Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 16/165/01. The contractual start date was in November 2017. The draft report began editorial review in February 2020 and was accepted for publication in December 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Flaherty et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Background and aims
The workHORSE project
This report is structured in chapters to provide a comprehensive description of the project. Chapter 1 provides an introduction to and the background of the project. Chapter 2 describes how we co-produced the workHORSE (working Health Outcomes Research Simulation Environment) tool and its user interface. Chapter 3 details how we updated the evidence base through interviews with the best-performing local authorities (LAs) and an umbrella review of strategies to improve uptake of screening programmes. Chapter 4 describes the workHORSE model’s methods, inputs and assumptions. Chapter 5 explains the use of the model, with some illustrative scenarios on how the tool can be used to explore the effectiveness, cost-effectiveness and equity impact of diverse programme implementation. Chapter 6 proposes ways to implement and use the tool in different settings in the future. Finally, Chapter 7 discusses the findings, next steps and report conclusions.
Background
Non-communicable diseases (NCDs) include heart disease, stroke, diabetes, dementia and common cancers. Together they account for > 90% of premature UK deaths. NCDs therefore impose a substantial and increasing burden on our society. 1 However, much of this premature disease burden is eminently preventable and therefore demands urgent attention. 2,3
Prevention is broadly recognised as the most effective and cost-effective way of reducing the NCD burden. How best to maximise the potential for prevention is still debated, as the specific interventions and ways of delivering them may have different degrees of effectiveness.
The NHS Long Term Plan recognised that cardiovascular disease (CVD) was the single most significant condition for which lives could be saved and proposed a target of preventing 150,000 cases of CVD [mainly coronary heart disease (CHD) and stroke] and dementia from 2019 to 2029. 4
The NHS Health Check programme (HCP) in England represents a key programme for achieving this prevention goal. The programme’s objective is the early identification and management of otherwise healthy people who are at high risk of CVD and diabetes. 2 The HCP is one of the largest nationwide CVD screening programmes in the world. NHS Health Checks are offered in a cycle, with people invited once every 5 years, starting from their 40th birthday. Most areas commission the NHS HCP from local general practitioners (GPs) (i.e. family doctors). To date, the programme has invited > 10 million people to participate (95% of all eligible participants thus far). The original modelling assumed an uptake of 75%. The current uptake of approximately 48% overall reflects significant variations at the LA level. The programme has improved its performance in terms of the invitation to participate, with higher participation among the socially disadvantaged. 5 However, this greater participation may not be sufficient to reduce inequalities in longer-term health outcomes.
A study in 2016 found that, based on a sample of approximately 10% of general practices in England, the programme had detected approximately 7800 new cases of hypertension (38/1000 checks), 1930 new cases of type 2 diabetes (9/1000 checks) and 800 new cases of chronic kidney disease (4/1000 checks) across England. 6
In 2008, the cost-effectiveness of the NHS HCP was prospectively modelled by the Department of Health and Social Care; it found an incremental cost-effectiveness ratio (ICER) of £2480 per quality-adjusted life-year (QALY), which might be regarded as being very cost-effective. However, the assumptions behind this model may not have been realistic (e.g. using effect size estimates from the US Diabetes Prevention Program, which was much more intensive and effective than most UK weight management programmes). 7 Other studies have found ICERs from £900 per QALY to around £23,000 per QALY. 8,9
Studies suggest that the NHS HCP, as currently implemented, does not reduce health inequalities. 10 However, our research, using data for Liverpool, suggested that a more targeted approach may produce more equitable results. 11
Programme costs are also substantial, with LAs spending around £57M in 2018/19 on commissioning the NHS HCP. 12 Although the NHS HCP is statutory,13 there is no specific earmarked budget for it and this means that funding for non-statutory public health services (e.g. smoking cessation) may be diverted to the NHS HCP. In terms of governance, the programme may appear entirely ‘top down’ as a national programme. However, LAs exercise a high degree of control over how they commission the programme, with innovative examples of implementation in some areas. The NHS Long Term Plan reconfirmed its commitment to the NHS HCP. 14 There is thus an urgent need to produce evidence to make the NHS HCP even more effective, efficient and equitable.
Most recent evaluations have focused on process measures and some intermediate outcomes. 5,6,15–20 However, there is no evaluation of the NHS HCP impact on disease incidence and mortality. Most studies have used quasi-experimental or natural experimental designs, such as before-and-after studies, propensity matching or inverse probability weighting. These studies often compare people who engaged with NHS HCP with people who did not engage. However, the studies cannot control for unobserved factors. For instance, people who attend NHS HCP may be more engaged with their health, and this level of engagement might be the mechanism that improves their health rather than the NHS Health Check itself.
Furthermore, observational data and randomised clinical trials for similar programmes have produced conflicting results, ranging from minimal to substantial efficacy and cost-effectiveness. 21–23 However, none of these evaluations is strictly comparable with the current implementation of the NHS HCP. They therefore have a limited role in helping to determine the programme’s effectiveness, cost-effectiveness and equity, particularly at the local level. Neither do they allow an exploration of different future implementation options.
Conducting an empirical evaluation of the entire programme might be challenging and would probably be unfeasible. Furthermore, it would not provide rapid insights within the urgent timescales needed for decision cycles on investment and changing public health priorities. Ideally, the programme would have been preceded by a pilot cluster randomised controlled trial (RCT), using a stepped-wedge design or similar, to establish its effectiveness before rolling it out and making it a statutory requirement. Computational modelling is the only feasible way of estimating the long-term impacts of programmes such as the NHS HCP; however, these are complex, multicomponent interventions that may take 10–20 years or more to produce measurable population health gains.
The Government Office for Science (London, UK) recently emphasised that computational models ‘are the only way to understand properties of many complex systems’ (contains public sector information licensed under the Open Government Licence v3.0),24 notably to help analyse and explain complex public health challenges, such as NCD prevention, for which head-to-head comparisons are impractical or impossible. Building on our successful research programme modelling across a spectrum of prevention activities in the UK and globally, we worked flexibly and dynamically with commissioners and decision-makers to ensure that the models and scenarios that can be implemented and analysed in workHORSE were relevant to their agendas and realities.
A simulation modelling approach might therefore provide rapid and useful insights to help commissioners and planners identify which specific aspects of the programme could increase its effectiveness, cost-effectiveness and equity, approaching the task in a non-normative way. Such modelling might also help to assess NHS HCP synergy with other preventative activities happening at local and national levels, such as programmes around tobacco control or promoting healthy weight. 25
Furthermore, given the complexity of the NHS HCP and its focus on implementation at the local level, any decision-support tool must provide a local perspective.
However, most previous modelling approaches to assess this type of programme have been ad hoc, short lived, proprietary, not comprehensive and not validated. Almost none of these approaches has provided equity outcome analysis, with a few exceptions. 11,26,27
Furthermore, existing tools lack the essential features to realistically model the changing population risk profile over time and the interaction between diseases that share common determinants that operate on different time scales, such as CVD and cancers. From around 1970 to 2011, CVD incidence showed a steady rate of decline in England, and so ignoring these secular trends in incidence or using data from some years ago can lead to an overestimation of the effectiveness of CVD screening. These crucial factors might have substantial implications for the overall cost-effectiveness of the programme. If diseases with common risk factors are modelled independently without considering time lags and competing risks of mortality and morbidity, there may be a risk of underestimating or, more likely, overestimating the effectiveness of interventions.
The Chartered Institute of Public Finance and Accountancy (London, UK) and Public Health England (PHE) (London, UK) have produced a report Evaluating Preventative Investments in Public Health: England28 that recommends that cost–benefit analysis methodology, and the UK Treasury Green Book/new economy model29 is well suited to judging the comparative merits of such investments. Furthermore, the model allows a whole-system view to facilitate decisions on a place-based basis. The report further recommended using the International Public Sector Accounting Standards Board guidance and the principles of the Prudential Code. Although the workHORSE project was well under way when this report was published, our approach aligns with these recommendations. 28,29 The workHORSE tool allows decision-makers to estimate the impact of NHS HCP over short, medium or long time horizons, and from a range of perspectives (i.e. NHS, social care, informal care, productivity and the value-of-health gains). It therefore enables a UK Treasury-type approach to be taken.
Aims
The key goal of the project was to develop a modelling tool to support decision-making of the NHS HCP that would be able to provide insights on the effectiveness, cost-effectiveness and equity of different implementations of the programme, co-produced with users.
Our model development followed four strategic principles:
-
co-production (i.e. joint model development with stakeholders to explicitly address their needs)
-
a robust evidence base (i.e. explicitly linking model parameters to best effectiveness evidence)
-
up-to-date information (i.e. exploiting the growing availability of local health surveillance data and new research)
-
openness (i.e. fostering analysis transparency and promoting the continuous development of the tool by interested stakeholders).
The process of co-production and joint model development with stakeholders is detailed in the next chapter.
Chapter 2 Co-producing the specifications of the workHORSE model
Introduction
Stakeholder engagement in conceptual model building is well established, and so are studies to explore why stakeholders do not use simulation models. 30,31 However, studies describing active engagement with stakeholders during computational model building are sparse. Freebairn et al. 32 explored the use of end-user decision-makers in participatory simulation modelling. The authors reported that the co-production element of the participatory approach was crucial in understanding the modelling process. Further benefits included trust in the model and its outputs, and simulating the effect of potential interventions. 32 Research councils are increasingly encouraging researchers to consider the broader impact of their research. Stakeholder engagement is a crucial component in public health research and is part of a strategy to ensure that research produces relevance and benefit in the ‘real world’ beyond academia, therefore ensuring the greatest impact for the end-user. 33,34
A key objective of workHORSE was to recruit and engage with a diverse group of stakeholders through workshops to powerfully strengthen the user perspective, which would inform desirable features of the user-friendly model and identify additional locally relevant future implementation scenarios.
Methods
Stakeholder mapping and recruitment
We developed a stakeholder recruitment grid based on our extensive public health networks at the local, regional and national level. The workHORSE project team identified relevant organisations and individuals from these organisations were added to the recruitment grid. The final recruitment grid contained a diverse group of stakeholders from different organisations, including PHE (national and regional levels), the British Heart Foundation (London, UK), Diabetes UK (London, UK), Alzheimer’s Research UK (Cambridge, UK), the National Institute for Health and Care Excellence (NICE) (London, UK), the British Medical Association (London, UK), Alcohol Change UK (London, UK), the North West Coast Strategic Clinical Networks (Warrington, UK), directors of public health, the Local Government Association (London, UK), Clinical Commissioning Groups (CCGs), LAs, GPs, pharmacies and academics. Inviting a cross-section of stakeholders representing local, regional and national perspectives provided a broad skill set and diverse perspectives for the process of co-producing the tool.
Stakeholders were sent an e-mail invitation to attend the workshops. If specific stakeholders were unable to attend, we used snowballing techniques to identify other individuals at their organisation to invite. Depending on the objectives of the specific workshop session, we would either group stakeholders from similar organisations or mix stakeholders from a broad range of perspectives (e.g. NHS HCP, local, regional and national decision-makers).
Workshop design
We delivered four workshops across the duration of the 2-year project. Workshops were delivered in months 4, 8, 16 and 24. We developed a systematic and pragmatic approach to the planning, development and delivery of all four workshops.
The organisation for each workshop commenced at least 3 months before delivery. Development and planning were undertaken in face-to-face research team meetings. The initial planning meeting would include reporting of stakeholder recruitment (i.e. number and organisation) and discussion about the duration, objectives, outcomes, format and activities for the workshop. Based on the initial discussion, the workshop co-ordinators (FLW and LH) would develop a draft workshop programme for further development and refinement at subsequent meetings. The final workshop plan included the purpose, aims, time required and allocated to each activity, materials required, roles and tasks for each team member during the workshop according to their skills and expertise, and outputs of the workshop. A week before the actual workshop, a full rehearsal took place to ensure that the workshop would be delivered efficiently and effectively to maximise the co-production process.
The design of the workshop programme was theory based, using the Cairney–Oliver key co-production principles. 35,36 These included co-identifying the requirements of the decision-support tool based on stakeholders’ current views and experience and future requirements, working iteratively over the lifespan of the project to co-steer the decision-support tool content and outputs, and co-developing interpretations of the decision-support tool and implications for dissemination and end use.
The workHORSE project workshops had the overall aim of co-producing the web-based decision-support tool with stakeholders and included a series of small-group exercises with specific objectives and outputs. Exercises were designed in the form of scripts. 37 We adapted previously validated scripts to our specific needs and context (Scriptapedia38), based on the work of Hovmand et al. ,39 as part of a general framework. This allowed the modelling team to engage with stakeholders in the co-design of qualitative and quantitative models. Each script contained a succession of elements, including descriptions of the exercise, purpose, time, materials needed, inputs, outputs, team roles required, steps and evaluation criteria. The scripts included a series of small-group exercises with specific objectives, questions, activities and outputs.
The workshops were iterative in their approach and involved an independent facilitator in their delivery. Immediate feedback was obtained using Post-it® Notes (3M, Cynthiana, KY, USA), flip charts and small-group and plenary discussions.
Group model building
The use of scripts enabled a better design of the workshops and more useful sessions, leading to a more comprehensive and user-friendly workHORSE modelling tool and ‘buy-in’ from stakeholders. Furthermore, the activities enabled the team to engage with stakeholders in the co-design of the decision-support tool, facilitating open discussion and opportunities for stakeholders to provide additional feedback afterwards. An example script for workshop 2 is provided as additional material [see workHORSE workshop programme and script examples via the NIHR Journals Library project web page URL: www.journalslibrary.nihr.ac.uk/programmes/hta/1616501/#/ (accessed 15 March 2021)].
A summary of the aims and activities for each workshop are shown in Table 1.
| Workshop | Aim | Activity |
|---|---|---|
| 1 |
|
Activity 1: group work to identify stakeholders’ expectations of the workHORSE project and identify what is working well/not so well and future hopes Activity 2: group work to elicit what features/specifications will make workHORSE a useful tool for the stakeholders |
| 2 |
|
Activity 1: group work to enable stakeholders to consider alternative NHS Health Check implementations and practice modelling, leading to a blueprint for co-produced scenario(s) Activity 2: group work to rank the importance of model outputs/visualisations that will make workHORSE a useful tool for stakeholders |
| 3 |
|
Activity 1: group work to enable stakeholders to discuss and co-produce realistic model scenarios, interpret model outputs and confirm their usefulness Activity 2: group work to explore the importance of model outputs and visualisations that will make workHORSE a useful tool for stakeholders |
| 4 |
|
Stakeholder demonstration of using the model and outputs interpretation |
Ethics approval
Ethics approval for the workshops was granted by the Health and Life Sciences Committee on Research Ethics (Psychology, Health and Society), University of Liverpool, Liverpool, UK, on 14 September 2017 (reference number 2242). Written consent was obtained from stakeholders before the workshop. All data were anonymised and stored in locked filing cabinets and on password-protected computers.
Evaluation
Both stakeholders and the modelling team completed questionnaires with open-ended and closed questions to evaluate the co-production process. At the end of each workshop, stakeholders completed stakeholder engagement questionnaires to explore their views and experiences throughout the process. Questions included their reasons for attending the workshops, their expectations and what they had gained from attending, and the perceived added value of their involvement.
The modelling team also completed questionnaires to explore their expectations before and their experiences after the workshops. Questions included the added value of having a series of workshops, the process of co-production and how the decision-support tool benefited from stakeholder involvement.
At the end of the project, the modelling team and stakeholders were e-mailed a final questionnaire that was tailored appropriately to identify their overall experience of the co-production process.
Thematic analysis
The qualitative information obtained from the questionnaires and notes from meetings with the lay advisers were analysed using the principles of thematic analysis, as described by Braun and Clarke. 40 Familiarisation of the data was carried out: reading through all of the data and generating initial codes based on the responses to the open questions. These data were then grouped into meaningful categories and further searched and reviewed for themes. The responses were then categorised into a sufficiently small set of broad categories, which were then coded and subsequently indexed.
Findings
Workshop 1
Workshop 1 took place in Liverpool in February 2018. Fifteen stakeholders participated, representing the local, regional and national perspective, with attendees from LAs, CCGs, general practices, academia, PHE and third-sector organisations.
We delivered two key activities. Activity 1 focused on developing a shared understanding of the NHS HCP and asked stakeholders to identify aspects of the programme that were working well or not that well, and their future hopes for the programme. During activity 2, stakeholders were asked to identify the key features that the workHORSE modelling tool should include that would make the tool useful for the decision-making process.
Each activity was completed individually, followed by both table and whole-group discussions. Each table had a mix of local, regional and national stakeholders to stimulate discussion. Stakeholders provided written feedback on Post-it Notes and the table and group discussions were audio-recorded and summarised on flip chart paper.
MoSCoW approach: prioritising stakeholders’ suggestions
Activity 2 resulted in stakeholders providing many suggestions. The feasibility of incorporating all stakeholders’ suggestions was limited because of the short 2-year timeline of the project. Therefore, we used the MoSCoW (Must have, Should have, Could have, Would have) approach to prioritise the suggestions made by the stakeholders to reach a common understanding of the importance of their proposals. 41 The MoSCoW prioritisation process uses the following categories:
-
Must have (i.e. the suggestions are critical to the project and without these the project will fail).
-
Should have (i.e. the suggestions are important, but are not as time dependent as the suggestions in the ‘Must have’ category).
-
Could have (i.e. the suggestions are desirable, but not necessary).
-
Would have (i.e. the suggestions are least valuable to the project and can be either dropped or incorporated at a later stage).
(Note that the ‘W’ in the MoSCoW approach stands for ‘Won’t have’; however, for this project, we changed it to ‘Would have, time permitting’.)
The project team initially utilised the MoSCoW approach to categorise the suggestions provided by the stakeholders. The results were then presented to the stakeholders and discussed until a consensus was reached.
The MoSCoW approach has proved valuable to the workHORSE project. We used the approach to successfully prioritise the features of the workHORSE modelling tool that are required to make the tool useful for the decision-making process.
Stakeholder feedback
The diverse stakeholders stated their continued financial and political support for the NHS HCP during the project. However, many stakeholders highlighted issues concerning lack of data on processes and outcomes, variability in the quality of delivery and suboptimal public engagement, and a lack of public understanding with regard to how to participate in the NHS HCP. Stakeholders’ hopes included maximising coverage, uptake and referrals; and producing additional evidence on population health, equity and economic impacts.
Essential suggestions for the decision-support model focused on developing good-practice template scenarios, use of accessible local data, analysis of broader prevention activities at the local level, broader economic perspectives and fit-for-purpose outputs. Stakeholders identified several modelling issues, including the lack of a quantitative evidence base regarding the effectiveness, cost-effectiveness and equity of the NHS HCP.
Workshop 1 evaluation: expectations of the co-production process
Most stakeholders indicated their anticipation of being able to learn about the workHORSE tool, the research process in tool development and having the opportunity to contribute actively. They also saw their knowledge, expertise and user perspective as potentially contributing to the components of the tool and ensuring that it was user-friendly and relevant to the end-user. Many stakeholders were enthused about the prospect of having a valuable tool that could lead to more effective and equitable Health Check delivery. Typical comments included:
To be included in creating a benefiting tool for the NHS Health Check programme.
SH2-2
I think it is a potentially hugely valuable tool that could help local areas design programmes which would make them less resistant to universal delivery.
SH12-2
Modelling team perspectives
This was the first time the modelling team had engaged with stakeholders regarding tool development. Prior to workshop 1, the team’s expectations of stakeholders’ contributions were mixed. Responses indicated that although they were hoping for some useful and innovative engagement, there was unfamiliarity with the process of stakeholder engagement and what could potentially be achieved:
I think that I was expecting the stakeholders to provide general ideas on scenario building features, but I wasn’t really expecting them to understand modelling details at the required level. My expectations were more about participation, being able to engage them in a fruitful and useful discussion.
M4
Although, initially, there was apprehension about the process of engaging with stakeholders, the team found that workshop 1 exceeded their expectations and provided added value to the tool development:
Yes, stakeholders were very engaged and came up with lots of ideas. I was particularly pleased with their enthusiasm and interest to participate . . . I think that as the first interaction with them, their understanding was better than I thought, as exemplified by the suggestion of the best practices templates tool.
M2
The modelling team were able to reflect on their usual process and approach to model building. Usually, decision-support tools would be developed with little consultation apart from internal consultation with colleagues and, perhaps, discussion with external modelling peers:
The modelling team would have made all the decisions without formal external input. After the end of the project, the users, including current stakeholders, would be able to provide feedback; but by then, we would have no resources and less flexibility to react to their feedback.
M3
Usually, we decide by ourselves what you should do to set up a scenario, and design the scenarios ourselves, and only consider discussing the results with third parties.
M4
Workshop 2
Workshop 2 took place in Liverpool in June 2018. Seventeen stakeholders participated, representing local, regional and national perspectives, with attendees from LAs, CCGs, general practices, academia, PHE, NICE and third-sector organisations.
The workshop activities commenced with a presentation reviewing the workshop 1 findings of ‘what will make workHORSE a useful tool’, based on the MoSCoW approach (i.e. what we must/should/could/would do). Stakeholders were reminded of their earlier comments and given a summary of the findings. This enabled stakeholders to understand how their proposals, so far, linked to the seven parameters of the model and what the model could provide, based on feedback, using the MoSCoW prioritisation approach.
We then delivered two activities. Activity 1 focused on enabling stakeholders to consider alternative NHS Health Check implementations and then practicing modelling these implementations on a laptop computer provided for each mixed-specialty group. Individual groups were invited to feedback on how they had modelled their NHS HCP scenario, followed by a plenary discussion.
In activity 2, stakeholders were asked to rank the importance of different model outputs and visualisations that would make workHORSE a useful tool. Again, feedback was provided by individual groups, followed by a plenary discussion.
Stakeholder feedback
Stakeholders provided detailed feedback regarding the usefulness, expressivity and clarity of the model. The stakeholders were very positive about the model’s ability to compare different scenarios and having mixed-model options that were easy to use. However, they felt that the model needed refining to ensure that users understood the interface to maximise outputs. Stakeholders suggested having flags/warnings if inputs were outside an expected or reasonable range to improve clarity. When using the model, some stakeholders commented that some terms required a more precise explanation. In the workshop setting, much of the model was self-explanatory; however, when used in the real world, the model would require clear guidance, notes and tutorials.
Workshop 2 evaluation: co-production as a process for tool development
By workshop 2, stakeholders expected the decision-support tool to have progressed because of their input during workshop 1. Stakeholders were eager to see a prototype of the tool and how they were contributing to the tool’s development:
To see progress and how engagement with stakeholders had contributed to that progress.
SH4-2
To see a prototype of the tool and how the last workshop has shaped developments so far and inform next steps.
SH9-2
Stakeholders expressed greater insight and understanding of what the model would include and how it would work. Furthermore, networking with other stakeholders provided the opportunity to gain different perspectives regarding what to include in the model and how various end-users would use it:
I was keen to see how the model had progressed and how it could be used to produce various scenarios to inform commissioning decisions potentially. It was also a great opportunity to network with people from other areas and organisations.
SH3-2
There was a consistent theme of co-production leading to a tool that would be relevant to the end-user, and of stakeholders being able to provide ‘real-world experience’ relating to actual work practices, a range of different perspectives and expectations of outputs:
Massive value – it’s been fascinating to watch academics extract from ‘real-world users’ the information they need to make the tool truly ‘useable’. If the project is to have a tangible outcome [the model tool], it will only be used if the end-users have had an input and ensured it is relevant to them.
SH12-2
The perceived value of co-production in model development was a continuous theme that was increasingly highlighted by the stakeholders as the workshops progressed, particularly in ensuring relevance for end-users:
To continue supporting the development of the tool and ensure it caters to the needs of localities that are not pushing boundaries of Health Checks, and to help ensure we end up with a product that’s going to work on the ground.
SH1-3
Modelling team perspectives
Researcher feedback after the delivery of workshop 2 demonstrated that the modelling team perceived co-production as providing validation for the decision-support tool in development and reassurance that its development would be of relevance to the end-user. Co-production ensured that all aspects relevant to the end-user were being considered, not just those the development team thought would be required.
By adopting this approach, the end-users would not only have a decision-support tool tailored to their needs, but also an in-depth understanding of the process involved in achieving the product. Likewise, the modelling team had a greater insight into why specific scenarios and outputs were necessary:
To make our research meaningful and helpful. To help us on focusing on what is really important for decision-makers.
M1
First and foremost, transparency. Most modelling exercises are opaque . . . Our approach put them at the centre of the model, responding to their needs, getting them engaged so that they help disseminate the work once it is finished and be local champions for it.
M4
The modelling team saw stakeholders as being able to contribute not only to what was required for the decision-support tool to be useful, but also to what should be excluded, therefore making the tool more refined and fit for purpose. Specifically, the team welcomed stakeholder contributions in terms of the required inputs, outputs and the graphical user interface (GUI):
I expect with their contributions to make the GUI useful and more intuitive for the users. I also hope to identify which model outputs are more useful to them so I can make them more easily accessible in the GUI.
M3
Workshop 3
Workshop 3 took place in Liverpool in February 2019. Ten stakeholders participated, representing the local, regional and national perspective, with attendees from LAs, CCGs, academia, PHE and third-sector organisations.
We delivered two key activities. Activity 1 focused on helping stakeholders understand how to create and interpret realistic scenarios. In activity 2, we wanted to obtain feedback on the alpha version of the model, focusing on output specifications. Stakeholders were asked to explore the importance of model outputs and visualisations that would make workHORSE a useful tool. Each activity was completed in small groups, followed by both table and plenary discussions. Each table had a mix of local, regional and national stakeholders to stimulate discussion.
Stakeholder feedback
Stakeholders were pleased to see the changes and improvements made to the model that were based on their comments in workshop 2. Stakeholders found the model user-friendly, but requested more detailed explanations of the user inputs and outputs. They requested clear guidance notes and language similar to what is used in the national NHS Health Checks scheme. Clarity issues included using terminology and definitions consistent with the NHS HCP, simple option explanations and straightforward explanations when using combined scenarios. Stakeholders also commented on having links to videos on the output graphs to explain outputs and written summaries of outputs to support the graphs.
Workshop 3 evaluation: consolidation of the decision-support tool via co-production
Many stakeholders attended workshop 3 to observe how the model had evolved from workshop 2. In addition, stakeholders wanted to understand how the model would work, especially in terms of outputs:
To further develop the tool in a positive, energetic, interactive workshop.
SH3-3
To see the next iteration of the tool. See how learning from the previous workshop has been used. Understand more about sustainability and future for the tool.
SH11-3
Stakeholders also commented on the added value of their involvement in the series of workshops. It provided them with more confidence in the tool, as they had observed and contributed to its development. Stakeholders’ comments indicated that they had felt that the iterative workshop process for model development was beneficial for both them and the modelling team:
Genuine proof these workshops and communications in-between have impact – mixed model and functionality now built-in, which is marvellous. A better understanding of reality of delivery for those on the ground.
SH3-3
Awareness that previous comments have been taken into account, and valuable insight and understanding of the tool, its benefits and capabilities.
SH5-3
All stakeholders were very positive regarding the advantage of having a series of workshops as opposed to one workshop. Most importantly, they saw it as an opportunity to learn about and reflect on the tool’s capacity, usage and usefulness as an end product:
Huge! It would be too much to take on over 1 day. Division months between workshops provided the opportunity to reflect and think of questions.
SH2-3
You end up with something truly co-produced, doing what people need it too. I worry this is not the case with other things we have commissioned development of recently.
SH8-3
Greater clarity and more sophisticated understanding of subsequent iterations of the model. The group was more aware of the detailed issues having attended previous workshops. More informed and detailed discussions.
SH10-3
Modelling team perspectives
The third workshop was a culmination of the co-production process. The modelling team felt that it provided an opportunity to refine the decision-support tool, achieve consensus and have the endorsement of the tool that had been created through the series of workshops:
Keep participants on board with the co-production process. Getting feedback before the interface is completed.
M2
Reassurance that we are travelling in the right direction. At that stage of the project, there was still time to improve the fundamentals if necessary.
M3
Because of the success of the experience, we gained valuable feedback re[garding] the user experience with the model, good discussions regarding the complexity and usefulness of the model, and very useful conversations on how the real LA setting in terms of analytical and modelling skills set can be enhanced by the model interface. This will be invaluable for the final design of the user interface.
M4
Furthermore, it was clear that the stakeholders added dimensions to the tool that would not have been identified by the modelling team alone:
There were many small additional improvements. Most of them very smart and useful that I would have never thought by myself.
M3
. . . particularly in how to help the user through the interface to understand some of the concepts and outputs of the model.
M4
Having co-produced the model with stakeholders, the modelling team expressed increased confidence in the decision-support tool that they were building. They received reassurance and endorsement from end-users that what was being created would be ‘fit for purpose’:
I am really pleased with how the model is looking. It is better than I thought it would be.
M2
I am now confident that the workHORSE model may fulfil its purpose to be useful and support policymakers to make better decisions . . .
M3
I am extremely pleased in viewing in action the principle of co-production. Features suggested in WS2 [workshop 2] and implemented and demonstrated in WS3 [workshop 3], providing an opportunity to iterate and incrementally improve the usefulness of the model.
M4
Workshop 4
Workshop 4 took place in in Liverpool in October 2019. Eleven stakeholders participated, representing local, regional and national perspectives, with attendees from LAs, CCGs, academia, PHE and third-sector organisations.
Workshop 4 was the concluding workshop and an opportunity to showcase the final model and provide stakeholders with information about the next steps. The aim of workshop 4 was to influence the adoption of the model, engage with influencers, exploit the model as an academic product and demonstrate its added value. Again, the programme was interactive and included (1) lay advisers talking about their experience and involvement in workHORSE and providing tips for lay involvement in future projects, (2) showcasing the model and talking through the changes made, including usage, understanding its application and maximising the use of tutorials, and (3) stakeholders (one from a local perspective and one from a national perspective) demonstrating the model’s capabilities, in terms of model usage and interpretation of results. Stakeholders were also informed about the implementation plan for the model and the next steps required to confirm support for the dissemination of the workHORSE model.
Workshop 4 evaluation: demonstrating proof of concept
Stakeholders attended the final workshop to support the development of the model and contribute to its final iteration:
To complete the participation in this programme and activity.
SH4-4
To contribute to stakeholder discussions supporting the development process of this model.
SH8-4
Stakeholders appreciated the opportunity to observe and discuss the tool with other stakeholders from different organisations and localities. They commented on the progression of the model and welcomed the opportunity to observe the final version and its use in practice:
Really great understanding around the tool/data/the art of the possible.
SH1-4
Better understanding of how the model will support me around future decisions for Health Checks.
SH4-4
Having different perspectives and needs from other stakeholders, seeing the progress and development of this model. Learning the capability of the new tool and how it can be applied.
SH8-4
Stakeholders expressed satisfaction with the decision-support tool that was presented to them. They enthused about the prospect of having a tool that would provide them with diverse scenarios and being able to demonstrate the capabilities of the NHS Health Check programme at various levels:
Opportunity to use the model to show the impact of various scenarios which wasn’t available before.
SH2-4
Being able to demonstrate HC [Health Check] effectiveness/HC programme evolving/cost-effectiveness of HC is still possible.
SH4-4
The new tool that will be publicly available will provide valuable information of the NHS Health Check at both national and local levels. It is also brilliantly flexible for all types of users in planning, managing and monitoring their local provision.
SH8-4
The modelling team valued the opportunity to demonstrate to stakeholders, and for stakeholders to demonstrate to their peers, how the co-production process had directly informed the decision-support tool, therefore resulting in a product that would be user-friendly:
Show the tool and how we have responded to stakeholder input into the project.
M2
To allow the stakeholders to demonstrate the use of a working version of the model and to get final comments and suggestions from them.
M3
Expectations were met in terms of the ‘lively and interesting conversations’ (M1) held between stakeholders, and stakeholders and the team. Workshop 4 enabled the modellers to demonstrate ‘proof of concept’ (M1) and also allowed modellers to ‘debrief stakeholders’ (M2). M3 commented on how one of the stakeholder’s tool demonstrations provided new ways of thinking for the modellers:
. . . they used the model in a way I have not previously thought of. I found this exciting.
M3
Modelling team perspectives
The process of co-production was deemed a success by the modelling team, with the right mix of stakeholders participating and their views incorporated into tool development. However, one modeller commented that input from practice nurses may have been useful (M2). In addition, M1 commented that ‘. . . of course, there is much more to do, as key aspects to be contemplated in the implementation plan might benefit from more interactions, but sadly we are not funded to do that work’. This comment was reiterated concerning changes they would have made to the process of co-production.
The modelling team felt that model development was only one component of enabling stakeholders to use the tool to inform decisions. Stakeholders would require training and support to ensure successful implementation in the workplace:
Develop their own use cases and modify/edit the tool for that purpose. We build the tool with that flexibility, so it will be the ultimate proof of concept.
M1
Some funding for training and ongoing support.
M2
We produced a prototype. Now we need the production pipeline and the training.
M3
End of project evaluation
We received responses from 11 out of 30 stakeholders who had attended the workHORSE workshops. Although the 37% response rate to the e-mailed questionnaire was suboptimal, the feedback obtained was incredibly insightful and valuable for the project team in terms of informing future co-production of decision-support tools.
The majority of respondents had attended the workshops to learn about the development of a decision-making tool for NHS Health Checks (n = 11), ensure that a useable decision-making tool was developed (n = 9), inform the research project of their organisation’s views on the NHS HCP (n = 8) and network with other stakeholders involved with NHS Health Checks (n = 8).
Respondents also valued being able to provide feedback by e-mail throughout the project, therefore enabling a continuous communication line to the research team (n = 10). Most respondents also felt that their input added value and had been addressed by the research team and incorporated into the workHORSE tool (n = 8).
Perceived benefits of participating in the workHORSE project
Respondents commented on what they had gained from attending the stakeholder workshops. The overarching theme was being able to meet, communicate and co-produce work with other stakeholders and the project team:
The interactive group exercises provided a platform for different ideas to be discussed among the stakeholders, and this is also more efficient in identifying key questions and in formulating the most helpful suggestions or recommendations.
SH2
Some of the comments on language and ease of usability – it was really useful to hear from others who are addressing these issues in the real world daily. Consultation is critical if the tool is to be fit for purpose and used, rather than another technical programme which does not get traction in the real world and its use is not maximised.
SH3
Ideas from other participants for offering and delivering Health Checks.
SH4
Opportunity to shape and develop a new resource and tool. Gave me a greater understanding of barriers to SROI [social return on investment] and modelling tools. Opportunity to network with others.
SH6
. . . being informed throughout the process and improving my understanding of the limitations of such a project and being able to ask questions in real time.
SH8
Benefits of being consulted were gaining insight into how and why the tool was being developed and having the opportunity to contribute towards shaping it, and having this knowledge and experience means I will be more likely to use it.
SH9
workHORSE e-lab online platform
Four of the respondents had used the workHORSE e-lab online platform, with mixed views about its value for the project. All agreed that it had enhanced their understanding of the workHORSE tool and three felt that it had enabled them to contribute to tool development. However, it was not perceived as the best platform for networking and discussing with other stakeholders or the project team about the workHORSE tool or the NHS HCP.
Seven respondents had not used the workHORSE e-lab. The reasons given were lack of time (n = 6) and not being relevant to their organisation (n = 2).
Stakeholder engagement for future research projects
Although there were mixed views about the e-lab, seven respondents commented that having a project website, such as e-lab, earlier in the project would have been beneficial. Other suggestions for improving stakeholder engagement included (1) having more workshops during the project (n = 3); (2) having workshops of shorter duration (n = 3); (3) having more opportunities to communicate with other stakeholders between workshops (n = 2); and (4) being consulted before and during the design of the research project (n = 2).
Planned usage of the workHORSE tool
Eight respondents commented on how the workHORSE tool will be used within their organisation for the NHS HCP. The tool was an asset for the commissioning process, especially relating to projecting the effectiveness and cost-effectiveness of different scenarios and future impact. SH5 commented on the potential power of the tool to provide evidence at the local level and provide an NHS Health Check service tailored to local population requirements:
To gain an understanding of the current effectiveness and cost-effectiveness projection of the NHS Health Check and how these could be affected by various scenarios (e.g. reduced funding).
SH2
To support awareness of its value in commissioning and delivering evidence-based practice through our national influencing work and also through the support that our regional teams give to local commissioners.
SH3
Projections.
SH4
In the south, there has never been an appetite or strategic leadership to embed the programme as a key enabler to CVD action at scale. I have long promoted the use of HEAs [health equity assessments] to understand the equity of access and outcomes and use of this data to work with local stakeholders to design approaches to implementation that suit local population need and local system priorities (e.g. detection of those with HT [hypertension]). This tool, if made available, could help LAs demonstrate and engage locally to do this. It could also be used to demonstrate how impact of the programme is massively restrained if primary care doesn’t provide appropriate clinical follow up in line with NICE guidance (e.g. offer of statins to all over 10% risk). Tool should make it possible to demonstrate clearly how increasing such take up impacts, which could be powerful when working with CCGs, STPs [sustainability and transformation partnerships] and ICSs [integrated care systems] on their NHS Long Term Plan CVD ambitions and 5-year plan commitments.
SH5
Have kept as a regular agenda item on NW [north west] network meetings and hope to demonstrate at a NW network meeting with NW Health Check commissioners.
SH6
To establish the cost-effectiveness of one version v[ersus] another version of the HC [Health Check] programme . . . this will inform future decisions based on funding available.
SH8
To model various scenarios to show impacts.
SH9
Inform decision-making, allow for easier projections to be made and possibly use the tool as an enabler for better coverage across our patch.
SH11
Stakeholder requirements for implementing the workHORSE tool
Eight respondents requested additional support for the implementation of the workHORSE decision-support tool. The requests included having a user manual, telephone/e-mail support and training:
User manual, FAQs [frequently asked questions], short video clips for the demonstration of key elements and possibly some sample scenarios.
SH2
Some clear promotional material to describe the value of the tool and some additional support/training materials (or workshops) to assist those using the tool.
SH3
Telephone support, online/e-mail support.
SH4
I need a better understanding of where the platform will be hosted, who and how it can be accessed and what guidance there will be around using it with LAs and other local stakeholders.
SH5
It would be wonderful to have someone to present the tool at my NW [north west] network meeting in February.
SH6
I would like a future training session on the various ways the tool can be used as we were only given a basic demonstration on how to use.
SH8
Adding some demo[nstration] scenarios such as those demonstrated at the last workshop might make the tool more user-friendly. The user could run these scenarios and then tweak as required and save as a custom report.
SH9
Accreditation of the programme tool may be vital to ensure confidence of the outputs are acknowledged and useful.
SH11
Stakeholder engagement: areas for improvement
Five respondents commented on how their experiences in shaping the workHORSE tool could have been improved. Two respondents commented on the location of the workshops (i.e. all four held in Liverpool) and the time commitment of travelling and attendance. The remaining feedback related to workshop delivery and the decision-support tool.
Workshop delivery
Feedback related to (1) the availability of briefing notes for stakeholders who did not attend all of the workshops, (2) being able to practice using the live tool in the workshops and (3) understanding of the decision-support tool outputs.
The decision-support tool
Feedback related to the (1) availability of the final decision-support tool; and (2) limitation of the tool concerning dementia and NHS Health Checks:
I can’t think of any – I came [to] the project after the first workshop so had a little catching up to do, so background info/briefings are clearly important.
SH3
Complicated and sometimes difficult to understand the output.
SH4
It was a long way to travel but not a lot you can do about that, and as much of our NHSHC [NHS Health Check] activity is in London, it felt entirely reasonable to travel up.
SH5
I am a little disappointed to learn only at this stage that the tool is unlikely to be available to commissioners. I thought this was the whole point and certainly the reason I have been involved – our stakeholders need to be able to consider how applying proportionate universalism principles to inform targeted allocation of resources (doing different things to different people) would impact on overall programme outcomes/equity. If it is only available to PHE, this will hugely limit the value of the tool. However, it will make it ‘safer’ in terms of facilitating approaches to modelling that are currently ‘off regs’. It will also be good for the green paper review.
SH5
Would have liked opportunity to use the tool in the workshops.
SH6
My expertise is around dementia and the NHS Health Check – the main limitation for me is that there is no ROI [return of investment] data around dementia risk reduction messaging within the NHS Health Check to enable the tool to meaningfully shape decision-making for services. That is not a fault of the project but a reflection on the lack of current evidence/data. Therefore, my contribution was always going to be limited.
Given that the consultation meetings took place in Liverpool it was a considerable time commitment for me to attend the workshops.
SH7
Patient and public involvement
The involvement of lay advisers has been an important component of the workHORSE study (from project preparation through to project dissemination). This section provides details about their involvement.
Aim of patient and public involvement in workHORSE
Lay advisers were recruited and involved in the workHORSE project to:
-
contribute to the design of the research
-
contribute to the management of the research through the Study Steering Committee
-
contribute to the content and delivery of the stakeholder workshops
-
contribute to the reporting of the research
-
summarise messages for lay audiences
-
ensure that the perspective of the public was represented.
Methods
We recruited four lay advisers through the National Institute for Health Research (NIHR) Patient and Public Involvement (PPI) Network and local Healthwatch. All of the lay advisers had personal experience of using the NHS HCP and were interested in how the development of the decision-support tool would benefit Health Check provision.
We continuously evaluated our PPI with our lay advisers throughout the project, at meetings and by e-mail, to assess its impact and to identify areas for improvement in lay adviser involvement.
Preparation of the workHORSE application for funding
We organised a meeting with all four lay advisers and research team members to (1) provide the opportunity for lay advisers to meet each other and the research team; (2) gather lay advisers’ views on the readability of the Plain English summary and Abstract; (3) provide the opportunity for lay advisers to share their early thoughts about the proposed research; and (4) obtain lay advisers’ observations about how the proposed research might be improved.
The lay advisers provided constructive feedback on the content of the Plain English summary and offered suggestions on additional stakeholders we should invite. The meeting enabled discussion and agreement of their role in the project (i.e. their own experiences and skills in helping interpret the results and acting as a voice to disseminate the research to a broad audience).
workHORSE project delivery
The lay advisers were active members of our Study Steering Committee, providing feedback on their perspectives (primarily on workshop development and delivery). We also ensured ongoing communication with the lay advisers between workshops by e-mail and at face-to-face meetings. The lay advisers provided advice on the content of the four workshops and attended workshops 1, 2 and 4, where, as members of the research team, they ensured the timely delivery of activities and recorded and fed back their observations. In workshop 4, the lay advisers presented on their experience of the workHORSE project and what a project needs for effective PPI.
Towards the end of the project, we held an event with the lay advisers and research team to discuss their perspectives on their experience and involvement in the workHORSE project, what went well and what could be carried out better in the future.
workHORSE project dissemination
The lay advisers have been involved in the writing of academic publications for peer-reviewed journals. They also helped us to write clear, understandable literature for dissemination within the research community and engagement with the wider public.
Study results
The lay advisers felt valued in their involvement in the project. They perceived their role as acting as public consultants and translators of information for a wider audience, and as advocates adding value from the public perspective and observing the ‘return on investment’ for the research funding provided from the public purse.
Lay advisers saw their involvement in writing the research proposal as a positive approach to co-producing with the public:
. . . beginning was excellent in terms of involvement. I loved commenting on the bid. The first meeting was taking our views and making a key contribution to how the bid would look . . . although it felt a bit over my head, we thought it was looking at NHS Health Checks, not specifically modelling. It took time to figure out the idea of modelling . . . but I did enjoy it.
LA1
However, as illustrated by the comment above, the lay advisers felt that the nature of the project inhibited their involvement. They commented that:
The project was quantitative, and we look more into the qualitative . . . PPI is important because it influences the care standard of what the patient is receiving.
LA2
Conversely, they did identify their valuable role in dissemination to ensure that the research findings reached the public realm, including co-producing summaries of the research for publication in, for example, local government newsletters.
Discussion
From the perspective of the workHORSE research team, the involvement of the lay advisers in the project provided valuable feedback in terms of having a public perspective on the NHS HCP and how the decision-support tool could improve the patient experience.
The lay advisers did comment that the workHORSE project was not typical of projects that they had previously been advisers for, as it was not research involving patients and the public. However, as the project progressed, they very much perceived their role as ‘translators’ of the research as being at the interface of a research project and the ultimate beneficiaries of the project outcomes.
As active members of the Study Steering Committee, the lay advisers were able to have ongoing involvement in and input to the content and delivery of the workshops. They also provided valuable input in the writing of project materials, especially those requiring plain English. The lay advisers’ participation in the workshops enabled us to gain perspectives of model usage from the public who would ultimately benefit from the model.
The workHORSE project was not a conventional project in terms of having patients and the public as the research subjects. Therefore, the role the lay advisers had envisaged (and had previous experience of) was not utilised to its full extent, leading to an initial mismatch of the project requirements and lay advisers’ expectations.
Researchers embarking on building decision-support tools need to look at the project aims, outcomes and delivery, and embed PPI activities that are best suited to the project to maximise their role and project impact.
Dissemination and outputs
The findings from the stakeholder engagement workshops have been disseminated to a broad audience and there has been considerable interest in our co-production approach to model development. Outcomes of workshop 1 and the co-production methodological process have been presented at conferences in both the UK and Europe. We also have two academic papers relating to workshop 1 outcomes and workshop co-production methodology. 42,43 Details of all dissemination can be found in the Acknowledgements.
Summary of findings
The workshop process established the added value of co-producing the decision-making tool. Workshop 1 provided the foundation for the future workshops, with stakeholders demonstrating a commitment to their involvement in creating a user-friendly decision-support tool, and the modelling team embracing the added value that the co-production process would provide for model development and the ultimate applicability and acceptability to the end-user. Workshop 2 provided the opportunity for stakeholders to experiment with the decision-support tool. This process enabled both the stakeholders and the modelling team to understand how end-users would utilise the decision-support tool. For example, stakeholders commented that in the workshop setting much of the model was self-explanatory, but when used in practice it would require clear guidance, notes and tutorials. Workshop 3 involved the fine-tuning of the decision-support tool based on the co-production process in workshop 2. It provided an opportunity for the stakeholders to approve the decision-support tool they had co-created. Issues were identified that the research team would probably not have considered without the iterative co-production process. These included refining the explanations for the inputs, outputs and combined scenarios, and informative video links on the output graphs. Workshop 4 represented the culmination of the iterative co-production process. Two stakeholders, one from a local and one from a national perspective, demonstrated how the decision-support tool could be employed by stakeholders who were enthusiastic about its capabilities in practice. Both stakeholders and the modelling team commented on the success of the co-production process, noting that having ongoing interaction in building the tool resulted in confidence in the end product.
Conclusions
The workHORSE dynamic simulation tool was developed to provide decision-makers and practitioners with a web-based decision-support tool to help identify the most effective, cost-effective and equitable interventions for the NHS HCP. Computational modellers rarely consult with end-users when developing tools to inform decision-making. Involving stakeholders in the co-production of tool development enabled productive and valuable dialogue, provided valuable learning about potential problems in practice and supported consensus building for effective end-use, adding substantial overall value. The resulting level of engagement resulted in modellers producing an operational tool that can be implemented in the ‘real world’, with the capacity to test an extensive range of scenarios to determine their likely short- and longer-term impacts. Likewise, stakeholders obtained increased confidence in the decision-support tool’s development and applicability in practice, with a robust basis for decisions on the delivery of the NHS HCP. However, when the tool is deployed and used for real-world decision-makers, an evaluation of the processes and experiences of the stakeholders and users is needed, including assessing the added value of the group model-building approach and refining the theories, processes and methods to develop decision tools jointly with stakeholders and users.
Chapter 3 Updating the evidence base to inform scenario design and model
How do the best-performing local authorities commission and implement the Health Check programme?
There has been national monitoring and publication of each LA’s performance on the NHS HCP since 2013. It was therefore possible to identify and contact the best-performing LAs from across England to potentially identify and share best practice. Variation in practice is common, as well as achievement against the key performance indicators of the programme. The overarching aim of this survey of best-performing LAs was to inform the workshops and scenario design features that the model needs to accommodate to support ‘what-if’ types of scenarios assessing the impact of locally adapting those best practices. For instance, if several best-performing LAs had outreach services in pharmacies, then we would want the workHORSE tool to be able to model these services.
Context
From April 2013, LAs became responsible for commissioning the risk assessment component of the NHS HCP. LAs can commission the risk assessment from any provider of their choice, but must work closely with their CCGs to ensure that there is a joined-up approach to the risk assessment, clinical follow-up and management. Although the NHS HCP is a national programme, there is variation in the implementation and delivery of NHS Health Checks within different LAs. Commissioners have some scope to adjust their delivery model to ensure that the programme is reaching their high risk and vulnerable communities. Therefore, different forms of delivery may have an impact on the uptake of NHS Health Checks among the eligible population.
Aim
We aimed to contact the best-performing LAs to find out how they were delivering the NHS HCP. This included identifying their success stories to develop best practice narratives and, if possible, templates for the workHORSE tool.
Methods
Sampling local authorities
We selected the best-performing LAs based on data from the NHS Health Checks Fingertips website [URL: https://fingertips.phe.org.uk/profile/nhs-health-check-detailed (accessed 4 November 2019)]. For the original sample, we looked at the performance of LAs during the complete 5-year cycle, from 2013 to 2017.
When considering the 5-year cycle, including the new quarters in 2018, the top-performing LAs remained the same. In addition, Gateshead joined the top-performing LAs, having improved most in the previous year, and was also included. The best-performing LAs were judged based on the percentage of eligible people who received an NHS Health Check. The percentage of eligible people who received an NHS Health Check varied from 17% to 95% across all LAs. PHE had an initial target for LAs to work towards 66% uptake of NHS Health Checks to improve coverage.
We used the 66% cut-off point for the best-performing LAs. In 2018, 12 LAs were reaching this target (Table 2), mapping well to the workHORSE model parameters and enabling scenario analysis, as described in Chapter 5.
| Number | LA | Percentage of eligible people who received an NHS Health Check |
|---|---|---|
| 1 | Walsall | 95.34 |
| 2 | Bolton | 87.12 |
| 3 | Westminster | 84.49 |
| 4 | Hammersmith and Fulham | 79.50 |
| 5 | Ealing | 76.09 |
| 6 | Leicester | 73.71 |
| 7 | Tower Hamlets | 70.78 |
| 8 | Bury | 70.71 |
| 9 | Newham | 69.24 |
| 10 | Hounslow | 67.16 |
| 11 | Islington | 66.92 |
| 12 | Wandsworth | 66.06 |
Additionally, we wanted to ensure that we had a representative sample of best-performing LAs. We identified five additional LAs that improved most over the previous year/four quarters (2017/18) compared with the previous year (2016/17) using the percentage of eligible people who received an NHS Health Check (Table 3).
| Number | LA | Per cent change (2016/17–2017/18) |
|---|---|---|
| 1 | Dudley | +10.5 |
| 2 | Rochdale | +8.5 |
| 3 | Westminster | +6.2 |
| 4 | Gateshead | +4.0 |
| 5 | Salford | +3.7 |
Model parameters and inclusion criteria
An objective was to produce real-world input parameters of NHS Health Checks for the workHORSE model. The model had six input parameters related to NHS Health Checks, including interventions, implementations or deliveries affecting:
-
eligibility criteria
-
coverage
-
uptake
-
average risk profile
-
diagnosis and treatment
-
referrals for brief interventions and lifestyle services.
To inform our workHORSE model, we selected the best-performing LAs based on their performance concerning coverage and uptake. These parameters were selected because what happens during and after an NHS Health Check is not routinely published. Only patchy data relating to the other parameters exist, and these are discussed in each LA summary in Results. Further information on the methodology can be found in Appendix 1.
Results
Each LA (n = 16) was contacted by e-mail with a request for a telephone call to explain the project. A telephone call was completed with all LAs apart from one (n = 15). After the telephone call, the questions were e-mailed and the LAs were asked to return them electronically. Thirteen LAs responded (81% response rate). One responding LA (LA C) was responsible for two LAs and provided combined information, as the same approach was utilised in both LAs.
Coverage
Invited population
Local authorities invited all 40- to 74-year-olds who had not already been diagnosed with heart disease, stroke, diabetes or kidney disease or were known to be at higher risk owing to already receiving treatment for high blood pressure or high blood lipid levels.
One LA, LA C, noted that, if possible, the focus should be on inviting high-risk patients, whereas LA K reported that invitations are stratified based on estimated QRISK®2 (ClinRisk Ltd, University of Nottingham, Nottingham and EMIS Health, Leeds; URL: https://qrisk.org/2017/). In the latter case, practices are responsible for running searches that had been developed to group people into priority groups for invitations (first QRISK 2 ≥ 20% and then QRISK 2 ≥ 15% < 20%). In addition, LA K developed searches to identify those who were eligible, but had never been invited to an NHS Health Check.
Local authority F provided their strategy for identifying people to invite. More manageable annual cohorts were obtained by inviting participants by birth month. In this way, all eligible participants were invited over the 5-year cycle.
Invitation methods
The LAs reported that their GPs tended to use a mix of invitation methods, depending on what worked for different practices. Common invitation methods used included letters, telephone calls, texts and e-mail. Seven LAs (A, B, D, E, G, J and L) also reported that their practices were using opportunistic methods, including on-screen reminders on general practice systems. Three LAs (E, G and L) used this as the primary method of invitation.
Six LAs (B, E, F, G, H and K) reported that some practices had changed their method of invitation since the beginning of the programme. LAs E and G had switched to opportunistic invitations, as using letters resulted in low uptake. They felt that the change in approach had improved uptake. However, LA E reported that it was more challenging to monitor invitations when using the opportunistic model. Other changes included using texts, e-mail, a standardised short letter and re-inviting those who were still eligible but had not had an NHS Health Check in the last 4 years.
Case examples
Local authority B
After changing to more opportunistic screening, coverage in LA B increased.
As of the end of 2017/18, 26.4% of the eligible population had been offered an NHS Health Check. This was significantly better than the overall England figure of 17.3%.
A total of 73,211 patients had been offered an NHS Health Check, which was 125% of the eligible population of 58,649. This was significantly better than the England figure of 90.9%.
Local authority F
In the final year of the cycle, searches were developed to re-invite all those who had not had a Health Check in the previous 4 years and remained eligible to increase the number of NHS Health Checks completed over the 5-year cycle.
Take-up
Approaches used to increase uptake
Local authorities reported a wide variety of different approaches to increase the uptake of NHS Health Checks.
Local authority A
Uptake increased by 17% (from 47% in 2013–14 to 64% in 2017–18).
In 2017/18, the number of NHS Health Check invitations was 14,672 and the number of NHS Health Checks completed was 9425. Twenty-eight health-care assistants/nurses attended NHS Health Checks training delivered in April 2018.
This LA had several approaches to increase uptake:
-
Free annual training for general practices (including refreshers training).
-
Systems and processes were in place to run reports for invoicing and make payments for NHS Health Checks more streamlined.
-
The public health team provided practices with eligible population reports, clinical templates, referral forms, information leaflets and associated crib sheets.
-
Practices were incentivised to reach their annual target by offering bonus payments according to their uptake.
In 2014, the public health team changed the way payments were made. Payments were split by Health Check invite, Health Check completed and Health Check bonus payment for achieving the target to increase uptake. The performance target for NHS Health Checks in this LA area was also increased in line with national targets.
Local authority B
In 2012/13, uptake varied between practices from 7% to 57%. The CCG average performance at this time was 24.3% (quartile 3). Slow uptake reflected a lack of resource and poor coding.
An increase in uptake was seen in 2013/14 from 27.2% to 40.6% of eligible individuals. In 2017/18, of the 15,478 people offered an NHS Health Check, 64.9% accepted. This was significantly better than the overall England figure of 47.9% and represented a relative increase of 35% from 2016/17 (48.1%).
The following numbers were reported over the 5-year period (2013–18):
-
Of the 73,211 people offered an NHS Health Check, 55.0% accepted. This was significantly better than the overall England figure of 48.7%.
-
Of the listed eligible population of 58,649 people, 68.7% (n = 40,270) received a Health Check, which was significantly better than the overall England figure of 44.3%.
Five approaches were used to increase uptake:
-
A joint initiative with the CCG to improve performance of the lowest-performing practices together with recognising the need to increase the number of NHS Health Checks for all of the LA B population. All practices developed an action plan focusing on two or three quality indicators. Actions within practices included NHS Health Checks training delivered by public health, use of point-of-care testing machines, holding additional clinics (including weekends), inclusion as a regular feature in practice newsletters and on the website, and working with practices to improve coding.
-
Use of a master template on the general practice clinical system.
-
Quarterly feedback on performance and key messages were sent to all providers.
-
An NHS Health Check Implementation Group to provide oversight to the programme.
-
A health and well-being intervention lead post, which provided training and support visits to Health Check providers to improve the quality of NHS Health Checks.
Local authority C
Local authority C reported on their approaches to increasing uptake of NHS HCP, but did not quantify that change. Their NHS HCP was delivered through primary care, primarily by health-care assistants. A three-pronged approach achieved this:
-
The LA developed a local template within the general practice clinical system and the associated reports. Alongside this, the LA provided all of its practices with point-of-care blood testing and developed a training programme. It also produced promotional materials and guidance on running the programme for practice managers and health-care assistants.
-
The LA actively engaged with their general practice teams, including attending quarterly CCG meetings and GP network meetings to promote the programme. It then visited general practices identified as needing additional support, providing training on using the template, equipment and running searches.
-
The LA’s payment structure ensured that practices were well remunerated for work delivery (recognising the financial pressures general practices face). Initially, it also offered a range of bonus incentive payments. Targets were set for practices and quarterly performance reports were produced. This helped to encourage natural competitiveness.
Local authority D
Local authority D had an eligible population of 75,038, of whom 53,434 (71.2%) had a Health Check over 5 years.
Several approaches were implemented to increase uptake, including:
-
practice visits
-
NHS Health Check events
-
health trainers
-
advertisements on buses
-
practice websites
-
campaigns using local celebrities (new since 2018).
Local authority E
The percentage of uptake increased from 8% of those eligible in 2010/11 to 30% of those eligible in 2012/13. Total number of screens completed increased from 7403 patients to 24,048 patients in 2012/13.
This LA altered their model of delivery and:
-
met with GPs to fully understand the barriers they faced
-
commissioned focus groups with the public to understand why people did not take up an invite
-
conducted a marketing campaign to increase awareness of the service.
Local authority F
Local authority F did not quantify recent changes. However, they did report the approaches they had used to increase uptake:
-
Pharmacies and optical practices were trained to undertake NHS Health Checks.
-
Three third-party providers and a team of sessional workers provided community and workplace NHS Health Checks.
-
In the final year, a pilot, using a private company formed by local practice clinicians, went into general practices where capacity was an issue to carry out NHS Health Checks.
In the year from April 2017 to March 2018, a performance-related bonus payment for practices was introduced. Practices reaching 66% coverage of the 5-year cohort received a bonus payment of £5 extra per check for all checks completed after that. To incentivise the practices to complete Health Checks, those that reached 75% coverage received a bonus payment of £10 for each check completed.
Local authority G
Local authority G did not report a precise percentage change, only their approaches to increase uptake. These had evolved and included:
-
professional training
-
general practice support
-
travelling NHS HCP sofas
-
a series of short NHS HCP promotional films
-
card-making project with children for loved ones
-
presentations at GP locality meetings
-
presenting to the local medical committee
-
practice meetings
-
promotion at local football matches
-
radio campaigns (both local and regional).
Local authority H
This LA provided monthly support to practices by (1) training clinical staff, (2) ringing patients to make an appointment and (3) sending out monitoring reports to practice managers to inform them of their completion and uptake figure. This information was benchmarked against other practices.
Local authority H did not report a specific percentage change in uptake.
Local authority I
Local authority I introduced a new model in April 2017, designed to increase activity, utilise the local system and integrate working across the LA and CCG.
Local authority I commission the NHS HCP through the broader primary care standards contract managed by their CCG. Public health was a specific domain area and to receive payment practices had to complete all areas within the domain. As a financial incentive to complete the whole domain, there was a threshold for each area, with a detailed performance management system for practices to monitor activity.
Local authority I did not report a specific percentage change in uptake.
Local authority J
During 2008–13, 45,275 NHS Health Checks were completed, and during 2013–18, 52,196 NHS Health Checks were completed, representing an increase of 15.3%.
The following approaches were introduced in 2014 and achieved a sharp increase in performance:
-
Introduction of monthly ‘activity’ dashboards that monitor invite and uptake, which are shared with GPs by e-mail. At quarterly ‘cluster meetings’, these data were presented to groups of GPs, comparing performances across practices, clusters and against neighbouring boroughs.
-
‘Outcome’ and ‘quality’ dashboards were presented at cluster meetings. For example, the number of diagnoses of type 2 diabetes, CVD and other conditions that had arisen in NHS Health Check patients within 3 months of a check (or year to date).
-
The quality dashboard monitored outcomes such as referrals into appropriate services for eligible patients, measures completed within a Health Check and invitation method.
The dashboards were feasible because (1) all GPs in LA J were on an electronic patient record system and (2) the LA had commissioned a third party that had data-sharing agreements in place with all GPs, and the analytical and technical capacity to offer the service.
Local authority J commented that the critical factors for improving uptake were access to regular high-quality data for purposes of contract monitoring and data sharing with GPs so that they could view their performance against their peers, thereby encouraging competition.
Local authority K
Between 2010 and 2015, 42,113 NHS Health Checks were delivered:
-
GPs delivered 64% of all NHS Health Checks.
-
Pharmacies delivered 6% of all NHS Health Checks.
-
Community outreach delivered 30% of all NHS Health Checks.
Local authority K reported that community outreach delivered the highest proportion of checks to younger age groups (i.e. those aged 35–49 years), accounting for 64% of all community NHS Health Checks delivered. However, the highest uptake rate was found in older age groups (i.e. those aged 60–74 years), with 53% of the eligible population receiving an NHS Health Check.
Local authority K had a multifaceted approach to NHS Health Check delivery. The approach was based on local analysis of where the most significant impact in reducing CVD-related inequalities could be achieved:
-
GPs focused on people with (1) a high estimated CVD risk (i.e. a QRISK 2 score of ≥ 10%) and (2) mental health/learning disabilities.
-
Pharmacies focused on (1) people who were not engaged with primary care and (2) deprived neighbourhoods.
-
Community outreach focused on (1) deprived communities, (2) ethnic minorities and (3) men.
Changes to the payment structure were implemented at the start of 2012/13 to incentivise GPs to target increasing uptake among people with a high estimated QRISK 2 score and those on mental health registers.
Local authority L
A total of 50,650 NHS Checks were carried out from 2009 to 2012, reaching in equal measure the local South Asian population, the socially deprived and the older-age population. In 2011/12 the uptake was 73%.
Individual general practices and GP networks organised how to invite their local patients to the NHS Health Check, resulting in a steady number of eligible registered people attending over the period. The GP networks were given a target to meet each year, which resulted in the LA continuing to meet the delivery targets for NHS Health Checks across the borough.
Community outreach
Approximately half of the LAs used community outreach activities for NHS Health Checks. Most of the community outreach activities were focused on improving uptake, specifically in more deprived communities where uptake was low. Three LAs (B, C and I) reported events in local community venues as their only community outreach activities. LAs F, G and K had more extensive outreach programmes with various activities.
Local authority F
-
NHS Health Checks in pharmacies and optical practices with ‘out-of-office’ hours appointments.
-
NHS Health Checks in local workplaces.
-
Third-party contracts offering NHS Health Checks. Advertised in local venues around boroughs of low uptake or high deprivation.
Local authority G
-
A travelling NHS HCP sofa visited local parks, supermarkets, general practices and town centres.
-
A series of short NHS HCP promotional films were developed to promote the programme.
-
A card-making project with children for loved ones who may be eligible for a check.
-
Promotion at football matches.
-
Radio campaigns (both local and regional).
Local authority K
-
A comprehensive communication programme to increase public awareness of availability and locations of NHS Health Checks delivery.
-
Programme delivery at accessible, high footfall locations, such as supermarkets and community events.
Half of the LAs included did not have any community outreach activities for NHS Health Checks (LAs A, D, E, H and J).
Lifestyle services
All but one LA had a directly commissioned lifestyle referral service. However, the one LA without a directly commissioned lifestyle referral service offered to signpost to a lifestyle service. Nine LAs reported the components of their lifestyle referral services, including health trainers, smoking cessation, weight management, physical activity, healthy eating, alcohol services, diabetes prevention and social prescribing. Two LAs (C and L) did not specify the components.
Cost per NHS Health Check
Nine LAs (A, B, C, E, F, G, H, J and K) paid practices for a completed NHS Health Check, varying from £18 to £47 per Health Check. Of these nine LAs, five indicated that they provided bonus payments if specific criteria were met (£4–28 per Health Check). Criteria included meeting the invite (100%) and uptake (66%) targets, placing patients on a management plan (one off) and testing high-risk patients. Only one LA (A) paid practices for sending out invites. LA E previously paid practices for invites but found it ineffective, with a very low percentage uptake. LAs D, I and L did not pay per NHS Health Check completed. However, they did have a fixed allocation budget, which depended on practices delivering on targeted numbers. LA I specified that this could amount to £37 per NHS Health Check completed.
Conclusions
The approaches adopted for improving coverage and uptake of NHS HCP varied across all of the top-performing LAs we contacted. The LAs varied in terms of population profile and numbers, and levels of social deprivation. These factors influenced how the LAs designed and implemented strategies to increase coverage and uptake. It was therefore not possible to establish a typical pattern to identify a set of effective approaches that can be recommended to LAs that are not performing as well. However, it was apparent that all of the LAs had taken a strategic and sometimes innovative approach based on their population profile to achieve the targets set.
In terms of the workHORSE project, the information obtained provided valuable case examples for possible scenarios. The information also suggested that uptake-based scenarios for analysis will provide support to model different ‘implementations’ or optimisation of the programme to analyse with the workHORSE tool and to inform the content of the stakeholder engagement workshops.
Umbrella review of approaches used to increase screening uptake
Introduction
Among the strategies developed to tackle the preventable burden of NCDs, screening programmes are proposed and in place for many conditions: primarily cancers and infectious diseases. Screening programmes are used to detect diseases in an earlier stage in asymptomatic people who may have an increased risk of disease and can lead to better chances of successful treatment. In turn, earlier diagnosis reduces premature morbidity and mortality. 44 However, several biases, relating to screening programmes, have been identified, including overdiagnosis, length time bias, lead time bias and reaching the worried well. 44
The introduction of screening programmes depends on the effectiveness, acceptability and cost of the intervention. Globally, there are many types of screening programmes that target different populations. Screening programmes for adults are mainly cancer related, including breast, cervical and colorectal cancer, as feasible and efficient evidence-based strategies exist for these diseases, which are cost-effective. 45,46 However, variability exists in approaches to screening in different countries, as shown in a report on cancer screening in the European Union. 47 Differences exist concerning the level at which the screening takes place (regional or national), the screening test used [i.e. an immunochemical faecal occult blood test (FOBT) vs. a guaiac-based FOBT for colorectal screening], the interval at which screening takes place and the population targeted (age range). 47
For screening programmes to be successful, there needs to be a system and not just a test. Programmes need to have an infrastructure to provide support throughout the entire process, from inviting people to attend the screening through to treatment and follow-up. 44 However, to maximise the impact of screening programmes, high uptake, compliance and diagnosis are also essential. Uptake represents the most important factor in determining the success of screening programmes. Unfortunately, for breast and cervical cancer screening, a trend is emerging internationally that a smaller proportion of eligible women are being screened. This trend is also observed in the UK, with none of the cancer screening programmes meeting its agreed standard targets in 2017/18. 48
Reasons for low uptake included low awareness of screening benefits, low acceptability of certain screening tests [e.g. Pap (Papanicolaou) test], difficulty in accessing services, language or cultural barriers, perceived costs and structural barriers. 44,48 Some ethnic minority groups have significantly lower uptake and people with disabilities or mental health problems also tend to have lower uptake than the general population. Screening has the potential to reduce health inequalities. However, this is not certain with the current design and therefore it is crucial to promote equitable access for underserved groups. 11,48,49
High participation rates in screening programmes targeting NCDs are instrumental in achieving full screening benefits; however, uptake enhancement in screening programmes remains underused, especially among vulnerable populations.
We conducted an umbrella review to assess the type of approaches screening programmes use to maximise uptake, the effectiveness of the approaches and the impact on equity.
Methods
Study design
We conducted an umbrella literature review (i.e. a review of systematic reviews and meta-analyses) of strategies intended to increase the uptake of screening programmes. To ensure proper conduct, we adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist (see Appendix 2, Figure 22). 50 A narrative synthesis was used to present the data by strategy, type of screening programme and strength of evidence. The protocol for this review was registered as PROSPERO CRD42019132087 [see the NIHR Journals Library project web page; URL: www.journalslibrary.nihr.ac.uk/programmes/hta/1616501/#/ (accessed 10 March 2020)].
Search strategy
Exemplar papers were used to develop the search terms and inform the search strategy. A pilot was conducted to determine appropriate databases, identify relevant papers and highlight potential issues to be addressed. Based on this, limits were applied to publication date only (1999–2019). Search terms included screening, uptake, participation, systematic reviews and meta-analysis. A full search strategy can be found in the file PROSPERO protocol [see the NIHR Journals Library project web page; URL: www.journalslibrary.nihr.ac.uk/programmes/hta/1616501/#/ (accessed 10 March 2020)].
We searched the following electronic bibliographic databases from 1999 to 2019 for both published and unpublished reports: MEDLINE, Cochrane Database of Systematic Reviews (CDSR), Cumulative Index to Nursing and Allied Health Literature (CINAHL), EMBASE, Web of Science, Healthcare Management Information Consortium (HMIC), Database of Promoting Health Effectiveness Reviews (DoPHER) (EPPI Centre) and the NIHR Journals Library. Targeted searches were also conducted in Google Scholar (Google Inc., Mountain View, CA, USA). The final search was conducted on 27 March 2019. Reference lists of included studies were screened for potential eligible papers and study authors were contacted if we were unable to access the paper.
Eligibility criteria and study selection
We included studies if they evaluated strategies to improve the uptake of screening programmes. We excluded studies focusing on shared decision-making or patient navigation interventions. Owing to time limitations and budget restrictions, only studies in English were included. The retrieved studies were evaluated using the PICOS (Participants, Interventions, Comparators, Outcomes and Study design) approach, summarised in Table 4.
| Include | Exclude |
|---|---|
| Participants | |
| Studies for adult age groups from all populations and high- and middle-income countries | Primary and secondary school children, pregnant women and low-income countries |
| Interventions | |
| Invitation method interventions aimed at increasing the uptake of screening programmes, including but not limited to (1) personalised risk communication and (2) invitation methods (e.g. letter of invitation, mailed educational material, letter of invitation plus telephone call, telephone call, training activities plus direct reminders, reminder letters, physician reminders, telephone reminders, home visits) |
Screening evaluation studies not including invitation method interventions Studies reporting on the effectiveness of different screening tools only Shared decision-making Patient navigation interventions |
| Comparators | |
| Systematic reviews or meta-analyses where interventions to improve uptake of screening programmes were evaluated (vs. usual care) or compared (vs. other interventions) | No comparisons of different invitation method interventions to improve uptake of screening programmes presented |
| Outcomes | |
| Uptake of screening programmes (i.e. participation rate) | Knowledge, informed decision, risk perception, patient acceptability/satisfaction, cost of the intervention, cost-effectiveness, incidence or prevalence of the disease screened |
| Study design | |
| Systematic reviews and meta-analyses of studies with the following study design: RCTs, qualitative studies, empirical observational studies, natural experiments, modelling studies, secondary analysis and before-and-after interventions | Primary studies |
The main outcome of this umbrella review was uptake of screening programmes (i.e. participation rate).
Michelle Maden conducted the searches and all papers identified by the searches were imported into Covidence (Melbourne, VIC, Australia) for screening. Duplicates were removed. Titles and abstracts were screened for eligibility independently by two reviewers (LH and FLW) and full-text papers were retrieved if papers were deemed potentially eligible. A full-text review was also carried out independently by two reviewers (LH and FLW). Any discrepancies were resolved by consensus or involving the senior author.
Data extraction and management
Data extraction forms were developed based on the recommendations made by Aromataris et al. 51 for the proper conduct of an umbrella review. These forms were pre-piloted and adapted for this review. The data extraction form included the following elements:
-
citation details
-
objectives of the included review
-
type of review
-
participant details
-
setting and context
-
number of databases sourced and searched
-
date range of database searching
-
publication date range of studies included in the review that inform each outcome of interest
-
the number of studies, types of studies and country of origin of studies included in each review
-
instruments used to appraise the primary studies and the quality rating
-
outcomes reported that are relevant to the umbrella review question
-
method of synthesis/analysis employed to synthesise the evidence
-
comments or notes the umbrella review authors may have regarding any included study.
The data extraction was initially carried out by one reviewer (LH). Each study was then checked by a second reviewer (AB or MO’F) for correctness and any potential missing information.
Risk of bias
The ROBIS (Risk of Bias in Systematic Reviews) tool was used to assess the risk of bias for each study. The tool assessed eligibility criteria, identification and selection of studies, data collection and study appraisal, and synthesis and findings. One reviewer (LH) assessed the risk of bias for all studies. A random sample (50%) of the studies was assessed independently by a second reviewer (MM or FLW). A second reviewer checked the remaining 50% of the studies for correctness (MM or ES). Discrepancies in the quality assessment were reconciled by consensus or involving a third senior member of the team.
Data synthesis
The evidence was summarised as a narrative synthesis according to intervention type, screening programme and strength of evidence to facilitate comparisons between the different interventions and screening programmes. (For summary tables of the studies included in this review, see Table 6.) The full data extraction tables are available in Appendix 3 with full references.
Results
In total, 5286 records were identified through the database searches. After removing 2207 duplicates, 3133 records were left for the title and abstract screening. During that process, 2955 records were excluded, leaving 178 records for full-text review. We included a total of 61 reviews in this umbrella literature review. Of these 61 reviews, 38 included more than two interventions or screening programmes (detailing 180 outcomes).
The main interventions identified included patient education, patient invitations and reminders, provider interventions, reducing out-of-pocket client costs, reducing structural barriers and multiple interventions. Definitions are provided in Table 5.
| Intervention | Definition |
|---|---|
| Patient education | The process by which health professionals and others provide information to patients that will alter their health behaviour |
| Patient invitations and reminders | The identification of eligible patients, inviting these patients to book or attend an appointment, reminding them to attend booked appointments and following up those who have not attended or who have not responded to invitations |
| Provider interventions | Interventions targeted at providers of screening services (e.g. training, financial incentives) to encourage improvement in provision and uptake by patients |
| Reducing out-of-pocket patient costs | Monetary incentives provided to patients to increase uptake of screening services (e.g. travelling expenses, one payment for attendance) |
| Reducing structural barriers | Making screening services more accessible to patients by, for example, providing transport, mailing kits and home visits |
| Multiple interventions | Utilising a combination of approaches (i.e. two or more) to increase the likelihood of uptake of screening services |
Most screening programmes identified focused on breast, cervical or colorectal cancer, with a few examples of other cancers (e.g. testicular) or infectious conditions [e.g. human immunodeficiency virus (HIV)].
Most of the systematic reviews and systematic reviews with meta-analyses included were reviewing RCTs, quasi-experimental and observational designs. In general, the individual reviews were at high risk of bias. The outcomes reported included screening uptake, participation, adherence and utilisation, test utilisation and some looked at guidelines’ adherence and compliance. A summary of the main findings is provided in Table 6.
| Intervention: screening programme | Number of reviews | Quality assessment: number at low risk of bias vs. at high risk of bias | Effectiveness |
|---|---|---|---|
| Patient invitations | |||
| Breast cancer | 5 | 1 vs. 4 | All reviews found patient invitations to be effective |
| Cervical cancer | 5 | 3 vs. 2 | All reviews found patient invitations to be effective |
| Colorectal cancer | 4 | 1 vs. 3 | All reviews found patient invitations to be effective |
| CVD risk factor screening | 1 | 0 vs. 1 | Not effective |
| Multiple screening programmes | 2 | 1 vs. 1 | Effective. One review reported greater effectiveness in cervical cancer screening vs. breast cancer screening |
| Patient reminders | |||
| Breast cancer | 4 | 1 vs. 3 | All reviews found patient reminders to be effective |
| Cervical cancer | 4 | 1 vs. 3 | All reviews found patient reminders to be effective |
| Colorectal cancer | 5 | 4 vs. 1 | Two reviews found modest improvements. Three reviews found larger effects among interventions with telephone component |
| Multiple screening programmes | 3 | 1 vs. 2 | Two reviews reported apparent effectiveness. One review found text reminders moderately effective |
| Access-enhancing interventions | |||
| Breast cancer | 5 | 1 vs. 4 | Generally effective. One review found strong evidence of effectiveness. Two reviews reported the largest effectiveness vs. individual-directed interventions, community education and mass media. One targeted ethnic minority women. One review found some evidence of effectiveness for mobile onsite mammography screening in certain women of Asian ethnicity. One review did not have enough evidence |
| Cervical cancer | 2 | 1 vs. 1 | Mixed results. One review reported that access-enhancing interventions were more effective than other interventions in ethnic minority women. One review found insufficient evidence to determine effectiveness |
| Colorectal cancer | 1 | 0 vs. 1 | Effective. Strong evidence |
| Multiple screening programmes | 1 | 0 vs. 1 | Effective for breast cancer, but less evidence for cervical and colorectal cancer |
| Mailed kits | |||
| Cervical cancer | 4 | 1 vs. 3 | Effective. All increased cervical screening rate, including underscreened women and non-responders |
| Colorectal cancer | 4 | 3 vs. 1 | Effective. All found an increase in colorectal cancer screening |
| Organisational change and procedures | |||
| Breast cancer | 1 | 0 vs. 1 | Modest effectiveness |
| Multiple screening programmes | 2 | 0 vs. 2 | Effective |
| Using dedicated personnel | |||
| CVD risk factor screening | 1 | 0 vs. 1 | Effective. Increased uptake of CVD risk factor screening |
| Interventions tailored for individuals | |||
| Breast cancer | 4 | 1 vs. 3 | Effective. Two reviews reported a small effect only |
| Cervical cancer | 3 | 2 vs. 1 | Effective. One review reported that in-reach interventions targeting health-care professionals and patients moderately improved screening rates |
| Individual: decision aids | |||
| Colorectal cancer | 1 | 0 vs. 1 | Decision aids and general colorectal cancer screening information had a similar impact on screening rates |
| Prostate cancer | 3 | 0 vs. 3 | Not effective |
| Individual: one-on-one education and counselling | |||
| Breast cancer | 4 | 0 vs. 4 | Effective. Three reviews suggested moderate effectiveness. One review suggested stronger evidence of effectiveness |
| Cervical cancer | 2 | 1 vs. 1 | Effective |
| Colorectal cancer | 1 | 0 vs. 1 | Effective. Sufficient evidence |
| Multiple screening programmes | 4 | 0 vs. 4 | Effective. All reported effectiveness |
| Individual home visits | |||
| Breast cancer | 1 | 0 vs. 1 | Not effective |
| Cervical cancer | 2 | 1 vs. 1 | Effective. One review found effectiveness. One review found effectiveness in some Asian populations |
| Individual: personalised risk communication/tailored messaging | |||
| Breast cancer | 1 | 1 vs. 0 | No effect on risk factor tailoring. Indicative findings for behavioural construct tailoring |
| Cervical cancer | 2 | 2 vs. 0 | Not effective |
| Colorectal cancer | 2 | 2 vs. 0 | Not effective |
| Multiple screening programmes | 2 | 1 vs. 1 | One review found it to be ineffective and one review found weak evidence of effectiveness |
| Mass campaign through community-based health workers | |||
| Breast cancer | 1 | 0 vs. 1 | Effective |
| Cervical cancer | 1 | 1 vs. 0 | Effective |
| Colorectal cancer | 1 | 1 vs. 0 | Effective |
| Multiple screening programmes | 1 | 0 vs. 1 | Effective. Greater uptake after using community-based health workers. Larger effect in previously non-adherent patients |
| Mass campaign through group education | |||
| Breast cancer | 5 | 2 vs. 3 | Mixed findings reported for effectiveness: one review reported limited effectiveness, one review reported some effectiveness, two reviews reported modest effectiveness and one review reported sufficient effectiveness |
| Cervical cancer | 3 | 1 vs. 2 | Two reviews found mixed findings and one review found favourable findings |
| Colorectal cancer | 1 | 0 vs. 1 | Limited effectiveness |
| Multiple screening programmes | 1 | 0 vs. 1 | Suggested most studies increased screening uptake in minority groups |
| Mass campaign through mass media | |||
| Breast cancer | 2 | 1 vs. 1 | Limited effectiveness |
| Cervical cancer | 3 | 2 vs. 1 | Limited effectiveness |
| Colorectal cancer | 2 | 0 vs. 2 | One review reported insufficient evidence. One review reported apparent effectiveness in ethnic minority groups |
| Mass campaign through small media | |||
| Breast cancer | 2 | 0 vs. 2 | Effective |
| Cervical cancer | 2 | 1 vs. 1 | One review reported mixed findings. One review suggested a positive effect on screening |
| Colorectal cancer | 3 | 2 vs. 1 | One review reported it to be ineffective. One review reported it to be inconsistent. One review reported it to be positive |
| Multiple screening programmes | 2 | 0 vs. 2 | Effective in all three cancers. One review reported it to be possibly effective in Asian people |
| Individual and mass campaign combined | |||
| Breast cancer | 4 | 0 vs. 4 | Effective. Three studies indicated a low to moderate effect. One study found inconsistent results |
| Cervical cancer | 6 | 0 vs. 6 | Effective. Three studies suggested some effectiveness. One study reported consistent results using culturally sensitive strategies. One study reported ineffective results among Latina women |
| Colorectal cancer | 3 | 1 vs. 2 | Effective. All studies found some evidence of effectiveness |
| Multiple screening programmes | 1 | 0 vs. 1 | Effective in some Asian populations |
| Provider reminders | |||
| Breast cancer | 2 | 1 vs. 2 | Effective |
| Cervical cancer | 1 | 0 vs. 1 | Modest effectiveness |
| Colorectal cancer | 4 | 2 vs. 2 | Effective |
| CVD risk factor screening | 1 | 0 vs. 1 | Effective |
| Multiple screening programmes | 3 | 0 vs. 3 | Effective |
| Education of health-care professionals | |||
| Colorectal cancer | 1 | 1 vs. 0 | Effective. Clinician education improved screening uptake |
| Multiple screening programmes | 1 | 0 vs. 1 | Effective. Some effectiveness |
| Provider assessment and feedback | |||
| Breast cancer | 2 | 0 vs. 2 | Effective |
| Cervical cancer | 1 | 0 vs. 1 | Effective |
| Colorectal cancer | 1 | 0 vs. 1 | Effective |
| Multiple screening programmes | 3 | 0 vs. 3 | Effective. Two studies found effectiveness and one found moderate effectiveness |
| Incentives for providers | |||
| Multiple screening programmes | 1 | 0 vs. 1 | Insufficient evidence |
| Combination of two or more provider interventions | |||
| Colorectal cancer | 1 | 1 vs. 0 | Limited effectiveness, inconsistent findings and few studies |
| Reducing out-of-pocket client costs and other financial incentives | |||
| Breast cancer | 1 | 0 vs. 1 | Effective |
| Cervical cancer | 1 | 0 vs. 1 | Insufficient evidence |
| Colorectal cancer | 2 | 1 vs. 1 | Not effective |
| CVD risk factor screening | 1 | 0 vs. 1 | Effective |
| Multiple screening programmes | 2 | 0 vs. 2 | Effective |
| Multiple component interventions: interventions with two or more components | |||
| Breast cancer | 10 | 3 vs. 7 | Mixed results, indicating no/low/modest/high effectiveness |
| Cervical cancer | 4 | 4 vs. 0 | Effective. Two studies reported effectiveness and two reported a modest positive effect |
| Colorectal cancer | 3 | 2 vs. 1 | Variable. One study reported effectiveness, one reported some effectiveness and one reported mixed findings |
| CVD risk factor screening | 1 | 0 vs. 1 | Effective. Low to moderate effectiveness |
| Multiple screening programmes | 4 | 1 vs. 3 | Effective in all four reviews |
Patient invitations
Thirteen studies46,52–63 investigated the effect of patient invitations, with two studies46,56 reporting outcomes on more than one screening programme separately (n = 17).
Breast cancer
Five reviews46,56,59,61,63 assessed the effect of different types of patient invitations on breast cancer screening uptake. All five reviews found patient invitations to be effective (one systematic review and meta-analysis,56, one meta-analysis59 and three systematic reviews46,61,63). Effective patient invitations included a letter of invitation or telephone call or both. The combination was the most effective intervention. Furthermore, adding appointments to invitation letters further increased uptake. One meta-analysis,59 deemed to have a low risk of bias, found a smaller intervention effect in underutilising populations than in the general population.
Cervical cancer
All reviews46,55–57,60 (three systematic reviews and meta-analyses,55–57 and two systematic reviews46,60) found invitation letters to be effective in increasing cervical cancer screening. Furthermore, open invitation letters, appointments on invitation letters, telephone invitations and personal invitations were also found to be effective. Invitation letters with a reminder telephone call were reported as the most effective intervention.
Colorectal cancer
Participation was increased by all invitation methods, including postal and telephone reminders, scheduled appointments on invitation letters, the addition of a kit to the invitation letter and GP involvement in two systematic reviews and meta-analyses. 56,58 Two further systematic reviews46,52 showed some positive impact of advance notification letters.
Multiple screening programmes
One systematic review,62 rated as having a low risk of bias, reported that invitation letters were more effective in cervical cancer screening than in breast cancer screening. One systematic review and meta-analysis,53 rated as having a high risk of bias, found that patient invitation was effective in increasing screening uptake.
Cardiovascular disease risk factor screening
Only one systematic review and meta-analysis,54 rated as having a high risk of bias, was included. This study54 suggested that patient invitations were not effective in increasing CVD risk factor screening.
Conclusions
Overall, there is strong evidence that patient invitations are effective in increasing screening uptake for breast, cervical and colorectal cancer. The combination of invitation letters plus a telephone reminder was even more effective. Evidence on the equity impact was limited and merits further research.
Patient reminders
Fourteen reviews52,62–74 were identified. Two systematic reviews67,69 presented outcomes for two screening programmes separately (n = 17).
Breast cancer
One systematic review and meta-analysis,71 one meta-analysis74 and one systematic review62 all found some evidence of the effectiveness of patient reminders. One systematic review,69 rated as having a high risk of bias, reported strong evidence of effectiveness. Finally, another systematic review67 investigated the effect of reminder letters on non-responders and reported consistent findings of increased uptake.
Cervical cancer
One meta-analysis,73 rated as having a high risk of bias, suggested significant effectiveness of reminder letters. They also found that those in lower socioeconomic groups had a lower uptake than those using mixed populations. A systematic review,66 rated as having a low risk of bias, and two systematic reviews,68,69 rated as having a high risk of bias,68,69 also found favourable results among lower socioeconomic groups.
Colorectal cancer
Five reviews52,64,65,67,69 were included, all of which were rated as having a low risk of bias, apart from Sabatino et al. 69 One systematic review and meta-analysis65 and one systematic review64 showed modest improvements in screening rates. Larger effects were seen among interventions with a telephone component (alone or in combination with a letter) (one systematic review and meta-analysis65 and two systematic reviews52,69).
Multiple screening programmes
Patient reminders appear to be effective in increasing breast, cervical and colorectal cancer based on one meta-analysis72 and one systematic review,70 both of which were rated as having a high risk of bias. Using text reminders appeared to have a moderate effect only. 67
Conclusions
Patient reminders were effective in increasing the uptake of breast, cervical and colorectal cancer screening. Patient reminders also seemed to increase uptake in non-responders for breast cancer screening. More research is needed, particularly to determine the effects on equity.
Reducing structural barriers
Reducing structural barriers for patients included five different types of interventions: (1) access-enhancing interventions, (2) mailing kits, (3) organisational change and procedures, (4) using dedicated personnel and (5) tailoring the interventions for individuals.
Access-enhancing interventions
Seven reviews63,69,70,75–78 were identified that included access-enhancing interventions, of which one systematic review69 presented outcomes for breast, cervical and colorectal cancer screening separately (n = 9).
Both meta-analyses,76,78 rated as having a low and high risk of bias, respectively, found that access-enhancing interventions had the largest effectiveness compared with individual-directed interventions, community education and mass media. One of the interventions targeted ethnic minority women. One systematic review,63 rated as having a high risk of bias, found no sufficient evidence, whereas another systematic review,69 also rated as having a high risk of bias, found strong evidence of effectiveness. Some evidence of effectiveness was found on mobile onsite mammography screening in certain Asian ethnic women in a systematic review,75 which was rated as having a high risk of bias.
Mixed results were found in one meta-analysis,77 rated as having a low risk of bias, which reported that access-enhancing interventions were more effective in increasing cervical cancer screening in ethnic minority women than other interventions. However, another systematic review,69 rated as having a high risk of bias, found insufficient evidence to determine the effectiveness on cervical cancer screening uptake because of the small number of studies.
Only one systematic review,69 rated as having a high risk of bias, was identified. Strong evidence was found for the effectiveness of access-enhancing interventions on colorectal cancer screening.
Only one systematic review70 included the effect of access-enhancing interventions on breast, cervical and colorectal screening combined. The review70 concluded that this intervention appears effective, but its role in cervical and colorectal cancer screening is less established.
Strong evidence was found that access-enhancing interventions increased breast cancer screening. Two meta-analyses76,78 even suggested that these interventions were more effective than other interventions (e.g. education, reminders, letters and mass media). Some evidence was found for effect on cervical and colorectal cancer; however, the number of reviews was limited and their role was less established. More research is needed to determine the effectiveness for cervical and colorectal cancer. Furthermore, some evidence suggested that these interventions may be effective in ethnic minority women for breast and cervical cancer. However, more research is needed to confirm this effect.
Mailed kits
For cervical and colorectal cancer, part of the screening programme can include sending out ‘do-it-yourself’ kits, as opposed to visiting a health-care professional to complete the screening.
Not applicable.
Three systematic reviews and meta-analyses,55,56,79 one rated as having a low risk of bias and two rated as having a high risk of bias, and one systematic review66 rated as having a high risk of bias, all showed an increase in cervical screening rates after mailing kits to patients homes, including in underscreened women and non-responders. 55,56
Three studies52,64,65 were rated as having a low risk of bias and one study80 was rated as having a low/unclear risk of bias. All four studies52,64,65,80 found an increase in colorectal cancer screening after mailing screening kits compared with controls. A similar effect was seen in underserved and minority populations.
Mailing kits to increase cervical cancer screening seems to be consistently effective in women invited for screening, underscreened women and non-responders. Similarly, mailing kits increased the uptake of colorectal cancer screening, with a potentially similar effect for underserved/minority populations.
Organisational change and procedures
Three reviews,53,72,81 all rated as having a high risk of bias, evaluated the effect of organisational change and procedures.
One meta-analysis81 found a modest effect of the reorganisation of the clinic and using nurse-based interventions.
No studies identified.
No studies identified.
One meta-analysis72 investigated the effect of organisational change to improve screening attendance for breast, cervical and colorectal cancer screening. Organisational change is most likely to improve cancer screening behaviour, compared with financial patient incentives, patient reminders, patient education and provider assessment and feedback, and it was the most potent intervention. A further systematic review and meta-analysis53 found clinical practice improvements to be effective in improving men’s screening uptake.
Using dedicated personnel
No studies identified.
No studies identified.
No studies identified.
One systematic review and meta-analysis54 found that using dedicated personnel significantly increased the uptake of CVD risk factor screening compared with the control groups.
The effect of using dedicated personnel to increase screening uptake remains unproven. Only one review54 of CVD risk factor screening was identified, with none on breast, cervical or colorectal cancer. More research is needed.
Interventions tailored for individuals
Mixed results were presented. One systematic review and meta-analysis82 and one systematic review,83 both rated as having high risk of bias, found a small effect on the uptake when using either simple interventions, or community education, clinical engagement and tailoring, respectively. Compared with other interventions, two meta-analyses76,78 found individual-directed interventions (e.g. counselling, letters and reminders) to be effective. One meta-analysis76 reported that the intervention effect was significant in one ethnic minority group, but not in others.
One meta-analysis,77 rated as having a low risk of bias, found individual-directed interventions (e.g. counselling, letters and reminders) to be effective; however, they were less effective when compared with access-enhancing and community education interventions. Combined intervention effects appeared significant for some ethnic minority groups, but not for others. A systematic review,84 rated as having a high risk of bias, found that individual-level interventions (e.g. education, letters and reminders) boosted uptake. In-reach interventions targeting both health-care professionals and patients seemed to moderately improve screening rates (low risk of bias). 66
Individual-directed interventions may have the potential to be effective. Further research is needed, particularly on equity.
Patient education
Patient education interventions were categorised based on their delivery mode, either at individual or group level. Interventions delivered to individuals included decision aids, one-on-one education or counselling, home visits and personalised risk communication or tailored messaging. Interventions delivered to groups of people included community health-based workers, group education, community education, small media and mass media. Small media are smaller-sized campaigns and include videos or printed materials (e.g. flyers, letters, newsletters and brochures) that contain educational messages to promote screening. Mass media includes larger-scale interventions (e.g. radio, television, newspapers, magazines and billboards). Some reviews combined individual and subpopulation interventions, and these are presented separately.
Individual level: decision aids
One systematic review and meta-analysis,85 rated as having an unclear risk of bias, was identified, which found that decision aids had a similar impact on colorectal cancer screening rates as general colorectal cancer screening information.
Three systematic reviews and meta-analyses,86–88 rated as having a high risk of bias, suggested that patients who received decision aids were less likely than controls to undergo screening. This perhaps reflected patients acquiring a better understanding of the associated uncertainties and limitations.
Evidence from all reviews indicated that decision aids have no impact on colorectal cancer screening rates and may even discourage uptake for prostate cancer.
Individual: one-on-one education and counselling
In total, nine reviews53,57,69–71,81,82,89,90 presented results on individual education interventions, of which one systematic review69 reported results for breast, cervical and colorectal cancer separately.
All four reviews69,71,74,82 were assessed as having a high risk of bias. Two systematic reviews and meta-analyses71,82 and one meta-analysis74 suggested moderate effectiveness at best for one-on-one education in improving breast cancer screening rates. One systematic review69 suggested more robust evidence of effectiveness.
One systematic review and meta-analysis,57 rated as having a low risk of bias, and one systematic review,69 rated as having a high risk of bias, found that one-on-one education can be effective in increasing cervical cancer screening rates.
Only one systematic review,69 rated as having a high risk of bias, was identified. It suggested sufficient evidence to support the use of one-on-one education in improving colorectal cancer screening.
One systematic review and meta-analysis53 and three systematic reviews,70,89,90 all rated as having a high risk of bias, reported on the effectiveness of one-on-one education for multiple screening programmes combined [including breast, cervical, colorectal, prostate, testicular and skin cancer, HIV, sexually transmitted infections (STIs) and hepatitis B virus]. Each review was slightly different in their target population and the way in which the intervention was structured. One systematic review and meta-analysis53 suggested educational interventions to be effective in men, but only when low methodological studies were excluded. Strong evidence was found for one-on-one education in Asian populations,90 but another systematic review89 found no link to ethnicity and reported inconsistent findings.
One-on-one education has the potential to be modestly effective for breast cancer and perhaps for cervical and colorectal cancer. However, the evidence is based on a limited number of studies. More research is needed, particularly to determine the impact on equity.
Individual: home visits
Three studies57,61,75 specified one-on-one education as home visits.
Only one systematic review,61 rated as having a high risk of bias, was identified for breast cancer screening and found home visits to be ineffective.
A systematic review and meta-analysis,57 rated as having a low risk of bias, found that home visits increased uptake significantly, whereas a systematic review75 found that home visits were effective in some Asian populations, but not in others.
There is insufficient evidence to determine the impact of home visits on breast cancer. Home visits may be effective in increasing rates of cervical cancer screening; however, this was based on a small number of studies and more research is needed.
Individual: personalised risk communication/tailored messaging
Five reviews57,64,91–93 investigated the effect of personalised risk communication or tailored messaging on screening uptake, of which one systematic review93 presented results for breast, cervical and colorectal cancer separately (n = 7). All studies were rated as having a low risk of bias, apart from Usher-Smith et al. 91
One systematic review93 investigated different types of tailoring risk and found indicative findings for behavioural construct tailoring, but no effect for risk factor tailoring.
Findings from both studies57,93 suggested that tailoring messages and enhanced risk assessment were ineffective, as no differences were found between the intervention and control or comparison groups.
Two systematic reviews and meta-analyses91,92 investigated the effect of personalised risk communication on more than two screening programmes (breast and colorectal cancer; breast, cervical and colorectal cancer). A study91 rated as having a low risk of bias found weak evidence of effectiveness, whereas a study91 rated as having a high risk of bias study found no effectiveness.
Personalised risk communication and tailored messaging do not appear to be effective for increasing the uptake of breast, cervical or colorectal screening.
Mass campaign: community-based health workers
Two systematic reviews and meta-analyses,94,95 rated as having a high risk of bias, and two systematic reviews,66,96 rated as having a low risk of bias, investigated the effect of community-based health workers.
Only one systematic review and meta-analysis95 was included. The review suggested a significantly increased uptake in breast cancer screening.
Only one systematic review66 was identified. This study found that community-based health worker interventions significantly increased the uptake of screening in lower socioeconomic groups.
One systematic review96 evaluated the use of community-based health worker interventions among Latino men. The review reported increased colorectal cancer screening uptake.
One systematic review and meta-analysis,94 rated as having a high risk of bias, combined findings for breast, cervical and colorectal cancer, and suggested a higher uptake after using community-based health workers and a larger effect in previously non-adherent patients.
Community-based health workers appear to be effective in increasing screening uptake for breast, cervical and colorectal cancer.
Mass campaign: group education
Eight reviews69,76–78,84,89,97,98 included group education, of which one systematic review69 presented results for breast, cervical and colorectal cancer separately (n = 10).
Five studies69,76,78,97,98 focused on breast cancer and mixed findings were found for the effectiveness of group education. Both meta-analyses,76,78 rated as having a low risk of bias and high risk of bias, respectively, reported modest effect at best. One systematic review,69 rated as having a high risk of bias, reported sufficient evidence for the effectiveness of group education, whereas limited effects were reported by another systematic review97 that was rated as having a high risk of bias. One systematic review,98 rated as having a low risk of bias, investigated the effect of group education in Turkish women and found some effectiveness.
The effectiveness of group education on cervical cancer screening rates generated mixed findings in a meta-analysis,77 which was rated as having a low risk of bias, and in a systematic review,69 which was rated as having a high risk of bias. One systematic review,84 rated as having a high risk of bias, found more favourable outcomes.
Only one systematic review,69 rated as having a high risk of bias, focused on colorectal cancer and found limited effect based on a small number of studies.
One systematic review,89 rated as having a high risk of bias, included breast, cervical, colorectal and prostate cancer, and hepatitis B virus screening programmes. The review89 suggested that most studies increased screening uptake in minority groups.
The effectiveness of group education on cancer screening uptake produced mixed and modest findings for breast cancer and cervical cancer. Insufficient evidence was available for colorectal cancer screening. Further research is needed.
Mass campaign: mass media
Seven studies60,69,76–78,89,99 investigated the effect of mass media on screening uptake.
Two meta-analyses,76,78 one rated as having a low risk of bias76 and one rated as having a high risk of bias,78 reported limited effectiveness of mass media on breast cancer screening.
Limited effectiveness was found for using mass media to increase cervical cancer screening based on one meta-analysis77 and two systematic reviews. 60,99
No review was identified that focused solely on colorectal cancer.
Two systematic reviews69,89 considered the effect of mass media on multiple screening programmes (breast, cervical, colorectal and prostate cancer, and hepatitis B virus). Both reviews69,89 were rated as having a high risk of bias. Sabatino et al. 69 reported insufficient evidence to determine effectiveness. Conversely, Kelly et al. 89 reported apparent effectiveness in ethnic minority groups.
Mass media to increase uptake has limited effectiveness in breast and cervical cancer screening. No evidence was identified for its effect on colorectal cancer screening. More research is needed, particularly to determine equity impact.
Mass campaign: small media
Seven reviews52,57,61,70,90,96,99 focused on the effect of small media, of which one systematic review99 reported outcomes for breast, cervical and colorectal cancer separately.
Two systematic reviews,61,99 rated as having a high risk of bias, suggested favourable effectiveness of small media on breast cancer screening uptake.
One systematic review and meta-analysis,57 rated as having a low risk of bias, reported mixed findings for using small media to highlight educational materials to increase cervical cancer screening. A systematic review,69 rated as having a high risk of bias, suggested a positive effect on screening.
Mixed findings were reported for systematic reviews,52,96 both of which were rated as having a low risk of bias. One review52 found no effectiveness, whereas the other review96 found mixed and inconsistent results. Conversely, a systematic review69 that was rated as having a high risk of bias found a positive effect of small media on colorectal cancer screening.
Two systematic reviews,70,90 rated as having a high risk of bias, reviewed multiple screening programmes (breast, cervical and colorectal cancer), with one70 suggesting that small media appeared to be effective in all three cancers. The other review90 suggested that small media might also be effective in Asian communities.
Modest effectiveness of small media was reported for breast cancer, and perhaps for cervical cancer. Results for colorectal cancer were mixed. More research is needed, especially examining equity.
Individual and mass campaign combined
Eleven reviews65,72,75,79,90,97,100–104 addressed the combination of two or more individual and mass campaign interventions. One meta-analysis72 and one systematic review102 focused on more than one screening programme (n = 14). All reviews were rated as having a high risk of bias, apart from one study by Dougherty et al. 65
One systematic review and meta-analysis,100 one meta-analysis72 and one systematic review97 all combined individual with mass campaign interventions (individual plus group education; individual education plus mass media; and education, message framing plus telephone calls, respectively). All studies indicated a low to moderate effect. The systematic review and meta-analysis found that Hispanic people had lower uptake levels than non-Hispanic white people. The use of culturally sensitive strategies produced inconsistent results. 102
One systematic review and meta-analysis,79 one meta-analysis72 and two systematic reviews75,101 suggested some effectiveness of combining mass media or small media with either individual or group education. Education and mass media did not seem effective among Latino populations. 103 Consistent results were reported for using culturally sensitive strategies. 102
One systematic review and meta-analysis,65 one meta-analysis72 and one systematic review104 found some evidence to indicate the effectiveness of combining individual with mass campaign interventions.
One systematic review suggested that lay health workers and mass education campaigns could be successful in some Asian populations. 90
Combining individual and mass campaign educational interventions achieved low to moderate effectiveness for increasing the uptake of breast, cervical and colorectal cancer screening. More research on equity is needed.
Provider interventions
Several interventions were targeted at providers and included reminders, education and incentives. Each will be presented separately below.
Provider reminders
Nine reviews were included. 46,54,64,65,81,89,99,105,106 One systematic review106 presented outcomes for multiple screening programmes (n = 11).
Both a meta-analysis81 and a systematic review,106 each rated as having a high risk of bias, suggested increases in uptake after provider reminders.
One systematic review,106 rated as having a high risk of bias, suggested that provider reminders modestly increased cervical screening rates.
All four reviews (i.e. two systematic reviews and meta-analyses65,105 and two systematic reviews64,106) found provider reminders to be effective in increasing colorectal cancer screening rates.
Three systematic reviews, all rated as having a high risk of bias, combined the effect of provider reminders on multiple screening outcomes (breast, cervical, colorectal and prostate cancer, Hepatitis B) and all were in favourable directions. 46,89,106 One focused on minority groups and found significant increases in uptake. 89
Provider reminders were stated to be effective in both pessimistic and optimistic scenarios in one meta-analysis and systematic review,54 which was rated as having a high risk of bias.
Provider reminder interventions seem to be effective in increasing breast, cervical and colorectal screening rates. Providers have the potential to increase uptake in minority groups, although the number of studies included was small and further research is needed to determine the impact on equity.
Education of health-care professionals
No studies were identified.
No studies were identified.
Only one systematic review and meta-analysis,65 rated as having a low risk of bias, found that clinician education improved screening uptake.
One systematic review and meta-analysis,53 rated as having a high risk of bias, focused on improving screening rates in men (e.g. prostate cancer, HIV, STIs, melanoma) and reported some effectiveness of health-care professional training in increasing men’s uptake compared with usual care.
No evidence was identified for breast and cervical cancer. Health-care professional training appeared to increase the uptake of colorectal cancer screening and health screening for men (prostate, HIV, STIs, melanoma). However, both reviews included only a small number of studies and were rated as having a high risk of bias. Further evidence is therefore needed.
Provider assessment and feedback
Four reviews69,70,72,81 were included, all of which were rated as having a high risk of bias. One systematic review69 provided outcomes for several screening programmes (n = 7).
One meta-analysis81 and one systematic review69 both indicated sufficient evidence that provider assessment and feedback increased uptake of breast cancer screening.
One systematic review69 suggested effectiveness.
One systematic review69 was included and offered evidence of effectiveness.
Three studies combined the effect of provider assessment and feedback on multiple screening programmes (i.e. breast, cervical and colorectal cancer). One meta-analysis72 found some effectiveness of provider feedback. However, this intervention was considered to be the least effective intervention when compared with other interventions, such as organisational change, patient reminders and patient education. The other two systematic reviews69,70 also reported sufficient evidence that this is an effective intervention.
Provider assessment and feedback interventions usually increase uptake of breast, cervical and colorectal cancer screening.
Incentives for providers
No studies identified.
No studies identified.
No studies identified.
Only one systematic review69 looked at the effect of provider interventions on breast, cervical and colorectal cancer screening. Insufficient evidence was found for each of the screening programmes because of the generally small and inconsistent results.
More evidence is needed to determine the effectiveness of provider incentives on breast, cervical and colorectal cancer screening uptake.
Combination of two or more provider interventions
No studies were identified that reported multiple provider interventions.
No studies identified.
Only one systematic review,52 rated as having a low risk of bias, investigated the effect of multiple provider interventions (e.g. GP involvement through reminders, letters and education) on the uptake of colorectal cancer screening. The review suggested limited effectiveness, reporting inconsistent findings and a small number of studies overall.
More research on multiple provider interventions is needed.
Reducing out-of-pocket client costs and other financial incentives
Four studies54,65,69,72 were identified in this domain, with one systematic review69 reporting on outcomes for breast, cervical and colorectal cancer separately (n = 6). All studies were rated as having a high risk of bias, apart from Dougherty et al. 65
Only one systematic review69 found sufficient evidence for reducing out-of-pocket client costs, but it found insufficient evidence for client incentives.
One systematic review69 found insufficient evidence for both reducing out-of-pocket client costs and client incentives.
One systematic review and meta-analysis,65 rated as having a low risk of bias, found that providing small financial incentives (US$5) slightly increased uptake. However, this effect did not occur with the financial incentive of US$10 and pooling both groups found no effectiveness. An earlier systematic review69 had failed to identify any studies.
One meta-analysis72 suggested that financial incentives for breast, cervical and colorectal cancer screening after an organisational change was effective.
One systematic review and meta-analysis54 reported that financial incentives significantly increased the uptake of CVD risk factor screening.
Financial incentives may increase the uptake of cancer screening and CVD risk factor screening; however, more research is needed.
Multiple component interventions
This section is divided into two subcategories. A distinction is made between the use of two or more interventions combined and studies combining the effect of several distinct single interventions.
Interventions with two or more components
Eighteen reviews54,60,61,65,66,74,78,79,81,82,89,90,104,107–111 investigated the effect of multiple component interventions and a wide variety of intervention combinations were evaluated. One review108 reported on outcomes for multiple screening programmes separately (n = 21).
Nine reviews were identified. 61,74,78,81,82,103,107–109 Mixed results were found for breast cancer screening rates, with some reviews indicating no to low effectiveness (one systematic review and meta-analysis,103 one meta-analysis74 and one systematic review61) and others reporting modest to high effectiveness (two systematic review and meta-analyses,82,107 two meta-analyses78,81 and one systematic review108). One systematic review109 reported the effect of multiple interventions being larger than that of single interventions.
Four reviews60,66,79,108 were included and all were rated as having a low risk of bias, apart from Musa et al. 79 A mix of interventions was evaluated (i.e. provider recommendations, in-reach and out-reach interventions with community education, mass media combined with invitation letters and/or education and education with reducing structural barriers or out-of-pocket client costs). Two systematic reviews60,108 found that multiple interventions were effective in increasing cervical cancer screening rates. The other systematic review and meta-analysis66 and systematic review79 found a modest positive effect.
One systematic review and meta-analysis,65 rated as having a low risk of bias, reported that multiple interventions were associated with larger increases in colorectal cancer screening rates (vs. single interventions). A systematic review108 that was rated as having a low risk of bias found mixed results, with some effectiveness of education, reducing structural barriers and out-of-pocket client costs. One systematic review104 that was rated as having a high risk of bias found mixed findings depending on the interventions identified. Patient mailings and telephone outreach were found to be effective, whereas multimedia interventions were not. 104
Four systematic reviews89,90,108,110 investigated the effectiveness of multiple interventions on breast, cervical, lung, prostate and colorectal cancer, and hepatitis B virus. All four systematic reviews89,90,108,110 reported some effectiveness of multicomponent interventions (e.g. education, small media and reminders; and special events reducing structural barriers, group and individual education, small media and reducing out-of-pocket client costs). For Asian groups, a range of interventions was identified to be effective, including the use of social networks, lay health workers, media education, community-based education, reminder notices, health-care provider assistance and health system changes. 90
Only one systematic review and meta-analysis54 was identified. The systematic review and meta-analysis54 investigated the effectiveness of both provider and patient interventions and reported a low to moderate level of effectiveness, depending on the pessimistic or optimistic scenario, respectively.
Despite the heterogeneity in the multiple interventions used, modest to high effectiveness was found for increasing breast, cervical and colorectal cancer screening uptake. Furthermore, some reviews60,109 reported that multiple interventions were more effective than single interventions. The impact on equity has not been adequately investigated and merits further study.
Single interventions combined
Mixed results were presented and were rated as having a high risk of bias. One systematic review and meta-analysis82 and one systematic review83 found a small effect on uptake when using simple interventions or community education, clinical engagement and tailoring, respectively. Two meta-analyses76,78 found individual-directed interventions (e.g. counselling, letters and reminders) to be effective compared with other interventions, with one meta-analysis76 reporting that the intervention effect was significant in one ethnic minority group, but not in others.
A meta-analysis77 that was rated as having a high risk of bias found individual-directed interventions (e.g. counselling, letters and reminders) to be effective. However, the interventions were less effective when compared with access-enhancing and community education interventions. Combined intervention effects appeared significant for some ethnic minority groups, but not for others. Another systematic review84 that was rated as having a high risk of bias found that individual-level interventions (e.g. education, letters and reminders) boosted uptake. In-reach interventions targeting both health-care professionals and patients seemed to moderately improve screening rates (low risk of bias). 66
Most interventions were heterogenous and therefore it was difficult to draw conclusions. Individual-directed interventions may have the potential to be effective. Further research is needed to determine the impact on equity.
Discussion
Summary of findings
This umbrella literature review identified 61 systematic reviews of interventions intended to increase the uptake of screening programmes. Almost all of the screening programmes that were identified focused on one of just three diseases – breast, cervical or colorectal cancer – and very few addressed screenings for high levels of cardiovascular risk factors.
The main targets spanned a spectrum from individuals and groups to communities, organisations or entire populations.
The potential interventions were numerous and diverse. Patient-focused interventions included education, media campaigns, invitations, reminders, mailed self-test kits, home visits, enhanced access or reduced costs. Provider-focused interventions included reminders, incentives, professional training, dedicated personnel, assessment and feedback, plus organisational changes to address structural barriers. Crucially, many involved multiple interventions targeted at multiple targets.
Surprisingly, few authors appeared to recognise that they were addressing complex, adaptive systems. In a review of the evidence, the Health Foundation advocate a complex adaptive systems approach in health care. 112 They suggest that doing so can challenge assumptions, focus on relationships rather than simple cause and effect models, provide a framework for categorising and analysing knowledge and agents, suggest new possibilities for change and provide a better picture of influences affecting change. The review also provides evidence of how patients can be understood as complex adaptive systems. By understanding the non-linear dynamics of internal and external features, patients can improve how health is defined; enhance professionals’ understanding of patients, disease and the systems in which they meet; help to develop future monitoring systems; and be used to support change. 113
The evidence on uptake effectiveness was often patchy and inadequate, and often rated as having a high risk of bias. The summary below is therefore tentative.
interventions considered in isolation
The most effective interventions considered in isolation were as follows:
-
There was strong and consistent evidence that patient invitations alone or reminders alone consistently increased screening uptake for breast, cervical and colorectal cancer. The combination of invitation letters plus a telephone reminder was even more effective.
-
Mailing kits to patients enhanced uptake for cervical and colorectal cancer screening.
-
Access-enhancing interventions increased screening in breast cancer.
-
Community-based health workers delivering patient education was effective in increasing screening uptake for breast, cervical and colorectal cancers.
Multiple interventions in combination
Multiple interventions involving very diverse combinations consistently appeared effective in increasing breast, cervical and colorectal cancer screening uptake. Furthermore, some reviews were able to make direct comparisons and report that multiple interventions were more effective than single interventions.
The ineffective interventions considered in isolation included:
-
decision aids and personalised risk communication/tailored messaging interventions.
Interventions with modest effectiveness included:
-
one-to-one patient education and counselling
-
group education
-
mass media and small media campaigns alone
-
media campaigns combined with individual education
-
financial incentives for patients.
Effective provider interventions included:
-
reminders to providers
-
provider assessment and feedback
-
training of health-care professionals.
The effectiveness evidence on several other interventions (i.e. individual home visits, provider incentives, using dedicated personnel, and organisational change and procedures) was inconclusive and requires further research.
Findings in the context of other literature
This review indicates the complexity of the evidence regarding interventions to encourage and increase the uptake of a specific screening programme. Interventions at the individual, health-care provider and the health-care system level all demonstrate varying degrees of success in different populations.
Interventions are used to promote uptake and optimal use of health-care services, including screening programmes. As demonstrated here, there are examples of successful interventions, those that have the potential to succeed and those that, although anticipated to be effective, did not prove successful.
Our review is very timely. The October 2019 Review of National Cancer Screening Programmes in England48 recently set out key recommendations for increasing the uptake and coverage of screening programmes. The review emphasised a high priority for spreading the implementation of evidence-based initiatives to increase uptake. 48 The review recommends an integrated system approach, including (1) implementing text message reminders for all screening programmes; (2) further pilots of social media campaigns, with formal evaluation and rollout if successful; (3) spreading good practice on physical and learning disabilities; (4) encouraging links with faith leaders, community groups and relevant voluntary, community and social enterprise organisations that work with the NHS at national, regional and local levels to reduce health inequalities and advance equality of opportunity; (5) increasing awareness of trans and gender-diverse issues among screening health professionals; and (6) consideration of financial incentives for providers to promote out-of-hours and weekend appointments.
Inequalities
Many studies have consistently reported lower uptake rates in disadvantaged individuals, groups and communities. However, our review found a striking lack of equity research comparing the differential response to an intervention intended to increase screening uptake. Some US studies76,77,89 did focus on equity in minority populations, but with no comparison population. Further research is required into the equitable uptake of screening programmes.
Research of interventions to increase the uptake of CVD screening programmes is also scarce and, likewise, necessitates further research.
The NHS England report48 also highlighted a current lack of equity in the uptake of NHS screening programmes. This was previously demonstrated by a review by Javanparast et al. 114 The review focused on the equity of participation in colorectal cancer screening among different population subgroups. The authors found that the provision of a single screening guideline for all groups within the population did not support equitable access, and individuals and some population subgroups may face a range of barriers hindering their actual utilisation of services. Interventions that resulted in improved participation rates included those that increased knowledge and influenced attitudes, engaged providers, and improved tracking, communication and support systems.
Might some interventions increase uptake more in affluent groups and therefore widen inequalities,115,116 for instance the recommendation to implement text message reminders by NHS England?48 This issue has been discussed by Asaria et al. ,117 who suggest a new methodological framework for undertaking distributional cost-effectiveness analysis to combine the objectives of maximising health and minimising unfair variation in health when evaluating population health interventions. The authors take the NHS bowel cancer screening programme as a case example, which was expected to improve population health, but had worsened population health inequalities associated with deprivation and ethnicity. The authors demonstrated the distributional cost-effectiveness analysis framework by examining two redesign options for the bowel cancer screening programme: (1) the introduction of an enhanced targeted reminder aimed at increasing screening uptake in deprived and ethnically diverse neighbourhoods and (2) the introduction of a basic universal reminder aimed at increasing screening uptake across the whole population. Asaria et al. 117 found that the universal reminder maximised population health, whereas the targeted reminder screening strategy minimised unfair variation in health. The framework can be used to demonstrate how the two objectives can be traded off against each other, and how alternative social value judgements can influence the assessment of which strategy is best, including judgements about which dimensions of health variation are considered unfair and societal levels of inequality aversion.
Behaviour change and nudge
We found that multiple interventions involving very diverse combinations consistently appeared effective in increasing cancer screening uptake. Furthermore, some reviews reported that multiple interventions appeared more effective than single interventions, consistent with current thinking regarding the need for multiple interventions that target key nodes within a complex system. 118
Behavioural approaches have therefore been highlighted to potentially help improve the translation of research into practice and enable the identification of interventions with maximum impact. Michie et al. 119 developed a framework called the ‘behaviour change wheel’. Elements of the behaviour change wheel provide a potential framework for the development of interventions to increase uptake and effectiveness of screening programmes at the health-care system, health-care provider and individual levels.
At the centre of Michie et al. ’s framework is a ‘behaviour system’ that involves three essential conditions: (1) capability, (2) opportunity and (3) motivation. This system forms the hub of the wheel, around which are positioned the nine intervention functions aimed at addressing deficits in one or more of these conditions: (1) education, (2) persuasion, (3) incentivisation, (4) coercion, (5) training, (6) enablement, (7) modelling, (8) environmental restructuring and (9) restrictions. Around this are placed seven categories of policy that could enable those interventions to occur: (1) communication/marketing, (2) legislation, (3) service provision, (4) regulation, (5) fiscal measures, (6) guidelines and (7) environmental/social planning.
Perry et al. 120 reviewed nudge-type interventions that have potential for changing behaviours in the broader context of increasing efficiency and reducing waste in health care. Perry et al. 120 suggest several approaches with potential, including framing health messages according to specific characteristics of a target audience; better information design, both in terms of text and language; framing and planning to enhance reminder content; financial micro-incentives; audit and feedback; and planning interventions, including ‘planning prompts’, action plans and implementation intentions.
Similar to our findings of the range of interventions with various degrees of effectiveness, the authors120 conclude that developing effective behaviour change interventions likely benefits from theory-based behavioural analysis, an appreciation of context, and structured selection of possible interventions, with consideration of acceptability and equity. However, what makes for effective combinations of nudge-type interventions remains mostly unexplored.
Strengths
The literature on interventions on improving screening programmes is extensive, complex and challenging. However, our umbrella literature review managed to identify and analyse 61 relevant reviews successfully. To our knowledge, this is the first comprehensive review of the evidence on invitation methods to improve uptake of screening programmes. It is particularly strong on interventions targeting breast, cervical and colorectal cancer screening programmes, and offers potentially valuable principles cautiously generalisable to CVD risk factor screening.
Limitations
This umbrella review has several limitations. First, we took the main message from the 61 reviews as published. We, therefore, did not go back to extract information related to individual studies. For some reviews, only one study was found for a specific intervention and we excluded this information in the summary tables and results section of this review. However, this more detailed information is available and is presented in the data extraction tables in Appendix 3. Second, we were unable to conduct a meta-analysis because the data were strikingly heterogeneous. Future researchers might wish to conduct a meta-analysis on a subgroup of interest. Third, we excluded two intervention areas during the pilot phase: (1) patient navigation interventions and (2) shared decision-making interventions. However, both were considered to fall outside our focus. Fourth, the evidence on interventions to increase the uptake of CVD screening was particularly patchy and poor. However, one might cautiously extrapolate some general principles from the ‘best-buy’ options that generally increased the uptake of three very different cancer screening programmes. Fifth, we did not consider economic analyses. However, substantial work might merit a separate review.
Conclusions
Strategies to improve the uptake of screening programmes have the potential to be effective. However, there are many components within these complex systems, at the individual, health-care professional or health-care system levels, that can influence the uptake of screening programmes. Single interventions may appear both plausible and attractive. However, within each screening programme, it is very likely that practitioners will need to implement multiple interventions to improve uptake maximally and therefore generate the most significant health gain.
Implications for the workHORSE model and tool
Our umbrella review is one approach that users can employ to interrogate the evidence base to design scenarios to use with the model. For parametrisation purposes, more detailed systematic reviews with meta-analysis may be required.
As a result of this umbrella review, we encourage users of the workHORSE tool to consider the costs of those methods of delivery that were found to be most effective and how these methods might enhance uptake for the NHS HCP. For example, users may model the impact of increasing text reminders or of having community outreach workers who would educate people on the benefits of participating in the NHS HCP. Furthermore, it is important to assess the studies’ outcomes to ensure that they match the model parameters used to set up scenarios (see Chapter 4, The graphical user interface).
Chapter 4 The workHORSE model
Introduction
The workHORSE is an application (app) consisting of a GUI that allows user interaction, an epidemiological engine, a health economics engine and the NHS HCP policy engine (Figure 1). In this section, we will describe all three engines and we will provide a brief overview of the GUI.
FIGURE 1.
The workHORSE architecture.

Epidemiological engine
High-level description
The epidemiological engine of workHORSE is a discrete-time dynamic stochastic microsimulation. The epidemiological engine of workHORSE consists of three modules: (1) the sociodemographic module, (2) the exposure module and (3) the disease module.
Within the workHORSE epidemiological engine, each unit is a synthetic individual (simulant) represented by a record containing a unique identifier and a set of associated attributes. The microsimulation then projects the life course of each synthetic individual.
The attributes for each synthetic individual include the sociodemographic characteristics, exposures to risk factors, acquired diseases and cause of death, if relevant.
Specific attributes included the following:
-
Age, sex, ethnicity, the LA of residence, education, quintile groups of equivalised income, Townsend Deprivation Index and Index of Multiple Deprivation (IMD) as sociodemographic exposures.
-
Alcohol intake, smoking status (i.e. current smoker/ex-smoker/never smoked), smoking duration, smoking intensity, environmental tobacco exposure, fruit consumption, vegetable consumption and physical activity as behavioural risk exposure variables.
-
Body mass index (BMI), systolic blood pressure (SBP), total serum cholesterol and high-density lipoprotein as biological risk exposures.
-
Type 2 diabetes mellitus (T2DM), atrial fibrillation (AF), CHD, stroke, post-stroke dementia, chronic obstructive pulmonary disease (COPD), lung cancer, colon cancer and breast cancer as diseases. It is worth noting that some diseases have dual roles, additionally acting as risk exposures for other diseases (e.g. T2DM and AF are risks for stroke).
-
Mortality from CHD, stroke, COPD, lung cancer, colon cancer, breast cancer or any other cause is recorded if it occurs.
-
Statin utilisation, antihypertension medication utilisation and corticosteroid medication utilisation are each quantified.
-
Family history of CVD, family history of diabetes, family history of cancer, personal history of any cancer, chronic kidney disease, rheumatoid arthritis and the additional number of comorbidities are also captured as auxiliary attributes.
All of these attributes are updated in discrete annual steps according to a set of stochastic rules. We structured these rules based on well-established epidemiological principles. Specifically, behavioural risk exposures are conditional on sociodemographic exposures, biological risk exposures are conditional on behavioural and sociodemographic exposures, and diseases are conditional on biological, behavioural and sociodemographic exposures. Finally, mortality is conditional on sociodemographic and disease exposures.
The life course of synthetic individuals is simulated as many times as the number of scenarios specified in the GUI, using the same random numbers for all policy scenarios to reduce stochastic noise. One of the scenarios is always the ‘baseline’ scenario with which all remaining policy scenarios are compared. The comparison of the disease outcomes under the life courses under the baseline scenario compared with the policy scenarios generates the health impact of the policy scenarios. The output of the epidemiological engine is a data set that contains the adult life course of the simulated synthetic individuals, with all of the attributes mentioned above recorded on an annual basis for every scenario.
The epidemiological engine of workHORSE consists of three modules: (1) the sociodemographic module, (2) the exposure module and (3) the disease module. In the below paragraphs, we will describe these three modules. Table 7 summarises the key assumptions and limitations of the workHORSE microsimulation model.
| Module | Key assumptions and limitations |
|---|---|
| Sociodemographic module | Migration is not modelled explicitly in the model. However, the model outputs are calibrated to ONS population projections by LA that take migration into account |
| Social mobility is not considered | |
| QIMD is a relative marker of (area) deprivation with several versions since 2003. We considered all version of QIMD to be identical | |
| Exposure module | We assume that the surveys used are truly representative of the population. For example, the adjustments for selection bias in the Health Surveys for England121 are adequate |
| Differences in exposures between LAs belonging to the same SHA are solely because of differences in their sociodemographic composition | |
| The linear correlations in exposures percentiles remain constant over time | |
| Disease module | We assume multiplicative risk effects |
| We assume log-linear dose–response for the continuous risk factors | |
| We assume that the effects of the risk factors on incidence and mortality are equal and that risk factors are not modifying survival | |
| We assume a 4-year mean lag time for CVD, 5-year mean lag time for COPD and 9-year mean lag time for cancers | |
| We assume 100% risk reversibility for all exposures, except smoking | |
| We assume that trends in disease incidence are attributable to trends of the relevant modelled risk factors only | |
| For cancers, we assume that survival 10 years after diagnosis equals remission | |
| NHS HCP policy engine | The decision of each synthetic individual to participate in the NHS HCP after an invitation is independent of previous decisions to participate or not participate in earlier invitations |
| When the lifestyle interventions inputs are used for a policy scenario, synthetic individuals who are affected have an 80% probability of retaining the healthier lifestyle every year after the intervention (user adjustable) | |
| As a consequence of the third assumption in the disease module, we quantify the impact of NHS HCP on primary prevention only |
Sociodemographic module
The first year of every simulation in workHORSE is 2013, reflecting that the LA commissioning of the NHS HCP has been a statutory requirement since 2013 as part of the Health and Social Care Act. 122 When the user selects the geographic area for simulation in the GUI, the algorithm in the module proceeds with the following:
-
The algorithm identifies the lower-layer super output areas (LSOAs) that constitute the user area selection.
-
The algorithm draws 200,000 synthetic individuals, aged 30–89 years, from the joint age and sex distribution of the identified LSOAs. This is a default value that can be modified by the user. The joint age and sex distribution for each LSOA for 2013 is informed by the Office for National Statistics (ONS) population estimates. 123
-
The algorithm assigns each synthetic individual an IMD score and a Townsend Deprivation Index score based on their LSOA.
-
The algorithm probabilistically assigns each synthetic individual an ethnicity based on their age group, sex and LSOA. The ethnicity mixture of each LSOA is informed by the 2011 Census. 124 We include nine ethnicities in the model: (1) white, (2) Indian, (3) Pakistani, (4) Bangladeshi, (5) other Asian, (6) black Caribbean, (7) black African, (8) Chinese and (9) other.
-
The algorithm assigns each synthetic individual a Strategic Health Authority (SHA) based on their LSOA. A SHA is the smallest geographical area that is accessible in the Health Survey for England (HSE) series. We use this in the exposure module, as described in the next section (see Exposure module).
-
The algorithm probabilistically assigns each synthetic individual their highest educational qualification conditional on their age, sex, quintile of the Index of Multiple Deprivation (QIMD), SHA and ethnicity. We used six levels for this variable [(1) National Vocational Qualification (NVQ) 4/NVQ 5/degree or equivalent, (2) higher education below degree level, (3) NVQ 3/General Certificate of Education (GCE) Advanced Level equivalent, (4) NVQ 2/GCE Ordinary Level equivalent, (5) NVQ 1/Certificate of Secondary Education other grade equivalent, (5) foreign/other and (6) no qualification], which was informed by the HSE ‘topqual3’ variable. 121 Specifically, we first fitted an ordinal regression model to the HSE data using topqual3 as the dependent variable and year, age, sex, QIMD, SHA and ethnicity as the independent variables. Then we sampled from the distribution of this model to simulate the highest educational qualification variable in the synthetic population. The approach binds the synthetic individuals to their highest educational qualification that remains constant over the simulated years. This is unlikely to introduce any substantial bias, as most adults aged > 30 years have already achieved their highest educational qualification.
So far, the algorithm has created a synthetic population that is a snapshot of the population in 2013. The following steps of the algorithm create backward and forward projections of the synthetic population that are essential to model exposure time trends and time lags between exposures and diseases.
The backward projection of the synthetic population goes back to 2003 and therefore the maximum time lag we allow in the model is 10 years. As everyone alive and aged > 30 years in 2013 was alive in 2003, the algorithm simply creates the back projections by appropriately reducing the age of the synthetic individuals, while keeping all other variables constant.
Similarly, for the forward projections, we project until the year 2041 and the algorithm increases the age of the synthetic individuals while keeping all other variables constant. For forward projections, mortality needs to be considered. We describe mortality within the disease module as disease-specific mortality, which is closely related to disease prevalence. workHORSE follows an open cohort approach. For every simulated year from 2013 onwards, a new cohort of 30-year-old synthetic individuals enters the model. The same sources inform the size of the cohort and the joint age, sex and ethnicity distribution that we described above. For example, in 2014, the new 30-year-old cohort will be informed by the population size and the joint age, sex and ethnicity distribution of those who were 29 years old in 2013. The approach may be crude; however, the final model outputs are directly standardised to ONS population projections estimates by LA or nationally. 125,126
Exposure module
This module simulates the adult life course exposures of the synthetic individuals based on the HSE series between 2003 and 2014. 127–138 For all simulated exposures, we follow the same general principles. First, we fit an appropriate statistical model to the HSE data, with the exposure of interest as the dependent variable and some functions of year, age, sex, QIMD, ethnicity and SHA as independent variables. Then, we use the statistical model to predict the exposure level of every synthetic individual in the simulation based on their sociodemographic characteristics that were estimated from the sociodemographic module.
The inclusion of year as an independent variable in our exposure model allows us to extract the trends from the HSE series and project them into the future. Furthermore, it allows us to make backward projections of exposures that we use when we simulate time lags. For example, for a synthetic female individual aged 30 years in 2013, we can estimate her BMI in 2003 when she was aged 20 years and in 2033 when she will be aged 50 years. To avoid excessively fast changes in exposure trends, and to reflect our belief that decays and growths in natural phenomena are rarely linear, we included the natural logarithm of years in the statistical models, assuming logarithmic trends.
The inclusion of SHA as an independent variable in our exposure models allow us to perform small-area estimation from the SHA level that is available in HSE to the LA level using individual-level modelling. 139 Essentially, we apply the exposure level observed in HSE at SHA level to the LA level weighted for the sociodemographic characteristics of the population at each LA. Therefore, we assume that differences in mean exposures between LAs belonging in the same SHA are because of differences in their sociodemographic composition.
For exposures that were recorded as ordinal categorical variables in HSE, we used logit ordinal regression to model them. For all other exposures, we used generalized additive model for location, scale and shape (GAMLSS). 140,141 These are flexible statistical models that can produce all parameters of an assumed distribution for the dependent variable, conditional to some function of the independent variables. For example, GAMLSS can model both the mean and the standard deviation of a dependent normally distributed variable, whereas a linear regression models only the mean. Appendix 4, Table 36 summarises our modelling approach for all of the exposures in the model.
The approach described above provides us with equations to estimate the distribution of an exposure for a given time and sociodemographic characteristics of a synthetic individual. When the synthetic individual enters the simulation, a set of random numbers between 0 and 1 and of size equal to the number of the modelled exposures is allocated to them. Each of them represents the percentile of the relevant exposure distribution. The principle is that synthetic individuals retain their percentiles throughout their life course (this is known as the rank stability assumption). 142 For example, in 2013, a 40-year-old male synthetic individual living in a QIMD 3 area with SBP of 120 mmHg has a SBP percentile of 0.52. Twenty years later, the same synthetic individual has retained his percentile score for SBP. However, his SBP is now estimated to be 137.6 mmHg because the SBP distribution has changed to reflect the SBP of 60-year-old men living in a QIMD 3 area in 2033 (Figure 2). In workHORSE, we allow the percentiles of the synthetic individuals to fluctuate every year using random walks to relax the rank stability assumption.
FIGURE 2.
Plot of the percentile against the SBP (cumulative distribution) of a male synthetic individual living in QIMD 3 area for aged 20 and 70 years.
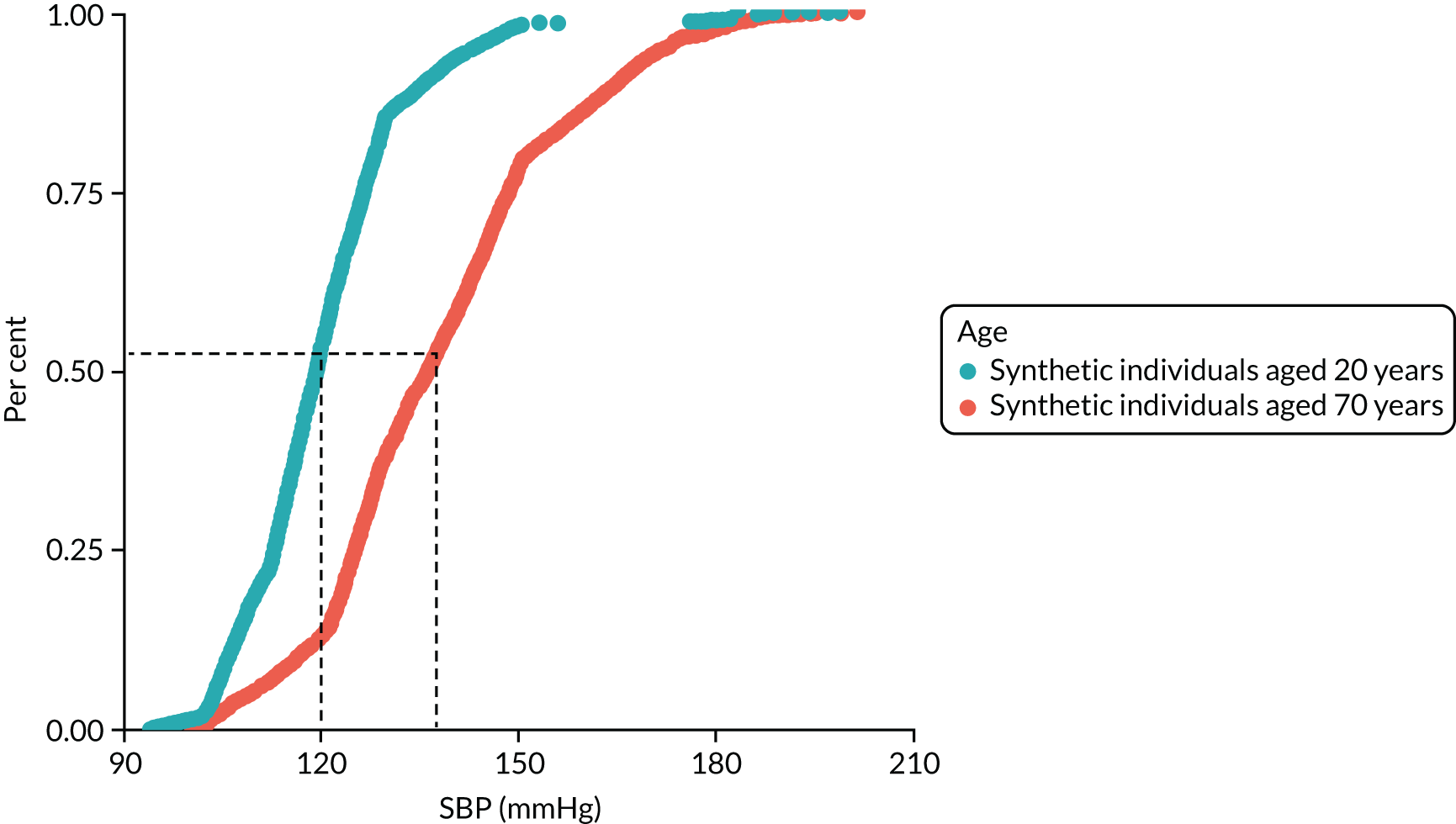
Clustering of risk factors
Finally, exposures in individuals are correlated. For example, people with a high BMI may also have high total cholesterol and hypertension. Some of these correlations reflect strong and well-established causal mechanisms, and some are biased by the cross-sectional nature of the HSE. The method we described above captures some of these correlations by including exposures as independent variables in the statistical models for estimating exposures. For example, we included BMI as a predictor for T2DM. Going a step further, we model the full linear correlation structure in HSE using the following approach:
-
We used the exposure models to impute missing variables in the HSE.
-
We used the quantile function of the distribution estimated by the exposure models to convert exposures in the HSE to percentiles. As the distributions were conditional on the independent variables used in each model, the percentiles are adjusted for these variables (i.e. age, sex, QIMD, etc.).
-
We estimated the linear correlation matrix of the percentiles of the exposures of interest in the HSE using Pearson’s correlation.
-
We used the linear correlation matrix from point 3 to generate streams of uniforms between 0 and 1 that had a correlation structure similar to that observed in the HSE. 143
-
We used the correlates streams of random numbers from point 4 as the exposure percentiles for the synthetic individuals.
For simplicity, we assumed that the correlation structure of the exposure percentiles remains constant over time.
Disease module
The previous two modules for demographics and exposure generate a dynamic close-to-reality synthetic population that is composed of the adult life course exposures of each of the synthetic individuals. The disease module then translates these exposures to disease incidence, using a population attributable risk approach [i.e. population attributable fraction (PAF)]. 144 We will first describe how the disease incidence is simulated in the model and then how the model simulates mortality.
Disease incidence
To estimate the individualised annual probability of a synthetic individual developing a specific disease conditional on their cumulative risk exposures, we follow a three-step approach:
-
The proportion of incidence attributable to each modelled risk factor by age, sex and QIMD is estimated, assuming a specific time lag between exposure and disease. The time lags in the model vary stochastically between 2 and 10 years, following a shifted binomial distribution. We set the mean lag time for CHD and stroke to 4 years, for COPD to 5 years and for cancers to 9 years, each reflecting the best possible empirical data based on the observation period of cohort studies and time to risk reversal in randomised clinical trials.
-
The proportion of the disease incidence attributable to all of the modelled risk factors is estimated and subtracted from the total incidence for 2013, assuming multiplicative risks.
The probability of developing the disease is estimated for each individual in the synthetic population and is used in an independent Bernoulli trial to select those who finally develop the disease.
The implementation of the above method is described in more detail using CHD as an example. The same process is used for all modelled diseases except T2DM, AF and post-stroke dementia, which are described separately.
Step 1
Population attributable fraction is an epidemiological measure that estimates the proportion of the disease attributable to an associated risk factor. It depends on the relative risk associated with the risk factor and the prevalence of the risk factor in the population. In a microsimulation context, where exposure to risk factors are known at the individual level and assuming multiplicative risk factors, PAF can be calculated using the formula:
where n is the number of synthetic individuals in the population and RRi1... ik are the relative risks of the risk factors associated with CHD for each individual, i. We calculated PAF based on the above formula stratified by age, sex and QIMD. Consistent with findings from the respective meta-analyses that were used for workHORSE (see Appendix 4, Table 38), SBP of < 115 mmHg, total cholesterol of < 3.8 mmol/l and a BMI of < 20 kg/m2 were considered to have a relative risk of 1. Similarly, consumption of eight or more portions of fruit and vegetables and 5 or more active days (i.e. > 30 minutes of moderate to vigorous activity) per week were also considered to have a relative risk of 1. All of the relative risks were taken from published meta-analyses and empirical studies (see Appendix 4, Table 38).
Step 2
The incidence of CHD not attributable to the modelled risk factors can be estimated by the formula:
where IObserved is the CHD incidence and PAF is from step 1. ITheoretical minimum represents CHD incidence if all of the modelled risk factors were at optimal levels. The theoretical minimum incidence is calculated by age, sex and QIMD in the initial year of the simulation only and it is assumed stable thereafter.
Stage 3
Assuming that ITheoretical minimum is the annual baseline probability of a synthetic individual to develop CHD for a given age, sex and QIMD due to risk factors not included in the model (e.g. genetics, etc.), the individualised annual probability of developing CHD, P(CHD/age, sex, QIMD, exposers), given his/her risk factors were estimated by the formula:
where RRi1... ik is the relative risks that are related to the specific risk exposures of the synthetic individual, same as in stage 1.
The method described above can only be used when the incidence of the disease in the population is known. For cancers, this information is available from the cancer registries. However, the true incidence of CHD (and stroke) is mostly unknown. Although several estimates exist, all have limitations. Therefore, for the estimation of CHD incidence by age and sex, we opted for a modelling solution to synthesise all of the available sources of information and minimise bias. Specifically, we used ONS CHD mortality [International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10)145 I20–I25] for England in 2013, self-reported prevalence of CHD from the HSE 2011135 and the incidence of first CHD event by QIMD146 to inform the World Health Organization DISMOD II model. 147 DISMOD II is a multistate life table model that can estimate the incidence, prevalence, mortality, fatality and remission of a disease when information about at least three of these indicators is available. A similar approach has been followed by the Global Burden of Disease team and other groups. 148,149 We considered CHD an incurable chronic disease (i.e. remission rate was set to 0) and therefore the derived DISMOD II incidence refers to the first-ever manifestation of angina or acute myocardial infarction, excluding any recurrent episodes. For the DISMOD II calculations, we assumed that incidence and case fatality had each been declining by 3% (relative) over the last 20 years, reflecting empirical observational studies. The derived CHD incidence and prevalence rates were used as an input for stroke. A similar approach was used for stroke and COPD. For cancers, we informed DISMOD II with cancer incidence, mortality and 5-year survival rates.
For the initial simulation year, some synthetic individuals need to be allocated as prevalent cases for each of the modelled diseases. We use DISMOD II prevalence estimates to identify prevalent disease cases by age, sex and QIMD.
Post-stroke dementia, type 2 diabetes mellitus and atrial fibrillation incidence
We modelled post-stroke dementia, T2DM and AF incidence differently, as the available data did not allow us to use the same approach in the model as with CVD, COPD and cancers. We modelled post-stroke dementia incidence as wholly attributed to stroke cases. In workHORSE, stroke cases have a probability of developing post-stroke dementia within the first year of having a stroke. Stroke and dementia share common risk factors. Therefore, to avoid overestimation of post-stroke dementia we restricted the period after a stroke for which dementia cases are attributed to the stroke to 1 year. We could not identify any useful evidence regarding AF incidence in the general population. Therefore, we model only AF prevalence in workHORSE. Finally, for T2DM incidence, we used the QDScore, which predicts the probability of incident T2DM. 150
Mortality
All synthetic individuals are exposed to the risk of dying from any of their acquired modelled diseases or any other non-modelled cause. We decomposed ONS-reported mortality rates by age, sex and QIMD for years 2003–16 to mortality rates from CHD, stroke, COPD, lung cancer, colon cancer, breast cancer (for women only) and any other non-modelled cause. We fitted functional demographic models by sex and QIMD to these data and we projected disease-specific mortality to the simulation horizon (2041) using the R package ‘demography’ (The R Foundation for Statistical Computing, Vienna, Austria). 151 Functional demographic models are generalisations of the Lee–Carter demographic model, influenced by ideas from functional data analysis and non-parametric smoothing. 152 Finally, using a similar approach to that used to model disease incidence, we allowed prevalent cases of T2DM, synthetic individuals with SBP > 140 mmHg, active smokers, those with one or fewer active days per week and those with excessive alcohol intake to experience higher non-modelled cause mortality rates. 153
NHS Health Check programme policy engine
The NHS HCP policy engine translates the user inputs regarding the implementation of an NHS HCP policy to changes in the exposure of the synthetic individuals. The change in exposures leads to a potentially different counterfactual life course for the synthetic individuals that are affected. The effectiveness of each policy scenario stems from the comparison of the disease-related events between the counterfactual life courses. The equity measures are derived from the comparison of the policy effectiveness by QIMD (see Health equity methods).
In modelling the NHS HCP, we made some assumptions:
-
The decision of each synthetic individual to participate in the NHS HCP after an invitation is independent of decisions of whether or not to participate in earlier invitations (we are not aware of any empirical evidence that could inform this assumption).
-
When the lifestyle interventions inputs are used for a policy scenario, synthetic individuals who are affected have an 80% probability of retaining the healthier lifestyle every year after the intervention (we are not aware of any empirical evidence on the long-term effect of NHS HCP on supporting healthier lifestyles). This is an influential parameter; however, it was evident from the workshops that users prefer a simpler, less-cluttered interface. As a compromise, we export this parameter in the GUI as an advanced setting that can only be seen and altered if the user explicitly requests this.
-
We assumed that people treated with a statin after a NHS Health Check would be prescribed Atorvastatin (20 mg). We model the effect of Atorvastatin (20 mg) on cholesterol using evidence from Law et al. 154 We also model the unwanted effect of statins on the T2DM incidence using evidence from Sattar et al. 155
-
We did not explicitly model the effect of every potential treatment or combination of treatments for hypertension. Instead, we assume that treatments for hypertension can potentially achieve SBP of 135 mmHg for every patient. However, discontinuation and poor adherence to treatment would decrease this effect (see next assumption).
-
Using evidence from Wales, we estimated that discontinuation of statins for primary prevention is 2.5% per year with no socioeconomic gradient. 156 Similarly, using evidence from Denmark, we assumed that adherence to statins for primary prevention is 90% with no socioeconomic gradient, after taking into account the very low discontinuation rate observed in Wales. We assumed a beta distribution with a mean of 0.9 and a shape2 of 0.2. The mean is user adjustable in the advanced options menu. 157 We applied the same values of discontinuation and adherence to antihypertension medication as with statins because of the lack of specific evidence.
Health economics and equity engine
Economics methods
The main economic analysis objective was to enable users to estimate the cost-effectiveness of each scenario within the model. The potential scenarios are a range of real-world and hypothetical scenarios around performance on NHS Health Checks in England as a whole or in individual LA areas. The eligible population is typically adults aged 40–74 years without pre-existing conditions. The broader context is to allow decision-makers to test scenarios around NHS Health Checks so that they can optimise the programme in their area in terms of understanding cost-effectiveness and equity impact; and informing budget allocation, payment and incentive strategies, and performance management.
Cost-effectiveness can be estimated within the tool with a range of ICER willingness-to-pay thresholds or QALY valuations (e.g. £30,000 based on NICE recommendations or £60,000 based on the UK Treasury Green Book29) and a range of perspectives (i.e. health care, health and social care, and societal).
Overall, the method was cost-effectiveness analysis. However, we enabled decision-makers to use a range of outcome measures and economic perspectives. First, the health-care perspective included intervention costs, health-care cost–consequences (e.g. disease costs) and net QALYs. Second, the health and social care perspective included the same as the health-care perspective with the addition of social care costs. Finally, the societal perspective included the same as the health and social care perspective with the addition of net informal care costs and production costs (i.e. household production and earnings).
The QALYs were calculated based on population norms from Janssen and Szende,158 adjusted using the equations from the UK EuroQol-5 Dimensions (EQ-5D) Medical Expenditure Panel Survey (MEPS) catalogue. 159 The workHORSE model adjusts for comorbidities, which is not possible with typical cohort or life table economic models.
The health-care disease costs were assembled based on searching for excess costs of diseases (as opposed to total running costs of individuals that do not separate out the costs of disease from other costs and comorbidities) and data obtained from recent high-quality UK studies.
Social care costs were based on Office for Budget Responsibility (OBR) estimates by age, adjusted for prevalence of dementia and stroke. 160,161 We did not find strong evidence for excess social care costs for other specific diseases. However, there are additional social care costs in the model that were associated with ageing.
Informal care costs were based on a study that predicted informal care costs using Health Outcomes Data Repository data for people discharged from hospital in Wales based on their age and EQ-5D scores. 162 Production costs (i.e. household production and earnings) were based on Appendix B of a paper by Claxton et al. 163 that presents a wealth of information about wider social benefits and has informed the approach taken by the UK Department of Health and Social Care in its impact assessments. The earnings and production estimates from this paper were updated with more recent data from the ONS. Unlike other costs that are deficits, production is a benefit measure in the model. Therefore, production decreases as rates of disease increase, whereas health, social and informal care costs all increase as rates of disease increase.
Costs were measured in Great British pounds in 2019 prices. The discount year was therefore 2019. Costs and QALYs that were gained or lost in years before 2019 were inversely discounted in the model. Costs and QALYs are given a range of potential discount rates in the model so that decision-makers can use 3.5% for both costs and QALYs (as typically used by NICE), 1.5% for QALYs and 3.5% for costs (as typically used by the UK Treasury) or 0% for both if they want to estimate the actual undiscounted costs and QALYs in constant prices.
More detail of costs and QALYs is given in Appendix 6.
Outcomes
The workHORSE simulation tool produces a range of outcomes, including disease case-years prevented or postponed (CYPP); economic outcomes, such as ICERs; and equity-related outcomes, such as a change in slope index of inequality and relative index of inequality. This approach enables distributive equity analysis, as we have demonstrated in our published papers using this model. 11,164
Overall results are presented as benefit–cost ratios, ICERs and net monetary benefit (NMB). This is to assist decision-makers in understanding both the ratio of costs and benefits, and the absolute magnitude of the benefits, as, for example, decision-makers may favour a programme that delivers slightly larger absolute benefits across the population over a programme that has a better cost–benefit ratio but smaller absolute returns, particularly if they have a fixed budget to allocate to NHS Health Checks.
Where the cumulative costs and QALYs are as follows, the benefit–cost ratio would be 10.6, representing a return on investment of £9.60 for every £1 spent. Strictly speaking, cost–benefit ratios have no units because they are a ratio, but they are often presented as ‘£10.60 in benefits for every £1 spent’.
The populations in the model are for England and 150 English upper-tier LAs, by age, sex, QIMD and ethnicity.
The decision-support tool allows a range of time horizons from 2013 to 2041, from 1 to 28 years. The reason the tool starts from 2013 is to allow decision-makers to model the cost-effectiveness of historical NHS Health Checks performance retrospectively, which they may want to compare with future scenarios.
Crucially, the model is a dynamic open-cohort microsimulation model (i.e. the model is trying to estimate the actual cost-effectiveness of the scenarios within a dynamic population where people are born, people age, people’s risk factors change and people die). The detailed modelling of the population dynamics in our model is therefore different from many economic models, which are often closed cohort, meaning that they follow the same population over time and often have a lifetime horizon.
The tool allows the user to filter outcomes for specific subgroups if they wish. This can be by age, gender, QIMD or ethnicity so that this can input into a subgroup analysis or be used for an equity audit.
Health equity methods
To estimate the impact of NHS Health Checks on existing absolute and relative socioeconomic inequalities in QALYs experienced across the population, we used two regression-based metrics inspired by the slope index of inequality: (1) the absolute equity slope index and (2) the relative equity slope index. 27,165 The absolute equity slope index measures the impact of an intervention on absolute inequality and the relative equity slope index considers the pre-existing socioeconomic gradient of disease burden and measures the impact of an intervention on relative inequality. For both metrics, positive values mean the intervention reduces inequality and negative values mean the intervention increases inequality. The impact on relative socioeconomic inequalities is therefore meaningful when the intervention tackles absolute inequalities (i.e. the absolute equity slope index is positive) only.
We used fifths of the national IMD as a marker of socioeconomic stratification. Some LAs have populations that are skewed towards certain deprivation quintiles. Each IMD fifth (quintile group) is characterised by a ridit value that corresponds to the average cumulative frequency of the IMD fifth to account for population size differences. This means that the regression line gradient is adjusted for the proportion of the population in each fifth and it represents an estimate of the population social gradient, rather than the most and least deprived group. In addition, within the user interface, we give users the option to use local IMD group quintiles in place of national quintiles (where each local quintile contains 20% of the LA population).
These calculations allow decision-makers to weigh up the equity benefits of a scenario against the health benefits generated, which are shown on the user interface on the health equity plane, as in Figure 3. In Figure 3, scenario 2 gains slightly greater monetary benefits and larger reductions in absolute inequalities. 166
FIGURE 3.
The health equity plane.

Uncertainty and probabilistic sensitivity analysis
workHORSE implements a second-order Monte Carlo approach to estimate uncertainty intervals (UIs) for each scenario. 167,168 For each iteration, a different set of input parameters is used by sampling from the respective distributions of input parameters. We assumed log-normal distributions for relative risks and hazard ratios, normal distributions for coefficients of linear regression equations, beta distributions for proportions, beta prime distribution for costs and PERT distributions for other parameters. Specifically, for relative risks and hazard ratios, the distributions were bounded above 1 when the mean was above 1 and vice versa.
In workHORSE, we minimise stochastic uncertainty by using the same random numbers for all scenarios, where appropriate, and drawing a different sample of the synthetic population. The user can perform one-way sensitivity analysis for all of the scenario inputs that are exported to the GUI.
workHORSE allows stochastic uncertainty, parameter uncertainty, individual heterogeneity and, to some extent, structural uncertainty to be propagated in the reported UI. The following example illustrates the different types of uncertainty that were considered in workHORSE. Let us assume that the annual risk for CHD is 5%. If we apply this risk to all individuals and randomly draw from a Bernoulli distribution with p = 5% to select those who will manifest CHD, we consider stochastic uncertainty only by using the same random numbers for all scenarios, where appropriate. If we allow the annual risk for CHD to be conditional on individual characteristics (i.e. age, sex, exposure to risk factors) then individual heterogeneity is considered. Finally, when the uncertainty of the relative risks due to sampling errors is considered in the estimation of the annual risk for CHD, the parameter uncertainty is considered. Of these three types of uncertainty, only the parameter uncertainty can be reduced from better studies in the future.
The structure of the model is grounded in fundamental epidemiological ideas and well-established causal pathways on which exposures are causally related with the specific NCDs that are explicitly modelled. For example, hypertension is causally related to CVD but not lung cancer. Hence, structural sensitivity analysis is not necessary to explore the possibility of hypertension being a risk factor for lung cancer. Therefore, we considered this type of uncertainty relatively small and did not study it in detail, with one exception. The discrete-time bias arises from the fact that time in workHORSE is not continuous. A synthetic individual within the model may die of multiple causes within 1 year; however, the discrete-time nature of the simulation does not allow the identification of the cause that ‘killed’ the simulant first. Each time this happens to a simulant, we randomly select a cause of death from the list of all of the terminal events that occurred for the simulant that year. Hence, we propagate discrete-time uncertainty to the output.
Dealing with uncertainty in health economics inputs
There are three main uncertainties around health state utility values.
The first uncertainty is around the model and the sample that was used to produce the indices. The MEPS data were modelled with censored least absolute deviation regression based on 79,000 individuals.
The second uncertainty is around the mean health state utility index value for a given condition (or age, gender, deprivation category), which would be driven by the sample size. Therefore, a small sample size may pick up people whose CVD is more or less severe than the general population in that category. As the sample gets bigger, the standard error gets smaller and tends towards zero, and the average should tend towards the population average. The standard errors in our sources are reassuringly small, which indicates a high degree of certainty around the mean utility decrements each disease.
The third uncertainty is the individual-level variation (unobserved heterogeneity), as described by the standard deviation. As the sample size gets bigger, this may be reduced, but with larger sample size the standard deviation will tend towards the true population standard deviation. In workHORSE, we have included the uncertainty around the mean decrement as measured by the standard error. In addition, because the initial sample was from the USA – albeit matched to UK preference scores – this may introduce another level of uncertainty that we cannot measure.
We estimate the probability over time for the scenario to be cost-effective, cost saving or for reducing health inequalities. We added a visual aid in the plane graphs (see Figure 10) to show that the high upfront cost of the checks is weighted against future health gains because of the time lags between exposure change and disease risk change.
Validation and calibration
We validated the workHORSE epidemiological engine using internal validation, plotting the modelled exposures prevalence and cancer incidence against the observed exposures prevalence and cancer incidence in the HSE and cancer registry, respectively. Mortality in the model is calibrated to mortality projections, as described above (see Mortality). We present the relevant validation plots in Appendix 5, Figure 23, stratified by year and age group. Additionally, we have produced and inspected plots for multiple combinations of stratification levels that are available on request. Overall, the plots suggest that workHORSE captures exposure trends and translates them to disease incidence and mortality reasonably well for the purpose of this project.
The graphical user interface
We built a prototype user interface to help users to interact with the model engine, co-produced with stakeholders (see Chapter 2).
The design replicates the workflow of a ‘scenario analysis exercise’ conducted in three steps: (1) setting the simulation parameters, (2) setting the scenario parameters and (3) analysing the outputs.
Simulation parameters tab: basic settings
To start using the workHORSE tool, the user needs to provide some necessary information in the simulation parameter sheet, including the geographical area of interest, the time horizon to simulate and how many scenarios to test (including the baseline or business-as-usual scenario). Finally, the user needs to decide on the perspective of the health economics analysis (i.e. societal, health and social care, or health care) (Figure 4).
FIGURE 4.
The simulation parameters tab.

In addition, the user can decide whether to use the national IMD quintiles or area-specific IMD quintiles. Local commissioners sometimes prefer area-specific quintiles because it means that there is an equal proportion (roughly 20%) of the population in each quintile, whereas areas will often have an uneven distribution of the population across national IMD quintiles.
Setting the scenario parameters tab
Once the user has defined the number of scenarios to evaluate, the user needs to define the scenarios in the scenario parameter sheet. To set any scenario, including the baseline scenario, the user needs to provide information about the model parameters. Each parameter has a tab in the scenario parameters sheet (Figure 5).
FIGURE 5.
The scenario parameters tab.

All scenario parameters have default values to give the user an indication of the magnitude of the value it would be expected under ‘normal’ use. For example, in the ‘Health Checks received’ parameter it is usually > 50% in most areas. However, the user is still allowed to input any plausible value for all parameters. We recommend that users have as much real-world data as possible to inform scenarios, particularly the baseline scenario.
Using the general parameters tab, the user can define (1) the name of the scenario (e.g. scenario A, baseline scenario), (2) whether or not this scenario is the baseline against which all other policy scenarios will be compared and (3) the starting year of implementation for this scenario.
Once the scenario has been defined, it can be saved so that it can be used later as a template for a new scenario. To do this, the user has to go to the general parameters tab and click the ‘save scenario’ button. If the user needs to use this scenario as a template later, it can be loaded by selecting ‘load scenario’ in the general parameters tab. All scenario parameters are saved in YAML (YAML Ain’t Markup Language) files that can be edited in any text editor.
Using the eligibility criteria tab (see Figure 5), the user can alter (1) the age of eligibility to be invited, (2) how often (in years) NHS Health Checks are offered and (3) whether known diabetics or hypertensive individuals are eligible. This tab already has default values to reflect current practice at the time that this report was written (2020).
In the appointments offered yearly tab, the user can specify changes related to coverage of the NHS HCP by changing the parameter ‘invitations’ (i.e. percentage of the eligible population) and the ‘cost per invitation’. If the user wishes to vary the above parameters by IMD, this can be carried out by clicking ‘detailed input’.
In the NHS Health Checks received tab, the user can specify changes related to uptake of the NHS HCP by changing the parameter ‘proportion of invitees attending an NHS Health Check’ and changes related to the payment providers received for each participant by changing the ‘cost per completed NHS Health Check’. This way, the user can accommodate different payment mechanisms for NHS Health Checks. For instance, they can select ‘block contract’ or ‘payment by results’ or a combination of both, provided that the user can derive these costs outside the workHORSE GUI. In the detailed input boxes, the user can define uptake by age group, sex, QIMD and risk.
The prescription rate tab (see Figure 5) relates to what happens after the NHS Health Check has taken place. Here, the user can specify changes to prescription rates of statins and antihypertensive medication for those participants with a QRISK 2 score > 10 and raised total cholesterol, or participants with hypertension, respectively. As can be done with the other parameters described above, the user can use the ‘detailed input’ button to vary these parameters by IMD and risk, if data are available.
In the impact on lifestyle tab, the user can evaluate the potential outcomes of referrals to lifestyle services, such as smoking cessation, weight management, alcohol consumption and physical activity programmes. The user would need to specify the percentage of people successfully achieving smoke cessation, losing weight, reducing alcohol consumption or increasing their physical activity, and the associated overhead and per-participant costs. Additionally, for the weight management, alcohol consumption and physical activity programmes, the user would need to specify the mean weight loss (kg), mean percentage reduction of alcohol consumption or the number of days physical activity increased, respectively.
Inspecting the model outputs
After setting up the scenarios, the user runs the simulation. The outputs tab will show on the dashboard. Here, the user will find, summarised in the banner at the top of the screen, the headline results for the scenarios analysed, a cost-effectiveness plane and an equity plane. During our stakeholder engagement workshops, the participants chose the type of graphs and other outputs to be presented and the way of presenting the information in the dashboard (Figure 6).
FIGURE 6.
The outputs tab ‘dashboard’.
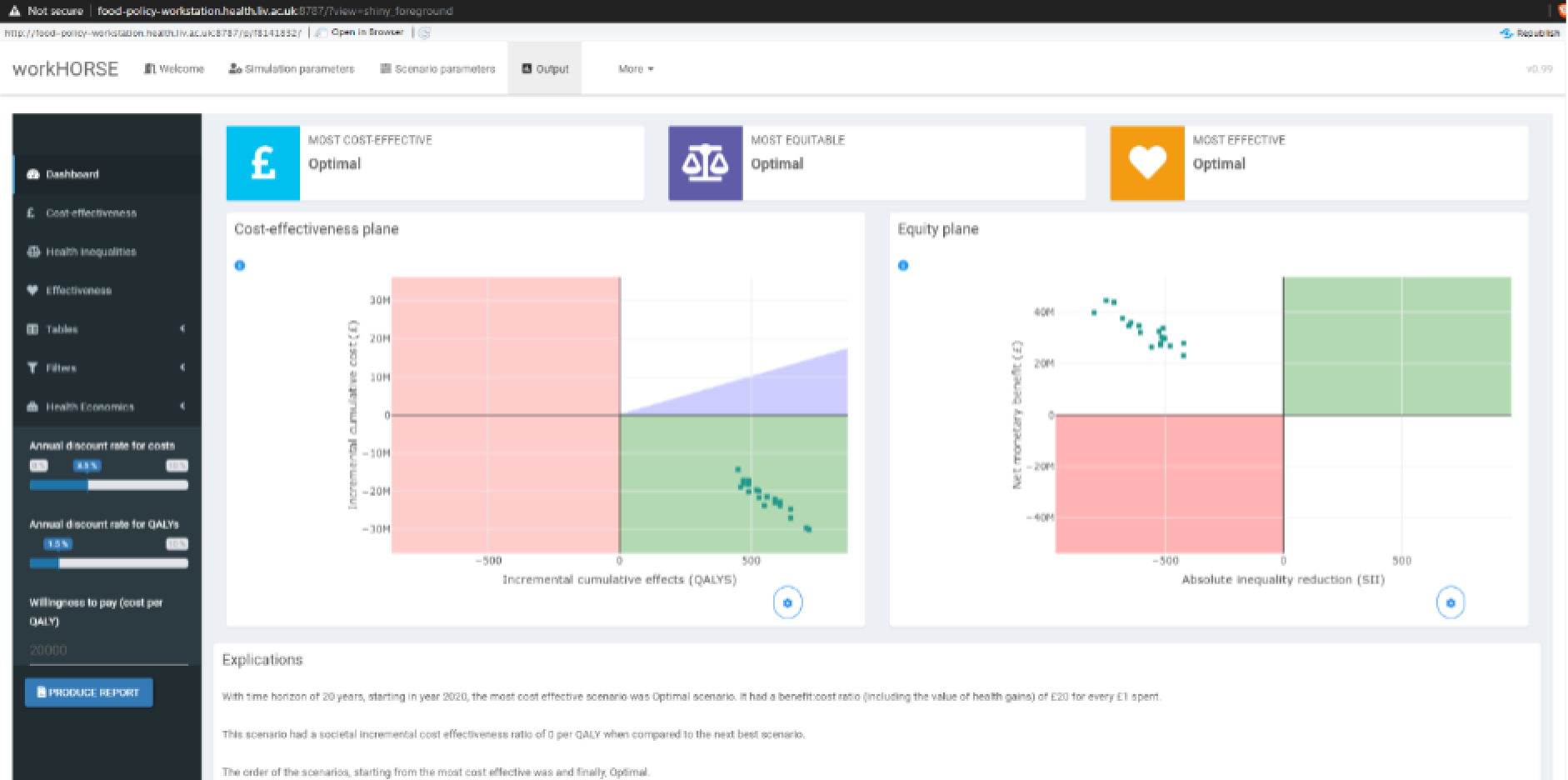
The model outputs dashboard provides more in-depth results for health economics, effectiveness and equity analysis. For example, the user can choose to inspect a breakdown of the disease cases prevented or postponed (CPP) in graphical form (Figure 7).
FIGURE 7.
Example of the advanced effectiveness tab.
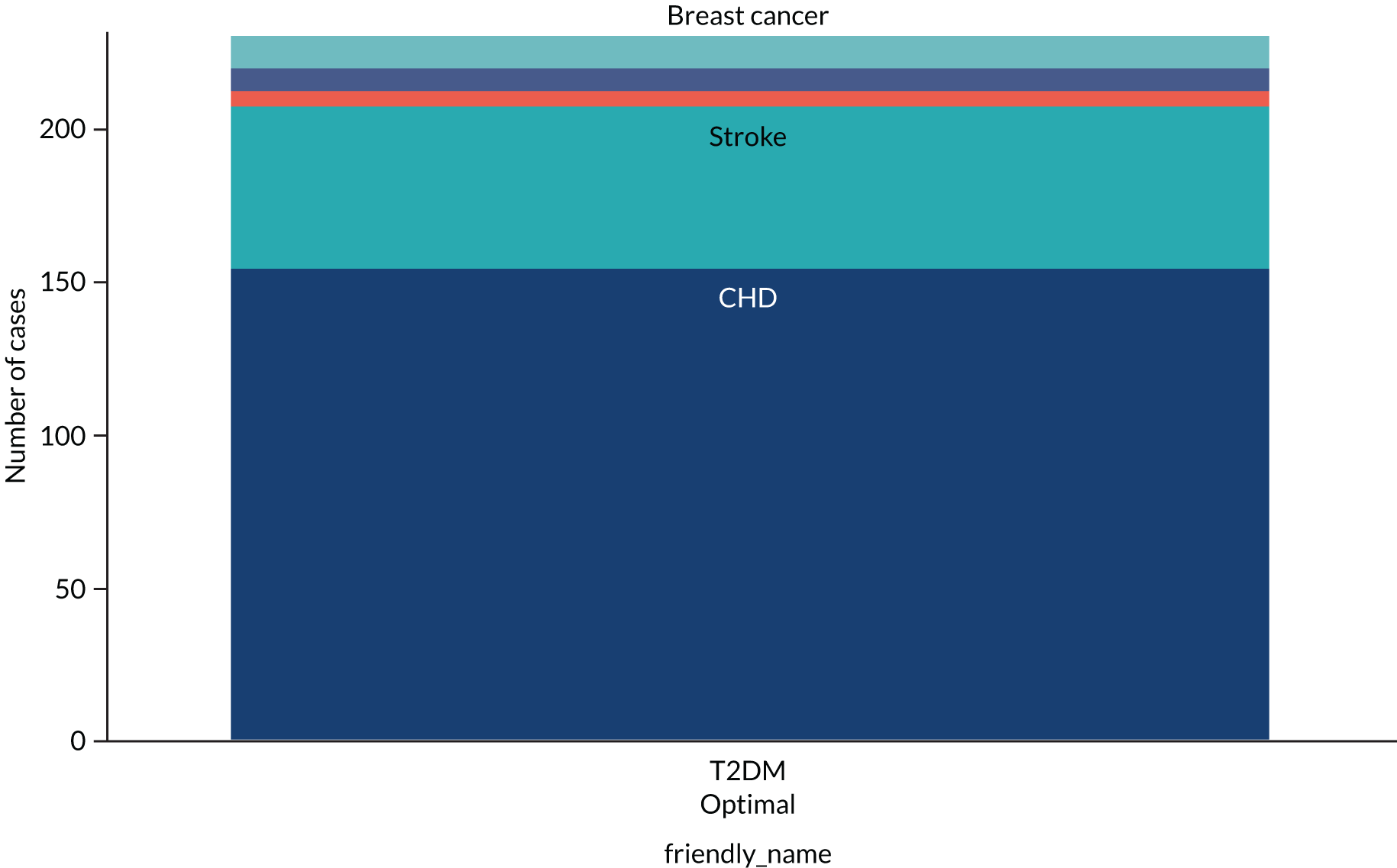
For advanced users, the results can be further tailored to specific age groups or results using ‘filters’.
The graph can also be exported for use in reports or presentations as high-quality Portable Network Graphics files.
Technical implementation
We developed the GUI in R Shiny (URL: https://shiny.rstudio.com/). For the plots, we used the R Plotly library (https://plot.ly/) and for the tables we used the R DT library, a wrapper for the JavaScript DataTables library. All of the dependencies of the workHORSE app can be found at the source code of the app [URL: https://github.com/ChristK/workHORSE/blob/master/dependencies.yaml (accessed 25 February 2021)].
Conclusions
This prototype GUI is an example of what can be achieved by building on top of the simulation engine. The user interface then represents a baseline scenario for a health-care preventative intervention: in this case, the NHS HCP.
The use of open-source technologies enables further development of the interface and adaptation to specific needs for a user.
Despite the simplicity, the approach used in the scenario parameter tab allows us to implement scenario analysis for all key aspects of programmes such as the NHS HCP. The key design decision was to use the basic parameters as the key entry point for user interaction. The user can then specify parameters that represent process and key performance indicators in the programme design [i.e. who will participate (eligibility), what mechanism will be used to optimise attendance (e.g. appointments offers or invitations), plus additional parameters describing participation (uptake) and provider delivery (prescriptions)]. Users are then empowered to develop scenarios to inform changes in these parameters ‘off-model’ and use the information to refine the scenarios or specify new ones.
Our approach provides an excellent balance to enable basic users to extract value from scenario analysis while providing flexibility to accommodate changes affecting programme design or provider delivery. Ideally, it might be useful to provide functionality for full-scenario specification. However, that would result in a less flexible modelling environment, particularly for programmes such as the NHS HCP, which are frequently reviewed, with subsequent nuanced changes to the remit or design.
Chapter 5 Using the workHORSE model to explore and compare the effectiveness, cost-effectiveness and equity impact of different implementations of the NHS Health Check programme
Introduction
In this chapter, we will explore several scenarios to assess the effectiveness, cost-effectiveness and equity impact of different implementations of the workHORSE model.
There is some controversy around whether or not NHS Health Checks are effective or cost-effective. NHS Health Checks were first modelled by the Department of Health and Social Care in 2008. 7 The Economic Modelling for Vascular Checks model suggested that having NHS Health Checks every 5 years starting at age 40 years would be cost-effective using QALY outcomes, which are a summary measure of length and quality of life, where 1 QALY is the equivalent of 1 year lived in full health. This modelling estimated a cost per QALY gained of £2480, which is less than the NICE threshold of willingness to pay for public health interventions of £20,000 per QALY gained. NICE recommends health and public health interventions based on whether or not they are best practice and are cost-effective. However, many of the modelling assumptions in 2008 were based on a limited and selective evidence base. For instance, it controversially used a highly effective intervention as a proxy for the effectiveness of a lifestyle intervention for people with impaired glucose regulation. The authors selected the US Diabetes Prevention Program when they could have selected from many examples of lifestyle interventions, most being less effective. Therefore, many of their modelling assumptions have been later questioned. A subsequent Cochrane systematic review and meta-analysis was published in 2012 and updated in 2019. 169 The review169 identified 16 studies of general Health Checks in adults and found that Health Checks most likely increased diagnosis and treatment rates, but did not significantly reduce mortality, morbidity or other key outcomes, such as hospital admissions, disability, worry, physician visits or absence from work. 169 However, many of the studies included in the Cochrane systematic review were conducted before 1980, when knowledge about risk factors and available treatment options was more limited. Many of the benefits now attributed to Health Checks are benefits from subsequent interventions, such as lifestyle advice or treatment with statin of antihypertensive medication.
Crossan et al. 9 suggested that typical implementation of NHS Health Checks resulted in an ICER of £23,276 per QALY, and a more optimal strategy of inviting only those who were already assessed as being high risk resulted in an ICER of £9257 per QALY gained. The cost-effectiveness of HCPs has been evaluated recently in a systematic review by Lee et al. 23 The authors identified 14 economic evaluations (five based on RCTs, seven on observational studies and two on modelling studies). The randomised evidence highlighted the need for sustained long-term risk factor changes to achieve cost-effectiveness. Most observational and modelling studies suggested that CVD screening programmes might be cost-effective. However, they relied on assumptions on costs, uptake, compliance and sustainability of the therapeutic interventions, which were not entirely consistent with empirical observations.
Furthermore, the equity impact of the NHS HCP has not been sufficiently studied. Robson et al. 6 looked at the implementation of NHS Health Checks from 2009 to 2012 and found that their uptake increased over the period, and that 19% of people identified as being high risk were newly prescribed statins and 8.8% were newly prescribed antihypertensives. This study found that attendance rates were higher in people from more deprived areas. However, this effect may be because of strategies to invite people from deprived areas earlier in the 5-year cycle, so that the gradient might change by the end of the 5 years. One study by Chang et al. 10 found slightly higher uptake in the most deprived quintile. However, several smaller but more detailed and precise studies17,170,171 showed significantly lower uptake in deprived areas. However, none of the studies determined whether or not the gradient in NHS Health Checks uptake was sufficient to improve inequalities in health outcomes, such as mortality or disease cases.
An essential aspect of evaluating the design of the NHS HCP since the initial 2008 assessment is to consider the additional impact on diseases that are causally linked to the prevention activities triggered by participation in the NHS HCP and the focus on equity impact.
Our earlier work suggested that reducing the overall burden and inequalities ideally requires a combination of approaches, including policies on smoking and diet that have an impact at the population level. 11,27 We will not be including such additional interventions in the analyses reported here. However, if so desired, the workHORSE model can easily incorporate these types of interventions when assessing an overall prevention strategy.
We developed the workHORSE model to enable users to explore some of the key questions that are relevant to the overall debate of NHS HCP effectiveness, cost-effectiveness and equity impact using a shared tool co-created with representative users. We do not provide a comprehensive assessment of the programme itself in this report. However, we will show the range of answers that the model can potentially provide by exploring an initial, indicative set of local scenarios, including assessing the potential value of more detailed local data inputs.
Our collaborative interactions with stakeholders (see Chapter 2), the review of exemplar local practices and the results of the umbrella literature review (see Chapter 3) each provided invaluable background to better explore the critical question of optimising the programme by increasing uptake, a key performance indicator, and look at its potential local impact. The first analysis will explore the potential for the NHS HCP if optimised as currently designed. We will then explore how to use the model to explore a variety of approaches to critically increase activity in the NHS HCP, quantifying the potential impact of optimising uptake by adopting ‘best practices’ to increase uptake utilising new evidence on invitation methods.
Scenario description and methods
Working with our stakeholders, we designed three initial analyses.
Analysis 1: optimising Liverpool implementation of the NHS Health Check programme to emulate the best-performing local authorities in the region
We first characterised a baseline scenario representing the current implementation of the NHS HCP in Liverpool in 2020, covering a population of some 552,000 people. We modelled the impact and costs if the NHS HCP continued unchanged in Liverpool throughout the modelled period to 2040. The parameters for this scenario, including costs, were informed from a previous research project we co-produced with the Public Health Department at the Liverpool City Council, but updated with new information when necessary and available. 11 We updated the annual coverage to 12.5% and the annual uptake to 57.5% (PHE Fingertips data for 2018–19172), and we assumed that they would remain stable until 2040. Prescription rates of statins would continue at 5% of the participants and antihypertensive treatments would continue at 6% of the participants, with different weights allocated to the prescription rates for low-, medium- and high-risk patients. The prescription rate for statins and antihypertensive medication use the number of participants as the denominator. Our stakeholders informed us that this is an easier indicator to extract from their data.
The workHORSE algorithm internally converts the prescription rate to one that has the number of eligible participants in the denominator. Eligibility is defined based on current NICE guidelines. Costs were updated to £6 per invitation and £15 per successful participation in the NHS HCP. This scenario also assumed that 1% of smokers would cease, 1% of those who are obese and overweight would lose about 1% of their weight, 1% of participants would increase their physical activity by 1 active day per week and that 1% of the participants who are heavy drinkers would reduce their alcohol intake by 1%.
The alternative ‘optimal’ scenario in this analysis represented the optimal implementation based on the best-performing LA in England in terms of invitation and uptake, plus assuming correspondingly better prescription rates and increased referrals to highly effective lifestyle services. The activities that the best-performing LAs described in our review in Chapter 3 provided a pragmatic justification for such a scenario. We therefore modelled the programme to invite 20% of the eligible population with 96.2% uptake (as achieved by the best-performing LA in England). Prescription rates in this scenario were assumed to be 10% and 12% to reflect current prescription guidelines and optimal provider compliance. We also assumed optimally effective lifestyle services, assuming that 10% of smokers would cease, 10% of obese and overweight participants would lose about 5% of their weight, 10% of participants would increase their physical activity by 1 active day per week and that 10% of the participants who are heavy drinkers would reduce their alcohol intake by 10%. The costs were estimated at £6 per invitation and £20 per participant (recognising that the additional activities would be more costly).
Analysis 2: improved uptake method based on a large randomised controlled trial
In this analysis, we used the model to explore the adoption of a better invitation method, using behaviourally informed invitation letters based on a published RCT. 173 In this pragmatic RCT there was one control arm and two intervention arms that used two types of behaviourally informed letters, one looking at arguments to counteract common barriers to attending the programme and one looking at sunk-cost information provision. 173 The counterargument letter proved to be the better of the two interventions, increasing uptake by 5.5%, whereas the sunk-cost letter increased uptake by 4.3%.
We therefore decided to use the more powerful of the two effective intervention letters (i.e. the counterargument letter). We assumed consistent effects by age, sex and deprivation, and assumed that there were no substantial extra costs associated with the improved invitation letter. This scenario was discussed and refined in workshop 4 with our stakeholders.
For the base case, we used a group of LAs in Northamptonshire, including Corby, Kettering, Daventry, Northamptonshire East and South, and Northampton, with a population of 747,622 people. We use invitation and uptake rates as reported on the Fingertips website for 2018–19,172 with an invitation proportion of 12.0% of the NHS HCP eligible population and an uptake of 51.0%.
For both the base case and the behaviourally informed letter scenario, we assumed that all of the scenario parameters were the same, except that uptake increased from 51.0% to 56.5%. We used the same costs for the implementation of the NHS HCP in both scenarios to see if varying the text in the invitation letter would make a negligible difference to costs.
In addition, we reran the scenario, assuming improved lifestyle services provisions, as in scenario 1, simulating a more comprehensive redesign of the implementation. In our experience of interacting with workHORSE, lifestyle changes were the key to substantially increasing programme effectiveness.
Analysis 3: using detailed versus rough input data to inform model equity assessments
The workHORSE model allows the scenarios to be specified using the risk profiles of the participants and those for whom medication is prescribed. However, almost all stakeholders requested that users should be able to define scenarios without this information. Therefore, when the risk profile of the participants is not explicitly specified, the workHORSE model assumes that, on average, their profile is that of the simulated area. When the risk profile for those for whom medication is prescribed is not explicitly specified, the workHORSE model assumes that their risk profile is like that of those who would be eligible for treatment, based on NICE guidelines. These additional assumptions introduce bias to the workHORSE model. The essence of this analysis is to identify the potential direction and quantify the potential magnitude of the bias. It does not necessarily reflect policy-relevant scenarios but may be used to emphasise the importance of detailed data about who is participating in the programme and how they react to it. Data regarding the risk profile of those participating in NHS HCP and those who are prescribed treatment, as a result, are not currently systematically collected. We obtained our data for these scenarios from Liverpool City Council and Liverpool CCG and have published policy-relevant scenarios in our PLOS Medicine paper. 11
The specific scenarios in this analysis were as follows.
Baseline
No Health Checks.
Current rough data
-
Appointments offered yearly: 12.5%.
-
Cost per invitation: £6.
-
Health Checks received: 57.5%.
-
Cost per Health Check: £15.
-
Prescription rates for statins: 5%.
-
Prescription rates for antihypertensive medication: 6%.
-
One per cent of smokers would cease.
-
One per cent of obese and overweight patients would lose about 1% of their weight.
-
One per cent of participants would increase their physical activity by one active day per week.
-
One per cent of the participants who are heavy drinkers would reduce their alcohol intake by 1%.
Current detailed data
Same as Current rough data, but we provide detailed weights for uptake and prescription based on real-world data from Liverpool. The data shows that approximately three out of four participants have a low cardiovascular risk (i.e. a QRISK 2 score < 10), approximately one in five have a moderate cardiovascular risk (i.e. a QRISK 2 of 10–20) and approximately 1 in 20 have a high cardiovascular risk (i.e. a QRISK 2 score > 20).
Improved services
Same as Current rough data, but with invitations up to 20% and uptake up to 80%, and prescriptions at 10% for statins and 12% for antihypertensive treatment.
Improved detailed data
Same as Current rough data, but with invitations up to 20% and uptake up to 80%.
For a detailed summary of the parameters for this scenario, see Appendix 7.
All analyses started in the year 2020 and the simulation horizon was 2040. The methods for the health economics analysis have been detailed earlier (see Chapter 4). In brief, we report costs from a societal perspective, we assumed a willingness to pay of £20,000 per QALY, and we discounted costs by 3.5% annually and QALYs by 1.5% annually. The workHORSE model also reports CPP and CYPP, as well as deaths prevented or postponed. For example, if a synthetic individual develops CHD at the age of 50 years in the baseline scenario and at the age of 60 years in the policy scenario then that is 10 CYPP but only one CPP. Finally, we ran 100 Monte Carlo iterations for each scenario.
Results
Analysis 1: optimising Liverpool implementation of the NHS Health Check programme to emulate the best-performing local authority in England
Compared with current NHS HCP implementation in Liverpool, the workHORSE model estimated that optimal implementation might result in health gains and that there is more than an 80% probability of becoming cost-effective by 2030 and cost saving by 2031, while reducing socioeconomic inequalities.
Effectiveness
The workHORSE model estimated that the optimal implementation of NHS HCP in Liverpool could prevent or postpone approximately 142 (95% UI 110 to 181) deaths by 2040. Table 8 presents CPP and CYPP by disease cumulatively up to 2040. Negative numbers denote additional cases and additional case-years. This is because, as the optimisation of NHS HCP prevents death and prolongs life, synthetic individuals then become susceptible to other diseases (competing risk framework). Furthermore, specifically for T2DM, the additional cases also reflect the increased risk of patients treated with statins to develop T2DM. Figure 8 depicts the dynamics of disease prevention. T2DM is of particular interest, as the initial beneficial effect of NHS HCP through lifestyle improvements is neutralised and reverts by 2040, primarily because of the increased statin utilisation.
| Disease | CPP | 95% UI | CYPP | 95% UI |
|---|---|---|---|---|
| CHD | 212 | 161 to 271 | 1339 | 861 to 1812 |
| Stroke | 60 | 40 to 88 | 380 | 213 to 588 |
| Post-stroke dementia | 5 | –1 to 13 | 32 | –6 to 74 |
| COPD | –1 | 13 to 13 | –80 | –185 to 10 |
| T2DM | –28 | –136 to 90 | –34 | –935 to 838 |
| Lung cancer | 1 | –7 to 6 | 1 | –27 to 19 |
| Colon cancer | 3 | –1 to 7 | 16 | –9 to 41 |
| Breast cancer | 5 | 0 to 11 | 27 | –11 to 74 |
FIGURE 8.
Analysis 1: CYPP by disease over the simulation period.

Cost-effectiveness
The workHORSE model estimated that under the optimal scenario, 610 (95% UI 455 to 845) QALYs could be gained over the simulation period. (For comparison, the total QALYs over the simulated period for the baseline scenario were 4.93 million.) Table 9 presents the cumulative net costs and NMB. Overall, this scenario was highly likely to be cost saving by 2040 (Figures 9 and 10).
| Output | GBP (£) | 95% UI (£) |
|---|---|---|
| Net policy costs | 5.09M | 5.05M to 5.11M |
| Net health-care costs | –2.44M | –3.72M to –1.27M |
| Net social care costs | –33,400 | –167,000 to 112,000 |
| Net informal care costs | 261,000 | –251,000 to 761,000 |
| Net productivity costs | 20.0M | 13.7M to 29.3M |
| NMB | 29.6M | 19.2M to 42.8M |
FIGURE 9.
Analysis 1: cost-effectiveness plane, optimal implementation scenario by the year 2040. Light orange, cost increasing or cost decreasing intervention with decreasing QALYs; light blue, cost saving intervention; light purple, cost-effective intervention.
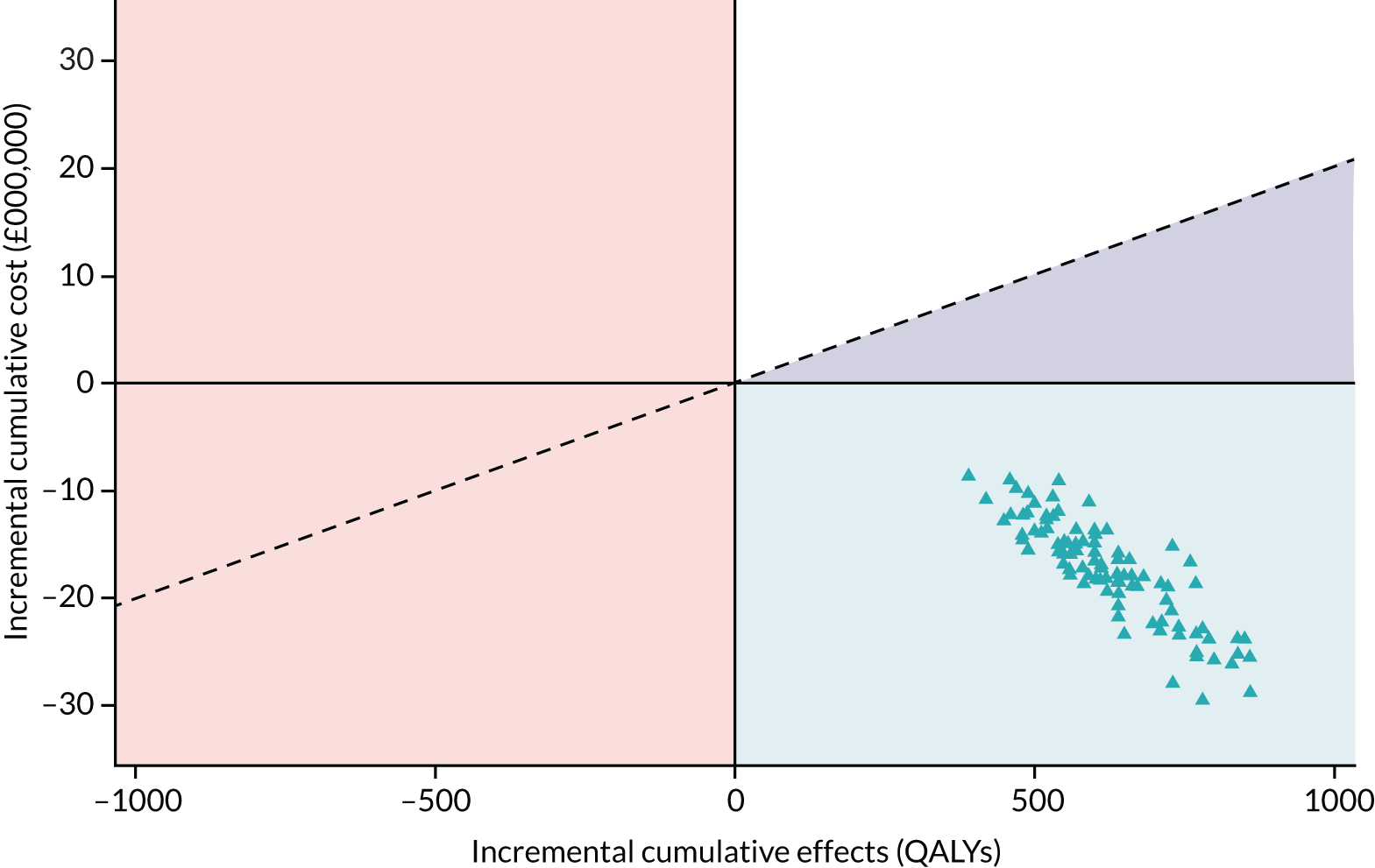
FIGURE 10.
Analysis 1: annual probability of optimal implementation scenario to be cost saving.
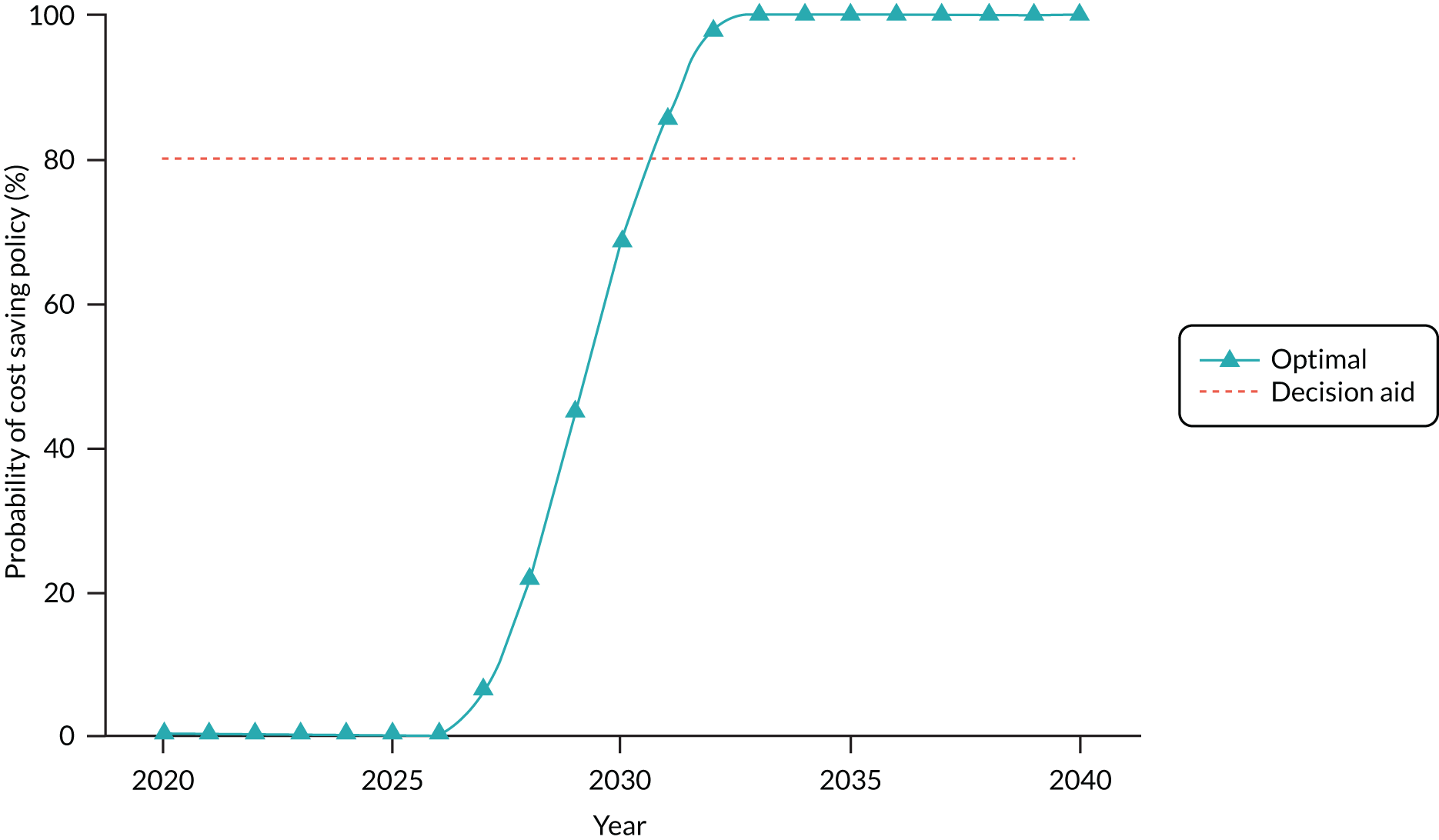
Equity
In terms of equity, the optimal scenario was more equitable than the current implementation, that is being more effective among the more deprived both in absolute and relative terms (Figure 11). Liverpool is a deprived LA, with most of the population living in areas in the most and second most deprived IMD quintile. Therefore, a highly effective NHS HCP, as this scenario assumes, would almost certainly reduce at least the absolute socioeconomic inequalities in health.
FIGURE 11.
Analysis 1: equity impact of the optimal implementation using the equity plane. SII, slope index of inequality.

Analysis 2: improved uptake method based on a large randomised controlled trial
Improving uptake using a behaviourally informed letter is unlikely to become cost-effective by the end of the simulation horizon (i.e. 2040), compared with the current implementation. However, adding an optimised lifestyle intervention will become cost-effective by 2028 and cost saving by 2029, with a probability higher than 80%. The effect on both scenarios on socioeconomic health inequalities is very uncertain.
Effectiveness
The workHORSE model estimated that the ‘behavioural’ scenario that assumes that a more effective invitation letter could increase uptake by 5.5% would have a negligible impact on mortality, preventing or postponing 1 (95% UI –22 to 36) death by 2040 in Northamptonshire. The addition of highly effective lifestyle interventions could prevent or postpone 28 (95% UI 7 to 62) deaths. Table 10 presents CPP and CYPP by disease cumulatively up to 2040 for both scenarios, and Figure 12 depicts the dynamics of disease prevention. Interestingly, unlike analysis 1, none of the scenarios is expected to increase T2DM cases because the additional prescription of statins resulting from the increased uptake is small in both scenarios.
| Scenario | Disease | CPP | 95% UI | CYPP | 95% UI |
|---|---|---|---|---|---|
| Behavioural | CHD | 7 | –19 to 34 | 53 | –202 to 254 |
| Stroke | 2 | –15 to 20 | 14 | –128 to 153 | |
| Post-stroke dementia | 0 | –5 to 6 | 0 | –40 to 42 | |
| COPD | 0 | –10 to 7 | –6 | –91 to 66 | |
| T2DM | 2 | –30 to 29 | 24 | –251 to 256 | |
| Lung cancer | 0 | –6 to 5 | –1 | –20 to 18 | |
| Colon cancer | 0 | –4 to 4 | 0 | –26 to 33 | |
| Breast cancer | 0 | –6 to 5 | 0 | –39 to 36 | |
| Behavioural plus lifestyle | CHD | 14 | –21 to 42 | 90 | –149 to 293 |
| Stroke | 10 | –13 to 31 | 71 | –102 to 228 | |
| Post-stroke dementia | 0 | –6 to 10 | 0 | –37 to 78 | |
| COPD | 1 | –10 to 10 | –14 | –94 to 94 | |
| T2DM | 34 | –9 to 72 | 324 | –92 to 701 | |
| Lung cancer | 0 | –6 to 7 | 0 | –23 to 26 | |
| Colon cancer | 2 | –5 to 8 | 9 | –30 to 45 | |
| Breast cancer | 2 | –5 to 8 | 9 | –38 to 55 |
FIGURE 12.
Analysis 2: CYPP by disease and by scenario over the simulation period. (a) Behavioural; and (b) behavioural plus lifestyle.


Cost-effectiveness
The workHORSE model estimated that approximately 16 (95% UI –83 to 150) QALYs could be gained under the behavioural scenario and 130 (95% UI 45 to 260) QALYs could be gained under the behavioural plus lifestyle scenario over the simulation period. (For comparison, the total QALYs over the simulated period for the baseline scenario were 7.45 million.) Table 11 presents the cumulative net costs and NMB. Overall, the workHORSE model estimated that the use of the improved invitation letter could only marginally improve the cost-effectiveness of the programme, whereas much larger benefits are expected by adding highly effective lifestyle interventions (Figures 13 and 14).
| Scenario | Output | GBP (£) | 95% UI (£) |
|---|---|---|---|
| Behavioural | Net policy costs | 342,000 | 320,000 to 365,000 |
| Net health-care costs | –57,100 | –796,000 to 587,000 | |
| Net social care costs | –10,300 | –133,000 to 150,000 | |
| Net informal care costs | 19,000 | –329,000 to 393,000 | |
| Net productivity costs | 399,000 | –3,190,000 to 4,960,000 | |
| NMB | 331,000 | –5,000,000 to 7,400,000 | |
| Behavioural plus lifestyle | Net policy costs | 340,746 | 312,000 to 365,000 |
| Net health-care costs | –413,906 | –1,280,000 to 260,000 | |
| Net social care costs | 10,523 | –229,000 to 170,000 | |
| Net informal care costs | 10,154 | –329,000 to 472,000 | |
| Net productivity costs | 4,007,957 | 1,010,000 to 10,700,000 | |
| NMB | 7.20M | 2.20M to 15.2M |
FIGURE 13.
Analysis 2: cost-effectiveness plane for behavioural and behavioural plus lifestyle scenarios by 2040. Light orange, cost increasing or cost decreasing intervention with decreasing QALYs; light blue, cost saving intervention; light purple, cost-effective intervention.
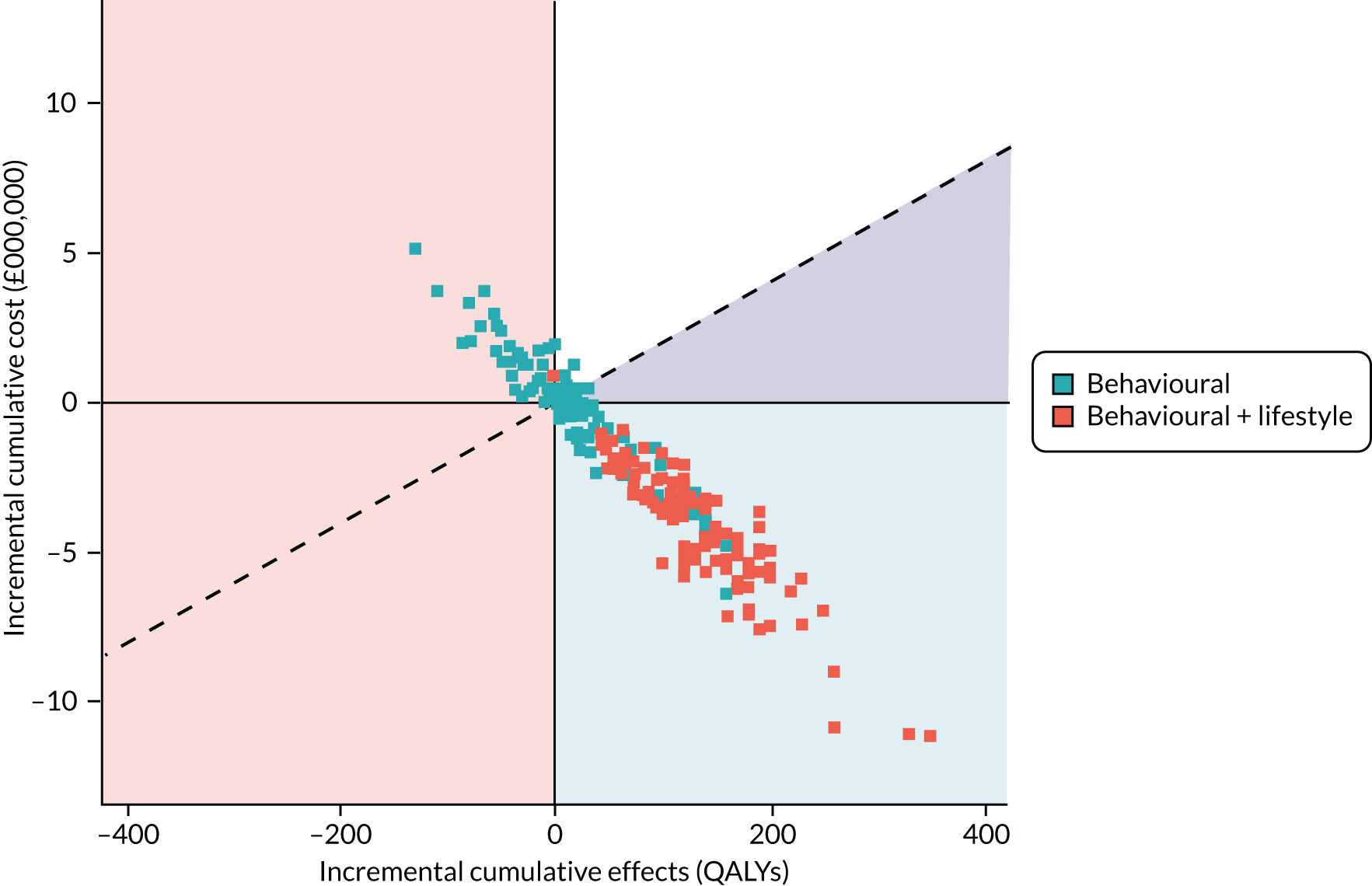
FIGURE 14.
Analysis 2: annual probability of the scenarios to be cost-effective.

Equity
Both scenarios are very uncertain in terms of reducing inequalities (Figure 15). Note, however, that we did not specify any of the scenario inputs by QIMD and this is reflected in the high estimated uncertainty.
FIGURE 15.
Analysis 2: equity plane for the comparison of the equity impact of the behavioural invitation method and the behavioural invitation method with improved lifestyles services scenarios. SII, slope index of inequality. Light blue, intervention reduce inequalities and results in positive net monetary benefit; light orange, intervention increase in equalities and reduces net monetary benefit.

Analysis 3: using detailed versus rough input data to inform the model
The aim of analysis 3 was to quantify the bias that is introduced by not specifying the detailed inputs for the scenarios. Therefore, the presentation of the results will focus on this aspect. As a reminder, the baseline scenario in this analysis assumes no NHS HCP. Moreover, the use of the detailed inputs that reflect the risk profiles of the participants in Liverpool is, on average, lower than the population in Liverpool.
Effectiveness
Figure 16 depicts the cumulative CYPP in each scenario over the simulated period. The figure gives the impression that scenarios that do not use the detailed inputs in their specification overestimate the effectiveness of NHS HCP. However, a closer look at Figure 17 reveals that scenarios that do not use the detailed inputs underestimate both positive and negative CYPP, and the net effect of this is an overall overestimation of CYPP.
FIGURE 16.
Analysis 3: cumulative CYPP in each scenario in Liverpool over the simulated period.

FIGURE 17.
Analysis 3: cumulative CYPP by disease in each scenario in Liverpool, over the simulated period.

Cost-effectiveness
Interestingly, the omission of the detailed scenario inputs seems to underestimate both the net utility of the intervention (using QALYs) and the incremental costs, and the underestimation increases as the coverage and uptake of the intervention increases (Figure 18). However, the probability of a scenario to be cost-effective appears less sensitive to whether or not detailed inputs are used (Figure 19).
FIGURE 18.
Analysis 3: cost-effectiveness plane for each scenario by 2040. We present only the median values for easier interpretation. Light orange, cost increasing or cost decreasing intervention with decreasing QALYs; light blue, cost saving intervention; light purple, cost-effective intervention.
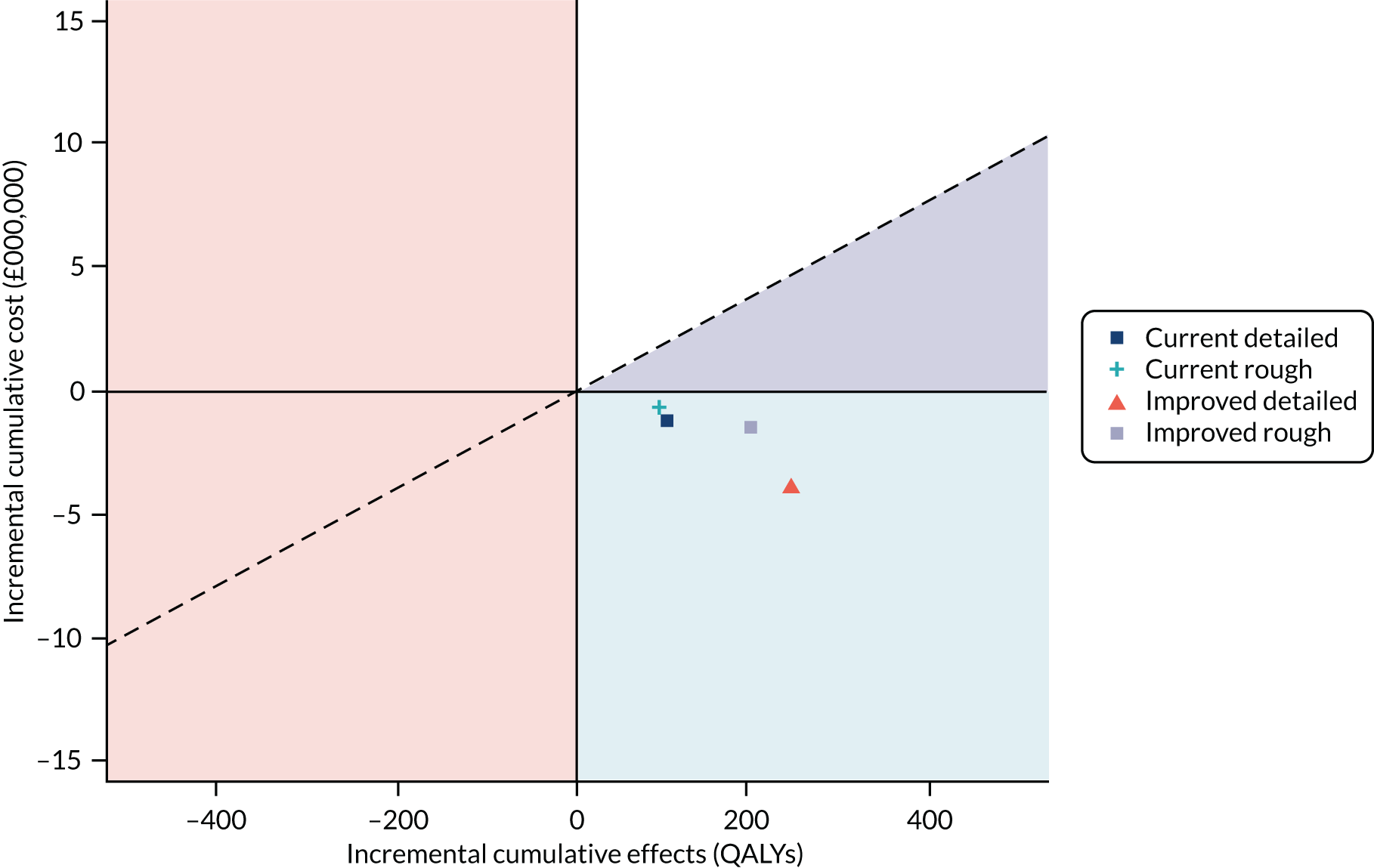
FIGURE 19.
Analysis 3: annual probability of the scenarios to be cost-effective.
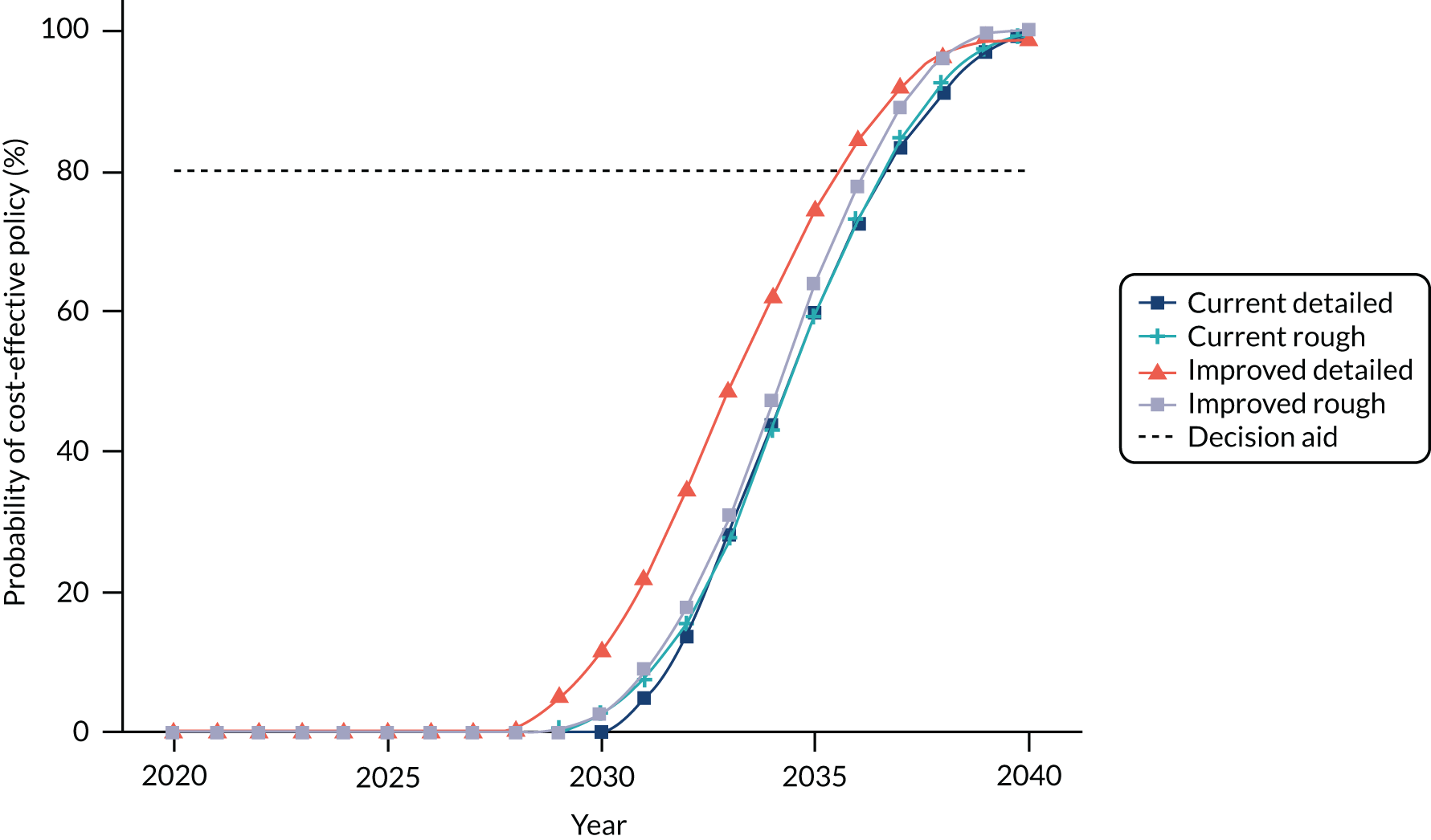
Equity
When equity is considered, it appears that improvements in absolute equity are underestimated when the detailed inputs are not used (Figure 20). Once again, the probability of a scenario being equitable appears less sensitive to whether or not detailed inputs are used (Figure 21; note that we present only median values to improve readability).
FIGURE 20.
Analysis 3: equity plane for the comparison of the equity impact of the scenarios. SII, slope index of inequality. Light blue, intervention reduce inequalities and results in positive net monetary benefit; light orange, intervention increase in equalities and reduces net monetary benefit.

FIGURE 21.
Analysis 3: annual probability of equitable policy (using absolute equity).
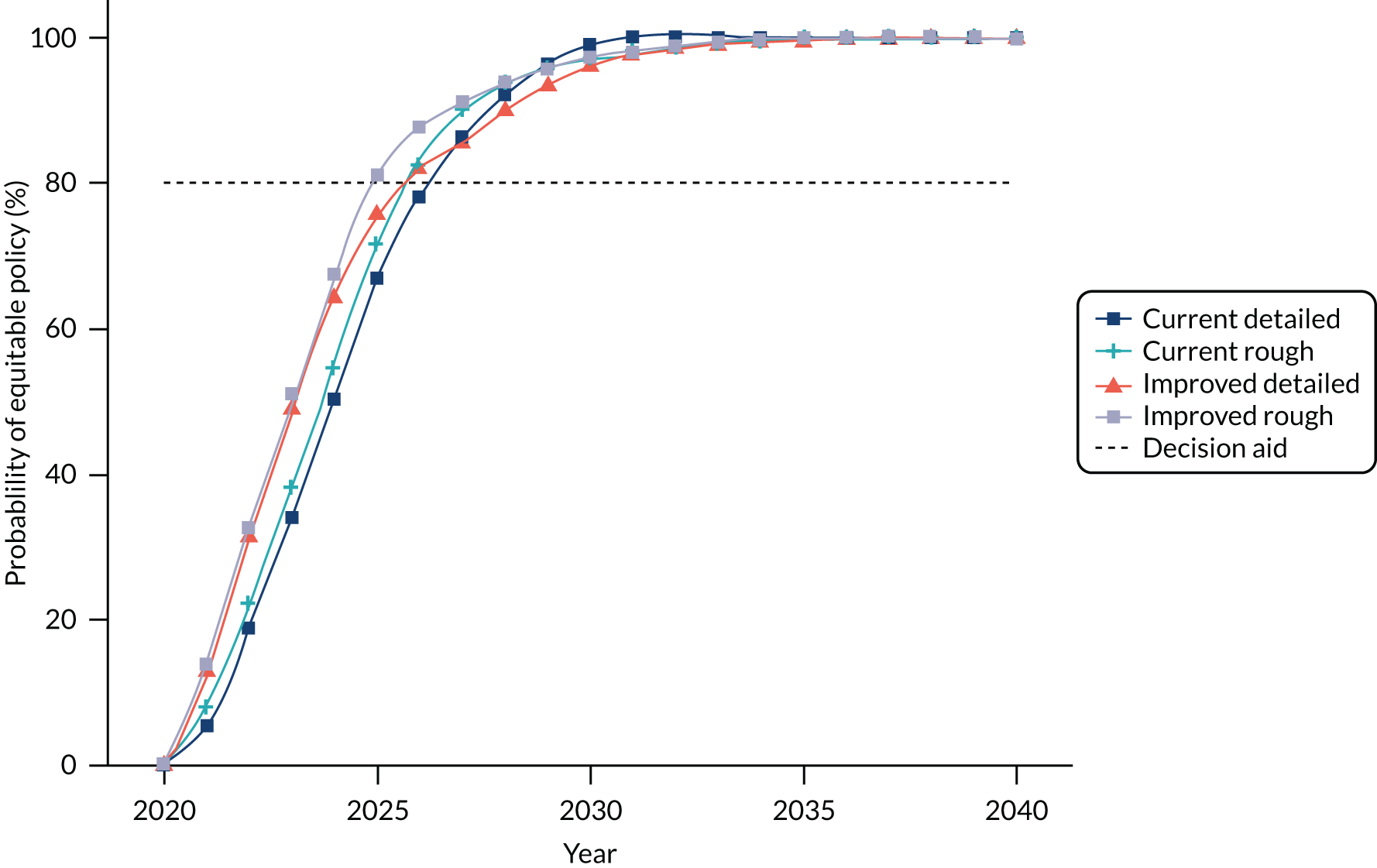
Discussion
To illustrate the uses of the workHORSE model, we conducted three analyses. The first two analyses estimated the health, economic and equity impact of the NHS HCP, illustrating how the workHORSE model can be used to assess the NHS HCP at the local level. The third analysis was conducted to assess the usefulness of striving to obtain more detailed data inputs to support decisions at the local level.
In general, redesigning the programme might result in modest health and economic gains. Our results (from analysis 2) echo our previous research, which highlighted the importance of additional lifestyle interventions to increase the effectiveness and cost-effectiveness of the programme. 11 However, this assumes that those lifestyle improvements are long-lasting. The programme has the potential to reduce socioeconomic health inequalities in Liverpool, a largely deprived area, but not in Northamptonshire.
In the first analysis, we evaluated an optimised version of the NHS HCP, focusing on increasing the level of participation and uptake in the programme, improving prescription rates and increasing referrals to optimally effective lifestyle services. We found that the improvements in implementation could prevent or postpone approximately 1700 case-years: mainly less CVD, with small gains in the other diseases. Increased policy costs would be offset by large productivity gains and reduced health-care costs, resulting in a substantial NMB of about £29.6M. This example of optimised performance of the NHS HCP will likely become cost-effective by 2028. The optimised NHS HCP has the potential to be more effective in the most deprived areas, reducing both absolute and relative socioeconomic inequalities in health. This was perhaps to be expected in a LA such as Liverpool, which already suffers from high levels of deprivation and high rates of NCDs, when the uptake and coverage of the programme reach higher levels. However, the real-world challenge is to implement policies that can make NHS HCP achieve such high coverage and uptake.
In the second analysis, we explored the potential effect of increasing the uptake of NHS HCP by using a more effective, behaviourally informed invitation letter, based on a RCT in Northamptonshire LAs, plus an additional scenario optimising the delivery of lifestyle services. We found that simply adopting a better invitation method would be unlikely to enhance health and economic gains unless the other key programme components were not simultaneously optimised. More importantly, in both cases, it is still unlikely that inequalities would be improved. This highlights the need to consider multiple scenarios when deciding on a redesign of the service, as most LAs might use multiple approaches simultaneously to improve the programme processes, suggesting that what is crucial is their likely combined effects.
Finally, in the third analysis, we assessed the sensitivity of the model to more detailed inputs when available at the local level, particularly in terms of CVD risk and socioeconomic distribution of risk. In the example for Liverpool, we found that not using the detailed inputs for scenario specification introduces bias for all point estimate outputs of the model, the direction and magnitude of which is hard to predict. However, the probability of a policy to be cost-effective or equitable was less sensitive to such bias. We expect that if we repeat this modelling exercise in another less-deprived area, the bias will be different. Therefore, we recommend that workHORSE model users make an effort to collect the required data to build scenarios using the detailed inputs whenever possible.
Overall, our results suggest a lower health and economic impact than some of the early evidence, and are more consistent with the more modest gains reported by Mytton et al. 26 and Hinde et al. 8 A direct comparison of these modelling exercises is unfortunately not feasible because the scenarios are not directly comparable, the geographies are different and the modelling methods differ substantially. Although such a comparative validity exercise was outside our remit, it could be useful as part of reviews of the NHS HCP programme174 and it would help considerably to better understand the importance of model methods, data and assumptions in explaining model-based decisions. Such comparative validation exercises have long been the tradition for modellers working on diabetes forecasting. 175
Despite these methodological difficulties, our analyses and these more recent studies8,26 alongside our own analysis suggest modest health and economic gains, mostly reflecting the postponement or prevention of CVD cases. This is not surprising, as the CVD burden can respond fairly rapidly to preventative interventions both at individual and population levels. However, other preventable NCDs generally have longer latency. 176,177 Therefore, the full benefit of increased Health Check interventions might only be realised beyond the 20 years horizon set in our simulations, and further preventative actions at individual and population level might be needed.
Strengths of this modelling approach
As presented in Chapter 4, the workHORSE model is an advanced, validated, flexible microsimulation of the dynamics of NCD in a population, including important NCDs that are amenable to prevention, and provides support to a range of capabilities to conduct state of the art effectiveness, cost-effectiveness and equity analysis.
Furthermore, including other diseases in the workHORSE model was valuable. The complex dynamic generated by reductions in CVD mortality needs to be modelled for a more realistic estimate of future costs from a societal perspective, which includes competing causes of illness and death. For instance, the model factors in that if CVD incidence is delayed, people may live longer and be more likely to develop any of the other modelled diseases. This was evident in our scenarios, where the number of COPD cases usually increased because of people living longer.
Limitations
All such modelling analyses have limitations. Several of the assumptions, particularly around the effectiveness of improved lifestyle services, might overestimate what could usually be achieved. However, we would suggest that these assumptions might usefully serve as ‘ideal or optimal’ targets to help the user better understand the maximal potential output of the exercise. Users have the option to perform one-way sensitivity analysis to identify influential assumptions and conduct targeted studies in their areas to collect more evidence and inform the workHORSE model. Using local effectiveness data for these services might be a way to improve the estimates when using the workHORSE model. We conducted a very simplistic assumption of the increased costs and benefits that might be observed over the full time course of the simulation. However, local commissioners might prefer to use the tool to refine the estimates by inputting their cost data.
For analysis 2, we considered modelling a multicomponent intervention as the most promising in increasing uptake, as identified in our umbrella literature review in Chapter 3. However, we decided that this was not feasible, as the review reported qualitative effect size only. In truth, any competent cost-effectiveness analysis would require an intervention that was very precisely costed to support a solid interpretation of the outputs. We believe that local commissioners have these detailed costings and the workHORSE model will be used as a guide for data collection. However, the optimal scenarios still provide a reasonable ballpark figure for the potential effectiveness of such interventions.
Diabetes prevalence can increase in some scenarios, consistent with the underlying population trends in obesity and diabetes, and the increased risk of incident diabetes in those patients using statins. 178,179 We explicitly modelled diabetes, including the associated increased risk of diabetes attributable to statins. It is not possible from our analyses to suggest changes in diabetes prevalence attributable to the interventions modelled, in part because of the small population numbers involved, resulting in considerable uncertainty. An in-depth analysis of this question might merit future exploration.
The costs of medications for CHD, stroke, diabetes and hypertension were all included in the broad health-care unit cost estimates. Although the costs of statins for people with no other diseases were not explicitly included, these costs are small, at around £20–40 per patient per annum. Furthermore, we did not measure or estimate the increased well-being and reassurance that some people might experience from undergoing NHS Health Checks that inform them that they are reasonably healthy. Finally, we, likewise, did not measure the ‘pill burden’ of being prescribed statins or other potential negative impacts that can be associated with interventions that involve inviting the general population to a health programme, such as discomfort, labelling or increased anxiety, or costs associated with overdiagnosis or misdiagnosis. Neither did we factor in private patient costs (e.g. cost of travel or childcare) or earnings lost for individuals to attend their Health Check. However, these are likely to be small. 180
When the impact of an intervention is modest and the local level is the focus of interest, the model results typically have a large degree of uncertainty in the cost-effectiveness of the decision, which will be revealed by large UIs that may cross zero. However, we consider that the median estimation still provides potentially valuable information as an indication of the potential for gains, rather than a conclusive statement on the equivalence between the compared scenarios. 11 Finally, we purposely used data from a publicly accessible repository reporting key performance indicators for the NHS HCP. However, there was no public or easy access to detailed data on local effectiveness of therapeutic and lifestyle referrals. This is concerning, given the considerable heterogeneity in the programme implementation that we found, even among the best-performing LAs (see Chapter 3).
As presented in Table 7, the workHORSE model considers only the benefits from primary prevention. We consider this to be appropriate, as NHS HCP is a primary prevention programme by design. However, one could reasonably argue that some of the interventions NHS HCP introduces may have an impact on the severity of the clinical disease. For instance, reducing the BMI of some participants may not only prevent or postpone T2DM, but, for those who will eventually develop the condition, clinical management would be better. Unfortunately, the argument that people leading healthier lifestyles might get less severe NCDs may be intuitive. However, this argument has weak evidence to support or refute it in most cases. The argument that people leading healthier lifestyles might experience more prolonged survival from NCDs is backed by more robust evidence in comparison, and that would reduce the cost-effectiveness of the NHS HCP from the health-care perspective.
Finally, we have not included a scenario where the different implementations of the NHS Health Checks were compared with other structural interventions that can reduce the burden of NCDs.
Conclusions
Our diverse scenario analyses suggest that the model can provide useful estimates of effectiveness, cost-effectiveness and equity, and therefore respond to questions around the implementation of the NHS HCP at the local level. Our scenarios further suggested that simply optimising the programme could be modestly effective and cost-effective.
Assessing the cost-effectiveness of the programme depends on scenario design based on reasonable assumptions of comparability. However, this is challenging, as the NHS HCP has varied its remit and processes over the last 7 years. Our results from the survey of best-performing LAs (see Chapter 3) showed a great heterogeneity of approaches. The workHORSE model can provide an analytical platform to explore their potential health, economic and equity impact in silico before implementation.
Furthermore, all such comparative analysis might usefully include other considerations, not least a focus on inequalities reduction and explicit consideration of the opportunity costs of potentially displaced interventions, as budgetary pressures on LAs are likely to continue.
The NHS HCP may be reviewed in the coming year, as heralded in the recent Prevention Green Paper. 174 The continuing evaluation of the effectiveness, cost-effectiveness and equity of this strategy is likely to be widely welcomed, especially if it involves a wide range of stakeholders in co-producing and interpreting relevant scenarios. The workHORSE tool will therefore be well placed to do this. It represents a potentially useful and user-friendly web-based decision-support model that is ready to be deployed at the local level mainly, if it is implemented as outlined in the next chapter.
Chapter 6 Implementation plan
Introduction
In this chapter, we propose a sustainability and implementation plan to deploy our user-friendly web-based decision-support model at the local level.
Models can have many purposes, including understanding or refining theory, system design or visualisation, forecasting and, crucially, the exploration and comparison of contrasting future scenarios. 24 Furthermore, computational modelling for decision support is not just a data and mathematical problem; it requires a closed collaboration between the commissioners, modellers, reviewers and users. 181–183
Trends in evidence used in local-level public health decision-making in England showed that key aspects are the importance of local evidence for local decisions, the critical role of local expertise in providing and interpreting the evidence and placing high value on local evaluation. 184 This was an issue that also came across firmly in our co-production exercises.
The workHORSE model seems to be particularly well-suited to support these types of evidence usage at the local level. These trends also highlight that it is not merely a case of having a tool available and distributed. A successful implementation will require people trained to use the tool, the resources to run and maintain it, and the skill sets to make the most of the analyses produced. In addition, the way that the tool will be implemented depends on what will be the ‘entry point’ of the tool in the organisation’s decision processes.
The workHORSE model has been developed with these fundamental principles in mind. The necessary limitations of a complex research project such as workHORSE, unfortunately, precluded a broader, national consultation that could have better informed the development of the workHORSE model and provided insights on how it could be deployed. However, the current stage of evolution of the tool can provide a solid foundation for such more comprehensive consultation and further evolution of the tool and its use.
The workHORSE model has been primarily developed for the exploration of future NCD prevention scenarios using the NHS HCP. The development phase was more prolonged than initially expected to engage as many stakeholders as practicable. However, this enabled us to accommodate as many different types of scenarios as possible. The tool is also flexible enough to allow the exploration of many NCD prevention questions at the national and local level, as well as ensure that the model does not become obsolete if the NHS HCP changes its remit, design or processes.
Key strategic implementation factors
The workHORSE model represents a different paradigm when compared with the decision-support tools generally deployed for use in LAs:
-
The workHORSE model can support powerful basic and advanced interface capabilities.
-
The workHORSE model can support scenario design features, supporting analysis of the current and alternative implementations of the NHS HCP.
-
The workHORSE model is provided as an open-source computer code with a permissive and copyleft licence. This feature enables external audit and quality improvement, customisation, adaptation and evolution.
However, using the tool requires skills in ‘scenario development’. Scenario development is an activity that results in the development of ‘scenario narratives’ that reflect the intervention being considered, prepares the necessary quantitative inputs to represent those interventions through changing parameters in the model and, finally, decides the scope of the analysis (i.e. in terms of time horizon and outcomes to evaluate), as illustrated in Chapter 5.
The prototype user interface described in Chapter 4 was built as an example of how scenario development can be supported by the tool. Therefore, developing more sophisticated and user-friendly interfaces will be an essential component of any implementation, as the interface will need to be able to adapt to the needs of local teams so that it can be used to represent the programme and policy to be assessed as the programme evolves.
We propose that there are five major areas to consider when strategically planning to implement the tool in an organisation: (1) the technical aspects of the implementation, (2) keeping the model updated, (3) training users in scenario development, implementation and interpretation, (4) the resources required in terms of people and expertise and (5) exploiting the possibilities of an open-source approach to future-proof the model. A summary is available in Table 12.
| Area | Role | Resources/skill set |
|---|---|---|
| Technical aspects of the implementation |
|
|
| Keeping the model updated |
|
|
| Training users |
|
|
| Developing the model |
|
|
| Implementation strategies |
|
|
Technical aspects and implementation feasibility
From a technical point of view, we designed and developed the workHORSE app to be easily adaptable to the available hardware. With minimal adjustments to the source code, the workHORSE model can support workstations, local network clusters or cloud computing facilities (e.g. Microsoft® Azure, Microsoft Corporation, Redmond, WA, USA). We tested the feasibility of local implementations and ‘cloud’ implementations, and we managed to run the tool both locally and in the cloud. For example, in workshop 4, we used the model in our internal local network remotely and we have also tested the model in a high-performance computer hosted at the University of Manchester, Manchester, UK. That said, the computational requirements are relatively high compared with everyday apps. The workHORSE model requires about 12GB of random-access memory per core and, ideally, more than 20 available cores per user. Nowadays, workstations that could host the workHORSE model cost around £5000; however, there is always the option of renting computational resources from a cloud computing service that is scalable and pay as you go, costing approximately £5 per hour.
Although any interested party can download the source code and run the workHORSE app, this requires the user to install all of the dependencies and resolve any potential incompatibilities. We recommend this option for advanced users and developers only. To make the installation of the workHORSE model hassle-free and adaptable, we built a Docker container (URL: www.docker.com/; Docker, Inc., Palo Alto, CA, USA). Docker is a technology that allows the containerisation of apps with all of their dependencies and an operating system. Therefore, the user can type a command into their terminal and the full app, including all of its dependencies, can be downloaded and run in an isolated sandbox using the available hardware. All main cloud computing services support Docker, which means that users can initialise virtual machines on the cloud running the workHORSE model within minutes. We provide specific instruction on how to do this for computers running Windows 10 Pro (Microsoft Corporation, Redmond, WA, USA) or Ubuntu Linux (Canonical Group Limited, London, UK) in Appendix 8. We additionally provide up-to-date detailed deployment instructions with or without Docker [URL: https://github.com/ChristK/workHORSE/blob/master/README.md (accessed 1 March 2021)].
All technologies used are open source and widely used; therefore, we do not expect licencing issues that could generate further costs for users in addition to the investment in hardware and information technology support needed. We have released the Docker container for use by any interested party at Docker Hub [URL: https://hub.docker.com/r/chriskypri/workhorse-app (accessed 1 March 2021)].
Our research project was time-limited and explicitly did not include the additional funding necessary to support the use of the tool or provide production-ready user interfaces for end-users. In fact, developing user interfaces requires a substantial investment in software engineering and user-interaction expertise. The process usually involves a design phase (during which functionality is elicited), a development phase (which usually results in a prototype and a production-level interface) and, finally, extensive testing of the user experience and interface. We ask the interested reader to contact us if they want technical details of the technologies used.
Keeping the model updated
The evidence supporting the core epidemiology in the model will need regular updating, particularly in the light of the recent changes in mortality trends in the UK population, with a likely slowdown in CVD mortality. 185
Evidence on the actual parameters representing the baseline scenarios (e.g. current implementation of the NHS HCP) will require a standardisation process to ensure its consistency over time, and therefore enable the most meaningful comparisons across place and time.
Information tools such as NHS England Fingertips can provide a user with data to implement a scenario for analyses in the workHORSE model, as definitions for key performance indicators are consistent with the model scenario design parameters. However, local cost and effectiveness data, and use of local services are not immediately available. Future efforts to provide dashboards or data repositories might wish to look at existing modelling tools and to our scenario design approach parameters to produce relevant data to be used directly or with minimal end-user processing.
The evidence informing the model described in Chapter 4 and Appendix 4 might benefit from regular updates. Rapid review methods can offer an efficient way to update key parameters. 186 Interestingly, given the flexibility and broad remit of a model such as workHORSE, approaches to review the different evidence needs of the programme, scenarios, epidemiology and effectiveness will require rapid, pragmatic synthesis methods more aligned with the nature of evidence needed in public health real-world decision-making.
We do not anticipate any data governance issues for basic users of the model, as equations, rather than data sets, represent most of the data. Scenario specification can be mostly carried out ‘outside’ the model, resulting in values that can be used to change the parameter sliders available in the interface. For example, the cost per invite for multiple interventions invitation strategies can be summarised in a single value. This might require obtaining local cost data to make the outputs of the model relevant to the local policy context.
The preparation of scenarios was usually carried out by our team ‘off-model’. Different data requirements and governance arrangements might be relevant if primary data are analysed to generate new, locally relevant scenarios. These issues will also need to be considered when creating or updating synthetic populations, effectiveness measures, cost data or developing more realistic inputs not contemplated by the interface.
Training future model users, including scenario development
The main training goals will be to enable a basic user to operate the beta interface competently, assuming a basic working knowledge of programme evaluation and health economics concepts. We have developed a new user tutorial as an example of the type of training materials that can be developed [see NIHR Journals Library project web page URL: www.journalslibrary.nihr.ac.uk/programmes/hta/1616501/#/ (accessed 10 March 2020)]. Mainly, this describes the primary use of the interface and is particularly suitable for smaller organisations to explore simple scenarios. The key features are to provide both visual orientations on the interface and worked examples.
However, to create a more flexible and powerful interaction with the model, the interface should be developed further. The user will need to interact with the ‘scripted’ code that instructs the model on how to run scenarios.
Training users in scenario development
Training is usually a key factor to improve the user experience with software.
We think that a critical skill is training users to develop and interpret scenarios. This includes understanding some theoretical principles on how the model works and what it can do, plus the user’s knowledge and expertise on evaluation. Our stakeholder group identified this issue as a critical need if the tool is to be widely adopted, alongside appropriate investment to support its use.
What is a scenario?
A scenario is the representation of the intervention that a user wishes to analyse with the model. It is not surprising that the word ‘scenario’ might need a definition when used in a modelling context and there is a substantial variation in what the definition means. At least 77 different definitions of what a scenario is have been reported in a systematic review. 187
The workHORSE model is designed to support the concept of ‘scenarios’ suggested by Spaniol and Rowland. 187 In summary, a scenario is a narrative of possible futures of our current situation where alternatives to the current situation can be explored and are plausible; it can be used comparatively and it allows exploratory uses as well as normative uses (e.g. making a decision based on a cost-effectiveness threshold). The work to specify the scenario is an iterative process that usually goes back and forth to the simulation to refine and test the scenario.
Scenario narratives
Scenario narratives require preparation, including data, evidence and costings for the specific interventions to be tested. The narrative explains the problem and intervention to be evaluated and the evidence base supporting it, plus the rationale for any assumptions made. These details must be thoroughly documented. However, there are no accepted formats to standardise this practice. The approach championed in foresight studies might be a practical approach to produce scenario narratives that enable better conversations when thinking of using this type of decision tool for non-normative purposes, for example when thinking about options to redesign the processes of the programme. 188
Simple scenarios
Simple scenarios [such as exercise 1 in the user tutorial, see NIHR Journals Library project web page URL: www.journalslibrary.nihr.ac.uk/programmes/hta/1616501/#/ (accessed 10 March 2020)] are straightforward to implement and are based on the ‘optimisation’ of existing parameters in the interface. Basic training, as described above, should be enough to exploit a basic interface for this purpose.
More complex scenarios
More complex scenarios would require interaction with more experienced users. We have co-produced an iterative scenario development exercise with Liverpool City Council colleagues, resulting in a peer-reviewed publication. 11 Bespoke solutions or implementation of additional interventions that are not already included in the current model will require more expert modelling input, or the ability to interact with the code and develop scripts or further model functionality. We encourage organisations and users with technical capabilities to build on the current version and shape the tool for specific uses, as this will benefit the entire modelling community, while enhancing the tool itself.
An important concept of exploiting the modelling approach is to understand the rich range of outputs that enable different types of strategies to be explored. For example, targeting the intervention by age, sex, ethnic group, geography or socioeconomic level allows the exploration of different types of implementation of the NHS Health Check programme. For instance, during this project, we explored how ‘universal’ compared with ‘targeted’ approaches differ in their effectiveness and equity impact for CVD prevention, using an earlier version of the engine. 164 In this paper, we found that, in Liverpool, the scenarios describing the implementation of the ‘universal plus targeted’ approach dominated the scenarios ‘increased’ and ‘current’ and reduced health inequalities. This paper also illustrates the possibility of conducting distributional cost-effectiveness analysis ‘off-model’. We refer the interested reader to this publication164 to gain additional insights on how the model can support more complex analysis strategies.
We consider that training users in scenario development and interpretation is likely to be more valuable if carried out at the local level, where the problems, policy and budgetary constraints are evident, and the need for decisions can benefit most from using the tool.
What resources are needed for local implementation?
We discussed above the technical resources required and here we will discuss the skill set that we consider necessary to use the model as intended. Although we have developed the tool with computing resources that can be available in most organisations, some aspects will require initial investments and funding to sustain its use over time. We will not discuss hardware or information technology resources, nor running costs for such a programme, as we think that will require a focus on a specific implementation project. Nor can we provide support for users willing to install and run the apps.
workHORSE is not a simple and intuitive tool. The complexity arises because of the need for flexibility to explore a broad set of scenarios that are difficult to anticipate. However, this, in turn, is making the tool more ‘future-proof’ in dealing with the inevitable future changes in the design of the programme and the evidence base informing the model. Developing more intuitive interfaces for such a model will require a substantial investment in GUI design and implementation: developments that were beyond the time and resources available in the project.
Interacting with the model, procuring and preparing inputs and interpreting outputs require users to have quantitative skills to operate the model. Furthermore, the user will need to know the specific characteristics of the local population and the issues relevant to the intervention programme being assessed. It is important to reflect all of these aspects in realistic scenarios so that the insights provided by the model are relevant.
Essential resources that a user would need to secure for effective use of the tool include the following.
Information technology and software engineering
Users would require information technology and software engineering to provide support in the local deployment of the software. Existing infrastructure might support local versions of the model, but it might require adaptation. In addition, maintenance and support might be required as needed, including third-party cloud deployment, which requires expertise in the technologies used (e.g. Docker). We have tested solutions using open-source software, reducing the need for a commercial software licence if the cloud provider protocols can interoperate with workHORSE code. Most cloud infrastructures are commercial and therefore operate on a pay-per-use basis.
The prototype user interface might need adaptation for specific user requirements, changes in the programme and to enable analyses that are not possible with the current functionality.
Modelling expertise
Additional technical expertise would be needed if users wished to update synthetic populations, incorporate new effectiveness and epidemiology data, or redesign the programme. A software engineering skill set is needed, including advanced knowledge of programming in R and C++ (Standard C++ Foundation; URL: https://isocpp.org) languages and advanced quantitative skills (i.e. to implement synthetic population approaches and other advanced statistical operations; see Chapter 4).
Data science expertise
Data science expertise is needed to conduct bespoke analysis and use or update additional data sets, mostly to keep the model epidemiology and parameters up to date. Models become obsolete very quickly (e.g. the initial cost-effectiveness analysis for the NHS HCP), usually because the intervention or the programme that the model addresses is changing.
We developed the workHORSE model to be flexible and accessible so that it can evolve alongside the issues addressed. The scenario parameters reflect the basic operations of a ‘detection and control’ individual-level intervention, primarily health care based, as it involves the prescription of drugs or intervention by a qualified professional at some point.
Specific programme expertise
Programme knowledge and expertise are essential for scenario design and interpretation. The tool provides a degree of flexibility in designing scenarios that is unique, requiring a thorough knowledge of the programme to fully exploit the tool’s capabilities. As shown in Chapter 2, working closely with stakeholders involved in the NHS HCP allowed us to develop specific interface features that are most relevant for local questions and decision-making.
Implementation approaches
Depending on resource availability and the mode of use of the tool by users and organisations, the implementation can be carried out in a ‘central to periphery’ strategy or a ‘local strategy’.
The ‘central to periphery’ strategy could be a centralised effort to develop an implementation programme that is adequately resourced centrally and in charge of the technical aspects. It could provide a bespoke standardised user interface, further maintenance and development of the tool, and training and support to users. The benefits of this strategy will include economies of scale, particularly around sourcing highly skilled staff.
The ‘local strategy’ (i.e. taking responsibilities for all the roles and areas at local or even regional level) will provide more flexibility. By implementing, developing and training local users to better satisfy local needs, it will respond best to the trends in evidence use at the local level in England. However, this will require the development of specific local or regional teams with modelling and analysis capabilities, or outsourcing of these activities with a dedicated budget. Furthermore, the increasing collaborative links between universities, local public health teams and public health training schemes can provide the necessary skills and research capabilities with a robust and local focus.
We estimate that a ‘central to periphery’ strategy can be a reasonable approach to resource the necessary skills, with a core team at a central level that is well resourced in modelling and software engineering capabilities and resources to support dedicated analysts at the local level. Alternatively, the ‘local’ implementation can serve local needs best with a small team composed of an analyst (with a data science background) and funded collaborations with local universities and software engineering providers.
The workHORSE model and code can be used for any of these levels of implementation.
Developing the model: exploiting the possibilities of an open-source approach
A Royal Society Open Science review on modelling has recommended open-source/open-access approaches to models used in policy decision-making. 181 Furthermore, the original NIHR call for this project indicated the need for an open-source tool. This approach has also been increasingly suggested to future-proof further enhancing transparency and providing commissioners and users with a starting platform to develop models more relevant to their users’ needs.
We have therefore licensed the code under General Public License (GPL) v3. GPL v3 is a widely used licence that guarantees and enables end-users and developers to use the software, share it or further modify and develop it to suit their own needs. It is a form of ‘copyleft’ licence, which encourages the evolution of this work by ensuring that all derivative work should be open source and distributed under the same or equivalent licence terms. As an illustration, any user can adapt or extend the code for other purposes. For instance, a user can be interested in exploring alternative designs for NHS Health Checks or when new evidence on novel interventions need to be evaluated and considered for inclusion. Therefore, ‘tinkering’ with the code is entirely allowable with the open-source licence used and encourages the academic and non-academic communities to use and expand the model and improve its methods, as long as it remains open source and under the GPL v3.
The open-source approach allows better integration with data sources and evolving linked data sets. Efforts in PHE and the increasing availability and access to linked data sets can result in better and more efficient ways to update the data and evidence used in the model.
A key aspect is that enhanced transparency allows detailed inspections of the code and the equations in the model, although it requires advanced coding expertise to judge it adequately. Therefore, even an open-source approach does not ensure full transparency of the modelling approach, an issue that merits further thinking on how to increase the confidence of end-users to modelling activities in general. Our stakeholders signalled this (see Chapter 2) and therefore more research is needed. The research should focus on finding ways of communicating the workings of these complex mathematical models in simpler terms, while preserving enough detail to judge their internal and external validity.
Finally, the adoption of open-source code removes the cost of commercially licenced software from the implementation strategy, while enabling commercial providers to provide specific services interacting with the model engine (e.g. the development of more advanced user interfaces). Further work might be required to facilitate this approach, including the development of ‘application programing interfaces’ (APIs). As is usual with open-source code, this software is provided without warranty and the authors cannot be held liable for any consequences arising from the use of this software. Unfortunately, we cannot support users on installing or using the model, as we are not funded to provide this activity.
The code is available in the GitHub repository (San Francisco, CA, USA) [URL: https://github.com/ChristK/workHORSE (accessed 1 March 2021)].
Chapter 7 Discussion, next steps and conclusions
The workHORSE project aimed to provide a validated open-source/open-access flexible model, enabling local commissioners to quantify the potential effectiveness, cost-effectiveness and equity for population health gain of the NHS HCP, by building on the solid foundation of our existing IMPACT NCDs model.
We recruited a diverse group of stakeholders to strengthen the user perspective, particularly to inform the desirable features of the user-friendly model and identify additional locally relevant scenarios to test (see Chapter 2).
We identified best-performing LAs and analysed the factors potentially contributing to their success, and then updated the published evidence base to support model and scenario development (see Chapter 3).
We then further developed our proven and tested computational model to allow for developments and changes to the NHS HCP and the diseases it addresses (see Chapter 4).
We then used this model to illustrate cases assessing the effectiveness, cost-effectiveness and equity of different strategies for implementing the NHS HCP (see Chapter 5).
We were therefore well placed to propose a way forward on what is needed for effective implementation to deploy the workHORSE model at the local level (see Chapter 6).
Main findings
The workHORSE dynamic simulation tool was developed to provide decision-makers and practitioners with a web-based decision-support tool to help identify the most effective, cost-effective and equitable interventions for the NHS HCP. The value of co-production when developing computational models is increasingly well recognised. 189–191 However, previous computational modellers have rarely involved end-users when developing tools to inform decision-making. The added value of involving stakeholders in the co-production of tool development enabled productive and valuable dialogue in this project and was powerfully apparent. It provided valuable learning about potential problems in practice and supported consensus building for effective end use.
Our first objective was therefore to recruit a diverse group of stakeholders to inform the desirable features of the user-friendly model and identify additional locally relevant scenarios to test. Our 30 stakeholders usefully represented a diverse range of local, regional and national perspectives. They provided detailed and positive feedback regarding the expressivity, clarity and potential usefulness of the model, particularly the ability to compare different scenarios.
Our fourth and final stakeholder workshop provided an opportunity to showcase the final model’s capabilities (in terms of model usage and interpretation of results), and discuss with stakeholders and lay advisers desirable next steps in terms of implementation and dissemination plans. Stakeholder feedback was generally positive, with the tool being a potential asset for commissioning, especially the ability to forecast the potential effectiveness, cost-effectiveness and equity of different future scenarios.
Many respondents highlighted the need for support during future implementation, including the importance of a user-friendly interface, supported by a clear user manual, guidance notes, tutorials, e-mail/telephone advice and training. This echoes longstanding advice on good practice from ISPOR and other leading groups. 192
The high level of stakeholder engagement therefore resulted in modellers producing a ‘real-world’ operational tool with the capacity to test a broad range of scenarios to determine their likely short- and longer-term impacts. Likewise, stakeholders obtained increased confidence in the decision-support tool’s development and applicability in practice, with a robust basis for decisions on the delivery of the NHS HCP.
Involving patients and the public was considered invaluable from project preparation through to project dissemination. All four of the lay advisers had a personal experience of using NHS Health Checks and were interested in how the development of the decision-support tool would benefit Health Check provision.
When preparing the workHORSE app for funding, the lay advisers provided constructive feedback on the content of the Plain English summary, offered suggestions on additional stakeholders we should invite and provided observations about how the proposed research might be improved.
The lay advisers were active members of our Study Steering Committee and provided feedback on their perspectives, primarily on workshop development and delivery. The advisers attended workshops 1, 2 and 4, where, as members of the research team, they ensured the timely delivery of activities and recorded and fed back their observations. As part of workshop 4, they presented on their experience of the workHORSE project and what a project needs for effective PPI. They have also been involved in the writing of academic publications for peer-reviewed journals and in ensuring the readability of literature for dissemination within the research community and engagement with the wider public.
The involvement of the lay advisers in the project provided valuable feedback in terms of having a public perspective on the NHS HCP and how the decision-support tool could improve the patient experience.
We contacted 16 of the best-performing (or most-improved) LAs across the country. Thirteen LAs responded and proved to be pleasingly diverse in terms of size, population profile and levels of social deprivation. The information obtained provided valuable material to inform the content of the stakeholder engagement workshops and generated excellent case examples for possible scenarios for the workHORSE modelling tool.
We were surprised by the wide range of approaches successfully adopted and adapted to maximise NHS HCP coverage and uptake. All 13 LAs had taken a strategic and sometimes innovative approach to achieve the targets set, while making adaptations based on their population profile. However, it was not possible to be more specific about defining a common set of effective approaches that could be recommended confidently and authoritatively to LAs that are not currently performing optimally.
To inform scenario developing, we undertook an umbrella review of the literature on strategies to increase uptake in screening programmes. As the workHORSE interface was developed around key parameters representing the processes involved in getting participants to participate in the programme, it might help in exploring scenario analysis (i.e. looking for the impact of the possible redesign of the processes in the NHS HCP to maximise participation).
Sixty-one systematic reviews of interventions that intended to increase the uptake of screening programmes were identified. From the reviews, we identified strategies to improve the uptake of screening programmes and strategies that have the potential to be effective. Surprisingly, few authors appeared to recognise that they were addressing complex, adaptive systems. However, many components within these complex systems, at the level of individual respondent, health-care professional or health-care system might influence screening uptake.
Many individual studies and systematic reviews focused on single interventions, with many appearing both plausible and attractive. However, very few studies addressed screening for elevated cardiovascular risk factors. Almost all of the studies identified focused on screening to detect breast, cervical or colorectal cancer, and the potential interventions were numerous and strikingly diverse.
Furthermore, the evidence on uptake effectiveness was often variable and rated as having a high risk of bias. The most significant single interventions appeared to include patient invitations and patient reminders, with the combination being even more effective.
Other useful interventions included mailing kits to patients (for cervical and colorectal cancer screening), interventions enhancing access (including community-based health workers), reminders to providers, assessment and feedback to providers, and training of health-care professionals.
Importantly, a variety of plausible interventions appear to be only weakly effective or ineffective. These include decision aids and personalised risk communication/tailored messaging interventions, one-to-one patient education and counselling, group education, mass and, surprisingly, financial incentives for patients. Most would depend on individual (agentic) responses and therefore on poorly sustained behaviour changes. 193,194
Fortunately, several reviews and studies evaluated multiple interventions targeted at multiple targets, which is a theoretically more attractive approach for tackling a complex adaptive system, although current complex intervention evaluation frameworks still need adaptation to complex system concepts. 118,195
Multiple interventions involving very diverse combinations consistently appeared effective in increasing breast, cervical and colorectal cancer screening uptake. Furthermore, some reviews were able to make direct comparisons and report that multiple interventions were more effective than single interventions.
The effectiveness evidence on several other interventions was inconclusive and required further research, including individual home visits, provider incentives, using dedicated personnel and organisational change and procedures.
The existing literature already offered potential candidates for ‘success factors’. The 2019 Review of National Cancer Screening Programmes in England48 recently set out key recommendations for increasing the uptake and coverage of screening programmes. The review advocated giving high priority to increasing the implementation of evidence-based initiatives to increase uptake and recommended an integrated system approach.
In conclusion, it is very likely that within each screening programme commissioners would need to implement multiple interventions to improve uptake maximally and therefore generate more health gains.
The epidemiological engine of the workHORSE model is an advanced discrete-time dynamic stochastic microsimulation. In addition to demographic and socioeconomic position attributes, the model takes into account behavioural and biological risk factor exposures, including alcohol intake, smoking status, fruit consumption, vegetable consumption, physical activity, BMI, SBP, total serum cholesterol and high-density lipoprotein.
The model produces a comprehensive set of estimates of prevalence (i.e. number of cases) and mortality from several diseases, including T2DM, AF, CHD, stroke, post-stroke dementia, COPD, lung cancer, colon cancer and breast cancer, in addition to mortality from any cause. Finally, the model includes information to assess CVD risk and key interventions in the NHS HCP.
The model includes costing for all health consequences and functionality to present outputs flexibly and comprehensively, enabling analyses on the distributional trade-offs when evaluating scenarios. It also provides outputs to be used in reports or publications, and the user can access all outputs of the model for further processing through the scripts.
The validation of the model is comprehensive, producing LA area-level outputs that can be used for simulation and scenario analyses.
We use the model to explore illustrative scenarios that explore the effectiveness, cost-effectiveness and equity impact of optimisation and adoption of different types of practices to improve key aspects of the design of the NHS HCP. We run several scenario analyses with the tool, looking at the effectiveness, cost-effectiveness and equity impact of typical scenarios to showcase key model capabilities. Optimising Liverpool implementation of the NHS HCP compared with the best-performing LA in the region will be effective, cost-effectiveness and equitable by 2030. The optimal implementation would prevent additional cases and increase QALYs, albeit modestly.
We also explored a type of intervention, a behavioural-informed strategy, that we identified in the umbrella review as a particularly feasible intervention, with improved effectiveness for lifestyle services. This illustrated the type of exercise that can be conducted with the workHORSE model to incorporate new evidence. Only improving the invitation method is unlikely to be cost-effective; however, it becomes cost-effective with the addition of improved provision of lifestyle interventions. This suggests that to maximise programme outputs it may be necessary to improve performance across many programme processes simultaneously. This more systemic intervention might result in increased costs and therefore its value for money will need to be reassessed. It will be possible to model more complex strategies for the invitation of participants, as our umbrella review showed that uptake is frequently reported as an outcome, regularly reported for the NHS HCP and maps well with the parameter representing uptake in the model. However, the cost-effectiveness might vary because of the different cost per invitation and subsequent resource used that needs to be factored into the analyses.
Finally, we explored how important it is to have local data to inform the model results. We found that when using local data, the model was moderately sensitive and that local data might, in some cases, be essential when assessing scenarios that might be borderline in terms of their cost-effectiveness or equity impact.
Strength and limitations
The views of our stakeholders chimed with the findings of other researchers around the preferences of public health decision-makers on economic evaluation. 196 This research found that public health decision-makers preferred economic evaluations to include costs and effects for different subgroups and different sectors (e.g. health, social care, productivity), as we have included in the workHORSE model. This research also found that decision-makers thought that including equity impacts was particularly important, as we have included in the model. Researching NHS Health Checks was particularly challenging, as there is a broad spectrum of opinions on the programme, with some people being NHS Health Check ‘evangelists’ and some being sceptics. 197 However, is it important to state that we had a limited number of participants in our stakeholder group, including only 30 participants. However, they were representative of all key groups and a mix of local regional and national perspectives, allowing us to capture key elements that influenced model and interface design.
The literature on interventions on improving screening programmes is extensive, complex and challenging. However, our umbrella review managed to identify and analyse 61 relevant reviews successfully. To our knowledge, this is the first comprehensive review of the evidence on invitation methods to improve uptake of screening programmes. It is particularly strong on interventions targeting breast, cervical and colorectal cancer screening programmes, and offers potentially valuable principles that are cautiously generalisable to CVD risk factor screening. We were unable to conduct a meta-analysis because the data were strikingly heterogeneous and therefore inform more direct scenario development, further compounded by the lack of detailed evidence directly applicable to CVD conditions. Future researchers might wish to conduct a meta-analysis on a subgroup of particular interest. However, one might cautiously extrapolate some general principles from the ‘best-buy’ options that generally increased the uptake of three very different cancer screening programmes, assuming that there are no ‘condition-specific’ determinants of uptake.
The workHORSE model uses microsimulation techniques and adjusts for comorbidities, and this is not possible with the typical cohort or life-table economic models.
The model is a dynamic, open-cohort microsimulation model designed to estimate the cost-effectiveness of the scenarios within a dynamic population where people are born, people age, people’s risk factors change and people die. By contrast, most traditional economic models are closed cohort, meaning that they follow the same population over time and often with a lifetime horizon of little relevance to most decision-makers.
This decision-support modelling tool also allows the user to filter outcomes for specific subgroups if desired (by age, gender, QIMD or ethnicity). This can therefore input into a subgroup analysis or be used for an equity audit. Furthermore, the prototype user interface, co-produced with the user and designed to reflect and provide flexibility to explore a range of effectiveness, economic and equity impact scenarios, is a blueprint for developing more advanced ways of supporting decision-making at the local level. However, it is unlikely that all necessary data will be available, mainly when the model is used for design rather than evaluation purposes. Techniques of expert elicitation are well developed198,199 and are used in the context of NCD prevention. 200 In addition, detailed toolkits with the implementation of the Bayesian expert elicitation approach do exist and are available in the public domain, such as SHELF (SHeffield ELicitation Framework) V4.0. 201 This structured approach to elicit model parameters can benefit from only one of the key workHORSE design principles (i.e. providing parameters for key processes representing the NHS Health Checks operations that are not ‘hardcoded’). Users who require expert elicitation to inform their analyses can therefore use their preferred elicitation approach to provide inputs for the existing parameters ‘off-model’.
Models require assumptions and have limitations. In Table 7, we have listed these assumptions and limitations. Notably, the model quantifies the impact of the NHS HCP on primary prevention only and not on the management of the conditions further down the line in secondary prevention.
We recognise that the model is complex and requires substantial amounts of data alongside technical expertise to both maintain and run. However, the flexibility that the approach offers is unparalleled, and the potential to tailor the interface to a particular use, facilitated by the open-source licencing, provides a solid foundation to build more user-friendly tools. This is particularly relevant for a programme such as the NHS HCP, as it has evolved as the remit changed over time. For example, the UK Government ‘Green paper’ said that the government would commission an evidence-based review of the programme and that it is likely that the scope, activities and key features of design will undoubtedly change after the review. 174
Possible future developments
Building on the considerable possibilities to further develop and evolve the code, we suggest that key priorities for future development may need to include the following:
-
Enhancing computing time performance to enable even more rapid interactive use of the model, enabling more flexible use of the model (including group-based model activities) and supporting long-term co-produced use of the model.
-
Further research and development of methods and approaches to maximise interactions between model designers and users, particularly on scenario design and interpretation. Furthermore, how these interactions are integrated into the decision process of an organisation. Finally, this aspect requires in-depth evaluation of the process and its added value.
-
Integrating the model with existing linked data resources for more streamlined or automatic updating.
-
Developing simulation modelling standards to enhance transparency, accountability and foster debate on public health issues. Recommendations for good practice for health economics purposes exist. 192 However, they do not cover the use of models for broader applications, such as public health use at the local level or using the models in ‘non-normative ways’ as thinking tools to facilitate and guide decision-making. The Brighton declaration represents a start, at least in bringing forward the issue. 202 Mainly, the Brighton declaration highlights the need to provide guidance and best practice for the nature of the evidence used in public health decision-making, highlighting that it is essential to look beyond pure cost-effectiveness. However, it offers no practical or operational principles to develop best practices in computational modelling for public health apps.
Conclusions
Our project has showed that developing a computer model with end-users to achieve a user-friendly and relevant model to contribute to, appraise, and provide a decision-support tool to redesign a programme such as the NHS HCP is feasible. More research is needed to further describe and evaluate the added value of interaction with stakeholders in co-producing such tools.
The survey of best-performing LAs revealed a diversity of effective approaches to maximise coverage and uptake of NHS Health Checks, with no single ‘best’ option.
The scoping literature review identified a wide range of single interventions that can increase screening uptake, and these should ideally be combined to improve uptake in these complex, adaptive systems.
Our project showed that the workHORSE model could be used to (1) estimate the health, economic and equity impact to provide comprehensive analysis at LA level, and (2) incorporate new evidence for redesigns for the implementation of the programme as new information or ideas appear, using the model as a design rather than as normative use.
The workHORSE approach demonstrated feasibility and offers substantial potential to be further developed, not only as a commissioning tool but also to stimulate a broader discussion on the role of such modelling tools in real-world decision-making.
Acknowledgements
National Institute for Health Research Health Technology Assessment programme and research design service
We would like to thank the NIHR Health Technology Assessment programme for providing the funding to conduct this project, especially the support provided during project development, delivery and the writing of this report. We would also like to thank the NIHR Research Design Service North West with whom we consulted during the application process.
Lay advisers who contributed to the workHORSE project
We would like to extend our grateful thanks to our four lay advisers, Mrs Kay Gallacher, Mr Jeff Goodman, Mr Peter Hale and Mrs Alison Shead, who provided ongoing support and advice for project development, delivery and dissemination.
Stakeholders
Grateful thanks are given to all of the stakeholders who gave their time and the benefit of their expertise at the co-production workshops, through the e-platform and between the workshops by e-mail. Their insights were invaluable for ensuring that the model was fit for purpose. We would also like to thank our stakeholders for completing the workshop evaluation questionnaires.
Local authorities
We would like to thank all of the LAs that shared their best practice for NHS HCP, which contributed to informing workshop content and model scenarios.
Study Steering Committee
We would like to extend our grateful thanks to the members of our Study Steering Committee who provided ongoing advice and guidance for the workHORSE project: Professor Christopher Millett (chairperson) Professor of Public Health, Imperial College London; Dr Chris Annus, Business and Service Development Manager, British Heart Foundation; Dr Kailash Chand, Honorary Vice President, British Medical Association; Mrs Kay Gallacher, lay adviser; Mr Jeff Goodman, lay adviser; Mr Peter Hale, lay adviser; Ms Jenny Hargrave, Head of Healthcare and Innovation, British Heart Foundation; Dr Catherine Lagord, Public Health Analyst, Health and Wellbeing, PHE; Dr Kay Nolan, Associate Director, NICE; Dr Ifeoma Onyia, Consultant in Public Health, Halton Borough Council; and Mrs Alison Shead, lay adviser.
Individuals who are not authors on this publication
We would like to extend our appreciation to the following individuals:
-
Peter Crowther and Melandra Ltd (Stockport, UK) for developing parts of the C++ code of the model.
-
Amandine Roberts for taking her internship with us and working on the project.
-
The open-source community who developed all of the upstream libraries and tools that the workHORSE model uses and on which it depends. It would be impossible to deliver the workHORSE model in such a short time without their work.
-
Sue Povall for being an excellent facilitator at our stakeholder co-production workshops.
-
Dr Eithne Sexton and the Post-Stroke Cognition Research Goup (StrokeCog project) for providing us with unpublished data from analysis carried out for Sexton et al. 203
Contributions of authors
Martin O’Flaherty (https://orcid.org/0000-0001-8944-4131) (Professor of Epidemiology, Department of Public Health and Policy, University of Liverpool) is the lead author for this report and wrote the first and subsequent drafts, with input from other authors.
Ffion Lloyd-Williams (https://orcid.org/0000-0002-9422-8174) (Research Fellow, Department of Public Health and Policy, University of Liverpool) conducted the qualitative work with stakeholders and Lirije Hyseni, wrote Chapter 2 and assisted with and commented on Chapter 3.
Simon Capewell (https://orcid.org/0000-0003-3960-8999) (Professor of Clinical Epidemiology, Fellow, Department of Public Health and Policy, University of Liverpool) supported and provided expertise for project delivery and commented on various drafts of the report.
Angela Boland (https://orcid.org/0000-0002-5435-8644) (Director, Liverpool Reviews and Implementation Group, University of Liverpool) provided expertise as part of the Project Planning Group and for the umbrella review, and contributed to the writing of Chapter 3.
Michelle Maden (https://orcid.org/0000-0003-4419-6343) (Postdoctoral Research Associate, Liverpool Reviews and Implementation Group, University of Liverpool) provided expertise and contributed to the analysis of the umbrella review, and contributed to the writing of Chapter 3.
Brendan Collins (https://orcid.org/0000-0002-3023-8189) (Health Economist – Tenure Track Fellow, Department of Public Health and Policy, University of Liverpool) conducted the health economic work and wrote Chapter 4 with Chris Kypridemos.
Piotr Bandosz (https://orcid.org/0000-0002-6395-6216) (Research Fellow, Department of Public Health and Policy, University of Liverpool) supported the testing of the code and code deployment techniques.
Lirije Hyseni (https://orcid.org/0000-0002-6620-9953) (Research Associate, Department of Public Health and Policy, University of Liverpool) conducted the qualitative work with stakeholders and with Ffion Lloyd-Williams, conducted the research with the best-performing LAs, conducted the umbrella review and wrote Chapter 3 as lead author.
Chris Kypridemos (https://orcid.org/0000-0002-0746-9229) (Senior Lecturer, Department of Public Health and Policy, University of Liverpool) built the workHORSE model, wrote Chapter 4 with Brendan Collins and wrote Chapter 5 with Martin O’Flaherty.
Publications
Kypridemos C, Collins B, McHale P, Bromley H, Parvulescu P, Capewell S, et al. Future cost-effectiveness and equity of the NHS Health Check cardiovascular disease prevention programme: microsimulation modelling using data from Liverpool, UK. PLOS Med 2018;15:e1002573.
Collins B, Kypridemos C, Cookson R, Parvulescu P, McHale P, Guzman-Castillo M, et al. Universal or targeted cardiovascular screening? Modelling study using a sector-specific distributional cost effectiveness analysis. Prev Med 2020;130:105879.
Hyseni L, Guzman-Castillo M, Kypridemos C, Collins B, Schwaller E, Capewell S, et al. Engaging with stakeholders to inform the development of a decision-support tool for the NHS health check programme: qualitative study. BMC Health Serv Res 2020;20:394.
Lloyd-Williams F, Hyseni L, Guzman-Castillo M, Kypridemos C, Collins B, Capewell S, et al. Evaluating stakeholder involvement in building a decision support tool for NHS Health Checks: co-producing the WorkHORSE study. BMC Med Inform Decis Mak 2020;20:182.
Presentations
Hyseni L, Lloyd-Williams F, Guzman-Castillo M, Collins B, Kypridemos C, Capewell S, et al. Engaging with Stakeholders to Inform the Development of a Computer Model for the NHS Health Check Programme: A Qualitative Study. Cardiovascular Disease Prevention Conference 2019: Saving Hearts and Minds Together Conference, Manchester, UK, 14 February 2019.
Hyseni L, Maden M, Boland A, Kypridemos C, Collins B, O’Flaherty M. PP Umbrella Review of Strategies to Improve Uptake of Screening Programmes. Pitch presentation: 12th European Public Health Conference, Marseille, November 2019.
Posters
Collins B, Lloyd-Williams F, Hyseni L, Guzman-Castillo M, Kypridemos C, Capewell S, et al. Evaluating Stakeholder Involvement in Building a Decision Support Tool for NHS Health Checks: Co-Producing workHORSE. PHE Annual Conference, Warwick University, UK, 10 and 11 September 2019, PHE ePoster Library. 9 December 2019; 274322; 131.
Lloyd-Williams F, Hyseni L, Guzman-Castillo M, Kypridemos C, Collins B, Capewell S, et al. P34 evaluating stakeholder involvement in building a decision support tool for NHS Health Checks: co-producing the workHORSE study. J Epidemiol Community Health 2019;73:A87.
Data-sharing statement
Most of the data sources used in building model are in the public domain. The model source code is available, as explained in Chapter 6 and Appendix 8. Requests for access to other data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health and Social Care.
References
- Murray CJ, Richards MA, Newton JN, Fenton KA, Anderson HR, Atkinson C, et al. UK health performance: findings of the Global Burden of Disease Study 2010. Lancet 2013;381:997-1020. https://doi.org/10.1016/S0140-6736(13)60355-4.
- NHS Expert Scientific and Clinical Advisory Panel . Emerging Evidence on the NHS Health Check: Findings and Recommendations n.d. www.healthcheck.nhs.uk/seecmsfile/?id=292 (accessed 10 October 2019).
- Kontis V, Mathers CD, Rehm J, Stevens GA, Shield KD, Bonita R, et al. Contribution of six risk factors to achieving the 25 × 25 non-communicable disease mortality reduction target: a modelling study. Lancet 2014;384:427-37. https://doi.org/10.1016/S0140-6736(14)60616-4.
- NHS England . Cardiovascular Disease (CVD) n.d. www.england.nhs.uk/ourwork/clinical-policy/cvd/ (accessed 17 January 2020).
- Usher-Smith J, Martin A, Harte E, MacLure C, Meads C, Saunders C, et al. NHS Health Check Programme Rapid Evidence Synthesis. Cambridge: University of Cambridge; 2017.
- Robson J, Dostal I, Sheikh A, Eldridge S, Madurasinghe V, Griffiths C, et al. The NHS Health Check in England: an evaluation of the first 4 years. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-008840.
- Department of Health Vascular Policy Team . Economic Modelling for Vascular Checks 2008 2008.
- Hinde S, Bojke L, Richardson G, Retat L, Webber L. The cost-effectiveness of population Health Checks: have the NHS Health Checks been unfairly maligned?. J Public Health 2017;25:425-31. https://doi.org/10.1007/s10389-017-0801-8.
- Crossan C, Lord J, Ryan R, Nherera L, Marshall T. Cost effectiveness of case-finding strategies for primary prevention of cardiovascular disease: a modelling study. Br J Gen Pract 2017;67:e67-e77. https://doi.org/10.3399/bjgp16X687973.
- Chang KC, Vamos EP, Palladino R, Majeed A, Lee JT, Millett C. Impact of the NHS Health Check on inequalities in cardiovascular disease risk: a difference-in-differences matching analysis. J Epidemiol Community Health 2019;73:11-8. https://doi.org/10.1136/jech-2018-210961.
- Kypridemos C, Collins B, McHale P, Bromley H, Parvulescu P, Capewell S, et al. Future cost-effectiveness and equity of the NHS Health Check cardiovascular disease prevention programme: microsimulation modelling using data from Liverpool, UK. PLOS Med 2018;15. https://doi.org/10.1371/journal.pmed.1002573.
- Ministry of Housing, Communities and Local Government . Local Authority Revenue Expenditure and Financing England: 2018 to 2019. Budget Individual Local Authority Data n.d. www.gov.uk/government/statistics/local-authority-revenue-expenditure-and-financing-england-2018-to-2019-budget-individual-local-authority-data (accessed 17 January 2020).
- UK Government . The Local Authorities (Public Health Functions and Entry to Premises by Local Healthwatch Representatives) Regulations 2013 n.d. www.legislation.gov.uk/uksi/2013/351/contents/made (accessed 17 January 2020).
- NHS England . NHS Long Term Plan n.d. www.longtermplan.nhs.uk (accessed 12 February 2020).
- Artac M, Dalton AR, Babu H, Bates S, Millett C, Majeed A. Primary care and population factors associated with NHS Health Check coverage: a national cross-sectional study. J Public Health 2013;35:431-9. https://doi.org/10.1093/pubmed/fdt069.
- McNaughton RJ, Shucksmith J. Reasons for (non)compliance with intervention following identification of ‘high-risk’ status in the NHS Health Check programme. J Public Health 2015;37:218-25. https://doi.org/10.1093/pubmed/fdu066.
- Baker C, Loughren EA, Crone D, Kallfa N. A process evaluation of the NHS Health Check care pathway in a primary care setting. J Public Health 2015;37:202-9. https://doi.org/10.1093/pubmed/fdv053.
- Dalton AR, Soljak M, Samarasundera E, Millett C, Majeed A. Prevalence of cardiovascular disease risk amongst the population eligible for the NHS Health Check Programme. Eur J Prev Cardiol 2013;20:142-50. https://doi.org/10.1177/1741826711428797.
- Dalton AR, Bottle A, Okoro C, Majeed A, Millett C. Uptake of the NHS Health Checks programme in a deprived, culturally diverse setting: cross-sectional study. J Public Health 2011;33:422-9. https://doi.org/10.1093/pubmed/fdr034.
- Robson J, Dostal I, Madurasinghe V, Sheikh A, Hull S, Boomla K, et al. The NHS Health Check programme: implementation in east London 2009–2011. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-007578.
- Ebrahim S, Taylor F, Ward K, Beswick A, Burke M, Davey Smith G. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database Syst Rev 2011;1. https://doi.org/10.1002/14651858.CD001561.pub3.
- Jørgensen T, Jacobsen RK, Toft U, Aadahl M, Glümer C, Pisinger C. Effect of screening and lifestyle counselling on incidence of ischaemic heart disease in general population: Inter99 randomised trial. BMJ 2014;348. https://doi.org/10.1136/bmj.g3617.
- Lee JT, Lawson KD, Wan Y, Majeed A, Morris S, Soljak M, et al. Are cardiovascular disease risk assessment and management programmes cost effective? A systematic review of the evidence. Prev Med 2017;99:49-57. https://doi.org/10.1016/j.ypmed.2017.01.005.
- Government Office for Science . Computational Modelling: Blackett Review n.d. www.gov.uk/government/publications/computational-modelling-blackett-review (accessed 31 January 2020).
- Davis SM. Chief Medical Officer Annual Report 2018: Better Health Within Reach n.d. www.gov.uk/government/publications/chief-medical-officer-annual-report-2018-better-health-within-reach (accessed 17 January 2020).
- Mytton OT, Jackson C, Steinacher A, Goodman A, Langenberg C, Griffin S, et al. The current and potential health benefits of the National Health Service Health Check cardiovascular disease prevention programme in England: a microsimulation study. PLOS Med 2018;15. https://doi.org/10.1371/journal.pmed.1002517.
- Kypridemos C, Allen K, Hickey GL, Guzman-Castillo M, Bandosz P, Buchan I, et al. Cardiovascular screening to reduce the burden from cardiovascular disease: microsimulation study to quantify policy options. BMJ 2016;353. https://doi.org/10.1136/bmj.i2793.
- Chartered Institute of Public Finance and Accountancy . Evaluating Preventative Investments in Public Health in England n.d. www.cipfa.org/policy-and-guidance/reports/evaluating-preventative-investments (accessed 26 November 2019).
- HM Treasury . The Green Book: Appraisal and Evaluation in Central Government n.d. www.gov.uk/government/publications/the-green-book-appraisal-and-evaluation-in-central-governent (accessed 31 January 2020).
- Allender S, Owen B, Kuhlberg J, Lowe J, Nagorcka-Smith P, Whelan J, et al. A community based systems diagram of obesity causes. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0129683.
- Frerichs L, Lich KH, Dave G, Corbie-Smith G. Integrating systems science and community-based participatory research to achieve health equity. Am J Public Health 2016;106:215-22. https://doi.org/10.2105/AJPH.2015.302944.
- Freebairn L, Atkinson JA, Kelly PM, McDonnell G, Rychetnik L. Decision makers’ experience of participatory dynamic simulation modelling: methods for public health policy. BMC Med Inform Decis Mak 2018;18. https://doi.org/10.1186/s12911-018-0707-6.
- van der Graaf P, Forrest LF, Adams J, Shucksmith J, White M. How do public health professionals view and engage with research? A qualitative interview study and stakeholder workshop engaging public health professionals and researchers. BMC Public Health 2017;17. https://doi.org/10.1186/s12889-017-4896-1.
- Morton KL, Atkin AJ, Corder K, Suhrcke M, Turner D, van Sluijs EM. Engaging stakeholders and target groups in prioritising a public health intervention: the Creating Active School Environments (CASE) online Delphi study. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013340.
- Cairney P, Oliver K. Evidence-based policymaking is not like evidence-based medicine, so how far should you go to bridge the divide between evidence and policy?. Health Res Policy Syst 2017;15. https://doi.org/10.1186/s12961-017-0192-x.
- Oliver K, Kothari A, Mays N. The dark side of coproduction: do the costs outweigh the benefits for health research?. Health Res Policy Syst 2019;17. https://doi.org/10.1186/s12961-019-0432-3.
- Andersen DF, Richardson GP. Scripts for group model building. Syst Dyn Rev 1997;13:107-29. https://doi.org/10.1002/(SICI)1099-1727(199722)13:2<107::AID-SDR120>3.0.CO;2-7.
- WikiBooks . Scriptapedia n.d. https://en.wikibooks.org/wiki/Scriptapedia (accessed 17 January 2020).
- Hovmand PS, Andersen DF, Rouwette E, Richardson GP, Rux K, Calhoun A. Group model-building ‘scripts’ as a collaborative planning tool. Syst Res Behav Sci 2012;29:179-93. https://doi.org/10.1002/sres.2105.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Barker D, Clegg R. Fast-Track: A Rad Approach (Case Method). Boston, MA: Addison–Wesley; 1994.
- Lloyd-Williams F, Hyseni L, Guzman-Castillo M, Kypridemos C, Collins B, Capewell S, et al. Evaluating stakeholder involvement in building a decision support tool for NHS Health Checks: co-producing the WorkHORSE study. BMC Med Inform Decis Mak 2020;20. https://doi.org/10.1186/s12911-020-01205-y.
- Hyseni L, Guzman-Castillo M, Kypridemos C, Collins B, Schwaller E, Capewell S, et al. Engaging with stakeholders to inform the development of a decision-support tool for the NHS health check programme: qualitative study. BMC Health Serv Res 2020;20. https://doi.org/10.1186/s12913-020-05268-5.
- Raffle AE, Mackie A, Gray JAM. Screening: Evidence and Practice. New York, NY: Oxford University Press; 2019.
- UK Government . Population Screening Programmes: Detailed Information n.d. www.gov.uk/topic/population-screening-programmes (accessed 2 December 2019).
- Ferroni E, Camilloni L, Jimenez B, Furnari G, Borgia P, Guasticchi G, et al. How to increase uptake in oncologic screening: a systematic review of studies comparing population-based screening programs and spontaneous access. Prev Med 2012;55:587-96. https://doi.org/10.1016/j.ypmed.2012.10.007.
- Ponti, A, Anttilla A, Ronco G, Senore C. Cancer Screening in the European Union. Report on the Implementation of Council Recommendation on Cancer Screening 2017.
- NHS England . Review of National Cancer Screening Programmes in England n.d. www.england.nhs.uk/publication/terms-of-reference-review-national-cancer-screening-programmes-england/ (accessed 31 January 2020).
- Public Health England . NHS Population Screening: Inequalities Strategy n.d. www.gov.uk/government/publications/nhs-population-screening-inequalities-strategy (accessed 13 February 2020).
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339. https://doi.org/10.1136/bmj.b2535.
- Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc 2015;13:132-40. https://doi.org/10.1097/XEB.0000000000000055.
- Rat C, Latour C, Rousseau R, Gaultier A, Pogu C, Edwards A, et al. Interventions to increase uptake of faecal tests for colorectal cancer screening: a systematic review. Eur J Cancer Prev 2018;27:227-36. https://doi.org/10.1097/CEJ.0000000000000344.
- Teo CH, Ling CJ, Ng CJ. Improving health screening uptake in men: a systematic review and meta-analysis. Am J Prev Med 2018;54:133-43. https://doi.org/10.1016/j.amepre.2017.08.028.
- Cheong AT, Liew SM, Khoo EM, Mohd Zaidi NF, Chinna K. Are interventions to increase the uptake of screening for cardiovascular disease risk factors effective? A systematic review and meta-analysis. BMC Fam Pract 2017;18. https://doi.org/10.1186/s12875-016-0579-8.
- Verdoodt F, Jentschke M, Hillemanns P, Racey CS, Snijders PJF, Arbyn M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: a systematic review and meta-analysis of randomised trials. Eur J Cancer Oxf Engl 1990 2015;51:2375-85. https://doi.org/10.1016/j.ejca.2015.07.006.
- Camilloni L, Ferroni E, Cendales BJ, Pezzarossi A, Furnari G, Borgia P, et al. Methods to increase participation in organised screening programs: a systematic review. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-464.
- Everett T, Bryant A, Griffin MF, Martin-Hirsch PP, Forbes CA, Jepson RG. Interventions targeted at women to encourage the uptake of cervical screening. Cochrane Database Syst Rev 2011;5. https://doi.org/10.1002/14651858.CD002834.pub2.
- Khalid-de Bakker C, Jonkers D, Smits K, Mesters I, Masclee A, Stockbrügger R. Participation in colorectal cancer screening trials after first-time invitation: a systematic review. Endoscopy 2011;43:1059-86. https://doi.org/10.1055/s-0031-1291430.
- Denhaerynck K, Lesaffre E, Baele J, Cortebeeck K, Van Overstraete E, Buntinx F. Mammography screening attendance: meta-analysis of the effect of direct-contact invitation. Am J Prev Med 2003;25:195-203. https://doi.org/10.1016/S0749-3797(03)00201-0.
- Black ME, Yamada J, Mann V. A systematic literature review of the effectiveness of community-based strategies to increase cervical cancer screening. Can J Public Health 2002;93:386-93. https://doi.org/10.1007/BF03404575.
- Bonfill X, Marzo M, Pladevall M, Martí J, Emparanza JI. Strategies for increasing women participation in community breast cancer screening. Cochrane Database Syst Rev 2001;1.
- Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J. The determinants of screening uptake and interventions for increasing uptake: a systematic review. Health Technol Assess 2000;4. https://doi.org/10.3310/hta4140.
- Sin JP, Leger AS. Interventions to Increase Breast Screening Uptake: Do They Make Any Difference. York: Centre for Reviews and Dissemination; 1999.
- Issaka RB, Avila P, Whitaker E, Bent S, Somsouk M. Population health interventions to improve colorectal cancer screening by fecal immunochemical tests: a systematic review. Prev Med 2019;118:113-21. https://doi.org/10.1016/j.ypmed.2018.10.021.
- Dougherty MK, Brenner AT, Crockett SD, Gupta S, Wheeler SB, Coker-Schwimmer M, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the united states: a systematic review and meta-analysis. JAMA Intern Med 2018;178:1645-58. https://doi.org/10.1001/jamainternmed.2018.4637.
- Rees I, Jones D, Chen H, Macleod U. Interventions to improve the uptake of cervical cancer screening among lower socioeconomic groups: a systematic review. Prev Med 2018;111:323-35. https://doi.org/10.1016/j.ypmed.2017.11.019.
- Uy C, Lopez J, Trinh-Shevrin C, Kwon SC, Sherman SE, Liang PS. Text messaging interventions on cancer screening rates: a systematic review. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.7893.
- Albrow R, Blomberg K, Kitchener H, Brabin L, Patnick J, Tishelman C, et al. Interventions to improve cervical cancer screening uptake amongst young women: a systematic review. Acta Oncol 2014;53:445-51. https://doi.org/10.3109/0284186X.2013.869618.
- Sabatino SA, Lawrence B, Elder R, Mercer SL, Wilson KM, DeVinney B, et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med 2012;43:97-118. https://doi.org/10.1016/j.amepre.2012.04.009.
- Brouwers MC, De Vito C, Bahirathan L, Carol A, Carroll JC, Cotterchio M, et al. What implementation interventions increase cancer screening rates? A systematic review. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-111.
- Vernon SW, McQueen A, Tiro JA, del Junco DJ. Interventions to promote repeat breast cancer screening with mammography: a systematic review and meta-analysis. J Natl Cancer Inst 2010;102:1023-39. https://doi.org/10.1093/jnci/djq223.
- Stone EG, Morton SC, Hulscher ME, Maglione MA, Roth EA, Grimshaw JM, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med 2002;136:641-51. https://doi.org/10.7326/0003-4819-136-9-200205070-00006.
- Tseng DS, Cox E, Plane MB, Hla KM. Efficacy of patient letter reminders on cervical cancer screening: a meta-analysis. J Gen Intern Med 2001;16:563-8. https://doi.org/10.1046/j.1525-1497.2001.016008567.x.
- Yabroff KR, Mandelblatt JS. Interventions targeted toward patients to increase mammography use. Cancer Epidemiol Biomark Prev Publ 1999;8:749-57.
- Lu M, Moritz S, Lorenzetti D, Sykes L, Straus S, Quan H. A systematic review of interventions to increase breast and cervical cancer screening uptake among Asian women. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-413.
- Han HR, Lee JE, Kim J, Hedlin HK, Song H, Kim MT. A meta-analysis of interventions to promote mammography among ethnic minority women. Nurs Res 2009;58:246-54. https://doi.org/10.1097/NNR.0b013e3181ac0f7f.
- Han HR, Kim J, Lee JE, Hedlin HK, Song H, Song Y, et al. Interventions that increase use of Pap tests among ethnic minority women: a meta-analysis. Psychooncology 2011;20:341-51. https://doi.org/10.1002/pon.1754.
- Legler J, Meissner HI, Coyne C, Breen N, Chollette V, Rimer BK. The effectiveness of interventions to promote mammography among women with historically lower rates of screening. Cancer Epidemiol Biomarkers Prev 2002;11:59-71.
- Musa J, Achenbach CJ, O’Dwyer LC, Evans CT, McHugh M, Hou L, et al. Effect of cervical cancer education and provider recommendation for screening on screening rates: a systematic review and meta-analysis. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0183924.
- Jager M, Demb J, Asghar A, Selby K, Mello EM, Heskett KM, et al. Mailed outreach is superior to usual care alone for colorectal cancer screening in the USA: a systematic review and meta-analysis. Dig Dis Sci 2019;64:2489-96. https://doi.org/10.1007/s10620-019-05587-6.
- Mandelblatt JS, Yabroff KR. Effectiveness of interventions designed to increase mammography use: a meta-analysis of provider-targeted strategies. Cancer Epidemiol Biomarkers Prev 1999;8:759-67.
- Gardner MP, Adams A, Jeffreys M. Interventions to increase the uptake of mammography amongst low income women: a systematic review and meta-analysis. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0055574.
- Anastasi N, Lusher J. The impact of breast cancer awareness interventions on breast screening uptake among women in the United Kingdom: a systematic review. J Health Psychol 2019;24:113-24. https://doi.org/10.1177/1359105317697812.
- Agide FD, Garmaroudi G, Sadeghi R, Shakibazadeh E, Yaseri M, Koricha ZB, et al. A systematic review of the effectiveness of health education interventions to increase cervical cancer screening uptake. Eur J Public Health 2018;28:1156-62. https://doi.org/10.1093/eurpub/cky197.
- Volk RJ, Linder SK, Lopez-Olivo MA, Kamath GR, Reuland DS, Saraykar SS, et al. Patient decision aids for colorectal cancer screening: a systematic review and meta-analysis. Am J Prev Med 2016;51:779-91. https://doi.org/10.1016/j.amepre.2016.06.022.
- Ivlev I, Jerabkova S, Mishra M, Cook LA, Eden KB. Prostate cancer screening patient decision aids: a systematic review and meta-analysis. Am J Prev Med 2018;55:896-907. https://doi.org/10.1016/j.amepre.2018.06.016.
- Volk RJ, Hawley ST, Kneuper S, Holden EW, Stroud LA, Cooper CP, et al. Trials of decision aids for prostate cancer screening: a systematic review. Am J Prev Med 2007;33:428-34. https://doi.org/10.1016/j.amepre.2007.07.030.
- Evans R, Edwards A, Brett J, Bradburn M, Watson E, Austoker J, et al. Reduction in uptake of PSA tests following decision aids: systematic review of current aids and their evaluations. Patient Educ Couns 2005;58:13-26. https://doi.org/10.1016/j.pec.2004.06.009.
- Kelly C, Pericleous M, Hendy J, de Lusignan S, Ahmed A, Vandrevala T, et al. Interventions to improve the uptake of screening across a range of conditions in ethnic minority groups: a systematic review. Int J Clin Pract 2018;72. https://doi.org/10.1111/ijcp.13202.
- Hou SI, Sealy DA, Kabiru CW. Closing the disparity gap: cancer screening interventions among Asians – a systematic literature review. Asian Pac J Cancer Prev 2011;12:3133-9.
- Usher-Smith JA, Silarova B, Sharp SJ, Mills K, Griffin SJ. Effect of interventions incorporating personalised cancer risk information on intentions and behaviour: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-017717.
- Edwards AG, Naik G, Ahmed H, Elwyn GJ, Pickles T, Hood K, et al. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev 2013;2. https://doi.org/10.1002/14651858.CD001865.pub3.
- Albada A, Ausems MG, Bensing JM, van Dulmen S. Tailored information about cancer risk and screening: a systematic review. Patient Educ Couns 2009;77:155-71. https://doi.org/10.1016/j.pec.2009.03.005.
- Bellhouse S, McWilliams L, Firth J, Yorke J, French DP. Are community-based health worker interventions an effective approach for early diagnosis of cancer? A systematic review and meta-analysis. Psychooncology 2018;27:1089-99. https://doi.org/10.1002/pon.4575.
- Wells KJ, Luque JS, Miladinovic B, Vargas N, Asvat Y, Roetzheim RG, et al. Do community health worker interventions improve rates of screening mammography in the United States? A systematic review. Cancer Epidemiol Biomarkers Prev 2011;20:1580-98. https://doi.org/10.1158/1055-9965.EPI-11-0276.
- Mojica CM, Parra-Medina D, Vernon S. Interventions promoting colorectal cancer screening among Latino men: a systematic review. Prev Chronic Dis 2018;15. https://doi.org/10.5888/pcd15.170218.
- Agide FD, Sadeghi R, Garmaroudi G, Tigabu BM. A systematic review of health promotion interventions to increase breast cancer screening uptake: from the last 12 years. Eur J Public Health 2018;28:1149-55. https://doi.org/10.1093/eurpub/ckx231.
- Secginli S, Nahcivan NO, Gunes G, Fernandez R. Interventions promoting breast cancer screening among Turkish women with global implications: a systematic review. Worldviews Evid Based Nurs 2017;14:316-23. https://doi.org/10.1111/wvn.12245.
- Baron RC, Rimer BK, Breslow RA, Coates RJ, Kerner J, Melillo S, et al. Client-directed interventions to increase community demand for breast, cervical, and colorectal cancer screening a systematic review. Am J Prev Med 2008;35:34-55. https://doi.org/10.1016/j.amepre.2008.04.002.
- Luque JS, Logan A, Soulen G, Armeson KE, Garrett DM, Davila CB, et al. Systematic review of mammography screening educational interventions for Hispanic women in the United States. J Cancer Educ 2019;34:412-22. https://doi.org/10.1007/s13187-018-1321-0.
- Saei Ghare Naz M, Kariman N, Ebadi A, Ozgoli G, Ghasemi V, Rashidi Fakari F. Educational interventions for cervical cancer screening behavior of women: a systematic review. Asian Pac J Cancer Prev 2018;19:875-84. https://doi.org/10.22034/APJCP.2018.19.4.875.
- Chan DN, So WK. A systematic review of randomised controlled trials examining the effectiveness of breast and cervical cancer screening interventions for ethnic minority women. Eur J Oncol Nurs 2015;19:536-53. https://doi.org/10.1016/j.ejon.2015.02.015.
- Corcoran J, Dattalo P, Crowley M. Cervical cancer screening interventions for U.S. Latinas: a systematic review. Health Soc Work 2012;37:197-205. https://doi.org/10.1093/hsw/hls035.
- Morrow JB, Dallo FJ, Julka M. Community-based colorectal cancer screening trials with multi-ethnic groups: a systematic review. J Community Health 2010;35:592-601. https://doi.org/10.1007/s10900-010-9247-4.
- Siddiqui MR, Sajid MS, Khatri K, Kanri B, Cheek E, Baig MK. The role of physician reminders in faecal occult blood testing for colorectal cancer screening. Eur J Gen Pract 2011;17:221-8. https://doi.org/10.3109/13814788.2011.601412.
- Baron RC, Melillo S, Rimer BK, Coates RJ, Kerner J, Habarta N, et al. Intervention to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers a systematic review of provider reminders. Am J Prev Med 2010;38:110-17. https://doi.org/10.1016/j.amepre.2009.09.031.
- Copeland VC, Kim YJ, Eack SM. Effectiveness of interventions for breast cancer screening in African American women: a meta-analysis. Health Serv Res 2018;53:3170-88. https://doi.org/10.1111/1475-6773.12806.
- Escribà-Agüir V, Rodríguez-Gómez M, Ruiz-Pérez I. Effectiveness of patient-targeted interventions to promote cancer screening among ethnic minorities: a systematic review. Cancer Epidemiol 2016;44:22-39. https://doi.org/10.1016/j.canep.2016.07.009.
- Donnelly TT, Hwang J. Breast cancer screening interventions for Arabic women: a literature review. J Immigr Minor Health 2015;17:925-39. https://doi.org/10.1007/s10903-013-9902-9.
- Escoffery C, Rodgers KC, Kegler MC, Haardörfer R, Howard DH, Liang S, et al. A systematic review of special events to promote breast, cervical and colorectal cancer screening in the United States. BMC Public Health 2014;14. https://doi.org/10.1186/1471-2458-14-274.
- Corcoran J, Dattalo P, Crowley M. Interventions to increase mammography rates among U.S. Latinas: a systematic review. J Womens Health 2010;19:1281-8. https://doi.org/10.1089/jwh.2009.1621.
- The Health Foundation . Complex Adaptive Systems n.d. www.health.org.uk/publications/complex-adaptive-systems (accessed 5 February 2020).
- Katerndahl DA. Lessons from Jurassic Park: patients as complex adaptive systems. J Eval Clin Pract 2009;15:755-60. https://doi.org/10.1111/j.1365-2753.2009.01228.x.
- Javanparast S, Ward P, Young G, Wilson C, Carter S, Misan G, et al. How equitable are colorectal cancer screening programs which include FOBTs? A review of qualitative and quantitative studies. Prev Med 2010;50:165-72. https://doi.org/10.1016/j.ypmed.2010.02.003.
- Macintyre S. Inequalities in Health in Scotland: What Are They and What Can We Do About Them 2007. http://eprints.gla.ac.uk/81903/ (accessed 17 January 2020).
- White M, Adams J, Heywood P. How and Why Do Interventions That Increase Health Overall Widen Inequalities Within Populations?. Bristol: Policy Press; 2009.
- Asaria M, Griffin S, Cookson R, Whyte S, Tappenden P. Distributional cost-effectiveness analysis of health care programmes – a methodological case study of the UK Bowel Cancer Screening Programme. Health Econ 2015;24:742-54. https://doi.org/10.1002/hec.3058.
- Rutter H, Savona N, Glonti K, Bibby J, Cummins S, Finegood DT, et al. The need for a complex systems model of evidence for public health. Lancet 2017;390:2602-4. https://doi.org/10.1016/S0140-6736(17)31267-9.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Perry C, Chhatralia K, Damesick D, Hobden S, Volpe L. Behavioural Insights in Health Care n.d. www.health.org.uk/publications/behavioural-insights-in-health-care (accessed 31 January 2020).
- Mindell J, Biddulph JP, Hirani V, Stamatakis E, Craig R, Nunn S, et al. Cohort profile: the Health Survey for England. Int J Epidemiol 2012;41:1585-93. https://doi.org/10.1093/ije/dyr199.
- Great Britain . Health and Social Care Act 2012 2012. https://doi.org/10.12968/eqhe.2012.1.7.5.
- Office for National Statistics . Lower Layer Super Output Area Population Estimates (Supporting Information) n.d. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/lowersuperoutputareamidyearpopulationestimates (accessed 14 December 2018).
- Office for National Statistics . Ethnic Group by Sex by Age 2013. www.nomisweb.co.uk/census/2011/lc2101ew (accessed 11 February 2020).
- Office for National Statistics . Population Projections – Local Authorities: SNPP Z1 n.d. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/datasets/localauthoritiesinenglandz1 (accessed 13 December 2018).
- Office for National Statistics . National Population Projections n.d. www.ons.gov.uk/ons/publications/all-releases.html?definition=tcm%3A77-21600 (accessed 13 March 2014).
- UK Data Service . Health Survey for England, 2003 n.d. https://doi.org/10.5255/UKDA-SN-5098-1 (accessed 1 May 2014).
- UK Data Service . Health Survey for England, 2004 n.d. https://doi.org/10.5255/UKDA-SN-5439-1 (accessed 1 May 2014).
- UK Data Service . Health Survey for England, 2005 n.d. https://doi.org/10.5255/UKDA-SN-5675-1 (accessed 1 May 2014).
- UK Data Service . Health Survey for England, 2006 n.d. https://doi.org/10.5255/UKDA-SN-5809-1 (accessed 1 May 2014).
- UK Data Service . Health Survey for England, 2007 n.d. https://doi.org/10.5255/UKDA-SN-6112-1 (accessed 1 May 2014).
- UK Data Service . Health Survey for England, 2008 n.d. https://doi.org/10.5255/UKDA-SN-6397-2 (accessed 1 May 2014).
- UK Data Service . Health Survey for England, 2009 n.d. https://doi.org/10.5255/UKDA-SN-6732-1 (accessed 1 May 2014).
- UK Data Service . Health Survey for England, 2010 n.d. https://doi.org/10.5255/UKDA-SN-6986-2 (accessed 1 May 2014).
- UK Data Service . Health Survey for England, 2011 n.d. https://doi.org/10.5255/UKDA-SN-7260-1 (accessed 1 May 2014).
- UK Data Service . Health Survey for England, 2012 n.d. https://doi.org/10.5255/UKDA-SN-7480-1 (accessed 1 May 2014).
- UK Data Service . Health Survey for England, 2013 n.d. https://doi.org/10.5255/UKDA-SN-7649-1 (accessed 1 May 2016).
- UK Data Service . Health Survey for England, 2014 n.d. https://doi.org/10.5255/UKDA-SN-7919-1 (accessed 1 May 2016).
- Rahman A. Estimating small area health-related characteristics of populations: a methodological review. Geospat Health 2017;12. https://doi.org/10.4081/gh.2017.495.
- Stasinopoulos MD, Rigby RA, Heller GZ, Voudouris V, De Bastiani F. Flexible Regression and Smoothing: Using GAMLSS in R. Boca Raton, FL: CRC Press/Taylor & Francis Group; 2017.
- Rigby RA, Stasinopoulos MD, Heller GZ, De Bastiani F. Distributions for Modelling Location, Scale, and Shape Using GAMLSS in R. New York, NY: CRC Press, LLC; 2019.
- Suen S, Goldhaber-Fiebert JD, Basu S. Matching microsimulation risk factor correlations to cross-sectional data: the shortest distance method. Med Decis Making 2018;38:452-64. https://doi.org/10.1177/0272989X17741635.
- Embrechts P, Lindskog F, Mcneil A, Rachev ST. Handbook of Heavy Tailed Distributions in Finance. Amsterdam: Elsevier; 2003.
- Levin ML. The occurrence of lung cancer in man. Acta – Unio Int Contra Cancrum 1953;9:531-41.
- International Statistical Classification Of Diseases and Related Health Problems – 10th Revision. Geneva; 2016.
- Pujades-Rodriguez M, Timmis A, Stogiannis D, Rapsomaniki E, Denaxas S, Shah A, et al. Socioeconomic deprivation and the incidence of 12 cardiovascular diseases in 1.9 million women and men: implications for risk prediction and prevention. PLOS One 2014;9. https://doi.org/10.1371/journal.pone.0104671.
- Barendregt JJ, Van Oortmarssen GJ, Vos T, Murray CJ. A generic model for the assessment of disease epidemiology: the computational basis of DisMod II. Popul Health Metr 2003;1. https://doi.org/10.1186/1478-7954-1-4.
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224-60. https://doi.org/10.1016/S0140-6736(12)61766-8.
- Boshuizen HC, Lhachimi SK, van Baal PH, Hoogenveen RT, Smit HA, Mackenbach JP, et al. The DYNAMO-HIA model: an efficient implementation of a risk factor/chronic disease Markov model for use in Health Impact Assessment (HIA). Demography 2012;49:1259-83. https://doi.org/10.1007/s13524-012-0122-z.
- Hippisley-Cox J, Coupland C, Brindle P. The performance of seven QPrediction risk scores in an independent external sample of patients from general practice: a validation study. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-005809.
- Hyndman RJ. R Package ‘Demography’: Forecasting Mortality, Fertility, Migration and Population Data 2017. https://cran.r-project.org/web/packages/demography/demography.pdf (accessed 10 October 2019).
- Hyndman RJ, Shahid Ullah M. Robust forecasting of mortality and fertility rates: a functional data approach. Comput Stat Data Anal 2007;51:4942-56. https://doi.org/10/c4cgvx.
- Stringhini S, Carmeli C, Jokela M, Avendaño M, Muennig P, Guida F, et al. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1.7 million men and women. Lancet 2017;389:1229-37. https://doi.org/10.1016/S0140-6736(16)32380-7.
- Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326. https://doi.org/10.1136/bmj.326.7404.1423.
- Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735-42. https://doi.org/10.1016/S0140-6736(09)61965-6.
- King W, Lacey A, White J, Farewell D, Dunstan F, Fone D. Socioeconomic inequality in medication persistence in primary and secondary prevention of coronary heart disease – a population-wide electronic cohort study. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0194081.
- Wallach-Kildemoes H, Andersen M, Diderichsen F, Lange T. Adherence to preventive statin therapy according to socioeconomic position. Eur J Clin Pharmacol 2013;69:1553-63. https://doi.org/10.1007/s00228-013-1488-6.
- Janssen B, Szende A, Szende A, Janssen B, Cabases J. Self-Reported Population Health: An International Perspective Based on EQ-5D 2014:19-30.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Mak 2011;31:800-4. https://doi.org/10.1177/0272989X11401031.
- Licchetta M, Stelmach M. Fiscal Sustainability Analytical Paper: Fiscal Sustainability and Public Spending on Health. London: Office for Budget Responsibility; 2016.
- Davies A, Bardsley M, Davies S, Georghiou T. Understanding Patterns of Health and Social Care at the End of Life. London: Nuffield Trust; 2017.
- Rowen D, Dixon S, Hernández-Alava M, Mukuria C. Estimating informal care inputs associated with EQ-5D for use in economic evaluation. Eur J Health Econ 2016;17:733-44. https://doi.org/10.1007/s10198-015-0718-5.
- Claxton K, Sculpher M, Palmer S, Culyer AJ. Causes for concern: is NICE failing to uphold its responsibilities to all NHS patients?. Health Econ 2015;24:1-7. https://doi.org/10.1002/hec.3130.
- Collins B, Kypridemos C, Cookson R, Parvulescu P, McHale P, Guzman-Castillo M, et al. Universal or targeted cardiovascular screening? Modelling study using a sector-specific distributional cost effectiveness analysis. Prev Med 2020;130. https://doi.org/10.1016/j.ypmed.2019.105879.
- Mackenbach JP, Kunst AE. Measuring the magnitude of socio-economic inequalities in health: an overview of available measures illustrated with two examples from Europe. Soc Sci Med 1997;44:757-71. https://doi.org/10.1016/S0277-9536(96)00073-1.
- Collins B, Griffin S, Asaria M, Capewell S, Love-Koh J, Kypridemos C, et al. How Do We Include Health Inequality Impacts in Economic Analysis of Policy Options n.d. www.york.ac.uk/che/news/news-2018/how-do-we-include-health-inequality-impacts/ (accessed 31 January 2020).
- Groot Koerkamp B, Stijnen T, Weinstein MC, Hunink MG. The combined analysis of uncertainty and patient heterogeneity in medical decision models. Med Decis Making 2011;31:650-61. https://doi.org/10.1177/0272989X10381282.
- Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. ISPOR-SMDM Modeling Good Research Practices Task Force . Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force – 6. Value Health 2012;15:835-42. https://doi.org/10.1016/j.jval.2012.04.014.
- Krogsbøll LT, Jørgensen KJ, Gøtzsche PC. General health checks in adults for reducing morbidity and mortality from disease. Cochrane Database Syst Rev 2019;1. https://doi.org/10.1002/14651858.CD009009.pub3.
- Gidlow C, Ellis N, Randall J, Cowap L, Smith G, Iqbal Z, et al. Method of invitation and geographical proximity as predictors of NHS Health Check uptake. J Public Health 2015;37:195-201. https://doi.org/10.1093/pubmed/fdu092.
- Attwood S, Morton K, Sutton S. Exploring equity in uptake of the NHS Health Check and a nested physical activity intervention trial. J Public Health 2016;38:560-8. https://doi.org/10.1093/pubmed/fdv070.
- Public Health England . Cardiovascular Disease Profiles n.d. https://fingertips.phe.org.uk/profile-group/cardiovascular-disease-diabetes-kidney-disease/profile/cardiovascular/data#page/0/page-options/ovw-do-0 (accessed 1 March 2021).
- Sallis A, Gold N, Agbebiyi A, James RJE, Berry D, Bonus A, et al. Increasing uptake of National Health Service Health Checks in primary care: a pragmatic randomized controlled trial of enhanced invitation letters in Northamptonshire, England. J Public Health 2021;43:e92-e99. https://doi.org/10.1093/pubmed/fdz134.
- Department of Health and Social Care . Advancing Our Health: Prevention in the 2020s – Consultation Document 2019.
- Palmer AJ, Si L, Tew M, Hua X, Willis MS, Asseburg C, et al. Computer modeling of diabetes and its transparency: a report on the eighth mount hood challenge. Value Health 2018;21:724-31. https://doi.org/10.1016/j.jval.2018.02.002.
- Bandosz P, Ahmadi-Abhari S, Guzman-Castillo M, Pearson-Stuttard J, Collins B, Whittaker H, et al. Potential impact of diabetes prevention on mortality and future burden of dementia and disability: a modelling study. Diabetologia 2020;63:104-15. https://doi.org/10.1007/s00125-019-05015-4.
- Capewell S, O’Flaherty M. Rapid mortality falls after risk-factor changes in populations. Lancet 2011;378:752-3. https://doi.org/10.1016/S0140-6736(10)62302-1.
- Crandall JP, Mather K, Rajpathak SN, Goldberg RB, Watson K, Foo S, et al. Statin use and risk of developing diabetes: results from the Diabetes Prevention Program. BMJ Open Diabetes Res Care 2017;5. https://doi.org/10.1136/bmjdrc-2017-000438.
- Casula M, Mozzanica F, Scotti L, Tragni E, Pirillo A, Corrao G, et al. Statin use and risk of new-onset diabetes: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis 2017;27:396-40. https://doi.org/10.1016/j.numecd.2017.03.001.
- Capewell S. Will screening individuals at high risk of cardiovascular events deliver large benefits? No. BMJ 2008;337. https://doi.org/10.1136/bmj.a1395.
- Calder M, Craig C, Culley D, de Cani R, Donnelly CA, Douglas R, et al. Computational modelling for decision-making: where, why, what, who and how. R Soc Open Sci 2018;5. https://doi.org/10.1098/rsos.172096.
- Gilbert N, Ahrweiler P, Barbrook-Johnson P, Narasimhan KP, Wilkinson H. Computational modelling of public policy: reflections on practice. J Artif Soc Soc Simul 2018;21. https://doi.org/10.18564/jasss.3669.
- Ruiz M, Zabaleta N, Elorza U. Decision Making Through Simulation in Public Policy Management Field n.d. https://doi.org/10.21125/inted.2016.0911.
- Kneale D, Rojas-García A, Raine R, Thomas J. The use of evidence in English local public health decision-making: a systematic scoping review. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0577-9.
- Public Health England . Recent Trends in Mortality in England: Review and Data Packs 2018.
- Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med 2015;13. https://doi.org/10.1186/s12916-015-0465-6.
- Spaniol MJ, Rowland NJ. Defining scenario. Futur Foresight Sci 2019;1. https://doi.org/10.1002/ffo2.3.
- Durance P, Godet M. Scenario building: uses and abuses. Technol Forecast Soc Change 2010;77:1488-92. https://doi.org/10.1016/j.techfore.2010.06.007.
- Brailsford SC, Bolt T, Connell C, Klein JH, Patel B. Stakeholder Engagement in Health Care Simulation n.d. https://doi.org/10.1109/WSC.2009.5429190.
- Jahangirian M, Taylor SJE, Eatock J, Stergioulas LK, Taylor PM. Causal study of low stakeholder engagement in healthcare simulation projects. J Oper Res Soc 2015;66:369-79. https://doi.org/10.1057/jors.2014.1.
- Jahangirian M, Borsci S, Shah SGS, Taylor SJE. Causal factors of low stakeholder engagement: a survey of expert opinions in the context of healthcare simulation projects. Simulation 2015;91:511-26. https://doi.org/10.1177/0037549715583150.
- Caro JJ, Briggs AH, Siebert U, Kuntz KM. ISPOR-SMDM Modeling Good Research Practices Task Force . Modeling good research practices – overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force – 1. Value Health 2012;15:796-803. https://doi.org/10.1016/j.jval.2012.06.012.
- Capewell S, Capewell A. An effectiveness hierarchy of preventive interventions: neglected paradigm or self-evident truth?. J Public Health 2018;40:350-8. https://doi.org/10.1093/pubmed/fdx055.
- Adams J, Mytton O, White M, Monsivais P. Why are some population interventions for diet and obesity more equitable and effective than others? The role of individual agency. PLOS Med 2016;13. https://doi.org/10.1371/journal.pmed.1001990.
- Moore GF, Evans RE, Hawkins J, Littlecott H, Melendez-Torres GJ, Bonell C, et al. From complex social interventions to interventions in complex social systems: future directions and unresolved questions for intervention development and evaluation. Evaluation 2019;25:23-45. https://doi.org/10.1177/1356389018803219.
- Frew E, Breheny K. Methods for public health economic evaluation: a Delphi survey of decision makers in English and Welsh local government. Health Econ 2019;28:1052-63. https://doi.org/10.1002/hec.3916.
- Mahase E. Changes to NHS health checks must be evidence based and beneficial, say GPs. BMJ 2019;366. https://doi.org/10.1136/bmj.l5201.
- Dias L, Morton A, Quigley J. Elicitation – The Science and Art of Structuring Judgement. New York, NY: Springer; 2018.
- O’Hagan A. Expert knowledge elicitation: subjective but scientific. Am Stat 2019;73:69-81. https://doi.org/10.1080/00031305.2018.1518265.
- Gillespie DO, Allen K, Guzman-Castillo M, Bandosz P, Moreira P, McGill R, et al. The health equity and effectiveness of policy options to reduce dietary salt intake in England: policy forecast. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0127927.
- O’Hagan T. SHELF: Version 4 n.d. www.tonyohagan.co.uk/shelf/SHELF4.html (accessed 28 September 2020).
- Webber L, Mytton OT, Briggs AD, Woodcock J, Scarborough P, McPherson K, et al. The Brighton declaration: the value of non-communicable disease modelling in population health sciences. Eur J Epidemiol 2014;29:867-70. https://doi.org/10.1007/s10654-014-9978-0.
- Sexton E, McLoughlin A, Williams DJ, Merriman NA, Donnelly N, Rohde D, et al. Systematic review and meta-analysis of the prevalence of cognitive impairment no dementia in the first-year post-stroke. Eur Stroke J 2019;4:160-71. https://doi.org/10.1177/2396987318825484.
- Public Health England . Technical Document for Sub-National English Atrial Fibrillation Prevalence Estimates n.d. www.gov.uk/government/publications/atrial-fibrillation-prevalence-estimates-for-local-populations (accessed 3 March 2021).
- Symmons D, Turner G, Webb R, Asten P, Barrett E, Lunt M, et al. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology 2002;41:793-800. https://doi.org/10.1093/rheumatology/41.7.793.
- Ezzati M, Henley SJ, Thun MJ, Lopez AD. Role of smoking in global and regional cardiovascular mortality. Circulation 2005;112:489-97. https://doi.org/10.1161/CIRCULATIONAHA.104.521708.
- Macacu A, Autier P, Boniol M, Boyle P. Active and passive smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res Treat 2015;154:213-24. https://doi.org/10.1007/s10549-015-3628-4.
- Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet 2011;378:1297-305. https://doi.org/10.1016/S0140-6736(11)60781-2.
- Wolf PA, D’Agostino RB, Kannel WB, Bonita R, Belanger AJ. Cigarette smoking as a risk factor for stroke: the Framingham study. JAMA 1988;259:1025-9. https://doi.org/10.1001/jama.1988.03720070025028.
- Forey BA, Thornton AJ, Lee PN. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm Med 2011;11. https://doi.org/10.1186/1471-2466-11-36.
- Liang PS, Chen T-Y, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer 2009;124:2406-15. https://doi.org/10.1002/ijc.24191.
- Tammemägi MC, Church TR, Hocking WG, Silvestri GA, Kvale PA, Riley TL, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLOS Med 2014;11. https://doi.org/10.1371/journal.pmed.1001764.
- He J, Vupputuri S, Allen K, Prerost MR, Hughes J, Whelton PK. Passive smoking and the risk of coronary heart disease – a meta-analysis of epidemiologic studies. N Engl J Med 1999;340:920-6. https://doi.org/10.1056/NEJM199903253401204.
- Oono IP, Mackay DF, Pell JP. Meta-analysis of the association between secondhand smoke exposure and stroke. J Public Health 2011;33:496-502. https://doi.org/10.1093/pubmed/fdr025.
- Fischer F, Kraemer A. Meta-analysis of the association between second-hand smoke exposure and ischaemic heart diseases, COPD and stroke. BMC Public Health 2015;15. https://doi.org/10.1186/s12889-015-2489-4.
- Kim CH, Lee Y-CA, Hung RJ, McNallan SR, Cote ML, Lim W-Y, et al. Exposure to secondhand tobacco smoke and lung cancer by histological type: a pooled analysis of the International Lung Cancer Consortium (ILCCO). Int J Cancer 2014;135:1918-30. https://doi.org/10.1002/ijc.28835.
- Global Health Data Exchange . Global Burden of Disease Study 2016 (GBD 2016) Data Resources n.d. http://ghdx.healthdata.org/gbd-2016 (accessed 3 March 2021).
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903-13. https://doi.org/10.1016/S0140-6736(02)11911-8.
- Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, . Prospective Studies Collaboration . Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet 2007;370:1829-39. https://doi.org/10.1016/S0140-6736(07)61778-4.
- The Emerging Risk Factors Collaboration . Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 2011;377:1085-95. https://doi.org/10.1016/S0140-6736(11)60105-0.
- The Emerging Risk Factors Collaboration . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215-22. https://doi.org/10.1016/S0140-6736(10)60484-9.
- Bull FC, Armstrong TP, Dixon T, Ham S, Neiman A, Pratt M. Comparative Quantification of Health Risks. Chapter 10: Physical Inactivity. Geneva: World Health Organization; 2004.
- Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr 2006;136:2588-93. https://doi.org/10.1093/jn/136.10.2588.
- Dauchet L, Amouyel P, Dallongeville J. Fruit and vegetable consumption and risk of stroke a meta-analysis of cohort studies. Neurology 2005;65:1193-7. https://doi.org/10.1212/01.wnl.0000180600.09719.53.
- Wang Y, Li F, Wang Z, Qiu T, Shen Y, Wang M. Fruit and vegetable consumption and risk of lung cancer: a dose–response meta-analysis of prospective cohort studies. Lung Cancer 2015;88:124-30. https://doi.org/10.1016/j.lungcan.2015.02.015.
- Vieira AR, Abar L, Vingeliene S, Chan DSM, Aune D, Navarro-Rosenblatt D, et al. Fruits, vegetables and lung cancer risk: a systematic review and meta-analysis. Ann Oncol 2016;27:81-96. https://doi.org/10.1093/annonc/mdv381.
- Vieira J, Mathur R, Shah AD, Pujades-Rodriguez M, Denaxas S, Smeeth L, et al. Ethnicity and the first diagnosis of a wide range of cardiovascular diseases: associations in a linked electronic health record cohort of 1 million patients. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0178945.
- Christiansen CB, Gerds TA, Olesen JB, Kristensen SL, Lamberts M, Lip GYH, et al. Atrial fibrillation and risk of stroke: a nationwide cohort study. Europace 2016;18:1689-97. https://doi.org/10.1093/europace/euv401.
- Singer DE, Chang Y, Borowsky LH, Fang MC, Pomernacki NK, Udaltsova N, et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc 2013;2. https://doi.org/10.1161/JAHA.113.000250.
- Wobus DZ, Olin GL. Health Care Expenses: Poor, Near Poor, and Low Income People in the United States Civilian Noninstitutionalized Population, 2002. Rockville, MD: Agency for Healthcare Research and Quality; 2005.
- HM Treasury . GDP Deflators at Market Prices, and Money GDP n.d. www.gov.uk/government/collections/gdp-deflators-at-market-prices-and-money-gdp (accessed 1 October 2019).
- National Institute for Health and Care Excellence . Hypertension Update: Appendix J Cost Effectiveness Analysis n.d. www.nice.org.uk/guidance/cg127/documents/hypertension-update-appendix-j-costeffectiveness-analysis-diagnosis2 (accessed 10 February 2020).
- Regional Drug and Therapeutics Centre . Cost Comparison Charts August 2019 2019.
- Stewart S, Murphy NF, Murphy N, Walker A, McGuire A, McMurray JJ. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart 2004;90:286-92. https://doi.org/10.1136/hrt.2002.008748.
- Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med 2012;29:855-62. https://doi.org/10.1111/j.1464-5491.2012.03698.x.
- Luengo-Fernández R, Leal J, Gray A, Petersen S, Rayner M. Cost of cardiovascular diseases in the United Kingdom. Heart 2006;92:1384-9. https://doi.org/10.1136/hrt.2005.072173.
- Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess 2007;11. https://doi.org/10.3310/hta11140.
- Punekar YS, Shukla A, Müllerova H. COPD management costs according to the frequency of COPD exacerbations in UK primary care. Int J Chron Obstruct Pulmon Dis 2014;9:65-73. https://doi.org/10.2147/COPD.S54417.
- Kerr M, Bray B, Medcalf J, O’Donoghue DJ, Matthews B. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant 2012;27:iii73-80. https://doi.org/10.1093/ndt/gfs269.
- Luengo-Fernandez R, Leal J, Gray AM. Cost of dementia in the pre-enlargement countries of the European Union. J Alzheimers Dis 2011;27:187-96. https://doi.org/10.3233/JAD-2011-102019.
- Laudicella M, Walsh B, Burns E, Smith PC. Cost of care for cancer patients in England: evidence from population-based patient-level data. Br J Cancer 2016;114:1286-92. https://doi.org/10.1038/bjc.2016.77.
- Lee S, Shafe AC, Cowie MR. UK stroke incidence, mortality and cardiovascular risk management 1999–2008: time-trend analysis from the General Practice Research Database. BMJ Open 2011;1. https://doi.org/10.1136/bmjopen-2011-000269.
- Prince M, Knapp M, Guerchet M, McCrone P, Prina M, Comas-Herrera A, et al. Dementia UK: Second Edition – Overview 2014 n.d. www.alzheimers.org.uk/sites/default/files/migrate/downloads/dementia_uk_update.pdf (accessed 10 October 2019).
- Miners A, Cairns J, Wailoo A. Department of Health Proposals for Including Wider Societal Benefits into Value Based Pricing: A Description and Critique. London: NICE Decision Support Unit; 2013.
- Office for National Statistics . Employee Earnings in the UK Statistical Bulletins n.d. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2018 (accessed 1 October 2019).
- Office of National Statistics . Unemployment n.d. www.ons.gov.uk/employmentandlabourmarket/peoplenotinwork/unemployment (accessed 21 November 2019).
- GOV.UK . Family Resources Survey n.d. www.gov.uk/government/collections/family-resources-survey--2 (accessed 2 September 2020).
Appendix 1 Best-performing local authorities survey methodology
Best-performing local authorities methodology
Each best-performing LA was initially contacted by telephone with the following information.
-
The workHORSE project background and aims.
-
The purpose of the contact: we are contacting the best-performing LAs to find out how they deliver their NHS Health Checks, find out their success stories regarding coverage and uptake and ask for documents supporting this (i.e. reports/audits) to record best-practice approaches and potentially develop best-practice templates for the tool.
An e-mail was sent requesting specific information. We were interested in receiving the most recent documents (if available also previous versions), including reports and audits produced regarding the coverage and uptake of NHS Health Checks and their outcomes, and any published individual case studies.
In addition, they were asked to answer the following questions.
Coverage
-
What are the processes around inviting people for NHS Health Checks?
-
Have these changed over time?
-
If so, please tell us (1) how (2) why and (3) when they have changed.
-
How have these different processes impacted on uptake?
-
Can you provide us with the most recent documents (if available also previous versions), including reports and audits produced regarding the coverage of NHS Health Checks and their outcomes, and any published individual case studies?
-
Take-up
-
What are/have been the approaches to increase take-up of NHS Health Checks?
-
Have the approaches changed since the start of the NHS HCP?
-
If so, please tell us (1) how (2) why and (3) when this changed.
-
Please report any changes in the % of uptake during this time.
-
If available, please include data by socioeconomic status.
-
-
Can you provide us with the most recent documents (if available also previous versions), including reports and audits produced regarding the take-up of Health Checks and their outcomes, and any published individual case studies?
-
-
If not included above, what are your approaches to invite patients?
-
Have these approaches to invite eligible people changed since the start of the HCP?
-
If so, please tell us (1) how (2) why and (3) when this changed.
-
Please report any changes in the % of uptake during this time.
-
If available, please include data by socioeconomic status.
-
Can you provide us with the most recent documents (if available also previous versions), including reports and audits produced regarding the take-up of Health Checks and their outcomes, and any published individual case studies?
-
-
Questions applicable for most improved local authorities only
Which approaches do you feel have contributed to the increase in the delivery of HCs, and to what extend?
Community outreach
-
Please describe your community outreach activities.
Lifestyle services
-
Do you have lifestyle referral services? Yes/No.
-
If no, why not?
-
If yes, which referral services do you have?
-
Cost per NHS Health Check
-
What is the cost per NHS Health Check?
You have been chosen for this study because your performance in terms of NHS Health Checks coverage is better than other areas. Is there anything else that either commissioner or provider has done that you believe has worked in terms of maximising NHS Health Checks coverage or uptake?
Data extraction and analysis
All data were extracted into a data extraction form, which was piloted before finalising. The data extraction form included LA, model parameter (i.e. coverage, uptake), approaches, outcomes (before and after) and time frame.
Data were analysed thematically.
-
Data were organised by model parameter.
-
Similarities and differences by LAs were explored.
-
Data were presented narratively, with tables and graphical displays (where appropriate).
If quantitative evidence was provided, these were analysed and, if feasible, used for best-practice scenarios for the tool.
Appendix 2 A PRISMA flow chart for the umbrella review on invitation methods to increase uptake in screening programmes
FIGURE 22.
A PRISMA flow chart for the umbrella review on invitation methods to increase uptake in screening programmes.

Appendix 3 Summary table of individual studies included in the umbrella review on invitation methods to increase uptake in screening programmes
Education (patients): individual interventions
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Volk et al. (2016)85 | SR and MA of RCTs, non-RCT and pre–post study | Colorectal cancer |
Aim: to describe studies evaluating patient decision aids for screening in average-risk adults and their impact on uptake Outcomes: uptake (RR) |
Patients exposed to a decision aid were more likely to be screened (pooled RR 1.3, 95% CI 1.1 to 1.4). The absolute group benefit was 8% (95% CI 6% to 11%) (i.e. in the decision aid group, 47 of 100 people completed their screening over 16–52 weeks, vs. 40 of 100 for the control group) | Unclear risk of bias |
| Evans et al. (2005)88 | SR and MA of RCTs and non-RCTs | Prostate cancer |
Aim: to identify and appraise PSA decision aids and evaluations Outcomes: uptake (RD, %) |
Findings showed a significantly reduced probability in PSA testing after a decision aid: –3.5% (95% CI 0.0% to 7.2%; p = 0.050; n = 4) | High risk of bias |
| Ivlev et al. (2018)86 | SR and MA of RCTs, non-RCT and BA | Prostate cancer |
Aim: to review the effect of decision aids on men’s screening utilisation Outcomes: screening utilisation (RR) |
Compared with the control group, the RCTs indicate that the use of decision aids leads to a potential decrease in the number of men who would undergo PSA testing during the first 3 weeks (RR 0.94, 95% CI 0.90 to 0.97; p = 0.02; n = 3, I2 = 0%, p = 0.91). However, this effect did not hold through the first year (two RCTs with three decision aids) | High risk of bias |
| Volk et al. (2007)87 | SR and MA of RCTs and quasi-experimental | Prostate cancer |
Aim: to examine the methods and findings of studies that have evaluated the impact of decision aids on patient outcomes Outcomes: screening behaviour (RR) |
The pooled RR for the fixed-effects model for patients with regular scheduled office visits was 0.88 (95% CI 0.81 to 0.97; p = 0.008). When studies (n = 2) of patients seeking screening services were added, the overall effect of the aids on screening behaviour remained statistically significant, with a pooled RR ratio from the fixed-effects model of 0.92 (95% CI 0.86 to 0.99; p = 0.028). Both results indicated that patients who received the aids were less likely to be screened than those who did not receive the aid | High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Vernon et al. (2010)71 | SR and MA of RCTs and quasi-experimental | Breast cancer |
Aim: to examine the effectiveness of intervention strategies that reported estimates of repeat mammography screening for intervention and control groups Outcomes: repeat mammography use (OR) |
The studies classified as education/motivation or counselling were homogeneous, with a summary OR of 1.27 (95% CI 1.17 to 1.37). The summary OR for reminder-only studies was greater than the summary OR for education/motivation and counselling | High risk of bias |
| Gardner et al. (2013)82 | SR and MA of RCTs | Breast cancer |
Aim: to estimate the magnitude of the effect of interventions used to increase uptake of mammography among low-income women Outcomes: uptake (RD) |
Face-to-face interventions (education or home visit) (n = 7) increased the uptake by a difference of 7.5% (CI 1.7% to 13.2%) compared with women in the control group | High risk of bias |
| Yabroff et al. (1999)74 | MA of randomised or concurrent control | Breast cancer |
Aim: to determine the effects of patient-based mammography screening strategies Outcomes: screening (difference, %) |
Interventions using generic education strategies had little impact on screening (1.1%, 95% CI –2.4% to 4.6%; n = 7), but those who used theory-based education increased screening rates by 23.6% (95% CI 16.4% to 30.1%) compared with usual care Interventions with active controls delivered by letter or videotape were ineffective, with an estimated increase in mammography utilisation of < 1% (0.4%, 95% CI –5.4% to 6.2%). However, interventions that were delivered interactively were effective, with a combined increase in mammography utilisation of 7.9% (95% CI 2.3% to 13.5%) |
High risk of bias |
| Sabatino et al. (2012)69 | SR | Breast cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake (percentage points) |
One-to-one education. The original review found strong evidence of effectiveness based on a median increase in mammography use of 9.2 percentage points across 23 studies (IQI 4.9 to 14.4 percentage points) and ORs from four additional study arms in the favourable direction Update: the median increase for seven intervention arms was 11.9 (95% CI 6.5 to 15.2) percentage points |
High risk of bias |
| Everett et al. (2011)57 | SR and MA of RCTs | Cervical cancer |
Aim: to assess the effectiveness of interventions aimed at women to increase the uptake of screening Outcomes: uptake (RR) |
Counselling vs. control (n = 2). The MA found that women given counselling to encourage attendance of a cervical screening programme had significantly higher uptake of screening than those given no counselling or patient prompts alone (RR 1.23, 95% CI 1.04 to 1.45) | Low risk of bias |
| Sabatino et al. (2012)69 | SR | Cervical cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
One-to-one education. The median post-intervention increase in Pap test completion over the eight study arms was 8.1 (IQI 5.7 to 17.3) percentage points. Overall, the magnitude of this effect and the consistent positive results across studies demonstrate that one-to-one education interventions are effective in increasing cervical cancer screening by Pap test | High risk of bias |
| Sabatino et al. (2012)69 | SR | Colorectal cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
One-to-one education. The median effect for FOBT was 19.1 (IQI 12.9 to 25.1) percentage points. Results for colorectal cancer changed from insufficient evidence (n = 2) to sufficient evidence (n = 7) | High risk of bias |
| Brouwers et al. (2011)70 | SR of RCTs | Breast cancer, cervical cancer and colorectal cancer |
Aim: to evaluate interventions designed to increase the rate of screening Outcomes: screening uptake |
One-on-one education appears effective, but their roles with colorectal cancer and cervical screening are less established | High risk of bias |
| Hou et al. (2011)90 | SR of RCT, quasi-experimental, BA | Breast cancer, cervical cancer and colorectal cancer |
Aim: to review published literature describing screening interventions for Asian populations in the USA to identify effective programmes for specific Asian ethnic groups Outcomes: screening |
There is strong evidence that one-on-one education, either by telephone or in person, and often conducted by lay health workers, can improve screenings in Asian communities | High risk of bias |
| Kelly et al. (2018)89 | SR of RCT, RT, quasi-experimental, pre–post, non-RCT, cohort | Breast cancer, cervical cancer, colon cancer, prostate cancer, hepatitis B virus |
Aim: to determine which interventions have successfully increased screening uptake among minorities Outcomes: screening uptake |
Lay health workers and navigators. Fourteen studies found statistically significant increases in screening. The remaining 10 studies were non-significant No link was found to ethnicity or screening modality in either the significant or non-significant categories |
High risk of bias |
| Teo et al. (2018)53 | SR and MA of RCTs and cluster RCTs | Prostate cancer, HIV, STIs, testicular cancer, melanoma |
Aim: to determine the effectiveness of interventions in improving men’s uptake of and intention to undergo screening Outcomes: uptake (RR) |
Educational interventions effective in increasing men’s screening by 11% vs. comparator (n = 5), and 37% vs. no usual care. With low methodological quality studies excluded (n = 2), there was no significant difference in screening uptake between educational interventions and comparators | High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Bonfill et al. (2001)61 | SR and MA of RCTs | Breast cancer |
Aim: to assess the effectiveness of strategies for increasing the participation rate of women invited to community screening activities or mammography programmes Outcomes: screening attendance (OR) |
Home visits did not prove to be effective (OR 1.06, 95% CI 0.80 to 1.40) | High risk of bias |
| Everett et al. (2011)57 | SR and MA of RCTs | Cervical cancer |
Aim: to assess the effectiveness of interventions aimed at women to increase the uptake, including informed uptake, of screening Outcomes: uptake (RR) |
Home visits. MA of three trials showed a significantly higher uptake of screening in women who received face-to-face home visits as a form of education than those in the control group (RR 2.33, 95% CI 1.04 to 5.23) | Low risk of bias |
| Lu et al. (2012)75 | SR of RCTs, non-equivalent control group, prospective cohort | Cervical cancer |
Aim: to update current knowledge on the effectiveness of existing intervention strategies to enhance screening uptake in Asian women Outcomes: screening uptake |
Evidence (n = 2) was found to support the effectiveness of home visit plus health education plus patient navigation among Chinese women in the USA and Canada | High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Albada et al. (2009)93 | SR of RCTs, randomised with comparison but no control group | Breast cancer |
Aim: to assess the effects of interventions that provided people with information about cancer risk and screening that is tailored to their personal characteristics on risk perception, knowledge and screening behaviour of these interventions Outcomes: screening behaviour |
Indicative findings for increased effects of tailoring based on behavioural constructs vs. a control group receiving no information. Overall, most studies reported that behavioural construct-tailored information significantly increased mammography adherence. Insufficient evidence for the effect of risk factor tailoring on mammography use. There are indicative findings that information tailored on behavioural as well as cultural constructs increases mammography screening rates | Low risk of bias |
| Everett et al. (2011)57 | SR and MA of RCTs | Cervical cancer |
Aim: to assess the effectiveness of interventions aimed at women to increase the uptake, including informed uptake, of screening Outcomes: uptake (RR) |
Message framing. No significant differences in uptake were seen between loss- or gain-framed messages for detection or prevention Enhanced risk assessment vs. control (n = 2) showed little difference in the uptake of screening between women who had an enhanced risk assessment and those in the control group (RR 1.52, 95% CI 0.58 to 3.95) |
Low risk of bias |
| Albada et al. (2009)93 | SR of RCTs, randomised with comparison but no control group | Cervical cancer |
Aim: to study interventions that provide people with information about cancer risk and about screening that is tailored to their personal characteristics. To assess the effects on risk perception, knowledge and screening behaviour of these interventions Outcomes: screening behaviour |
Found no evidence for effects on Pap test use of materials tailored on behavioural constructs or risk factors | Low risk of bias |
| Issaka et al. (2019)64 | SR of RCTs, quasi-experimental and observational | Colorectal cancer |
Aim: to determine the evidence of efficacy of interventions to improve FIT completion that could be scaled and utilised in population health management Outcomes: screening uptake (median efficacy, %) |
Tailored patient messaging (n = 3). No significant improvement in screening | Low risk of bias |
| Albada et al. (2009)93 | SR of RCTs, randomised with comparison but no control group | Colorectal cancer |
Aim: to assess the effects of interventions that provide people with information about cancer risk and screening that is tailored to their personal characteristics on risk perception, knowledge and screening behaviour of these interventions Outcomes: screening behaviour |
Found no evidence for the effect of tailored interventions on colorectal cancer screening uptake | Low risk of bias |
| Edwards et al. (2013)92 | SR and MA of RCTs | Breast cancer, colorectal cancer |
Aim: to assess the effects of personalised risk communication on informed decision-making by individuals taking screening tests Outcomes: screening uptake (OR) |
Overall, pooling of personalised risk communication and risk estimates categorised into high, medium or low strata of risk Yielded a combined OR of 1.15 (95% CI 1.02 to 1.29). Indicative of weak evidence and consistent with a small effect that personalised risk increases uptake of screening tests |
Low risk of bias |
| Usher-Smith et al. (2018)91 | SR and MA of RCTs | Breast cancer, cervical cancer, colorectal cancer |
Aim: to present the impact of interventions incorporating personalised information about cancer risk on behaviours Outcomes: screening attendance (RR) |
Except for one high-quality RCT, all showed no effect of the personalised risk-based interventions with a combined RR of 1.02 (95% CI 0.98 to 1.03; I2 = 61.6%) | High risk of bias |
Education (patients): mass campaign interventions
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Wells et al. (2011)95 | SR and MA of RCTs and quasi-experimental | Breast cancer |
Aim: to synthesise the effectiveness of community health worker programmes in improving screening mammography rates Outcomes: mammography rates (RR) |
Community health worker interventions were associated with a statistically significant increase in receipt of screening mammography [RR 1.06 (favouring intervention), 95% CI 1.02 to 1.11; p = 0.003] (I2 = 80%; p < 0.00001) | High risk of bias |
| Rees et al. (2018)66 | SR of RCTs and quasi-RCTs | Cervical cancer |
Aim: to assess the effectiveness of interventions to improve the uptake of screening among lower socioeconomic groups Outcomes: screening uptake |
Lay health advisors (n = 4). Community lay health advisor education intervention vs. usual care: all four studies showed a significant increase in the uptake of screening | Low risk of bias |
| Mojica et al. (2018)96 | SR of RCTs, experimental | Colorectal cancer |
Aim: to evaluate screening interventions among Latino men to characterise intervention components effective in increasing screening Outcomes: screening uptake |
Lay community health workers. Findings from this review further support the notion that community health workers can help increase colorectal cancer screening among Latino men | High risk of bias |
| Bellhouse et al. (2018)94 | SR and MA of RCTs and cluster RCTs | Breast cancer, cervical cancer, bowel cancer |
Aim: to assess the effectiveness of community-based health worker interventions for early detection of cancer Outcomes: uptake of screening (OR) |
Community-based health worker interventions resulted in greater uptake of breast, cervical and colon cancer screening than control conditions (OR 1.90, 95% CI 1.60 to 2.26; p < 0.001) Larger effect sizes were observed in participants previously non-adherent with recommended schedules of cancer screening (OR 2.4, 95% CI 1.85 to 3.11; p < 0.001) |
High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Han et al. (2009)76 | MA of (quasi-) experimental studies | Breast cancer |
Aim: to determine the effectiveness of the interventions for improving mammography screening among asymptomatic ethnic minority women Outcomes: mammography screening (overall MWES) |
Estimated effect sizes for approaches involving community education were not statistically significant (n = 4, MWES = 0.013, z = 0.324; p = 0.746) | High risk of bias |
| Legler et al. (2002)78 | MA of (quasi-) experimental studies | Breast cancer |
Aim: to determine which types of mammography-enhancing interventions are most effective for these diverse populations Outcomes: mammography use (difference, %) |
Community education yielded effects of 9.7%, based on 13 studies. Access-enhancing and individually directed interventions yielded larger effect sizes | High risk of bias |
| Secginli et al. (2017)98 | SR of (quasi-) experimental, pre–post | Breast cancer |
Aim: to review the scientific evidence on the effectiveness of various strategies aimed at improving screening behaviours in Turkish women Outcomes: screening rates (OR) |
Mammography (n = 5). Pooled results demonstrated a statistically significant increase in mammography rates at the 3-month follow-up (OR 10.08, 95% CI 3.87 to 26.28) and the 6-month follow-up (OR 2.18, 95% CI 1.19 to 4.02) among women who received group education compared with those who did not. Similar results at 12-month follow-up | Low risk of bias |
| Agide et al. (2018)84 | SR of RCTs, quasi-RCTs and non-RCTs | Breast cancer |
Aim: to provide evidence on the efficacy of the health promotion interventions to increase the uptake of screening Outcomes: screening uptake (%) |
Community-based interventions (n = 5). Inconsistent findings Group-based teachings and training. Interventions using video, visuals and audio–visuals (n = 3). Limited effect |
High risk of bias |
| Sabatino et al. (2012)69 | SR | Breast cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Group education yielded a post-intervention median absolute percentage point change of 11.5 (IQI 5.5 to 24.0). Results changed from insufficient evidence (n = 7) to sufficient evidence (n = 12) | High risk of bias |
| Han et al. (2011)76 | MA of RCTs and non-RCTs | Cervical cancer |
Aim: to examine the overall effectiveness of these interventions in increasing Pap test use by ethnic minority women in the USA Outcomes: Pap test use (overall MWES) |
Interventions using community education (n = 7) had the next largest effect size (after access-enhancing strategies) (d = 0.167, 95% CI 0.057 to 0.278), followed by individual-directed interventions and mass media approaches | High risk of bias |
| Agide et al. (2018)84 | SR of RCTs, quasi-RCTs and non-RCTs | Cervical cancer |
Aim: to see the effectiveness of health education interventions in screening uptake Outcomes: screening uptake (%) |
Community level (n = 7). Most studies improved screening behaviour (n = 3) and three studies significantly increased screening uptake. One study found no difference | High risk of bias |
| Sabatino et al. (2012)69 | SR | Cervical cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Group education (n = 4) yielded a post-intervention absolute median percentage point change in screening completed of 10.6 (95% CI 0 to 59.1). Insufficient evidence (small number of studies, methodologic limitations and inconsistent findings) | High risk of bias |
| Sabatino et al. (2012)69 | SR | Colorectal cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Group education (n = 2). The two studies included four intervention arms and yielded a median absolute percentage point change of 4.4 (95% CI –13 to 37). Insufficient evidence (small number of studies, methodologic limitations and inconsistent findings) | High risk of bias |
| Kelly et al. (2018)89 | SR of RCT, randomised trial, quasi-experimental, pre–post, non-RCT, cohort | Breast cancer, cervical cancer, colon cancer, prostate cancer, hepatitis b virus |
Aim: to determine which interventions have successfully increased screening uptake among minority groups Outcomes: screening uptake |
Group education approach (n = 8). Six studies had significant increases in screening uptake. Six studies involved Chinese Asian people and two studies involved African American people | High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Han et al. (2009)76 | MA of (quasi-) experimental studies | Breast cancer |
Aim: to determine the effectiveness of the interventions for improving mammography screening among asymptomatic ethnic minority women Outcomes: mammography screening (overall MWES) |
Estimated effect sizes for approaches involving mass media were not statistically significant (n = 4, MWES = 0.065, z = 1.759; p = 0.079) | High risk of bias |
| Legler et al. (2002)78 | MA of (quasi-) experimental studies | Breast cancer |
Aim: to determine which types of mammography-enhancing interventions are most effective for these diverse populations Outcomes: mammography use (difference, %) |
Media interventions yielded effects of 5.9% (n = 6). Access-enhancing and individually directed interventions yielded larger effect sizes | High risk of bias |
| Han et al. (2011)77 | MA of RCTs and non-RCTs | Cervical cancer |
Aim: to examine the overall effectiveness of interventions in increasing Pap test use by ethnic minority women in the USA Outcomes: Pap test use (overall MWES) |
Interventions using community education had the next largest effect size after access-enhancing strategies, followed by individual-directed interventions and mass media approaches (n = 6) (d = 0.119, 95% CI 0.055 to 0.183) | High risk of bias |
| Black et al. (2002)60 | SR of cohort analytic, CCT, pre–post, ITS | Cervical cancer |
Aim: to evaluate the effectiveness of interventions available to public health staff that could be used to increase screening to women Outcomes: screening rates |
Mass media alone (n = 4). Only one study was effective, which targeted a specific subpopulation with language-specific material | Low risk of bias |
| Baron et al. (2008)99 | SR of study designs with comparison group | Cervical cancer |
Aim: to present the effectiveness, applicability and economic efficiency of interventions designed to increase screening, by increasing community demand for these services Outcomes: completed screening (median post-intervention increase, percentage points) |
Mass media (n = 2). Evidence was insufficient to determine the effectiveness of mass media when used alone in increasing cervical cancer screening | High risk of bias |
| Sabatino et al. (2012)69 | SR | Breast cancer, cervical cancer, colorectal cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Mass media. There is insufficient evidence to determine the effectiveness of mass media interventions in increasing screening for breast, cervical and colorectal cancers | High risk of bias |
| Kelly et al. (2018)89 | SR of RCT, randomised trial, quasi-experimental, pre–post, non-RCT, cohort | Breast cancer, cervical cancer, colon cancer, prostate cancer, hepatitis B virus |
Aim: to determine which interventions have successfully increased screening uptake among minorities Outcomes: screening uptake |
Media intervention (n = 4). Three studies had significant results. These studies covered a variety of ethnicities and conditions | High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Bonfill et al. (2001)61 | SR and MA of RCTs | Breast cancer |
Aim: to assess the effectiveness of strategies for increasing the participation rate of women invited to community screening activities or mammography programme Outcomes: screening attendance (OR) |
The evidence favoured mailed educational material (OR 2.81, 95% CI 1.96 to 4.02) | High risk of bias |
| Baron et al. (2008)99 | SR of all types of study designs with comparison group | Breast cancer |
Aim: to present the results of effectiveness, applicability and economic efficiency of interventions designed to increase screening by increasing community demand for these services Outcomes: completed screening (median post-intervention increase, percentage points) |
Small media (n = 19). Median post-intervention increases in completed mammography was 7.0 (IQI 0.3 to 13.2) percentage points. Effect magnitude and consistent positive results across studies showed effectiveness of small media in increasing breast cancer screening by mammography. Median increases for tailored (n = 7) and untailored (n = 14) small media were 7.0 (IQI –4.5 to 11.2) percentage points and 4.7 (IQI 0.5 to 13.4) percentage points, respectively | High risk of bias |
| Everett et al. (2011)57 | SR and MA of RCTs | Cervical cancer |
Aim: to assess the effectiveness of interventions aimed at women to increase the uptake, including informed uptake, of screening Outcomes: uptake (RR) |
Education (printed material) vs. control (n = 3). Showed little difference in the uptake of screening between women who received printed material as a form of education and those in the control group (RR 1.11, 95% CI 0.88 to 1.41) Education (miscellaneous; educational materials) vs. control (n = 2). Showed a significantly higher uptake of screening in women in the education group than in women in the control group (RR 1.92, 95% CI 1.24 to 2.97) |
Low risk of bias |
| Baron et al. (2008)99 | SR of all types of study designs with comparison group | Cervical cancer |
Aim: to present the effectiveness, applicability and economic efficiency of interventions designed to increase screening by increasing community demand for these services Outcomes: completed screening (median post-intervention increase, percentage points) |
Small media (n = 12). Overall, the median post-intervention increase in Pap test completion for 12 intervention arms was 4.5 (IQI 0.2 to 9.0) percentage points. The magnitude of this effect and consistent positive results across studies demonstrate the effectiveness of small media in increasing cervical cancer screening by Pap test | High risk of bias |
| Rat et al. (2018)52 | SR of RCTs | Colorectal cancer |
Aim: to synthetize evidence on interventions aiming to increase uptake of faecal tests for screening Outcomes: screening uptake (OR) |
Video- or computer-based interventions (n = 2). No evidence of effectiveness | Low risk of bias |
| Mojica et al. (2018)96 | SR of RCTs, experimental | Colorectal cancer |
Aim: to evaluate the literature on screening interventions among Latino men to characterise intervention components effective in increasing screening Outcomes: screening uptake |
Small media. Results were mixed and inconsistent | High risk of bias |
| Baron et al. (2008)99 | SR of all types of study designs with comparison group | Colorectal cancer |
Aim: to present the effectiveness, applicability and economic efficiency of interventions designed to increase screening by increasing community demand for these services Outcomes: completed screening (median post-intervention increase, percentage points) |
Small media (n = 7). The median post-intervention increases in completed FOBT for eight intervention arms was 12.7 (IQI 0 to 26.4) percentage points. The magnitude of this effect and the consistent positive results across studies demonstrate the effectiveness of small media in increasing colorectal cancer screening by FOBT | High risk of bias |
| Brouwers et al. (2011)70 | SR of RCTs | Breast cancer, cervical cancer, colorectal cancer |
Aim: to evaluate interventions designed to increase the rate of screening Outcomes: screening uptake |
Small media appears to be an effective intervention to increase the uptake of screening for the three cancers | High risk of bias |
| Hou et al. (2011)90 | SR of randomised, quasi-experimental, BA | Breast cancer, cervical cancer, colorectal cancer |
Aim: to review screening interventions for cancers among Asian populations in the USA with a view to identifying effective programmes for specific Asian ethnic groups Outcomes: screening |
The use of small media (e.g. videos, printed materials, letters and brochures) to encourage breast and cervical cancer screenings is also applicable among Asian communities | High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Luque et al. (2019)100 | SR and MA of experimental and quasi-experimental | Breast cancer |
Aims: to synthesise the current literature on educational interventions to increase mammography screening among Hispanic women Outcomes: screening adherence (OR) |
Individual and group education. The summary OR was 1.67 (95% CI 1.24 to 2.26), suggesting a low to moderate intervention effect. Hispanic women exhibit lower levels of adherence to screening mammography than non-Hispanic white women | High risk of bias |
| Stone et al. (2002)72 | MA of randomised clinical trials, controlled clinical trials | Breast cancer |
Aim: to assess the relative effectiveness of previously studied approaches for improving adherence to adult immunisation and cancer screening guidelines Outcomes: use of services (OR) |
Patient education: individual education plus mass media (OR 1.31, 95% CI 1.12 to 1.52) | High risk of bias |
| Agide et al. (2018)97 | SR of RCTs, quasi-RCTs and non-RCTs | Breast cancer |
Aim: to provide evidence on the efficacy of the health promotion interventions to increase the uptake of screening and to develop effective interventions targeting women Outcomes: screening uptake (%) |
All four studies showed favourable outcomes of education, message framing and telephone calls on breast cancer screening uptake | High risk of bias |
| Chan and So (2015)102 | SR of RCTs | Breast cancer |
Aim: to examine the effect that screening programmes for ethnic minority women have on their screening intentions and uptake rates Outcomes: screening uptake |
Mammogram uptake (n = 4). Culturally relevant strategies (e.g. education, lay health adviser, language, print materials). Inconsistent results. Two studies did not find a significant difference between the groups | High risk of bias |
| Corcoran et al. (2012)103 | SR and MA of (quasi-) experimental | Cervical cancer |
Aim: to determine the association between participation in an intervention to increase cancer prevention behaviour among Latina women and the cervical screening rates Outcomes: screened vs. unscreened (OR) |
Education and mass media. Random-effects model: OR 0.778 (95% CI 0.576 to 1.049). Fixed-effects model: OR 0.783 (95% CI 0.661 to 0.928) The results show that interventions to improve cervical screening rates among Latina women do not seem to be effective |
High risk of bias |
| Musa et al. (2017)79 | SR and MA of RCTs and CBPR | Cervical cancer |
Aims: to understand the effect of provider recommendations for screening on eligible women at risk of cervical cancer Outcomes: screening rate (OR) |
Small media and telephone or text message education. The pooled summary effect of the interventions was two and a half times larger in comparison than that in the control (OR 2.46, 95% CI 1.88 to 3.21; n = 5), indicating some evidence of an increase in colorectal cancer screening rates | High risk of bias |
| Stone et al. (2002)72 | MA of randomised clinical trials, controlled clinical trials | Cervical cancer |
Aim: to assess the relative effectiveness of previously studied approaches for improving adherence to cancer screening guidelines Outcomes: use of services (OR) |
Individual education plus mass media (OR 1.53, 95% CI 1.30 to 1.81) | High risk of bias |
| Lu et al. (2012)75 | SR of RCTs, non-equivalent control group, prospective cohort | Cervical cancer |
Aim: to update current knowledge on the effectiveness of existing intervention strategies to enhance screening uptake in Asian women Outcomes: screening uptake |
Evidence (n = 2) was found to support the effectiveness of a mail campaign plus telephone calls in increasing cervical screening uptake among Chinese women in Taiwan | High risk of bias |
| Saei Ghare Naz et al. (2018)101 | SR of RCTs, quasi-experimental and pre–post | Cervical cancer |
Aim: to systematically assess the effects of educational interventions on cancer screening behaviour of women Outcomes: screening behaviour |
Mix of individual, group and mass media. The result showed that educational interventions based on health behaviour change theories could help to improve colorectal cancer screening behaviour of women in different parts of the world. The results showed that different health education methods are effective in modifying cervical cancer screening behaviour of women | High risk of bias |
| Chan and So (2015)102 | SR of RCTs | Cervical cancer |
Aim: to examine the effect that cancer screening programmes for ethnic minority women have on their screening intentions and uptake rates Outcomes: screening uptake |
Pap test uptake (n = 4). Culturally relevant strategies (e.g. education, lay health adviser, language, print materials). Consistent results. At 6-month follow-up, all studies with 21 participants or more reported that there was a statistically significant difference between groups, with more uptake reported in the intervention groups (p < 0.01) | High risk of bias |
| Dougherty et al. (2018)65 | SR and MA of RCTs | Colorectal cancer |
Aim: to identify interventions associated with increasing colorectal cancer screening rates and their effect sizes Outcomes: screening completion (RR and RD) |
Patient education (RR 1.20, 95% CI 1.06 to1.36; RD 4%, 95% CI 1% to 6%) increased colorectal cancer screening completion rates compared with usual care Among these studies, those with some additional component beyond patient education (e.g. clinician prompt or patient ability to request FBT directly) led to a significant increase in screening completion over usual care (RR 1.43, 95% CI 1.16 to 1.75; RD 8%, 95% CI 2% to 15%), while those without additional components did not (RR 1.08, 95% CI 0.97 to 1.20; RD 2%, 95% CI 0% to 4%) |
Low risk of bias |
| Stone et al. (2002)72 | MA of randomised clinical trials, controlled clinical trials | Colon cancer |
Aim: to assess the relative effectiveness of previously studied approaches for improving adherence to cancer screening guidelines Outcomes: use of services (OR) |
Patient education: individual education plus mass media (OR 1.38, 95% CI 0.84 to 2.25) | High risk of bias |
| Morrow et al. (2010)104 | SR of community-based RCTs | Colorectal cancer |
Aim: to summarise the current literature of community-based cancer screening RCTs with multiethnic groups Outcomes: screening adherence |
Counselling/community education (n = 5). Of five studies, four (80%) demonstrated significant differences between intervention and control groups | High risk of bias |
| Hou et al. (2011)90 | SR of randomised, quasi-experimental, BA | Breast cancer, cervical cancer, colorectal cancer |
Aim: to review screening interventions for breast, colorectal and cervical cancers among Asian populations in the USA Outcomes: screening |
Specifically, lay health worker and mass education campaigns that target delivery channels widely accessed by Asian American people, such as Asian grocery stores or churches (in the case of Korean people) are successful | High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Camilloni et al. (2013)56 | SR and MA of RCTs, quasi-experimental, prospective cohort, cross-sectional, pre–post | Breast cancer |
Aim: to assess the efficacy of interventions to increase participation in organised population-based screening programmes Outcomes: participation (RR) |
Effective interventions: postal (RR 1.37, 95% CI 1.25 to 1.51) and telephone reminders (with heterogeneous methods for implementation) plus GP’s signature on invitation letter (RR 1.13, 95% CI 1.11 to 1.16) Effective interventions included scheduled appointments instead of open appointments (RR 1.26, 95% CI 1.02 to 1.55) |
High risk of bias |
| Denhaerynck et al. (2003)59 | SR and MA of (quasi-) RCTs | Breast cancer |
Aim: to assess the overall effect of direct-contact recruitment Outcomes: mammogram participation (RR) |
High-quality studies: pooled RR was 1.26 (95% CI 1.11 to 1.44) Adding lower-quality studies: RR increased to 1.32 (95% CI 1.11 to 1.56), followed by a decrease in RR to 1.21 (95% CI 1.10 to 1.34) after the studies containing non-comparable control groups were entered. Direct-contact strategies improved attendance from 21% (95% CI 10% to 34%) to 46% (95% CI 32% to 61%). A smaller effect was recorded in underutilising populations (RR 1.05, 95% CI 1.01 to 1.08) than in a more general population (RR 1.27, 95% CI 1.19 to 1.35) (p = 0.003). Direct-contact interventions are effective in enhancing mammography participation |
High risk of bias |
| Bonfill et al. (2001)61 | SR and MA of RCTs | Breast cancer |
Aim: to assess the effectiveness of strategies for increasing the participation rate of women invited to community screening activities or mammography programmes Outcomes: screening attendance (OR) |
The evidence favoured active strategies for inviting women into community breast cancer screening services, including letter of invitation (OR 1.66, 95% CI 1.43 to 1.92), letter of invitation plus telephone call (OR 2.53, 95% CI 2.02 to 3.18) and telephone call (OR 1.94, 95% CI 1.70 to 2.23) | High risk of bias |
| Ferroni et al. (2012)46 | SR | Breast cancer |
Aim: to assess the effectiveness of population-based screening programmes in increasing coverage vs. spontaneous access Outcomes: screening participation (RR) |
Invitation letter vs. no intervention (usual care; n = 15): significantly more participation (RR 1.60, 95% CI 1.33 to 1.92) Telephone contact vs. no intervention (n = 7): the pooled effect was an increase of 29% (95% CI 20% to 39%) Invitation letter followed by telephone reminder vs. usual care (n = 2): significantly favoured the intervention. The pooled estimate of the effect was a RR of 3.22 (95% CI 1.24 to 8.41) Invitation letters vs. GP-based organised programmes: no significant differences were found between invitation letter- and GP-based organisation (RR 0.99, 95% CI 0.94 to 1.05) |
High risk of bias |
| Sin and Leger (1999)63 | SR of RCTs, BA | Breast cancer |
Aim: to evaluate the effectiveness of the different interventions to increase screening uptake Outcomes: screening uptake |
Person directed (n = 20). Appointments on the invitation letter increased uptake compared with open-ended invitations. From current evidence, endorsement of the invitation by a GP does not boost uptake | High risk of bias |
| Cheong et al. (2017)54 | SR and MA of RCTs, non-RCTs and pre–post | CVD |
Aim: to determine the effectiveness of existing intervention strategies to increase uptake of CVD risk factors screening Outcomes: uptake (RR) |
Patient invitations were not effective in increasing the uptake of CVD risk factors screening (RR 1.285, 95% CI 0.980 to 1.686) | High risk of bias |
| Everett et al. (2011)57 | SR and MA of RCTs | Cervical cancer |
Aim: to assess the effectiveness of interventions aimed at women to increase the uptake, including informed uptake, of cancer screening Outcomes: uptake (RR) |
Invitation letter vs. control (n = 12): women who received invitation letters had a significantly higher uptake of screening than women who received usual care or no invitation (RR 1.44, 95% CI 1.24 to 1.52) Letter with open invitation to make appointment vs. control (n = 4): women who received letters with an open invitation had significantly higher uptake of screening than women in the control group (RR 1.61, 95% CI 1.15 to 2.26) GP invitation letter vs. invitation letter from other authority sources (n = 2): mixed findings Letter with fixed appointment vs. letter with open invitation to make an appointment (n = 4): women who were given letters with a fixed appointment had a significantly higher uptake of screening than women who received letters with an open invitation (RR 1.57, 95% CI 1.43 to 1.72) Telephone invitation vs. control (n = 4): women who received a telephone invitation had a significantly higher uptake of screening than those in the control group (RR 2.16, 95% CI 1.70 to 2.74) Personal invitation vs. invitation letter (n = 2): women who received telephone invitations had a significantly higher uptake of screening than women given invitation letters (RR 1.32, 95% CI 1.15 to 1.53) |
Low risk of bias |
| Verdoodt et al. (2015)55 | SR and MA RCTs | Cervical cancer |
Aim: to evaluate the participation after an invitation, including a self-sampling device vs. an invitation to have a sample taken by a health professional, sent to underscreened women Outcomes: participation rates (absolute participation, %) |
Door to door (personal invitation): the participation rates were high in the two studies in a low-resource setting Opt-in self-sampling kit: the pooled participation was the same in the self-sampling as in the control arm (participation difference 0.2%, 95% CI –4.5% to 4.9%) |
Low risk of bias |
| Camilloni et al. (2013)56 | SR and MA of RCTs, quasi-experimental, prospective cohort, cross-sectional, pre–post | Cervical cancer |
Aim: to assess the efficacy of interventions to increase participation in organised population-based screening programmes Outcomes: participation (RR) |
Effective interventions: postal (RR 1.71, 95% CI 1.60 to 1.83) and telephone reminders (with heterogeneous methods for implementation); GP’s signature on invitation letter (RR 1.20, 95% CI 1.10 to 1.30); scheduled appointment instead of open appointment (RR 1.49, 95% CI 1.27 to 1.75) | High risk of bias |
| Black et al. (2002)60 | SR of cohort analytic, CCT, pre–post, ITS | Cervical cancer |
Aim: to evaluate the effectiveness of interventions available to public health staff that could be used to increase cancer screening to women Outcomes: screening rates |
Invitation letters (n = 5) were effective but required a centralised registry or survey to identify eligible women | Low risk of bias |
| Ferroni et al. (2012)46 | SR | Cervical cancer |
Aim: to assess the effectiveness of population-based screening programmes in increasing coverage vs. spontaneous access Outcomes: screening participation (RR) |
Invitation letter vs. no intervention (usual care; n = 12): showed significantly more participation (RR 1.52, 95% CI 1.28 to 1.82) Invitation letter followed by telephone reminder vs. usual care (n = 2): significantly in favour of the intervention (RR 2.26, 95% CI 1.19 to 4.29) Telephone contact vs. no intervention (n = 3): significant effect of telephone contact (RR 2.16, 95% CI 1.92 to 2.42) Invitation letters vs GP-based organised programmes: no significant differences were found between invitation letter- and GP-based organisation (RR 1.08, 95% CI 0.99 to 1.17) |
High risk of bias |
| Camilloni et al. (2013)56 | SR and MA of RCTs, quasi-experimental, prospective cohort, cross-sectional, pre–post | Colorectal cancer |
Aim: to assess the efficacy of interventions to increase participation in organised population-based screening programmes Outcomes: participation (RR) |
Effective interventions were: postal and telephone reminders (with heterogeneous methods for implementation) (RR 1.33, 95% CI 1.17 to 1.51); GP’s signature on invitation letter (RR 1.15, 95% CI 1.07 to 1.24); and scheduled appointment instead of open appointment (RR 1.79, 95% CI 1.65 to 1.93) | High risk of bias |
| Khalid-de Bakker et al. (2011)58 | SR and MA of prospective colorectal cancer screening studies | Colorectal cancer |
Aims: to review participation rate after first-time invitation for screening with FOBT, sigmoidoscopy, colonoscopy and/or CT colonography Outcomes: uptake (percentage point gain or reduction, %). |
Overall, pooled effects of invitation methods increased participation by 6%. Invitation methods that increased participation were the addition of a FOBT/FIT kit to an invitation letter (6%, n = 7), GP involvement (15%, n = 6) and invitation during a personal visit (19%, n = 3) Widely varying results were reported for adding an information brochure to the invitation letter (–1%, n = 6) and for inviting potential screeners to a personal visit (–2%, n = 5) (CI not presented because of inconsistent reporting in the original publication) |
High risk of bias |
| Rat et al. (2018)52 | SR of RCTs | Colorectal cancer |
Aim: to synthesise evidence on interventions aiming to increase uptake of faecal tests for screening and interventions that targeted GP involvement Outcomes: screening uptake (OR) |
Advance notification letter (n = 3): all studies reported a positive impact of advance notification letters (OR range 1.20–1.51), but one was not statistically significant Frames of invitation messages (n = 5): three studies showed increases in uptake, whereas two found no statistically significant effect |
Low risk of bias |
| Ferroni et al. (2012)46 | SR | Colorectal cancer |
Aim: to assess the effectiveness of population-based screening programmes in increasing coverage compared with spontaneous access Outcomes: screening participation (RR) |
Telephone contact vs. no intervention (n = 2): the results were homogeneous and showed a modest and non-significant 13% difference in the participation in FOBT screening (95% CI 7% to 37%) | High risk of bias |
| Teo et al. (2018)53 | SR and MA of RCTs and cluster RCTs | Prostate cancer, HIV, STIs, testicular cancer, melanoma |
Aim: to determine the effectiveness of interventions in improving men’s uptake of and intention to undergo screening Outcomes: uptake (RR) |
Interventions that used invitation (RR 1.78, 95% CI 1.17 to 2.68; k = 4) were shown to be more effective than comparators | High risk of bias |
| Jepson et al. (2000)62 | SR of RCTs, quasi-RCT and non-RCTs | Breast cancer, cervical cancer |
Aim: to examine factors associated with the uptake of screening programmes and to assess the effectiveness of methods used to increase uptake Outcomes: screening uptake |
Invitation methods Evidence of effectiveness of letters more effective in increasing the uptake of Pap smears than mammograms. Not enough evidence to detect whether or not GP letters are more effective than those from another source |
Low risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Vernon et al. (2010)71 | SR and MA of RCTs and quasi-experimental | Breast cancer |
Aim: to examine the effectiveness of various intervention strategies that reported estimates of repeat screening for intervention and control groups Outcomes: repeat mammography use (OR) |
For the heterogeneous reminder-only studies the summary OR was 1.79 (95% CI 1.41 to 2.29) The summary OR for reminder-only studies was significantly higher than the summary OR for education/motivation or counselling interventions |
High risk of bias |
| Yabroff and Mandelblatt (1999)74 | MA of randomised or concurrent control design | Breast cancer |
Aim: to determine the effects of patient-based mammography screening strategies Outcomes: screening (difference, %) |
Telephone reminders and letters increased screening by 13.2% (95% CI 4.7% to 21.2%; n = 6) compared with usual care, and by 5.6% (95% CI 0.6% to 10.6%; n = 7) when using a single intervention compared with active controls | High risk of bias |
| Jepson et al. (2000)62 | SR of RCTs, quasi-RCT and non-RCTs | Breast cancer |
Aim: to examine factors associated with the uptake of screening programmes and to assess the effectiveness of methods used to increase uptake Outcomes: screening uptake |
Reminders
|
Low risk of bias |
| Sin and Leger (1999)63 | SR of RCTs, BA | Breast cancer |
Aim: to evaluate the effectiveness of the different interventions to increase breast screening uptake Outcomes: screening uptake |
Encouraging non-attenders to attend (n = 7). Reminder letters increased uptake in different settings with consistent findings. Variations in content and GP endorsement showed higher response rates. The role for telephone reminders seems to be limited | High risk of bias |
| Sabatino et al. (2012)69 | SR | Breast cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Client reminders. The original review found strong evidence of effectiveness based on a median increase of 14.0 percentage points in recent mammography (n = 19; IQI 2.0 to 24.0 percentage points). In the update, six additional studies were included. Strong evidence | High risk of bias |
| Tseng et al. (2001)73 | MA of RCTs | Cervical cancer |
Aim: to investigate the efficacy of patient letter reminders on increasing cancer screening using Pap smears Outcomes: screening uptake (OR) |
Patients receiving reminder letters were found to be significantly more likely to undergo cancer screening than those under usual care (OR 1.64, 95% CI 1.49 to 1.80) The studies evaluating those in lower socioeconomic groups had a smaller response (OR 1.16, 95% CI 0.99 to 1.35) than those studies using mixed populations (OR 2.02, 95% CI 1.79 to 2.28) |
High risk of bias |
| Rees et al. (2018)66 | SR of RCTs and quasi-RCTs | Cervical cancer |
Aim: to assess the effectiveness of interventions to improve uptake of cancer screening among lower socioeconomic groups Outcomes: screening uptake |
Outreach strategies (n = 3). Screening rates increased (n = 2), but this was not significant. Simple messages were preferred over an extended letter with detailed information | Low risk of bias |
| Albrow et al. (2014)68 | SR of RCTs | Cervical cancer |
Aim: to summarise the evidence relating to interventions designed to increase screening uptake among women aged ≤ 35 years Outcomes: attendance (difference, %) |
Reminder letters (n = 3): there was some evidence to suggest that reminder letters have a positive effect on screening uptake in young women Telephone reminders (n = 2): the positive effect suggested that telephone reminders may be a candidate intervention for further evaluation in screening programmes |
High risk of bias |
| Sabatino et al. (2012)69 | SR | Cervical cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Client reminders. The original review found strong evidence of effectiveness based on a median increase in Pap test use across 14 intervention arms of 10.2 (IQI 6.3 to 17.9) percentage points. In the update, six additional qualifying studies were identified. Strong evidence | High risk of bias |
| Dougherty et al. (2018)65 | SR and MA of RCTs | Colorectal cancer |
Aim: to identify interventions associated with increasing screening rates and their effect sizes Outcomes: screening completion (RR and RD) |
Patient reminders (RR 1.20, 95% CI 1.02 to 1.41; RD 3%, 95% CI 0% to 5%) increased colorectal cancer screening completion rates compared with usual care. Larger associations were found among interventions with a telephone component | Low risk of bias |
| Rat et al. (2018)52 | SR of RCTs | Colorectal cancer |
Aim: to synthesise evidence on interventions aiming to increase uptake of faecal tests for screening groups Outcomes: screening uptake (OR) |
Reminders (n = 3). Showed increased uptake, ranging from an OR of 1.36 to an OR of 7.7 based on telephone and written reminders | Low risk of bias |
| Uy et al. (2017)67 | SR of RCTs and non-RCTs | Colorectal cancer |
Aim: to assess the effect of text messaging interventions on screening Outcomes: screening rates (%) |
Text reminders (n = 3) found much smaller effects on absolute screening rates, with increases ranging from 0.6% to 3.3% | Low risk of bias |
| Issaka et al. (2019)64 | SR of RCTs, quasi-experimental and observational studies | Colorectal cancer |
Aim: to determine the evidence of efficacy of interventions to improve FIT completion that could be scaled and utilised in population health management Outcomes: screening uptake (median efficacy, %) |
Pre-FIT patient reminders (n = 4) demonstrated small but consistent effect, with a median improvement of 4.1% (IQR 3.6–6.7%) in colorectal cancer screening Post-FIT patient reminders (n = 2) demonstrated modest efficacy with median 3.1% (IQR 2.9–3.3%) improvement in colorectal cancer screening |
Low risk of bias |
| Sabatino et al. (2012)69 | SR | Colorectal cancer |
Aim: to update a systematic review on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Client reminders. The original review found sufficient evidence of effectiveness for client reminders to increase colorectal cancer screening with FOBT based on a median increase of 11.5 (IQI 8.9 to 20.3) percentage points across four studies (n = 8 effect estimates). The update included three additional studies. Results for colorectal cancer changed from sufficient evidence to strong evidence | High risk of bias |
| Stone et al. (2002)72 | MA of randomised clinical trials, controlled clinical trials | Breast cancer, cervical cancer, colon cancer |
Aim: to assess the relative effectiveness of previously studied approaches for improving adherence to cancer screening guidelines Outcomes: use of services (OR) |
Organisational change was the most potent intervention type followed by patient incentives and patient reminders (adjusted OR, 95% CI 1.74 to 2.75) | High risk of bias |
| Uy et al. (2017)67 | SR of RCTs and non-RCTs | Breast cancer, cervical cancer, colorectal cancer |
Aim: to assess the effect of text messaging interventions on screening Outcomes: screening rates (%) |
Combined breast, cervical and colorectal cancer (n = 9): text messaging can moderately increase screening rates for breast and cervical cancers and may improve colorectal cancer screening to a lesser degree. Across all studies, text messaging interventions led to increases in absolute screening rates of 0.6–15% and relative screening rates of 4–63% vs. controls Breast cancer (n = 5) and cervical cancer (n = 1): increases in absolute screening rates ranged from 4.5% to 15% and relative screening rates found improvements of 20–63%. Although the smallest reported change in absolute screening rate was not statistically significant, both the overall direction and the magnitude of absolute effect for text messaging seem consistent for breast and cervical cancers |
Low risk of bias |
| Brouwers et al. (2011)70 | SR of RCTs | Breast cancer, cervical cancer, colorectal cancer |
Aim: to evaluate interventions designed to increase the rate of screening Outcomes: screening uptake |
Client reminders appear to be an effective intervention in increasing uptake of screening for the three cancers | High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Mandelblatt and Yabroff (1999)81 | MA of RCTs and concurrently controlled trials | Breast cancer |
Aim: to determine the effectiveness of interventions targeted at providers to enhance the use of mammography Outcomes: mammography utilisation (difference, %) |
Overall, the provider-targeted interventions with usual-care controls had an effect of increasing mammography by 13.2% (95% CI 7.8% to 18.4%). The interventions using active controls had an overall higher rate of mammography (6.8%, 95% CI 4.8% to 8.7%) | High risk of bias |
| Baron et al. (2010)106 | SR of all types of study designs with comparison group | Breast cancer |
Aim: to present the effectiveness, applicability and economic efficiency of provider reminder/recall interventions to increase screening Outcomes: screening completion (median post-intervention increase, percentage points) |
Mammography screening increased by a median of 10.0% (IQI 3.0% to 19.0%) | High risk of bias |
| Cheong et al. (2017)54 | SR and MA of RCTs, non-RCTs and pre–post | CVD |
Aim: to determine the effectiveness of existing intervention strategies to increase uptake of CVD risk factors screening Outcomes: uptake (RR) |
Using physician reminders (RR 1.392, 95% CI 1.192 to 1.625 in pessimistic analysis; RR 1.471, 95% CI 1.304 to 1.660 in optimistic analysis) for screening significantly increased the uptake of CVD risk factors screening vs. the controlled groups | High risk of bias |
| Baron et al. (2010)106 | SR of all types of study designs with comparison group | Cervical cancer |
Aim: to present the effectiveness, applicability and economic efficiency of provider reminder/recall interventions to increase screening Outcomes: screening completion (median post-intervention increase, percentage points) |
Pap screening increased by a median of 4.6% (IQI 2.4% to 9.2%) | High risk of bias |
| Dougherty et al. (2018)65 | SR and MA of RCTs | Colorectal cancer |
Aim: to identify interventions associated with increasing colorectal cancer screening rates and their effect sizes Outcomes: screening completion (RR and RD) |
Clinician reminders (RD 13%, 95% CI 8% to 19%) increased colorectal cancer screening completion rates vs. usual care | Low risk of bias |
| Siddiqui et al. (2011)105 | SR and MA of prospective randomised studies | Colorectal cancer |
Aim: to examine the uptake of FOBT after physician reminders as part of the colorectal cancer screening process Outcomes: uptake (%) |
All five studies obtained a higher percentage uptake when physician reminders were given. However, the combined increase was not statistically significant (random effects model: RD 6.6%, 95% CI –2% to 14.7%; z = 1.59; p = 0.112) | High risk of bias |
| Issaka et al. (2019)64 | SR of RCTs, quasi-experimental and observational | Colorectal cancer |
Aim: to determine the evidence of efficacy of interventions to improve FIT completion Outcomes: screening uptake (median efficacy, %) |
Provider alerts (n = 2). Modest improvement in FIT completion | Low risk of bias |
| Baron et al. (2010)106 | SR of all types of study designs with comparison group | Colorectal cancer |
Aim: to present the effectiveness, applicability and economic efficiency of provider reminder/recall interventions to increase screening Outcomes: screening completion (median post-intervention increase, percentage points) |
FOBTs and flexible sigmoidoscopy increased by a median of 15.3% (IQI, 1.0% to 24.2%). For FOBT alone, the median was 10.5% (IQI, 0.0% to 23.1%), whereas the single effect measure for flexible sigmoidoscopy was 24.3% | High risk of bias |
| Baron et al. (2010)106 | SR of all types of study designs with comparison group | Breast cancer, cervical cancer, colorectal cancer |
Aim: to present the effectiveness, applicability and economic efficiency of provider reminder/recall interventions to increase screening Outcomes: screening completion (median post-intervention increase, percentage points) |
All except four estimates were in a favourable direction for provider reminder interventions to increase screening for breast, cervical and colorectal cancers. The median post-intervention increase was 7.2% (IQI 2.4% to 19.7%) | High risk of bias |
| Ferroni et al. (2012)46 | SR | Breast cancer, cervical cancer, colorectal cancer |
Aim: to assess the effectiveness of population-based screening programmes in increasing coverage compared with spontaneous access Outcomes: screening participation (RR) |
GP-based organised screening (reminder) vs. no intervention (n = 8) showed a significant effect when compared with no intervention for breast cancer (RR 1.74, 95% CI 1.25 to 2.43), but not for cervical and colorectal cancer | High risk of bias |
| Kelly et al. (2018)89 | SR of RCT, randomised trial, quasi-experimental, pre–post, non-RCT, cohort | Breast cancer, cervical cancer, colon cancer, prostate cancer, hepatitis B virus |
Aim: to determine which interventions have successfully increased screening uptake among minorities Outcomes: screening uptake |
Physician targeted interventions (n = 2). Both studies were significant. The number of studies was small, limiting the ability to draw conclusions | High risk of bias |
Provider: educational and incentive interventions
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Dougherty et al. (2018)65 | SR and MA of RCTs | Colorectal cancer |
Aim: to identify interventions associated with increasing colorectal cancer screening rates and their effect sizes Outcomes: screening completion (RR and RD) |
Clinician interventions of academic detailing (RD 10%, 95% CI 3% to 17%; n = 6) increased colorectal cancer screening completion rates compared with usual care | Low risk of bias |
| Teo et al. (2018)53 | SR and MA of RCTs and cluster RCTs | Prostate cancer, HIV, STIs, testicular cancer, melanoma |
Aim: to determine the effectiveness of interventions in improving men’s uptake of and intention to undergo screening Outcomes: uptake (RR) |
Health-care professional training (RR 1.27, 95% CI 1.09 to 1.50) was shown to be more effective in increasing men’s screening uptake compared with usual care | High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Mandelblatt and Yabroff (1999)81 | MA of RCTs and concurrently controlled trials | Breast cancer |
Aim: to determine the effectiveness of interventions targeted at providers to enhance the use of mammography Outcomes: mammography utilisation (difference, %) |
The interventions included in this sample used audit with feedback and educational sessions or materials. Compared with usual care, cognitive interventions increase mammography by 18.6% (95% CI 12.8% to 24.4%) | High risk of bias |
| Sabatino et al. (2012)69 | SR | Breast cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Provider assessment and feedback. Findings for mammography varied from 3.4 to 20.6 percentage points | High risk of bias |
| Sabatino et al. (2012)69 | SR | Cervical cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Provider assessment and feedback. Findings for Pap varied from 4.0 to 29.5 percentage points | High risk of bias |
| Sabatino et al. (2012)69 | SR | Colorectal cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Provider assessment and feedback. Findings for FOBT varied from 12.3 to 23.0 percentage points | High risk of bias |
| Stone et al. (2002)72 | MA of randomised clinical trials, controlled clinical trials | Breast cancer, cervical cancer, colon cancer |
Aim: to assess the relative effectiveness of previously studied approaches for improving adherence to cancer screening guidelines Outcomes: use of services (OR) |
Provider feedback was one of the least effective interventions [mammography, OR 1.76 (95% CI 1.44 to 2.15); cervical cytology, OR 1.10; (95% CI 0.93 to 1.31); colon cancer, OR 1.18 (95% CI 0.98 to 1.43)] compared with organisational change, patient financial incentives, patient reminders and patient education | High risk of bias |
| Brouwers et al. (2011)70 | SR of RCTs | Breast cancer, cervical cancer, colorectal cancer |
Aim: to evaluate interventions designed to increase the rate of screening Outcomes: screening uptake |
Provider audit and feedback appears to be an effective intervention to increase the uptake of screening for three cancers | High risk of bias |
| Sabatino et al. (2012)69 | SR | Breast cancer, cervical cancer, colorectal cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Provider assessment and feedback. Findings across all screening sites led to a median increase in screening use of 13.0 (IQI 5.5 to 21.8) percentage points. There is sufficient evidence that provider assessment and feedback interventions are effective in increasing screening for breast cancer (mammography), cervical cancer (Pap test) and colorectal cancer (FOBT) | High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Sabatino et al. (2012)69 | SR | Breast cancer, cervical cancer, colorectal cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Provider incentives. The median change in screening use across the studies was 1.7 (IQI –0.1 to 3.6) percentage points. Findings for mammography varied from –2.0 to 1.7 percentage points, findings for Pap varied from 3.6 to 8.0 percentage points and findings for colorectal cancer screening varied from –0.1 to 2.8 percentage points. There is insufficient evidence to determine the effectiveness of provider incentives in increasing screening for breast, cervical or colorectal cancers. Evidence is insufficient because results were inconsistent and generally small | High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Rat et al. (2018)52 | SR of RCTs | Colorectal cancer |
Aim: to synthesise evidence on interventions aiming to increase uptake of faecal tests for screening and interventions that targeted GP involvement Outcomes: screening uptake (OR) |
Improving GP involvement (e.g. reminder, letter, training) (n = 3). Two studies showed increased uptake from 12.2% to 15.3% (each statistically significant), whereas one study was inconclusive | Low risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Sabatino et al. (2012)69 | SR | Breast cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Reducing out-of-pocket costs: the original review found sufficient evidence of effectiveness of interventions that reduce out-of-pocket costs to promote breast cancer screening, based on a median increase in completed mammography across eight intervention arms of 11.5 (IQI 6.0 to 28.5) percentage points. No additional studies were identified during the update Client incentives: insufficient evidence |
High risk of bias |
| Cheong et al. (2017)54 | SR and MA of RCTs, non-RCTs and pre–post | CVD |
Aim: to determine the effectiveness of existing intervention strategies to increase uptake of CVD risk factors screening Outcomes: uptake (RR) |
Providing financial incentives (RR 1.462, 95% CI 1.068 to 2.000) for screening significantly increased the uptake of CVD risk factors screening compared with the controlled groups | High risk of bias |
| Sabatino et al. (2012)69 | SR | Cervical cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Reducing out-of-pocket costs: one qualifying study. There is insufficient evidence to determine its effectiveness in increasing screening for cervical because too few studies were identified Client incentives: insufficient evidence |
High risk of bias |
| Dougherty et al. (2018)65 | SR and MA of RCTs | Colorectal cancer |
Aim: to identify interventions associated with increasing screening rates and their effect sizes Outcomes: screening completion (RR and RD) |
Pooling data across trials demonstrated slightly increased screening completion with US$5 incentives (RR 1.09, 95% CI 1.01 to 1.18; RD 3%, 95% CI 0% to 6%), but not US$10 incentives (RR 1.02, 95% CI 0.85 to 1.23; RD 1%, 95% CI −7% to 8%) or with pooling all financial incentive groups (RR 1.16, 95% CI 0.95 to 1.42; RD 6%, 95% CI −2% to 14%) | Low risk of bias |
| Sabatino et al. (2012)69 | SR | Colorectal cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Reducing out-of-pocket costs: no studies were identified. Insufficient evidence Client incentives: insufficient evidence |
High risk of bias |
| Stone et al. (2002)72 | MA of randomised clinical trials, controlled clinical trials | Breast cancer, cervical cancer, colon cancer |
Aim: to assess the relative effectiveness of previously studied approaches for improving adherence to cancer screening guidelines Outcomes: use of services (OR) |
The next most effective intervention components after organisational change was patient financial incentives (adjusted OR 1.82 to 3.42) | High risk of bias |
Reducing structural barriers: patients
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Han et al. (2009)76 | MA of (quasi-) experimental studies | Breast cancer |
Aim: to determine the effectiveness of the interventions for improving mammography screening among asymptomatic ethnic minority women Outcomes: mammography screening (overall MWES) |
Access-enhancing interventions had the largest MWES, at 0.155 (n = 6, z = 4.488; p < 0.001) compared with individual-directed interventions, mass media, community education and social network interventions | High risk of bias |
| Legler et al. (2002)78 | MA of (quasi-) experimental studies | Breast cancer |
Aim: to determine which types of mammography-enhancing interventions are most effective for these diverse populations Outcomes: mammography use (difference, %) |
Access-enhancing interventions (n = 14) had an estimated effect of 18.9% (95% CI 10.4% to 27.4%), which was the largest, followed by individual-directed interventions, community education, media campaigns and social network | High risk of bias |
| Lu et al. (2012)75 | SR of RCTs, non-equivalent control group, prospective cohort | Breast cancer |
Aim: to update current knowledge on the effectiveness of existing intervention strategies to enhance screening uptake in Asian women Outcomes: screening uptake |
Evidence (n = 2) was found to support onsite mobile mammography in increasing mammography intake among certain Asian women (and among Korean women in the USA) | High risk of bias |
| Sin and Leger (1999)63 | SR of RCTs | Breast cancer |
Aim: to evaluate the effectiveness of the different interventions to increase breast screening uptake Outcomes: screening uptake |
System directed/access enhancing (n = 2). No strong evidence exists in this category | High risk of bias |
| Sabatino et al. (2012)69 | SR | Breast cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Reducing structural barriers. The original review found strong evidence of effectiveness for reducing structural barriers to breast cancer screening, based on a median overall increase in mammography use of 17.7 (IQI 11.5 to 30.5) percentage points across seven studies. The update included one additional study, reaffirming original review results | High risk of bias |
| Han et al. (2011)76 | MA of RCTs and non-RCTs | Cervical cancer |
Aim: to examine the overall effectiveness of these interventions in increasing Pap test use by ethnic minority women in the USA Outcomes: Pap test use (overall MWES) |
Access-enhancing interventions (n = 6) increased compliance with cervical cancer screening to a greater extent than other types of interventions (e.g. community education, individual-directed or mass media) and yielded the largest effect size (d = 0.253, 95% CI 0.110 to 0.397) | High risk of bias |
| Sabatino et al. (2012)69 | SR | Cervical cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Reducing structural barriers (n = 3). For the overall body of evidence, the median increase in Pap screening was 13.6 (range of values 5.9–17.8) percentage points. Evidence was insufficient to determine the effectiveness of reducing structural barriers in increasing screening for cervical cancer | High risk of bias |
| Sabatino et al. (2012)69 | SR | Colorectal cancer |
Aim: to update a SR on the effectiveness of nine interventions to increase screening Outcomes: screening uptake |
Reducing structural barriers (n = 11). The original review found strong evidence of effectiveness of interventions to reduce structural barriers to colorectal cancer screening with FOBT. The median increase was 16.1 (IQI 12.1 to 22.9) percentage points. Five additional studies were included in the update. Based on four effect estimates in the update studies, there was a median 36.9 (IQI 16.3 to 41.1) percentage point increase across colorectal cancer screening tests | High risk of bias |
| Brouwers et al. (2011)70 | SR of RCTs | Breast cancer, cervical cancer, colorectal cancer |
Aim: to evaluate interventions designed to increase the rate of screening Outcomes: screening uptake |
Reduction of structural barriers appears effective, but their roles with colorectal cancer and cervical screening, respectively, are less established | High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Cheong et al. (2017)54 | SR and MA of RCTs, non-RCTs and pre–post | CVD |
Aim: to determine the effectiveness of existing intervention strategies to increase uptake of CVD risk factors screening Outcomes: uptake (RR) |
Using dedicated personnel (RR 1.510, 95% CI 1.014 to 2.247 in the pessimistic analysis; RR 2.536, 95% CI 1.297 to 4.960 in the optimistic analysis) for screening significantly increased the uptake of CVD risk factors screening, compared with the controlled groups | High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Verdoodt et al. (2015)55 | SR and MA of RCTs | Cervical cancer |
Aim: to evaluate the participation after an invitation including a self-sampling device vs. an invitation to have a sample taken by a health professional sent to underscreened women Outcomes: participation rates (absolute participation, %) |
The pooled participation in the self-sampling arm was 23.6% (95% CI 20.2% to 27.3%) when self-sampling kits were sent by mail to all women vs. 10.3% in the control arm (participation difference 12.6%, from 9.3% to 15.9%) | Low risk of bias |
| Musa et al. (2017)79 | SR and MA of RCTs and CBPR | Cervical cancer |
Aims: to understand the effect of provider recommendations for screening to eligible women population at risk of cancer Outcomes: screening rate (OR) |
Offering women the option of self-sampling for HPV testing increased cervical cancer screening rates nearly twofold (OR 1.71, 95% CI 1.32 to 2.22) | High risk of bias |
| Camilloni et al. (2013)56 | SR and MA of RCTs, quasi-experimental, prospective cohort, cross-sectional, pre–post | Cervical cancer |
Aim: to assess the efficacy of interventions to increase participation in organised population-based screening programmes Outcomes: participation (RR) |
Mailing a kit for self-sampling cervical specimens increased participation in non-responders (RR 2.37, 95% CI 1.44 to 3.90) | High risk of bias |
| Rees et al. (2018)66 | SR of RCTs and quasi-RCTs | Cervical cancer |
Aim: to assess the effectiveness of interventions to improve the uptake of screening among lower socioeconomic groups Outcomes: screening uptake |
HPV self-testing (n = 2). Both interventions showed statistically significant increases in attendance | Low risk of bias |
| Dougherty et al. (2018)65 | SR and MA of RCTs | Colorectal cancer |
Aim: to identify interventions associated with increasing screening rates and their effect sizes Outcomes: screening completion (RR and RD) |
FBT outreach (RR 2.26, 95% CI 1.81 to 2.81; RD 22%, 95% CI 17% to 27%) increased colorectal cancer screening completion rates compared with usual care | Low risk of bias |
| Jager et al. (2019)80 | SR and MA of RCTs | Colorectal cancer |
Aims: to compare the impact of a mailed outreach offering stool tests vs. usual care or clinic-based screening offers on colon cancer screening uptake in the USA Outcomes: screening completion (RR) |
Mailed outreach resulted in a 28% absolute (95% CI 25% to 30%; I2 = 47%) and a 2.8-fold relative (RR 2.65, 95% CI 2.03 to 3.45; I2 = 92%) increase in screening completion compared with usual care, with the number needed to invite estimated to be 3.6. Telephone reminders (27%) were associated with a similar increase as studies without telephone reminders (29%) The pooled absolute increase in screening focusing on underserved and/or minority populations (n = 6) was 27% (95% CI 23% to 30%; I2 = 49%) |
High risk of bias |
| Issaka et al. (2019)64 | SR of RCTs, quasi-experimental and observational | Colorectal cancer |
Aim: to determine the evidence of efficacy of interventions to improve FIT completion that could be scaled Outcomes: screening uptake (median efficacy, %) |
Mailed FIT outreach (n = 10). Among included studies, the median efficacy of mailed FIT outreach to improve colorectal cancer screening vs. controls was 21.5% (IQR 13.6–29.0%) | Low risk of bias |
| Rat et al. (2018)52 | SR of RCTs | Colorectal cancer |
Aim: to synthesise evidence on interventions aiming to increase uptake of faecal tests for colorectal cancer screening Outcomes: screening uptake (OR) |
Postal mailing of kits (n = 5). Mailing kits to screening invitees increased uptake (OR 1.31–2.89) | Low risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Mandelblatt and Yabroff (1999)81 | MA of RCTs and concurrently controlled trials | Breast cancer |
Aim: to determine the effectiveness of interventions targeted at providers to enhance the use of mammography Outcomes: mammography utilisation (difference, %). |
These provider-targeted interventions used nurse-based interventions or reorganisation of the clinic and improved mammography utilisation by 13.1% (95% CI 6.8% to 19.3%) | High risk of bias |
| Stone et al. (2002)72 | MA of randomised clinical trials, controlled clinical trials | Breast cancer, cervical cancer, colon cancer |
Aim: to assess the relative effectiveness of previously studied approaches for improving adherence to cancer screening guidelines Outcomes: use of services (OR) |
The most potent intervention types involved organisational change in process (the adjusted OR for increased use of services from organisational change ranged from 2.47 to 17.6) followed by patient financial incentives and patient reminders | High risk of bias |
| Teo et al. (2018)53 | SR and MA of RCTs and cluster RCTs | Prostate cancer, HIV, STIs, testicular cancer, melanoma |
Aim: to determine the effectiveness of interventions in improving men’s uptake of and intention to undergo screening Outcomes: uptake (RR) |
Clinical practice improvement interventions (RR 5.25, 95% CI 1.31 to 21.06) was shown to be more effective in increasing men’s screening uptake than usual care | High risk of bias |
Multiple strategies
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Corcoran et al. (2010)111 | SR and MA of (quasi-) experimental | Breast cancer |
Aim: to determine the effectiveness of interventions designed to increase mammography rates among US Latina women Outcomes: screened vs. unscreened (OR) |
Education plus reducing structural barriers. Random-effects model: OR 1.385 (95% CI 0.974 to 1.970). Fixed-effects model: OR 1.151 (95% CI 1.019 to 1.299) The overall effect indicated a low level of effectiveness |
High risk of bias |
| Gardner et al. (2013)82 | SR and MA of RCTs | Breast cancer |
Aim: to estimate the magnitude of the effect of interventions used to increase uptake of mammography among low-income women Outcomes: uptake (RD) |
Multiple interventions (n = 11) increased the uptake by a difference of 20.7% (CI 11.3% to 30.0%) compared with women in the control group | High risk of bias |
| Copeland et al. (2018)107 | MA of RCTs | Breast cancer |
Aim: to report on the effects of clinical trials in breast cancer screening for African American women Outcomes: uptake (OR) |
Psychosocial, behavioural and education interventions Findings indicated that screening interventions for African American women were significantly more likely to result in mammography than the control (OR 1.56, 95% CI 1.27 to 1.93; p < 0.0001). Average effect size was modest in magnitude and indicated that African American women were approximately 1.5 times more likely to receive a mammogram when they were randomised to pro-screening interventions |
Low risk of bias |
| Legler et al. (2002)78 | MA of (quasi-) experimental studies | Breast cancer |
Aim: to determine which types of mammography-enhancing interventions are most effective for these diverse populations Outcomes: mammography use (difference, %) |
The use of multiple intervention types was effective, with intervention effects averaging 13.3% overall (95% CI 8.6% to 18%; n = 26 studies). The most effective combination of intervention types appears to be access-enhancing interventions combined with individual-directed interventions. These studies had an estimated combined intervention effect of 26.9 (95% CI 9.9 and 43.9; n = 9 studies). The next largest effect was for combining access-enhancing and system-directed interventions (19.4, 95% CI 8.2 and 30.6; n = 5 studies) | High risk of bias |
| Mandelblatt and Yabroff (1999)81 | MA of RCTs and concurrently controlled trials | Breast cancer |
Aim: to determine the effectiveness of interventions targeted at providers to enhance the use of mammography Outcomes: mammography utilisation (difference, %) |
Behavioural and cognitive strategies to reach providers. The combined effect was a 21.0% increase in mammography utilisation (95% CI 8.8% to 33.6%) vs. usual care. Eliminating the study associated with heterogeneity led to a combined increase in mammography utilisation of 16.1% (95% CI 11.6% to 20.7%). Finally, when cognitive and behavioural strategies are targeted at patients and providers in communities, interventions are no longer effective (1.1% increase, 95% CI 26.8% to 9.0%) | High risk of bias |
| Yabroff and Mandelblatt (1999)74 | MA of randomised or concurrent control design | Breast cancer |
Aim: to determine the effects of patient-based mammography screening strategies Outcomes: screening (difference, %) |
Multicomponent interventions (n = 5): behavioural and cognitive interventions had variable effectiveness, ranging from little effect to a maximum effect of 33% | High risk of bias |
| Escribà-Agüir et al. (2016)108 | SR of RCTs and quasi-experimental studies | Breast cancer |
Aim: to identify, characterise and analyse the effectiveness of patient-targeted health-care interventions to promote cancer screening programmes in ethnic minorities Outcomes: screening participation |
Overall, the interventions (i.e. education, reminders, reducing structural barriers and out-of-pocket costs) were effective (n = 4) in increasing breast cancer screening participation and this was reflected in a statistically significant increase in cancer screening rates after the intervention | Low risk of bias |
| Bonfill et al. (2001)61 | SR and MA of RCTs | Breast cancer |
Aim: to assess the effectiveness of different strategies for increasing the participation rate of women invited to community cancer screening activities or mammography programmes Outcomes: screening attendance (OR) |
The evidence favoured five training activities plus direct reminders for women (OR 2.46, 95% CI 1.72 to 3.50) Letters of invitation to multiple examinations plus educational material favoured the control group (OR 0.62, 95% CI 0.32 to 1.20) |
High risk of bias |
| Donnelly and Hwang (2015)109 | SR of RCT and quasi-experimental studies | Breast cancer |
Aim: to improve the development of effective intervention programmes that promote breast cancer screening among Arabic women living in Qatar Outcomes: breast cancer screening |
Multilevel interventions that target general populations (especially women), health-care professionals and/or larger systems are more likely to be successful than single educational interventions or public awareness campaigns | High risk of bias |
| Cheong et al. (2017)54 | SR and MA of RCTs, non-RCTs and pre–post | CVD |
Aim: to determine the effectiveness of existing intervention strategies to increase uptake of CVD risk factors screening Outcomes: uptake (RR) |
Provider and patient interventions using multifaceted approaches were effective when optimistic analysis was performed (RR 2.268, 95% CI 1.401 to 3.672), but not when pessimistic analysis was performed (RR 1.549, 95% CI 0.978 to 2.453) | High risk of bias |
| Musa et al. (2017)79 | SR and MA of RCTs and Community Based Participatory Research Track | Cervical cancer |
Aims: to understand the effect of provider recommendations for screening on eligible women population at risk of cervical cancer Outcomes: screening rate (OR) |
Provider recommendations. A trend towards positive effects of the various provider-based interventions on cervical cancer screening rates was found | High risk of bias |
| Rees et al. (2018)66 | SR of RCTs and quasi-RCTs | Cervical cancer |
Aim: to assess the effectiveness of interventions to improve the uptake of cancer screening among lower socioeconomic groups Outcomes: screening uptake |
Mixed interventions (n = 7). In-reach, out-reach and community-based education. Potential to increase screening rates. Most increased screening rates, but only a few were statistically significant | Low risk of bias |
| Escribà-Agüir et al. (2016)108 | SR of RCTs and quasi-experimental studies | Cervical cancer |
Aim: to identify, characterise and analyse the effectiveness of patient-targeted health-care interventions to promote cancer screening programmes in ethnic minorities Outcomes: screening participation |
The use of the intervention strategies (i.e. education with reducing structural barriers and out-of-pocket cost; counselling with small media) was effective in promoting cervical cancer screening because the studies found a statistically significant improvement in cancer screening rates after the intervention (n = 4) | Low risk of bias |
| Black et al. (2002)60 | SR of cohort analytic, CCT, pre–post, ITS | Cervical cancer |
Aim: to evaluate and summarise evidence of the effectiveness of interventions available to public health staff that could be used to increase cancer screening rates in women Outcomes: screening rates |
Mass media (n = 9) combined with invitation letter and/or education. Studies that combined mass media campaigns with other strategies were effective at increasing either Pap smear rates or early cancer detection. The evidence suggests that a successful community programme combines a mass media campaign with direct tailored information/education to women and/or health-care providers | Low risk of bias |
| Dougherty et al. (2018)65 | SR and MA of RCTs | Colorectal cancer |
Aim: to identify interventions associated with increasing screening rates and their effect sizes Outcomes: screening completion (RR and RD) |
Interventions with multiple components were associated with greater increases in screening rates (vs. usual care) than those with single components (RR 1.92, 95% CI 1.69 to 2.19 vs. RR 1.43, 95% CI 1.19 to 1.71; RD 19%, 95% CI 16% to 23% vs. RD 6%, 95% CI 4% to 8%), albeit with high statistical and clinical heterogeneity. Meta-regression suggested that a screening test outreach component was more essential to the multicomponent effect than navigation, patient reminder or clinician reminder components | Low risk of bias |
| Escribà-Agüir et al. (2016)108 | SR of RCTs and quasi-experimental studies | Colorectal cancer |
Aim: to identify, characterise and analyse the effectiveness of patient-targeted health-care interventions to promote cancer screening programmes in ethnic minorities Outcomes: screening participation |
Overall, the interventions (i.e. education, reducing structural barriers and out-of-pocket costs) were effective in increasing cancer screening participation (n = 6), which was reflected in a statistically significant increase in cancer screening rates after the intervention | Low risk of bias |
| Morrow et al. (2010)104 | SR of community-based RCTs | Colorectal cancer |
Aim: to summarise the current literature of community-based screening RCTs in multiethnic groups Outcomes: screening adherence |
Patient mailings (e.g. reminders, educational material, tailored messages; n = 3): studies demonstrated significant differences between intervention and control groups in enhancing colorectal cancer screening adherence Telephone outreach (e.g. reducing structural barriers, education and invitations): all studies categorised as telephone outreach studies demonstrated significant screening rate improvements in the intervention group vs. controls Electronic/multimedia (e.g. provider reminders, education): one out of four studies demonstrated a significant difference between intervention and control groups. This study focused on FOBT screening only |
High risk of bias |
| Escribà-Agüir et al. (2016)108 | SR of RCTs and quasi-experimental | Breast cancer, cervical cancer, colorectal cancer, lung cancer, prostate cancer |
Aim: to identify, characterise and analyse the effectiveness of patient-targeted health-care interventions to promote cancer screening programmes in ethnic minorities Outcomes: screening participation |
Breast and cervical cancer (n = 3): the intervention strategies (i.e. education, small media and reminders) were effective in increasing breast and cervical cancer screening participation in only two out of the three interventions | Low risk of bias |
| Escoffery et al. (2014)110 | SR of pre–post, post | Breast cancer, cervical cancer, colorectal cancer |
Aim: to present findings from a SR on the impact of special events to promote cancer education and screening Outcomes: screening rates (%) |
Special events used two to four interventions, including reducing structural barriers, group education, individual education, small media and reducing out-of-pocket costs. Mammography screening rates ranged from 4.8% to 88%, Pap testing was 3.9% and clinical breast exams ranged from 9.1% to 100%. For colorectal screening, FOBT ranged from 29.4% to 76% and sigmoidoscopy was 100% at one event. The one event, which focused on breast and colorectal cancer screening, reported statistically significant changes in screening from pre- to post-test | High risk of bias |
| Hou et al. (2011)90 | SR of randomised, quasi-experimental, BA | Breast cancer, cervical cancer, colorectal cancer |
Aim: to review published literature describing screening interventions for cancers among Asian populations in the USA with a view to identifying effective programmes for specific Asian groups Outcomes: screening |
Effective interventions employed a variety of strategies, including the use of social networks, lay health workers, media education, community-based education, reminder notices, health-care provider assistance and health system changes. Specifically, lay health workers and mass education campaigns that target delivery channels widely accessed by Asian American people, such as Asian grocery stores or churches (in the case of Korean people), are successful | High risk of bias |
| Kelly et al. (2018)89 | SR of RCT, randomised trial, quasi-experimental, pre–post, non-RCT, cohort | Breast cancer, cervical cancer, colon cancer, prostate cancer, hepatitis B virus |
Aim: to determine which interventions have successfully increased screening uptake among minority groups Outcomes: screening uptake |
Multifaceted interventions (n = 15) with a variety of interventions used across all ethnicities and screening modalities. Thirteen studies had significant increases in screening uptake. We do note, however, that some studies found no additional improvement in screening for multiple interventions over a single intervention, which has financial implications | High risk of bias |
| Study | Study type | Screening programme | Aim and main outcome | Relevant results | Quality assessment |
|---|---|---|---|---|---|
| Gardner et al. (2013)82 | SR and MA of RCTs | Breast cancer |
Aim: to estimate the magnitude of the effect of interventions used to increase uptake of mammography among low-income women Outcomes: uptake (RD) |
Simple interventions (n = 15) increased the uptake by a difference of 6.9% (CI 1.8% to 11.9%) compared with women in the control group | High risk of bias |
| Han et al. (2009)76 | MA of (quasi-) experimental studies | Breast cancer |
Aim: to determine the effectiveness of the interventions for improving mammography screening among asymptomatic ethnic minority women Outcomes: mammography screening (overall MWES) |
Individually directed interventions (i.e. counselling, letters, reminders) (n = 19, MWES = 0.099, z = 6.552; p = 0.001) had the second biggest MWES after access-enhancing interventions. Mass media, community education and social networks had smaller, non-significant effects When combined intervention effects were examined for each ethnic group, the estimated intervention effect was significant for African American women, with a MWES of 0.098 (n = 9, z = 2.550; p = 0.011). Studies with other ethnic minority women yielded no significant findings, with a MWES of 0.094 for Asian and Pacific Islander women (n = 5, z = 1.955; p = 0.051) and 0.036 for Hispanic women (n = 5, z = 1.004; p = 0.315) |
High risk of bias |
| Legler et al. (2002)78 | MA of (quasi-) experimental studies | Breast cancer |
Aim: to determine which types of mammography-enhancing interventions are most effective for these diverse populations Outcomes: mammography use (difference, %) |
The impact of individual-directed interventions in health-care settings was nearly identical to that of access-enhancing strategies, with an estimated effect of 17.6% (95% CI 11.6% to 24%; n = 15). Efforts in community settings yielded effects of 6.8% (95% CI 1.8% to 11.8%; n = 13), followed by community education, social network and media interventions | High risk of bias |
| Anastasi and Lusher (2019)83 | SR of RCTs, cohort, cross-sectional pilot | Breast cancer |
Aim: to examine the impact of awareness campaigns on breast cancer awareness, breast self-examination and attendance at screening programmes Outcomes: screening attendance |
Breast cancer awareness interventions (i.e. community education, clinical engagement and tailoring) were found to increase the uptake of breast self-examination behaviours and increased the likelihood of breast cancer screening attendance | High risk of bias |
| Han et al. (2011)77 | MA of RCTs and non-RCTs | Cervical cancer |
Aim: to examine the overall effectiveness of interventions in increasing Pap test use by ethnic minority women in the USA Outcomes: Pap test use (overall MWES) |
Individually directed interventions (i.e. counselling, letters and reminders) (n = 10) (d = 0.132, 95% CI 0.069 to 0.195) increased compliance with cervical cancer screening to a lesser extent than access-enhancing and community education interventions Combined intervention effects were examined for each ethnic group. The effect size point estimates were significant for African American women (d = 0.146, 95% CI 0.028 to 0.265) and Asian women (d = 0.177, 95% CI 0.098 to 0.256). The interventions involving Hispanic women yielded a positive effect size of 0.116, but the lower bound of the 95% CI was negative (95% CI −0.008 to 0.240), suggesting no effect of the interventions on Pap test use in these women |
High risk of bias |
| Rees et al. (2018)66 | SR of RCTs and quasi-RCTs | Cervical cancer |
Aim: to assess the effectiveness of interventions to improve uptake of cancer screening among lower socioeconomic groups Outcomes: screening uptake |
In-reach strategies (n = 5) directed at health-care professionals and patients: all studies showed an increase in screening rates, but only three were statistically significant | Low risk of bias |
| Agide et al. (2018)84 | SR of RCTs, quasi-RCTs and non-RCTs | Cervical cancer |
Aim: to see the effectiveness of health education interventions in screening uptake Outcomes: screening uptake (%) |
Individual-level interventions (i.e. education, letters and reminders) (n = 6). Most studies (n = 5) boosted screening and/or intervention uptake | High risk of bias |
Appendix 4 workHORSE methods
| Exposure | Statistical modelling (distribution) | Independent variable | Comment |
|---|---|---|---|
| Equivalised income quintile groups | Logit ordinal regression | Age, sex, QIMD, SHA, ethnicity, education | Year was not included as this is a relative measure of income |
| Active days per week | Logit ordinal regression | Year, age, sex, QIMD, SHA, ethnicity | |
| Daily fruit consumption in grams | GAMLSS (zero-inflated Sichel) | Year, age, sex, QIMD, SHA, ethnicity | |
| Daily vegetable consumption in grams | GAMLSS (Delaporte) | Year, age, sex, QIMD, SHA, ethnicity | |
| Smoking status (never/ex, occasionally/ex, regularly/current) | GAMLSS (multinomial with four categories) | Year, age, sex, QIMD, SHA, ethnicity | All of the smoking-related variables are used in a smoking microsimulation subroutine that simulates smoking histories |
| Years of abstinence for ex-smokers | GAMLSS (double Poisson) | Year, age, sex, QIMD, SHA, ethnicity | Applies to the first year that a synthetic individual enters the simulation only. Then is estimated from the smoking subroutine |
| Smoking duration for ex-smokers | GAMLSS (double Poisson) | Year, age, sex, QIMD, SHA, ethnicity | Applies to the first year that a synthetic individual enters the simulation only. Then is estimated from the smoking subroutine |
| Smoking duration for current smokers | GAMLSS (negative binomial) | Year, age, sex, QIMD, SHA, ethnicity | Applies to the first year that a synthetic individual enters the simulation only. Then is estimated from the smoking subroutine |
| Smoking initiation probability | GAMLSS (binomial) | Year, age, sex, QIMD, SHA, ethnicity | |
| Smoking cessation probability | GAMLSS (binomial) | Year, age, sex, QIMD, SHA, ethnicity | |
| Smoking relapse probability | Exponential decay | Sex, QIMD, years since cessation | |
| Cigarettes per day for ex-smokers | GAMLSS (negative binomial) | Year, age, sex, QIMD, SHA, ethnicity | |
| Cigarettes per day for current smokers | GAMLSS (negative binomial) | Year, age, sex, QIMD, SHA, ethnicity | |
| Environmental tobacco smoking | GAMLSS (binomial) | Year, age, sex, QIMD, SHA, ethnicity | Currently, this is independent of smoking prevalence in an area |
| Ethanol consumption per day, based on average weekly consumption | GAMLSS (negative binomial) | Year, age, sex, QIMD, SHA, ethnicity, smoking status | Since HSE 2011135 |
| BMI | GAMLSS (Box–Cox power exponential) | Year, age, sex, QIMD, SHA, ethnicity, smoking status | |
| SBP | GAMLSS (Box–Cox power exponential) | Year, age, sex, QIMD, SHA, ethnicity, smoking status | |
| BP medication | GAMLSS (binomial) | Year, age, sex, QIMD, SHA, ethnicity, SBP | Since 2012 |
| Total cholesterol | GAMLSS (Box–Cox t) | Year, age, sex, QIMD, SHA, ethnicity | |
| HDL to total cholesterol ratio | GAMLSS (generalised beta type 1) | Year, age, sex, QIMD, SHA, ethnicity | |
| Statins | GAMLSS (binomial) | Year, age, sex, QIMD, SHA, ethnicity, total cholesterol | Since 2012 |
| AF diagnosed prevalence | GAMLSS (binomial) | Age, sex, QIMD, SHA, ethnicity | Self-reported prevalence of AF in HSE was ≈ 6.9%. This is higher than the QOF prevalence of 1.6% for 2019 and 1.7% for 2015/16. Therefore, we calibrated AF prevalence from HSE to that of QOF |
| AF prevalence (diagnosed and undiagnosed) | Calibrated to PHE estimates by CCG using probabilities from AF diagnosed prevalence |
We matched LSOAs to CCGs Source: PHE204 |
|
| Having a family member with CVD | GAMLSS (binomial) | Age, QIMD, SHA, ethnicity | |
| Chronic kidney disease | Ordered logit regression | Age | |
| Rheumatoid arthritis prevalence | Based on Symmons et al.205 | Age, sex | Source: Symmons et al.205 |
| Corticosteroid use prevalence | GAMLSS (binomial) | Age, sex | |
| T2DM prevalence (diagnosed and undiagnosed) | GAMLSS (binomial) | Age, sex, QIMD, SHA, ethnicity, BMI | This applies to 2013 only. Then T2DM is treated as a disease for which we explicitly model its incidence and mortality (see Chapter 4, Disease module) |
| T2DM diagnosed prevalence | GAMLSS (binomial) | Age, sex, QIMD, SHA, ethnicity, BMI | |
| T2DM duration for diagnosed cases | GAMLSS (generalised Poisson) | Age, sex | |
| Number of comorbidities | Calibrated to Sullivan et al.159 | Age | Source: Sullivan et al.159 |
| Parameter | Outcome | Details | Comments | Source |
|---|---|---|---|---|
| Relative risk for active smoking | CHD and stroke (ICD-10: I20–I25 and I60–I69) | Reanalysis of American Cancer Society’s Cancer Prevention Study II. Prospective cohort study, 6 years of follow-up | Stratified by age and sex. Adjusted for age, race, education, marital status, ‘blue collar’ employment in most recent or current job, weekly consumption of vegetables and citrus fruit, vitamin (A, C and E) use, alcohol use, aspirin use, BMI, exercise, dietary fat consumption, hypertension and diabetes at baseline | Ezzati et al.206 (table 1 model B) |
| Breast cancer (women only) (ICD-10: C50) | Random-effect meta-analysis of 27 prospective and 44 retrospective studies | The results were stable across different subgroup analyses, notably pre/post menopause and alcohol consumption adjustments, including/excluding passive smokers from the referent group | Macacu et al.207 (table 1) | |
| Other non-modelled mortality | Meta-analysis of 1.7 million men and women | Multiply adjusted. We used the non-CVD and non-cancer mortality effects | Stringhini et al.153 (figure 4) | |
| Relative risk for ex-smoking | CHD (ICD-10: I20–I25) | Meta-analysis. Multiply adjusted pooled estimates from 19 prospective studies | Multiply adjusted | Huxley and Woodward208 (figure 8, available in the web version of the original paper) |
| Stroke (ICD-10: I60–I69) | The Framingham Heart Study. Prospective cohort study | Stroke risk decreased significantly by 2 years and was at the level of non-smokers by 5 years after cessation of cigarette smoking | Wolf et al.209 | |
| Breast cancer (women only) (ICD-10: C50) | Random-effect meta-analysis of 27 prospective and 44 retrospective studies | The results were stable across different subgroup analyses, notably pre/post menopause and alcohol consumption adjustments, including/excluding passive smokers from the referent group | Macacu et al.207 (table 1) | |
| Relative risk for pack-years | COPD (ICD-10: J40–J47) | Very detailed random-effect meta-analysis | Smoking duration was not significant, but intensity and pack-years were significant. We used pack-years because they indirectly capture age effect. Most studies for pack-years were about incidence rather than mortality COPD. There was no differentiation between current and ex-smokers. This may dilute the effect | Forey et al.210 |
| Colon cancer (ICD-10: C18) | Meta-analysis of four studies | We used pack-years and considered the effect on incidence of colorectal cancer only, not mortality | Liang et al.211 (table III and text for confidence intervals) | |
| Relative risk for pack-years | Lung cancer (ICD-10: C33–C34 | RCT of 208,371 individuals | We used the PLCO2014 model | Tammemägi et al.212 (table S1) |
| Relative risk for environmental tobacco smoking | CHD (ICD-10: I20–I25) | Meta-analysis of 10 cohort and case–control studies | Adjusted for important CHD risk factors. The effect was applied to never regularly smokers | He et al.213 (table 3, adjusted relative risk) |
| Stroke (ICD-10: I60–I69) | Meta-analysis of 20 prospective, case–control and cross-sectional studies | Thirteen studies adjusted for important CHD risk factors. The overall effect from all 20 studies was used. The effect was applied to never regularly smokers | Oono et al.214 (figure 1) | |
| COPD (ICD-10: J40–J47) | Random-effect meta-analysis of 24 studies | The effect was applied to never regularly smokers | Fischer and Kraemer215 | |
| Lung cancer (ICD-10: C33–C34 | Meta-analysis of 18 case–control studies | The effect was applied to never regularly smokers | Kim et al.216 | |
| Breast cancer (women only) (ICD-10: C50) | GBD meta-analysis | Relative risk from the GBD 2016 study217 | GBD 2016 study217 | |
| Relative risk for SBP | CHD and stroke (ICD-10: I20–I25 and I60–I69) | Meta-analysis of individual data from 61 prospective studies | Stratified by age and sex. Adjusted for regression dilution, total blood cholesterol and, where available, lipid fractions (HDL and non-HDL cholesterol), diabetes, weight, alcohol consumption and smoking at baseline | Lewington et al.218 (figures 3 and 5) |
| Other non-modelled mortality | Meta-analysis of 1.7 million men and women | Multiply adjusted. We used the non-CVD and non-cancer mortality effects. We applied the effect to those with SBP > 140 mmHg | Stringhini et al.153 (figure 4) | |
| Relative risk for total cholesterol | CHD and stroke (ICD-10: I20–I25 and I60–I69) | Meta-analysis of individual data from 61 prospective studies | Stratified by age and sex. Adjusted for regression dilution and age, sex, study, SBP and smoking | Prospective Studies Collaboration219 (web, table 6 fully adjusted and figure 3) |
| Relative risk for BMI | CHD and stroke (ICD-10: I20–I25 and I60–I69) | Meta-analysis of 58 prospective studies | Stratified by age. Adjusted for age, sex, smoking status, SBP, history of diabetes and total and HDL cholesterol | The Emerging Risk Factors Collaboration220 (table 1 and figure 2) |
| Colon cancer (ICD-10: C18) | GBD meta-analysis | Relative risk from the GBD 2016 study217 | GBD 2016 study217 | |
| Breast cancer (women only) (ICD-10: C50) | GBD meta-analysis | Relative risk from the GBD 2016 study217 | GBD 2016 study217 | |
| Relative risk for diabetes mellitus | CHD and stroke (ICD-10: I20–I25 and I60–I69) | Meta-analysis of 102 prospective studies | Stratified by age. Adjusted for age, smoking status, BMI and SBP | The Emerging Risk Factors Collaboration221 (figure 2) |
| Other non-modelled mortality | Meta-analysis of 1.7 million men and women | Multiply adjusted. We used the non-CVD and non-cancer mortality effects | Stringhini et al.153 (figure 4) | |
| Colon cancer (ICD-10: C18) | GBD meta-analysis | Relative risk from the GBD 2016 study217 | GBD 2016 study217 | |
| Breast cancer (women only) (ICD-10: C50) | GBD meta-analysis | Relative risk from the GBD 2016 study217 | GBD 2016 study217 | |
| Relative risk for physical activity | CHD and stroke (ICD-10: I20–I25 and I60–I69) | Meta-analysis of 18 cohort studies for CHD and eight cohort studies for ischaemic stroke | Stratified by age and sex. Adjusted for measurement error, age, sex, smoking, blood pressure and cholesterol | Bull et al.222 (tables 10.19 and 10.20) |
| Colon cancer (ICD-10: C18) | Meta-analysis | Bull et al.222 (tables 10.19 and 10.20) | ||
| Breast cancer (women only) (ICD-10: C50) | Meta-analysis | Bull et al.222 (tables 10.19 and 10.20) | ||
| Other non-modelled mortality | Meta-analysis of 1.7 million men and women | Multiply adjusted. We used the non-CVD and non-cancer mortality effects. We applied the effect to those with ≤ 1 active day per week only | Stringhini et al.153 (figure 4) | |
| Relative risk for fruit and vegetable consumption | CHD (ICD-10: I20–I25) | Meta-analysis of nine cohort studies | Relative risk per portion of fruit and vegetables. Multiply adjusted | Dauchet et al.223 |
| Stroke (ICD-10: I60–I69) | Meta-analysis of seven cohort studies | Relative risk per portion of fruit and vegetables. Multiply adjusted | Dauchet et al.224 | |
| Relative risk for fruit consumption | Lung cancer (ICD-10: C33–C34) | Dose–response meta-analysis | The effect was like that estimated by Wang et al.225 | Vieira et al.226 |
| Relative risk for alcohol intake | CHD (ICD-10: I20–I25) | GBD meta-analysis | Relative risk from the GBD 2016 study217 | GBD 2016 study217 |
| Stroke (ICD-10: I60–I69) | GBD meta-analysis | Relative risk from the GBD 2016 study217 | GBD 2016 study217 | |
| Colon cancer (ICD-10: C18) | GBD meta-analysis | Relative risk from the GBD 2016 study217 | GBD 2016 study217 | |
| Breast cancer (women only) (ICD-10: C50) | GBD meta-analysis | Relative risk from the GBD 2016 study217 | GBD 2016 study217 | |
| Other non-modelled mortality | Meta-analysis of 1.7 million men and women | Multiply adjusted. We used the non-CVD and non-cancer mortality effects | Stringhini et al.153 (figure 4) | |
| Relative risk for ethnicity | CHD (ICD-10: I20–I25) | Cohort study | Vieira et al.227 (figure 3) | |
| Relative risk for untreated AF | Stroke (ICD-10: I60–I69) | Cohort study | Substantial bias is probable, as cases where they did not receive AF treatment were not randomly selected (which would be unethical) | Christiansen et al.228 |
| Relative risk for treated AF | Stroke (ICD-10: I60–I69) | Cohort study | Diagnosed cases on warfarin reduce risk by ≈ 66% | Singer et al.229 |
| Incident stroke | Post-stroke dementia | Meta-analysis of cohort studies | Probability of developing dementia a year after incident stroke. Personal communication with the authors of the study (Dr Eithne Sexton, Royal College of Surgeons of Ireland, 28 June 2019, personal communication) | Sexton et al.203 |
Appendix 5 workHORSE validation plots
FIGURE 23.
Validation: active days by year and age group.
FIGURE 24.
Validation: AF diagnosed by group.

FIGURE 25.
Validation: alcohol intake in grams per day.
FIGURE 26.
Validation: BMI by age.

FIGURE 27.
Validation: blood pressure treated by year and age.
FIGURE 28.
Validation: chronic kidney disease by age group.

FIGURE 29.
Validation: education by age group and year.

FIGURE 30.
Validation: exposure to environmental smoking by year and age group.
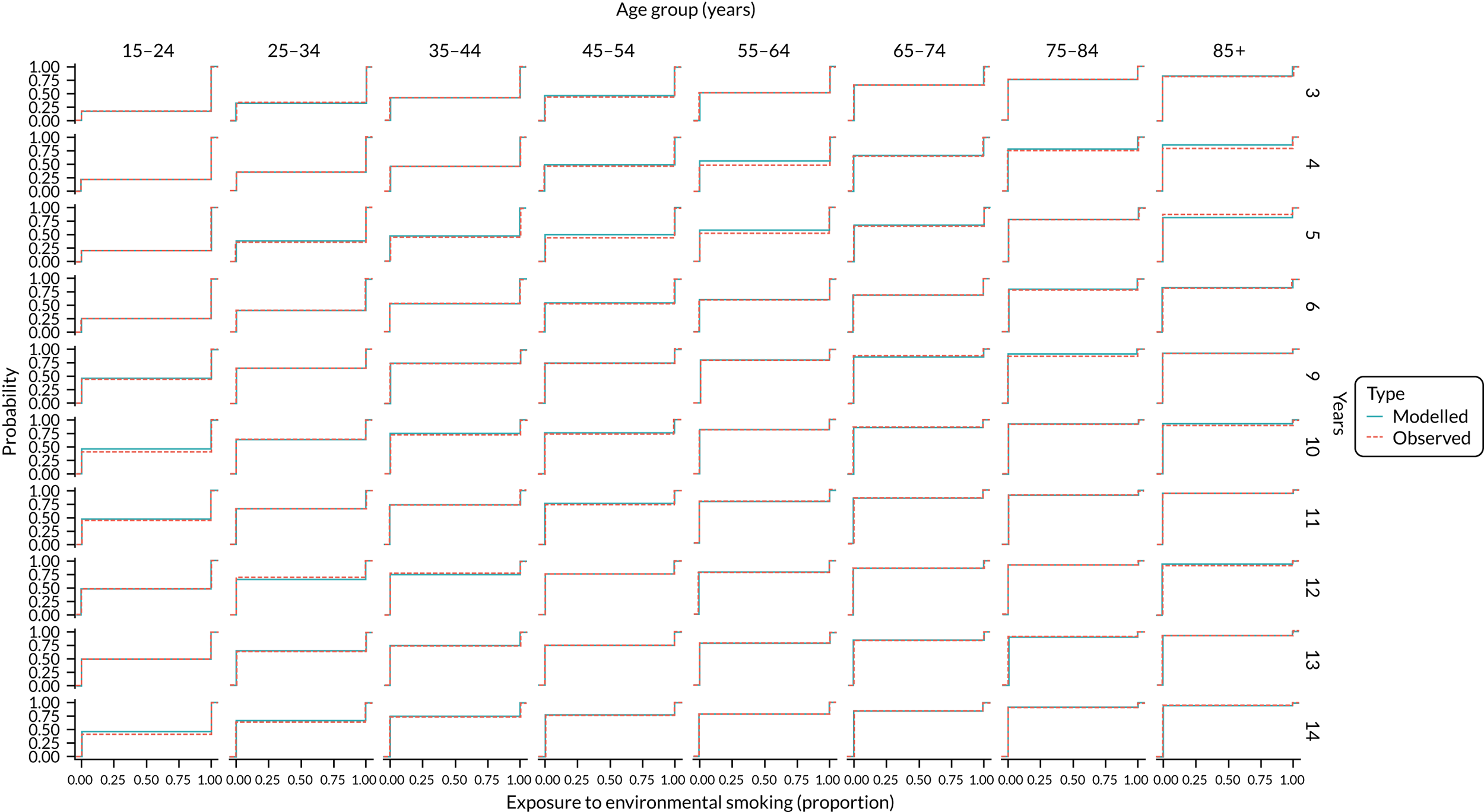
FIGURE 31.
Validation: family history of CVD by age group. For the x-axis values, 0 = no and 1 = yes.

FIGURE 32.
Validation: fruit intake by year and age group.

FIGURE 33.
Validation: high-density lipoprotein to total cholesterol ratio by year and age group.

FIGURE 34.
Validation: income by year and age group.

FIGURE 35.
Validation: number of cigarettes smoked by year and age group in ex-smokers.

FIGURE 36.
Validation: number of cigarettes smoked by year and age group in smokers.

FIGURE 37.
Validation: SBP by year and age group.
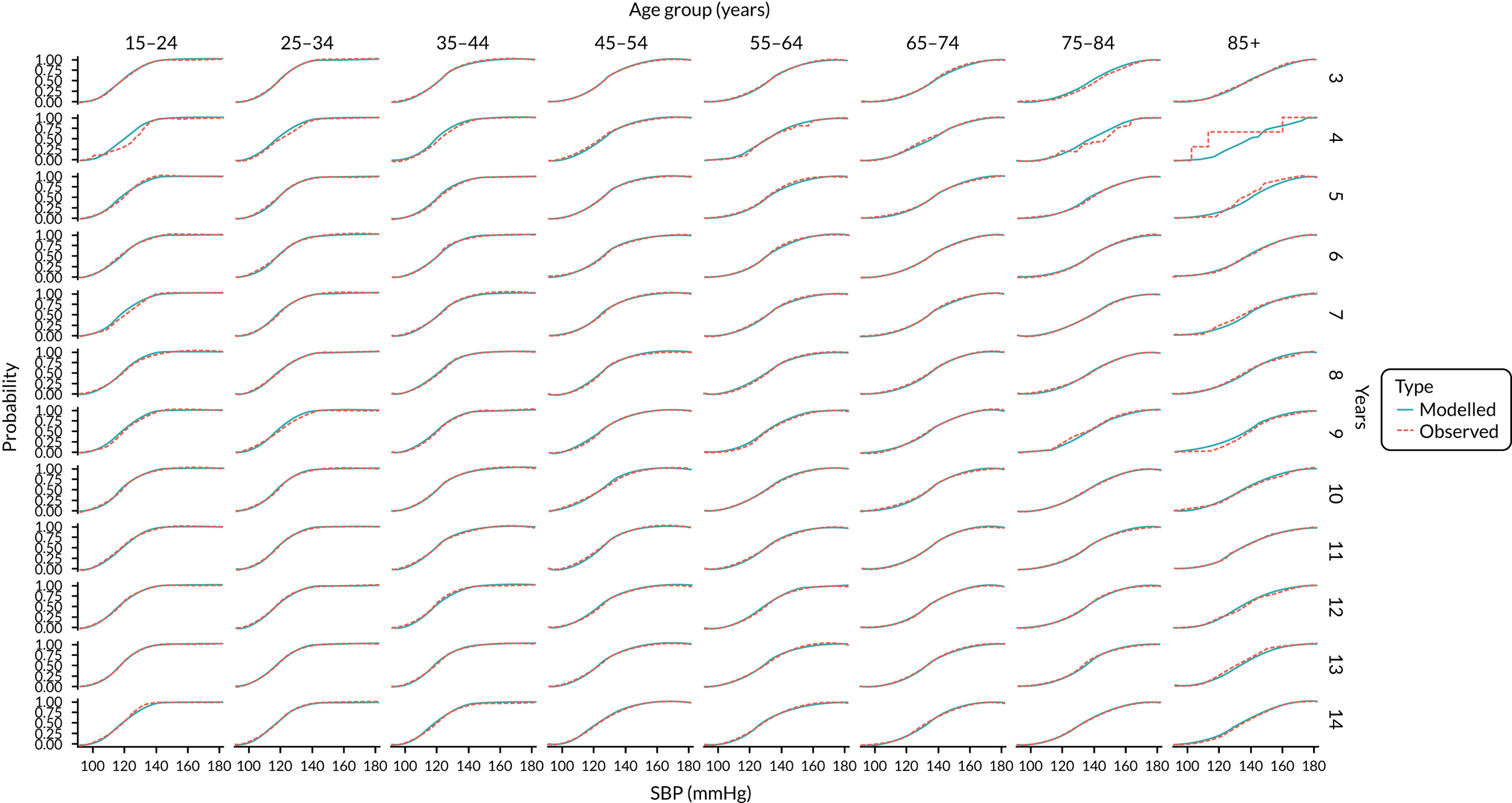
FIGURE 38.
Validation: smoking cessation by year and age group. For the x-axis values, 0 = no and 1 = yes.
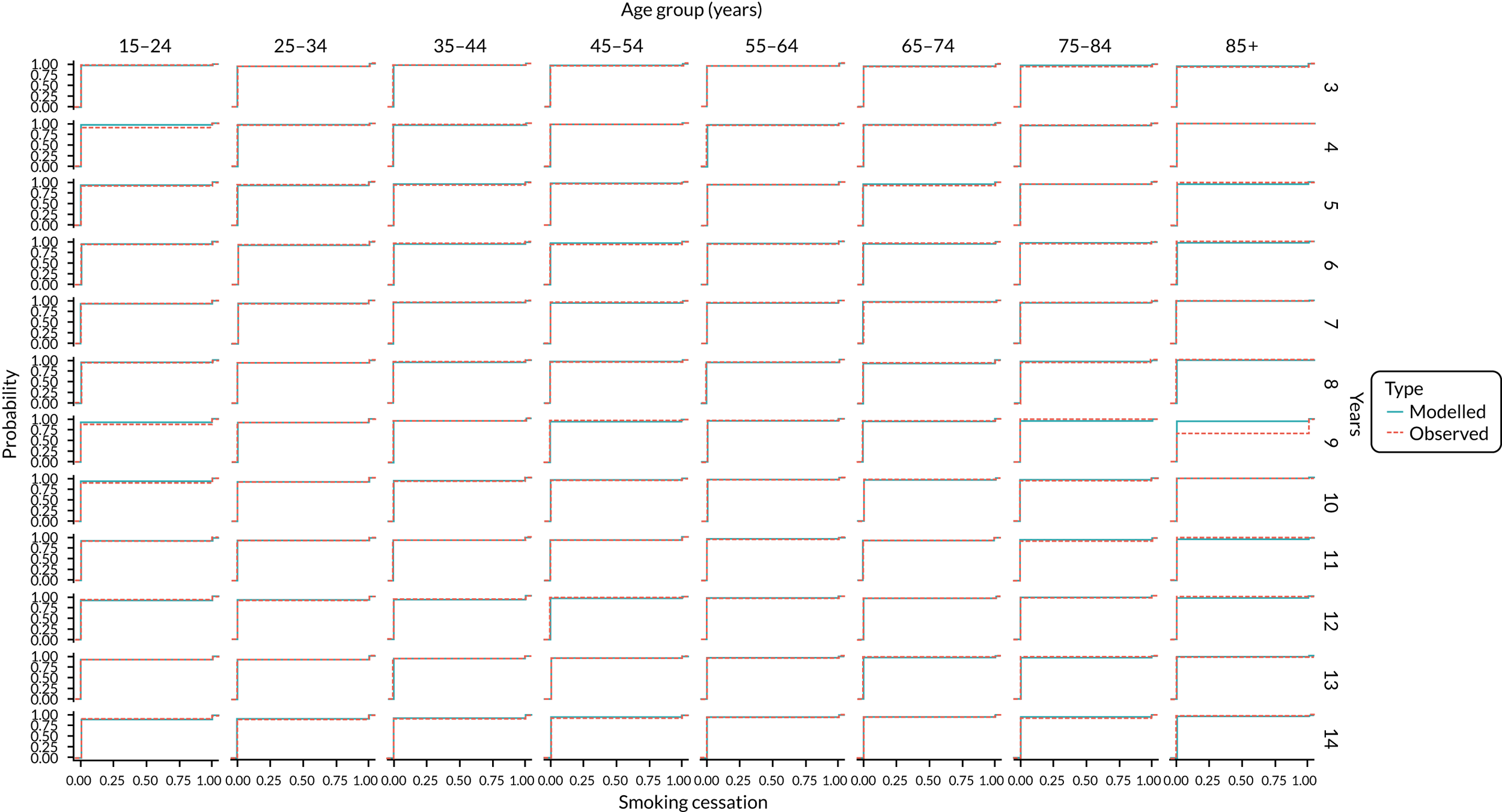
FIGURE 39.
Validation: smoking incidence by year and age group. For the x-axis values, 0 = no and 1 = yes.
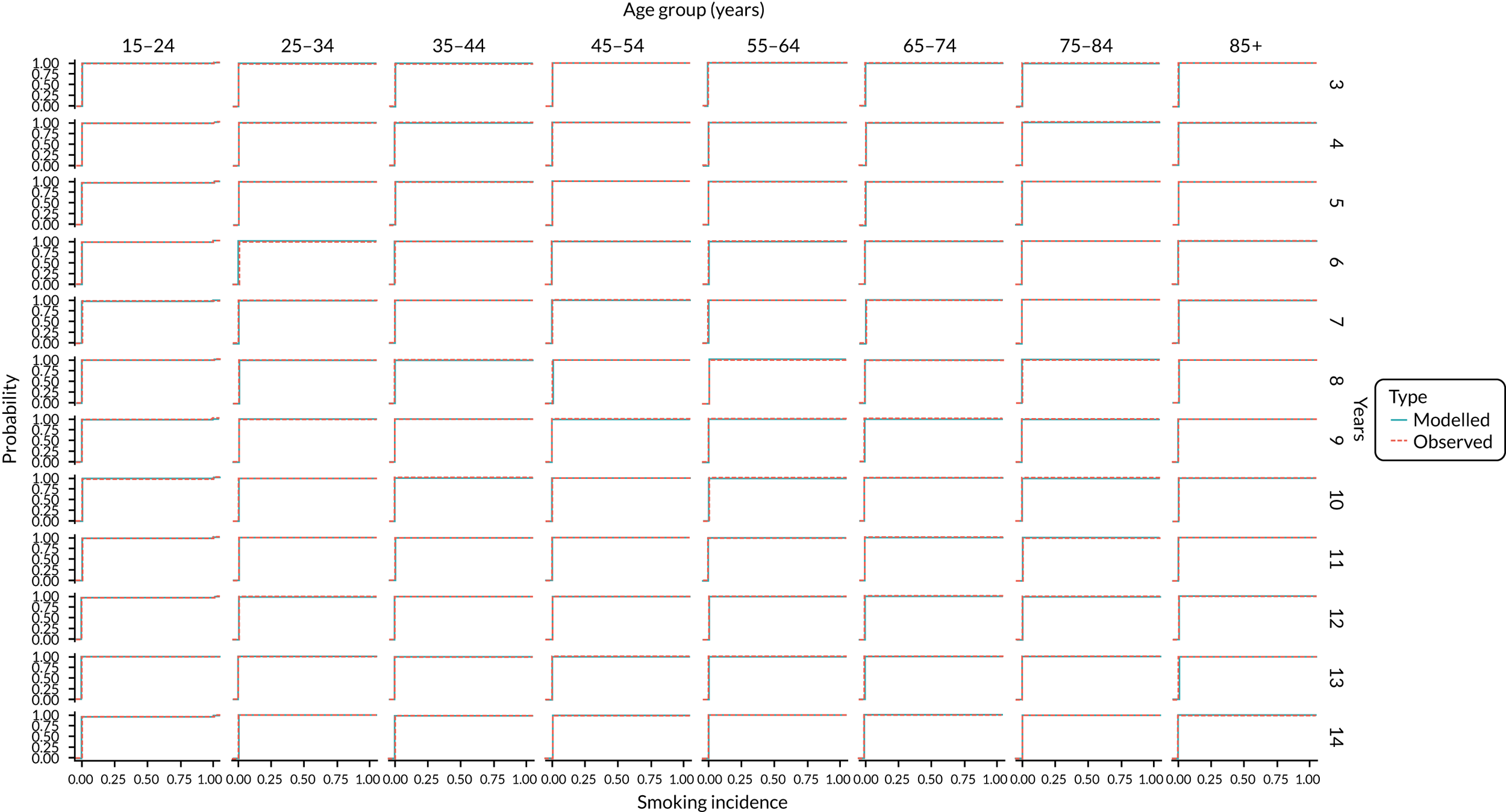
FIGURE 40.
Validation: smoking status by year and age group. Probability of smoking status lablels: 1, Never smoked cigarettes at all; 2, Used to smoke cigarettes occasionally; 3, Used to smoke cigarettes regularly; 4, Current cigarette smoker.

FIGURE 41.
Validation: statins prescriptions by year and age group. For the x-axis values, 0 = no and 1 = yes.
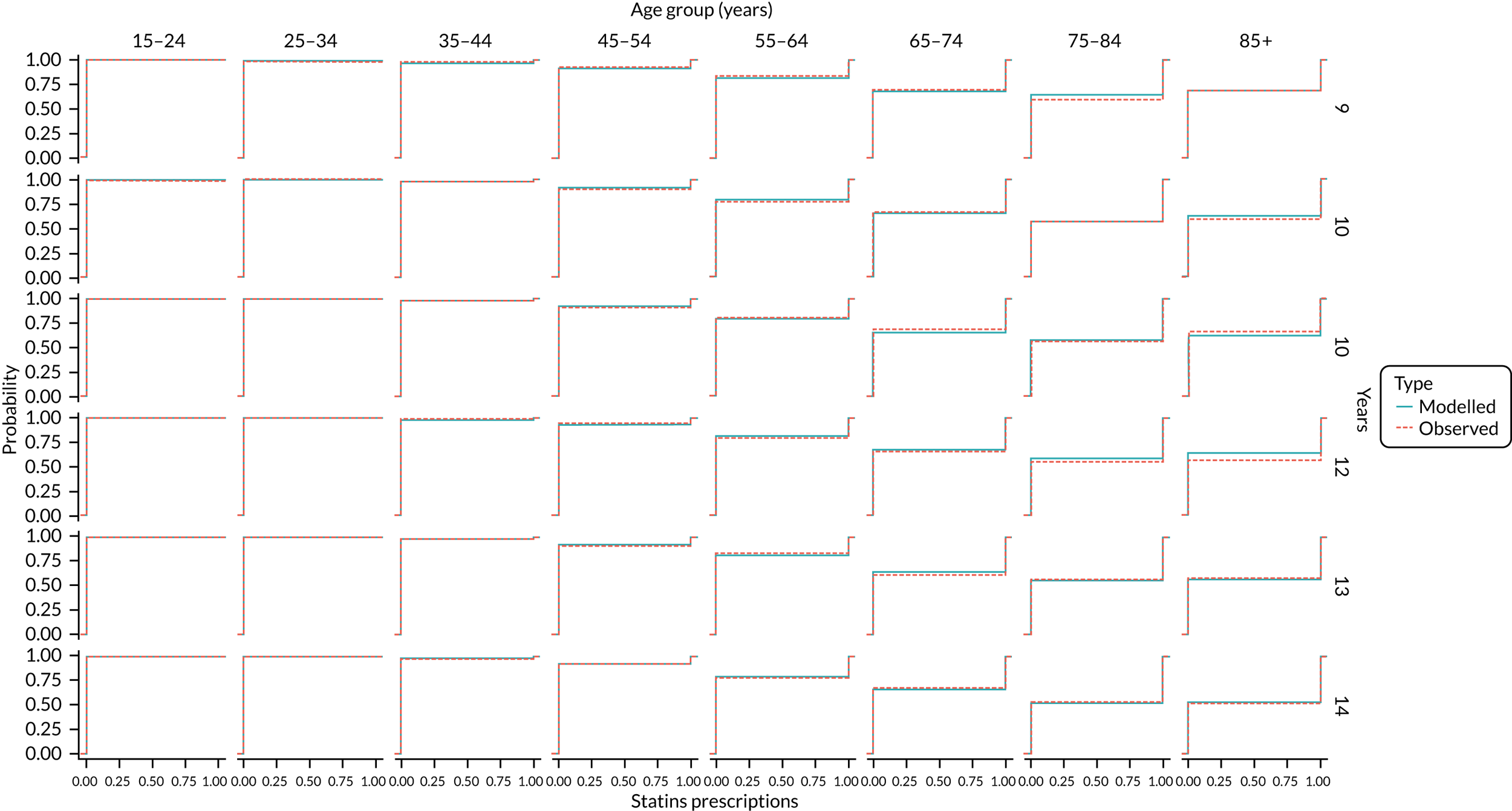
FIGURE 42.
Validation: diagnosed T2DM by year and age group. For the x-axis values, 0 = no and 1 = yes.
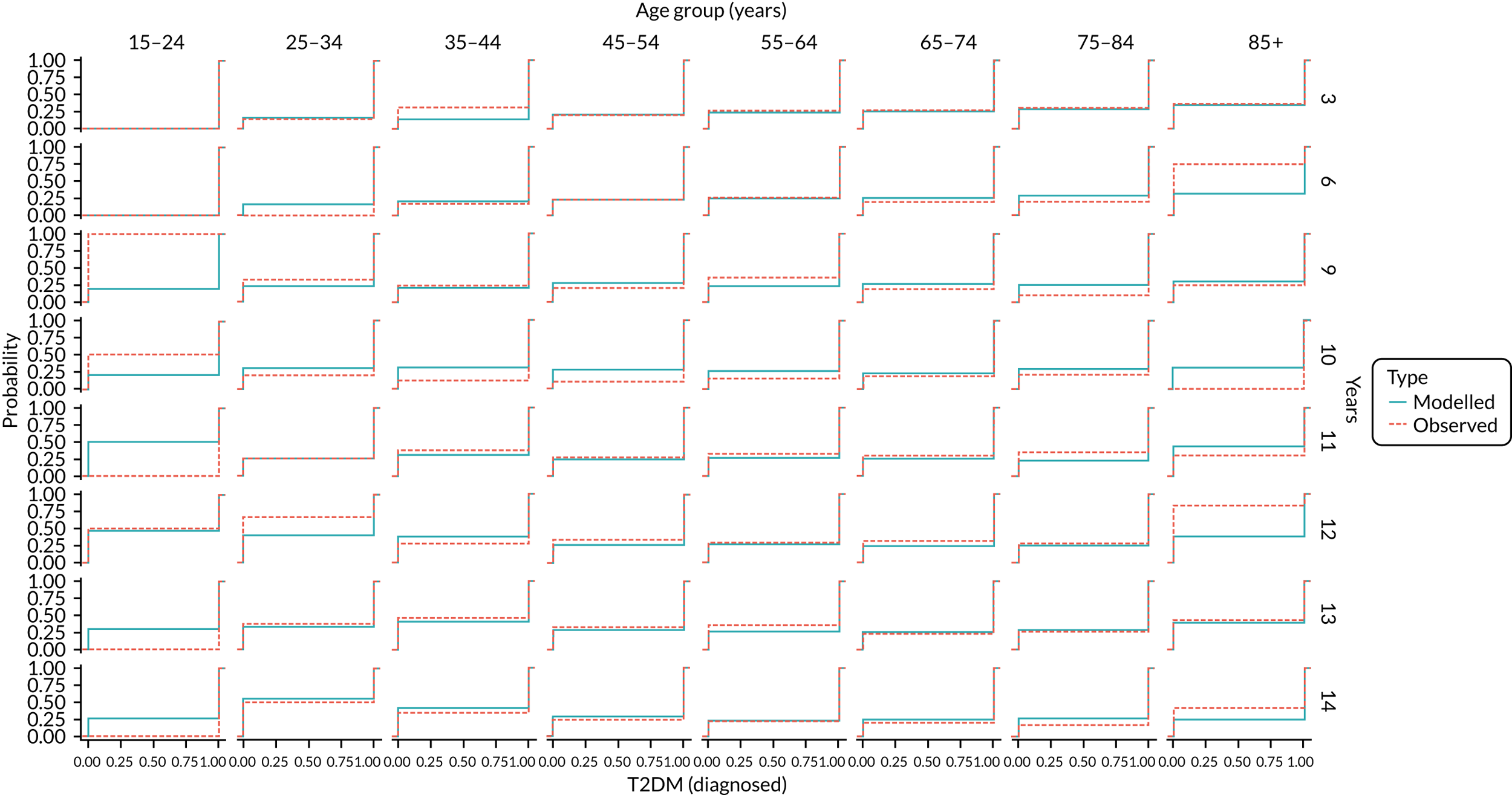
FIGURE 43.
Validation: T2DM prevalence by age group. For the x-axis values, 0 = no and 1 = yes.
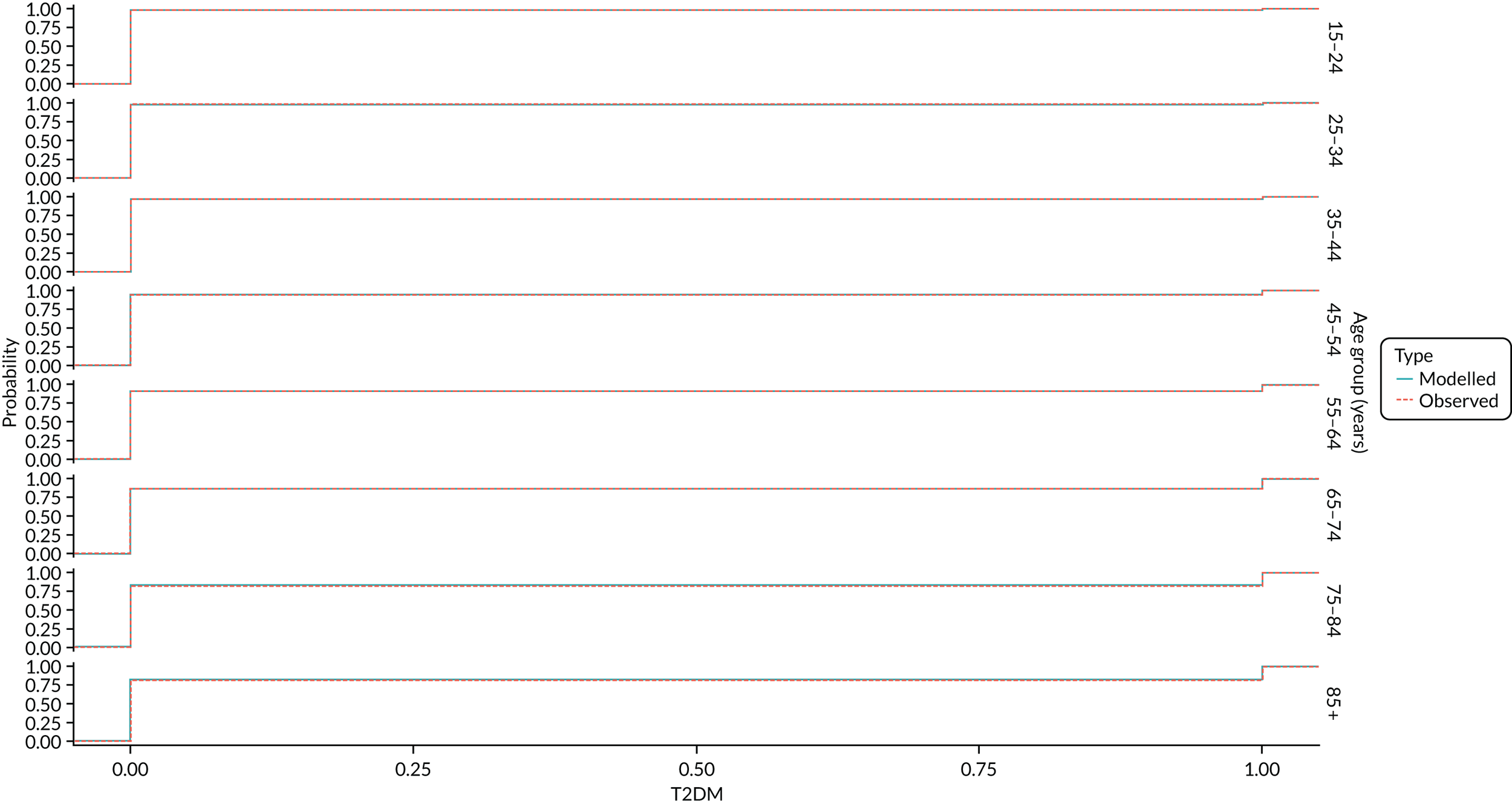
FIGURE 44.
Validation: total cholesterol (mmol/l) by year and age group.
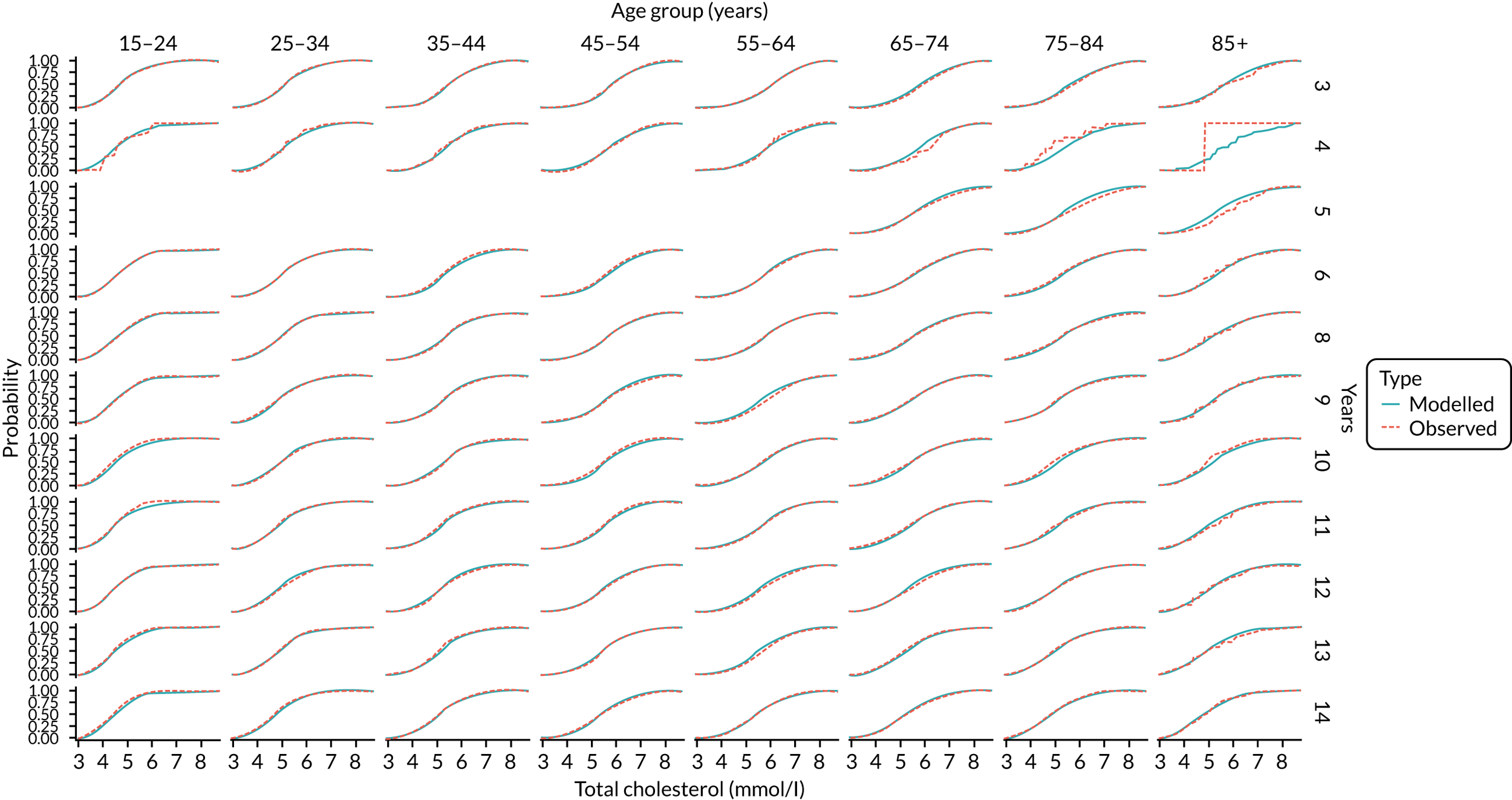
FIGURE 45.
Validation: vegetable intake by year and age group.
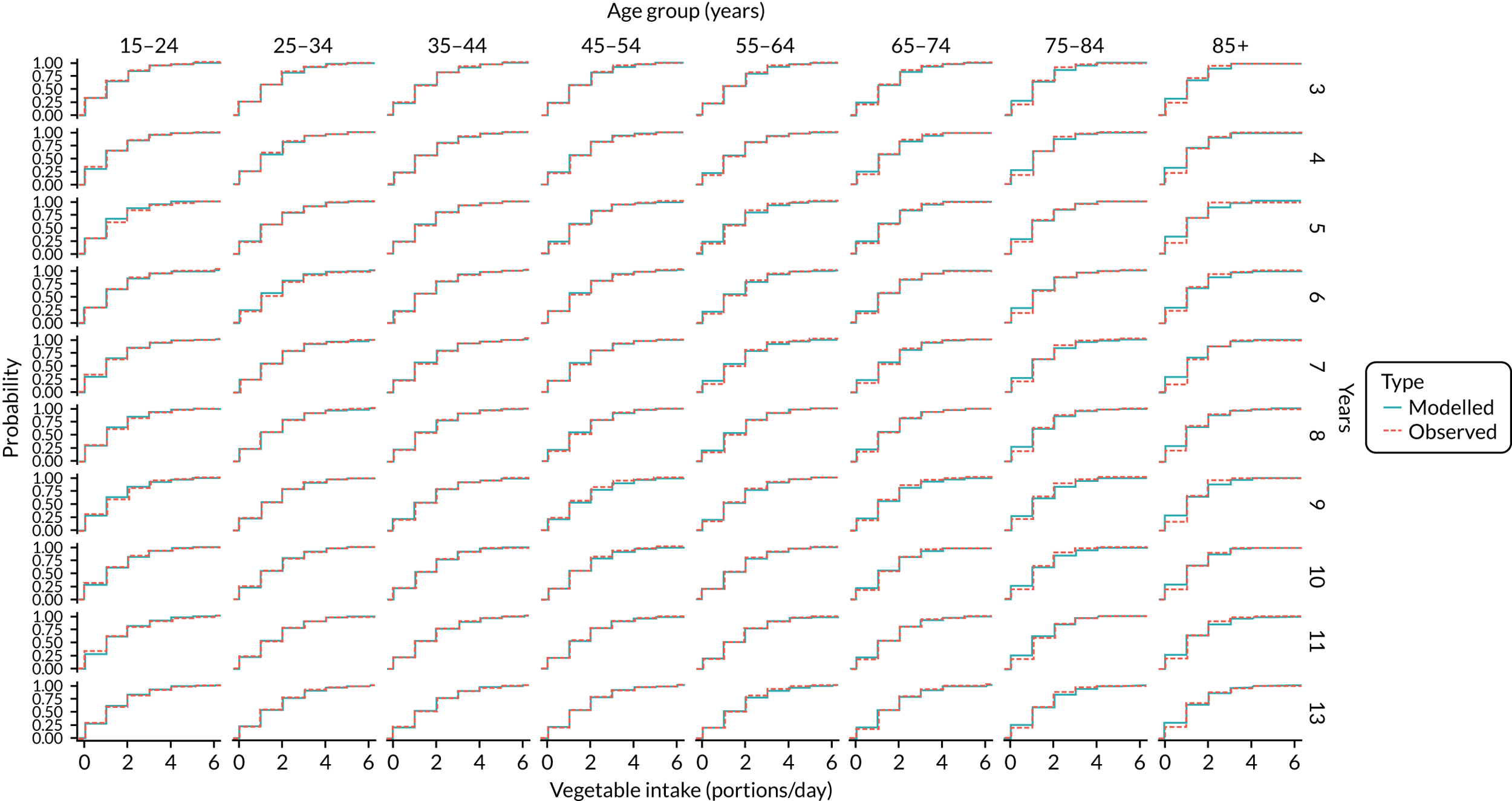
FIGURE 46.
Validation: years of smoking by year and age group, ex-smokers.
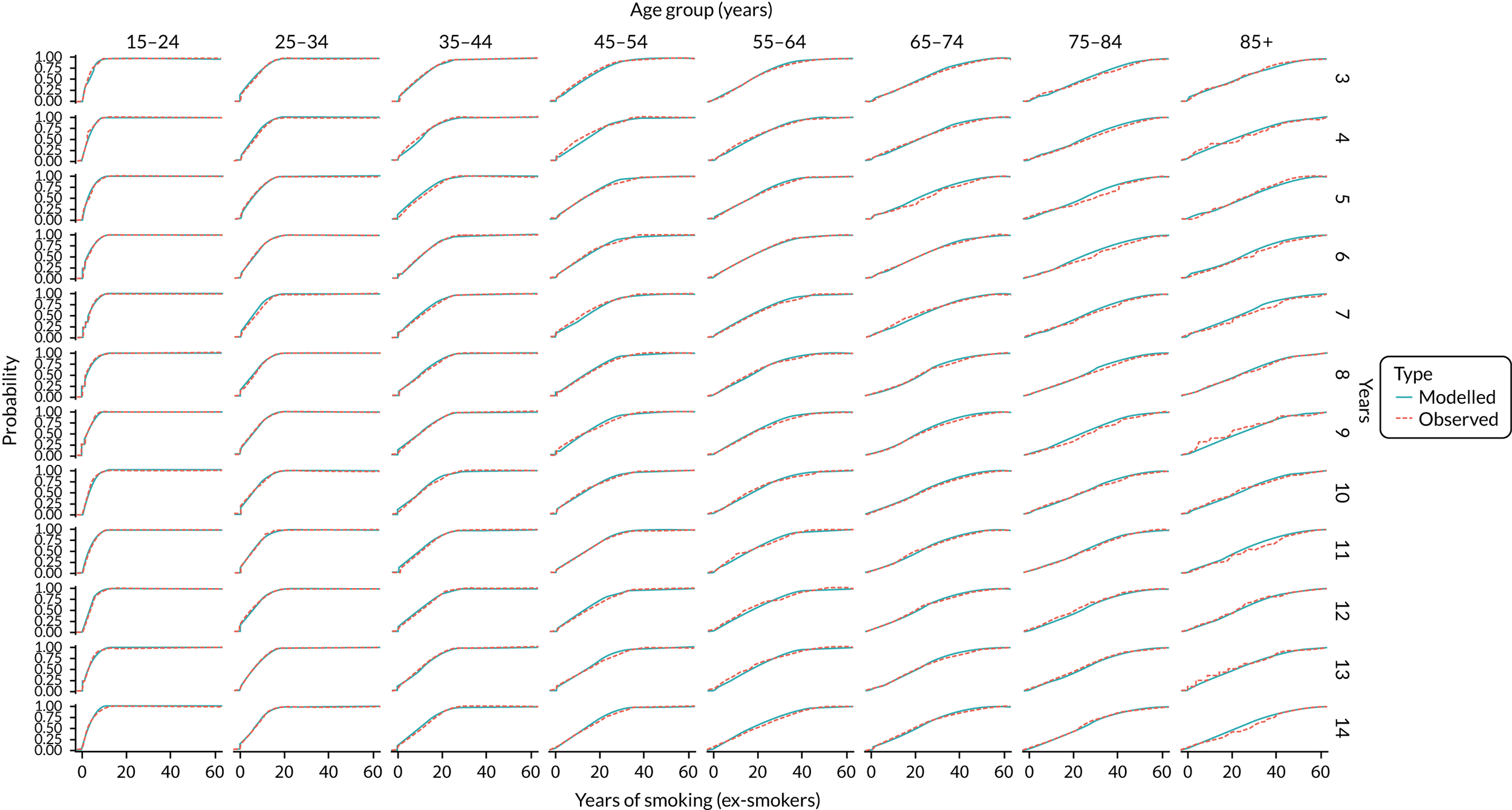
FIGURE 47.
Validation: years of smoking by year and age group, smokers.
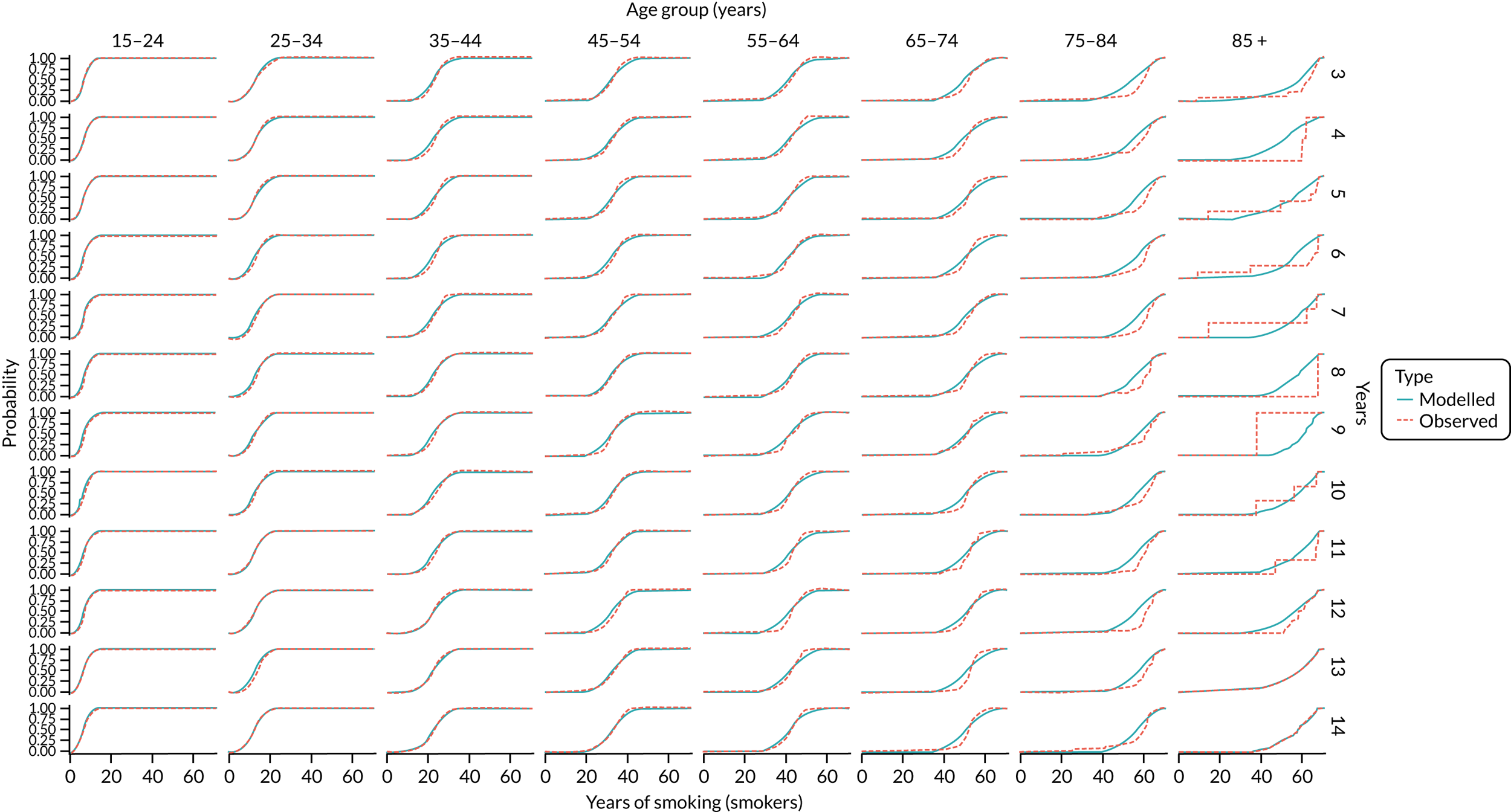
FIGURE 48.
Validation: years since smoking cessation by year and age group.

FIGURE 49.
Validation: lung cancer incidence rates by year and age group. Light orange shaded areas are 95% uncertainty intervals.

FIGURE 50.
Validation: colon cancer incidence rates by year and age group. Light orange shaded areas are 95% uncertainty intervals.
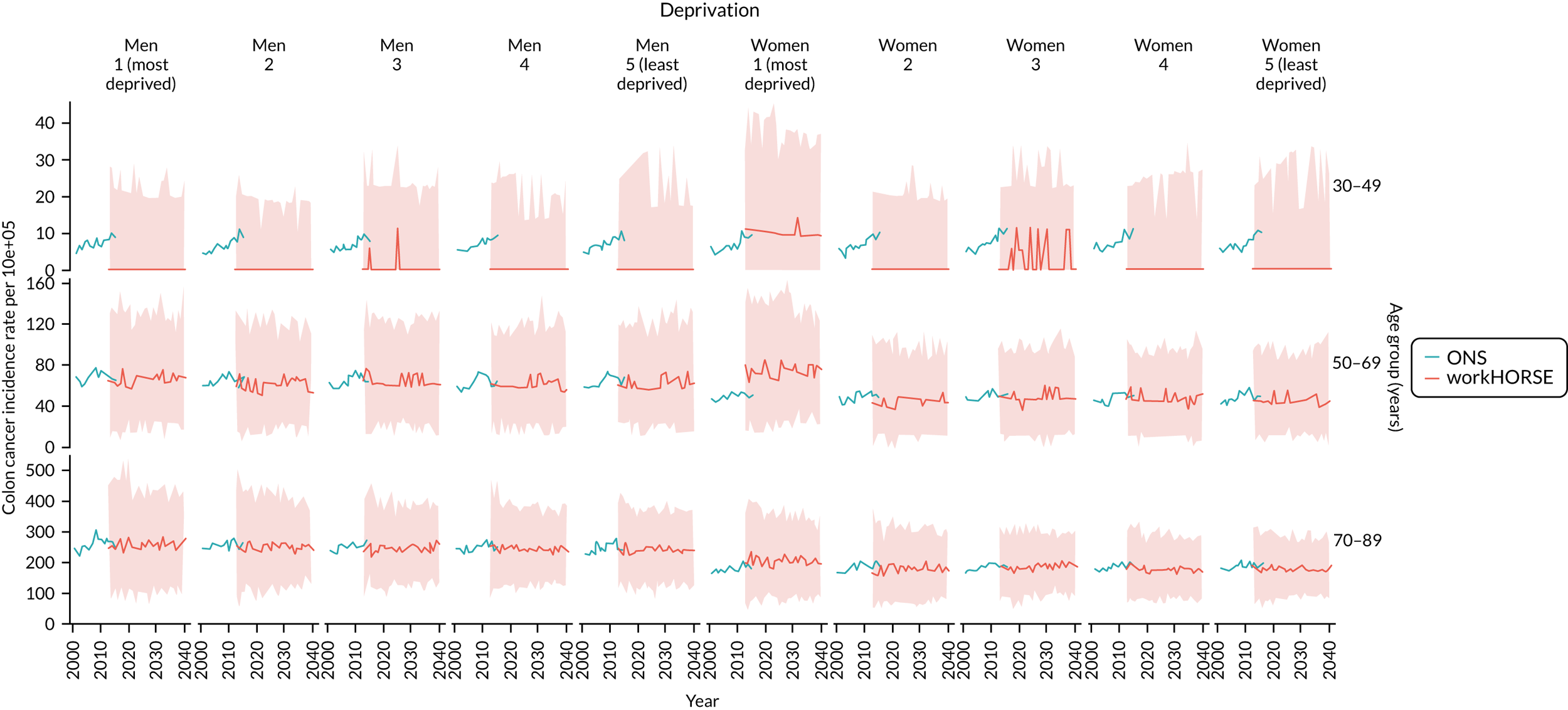
FIGURE 51.
Validation: breast cancer incidence per year and age group. Light orange shaded areas are 95% uncertainty intervals.
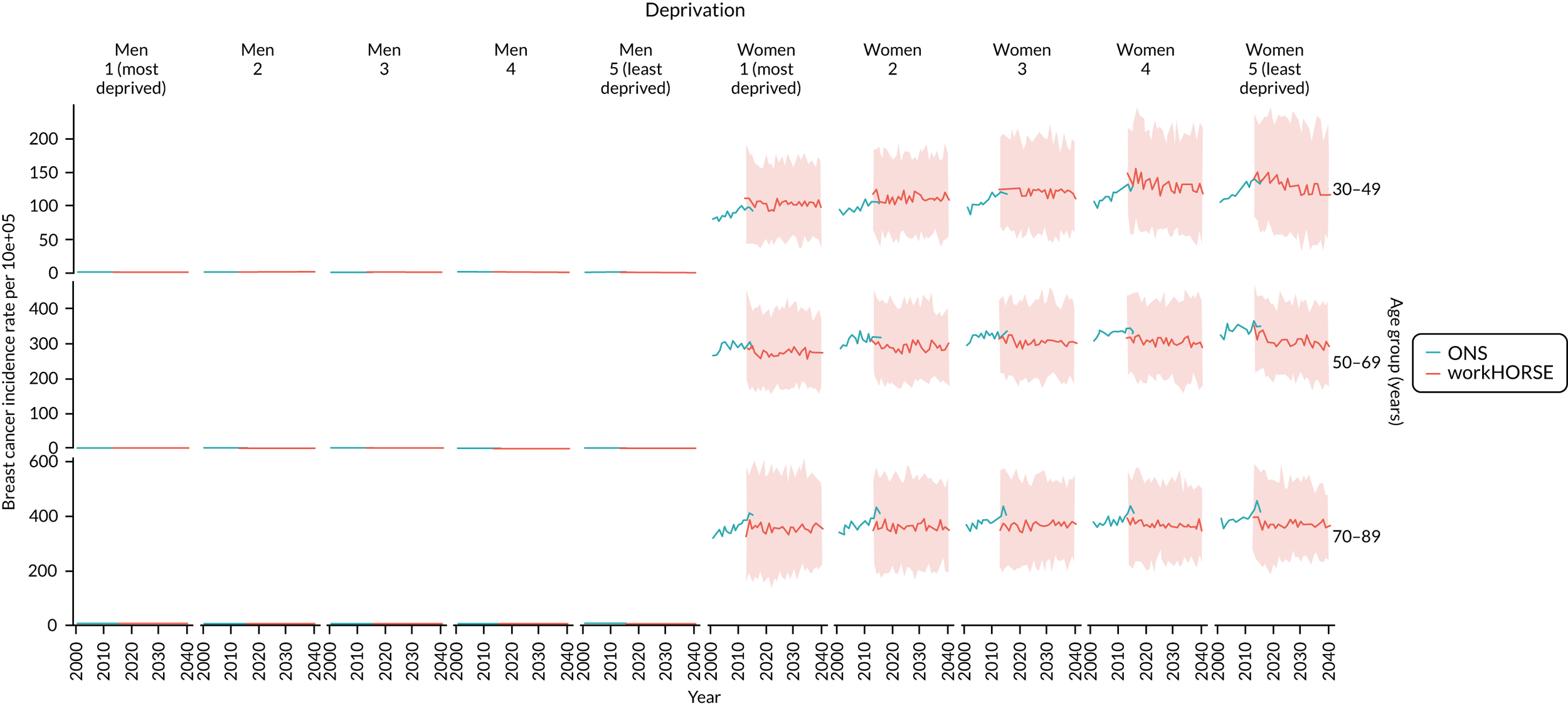
FIGURE 52.
Validation: CHD mortality rates. Light orange shaded areas are 95% uncertainty intervals.
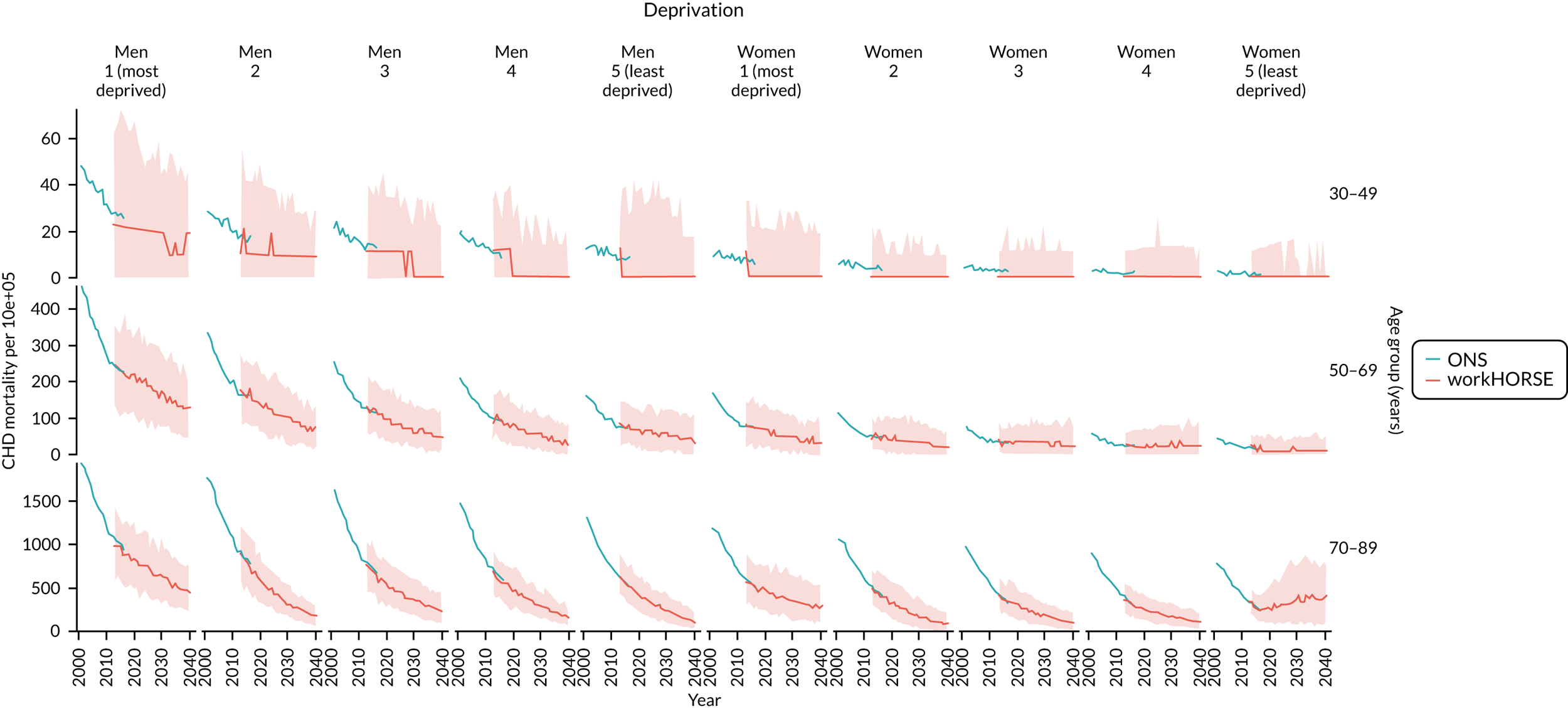
FIGURE 53.
Validation: stroke mortality rates. Light orange shaded areas are 95% uncertainty intervals.
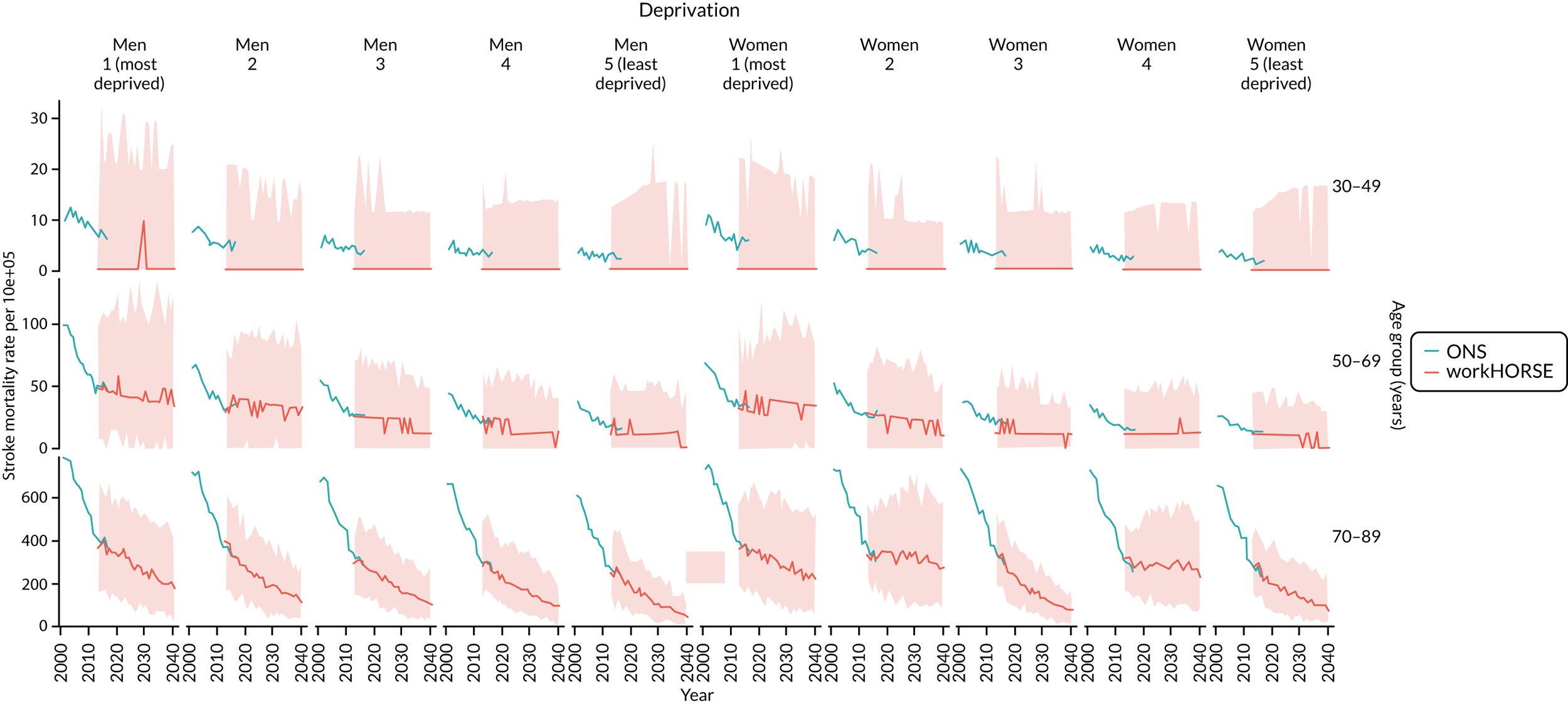
FIGURE 54.
Validation: COPD mortality rates. Light orange shaded areas are 95% uncertainty intervals.

FIGURE 55.
Validation: lung cancer mortality rate. Light orange shaded areas are 95% uncertainty intervals.

FIGURE 56.
Validation: colon cancer mortality rate. Light orange shaded areas are 95% uncertainty intervals.

FIGURE 57.
Validation: breast cancer mortality rate. Light orange shaded areas are 95% uncertainty intervals.
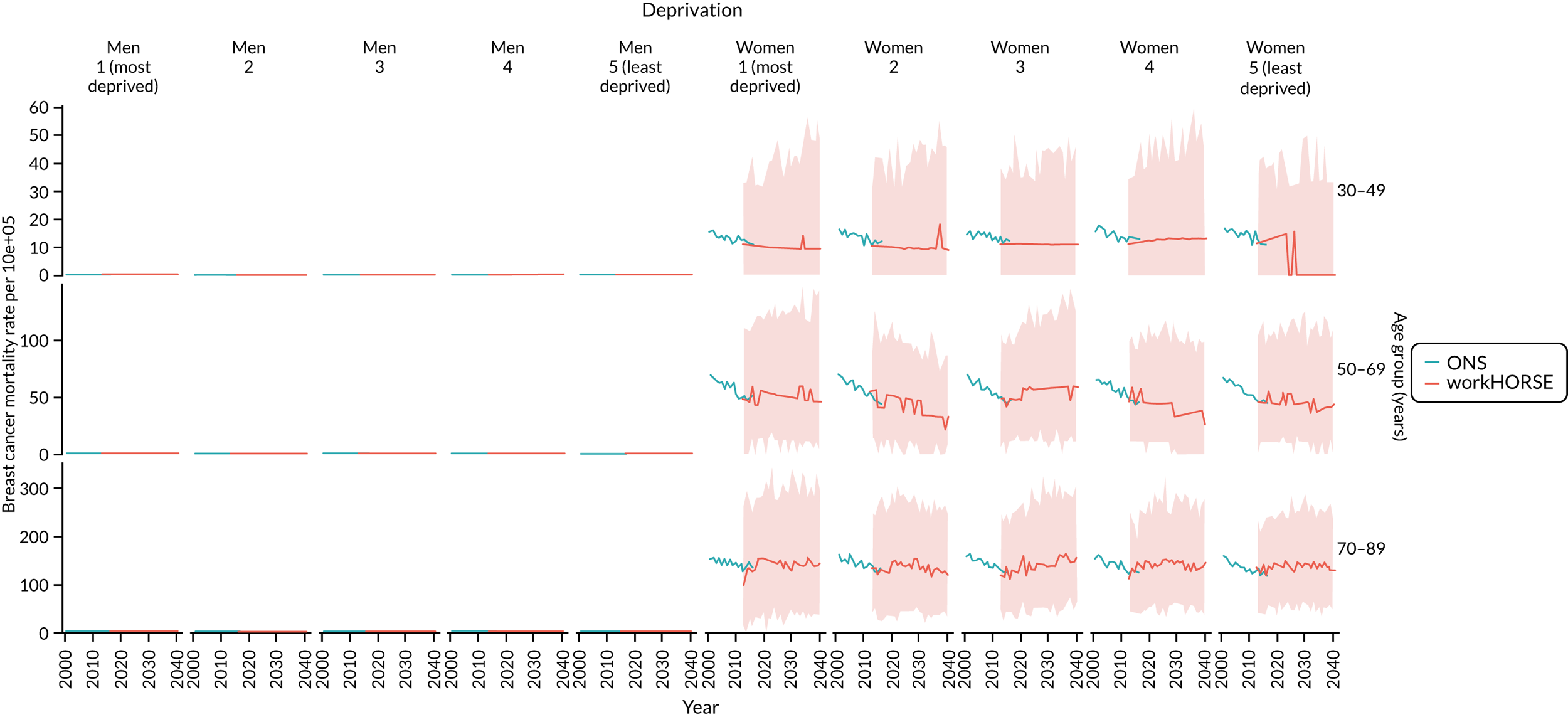
Appendix 6 Health economics methods
This appendix has been produced to add detail to the workHORSE model of NHS Health Checks. It includes information on how the utility coefficients and costs are estimated for the model.
Utility coefficients (EuroQol-5 Dimensions index scores)
Utility coefficients are multiplied by time lived to calculate QALYs experienced.
Individuals in the model were initially assigned to their England population norms based on Janssen and Szende158 (Table 38). After this, adjustments were applied based on income, education, age, specific diseases and numbers of comorbidities.
| Age group (years) | UK–England |
|---|---|
| 18–24 | 0.922 |
| 25–34 | 0.914 |
| 35–44 | 0.888 |
| 45–54 | 0.854 |
| 55–64 | 0.814 |
| 65–74 | 0.775 |
| 75 + | 0.706 |
| (All ages 18 +) | 0.853 |
For utility coefficients, International Classification of Diseases, Ninth Edition (ICD-9) codes from the EQ-5D MEPS catalogue159 were matched with diseases in the model. Where utility decrements for subcategories of disease were very similar (e.g. stroke), an unweighted average of relevant ICD-9 codes was used. The number of comorbidities was also included in the equation (see Utility coefficients for comorbidities and Table 40). These utility index scores are based on EuroQol-5 Dimensions, three-level version profiles from patients in the USA from 2000 to 2003 matched up to UK preference scores, based on the time trade-off method (Table 39).
| Disease | ICD-9 code | Utility coefficient | Standard error for utility |
|---|---|---|---|
| No diseases below | |||
| Hypertension | 401 | –0.0460 | 0.0042 |
| Hypercholesterolaemia | Assume no decrement | ||
| AF | 427 | –0.0384 | 0.0069 |
| T2DM (cost excluding complications) | 250 | –0.0714 | 0.0048 |
| CHD | 410–414 | –0.0679 | 0.0843 |
| Stroke | Used average of 433 precerebral occlusion, 435 transient cerebral ischemia, 436 CVA, 437 other cerebrovascular disease, 438 late effects cerebrovascular disease | –0.0578 | 0.0582 |
| COPD | Used average of 491 chronic bronchitis, 492 emphysema, 496 chronic airway obstruction NEC | –0.0957 | 0.0316 |
| CKD stage 3–5 | 586 | –0.1104 | 0.0212 |
| Dementia | 331 other cerebral degenerations (which includes Alzheimer’s) | –0.2166 | 0.0289 |
| Breast cancer | 174 breast cancer | –0.0194 | 0.0144 |
| Lung cancer | 162 lung cancer | –0.1192 | 0.0430 |
| Colorectal cancer | 153 colon cancer | –0.0674 | 0.0172 |
Utility coefficients for comorbidities
The MEPS catalogue has additional coefficients for the number of comorbidities. These coefficients are important to include, as they can have a much larger effect on the total utility score than the presence of the diseases themselves. The coefficients essentially have a smoothing effect where they amplify the effects of having fewer than five diseases (so the health-related quality-of-life decrement from having two to five diseases is more than the sum of its parts), whereas once someone has five or more diseases they reduce the additive effect and so the health-related quality-of-life decrement of having five or more diseases is less than the sum of its parts (Table 40 and Figure 58).
| Number of comorbidities | Utility coefficient |
|---|---|
| Two | –0.0528 |
| Three | –0.0415 |
| Four | –0.0203 |
| Five | 0.0083 |
| Six | 0.04087 |
| Seven | 0.06687 |
| Eight | 0.11589 |
| Nine | 0.13444 |
| Ten or more | 0.18361 |
FIGURE 58.
Utility coefficient by number of comorbidities.
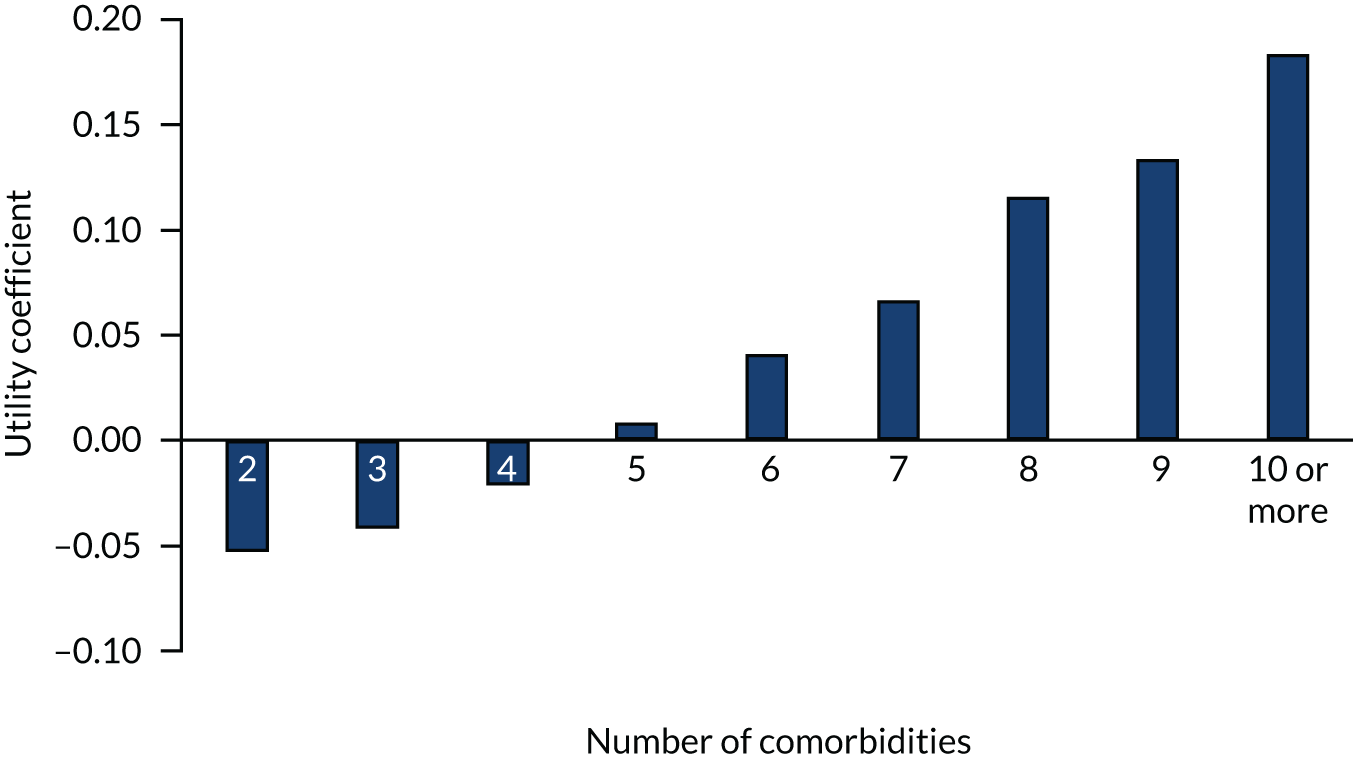
Utility coefficients for income
For income group, variable EQVINC (equivalised household income quintile) from HSE was used to match with the MEPS income definitions. This is based on quintile, whereas the US definitions are based on distance from the poverty line, so we have matched the cumulative position of the population on the income distribution. This is reasonably valid, as England and the USA have reasonably similar Lorenz (income distribution) curves. In 2002, when the MEPS data were collected, 16.7% of US citizens were classed as poor or near poor, 13.9% were classed as having low income, and the remainder (69.3%) were classed as having middle or high income. 230 In practice, the coefficients for low, middle and high income are very similar, so the main effect is between the lowest and second lowest quintile (Figure 59 and Table 41).
FIGURE 59.
Medical Expenditure Panel Survey income categories mapped to HSE income quintiles.

| HSE EQVINC | MEPS category | EQ-5D utility coefficient |
|---|---|---|
| 1. Lowest quintile (≤ £12,803) | Weighted average of poor and near poor (16.7/20%) and low income (3.3/20%) | 0.012572 |
| 2. Second lowest quintile (> £12,803–≤ 19,500) | Weighted average of low income (10.6/20%) and middle income (9.4/20%) | 0.038783 |
| 3. Middle quintile (> £19,500–≤ 29,865) | Middle income | 0.0396568 |
| 4. Second highest quintile (> £29,865–≤ 49,016) | Middle income | 0.0396568 |
| 5. Highest quintile (> £49,016) | High income | 0.0408501 |
Utility coefficients for education level
We matched the US education levels with utility decrements and we assumed that high school diploma in the USA was equivalent to NVQs 1–3 (Table 42).
| Topqual3 (HSE) variable (D) highest educational qualification | MEPS category to use | Utility coefficient |
|---|---|---|
| 1. NVQ 4/NVQ 5/degree or equivalent | Bachelor degree | 0.0060444 |
| 2. Higher education below degree | Other degree | 0.0056836 |
| 3. NVQ 3/GCE Advanced Level equivalent | High school | 0.0028418 |
| 4. NVQ 2/GCE Ordinary Level equivalent | High school | 0.0028418 |
| 5. NVQ 1/CSE other grade equivalent | High school | 0.0028418 |
| 6. Foreign/other | Other degree | 0.0056836 |
| 7. No qualification | 0 |
Utility coefficients for race (not included in the model)
The US MEPS data has coefficients for white, black, Hispanic, ‘aindian’ (presumably an old-fashioned term for native American people) and other ethnicities. In England, there are very small numbers of Hispanic and native American populations and these categories are not present in HSE ethnicity data. The coefficients for these race categories are all very small (all < 0.002 or less than a 0.2% change to the utility score) and so have been omitted from our equations.
Utility coefficient for gender
We included a utility coefficient of 0.0010046 for male gender, based on the Sullivan et al. ’s159 MEPS catalogue study.
Health-care costs
We carried out non-systematic reviews for health-care costs, looking for annual tariff-style excess costs of diseases. We aimed to find recent costs, that were for England or the UK, and favoured papers in academic journals recognised for health economics or by authors who are recognised for producing costs. We were aware that studies funded by pharmaceutical companies or charities working in particular disease areas may have unconscious biases that may inflate the total costs of diseases because there is pressure to produce media attention around the size of a disease burden.
Several papers were not used because they did not separate the costs of diseases from the costs of comorbidities, which may lead to double counting if these comorbidities are also included in the model. For example, diabetes is related to CVD and chronic kidney disease. All costs were inflated to 2019 prices using the Treasury GDP deflator. 231
For probabilistic sensitivity analysis, costs were all fitted to a generalized beta prime (GB2) [beta prime (3)] distribution with ± 20%. The GB2 distribution was chosen to reflect the typical shape of health-care cost distributions, which are skewed with a long right tail, meaning that a large number of people have close to the median costs, but a small number of people have very high costs (Table 43).
| Disease | Source | Original value (£) | Cost year | Value 2019 (£) | Notes |
|---|---|---|---|---|---|
| Other diseases not mentioned in the table | Licchetta and Stelmach160 | 1216 | 2018 | 1237 | Assumed that in absence of these diseases, health-care costs per year are same as a 35-year-old based on OBR estimates – most of these diseases do not happen until after aged 35 years |
| Hypertension | NICE232 | 61 | 2009 | 72 | Variation by age and gender is very small, so used only one value |
| Hypercholesterolaemia | Regional Drug and Therapeutics Centre233 | 9 | 2019 | 9 | Costs of 10 mg/day of Atorvastatin |
| AF | Stewart et al.234 | 764 | 2000 | 1102 | Preferred to more recent estimates as costs are for AF only, not including comorbidities that might be double counted |
| T2DM (cost excluding complications) | Hex et al.235 | 514 | 2011 | 586 | |
| CHD | Luengo-Fernández et al.236 | 1249 | 2004 | 1667 | Based on dividing gross CHD costs by prevalence from HSE 2003127 applied to the UK population |
| Stroke: acute event | Ward et al.237 | 7661 | 2009 | 9040 | Table 40. These costs are similar to those in Luengo-Fernández et al.236 |
| Stroke: year 1 | Ward et al.237 | 8986 | 2009 | 10,603 | Table 40 |
| Stroke: year 2 + | Ward et al.237 | 4720 | 2009 | 5569 | Table 40 |
| COPD | Punekar et al.238 | 2108 | 2011 | 2404 | |
| CKD stage 3 | Kerr et al.239 | 235 | 2010 | 273 | |
| CKD stage 4 | Kerr et al.239 | 235 | 2010 | 273 | |
| CKD stage 5 | Kerr et al.239 | 16,686 | 2010 | 19,391 | Weighted average of annualised costs of renal replacement therapy and transplant |
| Dementia | Luengo-Fernandez et al.240 | 1856 | 2007 | 2289 |
Cancer costs
Cancer costs were all drawn from one paper241 that had excess health-care costs of four common cancers for 3 years pre diagnosis and 9 years post diagnosis. For simplicity, we included the costs for the 3 years pre diagnosis with the year 1 costs. For lung cancer, the sample was too small to estimate costs after year 5, so we assumed that costs for years 6–9 were the same as those for year 5 (Tables 44 and 45).
| Cost years | Colorectal cancer (2010 £) | Breast cancer (2010 £) | Prostate cancer (2010 £) | Lung cancer (2010 £) | ||||
|---|---|---|---|---|---|---|---|---|
| Age group (years) | 18–64 | 65+ | 18–64 | 65+ | 18–64 | 65+ | 18–64 | 65+ |
| 3 years pre diagnosis | 201 | 435 | 165 | 439 | 162 | 375 | 344 | 544 |
| 2 years pre diagnosis | 262 | 471 | 183 | 398 | 224 | 517 | 310 | 542 |
| 1 year pre diagnosis | 1023 | 1760 | 484 | 1126 | 715 | 1430 | 1337 | 1979 |
| 1 year | 17,241 | 14,776 | 11,109 | 7788 | 5171 | 4699 | 12 ,083 | 9061 |
| 2 years | 5014 | 4231 | 3676 | 2675 | 1965 | 2705 | 4540 | 4320 |
| 3 years | 3687 | 3403 | 2176 | 2270 | 1927 | 2598 | 4002 | 3945 |
| 4 years | 2927 | 2821 | 1782 | 2283 | 1484 | 2529 | 2671 | 3365 |
| 5 years | 2388 | 2769 | 1708 | 2186 | 1559 | 2593 | 2551 | 3043 |
| 6 years | 1823 | 2741 | 1646 | 2222 | 1584 | 2536 | ||
| 7 years | 1960 | 2341 | 1459 | 2121 | 1414 | 3770 | ||
| 8 years | 1688 | 2630 | 1432 | 2144 | 1501 | 2782 | ||
| 9 years | 1370 | 2236 | 1316 | 2277 | 1451 | 2596 | ||
| Total (9 years) | 22,343 | 25,838 | 16,027 | 27,929 | 19,157 | 29,130 | 15,755 | 26,799 |
| Cost years | Colorectal cancer (2019 £) | Breast cancer (2019 £) | Prostate cancer (2019 £) | Lung cancer (2019 £) | ||||
|---|---|---|---|---|---|---|---|---|
| Age group (years) | 18–64 | 65+ | 18–64 | 65+ | 18–64 | 65+ | 18–64 | 65+ |
| Year 1 (including 3 pre-diagnosis years) | 21,764 | 20,270 | 13,877 | 11,332 | 7289 | 8159 | 16,356 | 14,092 |
| 2 years | 5827 | 4917 | 4272 | 3109 | 2284 | 3144 | 5276 | 5021 |
| 3 years | 4285 | 3955 | 2529 | 2638 | 2239 | 3019 | 4651 | 4585 |
| 4 years | 3402 | 3278 | 2071 | 2653 | 1725 | 2939 | 3104 | 3911 |
| 5 years | 2775 | 3218 | 1985 | 2540 | 1812 | 3013 | 2965 | 3536 |
| 6 years | 2119 | 3185 | 1913 | 2582 | 1841 | 2947 | 2965 | 3536 |
| 7 years | 2278 | 2721 | 1696 | 2465 | 1643 | 4381 | 2965 | 3536 |
| 8 years | 1962 | 3056 | 1664 | 2492 | 1744 | 3233 | 2965 | 3536 |
| 9 years | 1592 | 2599 | 1529 | 2646 | 1686 | 3017 | 2965 | 3536 |
Social care costs
Social care costs were based on estimates from the OBR,160 with excess costs for dementia and stroke only, as we did not find strong evidence for excess social care costs for other diseases, on top of those associated with age. Total costs for the whole population were taken from the OBR UK estimates of social care costs per person, adjusted to 2019 prices and multiplied by ONS England population estimates by single year of age for 2018. The OBR estimates by single year of age multiplied by population estimates for England (aged 18–90 years) gave a total of £22B. Estimates of excess costs of stroke (£943) and dementia (£6558) for the final year of life161 were added to these costs and multiplied by estimated prevalence of stroke242 and dementia243 by age. This summed to £29B for the whole population, at which point all of the costs were multiplied by 0.74 to calibrate them down to the £22B total for England (this was to adjust for the fact that social care costs of dementia and stroke were already part of the OBR totals). This meant that the additional cost of dementia was £4862 per year and the additional cost of stroke was £699 per year, in 2019 prices (Figure 60).
FIGURE 60.
Social care costs per person year by age, without dementia or stroke.
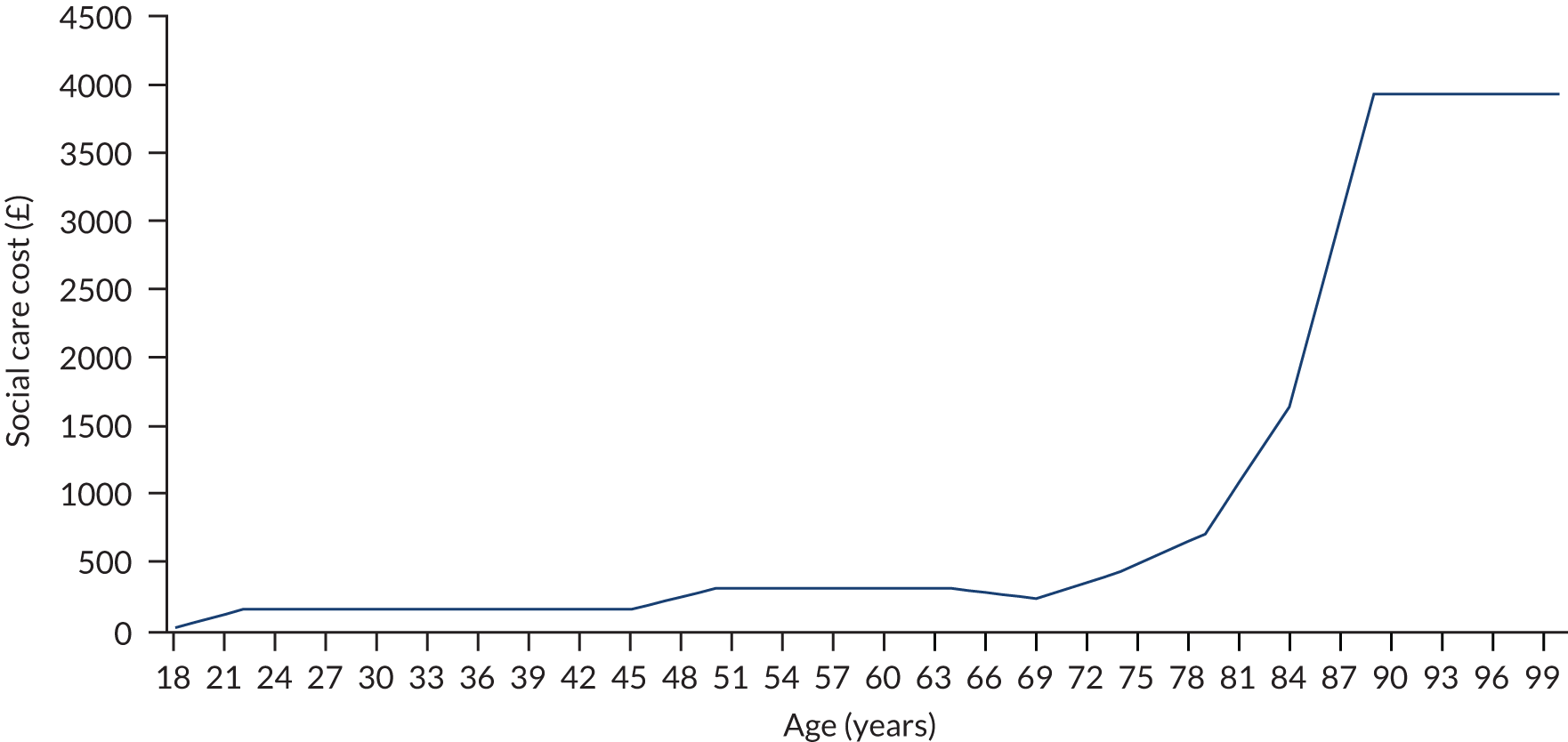
It is likely that the social care costs we have used mainly reflect public sector social care costs and not private social care costs, which are also significant at around £10B per year.
Workplace and household productivity
We used two types of production: (1) workplace earnings and (2) household production. Both were estimated for the whole population. Then, in the model, these are reduced for people who have had health problems or reduced to zero once people die. This enables comparison of productivity between scenarios; for instance, if one scenario has more people with CHD and a higher mortality rate, then productivity will be lower in that scenario. Productivity is estimated based on gender, age and health status, as measured by the EQ-5D.
Several of the assumptions for productivity are based on Appendix B of a paper by Claxton et al. ,163 which contains a lot of information about wider social benefits and has informed the approach taken by the UK Department of Health and Social Care in their impact assessments. In turn, this appendix was based on another report titled Department of Health Proposals For Including Wider Societal Benefits into Value Based Pricing: A Description and Critique. 244
Workplace earnings for the UK from the ONS annual survey of hours and earnings245 (provisional gross weekly pay estimates for 2018 divided by seven and multiplied by 365 days, inflated to 2019 prices) were multiplied by the probability of being employed (Labour Force Survey employment rate for UK for the most recent year, which was 2017)246 by age and gender to obtain overall average earnings, including those for people who are not in work. No additional uplift was applied for ‘oncosts’ or overheads for people in work. The employment data are open ended in terms of age, with a 60+ years age category for weekly pay and a 65+ years age category for employment (for males but not females, presumably because of differences in normal retirement age), so we do not know from this summary data at what age employment rates and earnings drop off significantly. In the absence of an estimate of this, we assume that workplace earnings are zero after aged 70 years (Figure 61).
FIGURE 61.
Average earnings per person year (£, 2019), including people who are unemployed, data for UK.

Household production hours per year were estimated from an equation from Claxton et al. , Appendix B163 that gave hours of unpaid production per month, which were multiplied by 12 to get hours per year:
For individuals aged 70–100 years, the hours were assumed to be the same as those of a 70-year-old (i.e. they do not increase beyond aged 70 years), as advised in the Claxton et al. appendix. 163
The value per hour of unpaid production was estimated using ONS estimates of unpaid production per person per day (183 minutes converted to 3.05 hours) multiplied by 365 days, multiplied by the UK population (65.13 million people) to get a total of 72.5 billion hours per year. The total gross value added (GVA) for household production for 2015 was £1,213,031,000,000 (£1.2T). This was divided by the total number of hours of unpaid work to get a GVA per hour of £16.73 in 2015 prices, which was inflated to £18.05 in 2019 prices. Figure 62 shows the value of household production. Unlike earnings, household production is larger for females than males.
FIGURE 62.
Average value of household production (£, 2019) per person year, males and females, data for UK.

Total production was calculated by combining earnings with the value of household production in each age and gender combination. Total production is larger for females, except for aged 30–59 years where it is larger for males (Figure 63).
FIGURE 63.
Average value of total production (£, 2019) per person year by age and gender, data for the UK.
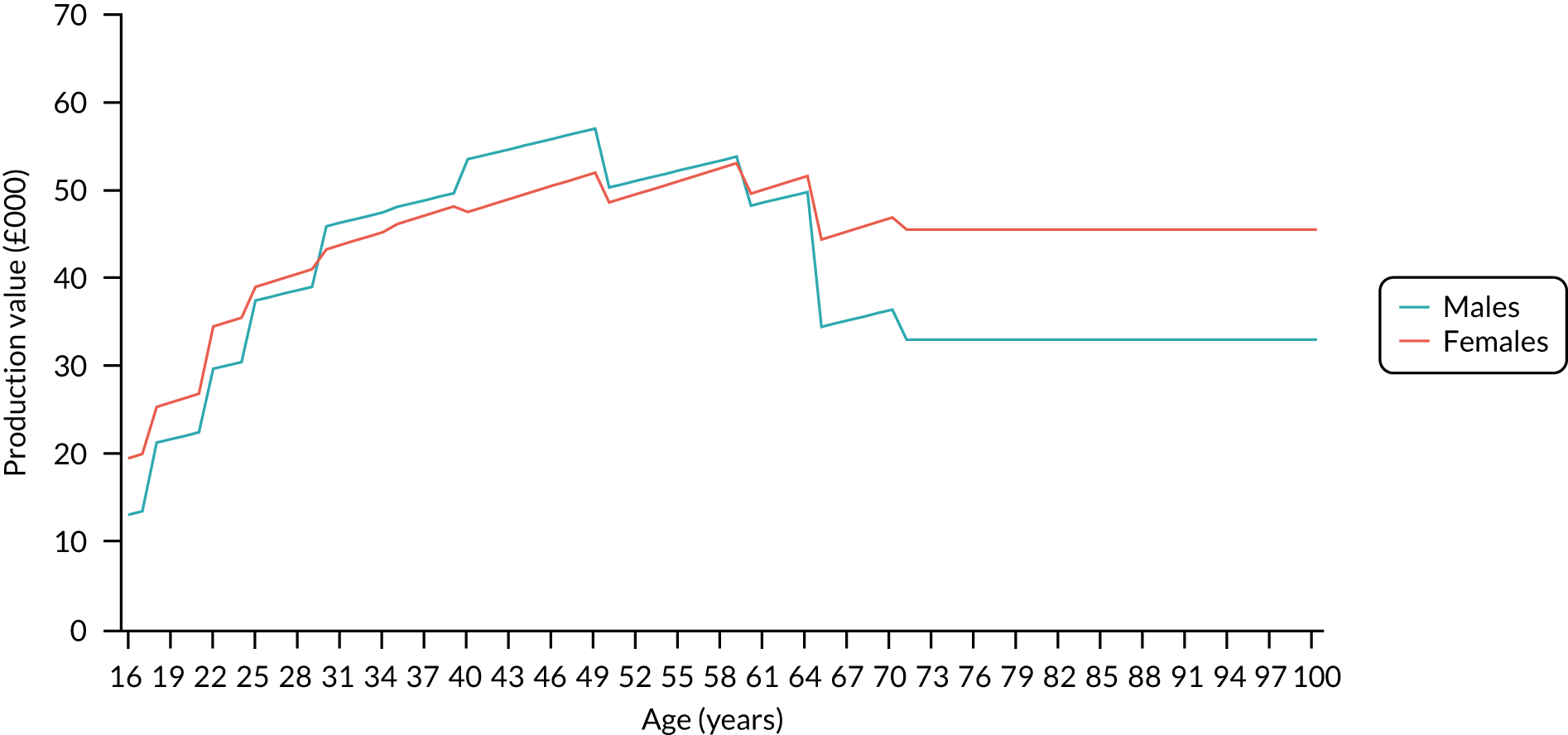
Equations from the Claxton et al. appendix163 were used to estimate relative production rates for age, gender and EQ-5D index score. The total productivity (i.e. earnings plus household production) was multiplied by hours of production for each combination of age, gender and EQ-5D index score (in 20 increments of 0.05 going from 0 to 1; e.g. 0, 0.05, 0.1, 0.15 . . ., etc). The production rates were calculated relative to those in the Claxton et al. appendix,163 but were applied to the more contemporary data on earnings (for 2017/18) and unpaid production (from 2015). The Claxton et al. appendix163 assumes that full health production falls to 0% after age 85 years; however, we changed this to 1% to make the equations work and avoid producing implausible results, given that we have assumed that unpaid production continues at a constant rate from age 70 to 100 years.
Therefore, in practice, this meant that someone aged 18 years with an EQ-5D index score of 1 (i.e. full health) would have a relative productivity of 98.5%, whereas someone aged 100 years with an EQ-5D index score of 0 would have a relative productivity of 0.02%. These productivity ratios were then applied to the total production in each single year of age for males and females.
In the model, production for each year will be calculated based on the age, gender and EQ-5D index score (to the nearest 0.05) of each person (Figures 64 and 65).
FIGURE 64.
Production by age and EQ-5D index score: males (£, 2019).
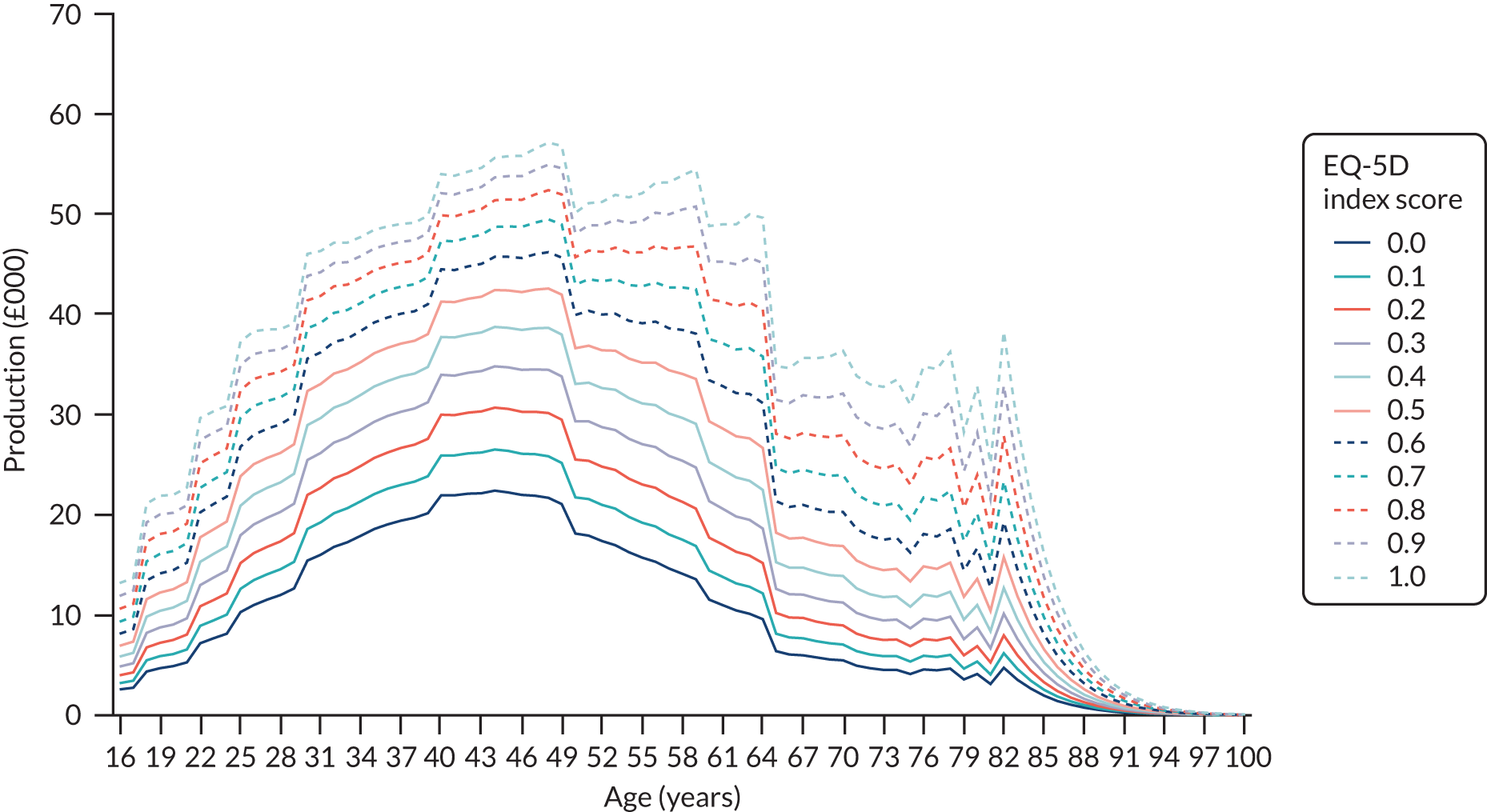
FIGURE 65.
Production by age and EQ-5D index score: females (£, 2019).

Caveats: productivity estimates
Clearly, earnings do not represent the full value of production (i.e. production in industry may be reinvested and may produce profit for shareholders or owners of businesses). One reason we included household production and not just workplace production is that only including workplace production may value women differently, as women are more likely to earn less or work part time. In fact, the GVA-based average value of household production that we use is larger than the average workplace wage that we use. Some of the data used is several years old, particularly the time use survey data. As we have assumed a constant cost for future years in the model, this may underestimate some productivity benefits, as the proportion of older people in work has increased over time. For instance, employment in females aged 50–64 years has increased from 47% in 1992 to 67% in 2017, and for males aged 50–64 years it has increased from 66% in 1992 to 76% in 2017. This is partly because of a healthier workforce, which is what we are measuring with productivity. Our main approach to productivity is to take current production and apply health-related decrements, rather than to see how better health might improve productivity. This might produce unreliable results, particularly when diseases affect a large proportion of the population.
Household production includes unpaid adult informal care (5% of GVA), so, arguably, when looking at results from a societal perspective, there can be some double counting of the effects of reducing or increasing disease prevalence and mortality, as having diseases reduces household production of informal care and increases consumption of informal care, but this effect will be small on the overall cost-effectiveness from a societal perspective.
Opportunity costs of informal (unpaid) care
There is no real consensus on how to include costs of informal (unpaid) care in economic evaluations. Ways to cost informal care may include using average hourly wages of all industries, hourly wages in the formal care sector or contingent valuation of receiving respite from informal care.
There are different types of informal care, including active care (e.g. bathing and helping people get dressed) and passive care (e.g. sitting with someone or being ‘on-call’ for when active care is required). The UK ONS uses four categories of informal care for its household satellite accounts, which are based on Family Resources Survey data:247
-
Continuous care, where individuals require a continuous carer for a maximum 168 hours per week.
-
Practical care, where care is not continuous, but involves physical help with paperwork, financial matters or other practical help, such as shopping, laundry, housework, gardening, doing odd-jobs, taking someone out for a walk and keeping an eye on someone.
-
Personal care is classified as help with personal care (e.g. dressing, bathing, washing, shaving, feeding and using the toilet), physical help (e.g. walking, getting up and down the stairs, and getting into and out of bed) and other personal help (e.g. preparing meals, giving medicines and change dressings).
-
Personal/practical, which is a mix of the two categories in the same hour.
The informal care hours were estimated by using a regression equation, which estimated days of informal care out of the last 42 days, based on Health Outcomes Data Repository data, which includes 59,512 observations across 44,494 individuals aged ≥ 18 years who were discharged from hospital at Cardiff and Vale University Health Board. 162 The regression equation was a zero-inflated negative binomial with a variable inflation model (which included age, gender, EQ-5D index score, primary diseases by ICD-10 chapter and presence of comorbidities) and a binary variable (defined as having diseases from more than one ICD-10 chapter). For our purposes, we included the coefficients for EQ-5D index score only and not for disease chapters or comorbidities.
The equation was:
where
and
where ICD-10 chapter and gender(1 = female) are binary variables.
Equation 6 was applied to EQ-5D index scores in increments of 0.05 from 0 to 1, as well as single year of age, and gender.
The estimated days of informal care were multiplied by the average hours per day of informal care for people in receipt of informal care based on ONS household satellite account data for 2014. This includes both passive and active informal care. The hours were multiplied by ONS estimates of total GVA for unpaid adult care for 2016 and divided by the total hours of GVA for 2014 to give an estimated GVA per hour of £8.91 in 2019 prices (inflated using Treasury GDP deflator231). The ONS GVA estimates are based on a weighted average of the four types of care (i.e. continuous, practical, personal and mixed practical/personal), which have different hourly rates that vary roughly from around £7 to £12, and are based on market rates for paid care staff.
Overall, this method produced a table of informal care costs per year by gender, single year of age (aged 35–100 years) and EQ-5D index score, which can then be used in the model. We present the values in graphical form in Figure 66.
FIGURE 66.
Annual estimated informal care costs by age and EQ-5D index score.

Appendix 7 Detailed inputs to inform analysis 3 scenarios (see Chapter 5)
| Sex | Age range (years) | Fifths of national IMD | QRISK range (%) | ||
|---|---|---|---|---|---|
| < 10.0 | 10.0–19.9 | 20.0 + | |||
| Men | 40–49 | 1 (least deprived) | 0.4 | 0.0 | 0.0 |
| 2 | 2.0 | 0.0 | 0.0 | ||
| 3 | 2.2 | 0.0 | 0.0 | ||
| 4 | 2.5 | 0.1 | 0.0 | ||
| 5 (most deprived) | 8.4 | 0.3 | 0.0 | ||
| 50–59 | 1 (least deprived) | 0.5 | 0.1 | 0.0 | |
| 2 | 2.0 | 0.3 | 0.0 | ||
| 3 | 2.0 | 0.5 | 0.1 | ||
| 4 | 2.3 | 0.6 | 0.0 | ||
| 5 (most deprived) | 5.5 | 2.0 | 0.4 | ||
| 60–69 | 1 (least deprived) | 0.1 | 0.3 | 0.0 | |
| 2 | 0.5 | 1.0 | 0.2 | ||
| 3 | 0.4 | 1.1 | 0.3 | ||
| 4 | 0.4 | 1.1 | 0.3 | ||
| 5 (most deprived) | 0.8 | 2.9 | 0.9 | ||
| 70–74 | 1 (least deprived) | 0.0 | 0.1 | 0.1 | |
| 2 | 0.0 | 0.3 | 0.3 | ||
| 3 | 0.0 | 0.3 | 0.3 | ||
| 4 | 0.0 | 0.2 | 0.4 | ||
| 5 (most deprived) | 0.1 | 0.4 | 1.1 | ||
| Women | 40–49 | 1 (least deprived) | 0.5 | 0.0 | 0.0 |
| 2 | 2.3 | 0.0 | 0.0 | ||
| 3 | 2.6 | 0.0 | 0.0 | ||
| 4 | 3.0 | 0.0 | 0.0 | ||
| 5 (most deprived) | 8.7 | 0.1 | 0.0 | ||
| 50–59 | 1 (least deprived) | 0.6 | 0.0 | 0.0 | |
| 2 | 2.9 | 0.0 | 0.0 | ||
| 3 | 3.1 | 0.1 | 0.0 | ||
| 4 | 3.5 | 0.1 | 0.0 | ||
| 5 (most deprived) | 8.0 | 0.6 | 0.1 | ||
| 60–69 | 1 (least deprived) | 0.4 | 0.1 | 0.0 | |
| 2 | 1.8 | 0.5 | 0.0 | ||
| 3 | 1.7 | 0.5 | 0.1 | ||
| 4 | 1.6 | 0.7 | 0.0 | ||
| 5 (most deprived) | 2.9 | 2.0 | 0.2 | ||
| 70–74 | 1 (least deprived) | 0.0 | 0.2 | 0.0 | |
| 2 | 0.1 | 0.8 | 0.1 | ||
| 3 | 0.1 | 0.7 | 0.2 | ||
| 4 | 0.1 | 0.7 | 0.2 | ||
| 5 (most deprived) | 0.2 | 1.2 | 0.7 | ||
| Fifth of national IMD | QRISK range (%) | ||
|---|---|---|---|
| < 10.0 | 10.0–19.9 | 20.0 + | |
| 1 (least deprived) | 6.0 | 23.9 | 25.0 |
| 2 | 7.7 | 21.8 | 36.8 |
| 3 | 8.3 | 21.8 | 40.3 |
| 4 | 8.9 | 24.8 | 38.4 |
| 5 (most deprived) | 10.1 | 28.8 | 45.0 |
| All IMD | 9.1 | 25.8 | 41.7 |
Appendix 8 workHORSE installation instructions
Appendix 9 Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist
| Section/item | Item number | Recommendation | Manuscript section |
|---|---|---|---|
| Title and abstract | |||
| Title | 1 | Identify the study as an economic evaluation or use more specific terms such as ‘cost-effectiveness analysis’, and describe the interventions compared | Chapter 5, chapter title |
| Abstract | 2 | Provide a structured summary of objectives, perspective, setting, methods (including study design and inputs), results (including base-case and uncertainty analyses) and conclusions | Abstract and Scientific summary |
| Introduction | |||
| Background and objectives | 3 | Provide an explicit statement of the broader context for the study | Chapters 1 and 4, Health economics and equity engine |
| Present the study question and its relevance for health policy or practice decisions | |||
| Methods | |||
| Target population and subgroups | 4 | Describe characteristics of the base-case population and subgroups analysed, including why they were chosen | Chapter 4, Economics methods and Chapter 5, Scenario description and methods |
| Setting and location | 5 | State relevant aspects of the system(s) in which the decision(s) need(s) to be made | Chapter 1, Chapter 4, Economics methods and Chapter 5, Introduction and Scenario description and methods sections |
| Study perspective | 6 | Describe the perspective of the study and relate this to the costs being evaluated | Chapter 4 , Health economics and equity engine |
| Comparators | 7 | Describe the interventions or strategies being compared and state why they were chosen | Chapter 5, Scenario description and methods |
| Time horizon | 8 | State the time horizon(s) over which costs and consequences are being evaluated and say why appropriate | Chapter 5, Scenario description and methods |
| Discount rate | 9 | Report the choice of discount rate(s) used for costs and outcomes and say why appropriate | Chapter 4 , Health economics and equity engine |
| Choice of health outcomes | 10 | Describe what outcomes were used as the measure(s) of benefit in the evaluation and their relevance for the type of analysis performed | Chapter 4 , Health economics and equity engine |
| Measurement of effectiveness | 11a | Single study-based estimates: describe fully the design features of the single effectiveness study and why the single study was a sufficient source of effectiveness data | Chapter 4 , Health economics and equity engine |
| 11b | Synthesis-based estimates: describe fully the methods used for identification of included studies and synthesis of effectiveness data | Chapter 4, Health economics and equity engine and Chapter 5, Scenario description and methods | |
| Measurement and valuation of preference-based outcomes | 12 | If applicable, describe the population and methods used to elicit preferences for outcomes | Chapter 4 , Health economics and equity engine |
| Estimating resources and costs | 13a | Single study-based economic evaluation: describe approaches used to estimate resource use associated with the alternative interventions. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs | N/A |
| 13b | Model-based economic evaluation: describe approaches and data sources used to estimate resource use associated with model health states. Describe primary or secondary research methods for valuing each resource item in terms of its unit cost. Describe any adjustments made to approximate to opportunity costs | Chapter 4, Health economics and equity engine | |
| Currency, price date and conversion | 14 | Report the dates of the estimated resource quantities and unit costs. Describe methods for adjusting estimated unit costs to the year of reported costs, if necessary. Describe methods for converting costs into a common currency base and the exchange rate | Chapter 4 , Health economics and equity engine |
| Choice of model | 15 | Describe and give reasons for the specific type of decision-analytical model used. Providing a figure to show model structure is strongly recommended | Chapter 1 and Chapter 4, Health economics and equity engine |
| Assumptions | 16 | Describe all structural or other assumptions underpinning the decision-analytical model | Chapter 4 , Health economics and equity engine |
| Analytical methods | 17 | Describe all analytical methods supporting the evaluation. This could include methods for dealing with skewed, missing or censored data; extrapolation methods; methods for pooling data; approaches to validate or make adjustments (such as half-cycle corrections) to a model; and methods for handling population heterogeneity and uncertainty | Chapter 4 , Health economics and equity engine |
| Results | |||
| Study parameters | 18 | Report the values, ranges, references and, if used, probability distributions for all parameters. Report reasons or sources for distributions used to represent uncertainty, where appropriate. Provide a table to show the input values is strongly recommended | Chapter 5, Results |
| Incremental costs and outcomes | 19 | For each intervention, report mean values for the main categories of estimated costs and outcomes of interest, as well as mean differences between the comparator groups. If applicable, report ICERs | Chapter 5, Results |
| Characterising uncertainty | 20a | Single study-based economic evaluation: describe the effects of sampling uncertainty for the estimated incremental cost and incremental effectiveness parameters, together with the impact of methodological assumptions (such as discount rate, study perspective) | N/A |
| 20b | Model-based economic evaluation: describe the effects on the results of uncertainty for all input parameters and uncertainty related to the structure of the model and assumptions | Chapter 5, Results | |
| Characterising heterogeneity | 21 | If applicable, report differences in costs, outcomes or cost-effectiveness that can be explained by variations between subgroups of patients with different baseline characteristics or other observed variability in effects that are not reducible by more information | N/A |
| Discussion | |||
| Study findings, limitations, generalisability and current knowledge | 22 | Summarise key study findings and describe how they support the conclusions reached. Discuss limitations and the generalisability of the findings and how the findings fit with current knowledge | Chapter 5, Discussion and Chapter 7 |
| Other | |||
| Source of funding | 23 | Describe how the study was funded and the role of the funder in the identification, design, conduct and reporting of the analysis. Describe other non-monetary sources of support | Title page |
| Conflicts of interest | 24 | Describe any potential for conflict of interest of study contributors in accordance with journal policy. In the absence of a journal policy, we recommend authors comply with International Committee of Medical Journal Editors recommendations | Completed ICMJE forms |
Glossary
- QRISK®2
- An online assessment tool for estimating the 10-year risk of having a cardiovascular event in people who do not already have heart disease.
- UK EuroQol-5 Dimensions Medical Expenditure Panel Survey
- A single-source catalogue of nationally representative EuroQol-5 Dimensions questionnaire scores for chronic conditions in the USA and the UK from the Medical Expenditure Panel Survey. Used extensively for public health and cost-effectiveness modelling.
List of abbreviations
- AF
- atrial fibrillation
- app
- application
- BMI
- body mass index
- CCG
- Clinical Commissioning Group
- CDSR
- Cochrane Database of Systematic Reviews
- CHD
- coronary heart disease
- COPD
- chronic obstructive pulmonary disease
- CPP
- cases prevented or postponed
- CVD
- cardiovascular disease
- CYPP
- case-years prevented or postponed
- EQ-5D
- EuroQol-5 Dimensions
- EQVINC
- equivalised household income quintile
- FOBT
- faecal occult blood test
- GAMLSS
- generalized additive model for location, scale and shape
- GCE
- General Certificate of Education
- GP
- general practitioner
- GPL
- General Public License
- GUI
- graphical user interface
- GVA
- gross value added
- HCP
- Health Check programme
- HIV
- human immunodeficiency virus
- HSE
- Health Survey for England
- ICD-9
- International Classification of Diseases, Ninth Edition
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- LA
- local authority
- LSOA
- lower-layer super output area
- MEPS
- Medical Expenditure Panel Survey
- MoSCoW
- Must have, Should have, Could have, Would have
- NCD
- non-communicable disease
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- NVQ
- National Vocational Qualification
- OBR
- Office for Budget Responsibility
- ONS
- Office for National Statistics
- PAF
- population attributable fraction
- Pap
- Papanicolaou
- PHE
- Public Health England
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- QIMD
- quintile of the Index of Multiple Deprivation
- RCT
- randomised controlled trial
- SBP
- systolic blood pressure
- SHA
- Strategic Health Authority
- STI
- sexually transmitted infection
- T2DM
- type 2 diabetes mellitus
- UI
- uncertainty interval
- workHORSE
- working Health Outcomes Research Simulation Environment
