Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/42/02. The contractual start date was in January 2012. The draft report began editorial review in March 2022 and was accepted for publication in August 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2024 Mackenzie et al. This work was produced by Mackenzie et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Mackenzie et al.
Chapter 1 Introduction
Parts of this chapter have been reproduced, with permission, from Logue et al. [Logue J, Stewart S, Munro J, Bruce J, Grieve E, Lean M, et al. SurgiCal Obesity Treatment Study (SCOTS): protocol for a national prospective cohort study of patients undergoing bariatric surgery in Scotland. BMJ Open 2015; doi.org/10.1136/bmjopen-2015-008106], Grieve et al. (Grieve E, Mackenzie RM, Munro J, O’Donnell J, Stewart S, Ali A, et al. Variations in bariatric surgical care pathways: a national costing study on the variability of services and impact on costs. BMC Obes 2018; doi.org/10.1186/s40608-018-0223-3) and Mackenzie et al. [Mackenzie RM, Greenlaw N, Ali A, Bruce D, Bruce J, Grieve E, et al. SCOTS investigators. SurgiCal Obesity Treatment Study (SCOTS): a prospective, observational cohort study on health and socioeconomic burden in treatment-seeking individuals with severe obesity in Scotland, UK. BMJ Open 2021; 11:e046441; doi.org/10.1136/BMJOPEN-2020-046441].
Obesity
Body mass index (BMI) is an indicator of body fat calculated by dividing a person’s weight in kilograms by their height in metres squared. Obesity is defined as BMI ≥ 30 kg/m2 and severe obesity as BMI ≥ 40 kg/m2. 1
Obesity and, in particular, severe obesity are associated with a variety of negative health outcomes, including increased risk of most major chronic diseases and premature death. 2 Obesity-related comorbidities are defined as conditions either directly caused by obesity or known to have their presence or severity affected by obesity. Consequently, it is anticipated that these comorbid conditions will improve or enter remission in the presence of effective and sustained weight loss. 3 A non-exhaustive list of known obesity-related comorbidities includes:
-
premature mortality
-
cardiovascular conditions
-
hypertension
-
atherosclerosis
-
myocardial infarction (MI)
-
stroke
-
congestive heart failure
-
cardiac arrhythmias
-
-
metabolic conditions
-
type 2 diabetes mellitus (T2DM)
-
prediabetes
-
dyslipidaemia
-
non-alcoholic fatty liver disease
-
-
pulmonary conditions
-
obstructive sleep apnoea
-
asthma
-
-
musculoskeletal conditions
-
degenerative arthritis
-
immobility
-
pain
-
-
reproductive conditions
-
polycystic ovary syndrome
-
infertility
-
sexual dysfunction
-
-
genito urinary conditions
-
impaired renal function
-
kidney stones (nephrolithiasis)
-
stress urinary incontinence
-
-
central nervous system conditions
-
impaired cognition
-
headache
-
idiopathic intracranial hypertension (pseudotumour cerebri)
-
-
psychosocial conditions
-
impaired health-related quality of life (HRQoL)
-
depression
-
anxiety
-
other psychopathy
-
-
cancers.
People with severe obesity are at increased risk of several of these comorbidities, including T2DM, cardiovascular disease (CVD) and depression. This can lead to the development of multiple morbidities, often at a young age, which, in turn, causes reduced HRQoL, increased healthcare costs and heightened risk of mortality. 4
In recent years, severe obesity has emerged as a major public health concern, with rates increasing rapidly in a number of countries across the world, including the United States of America (USA) where the prevalence of BMI > 40 kg/m2 among adults rose by 96% between 2000 and 2018, and around 9.2% of the adult population is now considered to have severe obesity. 5 Similarly, levels of severe obesity have risen in the United Kingdom (UK), with 3.3% of all adults in England6 and 3% of all adults in Scotland now estimated to have a BMI ≥ 40 kg/m2. 7
Obesity treatment
As the prevalence of severe obesity rises, effective treatment is a priority. Treatments for severe obesity may be surgical or non-surgical. Bariatric surgery is the collective term for a number of surgical interventions with the primary purpose of achieving large-scale weight loss. Non-surgical treatment usually involves a multicomponent approach comprising behavioural therapy, dietary change and increased physical activity, and can also involve pharmacotherapy. 8,9
Bariatric surgery
Bariatric surgery procedures can be restrictive, malabsorptive or a combination of the two and include Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG) and laparoscopic adjustable gastric banding (LAGB). 3
Roux-en-Y gastric bypass
RYGB is a restrictive-malabsorptive surgical procedure which involves transecting the stomach to create a small gastric pouch and connecting it to the small intestine. Ingested nutrients are thereby diverted from the body of the stomach, duodenum and proximal jejunum. 3,10 Consequently, less food is required for satiety and fewer calories are absorbed from food consumed.
Sleeve gastrectomy
In the restrictive SG procedure, a large portion of the stomach is removed, creating a tubular stomach based on the lesser curvature of the stomach. 3 As a result, patients are unable to consume as much food as they were prior to surgery and satiety is achieved sooner.
Laparoscopic adjustable gastric banding
LAGB is a minimally invasive restrictive bariatric procedure which involves using laparoscopic surgery to place an adjustable band around the top portion of the stomach. This creates a small pouch which results in less food intake and increased food transit time. The band is connected to a small device placed under the skin which allows post-surgical band tightening. 11,12
Endocrine changes associated with bariatric surgery
Many of the beneficial metabolic effects of bariatric surgery have been attributed to altered peptide hormone profiles, particularly gastrointestinal (GI) and pancreatic peptide hormones. These alterations include increases in peptides that increase satiety, such as glucagon-like peptide-1 (GLP-1), gastric inhibitory polypeptide, pancreatic peptide YY3-36, oxyntomodulin and gastrin. 3,13,14 Bariatric surgery is also known to cause an increase in peptides which reduce levels of the appetite-stimulating hormone, ghrelin. 3,13,14
Bariatric surgery and weight loss
A recent systematic review and meta-analysis of 33 published datasets of long-term (≥10 years) outcomes shows that all current bariatric surgical procedures are associated with substantial and enduring weight loss. 15 Eighteen reports of gastric bypass showed a weighted mean excess weight loss (EWL) of 56.7%, while 17 reports of LAGB showed 45.9% EWL and two reports of SG showed 58.3% EWL. 15 As such, there is high-quality evidence that bariatric surgery achieves sustained weight loss.
Clinical effectiveness and cost-effectiveness of bariatric surgery
A 2014 Cochrane systematic review of 22 randomised controlled trials (RCTs) for bariatric surgery found it to be more clinically and economically effective for the treatment of severe obesity than non-surgical measures after 2 years. 8 This finding was supported by data from trials with longer term follow-up, and cohort and modelling studies. 15,16 Indeed, in their 2019 systematic review and meta-analysis of 33 datasets reporting long-term (≥10 years) outcomes following bariatric surgery, O’Brien and colleagues reported that surgical procedures resulted in a weight loss effect three to four times greater than that of non-surgical therapy. 15 Similarly, Borisenko et al. found bariatric surgery to be cost-effective at 10 years post surgery16 and results from the Swedish Obese Subjects (SOS) study demonstrate cost-effectiveness of surgical procedures over 15 years, particularly in those with diabetes. 17
Clinical effectiveness and outcomes of bariatric surgery
Mortality
With regard to clinical outcomes following bariatric surgery, mortality rate has been one of the most extensively investigated to date. Observational studies have reported that patients undergoing bariatric surgery have a subsequent longer life expectancy than patients receiving non-surgical treatment for obesity; several large-scale cohort studies published in the last decade18–28 have reported a significant reduction in relative risk of long-term all-cause mortality for patients following bariatric surgery as compared to non-surgical controls. A recent meta-analysis29 of these studies (n = 269,818 bariatric surgery patients and 1,270,086 controls) revealed that bariatric surgery is associated with a 62% reduction of all-cause mortality for the whole operated population as compared to controls (pooled odds ratio = 0.62, p < 0.001).
Complications and hospitalisations
Complications following bariatric surgery have been poorly reported in the literature. However, a systematic review and meta-analysis conducted in 2017 assessed early (<30 days post surgery) major complications associated with bariatric surgery: anastomotic leak, MI and pulmonary embolism. 30 The review included 71 studies and 107,874 patients undergoing RYGB, LAGB or SG in the USA and reported that rates of the three major complications after either one of the procedures ranged from 0% to 1.6%. Mortality following these complications ranged from 0% to 0.6%. 30
Type 2 diabetes mellitus
Over the last 10 years, bariatric surgery has been shown to be an effective treatment for T2DM in patients with obesity. 9,15,31,32 A number of RCTs and cohort studies have demonstrated that surgery is associated with a greater improvement in hyperglycaemia as compared to alternative treatment and that this effect is sustained for at least 5 years postoperatively. 33–36 Improvement in hyperglycaemia is associated with a reduction in mortality37,38 and diabetes-related complications,39,40 including retinopathy, nephropathy and CVD. Improvement and remission of T2DM post bariatric surgery has been shown to be mediated by both weight-loss-dependent and weight-loss-independent mechanisms. 41,42
Cardiovascular disease
Bariatric surgery is associated with a reduction in cardiovascular mortality. 24,26,28,29 Two recent retrospective studies of patients with diabetes undergoing SG and RYGB report bariatric surgery is associated with significantly lower incidence of major cardiovascular events after 8 years. 43 Recently, Doumouras and colleagues44 demonstrated, in a population-based matched cohort study of 2638 patients with severe obesity and CVD, that bariatric surgery is associated with a significantly lower incidence of major adverse cardiovascular events, cardiovascular mortality, coronary events and heart-failure hospitalisations. While these results are yet to be confirmed in a large RCT, they suggest that bariatric surgery may be an effective intervention for patients with severe obesity and ischaemic heart disease or heart failure.
Health-related quality of life
It is currently well established that post-surgical HRQoL is improved on comparison with preop HRQoL for up to 10 years. 45–48 When patients who decide to proceed to surgery are compared to those who opt for non-surgical treatment only, baseline HRQoL is often far lower in those who select surgery, showing at least a perception of reduced HRQoL in these individuals. 49 A 2020 study by Poelemeijer et al. found that severe post-surgical complications and failure to achieve desired weight loss had a negative effect on postoperative (postop) HRQoL outcomes at 12 months. 48 There is a need for further research to examine correlations between HRQoL, weight loss, complications and clinical outcomes.
Anxiety and depression
The literature suggests that bariatric surgery is associated with long-term reductions in anxiety and depressive symptoms. In a systematic review of 14 prospective studies, 13 studies (93%) reported statistical and clinically significant reductions in the severity of patient-reported depressive symptoms up to 3 years after bariatric surgery. Similarly, there were reductions in overall anxiety symptom severity at ≥2 years post-surgical follow-up. 50
Cost-effectiveness of bariatric surgery
Obesity has an enormous economic impact; the total cost to the UK National Health Service (NHS) is estimated to be £6.1 billion per year. 51 Estimates of costs to the NHS include direct costs, indirect costs and the cost of treating obesity-related complications. More broadly, obesity has a serious impact on economic development; the overall cost of obesity to the UK economy is estimated at £27 billion. With increasing levels of obesity and severe obesity, these costs are set to rise, with the UK-wide NHS costs attributable to overweight and obesity projected to reach £9.7 billion per year by 2050 and the wider cost to UK society estimated to reach £49.9 billion. 52
Provision of bariatric surgery represents a relatively high upfront cost but surgical intervention is demonstrated to be cost-effective for adults with severe obesity when compared to non-surgical treatments. Cost savings arise from health benefits of a reduction in onset of incident diabetes, remission of existing diabetes and lower mortality. 4 Indeed, economic analysis for the National Institute for Health and Care Excellence (NICE) confirmed that the financial outlay for bariatric surgery is justified for the NHS. 53 In patients with diabetes, it was found that the cost of surgery would be negated within 3 years due to the reduction in prescriptions required. 54
A systematic review of the economic evidence suggests that RYGB surgery, compared with standard care for obesity, is associated with incremental cost-effectiveness ratios (ICERs) of between US$5400 (approximately £3172) and US$25,000 (approximately £20,779), with a cost per life-year gained of US$8171 (approximately £5000). 55 BMI change results from randomised trials of RYGB identified in the review were considered within a health economic simulation model using contemporary UK health data and appropriate modelled costs for NHS bariatric surgery follow-up, including complications. RYGB had an ICER of £10,126 compared to no intervention. These cost savings largely accrue from reduced resource use (including fewer outpatient clinic visits, hospitalisations, length of stay in hospital and medications) postoperatively. 56,57 These reductions in health service utilisation can partially be explained by patients undergoing definitive treatment for comorbid problems, such as total knee replacement or urology surgery, previously denied because of their obesity. The major reduction in utilisation, however, reflects improvement in medical conditions such as hypertension or diabetes, a direct result of weight loss. 56,58–60 Surgery also has indirect cost benefits; for example, state disability allowances are reduced if improved activity levels allow patients to return to paid employment. 61
Furthermore, bariatric surgery is comparably cost-effective to other public health interventions in the UK, including smoking cessation and the use of statins for primary prevention of CVD. 62 The 2022/23 NHS England Tariff Process for bariatric surgery was £8972 for RYGB, £5859 for SG and £2494 for LAGB.
UK bariatric surgery guidelines
UK NICE guidelines63 currently indicate that bariatric surgery is a treatment option for those with a BMI ≥ 40 kg/m2, or between 35 kg/m2 and 40 kg/m2 in the presence of other significant diseases (T2DM or hypertension, for example), which could be improved if they lost weight. People with a BMI of 30–34.9 kg/m2 with onset of T2DM within 10 years and people of Asian ethnicity with onset of T2DM at a lower BMI are also considered for assessment, as are any adults with a BMI > 50 kg/m2. In all cases, non-surgical weight management must have been attempted but not resulted in clinically beneficial weight loss before bariatric surgery is indicated. 53,63
In Scotland, Scottish Intercollegiate Guidelines Network guidance64 is followed. These guidelines are broadly similar to those of NICE, stipulating that bariatric surgery should be considered for those with a BMI ≥35 kg/m2 and one or more severe comorbidities which are expected to improve significantly with weight reduction, such as mobility problems, arthritis and T2DM. As per NICE guidelines, there is a requirement for evidence of completion of a multicomponent, structured weight-management programme that has not resulted in significant and sustained improvement in comorbidities. 64
Bariatric surgery rates
Despite evidence that bariatric surgery is a more clinically and economically effective intervention for the treatment of obesity than non-surgical options, for the UK NHS, like many other health systems, the volume of bariatric surgery procedures commissioned is very low. In fact, despite obesity prevalence being among the highest in the European Union, the UK performs only nine bariatric surgery procedures per 100,000 people,4,65 while Sweden, a country with a similar health service but lower obesity prevalence, performs 70–80 procedures per 100,000 people. 66 In North America, the rate of surgery is around 40–50 per 100,000 people, with the majority of these operations performed in the USA. 65 Moreover, despite escalating levels of severe obesity in the UK, numbers of NHS bariatric procedures are falling, with a reduction of 31% between 2011/2012 and 2014/2015. 4,6 Rates of surgery have also been observed to vary between countries in the UK; no NHS bariatric surgery is performed in Northern Ireland, while few NHS weight-loss operations take place in Wales and Scotland as compared to England. 4
It has been suggested that one reason for low bariatric surgery rates in the UK is that rather than general practitioners (GPs) referring directly to surgical services, patients must follow a complex pre-surgical tiered pathway and barriers are often encountered (see Table 1). 4 Other barriers include the perception among patients and clinicians that bariatric surgery is high risk, and the fact that commissioners restrict funding for bariatric operations, despite evidence of cost-effectiveness. 4 The latter may be due to the initial high cost of bariatric surgery, with savings recouped in subsequent years.
| Tier | Intervention | Barriers |
|---|---|---|
| 1 | Societal interventions to enhance weight loss (e.g. food tax, encouraging walking) |
|
| 2 | Primary care provision of advice or referral to community groups for lifestyle interventions (e.g. behavioural weight-management programmes) | |
| 3 | Secondary care-based medical management (e.g. dietary advice, medication) |
|
| 4 | Multidisciplinary team selection for bariatric surgery with follow-up for 2 years |
|
The low prioritisation of bariatric surgery within the UK and the strict criteria for access to surgery, including complex pre-surgical pathways and pre-surgical weight-loss requirements,67 results in low numbers of individuals with severe obesity actually receiving surgery. Those receiving surgery generally do so after many years of alternative conservative interventions, at a point when their mean BMI is extremely high, at around 45 kg/m2, and they are at a median age of 47 years. 68 To date, it is unclear how this delay in treatment impacts on health, physical functioning and HRQoL.
UK bariatric surgery care pathways
Pre- and postop care is a major component of the total cost of bariatric surgery. 67 However, anecdotal evidence suggests that bariatric surgery care pathways vary considerably, including clinical psychology provision. International bariatric guidance, while based on best practice, does not specify the optimal model of care69,70 and there is little evidence of whether intensive pre- and postop care improves outcomes and is cost-effective compared to less intensive care. Further investigation is therefore required as to the extent the intensity of pre- and postop bariatric surgical care is a factor affecting patient outcomes after surgery.
Surgical Obesity Treatment Study (SCOTS)
Background
In 2010, the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme issued an open call for research proposals for a long-term longitudinal cohort study of patients undergoing bariatric surgery in the UK, stating that, ‘Obesity is a growing problem in the UK, with a growing number of people among the morbidly obese. Concomitantly, there is an increase in requests for bariatric surgery but insufficient evidence of long-term effectiveness and safety of these procedures. There are existing registries of bariatric surgery, but these suffer from problems of collected data, completeness of follow-up, or data availability for secondary analysis. There is a need for a long-term study of bariatric surgery, so that the outcomes and complications of different procedures, their impact on QoL and nutritional status, and the effect on comorbidities can be monitored in both the short and the long term’. Following this call, the Surgical Obesity Treatment Study (SCOTS) was commissioned to address some of the uncertainties around the clinical effectiveness of bariatric surgery in the longer term.
The original SCOTS study design included a 10-year follow-up period. However, due to unforeseen recruitment issues, the result of reductions in both NHS and private surgical numbers in Scotland, both the study design and statistical plans were revised by the funder in 2016 (Report Supplementary Material 2). The study follow-up period was reduced from 10 years to 3 years. Objectives were revised to reflect research published between 2010 (the time of writing of the original SCOTS research proposal) and 2016 when the protocol was revised.
Study aims and objectives
The aim of SCOTS prospective observational cohort study was to investigate the short- and medium-term outcomes and complications following bariatric surgery in Scotland.
The specific objectives were to establish in a cohort of patients with obesity undergoing bariatric surgery:
-
the physical and mental health, and social burden of severe obesity;
-
incidence of acute and chronic postop complications (acute complications, defined as up to 3 months post surgery, include surgical site infection, chronic complications include revisional surgery, plastic surgery and chronic pain, for different bariatric surgical procedures);
-
the effect of surgical experience and the pre- and postop care pathway on complication rates and weight loss, for different bariatric surgical procedures;
-
the effect of the pre-surgical pathway and criteria on bariatric surgery patient selection
-
change in HRQoL, anxiety and depression over time pre- and postoperatively for a mean of 3 years from date of bariatric surgery;
-
the weight status pre- and postoperatively for 3 years after bariatric surgery;
-
the glycaemic control, lipids, blood pressure, medication prescription and rate of diabetes complications (microalbuminuria and renal disease, and retinopathy) in those who have pre-existing diabetes or develop diabetes during 3 years follow-up since bariatric surgery;
-
changes in socioeconomic factors (employment, benefit receipt, sick leave and healthcare use) for 3 years since bariatric surgery.
For the second objective outlined above, the specific definitions of acute and chronic postop pain have been disregarded due to the data collection at this time point being removed from the protocol in 2016. Complications were subsequently based on health record data and therefore have different definitions.
The effect of surgical experience on complication rates and weight loss, as per the third objective, was not examined as the information collected was not deemed appropriate for this objective. UK bariatric surgeons also perform laparoscopic upper GI surgery, which adds to their overall technical skills and experience. This more general experience was not collected.
With regard to the fourth objective, the effect of eligibility criteria on bariatric surgery patient selection was not examined as these criteria were standardised across Scotland in 2014.
Chapter 2 Methods
Parts of this chapter have been reproduced with permission from Logue et al. 71 and Mackenzie et al. 72
Patient involvement
Patients identified via bariatric surgery peer support groups in Scotland were involved in the design and conduct of this research. During the protocol development stage, patients provided input with regard to data collection and defining research questions. Two focus groups were held with patients to discuss methods of recruitment; they contributed to recruitment procedures and development of materials. Patients were included in a promotional recruitment video, with consent. A patient member was included on the independent study steering committee and patients were invited to a meeting to discuss plans for dissemination of study results.
Study design
SCOTS was a national, prospective, observational cohort study of adults aged over 16 years eligible for primary bariatric surgery in Scotland. Participants were recruited from 3 December 2013 to 28 February 2017. A mean of 3 years postop follow-up continued until October 2020. A detailed protocol for the SCOTS study was published71 but was amended in 2016 with follow-up reduced from 10 to 3 years.
Study setting
The study was conducted in 10 NHS-funded and 4 private hospitals in Scotland. Centres were eligible to participate if they performed bariatric surgery. Bariatric procedures included gastric banding, gastric bypass and SG. All patients’ healthcare interactions in Scotland are recorded by use of a single patient identification number. Information technology systems are common across all 14 health board areas, and a single government-funded department (Information Services Division) collates information for research purposes. This has allowed these systems to be utilised for post-surgical patient follow-up in SCOTS.
Study participants
Adult patients (aged 16 years and over) scheduled to undergo a primary bariatric procedure at any hospital in Scotland were eligible for invitation to the study.
Inclusion criteria
For inclusion in SCOTS, patients had to:
-
be aged 16 years or over and undergoing their first bariatric surgery in NHS hospitals or private practice in Scotland
-
have capacity to consent
-
be residents of Scotland
-
be able to provide written informed consent.
Exclusion criteria
Patients were excluded from SCOTS if they:
-
had previous weight-loss surgery or at the time of potential recruitment were undergoing a repeat procedure.
Patients with limited English language were eligible for participation in the data linkage aspect of the study only.
Participant screening
Eligibility checks were undertaken by clinical bariatric surgery teams or research nurses while patients attended preop bariatric assessment clinics. Patients were approached at least 4 weeks prior to surgery. Patients were consented in clinic or referred to the SCOTS research team, who provided further study information and then obtained fully informed consent. Patient information sheets were provided at least 24 hours before patients consented to the study. In the case of those patients who were to be recruited by the research team, a patient information sheet was provided when permission was sought to hand over contact details to the research team. An independent contact was provided on the patient information leaflet so that patients could discuss participation in research studies with someone independent of the study team, should they wish to do so.
Patients consented for clinical data linkage (part one), postal, electronic and/or telephone follow-up (part two) and whether they were interested in future research. Those requiring a translator were consented for clinical data linkage only.
Withdrawal of subjects
Participants could withdraw from the study at any point for any reason. Level of withdrawal was recorded. Data were retained unless complete withdrawal was requested.
Study procedures / data collection
Part one: health record linkage
The study collected data by record linkage to participants’ clinical outcomes. Information on participants’ operations was recorded by the clinical team. Participants were then followed using their medical records until 1 October 2020. This part of the study observed patients’ care, not altering their planned care in any way. No additional tests or treatments were given to patients who consented to be part of the study. If patients did not consent to participate in this part of the study, they were not asked to participate in part two or part three. Health record systems sources for all data and outcomes are summarised in Table 2.
| Planned analysis | Outcomes/data being presented | Potential predictors being considered/data being described |
|---|---|---|
| Baseline characteristics | Baseline data being described for the total SCOTS population and by age group, BMI group and separately by SIMD quintile | Summaries for the total SCOTS population and various subpopulations of baseline characteristics, including: |
| One-year surgical complications | These will include:
|
Predictors of non-progression to surgery using baseline data, including:
Predictors of surgical complications using baseline or other follow-up data, including: |
| Three-year outcomes | These will include: | Predictors of 3-year outcomes (all-cause mortality, readmissions, change in weight) using baseline or other follow-up data, including: |
|
Additional predictors for the 3-year diabetes outcomes and complications, to those noted above, for the population with diabetes:
Additional predictors to those noted above for the 3-year outcomes, for the QoL outcomes:
|
Part two: outcome data collection
Recruited participants completed questionnaires preoperatively and 3 years postoperatively. All participants were asked if they would be willing to be contacted about other research in the future.
Outcome measures
Patient-reported outcome measures (PROMs) questionnaires collected health-related information, including weight, medical history, smoking status, alcohol use, GI symptoms, urological health, depression, anxiety, HRQoL and obesity-specific QoL (O-QoL), life optimism, physical activity, health-care utilisation, employment and social security.
Questionnaires/instruments utilised for PROMs:
-
Comorbidity was assessed by self-report using a questionnaire designed specifically for this study (Report Supplementary Material 3).
-
GI reflux symptoms were evaluated using a questionnaire developed for the REFLUX trial. 73
-
Urological health was assessed using the International Prostate Symptom Score (IPSS),74 where a score ≥ 8 indicates moderate to severe symptoms, and the International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-UI SF), wherein a score ≥ 6 indicates moderate incontinence. 75
-
Female reproductive health data were obtained using a modified version of the questionnaire developed for the Longitudinal Assessment of Bariatric Surgery study. 76
-
Information on male erectile dysfunction was obtained using a modified version of the questionnaire developed for the Massachusetts Male Ageing Study. 77
-
Anxiety and depression were assessed using the Generalised Anxiety Disorder Assessment (GAD-7)78 and Patient Health Questionnaire (PHQ-9)79 instruments, respectively. A PHQ-9 score ≥ 10 is indicative of moderate to severe depression, while a GAD-7 score ≥ 6 is reflective of moderate to severe anxiety. 78,79
-
Smoking status was ascertained using a questionnaire specifically developed for this study (Report Supplementary Material 3).
-
Alcohol use was determined using a modified version of the Alcohol Use Disorders Identification Test (AUDIT). 80
-
HRQoL was assessed using the Rand 12-item Short Form Survey (SF-12)81 and Euro QoL 5-level EQ-5D version (EQ-5D-5L)82,83 instruments.
-
O-QoL was assessed using the Impact of Weight on Quality of Life-Lite (IWQOL-Lite) questionnaire. 84 Standardised scoring was used when interpreting IWQOL-Lite questionnaires. 85
-
Life optimism was determined using a modified version of the Life Orientation Test (LOT), wherein a score range of 0–13 reflects low optimism (high pessimism). 86
-
Data on physical activity were obtained using the International Physical Activity Questionnaire (IPAQ) – Short Form. 87
-
Information on participants’ employment, social security status and healthcare utilisation was obtained using questionnaires specifically developed for this study (Report Supplementary Material 3).
-
Questions relating skin excess following bariatric surgery were specifically developed for this study (Report Supplementary Material 3).
-
Information on postop plastic surgery was obtained using questions adapted from Ertelt et al. 88
Each participant’s quintile of the Scottish Index of Multiple Deprivation (SIMD), an area-based measure of socioeconomic status,89 was derived from their postcode. Combining a number of indicators of socioeconomic status across seven domains, the SIMD provides a relative measure of deprivation which can be used to compare data zones by ranking them from most to least deprived. The seven domains include income, employment, health, education, skills and training, housing, geographic access and crime. 89
Clinical data
Height and weight at the start of the weight-management programme were reported by clinical staff at the time of recruitment, allowing BMI to be calculated. Date of surgery, operation type, weight at operation and American Society of Anesthesiologists (ASA) grade were reported by the clinical teams. Weight at routine clinical follow-up visits and any revisional bariatric surgery procedures were also recorded.
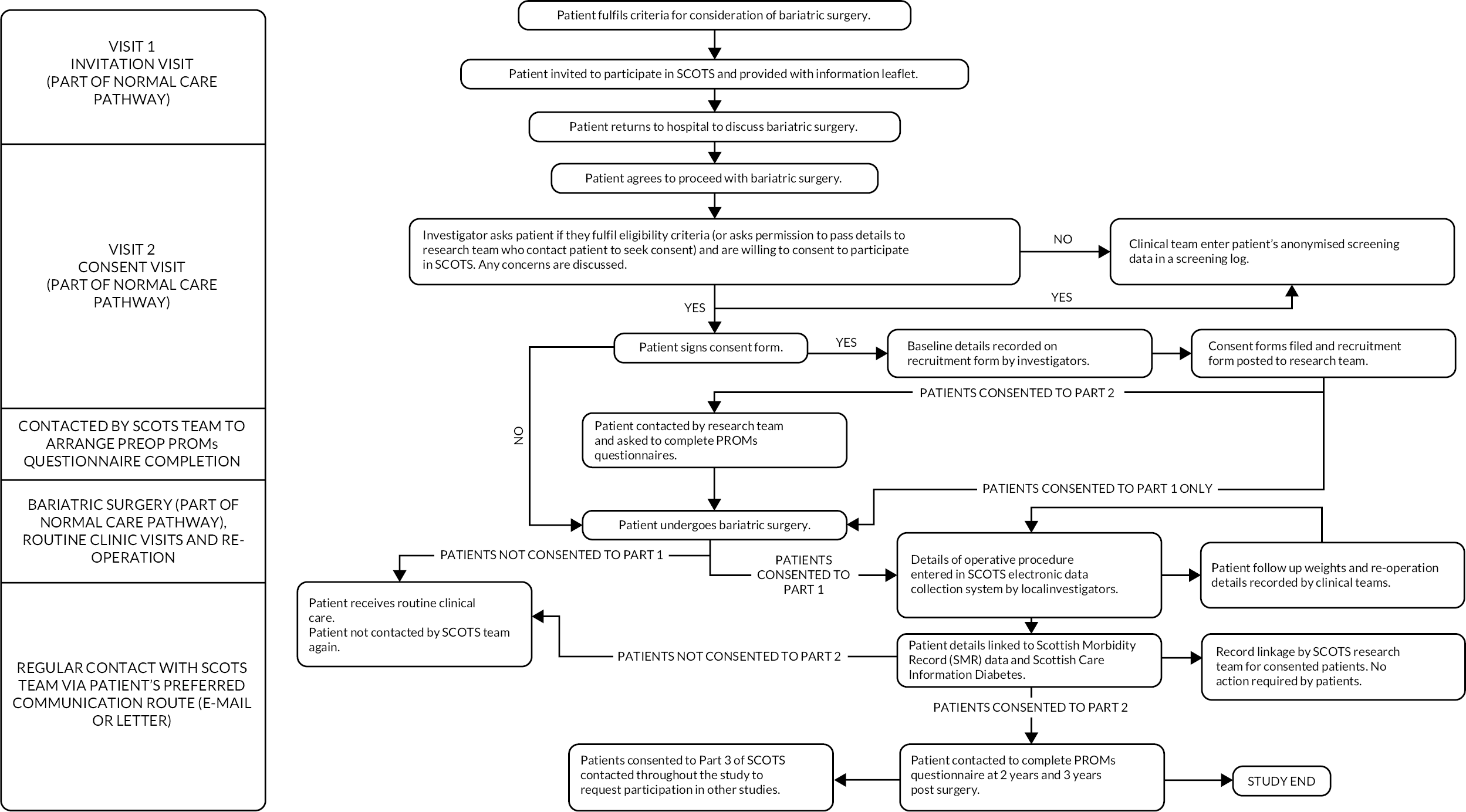
Outcomes and data sources are summarised in Table 2. Figure 1 summarises the patient journey through the study.
FIGURE 1.
SCOTS flow chart summarising patient journey through the study.
Procedures for data collection
Patient-reported outcome measures
Completion of questionnaires could be either by post or electronically via a secure link sent by e-mail. Two reminders were sent by the participant’s chosen method and a third reminder, if required, was sent by post to all participants. No further strategy was used after three reminders.
Where patients did not complete PROMs and there was no reliable clinical weight record, they were contacted after 3 years from their date of bariatric surgery requesting completion of a weight questionnaire (simply asking their current weight). The patients were offered an incentive (£30 high-street voucher) for completing both the year-2 and year-3 questionnaires. For those no longer in clinical follow-up / not completing PROMs, an incentive (£10 high-street voucher) for completing the year-3 weight questionnaire was offered.
Clinical data
A bespoke electronic data-collection system / web-based portal was developed for SCOTS. This was secure, password-protected and used to collect clinical data from participating sites. It also allowed patients to complete questionnaires online. Information from written questionnaires was entered into the database manually by the research team, as required.
Following their operation, patients had their weight-loss surgery details entered into the SCOTS electronic data collection system / web-based portal. The clinical teams then used the electronic data-collection system to include follow-up weights, gastric-band adjustments and reoperations (including reasons for reoperations).
A detailed breakdown of patient contact within the study is described in Table 3.
| Patient contact | Time | Routine care | SCOTS | Part 1 | Part 2 |
|---|---|---|---|---|---|
| 1 | 1 year to 6 weeks before surgery | Agree bariatric surgery | Patient given/sent invitation-to-participate letter and patient information sheet (and asked if they are willing to be contacted by SCOTS research team for further information and informed consent if no local recruitment). | 1 | 1 |
| 2 | 1 year to 6 weeks before surgery | Patient pre-surgical clinic visit | Patient asked if they are willing to consent to participate in SCOTS. If so, patient signs consent forms. Clinical team and patient complete contact details and baseline height and weight. | 1 | 1 |
| 3 | At least 4 weeks before surgery | Patient contacted by SCOTS team to complete preop questionnaire. | 1 | 1 | |
| 4 | Date of surgery | Patient has bariatric surgery | Clinical team enters details of bariatric surgery on SCOTS electronic data-collection system. | 1 | 1 |
| 5 | Patient admitted to hospital | If patient hospital admission is possibly related to bariatric surgery, identified by record linkage to SMR01. | 1 | 1 | |
| 6 | At routine follow-up visits | Patient attends routine clinical visits | Clinical teams enter weight and reoperation details. | 1 | |
| 7 | 2 years post surgery | Patient has routine annual diabetes care (if has diabetes) | Patient completes 2-year post-surgical questionnaire. Blood results available via SCI Diabetes. |
1 | |
| 8 | 3 years post surgery | Patient has routine annual diabetes care (if has diabetes) | Patient completes 3-year post-surgical questionnaire. Blood results available via SCI Diabetes. |
1 | |
| 9 | 3 years onward | Patient continues to have routine diabetes and post-bariatric surgery care | Patient contacted to thank them for completing PROMs. Record linkage continues. Patient informed about future SCOTS publications. | 1 | |
| Total | 5 | 9 |
Bariatric surgery care pathway site survey
Bariatric surgery care pathway update questionnaire
To establish preop assessment and postop care pathways used in bariatric surgery sites in Scotland, a questionnaire was distributed to each health centre (Report Supplementary Material 4). This covered pathways for referral, eligibility criteria, the different components of service delivery, the professionals involved and frequency and length of sessions and consultations. The questionnaire was distributed by e-mail and responses collected over a 2-year period which served as a consistency check for within-centre reporting over multiple years. Follow-up discussions by phone and e-mail were undertaken with centres where clarifications were required, on staffing grade for example. A limitation was that practice was not observed at any site to cross validate with the self-reported information.
Costing
Costs were based on publicly available information for staff time. Unit costs were taken from the Personal Social Services Research Unit 201590 and the Information and Statistics Division Scotland tariffs 2015. 91 Cost was calculated per person participating in the bariatric surgery care pathway by multiplying the salary costs of staff, according to grade or band, by the average number of annual sessions provided by that staff member and accounting for the length of session. Multidisciplinary team (MDT) costs were calculated from the number, type and grade of different specialists involved according to their time spent on delivering these sessions. All group sessions were cost per person by taking the average number of patients expected to participate. The assumption was made that costs such as those of equipment and instruments were constant, and the variability in costs was therefore in the staffing, which was more likely to affect patient outcomes.
Costings analyses
Descriptive statistics were used to present average cost per patient along with 95% confidence intervals (CIs), as well as the range of costs. Data were costed in Excel and statistical analyses were conducted using Stata version 12. A base case cost was calculated as the most likely average cost per person; a maximum cost was calculated based on optional or additional patient-dependent consultations. We assume zero optional or additional sessions in the base case and at least two for the maximum-cost scenario analysis. Where length of sessions or consultations was not provided, 30 minutes was assumed based on other responses received.
Statistical analyses
Data were analysed as available, without any imputation for missing data. All analyses were performed using SAS (version 9.3). Continuous data are reported as means and standard deviations (SD) or medians and lower (Q1) and upper (Q3) quartiles depending on data distribution, and counts and percentages are reported for categorical data. Comparisons between groups were made by Kruskal–Wallis test for continuous variables and Fisher’s exact test for categorial variables. Paired t-tests and Wilcoxon signed-rank test were used for change outcomes for continuous variables, McNemar test for dichotomous categorical variables and Bowker test for agreement for grouped categorical outcomes.
Binary logistic regression models were used for non-progression to surgery, admission to intensive-therapy unit (ITU) / high-dependency unit (HDU), readmission, <10% weight loss, reduction in diabetes medication, ‘need for specialist aids’ and ‘equipment in the home to assist with daily living’ outcomes. Length-of-stay outcomes were modelled with negative binomial regressions. Linear regression models were used for change in weight, change in HbA1c and change in QoL outcomes.
Regression model effect estimates, incidence rate ratios (IRRs) or odds ratios (ORs), and corresponding 95% CIs and associated p-values, are provided. The value of p < 0.05 is considered statistically significant.
A complete case analysis was performed with numbers of participants with available data listed. All results using health record data were subject to Public Health Scotland’s disclosure control protocol and outcomes affecting fewer than five participants cannot be reported (shown as xxx in tables).
Populations and outcome definitions
Five populations are considered within this report:
-
All operated – all operated patients of those consenting to part 1 of SCOTS. Patients in this population will have an operation type of gastric band, gastric bypass, SG or other.
-
Non-progression to surgery – patients who did not have an operation and had a completed non-progression to surgery form completed.
-
All operated and consented to PROMs – all operated patients as defined above who also consented to PROMs and had at least some data entered prior to their operation.
-
All operated and year 3 PROMs – all operated and consented to PROMs patients as defined above and have at least some 3-year data reported.
-
All operated and diabetes – all operated patients as defined above and who have at least one record in Scottish Care Information – Diabetes (SCI Diabetes) (i.e. regardless of patient-reported T2DM status in preoperative PROMs).
SMR01 and death record linkage outcomes
The matched initial bariatric operation was identified as the record in Scottish Morbidity Record 01 (SMR01), where the date of admission corresponding to a date of operation matches (on month and year) with the date of initial bariatric operation as entered into the electronic case-report form (eCRF). In instances where there may be more than one unique SMR01 admission with the same month and year as the initial bariatric operation as detailed in the eCRF, the Chief Investigator reviewed each SMR01 admission to note which ones were the initial bariatric operation. Note, for private patients the record corresponding to the initial bariatric operation may not have been provided. Admission to ITU/HDU during initial operation was identified from the SMR01 records where the initial bariatric operation occurred and has a ‘significant facility’ code for either ‘HDU’ or ‘Intensive Care Unit’.
Readmission was defined as any new stay admission record in SMR01 occurring after the initial bariatric operation where the admission was recorded as either urgent or emergency. If no matched bariatric operation occurred in the SMR01 data (i.e. for private patients), then the initial operation date as defined in the eCRF was used. Readmissions were considered within the same or subsequent calendar month, within the same or subsequent 11 calendar months or within the same or subsequent 35 calendar months. Different readmission codes (endocrine, circulatory, surgical) were defined using observed International Classification of Disease-10 codes (Report Supplementary Material 5).
Reoperations both within the period of ITU/HDU admission and up to 3 years post surgery were identified by OPCS Classification of Interventions and Procedures version 4 (OPCS-4) codes within SMR01. The following OPCS-4 codes were considered ‘bariatric surgery gastrointestinal complications or revisions’: G305 (maintenance of gastric band); G332 (revision of anastomosis); G387 (removal of gastric band); G436 (endoscopy and injection of lesion); G451 (upper GI endoscopy + biopsy); G459 (upper GI endoscopy); T309 (unspecified opening of abdomen); T315 (drainage of ant wall – laparoscopic); T413 (division of adhesions); T423 (closure of connection of stomach to jejunum).
Mortality was defined by any record within the deaths record. Exact date of death was provided, so mortality within 30 days of operation or within a year of operation was obtained using the exact date of initial bariatric operation as entered into the eCRF.
Diabetes record linkage outcomes
For all outcomes arising from the SCI Diabetes data, the preoperation value is the result available closest to the date of operation, including values entered on the date of operation or up to 18 months previously. The 3-year value is the value closest to the date of operation as entered in the eCRF + 3 years, and only includes values entered within the window of 27–45 months post operation. When more than one value is available, with one value occurring prior to the expected 3-year date and the other value occurring after the 3-year date, the value occurring prior to the expected 3-year date was used. Outlying values deemed implausible were removed, including HbA1c <12 mmol/mol and >348 mmol/mol and systolic blood pressure values of 0 mmHg and >1400 mmHg. Microalbuminuria was defined as an albumin : creatinine ratio ≥2.5 mg/mmol for men and ≥3.5 mg/mmol for women.
For retinopathy outcomes, data from the National Retinal Screening Programme were used, specifically focusing on the retinopathy and maculopathy data for the left and right eyes. Participants are categorised into two mutually exclusive groups at each time point (preoperation and 3 years post operation): no disease (bilateral R0 M0) or observable or referable disease in any eye. For the change in retinopathy status between preoperation and 3 years post operation, patients with retinopathy data at both time points are categorised into the four following mutually exclusive groups: no disease at both preoperation and 3 years post operation; no disease at preoperation but observable or referable disease at 3 years post operation; some disease at preoperation but no disease at 3 years post operation; observable or referable disease at both preoperation and 3 years post operation.
Prescribing Information System data were used to identify whether each participant with diabetes was prescribed any of the following specific categories of medications (British National Formulary paragraph drug code) at each time point (preoperation and 3 years post operation): insulin (6.1.1.1, 6.1.1.2); sulfonylureas (6.1.2.1); biguanides (6.1.2.2); glitazones (subset of 6.1.2.3); sodium-glucose cotransporter-2 Inhibitors (subset of 6.1.2.3); GLP-1 agonists (subset of 6.1.2.3); dipeptidyl-peptidase 4 (DPP-4) inhibitors (subset of 6.1.2.3); meglitinides (subset of 6.1.2.3); acarbose (subset of 6.1.2.3). Combination drugs and newer agents (6.1.2.3) were reviewed by the chief investigator and assigned to each individual category for the component medications. For the count of medications, the number of unique medication categories for each participant within the time point of interest was obtained.
Clinician- and participant- recorded outcomes
Weight was recorded at multiple time points by both the participant (self-reported if they consented to PROMs) and the clinician. Clinician-reported weight was used preferentially at each time point with participant-reported weight used when no clinician weight was available. For the purposes of obtaining a 12-month post-operation clinician-reported weight, a window of 9–18 months following the date of operation was used and the weight occurring closest to the 12 months following operation was used for analysis. Similarly, for the 36-month weight, a window of 33–42 months after the date of operation was used. For weight on date of operation, if no weight at operation was recorded, weight at the start of the weight-management programme was used instead. BMI was calculated for each source [weight (kg)/ height (m)67] and using the height reported upon recruitment into the study.
Sample size
At the time of study development, 230 operations were funded in NHS Scotland each year (of which approximately 60 were bypass). Bariatric surgeons performed an additional 270 private procedures per year and they were willing to commit to entering data (approximately 80 bypass). Therefore, 500 procedures per year were expected to be entered into the database with a belief that as numbers of people with severe obesity (BMI > 40) are rising rapidly, this number will increase despite financial constraints.
From previous studies,92,93 we expected a 10-year mortality of around 5% (100 deaths). This sample size would allow the mortality rate to be estimated with 95% CI to within ± 1% (i.e. for a 5% 10-year death rate, the 95% CI will be between 4% and 6%). We planned to compare this mortality rate with an age-sex-matched healthy population from the Registrar General of Scotland’s life tables (assumed known with no sampling error). One hundred deaths were sufficient to allow us to build a predictive model for death post surgery (conventionally one requires around 10 events per prognostic covariate considered). An initial sample size of 2000 was proposed as it would easily provide adequate power for the original outcomes under investigation.
However, there were a number of unforeseen recruitment issues that have impacted on the numbers stated above and in 2016 the sample size was revisited. In order to explore whether the sample at that time was likely to show meaningful results, the available statistical power for detecting 3-year difference in HbA1c and QoL (physical and mental components) was calculated using the numbers of participants available as of 29 July 2016. The majority of data to inform sample size were taken from papers where bariatric procedure was LAGB as this is recognised as the least effective of the bariatric procedures so will give a conservative estimate of measure of effect. This shows that there is >99% power to show differences in these outcomes at 3 years with the current sample size.
In order to explore the likely event rate for cardiovascular events and deaths, we performed health record linkage for currently recruited participants, linking with inpatient care and death records. Follow-up is from the date of surgery, so for those patients who went on to have surgery details entered (n = 180), there are 272 separate admissions. Of those 272, there were 72 ‘emergency’ admissions, and 4 of these, in three patients, are ‘circulatory disease’ using the main condition only. The codes included for these hospital admissions are:
-
angina pectoris (x1)
-
acute MI (x1)
-
pulmonary embolism without mention of acute cor pulmonale (x1)
-
orthostatic hypotension (x1).
Using date of operation as the starting point, we have a total (crude) follow-up time of 203.52 years (the mean is 1.13 years and the median is 1.04 years). As the number of cardiovascular events is so low, it is impossible to extrapolate this to a future event rate at this time. It should be noted that the participants have been cleared as healthy for elective surgery, meaning that it is unlikely that there would be many cardiovascular events in early follow-up.
Following discussions with the NIHR, it was agreed that recruitment will stop at approximately 400 patients and these numbers will be sufficient to answer the majority of objectives initially set.
Ethics, regulatory and reporting requirements
The study was performed according to the Research Governance Framework for Health and Community Care (second edition, 2006)94 and was registered prospectively at the International Standard Randomised Controlled Trials Number (ISRCTN) registry: ISRCTN47072588. A favourable ethical opinion for the study was obtained from the West of Scotland Research Ethics Committee 4 on 7 February 2013 (13/WS/0005).
Permission for linkage and access to data from participants’ electronic health records was granted by the Public Benefit and Privacy Panel for Health and Social Care on 11 October 2019.
Chapter 3 Variations in bariatric surgical care pathways: the variability of services and impact on costs
Parts of this chapter have been reproduced, with permission, from Grieve et al. 67
Introduction
With bariatric surgery care pathways known to vary considerably, the first step in obtaining better evidence of what works is to establish what is currently delivered. To this end, a survey of NHS-funded SCOTS study sites was undertaken in order to describe current services, to estimate their costs and explore differences in financial impact. This was necessary to facilitate further investigation as to what extent the intensity of preop and postop bariatric surgical care is a factor which may affect patient outcomes after surgery.
Results
A comparison of Scotland’s tier-four pathways by bariatric site
All 10 NHS-funded SCOTS study sites provided information on their bariatric surgery services. The questionnaires were completed, generally by the bariatric dietician or nurse, and returned by e-mail or hard copy to the investigator. Most patients were referred via GPs, diabetes clinics or consultants. Age range of patients was 18–60 years. Each site’s bariatric surgery preop and postop care pathways and eligibility criteria regarding glycaemic control and target weight loss pre-surgery were compared (see Table 4). It was assumed that BMI and comorbidity eligibility criteria would comply with NICE guidance. Note that one site (site 10) specified sleep apnoea treatment; this was not costed in calculations as a cost of surgery as it is considered a cost related to an obesity comorbidity, which would have been treated regardless of the bariatric surgery.
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 | Site 9 | Site 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-surgery targets – weight loss, glycaemic control (targets not specified by NICE) Most sites stated other factors such as attendance rates at clinics and lifestyle changes |
5% weight loss HbA1c < 64 mmol/mol |
5–10% weight loss HbA1c – control but no target Smoking cessation Remittance binge eating |
10% weight loss HbA1c < 69 mmol/mol |
5% weight loss in tier 3, further 5% weight loss tier 4 HbA1c – control but no target |
Weight loss > 5 kg in 6 months | 5–10% weight loss. If < 50 BMI, must reach target HbA1c < 64 mmol/mol |
Weight loss > 5 kg HbA1c < 75 mmol/mol |
Weight loss ≥ 5 kg | Weight loss > 5% HbA1c < 75 mmol/mol |
10% weight loss HbA1c < 75 mmol/mol Smoking cessation Sleep apnoea – 3-month treatment |
| Preop assessment a : | ||||||||||
| Assessment of any psychological or clinical factors that may affect adherence to postop care requirements (such as changes to diet) | MDT including clinical psychologist | Dietician, clinical psychologist | Dietician only | Dietician, clinical psychologist | Dietician, clinical psychologist | MDT including clinical psychologist | MDT including clinical psychologist | MDT includes diabetologist | MDT including clinical psychologist | MDT including clinical psychologist |
| Psychological support | Yes | Yes | No | Yes | If required | Yes | Yes | No | Yes | Yes |
| Session format (group or 1:1) | Group | 1:1 | Either | Both | 1:1 | Both | Both | 1:1 | Both | Both |
| Postop assessment: | ||||||||||
| Regular postop assessment, including specialist dietetic and surgical follow-up | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Psychological support | As required | Not stated | Not stated | Yes | As required | Yes | Yes | No | As required | Not stated |
| Session format (group or 1:1) | 1:1 | 1:1 | 1:1 | Both | 1:1 | 1:1 | Both | 1:1 | 1:1 | 1:1 |
Classification of Scotland’s tier-four pathway costs
Results of a sensitivity analysis (SA) show nearly a five fold difference in costs per patient for preop services (range £226–£1071) and more than a three fold difference for postop services (range £259–£896, see Table 5). The provision of services was variable regarding the format of delivery of sessions (group as one-to-one sessions), and frequency and length of access to psychology and dietetics before and after surgery. Access to psychological support was variable both preoperatively and postoperatively, with sessions lasting from 30 minutes to 2 hours, if this was actually provided. Similarly, for dieticians, some sites offered a one-off appointment pre-surgery, while others provided a regular group service over a number of weeks. Postop follow-up was more consistent, with regular reviews by dieticians, though this was far from standardised across sites. The full cost breakdown is provided in Report Supplementary Material 6.
| Site | Preop (base case) | Preop (SA) | Postop (base case) | Postop (SA) | Intensity |
|---|---|---|---|---|---|
| 1 | £681 | £1071 | £458 | £526 | High |
| 2 | £212 | £423 | £259 | £259 | Low |
| 3 | £185 | £231 | £452 | £458 | Medium |
| 4 | £340 | £359 | £225 | £261 | Low |
| 5 | £138 | £226 | £414 | £483 | Medium |
| 6 | £408 | £798 | £209 | £356 | Medium |
| 7 | £498 | £544 | £339 | £339 | Medium |
| 8 | £472 | £472 | £339 | £339 | Medium |
| 9 | £425 | £539 | £248 | £896 | High |
| 10 | £478 | £478 | £398 | £398 | Medium |
| Tier 4 summary costs | Mean | SE | 95% CI | Min/Max | |
| Preop (base case) | £384 | £53 | £264, £503 | £138, £681 | |
| Preop (SA) | £514 | £81 | £331, £697 | £226, £1071 | |
| Postop (base case) | £334 | £30 | £266, £402 | £209, £458 | |
| Postop (SA) | £432 | £59 | £299, £564 | £259, £896 |
Discussion
Bariatric surgery care pathways are widely regarded as varying considerably and international bariatric guidance is not specific with regard to the optimal model of care. 70 The results described in this chapter illustrate the large nationwide variability in preop and postop care, a likely consequence of widespread uncertainty regarding best practice and a lack of more detailed guidance with respect to service delivery. There is little evidence as to whether intensive preop and postop care improves outcomes and is cost-effective compared to less intensive care. This is likely to be more complicated than one standard pathway for all, with patient preferences also paramount in terms of type of provision (one-to-one or group sessions, for example). Furthermore, pre-surgery targets vary widely95 but are often low-cost group interventions and funded from a separate budget to surgery. Maximum cost is around £100–£200 per patient. However, these targets do add to the complexity of the pathway for the patients and variation in time and access to surgery, and therefore the usefulness of these targets is currently a subject of debate. 96–99
Impacts resulting from the benefits of dietician and psychological support prior to bariatric surgery have been published. Livhits et al. 100 undertook a systematic review which found that preop weight loss appears to be associated with greater weight loss postoperatively. In a more recent review, Gerber et al. 101 found the same beneficial effects from preop weight loss. On the other hand, it has been shown that psychological support before and after bariatric surgery had no impact on weight loss. 102 This study recommends further research to evaluate the longer-term implications for both weight loss and psychological support, and thereby the most effective timing for delivery of these interventions. As to why some sites offer more comprehensive services than others, decisions on staff resourcing are possibly being made on the basis of cost and availability of specialists, as there is currently no evidence as to whether these different models of care pathways improve outcomes. Indeed, this study illustrates how variable these costs are, even across health centres within the same country context, and this difference alone is worth highlighting. Therefore, it is important to evidence outcomes of these services.
Furthermore, there is a concern that bariatric surgery cost-effectiveness models may either omit pre-surgery and post-surgery care costs as part of their economic analyses or treat patients and the delivery of these services homogeneously by applying average costs. In a systematic review of a critical appraisal of economic evaluations of bariatric surgery,103 the considerable heterogeneity of what costs are included in economic studies and the frequent omission of different types of healthcare resource use were highlighted. Despite the identification of preoperative and postoperative costs, there was no detail reported on care pathways explicitly as an important cost component of an economic evaluation of bariatric surgery. A recent study by Gulliford et al. ,104 estimating the costs of bariatric surgery drawn from UK NHS tariffs, included preoperative weight management as part of the cost of the surgical procedure but only referred to the cost of medical weight-management services. There was no reference to bariatric surgery care pathway costs being included. 104 In the same model, a flat rate of £875 was also included for postoperative reviews. Procedure costs are not captured here and are assumed to be relatively standardised given the clear guidance on surgical procedures and, in Scotland, there is national procurement so device costs would also be standard across all sites. In their systematic review, Picot et al. 105 found the costs of bariatric surgery generally to be presented as standard unit costs with aggregate costs differing dependent on what is included in the total costs of surgery rather than any differences due to site variation. One study106 did find variation by gender but offered no explanation as to why.
The aim of this research was to understand whether differences in these care pathways are predictors of health outcomes, and thus influence cost-effectiveness from the benefit side. This study underlines the need to better understand the cost-effectiveness of bariatric surgery care pathways, and whether the varying level of intensity of services offered is an important factor in influencing outcomes. The SCOTS study provides the follow-up data required to assess whether this classification of preop and postop care pathways is a predictor of health outcomes. Classification of the intensity of preop and postop bariatric surgical care can now be considered for investigation as a factor which may affect patient outcomes after surgery. If further findings do demonstrate that more intensive (and expensive) services lead to better outcomes, it is not envisaged that this will change bariatric surgery from being cost-effective at the usual willingness-to-pay thresholds for reimbursement on the NHS given the modelled ICER of £10,126 per quality-adjusted life year. 55 However, budgetary impact is an important consideration and it is acknowledged that these costs do matter for payers, hospital resource use and more local-level decision-making. Should these pathways be found to be predictors of better health outcomes, the case for investment in these care pathways would be self-evident.
Conclusions
This study, focusing on preop costs and the first 12 months following surgery in which the majority of costs will occur, has illustrated the large nationwide variability in preop and postop care pathways across Scotland, and the subsequent financial impact on the provision of bariatric surgery services. This is a likely consequence of widespread uncertainty regarding best practice and a lack of more detailed guidance regarding service delivery. Health economic analyses do not always capture these costs103 or apply a flat rate. 104 There is a lack of evidence base and a clear requirement for the evaluation of bariatric surgical services to identify the care pathways preceding and following surgery which lead to the largest improvements in health outcomes and remain cost-effective to the health provider.
Chapter 4 Health and socioeconomic burden in treatment-seeking individuals with severe obesity: profile of the SCOTS national cohort
Parts of this chapter have been reproduced from Mackenzie et al. 72
Introduction
There is a lack of evidence to inform the delivery and follow-up of bariatric surgery for people with severe obesity. SCOTS is the first national epidemiological study established to investigate long-term outcomes following bariatric surgery. In addition, SCOTS collected clinical and patient-reported health outcomes from treatment-seeking individuals from across Scotland with severe obesity before they underwent bariatric surgery. This chapter describes the health-related characteristics of the recruited SCOTS cohort and examines relationships between age, preop BMI and other health-related factors.
Results
Recruitment
Participants were recruited over an approximate 3-year period from December 2013 to February 2017 with follow-up continuing until October 2020.
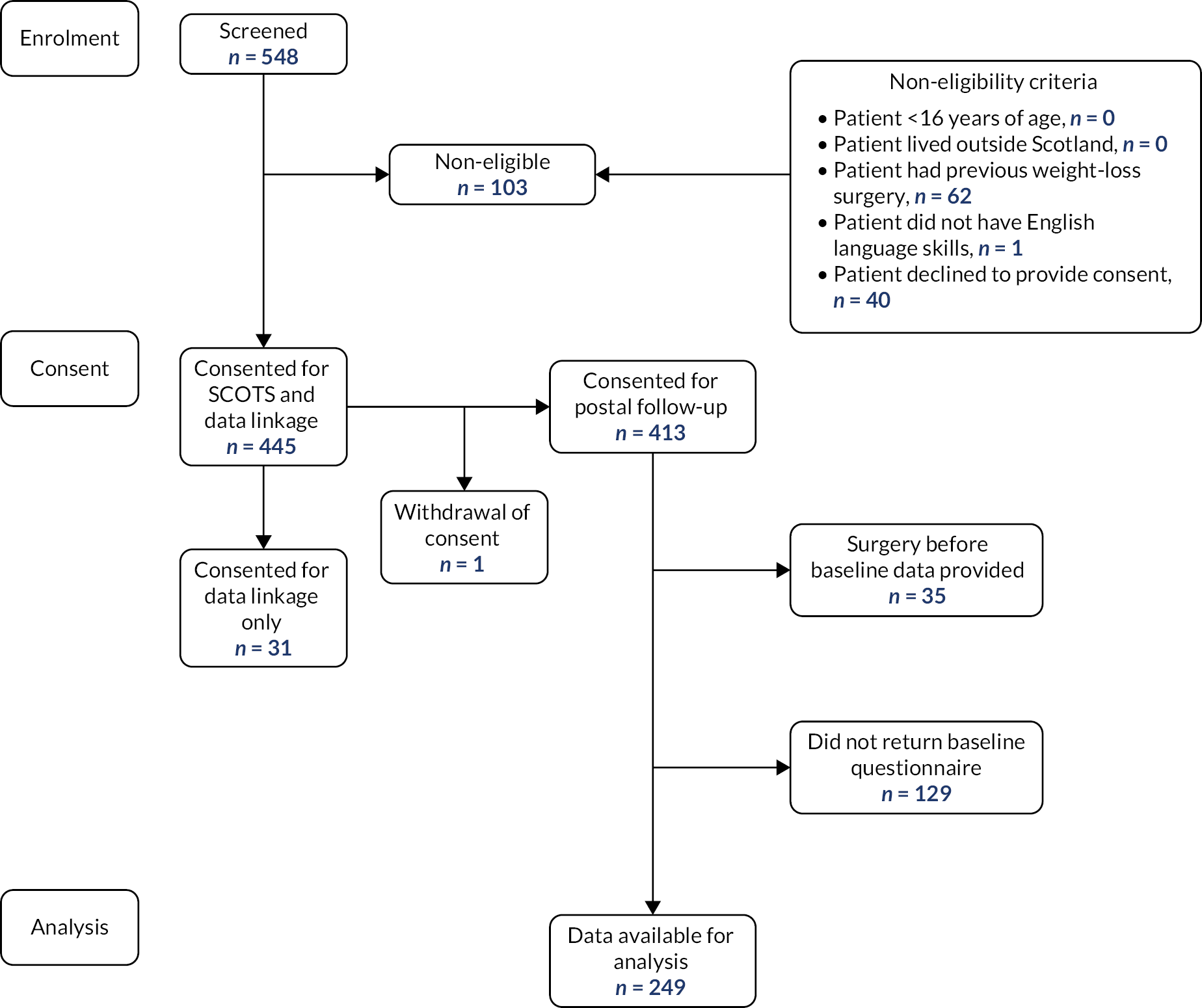
Over the recruitment period, a total of 548 patients were approached and screened for eligibility to participate. Of these, 103/548 (19%) were excluded or declined to participate (see Figure 2). We recruited 445/548 (81%) participants but one participant withdrew consent, leaving a recruited sample of 444 (81%). Of the recruited sample, 413/444 (93%) consented to data linkage and questionnaire follow-up, while 31/444 (7%) consented to data linkage only. Of these 413 participants, a total of 164/413 (40%) were not included in the subsequent analysis: 129 did not return a baseline questionnaire and 35 had bariatric surgery before their baseline PROMs questionnaires were completed. Of the 129 who did not return baseline questionnaires, 84/129 (65%) progressed to surgery, 43/129 (33%) did not progress to surgery and the status of 2/129 (2%) was unknown. Completed preop baseline PROMs data for 249/413 participants (60% of those consented) were available for analysis (see Figure 2).
FIGURE 2.
Screening, consent and follow-up.
Characteristics of recruited and analysed sample
Demographic data are summarised in Table 6. Participant characteristics were similar between the total recruited sample (n = 444) and the analysed subset (n = 249) with completed PROMs before bariatric surgery (see Table 6). Mean age was 46 years (±9.1 years), with a higher proportion of women than men (71% vs. 29%). Half of recruited participants were aged 35 to 49 years, with one-third being over 50 years. The median BMI was 47 kg/m2 (Q1 43; Q3 54), with more than 21% having a BMI of ≥55 kg/m2. Over half of the participants (55%) lived in areas of high socioeconomic deprivation (SIMD quintiles 1 and 2). There were no statistically significant differences between the analysed subset (n = 249) and the non-analysed subset (n = 195).
| Recruited sample N = 444 |
Analysed samplea N = 249 |
||
|---|---|---|---|
| Sex, N (%) | Male | 123 (27.7) | 72 (28.9) |
| Female | 321 (72.3) | 177 (71.1) | |
| Missing | 0 | 0 | |
| Age (years) | Mean (SD) | 46.2 (9.1) | 45.9 (9.1) |
| Missing | 0 | 0 | |
| Age group, N (%) | <35 years | 61 (13.7) | 36 (14.5) |
| 35–44 years | 116 (26.1) | 63 (25.3) | |
| 45–49 years | 109 (24.5) | 63 (25.3) | |
| 50–54 years | 79 (17.8) | 43 (17.3) | |
| 55+ years | 79 (17.8) | 44 (17.7) | |
| Missing | 0 | 0 | |
| BMI (kg/m2) | Median (Q1; Q3) | 47.2 (42.7; 53.6) | 47.6 (42.8; 53.8) |
| Missing | 1 | 0 | |
| BMI group, N (%) | BMI < 40 | 52 (11.7) | 24 (9.6) |
| BMI 40–44 | 115 (26.0) | 64 (25.7) | |
| BMI 45–49 | 116 (26.2) | 64 (25.7) | |
| BMI 50–54 | 71 (16.0) | 44 (17.7) | |
| BMI 55+ | 89 (20.1) | 53 (21.3) | |
| Missing | 1 | 0 | |
| SIMD quintile, N (%) | Quintile 1 (most deprived) | 135 (30.5) | 70 (28.3) |
| Quintile 2 | 108 (24.4) | 65 (26.3) | |
| Quintile 3 | 84 (19.0) | 51 (20.6) | |
| Quintile 4 | 68 (15.4) | 34 (13.8) | |
| Quintile 5 (least deprived) | 47 (10.6) | 27 (10.9) | |
| Missing | 2 | 2 | |
| Marital status, N (%) | Married/civil partnership/co-habiting | Not collected | 155 (63) |
| Single/separated/divorced/ widowed | 91 (37) | ||
| Missing | 3 | ||
| Ethnic group, N (%) | White | Not collected | 243 (97.6) |
| Mixed | 4 (1.6) | ||
| Asian/Asian Scottish/Asian British | 1 (0.4) | ||
| African Caribbean/black | 1 (0.4) | ||
| Other | 0 (0.0) | ||
| Missing | 0 | ||
| Education, N (%) | School only | Not collected | 58 (23.5) |
| Formal qualifications through training at work | 54 (21.9) | ||
| Qualification (other than a degree from college or university) | 64 (25.9) | ||
| Degree from college or university | 71 (28.7) | ||
| Missing | 2 | ||
| Current employment status, N (%) | Working full time | Not collected | 124 (50.0) |
| Working part time | 24 (9.7) | ||
| Unable to work because of illness or disability | 64 (25.8) | ||
| Student/unemployed and seeking employment/ unemployed and not seeking employment/ carer/other | 36 (14.5) | ||
| Missing | 1 |
Comorbidities
For the analysed sample (n = 249), self-reported medical comorbidities and physical, mental and functional measures are presented in Table 7. Over 40% reported having at least one of hypertension, T2DM, back problems, anxiety/depression and gastro-oesophageal reflux. Over 60% of the sample reported more than three comorbidities. Over 40% of male participants reported erectile dysfunction, while one-third of males described urinary incontinence. Half of female participants reported urinary incontinence. Mean depression scores reflected mild depression, although 44% of participants had scores indicating moderate to severe depression. Anxiety scores for all participants were indicative of mild anxiety (median 5.0), with half of participants having scores indicative of moderate to severe anxiety. The mean life optimism score for participants was reflective of low optimism (high pessimism). Very few participants smoked (5%) and, on average, alcohol consumption was moderate.
| N = 249 N (%) |
Missing N (%) |
||
|---|---|---|---|
| Comorbidity, self-report | Deep vein thrombosis | 8 (3.2) | 0 |
| Pulmonary embolism | 4 (1.6) | 0 | |
| Hypertension | 107 (43.0) | 0 | |
| T2DM | 124 (49.8) | 0 | |
| Angina/heart attack | 17 (6.8) | 0 | |
| Heart failure | 2 (0.8) | 0 | |
| Stroke/mini stroke | 6 (2.4) | 0 | |
| Arthritis | 73 (29.3) | 0 | |
| Back problems | 115 (46.2) | 0 | |
| Chronic bronchitis | 4 (1.6) | 0 | |
| Eczema/psoriasis | 33 (13.3) | 0 | |
| Asthma | 70 (28.1) | 0 | |
| Thyroid problems | 32 (12.9) | 0 | |
| Migraine | 49 (19.7) | 0 | |
| Anxiety/depression | 114 (45.8) | 0 | |
| Kidney disease | 7 (2.8) | 0 | |
| Liver disease | 2 (0.8) | 0 | |
| Cancer | 4 (1.6) | 0 | |
| Irritable bowel syndrome | 44 (17.7) | 0 | |
| Sleep apnoea | 66 (26.5) | 0 | |
| CVD | 20 (8.0) | 0 | |
| N (%) self-reported comorbidities | None | 9 (3.6) | 0 |
| 1–2 | 80 (32.1) | 0 | |
| ≥3 | 160 (64.3) | 0 | |
| Gastro-oesophageal reflux | Yes | 97 (40.4) | 9 (3.6) |
| Female reproductive health, N = 75a | Mean (SD) age years, last natural menstrual period | 39.4 (10.9) | 4 (5.3) |
| Female reproductive health, N = 177 | Polycystic ovarian syndrome, N (%) | 28 (16.8) | 10 (5.6) |
| Male reproductive health, N = 72 | Impotence, N (%) | 28 (41.2) | 4 (5.6) |
| IPSS score ≥ 8, N (%) | 34 (47.9) | 1 (1.4) | |
| Incontinence | Median (Q1; Q3) ICIQ-UI SF score | 4 (0.0; 10.0) | 10 (4.0) |
| ICIQ-UI SF score ≥ 6 | 105 (43.9) | 10 (4.0) | |
| Incontinence, females, N = 177 | ICIQ-UI SF score ≥ 6, N (%) | 83 (49.4) | 9 (5.1) |
| Incontinence, males, N = 72 | ICIQ-UI SF score ≥ 6, N (%) | 22 (31.0) | 1 (1.4) |
| Depression | Mean (SD) PHQ-9 score | 9.6 (6.3) | 5 (2.0) |
| N (%) PHQ-9 score ≥ 10 | 107 (43.9) | 5 (2.0) | |
| Anxiety | Median (Q1; Q3) GAD-7 | 5 (2.0; 9.0) | 6 (2.4) |
| N (%) GAD-7 score ≥ 6 | 114 (46.9) | 6 (2.4) | |
| Smoking status | Current | 13 (5.4) | 9 (3.6) |
| Former | 105 (43.8) | ||
| Never | 122 (50.8) | ||
| Alcohol use | Median (Q1; Q3) AUDIT | 3 (1.0; 6.0) | 20 (8.0) |
| Quality of life | |||
| SF-12 | Mean (SD) PCS | 37 (11.4) | 13 (5.2) |
| Mean (SD) MCS | 45.5 (10.3) | 13 (5.2) | |
| EQ-5D-5L | Median (Q1; Q3) | 0.6 (0.3; 0.8) | 12 (4.8) |
| Mean (SD) VAS | 55.3 (22.1) | 12 (4.8) | |
| IWQOL-Lite (Standardised Scoring) | Mean (SD) Physical Function | 56.9 (25.4) | 6 (2.4) |
| Mean (SD) Self Esteem | 70.7 (27.1) | 7 (2.8) | |
| Mean (SD) Sexual Life | 57.1 (31.7) | 18 (7.2) | |
| Mean (SD) Public Distress | 58.1 (27.2) | 6 (2.4) | |
| Mean (SD) Work | 43.6 (29.2) | 13 (5.2) | |
| Mean (SD) Total score | 58.5 (21.7) | 7 (2.8) | |
| Life optimism | Mean (SD) LOT score | 13 (4.9) | 14 (5.6) |
| Physical activity | ≥1 walking, moderate or vigorous activity in last 7 days | 201 (83.4) | 8 (3.2) |
| Median (Q1; Q3) IPAQ score (MET minutes/week) | 720 (40.0; 1800.0) | 6 (3.0) | |
| Healthcare utilisations | Using any aids or specialist equipment | 67 (28.9) | 17 (6.8) |
| Median (Q1; Q3) GP visits in last 3 months | 2 (1.0; 3.0) | 79 (31.7) | |
| Median (Q1; Q3) visits to other health/social care providers in last 3 months | 3 (1.0; 5.0) | 73 (29.3) | |
| Social security | Unable to work due to illness or disability | 64 (25.8) | 1 (0.4) |
| Receiving DLA (caring) | 44 (18.6) | 13 (5.2) | |
| Receiving DLA (mobility) | 47 (19.9) | 13 (5.2) | |
Health and obesity-related quality of life
Mean SF-12 Physical Component Summary (PCS) and Mental Component Summary (MCS) scores were low: PCS 37.0 (11.4), MCS 45.5 (10.3). The median EQ-5D-5L score of sample participants was 0.6 (Q1 0.3; Q3 0.8), while the mean EQ-5D-5L visual analogue scale (VAS) was 55.3 (±22.1). Participants had a mean IWQOL-Lite Physical Function score of 56.9 (±25.4) and a mean total score of 58.5 (±21.7, see Table 7), where an increase in IWQOL-Lite score indicates a worsening in QoL.
Physical activity
Over 80% of SCOTS participants reported undertaking at least 10 minutes of either walking, moderate or vigorous activity in the last 7 days and the median IPAQ score for the sample was 720.0 MET minutes/week. Almost one-third (29%) of participants reported using aids or specialist equipment to assist with their daily activities in the home (see Table 7).
Comorbidity by BMI and age
Comorbidity data are presented by BMI group and age group in Report Supplementary Material 7 and Report Supplementary Material 8, respectively. In order to further investigate the associations between BMI and age on physical, mental and functional measures, and healthcare utilisation within the SCOTS population, regression analyses were performed (see Table 8). There was no significant correlation between BMI and age (correlation = 0.01, p = 0.91). Higher BMI values and higher ages were negatively associated with physical, but not mental, HRQoL scores (see Table 8). For each 10 kg/m2 higher BMI there was a change of −5.2 (95% CI −6.9 to −3.5; p < 0.0001) in SF-12 PCS, −0.1 (95% CI −0.2 to −0.1; p < 0.0001) in EQ-5D-5L score and 14.2 (95% CI 10.7 to 17.7; p < 0.0001) in IWQOL-Lite Physical Function score (where an increase in score indicates a worsening). We observed a 3.1 times higher use of specialist aids and equipment in the home [odds ratio (OR) 3.1, 95% CI 1.9 to 5.0; p < 0.0001], adjusting for age, sex, smoking and socioeconomic deprivation. For each 10-year higher age, there was a change of −2.1 (95% CI −3.7 to −0.5; p < 0.01) in SF-12 PCS score, −0.1 (95% CI −0.1 to 0.0; p < 0.01) in EQ-5D-5L score and 5.01 (95% CI 1.8 to 8.3; p < 0.01) in IWQOL-Lite Physical Function score and a 3.4 (OR 3.4, 95% CI 1.9 to 5.0; p < 0.0001) times higher use of specialist aids or equipment in the home, adjusting for BMI, sex, smoking and socioeconomic status.
| QoL indicators and functional measures | Variable | Unadjusted modelsa | Adjusted modelb |
|---|---|---|---|
| Regression coefficient (95% CI)c | Regression coefficient (95% CI)c | ||
| SF-12 PCS | BMI | −4.91 (−6.55, −3.28) | −5.21 (−6.90, −3.52) |
| Age | −2.44 (−4.03, −0.85) | −2.14 (−3.73, −0.54) | |
| SF-12 MCS | BMI | −0.49 (−2.07, 1.09) | −0.40 (−2.06, 1.26) |
| Age | 0.42 (−1.04, 1.87) | 0.68 (−0.89, 2.25) | |
| EQ-5D-5L Score | BMI | −0.11 (−0.15, −0.06) | −0.11 (−0.16, −0.06) |
| Age | −0.08 (−0.12, −0.03) | −0.07 (−0.11, −0.02) | |
| EQ-5D-5L VAS | BMI | −7.08 (−10.38, −3.79) | −7.73 (−11.10, −4.36) |
| Age | −2.19 (−5.30, 0.92) | −0.65 (−3.77, 2.46) | |
| IWQOL-Lite physical function | BMI | 13.72 (10.28, 17.17) | 14.20 (10.69, 17.70) |
| Age | 5.77 (2.28, 9.26) | 5.01 (1.75, 8.27) | |
| IWQOL-Lite self esteem | BMI | 5.09 (1.02, 9.16) | 5.99 (1.86, 10.12) |
| Age | −4.74 (−8.51, −0.97) | −5.11 (−8.96, −1.25) | |
| IWQOL-Lite sexual life | BMI | 5.56 (0.71, 10.41) | 5.74 (0.82, 10.67) |
| Age | 3.86 (−0.75, 8.47) | 3.01 (−1.71, 7.73) | |
| IWQOL-Lite public distress | BMI | 15.36 (11.72, 19.00) | 16.07 (12.34, 19.80) |
| Age | −2.78 (−6.57, 1.02) | −3.04 (−6.51, 0.44) | |
| IWQOL-Lite work | BMI | 9.54 (5.25, 13.84) | 9.59 (5.16, 14.02) |
| Age | 1.95 (−2.25, 6.16) | 1.04 (−3.15, 5.24) | |
| IWQOL total score | BMI | 10.31 (7.29, 13.33) | 10.88 (7.79, 13.97) |
| Age | 1.20 (−1.84, 4.24) | 0.55 (−2.33, 3.43) | |
| Use of aids or specialist equipment | BMI | 2.34 (1.62, 3.39)* | 3.10 (1.94, 4.95)* |
| Age | 2.65 (1.76, 4.00)* | 3.40 (1.94, 4.95)* | |
| DLA (caring) | BMI | 1.19 (0.82, 1.73)* | 1.07 (0.72, 1.59)* |
| Age | 1.54 (1.03, 2.31)* | 1.62 (1.08, 2.46)* | |
| DLA (mobility) | BMI | 1.18 (0.82, 1.70)* | 1.13 (0.77, 1.65)* |
| Age | 1.64 (1.10, 2.45)* | 1.62 (1.08, 2.44)* |
Interactions were explored between smoking and both age and BMI [with smoking as a two-level variable (smoked or never smoked) due to small numbers in the current smoker group] and a borderline significant interaction between age and smoking status was observed (Report Supplementary Material 8). Further exploration in the subpopulations of smokers (current or former) and those who had never smoked revealed a significant effect of age on the use of specialist aids or equipment in the home in both subpopulations, but the OR suggests a trend towards a slightly larger odds in those participants who had never smoked (Report Supplementary Material 9). No significant effect of age or BMI on moderate to severe depression (PHQ-9) was observed in either the unadjusted or adjusted models. However, on extending that model to include the interactions between smoking and each of age and BMI, we observe a significant interaction between BMI and smoking status. Considering the subpopulations of smokers and those who had never smoked, there was no significant effect of BMI on smokers, but there was a significant effect of BMI in those who never smoked, with increasing BMI having increased odds of moderate to severe depression (Report Supplementary Material 9).
With regard to medical comorbidities, as shown in Report Supplementary Material 10, higher BMI had a significant association with higher prevalence of asthma in the SCOTS population, while older age was associated with higher prevalence of hypertension, arthritis and sleep apnoea.
Discussion
Despite escalating levels of severe obesity in Western society and the concomitant increase in bariatric surgical procedures being performed in some countries,107 there is a dearth of information on the health status of people living with severe obesity. This national Scottish cohort study of people seeking surgical treatment for severe obesity recruited 444 adults from 14 centres across Scotland over a 3-year period, including all NHS centres and major private hospitals undertaking bariatric surgery. We found that higher BMI and older age were associated with decreased physical QoL, increased use of specialist aids and equipment in the home, and a high prevalence of comorbidities.
There has been a significant increase in the prevalence of BMI ≥ 40 kg/m2 in recent decades; in Scotland, obesity prevalence has trebled in women age 16–64 years since 1995. However, it is hard to assess the global increase due to lack of reporting of BMI ≥ 40 kg/m2 in national health survey data. 108 While it is known that healthcare resource use increases in people with a BMI ≥ 30 kg/m2, with service use estimated to be over 25% higher than for those with a BMI in the normal weight range,109 few data exist for those with BMI 40 kg/m2 and above. In 2016, the Global BMI Mortality Collaboration110 conducted an individual-participant-data meta-analysis of 239 prospective studies and found a 2.8 times increased risk of all-cause mortality for people with a BMI of 40–60 kg/m2. Greive et al. 111 conducted a systematic review which focused on the economic cost of severe obesity (BMI ≥ 40 kg/m2) and found limited literature describing increased prescribing, outpatient utilisation and intensive care admission and hospital length of stays during critical illness. However, in neither study was there disaggregation of BMI beyond >40 kg/m2, meaning that the health consequences of severe obesity are not yet fully described.
There has been extensive research on the relationship between HRQoL and obesity. 112 Ul-Haq et al. 113 performed a meta-analysis of eight studies (43,086 participants) and found physical QoL, measured by the Rand 36-item Short Form Health Survey (SF-36), was reduced by 9.7 points in those with BMI 40 kg/m2 compared to those with a BMI in the normal range, although, again, there was no disaggregation above BMI 40 kg/m2. Van Nunen et al. 114 performed a meta-analysis to compare the general, non-treatment-seeking population to patients within weight-management programmes and those seeking bariatric surgery. They found that those seeking surgical treatment reported the most severely reduced HRQoL, perhaps reflecting their reasons for seeking definitive surgical treatment. Our cohort of treatment-seeking individuals, who completed a rich battery of patient-reported measures, provides data to show that HRQoL and O-QoL of those with the highest body mass is extremely poor and this is compounded by increasing age. QoL scores of SCOTS participants in both the upper BMI (≥55 kg/m2) and older age (≥55 years) groups included physical scores comparable with those reported by cancer patients receiving palliative care,115 patients with chronic heart failure expressing end-of-life preferences116 and patients with end-stage kidney disease. 117 Furthermore, patients with severe chronic obstructive pulmonary disease (COPD) report higher QoL scores, indicating a better QoL, than our cohort of treatment-seeking obese participants. 118 As far as we are aware, this is the first study to investigate physical and mental health in patients with severe obesity awaiting bariatric surgery with finer-level consideration of BMI up to ≥55 kg/m2.
As previously outlined, UK guidelines63 currently indicate that bariatric surgery is a treatment option for those with BMI ≥ 40 kg/m2, or between 35 kg/m2 and 40 kg/m2 in the presence of other significant diseases which could be improved if they lost weight. Non-surgical weight management must have been attempted but not resulted in clinically beneficial weight loss before surgery is specified. However, baseline SCOTS data appear to suggest that the low prioritisation of bariatric surgery and a lengthy preop pathway in the UK is associated with surgical treatment being reserved for individuals at an older age with very high BMI. Indeed, in 2018, the Global Registry initiative of the International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) reported a global median pre-bariatric surgery BMI of 41.7 kg/m2,119 as compared to 47.6 kg/m2 in the SCOTS cohort. Similarly, IFSO reported a median patient age of 42 years at the time of bariatric surgery,119 as compared to a median age of 47 years for SCOTS participants. This combination of higher BMI and older age means that, at the time of surgery, Scottish patients have high levels of comorbidity and poor physical functioning.
Bariatric surgery is considered a highly cost-effective intervention. 55 However, the health economic models rely on data primarily from US and Scandinavian studies,16,17,120–122 where BMI and age at the time of surgery are lower than in the UK. Higher BMI and older age are risk factors for postop complications123,124 and also associated with lower total weight loss. 125–127 T2DM remission rates are negatively correlated with age. 128 As such, focusing bariatric surgery provision on those with older age and higher BMI may result in higher costs of surgery with increased length of hospital stay, higher rates of postop complications, lower overall weight loss and lower rates of disease remission. Consequently, the impressive health benefits and resultant cost-savings of bariatric surgery observed in clinical trials and observational cohorts from other countries may not be fully realised for the UK/Scottish population.
The SCOTS dataset represents a unique and rich resource. A major strength of the study is its representativeness. Indeed, every clinical team providing publicly funded bariatric surgery in Scotland approached their patients for recruitment to the study, rendering it highly representative of the population in comparison to other studies undertaken in the field. However, the number of participants with valid baseline questionnaires was lower than anticipated. In many cases, this could be attributed to the participant undergoing surgery before completing the questionnaire, or the participant leaving the bariatric surgery pathway before surgery. The overall length of the questionnaire may have also played a role. Participants living in the most deprived areas were well represented in our cohort and the mean QoL findings were broadly similar to those of bariatric surgery cohorts from across the world. 129–131 A further strength of the study is that questionnaires were externally validated and wide-ranging, containing a number of unique questions covering medical, social, psychological and physical functioning domains. This wide range of self-reported health measures will allow us to account for a range of potentially mediating and confounding factors in future analyses. In addition, we have revealed the extent of comorbidities, including musculoskeletal, urinary and mental health problems affecting people with severe obesity. Low numbers of some comorbidities meant that this could not be a focus of this analysis.
A limitation of this study is that selection for bariatric surgery is often based on the presence of comorbidity so these results, while applicable to a treatment-seeking population, may not be directly applicable to the whole population with severe obesity in the wider society. While we will have access to medical records via electronic health record data linkage in follow-up, the current analyses are based on self-report of selected comorbidities. It is well known that self-reported weights are underreported, particularly by people with very high BMI. 132 However, we are confident of the accuracy of weight and height as these data were collected in clinic during the recruitment visit.
Conclusion
Obesity is a multisystem disease which affects every facet of a person’s life. Our data have shown that higher BMI combined with older age is associated with very poor physical functioning, and HRQoL and O-QoL. Indeed, QoL scores for those living with severe obesity in Scotland are akin to those seen in the end stage of diseases such as cancer and heart failure. The health consequences of severe obesity and the extent to which treatments such as bariatric surgery can improve these are not yet known. Researchers should ensure that they include people with severe obesity in population cohorts and treatment studies, and study the impact of severe obesity in more detail; there are substantial differences in the health status of those with a BMI >50 kg/m2 and those whose BMI is around 40 kg/m2. Policy-makers should consider the health and care needs of the growing numbers of individuals living with obesity. There will be considerable future demand for health care and services must be designed to accommodate the physical needs of the individuals. While primary prevention of obesity is clearly paramount to avoid more people developing such a debilitating, chronic condition, investment is urgently needed, both in the UK and globally, to provide increased access to bariatric surgery and other forms of effective weight management, directly targeting patient groups who will benefit from surgical intervention as early in the disease course as possible.
Chapter 5 Weight and complications outcomes up to 1 year post surgery
Introduction
As outlined in previous chapters, SCOTS obtained full data on pre-surgical and post-surgical pathways, criteria for progression to surgery, staffing and frequency of visits. This allowed services to be outlined by intensity and cost. Herein, we compare these factors, along with patient-related factors such as age, BMI and comorbidity, to patient outcomes at 1 year postoperatively, including weight loss, length of hospital stay, readmission and the need for ITU/HDU admission postoperatively.
Results
Progression to surgery
Of the recruited sample of SCOTS participants (recruited sample), 336/444 (76%) progressed to surgery (operated sample), 92/444 (21%) did not progress to surgery (non-progression to surgery sample) and 14/444 (3%) were still awaiting surgery at the end of the SCOTS study (awaiting surgery sample, see Figure 3). Baseline characteristics of these four samples are shown in Table 9. The cohort that did not progress to surgery had a higher proportion of males, a higher proportion of participants aged 55 years or older, a higher proportion of participants in the lowest SIMD quintile and a higher median BMI at the start of the weight-management programme than those who progressed to surgery.
FIGURE 3.
Screening, consent and follow-up to 1 year post initial bariatric surgery.

| Recruited sample N = 444 | All operated sample N = 336 | Non-progression to surgery sample N = 92 | Awaiting surgery sample N = 14 | ||
|---|---|---|---|---|---|
| Sex, N (%) | Male | 123 (27.7) | 85 (25.3) | 36 (39.1) | 1 (7.1) |
| Female | 321 (72.3) | 251 (74.7) | 56 (60.9) | 13 (92.9) | |
| Missing | 0 | 0 | 0 | 0 | |
| Age (years) | Mean (SD) | 46.2 (9.1) | 46.01 (9.15) | 47.10 (8.76) | 45.41 (9.06) |
| Missing | 0 | 0 | 0 | 0 | |
| Age group, N (%) | <35 years | 61 (13.7) | 47 (14.0) | 11 (12.0) | 2 (14.3) |
| 35–44 years | 116 (26.1) | 86 (25.6) | 25 (27.2) | 4 (28.6) | |
| 45–49 years | 109 (24.5) | 86 (25.6) | 20 (21.7) | 3 (21.4) | |
| 50–54 years | 79 (17.8) | 61 (18.2) | 15 (16.3) | 3 (21.4) | |
| 55 + years | 79 (17.8) | 56 (16.7) | 21 (22.8) | 2 (14.3) | |
| Missing | 0 | 0 | 0 | 0 | |
| SIMD quintile, N (%) | Quintile 1 (most deprived) | 135 (30.5) | 98 (29.3) | 32 (34.8) | 4 (28.6) |
| Quintile 2 | 108 (24.4) | 83 (24.9) | 25 (27.2) | 0 (0.0) | |
| Quintile 3 | 84 (19.0) | 56 (16.8) | 24 (26.1) | 4 (28.6) | |
| Quintile 4 | 68 (15.4) | 57 (17.1.) | 7 (7.6) | 3 (21.4) | |
| Quintile 5 (least deprived) | 47 (10.6) | 40 (12.0) | 4 (4.3) | 3 (21.4) | |
| Missing | 2 | 2 | 0 | 0 | |
| BMI at start of weight-management programme (kg/m2) | Median (Q1; Q3) | 47.2 (42.7; 53.6) | 46.4 (42.4; 52.0) | 50.2 (45.5; 59.4) | 42.3 (39.5; 45.2) |
| Missing | 1 | 1 | 0 | 0 | |
| BMI group at start of weight-management programme, N (%) | BMI < 40 | 52 (11.7) | 44 (13.1) | 4 (4.3) | 4 (28.6) |
| BMI 40–44 | 115 (26.0) | 91 (27.2) | 18 (19.6) | 6 (42.9) | |
| BMI 45-49 | 116 (26.2) | 92 (27.5) | 22 (23.9) | 1 (7.1) | |
| BMI 50–54 | 71 (16.0) | 55 (16.4) | 14 (15.2) | 2 (14.3) | |
| BMI 55+ | 89 (20.1) | 53 (15.8) | 34 (37.0) | 1 (7.1) | |
| Missing | 1 | 1 | 0 | 0 | |
| Weight at start of weight-management programme (kg) | Median (Q1; Q3) | 130 (117; 151) | 129 (115; 146) | 145 (126; 168) | 115 (98.6; 126) |
| Missing | 0 | 1 | 0 | 0 | |
| BMI at date of initial bariatric surgery (kg/m2) | Median (Q1; Q3) | — | 43.2 (39.7; 48.7) | — | — |
| Missing | 56 | ||||
| BMI group at date of initial bariatric surgery, N (%) | BMI < 40 | — | 80 (28.6) | — | — |
| BMI 40–44 | 85 (30.4%) | ||||
| BMI 45-49 | 59 (21.1%) | ||||
| BMI 50–54 | 34 (12.1%) | ||||
| BMI 55+ | 22 (7.9%) | ||||
| Missing | 56 | ||||
| Weight at date of initial bariatric surgery (kg) | Median (Q1; Q3) | — | 121 (107; 136) | — | — |
| Missing | 56 | ||||
| Change in weight from start of weight-management programme to date of initial bariatric surgery (kg) | Median (Q1; Q3) | — | −7.4 (−14.0; −2.0) | — | — |
| Missing | 57 |
The main reasons reported by sites for non-progression to surgery for SCOTS participants are shown in Table 10, with patient decision (for reasons other than weight loss/stress) being most frequently given (37% of participants with non-progression) followed by failure to achieve pre-surgical goals (31.5% of participants with non-progression).
| Reason for surgery not proceedinga | Number of participants (%) |
|---|---|
| Medical/surgical/anaesthetic reason | 16 (17.4) |
| Psychological contraindication | 7 (7.6) |
| Failure to achieve pre-surgical goals | 29 (31.5) |
| Achieved weight loss via other means | 7 (7.6) |
| Major life event or stressor | 1 (1.1) |
| Patient decision (for reasons other than weight loss/stress) | 34 (37.0) |
| Sought private surgery | 0 |
| Other | 7 (7.6) |
| Not known | 3 (3.3) |
| >1 of reasons listed above | 11 (12.0) |
As outlined in Table 11, BMI ≥ 55 kg/m2 and male sex were both associated with around twice the odds of non-progression to surgery as compared to BMI 45–49 kg/m2 and female sex, respectively.
| Variable | Explanatory variable groups | OR (95% CI) | Overall p-value |
|---|---|---|---|
| Age group (years) | <35 | 0.95 (0.40, 2.25) | |
| 35–44 | 1.49 (0.74, 3.00) | ||
| 45–49 | 1.00 (–) | 0.59 | |
| 50–54 | 1.22 (0.56, 2.69) | ||
| 55+ | 1.67 (0.78, 3.54) | ||
| BMI group at recruitment (kg/m2) | <40 | 0.34 (0.11, 1.08) | |
| 40–44 | 0.79 (0.39, 1.60) | ||
| 45–49 | 1.00 (–) | < 0.001 | |
| 50–54 | 0.91 (0.42, 1.95) | ||
| 55+ | 2.81 (1.45, 5.47) | ||
| Sex | Male | 1.96 (1.16, 3.32) | |
| Female | 1.00 (–) | 0.01 | |
| SIMD quintile | SIMD Q1 (most deprived) | 1.00 (–) | 0.03 |
| SIMD Q2 | 1.05 (0.55, 2.00) | ||
| SIMD Q3 | 1.54 (0.79, 2.98) | ||
| SIMD Q4 | 0.41 (0.17, 1.02) | ||
| SIMD Q5 (least deprived) | 0.36 (0.12, 1.14) |
Baseline characteristics of participants whose surgery was NHS or privately funded
Three hundred and twenty-one of 336 SCOTS (96%) participants undergoing bariatric surgery were NHS patients; 15/336 (4%) were patients receiving treatment privately (see Report Supplementary Material 11, supplementary table a). The cohort who underwent privately funded bariatric surgery had a lower median BMI at the start of the weight-management programme but less weight change pre-surgery, resulting in similar median BMIs at the time of surgery. The cohort who had privately funded bariatric surgery only resided in areas in SIMD quintiles 3–5 (more affluent areas, all p < 0.05).
Baseline characteristics of NHS SCOTS participants by pre-surgical pathway intensity cost category
Based on the calculated costs for the pre-surgery treatment pathway, each recruiting site in the NHS was assigned a category of low/medium/high intensity (see Chapter 3, Classification of Scotland’s tier-four pathway costs). Due to smaller numbers of participants in the medium- and low-intensity sites, these two categories were collapsed. One hundred and fifteen of 321 (36%) of SCOTS participants undergoing bariatric surgery on the NHS were on a high-intensity cost pre-surgical pathway; 206/321 (64%) were on a medium-/low-intensity pathway (see Report Supplementary Material 11, supplementary table b). Compared to those in the medium- and low-intensity pathway sites, those in the higher-intensity pathway sites were older and a lower proportion were from areas in the lowest (most deprived) SIMD quintile and had a lower BMI at the start of the weight-management programme (all p < 0.05). Those participants in the higher-intensity pathway sites also had a larger median change in weight pre-surgery with −11 kg (Q1 −17.0; Q3 −7.7) as compared to −5.1 kg (Q1 −11.0; Q3 −0.9) for those in in the low-intensity pathway sites (p < 0.001). This resulted in a large difference in median BMI at date of initial bariatric surgery, with the high-intensity pathway site group having a significantly lower median BMI: 41.5 kg/m2 (Q1 37.6; 45.3) versus 45.4 (Q1 40.4; Q3 50.2); p < 0.0001.
Baseline characteristics of all operated sample of SCOTS participants by bariatric operation type
Baseline characteristics of the operated sample, by operation type, are summarised in Table 12. Mean age of the all operated sample was 46 years (± 9.2 years), with a higher proportion of women than men (75% vs. 25%). Approximately half of the all operated sample of participants were aged 35 to 49 years, with one-third being 50 years or older. There was a median weight change of −7.4 kg (Q1 −14.0; Q3 −2.0) for the all operated sample from the start of the weight-management programme until the date of initial bariatric surgery. The median BMI at date of initial bariatric surgery for the all operated sample was 43.2 kg/m2 (Q1 39.7; Q3 48.7), with almost 8% having a BMI ≥ 55 kg/m2. Over half of the participants in the all operated group (54%) lived in areas of high socioeconomic deprivation (SIMD quintiles 1 and 2). Of the all operated sample of SCOTS participants, 42/336 (12.5%) had LAGB surgery, 128/336 (38.1%) had RYGB surgery, 165/336 (49.1%) had SG surgery and for 1/336 (0.3%) operation type data were missing (see Table 14).
| All operated sample N = 336a |
LAGB N = 42 |
RYGB N = 128 |
SG N = 165 |
||
|---|---|---|---|---|---|
| Sex, N (%) | Male | 85 (25.3) | 13 (31.0) | 33 (25.8) | 39 (23.6) |
| Female | 251 (74.7) | 29 (69.0) | 95 (74.2) | 126 (76.4) | |
| Missing | 0 | 0 | 0 | 0 | |
| Age (years) | Mean (SD) | 46.01 (9.15) | 46.95 (8.78) | 45.42 (8.35) | 46.18 (9.84) |
| Missing | 0 | 0 | 0 | 0 | |
| Age group, N (%) | <35 years | 47 (14.0) | 5 (11.9) | 18 (14.1) | 24 (14.5) |
| 35–44 years | 86 (25.6) | 10 (23.8) | 34 (26.6) | 42 (25.5) | |
| 45–49 years | 86 (25.6) | 9 (21.4) | 39 (30.5) | 38 (23.0) | |
| 50–54 years | 61 (18.2) | 11 (26.2) | 21 (16.4) | 28 (17.0) | |
| 55+ years | 56 (16.7) | 7 (16.7) | 16 (12.5) | 33 (20.0) | |
| Missing | 0 | 0 | 0 | 0 | |
| SIMD quintile, N (%) |
Quintile 1 (most deprived) |
98 (29.3) | 15 (35.7) | 29 (22.8) | 54 (32.9) |
| Quintile 2 | 83 (24.9) | 9 (21.4) | 33 (26.0) | 41 (25.0) | |
| Quintile 3 | 56 (16.8) | 8 (19.0) | 25 (19.7) | 23 (14.0) | |
| Quintile 4 | 57 (17.1) | 10 (23.8) | 21 (16.5) | 26 (15.9) | |
| Quintile 5 (least deprived) |
40 (12.0) | 0 (0.0) | 19 (15.0) | 20 (12.2) | |
| Missing | 2 | 0 | 1 | 1 | |
| BMI at start of weight-management programme (kg/m2) | Median (Q1; Q3) | 46.4 (42.4; 52.0) | 45.7 (42.2; 49.5) | 46.5 (41.9; 51.9) | 46.4 (42.7; 52.9) |
| Missing | 1 | 0 | 1 | 0 | |
| BMI group at start of weight-management programme, N (%) | BMI < 40 | 44 (13.1) | 4 (9.5) | 23 (18.1) | 16 (9.7) |
| BMI 40–44 | 91 (27.2) | 15 (35.7) | 28 (22.0) | 48 (29.1) | |
| BMI 45–49 | 92 (27.5) | 15 (35.7) | 35 (27.6) | 42 (25.5) | |
| BMI 50–54 | 55 (16.4) | 6 (14.3) | 23 (18.1) | 26 (15.8) | |
| BMI 55+ | 53 (15.8) | 2 (4.8) | 18 (14.2) | 33 (20.0) | |
| Missing | 1 | 0 | 1 | 0 | |
| Weight at start of weight-management programme (kg) | Median (Q1; Q3) | 129 (115; 146) | 130 (115; 143) | 127 (114; 146) | 129 (116; 148) |
| Missing | 1 | 0 | 1 | 0 | |
| BMI at date of initial bariatric surgery (kg/m2) | Median (Q1; Q3) | 43.2 (39.7; 48.7) | 42.8 (40.5; 47.9) | 42.6 (38.6; 48.9) | 43.9 (39.8; 48.8) |
| Missing | 55 | 1 | 32 | 22 | |
| BMI group at date of initial bariatric surgery, N (%) | BMI < 40 | 80 (28.6) | 9 (22.0) | 33 (34.4) | 38 (26.6) |
| BMI 40–44 | 85 (30.4) | 16 (39.0) | 26 (27.1) | 43 (30.1) | |
| BMI 45–49 | 59 (21.1) | 11 (26.8) | 16 (16.7) | 32 (22.4) | |
| BMI 50–54 | 34 (12.1) | 4 (9.8) | 14 (14.6) | 16 (11.2) | |
| BMI 55+ | 22 (7.9) | 1 (2.4) | 7 (7.3) | 14 (9.8) | |
| Missing | 55 | 1 | 32 | 22 | |
| Weight at date of initial bariatric surgery (kg) | Median (Q1; Q3) |
121 (107; 136) | 122 (111; 134) | 120 (106; 134) | 121 (107; 137) |
| Missing | 55 | 1 | 32 | 22 | |
| Change in weight from start of weight-management programme to date of initial bariatric surgery (kg) | Median (Q1; Q3) |
−7.4 (−14.0; −2.0) | −3.6 (−15.0; 0.0) | −9.4 (−16.0; −2.6) | −7.4 (−12.0; −2.5) |
| 56 | 1 | 33 | 22 |
LAGB surgery had the largest proportion of participants aged 50 years or older (42.9%) and the highest proportion of participants (35.7%) living in areas of the highest level of socioeconomic deprivation (SIMD quintile 1). SG surgery had the highest proportion of participants with a BMI ≥ 55 kg/m2 at the date of initial bariatric surgery (9.8%). RYGB surgery had the greatest median weight change from the start of weight management until initial bariatric surgery; −9.4 kg (Q1 −16.0; Q3 −2.6).
The proportion of each surgery type by year of operation is shown in Figure 4. LAGB decreases as a proportion of operations performed and SG increases over the period of the study, with 65% of operations being SG and only 7% LAGB by 2017.
FIGURE 4.
Number and type of initial bariatric procedure by year of operation.
Baseline sociodemographic characteristics of SCOTS participants (all operated with PROMs and available outcomes sample) by operation type
Of all SCOTS participants operated on, 189/336 (56%) had available outcome data from PROMs questionnaires. Baseline data are summarised in Table 15. Participant characteristics were comparable between the all operated sample (n = 336, Table 12) and the all operated with PROMs and available data sample (n = 189, Table 13). Of the 189 participants in the all operated with PROMs and available outcomes sample, 26/189 (13.8%) had LAGB surgery; 71/189 (37.6%) had RYGB surgery; 92/189 (48.7%) had SG surgery. Factors measured by patient-reported outcomes were broadly similar across the three surgery types (Tables 14 and 15).
| All operated with PROMs + available outcomes N = 189 |
LAGB N = 26 |
RYGB N = 71 |
SG N = 92 |
||
|---|---|---|---|---|---|
| Sex, N (%) | Male | 50 (26.5) | 8 (30.8) | 26 (36.6) | 16 (17.4) |
| Female | 139 (73.5) | 18 (69.2) | 45 (63.4) | 76 (82.6) | |
| Missing | 0 | 0 | 0 | 0 | |
| Age (years) | Mean (SD) | 45.5 (9.2) | 45.2 (8.5) | 45.81 (8.6) | 45.4 (9.9) |
| Missing | 0 | 0 | 0 | 0 | |
| Age group, N (%) | <35 years | 29 (15.3) | 4 (15.4) | 9 (12.7) | 16 (17.4) |
| 35–44 years | 47 (24.9) | 7 (26.9) | 16 (22.5) | 24 (26.1) | |
| 45–49 years | 49 (25.9) | 6 (23.1) | 24 (33.8) | 19 (20.7) | |
| 50–54 years | 35 (18.5) | 6 (23.1) | 12 (16.9) | 17 (18.5) | |
| 55 + years | 29 (15.3) | 3 (11.5) | 10 (14.1) | 16 (17.4) | |
| Missing | 0 | 0 | 0 | 0 | |
| SIMD quintile, N (%) | Quintile 1 (most deprived) |
46 (24.6) | 5 (19.2) | 12 (17.1) | 29 (31.9) |
| Quintile 2 | 53 (28.3) | 7 (26.9) | 24 (34.3) | 22 (24.2) | |
| Quintile 3 | 37 (19.8) | 7 (26.9) | 14 (20.0) | 16 (17.6) | |
| Quintile 4 | 29 (15.5) | 7 (26.9) | 9 (12.9) | 13 (14.3) | |
| Quintile 5 (least deprived) |
22 (11.8) | 0 (0.0) | 11 (15.7) | 11 (12.1) | |
| Missing | 2 | 0 | 1 | 1 | |
| Weight – start of weight-management programme (kg) | Median (Q1; Q3) | 130 (120; 145) | 130 (115; 137) | 133 (121; 150) | 129 (120; 144) |
| Missing | 0 | 0 | 0 | 0 | |
| Weight – initial bariatric surgery (kg) | Median (Q1; Q3) | 122 (109; 135) | 122 (109; 134) | 123 (108; 134) | 121 (109; 137) |
| Missing | 30 | 1 | 20 | 9 | |
| Change in weight to date of initial bariatric surgery (kg)a | Median (Q1; Q3) | −7.4 (−15.0; −1.0) | −5.2 (−21.0; 0.0) | −9.4 (−17.0; −3.8) | −6.7 (−12.0; −0.4) |
| Missing | 30 | 1 | 20 | 9 | |
| BMI at date of initial bariatric surgery (kg/m2) | Median (Q1; Q3) | 43.3 (40.2; 48.6) | 41.1 (40.5; 46.2) | 42.7 (38.3; 48.3) | 44.4 (40.2; 49.7) |
| Missing | 30 | 1 | 20 | 9 | |
| BMI group at date of initial bariatric surgery, N (%) | BMI < 40 | 39 (24.5) | 5 (20.0) | 16 (31.4) | 18 (21.7) |
| BMI 40–44 | 55 (34.6) | 11 (44.0) | 17 (33.3) | 27 (32.5) | |
| BMI 45–49 | 34 (21.4) | 7 (28.0) | 8 (15.7) | 19 (22.9) | |
| BMI 50–54 | 16 (10.1) | 1 (4.0) | 6 (11.8) | 9 (10.8) | |
| BMI 55+ | 15 (9.4) | 1 (4.0) | 4 (7.8) | 10 (12.0) | |
| Missing | 30 | 1 | 20 | 9 | |
| Marital status, N (%) | Marriedb | 115 (61.8) | 17 (65.4) | 46 (65.7) | 52 (57.8) |
| Other | 71 (38.2) | 9 (34.6) | 24 (34.3) | 38 (42.2) | |
| Missing | 3 | 0 | 1 | 2 | |
| Ethnic group, N (%) | White | 185 (97.9) | 25 (96.2) | 68 (95.8) | 92 (100.0) |
| Mixed | 2 (1.1) | 1 (3.8) | 1 (1.4) | 0 (0.0) | |
| Asian | 1 (0.5) | 0 (0.0) | 1 (1.4) | 0 (0.0) | |
| Black | 1 (0.5) | 0 (0.0) | 1 (1.4) | 0 (0.0) | |
| Missing | 0 | 0 | 0 | 0 |
| All operated with PROMs + available outcomes N = 189 |
LAGB N = 26 |
RYGB N = 71 |
SG N = 92 |
||
|---|---|---|---|---|---|
| Comorbidities | Diabetes N (%) | 95 (50.3) | 13 (50.0) | 44 (62.0) | 38 (41.3) |
| Missing | 0 | 0 | 0 | 0 | |
| N (%) Comorbidities: | |||||
| None | 8 (4.2) | 1 (3.8) | 2 (2.8) | 5 (5.4) | |
| 1–2 | 64 (33.9) | 11 (42.3) | 24 (33.8) | 29 (31.5) | |
| ≥3 | 117 (61.9) | 14 (53.8) | 45 (63.4) | 58 (63.0) | |
| Missing | 0 | 0 | 0 | 0 | |
| Depression | Median (Q1; Q3) preop PHQ-9 score | 8.0 (4.0; 13.0) | 9.0 (7.0; 14.0) | 8.0 (4.0; 12.0) | 8.0 (4.0; 14.0) |
| N (%) PHQ-9 score ≥ 10 (moderate to severe depression) |
77 (41.6) | 12 (48.0) | 24 (34.8) | 41 (45.1) | |
| Missing | 4 | 1 | 2 | 1 | |
| Anxiety | Median (Q1; Q3) preop GAD-7 score | 5.0 (2.0; 9.5) | 7.0 (4.0; 12.5) | 4.0 (1.0; 9.0) | 5.0 (1.0; 9.0) |
| N (%) GAD-7 score ≥ 6 (moderate to severe anxiety) | 80 (43.5) | 15 (62.5) | 24 (34.8) | 41 (45.1) | |
| Missing | 5 | 2 | 2 | 1 | |
| QoL | Preoperative mean (SD) SF-12 PCS score | 38.13 (11.7) | 41.91 (12.1) | 38.38 (11.1) | 36.91 (11.8) |
| Missing | 12 | 2 | 6 | 4 | |
| Mean (SD) SF-12 MCS score | 45.72 (10.3) | 43.51 (9.5) | 46.54 (10.4) | 45.72 (10.4) | |
| Missing | 12 | 2 | 6 | 4 | |
| Median (Q1; Q3) EQ-5D-5L score | 0.6 (0.4; 0.8) | 0.7 (0.6; 0.9) | 0.7 (0.4; 0.8) | 0.6 (0.3; 0.7) | |
| Mean (SD) EQ-5D-5L VAS | 57.3 (22.6) | 60.6 (27.4) | 58.7 (23.1) | 55.2 (20.8) | |
| Missing | 10 | 1 | 3 | 6 | |
| Mean (SD) IWQOL-Lite Physical Function scorea | 54.8 (25.7) | 53.7 (27.5) | 53.1 (25.1) | 56.4 (25.9) | |
| Missing | 5 | 1 | 2 | 2 | |
| Mean (SD) IWQOL-Lite Total scorea | 57.0 (22.1) | 59.8 (21.8) | 55.4 (22.3) | 57.5 (22.1) | |
| Missing | 6 | 1 | 2 | 3 | |
| All operated with PROMs + available outcomes N = 189 |
LAGB N = 26 |
RYGB N = 71 |
SG N = 92 |
||
|---|---|---|---|---|---|
| Life optimism | Preop mean (SD) LOT score | 12.9 (5.0) | 13.5 (5.0) | 12.8 (5.5) | 12.9 (4.5) |
| Missing | 12 | 1 | 4 | 7 | |
| Smoking status | Current, n (%) | 7 (3.8) | 0 (0.0) | 2 (2.9) | 5 (5.7) |
| Former, n (%) | 83 (45.6) | 13 (52.0) | 31 (44.9) | 39 (44.3) | |
| Never, n (%) | 92 (50.5) | 12 (48.0) | 36 (52.2) | 44 (50.0) | |
| Missing, n (%) | 7 | 1 | 2 | 4 | |
| Alcohol use | Preoperative median (Q1; Q3) AUDIT score | 3.0 (1.0; 6.0) | 4.0 (1.0; 6.0) | 2.5 (1.0; 6.0) | 3.0 (1.0; 6.0) |
| Missing | 16 | 2 | 7 | 7 | |
| Physical activity | N (%) ≥1 walking, moderate or vigorous activity in last 7 days | 163 (89.1) | 22 (88.0) | 62 (89.9) | 79 (88.8) |
| Missing | 6 | 1 | 2 | 3 | |
| Preoperative median (Q1; Q3) IPAQ score (MET minutes/week) | 693.0 (0.0; 1950.0) | 556.0 (198.0; 2622.0) | 767.3 (33.0; 1559.5) | 678.5 (0.0; 1872.0) | |
| Missing | 31 | 4 | 11 | 16 |
Hospitalisation, mortality and weight change outcomes up to 1 year post primary bariatric surgery
Outcomes from the primary bariatric surgery hospital admission by operation type (all operated sample)
Median length of stay in hospital during initial bariatric surgery was shortest for the LAGB surgery subsample at 1 (Q1 1.0; Q3 1.0) day as compared to 3.0 (Q1 2.0; Q3 5.0) days for the RYGB surgery subsample and 3 (Q1 2.0; Q3 4.0) days for the SG surgery subsample. For the all operated sample, the proportion admitted to ITU/HDU during the initial bariatric surgery admission was 33.4% and median length of stay in ITU/HDU was 1.0 (Q1 1.0; Q3 2.0) day (see Table 16). Admission to ITU/HDU was highest for the SG surgery subsample at 51%. There were <5 (0–4) participants who underwent any additional surgical procedures prior to or during their ITU/HDU admission.
| Time point | Outcome | All operated N = 336a |
LAGB N = 42 |
RYGB N = 128 |
SG N = 165 |
All operated + PROMs + outcomes N = 189 |
|
|---|---|---|---|---|---|---|---|
| During initial bariatric surgery | N (N missing) | 299 (37) | 29 (13) | 115 (13) | 155 (10) | 170 (19) | |
| Length of stay (days) | Median (Q1; Q3) | 3.0 (2.0, 4.0) | 1.0 (1.0; 1.0) | 3.0 (2.0; 5.0) | 3.0 (2.0; 4.0) | 3.0 (2.0; 4.0) | |
| Admission to ITU/HDU | Yes | 100 (33.4%) | XXX | XXX | 79 (51.0%) | 59 (34.7%) | |
| Length of stay in ITU/HDU if admitted (days) | Median (Q1; Q3) | 1.0 (1.0, 2.0) | XXX | XXX | 1.0 (1.0; 2.0) | 1.0 (1.0; 2.0) | |
| Within the same or subsequent calendar month of initial bariatric surgery | N (N missing) | 336 (0) | 42 (0) | 128 (0) | 165 (0) | 189 (0) | |
| ≥1 Readmissionb (any) | N (% total cohort) | 46 (13.7%) | XXX | 24 (18.8%) | XXX | 25 (13.2%) | |
| ≥1 Readmission (endocrine) | N (% total cohort) | XXX (<5%) | – | – | – | XXX | |
| ≥1 Readmission (circulatory) | N (% total cohort) | XXX (<5%) | – | – | – | – | |
| ≥1 Readmission (surgery-related) | N (% total cohort) | 35 (10.4%) | XXX | 21 (16.4%) | XXX | 20 (10.6%) | |
| ≥1 Readmission (other) | N (% total cohort) | 5 (1.5%) | XXX | XXX | 13 (7.9%) | 8 (4.2%) | |
| Within the same or subsequent 11 calendar months of initial bariatric surgery | N (N missing) | 336 (0) | 42 (0) | 128 (0) | 165 (0) | 189 (0) | |
| ≥1 Readmissionb (any) | N (% total cohort) | 89 (26.5%) | 12 (28.6%) | 38 (29.7%) | 39 (23.6%) | 47 (24.9%) | |
| ≥1 Readmission (endocrine) | N (% total cohort) | XXX (<5%) | – | – | – | – | |
| ≥1 Readmission (circulatory) | N (% total cohort) | 12 (3.6%) | XXX | XXX | 6 (3.6%) | XXX | |
| ≥1 Readmission (surgery-related) | N (% total cohort) | 61 (18.2%) | 8 (19.0%) | 32 (25.0%) | 21 (12.7%) | 35 (18.5%) | |
| ≥1 Readmission (other) | N (% total cohort) | 22 (6.5%) | XXX | XXX | 13 (7.9%) | XXX | |
| Within 30 days of operation | Mortality | Yes | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Within a year of operation | Mortality | Yes | XX (<2%) | – | – | – | – |
| 1 year from date of operation | Weight (kg) | N (N missing) | 273 (63) | 34 (8) | 102 (26) | 136 (29) | 165 (24)) |
| Median (Q1; Q3) |
94.1 (81.9; 107.0) | 105.0 (92.4; 115.0) | 90.0 (75.8; 100.0) | 96.1 (84.5; 109.0) | 93.9 (81.8; 107.0) | ||
| Change in weight from date of operation (kg) | Median (Q1; Q3) |
-29.0 (−39.0; −19.0) | −16.0 (−25.0; −11.0) | −34.0 (−46.0; −25.0) | −28.0 (−36.0; 18.0) | −30.0 (−41.0; −20.0) | |
| Percentage change in weight from date of operation (%) | Mean (SD) | −23.5 (10.1) | −13.4 (8.5) | −28.3 (8.6) | −22.3 (9.1) | −23.7 (10.3) | |
| Change in weight from start of weight-management programme (kg) | N (N missing) | 272 (64) | 34 (8) | 101 (27) | 136 (29 | 165 (24) | |
| Median (Q1; Q3) |
−36.0 (−48.0; −26.0) | −21.0 (−28.0; −13.0) | −44.0 (−53.0; −35.0) | −34.0 (−44.0; −26.0) | −39.0 (−49.0; −27.0) | ||
| Percentage change in weight from start of weight-management programme (%) | Mean (SD) | −27.9 (9.9) | −18.0 (8.5) | −32.9 (8.3) | −26.6 (9.0) | −28.3 (9.9) | |
| N (N missing) | 273 (63) | 34 (8) | 102 (26) | 136 (29) | 165 (24) | ||
| BMI (kg/m2) | Median (Q1; Q3) |
33.8 (30.2; 38.3) | 38.0 (32.1; 41.7) | 32.2 (27.3; 35.4) | 34.9 (31.1; 39.6) | 33.3 (30.0; 37.9) | |
| Change in BMI from date of operation (kg/m2) | Median (Q1; Q3) |
−10.0 (−14.0; −7.0) | −6.0 (−8.7; −3.9) | −13.0 (−16.0; −9.6) | −10.0 (−13.0; −6.6) | −11.0 (−14.0; −7.1) | |
| Change in BMI from start of weight-management programme (kg/m2) | N (N missing)) | 272 (64) | 34 (8) | 101 (27) | 136 (29) | 165 (24) | |
| Median (Q1; Q3) |
−13.0 (−17.0; −9.4) | −7.3 (−9.3; −5.0) | −17.0 (−19.0; 13.0) | −12.0 (−16.0; −9.5) | −14.0 (−17.0; −9.8) | ||
| < 10% weight loss | N (N missing) | 273 (63) | 34 (8) | 102 (26) | 136 (29) | 165 (24 | |
| Yes | 23 (8.4%) | 10 (29.4%) | 2 (2.0%) | 11 (8.1%) | 16 (9.7%) |
Outcomes from the primary bariatric surgery hospital admission by operation type (all operated with PROMs and available outcomes sample)
Results for participants in the all operated with PROMs and available outcomes sample were comparable to those of the all operated sample. By surgery type, median length of stay in hospital during initial bariatric surgery for the all operated with PROMs and available outcomes sample was 1.0 (Q1 0.0; Q3 1.0) day for the LAGB surgery subsample; 3.0 (Q1 2.0; Q3 4.0) days for the RYGB surgery subsample; 4.0 (Q1 2.0; Q3 4.0) days for the SG surgery subsample. The proportion admitted to ITU/HDU during the initial bariatric surgery admission was highest for the SG surgery subsample at 56.5%.
Readmission outcomes for all operated sample by operation type
For the all operated sample, 89/336 (26.5%) had one or more readmission(s) within the same or subsequent 11 calendar months of initial bariatric surgery, of which 46 (51.7%) were within the same or subsequent calendar month of initial bariatric surgery. Of the three surgery types, RYGB surgery had the highest proportion of participants with ≥one readmission(s), both within the same or subsequent calendar month (18.8%) and the same or subsequent 11 calendar months of initial bariatric surgery (29.7%, Table 16).
Surgery-related readmissions made up the highest proportion of readmissions; 10.4% of the operated cohort had one or more surgical readmission(s) within the same or subsequent calendar month, and 18.2% had one or more surgical readmission(s) within the same or subsequent 11 calendar months of initial bariatric surgery (see Table 16).
Twenty-eight (8.4%) of the operated cohort underwent an additional operation or procedure during their readmission; however, only eight of these (2.4% of the operated cohort) were considered to be related to bariatric surgery GI complications or revisions (operation codes of interest detailed in methods, Populations and outcome definitions).
Readmission outcomes for all operated with PROMs and available outcomes sample by operation type
For the all operated with PROMs and available outcomes sample, readmissions were broadly similar, with 47/189 (24.9%) participants having had one or more readmission(s) within the same or subsequent 11 calendar months of initial bariatric surgery, of which 25 (53.2%) were within the same or subsequent calendar month of initial bariatric surgery; 20/189 (10.6%) participants had one or more surgery-related readmission within the same or subsequent calendar month (see Table 16). RYGB surgery had the highest proportion of participants with ≥1 readmission(s) both within the same or subsequent calendar month (18.8%) and the same or subsequent 11 calendar months of initial bariatric surgery (29.6%), which is near identical to the all operated sample.
Mortality outcomes for all operated sample
There were zero deaths within 30 days of bariatric surgery. Within a year of bariatric surgery, mortality was <2% (see Table 16).
Weight outcomes for all operated sample by operation type
At 1 year from the date of initial bariatric surgery, there was a mean percentage weight change of −23.5% (±10.1) for the all operated sample. The LAGB surgery subsample had the lowest weight change [−13.4% (±8.5)] and the RYGB surgery subsample had the greatest [−28.3% (±8.6)]. Weight change for the SG surgery subsample was −22.3% (±9.1, see Table 16).
Weight change at 1 year post initial bariatric surgery was greater when the change from the start of the weight-management programme was included, increasing to −18.0% (±8.5) for the LAGB surgery subsample, −26.6% (±9.0) for the SG surgery subsample, and −32.9% (±8.3) for the RYGB surgery subsample, the greatest change of the three surgery types. A small proportion of participants (8.4%) experienced <10% weight loss at 1 year from the date of initial bariatric surgery, with 10/23 (43.4%) of these participants having undergone LAGB surgery (see Table 16).
Weight outcomes for all operated with PROMs and available outcomes sample by operation type
At 1 year post initial bariatric surgery, the mean percentage weight change for the all operated with PROMs and available outcomes sample was −23.7% (±10.3, see Table 16), −12.2% (±9.8) for the LAGB surgery subsample, −29.4% (±8.3) for the RYGB surgery subsample and −22.4% (±8.7) for the SG surgery subsample, in keeping with the all operated sample.
Mean percentage weight change at 1 year post initial bariatric surgery from the date participants commenced a weight-management programme was −28.3% (±9.9) for the all operated with PROMs and available outcomes sample (see Table 16), −17.7% (±8.9) for the LAGB surgery subsample, −34.0% (±7.6) for the RYGB surgery subsample and −26.8% (±8.8) for the SG surgery subsample.
Median change in BMI at 1 year from the date of initial bariatric surgery was −10.0 kg/m2 (Q1 −14.0; Q3 −7.0) for the all operated with PROMs and available outcomes sample (see Table 16), −6.0 kg/m2 (Q1 −8.7; Q3 −3.9) for the LAGB surgery subsample, −13.0 kg/m2 (Q1 −16.0; Q3 −9.6) for the RYGB surgery subsample and −10.0 kg/m2 (Q1 −13.0; Q3 −6.6) for the SG surgery subsample.
Median change in BMI at 1 year post initial bariatric surgery from the date participants commenced a weight-management programme was −13.0 kg/m2 (Q1 −17.0; Q3 −9.4) for the all operated with PROMs and available outcomes sample (see Table 16), −7.3 kg/m2 (Q1 −9.3; Q3 −5.0) for the LAGB surgery subsample, −17.0 kg/m2 (Q1 −19.0; Q3 −13.0) for the RYGB surgery subsample and −12.0 kg/m2 (Q1 −16.0; Q3 −9.5) for the SG surgery subsample.
For the all operated with PROMs and available outcomes sample, 16/165 (9.7%) participants reported <10% weight loss at 1 year from the date of initial bariatric surgery (see Table 16). As for the all operated sample, the LAGB surgery subsample had the highest number of participants reporting <10% weight loss at 10/42 (29.4%) participants.
Univariate and multivariable analyses to determine associations between surgical outcomes up to 1 year postoperatively and potential explanatory variables
Univariate analyses were performed to understand the associations between potential explanatory variables and bariatric surgery outcomes. Those variables that are routinely collected in practice were then tested in a multivariable model. Patient-reported outcomes were not included in the model as they are not routinely collected in practice so would present an additional burden, had smaller numbers of participants with available data and, other than the physical QoL variables and smoking status, none were statistically significantly associated with outcomes in the univariate analysis. Physical QoL variables are strongly associated with American Society of Anaesthesiologists (ASA) grade {all operated with PROMs; SF-12 PCS [mean (SD)] by ASA Grade I = 44.29 [9.53], II = 39.8 [11.7], III = 35.24 [11.24], p = 0.04} and, therefore, this was chosen for inclusion over QoL scores because the number of participants with data was higher, and they are collected in routine practice. Smoking status was excluded as it was only available for those who had completed PROMs and there were only seven current smokers in the cohort.
Postoperative outcomes for initial bariatric surgery by explanatory variables for all operated sample of SCOTS participants
Table 17 shows results of univariate analyses for initial bariatric surgery outcomes: length of stay, admission to ITU/HDU and length of stay in ITU/HDU. Older age was associated with increased length of stay and higher BMI was associated with increased odds of ITU/HDU admission. Higher ASA grade, lower EQ-5D-5L and SF-12 PCS scores and having a greater number of preop comorbidities were all associated with increased length of stay. All, with the exception of ASA grade, were associated with increased odds of ITU/HDU admission. Higher ASA grade was associated with increased length of ITU/HDU stay (all p < 0.05).
| Explanatory variable | Explanatory variable groups | Length of stay during initial bariatric surgery | Admission to ITU/HDU during initial bariatric surgery | Length of ITU/HDU stay during initial bariatric surgery (days) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of participants included | IRR (95% CI) | Overall p-value | Number of participants included | Odds ratio (95% CI) | Overall p-value | Number of participants included | IRR (95% CI) | Overall p-value | ||
| Age (per 10 years) | 299 | 1.13 (1.04, 1.23) | 0.005 | 336 | 0.76 (0.59, 0.98) | 0.03 | 100 | 0.92 (0.79, 1.07) | 0.29 | |
| Age group (years) | <35 | 299 | 0.87 (0.67, 1.14) | 336 | 1.75 (0.83, 3.71) | 100 | 1.50 (0.93, 2.42) | |||
| 35–44 | 0.91 (0.72, 1.14) | 1.18 (0.61, 2.28) | 1.19 (0.75, 1.89) | |||||||
| 45–49 | 1.00 (–) | <0.001 | 1.00 (–) | 0.44 | 1.00 (–) | 0.17 | ||||
| 50–54 | 0.85 (0.66, 1.09) | 0.84 (0.40, 1.78) | 1.51 (0.90, 2.53) | |||||||
| 55+ | 1.44 (1.12, 1.84) | 0.95 (0.44, 2.01) | 0.86 (0.50, 1.49) | |||||||
| Sex | Male | 299 | 0.91 (0.76, 1.10) | 336 | 1.14 (0.67, 1.93) | 100 | 0.82 (0.56, 1.20) | |||
| Female | 1.00 (–) | 0.33 | 1.00 (–) | 0.64 | 1.00 (–) | 0.30 | ||||
| SIMD | SIMD Q1 (most deprived) | 297 | 1.00 (–) | 0.04 | 334 | 1.00 (–) | <0.001 | 100 | 1.00 (–) | 0.02 |
| SIMD Q2 | 1.40 (1.13, 1.73) | 0.43 (0.23, 0.81) | 0.52 (0.34, 0.81) | |||||||
| SIMD Q3 | 1.11 (0.86, 1.43) | 0.31 (0.15, 0.65) | 0.776 (0.47, 1.29) | |||||||
| SIMD Q4 | 1.21 (0.93, 1.58) | 0.27 (0.13, 0.58) | 0.55 (0.32, 0.95) | |||||||
| SIMD Q5 (least deprived) | 1.11 (0.84, 1.48) | 0.28 (0.12, 0.67) | 0.65 (0.35, 1.19) | |||||||
| BMI group (kg/m2) | <40 | 299 | 0.81 (0.62, 1.07) | 335 | 0.38 (0.14, 1.09) | 100 | 0.58 (0.26, 1.30) | |||
| 40–44 | 0.93 (0.74, 1.16) | 1.07 (0.55, 2.09) | 0.57 (0.35, 0.93) | |||||||
| 45–49 | 1.00 (–) | 0.44 | 1.00 (–) | <0.0001 | 1.00 (–) | 0.05 | ||||
| 50–54 | 1.04 (0.82, 1.32) | 4.17 (2.04, 8.52) | 0.71 (0.46, 1.10) | |||||||
| 55+ | 0.88 (0.67, 1.14) | 1.30 (0.61, 2.75) | 1.11 (0.68, 1.82) | |||||||
| Preoperative BMI (per 10 kg/m2) | 299 | 1.03 (0.92, 1.16) | 0.59 | 335 | 1.42 (1.06, 1.91) | 0.02 | 100 | 1.31 (1.02, 1.68) | 0.04 | |
| Preoperative weight change (kg) | 242 | 1.01 (0.10, 1.02) | 0.19 | 274 | 1.05 (1.02, 1.09) | 0.001 | 79 | 0.99 (0.97, 1.02) | 0.62 | |
| Diabetes | Yes | 170 | 1.18 (0.99, 1.42) | 190 | 0.64 (0.34, 1.19) | 59 | 0.78 (0.52, 1.16) | |||
| No | 1.00 (–) | 0.07 | 1.00 (–) | 0.16 | 1.00 (–) | 0.22 | ||||
| Preoperative comorbidity | 170 | 1.07 (1.03, 1.12) | 0.001 | 190 | 1.25 (1.07, 1.45) | 0.005 | 59 | 0.99 (0.92, 1.06) | 0.75 | |
| Preoperative comorbidity (group) | None | 170 | 1.24 (0.80, 1.93) | 190 | 0.64 (0.12, 3.33) | 59 | 0.55 (0.20, 1.50) | |||
| 1–2 | 0.81 (0.66, 0.98) | 0.68 (0.35, 1.34) | 1.36 (0.88, 2.11) | |||||||
| ≥3 | 1.00 (–) | 0.05 | 1.00 (–) | 0.50 | 1.00 (–) | 0.13 | ||||
| Preoperative anxiety (GAD-7 score) | 165 | 0.10 (0.98, 1.01) | 0.63 | 185 | 1.03 (0.97, 1.09) | 0.35 | 57 | 0.99 (0.96, 1.03) | 0.72 | |
| GAD-7 score (grouped) | Mild anxiety | 165 | 1.00 (–) | 0.69 | 185 | 1.00 (–) | 0.26 | 57 | 1.00 (–) | 0.32 |
| Moderate anxiety | 0.98 (0.76, 1.26) | 1.27 (0.57, 2.85) | 1.36 (0.84, 2.18) | |||||||
| Moderately severe anxiety | 1.11 (0.86, 1.43) | 2.38 (1.01, 5.63) | 0.78 (0.45, 1.34) | |||||||
| Severe anxiety | 0.87 (0.61, 1.25) | 1.10 (0.32, 3.79) | 0.97 (0.38, 2.44) | |||||||
| Preoperative depression (PHQ-9) | 166 | 1.01 (0.10, 1.02) | 0.17 | 186 | 1.06 (1.01, 1.11) | 0.03 | 57 | 0.98 (0.95, 1.01) | 0.12 | |
| PHQ-9 score (grouped) | Minimal depression | 166 | 1.00 (–) | 0.65 | 186 | 1.00 (–) | 0.14 | 57 | 1.00 (–) | 0.35 |
| Mild depression | 0.94 (0.73, 1.21) | 1.02 (0.43, 2.41) | 1.18 (0.67, 2.07) | |||||||
| Moderate depression | 1.09 (0.84, 1.41) | 1.15 (0.45, 2.94) | 0.71 (0.38, 1.34) | |||||||
| Moderately severe depression | 1.16 (0.87, 1.56) | 2.09 (0.75, 5.83) | 0.86 (0.44, 1.67) | |||||||
| Severe depression | 1.09 (0.76, 1.56) | 3.90 (1.14, 13.36) | 0.67 (0.31, 1.42) | |||||||
| Preoperative SF-12 PCS score | (per 5 units) | 158 | 0.94 (0.90, 0.97) | 0.001 | 178 | 0.77 (0.66, 0.89) | <0.001 | 55 | 1.05 (0.95, 1.15) | 0.32 |
| Preoperative SF-12 MCS score | (per 5 units) | 158 | 0.98 (0.94, 1.02) | 0.36 | 178 | 0.82 (0.70, 0.96) | 0.0125 | 55 | 1.03 (0.93, 1.14) | 0.55 |
| Preoperative EQ-5D-5L score | 161 | 0.71 (0.54, 0.94) | 0.02 | 179 | 0.13 (0.05, 0.36) | <0.0001 | 55 | 1.30 (0.72, 2.35) | 0.39 | |
| Preoperative EQ-5D-5L VAS | (per 5 units) | 161 | 0.98 (0.96, 1.00) | 0.06 | 179 | 0.89 (0.82, 0.95) | 0.001 | 55 | 1.03 (0.98, 1.08) | 0.22 |
| Preoperative IWQOL Physical Function score | (per 5 units) | 165 | 1.02 (1.00, 1.04) | 0.07 | 185 | 1.10 (1.03, 1.18) | 0.003 | 57 | 0.99 (0.95, 1.04) | 0.73 |
| Preoperative IWQOL Total score | (per 5 units) | 164 | 1.01 (0.98, 1.03) | 0.64 | 184 | 1.11 (1.03, 1.20) | 0.007 | 57 | 1.00 (0.95, 1.06) | 0.88 |
| Preoperative optimism score | 158 | 0.99 (0.98, 1.01) | 0.55 | 178 | 0.94 (0.88, 1.00) | 0.05 | 55 | 1.00 (0.97, 1.04) | 0.87 | |
| Smoking status | Current | 163 | 1.06 (0.65, 1.73) | 183 | 1.58 (0.33, 7.49) | 57 | 0.65 (0.24, 1.80) | |||
| Former | 0.99 (0.82, 1.19) | 0.85 (0.45, 1.63) | 0.86 (0.57, 1.30) | |||||||
| Never | 1.00 (–) | 0.96 | 1.00 (–) | 0.71 | 1.00 (–) | 0.58 | ||||
| Preoperative alcohol use AUDIT | 155 | 0.98 (0.96, 1.01) | 0.24 | 174 | 0.97 (0.88, 1.06) | 0.48 | 54 | 1.02 (0.96, 1.08) | 0.54 | |
| Preoperative physical activity IPAQ | 141 | 1.00 (1.00, 1.00) | 0.13 | 159 | 1.00 (1.00, 1.00) | 0.32 | 47 | 1.00 (1.00, 1.00) | 0.49 | |
| Surgery type | Gastric band | 299 | 0.29 (0.20, 0.43) | 335 | 0.08 (0.02, 0.28) | 100 | 1.36 (0.43, 4.27) | |||
| Gastric bypass | 0.87 (0.74, 1.03) | 0.18 (0.10, 0.32) | 0.87 (0.58, 130) | |||||||
| SG | 1.00 (–) | <0.0001 | 1.00 (–) | <0.0001 | 1.00 (–) | 0.67 | ||||
| ASA grade | I | 250 | 1.12 (0.68, 1.83) | 282 | 0.25 (0.03, 2.01) | 85 | 0.33 (0.05, 2.39) | |||
| II | 1.00 (–) | <0.0001 | 1.00 (–) | 0.42 | 1.00 (–) | 0.04 | ||||
| III | 1.57 (1.32, 1.88) | 0.99 (0.57, 1.71) | 0.65 (0.44, 0.96) | |||||||
| Health board cost intensity | Low/medium | 299 | 1.00 (–) | 0.5004 | 321 | 1.00 (–) | <0.0001 | 100 | 1.00 (–) | 0.78 |
| High | 1.06 (0.90, 1.25) | 0.28 (0.16, 0.50) | 0.94 (0.62, 1.44) | |||||||
Surgery type was associated with length of stay and ITU/HDU admission, with LAGB surgery having the lowest length of stay and lower odds of ITU/HDU admission, and RYGB surgery having lower odds of ITU/HDU admission, all compared to SG surgery (all p < 0.05). The lowest quintile of SIMD was associated with increased odds of ITU/HDU admission and length of ITU/HDU admission. Of note, lower health board preop pathway intensity was associated with higher odds of ITU/HDU admission, yet increasing weight change (loss) preoperatively was also associated with higher odds of ITU/HDU admission (all p < 0.05).
Multivariable analyses of associations are shown in Table 18 and significant multivariable associations are summarised in Table 23.
| Explanatory variable | Length of stay, bariatric surgery (days) | Admission to ITU/HDU, bariatric surgery | Length of ITU/HDU bariatric surgery (days) | ||||
|---|---|---|---|---|---|---|---|
| IRR (95% CI) | p-value | OR (95% CI) | p-value | IRR (95% CI) | p-value | ||
| Age group (years) | <35 | 0.83 (0.61, 1.14) | 0.22 | 1.31 (0.34, 5.13) | 0.46 | 1.47 (0.78, 2.77) | 0.75 |
| 35–44 | 0.91 (0.71, 1.16) | 0.61 (0.19, 1.93) | 1.11 (0.63, 1.94) | ||||
| 45–49 | 1.00 (–) | 1.00 (–) | 1.00 (–) | ||||
| 50–54 | 0.93 (0.71, 1.21) | 0.39 (0.11, 1.39) | 1.39 (0.73, 2.63) | ||||
| 55+ | 1.21 (0.92, 1.60) | 0.69 (0.19, 2.44) | 1.01 (0.44, 2.29) | ||||
| Sex | Male | 0.88 (0.71, 1.09) | 0.23 | 1.95 (0.79, 4.80) | 0.15 | 0.91 (0.56, 1.49) | 0.71 |
| Female | 1.00 (–) | 1.00 (–) | 1.00 (–) | ||||
| SIMD | SIMD Q1 (most deprived) | 1.00 (–) | 0.04 | 1.00 (–) | 0.005 | 1.00 (–) | 0.19 |
| SIMD Q2 | 1.26 (1.00, 1.59) | 0.26 (0.09, 0.76) | 0.57 (0.32, 0.99) | ||||
| SIMD Q3 | 0.90 (0.68, 1.19) | 0.22 (0.07, 0.72) | 1.154 (0.53, 2.49) | ||||
| SIMD Q4 | 1.01 (0.75, 1.35) | 0.08 (0.02, 0.35) | 0.64 (0.31, 1.31) | ||||
| SIMD Q5 (least deprived) | 0.81 (0.57, 1.13) | 0.20 (0.05, 0.89) | 0.84 (0.33, 2.16) | ||||
| BMI group (kg/m2) | <40 | 0.96 (0.70, 1.32) | 0.72 | 0.58 (0.13, 2.59) | <0.0001 | 0.59 (0.23, 1.51) | 0.33 |
| 40-44 | 0.85 (0.68, 1.08) | 0.83 (0.30, 2.32) | 0.72 (0.39, 1.35) | ||||
| 45-49 | 1.00 (–) | 1.00 (–) | 1.00 (–) | ||||
| 50-54 | 0.98 (0.76, 1.27) | 14.94 (4.33, 51.54) | 1.13 (0.59, 2.15) | ||||
| 55+ | 0.92 (0.68, 1.25) | 0.67 (0.18, 2.55) | 1.18 (0.61, 2.28) | ||||
| Preoperative weight change (kg) | 1.01 (1.00, 1.02) | 0.33 | 1.10 (1.04, 1.16) | 0.001 | 1.02 (0.98, 1.05) | 0.43 | |
| Surgery type | Gastric band | 0.32 (0.22, 0.48) | <0.0001 | 0.01 (0.00, 0.07) | <0.0001 | 1.46 (0.40, 5.37) | 0.82 |
| Gastric bypass | 0.91 (0.74, 1.13) | 0.18 (0.07, 0.49) | 1.13 (0.57, 2.25) | ||||
| SG | 1.00 (–) | 1.00 (–) | 1.00 (–) | ||||
| ASA grade | I | 1.19 (0.73, 1.96) | <0.001 | 0.27 (0.02, 4.26) | 0.61 | 0.35 (0.04, 2.99) | 0.06 |
| II | 1.00 (–) | 1.00 (–) | 1.00 (–) | ||||
| III | 1.49 (1.22, 1.83) | 1.11 (0.44, 2.79) | 0.52 (0.29, 0.93) | ||||
| Health board cost intensity | Low/medium | 1.00 (–) | 0.60 | 1.00 (–) | <0.001 | 1.00 (–) | 0.50 |
| High | 0.95 (0.76, 1.17) | 0.16 (0.06, 0.43) | 1.28 (0.62, 2.66) | ||||
In this model, LAGB surgery was associated with a 68% (95% CI 0.22 to 0.48; p < 0.0001) shorter length of stay during initial bariatric surgery than SG surgery. Participants classified as ASA grade III had a 49% (95% CI 1.22 to 1.83; p < 0.001) increased length of stay during initial bariatric surgery as compared to participants classified as ASA grade II.
Admission to ITU/HDU during initial bariatric surgery was 74% (95% CI 0.09 to 0.76; p < 0.05) less likely for participants in SIMD Q2 than for those in SIMD Q1, and 14.9 times more likely (95 % CI 4.33 to 51.54; p < 0.0001) for participants with BMI 50–54 kg/m2 than for those with BMI 45–49 kg/m2. Participants having LAGB surgery and RYGB surgery were 99% (95% CI 0.00 to 0.07; p < 0.0001) and 82% (95% CI 0.07 to 0.49; p < 0.0001) less likely to be admitted to ITU/HDU during initial bariatric surgery than participants having SG surgery, respectively.
Every kilogram of weight lost by participants preoperatively was associated with a 10% (95% CI 1.04 to 1.16; p < 0.05) increased odds of admission to ITU/HDU. However, participants on high-cost-intensity preop care pathways had 84% lower odds (95% CI 0.06 to 0.43; p < 0.001) of being admitted to ITU/HDU during initial bariatric surgery than those on a medium-/low-cost-intensity pathway. No explanatory variables were significantly associated (p ≤ 0.05) with length of stay in ITU/HDU during initial bariatric surgery in the multivariable analysis.
Postoperative hospital readmission outcomes by explanatory variables for all operated sample of SCOTS participants
Table 19 shows results of univariate analyses for postop readmission outcomes: readmission within the same or subsequent calendar month of initial bariatric surgery and readmission within the same or subsequent 11 calendar months of initial bariatric surgery. A statistically significant association was observed between readmission within the same or subsequent calendar month and preoperatively alcohol use AUDIT score (p < 0.05). Readmission within the same or subsequent 11 calendar months of initial bariatric surgery was associated with preop smoking status (p < 0.05).
| Explanatory variable | Explanatory variable groups | Number of participants with data included | Readmission within same or subsequent calendar month of initial bariatric surgery | Readmission within same or subsequent 11 calendar months of initial bariatric surgery | ||
|---|---|---|---|---|---|---|
| OR (95% CI) |
Overall p-value | OR (95% CI) |
Overall p-value | |||
| Age (per 10 years) | 336 | 0.74 (0.53, 1.04) | 0.08 | 0.83 (0.64, 1.08) | 0.16 | |
| Age group (years) | <35 | 336 | 2.05 (0.79, 5.37) | 2.00 (0.91, 4.37) | ||
| 35–44 | 1.23 (0.50, 3.03) | 1.36 (0.68, 2.73) | ||||
| 45–49 | 1.00 (–) | 0.46 | 1.00 (–) | 0.50 | ||
| 50–54 | 1.32 (0.50, 3.46) | 1.15 (0.53, 2.49) | ||||
| 55+ | 0.75 (0.24, 2.31) | 1.18 (0.53, 2.59) | ||||
| Sex | Male | 336 | 0.92 (0.44, 1.90) | 1.13 (0.65, 1.95) | ||
| Female | 1.00 (–) | 0.82 | 1.00 (–) | 0.67 | ||
| SIMD – deprivation | SIMD Q1 (most) | 334 | 1.00 (–) | 0.39 | 1.00 (–) | 0.56 |
| SIMD Q2 | 1.11 (0.49, 2.53) | 0.92 (0.49, 1.75) | ||||
| SIMD Q3 | 0.46 (0.14, 1.48) | 0.69 (0.32, 1.46) | ||||
| SIMD Q4 | 1.43 (0.60, 3.42) | 0.81 (0.39, 1.68) | ||||
| SIMD Q5 (least) | 0.67 (0.21, 2.16) | 0.48 (0.19, 1.21) | ||||
| Diabetes | Yes | 190 | 1.94 (0.81, 4.63) | 1.49 (0.77, 2.89) | ||
| No | 1.00 (–) | 0.14 | 1.00 (–) | 0.24 | ||
| Preoperative BMI (per 10 kg/m2) | 335 | 0.79 (0.51, 1.22) | 0.29 | 0.91 (0.66, 1.25) | 0.55 | |
| BMI group (kg/m2) | <40 | 335 | 2.45 (0.97, 6.21) | 1.38 (0.64, 2.94) | ||
| 40–44 | 0.81 (0.32, 2.05) | 0.68 (0.35, 1.32) | ||||
| 45–49 | 1.00 (–) | 0.11 | 1.00 (–) | 0.27 | ||
| 50–54 | 1.64 (0.65, 4.15) | 0.99 (0.47, 2.06) | ||||
| 55+ | 0.77 (0.25, 2.34) | 0.56 (0.25, 1.27) | ||||
| Preoperative weight change (kg) | 274 | 1.00 (0.96, 1.03) | 0.83 | 1.00 (0.97, 1.03) | 0.97 | |
| Diabetes | Yes | 190 | 1.94 (0.81, 4.63) | 1.49 (0.77, 2.89) | ||
| No | 1.00 (–) | 0.14 | 1.00 (–) | 0.24 | ||
| Preoperative comorbidity | 190 | 1.04 (0.85, 1.28) | 0.69 | 1.04 (0.89, 1.22) | 0.64 | |
| Preoperative comorbidity (group) | <3 | 190 | 1.57 (0.68, 3.67) | 0.29 | 0.99 (0.50, 1.96) | 0.98 |
| ≥3 | 1.00 (–) | 1.00 (–) | ||||
| Preop anxiety (GAD-7 score) | 185 | 0.98 (0.90, 1.06) | 0.58 | 1.05 (0.99, 1.11) | 0.14 | |
| GAD-7 score (grouped) | Mild | 185 | 1.00 (–) | 0.51 | 1.00 (–) | 0.10 |
| Moderate | 0.31 (0.07, 1.41) | 0.63 (0.24, 1.69) | ||||
| Moderately severe | 0.93 (0.28, 3.03) | 1.35 (0.53, 3.45) | ||||
| Severe anxiety | 0.93 (0.19, 4.54) | 3.38 (1.08, 10.58) | ||||
| Preoperative depression (PHQ-9) | 186 | 1.00 (0.94, 1.07) | 0.92 | 1.02 (0.97, 1.07) | 0.50 | |
| PHQ-9 score (grouped) | Minimal | 186 | 1.00 (–) | 0.88 | 1.00 (–) | 0.52 |
| Mild | 1.20 (0.39, 3.72) | 0.68 (0.28, 1.70) | ||||
| Moderate | 0.86 (0.22, 3.27) | 0.75 (0.28, 2.05) | ||||
| Moderately severe | 1.07 (0.24, 4.70) | 1.46 (0.51, 4.21) | ||||
| Severe depression | 2.05 (0.44, 9.49) | 1.62 (0.46, 5.73) | ||||
| Preoperative SF-12 PCS score | (per 5 units) | 178 | 1.05 (0.86, 1.27) | 0.64 | 0.92 (0.79, 1.07) | 0.28 |
| Preoperative SF-12 MCS score | (per 5 units) | 178 | 0.93 (0.75, 1.15) | 0.51 | 0.92 (0.78, 1.09) | 0.34 |
| Preoperative EQ-5D-5L score | 179 | 1.83 (0.41, 8.13) | 0.43 | 0.51 (0.18, 1.42) | 0.20 | |
| Preoperative EQ-5D-5L VAS | (per 5 units) | 179 | 1.05 (0.95, 1.16) | 0.34 | 0.96 (0.89, 1.04) | 0.32 |
| Preoperative IWQOL Physical Function score | (per 5 units) | 185 | 0.94 (0.86, 1.02) | 0.15 | 0.99 (0.93, 1.06) | 0.75 |
| Preoperative IWQOL Total score | (per 5 units) | 184 | 0.97 (0.88, 1.06) | 0.49 | 1.00 (0.93, 1.08) | 0.98 |
| Preoperative optimism score | 178 | 0.98 (0.90, 1.07) | 0.67 | 0.94 (0.88, 1.01) | 0.11 | |
| Smoking status | Current | 183 | 0.80 (0.09, 7.13) | 7.61 (1.38, 41.91) | ||
| Former | 0.38 (0.14, 1.01) | 0.73 (0.35, 1.49) | ||||
| Never | 1.00 (–) | 0.15 | 1.00 (–) | 0.03 | ||
| Preoperative alcohol use AUDIT | 174 | 0.83 (0.69, 0.99) | 0.04 | 0.94 (0.84, 1.05) | 0.25 | |
| Preoperative physical activity IPAQ | 159 | 1.00 (1.00, 1.00) | 0.65 | 1.00 (1.00, 1.00) | 0.56 | |
| Surgery type | Gastric band | 335 | 0.59 (0.17, 2.10) | 1.29 (0.60, 2.76) | ||
| Gastric bypass | 1.77 (0.92, 3.40) | 1.36 (0.81, 2.30) | ||||
| SG | 1.00 (–) | 0.09 | 1.00 (–) | 0.49 | ||
| ASA grade | I | 282 | 0.66 (0.08, 5.45) | 0.59 (0.12, 2.86) | ||
| II | 1.00 (–) | 0.71 | 1.00 (–) | 0.26 | ||
| III | 0.74 (0.34, 1.60) | 0.62 (0.34, 1.13) | ||||
| Health board cost intensity | Low/medium | 321 | 1.00 (–) | 0.71 | 1.00 (–) | 0.96 |
| High | 0.88 (0.45, 1.71) | 0.99 (0.59, 1.65) | ||||
Multivariable models are shown in Table 20. No explanatory variables were significantly associated (p ≤ 0.05) with hospital readmission outcomes in the multivariable analysis.
| Explanatory variable | Explanatory variable groups | Readmission within same or subsequent calendar month of initial bariatric surgery | Readmission within same or subsequent 11 calendar months of initial bariatric surgery | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Age group (years) | <35 | 2.83 (0.73, 10.95) | 0.48 | 2.34 (0.83, 6.56) | 0.43 |
| 35–44 | 1.13 (0.36, 3.60) | 1.16 (0.50, 2.73) | |||
| 45–49 | 1.00 (–) | 1.00 (–) | |||
| 50–54 | 1.43 (0.40, 5.17) | 0.90 (0.34, 2.39) | |||
| 55+ | 0.75 (0.16, 3.43) | 0.94 (0.34, 2.57) | |||
| Sex | Male | 0.62 (0.22, 1.73) | 0.36 | 0.99 (0.48, 2.04) | 0.97 |
| Female | 1.00 (–) | 1.00 (–) | |||
| SIMD | SIMD Q1 (most deprived) | 1.00 (–) | 0.74 | 1.00 (–) | 0.56 |
| SIMD Q2 | 1.18 (0.40, 3.46) | 0.65 (0.29, 1.45) | |||
| SIMD Q3 | 0.55 (0.13, 2.35) | 0.69 (0.28, 1.72) | |||
| SIMD Q4 | 1.70 (0.47, 6.07) | 0.63 (0.23, 1.74) | |||
| SIMD Q5 (least deprived) | 0.93 (0.20, 4.36) | 0.36 (0.10, 1.32) | |||
| BMI group (kg/m2) | <40 | 3.87 (1.02, 14.64) | 0.12 | 1.84 (0.66, 5.11) | 0.31 |
| 40–44 | 1.18 (0.36, 3.87) | 0.92 (0.41, 2.06) | |||
| 45–49 | 1.00 (–) | 1.00 (–) | |||
| 50–54 | 2.34 (0.69, 7.96) | 1.36 (0.54, 3.43) | |||
| 55+ | 0.51 (0.10, 2.66) | 0.44 (0.14, 1.44) | |||
| Preoperative weight change (kg) | 0.98 (0.93, 1.03) | 0.37 | 1.00 (0.96, 1.04) | 0.97 | |
| Surgery type | Gastric band | 0.49 (0.11, 2.08) | 0.55 | 1.39 (0.55, 3.51) | 0.39 |
| Gastric bypass | 1.24 (0.46, 3.32) | 1.63 (0.76, 3.48) | |||
| SG | 1.00 (–) | 1.00 (–) | |||
| ASA grade | I | 0.82 (0.08, 8.36) | 0.96 | 0.73 (0.13, 4.13) | 0.52 |
| II | 1.00 (–) | 1.00 (–) | |||
| III | 0.88 (0.31, 2.46) | 0.64 (0.30, 1.39) | |||
| Health board cost intensity | Low/medium | 0.78 | 0.70 | ||
| High | 0.86 (0.30, 2.48) | 1.17 (0.53, 2.55) | |||
Weight outcomes by explanatory variables for all operated sample of SCOTS participants
Table 21 shows results of univariate analyses for weight outcomes: change in weight at 1 year and <10% weight loss at 1 year from the date of initial surgery. Statistically significant associations were observed between change in weight at 1 year and sex (p < 0.05), SIMD (p = 0.05), preop weight change (p < 0.01), preop IPAQ score (p < 0.05) and surgery type (p < 0.0001). Weight loss of <10% was associated with preop weight change (p < 0.05) and surgery type (p < 0.001).
| Explanatory variable | Explanatory variable groups | Number of participants included | Change in weight at 1 year adjusted for initial weight | <10% weight loss | ||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | Overall p-value | OR (95% CI) | Overall p-value | |||
| Age (per 10 years) | 272 | 0.61 (−1.01, 2.23) | 0.46 | 1.22 (0.76, 1.95) | 0.41 | |
| Age group (years) | <35 | 272 | 1.71 (−3.34, 6.77) | 0.48 (0.10, 2.46) | ||
| 35–44 | −0.48 (−4.75, 3.80) | 0.54 (0.15, 1.95) | ||||
| 45–49 | 0.00 (–) | 0.55 | 1.00 (–) | 0.73 | ||
| 50–54 | 2.17 (−2.44, 6.78) | 1.16 (0.37, 3.69) | ||||
| 55+ | 2.97 (−1.75, 7.70) | 0.81 (0.22, 2.94) | ||||
| Sex | Male | 272 | 3.88 (0.21, 7.56) | 1.64 (0.66, 4.06) | ||
| Female | 0.00 (–) | 0.04 | 1.00 (–) | 0.28 | ||
| SIMD (deprivation) | SIMD Q1 (most) | 270 | 0.00 (–) | 0.05 | 1.00 (–) | 0.85 |
| SIMD Q2 | −4.40 (−8.52, −0.27) | 0.74 (0.23, 2.38) | ||||
| SIMD Q3 | −3.11 (−7.61, 1.38) | 0.58 (0.15, 2.30) | ||||
| SIMD Q4 | −2.47 (−7.13, 2.18) | 1.14 (0.35, 3.72) | ||||
| SIMD Q5 (least) | −7.61 (−12.73, −2.49) | 0.57 (0.11, 2.85) | ||||
| Diabetes | Yes | 166 | 0.51 (−3.50, 4.52) | 0.55 (0.19, 1.60) | ||
| No | 0.00 (–) | 0.80 | 1.00 (–) | 0.28 | ||
| Preoperative BMI (per 10 kg/m2) | 272 | −0.04 (−2.62, 2.54) | 0.98 | 0.97 (0.56, 1.68) | 0.92 | |
| BMI group (kg/m2) | <40 | 272 | −4.42 (−9.81, 0.99) | 0.57 (0.11, 2.83) | ||
| 40–44 | −0.72 (−4.88, 3.43) | 0.89 (0.30, 2.59) | ||||
| 45–49 | 0.00 (–) | 0.22 | 1.00 (–) | 0.81 | ||
| 50–54 | −3.93 (−8.72, 0.87) | 0.40 (0.08, 1.95) | ||||
| 55+ | 0.61 (−4.53, 5.74) | 0.83 (0.23, 2.93) | ||||
| Preoperative weight change (kg) | 230 | −0.22 (−0.39, −0.06) | 0.007 | 0.95 (0.92, 0.99) | 0.007 | |
| Preoperative comorbidity | 166 | 0.37 (−0.60, 1.35) | 0.45 | 1.03 (0.81, 1.32) | 0.80 | |
| Preoperative comorbidity (group) | None | 166 | −7.51 (−17.59, 2.58) | – | ||
| 1–2 | −1.07 (−5.28, 3.14) | 0.99 (0.34, 2.87) | ||||
| ≥3 | 0.00 (–) | 0.33 | 1.00 (–) | 1.00 | ||
| Preoperative anxiety (GAD-7 score) | 161 | 0.07 (−0.30, 0.43) | 0.71 | 1.05 (0.96, 1.15) | 0.31 | |
| GAD-7 score (grouped) | Mild | 161 | 0.00 (–) | 0.47 | 1.00 (–) | 0.23 |
| Moderate | 3.54 (−1.57, 8.65) | 2.59 (0.73, 9.14) | ||||
| Moderately severe | −1.32 (−7.30, 4.67) | – | ||||
| Severe | −0.36 (−7.85, 7.12) | 4.35 (0.94, 20.14) | ||||
| Preoperative depression (PHQ-9) | 162 | 0.02 (−0.30, 0.34) | 0.91 | 0.99 (0.91, 1.08) | 0.82 | |
| PHQ-9 score (grouped) | Minimal depression | 162 | 0.00 (–) | 0.87 | 1.00 (–) | 0.71 |
| Mild depression | −1.57 (−6.94, 3.80) | 2.22 (0.54, 9.19) | ||||
| Moderate depression | −0.58 (−6.36, 5.20) | 0.80 (0.13, 5.09) | ||||
| Moderately severe depression | −0.74 (−7.70, 6.23) | 1.52 (0.23, 9.88) | ||||
| Severe depression | 2.91 (−5.50, 11.33) | 1.24 (0.12, 13.15) | ||||
| Preoperative SF-12 PCS score | (per 5 units) | 156 | −0.15 (−1.09, 0.79) | 0.75 | 0.96 (0.77, 1.21) | 0.75 |
| Preoperative SF-12 MCS score | (per 5 units) | 156 | 0.14 (−0.91, 1.19) | 0.80 | 1.09 (0.83, 1.42) | 0.54 |
| Preoperative EQ-5D-5L score | 156 | −0.01 (−6.41, 6.40) | 1.00 | 0.77 (0.16, 3.84) | 0.75 | |
| Preoperative EQ-5D-5L VAS | (per 5 units) | 156 | −0.08 (−0.54, 0.38) | 0.72 | 1.00 (0.89, 1.12) | 0.98 |
| Preoperative IWQOL Physical Function score | (per 5 units) | 161 | 0.05 (−0.37, 0.46) | 0.83 | 0.97 (0.87, 1.07) | 0.53 |
| Preoperative IWQOL Total score | (per 5 units) | 160 | 0.00 (−0.48, 0.48) | 0.99 | 0.97 (0.86, 1.09) | 0.61 |
| Preoperative optimism score | 154 | 0.04 (−0.39, 0.46) | 0.87 | 1.04 (0.94, 1.16) | 0.44 | |
| Smoking status | Current | 159 | −7.36 (−17.42, 2.69) | – | ||
| Former | 2.70 (−1.46, 6.85) | – | ||||
| Never | 0.00 (–) | 0.10 | – | – | ||
| Preoperative alcohol use AUDIT | 153 | −0.46 (−1.03, 0.11) | 0.11 | 0.89 (0.74, 1.08) | 0.23 | |
| Preoperative physical activity IPAQ | 140 | 0.00 (0.00, 0.00) | 0.04 | 1.00 (1.00, 1.00) | 0.17 | |
| Surgery type | Gastric band | 271 | 10.30 (6.03, 14.57) | 4.73 (1.81, 12.38) | ||
| Gastric bypass | −7.52 (−10.43, −4.60) | 0.23 (0.05, 1.06) | ||||
| SG | 0.00 (–) | <0.0001 | 1.00 (–) | <0.001 | ||
| ASA grade | I | 234 | −5.17 (−13.70, 3.36) | 1.05 (0.12, 8.92) | ||
| II | 0.00 (–) | 0.13 | 1.00 (–) | 0.42 | ||
| III | −3.27 (−6.85, 0.32) | 0.47 (0.15, 1.46) | ||||
| Health board cost intensity | Low/medium | 260 | 0.00 (–) | 0.13 | 1.00 (–) | 0.99 |
| High | −2.62 (−5.98, 0.74) | 1.01 (0.41, 2.48) | ||||
Multivariable models are shown in Table 22 and significant multivariable associations are summarised in Table 23.
| Explanatory variable | Explanatory variable groups | Change in weight at 1 yeara | <10% weight lossb | ||
|---|---|---|---|---|---|
| Estimate (95% CI) | p-value | OR (95% CI) | p-value | ||
| Age group (years) | <35 | 0.17 (−5.32, 5.66) | 0.43 | 0.27 (0.04, 2.01) | 0.34 |
| 35–44 | −1.72 (−6.13, 2.70) | 0.18 (0.03, 1.01) | |||
| 45–49 | 0.00 (–) | 1.00 (–) | |||
| 50–54 | 1.45 (−3.22, 6.12) | 0.49 (0.11, 2.19) | |||
| 55+ | 2.80 (−2.03, 7.63) | 0.41 (0.09, 1.98) | |||
| Sex | Male | −3.10 (−6.84, 0.65) | 0.10 | 1.49 (0.44, 5.02) | 0.52 |
| Female | 0.00 (–) | 1.00 (–) | |||
| SIMD | SIMD Q1 (most deprived) | 0.00 (–) | 0.16 | 1.00 (–) | 0.70 |
| SIMD Q2 | −4.79 (−9.02, −0.57) | 0.66 (0.14, 3.08) | |||
| SIMD Q3 | −1.64 (−6.28, 3.01) | 0.40 (0.07, 2.49) | |||
| SIMD Q4 | −4.72 (−9.84, 0.41) | 1.55 (0.32, 7.40) | |||
| SIMD Q5 (least deprived) | −3.04 (−8.92, 2.84) | 0.43 (0.03, 5.49) | |||
| BMI group (kg/m2) | <40 | 4.65 (−1.10, 10.40) | <0.0001 | 0.79 (0.11, 5.92) | 0.77 |
| 40–44 | 2.03 (−2.06, 6.11) | 0.60 (0.14, 2.68) | |||
| 45–49 | 0.00 (–) | 1.00 (–) | |||
| 50–54 | −6.82 (−11.70, −1.94) | 0.31 (0.04, 2.25) | |||
| 55+ | −11.91 (−17.25, −6.58) | 0.30 (0.03, 2.93) | |||
| Preoperative weight change (kg) | −0.47 (−0.65, −0.29) | <0.0001 | 0.92 (0.86, 0.97) | 0.006 | |
| Surgery type | Gastric band | 9.20 (4.26, 14.14) | <0.0001 | 6.79 (1.42, 32.53) | 0.010 |
| Gastric bypass | −7.58 (−11.42, −3.74) | 0.24 (0.04, 1.43) | |||
| SG | 0.00 (–) | 1.00 (–) | |||
| ASA grade | I | 2.64 (−5.08, 10.36) | 0.79 | 2.33 (0.15, 37.53) | 0.70 |
| II | 0.00 (–) | 1.00 (–) | |||
| III | 0.00 (−3.80, 3.80) | 0.66 (0.15, 2.91) | |||
| Health board cost intensity | Low/medium | 0.00 (–) | 0.57 | 0.50 | |
| High | −1.12 (−4.98, 2.74) | 1.74 (0.35, 8.81) | |||
| Outcome | Explanatory variable | Explanatory variable group | IRR/OR/Ea | p-value | |
|---|---|---|---|---|---|
| Length of stay during initial bariatric surgery | SIMD | SIMD Q1 (most deprived) | IRR | 1.00 (–) | 0.04 |
| SIMD Q2 | 0.26 (0.09, 0.76) | ||||
| SIMD Q3 | 0.22 (0.07, 0.72) | ||||
| SIMD Q4 | 0.08 (0.02, 0.35) | ||||
| SIMD Q5 (least deprived) | 0.20 (0.05, 0.89) | ||||
| Surgery type | Gastric band | IRR | 0.29 (0.20, 0.43) | <0.0001 | |
| Gastric bypass | 0.87 (0.74, 1.03) | ||||
| SG | 1.00 (–) | ||||
| ASA grade | I | IRR | 1.12 (0.68, 1.83) | <0.0001 | |
| II | 1.00 (–) | ||||
| III | 1.57 (1.32, 1.88) | ||||
| Admission to ITU/HDU during initial bariatric surgery | SIMD | SIMD Q1 (most deprived) | OR | 1.00 (–) | <0.001 |
| SIMD Q2 | 0.43 (0.23, 0.81) | ||||
| SIMD Q3 | 0.31 (0.15, 0.65) | ||||
| SIMD Q4 | 0.27 (0.13, 0.58) | ||||
| SIMD Q5 (least deprived) | 0.28 (0.12, 0.67) | ||||
| BMI group (kg/m2) | <40 | OR | 0.38 (0.14, 1.09) | <0.0001 | |
| 40–44 | 1.07 (0.55, 2.09) | ||||
| 45–49 | 1.00 (–) | ||||
| 50–54 | 4.17 (2.04, 8.52) | ||||
| 55+ | 1.30 (0.61, 2.75) | ||||
| Preoperative weight change | Per kg | OR | 1.1 | 0.001 | |
| Surgery type | Gastric band | OR | 0.29 (0.20, 0.43) | <0.0001 | |
| Gastric bypass | 0.872 (0.74, 1.03) | ||||
| SG | 1.00 (–) | ||||
| Health board cost intensity | Low/medium | OR | 1.00 (–) | <0.001 | |
| High | OR 0.16 | ||||
| Change in weight at 1 year | BMI group (kg/m2) | <40 | E | 4.65 (−1.10, 10.40) | <0.0001 |
| 40–44 | 2.03 (−2.06, 6.11) | ||||
| 45–49 | 0.00 (–) | ||||
| 50–54 | −6.82 (−11.70, −1.94) | ||||
| 55+ | −11.91 (−17.25, −6.58) | ||||
| Preoperative weight change | Per kg | E | 9.20 (4.26, 14.14) | <0.0001 | |
| Surgery type | Gastric band | E | −7.58 (−11.42, −3.74) | <0.0001 | |
| Gastric bypass | 0.00 (–) | ||||
| SG | 9.20 (4.26, 14.14) | ||||
| <10% weight loss | Preoperative weight change | Per kg | OR | 0.92 (0.86, 0.97) | 0.006 |
| Surgery type | Gastric band | OR | 6.79 (1.42, 32.53) | 0.01 | |
| Gastric bypass | 0.24 (0.04, 1.43) | ||||
| SG | 1.00 (–) | ||||
Higher BMI group was associated with larger change in weight at 1 year; for example, participants with a preop BMI ≥ 55 kg/m2 had 11.9 kg greater weight loss at 1 year than those with BMI 45−49 kg/m2 (95% CI −17.25 to −6.58; p < 0.0001). In comparison to participants having SG surgery, participants having LAGB surgery had 9.2 kg less weight loss (95% CI 4.26 to 14.14; p < 0.0001) at 1 year post surgery, while participants having RYGB surgery had an additional 7.6 kg weight loss (95% CI −11.42 to −3.74; p < 0.0001).
While each kilogram of weight lost preoperatively was associated with 0.47 kg less weight loss at 1 year postoperatively (95% CI −0.65 to −0.29; p < 0.0001), it was also associated with 9% lower odds of a weight loss <10% at 1 year postoperatively. In comparison to participants having SG surgery, participants having LAGB surgery were 6.8 times more likely to have a weight loss <10% at 1 year postoperatively.
Table 24 summarises total weight change from start of weight management to 1 year post surgery, by preop weight change category. Those who lost >10% of their body weight between starting weight management and date of surgery had the greatest total weight loss 1 year postoperatively. Those who lost >10% weight preoperatively had a mean weight loss of 32.7 kg (±9.6) compared to a total weight loss of 21.0 kg (±9.6) for those with 1% weight loss or less preoperatively (p < 0.0001).
| All operated + weight outcomes* | Preoperative weight loss | p-value | |||||
|---|---|---|---|---|---|---|---|
| >10% | 10–5.51% | 5.5–1.01% | ≤1% | ||||
| Total percentage weight loss at 1 year postop from start of weight management | N (N missing) |
233 (46) | 60 (14) | 57 (12) | 61 (14) | 55 (6) | |
| Mean (SD) | −27.5 (9.97) | −32.7 (9.67) | −30.0 (8.19) | −26.0 (8.48) | −21.0 (9.63) | <0.0001 | |
Discussion
We have described outcomes up to 1 year post bariatric surgery, including associations with baseline descriptive characteristics and patient-reported outcomes, in a contemporary cohort of patients from Scotland, UK.
Of the 444 recruited participants, 92 (21%) did not progress to bariatric surgery, with the most frequent reasons given being patient decision or failure to achieve pre-surgery goals. There is limited evidence as to the effect of pre-surgery goals on post-surgery outcomes133–135 and, as these goals are often specific to the individual rather than common across a programme, it is unclear what these goals were for each individual SCOTS participant. Often, goals are to achieve a 5% or 10% pre-surgery weight loss, or are related to maintaining a certain attendance rate at the pre-surgical programme.
For those participants who had bariatric surgery, SG was the predominant type and very few LAGB procedures were performed, in keeping with the international norm. 136 Those having SG surgery were older, had higher BMIs and resided in areas with higher levels of socioeconomic deprivation than those undergoing RYGB surgery. This is likely to reflect a choice made by the surgical team based on risks associated with each surgery type; RYGB surgery is known to have a slightly higher complication rate than other bariatric surgery types and is, therefore, less suitable for those who may have a greater severity of underlying comorbidity. 137
Weight change of SCOTS participants at 1 year post bariatric surgery was consistent with 1-year weight change data published from trials and other cohort studies, including registries where RYGB surgery was shown to result in the greatest weight loss. 138 Weight-loss failure, defined as a loss of less than 10% at 1 year post bariatric surgery, was an infrequent outcome for SCOTS participants, with the majority of cases seen in those who had LAGB surgery. While those SCOTS participants with the highest pre-surgical BMI had the greatest postop weight loss, higher preop BMI has not been shown to be a predictor of greater postop weight loss in other studies. 139
The role of preop weight loss within the bariatric surgery pathway is controversial, with many studies concluding that mandatory preop weight loss does not result in greater weight-loss outcomes. 140 Certainly, our data suggest that those with greater weight loss preoperatively have less weight loss postoperatively. However, for those SCOTS participants with greater preop weight loss, total weight loss from the start of the preop programme was greater and preop weight loss was associated with a far lower odds of having less than 10% weight loss at 1 year post surgery. Contrary to what may have been expected, greater pre-surgical weight loss was associated with increased odds of ITU/HDU admission post bariatric surgery. As this was an observational study, it is impossible to make a causal link but, rather than pre-surgical weight loss somehow necessitating ITU/HDU admission postoperatively, it may simply be a marker for patients whom the surgical team perceived as being at increased risk of post-surgical complications due to high body mass or multiple comorbidities and, therefore, for whom higher preop weight-loss targets were set in an attempt to reduce risk.
For SCOTS participants, the median length of stay for the initial bariatric procedure was 3 days, which is high compared to the international mean of 2 days. 136 Numbers of participants admitted to ITU/HDU postoperatively were also high, although it was noted that the median length of stay in ITU/HDU was only 1 day and very few of those admitted had a second operative procedure during their admission. Mortality was minimal, with no deaths within 30 days of bariatric surgery and very low numbers up to 1 year post surgery. Taken together, this suggests sites may be overly cautious when it comes to the postop management of patients who have undergone bariatric surgery.
Up to 1 year post bariatric surgery, readmissions for SCOTS participants were higher than the international mean. 136 However, very low numbers of operative procedures were performed during these admissions despite ‘surgical’ reasons for the readmissions being most common. The reasons for high rates of readmission without the need for surgery are not known. It may reflect the high baseline comorbidity of those having surgery or be a symptom of an inefficiency in the pathways for specialist care. We know that the volume of bariatric surgery procedures is low in Scottish hospitals compared to international practice and that may mean that there is an increased number of referrals and admissions by non-specialists.
SCOTS site intensity was defined by the high, medium or low cost of the preop programme. Lower site pathway intensity was associated with increased odds of admission to ITU/HDU during the initial bariatric surgery procedure for those SCOTS participants undergoing bariatric surgery on the NHS. Within the operated NHS-funded cohort, participants being treated at sites with a high-intensity preop surgical pathway had a lower median BMI at the start of the pathway and a far larger weight loss during the pre-surgical pathway, resulting in a significantly lower BMI at the time of initial bariatric surgery. This could certainly explain their lower admission rates to ITU/HDU postoperatively. However, the characteristics of a site with a high-intensity pre-surgical pathway should also be considered; implementation of a multidisciplinary preop pathway is suggestive of a site with a greater number of dedicated bariatric surgery staff and, potentially, a higher volume of bariatric surgery patients. It may, therefore, be this experience within the team that results in the difference in outcomes. The lower initial BMI in the high-intensity pathway cohort may reflect a higher volume of patients and a more transparent pathway for referral from primary care.
These results raise questions as to the efficiency of bariatric surgery in Scotland, with high numbers of ITU/HDU admissions and hospital readmissions but very limited reoperations suggesting that a cautious approach may be being adopted. The fact that weight loss is in keeping with international means and mortality rate is low serves to reassure that despite this apparent over-caution, the outcome aims of surgery are being achieved. However, there are many questions on bariatric surgery care that this study cannot answer and that is a major limitation. While we know the typical pathway of care in each site, we do not know what happened to each individual participant and the basis for each decision. An ethnographic study would add depth to our understanding of pre-surgical pathways and identify areas where decisions are made and the level of uncertainty and evidence on which those are based. The complexity of interpreting observational data means that it is impossible to make conclusions on the benefits (or otherwise) of preop weight loss, its use as a predictor of longer-term weight loss outcomes, and how it should be used in practice. Studies to date have either looked at those achieving significant weight loss preoperatively or have compared those with and without insurance-mandated weight loss. 97,141,142 What is required is a large randomised trial of weight loss versus weight stability in the population seeking bariatric surgery with follow-up for several years postoperatively.
Our results indicate that 21% of those starting a bariatric surgery pathway do not, ultimately, undergo surgery. This represents not only wasted resources and wasted opportunity to provide alternative treatments but a potential psychological cost to the individual associated with ‘failure’. Male sex and higher BMI were associated with increased odds of non-progression. This could offer some indication as to the population that may benefit from early review by an anaesthetist and/or other measures to inform decision-making by the individual patient earlier in the pathway. However, ‘failure to achieve preop goals’ was also a major reason for non-progression and this may reflect inequalities with regard to the preop pathway; weight-management programmes often require regular attendance at face-to-face classes and consultations which can be during working hours and, with men more likely to work full-time, attendance may have been an issue for them. We know that in our cohort, those with higher BMI had far poorer physical function, meaning that repeat attendance and, consequently, achieving pre-surgical weight loss goals may have proved difficult. The effects of multiple long-term comorbidity medications may have also contributed to lower pre-surgical weight loss. In addition, poorer mental health may have made regular class/consultation attendance and non-surgical weight loss difficult for participants. Poor attendance would have led to exclusion of individuals with complex comorbidities when evidence suggests that they would benefit significantly from bariatric surgery. 143
Conclusion
In conclusion, surgical outcomes for weight and mortality for the SCOTS cohort are in keeping with international results, but there are high numbers of ITU/HDU admissions and hospital readmissions that may be unnecessary. Questions about the optimal pre-surgery pathway to maximise safety and weight loss remain unanswered as current pathways may reflect volume and therefore specialist experience of staff and impact on patient selection.
Chapter 6 Weight and complication outcomes up to 3 years post surgery
Introduction
In this chapter we compare pre- and post-surgical pathway intensity and patient-related factors such as age, BMI and comorbidity, to patient outcomes at 3 years postoperatively, including weight loss, diabetes outcomes, length of hospital stay, readmission, reoperation and the need for ITU/HDU admission postoperatively.
Results
Follow-up to 3 years post surgery
Of the recruited sample of SCOTS participants (recruited sample), 336/444 (76%) progressed to surgery (operated sample), 92/444 (21%) did not progress to surgery (non-progression to surgery sample) and 14/444 (3%) were still awaiting surgery at the end of the SCOTS study (awaiting surgery sample, see Figure 3). Baseline characteristics of the all operated sample (n = 336) are shown in Table 9 and all operated with available preop PROMs in Table 15. Baseline characteristics for the all operated with available 3-year PROMs (n = 85) and all operated with T2DM (n = 192) are shown in Tables 25–27 and Report Supplementary Material 11, Table c, respectively. The sample with T2DM had a higher proportion of participants who were male, higher mean age and a higher proportion of participants aged ≥55 years, than the all operated cohort. Figure 5 outlines the follow-up to 3 years post bariatric surgery of the all-operated sample.
| All operated with PROMs N = 85 |
LAGB N = 13 |
RYGB N = 33 |
SG N = 39 |
||
|---|---|---|---|---|---|
| Sex, N (%) | Male | 25 (29.4%) | 3 (23.1%) | 14 (42.4%) | 8 (20.5%) |
| Female | 60 (70.6%) | 10 (76.9%) | 19 (57.6%) | 31 (79.5%) | |
| Missing | 0 | 0 | 0 | 0 | |
| Age (years) | Mean (SD) | 46.86 (8.46) | 46.16 (8.42) | 46.11 (7.84) | 47.74 (9.08) |
| Missing | 0 | 0 | 0 | 0 | |
| Age group, N (%) | <35 years | 9 (10.6%) | 2 (15.4%) | 4 (12.1%) | 3 (7.7%) |
| 35–44 years | 23 (27.1%) | 3 (23.1%) | 9 (27.3%) | 11 (28.2%) | |
| 45–49 years | 18 (21.2%) | 3 (23.1%) | 7 (21.2%) | 8 (20.5%) | |
| 50–54 years | 21 (24.7%) | 4 (30.8%) | 8 (24.2%) | 9 (23.1%) | |
| 55+ years | 14 (16.5%) | 1 (7.7%) | 5 (15.2%) | 8 (20.5%) | |
| Missing | 0 | 0 | 0 | 0 | |
| SIMD deprivation quintile, N (%) | Quintile 1 (most) | 17 (20.0%) | 1 (7.7%) | 4 (12.1%) | 12 (30.8%) |
| Quintile 2 | 25 (29.4%) | 4 (30.8%) | 13 (39.4%) | 8 (20.5%) | |
| Quintile 3 | 13 (15.3%) | 4 (30.8%) | 4 (12.1%) | 5 (12.8%) | |
| Quintile 4 | 18 (21.2%) | 4 (30.8%) | 7 (21.2%) | 7 (17.9%) | |
| Quintile 5 (least) | 12 (14.1%) | 0 (0.0%) | 5 (15.2%) | 7 (17.9%) | |
| Missing | 0 | 0 | 0 | 0 | |
| Weight at start of weight-management programme (kg) | Median (Q1; Q3) | 129 (115; 142) | 122 (115; 130) | 127 (120; 145) | 130 (113; 142) |
| Missing | 0 | 0 | 0 | 0 | |
| Weight at date of initial bariatric surgery (kg) | Median (Q1; Q3) | 122 (106; 134) | 113 (102; 124) | 123 (108; 134) | 121 (106; 139) |
| Missing | 16 | 1 | 10 | 4 | |
| Change in weight from start of weight-management programme to date of initial bariatric surgery (kg) | Median (Q1; Q3) | −7.1 (−13; −0.8) | −3.0 (−24; 0.0) | −7.7 (−17; −2.0) | −7.6 (−12; −0.3) |
| Missing | 15 | 1 | 10 | 4 | |
| BMI at date of initial bariatric surgery (kg/m2) | Median (Q1; Q3) | 42.6 (39.1; 48.6) | 41.1 (39.4; 47.4) | 40.8 (36.1; 48.3) | 44.2 (40.2; 49.8) |
| Missing | 15 | 1 | 10 | 4 | |
| BMI group at date of initial bariatric surgery, N (%) | BMI < 40 | 22 (31.4%) | 4 (33.3%) | 10 (43.5%) | 8 (22.9%) |
| BMI 40–44 | 19 (27.1%) | 3 (25.0%) | 4 (17.4%) | 12 (34.3%) | |
| BMI 45–49 | 17 (24.3%) | 5 (41.7%) | 5 (21.7%) | 7 (20.0%) | |
| BMI 50–54 | 7 (10.0%) | 0 (0.0%) | 2 (8.7%) | 5 (14.3%) | |
| BMI 55+ | 5 (7.1%) | 0 (0.0%) | 2 (8.7%) | 3 (8.6%) | |
| Missing | 16 | 1 | 10 | 4 | |
| Marital status, N (%) | Married/civil partnership/living as married | 53 (63.1%) | 8 (61.5%) | 24 (68.6%) | 23 (71.8%) |
| Other (single/separated/divorced widowed) | 31 (36.9%) | 5 (38.5%) | 11 (31.4%) | 9 (28.1%) | |
| Missing | 1 | 0 | 0 | 1 | |
| Ethnic group, N (%) | White | 81 (95.3%) | 12 (92.3%) | 30 (90.9%) | 39 (100.0%) |
| Mixed | 2 (2.4%) | 1 (7.7%) | 1 (3.0%) | 0 (0.0%) | |
| Asian/Asian Scottish/Asian British | 1 (1.2%) | 0 (0.0%) | 1 (3.0%) | 0 (0.0%) | |
| African Caribbean/black | 1 (1.2%) | 0 (0.0%) | 1 (3.0%) | 0 (0.0%) | |
| Other | 0 | 0 | 0 | 0 | |
| Missing | 0 | 0 | 0 | 0 |
| All operated PROMs N = 85 |
LAGB N = 13 |
RYGB N = 33 |
SG N = 39 |
||
|---|---|---|---|---|---|
| Comorbidities | Diabetes N (%) | 43 (50.6%) | 5 (38.5%) | 22 (66.7%) | 16 (41.0%) |
| Missing | 0 | 0 | 0 | 0 | |
| N (%) self-reported comorbidities: | |||||
| None | 4 (4.7%) | 0 (0.0%) | 1 (3.0%) | 3 (7.7%) | |
| 1–2 | 32 (37.6%) | 8 (61.5%) | 12 (36.4%) | 12 (30.8%) | |
| ≥3 | 49 (57.6%) | 5 (38.5%) | 20 (60.6%) | 24 (61.5%) | |
| Missing | 0 | ||||
| Depression | Median (Q1; Q3) preop PHQ-9 score | 8.0 (4.0; 12.0) | 8.0 (5.0; 11.0) | 8.0 (4.0; 12.0) | 9.0 (4.0; 13.0) |
| N (%) PHQ-9 score ≥ 10 (moderate to severe depression) |
34 (40.1%) | 5 (38.5%) | 11 (33.3%) | 18 (46.2%) | |
| Missing | 0 | 0 | 0 | 0 | |
| Anxiety | Median (Q1; Q3) preop GAD-7 score | 4.0 (1.0; 10.0) | 6.5 (3.5; 11.0) | 4.0 (1.0; 10.0) | 4.0 (1.0; 10.0) |
| N (%) GAD-7 score ≥ 6 | 35 (41.7%) | 8 (66.6%) | 10 (30.3%) | 17 (43.6%) | |
| Missing | 1 | 1 | 0 | 0 | |
| QoL | Preoperative mean (SD) SF-12 PCS score | 40.27 (12.27) | 45.40 (11.04) | 38.81 (12.11) | 39.61 (12.64) |
| Missing | 6 | 0 | 4 | 2 | |
| Preoperative mean (SD) SF-12 MCS score | 45.53 (9.47) | 43.41 (7.12) | 47.20 (10.41) | 44.96 (9.43) | |
| Missing | 6 | 0 | 4 | 2 | |
| Preoperative median (Q1; Q3) EQ-5D-5L score | 0.7 (0.4; 0.8) | 0.8 (0.6; 0.9) | 0.7 (0.4; 0.8) | 0.6 (0.2; 0.8) | |
| Missing | 1 | 0 | 1 | 0 | |
| Preoperative mean (SD) EQ-5D-5L VAS | 70.0 (40.0; 80.0) | 0.8 (0.6; 0.9) | 0.7 (0.4; 0.8) | 0.6 (0.2; 0.8) | |
| Missing | 1 | 0 | 1 | 0 | |
| Preoperative mean (SD) IWQOL-Lite Physical Function score (standardised scoring) | 48.28 (26.26) | 48.78 (28.33) | 48.42 (27.37) | 47.09 (24.95) | |
| Missing | 0 | 0 | 0 | 0 | |
| Preoperative mean (SD) IWQOL-Lite Total score (standardised scoring) | 45.50 (22.49) | 43.98 (23.24) | 48.22 (22.47) | 42.99 (22.39) | |
| Missing | 0 | 0 | 0 | 0 | |
| All operated PROMs N = 85 |
LAGB N = 13 |
RYGB N = 33 |
SG N = 39 |
||
|---|---|---|---|---|---|
| Life optimism | Preoperative mean (SD) LOT score | 13.24 (4.54) | 14.38 (4.91) | 13.41 (4.96) | 12.70 (4.05) |
| Missing | 3 | 0 | 1 | 2 | |
| Smoking status | Current n (%) | 13.24 (4.54) | 14.38 (4.91) | 13.41 (4.96) | 12.70 (4.05) |
| Former n (%) | 13.24 (4.54) | 14.38 (4.91) | 13.41 (4.96) | 12.70 (4.05) | |
| Never n (%) | 13.24 (4.54) | 14.38 (4.91) | 13.41 (4.96) | 12.70 (4.05) | |
| Missing n (%) | 0 | 0 | 0 | 0 | |
| Alcohol use | Preoperative median (Q1; Q3) AUDIT score | 3.0 (1.0; 5.0) | 4.0 (1.0; 4.0) | 2.0 (1.0; 5.0) | 3.0 (1.0; 5.5) |
| Missing | 6 | 0 | 3 | 3 | |
| Physical activity | N (%) ≥1 walking, moderate or vigorous activity in last 7 days | 79 (92.9%) | 13 (100.0%) | 29 (87.9%) | 37 (94.9%) |
| Missing | 0 | 0 | 0 | 0 | |
| Preoperative median (Q1; Q3) IPAQ score (MET minutes/week) | 1039.5 (462.0; 2479.5) | 1836.0 (462.0; 2622.0) |
1381.5 (462.0; 2820.0) |
808.5 (346.5; 1947.5) |
|
| Missing | 28 | 2 | 11 | 15 |
FIGURE 5.
Follow-up to 3 years post initial bariatric surgery.

Hospitalisation, mortality and weight change outcomes up to 3 years post primary bariatric surgery
Readmission outcomes for all operated sample by operation type
For the all operated sample, 139/335 (41.5%) had one or more readmission(s) within the same or subsequent 35 calendar months of initial bariatric surgery. Of the three surgery types, LAGBB surgery had the highest proportion of participants with ≥one readmission(s), both within the same or subsequent 35 calendar months of initial bariatric surgery (50.0%, see Table 28).
| Outcome | All operated N = 335 |
LAGB N = 42 |
RYGB N = 128 |
SG N = 165 |
All operated + PROMs + outcomes N = 86 |
|
|---|---|---|---|---|---|---|
| N (N missing) | 335 (0) | 42 (0) | 128 (0) | 165 (0) | 86 (0) | |
| ≥1 Readmission$ (any) | N (% total cohort) | 139 (41.5%) | 21 (50.0%) | 56 (43.8%) | 62 (37.6%) | 24 (27.9%) |
| ≥1 Readmission (endocrine) | N (% total cohort) | 11 (3.3%) | XXX | XXX | 6 (3.6%) | XXX |
| ≥1 Readmission (circulatory) | N (% total cohort) | 21 (6.3%) | 6 (14.3%) | 7 (5.5%) | 8 (4.8%) | XXX |
| ≥1 Readmission (surgery-related) | N (% total cohort) | 86 (25.7%) | 14 (33.3%) | 39 (30.5%) | 33 (20.0%) | 17 (19.8%) |
| ≥1 Readmission (other) | N (% total cohort) | 70 (20.9%) | 8 (19.0%) | 27 (21.1%) | 35 (21.2%) | 12 (14.0%) |
| Mortality | Yes | XXX (< 2%) | XXX | XXX | XXX | XXX |
| N (N missing) | 128 (207) | 20 (22) | 50 (78) | 58 (107) | 77 (9) | |
| Weight (kg)+ | Median (Q1; Q3) |
96.1 (84.3; 115.3) |
104.3 (89.4; 121.4) |
90.9 (76.6; 104.8) |
99.6 (86.6; 123.0) |
95.5 (80.7; 108.4) |
| Change in weight from date of operation (kg) | Median (Q1; Q3) |
−24.6 (−36.2; −11.4) |
−13.3 (−25.9; −3.5) |
−30.3 (−45.6; −20.4) |
−18.8 (−32.7; −10.0) |
−28.0 (−38.2; −15.2) |
| Percentage change in weight from date of operation (%) | Mean (SD) | −19.0 (14.10) | −12.3 (14.14) | −24.8 (12.60) | −16.3 (13.67) | −21.2 (14.26) |
| Change in weight from start of weight-management programme (kg) | Median (Q1; Q3) |
−30.0 (−45.6; −21.3) | −26.8 (−37.8; −12.6) | −40.9 (−53.0; −24.4) | −28.1 (−40.3; −18.7) |
−35.2 (−46.9; −24.9) |
| Percentage change in weight from start of weight-management programme (%) | Mean (SD) | −24.2 (12.8) | −19.7 (11.5) | −29.1 (12.9) | −21.5 (11.8) | −25.8 (12.6) |
| BMI (kg/m2) | Median (Q1; Q3) |
34.4 (29.9; 41.0) |
34.9 (32.0; 41.8) |
30.9 (27.9; 34.9) |
37.0 (32.2; 42.7) |
32.2 (29.3; 38.4) |
| Change in BMI from date of operation (kg/m2) | Median (Q1; Q3) |
−8.4 (−13.7; −4.1) |
−4.2 (−8.6; −1.2) | −10.4 (−15.6; −6.6) |
−6.9 (−11.7; −3.7) | −10.2 (−14.4; −5.6) |
| Change in BMI from start of weight-management programme (kg/m2) | Median (Q1; Q3) |
−10.9 (−15.9; −7.7) | −9.5 (−13.9; −4.3) |
−14.4 (−19.3; −8.8) |
−10.7 (−13.9; −6.0) |
−11.9 (−16.0; −8.6) |
| <10% weight loss | N (N missing) | 128 (207) | 20 (22) | 50 (78) | 58 (107) | 77 (9) |
| Yes | 33 (25.8%) | 9 (45.0%) | 5 (10.0%) | 19 (32.8%) | 17 (22.1%) |
Surgery-related readmissions made up the highest proportion of readmissions; 25.7% of the operated cohort had one or more surgical readmission within same or subsequent 35 calendar months of initial bariatric surgery (see Table 28).
Sixty (17.9%) of the operated cohort underwent an additional operation during their readmission; however, only 18 of these (5.4% of the operated cohort) were considered to be related to bariatric surgery GI complications or revisions (operation codes of interest detailed in SMR01 and death record linkage outcomes).
Readmission outcomes for all operated with PROMs and available outcomes sample by operation type
For the all operated with PROMs and available outcomes sample, readmissions were lower, with 24/86 (27.9%) participants having had one or more readmission within the same or subsequent 35 calendar months of initial bariatric surgery; 17/189 (19.8%) participants had one or more surgery-related readmission within the same or subsequent 35 calendar months of initial bariatric surgery (see Table 28).
Mortality outcomes for all operated sample
Within 3 years of bariatric surgery, mortality was <2% (see Table 28).
Weight outcomes for all operated sample by operation type
At 3 years from the date of initial bariatric surgery, there was a mean percentage weight change of −19.0% (±14.1) for the all operated sample. The LAGB surgery subsample had the lowest weight change [−12.3% (±14.4)] and the RYGB surgery subsample had the greatest [−24.8% (±12.6)]. Weight change for the SG surgery subsample was −16.3% (±13.7, see Table 28).
Weight change at 3 years post initial bariatric surgery was greater when the change from the start of the weight-management programme was included, increasing to −19.7% (±11.5) for the LAGB surgery subsample, −21.5% (±11.8) for the SG surgery subsample, and −29.1% (±12.9) for the RYGB surgery subsample, the greatest change of the three surgery types. A quarter of all participants (25.8%) experienced <10% weight loss at 3 years from the date of initial bariatric surgery, with 19/33 (57.6%) of these participants having undergone SG surgery, 32.8% of the SG surgery subsample with available outcome data (see Table 28).
Weight outcomes for all operated with PROMs and available outcomes sample by operation type
At 3 years post initial bariatric surgery, the mean percentage weight change for the all operated with PROMs and available outcomes sample was −21.2% (± 14.3, see Table 28), slightly higher than for the all operated sample.
Quality of life outcomes up to 3 years post primary bariatric surgery
For the all operated sample with available outcome data, there were significant improvements in self-reported QoL scores using EQ-5D-5L, all components of IWQOL-Lite and the physical component score of SF-12 (all p < 0.001). There was no change in the mental component score of SF-12 (see Table 29).
| Outcome | Pre-surgery/baseline | Three years post-surgery | Difference | p-value | |
|---|---|---|---|---|---|
| SF-12 PCS score* | N (N missing) | 69 (0) | |||
| Mean (SD) | 40.38 (11.89) | 48.70 (11.44) | 8.32 (8.95) | <0.001 | |
| SF-12 MCS score* | N (N missing) | 69 (0) | |||
| Mean (SD) | 45.99 (9.40) | 47.26 (11.93) | 1.27 (12.94) | 0.4817 | |
| EQ-5D-5L score** | N (N missing) | 65 (0) | |||
| Median (Q1; Q3) | 0.68 (0.48; 0.84) | 0.77 (0.59; 1.00) | 0.12 (0.00; 0.24) | <0.001 | |
| EQ-5D-5L VAS score* | N (N missing) | 79 (0) | |||
| Mean (SD) | 59.84 (24.22) | 69.77 (24.32) | 9.94 (19.88) | <0.001 | |
| IWQOL-Lite Physical Function score* | N (N missing) | 84 (0) | |||
| Mean (SD) | 47.67 (26.09) | 78.54 (25.00) | 30.87 (23.04) | <0.001 | |
| IWQOL-Lite Self Esteem score* | N (N missing) | 83 (0) | |||
| Mean (SD) | 32.23 (29.50) | 60.15 (33.93) | 27.93 (29.68) | <0.001 | |
| IWQOL-Lite Sexual Life score* | N (N missing) | 74 (0) | |||
| Mean (SD) | 45.35 (31.00) | 20.52 (28.34) | 20.52 (28.34) | <0.001 | |
| IWQOL-Lite Public Distress score* | N (N missing) | 83 (0) | |||
| Mean (SD) | 46.69 (25.40) | 65.88 (34.47) | 35.24 (27.93) | <0.001 | |
| IWQOL-Lite Work score* | N (N missing) | 81 (0) | |||
| Mean (SD) | 56.48 (28.22) | 84.34 (27.25) | 27.85 (28.78) | <0.001 | |
| IWQOL-Lite Total score* | N (N missing) | 82 (0) | |||
| Mean (SD) | 44.73 (21.12) | 74.04 (25.27) | 29.31 (20.92) | <0.001 | |
Type 2 diabetes mellitus outcomes up to 3 years post primary bariatric surgery
For those with available outcome, bariatric surgery was associated with a 5.72 mmol/mol (±16.71) reduction in HbA1c (p = 0.001), and a 4.6 mmHg (±16.6) reduction in systolic blood pressure after 3 years (p = 0.01, see Table 30). There was a decrease in prescribed diabetes medication in 84.9% of participants with 65.5% stopping all diabetes medications (p < 0.001). The proportion of prescribed insulin decreased from 13.6% to 4.0% (p < 0.001, see Table 31). Change in the prevalence of microalbuminuria could not be calculated as only 30 participants had a urine microalbumin result reported within 27–45 months of their primary bariatric surgery. In the 18 months prior to surgery, 124 participants with T2DM had a urine microalbumin result reported (58 missing) and 33 (26.6%) had a raised albumin : creatinine ratio. Diabetes remission was achieved in 58.6% at 3 years, with no difference observed between surgery types (p = 0.25).
| Outcome | Sample | Pre-surgery/ baseline | 3 years post surgery | Difference | p-value | |
|---|---|---|---|---|---|---|
| HbA1c (mmol/mol) | All operated, N = 182 |
N (N missing) | 93 (89) | |||
| Mean (SD) | 53.88 (13.40) | 48.16 (15.39) | −5.72 (16.71) | 0.001 | ||
| LAGB, N = 25 | N (N missing) | 10 (15) | ||||
| Mean (SD) | 53.20 (15.79) | 50.40 (10.10) | −2.80 (10.95) | 0.44 | ||
| RYGB, N = 83 | N (N missing) | 44 (39) | ||||
| Mean (SD) | 54.55 (15.03) | 45.07 (15.21) | −9.48 (19.39) | 0.002 | ||
| SG, N = 74 | N (N missing) | 39 (35) | ||||
| Mean (SD) | 53.31 (10.91) | 51.08 (16.31) | −2.23 (13.82) | 0.32 | ||
| Total cholesterol (mmol/l) | All operated, N = 182 |
N (N missing) | 79 (103) | |||
| Median (IQR) | 4.2 (3.5; 5.0) | 4.5 (3.8; 5.2) | 0.2 (−0.4; 0.8) | 0.01 | ||
| LAGB, N = 25 | N (N missing) | 13 (12) | ||||
| Median (IQR) | 4.7 (3.8; 5.4) | 4.6 (4.1; 5.5) | −2.80 (10.95) | 0.71 | ||
| RYGB, N = 83 | N (N missing) | 34 (49) | ||||
| Median (IQR) | 4.2 (3.3; 5.1) | 4.1 (3.7; 4.8) | −0.1 (−0.4; 0.5) | 0.96 | ||
| SG, N = 74 | N (N missing) | 32 (42) | ||||
| Median (IQR) | 4.2 (3.7; 4.5) | 4.8 (4.3; 5.8) | 0.5 (−0.0; 1.1) | <0.001 | ||
| Systolic blood pressure (mmHg) | All operated, N = 182 |
N (N missing) | 82 (100) | |||
| Mean (SD) | 131.1 (13.84) | 126.5 (14.40) | −4.63 (16.57) | 0.01 | ||
| LAGB, N = 25 | N (N missing) | 12 (13) | ||||
| Mean (SD) | 133.1 (11.84) | 127.9 (9.36) | −5.15 (15.75) | 0.44 | ||
| RYGB, N = 83 | N (N missing) | 35 (48) | ||||
| Mean (SD) | 129.9 (16.00) | 123.8 (16.61) | −6.06 (19.18) | 0.07 | ||
| SG, N = 74 | N (N missing) | 34 (40) | ||||
| Mean (SD) | 131.6 (12.34) | 128.6 (13.43) | −2.97 (14.14) | 0.23 | ||
| Diabetes remissiona | All operated N = 182 |
N (N missing) | 99 (83) | 0.25b | ||
| Yes (%) | – | 58 (58.6%) | – | |||
| LAGB, N = 25 | N (N missing) | 12 (13) | ||||
| Yes (%) | – | 5 (50.0%) | – | |||
| RYGB, N = 83 | N (N missing) | 46 (37) | ||||
| Yes (%) | – | 31 (67.4%) | – | |||
| SG, N = 74 | N (N missing) | 41 (33) | ||||
| Yes (%) | – | 21 (51.2%) | – | |||
| Outcome | Sample | Pre-surgery/baseline | Three years post surgery | p-value | |||
|---|---|---|---|---|---|---|---|
| N of unique diabetes medications (grouped) | All operated, N = 182 |
N (N missing) | 139 (43) | ||||
| N of unique diabetes medications (grouped) | 0 | XXX | 0 | 91 (65.5%) | <0.001 | ||
| 1 | 50 (36.0%) | 1 | 28 (20.1%) | ||||
| 2 | XXX | 2 | 12 (8.6%) | ||||
| 3+ | 47 (33.8%) | 3+ | 8 (5.8%) | ||||
| LAGB, N = 25 | N (N missing) | 17 (8) | |||||
| N of unique diabetes medications (grouped) | 0 | XXX | 0 | 10 (58.8%) | XXX | ||
| 1 | 7 (41.2%) | 1 | XXX | ||||
| 2 | 6 (35.3%) | 2 | XXX | ||||
| 3+ | XXX | 3+ | XXX | ||||
| RYGB, N = 83 | N (N missing) | 64 (19) | |||||
| N of unique diabetes medications (grouped) | 0 | XXX | 0 | 48 (75.0%) | <0.001 | ||
| 1 | 24 (37.5%) | 1 | XXX | ||||
| 2 | XXX | 2 | XXX | ||||
| 3+ | 21 (32.8%) | 3+ | XXX | ||||
| SG, N = 74 | N (N missing) | 58 (16) | |||||
| N of unique diabetes medications (grouped) | 0 | XXX | 0 | 33 (56.9%) | <0.001 | ||
| 1 | 19 (32.8%) | 1 | 13 (22.4%) | ||||
| 2 | XXX | 2 | 5 (8.6%) | ||||
| 3+ | 22 (37.9%) | 3+ | 7 (12.1%) | ||||
| Prescribed insulin | All operated, N = 182 |
N (N missing) | 176 (6) | ||||
| Yes (%) | 24 (13.6%) | 7 (4.0%) | <0.001 | ||||
| LAGB, N = 25 | N (N missing) | 25 (0) | |||||
| Yes (%) | XXX | XXX | XXX | ||||
| RYGB, N = 83 | N (N missing) | 79 (4) | |||||
| Yes (%) | 11 (13.9%) | XXX | 0.002 | ||||
| SG, N = 74 | N (N missing) | 72 (2) | |||||
| Yes (%) | 12 (16.7%) | 5 (6.9%) | 0.008 | ||||
Retinal screening showed observable or referable retinopathy preoperatively in 19.4% of participants with available data; however, there was no difference in the proportion having an improvement or worsening of retinopathy (8.6% in both groups) (see Table 32). The proportion with retinal screening outcomes available at 3 years post surgery was low (58/182; 31.9%).
| Retinal screening outcome | Pre-surgery/baseline | Three years post-surgery | Change category | N (%) |
|---|---|---|---|---|
| N (N missing) | 139 (43) | 58 (124) | 58 (124) | |
| No disease | 112 (80.6%) | 45 (77.6%) | No disease at both preop and 3 years | 40 (69.0%) |
| Observable or referable disease | 27 (19.4%) | 13 (22.4%) | No disease at preop but at least observable disease at 3 years | 5 (8.6%) |
| Some disease at preop but no disease at 3 years | 5 (8.6%) | |||
| At least observable disease at both preop and 3 years | 8 (13.8%) |
Change in participant-reported outcome measures (PROMs) from preoperative to 3 years post primary bariatric surgery
The only significant changes observed between preop and 3 years post-surgery time points were incontinence, where the proportion with symptomatic incontinence (ICIQ-UI SF score ≥ 6) decreased from 38.0% to 20.3% at 3 years (p = 0.003), physical activity, where there was a decrease in the proportion reporting having undertaken ≥1 of walking, moderate or vigorous physical activity in last 7 days (92.8% to 83.1%; p = 0.005), yet conversely an increase in reported physical activity of 918.0 (−655.0; 2194.5) MET minutes per week (p = 0.02, see Table 33).
| Outcome | Pre-surgery/baseline N = 86 |
Three years post surgery N = 86 |
Difference | p-value | ||
|---|---|---|---|---|---|---|
| Gastro-oesophageal reflux | N (N missing) | 81 (0) | ||||
| Yes | 29 (35.8%) | 25 (30.9%) | Started having reflux | 12 (14.8%) | 0.45 | |
| No change | 53 (65.4%) | |||||
| Stopped having reflux | 16 (19.8%) | |||||
| Male reproductive health – impotence | N (N missing) | 22 (0) | ||||
| IPPS score ≥ 8 | 14 (63.6%) | 10 (45.5%) | Increased Severity | 2 (9.1%) | 0.16 | |
| No change | 14 (63.6%) | |||||
| Reduced severity | 6 (27.3%) | |||||
| Incontinence | N (N missing) | 79 (0) | ||||
| ICIQ-UI SF score ≥ 6 | 30 (38.0%) | 16 (20.3%) | Increased Severity | 4 (5.1%) | 0.003 | |
| No change | 57 (72.2%) | |||||
| Reduced severity | 18 (22.8%) | |||||
| Incontinence females | N (N missing) | 58 (0) | ||||
| ICIQ-UI SF score ≥ 6 | 23 (39.7%) | 12 (20.7%) | Increased Severity | 3 (5.2%) | 0.008 | |
| No change | 41 (70.7%) | |||||
| Reduced severity | 14 (24.1%) | |||||
| Incontinence, males | N (N missing) | 21 (0) | ||||
| ICIQ-UI SF score ≥ 6 | 7 (33.3%) | 4 (19.0%) | Increased severity | 1 (4.8%) | 0.18 | |
| No change | 16 (76.2%) | |||||
| Reduced severity | 4 (19.0%) | |||||
| Depression | N (N missing) | 84 (0) | ||||
| PHQ-9 score ≥10) | 34 (40.5%) | 30 (35.7%) | Increased depression | 11 (13.1%) | 0.43 | |
| No change | 58 (69.0%) | |||||
| Reduced depression | 15 (17.9%) | |||||
| Anxiety | N (N missing) | 82 (0) | ||||
| GAD-7 score ≥6 | 35 (42.7%) | 29 (35.4%) | Increased anxiety | 9 (11.0%) | 0.22 | |
| No change | 58 (70.7%) | |||||
| Reduced anxiety | 15 (18.3%) | |||||
| Smoking status | N (N missing) | 83 (0) | ||||
| Current | 4 (4.8%) | 5 (6.0%) | Started smoking | 3 (3.6%) | 0.75 | |
| Former | 42 (50.6%) | 40 (48.2%) | No change | 78 (94.0%) | ||
| Never | 37 (44.6%) | 38 (45.8%) | Stopped smoking | 2 (2.4%) | ||
| Alcohol use | N (N missing) | 71 (0) | ||||
| Median (Q1; Q3) AUDIT score | 3.0 (1.0; 5.0) | 3.0 (1.0; 6.0) | 0.0 (−1.0; 2.0) | 0.19 | ||
| Life optimism | N (N missing) | 80 (0) | ||||
| Mean (SD) LOT score | 13.23 (4.58) | 12.86 (5.93) | –0.36 (5.12) | 0.55 | ||
| Physical activity | N (N missing) | 83 (0) | ||||
| ≥1 walking, moderate or vigorous physical activity in last 7 days | 77 (92.8%) | 69 (83.1%) | Started physical activity | 0 (0.0%) | 0.005 | |
| No change | 75 (90.4%) | |||||
| Stopped physical activity | 8 (9.6%) | |||||
| Physical activity | N (N missing) | 43 (0) | ||||
| Median (Q1; Q3) IPAQ score (MET minutes/ week) | 1377.0 (462.0; 2772.0) | 2133.0 (1314.0; 4026.0) | 918.0 (−655.0; 2194.5) | 0.02 | ||
| Healthcare utilisation | N (N missing) | 83 (0) | ||||
| Using any aids or specialist equipment | 17 (20.5%) | 17 (20.5%) | Started aid use | 3 (3.6%) | 1.00 | |
| No change | 77 (92.8%) | |||||
| Stopped aid use | 3 (3.6%) | |||||
| Median (Q1; Q3) GP visits in last 3 months | 2.0 (1.0; 4.0) | 2.0 (1.0; 4.0) | 0.0 (−2.0; 1.0) | 0.94 | ||
| Healthcare utilisation | N (N missing) | 83 (0) | ||||
| Median (Q1; Q3) visits to other health/social care providers in last 3 months | 2.0 (0.0; 3.0) | 2.0 (0.0; 3.0) | 0.0 (−1.0; 2.0) | 0.37 | ||
| Social security | N (N missing) | 83 (0) | ||||
| Receiving DLA (caring) | 17 (20.5%) | 16 (19.3%) | Started receiving DL | 3 (3.6%) | 0.71 | |
| No change | 76 (91.6%) | |||||
| Stopped receiving DLA | 4 (4.8%) | |||||
| Social security | N (N missing) | 83 (0) | ||||
| Receiving DLA (mobility) | 17 (20.5%) | 12 (14.5%) | Started receiving DLA | 1 (1.2%) | 0.06 | |
| No change | 76 (91.6%) | |||||
| Stopped receiving DLA | 6 (7.2%) | |||||
Postoperative hospital readmission and weight outcomes up to 3 years post surgery by explanatory variables for all operated sample of SCOTS participants
Table 34 shows results of univariate analyses for postop readmission and outcomes: readmission within the same or subsequent 35 calendar months of initial bariatric surgery, change in weight at 3 years and <10% weight loss at 3 years from the date of initial surgery. No significant associations were observed between preop/surgical variables and readmissions up to within the same or subsequent 35 calendar months of initial bariatric surgery.
| Explanatory variable | Explanatory variable groups | Readmission within same or subsequent 35 calendar months of initial bariatric surgery | Change in weight at 3 years post surgery adjusted for initial weight | <10% weight loss | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of participants with data included | OR (95% CI) | Overall p-value | Number of participants with data included | Estimate (95% CI) | Overall p-value | Number of participants with data included | OR (95% CI) | Overall p-value | ||
| Age (per 10 years) | 336 | 0.98 (0.78, 1.25) | 0.90 | 129 | 1.22 (−2.15, 4.58) | 0.48 | 129 | 0.99 (0.64, 1.55) | 0.98 | |
| Age group (years) | <35 | 336 | 1.18 (0.57, 2.41) | 129 | 3.86 (−6.10, 13.82) | 129 | 1.19 (0.35, 4.05) | |||
| 35–44 | 0.91 (0.49, 1.67) | −4.85 (−13.15, 3.45) | 0.32 (0.09, 1.15) | |||||||
| 45–49 | 1.00 (–) | 0.94 | 0.00 (–) | 0.33 | 1.00 (–) | 0.41 | ||||
| 50–54 | 1.16 (0.60, 2.25) | 2.02 (−6.81, 10.85) | 0.85 (0.27, 2.62) | |||||||
| 55+ | 1.02 (0.51, 2.01) | 3.56 (−5.74, 12.85) | 0.68 (0.20, 2.34) | |||||||
| Sex | Male | 336 | 0.9439 | 129 | 0.48 (−6.71, 7.67) | 129 | 0.96 (0.40, 2.32) | |||
| Female | 0.9439 | 0.97 | 0.00 (–) | 0.90 | 1.00 (–) | 0.93 | ||||
| SIMD | SIMD Q1 (most deprived) | 334 | 1.00 (–) | 0.43 | 129 | 0.00 (–) | 0.18 | 129 | 1.00 (–) | 0.61 |
| SIMD Q2 | 0.83 (0.46, 1.51) | −6.90 (−15.46, 1.67) | 0.48 (0.15, 1.55) | |||||||
| SIMD Q3 | 1.20 (0.62, 2.33) | −5.25 (−14.86, 4.37) | 0.80 (0.24, 2.69) | |||||||
| SIMD Q4 | 1.34 (0.70, 2.59) | −0.05 (−8.89, 8.80) | 0.80 (0.26, 2.45) | |||||||
| SIMD Q5 (least deprived) | 0.67 (0.31, 1.45) | −9.82 (−19.65, 0.01) | 0.38 (0.09, 1.60) | |||||||
| Diabetes | Yes | 224 | 1.42 (0.83, 2.42) | 107 | −0.97 (−7.58, 5.65) | 107 | 0.67 (0.28, 1.59) | |||
| No | 1.00 (–) | 0.20 | 0.00 (–) | 0.77 | 1.00 (–) | 0.36 | ||||
| Preoperative BMI (per 10 kg/m2) | 336 | 0.91 (0.69, 1.21) | 0.51 | 112 | 1.41 (−4.56, 7.38) | 0.64 | 129 | 1.48 (0.89, 2.46) | 0.13 | |
| BMI group (kg/m2) | <40 | 336 | 1.30 (0.63, 2.68) | 112 | −3.17 (−13.92, 7.57) | 129 | 0.80 (0.17, 3.69) | |||
| 40–44 | 0.68 (0.37, 1.22) | −2.41 (−12.42, 7.60) | 1.47 (0.47, 4.54) | |||||||
| 45–49 | 1.00 (–) | 0.22 | 0.00 (–) | 0.98 | 1.00 (–) | 0.69 | ||||
| 50–54 | 0.89 (0.46, 1.74) | 0.17 (−12.48, 12.81) | 1.85 (0.49, 6.89) | |||||||
| 55+ | 0.56 (0.28, 1.14) | 1.042 (−11.87, 13.96) | 2.00 (0.56, 7.15) | |||||||
| Preoperative weight change (kg) | 280 | 1.00 (0.98, 1.03) | 0.73 | 112 | −0.46 (−0.75, −0.16) | 0.003 | 112 | 0.97 (0.93, 1.01) | 0.10 | |
| Preoperative comorbidity | 224 | 1.09 (0.96, 1.24) | 0.20 | 107 | 0.98 (−0.71, 2.66) | 0.25 | 107 | 1.10 (0.88, 1.36) | 0.40 | |
| Preoperative comorbidity (group) | <3 | 224 | 0.64 (0.37, 1.13) | 107 | −4.21 (−10.91, 2.49) | 107 | 0.72 (0.30, 1.74) | |||
| ≥3 | 1.00 (–) | 0.12 | 0.00 (–) | 0.22 | 1.00 (–) | 0.46 | ||||
| Preoperative anxiety (GAD-7 score) | 93 | 1.00 (0.93, 1.06) | 0.90 | 105 | 0.11 (−0.51, 0.72) | 0.73 | 84 | 1.08 (1.01, 1.16) | 0.02 | |
| Preoperative depression (PHQ-9) | 94 | 0.99 (0.94, 1.04) | 0.67 | 106 | 0.10 (−0.44, 0.63) | 0.73 | 85 | 1.08 (1.02, 1.14) | 0.01 | |
| PHQ-9 score (grouped) | Minimal depression | 94 | 1.00 (–) | 0.67 | 106 | 0.00 (–) | 0.89 | 85 | 1.00 (–) | 0.05 |
| Mild depression | 0.86 (0.25, 2.93) | −3.09 (−11.70, 5.52) | 3.29 (0.73, 14.78) | |||||||
| Moderate depression | 0.41 (0.04, 3.89) | 0.89 (−8.26, 10.04) | 1.48 (0.14, 15.38) | |||||||
| Moderately severe depression | 0.35 (0.07, 1.76) | −1.02 (−12.61, 10.57) | 8.63 (2.05, 36.28) | |||||||
| Severe depression | 1.15 (0.32, 4.08) | 3.22 (−13.27, 19.72) | 4.23 (0.90, 19.78) | |||||||
| Preoperative SF-12 PCS score (per 5 units) | 212 | 0.89 (0.79, 1.01) | 0.07 | 101 | 0.87 (−0.65, 2.40) | 0.26 | 101 | 1.09 (0.91, 1.32) | 0.35 | |
| Preoperative SF-12 MCS score (per 5 units) | 212 | 1.02 (0.89, 1.16) | 0.79 | 101 | −0.82 (−2.47, 0.83) | 0.32 | 101 | 0.90 (0.73, 1.10) | 0.30 | |
| Preoperative EQ-5D-5L score | 75 | 2.28 (0.43, 12.17) | 0.34 | 71 | −5.84 (−16.66, 4.99) | 0.29 | 71 | 0.43 (0.10, 1.94) | 0.27 | |
| Preoperative EQ-5D-5L VAS (per 5 units) | 211 | 1.00 (0.94, 1.06) | 0.94 | 102 | −0.23 (−0.97, 0.51) | 0.54 | 102 | 0.99 (0.90, 1.09) | 0.89 | |
| Preoperative IWQOL Physical Function score (per 5 units) | 219 | 1.00 (0.95, 1.06) | 0.88 | 106 | −0.18 (−0.82, 0.47) | 0.59 | 106 | 0.98 (0.90, 1.07) | 0.66 | |
| Preoperative IWQOL Total score (per 5 units) | 218 | 1.00 (0.94, 1.07) | 0.92 | 106 | −0.48 (−1.21, 0.26) | 0.20 | 106 | 0.96 (0.87, 1.06) | 0.42 | |
| Preoperative optimism score | 212 | 0.96 (0.91, 1.02) | 0.19 | 103 | −0.52 (−1.25, 0.20) | 0.15 | 103 | 0.91 (0.82, 1.00) | 0.05 | |
| Smoking status | Current | 217 | 2.47 (0.59, 10.37) | 105 | −12.18 (−32.18, 7.82) | – | – | – | ||
| Former | 0.58 (0.33, 1.02) | −2.64 (−9.36, 4.08) | – | – | – | |||||
| Never | 1.00 (–) | 0.05 | 0.00 (–) | 0.40 | – | – | – | |||
| Preoperative alcohol use AUDIT score | 205 | 1.01 (0.93, 1.10) | 0.78 | 100 | −0.72 (−1.61, 0.16) | 0.11 | 100 | 0.92 (0.80, 1.05) | 0.21 | |
| Preoperative Physical Activity IPAQ score | 61 | 1.00 (1.00, 1.00) | 0.31 | 128 | 4.57 (−3.74, 12.87) | 58 | 1.00 (1.00, 1.00) | 0.44 | ||
| Surgery type | Gastric band | 335 | 1.66 (0.84, 3.29) | −10.40 (−16.54, −4.25) | 128 | 1.68 (0.59, 4.74) | ||||
| Gastric bypass | 1.29 (0.81, 2.07) | 0.00 (–) | <0.001 | 0.23 (0.08, 0.67) | ||||||
| SG | 1.00 (–) | 0.28 | 105 | −7.18 (−20.72, 6.36) | 1.00 (–) | 0.01 | ||||
| ASA grade | I | 282 | 0.84 (0.23, 3.08) | 0.00 (–) | 0.47 | 105 | 0.36 (0.04, 3.16) | |||
| II | 1.00 (–) | 0.46 | −3.10 (−10.55, 4.36) | 1.00 (–) | 0.55 | |||||
| III | 0.72 (0.43, 1.21) | 120 | 0.00 (–) | 0.79 | 0.71 (0.28, 1.85) | |||||
| Health board cost intensity | Low/medium | 321 | 1.00 (–) | 0.93 | −0.99 (−8.42, 6.44) | 120 | 1.00 (–) | 1.00 | ||
| High | 1.02 (0.64, 1.62) | 100 | −0.72 (−1.61, 0.16) | 0.11 | 1.00 (0.39, 2.55) | |||||
A statistically significant association was observed between change in weight at 3 years and surgery type with SG having 7.18 kg (95% CI −6.36 to 20.72) and LAGB −10.40 kg (95% CI 4.25 to 16.54) lower weight change compared to RYGB (p < 0.001). Each additional 1 kg of weight loss during preop weight management was associated with 0.46 kg (0.16, 0.75) lower weight loss at 3 years (p = 0.003). Having a weight loss of <10% at 3 years was associated with both increasing anxiety (GAD-7) and depression (PHQ-9) scores. For each unit increase of either score, the odds of <10% weight loss at 3 years increased by 8% [GAD-7 OR 1.08 (95% CI 1.01 to 1.16), p = 0.02; PHQ-9 OR 1.08 (95% CI 1.01 to 1.16), p = 0.01].
Multivariable models are shown in Table 35 and significant multivariable associations are summarised in Table 36. No explanatory variables were significantly associated (p ≤ 0.05) with hospital readmission outcomes in the multivariable analysis. Each kilogram of weight lost preoperatively was associated with 0.77 kg less weight loss at 3 years postoperatively (95% CI −1.16 to −0.37; p < 0.0001). In comparison to participants having RYGB surgery, participants having LAGB surgery had 10.4 kg less weight loss (95% CI 4.25 to 16.54) at 3 years post surgery, while participants having RYGB surgery had an additional 7.2 kg weight loss (95% CI 6.36 to 20.72; p < 0.001). In comparison to participants having SG surgery, participants having LAGB surgery were 1.7 times more likely to have a weight loss <10% at 1 year postoperatively (p = 0.01).
| Explanatory variable | Groups | Readmission same/subsequent 35 months | Change in weight at 3 years post surgery | <10% weight loss | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | Estimate (95% CI) | p-value | OR (95% CI) | p-value | ||
| Age group (years) | <35 | 1.16 (0.45, 3.04) | 0.97 | 0.185 (−13.52, 13.88) | 0.86 | 0.591 (0.09, 3.89) | 0.64 |
| 35–44 | 0.83 (0.39, 1.78) | −1.430 (−11.66, 8.80) | 0.339 (0.06, 1.80) | ||||
| 45–49 | 1.00 (–) | 0.00 (–) | 1.00 (–) | ||||
| 50–54 | 1.00 (0.44, 2.29) | 2.493 (−8.23, 13.21) | 0.810 (0.19, 3.55) | ||||
| 55+ | 0.97 (0.41, 2.29) | 4.291 (−6.53, 15.11) | 0.334 (0.06, 1.86) | ||||
| Sex | Male | 0.79 (0.41, 1.52) | 0.48 | −5.07 (−14.06, 3.91) | 0.26 | 0.792 (0.20, 3.11) | 0.74 |
| Female | 1.00 (–) | 0.00 (–) | 1.00 (–) | ||||
| SIMD | SIMD Q1 (most deprived) | 1.00 (–) | 0.22 | 0.00 (–) | 0.58 | 1.00 (–) | 0.45 |
| SIMD Q2 | 0.62 (0.30, 1.30) | −5.74 (−16.91, 5.42) | 0.91 (0.16, 5.27) | ||||
| SIMD Q3 | 1.13 (0.51, 2.52) | −0.97 (−12.62, 10.68) | 0.99 (0.20, 4.98) | ||||
| SIMD Q4 | 1.56 (0.65, 3.72) | 2.81 (−8.10, 13.72) | 1.46 (0.32, 6.72) | ||||
| SIMD Q5 (least deprived) | 0.47 (0.15, 1.42) | −5.71 (−18.69, 7.27) | 0.55 (0.07, 4.57) | ||||
| BMI group (kg/m2) | <40 | 2.02 (0.76, 5.36) | 0.15 | 12.57 (−2.76, 27.66) | 0.08 | 4.27 (0.36, 50.78) | 0.72 |
| 40–44 | 0.78 (0.38, 1.61) | −1.62 (−11.72, 8.48) | 0.85 (0.18, 4.15) | ||||
| 45–49 | 1.00 (–) | 0.00 (–) | 1.000 (–) | ||||
| 50–54 | 1.34 (0.59, 3.07) | −8.99 (−21.04, 3.058) | 1.06 (0.12, 7.41) | ||||
| 55+ | 0.50 (0.19, 1.34) | −8.52 (−21.01, 4.00) | 0.61 (0.08, 4.73) | ||||
| Preoperative weight change (kg) | 1.00 (0.96, 1.03) | 0.81 | −0.77 (−1.16, −0.37) | <0.001 | 0.95 (0.89, 1.01) | 0.09 | |
| Surgery type | Gastric band | 1.52 (0.65, 3.51) | 0.44 | 0.36 (−10.36, 11.02) | 0.006 | 1.23 (0.26, 5.71) | 0.03 |
| Gastric bypass | 1.39 (0.71, 2.71) | −15.49 (−24.99, −5.99) | 0.08 (0.01, 0.58) | ||||
| SG | 1.00 (–) | 0.00 (–) | 1.00 (–) | ||||
| ASA grade | I | 1.52 (0.66, 3.51) | 0.44 | 5.48 (−9.52, 20.486) | 0.75 | 2.26 (0.15, 33.92) | 0.67 |
| II | 1.39 (0.71, 2.72) | 0.00 (–) | 1.00 (–) | ||||
| III | 1.00 (–) | 1.82 (−7.06, 10.702) | 1.79 (0.44, 7.36) | ||||
| Health board cost intensity | Low/medium | 0.00 (–) | 0.60 | 0.00 (–) | 0.32 | 0.00 (–) | 0.69 |
| High | 1.20 (0.61, 2.38) | −4.57 (−13.59, 4.448) | 0.74 (0.17, 3.20) | ||||
| Outcome | Explanatory variable | Group | (IRR)/(OR)/(E)a | p-value | |
|---|---|---|---|---|---|
| Change in weight at 3 years post surgery | Preoperative weight change (kg) | E | −0.77 (−1.16, −0.37) | <0.001 | |
| Surgery type | Gastric band | 0.36 (−10.36, 11.02) | 0.006 | ||
| Gastric bypass | −15.49 (−24.99, −5.99) | ||||
| SG | 0.00 (–) | ||||
| <10% weight loss | Surgery type | Gastric band | OR | 1.23 (0.26, 5.71) | 0.03 |
| Gastric bypass | 0.08 (0.01, 0.58) | ||||
| SG | 1.00 (–) | ||||
| Change in SF-12 MCS – preop to 3 years post surgery |
Preoperative SF-12 MCS score | E | −0.70 (−1.24, −0.154) | 0.01 | |
| Change in SF-12 PCS – preop to 3 years post surgery |
Preoperative SF-12 MCS score | E | −0.36 (−0.59, −0.13) | 0.004 | |
| Change in HbA1c from baseline | HbA1c (baseline) | E | −0.59 (−0.92, −0.27) | <0.001 | |
Quality of life outcomes up to 3 years post surgery by explanatory variables for with PROMs and available outcomes sample of SCOTS participants
Table 37 shows results of univariate analyses for change in participant-reported SF-12 PCS and SF-12 MCS between baseline (preoperatively) and 3 years post surgery. No significant associations were observed between preop variables and SF-12 PCS. SF-12 MCS was associated with depression (PHQ) group, with moderate to severe depression having a smaller change in MCS score (p = 0.02). Each unit increase in optimism score was associated with a 0.69 unit increase in SF-12 MCS score between baseline and 3 years (p = 0.03). In the multivariable models (see Table 38), the only significant association was between baseline SF-12 PCS or MCS score and change in SF-12 PCS or MCS score respectively at 3 years.
| Explanatory variable | Explanatory variable groups | SF-12 PCSa | SF-12 MCSa | ||||
|---|---|---|---|---|---|---|---|
| Number of participants included | Estimate (95% CI) | Overall p-value | Number of participants included | Estimate (95% CI) | Overall p-value | ||
| Age (per 10 years) | 69 | −0.65 (−3.02, 1.71) | 0.58 | 69 | 2.17 (−1.09, 5.44) | 0.19 | |
| Age group (years) | <35 | 69 | −1.74 (−8.99, 5.50) | 69 | −12.28 (−22.00, −2.56) | ||
| 35–44 | 1.03 (−4.49, 6.56) | −2.54 (−10.04, 4.97) | |||||
| 45–49 | 0.00 (–) | 0.76 | 0.00 (–) | 0.16 | |||
| 50–54 | −2.56 (−8.56, 3.44) | −2.18 (−10.40, 6.05) | |||||
| 55+ | −1.12 (−7.72, 5.49) | −4.50 (−13.46, 4.53) | |||||
| Sex | Male | 69 | −1.29 (−5.60, 3.02) | 69 | 3.71 (−2.31, 9.73) | ||
| Female | 0.00 (–) | 0.55 | 0.00 (–) | 0.22 | |||
| SIMD | SIMD Q1 (most deprived) | 69 | 0.00 (–) | 0.77 | 69 | 0.00 (–) | 0.47 |
| SIMD Q2 | 0.37 (−5.62, 6.35) | −0.99 (−9.37, 7.40) | |||||
| SIMD Q3 | −0.32 (−7.30, 6.67) | −2.69 (−12.41, 7.03) | |||||
| SIMD Q4 | 2.64 (−3.58, 8.86) | 3.059 (−5.58, 11.69) | |||||
| SIMD Q5 (least deprived) | 3.06 (−3.77, 9.89) | 5.19 (−4.36, 14.74) | |||||
| Diabetes | Yes | 69 | 3.13 (−0.75, 7.01) | 69 | 1.59 (−3.98, 7.16) | ||
| No | 0.00 (–) | 0.11 | 0.00 (–) | 0.57 | |||
| Preoperative BMI (per 10 kg/m2) | 55 | −0.20 (−3.48, 3.07) | 0.90 | 69 | −3.00 (−6.97, 0.97) | 0.14 | |
| BMI group (kg/m2) | <40 | 55 | 3.25 (−2.63, 9.12) | 69 | −2.28 (−11.30, 6.74) | ||
| 40–44 | 0.73 (−5.36, 6.81) | −7.40 (−15.10, 0.29) | |||||
| 45–49 | 0.00 (–) | 0.45 | 0.00 (–) | 0.11 | |||
| 50–54 | 0.23 (−7.08, 7.54) | −4.40 (−14.34, 5.55) | |||||
| 55+ | 6.58 (−1.55, 14.71) | −11.94 (−21.21, −2.68) | |||||
| Preoperative weight change (kg) | 55 | −0.07 (−0.26, 0.12) | 0.47 | 55 | 0.23 (−0.08, 0.53) | 0.14 | |
| Preoperative comorbidity | 69 | 0.35 (−0.78, 1.465) | 0.54 | 69 | −0.55 (−2.08, 0.97) | 0.47 | |
| Preoperative comorbidity (group) | <3 | 69 | −2.21 (−6.36, 1.94) | 69 | 1.45 (−4.26, 7.16) | ||
| ≥3 | 0.00 (–) | 0.29 | 0.00 (–) | 0.61 | |||
| Preoperative anxiety (GAD-7 score) | 69 | −0.14 (−0.52, 0.25) | 0.49 | 69 | −0.47 (−1.14, 0.19) | 0.16 | |
| GAD-7 score (grouped) | Mild anxiety | 69 | 0.00 (–) | 0.40 | 69 | 0.00 (–) | 0.28 |
| Moderate anxiety | 0.07 (−5.02, 5.15) | −4.28 (−11.85, 3.30) | |||||
| Moderately severe anxiety | −4.26 (−9.78, 1.27 | −8.35 (−17.05, 0.34) | |||||
| Severe anxiety | 2.18 (−7.68, 12.01) | −3.30 (−18.03, 11.42) | |||||
| Preoperative depression (PHQ-9) | 69 | −0.14 (−0.53, 0.26) | 0.50 | 69 | −0.531 (−1.11, 0.05) | 0.07 | |
| PHQ-9 score (grouped) | Minimal depression | 69 | 0.00 (–) | 0.82 | 69 | 0.00 (–) | 0.02 |
| Mild depression | 2.58 (−2.97, 8.12) | 5.91 (−1.23, 13.05) | |||||
| Moderate depression | 0.36 (−5.66, 6.37) | −4.724 (−12.96, 3.51) | |||||
| Moderately severe depression | 1.30 (−6.45, 9.05) | −8.14 (−19.58, 3.29) | |||||
| Severe depression | −1.64 (−13.10, 9.83) | −4.77 (−19.09, 9.55) | |||||
| Preoperative optimism score | 69 | −0.03 (−0.46, 0.40) | 0.89 | 69 | 0.69 (0.07, 1.32) | 0.03 | |
| Smoking status | Current | 69 | 2.79 (−7.49, 13.07) | 69 | −5.42 (−19.44, 8.60) | ||
| Former | −0.07 (−4.19, 4.05) | −2.81 (−8.53, 2.92) | |||||
| Never | 0.00 (–) | 0.86 | 0.00 (–) | 0.53 | |||
| Preoperative alcohol use AUDIT score | 66 | 0.17 (−0.34, 0.69) | 0.50 | 66 | 0.36 (−0.34, 1.07) | 0.31 | |
| Surgery type | Gastric band | 68 | 3.73 (−2.28, 9.73) | 68 | 8.43 (0.15, 16.71) | ||
| Gastric bypass | 2.82 (−1.49, 7.12) | −0.18 (−6.21, 5.84) | |||||
| SG | 0.00 (–) | 0.30 | 0.00 (–) | 0.10 | |||
| ASA grade | I | 56 | 6.98 (−1.81, 15.77) | 56 | 3.12 (−9.81, 16.05) | ||
| II | 0.00 (–) | 0.27 | 0.00 (–) | 0.88 | |||
| III | −0.24 (−4.60, 4.13) | −0.09 (−6.43, 6.25) | |||||
| Health board cost intensity | Low/medium | 62 | 0.00 (–) | 0.78 | 62 | 0.00 (–) | 0.84 |
| High | −0.65 (−5.25, 3.96) | −0.65 (−7.13, 5.82) | |||||
| Explanatory variable | Groups | Change in SF-12 PCS preoperatively to 3 years post surgery | Change in SF-12 MCS preoperatively to 3 years post surgery | ||
|---|---|---|---|---|---|
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | ||
| Preoperative scorea | −0.36 (−0.59, −0.13) | 0.004 | −0.70 (−1.24, −0.154) | 0.01 | |
| Age group (years) | <35 | 9.66 (−1.33, 20.65) | 0.14 | −8.96 (−28.0, 10.07) | 0.89 |
| 35–44 | 9.30 (1.64, 16.96) | −1.85 (−15.74, 12.05) | |||
| 45–49 | 0.00 (–) | 0.00 (–) | |||
| 50–54 | 6.01 (−2.35, 14.35) | −1.44 (−16.11, 13.23) | |||
| 55+ | 9.24 (0.84, 17.64) | −0.59 (−15.19, 14.0) | |||
| Sex | Male | −0.94 (−7.15, 5.27) | 0.76 | 1.96 (−8.70, 12.63) | 0.71 |
| Female | 0.00 (–) | 0.00 (–) | |||
| SIMD | SIMD Q1 (most deprived) | 0.00 (–) | 0.06 | 0.00 (–) | 0.73 |
| SIMD Q2 | 1.02 (−6.64, 8.67) | 2.91 (−10.57, 16.37) | |||
| SIMD Q3 | −1.84 (−10.69, 7.01) | −4.53 (−20.11, 11.04) | |||
| SIMD Q4 | 8.22 (−0.12, 16.56) | 0.88 (−13.65, 15.40) | |||
| SIMD Q5 (least deprived) | 11.07 (0.34, 21.80) | 10.53 (−8.92, 29.99) | |||
| BMI group (kg/m2) | <40 | 5.82 (−6.28, 17.91) | 0.21 | 1.26 (−19.85, 22.37) | 0.92 |
| 40–44 | −2.42 (−9.60, 4.75) | −3.17 (−15.91, 9.57) | |||
| 45–49 | 0.00 (–) | 0.00 (–) | |||
| 50–54 | −8.48 (−18.10, 1.14) | −5.56 (−21.90, 10.77) | |||
| 55+ | −4.57 (−14.72, 5.58) | −6.24 (−23.93, 11.45) | |||
| Preoperative weight change (kg) | −0.14 (−0.39, 0.11) | 0.26 | 0.25 (−0.20, 0.70) | 0.26 | |
| Surgery type | Gastric band | 4.07 (−3.97, 12.11) | 0.32 | 12.48 (−1.55, 26.51) | 0.17 |
| Gastric bypass | 3.81 (−2.72, 10.34) | −2.94 (−14.40, 8.53) | |||
| SG | 0.00 (–) | 0.00 (–) | |||
| ASA grade | I | 4.41 (−5.69, 14.50) | 0.31 | −0.74 (−18.39, 16.90) | 0.97 |
| II | 0.00 (–) | 0.00 (–) | |||
| III | −3.66 (−10.36, 3.04) | 1.19 (−10.23, 12.60) | |||
| Health board cost intensity | Low/medium | 0.00 (–) | 0.59 | 0.00 (–) | 0.38 |
| High | 1.65 (−4.51, 7.81) | 4.77 (−6.23, 15.77) | |||
Type 2 diabetes mellitus outcomes up to 3 years post surgery by explanatory variables for all operated with type 2 diabetes mellitus and available outcomes sample of SCOTS participants
Table 39 shows results of univariate analyses change in HbA1c and reduction in medications between baseline (preop) and 3 years post surgery. Higher age was significantly associated with a smaller reduction in HbA1c, while higher preop BMI and greater preop weight loss were significantly associated with a greater reduction in HbA1c (all p < 0.05). Only surgery type had significant associations with decrease in number of diabetes medications from baseline to 3 years, with RYGB surgery associated with 4.3 times the odds of a reduction in number of diabetes medications compared to SG surgery (p = 0.04). In the multivariable model (see Table 40), a 1 mmol/mol higher HbA1c pre-surgery was associated with a 0.59 (95% CI 0.927 to 0.92) mmol/mol greater reduction in HbA1c at 3 years (p < 0.001).
| Explanatory variable | Groups | Change in HbA1c from baseline | Change in number of diabetes medications from baseline | ||||
|---|---|---|---|---|---|---|---|
| Number of participants included | Estimate (95% CI) |
Overall p-value | Number of participants included | OR (95% CI) |
Overall p-value | ||
| Age | (per 10 years) | 93 | 3.75 (0.26, 7.25) | 0.04 | 139 | 0.55 (0.29, 1.06) | 0.07 |
| Age group (years) | <35 | 93 | −7.21 (−19.06, 4.64) | ||||
| 35–44 | 3.65 (−5.25, 12.54) | ||||||
| 45–49 | 0.00 (–) | 0.26 | |||||
| 50–54 | 4.03 (−4.48, 12.54) | ||||||
| 55+ | 5.39 (−3.32, 14.11) | ||||||
| Sex | Male | 93 | 6.37 (−0.10, 12.84) | 139 | 1.65 (0.60, 4.57) | ||
| Female | 0.00 (–) | 0.05 | 1.00 (–) | 0.33 | |||
| SIMD (deprivation) | SIMD Q1 (most) | 93 | 0.00 (–) | 0.81 | 138 | 1.00 (–) | 0.12 |
| SIMD Q2 | 3.64 (−4.53, 11.82) | 0.31 (0.09, 1.09) | |||||
| SIMD Q3 | 3.60 (−6.26, 13.47) | 0.49 (0.12, 2.05) | |||||
| SIMD Q4 | −0.95 (−10.20, 8.31) | 2.71 (0.28, 25.78) | |||||
| SIMD Q5 (least) | −0.15 (−10.38, 10.07) | 1.53 (0.16, 14.99) | |||||
| Preoperative BMI | (per 10 kg/m2) | 83 | −6.25 (−11.31, −1.19) | 0.02 | 139 | 1.09 (0.53, 2.23) | 0.81 |
| BMI group (kg/m2) | <40 | 83 | 6.89 (−1.97, 15.76) | ||||
| 40–44 | 10.02 (1.71, 18.33) | ||||||
| 45–49 | 0.00 (–) | 0.06 | |||||
| 50–54 | 3.07 (−7.84, 13.99) | ||||||
| 55+ | −6.98 (−22.31, 8.35) | ||||||
| Preoperative weight change (kg) | 112 | −0.46 (−0.75, −0.16) | 0.003 | 118 | 0.96 (0.90, 1.02) | 0.17 | |
| Preoperative comorbidity | 63 | −0.18 (−2.12, 1.76) | 0.85 | 94 | 0.80 (0.62, 1.05) | 0.10 | |
| Preoperative comorbidity (group) | <3 | 63 | −4.05 (−12.60, 4.49) | 94 | 1.13 (0.33, 3.91) | ||
| ≥3 | 0.00 (–) | 0.35 | 1.00 (–) | 0.85 | |||
| Preoperative anxiety (GAD-7) | 61 | 0.10 (−0.64, 0.85) | 0.79 | 43 | 0.96 (0.85, 1.09) | 0.56 | |
| GAD-7 score (grouped) | Mild | 61 | 0.00 (–) | 0.93 | |||
| Moderate | 1.26 (−9.19, 11.71) | ||||||
| Moderately severe | 3.23 (−6.78, 13.24) | ||||||
| Severe | 1.40 (−20.54, 23.34) | ||||||
| Preoperative depression (PHQ-9) | 61 | −0.25 (−0.82, 0.32) | 0.38 | 43 | 1.05 (0.92, 1.19) | 0.49 | |
| PHQ-9 score (grouped) | Minimal | 61 | 0.00 (–) | 0.87 | |||
| Mild | −4.47 (−15.12, 6.19) | ||||||
| Moderate | −0.95 (−11.34, 9.43) | ||||||
| Moderately severe | −5.70 (−18.41, 7.01) | ||||||
| Severe | −2.46 (−17.59, 12.66) | ||||||
| Preoperative SF-12 PCS | (per 5 units) | 57 | 0.95 (−0.40, 2.30) | 0.17 | 87 | 1.05 (0.82, 1.35) | 0.69 |
| Preoperative SF-12 MCS | (per 5 units) | 57 | −1.26 (−2.92, 0.41) | 0.14 | 87 | 1.31 (0.95, 1.80) | 0.10 |
| Preoperative EQ-5D-5L score | 23 | 18.57 (−1.04, 38.18) | 0.06 | 33 | 0.06 (0.00, 24.53) | 0.35 | |
| Preoperative EQ-5D-5L VAS | (per 5 units) | 59 | 0.69 (−0.09, 1.46) | 0.08 | 89 | 0.98 (0.87, 1.11) | 0.79 |
| Preoperative IWQOL Physical Function score | (per 5 units) | 61 | 0.50 (−0.19, 1.19) | 0.15 | 92 | 1.04 (0.93, 1.15) | 0.54 |
| Preoperative IWQOL Total | (per 5 units) | 61 | 0.63 (−0.16, 1.42) | 0.12 | 92 | 1.03 (0.91, 1.18) | 0.61 |
| Preoperative optimism score | 59 | 0.03 (−0.91, 0.97) | 0.95 | 91 | 0.96 (0.84, 1.09) | 0.49 | |
| Smoking status | Current | 61 | – | – | – | – | |
| Former | 4.59 (−2.95, 12.12) | – | – | ||||
| Never | 0.00 (–) | 0.23 | – | – | – | ||
| Preoperative alcohol use AUDIT | 56 | −0.82 (−2.39, 0.75) | 0.30 | 88 | 1.20 (0.94, 1.53) | 0.13 | |
| Preoperative Physical Activity IPAQ | 93 | −0.64 (−10.77, 9.51) | 28 | 1.00 (1.00, 1.00) | 0.27 | ||
| Surgery type | Gastric band | −6.50 (−12.79, −0.20) | 139 | 0.94 (0.26, 3.37) | |||
| Gastric bypass | 0.00 (–) | 0.11 | 4.33 (1.32, 14.18) | ||||
| SG | 80 | −0.92 (−17.74, 15.90) | 1.00 (–) | 0.04 | |||
| ASA grade | I | 0.00 (–) | 0.97 | 119 | 0.35 (0.03, 4.24) | ||
| II | 0.66 (−5.80, 7.12) | 1.00 (–) | 0.48 | ||||
| III | 92 | 0.00 (–) | 0.79 | 1.54 (0.49, 4.84) | |||
| Health board cost intensity | Low/medium | −0.91 (−7.73, 5.90) | 138 | 1.00 (–) | 0.94 | ||
| High | 56 | −0.82 (−2.39, 0.75) | 0.30 | 1.04 (0.39, 2.78) | |||
| Explanatory variable | Groups | Change in HbA1c from baseline | |
|---|---|---|---|
| Estimate (95% CI) | p-value | ||
| HbA1c (baseline) | −0.59 (−0.92, −0.27) | <0.001 | |
| Age group (years) | <35 | −7.16 (−21.31, 6.98) | 0.33 |
| 35–44 | 8.68 (−2.80, 20.18) | ||
| 45–49 | 0.00 (–) | ||
| 50–54 | 3.25 (−7.07, 13.57) | ||
| 55+ | 5.08 (−5.3, 15.46) | ||
| Sex | Male | 7.83 (−0.44, 16.10) | 0.06 |
| Female | 0.00 (–) | ||
| SIMD | SIMD Q1 (most deprived) | 0.00 (–) | 0.28 |
| SIMD Q2 | −0.45 (−10.81, 9.92) | ||
| SIMD Q3 | 9.82 (−2.45, 22.09) | ||
| SIMD Q4 | 1.06 (−10.91, 13.04) | ||
| SIMD Q5 (least deprived) | −7.25 (−21.34, 6.85) | ||
| BMI group (kg/m2) | <40 | 7.10 (−6.37, 20.57) | 0.18 |
| 40–44 | 5.57 (−4.76, 15.91) | ||
| 45–49 | 0.00 (–) | ||
| 50–54 | −7.66 (−18.68, 3.36) | ||
| 55+ | −1.00 (−12.91, 10.91) | ||
| Preoperative weight change (kg) | −0.05 (−0.57, 0.46) | 0.83 | |
| Surgery type | Gastric band | 2.60 (−9.81, 15.01) | 0.92 |
| Gastric bypass | 0.59 (−8.35, 9.53) | ||
| SG | 0.00 (–) | ||
| ASA grade | I | 0.35 (−18.92, 19.62) | 0.80 |
| II | 0.00 (–) | ||
| III | −2.56 (−10.53, 5.41) | ||
| Health board cost intensity | Low/medium | 0.00 (–) | 0.91 |
| High | −0.57 (−10.60, 9.45) | ||
Discussion
We have described weight, complications, T2DM and patient-reported outcomes for up to 3 years post bariatric surgery, including associations with baseline descriptive statistics, in the SCOTS cohort of bariatric surgery patients from Scotland, UK. Three hundred and fifty-five participants underwent bariatric surgery with a recorded surgery type and 54.3% of them had a diagnosis of T2DM defined by inclusion in the Scottish diabetes electronic health record (SCI Diabetes).
Weight loss outcomes were comparable with international means, with 19% weight loss from the point of operation and 24.2% total weight loss from the start of the weight-management programme. 138 However, there are surgery type differences in weight loss outcomes, with RYGB having significantly higher weight loss of 29.1% from the start of the weight-management programme. Weight-loss failure (defined as <10% from day of operation) was more common by year 3, with 25.8% of the cohort affected. While this was common and expected in those who underwent LAGB, a third of those having had a SG were also affected and the loss in weight after 1 year of 22.3% had attenuated to 16.3% for SG, suggesting significant weight regain. Other than surgery type, no associations between <10% weight loss and baseline variables were found.
Weight regain after SG is well recognised,144 though hard to quantify due to lack of a consensus definition. 145 There is limited evidence on baseline and surgical factors associated with weight regain and no randomised studies of postop interventions to minimise weight regain. 146 A non-randomised study of 71 participants, 43 of whom had SG, compared typical 36-month follow-up (7 appointments) to more intensive follow-up (10 appointments) and found that the intensive follow-up group had greater weight loss at 3 years and fewer had significant weight regain, though this was not defined. 147 Our work found no association between health board programme intensity and the odds of <10% weight loss. It is clear that this is an area that requires further research, especially given the popularity of SG, which was the surgery type of half of our cohort.
Hospitalisations in the 3 years post surgery were high, with 41.5% of the cohort admitted at least once. Despite this high readmission rate, only 5.4% of the cohort had a reoperation or intervention that was considered to be related to a bariatric surgery complication or revision. This compares favourably to a reoperation rate (excluding endoscopy and biliary operations) that was reported recently from a large cohort of 33,560 adults who underwent SG or RYGB across 10 sites in the USA. In that cohort, operated between 2005 and 2015, 10% of the RYGB and 5.2% of the SG cohort had a further abdominal or revisional operation in the 3 years post surgery. 137
While participant-reported physical and obesity-related QoL scores improved in the 3 years after surgery, the lack of improvement in other measures was surprising. The mental component of SF-12 did not change, along with the prevalence of anxiety and depression; this is not what has been seen in other studies conducted in non-UK settings. Loh et al. conducted a recent systematic review and meta-analysis of cohort studies of individuals undergoing bariatric surgery and found a reduction in the prevalence of both anxiety and depression. The reviewed studies utilised the same PHQ-9 and GAD-7 questionnaires that we did; however, the main difference was the baseline prevalence of the conditions; 42.2% of the SCOTS cohort had anxiety and 40.5% depression at baseline compared to an overall mean of 24.5% with anxiety and 34.7% with depression in the reviewed studies. 148 These results fit with our observation in Chapter 4 that the cohort undergoing bariatric surgery in Scotland have higher levels of comorbidity than those having bariatric surgery in other countries and that may be resulting in less comorbidity resolution post surgery.
Type 2 diabetes outcomes have been a major focus of bariatric surgery research, with large reductions in HbA1c and medication requirements reported from high-quality randomised trials. 149 Our study reports a more modest reduction in HbA1c at 3 years (5.7 mmol/mol) than that reported in randomised trials, with the STAMPEDE trial reporting a reduction of 21.8 mmol/mol at 5 years for those having RYGB or SG. 150 Again this reflects underlying differences in the study populations, with the SCOTS cohort having a favourable mean HbA1c at baseline of 53.8 mmol/mol and only 13.6% requiring insulin, leaving limited scope for reduction, though the majority did stop all diabetes medication by year 3. Compared to STAMPEDE, the SCOTS cohort had a far higher BMI. The mean BMI in STAMPEDE was 37 kg/m2 in the RYGB group and 36 kg/m2 in the SG group, compared to 47.1 kg/m2 and 48.6 kg/m2, respectively, for the SCOTS cohort with T2DM.
Perhaps the most notable aspect of our findings on T2DM was not the expected reduction in glycaemia and medication requirements but the lack of follow-up data available for microalbuminuria and retinal screening. Over a quarter of participants had microalbuminuria prior to surgery yet at 3 years only 16% had a urine result reported and 32% had a retinal screening result reported. Data on nephropathy outcomes after bariatric surgery are limited151 and while bariatric surgery is associated with a reduced risk of developing diabetic retinopathy, there may be an initial worsening in the first year post surgery. 152 This lack of standard diabetes follow-up care is not a result of the coronavirus pandemic as the majority of cases pre-date 2020. It is recognised that there is a lack of guidance given to primary care and to patients on post-surgery diabetes care, where ‘diabetes remission’ is considered to have been achieved. Ensuring that healthcare records are appropriately coded so that recall for annual review and screening continues is necessary, as well as ensuring the patients understand the importance of continuing these reviews; it is well recognised that ‘diabetes remission’ is often a temporary state and the diagnostic threshold for T2DM and requirement for medication can recur within a short time, especially in those who have had T2DM for over 5 years prior to surgery. 128,153
Conclusion
Weight-loss outcomes for the SCOTS cohort are in keeping with international results; however, they show that poor weight loss and weight regain is a major problem for those undergoing SG. This is the predominant operation type in the UK and with specialist follow-up only funded for 2 years post surgery there will be many individuals who are not having intervention for this recognised complication of SG.
Improvements in comorbidities, particularly for mental health comorbidities, were not seen, at odds with international evidence. As well as raising concerns as to the selection (and access) to bariatric surgery in Scotland, research should consider how pre- and post-surgery follow-up and support could be improved to address this. Outcomes related to T2DM did improve, with reductions in medication requirements, but there appears to be an urgent issue related to post-surgery diabetes care where key opportunities for preventive screening are not being accessed.
Chapter 7 General discussion
Safety and efficacy of bariatric surgery in the SCOTS cohort
Overall, bariatric surgery appears to be a safe and effective procedure within the SCOTS cohort. Weight-loss outcomes up to 3 years post surgery are comparable to those reported internationally and reoperation rates and mortality are low. 136,137
However, there are differences between the selection and care of patients undergoing bariatric surgery recruited to this cohort (and therefore within Scotland) and those having bariatric surgery in other countries and that may be resulting in the decreased effectiveness, and therefore cost-effectiveness, of bariatric surgery. 154 The older, higher-BMI cohort in SCOTS had poor physical and mental QoL at baseline compared to other reported cohorts. While physical QoL improved 3 years post surgery, the high prevalence of comorbid mental health conditions did not. Those with type T2DM, on average, had fair glycaemic control prior to surgery and the majority stopped all diabetes medications 3 years after surgery. However, they did not appear to be getting full diabetes care with annual review and screening and therefore benefits from improved diabetes management may be negated by poor preventive care.
The immediate post-surgery management for participants in SCOTS showed a longer hospital stay and high HDU/ITU admission rate with no evidence of high complication rates in the form of subsequent operative procedures. This is an issue that does require further investigation and potentially corrective action in Scotland; regardless of not requiring surgical intervention, clearly patients were symptomatic and had life-disruption due to hospital admission. We have speculated that the low volume of bariatric surgery performed in the SCOTS sites may have led to cautious practice, especially as the median ITU/HDU stay was only 1 day. Subsequent readmissions over 3 years were also high, though also with low numbers of operative procedures suggestive of bariatric surgery complications. Potentially these may have been avoided or manageable as an outpatient were a specialist bariatric team available to review urgently. There is a need to understand the causes of this and improve pathways. Potential bariatric surgery patients in Scotland should be made aware of these risks as part of informed consent.
This combination of practice will mean higher costs for bariatric procedures while the decreased effectiveness, possibly due to restricting surgery to those with higher BMI and multiple comorbidities, may have major implications for the cost-effectiveness of bariatric surgery.
Strengths and limitations
The strengths of this study include the recruitment of a cohort from all bariatric surgery centres in Scotland and the high participation rate; we believe our sample is representative of the bariatric population in Scotland from the recruitment period. The collection of a wide variety of PROMs has meant that we can assess multiple outcomes and consider associated baseline variables. Linkage to national electronic health records has allowed the efficient collection of admission, operation, diabetes and mortality outcomes, reducing site and participant burden, though the lack of robust electronic health record data on outpatient and primary care visits may have resulted in some complications not being recorded. Collection of information on pre- and post-surgery pathways and costs have allowed unique analysis on the association of pathway intensity with outcomes.
This study does have a number of limitations. The first is the low numbers recruited and shortened follow-up compared to the original plan to recruit 2000 participants and follow-up for a mean of 10 years. The study recruitment plans had been based on figures supplied by surgeons at each site, based on previous operated numbers. However, while setting up the study, bariatric surgery guidance was standardised by the Scottish government and the number of operations subsequently performed was a fraction of what had been projected when planning SCOTS, including many who never progressed to having bariatric surgery. A widening of criteria for surgery was promised but never delivered by the Scottish government and in 2016 the decision was taken to stop recruitment and to simplify and shorten follow-up to 3 years. The recruited participants were white British in ethnicity, potentially limiting generalisability to other regions.
The second limitation is the low rate of follow-up of the SCOTS questionnaires at 3 years. The study was designed to be low-burden for participants and the completion of the questionnaires was optional. Although we did send reminder letters and e-mails, we did not have permission or resources to contact the participants in other ways or collect information during clinical visits. The need to recruit every bariatric surgery patient due to the overall low numbers in the eligible population meant that participants’ commitment to the longitudinal questionnaires could not be considered. This low rate of baseline data collection and subsequent non-completion will have potentially biased results; it could be expected that those with a positive outcome would have been more likely to respond. It also limited our ability to accurately define the impact of bariatric surgery on the patient-reported outcomes such as QoL.
The third limitation was the application of disclosure rules to health record data despite prospective informed consent; Public Health Scotland did not have a system that allowed them to differentiate information governance between consented prospective research studies and unconsented anonymised observational studies. This is a matter that requires urgent attention given the interest in data-enabled studies and trials in the UK. 155
Future research recommendations
Future research should consider the selection and pathways of care for people undergoing bariatric surgery. There should be consideration of a balance of outcomes and clarity around which non-surgical interventions, if any, should be considered prior to surgery for which groups. Focusing on pathways of care to achieve outcomes such as the greatest response in terms of diabetes outcomes or lowest risk of complications may result in the exclusion of individuals with more severe obesity who may benefit significantly from surgery. Randomised trials of pre- and post-surgery multidisciplinary interventions are required to ascertain the optimal care pathway to support safe and effective surgery.
Standardisation of outcomes in bariatric surgery is key within future research to allow comparisons and meta-analysis. Although SCOTS was designed prior to its publication, we ensured our final analysis plan focused on outcomes from the BARIACT Core Outcome Set for the benefits and adverse events of bariatric and metabolic surgery. 156 Similarly, the American Society for Metabolic and Bariatric Surgery released standardised outcome reporting standards in 2015. 157
Efficient study design is a priority for research funders and researchers, as its saves costs and reduces participant burden. Novel methods for non-randomised evaluation, specifically in surgical innovation, through repeated PROMs delivered through an online platform linked to electronic health records were explored as part of the NIHR-funded PROMiSe study. However, while this study explored statistical methodology and feasibility of electronic record linkage, the patient acceptability testing was conducted with a selected patient involvement group and no work focused on how to encourage completion in a real-world setting. Given the interest in this study design, alongside the need to have representative study cohorts and recruitment as part of routine care, urgent research is need on the best methods for ensuring data completeness from patient-reported outcomes.
Implications for decision-makers
This research has identified variation in bariatric surgical practice in Scotland, both between NHS sites performing surgery and from reported international practice. The older age and higher BMI of those having surgery in Scotland suggests that there may be a far greater need for bariatric surgery than the current provision allows for.
The high rate of hospital readmission up to 3 years after surgery is concerning and will be impacting on QoL and diminishing the cost-effectives of the procedures.
Policy and practice recommendations
The small number of participants with complete data collection has meant that recommendations for policy and practice are limited. However, bariatric surgery provision in Scotland should be re-evaluated, considering the health needs of the Scottish population living with obesity.
Postoperative bariatric surgery care should be investigated to identify possible causes for the high readmission rates. A clear pathway for the management of long-term postop complications of bariatric surgery should be developed and implemented, alongside ongoing audit of readmissions.
Equality, diversity and inclusion
By including all bariatric surgery centres in Scotland, having wide inclusion criteria and encouraging the recruitment of all people having bariatric surgery in Scotland, we have tried to be as inclusive as possible. We did not have funds to translate questionnaires but did have multilevel consent to allow participation even if the questionnaires were not possible due to reading ability, language, burden of disability or caring commitments. The main determinant of diversity in our sample was the selection of patients for bariatric surgery in Scotland. It is recognised that specialist weight management and bariatric surgery are accessed by a majority female, white British population aged 40–55, with areas with higher socioeconomic deprivation over-represented compared to the population living with severe obesity.
Patient and public involvement
This study was funded in 2011 when the NIHR guidance on public and patient involvement (PPI) was less well developed than it is now. We had planned, and therefore had funding available for, PPI at the start of the study and within the steering committee only. A group of six people with lived experience of bariatric surgery worked with us to develop the participant information sheet, corresponding video, website and questionnaires, having been recruited from bariatric surgery peer support groups across Scotland. They used their personal experience and those of their peers to suggest additional topics to study and provided vital expertise as to the phrasing of the questionnaire. They met with the study team twice in person at the start of the study and provided further comments and review by e-mail. They were paid for their time spent working on the study. An additional person with lived experience was an active member of the study steering committee for the course of the whole study. She provided insight, expertise and scrutiny to all aspects of study design, delivery and analysis. She was paid for her time attending meetings and reviewing documents.
Clearly the PPI on SCOTS was minimal compared to current standards but reflects best practice at the time of the award. Given the issues that SCOTS encountered with recruitment and questionnaire follow-up, it is clear where additional contributions from people with lived experience could have been utilised.
Acknowledgements
Contribution of authors
Ruth M Mackenzie (https://orcid.org/0000-0003-2041-5038) (research associate) wrote the first draft of this report and contributed to the acquisition of data from sites and development of the analysis plan.
Abdulmajid Ali (https://orcid.org/0000-0001-9473-4950) (consultant surgeon) provided specialist surgical expertise to the design, delivery and analysis of the study, acted as a site principal investigator and provided critical revisions of the report.
Duff Bruce (https://orcid.org/0000-0002-0838-9896) (consultant surgeon) provided specialist surgical expertise to the design, delivery and analysis of the study, acted as a site principal investigator and provided critical revisions of the report.
Julie Bruce (https://orcid.org/0000-0002-8462-7999) (professor of clinical trials and surgical epidemiology) contributed to the design and analysis plans for the study, particularly the data collection of surgical complications, and provided critical revisions to the report.
Ian Ford (https://orcid.org/0000-0001-5927-1823) (professor of biostatistics) provided senior statistical oversight to the study design, data collection and analysis and provided critical revisions to the overall report.
Nicola Greenlaw (https://orcid.org/0000-0003-3847-1126) (consultant biostatistican) led the development of the statistical analysis plan and conducted the analyses.
Eleanor Grieve (https://orcid.org/0000-0002-4115-2882) (lecturer in health economics) contributed to the design of data collection and designed and conducted the economic analysis and drafted the corresponding section of the report.
Mike Lean (https://orcid.org/0000-0003-2216-0083) (professor of human nutrition) contributed to the design of the study and analysis plans and provided and provided critical revisions to the report.
Robert Lindsay (https://orcid.org/0000-0002-9868-5217) (reader in diabetes medicine) contributed to the design of the study and analysis plans, particularly in relation to diabetes outcomes, and provided critical revisions to the report.
Joanne O’Donnell (https://orcid.org/0000-0002-1433-4226) (project research assistant) contributed to the project approvals, acquisition of data, quality assurance and site management and provided critical revisions to the report.
Naveed Sattar (https://orcid.org/0000-0002-1604-2593) (professor of metabolic medicine) contributed to the design of the study and analysis plans, acting and chief investigator when Jennifer Logue was on maternity leave, contributed to the design of the study and analysis plans, and provided critical revisions to the report.
Sally Stewart (https://orcid.org/0000-0002-9014-8601) (programme manager) led the design and set-up of the study, drafted the methods chapter of the report and provided critical revisions to the overall report.
Jennifer Logue (https://orcid.org/0000-0001-9549-2738) (professor of metabolic medicine) conceived the idea, designed the study and was chief investigator with overall responsibility for the design and delivery of the study and report.
The full list of SCOTS investigators at each site is listed in Report Supplementary Material 1.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data sharing
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Ethics
This research was conducted in accordance with the World Medical Association Declaration of Helsinki. A favourable ethical opinion for the study was obtained from the West of Scotland Research Ethics Committee 4 on 7 February 2013 (13/WS/0005).
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- World Health Organisation . BMI Classification. Global Database on Body Mass Index 2014.
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284-91. https://doi.org/10.1001/jama.2016.6458.
- Wolfe BM, Kvach E, Eckel RH. Treatment of obesity. Circ Res 2016;118:1844-55. https://doi.org/10.1161/CIRCRESAHA.116.307591.
- Welbourn R, Le Roux CW, Owen-Smith A, Wordsworth S, Blazeby JM. Why the NHS should do more bariatric surgery; how much should we do?. BMJ 2016;353. https://doi.org/10.1136/bmj.i1472.
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 2020;1.
- Baker C. House of commons library | obesity statistics 2021.
- Government S . Scottish Health Survery 2018: Main Report – Revised 2020 2020.
- Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev 2014;2014. https://doi.org/10.1002/14651858.CD003641.pub4.
- Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013;347. https://doi.org/10.1136/bmj.f5934.
- Naik RD, Choksi YA, Vaezi MF. Impact of weight loss surgery on esophageal physiology. Gastroenterol Hepatol 2015;11:801-9.
- Lo Menzo E, Szomstein S, Rosenthal RJ. Changing trends in bariatric surgery. Scand J Surg 2015;104:18-23. https://doi.org/10.1177/1457496914552344.
- Miller K. Obesity: surgical options. Best Pract Res Clin Gastroenterol 2004;18:1147-65. https://doi.org/10.1016/j.bpg.2004.06.003.
- Meek CL, Lewis HB, Reimann F, Gribble FM, Park AJ. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides 2016;77:28-37. https://doi.org/10.1016/j.peptides.2015.08.013.
- Langer FB, Reza Hoda MA, Bohdjalian A, Felberbauer FX, Zacherl J, Wenzl E, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg 2005;15:1024-9. https://doi.org/10.1381/0960892054621125.
- O’Brien PE, Hindle A, Brennan L, Skinner S, Burton P, Smith A, et al. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg 2019;29:3-14. https://doi.org/10.1007/s11695-018-3525-0.
- Borisenko O, Mann O, Duprée A. Cost-utility analysis of bariatric surgery compared with conventional medical management in Germany: a decision analytic modeling. BMC Surg 2017;17. https://doi.org/10.1186/s12893-017-0284-0.
- Keating C, Neovius M, Sjöholm K, Peltonen M, Narbro K, Eriksson JK, et al. Health-care costs over 15 years after bariatric surgery for patients with different baseline glucose status: results from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol 2015;3:855-65. https://doi.org/10.1016/S2213-8587(15)00290-9.
- Arterburn DE, Olsen MK, Smith VA, Livingston EH, Van Scoyoc L, Yancy WS, et al. Association between bariatric surgery and long-term survival. JAMA 2015;313:62-70. https://doi.org/10.1001/jama.2014.16968.
- Perry CD, Hutter MM, Smith DB, Newhouse JP, McNeil BJ. Survival and changes in comorbidities after bariatric surgery. Ann Surg 2008;247:21-7. https://doi.org/10.1097/SLA.0b013e318142cb4b.
- Moussa OM, Erridge S, Chidambaram S, Ziprin P, Darzi A, Purkayastha S. Mortality of the severely obese: a population study. Ann Surg 2019;269:1087-91. https://doi.org/10.1097/SLA.0000000000002730.
- Pontiroli AE, Zakaria AS, Fanchini M, Osio C, Tagliabue E, Micheletto G, et al. A 23-year study of mortality and development of co-morbidities in patients with obesity undergoing bariatric surgery (laparoscopic gastric banding) in comparison with medical treatment of obesity. Cardiovasc Diabetol 2018;17. https://doi.org/10.1186/s12933-018-0801-1.
- Singh P, Subramanian A, Adderley N, Gokhale K, Singhal R, Bellary S, et al. Impact of bariatric surgery on cardiovascular outcomes and mortality: a population-based cohort study. Br J Surg 2020;107:432-42. https://doi.org/10.1002/bjs.11433.
- Reges O, Greenland P, Dicker D, Leibowitz M, Hoshen M, Gofer I, et al. Association of bariatric surgery using laparoscopic banding, Roux-en-Y gastric bypass, or laparoscopic sleeve gastrectomy vs usual care obesity management with all-cause mortality. JAMA 2018;319:279-90. https://doi.org/10.1001/jama.2017.20513.
- Ceriani V, Sarro G, Micheletto G, Giovanelli A, Zakaria AS, Fanchini M, et al. Long-term mortality in obese subjects undergoing malabsorptive surgery (biliopancreatic diversion and biliointestinal bypass) versus medical treatment. Int J Obes 2019;43:1147-53. https://doi.org/10.1038/s41366-018-0244-5.
- Douglas IJ, Bhaskaran K, Batterham RL, Smeeth L. Bariatric surgery in the United Kingdom: a cohort study of weight loss and clinical outcomes in routine clinical care. PLOS Med 2015;12. https://doi.org/10.1371/journal.pmed.1001925.
- Eliasson B, Liakopoulos V, Franzén S, Näslund I, Svensson AM, Ottosson J, et al. Cardiovascular disease and mortality in patients with type 2 diabetes after bariatric surgery in Sweden: a nationwide, matched, observational cohort study. Lancet Diabetes Endocrinol 2015;3:847-54. https://doi.org/10.1016/S2213-8587(15)00334-4.
- Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg 2004;199:543-51. https://doi.org/10.1016/j.jamcollsurg.2004.06.014.
- Kauppila JH, Tao W, Santoni G, von Euler-Chelpin M, Lynge E, Tryggvadóttir L, et al. Effects of obesity surgery on overall and disease-specific mortality in a 5-country population-based study. Gastroenterology 2019;157:119-27.e1. https://doi.org/10.1053/j.gastro.2019.03.048.
- Wiggins T, Guidozzi N, Welbourn R, Ahmed AR, Markar SR. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and meta-analysis. PLOS Med 2020;17. https://doi.org/10.1371/journal.pmed.1003206.
- Chang SH, Freeman NLB, Lee JA, Stoll CRT, Calhoun AJ, Eagon JC, et al. Early major complications after bariatric surgery in the USA, 2003–2014: a systematic review and meta-analysis. Obes Rev 2018;19:529-37. https://doi.org/10.1111/obr.12647.
- Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev 2014;2014. https://doi.org/10.1002/14651858.CD003641.pub4.
- McGlone ER, Carey I, Velickovic V, Chana P, Mahawar K, Batterham RL, et al. Bariatric surgery for patients with type 2 diabetes mellitus requiring insulin: clinical outcome and cost-effectiveness analyses. PLOS Med 2020;17. https://doi.org/10.1371/journal.pmed.1003228.
- Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577-85. https://doi.org/10.1056/NEJMoa1200111.
- Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes – 5-year outcomes. N Engl J Med 2017;376:641-51. https://doi.org/10.1056/NEJMoa1600869.
- Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med 2013;273:219-34. https://doi.org/10.1111/joim.12012.
- Gulliford MC, Booth HP, Reddy M, Charlton J, Fildes A, Prevost AT, et al. Effect of contemporary bariatric surgical procedures on type 2 diabetes remission. A population-based matched cohort study. Obes Surg 2016;26:2308-15. https://doi.org/10.1007/s11695-016-2103-6.
- Billeter AT, Eichel S, Scheurlen KM, Probst P, Kopf S, Müller-Stich BP. Meta-analysis of metabolic surgery versus medical treatment for macrovascular complications and mortality in patients with type 2 diabetes. Surg Obes Relat Dis 2019;15:1197-210. https://doi.org/10.1016/j.soard.2019.04.029.
- Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753-61. https://doi.org/10.1056/nejmoa066603.
- Sjöström L, Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden A, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297-304. https://doi.org/10.1001/jama.2014.5988.
- Billeter AT, Scheurlen KM, Probst P, Eichel S, Nickel F, Kopf S, et al. Meta-analysis of metabolic surgery versus medical treatment for microvascular complications in patients with type 2 diabetes mellitus. Br J Surg 2018;105:168-81. https://doi.org/10.1002/bjs.10724.
- Batterham RL, Cummings DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care 2016;39:893-901. https://doi.org/10.2337/dc16-0145.
- Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339-50. https://doi.org/10.1097/00000658-199509000-00011.
- Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PR, et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA 2019;322:1271-82. https://doi.org/10.1001/jama.2019.14231.
- Doumouras AG, Wong JA, Paterson JM, Lee Y, Sivapathasundaram B, Tarride JE, et al. Bariatric surgery and cardiovascular outcomes in patients with obesity and cardiovascular disease: a population-based retrospective cohort study. Circulation 2021;143:1468-80. https://doi.org/10.1161/CIRCULATIONAHA.120.052386.
- Mathus-Vliegen EMH, De Wit LT. Health-related quality of life after gastric banding. Br J Surg 2007;94:457-65. https://doi.org/10.1002/bjs.5607.
- Raaijmakers LCH, Pouwels S, Thomassen SEM, Nienhuijs SW. Quality of life and bariatric surgery: a systematic review of short- and long-term results and comparison with community norms. Eur J Clin Nutr 2017;71:441-9. https://doi.org/10.1038/ejcn.2016.198.
- Flølo TN, Tell GS, Kolotkin RL, Aasprang A, Norekvål TM, Våge V, et al. Changes in quality of life 5 years after sleeve gastrectomy: a prospective cohort study. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-031170.
- Poelemeijer YQM, van der Knaap ETW, Marang-van de Mheen PJ, Demirkiran A, Wiezer MJ, Hazebroek EJ, et al. Measuring quality of life in bariatric surgery: a multicentre study. Surg Endosc 2020;34:5522-32. https://doi.org/10.1007/s00464-019-07350-4.
- Karlsson J, Taft C, Rydén A, Sjöström L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes 2007;31:1248-61. https://doi.org/10.1038/sj.ijo.0803573.
- Gill H, Kang S, Lee Y, Rosenblat JD, Brietzke E, Zuckerman H, et al. The long-term effect of bariatric surgery on depression and anxiety. J Affect Disord 2019;246:886-94. https://doi.org/10.1016/j.jad.2018.12.113.
- Department of Health and Social Care . Tackling Obesity: Empowering Adults and Children to Live Healthier Lives 2020.
- Public Health England . Health Matters: Obesity and the Food Environment 2017.
- Stegenga H, Haines A, Jones K, Professor JW. Identification, assessment, and management of overweight and obesity: summary of updated NICE guidance. BMJ 2014;349. https://doi.org/10.1136/bmj.g6608.
- Klein S, Ghosh A, Cremieux PY, Eapen S, McGavock TJ. Economic impact of the clinical benefits of bariatric surgery in diabetes patients with BMI ≥35 kg/m2. Obesity 2011;19:581-7. https://doi.org/10.1038/oby.2010.199.
- Avenell A, Robertson C, Skea Z, Jacobsen E, Boyers D, Cooper D, et al. Bariatric surgery, lifestyle interventions and orlistat for severe obesity: the rebalance mixed-methods systematic review and economic evaluation. Health Technol Assess 2018;22:1-46. https://doi.org/10.3310/hta22680.
- Karim MA, Ahmed J, Arneil C, Ali A. Utilization of hospital services by obese patients before and after bariatric surgery. Surg Today 2013;43:1129-33. https://doi.org/10.1007/s00595-013-0540-6.
- Karim MA, Clifton E, Ahmed J, Mackay GW, Ali A. Economic evaluation of bariatric surgery to combat morbid obesity: a study from the West of Scotland. Asian J Endosc Surg 2013;6:197-202. https://doi.org/10.1111/ases.12042.
- Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339-50. https://doi.org/10.1097/00000658-199509000-00011.
- Dixon JB, Pories WJ, O’Brien PE, Schauer PR, Zimmet P. Surgery as an effective early intervention for diabesity: why the reluctance?. Diabetes Care 2005;28:472-4. https://doi.org/10.2337/diacare.28.2.472.
- Levy P, Fried M, Santini F, Finer N. The comparative effects of bariatric surgery on weight and type 2 diabetes. Obes Surg 2007;17:1248-56. https://doi.org/10.1007/s11695-007-9214-z.
- Hawkins SC, Osborne A, Finlay IG, Alagaratnam S, Edmond JR, Welbourn R. Paid work increases and state benefit claims decrease after bariatric surgery. Obes Surg 2007;17:434-7. https://doi.org/10.1007/s11695-007-9073-7.
- Marsh K, Dolan P, Kempster J, Lugon M. Prioritizing investments in public health: a multi-criteria decision analysis. J Public Heal (United Kingdom) 2013;35:460-6. https://doi.org/10.1093/pubmed/fds099.
- National Institute for Health and Care Excellence . Weight Management: Lifestyle Services for Overweight or Obese Adults (PH53) 2014.
- Scottish Intercollegiate Guidelines Network (SIGN) . Management of Obesity. Edinburgh SIGN 2014.
- Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg 2015;25:1822-32. https://doi.org/10.1007/s11695-015-1657-z.
- Hedenbro JL, Näslund E, Boman L, Lundegårdh G, Bylund A, Ekelund M, et al. Formation of the Scandinavian Obesity Surgery Registry, SOReg. Obes Surg 2015;25:1893-900. https://doi.org/10.1007/s11695-015-1619-5.
- Grieve E, Mackenzie RM, Munro J, O’Donnell J, Stewart S, Ali A, et al. Variations in bariatric surgical care pathways: a national costing study on the variability of services and impact on costs. BMC Obes 2018;5. https://doi.org/10.1186/s40608-018-0223-3.
- Booth HP, Khan O, Fildes A, Prevost AT, Reddy M, Charlton J, et al. Changing epidemiology of bariatric surgery in the UK: cohort study using primary care electronic health records. Obes Surg 2016;26:1900-5. https://doi.org/10.1007/s11695-015-2032-9.
- Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, Kim J, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures – 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology. Surg Obes Relat Dis 2020;16:175-247. https://doi.org/10.1016/j.soard.2019.10.025.
- Busetto L, Dicker D, Azran C, Batterham RL, Farpour-Lambert N, Fried M, et al. Practical recommendations of the Obesity Management Task Force of the European Association for the Study of Obesity for the post-bariatric surgery medical management. Obes Facts 2017;10:597-632. https://doi.org/10.1159/000481825.
- Logue J, Stewart S, Munro J, Bruce J, Grieve E, Lean M, et al. SurgiCal obesity treatment study (SCOTS): protocol for a national prospective cohort study of patients undergoing bariatric surgery in Scotland. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-008106.
- Mackenzie RM, Greenlaw N, Ali A, Bruce D, Bruce J, Grieve E, et al. SCOTS Investigators . SurgiCal Obesity Treatment Study (SCOTS): a prospective, observational cohort study on health and socioeconomic burden in treatment-seeking individuals with severe obesity in Scotland, UK. BMJ Open 2021;11. https://doi.org/10.1136/BMJOPEN-2020-046441.
- Macran S, Wileman S, Barton G, Russell I. REFLUX trial group . The development of a new measure of quality of life in the management of gastro-oesophageal reflux disease: the Reflux questionnaire. Qual Life Res 2007;16:331-43. https://doi.org/10.1007/s11136-006-9005-3.
- Barry MJ, Fowler FJ, O’leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association Symptom Index for benign prostatic hyperplasia. J Urol 2017;197:S189-97. https://doi.org/10.1016/j.juro.2016.10.071.
- Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ . A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 2004;23:322-30. https://doi.org/10.1002/nau.20041.
- Gosman GG, King WC, Schrope B, Steffen KJ, Strain GW, Courcoulas AP, et al. Reproductive health of women electing bariatric surgery. Fertil Steril 2010;94:1426-31. https://doi.org/10.1016/j.fertnstert.2009.08.028.
- Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54-61. https://doi.org/10.1016/S0022-5347(17)34871-1.
- Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. https://doi.org/10.1001/archinte.166.10.1092.
- Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD: the PHQ Primary Care Study. Primary care evaluation of mental disorders. Patient Health Questionnaire. JAMA 1999;282:1737-44. https://doi.org/10.1001/jama.282.18.1737.
- Reinert DF, Allen JP. The alcohol use disorders identification test (AUDIT): a review of recent research. Alcohol Clin Exp Res 2002;26:272-9. https://doi.org/10.1111/j.1530-0277.2002.tb02534.x.
- Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). QualLife Res 2011;20:1727-36.
- Fermont JM, Blazeby JM, Rogers CA, Wordsworth S. The EQ-5D-5L is a valid approach to measure health related quality of life in patients undergoing bariatric surgery. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0189190.
- Kolotkin RL, Crosby RD. Psychometric evaluation of the impact of weight on quality of life-lite questionnaire (IWQOL-Lite) in a community sample. Qual Life Res 2002;11:157-71. https://doi.org/10.1023/A:1015081805439.
- Kolotkin R, Crosby R. The Impact of Weight on Quality of Life-Lite (IWQOL-Lite): User’s Manual. Durham, NC: Obesity and Quality of Life Consulting; 2002.
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol 1994;67:1063-78. https://doi.org/10.1037/0022-3514.67.6.1063.
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381-95. https://doi.org/10.1249/01.MSS.0000078924.61453.FB.
- Ertelt TW, Marino JM, Mitchell JE. Body Contouring. Berlin: Springer; 2010.
- Scottish Index of Multiple Deprivation 2016: Introductory Booklet 2016.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2015 2015.
- Edinburgh: Information Service Division . Scottish National Tariff 2013 2013.
- Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753-61.
- Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741-52.
- Scottish Executive Health Department . Research Government Framework for Health and Community Care 2006.
- Read S, Logue J. Variations in weight management services in Scotland: a national survey of weight management provision. J Public Health (Oxf) 2016;38:e325-35. https://doi.org/10.1093/pubmed/fdv132.
- Schneider A, Hutcheon DA, Hale A, Ewing JA, Miller M, Scott JD. Postoperative outcomes in bariatric surgical patients participating in an insurance-mandated preoperative weight management program. Surg Obes Relat Dis 2018;14:623-30. https://doi.org/10.1016/j.soard.2018.01.036.
- Keith C, Goss L, Blackledge C, Stahl R, Grams J. Insurance-mandated pre-operative diet and outcomes following bariatric surgery. Surg Obes Relat Dis 2016;12:S31-32. https://doi.org/10.1016/j.soard.2016.08.075.
- Houlden RL, Yen JL, Moore S. Effectiveness of an interprofessional glycemic optimization clinic on preoperative glycated hemoglobin levels for adult patients with type 2 diabetes undergoing bariatric surgery. Can J Diabetes 2018;42:514-9. https://doi.org/10.1016/j.jcjd.2017.12.011.
- Kalarchian MA, Marcus MD, Courcoulas AP, Cheng Y, Levine MD. Preoperative lifestyle intervention in bariatric surgery: initial results from a randomized, controlled trial. Obesity 2013;21:254-60. https://doi.org/10.1002/oby.20069.
- Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, et al. Does weight loss immediately before bariatric surgery improve outcomes: a systematic review. Surg Obes Relat Dis 2009;5:713-21. https://doi.org/10.1016/j.soard.2009.08.014.
- Gerber P, Anderin C, Thorell A. Weight loss prior to bariatric surgery: an updated review of the literature. Scand J Surg 2015;104:33-9. https://doi.org/10.1177/1457496914553149.
- Ogden J, Hollywood A, Pring C. The impact of psychological support on weight loss post weight loss surgery: a randomised control trial. Obes Surg 2015;25:500-5. https://doi.org/10.1007/s11695-014-1428-2.
- Campbell JA, Venn A, Neil A, Hensher M, Sharman M, Palmer AJ. Diverse approaches to the health economic evaluation of bariatric surgery: a comprehensive systematic review. Obes Rev 2016;17:850-94. https://doi.org/10.1111/obr.12424.
- Gulliford MC, Charlton J, Booth HP, Fildes A, Khan O, Reddy M, et al. Costs and outcomes of increasing access to bariatric surgery for obesity: cohort study and cost-effectiveness analysis using electronic health records. Val Health 2017;20:85-92. https://doi.org/10.1016/j.jval.2016.08.734.
- Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess (Rockv) 2009;13:1-190. https://doi.org/10.3310/hta13410.
- Craig BM, Tseng DS. Cost-effectiveness of gastric bypass for severe obesity. Am J Med 2002;113:491-8. https://doi.org/10.1016/S0002-9343(02)01266-4.
- Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ 2014;349. https://doi.org/10.1136/bmj.g3961.
- Williamson K, Nimegeer A, Lean M. Rising prevalence of BMI ≥40 kg/m2: a high-demand epidemic needing better documentation. Obes Rev 2020;21. https://doi.org/10.1111/obr.12986.
- le Roux CW, Chubb B, Nørtoft E, Borglykke A. Obesity and healthcare resource utilization: results from Clinical Practice Research Database (CPRD). Obes Sci Pract 2018;4:409-16. https://doi.org/10.1002/osp4.291.
- Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, de Gonzalez AB, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016;388:776-86. https://doi.org/10.1016/S0140-6736(16)30175-1.
- Grieve E, Fenwick E, Yang HC, Lean M. The disproportionate economic burden associated with severe and complicated obesity: a systematic review. Obes Rev 2013;14:883-94. https://doi.org/10.1111/obr.12059.
- Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health-related quality of life. Clin Obes 2017;7:273-89. https://doi.org/10.1111/cob.12203.
- Ul-Haq Z, Mackay DF, Fenwick E, Pell JP. Meta-analysis of the association between body mass index and health-related quality of life among adults, assessed by the SF-36. Obesity (Silver Spring) 2013;21:E322-7. https://doi.org/10.1002/oby.20107.
- Van Nunen AMA, Wouters EJM, Vingerhoets AJJM, Hox JJ, Geenen R. The health-related quality of life of obese persons seeking or not seeking surgical or non-surgical treatment: a meta-analysis. Obes Surg 2007;17:1357-66. https://doi.org/10.1007/s11695-007-9241-9.
- Radbruch L, Sabatowski R, Loick G, Jonen-Thielemann I, Kasper M, Gondek B, et al. Cognitive impairment and its influence on pain and symptom assessment in a palliative care unit: development of a minimal documentation system. Palliat Med 2000;14:266-76. https://doi.org/10.1191/026921600672986600.
- Brunner-La Rocca HP, Rickenbacher P, Muzzarelli S, Schindler R, Maeder MT, Jeker U, et al. End-of-life preferences of elderly patients with chronic heart failure. Eur Heart J 2012;33:752-9. https://doi.org/10.1093/eurheartj/ehr404.
- Iyasere O, Brown EA, Johansson L, Davenport A, Farrington K, Maxwell AP, et al. Quality of life with conservative care compared with assisted peritoneal dialysis and haemodialysis. Clin Kidney J 2018;12:262-8. https://doi.org/10.1093/ckj/sfy059.
- Voll-Aanerud M, Eagan TML, Wentzel-Larsen T, Gulsvik A, Bakke PS. Respiratory symptoms, COPD severity, and health related quality of life in a general population sample. Respir Med 2008;102:399-406. https://doi.org/10.1016/j.rmed.2007.10.012.
- Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, Ottosson J, et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the Fourth IFSO Global Registry Report 2018. Obes Surg 2019;29:782-95. https://doi.org/10.1007/s11695-018-3593-1.
- Alsumali A, Eguale T, Bairdain S, Samnaliev M. Cost-effectiveness analysis of bariatric surgery for morbid obesity. Obes Surg 2018;28:2203-14. https://doi.org/10.1007/s11695-017-3100-0.
- Maciejewski ML, Arterburn DE. Cost-effectiveness of bariatric surgery. JAMA 2013;310:742-3. https://doi.org/10.1001/jama.2013.276131.
- Hoerger TJ, Zhang P, Segel JE, Kahn HS, Barker LE, Couper S. Cost-effectiveness of bariatric surgery for severely obese adults with diabetes. Diabetes Care 2010;33:1933-9. https://doi.org/10.2337/dc10-0554.
- Khan MA, Grinberg R, Johnson S, Afthinos JN, Gibbs KE. Perioperative risk factors for 30-day mortality after bariatric surgery: is functional status important?. Surg Endosc 2013;27:1772-7. https://dx.doi.org/10.1007/s00464-012-2678-5.
- Quilliot D, Sirveaux MA, Nomine-Criqui C, Fouquet T, Reibel N, Brunaud L. Evaluation of risk factors for complications after bariatric surgery. J Visc Surg 2018;155:201-10. https://doi.org/10.1016/j.jviscsurg.2018.01.004.
- Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, et al. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg 2012;22:70-89. https://doi.org/10.1007/s11695-011-0472-4.
- Nickel F, De La Garza JR, Werthmann FS, Benner L, Tapking C, Karadza E, et al. Predictors of risk and success of obesity surgery. Obes Facts 2019;12:427-39. https://doi.org/10.1159/000496939.
- Parri A, Benaiges D, Schröder H, Izquierdo-Pulido M, Ramón J, Villatoro M, et al. Preoperative predictors of weight loss at 4 years following bariatric surgery. Nutr Clin Pract 2015;30:420-4. https://dx.doi.org/10.1177/0884533614568154.
- Jans A, Näslund I, Ottosson J, Szabo E, Näslund E, Stenberg E. Duration of type 2 diabetes and remission rates after bariatric surgery in Sweden 2007-2015: a registry-based cohort study. PLOS Med 2019;16. https://doi.org/10.1371/journal.pmed.1002985.
- Malone M, Alger-Mayer S, Polimeni JM. Health related quality of life after gastric bypass surgery. Appl Res Qual Life 2012;7:155-61. https://doi.org/10.1007/s11482-011-9157-3.
- Pristed SG, Omar HK, Kroustrup JP. Association between fulfilment of expectations and health-related quality of life after gastric bypass. Appl Res Qual Life 2013;8:101-11. https://doi.org/10.1007/s11482-012-9175-9.
- Julia C, Ciangura C, Capuron L, Bouillot JL, Basdevant A, Poitou C, et al. Quality of life after Roux-en-Y gastric bypass and changes in body mass index and obesity-related comorbidities. Diabetes Metab 2013;39:148-54. https://doi.org/10.1016/j.diabet.2012.10.008.
- Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr 2002;5:561-5. https://doi.org/10.1079/phn2001322.
- Eng V, Garcia L, Khoury H, Morton J, Azagury D. Preoperative weight loss: is waiting longer before bariatric surgery more effective?. Surg Obes Relat Dis 2019;15:951-7. https://doi.org/10.1016/j.soard.2019.03.012.
- Esquivel MM, Azagury D. Preoperative Weight Loss Before Bariatric Surgery-The Debate Continues. JAMA Netw Open 2020;3. https://doi.org/10.1001/jamanetworkopen.2020.4994.
- Brazil J, Finucane F. Structured lifestyle modification prior to bariatric surgery: how much is enough?. Obes Surg 2021;31:4585-91. https://doi.org/10.1007/s11695-021-05573-w.
- Brown WA, Kow L, Shikora S, Liem R, Welbourn R, Dixon J, et al. Sixth IFSO Global Registry Report 2021 2021.
- Courcoulas A, Coley RY, Clark JM, McBride CL, Cirelli E, McTigue K, et al. PCORnet Bariatric Study Collaborative . Interventions and operations 5 years after bariatric surgery in a cohort from the US National Patient-Centered Clinical Research Network Bariatric Study. JAMA Surg 2020;155:194-20. https://doi.org/10.1001/JAMASURG.2019.5470.
- O’Brien PE, Hindle A, Brennan L, Skinner S, Burton P, Smith A, et al. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg 2019;29:3-14. https://doi.org/10.1007/S11695-018-3525-0.
- Kourounis G, Kong CY, Logue J, Gibson S. Weight loss in adults following bariatric surgery, a systematic review of preoperative behavioural predictors. Clin Obes 2020;10. https://doi.org/10.1111/COB.12392.
- Tewksbury C, Williams NN, Dumon KR, Sarwer DB. Preoperative medical weight management in bariatric surgery: a review and reconsideration. Obes Surg 2017;27:208-14. https://doi.org/10.1007/S11695-016-2422-7.
- Horwitz D, Saunders JK, Ude-Welcome A, Parikh M. Insurance-mandated medical weight management before bariatric surgery. Surg Obes Relat Dis 2016;12:496-9. https://doi.org/10.1016/j.soard.2015.09.004.
- Talishinskiy T, Blatt M, Nyirenda T, Eid S, Schmidt H, Ewing D. Insurance-mandated medical weight management programs in sleeve gastrectomy patients do not improve postoperative weight loss outcomes at 1 year. Obes Surg 2020;30:3333-40. https://doi.org/10.1007/s11695-020-04692-0.
- Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: a review. JAMA 2020;324:879-87. https://doi.org/10.1001/jama.2020.12567.
- Lauti M, Kularatna M, Hill AG, MacCormick AD. Weight regain following sleeve gastrectomy – a systematic review. Obes Surg 2016;26:1326-34. https://doi.org/10.1007/S11695-016-2152-X.
- Lauti M, Lemanu D, Zeng ISL, Su’a B, Hill AG, MacCormick AD. Definition determines weight regain outcomes after sleeve gastrectomy. Surg Obes Relat Dis 2017;13:1123-9. https://doi.org/10.1016/J.SOARD.2017.02.029.
- Yu Y, Klem ML, Kalarchian MA, Ji M, Burke LE. Predictors of weight regain after sleeve gastrectomy: an integrative review. Surg Obes Relat Dis 2019;15:995-1005. https://doi.org/10.1016/J.SOARD.2019.02.009.
- Lombardo M, Bellia A, Mattiuzzo F, Franchi A, Ferri C, Padua E, et al. Frequent follow-up visits reduce weight regain in long-term management after bariatric surgery. Bariatr Surg Pract Patient Care 2015;10:119-25. https://doi.org/10.1089/BARI.2015.0021.
- Loh HH, Francis B, Lim LL, Lim QH, Yee A, Loh HS. Improvement in mood symptoms after post-bariatric surgery among people with obesity: a systematic review and meta-analysis. Diabetes Metab Res Rev 2021;37. https://doi.org/10.1002/DMRR.3458.
- Borgeraas H, Hofsø D, Hertel JK, Hjelmesæth J. Comparison of the effect of Roux-en-Y gastric bypass and sleeve gastrectomy on remission of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Obes Rev 2020;21. https://doi.org/10.1111/OBR.13011.
- Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567-76. https://doi.org/10.1056/NEJMoa1200225.
- Lee Y, Anvari S, Chu MM, Lovrics O, Khondker A, Malhan R, et al. Improvement of kidney function in patients with chronic kidney disease and severe obesity after bariatric surgery: a systematic review and meta-analysis. Nephrology (Carlton) 2022;27:44-56. https://doi.org/10.1111/NEP.13958.
- Yu CW, Park LJ, Pinto A, Ma ON, Lee Y, Gupta R, et al. The impact of bariatric surgery on diabetic retinopathy: a systematic review and meta-analysis. Am J Ophthalmol 2021;225:117-27. https://doi.org/10.1016/J.AJO.2020.12.033.
- McCombie L, Leslie W, Taylor R, Kennon B, Sattar N, Lean MEJ. Beating type 2 diabetes into remission. BMJ 2017;358. https://doi.org/10.1136/BMJ.J4030.
- Hachem A, Brennan L. Quality of life outcomes of bariatric surgery: a systematic review. Obes Surg 2016;26:395-409. https://doi.org/10.1007/S11695-015-1940-Z.
- Sydes MR, Barbachano Y, Bowman L, Denwood T, Farmer A, Garfield-Birkbeck S, et al. Data Enabled Trials Group Workshop Group members . Realising the full potential of data-enabled trials in the UK: a call for action. BMJ Open 2021;11. https://doi.org/10.1136/BMJOPEN-2020-043906.
- Coulman KD, Hopkins J, Brookes ST, Chalmers K, Main B, Owen-Smith A, et al. A Core outcome set for the benefits and adverse events of bariatric and metabolic surgery: the BARIACT project. PLOS Med 2016;13. https://doi.org/10.1371/JOURNAL.PMED.1002187.
- Brethauer SA, Kim J, El Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis 2015;11:489-506. https://doi.org/10.1016/j.soard.2015.02.003.
List of abbreviations
- ASA
- American Society of Anesthesiologists
- AUDIT
- Alcohol Use Disorders Identification Test
- BMI
- body mass index
- CI
- confidence interval
- CVD
- cardiovascular disease
- DLA
- disability living allowance
- eCRF
- electronic case report form
- EQ-5D-5L
- EuroQoL 5-level EQ-5D
- EWL
- excess weight loss
- GAD-7
- Generalised Anxiety Disorder Assessment
- GI
- gastrointestinal
- GLP-1
- glucagon-like peptide-1
- GP
- general practitioner
- HbA1c
- glycated haemoglobin
- HDU
- high-dependency unit
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICIQ-UI SF
- Incontinence Questionnaire-Urinary Incontinence Short Form
- IPAQ
- International Physical Activity Questionnaire
- IPSS
- International Prostate Symptom Score
- IRR
- incidence rate ratio
- ITU
- intensive-therapy unit
- IWQOL-Lite
- Impact of Weight on Quality of Life-Lite
- LAGB
- laparoscopic adjustable gastric banding
- LOT
- Life Orientation Test
- MDT
- multidisciplinary team
- MET
- metabolic equivalents of tasks
- MI
- myocardial infarction
- NAFLD
- non-alcoholic fatty liver disease
- NHS
- National Health Service
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OPCS-4
- OPCS Classification of Interventions and Procedures version 4
- O-QoL
- obesity-specific quality of life
- OR
- odds ratio
- PBPP
- Public Benefit and Privacy Panel for Health and Social Care
- PHQ-9
- Patient Health Questionnaire
- postop
- postoperative
- preop
- preoperative
- PPI
- public and patient involvement
- PROM
- patient-reported outcome measure
- QoL
- quality of life
- RCT
- randomised controlled trial
- RYGB
- Roux-en-Y gastric bypass
- SA
- sensitivity analysis
- SCI Diabetes
- Scottish Care Information – Diabetes
- SCOTS
- Surgical Obesity Treatment Study
- SF-12
- Rand 12-item Short Form Survey
- SF-12 MCS
- SF-12 Mental Component Summary
- SF-12 PCS
- SF-12 Physical Component Summary
- SF-36
- Rand 36-item Short Form Health Survey
- SG
- Sleeve gastrectomy
- SGLT2
- Sodium-glucose cotransporter-2
- SIGN
- Scottish Intercollegiate Guidelines Network
- SIMD
- Scottish Index of Multiple Deprivation
- SMR01
- Scottish Morbidity Record 01
- T2DM
- type 2 diabetes mellitus
- UK
- United Kingdom
- USA
- United States of America
Notes
-
List of SCOTS investigators
-
Preoperative assessment and postoperative care pathway questionnaire
-
Significant interactions with smoking as a two-level variable
-
Association of age and body mass index with self-reported comorbidities and mental health indicators at baseline
-
Baseline characteristics of NHS and privately-funded patients from the all operated sample of SCOTS participants
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/UNAW6331).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
