Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/111/01. The contractual start date was in May 2017. The draft report began editorial review in June 2021 and was accepted for publication in September 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Taylor et al. This work was produced by Taylor et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Taylor et al.
Chapter 1 Introduction
Scientific background
Smoking is the main cause of premature death and preventable morbidity in high-income nations. 1 In England, the broader annual cost to society of smoking is about £11B, including £2.5B to the NHS in England. 2 The smoking prevalence rate among the UK population has reduced to 14.7%,3 but proportions vary widely by socioeconomic and mental health status, which contributes to growing health inequalities.
According to the UK’s National Institute for Health and Care Excellence (NICE) Public Health No. 10 guidelines for smoking cessation,4 smokers are recommended to focus on identifying a quit date and abrupt cessation, using pharmacological and motivational support as appropriate. For those not immediately intending to quit or not wanting to quit, the limited evidence suggests that smoking reduction may lead to a greater likelihood of quitting and subsequent successful abstinence. 5–8 A wide range of approaches to reduction, such as using pharmacological and behavioural support and self-initiated approaches, have been suggested. Motivational support appears to have the potential to reduce smoking, and the more that is provided, the greater the likelihood of successful quitting. 9
Smoking reduction studies fall into two types: (1) those involving smokers who want to quit but are willing to reduce first rather than quit abruptly (see Lindson et al. 6 for a review) and (2) those involving smokers who do not want to quit (immediately) but are interested in smoking reduction or harm reduction (see Lindson-Hawley et al. 7 for a review). None of the studies included in these reviews explicitly involved an intervention to promote physical activity (PA) as part of the behavioural support. Most of the ‘motivational-phase intervention’ studies included in the latter review, which involved the administration of nicotine replacement therapy (NRT) in one form or another, and, to a lesser extent, vaping or a pharmacological aid, provided imprecise evidence of effects on smoking cessation or reduction because of limitations in trial methods. Only two studies (deemed to be of low quality) investigated the effects of behavioural support or advice; these also provided imprecise evidence of effects on smoking cessation or reduction. 10,11 Since then, the findings have been published from two US trials involving smokers not motivated to quit. In one, with 560 participants, there was some evidence that three sessions of two different forms of behavioural support (delivered over 4 weeks), compared with usual care, increased quit attempts and 12-month non-carbon monoxide (CO)-verified abstinence. 12 There was some evidence that changes in targeted constructs mediated the intervention effects on specific outcomes. 13 In the other study, with 255 participants, there was some evidence that four sessions of health education [and, less strongly, motivational interviewing (MI)] support increased smoking abstinence at 6 months. 14 Neither of these studies involved the promotion of PA as a way to manage smoking behaviour.
Data from the English Smoking Toolkit Study (2011–14) suggest that 50% of smokers are interested in smoking reduction and approximately 30% of UK smokers reported using e-cigarettes to achieve this. 15 With concerns about the safety of e-cigarettes and the unwillingness of some smokers to use them, there is a need for other evidence-based approaches to self-regulate smoking. 16
Physical activity as an aid for smoking cessation and reduction
There is considerable interest among smokers in using PA to manage smoking acutely and chronically,17,18 with acute moderate-intensity exercise being as efficacious as vigorous exercise. 19–21 However, a systematic review revealed that only one of 24 randomised controlled trials (RCTs) involving smokers attempting to quit showed that an exercise programme can increase abstinence for at least 6 months, relative to a passive control condition. 22 However, most studies were of low quality and focused on efficacy, rather than being pragmatic and offering an acceptable and feasible intervention for smokers wanting to quit more generally.
The logic behind PA being helpful for managing smoking involves both implicit and explicit processes. 17 For example, a smoker could focus on being physically active with associated emotional benefits, which may reduce cognitive and emotional triggers for smoking implicitly. Explicitly, exercise can acutely manage cigarette cravings and withdrawal symptoms,19,20 and chronically manage weight gain following changes in smoking or nudge smokers towards a healthier identity. 23
Prospective population surveys and trials show that weight gain and fear of weight gain can create a reluctance to quit smoking and remain abstinent; this may be especially true among women and initially heavier smokers. 24–26 A meta-analysis study reported an average gain of 4.67 kg [95% confidence interval (CI) 3.96 to 5.38 kg] after 12 months of abstinence. 27 Among a range of options for preventing long-term weight gain after smoking cessation, PA appears to be one of the most promising,28 by increasing energy expenditure and metabolic rate, and also by self-regulation of energy intake, particularly emotional snacking. 29
The potential for the TARS intervention
In a unique randomised pilot trial, Exercise Assisted Reduction then Stop (EARS), there was encouraging support for the short-term effects of a behavioural intervention for increasing PA and smoking reduction on number of cigarettes smoked and abstinence. 23,30 Previous studies13,14,31 have reported that health education, motivational support targeting perceived confidence and self-regulation to manage smoking can facilitate long-term smoking abstinence, and a mix of these approaches was embedded in the pilot intervention. In the EARS pilot trial,23,30 intervention participants had an average of 4.2 sessions, by telephone or face to face, with a health trainer (HT), with a range of 0 to 8 sessions. Compared with the control group, intervention participants were significantly more likely to reduce smoking by at least 50% (39% vs. 20%), to attempt to quit (22% vs. 6%), and to have CO-verified abstinence at 4 weeks (and up to 8 weeks) after their quit day (14% vs. 4%) and at 16 weeks (10% vs. 4%). More participants in the intervention group reported using PA for controlling smoking: 55% versus 22%, and 37% versus 16%, at 8 and 16 weeks, respectively. 23 A health economic analysis estimated that the intervention cost was approximately £192 per participant and preliminary cost-effectiveness modelling indicated that the intervention is cost-effective. 23
Following the encouraging EARS pilot trial,23 the Trial of physical Activity-assisted Reduction of Smoking (TARS) sought to establish the effectiveness and cost-effectiveness of behavioural support for increasing PA and reducing smoking on longer-term abstinence among smokers not immediately ready to quit.
Developing the TARS intervention
Following the work conducted in the EARS pilot trial, we refined the intervention to ensure that it addressed several issues raised in the process evaluation and that it was fully manualised for transparency and to be operational to guide HT training, supervision and intervention delivery across multiple sites. The intervention components and content and the delivery details have been described elsewhere. 32 Adaptations from the EARS pilot trial intervention included the following: (1) accommodating participants who were using or wanted to use vaping to manage smoking and align with guidance and practice in usual care; (2) improving the focus in the training and supervision of HTs on encouraging participants to manage social influence to increase PA and reduce smoking, which appeared to be the least evident HT competency;33 and (3) improving the focus in the training and supervision of HTs on promoting PA as well as smoking reduction (and quitting), which was also identified in an analysis of HT competencies. Additional patient and public involvement (PPI) work was conducted to assess smokers’ views of the intervention and opportunities for further refinement prior to finalising the intervention manual.
The intervention training manual was used to guide training and supervision of eight HTs across four sites. A group training session was held over 3 days for all HTs and their line managers at the University of Plymouth, the University of Oxford, the University of Nottingham and St George’s, University of London. The process evaluation involved a qualitative analysis of interviews with each HT after training to identify gaps in understanding of the intervention and scope for ongoing supervision. All HTs remained in post throughout the intervention delivery phase of the trial. Further interviews took place with all the HTs after they had had an opportunity to rehearse and deliver the early intervention sessions. Supervision sessions led by Tom P Thompson occurred less frequently as the HTs became more familiar with their role and operating procedures.
The trial was managed by the Peninsula Clinical Trials Unit (PenCTU), but a secure parallel bespoke online programme was also developed to manage the flow of intervention participants and their engagement with the HTs. This allowed Tom P Thompson to manage the HT resource (i.e. assign intervention participants to particular HTs at each site, subject to availability) and also view a live record of the number of sessions with intervention participants and HT notes of sessions to facilitate remote supervision.
Aims and objectives
The overarching aim of the TARS was to establish if an individually tailored behavioural intervention for smokers wanting to reduce but not immediately quit provided an effective and cost-effective approach to supporting increases in PA, smoking reduction, number of quit attempts and subsequent prolonged smoking abstinence.
The specific aims of the trial were to establish if the intervention, compared with support as usual (SAU), would:
-
increase the proportion of participants achieving CO-verified 6-month floating prolonged abstinence at 9 months post baseline
-
increase the proportion of participants reporting a ≥ 50% reduction in the number of cigarettes smoked (between baseline and 3 months, and baseline and 9 months)
-
increase the proportion of participants achieving CO-verified 12-month floating prolonged abstinence at 15 months post baseline
-
increase self-reported PA at 3 and 9 months post baseline, and accelerometer-assessed PA at 3 months post baseline
-
improve body mass index (BMI), quality of life, sleep, cigarette cravings and other beliefs about smoking and PA at 3 and 9 months post baseline.
Further aims were as follows:
-
to estimate the intervention, health-care and social care costs, compared with SAU, at 9 months post baseline and determine the cost-effectiveness of the intervention compared with SAU (1) at 9 months and (2) over a longer-term/lifetime horizon
-
to conduct an embedded mixed-methods process evaluation to explore the mechanisms of action of the intervention and acceptability of trial processes.
Chapter 2 Methods
Some text in this chapter has been reproduced from the study protocol, by Taylor et al. 32 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Trial design
The TARS was a multicentred, pragmatic, two-group, parallel, randomised controlled superiority clinical trial. It compared the effects of (1) tailored support to reduce smoking and increase PA with (2) brief advice to reduce or quit smoking on CO-verified 6-month floating prolonged abstinence and other smoking and PA outcomes. The trial included a mixed-methods embedded process evaluation and economic analysis. The trial design is summarised in Figure 1, from Taylor et al. 32
FIGURE 1.
Participant pathway. Reproduced from Taylor et al. 32 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/.

Recruitment to the trial took place over 16 months (January 2018 to May 2019). Follow-up measures were conducted at 3, 9 and 15 months post baseline. The 15-month assessment was conducted only with those participants who were CO-verified quitters at 9 months.
Ethics approval and research governance
The trial was approved by the South West – Central Bristol Research Ethics Committee (reference number 17/SW/0223) and the Health Research Authority. The trial was registered with the International Standard Randomised Controlled Trial Number register (reference number ISRCTN47776579) prior to commencement.
Patient and public involvement
A group of current and former smokers was convened at an early stage to act as PPI representatives for the trial. The group reviewed the proposed methods, the intervention and any implications for this trial of the use of e-cigarettes and NRT. Views differed on the merits of e-cigarettes and NRT to reduce smoking, and how various forms of PA may help; this was explored further in the set-up phase of the trial. A PPI representative contributed to the trial via their membership of the TARS Project Management Group and Trial Steering Committee (TSC). Prior to and during intervention development and implementation, the trial team also engaged with key stakeholders involved in commissioning and delivering research-type community interventions outside stop smoking services, to assess where the proposed intervention would best fit and its perceived value.
Patient and public involvement representatives provided valuable input into the training of HTs across each site and built on substantial experience gained during the EARS pilot trial. Notably, HTs rehearsed with PPI smokers how best to approach some of the intervention components that had been less well delivered in the EARS pilot trial, such as self-regulation of PA alongside smoking, integrating the concepts of PA and smoking, and working on social influence on the two behaviours.
Participants and settings
Adult smokers wanting to reduce the amount they smoke, but with no immediate plans to quit smoking, were recruited from general practices, secondary care and community settings around four collaborating university sites in the UK: Nottingham, Oxford, Plymouth, and St George’s, University of London (South London).
Inclusion criteria
-
Adult smokers wanting to reduce but not quit smoking in the next month.
-
Aged ≥ 18 years.
-
Smoke ≥ 10 cigarettes per day for at least 1 year. This was irrespective of use of other nicotine-containing products, for example e-cigarettes and/or NRT products.
-
Able to give informed consent.
Exclusion criteria
-
Unable to engage in at least 15 minutes of moderate-intensity PA (as judged by the potential participant).
-
Any illness or injury that might be exacerbated by exercise.
-
Unable to engage in the trial and/or the intervention because of a language barrier or for other reasons (e.g. if the person presents an unacceptable level of risk to the HT or research team members).
Participant identification and approach
The participant pathway is summarised in Figure 1.
A range of methods were employed to identify and approach potential participants, to ensure that the trial was as inclusive as possible:
-
An electronic record search using either EMIS Health (EMIS Health, Leeds, UK) or SystmOne (The Phoenix Partnership Ltd, Leeds, UK) at participating NHS general practices, and, in Plymouth, an electronic record search of stop smoking services records followed by an invitation sent by the general practice.
-
Opportunistically by NHS primary care practitioners, NHS secondary health-care practitioners, NHS Stop Smoking Services, community-based routes (including pharmacies, dental practices, local businesses, adverts in the local media and via social media). Potential participants were then invited to contact the local researcher for further details about the trial.
The strategy used to approach potential participants via general practices was informed by the findings of a study within a trial (SWAT) conducted in the early phase of recruitment to the TARS;34,35 see Report Supplementary Material 1 for details. In brief, the efficiency and value for money of three different invitation methods were compared. These methods were as follows: (1) a full postal information pack sent to potential participants via Docmail® (CFH Docmail Ltd, Radstock, UK) (the commercial communication service embedded in the general practice), (2) a single-page postal invitation via Docmail and (3) a text message sent from the general practice. Despite being the most expensive invitation method, the full postal information pack was the most efficient recruitment method; hence, this was predominantly used by general practices thereafter.
Non-responders to the invitation sent by the general practice were initially contacted by the local researcher, either by post (postcard or letter) or via e-mail, telephone call or text message, and (when possible) subsequently telephoned by a member of the general practice staff, so as not to disadvantage those with low literacy levels.
Screening and informed consent
On receipt of an expression of interest, received by post, e-mail, text message or telephone, the local researcher contacted the potential participant by telephone to discuss the trial and assess eligibility. At this point, the trial-specific participant information sheet was provided (by post or e-mail) to those who had not already received this (e.g. those self-referring via the community-based routes). Subsequently, the local researcher confirmed eligibility and sought informed consent from eligible and willing individuals, verbally (in person or by telephone), and a copy of the completed consent form was then posted to the participant and saved in the PenCTU’s participant records. All ineligible participants were advised to seek smoking reduction advice in line with local usual practice.
Intervention
The intervention was initially designed and evaluated for the EARS pilot trial. 23 There was acceptable training and delivery fidelity for the three HTs involved, and acceptable intervention engagement. There were some signals of effectiveness. The need to make some small adaptations was identified and incorporated into the HT manual,36 and into the training and supervision of eight HTs who delivered the intervention across four sites.
The intervention is described in detail elsewhere. 32 Briefly, participants in the intervention arm were offered individually tailored behavioural support from a HT in up to eight weekly sessions, the goal being to build a participant’s motivation and confidence to reduce smoking and increase PA, and possibly to make a quit attempt. An additional six weekly sessions were offered to those participants who wanted support after quitting smoking. The intervention was based on self-determination theory37 and the use of evidence-based health behaviour change techniques. Self-determination theory helps to link the basic human need to feel competent, autonomous and in control with motives that drive human behaviour. The TARS intervention aimed to enhance participants’ sense of competence, control and connectedness related to reducing smoking and increasing PA. The content had some overlap with interventions included in similar studies with a focus on smoking reduction for those smokers not wanting to immediately quit. 9–11,14 The components of the intervention are summarised in Appendix 1 and were used to assess delivery fidelity in the EARS pilot trial. 33
Support as usual
Following randomisation, all participants (intervention and SAU arms) received standardised written guidance on smoking reduction and cessation from the local researcher, with signposting to the support offered at local level (see Appendix 2). In the absence of formal programmes for use of e-cigarettes or licensed nicotine-containing products (LNCPs) to support reduction, for participants not wanting to immediately quit, participants in both arms purchased their own NRT or e-cigarette product.
Randomisation, allocation concealment and blinding
Following completion of baseline measures, participants were randomised by a member of the PenCTU by means of a web-based system created by the PenCTU in conjunction with a statistician independent of the trial team (ensuring allocation concealment from the research staff).
Participants were individually randomised to either the intervention or the control group (1 : 1 ratio) using random permuted blocks, with stratification by recruitment site and the two-item Heaviness of Smoking Index (HSI). 38 For the purposes of stratification, HSI total scores were categorised as 0–4 (low) or 5 and 6 (high).
It was not possible to blind participants to their allocated group. Every effort was made to ensure that the local researchers conducting follow-up assessments remained blind to a participant’s allocation. Participant self-report questionnaire booklets and accelerometers were posted out from, and returned to, the PenCTU without knowledge of the trial arm allocation. However, it was possible that participants disclosed the nature of any support received to reduce smoking during contact with the researcher. In accordance with the statistical analysis plan (SAP), primary analysis of the primary and secondary outcomes was undertaken by the trial statisticians blinded to allocation group, after the 9-month follow-up. Analyses that necessitated unblinding (i.e. repeats of the primary analysis allowing for partial clustering, sensitivity analyses and analysis of 15-month data) were conducted unblinded.
Data collection and management
Data were collected and maintained at the PenCTU in accordance with the current legal and regulatory requirements [the Data Protection Act 1998,39 the General Data Protection Regulation (GDPR) 201640 and later the Data Protection Act 201841].
Participant numbering
Following receipt of an expression of interest, each individual was allocated a unique number and was subsequently identified in all trial-related documentation by their identification number and initials only. A record of names, addresses, telephone numbers and e-mail addresses linked to participants’ identification numbers was stored securely on the trial database at the PenCTU for the purposes of the trial only.
Data processing
All data recorded in case report forms and questionnaire booklets were double-entered by PenCTU staff into a bespoke password-protected Microsoft SQL Server database (Microsoft Corporation, Redmond, WA, USA) and encrypted using Secure Sockets Layer version 3 (QuoVadis Online Ltd, London, UK). Access to identifiable information was restricted and permission based.
A parallel, linked, secure, bespoke, online data system was used to manage intervention engagement post randomisation. This GDPR-compliant system captured all HT attempted and actual contact with intervention participants in real time to produce summary data and to aid supervision sessions and intervention management (e.g. for use should a HT be unavailable).
Raw data from the accelerometer were downloaded using the GENEActiv personal computer software (version 3.0_09.02.2015) (Activinsights Ltd, Kimbolton, UK) and analysed in R (The R Foundation for Statistical Computing, Vienna, Austria) using package GGIR version 1.2-8 (https://cran.r-project.org/web/packages/GGIR/index.html). GGIR performs autocalibration with the reference of local gravity. 42,43 Raw acceleration data are used to compute Euclidean norm minus one [measured in milligravity units (mg)44]. Data were analysed from the first to the final midnight using 5-second epochs.
Non-wear was detected if the standard deviation (SD) of two axes was < 13 mg with a range of < 50 mg in windows of 60 minutes. Time spent in activity intensities was established using published thresholds. 45
Computed variables included average daily moderate to vigorous physical activity (MVPA) accumulated in any 5-second epochs, and diurnal inactivity.
Data cleaning
Local researchers aided the completion of the baseline questionnaire over the telephone or face to face, but at follow-up the questionnaire was posted to participants. Possible confusion over the reporting of grams or ounces of loose tobacco led to some extreme and implausible values in self-reported smoking for some participants. Hence, loose tobacco amounts of ≥ 2 ounces were reassigned as grams (of relevance to 9 and 16 participants at 3 and 9 months, respectively). However, even after this change, at 3 and 9 months, 28 and 23 participants, respectively, smoked the equivalent of > 100 cigarettes, on average, per day over the preceding week. Therefore, data for the total number of daily cigarettes smoked (derived from self-reported number of cigarettes, cigars and amount of loose tobacco) were recalculated using a truncated upper limit of 100 cigarettes per day.
Some self-reported total weekly minutes of MVPA and sleep also included implausible values. In line with other surveys (e.g. International Physical Activity Questionnaire46), we opted to cap all values of MVPA to 1260 minutes per week (equivalent to 3 hours per day). At 3 months and 9 months, data from 43 and 38 participants, respectively, were truncated. Similarly, the sleep data were truncated to no more than a daily average of 12 hours in the previous week: at 3 months and 9 months, 7 and 6 responses, respectively, were truncated as a result. Values of sleep of < 3 hours were set as missing: at 3 months and 9 months, 16 and 19 responses, respectively, were classed as missing.
Baseline assessment
The baseline assessment booklet was administered to consented participants by the local researcher, over the telephone or in person, depending on local practice and a participant’s preference. Baseline measures are summarised in Table 1.
| Measure | Screening and baseline | Month | ||
|---|---|---|---|---|
| 3 | 9 | 15a | ||
| Demographics (date of birth, gender, ethnic group, relationship status, partner’s smoking status, employment status, education completed) | ✗ | |||
| Self-reported measures | ||||
| Weight and height (to derive BMI) | ✗ | ✗ | ✗ | |
| HSI38 | ✗ | |||
| Number of cigarettes (or equivalent) smoked in a typical day in the previous week | ✗ | ✗ | ✗ | ✗ |
| Frequency of use of smoking management products (LNCPs or e-cigarettes) in a typical day in the previous week | ✗ | ✗ | ✗ | ✗ |
| Quit attemptb (and date of quit attempt) | ✗ | ✗ | ||
| Prolonged abstinence since quitting smoking (at least 6 months) | ✗ | ✗ | ||
| PA and sleep (7-day recall)47 | ✗ | ✗ | ✗ | |
| Health and social care utilisation (resource use questionnaire) | ✗ | ✗ | ✗ | |
| Health-related quality of life (EQ-5D-5L48 and SF-12v249,50) | ✗ | ✗ | ✗ | |
| Serious adverse events | ✗ | ✗ | ||
Process measures:c
|
✗ | ✗ | ||
| Accelerometer-assessed minutes of MVPA (in a sample) | ✗ | |||
| CO-verified abstinence (for self-reported quit attempts at 3 and/or 9 months) or self-reported abstinence (at 15 monthsa) | ✗d | ✗e | ✗f | |
| Qualitative process evaluation in parallel (in a sample) | ✗ | ✗ | ✗ | ✗ |
Follow-up assessments
Follow-up assessments, scheduled at 3, 9 and 15 months post baseline, are summarised in Table 1.
At 3 and 9 months, all participants were posted a self-report questionnaire booklet to complete and return to the PenCTU using prepaid, pre-addressed envelopes. See the questionnaire on the project web page (www.journalslibrary.nihr.ac.uk/programmes/hta/1511101/#/documentation). At 15 months, all participants with CO-verified abstinence at 9 months were posted a self-report questionnaire booklet to complete and return to the PenCTU. Participants received a £20 shopping voucher on receipt of a questionnaire booklet at the PenCTU. To increase response rates, motivational postcards (in sealed envelopes) were posted to participants before the questionnaires were sent out. Standard reminder letters were sent and telephone calls made to remind non-responders to return the questionnaire booklet. To maximise follow-up data on key outcomes, non-responders were then given the option to complete only the key questions about smoking behaviour and to submit these responses by e-mail, telephone or text, if preferred.
Participants who self-reported that they had quit smoking were invited to attend a face-to-face visit with the local researcher to verify abstinence. In making arrangements for the visit, the researcher checked whether or not a participant had restarted smoking in the interim; the visit went ahead only with participants who confirmed that they had not smoked a puff in the 7 days preceding the day of the visit. At the visit, abstinence was verified by expired CO, measured using a CareFusion MicroCO Meter (Williams Medical Supplies Ltd, Rhymney, UK). Expired CO of < 10 parts per million (p.p.m. ) indicated abstinence.
At 3 months, an objective measure of total weekly minutes of MVPA was collected in a sample of participants. Participants were asked to wear a waterproof GENEActiv accelerometer51 on the wrist of the non-dominant hand constantly for 1 week and then return the device to the PenCTU. The finite supply of accelerometers determined which participants were posted an accelerometer (i.e. if an accelerometer was available at the PenCTU to be posted, this was posted to the next participant due a 3-month questionnaire).
Contingency measure for biochemical verification during the coronavirus pandemic in 2020
In accordance with the UK government advice on reducing COVID-19 transmission, verification of self-reported abstinence by expired CO was discontinued from 20 March 2020, to avoid direct contact between participants and local researchers. As a contingency measure, biochemical verification of self-reported abstinence from smoking was achieved by a posted self-test saliva cotinine test, removing the requirement for participants to meet with a researcher face to face, as is the case with the expired CO assessment. This contingency measure applied to a minority of participants (see Chapter 3, Analyses of primary outcome).
The self-test saliva kit was supplied by ABS Laboratories (York, UK). A swab was provided for participants to place under the tongue. Once the swab had become soaked with saliva, the swab was placed into a tube provided, and then into a second (outer) tube ready for posting direct to ABS Laboratories for analysis using the prepaid, pre-addressed envelope provided. Cotinine concentration in the saliva sample was quantified using a validated in-house method (by ABS Laboratories) using protein precipitation with a deuterated cotinine internal standard and analysis by liquid chromatography with tandem mass spectrometry. A saliva cotinine concentration of < 12 ng/ml indicated abstinence. 52
Process evaluation
A mixed-methods process evaluation focused on trial methods (in an internal pilot study), and if and how the intervention was working, using data from the trial database; audio-recordings of intervention sessions; and audio-recorded and transcribed interviews with participants, HTs, research assistants and general practitioners (GPs)/practice managers (PMs). The methods, procedures, data analysis and findings are reported in more detail in Chapter 4.
Measures
Primary outcome measure
The primary outcome measure was CO-verified 6-month floating prolonged abstinence53 derived from CO-verified (following self-reported abstinence) point prevalence abstinence at both 3 and 9 months post baseline.
Secondary outcome measures
Only participants who were CO-verified abstinent at 9 months were followed up at 15 months.
Abstinence measures
-
Self-reported point prevalence abstinence at 3, 9 and 15 months post baseline.
-
Self-reported abstinence at 3 months was defined as follows: participant reported having made a quit attempt (going at least 24 hours without even a puff) since joining the trial and smoked not even a puff of a tobacco product (excluding LNCPs) since the reported quit date.
-
Self-reported abstinence at 9 months was defined as follows: participant reported either not having smoked a puff of a tobacco product (excluding LNCPs) since a quit attempt in the previous 6 months or having smoked fewer than five cigarettes since having made a quit attempt in the first 3 months and had a CO monitoring visit at 3 months.
-
Self-reported abstinence at 15 months was defined as follows: participant reported smoking fewer than five cigarettes since the 9-month CO assessment and not a puff of a tobacco product (excluding LNCPs) in the 7 days preceding the day of CO assessment.
-
-
CO-verified point prevalence abstinence at 3, 9 and 15 months was defined as self-reported abstinence and expired CO of < 10 p.p.m. Note that (1) at 3 and 9 months, only those reporting abstinence were contacted for expired CO assessment (to verify self-reported abstinence) and (2) at 15 months, only those who were CO-verified abstinent at 9 months and who self-reported abstinence at 15 months were contacted for an expired CO assessment (see Figure 1).
-
CO-verified floating prolonged abstinence over any 6-month period between either 3 and 9 months or 9 and 15 months post baseline.
-
CO-verified prolonged abstinence for at least 12 months, derived from CO-verified self-reported point prevalence abstinence at all three follow-up time points.
Non-abstinence smoking measures at 3, 9 and 15 months post baseline
-
Self-reported number of cigarettes smoked on a normal day in the previous week. The number of cigars and weight of loose tobacco were converted into an equivalent number of cigarettes (i.e. 0.45 g of tobacco was the equivalent of one cigarette). 23,54
-
Frequency of use of smoking management products (LNCPs or e-cigarettes) in a typical day in the previous week.
-
Reduction of number of cigarettes smoked (or equivalent) by at least 50% between (1) baseline and 3 months and (2) baseline and 9 months.
Self-reported physical activity at baseline and at 3 and 9 months using the 7-day Physical Activity Recall questionnaire47
-
Total MVPA over the previous 7 days.
-
Daily average time spent sleeping over the previous week.
Objectively assessed physical activity (by wrist-worn accelerometer) at 3 months (in a sample)
-
Average time spent in MVPA each day over 1 week.
Body mass index
-
Body mass index was derived from self-reported height and weight.
Health-related quality of life at baseline and at 3 and 9 months
-
EuroQol-5 Dimensions, five-level version (EQ-5D-5L). 48
-
Short Form questionnaire-12 items, version 2 (SF-12v2). 49,50
An economic evaluation was conducted alongside the trial to estimate (1) the intervention cost, (2) the trial-based cost-effectiveness analysis over 9 months and (3) a model-based long-term cost-effectiveness of the intervention over a lifetime horizon. The methods are described in more detail in Chapter 5.
Sample size
The sample size was calculated based on a two-sided Fisher’s exact test. For an increase of 6% in the CO-verified abstinence rate in the intervention group, compared with the SAU group, between 3 and 9 months post baseline [i.e. from 5% in the SAU group to 11% in the intervention group, corresponding to an odds ratio (OR) of 2.35 or a relative risk of 2.2; number needed to treat 17], 450 participants were required per group, and therefore 900 participants were required in total, to detect this difference at the 5% significance level with 90% power (Table 2). The abstinence rates used to calculate the sample size were conservative estimates, consistent with those from the preceding pilot trial23 and those reported from a systematic review of pharmacological interventions. 55,56
| Assumed relative risk | Percentage of participants achieving CO-verified prolonged abstinence | Power (%) | |
|---|---|---|---|
| SAU group | Intervention group | ||
| 2.20 | 5 | 11 | 90 |
| 2.43 | 3 | 7.3 | 80 |
| 2.70 | 3 | 8.1 | 90 |
| 2.20 | 4 | 8.8 | 81 |
| 2.40 | 4 | 9.6 | 90 |
| 2.04 | 5 | 10.2 | 81 |
| 1.92 | 6 | 11.5 | 80 |
| 2.08 | 6 | 12.5 | 90 |
| 1.76 | 8 | 14.1 | 80 |
| 1.89 | 8 | 15.1 | 90 |
| 1.66 | 10 | 16.6 | 80 |
| 1.77 | 10 | 17.7 | 90 |
As the primary analyses followed the Russell Standard on handling missing cessation outcome data due to loss to follow-up,57 with only participants who were unavoidably lost to follow-up not included (expected to be < 5% of recruited participants), the sample size was not inflated to allow for loss to follow-up.
Table 2 illustrates the range of likely effect sizes detectable, based on recruiting 450 smokers to each group, across plausible values for the control abstinence rate. Under these various scenarios, our sample size would allow us to detect a relative risk ranging, at best, from 1.66 to 2.70.
Statistical methods
The reporting and presentation of this trial is in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines. 58,59 The prespecified analyses were detailed in the SAP, approved by the chief investigator and independent statisticians on the TARS oversight committees; see the SAP on the project web page (www.journalslibrary.nihr.ac.uk/programmes/hta/1511101/#/documentation). The primary analyses of the primary and secondary outcomes were performed following the intention-to-treat principle. All analyses were undertaken using Stata® version 14 (StataCorp LP, College Station, TX, USA), with statistical programming for the primary analyses independently double-coded in R and analyses cross-checked.
Adjustments
No adjustments for multiple analyses were made. Adjustments were made for the stratification variables, namely site as a fixed effect and HSI as a binary factor, as well as the corresponding baseline measure of the outcome being modelled, when appropriate, in fully adjusted models.
Interim analysis
No interim inferential analysis was planned, and none was conducted.
Inferential analyses
Primary analysis of the primary outcome
In line with the Russell Standard schedule,57 participants with missing responses were considered to still be smokers, with the exception of those unavoidably lost to follow-up (defined as participants who had died or moved to an untraceable address). The primary analysis used a multivariable logistic regression model to compare the CO-verified 6-month floating prolonged abstinence rate between 3 and 9 months between allocated groups, with adjustment for the stratification variables. Both adjusted and unadjusted ORs and corresponding 95% CIs are presented, along with the absolute between-group differences in floating prolonged abstinence rates, as recommended in the CONSORT guidelines for parallel-group randomised trials. 60 The interpretation of the primary effectiveness analysis was based on the adjusted OR from the logistic regression model. Intervention effectiveness is also presented as a relative risk, calculated from the estimated OR for the intervention and the baseline rate among the control group, along with the corresponding 95% CI.
Sensitivity analysis of the primary outcome
The following sensitivity analyses of the primary outcome were planned:
-
Participants with missing primary outcome data at 3 or 9 months, otherwise interpreted as still smoking, will be assumed to have quit for the months for which their responses are missing, and the primary analysis will be re-run on a new derivation of the primary outcome from the 3- and 9-month responses, assuming that missing denotes having quit. This may be considered a ‘best-case scenario’.
-
A complier-average causal effect analysis, if > 20% of participants allocated to the intervention group are categorised as not having completed at least two intervention sessions with a HT. Participants in the control group and non-compliers in the intervention group will be compared with compliers in the intervention group.
-
Additional analyses adjusting sequentially for potential confounding variables at baseline [Index of Multiple Deprivation (IMD), self-reported MVPA, use of LNCPs], in addition to the two stratification factors, if imbalance between allocated groups at baseline [> 10% for binary outcomes or > 0.5 SDs for numeric outcomes] is observed.
-
Additional analyses to explore any effect of changing the method of biochemical verification of abstinence from exhaled air testing to saliva testing.
Planned secondary analyses of the primary outcome
Although the trial was not powered to detect the influence of potential moderating variables on the primary outcome, secondary analyses were planned to explore whether or not the intervention effect was modified by any of the following baseline measures: (1) IMD, (2) the use of a smoking cessation medication or a vaping product, (3) self-reported MVPA level, (4) confidence to quit, (5) HSI and (6) recruiting site. The primary analysis multivariable logistic regression model was extended to include the interaction term of allocated group and each of the potential modifying variables. The statistical significance of intervention modification was determined by the p-value of the interaction term between the allocated group and each potential modifying variable and was set at the 1% level of significance.
An exploratory analysis of the HT effect was planned, using a multilevel, mixed-modelling approach, to allow for the partially nested data: participants allocated to the intervention group were partially clustered within the HT, in turn nested within sites.
To explore whether or not the primary outcome was influenced by the intervention dose actually received (i.e. number of HT sessions attended), the primary outcome was modelled on the number of HT sessions attended in the intervention group only, adjusting also for the stratification variables.
Analysis of secondary outcomes
The primary analyses of the secondary outcomes followed a similar approach to that for the primary outcome, using multivariable logistic or linear regression modelling as appropriate, with both adjusted and unadjusted results presented. Additional planned analyses included exploration of whether or not intervention effects were modified by any prespecified baseline characteristics, as outlined for the primary outcome, and exploration of the concordance between self-reported and objective measures of PA.
Adverse events
The recording and reporting of non-serious adverse events in this low-risk trial were not required. Serious adverse events (SAEs) were documented from the time of participant consent until a maximum of 8 weeks after the follow-up assessment at 9 months. Reportable SAEs were detected through self-reported hospital admissions or reported directly by local researchers or HTs. Participant-reported hospitalisations were reviewed by the chief investigator in the first instance, to assess the need for on-reporting, for example to avoid reporting hospitalisations for pre-elective procedures, and similar, as SAEs in this trial. SAEs were listed descriptively by group and included details of the event, and the likely relatedness to either treatment.
Model checking and validation
Checks were undertaken to assess the robustness of statistical models, including visual assessment of model residual normality and heteroscedasticity.
Changes to the project protocol
Omission of Short Form questionnaire-12 items, version 2, data at one site
Owing to an error in the production of the baseline questionnaire booklet, the response options for a number of the items in the SF-12v2 were incorrectly displayed in the booklet used by one of the four sites. Consequently, statistical analysis of SF-12v2 data could not be undertaken as intended and has therefore not been conducted.
Contingency measure for biochemical verification during the coronavirus pandemic
Because of COVID-19, the original method of verification of self-reported abstinence (expired CO) was replaced with a posted self-test for saliva cotinine level.
Participants who had been due to attend a face-to-face CO test for verification of self-reported abstinence from 20 March 2020 onwards were contacted by a researcher who explained the changes to the procedure and introduced the posted self-test for saliva cotinine as an alternative. Verbal consent to the alternative test was taken and documented prior to dispatch of the self-test kit to participants. Along with the kit, consented participants received a participant information sheet that described the new arrangements for verifying self-reported abstinence in this trial, and a step-by-step instruction sheet on how to use the kit. The number of participants for whom this was relevant is reported in Chapter 3.
Chapter 3 Quantitative trial results
Participant recruitment and flow through the trial
A total of 915 participants were recruited to the trial and randomised between January 2018 and May 2019 (16 months).
Figure 2 shows the CONSORT flow diagram for recruitment to the trial. Of the initial 1441 smokers expressing an interest in participating, 915 (63.5%) individuals were contactable and eligible, and eventually randomised. The main reasons for ineligibility were smoking < 10 cigarettes per day (n = 92, 6.4% of those showing an interest), being unable to engage in at least 15 minutes of moderate-intensity PA (n = 16, 1.1%) and wanting to quit immediately (n = 7, < 1%). We were unable to contact 385 (26.7%) smokers who showed an initial interest in the trial.
FIGURE 2.
The TARS CONSORT flow diagram. Follow-up at 15 months was contingent on CO-verified abstinence at 9 months. Reasons for ineligibility, losses to follow-up and not completing verification of self-reported abstinence are given in the following tables in Appendix 4. a, Table 30; b, Table 31; c, Table 32; d, Table 33; e, Table 34; f, Table 35; g, Table 36; h, Table 37; i, Table 38; j, Table 39. QB, questionnaire booklet.

Appendix 3 shows the number and percentage of participants who entered the trial via the different recruitment methods, by allocated group and overall. The findings from a SWAT to examine the effectiveness and associated costs of different approaches to primary care recruitment (i.e. full Docmail invitation, single letter via Docmail, and text message) have been reported elsewhere34,35 (see also Report Supplementary Material 1). The majority of participants (n = 659, 72.0%) were recruited via a general practice, via invitation letter (which may or may not have contained full details of the trial; some participants received full details and some received a brief version and an invitation to read more on the website or by contacting a researcher), text message or opportunistically.
Of the 915 randomised participants, 624 (68.2%) provided follow-up data at 3 months, and 583 (63.7%) provided follow-up data at 9 months (see Figure 2). There was little difference in the proportions followed up between allocated groups.
Baseline participant characteristics and allocated group comparability
Appendix 5 shows the success of the stratified randomisation algorithm in achieving balance between the allocated groups in terms of the stratification factors (HSI and recruiting site). Overall, the percentages of participants recruited in London, Plymouth, Oxford and Nottingham were 31.1%, 27.0%, 23.7% and 18.1%, respectively. Trial outcomes at baseline and follow-up are reported below.
Table 3 shows the baseline sample demographics and smoking indicators by allocated group and for the whole sample. There was good balance between allocated groups at baseline. The mean age of participants was 49.8 (SD 13.9) years, with a wide range (18–82 years). Overall, 55.4% of participants identified as female and 84.9% identified as being white. The majority of the participants (41.9%) were either married or had a partner. The proportion of participants with an advanced level of education (first or higher degree) was 28.6%, and 45.7% of participants were in paid employment. Just under one-third of participants (32.6%) reported having their first cigarette within 5 minutes of waking. The majority of the participants (59.3%) reported smoking 11–20 cigarettes per day at baseline, with a derived 18.5% having a high baseline HSI. The overall mean number of daily cigarettes (derived from self-reported bought cigarettes, loose tobacco and cigars) smoked at baseline was 18.0 (SD 13.4) (Table 4).
| Characteristic | SAU group (N = 458) | Intervention group (N = 457) | Both groups (N = 915) |
|---|---|---|---|
| Age (years), mean (SD) [minimum, maximum] | 50.0 (13.6) [18.0, 82.0] | 49.5 (14.1) [18.0, 81.0] | 49.8 (13.9) [18.0, 82.0] |
| Female, n (%) | 263 (57.4) | 244 (53.4) | 507 (55.4) |
| IMD rank (derived from postcode), mean (SD) [minimum, maximum] | 14,467.6 (8655.3) [36.0, 32,745.0] | 14,393.1 (8823.2) [36.0, 32,844.0] | 14,430.4 (8734.8) [36.0, 32,844.0] |
| Ethnicity, n (%) | |||
| White | 390 (85.2) | 387 (84.7) | 777 (84.9) |
| Black Caribbean | 19 (4.1) | 9 (2.0) | 28 (3.1) |
| Black African | 6 (1.3) | 9 (2.0) | 15 (1.6) |
| Black other | 11 (2.4) | 13 (2.8) | 24 (2.6) |
| Indian | 3 (0.7) | 4 (0.9) | 7 (0.8) |
| Pakistani | 2 (0.4) | 6 (1.3) | 8 (0.9) |
| Bangladeshi | 0 (0.0) | 2 (0.4) | 2 (0.2) |
| Other | 27 (5.9) | 26 (5.7) | 53 (5.8) |
| Missing | 0 (0.0) | 1 (< 0.1) | 1 (< 0.1) |
| Relationship status, n (%) | |||
| Single (never married or civil partnered) | 190 (41.5) | 200 (43.8) | 390 (42.6) |
| Married | 136 (29.7) | 119 (26.0) | 255 (27.9) |
| In a civil partnership | 5 (1.1) | 3 (0.7) | 8 (0.9) |
| Divorced or civil partnership dissolved | 57 (12.4) | 54 (11.8) | 111 (12.1) |
| Widowed or surviving civil partner | 9 (2.0) | 13 (2.8) | 22 (2.4) |
| Common-law partner (living together as if married) | 61 (13.3) | 67 (14.7) | 128 (14.0) |
| Missing | 0 (0.0) | 1 (< 0.1) | 1 (< 0.1) |
| Work situation, n (%) | |||
| Working full time in paid employment | 145 (31.7) | 155 (33.9) | 300 (32.8) |
| Working part time in paid employment | 67 (14.6) | 51 (11.2) | 118 (12.9) |
| Working as a volunteer | 15 (3.3) | 11 (2.4) | 26 (2.8) |
| In full-time education | 14 (3.1) | 21 (4.6) | 35 (3.8) |
| Looking after the home | 27 (5.9) | 18 (3.9) | 45 (4.9) |
| Retired | 76 (16.6) | 70 (15.3) | 146 (16.0) |
| Unemployed | 73 (15.9) | 83 (18.2) | 156 (17.0) |
| Other | 41 (9.0) | 48 (10.5) | 89 (9.7) |
| Education status,a n (%) | |||
| No qualifications | 95 (20.7) | 102 (22.3) | 197 (21.5) |
| GCSE | 259 (56.6) | 256 (56.0) | 515 (56.3) |
| A level | 125 (27.3) | 116 (25.4) | 241 (26.3) |
| First degree | 104 (22.7) | 83 (18.2) | 187 (20.4) |
| Higher degree | 38 (8.3) | 37 (8.1) | 75 (8.2) |
| Partner smokes, n (%) | |||
| No | 285 (62.2) | 264 (57.8) | 549 (60.0) |
| Yes | 132 (28.8) | 145 (31.7) | 277 (30.3) |
| N/A | 40 (8.7) | 46 (10.1) | 86 (9.4) |
| Missing | 1 (< 0.1) | 2 (< 0.1) | 3 (< 0.1) |
| Time after waking of smoking first cigarette (minutes), n (%) | |||
| > 60 | 45 (9.8) | 46 (10.1) | 91 (9.9) |
| 31–60 | 59 (12.9) | 55 (12.0) | 114 (12.5) |
| 6–30 | 205 (44.8) | 207 (45.3) | 412 (45.0) |
| ≤ 5 | 149 (32.5) | 149 (32.6) | 298 (32.6) |
| Number of cigarettes smoked per day, n (%) | |||
| ≤ 10 | 59 (12.9) | 61 (13.3) | 120 (13.1) |
| 11–20 | 272 (59.4) | 271 (59.3) | 543 (59.3) |
| 21–30 | 105 (22.9) | 84 (18.4) | 189 (20.7) |
| > 30 | 22 (4.8) | 41 (9.0) | 63 (6.9) |
| Descriptive data | Baseline | 3-month follow-up | 9-month follow-up | |||
|---|---|---|---|---|---|---|
| SAU | Intervention | SAU | Intervention | SAU | Intervention | |
| Total number of cigarettes smoked dailya | ||||||
| n | 454 | 452 | 283 | 275 | 240 | 244 |
| Mean (SD) | 17.4 (9.9) | 18.2 (13.2) | 26.8 (27.0) | 21.1 (23.6) | 24.2 (23.9) | 22.6 (25.8) |
| Median | 15.6 | 15.0 | 16.0 | 12.0 | 15.0 | 12.0 |
| Minimum, maximum | 2.2, 100.0 | 4.0, 100.0 | 1.0, 100.0 | 1.0, 100.0 | 1.0, 100.0 | 1.0, 100.0 |
| BMI (kg/m2) | ||||||
| n | 448 | 443 | 288 | 301 | 265 | 262 |
| Mean (SD) | 26.4 (5.8) | 26.4 (5.8) | 26.7 (6.1) | 26.1 (5.8) | 26.7 (5.9) | 26.4 (6.1) |
| Median | 25.4 | 25.3 | 25.9 | 25.1 | 25.9 | 25.0 |
| Minimum, maximum | 14.3, 49.8 | 15.2, 51.1 | 15.2, 50.5 | 14.1, 49.5 | 15.4, 50.5 | 14.4, 53.7 |
| Weight (kg) | ||||||
| n | 450 | 443 | 288 | 301 | 265 | 262 |
| Mean (SD) | 76.4 (19.2) | 76.7 (18.7) | 77.7 (19.5) | 76.3 (19.0) | 77.7 (19.4) | 76.9 (20.2) |
| Median | 73.0 | 75.0 | 75.2 | 73.0 | 75.0 | 75.7 |
| Minimum, maximum | 38.1, 175.0 | 39.4, 139.7 | 39.0, 160.0 | 39.0, 142.3 | 38.1, 160.0 | 40.0, 170.1 |
| Self-reported total weekly minutes of MVPAb | ||||||
| n | 458 | 457 | 300 | 308 | 269 | 273 |
| Mean (SD) | 462.4 (419.2) | 456.1 (434.0) | 319.1 (354.9) | 397.7 (389.9) | 330.7 (360.6) | 352.9 (375.5) |
| Median | 360.0 | 315.0 | 210.0 | 274.5 | 210.0 | 240.0 |
| Minimum, maximum | 0.0, 1260.0 | 0.0, 1260.0 | 0.0, 1260.0 | 0.0, 1260.0 | 0.0, 1260.0 | 0.0, 1260.0 |
| Self-reported daily hours spent sleepingc | ||||||
| n | 454 | 452 | 278 | 287 | 247 | 260 |
| Mean (SD) | 6.7 (1.5) | 6.9 (1.6) | 6.9 (1.7) | 7.1 (1.6) | 6.7 (1.6) | 7.0 (1.8) |
| Median | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 |
| Minimum, maximum | 3.0, 12.0 | 3.0, 12.0 | 3.0, 12.0 | 3.0, 12.0 | 3.0, 12.0 | 3.0, 12.0 |
| Accelerometer-measured average daily minutes of MVPAd | ||||||
| n | N/A | N/A | 51 | 54 | N/A | N/A |
| Mean (SD) | 77.1 (55.2) | 91.9 (55.2) | ||||
| Median | 74.3 | 86.6 | ||||
| Minimum, maximum | 0.0, 210.0 | 0.0, 264.9 | ||||
| Accelerometer-measured average daily minutes of MVPAe | ||||||
| n | N/A | N/A | 45 | 42 | N/A | N/A |
| Mean (SD) | 82.4 (53.6) | 95.2 (43.6) | ||||
| Median | 78.5 | 92.3 | ||||
| Minimum, maximum | 10.4, 210.0 | 20.2, 195.3 | ||||
Summary statistics for smoking outcomes, physical activity, sleep and weight/body mass index at baseline and at 3 and 9 months, by allocated group
Table 4 shows descriptive data by allocated group for self-reported smoking level, BMI, weight, and PA and sleep at baseline and at 3 and 9 months, together with accelerometer-recorded PA at 3 months. We do not report the number of cigarettes smoked at 15 months because there are too few participants for meaningful interpretation of these data.
The descriptive data for the total number of cigarettes smoked underwent data cleaning, described in Chapter 2. The summary statistics for the derived daily equivalent total number of cigarettes smoked, prior to truncation, are shown in Report Supplementary Material 2. At baseline, in both the cleaned/truncated and non-truncated data, there was good balance between allocated groups in the total number of cigarettes smoked daily, with a broad range. Among participants providing data at 3 and 9 months, the median number of total cigarettes smoked per day remained fairly stable for participants in the SAU group whereas, on average, intervention participants reduced their consumption compared with baseline. The summary statistics in Table 4 are produced from PA and sleep data that have also undergone data cleaning, described in Chapter 2. Descriptive data before cleaning/truncation for reported PA and sleep are reported in Report Supplementary Material 2.
The descriptive data captured from the separate self-reporting of cigarettes, cigars and loose tobacco consumed are shown in Appendix 6. The percentage of the overall sample who at baseline reported smoking even a puff of bought cigarettes, loose tobacco or cigars was 54.2%, 49.7% and 1.0%, respectively (with some participants smoking more than one tobacco product). Appendix 7 shows the descriptive data when participants with missing data were removed from the calculation of the summary statistics: the percentage of the overall sample who at baseline reported smoking even a puff of bought cigarettes, loose tobacco or cigars was then 64.5%, 62.0% and 1.5%, respectively. The number and percentage of participants who reported using any LNCP at each time point, by allocated group, are also shown in Appendix 7. Additional information about the reported weekly and daily use of LNCPs is shown in Report Supplementary Material 3. At baseline, overall, only 14.0% of participants used any LNCP, increasing to 40.8% and 39.0% overall at 3 months and 9 months, respectively, among participants who were followed up (see Appendix 7) (or 26.0% and 22.8% if we assume that those who were missing at follow-up were not using LNCPs).
Table 5 shows the self-reported, CO-verified point prevalence and 6-month floating prolonged abstinence outcomes across time points by allocated group. At 3 months, 5.5% of participants in the intervention group self-reported being abstinent, compared with 2.8% in SAU group, which dropped to 3.7% and 1.7%, respectively, based on CO-verified abstinence. At 9 months, 8.3% self-reported being abstinent in the intervention group, compared with 7.9% in SAU group, which dropped to 5.5% and 4.6%, respectively, based on CO-verified abstinence. Descriptive data for CO-verified 6-month floating prolonged abstinence (between different time points) are also shown in Table 5. The number and percentage of participants who reported a quit attempt of at least 24 hours, by allocated group, are shown in Appendix 8. At 3 months, 19.2% of responding participants in the intervention group reported a quit attempt, compared with 13.5% in the SAU group. At 9 months, 27.6% of participants in the intervention group who responded reported one or more quit attempts in the previous 6 months, compared with 25.3% in the SAU group.
| Abstinence outcomes | Follow-up, n (%) | |||||
|---|---|---|---|---|---|---|
| 3 months | 9 months | 15 monthsa | ||||
| SAU | Intervention | SAU | Intervention | SAU | Intervention | |
| Self-reported point prevalence abstinence | ||||||
| Yes | 13 (2.8) | 25 (5.5) | 36 (7.9) | 38 (8.3) | 14 (3.1) | 16 (3.5) |
| No | 443 (96.7) | 431 (94.3) | 415 (90.6) | 412 (90.2) | 437 (95.4) | 434 (95.0) |
| Irretrievably lost to follow-upb | 2 (0.4) | 1 (0.2) | 7 (1.5) | 7 (1.5) | 7 (1.5) | 7 (1.5) |
| CO-verified point prevalence abstinence | ||||||
| Yes | 8 (1.7) | 17 (3.7) | 21 (4.6) | 25 (5.5) | 7 (1.5) | 11 (2.4) |
| No | 448 (97.8) | 439 (96.1) | 430 (93.9) | 425 (93.0) | 444 (96.9) | 439 (96.1) |
| Irretrievably lost to follow-upb | 2 (0.4) | 1 (0.2) | 7 (1.5) | 7 (1.5) | 7 (1.5) | 7 (1.5) |
| CO-verified 6-month floating prolonged abstinence between 3 and 9 months | ||||||
| Yes | 4 (0.9) | 9 (2.0) | ||||
| No | 447 (97.6) | 441 (96.5) | ||||
| Irretrievably lost to follow-upb | 7 (1.5) | 7 (1.5) | ||||
| CO-verified 6-month floating prolonged abstinence between 3 and 9 months or 9 and 15 monthsa | ||||||
| Yes | 10 (2.2) | 14 (3.1) | ||||
| No | 441 (96.3) | 436 (95.4) | ||||
| Irretrievably lost to follow-upb | 7 (1.5) | 7 (1.5) | ||||
| CO-verified 12-month floating prolonged abstinence between 3 and 15 monthsa | ||||||
| Yes | 1 (0.2) | 6 (1.3) | ||||
| No | 450 (98.3) | 444 (97.2) | ||||
| Irretrievably lost to follow-upb | 7 (1.5) | 7 (1.5) | ||||
Descriptive summary of serious adverse events, by allocated group
Seven SAEs were reported (seven participants). Summaries are shown in Appendix 9. No SAEs were linked to the intervention or trial methods. Self-reported hospitalisations for three participants could not be followed up, as we were unable to make contact with the participants concerned and their GP did not respond to our enquiries.
Data collected at 15 months
Descriptive data collected for the self-reported number of cigarettes smoked per day and LNCP use at 15 months are not reported in any of the tables or appendices because of the small numbers of participants who provided these data at this time point, given that only those who were CO-verified abstinent at 9 months were contacted for follow-up at 15 months. As Table 5 shows, a higher proportion of intervention participants than SAU participants had CO-verified point prevalence abstinence at 15 months (2.4% vs. 1.5%) and CO-verified floating prolonged abstinence rates, when prolonged abstinence rates including 15-month data were considered.
Analyses of primary outcome
Under the Russell Standard criteria,61 assuming all non-responding participants were still smoking except those irretrievably lost to follow-up (shown in Table 5), the allocated groups contributing to the primary analysis differed in size by only one participant (Table 6). As Table 6 shows, 2.0% (n = 9) of intervention participants had CO-verified 6-month floating prolonged abstinence between 3 and 9 months, compared with 0.9% (n = 4) of SAU participants. From the prespecified primary analysis, the adjusted OR is 2.30 (95% CI 0.70 to 7.56). In other words, the odds of achieving the primary outcome in the intervention group were, on average, more than double the odds in the SAU group. However, the difference was not statistically significant. Table 6 also presents the adjusted absolute difference and relative risk. The unadjusted analyses gave very similar results to the adjusted analyses, across the different methods of estimating the effect of the intervention (see Table 6).
| Analysis | Number of participants in analysis | Outcomes per group, n (%) | Unadjusted analysis | Adjusted analysisa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SAU | Intervention | SAU | Intervention | ORb (95% CI); p-value | Absolute between-group differences in riskc (95% CI); p-value | Relative riskd (95% CI); p-value | ORb (95% CI); p-value | Absolute between-group differences in riskc (95% CI); p-value | Relative riskd (95% CI); p-value | |
| Primary analysis | 451 | 450 | 4 (0.9) | 9 (2.0) | 2.28 (0.70 to 7.46); 0.173 | 0.01 (–0.00 to 0.03); 0.161 | 2.25 (0.70 to 7.27); 0.173 | 2.30 (0.70 to 7.56); 0.169 | 0.01 (–0.00 to 0.03); 0.157 | 2.27 (0.71 to 7.29); 0.169 |
| Best-case scenario sensitivity analysis | 451 | 450 | 124 (27.5) | 109 (24.2) | 0.84 (0.63 to 1.14); 0.262 | –0.03 (–0.09 to 0.02); 0.262 | 0.88 (0.71 to 1.10); 0.263 | 0.84 (0.62 to 1.14); 0.260 | –0.03 (–0.09 to 0.02); 0.259 | 0.88 (0.71 to 1.10); 0.261 |
Applying the prespecified best-case scenario to the primary outcome, the direction of effect switched to favouring the SAU group, with 27.5% of participants (n = 124) categorised as being CO-verified 6-month floating prolonged abstainers in the SAU group, compared with 24.2% (n = 109) in the intervention group, with an adjusted OR of 0.84 (95% CI 0.62 to 1.14). This was reflected in all estimates of the intervention effect, both adjusted and unadjusted, and none of these results was statistically significant.
Owing to the sparse primary outcome data, the planned complier-average causal effect analysis, to estimate the intervention effect among participants attending at least two HT sessions, was not performed. Instead, descriptive statistics of the primary outcome by the prespecified categories of engagement are presented in Appendix 10. All nine participants in the intervention group who achieved the primary outcome attended at least two HT sessions.
The SAP prespecified a sensitivity analysis including additional adjustment for selected baseline characteristics if any were imbalanced between allocated groups. As there was no evidence of imbalance, this sensitivity analysis was not required.
The SAP also detailed planned sensitivity analyses to explore the impact of the required changes in verifying self-reported abstinence as a result of COVID-19; however, again because of the sparse primary outcome data, only descriptive statistics are reported. At the 9-month follow-up, 46 CO tests and two saliva tests were carried out among participants who had self-reported being abstinent from smoking. There were 24 out of 25 (96.0%) subsequent CO-verified abstinences in the intervention group and 20 out of 21 (95.2%) in the SAU group. Both saliva cotinine tests (one in each allocated group) verified the self-reported abstinence at 9 months. At the 15-month follow-up, 12 CO tests and nine saliva cotinine tests were carried out in participants who self-reported being abstinent from smoking. All 12 CO tests confirmed self-reported abstinences (eight in the intervention group, four in the SAU group). Of the nine saliva cotinine tests, three out of five (60.0%) confirmed the self-reported abstinences in the intervention group and three out of four (75.0%) confirmed the self-reported abstinences in the SAU group.
Secondary analyses of the primary outcome
The planned formal interaction analyses of the primary outcome with respect to prespecified potential moderating variables were not undertaken as there were too few instances of CO-verified 6-month floating prolonged abstinence between 3 and 9 months. However, the distributions of the 13 participants who were CO-verified abstinent across the prespecified moderating variables are shown in Appendices 11 and 12. The sparseness of the primary outcome data also meant that there was inadequate information to support the planned mixed-effects modelling with partial clustering by HT and the exploration of whether or not the number of HT sessions attended was associated with the primary outcome in the intervention group.
Primary analyses of the secondary outcomes
We described how self-reported smoking data were cleaned/truncated in Chapter 2; descriptive data are presented for cleaned/truncated data in this chapter and for non-truncated data in Appendices 13, 14 and 17, and Report Supplementary Material 2. Sensitivity analyses revealed no effects on the analyses as a result of these data-cleaning procedures.
Table 7 shows the estimated intervention effects on the 3-, 9- and 15-month point prevalence and 6-month floating prolonged abstinence smoking outcomes. Only self-reported point prevalence abstinence at 3 months was (marginally) statistically significant, in favour of the intervention group (5.5% vs. 2.9% for the intervention and SAU groups, respectively). The adjusted OR was 1.99 (95% CI 1.00 to 3.94). This effect was weaker and marginal, again in favour of the intervention group, for CO-verified point prevalence abstinence at 3 months (adjusted OR 2.19, 95% CI 0.93 to 5.14). There was no evidence of an intervention effect on point prevalence abstinence (from self-reported or CO-verified measures) at 9 or 15 months. When expressed as a risk difference, the estimated intervention effect on CO-verified 12-month floating prolonged abstinence between 3 and 15 months was marginal (adjusted analysis: risk difference 0.02, 95% CI 0.00 to 0.04), whereas, despite a point estimate adjusted OR of 6.33, the OR was not statistically significant (95% CI 0.76 to 53.10).
| Outcome | Number of participants in analysis | Outcomes per group, n (%) | Unadjusted analysis | Adjusted analysisa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SAU group | Intervention group | SAU group | Intervention group | ORb (95% CI); p-value | Absolute between-group differences in riskc (95% CI); p-value | Relative riskd (95% CI); p-value | ORc (95% CI); p-value | Absolute between-group differences in riskc (95% CI); p-value | Relative riskd (95% CI); p-value | |
| CO-verified abstinence at 3 months | 456 | 456 | 8 (1.8) | 17 (3.7) | 2.17 (0.93 to 5.08); 0.074 | 0.02 (–0.00 to 0.04); 0.067 | 2.12 (0.93 to 4.87); 0.075 | 2.19 (0.93 to 5.14); 0.071 | 0.02 (–0.00 to 0.04); 0.064 | 2.14 (0.93 to 4.90); 0.072 |
| CO-verified abstinence at 9 months | 451 | 450 | 21 (4.7) | 25 (5.6) | 1.20 (0.66 to 2.18); 0.540 | 0.01 (–0.02 to 0.04); 0.540 | 1.19 (0.68 to 2.10); 0.540 | 1.21 (0.66 to 2.19); 0.539 | 0.01 (–0.02 to 0.04); 0.539 | 1.19 (0.68 to 2.10); 0.539 |
| CO-verified abstinence at 15 months | 451 | 450 | 7 (1.6) | 11 (2.4) | 1.59 (0.61 to 4.14); 0.343 | 0.01 (–0.01 to 0.03); 0.338 | 1.57 (0.62 to 4.03); 0.343 | 1.61 (0.62 to 4.21); 0.332 | 0.01 (–0.01 to 0.03); 0.328 | 1.59 (0.62 to 4.04); 0.332 |
| Self-reported abstinence at 3 months | 456 | 456 | 13 (2.9) | 25 (5.5) | 1.98 (1.00 to 3.91); 0.051 | 0.03 (0.00 to 0.05); 0.046 | 1.92 (1.00 to 3.71); 0.051 | 1.99 (1.00 to 3.94); 0.049 | 0.03 (0.00 to 0.05); 0.045 | 1.93 (1.00 to 3.72); 0.050 |
| Self-reported abstinence at 9 months | 451 | 450 | 36 (8.0) | 38 (8.4) | 1.06 (0.66 to 1.71); 0.801 | 0.00 (–0.03 to 0.04); 0.801 | 1.06 (0.68 to 1.64); 0.801 | 1.07 (0.66 to 1.72); 0.794 | 0.00 (–0.03 to 0.04); 0.794 | 1.06 (0.69 to 1.64); 0.794 |
| Self-reported abstinence at 15 months | 451 | 450 | 14 (3.1) | 16 (3.6) | 1.15 (0.55 to 2.39); 0.706 | 0.00 (–0.02 to 0.03); 0.706 | 1.15 (0.57 to 2.32); 0.706 | 1.15 (0.56 to 2.40); 0.700 | 0.00 (–0.02 to 0.03); 0.700 | 1.15 (0.57 to 2.32); 0.700 |
| CO-verified 6-month floating prolonged abstinence between 3 and 9 months or 9 and 15 monthse | 451 | 450 | 10 (2.2) | 14 (3.1) | 1.42 (0.62 to 3.22); 0.407 | 0.01 (–0.01 to 0.03); 0.405 | 1.40 (0.63 to 3.13); 0.407 | 1.43 (0.62 to 3.26); 0.398 | 0.01 (–0.01 to 0.03); 0.396 | 1.41 (0.64 to 3.13); 0.399 |
| CO-verified 9-month floating prolonged abstinence between 3 and 15 monthse | 451 | 450 | 1 (0.2) | 6 (1.3) | 6.08 (0.73 to 50.72); 0.095 | 0.01 (–0.00 to 0.02); 0.057 | 6.01 (0.73 to 49.75); 0.096 | 6.33 (0.76 to 53.10); 0.089 | 0.02 (–0.00 to 0.04); 0.053 | 6.17 (0.75 to 50.84); 0.091 |
Additional information on the estimated intervention effects on the proportion of participants self-reporting at least one quit attempt of at least 24 hours’ duration, reported at 3 and 9 months, is provided in Report Supplementary Material 4.
Table 8 shows that there was a statistically significant intervention effect in terms of the proportion of participants reducing their smoking level by at least 50%, relative to baseline, at both 3 months (18.9% in the intervention group vs. 10.5% in the SAU group, adjusted OR 1.98, 95% CI 1.35 to 2.90) and 9 months (14.4% in the intervention group vs. 10.0% in the SAU group, adjusted OR 1.52, 95% CI 1.01 to 2.29).
| Outcome | Number of participants | Outcomes per group, n (%) | Unadjusted analysis | Adjusted analysisa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SAU group | Intervention group | SAU group | Intervention group | ORb (95% CI); p-value | Absolute between-group differences in riskc (95% CI); p-value | Relative riskd (95% CI); p-value | ORb (95% CI); p-value | Absolute between-group differences in riskc (95% CI); p-value | Relative riskd (95% CI); p-value | |
| Reduced smoking by ≥ 50% between baseline and 3 months | 456 | 456 | 48 (10.5) | 86 (18.9) | 1.98 (1.35 to 2.89); < 0.001 | 0.08 (0.04 to 0.13); < 0.001 | 1.79 (1.29 to 2.49); < 0.001 | 1.98 (1.35 to 2.90); < 0.001 | 0.08 (0.04 to 0.13); < 0.001 | 1.79 (1.29 to 2.49); < 0.001 |
| Reduced smoking by ≥ 50% between baseline and 9 months | 451 | 450 | 45 (10.0) | 65 (14.4) | 1.52 (1.02 to 2.28); 0.042 | 0.05 (0.00 to 0.09); 0.040 | 1.45 (1.01 to 2.07); 0.042 | 1.52 (1.01 to 2.29); 0.043 | 0.04 (0.00 to 0.09); 0.042 | 1.44 (1.01 to 2.05); 0.044 |
Table 9 shows that there was a statistically significant intervention effect on the total number of cigarettes smoked per day at 3 months (derived from self-reported cigarettes, cigars and loose tobacco smoked), with the intervention group reporting smoking, on average, almost six fewer cigarettes per day than the SAU group, relative to baseline (adjusted mean difference –5.62, 95% CI –9.80 to –1.44). There was no evidence of a difference at 9 months. These conclusions were consistent when the analyses were re-run on the pre-truncated data (see Appendix 13).
| Cigarettes smoked | SAU group | Intervention group | Unadjusted analysis | Adjusted analysisa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | n (%) | Mean (SD) | Mean differenceb (95% CI) | p-value | SAU group (n) | Intervention group (n) | Mean differenceb (95% CI) | p-value | |
| Number of cigarettes smoked daily from cigarettes, cigars and loose tobacco at 3 months | 283 (61.8) | 26.8 (27.0) | 275 (60.2) | 21.1 (23.6) | –5.69 (–9.92 to –1.46) | 0.008 | 280 | 274 | –5.62 (–9.80 to –1.44) | 0.009 |
| Number of cigarettes smoked daily from cigarettes, cigars and loose tobacco at 9 months | 240 (52.4) | 24.2 (23.9) | 244 (53.4) | 22.6 (25.8) | –1.55 (–6.00 to 2.90) | 0.494 | 237 | 243 | –0.95 (–5.37 to 3.46) | 0.671 |
Table 10 shows that there was a significant intervention effect on self-reported weekly MVPA at 3 months, but not at 9 months. At 3 months, the intervention group reported doing an average of 82 minutes more MVPA per week (95% CI 29 to 134 minutes) than the SAU group, having adjusted for baseline MVPA and stratification factors, based on the analysis of the truncated data. These conclusions were consistent when the analyses were re-run on the pre-truncated data (see Appendix 14). There was no evidence of a significant between-group difference in accelerometer-recorded MVPA among the subsample of those wearing an accelerometer at 3 months (regardless of whether or not this analysis was adjusted for baseline self-reported MVPA). The correlation between self-reported and accelerometer-measured MVPA (with at least 1 day of 10 hours of wear-time) at 3 months among the subcohort receiving the accelerometer devices was 0.204 (95% CI –0.002 to 0.409). This was too low, according to the prespecified cut-off point (of > 0.3) stipulated in the SAP, to justify further analysis comparing agreement between the two measures of MVPA at 3 months.
| MVPA | SAU group | Intervention group | Unadjusted analysis | Adjusted analysisa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | n (%) | Mean (SD) | Mean differenceb (95% CI) | p-value | SAU group (n) | Intervention group (n) | Mean differenceb (95% CI) | p-value | |
| Self-reported total weekly minutes of MVPA at 3 months | 300 (65.5) | 319.1 (354.9) | 308 (67.4) | 397.7 (389.9) | 78.60 (19.17 to 138.03) | 0.010 | 300 | 308 | 81.61 (28.75 to 134.47) | 0.003 |
| Self-reported total weekly minutes of MVPA at 9 months | 269 (58.7) | 330.7 (360.6) | 273 (59.7) | 352.9 (375.5) | 22.16 (–39.97 to 84.29) | 0.484 | 269 | 273 | 23.70 (–33.07 to 80.47) | 0.413 |
| Average daily minutes of accelerometer-measured MVPA at 3 monthsc | 51 (11.1) | 77.1 (55.2) | 54 (11.8) | 91.9 (55.2) | 14.80 (–6.58 to 36.18) | 0.173 | 51 | 54 | 14.24 (–7.88 to 36.37) | 0.204 |
| Average daily minutes of accelerometer-measured MVPA at 3 monthsd | 45 (9.8) | 82.4 (53.6) | 42 (9.2) | 95.2 (43.6) | 12.80 (–8.11 to 33.71) | 0.227 | 45 | 42 | 13.88 (–7.74 to 35.50) | 0.205 |
As the results in Appendices 15–17 show, there was no evidence of a statistically significant intervention effect on any of the remaining secondary outcomes (LNCP use, BMI or sleep) at 3 or 9 months.
Secondary analyses (potential modifiers) of secondary outcomes
Similarly to the primary outcome, the number of 6-month floating prolonged abstinences between 9 and 15 months was too small to enable us to undertake the planned analysis of interaction effects between the allocated group and the prespecified potential modifiers (e.g. IMD). Interaction analyses were performed for the reduction in smoking outcomes as planned. The potential interaction effects were also explored for any CO-verified 6-month floating prolonged abstinence (i.e. between 3 and 9 months or 9 and 15 months) and point prevalence abstinence secondary outcome measures, as well as self-reported amount smoked daily in equivalent number of cigarettes, LNCP use, MVPA and BMI. The interaction models could not be fitted at all to the prolonged 12-month abstinence outcome owing to the sparseness of the data. Appendix 18 shows the p-values for the interaction effects when the model ran successfully.
None of the interactions between the allocated group and each potential modifier was found to be statistically significant for any of the reduction in smoking outcomes, any of the point prevalence abstinence outcomes or other prolonged abstinence secondary outcomes.
The IMD was found to have a statistically significant modifying effect on the intervention effect for the number of cigarettes smoked daily at both 3 months (p = 0.004) and 9 months (p = 0.001), but in differing ways. To illustrate this modifying effect, the mean numbers of cigarettes smoked daily for those in the 10th and 90th centiles of IMD rank are displayed in Figures 3 (at 3 months) and 4 (at 9 months), with the intervention group in light blue and the SAU group in dark blue. At 3 months, it appears that the intervention was most effective, compared with SAU, for participants in areas with lower IMD values (more deprivation) and became increasingly less effective up the social gradient to a point where it was not more effective than SAU among participants in areas with the least deprivation. At 9 months, the intervention appeared to be more effective than SAU in the most deprived areas, but SAU was more effective than the intervention in the least deprived areas.
FIGURE 3.
Interaction plot of allocated group and IMD rank for number of equivalent cigarettes smoked daily at 3 months. Predictive margins with 95% CIs.

FIGURE 4.
Interaction plot of allocated group and IMD rank for number of equivalent cigarettes smoked daily at 9 months. Predictive margins with 95% CIs.
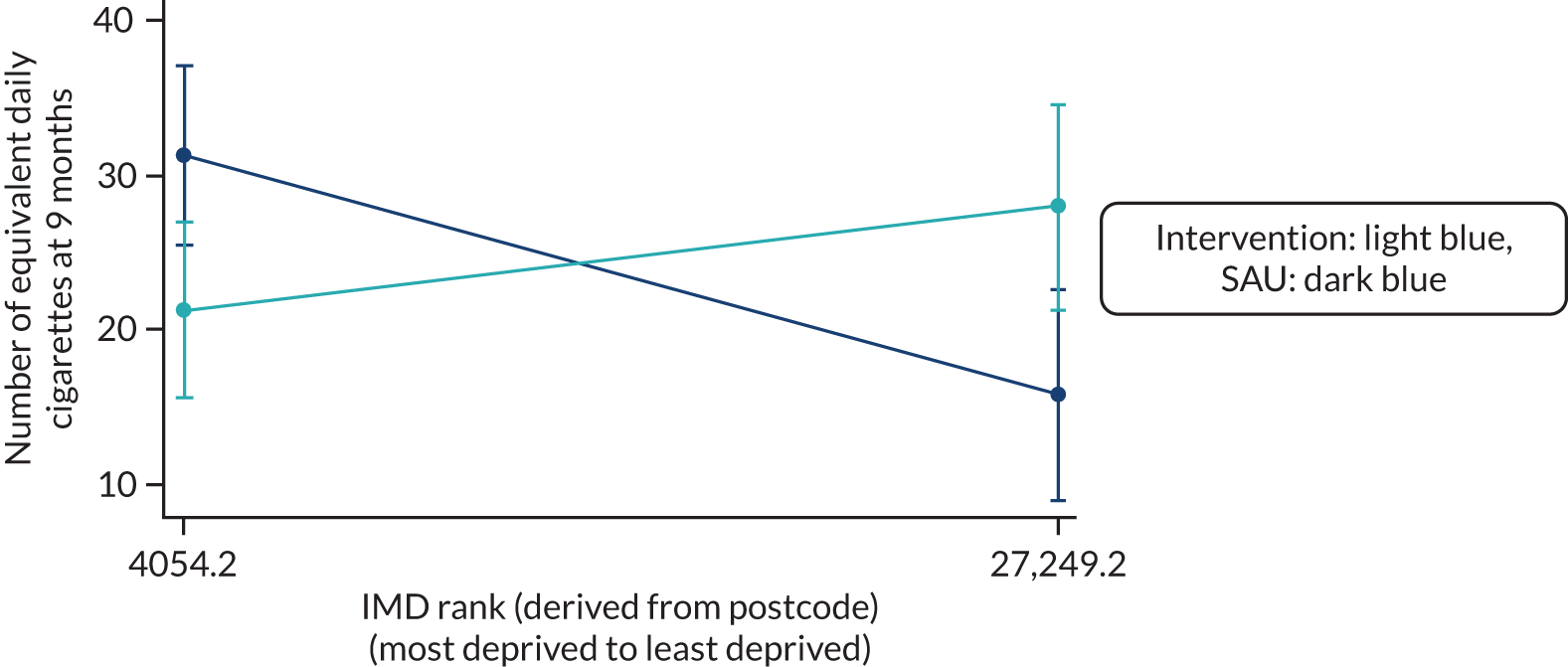
Chapter 4 Process evaluation
Introduction
The TARS intervention was designed to promote a decrease in smoking behaviour (cessation and reduction) and an increase in PA via changes in targeted processes outlined in our intervention process model (Figure 5). It was intended that changes in the two behaviours (smoking and PA) would be explicitly and/or implicitly integrated.
FIGURE 5.
Intervention process model.
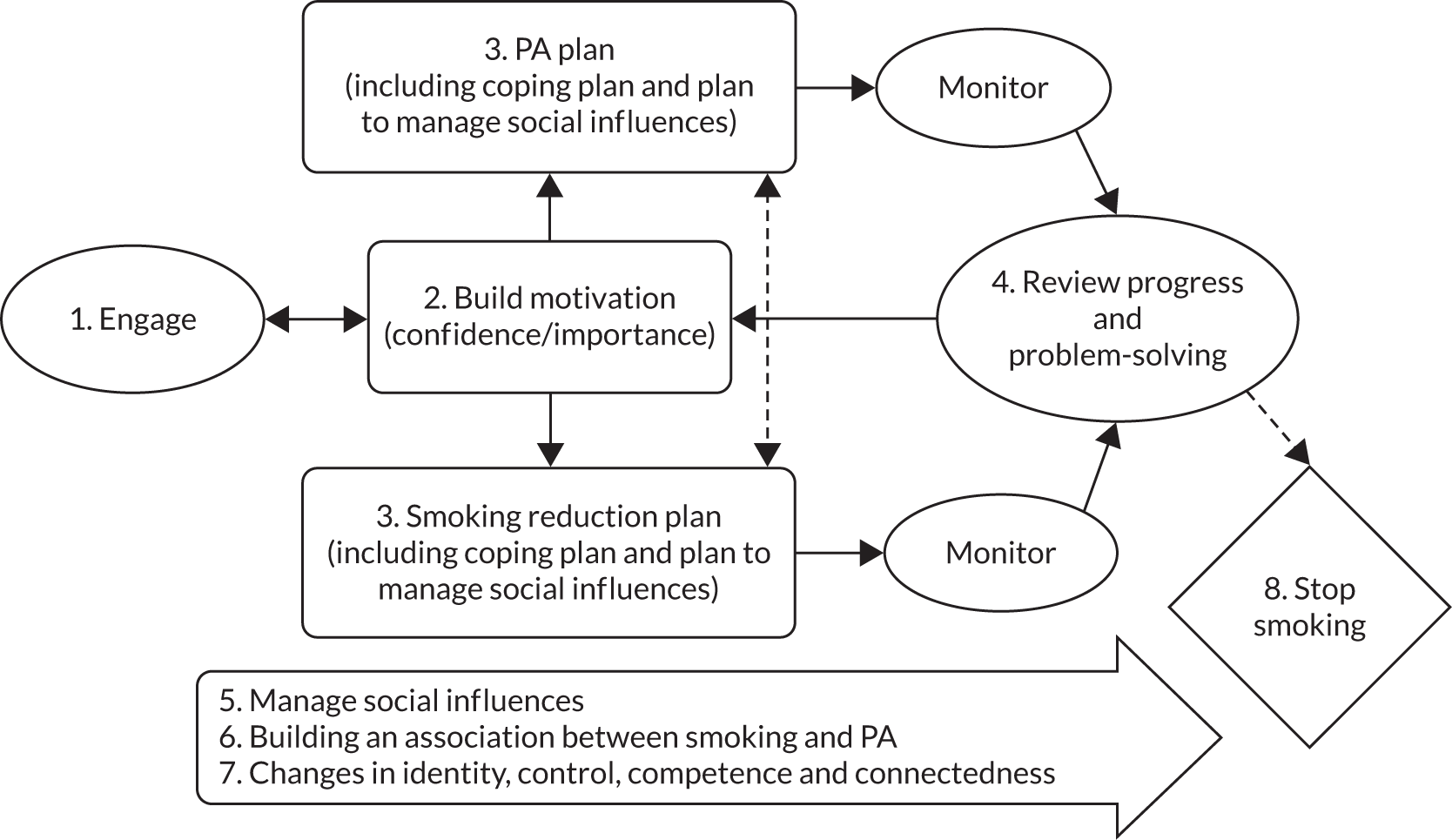
The process evaluation aimed to:
-
assess the extent to which participants engaged with the intervention
-
assess whether or not key intervention processes were delivered, received and enacted as intended
-
illustrate how (and if) the intervention processes worked
-
understand how people engaged with the intended intervention processes.
This mixed-methods process evaluation was designed with multiple complementary workstreams embedded within the delivery of the TARS. The intervention was designed around a process model that embedded a set of intervention components and core competencies (CCs) developed through the pilot trial (see Appendix 1). The model and CCs also underpinned the development of the HT manual;36 the HT training; and the delivery of the intervention, including ongoing supervision.
Methods
The embedded mixed-methods process evaluation was split into two phases: (1) an initial evaluation linked to the internal pilot phase (months 1–4) and (2) the subsequent main trial phase consisting of four workstreams to support the aims of the process evaluation.
The workstreams for the main trial consisted of (1) data related to levels of intervention engagement (aim 1); (2) assessment of delivery, receipt and enactment fidelity (aim 2); (3) mediation analyses of changes in PA and process measures on outcomes (aim 3); and (4) an embedded qualitative study (aims 3 and 4).
Data collection for the internal pilot phase
Semistructured interviews were conducted with HTs (n = 8), intervention participants (n = 3), control participants (n = 4), study decliners (n = 7), general PMs (n = 2) and research assistants (n = 5) within 3 months of commencing the trial.
Data collection and sources for the main trial process evaluation
The data corpus for the process evaluation consisted of a range of data types collected from multiple sources and split into data sets to meet the aims and workstreams within the process evaluation, as shown in Table 11.
| Data source | Process evaluation workstream and aim | |||
|---|---|---|---|---|
| Intervention engagement (aim 1) | Intervention delivery, receipt and enactment fidelity (aim 2) | Mediation analyses (aim 3) | Qualitative study (aims 3 and 4) | |
| Participant interviews (n = 24) | ✗ (R, E) (n = 24) | ✗ (n = 16) | ||
| HT interviews (n = 8) | ✗ | |||
| Main trial data set | ✗ | ✗ | ||
| Quantitative process measures | ✗ (E) | ✗ | ||
| HT database | ✗ | |||
| Audio-recordings of intervention sessions (n = 72) | ✗ (D) | |||
Participant interviews
Interviews were conducted with 24 intervention participants within 4 months of completing the intervention, and again at up to 6 months post intervention. Topic guides (see Appendix 19) were used to guide semistructured interviews. The comprehensive topic guides generated data that could be used for multiple components of work within and beyond this process evaluation. Demographics of those sampled for interview can be found in Table 12.
| Demographic | Value |
|---|---|
| Total sample (n) | 24 |
| Age (years), mean (SD) | 52.5 (12.54) |
| Number of cigarettes smoked per day, mean (SD) | 19.1 (14.9) |
| Gender, n (%) | |
| Male | 17 (70.8) |
| Female | 7 (29.2) |
| Site, n (%) | |
| Plymouth | 6 (25) |
| Nottingham | 6 (25) |
| London | 6 (25) |
| Oxford | 6 (25) |
| Ethnic group, n (%) | |
| White | 21 (87.5) |
| Pakistani | 1 (4.2) |
| Black (other) | 1 (4.2) |
| Other | 1 (4.2) |
Health trainer interviews
All HTs (n = 8) were interviewed twice: once following training before commencing intervention delivery (for the internal pilot) and again towards the end of the trial when they were well experienced in intervention delivery. Topic guides (see Appendix 20) were used to guide semistructured interviews.
Main trial data set
The main trial data were utilised to support the aims of the process evaluation as necessary (i.e. mediation analyses).
Quantitative process measures
Quantitative process measures were collected at baseline and at 3 and 9 months simultaneously with the main trial data set.
Health trainer database
A bespoke online database was created for HTs to record details of all sessions with participants, including details of date, time, location, duration and format.
Audio-recordings of intervention sessions
With consent, all intervention sessions between HTs and participants were audio-recorded and recordings were stored securely.
Structure of this chapter
A summary of the internal pilot phase is presented, followed by sections for each workstream. Each subsequent section has a brief description of the methods for that section/workstream, followed by the results. The overall discussion brings together the results to describe a narrative of the processes triangulated from multiple data sources.
Internal pilot phase
Summary
Qualitative methods were used to evaluate processes, methods, acceptability and implementation related to recruitment, implementation and engagement of the intervention and trial methods in the early phases of the trial. A detailed analysis of the data from this phase can be found in Appendix 21. In summary, the HT training, manual and HT perceptions of delivering the intervention were found to be acceptable, with a few recommendations for improvement. The main concern in this phase was the lower than expected uptake from posted invitations to patients in primary care. A SWAT is described in Chapter 2, which informed the most efficient primary care recruitment method. Initial engagement with the intervention was also found to be acceptable, with participants responding positively to the materials, core components and structure of the delivery.
A substudy was also conducted by a member of the research team (Mary George) as part of her work towards a Doctor of Psychology (DPsych) in health psychology. The substudy examined the acceptability of the trial methods, a summary of which can be found in Appendix 22. In summary, trial methods were deemed to be acceptable to participants.
Intervention engagement
Methods
Intervention records were collected by a bespoke online system, developed and maintained in the PenCTU alongside the bespoke trial database, which allowed HTs to record descriptive data on sessions, including the following: site, date, time, location, duration and format (post-session case notes could also be uploaded and sessions that were not attended could be recorded). Data for looking at predictors of intervention engagement were taken from the main trial data set. The criterion of attending two or more intervention sessions was used to represent an acceptable level of engagement with the intervention (as used in the internal pilot phase progression criteria).
Results
Details of intervention engagement levels by site and type of session can be found in Table 13.
| Site | Number of sessions, mean (SD) | Face to face vs. telephone (%) | Session duration (minutes), mean (SD) | ||
|---|---|---|---|---|---|
| Telephone | Face to face | Overall | |||
| Plymouth | 3.9 (2.8) | 51.7 vs. 47.2 | 28.2 (14.5) | 52.4 (17.3) | 40.6 (20.1) |
| Nottingham | 5.7 (2.7) | 34.9 vs. 64.8 | 22.1 (12.1) | 41.2 (16.2) | 28.8 (16.4) |
| Oxford | 6.8 (3.5) | 68.4 vs. 31.1 | 25.5 (12.1) | 51.9 (15.7) | 43.4 (19.3) |
| London | 3.5 (3.2) | 34.1 vs. 65.3 | 11.5 (6.7) | 26.4 (9.8) | 16.3 (10.7) |
| Across all sites | 4.8 (3.4) | 49.6 vs. 49.9 | 20.9 (13.1) | 46.3 (18.0) | 33.5 (20.3) |
Overall, 76% of participants had two or more sessions (range across sites 58–89%). The mean number of sessions across sites varied from 3.5 to 6.8 and overall the numbers of face-to-face and telephone interiews were approximately equal. The face-to-face sessions lasted over twice as long as the telephone sessions; the average duration of all sessions was 33.5 minutes.
Predictors of intervention engagement levels
Baseline (self-reported MVPA) demographic characteristics (age, gender) and trial site were checked for associations with level of intervention engagement (fewer than two sessions vs. two or more sessions). The only noticeable association was with site: 59.5% and 90.4% of participants in London and Nottingham, respectively, completed at least two sessions with a HT, as shown in Appendix 23.
Intervention fidelity
A comprehensive assessment of fidelity, beyond the scope of this report, is being completed by a member of the research team (Jane Horrell) as part of their ongoing Doctor of Philosophy (PhD) course of study. The work will provide a detailed assessment of the five Behavior Change Consortium domains of fidelity (design, training, delivery, receipt and enactment)62 while also developing and testing novel methods for assessing fidelity. Outputs from the PhD will be published elsewhere.
This report provides an assessment of how well the intervention was delivered in line with intended processes (delivery fidelity), how well the participants understood the processes (receipt) and whether or not the participants showed any evidence of enacting the processes and behaviours after receiving the intervention (enactment).
Intervention delivery fidelity assessment
Delivery fidelity62 assesses the extent to which the TARS intervention was delivered as intended and enhances the reliability of findings by increasing confidence that changes in dependent variables can be attributed to the intervention, should fidelity be high. 62,63 Identifying if elements of an intervention have been delivered below an expected standard also allows for the identification of areas for future improvement. A detailed description of the 11 intervention competencies (CC 1–11) intended for delivery in the TARS intervention are provided in Appendix 1.
Methods
Twenty-four participants (three per HT) were purposively sampled equally across the four sites, to represent a range of demographics. Three audio-recordings of delivered sessions (n = 72) were assessed for each participant (the first session, a subsequent session and the final session) to ensure that all the CCs were evidenced as participants progressed through the intervention. The coding methods have been used previously33 and involved two trained independent researchers who provided an overall score for each HT–participant based on all three recorded sessions. The Dreyfus model of skill acquisition64 was used to assign a score for the HT delivery of each of the 11 CCs on a 7-point Likert scale (0–6, from incompetent to expert) as used previously. 33
To standardise coding, two recorded sessions were listened to by both researchers simultaneously. Independent scores were then discussed, and agreement reached on levels of competence. A further six sessions (across two HTs) were scored independently and subsequently discussed to further ensure inter-rater agreement before the remaining sessions were reviewed and scored independently by both researchers. A score of 3 (representing ‘competent delivery’) was judged to represent acceptable delivery of the TARS intervention.
Results
Mean (SD) scores for the 11 CCs drawn from coding all 72 recorded sessions are shown in Appendix 24 for each coder and overall.
Inter-rater reliability across competencies was assessed across all items using a two-way mixed, absolute agreement, intraclass correlation coefficient (ICC). The resulting ICC was in the excellent range (ICC = 0.95), suggesting that coders had a very high level of agreement.
Delivery fidelity scores for the 11 CCs are shown in Figure 6. The mean score for overall delivery was 3.2 (SD 1.4), suggesting overall competent delivery. Active participant involvement (CC 1) was the highest scoring and was delivered at a proficient standard [mean 4.1 (SD 1.2)]. All other competencies were delivered at an advanced beginner/competent level except managing social influence on PA, which was delivered at a novice level [mean 1.7 (SD 1.6)]. The smoking-related competencies (i.e. CCs 2, 4, 6 and 10) tended to be delivered more proficiently than their PA equivalents (i.e. CCs 3, 5, 7 and 11), as shown in Figure 6.
FIGURE 6.
Box plot of fidelity scores by CC. CC 1, active participant involvement; CC 2, build motivation for cutting down; CC 3, build motivation to increase PA; CC 4, self-monitor and set goals to reduce smoking; CC 5, self-monitor and set goals to increase PA; CC 6, review efforts/problem-solving for reducing smoking; CC 7, review efforts/problem-solving for increasing PA; CC 8, integrate idea of changing smoking and PA; CC 9, reinforce health identity shift; CC 10, manage social influences on smoking; and CC 11, manage social influences on PA. Outlier circles are labelled with the corresponding participant identification number.

Intervention receipt fidelity
Receipt fidelity assesses the extent to which participants received and understood the intervention as intended. 62 The fidelity assessment focused on evaluating whether or not those receiving the intervention had understood the skills and cognitive strategies hypothesised to support behaviour change in terms of (1) reducing smoking, (2) increasing PA and (3) how the two behaviours may influence and relate to each other.
Methods
Twenty-four participants were purposively sampled from across the four sites to correspond with those included in the delivery fidelity analysis to allow possible triangulation. Semistructured interviews were conducted within 4 months of completing the intervention.
Thematic analysis65 was used to develop a priori themes relating to participant understanding of (1) techniques for changing the two behaviours (e.g. using hierarchical reduction techniques) and (2) how the two behaviours may influence each other (e.g. how increasing activity may affect amount smoked or how PA may support reducing smoking). The template emerged through an iterative discussion process involving two researchers. Initial coding identified relevant sections of transcripts and additional inductive codes were added when relevant data were not attributable to a priori themes. An initial template was produced after a subset of eight interviews had been analysed and this was then applied to the whole data set (with additional inductive codes directing adaptations of the template when relevant).
Results
Generally, the interviewed participants demonstrated good understanding of the skills and cognitive strategies that could be utilised in supporting them to help change their behaviours.
Understanding of smoking reduction techniques was widespread, with a range of approaches to reduction being understood (scheduled/hierarchical/planned reduction). Participants also highlighted the value of monitoring the amount smoked to identify triggers and set goals (see Appendix 25, Quotation 1).
Understanding of techniques and strategies to increase levels of PA was less represented in the data than understanding relating to smoking behaviours. Participants who did increase their activity levels showed understanding of how monitoring helped build motivation, how to increase levels at work, the importance of finding activities that they would find enjoyable and engaging social support (see Appendix 25, Quotation 2).
However, some participants talked less of techniques to increase activity as they felt unable to do so owing to physical illness/impairment, had no interest in increasing their activity levels and/or felt that they were already active (see Appendix 25, Quotation 3).
Participants also verified that they understood how the two behaviours might support each other in making changes. They spoke of a number of ways in which this might happen, including using PA as a distraction, exercising without having cigarettes on their person, understanding the influence of smoking on their ability to exercise and supporting a psychological shift towards being healthier (see Appendix 25, Quotation 4).
However, some felt that the two behaviours were unconnected and that the addiction to tobacco was too strong to be counteracted by PA (see Appendix 25, Quotation 5).
Intervention enactment fidelity
Enactment fidelity assesses the extent to which participants enact the behavioural skills and cognitive strategies designed to support behaviour change in real-life settings. 62 The fidelity assessment focused on evaluating whether or not those receiving the intervention had performed the skills and behaviours to reduce smoking and increase PA, and also evaluated to what extent they used PA to control smoking. This was done qualitatively and quantitatively.
Methods
Qualitative data
Twenty-four participants were purposely sampled from across the four sites. Semistructured interviews were conducted at two time points: within 4 months of completing the intervention and 5 or 6 months after completing the first interview. This enabled an assessment of ongoing enactment.
Thematic analysis65 was used to develop a priori themes relating to evidence of (1) enactment of skills related to reducing smoking/increasing activity (e.g. monitoring and goal-setting) and (2) enactment of behaviours (e.g. reducing smoking/increasing PA). The development of the template was an iterative process developed and discussed by two researchers. Initial coding identified relevant sections of transcripts and additional inductive codes were added when relevant data were not attributable to a priori themes. An initial template was produced after a subset of eight interviews had been analysed and this was then applied to the whole data set (with additional inductive codes directing adaptations of the template when relevant).
Quantitative data
Intervention effects on process survey items, with the same analytical model used in the primary analysis, were assessed to triangulate with qualitative findings. A significant change in the use of patient-enacted intervention techniques (represented by the quantitative process measures) from baseline to 3 months in the intervention group, compared with the control group, would indicate successful enactment of some of the key intervention processes. Quantitative process data were collected at baseline and 3 months alongside the main trial data set. Individual questions related to key psychological and behavioural processes of the intervention were captured using a five-point Likert scale (strongly disagree to strongly agree) for both smoking and PA behaviours (Tables 14 and 15). As these questions were intended for participants who were still smoking, they are not applicable to those who reported quitting at 3 months. A derived variable was denoted as missing if a response to any one of the constituent questions was missing.
| Statement | Value | Baseline | 3 months | Coefficient (difference at 3 months) (95% CI); p-valuea | ||
|---|---|---|---|---|---|---|
| SAU group | Intervention group | SAU group | Intervention group | |||
| Important I reduce my smoking | Participants (n) | 458 | 456 | 291 | 294 | 0.20 (0.08 to 0.32); 0.001 |
| Mean (SD) | 4.7 (0.5) | 4.7 (0.6) | 4.4 (0.8) | 4.6 (0.6) | ||
| Median | 5.0 | 5.0 | 5.0 | 5.0 | ||
| Minimum, maximum | 2.0, 5.0 | 2.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | ||
| Important I quit smoking | Participants (n) | 458 | 457 | 289 | 291 | 0.06 (–0.08 to 0.19); 0.402 |
| Mean (SD) | 4.2 (1.0) | 4.2 (0.9) | 4.1 (1.0) | 4.3 (0.9) | ||
| Median | 4.0 | 4.0 | 4.0 | 5.0 | ||
| Minimum, maximum | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | ||
| Confident I can reduce my smoking | Participants (n) | 458 | 457 | 291 | 291 | 0.80 (0.64 to 0.97); 0.001 |
| Mean (SD) | 3.6 (1.0) | 3.6 (1.0) | 3.4 (1.1) | 4.1 (1.0) | ||
| Median | 4.0 | 4.0 | 4.0 | 4.0 | ||
| Minimum, maximum | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | ||
| Confident I can quit smoking | Participants (n) | 458 | 457 | 289 | 296 | 0.49 (0.33 to 0.65); 0.001 |
| Mean (SD) | 2.9 (1.1) | 3.0 (1.1) | 2.9 (1.1) | 3.5 (1.1) | ||
| Median | 3.0 | 3.0 | 3.0 | 3.0 | ||
| Minimum, maximum | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | ||
| People close to me support me reducing smoking | Participants (n) | 458 | 457 | 287 | 294 | 0.42 (0.25 to 0.59); 0.001 |
| Mean (SD) | 3.8 (1.3) | 3.7 (1.2) | 3.4 (1.2) | 3.8 (1.1) | ||
| Median | 4.0 | 4.0 | 4.0 | 4.0 | ||
| Minimum, maximum | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | ||
| Smoking action planningb | Participants (n) | 457 | 457 | 286 | 287 | 1.55 (1.23 to 1.88); 0.001 |
| Mean (SD) | 3.8 (2.1) | 3.9 (2.0) | 5.5 (2.2) | 7.1 (1.8) | ||
| Median | 3.0 | 3.0 | 6.0 | 7.0 | ||
| Minimum, maximum | 1.0, 9.0 | 1.0, 9.0 | 1.0, 9.0 | 1.0, 9.0 | ||
| Smoking coping planningb | Participants (n) | 458 | 457 | 284 | 284 | 1.34 (1.01 to 1.67); 0.001 |
| Mean (SD) | 3.2 (1.9) | 3.2 (1.8) | 4.5 (2.1) | 5.9 (2.0) | ||
| Median | 3.0 | 3.0 | 5.0 | 6.0 | ||
| Minimum, maximum | 1.0, 9.0 | 1.0, 9.0 | 1.0, 9.0 | 1.0, 9.0 | ||
| Plans for quitting smoking | Participants (n) | 457 | 457 | 284 | 291 | 0.48 (0.29 to 0.68); 0.001 |
| Mean (SD) | 2.3 (1.2) | 2.3 (1.1) | 2.9 (1.3) | 3.4 (1.2) | ||
| Median | 2.0 | 2.0 | 3.0 | 3.0 | ||
| Minimum, maximum | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | ||
| Self-monitoring smokingb | Participants (n) | 458 | 457 | 278 | 290 | 0.98 (0.71 to 1.25); 0.001 |
| Mean (SD) | 5.7 (1.9) | 5.5 (1.8) | 6.5 (1.7) | 7.5 (1.6) | ||
| Median | 6.0 | 6.0 | 7.0 | 8.0 | ||
| Minimum, maximum | 1.0, 9.0 | 1.0, 9.0 | 1.0, 9.0 | 1.0, 9.0 | ||
| Frequency of urges to smoke | Participants (n) | 458 | 457 | 289 | 294 | –0.55 (–0.72 to–0.39); 0.001 |
| Mean (SD) | 3.3 (1.1) | 3.2 (1.2) | 3.3 (1.1) | 2.7 (1.1) | ||
| Median | 3.0 | 3.0 | 3.0 | 3.0 | ||
| Minimum, maximum | 0.0, 5.0 | 0.0, 5.0 | 1.0, 5.0 | 0.0, 5.0 | ||
| Strength of urges to smoke | Participants (n) | 458 | 457 | 290 | 295 | –0.47 (–0.63 to–0.31); 0.001 |
| Mean (SD) | 3.2 (1.1) | 3.2 (1.1) | 3.3 (1.0) | 2.8 (1.1) | ||
| Median | 3.0 | 3.0 | 3.0 | 3.0 | ||
| Minimum, maximum | 0.0, 5.0 | 0.0, 5.0 | 0.0, 5.0 | 0.0, 5.0 | ||
| Statement | Value | Baseline | 3 months | Coefficient (difference at 3 months) (95% CI); p-valuea | ||
|---|---|---|---|---|---|---|
| SAU group | Intervention group | SAU group | Intervention group | |||
| Important I am physically active | n | 458 | 457 | 302 | 311 | 0.08 (–0.03 to 0.19); 0.158 |
| Mean (SD) | 4.4 (0.8) | 4.3 (0.8) | 4.2 (0.7) | 4.2 (0.8) | ||
| Median | 5.0 | 4.0 | 4.0 | 4.0 | ||
| Minimum, maximum | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | ||
| Confident I can be physically active | n | 458 | 457 | 303 | 314 | 0.21 (0.07 to 0.34); 0.002 |
| Mean (SD) | 4.2 (0.9) | 4.1 (0.9) | 3.8 (1.0) | 4.0 (0.9) | ||
| Median | 4.0 | 4.0 | 4.0 | 4.0 | ||
| Minimum, maximum | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | ||
| People close support me being physically active | n | 457 | 456 | 302 | 311 | 0.13 (–0.02 to 0.27); 0.089 |
| Mean (SD) | 3.8 (1.2) | 3.8 (1.2) | 3.5 (1.1) | 3.7 (1.0) | ||
| Median | 4.0 | 4.0 | 4.0 | 4.0 | ||
| Minimum, maximum | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | ||
| PA is a way of controlling smoking | n | 458 | 457 | 302 | 314 | 0.48 (0.31 to 0.64); 0.001 |
| Mean (SD) | 2.2 (1.2) | 2.1 (1.1) | 2.8 (1.1) | 3.2 (1.1) | ||
| Median | 2.0 | 2.0 | 3.0 | 3.0 | ||
| Minimum, maximum | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | 1.0, 5.0 | ||
| PA action planningb | n | 458 | 457 | 292 | 307 | 1.27 (0.81 to 1.73); 0.001 |
| Mean (SD) | 7.1 (3.9) | 6.7 (3.6) | 7.9 (3.2) | 9.1 (2.9) | ||
| Median | 7.0 | 7.0 | 8.0 | 10.0 | ||
| Minimum, maximum | 1.0, 13.0 | 1.0, 13.0 | 1.0, 13.0 | 1.0, 13.0 | ||
| PA coping planningb | n | 458 | 456 | 294 | 309 | 0.78 (0.48 to 1.09); 0.001 |
| Mean (SD) | 3.8 (2.2) | 3.7 (2.1) | 4.5 (2.0) | 5.3 (2.0) | ||
| Median | 3.0 | 3.0 | 5.0 | 5.0 | ||
| Minimum, maximum | 1.0, 9.0 | 1.0, 9.0 | 1.0, 9.0 | 1.0, 9.0 | ||
| Self-monitoring of PA | n | 458 | 457 | 294 | 311 | 0.85 (0.55 to 1.15); 0.001 |
| Mean (SD) | 4.8 (2.4) | 4.6 (2.1) | 5.4 (2.1) | 6.2 (2.0) | ||
| Median | 5.0 | 5.0 | 5.0 | 7.0 | ||
| Minimum, maximum | 1.0, 9.0 | 1.0, 9.0 | 1.0, 9.0 | 1.0, 9.0 | ||
Results
Qualitative
Results show that the intended behaviour change techniques were enacted for both smoking and PA both during and after the intervention.
Interviews with participants revealed that many had, as a result of the intervention, managed to halve the number of cigarettes they smoked daily (see Appendix 25, Quotation 6).
Although some reported returning to previous levels post intervention, others had plans to make a quit attempt in the future, some with the assistance of nicotine substitute patches from their GP (see Appendix 25, Quotation 7).
A range of approaches for reduction included smoking half the number of cigarettes, distraction, goal-setting and buying fewer cigarettes, but self-monitoring was commonly utilised and evidenced in the data (see Appendix 25, Quotation 8).
Although there was evidence that participants understood the strategies and techniques for increasing PA (receipt), the enactment of these skills varied across participants. The main reasons for this were that a participant had health-related restrictions on how much exercise they could do or that they were already physically active enough (e.g. employed in jobs that demanded that they were physically active) and did not feel that they needed/wanted to increase this further (see Appendix 25, Quotation 9).
Among those who did engage, there was evidence that they enacted a number of techniques and strategies, including engaging social support (exercising with others) and goal-setting. Monitoring of PA was perceived as particularly motivating, with many using the pedometer supplied by the intervention (see Appendix 25, Quotation 10).
Participants talked of using PA in a number of ways, for example as a replacement for smoking, as a means to help control weight and to assist in managing cravings (see Appendix 25, Quotation 11).
Participants who had increased their activity levels largely reported maintaining this post intervention (see Appendix 25, Quotation 12).
Quantitative
The descriptive data for the process survey items at baseline and 3 months in each group, and the intervention effects for smoking- and PA-related process measures, are shown Tables 14 and 15, respectively (descriptive data for all individual process item measures can be found in Appendices 26 and 27). The intervention had positive significant effects on all measures with the exception of the importance of quitting smoking, the importance of being more physically active and a social support-related measure for PA.
The mediating role of process measures on physical activity and smoking outcomes, and of physical activity on smoking outcomes
The aims were to examine if changes in PA mediate intervention effects on smoking outcome effects, and if changes in smoking beliefs and PA beliefs mediate intervention effects on smoking and PA behaviour outcomes, respectively.
Methods
To limit the number of mediation analyses, we examined mediation effects only when there were significant (p ≤ 0.05) intervention effects on the outcome. Three exploratory mediation models were considered: (1) the mediating effects of changes from baseline to 3 months in self-reported MVPA on intervention effects on smoking outcomes; (2) the mediating effect of changes in smoking-related process measures on intervention effects on smoking outcomes; and (3) the mediating effect of changes in PA-related process measures on intervention effects on MVPA. The a priori models are represented diagrammatically in Figure 7.
FIGURE 7.
Mediation analyses diagrams.
The candidate mediators from the smoking process measures comprised the single items:
-
importance of reducing
-
importance of quitting
-
confidence in reducing
-
confidence in quitting
-
support
-
quitting planning
-
frequency of urges to smoke
-
strength of urges to smoke.
They also comprised additional multi-item scales:
-
action planning (two items) for changes in smoking
-
coping planning (two items) for changes in smoking
-
self-monitoring (two items) of smoking.
The candidate mediators from the PA process measures comprised the single items:
-
importance of PA
-
confidence
-
support
-
PA for controlling smoking.
They also comprised additional factors constructed from single items:
-
action planning (derived from three items) for changes in PA
-
coping planning (derived from two items) for changes in PA
-
self-monitoring (derived from two items) of PA.
The mediation analysis was carried out using structural equation models:66 this simultaneously fitted two regression equations. In one, the mediator (change in process measure from baseline to 3 months) was regressed on the intervention, and adjusted for baseline values of the process measure and stratification variables, with the intervention effect on the mediator providing the coefficient for the ‘A path’. In the other, the outcome was regressed on the mediator and on the intervention, and adjusted for baseline values of the outcome, when appropriate, and stratification variables. This part of the structural equation model provided an estimate of the natural direct effect of the intervention adjusted for the effect of the mediator. The latter path may be considered the ‘B path’, and the product of this and the ‘A path’ provided an estimate of the natural indirect effect, the so-called mediated effect, of the intervention on the outcome. The remaining direct influence of the intervention on the outcome is the ‘C path’. Because the sampling distribution of the product of the two coefficients can be assumed to be normally distributed only in certain cases, the calculation of the standard error may lead to lower power in the test of the product. 67,68 Therefore, the CIs for the mediated path were estimated through the bootstrap resampling method, with 1000 replications.
Results from the mediation analysis
There was no evidence from the mediation analysis that self-reported changes in PA mediated changes in smoking-related outcomes at 3 or 9 months, as shown in the bottom rows of Tables 16–18.
| Mediator | n | A path, coefficient (SE) | B path, coefficient (SE) | Mediated effect | C path, coefficient (SE) | |
|---|---|---|---|---|---|---|
| Coefficient (SE) | 95% CI | |||||
| Important I reduce my smoking | 584 | 0.201 (0.060) | –3.526 (1.328) | –0.709 (0.407) | –1.690 to –0.101 | –5.081 (2.145) |
| Confident I can reduce my smoking | 582 | 0.802 (0.083) | –1.922 (0.912) | –1.541 (0.694) | –3.071 to –0.243 | –4.184 (2.262) |
| Confident I can quit smoking | 585 | 0.489 (0.080) | –0.132 (0.980) | –0.065 (0.473) | –0.930 to 0.871 | –5.488 (2.188) |
| People close to me support me reducing smoking | 581 | 0.420 (0.084) | –1.636 (0.870) | –0.687 (0.412) | –1.595 to 0.077 | –4.960 (2.183) |
| Action planning for changes in smoking | 573 | 1.554 (0.165) | –1.220 (0.421) | –1.896 (0.711) | –3.471 to –0.689 | –3.754 (2.251) |
| Coping planning for changes in smoking | 568 | 1.343 (0.167) | –0.905 (0.447) | –1.215 (0.575) | –2.398 to –0.142 | –4.965 (2.232) |
| Self-monitoring of smoking | 568 | 0.981 (0.135) | –1.586 (0.505) | –1.556 (0.519) | –2.736 to –0.660 | –3.715 (2.257) |
| Plans for quitting smoking | 575 | 0.483 (0.099) | –0.941 (0.761) | –0.455 (0.348) | –1.207 to 0.157 | –5.292 (2.192) |
| Urge to smoke in previous week | 583 | –0.553 (0.084) | 0.890 (0.876) | –0.492 (0.540) | –1.706 to 0.422 | –5.145 (2.176) |
| Strength of urges to smoke in previous week | 585 | –0.466 (0.081) | 0.959 (0.925) | –0.447 (0.437) | –1.358 to 0.430 | –5.164 (2.172) |
| Standardised difference in self-reported MVPA (truncated), baseline to 3 months | 608 | 0.195 (0.064) | 0.841 (1.102) | 0.164 (0.265) | –0.228 to 0.943 | –5.470 (2.162) |
| Mediator | n | A path, coefficient (SE) | B path, coefficient (SE) | Mediated effect | C path, coefficient (SE) | |
|---|---|---|---|---|---|---|
| Coefficient (SE) | 95% CI | |||||
| Important I reduce my smoking | 584 | 0.201 (0.060) | 0.091 (0.126) | 0.018 (0.026) | –0.020 to 0.086 | 0.725 (0.208) |
| Confident I can reduce my smoking | 582 | 0.802 (0.083) | 0.272 (0.087) | 0.218 (0.072) | 0.083 to 0.362 | 0.498 (0.217) |
| Confident I can quit smoking | 585 | 0.489 (0.080) | 0.229 (0.091) | 0.112 (0.055) | 0.015 to 0.234 | 0.591 (0.210) |
| People close to me support me reducing smoking | 581 | 0.420 (0.084) | 0.011 (0.081) | 0.005 (0.034) | –0.062 to 0.072 | 0.685 (0.210) |
| Action planning for changes in smoking | 573 | 1.554 (0.165) | 0.150 (0.042) | 0.233 (0.070) | 0.110 to 0.380 | 0.498 (0.218) |
| Coping planning for changes in smoking | 568 | 1.343 (0.167) | 0.154 (0.044) | 0.207 (0.068) | 0.085 to 0.353 | 0.529 (0.217) |
| Self-monitoring of smoking | 568 | 0.981 (0.135) | 0.156 (0.049) | 0.153 (0.057) | 0.059 to 0.290 | 0.480 (0.216) |
| Plans for quitting smoking | 575 | 0.483 (0.099) | 0.188 (0.073) | 0.091 (0.039) | 0.026 to 0.177 | 0.641 (0.212) |
| Urge to smoke in previous week | 583 | –0.553 (0.084) | –0.087 (0.079) | 0.048 (0.046) | –0.037 to 0.146 | 0.637 (0.209) |
| Strength of urges to smoke in previous week | 585 | –0.466 (0.081) | –0.076 (0.082) | 0.035 (0.041) | –0.039 to 0.121 | 0.645 (0.208) |
| Standardised difference in self-reported MVPA (truncated), baseline to 3 months | 608 | 0.195 (0.064) | 0.044 (0.102) | 0.009 (0.021) | –0.032 to 0.053 | 0.648 (0.205) |
| Mediator | n | A path, coefficient (SE) | B path, coefficient (SE) | Mediated effect | C path, coefficient (SE) | |
|---|---|---|---|---|---|---|
| Coefficient (SE) | 95% CI | |||||
| Important I reduce my smoking | 584 | 0.201 (0.060) | 0.179 (0.151) | 0.036 (0.032) | –0.012 to 0.110 | 0.380 (0.237) |
| Confident I can reduce my smoking | 582 | 0.802 (0.083) | 0.184 (0.099) | 0.148 (0.079) | 0.011 to 0.330 | 0.257 (0.246) |
| Confident I can quit smoking | 585 | 0.489 (0.080) | 0.129 (0.104) | 0.063 (0.066) | –0.057 to 0.198 | 0.268 (0.237) |
| People close to me support me reducing smoking | 581 | 0.420 (0.084) | 0.065 (0.092) | 0.027 (0.043) | –0.061 to 0.110 | 0.287 (0.236) |
| Action planning for changes in smoking | 573 | 1.554 (0.165) | 0.033 (0.045) | 0.051 (0.075) | –0.090 to 0.197 | 0.295 (0.244) |
| Coping planning for changes in smoking | 568 | 1.343 (0.167) | 0.040 (0.048) | 0.054 (0.069) | –0.081 to 0.199 | 0.273 (0.244) |
| Self-monitoring of smoking | 568 | 0.981 (0.135) | 0.096 (0.056) | 0.094 (0.053) | 0.002 to 0.205 | 0.170 (0.246) |
| Plans for quitting smoking | 575 | 0.483 (0.099) | 0.032 (0.081) | 0.015 (0.039) | –0.060 to 0.098 | 0.301 (0.236) |
| Urge to smoke in previous week | 583 | –0.553 (0.084) | –0.160 (0.090) | 0.088 (0.051) | –0.002 to 0.199 | 0.254 (0.237) |
| Strength of urges to smoke in previous week | 585 | –0.466 (0.081) | –0.117 (0.093) | 0.055 (0.048) | –0.033 to 0.151 | 0.280 (0.236) |
| Standardised difference in self-reported MVPA (truncated), baseline to 3 months | 608 | 0.195 (0.064) | –0.056 (0.116) | –0.011 (0.024) | –0.068 to 0.033 | 0.251 (0.232) |
Changes between baseline and 3 months in 5 of the 10 smoking process measures mediated intervention effects on the self-reported number of cigarettes smoked daily at 3 months, and in 6 of the 10 measures mediated intervention effects on whether or not participants reduced their smoking by ≥ 50% from baseline to 3 months. Increasing beliefs in being able to reduce smoking, action planning, coping with setbacks and self-monitoring smoking all mediated intervention effects on both the number of cigarettes smoked daily and ≥ 50% reduction up to 3 months. At 9 months, there were no mediating effects on smoking reduction other than confidence in reducing and self-monitoring, which had weakened and become marginally significant since the 3-month visit.
Changes in both confidence in being physically active and self-monitoring mediated intervention effects on self-reported MVPA at 3 months, as shown in Table 19.
| Mediator | n | A path, coefficient (SE) | B path, coefficient (SE) | Mediated effect | C path, coefficient (SE) | |
|---|---|---|---|---|---|---|
| Coefficient (SE) | 95% CI | |||||
| Important I am physically active | 613 | 0.080 (0.056) | 16.302 (15.180) | 1.304 (1.651) | –0.389 to 7.581 | 78.157 (26.947) |
| Confident I can be physically active | 617 | 0.208 (0.068) | 34.939 (13.544) | 7.267 (3.432) | 1.963 to 16.121 | 72.658 (26.905) |
| People close support me being physically active | 612 | 0.128 (0.074) | 22.219 (11.394) | 2.844 (2.189) | –0.243 to 9.234 | 76.806 (26.805) |
| PA is a way of controlling smoking | 616 | 0.476 (0.085) | 17.749 (10.045) | 8.449 (5.448) | –0.794 to 20.625 | 70.974 (27.402) |
| Action planning for changes in PA | 599 | 1.270 (0.231) | 6.828 (3.598) | 8.672 (5.057) | –0.648 to 19.398 | 72.325 (27.962) |
| Coping planning for changes in PA | 603 | 0.784 (0.155) | 5.543 (5.497) | 4.346 (4.583) | –5.664 to 13.551 | 78.971 (27.682) |
| Self-monitoring of PA | 598 | 0.854 (0.152) | 12.957 (5.817) | 11.065 (5.421) | 1.491 to 23.460 | 68.012 (27.957) |
Qualitative study
The embedded qualitative study aimed to promote understanding from the perspectives of both the participants and the HTs as to how the key intervention processes portrayed in the intervention process model (see Figure 5) were being used, and which offered the most benefit, and to identify any possible changes or areas where the intervention processes were not working and could be improved.
Methods
All HTs were invited to take part in two sets of semistructured interviews, one soon after training and in the early stages/while in preparation for intervention delivery and the second later in delivery. The first HT interviews were used to inform the pilot trial and the second interviews were used to inform the main trial. Twenty-four intervention participants took part in semistructured interviews following completion of the intervention. Participants were selected to ensure equal numbers across sites and HTs, and analysis continued until data saturation was reached. Sixteen participant interviews and eight HT interviews were selected for analysis for the main trial in order to cross-validate and strengthen the data related to each theme. Interviews lasted up to 60 minutes and were guided by a semistructured interview schedule. Audio-recordings were transcribed verbatim, and data were analysed and organised using NVivo 12 (QSR International, Warrington, UK). Data were analysed using thematic analysis. 69
Results
Seven key higher-level themes that are of relevance to the aims of the process evaluation are presented in this section. These are as follows:
-
approaches to smoking reduction
-
approaches to increasing PA
-
multiple behaviour change
-
progression to cessation
-
other effective intervention components
-
barriers and challenges
-
suggested improvements to the intervention.
The data are presented under each theme from the perspective of both the intervention participant (referred to hereafter as ‘participant’) and the HT to support triangulation under each theme.
Approaches to smoking reduction
Almost half of the HTs spoke about the offer of reduction, rather than solely that of quitting, as being motivating, increasing a sense of confidence that change was possible. Participants, particularly those who had attended traditional stop smoking services, were used to being told or expected to quit smoking, rather than reduce. Not only was this considered less daunting, but it also felt supportive for people who were not yet at the stage of feeling ready to quit (see Appendix 28, Quotations 1 and 2).
HTs spoke about presenting the four main reduction techniques that are contained in the manual during the early intervention sessions with participants, and how important these were in supporting goal-setting. It was also recognised that participants also had their own strategies that they had tried before, which the HT supported them to develop (see Appendix 28, Quotation 3).
Scheduled reduction was described as a useful approach to reducing smoking by a number of participants. Participants spoke about a number of ways in which they scheduled their smoking in order to reduce the number of cigarettes they smoked. Strategies included using distracting activities so that they could delay when they smoked (see Appendix 28, Quotation 4).
One participant found that by using an activity to initially delay the time when they would usually smoke, they were able to build in further ways to delay smoking, thereby helping them to reduce overall (see Appendix 28, Quotation 5). Furthermore, although some participants did not closely monitor the number of cigarettes they were smoking, they were able to assess progress in terms of reduction by the lengthening of time periods between cigarettes (see Appendix 28, Quotation 6).
Some participants focused on planning their reduction in terms of the number of cigarettes they smoked. For example, some participants set a gradually reducing number of cigarettes to smoke throughout each day (see Appendix 28, Quotation 7).
Other participants found hierarchical reduction beneficial, reflecting on which cigarettes they would find most difficult to stop. By doing this, they were able to stop smoking the less ‘important’ cigarettes first, to start building their confidence to reduce smoking (see Appendix 28, Quotation 8).
Linked to the strategies, two HTs discussed how they used the worksheets and paperwork linked to the hierarchical reduction strategy, which participants found to be helpful (see Appendix 28, Quotation 9).
Approaches to increasing physical activity
The main way in which the intervention supported participants to increase PA was through monitoring. Participants used the step counter that was given to them by the HT to record their steps during the day and monitor the number of steps over time. This, in turn, led some participants to set and achieve goals linked to their PA (see Appendix 28, Quotations 10 and 11).
The majority of the HTs also spoke about self-monitoring as a key approach to support participants to increase their PA. Use of the pedometer provided a useful aid for monitoring PA, both in terms of facilitating discussion about PA between the HT and the participant and by providing the participant with a visual representation of their PA levels (see Appendix 28, Quotations 12 and 13).
As well as enjoying using the pedometer to count the number of steps that they did, the pedometer also provided some participants with a baseline that not only enabled them to recognise their low level of activity, but also gave them a starting point to work from to increase their activity levels (see Appendix 28, Quotation 14).
Other participants found that the pedometer was not always accurate, so they used devices that they already had such as their mobile phone or a Fitbit device (Fitbit LLC, San Francisco, CA, USA) to record their steps or the time that they spent engaging in PA. Some reported using an app on their phone to record their running, to set goals and to monitor progress (see Appendix 28, Quotation 15).
Progressive goal-setting was recognised as a powerful technique, whereby HTs focused on supporting participants to set small goals. Although this was seen as an advantageous approach in supporting participants to take a small step to increasing their PA, it was recognised that, in setting small goals, if a participant struggles to attain or nearly attain the goal, they are often back in the position in which they started without having moved forward (see Appendix 28, Quotations 16 and 17).
Health trainers recognised that participants often perceived barriers to engaging in PA that needed to be addressed so that they could work on increasing PA. HTs recognised that discussing barriers to PA engagement and ways to overcome barriers was a fruitful exercise (see Appendix 28, Quotations 18 and 19).
Social support was also key to some participants increasing their PA. People close to participants supported their engagement in PA in a range of ways including joining in an activity with them, inviting them to take part in an activity, making new friends through exercise and encouraging them to continue to engage in whatever type of PA they had chosen (see Appendix 28, Quotations 20 and 21).
Health trainers also spoke about the social aspect of PA. Engaging friends and family in PA was viewed by HTs as beneficial to enhancing motivation to increase PA, particularly if the participant was new to PA and/or taking up a new activity (see Appendix 28, Quotation 22). Encouraging engagement in activities with new people was also seen as beneficial to supporting participants to take up new group activities (see Appendix 28, Quotation 23).
Other approaches taken by HTs to support PA increase included talking about activity rather than exercise so that participants could recognise other activities such as hoovering that could be included as PA rather than ‘formal’ exercise. It was noted that it was important to encourage activity that participants wanted to engage in and, furthermore, that they would enjoy (see Appendix 28, Quotation 24).
Health trainers were concerned not only with encouraging existing exercise, but also with encouraging participants to experiment with new activities in order to develop motivation (see Appendix 28, Quotation 25).
Multiple behaviour change
Health trainers spoke about how promoting the role of PA for managing smoking was received by participants. Most HTs spoke of a mixed reaction from participants, with some participants being very receptive to the idea and others being more resistant (see Appendix 28, Quotations 26 and 27).
There was a sense that HTs tested the water with raising the idea of PA to see if it was acceptable to participants, but did not push the link if there was a suggestion of resistance (see Appendix 28, Quotation 28).
A number of participants discussed the link between smoking and PA in one of three ways. First, participants spoke about the negative impact that smoking has on an individual’s ability to engage in PA. Second, it was noted that it can be used as a distraction for reducing or stopping smoking by managing cravings through engaging in a form of PA. Third, it was noted that it can be used to counter potential weight gain, which concerns some participants when they are considering reducing or stopping smoking (see Appendix 28, Quotations, 29–31).
However, although participants generally had some understanding of a link between smoking reduction and PA and the importance of this link, this was not always perceived as important to the participant personally (see Appendix 28, Quotation 32). A strong dependence on tobacco could stop participants from seeing the link between smoking and PA and from seeing how PA might help (see Appendix 28, Quotation 33). HTs also suggested that, for some participants, there was a lack of fundamental understanding of the link between the two behaviours and how one might affect the other (see Appendix 28, Quotation 34).
Health trainers reflected on how participants explicitly linked smoking and PA to support smoking reduction. Almost all the HTs spoke about the way in which participants used PA as a distraction (see Appendix 28, Quotations 35 and 36).
As seen previously, HTs also spoke about how participants used PA to manage cravings and how they supported participants to do this by encouraging the use of short bursts of PA as an experiment to see how it affected their cravings (see Appendix 28, Quotation 37).
The HTs also spoke of how participants would use PA to fill time and delay when they would smoke (see Appendix 28, Quotation 38).
Some participants who recognised the link between smoking and PA decided to tackle each separately, rather than explicitly using PA to control their smoking (see Appendix 28, Quotation 39).
One participant had intended to increase their PA through swimming, but was about to receive a hip replacement and so decided to concentrate on strengthening exercises to support their hip prior to surgery, rather than increase swimming. Others felt that they were already sufficiently engaged in PA or did not have time to make this increase.
As mentioned previously, one of the main benefits of PA discussed by participants who were engaging in increasing PA and reducing smoking simultaneously was managing cravings. Identifying an activity that they could use to manage cravings when they happened, or that could be plugged into their day at a point at which they knew they would be likely to crave a cigarette, either stopped them smoking that cigarette at the usual time or created a longer gap between cigarettes, supporting overall reduction (see Appendix 28, Quotation 40).
Furthermore, some participants took up a new form of exercise not only as a way of increasing their PA, but also to occupy them and prevent them from smoking (see Appendix 28, Quotation 41).
Participants who increased their PA at the same time as reducing smoking noticed changes in how they felt when they were exercising (e.g. less breathless) and other physical symptoms that had been caused by smoking (e.g. one participant reported reduced crackling in their chest). A ‘tipping point’ with regard to PA and smoking was described: when benefits of PA begin to feel important and noticeable, it directly highlights the negative effects of smoking (see Appendix 28, Quotation 42).
Some interview participants, although they addressed both behaviours, made changes consecutively or separately. For most of these participants, smoking was the most important behaviour for them to address and, in some cases, they had not realised that increasing PA was a component of the intervention. Although some participants may have been interested in increasing their level of PA, as a result of the conflict that they saw in engaging in both behaviours, they could not envisage engaging in or increasing their PA while they continued to smoke (see Appendix 28, Quotation 43).
Health trainers described the importance of recognising the different motivations for each behaviour. Half of the HTs felt that most participants were more interested in reducing smoking than they were in increasing PA. As a result, some HTs initially focused solely on supporting smoking reduction, with approaches to increasing PA brought in later in the intervention, dependent on the participant (see Appendix 28, Quotation 44).
Some HTs stated that they needed to use different approaches for smoking and PA. One HT reflected that the use of monitoring was more useful for smoking reduction than for increasing PA (see Appendix 28, Quotation 45).
Health trainers raised several challenges to supporting multiple behaviour change, a key one being that participants were often interested only in reducing smoking, and not in increasing PA (see Appendix 28, Quotation 46). Another challenge faced by a number of HTs was that of participants reporting that they smoked while they walked (see Appendix 28, Quotation 47). HTs tried to highlight the discrepancy between the behaviours described by some participants as a strategy to try to address this (see Appendix 28, Quotations 48 and 49).
Progression to cessation
Health trainers talked about a range of approaches to supporting progression to cessation. Approaches used sought to build confidence to support motivation, and included framing quitting as temporary as well as practising quitting by trying it out without having to make a commitment to it (see Appendix 28, Quotation 50).
One HT found that use of substitution, particularly in the form of vaping, was useful in supporting quitting (see Appendix 28, Quotation 51).
Health trainers also spoke about the challenges of quitting, one of the most common being the extent to which smoking was a part of each participant’s life and also how it is bound up with their identity, so quitting would represent a fundamental change in who they are (see Appendix 28, Quotation 52).
One HT stated that they would not initiate a conversation about quitting, but rather would focus on reduction and wait for participants to raise quitting themselves before discussing it. Another HT observed that participants found it hard to quit the last few cigarettes when they had reduced the amount they smoked. Linked to this, another HT said that they were not confident in talking to participants about quitting, particularly if participants had already significantly reduced the number of cigarettes they smoked, so as not to undermine a participant’s achievement (see Appendix 28, Quotation 53).
Discussing approaches that have not worked to support quitting in the past raises further challenges for HTs in supporting participants to progress to cessation. For example, having been told to quit in the past was observed as making participants resistant to quit. Furthermore, it was recognised by HTs that, for some participants, traditional stop smoking services had not worked in the past, in part because they felt that they were being told what to do and that the reduction offered by the TARS was probably what was appealing after this experience (see Appendix 28, Quotation 54).
Health trainers observed that some participants who were not considering a quit attempt at the start of the intervention, because they were focused on reducing smoking, were considering making a future quit attempt in later sessions (see Appendix 28, Quotation 55).
Although participants did not always make a quit attempt while they were receiving the intervention, the intervention provided skills to build confidence to make a quit attempt beyond the trial. One participant had set a date to quit and felt positive about this because of the skills gained during the intervention, in particular using PA to manage cravings (see Appendix 28, Quotation 56).
Other effective intervention components
Self-monitoring was viewed as useful for supporting smoking reduction, particularly with the use of fridge magnet notebooks. HTs described how just being made aware of how much they are currently smoking was enough to develop participants’ motivation to make changes and reduce the number of cigarettes they smoked (see Appendix 28, Quotation 57). Monitoring the number of cigarettes smoked was used to support reduction by most participants whose interviews were analysed. Most participants wrote down when they smoked a cigarette and why they had it, and some used the fridge magnet notebook to organise monitoring (see Appendix 28, Quotation 58).
Participants also reported that monitoring triggered mechanisms that supported reduction in the number of cigarettes that they smoked. For example, by monitoring their smoking, one participant started to question why they were having each cigarette, which in and of itself led them to reduce the number of cigarettes that they smoked (see Appendix 28, Quotation 59). Furthermore, by keeping track of the number of cigarettes smoked across a period of time, one participant talked about how monitoring helped them to visualise where there were peaks and troughs in their smoking, which they could reflect on and use to help them to identify times to reduce (see Appendix 28, Quotation 60). This awareness of the reasons why they did and did not smoke was found to be helpful for most interview participants. Furthermore, the intervention itself and the role of the HTs delivering the intervention provided the space and opportunity to facilitate the development of participants’ awareness (see Appendix 28, Quotation 61).
Some participants talked about how the intervention supported them to learn from setbacks when they were trying to reduce and/or stop smoking. Examples provided included HTs helping participants to modify the language that they used when they hit a setback away from ‘failure’, and instead, reflecting on why they had a cigarette, to simply being able to speak openly about any setbacks with their HT (see Appendix 28, Quotations 62 and 63).
Health trainers talked about how they supported participants to make specific, measurable, achievable, relevant and time-bound (SMART) goals to reduce their smoking. Ensuring that goals were specific was important in supporting participants to make a reduction. It was noted by HTs that some participants were initially resistant or reticent to make their goals specific regarding reduction, but, through encouragement to develop SMART goals, participants started to make changes (see Appendix 28, Quotation 64). Furthermore, ensuring that goals were realistic was important for participants to develop the confidence to achieve their goals (see Appendix 28, Quotation 65).
Approximately half of the participants spoke about goal-setting in relation to smoking reduction. Participants spoke about flexibility in goal-setting to ensure that goals could be achieved and also the positive impact of goal-setting and achievement on their confidence (see Appendix 28, Quotation 66). Furthermore, both reflecting on and planning goals usefully contributed to the structure of the intervention sessions, focusing on what a participant wanted to achieve (see Appendix 28, Quotation 67).
Participants spoke about activities that they were able to use to distract them from thinking about smoking, including gardening, doing puzzles, cleaning the house, visiting family who do not smoke, playing video games or watching a movie. Activities were often incompatible with smoking or took place where they were unable to smoke or participants did not take their cigarettes with them, for example when they went shopping (see Appendix 28, Quotation 68).
Participants also spoke about how they changed their routine to fill times when they would usually smoke with activities so that they reduced the number of cigarettes they smoked (see Appendix 28, Quotation 69).
Social support was raised as being helpful for smoking reduction in two ways. First, participants told people in their lives who they knew would be supportive in thinking of strategies to support reduction or who they could talk to when they were finding reduction challenging. Second, one participant talked with their HT about social support and made the decision to spend more time with people who did not smoke, which they directly attributed to reduction (see Appendix 28, Quotation 70).
Social influence was also viewed as being facilitative in supporting participants’ smoking reduction, both in terms of when people who are important to them notice that they have made changes to their smoking behaviour and by encouraging participants to spend time with people who engage in a healthy lifestyle (see Appendix 28, Quotations 71 and 72).
It was also noted that another important facilitator for supporting motivation to change, particularly for smoking, is that of changes to health. Although some participants with conditions such as chronic obstructive pulmonary disease (COPD) were able to recognise improvements in their health owing to health assessments linked to their condition, all participants could be supported to take notice of improvements to their breathing as they reduced their smoking (see Appendix 28, Quotation 73).
Almost half of the participants spoke about using substitution to support their smoking reduction. Non-NRT examples provided by participants included using chewing gum, eating celery sticks and sucking mints. NRT examples included both vapes and inhalators. One participant who tried sucking mints did not find this useful, and so switched to an inhalator, which they felt worked better for them owing to their craving for nicotine (see Appendix 28, Quotation 74).
Participants raised aspects of the MI approach as being important in their efforts to reduce smoking. The development of a relationship built on trust and enabling a relaxed environment for intervention delivery were seen as crucial in participants’ experiences of the intervention (see Appendix 29, Quotation 75). Furthermore, engendering an equal relationship between the HT and the participant was seen as key to developing motivation to change behaviour (see Appendix 28, Quotation 76).
For one participant, being able to be honest with the HT about setbacks and the HT not being judgemental was pivotal in supporting a change in their own view of themselves and their behaviour to support behaviour change (see Appendix 28, Quotation 77).
Health trainers spoke about a range of MI techniques that they found useful in working with participants to support them to reduce smoking. HTs recognised the importance of using open questions in encouraging participants to speak. Reflection was raised as a useful technique by over half of the HTs. The utility of reflection was discussed in terms of both enabling HTs to clarify what a participant meant when they said something and reinforcing progress that they had made with either smoking reduction or increasing PA (see Appendix 28, Quotations 78 and 79).
Other techniques discussed included identifying the discrepancy between their behaviour and where they wanted to be in the future to encourage participants to develop motivation for change (see Appendix 28, Quotation 80).
One HT spoke about the technique of rolling with resistance, not only in supporting behaviour change, but also in developing a trusting relationship between the HT and the participant by not appearing threatening or confrontational (see Appendix 28, Quotation 81).
The intervention’s non-directive and non-judgemental approach was viewed by HTs as being central to its success in supporting reduction. Not telling participants what to do and, instead, supporting them to develop their own plans and goals were key to developing confidence and motivation to change (see Appendix 28, Quotations 82 and 83).
Through listening, guidance and support, rather than direction, HTs recognised that participants came up with their own strategies that were supportive of behaviour change, thereby making them feel empowered (see Appendix 28, Quotation 84).
Barriers and challenges
The most cited external challenge was that of social influence. Some of the ways that social influence was not supportive of participants was through the lack of understanding some people had of the challenge of quitting smoking and the lack of understanding of reduction as a pathway to cessation (see Appendix 28, Quotations 85 and 86).
Not having time or financial resources for PAs of interest to participants was viewed as a barrier to taking up and increasing PA. Participants’ poor health was also seen as a barrier to being able to work with them to support PA increase (see Appendix 28, Quotation 87).
Health trainers raised a number of challenges of their role in two areas: role practicalities and participant-centred challenges, each of which will be summarised in turn.
First, HTs spoke about a range of practical challenges that they faced in their role. Some HTs felt that there were not enough intervention sessions, particularly to support participants to make a quit attempt (see Appendix 28, Quotation 88). Using the telephone, as opposed to meeting participants in person, was also raised as a challenge in terms of being able to review smoking and PA self-monitoring, picking up on non-verbal cues and applying MI techniques (see Appendix 28, Quotation 89). Other practical challenges raised included some difficulties meeting participants when their children were present, building knowledge of PA opportunities in the local community that could be used to signpost participants and not being able to communicate with participants’ GPs when they had complex comorbidities. One HT also said that they found the role isolating, particularly when working with participants over the telephone. Finally, two HTs stated that they found it challenging to write end-of-intervention letters to their participants when there had been little change in their behaviour (see Appendix 28, Quotation 90).
Second, challenges centred on participants included participant characteristics, rapport, barriers to engagement, resistance to intervention approaches and techniques, lack of intervention receipt/understanding, and participant attitude towards PA. HTs spoke about a range of participant characteristics and behaviours that challenged intervention delivery in a number of ways. HTs described the challenges of working with participants who were not ready for change, perceived as unreliable or not psychologically aware. Furthermore, three HTs discussed how they worked with less talkative participants in order to ensure that they encouraged and achieved active participant involvement, rather than filling the silence and making suggestions about strategies and activities that would not have come from the participant themselves (see Appendix 28, Quotation 91).
Lack of rapport was discussed as challenging when a HT has a sense that they are not the right person to support the individual or they cannot understand or relate to what drives a participant in order to be able to provide appropriate support (see Appendix 28, Quotation 92).
Barriers to engagement raised by HTs included a participant’s enthusiasm dropping off, participants not prioritising the intervention, a participant being busy or having a chaotic lifestyle and the impact of unexpected events in the lives of participants. Although HTs maintained a flexible approach with participants to support engagement, this did not always ensure that participants maintained engagement with the intervention and some participants stopped attending appointments despite the efforts of the HTs (see Appendix 28, Quotation 93).
Health trainers also raised the challenge of participants’ resistance to intervention approaches and techniques, including participant resistance to the four smoking reduction strategies, resistance to goal-setting and general resistance to change. Over half of the HTs experienced resistance to setting goals among the participants they supported (see Appendix 28, Quotation 94).
There was also discussion of some participants not fully understanding the trial or the intervention. One HT talked about some participants thinking that they were going to be provided with NRT on entering the trial, whereas others described participants’ lack of understanding becoming apparent in their expectations of being told what to do in order to make changes (see Appendix 28, Quotation 95).
Participants’ attitudes to and experiences of PA were also viewed as a challenge in terms of supporting increased PA. Although some participants were reluctant to do PA and were not engaged with monitoring PA, others were already active, either through taking part in activities or because they had a very active job, which provided challenges for the HT in supporting further increases in PA to aid smoking reduction (see Appendix 28, Quotation 96).
Suggested improvements to the intervention
Both participants and HTs highlighted several suggestions for improving the intervention, which are shown in Table 20. For supporting quotations from the data source, see Appendix 28, Quotations 97–112.
| Group | Suggested improvement | Rationale |
|---|---|---|
| Intervention participants | Increase intervention time frame and gaps between contact sessions | Not currently long enough to support people to change target behaviours |
| Top-up sessions following main delivery | Reinforce skills learned to use when they met challenges with reduction or cessation | |
| Increase in-person contact | In-person sessions created a greater sense of accountability; longer, more relaxed and more motivating than phone sessions | |
| Exercise vouchers | Enable participants to afford to take up certain forms of PA | |
| Integrate and directly offer NRT | Better support of quit attempt, compared with current signposting to access own NRT | |
|
Promote and support motivation | |
| Change in style of delivery by being more directive with use of CO reader | Enhance accountability | |
| More emphasis on and more evidence provided for the link between smoking and PA | Provide clarity and evidence for the link and potential benefits of making the link | |
| More local signposting | Enhance access to and knowledge of local services and support | |
| HTs | Summary leaflet about the TARS and multiple behaviour change to provide to participants early in the intervention | Provide a visual aid and evidence to support HTs in explaining intervention processes to participants |
| Check-in point post main delivery | Let the HT know how they were getting on and if they had continued to reduce or quit | |
| Provision of more information and access to NRT | Enhance provision of support | |
| Training: | ||
|
|
|
| Supervision: | ||
|
|
|
Discussion
The internal pilot phase of the process evaluation demonstrated acceptability of the intervention and trial methods, which was supported by the trial subsequently exceeding its recruitment target and demonstrating good levels of intervention engagement. Overall, participants in the intervention attended an average of 4.8 sessions across all sites, with 76% of participants receiving the a priori ‘dose’ of the intervention (i.e. two or more sessions), which exceeded the levels observed in the pilot trial. 30 The high levels of engagement support the intervention design and delivery for engaging with people who smoke but do not want to immediately quit. However, as shown by the primary statistical analysis and supported by the qualitative work of this process evaluation, the intervention had less of an effect than expected on smoking cessation induction. Although the intervention successfully supported a reduction of smoking (evidenced from all data sources), it failed to translate this into increased smoking cessation, and the qualitative work demonstrated barriers to this in terms of the participants (e.g. low and unchanged importance of quitting smoking, motivation linked solely to reduction), the HTs (not feeling confident to push the message of cessation and concerns about undermining achievements related to reduction) and the intervention design (e.g. described as not long enough to affect changes related to cessation). The EARS pilot trial excluded people wanting to use LNCPs, but with the increased prevalence of LNCP use over time, participants were not excluded for wanting to use LNCPs in the TARS. The HTs were trained to provide advice on and referrals to other services should an individual be interested. However, other services were not a formal part of the intervention. Limited qualitative data showed that this may have been a valued addition to the intervention. There was an overall increase in the proportion of participants who reported using LNCPs at baseline from 14.0% to 40.8% and 39.0% at 3 and 9 months, respectively (based on a denominator of number of participants followed up) (or 26.0% and 22.8%, assuming that those who were missing at follow-up were not using LNCPs, at least among those followed up). However, there were no significant differences in LNCP use between the groups at the follow-up assessments and LNCP use did not modify the intervention effects for any of the smoking outcomes, albeit the data were too sparse to fully investigate this.
There appeared to be some variation between sites in terms of number and duration of sessions provided, but the main statistical analysis showed no effect of site, or whether or not at least two sessions took place with a HT, on intervention effects, suggesting that variation in intervention dose did not have an impact on its effects. Further work is needed to understand if delivery fidelity (i.e. the standard and quality of delivery), and not just the number and duration of sessions, influenced smoking outcomes; this will be completed as part of Jane Horrell’s PhD.
Fidelity assessment demonstrated that the core components of the intervention were delivered to a predefined level of acceptability overall, but smoking-related HT competencies were delivered to a higher standard than the related competencies for PA. Results from enactment fidelity assessment indicate that behaviour change relating to PA was also lower than that relating to smoking reduction. These findings were also reflected by the results from the delivery and receipt fidelity checks. The PA element may have been delivered to a lesser extent because a proportion of participants were less motivated and/or amenable to increasing their PA, and therefore influenced the HT to be more smoking focused. This difference was also supported in the qualitative work, which highlighted some of the different challenges and barriers faced in addressing the different behaviours. A similar difference was found in the fidelity assessment of the pilot trial,33 and although the standard of delivery did improve comparatively (following learning from the pilot trial, including adaptations in training and delivery) in the current trial, and although the difference in standard of delivery between smoking and PA components is not as pronounced, more investigation of the barriers to addressing PA alongside smoking behaviour is needed.
Changing the two behaviours simultaneously did not appear to be a barrier for those participants who were able to increase their PA levels and open to increasing them, and there is evidence that this operated on both practical (distraction from cravings, removal from the opportunity to smoke) and psychological (feeling healthier) levels. As shown in the fidelity and qualitative work, motivation, past experience of PA and perceived fitness influenced participants’ willingness to incorporate PA into their behaviour change goals. Related to this, there was some evidence from the qualitative data that experiencing the incompatible nature of the two behaviours (for those who engaged in PA) did drive motivation for changing smoking behaviour and could lead to multiple behaviour change. Data from the process measures collected at baseline and 3 months also supported an increase in participants’ beliefs about using PA as a way to control smoking behaviour, and qualitative data demonstrated the perceived utility of using PA to manage smoking behaviour; however, changes in PA were shown not to mediate smoking outcomes, further demonstrating the complex nature of the two behaviours.
Active participant involvement, and the non-judgemental and empowering client-led nature of the intervention (drawing on MI techniques) were shown to be delivered to a high standard and to be fundamentally important parts of the intervention for both initiating and maintaining engagement with participants and effecting change. Linked to this, the focus on reduction rather than cessation promoted a sense of confidence among participants who may have previously had negative experiences with traditional cessation services, including feeling judged should they fail to quit.
The data showed that the intervention successfully increased participants’ self-reported levels of confidence for both changing PA and reducing smoking, as well as showing that it increased perceived importance of reducing smoking. However, it did not support significant changes in the perceived importance of quitting smoking or in the importance of PA. These data support both the fidelity work and the qualitative work that highlighted difficulties with progressing to cessation, as well as some resistance and difficulty in delivering and supporting changes in PA. This could be a function of the intervention (which was not designed around a ‘fear’ message for either behaviour) of the focus of the intervention on reduction rather than cessation and of PA being seen as secondary target for change after smoking reduction.
The process data demonstrated significant changes at 3 months in all but one process measure related to smoking, suggesting that the intervention successfully supported change in the targeted processes. For PA, all but two process measures showed significant improvement. Those processes that failed to improve were again linked to perceived importance, and to that of social support for PA. Managing social influence for PA was the least well-delivered component of the intervention, which may explain the failure to observe improvements in the process data.
Self-monitoring of behaviour was identified as an important process for changing both behaviours, and qualitative data supported it as important from the perspectives of both the participants and the HTs. Three-month process data showed that intervention participants successfully increased the amount of self-monitoring for both behaviours, and self-monitoring was shown to mediate the effects on smoking reduction and PA at 3 months, highlighting it as a fundamental process in the intervention.
It was repeatedly observed in the data that the duration of the programme was not considered long enough by either the participants or the HTs. Although the mediation analyses demonstrated some success in changes in the processes mediating the behaviours at 3 months, this was not observed at 9 months. It is therefore reasonable to suggest that a longer intervention may produce longer-lasting effects. Linked to this, the qualitative data showed that some participants and HTs did not think 8 weeks was long enough to get somebody who does not want to quit initially to a point where they are motivated to quit, and booster sessions would have been valued to increase rates of cessation.
Overall, there was evidence that the components of the intervention were delivered to an acceptable level and that the intervention successfully resulted in positive change in most of the key psychological and behavioural processes, although this did not translate into increased rates of quitting. There was difficulty in progressing participants to cessation, and PA was often shown to be a ‘secondary’ target after managing smoking consumption, as demonstrated by relatively poorer delivery, fewer successful changes in the process data and support for smoking reduction being the primary target in the qualitative data. Further work is needed to understand if those participants who reduced their smoking up to 3 months were also more likely to report a quit attempt and point prevalence abstinence (at 3 months) from TARS data, in line with other studies. 5,9
Chapter 5 Economic evaluation
Introduction
In this chapter, we present an estimation of the direct cost associated with the delivery of the TARS intervention, the incremental impacts of the intervention on costs and quality of life as observed in the TARS, and the translation of the primary clinical effectiveness outcome (smoking cessation) on lifetime costs and quality-adjusted life-years (QALYs). Two cost-effectiveness analyses are presented, jointly answering the following research questions:
-
What is the estimated resource use and cost associated with the TARS intervention?
-
Is the TARS intervention a cost-effective use of NHS resources, versus brief advice (control), based on economic outcomes (health and social care resource use and quality of life), as measured in the TARS?
-
Is the TARS intervention a cost-effective use of NHS resources, versus brief advice (control), over the long term, based on the effectiveness of the TARS on smoking cessation when modelling the effectiveness, lifetime costs and QALYs?
In all analyses, costs are given in 2018/19 Great British pounds. An NHS and Personal Social Services (PSS) perspective is used.
Estimating the cost of delivering the TARS intervention
The TARS intervention has been described in detail in Chapter 2. In the TARS RCT, data have been collected to measure the resource use associated with delivering the intervention, compared with brief advice (control).
The main components of resource use for the TARS intervention, informed by the pilot trial,23 are as follows:
-
HT time, which comprises contact time with clients as well as non-contact time directly attributable to the TARS intervention
-
training and supervision of HTs to support the delivery of the TARS intervention
-
venues/locations for face-to-face contacts with clients and HT non-contact time
-
pedometers and notebooks given to all clients.
Methods
Measuring resource use
Health trainer contact time
In the TARS, HTs recorded all client sessions and other client contacts for each participant, describing the following:
-
the nature of the contact – session (delivery of the intervention), contact (e.g. checking on progress, scheduling sessions), other, did not attend
-
the method of the contact – face to face, telephone, Short Message Service (SMS), e-mail, letter
-
the date and time of the contact
-
the duration of the contact
-
the location of the contact
-
the time for a HT to travel to and from the contact (when applicable).
In total, 7558 such contacts were recorded; these form the basis for the costing of HT contact time.
We included the duration of the contact and the time for a HT to travel to and from contacts in the estimation of contact time.
Health trainer non-contact time
Health trainer non-contact time was not directly measured in the TARS, but was informed by trial data and input from the trial co-ordinators. Investigators estimated that the maximum caseload for a full-time HT delivering solely the TARS intervention would be 25 concurrent clients. We therefore estimated the non-contact time per participant by apportioning to non-contact time the remaining time for a full-time HT delivering solely the TARS intervention after accounting for contact time and supervision.
Supervision
It was assumed that if the TARS intervention were to be implemented then HTs would require TARS-specific supervision from a senior HT to ensure fidelity of the intervention. Investigators estimated that a monthly group supervision session lasting 2 hours would be appropriate, and that this could be conducted virtually, with one senior HT supervising six HTs. Therefore, for each HT each year, there would be 1440 minutes of HT time to be supervised and 240 minutes of senior HT time for supervision. Investigators estimated that the average number of annual cases per HT (given that not all HTs would deliver solely the TARS intervention) would be 80. This means that for each client there is an allocation of 18 minutes’ HT time and 3 minutes’ supervisor time for supervision purposes.
It was assumed that supervision not relating to the TARS was included in the unit cost for HTs.
Training
Based on discussions with investigators, we assumed that for a HT to deliver the TARS intervention they would require an initial training period of 5 days (37.5 hours). They would then be able to continue delivering the TARS intervention provided they had 1 day (7.5 hours) of self-directed refresher training each year.
We assumed that the initial training would be led by a senior HT and that three HTs would be trained at a time. We included 5 days of use of a suitable training venue and training materials in our estimates, and we assumed that the senior HT would be eligible for 50 miles per day in travel expenses. 23
We assumed that HTs would stop delivering the TARS intervention after 3 years (e.g. owing to promotion, redeployment, career change), so that there would be one period of initial training and 2 refresher training days.
We amortised the costs of training over 3 years using a discount rate of 3.5% per year. We finally divided the amortised annual costs per HT by the assumed annual caseload of 80 cases to obtain the training cost per client.
Consumables
Investigators stated that the only consumables used as part of the TARS intervention were pedometers and fridge magnet notebooks. One of each was given to each client and they were not returned.
We also included costs for any letters sent as recorded by HTs in their contact sheets.
Unit costs
Health trainer and senior health trainer
Previous research23,70 has assumed that NHS Agenda for Change band 4 pay is a suitable proxy for the cost of HTs, even though they would not necessarily be employed by the NHS. As HT costs are central to the estimation of the cost of the TARS, we judged it important to validate this approach to costing.
We identified that the Standard Occupational Classification for a HT is 3219 ‘Health associate professionals n.e.c. [not elsewhere classified]’. In the 2019 provisional results of the Annual Survey of Hours and Earnings, the median gross hourly pay in this Standard Occupational Classification was £12.20 and the mean gross hourly pay was £13.18. 71
For comparison, the mean annual wage for Agenda for Change band 4 (scientific and professional staff) is estimated to be £22,256 in the Personal Social Services Research Unit (PSSRU) Unit Costs of Health and Social Care 2019. 72 The hourly wage, therefore, is somewhere between £12.36 (assuming 1800 working hours per year) and £13.76 (assuming 1618 working hours per year).
Therefore, we judged that the salary costs of Agenda for Change band 4 would be a good proxy for the salary costs of HTs. We further assumed that other costs (employer’s National Insurance and pension contributions, buildings, equipment, expenses, management) would be well approximated by the PSSRU Unit Costs of Health and Social Care 2019.
The cost per hour of a HT was ultimately estimated to be £29.43. 72
We assumed that supervision and training would be conducted by a HT with more experience, and therefore used Agenda for Change band 5 for this cost (£33.83 per hour).
Training costs
The cost of the venue was estimated to be £100 per day. 23 Investigators estimated that the training materials would cost £200, and we allowed for £0.20 per mile for travel expenses for the senior HT.
Consumables
Investigators provided costs of £7 for pedometers and £3 for fridge magnet notebooks. We estimated that the cost of stationery and postage for a letter would be £1.
Analysis of uncertainty
We explored the uncertainty around contact time by conducting bootstrapping of the per-participant costs (1000 iterations) and producing bias-corrected and accelerated 95% CIs.
We explored the uncertainty around non-contact time by varying the assumed maximum caseload for a HT (25 in the base-case analysis) from 20 to 30.
We explored the uncertainty around supervision and training costs by varying the assumed annual caseload for a HT (80 in the base-case analysis) from 40 to 160.
We explored the uncertainty around HT and senior HT time unit costs by varying these by ± 10%.
Results
The estimated intervention costs for the TARS intervention are given in Table 21. It is estimated that the total mean cost per participant is £239.18.
| Resource | Mean per participant | Unit cost (£) | Mean cost per participant (£) |
|---|---|---|---|
| HT contact time (resource use in minutes) | |||
| Sessions | 162.0 | 29.43 per hour | 79.46 |
| Contacts | 24.5 | 29.43 per hour | 12.02 |
| DNA | 1.2 | 29.43 per hour | 0.58 |
| Other | 1.6 | 29.43 per hour | 0.78 |
| Total | 189.3 | 29.43 per hour | 92.84 |
| HT travel time (resource use in minutes) | |||
| Sessions | 102.8 | 29.43 per hour | 50.41 |
| Contacts | 1.2 | 29.43 per hour | 0.58 |
| DNA | 3.7 | 29.43 per hour | 1.82 |
| Other | 0.4 | 29.43 per hour | 0.20 |
| Total | 108.1 | 29.43 per hour | 53.02 |
| HT non-contact time (resource use in minutes) | |||
| Client related | 128.2 | 29.43 per hour | 62.86 |
| Supervision | 18.0 | 29.43 per hour | 8.83 |
| Total | 146.2 | 29.43 per hour | 71.69 |
| Consumables | |||
| Pedometers | 1 | 7 | 7.00 |
| Fridge magnet notebooks | 1 | 3 | 3.00 |
| Letters | 0.466 | 1 | 0.47 |
| Total | 10.47 | ||
| Supervisor time (resource use in minutes) | |||
| Monthly supervision | 3.00 | 33.83 per hour | 1.69 |
| Traininga | |||
| Initial training | 7.66 | ||
| Refresher training | 1.81 | ||
| Total | 9.47 | ||
| Total cost per participant | 239.18 | ||
Health trainer contact time
The contact data recorded in the trial showed that, on average, participants had the following:
-
4.8 sessions, each session lasting 33.5 minutes, on average (not including travel time)
-
10.9 non-session contacts, each lasting 2.2 minutes, on average
-
0.5 non-attendances, each lasting 2.2 minutes, on average (not including travel time)
-
0.2 other interactions, each lasting 10.0 minutes, on average.
After including travel time, there were 297.4 minutes of HT input time per participant, resulting in a cost of £145.87 for HT contact time (95% CI £134.80 to £160.70).
Health trainer non-contact time
The average contact time per session (dividing the total contact time by the total number of sessions) was 62.4 minutes. For 25 clients, this would result in total weekly contact time of 1561 minutes. After adding 27.6 minutes per week for supervision, this leaves 667 minutes to allocate across the client workload for non-contact activities, that is 26.7 minutes per week. The average number of sessions delivered per client was 4.8, so we calculated 128 minutes per client for non-contact time.
Supervision and training
The cost of supervision per client was £10.52 (£8.83 of HT time and £1.69 of senior HT time).
The cost of delivering initial training to three HTs was estimated to be £5329 (£7.66 per client), and the cost of each day of refresher training was estimated to be £221 (£1.81 per client), giving a total per-client cost of £9.47. Further details are given in Appendix 29, Table 42.
Sensitivity analyses
The results of the sensitivity analyses for the cost of delivering the TARS intervention are shown in Appendix 29, Table 43.
The cost estimate was most sensitive to assumptions regarding the maximum caseload (varied from 20 to 30 in sensitivity analyses). When a high maximum caseload of 30 concurrent clients was assumed, the cost of the TARS intervention was estimated to be £204, whereas a low maximum caseload assumption of 20 concurrent clients led to a cost for the TARS intervention of £292. The cost estimates were also sensitive to the unit cost of HT time: when this was varied by ± 10%, the costs of the TARS intervention ranged from £217 to £262.
Trial-based cost-effectiveness analysis of the TARS intervention
Introduction
In this section, we answer the following research question: is TARS cost-effective, compared with control, based on data measured in TARS study?
This cost-effectiveness analysis was conducted by estimating the costs and QALYs for each participant in TARS over the follow-up period of 9 months and then using regression methods to estimate the effect of TARS on costs and QALYs.
There were two components to costs: the cost of delivering the TARS intervention (described previously, see Estimating the cost of delivering the TARS intervention) and the cost of NHS and PSS resources used by participants. To estimate QALYs, we used the EQ-5D-5L self-reported by participants.
The analysis was conducted in line with a prespecified health economics analysis plan [see the health economics plan on the project web page (www.journalslibrary.nihr.ac.uk/programmes/hta/1511101/#/documentation)].
We used generalised linear models (GLMs) (which are flexible and do not require errors to be normally distributed), including the treatment allocation as a covariate, as well as stratification variables used in randomisation (HSI and centre) and predictors (age, gender and baseline costs/EQ-5D-5L score).
The base-case analysis used complete cases, that is those providing data for all variables required in the regression models.
The primary outcomes were the incremental cost-effectiveness ratio (ICER), which is the incremental costs divided by the incremental QALYs, and the incremental net monetary benefit (INMB), which is obtained by valuing incremental QALYs according to a willingness-to-pay (monetary) value and offsetting incremental costs.
As the follow-up period was 9 months, we did not discount costs or QALYs.
Methods
Use of NHS/Personal Social Services resources
The use of NHS and PSS resources was estimated with a resource use questionnaire administered at baseline and at 3 and 9 months post randomisation. At baseline and at 9 months post randomisation, participants were asked to recall usage over the previous 6 months, whereas at 3 months post randomisation, participants were asked to recall usage over the previous 3 months. The total within-trial resource use was therefore estimated by summing the responses at 3 and 9 months post randomisation.
When a participant partially completed the resource use questionnaire, we assumed that other resources had not been used. When a participant indicated that they had used a service but did not indicate how many times they used the service, we assumed that they had used it once.
Free-text responses were allowed in the resource use questionnaire and we examined the responses to identify if any important NHS/PSS resources had been omitted from the resource use questionnaire.
Resource values (unit costs) were generally obtained from the PSSRU Unit Costs of Health and Social Care 201972 and the 2019 National Cost Collection for the NHS. 73 Unit costs are shown in Table 22.
| Item | Unit cost (£) | Source |
|---|---|---|
| Primary and/or community-based services (per contact or consultation) | ||
| GP at surgery/health centre | 39.19 | PSSRU 201972 (general practitioner cost per surgery consultation lasting 9.22 minutes, including direct care staff costs, including training) |
| GP via telephone | 15.52 | PSSRU 201972 |
| GP home visit | 78.92 | PSSRU 201474 and PSSRU 201972 [£156 per hour of GMS activity, 11.4-minute visit, 1 : 0.61 ratio of direct time to indirect time (7.0 minutes’ indirect time), 12 minutes’ travel] |
| Practice nurse at surgery/health centre | 12.43 | PSSRU 201474 and PSSRU 201972 (£37 per hour, 15.5 minutes per contact, 1 : 0.30 ratio of direct time to indirect time) |
| Practice nurse via telephone | 7.80 | PSSRU 201972 |
| Practice nurse at home | 39.68 | National Cost Collection 2018/1973 (N02AF – district nurse, face to face) |
| Physiotherapist | 62.90 | National Cost Collection 2018/1973 (A08A1 – physiotherapist, adult, one to one) |
| Occupational therapist | 83.17 | National Cost Collection 2018/1973 (A06A1 – occupational therapist, adult, one to one) |
| Social worker | 65.94 | PSSRU 201972 (£1648 per week, estimated caseload of 25, with one visit per case per week) |
| Care worker | 10.79 | PSSRU 201972 (£28.14 per weekday hour face to face, assume 23-minute visit70) |
| NHS Stop Smoking Service | 137.33 for one or more contacts | Walker et al.75 (average cost per client) |
| Walk-in centre | 21.00 | PSSRU 201972 (assume 15 minutes of b nurse time) |
| Secondary care services | ||
| Hospital inpatient | 385.16 per admission, plus 503.96 per day | NHS Reference Costs 2017/1876,77 |
| Outpatient appointment | 126.85 | National Cost Collection 2018/1973 (weighted average of all consultant-led and non-consultant-led outpatient attendances) |
| Day-case treatment | 751.90 | National Cost Collection 2018/1973 (weighted average of all day-case episodes) |
| A&E attendance | 182.81 | National Cost Collection 2018/1973 (weighted average of all emergency medicine episodes except those for which no investigation or significant treatment was required or the patient was dead on arrival) |
At each time point (baseline and 3 and 9 months post randomisation), the number of contacts for each resource was multiplied by the relevant unit cost, and these were summed to obtain the costs at each point. The total within-trial costs were calculated as the sum of the costs at 3 months (3-month recall) and at 9 months (6-month recall). The costs at baseline were log-transformed (after adding £1 to avoid zeros) and used as a covariate in cost regressions.
Quality-adjusted life-years
Health-related quality of life was measured with the EQ-5D-5L instrument48 and valued using the crosswalk78 to the EuroQol-5 Dimensions, three-level version (EQ-5D-3L), tariff,79 as currently recommended by NICE. 80
Quality-adjusted life-years were calculated for each participant using the area under the curve method. 81 QALYs were treated as missing if EQ-5D-5L data were missing at baseline or at 9 months post randomisation.
Analysis
A naive comparison of the mean costs in the two groups is inappropriate for a number of reasons. It is important to adjust analyses for the use of stratification in the randomisation process,82 and adjusting for covariates that are predictive of costs and/or QALYs can improve the power of statistical comparisons. It is necessary, therefore, to use regression methods that allow for multiple independent variables. Standard linear regression is not appropriate either, because the assumption of normally distributed errors is violated for costs and QALYs. The costs of NHS and PSS resource use cannot be negative, and are expected to be right-skewed, with a small minority of participants incurring large costs. In this trial, QALYs are bounded above by 0.75, because participants were followed up for 9 months. QALYs may also demonstrate skewness.
For the analysis, we used GLMs,83 which take the form shown in Equation 1, where yi is the dependent variable (the outcome) for unit (participant) i, xi is the independent variables (predictors) for unit i, β is a vector of coefficients, g is the link function and F is a statistical distribution from the exponential family.
Following fitting a GLM, adjusted mean outcomes are estimated by calculating the fitted values with the treatment allocation variable set to TARS intervention for all participants (leaving other covariates at their original levels), and then doing the same but setting the treatment allocation variable to control for all participants. These are estimates of the counterfactual mean outcomes if all participants had received the TARS intervention and if all participants had received control. These estimates were produced using the Stata command margins.
In addition, we used the Stata command suest to estimate the correlations between parameters of the cost and QALY models, allowing for estimation of the ICER and INMB and accompanying CIs.
For costs, we used the gamma family and the log-link. For QALYs, we also used the gamma family and the log-link, but the outcome variable was not QALYs (which are bounded above by 0.75), but the QALY loss from 0.75 (bounded below by 0). We added £239.18 (the cost of delivering the TARS intervention) to the costs for all participants in the intervention group.
Baseline costs were used as a covariate in the model for costs and baseline EQ-5D-5L data were used as a covariate in the model for QALYs. In addition, the stratification variables HSI (0–4 vs. 5 and 6) and centre (Plymouth, London, Oxford, Nottingham) and the predictors age and gender were used as covariates in both models.
In the primary (base-case) analysis, we included only complete cases, that is participants for whom we could calculate total costs and QALYs and for whom we had all baseline covariates.
Prior to unblinding, we examined the data for possible outliers, and found one participant who reported a 6-month inpatient hospital stay. We decided, prior to unblinding, that this participant would be excluded if in the control group and included if in the intervention group. Following unblinding, it was determined that the participant was in the control group; they were therefore excluded from all analyses.
Subgroup analyses were conducted by repeating regression analyses while only including participants who were members of each subgroup. Subgroup analyses were conducted according to age, gender and HSI score.
Results
Costs
Descriptive statistics for resource use and costs across the 9-month follow-up period are given in Tables 23 and 24. These statistics are for all available cases, that is participants for whom resource use data at 3 and 9 months were available. Descriptive statistics for resource use and costs at each individual time point (baseline, 3 months and 9 months) are given in Appendix 30, NHS and Personal Social Services resource use.
| Item | SAU group | Intervention group | ||
|---|---|---|---|---|
| Participants (n) | Mean (SD) [range] | Participants (n) | Mean (SD) [range] | |
| Primary care and community services | ||||
| GP at surgery/health centre | 241 | 4.87 (6.70) [0–47] | 248 | 3.80 (3.82) [0–24] |
| GP via telephone | 241 | 1.68 (3.53) [0–30] | 248 | 1.56 (2.66) [0–18] |
| GP at home | 241 | 0.07 (0.64) [0–7] | 248 | 0.00 (0.00) [0–0] |
| Nurse at surgery/health centre | 241 | 1.59 (2.68) [0–20] | 248 | 1.56 (3.04) [0–26] |
| Nurse via telephone | 241 | 0.27 (1.47) [0–15] | 248 | 0.13 (0.58) [0–6] |
| Nurse at home | 241 | 0.01 (0.09) [0–1] | 248 | 0.01 (0.14) [0–2] |
| Physiotherapist at surgery/health centre | 241 | 0.81 (2.91) [0–29] | 248 | 0.62 (1.80) [0–12] |
| Physiotherapist at home | 241 | 0.01 (0.09) [0–1] | 248 | 0.01 (0.11) [0–1] |
| Occupational therapist at surgery/health centre | 241 | 0.22 (1.12) [0–12] | 248 | 0.15 (0.79) [0–8] |
| Occupational therapist at home | 241 | 0.06 (0.45) [0–5] | 248 | 0.15 (1.63) [0–24] |
| Social worker | 241 | 0.35 (2.42) [0–27] | 248 | 0.34 (2.73) [0–40] |
| Care worker | 241 | 0.66 (8.04) [0–124] | 248 | 3.46 (26.00) [0–238] |
| NHS Stop Smoking Service | 241 | 0.67 (1.92) [0–13] | 248 | 0.81 (2.30) [0–15] |
| Walk-in centre | 241 | 0.19 (0.96) [0–9] | 248 | 0.28 (0.99) [0–9] |
| Secondary care services | ||||
| Outpatient attendances | 241 | 1.74 (3.23) [0–26] | 247 | 2.13 (5.78) [0–60] |
| Overnight stays | 241 | 0.17 (0.70) [0–7] | 248 | 0.14 (1.06) [0–16] |
| Inpatient days/nights | 241 | 0.78 (5.29) [0–66] | 248 | 0.17 (0.86) [0–9] |
| A&E attendances | 241 | 0.31 (1.03) [0–9] | 248 | 0.29 (0.90) [0–8] |
| Day-case procedures | 241 | 0.57 (1.54) [0–15] | 247 | 0.59 (1.70) [0–18] |
| Item | SAU group | Intervention group | ||
|---|---|---|---|---|
| Participants (n) | Mean (SD) [range] | Participants (n) | Mean (SD) [range] | |
| Primary care and community services | ||||
| GP at surgery/health centre | 241 | 190.91 (262.44) [0–1842] | 248 | 149.02 (149.60) [0–941] |
| GP via telephone | 241 | 26.08 (54.82) [0–466] | 248 | 24.16 (41.28) [0–279] |
| GP at home | 241 | 5.24 (50.67) [0–552] | 248 | 0.00 (0.00) [0–0] |
| Nurse at surgery/health centre | 241 | 19.75 (33.30) [0–249] | 248 | 19.40 (37.82) [0–323] |
| Nurse via telephone | 241 | 2.07 (11.49) [0–117] | 248 | 1.04 (4.51) [0–47] |
| Nurse at home | 241 | 0.33 (3.61) [0–40] | 248 | 0.48 (5.63) [0–79] |
| Physiotherapist at surgery/health centre | 241 | 50.89 (182.99) [0–1824] | 248 | 38.81 (113.38) [0–755] |
| Physiotherapist at home | 241 | 0.52 (5.72) [0–63] | 248 | 0.76 (6.89) [0–63] |
| Occupational therapist at surgery/health centre | 241 | 17.95 (93.11) [0–998] | 248 | 12.07 (65.41) [0–665] |
| Occupational therapist at home | 241 | 5.18 (37.22) [0–416] | 248 | 12.74 (135.97) [0–1996] |
| Social worker | 241 | 22.98 (159.53) [0–1780] | 248 | 22.33 (180.15) [0–2638] |
| Care worker | 241 | 7.12 (86.72) [0–1338] | 248 | 37.37 (280.51) [0–2568] |
| NHS Stop Smoking Service | 241 | 24.50 (52.69) [0–137] | 248 | 28.24 (55.62) [0–137] |
| Walk-in centre | 241 | 4.01 (20.25) [0–189] | 248 | 5.93 (20.80) [0–189] |
| Secondary care services | ||||
| Outpatient attendances | 241 | 221.07 (409.25) [0–3298] | 247 | 269.62 (733.58) [0–7611] |
| Overnight stays | 241 | 65.53 (267.80) [0–2696] | 248 | 54.36 (408.66) [0–6163] |
| Inpatient days/nights | 241 | 393.13 (2663.80) [0–33,261] | 248 | 83.32 (432.86) [0–4536] |
| A&E attendances | 241 | 56.89 (187.94) [0–1645] | 248 | 53.07 (164.13) [0–1462] |
| Day-case procedures | 241 | 427.43 (1157.86) [0–11,279] | 247 | 444.44 (1275.31) [0–13,534] |
The most apparent differences in resource use [mean number of contacts (mean cost)] between the groups are as follows:
-
GP at surgery/health centre [3.80 (£149) in TARS intervention group vs. 4.87 (£191) in control group]
-
care worker [3.46 (£37) in TARS intervention group vs. 0.66 (£7) in control group]
-
outpatient attendances [2.13 (£270) in TARS intervention group vs. 1.74 (£221) in control group]
-
inpatient days/nights [0.17 (£83) in TARS intervention group vs. 0.78 (£393) in control group].
We have not examined the statistical significance of any of these comparisons because of concerns of multiple testing.
Outcomes
Sustained quits to 9 months (primary trial outcome)
As shown in Chapter 3, there was a relative risk of sustained abstinence at 9 months of 2.267 (95% CI 0.705 to 7.288), and an absolute risk difference of 0.011 (95% CI −0.004 to 0.027).
EuroQol-5 Dimensions, five-level version
The EQ-5D-5L measurements are summarised in Table 25 for all available cases. There is a general pattern that the proportion of respondents reporting no problems on the different domains decreases from baseline. This may be due to reversion to the mean following recruitment to the trial or could be due to differential non-response at subsequent time points. Self-assessed health by the visual analogue scale suggests that there may have been some improvement in health-related quality of life in the TARS intervention group.
| Health-related quality of life | SAU group | Intervention group | ||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 9 months | Baseline | 3 months | 9 months | |
| EQ-5D-5L dimensions, n (%) | ||||||
| Mobility (N) | 453 | 300 | 271 | 453 | 310 | 281 |
| 1, n (%) | 344 (76) | 188 (63) | 167 (62) | 322 (71) | 201 (65) | 165 (59) |
| 2, n (%) | 58 (13) | 57 (19) | 48 (18) | 66 (15) | 52 (17) | 50 (18) |
| 3, n (%) | 37 (8) | 34 (11) | 41 (15) | 54 (12) | 37 (12) | 42 (15) |
| 4, n (%) | 13 (3) | 21 (7) | 14 (5) | 8 (2) | 18 (6) | 22 (8) |
| 5, n (%) | 1 (0) | 0 (0) | 1 (0) | 3 (1) | 2 (1) | 2 (1) |
| Self-care (N) | 453 | 299 | 269 | 453 | 310 | 279 |
| 1, n (%) | 409 (90) | 242 (81) | 208 (77) | 411 (91) | 271 (87) | 232 (83) |
| 2, n (%) | 23 (5) | 25 (8) | 35 (13) | 29 (6) | 22 (7) | 24 (9) |
| 3, n (%) | 12 (3) | 18 (6) | 18 (7) | 9 (2) | 15 (5) | 18 (6) |
| 4, n (%) | 7 (2) | 13 (4) | 7 (3) | 3 (1) | 2 (1) | 4 (1) |
| 5, n (%) | 2 (0) | 1 (0) | 1 (0) | 1 (0) | 0 (0) | 1 (0) |
| Usual activities (N) | 453 | 299 | 272 | 453 | 310 | 281 |
| 1, n (%) | 349 (77) | 163 (55) | 141 (52) | 346 (76) | 187 (60) | 152 (54) |
| 2, n (%) | 49 (11) | 71 (24) | 66 (24) | 58 (13) | 66 (21) | 68 (24) |
| 3, n (%) | 41 (9) | 38 (13) | 37 (14) | 39 (9) | 43 (14) | 43 (15) |
| 4, n (%) | 9 (2) | 25 (8) | 25 (9) | 8 (2) | 12 (4) | 13 (5) |
| 5, n (%) | 5 (1) | 2 (1) | 3 (1) | 2 (0) | 2 (1) | 5 (2) |
| Pain/discomfort (N) | 453 | 301 | 274 | 453 | 314 | 281 |
| 1, n (%) | 217 (48) | 97 (32) | 96 (35) | 221 (49) | 112 (36) | 92 (33) |
| 2, n (%) | 124 (27) | 104 (35) | 94 (34) | 120 (26) | 103 (33) | 97 (35) |
| 3, n (%) | 78 (17) | 52 (17) | 47 (17) | 86 (19) | 68 (22) | 57 (20) |
| 4, n (%) | 26 (6) | 30 (10) | 27 (10) | 16 (4) | 24 (8) | 22 (8) |
| 5, n (%) | 8 (2) | 18 (6) | 10 (4) | 10 (2) | 7 (2) | 13 (5) |
| Anxiety/depression (N) | 455 | 301 | 273 | 456 | 314 | 281 |
| 1, n (%) | 215 (47) | 101 (34) | 84 (31) | 210 (46) | 120 (38) | 112 (40) |
| 2, n (%) | 98 (22) | 90 (30) | 89 (33) | 122 (27) | 101 (32) | 81 (29) |
| 3, n (%) | 83 (18) | 58 (19) | 52 (19) | 81 (18) | 59 (19) | 52 (19) |
| 4, n (%) | 32 (7) | 22 (7) | 31 (11) | 24 (5) | 21 (7) | 23 (8) |
| 5, n (%) | 27 (6) | 30 (10) | 17 (6) | 19 (4) | 13 (4) | 13 (5) |
| EQ VAS | ||||||
| n | 457 | 298 | 268 | 457 | 306 | 275 |
| Mean | 65.3 | 64.3 | 65.5 | 64.3 | 67.8 | 67.0 |
| SD | 20.0 | 21.2 | 21.8 | 19.9 | 20.3 | 21.5 |
| Range | 0–100 | 5–100 | 10–100 | 0–100 | 0–100 | 0–100 |
EuroQol-5 Dimensions utility values and quality-adjusted life-years
Table 26 shows the EuroQol-5 Dimensions (EQ-5D) utilities (measured using EQ-5D-5L, valued using crosswalk to EQ-5D-3L) among available cases at each time point, and the mean QALYs over 9 months among available cases. These results suggest that a difference in utility values at 3 months is largely responsible for an overall difference in raw mean QALYs of 0.017 in favour of the TARS intervention group.
| Measure: time point | SAU group | Intervention group | ||
|---|---|---|---|---|
| Participants (n) | Mean (SD) [range] | Participants (n) | Mean (SD) [range] | |
| EQ-5D: baseline | 451 | 0.76 (0.25) [–0.25 to 1.00] | 452 | 0.77 (0.24) [–0.25 to 1.00] |
| EQ-5D: month 3 | 297 | 0.66 (0.31) [–0.25 to 1.00] | 306 | 0.72 (0.25) [–0.25 to 1.00] |
| EQ-5D: month 9 | 267 | 0.67 (0.29) [–0.25 to 1.00] | 279 | 0.68 (0.27) [–0.40 to 1.00] |
| EQ-5D QALYs | 263 | 0.51 (0.20) [–0.12 to 0.75] | 276 | 0.53 (0.17) [–0.23 to 0.75] |
Data completeness
There were a considerable number of participants for whom it was not possible to estimate costs and/or QALYs, resulting in a total of 470 out of 915 (51.4%) being included in the base-case analysis (complete-case analysis). See Appendix 30, Table 50, for further details.
Restricting to complete cases had the effect of decreasing the costs and QALYs in the control group but increasing the costs and QALYs in the intervention group. See Appendix 30, Table 51, for further details.
The assumption that data were missing completely at random was examined using Little’s test84 (with the Stata command mcartest85). Specifically, we applied Little’s test to the log-transformed costs at 3 and 9 months and the EQ-5D utility values at 3 and 9 months. This resulted in p = 0.15, suggesting insufficient evidence to reject the null hypothesis of missing completely at random. We further examined whether missingness could be predicted from the covariates to be used in regressions (covariate-dependent missingness) and obtained p = 0.99. This suggests that the complete-case analysis including the specified covariates may be unbiased.
Cost-effectiveness analysis
The primary cost-effectiveness analysis results are shown in Table 27. The TARS intervention is estimated to lead to a net cost increase of £173.50 (95% CI £354 saving to £514 increase) and to a QALY loss of 0.006 (95% CI 0.033 loss to 0.021 gain). The TARS intervention is therefore estimated to be dominated by (i.e. be less effective and more costly than) the control. The INMB for the TARS intervention is −£362 (95% CI −£1288 to £694), confirming that the TARS intervention is not expected to be cost-effective at a threshold of £30,000 per QALY, but that there is uncertainty around this, because the 95% CI for the INMB crosses zero. The cost per additional quit (sustained abstinence from 3 months to 9 months) was £15,500.
| Economic end points | Participants (n) | SAU group | Intervention group | Difference (Intervention − SAU) | |||
|---|---|---|---|---|---|---|---|
| Mean | 95% CIa | Mean | 95% CIa | Mean | 95% CIa | ||
| Costs (£) | 470 | 1385 | 1077 to 1795 | 1559 | 1277 to 1874 | + 173.50 | −354 to 514 |
| QALYs | 470 | 0.508 | 0.486 to 0.531 | 0.502 | 0.476 to 0.525 | −0.006 | −0.033 to 0.021 |
| ICER (cost per additional QALY) | Dominated | b | |||||
| Net monetary benefit (1 QALY valued at £30,000) (£000s) | 13.87 | 13.11 to 14.72 | 13.51 | 12.68 to 14.33 | −0.362 | −1.288 to 0.694 | |
The uncertainty around cost-effectiveness is demonstrated in the cost-effectiveness acceptability curve in Figure 8.
FIGURE 8.
Cost-effectiveness acceptability curve according to willingness to pay for 1 additional QALY.

Sensitivity analyses
Unit costs
When the lowest TARS intervention delivery cost from a sensitivity analysis (£203.99) was used, incremental costs reduced to £126.13. The TARS intervention was still dominated: as expected, incremental QALYs were negative, but the INMB improved from −£362 to −£315. When the highest cost from a sensitivity analysis (£291.96) was used, the incremental cost of the TARS intervention increased to £243.25 and the INMB worsened to −£432.
One-way sensitivity analyses were conducted for the unit costs of all NHS and PSS resources and are presented in Figure 9. Unit costs were increased or decreased by 20%. No single unit cost was sufficiently important for a variation of 20% to change the sign of the INMB (i.e. to make the TARS intervention cost-effective).
FIGURE 9.
Tornado diagram for one-way sensitivity analyses of NHS/PSS resource unit costs.

Incremental costs were most sensitive to the inpatient length of stay. In the base-case analysis, the cost per inpatient day was £503.96, and the mean resource use was 0.17 days in the TARS intervention group, versus 0.78 days in the control group (even after the exclusion of an outlier). This is partially caused by hospital stays of > 1 week in the control arm (four in the control group vs. zero in the TARS intervention group in the complete-case analysis), although there would still be a mean of 0.36 inpatient days in the control group if length of stay was Winsorised to 7 days.
Regression model specification
We explored the impact of the specification of the regression models on costs and QALYs by using alternative families and link functions in the GLM, and by excluding baseline costs and QALYs from the predictors. For costs and QALYs, we explored combinations of families (gamma, Gaussian and inverse-Gaussian), link functions (log, identity, inverse, inverse-squared) and transformation of baseline costs/QALYs (log, identity, inverse). We calculated the Akaike information criterion (AIC) for each model.
For costs, the lowest AIC (7681.0) was obtained using a gamma family with the log-link and log-transformed baseline costs (i.e. the base case). The second lowest AIC (7682.5) was obtained using a gamma family with identity link and untransformed baseline costs. The AIC for a linear model (Gaussian family with identity link and untransformed baseline costs) was 8758.8. Further details are given in Appendix 30, Regression model specifications for costs and quality-adjusted life-years.
For QALYs, the lowest AIC (−694.4) was obtained using a linear model. The second lowest AIC (−625.5) was obtained using a gamma family with identity link and log-transformed baseline EQ-5D-5L scores. The base case (gamma family with log-link and untransformed baseline EQ-5D-5L scores) had an AIC of −599.5. Further details are given in Appendix 30, Regression model specifications for costs and quality-adjusted life-years.
The choice of GLM family, link function and transformation of baseline costs/quality of life had a moderate effect on cost-effectiveness (see Appendix 30, Table 56). In all analyses, costs were estimated to be higher in the TARS intervention group, but incremental costs varied from £51 to £227 (base case £174). In the base-case analysis, incremental QALYs were small and negative (−0.006), whereas, in sensitivity analyses, incremental QALYs were small and positive (0.002 to 0.007).
The inclusion or exclusion of baseline EQ-5D-5L scores had a significant impact on the estimation of incremental QALYs: when these were included (base case), we estimated small and negative incremental QALYs (−0.006), but, when these were excluded, we estimated modest and positive incremental QALYs (0.026). This corresponds to losing 11% fewer QALYs from the maximum possible 0.75 QALYs than in the control arm. It is generally considered important to include baseline health state values in regression models for QALYs,86 which is why they are included in the base-case analysis. Fitting the models with and without baseline health state values helps us to see how much of the apparent difference in QALYs can be explained by differences in baseline health between the groups.
The mean baseline EQ-5D-5L scores were 0.744 in the control group and 0.780 in the TARS intervention group when restricting to complete cases. This difference was not primarily caused by a failure of randomisation: the baseline EQ-5D-5L scores were 0.756 in the control group and 0.766 in the TARS intervention group when all 904 participants providing baseline EQ-5D-5L data are included. The restriction to complete cases led to a disproportionate elimination of participants in the TARS intervention group with poor baseline EQ-5D-5L data. Overall, 13% (115/904) of participants had a baseline EQ-5D-5L score of < 0.5, and, when restricting to complete cases, again 13% (60/470) of participants had a baseline EQ-5D-5L score of < 0.5. Among participants in the TARS intervention group, 11% (50/452) had a baseline EQ-5D-5L score of < 0.5, but this reduced to 8% (20/239) when restricting to complete cases.
Handling of missing data
As noted previously, there is some reason to suspect that missingness is related to baseline quality of life and treatment allocation. Combined with the moderately high rate of missing data (only 51.4% of participants were included in the complete-case analysis), this gives some concern that missing data may bias the analysis results.
We conducted a multiple imputation analysis using multiple imputation by chained equations. The conditional models used predictive mean matching and included all analysis variables plus relationship status, employment status, EuroQol Visual Analogue Scale scores and SF-12v2 scores. We generated 50 imputation sets.
The results of the analysis (Table 28) show that there is still no statistically significant difference in either the costs or the QALYs between the control and intervention groups, but that the expected incremental costs are now negative (small saving of £38) and incremental QALYs are now positive (small gain of 0.009). Therefore, based on expected values, the TARS intervention is now dominant, rather than dominated by the control, and the INMB is now positive (although the 95% CI for the INMB still crosses zero).
| Economic end points | Participants (n) | SAU group | Intervention group | Difference (Intervention − SAU) | |||
|---|---|---|---|---|---|---|---|
| Mean | 95% CIa | Mean | 95% CIa | Mean | 95% CIa | ||
| Costs (£) | 914 | 1538 | 1235 to 1842 | 1500 | 1269 to 1731 | −38 | −413 to 336 |
| QALYs | 914 | 0.499 | 0.481 to 0.517 | 0.508 | 0.491 to 0.526 | 0.009 | −0.014 to 0.033 |
| ICER (cost per additional QALY) | Dominant | b | |||||
| Net monetary benefit (1 QALY valued at £30,000) (£000s) | 0.317 | −0.535 to 1.169 | |||||
The cost-effectiveness acceptability curve for the multiple imputation analysis (Figure 10) shows that there is still no value of willingness to pay for 1 QALY at which we are confident (at the 0.05 significance level) that the TARS intervention or control is economically superior, but suggests a greater probability that the TARS intervention is cost-effective than was suggested by the complete-case analysis.
FIGURE 10.
Cost-effectiveness acceptability curve with multiple imputation.
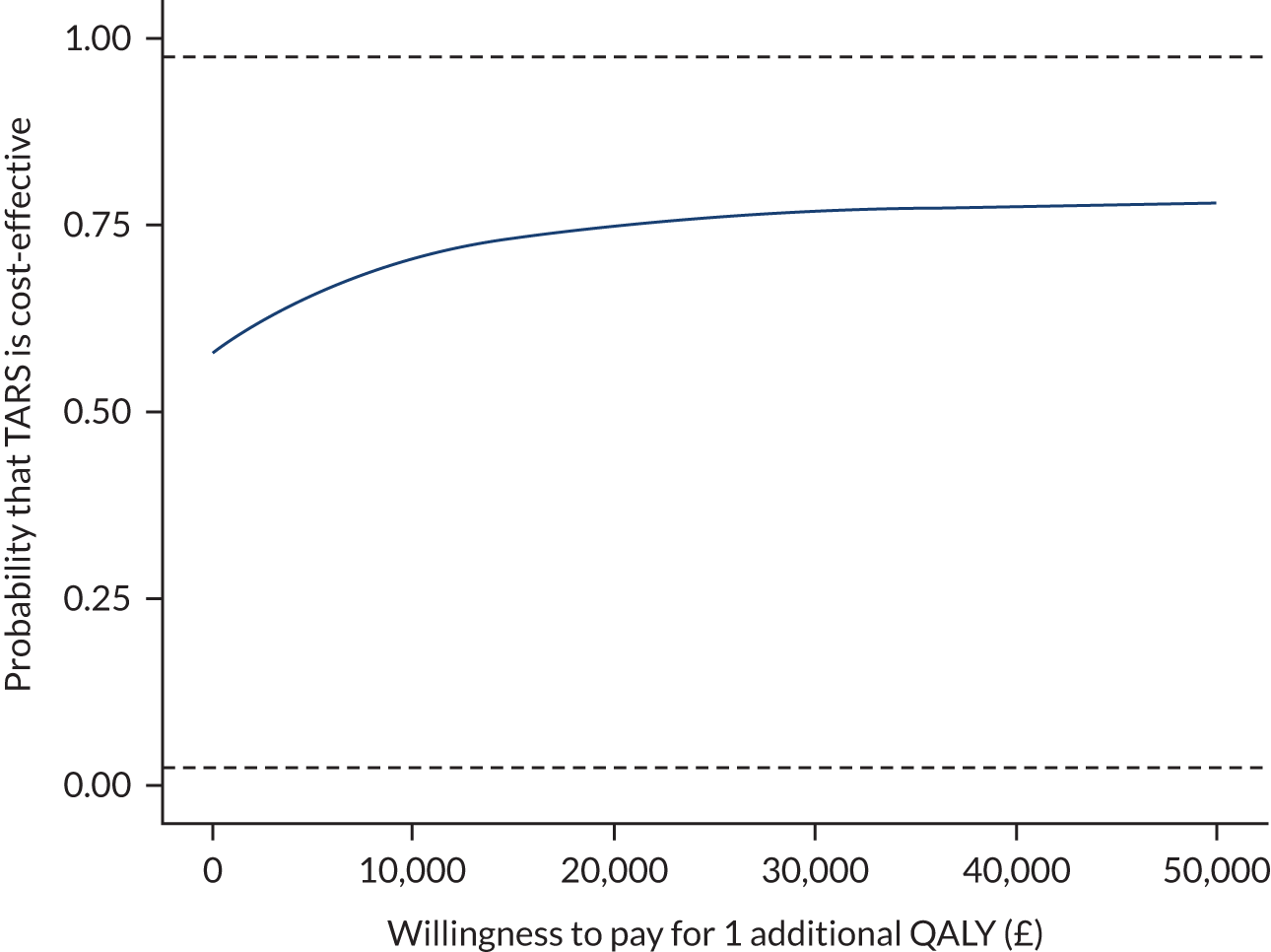
Subgroup analyses
The results of subgroup analyses are shown in Appendix 30, Table 56. Although many of these comparisons are underpowered, we did find statistical significance for incremental costs (null hypothesis being zero) in the two younger age subgroups. Those with high HSI scores are expected to result in cost savings, but also QALY losses, for an ICER of £20,200 per QALY. As this is in the south-west quadrant, the ICER must be greater than the cost-effectiveness threshold for the TARS intervention to be cost-effective. Generally, there are no strong signals for participant groups in which the TARS intervention would be expected to be more or less cost-effective.
Review of model-based approaches to estimating cost-effectiveness of smoking cessation interventions
We conducted a review of the published cost-effectiveness literature for smoking cessation to support the development of a modelling framework to evaluate the TARS intervention.
Full details of the review are provided in Appendix 31, with the methods and results summarised in this section.
Objectives
The objectives of the review were to identify:
-
existing health economic evaluations of interventions aimed at helping people to reduce or quit smoking
-
key structural features of models used for health economic evaluations
-
key model input parameters affecting the cost-effectiveness of smoking reduction/cessation interventions.
Methods
Bibliographic database searches were conducted to retrieve economic evaluations of smoking cessation published since 2012 (as a review of studies up to 2012 was conducted for the pilot trial23). Data were abstracted into a bespoke form based on, but extending, the data abstraction elements in the pilot trial. We included studies of individual-level support for smoking cessation (i.e. not public health interventions such as media campaigns, tax policies or changes to packaging), including pharmacological and behavioural interventions. Formal quality appraisal was not conducted, as the aim of the review was to consider different modelling approaches, rather than to weigh the results of studies. Narrative synthesis was conducted, supported by cross-tabulation of trial methods.
Results
Bibliographic database searches returned 9304 titles and abstracts. After automatic identification and removal of duplicates (n = 1814) and studies published before 2012 (n = 4804), 2686 titles and abstracts were screened.
Forty-four studies were ultimately included in the review. These included a mixture of intervention types (pharmacological aids, counselling, bespoke programmes, electronic aids, exercise, financial incentives and various combinations of these), and they were conducted in a wide range of countries, although the most studies were from the UK (n = 9) and the USA (n = 8).
Most models were Markov cohort simulations or other cohort/population simulations. Four studies used individual patient simulations (Markov microsimulation or discrete event simulation).
Few models included significant structural detail for smoking-related morbidities: generally patients were either unaffected or affected by a morbidity. Exceptions to this (i.e. with more complex model structures for a morbidity) were studies modelling smoking cessation in smokers already affected by COPD87,88 or Crohn’s disease,89 or at high risk of tuberculosis. 90
We categorised studies according to whether they modelled subsequent smoking behaviour (relapse and spontaneous quitting), specific smoking-related morbidities or smoking-related excess morbidity and mortality (captured in utility values and all/other-cause mortality rates). Six models incorporated all three of these, and all models incorporated at least one of these. These categories were further broken down, and models incorporated these features to greater or lesser extents (e.g. many models included relapse, but not spontaneous quitting).
We abstracted the data sources for key model inputs, including smoking behaviour, morbidities, utility values and excess mortality. The strengths and weaknesses of these data sources were considered as the choice of data sources can have a significant impact on the results of economic evaluations. We found that different data sources were used in many studies, and that few (if any) studies reported their process for identifying and appraising possible data sources.
We examined the results of sensitivity analyses conducted in models, as such results can indicate which model inputs should receive particular attention in terms of seeking and appraising data sources. Generally, the effectiveness of interventions (in terms of quit rates), the discount rates, the relative risks and costs of morbidities and intervention costs had the strongest effects on cost-effectiveness.
Age was an important determinant of cost-effectiveness, but different studies found different patterns in incremental costs and incremental QALYs as a function of age. This may be a result of discounting being applied differently, or different assumptions about the risks of relapse or spontaneous quitting.
Model-based cost-effectiveness analysis of the TARS intervention
The TARS has not demonstrated superiority of the TARS intervention versus control in the primary outcome to the prespecified significance level of the study (0.05), as explained previously. Although it has been argued that demonstrating statistical significance in a clinical effectiveness outcome should not be a prerequisite for deciding to introduce a new treatment (e.g. Claxton91), and it is not uncommon for economic evaluations to find in favour of a new treatment even if neither clinical outcomes nor costs have been shown to be different,92 it is nevertheless important to ensure that the fundamental message of the trial – a negative result – is not obscured by an economic evaluation, especially given all the possible sensitivity analyses that can be included in a model-based analysis.
The model-based analysis presented in this subsection explores the potential cost-effectiveness of the TARS intervention, and incorporates the uncertainty surrounding treatment effectiveness using standard decision-analytic modelling methods. The decision to conduct a model-based economic evaluation was prespecified and is not an indication that the effectiveness of the TARS intervention is considered to be proven.
Methods
Statement of the problem/objective of the research
The benefits of smoking cessation are not expected to be realised immediately and cannot be fully captured by RCTs, whose follow-up periods are generally long enough to establish whether or not a quit attempt has been successful, but not long enough to see benefits in terms of reducing the incidence of smoking-related disease and smoking-related mortality. A modelling framework is needed to translate the effectiveness data from the TARS into lifetime estimates of QALYs and costs that fall on the NHS and PSS.
Perspective
The cost-effectiveness analysis adopts the perspective of the NHS and PSS, in line with the NICE reference case,93 which is typically followed in health economic evaluations in the UK.
Interventions
The interventions compared in the cost-effectiveness analysis are the TARS intervention and brief advice (i.e. a standard care control).
Model type and rationale for structure
A proportional multistate life table (PMSLT) cohort simulation model has been developed,94 using a cycle length of 3 months. This is similar to a Markov cohort simulation, but it makes a number of assumptions to allow the modelling of multiple (co)morbidities without leading to a combinatorial explosion of health states. This model approach has been used in the World Health Organization Global Burden of Disease studies and is well suited to applications where a risk factor for multiple diseases is modified. 95 Higashi and Barendregt96 have previously used the PMSLT methodology to evaluate the cost-effectiveness of personal smoking cessation support.
A key assumption of the PMSLT approach is that, after the population has been separated according to the risk factor(s) of interest, the diseases being modelled are independent of each other (apart from competing mortality). Specifically:
-
Having one morbidity does not put an individual at greater or lesser risk of incidence of another morbidity.
-
The mortality rates of diseases are additive (e.g. if disease A alone contributes an additional 100 deaths per 100,000 person-years and disease B alone contributes an additional 200 deaths per 100,000 person-years, then the two diseases together will contribute an additional 300 deaths per 100,000 person-years).
The model follows two identical cohorts, one of which receives the TARS intervention and the other receives a control (brief advice). The relative effectiveness of the TARS intervention is introduced in the first cycle of the model, and thereafter the cohorts are modelled identically.
Our modelling approach incorporates smoking as a risk factor for morbidity and mortality and separates the population into three groups according to whether they are currently smoking, or, if they have quit, whether they quit after receiving the intervention (see Short-term quitting). The model allows for individuals to relapse after quitting, and to spontaneously quit.
The model predicts the risk of incidence and mortality of four smoking-related diseases: COPD, coronary heart disease, lung cancer and stroke. These were selected by consideration of the role these diseases play in the burden of smoking (health and economic),97,98 and the systematic review of existing economic models of smoking cessation (see Review of model-based approaches to estimating cost-effectiveness of smoking cessation interventions).
The model also predicts excess mortality due to smoking that is not already realised through the four smoking-related diseases.
Figure 11 gives an overview schematic of the model.
FIGURE 11.
Overview model schematic.
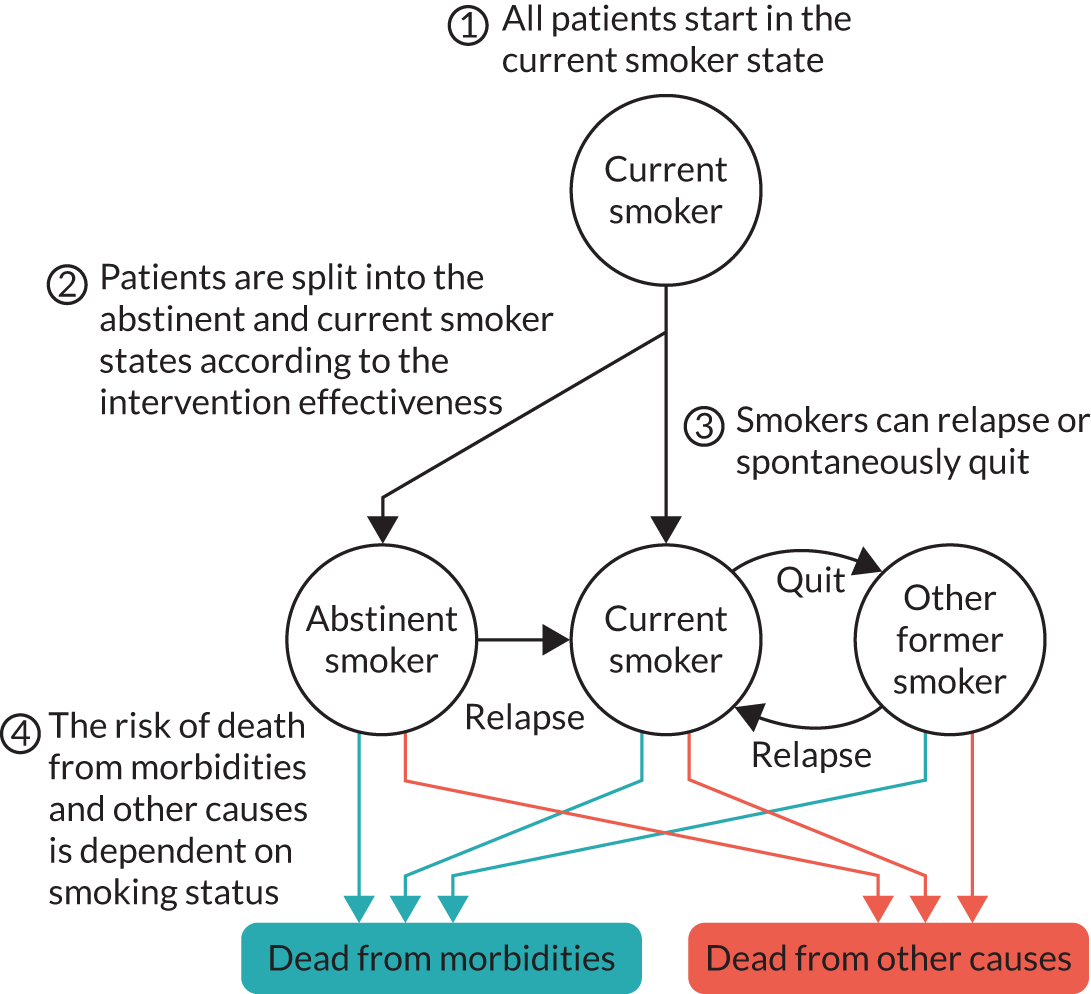
The model uses the life-table method (an alternative to the half-cycle correction) to account for transitions occurring during cycles. 99
Base-case analysis cohort characteristics
In the base-case analysis, we consider men who are aged 50 years and are current smokers. In heterogeneity analyses, we consider men and women (current smokers) aged 25–85 years. The mean age in the TARS was 49.8 years, and, although women made up the majority of participants in the TARS (55%), men are more likely to be current smokers in the general population.
Modelling smoking status over time
In the model, the population is divided into current smokers and former smokers. At the start of the model, the whole cohort is a current smoker. Over time, current smokers may spontaneously quit, and former smokers may relapse. We separate former smokers into those who quit in the first cycle (at the time the TARS intervention is delivered) and remain abstinent, and those who quit at a subsequent cycle. The states are labelled ‘current smoker’, ‘abstinent smoker’ and ‘other former smoker’. As it is only possible to enter the ‘abstinent smoker’ state in the first cycle, the time since quitting is known (subject to rounding to the cycle length). In the ‘other former smoker’ state, it is expected that there will be a distribution of times since quitting, but we track the mean time since quitting. 100
Short-term quitting
The TARS intervention is expected to be delivered in eight sessions across 8 weeks, with the intention that any quit attempts that are made as a result of the intervention are made by 3 months after initiation. We assume that the effect of the TARS intervention is restricted to the first cycle (which ends 3 months after the model starts).
In the first cycle, there is a baseline probability of smoking cessation of 3.9% (for a cohort aged 50 years; for a cohort aged 30 years the probability is 5.6% and for a cohort aged 70 years the probability is 3.7%). This probability is derived from the rate of spontaneous quitting described in Long-term relapse and spontaneous quits. Those quitting smoking in the first cycle transition to the ‘abstinent smoker’ health state. In the control arm, the baseline rate is applied, whereas, in the TARS intervention arm, the relative effectiveness of the TARS intervention is introduced as a relative risk. A relative risk was selected because, in the absence of differential mortality by smoking status (which is not expected to occur immediately), the relative risk of being in the ‘abstinent smoker’ health state is preserved in the presence of relapse (it applies equally in both arms) and subsequent quits (they enter the ‘other former smoker’ state rather than the ‘abstinent smoker’ state).
Those who remain in the ‘abstinent smoker’ state to the end of the third cycle (to 9 months from the start of the model) will have achieved the ‘primary outcome’ of TARS (prolonged abstinence from 3 to 9 months post randomisation). At the end of the third cycle, the relative risk of being in the ‘abstinent smoker’ state will be the same as was introduced in the first cycle.
The relative risk itself was calculated to be 1.34 on the basis of the absolute risk difference from TARS (1.12%). We chose to use the absolute risk difference from TARS as opposed to the relative risk (2.27) because it is the absolute risk difference that will drive incremental costs and QALYs and because the uncertainty distribution for the relative risk is asymmetric and extends to implausibly high effectiveness values.
Because TARS has not demonstrated statistical significance in the primary outcome, the model does not assume that the risk difference of 1.12% is known precisely. Instead, as with virtually all model inputs, it is associated with uncertainty: in this case, the uncertainty distribution is informed by TARS. In sensitivity analyses, the risk difference is varied, and, in some instances, the risk difference is zero or even negative. The input distribution for the risk difference is normally distributed, with a mean of 0.0112 (SD 0.0079), such that 95% of the distribution is between −0.0040 and 0.0267 (the 95% CI for the risk difference).
Long-term relapse and spontaneous quits
Hawkins et al. 101 used data from multiple waves of the British Household Panel Survey to identify longitudinal data on smokers who had attempted to quit smoking, and to assess the risk of relapse. We used Bayesian methods102 to fit a Gompertz model to the risk of relapse according to time since quitting, accounting for left truncation, interval censoring and right censoring present in the data reported. The Gompertz model suggests that just over 60% of quit attempts ultimately end with relapse. Full details are provided in Appendix 32, Smoking behaviour.
The annual probability of spontaneously quitting smoking was estimated from the Understanding Society study, waves 6–9. 103 Flexible regression splines were used to account for the relationship between age and spontaneous quitting. As it was only possible from the data to identify individuals who were non-smokers 1 year after previously describing themselves as current smokers, it was necessary to account for those smokers who attempted to quit but relapsed before being surveyed the following year. We estimate relatively high rates of quit attempts (15–25 quit attempts per 100 person-years), compared with the existing literature104–108 (annual quit probabilities of around 4%). Full details are provided in Appendix 32, Smoking behaviour.
Modelling smoking-related morbidities
We modelled each of the four smoking-related morbidities in a similar way. For each, we had a health state for being alive and unaffected by the disease, a health state for being alive and affected by the disease, and a health state for having died as a result of the disease. Each of these health states was further composed of abstinent smokers, current smokers and other former smokers (Figure 12). Owing to the structure of the PMSLT model, each of these models is not concerned with tracking how many have died from other causes.
FIGURE 12.
Example of morbidity model (for COPD).
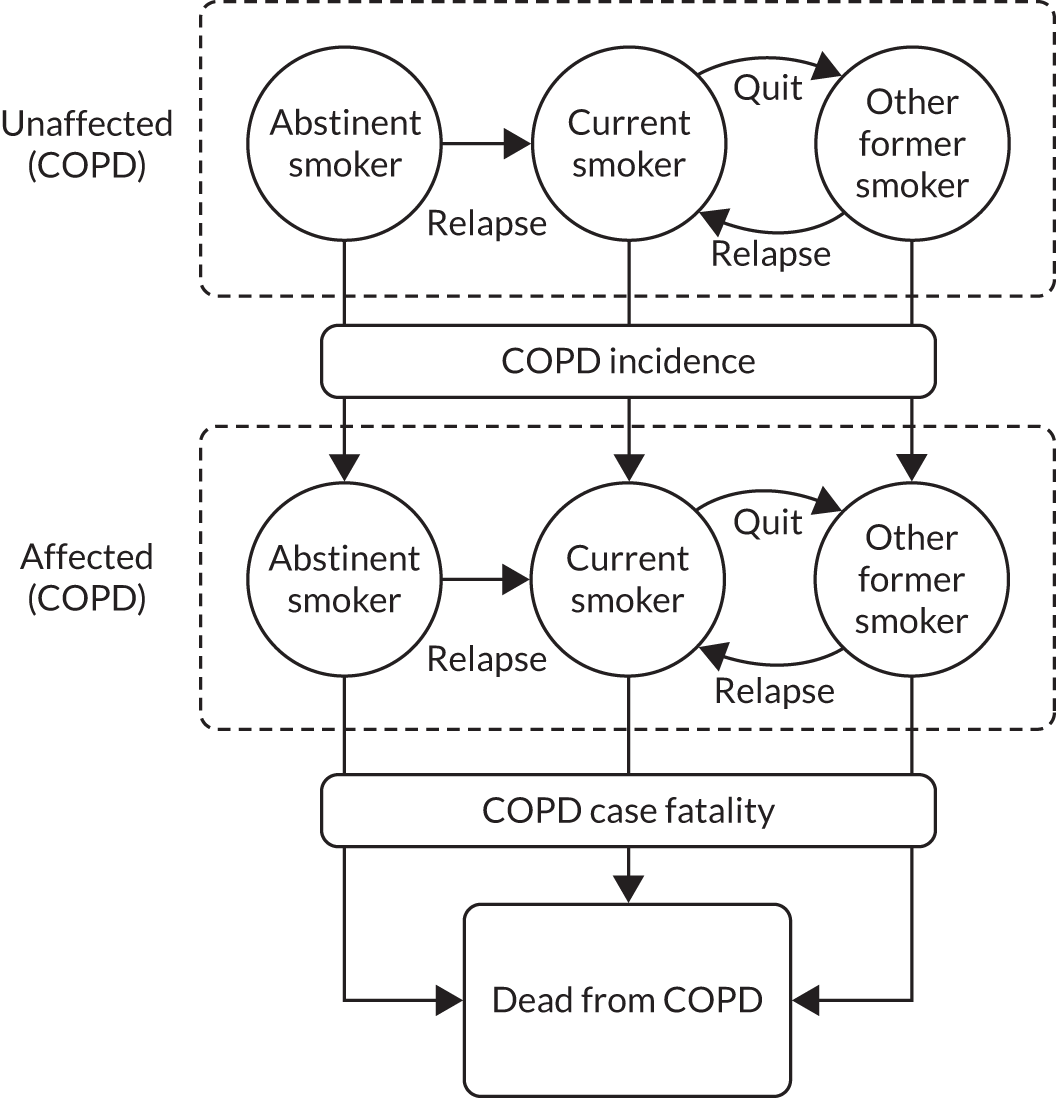
For COPD and lung cancer, we assumed that it was not possible to go directly from being alive and unaffected by the disease to having died as a result of the disease, and that individuals would need to first visit the alive and affected health state prior to dying from the disease. Therefore, there were three parameters of interest (for each combination of smoking status, age and gender): the prevalence at the start of the model (when the whole cohort is current smokers), the incidence of the disease and the case fatality rate for the disease.
For coronary heart disease and stroke, we assumed that it would be possible to go directly from being alive and unaffected by the disease (or at least to have not had the disease diagnosed) to having died as a result of the disease. We therefore had an additional parameter for these diseases, which was the probability that disease incidence would be fatal.
Chronic obstructive pulmonary disease
Rodriguez et al. 109 estimated that the OR for COPD incidence would be 6.15 for current smokers versus never smokers, and 3.45 for former smokers versus never smokers. They also estimated an OR of 0.61 for former smokers versus current smokers. From the CIs presented, we calculated that, on a logarithmic scale, the ORs for current and former smokers versus never smokers would have a correlation of 0.4. Rodriguez et al. 109 did not separate former smokers by time since quitting, so we incorporated the effect of time since quitting using estimates from Hoogenveen et al. ,110 which led to an estimate that 78% of the excess risk due to smoking would be eliminated 10 years after quitting.
We estimated the prevalence of COPD among smokers from the 2018 Health Survey for England. 111 Further details are provided in Appendix 32, Prevalence of morbidities at start of model.
Table S5 of McLean et al. 112 reports data from the Clinical Practice Research Datalink 2011. The number of events and the exposure (person-years) were inferred from the rates and CIs. A restricted cubic spline regression with interaction between age and gender was used to predict population-level incidence. Estimates for the OR for developing COPD within 1 year according to smoking status from Rodriguez et al. 109 were used to adjust these to incidence rates for current smokers.
We determined the case fatality rate by using data presented by Shavelle et al. 113 For each combination of smoking status, gender and age group, we calculated a weighted average of the excess mortality over the distribution of different COPD stages.
Lung cancer
Remen et al. 114 estimated the association between smoking (including time since cessation for former smokers) and lung cancer incidence. Compared with never smokers, the OR was 17.6 for current smokers and 4.47 for former smokers. We used the estimates from model 7 of table 4 in Remen et al. 114 to incorporate the effect of cessation, including the log-transformed time since cessation, to adjust the OR for lung cancer incidence. As incidence is generally low, we assumed that the ratio of incidence rates would be well approximated by an OR.
The prevalence at the start of the model (when the whole cohort is made up of current smokers) varies from < 1% for those aged < 60 years to around 4% for those aged ≥ 80 years. For further details, see Appendix 32, Prevalence of morbidities at start of model.
Population lung cancer incidence estimates were taken from the Office for National Statistics’ cancer registration statistics115 and were then adjusted according to smoking status using the estimates from Remen et al. 114
A Weibull survival model was fitted to UK lung cancer survival estimates116 (5-year survival by gender and age group, and 1-, 5- and 10-year survival by gender across age groups). The shape parameter for this model was 0.44, which indicates a decreasing hazard of mortality over time. To estimate a suitable average hazard of mortality (case fatality rate), we calculated the discounted life expectancy for each combination of age group and gender and used this to identify the exponential survival model with the same discounted life expectancy. As it is not possible within the model to know at what age an individual developed lung cancer (and as survival is generally poor), it was assumed that the average case fatality rate could be determined from the current age in the model, despite the survival estimates coming from the age at diagnosis.
Coronary heart disease
Shields and Wilkins117 estimated the effect of smoking on heart disease, including estimating the effect of time since quitting among former smokers. The relative risk for current smokers versus never smokers was 1.6 for men and 1.7 for women.
Prevalence, incidence and mortality data for ischaemic heart disease were sourced from the 2019 Global Burden of Disease study data. 118
To estimate the probability of incident disease being fatal, we relied on incidence and case fatality data from Smolina et al. ,119 who studied hospital admissions and deaths due to acute myocardial infarction. They identify which of these are ‘first’ acute myocardial infarctions (no record of acute myocardial infarction in prior 12 years), but this does not rule out that these individuals would have already been diagnosed with angina. We assumed that 50% of these would be in individuals with no prior diagnosis of angina and estimated that 14–48% would be fatal (depending on age). 119 We then estimated the case fatality rate among the prevalent population by attributing the remaining mortality not explained by fatal incident myocardial infarction to mortality in the prevalent population, as shown in Equation 2, where β is the probability that an incident case is fatal.
Stroke
Myint et al. 120 estimated the relative risk for stroke according to smoking status. For current smokers versus non-current smokers (i.e. including never smokers and former smokers), the relative risk was 2.25 for those aged < 65 years and 1.35 for those aged ≥ 65 years. They also found that previous smoking versus never smoking was not associated with stroke risk. We instead incorporated the delayed effects of smoking cessation using the model estimated by Hoogenveen et al. ,110 which predicts that 93% of the excess risk due to smoking is eliminated by 10 years after cessation.
Prevalence, incidence and mortality data for stroke were sourced from the 2019 Global Burden of Disease study data. 118
For stroke, we incorporated both a probability that an incident stroke would be fatal and the excess rate of death among prevalent stroke cases. The probability that an incident stroke would be fatal was estimated according to age from a study by Seminog et al. ,121 and the fatality rate among prevalent cases was estimated using Equation 2.
Smoking-related excess mortality
Smoking is a risk factor for many conditions beyond COPD, lung cancer, coronary heart disease and stroke; therefore, our model incorporates smoking-related excess mortality not already captured by the specific modelling of morbidities. The model was structured so that, in the control arm, the mortality due to COPD, lung cancer, coronary heart disease and stroke was subtracted from excess mortality, to avoid double-counting. In the TARS intervention arm, the excess mortality was taken from the control arm, meaning that overall mortality would be lowered if mortality due to the modelled morbidities was decreased. All calculations were stratified by smoking status (current smoker, abstinent smoker, other former smoker).
As part of our review of existing models (see Appendix 31), we considered a number of alternative data sources for the excess mortality due to smoking, and, in particular, how smoking cessation can reduce the rate of excess mortality. We concluded that the study by Jha et al. 122 was the most appropriate study, as it was based on comparatively recent data from a (US) nationally representative longitudinal survey, and it distinguishes between short-term and long-term quitters by using the age at quitting as a predictor for excess mortality. To improve the precision of the estimate for the risks of current smokers versus those of never smokers, we also incorporated data from Prescott et al. 123 Full details of the estimation are given in Appendix 32, Smoking-related excess mortality.
The resulting excess mortality used in the model is shown in Figure 13, demonstrating that smokers quitting in their 30s and 40s could expect to avoid most excess mortality, but that there would be less benefit obtained by smokers quitting at older ages (although at any age it was still expected to be beneficial to quit smoking). Specifically, by age 61 years, it is estimated that half of the excess mortality (in terms of the hazard ratio vs. never smokers) is ‘baked in’ and cannot be reversed by quitting.
FIGURE 13.
Excess mortality due to smoking.

We have described how we estimated the relative risks of mortality for individuals according to their smoking status, but further steps were necessary to estimate the absolute mortality rates. Mortality rates are published for the population as a whole (combining current smokers, former smokers and never smokers); it is erroneous to assume that these represent the rates for never smokers. To estimate the mortality rate among never smokers, we used the proportion of the population with each smoking status (current, former and never),124 and assumed that former smokers quit at the mid-point between age 16 years and their current age (e.g. we assumed that former smokers aged 56 years would have quit at age 36 years). We then calculated the appropriate hazard ratios using the sigmoid function described previously and used these with gender-specific period life tables125 to infer the mortality rate among never smokers.
Health state utility values
To estimate the effects of smoking status and morbidities on preference-based health-related quality of life, we used estimates from Vogl et al. ,126 who used data from the 2006 round of the Health Survey for England to estimate the effects of smoking status, age, the number of limiting conditions and a number of other covariates on EQ-5D-3L utility values. Ordinary least squares regression was conducted and reported in supplementary materials. From this, we assigned utility values as shown in Equation 3.
Costs
The key costs included in the model are the cost of delivering the TARS intervention (£239.18; see Estimating the cost of delivering the TARS intervention) and the annual costs associated with prevalent morbidities.
For lung cancer, coronary heart disease and stroke, we adopted costs from Aveyard et al. 127 and inflated them to 2018/19 prices. The resulting cost estimates were £7248 for lung cancer, £1291 for coronary heart disease and £6093 for stroke.
For COPD, we took estimates from McLean et al. 112 and inflated them to 2018/19 prices. This resulted in estimated maintenance costs (excluding exacerbations) of £799 for mild COPD, £905 for moderate COPD, £1308 for severe COPD and £1811 for very severe COPD. The estimated cost of an exacerbation not leading to hospitalisation was £133, whereas the cost of an exacerbation leading to hospitalisation was £4186. Averaging over the distribution of COPD severity and accounting for the rates of exacerbations, we calculated an annual cost of £1785 for COPD.
Discounting
Future QALYs and costs were discounted at 3.5% per year, in line with the NICE reference case. 93 Life-years are presented without discounting.
Analysis and presentation of results
The lifetime costs and QALYs for those receiving the TARS intervention and those receiving brief advice (control) were estimated from the model up to a maximum age of 100 years. From these, we calculated the ICER, as shown in Equation 6, and the INMB, as shown in Equation 7. The INMB requires the specification of λ, the willingness to pay for 1 QALY. Throughout this report, we use a value of £30,000 per QALY for λ, such that if the INMB is positive, this indicates that the TARS intervention is cost-effective at a threshold of £30,000 per QALY.
We conducted a base-case deterministic analysis in which all parameters took their ‘base-case’ values (typically maximum likelihood estimates), and in which the cohort comprised men aged 50 years. We also conducted a heterogeneity analysis, in which the age and gender of the cohort were varied to observe the relationship between age and gender and cost-effectiveness. We conducted one-way sensitivity analyses on all parameters, generally varying them between the limits of their 95% uncertainty intervals. We conducted detailed sensitivity analyses on parameters with a significant effect on cost-effectiveness. We conducted two probabilistic sensitivity analyses: in the first, the cohort was fixed as men aged 50 years (as in the base-case deterministic analysis); in the second, the joint distribution of age and gender was taken from TARS.
Results
In the base-case analysis, it was estimated that the TARS intervention would lead to marginal improvements in life expectancy and QALYs, at an increased cost of £236 (Table 29), leading to an ICER of £37,100 per QALY. The main contribution to increased costs was the cost of delivering the TARS intervention (£239).
| Trial arm | Life-years | QALYs | Cost (£) | INMB (£000s) |
|---|---|---|---|---|
| SAU | 27.69 | 14.03 | 10,575 | 410.40 |
| Intervention | 27.71 | 14.04 | 10,811 | 410.36 |
| Difference | 0.017 | 0.006 | 236 | −0.0453 |
It is important to note that, in the base-case analysis, all input values are fixed at their best estimates. This means that it is assumed that the TARS intervention leads to a 1.12% absolute increase in quit probability. The results in Table 29 are conditional on the true effectiveness of the TARS intervention being 1.12% (and all the true values of other inputs being equal to their best estimates), even though the TARS has not established the effectiveness of the TARS intervention with sufficient precision to even conclude that it is positive.
To demonstrate the effect of smoking cessation in the model, we ran the model with a relative risk of 20 for sustained abstinence to 9 months. As shown in Figure 14, this means a very significant difference in the prevalence of smoking early in the model, but the prevalence tapers quite quickly as a result of the rate of spontaneous quitting and the risk of relapse, which is initially high. This translates into an improvement in life expectancy of 0.95 years (Figure 15), and affects the prevalence of the four modelled morbidities (Figure 16).
FIGURE 14.
Prevalence of smoking when relative risk for sustained abstinence at 9 months is 20.
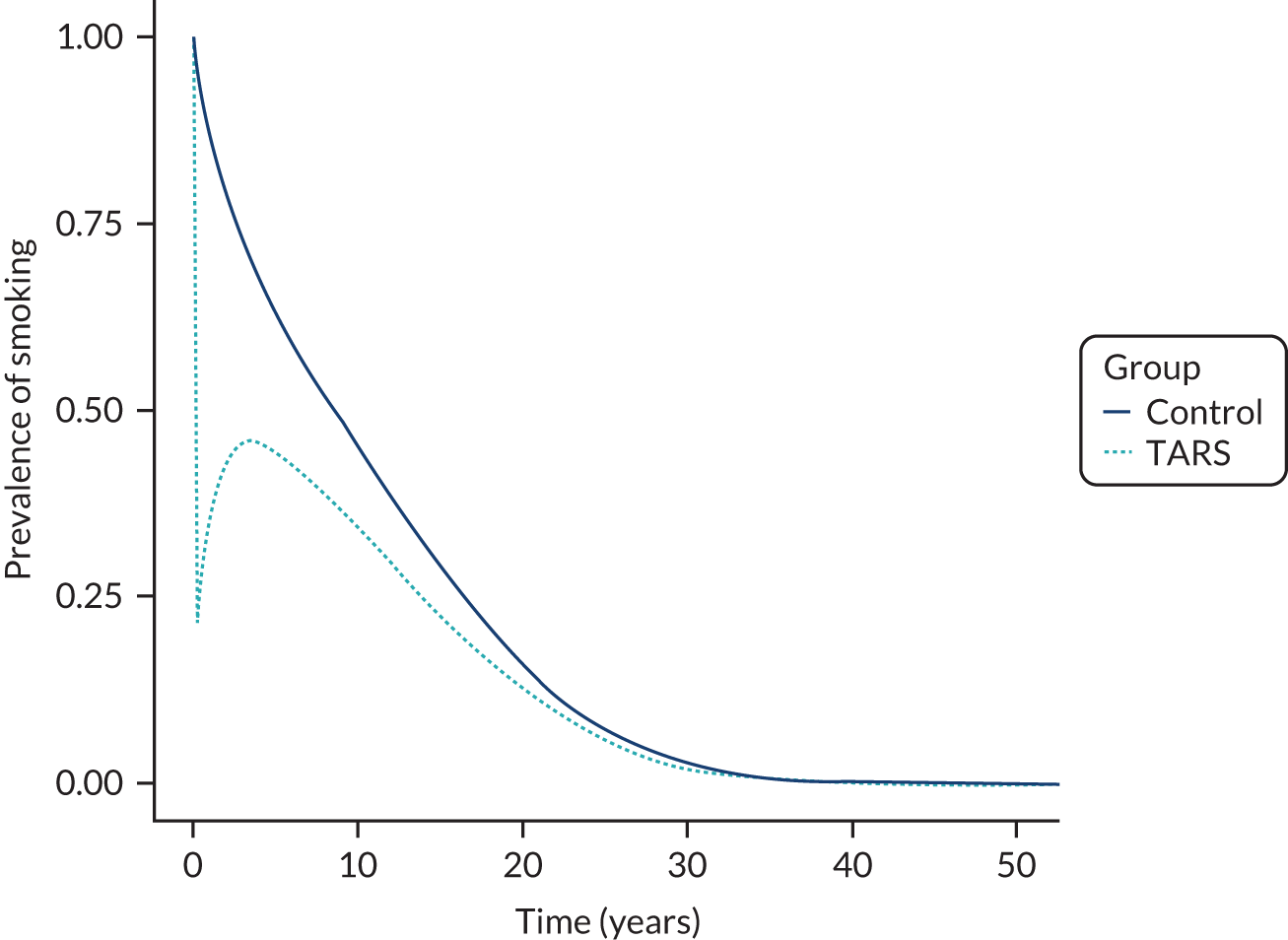
FIGURE 15.
Overall survival when relative risk for sustained abstinence at 9 months is 20.
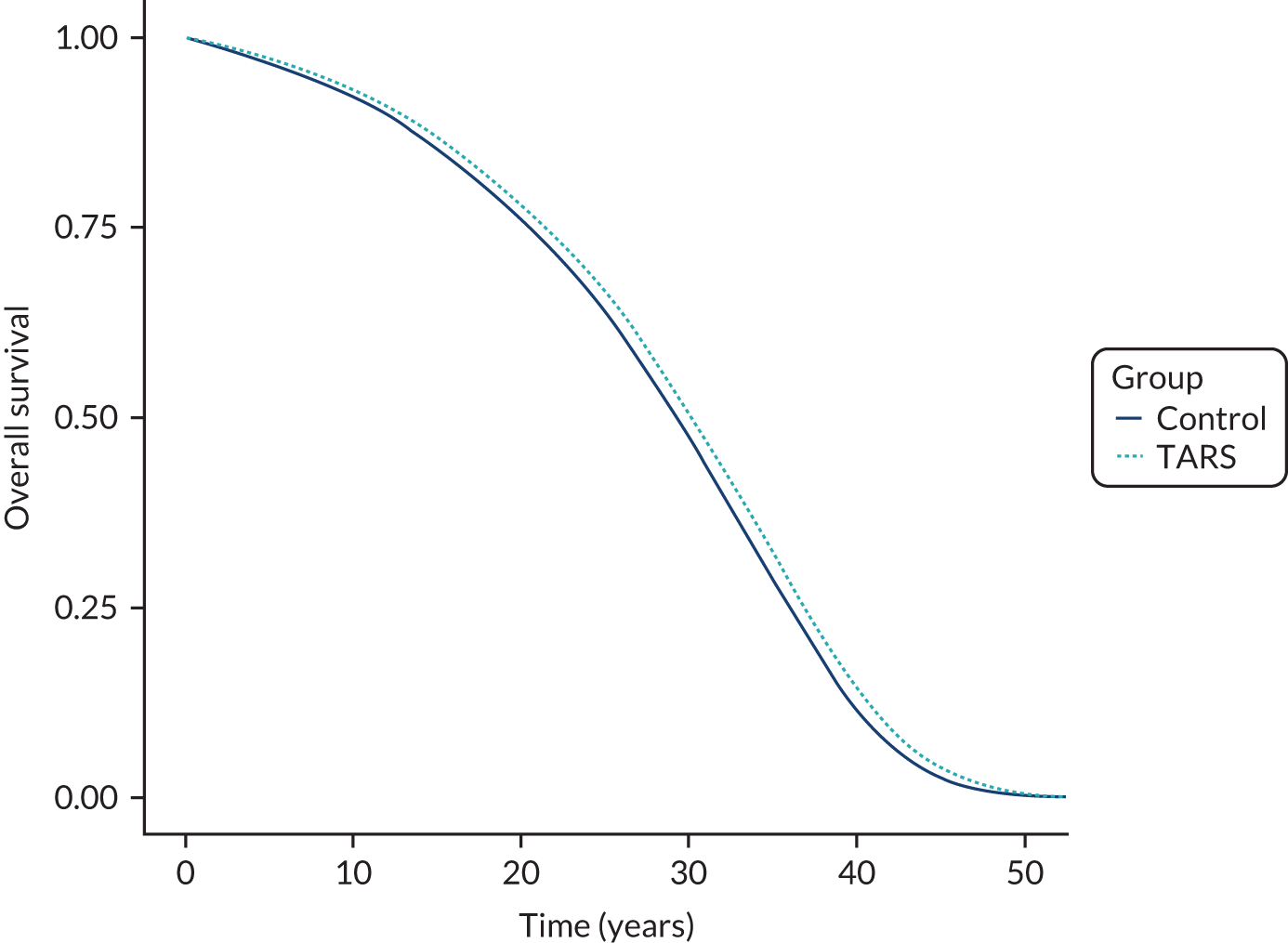
FIGURE 16.
Prevalence of modelled morbidities when relative risk of sustained abstinence at 9 months is 20. (a) Coronary heart disease; (b) COPD; (c) lung cancer; and (d) stroke.

Heterogeneity analyses
As shown in Figure 17, age has a significant effect on the cost-effectiveness of the TARS intervention, whereas gender has a smaller effect. The TARS intervention is most cost-effective for those aged 45–60 years, and least cost-effective for those younger or older than this range. This result is expected because younger smokers have more subsequent opportunities to quit (or relapse if they initially quit) before the morbidity and mortality risks of smoking increase sharply. For older smokers, there is less potential to benefit from quitting because less of the increased risk due to smoking can be reversed.
FIGURE 17.
Analysis of the effects of age and gender on the cost-effectiveness of the TARS intervention.
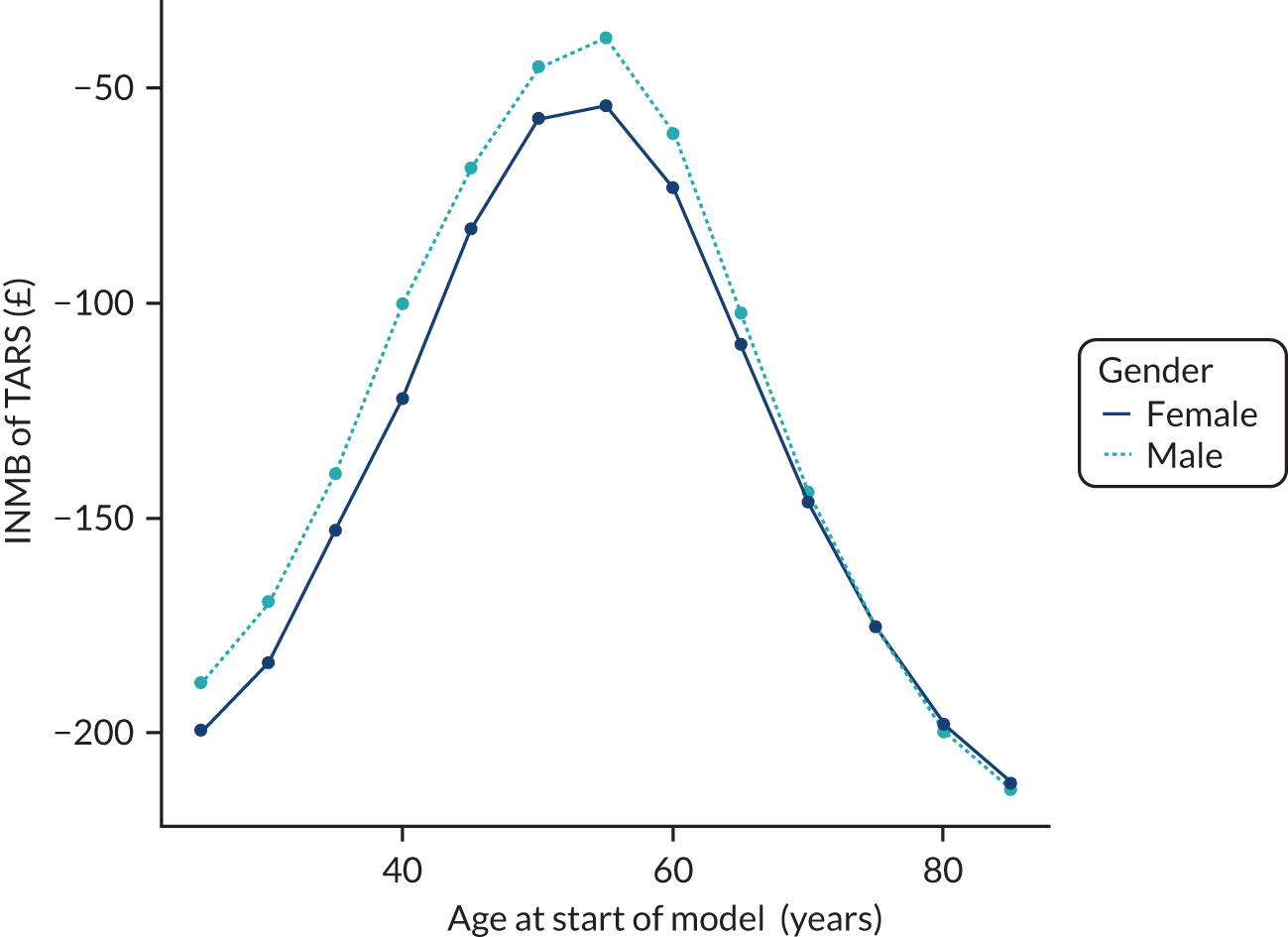
Sensitivity analyses
One-way sensitivity analyses were conducted for all parameters (generally varying between the limits of the 95% CIs). When a parameter was correlated with other parameters in the model, we recalculated their values in accordance with the parameter that was directly varied using the conditional expectation.
The results of the one-way sensitivity analyses are presented in a tornado diagram in Figure 18. The 20 parameters with the biggest impact on cost-effectiveness are shown. The risk difference for sustained abstinence (parameter name TARS_RD) has the biggest impact on cost-effectiveness, followed by the age at which people receive the TARS intervention, the discount rate for QALYs and the cost of the TARS intervention. Two parameters relating to how quickly the risk of coronary heart disease declines following smoking cessation were important (chd_rr_m_under5 and chd_rr_m_10_14). Two of the parameters for excess mortality due to smoking (xsm_beta_0 and xsm_beta_1) are also important for cost-effectiveness, but these parameters determine the quitting age at which 50% of excess mortality remains and the slope of the excess mortality curve at this point, rather than the magnitude of excess mortality (xsm_beta_2), which has less impact on cost-effectiveness over the range in which it is varied.
FIGURE 18.
Tornado diagram of one-way sensitivity analyses.
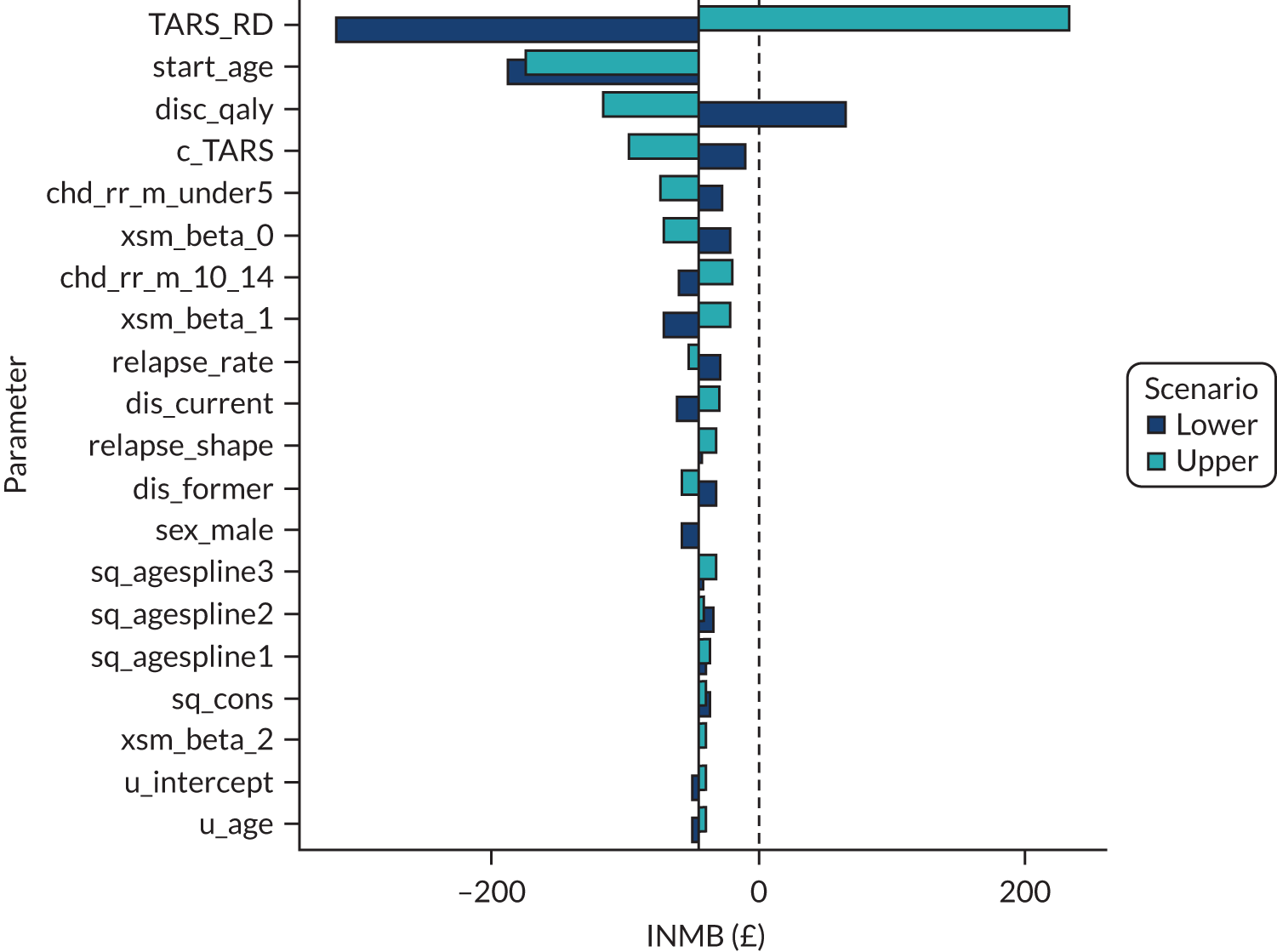
The relationship between the risk difference for prolonged abstinence and cost-effectiveness was explored further. We found an almost linear relationship between the risk difference and the INMB for the TARS intervention. For a man aged 50 years (the base case), the risk difference would need to be 1.35% for the TARS intervention to be marginally cost-effective at a cost-effectiveness threshold of £30,000 per QALY.
We estimated the slope of INMB to be £189 per 1% risk difference in the base-case analysis; however, different slopes were calculated for men and women of different ages. For women aged 30 years, the TARS intervention would need to result in a risk difference of at least 4.83% to be cost-effective at a threshold of £30,000 per QALY.
If the relative risk from TARS (2.267) is used instead of the risk difference, the model predicts that the TARS intervention will be significantly more cost-effective (see Appendix 32, Table 63), although it would not be considered cost-effective at a threshold of £20,000 per QALY for women aged 30 or 70 years, or men aged 70 years.
The effect of the rate of spontaneous quitting on cost-effectiveness was also examined, as the TARS intervention was investigated in a population of smokers not willing to quit at the point of recruitment. In this scenario, we removed the age-related components of the spontaneous quit curve and set the rate so that the proportion abstinent in the control arm at 9 months would be 0.9% (as in TARS).
In this scenario, the life expectancy and QALYs are reduced in both groups, compared with the base-case analysis, and costs are slightly increased (see Appendix 32, Table 64). The difference in QALYs between the control and TARS intervention groups expanded from 0.006 to 0.010, which led to the ICER reducing from £37,100 to £23,100 per QALY.
Probabilistic sensitivity analysis
The combined effect of uncertainty in all parameters was assessed through a probabilistic sensitivity analysis (Figure 19). The population was kept as men aged 50 years (as in the base-case analysis). The probability of the TARS intervention being cost-effective at £20,000 per QALY was estimated to be 0.158, increasing to 0.388 at a cost-effectiveness threshold of £30,000 per QALY.
FIGURE 19.
Probabilistic sensitivity analysis for men aged 50 years.

To assess the probability of cost-effectiveness in a representative population, we conducted a probabilistic sensitivity analysis in which the ages and genders were drawn from the 915 TARS participants (Figure 20). As expected, this resulted in a worsening of the cost-effectiveness of the TARS intervention.
FIGURE 20.
Probabilistic sensitivity analysis with age and gender varied according to the TARS population.
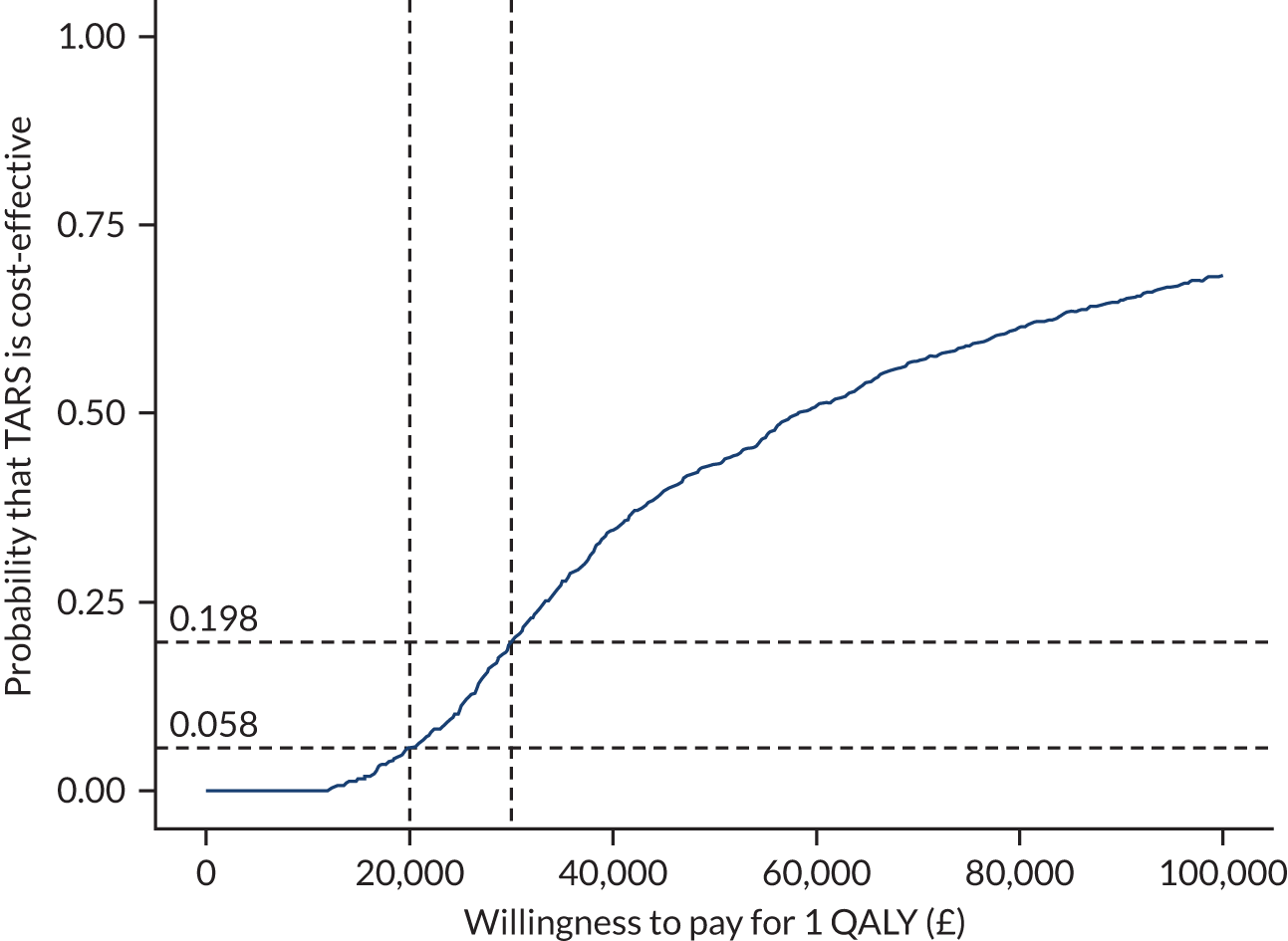
Chapter summary
Principal findings
Cost of delivering the TARS intervention
We have estimated that the cost of delivering the TARS intervention is £239.18, although there is uncertainty in this estimate, and it could reasonably be expected to cost between £200 and £300. The main source of uncertainty surrounds the amount of non-contact time for HTs related to delivering the TARS intervention, which was estimated indirectly based on investigator estimates for the maximum caseload for a HT delivering only the TARS intervention.
Trial-based economic evaluation
In a trial-based cost-effectiveness analysis, we estimated that the TARS intervention would lead to a non-statistically significant increase in costs (combining the cost of delivering the TARS intervention with the impact on NHS/PSS resource use) of £173.50 (95% CI −£353.82 to £513.77) and a non-statistically significant decrease in QALYs of 0.006 (95% CI 0.033 QALY decrease to 0.021 QALY increase). The probability that the TARS intervention is cost-effective over the 9-month trial duration was estimated to be 17% at a threshold of £20,000 per QALY, rising to 20% at a threshold of £30,000 per QALY.
The values (unit costs) of NHS/PSS resources did not substantially affect the cost-effectiveness conclusions. The specification of the regression model did have an impact on cost-effectiveness: if a linear model for QALYs is used (which had the lowest AIC), then incremental QALYs are expected to be small and positive (0.007), rather than small and negative (−0.006). The inclusion of baseline EQ-5D-5L scores also had a significant effect on expected incremental QALYs, but this was suspected to be due to patterns of missing data rather than an imbalance at baseline.
There was a significant number of missing data in the trial-based analysis, so a sensitivity analysis with multiple imputation was conducted. In this sensitivity analysis, the TARS intervention was estimated to lead to a non-statistically significant saving of £38.02 (95% CI £412.51 saving to £336.47 additional cost) and a non-statistically significant increase in QALYs of 0.009 (95% CI −0.014 to 0.033). The probability that the TARS intervention is cost-effective in this sensitivity analysis was estimated to be 75% at a threshold of £20,000 per QALY, rising to 77% at a threshold of £30,000 per QALY.
Model-based economic evaluation
In a model-based economic evaluation, we estimated the effect of a 1.1% absolute difference in the probability of a sustained quit to 9 months on lifetime costs and QALYs through the effects of smoking and smoking cessation on COPD, coronary heart disease, stroke and lung cancer, as well as on health-related quality of life and excess mortality risk.
We estimated that the TARS intervention would lead to a small gain in lifetime QALYs and a small reduction in lifetime costs as a result of smoking-related diseases, but that these would not be enough to make the TARS intervention cost-effective at a threshold of £20,000–30,000 per QALY, with an ICER of £37,100 per QALY. We predicted that the TARS intervention would be most cost-effective in those aged 50–55 years (although still not being cost-effective at a threshold of £20,000–30,000 per QALY), and less cost-effective in those younger or older than this. This is a function of the pattern of spontaneous quitting according to age, as well as the age at which the risks of smoking increase and when quitting needs to take place to substantially reduce the risks of smoking. It is not a function of the expected effectiveness of the TARS intervention according to age, as TARS has not established that the TARS intervention is clinically effective.
We found that there was substantial uncertainty in the cost-effectiveness of the TARS intervention owing to imprecision in the estimate of the absolute difference in quit rates, with this being the parameter to which the cost-effectiveness results were most sensitive. After establishing that the relationship between the absolute difference in quit rates and the INMB (used as a measure of cost-effectiveness) was almost exactly linear, we estimated the required absolute differences in quit rates that would be required for the TARS intervention to be cost-effective for men and women of different ages.
We also found that assumptions around the rate of spontaneous quitting had a strong impact on cost-effectiveness. When the rate of spontaneous quitting was reduced to match the quit rate in the control arm of TARS (and maintained at this low level when projected over the lifetimes of the recipients), this improved the cost-effectiveness of the TARS intervention so that it would be cost-effective at a threshold of £30,000 per QALY, but still not cost-effective at a threshold of £20,000 per QALY (ICER £23,100 per QALY).
Strengths and limitations
The principal limitation of the cost-effectiveness analyses presented is that TARS has not demonstrated that the TARS intervention is superior to control (no statistically significant difference was demonstrated in the primary outcome). Power calculations for the trial assumed a higher rate of quitting than was observed in the trial. This has meant that the relative effectiveness of the TARS intervention versus control is highly uncertain, and there is a non-trivial probability that the TARS intervention is not effective, and therefore cannot be cost-effective.
The measure of health benefit in the cost-effectiveness analyses was QALYs, which incorporate the effects of interventions on quality of life and survival, and which permit the use of established cost-effectiveness thresholds of £20,000–30,000 per QALY. For the trial-based analysis, this made it possible to measure the effect of the TARS intervention on health-related quality of life, rather than the narrow measure of sustained abstinence from smoking. It therefore had the potential to detect benefits from exercise and from reductions in smoking among those not quitting. The assessment of QALYs in the trial-based analysis was somewhat hindered by missing data: only 52% completed the EQ-5D-5L instrument at baseline, 3 months and 9 months, and a further 7% missed only the 3-month measurement (QALYs were calculated for these participants, but 87% of them also had missing costs, so they were not included in the complete-case analysis). In the complete-case analysis, it was estimated that the TARS intervention would result in a small and non-statistically significant loss of QALYs, whereas, in the multiple imputation analysis, it was estimated that the TARS intervention would result in a small and non-statistically significant gain in QALYs. The overall conclusion though is that the effect on QALYs was small and non-statistically significant.
We have avoided a number of inappropriate assumptions that are prevalent in the literature of cost-effectiveness modelling for smoking cessation, chiefly around future smoking behaviour. Many models, including those based on the Benefits of Smoking Cessation on Outcomes (BENESCO) model and used to demonstrate the cost-effectiveness of the pharmacological therapy varenicline, have not included future quit attempts (spontaneous quitting), even though population data and surveys show that smokers do make further quit attempts if initial attempts are unsuccessful and that most smokers quit during their lifetime. Failure to model spontaneous quitting leads to bias in favour of cessation interventions in cost-effectiveness analyses. We have not assumed that the benefits of smoking cessation apply immediately, which has been demonstrated to bias estimates of QALY gains. 110
Our modelling has incorporated the costs of only four morbidities (coronary heart disease, COPD, lung cancer and stroke), and has not attempted to otherwise estimate the effect of smoking or smoking cessation on lifetime health-care resource use.
Our modelling has largely relied on observational data comparing current with former smokers for the risks of morbidity and mortality. Even though, in most instances, we sourced data from studies that controlled for many potential confounders, it is still possible that confounding remains and that the estimated effects of smoking cessation are not the causal effects of smoking cessation.
A significant area of uncertainty relates to how effective the TARS intervention should be assumed to be in leading to sustained abstinence from smoking. In TARS, the rate of quitting was substantially lower than would be expected from a general population of smokers (perhaps, in part, because any smokers who were ready to quit smoking were excluded); as a result, low event counts were observed (leading to sampling uncertainty) and there was uncertainty about which measure of effectiveness (absolute or relative) was more appropriate to include in the modelling.
Finally, our modelling approach estimated the effect of the intervention only as mediated through sustained abstinence from smoking (the trial primary outcome). We did not include potential benefits from the TARS intervention such as increasing PA (which could improve long-term health outcomes if sustained) or reduction in smoking intensity.
Relation to existing work
Wu et al. 128 reported cost-effectiveness analyses of computer-tailored smoking cessation advice in primary care. The absolute difference in prolonged abstinence (3-month prolonged abstinence at 6-month follow-up) between the treatment groups was approximately 0.5%, and this was projected to an expected QALY gain of 0.0008 QALYs. In this study, an absolute difference in prolonged abstinence (6-month prolonged abstinence at 9-month follow-up) of 1.1% was projected to an expected QALY gain of 0.006 QALYs. This suggests that our model predicts roughly four times as many QALY gains per quitter: this can be explained by the higher bar for quitting (6 months of prolonged abstinence vs. 3 months) and the inclusion of smoking-related morbidities (which result in reductions in quality of life). Wu et al. 128 conclude that their intervention is cost-effective only on the basis of very small incremental costs (£9).
Other studies have generally not considered absolute differences in quit rates as low as 1.1%.
Stapleton and West129 have presented a look-up table for estimating the cost-effectiveness of smoking cessation interventions. According to table 2 of Stapleton and West,129 a 1.0% incremental intervention effect should result in 0.0077 to 0.0103 life-years gained (discounted at 3.5%), depending on whether final follow-up is at 6 or 12 months. This is reassuringly close to the 0.008 QALYs predicted in our model. Incremental QALYs could be lower than life-years gained because population norms for QALY weights are generally < 1; however, incremental QALYs could also be higher than life-years gained because avoiding morbidities can improve quality of life. According to table 3 of Stapleton and West,129 a 1.0% incremental intervention effect and an intervention cost of £250 should lead to an ICER of £24,317–32,422 per life-year gained; however, this does not appear to adjust the incremental cost in the ICER calculation depending on the intervention cost, that is there is no attempt to account for cost savings resulting from morbidity avoided or additional costs resulting from extended survival with morbidities. Finally, we note that Stapleton and West129 estimate that those aged < 35 or > 55 years gain fewer life-years than those aged 35–54 years, a finding similar to the findings of our analysis in which those aged 50–55 years gain the most QALYs from the intervention.
Chapter 6 Discussion
Summary of findings
Of the sample recruited, 55% identified as female, the average age was 50 years and high levels of unemployment were reported. Sixty per cent of participants lived in one of the four most deprived UK deciles. The sample participants were moderately heavy smokers and fairly active, roughly comparable to the EARS pilot trial.
TARS was powered to detect a difference of 6% (i.e. 5% vs. 11%) in the proportion of smokers who achieved CO-verified 6-month floating prolonged abstinence, with a corresponding OR for this target effect size of 2.3, based on the EARS pilot trial and existing literature. Although the TARS primary analysis showed that the estimated odds of achieving the primary outcome in the intervention group were in line with the target OR (fully adjusted estimated OR of 2.30), the between-group difference was not statistically significant (95% CI 0.70 to 7.56), owing to the much lower than anticipated proportion of participants in both groups achieving CO-verified abstinence. We recorded 0.9% (n = 4) of participants in the SAU group with CO-verified 6-month floating prolonged abstinence between 3 and 9 months, compared with 2.0% (n = 9) of participants in the intervention group. When we also included participants who had not been CO-verified abstinent at 3 months but were at both 9 and 15 months, we found that the SAU and intervention arms had 2.2% (n = 10) and 3.1% (n = 14) CO-verified abstinence, respectively, which was also not significantly different.
We also considered the proportion of participants who achieved CO-verified 12-month floating prolonged abstinence. The estimated adjusted OR was 6.33, but with 0.2% (n = 1) and 1.3% (n = 6) in the SAU and intervention groups, respectively, this between-group difference also proved statistically not significant (95% CI 0.76 to 53.10; p = 0.089), which is unsurprising given the small numbers of participants involved in this analysis.
Secondary outcomes (smoking)
Although the TARS was powered to primarily detect differences in CO-verified 6-month floating prolonged abstinence, we also investigated intervention effects on a number of secondary smoking outcomes, which have been reported previously in studies focused on harm and smoking reduction involving behavioural support for smokers who do not want to quit. Given the focus of the TARS intervention on multiple behaviour changes, we also examined the intervention effects on PA.
The intervention had weak effects on both self-reported 7-day point prevalence and CO-verified abstinence at 3 months, but not at 9 or 15 months, compared with SAU. Between baseline and 3 months, the intervention group reduced the number of cigarettes smoked by 5.6, compared with the control group. There were stronger, statistically significant, intervention effects, compared with SAU, on the proportion of participants who reduced the number of cigarettes smoked per day by at least 50%, between baseline and 3 months (18.9% vs. 10.5%) and between baseline and 9 months (14.4% vs. 10%). Assuming that those lost to follow-up did not make a quit attempt, the proportion of participants who reported making a quit attempt of at least 24 hours was fairly similar in the intervention and SAU groups between both baseline and 3 months (11.8% vs. 8.1%) and 3 and 9 months (16.9% vs. 15.1%).
Moderators of intervention effects
When possible (i.e. with sufficient available data), modifiers of the intervention effect on smoking outcomes were explored. The only significant interaction (p < 0.01) involved the IMD (comparing effects for those in the lowest and those in the highest deciles), with the intervention effect on number of cigarettes smoked per day at 3 months being present for those in the most, but not in the least, deprived neighbourhoods. At 9 months, the intervention was still effective for those in the most deprived decile, but SAU became more effective in the least deprived neighbourhood. The TARS intervention, including during the EARS pilot trial, was designed to support smokers from more disadvantaged communities, perhaps who find changing smoking habits more difficult. The focus of the client-centred intervention on increasing a sense of competence, control and connectedness, while building trust and demonstrating empathy, were all valued aspects, based on interviews with participants and HTs in our process evaluation. We have not yet explored any moderator effects on our process survey items, but, if identified, this would link well to the logic model in which the intervention may be most useful for those who need most support to change without the pressure of more directive approaches from health professionals. Overall, however, the intervention effects on various outcomes were robust across different potential moderators.
Comparisons with previous studies
Only a few studies10–12,14,23 have considered the effects on quitting and smoking abstinence of behavioural motivational support for smoking reduction among smokers who are not ready to quit. A summary table of four randomised trials involving behavioural support to reduce smoking for smokers not wanting to quit is shown in Appendix 33. No other study has used such a rigorous primary outcome (prolonged, CO-verified abstinence) as TARS, but it is worth comparing the present findings and the extent to which the population, intervention, control condition and outcomes may have influenced the comparative effects.
Population
TARS was the first definitive trial in the UK, following the EARS pilot trial, to examine the effects of motivational support on smoking reduction for smokers not wanting to quit but smoking at least 10 cigarettes per day, after four other trials in the USA. All the studies had similar trial participants, based on average age (range 40–55 years), gender (mainly female) and ethnicity (mainly white, with the exception of the Catley et al. 14 trial, which had predominantly African American participants), with participants typically being moderate smokers (≈20 cigarettes per day) at baseline. Only one study (Glasgow et al. 11) involved patients with chronic health conditions who may have been restricted in their level of daily PA.
Intervention
The components and theoretical basis for the TARS intervention is described in Chapters 1 and 2, and were drawn from a review of related studies, extensive process evaluation in the EARS pilot trial and PPI throughout intervention development. Not surprisingly, there were considerable similarities to aspects of the US trials, in terms of targeted behavioural change processes, but only one previous trial (Catley et al. 14) involved face-to-face support, with the other study interventions delivered by telephone. All interventions involved three or four sessions over periods ranging from 4 weeks to 26 weeks, and focused on facilitating the setting of reduction targets, except the Catley et al. 14 trial, in which the MI intervention adopted a more client-focused approach, similar to the TARS intervention. The TARS intervention effects on CO-verified point prevalence abstinence rates at 3 months were marginally not significant and not significant at 9 months, and the Catley et al. 14 trial also showed no significant MI effect (at 6 months). In the Catley et al. 14 three-arm trial, the health education intervention was shown to be more effective than the control group, raising questions about the balance between more and less directive approaches in this context.
It is challenging to compare intervention engagement across the studies considered above with a focus on smoking reduction because the TARS sessions were 50% face to face and 50% by telephone, and not as structured or of the same duration. However, the TARS intervention may have had slightly more engagement, in terms of overall frequency and duration of sessions, compared with other interventions, with a mean of 4.8 (SD 3.4) sessions, lasting, on average, 33 minutes, and 76% of intervention participants having two or more sessions. Glasgow et al. 11 reported that 79% of intervention participants received at least three of the four phone calls, while Klemperer et al. 12 reported that 84% and 77% had completed all three motivational calls and smoking reduction intervention calls (lasting up to 15 minutes each). Catley et al. 14 reported that 98% did at least two of the four MI or health education sessions.
Only the Catley et al. 14 trial involved a detailed attempt to assess intervention delivery fidelity, using the MI Treatment Integrity Code,130 to ensure that the MI and health education interventions did differ, as they were delivered by the same person. It is, therefore, not clear if all intervention components were delivered as intended in the other studies, but, in TARS, the HTs showed an acceptable level of intervention delivery fidelity, as described in Chapter 4. Subjectively, delivery fidelity across the 11 components assessed by independent coders of a sample of intervention sessions (delivered by all eight HTs) was better in the TARS than in the EARS pilot trial,33 with the exception of ‘integration of concepts’ and ‘managing social influence on PA’. Qualitative interviews with intervention participants and HTs also highlighted the challenges faced in supporting participants to increase PA as a way of managing smoking for some people, but not everyone; we will return to discuss intervention effects on PA in Effects on physical activity.
Unlike in the EARS pilot trial, participants in TARS were supported to use LNCPs if they wanted. At baseline, 14% of participants overall reported using any LNCP, increasing to 40.8% and 39.0% (or 26.0% and 22.8% if we assume that those who were missing at follow-up were not using LNCPs) at 3 and 9 months, respectively, with no differences between the groups. Because of the small numbers of participants who achieved the primary outcome and lack of a significant intervention effect, we did not explore if change in LNCP use mediated intervention effects on the primary outcome, as originally planned. Given similar LNCP use across the allocated groups, it seems unlikely that differences in any smoking outcome were mediated by LNCP use.
Control group changes in smoking outcomes
The failure of TARS to show a statistically significant effect on the primary outcome of CO-verified 6-month (and 12-month) floating prolonged abstinence was possibly due to fewer participants than expected stopping smoking. In particular, the sample size calculation assumed that 5% of the SAU group would achieve the primary outcome. Although the sample size scenarios considered at trial design went as low as 3% for the SAU group, the observed 0.9% was much lower than anticipated (see Table 2).
Although there are no comparative data from previous studies for the primary outcome, a number of comparisons can be made for secondary outcomes for control/SAU group participants. Catley et al. 14 reported that, among control participants, 0% had 6-month CO-verified abstinence, compared with 1.8% and 4.7% with CO-verified 3- and 9-month point prevalence abstinence in TARS, respectively. Carpenter et al. 10 reported that 4% of control participants self-reported 7-day point prevalence abstinence at 6 months, compared with 2.9% (at 3 months) and 8.0% (at 9 months) in TARS. Klemperer et al. 12 reported control rates for self-reported 7-day point prevalence abstinence of 5% and 4% at 6 and 12 months, respectively.
Similarly, Carpenter et al. 10 reported that 16% of control participants had reported making a 24-hour quit attempt at 6 months, whereas Klemperer et al. 12 reported that 34% of control participants made a quit attempt lasting at least 24 hours up to the 6-month follow-up. In Catley et al. ,14 31% and 43% of control participants had made a quit attempt (in the previous 30 days) by the 3-month and 6-month follow-ups, but this was the only trial that included participants who smoked as few as one cigarette per day at the time of recruitment (compared with at least 10 daily cigarettes in the other studies and in TARS). In comparison, in TARS, 14% of control participants reported having made a quit attempt (of ≥ 24 hours) between baseline and 3 months, and 25% reported having made a quit attempt between 3 and 9 months.
Compared with the study by Glasgow et al. ,11 in which 19% of control participants achieved a ≥ 50% reduction in the number of cigarettes smoked per day between baseline and 12-month follow-up, TARS found that 10.5% of control participants did so at 3 and 9 months, respectively.
The EARS pilot trial reported that 4% of control participants had expired CO-verified abstinence at least 4 weeks after quitting; 6% made a quit attempt; 4% achieved self-reported point prevalence abstinence at 16 weeks; and 16% and 20% achieved at least a 50% reduction in the number of daily cigarettes smoked by weeks 8 and 16, respectively.
In summary, TARS reports less change in smoking outcomes in the control/SAU group than in other studies on roughly comparable measures.
Outcomes
Although the outcomes in other studies on behavioural support for smoking reduction were not as rigorous as the primary outcome used in TARS, they may help us to understand whether or not our expectations for differences between the control and intervention groups in the TARS were realistic, especially as there is some agreement between measures of prolonged abstinence and point prevalence abstinence. 131
In terms of quit attempts, TARS and trials by Catley et al. 14 and Klemperer et al. 12 have shown that their interventions did not induce more quit attempts than SAU. Further examination of the behavioural support provided in the Carpenter et al. 10 study suggests that there may have been a greater emphasis on quitting once participants had achieved a 50% reduction in the number of cigarettes smoked, than perhaps in the interventions in TARS and other studies. In the EARS pilot trial, we reported that 22% made a quit attempt, compared with 6% in the control group, within 4–8 weeks of follow-up.
In terms of the number of cigarettes smoked daily, TARS did report an intervention effect at 3 months, but not at 9 months, whereas Glasgow et al. 11 reported no effect at 3 and 12 months, for no obvious reason.
In terms of smoking reduction, although TARS showed a significant intervention effect on the proportion who had reduced the number of cigarettes smoked daily by ≥ 50% at both 3 months (18.9% vs. 10.5% in the intervention and control groups, respectively) and 9 months (14.4% vs. 10.0% in the intervention and control groups, respectively), Glasgow et al. reported an intervention effect at 3 months (11% vs. 5.8% in the intervention and control groups, respectively), but not at 12 months (14% vs. 14% in the intervention and control groups, respectively). In the EARS pilot trial, we reported that more intervention than control participants were estimated to have reduced their smoking by ≥ 50% (39% vs. 20%) by 16 weeks.
In terms of self-reported 7-day point prevalence and CO-verified abstinence rates, although TARS supported a weak intervention effect at 3 months (5.5% vs. 2.9% for self-reported 7-day point prevalence abstinence and 3.7% vs. 1.8% for CO-verified abstinence in the intervention and control groups, respectively), other studies have shown mixed results. Carpenter et al. ,10 Catley et al. 14 and Klemperer et al. 12 all reported an effect of the motivational intervention on the proportion smoking at 6 months, but Glasgow et al. 11 did not at either 6 or 12 months. In the Klemperer et al. 12 study, the motivational intervention effects were marginally more apparent at 12 months (than at 6 months) on self-reported abstinence, but this was probably a result of fewer control participants reporting abstinence later in the study. In the EARS pilot trial, 14% versus 4% at 4 weeks, and 10% versus 4% at 16 weeks, in the intervention and control groups, respectively, had CO-verified point prevalence abstinence, which was clearly more encouraging than in the present trial.
Trends in smoking
Across the different smoking outcomes, there have been too few studies to identify support for the idea that smokers are becoming more resistant to quit as population prevalence of smoking has reduced over the previous 20 years (i.e. hardening). 132
Effects on physical activity
TARS is unique in that it had an additional focus on supporting changes in PA to complement behavioural support for smoking reduction. Clearly, the TARS intervention, with a multiple behaviour change focus, has not had adverse effects on smoking outcomes for smokers who are not motivated to quit. Although the EARS pilot trial,30 with 99 participants, showed no intervention effect on self-reported or accelerometer-recorded MVPA in the exploratory analysis, it appeared that a greater focus in training the HTs to support changes in PA may have led to the significant intervention effect on self-reported minutes of MVPA at 3 months; this equated to a weekly average of 79 minutes more than the control group. However, these effects were not evident at 9 months (mean difference 22 minutes), and it would appear that additional support for participants would be needed for longer-term change in PA.
Mediation of physical activity on smoking
A key question for TARS was if changes in MVPA would mediate intervention effects on smoking outcomes. As the results in Chapter 4 show, MVPA minutes did not significantly mediate intervention effects on the number of cigarettes smoked daily at 3 months or on achievement of a ≥ 50% reduction in the number of cigarettes smoked daily between baseline and 3 months or baseline and 9 months. There have been 24 randomised trials, of varying quality, on the effects of exercise interventions on smoking cessation22 for smokers wanting to quit, with only very limited certainty that exercise can be effective. TARS was not designed to test the separate effects of PA on smoking outcomes, but the analysis does not support the hypothesis that increasing PA mediated the effects on smoking; however, the very modest-sized effects overall limit our ability to test this. Subjective evidence from interviews with HTs and participants did indicate that some participants were finding various effective ways for using PA to help changes in smoking.
Intervention effects on smoking and physical activity process survey measures
The logic model informed the design of the HT-led intervention and the process survey measures we collected. It was encouraging to see that the intervention had positive effects on 10 out of 11 smoking process survey items, and on five out of seven PA process survey items, including one item that suggested that intervention participants became more positive about PA for controlling smoking. This is contrary to the mediational analysis discussed previously, in which PA did not mediate smoking outcomes, and contrary to the rating of HT sessions in which the integration of behaviours appeared to be less easy to deliver than some of the other competencies. The only smoking-related process measure that was not influenced by the intervention was the importance attached to quitting, and the dilemma for the HTs in supporting reduction and not pushing cessation was evident in qualitative work.
Process measures as mediators of intervention effects on smoking and physical activity
The increase between baseline and 3 months in smoking beliefs about the importance of smoking reduction; confidence to reduce; and use of action planning, coping planning and self-monitoring mediated changes in intervention effects on the number of cigarettes smoked daily up to 3 months. Changes in confidence to reduce and to quit, action planning, coping planning, self-monitoring and thoughts about quitting also mediated intervention effects on whether or not participants reduced their smoking by ≥ 50% up to 3 months, but, beyond 3 months, the effect of these process measures weakened, with only confidence in reducing and self-monitoring being marginally significant. Changes in PA beliefs, up to 3 months, regarding confidence to be active and self-monitoring PA mediated intervention effects on MVPA minutes at 3 months. Klemperer et al. 13 also explored possible mediators of changes in smoking outcomes and found that a 1-unit (on a scale of 0–5, from low to high) change in self-efficacy to quit from baseline to 4 weeks mediated intervention effects on whether or not a quit attempt (lasting ≥ 24 hours) took place over 6 months, but had no effect on the self-reported point prevalence abstinence rate at 6 months.
Health economics
The TARS intervention has been estimated to cost £239.18 per participant, on average. The majority of this cost is HT time, including contact time (£92.84), travel time (£53.02) and non-contact time (£71.69). The assumptions required to estimate non-contact time mean that it is plausible that the cost could vary between £200 and £300.
The review of cost-effectiveness analyses of targeted smoking cessation interventions (i.e. not using mass marketing or changes to taxation) found a number of studies evaluating pharmacological aids, counselling, electronic aids and complex interventions. Two published studies incorporated exercise. Taylor et al. 23 reported the results of the EARS trial, which was a pilot for this trial. The cost of the EARS trial intervention was estimated to be £192, with the principal reason for the cost difference being improved estimates of HT time associated with the intervention. Leung et al. 108 reported the incremental costs of an exercise–counselling intervention as NZ$428 [£228 when converted from 2012 New Zealand Organisation for Economic Co-operation and Development (OECD) purchasing power parity (PPP) to 2018 UK OECD PPP133]. Glasgow et al. 11 reported the costs of a counselling programme for smoking reduction as US$652 (excluding recruitment costs, approximately £526 when converted to 2018 UK OECD PPP); the differences in costs between the study by Glasgow et al. 134 and TARS are likely to be explained by the presence of additional components (tailored newsletters) and more intensive training and supervision (including supplementary training and live supervision).
The NHS and PSS resource use measured in TARS suggests that those receiving the TARS intervention may have incurred somewhat lower costs than those in the control arm, but after accounting for the cost of the intervention, total costs are expected to be higher for those receiving the TARS intervention by £173.50 over 9 months, starting from treatment allocation. This difference was not statistically significant.
Preference-based health-related quality of life was measured, which allowed QALYs to be estimated for trial participants. There was no statistically significant difference in QALYs between the groups.
A significant number of data for health economic analysis were missing. A sensitivity analysis using multiple imputation found that the TARS intervention resulted in net savings of £38.02 and a gain of 0.009 QALYs, but neither of these findings were statistically significant.
A decision-analytic model was constructed to estimate the lifetime costs and health outcomes based on the trial outcome of smoking cessation. The model incorporated the effects of smoking cessation on COPD, coronary heart disease, stroke and lung cancer; on excess mortality not due to those four conditions; and on quality of life.
It was estimated that the 1.1% absolute difference in the probability of prolonged abstinence to 9 months would translate into a small gain in lifetime QALYs, and small savings in lifetime costs from smoking-related diseases, but not enough to offset the cost of the intervention and lead to TARS being considered cost-effective (at a threshold of £20,000–30,000 per QALY).
The absolute difference in quit rates between the model arms and the assumptions around the rate of spontaneous quitting had a strong effect on cost-effectiveness. In a sensitivity analysis (in which spontaneous quitting was reduced to match the quit rate in the control arm of the TARS and maintained at this low level over the lifetime of the cohort), the TARS intervention was estimated to be cost-effective at a threshold of £30,000 per QALY, but not at £20,000 per QALY.
Strengths
TARS has a number of important strengths including the following: it involved a very robust primary outcome, a detailed mixed-methods process evaluation and a health economic analysis; rigorous intervention development with PPI input; manualisation of the intervention and practitioner training; good intervention engagement and fidelity (content, training, delivery and signs of enactment); and a good proportion of the sample was from socially deprived neighbourhoods. The logic model seemed to be working based on process measures (and interviews/session recordings). This is the first UK definitive trial of behavioural support for smokers not wanting to quit and adds to the research on multiple behaviour change interventions, especially involving PA and smoking. Given the prevalence of clusters of poor health behaviours, linked to low socioeconomic status, TARS is unique in its contribution to the understanding of intervention engagement and effects in this population.
Limitations
Although we identified an adjusted OR close to our pre-planned rates for the primary outcome, very low abstinence rates for the whole sample made it impossible to be confident in this effect in favour of the intervention. However, the low absolute quit rates achieved mean that, even if we had detected this effect, the TARS intervention does not represent a worthwhile addition to tobacco control interventions because it was not cost-effective.
We collected data mostly during face-to-face meetings with participants at baseline, and then relied on remote data capture at the follow-up assessments; it is unknown what effects this had on the results. To reduce participant burden in recruitment and completion of baseline data, we collected accelerometer data from only a sample of participants at 3 months, which made the assessment of intervention effects on PA less robust.
To detect intervention effects on secondary outcomes and to detect moderators and mediators only exploratory analyses were conducted. Although we calculated Cronbach’s alpha for the composite scales involving process survey items drawn from previous measures used with our randomised trials, we did not examine the factor structure of the scales.
A considerable proportion of participants did not return follow-up assessments; in this case, we applied the Russell Standard and the assumption that those with missing data were still smoking. We conducted a sensitivity analysis to check this assumption, but it is less clear how missing data influence other secondary outcomes.
The small number of participants achieving the primary outcome precluded many of the planned sensitivity analyses, with only the prespecified ‘best-case scenario’ analysis possible, which did not change the overall conclusion. Similarly, the effects of COVID-19 restrictions, which forced us to switch to saliva cotinine verification of self-reported abstinence, were considered descriptively, but the numbers tested using saliva cotinine-verified abstinence were too small to conduct any worthwhile further analysis.
In terms of the process evaluation, one limitation of note was that interviews with intervention participants took place after receipt of the intervention, but before the final follow-up; this could have influenced some of the trial outcomes. Moreover, males were slightly over-represented in the interviews as a result of the sampling methods, although it is unclear how this may have affected the analysis and reflections.
Implications for health care
It is clear from the perspective of the primary outcome (CO-verified 6- and 12- month floating prolonged abstinence) and associated health economic analysis that there was no evidence that the intervention was effective or cost-effective.
For smokers not wanting to quit, the HT intervention can change both smoking behaviours and PA for at least up to 3 months, with some evidence that the multiple behaviour change intervention can increase the proportion who report reducing their smoking by at least 50%.
TARS involved a detailed analysis of what the intervention involved, how the HTs were trained and delivered the intervention, and what effects this had on beliefs about smoking, PA and multiple behaviours for > 450 participants. Overall, there was good evidence of acceptability to participants and feasibility of scaling up the delivery of this intervention through standardised training and supervision.
The limited long-term intervention effect on CO-verified abstinence and qualitative work suggest that, for some participants, booster sessions to maintain harm reduction and smoking abstinence would have been valued. Delivering motivational support to reduce smoking, while at the same time encouraging quitting and abstinence, was highlighted as a challenge.
The data suggested that, although the sample participants were initially physically active (possibly from a greater proportion in manual occupations, and lower levels of car ownership, than the general population), the HT intervention still managed to increase short-term self-reported MVPA. However, as far as we can tell from the present preliminary analyses, intervention effects on MVPA do not appear to then modify changes in smoking behaviour, although some participant interviews did provide support that this was happening. The qualitative data provide some rich detail on how some, but clearly not all, participants used PA to support changes in smoking behaviour, both acutely and chronically.
There are a variety of UK community-based practitioners with overlapping roles with HTs, such as link workers, health coaches and those working in social prescribing initiatives, who may all be faced with providing support for multiple behaviour change, especially among disadvantaged groups. For smokers not wanting to immediately quit, it is possible to support short-term changes in PA and smoking, but we found no evidence that these changes result in sustained abstinence in smoking and longer-term PA, or that this intervention would be cost-effective. The findings should be integrated into future reports that follow The King’s Fund work on multiple health behaviour change. 135
The model-based cost-effectiveness analysis did not incorporate the effects of sustained reduction in smoking intensity or sustained increase in PA on long-term costs and health benefits. These effects would probably be in favour of the TARS intervention, as reduced smoking intensity and increased PA would lower the risks of smoking-related morbidity and mortality (reducing costs and improving quality-adjusted life expectancy). If these effects can be realised and sustained in real-life practice, then the value for money of the TARS intervention will be improved.
Future research implications
In seeking to recruit participants to the trial, we clearly advertised that the trial was not about quitting, and the trial was not overtly linked to NHS Stop Smoking Services. Further research is needed to explore the role of multiple behaviour change support (building on the present HT intervention) for smokers who want to reduce prior to quitting.
Further interventions should be tested in which approaches to aid longer-term changes in smoking and PA are added. The present intervention was client centred in that participants could elect to work on changing smoking and/or PA behaviours, in their own time and way (with manualised HT support). Although some TARS participants appreciated the opportunity to receive support for smoking reduction without the pressure to quit, this approach was insufficient to offer an overall effective and cost-effective approach to achieve sustained abstinence; alternative forms of support will need to be explored.
In populations who are resistant to quitting smoking but are open to reducing the amount they smoke and engaging in other positive health behaviours (such as increasing PA), an alternative modelling framework for health economic evaluation may be necessary that is less driven by prolonged abstinence and more driven by smoking intensity and measures of physical health. In such modelling, it will be important to consider the possibility of relapse towards prior health behaviours (i.e. returning to pre-intervention levels of smoking and PA), just as our economic evaluation considered the possibility of relapsing following smoking cessation.
Conclusions
TARS showed that the intervention was delivered with sufficient fidelity and influenced theoretically relevant constructs that led to short-term behaviour change in smoking and PA. However, we found no evidence that the intervention was effective in promoting long-term smoking cessation and it was not cost-effective in the short term or in terms of long-term health. We had assumed that the baseline abstinence rate would be much higher, so that a relative effect equivalent to the point estimate we observed would have given us 90% power. In the event, achieved power was much lower, but the trial evidence was sufficient to show that the intervention was not cost-effective. Consequently, this intervention cannot be recommended to health and social care providers as a way to promote prolonged smoking abstinence.
Acknowledgements
We appreciate the support of the trial sponsor: University Hospitals Plymouth NHS Trust.
The trial was led by researchers and managed by the PenCTU at the University of Plymouth. We are indebted to Dr Helen Hancocks for her contribution to the TARS as Clinical Trial Manager at PenCTU; sadly, Helen passed away suddenly on 24 January 2019. We thank Doug Webb (Assistant Trial Manager, PenCTU) for helping to develop the protocol.
Our thanks go to all organisations involved in recruitment, and especially to those working in primary and secondary care and in local stop smoking services. We thank James Davies and Will Moyle for preparing the search code for identifying potential participants in primary care.
We acknowledge the contribution of the HTs in delivering the intervention: Alix Covenant, Stephanie Erivo, Gemma Fox, Alex Gude, Sarah Kennedy, Amanda Perry, Lucy Porter and Charlotte Wahlich. We acknowledge the contribution of site researchers in recruitment activities and data collection: Jennie King, Mary George, Louise Hamilton and Kelisha Cheema.
We gratefully acknowledge the members of the TSC and Data Monitoring Committee for their valuable support and guidance. TSC members: Professor Marcus Munafo (chairperson), Dr Stephanie MacNeill, Professor Amanda Amos and Dr Michael Callaghan (PPI representative). Sadly, Michael died having made a valuable contribution to the trial. Data Monitoring Committee members: Dr Rebecca Playle (chairperson), Dr Charlie Foster and Dr Jamie Brown.
Our sincerest thanks go to all the participants involved in the trial.
Contributions of authors
Adrian H Taylor (https://orcid.org/0000-0003-2701-9468) (Professor in Health Services Research) conceived the idea for the study with Tom P Thompson (https://orcid.org/0000-0002-6366-9743) (Senior Research Fellow, Addiction and Health Behaviour Research), Michael Ussher (https://orcid.org/0000-0002-0995-7955) (Professor of Behavioural Medicine), Paul Aveyard (https://orcid.org/0000-0002-1802-4217) (Professor of Behavioural Medicine), Colin Green (https://orcid.org/0000-0001-6140-1287) (Professor of Health Economics), Colin J Greaves (https://orcid.org/0000-0003-4425-2691) (Professor of Psychology Applied to Health) and Siobhan Creanor (https://orcid.org/0000-0002-7373-8263) (Professor of Medical Statistics and Clinical Trials).
Adrian H Taylor, Tom P Thompson, Michael Ussher, Paul Aveyard, Rachael L Murray (https://orcid.org/0000-0001-5477-2557) (Professor of Population Health), Colin Green, Colin J Greaves and Siobhan Creanor contributed to the final trial design and development of the protocol.
Adrian H Taylor, Tom P Thompson and Colin J Greaves developed the intervention, with Lynne Callaghan (https://orcid.org/0000-0002-0766-645X) (Senior Research Fellow, Psychological Research Methods), who supported PPI input.
Adrian H Taylor, Michael Ussher, Paul Aveyard, Rachael L Murray and Tess Harris (https://orcid.org/0000-0002-8671-1553) (Professor of Primary Care Research) were the principal investigators at participating sites.
Tom P Thompson led the process evaluation data collection, analysis and write-up with Adam Streeter (https://orcid.org/0000-0001-9921-2369) (Research Fellow in Medical Statistics) (mediation analysis), Jane Horrell (https://orcid.org/0000-0001-7284-7647) (research assistant), Lynne Callaghan (lead) and Lucy Cartwright (https://orcid.org/0000-0001-8733-1379) (research assistant) (qualitative methods), and with further input from Adrian H Taylor and Colin J Greaves.
Adam Streeter, Jade Chynoweth (https://orcid.org/0000-0002-2516-5923) (Research Assistant in Medical Statistics) and Siobhan Creanor provided the SAP, and Adam Streeter and Jade Chynoweth performed the statistical analysis.
Wendy Ingram (https://orcid.org/0000-0003-0182-9462) (Clinical Trials Manager) and Sarah Campbell (https://orcid.org/0000-0002-5974-5969) (Senior Clinical Trials Manager) were the trial managers at the PenCTU.
Colin Green developed the economic evaluation analysis plan with Tristan Snowsill (https://orcid.org/0000-0001-7406-2819) (Senior Research Fellow, Health Economics). Tristan Snowsill performed the health economic analysis with Colin Green.
Lisa Price (https://orcid.org/0000-0001-9478-3488) (Lecturer in Physical Activity and Health) was consulted on accelerometer data capture and processed the accelerometer data.
Jonny Wilks (https://orcid.org/0000-0002-0545-4146) (Clinical Trials Data Manager) was the data manager at the PenCTU.
Dan Preece (https://orcid.org/0000-0002-0559-7948) (Advanced Public Health Practitioner, Plymouth City Council) provided a public health perspective on usual community stop smoking service support.
All authors critically revised successive drafts of the manuscript and approved the final version.
This research has been conducted independently by researchers at the University of Plymouth, the University of Oxford, the University of Nottingham and St George’s, University of London, with the support of clinical research networks (CRNs) at each site and the Applied Research Collaboration South West Peninsula.
Data-sharing statement
Following publication of the primary results of the trial, access to available anonymised data may be granted depending on review of the data request and appropriate agreements being in place. Members of the Project Management Group, in particular the chief investigator in the first instance, with members of the PenCTU, medical statistics and health economics team, a representative of the sponsor and site principal investigators, will consider applications. Any disagreement will be considered by members of the TSC.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Global Burden of Disease 2015 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1659-724.
- Department of Health and Social Care . Towards a Smokefree Generation: A Tobacco Control Plan for England 2017. www.gov.uk/government/publications/towards-a-smoke-free-generation-tobacco-control-plan-for-england (accessed 27 July 2021).
- Office for National Statistics . Adult Smoking Habits in the UK: 2018 n.d. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/bulletins/adultsmokinghabitsingreatbritain/2018 (accessed 14 May 2021).
- National Institute for Health and Care Excellence (NICE) . Smoking Cessation Services. Public Health Guideline [PH10] 2008.
- Klemperer EM, Hughes JR, Naud S. Reduction in cigarettes per day prospectively predicts making a quit attempt: a fine-grained secondary analysis of a natural history study. Nicotine Tob Res 2019;21:648-54. https://doi.org/10.1093/ntr/nty056.
- Lindson N, Klemperer E, Hong B, Ordóñez-Mena JM, Aveyard P. Smoking reduction interventions for smoking cessation. Cochrane Database Syst Rev 2019;9. https://doi.org/10.1002/14651858.CD013183.pub2.
- Lindson-Hawley N, Hartmann-Boyce J, Fanshawe TR, Begh R, Farley A, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst Rev 2016;10. https://doi.org/10.1002/14651858.CD005231.pub3.
- National Institute for Health and Care Excellence (NICE) . Tobacco: Harm-Reduction Approaches to Smoking. Public Health Guideline [PH45] 2013.
- Klemperer EM, Hughes JR. Does the magnitude of reduction in cigarettes per day predict smoking cessation? A qualitative review. Nicotine Tob Res 2016;18:88-92. https://doi.org/10.1093/ntr/ntv058.
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol 2004;72:371-81. https://doi.org/10.1037/0022-006X.72.3.371.
- Glasgow RE, Gaglio B, Estabrooks PA, Marcus AC, Ritzwoller DP, Smith TL, et al. Long-term results of a smoking reduction program. Med Care 2009;47:115-20. https://doi.org/10.1097/MLR.0b013e31817e18d1.
- Klemperer EM, Hughes JR, Solomon LJ, Callas PW, Fingar JR. Motivational, reduction and usual care interventions for smokers who are not ready to quit: a randomized controlled trial. Addiction 2017;112:146-55. https://doi.org/10.1111/add.13594.
- Klemperer EM, Hughes JR, Callas PW, Solomon LJ. A mediation analysis of motivational, reduction, and usual care interventions for smokers who are not ready to quit. Nicotine Tob Res 2017;19:916-21. https://doi.org/10.1093/ntr/ntx025.
- Catley D, Goggin K, Harris KJ, Richter KP, Williams K, Patten C, et al. A randomized trial of motivational interviewing: cessation induction among smokers with low desire to quit. Am J Prev Med 2016;50:573-83. https://doi.org/10.1016/j.amepre.2015.10.013.
- West R, Mohr G, Proudfoot H, Brown J. Top-Line Findings on Smoking in England from the Smoking Toolkit Study n.d. https://smokinginengland.info/graphs/top-line-findings (accessed 15 November 2021).
- Gotts JE, Jordt SE, McConnell R, Tarran R. What are the respiratory effects of e-cigarettes?. BMJ 2019;366. https://doi.org/10.1136/bmj.l5275.
- Taylor A, Thompson TP, Budde H, Wegner M. The Exercise Effect on Mental Health: Neurobiological Mechanisms. New York, NY: Taylor and Francis; 2018.
- US Department of Health and Human Services . Treating Tobacco Use and Dependence: 2008 Update 2008.
- Haasova M, Warren FC, Ussher M, Janse Van Rensburg K, Faulkner G, Cropley M, et al. The acute effects of physical activity on cigarette cravings: systematic review and meta-analysis with individual participant data. Addiction 2013;108:26-37. https://doi.org/10.1111/j.1360-0443.2012.04034.x.
- Haasova M, Warren FC, Ussher M, Janse Van Rensburg K, Faulkner G, Cropley M, et al. The acute effects of physical activity on cigarette cravings: exploration of potential moderators, mediators and physical activity attributes using individual participant data (IPD) meta-analyses. Psychopharmacology 2014;231:1267-75. https://doi.org/10.1007/s00213-014-3450-4.
- Taylor AH, Everson-Hock ES, Ussher M. Integrating the promotion of physical activity within a smoking cessation programme: findings from collaborative action research in UK Stop Smoking Services. BMC Health Serv Res 2010;10. https://doi.org/10.1186/1472-6963-10-317.
- Ussher MH, Faulkner GEJ, Angus K, Hartmann-Boyce J, Taylor AH. Exercise interventions for smoking cessation. Cochrane Database Syst Rev 2019;10. https://doi.org/10.1002/14651858.CD002295.pub6.
- Taylor AH, Thompson TP, Greaves CJ, Taylor RS, Green C, Warren FC, et al. A pilot randomised trial to assess the methods and procedures for evaluating the clinical effectiveness and cost-effectiveness of Exercise Assisted Reduction then Stop (EARS) among disadvantaged smokers. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18040.
- Pisinger C, Jorgensen T. Waist circumference and weight following smoking cessation in a general population: the Inter99 study. Prev Med 2007;44:290-5. https://doi.org/10.1016/j.ypmed.2006.11.015.
- Filozof C, Fernández Pinilla MC, Fernández-Cruz A. Smoking cessation and weight gain. Obes Rev 2004;5:95-103. https://doi.org/10.1111/j.1467-789X.2004.00131.x.
- Meyers AW, Klesges RC, Winders SE, Ward KD, Peterson BA, Eck LH. Are weight concerns predictive of smoking cessation? A prospective analysis. J Consult Clin Psychol 1997;65:448-52. https://doi.org/10.1037//0022-006x.65.3.448.
- Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ 2012;345. https://doi.org/10.1136/bmj.e4439.
- Parsons AC, Shraim M, Inglis J, Aveyard P, Hajek P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst Rev 2009;1. https://doi.org/10.1002/14651858.CD006219.pub2.
- Oh H, Taylor AH. Self-regulating smoking and snacking through physical activity. Health Psychol 2014;33:349-59. https://doi.org/10.1037/a0032423.
- Thompson TP, Greaves CJ, Ayres R, Aveyard P, Warren FC, Byng R, et al. An exploratory analysis of the smoking and physical activity outcomes from a pilot randomized controlled trial of an exercise assisted reduction to stop smoking intervention in disadvantaged groups. Nicotine Tob Res 2016;18:289-97. https://doi.org/10.1093/ntr/ntv099.
- Williams GC, Niemiec CP, Patrick H, Ryan RM, Deci EL. The importance of supporting autonomy and perceived competence in facilitating long-term tobacco abstinence. Ann Behav Med 2009;37:315-24. https://doi.org/10.1007/s12160-009-9090-y.
- Taylor AH, Thompson TP, Ussher M, Aveyard P, Murray RL, Harris T, et al. A randomised controlled trial of tailored support to increase physical activity and reduce smoking in smokers not immediately ready to quit: protocol for the Trial of physical Activity assisted Reduction of Smoking (TARS) study. BMJ Open 2020;10.
- Thompson TP, Lambert J, Greaves C, Taylor A. Intervention delivery fidelity assessment of a counselling based intervention for multiple behaviours. Health Psychol 2018;37:627-37. https://doi.org/10.1037/hea0000613.
- Hancocks H, King J, Gude A, Treweek S, Ingram W, Thompson T, et al. The Effect of the Method of Invitation on Recruitment of Participants from GP Practices to a Trial of a Smoking Reduction Intervention: A Study Within a Trial n.d.
- Taylor AH, Hancocks H, Creanor S. SWAT 115: Comparisons of Invitation Methods used to Recruit Participants to a Trial of a Smoking Reduction Intervention. The Northern Ireland Network for Trials Methodology Research; 2017.
- Thompson T, Taylor A, Callaghan L, Greaves C. TARS Health Trainer Manual. University of Plymouth; 2021.
- Deci EL, Ryan RM. Handbook of Self-determination Research. Rochester, NY: University of Rochester Press; 2002.
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict 1989;84:791-9. https://doi.org/10.1111/j.1360-0443.1989.tb03059.x.
- Great Britain . Data Protection Act 1998 1998.
- European Parliament, Council of the European Union . General Data Protection Regulation n.d. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32016R0679 (accessed 11 November 2021).
- Great Britain . Data Protection Act 2018 2018.
- van Hees V, Fang Z, Zhao J, Heywood J, Mirkes E, Sabia S, et al. GGIR: Raw Accelerometer Data Analysis. R Package Version 12-8 n.d. https://cran.r-project.org/web/packages/GGIR/vignettes/GGIR.html (accessed 10 December 2021).
- van Hees VT, Fang Z, Langford J, Assah F, Mohammad A, da Silva IC, et al. Autocalibration of accelerometer data for free-living physical activity assessment using local gravity and temperature: an evaluation on four continents. J Appl Physiol 2014;117:738-44. https://doi.org/10.1152/japplphysiol.00421.2014.
- Hildebrand M, VAN Hees VT, Hansen BH, Ekelund U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc 2014;46:1816-24. https://doi.org/10.1249/MSS.0000000000000289.
- Esliger DW, Rowlands AV, Hurst TL, Catt M, Murray P, Eston RG. Validation of the GENEA accelerometer. Med Sci Sports Exerc 2011;43:1085-93. https://doi.org/10.1249/MSS.0b013e31820513be.
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381-95. https://doi.org/10.1249/01.MSS.0000078924.61453.FB.
- Blair SN, Haskell WL, Ho P, Paffenbarger RS, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 1985;122:794-80. https://doi.org/10.1093/oxfordjournals.aje.a114163.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. How to Score Version 2 of the SF-12® Health Survey (with a Supplement Documenting Version 1). Boston, MA, and Lincoln, RI: QualityMetric Incorporated; 2002.
- Powell C, Carson BP, Dowd KP, Donnelly AE. Simultaneous validation of five activity monitors for use in adult populations. Scand J Med Sci Sports 2017;27:1881-92. https://doi.org/10.1111/sms.12813.
- Jarvis MJ, Fidler J, Mindell J, Feyerabend C, West R. Assessing smoking status in children, adolescents and adults: cotinine cut-points revisited. Addiction 2008;103:1553-61. https://doi.org/10.1111/j.1360-0443.2008.02297.x.
- Aveyard P, Wang D, Connock M, Fry-Smith A, Barton P, Moore D. Assessing the outcomes of prolonged cessation-induction and aid-to-cessation trials: floating prolonged abstinence. Nicotine Tob Res 2009;11:475-80. https://doi.org/10.1093/ntr/ntp035.
- Laugesen M, Epton M, Frampton CM, Glover M, Lea RA. Hand-rolled cigarette smoking patterns compared with factory-made cigarette smoking in New Zealand men. BMC Public Health 2009;9. https://doi.org/10.1186/1471-2458-9-194.
- Asfar T, Ebbert JO, Klesges RC, Relyea GE. Do smoking reduction interventions promote cessation in smokers not ready to quit?. Addict Behav 2011;36:764-8. https://doi.org/10.1016/j.addbeh.2011.02.003.
- Hughes JR, Carpenter MJ. The feasibility of smoking reduction: an update. Addiction 2005;100:1074-89. https://doi.org/10.1111/j.1360-0443.2005.01174.x.
- West R. Assessing Smoking Cessation Performance in NHS Stop Smoking Services: The Russell Standard (Clinical). Dorchester: National Centre for Smoking Cessation and Training; 2005.
- Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P. Qualitative research methods in health technology assessment: a review of the literature. Health Technol Assess 1998;2. https://doi.org/10.3310/hta2160.
- Schulz KF, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c332.
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c869.
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299-303. https://doi.org/10.1111/j.1360-0443.2004.00995.x.
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23:443-51. https://doi.org/10.1037/0278-6133.23.5.443.
- Lambert JD, Greaves CJ, Farrand P, Cross R, Haase AM, Taylor AH. Assessment of fidelity in individual level behaviour change interventions promoting physical activity among adults: a systematic review. BMC Public Health 2017;17. https://doi.org/10.1186/s12889-017-4778-6.
- Dreyfus H, Burke JW. Competency Based Education and Training. Abingdon-on-Thames: Routledge; 1989.
- King N, Cassell C, Symon G. Essential Guide to Qualitative Methods in Organizational Research. London: SAGE Publications; 2004.
- Rijnhart JJM, Twisk JWR, Eekhout I, Heymans MW. Comparison of logistic-regression based methods for simple mediation analysis with a dichotomous outcome variable. BMC Med Res Methodol 2019;19. https://doi.org/10.1186/s12874-018-0654-z.
- Fairchild AJ, McDaniel HL. Best (but oft-forgotten) practices: mediation analysis. Am J Clin Nutr 2017;105:1259-71. https://doi.org/10.3945/ajcn.117.152546.
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods 2002;7:83-104. https://doi.org/10.1037/1082-989X.7.1.83.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Callaghan L, Thompson TP, Creanor S, Quinn C, Senior J, Green C, et al. Individual health trainers to support health and well-being for people under community supervision in the criminal justice system: the STRENGTHEN pilot RCT. Public Health Research 2019;7. https://doi.org/10.3310/phr07200.
- Office for National Statistics . Earnings and Hours Worked, Occupation by Four-Digit SOC: ASHE Table 14 2019. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/datasets/occupation4digitsoc2010ashetable14 (accessed 7 February 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: Personal Social Services Research Unit, University of Kent; 2019.
- NHS Improvement . National Cost Collection for the NHS n.d. www.england.nhs.uk/national-cost-collection/ (accessed 27 August 2020).
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Walker N, Yang Y, Kiparoglou V, Pokhrel S, Robinson H, van Woerden H. An examination of user costs in relation to smokers using a cessation service based in the UK. BMC Health Serv Res 2018;18. https://doi.org/10.1186/s12913-018-2985-1.
- Department of Health and Social Care . NHS Reference Costs 2017 18 2018. https://webarchive.nationalarchives.gov.uk/ukgwa/20200501111106/https://improvement.nhs.uk/resources/reference-costs/ (accessed 10 June 2020).
- Stathi A, Medina-Lara A, Fox K, Greaves C, Green C, Taylor G, et al. A Randomised Controlled Trial and Economic Evaluation of a Community-Based Physical Activity Intervention to Prevent Mobility-Related Disability for Retired Older People. The REACT (REtirement in ACTion) Study n.d. www.journalslibrary.nihr.ac.uk/programmes/phr/1316451/#/ (accessed 16 November 2021).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- National Institute for Health and Care Excellence . Position Statement on Use of the EQ-5D-5L Valuation Set for England (Updated October 2019) n.d. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/EQ-5D-5L (accessed 14 May 2021).
- Brazier J, Ratcliffe J, Salomon J, Tsuchiya A. Measuring and Valuing Health Benefits for Economic Evaluation. Oxford: Oxford University Press; 2007.
- Kahan BC, Morris TP. Improper analysis of trials randomised using stratified blocks or minimisation. Stat Med 2012;31:328-40. https://doi.org/10.1002/sim.4431.
- McCullagh P, Nelder JA. Generalized Linear Models. London: Chapman & Hall/CRC; 1989.
- Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc 1988;83:1198-202. https://doi.org/10.1080/01621459.1988.10478722.
- Li C. Little’s test of missing completely at random. Stata J 2013;13:795-809. https://doi.org/10.1177/1536867X1301300407.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Chandra K, Blackhouse G, McCurdy BR, Bornstein M, Campbell K, Costa V, et al. Cost-effectiveness of interventions for chronic obstructive pulmonary disease (COPD) using an Ontario policy model. Ont Health Technol Assess Ser 2012;12:1-61.
- Menn P, Leidl R, Holle R. A lifetime Markov model for the economic evaluation of chronic obstructive pulmonary disease. PharmacoEconomics 2012;30:825-40. https://doi.org/10.2165/11591340-000000000-00000.
- Coward S, Heitman SJ, Clement F, Negron M, Panaccione R, Ghosh S, et al. Funding a smoking cessation program for Crohn’s disease: an economic evaluation. Am J Gastroenterol 2015;110:368-77. https://doi.org/10.1038/ajg.2014.300.
- Kowada A. Cost-effectiveness of tobacco cessation support combined with tuberculosis screening among contacts who smoke. Int J Tuberc Lung Dis 2015;19:857-63. https://doi.org/10.5588/ijtld.14.0518.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64.
- Raftery J, Williams HC, Clarke A, Thornton J, Norrie J, Snooks H, et al. ‘Not clinically effective but cost-effective’ – paradoxical conclusions in randomised controlled trials with ‘doubly null’ results: a cross-sectional study. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019-029596.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 – 5: The Reference Case 2013. www.nice.org.uk/process/pmg9/chapter/the-reference-case (accessed 14 May 2021).
- Barendregt JJ, Van Oortmarssen GJ, Van Hout BA, Van Den Bosch JM, Bonneux L. Coping with multiple morbidity in a life table. Math Popul Stud 1998;7:29-4. https://doi.org/10.1080/08898489809525445.
- Barendregt JJ, Van Oortmarssen GJ, Vos T, Murray CJ. A generic model for the assessment of disease epidemiology: the computational basis of DisMod II. Popul Health Metr 2003;1. https://doi.org/10.1186/1478-7954-1-4.
- Higashi H, Barendregt JJ. Cost-effectiveness of tobacco control policies in Vietnam: the case of personal smoking cessation support. Addiction 2012;107:658-70. https://doi.org/10.1111/j.1360-0443.2011.03632.x.
- Allender S, Balakrishnan R, Scarborough P, Webster P, Rayner M. The burden of smoking-related ill health in the UK. Tob Control 2009;18:262-7. https://doi.org/10.1136/tc.2008.026294.
- Ezzati M, Lopez AD, Ezzati M, Lopez AD, Rodgers A, Murray CJL. Comparative Quantification of Health Risks. Geneva: World Health Organization; 2004.
- Barendregt JJ. The half-cycle correction: banish rather than explain it. Med Decis Making 2009;29:500-2. https://doi.org/10.1177/0272989X09340585.
- Snowsill T. Cross-Sectional Sojourn Time Distribution 2020. https://observablehq.com/@tristansnowsill/cross-sectional-sojourn-time-distribution (accessed 6 August 2020).
- Hawkins J, Hollingworth W, Campbell R. Long-term smoking relapse: a study using the British household panel survey. Nicotine Tob Res 2010;12:1228-35. https://doi.org/10.1093/ntr/ntq175.
- Stan Development Team . Stan Modelling Language Users Guide and Reference Manual, v2.23 2020. https://mc-stan.org/ (accessed 14 July 2020).
- University of Essex, Institute for Social and Economic Research . Understanding Society: Waves 1–9, 2009–2018 and Harmonised BHPS: Waves 1–18, 1991–2009 [data Collection] 2020. https://doi.org/10.5255/UKDA-SN-6614-13.
- Barnett PG, Jeffers A, Smith MW, Chow BK, McFall M, Saxon AJ. Cost-effectiveness of integrating tobacco cessation into post-traumatic stress disorder treatment. Nicotine Tob Res 2016;18:267-74. https://doi.org/10.1093/ntr/ntv094.
- Barnett PG, Wong W, Jeffers A, Hall SM, Prochaska JJ. Cost-effectiveness of smoking cessation treatment initiated during psychiatric hospitalization: analysis from a randomized, controlled trial. J Clin Psychiatry 2015;76:e1285-91. https://doi.org/10.4088/JCP.14m09016.
- Barnett PG, Wong W, Jeffers A, Munoz R, Humfleet G, Hall S. Cost-effectiveness of extended cessation treatment for older smokers. Addiction 2014;109:314-22. https://doi.org/10.1111/add.12404.
- Guerriero C, Cairns J, Roberts I, Rodgers A, Whittaker R, Free C. The cost-effectiveness of smoking cessation support delivered by mobile phone text messaging: Txt2stop. Eur J Health Econ 2013;14:789-97. https://doi.org/10.1007/s10198-012-0424-5.
- Leung W, Roberts V, Gordon LG, Bullen C, McRobbie H, Prapavessis H, et al. Economic evaluation of an exercise-counselling intervention to enhance smoking cessation outcomes: the Fit2Quit trial. Tob Induc Dis 2017;15. https://doi.org/10.1186/s12971-017-0126-y.
- García Rodríguez LA, Wallander MA, Tolosa LB, Johansson S. Chronic obstructive pulmonary disease in UK primary care: incidence and risk factors. COPD 2009;6:369-79. https://doi.org/10.1080/15412550903156325.
- Hoogenveen RT, van Baal PH, Boshuizen HC, Feenstra TL. Dynamic effects of smoking cessation on disease incidence, mortality and quality of life: the role of time since cessation. Cost Eff Resour Alloc 2008;6. https://doi.org/10.1186/1478-7547-6-1.
- National Centre for Social Research (NatCen), University College London, Department of Epidemiology and Public Health . Health Survey for England, 2018 2020. https://doi.org/10.5255/UKDA-SN-8649-1.
- McLean S, Hoogendoorn M, Hoogenveen RT, Feenstra TL, Wild S, Simpson CR, et al. Projecting the COPD population and costs in England and Scotland: 2011 to 2030. Sci Rep 2016;6. https://doi.org/10.1038/srep31893.
- Shavelle RM, Paculdo DR, Kush SJ, Mannino DM, Strauss DJ. Life expectancy and years of life lost in chronic obstructive pulmonary disease: findings from the NHANES III follow-up study. Int J Chron Obstruct Pulmon Dis 2009;4:137-48. https://doi.org/10.2147/COPD.S5237.
- Remen T, Pintos J, Abrahamowicz M, Siemiatycki J. Risk of lung cancer in relation to various metrics of smoking history: a case–control study in Montreal. BMC Cancer 2018;18. https://doi.org/10.1186/s12885-018-5144-5.
- Office for National Statistics . Cancer Registration Statistics, England, 2017 2019. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancerregistrationstatisticscancerregistrationstatisticsengland (accessed 25 September 2020).
- Cancer Research UK . Lung Cancer Survival Statistics 2020. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/survival (accessed 26 September 2020).
- Shields M, Wilkins K. Smoking, smoking cessation and heart disease risk: a 16-year follow-up study. Health Rep 2013;24:12-2. https://doi.org/10.1017/jsc.2015.9.
- Global Burden of Disease Collaborative Network . Global Burden of Disease Study 2019 (GBD 2019) Results 2020. http://ghdx.healthdata.org/gbd-results-tool (accessed 21 October 2020).
- Smolina K, Wright FL, Rayner M, Goldacre MJ. Incidence and 30-day case fatality for acute myocardial infarction in England in 2010: national-linked database study. Eur J Public Health 2012;22:848-53. https://doi.org/10.1093/eurpub/ckr196.
- Myint PK, Sinha S, Luben RN, Bingham SA, Wareham NJ, Khaw KT. Risk factors for first-ever stroke in the EPIC-Norfolk prospective population-based study. Eur J Cardiovasc Prev Rehabil 2008;15:663-9. https://doi.org/10.1097/HJR.0b013e32830fe465.
- Seminog OO, Scarborough P, Wright FL, Rayner M, Goldacre MJ. Determinants of the decline in mortality from acute stroke in England: linked national database study of 795-869 adults. BMJ 2019;365. https://doi.org/10.1136/bmj.l1778.
- Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 2013;368:341-50. https://doi.org/10.1056/NEJMsa1211128.
- Prescott E, Osler M, Andersen PK, Hein HO, Borch-Johnsen K, Lange P, et al. Mortality in women and men in relation to smoking. Int J Epidemiol 1998;27:27-32. https://doi.org/10.1093/ije/27.1.27.
- Office for National Statistics, Public Health England . Adult Smoking Habits in Great Britain (2019) 2020. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/drugusealcoholandsmoking/datasets/adultsmokinghabitsingreatbritain (accessed 15 July 2020).
- Office for National Statistics . 2018-Based National Population Projections Lifetable Template 2019. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/lifetablesprincipalprojectionunitedkingdom (accessed 18 September 2020).
- Vogl M, Wenig CM, Leidl R, Pokhrel S. Smoking and health-related quality of life in English general population: implications for economic evaluations. BMC Public Health 2012;12. https://doi.org/10.1186/1471-2458-12-203.
- Aveyard P, Lindson N, Tearne S, Adams R, Ahmed K, Alekna R, et al. Nicotine preloading for smoking cessation: the Preloading RCT. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22410.
- Wu Q, Parrott S, Godfrey C, Gilbert H, Nazareth I, Leurent B, et al. Cost-effectiveness of computer-tailored smoking cessation advice in primary care: a randomized trial (ESCAPE). Nicotine Tob Res 2014;16:270-8. https://doi.org/10.1093/ntr/ntt136.
- Stapleton JA, West R. A direct method and ICER tables for the estimation of the cost-effectiveness of smoking cessation interventions in general populations: application to a new cytisine trial and other examples. Nicotine Tob Res 2012;14:463-71. https://doi.org/10.1093/ntr/ntr236.
- Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. J Subst Abuse Treat 2005;28:19-26. https://doi.org/10.1016/j.jsat.2004.11.001.
- Hughes JR, Carpenter MJ, Naud S. Do point prevalence and prolonged abstinence measures produce similar results in smoking cessation studies? A systematic review. Nicotine Tob Res 2010;12:756-62. https://doi.org/10.1093/ntr/ntq078.
- Hughes JR. An update on hardening: a qualitative review. Nicotine Tob Res 2020;22:867-71. https://doi.org/10.1093/ntr/ntz042.
- Shemilt I, James T, Morciano M. A web-based tool for adjusting costs to a specific target currency and price year. Evid Policy 2010;6:51-9. https://doi.org/10.1332/174426410X482999.
- Glasgow RE, Estabrooks PA, Marcus AC, Smith TL, Gaglio B, Levinson AH, et al. Evaluating initial reach and robustness of a practical randomized trial of smoking reduction. Health Psychol 2008;27:780-8. https://doi.org/10.1037/0278-6133.27.6.780.
- The King’s Fund . Tackling Multiple Unhealthy Risk Factors: Emerging Lessons from Practice n.d. www.kingsfund.org.uk/publications/tackling-multiple-unhealthy-risk-factors (accessed 1 December 2021).
- Cochrane Tobacco Addiction Group . The Cochrane TAG Specialised Register 2020. https://tobacco.cochrane.org/resources/cochrane-tag-specialised-register (accessed 27 November 2020).
- Glanville J, Kaunelis D, Mensinkai S. How well do search filters perform in identifying economic evaluations in MEDLINE and EMBASE. Int J Technol Assess Health Care 2009;25:522-9. https://doi.org/10.1017/S0266462309990523.
- Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al. The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation. Health Technol Assess 2002;6. https://doi.org/10.3310/hta6160.
- Pennington B, Filby A, Owen L, Taylor M. Smoking cessation: a comparison of two model structures. PharmacoEconomics 2018;36:1101-12. https://doi.org/10.1007/s40273-018-0657-y.
- Virtanen SE, Galanti MR, Johansson PM, Feldman I. Economic evaluation of a brief counselling for smoking cessation in dentistry: a case study comparing two health economic models. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2017-016375.
- Annemans L, Marbaix S, Nackaerts K, Bartsch P. Cost-effectiveness of retreatment with varenicline after failure with or relapse after initial treatment for smoking cessation. Prev Med Rep 2015;2:189-95. https://doi.org/10.1016/j.pmedr.2015.03.004.
- Athanasakis K, Igoumenidis M, Karampli E, Vitsou E, Sykara G, Kyriopoulos J. Cost-effectiveness of varenicline versus bupropion, nicotine-replacement therapy, and unaided cessation in Greece. Clin Ther 2012;34:1803-14. https://doi.org/10.1016/j.clinthera.2012.07.002.
- Baker CL, Pietri G. A cost-effectiveness analysis of varenicline for smoking cessation using data from the EAGLES trial. Clinicoecon Outcomes Res 2018;10:67-74. https://doi.org/10.2147/CEOR.S153897.
- Kautiainen K, Ekroos H, Puhakka M, Liira H, Laine J, Linden K, et al. Re-treatment with varenicline is a cost-effective aid for smoking cessation. J Med Econ 2017;20:246-52. https://doi.org/10.1080/13696998.2016.1249485.
- Knight C, Marbaix S, Annemans L, Prignot J, Bowrin K. The cost-effectiveness of an extended course (12+12 weeks) of varenicline plus brief counselling compared with other reimbursed smoking cessation interventions in Belgium, from a public payer perspective. Acta Clin Belg 2012;67:416-22. https://doi.org/10.2143/ACB.67.6.2062706.
- Leaviss J, Sullivan W, Ren S, Everson-Hock E, Stevenson M, Stevens JW, et al. What is the clinical effectiveness and cost-effectiveness of cytisine compared with varenicline for smoking cessation? A systematic review and economic evaluation. Health Technol Assess 2014;18. https://doi.org/10.3310/hta18330.
- Lutz MA, Lovato P, Cuesta G. Cost analysis of varenicline versus bupropion, nicotine replacement therapy, and unaided cessation in Nicaragua. Hosp Pract 2012;40:35-43. https://doi.org/10.3810/hp.2012.02.946.
- Lutz MA, Lovato P, Cuesta G. Cost-effectiveness analysis of varenicline versus existing smoking cessation strategies in Central America and the Caribbean using the BENESCO model. Hosp Pract 2012;40:24-3. https://doi.org/10.3810/hp.2012.02.945.
- von Wartburg M, Raymond V, Paradis PE. The long-term cost-effectiveness of varenicline (12-week standard course and 12 + 12-week extended course) vs. other smoking cessation strategies in Canada. Int J Clin Pract 2014;68:639-46. https://doi.org/10.1111/ijcp.12363.
- Wilson K, Hettle R, Marbaix S, Diaz Cerezo S, Ines M, Santoni L, et al. An economic evaluation based on a randomized placebo-controlled trial of varenicline in smokers with cardiovascular disease: results for Belgium, Spain, Portugal, and Italy. Eur J Prev Cardiol 2012;19:1173-83. https://doi.org/10.1177/1741826711420345.
- Howard P, Knight C, Boler A, Baker C. Cost-utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO Simulation model: application to a population of US adult smokers. PharmacoEconomics 2008;26:497-511.
- Boyd KA, Briggs AH, Bauld L, Sinclair L, Tappin D. Are financial incentives cost-effective to support smoking cessation during pregnancy?. Addiction 2016;111:360-70. https://doi.org/10.1111/add.13160.
- Maciosek MV, LaFrance AB, Dehmer SP, McGree DA, Xu Z, Flottemesch TJ, et al. Health benefits and cost-effectiveness of brief clinician tobacco counseling for youth and adults. Ann Fam Med 2017;15:37-4. https://doi.org/10.1370/afm.2022.
- Mullen KA, Coyle D, Manuel D, Nguyen HV, Pham B, Pipe AL, et al. Economic evaluation of a hospital-initiated intervention for smokers with chronic disease, in Ontario, Canada. Tob Control 2015;24:489-96. https://doi.org/10.1136/tobaccocontrol-2013-051483.
- Sonntag D, Gilbody S, Winkler V, Ali S. German EstSmoke: estimating adult smoking-related costs and consequences of smoking cessation for Germany. Addiction 2018;113:125-36. https://doi.org/10.1111/add.13956.
- Cadier B, Durand-Zaleski I, Thomas D, Chevreul K. Cost effectiveness of free access to smoking cessation treatment in France considering the economic burden of smoking-related diseases. PLOS One 2016;11. https://doi.org/10.1371/journal.pone.0148750.
- Cantor SB, Deshmukh AA, Luca NS, Nogueras-González GM, Rajan T, Prokhorov AV. Cost-effectiveness analysis of smoking-cessation counseling training for physicians and pharmacists. Addict Behav 2015;45:79-86. https://doi.org/10.1016/j.addbeh.2015.01.004.
- Chen YF, Madan J, Welton N, Yahaya I, Aveyard P, Bauld L, et al. Effectiveness and cost-effectiveness of computer and other electronic aids for smoking cessation: a systematic review and network meta-analysis. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16380.
- Chevreul K, Cadier B, Durand-Zaleski I, Chan E, Thomas D. Cost effectiveness of full coverage of the medical management of smoking cessation in France. Tob Control 2014;23:223-30. https://doi.org/10.1136/tobaccocontrol-2012-050520.
- Gilbert H, Sutton S, Morris R, Petersen I, Wu Q, Parrott S, et al. Start2quit: a randomised clinical controlled trial to evaluate the effectiveness and cost-effectiveness of using personal tailored risk information and taster sessions to increase the uptake of the NHS Stop Smoking Services. Health Technol Assess 2017;21. https://doi.org/10.3310/hta21030.
- Wu Q, Gilbert H, Nazareth I, Sutton S, Morris R, Petersen I, et al. Cost-effectiveness of personal tailored risk information and taster sessions to increase the uptake of the NHS Stop Smoking Services: the Start2quit randomized controlled trial. Addiction 2018;113:708-18. https://doi.org/10.1111/add.14086.
- Igarashi A, Goto R, Suwa K, Yoshikawa R, Ward AJ, Moller J. Cost-effectiveness analysis of smoking cessation interventions in Japan Using a discrete-event simulation. Appl Health Econ Health Policy 2016;14:77-8. https://doi.org/10.1007/s40258-015-0204-3.
- Lal A, Mihalopoulos C, Wallace A, Vos T. The cost-effectiveness of call-back counselling for smoking cessation. Tob Control 2014;23:437-42. https://doi.org/10.1136/tobaccocontrol-2012-050907.
- López-Nicolás A, Trapero-Bertran M, Muñoz C. Cost-benefit of medical advice for quitting smoking in the Region of Murcia. Aten Primaria 2017;49:407-16. https://doi.org/10.1016/j.aprim.2016.11.006.
- Meeyai A, Yunibhand J, Punkrajang P, Pitayarangsarit S. An evaluation of usage patterns, effectiveness and cost of the national smoking cessation quitline in Thailand. Tob Control 2015;24:481-8. https://doi.org/10.1136/tobaccocontrol-2013-051520.
- Nohlert E, Helgason AR, Tillgren P, Tegelberg A, Johansson P. Comparison of the cost-effectiveness of a high- and a low-intensity smoking cessation intervention in Sweden: a randomized trial. Nicotine Tob Res 2013;15:1519-27. https://doi.org/10.1093/ntr/ntt009.
- Popp J, Nyman JA, Luo X, Bengtson J, Lust K, An L, et al. Cost-effectiveness of enhancing a Quit-and-Win smoking cessation program for college students. Eur J Health Econ 2018;19:1319-33. https://doi.org/10.1007/s10198-018-0977-z.
- Rasmussen SR. The cost effectiveness of telephone counselling to aid smoking cessation in Denmark: a modelling study. Scand J Public Health 2013;41:4-10. https://doi.org/10.1177/1403494812465675.
- Tosanguan J, Chaiyakunapruk N. Cost-effectiveness analysis of clinical smoking cessation interventions in Thailand. Addiction 2016;111:340-50. https://doi.org/10.1111/add.13166.
- Tran K, Asakawa K, Cimon K, Moulton K, Kaunelis D, Pipe A, et al. Pharmacologic-based Strategies for Smoking Cessation: Clinical and Cost-effectiveness Analyses. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2010.
- Tran K, Asakawa K, Cimon K, Moulton K, Kaunelis D, Pipe A, et al. Pharmacologic-based strategies for smoking cessation: clinical and cost-effectiveness analyses. CADTH Technol Overv 2012;2.
- Villanti AC, Jiang Y, Abrams DB, Pyenson BS. A cost–utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLOS One 2013;8. https://doi.org/10.1371/journal.pone.0071379.
- Krall EA, Garvey AJ, Garcia RI. Smoking relapse after 2 years of abstinence: findings from the VA Normative Aging Study. Nicotine Tob Res 2002;4:95-100. https://doi.org/10.1080/14622200110098428.
- Wetter DW, Cofta-Gunn L, Fouladi RT, Cinciripini PM, Sui D, Gritz ER. Late relapse/sustained abstinence among former smokers: a longitudinal study. Prev Med 2004;39:1156-63. https://doi.org/10.1016/j.ypmed.2004.04.028.
- Etter JF, Stapleton JA. Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob Control 2006;15:280-5. https://doi.org/10.1136/tc.2005.015487.
- Stapleton J. Cigarette smoking prevalence, cessation and relapse. Stat Methods Med Res 1998;7:187-203. https://doi.org/10.1177/096228029800700206.
- Levy DT, Graham AL, Mabry PL, Abrams DB, Orleans CT. Modeling the impact of smoking-cessation treatment policies on quit rates. Am J Prev Med 2010;38:364-72. https://doi.org/10.1016/j.amepre.2009.11.016.
- Taylor DH, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of smoking cessation for longevity. Am J Public Health 2002;92:990-6. https://doi.org/10.2105/AJPH.92.6.990.
- Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ 1994;309:901-11. https://doi.org/10.1136/bmj.309.6959.901.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328. https://doi.org/10.1136/bmj.38142.554479.AE.
- LaCroix AZ, Lang J, Scherr P, Wallace RB, Cornoni-Huntley J, Berkman L, et al. Smoking and mortality among older men and women in three communities. N Engl J Med 1991;324:1619-25. https://doi.org/10.1056/NEJM199106063242303.
- Jha P, Jacob B, Gajalakshmi V, Gupta PC, Dhingra N, Kumar R, et al. A nationally representative case-control study of smoking and death in India. N Engl J Med 2008;358:1137-47. https://doi.org/10.1056/NEJMsa0707719.
- Rogers RG, Powell-Griner E. Life expectancies of cigarette smokers and nonsmokers in the United States. Soc Sci Med 1991;32:1151-9. https://doi.org/10.1016/0277-9536(91)90092-q.
- Rogers RG, Hummer RA, Krueger PM, Pampel FC. Mortality attributable to cigarette smoking in the United States. Popul Dev 2005;31:259-92. https://doi.org/10.1111/j.1728-4457.2005.00065.x.
- Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. Lung Health Study Research Group . The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 2005;142:233-9. https://doi.org/10.7326/0003-4819-142-4-200502150-00005.
- Peto R, Lopez AD, Boreham J, Thun M, Heath C. Mortality from tobacco in developed countries: indirect estimation from national vital statistics. Lancet 1992;339:1268-78. https://doi.org/10.1016/0140-6736(92)91600-D.
- Friedman G, Tekawa I, Salder M, Sidney S. Smoking and mortality: the Kaier Permanente experience. Changes in cigarette-related disease risks and their implications for prevention and control. Smoking and Tobacco Control Monographs, National Cancer Institute 1997;8:472-99.
- Uno F, Ishikawa S, Nakamura Y, Gotoh T, Nago N, Kayaba K, et al. Smoking and risk of all-cause mortality: the Jichi Medical School (JMS) Cohort Study. J Epidemiol 2005;15:173-9. https://doi.org/10.2188/jea.15.173.
- Thun MJ, Apicella LF, Henley SJ. Smoking vs other risk factors as the cause of smoking-attributable deaths: confounding in the courtroom. JAMA 2000;284:706-12. https://doi.org/10.1001/jama.284.6.706.
- Freedman ND, Leitzmann MF, Hollenbeck AR, Schatzkin A, Abnet CC. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol 2008;9:649-56. https://doi.org/10.1016/S1470-2045(08)70154-2.
- Pesch B, Kendzia B, Gustavsson P, Jöckel KH, Johnen G, Pohlabeln H, et al. Cigarette smoking and lung cancer – relative risk estimates for the major histological types from a pooled analysis of case–control studies. Int J Cancer 2012;131:1210-19. https://doi.org/10.1002/ijc.27339.
- Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med 2006;354:333-42. https://doi.org/10.1056/NEJMoa033250.
- Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ 2000;321:323-9. https://doi.org/10.1136/bmj.321.7257.323.
- US Department of Health and Human Services . The Health Consequences of Smoking: A Report of the Surgeon General 2004.
- Løkke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax 2006;61:935-9. https://doi.org/10.1136/thx.2006.062802.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D (Discussion Paper 172). York: Centre for Health Economics, University of York; 1999.
- Tillmann M, Silcock J. A comparison of smokers’ and ex-smokers’ health-related quality of life. J Public Health Med 1997;19:268-73. https://doi.org/10.1093/oxfordjournals.pubmed.a024629.
- Fiscella K, Franks P. Cost-effectiveness of the transdermal nicotine patch as an adjunct to physicians’ smoking cessation counseling. JAMA 1996;275:1247-51.
- Jia H, Lubetkin EI. Trends in quality-adjusted life-years lost contributed by smoking and obesity. Am J Prev Med 2010;38:138-44. https://doi.org/10.1016/j.amepre.2009.09.043.
- Office for National Statistics . Population Estimates for UK, England and Wales, Scotland and Northern Ireland: Mid-2018, Using April 2019 Local Authority District Codes 2020. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (accessed 24 September 2020).
- Office for National Statistics . Mid-Year Population Estimates of the Very Old, Including Centenarians: England 2020. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/ageing/datasets/midyearpopulationestimatesoftheveryoldincludingcentenariansengland (accessed 24 September 2020).
Appendix 1 Intervention components and core competencies of the health trainers
| Intervention component (CC) | Aim | Content | Indicative change in processes |
|---|---|---|---|
| Active participant involvement (1) | Develop rapport and build trust; shared respect | Effective communication skills. Build autonomous support | Participant feedback on HT-led support |
| Build motivation to reduce smoking (2) and increase PA (3) | Identify ambivalence towards reduction and quitting. Build self-awareness and confidence to cut down smoking and increase PA | Help smoker to identify importance and challenges of reduction and cessation, and implicit and explicit roles of PA (MI techniques) | Smoker has desire and confidence to cut down and perhaps quit over the early sessions, and increase PA. Smoker engages in more self-monitoring of smoking and PA behaviour |
| Self-monitor smoking and PA and set goals to reduce smoking (4) and increase PA (5) | Develop strategies to reduce smoking and increase PA | Set SMART goals to reduce smoking and increase PA. Signpost to PA opportunities and remove barriers to doing PA | Goals identified and action plans developed. Smoker engages in more goal-setting to reduce smoking and increase PA behaviour |
| Review/problem-solving for smoking (6) and PA (7) | Build confidence, perceptions of control, and self-regulation skills | Smoker reflects on smoking reduction and PA, identifies barriers and possible solutions, increases and sets new targets, perhaps to quit | Goals revised to reflect confidence to increase PA, reduce smoking and possibly quit |
| Integrating idea of changing smoking and PA (8) | To help smoker to identify any links between smoking and PA | Explore with smoker how PA may influence smoking (and vice versa) (person-centred exchange of information (ask–tell–discuss) | Smoker increases use of PA as an aid in smoking reduction |
| Reinforce health identity shift (9) | To help identify shift from smoker to healthier identity | Smoker reflects on label as heavy, moderate, light or non-smoker status, and more active person | Decrease in importance of smoking and increase in importance of doing PA identified |
| Manage social influences on smoking (10) and PA (11) | To involve others in process of reducing smoking and increasing PA. Manage negative or undermining social influences | Smoker identifies key people who can support reduced smoking (or cessation) and increasing PA, and engages with them in preferred ways. Uses negotiation and discussion to manage negative social influences | Support from others identified as important and used for smoking reduction or cessation, and increasing PA |
Core competency 1: active participant involvement
Key features
The HT should ensure that the client is actively involved in the consultation. The idea is to maximise a smoker’s autonomy as the main agent of change, developing intrinsic, rather than extrinsic, motivation, and encouraging her/him to be the person coming up with the desire to change behaviour. However, the smoker should not be allowed to ramble in an unstructured way and the consultation should be guided. A collaborative/shared decision-making style is appropriate and the HT may share his/her own expertise and ideas, using techniques such as elicit–provide–elicit (see next section). Overall, a smoker should be increasingly empowered to take control of her/his smoking and related PA behaviour. Interactions should be encouraging, respectful and non-judgemental (the opposite of a didactic, telling or persuading style of interaction). The smoker should ideally talk for at least half of the time. The interaction should also be individually tailored to a participant’s specific information needs, beliefs, motivations and barriers. The HT should engender a clear sense of warmth, genuineness and empathy (within professional boundaries).
Intervention techniques
Open questions, affirmation, reflective listening and summaries (OARS) were used. Reflective listening may include simple reflections of content, but may also be more sophisticated (e.g. amplified reflection; reflection with a twist) and used to direct the conversation or highlight key strengths or barriers. The ask–tell–discuss (elicit–provide–elicit) technique should be used to exchange information (e.g. to address misconceptions or offer helpful new information). The above empathy-building techniques and individual tailoring should be used throughout the consultations, from the initial consultation through action planning to review/maintenance sessions.
Using whole and half numbers, score the level to which you think the HT has delivered this intervention process.
0 Absence of active participant involvement techniques. A highly didactic/practitioner-led or ‘lecturing’ style of interaction, which may increase or sustain client’s resistance.
1 Minimal participant involvement or use of active participant involvement techniques. The practitioner dominates the discussion.
2 Appropriate use of participant involvement techniques, but not frequent enough. The practitioner sometimes dominates the discussion.
3 Appropriate and frequent use of participant involvement techniques. Teamwork evident, but some difficulties in content or method of delivery.
4 Appropriate and frequent use of participant involvement techniques. Minor problems evident (e.g. some reflection opportunities missed).
5 Highly appropriate and regular use of participant involvement techniques, facilitating shared understanding and decision-making. Minimal problems.
6 Excellent/expert use of participant involvement techniques throughout all consultations. A clear sense of collaborative alliance is developed.
Core competency 2: motivation-building for cutting down (quitting)
Key features
The HT should work with the smoker to explore initial beliefs about cutting down, and quitting (importance and confidence, triggers for smoking). A smoker’s motivation and confidence for cutting down is built up/enhanced through the exchange of information and techniques to assess and enhance motivation, that is to enhance the perceived benefits (importance) of cutting down/quitting and confidence (self-efficacy) to take the actions needed.
Intervention techniques
The OARS should be used specifically to explore current and past smoking behaviour and the pros and cons of cutting down, and to develop discrepancies between current behaviour and desired behaviour (or outcomes). The decisional balance technique or 0–10 questions may be used to explore importance and confidence. Information should be exchanged on the pros and cons of cutting down and this and other techniques (exploring possible futures, discussing past quitting attempts) should be used to explore barriers, and possible solutions, to increasing confidence about cutting down/quitting. Motivation-building should ideally happen around the start of the intervention process, although it can be further explored and reinforced at later (action planning, review and maintenance) stages. Establishing self-rewards or incentives (e.g. saving money in a jar, planning rewards) may be part of the process for maintaining motivation.
Using whole and half numbers, score the level to which you think the HT has delivered this intervention process. Note that achieving a strong motivation is not necessary to score highly here: the aim is to explore motivation sufficiently to allow a client to be able to make an informed choice (which may be not to make any changes at this point in time).
0 Absence (or very poor delivery) of motivation-building techniques. Motivation to cut down or quit smoking is assumed or not discussed.
1 Minimal use of (or poor delivery of) motivation-building techniques. Minimal exploration of either reasons for change or confidence about making changes.
2 Some use of motivation-building techniques, but the exploration of motivation to cut down or quit is not of sufficient depth or detail.
3 Appropriate use of motivation-building techniques. However, some difficulties evident (e.g. moving on to change talk before motivation is fully established).
4 Appropriate and frequent use of motivation-building techniques relating to cutting down or quitting smoking. Minor problems evident (e.g. some inconsistencies).
5 Highly appropriate and sufficient use of motivation-building techniques, facilitating a clear understanding of reasons for change and confidence issues. Minimal problems.
6 Excellent/expert use of motivation-building techniques, facilitating a clear understanding of reasons for change and confidence issues. No real problems.
Core competency 3: motivation-building for physical activity
Key features
The HT should work with the smoker to introduce PA as an aid to cutting down and quitting. They should explore initial beliefs about increasing PA (importance and confidence). A smoker’s motivation and confidence for introducing new PA behaviours should be built up through the exchange of information and techniques to assess and enhance motivation, that is to enhance a smoker’s perceived benefits and usefulness (importance) of PA and confidence (self-efficacy) to take the actions needed.
Intervention techniques
The OARS should be used specifically to explore current and past PA behaviour and the pros and cons of increasing PA, and to develop discrepancies between current behaviour and desired behaviour (or outcomes). The decisional balance technique or 0–10 questions may be used to explore importance and confidence. Information should be exchanged on the pros and cons of PA and this and other techniques (exploring possible futures, discussing past attempts to increase PA) should be used to explore barriers, and possible solutions, to adopting PA strategies/increasing PA. Motivation-building should ideally happen around the start of the intervention process, although it can be further explored and reinforced at later (action planning, review and maintenance) stages.
Using whole and half numbers, score the level to which you think the HT has delivered this intervention process. Note that achieving a strong motivation or any changes is not necessary to score highly: the aim is to explore motivation sufficiently to allow a client to be able to make an informed choice about whether or not to change.
0 Absence (or very poor delivery) of motivation-building techniques. Motivation to adopt PA strategies is assumed or not discussed.
1 Minimal use of (or poor delivery of) motivation-building techniques. Minimal exploration of either reasons for change or confidence about making changes.
2 Some use of motivation-building techniques, but the exploration of motivation for PA is not of sufficient depth or detail.
3 Appropriate use of motivation-building techniques. However, some difficulties evident (e.g. moving on to change talk before motivation is fully established).
4 Appropriate and frequent use of motivation-building techniques relating to PA. Minor problems evident (e.g. some inconsistencies).
5 Highly appropriate and sufficient use of motivation-building techniques, facilitating a clear understanding of reasons for change and confidence issues. Minimal problems.
6 Excellent/expert use of motivation-building techniques, facilitating a clear understanding of reasons for change and confidence issues. No real problems.
Core competency 4: self-monitor, set goals and discuss strategies to reduce smoking
Key features
The HT should work with the smoker to discuss a range of strategies for reducing the number of cigarettes smoked. They should agree a verbal plan of action, seeking to make this as specific as possible. They should discuss the use of self-monitoring to keep track of progress.
Intervention techniques
Goal-setting (with gradual/graded progression), action planning, self-monitoring and deconditioning strategies can all be used. Any or all of the four distinct EARS pilot trial strategies for cutting down (based on breaking the conditioned/automated link between smoking and reward and replacing this with consciously mediated strategies) may be presented and discussed. The action plan should normally be made verbally, but the HT should seek to make this as specific as possible in terms of what, where, when and who with, and to make a goal as SMART as possible. The HT should introduce and discuss with the smoker the usefulness of self-monitoring of behaviours (number of cigarettes smoked, pattern of use). A specific plan for self-monitoring should be included in the action plan. The HT may also encourage self-monitoring of the contexts (social or environmental or emotional circumstances) in which problems/relapses might occur. Pre-empting and thinking of solutions for possible problems (making a coping plan) is also appropriate here and may involve the use of other recognised behaviour change techniques (e.g. engaging social support, stress management).
Using whole and half numbers, score the level to which you think the HT has delivered this intervention process.
0 Absence (or very poor delivery) of action planning techniques or discussion of smoking reduction strategies.
1 Minimal use (or poor delivery) of action planning techniques or discussion of smoking reduction strategies.
2 Some use of action planning techniques or discussion of smoking reduction strategies, but not in sufficient depth or detail.
3 Appropriate use of action planning techniques and discussion of smoking reduction strategies. However, some difficulties evident (e.g. not setting up self-monitoring, plan generated more by the HT than by the smoker).
4 Appropriate use of action planning techniques and discussion of strategies. Minor problems evident (e.g. the plan is a bit less specific than it could be).
5 Highly appropriate and sufficient use of action planning techniques and discussion of smoking reduction strategies. Minimal problems.
6 Excellent/expert use of action planning techniques and discussion of smoking reduction strategies. No real problems.
Core competency 5: self-monitor, set goals and discuss strategies to set goals to increase physical activity
Key features
The HT should work with the smoker to discuss ideas for introducing new PAs that might help to reduce smoking. They should agree a verbal plan of action, seeking to make this as specific as possible. They should discuss the use of self-monitoring to keep track of progress, including offering a pedometer as a means of monitoring walking activity, if appropriate.
Intervention techniques
Goal-setting (with gradual/graded progression), action planning and self-monitoring can be used. Ideas for introducing relevant PAs should be discussed. The action plan should normally be made verbally, but the HT should seek to make this as specific as possible in terms of what, where, when and who with, and to make a goal as SMART as possible. The HT should introduce and discuss with the smoker the usefulness of self-monitoring of behaviours (using memory, a diary and/or a pedometer). A specific plan for self-monitoring should be included in the action plan. Pre-empting and thinking of solutions for possible problems (making a coping plan) is also appropriate here and may involve the use of other recognised behaviour change techniques (e.g. establishing prompts or cues to do PA).
Using whole and half numbers, score the level to which you think the HT has delivered this intervention process.
0 Absence (or very poor delivery) of action planning techniques in relation to PA.
1 Minimal use (or poor delivery) of action planning techniques.
2 Some use of action planning techniques relating to PA, but not in sufficient depth or detail.
3 Appropriate use of action planning techniques. However, some difficulties evident (e.g. no self-monitoring, plan generated more by the HT than by the smoker.
4 Appropriate use of action planning techniques relating to PA. Minor problems evident (e.g. the plan is a bit less specific than it could be).
5 Highly appropriate and sufficient use of action planning techniques. Minimal problems.
6 Excellent/expert use of action planning techniques relating to PA. No real problems.
Core competency 6: review efforts to cut down smoking/problem-solving
Key features
The HT should work with the smoker to reflect on progress with smoking reduction. The HT should affirm/reinforce any successes. The smoker and HT should discuss any setbacks (reframing to normalise them, identifying barriers and exploring ways to overcome them). The HT and smoker should then set new targets (possibly including making an attempt to quit).
Intervention techniques
Use of OARS specifically to reinforce successes, to discuss setbacks, to identify barriers (including social or environmental contexts that increase cravings) and to explore ways to overcome them (problem-solving). Reframing should be used to normalise setbacks. Goals/action plans should then be reviewed. There may also be some reflection on, and reinforcement of, a smoker’s skills in avoiding or managing relapse (building skills and self-efficacy). Problem-solving may involve the use of other recognised behaviour change techniques (e.g. engaging social support, stress management).
Using whole and half numbers, score the level to which you think the HT has delivered this intervention process.
0 Absence (or very poor delivery) of progress review or problem-solving techniques in relation to smoking reduction.
1 Minimal use (or poor delivery) of progress review or problem-solving techniques.
2 Some use of progress review and problem-solving techniques in relation to smoking reduction, but lacking sufficient depth or detail.
3 Appropriate use of progress review and problem-solving techniques. However, some difficulties evident (e.g. not reinforcing successes, providing rather than eliciting possible solutions to problems).
4 Appropriate and frequent use of progress review and problem-solving techniques in relation to smoking reduction. Minor problems evident.
5 Highly appropriate and sufficient use of progress review and problem-solving techniques, facilitating a clear understanding of the current situation and how to move forward. Minimal problems.
6 Excellent/expert use of progress review and problem-solving techniques in relation to smoking reduction, facilitating a clear understanding of the current situation and how to move forward. No real problems.
Core competency 7: review efforts to increase physical activity/problem-solving
Key features
The HT should work with the smoker to reflect on progress with introducing relevant PAs. The HT should affirm/reinforce any successes. The smoker and HT should discuss any setbacks (reframing to normalise them, identifying barriers and exploring ways to overcome them). The HT and smoker should then revise the smoker’s PA-related goals.
Intervention techniques
Use of OARS specifically to reinforce successes, to discuss setbacks, to identify barriers and to explore ways to overcome them (problem-solving). Reframing should be used to normalise setbacks. Goals/action plans should then be reviewed. There may also be some reflection on, and reinforcement of, a smoker’s skills in avoiding or managing relapse (building skills and self-efficacy).
Using whole and half numbers, score the level to which you think the HT has delivered this intervention process.
0 Absence (or very poor delivery) of progress review or problem-solving techniques in relation to the PA component of the intervention.
1 Minimal use (or poor delivery) of progress review or problem-solving techniques.
2 Some use of progress review and problem-solving techniques in relation to PA, but lacking sufficient depth or detail.
3 Appropriate use of progress review and problem-solving techniques. However, some difficulties evident (e.g. not reinforcing successes, providing rather than eliciting possible solutions to problems).
4 Appropriate and frequent use of progress review and problem-solving techniques in relation to PA. Minor problems evident.
5 Highly appropriate and sufficient use of progress review and problem-solving techniques, facilitating a clear understanding of the current situation and how to move forward. Minimal problems.
6 Excellent/expert use of progress review and problem-solving techniques in relation to PA, facilitating a clear understanding of the current situation and how to move forward. No real problems.
Core competency 8: integration of concepts – building an association between physical activity and smoking reduction
Key features
The HT should work with the smoker specifically to help her/him gain an appreciation of the relationship between PA and smoking. A clear rationale should be presented for how PA might be relevant to reducing smoking (e.g. as a distraction, as a way to reduce withdrawal symptoms such as stress or cravings, as a way to prevent weight gain when reducing smoking). However, both explicit processes (explanations) and implicit processes (learning from experience, disrupting usual patterns of smoking behaviour, reductions in withdrawal symptoms that the smoker is not consciously aware of) should be facilitated by the HT.
Intervention techniques
Explicit integration techniques might include (1) developing (ideally using the elicit–provide–elicit information-exchange technique) an appropriate conceptualisation or rationale for increasing PA as an aid to reducing smoking and (2) setting up an experiment (to do some extra PA) and encouraging self-monitoring of links between PA and cigarette cravings, as well as on cigarette use. Implicit techniques might include setting up an experiment to see if it helps reduce smoking, with monitoring only of outcomes (cigarette use) and without trying to make a conscious link between PA and strength of cravings. Review of experiences with using PA and its impact on cravings or smoking behaviour may also be used in later sessions.
Using whole and half numbers, score the level to which you think the HT has delivered this intervention process.
0 The absence (or very poor delivery) of techniques to link PA to cravings or amount smoked.
1 Minimal use (or poor delivery) of techniques to link PA to cravings or amount smoked. No clear rationale linking PA to smoking reduction is understood by the client.
2 Some use of techniques to link PA to cravings or number of cigarettes smoked, but not of sufficient depth or detail. Only a limited rationale linking PA to smoking reduction is understood by the client.
3 Appropriate use of techniques to link PA to cravings or number of cigarettes smoked. The rationale is at least partly understood by the client. Some difficulties evident (e.g. not addressing misconceptions, not using ask–tell–discuss).
4 Appropriate use of techniques to link PA to cravings or number of cigarettes smoked. The rationale is understood by the client. Minor problems evident (e.g. minor inconsistencies).
5 Highly appropriate use of techniques to link PA to cravings or number of cigarettes smoked. The rationale is well developed and understood. Minimal problems.
6 Excellent/expert use of techniques to link PA to cravings or number of cigarettes smoked. The rationale is well developed and understood. No real problems.
Core competency 9: identify and reinforce any identity shifts towards being a more ‘healthy person’ or ‘healthy living’
Key features
The HT should pick up on any opportunity to reflect or reinforce statements that the smoker makes relating to becoming or wanting to become a more healthy person in general.
Intervention techniques
Open questions, affirmation and reflective listening should be used. Reflective listening may include simple reflections of content, but may also be more sophisticated (e.g. amplified reflection, reflection with a twist) and used to direct the conversation or highlight key changes in thinking that may generalise to a change in a client’s concept of self or their identity, particularly with regard to being a healthy person or living a healthy lifestyle.
Using whole and half numbers, score the level to which you think the HT has delivered this intervention process. It is recognised that there may be only a few, if any, opportunities to deliver this aspect of the intervention. Hence, we expect scores to be relatively low for this item.
0 Absence (or very poor delivery) of identity-building interactions.
1 Minimal (or poorly delivered) identity-building interaction.
2 Some identity-building interaction.
3 Several examples of identity-building interaction. However, some difficulties evident (e.g. missed opportunities, talking at odds with the participant).
4 Appropriate use of identity-building interactions, taking almost all opportunities. Minor problems evident.
5 Highly appropriate and sufficient use of identity-building interactions. Minimal problems.
6 Excellent/expert use of identity-building interactions. No real problems.
Core competency 10: managing social influences on smoking reduction
Key features
The HT should encourage the smoker to engage social support (to assist in making or carrying out plans) or manage social influences on smoking behaviour. Social support can be informational (helping to make plans, providing ideas), emotional (not putting pressure on the person to smoke/accepting their decision to cut down or quit) or practical (e.g. helping to monitor progress).
Intervention techniques
The OARS may be used to explore social influences and to identify possible problems and solutions relating to social influences.
Using whole and half numbers, score the level to which you think the HT has delivered this intervention process.
0 Absence (or very poor delivery) of interactions around engaging social support or managing social influences on smoking behaviour.
1 Minimal (or poorly delivered) interaction around engaging social support or managing social influences.
2 Some interaction around engaging social support or managing social influences on smoking behaviour, but not in sufficient depth or detail.
3 Several examples of interaction around engaging social support or managing social influences. However, some difficulties evident (e.g. missed opportunities, talking at odds with the participant).
4 Appropriate use of interactions to engage social support or manage social influences on smoking behaviour, taking almost all opportunities. Minor problems evident.
5 Highly appropriate and sufficient use of interactions to engage social support or manage social influences. Minimal problems.
6 Excellent/expert use of interactions to engage social support or manage social influences on smoking behaviour. No real problems.
Core competency 11: managing social influences on physical activity
Key features
The HT should encourage the smoker to engage social support (to assist in making or carrying out plans) or manage social influences on PA. Social support can be informational (helping to make plans, providing ideas), emotional (not putting pressure on the person to smoke/accepting their decision to cut down or quit) or practical (e.g. helping to monitor progress).
Intervention techniques
The OARS may be used to explore social influences and to identify possible problems and solutions relating to social influences.
Using whole and half numbers, score the level to which you think the HT has delivered this intervention process.
0 Absence (or very poor delivery) of interactions around engaging social support or managing social influences on PA.
1 Minimal (or poorly delivered) interactions around engaging social support or managing social influences.
2 Some interaction around engaging social support or managing social influences on PA, but not in sufficient depth or detail.
3 Several examples of interaction around engaging social support or managing social influences. However, some difficulties evident (e.g. missed opportunities, talking at odds with the participant).
4 Appropriate use of interactions to engage social support or manage social influences on PA, taking almost all opportunities. Minor problems evident.
5 Highly appropriate and sufficient use of interactions to engage social support or manage social influences. Minimal problems.
6 Excellent/expert use of interactions to engage social support or manage social influences on PA. No real problems.
Core competency 12: referral to smoking cessation services
Was the issue of making an attempt to stop smoking raised and the response appropriately addressed (i.e. if desired, to make a referral/support access to NHS Stop Smoking Service or other specialist support available in the local area)?
Yes □ No □
_______________________________________________________________________
Below is some guidance on how these CCs may be scored as part of the research process when session recordings are being reviewed. If it is helpful, a scoring approach could also be used in supervision sessions.
The rating scale
The present 7-point scale (i.e. a 0–6 Likert scale) extends from 0, meaning the HT did not deliver the intervention element appropriately, either they did not do it well or did not do it sufficiently (low fidelity), to 6, meaning the element was delivered appropriately (high fidelity). Thus, the scale assesses a composite of adherence to the intended intervention method and skill of the HT. To aid with the rating of items, an outline of the key features of each item is provided at the top of each section above. A description of the various rating criteria is given in Figure 21. The examples are intended to be used as useful guidelines only, providing illustrative anchor points, rather than prescriptive scoring criteria.
FIGURE 21.
Description of the various rating criteria. The scale incorporates the Dreyfus64 system for denoting competence. Please note that the ‘top’ marks (i.e. near the ‘expert’ end of the continuum) are reserved for those HTs demonstrating highly effective skills, particularly in the face of difficulties (i.e. clients with high resistance to change, high levels of emotional expression and complex situational barriers). Please note that there are six competency levels but seven potential scores.

Adjusting for the presence of participant difficulties
Adjustments may be needed when participant difficulties are evident (e.g. excessive avoidance or resistance). In such circumstances, the rater needs to assess the HT’s therapeutic skills in the application of the methods. Even though the HT may not facilitate change, credit should be given for demonstrating appropriate skilful interaction.
When rating the item, you should first identify whether some of the ‘key features’ are present. If the HT includes most of the key features and uses them appropriately (i.e. misses few relevant opportunities to use them and delivers them well), the HT should be rated very highly. It is also possible that not every item will be applicable in every consultation. It is important to remember that the scoring profile for this scale should approximate to a normal distribution (i.e. mid-point 3), with relatively few scoring at the extremes.
Appendix 2 Information to all intervention and support as usual TARS participants about quitting
Appendix 3 The number and percentage of participants recruited by the different approach methods, by allocated group and overall
| Recruitment method | Group, n (%) | ||
|---|---|---|---|
| SAU (N = 458) | Intervention (N = 457) | Both (N = 915) | |
| General practice postal invite, single letter | 141 (30.8) | 129 (28.2) | 270 (29.5) |
| General practice postal invite, full pack | 124 (27.1) | 141 (30.9) | 265 (29.0) |
| General practice opportunistic invite | 47 (10.3) | 44 (9.6) | 91 (9.9) |
| GP text message invite | 17 (3.7) | 16 (3.5) | 33 (3.6) |
| Community advertisement, local paper, newsletter, invitation from stop smoking services (to those who had previously failed to quit), etc. | 75 (16.4) | 80 (17.5) | 155 (16.9) |
| Community social media or radio | 24 (5.2) | 22 (4.8) | 46 (5.0) |
| Community word of mouth | 17 (3.7) | 11 (2.4) | 28 (3.1) |
| Other | 13 (2.8) | 14 (3.1) | 27 (3.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Appendix 4 The CONSORT flow diagram: breakdown of reasons for exclusions and losses to follow-up
| Reason | People (n) |
|---|---|
| Smoke < 10 cigarettes per day | 70 |
| Unable to give informed consent | 3 |
| Smoker wanted to quit | 2 |
| Unable to walk unaided for > 15 minutes | 2 |
| Unable to engage (language) | 1 |
| Total | 78 |
| Reason | Participants (n) |
|---|---|
| Smoke < 10 cigarettes per day | 20 |
| Unable to walk unaided for > 15 minutes | 14 |
| Smoker wanted to quit | 4 |
| Unable to engage (language) | 3 |
| Unable to give informed consent | 3 |
| Injury/illness may be exacerbated | 1 |
| Aged < 18 years | 1 |
| Total | 46 |
| Reason | Participants (n) |
|---|---|
| Unable to give informed consent | 8 |
| Smoke < 10 cigarettes per day | 1 |
| Unacceptable level of risk to HT | 1 |
| Total | 10 |
| Reason | Participants (n) |
|---|---|
| Declined | 5 |
| Non-contactable | 4 |
| Ineligible: smoke < 10 cigarettes per day | 1 |
| Two expressions of interest registered for the same individual (in error); one expression of interest withdrawn | 1 |
| Total | 11 |
| Reason | Group (n) | ||
|---|---|---|---|
| SAU (N = 458) | Intervention (N = 457) | Both (N = 915) | |
| Declined (n = 13) | 3 | 10 | 13 |
|
0 | 4 | 4 |
|
0 | 2 | 2 |
|
2 | 0 | 2 |
|
1 | 1 | 2 |
|
0 | 1 | 1 |
|
0 | 1 | 1 |
|
0 | 1 | 1 |
| Untraceable (n = 2)a | 1 | 1 | 2 |
| Died (n = 1)a | 1 | 0 | 1 |
| Total (% of randomised) | 5 (1) | 11 (2) | 16 (2) |
| 3 months | Group (n) | ||
|---|---|---|---|
| SAU (N = 458) | Intervention (N = 457) | Both (N = 915) | |
| Eligible for CO verification | 13 | 25 | 38 |
| CO verification completed | 8 | 17 | 25 |
| CO verification not completed | 5 | 8 | 13 |
|
5 | 4 | 9 |
|
0 | 4 | 4 |
| Reason | Group (n) | ||
|---|---|---|---|
| SAU (N = 458) | Intervention (N = 457) | Both (N = 915) | |
| Declined (n = 23) | 18 | 5 | 23 |
|
7 | 2 | 9 |
|
2 | 1 | 3 |
|
3 | 0 | 3 |
|
2 | 0 | 2 |
|
2 | 0 | 2 |
|
0 | 1 | 1 |
|
1 | 0 | 1 |
|
1 | 0 | 1 |
|
0 | 1 | 1 |
| Total (% of randomised) | 18 (4) | 5 (1) | 23 (3) |
| 9 months | Group | ||
|---|---|---|---|
| SAU (N = 458) | Intervention (N = 457) | Both (N = 915) | |
| QBs received, n (% of randomised) | 285 (62) | 289 (63) | 574 (63) |
| QBs received, n (% of QBs sent) | 285 (66) | 289 (66) | 574 (66) |
| Eligible for CO verification, n (% of QBs received) | 34 (12) | 36 (12) | 70 (12) |
| CO verification completed,a n (% of eligible) | 22 (65) | 26 (72) | 48 (69) |
| CO verification not completed (N = 22) (n) | 12 | 10 | 22 |
|
11 | 9 | 20 |
|
1 | 1 | 2 |
| Reason | Group (n) | ||
|---|---|---|---|
| SAU (N = 458) | Intervention (N = 457) | Both (N = 915) | |
| No self-reported quit attempt at 9 months (N = 791) | 393 | 398 | 791 |
|
250 | 262 | 512 |
|
143 | 136 | 279 |
| Quit attempt reported, but restarted smoking prior to CO verification visit (N = 22) | 12 | 10 | 22 |
| Quit attempt reported, but ≥ 10 p.p.m. of CO at the 9-month CO verification visit (N = 1) | 1 | 0 | 1 |
| Declined (N = 3) | 2 | 1 | 3 |
|
1 | 1 | 2 |
|
1 | 0 | 1 |
| Untraceable (N = 11)a | 5 | 6 | 11 |
| Total (% of randomised) | 413 (90) | 415 (91) | 828 (90) |
| 15 months | Group | ||
|---|---|---|---|
| SAU (N = 458) | Intervention (N = 457) | Both (N = 915) | |
| QBs received, n (% of randomised) | 19 (4) | 20 (4) | 39 (4) |
| QBs received, n (% of QBs sent) | 19 (86) | 20 (77) | 39 (81) |
| Eligible for CO verification, n (% of QBs received) | 14 (73) | 18 (90) | 32 (82) |
| CO verification completed,a n (% of eligible) | 8 (53) | 13 (68) | 21 (62) |
| CO verification not completed (N = 11) | 6 | 5 | 11 |
|
3 | 1 | 4 |
|
2 | 2 | 4 |
|
1 | 1 | 2 |
|
0 | 1 | 1 |
Appendix 5 The number and percentage of participants recruited for the two stratification variables (dichotomised Heaviness of Smoking Index and recruiting site), by allocated group and overall
| Stratification variable | Group, n (%) | ||
|---|---|---|---|
| SAU (N = 458) | Intervention (N = 457) | Both (N = 915) | |
| HSI ordinal level | |||
| Low HSI strata | |||
| 0 | 15 (3.3) | 17 (3.7) | 32 (3.5) |
| 1 | 39 (8.5) | 36 (7.9) | 75 (8.2) |
| 2 | 69 (15.1) | 73 (16.0) | 142 (15.5) |
| 3 | 150 (32.8) | 146 (31.9) | 296 (32.3) |
| 4 | 102 (22.3) | 99 (21.7) | 201 (22.0) |
| High HSI strata | |||
| 5 | 69 (15.1) | 54 (11.8) | 123 (13.4) |
| 6 | 14 (3.1) | 32 (7.0) | 46 (5.0) |
| HSI | |||
| Low | 375 (81.9) | 371 (81.2) | 746 (81.5) |
| High | 83 (18.1) | 86 (18.8) | 169 (18.5) |
| Site | |||
| Plymouth | 124 (27.1) | 123 (26.9) | 247 (27.0) |
| Nottingham | 83 (18.1) | 83 (18.2) | 166 (18.1) |
| London | 142 (31.0) | 143 (31.3) | 285 (31.1) |
| Oxford | 109 (23.8) | 108 (23.6) | 217 (23.7) |
Appendix 6 Consumption of cigarettes, cigars and loose tobacco, and licensed nicotine-containing product use, across time points, by allocated group
| Outcome | Baseline | 3-month follow-up | 9-month follow-up | |||
|---|---|---|---|---|---|---|
| SAU | Intervention | SAU | Intervention | SAU | Intervention | |
| Cigarettes (even a puff) smoked over previous week, n (%) | ||||||
| Yes | 253 (55.2) | 243 (53.2) | 179 (39.1) | 159 (34.8) | 147 (32.1) | 161 (35.2) |
| No | 139 (30.3) | 134 (29.3) | 62 (13.5) | 91 (19.9) | 74 (16.2) | 82 (17.9) |
| Missing | 66 (14.4) | 80 (17.5) | 217 (47.4) | 207 (45.3) | 237 (51.7) | 214 (46.8) |
| How many cigarettes smoked over previous week | ||||||
| n | 250 | 238 | 178 | 158 | 147 | 159 |
| Mean (SD) | 17.6 (8.2) | 17.5 (9.3) | 15.1 (11.0) | 9.9 (8.0) | 14.1 (9.3) | 11.0 (9.9) |
| Median (minimum, maximum) | 16.0 (2.0, 80.0) | 15.0 (0.0, 80.0) | 15.0 (1.0, 100.0) | 8.0 (0.0, 50.0) | 12.0 (1.0, 60.0) | 10.0 (0.0, 60.0) |
| Cigars (even a puff) smoked over previous week, n (%) | ||||||
| Yes | 2 (0.4) | 7 (1.5) | 3 (0.7) | 8 (1.8) | 1 (0.2) | 5 (1.1) |
| No | 295 (64.4) | 288 (63.0) | 170 (37.1) | 194 (42.5) | 171 (37.3) | 183 (40.0) |
| Missing | 161 (35.2) | 162 (35.4) | 285 (62.2) | 255 (55.8) | 286 (62.4) | 269 (58.9) |
| How many cigars smoked over previous week | ||||||
| n | 2 | 7 | 3 | 9 | 1 | 5 |
| Mean (SD) | 10.0 (0.0) | 6.7 (4.3) | 3.0 (2.6) | 1.6 (1.2) | 5.0 | 7.2 (5.0) |
| Median (minimum, maximum) | 10.0 (10.0, 10.0) | 10.0 (1.0, 10.0) | 2.0 (1.0, 6.0) | 1.0 (0.0, 4.0) | 5.0 (5.0, 5.0) | 7.0 (2.0, 14.0) |
| Smoked (even a puff) loose tobacco, n (%) | ||||||
| Yes | 220 (48.0) | 235 (51.4) | 132 (28.8) | 141 (30.9) | 114 (24.9) | 110 (24.1) |
| No | 143 (31.2) | 136 (29.8) | 109 (23.8) | 125 (27.4) | 121 (26.4) | 131 (28.7) |
| Missing | 95 (20.7) | 86 (18.8) | 217 (47.4) | 191 (41.8) | 223 (48.7) | 216 (47.3) |
| Loose tobacco smoked (g) | ||||||
| Participants (n) | 217 | 231 | 129 | 137 | 112 | 106 |
| Mean (SD) | 7.2 (5.4) | 8.0 (8.4) | 20.3 (23.2) | 14.2 (13.8) | 15.8 (14.9) | 17.1 (16.4) |
| Median (minimum, maximum) | 6.3 (1.0, 60.0) | 6.7 (0.5, 75.0) | 10.0 (0.5, 150.0) | 10.0 (1.0, 60.0) | 10.0 (0.5, 60.0) | 10.0 (0.5, 75.0) |
| Cigarettes, cigars or loose tobacco (even a puff) smoked over previous week, n (%) | ||||||
| Yes | 458 (100.0) | 457 (100.0) | 287 (62.7) | 278 (60.8) | 242 (52.8) | 249 (54.5) |
| No | 0 (0.0) | 0 (0.0) | 16 (3.5) | 36 (7.9) | 40 (8.7) | 46 (10.1) |
| Missing | 0 (0.0) | 0 (0.0) | 155 (33.8) | 143 (31.3) | 176 (38.4) | 162 (35.4) |
| LNCP use, n (%) | ||||||
| Yes | 59 (12.9) | 69 (15.1) | 113 (24.7) | 125 (27.4) | 96 (21.0) | 114 (24.9) |
| No | 398 (86.9) | 386 (84.5) | 175 (38.2) | 171 (37.4) | 172 (37.6) | 156 (34.1) |
| Missing | 1 (0.2) | 2 (0.4) | 170 (37.1) | 161 (35.2) | 190 (41.5) | 187 (40.9) |
Appendix 7 Indication (use vs. non-use) of cigarettes, cigars, loose tobacco and licensed nicotine-containing products across time points, by allocated group
| Outcome | Baseline | 3-month follow-up | 9-month follow-up | |||
|---|---|---|---|---|---|---|
| SAU | Intervention | SAU | Intervention | SAU | Intervention | |
| Cigarettes (even a puff) smoked over previous week | ||||||
| Yes, n (%) | 253 (64.5) | 243 (64.5) | 179 (74.3) | 159 (63.6) | 147 (66.5) | 161 (66.3) |
| No, n (%) | 139 (35.5) | 134 (35.5) | 62 (25.7) | 91 (36.4) | 74 (33.5) | 82 (33.7) |
| Missing (n) | 66 | 80 | 217 | 207 | 237 | 214 |
| Cigars (even a puff) smoked over previous week | ||||||
| Yes, n (%) | 2 (0.7) | 7 (2.4) | 3 (1.7) | 8 (4.0) | 1 (0.6) | 5 (2.7) |
| No, n (%) | 295 (99.3) | 288 (97.6) | 170 (98.3) | 194 (96.0) | 171 (99.4) | 183 (97.3) |
| Missing (n) | 161 | 162 | 285 | 255 | 286 | 269 |
| Smoked (even a puff) loose tobacco | ||||||
| Yes, n (%) | 220 (60.6) | 235 (63.3) | 132 (54.8) | 141 (53.0) | 114 (48.5) | 110 (45.6) |
| No, n (%) | 143 (39.4) | 136 (36.7) | 109 (45.2) | 125 (47.0) | 121 (51.5) | 131 (54.4) |
| Missing (n) | 95 | 86 | 217 | 191 | 223 | 216 |
| Any cigarettes, cigars or loose tobacco (even a puff) smoked over previous week | ||||||
| Yes, n (%) | 458 (100.0) | 457 (100.0) | 287 (94.7) | 278 (88.5) | 242 (85.8) | 249 (84.4) |
| No, n (%) | 0 (0.0) | 0 (0.0) | 16 (5.3) | 36 (11.5) | 40 (14.2) | 46 (15.6) |
| Missing (n) | 0 | 0 | 155 | 143 | 176 | 162 |
| LNCP use | ||||||
| Yes, n (%) | 59 (12.9) | 69 (15.2) | 113 (39.2) | 125 (42.2) | 96 (35.8) | 114 (42.2) |
| No, n (%) | 398 (87.1) | 386 (84.8) | 175 (60.8) | 171 (57.8) | 172 (64.2) | 156 (57.8) |
| Missing (n) | 1 | 2 | 170 | 161 | 190 | 187 |
Appendix 8 Self-reported quit attempts of at least 24 hours in the previous 3 or 6 months (reported at 3 and 9 months), by allocated group
| Self-reported quit attempt (of ≥ 24 hours) | SAU | Intervention |
|---|---|---|
| Reporting a quit attempt since baseline at the 3-month follow-up | ||
| Participants, N,a n (%) | 275, 37 (13.5) | 281, 54 (19.2) |
| Participants, N,b n (%) | 456, 37 (8.1) | 456, 54 (11.8) |
| Reporting a quit attempt in the previous 6 months, at the 9-month follow-up | ||
| Participants, N,a n (%) | 269, 68 (25.3) | 275, 76 (27.6) |
| Participants, N,b n (%) | 451, 68 (15.1) | 450, 76 (16.9) |
Appendix 9 Serious adverse events
| Allocation | Classification | Summary of event | MedDRA System Organ Class | Principal investigator’s assessment of relatedness | Outcome of SAE at time of report |
|---|---|---|---|---|---|
| SAU | Hospitalisation | Hospitalised with cardiac arrest | Cardiac disorders | Unrelated | Recovered |
| Intervention | Hospitalisation | Admission to hospital following cardiac arrest | Cardiac disorders | Unrelated | Recovered |
| Intervention | Significant medical event | Attempted suicide | Psychiatric disorders | Unlikely | Ongoing |
| Intervention | Persistent/significant disability/incapacity | Participant cited a ‘mental breakdown’ and unable to leave the house | Psychiatric disorders | Unlikely | Ongoing |
| SAU | Death | Sudden and unexpected death. Cause of death given as ‘bleed to the brain’ | Nervous system disorders | Unrelated | Death |
| Intervention | Hospitalisation | In hospital for caesarean delivery of twins | Pregnancy, puerperium and perinatal conditions | Unrelated | Recovered |
| Intervention | Hospitalisation | Planned elective surgery for a hernia, which subsequently became infected | Surgical and medical procedures | Unrelated | Recovered |
The chief investigator reviewed 67 participant-reported hospital admissions (62 participants) in the first instance (Table 41). Additional information on the hospitalisation was required for 13 events. None of the 67 events warranted onward SAE reporting in this trial.
| MedDRA System Organ Class (version 22.0) | 3 months | 9 months | Total | ||||
|---|---|---|---|---|---|---|---|
| SAU | Intervention | Total | SAU | Intervention | Total | ||
| Surgical and medical procedures | 3 | 2 | 5 | 4 | 2 | 6 | 11 |
| Investigations | 2 | 2 | 4 | 2 | 1 | 3 | 7 |
| Psychiatric disorders | 3 | 1 | 4 | 2 | 1 | 3 | 7 |
| Injury, poisoning and procedural complications | 2 | 1 | 3 | 2 | 1 | 3 | 6 |
| Infections and infestations | 3 | 1 | 4 | 1 | 1 | 5 | |
| Cardiac disorders | 1 | 1 | 2 | 2 | 1 | 3 | 5 |
| Nervous system disorders | 1 | 1 | 1 | 2 | 3 | 4 | |
| Hepatobiliary disorders | 1 | 1 | 1 | 1 | 2 | ||
| Musculoskeletal and connective tissue disorders | 1 | 1 | 1 | 1 | 2 | ||
| Renal and urinary disorders | 1 | 1 | 1 | 1 | 2 | ||
| Gastrointestinal disorders | 1 | 1 | 2 | 2 | |||
| Psychiatric disorders | 2 | 2 | 2 | ||||
| Social circumstances | 2 | 2 | 2 | ||||
| Unknown | 2 | 2 | 2 | ||||
| Eye disorders | 1 | 1 | 1 | ||||
| Gastrointestinal disorders/investigation | 1 | 1 | 1 | ||||
| Infections and infestations/surgical and medical procedures | 1 | 1 | 1 | ||||
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 1 | 1 | 1 | ||||
| Pregnancy, puerperium and perinatal conditions | 1 | 1 | 1 | ||||
| General disorders and administration site conditions | 1 | 1 | 1 | ||||
| Respiratory, thoracic and mediastinal disorders | 1 | 1 | 1 | ||||
| Vascular disorders | 1 | 1 | 1 | ||||
| Total | 19 | 12 | 31 | 22 | 14 | 36 | 67 |
Appendix 10 Descriptive complier-average causal effect analysis of carbon monoxide-verified 6-month floating prolonged abstinence between 3 and 9 months
| CO-verified 6-month floating prolonged abstinence between 3 and 9 months | Group (n) | |||
|---|---|---|---|---|
| SAU | Intervention non-compliers (fewer than two HT visits) | Intervention compliers (two or more HT visits) | Both | |
| Yes | 4 | 0 | 9 | 13 |
| No | 447 | 103 | 338 | 888 |
| Total | 451 | 103 | 347 | 901 |
Appendix 11 Stratification variables, licensed nicotine-containing product use at baseline and confidence to quit at baseline by allocated group for participants who had carbon monoxide-verified 6-month floating prolonged abstinence between 3 and 9 months
| Variable | Group, n (%) | |
|---|---|---|
| SAU (N = 4) | Intervention (N = 9) | |
| HSI strata | ||
| Low (0–4) | 3 (75) | 9 (100) |
| High (5 or 6) | 1 (25) | 0 (0) |
| Centre | ||
| Plymouth | 2 (50) | 2 (22) |
| Nottingham | 1 (25) | 3 (33) |
| London | 0 (0) | 1 (11) |
| Oxford | 1 (25) | 3 (33) |
| LNCP use at baseline | ||
| No | 3 (75) | 4 (44) |
| Yes | 1 (25) | 5 (56) |
| Confident I can quit smoking, at baselinea | ||
| Strongly disagree | 0 (0) | 1 (11) |
| Disagree | 0 (0) | 0 (0) |
| Neither agree or disagree | 2 (50) | 2 (22) |
| Agree | 0 (0) | 3 (33) |
| Strongly agree | 2 (50) | 3 (33) |
Appendix 12 Summary statistics of continuous modifying variables by allocated group for participants who had carbon monoxide-verified 6-month floating prolonged abstinence between 3 and 9 months
| Variable | Group | |
|---|---|---|
| SAU (n = 4) | Intervention (n = 9) | |
| IMD (derived from postcode), mean (SD) | 8201 (9587) | 17,694 (10,967) |
| Median (minimum, maximum) | 5290 (321, 21,902) | 23,778 (2190, 32,353) |
| Self-reported total weekly minutes of MVPA at baseline, mean (SD) | 394 (282) | 742 (987) |
| Median (minimum, maximum) | 310 (180, 775) | 135 (30, 2520) |
| Confident I can quit smoking, at baseline,a mean (SD) | 4.0 (1.2) | 3.8 (1.3) |
| Median (minimum, maximum) | 4.0 (3.0, 5.0) | 4.0 (1.0, 5.0) |
Appendix 13 Estimated intervention effects on total number of cigarettes smoked daily at 3 and 9 months using pre-truncated data
| Number of cigarettes smoked daily from cigarettes, cigars and loose tobacco | SAU | Intervention | Unadjusted analysis | Adjusted analysisa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | n (%) | Mean (SD) | Mean differenceb (95% CI) | p-value | SAU (n) | Intervention (n) | Mean differenceb (95% CI) | p-value | |
| At 3 months | 283 (61.8) | 30.1 (39.8) | 275 (60.2) | 21.5 (25.1) | –8.60 (–14.15 to –3.04) | 0.002 | 280 | 274 | –8.49 (–13.97 to –3.00) | 0.002 |
| At 9 months | 240 (52.4) | 25.1 (27.2) | 244 (53.4) | 24.0 (30.8) | –1.06 (–6.25 to 4.13) | 0.688 | 237 | 243 | –0.43 (–5.59 to 4.74) | 0.872 |
Appendix 14 Estimated intervention effects on self-reported moderate to vigorous physical activity at 3 and 9 months using pre-truncated data
| MVPA | SAU | Intervention | Unadjusted analysis | Adjusted analysisa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | n (%) | Mean (SD) | Mean differenceb (95% CI) | p-value | SAU (n) | Intervention (n) | Mean differenceb (95% CI) | p-value | |
| Self-reported total weekly minutes of MVPA at 3 months | 300 (65.5) | 356.7 (500.7) | 308 (67.4) | 460.4 (569.8) | 103.72 (18.20 to 189.23) | 0.018 | 300 | 308 | 97.61 (21.27 to 173.94) | 0.012 |
| Self-reported total weekly minutes of MVPA at 9 months | 269 (58.7) | 380.9 (530.5) | 273 (59.7) | 392.5 (501.9) | 11.54 (–75.58 to 98.66) | 0.795 | 269 | 273 | 8.93 (–72.11 to 89.98) | 0.829 |
| Average daily minutes of accelerometer-measured MVPA at 3 monthsc | 51 (11.1) | 77.1 (55.2) | 54 (11.8) | 91.9 (55.2) | 14.80 (–6.58 to 36.18) | 0.173 | 51 | 54 | 15.09 (–7.10 to 37.27) | 0.180 |
| Average daily minutes of accelerometer-measured MVPA at 3 monthsd | 45 (9.8) | 82.4 (53.6) | 42 (9.2) | 95.2 (43.6) | 12.80 (–8.11 to 33.71) | 0.227 | 45 | 42 | 15.20 (–6.33 to 36.72) | 0.164 |
Appendix 15 Estimated intervention effects on reported licensed nicotine-containing product use at 3 and 9 months
| LNCP use | Participants (n) | Outcomes per group, n (%) | Unadjusted analysis | Adjusted analysisa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SAU | Intervention | SAU | Intervention | ORb (95% CI); p-value | Absolute between-group differences in riskc (95% CI); p-value | Relative riskd (95% CI); p-value | ORb (95% CI); p-value | Absolute between-group differences in riskc (95% CI); p-value | Relative riskd (95% CI); p-value | |
| At 3 months | 288 | 296 | 113 (39.2) | 125 (42.2) | 1.13 (0.81 to 1.58); 0.462 | 0.03 (–0.05 to 0.11); 0.461 | 1.08 (0.88 to 1.31); 0.462 | 1.05 (0.74 to 1.49); 0.797 | 0.01 (–0.07 to 0.09); 0.797 | 1.02 (0.85 to 1.23); 0.798 |
| At 9 months | 268 | 270 | 96 (35.8) | 114 (42.2) | 1.31 (0.93 to 1.85); 0.128 | 0.06 (–0.02 to 0.15); 0.127 | 1.18 (0.95 to 1.46); 0.129 | 1.27 (0.88 to 1.83); 0.195 | 0.05 (–0.03 to 0.13); 0.194 | 1.14 (0.93 to 1.40); 0.196 |
Appendix 16 Estimated intervention effects on self-reported body mass index at 3 and 9 months
| Outcome | SAU | Intervention | Unadjusted analysis | Adjusted analysisa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | n (%) | Mean (SD) | Mean differenceb (95% CI) | p-value | SAU (n) | Intervention (n) | Mean differenceb (95% CI) | p-value | |
| BMI at 3 months (kg/m2) | 288 (62.9) | 26.7 (6.1) | 301 (65.9) | 26.1 (5.8) | –0.58 (–1.54 to 0.38) | 0.239 | 284 | 294 | –0.17 (–0.50 to 0.16) | 0.323 |
| BMI at 9 months (kg/m2) | 265 (57.9) | 26.7 (5.9) | 262 (57.3) | 26.4 (6.1) | –0.30 (–1.32 to 0.73) | 0.568 | 263 | 256 | –0.26 (–0.64 to 0.11) | 0.166 |
Appendix 17 Estimated intervention effects on self-reported hours of sleep at 3 and 9 months (truncated and pre-truncated data)
| Outcome | SAU | Intervention | Unadjusted analysis | Adjusted analysisa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | n (%) | Mean (SD) | Mean differenceb (95% CI) | p-value | SAU (n) | Intervention (n) | Mean differenceb (95% CI) | p-value | |
| Truncated data | ||||||||||
| Sleep at 3 months | 278 (60.7) | 6.9 (1.7) | 287 (62.8) | 7.1 (1.6) | 0.19 (–0.08 to 0.45) | 0.168 | 276 | 284 | –0.02 (–0.26 to 0.22) | 0.855 |
| Sleep at 9 months | 247 (53.9) | 6.7 (1.6) | 260 (56.9) | 7.0 (1.8) | 0.26 (–0.04 to 0.56) | 0.084 | 245 | 257 | 0.09 (–0.19 to 0.36) | 0.533 |
| Original data | ||||||||||
| Sleep at 3 months | 287 (62.7) | 6.8 (2.1) | 294 (64.3) | 7.7 (11.5) | 0.89 (–0.46 to 2.24) | 0.194 | 285 | 292 | 0.77 (–0.60 to 2.15) | 0.270 |
| Sleep at 9 months | 258 (56.3) | 6.6 (2.2) | 268 (58.6) | 6.9 (2.3) | 0.30 (–0.08 to 0.68) | 0.124 | 256 | 265 | 0.10 (–0.27 to 0.46) | 0.599 |
Appendix 18 The p-values of interaction effects with allocated group for each prespecified potential baseline moderator
| Self-reported total weekly MVPA | p-value for interaction term (baseline moderator × allocated group) | |||||
|---|---|---|---|---|---|---|
| Self-reported total weekly minutes of MVPA | IMD rank | LNCP use | Confidence to quit | HSI strata | Site | |
| Self-reported abstinence at 3 months | 0.103 | 0.178 | 0.594 | 0.226 | 0.141 | 0.286 |
| Self-reported abstinence at 9 months | 0.085 | 0.997 | 0.317 | 0.720 | 0.240 | 0.531 |
| CO-verified abstinence at 3 months | 0.145 | 0.081 | 0.606 | 0.966 | 0.180 | 0.805 |
| CO-verified abstinence at 9 months | 0.595 | 0.647 | 0.789 | 0.455 | 0.133 | 0.900 |
| CO-verified 6-month floating prolonged abstinence between 3 and 9 months or between 9 and 15 months | 0.423 | 0.787 | 0.605 | 0.657 | 0.342 | 0.732 |
| Reduced smoking by ≥ 50% between baseline and 3 months | 0.568 | 0.409 | 0.312 | 0.750 | 0.194 | 0.424 |
| Reduced smoking by ≥ 50% between baseline and 9 months | 0.479 | 0.569 | 0.292 | 0.213 | 0.951 | 0.580 |
| Number of cigarettes smoked daily from cigarettes, cigars and loose tobacco at 3 months | 0.448 | 0.004 | 0.102 | 0.054 | 0.573 | 0.229 |
| Number of cigarettes smoked daily from cigarettes, cigars and loose tobacco at 9 months | 0.717 | 0.001 | 0.460 | 0.294 | 0.866 | 0.235 |
| LNCP use at 3 months | 0.212 | 0.357 | N/A | 0.111 | 0.825 | 0.030 |
| LNCP use at 9 months | 0.842 | 0.285 | N/A | 0.423 | 0.919 | 0.992 |
| Self-reported total weekly minutes of MVPA at 3 months | N/A | 0.842 | 0.918 | 0.652 | 0.369 | 0.185 |
| Self-reported total weekly minutes of MVPA at 9 months | N/A | 0.958 | 0.169 | 0.999 | 0.451 | 0.471 |
| BMI at 3 months (kg/m2) | 0.073 | 0.921 | 0.211 | 0.669 | 0.039 | 0.950 |
| BMI at 9 months (kg/m2) | 0.791 | 0.269 | 0.346 | 0.107 | 0.061 | 0.412 |
Appendix 21 The TARS process evaluation (internal pilot)
Interviews with health trainers
Eight interviews were conducted with HTs approximately 4 weeks post training (3 days of face-to-face training in Oxford). Some interview questions related to the comprehensiveness of training were not relevant to the two HTs who had not received the full 3 days of training. However, even though one would have preferred the full training, both expressed feeling that the training they had received and the manual were sufficient and acceptable to enable them to start delivery:
And I don’t feel like, although I know I missed the 3 days or whatever it was in Oxford, I don’t feel like I missed anything important because we had that opportunity to go through everything.
HT9
The HTs’ relevant backgrounds and experiences showed them to be more qualified than traditional HTs. There was a range of education at undergraduate, master’s and doctorate levels, and experience of working on other health interventions and for mental health organisations, and two HTs had direct experience of the role. HTs had mixed experience of the use of MI, although mostly it had been either not used or used informally. Whether or not HTs had MI experience, it was described positively, suggesting acceptability.
Themes generated were originally intended to be deductive, as per protocol; however, owing to recruitment delays, HTs had not commenced sessions with participants at the time of interview, and so not all deductive themes could be satisfied at this stage (perceptions of supervision, experience of participant allocation, and perceptions of experience of delivering the intervention including both practical issues and delivery of CCs).
Training
Perception of acceptability
The general feeling from all interviews, whether all 3 days were attended or training had been received in a reduced/other format, was one of positivity, in terms of content, format and feelings of empowerment:
But by breaking it down into, kind of, didactic teaching and, like, Post-it note [3M, Maplewood, MN, USA] exercises as well as the role play, like I said before, is quite good to make it enjoyable as well as memorable by having different activities.
HT3
I’ve learned a lot, it’s just given me confidence to deliver what I was supposed to deliver.
HT2
There were some reports of not knowing what to expect of the training or what the format would be, but this did not seem to affect acceptability of the training itself:
Before I came to the training, I was just thinking ‘how are other people gonna be?’, like ‘is this going to be a formal one?’. Cause for me it’s like a new job and so I thought, ‘oh, meeting at Oxford, is it going to be really strict and formal?’, ‘you have to behave decently’, it was completely opposite but then you’ve got the training you went for, that was gained, but it was just a nice bunch of people where you can just casually talk to as a friend and just ask any questions that you feel like, even if you think it’s a silly question, it’s not, but everything was just really comfortable and beyond my expectations I would say, really good people and just gained good training.
HT2
Perception of utility
There was a range of perceived utility, particularly in relation to the use of Post-it notes for both HT queries and possible questions for participants to use during role play, and the role-play/practical elements generally:
The board of putting all the questions that could be used, I think all the practical stuff in terms of what the sessions are meant to cover, that final day of like rounding everything up, it all kind of made it all come together.
HT1
But just by reading the protocol, it doesn’t make sense to me unless I put it into practice, so there were loads of role-playing and practical kind of activities that really gave me good training because for me, I have [to] practise in order to understand things, so, from my perspective, I thought that was really, really good.
HT2
Receipt of training objectives
When asked, all HTs understood the TARS objectives, including multiple behaviour change (smoking reduction and PA), the emphasis on reducing rather than stopping smoking altogether, and having a person-centred understanding:
So the aim of TARS is to support people to reduce their smoking . . . and kind of changing two behaviours at one time, so it’s a complex intervention, so we want to reduce smoking by increasing physical activity as well, so the two kind of go hand in hand.
HT3
Suggested improvements
Health trainers perceived that it would be easier to suggest improvements once they were able to deliver the intervention to participants, but they were able to offer suggestions for future training. The emphasis on the perception of utility is reflected here in that the suggestions were based mostly around wanting more practical elements incorporated, particularly more examples of how interactions should look, with suggestions of videos, more role-play examples, more time for role play and having more discussion/practical elements:
So I think in the final exercise that we did, there was three of us and the HT was the one encouraged to have a go obviously, and then we went round and got people’s feedback, but I think it would have just felt useful to do it again. You know, because you do it the first time and you’re like, ‘oh god, yeah, I did this and I did this’, and then only one person in the group got to have a go . . . I just think that would have been more useful.
HT4
Manual
Perception of acceptability
Acceptability of the manual was demonstrated by the views of the HTs, with it being described as ‘comprehensive’, ‘instructive’, ‘informative’, ‘logical’, ‘easy to read’ and ‘user friendly’. There were no negative accounts related to acceptability.
Perception of utility
Most HTs had not read the entire manual cover to cover, but had dipped in and out to gain further understanding of certain topics. There was, again, a focus on seeking out practical guidance or having only read it/intending to read it once a participant/practice participant was imminent, with the most useful elements being reported as the worksheets, example questions for participants, case studies, transcripts and ways to stop smoking:
I’ve been looking at the worksheets, I’m planning this week to print some of them out, just so I’ve got some in case people do want to use them. Got a variety of different ones just in case people might find those helpful. So I might make my own kind of pack to take with me when I see people, so I’ve got a variety of things to offer if they want them.
HT5
Suggested improvements
As with training, there was again mention of not being able to suggest improvements before the intervention had really started. However, unlike the training, no suggested improvements were made, perhaps as a result of HTs not having read it all the way through.
Perception of delivering the intervention
Expected participants
All HTs were either awaiting allocation or had been allocated participants but had not yet seen any of them; one had utilised a practice participant, and one was intending to use their partner for practice. Some HTs described other preparation they had attempted before seeing participants, including scoping out locations and making an ‘activities sheet’. Two HTs described discussing ideas and potential problems with other HTs and finding that useful:
I had a discussion with [name] the other here, the HT last week. And we went through things which we thought were going to be the sort of trickiest. So we’ve sort of done a bit of a, sort of, work-through ourselves.
HT8
Practical issues
There was repeated mention of feeling confident and prepared to take on delivery of the TARS intervention, suggesting that empowerment was gained from training and the manual:
I feel kind of quite confident, in terms of going out and seeing people . . . I feel reasonably confident about seeing people, talking to them, opening up the kind of the conversation . . . finding out what they want to do, what they’ve done before, what they feel their strengths are. That sort of thing, I think that should be OK.
HT7
Some HTs also reported feeling uncertain or concerned about some factors of delivery, mostly in relation to the practicalities of sessions with participants:
I need to . . . feel in control in order to be able to kind of give that sense of containment to the participants. So if you’re in a flap because you can’t get into the room you’re supposed to be in or whatever, then it’s not going to create a good sort of first impression. And I guess that’s the issue, certainly for me at the moment, if I was going to see a TARS participant tomorrow, I’d want to know where am I gonna be there? How do I get there? Where am I gonna park?
There were other potential concerns reported, including wanting to achieve clarity with participants, being unsure about the format of later sessions and concern that they might not know what to say. However, these seem to be mostly natural insecurities rather than factors highlighting gaps in training or the manual.
Interviews with intervention participants
Three intervention participants were interviewed towards the end of their HT sessions, something which may have had an impact on memory of initial recruitment and engagement, as reported below. Themes were generated deductively from topics set out in the protocol.
Understanding of what the trial/intervention is about based on the trial information pack materials
Participant responses did not cover this area to a large degree, with answers mostly based on what they had received from their HT, rather than from the trial information pack (TIP). As mentioned previously, this could be because of the timing of interviews; all three had received several HT sessions and so could have been answering based on their knowledge and experience to date, rather than just from TIP content. Of the limited responses that did relate to understanding based on the TIP, one participant had not understood fully (although did find it helpful) and another had still assumed that they would be told to quit. The third had understood the trial better and wanted to cut down smoking but not quit.
Perceptions of materials contained in the trial information pack
There seemed to be mixed opinions about the TIP materials, with views that they were clear and well-presented, but also that there was too much to read and a suggestion that a summary box with key points might have been useful:
It was fine, it was quite clear, the name was good, TARS, it was, you know, tar, you know, which is, we kind of like tar obviously. I think that helps, just in that alone, the graphics, of course we’re living in that kind of world where graphics and advertising is everything. So, yeah, I thought it was fine, it was really good.
Internal pilot phase participant (IP) 2
I think, it’s one of those things, you know where banks have all this cobblers that they write, with their terms and conditions, and they have the summary box. I think the summary box is a good thing, you know, let’s have a summary box which has all the boiled down to the nuggets of what it’s about, and you can read that and be covered. And also you’ve got all the, all the technical details at length.
IP1
Motivation for taking part in the trial
Two of the three participants were attracted to the trial because it was aimed at reducing, but not stopping, smoking:
And at the time, I did want to cut down, rather than give up smoking. And everyone in the medical profession who ever brought up my smoking beforehand were always sort of a little bit judgy, a little bit giving up. And this was the first people who weren’t sort of like that, and I thought, ‘Well actually, I do want to reduce’. But I didn’t want to cease smoking. So that was, that laid behind my motivation.
IP1
Other motivations included receiving a voucher incentive, the possibility of receiving nicotine patches, wanting to be fitter and being attracted by the PA element, and not wanting to let their GP down by not accepting the invitation.
Perceptions of being informed of their allocation to the intervention group
Although understanding appeared somewhat vague for some participants, which again could be a result of the time period between allocation and interview, all three seemed aware of the possibility of being allocated to the control group and did not see it as a barrier to engagement:
And how were you told about your allocation to the group? That you were in the intervention group?
Gosh I don’t remember?
OK, were you aware that there was a chance of not getting the intervention?
Oh yes, I did, I think it was something like 50 : 50.
Right, yep, OK, and how did you feel about that?
Well, I thought that was fine, I thought that was fair.
One participant felt that the voucher incentive was enough to maintain engagement, even if they had been allocated to the control group:
And were you aware when you signed up that there was a 50 : 50 chance that you’d get the health trainer intervention or you’d get control?
Yes, I was. I was made aware. Because I remember commenting to the person, that first person that sort of interviewed me, I basically said, ‘Well if I don’t get the health trainer, then nothing ain’t gonna happen to me’. You know, I remember saying it was very clear to me. That was the case and I’m very pleased and very happy that I got the health trainer, because of the change I’ve been able to make.
Yeah, OK, so did, how did you feel about the chance, that when you signed up for this, that there was a chance that you weren’t going to get anything?
Well, [pause] to be honest if I weren’t going to get anything, I’d have gone through the motions and got my voucher, and at least got something.
Perceptions of engaging with the intervention
Initial engagement
The process of initial engagement appeared acceptable, with participants reporting being invited via their GP surgeries by text or post. All three participants described being very happy with the mutually agreed locations for their HT appointments:
OK, and were you happy to go to the surgery to meet her, or would you have preferred somewhere else?
Yes, very happy, delighted.
Acceptability of core components of the intervention
Participants positively described the core components of the intervention, with an emphasis on liking the person-centred model, the use of techniques and being made aware of their health behaviours:
And . . . I haven’t had to justify myself in any way, shape or form with this trial because I was given the opportunity of participating. I chose to participate, I chose to tell my trainer whatever I wanted to, so I was able to give up, I had control, but I was guided. So it’s been, it’s great.
IP1
And like I say for 1 hour I’m not going to smoke, make a time, like, the next half hour I’m not going to smoke, and try and do that, and I spoke to her about, I did cut down once and I used to avoid one in the morning, half an hour, then an hour the next, and that way I could do like an hour, and then I’d get up without having a cigarette.
IP2
I wasn’t aware that there were some triggers to my smoking, which you know, I could alter.
IP3
Acceptability of materials
There were no negative accounts of materials suggested for smoking or exercise. Two participants had used a pedometer and found it useful for monitoring and increasing their exercise levels. The third participant had used a smoking diary, which they had also found useful:
Yeah, so the, actually the smoking diary you found, you find useful for kind of monitoring yourself?
I think it’s a very useful thing to do. Yeah, and that’s a good skill learnt.
OK, and how do you think that in itself helps you, what kind of?
Because it becomes something you think about for a bit, rather than just do.
Use of other aids to self-regulate smoking
Two participants mentioned other aids to self-regulate smoking: one was using an aid as a substitute for when they are unable to smoke, something which they had discussed with their HT, and the other stated that they were using an ‘anti-smoking pill’, but this seemed unrelated to the intervention.
Interviews with control participants
One control participant from each of the four trial sites participated in an interview about their experience of taking part in the trial.
Understanding of what the trial/intervention is about based on the trial information pack materials
Interview participants spoke about their understanding of aspects of the trial and intervention, although it was not always clear if this understanding was due to their reading and comprehension of the TIP materials. In the case of the following participants, they understood that engaging in PA was a component of the intervention:
Yeah, I mean I did speak to somebody about it, I don’t know if it was yourself or not? But I mean I do do a lot of activity anyway, my husband’s a marathon runner.
010100
I was and I was interested, because I’m someone that does a huge amount of physical activity.
Right.
And yet still carries on smoking.
030126
Well, I thought it would be part of the programme and I was willing to take part in it.
040052
Participants also understood that the intervention was to be delivered one to one and in person:
Um? Well, from what it seemed, you were going to get, like, a one-to-one person, weren’t you?
010100
I know it was going, seeing somebody face to face and then doing like a programme of things to help you stop, and then you go back and see that person again.
Right.
What, what I was led to believe.
020045
One participant spoke of possible confusion about whether or not they would need to be able to walk for at least 15 minutes to be eligible to take part in the trial. It appears that there was a discrepancy between the information provided in the trial materials and that provided on the telephone, which led to the confusion for this participant.
Lack of understanding regarding mobility:
That my mobility isn’t so good.
OK.
I was told that that wasn’t a problem where, obviously is a problem.
OK, can I just ask why you think that?
Why I think it is a problem?
Yes, yeah.
Because you, they said you didn’t have to be able to walk to 15 minutes and, when I was on phone, I was told that you did.
020045
Motivation for taking part in the trial
Control participants suggested a range of reasons for wanting to take part in the trial, and that encouraged them to step forward to participate. One reason stated was that of accountability, with participants being particularly interested in having a practitioner to whom they were accountable to support smoking reduction and/or cessation:
Yeah. That’s right, and I just feel that if, I mean I don’t know how that part of it actually worked, but just say, for example, if you actually report to somebody once a week, once a month, and how many cigarettes have you had. I think in my mind then, that would have made me start reducing again, because I would feel as if I failed if I had to report to somebody, if you know what I mean [laughs].
010100
You know that you’d have like, a one to one where, you know, you’d be monitored. Whereas with no intervention, you’re not monitored so you could smoke, like I said you could smoke a hundred fags a day and, by doing that you actually, and then you could lie, you know.
020045
Control interview participants were also motivated by the offer of support provided by HTs delivering the intervention:
So it was, it was sort of that that got me intrigued.
Yep.
Because I thought, ‘maybe I could do it with that’.
020045
I suppose just somebody to listen, and maybe encourage.
040052
Participants were also drawn to the trial and motivated to take part because they wanted to reduce and/or stop smoking and thought that the intervention offered could help them to achieve this:
I wanted to be a part of the project because I really should give up smoking, had a scare last year, this, I had an enlarged lymph node behind my lungs, I had a really bad chest infection for nearly 10 months.
Right.
And they thought it may have been cancer, which obviously scared me. But, because my willpower is so terrible, I did try to reduce my smoking like, because I’ve smoked for the last 40 years. I’ve reduced my smoking right down, but as soon as I got the all clear, I went back to smoking normal.
Right, OK.
So, but I know, and I know I should really give up. But I’m rubbish [laughs].
010100
Well, I want to pack up smoking.
020045
Well, I suppose looking back to when I first applied, I thought if it was support to cut down, I might even manage to stop.
Right.
I suppose that that is what I was hoping for.
040052
One participant was motivated to take part by the goal-setting to support smoking reduction during the intervention:
Well it would have given me other, there would have been a goal constantly at the forefront of my mind, I suppose.
030126
Finally, one participant spoke positively about the idea of reduction as a novel way of supporting smoking cessation, as they had tried a variety of methods in the past that had not worked for them:
Tried with all the patches, and the tablets and all the rest of it.
Right.
And nothing was working for me, then I thought, ‘Maybe if I cut down, and then cut down more, and then cut down more.’.
Yep.
That that might be the way to go.
020045
Perceptions of materials contained in the trial information pack
Interview participants did not discuss the materials contained in the TIP in detail. In general, participants found the materials clear and easy to understand:
Yeah, no, there was nothing difficult to understand, and when I first read it, and I think, I can’t remember if I’d actually spoken to someone about it?
010100
OK, and what did you think of the information pack? Did you, what did you think about the information, was there anything that you thought could be improved, or anything that you didn’t kind of, when you think back you didn’t really understand?
No, no. I understood it all, but, I just think that having intervention, having someone, you know monitoring you, your smoking was more of a, you know, ‘Let’s do this’.
020045
Perceptions of being informed of their allocation to the control group
All control participants who took part in an interview expressed their disappointment at being allocated to the control group and not receiving the intervention. It was clear from this that participants were aware that they would be allocated to either the control or intervention groups prior to allocation:
It said you would be chosen to be in two different categories, wasn’t it? One is with support and one sort of not. You know?
Yeah.
And I was really disappointed when I got the one without the health trainer support type thing, because that, I know from like losing weight and things before, if I went to a class I know I would stick to it, do you know what I mean?
010100
Um, I was disappointed really, because, you know, I thought it was a bit more like getting me down on the cigarettes, and really it wasn’t.
020045
OK, OK, and how did you feel about being allocated to the control group?
A bit disappointed.
030126
One participant was particularly disappointed by their allocation, which then affected their enthusiasm and motivation to continue their engagement in the trial:
Well initially I was quite enthusiastic until I realised I was going to be in the control group, and then I lost interest altogether.
040052
Interviews with decliners
Seven individuals who declined to take part in the trial consented to take part in a short semistructured interview about their perceptions of the trial and the intervention, and their decision not to participate. Two individuals each were interviewed from Plymouth, London and Oxford, and one from Nottingham. Data were coded deductively under each of the objectives presented in the protocol. Themes are presented with reference to illustrative quotations.
Understanding of the trial
Most interview participants understood that the focus of the trial was on reducing smoking:
Um? It was more about, not the fact this, this just calling it quits and quitting smoking straight away, it was more about the, if you wanted to do it gradually and you wanted the help.
030000
Others recognised that it could also support quitting smoking:
They would try to help you stop smoking.
030043
Although several participants discussed PA as a component of the trial, only one described the intervention as using PA to support smoking reduction:
It’s . . . it’s a programme via physical exercise you might be able to reduce your smoking.
010057
Two participants perceived the intervention as providing something more than usual care in terms of health/stop smoking services, although one participant thought that group work was an element of the intervention:
They had group work and things like that, and that might be more supportive than just seeing or the, the, the nurse you know, or trying to do it on your own.
030043
And, I thought, ‘This is something totally different’, which is lovely.
010047
Barriers to taking part in the trial
Participants discussed a range of barriers to, or reasons for not, taking part in the trial. Current smoking behaviour was the primary reason why some of the seven participants made the decision to decline to take part. Some participants did not currently smoke a sufficient number of cigarettes to meet the threshold set for the trial (≥ 10).
But it was just the fact that, mean, I’ve only ever smoked an average of maybe 10 cigarettes a day, it’s not worse than that, I think so my, I smoked about five when I smoked . . ..Um, on occasion maybe seven, so it felt kinda, you know, a bit difficult . . ., I mean I, wouldn’t that must I think [inaudible 1.14].
030043
Yeah, so you smoke fewer than 10 a day, is that it?
Um, one or two or . . .
Yeah.
Never.
020074
So yeah, that’s why I didn’t have an issue with this sheet, with this sheet now, I’m just scanning back through it, because there was no real reason for me to cut down, because I do that myself.
030000
In addition, one participant stated that they currently smoked cigars rather than cigarettes, and that they declined to participate because the participant information sheet clearly stated cigarettes in the criteria, and did not include cigars:
Right? Now it goes on to say, ‘The number of cigarettes they smoke’.
Yeah.
I don’t smoke cigarettes; I smoke cigars.
010057
Another reason provided for not wanting to participate was the participant’s current health status:
No, and then as I said to you, said to you briefly the other day, I think I’ve been hit with multiple life-changing diagnoses, and so I’ve got no intention and there’s no way I’m going to stop smoking now.
030000
Another participant was concerned about the time commitment required for participation and did not feel that they would be able to spare the time needed:
Yes I do, yes and I thought some of it was quite long and drawn out, you know, over a time period. Which I know it has to be . . . You know, I didn’t want to feel committed to that amount of time.
Lack of clarity in trial information may have contributed to two individuals declining to take part in the trial. One potential participant was under the impression that they would need access to the internet to take part:
But I thought I’ll talk because I have no internet, I’m one of the old-fashioned people, that is really my main reason, I think what you’re doing is wonderful.
010047
This individual was also concerned that the PA component of the intervention would mean they needed to attend a gym, which would not have suited them:
Um, I think that, you know, when it’s just, like, activities . . .
Yeah.
You know, you can expect that, to me that would have been like a gym thing.
OK.
And people who are slow haven’t got that much lung capacity.
Yeah.
If anything, I thought it would be rigorous.
Yeah.
Yes, that was kind of a little bit off-putting.
010047
Furthermore, on reading the participant information sheet, one interview participant did not believe that they met the criteria for the trial:
Right OK, the first thing on the form is the purpose of the study.
Yep.
And it says, ‘You were asked to take part in a research study, if you want to you can cut down’.
Yep.
Well I don’t wish to cut down, I want to give up.
010057
Perceptions of materials
Interview participants were asked for their views about the trial documentation and materials that they were sent about their potential participation in the trial. This included the participant information sheet and informed consent form. In general, interview participants viewed the trial materials positively in terms of the content and levels of information. In particular, participants spoke about clarity of information to enable them to assess if they were eligible to take part and the voluntary nature of the research:
It gave plenty of information.
010047
No, I think the leaflet was quite good, [inaudible 10:52] people who would ask questions about it [inaudible 10:56] I was in the health service until I retired [inaudible 11:04] basically what you’re after. That’s why I’m trying to help.
010057
It, was, there was nothing wrong with the way that it was written.
030000
Um, it was fine.
030043
Yeah, very, very straightforward.
Yeah, OK very straightforward.
That’s when I knew I didn’t fit the criteria, because, you know . . .
It was easy to see what the eligibility criteria was, and you didn’t kind of meet it?
Yeah, exactly.
030043
Yeah, going through, so that’s why I, again, like I said, put myself forward for the interview because I didn’t have an issue with it, it wasn’t in your face, it wasn’t being rammed down your throat, it was just your choice.
030000
One participant perceived sending out the invitation letters through the GP surgery as a waste of resource and another stated that they did not want the letter:
Yeah, it was OK, it was OK, it’s like I say, it’s just more about the resources, and that’s the only thing. I mean I could have, I could have, in what I’m going through at the moment, I could have just put that in the bin.
030000
I didn’t want the letter.
010047
In addition, one participant, perceived that the trial documentation needed more clarity and detail for them to make an informed decision about participation:
It didn’t really say what it would involve, I mean, like, the activity thing.
010047
Perceptions of what would influence them to take part in the trial
There were mixed views about the need for incentives to take part. The following participant spoke about this and voiced the view that if you want to take part and reduce smoking, you should not need a tangible incentive such as shopping vouchers:
I mean, the shopping vouchers, yeah, I mean if people are, they think that’s an incentive, but that wouldn’t be an incentive for me. I think if you want to do it, you should do it without having an incentive.
03000
Another participant felt that the rationale for taking part should be more than only because of health and, furthermore, that the person needs to be ready to make a change:
And it has to be not only health reasons.
030043
No, but also too I think, the person has to be ready.
030043
How to let people know about the trial
Interview participants provided a range of ideas to promote the trial and improve recruitment. One participant felt that it was important to raise awareness of the novel aspects of the trial and the intervention to get people’s attention:
For people, something totally different from what’s on the market now, I expect people would really sit up and think ‘I’m going to give that a try’.
010047
Another participant suggested going into schools, and when reminded about the eligibility criteria, felt that targeting young adults would be beneficial:
I don’t know . . . I mean, it’s strange, the first thing on my mind was about children. Or children and young adults.
010057
Finally, participants also suggested enhancing recruitment in hospitals and GP surgeries by targeting departments in the hospital that people who smoke are more likely to attend, and GP surgeries that have a higher proportion of registered patients who smoke:
Or in, or only around certain departments, to do with where someone is going to have an issue with smoking. Whereas you have to walk through so many corridors to get to the departments, it, it would be something that would need to be quite prevalent in the main areas.
030000
Um, they have a wide range of posters but I think, targeting certain GP practices where there’s a high problem with smokers . . . Certain ones you know have a high problem, with people coming in with, you know, with smoking-related illnesses, and things like that, you know to pull that to target certain people, you know, I suppose they won’t pick up the target, but you know you can . . .
030043
Interviews with practice managers and staff
One PM and one administrator working closely with the PM on recruitment for this project were interviewed from two different localities (Plymouth and London). PMs and their colleagues from other sites were invited to participate, but either declined the invitation or did not respond. Deductive themes from these interviews as per protocol are described in the following sections.
Acceptability and feasibility of using read codes as proxy indicators
Although this was not covered in detail, one interviewee did describe the coding process; neither PM interviewee mentioned that this process was unacceptable or unfeasible:
Yes, we added a code to those that have [already responded], and when we looked at the original search, and we ran it having taken out the coded ones, and I think we lost another one or two, but then just coded it as, ‘Patient participating in a trial’. And took those 18 patients out again.
PM2
Resources needed/burden on practices
Both PM interviewees had consulted with one of their GPs before agreeing that the trial was feasible for them to take on. One interviewee confirmed that the trial was feasible for them and both described that the time burden was not great:
They don’t take long once you’ve got all the criteria and it’s just running it and double-checking that it’s captured, you know, all the information . . . the exclusions, to make sure that none of the exclusions are in there as well, just double-checking it really. I’d say probably 30 minutes.
PM1
Barriers to and facilitators of effective pre screening and working with the research team
Overall, it seemed that the screening/searching process had worked well, with large numbers of patients being contacted based on the inclusion/exclusion criteria in an iterative process of screening refinement:
And then I went back to her with the number of people and we sort of refined that a bit and actually yesterday, this time we sent out text messages to 1029 people.
PM2
Although no barriers to or facilitators of working with the research team specifically were identified in these interviews, some barriers to the process of pre screening were identified: not feeling confident in identifying search criteria as non-clinical staff, patients being missed if the search criteria were not correct, being concerned that practices are setting up their own searches and that this may result in inconsistency, and potential issues with text messages as the form of invitation (e.g. some people do not have mobile phones or have not updated their contact details):
No, no, as long as they’ve got a mobile and they’ve signed up to receive text messaging. I mean, you know for an example, out of probably the first lot, 570-odd, probably only 300 would have text messages, you know, that we could send to, that’s about the average when we’re sending out other things, we do find it’s possibly not 100%, but we will target.
PM1
There was the suggestion by one interviewee that having pre-assigned searches is helpful and saves time; this may be one solution to the search issues mentioned previously:
If you send us a search to work then it makes life very easy for me. If I’ve got to set up the search myself, it’s not my favourite thing to do. And I used to work with the practice manager on that to make sure we get it right, and it would take a little bit longer. But if you send us a search, then it’s a bit of a doddle really.
PM2
Interviews with researchers
Five researchers took part in interviews about recruitment and retention, with representation from all four sites.
Recruitment
Barriers to recruitment
One of the main barriers to recruitment perceived by researchers was not having direct contact with potential participants. Individuals who requested further information, having seen advertisements for the trial on posters or on social media, usually contacted the site research teams via e-mail. Although teams received a large number of expressions of interest, once the trial information had been sent, they did not hear from the majority of individuals again:
It feels to me like [pause] if, it, it feels like the kind of the community stuff and [principal investigator] has already said this anyway about this, you know from EARS, the community stuff, having a poster up just, it’s not personal enough, it’s not a direct enough approach, so then he has to, you know, it’s like the difference between seeing a poster and getting something on your phone. If you saw the poster, it might not have the same impact as getting something on your phone, even though you’re the same person, with the same level of smoking, or the same job, you know.
Research assistant (RA) 1
It’s mainly via e-mail, so we’ll get an e-mail, a lot of them have come from, a lot of them are from daily info[rmation], we get a few from the council, like staff mail-out. And so then I’ll respond to them and send them the patient information leaflet and say, ‘If you’ve got any questions and you’re interested, then get back to me and let me know.’. And then a lot of them just don’t respond, I do try and chase them up. Give them a week or two, and try and chase them up again. But I haven’t, yeah, we don’t get many back once they yeah, after the initial.
RA4
Researchers perceived a difference in conversion to recruitment when they had the telephone number of the individual:
Yeah, I think it might be to do with, well maybe if they saw it on Twitter [Twitter, Inc., San Francisco, CA, USA] or Facebook [Facebook, Inc., Menlo Park, CA, USA], I think, outside of social media as well, so if they’ve seen kind of a poster maybe up somewhere, different groups that aren’t, community groups that aren’t on the Docmail practice routes, people then e-mail us rather than call us, that conversion rate isn’t very good.
RA2
Delays were also viewed as being a barrier to recruitment. Delays occurred at different points in the recruitment process, including during the search process in GP surgeries:
Yeah, yeah, so initially things were delayed because after we did the search at the first practice, we realised that it was kind of excluding a lot of people that would probably be eligible to receive a letter. And so while the search was kind of being looked at again, because we didn’t do any other mail-outs, any other searches at practices in the meantime because we wanted to get the search right. And then since, we’ve kind of, we’re asking the GPs to do their own search now, to kind of do it themselves. But, yeah, so since we’ve been doing that they’ve just not, there is one practice that was supposed to send it out quite a few weeks ago, and they still haven’t done it.
RA3
And between screening and consenting:
Um, and then I had another person who, well the first one I, the first one I did, the first baseline, I’d spoken to him on, I don’t know, the Thursday or whatever, and then would, just, agreed that I’d send him out the information, the PIS [participant information sheet] and then we’d made a time to speak again the following Tuesday or something. And within that time, he’d decided to stop smoking [laughs].
Right.
So by the time we got hold of, you know, actually spoke to him, he had become, he had a [inaudible 5:18], which was kind of frustrating. Sort of made me realise that, you know, we do kind of need to act quite quickly in that, in that respect.
Researchers also noted that potential participants were put off by the time commitment required to take part in the trial:
Yeah, I think it might be because they might have misunderstood what it’s about because when we’re advertising on the poster or on a Tweet, you have a limited amount of, like, text or characters you can use to explain it. And if they haven’t seen a patient information sheet, and when you send it they realise, ‘Oh, it’s not just a survey. It’s actually a bit more of a commitment’. And they’re not [laughs], they’re not ready for that, I had one person, well a few people actually, saying, ‘Can you tell me a bit more about the survey?’. And when you explain it’s kind of, it could be up to 8-week block, it could be even more than 8 weeks if they want to quit smoking and they’re not there after 6 weeks. Yeah, I think it might be too, they don’t appreciate how intensive it could be.
RA2
Researchers also reflected on the number of potential participants who were screened out because of the number of cigarettes they smoked and questioned the need for the threshold of 10 cigarettes a day for participation:
And you know, so, it, it’s, it’s giving that really, like we’re saying about that kind of people who don’t smoke 10 a day, but still maybe, you know, you’re, that they will, if they’ve just read that and have screened themselves out we don’t know that that was the issue, so then we can’t sort of think, Well maybe we should be offering it to people who smoke five a day. Or, as many, you know, because actually I don’t know where that 10 a day came from, do you know where the 10 a day, what’s the principle for the, for it? So, so, you know.
RA1
Researchers were also aware of the added burden of recruitment processes on already busy general practices, including conducting searches and sending Docmail:
I think so, yeah, a lot of them have got back to me and said, ‘Well, sorry we were really busy with X, Y, Z.’. You know, so probably fall into the bottom of their list of priorities when they’ve got all the GP practice related stuff to do really . . . But, they’ve been really helpful re . . . generally, you know, we met one of the practice managers, she arranged a meeting for us to go and have a chat with her, and you know, she arranged for it to be put on their website, chuck a leaflet and things like that, so they’ve been quite good in that respect, but I suppose, you know, if they’re busy then there’s not much that they can do about it.
RA5
Other barriers to recruitment perceived by researchers included the following:
-
wording of trial information, which was not clear enough to ensure that participants had the information required to support a decision to participate
-
lack of patient relationship with GPs, meaning that the letter received from the general practice did not have impact
-
lack of time for potential participants to respond owing to busy lives
-
organisations not allowing use of social media to advertise the trial.
Facilitators of recruitment
Researchers spoke about their experiences of recruitment and the factors that had aided or improved recruitment to the trial. Linked, and in contrast, to the barriers presented previously, researchers viewed having direct contact with potential participants as strengthening their ability to successfully recruit. Although, at this stage, researchers had not examined this specifically, they believed that when they were able to speak to potential participants and answer any queries they may have, participants were less likely to decline to participate and more likely to make the decision to take part:
I think, I don’t know? I don’t know how many, I don’t know how many people have, because I guess then you’re looking at the numbers of people who may have had an initial contact, and then decided against it? But I don’t think there’s very many of those, who’ve kind of had spoken to us and then said, ‘Actually no, this isn’t for me’. I don’t think there have been many of those. I think there’s, it’s more people who haven’t had that kind of, as soon as, I think once we’ve spoken to people if they’re eligible, they’ve gone on to be randomised.
RA2
Not only was a telephone call viewed as harder to ignore, and therefore encouraged a response from potential participants, compared with an e-mail, telephone calls also afforded researchers the opportunity to start to build an initial rapport with potential participants, which facilitated recruitment:
I think it’s, that in that phone you call you can build up that kind of initial rapport with an individual, and you can answer any queries that they might have. And also if you capture them on that first phone call, and you get them to fill out all the baseline information then, kind of, say from start, ‘This phone call could take up to half an hour if you’re eligible to take part, but we agree to ask you a few questions first.’. I just think it makes it just a lot quicker as well, rather than e-mail, you know, you might have to find the time to call them, or you know, you have to chase for a telephone number. So I think it’s like the building rapport with an individual that you have on a telephone call.
RA2
Researchers recognised the impact of having the support of an individual and/or organisation that endorses the trial through letters or by allowing advertisements on websites and newsletters or via staff from that organisation who directly approach potential participants to inform them about the trial and how to participate:
But thinking about, kind of, individuals who are endorsing the study, I don’t really know to be honest? I think it’s, yeah, it’s mainly the GP letters. And kind of the mailings that go out from the university to staff, from the hospital to staff, that’s kind of going out, you know, in the weekly news bulletins. I think that’s helpful as well.
RA2
Yeah, I think so and I guess if I was to bring in, I think [place] they suggested going to Slimming World [Alfreton, UK] and Weight Watchers [WW International, Inc., New York, NY, USA] as well, so we’ve started to do that. And then we said, we asked if we could actually come along and give a talk, or hand out leaflets at the group. But I think the one that we’re actually, the one that we’re dropping leaflets off to today, they wanted to give them out themselves. But then it, then someone else just disseminating our, the research study, and that’s someone that the people they’re disseminating it to trust and you know, they’ve got their rapport with them. So yeah, I think that’s hopefully going to be helpful. We’ll see how many recruits we get through that.
RA2
When I was doing the [name of project], they worked because you were working through a trusted relationship, so, and once you’ve, you know, once you’ve had the buy-in.
RA1
The researchers also talked about how tightening recruitment processes improved and ensured recruitment. For example, one researcher stressed the need for timely response to potential participants enquiring about the trial:
Just the process of thinking about it and making the initial kind of enquiry, they’ve star– . . . they could, potentially have started that process.
Yep.
So capturing them as early as possible is kind of important.
In addition, another researcher spoke about the utility of regular site meetings, not only to keep on top of recruitment, but to discuss different strategies to improve recruitment:
We, yeah, I mean that’s something that we were speaking about, is how these weekly meetings are really helpful, because I know [name] and [name], they’re just really busy people, and they’re on various studies as well. So it kind of keeps them going weekly, and for our topic recruitment every week, and you know, they give out, they give some hints and tips on how things should be done. So weekly meetings are really, really helpful in my opinion.
RA4
Enhancing recruitment
The researchers interviewed discussed a variety of ways in which they felt that recruitment could be enhanced, some of which were about to be implemented at the time of interview. It was suggested that changing the wording on the participant information sheet to provide more clarity about the next stages of the trial and what it expected of participants would help people to make the decision to take part in the trial:
But maybe we’re kind of putting people off there? And maybe we need to do a more generic, ‘Please contact, you know here’s the PIS [participant information sheet], let us know if you’d like to take it forward, or if you’re not eligible’. Maybe if we have a more, uumm, I don’t know, just, well we’re asking them to let us know what they want us to do next, what they want to do next.
Yeah.
So we’re asking for them to tell us what they want to do next, and then we would say, ‘Well, this is what happens after that’. Do you know what I mean? So, we’re giving less information about what we do next, but asking them to tell us what their next steps would be. So, ‘Yes, I want to progress’ or ‘No, I don’t want to progress for this reason’. I don’t know, maybe we just need to.
Linked to the barriers and facilitators sections regarding direct contact, researchers also felt that increasing opportunities for direct contact with potential participants would enhance recruitment:
The kind of gut feeling is that if you could speak to those people, you could at least talk through what, any questions they may have more explicitly than maybe them sending a text or e-mail.
RA1
We’ve had approval from one, so they’ve booked us a slot on Monday the 23rd of April, where we’re going to go down, they said, you know, ‘You can have a table, set up what you want there for the day, and then kind of approach people as they’re coming through’.
Great.
So, I think what we want to try and do is look at areas where we can actually go to people ourselves, and have that chat with them face to face.
OK.
Because, you know, it takes away the effort on their half, with having to kind of look at the posters, make a note of the number, and then getting in touch with us and . . .
Yeah.
This way we can get contact details face to face, we can give them the information face to face, at the same exact time as kind of mailing stuff out to them, and then hopefully follow up with them like that.
Being known and having a presence in the community was also seen as having the potential to enhance recruitment. Not only would this bring researchers into more frequent direct contact with potential participants, but it would also ensure that they stayed informed of potential opportunities and routes for recruitment:
You know, that seems to have been something, it feels like it should, it feels like we should be able to get, you know to do a bit more like in [other site] with their, kind of their hubs, they found . . . that sounds like an ideal place to kind of try and base yourself and it is frustrating because it almost feels like you need a presence in the surgeries, you need, you need to be somebody who is there so that, you know, people can just kind of have a quick chat with you. But we can’t be in all of those places all of the time, it’s just not possible.
RA1
Yes, also yesterday in the morning we spoke to the mental health team here at [hospital], where we’re working, we switched departments, and they gave us new contact details of the department who works with people who are trying to get back in to work. And maybe it’s a good gap for them to be able to join in studies like this, to keep themselves busy, so we’ve got a few contact details out of, we’re trying to get in touch with as well.
RA4
Site teams were starting to put more plans in place to fully utilise social media to enhance recruitment. Researchers recognised the potential of social media to extend the reach of trial recruitment information:
Well it sounds like we could do a lot more with Facebook and with Twitter, and all those sorts of things, so the social media element I think.
RA1
And Facebook is still new for us so we’re still exploring Facebook, maybe creating, like, a community page, because, we don’t, we have an account, and we’ve had quite a number of interest from the account itself. So we’re probably planning to create a community page and seeing if that’s making a difference or if that’s going to boost up the recruitment, or expression of interest. Yep, that’s our plan for now.
RA4
So I think whatever we do has to be something that reaches kind of larger, like a large number of people. So I think like, things like social media could potentially, well they could depending on where you’re putting them and who’s sharing it and stuff, could go out to quite a lot of people.
RA3
Follow-up
Researchers were interviewed early in the recruitment process at a time when participants were only just starting to be followed up. Therefore, researchers’ perspectives on follow-up were based on what they believed might happen, some of which was based on their experience of working on other projects and trials.
Barriers to follow-up
Researchers raised two potential barriers to successful follow-up of participants: first, participants being in the control group and, second, the use of self-completed measures. Researchers were sceptical about participants in the control group, who were not assigned a HT, completing measures at follow-up points. Researchers thought that those in the control group would be less likely to return measures than those in the intervention group because they had not been assigned a HT and would not be as engaged with the trial as a whole because there was less contact with the control group:
So, I wonder what sort of a response rate we’ll get, particularly from those people who haven’t had the health trainer.
RA1
I just, yeah, I worry that people in the control group might have lost all kind of engagement with the study, and might not be as willing to send it back.
RA2
I think particularly, maybe for the people that are not in the intervention group, as they’ve kind of received, you know, letter or e-mail or whatever with some advice in it months ago, they might be a bit less motivated to complete it and send it back.
RA3
Researchers were also concerned that self-completion of measures at a distance, thereby necessitating participants to return the measures by post, may also negatively affect follow-up rates:
Well, um? You, you’d like to think that at least those people who have had the health trainer intervention are, will, will engage with that process. I wonder if we’re going . . . you know, because it’s kind of a, you know, self-completing thing, I think you know, the questionnaires get sent out from CTU [clinical trials unit] and we don’t really have very much involvement, so, I wonder what sort of a response rate we’ll get, particularly from those people who haven’t had the health trainer.
RA1
I, because we’ve, from the beginning have just been doing the baseline questionnaires over the phone, we never posted any out, but I know like some of the other centres had posted them out and then were kind of struggling to chase people up to send them back, and I think again when you’re, if you can just talk to someone and get it done there and then, you’re going to get it rather than rely on them. I mean they might, they might have, they might intend to do it, but you know, just life gets in the way and they get busy.
RA3
Um, yep, I mean there is some barriers to it because, like I said, time, the timings, and just people forgetting, people at 3 months maybe not just interested. I mean things could change within 3 months, after the 8-week sessions, people may forget, yeah pe– . . . I mean, like, practical things, like, possible things, like, you know, papers getting missed out, or, you know, them misplacing the document. I think that rather than, yeah those kind of, just practical possible things that could happen to get from there, so timing and. It’s hard to, it’s hard to even call on the participants over the phone, I guess it would be more harder with the phone, yeah.
RA4
Facilitators of follow-up
Researchers perceived that one of the main facilitators to ensuring completion of measures at follow-up was the shopping voucher incentive, of which participants were made aware at recruitment:
I guess they’ve got the £20 voucher if they complete the second and third.
Yep.
So hopefully that will be an incentive but I think, you know, it may well end up not being used.
One researcher also saw the freepost envelope provided with the pack sent to participants as removing a potential barrier to returning the completed measures:
But I guess, so now they’re sent the questionnaires and they have to fill it out and then post it back, and they’ve got the freepost envelope so hopefully, you know, there’s not that barrier there.
RA2
Enhancing follow-up
Researchers provided a number of ways in which they believed follow-up rates could be enhanced. The most popular method to enhance follow-up links with the previous sections regarding direct contact and how, conversely, lack of direct contact was perceived as a barrier to both recruitment and follow-up. Increasing direct contact with participants, via telephone, was viewed as being able to improve follow-up rates. This was seen as enabling researchers to not only remind participants to complete the measures, but also to answer questions and provide support to complete some or all of the items:
I think it might be worth, that once they’ve got their questionnaire, or kind of week after it’s been sent, or a couple of days after it’s been sent, allow time for it to get there, to then to call the participant and ask if they have any problems or if they’ve got any questions about it, if they want to go through it over the phone. Just kind of giving that support to them and kind of reminding them to fill it out.
RA2
Um? I think phone calls from an RA, from the RA would be a better option from my point of view. Because you have their number, and these people [inaudible 19:11]. I mean they could happen, but you have the number, and give them a ring, and find out. Because why I say that, is that at the beginning of our recruitment we were really struggling to get hold of our baseline questionnaires that we sent out to our participants. The delays were unbelievable, we lost about one or two participants because of that. One or two, maybe three or four participants because of that. Because they just don’t get the time to, you know, fill out the questionnaires and get them back to us, then we switched our approach to us calling them and filling out the questionnaire over the phone, and we never missed out a participant after that. So that’s the only reason why I say the RAs calling the participants would be a better option.
RA4
Working with general practices and other recruitment routes
Researchers had varying levels of experience of working with general practices at the point in the trial when they were interviewed. Some had not had direct involvement in working with practices, but were aware of the work of other members of the team alongside the CRN that was supporting aspects of recruitment:
Not directly, only, not so far no, I think [researcher] went with [principal investigator] to see the other, the two GPs who are already kind of . . .
OK, on board.
They were already on board, yeah, yeah. I’ve had contact with, with, when we went to the CRN, the [area] CRN kind of conference, we spoke to quite a lot of people there and so . . . I spoke to a couple of the GPs, you know, a couple of GPs there, we spoke to, we spoke to someone from [GP surgery] at that, so.
Oh, OK, yep.
But no, I haven’t actually, I, like I say, I’ve kind of met with [name] at the stop smoking service, and [name] who is her boss, but, apart from that I haven’t had any contact with her GPs.
Other researchers had more direct involvement and described the process by which general practices were identified and negotiations took place to decide if the practices or the CRN would conduct the searches and arrange for Docmail to be sent out:
It was, the same, the same procedure, so the CRN, [name] she would go to the practices with [name] and [name]. So if [name] wasn’t available, [name] is, well it would have been [name] because obviously they have full on with all the meetings, so they weren’t always available, then [name] would take them, and go and have a face-to-face meeting with the GP practice manager. And if they’re happy, yes happy to go along, some GP, GP practice managers would say they are happy to do the searching themselves and send the Docmail themselves or, but other GPs may not do that. Sometimes [name] would do the search herself and send the Docmails out. But it was the thing in thinking in terms of recruiting them, I think the first step would be for [name] to find out and if GPs are interested and then she would contact them. And then if they agreed for a face-to-face visit, then [name] or [name] would go with [name] or myself and the other RA and the health trainer would go, and have the face-to-face meeting, if they are interested and happy to go along, and the search would be done and the Docmails would be sent out.
RA4
Recruitment routes other than general practices and social media, discussed previously, that were stated by researchers as having been utilised by site research teams or through which potential participants contacted teams up to the point of interview included:
-
football clubs
-
routine health checks
-
local authorities
-
newsletters and media
-
posters and leaflets in a range of settings
-
stop smoking service
-
trial registry.
Appendix 22 Acceptability of methods: trial summary
The acceptability of a client-centred smoking reduction and physical activity intervention in comparison to usual care
Trainee
Mary George, DPsych Health Psychology, City, University of London.
Academic supervisor
Dr Triece Turnbull, Visiting Lecturer in Health Psychology, City, University of London.
Research team
Professor Adrian H Taylor, Chair in Health Services Research, Peninsula Medical School, Faculty of Health, University of Plymouth (chief investigator and local principal investigator).
Dr Tom P Thompson, Research Fellow, Plymouth University Peninsula Schools of Medicine and Dentistry (intervention lead).
Dr Lynne Callaghan, Senior Research Fellow, Plymouth University Peninsula Schools of Medicine and Dentistry (process evaluation lead).
Background
It is increasingly acknowledged that ‘acceptability’ assessment is an important process when designing, implementing and evaluating health-care interventions. The purpose of this qualitative study was to explore retrospective acceptability of a client-centred smoking reduction and PA intervention plus usual care in comparison with usual care alone, from the perspective of trial participants.
Method
This was a qualitative study, using semistructured one-to-one interviews. Participants were those who had consented to take part in the trial. Participants were recruited from both intervention and control arms using consecutive sampling methods across two sites: St George’s, University of London, and the University of Plymouth. Questions focused on acceptability of what the participant was randomised to receive (intervention or control arm) across the seven components of the theoretical framework of acceptability.
Results
Participants in the intervention arm cited that the trial was acceptable; few participants in the control arm deemed the trial to be unacceptable, and cited that they were left to their own devices and not provided with any follow-up. Furthermore, some participants highlighted that increase of PA had contributed to their reduction of smoking; however, some who were already physically active found it quite hard to judge the change.
Conclusion
Research exploring acceptability in smoking reduction and PA allows health policy-makers and practitioners to implement interventions effectively to encourage long-term behaviour change. This research highlights specific areas where future intervention could be improved. Future research needs to address the barriers to smoking reduction and increasing PA.
Appendix 23 Baseline predictors of intervention engagement
The following table shows selected baseline variables for those who attended fewer than two versus those who attended two or more intervention sessions.
| Baseline variable | Number of HT sessions attended | |
|---|---|---|
| < 2 (n = 106) | ≥ 2 (n = 351) | |
| Age (years), mean (SD) [minimum, maximum] | 47.5 (13.1) [19, 75] | 50.1 (14.4) [18, 81] |
| Self-reported total weekly minutes of MVPA (truncated), mean (SD) [minimum, maximum] | 442.4 (441.5) [0, 1260] | 460.2 (432.3) [0, 1260] |
| Number of HT sessions attended, mean (SD) (% of participants) | ||
| Gender | ||
| Male | 52 (49.1) (24.4) | 161 (45.9) (75.6) |
| Female | 54 (50.9) (22.1) | 190 (54.1) (77.9) |
| HSI strata | ||
| Low (0–4) | 81 (76.4) (21.8) | 290 (82.6) (78.2) |
| High (5 or 6) | 25 (23.6) (29.0) | 61 (17.4) (71.0) |
| Site | ||
| Plymouth | 27 (25.5) (21.9) | 96 (27.4) (78.1) |
| Nottingham | 8 (7.5) (9.6) | 75 (21.4) (90.4) |
| London | 58 (54.7) (40.5) | 85 (24.2) (59.5) |
| Oxford | 13 (12.3) (12.0) | 95 (27.1) (88.0) |
Appendix 24 Delivery fidelity core competency scores by coder and overall
The following table shows the mean (SD) intervention fidelity scores for each CC, overall and by coder.
| Coder | Intervention fidelity CC mean score (SD) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CC1 | CC2 | CC3 | CC4 | CC5 | CC6 | CC7 | CC8 | CC9 | CC10 | CC11 | |
| Coder 1 | 4.5 (1.0) | 4.0 (1.1) | 3.3 (.9) | 3.6 (1.1) | 2.9 (.9) | 3.5 (1.2) | 3.0 (1.1) | 2.6 (1.2) | 2.5 (1.2) | 3.0 (1.4) | 1.7 (1.6) |
| Coder 2 | 4.5 (1.1) | 4.2 (1.3) | 3.4 (1.1) | 3.8 (1.2) | 3.0 (1.1) | 3.6 (1.2) | 2.9 (1.2) | 2.7 (1.2) | 2.6 (1.2) | 3.1 (1.3) | 1.7 (1.7) |
| Overall | 4.5 (1.0) | 4.1 (1.2) | 3.3 (1.0) | 3.7 (1.1) | 2.9 (1.0) | 3.6 (1.2) | 2.9 (1.1) | 2.7 (1.2) | 2.5 (1.2) | 3.1 (1.4) | 1.7 (1.6) |
Appendix 25 Quotations on receipt and enactment fidelity
Quotations from participants regarding intervention receipt fidelity
Quotation 1
I, I realised which ones were, were most important to me and, and most physically needy for me . . . And they were the morning ones. So I didn’t attempt to have a go at them . . . So the ones sort of, in the afternoon when I was kind of maintaining a level of nicotine and those were the first ones and the evening ones, they were the ones I attacked first.
Participant 3, Plymouth, female
Quotation 2
I mean (inaudible, 10.45) friend of mine’s run the London Marathon and stuff and he was like, ‘ooh you should come out running’, I was always like, ‘no, I hate it’ and actually I really enjoyed it.
Participant 23, Oxford, male
Quotation 3
In ways, yeah. Like obviously she didn’t really concentrate on my, on my activity and stuff because that wasn’t an issue.
Participant 9, Nottingham, female
Quotation 4
I’d just say what you’ve got to do is, is do something else other than smoking, either exercise or go for a walk. Try not to, you know, try to be more mindful of when you’re smoking and how you’re smoking.
Participant 22, Oxford, male
Quotation 5
I think in my mind they were two separate things . . .. Yeah, because I have such a strong psychological attachment to, to tobacco . . . And that no amount of physical activity would actually change that.
Participant 10, Nottingham, female
Quotations from participants regarding intervention enactment fidelity
Quotation 6
I probably cut down maybe, a, to half, I was on probably on 20 a day you know, I probably, I’d go down to 10.
Participant 7, Nottingham, male
Quotation 7
So I will, I will be going down to my doctor’s and saying, ‘look, I’ve got this far, yeah, can you give me a prescription for a month’s supply of the 24-hour ones?’, like, do you know?
Participant 15, London, male
Quotation 8
I suppose, yeah, you said about like, you know, you don’t enjoy all of them and I’d, when I was doing my diary I tried to put that in, like the ones that were just, you know, where I’d light one and then just straight away put it out, just thinking ‘why am I doing that?’, ’cause I’m not even enjoying it.
Participant 4, Plymouth, male
Quotation 9
Well I, as, only really the sort of physical activity side of things because as I say I’m not a very physical, you know, I, my job was physical so I, when I finish work the last thing I wanna do is anything more physical.
Participant 4, Plymouth, male
Quotation 10
You know, and, and it, it was another, it’s like another tool, like, you know, you can use . . . And you’re thinking about that rather than thinking about that, like, do you know . . . And it discourages you to go back the way you was, like, do you know?
Participant 16, London, male
Quotation 11
Just booked that Parkrun and I booked meself to do that again. So that was at least once a week. And then weekdays I would just, when I wasn’t working or, well depending on me work then, yeah I would, I would do some exercise rather than sitting in me house, you know, smoking.
Participant 7, Nottingham, male
Quotation 12
And now that I’ve completed TARS I’m probably even more active . . . Because I, I do a little bit more with the, the boys rugby. Both of my sons play rugby as well, so I do a bit more with them.
Participant 23, Oxford, male
Appendix 26 Itemised descriptive data of smoking process measures
| Question | Baseline | 3-month follow-up | Coefficient (difference at 3 months) (95% CI); p-value | ||
|---|---|---|---|---|---|
| SAU | Intervention | SAU | Intervention | ||
| Important I reduce my smoking | |||||
| n | 458 | 456 | 291 | 294 | 0.20 (0.08 to 0.32); 0.001 |
| Mean (SD) | 4.7 (0.5) | 4.7 (0.6) | 4.4 (0.8) | 4.6 (0.6) | |
| Median (minimum, maximum) | 5.0 (2.0, 5.0) | 5.0 (2.0, 5.0) | 5.0 (1.0, 5.0) | 5.0 (1.0, 5.0) | |
| Important I quit smoking | |||||
| n | 458 | 457 | 289 | 291 | 0.06 (–0.08 to 0.19); 0.402 |
| Mean (SD) | 4.2 (1.0) | 4.2 (0.9) | 4.1 (1.0) | 4.3 (0.9) | |
| Median (minimum, maximum) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 5.0 (1.0, 5.0) | |
| Confident I can reduce my smoking | |||||
| n | 458 | 457 | 291 | 291 | 0.80 (0.64 to 0.97); < 0.001 |
| Mean (SD) | 3.6 (1.0) | 3.6 (1.0) | 3.4 (1.1) | 4.1 (1.0) | |
| Median (minimum, maximum) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | |
| Confident I can quit smoking | |||||
| n | 458 | 457 | 289 | 296 | 0.49 (0.33 to 0.65); < 0.001 |
| Mean (SD) | 2.9 (1.1) | 3.0 (1.1) | 2.9 (1.1) | 3.5 (1.1) | |
| Median (minimum, maximum) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | |
| People close to me support me reducing smoking | |||||
| n | 458 | 457 | 287 | 294 | 0.42 (0.25 to 0.59); < 0.001 |
| Mean (SD) | 3.8 (1.3) | 3.7 (1.2) | 3.4 (1.2) | 3.8 (1.1) | |
| Median (minimum, maximum) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | |
| Plans for how much I will smoke | |||||
| n | 458 | 457 | 289 | 291 | Action planning: 1.55 (1.23 to 1.88); < 0.001 |
| Mean (SD) | 2.3 (1.2) | 2.3 (1.2) | 3.2 (1.2) | 4.0 (1.1) | |
| Median (minimum, maximum) | 2.0 (1.0, 5.0) | 2.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | |
| Plans for strategies to reduce my smoking | |||||
| n | 457 | 457 | 286 | 288 | |
| Mean (SD) | 2.5 (1.2) | 2.5 (1.2) | 3.3 (1.2) | 4.0 (1.0) | |
| Median (minimum, maximum) | 2.0 (1.0, 5.0) | 2.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | |
| Plans for what to do if something interferes with my plans | |||||
| n | 458 | 457 | 284 | 285 | Coping planning: 1.34 (1.01 to 1.67); < 0.001 |
| Mean (SD) | 2.1 (0.9) | 2.1 (0.9) | 2.7 (1.1) | 3.4 (1.1) | |
| Median (minimum, maximum) | 2.0 (1.0, 5.0) | 2.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | |
| Plans for coping with possible setbacks | |||||
| n | 458 | 457 | 285 | 287 | |
| Mean (SD) | 2.1 (1.0) | 2.1 (0.9) | 2.8 (1.1) | 3.5 (1.1) | |
| Median (minimum, maximum) | 2.0 (1.0, 5.0) | 2.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | |
| Plans for quitting smoking | |||||
| n | 457 | 457 | 284 | 291 | 0.48 (0.29 to 0.68); < 0.001 |
| Mean (SD) | 2.3 (1.2) | 2.3 (1.1) | 2.9 (1.3) | 3.4 (1.2) | |
| Median (minimum, maximum) | 2.0 (1.0, 5.0) | 2.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | |
| Monitored amount I have smoked | |||||
| n | 458 | 457 | 283 | 293 | Self-monitoring: 0.98 (0.71 to 1.25); < 0.001 |
| Mean (SD) | 2.7 (1.3) | 2.7 (1.2) | 3.4 (1.1) | 4.1 (1.0) | |
| Median (minimum, maximum) | 2.0 (1.0, 5.0) | 2.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | |
| Regularly thought about how much I am smoking | |||||
| n | 458 | 457 | 283 | 291 | |
| Mean (SD) | 4.0 (1.0) | 3.9 (1.0) | 4.2 (0.9) | 4.4 (0.8) | |
| Median (minimum, maximum) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 5.0 (1.0, 5.0) | |
| Frequency of urges to smoke | |||||
| n | 458 | 457 | 289 | 294 | –0.55 (–0.72 to–0.39); 0.001 |
| Mean (SD) | 3.3 (1.1) | 3.2 (1.2) | 3.3 (1.1) | 2.7 (1.1) | |
| Median (minimum, maximum) | 3.0 (0.0, 5.0) | 3.0 (0.0, 5.0) | 3.0 (1.0, 5.0) | 3.0 (0.0, 5.0) | |
| Strength of urges to smoke | |||||
| n | 458 | 457 | 290 | 295 | –0.47 (–0.63 to–0.31); 0.001 |
| Mean (SD) | 3.2 (1.1) | 3.2 (1.1) | 3.3 (1.0) | 2.8 (1.1) | |
| Median (minimum, maximum) | 3.0 (0.0, 5.0) | 3.0 (0.0, 5.0) | 3.0 (0.0, 5.0) | 3.0 (0.0, 5.0) | |
Appendix 27 Itemised descriptive data of physical activity process measures
| Question | Baseline | 3-month follow-up | Coefficient (difference at 3 months) (95% CI); p-value | ||
|---|---|---|---|---|---|
| SAU | Intervention | SAU | Intervention | ||
| Important I am physically active | |||||
| n | 458 | 457 | 302 | 311 | 0.08 (–0.03 to 0.19); 0.158 |
| Mean (SD) | 4.4 (0.8) | 4.3 (0.8) | 4.2 (0.7) | 4.2 (0.8) | |
| Median (minimum, maximum) | 5.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | |
| Confident I can be physically active | |||||
| n | 458 | 457 | 303 | 314 | 0.21 (0.07 to 0.34); 0.002 |
| Mean (SD) | 4.2 (0.9) | 4.1 (0.9) | 3.8 (1.0) | 4.0 (0.9) | |
| Median (minimum, maximum) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | |
| People close support me being physically active | |||||
| n | 457 | 456 | 302 | 311 | 0.13 (–0.02 to 0.27); 0.089 |
| Mean (SD) | 3.8 (1.2) | 3.8 (1.2) | 3.5 (1.1) | 3.7 (1.0) | |
| Median (minimum, maximum) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | |
| Using PA as a way of controlling smoking | |||||
| n | 458 | 457 | 302 | 314 | 0.48 (0.31 to 0.64); 0.001 |
| Mean (SD) | 2.2 (1.2) | 2.1 (1.1) | 2.8 (1.1) | 3.2 (1.1) | |
| Median (minimum, maximum) | 2.0 (1.0, 5.0) | 2.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | |
| Planning when to be physically active | |||||
| n | 458 | 457 | 296 | 311 | Action planning: 1.27 (0.81 to 1.73); < 0.001 |
| Mean (SD) | 3.0 (1.4) | 2.8 (1.3) | 3.3 (1.1) | 3.6 (1.1) | |
| Median (minimum, maximum) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 3.5 (1.0, 5.0) | 4.0 (1.0, 5.0) | |
| Planning where to be physically active | |||||
| n | 458 | 457 | 292 | 308 | |
| Mean (SD) | 3.1 (1.4) | 2.9 (1.3) | 3.3 (1.1) | 3.8 (1.0) | |
| Median (minimum, maximum) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | |
| Planning how often to be physically active | |||||
| n | 458 | 457 | 295 | 311 | |
| Mean (SD) | 3.0 (1.4) | 2.9 (1.2) | 3.2 (1.1) | 3.7 (1.0) | |
| Median (minimum, maximum) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | |
| Planning what I will do if something interferes | |||||
| n | 458 | 457 | 294 | 309 | Coping planning: 0.78 (0.48 to 1.09); < 0.001 |
| Mean (SD) | 2.4 (1.2) | 2.4 (1.1) | 2.8 (1.0) | 3.1 (1.0) | |
| Median (minimum, maximum) | 2.0 (1.0, 5.0) | 2.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | |
| Planning how to cope with possible setbacks | |||||
| n | 458 | 456 | 294 | 309 | |
| Mean (SD) | 2.4 (1.1) | 2.3 (1.1) | 2.8 (1.0) | 3.2 (1.0) | |
| Median (minimum, maximum) | 2.0 (1.0, 5.0) | 2.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | |
| Consistently monitored amount of PA | |||||
| n | 458 | 457 | 294 | 311 | Self-monitoring: 0.85 (0.55 to 1.15); < 0.001 |
| Mean (SD) | 2.7 (1.3) | 2.5 (1.2) | 3.0 (1.2) | 3.5 (1.1) | |
| Median (minimum, maximum) | 2.0 (1.0, 5.0) | 2.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | |
| Regularly thought about how much PA | |||||
| n | 457 | 457 | 291 | 310 | |
| Mean (SD) | 3.2 (1.3) | 3.1 (1.2) | 3.5 (1.1) | 3.8 (1.0) | |
| Median (minimum, maximum) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | 4.0 (1.0, 5.0) | |
Appendix 28 Qualitative study quotations regarding intervention processes
Quotations regarding approaches to smoking reduction
Quotation 1
So I think the idea of being able to reduce rather than quit makes it seem more achievable to the individuals, it’s not kind of, we’re telling them to go cold turkey and that’s quite daunting and quite scary.
HT3
Quotation 2
And they’ve all been, there’s a lot of positivity about it, a lot of real positivity about having support to cut down, not to stop.
HT1
Quotation 3
Yeah, with the, the reduction I’d try and go through kind of, the four main strategies that are in the manual, the kind of planned reductions . . . scheduled reduction. But then often it’s more about, people don’t necessarily stick to those, it’s more about what they, what they’ve come up with or the strategies they’ve, they’re thinking of, kind of like, well I’ve leave my tobacco in a different room or I’ll only smoke outside or I won’t smoke in my car, I won’t take any out with me.
HT8
Quotation 4
Putting a task in, in front of a cigarette was always, like, make, don’t, if I was having a cup of tea I would have a cigarette before and then a cigarette after . . . So make a cup of tea, drink your cup of tea and have that cig, you know, have a cigarette then. Delaying . . . Helped me . . . You know, reduce.
Participant 3, Plymouth, female
Quotation 5
I did it by putting ones off, that was the way I did it. Like instead of thinking, say I was thinking, ‘oh, I want a cigarette now’, I’d think, ‘oh no, I’ll do this first’ and then after I’d done that it’s almost like, well I’ll just do, you know, and keep putting it off, like delaying it . . . And that’s helped.
Participant 5, Plymouth, female
Quotation 6
But, but yeah, I wasn’t tallying anything or, but I know that I was reducing, well I wasn’t smoking as much because the time frames between each cigarette were quite extended.
Participant 12, Nottingham, male
Quotation 7
The, and the other thing was if [name] had said, this week’s target is eight . . . Eight per day that is, then that was the target I had to hit . . . So if I got to seven and it was only eight o’clock in the evening I’d stop.
Participant 17, London, male
Quotation 8
I, I realised which ones were, were most important to me and, and most physically needy for me . . . And they were the morning ones. So I didn’t attempt to have a go at them . . . So the ones sort of, in the afternoon when I was kind of maintaining a level of nicotine and those were the first ones and the evening ones, they were the ones I attacked first.
Participant 3, Plymouth, female
Quotation 9
Yeah, I think the hierarchical paperwork was really handy, really useful.
HT1
Quotations regarding approaches to increasing physical activity
Quotation 10
Yeah, yeah. I’d, it’s, it was sort of like, I was surprised sometimes and I, I became, not obsessive, but sort of like, I was like, ooh, that’s like, so many steps to the school and back and, ooh, if I walk over there and stuff it’s like, oh, it’s that many steps and oh, I haven’t quite hit my target I better run up and down the stairs [laugh].
Participant 2, Plymouth, female
Quotation 11
So, but I, I always set it to see if I could do 7000 steps a day . . . Which I mostly did.
Participant 3, Plymouth, female
Quotation 12
And then having the pedometer as a tool to then give to people . . . Has been, like, a brilliant way to kind of, talk about physical activity.
HT3
Quotation 13
For people who aren’t already very active I find the pedometers a really useful way to do that so that they can clearly see.
HT4
Quotation 14
I think probably first of all with the, with the physical activity I tend to encourage them to monitor the steps, if you see what I mean . . . and see where they go from there. Some people become really like, oh my god, I didn’t realise how few steps I did. Or, oh I could just do this.
HT6
Quotation 15
So it monitored my running and stuff . . . And then the next week I, I just went. It, I didn’t even think about it, I just went . . . And the, and the week after that and, and then all of a sudden I was going, I’m enjoying this, I’m gonna beat my time . . . Or I’m gonna, mm, you know, write down the, the, what I’m doing running and walking better and stuff like this and, yeah, and I actually enjoyed it [laugh].
Participant 23, Oxford, male
Quotation 16
Just getting people to do a small amount. So often it’s been about just getting them to go for a walk. So just doing something small.
HT7
Quotation 17
I tend to try and, and encourage people to start small anyway, if they’re not physically, if they’re not massively physically active I tend to suggest to people that they try small bites first because the mm, if it’s not scary and it’s a small amount then it’s easier to do . . . Which, on some levels is great but the other level doesn’t give you much of a point to drop to if they (inaudible, 34.38) . . . So there’s, there’s definite disadvantages to starting small.
HT6
Quotation 18
And also having ideas about what people’s barriers are to physical activity. So a conversation around that to then discuss strategies to overcome barriers, whether that’d be, they don’t have enough time in the day, or they don’t know where the local yoga classes are. So then kind of, helping them overcome the barriers.
HT4
Quotation 19
But I think what’s also quite interesting is the way that I talk about physical activity and introduce it ’cause lots of people think, ‘ooh the gym, oh no. I can’t do the, I can’t go to the gym, I don’t want to do that’ . . . But it’s about talking about all the other options and actually is really good, really good for the, really good activity.
HT7
Quotation 20
And I was walking [with] friends is what, well I was with a friend as well and he was, he was looking to, to increase his physical activity ’cause he’s slightly overweight, so I was using him as motivation to, to walk as well.
Participant 12, Nottingham, male
Quotation 21
My lad, that was just, come in and asked if I wanted a coffee, like he was really happy that I was doing it and he came out running with me as well . . . Which was good for him as well as me . . . So we did that together, it just kind of became a father and son thing.
Participant 23, Oxford, male
Quotation 22
Yeah, and with physical activity I think a big kind of, confidence factor is about kind of, going along with other people, especially if, yeah, it’s not something they’ve done before, I think people seem a lot more keen if they plan to go to a group or if they plan to do something with friends . . . So it’s about encouraging them to make those kind of plans where they can involve other people . . . if they can.
HT8
Quotation 23
Yeah, so I think with physical activity, that’s more about, I guess it is about the people in their lives but maybe it’s more about engaging with kind of, new people . . . In terms of doing activity maybe in a group of people that they’ve not met before and to kind of encourage that, yeah, them to do something different so they’re not having to do it on their own.
HT8
Quotation 24
So, quite often again that’s a conversation around what people enjoy, to identify something that they’re more likely to engage in.
HT4
Quotation 25
. . . with physical activity kind of, if they’ve never really been active . . . Or it’s about thinking about, kind of, a totally new activity . . . Then sometimes that’s just a case of looking at things as a bit of an experiment and saying ‘well [why] don’t you go along to this class or do this activity and just see what it’s like, just do it the once and see if you like it or not’.
HT8
Quotations regarding multiple behaviour change
Quotation 26
And that’s a funny one because some people are very keen, but some people don’t want to go anywhere near it.
HT7
Quotation 27
So, so sort of, for some people bringing in the physical activity thing was, was really motivating, in the sense of feeling healthy and stuff but it just didn’t work for . . . for everybody, unfortunately.
HT2
Quotation 28
So, if they’re keen, obviously that’s great, so we’ll talk about that but if not, I’ll, I’ll kind of encourage them a little bit, but I guess sometimes there’s only so far I can go, with bringing it up, if people are very resistant . . . Then I just do tend to talk to just focus on the smoking and perhaps the physical activity will naturally come in later on and then we’ll pick up on it then.
HT7
Quotation 29
Well I think you do get fitter, don’t you? Your body probably, and then you realise, you know, smoking’s kind of holding you back from probably being as fit as you could be.
Participant 7, Nottingham, male
Quotation 30
Mm, well the less I smoked the better, you know, easier I found it to exercise and stuff like that, and, you know, and then vice versa, getting a bit more active would keep my mind off not smoking and yeah, so they kind of went hand in hand.
Participant 5, Plymouth, female
Quotation 31
And then, so, and then I lost some weight and it went. So I was so worried about quit, cutting down on smoking and eating more, and putting weight on because I’d cut down on smoking . . . I was so worried about that happening . . . That was the only reason, really, with the activity, that I wanted to kind of increase it a little bit.
Participant 9, Nottingham, female
Quotation 32
So I, I think the two going hand in hand is important. How important is was to me as an individual, potentially less so than others.
Participant 19, Oxford, female
Quotation 33
I think in my mind they were two separate things . . . Yeah, because I have such a strong psychological attachment to, to tobacco . . . And that no amount of physical activity would actually change that.
Participant 10, Nottingham, female
Quotation 34
So, and that’s the funny thing, it’s sometimes I think that there’s a disconnect, even with the people that were doing it. So they could see it as being healthier and stuff, but they still couldn’t quite connect it to the smoking, even if they realised that they were cutting down their smoking . . . kind of thing. So, there sometimes was a bit of, I think, a bit of a disconnect, with people.
HT2
Quotation 35
So I’d say, I’d say the majority of people I’ve talked to are kind of using it as a distraction.
HT3
Quotation 36
And a surprising number of people who have actually started using exercise to stop cravings or to distract themselves . . . And it’s lovely when they do that.
HT6
Quotation 37
I often suggest that people kind of experiment with doing like a short burst of activity before they have a cigarette or when the craving comes and just kind of, being aware of how they then feel after doing that activity and whether they still want to have a cigarette.
HT4
Quotation 38
Or could they do an activity and, like go for a walk first and you know, put, put off having a cigarette, you know, just do that first. ’Cause often we’d talk about putting it off.
HT2
Quotation 39
Well they’re, they’re related because there’s no way that I would even entertain trying to increase my activity, well I couldn’t have done, if I’d have carried on smoking cigarettes . . . That was it . . . (inaudible, 37.05) . . . And I felt it better to do it in two completely separate things . . . rather than try to combine the two.
Participant 6, Plymouth, male
Quotation 40
Well when I was sort of getting a, getting a craving I would like, at the time I could whack music on and dance around and do some exercises and things, which really, sort of, helped ’cause I’d get out of breath and not want one for another hour or so.
Participant 2, Plymouth, female
Quotation 41
Just booked that Parkrun and I booked meself to do that again. So that was at least once a week. And then weekdays I would just, when I wasn’t working or, well depending on me work then, yeah I would, I would do some exercise rather than sitting in me house, you know, smoking.
Participant 7, Nottingham, male
Quotation 42
Just because, you know, when you’re, when you are, when you have taken part in activity, physical . . . activity afterwards, you know, you, you feel cleaner, if you know what I mean, from the inside out . . . And then smoking, you know, you just have that feeling of, of being poisoned. Basically, which you’re looking for, but you know full well is bad for you . . . But I think the more exercise you do the more it reminds your body that actually you know, it’s a, it, it’s a good feeling . . . to, to be fit and healthy.
OK. So it, so it kind of helped in, kind of, make a link between that the, the two behaviours didn’t go well together, would that be –
That’s right, yeah . . . Yeah, yeah, . . . after a while. I think the more, the more physically active you are, you know, the more it reminds you to, you know, to look at your smoking more consciously.
Quotation 43
You know, so, yes, you know, other people may disagree with me, but as far as I’m concerned there is no way I could start leading an active health life with smoking cigarettes, no way.
Participant 6, Plymouth, male
Quotation 44
But that’s where, if someone’s come into the programme and their main focus is ’cause they want to kind of, reduce their smoking and then that conversation about physical activity might come a bit later on in the sessions.
HT4
Quotation 45
So, so, it wasn’t, ’cause we did talk with some people about writing it down, but like I said, it was quite difficult to get people motivated for the exercise stuff. So, so the lady that was doing the planks, she kind of knew that she was doing, doing a 5-minute plank every night . . . So, ’cause she enjoyed it so much she knew she was doing it, kind of, you know . . . So she didn’t feel the need to record it, as such . . . And the people going for walks knew when they were going for walks . . . kind of thing. So it’s a different, it was kind of different.
HT2
Quotation 46
[. . .] think, well it’s a funny one ’cause I probably would have done it slightly differently because I know that with the TARS model it wanted us to look at physical activity as much as really smoking reduction plan . . . kind of, that, that was always the essence I got from, from the training . . . and stuff . . .. But the reality for the vast majority of people we saw is that they just wanted to do the smoking reduction plan and see the physical activity as an add-on, as opposed to the same level of importance.
HT2
Quotation 47
I would say I probably struggled more though with, or rather I’ve, I’ve had more success or less success depending on the, on the person, with persuading people not to walk and smoke . . . which has been, because if you’re gonna use walking . . . and you would not believe the amount of people who walk and smoke.
HT5
Quotation 48
But yeah, I do, I do try and also get them to think about their lungs and how it is to have clean lungs with clean air in . . . which I know is probably a really weird thing to say but it does seem to get them to think that maybe smoking and walking is not the best combination.
HT6
Quotation 49
Yeah, just looking at how it could be different or getting them to kind of see the disparity between, oh well, you’re doing something really active and you’re smoking at the same time, or you’ve just come out of the gym and you’ve lit up a cigarette straight away, what do you think, kind of, yeah, can you see that that’s a bit of a, that doesn’t really go together?
HT8
Quotations regarding progression to cessation
Quotation 50
Now I mean, the one that’s working with my, with my lady who’s (inaudible, 31.14), it was [name]’s suggestion that they try practice days . . . You know, you don’t go for a quit attempt, you have a practice day . . . And see how you find it, and learn from it . . .. And that has helped people because they feel that they can have a go at it without necessarily committing . . . It’s a dip toe in water without sticking your whole, up to your neck in it [. . .] they can try it and you know, have a day without smoking and then, but go back to smoking without feeling like they’ve failed . . .
HT5
Quotation 51
What facilitates someone to quit? Oh, it, in terms of physical things I know quite a lot of participants have found vaping helpful . . . To kind of have, have a substitute initially, and we’ve spoken about obviously coming off that longer term.
HT7
Quotation 52
I think some of it is letting them know that they can stay the same person inside and be healthier and let go. I think quite a lot of smokers feel like if they give up the smoking they’re going to change, their basic . . . personality is going to change, do you, do you know what I mean?
HT6
Quotation 53
I think I have the skills, I think maybe I lack the confidence a bit in kind of, delivering that . . . I think, so I had someone who came into the first session and they’d already cut down to, from 20 to like, five a day . . . which (inaudible, 58.27) they’d managed to do that themselves, and then they were saying that those five, they actually really enjoy smoking those five cigarettes . . . And that’s quite a difficult conversation to have. ’Cause you normally have a conversation about, well, what do you get out of those like, last cigarettes? Why, why is it you’re still smoking them? And this person just really enjoyed them . . . And you don’t want to take away what they’ve done, their, the success they’ve already had in reducing . . . They’re down to five which was incredible, so that, yeah, that was quite difficult.
HT4
Quotation 54
But I think most people are quite reluctant or they’ve had bad experiences with their stop smoking service for whatever reason. And I wonder whether maybe that’s the kind of participants that sign up to the study . . . they want to do something different or something new, maybe they’ve tried the stop smoking service in the past or the different products and it’s not worked for them . . .. So they wanted to try a different angle.
HT8
Quotation 55
I think it’s very different for lots of people so some people it’s really interesting that at the beginning they never even contemplated quitting, it wasn’t on their radar, but as they cut down and down and down, they start to think about it and think, ‘oh well actually, maybe in the near future I’ll be able to make a quit attempt and it won’t be as bad as I thought’.
HT8
Quotation 56
Yeah. At the time, when I was taking part in the study, physically I really wasn’t in the right place for it . . . But it, it has helped, ’cause the stuff that I sort of talked through with [name], it was really helpful and I’m putting, starting to put that into practice now . . . I’ve got the, got the proper motivation for it [. . .] when I think about it, I think, nah, it’s gonna be positive ’cause I’m gonna be able to, I’ll be out on my bike more, I’ll be doing this a bit more, just to distract myself and eventually the, the cravings will go.
Participant 2, Plymouth, female
Quotations regarding other effective intervention components
Quotation 57
The mon–, yeah, the self-monitoring I think, people have said that’s been really useful ’cause for a lot of people, even just doing that and monitoring kind of, a natural week of smoking . . . They’ve realised, ‘oh my goodness, I smoke way [more] than I should’ and even in that first week they’ve started to reduce ’cause they’re very conscious of, yeah, a lot more conscious of when they’re smoking. So just that awareness really of how much they’re actually smoking or how active they actually are . . .
HT7
Quotation 58
I had a, a list on the fridge . . . and I’d, I’d mark each cigarette and the reason why I had it . . . And it worked very well. So even if I was out I would, I would count. It became a, it became a thing with me.
Participant 3, Plymouth, female
Quotation 59
Even by watching my cigarettes, you know, by monitoring, that actually in itself I felt, decreased my smoking because it wasn’t just something I went to every time, it was like, ‘ooh, why are you having this one then?’. It made it a big thing in my head . . . So that, that helped in itself.
Participant 3, Plymouth, female
Quotation 60
And I could see how, you know, I mean some days I’d have a peak but most of, really low and yeah, just visually seeing that would help me.
Participant 5, Plymouth, female
Quotation 61
It was, it was quite nice speaking to the health coach and talking about smoking and it kind of opened up my eyes a little more to, to how much I was smoking and my behaviours and like what, what kind of urged me on to, to smoke and why I might, why I smoke at the time.
Participant 12, Nottingham, male
Quotation 62
And sort of, just not, it was also, she did a lot of sort of like, the language that I, I used. Sort of like, I haven’t failed, I’m not, by having a cigarette I’m not failing myself, I’m not letting myself down, it’s not really bad, as I, sort of, was, sort of, talking to myself about, it was more about, you know, well I haven’t failed, you know, why am I having this cigarette? What is it that I can change next time I want one and things like that, so.
Participant 2, Plymouth, female
Quotation 63
That’s when, you know, I could discuss that with her . . . you know, every week and you know, not beat myself up too much . . . And you know, I would, I’d be honest and say, ‘oh I had one day where, you know, I had 21 and you know, that’s not a, you know, really backwards for me’ . . . and, but I, the, the every week talking about it, it, it, it just made a difference in the sense that I was offloading.
Participant 3, Plymouth, female
Quotation 64
Setting goals, so the SMART goals . . . I think is the most, one of the most helpful things. So, what I find interesting is sometimes people don’t want to set goals . . . especially SMART goals, they say, ‘oh this week I’m just going to try and reduce and see what happens’ and then, especially if they do that for a couple of weeks, I try and come back and say, ‘oh well actually you’ve, you’ve tried to set this goal to reduce for a couple of weeks and it’s not really happening, why do you think that is? What do you think it would be like if you said I was gonna reduce by x amount or I was gonna reduce by doing this? . . . How different do you think it would be?’. And then kind of encouraging them to set a more specific goal . . . And I think that’s really helpful and they find, oh actually, if I set a specific goal, but also if I write it down, kind of, in a session with me . . . Or if they agree to write it down after they’ve finished and kind of revisit it . . . I think that really helps people to make changes.
HT7
Quotation 65
So, yeah, so making achievable goals, so make the SMARTer [sic] goals really, talking to them, I did talk to a few people about SMARTer [sic] goals. And realising about the achievability, your confidence levels about it and setting a realistic goal, a relevant goal to you.
HT2
Quotation 66
Yeah, yeah. So, so with the goals, I think the goal-setting was, was vital in, yeah in, in the whole process, sort of, of confidence and conf–, the goal-setting was the most important for (inaudible, 21.45) my confidence I’d say.
Participant 12, Nottingham, male
Quotation 67
We were always discussing, sort of, hopes for next week or hopes for the sort of month, shooting forwards, that was, that was very much kind of driven by me.
Participant 19, Oxford, female
Quotation 68
A couple of things was actually being in a cinema, for 2 hours . . . There was actually, going shopping and not actually taking my cigarettes with me . . . Because I know I’d only be a couple of hours . . . So, that, that worked.
Participant 22, Oxford, male
Quotation 69
So, rather than putting a cigarette in my mouth as soon as I’ve walked out the door at work I’d walk to my car and get my lunch box and I’d have a sandwich and I’d sit and look at my phone or, you’d get some, [name] said about getting these, I’ve forgot what they’re called now, books that talk to you, you put your earphones in and they read books to you [audiobooks] [. . .] And I would just pass some time and before I knew it I, my break was over and I’d quickly have a cigarette and go in and then I’d, I’d, I’d instantly taken my break down to one . . . instead of three.
Participant 23, Oxford, male
Quotation 70
I guess like, some, something that I’d like, was to, to spend more time with people who, what, weren’t smoking . . . So, so like, l knew like, with my girlfriend [name] she doesn’t like, she didn’t like the smell of smoke so when I’m around her I just automatically, subconsciously just smoke less because if she doesn’t, she doesn’t like the smell of it then . . . I’m gonna smoke less when I’m around her . . .
Participant 12, Nottingham, male
Quotation 71
And facilitators, I think I, often the way that it kind of builds someone’s motivation is whether or not their friends and family have noticed that they’ve reduce[d] their smoking . . . so if they get kind of comments from their colleagues or friends that they look better or, they, yeah, so sometimes I would ask whether kind of, any family and friends have noticed any differences in them . . .. So that, I find, helps increase motivation if they have.
HT4
Quotation 72
I guess again kind of bringing in other people is a real facilitator, it’s kind of, the people around them maybe change their behaviour too or are on board with it . . .
Especially kind of with the smoking, if it’s easier for them to kind of, be a healthier person if they’re spending time I guess with other healthier, like, people that are healthier and not doing, yeah, not smoking or maybe sitting around all day . . . I think that’s a bit easier.
HT8
Quotation 73
What was quite interesting, which helped reinforce identity shifts, was actually those people that had cut down at least by half, quite quickly, had already noticed that they started breathing better . . . That was a big thing for a few people. ‘God, I’m breathing better already’, kind of thing.
HT2
Quotation 74
We, I tried sucking a Polo Mint [Nestlé, Vevey, Vaud, Switzerland], tried having a glass of coconut water. I tried reading for a little bit. I’m sure there was something else we tried as well. But nothing worked . . . It was the nicotine I wanted . . . Once I started using the nicotine inhalers, it was situations like that that they were a really big boon. ’Cause all I needed was a little hit of nicotine.
Participant 20, Oxford, male
Quotation 75
I mean there was a bit of me that was, that was disappointed that it finished . . . But that’s because I’d, you know, I think, me and [name] had almost become friends . . . That’s how it felt to me . . . You know, I trusted her completely. You know, obviously she has to keep a professional direction on that, and I completely get that but she . . . You know, it was made, it was, you know, it was a neutral environment . . . You know, and it was very relaxed. It just, it made you comfortable . . . So there was a bit of disappointment really, ’cause, you know, I, I, I really liked [name] and I wish her all the best and I’m grateful for the time she gave to me . . . ’cause it worked [laugh].
Participant 23, Oxford, male
Quotation 76
Whereas if I was just doing it off my own bat, I wouldn’t have [. . .] But being part of something made me do it . . . I, I always need to be part of a team, I’m a team player rather than trying to do stuff on my own.
Participant 20, Oxford, male
Quotation 77
[. . .] not feeling, you know, mm, you know, the person who’s asking me questions isn’t being condemning of, urh, ‘you’ve let yourself down then, having one’. You know, they’re being supportive, ‘well that’s really good that you left it that period of time and it’s really good that you did that’, sort of thing . . . So, yeah, and it sort of changes your inner language as well ’cause it was sort of like, for me I was like, yeah, it was, it was really good that I left it, you know, 3 hours and didn’t have one, you know, and when I did want one I did a little bit of exercise to prolong it and, you know, it, you know, it was really, it was really helpful.
Participant 2, Plymouth, female
Quotation 78
Yeah, I guess, kind of using reflection as well, as a technique. So with this if someone kind of, has an idea, reflecting it back to them to kind of make sure that I understood what they meant, and they can . . . have the option then to kind of, clarify and yeah. Yeah, I think that’s another one.
HT4
Quotation 79
Yeah, so, if people kind of, come back to me and say, ‘oh well, that didn’t go very well’, everything, yeah, ‘I didn’t achieve anything that I wanted to achieve’, that’s trying to reflect any really small changes and kind of focus on the positives . . .
And, and looking at what, what went well, even if it was one occasion where they didn’t smoke where they wanted to or if it was one day where they achieved what they wanted to, and kind of, reflecting that.
HT8
Quotation 80
And then kind of, imagining them, getting them to imagine how their life would be different or what would be different if they did make those changes, and the benefits of that . . . but also getting them to see, kind of, the difference between where they’re at now, in their life, with their smoking and their activity, and where they’d ideally like to be to kind of, encourage them to see that difference and that discrepancy between what’s happening now and what they’re doing now . . . but what they would rather be doing.
HT7
Quotation 81
[. . .] and trying to roll with resistance with people, which is less threatening often to people than trying to be a bit challenging . . . to them, about, about where they’re, they’re at. So I think that helps with the, the relationship-building . . . element of it.
HT2
Quotation 82
It is completely different . . . And that’s what they like, they like the, ‘I can do it my way’ . . . And this person will help me, rather than tell me how to do it . . . She’ll give me ideas and she’ll give me hints and she’ll give me tips and she’ll say, ‘well maybe that’s gonna make it difficult’, but she won’t tell me what to do.
HT5
Quotation 83
Kind of thing, because I think having somebody to talk to, having somebody that wasn’t gonna judge them, that was just gonna talk to them about stuff.
HT1
Quotation 84
[. . .] where you explore things and where you let them find their own answers is probably likely to be more powerful . . . in terms of making change.
HT1
Quotations regarding barriers and challenges
Quotation 85
In most cases the people around them are supportive, not always in a way that’s helpful to the person [laugh] . . . If you know, well I think the problem is that because they’re people we care about that, if it’s somebody we care we tend to want them to be healthy and just tell them to stop, whereas actually that doesn’t, in most cases, help people . . . So I, I think that understanding, but I, so in that case I will try and help the person understand that the person who is annoying them and pushing their buttons and making them [think] ‘no stuff you, I’m gonna smoke more’, they’re doing it because they’re concerned for them rather than because they want to annoy them.
HT6
Quotation 86
Yeah, and whilst it, I try and reframe that to say, you know, it’s ’cause they care that that’s how they feel but it’s always like, family members and friends get frustrated if they haven’t been smokers themselves and they don’t understand that it’s an addiction and they expect you to be able to change straight away, type of thing . . . So I think, yeah, with smoking reduction they’ve maybe come up against a bit more kind of, animosity in their social support.
HT4
Quotation 87
The barrier, the massive barrier for people, a, I don’t, I don’t, ’cause I don’t always think, a, I was working with some people that were physically very poorly . . . And, and as much they might have said they could have done something to get onto the study it was very evident that they couldn’t.
HT2
Quotation 88
But I think a lot of the time if you can tip them over the balance then, I don’t think, eight sessions is quite, if they’re not quite where they need to be it’s, it’s possibly not quite enough to get them to the point where they’re ready to quit, if you see what I mean.
HT5
Quotation 89
I think, I mean but it, don’t get wrong, being on the phone has worked quite, quite well with a lot of people but you, you can’t pick up on cues and it’s, it’s a bit, I think it’s a bit harder to roll with resistance and stuff over the phone than it is face to face.
HT1
Quotation 90
I do find it hard, especially, I know at the end of sessions I send all participants a letter just to kind of summarise what we’ve been, what we spoke about and kind of, what they’ve achieved . . . And I find it quite hard to, summarise in a way, if they’ve not, it sounds a bit cruel to say but if they’ve not actually changed that much or done that much . . . for whatever reason, but it’s just a case of reinforcing that they have started to make changes or even the fact they’ve thought about making changes and took part in the study.
HT7
Quotation 91
I think sometimes with people that are a bit quieter it can be easy to kind of make suggestions and things that probably they’re not really that interested in . . . So with those kind of people it is a bit more challenging . . . I think that’s the right word, to kind of get them involved and to get them doing what they want to do . . . We’re encouraging them to think of their own ideas.
HT8
Quotation 92
I guess personality has to come into it, and there are some people who you naturally bond, for the want of a better word, with better . . . than others, if you know what I mean . . . And I’m aware that some people I possibly struggle with more than others because I’m not quite so sure what makes them tick and it’s not so easy to then, get them to actively participate.
HT6
Quotation 93
There was one participant in particular that I think we had three or four sessions and the first session she DNAed [did not attend], she didn’t turn up to the GP surgery . . . And she’d told me, ‘oh I’m very anxious, I don’t like going out of the house; can I bring somebody with me?’. So I said, ‘yeah, that’s absolutely fine if you want to bring somebody with you you can’. But then in the call she wasn’t really, she just didn’t care, she just did not care at all. So I could tell with her that, yeah, she probably wasn’t going to and she did in the end turn around and say this is wasting my time, I don’t want to do it, so . . . with her, I could tell.
HT7
Quotation 94
Sometimes I kind of feel like people, not everyone, but some people don’t, they’re not really that bothered about kind of, setting them goals, setting themselves goals. Or sometimes they’re a bit scared to set themselves goals ’cause they don’t want to fail. So again that’s kind of, looking at it as an experiment and just seeing what happens.
HT8
Quotation 95
I think sometimes some people come to you wanting to have, for you to tell them what to do . . . (inaudible, 2.50). And I think that can sometimes be a barrier, if they kind of come to you looking for the answers, like you have a magic pill almost.
HT4
Quotation 96
[. . .] for example if they only do activity with, if they’re, have a very active job that’s kind of their feet doing kind of manual work or whatever where they’re very active . . . And they come home and they don’t wanna do any more activity . . . That’s quite hard.
HT8
Quotations regarding suggested improvements to the intervention
Quotation 97
I, you know, the other negative that I’d ever give about the course was that I actually feel 6 weeks is not long enough and I know that that is essentially the time that has been decided that it takes to break a habit in your mind but I think, I think a 3 months, you know, if it, if it’s going to run to out I, I just feel the 6 weeks is not long enough.
Participant 19, Oxford, female
Quotation 98
I, I’d say it was too short really, I think, maybe . . . that’s also the thing. If it had carried on and embedded itself and I could see, you know, if, over a longer period I might have been able to kind of build on that.
Participant 18, London, female
Quotation 99
I mean maybe, (inaudible, 34.15) maybe to have more, or the option to kind of, you know, if, if you have had like, a little slip-up that you had an option to kind of go back and have a few more, just to kind of reinforce things maybe.
Participant 5, Plymouth, female
Quotation 100
I mean it was only a study, if, if you were sort of doing it live a longer, sort of, a longer period of time . . . Or, sort of thing, maybe, ’cause I think it was, was it weekly that I was speaking with [name], so maybe initially weekly but then go on to sort of monthly or 3-monthly, things like that, just to have that, someone checking in on you, if that . . .
Participant 2, Plymouth, female
Quotation 101
More, more one-to-one face-to-face chats. But that, that’s me as a person, again, like I like to see a face when I’m speaking to someone . . . as opposed to just over the phone. Because I guess like, over the phone you can kind of, you kind of feel like you can get off the hook, so if you don’t meet, meet a goal then you feel like you’re getting away with not meeting a goal. But if you’re one to one and you’re seeing the person that you’re speaking to and you’ve built like, I guess a little, you’ve built rapport with the, with the heath coach, you also feel like you don’t, you’re letting the health coach down as well, but if you’re over the phone you, it’s, yeah, you’re getting away with a bit more so.
Participant 12, Nottingham, male
Quotation 102
Maybe more of a face-to-face thing. I mean it’s alright doing it on the phone or Skype [™ Microsoft Corporation, Redmond, WA, USA] or things like that but . . . I think they’ve lacked the, the, the human touch, the, maybe the empathy kind of thing. You know, actually being, you’re, you’re using, you’re using that hour to, well I know it’s only like, 15 minutes, you know, or, or, when, when she’d ring . . . But if we, if they (inaudible, 43.45) 6, do it over 6 weeks . . . and have an hour face to face . . . sit in a coffee shop or something like that . . . and then we’d discuss things, write things down, you know, get more motivated that way.
Participant 22, Oxford, male
Quotation 103
[Laugh] maybe sort of some vouchers for like, swimming and stuff, so that, you know, could afford to go a bit, bit more or something like that.
Participant 2, Plymouth, female
Quotation 104
I’d say like, so, so maybe like, with the help of like, certain medications, I don’t know (inaudible, 57.04) that could help people. Like, I know that there’s, so if you use it like alongside with, with the whole one-to-one chats and stuff, with the health coach, like if the health coach could maybe explore some sort of like, Nicorette patches [Johnson & Johnson, New Brunswick, NJ, USA] or other vaping methods, so, I don’t know, that could help you reduce smoking . . . if they could use it alongside it then, and then also maintain that support that they, that they’d shown in my time with the TARS, with the health coach then I guess that could possibly, that could possibly help.
Participant 12, Nottingham, male
Quotation 105
So the quit attempt, I think it would have, I think it would be helpful if you were in a position to issue people with aids like the, the nicotine inhalers, rather than people having to find another source of those [. . .] So, I dunno whether it’s something you could maybe do in the future . . . Have these things, even if you had to charge for them. Just having a reliable supply . . . of these things that people can use to help themselves with.
Participant 20, Oxford, male
Quotation 106
I think if next time someone, bring in three or four people, sat down, have a chat, and maybe half hour or so and get these people to, how can I put it? Help each other maybe . . . if, you know, or you know, like, say, because they can see, I mean it’s kind of like a, be, competition, who could do best and it, and it’s for their health anyway, innit, you know . . . So I think, talk, sitting down and talking to her and maybe three other people, four other people, you know. In a room and you know, we all look at how, the best way to try, you know, stop smoking (inaudible, 14.25).
Participant 15, London, male
Quotation 107
It would have been handy to have a leaflet. Just a really little leaflet about a summary over the study and how activity, and what they found already for how physical activity can help cravings.
HT1
Quotation 108
But also, yeah, I think a top-up in terms of, I don’t know how, maybe another day or something, just to go back through everything [. . .] just to remind us all . . . See if we are still on track . . . with where, what we’re meant to be doing.
HT7
Quotation 109
I know everybody hates it, and I would cringe at it as well, but to have a practice with each other . . . sort of, with some scenarios and stuff . . . or maybe do a little revisit. I think it’s a shame that we never met again.
HT1
Quotation 110
I think, again, social influence, influence of other people, I don’t know whether that is just one of the biggest barriers or whether I don’t, maybe, tackle it as well as I could do . . . So maybe some more support around how to help people, especially if, yeah, other people in their household are smokers and aren’t willing to reduce or don’t want to support that person in reducing.
HT7
Quotation 111
Might have been useful to have some as kind of, Skype sessions maybe, to make them a bit more interactive.
HT3
Quotation 112
[. . .] think, at the beginning they’d said that we would listen to our recordings back together, or maybe before a supervision and then discuss it . . . And I think actually, we haven’t done anything like but I think doing something like that would be really helpful so we could reflect altogether . . . and get feedback from everybody else on what we’re doing and if we’re doing it right or what we’re not doing . . . So I think that would have been helpful.
HT7
Appendix 29 Intervention costing details
Table 42 shows how training costs were estimated. Amortisation is the process by which long-term investments are revalued as annual expenditures, taking into account discounting.
| Item | Resource use | Unit cost (£) | Cost (£) |
|---|---|---|---|
| Initial training | |||
| HT being trained | Three HTs × 37.5 hours | 29.43 per hour | 3310.68 |
| Lead trainer | 37.5 hours | 33.83 per hour | 1268.81 |
| Venue opportunity cost | 5 days | 100 per day | 500.00 |
| Materials | 200.00 | ||
| Travel | 50 miles per day (trainer) | 0.20 per mile | 50.00 |
| Course total | 5329.49 | ||
| (Per trainee) | (÷ 3) | 1776.50 | |
| (Annual cost per trainee when amortised over 3 years) | (× 0.345) | 612.65 | |
| (Cost per client) | (÷ 80) | 7.66 | |
| Refresher training | |||
| HT being trained | 7.5 hours | 29.43 per hour | 220.71 |
| (Annual cost per trainee when two refresher trainings amortised over 3 years) | 4.9 hours | 29.43 per hour | 144.60 |
| (Cost per client) | 3.68 minutes | 29.43 per hour | 1.81 |
Table 43 shows the results of sensitivity analyses for the cost of delivering the TARS intervention.
| Sensitivity analysis | Cost per person of delivering the TARS intervention (£) |
|---|---|
| Maximum caseload: 30 | 203.99 |
| HT cost – 10% | 216.76 |
| Contact time 95% lower confidence limit | 228.11 |
| Annual caseload: 160 | 229.18 |
| Senior HT cost – 10% | 238.82 |
| Base case | 239.18 |
| Senior HT cost + 10% | 239.53 |
| Contact time 95% upper confidence limit | 254.01 |
| Annual caseload: 40 | 259.16 |
| HT cost + 10% | 261.59 |
| Maximum caseload: 20 | 291.96 |
Appendix 30 Trial-based cost-effectiveness analysis details
Results
NHS and Personal Social Services resource use
Number of contacts
| Item | SAU group | Intervention group | ||
|---|---|---|---|---|
| Participants (n) | Mean (SD) [range] | Participants (n) | Mean (SD) [range] | |
| Primary care and community services | ||||
| GP at surgery/health centre | 457 | 3.12 (4.36) [0–32] | 457 | 2.85 (3.98) [0–60] |
| GP via telephone | 457 | 0.89 (2.42) [0–30] | 457 | 0.76 (1.77) [0–13] |
| GP at home | 457 | 0.01 (0.11) [0–2] | 457 | 0.12 (2.43) [0–52] |
| Nurse at surgery/health centre | 457 | 1.05 (2.16) [0–24] | 457 | 1.04 (1.88) [0–20] |
| Nurse via telephone | 457 | 0.11 (0.70) [0–10] | 457 | 0.09 (0.49) [0–5] |
| Nurse at home | 457 | 0.00 (0.05) [0–1] | 457 | 0.01 (0.23) [0–5] |
| Physiotherapist at surgery/health centre | 457 | 0.26 (1.27) [0–20] | 457 | 0.24 (0.98) [0–8] |
| Physiotherapist at home | 457 | 0.01 (0.19) [0–4] | 457 | 0.00 (0.09) [0–2] |
| Occupational therapist at surgery/health centre | 457 | 0.03 (0.32) [0–5] | 457 | 0.03 (0.32) [0–6] |
| Occupational therapist at home | 457 | 0.04 (0.59) [0–12] | 457 | 0.00 (0.07) [0–1] |
| Social worker | 457 | 0.21 (1.94) [0–26] | 457 | 0.18 (1.61) [0–26] |
| Care worker | 457 | 1.97 (16.07) [0–182] | 457 | 1.54 (19.86) [0–365] |
| NHS Stop Smoking Service | 457 | 0.06 (0.24) [0–1] | 457 | 0.10 (0.30) [0–1] |
| Walk-in centre | 457 | 0.14 (1.15) [0–20] | 457 | 0.15 (0.58) [0–6] |
| Secondary care services | ||||
| Outpatient attendances | 457 | 1.12 (2.30) [0–20] | 457 | 1.03 (2.05) [0–24] |
| Overnight stays | 457 | 0.10 (0.37) [0–3] | 457 | 0.09 (0.48) [0–7] |
| Inpatient days/nights | 457 | 0.32 (1.69) [0–23] | 457 | 0.41 (2.38) [0–28] |
| A&E attendances | 457 | 0.23 (1.48) [0–30] | 457 | 0.19 (0.81) [0–14] |
| Day-case procedures | 457 | 0.42 (2.84) [0–50] | 457 | 0.36 (2.90) [0–60] |
| Item | SAU group | Intervention group | ||
|---|---|---|---|---|
| Participants (n) | Mean (SD) [range] | Participants (n) | Mean (SD) [range] | |
| Primary care and community services | ||||
| GP at surgery/health centre | 297 | 1.99 (3.14) [0–34] | 314 | 1.65 (2.17) [0–20] |
| GP via telephone | 297 | 0.72 (1.57) [0–15] | 314 | 0.76 (1.81) [0–15] |
| GP at home | 297 | 0.02 (0.20) [0–3] | 314 | 0.00 (0.06) [0–1] |
| Nurse at surgery/health centre | 297 | 0.64 (1.30) [0–10] | 314 | 0.59 (1.50) [0–16] |
| Nurse via telephone | 297 | 0.12 (0.95) [0–15] | 314 | 0.06 (0.34) [0–3] |
| Nurse at home | 297 | 0.02 (0.30) [0–5] | 314 | 0.01 (0.11) [0–2] |
| Physiotherapist at surgery/health centre | 297 | 0.20 (0.69) [0–4] | 314 | 0.14 (0.51) [0–4] |
| Physiotherapist at home | 297 | 0.00 (0.06) [0–1] | 314 | 0.00 (0.00) [0–0] |
| Occupational therapist at surgery/health centre | 297 | 0.09 (0.59) [0–6] | 314 | 0.04 (0.30) [0–3] |
| Occupational therapist at home | 297 | 0.02 (0.17) [0–2] | 314 | 0.04 (0.52) [0–9] |
| Social worker | 297 | 0.13 (0.93) [0–12] | 314 | 0.11 (0.87) [0–10] |
| Care worker | 297 | 0.57 (6.29) [0–90] | 314 | 0.87 (12.04) [0–208] |
| NHS Stop Smoking Service | 297 | 0.11 (0.31) [0–1] | 314 | 0.15 (0.36) [0–1] |
| Walk-in centre | 297 | 0.11 (0.59) [0–8] | 314 | 0.10 (0.45) [0–3] |
| Secondary care services | ||||
| Outpatient attendances | 297 | 0.80 (2.38) [0–24] | 314 | 0.75 (1.65) [0–15] |
| Overnight stays | 297 | 0.08 (0.37) [0–4] | 314 | 0.04 (0.21) [0–2] |
| Inpatient days/nights | 297 | 0.32 (2.54) [0–35] | 314 | 0.11 (0.72) [0–10] |
| A&E attendances | 297 | 0.14 (0.47) [0–3] | 314 | 0.13 (0.54) [0–5] |
| Day-case procedures | 297 | 0.33 (2.00) [0–32] | 314 | 0.21 (0.75) [0–6] |
| Item | SAU group | Intervention group | ||
|---|---|---|---|---|
| Participants (n) | Mean (SD) [range] | Participants (n) | Mean (SD) [range] | |
| Primary care and community services | ||||
| GP at surgery/health centre | 274 | 2.94 (4.69) [0–40] | 280 | 2.21 (2.61) [0–20] |
| GP via telephone | 274 | 1.03 (2.73) [0–30] | 280 | 0.87 (1.61) [0–10] |
| GP at home | 274 | 0.04 (0.44) [0–6] | 280 | 0.00 (0.00) [0–0] |
| Nurse at surgery/health centre | 274 | 1.00 (2.15) [0–20] | 280 | 0.93 (2.08) [0–20] |
| Nurse via telephone | 274 | 0.14 (0.84) [0–10] | 280 | 0.06 (0.41) [0–6] |
| Nurse at home | 274 | 0.00 (0.06) [0–1] | 280 | 0.00 (0.06) [0–1] |
| Physiotherapist at surgery/health centre | 274 | 0.54 (2.34) [0–25] | 280 | 0.46 (1.55) [0–12] |
| Physiotherapist at home | 274 | 0.00 (0.06) [0–1] | 280 | 0.05 (0.72) [0–12] |
| Occupational therapist at surgery/health centre | 274 | 0.14 (0.78) [0–8] | 280 | 0.10 (0.67) [0–8] |
| Occupational therapist at home | 274 | 0.04 (0.34) [0–5] | 280 | 0.10 (1.44) [0–24] |
| Social worker | 274 | 0.22 (1.87) [0–24] | 280 | 0.19 (1.87) [0–30] |
| Care worker | 274 | 0.32 (3.92) [0–64] | 280 | 2.10 (18.29) [0–180] |
| NHS Stop Smoking Service | 274 | 0.14 (0.35) [0–1] | 280 | 0.10 (0.31) [0–1] |
| Walk-in centre | 274 | 0.11 (0.70) [0–8] | 280 | 0.16 (0.67) [0–6] |
| Secondary care services | ||||
| Outpatient attendances | 274 | 1.07 (2.15) [0–16] | 279 | 1.34 (4.92) [0–60] |
| Overnight stays | 274 | 0.13 (0.62) [0–7] | 280 | 0.10 (0.98) [0–16] |
| Inpatient days/nights | 274 | 0.42 (3.07) [0–45] | 280 | 0.10 (0.59) [0–7] |
| A&E attendances | 274 | 0.22 (0.83) [0–8] | 280 | 0.18 (0.52) [0–3] |
| Day-case procedures | 274 | 0.35 (1.30) [0–15] | 279 | 0.32 (1.07) [0–12] |
Costs
| Item | SAU group | Intervention group | ||
|---|---|---|---|---|
| Participants (n) | Mean (SD) [range] | Participants (n) | Mean (SD) [range] | |
| Primary care and community services | ||||
| GP at surgery/health centre | 457 | 122.29 (170.85) [0–1254] | 457 | 111.65 (155.90) [0–2351] |
| GP via telephone | 457 | 13.79 (37.58) [0–466] | 457 | 11.75 (27.46) [0–202] |
| GP at home | 457 | 0.69 (9.03) [0–158] | 457 | 9.15 (192.00) [0–4104] |
| Nurse at surgery/health centre | 457 | 13.08 (26.91) [0–298] | 457 | 12.92 (23.38) [0–249] |
| Nurse via telephone | 457 | 0.84 (5.44) [0–78] | 457 | 0.73 (3.81) [0–39] |
| Nurse at home | 457 | 0.09 (1.86) [0–40] | 457 | 0.43 (9.28) [0–198] |
| Physiotherapist at surgery/health centre | 457 | 16.38 (79.69) [0–1258] | 457 | 15.28 (61.93) [0–503] |
| Physiotherapist at home | 457 | 0.69 (12.13) [0–252] | 457 | 0.28 (5.88) [0–126] |
| Occupational therapist at surgery/health centre | 457 | 2.55 (26.29) [0–416] | 457 | 2.55 (26.86) [0–499] |
| Occupational therapist at home | 457 | 3.28 (48.85) [0–998] | 457 | 0.36 (5.50) [0–83] |
| Social worker | 457 | 14.00 (127.63) [0–1714] | 457 | 11.54 (106.07) [0–1714] |
| Care worker | 457 | 21.30 (173.43) [0–1964] | 457 | 16.65 (214.26) [0–3938] |
| NHS Stop Smoking Service | 457 | 8.41 (32.97) [0–137] | 457 | 14.12 (41.76) [0–137] |
| Walk-in centre | 457 | 2.89 (24.18) [0–420] | 457 | 3.08 (12.09) [0–126] |
| Secondary care services | ||||
| Outpatient attendances | 457 | 142.12 (291.40) [0–2537] | 457 | 130.46 (259.52) [0–3044] |
| Overnight stays | 457 | 38.77 (141.30) [0–1155] | 457 | 36.24 (184.78) [0–2696] |
| Inpatient days/nights | 457 | 162.11 (850.17) [0–11,591] | 457 | 207.32 (1201.21) [0–14,111] |
| A&E attendances | 457 | 42.80 (270.98) [0–5484] | 457 | 35.60 (148.20) [0–2559] |
| Day-case procedures | 457 | 319.19 (2132.99) [0–37,595] | 457 | 268.18 (2183.89) [0–45,114] |
| Total | 457 | 925.24 (2663.15) [0–38,185] | 457 | 888.30 (2721.45) [0–45,400] |
| Item | SAU group | Intervention group | ||
|---|---|---|---|---|
| Participants (n) | Mean (SD) [range] | Participants (n) | Mean (SD) [range] | |
| Primary care and community services | ||||
| GP at surgery/health centre | 297 | 77.85 (122.88) [0–1332] | 314 | 64.53 (85.21) [0–784] |
| GP via telephone | 297 | 11.18 (24.30) [0–233] | 314 | 11.81 (28.09) [0–233] |
| GP at home | 297 | 1.59 (15.81) [0–237] | 314 | 0.25 (4.45) [0–79] |
| Nurse at surgery/health centre | 297 | 7.95 (16.19) [0–124] | 314 | 7.32 (18.59) [0–199] |
| Nurse via telephone | 297 | 0.97 (7.40) [0–117] | 314 | 0.50 (2.67) [0–23] |
| Nurse at home | 297 | 0.80 (11.73) [0–198] | 314 | 0.25 (4.48) [0–79] |
| Physiotherapist at surgery/health centre | 297 | 12.50 (43.46) [0–252] | 314 | 8.61 (32.02) [0–252] |
| Physiotherapist at home | 297 | 0.21 (3.65) [0–63] | 314 | 0.00 (0.00) [0–0] |
| Occupational therapist at surgery/health centre | 297 | 7.56 (49.43) [0–499] | 314 | 3.18 (24.67) [0–250] |
| Occupational therapist at home | 297 | 1.40 (14.43) [0–166] | 314 | 2.91 (43.24) [0–749] |
| Social worker | 297 | 8.66 (61.31) [0–791] | 314 | 7.56 (57.24) [0–659] |
| Care worker | 297 | 6.10 (67.89) [0–971] | 314 | 9.42 (129.93) [0–2244] |
| NHS Stop Smoking Service | 297 | 14.80 (42.65) [0–137] | 314 | 20.99 (49.50) [0–137] |
| Walk-in centre | 297 | 2.26 (12.48) [0–168] | 314 | 2.14 (9.40) [0–63] |
| Secondary care services | ||||
| Outpatient attendances | 297 | 101.65 (302.14) [0–3044] | 314 | 95.34 (209.61) [0–1903] |
| Overnight stays | 297 | 32.42 (143.16) [0–1541] | 314 | 15.95 (82.79) [0–770] |
| Inpatient days/nights | 297 | 159.50 (1280.25) [0–17,639] | 314 | 52.96 (364.27) [0–5040] |
| A&E attendances | 297 | 25.24 (85.89) [0–548] | 314 | 23.87 (97.84) [0–914] |
| Day-case procedures | 297 | 245.57 (1500.83) [0–24,061] | 314 | 158.04 (567.22) [0–4511] |
| Total | 297 | 718.22 (2362.79) [0–28,916] | 314 | 485.64 (967.79) [0–7176] |
| Item | SAU group | Intervention group | ||
|---|---|---|---|---|
| Participants (n) | Mean (SD) [range] | Participants (n) | Mean (SD) [range] | |
| Primary care and community services | ||||
| GP at surgery/health centre | 274 | 115.28 (183.89) [0–1568] | 280 | 86.50 (102.22) [0–784] |
| GP via telephone | 274 | 16.03 (42.33) [0–466] | 280 | 13.52 (24.96) [0–155] |
| GP at home | 274 | 3.17 (34.63) [0–474] | 280 | 0.00 (0.00) [0–0] |
| Nurse at surgery/health centre | 274 | 12.48 (26.69) [0–249] | 280 | 11.59 (25.86) [0–249] |
| Nurse via telephone | 274 | 1.05 (6.54) [0–78] | 280 | 0.47 (3.23) [0–47] |
| Nurse at home | 274 | 0.14 (2.40) [0–40] | 280 | 0.14 (2.37) [0–40] |
| Physiotherapist at surgery/health centre | 274 | 33.98 (147.15) [0–1573] | 280 | 29.20 (97.59) [0–755] |
| Physiotherapist at home | 274 | 0.23 (3.80) [0–63] | 280 | 3.37 (45.53) [0–755] |
| Occupational therapist at surgery/health centre | 274 | 11.23 (64.46) [0–665] | 280 | 8.02 (55.54) [0–665] |
| Occupational therapist at home | 274 | 3.04 (28.31) [0–416] | 280 | 8.61 (120.12) [0–1996] |
| Social worker | 274 | 14.68 (123.23) [0–1583] | 280 | 12.48 (123.52) [0–1978] |
| Care worker | 274 | 3.43 (42.30) [0–691] | 280 | 22.62 (197.39) [0–1942] |
| NHS Stop Smoking Service | 274 | 19.55 (48.07) [0–137] | 280 | 14.22 (41.92) [0–137] |
| Walk-in centre | 274 | 2.22 (14.60) [0–168] | 280 | 3.45 (14.02) [0–126] |
| Secondary care services | ||||
| Outpatient attendances | 274 | 135.65 (273.13) [0–2030] | 279 | 170.50 (623.53) [0–7611] |
| Overnight stays | 274 | 49.20 (238.33) [0–2696] | 280 | 39.89 (376.08) [0–6163] |
| Inpatient days/nights | 274 | 213.36 (1545.92) [0–22,678] | 280 | 52.20 (298.68) [0–3528] |
| A&E attendances | 274 | 39.36 (152.63) [0–1462] | 280 | 31.99 (94.42) [0–548] |
| Day-case procedures | 274 | 263.44 (978.52) [0–11,279] | 279 | 242.55 (805.40) [0–9023] |
| Total | 274 | 937.50 (2330.52) [0–26,165] | 278 | 705.97 (1257.55) [0–10,639] |
Data completeness
| Group | SAU group | Intervention group | Overall |
|---|---|---|---|
| Number randomised (n) | 458 | 457 | 915 |
| Costs missing (n) | 216 | 211 | 427 |
| QALYs missing (n) | 195 | 181 | 376 |
| Complete cases, n (%) | 231 (50.4) | 239 (52.3) | 470 (51.4) |
| Analysis set, outcome | SAU group | Intervention group |
|---|---|---|
| Available cases | ||
| Mean costs (£) (n participants) | 1548 (241) | 1450 (246) |
| Mean QALYs (n participants) | 0.513 (263) | 0.530 (276) |
| Complete cases | ||
| Mean costs (£) (n participants) | 1494 (231) | 1475 (239) |
| Mean QALYs (n participants) | 0.508 (231) | 0.539 (239) |
Available case analyses of costs and quality-adjusted life-years
| Group | Costs (£) (n = 487) | QALYs (n = 539) | ||
|---|---|---|---|---|
| Adjusted mean | 95% CI | Adjusted mean | 95% CI | |
| SAU | 1421 | 1107 to 1736 | 0.502 | 0.480 to 0.525 |
| Intervention | 1545 | 1185 to 1906 | 0.502 | 0.479 to 0.525 |
| Difference | 124.04 | −336.94 to 585.01 | −0.0004 | −0.026 to 0.025 |
Regression model specifications for costs and quality-adjusted life-years
| Family | Link function | Inclusion of baseline costs as predictor | AIC |
|---|---|---|---|
| Gaussian | Inverse | Inverse | 8851.331 |
| Identity | 8918.287 | ||
| Log | 8918.287 | ||
| Log | Inverse | 8790.007 | |
| Identity | 8776.709 | ||
| Log | 8760.236 | ||
| Identity | Inverse | 8805.304 | |
| Identity | 8758.814 | ||
| Log | 8784.140 | ||
| Gamma | Inverse | Inverse | 7745.06 |
| Identity | 6.45 × 1012 | ||
| Log | 6.39 × 1012 | ||
| Log | Inverse | 7747.628 | |
| Identity | 7703.817 | ||
| Log (base case) | 7681.019 | ||
| Identity | Inverse | 7741.512 | |
| Identity | 7682.524 | ||
| Log | 7.82 × 1010 |
| Family | Link function | Inclusion of baseline EQ-5D as predictor | AIC |
|---|---|---|---|
| Gaussian | Inverse | Inverse | –274.643 |
| Identity | 172.615 | ||
| Log | –336.1173 | ||
| Log | Inverse | –273.8452 | |
| Identity | –605.3866 | ||
| Log | –422.9234 | ||
| Identity | Inverse | –273.4791 | |
| Identity | –694.4329 | ||
| Log | –534.2838 | ||
| Gamma | Inverse | Inverse | –456.7708 |
| Identity | 9.39 × 108 | ||
| Log | 4.70 × 108 | ||
| Log | Inverse | –457.1944 | |
| Identity (base case) | –599.4751 | ||
| Log | –569.4504 | ||
| Identity | Inverse | –457.6132 | |
| Identity | –623.3385 | ||
| Log | –625.5415 |
| Costs | QALYs | Baseline costs/EQ-5D-5L data included | Incremental costs (£) | Incremental QALYs | ICER | INMB |
|---|---|---|---|---|---|---|
| Base case | ||||||
| Gamma–log | Gamma–log | Yes | 173.50 | −0.006 | Dominated | −361.94 |
| Sensitivity analyses | ||||||
| Gaussian–identity | Gaussian–identity | Yes | 50.65 | 0.007 | 6800 | 171.40 |
| Gaussian–identity | Gamma–log | Yes | 227.38 | −0.006 | Dominated | −415.82 |
| Gamma–log | Gaussian–identity | Yes | 173.50 | 0.007 | 24,600 | 38.35 |
| Gamma–log | Gaussian–identity | Yes | 173.50 | 0.002 | 75,600 | −104.7 |
| Gamma–log | Gamma–log | No | 157.70 | 0.026 | 6100 | 615.18 |
Cost-effectiveness subgroup analyses
| Subgroup | Incremental costs (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|
| Age (years) | |||
| 18–39 (n = 97) | 650* | 0.021 | 30,500 |
| 40–50 (n = 112) | 888* | −0.050 | Dominated |
| 51–59 (n = 130) | −612 | −0.003 | 209,000 |
| 60–82 (n = 131) | 514 | −0.018 | Dominated |
| Gender | |||
| Male (n = 210) | 113 | −0.023 | Dominated |
| Female (n = 260) | 376 | 0.009 | 41,100 |
| HSI (score) | |||
| 0–4 (n = 384) | 341 | 0.001 | 481,800 |
| 5 or 6 (n = 86) | −987 | −0.049 | 20,200 |
Appendix 31 Review of model-based approaches to estimating the cost-effectiveness of smoking cessation interventions
Objectives
-
To identify existing health economic evaluations of interventions aimed at helping people to reduce or quit smoking.
-
To identify key structural features of models used for health economic evaluations.
-
To identify key model input parameters affecting the cost-effectiveness of smoking reduction/cessation interventions.
Methods
A descriptive review of published literature (including bibliographic databases and literature cited by known existing reviews) was conducted, including data abstraction and narrative synthesis. Formal critical appraisal was not performed, as the purpose of the review was to inform model development.
Search strategy
The following bibliographic databases were searched:
-
MEDLINE and MEDLINE In-Process & Non-Indexed Citations (both via Ovid)
-
EMBASE (via Ovid)
-
Health Technology Assessment database (via The Cochrane Library)
-
NHS Economic Evaluations Database (via The Cochrane Library).
Search terms for smoking cessation were based on the searches for the Cochrane Tobacco Addiction Group. 136
The Centre for Reviews and Dissemination filters for economic studies were used for the MEDLINE and EMBASE searches. 137
Studies published since 2012 were searched, as the previous review23 included searches to August 2012. The prior review was the point of departure for this review.
Forward citation chasing (by Scopus) was used to identify any studies that have cited the previous review and other reviews. 23
Study selection
The study inclusion criteria were as described in Table 57.
| Criteria | Include | Exclude |
|---|---|---|
| Population | Current smokers, including smokers of cigarettes, cigars and pipes, regardless of intention to quit or past attempts to quit |
|
| Interventions | Any direct intervention designed to assist in the reduction or cessation of smoking | Public health interventions (e.g. media campaigns, tax policies, changes to packaging) |
| Comparator(s) | Other interventions, or no intervention (control group) | Studies in which no comparators are included |
| Outcome | Costs, life-years, health-related quality of life, QALYs, disability-adjusted life-years | |
| Study design |
|
|
| Other | Studies published only in abstract form |
Titles and abstracts were screened by a health economist with experience of reviews of economic evaluations (TS). Full texts were retrieved for all records that could not be excluded on the basis of title and abstract. Linked publications from the same studies were collated. A random 10% sample of titles and abstracts was independently screened by a professor in health economics (CG), with full texts similarly retrieved, to assure that no studies were omitted that met the inclusion criteria.
Data abstraction
Data were abstracted using a template used in the previous review,23 and additional tables were designed for this review.
Synthesis
A narrative synthesis was conducted on the methods and results of the included studies, facilitated by tabulation across studies.
Results
Study selection
Database searches produced 9304 records, which were combined with 170 records from forward citation chasing (the majority of which cited Woolacott et al. 138). After screening of titles, abstracts and full texts (Figure 22), we included 44 studies reported in 45 publications.
FIGURE 22.
The PRISMA flow diagram for the review of model-based economic evaluations of smoking cessation interventions.

Summary of model-based cost-effectiveness studies in smoking cessation
As shown in Table 58, a significant number of studies evaluated pharmacological aids (generally NRT, bupropion and/or varenicline), as well as counselling (in various forms including telephone counselling) and smoking cessation programmes in their entirety. Approximately one-third of the studies were economic evaluations alongside clinical trials, with the remainder relying on evidence synthesis. The vast majority of studies used a lifetime time horizon and QALYs as the measure of health benefit. Most studies were conducted in high-income countries in Europe and North America, but there were also five studies in East Asia. Markov cohort simulation was the most common modelling methodology; four studies used individual patient simulations.
| Characteristic | Number of studies |
|---|---|
| Type of intervention (any intervention that was common across options considered is not included here) |
|
| Population |
|
| Economic evaluation alongside a clinical trial |
|
| Time horizon |
|
| Country |
|
| Measure of health benefit |
|
| Model typea |
|
Ten studies141–150 were applications and/or minor extensions of the BENESCO model,151 which was described in the previous review;23 therefore, these studies will not be discussed in detail.
Nine studies87,89,90,104,105,152–155 did not use a population of general smokers; therefore, they are less likely to be generalisable and may include model features that would be irrelevant in the context of general smokers. These studies are also not discussed in detail.
Model structures
Three model features can be used to categorise the modelling approaches of the included studies: modelling subsequent smoking behaviour (e.g. relapse, spontaneous quit), explicitly modelling smoking-related morbidities (e.g. cardiovascular and respiratory diseases), and incorporating smoking-related excess morbidity (impact on health-related quality of life and/or health-care usage) and/or mortality. Table 59 shows these features across the studies.
| Study | Subsequent smoking behaviour | Explicitly modelled smoking-related morbidity | Smoking-related excess morbidity and/or mortality |
|---|---|---|---|
| Annemans 2015141 | Yes | Yes | No |
| Athanasakis 2012142 | Yes | Yes | No |
| Baker 2018143 | Yes | Yes | No |
| Barnett 2016104 | Yes | No | Yes |
| Barnett 2015105 | Yes | No | Yes |
| Barnett 2014106 | Yes | No | Yes |
| Boyd 2016152 | Yes | No | Yes |
| Cadier 2016156 | Yes | Yes | No |
| Cantor 2015157 | Yes | No | Yes |
| Chandra 201287 | No | Yes | Yes |
| Chen 2012158 | No | No | Yes |
| Chevreul 2014159 | Yes | No | Yes |
| Coward 201589 | No | Yes | No |
| Gilbert 2017160,161 | No | Yes | Yes |
| Guerriero 2013107 | Yes | Yes | Yes |
| Higashi 201296 | Yes | Yes | Yes |
| Igarashi 2016162 | Yes | Yes | No |
| Kautiainen 2017144 | Yes | Yes | No |
| Knight 2012145 | No | Yes | No |
| Kowada 201590 | No | Yes | Yes |
| Lal 2014163 | Yes | Yes | No |
| Leaviss 2014146 | Yes | Yes | No |
| Leung 2017108 | Yes | Yes | No |
| López-Nicolás 2017164 | Yes | Yes | No |
| Lutz 2012147 | Yes | Yes | No |
| Lutz 2012148 | Yes | Yes | No |
| Maciosek 2017153 | Yes | Yes | Yes |
| Meeyai 2015165 | No | No | Yes |
| Menn 201288 | No | Yes | Yes |
| Mullen 2015154 | No | No | Yes |
| Nohlert 2013166 | No | Yes | No |
| Pennington 2018 (population cohort model)139 | Yes | Yes | Yes |
| Pennington 2018 (discrete event simulation)139 | Yes | Yes | Yes |
| Popp 2018167 | Yes | No | Yes |
| Rasmussen 2013168 | No | No | Yes |
| Sonntag 2018155 | No | Yes | Yes |
| Taylor 201423 | Yes | No | Yes |
| Tosanguan 2016169 | No | Yes | No |
| Tran 2012170,171 | Yes | Yes | Yes |
| Villanti 2013172 | No | Yes | Yes |
| Virtanen 2017 (population simulation)140 | No | Yes | No |
| Virtanen 2017 (Markov microsimulation)140 | No | Yes | No |
| von Wartburg 2014149 | Yes | Yes | No |
| Walker 201875 | Yes | Yes | Yes |
| Wilson 2012150 | Yes | Yes | No |
| Wu 2014128 | Yes | No | Yes |
In summary:
-
four studies154,158,165,168 modelled smoking-related excess morbidity and/or mortality only
-
five studies89,140,145,166,169 explicitly modelled one or more smoking-related morbidities, but did not model subsequent smoking behaviour or smoking-related excess morbidity or mortality
-
six studies87,88,90,155,160,172 explicitly modelled one or more smoking-related morbidities and smoking-related excess morbidity and/or mortality, but did not model subsequent smoking behaviour
-
nine studies23,104–106,128,152,157,159,167 modelled subsequent smoking behaviour and smoking-related excess morbidity and/or mortality, but did not explicitly model smoking-related morbidity
-
fourteen studies108,141–144,146–150,156,162–164 modelled subsequent smoking behaviour and explicitly modelled one or more smoking-related morbidities, but did not include smoking-related excess morbidity or mortality
-
six studies75,96,107,139,153,170,171 modelled subsequent smoking behaviour and explicitly modelled one or more smoking-related morbidities and included smoking-related excess morbidity and/or mortality.
It is important to note that the complexity of a model is not necessarily a sign of quality or freedom from bias, and that these three modelling features can be included to a greater or lesser extent. For example, in some cases, the modelling of subsequent smoking behaviour was limited to modelling future relapse, whereas, in other studies, spontaneous quit attempts were also included.
Modelling subsequent smoking behaviour is important because relapsing and spontaneous quitting lead to ‘mixing’ and a dilution of any absolute treatment effect over time. Failure to include these factors will generate overly optimistic cost-effectiveness estimates.
It is also important to note that, if specific morbidities are modelled, and smoking-related excess morbidity and/or mortality is included, studies should be careful to avoid ‘double-counting’ of smoking-related disease.
Many models used Markov cohort simulation, but these typically did not allow for comorbidities, that is all individuals in the cohort could have zero or one smoking-related disease. The model would typically allow transitions from ‘less serious’ to ‘more serious’ smoking-related diseases, but not vice versa.
Four studies used other population/cohort simulation methods,96,139,140,172 although one of these172 considered only lung cancer as a smoking-related disease. Two of the other models139,140 used smoking status to estimate either smoking-related disease prevalence or incidence. One of these139 estimated prevalence, and also allowed for relapse and spontaneous quitting of smoking; however, it appears that relapse would result in instantaneous incidence of disease in those relapsing (if the prevalence of the disease was higher among current smokers than among former smokers) or instantaneous cure of disease in those quitting (even for incurable diseases). The other140 did not allow for relapse or spontaneous quitting. Higashi et al. 96 used smoking status to determine incidence, but also allowed for relapse and spontaneous quitting.
Four studies139,153,162,167 used individual patient simulation (Monte Carlo) methods, which require separate model runs to simulate an individual patient, and simulate a large number (ranging from 5000 to > 1 million) patients obtain stable population-level estimates. Such models are typically more flexible than cohort simulation models, but the computational cost of Monte Carlo simulation often means that exploration of uncertainty is more challenging.
Model data sources
Data on smoking behaviour
A number of different studies underpinned assumptions regarding future smoking behaviour. Relapse was more commonly modelled than spontaneous quitting. Studies by Krall et al. 173 and Wetter et al. 174 underpinned the BENESCO models. Three other studies153,162,170,171 also used data from Wetter et al. 174 Krall et al. 173 describe the results of a longitudinal study that identified 483 men in the Greater Boston metropolitan area (mostly veterans) who were identified as smokers at baseline and had been abstinent from smoking for 2 years. In the first 6 years since quitting, the relapse rate was between 2% and 4% per year. By 10 years, the relapse rate had fallen to < 1%. A cumulative 19% of those who were abstinent for at least 2 years eventually relapsed. Wetter et al. 174 describe the results of a study that identified 1143 former smokers (mostly white men) who were followed for 4 years; the relationship between their duration of abstinence at baseline and subsequent relapse was investigated. A total of 90.6% of those who had been abstinent for > 5 years at baseline had also been abstinent for at least 2 years at the 4-year follow-up, compared with 83.6% of those who had been abstinent for 2–5 years at baseline, 68.4% of those who had been abstinent for 1–2 years at baseline, 50.0% of those who had been abstinent for 4–12 months at baseline and 31.3% of those who had been abstinent for up to 3 months at baseline.
Etter and Stapleton175 conducted a meta-analysis of RCTs of nicotine replacement therapy with final follow-up > 1 year after the start of treatment. Twelve RCTs with a combined total of 4792 participants included a final follow-up of 2–8 years after treatment initiation (average 4.3 years). The overall relapse rate between 12 months and final follow-up was 30%, and this was independent of the treatment arm and duration of treatment. Trials with longer follow-up (> 4 years) did not systematically have higher relapse rates than trials with shorter follow-up (≤ 4 years). Participants lost to follow-up were assumed to be smoking, which may inflate the relapse rate estimates. This study informed a number of modelling studies included in this review. 23,75,107,108,128
Hawkins et al. 101 reported an analysis of the British Household Panel Survey in which they identified 1578 individuals who reported not smoking in two consecutive waves after formerly reporting smoking in at least one wave. Waves of the survey were conducted annually. It was estimated that 37.1% of individuals would relapse within 10 years, although the annual relapse probability was much higher in the first period (15.1%) than in subsequent periods (declining from 7.9% in the second period to < 2% in periods 6–10). This study informed four modelling studies in this review,104–106,146 although three of these had the same first author.
Spontaneous quit rates were less frequently used in modelling. Stapleton176 estimated from the British General Household Survey that cessation rates for those aged 20–34 years in 1972 rose from around 2% per year to 3.5–4% per year over 25 years of survey data. As this is from a single age cohort, it is not possible to separate age and period effects. Two studies107,108 in this review were informed by this study. Levy et al. 177 used modelling and data from the 2003 (US) Tobacco Use Supplement, the Current Population Survey and Cochrane reviews of treatment effectiveness to estimate a population quit rate of 4.3% per year. Three studies104–106 in this review (all sharing the same first author) used this estimate.
Data on excess mortality
All the models of smoking cessation included in this review include some mechanism for smoking cessation to lead to improved life expectancy. As described previously, for some models this is indirect, through smoking cessation reducing the risk of smoking-related morbidity, which is, in itself, associated with increased mortality. Other models explicitly set life expectancy for current and former smokers, and then make suitable adjustments to avoid double-counting if they also model morbidities.
Smoking-related excess mortality has been investigated in a number of studies and these have informed many of the models included in this review.
American Cancer Society Cancer Prevention Study II
Taylor et al. 178 reported the results of the Cancer Prevention Study II (CPS-II), which followed up 877,243 respondents for mortality from 1982 to 1996. They found that current smokers were generally at least twice as likely to have died as never smokers. Former smokers were less likely to have died, and the relative risk of death generally dropped with increasing quit duration. The CPS-II underpinned five studies in this review, although three of these shared the same first author. 96,104–106,154 The principal limitations of the CPS-II are that it is not fully representative of the US population (respondents were more likely to have a college degree than the general population, and more likely to be white), the analysis excluded participants who ever smoked a pipe or cigar (18%) and the study did not collect smoking status during the 14-year follow-up period (a sensitivity analysis was conducted to attempt to adjust for this).
US National Health Interview Survey
Jha et al. 122 reported the results of an analysis based on the US National Health Interview Survey. Smoking histories were obtained from 202,248 respondents aged ≥ 25 years between 1997 and 2004, who were then followed up for mortality to the end of 2006. The study found that, compared with never smokers, current smokers lost 11–12 years of life expectancy, and that those who quit aged < 35 years had similar mortality outcomes to those who never smoked. The hazard ratio for mortality was 2.9 for continuing smokers versus never smokers. This study underpins one economic evaluation in this review. 167 The main limitations of this study are that the follow-up was relatively limited (mean follow-up of 7 years) and did not include subsequent observations of smoking status.
UK prospective study of male doctors
Doll et al. 179,180 have reported the results of a prospective study of 34,439 male British doctors who were identified in 1951 and followed up for cause-specific mortality over 50 years. Their smoking behaviour was elicited in 1951 and periodically thereafter. In addition to demonstrating that lifelong non-smokers had a life expectancy that was ≈10 years longer than continuing cigarette smokers, the study also showed that those who stopped smoking when aged 25–44 years had a pattern of survival similar to that of men who never smoked. This study has underpinned five studies in the review. 23,107,128,139,159 The principal limitations of this study are that it is based on data from men only, in professional careers (doctors), who were born before 1930, and in many cases before 1900.
Copenhagen prospective studies
Prescott et al. 123 reported the results of a pooled analysis of three prospective studies in Copenhagen, representing a total 30,917 participants, who were followed up for up to 30 years. The study showed that current smokers who smoked ≥ 15 g of tobacco per day had mortality rates that were 2.1 times higher than never smokers, with less heavy current smokers (< 15 g of tobacco per day) having mortality rates that were 1.7 times higher than never smokers, and former female smokers having a mortality rate ratio of 1.2 (1.1 for former male smokers), compared with never smokers. There was some evidence that women face a higher risk of respiratory disease and vascular disease. This study has underpinned five economic evaluations in this review, although three of these share the same first author. 88,104–106,168 The principal limitation of this study is that it does not distinguish between recent and long-term quitters.
US prospective three communities study
LaCroix et al. 181 have reported the results of a prospective study of 7178 women and men aged ≥ 65 years with no history of myocardial infarction, stroke or cancer living in three communities (East Boston, MA; Iowa and Washington counties, IA; New Haven, CT). Their smoking statuses were elicited and they were followed up for 5 years for cause-specific mortality. The study showed that rates of total mortality for current smokers were twice what they were among participants who had never smoked, and that mortality from cardiovascular disease was not significantly different between never smokers and former smokers, whereas mortality from smoking-related cancers remained higher for former smokers than for never smokers (not statistically significant for women). This study has underpinned three studies in this review, all with the same first author. 104–106 The principal limitations of this study are that it is limited to men and women aged ≥ 65 years, but with no history of myocardial infarction, stroke or cancer. This resulted in the exclusion of approximately 30% of potentially eligible participants. This exclusion criterion is likely to be partly responsible for the findings that smoking has a lower impact on mortality for those aged > 75 years, as participants were selected who may have protective characteristics. Furthermore, all study participants were born before 1920, so the relevance to current cohorts is diminished.
Other studies
Jha et al. 182 reported the results of an unmatched case–control study conducted in India, which was used in one economic evaluation in this review. 155 Rogers and Powell-Griner183 used data from two different US surveys to construct lifetables for white smokers, former smokers and non-smokers, which were used in one economic evaluation in this review. 172 Rogers et al. 184 reported the results of a study using data from the US National Health Interview Survey (1990), linked to the Multiple Causes of Death database through to the end of 1997. This was used in one economic evaluation in this review. 157 Anthonisen et al. 185 reported the results of a RCT of a smoking cessation intervention in individuals with asymptomatic airway obstruction, demonstrating a hazard ratio for all-cause mortality of 1.18 for the control group versus the intervention group, with 5-year smoking cessation rates of 5.4% and 21.7% in the control and intervention groups, respectively. This was used by one economic evaluation in this review. 87 Peto et al. 186 indirectly estimated mortality attributable to smoking in a number of countries by reference to the lung cancer incidence rates in those countries and extrapolating, by means of interim data from CPC-II, these to total mortality attributable to smoking. An update of this study was used by one economic evaluation in the review. 152 Friedman et al. 187 reported the results of a study conducted in a medical care programme in which smoking habits were obtained for > 60,000 individuals between 1979 and 1986, who were then followed up to 1987 for mortality. This was used by one economic evaluation in this review. 104 Uno et al. 188 reported the results of a Japanese cohort study in which 10,873 Japanese men and women were followed up for a mean of 8.2 years for mortality. The study found that current smokers had a hazard ratio for all-cause mortality of 1.65 versus never smokers. When limiting to women, there was no evidence of harm from smoking, but smoking was rare among women so the study lacked statistical power in this regard.
Data on morbidities
Lung cancer
Many studies,75,96,107,141–150,170,171 including those based on the BENESCO model,151 used estimates for the risk of lung cancer attributable to smoking from the CPS-II, as reported by Thun et al. 189 This study gives hazard ratios for cause-specific mortality, but the assumption is made that, as survival from lung cancer is poor, this can be a suitable proxy for the hazard ratio for incidence. It is further claimed that, as not all individuals with lung cancer die, that applying the hazard ratio to incidence is conservative. This study estimates hazard ratios of 21.3 (95% CI 17.7 to 25.6) for men who currently smoke, 8.3 (95% CI 6.9 to 10.0) for men who no longer smoke (more specifically, who reported having quit smoking in 1982; vital status was followed up to 1988), 12.5 (95% CI 10.9 to 14.3) for women who currently smoke and 4.8 (95% CI 4.1 to 5.6) for women who no longer smoke. The CPS-II has underpinned a series of publications of estimates of deaths attributable to smoking186 that have, in turn, underpinned a number of modelling studies in this review. 140,163,166
Freedman et al. 190 investigated the effect of intensity of cigarette smoking and time since quitting on lung cancer incidence in another US prospective cohort [National Institutes of Health (NIH)-American Association of Retired Persons (AARP) Diet and Health] that included nearly half a million participants from eight US states and followed them up for ≈8 years. They identified a clear dose–response relationship between the intensity of cigarette smoking and lung cancer incidence, and also that greater time since quitting decreased the relative risk of lung cancer. They identified that women who have never smoked have a higher rate of lung cancer than men who have never smoked, although this is not adjusted for the risks of second-hand smoke from a spouse. One cost-effectiveness model108 made use of estimates from this study.
Doll et al. 179 reported the mortality by smoking habits from neoplastic disease among a cohort of male British doctors. Between 1951 and 1991, there were 893 lung cancer deaths in the cohort, and the rate of lung cancer mortality was much higher among current smokers than among former or never smokers; there was also a relationship between the current number of cigarettes smoked and the annual lung cancer mortality rate. One cost-effectiveness study156 adapted estimates from this study.
Pesch et al. 191 pooled the results of a number of case–control studies investigating the link between smoking and lung cancer. They found that, as time since quitting increased, the OR for lung cancer decreased. They also found that adenocarcinoma is less associated with smoking than squamous cell cancer and small cell lung cancer. One cost-effectiveness study139 made use of estimates from this study.
Haiman et al. 192 reported the association of smoking with lung cancer in the US Multiethnic Cohort Study. They found ethnic/racial differences in quitting rates and found that, for smokers who smoked < 30 cigarettes per day, the risks of lung cancer were higher among African Americans and Native Hawaiians than among Japanese Americans, Latinos and whites. One cost-effectiveness study169 made use of estimates from this study.
Cardiovascular disease
Again, the CPS-II, as published by Thun et al. ,189 was the data source for studies based on the BENESCO model. 151 One economic evaluation156 relied on the Doll et al. 179 cohort study described previously and another166 relied on a publication reporting estimates based on two British case–control studies (conducted in 1950 and 1990). 193 A report by the US Office of the Surgeon General (a review of published studies)194 underpinned a single economic evaluation. 107
Shields and Wilkins117 reported the results of a study of the longitudinal Canadian National Population Health Survey over 16 years. They found evidence that being a current smoker increased the risk of heart disease, and, for women, being a former smoker also increased the risk of heart disease. One economic evaluation139 used estimates from this study.
Stroke
Once again, the CPS-II, as published by Thun et al. ,189 underpinned many models, including the BENESCO models. Myint et al. 120 report the risk factors for stroke based on > 20,000 men and women in Norfolk who were evaluated for risk factors in 1993–1996 and followed up for disease end points to 2005. Being a current smoker was associated with an increased risk of stroke, compared with not being a current smoker. Having previously smoked was not associated with an increased risk of stroke. One economic evaluation139 used estimates from this study.
Chronic obstructive pulmonary disease
The CPS-II as published by Thun et al. 189 was used by many models, including the BENESCO models. The Doll et al. 179 cohort study of British doctors was adapted for use by one economic evaluation. 156 Rodriguez et al. 109 reported the results of a case–control analysis based on the UK General Practice Research Database. Current smokers and former smokers both had a significantly increased risk of COPD, compared with never smokers, but former smokers had a significantly lower risk than current smokers. One economic evaluation160 used estimates from this study. Løkke et al. 195 reported the results of the Copenhagen City Heart Study, which found that the OR of developing COPD was 6.3 for current smokers versus never smokers, which corresponded to an absolute risk of 24.3% for developing clinically significant (stage 2 or worse) COPD. One economic evaluation139 used estimates from this study.
Summary
There are several studies that can inform the risks of smoking-related diseases. These are frequently cohort studies or case–control studies. The CPS-II has been frequently used as a source for the effect of smoking on related diseases, although this (and another cohort study of British doctors179) report the effect on cause-specific mortality only, and not incidence. Not all studies follow up for smoking status (e.g. it is measured only at baseline); therefore, they may underestimate the effect of smoking cessation.
Effect of smoking on health-related quality of life
Beyond the inclusion of health state utility values for smoking-related diseases, a number of studies modelled a difference between the utility for current smokers and for former smokers.
Vogl et al. 126 used data from the 2006 Health Survey for England to examine the impact of smoking status on EQ-5D-3L utility. They produced age- and gender-dependent utility values for current smokers (split into heavy, moderate and light), ex-smokers (ex-occasional and ex-regular) and never smokers. Estimates were adjusted for the presence of cardiovascular disease and ‘limiting conditions’. Additional file 4 of Vogl et al. 126 gives linear regression coefficients that show that, compared with never smokers, the EQ-5D is 0.0169 lower for those who used to smoke regularly, is 0.0209 lower for those who currently smoke < 10 cigarettes per day, is 0.0327 lower for those who currently smoke 10–19 cigarettes per day and is 0.0516 lower for those who currently smoke ≥ 20 cigarettes per day. Eight economic evaluations23,75,104–106,139,160,167 used estimates from this study.
Kind et al. 196 used data from the UK Measurement and Valuation of Health project to examine the impact of smoking status on EQ-5D-3L utility. They produced age- and gender-dependent utility values for non-smokers, those who smoke < 20 cigarettes per day and those who smoke ≥ 20 cigarettes per day. The utility for those smoking < 20 cigarettes per day was 0.01–0.07 lower than the utility for non-smokers. Two economic evaluations128,152 in this review used data from this study.
Tillman and Silcock197 administered a questionnaire to smokers and ex-smokers registered with general practices in Aberdeen, including the EuroQol questionnaire. An unspecified EuroQol tariff was applied; it may have been the standard UK EQ-5D-3L time trade-off tariff. Smokers had a mean utility of 0.75, compared with a mean utility of 0.78 for ex-smokers. Without adjustment, this difference was statistically significant; however, it was no longer statistically significant after adjusting for age, sex and socioeconomic status variables.
Fiscella and Franks198 analysed data from the 1991 US National Health Interview Survey, which utilised the Years of Healthy Life measure to assess health-related quality of life. This is not a preference-based measure, but it does produce a value between 0 (death) and 1 (optimal health). They produced age- and gender-dependent quality-of-life values for current smokers and for former smokers who quit at least 15 years previously. The utility for current smokers was 0.02–0.09 lower than for those who quit at least 15 years ago. One economic evaluation157 in this review used estimates from this study.
Jia and Lubetkin199 analysed data from the 1993–2008 US Behavioral Risk Factor Surveillance System to assess the association between smoking status and health-related quality of life. The Behavioral Risk Factor Surveillance System does not use a preference-based health-related quality-of-life measure, but asks about the number of days in the preceding 30 days affected by ill health. This was mapped to EQ-5D-3L values from a US population time trade-off study. They estimated age-dependent utility values for individuals who smoke and individuals who do not smoke. The predicted EQ-5D was 0.033 higher, on average, among non-smokers. One study167 made use of this estimate, although it also used estimates from Vogl et al. 126
One study170,171 in the review reported the results of an analysis of the 2005 Canadian Community Health Survey, which uses the Health Utilities Index 3 as a measure of health-related quality of life. The authors analysed utilities among respondents without any chronic conditions and estimated age- and sex-dependent utility values for current and former smokers.
Summary
There are a number of studies that have investigated the relationship between smoking status and health-related quality of life. These generally found that smokers had lower health-related quality of life than non-smokers, and there was evidence in some studies that heavier smokers had worse health-related quality of life. Not all studies used a preference-based instrument to measure quality of life: Fiscella and Franks198 used a non-preference-based measure (Years of Healthy Life), and Jia and Lubetkin199 mapped a non-preference-based measure (healthy days) to EQ-5D. Not all studies adjusted for health conditions (or excluded those with health conditions), and so would not be suitable for use in a model with explicitly modelled smoking-related diseases with an impact on utilities.
Results of studies
As this is primarily a review of modelling methodology, there is less focus on describing and synthesising the results of the included studies than there is on their methods. Although probabilistic sensitivity analyses are more useful than one-way sensitivity analyses for decision-makers, we focus on the reported results of one-way sensitivity analyses and structural sensitivity analyses as these can help to identify which model inputs and assumptions have a strong effect on the model results.
The Benefits of Smoking Cessation on Outcomes model
Nine141–145,147–150 of the studies based on the BENESCO model found that varenicline was cost-effective, compared with alternatives, or that using an extended course, or attempting a second quit attempt, with varenicline was cost-effective. These studies were all funded by Pfizer Inc. (New York, NY, USA) (which produces varenicline). One other study146 based on the BENESCO model, but not funded by Pfizer Inc., found that cytisine was cost-effective, compared with varenicline.
Seven141–144,146,149 of the studies based on the BENESCO model reported the results of one-way or structural sensitivity analyses.
Annemans et al. 141 and Kautiainen et al. 144 did not comprehensively report which parameters were subjected to one-way sensitivity analysis, or what range of variation was examined for each parameter. They reported that the model results were most sensitive to discount rates, the cost of NRT and the relative risks for smoking-related diseases among long-term quitters. Athanasakis et al. 142 reported which parameters were subjected to one-way sensitivity analysis (although not why any other parameters were excluded), and generally varied parameters by ± 10%. They produced a tornado diagram in which the most influential parameters were the utilities after a smoking-related disease event, the discount rate and the costs of events. von Wartburg et al. 149 give only qualitative descriptions of the results of a one-way sensitivity analysis of the discount rate. Leaviss et al. 146 did not report how they selected variables for one-way sensitivity analysis, but they did report comprehensive results in a table and the majority of parameters were varied between their respective confidence/credible interval limits. The most influential parameter was the relative effectiveness of cytisine compared with varenicline. All other parameters had very limited impact on cost-effectiveness, which may reflect the precision to which they are known or the insensitivity of the model to those parameters.
Baker et al. 143 presented structural sensitivity analyses in which the time horizon was varied from 2 years to lifetime (the base case). After 2 years, varenicline is predicted to lead to increased QALYs, versus comparators, but the costs of varenicline exceed those of bupropion and placebo to at least 20 years. Wilson et al. 150 conducted a subgroup analysis, a time horizon sensitivity analysis and sensitivity analyses regarding the costs of comorbidities. Only the subgroup analyses had a significant impact on cost-effectiveness.
Other studies
Barnett et al. 104 explored a number of assumptions in their evaluation of integrated care compared with a smoking cessation clinic for smoking veterans with post-traumatic stress disorder. They found that, if they excluded health-care costs unrelated to the intervention, the ICER would improve from US$32,000 per QALY to US$15,000 per QALY. If they used an alternative assumption about resource use post smoking cessation, the ICER worsened to US$64,000 per QALY. Increasing age was associated with worsening cost-effectiveness (from $23,000 per QALY when initial age was 45 years to $41,000 per QALY when initial age was 65 years).
In the evaluation of financial incentives for smoking cessation among pregnant women, Boyd et al. 152 found that assumptions around postnatal relapse rates had a significant impact on the probability of cost-effectiveness at a threshold of £30,000 per QALY. They also found that using self-reported (rather than biochemically verified) quit rates reduced the probability that financial incentives would be cost-effective, and that increasing the maximum incentive from £400 to £800 or £1800 would improve its effectiveness, but worsen cost-effectiveness.
Cadier et al. 156 investigated the cost-effectiveness of providing free access to smoking cessation. They found that younger or older ages of patients were associated with worse cost-effectiveness compared with middle age, and that providing free access to a greater number of quit attempts would improve effectiveness, but marginally worsen cost-effectiveness.
Cantor et al. 157 estimated that the cost-effectiveness of training physicians and pharmacists to deliver smoking cessation advice would improve with the increased age of the patients.
Guerriero et al. 107 predicted that increasing age (from 25 to 48 years) improved the cost-effectiveness of their intervention (further reducing incremental costs and increasing incremental QALYs).
Igarashi et al. 162 found that the most optimal age group in terms of cost-effectiveness in which to introduce varenicline would be 50–59 years, with progressively lower INMB among younger and older age groups. This resulted from incremental QALYs peaking in the 40–49 years’ age group and cost savings peaking among those aged ≥ 60 years.
Leung et al. 108 found that incremental costs were lower for older-aged cohorts (from 30 up to 60 years of age) and that incremental QALYs were highest for the cohorts aged 60 years (men) or 50 years (women). The ICER was lowest for those aged 60 years (men and women).
Menn et al. 88 found in their evaluation of a smoking cessation programme for COPD patients that cost-effectiveness was linked to age: as initial age was varied from 45 to 75 years, the incremental costs of the programme increased, compared with usual care, whereas the incremental QALYs decreased. They also found that the risks of COPD progression to stage II for current and former smokers had a bigger impact on net monetary benefit than other parameters investigated, although the effect on INMB was not presented.
Nohlert et al. 166 found that the cost-effectiveness of high-intensity versus low-intensity treatment for smoking cessation was most sensitive to the costs in added years of life, disease risks, the effect of quitting on disease risks and the intervention costs. They also estimated that the effects of smoking cessation were most favourable in the age range 30–44 years (combining estimated QALYs and cost savings at SEK100,000 per QALY), with QALY gains and cost savings diminishing in older age groups.
Pennington et al. 139 considered a range of alternative structural assumptions for modelling NRT versus no treatment. The most significant assumptions for cost-effectiveness were whether or not long-term relapse was incorporated (it has the effect of substantially reducing the incremental QALYs) and whether or not morbidities were directly associated with mortality. They found that the choice of modelling approach (state-transition model vs. discrete event simulation) did not directly influence cost-effectiveness, but that certain long-term assumptions did and that state-transition models may lack the flexibility for exploring these.
Rasmussen168 estimated the life-years saved by quitting smoking according to age when quitting and found that life-years saved diminished as the age at quitting increased.
Tran et al. 170,171 did not discern a clear relationship between age and cost-effectiveness. They found that results were sensitive to the definition of quit rates (point prevalence vs. continuous abstinence).
Discussion
In this review, and in the previous review of model-based economic evaluations of smoking cessation therapies,23 we have found a significant number of different models being used to investigate the cost-effectiveness of a variety of interventions. Apart from the studies utilising the BENESCO model to evaluate the cost-effectiveness of varenicline,151 most models were de novo for the particular study. Model structures varied substantially, but all of the models assumed that quitting smoking would lead to a change in life expectancy, directly and/or indirectly through the risk of morbidities with associated mortality. Many models failed to adequately represent realistic smoking behaviour, that is that individuals may relapse after quitting, and that smokers may make further attempts to quit later in life. It is clear that omitting either or both of these features will artificially improve the cost-effectiveness of an intervention for smoking cessation.
Studies have relied on a number of alternative data sources to estimate the effect of smoking on morbidity and mortality. In some cases, there are significant threats to the generalisability of the data sources to the decision problems. For example, Doll et al. 179,180 investigated the effect of smoking on mortality, but their cohort was male doctors born before 1930, and, in many cases, in the 19th century. As another example, Thun et al. 189 estimated the effect of smoking on lung cancer mortality, but this has been widely applied as the effect of smoking on lung cancer incidence.
Studies did not generally report how they identified and appraised data sources for model inputs, which places them at higher risk of bias.
Studies also generally assumed that the causal effect on health outcomes (e.g. incidence of morbidities, mortality) of quitting smoking as a result of a particular intervention (e.g. pharmacotherapy, behavioural therapy) could be estimated from observational studies comparing current smokers with former smokers and/or never smokers. This assumption may be unreasonable if, for example, those who quit in observational studies also had other positive health behaviours (e.g. if they also took up exercise or reduced their alcohol consumption), which would not be generated by the intervention for smoking cessation.
No studies in the review considered any externalities of smoking, for example health consequences for other people living in the same household. Studies also did not analyse any ethical or distributional impact of smoking cessation interventions, except Walker et al. ,75 who included deprivation in a cost-per-quit (but not cost-per-QALY) analysis.
Search strategies
EMBASE
| Database | EMBASE |
| Issue | 22 May 2018 |
| Platform | Ovid |
| Date searched | 23 May 2018 |
| Searcher | Tristan Snowsill |
| Total hits (n) | 5505 |
| # | Searches | Results (n) |
|---|---|---|
| 1 | Health Economics/ | 35,868 |
| 2 | exp Economic Evaluation/ | 273,861 |
| 3 | exp Health Care Cost/ | 263,039 |
| 4 | Pharmacoeconomics/ | 7845 |
| 5 | (econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. | 884,286 |
| 6 | (expenditure$ not energy).ti,ab. | 34,282 |
| 7 | (value adj2 money).ti,ab. | 2086 |
| 8 | budget$.ti,ab. | 33,065 |
| 9 | or/1-8 | 1,129,802 |
| 10 | (metabolic adj cost).ti,ab. | 1311 |
| 11 | ((energy or oxygen) adj cost).ti,ab. | 3890 |
| 12 | ((energy or oxygen) adj expenditure).ti,ab. | 27,952 |
| 13 | or/10-12 | 32,148 |
| 14 | 9 not 13 | 1,123,190 |
| 15 | letter.pt. | 1,021,261 |
| 16 | editorial.pt. | 566,438 |
| 17 | note.pt. | 714,289 |
| 18 | conference abstract.pt. | 3,012,020 |
| 19 | or/15-18 | 5,314,008 |
| 20 | 14 not 19 | 871,280 |
| 21 | animal/ | 1,847,887 |
| 22 | exp animal experiment/ | 2,222,997 |
| 23 | nonhuman/ | 5,451,924 |
| 24 | (rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. | 5,769,128 |
| 25 | or/21-24 | 8,551,341 |
| 26 | exp human/ | 19,660,148 |
| 27 | human experiment/ | 407,460 |
| 28 | or/26-27 | 19,661,675 |
| 29 | 25 not (25 and 28) | 6,413,022 |
| 30 | 20 not 29 | 784,532 |
| 31 | smoking cessation.mp. | 57,588 |
| 32 | exp Smoking cessation/ | 51,772 |
| 33 | ((quit$ or stop$ or ceas$ or giv$) adj5 smok$).ti,ab. | 21,844 |
| 34 | or/31-33 | 63,973 |
| 35 | 30 and 34 | 5505 |
MEDLINE
| Database | MEDLINE |
| Issue | May Week 2 2018 |
| Platform | Ovid |
| Date searched | 23 May 2018 |
| Searcher | Tristan Snowsill |
| Total hits | 3195 |
| # | Searches | Results (n) |
|---|---|---|
| 1 | economics/ | 26,924 |
| 2 | exp “costs and cost analysis”/ | 215,318 |
| 3 | economics, dental/ | 1892 |
| 4 | exp “economics, hospital”/ | 22,857 |
| 5 | economics, medical/ | 8955 |
| 6 | economics, nursing/ | 3979 |
| 7 | economics, pharmaceutical/ | 2762 |
| 8 | (economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. | 551,958 |
| 9 | (expenditure$ not energy).ti,ab. | 22,078 |
| 10 | value for money.ti,ab. | 1204 |
| 11 | budget$.ti,ab. | 21,187 |
| 12 | or/1-11 | 689,878 |
| 13 | ((energy or oxygen) adj cost).ti,ab. | 3129 |
| 14 | (metabolic adj cost).ti,ab. | 1048 |
| 15 | ((energy or oxygen) adj expenditure).ti,ab. | 19,752 |
| 16 | or/13-15 | 23,096 |
| 17 | 12 not 16 | 684,722 |
| 18 | letter.pt. | 933,150 |
| 19 | editorial.pt. | 408,918 |
| 20 | historical article.pt. | 344,882 |
| 21 | or/18-20 | 1,669,405 |
| 22 | 17 not 21 | 652,628 |
| 23 | Animals/ | 6,209,917 |
| 24 | Humans/ | 17,085,445 |
| 25 | 23 not (23 and 24) | 4,425,870 |
| 26 | 22 not 25 | 605,607 |
| 27 | smoking cessation.mp. | 31,789 |
| 28 | exp Smoking cessation/ | 25,621 |
| 29 | ((quit$ or stop$ or ceas$ or giv$) adj5 smoking).ti,ab. | 12,743 |
| 30 | exp Smoking/pc, th | 1935 |
| 31 | or/27-30 | 36,586 |
| 32 | 26 and 31 | 3195 |
MEDLINE In-Process & Other Non-Indexed Citations
| Database | MEDLINE In-Process & Other Non-Indexed Citations |
| Issue | 22 May 2018 |
| Platform | Ovid |
| Date searched | 23 May 2018 |
| Searcher | Tristan Snowsill |
| Total hits | 283 |
| # | Searches | Results (n) |
|---|---|---|
| 1 | economics/ | 0 |
| 2 | exp “costs and cost analysis”/ | 0 |
| 3 | economics, dental/ | 0 |
| 4 | exp “economics, hospital”/ | 0 |
| 5 | economics, medical/ | 0 |
| 6 | economics, nursing/ | 0 |
| 7 | economics, pharmaceutical/ | 0 |
| 8 | (economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. | 97,280 |
| 9 | (expenditure$ not energy).ti,ab. | 2785 |
| 10 | value for money.ti,ab. | 185 |
| 11 | budget$.ti,ab. | 3856 |
| 12 | or/1-11 | 101,161 |
| 13 | ((energy or oxygen) adj cost).ti,ab. | 484 |
| 14 | (metabolic adj cost).ti,ab. | 134 |
| 15 | ((energy or oxygen) adj expenditure).ti,ab. | 2022 |
| 16 | or/13-15 | 2581 |
| 17 | 12 not 16 | 100,421 |
| 18 | letter.pt. | 48,233 |
| 19 | editorial.pt. | 46,837 |
| 20 | historical article.pt. | 1 |
| 21 | or/18-20 | 95,070 |
| 22 | 17 not 21 | 99,461 |
| 23 | Animals/ | 4 |
| 24 | Humans/ | 9 |
| 25 | 23 not (23 and 24) | 0 |
| 26 | 22 not 25 | 99,461 |
| 27 | smoking cessation.mp. | 2131 |
| 28 | exp Smoking cessation/ | 0 |
| 29 | ((quit$ or stop$ or ceas$ or giv$) adj5 smoking).ti,ab. | 1149 |
| 30 | exp Smoking/pc, th | 0 |
| 31 | or/27-30 | 2689 |
| 32 | 26 and 31 | 283 |
NHS Economic Evaluation Database
| Database | NHS Economic Evaluation Database |
| Issue | Issue 2 of 4, April 2015 |
| Platform | The Cochrane Library |
| Date searched | 23 May 2018 |
| Searcher | Tristan Snowsill |
| Total hits | 214 |
| # | Searches | Results (n) |
|---|---|---|
| 1 | smoking cessation | 214 |
| 2 | [mh “Smoking Cessation”] | 0 |
| 3 | (quit or stop or cease or give) near/5 smoking | 67 |
| 4 | {OR #1-#3} | 214 |
Health Technology Assessment
| Database | Health Technology Assessment database |
| Issue | Issue 4 of 4, October 2016 |
| Platform | The Cochrane Library |
| Date searched | 23 May 2018 |
| Searcher | Tristan Snowsill |
| Total hits | 107 |
| # | Searches | Results (n) |
|---|---|---|
| 1 | smoking cessation | 104 |
| 2 | [mh “Smoking Cessation”] | 0 |
| 3 | (quit or stop or cease or give) near/5 smoking | 21 |
| 4 | {OR #1-#3} | 107 |
Appendix 32 Health economic modelling details
Methods
Smoking behaviour
Relapse
The risk of relapse for individuals in the ‘abstinent smokers’ state was estimated using data from Hawkins et al. 101
The data from table 2 of Hawkins et al. 101 were used to produce data suitable for survival analysis with interval censoring (Table 60).
| t l | t r | Relapsing in period (tl, tr) | Lost to follow-up in period (tl, tr) |
|---|---|---|---|
| 1 | 2 | 227 | 232 |
| 2 | 3 | 95 | 201 |
| 3 | 4 | 48 | 139 |
| 4 | 5 | 25 | 85 |
| 5 | 6 | 16 | 109 |
| 6 | 7 | 6 | 87 |
| 7 | 8 | 6 | 57 |
| 8 | 9 | 1 | 40 |
| 9 | 10 | 4 | 29 |
| 10 | 11 | 0 | 25 |
| 11 | ∞ | 155 |
Owing to the study design of Hawkins et al. ,101 individuals would have quit smoking at some point in the interval (−1,0), that is at some point in the year prior to the time origin. In addition, owing to the design of Hawkins et al. ,101 any individuals not reported as not smoking for two consecutive annual waves would be excluded, so the distribution is left-truncated at 1 + ui where –ui ϵ (–1,0) is the (unobserved) time at which the individual i quit.
We assumed that ui follows a uniform distribution, and therefore performed survival analysis with all observations left-truncated at an unobserved time and interval- or right-censored.
A Bayesian model was constructed in Stan102 using a Gompertz distribution for relapse times. The prior distribution for the shape parameter was N(0,1) and the prior distribution for the rate parameter was γ(2,0.1). Weibull and log-normal distributions were also investigated, but did not achieve good fits to the data. The default No U-Turn (Hamiltonian) Sampler was used, with four chains, each with a warm-up of 1000 iterations and sampling of 1000 iterations.
Let 1 + ui be the duration of left truncation, where ui ∼ Uniform (0,1). We observe that the individual relapsed later than tiL+ui , and we may observe that they relapsed earlier than tiR+ui (interval-censored) or we may not know this (right-censored).
If right-censored:
If interval-censored:
Trace and autocorrelation function plots were examined to ensure appropriate mixing of the chains.
| Parameter | Posterior mean | Posterior SD | Posterior correlation |
|---|---|---|---|
| Shape | −0.4184616 | 0.0346951 | −0.9027895 |
| Rate | 0.4070777 | 0.04612629 | |
| Lead time | 0.500009 | 0.2888457 |
A Gompertz model has previously been used for relapse rates,110 although it had a different parameterisation and measured time in months rather than years.
As shown in Figure 23, these two estimates ultimately lead to quite similar rates of permanent abstinence, but the risk of relapse lasts longer in the data from Hawkins et al. 101
FIGURE 23.
Relapse curves.
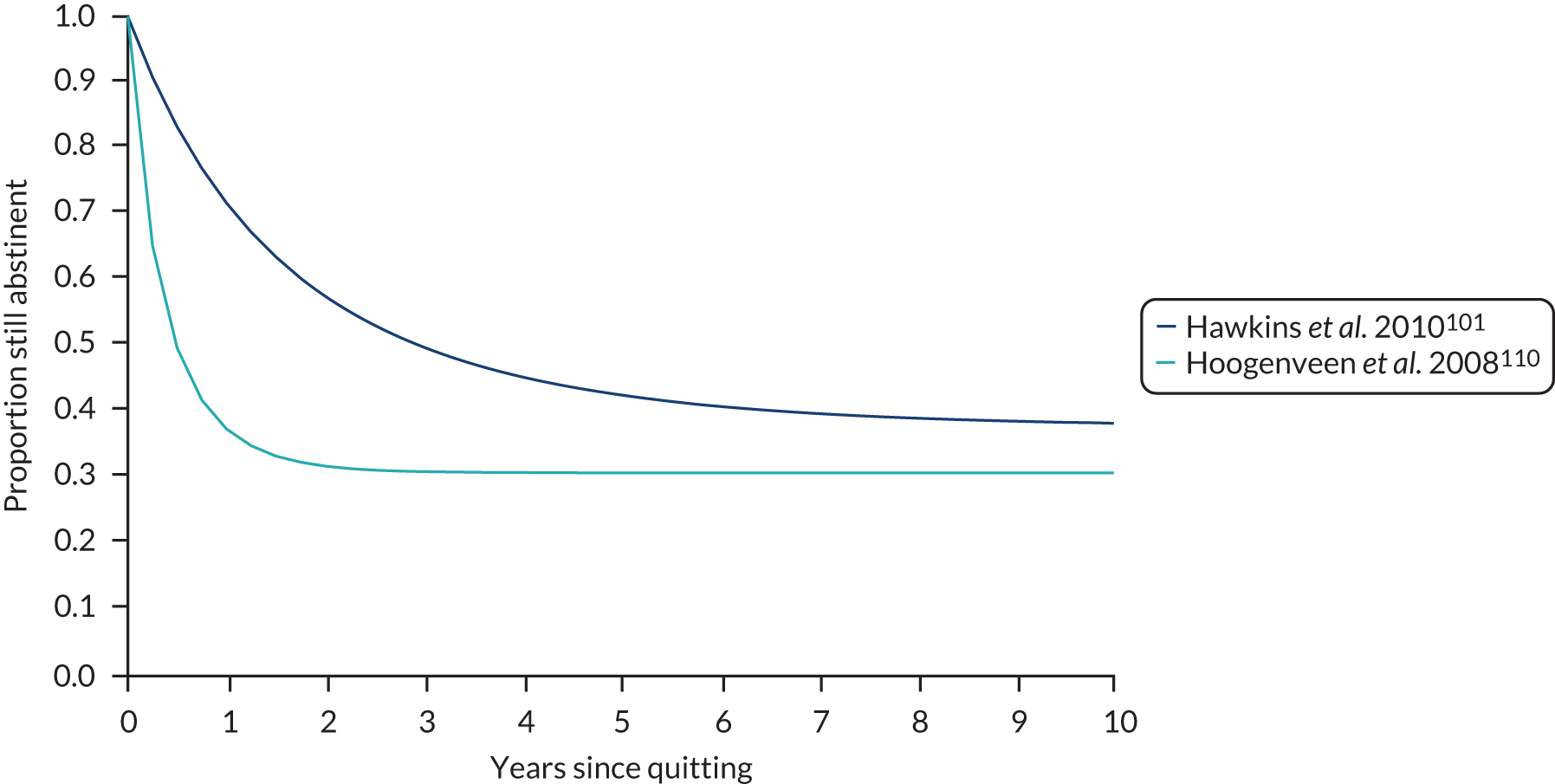
Spontaneous quitting
The annual probability of spontaneously quitting smoking was estimated from the Understanding Society study, waves 6–9. This study replaces the British Household Panel Survey (which was the data source that Hawkins et al. 101 used to estimate relapse curves).
Individuals were identified who described themselves as a smoker in at least one wave and were then present in the subsequent wave. Logistic regression was conducted to estimate the probability of not currently smoking, having identified as smoking in the previous wave, conditional on age. Non-linear age dependence was accounted for by the inclusion of restricted cubic splines.
This logistic regression gave a probability of being a non-smoker 1 year after being a smoker. This cannot be directly converted to a hazard rate for quitting. An individual can still be a smoker after a year because they continued smoking or because they had one or more unsuccessful quit attempts. If they are a non-smoker, it means that they quit and avoided relapse.
We use an exponential distribution to approximate the Gompertz distribution for relapse in the short term (as we need to approximate it for only 1 year).
We chose the rate by the 6-month relapse risk as shown in Equation 10:
Given this, we have a two-state ordinary differential equation system, as shown in Equation 11, which is solved in Equation 14. We set λq to obtain the relevant probability of having quit 1 year later, as shown in Equation 15.
This does not have an analytical solution, so we use the Newton method to find a numerical solution. The initial estimate for λq is λq(0)=−ln(1−p) , and we perform 10 Newton method iterations.
Figure 24 shows the results of our analyses. The probability of being a non-smoker 1 year after reporting smoking ranges from 11.5% to 20.3%. It is high at young ages, then dips in middle age, before rising again in older age. The rate without adjustment for relapse and the rate with adjustment for relapse are also shown in the figure.
FIGURE 24.
Annual probability and rate of spontaneous quitting.

Smoking-related excess mortality
Two data sources were used to estimate smoking-related excess mortality, which is included in the model to represent smoking-related mortality other than the four explicitly modelled morbidities. The primary data source was the study by Jha et al. ,122 which estimated the impact of smoking cessation on all-cause mortality, using age of quitting as the factor of interest. A secondary data source, Prescott et al. ,123 provided a further estimate of the excess mortality for current smokers compared with former smokers.
These data were synthesised using a non-linear model for the hazard ratio. It was assumed that, for former smokers, the hazard ratio would have a sigmoid shape as a function of age at quitting, and that the hazard ratio would never be < 1 (the mortality rate for former smokers cannot be lower than the mortality rate for never smokers). The hazard ratio for former smokers was assumed to have an asymptote of the hazard ratio for current smokers.
We fitted the model shown in Equation 14 using maximum likelihood estimation (non-linear least squares). In this model, exp(β2) is the hazard ratio for current smokers (and the asymptotic hazard ratio for former smokers as age at quitting increases), whereas β0 and β1 determine the position and slope of the sigmoid curve for former smokers.
Prevalence of morbidities at start of model
Chronic obstructive pulmonary disease
The Health Survey for England 2018111 provides data on the smoking and COPD status for 8108 adults, and also provides their ages in 5-year intervals. To fit a flexible regression spline for age, we created 25 imputation sets, with age for each individual randomly sampled in each of the imputation sets. The probability distribution for age, given age group, was estimated using mid-2018 population estimates for England. 200,201 For each imputation set, we fitted a logistic model for COPD status among current smokers (n = 1316) with a restricted cubic regression spline with knots at 19, 40, 56 and 79 (reusing the knots from the estimation of spontaneous quitting behaviour). The models from the imputation sets were then combined using Rubin’s rules. The resulting estimates are shown in Figure 25. The prevalence of COPD is expected to be near zero for those aged < 40 years and to rise to 15–20% for those aged ≥ 70 years.
FIGURE 25.
Estimated prevalence of COPD among current smokers.
Lung cancer
We obtained historical cancer registrations in England for lung cancer (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, codes C33–34) and population estimates from 1998 to 2017. 115 We fitted an age–period–cohort model with restricted cubic splines to estimate the incidence of lung cancer primarily from age and birth cohort. A reference cohort of those born in 1970 was used and the number of knots for each spline was chosen by inspection for face validity (five for age, two for period, five for cohort). Separate models were fitted for men and women, as smoking patterns have differed significantly for men and women.
These rates were used to estimate the probability density function for lung cancer incidence according to age for each birth cohort. These were then combined with a Weibull model for lung cancer survival (which was fitted to recent estimates of lung cancer survival with age group and gender as covariates116) to estimate the prevalence of lung cancer at each age.
This was then adjusted by the probability of each smoking status (never smoker, current smoker, former smoker) and the relative risk of developing lung cancer according to smoking status taken from Remen et al. 114 Remen et al. 114 estimate (in table 5) ORs of 4.47 and 17.59 for former and current smokers, respectively, for the development of lung cancer, compared with never smokers. As the absolute risk of lung cancer is low (especially for never smokers), the ORs were assumed to closely approximate relative risks, and it was also assumed that these relative risks for incidence would approximate the relative risks for prevalence.
The resulting estimates for lung cancer prevalence are shown in Figure 26; the prevalence of lung cancer is < 1% for those aged < 60 years, and then rises steadily to around 4% for those aged ≥ 80 years.
FIGURE 26.
Estimated prevalence of lung cancer among current smokers.

Results
| Age (years) | Gender | Slope of INMB vs. risk difference | ‘Break-even’ risk difference |
|---|---|---|---|
| 30 | Female | 49.5 | 4.83 |
| 50 | Female | 162.3 | 1.47 |
| 70 | Female | 83.0 | 2.88 |
| 30 | Male | 62.0 | 3.86 |
| 50 | Male | 179.7 | 1.35 |
| 70 | Male | 84.9 | 2.82 |
| Age (years) | Gender | QALYs | Costs (£) | ICER (£ per QALY) | INMB | ||
|---|---|---|---|---|---|---|---|
| SAU | Intervention | SAU | Intervention | ||||
| 30 | Female | 20.76 | 20.77 | 3630 | 3859 | 24,300 | 53.8 |
| 50 | Female | 14.92 | 14.94 | 11,643 | 11,860 | 9900 | 439.8 |
| 70 | Female | 7.54 | 7.55 | 11,488 | 11,721 | 21,500 | 91.7 |
| 30 | Male | 20.20 | 20.21 | 3479 | 3710 | 19,300 | 127.2 |
| 50 | Male | 14.03 | 14.06 | 10,573 | 10,798 | 9200 | 506.78 |
| 70 | Male | 6.70 | 6.71 | 9358 | 9595 | 21,100 | 99.8 |
| Arm | Life-years | QALYs | Costs (£) | INMB (£000) |
|---|---|---|---|---|
| SAU | 26.12 | 13.45 | 10,777 | 392.59 |
| Intervention | 26.14 | 13.46 | 11,011 | 392.66 |
| Difference | 0.026 | 0.010 | 233 | 0.0693 |
Appendix 33 Comparison of randomised controlled trial outcomes
The following table shows the summaries of four parallel-group randomised trials of the effects of reduction-focused behavioural support for smokers not wanting to quit.
| Study | Population and design | Intervention group(s) | Control/SAU | Outcomes | Results |
|---|---|---|---|---|---|
| Carpenter et al.10 | 616 community-dwelling smokers randomised to three groups | 6-week telephone-based (a) three sessions of reduction counselling plus NRT plus brief advice to quit, or (b) three sessions of motivational advice plus brief advice | (c) SAU |
|
(a) and (b) > (c) for quit attempts and point prevalence |
| Glasgow et al.11 | 320 (patients scheduled for outpatient surgery or a diagnostic procedure) randomised to two groups | 6-month theory-based (a) smoking graduated-reduction intervention with both four telephone counselling and four tailored newsletter contacts | (b) Enhanced SAU |
|
|
| Catley et al.14 | 255 community-dwelling smokers randomised to three groups | 18-week face-to-face (with additional sessions for quitters) (a) four sessions of MI, or (b) four sessions of health education | (c) One session of brief advice |
|
|
| Klemperer et al.12 | 560 community-dwelling smokers randomised to three groups | 4 weeks by telephone (a) three calls with brief motivational support, or (b) three calls with smoking reduction support | (c) SAU |
|
|
List of abbreviations
- AIC
- Akaike information criterion
- BENESCO
- Benefits of Smoking Cessation on Outcomes
- BMI
- body mass index
- CC
- core competency
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- CPS-II
- Cancer Prevention Study II
- CRN
- clinical research network
- DPsych
- Doctor of Psychology
- EARS
- Exercise Assisted Reduction then Stop
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GDPR
- General Data Protection Regulation
- GLM
- generalised linear model
- GP
- general practitioner
- HSI
- Heaviness of Smoking Index
- HT
- health trainer
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- INMB
- incremental net monetary benefit
- IP
- internal pilot phase participant
- LNCP
- licensed nicotine-containing product
- MI
- motivational interviewing
- MVPA
- moderate to vigorous physical activity
- NICE
- National Institute for Health and Care Excellence
- NRT
- nicotine replacement therapy
- OARS
- open questions, affirmation, reflective listening, summaries
- OR
- odds ratio
- PA
- physical activity
- PenCTU
- Peninsula Clinical Trials Unit
- PhD
- Doctor of Philosophy
- PM
- practice manager
- PMSLT
- proportional multistate life table
- p.p.m.
- parts per million
- PPI
- patient and public involvement
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RA
- research assistant
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SAP
- statistical analysis plan
- SAU
- support as usual
- SD
- standard deviation
- SF-12v2
- Short Form questionnaire-12 items, version 2
- SMART
- specific, measurable, achievable, relevant, time-bound
- SWAT
- study within a trial
- TARS
- Trial of physical Activity-assisted Reduction of Smoking
- TIP
- trial information pack
- TSC
- Trial Steering Committee
Notes
-
TARS SWAT: the effect of the method of invitation on recruitment of participants from general practices to a trial of a smoking reduction intervention
-
Summary statistics of self-reported smoking, physical activity and sleeping prior to truncation at baseline and at 3 and 9 months, by allocated group
-
Weekly and daily use of licensed nicotine-containing products at baseline and at 3 and 9 months, by allocated group
-
Estimated intervention effects on self-reported quit attempts of at least 24 hours in the previous 3 or 6 months (reported at 3 and 9 months, respectively)
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/KLTG1447).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
