Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 14/140/80. The contractual start date was in December 2015. The draft report began editorial review in December 2019 and was accepted for publication in July 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2022. This work was produced by Mountain et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health and Care Research, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2022 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
Scientific and clinical background
According to the World Health Organization, approximately 82 million people will be living with dementia across the world by 2030, and that figure is estimated to rise to 152 million people by 2050. 1 It is, therefore, not surprising that dementia is a global and national priority. In 2015, the estimated worldwide cost of dementia was US$818B2 and it is predicted that by 2026 costs to the UK alone will be £24B. 3
Population prevalence increases with age, rising from 1 in 14 for people aged ≥ 65 years to 1 in 6 for people aged ≥ 80 years. 4 In the UK, dementia affects approximately 850,000 people, with 61% of those affected living in the community5,6 and half requiring some form of support. 7 The high prevalence of dementia and related costs has a significant impact on individuals living with the condition, their family carers, services and the economy.
Dementia is a significant cause of illness and disability in older adults. However, in the UK, overall funding for dementia still lags behind that for other common conditions of later life, such as cancer and stroke. 8 Globally, investment in dementia research (both cure and care) has been significantly less than what is indicated by the burden of the disease and the dependency it can create. Therefore, an urgent need for funding for dementia research worldwide has been highlighted. 9
In the last decade there has been a number of UK initiatives to begin to redress the previous neglect of dementia by policy-makers, services and society. In 2009, the UK Government announced the first National Dementia Strategy, which had a number of priorities, including increasing the rates of early diagnosis and improving support for people recently diagnosed with dementia. 10 As part of this strategy, the UK government mandated the establishment of memory services in each health locality. The aim of these services is to enable people experiencing symptoms of dementia to access expert diagnosis, help and, particularly, earlier diagnosis. 10,11 A national audit of memory services conducted in 2013 found a fourfold increase in numbers of dementia patients presenting to such services since 2010/11, with services seeing an average of 1206.2 patients annually compared with 317 in 2010/11. 12 It also highlighted that 49% of patients were in the early stages of the condition. 12 A subsequent audit in 2015 found that access to post-diagnostic services had increased since the previous audit. However, the assistance that people could expect to receive following diagnosis was patchy and inconsistent. 13
UK policy is now focused on the treatment and support required by people following diagnosis,14 with this being echoed by global recommendations. 1 Services are being strongly encouraged to provide post-diagnostic treatment and support to enable people to live well with the condition from diagnosis to end-of-life care. 15 Refreshed national guidance states that people with dementia should be offered a range of tailored activities to promote well-being, and that cognitive rehabilitation and occupational therapy should be considered to support functional abilities. 16 The World Health Organization’s Global Action Plan on Dementia1 confirms the importance of post-diagnostic interventions.
The value of psychosocial interventions for people recently diagnosed with dementia is, therefore, recognised and is also driven by the knowledge that a cure is unlikely in the near future. 17 Additionally, early diagnosis has led to the voice of people with dementia being more readily articulated in books and social media (e.g. Mitchell18), with individuals emphasising that they wish to be offered psychosocial treatment and support from the point of diagnosis. Psychosocial interventions can promote independence and self-efficacy, decrease reliance on carers for longer and can help people to continue to enjoy life. 19 However, this is a neglected area for research and for practice and, as a consequence, little investment (until recently) has been made into intervention development and testing.
Self-management is one example of a psychosocial intervention that might be provided post diagnosis to people with dementia. It is an established concept for those living with long-term conditions and is a cornerstone of health policy in the UK and internationally. 20 It involves people with long-term health conditions identifying strategies and knowledge (in partnership with professionals) that can enable them to take responsibility for their own health as far as they are able to. People with dementia were excluded from the 2005 UK Self-Management for Long-term Conditions policy. 21 In recent years, however, there has been a radical shift in thinking and work is now taking place to explore how people with dementia might be helped to manage their symptoms for as long as possible. There is a growing body of evidence to demonstrate how individuals with dementia can be supported to use self-management-based techniques (sometimes in combination with other interventions, such as cognitive rehabilitation and occupational therapy). 22–27 There is also increased interest in how people with dementia and other comorbid conditions might be enabled to self-manage their health. 28
A qualitative study that interviewed people with dementia who attended a self-management programme found that they considered the programme enjoyable and useful. 23 However, they felt that the programme could have been improved by having more emphasis on maintaining activities and relationships that improve positive well-being. 23 This work also found that people value support from others facing similar challenges. 23 The value of peer support is echoed in studies that have found that people with dementia and their carers benefit from peer support. 29,30
This research aimed to add to the body of evidence through research into the effectiveness of a mixed intervention for people in the early stages of dementia that draws on self-management, occupational therapy treatment techniques and peer support.
Study aims and objectives
The primary aim of the trial was to determine the clinical effectiveness and cost-effectiveness of the Journeying through Dementia (JtD) intervention for people in the early stages of dementia.
To meet this aim, the objectives were to:
-
conduct an internal pilot randomised controlled trial (RCT) of the intervention to check the feasibility of rates of recruitment at scale
-
proceed to a full pragmatic RCT evaluating the clinical effectiveness and cost-effectiveness of the JtD intervention
-
conduct fidelity checks regarding the delivery of the JtD intervention
-
undertake an embedded qualitative substudy to explore issues concerned with intervention delivery
-
identify how the intervention might be realistically delivered through services.
Intervention: rationale, methods and delivery
This summary of the intervention has been written in accordance with the Template for Intervention Description and Replication (TIDier) framework for reporting intervention development and testing. 31
Rationale, theory or goal of the elements essential to the intervention
Journeying through Dementia is a manualised psychosocial intervention for people with early-stage dementia. It was designed through consultation with people living with dementia and was intended to equip individuals with the necessary knowledge, skills and understanding to successfully self-manage their dementia symptoms and live well with dementia. 32 It was created in response to earlier diagnosis of dementia by memory services and the consequent requirement for post-diagnostic interventions. A key distinguishing feature of this intervention is that people with dementia do not necessarily need to involve a supporting carer to take part, thereby addressing the potential needs of the estimated one-third of people with this diagnosis who live alone in the community. 33
The intervention comprised the following two elements: (1) self-management and (2) occupational therapy principles.
Self-management in dementia
Dementia is a constant process of change and readjustment, with one core aspect being capacity to manage the symptoms of the condition. Self-management is one form of psychosocial intervention whereby those with a long-term condition are encouraged to manage their physical and mental health by identifying solutions that meet their own needs, usually in partnership with professionals. Self-management programmes are well established for people with long-term conditions, but their use with people with early-stage dementia is a relatively recent concept. 24
Occupational therapy principles
The JtD intervention is based on the occupational therapy programme Lifestyle Matters,34 which was inspired by an efficacious US intervention called Lifestyle Redesign. 35,36 In common with Lifestyle Redesign and Lifestyle Matters, the JtD intervention operationalises the understanding that individuals are occupational beings and that continued engagement in meaningful activity is key to well-being. 37
Underpinning theoretical framework
The JtD intervention is underpinned by social cognitive theory,38 thereby aiming to increase self-efficacy and effective problem-solving (using the knowledge and techniques described above), which in turns leads to positive emotions, ability to self-manage and life satisfaction.
The content of intervention and processes associated with being in a group target a number of key domains that have been shown to be associated with and/or predict quality of life in dementia. 39–42
-
Mood: the intervention leads to improvements in mood either directly through behavioural activation and learning new coping strategies or indirectly by improving sense of self-efficacy.
-
Relationships: the intervention helps the individual to build relationships and enhances their sense of being connected to others and the community.
-
Beliefs: the intervention helps to promote the understanding that life is meaningful despite dementia.
-
Functional skills: the intervention helps the individual retain skills in activities of daily living and cognitive functioning for as long as possible.
The intervention delivery was also informed by Mitchie et al. ’s43 theory of behaviour change, which emphasises the importance of capability, motivation and opportunity in driving change. Within the theoretical framework of the JtD intervention, behaviour change is associated with improved self-management and engagement in meaningful activity. Capability is addressed by imparting knowledge and training in emotional, cognitive and behavioural skills. Motivation is addressed by increasing knowledge and understanding, eliciting feelings about change and experiential learning to demonstrate positive feelings associated with behaviour change. Opportunity is addressed by making both physical and social environmental changes to enable participants to not only experience the behaviour change in situ (i.e. within the groups and one-to-one sessions), but also by incorporating change into their lives.
The Journeying through Dementia intervention
The manualised intervention contains the following menu of topics that map onto the elements described above. Included topics were identified through consultation with people with dementia prior to the study:32
Reproduced with permission from Craig44
The goals of the intervention are achieved in several ways. Participants may adapt the way an activity is performed, make adjustments to the wider environment or use compensatory techniques. The programme supports individuals to recognise, build on and use existing skills, and to develop new interests that might include drawing upon wider resources.
The intervention content and modes of delivery were tested in a feasibility study25 and found to be acceptable to both people with dementia and their supporters/carers. Reported benefits in people with dementia included increased confidence and self-efficacy, participation in activities and re-engagement in fun and friendships.
Procedures
Who provides: staff and training
To meet service capacity and resources, the intervention was designed for delivery by clinicians who are NHS Agenda for Change (AfC) band 4 or above. All facilitators received a 2-day training course prior to delivering the intervention.
It was intended that the intervention be delivered by a minimum of two trained facilitators at each site and that each trained facilitator would be expected to be involved with the JtD intervention for 1 day per week during the 12-week intervention delivery. This would include preparation of activities, delivery of the group sessions and one-to-one sessions, record keeping and supervision. It was also recommended that more than two facilitators should be trained and be available for intervention delivery at any one site to take account of staff absence.
Delivery methods
The following dimensions were woven into intervention operationalisation, both through the topics within the manualised intervention and the approaches encouraged by facilitators during training and subsequent supervision:
-
content that promotes individuals as occupational beings
-
the premise that continued engagement in meaningful activity is key to experiencing quality of life and well-being
-
menu-led content that allows participants and facilitators to select the topics of most relevance to them from a list of potential options, rather than being required to cover all aspects
-
an asset-based philosophy where group participants are a valued resource for each other and for the group
-
a focus on building self-efficacy within a supportive environment
-
maintaining the locus of control with the person with dementia
-
viewing participants holistically rather than solely focusing on their dementia
-
relationship centred
-
challenges to preconceptions regarding what people with dementia can achieve
-
enactment of activities in real-world settings.
Intervention delivery comprised a mix of facilitated group and individual sessions, with individual sessions feeding into group sessions and group sessions supporting individual sessions.
To meet intervention goals it was recommended that each intervention group involve a maximum of 12 people with early-stage dementia (to account for attrition) who would meet together on a weekly basis for 12 consecutive weeks. It was advised that all participants commence their involvement at week 1, as joining later in the 12-week programme would likely erode group cohesion. Each participant received four one-to-one sessions with one of the facilitators at intervals during the 12 weeks and, as far as possible, the same facilitator conducted all four one-to-one sessions with a participant.
Format of the sessions
Each group session involved the same familiar structure:
-
a welcome and sharing of aims
-
information giving to set the context
-
group discussion topics to build a shared understanding and draw on the strengths and skills of participants
-
a practical activity to provide an opportunity for active experimentation, particularly through out-of-venue activities
-
a summary of key messages and an opportunity to plan for the next session.
Each group was encouraged to select the content of their sessions from a range of topics. An essential component of each session was the enactment of activities in the community, which would involve support from each other and the facilitators.
The nature and content of the one-to-one sessions were guided by the participant’s expressed needs, interests and aspirations, and not those of others. The one-to-one sessions were not to be solely used as an aide memoire of the groups. The sessions involved some discussion and also enactment of activities in the home and/or community, depending on the participant’s goals. Examples of goals taken forward during the feasibility study25 included introducing a method to read recipes, maintaining a diary, attending a community group, engaging in physical activity and preparing resources to take to a forthcoming group session.
Participants were not required to nominate a supporting carer/supporter to take part, but if they did, supporter involvement was limited to certain sessions. This was to promote independence and self-efficacy in the person with dementia and to take account of the needs of participants without supporters/carers.
Location and necessary infrastructure
A local accessible venue was required for the 12 group sessions to assist people to maintain citizenship and community links, rather than promote a model of ill health. Facilitators were advised to identify such a location in advance of intervention delivery commencing and in sufficient time to inform participants of both the venue and the timing of group sessions. The venue was to be readily accessible by public transport, part of the local community, warm and inviting, have sufficient space for group activities and include a kitchen and good toilet facilities (e.g. a library, theatre, leisure centre or community hall).
When and how much
The 12-week intervention duration was based on clinical recommendations that advised that this is the maximum that is affordable for routine service delivery.
Supporter/carer involvement was invited at group sessions 1, 6 and 12 and in one-to-one sessions with the person with dementia, if this was requested by the individual participant.
Sessions: number of times delivered, duration and intensity/dose
Each group session lasted 2 hours and included breaks for refreshments and socialising.
Facilitators were asked to incorporate a minimum of three out-of-venue activities within the 12-week group intervention delivery to enable enactment of activities in real-life settings to take place.
The duration of the one-to-one sessions was not specified and facilitators were advised that they should take place at a venue agreed between the participant, facilitator and supporter/carer, if the person asked for supporter/carer involvement.
Prior to intervention delivery commencing for the trial, we determined that 10 sessions attended out of the potential 16 (irrespective of whether they were group sessions or one-to-one sessions) would constitute a therapeutic dose. This was a pragmatic decision taken by the study team, which took account of past experience of delivering similar interventions.
Intervention tailoring
Facilitators were encouraged to work with other topics that groups and individuals identified, as well as those in the intervention manual. However, they were asked to deliver the intervention as described in the manual and training.
Out-of-venue activities were tailored to the expressed needs of the group and the individuals within it, and they could be simple or more elaborate, depending on the group. For example, moving to a public cafe area within the same building so that participants support each other with the activities they find challenging. Within the group, the aims of individuals will differ. For one person it could be ordering a drink, for another it might be handling money and for a third it might be socialising in a public area. Another group could be enabled to practise their individual skills and nurture their interests in a more demanding out-of-venue activity.
The one-to-one sessions were in place to enable participants to work towards meeting individual goals, with or without a supporter/carer depending on wants, needs and circumstances. Goals could be very small and their achievement incorporated some degree of enactment. On occasion, one-to-one sessions were used to discuss issues of concern to individuals to help identify ways of managing the challenge (e.g. using public transport or continuing to drive). As with the group sessions, the overall aim was to enable participants to articulate how they wanted to use these sessions and be supported to identify and achieve realistic goals. Facilitators were advised that the first one-to-one session should be organised before commencement of the group so that they could introduce themselves to the participant and discuss the forthcoming 12 weeks, including how the participant would access the group venue (transport was not provided). We were also aware (from our previous Lifestyle Matters research45) that this initial face-to-face meeting was important for establishing one-to-one sessions in this form of mixed intervention.
Facilitator/supervisor training
People living with dementia played an integral role in the development of the training for the intervention, as well as in intervention development. Initial consultation led to insights that informed the training. This was then informed by consultation with the Scottish Dementia Working Group (a national campaigning group comprising people with dementia), which assisted with the design of the materials for the intervention and added to our understanding of what would make good facilitation.
Facilitator training was designed to mirror the modes of intervention delivery, in that it was group based (i.e. involving facilitators from more than one site at an independent venue), it sought to impart necessary didactic information to facilitators to achieve a shared understanding and it included experiential work, using exercises taken from the manualised intervention. For example, two trainers modelled group facilitation techniques and trainee facilitators were given the opportunity to rehearse and role play responses to potential scenarios that might arise in the groups they were to facilitate. Training took place as near as possible to commencement of intervention delivery.
Taught sessions within the training (didactic information) included the following:
-
the background, context and development of the JtD intervention
-
how the intervention was underpinned by evidence regarding needs for, benefits of and nature of post-diagnostic support for people affected by dementia
-
the underpinning philosophy and approach of the intervention
-
information on the models and modes of group work
-
information on the role of individual sessions
-
information on environment and resource management
-
information on methods of record-keeping and reflection through use of reflective diaries.
Discussion topics included the following:
-
prior experience, interest and motivations
-
understanding the experiences of people living with dementia (with responses to quotes by people with dementia and a film made by people living with dementia)
-
a walk through of the manual (identifying the components)
-
details on the preferred approaches to group facilitation
-
information on how the environment can support and inhibit interaction and self-efficacy
-
information on managing groups (including a discussion based around a series of personas).
Experiential sessions included the following:
-
what makes a good post-diagnostic support programme (using an interactive activity)
-
community resources (including a pictorial and mapping activity)
-
guidance on the planning, and facilitation of sessions (with trainees acting as group participants)
-
guidance on managing groups and delivering individual sessions (using role play)
-
information on methods of record-keeping and completion of other intervention-related documentation for the purposes of delivery and for research.
Facilitators were asked to document all group and individual sessions on a pro forma provided by the research team. They were asked to write records primarily for the benefit of participants and in accordance with Dementia Engagement and Empowerment Project (DEEP) guidance for best practice in providing documentation for people with dementia. 46
Facilitators were asked to write session records as soon after the group as possible and post to all participants before the next session.
Facilitators were also asked to maintain registers of participant attendance and records of the supervision sessions they attended on other pro formas provided to them by the trial manager.
Facilitators were asked to post copies of all these records to the trial manager. These records were requested at the end of the 12 weeks for the first rounds of intervention delivery at all participating sites. For subsequent rounds, intervention delivery facilitators were asked to return records every 3 weeks. The reason for this change was to improve delivery of the intervention in accordance with the manualised intervention on an ongoing basis.
Facilitator supervision (for the purposes of this study)
Once intervention delivery commenced for the trial, facilitators all received weekly supervision from a suitably knowledgeable and experienced clinical professional (AfC band 7 and above) from their place of work, identified and nominated by the research site.
Ideally, people supervising would have themselves previously facilitated the intervention. For the purposes of this study, however, this was not possible, and so a ‘train the trainers’ model was adopted. This involved suitably experienced individuals being nominated as supervisors. Members of the trial team, who were experienced in delivering and supervising psychosocial interventions within trials, then delivered regular supervision to these supervisors.
The supervision protocol identified for the purposes of the study is outlined below (see Report Supplementary Material 1 for the full supervision protocol):
-
Suitably qualified professionals who agreed to supervise facilitators at site were invited to take part in the first day of the 2-day facilitator training, ideally with the facilitators they were to supervise, as well as with other supervisors and facilitators from different sites.
-
A second half-day session with other supervisors and the trial team member nominated to supervise them was then convened to discuss perceptions and any concerns regarding both intervention delivery and the associated research, as well as to go through practical details, including completion of necessary trial documentation for supervision (see Report Supplementary Material 2 for the supervisor booklet). This second session wase scheduled as close as possible to the initial training.
-
On commencement of intervention delivery, each facilitator then received weekly supervision in a format agreed between themselves and the person nominated at site to supervise them [e.g. face to face, Skype™ (Microsoft Corporation, Redmond, WA, USA), FaceTime (Apple Inc., Cupertino, CA, USA) or telephone]. These supervisions included both individual and group supervisions.
-
Facilitators and those supervising them maintained records of the nature and duration of each supervision record, using the trial pro formas.
Modifications to facilitator identification and training, intervention delivery and supervision
Slower rates of recruitment at sites than those originally envisaged led to the need for a greater number of delivery sites (n = 13) and, hence, a greater number of facilitators and supervisors. Facilitator and supervisor attrition and absence also led to increased numbers being trained. Overall, we trained 69 facilitators (not all of whom delivered sessions) and 21 supervisors.
Identification of intervention facilitators
The intention was to recruit NHS band 4 staff with appropriate experience to deliver the intervention, but in practice a range of staff were recruited. Staff included experienced grade 8 occupational therapists and (in one instance) a grade 2 assistant (see Report Supplementary Material 3 for details of the numbers and pay grades of facilitators and supervisors).
Facilitator and supervisor training and supervision
The total number of 2-day facilitator training sessions delivered as originally intended was six. The number of facilitators trained as intended was 53 (of the overall 69 facilitators trained). The total number of supervisors’ training sessions delivered as intended was eight. The number of supervisors trained as intended was 17 (of the overall 21 supervisors trained).
Facilitator attrition occurred because of a significant number of assistant psychologists who fulfilled this role who then moved to different posts or higher training, and also because of staff sickness or pregnancy in other cases. In addition, new research sites had to be involved over time to meet the recruitment target. This led to supervisor and facilitator training being repeated and modified to meet the needs of those new to the study. Sixteen facilitators and four supervisors subsequently received a modified version of the training. The following modifications were delivered:
-
Site-based training to facilitators at four sites, rather than working with a group of other facilitators and supervisors at a central university venue.
-
Site-based training to supervisors at five sites, rather than working with a group of others at a university venue.
-
Shortened facilitator training from 2 days to 1.5 days, complemented by provision of online training presentations.
-
Shortened facilitator training from 2 days to 1 day, complemented by online presentations and resources in four instances.
-
In one case, for a relief facilitator who the site felt was unlikely to facilitate but wanted trained just in case, the full course was provided remotely via online presentations, accompanied by a telephone call.
-
Refresher training at local sites was delivered 10 times because of a protracted period between initial training and delivery.
Fidelity and consistency across selected facilitator training sessions were formally assessed (see Chapter 3, Fidelity study for more details).
Intervention delivery and attendance
The numbers of participants registered to receive the intervention for each round of delivery per site was < 12 participants for the majority of sites, with the range being between 4 and 12 participants (Table 1). Only two intervention groups achieved 12 participants. This led to 28 rounds of intervention delivery for the trial, whereas the original estimate was 20 groups (i.e. two rounds of intervention delivery at 10 sites). For further information about intervention delivery and attendance see Chapter 3, Journeying through Dementia intervention and Appendix 5, Tables 34 and 35.
| Site IDa | Intervention delivery round | Participants (n = 239b) |
|---|---|---|
| S01 | 1 | 7 |
| 2 | 8 | |
| 3 | 7 | |
| S02 | 1 | 7 |
| 2 | 7 | |
| 3 | 8 | |
| S04 | 1 | 9 |
| 2 | 6 | |
| S05: delivery site 1 | 1 | 12 |
| 2 | 7 | |
| S05: delivery site 2 | 1 | 8 |
| 2 | 7 | |
| S06 | 1 | 9 |
| 2 | 10 | |
| S07 | 1 | 12 |
| 2 | 11 | |
| S08 | 1 | 9 |
| 2 | 10 | |
| S09 | 1 | 8 |
| 2 | 9 | |
| S10 | 1 | 9 |
| 2 | 11 | |
| 3 | 11 | |
| S11 | 1 | 10 |
| S12 | 1 | 6 |
| 2 | 4 | |
| S13 | 1 | 6 |
| S15 | 1 | 11 |
Extent to which intervention was delivered as planned
As well as providing records for participants, the group and individual records maintained by facilitators were also used to enable ongoing review of intervention delivery.
The chief investigator and leads for supervision (KB/Jasper Palmier-Claus) aimed to review returned records in a timely manner and provide feedback to site supervisors on facilitator compliance with the intended intervention and identify where any adjustments were required. Feedback was provided by e-mail communication to all sites actively delivering the intervention at any time and was not site specific.
Issues identified through review of records maintained by facilitators included examples of good practice that matched the manualised intervention, needing improvement and examples of practice that was poor and also non-adherent to the intervention. Examples of good practice include:
-
records written in lay language and in the first person, focussing on key learning points for participants
-
records typewritten in sufficiently large font with text well spaced on the page
-
providing evidence covering all components of the intervention (including a discussion, goal-setting and activities)
-
documented adherence to out-of-venue activities
-
provision of additional, well-presented information (e.g. information on locations used for out-of-venue activities)
-
facilitators engaging in one-to-one activities chosen by participants
-
additional positive elements delivered above and beyond the manualised intervention (e.g. skill-sharing and having shared lunches among participants).
Examples of practice that needed improvement included:
-
poorly documented records or records that were too dense
-
no concrete examples documented within records
-
over-reliance on clinical terminology or professional language, including use of abbreviations
-
overly prescribed intervention content or not letting participants choose content and not responding to participant ideas for activities
-
different facilitators involved in one-to-one sessions with the same participant
-
facilitators focusing more on reviewing the groups in one-to-one sessions and encouraging group attendance rather than engaging in new purposeful activities
-
records recording referrals to health care
-
signposting only, rather than following up with activity
-
unrealistic goal-setting.
Examples of practice that was poor and also non-adherent to the intervention include:
-
use of NHS premises for the group
-
supervisors routinely adopting a facilitation role (our facilitator absence guidance stated that supervisors could occasionally, in unavoidable circumstances, step in as facilitators, but it was not appropriate for supervisors to take on the role of facilitator on a regular basis)
-
unclear which facilitators had been involved
-
group sessions conducted with only one facilitator
-
not conducting the three out-of-venue activities
-
supporters/carers attending out-of-venue activities and/or more than the three allocated supporter sessions (i.e. sessions 1, 6 and 12)
-
facilitators not undertaking one-to-one sessions
-
facilitators undertaking one-to-one sessions but not the group sessions
-
one-to-one sessions conducted with two facilitators rather than one
-
telephone-delivered one-to-one sessions
-
supervisors not providing opportunities for individual, as well as joint, supervision for facilitators
-
use of additional bespoke questionnaires to determine needs and assess risks.
Chapter 2 Methods
The study design was a pragmatic superiority two-arm parallel-group individually randomised controlled trial. It involved an intention-to-treat (ITT) comparison of the JtD intervention with usual care. 47 The full trial protocol is available via the funder website48 or as a publication. 47 The trial proceeded to a full trial after conducting an internal pilot RCT of the intervention to check the feasibility of rates of recruitment at scale. This chapter describes the detailed methods for the main trial and for two of the substudies: (1) an examination of intervention fidelity and (2) a qualitative study of experiences from those who took part. Methods relating to the health economic analysis and participant and public involvement (PPI) are described separately in Chapters 4 and 6, respectively.
Methods for the main trial
Important changes to methods after trial commencement
The most significant study changes after trial commencement are listed below. Study protocol amendments are listed in full in Appendix 2.
Recruitment period changes
The original planned recruitment period was from October 2016 to end of September 2017 (12 months). However, because of issues with recruitment, the final recruitment period was November 2016 to August 2018 (22 months). The study was open to recruitment for 10 months more than originally planned. Recruitment was stopped in August 2018 to ensure that all 8-month follow-ups could be conducted within project timelines.
Number of recruiting sites changes
The project originally aimed to have 10 recruiting sites; however, because of issues with recruitment, 13 recruiting sites (across 14 NHS trusts) were involved. More NHS trusts than recruiting sites were involved because two neighbouring NHS trusts worked together to recruit participants and run the intervention.
Data analysis changes
In the event that there were > 10 couples living in the same household, where both individuals had a dementia diagnosis and both were enrolled in the study, the primary and secondary analyses were to be changed to take into account the hierarchical or clustered nature of the data. A multilevel mixed-effects model would be used [the random effects were the JtD intervention groups (top level) and couple/singles (lower level)]. Individual participants who were not part of a couple were to be treated as clusters of size 1. This analysis approach was not implemented in the study, as there were not > 10 couples living in the same household, where both individuals were diagnosed with dementia.
Changes to facilitator and supervisor training
Three changes to the plan for training of facilitators and supervisors were made. The first was that one of the initial training sessions (on 23 and 24 January 2017) had to be significantly modified during delivery because of the large numbers of attendees. Modifications included reducing time spent on a topic/activity or excluding it altogether (see Chapter 3, Training fidelity for further information on modifications). The second was that online resources were developed to assist with training part-way through the study. The third change was that many training sessions had to be delivered at the local sites, rather than centrally as planned (see Chapter 1, Facilitator/supervisor training for further information).
Follow-up window changes
In the final months of the trial a decision was made by the study team, with support from the Trial Steering Committee (TSC), to extend the time window in which the outcome data could be collected. The window already included the period 8 weeks after the 8 or 12 months post randomisation date. This was unchanged. The period in which data could be collected prior to the 8- and 12-month follow-ups was originally only 2 weeks. This was extended to 8 weeks. A gap of at least 52 days was preserved, when possible, between the 8- and 12-month follow-ups. This change allowed 12-month data to be collected from participants before the end of the trial where it may have otherwise been missed. The change meant that approximately 41 participants had their 12-month follow-up earlier than originally planned. Despite this change, 13 participants were still unable to receive their 12-month follow-up appointment.
Data collection procedure changes
To retain participants in the study and to be able to collect the maximum number of participant data, a decision was made that when it was the only feasible way (i.e. no physical visit was possible), outcome measures could be collected from participants over the telephone for the 8- and 12-month follow-ups. This included the primary outcome Dementia-Related Quality of Life (DEMQOL) measure, the reduced Health and Social Care Resource Use Questionnaire (HSCRU) and a telephone version of the EuroQol-5 Dimensions, five-level version (EQ-5D-5L). These measures were chosen as the most important to assess the effectiveness of the intervention, judged to not place a high burden on participants and were the most feasible to collect over the telephone. The telephone measures for participants were used only twice for the DEMQOL, once for the EQ-5D-5L (telephone version) and four times for the reduced HSCRU.
Further study document amendments
Amending the instrumental activities of daily living questionnaire (approved 24 January 2017)
An amended version of the published instrumental activities of daily living (IADL) was approved by the Research Ethics Committee, which re-phrased some of the questions and gave an additional option for one of the activities covered in the questionnaire (travel) that the activity was not done at all. All participants used the updated version of the form during the baseline and 8-month assessments.
Amending the Sense of Competence Questionnaire (approved 13 March 2017)
This was a minor change to the Sense of Competence Questionnaire (SCQ), which we used at baseline and 8 months for the participating supporter (i.e. a supporter/carer who had signed up to be part of the trial). The study team decided to remove all headers/taglines at the top of the printed document that referred to demented persons. This was approved by the Health Research Authority as a minor amendment. This did not affect the questions.
Changes aimed at participant retention
In October 2018, after advice from the TSC and our PPI Advisory Group, the suite of documents used by the study to contact participants to arrange and confirm appointments was revised after a review of the live trial data indicated that withdrawal rates were high.
Participant and Public Involvement Advisory Group: supporting qualitative analysis
To assist with interpretation of the qualitative analysis, people with lived experience of dementia, from the University of Bradford Experts by Experience Group, were invited to take part in two successive workshops. Some of the individuals who took part were members of the Study Advisory Group and others were new to the study. Quotation/extracts from the interview data, which researchers identified as being representative of the categories identified during the analysis, were presented to group members for discussion. The workshop outcomes were used to refine and, when necessary, revisit the qualitative analysis. The study protocol was amended on 5 December 2018 to reflect the PPI role in this respect.
Changes to the fidelity substudy
The protocol was amended on 14 June 2017 to change methods from the use of filming to observation to assess facilitator adherence to the manualised intervention and participant receipt of the intervention. The decision was made to use observations as this method was more practical and would still achieve the aims of the fidelity substudy.
Changes to the qualitative substudy
Originally, qualitative interviews were planned with staff at the fidelity sites at the beginning and end of their delivery of the intervention to monitor their change in outlook based on their experience of delivering the intervention. However, as many of the facilitators to be interviewed had delivered the intervention in a previous wave within the study, there was deemed to be less value in conducting two interviews. The protocol was therefore amended on 28 November 2017, so that one interview was conducted with individuals to look more broadly at the facilitator experience of delivering the intervention.
The purposive sample for qualitative semistructured interviews with participants and participating supporters was changed to participants at sites who were part of the fidelity assessment. This change was to ensure meaningful triangulation of qualitative results with other fidelity assessments.
Participants and eligibility criteria
Participants in the trial were individuals with a formal diagnosis of dementia and their supporters where these consented to be involved. Supporters included family members, friends and neighbours named as supporters by the person with dementia. Where supporters were trial participants they were referred to as ‘participating’ supporters. Participants and participating supporters were selected based on the eligibility criteria outlined below.
People diagnosed with dementia
Inclusion criteria
-
People diagnosed with all forms of dementia.
-
A Mini Mental State Examination (MMSE) score of ≥ 18 (taken < 2 months pre consent). MMSE is measured on a scale from 0 to 30 and higher scores indicate better cognitive function.
-
People with the ability to make informed decisions.
-
People living in the community or in sheltered accommodation, alone or with others.
-
People with the ability to converse and communicate in English.
-
People willing to engage in a 12-week group self-management intervention.
Exclusion criteria
-
People not diagnosed with a form of dementia.
-
People with a moderate or severe dementia with a MMSE score of < 18.
-
People assessed as lacking capacity.
-
People living in residential or nursing care.
-
People who are not able to converse or communicate in English.
-
People taking part in any other pharmacological or psychosocial intervention studies.
Participating supporters
Inclusion criteria
-
People aged ≥ 18 years.
-
People named by the person with dementia as their supporter.
-
People with the ability to converse and communicate in English.
-
People with the ability to give informed consent.
Exclusion criteria
-
People aged < 18 years.
-
The person with dementia for whom they provide support to is not participating in the trial.
-
People who are not able to converse or communicate in English.
-
People who are not able to give informed consent.
Participant identification and recruitment
Recruiting people living with dementia to clinical trials is known to be challenging and, therefore, a variety of participant identification methods were used to maximise recruitment. The recruitment strategies used varied between sites because of the different configurations of dementia care within NHS trusts (see Report Supplementary Material 4 for the overall participant identification and recruitment process within the JtD intervention).
Regardless of the method of identification, care was taken to cater to the needs of the individual, given the potential for cognitive and communication difficulties. Although there was a standardised identification and recruitment process, it was important that it allowed some degree of flexibility to cater to the specific needs potential participants may have had. Alongside the participant information sheet, a shorter information leaflet, which had been reviewed by the PPI Advisory Group, was used to provide a simplified overview of the trial. This was used before going into more detail with the full-length participant information sheet if the individual was still interested after receiving the initial information. In the first instance, any information about the trial was communicated to the person living with dementia; however, if the person with dementia requested, researchers could communicate with someone they identified as a participating supporter.
Anyone who expressed interest received a face-to-face visit from a researcher who had been offered training on the additional communication needs of people living with dementia.
Researchers were provided with suggestions for recruitment methods, but implementation of these methods was at the discretion of the researchers because of their knowledge of the individual NHS trust. Recruitment was continually kept under review and support was provided from the central team if required. The different methods used are described below.
Recruitment via secondary care services
Patients attending post-diagnostic appointments who were potentially eligible were informed about the JtD intervention by the health-care professionals or other relevant staff members while at the clinic. They were given brief written information about the trial and if they were interested verbal consent was taken to pass their contact details to a member of the research team who would then contact them and follow the recruitment procedure. On occasion, a member of the research team attended post-diagnostic clinics so that they were on hand to provide further information and answer questions. This was also helpful in reminding health-care professionals of the study and bringing it to patients’ attention.
Some NHS trusts screened patient lists for potentially eligible people and sent them an information pack containing a participant invitation letter and free-post response card. If the person with dementia was interested, they completed and returned the response card to the research team who then followed the recruitment process. As it is known that people with dementia may find it challenging to return the response card by post, if appropriate, those sent the initial recruitment pack would also be followed up with a telephone call by an NHS staff member.
Recruitment via primary care
Primary care general practitioners (GPs) were approached to recruit participants, namely by carrying out mail-outs to appropriate registered patients.
Recruitment through the Join Dementia Research database
The JtD trial was registered on the Join Dementia Research (JDR) database, which is a database hosted by the National Institute for Health and Care Research (NIHR) to help people with dementia identify recruiting research studies that they may be interested in joining. The JDR database was used for recruitment to the JtD trial in two ways. First, those registered to the JDR database who were interested in the JtD trial were able to contact the research team directly. Second, the research team were able to search for potentially eligible people and contact them, provided permission was given to do so. Once the research team had details of the person with dementia, the JtD trial standard recruitment pathway was then followed.
Recruitment through service user groups
Service user groups, such as the Alzheimer’s Society support groups, were used to identify potentially eligible participants. Researchers visited such groups to explain the trial and anyone interested was given a response card to complete or return. Once researchers had details of the interested individuals, the recruitment process was followed.
Recruitment through general promotion
The JtD trial was promoted using posters and leaflets in general practices and community venues. Social media was also used to make people aware of the trial. For these general promotional methods, the researcher’s details were made available for those interested to contact them directly.
Baseline and follow-up visits
Visits types and timings
The aim was to conduct baseline visits for participants and participating supporters no more than 2 months before the intervention was due to start. Follow-up visits were conducted with participants and participating supporters at 8 months after the date of randomisation, and with only participants at 12 months after the date of randomisation. Follow-up visits were conducted in a period between 2 weeks prior to the target date and 8 weeks after. Later in the study, this period was extended to 8 weeks prior to the target date. However, in circumstances where visits within this period were not possible, attempts were made to collect outcome measures outside this period.
Staff conducting visits
Visits were conducted by one or two outcome assessors who were blinded to allocation for the 8- and 12-month follow-up visits. Staff followed lone working policies when conducting visits alone.
Planning visits
Baseline visits were arranged with participants and participating supporters at the time of consent being taken. The participant and participating supporter visits could be carried out at the same time or separately, depending on preferences. Additionally, when close to the intervention starting, consent and eligibility visits could be combined with baseline visits.
Four weeks prior to follow-up visits commencing, participants and participating supporters were contacted by post to let them know that a member of the study team would shortly be contacting them by telephone to arrange a visit. Outcome assessors would then telephone participants and participating supporters to request and arrange the visit. Written confirmation of the visit would be sent to the participants and, if participants wished, outcome assessors would make a brief telephone call shortly before the visit to confirm with the participant that it was still acceptable to go ahead. After introducing new retention procedures, researchers aimed to organise the 12-month visit time during the 8-month visit.
Consenting and assessing capacity
Written consent was obtained when participants joined the trial and confirmed verbally at subsequent visits. Capacity to consent was formally assessed when participants joined the trial and was assessed informally by researchers at each visit. At visits where the participant was felt not to have capacity to consent, attempts were made to support the person to demonstrate capacity, for instance by presenting information on the study in a different format or returning for a visit at a later date. When these attempts were unsuccessful during follow-up visits, the consultee process was enacted. The consultee process is described in Ethics arrangements and regulatory approvals.
Conducting visits
Visits were principally conducted in the participants’ homes or in other public locations, such as cafes, if the participant requested this. Measures were administered by the assessors using a verbal structured interview technique. Available responses were presented in an A4 format to aid participants, with large black print on a yellow background to assist those with visual impairments. The measures used are outlined in Outcomes.
In some circumstances, participants did not feel able to complete all the measures (e.g. because of fatigue). In these cases the researchers prioritised the DEMQOL, EQ-5D-5L and HSCRU (during follow-ups) as the key outcome measures. Additional visits could be made to complete measures when it was not possible to complete them in a single visit and participants were happy to be visited again.
Telephone measures
When it was not possible to arrange face-to-face follow-up visits with participating supporters, for example because of work commitments, data could be collected over the telephone. In specific circumstances a reduced set of measures (i.e. the DEMQOL, EQ-5D-5L and shortened HSCRU) could be collected by telephone from participants when it was not feasible to collect these data in any other way.
Outcomes
Outcome measure selection
The outcome measures selected were used to quantify the following key components of the intervention: mental well-being and mood, building relationships, a sense of connectedness, belief that life is meaningful despite a diagnosis of dementia, instrumental activities of daily living and strategies to maintain cognitive functioning. They also support analysis of the participating supporters’ perceptions of competence of caring for the person with dementia. Dementia-specific outcome measures were selected based on recommendations for research across Europe. 49 When there were no appropriate dementia-specific measures available, measures for the general population were used.
Primary outcome measure
Health-related quality of life of the person with dementia at 8 months post randomisation, measured using the DEMQOL measure,50,51 was the primary outcome. The DEMQOL measure contains 28 items and covers 5 domains: (1) daily activities and looking after yourself, (2) health and well-being, (3) cognitive functioning, (4) social relationships and (5) self-concept. It is completed by the person with dementia, with a higher score indicating a better health-related quality of life. Health-related quality of life of the person with dementia at the 12-month follow-up interval, measured using the DEMQOL measure, was a secondary outcome measure. The DEMQOL is measured on a scale from 28 to 112 and higher scores represent higher health-related quality of life.
Secondary outcome measures
The secondary outcome measures for the trial are listed below.
-
Generic health status was assessed for both the person with dementia and any participating supporters using the EQ-5D-5L. 52,53 The measure comprises an assessment of five dimensions of health: (1) mobility, (2) self-care, (3) usual activities, (4) pain and discomfort and (5) anxiety and depression. A score closer to 1.00 represents full health. A visual analogue scale (VAS) also rates overall current health, with 0 representing the worst health imaginable and 100 representing the best health imaginable.
-
Depression, assessed for both the person with dementia and their participating supporter, was measured using Patient Health Questionnaire-9 items (PHQ-9). 54,55 The PHQ-9 measures the severity of depressive symptoms based on the symptoms of major depressive disorder. A higher score represents more depressive symptoms.
-
Anxiety was assessed for both the person with dementia and their participating supporter using the Generalised Anxiety Disorder-7 (GAD-7). 56 The GAD-7 comprises seven items and assesses the severity of the symptoms of anxiety based on recognised symptoms of generalised anxiety disorder. A higher score indicates greater levels of anxiety.
-
The person with dementia’s perceived ability to feel self-efficient and manage day-to-day challenges was assessed using the 10-item General Self-Efficacy Scale (GSE). 57 A higher score on the scale indicates greater self-efficacy.
-
The perception of a positive state of well-being of the person with dementia was measured using Diener’s Flourishing Scale (DFS). 58 This eight-item scale assesses key aspects of positive social and psychological functioning. A higher score corresponds to a more positive state of well-being.
-
The person with dementia’s perceived ability to self-manage was measured using the Self-Management Ability Scale (SMAS). 59 The SMAS is a 30-item scale for ageing individuals, measuring central cognitive and behavioural abilities that are presumed to contribute to successful self-management of ageing. Higher scores correspond to higher levels of ability to self-manage.
-
The functional ability of the person with dementia was measured using the IADL. 60 The IADL assesses functional ability in eight domains of complex activity by the selection of statements of capability. A higher score represents better functional ability and greater independence.
-
Health and social care resource use of the person with dementia was measured using a bespoke measure, the HSCRU. 61 The HSCRU records four key areas of resource use: (1) hospital episodes, (2) use of community health resources, (3) use of day services and (4) medication use. Hospital episode records details of attendance at accident and emergency departments, inpatient admissions, outpatient clinics and hospital transport use. Use of community health resources records the use of a wide range of health and social care provision, including frequency and location of use and who provides the service. Day service use includes name, location and provider of the service, and the frequency of use. Medication use information includes dosage, frequency and duration of use of memory enhancers, medication for mood, pain relief and hypnotics. The information gathered in the HSCRU supports the cost-effectiveness evaluation of the intervention.
-
Satisfaction of the supporter with their perceived competence in providing care to the person with dementia was measured by the SCQ. 62,63 The SCQ is a 27-item scale with three domains: (1) satisfaction with the care recipient, (2) satisfaction with one’s own performance and (3) consequences of involvement in care for the personal life of the caregiver. Higher scores indicate a better sense of competence. The SCQ has been shown to have good validity for supporters/carers of those with diagnosed dementia, but insufficient validity in a population of carers of persons with dementia in its early stages. 62,64 However, the SCQ was identified as an appropriate measure based on recommendations for dementia research across Europe, despite these concerns. 49
Scoring and interpretation of outcome measures
The DEMQOL score was calculated by summing the response to the first 28 items when at least half of the items were answered. Any missing items were imputed with the mean of the completed items. 65 The PHQ-9 and GAD-7 were scored by summing the responses to items if no more than two items were missing. 66,67 The total SMAS score was calculated by summing responses across the six subdomains (each subdomain was classed as valid if no more than one item was missing and the total score was calculated if all subdomain scores were valid). The GSE was calculated by summing the response to items if at least seven items were complete. 68 The DFS, IADL, MMSE and SCQ were all calculated by summing the responses to the items on each scale, only if all items of the scale were completed. The EQ-5D-5L was scored using the mapping function developed by van Hout et al. 69 and no score was calculated if any item was missing. This was different from our intended scoring prespecified in the statistical analysis plan because of an updated positional statement by the National Institute for Health and Care Excellence (NICE) published while the trial was ongoing. 70 For all outcome measures scored with missing items, the missing items were imputed with the mean of completed items for that scale.
Measures taken
The measures taken with both the participants and participating supporters at each of the visits are detailed in Tables 2 and 3.
| Measure | Baseline visit | 8-month follow-up visit | 12-month follow-up visit |
|---|---|---|---|
| DEMQOL | ✓ | ✓ | ✓ |
| EQ-5D-5L | ✓ | ✓ | ✓ |
| PHQ-9 | ✓ | ✓ | |
| GAD-7 | ✓ | ✓ | |
| GSE | ✓ | ✓ | |
| DFS | ✓ | ✓ | |
| SMAS | ✓ | ✓ | |
| IADL | ✓ | ✓ | |
| HSCRU | ✓ | ✓ |
| Measure | Baseline visit | 8-month follow-up visit | 12-month follow-up visit |
|---|---|---|---|
| EQ-5D-5L | ✓ | ✓ | |
| PHQ-9 | ✓ | ✓ | |
| SCQ | ✓ | ✓ |
Sample size
The sample size was calculated assuming a standard deviation (SD) of 11 points and that a difference of ≥ 4 points in mean DEMQOL score 8 months post randomisation was clinically and practically important. 51 The sample size was calculated to have 90% power for detecting this 4-point difference (or more) if it truly exists, which is equivalent to a standardised effect size of 0.36, as statistically significant at the 5% two-sided level. As the JtD intervention was a facilitator-led group intervention, the outcomes of the participants in the same group with the same facilitators may have been clustered. For an individual RCT without adjustment for clustering, the target sample size would be 160 participants per arm (i.e. 320 participants in total). Assuming an average cluster size of eight participants per facilitated group45 and an intracluster correlation of 0.03 this inflated the sample size, by a design effect of 1.21, to 194 participants per group (i.e. 388 participants in total) with valid primary outcome data. We assumed a 20% loss to follow-up and therefore the trial target sample size was to randomise to 243 participants in each arm (i.e. 486 participants in total).
There is no reported or established minimum clinically important difference (MCID) for the DEMQOL measure. Data from the development and validation of the preference/utility-based DEMQOL-Utility (DEMQOL-U) score,51 which compared the performance of the DEMQOL (and DEMQOL-U) with other patient-reported outcome measures for people with dementia (e.g. the MMSE,71 the Bristol Activity of Daily Living Scale72 and the Neuropsychiatric Inventory)73 with established MCIDs, reported changes on the DEMQOL of 5.4, 7.5 and 6.4 points, respectively, for patients who improved by more than the MCID on the anchor measure. This suggests that our proposed 4-point difference, although small, is likely to be of clinical and practical importance.
Internal pilot trial
The following stop/go criteria were reviewed after 8 months of active recruitment to an internal pilot trial:
-
recruitment of a minimum of 113 participants across the six pilot sites by the end of the fifth month of active recruitment (i.e. 75% of the 150 participant target)
-
recruitment of a minimum of 12 facilitators (i.e. two facilitators identified at each of the six pilot sites by the start of active recruitment to deliver the intervention)
-
no more than two of the six planned groups in the internal pilot with fewer than four participants registered for the group by the sixth month of active recruitment.
The TSC assessed whether or not they could recommend that the trial continue in the light of the feasibility results against the stop/go criteria. The results and recommendations were communicated to the funder [see Chapter 3, Internal pilot (stop/go assessment)].
Method used to generate and assign the random allocation
Randomisation was overseen by the Sheffield Clinical Trials Research Unit (CTRU), using a computerised randomisation sequence generated before the start of recruitment. An unblinded member of the research team who did not conduct any outcome assessments entered the participants’ details into the remote web-based randomisation system. Participants were then randomly allocated to receive either the intervention or usual care. Owing to the group-based intervention, participants were randomised after the baseline outcome measures had been collected, which ideally occurred < 2 months before the intervention wave began at each site. In instances when a couple living in the same household both consented, they were randomised as a couple rather than individually. Participating supporters were allocated to the same group as the linked participant.
The participants and, if relevant, their participating supporters were informed of their allocation by letter. Participants allocated to the intervention were told that an intervention facilitator would contact them to discuss the next steps of the trial.
Following allocation of a participant to the intervention group, an unblinded member of the research team informed the appropriate intervention facilitators so that they could contact participants to arrange intervention delivery.
Type of randomisation and details of any restriction
Participants were randomised 1 : 1 to receive either the JtD intervention or usual care. The computer-generated randomisation was stratified by a stratification site and restricted by a fixed block of size 4. The stratification site was either the recruiting site or the group delivery site if the recruiting site was delivering more than one group simultaneously in different locations. The JtD intervention had 16 stratification sites across 13 recruiting sites. A fixed block size was used to ensure that the imbalance between treatment groups at each delivery site was controlled and minimal. Two intervention groups were planned at each site and a maximum number of 13 participants were allowed in each intervention group. The risk of predicting the allocation was deemed minimal, as it was performed by central unblinded members of the trial team and members of the trial team responsible for recruiting participants at site were blinded to treatment allocation.
Blinding and methods used to control bias
Blinding in the context of research studies refers to the concealment from individuals involved in a clinical trial of the allocation to an intervention or control group of participants in the trial. It is used to minimise or prevent differential treatment or outcome assessment of groups in the trial and reduce bias. 74 The trial was single blind as participants were aware of their allocation to either the JtD intervention group or the usual-care control group. Outcome assessments were completed by assessors who were blind to group allocation. The sections below on allocation concealment, blinding and other methods provide further information on the study’s approach to reducing bias.
Allocation concealment mechanism
Allocation concealment was maintained by using a centralised web-based randomisation service. A member of the trial team, unblinded to treatment allocation, informed participants of their allocation. Participants were not blinded to their allocation because of the nature of the intervention; however, they were advised that the outcome assessors were blinded to their allocation. If outcome assessors suspected that they had been unblinded, this was recorded in the case report form (CRF) and reported to the trial oversight committees.
Blinding
Outcome assessment
Most research sites nominated an unblinded researcher to facilitate communication with the central study team regarding the allocation of participants to the intervention. Baseline measures were completed prior to the randomisation of participants and so were completed by both blinded and unblinded researchers. Subsequent allocation to the intervention or control group was communicated to participants and group facilitators by an unblinded researcher, or by the central study team when there was no unblinded researcher at a site. Eight- and 12-month outcome measures were completed by blinded researchers. Facilitators of the intervention were not involved in outcome assessment.
When blinded research staff became unintentionally unblinded (e.g. a participant unintentionally disclosed their study arm during a visit) this was recorded on a paper unblinding form and then entered onto the research database by an unblinded researcher. The unblinding form recorded information from the assessors regarding whether they were sure or unsure that they had been unblinded, and to which arm of the trial they suspected the participant was allocated. Paper records of instances of unblinding were stored in a separate location to the CRFs to prevent blinded researchers viewing this information. To prevent unblinding, the study database was designed such that researchers were unable to view unblinding forms for sites at which they were blind.
During the trial, a system was implemented so that a person who was not conducting the outcome measures visit would make the telephone call to arrange the visit to lessen the chance of unblinding. A telephone script was used during the telephone call. The script asked the participant and supporter not to tell the outcome assessor what treatment they did or did not receive during the visit. The visit confirmation letter also reaffirmed the requisite not to disclose information about treatment to the outcome assessor. If unblinding occurred prior to assessment, for instance on the telephone while arranging an assessment visit, another blinded researcher would conduct the assessment. If unblinding occurred during an outcome visit, the visit continued; however, future assessments were conducted by a different blind assessor.
Data analysis
The trial statistician and the health economist were blinded during the intervention delivery and outcome assessment, but not blinded for the main analysis. The TSC was blinded throughout the study.
Methods used to control bias
The study used methods to protect against both facilitator bias and the risk of cross-contamination between the two study arms. Facilitator bias is when the same facilitators might also provide any interventions in the usual-care arm. Two approaches were used to address this. First, usual care (see Chapter 3, Usual care) is limited and most often restricted to NICE-recommended cognitive stimulation therapy (CST), which would not be readily influenced by training in delivery of the JtD intervention. CST follows a detailed and prescriptive session-by-session plan of group exercises and activities that are facilitator led and take place wholly within the delivery setting. In the JtD trial sites, other post-diagnostic services offered included Living Well with Dementia groups (three sites) and memory groups (two sites); however, these were not common. Living Well with Dementia groups vary in content, but may have contained some similar material to the JtD intervention. However, they do not include enactment of learnt skills in the community or the mix of individual and group sessions that the JtD intervention incorporates. Additionally, analysis indicates that at 8 months only one JtD trial participant was attending an NHS-run Living Well with Dementia group, indicating that these are rare (see Chapter 3, Usual care). Second, a proportion of the facilitators recruited were not those who would deliver usual care (e.g. approximately 25% of facilitators were trained research staff).
Cross-contamination could also occur between the two study arms, when, for example, participants in the intervention arm meet those in the control arm by attending memory clinics and discuss the intervention. As post-diagnostic services for people living with dementia are currently limited and often only involve CST, which is more likely to be offered later in the dementia trajectory, it is unlikely that participants met at these courses. Extended post-diagnostic follow-up is not common and so it is unlikely that participants from different arms of the study would meet up and discuss their involvement in the study during routine appointments.
Analysis populations
The ITT data set includes all participants who consented and were randomised according to randomised treatment assignments (ignoring any occurrences post randomisation, such as protocol or treatment non-compliance and withdrawals) and who had complete DEMQOL data at 8 months post randomisation. This excludes participants who withdrew before randomisation and includes participants found to be ineligible post randomisation. 75
The complier-average causal effect (CACE) data set is a subset of the ITT set. It included a subgroup of participants who were believed to comply with the JtD intervention (i.e. attending at least 10 of 16 sessions), excluded ineligible participants randomised in error and included participants who were randomised to usual care but received and complied with the JtD intervention.
The analysis population for participating supporters included participating supporters who met eligibility criteria, provided informed consent and provided follow-up data.
Statistical analysis
General considerations
All statistical analyses were performed in Stata® v15 statistical software (StataCorp LP, College Station, TX, USA). The usual-care arm was the reference group for all analyses. A comprehensive statistical analysis plan was developed while the statistician was blinded to treatment allocation. Data were reported and presented in accordance with the revised Consolidated Standards of Reporting Trials (CONSORT) statement. 76 All statistical tests were two tailed at a 5% significance level and confidence intervals (CIs) were two sided, with 95% intervals. No adjustment was made for multiple testing.
Data completeness
A CONSORT flow diagram was used to display data completeness and participant throughput from first contact to final follow-up.
Baseline characteristics
The baseline characteristics, medical history and quality-of-life data for the participants were summarised and assessed for comparability between the intervention and control groups. No statistical significance testing was carried out to test imbalances between the treatment groups, but any noted differences were reported descriptively.
Primary effectiveness analysis
The mean DEMQOL total score at 8 months post randomisation was compared between participants in the JtD intervention group and participants in the usual-care group using a mixed-effects linear regression model adjusted for DEMQOL baseline total score and stratification site (fixed effect) and allowing for the clustering of the outcome by the JtD intervention groups (random effect). 77–79 A partially clustered mixed-effects linear regression model with homoscedastic errors was used to model clustering in the intervention arm. Degrees of freedom were computed using the Satterthwaite approximation. 80
Sensitivity analyses on primary outcome
The below pre-planned sensitivity analyses were undertaken on the primary outcome and displayed alongside the primary analysis results.
To truly implement ITT analysis, imputation was performed on all participants who were consented and randomised, including those with missing primary outcome data, but excluding those who died before the 8-month follow-up. Participants’ baseline characteristics were summarised and compared between completers and non-completers. Missing 8-month DEMQOL data were imputed using regression imputation and multiple imputation. The regression imputation equation accounted for participant baseline data (i.e. age, gender, presence of participating supporter, type of dementia, site, and baseline DEMQOL, GAD-7 and PHQ-9 scores). Multiple imputation imputed missing outcome data using chained equations (regression) with 100 imputations, using baseline data, prognostic factors and predictors of missing data (i.e. age, gender, presence of participating supporter, type of dementia, duration of dementia, site, and baseline DEMQOL, GAD-7 and PHQ-9 scores) as covariates in the imputation equation, and excluding treatment group. The model described in the primary outcome analysis was applied to the multiply imputed data using residual degrees of freedom.
A sensitivity analysis on the primary outcome was conducted excluding participants with data collected > 2 weeks pre the 8-month follow-up and > 10 weeks post the 8-month follow-up.
The analysis of outcome in relation to JtD intervention compliance was conducted via CACE analysis using a two-stage least squares regression, excluding clustering adjustment but including site and baseline covariates (i.e. age, gender, presence of participating supporter and baseline DEMQOL score). Compliance was defined as attending at least 10 of the possible 16 sessions. Exploratory descriptive analysis compared compliers with non-compliers in the intervention arm with respect to baseline data and socioeconomic status. (Socioeconomic status was calculated from postcode data using the Office for National Statistics Index of Multiple Deprivation.)81
A process of delayed baseline and randomisation was implemented to ensure that the delay between baseline and a JtD intervention group starting was < 2 months, hence the time between baseline and course starting varied across participants. The following two sensitivity analyses were conducted:
-
a sensitivity analysis including a covariate of days between baseline and the group starting in the primary analysis model
-
a sensitivity analysis including a covariate of days between baseline and the 8-month follow-up visit in the primary analysis model.
Secondary effectiveness analysis
Secondary outcomes measured on the participant at 8 and 12 months were analysed using a mixed-effects regression model, as for the primary outcome, including the baseline measurement of the respective outcome. Secondary outcomes measured on the participating supporter were analysed using least squares linear regression models.
Subgroup analysis
Pre-planned subgroup analyses were undertaken and regarded as exploratory. The following subgroups were investigated:
-
type of dementia (Alzheimer’s disease vs. any vascular dementia vs. other)
-
presence of participating supporter (as asked at eligibility assessment).
The subgroup analysis used mixed-effects linear regression with total DEMQOL score at 8 months as the response. The model included the main effects of treatment and subgroup, and an interaction term between treatment and subgroup, and covariates of stratification site, baseline DEMQOL score (fixed effects) and JtD intervention groups (random effect). The evidence for treatment effect varying between subgroup was investigated using a statistical test for interaction between the randomised intervention group and the subgroup.
Safety and harms analysis
Serious adverse events (SAEs) were summarised and assessed for similarity between treatment groups. SAEs were reported on an ITT basis for randomised participants only. The proportion of participants and participating supporters considered to be suffering moderately severe depression symptoms (i.e. a PHQ-9 score of ≥ 15)55 or moderately/severe levels of anxiety (i.e. a GAD-7 score of ≥ 10)82 were summarised by treatment group and time point and assessed for similarity.
Study oversight and management
Trial committees
The trial conduct was governed by three oversight committees: (1) the TSC, (2) the Data Monitoring and Ethics Committee (DMEC) and (3) the Trial Management Group (TMG). The trial was conducted in line with Sheffield CTRU’s standard operating procedures and the committees assembled at intervals dictated by standard operating procedures and study requirements. For names of those on the committees please see Appendix 1.
Trial Steering Committee
The TSC comprised a person with dementia, researchers and clinicians, all of whose membership was approved by the NIHR Health Technology Assessment (HTA) programme (i.e. the trial funder). The TSC was chaired by Professor Catherine Hewitt, Deputy Director of York Clinical Trials Unit, University of York, York, UK. Professor Hewitt is a statistician and is independent of the trial. The TSC was responsible for advising the chief investigator, supervising the trial protocol, CRF and statistical analysis, as well as monitoring the trial progress. The TSC also reviewed relevant trial-related information from external sources, trial outputs and reports. The TSC met approximately 6-monthly.
Data Monitoring and Ethics Committee
The DMEC comprised a chairperson, a statistician and a clinical researcher, all of whom were independent of the trial. Membership was approved by the NIHR HTA programme. The DMEC was chaired by Dr Mona Kanaan, Senior Lecturer, University of York, York, UK, who is a statistician. The DMEC was responsible for reviewing the trial protocol and study materials, monitoring participant safety and advising the TSC when trial protocol should be changed. The DMEC met approximately 6-monthly.
Trial Management Group
The TMG was made up of individuals directly involved in trial development and delivery. This includes the chief investigator, the trial manager and collaborators. The role of the TMG was to implement the trial, as well as identify and resolve problems regarding the intervention and associated research. The TMG met on a monthly or bi-monthly basis, depending on the stage of the trial.
Project management
The trial sponsor, the Sheffield Health and Social Care NHS Foundation Trust, selected the University of Sheffield’s CTRU to oversee the day-to-day management of the trial. This included the trial manager, whose role was to co-ordinate the trial, supervised by the director of the CTRU. The research team carried out monitoring in accordance with the trial’s monitoring plan.
Patient and public involvement
The trial received input from people with dementia and people supporting individuals with dementia throughout. An individual living with dementia sat on the TSC. Aside from this, a separate JtD trial PPI Advisory Group met regularly throughout the trial. A member of the study team who took the lead for PPI activities was key to facilitating the involvement of individuals with dementia and supporters/carers in this trial (see Chapter 6 for further information).
Ethics arrangements and regulatory approvals
Ethics and regulatory approvals
A protocol was submitted for scrutiny to a UK NHS Research Ethics Committee in May 2016 (reference 16/YH/0238), with approval being granted in July 2016. UK Health Research Authority approval was given in August 2016 (Integrated Research Application System reference 199383). Participating sites gained permission from the local NHS trust research and development department prior to commencing recruitment as a study site.
Ethics concerns
Two principal ethics issues were identified: (1) the need to break confidentiality in the case of a risk of harm being identified and (2) the potential for participants to lose capacity during the trial.
Breaking confidentiality
Participants had extensive contact with facilitators of the intervention, as well as home visits from facilitators and outcome assessors, during which time the identification of risk to participants or participating supporters was possible. To safely manage this risk and prevent harm to participants, it was possible that trial staff would need to break the normal boundaries of confidentiality. This principle was explained to participants during the consent process and information was included on the participant information sheet for both participants and participating supporters. The person with dementia and participating supporters then explicitly consented to researchers contacting their GP or dementia worker in the case of a concern about risk as part of the study consent process.
Loss of capacity during the trial
The capacity of participants to continue in the trial was assessed throughout the trial process. If it was felt that a participant or participating supporter lacked capacity during a contact, attempts were made to support the person to demonstrate capacity, for instance by presenting information on the study in a different format or returning for a visit at a later date. The degenerative nature of dementia meant that it was possible that participants who had initially given informed consent could lose the capacity to do so later in the trial. To manage this possibility, a consultee was identified at the point of the person with dementia consenting to the trial, in line with guidance from the Mental Capacity Act 2005. 83
The consultee was an individual involved in the care of the person with dementia, who was not involved in the trial and who could be consulted were the person with dementia to lose capacity. If a person with dementia lost the capacity to give informed consent to continue, the consultee was asked what, in their opinion, the person with dementia’s wishes about continuing in the study would likely be if they did have the capacity to consent. Prior to making this decision, the consultee was provided with written information about the role of the consultee and the consultee’s decision on whether or not to take part was given in writing on a study-designed form. If the consultee felt that the participant would wish to continue, the participant expressed the wish to continue and the researcher felt that this would be possible, then the person with dementia continued in the study. If necessary, adjustments were made to facilitate the continued involvement of the person with dementia (e.g. by administering a reduced set of outcome measures).
A person with dementia was withdrawn from the trial if they lost capacity and did not wish to continue. Participating supporters could not act as consultee. If participating supporters lost capacity then there was no provision for them to continue in the trial and they were withdrawn.
Information about the activation of consultee pathways and outcomes were entered on the study database.
Safety
Concerns
Safety concerns could be raised at any point during the trial (e.g. because of a risk to self or others, deterioration in mental state of a participant or if there is a safeguarding issue). A concerns procedure was in place to allow study staff to report concerns to the health-care professional involved in the care of the person with dementia, or their participating supporter. The concerns process was also triggered automatically by high overall scores on the PHQ-9 and GAD-7 (indicating moderate or severe depression or anxiety) or a high score on one item of the PHQ-9 that refers to thoughts of being better off dead or of harming yourself (indicating thoughts of this nature occurring on more than half the days or nearly every day of the past 2 weeks). Participants were not automatically withdrawn based on high questionnaire scores.
In the event of a concern, the participant’s or participating supporter’s GP or a health-care professional at their memory service was contacted in writing with details of the concern. The GP or health-care professional was able to recommend that the participant or participating supporter be withdrawn from the study if they felt it appropriate. The local principal investigator was also often consulted to determine further steps to be taken regarding risk, and any immediate risk management was carried out as per the local NHS site procedures. Details of the concern and the actions taken as a result were recorded on the study database.
Serious adverse events
Additional monitoring of patient safety was completed using a SAE reporting system for the participants with dementia. A SAE was defined as an event that:
-
resulted in death
-
was life-threatening (i.e. the subject was at immediate risk of death)
-
required hospitalisation or prolonged existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
consisted of a congenital anomaly or birth defect
-
was otherwise considered medically significant by the investigator.
Serious adverse events were reported to the trial manager within 24 hours of being identified, using a centralised reporting system. Another person was delegated to receive and record SAEs in the absence of the trial manager. The reporting form was submitted to the local principal investigator for assessment of the severity and whether or not it was related to the intervention. Adverse events not identified as serious were not recorded or reported.
Other safety concerns
There was potential for participants to become distressed while discussing their experiences in intervention sessions or as part of the follow-up process. It was felt that this could be managed by the presence of health-care staff delivering the intervention or researchers conducting follow-ups who could provide reassurance or direct participants to appropriate sources of support.
As there was a potential safety risk to researchers who were working alone in participants’ homes, a lone worker policy was developed. The researcher completed a form detailing information about any participant visits and their contact information, and provided this to a ‘buddy’ who ensured the safety of the researcher. The researcher checked in with the buddy before and after a visit finished or the buddy followed escalation procedures. Checklists provided guidance on what to do before and during the visits (e.g. ensuring that mobile phones are fully charged and being prepared to leave in an emergency if there are concerns about safety). A phrase was provided to enable the researcher to report an emergency during the visit. Guidance was provided for general safe travelling (e.g. to keep to well-lit paths and driveways).
Data collection and management
The follow-up of participants and data collection was co-ordinated by the CTRU in conjunction with the participating NHS sites and collaborating universities. Data were collected by researchers employed by The University of Sheffield, University of Hull, University of Bradford and University of Nottingham, as well as authorised individuals at participating NHS sites. Information, such as outcome measures, was gathered using the JtD trial CRF, an ad hoc record with both paper and electronic components designed specifically for this trial. The CRFs were made up of paper and electronic parts. During the trial, paper documents were retained in secure locations.
The CTRU’s in-house web-based data management system (Prospect; University of Sheffield, Sheffield, UK, and epiGenesys, Sheffield, UK) was used to capture and store participant data. Data were inputted by trained and authorised members of the trial team whose access was controlled by individual usernames and encrypted passwords. A privilege management feature was used to ensure that users of Prospect had an appropriate level of access to data to allow them to complete their tasks. Participant confidentiality was respected at all times. Access to names and contact details entered into Prospect was restricted to only users with certain privileges. All other data were pseudonymised, identifiable only through a participant identification number.
Validation features of Prospect were used to monitor study data quality. When clarification of data were needed, error reports were generated detailing queries.
Usual care
Information regarding usual care available to participants at intervention sites was recorded with the use of a usual-care template, the format of which was agreed by the study’s health economist. The template was sent to principal investigators at each site, who either completed templates themselves or nominated a person with appropriate knowledge for the task. When a site ran interventions in two or more areas, when usual care differed, usual-care information was requested for each of the intervention areas.
The templates asked for details about care and the care pathway that was usually offered to people who had recently been diagnosed as living with dementia, including where any clinic or group attendance was offered after diagnosis. The template also asked for the method and frequency of review post diagnosis and what action would be taken if a person’s condition worsened. To view analysis of the usual care at intervention sites please see Chapter 3, Usual care.
Additionally, the 8-month HSCRU sections on day service use and community-based service use were analysed and are summarised in Chapter 3, Usual care to provide an insight into the types of services routinely being accessed by people living with dementia over the month prior to being asked.
Fidelity assessment
Rationale
The need to improve and expand on the evidence base for complex interventions has been recognised by health-care providers, commissioners, researchers and the public. 84 Complex interventions present a number of methodological challenges to evaluation, including standardisation of multicomponent interventions, contextual impact and complexity of associating change with the intervention. 85 Trial designs that include mixed methods have been widely recommended to capture such changes and explain the reasons for intervention efficacy or lack of efficacy. 85,86
Researchers need to be able to demonstrate with confidence that their study results are due to the impact of the intervention under investigation and not other extraneous factors. Fidelity assessment uses multiple methods to evaluate validity and reliability of complex interventions. 87,88 For this study, we employed observations and facilitator reports to explore training, delivery and receipt of the group aspect of the intervention.
Aims
The main aim of the fidelity assessment was to assess how well the intervention was delivered in accordance with the protocol and intervention manual, as outlined in the assessment framework presented in Table 4. The framework was designed specifically for this intervention and is based on criteria identified by the Behaviour Change Consortium87 and NICE guidance on behaviour change,89 providing quality assurance parameters for intervention design, training, delivery, receipt and enactment.
| Element | Fidelity criterion | Fidelity standard |
|---|---|---|
| Trial design | ||
| Comparable treatment | All participants receive the same intervention tailored to the needs of the group/setting |
|
| Risk to implementation | Plan for potential issues that could affect the delivery of the intervention |
|
| Monitoring facilitator training | ||
| Standardised training | All facilitators and supervisors receive the same training in a similar way |
|
| Facilitator skill acquisition | All facilitators understand and engage with the intervention training in a similar way |
|
| Monitoring intervention delivery | ||
| Standardised delivery | All facilitators using the same techniques and content from the intervention |
|
| Minimise drift in skills/delivery | Adherence to training content and delivery across sites |
|
| Monitoring receipt of intervention | ||
| Participant attendance and engagement |
Numbers of participants attending the intervention each week All participants taking part in the group meetings and activities Impact of intervention on participant in terms of well-being |
|
Research team and reflexivity
Throughout the fidelity study, two researchers were engaged in data collection and analysis (i.e. the fidelity lead and a second researcher). The fidelity lead remained consistent throughout the duration of the substudy, whereas the second researcher role was undertaken by four successive researchers.
Sampling
A convenience sample of four of the total number of sites participating in the study were invited and agreed to take part in the fidelity assessment and all four sites agreed to participate. A pragmatic approach was taken towards site selection (including local research governance approvals being in place, sites being open and running the intervention and sites having the capacity to participate in the fidelity work). Consideration was also given to geographical location and population size to represent services across the totality of field work sites.
Methods
The fidelity lead and members of the research team developed bespoke fidelity assessment checklists and intervention delivery registers to collect data. The TMG and authors of the manualised intervention reviewed draft versions of several iterations before reaching agreement on the final content (see Report Supplementary Material 5–12 for copies of all registers and checklists).
Observations and checklists
Non-participatory observations were used to explore intervention training for facilitators and supervisors, as well as delivery and receipt of the intervention, during a sample of four group meetings at each of the four sites. Purposely designed checklists listing core skills and anticipated observed behaviours [e.g. role-play (for training fidelity) and enactment (for group intervention fidelity)] were identified from the training materials, intervention manual and trial protocols. These checklists were piloted by the researchers during the first training session and the first group meeting at two different sites to identify any issues and undertake revisions before application to remaining observations.
The fidelity lead and a second researcher attended and coded three training sessions and eight group meetings (two group meetings per fidelity site at approximately weeks 3 and 8 of the 12-week programme). Group meeting observations took place within the location and venue of the given group. Out-of-venue activities were not observed, as it would not be practical to do so given the observation methods. Observations of the group meetings were planned to help identify any facilitator drift over the intervention delivery lifespan.
To provide comparisons with the researcher observations, the facilitators and supervisors were also asked to complete a simplified version of the intervention training checklist. In addition, facilitators were asked to complete a simplified version of the group meeting checklist.
Observations of one-to-one sessions were considered too intrusive. Instead, all facilitators taking part in the fidelity assessment were asked to complete one-to-one session checklists to evaluate their experience of these sessions. These were completed at the end of each of the four one-to-one sessions delivered to the participant.
Facilitators and supervisors both completed a supervision checklist to assess adherence to, and the delivery and quality of, supervision. This was completed at the end of the first, fifth and twelfth weeks of supervision.
Attendance registers and intervention worksheets
Facilitators and supervisors were requested to maintain a number of records for the trial and these were also used as part of the fidelity assessment at the four sites. These included weekly registers of group and one-to-one session attendance. A supervision log recorded by the research supervisor was maintained to record adherence to the intervention (see Report Supplementary Material 2).
Analysis
Frequencies
The extent to which the intervention training and the delivery and receipt of the intervention were implemented as intended was assessed using the previously described data sources (checklists, registers and logs). First, the items on the observation checklists were scored (by two researchers). The categories for scoring were ‘0’ (never observed), ‘1’ (sometimes observed) and ‘2’ (observed most of the time). The scores were then analysed to describe frequencies.
Completion rates
Completion rates of the checklists and registers determined how well the intervention was delivered in accordance with the protocol and the intervention manual.
Inter-rater reliability
Inter-rater reliability between the two observing researchers was examined to establish the extent to which coders attributed the same score to the same checklist item. 90 Cohen’s kappa does not take into account the degree of disagreement between coders when using an ordinal scale as the 3-point scale for the checklists; therefore, a weighted kappa using a predefined table of weights was applied to provide estimates of the degree of disagreement between the coders.
Qualitative study
Rationale
Qualitative research can provide a richer understanding of people’s experience, thoughts and motivations in a real-world context. An embedded qualitative substudy was conducted as an adjunct to the main trial to explore insights from those involved in delivering and receiving the intervention to evaluate and inform future development of the intervention.
Aims
The aim of the qualitative study was to enable exploration of intervention facilitation, delivery and receipt, specifically how the intervention produces change in a particular context. 91
Sample
Interviews were conducted with a purposive sample of participants and their participating supporters (when one was present). The participant sampling frame was based on a range of characteristics, including diagnosis, age, ethnicity, gender, MMSE score, living arrangements and extent of participation (including participants who had withdrawn from the intervention) (see Table 32 for further details on the participant sample).
Facilitator and supervisor sample
Interviews were also conducted with facilitators and supervisors at each of the four fidelity sites. Interviews were conducted by three members of the research team, including the fidelity lead. All interviews were conducted individually over the telephone using a topic guide. Interviews took place at the end of a cycle of delivery (post completion of a group session at approximately week 13). All facilitators and supervisors involved in delivering the intervention from the four fidelity sites were invited to be interviewed.
Methods
A critical realist epistemological position was taken, which assumes that from a social science perspective we are able to identify and describe real properties and causal relationships in the world around us. 92 However, it also assumes that not all knowledge is equal when trying to understand the real world. 93 We therefore assumed a level of truth about the effectiveness of the intervention based on participant, participating supporter, facilitator and supervisor perspectives, thoughts and experiences of delivery and receipt of that intervention, and by collecting a range of views that could be deemed an approximation of a wider understanding.
Participant and participating supporter interviews
Individual semistructured interviews were conducted with participants and participating supporters (face to face) from the four sites participating in the fidelity assessments. The fidelity lead and members of the research team initially developed interview schedules independently. The wider TMG, including PPI input and authors of the intervention manual, then reviewed the schedules for several iterations before reaching agreement on the final content. The final schedules for the participant and participating supporter interviews were also reviewed by the JtD trial PPI Advisory Group whose members had lived experience of dementia. With their advice, the schedules were shortened and language simplified.
Interviews were conducted until saturation of the data was reached. The participants were known to the researchers conducting the interviews, as the researchers had observed the participants’ group sessions as part of the fidelity assessment.
All interviews were conducted after the last meeting for each of the four groups that took part. Owing to administrative issues and capacity to arrange suitable times, some of the interviews were conducted several months after the intervention finished. Our sampling strategy sought to interview participating supporters of participants who were also being interviewed so that their accounts could be triangulated for a deeper understanding of their experience. We sought to conduct the interviews separately when possible. Participants could ask for a friend or relative to be present at the interview, and, if the friend or relative shared their views, they would be asked for verbal permission to include them in the analysis.
The interview schedules explored the following themes:
-
the range and nature of issues that influence experiences of the intervention
-
the perceived advantages and disadvantages of taking part in the intervention
-
factors that may mediate or moderate the effectiveness of the intervention
-
the perceived skills and competencies required to facilitate the intervention
-
the barriers to and facilitators of uptake and continued use of the intervention
-
the effect of the intervention on participation and living with the diagnosis of dementia
-
the impact of support for someone with dementia.
The researchers used prompts to aid participant recall on some of the interview questions in terms of giving examples of activities conducted or topics discussed in the group sessions the researchers had observed.
Interviews were conducted in a convenient location for the participant; all participants requested for the interviews to take place at their home. The interviews were audio-recorded with consent. Researchers undertaking the interviews were trained to use enhanced methods of communication with people with dementia to ensure that meaningful findings were obtained. All participants and participating supporters were sent a copy of their interview transcript for respondent validation. No responses were received.
Facilitator and supervisor interviews
Individual semistructured interviews were conducted with facilitators and supervisors by telephone from the four sites participating in the fidelity assessments. The interview schedule, reviewed and approved by the TMG, explored the following themes:
-
what issues promote the effectiveness of intervention facilitation
-
the skills and competencies required to facilitate the intervention
-
the barriers to and facilitators of uptake and continued use of the intervention
-
factors that may mediate or moderate the effectiveness of the intervention
-
perceptions of growth into the role (or lack of growth)
-
participant receipt of the intervention
-
satisfaction with supervision
-
potential drift
-
impact on own practice.
Copies of the interview transcripts were sent for respondent validation. Several responses were received and the information was included in the analysis.
Analysis
The same methods of analysis were applied to all interview transcripts. This involved the trial qualitative lead and a second researcher throughout. The trial qualitative lead and a second researcher first read through all the transcripts to embed themselves in the data. A purposive sample of transcripts were then selected for initial thematic open coding. Selection of the facilitator, participant and participating supporter transcripts for this first open coding included representation from each site and a cross-section of viewpoints. As there were only four supervisors, all transcripts were included in the open coding process.
These transcripts were coded independently by the two researchers before a joint review to establish an initial framework of overarching themes and subcategories. Positive, negative and ambivalent cases were considered and the use of two coders ensured that coding did not bias any one point of view.
Framework analysis,94 a theoretical form of thematic analysis, was used to analyse the full data set. 95 NVivo software version 12 (QSR International, Warrington, UK) was used to assist with analysis, which involved the confirmation of the final thematic framework and the development of an index of subcategories that were then applied to all transcripts. Further iteration of the framework was agreed during the coding process for any new subcategories arising from the full data set. All interview data were then charted using the framework, and the resulting data map was used to identify explanations and processes underlying the intervention. Results were also used to identify emergent factors that could influence the uptake and impact of the intervention.
The participant and participating supporter interviews, and the staff interviews were analysed separately, therefore producing two separate frameworks (one for participants and participating supporters, and one for facilitators and supervisors). Finally, the two frameworks were triangulated to identify common themes and divergent cases.
For the purposes of reporting, confidentiality was maintained by using unique participant identifiers and removing identifiable information.
Co-researcher qualitative analysis validation workshop
People living with dementia and their supporters took part in two co-researcher data validation workshops held in February and July 2019 to support the analysis of the participant and supporter interview data. Some of those who were members of the JtD trial PPI Advisory Group took part in the workshops, and others were recruited for these sessions from the University of Bradford Experts by Experience cohort. 96 The first workshop was attended by six co-researchers (two couples, one person living with dementia and one supporter/carer) and the second workshop was attended by 12 co-researchers (five couples, one person living with dementia and one supporter/carer). Both workshops were delivered in a community venue and co-researchers were recompensed for their time and expenses. The purpose of the workshops was to identify similarities and differences between researcher interpretations of the data and the interpretations of people with lived experience of dementia. A total of 20 quotations (nine quotations in workshop 1 and 11 quotations in workshop 2) were selected from the participant and supporter interview data as being examples from the range of themes and subcategories in the framework analysis. These were presented to the workshop attendees, who were then asked to review and respond to the quotations, with particular focus on language, tone and overall meaning of content. The activity was supported by two facilitators who were experienced in delivering workshops and working with people with dementia. Written notes were taken during the workshop and the sessions were also audio-recorded to ensure accuracy of interpretations. The interpretations of people living with dementia were compared with those of the researchers and used to refine the final qualitative analysis.
Triangulation of qualitative findings
Rationale
Triangulation enables the comparison of concurrently collected data obtained using different methodologies and from different researchers to be explored for interaction, thereby adding validity to research findings. 97
Methods
Findings from the qualitative study were explored for inter-method convergence, discrepancy or complementary information. 97 The below methods of triangulation were employed.
-
Methodological: results from the two data collection methods were compared (i.e. interviews and checklists).
-
Data source: a range of perspectives were represented, including participants, participating supporters, facilitators and supervisors.
-
Investigator: researchers and a PPI Advisory Group recruited from the University of Bradford Experts by Experience cohort were involved in the interpretation of the interview data.
Analysis
The triangulation protocol for analysis (Table 5) was based on that described by Farmer et al. 98
| Step | Activity |
|---|---|
| 1. Sorting | Sort findings from each data source, in this case the interviews and the observations/checklists |
| 2. Convergence coding | Identify key themes from each data source and compare findings |
| 3. Convergence agreement | Identify any full or partial agreement and any divergent or dissonant themes between the data sources |
| 4. Convergence assessment | Global assessment for level of agreement |
| 5. Completeness | How results of the global assessment enhance the completeness of the overall findings |
Chapter 3 Results
Internal pilot (stop/go assessment)
In May 2017, the study team reported to the funder that the trial had met the stop/go criteria for the internal pilot (see Chapter 2, Internal pilot trial for criteria).
-
As of 30 April 2017, the trial had recruited 127 participants across the six pilot sites.
-
At least two facilitators were identified for all six pilot sites at the start of active recruitment. Many of the sites had identified three or more facilitators and all sites had a supervisor.
-
None of the first six planned groups had fewer than four participants registered as of the end of May 2017.
The trial was allowed to progress to the full, definitive trial.
Recruitment
Participant recruitment commenced on 30 November 2016. The original recruitment period ran until 31 October 2017 and involved nine sites. However, because of slower than expected recruitment rates and smaller groups running than planned, the recruitment period was extended. Existing sites ran additional groups and four new recruiting sites were added. Recruitment continued until August 2018, when recruitment targets were met (see Appendix 4, Figure 13). The follow-up period on the trial ran from October 2017 to May 2019.
Participant flow
Figure 1 shows the CONSORT flow of participants through the trial. In total, 519 participants consented to be part of the study and 39 dropped out prior to randomisation. Four hundred and eighty participants were randomised, of whom 388 (81%) had primary outcome data at the 8-month follow-up. The number of participants randomised was just below our target of 488 participants; however, retention was better than accounted for in the sample size, and so the target number of participants with evaluable primary outcome data was met (i.e. 388 participants). Table 6 displays withdrawal reasons for the 87 participants who withdrew post randomisation. Most (79%) incidences of withdrawal were participant choice. Figure 2 shows the flow of participating supporters in the trial. Three hundred and fifty of the 480 randomised participants had a participating supporter and 285 of the participating supporters contributed follow-up data at 8 months.
FIGURE 1.
Participant flow for the JtD trial. a, Two participants missed the 8-month follow-up but completed the DEMQOL at 12-month follow-up. b, Three participants missed the 8-month follow-up but completed the DEMQOL at 12-month follow-up. DnA, did not attend; Ltf, lost to follow-up.
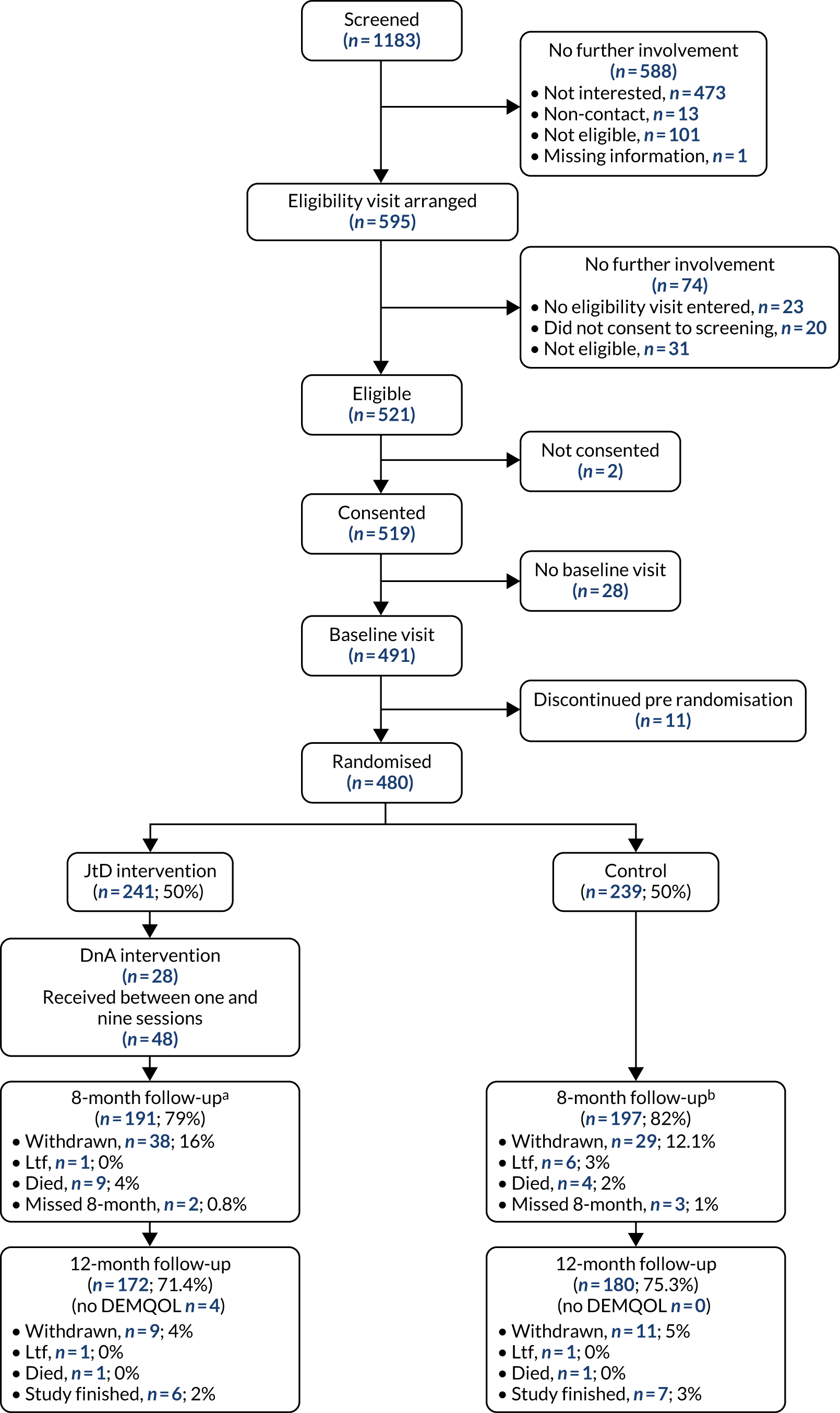
| Reasons for post-randomisation withdrawal | Intervention, n | Control, n | Total, n (%) |
|---|---|---|---|
| Decision by clinician/study team | 3 | 5 | 8 (9) |
| Consultee/carer choice | 3 | 7 | 10 (11) |
| Participant choice | 39 | 30 | 69 (79) |
| Participant’s reason (multiple reasons per participant) | |||
| Not stated | 3 | 5 | 8 |
| Preference for intervention | 1 | 1 | 2 |
| Preference for control | 1 | 0 | 1 |
| Too time-consuming/too much effort | 7 | 4 | 11 |
| No longer interested | 17 | 14 | 31 |
| No longer able | 7 | 3 | 10 |
| Non-dementia physical/psychological health problem | 1 | 2 | 3 |
| Other reason | 2 | 2 | 4 |
FIGURE 2.
Flow diagram of participating supporters of the randomised participants.

Baseline characteristics
Participant baseline characteristics
The characteristics of the randomised participants are given in Tables 7 and 8, and are well balanced between the randomised groups. Thirteen sites were recruited to the study and contributed between 12 and 66 participants per site. There were slightly more male participants (58%) and participants were generally white British (95%). The average age of participants was 77 years and 26% of the participants lived alone. Alzheimer’s contributed 60% of the dementia diagnoses and the median length of time since dementia diagnosis was 0.7 years. The baseline MMSE and patient-reported outcome measures are given in Table 9. The participant-reported outcomes were well matched across the randomised groups; the majority of participants (80%) rated their quality of life overall as either good or very good on the DEMQOL measure. See Appendix 8, Tables 37 and 38 for categorical and continuous baseline characteristics by intervention group and missing data status.
| Characteristic | Intervention (N = 241) | Control (N = 239) | Total (N = 480) |
|---|---|---|---|
| Site ID, n (%) | |||
| S01 | 23 (10) | 24 (10) | 47 (10) |
| S02 | 21 (9) | 20 (8) | 41 (9) |
| S04 | 14 (6) | 15 (6) | 29 (6) |
| S05 | 33 (14) | 33 (14) | 66 (14) |
| S06 | 19 (8) | 19 (8) | 38 (8) |
| S07 | 23 (10) | 23 (10) | 46 (10) |
| S08 | 20 (8) | 19 (8) | 39 (8) |
| S09 | 18 (7) | 17 (7) | 35 (7) |
| S10 | 32 (13) | 32 (13) | 64 (13) |
| S11 | 11 (5) | 10 (4) | 21 (4) |
| S12 | 10 (4) | 10 (4) | 20 (4) |
| S13 | 6 (2) | 6 (3) | 12 (3) |
| S15 | 11 (5) | 11 (5) | 22 (5) |
| Gender, n (%) | |||
| Male | 136 (56) | 143 (60) | 279 (58) |
| Female | 105 (44) | 96 (40) | 201 (42) |
| Ethnicity, n (%) | |||
| English/Welsh/Scottish/Northern Irish/British | 228 (95) | 230 (96) | 458 (95) |
| Irish | 5 (2) | 2 (1) | 7 (1) |
| Any other white background | 5 (2) | 0 (0) | 5 (1) |
| Indian | 0 (0) | 3 (1) | 3 (1) |
| Any other Asian background | 1 (0) | 0 (0) | 1 (0) |
| Any other mixed/multiple ethnic background | 0 (0) | 2 (1) | 2 (0) |
| Caribbean | 1 (0) | 1 (0) | 2 (0) |
| Any other ethnic group | 0 (0) | 1 (0) | 1 (0) |
| Prefer not to say | 1 (0) | 0 (0) | 1 (0) |
| Lives with others? n (%)a | |||
| No | 62 (26) | 63 (26) | 125 (26) |
| Yes | 178 (74) | 176 (74) | 354 (74) |
| Lives with, n (%) | |||
| Spouse/partner | 156 (65) | 157 (66) | 313 (65) |
| Child/children | 15 (6) | 6 (3) | 21 (4) |
| Both partner and children | 5 (2) | 10 (4) | 15 (3) |
| Other | 2 (1) | 3 (1) | 5 (1) |
| Accommodation type, n (%) | |||
| Sheltered or retirement housing | 27 (11) | 16 (7) | 43 (9) |
| Own home | 207 (86) | 218 (91) | 425 (89) |
| Friend’s/relative’s home | 7 (3) | 3 (1) | 10 (2) |
| Other | 0 (0) | 2 (1) | 2 (0) |
| Age (years) | |||
| n (%) | 241 (100) | 239 (100) | 480 (100) |
| Mean (SD) | 77 (7.0) | 77 (7.7) | 77 (7.3) |
| Median (IQR) | 78 (73–82) | 78 (72–82) | 78 (73–82) |
| Minimum, maximum | 56, 93 | 39, 91 | 39, 93 |
| Characteristic | Intervention (N = 241) | Control (N = 239) | Total (N = 480) |
|---|---|---|---|
| Type of dementia diagnosed, n (%) | |||
| Alzheimer’s | 142 (59) | 148 (62) | 290 (60) |
| Vascular dementia | 31 (13) | 19 (8) | 50 (10) |
| Mixed Alzheimer’s/vascular dementia | 51 (21) | 58 (24) | 109 (23) |
| Dementia in Parkinson’s disease | 3 (1) | 3 (1) | 6 (1) |
| Frontotemporal dementia | 5 (2) | 2 (1) | 7 (1) |
| Lewy body dementia | 1 (0) | 3 (1) | 4 (1) |
| Unspecified dementia | 7 (3) | 5 (2) | 12 (3) |
| Other | 1 (0) | 1 (0) | 2 (0) |
| Length of time since dementia diagnosis (years) | |||
| n (%) | 241 (100) | 238 (100) | 479 (100) |
| Mean (SD) | 1.3 (1.5) | 1.3 (1.7) | 1.3 (1.6) |
| Median (IQR) | 0.7 (0.3–1.8) | 0.8 (0.3–1.8) | 0.7 (0.3–1.8) |
| Minimum, maximum | 0.0, 7.9 | 0.0, 13.0 | 0.0, 13.0 |
| Medical history | |||
| Stroke | 33 (13.7) | 37 (15.5) | 70 (14.6) |
| Diabetes | 40 (16.6) | 41 (17.2) | 81 (16.9) |
| Heart or chest problems | 75 (31.1) | 74 (31.0) | 149 (31.0) |
| Arthritis/mobility problems | 102 (42.3) | 105 (43.9) | 207 (43.1) |
| Falls/dizziness/blackouts | 61 (25.3) | 58 (24.3) | 119 (24.8) |
| Anxiety/depression | 43 (17.8) | 49 (20.5) | 92 (19.2) |
| High blood pressure | 82 (34.0) | 72 (30.1) | 154 (32.1) |
| Other | 91 (37.8) | 117 (49.0) | 208 (43.3) |
| Participant outcome measure | Intervention (N = 241) | Control (N = 239) | Total (N = 480) |
|---|---|---|---|
| MMSE (total score) | |||
| Mean (SD) | 24.5 (3.1) | 24.6 (3.2) | 24.6 (3.1) |
| n (%) | 241 (100) | 239 (100) | 480 (100) |
| MMSE cognitive impairment | |||
| Mild | 92 (38) | 87 (36) | 179 (37) |
| Normal cognition | 149 (62) | 152 (64) | 301 (63) |
| DEMQOL (total score) | |||
| Mean (SD) | 90.8 (13.0) | 90.3 (13.2) | 90.6 (13.1) |
| n (%) | 241 (100) | 239 (100) | 480 (100) |
| DEMQOL quality of life overall (question 29), n (%) | |||
| Poor | 5 (2) | 5 (2) | 10 (2) |
| Fair | 43 (18) | 45 (19) | 88 (18) |
| Good | 123 (51) | 109 (46) | 232 (48) |
| Very good | 70 (29) | 80 (33) | 150 (31) |
| PHQ-9 (total score) | |||
| Mean (SD) | 4.2 (4.4) | 4.0 (4.4) | 4.1 (4.4) |
| n (%) | 241 (100) | 239 (100) | 480 (100) |
| PHQ-9 depression severity, n (%) | |||
| None (0–4) | 156 (65) | 152 (64) | 308 (64) |
| Mild (5–9) | 52 (22) | 58 (24) | 110 (23) |
| Moderate (10–14) | 25 (10) | 23 (10) | 48 (10) |
| Moderately severe (15–19) | 6 (2) | 3 (1) | 9 (2) |
| Severe (20–27) | 2 (1) | 3 (1) | 5 (1) |
| GAD-7 (total score) | |||
| Mean (SD) | 2.8 (3.6) | 2.8 (3.5) | 2.8 (3.5) |
| n (%) | 241 (100) | 238 (100) | 479 (100) |
| GAD-7 anxiety severity, n (%) | |||
| No anxiety (0–4) | 192 (80) | 185 (77) | 377 (79) |
| Mild anxiety (5–9) | 38 (16) | 40 (17) | 78 (16) |
| Moderate anxiety (10–14) | 5 (2) | 11 (5) | 16 (3) |
| Severe anxiety (15–21) | 6 (2) | 2 (1) | 8 (2) |
| EQ-5D-5L (crosswalk value index) | |||
| Mean (SD) | 0.77 (0.21) | 0.78 (0.19) | 0.77 (0.20) |
| n (%) | 241 (100) | 239 (100) | 480 (100) |
| EQ-5D VAS | |||
| Mean (SD) | 75.6 (16.7) | 73.8 (17.8) | 74.7 (17.3) |
| n (%) | 241 (100) | 238 (100) | 479 (100) |
| GSE (total score) | |||
| Mean (SD) | 30.4 (5.5) | 30.9 (5.4) | 30.6 (5.5) |
| n (%) | 239 (99) | 238 (100) | 477 (99) |
| DFS | |||
| Mean (SD) | 45.3 (6.7) | 45.6 (7.2) | 45.5 (6.9) |
| n (%) | 240 (100) | 233 (97) | 473 (99) |
| SMAS | |||
| Mean (SD) | 124.6 (20.7) | 125.6 (19.5) | 125.1 (20.1) |
| n (%) | 235 (98) | 236 (99) | 471 (98) |
| IADL (total score) | |||
| Mean (SD) | 5.7 (1.8) | 5.8 (1.9) | 5.7 (1.8) |
| n (%) | 237 (98) | 234 (98) | 471 (98) |
Participating supporter baseline characteristics
Table 10 gives the baseline characteristics for the participating supporters. The majority of participating supporters were female (74%) and were the spouse/partner of the participant (79%).
| Characteristic | Intervention (N = 177) | Control (N = 173) | Total (N = 350) |
|---|---|---|---|
| Age (years) | |||
| n (%) | 171 (97) | 166 (96) | 337 (96) |
| Mean (SD) | 70 (11) | 68 (12) | 69 (11) |
| Median (IQR) | 73 (65–77) | 70 (60–76) | 71 (62–76) |
| Minimum, maximum | 37, 89 | 26, 88 | 26, 89 |
| 52 (29%) | 39 (23%) | 91 (26%) | |
| Gender, n (%) | |||
| Male | 125 (71) | 134 (77) | 259 (74%) |
| Female | 52 (29) | 39 (23) | 91 (26) |
| Relationship to person with dementia, n (%) | |||
| Spouse/partner | 140 (79) | 136 (79) | 276 (79) |
| Child | 31 (18) | 32 (18) | 63 (18) |
| Sibling | 1 (1) | 1 (1) | 2 (1) |
| Other family member | 3 (2) | 2 (1) | 5 (1) |
| Friend | 0 (0) | 2 (1) | 2 (1) |
| Neighbour | 1 (1) | 0 (0) | 1 (0) |
| Other | 1 (1) | 0 (0) | 1 (0) |
| Length of time caring for person with dementia, n (%)a | |||
| 0–6 months | 35 (20) | 24 (14) | 59 (17) |
| 7–12 months | 19 (11) | 12 (7) | 31 (9) |
| 13–18 months | 18 (10) | 20 (12) | 38 (11) |
| 19–24 months | 10 (6) | 19 (11) | 29 (8) |
| > 2 years | 92 (52) | 97 (56) | 189 (54) |
| Lives with person with dementia? n (%) | |||
| No | 26 (15) | 31 (18) | 57 (16) |
| Yes | 151 (85) | 142 (82) | 293 (84) |
| EQ-5D-5L (crosswalk value index) | |||
| Mean (SD) | 0.81 (0.18) | 0.82 (0.18) | 0.81 (0.18) |
| n (%) | 168 (95) | 168 (97) | 336 (96) |
| EQ-5D VAS | |||
| Mean (SD) | 77.8 (18.9) | 79.7 (16.2) | 78.8 (17.6) |
| n (%) | 169 (95) | 168 (97) | 337 (96) |
| SCQ (total score) | |||
| Mean (SD) | 100.3 (16.7) | 102.5 (16.3) | 101.4 (16.6) |
| n (%) | 159 (90) | 160 (92) | 319 (91) |
| PHQ-9 (total score) | |||
| Mean (SD) | 4.1 (4.2) | 3.9 (4.4) | 4.0 (4.3) |
| n (%) | 167 (94) | 168 (97) | 335 (96) |
Journeying through Dementia intervention
Table 11 shows a summary of the JtD intervention. There were 28 intervention groups across the 13 sites. See Chapter 1, Modifications to facilitator identification and training, intervention delivery and supervision for an overview of facilitator characteristics and grades, and registered numbers of participants by site. The median session size was five participants [interquartile range (IQR) 4–6]. Of the 241 participants randomised to receive the JtD intervention, 165 attended at least 10 of the 16 available sessions (defined in the protocol as the per-protocol treatment threshold), three participants randomised to the control group received the JtD intervention and all participants attended at least 10 sessions. The median number of group sessions attended (out of a possible 12) was 10 (IQR 1–11) and 153 out of 244 participants attended all four individual meetings. Figure 3 shows the group size (number of participants) across each of the group sessions. Attendance at the first session was mostly between four and seven participants, but was as small as two participants for some groups. The groups’ sizes were broadly consistent over the 12 weeks of sessions. Of those who attended at least one group session, but < 12, the majority (n = 130) had intermittent session non-attendance and a minority (n = 23) had monotone session non-attendance (i.e. having missed a session, the participant did not attend future sessions).
| Intervention | Summary | |
|---|---|---|
| Number of JtD intervention groups | 28 | |
| Session size | ||
| n (total number of possible sessions) | 336 | |
| Mean (SD) | 5.3 (1.8) | |
| Median (IQR) | 5 (4–6) | |
| Minimum, maximum | 1, 9 | |
| Meetings attended (out of a possible 16) | ||
| n | 244 | |
| Mean (SD) | 10 (6) | |
| Median (IQR) | 13 (3–15) | |
| Minimum, maximum | 0, 16 | |
| Did not attend intervention, n (%) | 28 (11) | |
| Attended between one and nine sessions, n (%) | 48 (20) | |
| Attended between 10 and 16 sessions (per protocol), n (%) | 168 (69) | |
| Group meetings attended (out of a possible 12) | ||
| n | 244 | |
| Mean (SD) | 7.3 (4.6) | |
| Median (IQR) | 9 (1–11) | |
| Minimum, maximum | 0, 12 | |
| Individual meetings attended, n (%) | ||
| Zero | 28 (11) | |
| One | 33 (14) | |
| Two | 12 (5) | |
| Three | 18 (7) | |
| Four | 153 (63) | |
| Meetings accompanied, n (%)a | Group | Individual |
| Zero | 102 (42) | 75 (31) |
| One | 47 (19) | 42 (17) |
| Two | 45 (18) | 38 (16) |
| Three | 50 (20) | 30 (12) |
| Four | n/a | 59 (24) |
| Accompanied by, n (%)b | ||
| Participating supporter | 523 (78) | |
| Non-participating supporter | 32 (5) | |
| Consultee | 9 (1) | |
| Other | 108 (16) | |
FIGURE 3.
Box plot showing number of participants attending a session by session number.
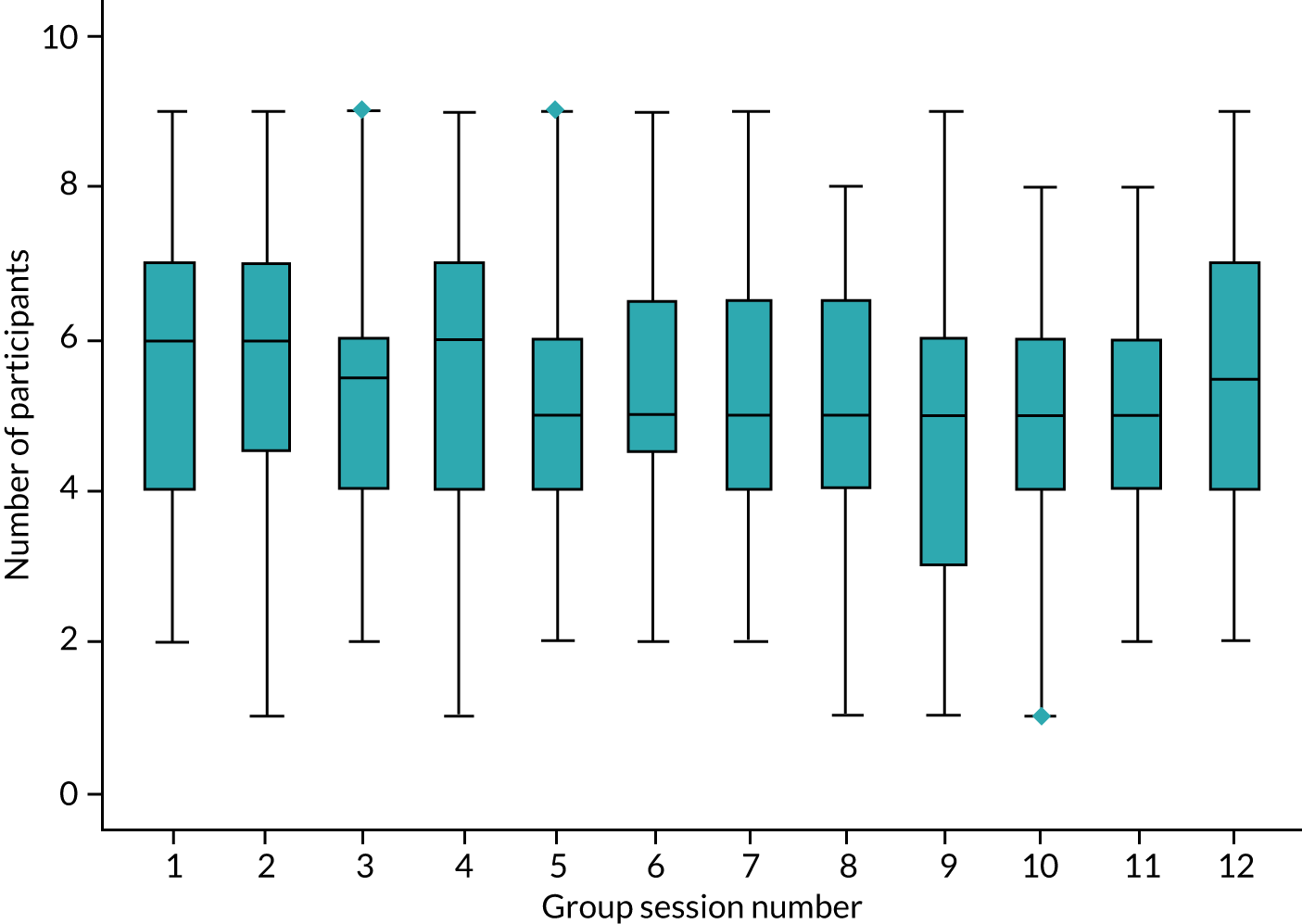
Of the three group sessions that could be accompanied, 50 participants (20%) were accompanied for all three group sessions and 59 participants (24%) were accompanied at all four individual sessions. The accompanying person was most likely to be the participating supporter (78%).
Table 12 displays the reason for session non-attendance and intervention withdrawal. A small number of participants were removed from the intervention owing to investigator decision (n = 3) or consultee choice (n = 2); however, the majority of intervention withdrawals were because of participant choice or the participant being unable to attend the intervention. Travel limitations, finding the group too time-consuming and not finding the group useful were given as reasons for a participant choosing to withdraw from the intervention.
| Intervention non-attendance and withdrawal | n (%) |
|---|---|
| Reason not attended intervention session | |
| Illness | 122 (10) |
| Transport problems | 15 (1) |
| Too busy | 53 (4) |
| Holiday | 92 (8) |
| Deceased | 11 (1) |
| Withdrawn from intervention | 702 (59) |
| Supporter unavailable | 17 (1) |
| Other | 117 (10) |
| Unknown | 69 (6) |
| Reason for intervention withdrawal | |
| Participant choicea | 38 (45) |
| Unable to attend interventionb | 20 (24) |
| Investigator decision | 3 (4) |
| Consultee choice | 2 (2) |
| Other | 2 (2) |
| Not given | 1 (1) |
Table 13 shows intervention attendance across various demographics and socioeconomic status. Intervention ‘attenders’ (i.e. those attending at least 10 of a possible 16 sessions) were more likely to be male (61% vs. 48% in the ‘non-attenders’) and have a participating supporter (80% vs. 58% in the ‘non-attenders’). Participants’ age, type and length of dementia diagnosis and socioeconomic status (based on Index of Multiple Deprivation decile) were similar for those who attended at least 10 sessions and those who attended < 10 sessions in the intervention arm.
| Characteristic | Intervention attendance | Total (N = 241) | |
|---|---|---|---|
| At least 10 sessions (N = 165) | < 10 sessions (N = 76) | ||
| Gender, n (%) | |||
| Male | 100 (61) | 36 (47) | 136 (56) |
| Female | 65 (39) | 40 (53) | 105 (44) |
| Participating supporter, n (%) | |||
| No | 32 (19) | 32 (42) | 64 (27) |
| Yes | 133 (81) | 44 (58) | 177 (73) |
| Type of dementia diagnosed, n (%) | |||
| Alzheimer’s | 101 (61) | 41 (54) | 142 (59) |
| Vascular dementia | 19 (12) | 12 (16) | 31 (13) |
| Mixed Alzheimer’s/vascular dementia | 32 (19) | 19 (25) | 51 (21) |
| Dementia in Parkinson’s disease | 3 (2) | 0 (0) | 3 (1) |
| Frontotemporal dementia | 4 (2) | 1 (1) | 5 (2) |
| Lewy body dementia | 1 (1) | 0 (0) | 1 (0) |
| Unspecified dementia | 5 (3) | 2 (3) | 7 (3) |
| Other | 0 (0) | 1 (1) | 1 (0) |
| Age (years) | |||
| n (%) | 165 (100) | 76 (100) | 241 (100) |
| Mean (SD) | 77.2 (6.9) | 78.2 (7.1) | 77.5 (7.0) |
| Median (IQR) | 78.0 (73.0–81.0) | 78.5 (73.5–83.0) | 78.0 (73.0–82.0) |
| Minimum, maximum | 56.0, 93.0 | 58.0, 91.0 | 56.0, 93.0 |
| Index of Multiple Deprivation Decile | |||
| n (%) | 164 (99) | 76 (100) | 240 (100) |
| Mean (SD) | 6.3 (2.9) | 5.9 (3.1) | 6.2 (3.0) |
| Median (IQR) | 7.0 (4.0–9.0) | 6.0 (3.0–9.0) | 7.0 (4.0–9.0) |
| Minimum, maximum | 1.0, 10.0 | 1.0, 10.0 | 1.0, 10.0 |
| Length of time since dementia diagnosis (years) | |||
| n (%) | 165 (100) | 76 (100) | 241 (100) |
| Mean (SD) | 1.3 (1.4) | 1.3 (1.5) | 1.3 (1.5) |
| Median (IQR) | 0.8 (0.3–1.8) | 0.6 (0.2–1.9) | 0.7 (0.3–1.8) |
| Minimum, maximum | 0.0, 7.7 | 0.0, 7.9 | 0.0, 7.9 |
Primary outcome analysis
Among the participants completing 8-month follow-up, DEMQOL score was very similar across the treatment groups (Table 14). The mean DEMQOL score at 8 months was 93.3 and 91.9 in the treatment and control groups, respectively (adjusted mean difference 0.9, 95% CI –1.2 to 3.0; p = 0.380). The 95% CI for the difference was wholly below the target difference defined as clinically meaningful specified in the sample size (i.e. 4 points). Figures 4 and 5 show the unadjusted mean DEMQOL score by time point and group. The difference between the treatments at 12 months was smaller than that at 8 months.
| Outcome | Treatment group | Adjusteda mean difference | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| DEMQOL score | 191 | 93.3 (13.0) | 197 | 91.9 (14.6) | 0.9 | –1.2 to 3.0 | 0.380 |
FIGURE 4.
Mean DEMQOL score for ITT participants over time by randomised treatment group with truncated vertical scale.
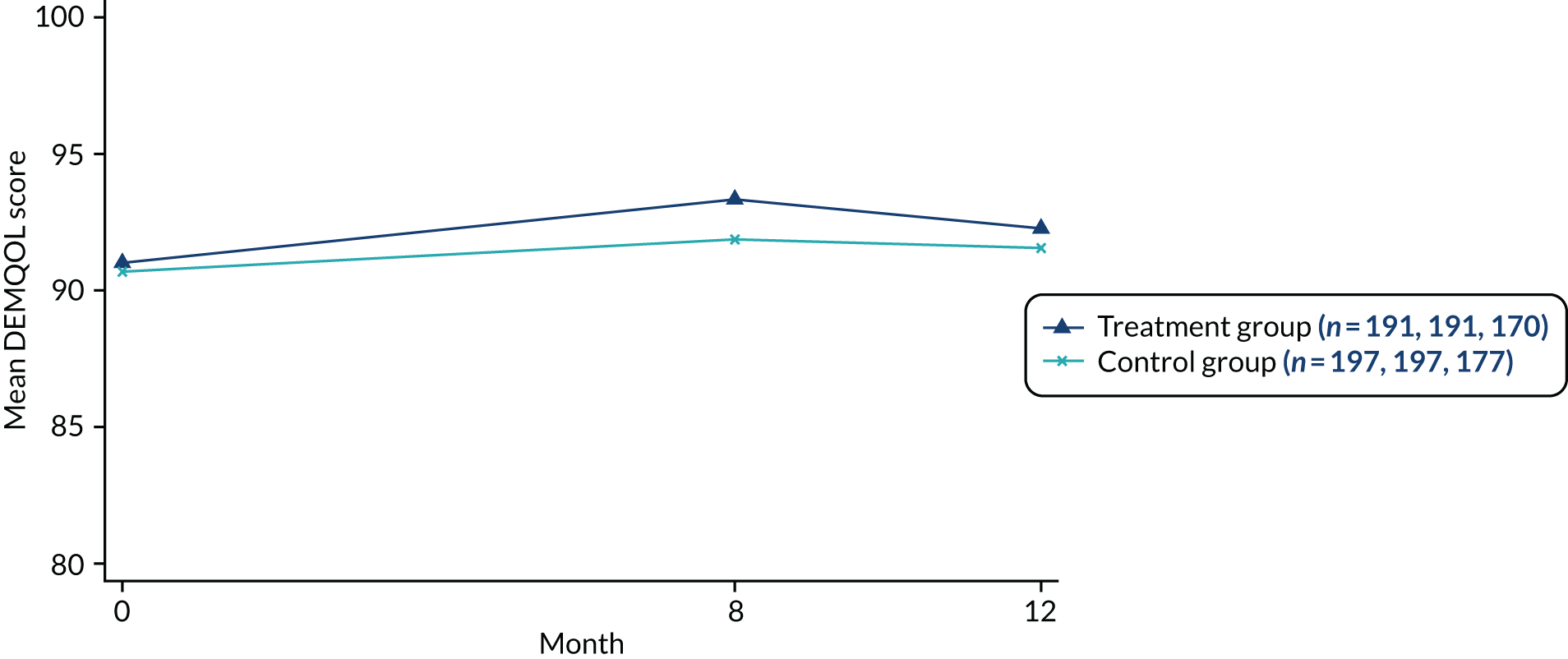
FIGURE 5.
Mean DEMQOL score for ITT participants over time by randomised treatment group with expanded vertical scale.
Sensitivity analysis on primary outcome
A number of sensitivity analyses were undertaken on 8-month DEMQOL score with regard to alternative missing data assumptions, removing mistimed measurements and treatment adherence (Table 15 and Figure 6). In all cases, the treatment difference remained similar, partially because dropout was low and adherence was high, and so the populations included in the sensitivity analyses differ only slightly to those included in the primary analysis. CACE analysis (i.e. a measure of the causal effect of the intervention on the people who received it) estimated the treatment difference as slightly larger than the ITT primary analysis (1.5 points), but the 95% CI for the treatment difference was still below 4 points (95% CI –0.6 to 3.7; p = 0.158).
| Outcome: DEMQOL score at 8 months | Treatment group | Adjusteda mean difference | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| Observed data (primary analysis) | 191 | 93.3 (13.0) | 197 | 91.9 (14.6) | 0.9 | –1.2 to 3.0 | 0.380 |
| Removing mistimed measurementsb | 182 | 93.3 (13.1) | 188 | 92.0 (14.5) | 1 | –1.1 to 3.1 | 0.373 |
| Simple regression imputationc | 235 | 93.3 (12.3) | 236 | 91.8 (13.9) | 0.8 | –0.9 to 2.6 | 0.330 |
| Multiple imputationd | 235 | 93.3 (13.0) | 236 | 91.8 (14.6) | 0.8 | –1.3 to 2.9 | 0.467 |
| Per protocole | 155 | 93.7 (13.2) | 197 | 91.9 (14.6) | 1.2 | –0.9 to 3.3 | 0.255 |
| CACEf | 1.5 | –0.6 to 3.7 | 0.158 | ||||
FIGURE 6.
Forest plot of mean difference in 8-month post-randomisation DEMQOL score for the sensitivity analysis samples, with the target difference of 4 points.
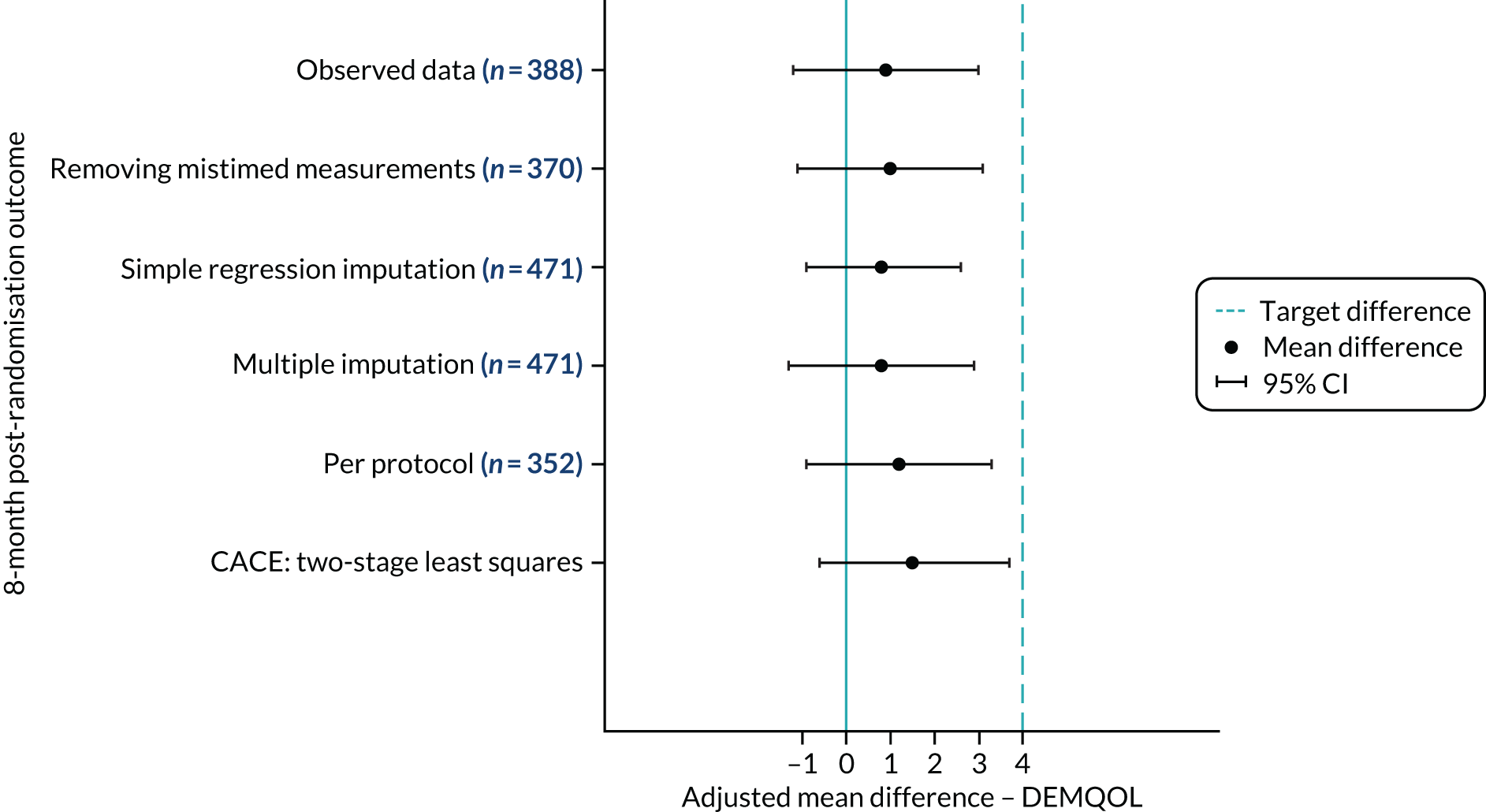
Relationship between group attendance and primary outcome
The relationship between number of JtD sessions attended and DEMQOL change in score from baseline at 8 months for the intervention group is shown in Figure 7. There is considerable variation in DEMQOL change in score across each level of session attendance and there is no clear association between total session attendance and 8-month DEMQOL change in score.
FIGURE 7.
Change from baseline in 8-month DEMQOL score by number of JtD intervention group sessions attended (n = 191).
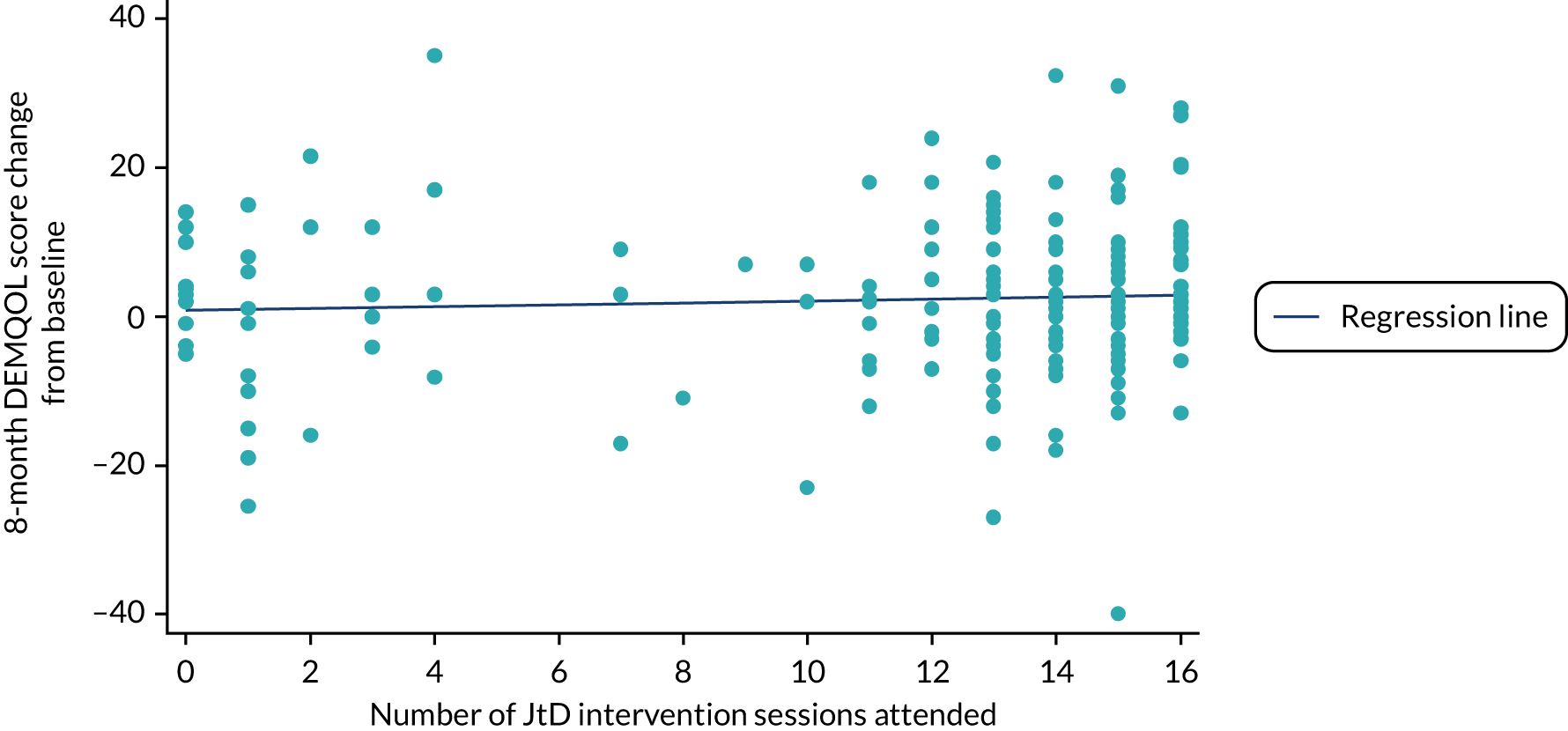
Secondary outcomes analysis
Participant secondary outcomes
Comparisons of secondary participant-reported outcomes at 8 months are presented in Table 16. Overall, the treatment differences were in favour of the intervention group (excluding GAD-7), but the differences were small (i.e. unlikely to be clinically significant) and only the difference in DFS was statistically significant. There were no differences in secondary outcomes measured at 12 months (Table 17).
| Outcome | Treatment group | Adjusteda mean difference | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| PHQ-9 (total score) | 186 | 3.4 (4.2) | 193 | 3.6 (4.8) | –0.3 | –1.1 to 0.5 | 0.412 |
| GAD-7 (total score) | 185 | 2.4 (3.5) | 192 | 2.4 (3.8) | 0.1 | –0.5 to 0.7 | 0.757 |
| EQ-5D-5L (crosswalk value index) | 190 | 0.78 (0.21) | 195 | 0.78 (0.22) | 0.01 | –0.03 to 0.05 | 0.672 |
| EQ-5D VAS | 188 | 74.6 (18.3) | 193 | 72.1 (18.0) | 2.1 | –1.7 to 5.9 | 0.284 |
| GSE (total score) | 178 | 30.1 (5.5) | 185 | 29.5 (5.8) | 0.9 | –0.1 to 1.9 | 0.066 |
| DFS | 169 | 46.0 (6.3) | 177 | 45.1 (7.1) | 1.2 | 0.1 to 2.3 | 0.028 |
| SMAS | 171 | 124.8 (20.2) | 176 | 123.7 (18.1) | 1.5 | –2.3 to 5.3 | 0.446 |
| IADL (total score) | 181 | 5.2 (1.8) | 190 | 5.2 (1.9) | 0.1 | –0.3 to 0.4 | 0.747 |
| Outcome | Treatment group | Adjusteda mean difference | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| DEMQOL (total score) | 172 | 92.3 (14.3) | 180 | 91.7 (13.9) | 0.4 | –1.6 to 2.5 | 0.687 |
| EQ-5D-5L (crosswalk value index) | 170 | 0.79 (0.22) | 178 | 0.78 (0.22) | 0.02 | –0.02 to 0.06 | 0.306 |
| EQ-5D VAS | 173 | 70.8 (19.1) | 177 | 70.9 (19.1) | –0.4 | –4.3 to 3.6 | 0.857 |
Participating supporter secondary outcomes
The comparison of 8-month outcome measures for the participating supporters is presented in Table 18. Mean differences were small and not statistically significant, with the exception of EQ-5D-5L, for which the participating supporters in the control group reported better health (adjusted mean difference –0.06, 95% CI –0.09 to –0.0.2; p = 0.002). This difference was not borne out in the EuroQol-5 Dimensions (EQ-5D) VAS, which showed no difference between the intervention and control groups (mean difference 0.1, 95% CI –2.8 to 3.1).
| Outcome | Treatment group | Adjusteda mean difference | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| n | Mean (SD) | n | Mean (SD) | ||||
| EQ-5D-5L (crosswalk value index) | 138 | 0.76 (0.22) | 135 | 0.80 (0.17) | –0.06 | –0.09 to –0.02 | 0.002 |
| EQ-5D VAS | 138 | 77.9 (15.5) | 136 | 77.7 (16.8) | 0.1 | –2.8 to 3.1 | 0.942 |
| SCQ (total score) | 128 | 97.8 (17.3) | 125 | 101.3 (16.6) | –1.4 | –4.3 to 1.5 | 0.344 |
| PHQ-9 (total score) | 139 | 4.7 (4.6) | 135 | 4.1 (4.6) | 0.4 | –0.4 to 1.2 | 0.347 |
Assessment of follow-up time in relation to outcome
A process of delayed baseline and randomisation was implemented on this study to ensure that the delay between baseline and a group starting was within 2 months. Hence, the time between baseline measure and a course starting varied across participants. Figure 8 shows the time between baseline and randomisation, which is distributed similarly across the treatment groups. Figure 9 shows the time between randomisation and a JtD intervention group starting. For participants in the intervention arm, the median number of days was 36 (IQR 20–56 days). The two participants who had a > 150-day wait between baseline and the group intervention starting had decided to delay starting a group to the next wave of the intervention at that site. Adjusting the primary outcome mixed-effects model for (1) the number of days between baseline and group starting and (2) the number of days between baseline and 8-month outcome had minimal impact of the estimation of the difference in DEMQOL score between the treatment groups.
FIGURE 8.
Days between baseline and randomisation for participants in the (a) intervention arm (n = 191); and (b) control arm (n = 197).
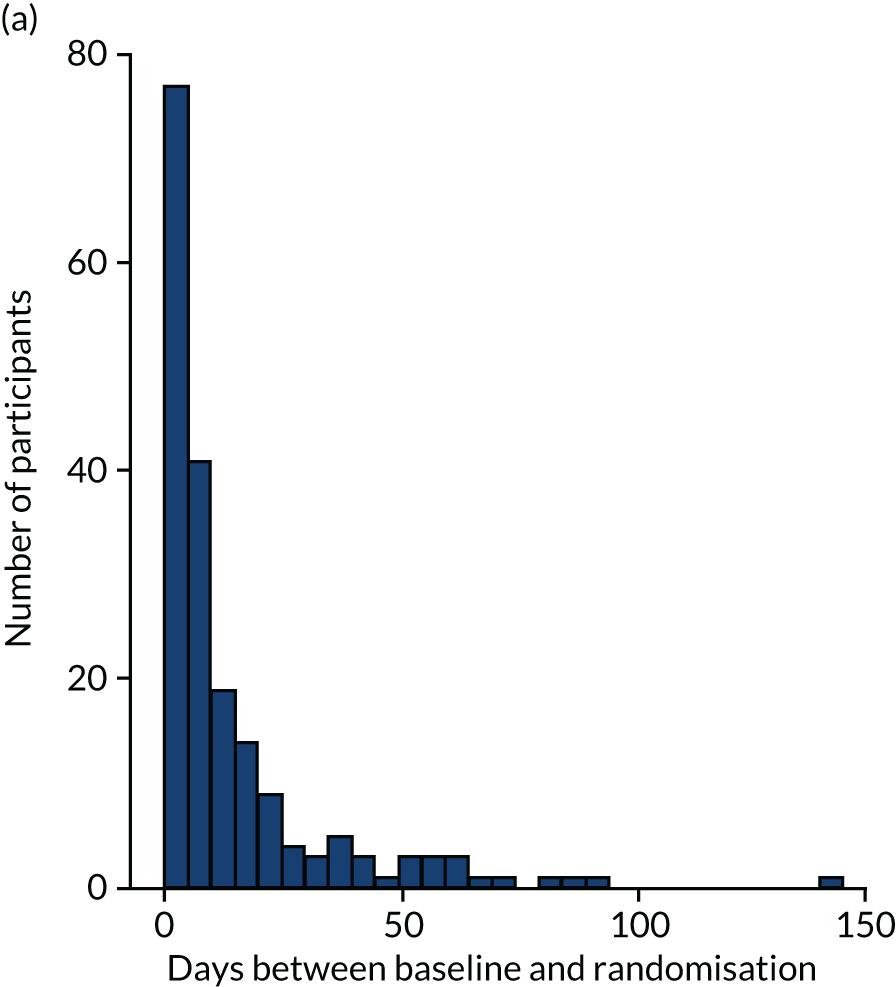
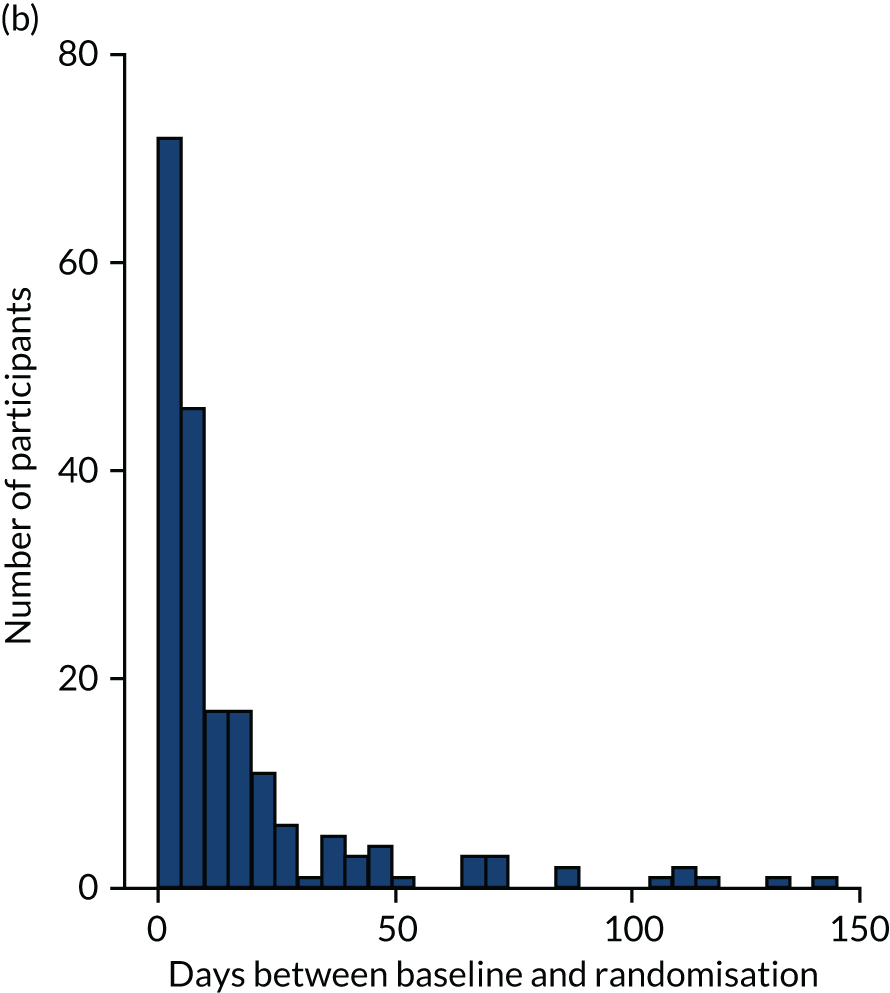
FIGURE 9.
Days between baseline and first group session for participants in the intervention arm (n = 191).
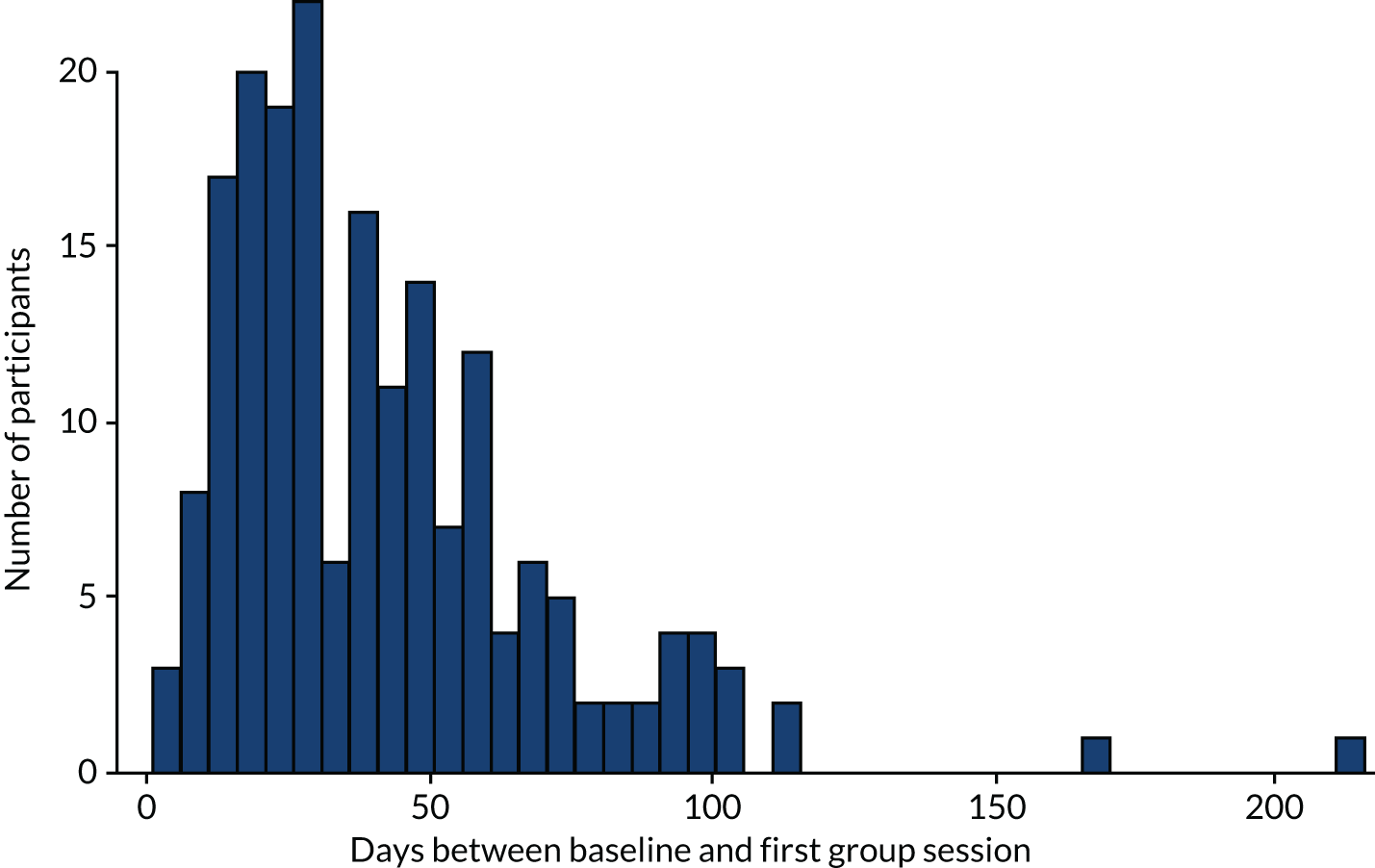
Subgroup analysis
The potential moderating effect of two predefined subgroups was explored by including an interaction between treatment and subgroup in the mixed-effects primary outcome regression model. Results of the subgroup analyses are presented in Table 19 and summarised in Figures 10 and 11. No reliable statistical evidence of subgroup effects or interactions were found between the treatment groups.
| Outcome | Treatment group | Mean difference | 95% CI | Interaction coefficienta | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||||
| n | Mean (SD) | n | Mean (SD) | ||||||
| Is there a participating supporter? | |||||||||
| Yes | 145 | 93.6 (13.2) | 139 | 92.8 (14.3) | 0.1 | –2.4 to 2.5 | |||
| No | 46 | 92.5 (12.4) | 58 | 89.7 (15.2) | 3.4 | –0.5 to 7.2 | 3.3b | –1.2 to 7.7 | 0.149 |
| Type of dementia | |||||||||
| Alzheimer’s | 117 | 94.1 (14.2) | 126 | 93.4 (14.0) | 0.8 | –1.8 to 3.4 | |||
| Any vascular dementia | 58 | 92.7 (9.9) | 62 | 91.1 (14.8) | 0.5 | –3.1 to 4.1 | |||
| Other | 16 | 90.1 (14.5) | 9 | 76.5 (13.8) | 6.9 | –1.2 to 15.1 | 0.341 | ||
| Type of dementia: sensitivity 1 | |||||||||
| Alzheimer’s | 117 | 94.1 (14.2) | 126 | 93.4 (14.0) | 0.8 | –1.8 to 3.4 | |||
| Vascular dementia | 20 | 91.3 (10.5) | 14 | 89.5 (11.4) | 2.8 | –3.9 to 9.5 | |||
| Mixed and other dementia | 54 | 92.5 (11.3) | 57 | 89.2 (16.3) | 1 | –2.8 to 4.8 | 0.855 | ||
| Type of dementia: sensitivity 2 | |||||||||
| Alzheimer’s and mixed dementia | 155 | 93.9 (13.2) | 174 | 92.9 (14.4) | 0.6 | –1.7 to 2.8 | |||
| Vascular dementia | 20 | 91.3 (10.5) | 14 | 89.5 (11.4) | 2.8 | –3.9 to 9.5 | |||
| Other dementia | 16 | 90.1 (14.5) | 9 | 76.5 (13.8) | 7 | –1.1 to 15.2 | 0.279 | ||
FIGURE 10.
Mean 8-month DEMQOL score by (a) subgroup; and (b) treatment group (n = 388).
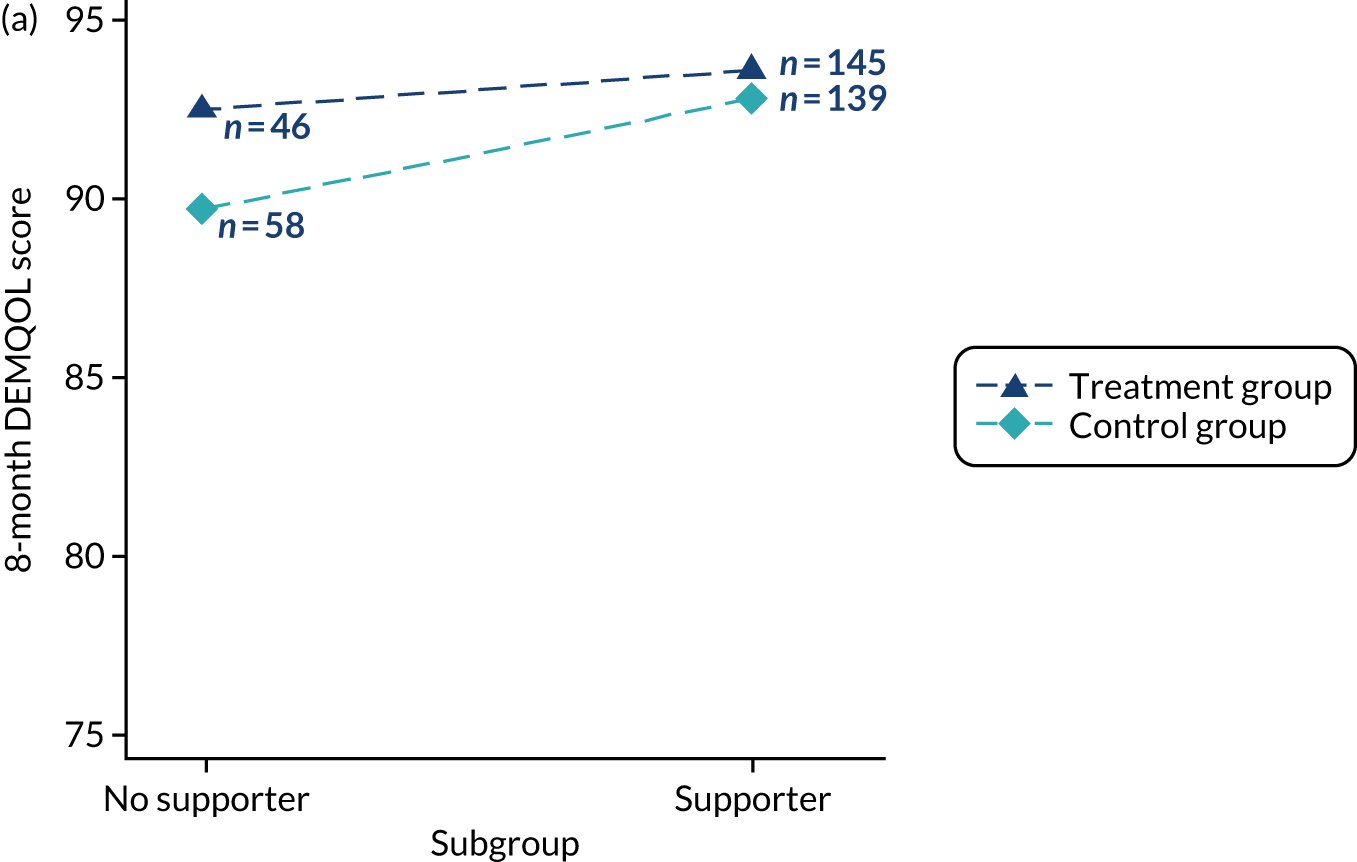
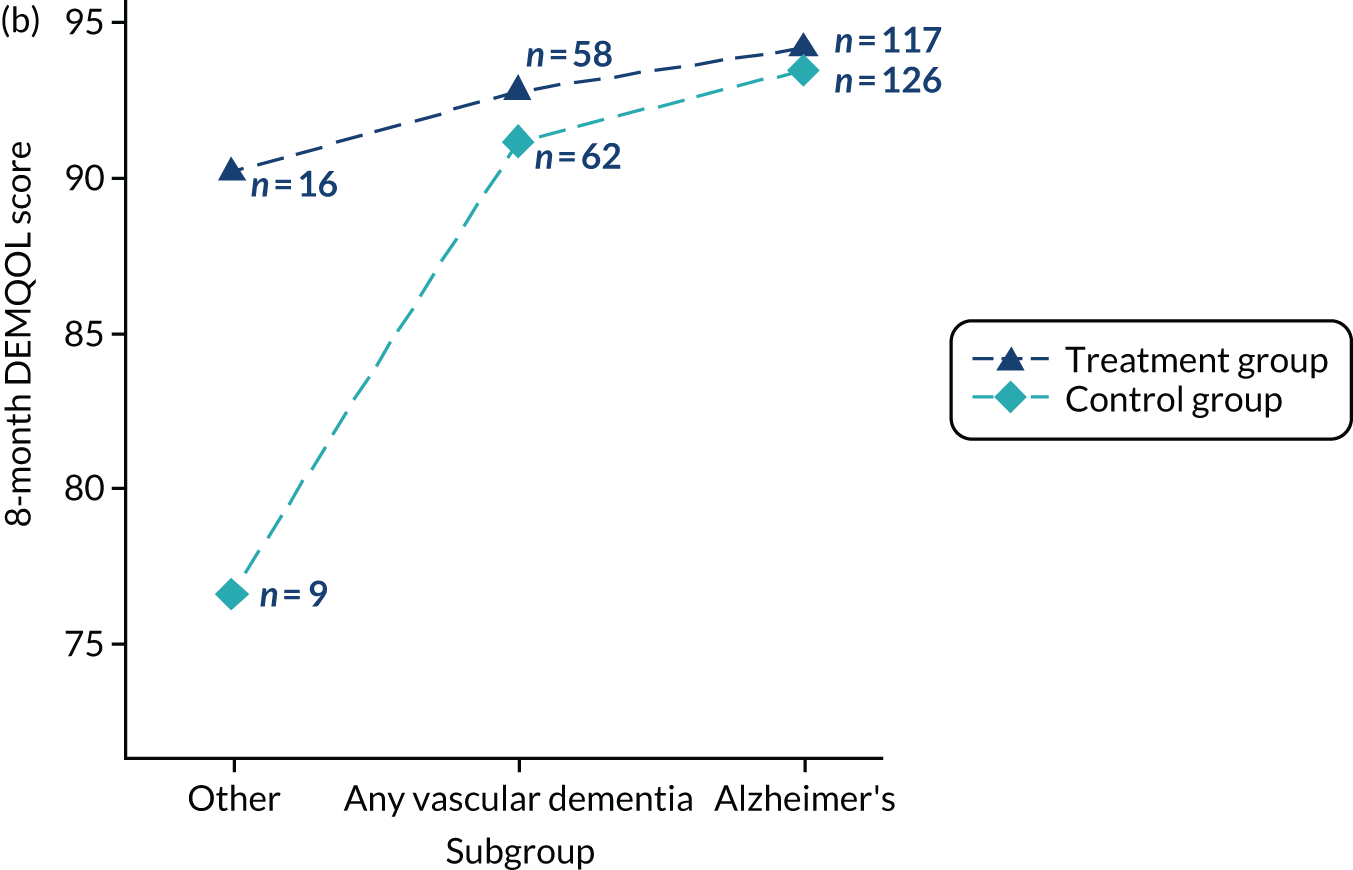
FIGURE 11.
Forest plot of mean differences in 8-month DEMQOL score for the treatment groups by subgroup (n = 388).
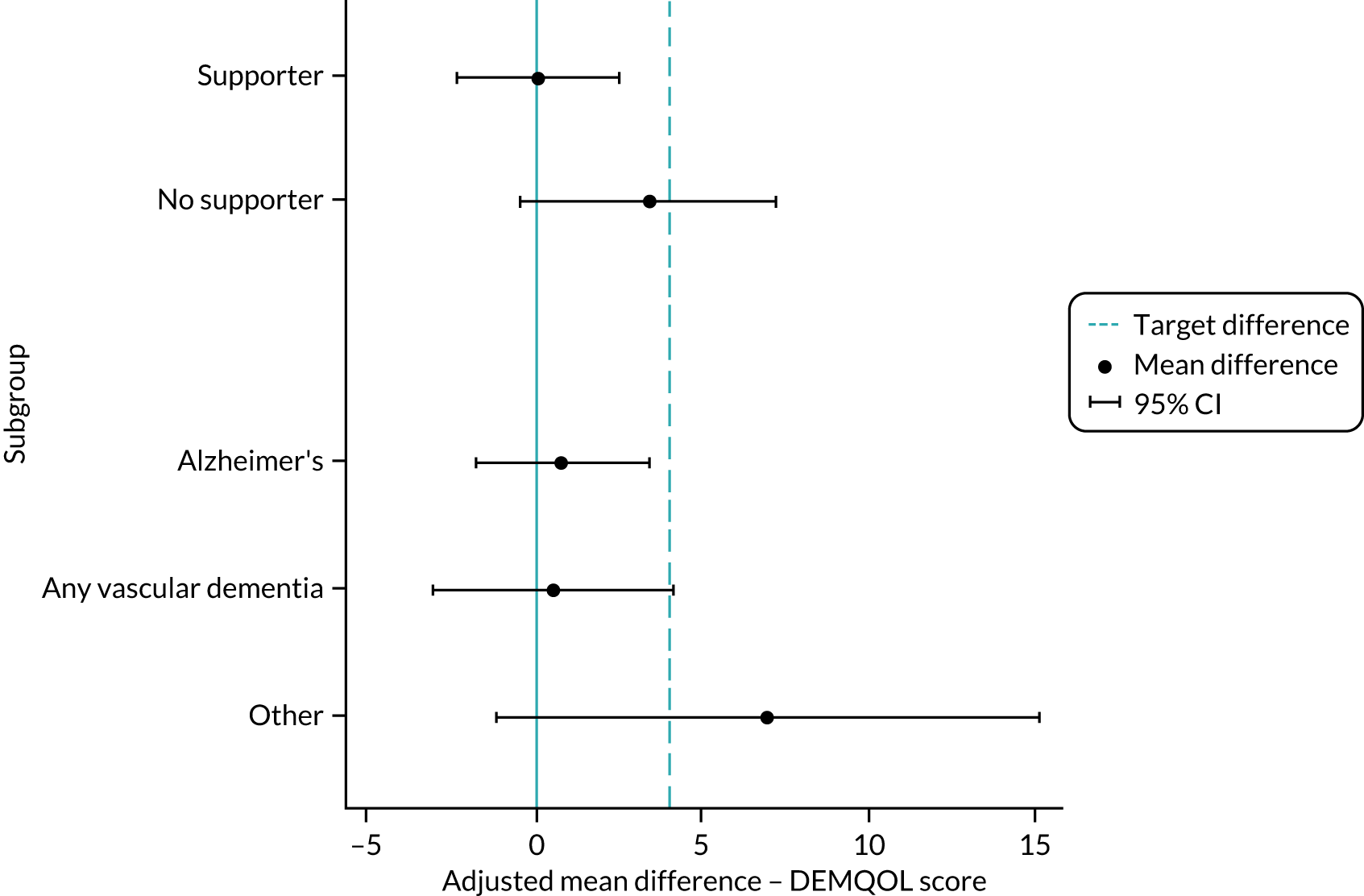
Fidelity study
The results presented below are for the four fidelity assessment sites.
Training fidelity
As per protocol, a number of formal 2-day face-to-face intervention training sessions were conducted during 2017, prior to sites opening for recruitment. Details of the training are provided in Chapter 1, Intervention: rationale, methods and delivery. The author of the manual led all of the sessions, accompanied by a second trainer with experience working with people with dementia and delivering similar group interventions. Three of these sessions were included in the fidelity assessment (Table 20). Fifty trainees attended. On average, the time from training to delivering the first group was 106 days (range 79–134 days).
| Date of training | Fidelity site(s) attended | Date of first group meeting | Trainers | Numbers of attendees by role | Researchers | |||
|---|---|---|---|---|---|---|---|---|
| Facilitators | Supervisors | Research assistants | Total | |||||
| 17 January 2017 | Site 4 | 6 April 2017 | CC/SB | 10 | 1 | 0 | 11 | KS/SM |
| 19 January 2017 | CC/SB | 10 | 1 | 0 | 11 | KS/SM | ||
| 23 January 2017 | Site 1 | 24 May 2017 | CC/SB | 21 | 6 | 4 | 31 | KS/SM |
| Site 2 | 24 April 2017 | |||||||
| Site 3 | 6 June 2017 | |||||||
| 24 January 2017a | CC/SB | 21 | 1 | 0 | 22 | KS/SM | ||
| 22 August 2017 | CC/GMb | 7 | 1 | 0 | 8 | KS/JBDc | ||
| 23 August 2017 | CC | 7 | 1 | 0 | 8 | KS/JBD | ||
Two researchers were present during each intervention training session, with coding shared between three members of the research team, one of whom was present at all three intervention training sessions (i.e. the fidelity lead). Fidelity scored by the researchers on the checklist items was high overall across the three sessions, averaging 95% achievement (range 91–97%) of checklist items. Attendees’ scores supported this, averaging 94% achievement (range 88–97%).
The second training session (on the 23 and 24 January 2017) scored the lowest fidelity. Ninety-one per cent compliance with checklist items was scored by the researchers and 88% compliance was scored by the trainees, which may be explained by the changes made to day 2 of the training. The session had to be significantly modified during delivery because of the large numbers of attendees. On day 1 of the training, supervisors were asked not to attend day 2 to ensure that the facilitators had enough time and opportunity to practise elements of the intervention with their peers. Modifications included reducing time spent on a topic/activity or excluding it altogether. Items identified by the researchers as affected included:
-
how the participant and supporter/carer work together on the intervention
-
transferring skills from the safety of the group to their wider lives
-
reflecting on and sharing their own facilitation style and skills
-
exploring the value of out-of-venue activities and how to help group members to plan them
-
discussing the value and principles of supervision.
The same pattern was reflected in the trainee checklist scores, with high agreement on items scored as delivered or not delivered overall between trainees, averaging 93% (range 88–96%). There was 100% response rate for trainee checklists.
Eighteen out of 50 trainees felt that at least one item of the trainee checklist (range 1–8 items) had not been delivered at the training. There was agreement between the researchers and trainees on the top three items not delivered. These were:
-
Item 7: ‘did the trainer discuss the supporter attended sessions and relationship dynamics?’ (8 out of 18 trainees).
-
Item 11: ‘were you able to reflect on and share your own facilitation style and skills?’ (8 out of 18 trainees).
-
Item 17: ‘did you discuss the value and principles of supervision?’ (12 out of 18 trainees). This rating related predominantly to the second training session (on 23 and 24 January 2017) and was one of the topics affected by the modifications made to day 2 of the training.
Intervention fidelity
Overall, attendance was good for those who completed the 12-week intervention, with 25 out of 35 (71%) participants from the four sites used to assess fidelity attending 10 out of 16 sessions. Five (14%) participants attended all 16 sessions. This was representative of attendance in the trial (see Journeying through Dementia intervention).
Group meeting fidelity
Of the total 331 available group sessions (i.e. the number of group meetings × number of engaged participants in the four fidelity sites, note that sessions were counted if the participant missed them, but if the participant had withdrawn, then future sessions were not counted), 264 (80%) were attended. Fidelity to the intervention observed during group meetings was very high and achieved a score of between 85% and 95%.
For all four groups combined, a Cohen’s kappa score of 0.68 demonstrated substantial inter-rater reliability (95% CI 0.58 to 0.78) between researchers coding the group meetings. A site breakdown of scores can be found in Appendix 6, Table 36.
One hundred per cent of facilitator group meeting fidelity checklists were completed. Overall, facilitators reported that they had delivered the intervention as intended during the observed group meetings and fidelity averaged 93% (range 84–100%). This reflects the observations of the researchers.
One-to-one session fidelity
Three out of the four fidelity sites returned one-to-one session checklists. Overall, 20 out of a possible 35 checklists were returned. Each participant initially agreed to attend the intervention and completed the first one-to-one session. Eight participants withdrew between session 1 and session 2. Of the remaining 26 participants, only one participant missed a one-to-one session.
Three of the four fidelity sites returned one-to-one session checklists for 20 participants. Each participant had a maximum of four records, one per session. Seven participants had incomplete records and six of these participants were from the same site.
Facilitators recorded that they were able to deliver most items on the checklist during each session, averaging 77% achievement (range 22–100%). The items with the highest rates of non-delivery were item 5 ‘did you help the participant set any goals?’, with 40% achievement, and item 8 ‘did you enable the participant to rehearse skills learned in their everyday life?’, with 22% achievement. This differs from overall adherence to the intervention by all sites, as demonstrated through scrutiny of facilitator records, which revealed that they were not always able to deliver the intervention as intended. One rationale for this disparity may be facilitator awareness of being observed for fidelity assessment.
Supervision fidelity
Site supervision at fidelity sites
The four fidelity sites undertook 10 groups in total. Supervision registers and checklists were completed and returned, covering nine groups (at least two per site) and included 11 facilitators and five supervisors. These registers and checklists covered the majority of groups that took place at these sites over the full period of the trial. One site had two supervisors to support the intervention who shared supervision of the facilitators. There were no staff changes to the facilitators or supervisors during the fidelity assessment.
Only one site conducted a minimum of four individual supervision sessions with each facilitator, as per protocol. Two sites failed to do so during delivery of their first group, but did achieve this during delivery of their second group, which was perhaps as a consequence of encouragement during research supervision. One site did not achieve this during any of the three groups they delivered. One facilitator had no recorded individual sessions and, out of the 36 supervision sessions offered, attended only joint supervision on 15 occasions.
Of 111 opportunities for supervision across the four sites (including additional sessions pre or post groups starting), 105 were delivered. Of the six supervision sessions not carried out, five were because of the supervisor being on leave and one was cancelled by the facilitators. The average length of time for a joint supervision session was 61 (range 30–125) minutes and the average length of time for an individual supervision session was 51 (range 25–70) minutes. Joint supervision was carried out on 59 occasions and individual supervision on 42 occasions. Four sessions were delivered as a combination of joint and individual time. The majority of sessions (n = 97) were conducted face to face and eight were conducted via telephone or Skype.
Ninety per cent of supervision checklists at weeks 1, 5 and 11 were completed. Fidelity, overall, was high across the three checklists and five supervisors, averaging 82% (range 77–86%) achievement. Supervisors recorded that they were able to deliver most items during each supervision session. The most common item not delivered was ‘did you use a reflective diary as part of the supervision session?’. This was not unexpected, as maintaining a reflective diary was optional.
Fidelity of training and supervision of supervisors at fidelity sites
All supervisors at the four fidelity sites had access to the supervision protocol (see Report Supplementary Material 1). They either attended the 2-day formal training attended by the facilitators (apart from the second day of the 23 and 24 January 2017 training session, when supervisors were requested not to attend) or received informal training from the trial team. They also received a separate half-day, bespoke supervision training session (see Chapter 1, Intervention: rationale, methods and delivery, for details of the training provided).
As per protocol, a member of the research team experienced in the intervention and the ‘train the trainer’ model provided regular research supervision to each of the fidelity sites supervisors. The four fidelity sites received an average of 7.25 (range 5–10) sessions lasting an average of 36 (range 20–90) minutes. The majority of sessions were by telephone (68%), 22% were face to face and 10% were by e-mail. All four fidelity sites had at least one face-to-face supervision session. In July 2017, further guidance was provided by the research supervisor to the fidelity site supervisors on managing annual leave or sick leave for facilitators and supervisors.
Changes to group meeting fidelity researcher checklist
A number of changes were made to the researcher checklist after testing at the first group meeting was conducted at each site. These changes were based on researcher debriefing after the meetings and observations made about the usability of the checklist scale. Questions 4, 5, 6 and 23 were converted to a ‘yes/no’ response and the overall scale was converted from 0–3 (none/some/most/all of the time) to 0–2 (none/some/most of the time). It was considered unlikely to observe group members receiving or enacting checklist criteria ‘all of the time’ because of participant preferences and characteristics. Scores on the checklists from the first group meetings were converted to the revised scale. Any identified as ‘all’ were combined with the ‘most’ category.
Safety and harms
Serious adverse events are summarised in Table 21. All SAEs were assessed for relatedness and were all considered either unrelated or unlikely to be related to the intervention. The numbers of participants and participating supporters reporting symptoms of anxiety or depression at baseline and 8 months are presented in Table 22. The proportion reporting anxiety and depression at each time point was low and similar across the treatment groups.
| SAE | Intervention (N = 241) | Control (N = 239) | Total (N = 480) |
|---|---|---|---|
| Number of participants who experienced one or more SAE | 40 | 35 | 75 |
| Number of SAEs (including repeated events) | 61 | 39 | 100 |
| Occurred, n (%) | |||
| Before randomisation | 3 (5) | 1 (3) | 4 (4) |
| After randomisation | 58 (95) | 38 (97) | 96 (96) |
| Seriousness, n (%) | |||
| Death | 10 (16) | 5 (13) | 15 (15) |
| Life-threatening | 3 (5) | 2 (5) | 5 (5) |
| Inpatient hospitalisation | 45 (74) | 31 (79) | 76 (76) |
| Prolonged hospitalisation | 1 (2) | 0 (0) | 1 (1) |
| Persistent or significant disability/incapacity | 2 (3) | 1 (3) | 3 (3) |
| Intensity, n (%)a | |||
| Mild | 6 (10) | 5 (13) | 11 (11) |
| Moderate | 35 (57) | 25 (64) | 60 (60) |
| Severe | 17 (28) | 9 (23) | 26 (26) |
| Relationship to intervention, n (%) | |||
| Unlikely | 13 (21) | 5 (13) | 18 (18) |
| Unrelated | 48 (79) | 34 (87) | 82 (82) |
| Reported symptom | Participant | Participating supporter | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 191) | Control (N = 197) | Total (N = 388) | Intervention (N = 142) | Control (N = 141) | Total (N = 283) | |
| Number (%) with symptoms of anxiety (GAD-7 score ≥ 10) | ||||||
| Baseline | 11 (6) | 12 (6) | 23 (6) | |||
| 8 months | 12 (6) | 12 (6) | 24 (6) | |||
| Number (%) with symptoms of depression (PHQ-9 score ≥ 15) | ||||||
| Baseline | 7 (4) | 6 (3) | 13 (3) | 3 (2) | 4 (3) | 7 (2) |
| 8 months | 4 (2) | 8 (4) | 12 (3) | 7 (5) | 5 (4) | 12 (4) |
Unblinding
Researchers conducting the outcome assessments/interviews were blind to treatment group allocation. In cases where treatment group was revealed or suspected, a new blinded outcome assessor was sought to conduct future outcome assessments. Table 23 summarises the cases of unblinding in the trial [202 cases of unblinding occurred, 134 (over 92 participants) for the intervention group and 68 (over 56 participants) for the control]. The majority of unblinding occurred via disclosure from the participant (42%) and the supporter/carer (37%).
| Characteristic | Intervention (N = 134), n (%) | Control (N = 68), n (%) | Total (N = 202), n (%) |
|---|---|---|---|
| Unblinded case | |||
| Yes | 125 (93) | 59 (87) | 184 (91) |
| Unsure | 9 (7) | 9 (13) | 18 (9) |
| Role | |||
| Outcome assessor | 131 (98) | 67 (99) | 198 (98) |
| Other | 3 (2) | 1 (1) | 4 (2) |
| Source of unblinding | |||
| Facilitator | 13 (10) | 2 (3) | 15 (7) |
| Participant | 56 (42) | 28 (41) | 84 (42) |
| Supporter | 43 (32) | 32 (47) | 75 (37) |
| Medical professional | 2 (1) | 0 (0) | 2 (1) |
| Other | 20 (15) | 5 (7) | 25 (12) |
| Reason for unblinding | |||
| Accidental | 124 (93) | 65 (96) | 189 (94) |
| Safety | 1 (1) | 0 (0) | 1 (0) |
| Other | 9 (7) | 3 (4) | 12 (6) |
| Method of unblinding | |||
| Face to face | 87 (65) | 43 (63) | 130 (64) |
| 12 (9) | 2 (3) | 14 (7) | |
| By observation | 3 (2) | 0 (0) | 3 (1) |
| Over the telephone | 26 (19) | 21 (31) | 47 (23) |
| Other | 6 (4) | 2 (3) | 8 (4) |
| Time pointa | |||
| 8 months | 76 (57) | 45 (66) | 121 (60) |
| 12 months | 14 (10) | 10 (15) | 24 (12) |
| Other | 44 (33) | 13 (19) | 57 (28) |
The 202 cases of unblinding referred to in Table 23 relate to 148 trial participants over 740 trial visits. Multiple outcome assessors became unblinded to the same participants, in some cases up to four outcome assessors, despite trial procedures designed to avoid this. Twenty-four unblinding cases occurred during the 12-month visit.
Analysis of the trial database showed that, among 58 cases of unblinding that occurred before the primary 8-month outcome measure visit, there were five cases where potentially unblinded outcome assessors still conducted the visit. Reasons for this included (1) the unblinding event happened a long time before the appointment (n = 3) and (2) pragmatic reasons, including lack of availability of other staff, as to why that person still needed to attend (n = 2). None of the five potentially unblinded staff went on to conduct the 12-month visits.
A further 94 cases of unblinding that may have affected the 12-month visit occurred either at the 8-month visit or before the 12-month visit. There were 19 cases where the potentially unblinded outcome assessor still went on to conduct the 12-month visit, most likely for pragmatic reasons, as in many cases other members of the local outcome assessment team were also unblind to that participant’s allocations. In some cases, the outcome assessors had stated that they were unsure about the participant’s treatment allocation.
In 24 cases, in total, potentially unblinded outcome assessors went on to conduct further outcome assessment visits. In all other cases, different assessors conducted future visits. However, the outcome assessors did not always correctly identify the participant’s treatment allocation. Of the 202 unblinding events, the outcome assessor was wrong in 22 (11%) cases (20 cases thought to be in the intervention arm were in the control arm and two cases thought to be in the control arm were offered the intervention). Additionally, in 18 (9%) cases the outcome assessor was unsure if they were unblind.
Usual care
Usual care at delivery sites
All participants in the study, whether allocated to the intervention or control arm, received treatment as usual from their service providers at the intervention delivery sites (which were all NHS trusts). The nature of treatment as usual varied across intervention delivery sites. There was also variation in the provision of treatment as usual for participants within some delivery sites because of the size of some NHS trusts and recruitment having a large geographical spread. These variations were the same for both the intervention and control arms.
Treatment as usual across delivery sites for people with dementia post diagnosis included:
-
prescription of medication for dementia (i.e. cholinesterase inhibitors and/or memantine) or other medication to control symptoms (e.g. depression)
-
assessment of needs and, in some instances, referral to various individual and/or group-based sessions, and/or other health and social care services (e.g. Community Mental Health Teams)
-
referral to third-sector services or voluntary services (e.g. the Alzheimer’s Society)
-
provision of educational material.
Analysis of usual-care pathways within participating sites revealed variance in the approaches taken following diagnosis. Some sites retained patients on their lists, while others referred to alternative services. The economy of care within the trusts was very different, for example, the availability of local services and service providers, and the role of third-sector organisations in providing care and support services.
Immediately post diagnosis, all trusts reported that they conducted some form of assessment of needs as part of usual care, followed by referral to support services (e.g. Community Mental Health Teams or third-sector support). Participating trusts provided limited follow-on support to people with dementia and their supporters. In almost all instances (with the exception of one site) patients were referred back to primary care if there were no complicating factors, or when they completed support groups or therapy provided by memory clinics. Two sites described using a ‘shared care protocol’ with primary care. Referrals back to the memory clinic were made by GPs, a member of a Community Mental Health Team or, if present, a memory support worker when a person with dementia’s condition had deteriorated.
The provision of group-based interventions as usual care was common across the 20 trial sites (Table 24). CST was the most commonly provided group-based intervention (provided at nine sites). CST groups were provided for 7–14 weeks with either one or two sessions per week. CST groups were provided as post-diagnosis support, with one site reporting that this was typically in the first 6–12 months post diagnosis. Several sites reported that CST groups were not available to all people diagnosed with dementia because of a high demand for the service. CST differs from the JtD programme, as it follows a detailed and prescriptive session-by-session plan of group exercises and activities that are facilitator led and takes place wholly within the delivery setting.
| JtD trial site ID | Treatments provided as usual care | ||||||
|---|---|---|---|---|---|---|---|
| Conducting needs assessment | Drug therapy | Referral back to primary care | CST group | Educational group | Memory or Living Well with Dementia group | Third sectora/otherb | |
| S01 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| S02 | ✓ | ✓ | ✓ | ✓ | |||
| S04 | ✓ | ✓ | ✓ | ✓ | |||
| S05 | ✓ | ✓ | ✓ | ✓ | ✓c | ✓ | |
| S06 | ✓ | ✓ | ✓ | ✓ | |||
| S07 | ✓ | ✓ | ✓ | ✓ | ✓d | ||
| S08 | ✓ | ✓ | ✓ | ✓ | |||
| S09 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| S10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| S11 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| S12: delivery site 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| S12: delivery site 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| S13 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| S15 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
Memory or Living Well with Dementia groups were run at five sites (two and three sites, respectively) and a psychosocial intervention was provided by a third-sector organisation (the Alzheimer’s Society) at a further site through referrals from memory clinics. In one of the five sites, the group was not run at the same time as JtD groups, as the usual facilitators were delivering the JTD intervention. The groups provided by sites varied in length from four sessions of 90 minutes to 10 sessions of 2 hours. All were shorter than the JtD programme. Educational groups were additionally provided at three sites. Memory groups may focus on reminiscence, which is not a central theme of the JtD programme. The Living Well with Dementia groups may have had some of the features of the JtD programme; however, they do not generally include enactment of learnt skills in the community or the mix of individual and group sessions that the JtD programme incorporates.
The post-diagnostic review approach varied greatly at sites (Table 25). GPs were most often tasked with the provision of post-diagnostic review of patients (seen at eight sites). Usual post-diagnostic care at seven sites was to increase the frequency of patient reviews, depending on a patient’s need and/or deterioration. Six sites carried out patient reviews at least once in the first 3 months post diagnosis, with four sites conducting annual reviews of patients as part of their provision of usual care. Only two sites provided a path by which patients could refer themselves back to their local memory clinic.
| JtD trial site ID | Post-diagnostic review | |||||
|---|---|---|---|---|---|---|
| GP provision of post-diagnostic review | Medication stabilisation review | Self-referral by patient available | More regular follow-ups with patient deterioration/need | Patient review 1–3 months post diagnosis | Regular/annual memory service post-diagnostic review | |
| S01 | ✓ | ✓ | ||||
| S02 | ✓ | ✓ | ||||
| S04 | ✓ | |||||
| S05 | ✓ | ✓ | ||||
| S06 | ✓ | ✓ | ||||
| S07 | ✓ | ✓ | ✓ | |||
| S08 | ✓ | ✓ | ||||
| S09 | ✓ | ✓ | ||||
| S10 | ✓ | |||||
| S11 | ✓ | ✓ | ✓ | ✓ | ||
| S12: delivery site 1 | ✓ | ✓ | ||||
| S12: delivery site 2 | ✓ | ✓ | ✓ | |||
| S13 | ✓ | ✓ | ||||
| S15 | ✓ | ✓ | ||||
As usual care, appropriate medication was provided to patients depending on the type of dementia, progression, and behavioural and psychological symptoms of dementia. Annual patient reviews were commonly used to assess medication regimes and a limited number of memory clinics assessed medication stability within the first few months of diagnosis. As patients were commonly referred back to primary care after diagnosis, GPs became responsible for monitoring patients’ tolerance of and adherence to medication.
Services used at 8 months post randomisation
A review of the services used at 8 months post randomisation from sections within the HSCRU on day and community-based service use shows that although dementia-specific day services were used, the type of services and groups varied greatly in content and were not often provided by the NHS. Additionally, although one-to-one health-care professional home visits did take place, these were rare.
Review of day service use data indicates that 100 of the 371 (27%) activities reported were clearly dementia-specific activities that varied in type, from voluntary memory cafes (n = 31), dementia groups (n = 29), singing groups (n = 13), Dementia Forward (n = 8), Alzheimer’s Society clubs (n = 6) and other groups (n = 13; e.g. Age UK, Age Concern, exercise and other groups). Five participants reported attending memory groups run through voluntary services and one reported attending an NHS-run Living Well with Dementia group.
Overall, 23 dementia groups were reported relating to 29 of the 371 (8%) reported activities and these ranged from church groups for people with dementia to lunch and reminiscence groups. Of note, three of the groups referred specifically to young-onset groups and one to a young-onset group theatre trip. At most sites there was only one participant attending the groups, but at some sites there were more popular groups. These included the York Minds and Voices99 (a peer support and activism group), the Humber Butterflies group100 (a group that involves speakers, quizzes and music) and activities run by the Essence Service101 in Sunderland (which includes outreach drop-ins and a well-being group). Very few groups of any kind were offered by the NHS (four in total) and the majority were voluntary. These examples of dementia groups appear to be open-ended rather than a course, and they highlight the variation in what is available to people with dementia across different areas.
Review of community-based service use data provided further information about which participants attended clinics to receive CST (n = 4) and which health-care professionals visited the participants at home. The following health-care professionals visited participants at home:
-
memory support workers (two participants), a memory nurse (one participant) or others from the memory clinic (four participants)
-
mental health nurses (seven participants) and community mental health nurses (three participants)
-
occupational therapists (six participants)
-
psychiatrists (two participants), community psychiatrists (two participants) and community psychiatric nurses (two participants).
The information also indicates that in one case there was an Age UK home visit and in another a befriender visited the participant’s home. Although these data show that there may be one-to-one appointments with professionals, such services are very rare (n = 31, 6%) compared with the overall number of community-based services reported (n = 536).
Chapter 4 Health economics analysis
Study question
Was the JtD intervention cost-effective for people in the early stages of dementia compared with usual care over a 1-year period from an NHS and social care perspective?
Form of evaluation
This study was a pragmatic two-arm parallel-group superiority individually and equally randomised controlled trial comparing the JtD intervention with usual care to determine benefit for people in the early stages of dementia. Although the study was an individually randomised controlled trial, the delivery of the JtD intervention in one arm of the trial was group based (i.e. groups of 8–12 participants randomised to the JtD intervention attended 12 weekly facilitated meetings in local venues to receive the intervention).
An economic evaluation (i.e. a cost–utility analysis) was undertaken from an NHS and Personal Social Service perspective, for which results are expressed in terms of incremental cost per quality-adjusted life-year (QALY) gained. Cost–utility analysis is a method used to carry out an economic evaluation. The benefits are assessed in terms of both quality and duration of life, and are expressed as QALYs. A QALY is a measure of the health of a person in which length of life is adjusted to reflect the quality of life. Length of life is measured in years, whereas utility is a measure of morbidity or health-related quality of life. Utility is measured on a scale anchored on 1 (perfect health) and zero (death). Negative values are possible for health states considered to be worse than death. The values given are based on patient or societal preferences. Utilities are essential for the calculation of QALYs.
The study analysis presents the results over the 1-year study period and, therefore, no discounting is applied. The analysis followed recommended methods and good practice guides. 102–104
Effectiveness data
The EQ-5D-5L and DEMQOL measures were administered at baseline, 8 and 12 months. In the primary analysis, van Hout et al. ’s69 mapping algorithm was used to obtain utility values in accordance with NICE’s position statement on the EQ-5D-5L valuation tariffs for England70 and further sensitivity analysis was applied to estimate utilities for the EQ-5D-5L values for the Devlin et al. algorithm. 105 Utilities were calculated at baseline, 8 and 12 months, and QALYs were estimated using the trapezium rule. Therefore, a QALY of 1 would imply good health over the entire 12 months of follow-up. The data were collected using EQ-5D-5L, which was mapped to three-level utility scores using the algorithm mentioned above for the primary analysis.
A preference-based version of the DEMQOL (i.e. the DEMQOL-U) has been developed and can be used in cost-effectiveness analysis, with preference weights for use in cost-effectiveness analysis available. 51 A further analysis using DEMQOL-U was undertaken.
Resource use
Intervention costs and subsequent resource use costs for routine health and social care services were estimated for individuals. Intervention costs included cost of training materials and delivery of training to facilitators and supervisors, staff time for delivery of the intervention, intervention materials, preparation time and travel time to the training courses. Trainers provided details of the number of courses they delivered and the length of the courses, any preparation time, travel time to courses and overnight stay, if applicable. Trainers provided details of their staff grade using the AfC pay scale to cost their time. 106 The costs of materials was provided by the study team and these included printing of manuals, catering, room hire, and administration time for the arrangement of dates and co-ordination of training delivery. Table 26 presents a breakdown of the intervention costs overall and per participant receiving the intervention.
| Resource | Overall cost (£) | Cost (£) per participant (JtD arm n = 191)a |
|---|---|---|
| Training | ||
| Trainer time, travel time and preparation time | 13,565 | 71 |
| Manuals and administration | 3080 | 16 |
| Facilitators and supervisors receiving training | 22,641 | 119 |
| Supervisory meetings between supervisors and facilitators | 15,221 | 80 |
| Intervention delivery by facilitators | 67,686 | 354 |
| Overall intervention costs | 122,193 | 640 |
The overall cost of training for the trainers was £13,564.56 for > 66 hours of training. The cost of the printing of materials, administration costs for the co-ordination of training, catering and room hire was £3080.27, with an overall cost of running the training of £16,644.83.
Information was gathered on the overall cost of training facilitators and supervisors, and the cost of travel to training to NHS sites. The overall cost of training to facilitators and supervisors was £22,641. Supervisors provided extra weekly support to facilitators throughout the study; they met with facilitators weekly, either as a group or in one-to one sessions, and the time spent on these sessions was recorded. The cost of providing supervision during delivery of the intervention was £15,221, which was £79.69 per participant or £217.987 per facilitator in the study.
The costs of the delivery of the intervention were taken from records of the number of facilitators at each session and the time spent at each session, and includes any expenses for room hire, catering and outings. This cost was £67,686 overall or £354.38 per participant in the study.
The overall costs of the intervention training and delivery were summed. The intervention costs £122,193 overall or £639.75 per participant in the intervention arm.
Other resource use costs were obtained from Department of Health and Social Care (DHSC) reference costs,107 Personal Social Services Research Unit (PSSRU) unit costs108 and the British National Formulary for medication costs. 109 Costs are presented in 2018/19 prices and were summed to obtain a total cost for each arm of the trial.
Allowance for uncertainty
Bootstrapping was carried out to allow for uncertainty. A total of 5000 bootstrap replicates were run and bias-corrected bootstrap 95% CIs were estimated.
One-way sensitivity analysis was performed (1) on utility estimates, using alternative preference-based weights or measures and (2) using alternative ways of delivering the intervention (e.g. without supervision and with facilitators being band 4 or above) were used. A further sensitivity analysis used multiple imputation to impute missing cost and QALY values. 110 A total of 20 imputations were run.
All analysis was carried out in Stata.
Presentation of results
Table 27 presents the completeness of data for the EQ-5D and DEMQOL, QALYs derived from the EQ-5D and DEMQOL, and costs. Data completeness was high for the study, with only 10% of data being incomplete at 12 months. There were no differences in completeness between the two arms.
| Parameter | JtD intervention (N = 191), n (%) | Usual care (N = 197), n (%) | JtD intervention vs. usual care: difference in % missing |
|---|---|---|---|
| Baseline utility: EQ-5D-3L van Hout et al.69 | 191 (100) | 197 (100) | 0.0 |
| Baseline utility: EQ-5D-5L Devlin et al.105 | 191 (100) | 197 (100) | 0.0 |
| Baseline utility: DEMQOL-U | 191 (100) | 197 (100) | 0.0 |
| QALYs based on EQ-5D van Hout et al.69 | 173 (91) | 176 (89) | 1.3 |
| QALYs based on EQ-5D Devlin et al.105 | 168 (88) | 173 (88) | 0.2 |
| QALYs based on DEMQOL-U | 173 (91) | 177 (90) | 0.8 |
| Total cost | 174 (91) | 176 (89) | 1.8 |
Table 28 presents the EQ-5D and DEMQOL-U values at baseline, and QALYs over 1 year. There was very little difference in both utility scores at baseline and QALYs between the two arms of the study. Figure 12 illustrates the small difference between the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), EQ-5D-5L and DEMQOL-U measures over time. It is also difficult to discern difference in the values between the JtD and control groups.
| Parameter | JtD intervention | Usual care | JtD intervention vs. usual care: difference in mean | ||
|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | ||
| Baseline utility: EQ-5D-3L van Hout et al.69 | 191 | 0.764 (0.22) | 197 | 0.773 (0.19) | –0.009 |
| Baseline utility: EQ-5D-5L Devlin et al.105 | 191 | 0.830 (0.19) | 197 | 0.840 (0.19) | –0.010 |
| Baseline utility: DEMQOL-U | 191 | 0.792 (0.10) | 197 | 0.805 (0.10) | –0.013 |
| QALYs based on EQ-5D van Hout et al.69 | 168 | 0.774 (0.19) | 173 | 0.777 (0.19) | –0.003 |
| QALYs based on EQ-5D Devlin et al.105 | 173 | 0.837 (0.16) | 176 | 0.840 (0.17) | –0.003 |
| QALYs based on DEMQOL-U | 173 | 0.802 (0.08) | 177 | 0.818 (0.07) | –0.016 |
FIGURE 12.
Mean utility scores over time by treatment group. (a) EQ-5D-3L; (b) EQ-5D-5L; and (c) DEMQOL-U.
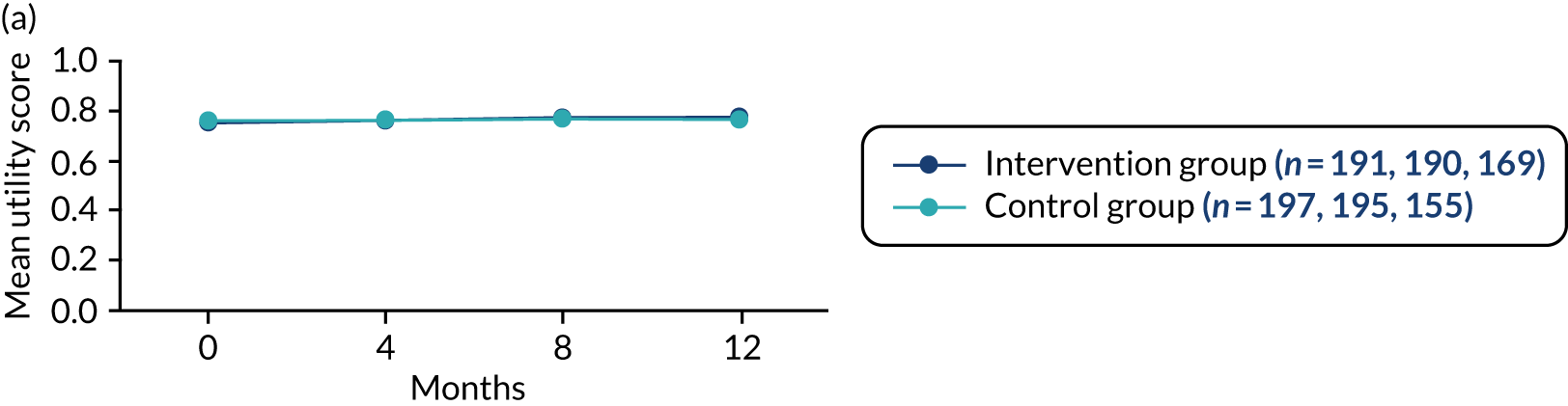
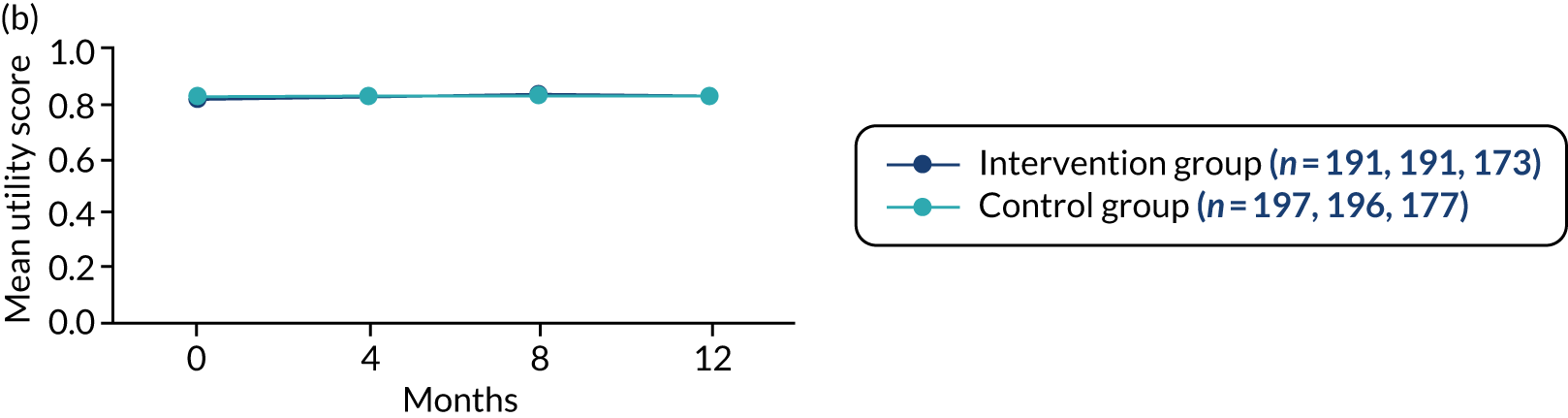
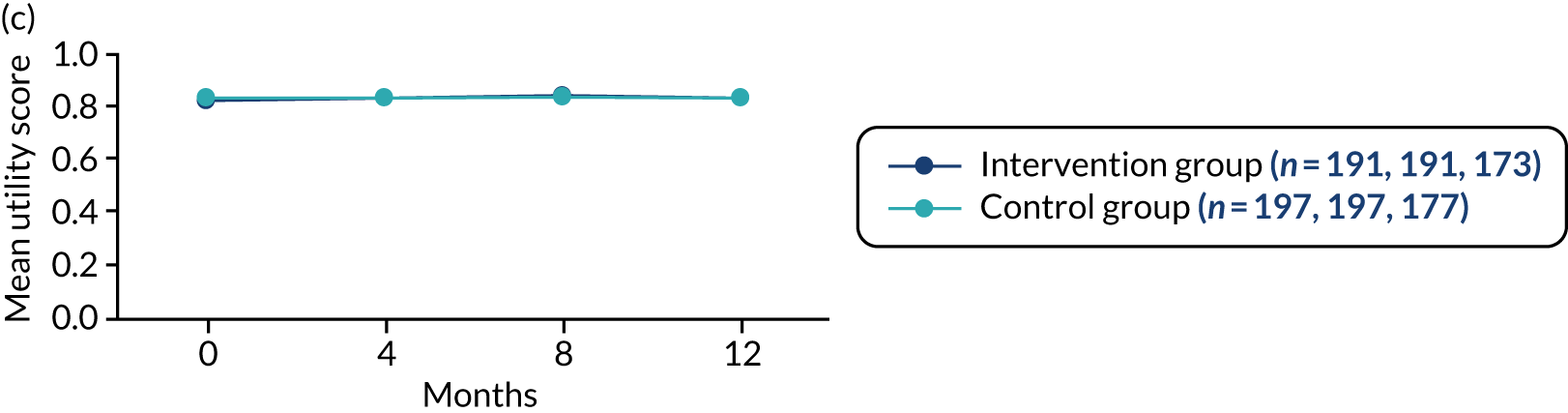
Resource use
Table 29 summarises the resource use costs for the JtD intervention and usual care. A detailed summary of all resource use, including unit cost sources, is provided in Appendix 9, Tables 39–44. Bootstrapped CIs are presented to depict uncertainty.
| Resource use | JtD intervention (n = 174) cost (£) | Usual care (n = 176) cost (£) |
|---|---|---|
| Intervention | 122,193 | 640 |
| Outpatients | 192 (149 to 236) | 199 (159 to 248) |
| A&E attendances | 40 (23 to 60) | 29 (16 to 48) |
| Hospitalisations | 323 (72 to 866) | 330 (105 to 708) |
| Other hospital services | 14 (2 to 34) | 17 (5 to 36) |
| Transport | 24 (10 to 48) | 25 (12 to 45) |
| Community services | 181 (155 to 212) | 211 (180 to 243) |
| Day services | 50 (24 to 88) | 82 (28 to 167) |
| Medications | 210 (123 to 382) | 164 (121 to 219) |
| Total costs | 1676 (1367 to 2227) | 1067 (792 to 1484) |
Outpatient visits
Overall, there were slightly more outpatient visits in the usual-care arm than in the JtD arm; however, overall costs are similar in the two arms of the trial.
Accident and emergency attendance
There were a total of 41 visits to accident and emergency. Nineteen participants had one visit and two participants had two visits in the JtD arm. Fourteen participants had one visit and two participants had two visits in the usual-care arm. The costs of accident and emergency visits are slightly higher in the JtD arm.
Hospitalisations
Overall, there were 22 hospital admissions (nine in the JtD arm and 13 in the usual-care arm). Reasons for admissions varied and included stroke, chronic obstructive pulmonary disease, pneumonia, heart attack and breathing problems. The costs between the two groups were similar.
Other hospital services
Overall, there were 15 accesses to other hospital services in the study (seven in the JtD arm and eight in the usual-care arm). These included day services and community hospitals. Costs were slightly higher in the usual-care group, although they were still similar.
Transport
There were 26 emergency journeys (15 in the JtD arm and 11 in the usual-care arm) and 38 non-emergency ambulance journeys (10 in the JtD arm and 28 in the usual-care arm, with one person in the usual-care arm having regular journeys for dialysis). The overall cost of transportation over 12 months was similar in both arms of the study.
Community services
The top community services accessed are presented in Appendix 9, Table 42. The most common services accessed were general practices (GP and practice nurse), memory clinics and chiropodists. The usual-care arm accessed community services slightly more than the JtD arm and their costs were slightly higher.
Day services
The majority of participants in the study accessed day care, lunch clubs, social clubs or other services, although only 8% accessed these services via either the NHS or the local authority. The remainder of these services were accessed through charities or private sources. Given that the aim of the cost-effectiveness analysis is to present the costs from an NHS and social care perspective, services accessed only via the NHS or local authority are presented here. Examples of other day services accessed included exercise classes, gym sessions, dementia carers groups and walking groups, and these activities were assumed to last 1 hour. Table 29 shows the average costs with bootstrap CIs. Participants were more likely to incur higher day care costs at month 12 than month 8 of the study. Participants in the control arm incurred higher day care costs than participants in the JtD arm.
Medications
Medication costs were slightly higher in the JtD arm.
The overall cost of the JtD intervention, including intervention and resource use costs, was £1676, whereas the overall cost of usual care was £1067. Therefore, the JtD intervention was more expensive by an average of £609.
Cost-effectiveness
The incremental cost-effectiveness ratio (ICER) was approximately –£203,000 (Table 30). This indicates that the JtD programme is both more costly and less effective than usual care (dominated), with over half (55%) of the bootstrap points being in the top-left quadrant of the cost-effectiveness plane (see Appendix 7, Figure 14). Although the bootstrap estimates of the paired differences in costs and effects cover all four quadrants of the plane, they are predominantly in the northern quadrants (indicating that JtD treatment is more costly than the usual-care control treatment). The uncertainty lies in the QALYs, for which there is a small loss for JtD participants, causing uncertainty in the results. The cost-effectiveness acceptability curve (see Appendix 7, Figure 15) presents the probability of JtD being cost-effective and different willingness-to-pay thresholds. The probability at a willingness-to-pay threshold of £20,000 is 10.3% and, up to a threshold of £100,000, it never exceeds 35%.
| Mean costs and QALYs | JtD (n = 166) | Usual care (n = 173) | Incremental costs and QALYs | ICER |
|---|---|---|---|---|
| QALYs (95% CI) | 0.774 (0.744 to 0.802) | 0.777 (0.748 to 0.803) | –0.003 (–0.044 to 0.038) | |
| Overall costs (£) (95% CI) (over 12 months) | 1676 (1367 to 2227) | 1067 (792 to 1484) | 609 (105 to 1179) | –202,857 (–534,733 to 483,739) |
Sensitivity analysis
The sensitivity analyses are presented in Table 31. Two further ways of estimating QALYs were explored, the Devlin et al. 105 algorithm and the DEMQOL-U, and in both cases the utility loss was small and in favour of usual care. For the EQ-5D-5L, the Devlin et al. 105 algorithm was non-significant; however, for the DEMQOL-U, there may be a QALY lost from the JtD intervention. In both cases, the incremental cost-effectiveness of the JtD intervention remained dominated.
| Mean costs and QALYs | JtD (n = 166) | Usual care (n = 173) | Incremental costs and QALYs | ICER |
|---|---|---|---|---|
| Main analysis | ||||
| QALYs (95% CI) | 0.774 (0.744 to 0.802) | 0.777 (0.748 to 0.803) | –0.003 (–0.044 to 0.038) | |
| Overall costs (95% CI) (£) (over 12 months) | 1676 (1367 to 2227) | 1067 (792 to 1484) | 609 (105 to 1179) | –202,857 (–534,733 to 483,739) |
| Devlin et al.105 algorithm | ||||
| QALYs (95% CI) | 0.838 (0.813 to 0.861) | 0.841 (0.815 to 0.864) | –0.003 (–0.038 to 0.032) | |
| Overall costs (95% CI) (£) (over 12 months) | 1676 (1367 to 2227) | 1067 (792 to 1484) | 609 (105 to 1179) | –202,857 (–615,142 to 483,402) |
| DEMQOL-U | ||||
| QALYs (95% CI) | 0.802 (0.789 to 0.814) | 0.818 (0.809 to 0.828) | –0.016 (–0.032 to –0.0004) | |
| Overall costs (95% CI) (£) (over 12 months) | 1676 (1367 to 2227) | 1067 (792 to 1484) | 609 (105 to 1179) | –38,036 (–224,443 to 1088) |
| Excluding the supervisor role from the intervention | ||||
| QALYs (95% CI) | 0.774 (0.744 to 0.802) | 0.777 (0.748 to 0.803) | –0.003 (–0.044 to 0.038) | |
| Overall costs (95% CI) (£) (over 12 months) | 1565 (1256 to 2116) | 1067 (792 to 1484) | 498 (–6 to 1068) | –165,860 (–458,500 to 403,893) |
| Assuming all facilitators are employed at grade 4 | ||||
| QALYs (95% CI) | 0.774 (0.744 to 0.802) | 0.777 (0.748 to 0.803) | –0.003 (–0.044 to 0.038) | |
| Overall costs (95% CI) (£) (over 12 months) | 1680 (1372 to 2232) | 1067 (792 to 1484) | 613 (110 to 1168) | –204,490 (–539,020 to 489,003) |
| Imputing missing values | n = 174 | n = 176 | ||
| QALYs (95% CI) | 0.768 (0.739 to 0.796) | 0.775 (0.745 to 0.801) | –0.007 (–0.047 to 0.033) | |
| Overall costs (95% CI) (£) (over 12 months) | 1675 (1380 to 2256) | 1058 (790 to 1447) | 617 (109 to 1183) | –88,187 (–475,761 to 458,153) |
If the JtD intervention was rolled out in the NHS, then the supervisor role provided as part of the intervention may not be needed. Therefore, an alternative cost for the intervention was examined excluding the cost of supervisors. This reduced the cost difference between the JtD intervention and usual care from £609 to £498, but the JtD intervention remained dominated. We also explored the assumption that all facilitators would be band 4, as this was assumed at the beginning of the study, although in practice some facilitators were band 3. This analysis increased the incremental cost by £5 to £613 and the JtD intervention remained dominated.
Finally, missing QALYs and costs were imputed using multiple imputation and, again, the JtD intervention remained dominated by usual care.
Summary
In this analysis we have explored the cost-effectiveness of the JtD intervention. The QALY effect from comparing the JtD intervention with usual care was small and non-significant, and the JtD intervention cost approximately £600 more than usual care. Overall, owing to the small effect size, the JtD intervention was dominated by usual care. However, the results were uncertain and covered all four quadrants of the cost-effectiveness plane. A range of sensitivity analyses were carried out, including use of alternative algorithms and measures for measuring utility and costing an alternative delivery of the JtD intervention. The incremental cost per QALY ranged from –£88,000 to –£205,000, indicating that the JtD intervention may not be cost-effective.
Chapter 5 Qualitative substudy
Aims and methods
This embedded qualitative substudy was conducted to understand the experience of taking part in the JtD intervention (see Chapter 2, Qualitative study for a detailed description of the methods of the qualitative study).
Sample
Nineteen participants and 14 participating supporters from the four sites identified for fidelity assessment were purposefully identified, contacted by telephone and invited to take part. This led to 15 participants and 10 participating supporters being interviewed in their homes. All interviews followed a semistructured format and were audio-recorded after permission was obtained for this. Numbers were smaller than originally planned because of smaller numbers of participants per intervention group at the four fidelity sites (i.e. an average of seven participants rather than 12). The intention was to interview participants and supporters separately to allow the voice of each to be heard. However, four dyads (i.e. participants and participating supporters) were interviewed together at the request of the participant. Table 32 describes the characteristics of those who took part.
| Characteristic | Participants (N = 15) | Participating supporters (N = 10) |
|---|---|---|
| Dementia type (n) | ||
| Alzheimer’s | 6 | n/a |
| Frontotemporal | 2 | |
| Vascular | 3 | |
| Mixed | 3 | |
| Other: mild dementia | 1 | |
| Age (years) | ||
| Range | 67–90 | 50–89 |
| Average | 77 | 71 |
| Ethnicity | ||
| White British | 14 | Data not available |
| African/Asian | 1 | Data not available |
| Gender (n) | ||
| Female | 7 | 7 |
| Male | 8 | 3 |
| MMSE score | ||
| Range | 20–29 | n/a |
| Average | 26 | |
| Living arrangements | ||
| Living alone | 2 | n/a |
| Living with others | 13 | |
Those interviewed were representative of the overall trial population, except participant age (range 39–93 years in the trial).
All facilitators (n = 10) and four of the five supervisors from the four fidelity sites were interviewed individually over the telephone in a semistructured format using a topic guide. All were employed in NHS recruitment and delivery sites for the study. Facilitators could be from any professional background and, for these interviews, included those in junior posts (i.e. AfC bands 3 and 4) as well as those in more senior posts. Supervisors were all experienced clinicians from a range of backgrounds and employed at a senior level (i.e. AfC band 7).
Findings
Interviews with participants, participating supporters, facilitators and supervisors were analysed separately and then triangulated to highlight areas of agreement and divergence. Quotations have been selected to illustrate the findings.
Three main themes could be identified from the analysed interview data (Table 33).
| Theme | Subtheme |
|---|---|
| Being prepared |
|
| Engaging with the intervention |
|
| Outcomes for participants |
|
Theme 1: being prepared
Expectations: participants and participating supporters
Both participants and participating supporters talked about experiencing a range of emotions following diagnosis, including anger, depression and fear, while at the same time giving examples of how they had sought to manage this positively. Various strategies were described, including using diaries to support memory, and looking after pets to give focus and maintain a sense of responsibility. Failing memory was significant for the majority:
Yes, my initial reaction was it’s a life sentence, or a death sentence and I was very, very depressed . . . then I thought to myself well, I’ve got to keep going because they might find a cure . . .
Participant 14, individual
Yes, it sounds a bit silly but it’s like if you’ve had a book and some pages have been torn out . . .
Participant 09, individual (talking about impact on memory)
Those participants who did report a change in their daily routines and living situation discussed that sometimes this was in response to concerns of family members regarding safety and ability to cope:
I used to cook and this sort of thing and now she [sister] certainly won’t let me iron my clothes. And she won’t let me cook because I could have caused accidents with someone . . .
Participant 09, individual
Two dyads described loss of friendships and social activity since diagnosis. For example, one couple had stopped playing bridge, which led to loss of contact with a friendship group. However, most participants and participating supporters described remaining reasonably active and independent. Notably, some participants were driving and/or using public or community transport unaccompanied:
. . . I drive, I do voluntary work on a Monday, I go with my friends for coffee, I can go do the shopping . . . so I’m quite independent.
Participant 11, individual
When asked what expectations they had of taking part in the intervention, a reiterated desire was hoping that it would not just potentially help them, but also help others going through the same experience.
The majority of participants and their supporters talked about wanting to know more about the condition, and wanting to share knowledge and problem-solving with others in the same situation. Several participants wanted to improve their mental and physical well-being:
I’ve got to be honest and say I’m a little bit ashamed . . . but I’ve got into a rut . . . I should maybe be doing a bit more activity in my life than what I am.
Participant 05, individual
Only one supporter indicated that they had not expected to see any benefit for the person they cared for:
I must admit, not a lot. You know, because it’s not something that’s going to improve.
Supporter 06, individual
Some participating supporters described the challenges of understanding dementia and its consequences for them and the person they cared for. This could lead to unrealistically high expectations, as observed by a facilitator:
I think that’s been quite hard for her [supporter] and it became a little bit more about her at times, and that was hard to manage . . . and I think that they think that they’re [participant] just going to get better, often carers can just think that, you know by coming to the group it’s going to improve their memory and make them better . . .
Facilitator 10
Intervention training for facilitators and supervisors
NHS staff who delivered the intervention attended a 2-day training workshop to prepare them. The timing of this was significant, as receiving training too early could be detrimental:
[I]t [training] would have been a few months before we were actually going to be doing our first session. So, I guess lots of what we do when we practise in the training, we’ve kind of forgotten about by the time we think of doing it.
Facilitator 02
Two facilitators described how the training had not met all of their needs, resulting in them undertaking ‘top-up’ training:
[A researcher] came out to our base and talked us through it a little bit more again [after the training], and we understood it a bit better after that and I don’t know whether it was just because there was quite a lot of information over the 2 days . . . we came away [from the training] just a little bit confused.
Facilitator 10
The opportunity within the 2-day training to practise components with colleagues and share learning with others was highlighted by all interviewed staff as being particularly helpful:
[D]oing the practical side of it, where they make you get up and almost run a group in front of the other people, they said you will hate it but it will be the most vital part of the training . . . and I did hate it, but it was the most vital part of the training.
Facilitator 10
Most facilitators and supervisors did not feel that a professional qualification was required to deliver the intervention, but experience in leading groups and of working with people with dementia was necessary.
Resources (staffing, manual and materials)
Most facilitators and supervisors found that the manualised intervention and accompanying online resources added to their understanding of the intervention and were suitable for the wide range of staff who would deliver it. Some facilitators found the manual to be a helpful ‘toolbox’ to dip in to, whereas others told us that the manual was overly comprehensive:
[T]here’s so many different topics and you’re trying to encourage the group to think for themselves what they want to do. And there’s so much ‘how do we pick from all of these?’ So, it’s got an advantage and a disadvantage I guess having a lot of choice . . . but then it can also be a bit overwhelming for them.
Facilitator 02
All of the people with dementia who were interviewed talked about being involved, to some extent, in selecting topics for their group. Facilitators also recounted how participants had identified additional topics (e.g. ‘confidence’ and ‘getting to know you’).
Facilitators described how their use of the manual changed over time and, in particular, with improved understanding of the participants they were working with. Several facilitators considered that some activities and concepts within the manual were not accessible for people with more advanced impairments:
I think the conceptual stuff around the importance of engaging with community . . . not sure that was taken on board by a lot of people.
Facilitator 08
[W]e had a couple [of participants] who were quite further on in their [dementia] journey and so I guess lots of the materials and lots of the discussion points and activities were really extremely difficult for them to do and engage in.
Facilitator 02
Most of the facilitators and supervisors suggested that some of the topics and supporting activities presented in the manualised intervention were repetitive or ‘dated’, which they felt made planning and preparing for sessions difficult. However, most described being able to adapt the resources:
[W]e found they [participants] wanted to do things more, they didn’t want to sit and discuss, they wanted to do things and in the manual there wasn’t a lot of doing for some of the topics.
Facilitator 05
Time commitment and workload management
All interviewed facilitators told us that the estimated time to undertake the intervention (i.e. 1 day per week) was inadequate and some were given extra time by their employers, particularly for the first intervention group. The time required reduced with experience of delivering the intervention, but it still remained in excess of 1 day:
[I]t’s been a good 2 days a week if not 2 and a bit days a week. With all the one to ones, the preparation, the actions required . . .
Facilitator 06
. . . [W]e had a bit of insight into what had worked for that first group and what had not. So we could be a little bit more canny about what we were trying to deliver . . . So yes it took less time second time around definitely. However, it still took a lot of time . . .
Facilitator 08
The intervention protocol stipulated that two facilitators were required to deliver the intervention. However, several facilitators said that the number of staff required was related to the size of the group, the needs of the individuals within each group and the type of activities being undertaken. They described accessing additional support (e.g. administrative support for preparing and printing materials, and extra staff/students to assist with out-of-venue activities):
Three [staff] as a minimum, but equally sometimes three was difficult particularly when we were going on outings. When we went on outings I felt that we needed probably at least four depending on the outing really.
Facilitator 06
Supervision
All facilitators appreciated having regular, flexible supervision sessions tailored to their experience and individual needs. Supervision was described as a safe space for reflection to raise issues or concerns and to ask questions:
I felt she [the supervisor] led us in the right direction especially in the beginning . . . so she was very helpful and I think that is what you just need, somebody who is not in the group, not immersed in the group but is there from an outside point?
Facilitator 05
Both facilitators and supervisors described how some groups were ‘complex’ and required more supervision. Three of the four interviewed supervisors described a ‘hands-on’ approach, which included attending a group session to meet participants to understand their needs and the group dynamics. The same three supervisors also speculated that participants may have felt reassured that facilitators had this senior support:
[I]t’s being aware of the individuals who are participating in the group and some of the issues that might come up in supervision about how that person presents . . . so getting a sense of their personalities.
Supervisor 04
The opportunity to have a mix of individual and group supervision was considered useful by all facilitators and supervisors. One facilitator said that individual supervision sessions were important early on to allow time and space to explore personal concerns. At other times, group supervision was preferred, as facilitators wanted to talk through the same issues. All facilitators and supervisors described finding the most beneficial ways of managing supervision.
Co-location of the supervisor and facilitator could have an impact on relationship building and easier access to ad hoc support:
I can’t think of anything unhelpful other than [we] worked part time and we work at different sites . . . So more logistical than anything.
Facilitator 08
In contrast to the benefits described by other interviewed staff, one facilitator did not feel that their supervisor understood their concerns because of differing attitudes to risk:
First I had a discussion with [supervisor] and she couldn’t understand why I was concerned, she just could not understand . . . she felt that the NHS was too risk averse and that was the main problem and it was a problem that we had to manage, basically.
Facilitator 06
Theme 2: ability to engage with the intervention
Eligibility
All facilitators and supervisors discussed concerns that the abilities and impairments that some participants presented with were different from what they had anticipated:
[A] lot of the training, I think had given us a picture of people who perhaps were more able and more at an earlier stage of dementia . . .
Facilitator 02
[I]n my head I thought we were going to end up with people who were newly diagnosed and quite cognitively intact in those with the mild dementia. That’s not how it was.
Facilitator 08
Challenges to participation recounted by facilitators included the impact of poor memory and cognitive capacity on ability to understand the intervention and enact and take forward learning:
The people who were too cognitively impaired to get anything much out of it other than it was a social activity thing.
Facilitator 08
One participant described observing this in fellow group members:
That is why I was a little bit disappointed with the group I think . . . I think it [dementia] was more advanced with the vast majority of them than it was with me, shall I say, and I found that there was quite a big gap.
Participant 03, individual
It was also observed by several of the participating supporters:
I believe in the moment she [participant] probably got a lot but she’s not taken it from the moment . . . I’ve heard the facilitators trying to enable it to be heard . . . I’ve observed it and they couldn’t have done a better job . . . but it still got missed.
Supporter 01, individual
However, interviewed participants described a range of positive experiences and outcomes, suggesting that they had some engagement.
All interviewed facilitators and supervisors raised how differing participant abilities made planning, organising and delivering the intervention complex. Adhering to the intervention ethos of self-management was particularly challenging. One facilitator described how attempting to meet the needs of the whole group could lessen the experience of individuals:
Because we were always holding back for those [participant’s] that couldn’t [participate] in other respects? Yes, the outings would have been better, the actual topics that came up would have been better and more fully explored for people.
Facilitator 08
Venue, transport and locality
Some facilitators described the challenges of identifying suitable, affordable community venues (as was intended) for the group sessions. Therefore, some interventions were hosted on NHS premises. One participant thought that this was a better approach:
[Y]ou also need a setting that is private because of the sensitive nature of the subject . . . personalised to the people who are taking part it in the group it has to be in a place where we all knew it was safer, rather than public.
Participant 11, individual
However, the same person also thought that use of NHS premises could be stigmatising. One facilitator talked about the benefits of using NHS premises for them, as well as for the participants:
. . . the groups ideally weren’t to be in a hospital setting but we’ve used one of our day rooms [community hospital] . . . people feel comfortable, they feel safe there . . . it’s been nice to know that we’ve got everything we need . . . if we were in the community renting somewhere, you feel a little bit lost because it’s not your familiar environment.
Facilitator 10
Some participants drove or were driven to the venue, whereas others used community or public transport. Some staff described providing and paying for taxis. Everyone interviewed talked, to some extent, about the importance of access to reliable and suitable transport:
Oh if I hadn’t [have] been driving I think I should’ve had to have pulled out altogether.
Participant 01, Individual
Transport has been an issue, there has been a few people . . . decided they would withdraw before the group started because of transport problems.
Facilitator 08
Participants who used community or public transport described several limitations, including poor availability, lack of ‘dementia-friendly’ trained drivers and poor service coverage in rural areas:
[N]ow I try and get the access bus to pick me up . . . if I want it for Friday, [I have to] ring up Monday morning or else you will not get it. I tried to get them to take me to [place not far away] ‘out the area’.
Participant 10, individual
This was echoed by facilitators:
The [participants] who were randomised to the group lived in smaller villages quite far out so that took some organising which we ended up doing through our voluntary services but there were a few times when they couldn’t provide the support.
Facilitator 05
Several facilitators described feeling responsible for assisting with transport, either by driving participants or helping to organise transport. The view was expressed that expecting participants to organise their own transport was difficult:
I’ve filled in some gaps where there’s been transport problems . . . which I know goes against the philosophy behind Journeying through Dementia, but I think in terms of helping people to maintain their attendance, I’ve seen no other option but to fill in the gaps.
Facilitator 08
One supervisor also acknowledged that attending the group with their relative could present logistical challenges for the carer, especially if they were working:
[T]hey’re having to give up a day’s work or to book an annual leave day or shift their working around to be able to be involved in the programme.
Supervisor 04
Several participants, participating supporters and facilitators expressed how the locality of the venue for group sessions was important. Local venues would have improved attendance at groups and out-of-venue activities, and also supported continuation post intervention. However, this was not possible for the majority because of the catchment area for recruitment:
The logistics were difficult because of where mum lived, it would have been easier if she had been closer as you said, but I still think it was important that she did it rather than not.
Supporter 01, individual
Several facilitators highlighted the benefits of developing knowledge of community resources for planning sessions and signposting:
[N]etworking has been brilliant. We’ve been able to get involved with different organisations . . . we’ve developed quite a few links by doing this group that we perhaps didn’t have before.
Facilitator 10
Group dynamics
All the interviewees spoke about the impact of group dynamics on their experience. Influencing factors included group size, gender balance, age range and stage of dementia of participants:
[A] smaller group for me it’s important, if you have a larger group, people may not share so much but if it’s a smaller group.
Participant 11, individual
[I]t was useful because in fact although there were one or two men there similar to myself . . . you do meet people that are not within your same sphere.
Participant 02, individual
[T]hey were a group that just naturally gelled, they all had lots in common, they all lived nearby . . . in the second group it was a complete contrast . . . they were from all over and they didn’t have as much in common.
Supervisor 02
Having common ground was important, but many participants also recognised that meeting people with different interests and experiences was valuable for sharing and learning:
[I]t did a terrific amount of good to you, to find that there was other people, you were not the only one . . . it wasn’t a frightening or sad thing when you found that there’s other people with you with sort of similar problems.
Participant 01, individual
I think that is important because people from different walks of life bring different experiences, everybody has got a different lifestyle and different life experience and we all react to things in a different way. So I think it was a good group.
Participant 11, individual
I think there was a few people within the group reflected on peoples’ different ways of approaching things. And if there hadn’t been the group they would have never seen life in that different perspective.
Supervisor 01
However, perceptions of two participants were that different personalities could lead to negative experiences:
[T]here were a couple of them that were ‘blah, blah, blah’ . . . I mean there was nothing wrong but they thought they were prime ministers sort of thing.
Participant 09, individual
[W]hen I went in through the door some of them would snub you, I mean there’s two sides to every story isn’t there with people, but I always talk to people whether they talk to me or not.
Participant 07, dyad
For one facilitator, participant attitudes were recalled as being a significant factor affecting group dynamics:
[T]he second group . . . were quite negative, like ‘how long have I got’ . . . and we wanted to address those things but not bring the whole group down . . . it was quite difficult to manage that . . . whereas the first group were ‘yes let’s do this’ quite proactive.
Facilitator 04
Several facilitators described the impact of carers taking part in groups:
Yes and no. Yes, because the supporter [knows] how they can be better at caring for them [participants] or if there’s anything that they’re doing that they can do differently at home. And no because I think they need that independence when they come to the group sessions. Sometimes having the carers there can stop them from saying things because they probably don’t want to upset them. It would be difficult for them to be more open about their experiences.
Facilitator 03
Out-of-venue activities
Most facilitators and participants talked positively about out-of-venue activities:
Oh it was better being out and about and seeing different things.
Participant 03, individual
[T]o be able to have the freedom to go out and about into the community with people, again that is something that we don’t ordinarily get to do within our service. So, I think that has been a real privilege.
Facilitator 09
However, there were caveats. For one participant, some of the out-of-venue activities lacked purpose:
We met there [garden centre] . . . and I thought, well what are we doing here? . . . there was nothing, I didn’t think organised for what it was . . . and I thought what a waste of an afternoon, to my knowledge, I couldn’t see the point of it.
Participant 06, individual
For one supervisor, the out-of-venue activities were the most challenging aspect of the intervention and they thought that training for this had been inadequate:
[W]hat wasn’t made clear at the outset were the outings, the expectations, how many you would have to do and the circumstances . . . and considerations before you carry them out. So it’s been those that have been probably the most challenging aspects of it.
Supervisor 03
Some facilitators and supervisors were concerned about the risks involved in accompanying people in the community and, in particular, organisational accountability for this. There was tension between duty of care to patients and the ‘self-management’ community ethos of the intervention:
We are professionals, we are entering into this with service users. We have a responsibility and a duty of care. We need to do things properly. We do need to risk assess because we’re in paid employment and we work for an organisation that would expect that of us . . . it was felt that the response [from the research team] was a little bit woolly.
Supervisor 03
One facilitator described how they investigated potential activities prior to suggesting them to identify risks and make decisions about whether or not to proceed. Using the first outing as a test of abilities of the group, as well as a learning tool, was raised by several facilitators. Facilitators from one site related undertaking formal risk assessments for out-of-venue activities.
Selection of and expectations of, out-of-venue activities had to be managed because of the physical as well as cognitive abilities of group members:
Because of the level of physical problems . . . it limited the range of the outings . . . until we got people out of there we didn’t realise how impaired they were both cognitively and physically.
Facilitator 08
There was still a couple of participants who were restless because they wanted to do more but we were almost having to tailor it for the slowest person.
Facilitator 06
Several facilitators perceived out-of-venue activities as a positive experience for participants:
But the outings have been absolutely, really vital parts of the group actually, a bonding session . . . after [the first outing], it just felt much more bonded because I suppose you’re in a social setting aren’t you and it’s a little bit more relaxed.
Facilitator 10
Out-of-venue activities held in dementia-friendly venues or as part of dementia-friendly services that included trained community staff were particularly well received by participants and improved the experience of the out-of-venue activity. Several supporters also expressed being reassured by this:
Oh that was great that was . . . again you were treated like somebody, you’re not patronised, you’re not treated like a cripple.
Participant 14, dyad (Singing in the Rain at the cinema)
And like at the leisure centre you’ve got somebody there [trainer] . . . who knows the limitations . . . of the dementia.
Supporter 09, dyad
One facilitator noted that some participants and some participating supporters preferred the safe routine of the weekly group meetings compared with the novelty of the out-of-venue activities:
[M]ainly it was the sort of anxiety of changing the routine. Even going on the out-of-venue activities it seemed like ‘do we have to go on an outing’ . . . I think they would have been quite happy to meet for 12 weeks in the room and do things in the room.
Facilitator 06
One-to-one sessions
Facilitators talked about how they had used the one-to-one sessions:
We used the individual sessions so, for the first group there were clearly some dynamics and we used the individual sessions to try and talk through how to support those particular individuals who are having difficulties and try to find a way forward.
Facilitator 09
All facilitators and supervisors described the challenges of delivering these sessions, with two facilitators expressing lack of clarity regarding purpose. The same two facilitators also said that, in their opinion, some participants did not understand the purpose of the sessions either:
I think when it was a matter of us going out to initiate something practical with them there was something tangible . . . whether they had any suggestions about the [goal-setting] . . . that was a little bit more abstract So difficult for them.
Facilitator 08
One participating supporter considered that the person they supported would have benefited from a direct approach, with clear expectations of actions between one-to-one sessions:
[I]t’s almost as if there is no expectation set . . . but I think they [participant] forget so quickly . . . if it’s an agreement that’s on the table, this is what you said you were going to do.
Supporter 01, individual
Two facilitators expressed feeling awkward when pursuing one-to-one sessions:
[A]nd it was so difficult because this gentleman did not want to do anything [in the one to ones], he was more than happy with the way things are and it got to the point where he was getting quite irritated with me trying to help him.
Facilitator 10
[S]ometimes it felt like I was trying to force a goal on them.
Facilitator 04
One participant suggested an alternative schedule of one-to-one sessions:
I still feel that the group finished and there’s a cut-off point, there is no, well look there’ll be a one-to-one session at the end after 3 months or 4 months to see how you’re doing, now that would have been more beneficial.
Participant 11, individual
All participants who recalled one-to-one sessions during interviews described them as an opportunity for relationship building and provision of support to meet their needs:
I valued those one-to-one sessions quite a lot because that was ‘me’ time with [facilitator] . . . that was really good, because I knew that was just me and her talking about the things that I felt uneasy about or something that I felt strongly about, yes so to me they were quite valuable sessions.
Participant 11, individual
Only to be almost like a normal person having a normal life and going out for a cup of coffee [with the facilitator] and not having to think of it as just Alzheimer’s.
Participant 13, individual
Several facilitators felt that one-to-one sessions were used by participants as an opportunity for conversation and socialisation rather than to pursue specific goals, and they considered this a positive outcome. One facilitator referred to the lack of resources within health care to provide time to listen:
I think a lot of them just enjoyed chatting to you . . . just having company which isn’t really what it’s meant for but they enjoy just having that chat, talking about themselves, having someone from the NHS listen to what they’re thinking. Because often they say ‘oh I go to my GP and I’ve got 10 minutes and nothing came of it’.
Facilitator 04
Several supervisors and facilitators described using the one-to-one sessions to consolidate observations from the group sessions or to signpost participants to new activities. In addition, they also talked about the ways in which one-to-one sessions could be used for managing the end of the intervention for themselves, as well as for the participant:
[I]t’s almost like a big thing for us in a way that the group is coming to an end, because you develop professional relationships with people . . . and obviously we want the best for them so, we’ve been conscious that things are going to draw to a close and used the individual sessions as well to talk about how they feel.
Facilitator 09
Although the majority of interviewed facilitators recognised the importance of goal-setting, this was frequently described as being challenging for participants to understand and undertake:
I didn’t get a goal from anyone, I mean most of them said I just want to see what it’s all about, which isn’t a goal . . . nobody actually said, ‘oh, this is really what I’d like to get out of this group’.
Facilitator 06
However, several facilitators also described how goals could be identified and explored:
[Q]uite a few participants have struggled to know what goals they would like to achieve, particularly in the first session . . . however, I have just reassured them . . . and on subsequent sessions, we’ve been able to nurture those ideas into tangible goals.
Facilitator 09
Facilitators and supervisors all talked about the impact that a supporter being present could have:
[S]ometimes we’ve looked at issues where the carer has been really helpful and allowed the individual to speak for themselves and other times where they’ve found it quite difficult to manage with the carer there because they’re tending to speak on behalf of the participant.
Supervisor 04
Intervention facilitation
Good relationships between co-facilitators, participants, facilitators and participants, and facilitators and supervisors, were described by all those interviewed as being vital for successful intervention delivery:
[W]e’ve [co-facilitators] got a good working relationship so we’re able to put our hands up to do certain bits and we recognise each other’s skill set and what’s worked well in previous groups. So we will bring those skills together.
Facilitator 09
One facilitator of a lower staffing grade acknowledged how they had needed more support than their professional trained colleagues to gain the confidence and skills to deliver the intervention.
All those interviewed, both staff and those in receipt of the intervention, provided illustrations of how the facilitator–participant relationship was a key factor in promoting participant engagement:
They [facilitators] were brilliant, they let you talk, they shut you up when they had to . . . they didn’t tell you what to do, they guided you, signposted you, they were brilliant. Yes I didn’t feel as if they were patronising me in any way.
Participant 14, dyad
They made the group feel safe to talk . . . Yes and a couple of people were able to express something about themselves because they must have felt safe.
Participant 11, individual
However, one participant described how, for them, poor facilitation negatively affected their experience:
I thought they [some group meetings] were rather boring because I don’t think the leaders put in any effort to run them.
Participant 13, individual
All the facilitators and supervisors talked about how they had broadened their skills or learned something new from their involvement (e.g. building confidence, expanding skills and challenging perceptions of people living with dementia). All facilitators proposed that this enabled them to deliver the intervention as intended.
Several facilitators and supervisors reflected on existing interventions commonly provided to people post dementia diagnosis. They described the JtD intervention as a more proactive and interactive intervention that offers professional input early, and supports enactment through out-of-venue activities and being more community focused:
I’m working in a situation we haven’t got OTs [occupational therapists] in at that earlier stage of someone’s journey and I really see the significance and the value. So for me it’s really reminded me of what difference some of this work can make on peoples’ journey in life and to be able to take some control over their own situation and what benefit that is.
Supervisor 01
So I think the group activities, to be able to have the freedom to go out and about into the community with people, again that is something that we don’t ordinarily get to do within our service.
Facilitator 09
Participant-facing communication
There were mixed views from both participants and participating supporters regarding the usefulness and accessibility of the intervention folder and paperwork given to participants throughout their attendance. Some participants remembered the intervention folder and said that they found it helpful, whereas others had no memory of it at all and/or had not used it. Most participating supporters said that they had at least looked at the folder contents to understand what the participant was doing and one participant described how the communications had kept them informed when they had missed sessions:
I missed two because we booked a holiday . . . I still got what we’d done each week, we got these little things [summary of each session] back saying what we’ve done and I even got the ones [session summaries] through when I’d been on holiday . . . so I missed nothing really.
Participant 09, individual
Two facilitators reflected on feedback they had received from participating supporters that suggested that at least some of the supporters were not as informed as they would like to be:
I think that the carers feel that they need to know more about the groups and what they actually did. Because I think the group members come out of the session and then they just don’t talk about it or what they’ve learnt from it.
Facilitator 03
A supporter and several participants proposed the value of shared information:
I could have shared it with [sister] and my brother-in-law and then there’s three of us that have the opportunity to say so, what about this, what about that.
Supporter 01, individual
But it was also what you [participating supporter] could hear there [at the group] you wouldn’t have known if you hadn’t been there, because me not remembering . . . that then gave us a reason to talk about it [the group] when we got home . . .
Participant 12, dyad
Theme 3: outcomes for participants
When asked if they thought attending the intervention had made a difference to their lifestyle, some participants indicated that they could not identify any tangible difference. This was despite almost all participants reporting some positive impact from taking part, including increased confidence, starting new activities and making friendships.
Confidence through knowledge and gaining perspective
Most participants talked about how they had gained confidence because of their involvement:
It [the group] brings things into perspective about the reality of life. See it wasn’t all about how bad my situation is, I’m going to lose my memory, I’m just going to lose it – no it was just a good thing, it was kind of building confidence not destroying people, but building them up. That there is life after this.
Participant 11, individual
This extended to reports from facilitators about participating supporters seeing the participant developing confidence and maintaining independence:
He actually had the confidence to go out and join new things by himself. He actually started doing a walking football group and going to a day centre every week. So I think she [supporter] was quite amazed at how he’d developed this confidence and been able to make relationships with people. So, I guess she was a bit more encouraged as well to support him in going out and trying new things.
Facilitator 02
The majority of participants described how they valued the opportunity to share experiences and knowledge, and learn about dementia and how other people live with dementia:
I think I got more information about the difference between Alzheimer’s and dementia . . . and it was interesting listening to the other people as well, how they felt about their diagnoses and how people reacted to it.
Participant 11, individual
However, one facilitator recounted how, for one participant, seeing others at different stages of the dementia journey was a negative experience:
I think the guy that withdrew he felt like he was too mild for the group because he had very recently been diagnosed and he was incredibly independent . . . I think he maybe found it a bit negative because he thought ‘oh wow am I going to get like this’.
Facilitator 04
Enjoyment, feeling valued or empowered
Participants talked about enjoying various activities, sharing this with others and bonding. Examples included ‘bat and chat’ (i.e. social table tennis), bowls, dominoes, quizzes, games and role play. Out-of-venue activities that were recounted included visits to local libraries, cinemas, theatres, leisure centres, steam railways, garden centres, National Trust properties, cafes and markets.
Several participants recalled taking part in out-of-venue activities that they would not have considered otherwise:
[T]he [National Trust property] I thought was something else. Somewhere I would never have gone to in my life because I thought ‘what do I want to go there for’. It was out of this world.
Participant 03, individual
Two interviewed participants expressed disappointment that the intervention had not been as they had expected:
I thought it was quite a flippant intervention and I expected it to be a bit more digging deep and it didn’t do that.
Participant 13, individual
I didn’t think that you could get anything really concrete out of what the questions came through [group meeting activities], we weren’t getting it, I didn’t think there was anything there at the end of it.
Participant 06, dyad
Intervention topics were generally selected week by week. Facilitators talked about how the time to forward plan for speakers to attend or to organise activities was very limited and could lead to disappointment:
The odd meeting she [participant] went to, I’d pick her up and she’d say ‘waste of time today’. They were supposed to do so and so and it didn’t happen . . . And that can be a bit disappointing.
Supporter 07, individual
Several participants talked about how the intervention had enabled them to contribute to their community. For example, one participant started volunteering at the local Alzheimer’s Society, undertaking accessibility checks of public venues to see if they were dementia friendly.
Several participants talked about how taking part in group discussions and one-to-one sessions had empowered them:
It’s making me a bit more doing it myself not just sitting in and letting everybody else do it.
Participant 09, individual
One participating supporter also reflected on this in the person they supported:
[T]he real positive thing is that having done that group he then decided he would go to respite care for a day . . . it made him decide that he would go somewhere, that was the really positive thing that came out of it. Having gone out [to JtD], you know we continued the conversation.
Supporter 02, individual
Activity, hobbies and skills
Most participants said that they had found out about and explored various groups or started new activities during their involvement in the intervention. Examples included Singing for the Brain (Alzeimer’s Society, London, UK), working with the Alzheimer’s Society, going swimming, bowls, walking, meditation, attending dementia-friendly theatre and cinema performances, and joining local support groups:
It’s quite interesting really, they woke me up in some ways. And there was people who used to read a lot, I used to do, I don’t read much now, I’ve been trying to start reading again.
Participant 09, individual
Several participating supporters also identified increased engagement in meaningful activity by the person with dementia they were caring for.
Learning ways to adapt activities was described as helpful by some. For example, one participant started using digital devices to do puzzles and jigsaws after being introduced to this during a group. Another shared how they used rowing machines at a local gym (that supports people living with dementia) as an alternative to open water rowing.
One participant described learning from seeing other people living with dementia being active:
I’ve learned how to swim again. I didn’t have the confidence to swim . . . Well it feels like you’ve achieved something again, something worthwhile. I thought these groups was going to be all sort of bingo and nostalgia . . . but when I went to the group and I met these people and they weren’t just talking . . . they were doing these things.
Participant 14, individual
One facilitator described participants continuing with activities post intervention:
[W]e know that a couple of people have kept up with the activity, you know one person has continued . . . to go swimming, table tennis . . . Another person has started something called Men in Sheds and . . . as far as I’m aware at the moment they’ve continued to do that so it has had I feel a positive impact on the participants.
Facilitator 10
However, successfully delivering an activity within the constraints of a group could be challenging, as discussed by one participant:
[T]hey had a ping pong set in the room and they only had it for a very limited time . . . you hardly had a game not a serious game . . . I would have thought more time for something like that, was very popular, could have been given to that.
Participant 13, individual
Social contact and friendships
Nearly all of the participants interviewed talked about friendships made during group sessions and how these relationships were important for attendance, group cohesion and bonding. Nearly all the participating supporters and several facilitators interviewed had similar observations:
You felt you were getting support there from each individual who was in the room.
Participant 06, individual
[S]he was looking forward to it, she enjoyed the company and the people. They got to become friends as such.
Supporter 03, individual
[T]he biggest thing that they’ve taken away from it is the social side . . . they feel a bit more comfortable and a bit more confident in themselves, with how they are because they’ve been around other people in the same situation?
Facilitator 01
One facilitator described how strong friendships between participants and participating supporters who had met at the group had led to them continuing to meet:
I do know however that so many couples from the first group are still meeting up once a week. They’ve made quite a strong friendship group and they now go to some of the Alzheimer’s groups together and meet up and then they meet up once a week at a little cafe in the town. So I’ve seen them out and about at different other venues.
Facilitator 05
Most participants described the immediate sense of loss of social contact or friendships once the intervention had finished, with two participants expressing disappointment that their expectations of continuing friendship had not been met:
I mean you know there was one or two that, you felt that they wanted to be a friend sort of thing . . . but that’s what made me not upset because we all have our own things, but that we haven’t kept in touch.
Participant 12, dyad
For one facilitator, this sense of loss was related to the group being focused on the ‘here and now’ and less so on the future. Participants described receiving no further contact from fellow group members, despite expressed intentions to continue meeting post intervention and having been given the tools to keep in touch with their group by the facilitators:
They [the group] all wanted to see each other again so, if they don’t I won’t believe it . . . because they were all so positive, they wanted it to happen and I think it will. It’s just a waiting game isn’t it? We’ve all got each other’s numbers.
Participant 03, individual
Fear of rejection, logistics or someone taking responsibility for facilitating the group or activity were all cited as reasons for possible lack of continuation:
I thought well I’m sitting here saying nobody’s done it to me [contacted], but I haven’t done it to anybody else either so . . . it’s a different environment though isn’t it, I think you feel safe if you’re in a group, rather than [contacting] somebody and perhaps get a negative [response].
Participant 12, dyad
One dyad (a participant and their participating supporter) considered that without the structure of a facilitated group then people would not be motivated to attend:
[P]ersonally I think that people would just drop out and not bother.
Participant 14, dyad
Several facilitators considered participating supporters or other carers to be a potential significant factor in participants being able to continue activities beyond the intervention, for example by organising or taking a lead:
I think it’s the ones where the supporters are keen in this group, they might keep in touch but if the supporters aren’t then they might not.
Facilitator 04
One participating supporter indicated how they had considered offering to manage future meetings post intervention. However, concerns over safety and taking responsibility had prevented them doing so:
I think leaving it to the last session, to really make people aware that it’s the last one is, I mean and I’m sure it was very clear that there were only so many but I really felt the people’s loss at the time.
Supporter 01, individual
One facilitator speculated that the length of the intervention may have created a dependency among participants and perhaps contributed to the sense of loss they perceived that group members had felt:
[P]eople have said in the group towards the end is a sense of loss and grief . . . and that doesn’t normally happen with our own groups which are about 6 weeks long. We wondered whether 12 weeks was building a dependency for these people?
Facilitator 08
Supporter engagement
Most facilitators and supervisors spoke of the potential benefit to participants of participating supporters or other carers attending the three identified group sessions. One facilitator reflected that they had received feedback from several participating supporters that although their relative was enjoying being involved, often they could not recall what they had done and therefore could not continue at home unaided. However, they also acknowledged how carer attendance could change the dynamics of a session:
So [participant] said I want to be able to use the bus, I didn’t know any of the background . . . I had to do all the groundwork and I came away thinking, is this really practical and that’s when I talked to the husband and he said you’re not doing it.
Facilitator 06
Several participants, their participating supporters and facilitators also suggested that participating supporter involvement could be instrumental in the participant attending the group or not. This was a challenge for those participants without a supporter:
Where they [participant] are by themselves with no supporter it’s a very different picture both on the one to ones and . . . actual attendance to the sessions.
Facilitator 08
Several participating supporters and facilitators described how attendance could offer tangible benefits to carers:
[I]f I hadn’t got friendly with these women and networked with them I wouldn’t have known anything about what we were entitled to . . . talking to them you learned something every week.
Supporter 09, dyad
One supervisor recognised the role that the participating supporters play as a carer, often to their spouse or parent, and the stress that this can cause the individual. They considered that this could present challenges for the facilitators when trying to meet the needs of the participant and the participating supporter or other carer in sessions when carers attended:
[Q]uite often I guess a carer will be a spouse and probably have their own health problems and trying to facilitate their needs as well in those carer sessions can sometimes prove quite difficult.
Supervisor 04
Chapter 6 Patient and public involvement
The involvement of people with dementia and their supporters has extended through all aspects of the JtD trial. It commenced with intervention development. 32 Those people involved with intervention development, who then participated in the subsequent feasibility study, were invited to give their views on how the intervention might be further developed. 25 For this study of intervention effectiveness, people with dementia and their supporters have participated as study advisors and as co-researchers.
Recruitment to patient and public involvement roles within the study
Recruitment to the JtD PPI Advisory Group was predominantly through a cohort of people affected by dementia (i.e. people with the diagnosis and current and past family carers) called Experts by Experience. 96 Experts by Experience are recruited and supported with their involvement in dementia research, education and training by the Centre for Applied Dementia Studies, University of Bradford, Bradford, UK. Some of these people have mild dementia, whereas others have more moderate disease. Several are accompanied by a carer, but others attend by themselves.
Through the member of staff who is responsible for recruiting to and supporting Experts by Experience, we were able to attract people with relevant experience of early-stage dementia to take part in different aspects of the study. Each potential volunteer was visited at home prior to taking part so that we could explain the study and what their involvement might entail. It was emphasised that they could choose to engage in as much or as little as they chose, so, for example, involvement in just one event would be valuable. A letter was then sent to each volunteer to confirm what had been discussed.
Principles of patient and public involvement
Our working principles were informed by NIHR INVOLVE,111 as well as standard working practices for participation of people with dementia established by the Centre of Applied Dementia Studies. Designated members of the study team took the lead for PPI activities, thereby ensuring consistency. All meetings were convened at accessible venues, meeting schedules were carefully thought through to enable participation and all written materials complied with dementia-friendly guidance, such as that recommended by the Dementia Engagement and Empowerment Project. 46
In line with national guidance,111 PPI members were paid £20 per hour in high street shopping vouchers to compensate them for their time spent in any form of PPI activity. All PPI activity was recorded and continuously updated, with PPI being a standing item in TMG meetings. Reporting of all PPI work for this study adheres to the Guidance for Reporting Involvement of Patients and the Public framework. 112
The patient and public involvement advisor role
Two such roles were identified by the TMC at the outset and were then immediately operationalised:
-
membership of the TSC and thereby advising on study governance
-
membership of an advisory group, with the deliberations of that group being relayed to the TMG by the study PPI lead.
Patient and public involvement within the Trial Steering Committee
Recruitment to the TSC did not meet agreed best practice, in that only one person with a diagnosis of early-onset dementia was recruited to take part. However, this person was highly able, sustained her involvement (attending every meeting) and made a significant contribution. All materials were sent to the PPI representative in hard copy (at her request) well in advance of each meeting. The majority of meetings were face to face in accessible venues. One meeting was held by video teleconference and a member of the study team went to the PPI representative’s home to support her with use of the technology.
The PPI representative contributed to all discussions. A key example of where a significant contribution was made pertained to retaining participants in the study. Suggestions included sending researcher photos with the follow-up appointment reminder letters and a personal message from the researcher to the person. She also advised that the trial include details about the value of being in the control arm or intervention arm, invite people to continue to provide input into the trial and to include a message to the carer about how important they are to the trial. Her feedback was combined with that received from the PPI Advisory Group and taken forward.
The Journeying through Dementia Patient and Public Involvement Advisory Group
The JtD PPI Advisory Group of five couples (i.e. people diagnosed with dementia and their care partners) was established and an inaugural meeting was organised in July 2017. Membership increased to a group of around 12 core members, which was maintained for the duration of the study, supported by members of the study team from the University of Bradford. As with all other long studies, the membership of the PPI Advisory Group altered over time because of other commitments, illness and the progression of dementia.
We convened meetings at regular intervals, roughly every 6–9 months, so that members could remain actively engaged in the study. Additionally, meetings were organised on an ad hoc basis to meet the needs of the study and, on occasion, individual members were also consulted about specific aspects for which we required guidance.
Examples of activities the advisory group engaged with are below.
The content and formatting of study newsletters intended for study participants
One suggestion was to produce the newsletter in a narrative rather than newspaper (i.e. columned) format, as people with dementia find reading across the page (rather than up and down) to be more accessible.
Formatting and content of study materials intended for participants
Feedback was sought on drafts of the recording sheets facilitators would be asked to complete and send to participants after each group session. They were also asked about the formatting and content of information sheets for potential recruits to the study.
How to retain participants at the 8- and 12-month follow-up phases of the study
Group discussions were stimulated through a small number of alternative scenarios of participant involvement in the study. Various suggestions were provided, including emphasising the contributions made by participants in both arms of the study in the participant newsletter. Members also suggested booking researcher visits in advance and face to face at a previous visit, so that follow-up visits would just need to be confirmed. This was included in study follow-up researcher guidance. The idea of sending a reminder card (rather than letter or sheet) before booking follow-up appointments was also proposed, as it was considered that a card would be bright, more visually appealing and less anxiety-provoking than formal letters or information sheets. A reminder card was subsequently designed, approved and used.
The accessibility of questions for qualitative interviews with participants
Members were asked for their feedback on how researchers could make the questions intended for the qualitative substudy with participants more relatable and accessible. As a consequence, the language and wording were changed to make them simpler and clearer. It was also suggested that giving participants prompts and reminders during the interview about what had taken place in group intervention sessions might help with recall. As a consequence, the researchers who observed group intervention sessions as part of the fidelity assessment noted down some of the group activities so that they might prompt recall at the later interviews.
Patient and public involvement members as co-researchers
The TMG identified the potential value of involving people with dementia in certain aspects of the qualitative analysis and, specifically, in the validation of researcher interpretation of quotes from the qualitative interviews with participants. This was inspired by the work of an external collaborator who subsequently advised the study team on how this might be achieved. 113,114 Endorsement for this work was obtained from the study TSC and DMEC before taking it forward.
The opportunity to engage with the study as co-researchers was advertised to people affected by dementia through the Experts by Experience group and the first workshop was organised in February 2019. As described above, best practice principles for PPI involvement were adhered to. This first workshop was followed by a second workshop in July 2019. The methods are described further above in Chapter 2, Co-researcher qualitative analysis validation workshop. Learning points from the first workshop were taken forward into the second workshop. The outputs from these workshops are incorporated into the qualitative substudy results (see Chapter 5).
Chapter 7 Discussion
Main findings
The primary aim of the trial was to determine the clinical effectiveness and cost-effectiveness of the JtD intervention, which was designed for people in the early stages of dementia. To meet this objective, we conducted a full pragmatic RCT, which involved a health economics evaluation substudy. Additionally, there was a fidelity assessment of intervention delivery and a qualitative substudy to explore participant and facilitator experiences.
The study was robustly conducted, delivering the intervention across 13 English sites and following up 388 participants at 8 months post randomisation. A total of 480 people (intervention, n = 241; usual care, n = 239) were randomised. There were 28 intervention groups across the 13 sites. The median number of participants attending each group session was five. Of the 241 participants randomised to the intervention, 165 participants attended at least 10 of the 16 available sessions (the per-protocol treatment threshold). Therefore, intervention attendance was good.
The fidelity assessment showed that the facilitator training was assessed as having excellent fidelity. Assessment of the group aspect of the intervention in four fidelity sites confirmed good intervention adherence [25 out of 35 (71%) participants attended ≥ 10 sessions and five (14%) participants attended all 16 sessions]. The facilitators maintained very good fidelity to the intervention during the observed group sessions. The findings of the qualitative study are discussed separately in Summary of qualitative findings.
Of those randomised, 388 participants provided primary outcome data for the ITT analysis, achieving the sample size target of 388 participants with available primary outcome data at 8 months. The mean score on the primary outcome (i.e. DEMQOL score at 8 months, where higher scores equate to higher health-related quality of life) was 93.3 (SD 13.0) in the intervention arm and 91.9 (SD 14.6) in the usual-care arm [a difference in means of 0.9 (95% CI –1.2 to 3.0) after adjustment for covariates]. The estimated mean difference was therefore small (0.9) and the 95% CI for this difference was below that defined as being clinically meaningful in the sample size calculation. Of the other eight secondary outcomes, the only difference was found in the DFS (for which a higher score represents more psychological resources and strengths), which was statistically significant in favour of the intervention group. At 12 months post randomisation, the mean DEMQOL score was 92.3 (SD 14.3) in the intervention group and 91.7 (SD 13.9) in the usual-care group [a difference in means of 0.4 (95% CI –1.6 to 2.5) after adjustment].
The adverse event rate was similar across the groups, with 17% (40/241) of participants in the intervention arm and 15% (35/239) in the control arm experiencing at least one SAE. All SAEs were considered as either unrelated or unlikely to be related to the intervention.
The health economic evaluation explored the cost-effectiveness of JtD intervention compared with the usual-care control treatment. The observed difference in QALYs between the JtD and usual-care groups was small (and non-significant) and favoured the control group (–0.003, 95% CI –0.044 to 0.038). In addition, the JtD intervention cost on average approximately £600 per patient (95% CI £105 to £1179) more than usual care. Overall, owing to the small effect size, the JtD intervention was dominated by usual care (i.e. being more costly and less effective) with an incremental cost per QALY of approximately –£203,000 (95% CI –£534,733 to £483,739), with results that covered all four quadrants of the cost-effectiveness plane.
Summary of qualitative findings
The professional (e.g. the occupational therapist) or organisational (e.g. NHS) approach to maintaining safety or managing risk at times sat uncomfortably with the participant-led ethos of the JtD intervention. For example, although enactment as part of out-of-venue activities was deemed valuable, how to implement this within an NHS culture was problematic. The current pattern of psychosocial services post dementia diagnosis tends to be that of short-term interventions within NHS premises or provision through the voluntary sector.
Staff involved in intervention delivery described the length and multilayered complexity of the intervention as being time-consuming and resource-hungry compared with other intervention options. Work experience was cited by facilitators as being a significant enabling factor for their confident delivery of the intervention. Although there was an intensive 2-day training package delivered to facilitators, further training was requested by some, specifically for delivery of out-of-venue activities and one-to-one sessions. An understanding of these components and how to grade and layer activities to be able to deliver them was lacking in some facilitators, which may have affected the participant experience. This research raises questions regarding whether or not staff are ready, skilled and able to deliver this form of community-based intervention. Regular supervision was considered helpful when managing groups with more complex needs. If implemented in practice, consideration should be given to supervisor role and responsibilities, particularly if the supervisor has not delivered the intervention previously.
Memory and abilities (i.e. physical and cognitive) are individual, and how some participants were more impaired was reported. The extent to which some participants fully understood the intervention was discussed, including how the impact of the intervention may have been limited to the ‘here and now’ for those with greater functional memory deficits. However, the population who were recruited to take part had mild symptoms of dementia, as determined by the screening tool. Therefore, who might benefit from this form of intervention may warrant further consideration and extend beyond assessed cognition to a range of other factors, for example whether or not they live alone or are socially isolated.
Accessibility and transport to group sessions was raised as an issue by all those interviewed and were perceived as being the greatest barriers to maintaining attendance at the group sessions and out-of-venue activities, particularly in the absence of supporter/carer or facilitator involvement. Questions remain regarding how such barriers can be used as rationales for other, less tangible, challenges. Resolution is unclear, given that a venue for group sessions will never be convenient for all participants because of the geographical spread of people diagnosed with dementia. In addition, NHS services are not able to provide transport for such interventions.
Diversity within groups was viewed as being a strength by some of those interviewed, supporting greater learning opportunities from a wider pool of experience. Conversely, it was also viewed as being a weakness by others, particularly when diversity was expressed in terms of varying cognitive abilities and could lead to visualisation of a path of decline.
The value of social contact was discussed by all participants. The intervention had enabled them to meet people and create connections. The group sessions provided a structure within which contacts could be retained. When this structure was gone, contact was lost and some participants described a sense of loss. This raised questions about sustainability beyond intervention delivery, although this was never an articulated goal of the programme.
Communications designed for participants to use as an aide memoire for both group and one-to-one sessions were helpful for some participants and supporters, but not utilised by others. Further consideration needs to be given to the structure, frequency and content of communications to ensure that they are useful for participants. Further consideration also needs to be given to the design of such communications so that they might assist with recall and enactment beyond the group sessions and intervention cessation. Training and encouraging facilitators to provide these communications is essential for success.
Despite the complexities of intervention delivery and the experienced limitations, participants described experiencing a range of positive outcomes as a result of taking part. Secondary to this was a sense of reassurance through commonality and acceptance they found in the group aspect of the intervention.
The JtD intervention was primarily designed for the person living with dementia, with only minimal involvement of supporters to support the participant journey. However, similar benefits experienced by the participants were also described by participating supporters, including friendships, sharing knowledge and gaining perspective through meeting other supporters/carers. Participating supporter attitude and their approach to perceived risk were factors that contributed to the participant experience of the intervention. Although not all participants had an identified participating supporter involved, many described the need for some support, particularly for transport and to scaffold memory. This research highlights the balance between supporter/carer engagement as a motivator for the participant, but also how supporter engagement can undermine participant independence in some instances.
Strengths and limitations
Strengths
Study design and implementation
A strength of this study is that it demonstrates that it is possible to undertake a large-scale, rigorously implemented, definitive trial of a complex intervention for dementia, involving people with dementia and supporters. The JtD trial was a fully powered pragmatic multicentre RCT that included a health economic evaluation, fidelity assessment and qualitative study. The trial recruited 519 participants (including supporters), making it, to the best of our knowledge, one of the largest psychosocial dementia trials in the UK. The trial met the calculated recruitment and retention targets, and many of the study processes successfully used in this study can be incorporated into the design of future trials in dementia. These include enhanced procedures to enable researchers to identify capacity to consent, assist with participant retention and follow-up, undertake data collection and draw on methods to address bias. The success of this trial in collecting a relatively large battery of outcome measures from participants with good completion rates up to 12 months post randomisation can be attributed to the support given to the research workforce, the content of which can be shared with other studies. Enhanced training was provided to all researchers, including non-clinical research staff, by the core research team. This was to support researchers to work in dementia-friendly ways and ensure that all data collection was conducted face to face with people with dementia, only using telephone calls for outcome measure completion by participating supporters if necessary and in very limited circumstances for participants.
People living with dementia (i.e. both people with a diagnosis and their supporters) were engaged in meaningful ways throughout the trial, for example, ensuring dementia-friendly feedback of trial progress and results to participants and supporters. It extended to contributions to qualitative analysis of participant interviews by providing interpretations from the perspectives of those living with dementia. This latter example of involvement is highly innovative.
Scientific rigour was maintained throughout to avoid bias, including the blinding of study staff involved in outcome assessment to group allocation and the blinding of study statisticians during study implementation. In addition, processes were used to identify (when unblinding occurred, this was recorded on a form) and address incidences of unblinding (e.g. where outcome assessors were unblinded, other staff were used for future follow-up time points).
The embedded fidelity and qualitative substudies have illuminated the possible processes and mechanisms that contributed to the quantitative results.
This study has facilitated the promulgation of higher-level skills in applied dementia research among the research community, which will benefit future trials.
Generalisability
The study recruited participants from a range of sources, including NHS services, dementia cafes and self-referral routes. All possible referral routes were exploited at every site (e.g. local voluntary sector services were approached for engagement). The majority of facilitators were working in NHS clinical practice within participating sites and were involved in the delivery of services to people with dementia in a variety of ways, therefore supporting the generalisability of findings to the NHS.
The study included substantial work to identify and describe the range of usual-care services within the participating trusts, enabling comparison of these services with the JtD intervention and thereby demonstrating a distinction between the services available to participants in the two arms of the trial.
The intervention
The JtD intervention was designed to include sessions without participating supporters present, in response to what people with dementia said would assist them to develop independence and well-being. It is one of the few interventions that people with dementia can participate in without supporters. Adherence to the intervention was high, with good attendance that met the specified per-protocol dose for the majority of participants.
Participant and public involvement
Patient and participant involvement was embedded in the study, and it influenced how the study was conducted and the associated study processes. The JtD PPI Advisory Group comprised people with a dementia diagnosis and their supporters or carers. The group advised on the presentation of all study materials for participants and on study events involving members of the public to ensure accessibility. There was strong PPI expertise on the TSC, which significantly contributed towards study governance. A particular strength and innovative feature of PPI involvement in this study was the workshops involving people with dementia and supporters in the analysis of qualitative data from study participants.
Limitations
Methods
A significant limitation of this study was that the primary outcome measure may not have been sufficiently sensitive to detect the changes in living well with dementia that people may experience. The DEMQOL measure was selected as the primary outcome in the absence of a measure of well-being for people with dementia. Some of the secondary outcome measures have not been validated in dementia populations and may be insensitive to differences or changes in the outcome over time or following a change in health status. This may explain some of the small differences observed between the randomised groups. The outcome measures were selected based on published empirical work,49,115,116 which also acknowledges the lack of available instruments to measure positive outcomes for people with dementia, such as well-being, self-management and positive attitude.
We overestimated how fast participants would be recruited at some sites, which was a particular issue in the light of the need to recruit sufficient individuals to establish a group from those allocated to the intervention. Group size was, therefore, smaller than anticipated, leading to the need to identify additional sites to deliver extra waves of intervention delivery. Consequently, a funded study extension was required.
The processes for collecting outcome measures from participants entailed home visits (at least three per participant, but on occasion more than that), which were resource intensive. The need to undertake face-to-face data collection led to the extension of the time frame within which 8-month data collections were conducted. Twelve-month follow-up appointments were missed for 13 participants.
There were a small number of treatment allocation errors that resulted in participants randomised to the control arm being offered and attending the intervention. However, sensitivity analyses demonstrated that this did not affect the study results.
Bias
The JtD trial included comprehensive systems to maintain blinding for research staff involved in outcome assessment, but some unblinding was unavoidable and perhaps more likely to occur than in other clinical trials. All incidents of unblinding were documented and the majority were dealt with by sending a new researcher to any subsequent visits to collect outcome measures. Nevertheless, during 115 of 740 outcome measure visits an outcome assessor thought that they had potentially been unblinded during the visit (note that there could be two assessors at one visit). Additionally, assessors who had reported that they were previously unblinded during an outcome assessment (24 instances) could go on to conduct a further outcome assessment with these participants, for pragmatic reasons. Although this seems high, it is important to consider that (1) in > 10% of cases, the outcome assessor was incorrect as to which arm they thought the participant was in and most commonly they thought that participants on the intervention arm were on the control arm; (2) in a further 9% of cases, the outcome assessor was unsure if they had been unblinded; and (3) the risk of detection bias was low, as none of the scales at the follow-up appointments included subjective observations or measurements, and the participants were always asked to rate the answers to set questions and showed scales on paper on which to do this. Overall, the effect of detection bias on the outcome measures should have been low. Any bias that may have resulted from positive conversations about the intervention taking place clearly did not have a large effect on the outcomes, given the results of the trial.
There was some potential for facilitator bias, despite rigorous efforts to eliminate it. At some sites, facilitators trained in the JtD trial may also have been engaged in delivery of other interventions delivered as part as usual care.
Intervention
Facilitator training had to be adapted from the original plan for practical purposes (e.g. telephone-based training was used for facilitators who joined later in the study, in addition to face-to-face training). Although this meant that facilitator training was not uniform throughout the study and across sites, it did reflect a realistic model for delivery in the NHS. It also enabled reconsideration of the formats in which the necessary training might be effectively provided.
Analysis of usual-care pathways provided by trusts and 8-month service use information indicated that other dementia support groups were being offered to and attended by JtD trial participants. In addition, health-care professionals were visiting participants’ homes for separate appointments. However, in the majority of cases, the types of groups offered (e.g. CST, open-ended memory and other support groups for people diagnosed with dementia) were substantially different from the design and content of the JtD intervention, and so overlap between interventions was unlikely. Although three NHS trusts reported offering Living Well with Dementia groups, which may (or may not) have contained some similar content to the JtD intervention, at 8 months, only one JtD trial participant reported attending this type of group, indicating that they were not widely available. We have no evidence that the other groups that participants attended included the mix of group and one-to-one sessions or enactment of learnt skills in the community that constituted the JtD intervention. Although participants reported having appointments with varied health-care professionals in their homes, instances of this occurring were few (i.e. 6% of the community-based services reported). Owing to the variation in dementia services offered across the UK, pragmatic clinical trials of interventions such as this will inevitably face the possibility of a very small minority of participants potentially attending or receiving similar forms of care and support at any one time.
Generalisability
The study involved sites across the Midlands and the north of England, which may be a geographical limitation. However, the sites reflected a wide range of services and the diversity of usual care they provided was recorded. Nevertheless, the demographic profile of the study population was restricted in ethnic diversity, which could also have implications for generalisability of the findings. It is also notable that those who were most likely to attend ≥ 10 intervention sessions were male participants with co-resident participant supporters. This is unlikely to differ from what occurs in routine service delivery.
Limitations of the health economics approach
There is evidence that generic preference-based health-related quality-of-life measures, such as the EQ-5D, may not be sensitive to changes in health-related quality of life in people with dementia. 53,117–119 However, we also included a dementia-specific preference-based measure, the DEMQOL-U, as a sensitivity analysis, which did increase the QALY deficit from the JtD intervention, although the JtD intervention remained more costly and less effective.
Strengths and limitations of the qualitative approach
The researchers who conducted the interviews observed a number of group meetings as part of the fidelity assessment, which were the same meetings attended by the participants interviewed, thereby providing consistency in approach. The interviewers were, therefore, better placed to probe during interviews to maximise engagement, using knowledge of activities from these observed groups. This was also the case when supporters were present during dyad interviews. Some of the participants and supporters recognised the researchers from the observations, which also created a more relaxed environment for the interviews as the researchers were already known to them. When possible, we encouraged separate interviews with participants and supporters, and the majority seemed happy to do this. When we did have dyads interviewed, this was at the request of the participant and was based on saving the interviewer time, the participant wanting reassurance or couples expressing that they were a ‘partnership’.
Significant challenges in planning and conducting interviews with people living with dementia are the lack of abstract thought, difficulty following sentences and recall due to memory problems, both short and long term. The JtD PPI Advisory Group (i.e. people with lived experience of dementia) was consulted on the language and content of the interviews and the approaches that might be used to aid recall, and we followed its advice to make the interviews as accessible as possible for people living with dementia. Interviews were planned to be around 30 minutes in duration, but some interviews took ≥ 45 minutes. Although none of the participants asked to stop an interview, fatigue could have been an issue for some, which can further impair memory. The time lag between attendance at the intervention and taking part in the subsequent interviews may also have contributed to poor recall. Therefore, the findings should be read and interpreted in the light of this.
Important components of the intervention were the out-of-venue activities and one-to-one sessions. It was not possible to observe these components because of practicalities, methods and the comfort of participants. Therefore, we were not able to prompt and aid recall of these aspects in the way that we were able for the group sessions. This may have limited the discussion of the receipt of these elements of the intervention.
We involved people living with dementia as co-researchers in the analysis of the qualitative data to help interpret findings from the participant and supporter interviews and thereby validate and enhance the analyses conducted by researchers. This was helpful when people living with dementia were still cognitively able to understand and engage with the task presented, which involved commenting on individual quotations taken from the interviews. However, when people had more severe capacity issues, it proved more difficult for them to engage and contribute in a meaningful way.
Strengths and limitations of the fidelity assessment approach
To support reliability and validity of our findings regarding delivery of the intervention, we incorporated data from multiple sources, including independent observers (i.e. researchers) and from a novel perspective (i.e. that of self-report by facilitators and supervisors at each of the sites selected for fidelity assessment). However, presence of the observers at group meetings and the direct involvement of facilitators and supervisors in the fidelity assessment by asking them to self report may have led to heightened awareness and wanting to please the research team. Whether or not the delivery at the four fidelity sites was reflective of all the sites that took part in the trial might, therefore, be questioned.
Evaluation of the integrity of the intervention required a comprehensive and detailed understanding of the manualised intervention and facilitator training programme to maximise impact in delivery and receipt of the intervention for the fidelity assessment, which also had to be objective. Members of the research team (CC and GM) had extensive experience through developing and testing the intervention prior to the trial but did not participate in any aspect of the fidelity assessment.
The observation checklists used for fidelity assessment were formulated based on the underpinning ethos of the intervention, as well as the materials designed to deliver the intervention (e.g. training materials, the manual and other resources). Whether or not such tools are sensitive enough to identify the complex constructs within this type of intervention and the existence, or alternatively absence, of mechanisms for behaviour change needs further exploration.
Although observations were made for one group at two different time points at each of the four fidelity sites, observing only one group at each site may have resulted in the researchers missing potential facilitator drift. Observing multiple groups would have addressed but would have required additional resources.
Implementing the trial
Recruitment and retention of participants
The challenges of recruiting to dementia research studies are beginning to be documented. 120 Overall, recruitment to the JtD trial was successful in that the number of required participants was achieved. However, the need to recruit to a group-based intervention and slower than expected recruitment meant that the recruitment period and number of recruiting sites was extended. A mixed toolkit of methods was used to recruit participants, for example, recruitment via secondary care, primary care, the JDR database, third-sector organisations and general promotion at sites. The majority of recruits came from referrals from secondary care, identifying participants via the JDR database and visiting groups such as dementia cafes run by third-sector organisations. Mailouts from general practices were also run successfully at many sites in conjunction with the NIHR Clinical Research Network. The lessons learnt from recruiting to the JtD trial will be disseminated to add to the existing body of knowledge.
In terms of retention, during the of summer 2018, the study team identified that the active withdrawal rate was higher than anticipated, at > 20% of participants at the primary outcome measure time point of 8 months. The trial sample size calculation was based on an expected withdrawal rate of 20%, and so it was critical that the active withdrawal rate be brought below this figure. In consultation with study sites, the TMG, TSC and PPI representatives, the study put in place the following four measures to attempt to reduce the withdrawal rate.
-
Continuity with who was contacting and visiting participants (e.g. those who recruited the participant should also attend further visits, if possible).
-
Purposely designed participant correspondence that was more visually appealing and less anxiety-provoking compared with formal letters or information sheets (e.g. a new document was an appointment card, rather than a letter).
-
An updated script was produced for researchers to follow when calling participants. The script was less formal and emphasised the value of participant contribution to the overall study.
-
A limited number of measures were approved to be collected over the telephone, as a last resort, to capture outcome information when the participant seemed likely to withdraw.
After putting these measures into place, the study team observed a continuous drop in the withdrawal rate to 19.2% by the end of the study.
Delivery of the intervention
The study provided strong support to facilitators and supervisors to equip and support them to deliver the intervention. This included training, ongoing feedback on intervention delivery and provision of timely responses to any issues raised. Some difficulties did arise because of an unavoidable gap between training and intervention delivery for some facilitators. However, this was, in many cases, addressed with refresher training provided to sites before intervention delivery commenced.
Resultant findings regarding intervention delivery demonstrate how well this complex intervention can be delivered in practice and, alternatively, the ways in which it is readily eroded, as illustrated by the facilitator records of intervention delivery. It raises questions about workforce readiness to work with people with dementia post diagnosis in a meaningful way. Issues that might militate against optimum delivery included lack of experience of working with people with dementia, an overmedicalised approach and facilitator perceptions of risk.
The difficulty that facilitators appeared to have in creating meaningful written materials for people with dementia is significant and worthy of concern. The findings raise questions about the nature of dementia education that the workforce might benefit from, both those who were more experienced and those without previous experience of working with people with dementia. The findings also question the ability of the existing workforce to deliver all aspects of the intervention as intended and, in particular, the more innovative components that involved taking action in partnership with the person with dementia, rather than limiting sessions to discussion and verbal support. This is important given the proliferation of such programmes for people with dementia and also for those with other long-term conditions.
The trial in context
Emergent findings from a large longitudinal study of people with dementia and their carers to determine the factors that can promote living well with the condition include promoting an integrated approach to the exploration of living well with dementia. 121 Our selection of outcome measures for this trial aimed to reflect living well in the absence of a specific measure of well-being.
The mean baseline DEMQOL scores among participants in the JtD trial, although apparently high, was similar to other studies. The mean baseline DEMQOL score of 90.6 (SD 13.1) in our sample was similar to the mean DEMQOL score observed in the original study used to develop the DEMQOL50 (mean 91.2, SD 11.1) and the baseline DEMQOL score in the Goal-oriented cognitive Rehabilitation in Early-stage Alzheimer’s and related dementias: multicentre single-blind randomised controlled Trial122,123 (GREAT) (mean 92.3, SD 12.3). These two studies included patients with mild to moderate dementia, similar to those recruited for this study.
Not all reported randomised controlled clinical trials of psychosocial interventions for people living with dementia have measured quality-of-life scores. Until recently, the primary outcome for such studies tended to be activities of daily living, as for pharmacological studies. Three dementia trials and one feasibility study,123–126 however, have used various quality-of-life measures, including DEMQOL, a quality-of-life scale for older French people (i.e. the 15D, a 15-dimensional self-administered instrument) and the Quality of Life in Alzheimer’s Disease scale. None of these found a significant improvement in the intervention group and none reported a decrease in scores. 123–126 Therefore, the findings from the JtD trial are consistent with results from other trials in terms of quality-of-life scores for people with dementia.
One trial124 reported a positive change in the quality of life of spouses. Participants in the intervention arm of the Laakkonen et al. 124 trial received 8 weeks of self-management group rehabilitation. Separate group rehabilitation sessions were offered concurrently to the spouses. When measured at 3 months (it is assumed that this means post baseline, as this is not explained) using the 36-Item Short Form Health Survey (SF-36) measure of quality of life, spouses showed a significant difference of 1.0 on the physical component summary part of the questionnaire, compared with the control group. 124 The difference may be linked to the spouses also receiving group sessions, but higher quality-of-life scores were not replicated at the 9-month follow-up point.
The JtD trial found a very slight statistically significant adjusted negative mean difference of –0.06 in quality of life of spouses of those receiving the intervention compared with usual care, measured by the EQ-5D-5L crosswalk value index. The REMiniscence groups for people with dementia and their family CAREgivers (REMCARE) trial similarly found a measured increase in caregiver stress. 127 The impact of changes to established routines and the additional demands arising from the engagement of the person they support in an additional intervention such as the JtD intervention can have an adverse consequence. The GREAT123 reported no significant difference for EQ-5D-3L scores or World Health Organization Quality of Life-BREF scores of carers between study arms at any time point. 123 The difference may be that cognitive rehabilitation in GREAT was delivered in people’s homes, whereas the JtD and REMCARE interventions included group sessions conducted outside the home.
Few relevant UK trials, reported at the time of writing, have included any of the secondary outcome measures used in the JtD trial. One exception was GREAT,122,123 which included use of the GSE. Similarly to the JtD trial, GREAT also reported no difference in GSE scores between study arms. 27 A small pilot RCT of an 8-week self-management group intervention for people with early-stage dementia used the GSE as the primary outcome measure and found a small difference in mean scores of 0.35 in favour of the intervention arm at 3 months; however, this was not statistically significant. 128
A feasibility trial of the PRIDE (Promoting Independence in Dementia) intervention in the same population of people with mild dementia129 used three of the same outcome measures as the JtD trial. The PRIDE trial used the DEMQOL, EQ-5D-5L and SMAS as secondary outcome measures. Results were not available at the time of publication.
Although no relevant clinical trials have reported an improved quality-of-life score, several trials have reported a reduction in cognitive decline or functional ability in people with dementia who receive an intervention than those in control or other groups who do not receive an intervention. These aspects were not measured on the JtD trial. The ETNA3 trial reported that individualised cognitive rehabilitation led to better functional ability. 125 A trial by Laakkonen et al. 124 found that cognitive decline (measured by the clock-drawing test) and verbal fluency had not declined as fast in participants in the intervention arm. However, a critique of this trial by Mountain130 identified significant limitations, including the need to accurately record the level of impairment of participants and the selection of appropriate outcome measures.
A systematic review of psychosocial interventions has also revealed that group activities could be important for improving social integration for people with dementia, therefore measures of social integration could have been included in the JtD trial. 131
As far as is known, no other relevant psychosocial interventions have been seen to be cost-effective when measured using the DEMQOL-U. GREAT reported that the cost per QALY using the DEMQOL-U was unlikely to be cost-effective, which matches our findings on cost-effectiveness of the JtD intervention. 123 However, GREAT reported that when considering willingness to pay, cognitive rehabilitation could be cost-effective from the point of view of Bangor Goal-Setting Interview scores. 123 Overall, the GREAT intervention average cost per participant was £1736, and so was considerably more expensive than the JtD intervention. 123 The self-management groups in the Laakkonen et al. 124 trial cost –€436 (–£394) per person per year and were seen as cost neutral.
Graff et al. 132 compared a community intervention programme with usual care in the Netherlands over a 3-month period. The authors found an intervention cost of £848 per participant, which is comparable to the JtD intervention. However, their study was over a shorter 3-month time frame and was from a societal rather than health-care provider perspective, and so is not comparable with our study. In addition, a US study that evaluated an 8-week occupational therapy intervention presented costs per day for the intervention of US$941.43 (i.e. £729.43). 133 This study measured effects in terms of the time care givers were able to spend doing usual activities; however, owing to differences between the US and UK health-care systems, it is difficult to compare it with the results of our trial. 133
Findings from the qualitative study of GREAT123 echoed our findings in three aspects. First, therapists in GREAT123 divided participants into groups of ‘lower and higher cognitive and functional ability’ and tailored the way in which they delivered the intervention accordingly. A key finding of the JtD trial was that a wide spectrum of abilities in the groups made delivery of the group aspect of the intervention complex and may have led to some participants having less positive experiences. Second, participants in the GREAT qualitative study were disappointed that they did not see improvements in memory as a consequence of attending the group. 123 The JtD trial findings also showed that some participants had higher expectations than the intervention was able to deliver in terms of treatment and, in particular, improvements in cognition. Third, the GREAT123 qualitative findings showed that there were reported positive outcomes for participants, including improved confidence, empowerment, anxiety reduction and well-being improvement, alongside a desire for social contact and support. People receiving the JtD intervention also reported a range of positive outcomes, including all of those mentioned by GREAT,123 plus an increased knowledge of dementia and resources available for them in the community.
Meaning and implications of the study
For clinicians and practice
This multicomponent intervention, which was designed taking into account what people with dementia said would be helpful post diagnosis, proved too demanding for existing services to deliver. Some of the complexity expressed by intervention facilitators and supervisors could be attributed to the challenges of responding to the diversity of needs that people with dementia present with, which can be amplified in a group setting. Furthermore, the complexity of dementia, the diversity of populations living with dementia and the additional comorbidities that people often experience means that it is unlikely that there will ever be one intervention that is suitable for all, and tailoring to individual needs must be a key component. Despite this intervention being designed primarily for people with the diagnosis, participating supporters also articulated receiving benefit from being involved and, in particular, from meeting others in the same situation.
For policy-makers
How to provide the best treatments, care and support for people diagnosed with dementia is not resolved and requires continuing investment in services and in research, with this being supported by robust, evidence informed policy frameworks.
For society
Societal awareness of people living with dementia is increasing and positive messages about how to live as well as possible with the condition are helpful. The JtD intervention aimed to enable people to live well through practical strategies to support self-management and continued participation in life. Even if the intervention cannot be delivered as intended through services, society should seek to support people in these ways so that they can live a meaningful life.
For researchers and academics
It is important for researchers involved in all forms of dementia research to be transparent when reporting the processes and findings of studies, for example, the challenges of recruitment and how they might be overcome; whether or not designed interventions meet requirements and can be delivered as intended; and how we can develop the science of outcome measurement so that instruments to measure positive effects are validated for use with people with dementia and do not rely solely upon recall. As a priority, the research community needs to interrogate the reasons for almost all studies of interventions designed for people with dementia (both pharmacological and psychosocial) reporting similar neutral results to the findings produced by this trial (see Recommendations for future research).
Economic implications
The economic results are clear. The intervention delivered in its entirety is unlikely to be cost-effective. This finding is set against a backdrop of uncertain cost-effectiveness for the majority, if not all, psychosocial interventions delivered through existing services.
On services and usual care
We recommend that elements of the JtD intervention and, in particular, opportunities for people with dementia to practise new and neglected skills should be adopted by clinical practice and provided to individuals who might benefit. Given the additional demands of the group aspect of the intervention, rather than trying to manage the challenges of delivering this intervention in a group setting, certain elements intended to be delivered through the group could be provided on a one-to-one basis. However, it must be acknowledged that participants described benefiting from the friendships and camaraderie provided through the group sessions. Social care and third-sector organisations should be encouraged to provide this form of ongoing support for people in the earlier stages of dementia and separate groups for those supporting them (i.e. their carers).
Recommendations for future research
Four recommendations for future research are outlined below:
-
To undertake a detailed analysis of the methodologies employed for similar studies to identify the reasons for poor overall success and make recommendations for future programmes. Reasons may include populations recruited and speed of recruitment; the match between the intervention and what people want and need, and also what can be reasonably delivered through existing services; limitations of the measures applied in such studies; and overall study design. Each of these factors warrants scientific interrogation.
-
To create a validated, dementia-specific measure of well-being that will enable measurement of interventions created to support living well with dementia in the manner described in current policy. 15
-
To use the data collected through the JtD trial to inform other studies (e.g. further work on development of outcome measures that reflect the ability of individuals).
-
This study examined the cost-effectiveness of the JtD intervention from an NHS and social care perspective. However, it is recognised that there are some costs that are borne by people with dementia and their families, including community services, informal care, and travelling to and from outpatient appointments, hospital appointments and other appointments. A further study could account for these resources by taking a perspective wider than the NHS. Furthermore, the impact of dementia on family and carers has not been included in the quality-of-life estimates and future studies could explore this further.
Conclusions
This study was robustly conducted and executed. The results are therefore reliable. What is needed now is a concerted effort to identify the future direction of psychosocial dementia research and from this the interventions that people at different stages of the dementia trajectory might benefit from. People with dementia deserve to benefit from high-quality services following diagnosis. We must, therefore, seize the opportunity to undertake further work to determine what this might be and how it might be most accurately recorded.
Acknowledgements
We are grateful to Clare Mason, who acted as PPI lead on the study, and the members of the JtD PPI Advisory Group and Experts by Experience group (based at the University of Bradford). Additionally, we would like to thank Nicholas Bell, Sheffield Health and Social Care NHS Foundation Trust, for acting on behalf of the sponsor and advising on trial procedures. We would also like to thank Jasper Palmier-Claus, Division of Health Research, Lancaster University, for conducting research supervision on the project while Katherine Berry was unavailable.
We would like to thank the following people for assisting with the original project application: Martin Orrell, Institute of Mental Health, The University of Nottingham; Daniel Blackburn, Sheffield Institute for Translational Neuroscience, The University of Sheffield; and Diana Papaioannou, Sheffield CTRU, School of Health and Related Research, The University of Sheffield.
Finally, we would like to thank all of the study participants, and the committed team of researchers and administrative staff across the participating universities and trusts who made this research possible. In particular, we would like to thank Zoe Furniss, Trial Support Officer, Sheffield CTRU, for expert and continued support of the trial. University research assistants who played a pivotal part in data collection and trial work but who are not listed as co-authors include Shaz Majid, Faye Shelton, Kate Thresher, Lucy Musson, Holly Griffiths, Alisha Patel, Amy Pearce and Michelle Drury.
Contributions of authors
Gail Mountain (https://orcid.org/0000-0002-5417-7691) (Professor of Applied Dementia Research, chief investigator) initially wrote the abstract, Chapter 1 and much of Chapter 7 and extensively reviewed the full report.
Jessica Wright (https://orcid.org/0000-0002-1814-3697) (Research Associate, trial manager) wrote parts of all chapters and extensively copy-edited and reviewed the full report.
Cindy L Cooper (https://orcid.org/0000-0002-2995-5447) (Director of Sheffield CTRU, CTRU lead) wrote part of Chapter 7 and extensively reviewed the full report.
Ellen Lee (https://orcid.org/0000-0003-4529-7410) (Medical Statistician, trial statistician) wrote sections on statistical methods and results in Chapters 2 and 3 and reviewed the full report.
Kirsty Sprange (https://orcid.org/0000-0001-6443-7242) (Assistant Professor of Clinical Trials, trial fidelity and qualitative lead) co-wrote the sections on fidelity and the qualitative study in Chapters 2, 3 and 7 and reviewed the full report.
Jules Beresford-Dent (https://orcid.org/0000-0002-3316-2191) (Research Assistant, trial fidelity and qualitative researcher) co-wrote the sections on fidelity and the qualitative study in Chapters 2, 3 and 7 and reviewed the full report.
Tracey Young (https://orcid.org/0000-0001-8467-0471) (Health Economist, lead health economist) wrote the sections on health economics in Chapters 2, 3 and 7 and reviewed the full report.
Stephen Walters (https://orcid.org/0000-0001-9000-8126) (Professor of Medical Statistics and Clinical Trials, lead trial statistician) extensively reviewed sections on statistical methods, results, health economics and many other parts of the report.
Katherine Berry (https://orcid.org/0000-0002-7399-5462) (Senior Clinical Lecturer, research supervisor) extensively reviewed sections of the report, including Chapter 1, and reviewed the full report.
Tom Dening (https://orcid.org/0000-0003-3387-4241) (Professor of Dementia Research, co-applicant) contributed to the discussion and interpretation of the results and extensively reviewed the full report.
Amanda Loban (https://orcid.org/0000-0001-7089-1580) (Head of Data Management, data manager) provided data for the report and reviewed the full report.
Emily Turton (https://orcid.org/0000-0001-5763-9604) (Data Management Officer, data manager) provided data for the report and reviewed the full report.
Benjamin D Thomas (https://orcid.org/0000-0002-6659-6930) (Research Assistant) co-wrote sections of Chapter 2, co-wrote sections on usual care in Chapter 3 and reviewed the full report.
Emma L Young (https://orcid.org/0000-0001-9797-0123) (Research Assistant) wrote the participant identification and recruitment section of Chapter 2, compiled information on other studies and results and reviewed the full report.
Benjamin J Thompson (https://orcid.org/0000-0002-5516-8797) (Research Assistant) co-wrote sections on outcome measures, blinding, baseline and follow-up visits, ethics arrangements and regulatory approvals in Chapter 2 and reviewed the full report.
Bethany Crawford (https://orcid.org/0000-0003-4549-3948) (Research Assistant) co-wrote sections on study oversight, randomisation and data collection in Chapter 2 and reviewed the full report.
Claire Craig (https://orcid.org/0000-0002-3475-3292) (Reader in Design and Creative Practice in Health, designer of the intervention and co-applicant) extensively reviewed the intervention description in Chapter 1 and reviewed the full report.
Peter Bowie (https://orcid.org/0000-0002-3022-2275) (Consultant in Old Age Psychiatry, co-applicant) contributed to the discussion and interpretation of the results in Chapter 7 and reviewed the full report.
Esme Moniz-Cook (https://orcid.org/0000-0002-7232-4632) (Professor of Psychology Ageing and Dementia Care Research, co-applicant) contributed to the discussion and interpretation of the results in Chapter 7 and reviewed the full report.
Alexis Foster (https://orcid.org/0000-0002-7978-2791) (NIHR Doctoral Research Fellow, initial trial manager) drafted initial methods sections in the trial protocol and extensively reviewed the full report.
Publications
Wright J, Foster A, Cooper C, Sprange K, Walters S, Berry K, et al. Study protocol for a randomised controlled trial assessing the clinical and cost-effectiveness of the Journeying through Dementia (JtD) intervention compared to usual care. BMJ Open 2019;9:e029207.
Mountain GA, Cooper CL, Wright J, Walters SJ, Lee E, et al. The Journeying through Dementia psychosocial intervention versus usual care study: a single-blind, parallel group, phase 3 trial. Lancet Healthy Longevity 2022;3:e276–85.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- World Health Organization . Global Action Plan on the Public Health Response to Dementia 2017–2025 2017. www.who.int/mental_health/neurology/dementia/action_plan_2017_2025/en/ (accessed 16 December 2019).
- Wimo A, Guerchet M, Ali GC, Wu YT, Prina AM, Winblad B, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 2017;13:1-7. https://doi.org/10.1016/j.jalz.2016.07.150.
- McCrone P, Dhanasiri S, Patel A, Knapp M, Lawton-Smith S. Paying the Price: The Cost of Mental Health Care in England to 2026 2008. www.kingsfund.org.uk/sites/default/files/Paying-the-Price-the-cost-of-mental-health-care-England-2026-McCrone-Dhanasiri-Patel-Knapp-Lawton-Smith-Kings-Fund-May-2008_0.pdf (accessed 26 September 2018).
- Alzheimer’s Research UK. Prevalence by Age in the UK 2018. www.dementiastatistics.org/statistics/prevalence-by-age-in-the-uk/ (accessed 7 October 2019).
- Alzheimer’s Research UK. Numbers of People in the UK 2018. www.dementiastatistics.org/statistics/numbers-of-people-in-the-uk/ (accessed 7 October 2019).
- Alzheimer’s Research UK. Care Services 2019. www.dementiastatistics.org/statistics/care-services/ (accessed 7 October 2019).
- Knapp M, Comas-Herrera A, Somani A, Banerjee S. Dementia: International Comparisons – Summary Report to the National Audit Office 2007.
- Alzheimer’s Society . Facts for the Media 2019. www.alzheimers.org.uk/about-us/news-and-media/facts-media (accessed 17 December 2019).
- Alzheimer’s Disease International . World Alzheimer Report 2018. The State of the Art of Dementia Research: New Frontiers 2018.
- Department of Health and Social Care . Living Well With Dementia: A National Dementia Strategy 2009. www.gov.uk/government/publications/living-well-with-dementia-a-national-dementia-strategy (accessed 26 September 2018).
- Department of Health and Social Care . Prime Minister’s Challenge on Dementia – Delivering Major Improvements in Dementia Care and Research by 2015 2012. www.gov.uk/government/publications/prime-ministers-challenge-on-dementia (accessed 26 September 2018).
- Hodge S, Hailey E. English National Memory Clinics Audit Report 2013.
- Hodge S, Hailey E. Second English National Memory Clinic Audit Report 2015.
- Department of Health and Social Care . Prime Minister’s Challenge on Dementia 2020 2015. www.gov.uk/government/uploads/system/uploads/attachment_data/file/406076/Dementia_vision.pdf (accessed 26 September 2018).
- NHS England . Well Pathway for Dementia 2017. www.england.nhs.uk/mentalhealth/wp-content/uploads/sites/29/2016/03/dementia-well-pathway.pdf (accessed 30 October 2018).
- National Institute for Health and Care Excellence . Dementia: Assessment, Management and Support for People Living With Dementia and Their Carers. NICE Guideline NG97 2018. www.nice.org.uk/guidance/ng97 (accessed 17 December 2019).
- Moniz-Cook E, Vernooij-Dassen M, Woods B, Orrell M. Psychosocial interventions in dementia care research: the INTERDEM manifesto. Aging Ment Health 2011;15:283-90. https://doi.org/10.1080/13607863.2010.543665.
- Mitchell W. Somebody I Used to Know. London: Bloomsbury Publishing; 2018.
- Keogh F, Mountain G, Joddrell P, Lord K. Psychosocial interventions for community-dwelling people following diagnosis of mild to moderate dementia: findings of a systematic scoping review. Am J Geriatr Psychiatry 2019;27:641-51. https://doi.org/10.1016/j.jagp.2018.12.027.
- Department of Health and Social Care . Supporting People With Long Term Conditions: An NHS and Social Care Model to Support Local Innovation and Integration 2005.
- Mountain GA. Self-management for people with early dementia: an exploration of concepts and supporting evidence. Dement Int J Soc Res Pract 2006;5:429-47. https://doi.org/10.1177/1471301206067117.
- Graff MJ, Vernooij-Dassen MJ, Thijssen M, Dekker J, Hoefnagels WH, Rikkert MG. Community based occupational therapy for patients with dementia and their care givers: randomised controlled trial. BMJ 2006;333. https://doi.org/10.1136/bmj.39001.688843.BE.
- Martin F, Turner A, Wallace LM, Stanley D, Jesuthasan J, Bradbury N. Qualitative evaluation of a self-management intervention for people in the early stage of dementia. Dementia 2015;14:418-35. https://doi.org/10.1177/1471301213498387.
- Quinn C, Toms G, Anderson D, Clare L. A review of self-management interventions for people with dementia and mild cognitive impairment. J Appl Gerontol 2016;35:1154-88. https://doi.org/10.1177/0733464814566852.
- Sprange K, Mountain GA, Shortland K, Craig C, Blackburn D, Bowie P, et al. Journeying through dementia, a community-based self-management intervention for people aged 65 years and over: a feasibility study to inform a future trial. Pilot Feasibility Stud 2015;1. https://doi.org/10.1186/s40814-015-0039-6.
- Toms GR, Quinn C, Anderson DE, Clare L. Help yourself: perspectives on self-management from people with dementia and their caregivers. Qual Health Res 2015;25:87-98. https://doi.org/10.1177/1049732314549604.
- Clare L, Kudlicka A, Oyebode JR, Jones RW, Bayer A, Leroi I, et al. Individual goal-oriented cognitive rehabilitation to improve everyday functioning for people with early-stage dementia: a multicentre randomised controlled trial (the GREAT trial). Int J Geriatr Psychiatry 2019;34:709-21. https://doi.org/10.1002/gps.5076.
- Ibrahim JE, Anderson LJ, MacPhail A, Lovell JJ, Davis MC, Winbolt M. Chronic disease self-management support for persons with dementia, in a clinical setting. J Multidiscip Healthc 2017;10:49-58. https://doi.org/10.2147/JMDH.S121626.
- Clarke CL, Keyes SE, Wilkinson H, Alexjuk J, Wilcockson J, Robinson L, et al. Healthbridge: The National Evaluation of Peer Support Networks and Dementia Advisers in Implementation of the National Dementia Strategy for England. Department of Health Policy Research Programme Project. London: Department of Health and Social Care; 2013.
- Keyes SE, Clarke CL, Wilkinson H, Alexjuk EJ, Wilcockson J, Robinson L, et al. ‘We’re all thrown in the same boat . . .’: a qualitative analysis of peer support in dementia care. Dementia 2016;15:560-77. https://doi.org/10.1177/1471301214529575.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Mountain GA, Craig CL. What should be in a self-management programme for people with early dementia?. Aging Ment Heal 2012;16:576-83. https://doi.org/10.1080/13607863.2011.651430.
- Knapp M, Comas-Herrera A, Somani A, Banerjee S. Dementia: International Comparisons. Summary Report for the National Audit Office 2007. www.nao.org.uk/wp-content/uploads/2007/07/0607604_International_Comparisons.pdf (accessed 16 December 2019).
- Craig C, Mountain G. Lifestyle Matters: An Occupational Approach to Healthy Ageing. Bicester: Speechmark Publishing Ltd; 2007.
- Clark F, Azen SP, Zemke R, Jackson J, Carlson M, Mandel D, et al. Occupational therapy for independent-living older adults. A randomized controlled trial. JAMA 1997;278:1321-6. https://doi.org/10.1001/jama.1997.03550160041036.
- Clark F, Jackson J, Carlson M, Chou CP, Cherry BJ, Jordan-Marsh M, et al. Effectiveness of a lifestyle intervention in promoting the well-being of independently living older people: results of the Well Elderly 2 Randomised Controlled Trial. J Epidemiol Community Health 2012;66:782-90. https://doi.org/10.1136/jech.2009.099754.
- Law M, Cooper B, Strong S, Stewart D, Rigby P, Letts L. The person-environment-occupation model: a transactive approach to occupational performance. Can J Occup Ther 1996;63:9-23. https://doi.org/10.1177/000841749606300103.
- Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: Freeman; 1997.
- Banerjee S, Samsi K, Petrie CD, Alvir J, Treglia M, Schwam EM, et al. What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and explanatory value of disease specific measures of health related quality of life in people with dementia. Int J Geriatr Psychiatry 2009;24:15-24. https://doi.org/10.1002/gps.2090.
- Smith SC, Lamping DL, Banerjee S, Harwood RH, Foley B, Smith P, et al. Development of a new measure of health-related quality of life for people with dementia: DEMQOL. Psychol Med 2007;37:737-46. https://doi.org/10.1017/S0033291706009469.
- Lamb SE, Sheehan B, Atherton N, Nichols V, Collins H, Mistry D, et al. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ 2018;361. https://doi.org/10.1136/bmj.k1675.
- O’Rourke HM, Duggleby W, Fraser KD, Jerke L. Factors that affect quality of life from the perspective of people with dementia: a metasynthesis. J Am Geriatr Soc 2015;63:24-38. https://doi.org/10.1111/jgs.13178.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. https://doi.org/10.1186/1748-5908-6-42.
- Craig C. Re-Designing Dementia 2017. http://shura.shu.ac.uk/15647/ (accessed 24 April 2020).
- Mountain G, Windle G, Hind D, Walters S, Keertharuth A, Chatters R, et al. A preventative lifestyle intervention for older adults (lifestyle matters): a randomised controlled trial. Age Ageing 2017;46:627-34. https://doi.org/10.1093/ageing/afx021.
- DEEP . DEEP Guides 2019. www.dementiavoices.org.uk/deep-guides/ (accessed 17 December 2019).
- Wright J, Foster A, Cooper C, Sprange K, Walters S, Berry K, et al. Study protocol for a randomised controlled trial assessing the clinical and cost-effectiveness of the Journeying through Dementia (JtD) intervention compared to usual care. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-029207.
- National Institute for Health and Care Research . Journeying Through Dementia; Randomised Controlled Trial of Clinical and Cost Effectiveness 2019. https://fundingawards.nihr.ac.uk/award/14/140/80 (accessed 20 December 2019).
- Moniz-Cook E, Vernooij-Dassen M, Woods R, Verhey F, Chattat R, De Vugt M, et al. A European consensus on outcome measures for psychosocial intervention research in dementia care. Aging Ment Health 2008;12:14-29. https://doi.org/10.1080/13607860801919850.
- Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9100.
- Mulhern B, Rowen D, Brazier J, Smith S, Romeo R, Tait R, et al. Development of DEMQOL-U and DEMQOL-PROXY-U: generation of preference-based indices from DEMQOL and DEMQOL-PROXY for use in economic evaluation. Health Technol Assess 2013;17. https://doi.org/10.3310/hta17050.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Hounsome N, Orrell M, Edwards RT. EQ-5D as a quality of life measure in people with dementia and their carers: evidence and key issues. Value Health 2011;14:390-9. https://doi.org/10.1016/j.jval.2010.08.002.
- Spitzer RL, Williams JBW, Kroenke K. Test review: Patient Health Questionnaire-9 (PHQ-9). Rehabil Couns Bull 2014;57:246-8. https://doi.org/10.1177/0034355213515305.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Wild B, Eckl A, Herzog W, Niehoff D, Lechner S, Maatouk I, et al. Assessing generalized anxiety disorder in elderly people using the GAD-7 and GAD-2 scales: results of a validation study. Am J Geriatr Psychiatry 2014;22:1029-38. https://doi.org/10.1016/j.jagp.2013.01.076.
- Schwarzer R, Jerusalem M, Weinman J, Wright S, Johnston M. Measures in Health Psychology: A User’s Portfolio Causal and Control Beliefs. Windsor: NFER-Nelson; 1995.
- Diener E, Wirtz D, Tov W, Kim-Prieto C, Choi D, Oishi S, et al. Social Indicators Research Series. New York, NY: Springer Science; 2009.
- Schuurmans H, Steverink N, Frieswijk N, Buunk BP, Slaets JP, Lindenberg S. How to measure self-management abilities in older people by self-report. The development of the SMAS-30. Qual Life Res 2005;14:2215-28. https://doi.org/10.1007/s11136-005-8166-9.
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living 1. Gerontologist 1969;9:179-86. https://doi.org/10.1093/geront/9.3_Part_1.179.
- Database of Instruments for Resource Use Measurement . About DIRUM 2018. www.dirum.org/about (accessed 16 December 2019).
- Vernooij-Dassen MJ, Persoon JM, Felling AJ. Predictors of sense of competence in caregivers of demented persons. Soc Sci Med 1996;43:41-9. https://doi.org/10.1016/0277-9536(95)00332-0.
- Jansen AP, van Hout HP, van Marwijk HW, Nijpels G, Gundy C, Vernooij-Dassen MJ, et al. Sense of competence questionnaire among informal caregivers of older adults with dementia symptoms: a psychometric evaluation. Clin Pract Epidemiol Ment Health 2007;3. https://doi.org/10.1186/1745-0179-3-11.
- Vernooij-Dassen M. Dementia and Home-Care 1993.
- Banerjee S. DEMQOL: Dementia Quality of Life Measure 2019. www.bsms.ac.uk/research/neuroscience/cds/research/demqol.aspx (accessed 15 October 2019).
- NHS . Patient Health Questionnaire 2019. www.datadictionary.nhs.uk/data_dictionary/nhs_business_definitions/p/patient_health_questionnaire-9_de.asp?shownav=1 (accessed 15 October 2019).
- NHS . Generalised Anxiety Disorder Questionnaire 2019. www.datadictionary.nhs.uk/data_dictionary/nhs_business_definitions/g/generalised_anxiety_disorder_questionnaire_de.asp?shownav=1 (accessed 15 October 2019).
- Schwarzer R. Documentation of the General Self-Efficacy Scale 2014. http://userpage.fu-berlin.de/∼health/faq_gse.pdf (accessed 15 October 2019).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Value Set for England (Updated October 2019) 2019. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 16 December 2019).
- Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. https://doi.org/10.1016/0022-3956(75)90026-6.
- Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: development of the Bristol Activities of Daily Living Scale. Age Ageing 1996;25:113-20. https://doi.org/10.1093/ageing/25.2.113.
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308-14. https://doi.org/10.1212/wnl.44.12.2308.
- Karanicolas PJ, Farrokhyar F, Bhandari M. Blinding: who, what, when, why, how?. Can J Surg 2010;53:345-8.
- Altman DG. Practical Statistics for Medical Research. Boca Raton, FL: CRC Press; 1990.
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. CONSORT Group . Methods and processes of the CONSORT Group: example of an extension for trials assessing nonpharmacologic treatments. Ann Intern Med 2008;148:W60-6.
- Baldwin SA, Bauer DJ, Stice E, Rohde P. Evaluating models for partially clustered designs. Psychol Methods 2011;16:149-65. https://doi.org/10.1037/a0023464.
- Roberts C, Batistatou E, Roberts SA. Design and analysis of trials with a partially nested design and a binary outcome measure. Stat Med 2016;35:1616-36. https://doi.org/10.1002/sim.6828.
- Flight L, Allison A, Dimairo M, Lee E, Mandefield L, Walters SJ. Recommendations for the analysis of individually randomised controlled trials with clustering in one arm – a case of continuous outcomes. BMC Med Res Methodol 2016;16. https://doi.org/10.1186/s12874-016-0249-5.
- Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics 1946;2:110-14. https://doi.org/10.2307/3002019.
- Department for Communities and Local Government . The English Indices of Deprivation 2015: Statistical Release 2015.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. https://doi.org/10.1001/archinte.166.10.1092.
- Great Britain . Mental Capacity Act 2005 2005.
- Datta J, Petticrew M. Challenges to evaluating complex interventions: a content analysis of published papers. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-568.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and Evaluating Complex Interventions: New Guidance. London: Medical Research Council; 2008.
- O’Cathain A, Murphy E, Nicholl J. Why, and how, mixed methods research is undertaken in health services research in England: a mixed methods study. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-85.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51. https://doi.org/10.1037/0278-6133.23.5.443.
- Spillane V, Byrne MC, Byrne M, Leathem CS, O’Malley M, Cupples ME. Monitoring treatment fidelity in a randomized controlled trial of a complex intervention. J Adv Nurs 2007;60:343-52. https://doi.org/10.1111/j.1365-2648.2007.04386.x.
- National Institute for Health and Care Excellence . Behaviour Change at Population, Community and Individual Levels. Public Health Guidance 6 2007.
- McHugh ML. Interrater reliability: the kappa statistic. Biochem Med 2012;22:276-82. https://doi.org/10.11613/BM.2012.031.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Bhaskar R. A Realist Theory of Science. Hemel Hempstead: Harvester; 1978.
- Fletcher AJ. Applying critical realism in qualitative research: methodology meets method. Int J Soc Res Methodol 2017;20:181-94. https://doi.org/10.1080/13645579.2016.1144401.
- Ritchie J, Spencer L, Humberman AM, Miles MB. The Qualitative Researcher’s Companion. London: Sage; 2002.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- University of Bradford . Experts by Experience 2019. www.bradford.ac.uk/dementia/ (accessed 20 December 2019).
- O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ 2010;341. https://doi.org/10.1136/bmj.c4587.
- Farmer T, Robinson K, Elliott SJ, Eyles J. Developing and implementing a triangulation protocol for qualitative health research. Qual Health Res 2006;16:377-94. https://doi.org/10.1177/1049732305285708.
- Dementia Action Alliance . York Minds &Amp; Voices 2019. www.dementiaaction.org.uk/members_and_action_plans/9872-york_minds_voices (accessed 23 April 2020).
- Butterflies . Butterflies Memory Loss Support Group 2020. www.butterflies.org.uk/ (accessed 24 April 2020).
- Essence Service . Essence Service 2020. https://essenceservice.org.uk/ (accessed 24 April 2020).
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 16 December 2019).
- Briggs AH, Drummond M, McGuire A. Economic Evaluation in Health Care: Merging Theory with Practice. Oxford: Oxford University Press; 2001.
- Drummond M, Brandt A, Luce B, Rovira J. Standardizing methodologies for economic evaluation in health care. Practice, problems, and potential. Int J Technol Assess Health Care 1993;9:26-3. https://doi.org/10.1017/s0266462300003007.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22. https://doi.org/10.1002/hec.3564.
- Royal College of Nursing . NHS Pay Scales 2017–18 2019. www.rcn.org.uk/employment-and-pay/nhs-pay-scales-2017-18 (accessed 16 December 2019).
- NHS Improvement . Reference Costs 2017 18 2018. https://improvement.nhs.uk/resources/reference-costs/ (accessed 15 January 2019).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2018. Canterbury: PSSRU, University of Kent; 2018.
- National Institute for Health and Care Excellence . British National Formulary 2019. https://bnf.nice.org.uk/ (accessed 16 December 2019).
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 2004.
- INVOLVE . Public Involvement in Research; Values and Principles Framework 2015. www.invo.org.uk/wp-content/uploads/2017/08/Values-Principles-framework-Jan2016.pdf (accessed 16 December 2019).
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ 2017;358. https://doi.org/10.1136/bmj.j3453.
- Poland F, Charlesworth G, Leung P, Birt L. Embedding patient and public involvement: managing tacit and explicit expectations. Health Expect 2019;22:1231-9. https://doi.org/10.1111/hex.12952.
- Birt L, Poland F, Csipke E, Charlesworth G. Shifting dementia discourses from deficit to active citizenship. Sociol Health Illn 2017;39:199-211. https://doi.org/10.1111/1467-9566.12530.
- Moody CJ, Mitchell D, Kiser G, Aarsland D, Berg D, Brayne C, et al. Maximizing the potential of longitudinal cohorts for research in neurodegenerative diseases: a community perspective. Front Neurosci 2017;11. https://doi.org/10.3389/fnins.2017.00467.
- Øksnebjerg L, Diaz-Ponce A, Gove D, Moniz-Cook E, Mountain G, Chattat R, et al. Towards capturing meaningful outcomes for people with dementia in psychosocial intervention research: a pan-European consultation. Health Expect 2018;21:1056-65. https://doi.org/10.1111/hex.12799.
- Coucill W, Bryan S, Bentham P, Buckley A, Laight A. EQ-5D in patients with dementia: an investigation of inter-rater agreement. Med Care 2001;39:760-71. https://doi.org/10.1097/00005650-200108000-00003.
- Kavirajan H, Hays RD, Vassar S, Vickrey BG. Responsiveness and construct validity of the health utilities index in patients with dementia. Med Care 2009;47:651-61. https://doi.org/10.1097/MLR.0b013e31819241b9.
- Karlawish JH, Zbrozek A, Kinosian B, Gregory A, Ferguson A, Glick HA. Preference-based quality of life in patients with Alzheimer’s disease. Alzheimers Dement 2008;4:193-202. https://doi.org/10.1016/j.jalz.2007.11.019.
- Field B, Mountain G, Burgess J, Di Bona L, Kelleher D, Mundy J, et al. Recruiting hard to reach populations to studies: breaking the silence: an example from a study that recruited people with dementia. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-030829.
- Clare L, Wu YT, Jones IR, Victor CR, Nelis SM, Martyr A, et al. A comprehensive model of factors associated with subjective perceptions of ‘living well’ with dementia: findings from the IDEAL study. Alzheimer Dis Assoc Disord 2019;33:36-41. https://doi.org/10.1097/WAD.0000000000000286.
- Clare L, Bayer A, Burns A, Corbett A, Jones R, Knapp M, et al. Goal-oriented cognitive rehabilitation in early-stage dementia: study protocol for a multi-centre single-blind randomised controlled trial (GREAT). Trials 2013;14. https://doi.org/10.1186/1745-6215-14-152.
- Clare L, Kudlicka A, Oyebode JR, Jones RW, Bayer A, Leroi I, et al. Goal-oriented cognitive rehabilitation for early-stage Alzheimer’s and related dementias: the GREAT RCT. Health Technol Assess 2019;23. https://doi.org/10.3310/hta23100.
- Laakkonen ML, Kautiainen H, Hölttä E, Savikko N, Tilvis RS, Strandberg TE, et al. Effects of self-management groups for people with dementia and their spouses – randomized controlled trial. J Am Geriatr Soc 2016;64:752-60. https://doi.org/10.1111/jgs.14055.
- Amieva H, Robert PH, Grandoulier AS, Meillon C, De Rotrou J, Andrieu S, et al. Group and individual cognitive therapies in Alzheimer’s disease: the ETNA3 randomized trial. Int Psychogeriatr 2016;28:707-17. https://doi.org/10.1017/S1041610215001830.
- Djabelkhir L, Wu YH, Vidal JS, Cristancho-Lacroix V, Marlats F, Lenoir H, et al. Computerized cognitive stimulation and engagement programs in older adults with mild cognitive impairment: comparing feasibility, acceptability, and cognitive and psychosocial effects. Clin Interv Aging 2017;12:1967-75. https://doi.org/10.2147/CIA.S145769.
- Woods RT, Orrell M, Bruce E, Edwards RT, Hoare Z, Hounsome B, et al. REMCARE: pragmatic multi-centre randomised trial of reminiscence groups for people with dementia and their family carers: effectiveness and economic analysis. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0152843.
- Quinn C, Toms G, Jones C, Brand A, Edwards RT, Sanders F, et al. A pilot randomized controlled trial of a self-management group intervention for people with early-stage dementia (The SMART study). Int Psychogeriatr 2016;28:787-800. https://doi.org/10.1017/S1041610215002094.
- Csipke E, Yates L, Cook EM, Leung P, Charlesworth G, Walton H, et al. Promoting independence in dementia: protocol for a feasibility trial of the PRIDE intervention for living well with dementia. Int J Clin Trials 2018;5:177-85. https://doi.org/10.18203/2349-3259.ijct20184399.
- Mountain G. Self-management programme for people with dementia and their spouses demonstrates some benefits, but the model has limitations. Evid Based Nurs 2017;20:26-7. https://doi.org/10.1136/eb-2016-102408.
- McDermott O, Charlesworth G, Hogervorst E, Stoner C, Moniz-Cook E, Spector A, et al. Psychosocial interventions for people with dementia: a synthesis of systematic reviews. Aging Ment Health 2019;23:393-40. https://doi.org/10.1080/13607863.2017.1423031.
- Graff MJ, Adang EM, Vernooij-Dassen MJ, Dekker J, Jönsson L, Thijssen M, et al. Community occupational therapy for older patients with dementia and their care givers: cost effectiveness study. BMJ 2008;336:134-8. https://doi.org/10.1136/bmj.39408.481898.BE.
- Gitlin LN, Hodgson N, Jutkowitz E, Pizzi L. The cost-effectiveness of a nonpharmacologic intervention for individuals with dementia and family caregivers: the tailored activity program. Am J Geriatr Psychiatry 2010;18:510-19. https://doi.org/10.1097/JGP.0b013e3181c37d13.
- Loud F, Cockwell P. Kidney Care UK, Renal Association, British Renal Society and National Kidney Federation . Dialysis Transport: Finding a Way Together 2019. https://renal.org/wp-content/uploads/2019/02/Dialysis-transport-PDF.pdf (accessed 2 September 2020).
Appendix 1 Members of the Trial Management Group, Data Monitoring and Ethics Committee and Trial Steering Committee
Trial Management Group
-
Gail Mountain, chairperson.
-
Cindy Cooper, CTRU lead.
-
Jessica Wright, trial manager.
-
Kirsty Sprange, qualitative and fidelity lead.
-
Stephen Walters, lead statistician.
-
Ellen Lee, trial statistician.
-
Katherine Berry, research supervisor.
-
Jules Beresford-Dent, researcher.
-
Amanda Loban, data management.
-
Emily Turton, data management.
-
Esme Moniz-Cook, co-applicant.
-
Tom Dening, co-applicant.
-
Claire Craig, co-applicant.
-
Tracey Young, health economist.
-
Nicholas Bell, sponsor representative.
-
Peter Bowie, co-applicant.
-
Daniel Blackburn, co-applicant.
Data Monitoring and Ethics Committee
-
Mona Kanaan (chairperson), Department of Health Sciences, University of York.
-
Jane Burgess, North East London NHS Foundation Trust.
-
Emily Robinson, Institute of Psychiatry, Psychology and Neuroscience, King’s College London.
Trial Steering Committee
-
Catherine Hewitt (chairperson), Department of Health Sciences, University of York.
-
Wendy Mitchell, PPI representative.
-
Jennifer Wenborn, School of Life and Medical Sciences, University College London.
Appendix 2 Changes to the protocol
The initial JtD protocol version 1.0 was dated 16 May 2016. It obtained Research Ethics Committee approval on 1 July 2016. Updates to the protocol are described below.
Journeying through Dementia protocol V2.0, 20 September 2016, Health Research Authority approval 11 October 2016
This protocol had the following changes:
-
Contact details were updated.
-
Professor Tom Dening replaced Professor Martin Orrell as lead at the University of Nottingham.
-
Throughout the protocol, the ordering of outcome measures was changed to the order in which they were administered.
-
The IADL measure was added to the trial summary, as it had been omitted in error.
-
The planned analysis part of the summary was updated to reflect what was contained in the main part of the protocol.
-
The stop/go criteria were altered at the request of the HTA programme (funder).
-
Figures and text were changed to provide more flexibility for participating supporters to be consented and have their baseline outcome measures measured at separate visits.
-
The reference to health care professionals only discussing the trial at post-diagnostic appointments was removed.
-
More options were provided for how health-care professionals can signpost people to the trial.
-
Collection of written permission to conduct the eligibility assessment was added.
-
It was proposed that an unblinded member of the research team can conduct randomisation rather than naming the specific roles.
-
Trial support officer was changed to an unblinded member of the research team who would inform the intervention staff that a participant has been randomised to receive the intervention.
-
The requirement that supervisors must be trained in the JtD intervention was added.
-
Provision for outcome measures from participating supporters to be collected via the telephone was added.
-
The assessment table for participating supporters was corrected because the EQ-5D-5L had been missed from the table (it had been specified in the text).
-
The PPI section was updated, in that the JtD PPI Advisory Group would link in with the TMG.
-
In the qualitative section, more flexibility was given for the timing of the interviews.
-
Flagging of the PHQ-9 score was changed from ‘3 and 4’ to ‘2 and 3’ to reflect the scoring on the outcome measure.
-
The sequencing used for randomisation was amended to reflect that randomisation needs to be by delivery site.
-
Minor reference updates.
Journeying through Dementia protocol V3.0, 7 December 2016, Research Ethics Committee approval 24 January 2017
This protocol had the following changes:
-
Inserted ISRCTN registration details.
-
The addresses of some members of the TMG were changed.
-
Ellen Lee replaced Munya Dimairo as the trial statistician. The senior trial statistician and co-applicant, Professor Stephen Walters, remained the same.
-
Katherine Ludwin left the study, and so was removed from the TMG.
-
Section 6.1: the wording was changed to explain that the randomisation schedule would be produced before recruiting participants and not before the trial starts.
-
Section 7.1: the wording was changed to make it clear that one of the individual sessions would be held after the group sessions finished.
-
Section 7.1: reference to specific AfC bands for facilitators were caveated with ‘usually’ to take into account that each NHS trust had a different configuration of staff.
-
Section 9.2: a paragraph was removed regarding secondary outcomes, as this paragraph was repeated later in the section.
-
Minor reference updates.
Journeying through Dementia protocol V4.0, 14 June 2017, Research Ethics Committee approval 25 July 2017
This protocol had the following changes:
-
Members of staff on the TMG were updated.
-
It was outlined that the gap between baseline and intervention would ideally be < 2 months, rather than a strict requirement, noting that central research team approval is required to start conducting baseline visits.
-
Process for completion of MMSE for visually impaired individuals outlined.
-
Details of a further substudy on developing the JtD intervention was added.
-
It was clarified that the research supervisor would receive individual/group sheets during the intervention for supervision purposes.
-
The fidelity section was updated to advise that it would not include video-recordings.
-
Information was added to the risk section about actions to be taken when there is a score signalling moderate/severe anxiety or depression.
-
The randomisation and statistical analysis sections were updated in relation to inclusion of couples, both with dementia, into the study.
-
Minor text corrections.
Journeying through Dementia protocol v5.0, 25 October 2017, Research Ethics Committee approval 28 November 2017
This protocol contained an update to the fidelity substudy. Originally, interviews were planned for staff at the fidelity sites at the beginning and end of them delivering the intervention to monitor their change in outlook based on their experience of delivering the intervention. However, as many of the facilitators they proposed to interview had actually delivered the intervention before in a previous wave, we considered there to be less value in doing a second interview. The protocol was amended to state that one interview would now be conducted and it would look more broadly at the facilitator experience of delivering the intervention. It was clarified that staff would be interviewed at one time point at the four fidelity sites and three additional sites.
Journeying through Dementia protocol v6.0, 25 July 2018, Research Ethics Committee approval 24 October 2018
This protocol had the following changes:
-
Members of staff on the TMG were updated.
-
There were changes in relation to a small number of participants potentially not receiving the 12-month follow-ups. The protocol was updated to indicate that follow-ups will now take place only when possible within project time scales, through changes to different sections of the protocol.
-
There were updates to the fidelity substudy. Minor changes included a change to who completes individual session checklists (amended to apply to only facilitators), a clarification on where participants are sampled from for the qualitative interviews and a clarification that the qualitative findings will be triangulated with results obtained from the fidelity assessment, as well as the qualitative analysis.
-
Clarification on who may deliver the training course was added. Sentence added: ‘In some cases, for example, if the individual is to be a reserve facilitator, or the group to be trained is small, they may receive a shortened course supported by online resources created for this purpose’.
-
The text was updated to explain that some measures may be taken from the participant on the telephone at the 8- and 12-month follow-up visits if this is the only feasible way of collecting the data.
-
SAE procedures were updated to indicate that the local principal investigator, not the chief investigator, will assess SAEs to judge whether or not they are unexpected and related.
Journeying through Dementia protocol v7, 5 December 2018, Research Ethics Committee approval 10 December 2018
This update included information on the plans for involving people with dementia in validating qualitative analyses.
Appendix 3 Recruiting centres
These are the 14 NHS trusts that recruited across 13 JtD sites in the UK:
-
Bradford District Care NHS Foundation Trust
-
Humber Teaching NHS Foundation Trust
-
Leeds and York Partnership NHS Foundation Trust
-
Leicestershire Partnership NHS Trust
-
Lincolnshire Partnership NHS Foundation Trust
-
North Staffordshire Combined Healthcare NHS Trust
-
Northamptonshire Healthcare NHS Foundation Trust
-
Northumberland, Tyne and Wear NHS Foundation Trust
-
Nottinghamshire Healthcare NHS Foundation Trust
-
Pennine Care NHS Foundation Trust
-
Sheffield Health and Social Care NHS Foundation Trust (sponsor)
-
South West Yorkshire Partnership NHS Foundation Trust
-
Tees, Esk and Wear Valleys NHS Foundation Trust
-
University Hospitals of North Midlands NHS Trust (partnered with North Staffordshire Combined Healthcare NHS Trust for the purposes of recruitment and delivery).
Appendix 4 Participant recruitment rates and targets
FIGURE 13.
Participant recruitment rates and targets.
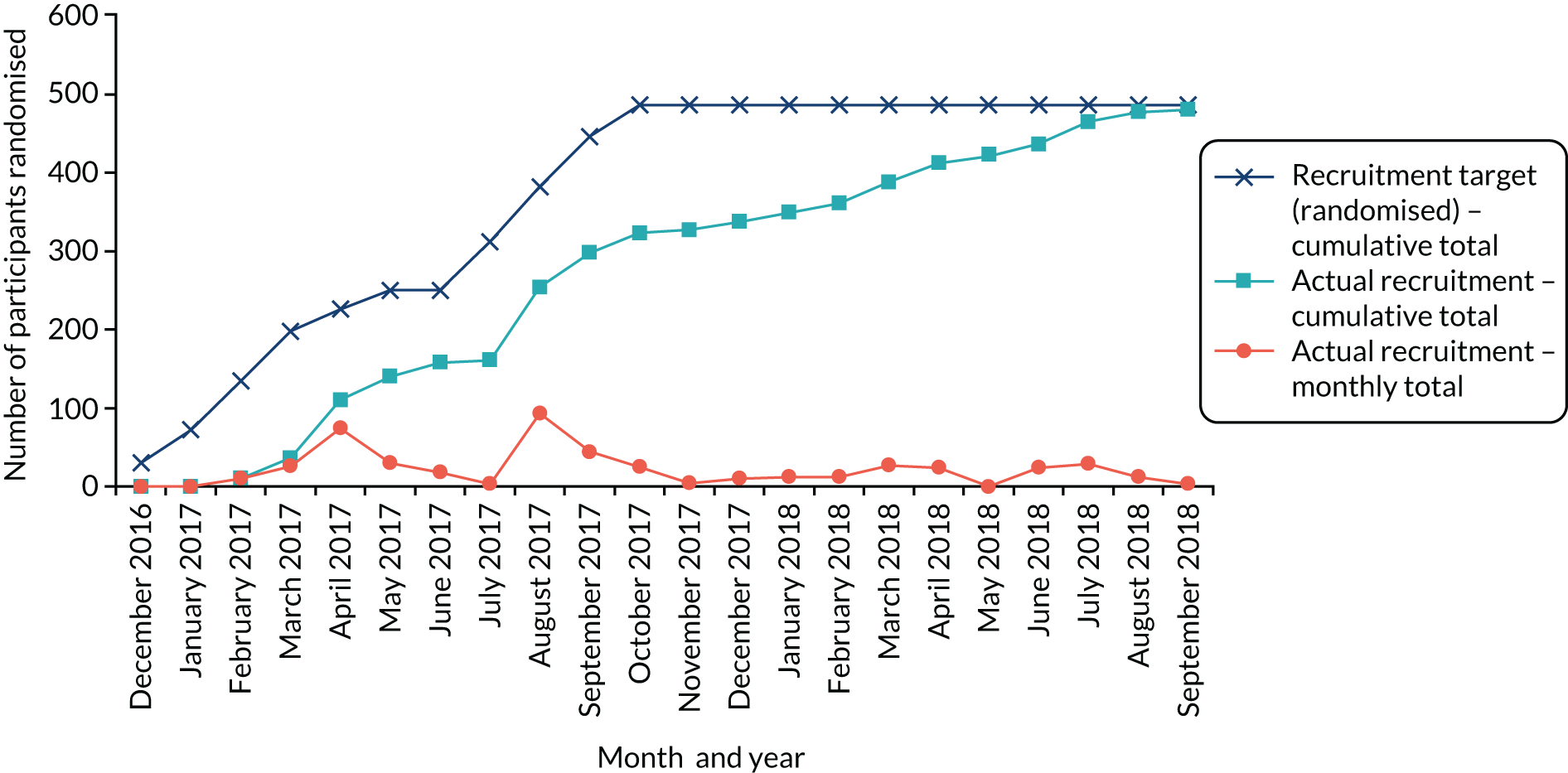
Appendix 5 Attendance at Journeying through Dementia trial intervention sessions
| Site ID | Group | Participants registered, na | Participants attending any session, n | Week, n (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1b | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||||
| S01 | S01/G1 | 7 | 6 | 5 (83.3) | 5 (83.3) | 6 (100.0) | 6 (100.0) | 6 (100.0) | 6 (100.0) | 4 (66.7) | 3 (50.0) | 6 (100.0) | 6 (100.0) | 4 (66.7) | 6 (100.0) |
| S01/G2 | 8 | 5 | 4 (80.0) | 1 (20.0) | 3 (60.0) | 4 (80.0) | 2 (40.0) | 4 (80.0) | 2 (40.0) | 3 (60.0) | 3 (60.0) | 4 (80.0) | 3 (60.0) | 4 (80.0) | |
| S01/G3 | 7 | 6 | 5 (83.3) | 5 (83.3) | 5 (83.3) | 4 (66.7) | 3 (50.0) | 4 (66.7) | 3 (50.0) | 4 (66.7) | 4 (66.7) | 2 (33.3) | 3 (50.0) | 4 (66.7) | |
| S02 | S02/G1 | 7 | 7 | 6 (85.7) | 7 (100.0) | 7 (100.0) | 7 (100.0) | 7 (100.0) | 6 (85.7) | 6 (85.7) | 7 (100.0) | 6 (85.7) | 7 (100.0) | 6 (85.7) | 7 (100.0) |
| S02/G2 | 7 | 7 | 7 (100.0) | 6 (85.7) | 6 (85.7) | 6 (85.7) | 6 (85.7) | 6 (85.7) | 3 (42.9) | 5 (71.4) | 4 (57.1) | 4 (57.1) | 5 (71.4) | 5 (71.4) | |
| S02/G3 | 8 | 8 | 7 (87.5) | 6 (75.0) | 6 (75.0) | 5 (62.5) | 6 (75.0) | 7 (87.5) | 5 (62.5) | 5 (62.5) | 6 (75.0) | 6 (75.0) | 7 (87.5) | 7 (87.5) | |
| S04 | S04/G1 | 9 | 8 | 6 (75.0) | 7 (87.5) | 7 (87.5) | 5 (62.5) | 6 (75.0) | 8 (100.0) | 8 (100.0) | 6 (75.0) | 7 (87.5) | 7 (87.5) | 8 (100.0) | 4 (50.0) |
| S04/G2 | 6 | 5 | 4 (80.0) | 4 (80.0) | 4 (80.0) | 5 (100.0) | 5 (100.0) | 5 (100.0) | 4 (80.0) | 5 (100.0) | 5 (100.0) | 4 (80.0) | 5 (100.0) | 4 (80.0) | |
| S05 | S05/G1 | 12 | 9 | 7 (77.8) | 8 (88.9) | 6 (66.7) | 7 (77.8) | 6 (66.7) | 6 (66.7) | 7 (77.8) | 7 (77.8) | 5 (55.6) | 6 (66.7) | 4 (44.4) | 6 (66.7) |
| S05/G2 | 7 | 7 | 5 (71.4) | 5 (71.4) | 4 (57.1) | 6 (85.7) | 5 (71.4) | 6 (85.7) | 6 (85.7) | 6 (85.7) | 5 (71.4) | 5 (71.4) | 6 (85.7) | 6 (85.7) | |
| S05/G3 | 8 | 8 | 3 (37.5) | 4 (50.0) | 4 (50.0) | 5 (62.5) | 5 (62.5) | 5 (62.5) | 6 (75.0) | 5 (62.5) | 2 (25.0) | 3 (37.5) | 4 (50.0) | 6 (75.0) | |
| S05/G4 | 7 | 6 | 5 (83.3) | 4 (66.7) | 5 (83.3) | 5 (83.3) | 3 (50.0) | 5 (83.3) | 4 (66.7) | 5 (83.3) | 5 (83.3) | 5 (83.3) | 5 (83.3) | 5 (83.3) | |
| S06 | S06/G1 | 9 | 9 | 9 (100.0) | 9 (100.0) | 9 (100.0) | 9 (100.0) | 6 (66.7) | 9 (100.0) | 9 (100.0) | 7 (77.8) | 8 (88.9) | 8 (88.9) | 7 (77.8) | 8 (88.9) |
| S06/G2 | 10 | 9 | 6 (66.7) | 5 (55.6) | 7 (77.8) | 6 (66.7) | 3 (33.3) | 5 (55.6) | 5 (55.6) | 5 (55.6) | 6 (66.7) | 5 (55.6) | 6 (66.7) | 7 (77.8) | |
| S07 | S07/G1 | 12 | 12 | 7 (58.3) | 6 (50.0) | 6 (50.0) | 7 (58.3) | 5 (41.7) | 5 (41.7) | 7 (58.3) | 6 (50.0) | 7 (58.3) | 6 (50.0) | 6 (50.0) | 5 (41.7) |
| S07/G2 | 11 | 11 | 6 (54.5) | 7 (63.6) | 6 (54.5) | 7 (63.6) | 5 (45.5) | 3 (27.3) | 7 (63.6) | 6 (54.5) | 7 (63.6) | 6 (54.5) | 6 (54.5) | 6 (54.5) | |
| S08 | S08/G1 | 9 | 8 | 4 (50.0) | 6 (75.0) | 5 (62.5) | 7 (87.5) | 4 (50.0) | 5 (62.5) | 4 (50.0) | 4 (50.0) | 3 (37.5) | 4 (50.0) | 3 (37.5) | 4 (50.0) |
| S08/G2 | 10 | 9 | 2 (22.2) | 5 (55.6) | 4 (44.4) | 4 (44.4) | 4 (44.4) | 4 (44.4) | 4 (44.4) | 1 (11.1) | 4 (44.4) | 2 (22.2) | 3 (33.3) | 3 (33.3) | |
| S09 | S09/G1 | 8 | 7 | 6 (85.7) | 5 (71.4) | 5 (71.4) | 4 (57.1) | 5 (71.4) | 5 (71.4) | 5 (71.4) | 4 (57.1) | 3 (42.9) | 4 (57.1) | 5 (71.4) | 5 (71.4) |
| S09/G2 | 9 | 9 | 6 (66.7) | 4 (44.4) | 4 (44.4) | 4 (44.4) | 5 (55.6) | 5 (55.6) | 4 (44.4) | 3 (33.3) | 4 (44.4) | 5 (55.6) | 5 (55.6) | 3 (33.3) | |
| S10 | S10/G1 | 9 | 9 | 6 (66.7) | 7 (77.8) | 5 (55.6) | 7 (77.8) | 6 (66.7) | 7 (77.8) | 5 (55.6) | 7 (77.8) | 5 (55.6) | 6 (66.7) | 6 (66.7) | 6 (66.7) |
| S10/G2 | 11 | 8 | 7 (87.5) | 8 (100.0) | 6 (75.0) | 7 (87.5) | 5 (62.5) | 7 (87.5) | 5 (62.5) | 7 (87.5) | 3 (37.5) | 7 (87.5) | 4 (50.0) | 8 (100.0) | |
| S10/G3 | 11 | 7 | 6 (85.7) | 7 (100.0) | 6 (85.7) | 6 (85.7) | 6 (85.7) | 6 (85.7) | 4 (57.1) | 6 (85.7) | 4 (57.1) | 5 (71.4) | 6 (85.7) | 7 (100.0) | |
| S11 | S11/G1 | 10 | 10 | 9 (90.0) | 9 (90.0) | 9 (90.0) | 8 (80.0) | 9 (90.0) | 9 (90.0) | 9 (90.0) | 7 (70.0) | 5 (50.0) | 7 (70.0) | 7 (70.0) | 8 (80.0) |
| S12 | S12/G1 | 6 | 6 | 4 (66.7) | 6 (100.0) | 4 (66.7) | 4 (66.7) | 5 (83.3) | 2 (33.3) | 5 (83.3) | 4 (66.7) | 3 (50.0) | 4 (66.7) | 4 (66.7) | 4 (66.7) |
| S12/G2 | 4 | 4 | 4 (100.0) | 3 (75.0) | 2 (50.0) | 1 (25.0) | 2 (50.0) | 2 (50.0) | 2 (50.0) | 2 (50.0) | 1 (25.0) | 1 (25.0) | 2 (50.0) | 2 (50.0) | |
| S13 | S13/G1 | 6 | 6 | 4 (66.7) | 3 (50.0) | 4 (66.7) | 3 (50.0) | 3 (50.0) | 2 (33.3) | 4 (66.7) | 4 (66.7) | 3 (50.0) | 3 (50.0) | 3 (50.0) | 3 (50.0) |
| S15 | S15/G1 | 11 | 11 | 9 (81.8) | 8 (72.7) | 9 (81.8) | 7 (63.6) | 9 (81.8) | 7 (63.6) | 8 (72.7) | 8 (72.7) | 9 (81.8) | 7 (63.6) | 8 (72.7) | 9 (81.8) |
| Total | 239 | 217 | 159 (73.3) | 160 (73.7) | 154 (71.0) | 156 (71.9) | 142 (65.4) | 151 (69.6) | 145 (66.8) | 142 (65.4) | 133 (61.3) | 139 (64.1) | 141 (65.0) | 152 (70.0) | |
| Site ID | Group | Participants registered, na | Participants attending any session, n | Week, n (%) | |||
|---|---|---|---|---|---|---|---|
| 1b | 2 | 3 | 4 | ||||
| S01 | S01/G1 | 7 | 6 | 6 (100.0) | 6 (100.0) | 6 (100.0) | 6 (100.0) |
| S01/G2 | 8 | 5 | 3 (60.0) | 4 (80.0) | 4 (80.0) | 4 (80.0) | |
| S01/G3 | 7 | 6 | 6 (100.0) | 5 (83.3) | 3 (50.0) | 5 (83.3) | |
| S02 | S02/G1 | 7 | 7 | 7 (100.0) | 7 (100.0) | 7 (100.0) | 7 (100.0) |
| S02/G2 | 7 | 7 | 7 (100.0) | 6 (85.7) | 5 (71.4) | 5 (71.4) | |
| S02/G3 | 8 | 8 | 8 (100.0) | 7 (87.5) | 7 (87.5) | 7 (87.5) | |
| S04 | S04/G1 | 9 | 8 | 8 (100.0) | 6 (75.0) | 8 (100.0) | 6 (75.0) |
| S04/G2 | 6 | 5 | 5 (100.0) | 5 (100.0) | 5 (100.0) | 5 (100.0) | |
| S05 | S05/G1 | 12 | 9 | 9 (100.0) | 7 (77.8) | 7 (77.8) | 7 (77.8) |
| S05/G2 | 7 | 7 | 7 (100.0) | 6 (85.7) | 6 (85.7) | 6 (85.7) | |
| S05/G3 | 8 | 8 | 8 (100.0) | 6 (75.0) | 6 (75.0) | 6 (75.0) | |
| S05/G4 | 7 | 6 | 6 (100.0) | 6 (100.0) | 4 (66.7) | 4 (66.7) | |
| S06 | S06/G1 | 9 | 9 | 9 (100.0) | 9 (100.0) | 9 (100.0) | 9 (100.0) |
| S06/G2 | 10 | 9 | 9 (100.0) | 6 (66.7) | 7 (77.8) | 7 (77.8) | |
| S07 | S07/G1 | 12 | 12 | 12 (100.0) | 8 (66.7) | 8 (66.7) | 8 (66.7) |
| S07/G2 | 11 | 11 | 11 (100.0) | 7 (63.6) | 7 (63.6) | 7 (63.6) | |
| S08 | S08/G1 | 9 | 8 | 8 (100.0) | 6 (75.0) | 4 (50.0) | 3 (37.5) |
| S08/G2 | 10 | 9 | 9 (100.0) | 5 (55.6) | 4 (44.4) | 4 (44.4) | |
| S09 | S09/G1 | 8 | 7 | 7 (100.0) | 6 (85.7) | 1 (14.3) | 5 (71.4) |
| S09/G2 | 9 | 9 | 9 (100.0) | 9 (100.0) | 9 (100.0) | 8 (88.9) | |
| S10 | S10/G1 | 9 | 9 | 9 (100.0) | 7 (77.8) | 6 (66.7) | 6 (66.7) |
| S10/G2 | 11 | 8 | 8 (100.0) | 8 (100.0) | 8 (100.0) | 8 (100.0) | |
| S10/G3 | 11 | 7 | 7 (100.0) | 7 (100.0) | 7 (100.0) | 7 (100.0) | |
| S11 | S11/G1 | 10 | 10 | 10 (100.0) | 9 (90.0) | 9 (90.0) | 9 (90.0) |
| S12 | S12/G1 | 6 | 6 | 5 (83.3) | 6 (100.0) | 6 (100.0) | 6 (100.0) |
| S12/G2 | 4 | 4 | 4 (100.0) | 2 (50.0) | 2 (50.0) | 2 (50.0) | |
| S13 | S13/G1 | 6 | 6 | 6 (100.0) | 4 (66.7) | 2 (33.3) | 0 (0.0) |
| S15 | S15/G1 | 11 | 11 | 11 (100.0) | 9 (81.8) | 8 (72.7) | 9 (81.8) |
| Total | 239 | 217 | 214 (98.6) | 179 (82.5) | 165 (76.0) | 166 (76.5) | |
Appendix 6 Fidelity and kappa scores by site: fidelity substudy
| Site | Researcher | Agreed score | % | κ score | CI | |
|---|---|---|---|---|---|---|
| 1 | 2 | |||||
| 1 | 127 | 124 | 127/144 | 88 | 0.77 | 0.43 to 0.84 |
| 2 | 137 | 136 | 137/144 | 95 | 0.64 | 0.39 to 0.78 |
| 3 | 123 | 122 | 123/144 | 85 | 0.66 | 0.45 to 0.86 |
| 4 | 128 | 132 | 130/144 | 90 | 0.59 | 0.57 to 0.96 |
Appendix 7 Cost-effectiveness plane and cost-effectiveness acceptability curve
FIGURE 14.
Incremental cost-effectiveness plane (5000 bootstrapped replicates).
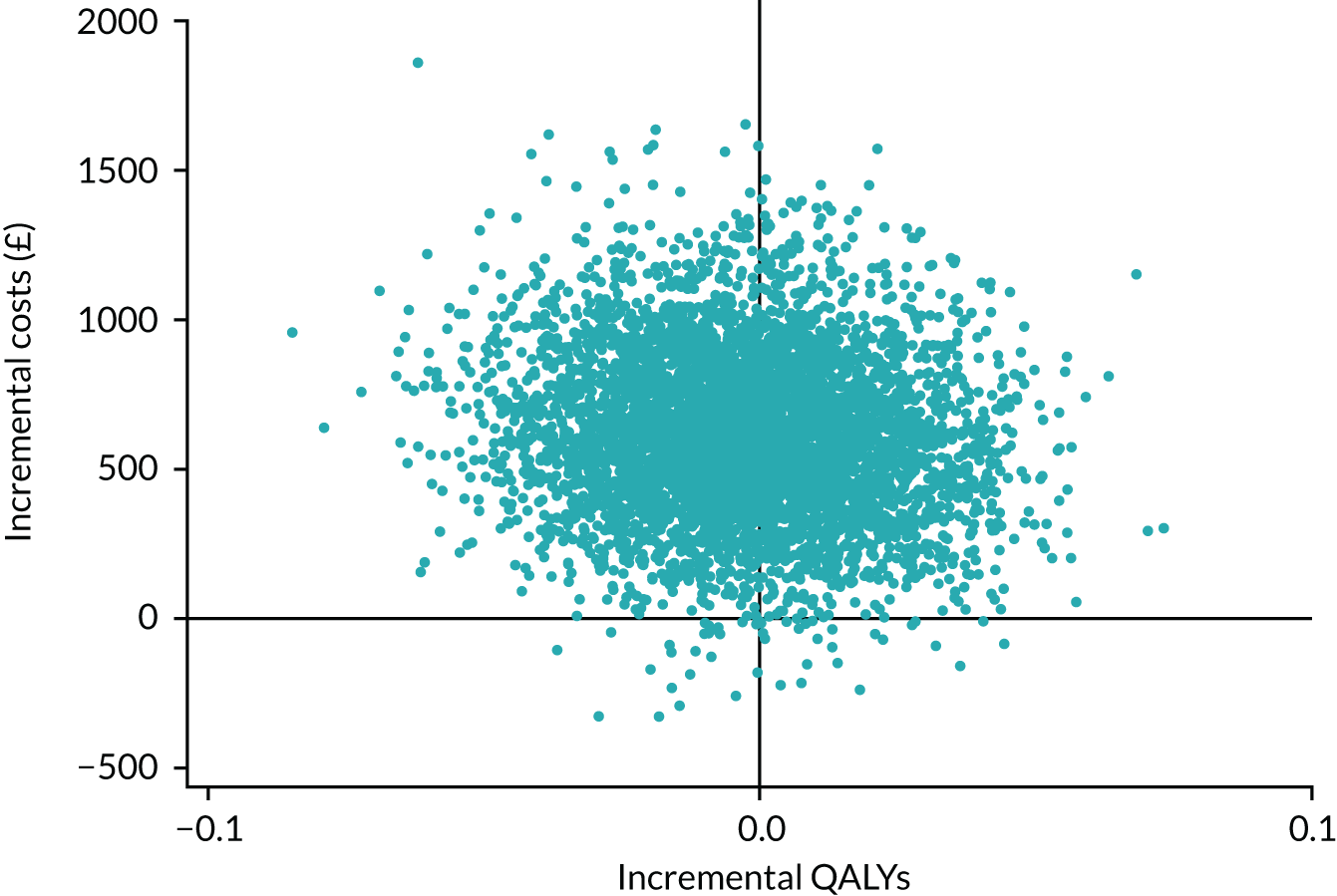
FIGURE 15.
Cost-effectiveness acceptability curve.
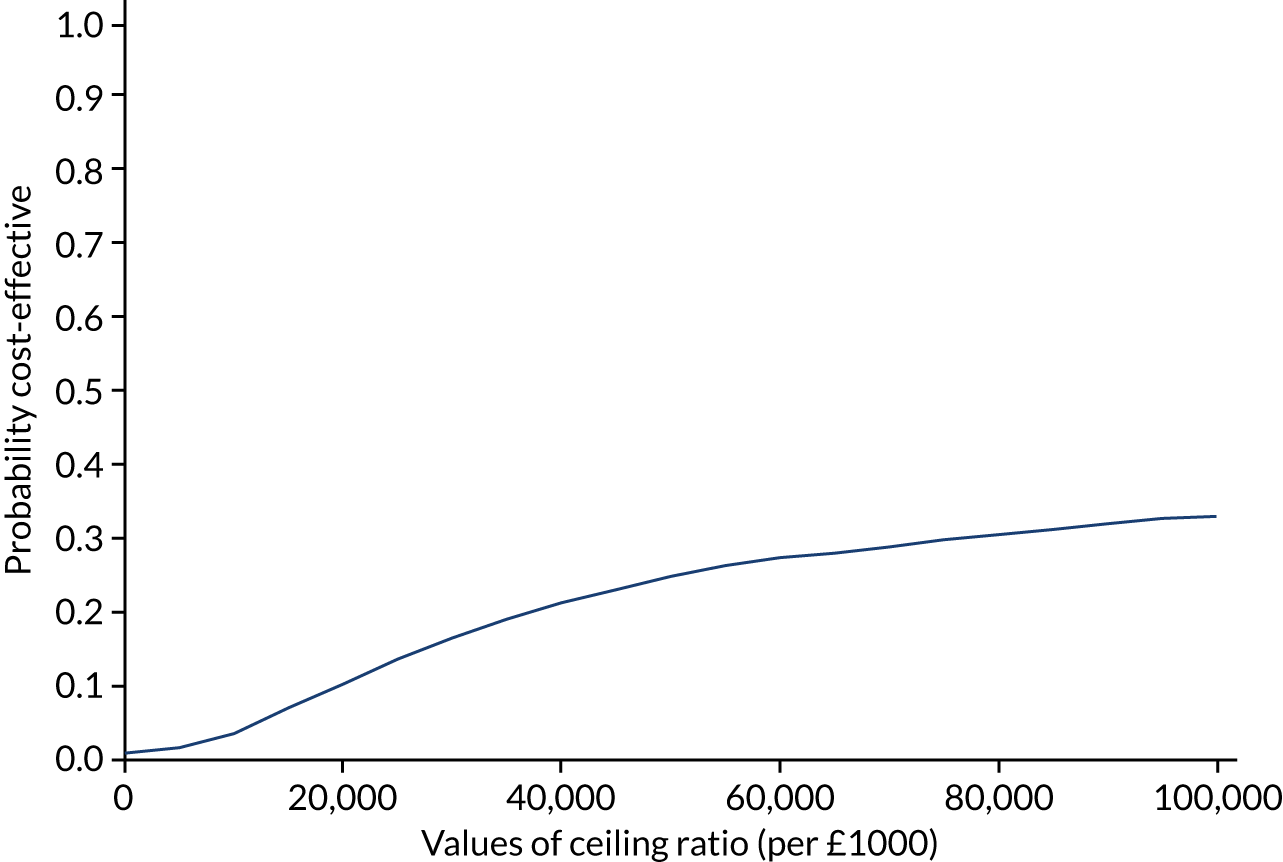
Appendix 8 Baseline characteristics by missing data status
| Characteristic | Missing 8-month DEMQOL data, n (%) | Complete 8-month DEMQOL data, n (%) | ||||
|---|---|---|---|---|---|---|
| Intervention (N = 44) | Control (N = 39) | Total (N = 83) | Intervention (N = 191) | Control (N = 197) | Total (N = 388) | |
| Site ID | ||||||
| S01 | 2 (5) | 6 (15) | 8 (10) | 20 (10) | 18 (9) | 38 (10) |
| S02 | 3 (7) | 2 (5) | 5 (6) | 18 (9) | 18 (9) | 36 (9) |
| S04 | 0 (0) | 2 (5) | 2 (2) | 12 (6) | 13 (7) | 25 (6) |
| S05 | 4 (9) | 5 (13) | 9 (11) | 29 (15) | 28 (14) | 57 (15) |
| S06 | 0 (0) | 1 (3) | 1 (1) | 19 (10) | 18 (9) | 37 (10) |
| S07 | 5 (11) | 2 (5) | 7 (8) | 18 (9) | 21 (11) | 39 (10) |
| S08 | 5 (11) | 8 (21) | 13 (16) | 14 (7) | 9 (5) | 23 (6) |
| S09 | 6 (14) | 5 (13) | 11 (13) | 12 (6) | 12 (6) | 24 (6) |
| S10 | 11 (25) | 5 (13) | 16 (19) | 19 (10) | 27 (14) | 46 (12) |
| S11 | 2 (5) | 0 (0) | 2 (2) | 9 (5) | 10 (5) | 19 (5) |
| S12 | 2 (5) | 0 (0) | 2 (2) | 8 (4) | 10 (5) | 18 (5) |
| S13 | 1 (2) | 1 (3) | 2 (2) | 5 (3) | 5 (3) | 10 (3) |
| S15 | 3 (7) | 2 (5) | 5 (6) | 8 (4) | 8 (4) | 16 (4) |
| Gender | ||||||
| Male | 22 (50) | 23 (59) | 45 (54) | 110 (58) | 118 (60) | 228 (59) |
| Female | 22 (50) | 16 (41) | 38 (46) | 81 (42) | 79 (40) | 160 (41) |
| Ethnicity | ||||||
| English/Welsh/Scottish/Northern Irish/British | 43 (98) | 39 (100) | 82 (99) | 181 (95) | 188 (95) | 369 (95) |
| Irish | 1 (2) | 0 (0) | 1 (1) | 4 (2) | 2 (1) | 6 (2) |
| Any other white background | 0 (0) | 0 (0) | 0 (0) | 4 (2) | 0 (0) | 4 (1) |
| Indian | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (2) | 3 (1) |
| Any other Asian background | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 1 (0) |
| Any other mixed/multiple ethnic background | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (1) | 2 (1) |
| Caribbean | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (1) | 2 (1) |
| Any other ethnic group | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (0) |
| Lives with others | ||||||
| No | 14 (32) | 14 (36) | 28 (34) | 45 (24) | 48 (24) | 93 (24) |
| Yes | 30 (68) | 25 (64) | 55 (66) | 145 (76) | 149 (76) | 294 (76) |
| Participating supporter | ||||||
| No | 15 (34) | 8 (21) | 23 (28) | 46 (24) | 58 (29) | 104 (27) |
| Yes | 29 (66) | 31 (79) | 60 (72) | 145 (76) | 139 (71) | 284 (73) |
| Type of dementia diagnosed | ||||||
| Alzheimer’s | 21 (48) | 21 (54) | 42 (51) | 117 (61) | 126 (64) | 243 (63) |
| Vascular dementia | 11 (25) | 5 (13) | 16 (19) | 20 (10) | 14 (7) | 34 (9) |
| Mixed Alzheimer’s/vascular dementia | 11 (25) | 8 (21) | 19 (23) | 38 (20) | 48 (24) | 86 (22) |
| Dementia in Parkinson disease | 0 (0) | 1 (3) | 1 (1) | 3 (2) | 2 (1) | 5 (1) |
| Frontotemporal dementia | 0 (0) | 1 (3) | 1 (1) | 5 (3) | 1 (1) | 6 (2) |
| Lewy body dementia | 0 (0) | 1 (3) | 1 (1) | 1 (1) | 2 (1) | 3 (1) |
| Unspecified dementia | 0 (0) | 2 (5) | 2 (2) | 7 (4) | 3 (2) | 10 (3) |
| Other | 1 (2) | 0 (0) | 1 (1) | 0 (0) | 1 (1) | 1 (0) |
| Characteristic | Missing 8-month DEMQOL data | Complete 8-month DEMQOL data | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (N = 44) | Control (N = 39) | Total (N = 83) | Intervention (N = 191) | Control (N = 197) | Total (N = 388) | |||||||
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Age (years) | 44 | 80 (6) | 39 | 79 (6) | 83 | 80 (6) | 191 | 77 (7) | 197 | 76 (8) | 388 | 76 (8) |
| Length of time since dementia diagnosis (years) | 44 | 1.2 (1.7) | 39 | 1.3 (1.3) | 83 | 1.2 (1.6) | 191 | 1.3 (1.4) | 197 | 1.4 (1.8) | 388 | 1.3 (1.6) |
| DEMQOL (total score) | 44 | 91.2 (11.8) | 39 | 89.1 (13.9) | 83 | 90.2 (12.8) | 191 | 91.0 (13.3) | 197 | 90.7 (13.0) | 388 | 90.8 (13.1) |
| EQ-5D-5L (value index) | 44 | 0.9 (0.1) | 39 | 0.9 (0.1) | 83 | 0.9 (0.1) | 191 | 0.8 (0.2) | 197 | 0.8 (0.2) | 388 | 0.8 (0.2) |
| EQ-5D VAS | 44 | 76.1 (15.6) | 39 | 69.6 (16.9) | 83 | 73.1 (16.5) | 191 | 76.0 (16.9) | 197 | 74.5 (17.9) | 388 | 75.2 (17.4) |
| DFS | 44 | 45.1 (7.2) | 39 | 44.9 (7.3) | 83 | 45.0 (7.2) | 191 | 45.4 (6.6) | 197 | 45.7 (7.2) | 388 | 45.6 (6.9) |
| GAD-7 (total score) | 44 | 2.6 (3.3) | 39 | 2.8 (3.4) | 83 | 2.7 (3.3) | 191 | 2.8 (3.6) | 197 | 2.8 (3.6) | 388 | 2.8 (3.6) |
| PHQ-9 (total score) | 44 | 3.5 (3.4) | 39 | 4.2 (3.8) | 83 | 3.8 (3.6) | 191 | 4.2 (4.5) | 197 | 3.9 (4.5) | 388 | 4.1 (4.5) |
| GSE (total score) | 44 | 29.8 (6.0) | 39 | 30.5 (4.9) | 83 | 30.2 (5.5) | 191 | 30.4 (5.4) | 197 | 30.9 (5.5) | 388 | 30.7 (5.5) |
| IADL (total score) | 44 | 5.6 (2.0) | 39 | 5.1 (2.2) | 83 | 5.4 (2.1) | 191 | 5.8 (1.8) | 197 | 5.9 (1.7) | 388 | 5.8 (1.8) |
| SMAS | 44 | 121.1 (23.2) | 39 | 120.8 (16.9) | 83 | 121.0 (20.3) | 191 | 125.6 (20.1) | 197 | 126.6 (19.9) | 388 | 126.1 (20.0) |
| MMSE (total score) | 44 | 23.0 (3.0) | 39 | 23.5 (2.9) | 83 | 23.2 (3.0) | 191 | 24.9 (3.0) | 197 | 24.9 (3.1) | 388 | 24.9 (3.1) |
Appendix 9 Resource use details
| Specialty | Source | Unit cost (£) | JtD intervention, n | Usual care, n | Total, n |
|---|---|---|---|---|---|
| Anticoagulation | DHSC OP 324107 | 34 | 1 | 1 | 2 |
| Audiology | DHSC OP 310107 | 108 | 9 | 6 | 15 |
| Brain scan | DHSC IMAGOP107 | 141 | 1 | 0 | 1 |
| Breast screening | 12 | 0 | 1 | 1 | |
| Plastic surgery | DHSC OP 104107 | 150 | 1 | 0 | 1 |
| Burns unit | DHSC OP107 | 156 | 1 | 0 | 1 |
| Cardiology | DHSC OP 320107 | 134 | 13 | 10 | 23 |
| Chiropodist | PSSRU108 | 44 | 1 | 0 | 1 |
| General outpatient | DHSC OP107 | 125 | 1 | 7 | 8 |
| CT | DHSC IMAGOP107 | 228 | 1 | 0 | 1 |
| Dental | DHSC OP 141107 | 152 | 1 | 0 | 1 |
| Dermatology | DHSC OP 330107 | 111 | 9 | 8 | 17 |
| Diabetes | DHSC OP107 | 147 | 0 | 4 | 4 |
| Dialysis | DHSC RENAL CKD107 | 151 | 0 | 3 | 3 |
| Dietitian | PSSRU108 | 86 | 0 | 1 | 1 |
| Endocrinology | DHSC OP 302 | 164 | 0 | 2 | 2 |
| ENT | DHSC OP 120 | 104 | 4 | 3 | 7 |
| Epidural | DHSC | 412 | 1 | 0 | 1 |
| Gastroenterology | DHSC OP 301107 | 149 | 8 | 6 | 14 |
| Geriatric outpatients | DHSC OP 430107 | 257 | 1 | 0 | 1 |
| GI physiology | DHSC OP 106107 | 147 | 1 | 0 | 1 |
| Gynaecology | DHSC OP 502107 | 142 | 0 | 2 | 2 |
| Haematology | DHSC OP 303107 | 160 | 4 | 6 | 10 |
| Limb fitting | DHSC OP107 | 142 | 0 | 1 | 1 |
| Maxillofacial surgery | DHSC OP 144107 | 126 | 1 | 0 | 1 |
| Memory clinic | DHSC OP 727107 | 136 | 2 | 5 | 7 |
| Neurology | DHSC OP 400107 | 167 | 8 | 9 | 17 |
| Occupational Health | DHSC OP 651107 | 73 | 2 | 0 | 2 |
| Oncology | DHSC OP 370107 | 162 | 7 | 3 | 10 |
| Ophthalmology | DHSC OP 130107 | 98 | 15 | 23 | 38 |
| Orthopaedics | DHSC OP 111107 | 124 | 4 | 7 | 11 |
| Orthotics | DHSC OP 658107 | 126 | 3 | 0 | 3 |
| Outpatient nurse | PSSRU108 | 24 | 1 | 0 | 1 |
| Pain clinic | DHSC OP107 | 145 | 1 | 2 | 3 |
| Phlebotomy | DHSC OP107 | 34 | 2 | 1 | 3 |
| Physiotherapy | DHSC OP 304107 | 74 | 1 | 3 | 4 |
| Plastics | DHSC OP107 | 107 | 0 | 1 | 1 |
| Podiatry | DHSC OP 653107 | 51 | 3 | 2 | 5 |
| Pulmonology | DHSC OP107 | 150 | 0 | 1 | 1 |
| Radiology | DHSC OP107 | 145 | 6 | 8 | 14 |
| Respiratory | DHSC OP107 | 24 | 2 | 2 | 4 |
| Rheumatology | DHSC OP 410107 | 146 | 2 | 6 | 8 |
| Scan | DHSC OP107 | 141 | 1 | 0 | 1 |
| Speech and language therapy | PSSRU108 | 55 | 1 | 0 | 1 |
| Stroke/seizures | DHSC OP 328107 | 216 | 1 | 0 | 1 |
| Surgical | DHSC OP107 | 140 | 1 | 0 | 1 |
| Ultrasound | DHSC IMAGOP107 | 54 | 0 | 1 | 1 |
| Urgent care | DHSC UC107 | 160 | 1 | 0 | 1 |
| Urology | DHSC OP 107107 | 110 | 9 | 8 | 17 |
| Vascular | DHSC OP107 | 147 | 1 | 1 | 2 |
| Walk-in clinic | PSSRU GP108 | 31 | 0 | 1 | 1 |
| X-ray | DHSC IMAGOP107 | 71 | 3 | 3 | 6 |
| Total | 136 | 148 | 284 |
| Reason | JtD intervention, n | Usual care, n | Total, n |
|---|---|---|---|
| Increased confusion | 1 | 0 | 1 |
| Ankle | 1 | 0 | 1 |
| Breathing problems | 2 | 2 | 4 |
| Broken hip | 0 | 1 | 1 |
| COPD | 1 | 0 | 1 |
| Cancer: acute pain | 1 | 0 | 1 |
| Chest pain | 1 | 1 | 2 |
| Collapsed | 1 | 0 | 1 |
| Fall | 9 | 2 | 11 |
| Grazed leg | 1 | 0 | 1 |
| Haemorrhage | 1 | 0 | 1 |
| Heart attack | 0 | 1 | 1 |
| INR excessively high | 1 | 0 | 1 |
| Inflamed tendon and displaced Achilles | 1 | 0 | 1 |
| Pain: compacted bowel | 0 | 1 | 1 |
| Pain in back | 0 | 1 | 1 |
| Pain in chest/abdominal | 0 | 1 | 1 |
| Pain in leg | 1 | 0 | 1 |
| Pneumonia | 0 | 1 | 1 |
| Problems with catheter | 0 | 2 | 2 |
| Severe constipation | 0 | 1 | 1 |
| Suspected atypical TIA | 0 | 1 | 1 |
| Swollen leg | 1 | 0 | 1 |
| Dog bite | 0 | 1 | 1 |
| Urinary issues | 0 | 2 | 2 |
| Total | 23 | 18 | 41 |
| Resource use | Source | Unit cost (£) | JtD intervention, n | Usual care, n |
|---|---|---|---|---|
| Accident and emergency visits | ||||
| 0 | DHSC reference costs AE107 | 160 | 144 | 181 |
| 1 | 19 | 14 | ||
| 2 | 2 | 2 | ||
| Emergency transport | ||||
| 0 | DHSC reference cost (mean of see treat or refer and see treat and convey)107 | 222 | 157 | 187 |
| 1 | 1 | 9 | ||
| 2 | 7 | 1 | ||
| Non-emergency transport | ||||
| 0 | Dialysis Transport: Finding a Way Together (p. 20)134 | 22 | 158 | 194 |
| 1 | 4 | 2 | ||
| 2 | 3 | 0 | ||
| 26a | 0 | 1 | ||
| Day care | PSSRU Table 1.4 (p. 30)108 | 58 | 2 | 2 |
| Lunch clubs, social clubs and other clubs | PSSRU Table 1.4 (p. 30)108 | 13/hour | 17 | 12 |
| Community service | Source | Unit cost (£) | JtD intervention, n | Usual care, n | Overall, n |
|---|---|---|---|---|---|
| GP | PSSRU108 | 31 | 157 | 161 | 318 |
| Practice nurse | PSSRU Table 10.2108 | 36 | 76 | 81 | 157 |
| Memory clinic | DHSC reference costs outpatient107 | 136 | 26 | 21 | 47 |
| Chiropodist | PSSRU band 5108 | 34 | 21 | 22 | 43 |
| District nurse | PSSRU108 | 53 | 13 | 17 | 30 |
| Social worker | PSSRU108 | 44 | 8 | 14 | 22 |
| Physiotherapist | PSSRU Table 17.1108 | 46 | 6 | 15 | 21 |
| Home care worker | PSSRU108 | 22 | 9 | 8 | 17 |
| Community MH nurse | PSSRU per contact108 | 9 | 4 | 12 | 16 |
| Occupational therapy | PSSRU band 5108 | 34 | 10 | 3 | 13 |
| Dentist | PSSRU Table 10.6108 | 104 | 3 | 7 | 10 |
| Nurse | PSSRU nurse band 5108 | 37 | 4 | 4 | 8 |
| Diabetes nurse | PSSRU hospital nurse108 | 54 | 1 | 4 | 5 |
| Pharmacist | PSSRU band 6108 | 45 | 3 | 2 | 5 |
| Speech and language therapy | PSSRU band 5108 | 34 | 3 | 2 | 5 |
| Dietitian | PSSRU Table 7.1108 | 86 | 2 | 2 | 4 |
| Optician | PSSRU108 | 31 | 3 | 1 | 4 |
| Podiatrist | PSSRU band 5108 | 34 | 4 | 0 | 4 |
| Psychologist | PSSRU band 7108 | 54 | 2 | 2 | 4 |
| Phlebotomist | PSSRU mean cost per contact108 | 0.48 | 1 | 2 | 3 |
| Audiology | DHSC reference costs outpatient107 | 108 | 1 | 1 | 2 |
| Community psychiatrist | PSSRU per contact108 | 9.43 | 6 | 1 | 7 |
| First responder | Assume same as paramedic PSSRU108 | 52 | 1 | 1 | 2 |
| Heart nurse | PSSRU band 7108 | 53 | 0 | 2 | 2 |
| Balancing group | DHSC reference costs outpatient107 | 104 | 0 | 1 | 1 |
| Cardiac nurse | PSSRU band 7108 | 53 | 0 | 1 | 1 |
| Care allowance assessment | Assume social worker per hour PSSRU108 | 44 | 0 | 1 | 1 |
| Carers | PSSRU per hour108 | 22 | 1 | 0 | 1 |
| Cognitive stimulation | PSSRU Table 6.7108 | 52 | 1 | 0 | 1 |
| Counsellor | PSSRU band 5108 | 36 | 2 | 0 | 2 |
| CST | PSSRU Table 6.7108 | 52 | 0 | 1 | 1 |
| Health visitor | Assume the same as home care worker PSSRU108 | 22 | 0 | 1 | 1 |
| Home assessment | Assume the same as home care worker PSSRU108 | 22 | 0 | 1 | 1 |
| Hospice | PSSRU Table 7.6, per hour of service108 | 76 | 0 | 1 | 1 |
| Integrated care centre | Costed as social worker PSSRU108 | 44 | 0 | 1 | 1 |
| Paramedic | Assume part of ambulance as also ticked ambulance required | n/a | 0 | 1 | 1 |
| Psychiatrist | PSSRU108 | 341 | 0 | 1 | 1 |
| Sensor team | Costed as social worker PSSRU108 | 44 | 0 | 1 | 1 |
| Thyroid clinic | PSSRU nurse band 5108 | 37 | 1 | 0 | 1 |
| Walk-in centre | PSSRU (assume same as GP)108 | 31 | 0 | 1 | 1 |
| Warfarin clinic | PSSRU nurse band 5108 | 37 | 0 | 1 | 1 |
| Reason for admission | Sourcea | Unit cost (£) | Average LOS (days) | JtD intervention, n | Usual care, n |
|---|---|---|---|---|---|
| Abdominal pain | Elective Inpatient FD05A | 3075 | 4 | 1 | 0 |
| Acute neuropathic pain | Elective Inpatient WH08A | 2063 | 5 | 0 | 1 |
| Breathing problems | Elective Inpatient DZ18D | 1351 | 2 | 0 | 2 |
| Broken hip | Elective Inpatient HE11C | 9733 | 14 | 0 | 1 |
| COPD | Elective Inpatient DZ65E | 3737 | 5 | 1 | 0 |
| Chest pains | Elective Inpatient EB12B | 728 | 1 | 1 | 0 |
| Back issues | Elective Inpatient HC32G | 2526 | 2 | 1 | 0 |
| Detox (alcohol) | Elective Inpatient WH21B | 495 | 1 | 0 | 1 |
| Diarrhoea/dehydration | Elective Inpatient FD01D | 3747 | 6 | 1 | 0 |
| Endoscopy/investigation | Elective Inpatient FE12A | 1015 | 1 | 1 | 0 |
| Haemorrhage | Elective Inpatient AA23F | 2367 | 3 | 1 | 0 |
| Heart attack | Elective Inpatient EB10A | 3940 | 5 | 0 | 1 |
| Increased confusion, possible UTI | Elective Inpatient LA04K | 4487 | 11 | 1 | 0 |
| Pneumonia | Elective Inpatient DZ11P | 4993 | 10 | 1 | 1 |
| Stroke | Elective Inpatient AA35C | 4511 | 11 | 0 | 1 |
| Suspected atypical TIA | Elective Inpatient AA29D | 1109 | 2 | 0 | 1 |
| Urinary issues | Elective Inpatient LA04K | 4487 | 11 | 0 | 2 |
| Compacted bowel | Elective Inpatient FD02C | 5447 | 10 | 0 | 1 |
| Prolapse operation | Elective Inpatient MB09C | 3171 | 3 | 0 | 1 |
| Total | 9 | 13 | |||
| Service used | Source | Unit cost (£) | JtD intervention, n | Usual care, n |
|---|---|---|---|---|
| Community hospital | DHSC reference costs Community Health Services107 | 296 | 1 | 0 |
| Day hospital | DHSC reference costs107 | 327 | 1 | 5 |
| Eye clinic | DHSC reference costs107 | 98 | 1 | 0 |
| GP/nurse | PSSRU108 | 31 | 1 | 1 |
| Podiatry | DHSC reference costs Community Health Services107 | 53 | 0 | 1 |
| Vascular surgery | DHSC reference costs day case VQ50C107 | 481 | 1 | 0 |
| Warfarin clinic | DHSC reference costs consultant-led pain management107 | 101 | 0 | 1 |
| Phlebotomy | DHSC reference costs107 | 34 | 1 | 0 |
| Walk-in centre | PSSRU (assume same as GP)108 | 31 | 1 | 0 |
| Total | 7 | 8 | ||
List of abbreviations
- AfC
- Agenda for Change
- CACE
- complier-average causal effect
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CST
- cognitive stimulation therapy
- CTRU
- Clinical Trials Research Unit
- DEMQOL
- Dementia-Related Quality of Life
- DEMQOL-U
- DEMQOL-Utility
- DFS
- Diener’s Flourishing Scale
- DHSC
- Department of Health and Social Care
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GAD-7
- Generalised Anxiety Disorder-7
- GP
- general practitioner
- GREAT
- Goal-oriented cognitive Rehabilitation in Early-stage Alzheimer’s and related dementias: multicentre single-blind randomised controlled Trial
- GSE
- General Self-Efficacy Scale
- HSCRU
- Health and Social Care Resource Use Questionnaire
- HTA
- Health Technology Assessment
- IADL
- instrumental activities of daily living
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ITT
- intention to treat
- JDR
- Join Dementia Research
- JtD
- Journeying through Dementia
- MCID
- minimum clinically important difference
- MMSE
- Mini Mental State Examination
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- PHQ-9
- Patient Health Questionnaire-9 items
- PPI
- participant and public involvement
- PRIDE
- Promoting Independence in Dementia
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REMCARE
- REMiniscence groups for people with dementia and their family CAREgivers
- SAE
- serious adverse event
- SCQ
- Sense of Competence Questionnaire
- SD
- standard deviation
- SMAS
- Self-Management Ability Scale
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
Notes
-
JtD training delivery checklist: researchers V2.0 20 January 2017
-
JtD training delivery checklist: trainees V2.0 20 January 2017
-
JtD programme delivery checklist: researchers V3.0 12 December 2017
-
JtD programme delivery checklist: facilitators V1.0 19 February 2017
-
JtD individual session checklist: facilitators V1.0 19 February 2017
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/KHHA0861).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
