on functional ability in people with dementia living in nursing homes: research protocol of the LEDEN study, a cluster randomised controlled trial. Contemp Clin Trials 2016;47:289-95. https://doi.org/10.1016/j.cct.2016.02.004.
- Pitkälä KH, Pöysti MM, Laakkonen ML, Tilvis RS, Savikko N, Kautiainen H, et al. Effects of the Finnish Alzheimer disease exercise trial (FINALEX): a randomized controlled trial. JAMA Intern Med 2013;173:894-901. https://doi.org/10.1001/jamainternmed.2013.359.
- Galasko D, Schmitt F, Thomas R, Jin S, Bennett D. Alzheimer’s Disease Cooperative Study: detailed assessment of activities of daily living in moderate to severe Alzheimer’s disease. J Int Neuropsychol Soc 2005;11:446-53. https://doi.org/10.1017/S1355617705050502.
- Haralambous B, Haines TP, Hill K, Moore K, Nitz J, Robinson A. A protocol for an individualised, facilitated and sustainable approach to implementing current evidence in preventing falls in residential aged care facilities. BMC Geriatr 2010;10. https://doi.org/10.1186/1471-2318-10-8.
- Close JC, Wesson J, Sherrington C, Hill KD, Kurrle S, Lord SR, et al. Can a tailored exercise and home hazard reduction program reduce the rate of falls in community dwelling older people with cognitive impairment: protocol paper for the i-FOCIS randomised controlled trial. BMC Geriatr 2014;14. https://doi.org/10.1186/1471-2318-14-89.
- Farag I, Sherrington C, Ferreira M, Howard K. A systematic review of the unit costs of allied health and community services used by older people in Australia. BMC Health Serv Res 2013;13. https://doi.org/10.1186/1472-6963-13-69.
- Parry SW, Bamford C, Deary V, Finch TL, Gray J, MacDonald C, et al. Cognitive–behavioural therapy-based intervention to reduce fear of falling in older people: therapy development and randomised controlled trial – the Strategies for Increasing Independence, Confidence and Energy (STRIDE) study. Health Technol Assess 2016;20. https://doi.org/10.3310/hta20560.
- The EuroQol Group . EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Janssen MF, Bonsel GJ, Luo N. Is EQ-5D-5L better than EQ-5D-3L? A head-to-head comparison of descriptive systems and value sets from seven countries. PharmacoEconomics 2018;36:675-97. https://doi.org/10.1007/s40273-018-0623-8.
- Easton T, Milte R, Crotty M, Ratcliffe J. An empirical comparison of the measurement properties of the EQ-5D-5L, DEMQOL-U and DEMQOL-Proxy-U for older people in residential care. Qual Life Res 2018;27:1283-94. https://doi.org/10.1007/s11136-017-1777-0.
- Orgeta V, Edwards RT, Hounsome B, Orrell M, Woods B. The use of the EQ-5D as a measure of health-related quality of life in people with dementia and their carers. Qual Life Res 2015;24:315-24. https://doi.org/10.1007/s11136-014-0770-0.
- Bryan S, Hardyman W, Bentham P, Buckley A, Laight A. Proxy completion of EQ-5D in patients with dementia. Qual Life Res 2005;14:107-18. https://doi.org/10.1007/s11136-004-1920-6.
- Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219-36. https://doi.org/10.1002/1097-0258(20001215)19:23<3219::AID-SIM623>3.0.CO;2-P.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212-14. https://doi.org/10.1177/0272989X9001000308.
- Kellogg International Work Group . The prevention of falls in later life: a report of the Kellogg International Work Group on the prevention of falls by the elderly. Dan 1987;34:1-24.
- World Health Organization . ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision 2004. https://apps.who.int/iris/handle/10665/42980 (accessed August 2019).
- World Medical Association . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. https://doi.org/10.1001/jama.2013.281053.
- Department of Health and Social Care . Mental Capacity Act 2005.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695-9. https://doi.org/10.1111/j.1532-5415.2005.53221.x.
- Bamford C, Wheatley A, Shaw C, Allan LM. Equipping staff with the skills to maximise recovery of people with dementia after an injurious fall [published online ahead of print 15 November 2018]. Aging Ment Health 2018. https://doi.org/10.1080/13607863.2018.1501664.
- Wheatley A, Bamford C, Shaw C, Boyles M, Fox C, Allan L. Service organization for people with dementia after an injurious fall: challenges and opportunities. Age Ageing 2019;48:454-8. https://doi.org/10.1093/ageing/afz010.
- Rapley T, Flick U. The SAGE Handbook of Qualitative Data Analysis. London: Sage Publications; 2013.
- Hammersley M, Atkinson P. Ethnography: Principles in Practice. London: Routledge; 2007.
- Abbey J, Piller N, De Bellis A, Esterman A, Parker D, Giles L, et al. The Abbey pain scale: a 1-minute numerical indicator for people with end-stage dementia. Int J Palliat Nurs 2004;10:6-13. https://doi.org/10.12968/ijpn.2004.10.1.12013.
- Schrijvers G, van Hoorn A, Huiskes N. The care pathway: concepts and theories – an introduction. Int J Integr Care 2012;12. https://doi.org/10.5334/ijic.812.
- Deneckere S, Euwema M, Van Herck P, Lodewijckx C, Panella M, Sermeus W, et al. Care pathways lead to better teamwork: results of a systematic review. Soc Sci Med 2012;75:264-8. https://doi.org/10.1016/j.socscimed.2012.02.060.
- Morse A. Health and Social Care Integration. London: National Audit Office; 2017.
- Wesson J, Clemson L, Brodaty H, Lord S, Taylor M, Gitlin L, et al. A feasibility study and pilot randomised trial of a tailored prevention program to reduce falls in older people with mild dementia. BMC Geriatr 2013;13. https://doi.org/10.1186/1471-2318-13-89.
- Hall AJ, Watkins R, Lang IA, Endacott R, Goodwin VA. The experiences of physiotherapists treating people with dementia who fracture their hip. BMC Geriatr 2017;17. https://doi.org/10.1186/s12877-017-0474-8.
- Staples WH, Killian CB. Development of an instrument to measure attitudes of physical therapy providers working with people with dementia. Am J Alzheimers Dis Other Demen 2012;27:331-8. https://doi.org/10.1177/1533317512452041.
- Gill N, Hammond S, Cross J, Smith T, Lambert N, Fox C. Optimising care for patients with cognitive impairment and dementia following hip fracture. Z Gerontol Geriatr 2017;50:39-43. https://doi.org/10.1007/s00391-017-1224-4.
- Patel M, Lee S, Hammond S, Fox C, Poland F, Lambert N, et al. Caring for People With Hip Fracture and Cognitive Impairments: Qualitative Findings from the PERFECTED Research Programme n.d.
- Isbel ST, Jamieson MI. Views from health professionals on accessing rehabilitation for people with dementia following a hip fracture. Dementia 2017;16:1020-31. https://doi.org/10.1177/1471301216631141.
- Bowling A. An ‘inverse satisfaction law’? Why don’t older patients criticise health services?. J Epidemiol Community Health 2002;56. https://doi.org/10.1136/jech.56.7.482-a.
- Wheatley A, Bamford C, Shaw C, Flynn E, Smith A, Beyer F, et al. Developing an Intervention for Fall-Related Injuries in Dementia (DIFRID): an integrated, mixed-methods approach. BMC Geriatr 2019;19. https://doi.org/10.1186/s12877-019-1066-6.
- Pawson R, Tilley N. Realistic Evaluation. London: Sage Publications; 1997.
- Wong G, Westhorp G, Manzano A, Greenhalgh J, Jagosh J, Greenhalgh T. RAMESES II reporting standards for realist evaluations. BMC Med 2016;14. https://doi.org/10.1186/s12916-016-0643-1.
- Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review: a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10:21-34. https://doi.org/10.1258/1355819054308530.
- Rycroft-Malone J, McCormack B, Hutchinson AM, DeCorby K, Bucknall TK, Kent B, et al. Realist synthesis: illustrating the method for implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-33.
- Pluye P, Robert E, Cargo M, Bartlett G, O’Cathain A, Griffiths F, et al. Proposal: A Mixed Methods Appraisal Tool for Systematic Mixed Studies Reviews 2011. www.mixedmethodsappraisaltoolpublic.pbworks.com (accessed 3 June 2017).
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Schepker CA, Leveille SG, Pedersen MM, Ward RE, Kurlinski LA, Grande L, et al. Effect of pain and mild cognitive impairment on mobility. J Am Geriatr Soc 2016;64:138-43. https://doi.org/10.1111/jgs.13869.
- Kolanowski A, Mogle J, Fick DM, Hill N, Mulhall P, Nadler J, et al. Pain, delirium, and physical function in skilled nursing home patients with dementia. J Am Med Dir Assoc 2015;16:37-40. https://doi.org/10.1016/j.jamda.2014.07.002.
- Kress HG, Ahlbeck K, Aldington D, Alon E, Coaccioli S, Coluzzi F, et al. Managing chronic pain in elderly patients requires a CHANGE of approach. Curr Med Res Opin 2014;30:1153-64. https://doi.org/10.1185/03007995.2014.887005.
- Husebo BS, Ballard C, Fritze F, Sandvik RK, Aarsland D. Efficacy of pain treatment on mood syndrome in patients with dementia: a randomized clinical trial. Int J Geriatr Psychiatry 2014;29:828-36. https://doi.org/10.1002/gps.4063.
- Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ 2011;343. https://doi.org/10.1136/bmj.d4065.
- Ahn H, Horgas A. Does pain mediate or moderate the effect of cognitive impairment on aggression in nursing home residents with dementia?. Asian Nurs Res 2014;8:105-9. https://doi.org/10.1016/j.anr.2014.03.003.
- Ahn H, Horgas A. The relationship between pain and disruptive behaviors in nursing home residents with dementia. BMC Geriatr 2013;13. https://doi.org/10.1186/1471-2318-13-14.
- Flo E, Gulla C, Husebo BS. Effective pain management in patients with dementia: benefits beyond pain?. Drugs Aging 2014;31:863-71. https://doi.org/10.1007/s40266-014-0222-0.
- Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc 2001;49:516-22. https://doi.org/10.1046/j.1532-5415.2001.49108.x.
- McIntyre A, Reynolds F. There’s no apprenticeship for Alzheimer’s: the caring relationship when an older person experiencing dementia falls. Ageing Soc 2012;32:873-96. https://doi.org/10.1017/S0144686X11000699.
- Valeriani L. Management of demented patients in emergency department. Int J Alzheimers Dis 2011;2011. https://doi.org/10.4061/2011/840312.
- Gonski PN, Moon I. Outcomes of a behavioral unit in an acute aged care service. Arch Gerontol Geriatr 2012;55:60-5. https://doi.org/10.1016/j.archger.2011.06.013.
- Rösler A, von Renteln-Kruse W, Mühlhan C, Frilling B. Treatment of dementia patients with fracture of the proximal femur in a specialized geriatric care unit compared to conventional geriatric care. Z Gerontol Geriatr 2012;45:400-3. https://doi.org/10.1007/s00391-012-0299-1.
- Giusti A, Barone A, Pioli G. Rehabilitation after hip fracture in patients with dementia. J Am Geriatr Soc 2007;55:1309-10. https://doi.org/10.1111/j.1532-5415.2007.01258.x.
- Taylor ME, Lord SR, Brodaty H, Kurrle SE, Hamilton S, Ramsay E, et al. A home-based, carer-enhanced exercise program improves balance and falls efficacy in community-dwelling older people with dementia. Int Psychogeriatr 2017;29:81-9. https://doi.org/10.1017/S1041610216001629.
- Seitz DP, Gill SS, Austin PC, Bell CM, Anderson GM, Gruneir A, et al. Rehabilitation of older adults with dementia after hip fracture. J Am Geriatr Soc 2016;64:47-54. https://doi.org/10.1111/jgs.13881.
- Suttanon P, Hill KD, Said CM, Byrne KN, Dodd KJ. Factors influencing commencement and adherence to a home-based balance exercise program for reducing risk of falls: perceptions of people with Alzheimer’s disease and their caregivers. Int Psychogeriatr 2012;24:1172-82. https://doi.org/10.1017/S1041610211002729.
- Tarazona-Santabalbina FJ, Domenech-Pascual JR, Belenguer-Varea AA, Rovira Daudi E. The approach to patients with cognitive impairment and hip fracture: the role of orthogeriatric care. Rev Clin Gerontol 2014;24:219-27. https://doi.org/10.1017/S0959259814000100.
- Aharony L, Sela-Katz P. Depression, falls and cognitive changes among community-dwelling elderly people. Alzheimer’s Dement 2011;7. https://doi.org/10.1016/j.jalz.2011.05.1777.
- Goldstein FC, Strasser DC, Woodard JL, Roberts VJ. Functional outcome of cognitively impaired hip fracture patients on a geriatric rehabilitation unit. J Am Geriatr Soc 1997;45:35-42. https://doi.org/10.1111/j.1532-5415.1997.tb00975.x.
- Faes MC, Reelick MF, Joosten-Weyn Banningh LW, Gier Md, Esselink RA, Olde Rikkert MG. Qualitative study on the impact of falling in frail older persons and family caregivers: foundations for an intervention to prevent falls. Aging Ment Health 2010;14:834-42. https://doi.org/10.1080/13607861003781825.
- Nilsson I, Rogmark C. Hemiarthroplasty for displaced femoral neck fracture: good clinical outcome but uneven distribution of occupational therapy. Disabil Rehabil 2011;33:2329-32. https://doi.org/10.3109/09638288.2011.570412.
- Luxford K, Axam A, Hasnip F, Dobrohotoff J, Strudwick M, Reeve R, et al. Improving clinician–carer communication for safer hospital care: a study of the ‘TOP 5’ strategy in patients with dementia. Int J Qual Health Care 2015;27:175-82. https://doi.org/10.1093/intqhc/mzv026.
- Raivio M, Korkala O, Pitkala K, Tilvis R. Rehabilitation outcome in hip-fracture: impact of weight-bearing restriction – a preliminary investigation. Phys Occup Ther Geriatr 2004;22:1-9. https://doi.org/10.1300/J148v22n04_01.
- Toulotte C, Fabre C, Dangremont B, Lensel G, Thévenon A. Effects of physical training on the physical capacity of frail, demented patients with a history of falling: a randomised controlled trial. Age Ageing 2003;32:67-73. https://doi.org/10.1093/ageing/32.1.67.
- de Rotrou J, Cantegreil I, Faucounau V, Wenisch E, Chausson C, Jegou D, et al. Do patients diagnosed with Alzheimer’s disease benefit from a psycho-educational programme for family caregivers? A randomised controlled study. Int J Geriatr Psychiatry 2011;26:833-42. https://doi.org/10.1002/gps.2611.
- Nyman SR, Victor CR. Older people’s participation in and engagement with falls prevention interventions in community settings: an augment to the Cochrane systematic review. Age Ageing 2012;41:16-23. https://doi.org/10.1093/ageing/afr103.
- Ritter MA, Harty LD. Total joint replacement in patients with dementia syndromes: a report of thirteen cases. Orthopedics 2004;27:516-17.
- Murphy MR, Escamilla MI, Blackwell PH, Lucke KT, Miner-Williams D, Shaw V, et al. Assessment of caregivers’ willingness to participate in an intervention research study. Res Nurs Health 2007;30:347-55. https://doi.org/10.1002/nur.20186.
- Keith PM, Wacker R, Collins SM. Family influence on caregiver resistance, efficacy, and use of services in family elder care. J Gerontol Soc Work 2009;52:377-400. https://doi.org/10.1080/01634370802609304.
- Dow B, Moore K, Russel M, Ames D, Cyarto E, Haines T, et al. Improving mood through physical activity for carers and care recipients (IMPACCT): protocol for a randomised trial. J Physiother 2013;59. https://doi.org/10.1016/S1836-9553(13)70165-6.
- Comans TA, Currin ML, Brauer SG, Haines TP. Factors associated with quality of life and caregiver strain amongst frail older adults referred to a community rehabilitation service: implications for service delivery. Disabil Rehabil 2011;33:1215-21. https://doi.org/10.3109/09638288.2010.525288.
- Aminzadeh F, Byszewski A, Dalziel WB. A prospective study of caregiver burden in an outpatient comprehensive geriatric assessment program. Clin Gerontol 2006;29:47-60. https://doi.org/10.1300/J018v29n04_04.
- Barbosa A, Figueiredo D, Sousa L, Demain S. Coping with the caregiving role: differences between primary and secondary caregivers of dependent elderly people. Aging Ment Health 2011;15:490-9. https://doi.org/10.1080/13607863.2010.543660.
- Sutcliffe CL, Giebel CM, Jolley D, Challis DJ. Experience of burden in carers of people with dementia on the margins of long-term care. Int J Geriatr Psychiatry 2016;31:101-8. https://doi.org/10.1002/gps.4295.
- Kim H, Chang M, Rose K, Kim S. Predictors of caregiver burden in caregivers of individuals with dementia. J Adv Nurs 2012;68:846-55. https://doi.org/10.1111/j.1365-2648.2011.05787.x.
- Buri H, Dawson P. Caring for a relative with dementia: a theoretical model of coping with fall risk. Health Risk Soc 2000;2:283-93. https://doi.org/10.1080/713670166.
- Cristancho-Lacroix V, Wrobel J, Cantegreil-Kallen I, Dub T, Rouquette A, Rigaud AS. A web-based psychoeducational program for informal caregivers of patients with Alzheimer’s disease: a pilot randomized controlled trial. J Med Internet Res 2015;17. https://doi.org/10.2196/jmir.3717.
- Zarit SH, Lee JE, Barrineau MJ, Whitlatch CJ, Femia EE. Fidelity and acceptability of an adaptive intervention for caregivers: an exploratory study. Aging Ment Health 2013;17:197-206. https://doi.org/10.1080/13607863.2012.717252.
- Shim B, Barroso J, Davis LL. A comparative qualitative analysis of stories of spousal caregivers of people with dementia: negative, ambivalent, and positive experiences. Int J Nurs Stud 2012;49:220-9. https://doi.org/10.1016/j.ijnurstu.2011.09.003.
- Lach HW, Chang YP. Caregiver perspectives on safety in home dementia care. West J Nurs Res 2007;29:993-1014. https://doi.org/10.1177/0193945907303098.
- Reuben DB, Ganz DA, Roth CP, McCreath HE, Ramirez KD, Wenger NS. Effect of nurse practitioner comanagement on the care of geriatric conditions. J Am Geriatr Soc 2013;61:857-67. https://doi.org/10.1111/jgs.12268.
- Ganz DA, Koretz BK, Bail JK, McCreath HE, Wenger NS, Roth CP, et al. Nurse practitioner comanagement for patients in an academic geriatric practice. Am J Manag Care 2010;16:e343-55.
- Lichtenstein BJ, Reuben DB, Karlamangla AS, Han W, Roth CP, Wenger NS. Effect of physician delegation to other healthcare providers on the quality of care for geriatric conditions. J Am Geriatr Soc 2015;63:2164-70. https://doi.org/10.1111/jgs.13654.
- Al-Ani AN, Flodin L, Söderqvist A, Ackermann P, Samnegård E, Dalén N, et al. Does rehabilitation matter in patients with femoral neck fracture and cognitive impairment? A prospective study of 246 patients. Arch Phys Med Rehabil 2010;91:51-7. https://doi.org/10.1016/j.apmr.2009.09.005.
- Dalkey N. The Delphi Method: An Experimental Study of Group Opinion. Santa Monica, CA: Rand Corporation; 1969.
- Dalkey N, Helmer O. An experimental application of the DELPHI method to the use of experts. Manag Sci 1963;9:458-67. https://doi.org/10.1287/mnsc.9.3.458.
- McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm 2016;38:655-62. https://doi.org/10.1007/s11096-016-0257-x.
- Kellogg Foundation . Logic Model Development Guide 2004.
- Dwyer JJ, Makin S. Using a program logic model that focuses on performance measurement to develop a program. Can J Public Health 1997;88:421-5.
- Skills for Health, Health Education England, Skills for Care . Dementia Training Standards Framework 2018.
- Elia M. Screening for Malnutrition: A Multidisciplinary Responsibility – Development and Use of the Malnutrition Universal Screening Tool (‘MUST’) for Adults. Redditch: BAPEN; 2003.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Allan LM, Wheatley A, Flynn E, Smith A, Fox C, Howel D, et al. Is it feasible to deliver a complex intervention to improve the outcome of falls in people with dementia? A protocol for the DIFRID feasibility study. Pilot Feasibility Stud 2018;4. https://doi.org/10.1186/s40814-018-0364-7.
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med 2002;64:510-19. https://doi.org/10.1097/00006842-200205000-00016.
- Hill KD, Schwarz JA, Kalogeropoulos AJ, Gibson SJ. Fear of falling revisited. Arch Phys Med Rehabil 1996;77:1025-9. https://doi.org/10.1016/S0003-9993(96)90063-5.
- Kiresuk TJ, Sherman RE. Goal attainment scaling: a general method for evaluating comprehensive community mental health programs. Community Ment Health J 1968;4:443-53. https://doi.org/10.1007/BF01530764.
- Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142-8. https://doi.org/10.1111/j.1532-5415.1991.tb01616.x.
- Gélinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Ther 1999;53:471-81. https://doi.org/10.5014/ajot.53.5.471.
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649-55. https://doi.org/10.1093/geront/20.6.649.
- Stolee P, Stadnyk K, Myers AM, Rockwood K. An individualized approach to outcome measurement in geriatric rehabilitation. J Gerontol A Biol Sci Med Sci 1999;54:M641-7. https://doi.org/10.1093/gerona/54.12.M641.
- Rodgers H, Shaw L, Cant R, Drummond A, Ford GA, Forster A, et al. Evaluating an eXTended rehAbilitation service for Stroke patients (EXTRAS): study protocol for a randomised controlled trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-0704-3.
- Rodgers H, Shaw L, Bosomworth H, Aird L, Alvarado N, Andole S, et al. Robot Assisted Training for the Upper Limb after Stroke (RATULS): study protocol for a randomised controlled trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-2083-4.
- Colver AF, Merrick H, Deverill M, Le Couteur A, Parr J, Pearce MS, et al. Study protocol: longitudinal study of the transition of young people with complex health needs from child to adult health services. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-675.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Research Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Shanyinde M, Pickering RM, Weatherall M. Questions asked and answered in pilot and feasibility randomized controlled trials. BMC Med Res Methodol 2011;11. https://doi.org/10.1186/1471-2288-11-117.
- Bugge C, Williams B, Hagen S, Logan J, Glazener C, Pringle S, et al. A process for Decision-making after Pilot and feasibility Trials (ADePT): development following a feasibility study of a complex intervention for pelvic organ prolapse. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-353.
- Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 2008;19:385-97. https://doi.org/10.1007/s00198-007-0543-5.
- Li SA, Jeffs L, Barwick M, Stevens B. Organizational contextual features that influence the implementation of evidence-based practices across healthcare settings: a systematic integrative review. Syst Rev 2018;7. https://doi.org/10.1186/s13643-018-0734-5.
- Read ST, Toye C, Wynaden D. The participation of people with dementia in the planning of their care and support: an integrative literature review [published online ahead of print 1 January 2018]. Dementia 2018. https://doi.org/10.1177/1471301218784806.
- Vermunt NPCA, Harmsen M, Westert GP, Olde Rikkert MGM, Faber MJ. Collaborative goal setting with elderly patients with chronic disease or multimorbidity: a systematic review. BMC Geriatr 2017;17. https://doi.org/10.1186/s12877-017-0534-0.
- Delbaere K, Close JC, Taylor M, Wesson J, Lord SR. Validation of the Iconographical Falls Efficacy Scale in cognitively impaired older people. J Gerontol A Biol Sci Med Sci 2013;68:1098-102. https://doi.org/10.1093/gerona/glt007.
- Chan PPW, Chan APS, Lau E, Delbaere K, Chan YH, Jin XK, et al. Translation and validation study of the Chinese version Iconographical Falls Efficacy Scale-Short Version (Icon-FES). Arch Gerontol Geriatr 2018;77:1-7. https://doi.org/10.1016/j.archger.2018.03.008.
- Harwood RH, van der Wardt V, Goldberg SE, Kearney F, Logan P, Hood-Moore V, et al. A development study and randomised feasibility trial of a tailored intervention to improve activity and reduce falls in older adults with mild cognitive impairment and mild dementia. Pilot Feasibility Stud 2018;4. https://doi.org/10.1186/s40814-018-0239-y.
- Russell P, Banerjee S, Watt J, Adleman R, Agoe B, Burnie N, et al. Improving the identification of people with dementia in primary care: evaluation of the impact of primary care dementia coding guidance on identified prevalence. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-004023.
- Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med 1986;80:429-34. https://doi.org/10.1016/0002-9343(86)90717-5.
- Berg K, Wood-Dauphine S, Williams JI, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiother Can 1989;41:304-11. https://doi.org/10.3138/ptc.41.6.304.
- Smith R. Validation and reliability of the Elderly Mobility Scale. Physiotherapy 1994;80:744-7. https://doi.org/10.1016/S0031-9406(10)60612-8.
- Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998.
- Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for predicting pressure sore risk. Nurs Res 1987;36:205-10. https://doi.org/10.1097/00006199-198707000-00002.
- Tinetti ME, Richman D, Powell L. Falls efficacy as a measure of fear of falling. J Gerontol 1990;45:P239-43. https://doi.org/10.1093/geronj/45.6.P239.
Appendix 1 Review of approaches to economic evaluation search strategy
MEDLINE, EMBASE and NHS Economic Evaluation Database
Date searched: August 2016.
Search strategy
-
#1 exp dementia/or exp cognition disorders/
-
#2 exp Supranuclear Palsy, Progressive/or exp Hydrocephalus, Normal Pressure/
-
#3 (Dementia? or Amentia? or Alzheimer* or cogniti* impair*).ti,ab,hw,kw.
-
#4 ((Creutzfeldt-Jakob or huntington? or kluver-bucy or lewy-bod* or (lewy adj2 bod*)) adj3 (Syndrome or disease or disorder or dementia?)).ti,ab,hw,kw.
-
#5 ((normal adj2 hydrocephalus) or (supranuclear adj1 palsy) or (picks adj1 (disorder or disease))).ti,ab,hw,kw.
-
#6 or/1-5
-
#7 ((Accidental* adj3 Fall?) or Falls or Fall-related or Fracture? or ((bone? or hip or femur or tibia or arm?) adj3 broken)).mp.
-
#8 (fall* adj3 injur*).mp.
-
#9 exp fractures, bone/
-
#10 accidental falls/
-
#11 or/7-10
-
#12 (QOL or (quality adj2 life)).mp.
-
#13 exp Activities of Daily Living/
-
#14 HRQoL.mp.
-
#15 or/12-14
-
#16 (utiliti* or disutili*).mp.
-
#17 economics/
-
#18 exp ‘costs and cost analysis’/
-
#19 economics, dental/
-
#20 exp economics, hospital/
-
#21 Economics, Medical/
-
#22 economics, nursing/
-
#23 economics, pharmaceutical/
-
#24 (economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab.
-
#25 (expenditure not energy).ti,ab.
-
#26 value for money.ti,ab.
-
#27 budget$.ti,ab.
-
#28 or/16-28
-
#29 ((energy or oxygen) adj cost).ti,ab.
-
#30 (metabolic adj cost).ti,ab.
-
#31 ((energy or oxygen) adj expenditure).ti,ab.
-
#32 or/29-31
-
#33 28 not 32
-
#34 letter.pt.
-
#35 editorial.pt.
-
#36 historical article.pt.
-
#37 or/34-36
-
#38 33 not 37
-
#39 exp animals/not humans/
-
#40 38 not 39
-
#41 6 and 11 and (15 or 40)
Appendix 2 Read codes for general practitioner dementia Quality Outcomes Framework register
Parts of this appendix are reproduced from Russell et al. 170 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 3.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/3.0/.
Where some diagnostic data are available, the code ‘Eu00.’ can be used for Alzheimer’s disease, ‘Eu002’ can be used for mixed dementia and ‘Eu01.’ can be used for vascular dementia. All others can be given ‘Eu02z’.
Recommended Read codes
| ICD-10 code | Diagnosis | Read code |
|---|---|---|
| F00 | Dementia in Alzheimer’s disease | Eu00. |
| F00.2 | Dementia in Alzheimer’s disease, atypical or mixed type (‘Mixed Dementia’) | Eu002 |
| F01 | Vascular dementia | Eu01. |
| F03 | Unspecified dementia | Eu02z |
Where detailed information on subtype of dementia is available, then the Read codes below can be used. The below table matches the International Classification of Diseases, Tenth Edition (ICD-10),69 codes to recognised general practice dementia Read codes.
All Read codes
| ICD-10 code | Diagnosis | Read code |
|---|---|---|
| F00 | Dementia in Alzheimer’s disease | Eu00. |
| F00.0 | Dementia in Alzheimer’s disease with early onset | Eu000 |
| F00.1 | Dementia in Alzheimer’s disease with late onset | Eu001 |
| F00.2 | Dementia in Alzheimer’s disease, atypical or mixed type | Eu002 |
| F00.9 | Dementia in Alzheimer’s disease, unspecified | Eu00z |
| F01 | Vascular dementia | Eu01. |
| Arteriosceloritic dementia | E004 | |
| F01.1 | Multi-infarct dementia | Eu011 |
| F01.2 | Subcortical vascular dementia | Eu012 |
| F01.3 | Mixed cortical and subcortical vascular dementia | Eu013 |
| F01.8 | Other vascular dementia | Eu01y |
| F01.9 | Vascular dementia, unspecified | Eu01z |
| Uncomplicated arteriosclerotic dementia | E0040 | |
| Arteriosclerotic dementia with delirium | E0041 | |
| Arteriosclerotic dementia with paranoia | E0042 | |
| Arteriosclerotic dementia with depression | E0043 | |
| Arteriosclerotic dementia NOS | E004z | |
| F02 | Dementia in other diseases classified elsewhere | Eu02. |
| F02.0 | Dementia in Pick’s disease | Eu020 |
| F02.1 | Dementia in Creutzfeldt–Jakob disease | Eu021 |
| F02.2 | Dementia in Huntington’s disease | Eu022 |
| F02.3 | Dementia in Parkinson’s disease | Eu023 |
| F02.4 | Dementia in HIV disease | Eu024 |
| F02.8 | Dementia in other disease classified elsewhere | Eu02y |
| Dementia in conditions | E041 | |
| F03 | Unspecified dementia | Eu02z |
| Presenile dementia | E001. | |
| Uncomplicated presenile dementia | E0010 | |
| Presenile dementia with delirium | E0011 | |
| Presenile dementia with paranoia | E0012 | |
| Presenile dementia with depression | E0013 | |
| Presenile dementia NOS | E001z | |
| Uncomplicated senile dementia | E000 | |
| Senile dementia with depressive or paranoid features | E002 | |
| Senile dementia with paranoia | E0020 | |
| Senile dementia with depression | E0021 | |
| Senile dementia with depressive or paranoid features NOS | E002z | |
| F05.1 | Delirium superimposed on dementia | Eu041 |
| Senile dementia with delirium | E003 | |
| F05.9 | Delirium, unspecified | Eu04z |
| F06.0 | Organic hallucinosis | Eu050 |
| Other senile and presenile organic psychoses | E00y | |
| Senile or presenile psychoses | E00z | |
| F06.7 | Mild cognitive disorder | Eu057 |
| F10.7 | Residual and late-onset psychotic disorder due to alcohol. Including: | Eu107 |
| • Alcoholic dementia | Eu10711 | |
| • Other alcoholic dementia | E012 | |
| • Chronic alcoholic brain syndrome | E0120 | |
| G30 | Alzheimer’s disease | F110. |
| G30.8 | Other Alzheimer’s disease | |
| G30.9 | Alzheimer’s disease, unspecified | |
| G30.0 | Alzheimer’s disease with early onset | F1100 |
| G30.1 | Alzheimer’s disease with late onset | F1101 |
| G31.0 | Circumscribed brain atrophy. Including: | |
| • Frontotemporal dementia | No code | |
| • Pick’s disease | F111. | |
| • Progressive isolated aphasia | ||
| G31.1 | Senile degeneration of the brain, not elsewhere classified | F112. |
| G31.8 | Other specified degenerative disease of the nervous system. Including: | |
| • Grey matter degeneration | ||
| • Lewy body disease | F116 | |
| • Lewy body dementia | Eu025 | |
| • Subacute necrotising encephalopathy |
Appendix 3 Falls diary for work package 2
Newcastle University logo used with permission.
Appendix 4 Responses to the Health Utilisation Questionnaire in work package 2
| Area of resource use | Number of people who have seen that service | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diary 1 (n = 9) | Diary 2 (n = 6) | Diary 3 (n = 6) | ||||||||||
| Week | ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Used an NHS service? | 8 | 9 | 9 | 8 | 6 | 6 | 6 | 6 | 6 | 5 | 5 | 5 |
| Have you seen a GP – general practice? | 7 | 9 | 8 | 8 | 5 | 5 | 6 | 6 | 5 | 5 | 5 | 5 |
| Have you seen a GP – home? | 7 | 9 | 8 | 8 | 4 | 5 | 6 | 6 | 5 | 5 | 5 | 5 |
| Have you spoken to a GP (phone)? | 7 | 9 | 8 | 8 | 4 | 5 | 6 | 6 | 5 | 5 | 5 | 5 |
| Have you seen a nurse – general practice? | 7 | 9 | 8 | 8 | 4 | 5 | 6 | 6 | 5 | 5 | 5 | 5 |
| Have you seen a nurse – home? | 7 | 9 | 8 | 8 | 4 | 5 | 6 | 6 | 5 | 5 | 5 | 5 |
| Have you spoken to a nurse (phone)? | 7 | 9 | 8 | 8 | 5 | 5 | 6 | 6 | 5 | 5 | 5 | 5 |
| Have you seen an OT – general practice? | 7 | 9 | 7 | 8 | 4 | 5 | 6 | 6 | 6 | 5 | 5 | 5 |
| Have you seen an OT – home? | 7 | 9 | 7 | 8 | 4 | 5 | 6 | 6 | 5 | 5 | 5 | 5 |
| Have you seen an OT – hospital? | 7 | 9 | 8 | 8 | 4 | 5 | 6 | 6 | 5 | 5 | 5 | 5 |
| Have you seen a physiotherapist – general practice? | 8 | 8 | 8 | 8 | 4 | 5 | 5 | 6 | 5 | 5 | 5 | 5 |
| Have you seen a physiotherapist – home? | 8 | 8 | 8 | 8 | 5 | 5 | 5 | 6 | 6 | 5 | 5 | 5 |
| Have you spoken to a physiotherapist (phone)? | 8 | 8 | 8 | 8 | 4 | 5 | 5 | 6 | 5 | 5 | 5 | 5 |
| Have you seen a physiotherapist – hospital? | 8 | 9 | 8 | 8 | 4 | 5 | 6 | 6 | 5 | 5 | 5 | 5 |
| Have you seen a physiotherapist – day unit? | 8 | 8 | 8 | 8 | 4 | 5 | 5 | 6 | 5 | 5 | 5 | 5 |
| Have you seen a physiotherapist (connect health care)? | 8 | 8 | 8 | 8 | 4 | 5 | 5 | 6 | 5 | 5 | 5 | 5 |
| Have you attended an outpatient appointment? | 8 | 9 | 9 | 8 | 5 | 5 | 6 | 6 | 6 | 5 | 5 | 5 |
| Have you used an emergency ambulance? | 8 | 9 | 9 | 8 | 5 | 5 | 6 | 6 | 6 | 5 | 5 | 5 |
| Have you attended a day hospital (rehabilitation unit)? | 8 | 9 | 9 | 8 | 5 | 5 | 6 | 6 | 5 | 5 | 5 | 5 |
| Have you been to a rehabilitation class? | 8 | 9 | 9 | 8 | 6 | 6 | 6 | 6 | 6 | 5 | 5 | 5 |
| Have you attended ED? | 6 | 7 | 7 | 8 | 5 | 6 | 6 | 6 | 5 | 5 | 5 | 5 |
| Have you stayed on a hospital ward for a day only? | 6 | 8 | 7 | 8 | 5 | 6 | 6 | 6 | 4 | 5 | 5 | 5 |
| Have you stayed on a hospital ward overnight? | 6 | 8 | 7 | 8 | 5 | 6 | 6 | 6 | 4 | 5 | 5 | 5 |
| Resource | n | Resource use | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Minimum | Maximum | ||
| Number of weeks reporting NHS resource use | 9 | 2.89 (2.52) | 3 (0–5) | 0 | 6 |
| Number of GP visits – general practice | 9 | 0.77 (1.39)a | 0 (0–1) | 0 | 4 |
| Number of GP visits – home | 9 | 0.11 (0.33) | 0 (0–0) | 0 | 1 |
| Number of GP visits – phone | 9 | 0.11 (0.33) | 0 (0–0) | 0 | 1 |
| Number of nurse visits – practice | 9 | 1.00 (1.66) | 0 (0–1) | 0 | 5 |
| Number of nurse visits – home | 9 | 0.33 (0.71) | 0 (0–0) | 0 | 2 |
| Number of nurse visits – phone | 9 | 0.33 (0.71) | 0 (0–0) | 0 | 2 |
| Number of OT visits | 9 | 0.11 (0.33) | 0 (0–0) | 0 | 1 |
| Number of OT visits – home | 9 | 0.33 (1.00) | 0 (0–0) | 0 | 3 |
| Number of OT visits – hospital | 9 | 0.22 (0.67) | 0 (0–0) | 0 | 2 |
| Number of physiotherapist visits | 9 | 0.00 (0.00) | 0 (0–0) | 0 | 0 |
| Number of physiotherapist visits – home | 9 | 0.11 (0.33) | 0 (0–0) | 0 | 1 |
| Number of physiotherapist visits – phone | 9 | 0.00 (0.00) | 0 (0–0) | 0 | 0 |
| Number of physiotherapist visits – hospital | 9 | 0.44 (1.33) | 0 (0–0) | 0 | 4 |
| Number of physiotherapist visits – day unit | 9 | 0.22 (0.67) | 0 (0–0) | 0 | 2 |
| Physiotherapist visits – connect health care | 9 | 0.00 (0.00) | 0 (0–0) | 0 | 0 |
| Number of outpatient visits | 9 | 0.44 (0.53) | 0 (0–1) | 0 | 1 |
| Number of ambulance visits | 9 | 0.33 (0.71) | 0 (0–0) | 0 | 2 |
| Number of ambulance visits – hospital | 9 | 0.22 (0.44) | 0 (0–0) | 0 | 1 |
| Number of rehabilitation visits | 9 | 0.56 (0.88) | 0 (0–1) | 0 | 2 |
| Number of ED visits | 9 | 0.33 (0.71) | 0 (0–0) | 0 | 2 |
| Number of day-case visits | 9 | 3.11 (9.33) | 0 (0–0) | 0 | 28 |
| Number of inpatient nights | 9 | 3.33 (10.00) | 0 (0–0) | 0 | 30 |
Appendix 5 Work package 2 self-reported paid help
| Participant | Diary | Type of paid help | Total cost per month (£) | Total cost per participant (£) |
|---|---|---|---|---|
| 1 | 1 | Cleaning | 25 | 25 |
| 3 | Cleaning | 0 | ||
| 2 | 1 | Cleaning (one morning) | 30 | 30 |
| 3 | 1 | Emergency call pendant | 30 | 30 |
| 4 | 1 | Private cleaning, transport and support | 90 | 256 |
| 2 | Cleaner for 2 hours, HCA for 3 hours | 76 | ||
| 3 | Call line, cleaner, welfare check | 90 | ||
| 5 | 1 | Cleaner | 50 | 150 |
| 2 | Cleaner | 50 | ||
| 3 | Cleaner | 50 | ||
| Total | 491 | 491 | ||
Appendix 6 Qualitative topic guides
Work package 2: patient and carer interviews
-
Introduction.
-
Your recent fall.
-
Experience of <name of service>.
-
Suitability of service for people with dementia.
-
-
Recovering from the fall.
-
Improving services and developing a new approach for people with memory problems who have fallen and hurt themselves.
Work package 2: professional interviews and focus groups
-
Role and service.
-
Clientele.
-
Referral routes into the service.
-
Onward trajectory.
-
‘Fit’ of people with dementia within the service (including group activities).
-
Views on perceived value of specific intervention for people with dementia.
-
Views on key components of an intervention for people with dementia.
-
Specific training needs in relation to people with dementia with fall-related injuries.
-
Facilitators and barriers to implementing change.
-
Use of outcome measures.
Work package 4: patient interviews
-
Did you feel this was a good intervention for you?
-
Tell me more about that.
-
-
What did you like about the intervention sessions?
-
What did you dislike? What could have been different?
-
Which aspects of the DIFRID intervention have been most useful?
-
Which aspects of the DIFRID intervention have been least useful?
-
How did you feel about the activities that you were asked to do? (Were they personalised enough?)
-
Has the intervention made any difference to you?
-
Thinking about the intervention materials such as the diary and the manual, are there any changes that you think we should make?
-
Could you tell me a bit about the staff delivering the intervention?
-
Can you tell me a bit about the goal you have been working towards?
-
What kinds of patients do you think would benefit most from this type of intervention? Are there patients for whom it would not be useful?
-
Is there anything else that we haven’t covered about the DIFRID intervention?
Work package 4: carer interviews
-
What were/are your expectations about the DIFRID intervention?
-
Did you feel this was a good intervention for <name>?
-
Tell me more about that.
-
What did you like about the intervention sessions?
-
What did you dislike? What could have been different?
-
How engaged did <name> seem to be in the intervention?
-
Which aspects of the DIFRID intervention have been most useful?
-
Which aspects of the DIFRID intervention have been least useful?
-
How did you feel about the goals that <name> has been working towards?
-
-
Has the intervention made any difference to <name>?
-
What about yourself, how involved have you been in the intervention?
-
Thinking about the intervention materials such as the diary and the manual, are there any changes that you think we should make?
-
The DIFRID intervention is delivered by physiotherapists, occupational therapists and rehabilitation assistants. From your perspective what are the advantages and disadvantages of using staff with this skill mix?
-
Could you talk about your perception of the staff delivering the intervention?
-
What kinds of patients do you think would benefit most from this type of intervention? Are there patients for whom it would not be useful?
-
Is there anything else that we haven’t covered about the DIFRID intervention?
Work package 4: interviews with staff responsible for recruitment and assessment of outcomes
-
Can you start by telling me about how the recruitment process has worked in practice?
-
How interested do patients seem to be in the intervention?
-
Have there been any patients who met the inclusion criteria but you felt were not appropriate for the study?
-
What sense do you have of how feasible it would be to proceed to a full trial with a control group etc.?
-
Can you tell me about how the assessment processes and outcome measures have worked in practice?
-
What sense do you have of how useful the intervention is?
Work package 4: interviews with staff responsible for developing the intervention and training and supervising intervention delivery
-
How do you think the training went?
-
How has the supervision process been?
-
How do you think the study/intervention is going so far?
-
How confident are you in the intervention?
-
What reservations do you have about the intervention?
-
Which aspects of the DIFRID intervention do you feel most confident with/have been most useful?
-
Which aspects of the DIFRID intervention do you find most challenging/have been least useful?
-
Overall, what sense do you have of how useful the intervention is?
-
In this study, the DIFRID intervention is delivered by physiotherapists, occupational therapists and rehabilitation assistants. From your perspective what are the advantages and disadvantages of using staff with this skill mix?
-
What kinds of patients do you think would benefit most from this type of intervention? Are there patients for whom it would not be useful?
-
Based on feedback from supervision, how interested do patients seem to be in the intervention?
-
From your perspective, what are the facilitators and barriers to getting people/patients engaged in the study?
-
From your perspective, what are the facilitators and barriers to implementing the intervention?
-
From your perspective, what are the facilitators and barriers to evaluating the acceptability and impacts of the DIFRID intervention?
-
Are there any changes we should make to the DIFRID intervention?
-
Is there anything else that we haven’t covered about the DIFRID intervention?
Work package 4: interviews and focus groups with staff delivering the intervention
-
What are/were your expectations about the DIFRID intervention?
-
How do you think the study/intervention is going so far?
-
How confident are you in the intervention?
-
What reservations do you have about the intervention?
-
Which aspects of the DIFRID intervention do you feel most confident with/have been most useful?
-
Which aspects of the DIFRID intervention do you find most challenging/have been least useful?
-
What opportunities have you had to discuss the value of the intervention with your colleagues?
-
How might we modify the DIFRID intervention?
-
From your perspective, what are the facilitators and barriers to implementing the intervention?
-
Overall, what sense do you have of how useful the intervention is?
-
In this study, the DIFRID intervention is delivered by physiotherapists, occupational therapists and rehabilitation assistants. From your perspective what are the advantages and disadvantages of using staff with this skill mix?
-
What kinds of patients do you think would benefit most from this type of intervention? Are there patients for whom it would not be useful?
-
Based on feedback from supervision, how interested do patients seem to be in the intervention?
-
From your perspective, what are the facilitators and barriers to getting people/patients engaged in the study?
-
Could you describe the process of tailoring the intervention to the individual patient?
-
How helpful were different components of the intervention (e.g. training, manual, MDT meetings, supervision)?
-
Are there any changes we should make to the intervention materials (e.g. the assessment form)?
-
From your perspective, how well did the intervention ‘fit’ with other services?
-
Do you feel you have the support you need to deliver the intervention?
-
What dementia training had you previously received?
-
How do you think the training went?
-
How well has the supervision process gone?
-
In terms of taking this work forward, how could we improve the initial training session(s)?
-
How will your experience with the DIFRID intervention influence your usual practice in the future?
-
Is there anything else that we haven’t covered about the DIFRID intervention?
Appendix 7 Outcome measures reported in the qualitative study
| Domain | Number of times mentioned |
|---|---|
| Quality of life | 3 |
| Functional ability | |
| Barthel Index31 | 4 |
| Tinetti Balance Assessment171 | 5 |
| Berg Balance Scale172 | 3 |
| Elderly Mobility Scale173 | 2 |
| Rated Perceived Exertion Scale174 | 1 |
| Braden score175 | 1 |
| Goal-setting | 5 |
| Psychological well-being | |
| MFES/FES176 | 1 |
| Carer well-being | 2 |
| Patient and/or carer satisfaction | 8 |
Appendix 8 Example initial realist review search strategy
MEDLINE, Cochrane Central Register of Controlled Trials, Health Management Information Consortium, EMBASE, Cumulative Index to Nursing and Allied Health Literature, Web of Science, Allied and Complementary Medicine Database and Physiotherapy Evidence Database
Date searched: November 2015.
The MEDLINE literature search strategy is provided here. This strategy was translated as necessary for each of the resources searched. This is one example of the initial broad search strategy. Parallel research strategies were created for other databases; the one shown in here was for MEDLINE (Ovid).
Search strategy
-
exp dementia/
-
exp Supranuclear Palsy, Progressive/or exp Hydrocephalus, Normal Pressure/
-
(Dementia? or Amentia? or Alzheimer*).ti,ab,hw,kw.
-
((Creutzfeldt-Jakob or huntington? or kluver-bucy or lewy-bod* or (lewy adj2 bod*)) adj3 (Syndrome or disease or disorder or dementia?)).ti,ab,hw,kw.
-
((normal adj2 hydrocephalus) or (supranuclear adj1 palsy) ((picks adj1 (disorder or disease)).ti,ab,hw,kw.
-
or/1-5
-
((Accidental* adj3 Fall?) or Falls or Fall-related or Fracture? or ((bone? or hip or femur or tibia or arm?) adj3 broken)).mp.
-
(fall* adj3 injur*).mp.
-
exp fractures, bone/
-
accidental falls/
-
or/7-10
-
exp accident prevention/
-
(preventi* or prevent).mp.
-
intervention?.mp.
-
exp Rehabilitation/
-
rehabilitat*.mp.
-
exp Nutrition Therapy/
-
((nutrition* or ergonomic or exercise or occupational or physical) adj3 (support* or therap*)).mp.
-
physiotherap*.mp.
-
(improv* adj5 (outcome? or care)).mp.
-
management.mp.
-
((psycho* or physical* or mobility) adj5 (outcome? or improv*)).mp.
-
(decreas* adj2 risk?).mp.
-
((improv* or increas*) adj5 (social* or participation or independence or activit* or well?being or QOL or (quality adj2 life))).mp.
-
exp Activities of Daily Living/
-
((multifactorial or multicomponent or multidisciplinary) adj3 (team? or assessment or intervention?)).mp.
-
recovery.mp.
-
HRQoL.mp.
-
or/12-28
-
6 and 11 and 29
Appendix 9 Example targeted realist review search strategy
The following search strategy was conducted to identify studies providing evidence for a connection between pain relief and rehabilitation outcomes (CMOc1). No such studies were found; only studies providing evidence for a link between pain relief and aggressive/challenging behaviour and studies reporting methods of assessing pain were found.
MEDLINE and Cumulative Index to Nursing and Allied Health Literature
Date searched: February and March 2017.
Search strategy
| # | Search terms | Records | Notes |
|---|---|---|---|
| #9 | MM Pain AND (MM Dementia OR MM Aged+) AND MM Rehabilitation | 0 | |
| #10 | (MM Dementia OR MM Aged+) AND MM Rehabilitation | 13 | |
| #11 | (MM Dementia OR MM Aged+) AND TI (pain N2 (relie* or medic* or manag* or assess*)) OR AB (pain N2 (relie* or medic* or manag* or assess*)) AND MM Rehabilitation | 6 | Using keywords instead of thesaurus heading for pain |
| #12 | (MM Dementia OR MM Aged+) AND TI (pain N2 (relie* or medic* or manag* or assess*)) OR AB (pain N2 (relie* or medic* or manag* or assess*)) AND TI (outcome* or benefit* or effect* or recover*) OR AB (outcome* or benefit* or effect* or recover*) | 73 | Using some keyword synonyms instead of thesaurus heading for rehabilitation |
| #13 | (MM Dementia OR MM Aged+) AND MM Rehabilitation+ AND MM Pain+/pc | 2 | Using ‘explode’ for rehabilitation; using ‘prevention & control’ subheading for pain |
| #14 | MM Pain+/pc AND MH Dementia/rh | 0 | Using ‘rehabilitation’ subheading for dementia |
Appendix 10 Consensus statements
| Statement | Outcome | Round | Percentage | Final selection |
|---|---|---|---|---|
| Feasibility, design and inclusion criteria of the study | ||||
| The brief requires us to design a complex intervention | Agreed | 1 | 92.9 | As statement |
| Patients with non-injurious falls should be eligible for the intervention | Agreed | 1 | 100.0 | As statement |
| Fallers with an acute medical illness causing their fall, e.g. pneumonia or stroke, are included | No consensus | 2 | 46.2 | Include |
| Fallers should be recruited either within 1 week of the fall or 1 month of the fall | No consensus | 2 | 53.8, 46.2 | 1 month |
| A feasible and useful sample size would be (% given for range of choices up to 39 participants) | No consensus | 2 | 61.5 | Up to 30 participants |
| The number of sites included should be three sites | Agreed | 2 | 76.9 | Three sites |
| It is feasible to recruit to WP4 | Agreed | 2 | 100.0 | As statement |
| Setting of the study | ||||
| It would be useful to recruit participants presenting with a fall in the emergency department | Agreed | 1 | 92.9 | As statement |
| It would be useful to recruit participants presenting with a fall to paramedics if single ambulance stations are targeted | Agreed | 1 | 92.9 | As statement |
| It would be useful to recruit participants presenting with a fall in the primary care setting | Agreed | 1 | 85.8 | As statement |
| If we are recruiting participants who have had a fall within the last week, it would be useful for GPs to write to all patients on their QOF dementia register | No consensus | 2 | 30.8 | Rejected as we will not be recruiting patients up to 1 week after a fall |
| If we are recruiting participants who have had a fall within the last month, it would be useful for GPs to write to all patients on their QOF dementia register | Agreed | 2 | 84.6 | As statement |
| It would be useful to recruit participants in another setting | Agreed | 1 | 78.6 | As statement |
| Mean priorities for alternative settings | ||||
| Community services e.g. multidisciplinary outreach teams | 3.1 | Include | ||
| Domiciliary physiotherapy | 4.6 | Exclude | ||
| Supported discharge teams | 3.4 | Include | ||
| Telecare services | 3.4 | Include | ||
| Social services re-enablement teams | 4.4 | Exclude | ||
| Memory clinics | 4.4 | Exclude | ||
| Dementia cafes | 5.5 | Exclude | ||
| Social media | 7.2 | Exclude | ||
| The intervention should primarily take place in the patient’s home | Agreed | 1 | 85.7 | As statement |
| The setting of the intervention should make use of existing pathways only when referral from the team deems it would be useful for the individual | Agreed | 1 | 85.7 | As statement |
| Content of the intervention (staff) | ||||
| A physiotherapist should be routinely involved | Agreed | 1 | 71.4 | As statement |
| An occupational therapist should be routinely involved | Agreed | 1 | 71.4 | As statement |
| A geriatrician should be routinely involved via multidisciplinary team meeting and available for face-to-face consultation if required | No consensus | 2 | 61.5 | As statement |
| A rehabilitation support worker should be routinely involved | Agreed | 1 | 71.4 | As statement |
| A registered general nurse should be routinely involved via multidisciplinary team meeting and available for face-to-face consultation if required | No consensus | 2 | 61.5 | As statement |
| A community psychiatric nurse should be available on referral | Agreed | 1 | 71.4 | As statement |
| A social worker should be available on referral | Agreed | 1 | 71.4 | As statement |
| Re-enablement workers should be available on referral | Agreed | 1 | 71.4 | As statement |
| An old age psychiatrist should be available on referral | Agreed | 2 | 84.6 | As statement |
| A podiatrist should be available on referral | Agreed | 2 | 92.3 | As statement |
| Content of the intervention (assessment) | ||||
| Assessment should involve multiple sources of information including information from carers | Agreed | 1 | 100.0 | As statement |
| Assessment should include direct observation | Agreed | 1 | 100.0 | As statement |
| Formal assessments of gait and balance should be carried out by the Timed Up and Go Test | No consensus | 2 | 61.5 | As statement |
| A home hazard assessment should include a walk around the house to determine where actual falls have occurred and negotiate how these might be reduced | Agreed | 1 | 92.9 | As statement |
| An assessment of comorbidities is required | Agreed | 1 | 100.0 | As statement |
| An osteoporosis risk assessment is required | Agreed | 1 | 92.9 | As statement |
| A vision assessment is required | Agreed | 1 | 100.0 | As statement |
| A medication review is required | Agreed | 1 | 100.0 | As statement |
| All patients require attendance for a lying and standing BP | No consensus | 2 | 53.8 | As statement: to be carried out by therapist in the patient’s home |
| A continence assessment is required | Agreed | 1 | 78.6 | As statement |
| An assessment of challenging behaviour is required | Agreed | 1 | 92.9 | As statement |
| Tools which assess non-verbal signs of pain should be used | Agreed | 1 | 92.9 | As statement |
| A multidisciplinary team meeting should be available if needed | Agreed | 1 | 92.9 | As statement |
| Carer stress should be routinely assessed | Agreed | 1 | 92.9 | As statement |
| Content of the intervention (methodology and quantity) | ||||
| Interventions should be based on goals set by the patient and carer | Agreed | 1 | 85.7 | As statement |
| Therapists should work with service users to minimise the risk of falling, as this may improve confidence and enable realistic risk taking | Agreed | 1 | 100.0 | As statement |
| Therapists should facilitate caregivers, family and friends to adopt a positive approach to risk | Agreed | 1 | 100.0 | As statement |
| Exercise interventions should be informed by evidence-based formats such as the Otago programme but tailored to the circumstances of people with dementia and embedded in their daily life | Agreed | 2 | 69.2 | As statement |
| The total number of physiotherapy sessions available in the first 3 months (including sessions delivered by a support worker) should be 16, 20 or 24 | No consensus | 2 | 30.8, 38.5, 30.8 | 20 sessions: twice weekly (weeks 0–8) tapering to once weekly (weeks 9–12) |
| The total number of occupational therapy sessions available in the first 3 months should be 3–4 | No consensus | 2 | 61.5 | Four sessions |
| Therapists should offer service users information on assistive devices and facilitate delivery | Agreed | 1 | 100.0 | As statement |
| Therapists should help the service user and caregiver to develop a meaningful programme of activities | Agreed | 1 | 100.0 | As statement |
| Therapists should undertake observed activities with the service user to facilitate new learning | Agreed | 1 | 92.9 | As statement |
| Intervention staff should be able to provide basic carer education and support, referring to other agencies as needed | Agreed | 2 | 76.9 | As statement |
| Staff training | ||||
| Tier 2 training is required for intervention staff | Agreed | 2 | 84.6 | As statement |
| Training needs to include how to tailor an intervention for a person with dementia | Agreed | 1 | 100.0 | As statement |
| Training needs to include advice on how to engage and motivate a person with dementia | Agreed | 1 | 100.0 | As statement |
| Training should include on-the-job role modelling | Agreed | 1 | 100.0 | As statement |
| Outcome measures for the intervention | ||||
| The primary outcome measure be a numerical measure of falls | Agreed | 2 | 76.9 | As statement |
| Secondary outcomes should include health-related quality-of-life measure | Agreed | 1 | 100.0 | As statement |
| The best health-related quality-of-life measure would be Quality of Life in Alzheimer’s Disease (QOL-AD) | Agreed | 2 | 69.2 | As statement |
| Secondary outcomes should include activities of daily living measure | Agreed | 1 | 92.9 | As statement |
| The best activities of daily living measure would be Disability Assessment in Dementia (DAD) | Agreed | 2 | 84.6 | As statement |
| Secondary outcomes should include carer burden measure | Agreed | 1 | 92.9 | As statement |
| The best carer burden measure would be Zarit Burden Interview | Agreed | 2 | 69.2 | As statement |
| Secondary outcomes should include psychological consequences of falling measure | Agreed | 1 | 85.7 | As statement |
| The best psychological consequence measure e.g. fear of falling would be the Modified Falls Efficacy Scale | Agreed | 1 | 71.4 | As statement |
| Secondary outcomes should include physical activity measure | No consensus | 1 | 64.2 | As statement |
| The best physical activity measure would be a wearable physical activity monitor | Agreed | 1 | 78.6 | As statement |
| Secondary outcomes should include strength and balance measure | No consensus | 1 | 57.1 | As statement – this would be TUG as in initial assessment |
| Secondary outcomes should include goal-setting or performance measure | No consensus | 1 | 35.7 | As statement |
| The best goal-setting or performance measure would be goal attainment scaling | Agreed | 2 | 84.6 | As statement |
| The best carer quality-of-life measure would be EQ-5D-5L | No consensus | 1 | 57.1 | Exclude – see next statement |
| The most popular carer quality-of-life measure was EQ-5D-5L, but it was suggested that a measure of carer burden would be sufficient | No consensus | 2 | 53.8 | As statement |
| Prioritise the remaining domains where consensus was not achieved: 1 (highest) to 4 (lowest) | ||||
| Goal-setting measure | 2 | 2.0 | Include | |
| Physical activity measure | 2 | 2.5 | Include | |
| Strength and balance measure | 2 | 2.5 | Include | |
| Carer quality of life | 2 | 3.0 | Exclude | |
Appendix 11 Results of prioritisation of potential intervention components
| Potential component | Numbers of consensus panel members | |||||
|---|---|---|---|---|---|---|
| Should this be part of the intervention? | Is this feasible? | |||||
| Essential | Desirable | Undesirable | Yes | No | Don’t know | |
| Develop and disseminate information on local care pathways and eligibility criteria (e.g. by introducing a central point of contact) | 3 | 3 | 4 | 2 | ||
| Provide more flexibility in the duration and frequency of intervention delivery | 2 | 3 | 2 | 4 | ||
| Introduce proactive maintenance/follow-up | 2 | 4 | 1 | 2 | 3 | |
| Improve access to telecare and dedicated first response services | 3 | 2 | 4 | 2 | ||
| Use dementia-friendly design principles to improve the ED environment | 3 | 3 | 3 | 3 | ||
| Extend facilitated discharge services to provide 24-hour cover and include non-hospital recuperation settings | 2 | 3 | 5 | 1 | ||
| Increase opportunities for holistic assessment | 4 | 2 | 3 | 3 | ||
| Identify ways of improving information-sharing across service boundaries | 1 | 2 | 3 | 3 | ||
| Clarify responsibilities for actions (e.g. equipment provision) to ensure that recommendations are put into practice | 3 | 3 | 5 | 1 | ||
| Identify local specialist to provide advice and/or joint working | 2 | 3 | 2 | 4 | ||
| Ensure routine assessment of cognitive function | 5 | 5 | 1 | |||
| Emphasise social support networks as part of discharge procedures | 3 | 2 | 5 | 1 | ||
| Deliver intervention through multidisciplinary, collaborative teams | 5 | 1 | 2 | 2 | 2 | |
| Ensure consistency in staffing for intervention | 2 | 3 | 1 | 2 | 3 | |
| Potential component | Numbers of consensus panel members | |||||
|---|---|---|---|---|---|---|
| Should this be part of the intervention? | Is this feasible? | |||||
| Essential | Desirable | Undesirable | Yes | No | Don’t know | |
| Explore ways of addressing negative attitudes to dementia | 3 | 1 | 3 | 1 | ||
| Increase understanding of dementia including challenging behaviour | 3 | 1 | 3 | 1 | ||
| Provide communication skills training to appropriate staff | 4 | 3 | 1 | |||
| Provide training in person-centred care to appropriate staff | 4 | 3 | 1 | |||
| Provide training for staff on recognising pain and/or permissions to prescribe pain relief | 4 | 3 | 1 | |||
| Implement strategies to increase staff co-operation and engagement with interventions | 3 | 1 | 2 | 2 | ||
| Potential component | Numbers of consensus panel members | |||||
|---|---|---|---|---|---|---|
| Should this be part of the intervention? | Is this feasible? | |||||
| Essential | Desirable | Undesirable | Yes | No | Don’t know | |
| Focus interventions on enjoyable and meaningful activities | 3 | 3 | 5 | 1 | ||
| Explore ways of embedding exercises or activities into daily routines | 2 | 3 | 3 | 1 | 2 | |
| Consider the most appropriate location for the intervention | 5 | 1 | 5 | 1 | ||
| Identify and address barriers to engagement | 5 | 1 | 3 | 3 | ||
| Ensure that basic comfort needs of people with dementia are met prior to assessment or intervention sessions (e.g. pain, food, water) | 6 | 6 | ||||
| Ensure that modifiable risk factors for falls have been addressed | 5 | 4 | 1 | 1 | ||
| Use multiple sources to gather information required to deliver person-centred care (e.g. direct observation, adoption of ‘This is Me’) | 3 | 3 | 4 | 2 | ||
| Time intervention to fit with their routines and daily rhythms | 5 | 3 | 3 | |||
| Identify ways of making the environment for rehabilitation supportive | 4 | 2 | 4 | 1 | 1 | |
| Identify alternative resources to support the person with dementia (e.g. buddy) | 2 | 4 | 3 | 1 | ||
| Implement a model similar to the TOP 5 strategy118 to make use of caregiver expertise and ensure that care is person-centred | 3 | 3 | 5 | 1 | ||
| Potential component | Numbers of consensus panel members | |||||
|---|---|---|---|---|---|---|
| Should this be part of the intervention? | Is this feasible? | |||||
| Essential | Desirable | Undesirable | Yes | No | Don’t know | |
| Assess and address carer burden and stress levels (e.g. referral for carer assessment) | 4 | 4 | ||||
| Identify and activate appropriate carer support services | 4 | 4 | ||||
| Identify and address carer education needs (e.g. positive risk) | 4 | 3 | 1 | |||
| Provide appropriate training for carers involved in delivering interventions | 4 | 3 | 1 | |||
| Involve carers in the decision-making process | 4 | 4 | ||||
Appendix 12 Falls diary for work package 4
Newcastle University logo used with permission.
List of abbreviations
- AD
- Alzheimer’s disease
- ADL
- activities of daily living
- CCG
- Clinical Commissioning Group
- CEAC
- cost-effectiveness acceptability curve
- CGA
- Comprehensive Geriatric Assessment
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CMOc
- context–mechanism–outcome configuration
- CONSORT
- Consolidated Standards of Reporting Trials
- CTA
- clinical trials assistant
- DAD
- Disability Assessment for Dementia
- DIFRID
- Developing an Intervention for Fall related Injuries in Dementia
- EBPRAC
- Encouraging Best Practice in Residential Aged Care
- ED
- emergency department
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- FINALEX
- FINnish ALzheimer disease EXercise trial
- GAS
- goal attainment scaling
- GP
- general practitioner
- HUQ
- Health Utilisation Questionnaire
- ICD-10
- International Classification of Diseases, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- LEDEN
- Effects of a long-term exercise programme on functional ability in people with dementia living in nursing homes
- MDT
- multidisciplinary team
- MFES
- Modified Falls Efficacy Scale
- MMSE
- Mini Mental State Examination
- MoCA
- Montreal Cognitive Assessment
- MRC
- Medical Research Council
- NPT
- normalisation process theory
- OT
- occupational therapist
- PIS
- participant information sheet
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- ProFaNE
- Prevention of Falls Network Europe
- PWD
- people with dementia
- QALY
- quality-adjusted life-year
- QOF
- Quality and Outcomes Framework
- QOL-AD
- Quality of Life in Alzheimer’s Disease
- RCT
- randomised controlled trial
- SD
- standard deviation
- TIDieR
- Template for Intervention Description and Replication
- TOC
- Trial Oversight Committee
- TUG
- Timed Up and Go Test
- VAD
- vascular dementia
- WP
- work package
