Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 14/192/109. The contractual start date was in May 2016. The draft manuscript began editorial review in September 2022 and was accepted for publication in September 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Crawley et al. This work was produced by Crawley et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Crawley et al.
Chapter 1 Introduction
Chronic fatigue syndrome or myalgic encephalomyelitis in children
Paediatric myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is common in the UK, with an estimated prevalence of 0.5%. 1 Before and during this study, ME/CFS was defined as generalised fatigue, causing disruption of daily life, persisting after routine tests and investigations have failed to identify an obvious underlying cause. 2,3 This definition changed in 2021, and a diagnosis of ME/CFS now requires the following additional symptoms: post-exertional malaise, unrefreshing sleep or sleep disturbance and cognitive difficulties. 4 Adolescents with ME/CFS are disabled,5,6 and use significant healthcare resources over a considerable period prior to accessing ME/CFS treatment. 7 Only 8% of adolescents appear to recover within 6 months with usual care,8 and this is consistent with adult data. 9 Usual care includes no treatment, treatment delivered by general practitioners (GPs) or by therapists that are not specialised in ME/CFS. Parents often stop or reduce time at work in order to care for their affected children. 10
At the time that Fatigue In Teenagers on the interNET in the National Health Service (FITNET-NHS) was conducted, the National Institute for Health and Care Excellence (NICE) guidelines recommended a minimum 3-month duration of fatigue before making a diagnosis in adolescents. 3 NICE recommended that adolescents with ME/CFS were offered either cognitive–behavioural therapy (CBT), which focuses on cognitive behavioural strategies to identify, challenge and change cognitive processes and resume activities); graded exercise therapy (GET), which stabilises physical activity levels, before gradually increasing at a manageable rate; or Activity Management, a goal-oriented and person-centred approach tailored to the needs of the person, which establishes a baseline for all activity, mainly cognitive, such as school and homework, in adolescents, which is then increased. 3,11 There is good evidence that CBT and GET are moderately effective in adults with ME/CFS. Four systematic reviews have shown that CBT and GET are moderately effective in improving function and reducing fatigue. 12–15 In particular, Pacing, graded Activity and Cognitive behavioural therapy, a randomised Evaluation trial (PACE) showed that both CBT and GET were more effective than specialist medical care or specialist medical care plus adaptive pacing therapy (a form of Activity Management that does not routinely increase activity but uses the envelope theory (patients work within their envelope of energy). 16
There is less evidence for the treatment of paediatric ME/CFS. However, when adolescents are offered treatment, the outcomes appear to be better than those seen in adult trials. We have conducted two systematic reviews8,17 to investigate treatment outcomes for paediatric ME/CFS, as well as a systematic review investigating recovery in paediatric ME/CFS using observational and trial data. 18 These supplement two previous systematic reviews on interventions in paediatric ME/CFS. 12,19 All five reviews identified good evidence from four randomised controlled trials (RCTs) (including the Dutch FITNET) that CBT is effective for paediatric ME/CFS. 8,20–22 Only the FITNET trial investigated internet-delivered CBT, and none of the published paediatric trials reported on cost-effectiveness. A search of trial registries located no other relevant trials prior to starting FITNET-NHS.
The FITNET trial, which was conducted in adolescents with ME/CFS in the Netherlands,8 showed that internet-based CBT was effective compared to usual care at 6 months. Usual care in the FITNET trial was not quantified, but participants probably had access to individual or group-based rehabilitation programmes, CBT face-to-face, or graded exercise treatment; these interventions being provided by therapists who were often not specialists in ME/CFS. Compared with usual care, adolescents in the FITNET group were more likely to attend school full-time [75% vs. 16%, relative risk 4.8, 95% confidence interval (CI) 2.7 to 8.9; p < 0.0001], less likely to have severe fatigue (85% vs. 27%, relative risk 3.2, 95% CI 2.1 to 4.9; p < 0.0001), and more likely to have normal physical functioning (78% vs. 20%, relative risk 3.8, 95% CI 2.3 to 6.3; p < 0.0001) at 6 months. Adolescents in the FITNET group were also more likely to have recovered at 6 months (defined as no longer severely fatigued or physically impaired, attending school, and perceiving themselves as completely/nearly completely recovered) (63% vs. 8%, relative risk 8.0, 95% CI 3.4 to 19.0; p < 0.0001). Improvement was maintained at 12 months. The FITNET-NHS intervention has been developed based on the Dutch FITNET8 and tailored to deliver specialist CBT treatment for adolescents with ME/CFS over the internet in the UK, and its effectiveness and cost-effectiveness will be assessed in this study.
Since we started FITNET-NHS, a large observational implementation study of FITNET in the Netherlands was conducted. 23 In this study, the 244 participants were allowed to be seen face-to-face (unlike in FITNET-NHS) and 41% were seen face-to-face at least once. Participants had improved fatigue, physical function and school attendance after treatment, and deterioration in fatigue and physical function was low (1.2% and 4.1%, respectively). A cost-effectiveness study was not conducted.
ME/CFS and comorbid depression and/or anxiety
Comorbid anxiety and depression affect more than 30% of adolescents with ME/CFS. 24–26 Most, but not all,27 studies in adults suggest that CBT for the treatment of ME/CFS is less effective in patients with comorbid depression16,28 compared to those without depression. The only study investigating predictors of outcome in adults treated with internet-delivered CBT for the treatment of ME/CFS is less effective in those with than without comorbid depression. 29 However, CBT appears to be a more effective treatment than GET for adults with ME/CFS and depression. 15,16 The paediatric trials conducted to date have either excluded adolescents with elevated comorbid depression and/or anxiety symptoms20 or not been powered to investigate treatment efficacy in this group. 8,21,22 As a substantial proportion of adolescents diagnosed with ME/CFS have comorbid depression and/or anxiety, FITNET-NHS was designed to treat those with comorbid depression and/or anxiety as well as ME/CFS and to examine if the effects of FITNET-NHS differ in this subgroup of adolescents as a secondary outcome. Negative thinking patterns contribute to the development and maintenance of depression and had not been investigated in paediatric ME/CFS prior to commencement of the FITNET-NHS trial. We embedded a substudy to investigate whether adolescents with ME/CFS and comorbid depression differed from those who are not depressed on cognitive errors and cognitive and behavioural responses to symptoms of ME/CFS. 30
National Health Service policy and practice
National Institute for Health and Care Excellence guidance in 2007 stated that adolescents with ME/CFS should be offered referral to a specialist service immediately if they are severely affected, within 3 months if they are moderately affected and within 6 months if they are mildly affected. 3 Current NICE guidance recommends that children and young people should have access to a ME/CFS specialist team ‘who they can contact with any concerns about the child’ (recommendation 1.10.4). 4 However, only around 10% of UK adolescents have access to a local NHS ME/CFS specialist service, and most adolescents cannot access the treatment they require because they live too far away from a specialist service. For those that do access a service, few are assessed within NICE-recommended time scales. 7 In some cases, GPs and paediatricians (or equivalent specialist doctors) advise on sleep, symptom control and Activity Management, but the specialist CBT for ME/CFS, for which there is an evidence base, is not available other than through specialist services.
Internet-delivered CBT is not only important for children who are unable to access specialist services, but has been vital during the COVID-19 pandemic. It has the potential to be adapted to other long-term conditions; however, few studies have investigated the acceptability, efficacy and cost-effectiveness. 31–33 Further understanding of internet-delivered CBT, particularly around how it is received by adolescents, will inform future treatment studies.
Justification of research
There is good evidence that CBT is effective in the treatment of paediatric ME/CFS. 20–22 However, most adolescents in the UK are unable to access specialist CBT for ME/CFS delivered face-to-face. Therefore, delivery of specialist CBT using the internet is an attractive option. In this study, we set out to demonstrate whether implementation of FITNET-NHS in the UK is feasible and acceptable during the internal pilot study, and whether it is effective and cost-effective during the full trial. We chose Activity Management, delivered via videoconference, as the comparator intervention because it was the only NICE (2007)-recommended3 approach offered by some paediatricians (or equivalent specialist doctors) outside specialist services. Although standard care and Activity Management were usually delivered face-to-face, videoconferencing was becoming more routine before FITNET-NHS started and became routine during the pandemic. For this trial, every aspect was delivered remotely. The study set out to find out if FITNET-NHS is effective and cost-effective because if so, its provision by the NHS has the potential to deliver substantial health gains for the large number of adolescents with ME/CFS but unable to access treatment because there is no local specialist service.
Objectives for the full trial
The overall aim of this study was to investigate whether CBT specifically designed for ME/CFS and delivered over the internet (FITNET-NHS) is effective and cost-effective compared to remotely delivered Activity Management for adolescents with ME/CFS who do not have access to a local specialist ME/CFS service.
Primary objective
Specifically, the primary objective of the full trial was to estimate the effectiveness of FITNET-NHS compared to Activity Management in the NHS for paediatric ME/CFS.
Secondary objectives
-
Estimate the effectiveness of FITNET-NHS compared to Activity Management for those with mild/moderate comorbid mood disorders (anxiety/depression).
-
Estimate the cost-effectiveness of FITNET-NHS compared to Activity Management.
-
Estimate the cost-effectiveness of FITNET-NHS compared to Activity Management for those with mild/moderate comorbid mood disorders (anxiety/depression).
The objectives for the pilot study are described in Chapter 3.
Chapter 2 Methods
Trial design
This was a RCT comparing FITNET-NHS with Activity Management when remotely delivered in the NHS for adolescents with ME/CFS. Participants were allocated in a 1 : 1 ratio, minimised by age and gender, between the two interventions. The first 12 months of the trial formed an internal pilot study (with continuation of the trial based on achieving defined stop/go criteria) including integrated qualitative methods to optimise recruitment and retention.
The study methods were prespecified in a published protocol. 34 A key change to the trial was published in a protocol update paper. 35 Protocol changes are presented in detail in Chapter 4.
Participants
Patients aged 11–17 years, with ME/CFS, who did not have a local specialist ME/CFS service were recruited at the specialist paediatric ME/CFS service, Bath Royal United Hospital.
Inclusion criteria
-
Aged 11–17 years.
-
ME/CFS diagnosis (defined using NICE guidance,3 see Table 1).
-
No local specialist ME/CFS service.
| NICE 2007 guidance | NICE 2021 guidance | CDC criteria | IOM criteria | CC criteria | |
|---|---|---|---|---|---|
| Duration of fatigue | 3 months (paediatric) | 4 weeks (paediatric) | 6 months | 6 months | 6 months |
| Symptoms | 1 or more of:
|
All of:
|
4 or more of:
|
3 or more of:
|
4 or more of:
|
|
|
|
|||
|
|||||
| Exclusionary diagnoses | Conditions that explain the fatigue | Conditions that explain the fatigue | Any active medical condition that may explain the presence of chronic fatigue. Any past or current diagnosis of: |
If patients do not have these symptoms at least half of the time with moderate, substantial or severe intensity | Active disease processes that explain most of the major symptoms of fatigue, sleep disturbance, pain, cognitive dysfunction. |
|
It is essential to exclude certain diseases: Addison’s disease, Cushing syndrome, hypothyroidism, hyperthyroidism, iron deficiency, other treatable forms of anaemia, iron overload syndrome, diabetes mellitus, cancer | ||||
|
It is essential to exclude treatable sleep disorders such as upper airway resistance syndrome and obstructive or central sleep apnoea; rheumatological disorders such as rheumatoid arthritis, lupus, polymyositis and polymyalgia rheumatica; immune disorders such as AIDS; neurological disorders such as multiple sclerosis, parkinsonism, myasthenia gravis and B12 deficiency; infectious diseases such as tuberculosis, chronic hepatitis, Lyme disease, etc.; primary psychiatric disorders, substance abuse | ||||
| Alcohol or other substance abuse within 2 years. Severe obesity (BMI > 45) |
Exclusion criteria
-
Not disabled by fatigue.
-
Fatigue was due to another cause.
-
Patients or parents unable to complete video calls or FITNET-NHS modules, for example, being unable to read FITNET-NHS material, having significant development problems, limited internet access, or being unwilling/unable to set up personal e-mail address/video call account.
-
Patients reporting pregnancy at assessment.
Setting
Patients (aged 11–17 years) were assessed by their GP, referred for local paediatric assessment and had blood tests (see Table 2) to exclude other causes of fatigue, in accordance with NICE guidance. 3 Where there was no local specialist paediatric ME/CFS service (about 90% of the UK), GPs and paediatricians (or equivalent specialist doctors) were able to refer those with ME/CFS to the Bath Specialist Paediatric ME/CFS Service.
| Full blood count | Screening blood tests for gluten sensitivity |
|---|---|
| Creatinine, urea and electrolytes | Serum calcium |
| Thyroid function | Creatine kinase |
| Erythrocyte sedimentation rate/plasma viscosity | Ferritin levels |
| C-reactive protein | Liver function tests |
| Random blood glucose | Vitamin D (if housebound) |
Patients were referred using normal clinical referral pathways from GPs across the UK to the Bath Royal United Hospital (via NHS e-referral service).
Between September 2018 and March 2020, GP surgeries in some areas of the UK were also offered the option of being set up as patient identification centre (PIC) sites (see Protocol Changes for more details). The PIC site work involved: (1) a database search to identify potentially eligible patients; (2) GP approval of a list of patients for mailing out an invitation letter; (3) letter mailout (directly to the patient if aged 16–17 years or to the parent/carer if aged 11–15 years) to invite families to consider the trial and ask them to make a GP appointment for a clinical referral to the Bath Royal United Hospital if they were interested.
At regular intervals, the trial was publicised to UK GPs via e-mails distributed by local National Institute for Health and Care Research (NIHR) Clinical Research Networks (CRNs), including regular bulletins and magazines released to GPs and other health professionals, supplying e-mailed referral flyers to disseminate to clinical staff (example flyer in Report Supplementary Material 1), and (from April 2018) a brief video for clinicians (a summary of a video is given in Report Supplementary Material 4), and (from November 2018) patient posters/flyers for display in GP surgeries (an example poster for patients is given in Report Supplementary Material 1).
The clinical team at the Bath Specialist ME/CFS Service reviewed referrals and performed initial screening to identify patients aged 11–17 years who were thought to have ME/CFS and no local specialist service. Referrals were accepted by the service if the patient had been assessed by a paediatrician (or equivalent specialist doctor) and had the NICE 2007 guidance screening blood tests (Table 2). 3
Recruitment
Potentially eligible patients were contacted via telephone by the clinical team to discuss ME/CFS treatment from Bath Services and the possibility of taking part in this research. Patients interested in the FITNET-NHS study were sent study information via e-mail to include: (1) a participant information leaflet (see Report Supplementary Material 5), (2) a link to the Revised Children’s Anxiety and Depression Scale (RCADS) questionnaire36 to complete (with an additional question for them consenting to the data being used for research purposes if they later decide to consent to the study), (3) a link to an online ‘consent to contact’ form (for electronic signing). Online forms were stored on a secure electronic system used for data capture called research electronic data capture37 (REDCap: www.project-redcap.org/).
The specialist nurse offered a final eligibility assessment via telephone/video call with both the referred patient and their parent/carer (hereafter ‘parent’) to confirm the patient’s ME/CFS diagnosis. This was completed using questions routinely used by the Bath Specialist ME/CFS Service on length of illness and other symptoms. These include four questions on fatigue: (1) debilitating persistent or relapsing fatigue for at least 3 months, but not life-long; (2) not the result of ongoing exertion and not substantially alleviated by rest; (3) post-exertional malaise; and (4) severe enough to cause substantial reduction in previous levels of occupational, educational, social or personal activities, one on length of illness and twelve on symptoms. Patients who answered ‘yes’ to these four questions and therefore had 3 months of disabling fatigue plus one further symptom were assessed as having ME/CFS. 3 The specialist nurse also identified patients who fulfilled the Centres for Disease Control and Prevention (CDC) diagnostic criteria at the eligibility assessment as this enables a comparison of our results with those of other trials.
During the eligibility assessment the specialist nurse ensured that patients did not have depression or anxiety sufficiently severe to cause their fatigue by reviewing RCADS responses. 38,39 The RCADS has 47 items with subscales that assess obsessive-compulsive disorder, social anxiety, panic, generalised anxiety, separation anxiety and depression with age- and sex-adjusted thresholds for each subscale. 40 Patients who scored above the threshold were considered to have a comorbid mental health problem. Those scoring above the borderline threshold answered further screening questions to determine whether they were at risk of harm and/or whether their mental health problem was sufficiently severe to explain the fatigue. This included questions about whether they wanted their low mood or anxiety or their fatigue treated first as well as questions about hopelessness, their sleep pattern, eating and whether they were experiencing anhedonia. Those who were considered as primarily having a mood problem or other cause for their fatigue (rather than ME/CFS) were told about the provisional diagnoses, offered referral to the appropriate provider and were not eligible for this trial. The referring clinician was informed about alternative diagnoses and signposted to relevant services.
If the patient was eligible and they and their parent were willing, the recruiting team at Bath Specialist ME/CFS Service arranged a telephone/video call recruitment consultation. The research team explained the trial design and interventions; ensured the patient and parent had had an opportunity to read the age-appropriate patient information leaflet (PIL) and answered any questions about the research project. Recruitment discussions were audio-recorded with families’ consent.
If patients were willing to take part in the FITNET-NHS trial, the research nurse requested consent/assent from both the patient and parent during the recruitment consultation using an online consent form (signed electronically) on the secure electronic system, REDCap. Patients aged 11–15 years completed an assent form, while patients aged 16–17 years and parents completed a consent form. Patients deciding not to take part in the FITNET-NHS trial were still offered treatment from the service. This could be remote or face-to-face depending on clinical need and geographical location/transport.
Randomisation
The research team performed randomisation while the participant was on the telephone/video call. An automated web randomisation service operated by the Bristol Trials Centre was used. The web randomisation service automatically allocated participants in a 1 : 1 ratio to receive either FITNET-NHS or Activity Management. Allocation used minimisation to facilitate balance by age (11–14 or 15–17 years) and gender assigned at birth (male or female) and retained a random component to prevent accurate prediction of allocation (i.e. allocation concealment was preserved).
Recruited patients (hereafter ‘participants’) and their parents were informed of their allocated intervention at the end of the recruitment consultation. GPs were informed (via letter) about the intervention to which their patient had been allocated.
Blinding
Because of the nature of the interventions, it was not practical to blind either the participant, family or the clinical service to treatment allocation. The investigators who wrote the statistical analysis plan and analysed the clinical and economic outcomes were blinded to treatment allocation (this did not include the trial statistician who presented unblinded data to the confidential Data Safety and Monitoring Committee).
Interventions
In this section we use the TIDieR subheadings to describe the two study interventions. 41
Fatigue In Teenagers on the interNET in the National Health Service
Rationale
FITNET-NHS is a web-based modular ME/CFS-specific CBT programme designed to be used by adolescents and their parents. It is a UK version of the Dutch FITNET platform (which was shown to be highly effective8 or paediatric ME/CFS in the Netherlands. FITNET-NHS has been translated into English and adapted to be a fully remote service for use within the NHS. As a web-based intervention, it can be provided for adolescents who do not have a local service.
Theory
Content for participants was designed to encourage active collaboration and self-discovery. Participants were encouraged to monitor their activity, establish a manageable baseline and build on this gradually. Content for parents is designed to encourage parents to explore and address their beliefs and behaviours towards their child with ME/CFS focussing on their role as carers, and complement the patient sessions.
Materials
The programme has psycho-educational and CBT sections for patients and a parallel programme for their parents. There are 19 chapters in total for patients and parents to work through in their own time (see Appendix 1 for chapter titles). The initial psycho-educational chapters are available (on logging into the web-based platform) to all patients and parents immediately after receiving log-in codes. Chapters 1, 2 and 3 introduce CBT and explain the role of therapists and parents, present ME/CFS as a multifactorial model with predisposing, precipitating and maintaining factors and discuss the role of the family. Chapter 4 focuses on treatment goals, including the goal of full-time education. Chapters 5–19 focus on cognitive and behavioural strategies, starting with addressing sleep patterns, with exercises designed to notice and change behaviour patterns and on identifying unhelpful thinking processes and changing these to more helpful ones, with a focus on goal attainment. These chapters are unlocked by the therapist based on individual patient’s needs, goals and formulation.
There are diaries included within the web-based platform which are visible to the therapist so that patients can record their sleep, activity levels, and helpful thoughts, and then discuss these with their therapist (Report Supplementary Material 6 presents a selection of screenshots from the FITNET-NHS platform).
Procedures
FITNET-NHS treatment is delivered using asynchronous individualised e-consultations (comprising written messages sent separately to the patient and their parent) delivered within the web-based platform itself. Each patient and their parent are set up on the platform by the therapist. The patient and parent then each set up an independent password-protected account. The therapist works with patients and parents separately and responding together is discouraged. Parents can read the content of the patient chapters but cannot see their child’s answers to the questions. Therapists can view patients’ question responses and diaries and they use e-consultations to help the patient through the programme. Therapists request that their patient responds via message within the platform before a specific date at which the therapist delivers their next detailed and tailored message.
Therapist messages would include comments on the information the participant provided in their diaries, completed chapters, and e-messages on the platform. Therapists would highlight achievements (such as the participant reaching a set goal/task during the week), would notice patterns (such as an increase in activity, or an improvement in sleep onset time) and would prompt further reflection or thought for them (such as asking further questions about anything the patient had mentioned). Therapist messages would also seek to answer any questions the participant may have raised, or address any difficulties with tasks that the patient may have mentioned. The same processes occurred between participants’ parents, and therapists.
When treatment was complete, participants could opt for further treatment in the clinical service (after a medical review), or organise further treatment locally.
Delivered by
FITNET-NHS was delivered by clinical psychologists or CBT therapists. At the start of the study, therapists received four days of intensive training held over 2 months. The training sessions were video-recorded, and training materials (PowerPoint presentation, and supplementary written material) were developed for new members of staff. The therapists received at least monthly supervision by the Dutch FITNET team, including reviews of e-consultations sent to ensure intervention fidelity until 2010. Supervision was then provided within the UK team without oversight from the Dutch FITNET team. Therapists who joined after the trial started received similar intensive training using the training material and supervision from experienced members of the UK team who had attended the initial training.
When and how much?
Contacts were initially weekly and tended to be spaced out towards the later stages of therapy, but frequency varied according to need. FITNET-NHS Treatment was designed to last approximately 6 months, though this was expected to be variable between participants.
Tailoring
Chapters 5–19 are unlocked (i.e. made visible and accessible) to patients (with the corresponding section unlocked for the parent) based on clinical presentation, needs and clinical formulation. For example, Chapter 16: Plan for work would only be opened for somebody who wanted to work. Therefore, each patient is likely to have a different combination of chapters at different points and not all will complete every chapter. The language used in the e-consultations is also tailored to the participants and to the questions/responses raised in the e-consultations.
Modifications were made during the study. Please see Chapter 4.
Fidelity was assessed in FITNET-NHS supervisions (see above).
Activity Management via videocall
Rationale
Activity Management is a behavioural treatment offered throughout the UK in paediatric services. Patients and their families often call this Pacing. It was recommended by NICE in 2007 and in 2021. 3,4
Materials
Participants received information on ME/CFS, Activity Management, sleep and symptom management. Participants could use an online app (ActiveMe) to record activity.
Procedures
Activity Management was delivered via videocall (using Skype). During the initial appointment, the clinician carried out a detailed assessment of physical and cognitive activity with the participant. Clinicians discussed the different types of physical activity and the different types of cognitive activity (high-concentration and low-concentration), which varied according to age. High-concentration cognitive activities include time at school or doing schoolwork, reading, some craft/hobbies, socialising and screen time (phone, TV, computer, other devices). The participant and therapist agreed a ‘baseline’, which is the average level of activity. For severely affected adolescents, this might include sitting up in bed; for those with mild ME/CFS, this might include walking fast. The baseline could either be estimated in collaboration with the specialist therapist or calculated after a period of recording activity. Using a baseline for activity, usually means limiting activity on good days. Once the baseline was agreed with participants, they were asked to record the total number of minutes spent each day doing high-concentration cognitive activities using paper diaries or the iPhone/iPad app ‘ActiveME’. Participants were asked to scan and e-mail or post the paper diaries or e-mail outputs from ‘ActiveME’ to the therapists. Recording activity was used to help participants understand whether they are doing the same each day or varying their activity and whether the baseline has been set at the correct level. Participants were then encouraged to increase their activity gradually. When participants managed the baseline for 1–2 weeks, they were encouraged to increase this gradually (by no more than 10–20%) each week in a flexible and individualised way. During the follow-up video calls, the clinician reviewed physical and cognitive activity and sleep and helped participants problem-solve. Participants were encouraged to increase activity until they were able to do up to 8 hours of cognitive and physical activity a day.
Following the course of video calls, the participant’s clinician handed over to the participant’s nominated local therapist or doctor to deliver care (offering phone call hand-over as well as treatment summary letter).
Delivered by a ME/CFS clinician (usually a physiotherapist/occupational therapist) at the Bath Specialist ME/CFS Service.
When and how much
The initial assessment took up to 90 minutes, and an option was added to enable this to be split into two sessions if needed (due to the energy level of the participant). The first follow-up video call was arranged 2–6 weeks after assessment depending on participant and their parent preference. Further follow-up video calls were organised with gaps of 2–6 weeks between them. Follow-up calls were designed to take approximately 60 minutes each time. The total time in treatment varied depending on the number of sessions and the gap between sessions but was designed to last about 6 months.
Tailoring
Clinicians could individualise sessions but were prohibited from exploring participant cognitions and emotions in detail. Sessions were guided using a checklist (see Appendix 1 for details) with flexible elements that could be used. The speed and timing of activity increase (or decrease), as well as the time between sessions were individualised with participants.
Modifications
Please see Chapter 4 for details on the modifications. The most important modification was increasing the number of Activity Management sessions from 3 to 6 in November 2017 in response to feedback from families and clinicians.
Fidelity was assessed using the intervention checklists of mandatory, flexible and prohibited elements (see Appendix 1 for details).
Outcomes
The primary outcome for the full trial was disability measured using the 36-item Short Form Health Survey Physical Function subscale (SF-36-PFS)42 reported 6 months after randomisation. This subscale has 10 items and scoring ranges from 0 to 100, with higher scores indicating better physical function.
The secondary outcomes are listed below and were measured at baseline (shortly after randomisation, and before treatment began) and at 3, 6 and 12 months after randomisation unless otherwise specified:
-
SF-36-PFS42 measures at 3 and 12 months after randomisation (the 6-month measure formed the primary outcome)
-
fatigue [Chalder fatigue scale43 and checklist individual strength (CIS) fatigue severity subscale]44
-
school attendance (self-report days per week attendance at school and/or receiving home tuition)
-
mood (RCADS)36
-
pain visual analogue scale (VAS)45
-
Clinical Global Impression Scale46
-
quality of life [(EuroQol-5 Dimensions Youth (EQ-5D-Y)]47
-
Parent-completed child’s healthcare use and parent’s out-of-pocket resource use questionnaire (RUQ)
-
parent-completed work productivity and activity impairment questionnaire general health (WPAI:GH)48 (parent-related productivity and activity).
These measures were chosen as being important and relevant domains49 used in UK services, child and adolescent mental health services (CAMHS) and/or tested in previous trials. 8,50,51
The following measures were also collected from participants at the eligibility assessment: age, sex, postcode (for data on deprivation – indices of multiple deprivation), ethnicity, symptoms (both CDC and NICE criteria to enable comparison of results with those of other studies using CDC criteria), months of illness, and diagnoses of comorbid illnesses.
At baseline, the first 205 participants completed the following additional questionnaires (not repeated at follow-up):
-
cognitive behavioural responses to symptoms questionnaire (CBRSQ)28,29
-
children’s negative cognitive error questionnaire – revised (CNCEQ-R). 31
These additional questionnaires were included to explore differences in negative thinking between those participants with ME/CFS and comorbid mood disorders and those without. The CBRSQ has previously been used with adults with ME/CFS in the PACE trial,52 and with adolescents. 53 The results from these questionnaires will be reported elsewhere. Table 3 shows the full schedule of data collection at baseline and at follow-up.
| Data item | Baseline | Follow-up (months) | |||||
|---|---|---|---|---|---|---|---|
| Referral letter | Eligibility assessment | Following recruitment | 3 | 6 | 12 | ||
| Assessment data | Age and postcode | X | |||||
| Sex and ethnicity | X | ||||||
| Symptoms list (CDC and NICE criteria) | X | ||||||
| Months of illness | X | ||||||
| Comorbid conditions | X | ||||||
| Questionnaires (child) | SF-36-PFS | X | X | X | X | ||
| Chalder fatigue and CIS fatigue | X | X | X | X | |||
| School attendance | X | X | X | X | |||
| RCADS | X | X | X | X | |||
| Pain VAS | X | X | X | X | |||
| Clinical Global Impressions Scale | X | X | |||||
| EQ-5D-Y | X | X | X | X | |||
| CNCEQ-R | X | ||||||
| CBRSQ | X | ||||||
| Questionnaires (parent/carer) | Healthcare resource use | X | X | X | |||
| WPAI:GH | X | X | X | X | |||
Clinicians rated each participant’s treatment adherence on discharge from trial treatment, rating these on a 3-point scale: (1) non-starter (never began treatment/not contactable after enrolment); (2) started then stopped (made a start but then discontinued treatment/became uncontactable); (3) 80% + completion (majority of clinically relevant modules/sessions required had been attended/completed).
Data collection methods
All baseline and follow-up questionnaire data were collected on REDCap, a secure system used by many institutions for large multicentre studies. Participants were sent a web link to access their REDCap forms asking these to be filled in (Report Supplementary Material 2 gives examples of how the participant and parent questions appeared). Completed forms that had been submitted could not be re-accessed by participants, and forms that had been partially completed and exited could be re-accessed by the participant for completion only via entering a code unique to that specific survey, generated by REDCap, to ensure data security. An automated reminder e-mail was sent to participants who had not filled in their baseline questionnaire 7 days after it was first sent, with a further automated reminder e-mail after 14 days if it was still incomplete. Newly recruited participants were also contacted by phone, text and/or letter to try to gain the baseline data prior to commencing treatment if the baseline remained incomplete.
At the follow-up time points, an e-mail was sent to participants automatically with a link to complete questionnaires online. If these were not completed, automated reminders were sent at 2 weeks and then again at 4 weeks after the questionnaire was due. If questionnaires were not completed, we tried to contact participants by telephone or text. An e-mail with a link to a reduced set of questionnaires (SF-36-PFS, Chalder fatigue, school attendance, EQ-5D-Y and Clinical Global Impression Scale only) was also sent 6 weeks after the questionnaire was due for participants who had not completed their questionnaires, with the aim of capturing a minimal data set. If participants were contacted by phone and did not want to complete the minimum data set, we asked if they would be willing to complete the primary outcome only.
Harms/adverse events assessment
The FITNET-NHS trial investigated whether adolescents randomised to one treatment group were at higher risk of having a serious deterioration in health compared to the other treatment group. We defined a serious deterioration in health as either: (1) clinician-reported serious deterioration in health (reported during FITNET-NHS or Activity Management session) reported as an adverse event; (2) a decrease of ≥ 20 in SF-36-PFS between baseline and 3, 6 or 12 months or scores of ‘much’ or ‘very much’ worse on the Clinical Global Impression Scale; or (3) withdrawal from treatment because of feeling worse. Safety outcomes were reported to the Data Safety Monitoring Committee.
An adverse event is any untoward medical occurrence in a patient which does not necessarily have a causal relationship with the treatment. Data on adverse events were collected for each participant from the point at which they consented to take part in the study until the end of the follow-up period (12 months). On being alerted about an adverse event from a member of the clinical team, the research team provided the person reporting the event with a link to an online adverse event questionnaire (within REDCap) to record these details in accordance with Health Technology Assessment (HTA) and University of Bristol guidelines on reporting.
ME/CFS is by its nature a fluctuating illness. The description of activity and function in ME/CFS is one of boom–bust, which usually occurs over several days and sometimes weeks. ‘Payback’ or ‘crashes’ or ‘flares’ are to be expected in young people whether or not they are undergoing treatment. Payback, crashes or flares can mean that an adolescent who was previously mobile becomes bed-bound or is unable to go to school. Episodes can last days or occasionally weeks. Treatment is designed to reduce these over time but the risk of flares without treatment, during or post treatment is not known. Between 30% and 40% of adolescents were expected to experience significant comorbid anxiety and depression. In most cases, this is because of the prolonged disabling nature of ME/CFS. This means that it is not unexpected for adolescents with ME/CFS to be referred to CAMHS for treatment. It was expected that some adolescents would be started on medical treatment, including selective serotonin reuptake inhibitors. Data on medication for mood were collected via parent questionnaires and review of medical records of the participant. Other medication expected to be used by this patient group is melatonin (to improve sleep) and amitriptyline (to improve chronic pain and sleep).
All adverse events were recorded on an electronic case record form (stored on REDCap), including an assessment of whether it was expected or not expected and if it was (or possibly was) related to trial treatment. All adverse events were reported and reviewed by the Data Safety and Monitoring Committee during the study.
Any adverse event was to be defined as serious if: it resulted in death, was life-threatening, required unplanned inpatient hospitalisation (overnight) or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity or resulted in congenital anomaly or birth defect. Any adverse event meeting any one of these criteria was reported by the clinical team/participant or parent to the research team, recorded in detail on an online case record form (via REDCap) and reported to the Sponsor and Principal Investigator by the research team within 24 hours of being notified, in accordance with the Sponsor’s protocol.
For all serious adverse events, the subject was actively followed up, and the investigator (or delegated person) provided follow-up details every 5 working days and submitted a Serious Adverse Event Follow-up report to the sponsor and the Principal Investigator until the serious adverse event had resolved or a decision for no further follow-up had been taken.
Sample size
We planned to randomise 314 participants, assuming 15% attrition (withdrawal or non-provision of primary outcome data); see Chapter 4, Sample size for the original sample size calculation. Therefore, we expected data to be available for 266 participants for the primary analysis. This sample size gives 90% power at 5% significance to detect a 10-point [approximately 0.4 standard deviation (SD)] difference for the SF-36-PFS for our primary outcome. This is the minimally clinically important difference (MCID) defined previously in paediatric ME/CFS using triangulation of three methods: the anchor method, the distribution method and qualitative methods. 54
Statistical methods
Full details of the statistical methods have been presented in the FITNET-NHS statistical analysis plan, written without access to the accumulating outcome data, and made publicly available on 6 October 2021. 55 All analyses were conducted using Stata version® 17.0 statistical software (StataCorp, College Station, TX, USA, 2021).
For the primary outcome, the null hypothesis tested was that the population mean SF-36-PFS score at 6 months’ follow-up was equal between groups allocated to FITNET-NHS or to Activity Management. We used an intention-to-treat analysis, comparing study participants who completed the required measures, in the treatment groups to which they were allocated (the full analysis population). We used multivariable linear regression adjusting for baseline values of the outcome, baseline age and gender. The treatment effect was estimated as an adjusted difference between sample means.
The outcome variable (yi) was patient response at 6 months post randomisation. Treatment covariates were: treatment allocation (x1i = 1: FITNET-NHS; x1i = 0: Activity Management), baseline SF-36-PFS (x2i), age at recruitment (x3i as a continuous measure), and gender (x4i = 1: male; x4i = 0: female). Finally, a dummy variable distinguishing those participants without a baseline assessment of outcome (x5i = 1: no baseline assessment; x5i = 0: baseline assessment available) was used. 56 A normal distribution was assumed for the residual errors: ei ~ N(0,σe). The coefficient for the treatment allocation covariate (β1) is the intention-to-treat estimate of treatment effectiveness, comparing FITNET-NHS to Activity Management. In statistical notation:
The residuals from the model were checked for a normal distribution, and as having a similar SD in the two treatment groups.
The statistical analysis plan pre-specified the following sensitivity analyses to aid interpretation of the results. We did not need to adjust our primary analysis model for prognostic variables (baseline variables) for which there was a baseline imbalance between treatment arms of more than half a SD between means (or more than 0.1 between proportions). We also adjusted for any variation across participants in the time between randomisation and the 6-month outcome assessment.
Because of changes in the first lockdown during the COVID pandemic (for example, school, social activities), we prospectively decided to repeat the analysis with the addition of a binary covariate distinguishing participants recruited before and after 1 September 2019 as this defines those with a 6-month primary outcome before or after the start of the first lockdown. Furthermore, during the pandemic, we noticed an increase in time between randomisation and treatment. We therefore repeated the analysis using 12-month assessment (rather than 6-month assessment) for those who did not start treatment until after the 3-month assessment.
We estimated the effectiveness of FITNET-NHS compared with Activity Management for the SF-36-PFS primary outcome in participants completing one or more modules/sessions of their allocated intervention. This is a change from the corresponding sensitivity analysis described in the published protocol paper,35 which can more easily be applied in an equivalent manner to participants irrespective of their allocation.
We explored the robustness of the findings from the analysis of the primary outcome to assumptions about the missing outcome data using a pattern mixture model approach. 57 This was used to indicate how different the missing and observed measurements would need to be on average, for the observed treatment comparison to be considered an artefact of the missing data.
The primary analysis was adapted to each of the other questionnaire measures at 6- and 12-month follow-up assessments in turn (with the twelve-month assessment of SF-36-PFS included as a secondary outcome). The corresponding baseline measure of the questionnaire being analysed was included. The primary analysis was adapted to the Clinical Global Impression Scale, with an ordered logistic regression model being employed. The seven response categories were kept separate when included in this model. There was no baseline assessment of this measure.
In a single pre-specified subgroup analysis, we estimated the effectiveness of FITNET-NHS compared with Activity Management on the primary outcome in participant subgroups defined by the presence or absence of baseline anxiety or depression, defined by using the age- and gender-specific clinical thresholds for each subscale on the RCADS. Evidence that the intervention effect differs between subgroups was examined by adding interaction terms to the multivariable linear regression model for the SF-36-PFS primary outcome only.
Measures of harm and adverse events were tabulated, along with a count of the number of participants who met one or more of the above measures.
Economic evaluation methods
The primary objective of the economic evaluation was to estimate the cost-effectiveness of FITNET-NHS compared to Activity Management delivered by videocall to treat children aged 11–17 years with CFS/ME, from the perspective of the NHS in the UK over a 12-month follow-up period.
The secondary objectives were to:
-
estimate the cost-effectiveness of FITNET-NHS compared to Activity Management for subgroups with and without mild/moderate comorbid anxiety/depression
-
estimate the cost-effectiveness of FITNET-NHS compared to Activity Management from a wider perspective (including the participant and family costs, and impacts on participant education).
A health economics analysis plan (HEAP), which followed intention-to-treat principles, was pre-specified by the study team. 55 The trial was conducted in the UK, where the health system is predominantly publicly funded, provided by the NHS, and care for UK residents is free at the point of access. The primary analysis was conducted from the NHS perspective, which involved assessing the impact on secondary, primary and community care. A within-trial cost–utility analysis (CUA) was conducted for the primary analysis. This involved comparing mean incremental differences in costs and quality-adjusted life-years (QALYs) over the first 12 months from randomisation.
Measurement of resources
Resource use identified as relevant from an NHS perspective included: (1) training for FITNET NHS; (2) delivery of FITNET and Activity Management; (3) primary and community care visits; (4) prescribed medications; and (5) secondary care. Relevant resource use from a wider perspective, in addition, included: (1) participant and family out-of-pocket costs for private tuition, average travel cost incurred per return journey for a secondary, primary or community care visit, over-the-counter medication costs; (2) loss in productivity and time in education; (3) school counsellor costs; and (4) any other patient and family costs incurred due to the child’s CFS/ME.
Study records captured training costs for FITNET-NHS. Electronic health records were used to capture treatment delivery costs as well as secondary care use. All other costs were captured via a RUQ, which parents completed on behalf of their child. The RUQ had been piloted prior to the trial. It captured data on: primary and community care, medication use, participant and family expenses and productivity loss, and education impacts. The RUQ was completed online at 3, 6 and 12 months post randomisation. Table 4 summarises the key resources measured.
| Resource category | Unit cost (£) | Source of unit cost |
|---|---|---|
| Intervention training | ||
| Non-NHS staff time and travel to deliver training | Variesa | Study records |
| NHS staff time to deliver training | Variesa | Study records |
| NHS staff time to receive training | Variesa | Study records |
| Car mileage | 0.45 | HM Revenue and Customs, 202064 |
| Treatment delivery costs | ||
| Initial consultation (90 minutes) | Varies | Curtis and Burns, 202058 |
| Follow-up consultation (60 minutes) | Varies | Curtis and Burns, 202058 |
| Additional consultation (30 minutes) | Varies | Curtis and Burns, 202058 |
| Primary and community care | ||
| GP contact | 34b,c,d | Curtis and Burns, 202058 |
| GP Home visit | 104b,c,e | Curtis and Burns, 201361 |
| Nurse contact | 10.85c | Curtis and Burns, 202058 |
| NHS 111 call | 12.26 | Pope et al., 201762 |
| Walk-in centre | 39.76f | National Cost Collection, 202059 |
| Child and adolescent mental health contact | 97g | Curtis and Burns, 202058 |
| Other contact | 51.45c,h | Curtis and Burns, 202058 |
| Medications | Varies | Prescription Cost Analysis, 202063 |
| Secondary care | ||
| Outpatient visits | 234.15i | National Cost Collection, 202059 |
| Accident and Emergency visits | Variesj | National Cost Collection, 202059 |
| Inpatient admissions | Variesk | National Cost Collection, 202059 |
Data on staff training for FITNET-NHS between 2016 and 2020 were logged by a senior clinician at Bath Specialist CFS/ME service. Records included dates when the training took place and the number of clinicians and trainers who attended the training. A clinician involved in the delivery of the training estimated the duration for a typical training session.
Patient-level data on the delivery of FITNET-NHS and Activity Management were accessed via Bath Royal United Hospitals NHS Trust’s electronic patient record system (known as Millennium). These data included the number and type of appointments provided (e.g. staff type and whether it was an initial or follow-up appointment). A clinician involved in the delivery of the CFS/ME service at Bath Royal United Hospital estimated the typical consultation duration for each appointment type (e.g. initial, follow-up and additional e-consultations or videocalls). Two further clinicians from the service verified these estimates. It was estimated that the first appointment for both FITNET-NHS and Activity Management took 90 minutes and follow-up consultations took 60 minutes. In addition, for the FITNET-NHS group, if a patient required substantially more support on a particular week, then an additional 30-minute time slot was logged in the Millennium system.
Patient-level records on admitted care, outpatient visits, emergency department attendances and specialist secondary mental health care at NHS hospitals in England were requested from NHS Digital. Specifically, hospital episode statistics (HES) collates hospital care paid for by the NHS and provided by any acute NHS or independent hospitals in England. Due to delays in receiving data from NHS Digital, we were not able to include secondary mental health care costs in this report. In addition, NHS Digital were only able to provide the emergency care data set (ECDS) from April 2018. As the ECDS only became the official data source for Emergency Care in England from April 2020, our measure of patient Emergency Care visits may be incomplete between April 2018 and March 2020.
Data linkage was carried out by NHS Digital. The FITNET-NHS research team provided NHS Digital with patient identifiers (NHS number and date of birth) and a pseudonymised participant ID number. Data were only requested for the 306 (97.45%) participants who provided consent for their medical records to be linked. NHS Digital were able to link 296 (94.27%) participants. NHS Digital removed the patient identifiers and returned the pseudonymised linked data to the FITNET-NHS research team. The Millennium data set was used as the primary source for outpatient visits taking place at the CFS/ME service at Bath Royal United Hospital. Therefore, in order to avoid duplicates, outpatient visits that took place at Bath Royal United Hospital were dropped from the HES data set before analysis.
Please refer to Appendix 2 for an overview and timeline of the data access request service (DARS) application process. In summary, we began the application process on 14 March 2019 and eventually received most of the data requested in February 2022.
It was intended that routine data from GP electronic patient record system providers would be used as the main data source for primary and community care. The system providers were unable to provide access to pseudonymised data and so the RUQ was used as the sole source for primary and community care resource use. The RUQ was completed by parents on behalf of their child, and included: (1) all types of GP surgery and telephone consultations with the GP and Practice Nurse/Nurse Practitioner; (2) all types of GP home visits; and (3) all types of other primary and community-based contacts (e.g. walk-in centre visits, telephone calls to 111). Medications include any prescribed medications as well as a list of specific medications (e.g. Amitriptyline, Melatonin, Paracetamol, Ibuprofen, Codeine) commonly used for ME/CFS symptoms.
The RUQ asked parents to provide data on the costs they had incurred as a result of their child’s CFS/ME. Parents were asked to report on out-of-pocket costs they incurred for the following: (1) return journey to primary or community care centre, or hospital (for public transport, this included the cost of a return fare for the child and parent; for private vehicle, this included the cost of parking and fuel costs); (2) any over-the-counter medications purchased for their child; (3) any other out-of-pocket expenses the parent or the immediate family have incurred due to child’s illness; and (4) hours absent from work and regular activities due to child’s health problems. Parents were also asked to report any impact on their productivity in the past seven days using an adapted six-item WPAI:GH V2.0. 48 The WPAI:GH V2.0 questionnaire was adapted so that parents were asked about how their child’s health impacted on their productivity. More specifically, they were asked to report on: how many hours of work they missed; how much their productivity was affected on a scale of 0–10; and how much their usual activities were affected. Lastly, parents were asked to report whether children had received support from a school counsellor.
Children were asked whether they were currently receiving home tuition, and if so, they were asked to specify how many hours of home tuition they had received in the previous week. Children were also asked to report on the proportion of the week they typically attended school in the previous school term.
Valuation of resources
Resources were valued using 2019/20 prices. Where a unit cost was not available for 2019/20, it was inflated using the NHS cost inflation index (NHSCII). 58 Hospital-based healthcare staff delivering FITNET-NHS and Activity Management, as well as care provided by primary and community-based healthcare staff was valued using the 2020 Unit Costs published by the Personal Social Services Research Unit (PSSRU). 58 Secondary care resource use, excluding the FITNET-NHS and Activity Management interventions, was costed using the 2020 NHS reference costs from the National Cost Collection. 59 When a unit cost was unavailable for a specific inpatient or emergency care visit, simple mean imputation was used. This involved us using the mean costs of the children in our study who had an inpatient or emergency care visit. Prescribed medications were assigned a unit cost based on the prescription cost analysis (PCA) for 2020. 59 Actual costs reported by the parents were used for out-of-pocket costs. Productivity costs were derived from the Annual Survey of Hours and Earnings using median pay per hour. 60 Table 458,59,61–64 and Table 558 summarise the resources collected and their valuation from the NHS and wider perspective, respectively.
| Resource category | Unit cost (£) | Source of unit cost |
|---|---|---|
| School counsellor | 97a | Curtis and Burns, 202058 |
| Out-of-pocket costs | Varies | Cost reported by participants |
| Productivity: absenteeism | 15.14 | ONS AHSE, 2020b,c |
| Productivity: presenteeism | 15.14 | ONS AHSE, 2020b,d |
| Tuition costs | 28 | Varies, 2021e |
Measurement and valuation of outcomes
The EQ-5D-Y was collected at baseline, 3, 6 and 12 months, using a participant self-completed questionnaire completed online. At the time of study design, it was anticipated that an EQ-5D-Y UK child scoring algorithm (value set) would be available by the time of analysis. However, a value set for the EQ-5D-Y is not yet available for the UK and it is not recommended to use the UK adult EQ-5D-3L value set as a proxy for children. 65 Instead, a proxy EQ-5D-Y value set derived for the German population was used to calculate utility scores for each participant. 66
Economic analysis
The analysis took an intention-to-treat approach, whereby all participants who did not withdraw their consent to have their data used in the study were analysed according to group they were randomised to. As costs and outcomes were not assessed beyond 12 months, discounting was not required.
Mean and SD resource use and number of respondents were estimated and presented by group for each resource use category (e.g. outpatient visits, medication use, etc.). Utility scores derived from the EQ-5D-Y were used to calculate the QALYs for each patient using an area-under-the-curve approach. 67 Seemingly unrelated regression (SUR) was used to estimate mean costs and QALYs and 95% CIs in each group and the incremental difference in costs and QALYs between the groups. SUR accounted for the correlation between costs and effects. 19 In addition, costs and QALYs were estimated with baseline age and gender as covariates; baseline EQ-5D-Y score was also a covariate in the QALY regression.
In order to reduce possible bias due to missing data, our primary analysis used multiple imputation by chained equation (MICE) using predictive mean matching. We assumed data were missing at random (MAR).
All cost and outcome variables were included in the imputation model as well as the covariates baseline age and gender. The imputation model was stratified by treatment allocation group and a random seed was set to provide reproducible imputations. We created 50 imputed data sets. Rubin’s rule was used to pool and analyse multi-imputed data sets. Total utility scores, primary care costs and medication costs were imputed for each data collection timepoint (baseline, 3, 6, and 12 months) due to questionnaire data not being returned. In addition, secondary care costs were imputed for a minority of participants (n = 19) due to data linkage not being possible. Imputed utility scores at baseline, 3, 6, and 12 months were used to calculate mean QALYs and the mean incremental difference in QALYs per group. Similarly, imputed costs at 3, 6 and 12 months for the various cost categories were summed up to calculate total mean costs, and the mean incremental difference in costs per group.
The primary analysis combined cost and QALY data to calculate an incremental cost-effectiveness ratio and an incremental net monetary benefit (iNMB) statistic. 68 INMB was used to estimate cost-effectiveness at the UK NICE-recommended cost-effectiveness thresholds of £20,000 and £30,000 per QALY. We used a willingness-to-pay threshold of £20,000 per QALY in the primary analysis. Uncertainties in the point estimates of iNMB were quantified using 95% CIs estimated from the regression. A cost-effectiveness acceptability curve (CEAC) was used to illustrate the probability of FITNET-NHS being cost-effective compared to Activity Management across a range of willingness-to-pay thresholds.
In line with the clinical effectiveness analysis, a subgroup analysis was performed to explore the interaction between comorbid anxiety/depression disorder and the cost-effectiveness of FITNET-NHS. Comorbid anxiety/depression at baseline was included as an interaction term with treatment allocation to assess any modification of cost-effectiveness in these subgroups.
Uncertainty in the primary analysis was explored through sensitivity analyses. Firstly, we conducted sensitivity analyses that were pre-specified in the HEAP:
-
Handling missing data: a complete-case analysis was performed, where only participants who had complete cost and QALY data were included in the analysis. This analysis assumed data were missing completely at random (MCAR).
-
Intervention costing: the primary analysis was repeated using the 2019/20 tariff paid by the Clinical Commissioning Groups (CCGs) (instead of the reference cost used in the primary analysis) for FITNET-NHS and Activity Management consultations.
Further, post hoc sensitivity analyses were performed to explore the impact of alternative methodological choices:
-
We repeated the analysis including participants with ME/CFS as redefined in the NICE (2021) criteria.
-
Handling missing data: data may be missing due to unobserved factors that may be correlated with both treatment and outcome. We therefore conducted a sensitivity analysis assuming health-related quality-of-life data were missing not at random (MNAR). Following Laurent et al. (2018),69 we fitted pattern mixture models using multiple imputation. Specifically, in each treatment group we assumed missing health-related quality-of-life values were 0%, 5% or 10% lower than participants with similar characteristics who do not have missing values. We allowed the missing values to differ by group. We expected any between group difference to be small, and so we assumed the between group difference would not be greater than 5%. This results in six MNAR scenarios.
-
Excluding training costs: training all staff on how to deliver FITNET-NHS was a one-off cost. If FITNET-NHS were to be implemented widely across the NHS, it is likely that training in delivery would become a routine element of clinical training in ME/CFS care.
-
Intervention costing: the FITNET-NHS intervention was primarily delivered by Band 7 clinicians. It is possible that other clinicians could be trained to deliver FITNET-NHS. We conducted an analysis to explore how total intervention delivery costs would change if FITNET-NHS was delivered by Band 6 clinicians.
-
Valuation set: the impact of using an alternative value set was explored given there is no UK-value set. The proxy EQ-5D-Y value set derived for the Spanish population was applied. 70
General practitioner data extraction
We initially intended to visit GP surgeries and extract data on the number of GP visits, referrals and tests. 34 However, conversations with EMIS (Egton Medical Information System) Health suggested that we could obtain all of these data reliably through them. EMIS Health provides electronic patient record systems and software for the majority of general practices for FITNET-NHS participants. Key primary care resource use categories extracted for the health economic analysis included: consultations, medications and tests.
Negotiations with EMIS Health started in the summer of 2019 and continued until the Spring of 2022. Data extraction was planned in February 2020 but on 14 April 2022, EMIS informed us that data extraction was not going to be possible before this report was written. Working with EMIS was therefore abandoned. For a full description of the process and difficulties experienced, please see Appendix 2.
Qualitative methods
Qualitative methods inspired by those used in the QuinteT Recruitment Intervention to optimise RCT recruitment71 were integrated into the pilot and main phase of the trial to explore trial conduct and acceptability of the recruitment process and interventions through analysis of recruitment to trial consultations and in-depth interviews with recruiters, trial therapists and participants (adolescents and their parents). Findings were fed back to the Trial Management Group (TMG) with suggestions to change aspects of the design, conduct, and organisation of the trial to optimise recruitment particularly during the pilot phase and beyond as appropriate.
Families interested in taking part in the trial were contacted by a research nurse by telephone to briefly introduce the trial. They were e-mailed the PILs and later followed up with a second in-depth call (telephone/videocall options offered) to conduct a full eligibility assessment, discuss the trial in further depth and answer questions. We aimed to audio-record (with consent) all the second in-depth recruitment consultations in the pilot phase, and continue to record as needed in the main trial, to identify any areas for improving informed consent processes to optimise recruitment. 71,72 A member of the research team (RP) analysed the majority (69/89) of recordings in the pilot phase of the trial (November 2016 to October 2017). This was followed by smaller samples at 3 further time points in the main trial: (1) to ensure adherence to training (February 2018); (2) when a new recruiter joined the trial (May 2019); and (3) when recruitment slowed (October–December 2019). Information provision by the recruiters, recruitment techniques, patient intervention preferences, and trial participation decisions in particular were scrutinised in the recordings. Findings were presented to the TMG and actions were taken to address identified recruitment problems such as training recruiters.
Trial staff (recruiters and therapists) for both treatment arms were interviewed during the pilot and early phase of the main trial to ascertain their views on: recruiting into the trial (eligibility criteria, decisions, recruitment pathway), provision of trial information, treatment preferences, the feasibility of delivering the intervention to adolescents, treatment effectiveness and potential changes needed to trial processes and interventions. We had a limited pool of therapists delivering the intervention and aimed to interview them all. Trial staff were interviewed in person and participants were interviewed via videoconference (Skype) or telephone.
We undertook one off in-depth interviews with participants and their parents to understand their experiences and views of trial processes, provision and acceptability of patient information, reasons for accepting or declining participation, treatment preferences and acceptability of both the content and delivery of treatments. The majority of families were interviewed in the pilot and early phase of the main trial. A few families that withdrew were interviewed later in the trial. Participants were purposively selected for maximum variation (intervention, age and gender). 73 Families were given a choice of being interviewed over videocall (Skype) or telephone, together or alone.
Interviews followed a checklist of topics to ensure that key areas described above were explored but was sufficiently flexible to allow new issues of importance to participants to emerge (Report Supplementary Material 3 gives examples of the interview topics). Both the recruitment consultations and interviews were audio-recorded with consent using encryption software, transcribed verbatim and anonymised.
Transcriptions were prepared by a professional service (Bristol Transcription and Translation Services Limited, Bristol, UK). The data were imported into NVivo (QSR International, Warrington, UK) to provide a visible audit trail of the data analysis. A reflective journal was kept in NVivo to note down any emerging findings and initial observations of differences between subgroups. These were then explored moving through the whole data set, searching within and between participant groups, and revised based on supportive or disconfirming evidence in the data, facilitating a robust analysis. We ensured quotes were selected and presented to ensure they represented a range of participants.
A proportion of transcripts were double-coded: 10% of recruitment consultations, 100% therapist interviews, 32% of participant interviews, and compared in order to improve the trustworthiness of the analysis. 74 Any discrepancies were identified and discussed with reference to the raw data. Qualitative findings of issues arising and potential solutions were discussed and agreed with the TMG to improve aspects of the conduct of the trial, provision of patient information and training of recruiters.
Qualitative data analysis
Qualitative data analysis was an ongoing and iterative process commencing soon after data collection to inform further data collection. 75 Audio recordings were transcribed verbatim in full or part, checked for accuracy and imported into NVivo to aid data organisation and analysis.
Only relevant sections of recruitment consultations were transcribed verbatim: where the recruiter described the trial, treatments, randomisation and explored patient preferences. Later in the trial, calls were listened to, to identify examples of good practice, areas for improvement and any new findings. Only quotes to illustrate findings for training were transcribed verbatim. Participant interviews were transcribed in entirety.
The data were systematically assigned codes and analysed thematically using techniques of constant comparison. 76 The data were examined for patterns and themes incorporating a mixture of deductive and inductive coding, to enable development of both anticipated (e.g. themes around equipoise) and emergent themes (specific to the FITNET-NHS trial) as interviews progressed. Transcripts were read line-by-line for content and meaning, and a provisional coding framework developed, with new codes added and existing codes merged or split. Through this process, broader categories and higher-level recurring themes were developed.
What and how trial information was conveyed by recruiters was a particular focus of the analysis such as: equipoise, language use (e.g. avoiding terms such as ‘standard vs. experimental treatment’), use of open questions, explaining randomisation, checking patient understanding and exploring patient preferences. Participant responses and any misconceptions about the trial and treatments as well as patient preferences were explored. Examples of difficult communication (e.g. participant confusion) were studied in detail to identify patterns relating to the success or failure of conveying trial information. The findings were used to help improve informed consent and trial recruitment.
Anticipated areas for exploration based on the topic guide formed the initial coding framework and included: reasons for participating in the trial, acceptability of patient information and the recruitment process, acceptability of treatment and perceived treatment effectiveness. Inductive coding was subsequently undertaken to construct subthemes and expand the coding framework, identifying themes around benefits and disadvantages of online treatment, facilitators and barriers to treatment, suggested practical changes to the trial and treatments. The data were compared between subgroups (age, gender, treatment arms) to explore any differences. Interviews with participants continued until data saturation was reached, where new interviews produced little or no change in themes in the data. 77
Trial governance
The trial was supported throughout by The Trial Steering Committee (TSC) and the Data Safety and Monitoring Committee and the TMG. Appendix 3 provides details of these committees.
Further award information is available from the National Institute for Health and Care Research Journals Library website including all versions of the study protocol:
www.journalslibrary.nihr.ac.uk/programmes/hta/14192109#/
The trial was registered in the ISRCTN registry (number: 18020851) and the International Registered Report Identifier (IRRID): RR2-10.1186/s13063-018-2500-3.
Chapter 3 Results – internal pilot
Internal pilot results
Full details of the within-pilot phase results are presented in our publication78 (see Publications). A brief summary of the main within-pilot results is presented below.
The outcome for the pilot phase was the viability to continue to full trial, based on stop/go criteria agreed with the TSC prior to commencing recruitment. The criteria for not proceeding to full trial were:
-
if the recruitment rate was substantially below target (less than an average of 15 adolescents per month) during the last 6 months of the internal pilot study (allowing for seasonal variation) AND if the qualitative data collected suggest that these rates could not be improved by changing recruitment methods, OR
-
the qualitative data suggest the interventions are not acceptable to adolescents and/or their parents.
A total of 89 out of 150 (59% of potentially eligible referrals) young people and their parents were recruited, with 75 out of 89 (84%) providing 6-month outcome data.
Qualitative interviews found that overall, recruitment, consent and randomisation processes were acceptable to participants and their parents. Some issues with recruitment were identified and addressed. Remote treatment was acceptable; however, participants and clinicians described both advantages and disadvantages of remote methods with some families preferring to travel for face-to-face treatment. No serious adverse events were reported.
While the recruitment rates in the within-pilot phase were lower than initial projections (see Appendix 4, Figure 7 for pilot phase recruitment graph), consultation with the TSC, Data Safety and Monitoring Committee, TMG, the funder and the Sponsor confirmed that the stop criteria had not been met and the study should proceed to full trial. The TSC made the following specific recommendation on 26 October 2017:
After a period where the referral rate was very high, communication about the trial was reduced. This coincided with the summer months and this unfortunately led to cumulative recruitment falling below target, to 68% by July. (It has been suggested that ME/CFS treatment is seasonal.) The TSC were not unduly concerned about the fall in the recruitment rate and recognised that [the] FITNET [-NHS trial] has recruited faster than any other trial in this area. Qualitative data have been collected which suggest that recruitment can be improved and they also indicate that the interventions are acceptable to participants. As such the STOP criteria are not met and our recommendation is that the study should continue. We will of course wish to monitor the situation closely.
The TSC have also received communication from the DMSC who are also satisfied with the current state of the trial.
The TSC is referring to the seasonal pattern of referrals to ME/CFS services, rising in the winter and falling in the summer. In November 2017, the pilot study continued into the full trial.
Qualitative findings: optimising recruitment and informed consent
Of the 351 total recruitment consultations (November 2016 to November 2020), 316 were audio-recorded and 107 were analysed (November 2016 to December 2019). Only seven patients declined to have the call recorded. The majority (n = 69) were analysed in the pilot phase (November 2016–October 2017) to identify potential problems early on and reduce any recruitment difficulties moving into the full trial. Further recruitment consultations were analysed as the trial progressed: 17 to ensure training was being adhered to (February 2018); 12 when a new recruiter joined the trial (May 2019), and nine when recruitment slowed (October–December 2019). Recruitment consultations were reviewed for five different recruiters. Seven training sessions were undertaken with recruiters quarterly to address identified recruitment issues; four in the pilot phase and three subsequently to review progress and train a new recruiter.
Ten interviews were undertaken with trial staff in person on hospital premises (April 2017–November 2017): two recruiters, four Activity Management therapists and four FITNET-NHS therapists. Only one health professional invited did not respond to the invitation to participate. Interviews were mainly undertaken during the pilot phase but did extend into the main trial. Interviews were undertaken with 32 families (February 2017–July 2019): 17 in the FITNET-NHS, group, 15 in Activity Management. These included 26 participants (15 females and 11 males, aged 12–17 years) and 34 parents (30 mothers and 4 fathers; 2 interviews included both parents). Eight families declined to be interviewed as the young person was too ill (n = 1), they wanted to focus on school (n = 3), their treatment had been delayed (n = 1), or they did not want to take part (n = 3). Five families cancelled their interview, and we were unable to reschedule. Families were given the choice of being interviewed alone or together. The majority (n = 22) were interviewed together, four separately and six were with the mother only. Seventeen families chose to be interviewed via videocall and 15 via telephone.
Participating families had often struggled to gain a ME/CFS diagnosis and reported a lack of specialist treatment and support. Some received ‘generic’ advice on managing activity, medication (e.g. citalopram, pain killers) or counselling within the NHS: from GPs, paediatricians, physiotherapists and CAMHS. Others had tried alternative approaches: the Lightning Process, osteopathy, hydrotherapy, reflexology, oxygen therapy, homeopathy, herbalism, food intolerances, vitamins, and self-management of activity. General advice on managing activity helped participants develop coping strategies, manage and build up energy and activity to a moderate level. Counselling helped them come to terms with the illness, think positively and build confidence. However, most families reported a lack of access to treatment and more often reported negative experiences from previous treatment including: no improvement, being wrongly labelling with mood problems, relapsing and plateauing.
M1000019:…the paediatrician wasn’t really offering her any options other than pacing and taking time off school.
M1000128:…well there’s very little helpful out there so we’ve done things like check (child) for food intolerances. We did an anti-candida diet. He does – he sees a cranial osteopath. We’ve tried food supplements. We’ve tried all sorts of things to try and help him with his sleep. He gets really bad abdominal pain and we’ve tried lots of pain killers specifically for that region but he’s quite sensitive to medicine so nothing’s really helped there.
M1000072:You know, ups and downs, but we’d kind of learnt enough now to get her better and keep her moderately well, but we didn’t get any better.
Participants were positive about being offered an opportunity to take part in the trial as there was most commonly ‘absolutely no treatment’ available and they were ‘willing to try anything’. They encountered funding cuts, long waiting lists and great distances to travel for specialist services. Families described the trial as a ‘lifeline’, participants felt ‘lucky’ and ‘excited’ and parents were ‘thrilled’ and ‘hopeful’. Taking part in research to help participants with ME/CFS in the future was also seen as a benefit. Participants and their parents liked the idea of online treatment as travelling and meeting new people in hospital environments with bright lights can exacerbate symptoms. A few families were ‘sceptical’ about online treatment and participants worried about how much work the treatment would involve, possibly making them ‘feel even more tired’ (C1000029).
M1000029:I think because (child) has had absolutely no treatment at all or help really from anywhere, she saw this as an opportunity so she was going to take it whichever she was given.
C1000002:I liked the idea of it all being online, I think going to the appointments of different things can be difficult. Whereas this I can just do whenever I fancy.
M1000120:…if this trial can’t make her better but in five years’ time it can make another teenager better then – then you know, why would, you know, why would you not do it?
Interview data suggested that trial information leaflets, recruitment/consent and randomisation processes were acceptable to participants and their parents. The written PILs were found to be ‘clear’, ‘helpful’ and ‘comprehensive’. Families wanted to know how long the treatment would last which was not clear early in the trial. Some families felt there was too much information and ‘quite a lot of reading’ (M1000093), meaning it was difficult to get the young person to concentrate. Shorter leaflets or links to a website with more images were suggested as an additional source of information, particularly for younger adolescents. The FITNET-NHS website79 and its frequently asked questions (FAQ) section was developed to help this.
C1000107:Yeah, I understood everything that was on the leaflets, it made sense.
M1000093:… there was quite a lot of reading, but that was good. I mean I don’t mind that. It was, it was quite difficult to get (child) to sit and concentrate because there was stuff for him to read as well I seem to recall.
Analysis of recruitment consultations highlighted that some participants had misconceptions about what was offered in both treatments. ‘Activity Management’ was perceived to only focus on physical activity, whilst the ‘FITNET’ group was thought to only involve CBT, ‘the CBT arm of it and the physical arm of it?’ (M100131). In fact, they both give advice on: sleep, building up activity and addressing individual goals. Review of the written patient information showed that it did not fully explain the similarities and subtle differences between the treatments and recruiters were also failing to explain this on recruitment calls. The PILs and the FITNET-NHS website including the FAQs were updated, as well as training recruiters to provide more balanced information about the trial treatments. Following this change, participants had a more balanced view of the treatments:
M1000025:I thought that the treatment [FITNET] was involving only cognitive therapy while it does involve also the exercise programme, which in our case is a benefit for (child).
Families were particularly happy with telephone recruitment and described the research nurses as ‘positive’, ‘understanding’, ‘empathic’, and ‘helpful’, allowing them several opportunities to ask questions and time to decide whether to take part. Most participants accepted randomisation as part of the research process and understood the need for a ‘fair’ comparison. Although some families expressed a treatment preference, they were often willing to try anything as ‘anything’s better than nothing’ (M1000041). However, some would have liked to choose their treatment as parents felt they knew what would benefit their own child and felt preference should be taken into account. A few families did not seem to understand randomisation, which was fed back to research nurse training for recruitment calls. Participants preferred remote consenting and data collection as it was ‘easy’ and there was no need to post paper forms.
M1000093:I think we understood that there were two different elements to the trial and it would be a random choice as to which one we got. We had our initial discussion with one of the members of the team who was lovely and very informative and gave (child) enough time. I think we spoke once and then she phoned back and then we spoke again, so (child) managed to get a proper conversation in. So, no, I feel like it, it was very well prepared and laid out.
M1000020:But I think if you’ve got a child that has got a preference for that, then that might be something if that could be taken into account.
Some families expressed a preference for one of the trial treatment arms in recruitment consultations. In the pilot phase of the trial, parents often preferred FITNET-NHS as they had seen it publicised on the news and were aware of the previous trial ‘in Holland with some good results’ (M1000029). Some families had tried Activity Management before with little improvement and saw FITNET-NHS as a ‘new’ treatment. Some participants liked the idea of online treatment allowing them flexibility to complete it, ‘whenever I fancy’ (C1000002). Parents, particularly those who felt their child had mood issues, felt CBT would be useful to provide them with ‘mental strategies’ as they had become low in mood as a result of the illness. However, other families were worried about the amount of reading that FITNET-NHS involved, ‘The Activity Management sounds like it’s not as intensive’ (D1000077). A few parents were concerned that FITNET-NHS indicated that their child’s ME/CFS is a psychological rather than physical condition, and had the impression that the FITNET-NHS group was more appropriate for those with mood problems.
C1000002:…if I was on the other one [Activity Management], it might have been more repetition of what I’ve already done. Whereas, what I’m doing now is I would say more interesting and new to me.
M1000107:…she [child] definitely does suffer with anxiety and I think that makes her seem worse. So, I kind of think the two things are connected anyway, sort of your physical and your mental stage. So, I think perhaps I’d thought that that approach [FITNET-NHS] might be good for her.
M1000039:It worries me that it [FITNET-NHS] makes it out that it’s more of a psychological condition when you know, obviously if you’re seeing it first hand and I know hand physically it affects the body.
Families that preferred Activity Management felt it would address activity more than FITNET-NHS, the participant was used to using Skype and they would receive more face-to-face therapist contact. However, some parents were not familiar with Skype and a few participants favoured the idea of e-consultations as they preferred not to talk to anyone.
C1000076:Well to be honest I feel like the activity management would be more relevant to me because I feel like that’s what I need to sort out at the moment …I should becoming active and doing it.
C1000029:Because I thought that with it being on Skype it would be more personal and easy to talk about, if you know what I mean.
Early in the pilot phase, recruitment nurses usually did not ask about patient preferences, asked closed questions about preference and/or did not explore why participants had a preference. Exploring patient preferences is important to elicit participants’ understanding of treatments so that any misconceptions about the trial and/or treatments can be corrected in order for the family to consider the trial based on full and balanced information. 80,81
PARENT 1000029:I thought that the Skype one would be more personal. I don’t know
RECRUITER:Yep, yeah, I think that’s a very good point. Yep absolutely. Ok, ok, but you understand that [child] will have an equal chance of being allocated to either group?
PARENT 1000029:Yes absolutely.
Review of the recruitment consultations highlighted that recruiters were less familiar and comfortable with online treatment and often implied that face-to-face treatment offered as standard outside of the trial was better. The two main recruiters were both nurses by background and they were more familiar and comfortable with face-to-face treatment due to their own experiences.
Recruiter HP70001: I think it might. For me, my experience of hospital appointments are face-to-face in a clinic and I’m not used to having Skype appointments, for example, or treatment over the internet. It doesn’t feel what I’m used to, what I’ve grown up with and bearing in mind a lot of the parents that we’re talking to are the same age as me, from my generation, they will be the same. They haven’t grown up with IT, with computers and Skype and all that and so I think most parents will be expecting to be seen in a clinic face-to-face with somebody.
Recruiters were also referring to Activity Management as ‘our standard’ treatment and the FITNET-NHS arms as ‘this CBT type treatment’. Recruiters also revealed that they felt they knew more about the Activity Management group of the trial than the FITNET-NHS group and this affected how they explained it to patients during recruitment calls. They were unsure how to explain how CBT can work in practice during the recruitment discussions with patients.
Recruiter:…we’re trialling is a CBT type treatment which is delivered over the internet and comparing that with our standard activity management treatment which we would give over Skype and seeing whether one is better than the other.
Recruiter HP70001:Yes I can explain quite a lot about that [Activity Management] if anybody asks…but the FITNET was completely different.
Recruiters were encouraged to use open questions to elicit patient concerns or preferences and explore the reasons for preferences: for example ‘What were your thoughts when you first heard about the study/treatments?’ This was then followed by specific questions to understand reasons for preference so that balancing information could be offered to improve informed consent. Following training, research nurses explored patient preferences and corrected any misconceptions patients had about the trial or treatments:
RECRUITER:… why do you think that [Activity Management group] sounds better than the other treatment?
C1000043:Umm, because it’s more individually face to face, well as face to face as it can be over the internet, that’s sort of good.
REC:…With the other group, with the CBT, you will have an individual clinician delivering your treatment, delivering your care and you will have a lot of communication with that individual so you will have an individual relationship with the clinician giving the FITNET treatment as well.
The bias for face-to-face treatment was addressed in recruitment training. Recruiters were provided with examples of bias from the audio-recorded recruitment consultations. The potential benefits of online treatment for ME/CFS patients were discussed. Recruiters fed back that they valued the training and they often asked for individual feedback on specific consultations they felt had been ‘tricky’. Recruiters expressed a more balanced view of the treatments following training. Recruiters were also advised to avoid terms such as ‘standard’ treatment in the recruitment discussions, as it could imply ‘tried and tested’ or ‘safer’ of the two options. They were also familiarised in more detail on what both treatment arms involved. They were given access to a dummy FITNET-NHS platform and/or a PDF of the platform content to read through. A document of FITNET-NHS and Activity Management treatment chapter headings and content was also produced to enable recruitment nurses to go through these on recruitment calls with patients.
In November 2019, the qualitative reasons families gave to therapists or trial staff for withdrawing from the trial were analysed using thematic analysis for 35 patients. 76 Coding was undertaken by two researchers and compared and any disagreements discussed and resolved. The analysis resulted in a comprehensive and representative set of ‘withdrawal codes’ and the existing codes on REDCap were amended to reflect this allowing the accurate quantitative capture of withdrawal reasons (see Table 6).
| Original REDCap codes (Jan 2020) | RP code (qual analysis) | New REDCap code |
|---|---|---|
| Not confident in treatment approach | Not confident in treatment approach | |
| School (GCSEs, A-levels) | Competing interest: education | Education priority |
| Trial burden | Trial burden (does not want to complete questionnaires) | |
| Treatment does not suit them | Treatment burden (too much work) | |
| Does not like online approach | Does not like online approach | |
| Getting better | Recovering (allocated group) | |
| Getting better – alternative treatment | Recovering (alternative treatment) | |
| Getting better (non-starter) | Recovering (non-starter) | |
| Child too severe | Child severely affected | |
| Not recovering in allocated group | No improvement | Not recovering |
| Deteriorating in allocated group | Patient deteriorating/flare ups | Deteriorating/flare ups |
| Received treatment (not FITNET/Activity Management) outside of the trial | Alternative treatment | Received treatment (not FITNET/Activity Management) outside of the trial |
| Mental health | Mental health priority | |
| External events (holidays) | External events (holidays) | |
| Does not want any further clinical treatment with the service | Does not want any further clinical treatment with the service | |
| Preference for other group | Preference for other group | |
| Negative information about FITNET | Negative information about FITNET | |
| Reason unclear | Unclear | |
| Other (please provide details) | ||
| *Hidden on REDCap | *Yellow remain the same | *New codes added |
Chapter 4 Protocol changes
Changes to trial treatments based on qualitative data (pilot phase)
Full details of the qualitative results from the pilot phase and changes made to treatments as a result are presented in our publication78 (see Publications). The key changes are also presented below.
FITNET-NHS
In response to feedback refinements were made to the FITNET-NHS platform to improve user experience. For example, we introduced pop-up messages to warn participants prior to the automatic time-out function activating when they were writing long messages. We also introduced avatars that young people could chose to personalise the platform.
Some young people were not sure how to complete the diaries and found them difficult therefore written guidance for the diaries was integrated into the platform. Data entry problems such as the inability to enter zero for no school attendance were also addressed. Changes to wording on the platform were made to clarify meaning.
M1000117:No, it timed out after 30 minutes, so I would have loads of times where I was writing, and then I’d go to send it and I wasn’t aware that it was timing out and I’d lose the lot.
M1000009:Oh, that was really complicated setting out how to do the diary, wasn’t it, to start with?
M1000002:If there had been an online tutorial, even a couple of screen-shots just saying “this is what this [diary] screen will look like, this is what this screen will look like, it is the difference between what your son will see and what you will see. These are the different tabs you’ll have access to”, that would have been extremely helpful.
M1000041:Only when he doesn’t do school attendance you can’t put a zero in [the diary] for saying like no hours on school attendance when they’re meant to be there.
Activity Management
The qualitative interviews with both families and health professionals indicated that most felt that three videocall appointments were not enough. Clinicians felt that the two treatment arms were unequal as Activity Management only included three videocalls and FITNET-NHS included up to 28 e-consultations. More importantly, patients outside the trial were receiving, on average, six clinical appointments for Activity Management when the study was being conducted (an increase from when the trial was designed).
I do worry a little bit about how equal the two arms are. It does feel like people do FITNET-NHS for good or ill really, have a lot more to do...the activity management arm is three Skype sessions...But it feels like they don’t feel comparable in terms of therapist input, which can be a factor in itself in terms of outcomes I would imagine.
Therapist 70004
M1000007:I feel overall, my experience as a parent has been... 75% has been giving information, and 25% is intervention, so it’s been a lot of information giving, a lot of questions.
In response, the Activity Management group was changed to allow up to six sessions [submitted as a first substantial amendment (SA1), gaining ethical approval in October 2017 – see Appendix 5]. The first videocall appointment was intended to be 90 minutes for therapists to undertake a clinical assessment and explain and start treatment. However, this was found to be too long by both families and therapists and was therefore split into two sessions. Therapists found this helpful as participants weren’t as tired and the shorter follow-up appointment enabled them to check if the family had understood the treatment concepts. The recruitment and treatment process was streamlined to ensure the initial assessment by recruiting research nurses was passed onto therapists rather than being repeated. Information about Activity Management as well as paper diaries were sent to participants in advance of their first appointment. This was felt to speed up treatment, and one therapist felt it should be carried over from the trial into usual care.
HP70005:You know, so for example people are being sent out activity management programmes and explanations of how to fill it in….before their first session. So you still have the odd person who is still not quite sure, haven’t quite got it and so the first session is very much about talking about that concept. But those which have been able to read it and understand fully and write the information down, then we are starting from a running point as opposed to wasting time really. So I think that could be transferred into general practice.
HP70005:It’s just that the 90 minutes is a bit much. So yes, it is. The 30 minutes I tended to use as, making sure that they have got the concepts. And if they have got the concept, which some of them have, I’ve been able to progress things on a little bit.
Allowing analysis of therapeutic e-consultations within the FITNET-NHS platform
The written format of the individualised therapeutic sessions within the FITNET-NHS platform, provided rich data which we decided to analyse. We submitted a request to enable analysis of the FITNET-NHS e-consultations in the first substantial amendment (SA1), and changed the consent forms for newly recruited participants to give consent for this. This amendment was given a favourable opinion on the 23 October 2017 (see Appendix 5).
A subproject was subsequently set up to explore whether the linguistic content of participants’ e-messages could identify those with comorbid mood disorders82 (see Publications).
Changes to recruitment methods
In response to slower than anticipated recruitment (see our pilot paper),78 we worked directly with some large GP practises to conduct searches of their record. We set-up GP surgeries as PICs (see Appendix 5), and for a patient-facing leaflet/poster to be displayed in GP surgeries. This second substantial amendment (SA2) gained full Research Ethics Committee (REC) and Health Research Authority (HRA) approval on 9 August 2018 (see Appendix 5). The database searches for ME/CFS yielded few patients. On advice, we broadened the search criteria to include ‘tired all of the time’ and checked the diagnosis of ME/CFS after identification as in the protocol [see third substantial amendment (SA3) – see Appendix 5].
Sample size
In late 2018, it became clear that the recruitment rate would not allow us to achieve our recruitment target set around the secondary aims of testing the effectiveness and cost-effectiveness in adolescents with ME/CFS and comorbid mood disorders (which assumed a 30% prevalence of comorbid mood disorders). We therefore: consulted with the TSC (28 November 2018), Data Safety and Monitoring Committee (10 October 2018 and by e-mail report on 11 March 2019) and TMG (18 October 2018) and reviewed the trial with the funders (HTA) (10 July 2018). The primary aim for the full FITNET-NHS trial was the effectiveness of FITNET-NHS in adolescents with ME/CFS. The trial was originally powered on the subgroup analysis (secondary outcome) to test the effectiveness and cost effectiveness of FITNET-NHS in adolescents with ME/CFS and comorbid mood disorders. This meant that to achieve 80% power to detect a 0.4 SD difference at 5% significance, assuming 30% of participants had a comorbid mood disorder, we needed to randomise 734 participants. This then provided 97% power at 1% significance to detect 0.35 SD difference on our primary outcome.
In September 2018, we calculated the sample size required for the primary outcome/aim of assessing the effectiveness of FITNET-NHS in adolescents with ME/CFS as follows: Data on 266 participants would give us 90% power at 5% significance to detect a 0.4 SD difference on the SF-36-PFS. With attrition set at 15% (a realistic target according to follow-up rates achieved at the time), we needed to recruit 314 participants. This was assessed as achievable (based on recruitment rates to date) by November 2020.
Hence, the revised sample size target of 314 participants in total would give conclusive evidence to address the primary hypothesis of the FITNET-NHS trial.
After consultation with the HTA, TSC and Data Safety and Monitoring Committee, the decision was made by the TMG, on 30 January 2019 to adjust the recruitment targets and extend the recruitment time by 6 months so that the trial was able to achieve the primary aim of testing the effectiveness and cost-effectiveness of FITNET-NHS compared to Activity Management. We, therefore, aimed to recruit 314 participants by November 2020 with follow-up finishing a year later.
We followed standard procedure for updating HTA, REC and trial registration (ISRCTN) regarding these changes. HTA approval for the revised recruitment target and the contract variation to include a 6-month extension to the project timeline was received on 24 April 2019 further subjected to ratification by the Department of Health and Social Care. See Appendix 5 for details.
These changes were published as an amendment to the published trial protocol33 (see Publications).
COVID-19-pandemic-related changes to the trial
The COVID-19 pandemic caused disruption to many research processes and clinical services from March 2020. Because FITNET-NHS was set up to recruit participants via entirely remote (telephone and online) methods and online delivery of treatment and research data collection, the trial was able to continue throughout the pandemic. Some changes were made to treatment pathways at the Bath ME/CFS service as detailed below:
Prior to the pandemic, adolescents with ME/CFS in the Bath/Bristol region were not eligible to access the FITNET-NHS trial because they had access to a specialist local service. The service was provided with a face-to-face assessment. Follow-ups were either face to face or using video (Skype). In addition, participants who withdrew from FITNET-NHS treatment, and those who had finished treatment but wanted further treatment, were offered face-to-face appointments to review the clinical diagnosis and consider treatment options.
In March 2020, the Royal United Hospital foundation trust moved all clinics to virtual clinics and face-to-face clinic appointments were suddenly no longer available for our regional patients. Neither were they available for FITNET-NHS participants who had previously been reviewed face-to-face.
In May 2020, the service reviewed the care pathways to ensure video assessment of patients referred to the service was as safe as possible. A risk assessment was undertaken and a care pathway was created to describe the flow of patients in different parts of the service.
Initially we assumed that face-to-face clinics would resume and the regional patients would be seen however, it became increasingly clear that face-to-face assessments would not resume for some time. Patients in the Bath regional service were therefore unable to access treatment and were not being seen. They therefore became eligible for FITNET-NHS and were offered recruitment to the trial.
From May 2020 the service (in relation to FITNET-NHS) operated as follows:
Those who withdrew from treatment and those who had completed treatment but wanted further treatment were considered to be safe to have a video medical assessment online. This is because they already had an assessment and had a local paediatrician. There were risks associated with this; therefore, patients went through safety triage for video assessment.
GP surgeries and CRNs across the country halted much research activity in order to reserve resources for managing the COVID-19 pandemic. This meant that the PIC site mailouts and new PIC sites planned from March 2020 onwards were cancelled. We sent messages to all the CRNs and GPs across the country which we were in touch with to publicise that the trial was continuing and would be accepting opportunistic referrals. The FITNET-NHS website was also updated with this message.
Chapter 5 Clinical findings
Participant recruitment and flow through the trial
Recruitment and retention
Figure 1 describes the patient flow. 892 out-of-area patients were referred to the Bath Royal United Hospital ME/CFS services between 1 November 2016 and 31 October 2020, of which 550 were eligible to take part. Of these, 314 (57% of eligible) patients (and their parents) were recruited into the trial; 155 were randomised to FITNET-NHS and 159 to Activity Management treatment, meeting the revised recruitment target agreed with the funders (see Appendix 4, Figure 8). Retention was good and 265 (84% of participants) were included in the primary analysis (127 for FITNET-NHS and 138 for Activity Management). Follow-up was concluded on 11 November 2021.
FIGURE 1.
Consolidated Standards of Reporting Trials participant flow diagram. a, Treatment withdrawals between the completion of baseline, 6-month and 12-month assessments, substituted with time point since random allocation if an assessment was not completed.
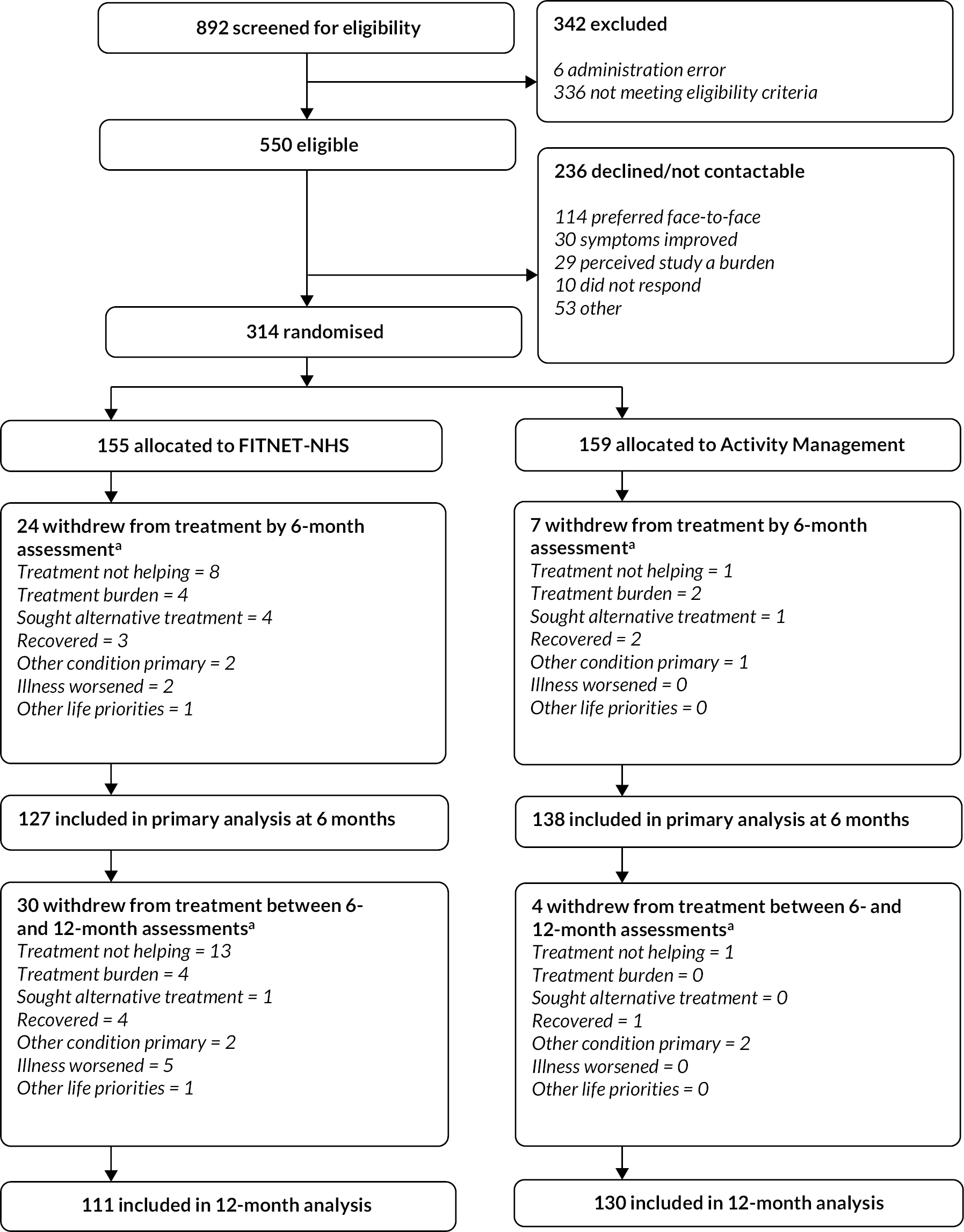
Exclusions and declines
Of the 892 referrals, 342 (38%) were excluded at eligibility assessment because: 54 did not meet the age criteria (were either < 11 or > 17 years of age); 47 had access to a local specialist service; 113 did not have a confirmed ME/CFS diagnosis (of whom 92 were patients referred to for face-to-face assessment to confirm ME/CFS diagnosis); 25 had not had the diagnostic blood tests necessary to rule out other causes of fatigue (5 had needle phobia and 20 were unresponsive to requests by the research nurses to arrange blood tests); 6 were not disabled by their fatigue; 10 were unable to complete online modules due to learning difficulties or unable to attend to video calls due to poor internet connection or had no access to computers; 52 had a range of reasons (including: already completed treatment in trial, did not have a clear confirmed diagnosis of ME/CFS, significant mental health issues requiring face-to-face assessment).
Within the initial period after trial launch, six potentially eligible patients were incorrectly excluded (by the clinical team) before reaching eligibility assessment by a research nurse. These patients were offered face-to-face clinical treatment as for the normal treatment pathway (outside of the trial). On discovering this, the clinical team was offered extra training, and standard operating procedures for the administrative handling of out-of-area referrals were improved, which ensured no further incorrect exclusions. A total of 29 patients were referred by GPs in Wales, Scotland or Ireland where treatment funding arrangements (between the Welsh Health Boards and the Bath Royal United Hospital) prevented these patients from entering the trial. After these exclusions, a total of 550 potentially eligible referrals remained.
Of the 550 potentially eligible patients, 236 (42.9%) declined to participate. The main reason was that they wanted to be seen face-to-face, with 114 (20.7% of those eligible) preferring to travel to the hospital to be seen face-to-face instead of taking part in the trial. Some of these patients travelled because they were unable to assess general paediatric services to get a diagnosis of ME/CFS but the majority wanted to travel because they did not wish to receive online treatment. 30 out of 550 (5.5%) patients declined because of symptom improvement, or because they were receiving treatment from local support groups for significant health issues other than fatigue; 29 declined due to perceived study burden; and 10 were unresponsive to communications about the study (or the child was in boarding school and the parents were uncontactable). The remaining 53 patients declined for a variety of reasons (unwillingness to use videocall such as Skype, unwilling to wait for the local paediatrician to confirm ME/CFS diagnosis, preferred one treatment group over another) or did not provide a reason for declining.
Patient identification centre site activity and recruitment
Five of the 15 national CRNs supported PIC site activity, facilitating 26 GP surgeries to be set up as PIC sites across England before the pandemic halted these activities. Of the 144 letters sent to patients, 18 were to those with a recent ME/CFS or post-viral fatigue entry in their medical records, and 126 were sent to patients with a recent ‘tired all the time’ entry. Six of the enrolled participants were recruited as a result of these (none of whom were identified via ‘tired all the time’ searches).
Baseline data
At the point of random allocation, the two groups were comparable in terms of demographic factors and clinical characteristics (see Table 7). The typical participant was 14 years old, White British, and a little more likely to be female. Participants joined the study about 15 months following illness onset, with about half having a comorbid mood disorder and most having a reduced school timetable. About one-fifth of participants joined the study during the COVID-19 pandemic and associated school closures.
| FITNET-NHS (n = 155) |
Activity Management (n = 159) |
|
|---|---|---|
| Mean age (SD) | 14.3 (1.6) | 14.1 (1.8) |
| Number female (%) | 98 (63.2%) | 100 (62.9%) |
| Number white/British/English ethnicitya (%) | 142 (91.6%), 150 | 144 (90.6%), 151 |
| Median months since illness onset (25th, 75th percentiles) | 16 (9, 30) | 18 (11, 30) |
| Number comorbid anxietyb (%) | 15 (9.7%) | 19 (11.9%) |
| Number comorbid depressionb (%) | 69 (44.5%) | 76 (47.8%) |
| Number recruited during school closurec (%) | 29 (18.7%) | 33 (20.8%) |
| School attendance in the previous week | ||
| None | 35 (22.6%) | 37 (23.3%) |
| About 10% (e.g. 1 half-day) | 12 (7.7%) | 12 (7.5%) |
| About 20% (e.g. 1 day) | 14 (9.0%) | 12 (7.5%) |
| About 40% (e.g. 2 days) | 17 (11.0%) | 18 (11.3%) |
| About 60% (e.g. 3 days) | 24 (15.5%) | 25 (15.7%) |
| About 80% (e.g. 4 days) | 29 (18.7%) | 28 (17.6%) |
| Full-time (100%) | 13 (8.4%) | 14 (8.8%) |
| Not applicable (N/A) | 6 (3.9%) | 5 (3.1%) |
| Not answered | 5 (3.2%) | 8 (5.0%) |
Adherence
The vast majority of participants started their allocated treatment (see Table 8), although for 14% of the FITNET-NHS group and 26% of the Activity Management group, treatment was not initiated until after 3 months following random allocation. Relatively few FITNET-NHS group participants were considered by their therapist as having completed 80% or more of the expected activities for their allocated intervention (Report Supplementary Material 7 presents the adherence assessment completed by therapists). In contrast, a majority of Activity Management group participants were considered to have completed 80% or more of the activities for their allocated intervention.
| FITNET-NHS (n = 155) | Activity Management (n = 159) | |
|---|---|---|
| Number not starting allocated treatment (%) | 3 (1.9%) | 9 (5.7%) |
| Number completing 80% or more of expected modules/sessions of allocated treatment (%) | 58 (37.4%) | 124 (78.0%) |
| Number starting allocated treatment more than 3 months after allocation (%) | 22 (14.2%) | 42 (26.4%) |
Outcomes and estimation
Primary analysis
At recruitment, participants were physically disabled with low mean scores for the SF-36-PFS which was 49.8 (SD 21.9) for FITNET-NHS participants and 47.1 (SD 23.6) for Activity Management participants. There was greater improvement for those allocated to FITNET-NHS at 6 months (mean 60.5, SD 29.5) compared to Activity Management (mean 50.3, SD 26.5). The adjusted mean difference was 8.2 (95% CI 2.7 to 13.6; p = 0.003). The observed magnitude of improvement in the FITNET-NHS group exceeded the minimally clinical important difference of 10 points, although the adjusted difference in means between the two groups was less than 10 points. Individual participant changes on the SF-36-PFS from baseline to 6 months assessments are presented in Appendix 6, Figure 9. Table 9 also presents the results of the sensitivity analyses, all of which indicated the primary analysis results are robust and unchanged to these variations in the conduct of the statistical analysis.
| FITNET-NHS | Activity Management | Difference in means (95% CI) | p-value | |
|---|---|---|---|---|
| Mean (SD), N | Mean (SD), N | |||
| Baseline measurement | 49.8 (21.9), 150 | 47.1 (23.6), 151 | ||
| Primary outcome at 6 months | 60.5 (29.5), 127 | 50.3 (26.5), 138 | 8.2 (2.7 to 13.6) | 0.003 |
| Sensitivity analyses | ||||
| Covariate added: days after randomisation outcome completeda | 60.5 (29.5), 127 | 50.3 (26.5), 138 | 8.6 (3.2 to 14.1) | 0.002 |
| Covariate added: randomised before/after 1st September 2019 | 60.5 (29.5), 127 | 50.3 (26.5), 138 | 8.2 (2.7 to 13.6) | 0.003 |
| 6- or 12-month assessment used according when intervention started | 59.5 (29.6), 131 | 50.6 (27.2), 141 | 7.2 (1.8 to 12.5) | 0.009 |
| Participants included if attending 1 + sessions | 60.9 (29.3), 126 | 50.7 (26.0), 134 | 8.5 (3.1 to 14.0) | 0.002 |
| Post hoc sensitivity analysis new definition ME/CFS (NICE 2021) | 60.5 (29.5), 127 | 50.5 (26.4), 137 | 8.0 (2.5 to 13.4) | 0.004 |
| Subgroup analysis | ||||
| Baseline assessment | ||||
| Comorbid anxiety or depression | 43.8 (20.1), 69 | 42.1 (21.0), 73 | ||
| No comorbid anxiety or depression | 54.8 (22.2), 81 | 51.7 (25.0), 78 | ||
| 6-month assessment | ||||
| Comorbid anxiety or depression | 52.1 (30.3), 55 | 45.3 (25.3), 64 | ||
| No comorbid anxiety or depression | 67.0 (27.3), 72 | 54.5 (27.0), 74 | ||
| Interaction effect b | −4.9 (−15.9 to 6.1) | 0.38 | ||
We conducted two post hoc sensitivity analyses. We repeated the analysis for those participants defined as having ME/CFS using the new NICE definition (2021). One participant was excluded in this analysis because of missing data, and the results supported the same conclusions as the primary analysis (see Table 9). Participants could indicate if a parent had assisted with the completion of the primary outcome measure; a second post hoc sensitivity analysis (see Appendix 6, Table 23) showed participants who were helped by their parents had, on average, poorer physical function at baseline and showed less improvement on average irrespective of their treatment allocation.
The pre-specified subgroup analysis distinguished participants with comorbid anxiety or depression at the baseline assessment from those who did not (see Table 9). The baseline assessment of the SF-36-PFS indicates poorer physical function among those with comorbid anxiety or depression in both allocated groups. At the 6-month assessment of the primary outcome, less of an advantage of FITNET-NHS over Activity Management is observed in the group with comorbid anxiety or depression, but this difference could have arisen by chance (interaction p-value = 0.38).
The primary outcome measure was missing for 28 (18%) participants in the FITNET-NHS group, and 21 (13%) in the Activity Management group. Looking at the baseline measures of SF-36-PFS, those allocated to the FITNET-NHS group and providing the primary outcome at 6 months had a mean baseline score of 50.7 (n = 125), compared to a mean baseline score of 45.0 (n = 25) in participants missing the primary outcome. Among those allocated to the Activity Management group, those providing the primary outcome at 6 months had a mean baseline score of 48.2 (n = 134), compared to a mean baseline score of 38.2 (n = 17) in participants missing the primary outcome.
Figure 2 presents a sensitivity analysis of the potential impact of the missing data on the observed results. Delta on the x-axis looks at a range of assumed mean scores for those missing the primary outcome measure, minus the observed mean score in those for whom the primary outcome was observed (see Table 9). The ascending line assumes that delta describes the difference between the missing and observed primary outcome data in those allocated to the FITNET-NHS group, with missing data occurring at random in the Activity Management group (i.e. delta is fixed at zero for the Activity Management group). These sensitivity analyses indicate that only if those with missing data in the FITNET-NHS group had a mean SF-36-PFS score at 6 months of 40 points lower (poorer functioning) than those with primary outcome data, could the observed treatment benefit of FITNET-NHS over Activity Management be entirely due to bias caused by the missing data. The descending line assumes that that delta describes the difference between the missing and observed primary outcome data in the Activity Management group, with missing data occurring at random in the FITNET-NHS group. If it is considered feasible that those with missing data in the Activity Management group score on average 50 points higher (better functioning) than those providing primary outcome data, then the observed treatment effect may be spurious. These analyses indicate that our finding of a benefit of FITNET-NHS compared to Activity Management at 6 months is robust to all but the strongest biases due to missing data.
FIGURE 2.
Sensitivity analysis for the primary treatment difference. Note: y-axis (thick line = point estimate, vertical line = 95% CI) under different assumptions about the missing data. Delta on the x-axis is assumed mean for missing values – observed mean. The blue line (panel B) is varying delta in the FITNET-NHS group whilst assuming data are MAR (delta = 0) in the Activity Management group, and vice versa for the green line (panel A).
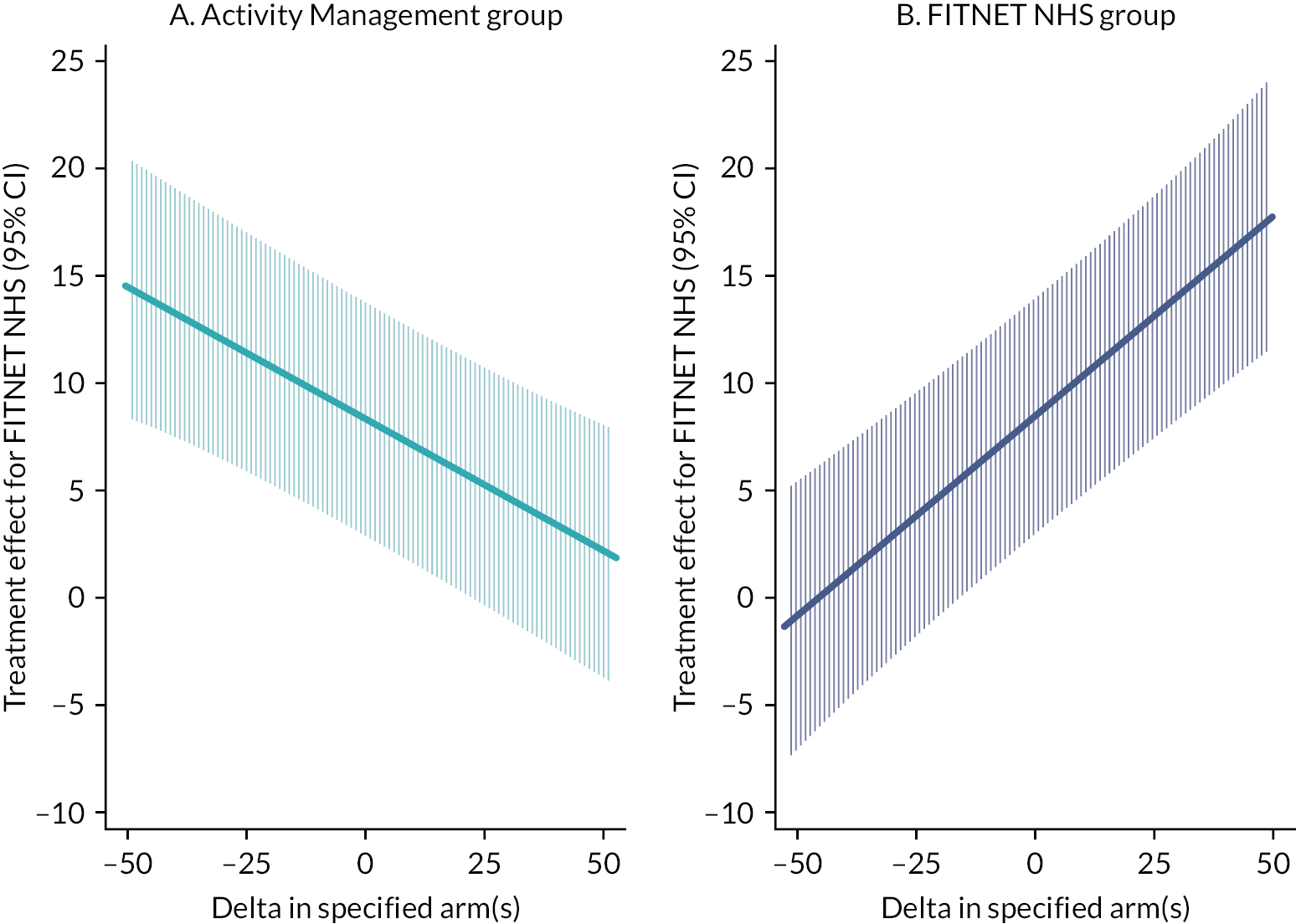
Secondary analyses
Table 10 shows that participants in both arms continued to improve at 12 months. At 12 months, there was no longer evidence of a difference in physical functioning (SF-36-PFS) between FITNET-NHS and Activity Management; this appeared to be due to participants in the Activity Management group catching up much of the difference observed at 6 months.
| FITNET-NHS | Activity Management | Difference in means (95% CI) | p-value | |
|---|---|---|---|---|
| Mean (SD), N | Mean (SD), N | |||
| SF-36 PFS 12 months | 62.9 (29.1), 111 | 57.8 (27.9), 130 | 4.4 (−1.7 to 10.5) | 0.16 |
| Chalder fatigue: score range 0 to 33, high scores = greater fatigue | ||||
| Baseline | 25.4 (4.6), 150 | 24.7 (5.3), 150 | ||
| 6 months | 20.1 (7.7), 118 | 20.0 (7.6), 132 | −0.5 (−2.2 to 1.3) | 0.60 |
| 12 months | 19.3 (8.0), 105 | 19.5 (8.1), 124 | −0.8 (−2.7 to 1.2) | 0.44 |
| CIS subjective fatigue 8-item subscale: score range 8 to 56, high scores = greater fatigue | ||||
| Baseline | 48.8 (6.4) 150 | 47.7 (7.6) 151 | ||
| 6 months | 41.2 (12.6) 77 | 43.7 (9.4) 101 | −3.9 (−6.8 to −1.0) | 0.009 |
| 12 months | 37.6 (13.2) 74 | 40.7 (11.0) 88 | −3.9 (−7.4 to −0.4) | 0.029 |
| Pain VAS: score range from 0 to 100, high scores = greater pain intensity | ||||
| Baseline | 47.5 (28.1), 150 | 49.5 (27.1), 151 | ||
| 6 months | 35.3 (27.9), 81 | 43.8 (26.7), 102 | −5.9 (−12.2 to 0.5) | 0.072 |
| 12 months | 35.2 (29.1), 74 | 37.8 (27.3), 88 | −0.4 (−7.9 to 7.2) | 0.92 |
| School attendance: as a percentage of full-time, high scores = greater school attendance | ||||
| Baseline | 42.6 (34.9), 144 | 42.6 (35.1), 146 | ||
| 6 months | 52.2 (37.4), 116 | 41.8 (36.5), 121 | 12.0 (4.9 to 19.0) | < 0.001 |
| 12 months | 56.7 (38.8), 97 | 46.7 (39.8), 111 | 12.4 (3.3 to 21.5) | 0.008 |
Participants in both groups had less fatigue at both 6 months and 12 months on both the CIS-fatigue scale and the Chalder fatigue scale. For the Chalder fatigue score, both groups improved by more than 4 points (widely considered to be the MCID) but the improvement was similar between treatment arms (difference in means −0.5, 95% CI −2.2 to 1.3). The improvement was maintained at 12 months. Evidence of a greater improvement of fatigue symptoms in the FITNET-NHS group compared to the Activity Management group was apparent on the CIS Fatigue scale at 6 months (difference −3.9, 95% CI −6.8 to −1.0; p = 0.009). At 12 months, both arms improved further, but those in the FITNET-NHS group improved more than Activity Management.
A similar pattern of results to the SF-36-PFS was observed for the pain VAS, with modest evidence of a greater improvement in the FITNET-NHS group at 6 months, and the Activity Management group catching up most of that advantage by 12 months.
Finally, only the FITNET-NHS group was seen to recover time at school during the first 6 months, with the difference of just over half a day per week at 6 months. At 12 months, the FITNET-NHS group continued to show improvement in school attendance. Although school attendance improved slightly in the Activity Management group at 12 months, it was still greater in the FITNET-NHS group by just over half a day (mean difference 12.4%, 95% CI 3.3 to 21.5).
Participants were asked at the 6- and 12-month assessments to rate their overall improvement on a 7-point scale, from very much worse (score = 7) to very much better (score = 1), with these responses being presented in simplified form in Table 11. Similar levels of improvement were observed between the two groups at 6 months, and whilst the odds ratios greater than one suggested greater improvement with FITNET-NHS at 6 and 12 months, this difference between allocated groups was consistent with a chance result (p > 0.5).
| FITNET-NHS | Activity Management | Odds ratio (95% CI) | p-value | |
|---|---|---|---|---|
| Change from baseline (6 months) | ||||
| Much better or very much better (%) | 40 (33%a) | 38 (30%) | ||
| Minimal change (%)b | 69 (57%) | 85 (64%) | ||
| Much worse or very much worse (%) | 12 (10%) | 9 (7%) | 1.13 (0.73 to 1.77) | 0.58 |
| Missing | 34 | 27 | ||
| Change from baseline (12 months) | ||||
| Much better or very much better (%) | 47 (43%) | 44 (34%) | ||
| Minimal change (%)a | 54 (50%) | 74 (58%) | ||
| Much worse or very much worse (%) | 8 (7%) | 10 (8%) | 1.15 (0.73 to 1.83) | 0.54 |
| Missing | 46 | 31 | ||
Harms
The small number of reports from participants and clinicians of a worsening condition at the point of reporting of an adverse event or treatment withdrawal were predominantly in the FITNET-NHS group (see Table 12), perhaps due to the more frequent contact in the FITNET-NHS intervention leading to the more adverse events being reported (one or more adverse events or serious adverse events reported for 28 participants in the FITNET-NHS group and 18 participants in the Activity Management group), and more treatment withdrawals (61 participants in the FITNET-NHS group and 12 participants in the Activity Management group). In contrast, participants meeting the pre-defined criteria for a worsening condition on the SF-36-PFS or CGI were evenly distributed between the two groups, with a composite of these different measures indicating about one-quarter of participants in each group experiencing a worsening condition at some point in the 12-month follow-up period.
| FITNET-NHS (n = 159) |
Activity Management (n = 155) | |
|---|---|---|
| Number of participants with clinician report of worsening condition (%) | 3 (2) | 1 (1) |
| Number of participants reporting worsening condition on withdrawing from treatment (%) | 7 (5) | 0 |
| Number of participants with evidence of worsening condition from SF-36-PF or the Clinical Global Impression Scale (%)a | 36 (23%) | 41 (26%) |
| Number of participants with any evidence of worsening condition – one or more of the above (%) | 39 (25%) | 42 (26%) |
Chapter 6 Results of the economic evaluation
Overview of data set
At 3 months, 84% had complete EQ-5D-Y data. This reduced to 81% at 6 months, and to 74% at 12 months. At 3 months, 87% had complete RUQ data; this reduced to 75% at 6 months and 72% at 12 months. Due in part to incomplete routine data on emergency department visits, both the FITNET-NHS and Activity Management groups had at least two-thirds of participants missing one or more data items, 75% (n = 116) and 67% (n = 106), respectively. Participants with missing data had a baseline utility score that was slightly lower (−0.024, 95% CI −0.088 to 0.041) than the complete cases. Groups with missing and complete data were similar in terms of baseline age. Those with missing data had a mean age of 14 years (95% CI 14.0 to 14.4) while complete cases had a mean age of 14 years (95% CI 13.9 to 14.5). The prevalence of missing data was similar for males and females (odds ratio 1.00, 95% CI 0.61 to 1.66).
Costs
Table 13 reports mean costs for the main NHS resource categories for those participants where data were available. All training for FITNET-NHS took place between 2016 and 2019 and resulted in an additional £330 per participant. Training comprised of staff time and travel costs, for both non-NHS and NHS staff. More specifically, three non-NHS staff, with expertise in design and delivery of FITNET-NHS, delivered training to four NHS clinicians. This training was made up of four full-day (8 hour) face-to-face training days and 6 months of follow-up support which entailed fortnightly/monthly 1-hour online group supervisions. Three of these four NHS clinicians replicated this training package for a further seven NHS clinicians.
| Resource category | Measurement (data source) | FITNET-NHS study both arms total cohort (n = 155) | Activity Management (n = 159) | ||||
|---|---|---|---|---|---|---|---|
| n a | Resource use, mean (SD) | Cost, mean (SD) (£) | n a | Resource use, mean (SD) | Cost, mean (SD) (£) | ||
| Total intervention training | - | 155 | - | £330.08 | 159 | - | £0 |
| Total intervention delivery | Number of consultations (Millennium data set)b | 154 | 19.83 (9.16) | 990.48 (503.41) | 152 | 5.11 (1.99) | 316.70 (131.75) |
| Outpatient visits outside of RUH | Number of visits (HES) | 150 | 5.06 (5.95) | 721.67 (779.40) | 146 | 4.86 (6.86) | 770.47 (1206.55) |
| Outpatient visits at for CFS RUH not FITNET-NHS/AM | Number of visits (RUH NHS Trust Millennium data set) | 154 | 0.08 (0.43) | 19.75 (99.93) | 152 | 0.14 (0.60) | 32.33 (140.06) |
| Outpatient visits for other paediatric visits at RUH | Number of visits (RUH NHS Trust Millennium data set) | 154 | 0.01 (0.11) | 2.87 (25.10) | 152 | 0.03 (0.26) | 6.07 (59.58) |
| Inpatient stays | Number of nights (HES) | 150 | 0.25 (0.74) | 338.13 (1074.13) | 146 | 0.18 (0.59) | 258.92 (1024.79) |
| Emergency care visits | Number of visits (HES) | 93 | 0.50 (1.12) | 80.87 (201.09) | 93 | 0.43 (0.79) | 70.89 (130.78) |
| GP surgery contacts | Number of visits (self-report) | 95 | 3.74 (8.30) | 127.05 (282.09) | 101 | 3.24 (3.89) | 110.08 (132.31) |
| Home visits | Number of visits (self-report) | 95 | 0.02 (0.21) | 2.39 (23.34) | 101 | 0.01 (0.10) | 1.13 (11.32) |
| Nurse surgery contacts | Number of visits (self-report) | 95 | 1.09 (2.58) | 11.88 (28.00) | 101 | 1.01 (1.63) | 10.95 (17.73) |
| NHS 111 phone calls | Number of calls (self-report) | 95 | 0.14 (0.50) | 1.75 (6.37) | 101 | 0.15 (0.50) | 1.90 (6.38) |
| Walk-in centre visits | Number of visits (self-report) | 95 | 0.02 (0.14) | 0.84 (5.74) | 101 | 0.11 (0.40) | 4.33 (15.81) |
| CAMHS | Number of visits (self-report) | 95 | 0.14 (0.68) | 13.27 (65.77) | 101 | 0.25 (1.40) | 24.01 (135.39) |
| Other primary and community care visits/calls | Number of visits/calls (self-report) | 95 | 0.91 (2.41) | 46.58 (124.02) | 101 | 1.45 (3.27) | 74.37 (168.37) |
| Amitriptyline | Number of prescriptions (self-report) | 95 | 0.22 (0.70) | 0.77 (2.41) | 101 | 0.19 (0.61) | 0.60 (1.99) |
| Melatonin | Number of prescriptions (self-report) | 95 | 0.42 (0.95) | 35.43 (79.63) | 101 | 0.44 (0.99) | 35.75 (81.77) |
| Paracetamol | Number of prescriptions (self-report) | 95 | 0.33 (0.71) | 0.65 (1.41) | 101 | 0.35 (0.68) | 0.69 (1.36) |
| Ibuprofen | Number of prescriptions (self-report) | 95 | 0.20 (0.52) | 0.84 (2.17) | 101 | 0.24 (0.53) | 0.99 (2.23) |
| Codeine | Number of prescriptions (self-report) | 95 | 0.05 (0.22) | 0.06 (0.25) | 101 | 0.13 (0.46) | 0.14 (0.51) |
| Other prescribed medications | Number of prescriptions (self-report) | 95 | 0.99 (1.50) | 58.50 (165.49) | 101 | 1.25 (1.81) | 48.65 (121.96) |
| Total NHS costs | - | 50 | - | 2561.88 (992.32) | 60 | - | 1503.90 (1104.29) |
On average, participants in the FITNET-NHS treatment group had almost four times more intervention delivery consultations than participants in the Activity Management group (19.8 and 5.1, respectively). This is reflected in the treatment delivery costs, which were on average £990.48 per person for the FITNET-NHS group and £316.70 for the Activity Management group. During the 12-month follow-up period, resource use was similar across the majority of primary, community and secondary care cost categories. Activity Management participants had slightly more visits for outpatient, CAMHS, other primary and community care services. Overall, the more intensive treatment provided by FITNET-NHS did not result in substantial NHS savings elsewhere during the first 12 months. Total NHS unadjusted costs for the participants with complete resource use data were £2562 (n = 50) in the FITNET-NHS group and £1504 (n = 60) in the Activity Management group.
Utility scores and quality-adjusted life-years (QALYs)
Complete EQ-5D-Y scores at all timepoints were available for just over half (55.48%, n = 86) of the FITNET-NHS group and two-thirds (66.04%, n = 105) of the Activity Management group. Figure 3 shows the change in mean domain responses from baseline to 12 months by group. In both groups, domain scores generally decreased over time which demonstrated small improvements in function across several of the EQ5D domains. As shown in Figure 3, the largest improvement was observed in responses to the ‘Usual Activities’ domain.
FIGURE 3.
Mean EQ-5D-Y domain scores at baseline and 12-month follow-up by group. AM, Activity Management.
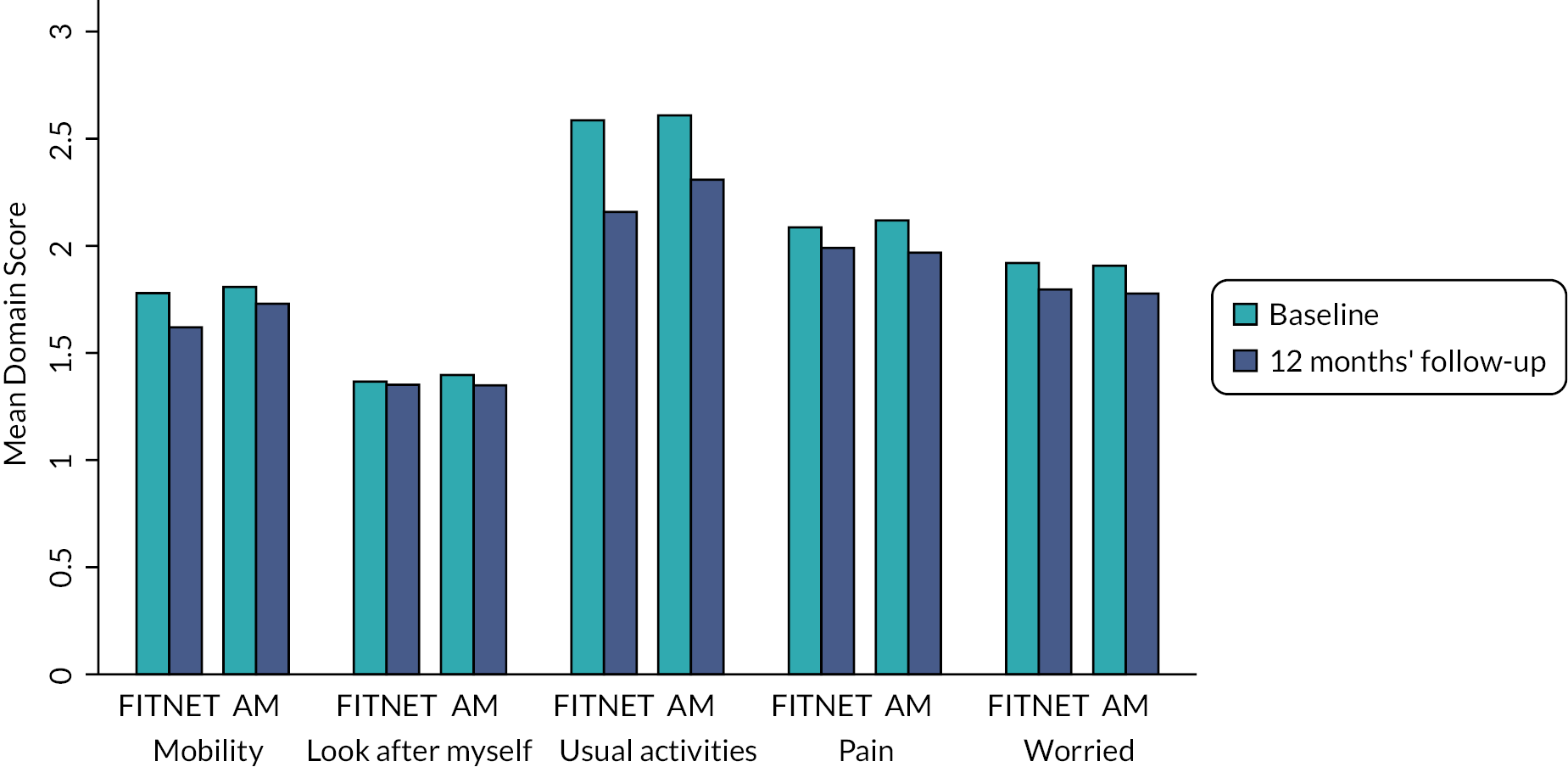
Similarly, EQ-5D-Y scores improved over the first 6 months and levelled off thereafter. The FITNET-NHS group had slightly higher EQ-5D-Y scores, most notably at 6 months, but differences were small and not statistically significant (see Figure 4). Overall, total QALYs for the 12-month period, prior to multiple imputation and adjustment for covariates, were slightly larger for the FITNET-NHS group (see Table 14).
FIGURE 4.
Mean utility scores with 95% CIs over 12-month time horizon. AM, Activity Management.
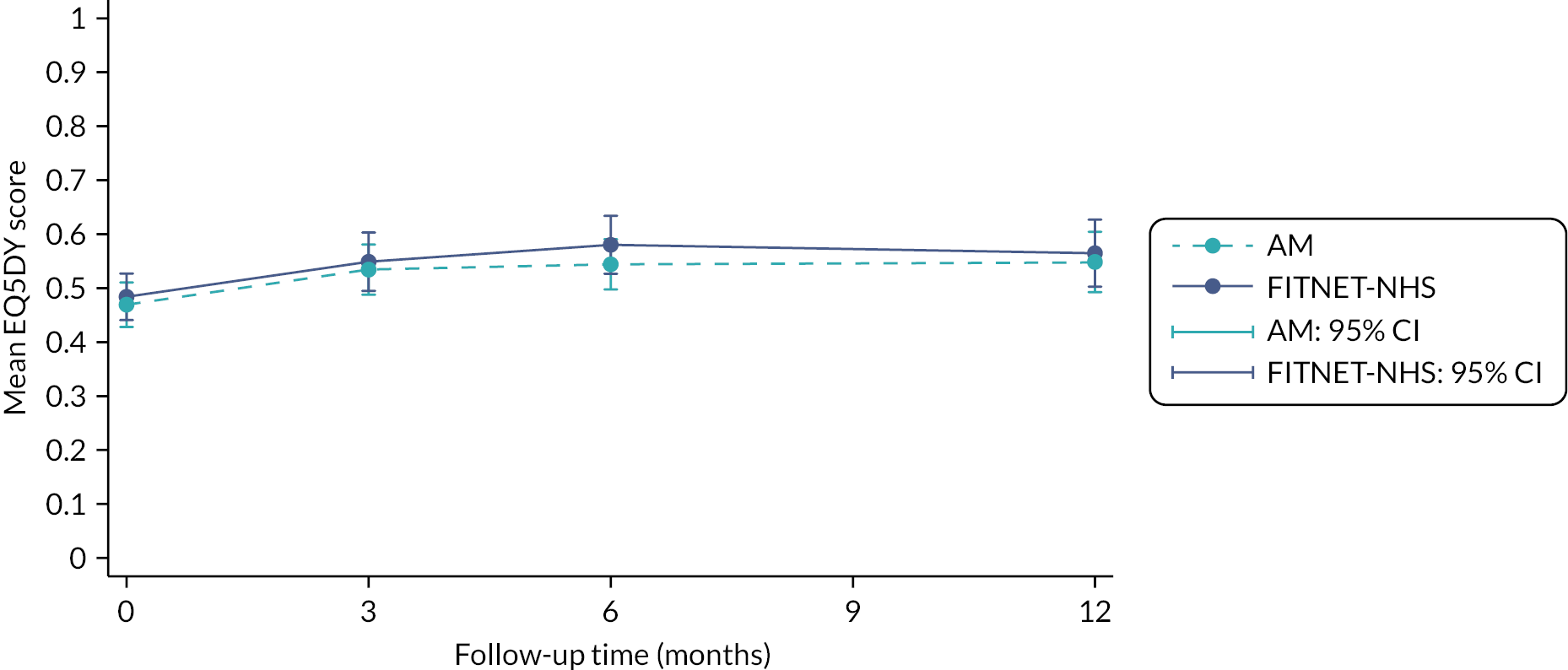
| Outcome | FITNET-NHS | Activity Management | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| Total QALYs | 86 | 0.597 (0.230) | 105 | 0.549 (0.228) |
Health economic primary analysis and sensitivity analyses for missing data
Results of the primary analysis are presented in Table 15. After multiple imputation and adjusting for age, gender and baseline EQ-5D-Y score, the FITNET-NHS group had a small gain in QALYs (0.002, 95% CI −0.040 to 0.045) compared to Activity Management. Compared to Activity Management, the FITNET-NHS group had substantially higher mean costs (£1047.51, 95% CI £624.61 to £1470.41). In the primary analysis, from an NHS perspective, at a threshold of £20,000 per QALY, the iNMB was −£1001.74 (−£2041.31 to £37.83), indicating the intervention is unlikely to be cost-effective. The wide CIs show there is considerable uncertainty in this result.
| Trial group | n | Adjusteda mean (95% CI) | Incremental adjusteda mean (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | ICER (£/QALY) | iNMB (£) at £20,000/QALY (95% CI) | ||
| Primary analysis: SUR with MI and MAR assumption | |||||||
| FITNET-NHS | 155 | £2825.84 (£2525.41 to £3126.27) | 0.532 (0.501 to 0.5644) | £1047.51 (£624.61 to £1470.41) | 0.002 (−0.041 to 0.045) | £457,721.18 | −£1001.74 (−£2041.31 to £37.83) |
| Activity Management | 159 | £1778.33 (£1481.19 to £2075.47) | 0.530 (0.501 to 0.558) | ||||
| Sensitivity analysis: SUR with complete-case and MCAR assumption | |||||||
| FITNET-NHS | 39 | £2911.62 (£2490.26 to £3332.969) | 0.582 (0.529 to 0.636) | £1287.40 (£731.21 to £1843.59) | 0.020 (−0.051 to 0.091) | £63,768.11 | −£883.63 (−£2477.01 to £709.75) |
| Activity Management | 53 | £1624.22 (£1263.02 to £1985.41) | 0.562 (0.516 to 0.608) | ||||
The CEAC (see Figure 5) illustrates the uncertainty around our estimates. As the willingness-to-pay threshold for one additional QALY gained increases from £20,000 to £30,000, the probability of FITNET-NHS being cost-effective increases from 3% to 9%. However, the additional benefits of FITNET-NHS, as measured by the QALY, are not large enough to clearly justify the additional cost at any willingness-to-pay threshold. The CEAC asymptotes below one (where a value of one would suggest the probability of FITNET-NHS being cost-effective is 100%) as there is uncertainty in whether the intervention will result in a gain in QALYs. By contrast as the CEAC cuts the y-axis at zero, this suggests FITNET-NHS is unlikely to cost less than Activity Management. This uncertainty in our results can also be observed in the CIs presented in Table 15.
FIGURE 5.
Primary analysis: NHS perspective with multiple imputation (n = 314).
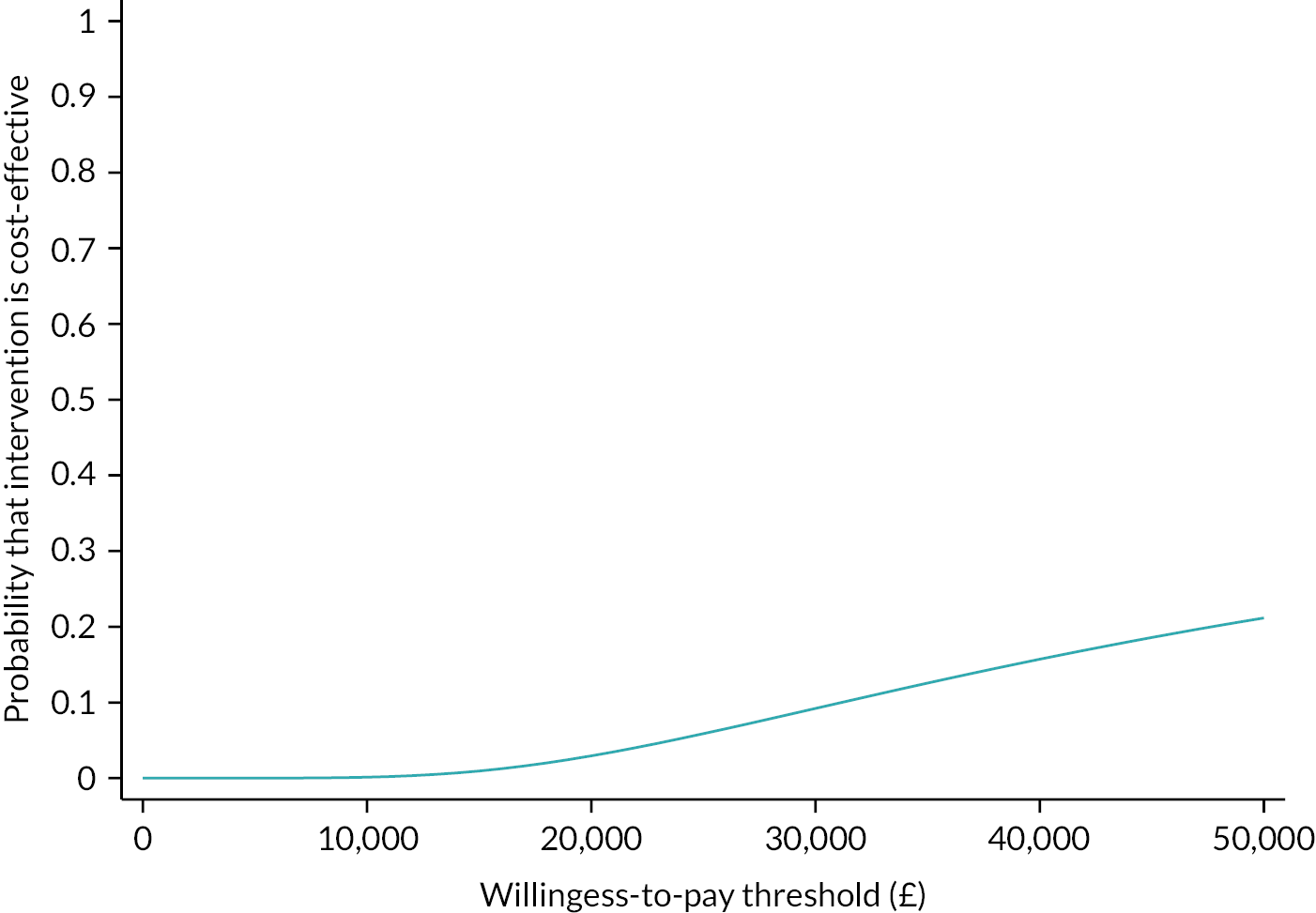
In accordance with the primary analysis, the complete-case analysis (n = 92) resulted in a negative iNMB estimate −£803.10 (−£1899.58 to £293.39). More specifically, there was a small mean incremental difference in QALYs (0.020, 95% CI −0.051 to 0.091) and a large mean incremental difference in costs (£1287.40, £731.21 to £1843.59).
The results of the sensitivity analysis assuming data are MNAR are presented in Table 16. Across all scenarios, the MNAR results remained similar to the primary analysis. Even under the scenario most favourable to FITNET-NHS, the probability that it was more cost-effective than Activity Management did not exceed 5%.
| Scenario number | MNAR rescaling parametersa | Incremental costb (£) (95% CI) | Incremental QALYs (95% CI) | INMBc (£) (95% CI) | Probability cost-effective (%) | |
|---|---|---|---|---|---|---|
| AM | FITNET-NHS | |||||
| MAR | 1 | 1 | £1047.51 (624.61 to £1470.41) | 0.002 (−0.041 to 0.045) | −£1001.74 (−£2041.31 to £37.83) | 2.95 |
| 1 | 1 | 0.95 | £1047.51 (624.61 to £1470.41) | −0.002 (−0.045 to 0.040) | −£1096.76 (−£2128.31 to −£65.22) | 1.86 |
| 2 | 0.95 | 1 | £1047.51 (624.61 to £1470.41) | 0.006 (−0.037 to 0.049) | −£929.97 (−£1967.29 to £107.35) | 3.94 |
| 3 | 0.95 | 0.95 | £1047.51 (624.61 to £1470.41) | 0.001 (−0.041 to 0.044) | −£1025.00 (−£2054.28 to £4.29) | 2.54 |
| 4 | 0.95 | 0.90 | £1047.51 (624.61 to £1470.41) | −0.004 (−0.046 to 0.039) | −£1120.02 (−£2142.08 to £−97.96) | 1.59 |
| 5 | 0.90 | 0.95 | £1047.51 (624.61 to £1470.41) | 0.005 (−0.038 to 0.047) | £−953.23 (−£1980.81 to £74.35) | 3.45 |
| 6 | 0.90 | 0.90 | £1047.51 (624.61 to £1470.41) | −0.000 (−0.042 to 0.042) | −£1048.25 (−£2068.61 to −£27.89) | 2.20 |
Subgroup analysis
The subgroup analysis (see Figure 6) indicated that the probability that FITNET-NHS is cost-effective compared to Activity Management was higher in the subgroup of patients with comorbid anxiety/depression (probability ≈ 0.5 at the NICE £30,000 per QALY threshold) than in the subgroup of patients without comorbid anxiety/depression (probability < 0.05 at the NICE £30,000 per QALY threshold). However, the interaction with comorbidity was not statistically significant for either costs (−£245.05, 95% CI −£1090.04 to £599.94) or QALYs (0.064, 95% CI −0.018 to 0.147), so we cannot be certain of this difference in cost-effectiveness between subgroups. A breakdown of the results by subgroup is presented in Table 17.
FIGURE 6.
Subgroup analysis with and without comorbid anxiety/depression.
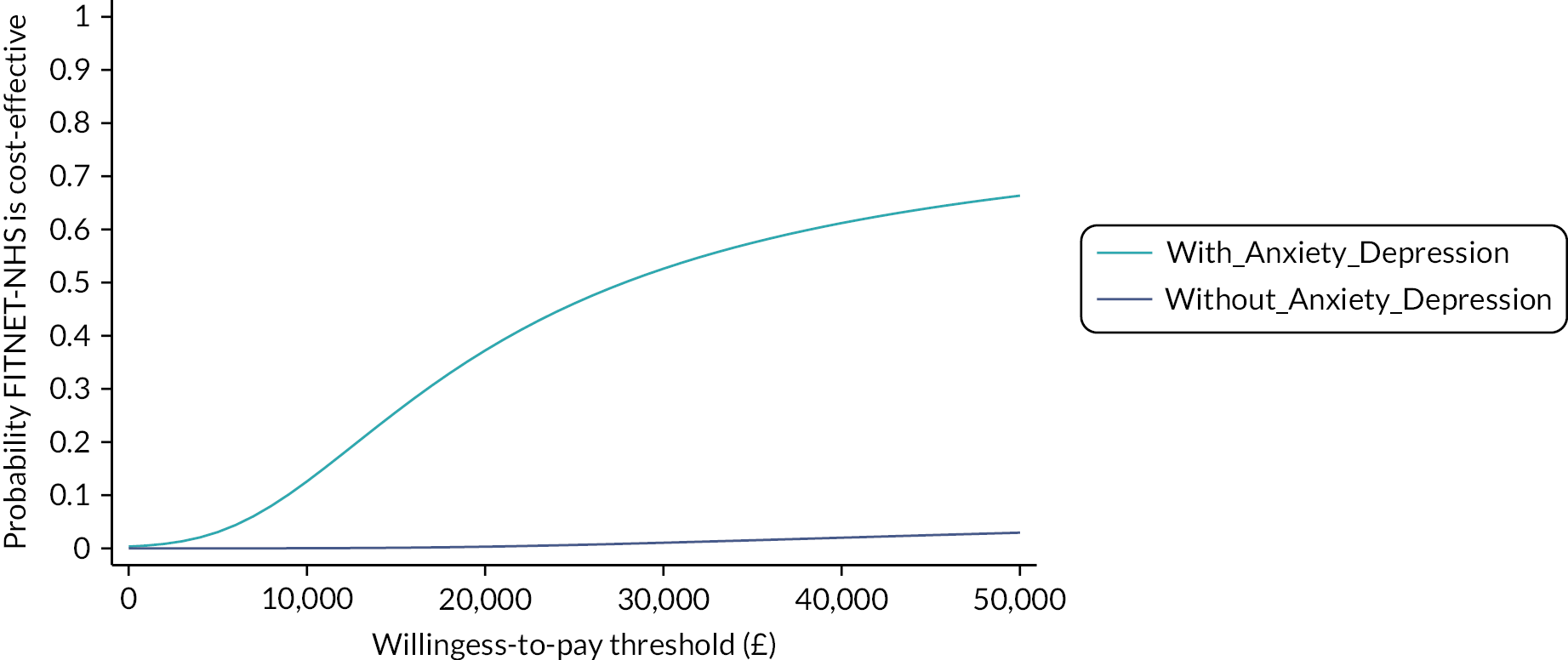
| Trial group | n | Adjusteda mean (95% CI) | Incremental adjusteda mean (95% CI) | ICER (£/QALY) | iNMB (£) at £20,000/QALY (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | ||||
| Subgroup analysis: patients with comorbid anxiety/depression | |||||||
| FITNET-NHS | 69 | £2972.76 (£2475.42 to £3470.09) | 0.464 (0.415 to 0.514) | £949.58 (£264.79 to £1634.37) | 0.034 (−0.032 to 0.101) | £27,822.03 | −£266.97 (−£1872.88 to £1338.94) |
| Activity Management | 78 | £2023.17 (£1553.60 to £2492.74) | 0.430 (0.386 to 0.474) | ||||
| Subgroup analysis: patients without comorbid anxiety/depression | |||||||
| FITNET-NHS | 86 | £2720.28 (£2364.32 to £3076.24) | 0.590 (0.585 to 0.657) | £1190.80 (£678.09 to £1703.50) | −0.031 (−0.083 to 0.022) | −£38,994.63 | −£1801.55 (−£3091.24 to −£511.86) |
| Activity Management | 81 | £1529.48 (£1162.31 to £1896.65) | 0.621 (0.585 to 0.657) | ||||
Additional analyses
In the wider perspective analysis (see Table 18), costs were substantially higher in both groups. However, the incremental difference in costs and iNMB estimate were similar to the primary analysis. For both groups, the greatest contribution to the increase in costs was due to parent productivity loss (see Table 19). While the FITNET-NHS group had greater absenteeism rates compared to the Activity Management group, the Activity Management group reported greater presenteeism.
| Trial group | n | Adjusteda mean (95% CI) | Incremental adjusted mean (95% CI) | ICER (£/QALY) | iNMB (£) at £20,000/QALY (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Costs (£) | QALYs | Costs (£) | QALYs | ||||
| Wider perspective | |||||||
| FITNET-NHS | 155 | £8458.38 (£7338.34 to £9578.42) | 0.536 (0.506 to 0.566) | £1214.15 (−£259.62 to £2687.93) | 0.006 (−0.037 to 0.048) | £215,276.38 | −£1101.35 (−£2910.20 to £707.50) |
| Activity Management | 159 | £7244.23 (£6241.90 to £8246.56) | 0.530 (0.501 to 0.566) | ||||
| Sensitivity analysis: assuming the fee per patient paid by the CCGs represents the intervention cost | |||||||
| FITNET-NHS | 155 | £4545.71 (£4213.90 to £4877.53) | 0.535 (0.504 to 0.565) | £2079.75 (£1613.44 to £2546.06) | 0.005b (−0.036 to 0.049) | £355,656.33 | −£1962.80 (−£3006.20 to −£919.39) |
| Activity Management | 159 | £2465.96 (£2138.35 to £2793.58) | 0.529 (0.499 to 0.559) | ||||
| Sensitivity analysis: excluding FITNET-NHS training costs | |||||||
| FITNET-NHS | 155 | £2495.76 (£2195.33 to £2796.19) | 0.532 (0.500 to 0.564) | £717.43 (£294.53 to £1140.33) | 0.002 (−0.041 to 0.045) | £313,489.04 | −£671.66 (−£1711.23 to £367.91) |
| Activity Management | 159 | £1778.33 (£1481.19 to £2075.47) | 0.530 (0.501 to 0.558) | ||||
| Sensitivity analysis: FITNET-NHS delivered by Band 6 clinicians | |||||||
| FITNET-NHS | 155 | £2653.21 (£2353.63 to £2952.79) | 0.533 (0.502 to 0.564) | £874.83 (£453.91 to £1295.75) | 0.003b (−0.038 to 0.046) | £258,839.40 | −£807.23 (−£1836.95 to £222.49) |
| Activity Management | 159 | £1778.38 (£1482.58 to £2074.18) | 0.530 (0.501 to 0.558) | ||||
| Sensitivity analysis: value set from Spain | |||||||
| FITNET-NHS | 155 | £2821.95 (£2521.28 to £3122.62) | 0.410 (0.373 to 0.448) | £1047.36 (£623.97 to £1470.74) | 0.010 (−0.041 to 0.061) | £100,805.03 | −£839.56 (−£2023.70 to 344.59) |
| Activity Management | 159 | £1774.59 (£1476.32 to £2072.87) | 0.400 (0.3648548 to 0.435) | ||||
| Resource category | Measurement | FITNET-NHS (n = 155) | Activity Management (n = 159) | ||||
|---|---|---|---|---|---|---|---|
| n a | Resource use mean (SD) | Cost, mean (SD) (£) | n a | Resource use mean (SD) | Cost, mean (SD) (£) | ||
| School counsellor | Number of visits | 95 | 1.33 (4.19) | 128.65 (406.26) | 101 | 1.30 (3.69) | 125.81 (358.39) |
| Travel for healthcare visits (primary and community care, hospital care) | Pay for one return journey | 95 | n/a | 28.40 (48.12) | 101 | n/a | 21.74 (37.34) |
| Home tuition | Hours of home tuition | 89 | 15.96 (28.44) | 446.82 (796.55) | 110 | 22.20 (36.01) | 621.47 (1008.42) |
| Productivity loss of parents/carers involved in the care of the child | Hours missed from work due to child’s health problems | 87 | 110.58 (210.73) | 1674.21 (3190.42) | 90 | 78.75 (141.73) | 1192.28 (2145.77) |
| Productivity loss of parent/carer who completed the RUQ | Work productivity loss (hours) due to child’s health problems | 95 | 127.30 (207.49) | 1927.26 (3141.35) | 101 | 159.34 (222.54) | 2412.44 (3369.20) |
| Productivity loss (non-working activities) | Scale of impact (0–10) on non-work daily activities due to child’s health problems | 95 | 33.19% (26.39%) | Not valued | 101 | 39.71% (26.77%) | Not valued |
| Out-of-pocket costs | Free text | 92 | Varies | 500.54 (791.00) | 100 | Varies | 432.50 (888.92) |
| Attendance at school/college | Attendance in a typical week during the last term | 73 | 58.01% (33.89%) | Not valued | 87 | 46.26% (35.82%) | Not valued |
| Over-the-counter medication | One-off purchases of paracetamol or ibuprofen per time point | 95 | 2.35 (1.66) | 6.36 (5.13) | 101 | 2.32 (1.77) | 6.31 (5.44) |
Additional sensitivity analyses (see Table 18) excluding training costs and using the Spanish EQ-5D-Y value set did not change our overall interpretation of the primary results, which are dominated by the higher costs of delivering the FITNET-NHS intervention. In all analyses, the iNMB remained negative with FITNET-NHS resulting in substantially more costs and a small gain in QALYs. The greatest incremental difference in costs was observed in the analysis where the CCG fees were used to estimate treatment delivery costs.
Chapter 7 Qualitative results
Participants’ views and experiences of FITNET-NHS platform and e-consultations
Table 20 summarises the main themes and subthemes on the acceptability of the FITNET-NHS treatment from participants and their parents and therapists. Fuller details of the qualitative analysis are given in Appendix 7.
| Participants’ views and experiences | Therapists’ views and experiences |
|---|---|
Benefits of online treatment:
|
Benefits of online treatment:
|
Disadvantages of online treatment
|
Challenges of online treatment
|
FITNET-NHS platform usability
|
A different set of clinical skills is required
|
FITNET-NHS Treatment content and advice
|
Treatment approach and content
|
|
Suggested changes to treatment content |
| Parental involvement | Variable patient engagement |
| Contact with a therapist is essential | |
| Treatment effectiveness | Treatment effectiveness
|
The majority of participants (both adolescents and parents) were positive about the FITNET-NHS treatment. They liked the platform, felt that it was as good as face-to-face treatment and they got individualised advice through e-consultations. Families particularly praised the support and encouragement they got from therapists. Some families found the amount of reading and work on the platform difficult to keep up with alongside school and exams and parents were often ‘nagging’ their child to complete the chapters. Families naturally talked about the benefits and disadvantages of online treatment and their views on the structure, content, relationship with the therapist and treatment effectiveness are discussed below.
The majority of participants felt that the FITNET-NHS treatment was convenient, flexible and liked that they could complete treatment (reading and answering questions on the platform) in their own time rather than having to attend appointments. Parents felt it allowed treatment to fit in around school and work, ‘… the thing about the e-mail is, is that you can just pick it up when you, when it fits in’ (M1000049). E-consultations from therapists acted as prompts for participants to think about what they needed to do. Participants felt e-consultations were easy to follow and allowed them to go back and re-read what had been mentioned and gave them time to think about their answers. Some participants found it easier to talk about sensitive topics like ‘unhelpful’ thoughts over e-mail. Parents of shy or less talkative participants felt online treatment worked well as they would have ended up talking more during videocall appointments ‘ … with (child) being shy I think it would’ve probably have ended up being me doing more of the talking than her’ (M1000019).
C1000064:Good. When I email (therapist) she lays them out very… lays them out in sections so each goal will be a paragraph and it’s very easy to follow and you know, you can always go back and re-read the email, make sure you’re doing everything that was mentioned in the email.
M1000273:Yeah, I think he didn’t mind it. He’s not very outgoing and talkative a person so I think he found it easier rather than having to go and face somebody. He didn’t worry about it at all, not a problem there.
Some participants found it difficult to express themselves in writing and would have preferred some face-to-face contact. One young person reported that the e-consultations were too long with too many questions. Parents also described a lack of rapport and connection through e-consultations. One child felt you are ‘more obliged to be truthful’ (C100064) face-to-face than in writing. Where therapists only replied to e-mails at set times, some families found this ‘annoying’ particularly related to more urgent content, ‘… like something’s that’s like urgent, you don’t get a reply to like the next week’ (C1000115). Some parents felt it would be useful to have the flexibility to e-mail therapists spontaneously for clarifications or when their child was relapsing. Families that had received a therapist phone call at the start of treatment felt this helped build rapport, ‘… we had the chance to chat [phone call] before it all started, we still, we kind of built up a rapport between us as well’ (M1000273).
C1000008:Over an email, sometimes it’s quite hard to portray how I feel personally or how I am and how I feel. It’s like very hard because you’re only writing in an email. It’s okay, it’s really convenient, but like I say, face to face you get the whole how I actually am rather than just words how I am.
C1000117:Just the lack of contact really, I don’t know, I just really struggle with it just being online, just messages without actually seeing anyone.
All participants liked the simple layout of the online platform, ‘it’s not too busy’ (C1000064) and felt it was ‘easy to access’ and ‘easy to use’ (M1000273). However, whilst some found the content and diaries helpful others struggled to complete them. Parents liked the gradual opening of chapters: ‘they don’t bombard you and they only up the chapters as you go and I think that’s a really positive thing to do because it’s not too daunting’. (M1000149). Participants liked the diaries as they acted as a reminder to complete agreed activities and enabled them to see progress, motivating them to improve further. However, a few participants found it hard to motivate themselves to complete the diaries as well as the reading required and answering questions on the platform. Parents reported that participants found it ‘draining’ and they had to ‘nag’ them to complete them.
C1000015:Um, yes, they [the diaries] were, they were helpful to me so like um, I was like um, I just see like I was improving and so like that was a, and like put me into a good mind-set. And I, and, and and, and it made me want to improve.
M1000273:The treatment that he had was very well structured, he had to do a sleep log and then an activity log which he did every day. I think that he found sometimes draining, to have to type everything in and do the online side of things…
The treatment approach was felt to be ‘pushy’ and hard at first, but many participants reported that it got easier. While many found the content relatable, some patients felt it was ‘nothing new’ and the amount of reading was particularly difficult for younger adolescents and those with cognitive difficulties.
Most participants were positive about the FITNET-NHS treatment content and advice, ‘I am really happy with it, I am really glad I am doing it’ (C1000029), ‘I can’t think of anything I’d change’ (M1000009). Families reported that the treatment was ‘hard’, ‘intense’, ‘difficult’ at first. They felt there was a lot of reading and routines such as sleep and exercise to change and implement, it required 100% commitment but it got easier as they got used to it, built up stamina and they felt they could see the benefits of the small changes they had been making. A few parents described the treatment approach as more ‘positive’, ‘pushy’, ‘no nonsense’ and ‘ambitious’ compared to their previous experience of treatment, ‘The paediatrician was just, “Cut down, cut down,” [ … ] whereas this [FITNET-NHS] [ … ] was a more positive approach to what you previously had’ (D1000019).
M1000002:I think it’s [FITNET-NHS] quite pushy but in a good way, so you’re still very supported, and you feel very still very safe, and you know that what you’re being asked to do is the right thing to do, but as I say, it’s quite pushy like, “Right, this week you’ve got to do this”. And for the next three weeks I want you to increase “blah-di-blah”. I don’t think (child) would had someone say that to him before, I think his physiotherapist, the hydrotherapist, and the CBT therapist had been a bit gentler, and working at his pace; rather than pushing him to work at a different pace.
C1000029:It has been hard. At the beginning because it said that I need to commit myself 100%, obviously I was up for that but I didn’t realise quite how intense it would be, I really liked it and I can tell that it is really good and that its logical, but it is very, it’s changed a lot of my routines and my everyday life, but I really liked it.
Participants described how they were reassured by the advice on the platform as they were able to relate to the ‘really good examples’ (C1000008) that summed up how they felt. One child, who had begun to lose motivation, described how a chapter on the platform reflected that they ‘would likely to be feeling like this’ and this motivated them to continue; ‘every week when I am starting to feel something, it somehow, it’s like it knows how I will feel’ (C100029). They liked the different sections on sleep, activity and helpful thoughts that facilitated them to change routines. Participants seemed to enjoy the new activity routines such as walking and cycling, and felt it gave them something to do rather than ‘sitting in the house’ (C1000009). They felt goals gave them ‘light at the end of the tunnel’ (C1000002) and they were reassured by the advice on the platform that they were doing the right thing. Participants also found the chapters recommending reduced focus on symptoms and fatigue helpful.
C1000008:Each example’s relevant to how you cope with it, how you feel about it, what other people think, it’s invisible condition and it’s not. It just sums up how I feel.
C1000009:I’d say the sleeping one because it’s stopped me from like getting more tired during the day when I sleep cos it’s sorted out my sleeping. So that’s probably the most helpful one…
Parents felt that the structured advice on building activity and ‘keeping to a routine’ worked well and ‘The fact that it’s measured, paced carefully, that you have confidence’ (M1000015). Many parents particularly liked the chapters on ‘shifting attention away from fatigue’ as they had got into unhelpful patterns over the course of their child’s illness.
M1000002:…it was one of the most helpful in terms of I think it moved us forward, but it was one of the most terrifying, was the chapter about “stop asking your child how they are”.
M1000040:…I think the CBT has shown that it is really about giving your child the independence to think for themselves, make their own choices sort of move away from the negative thinking almost that draining thinking which is very much focussed on how tired are you, do you think you’ve done too much? Those sorts of limiting ideas rather than encouraging them to think about other things that – rather than looking at the limitations almost right think about what are the opportunities. So it’s been really empowering actually.
By contrast, for some families, the advice was no different to what they had previously tried: ‘It was just stuff that we’d already done, like the bedtime routines, the waking up the same time every day, no screens an hour before bed, that kind of thing’. (M1000273). Advice on outdoor activities such as ‘walking’ were limited by the weather and were felt to be ‘an unrealistic expectation, you know, of going walking every day …’ (D1000115). Some participants found the advice on sleep and activity difficult to implement. One child felt that starting with a short activity such as walking for two minutes was ‘pointless’ (C1000117). They did not always believe the ‘positive talk’ they were encouraged to come up with as part of treatment. Sections on the platform (e.g. washing/self-care) were also felt to be less relevant to those who were not severely affected.
C1000019:When you think about doing an activity, while you might try to think positively and you might come up with positive talk it’s not always what you think.
M1000041:…some of it wasn’t relevant to him and like going to school like or getting up out of bed and just having a wash. I mean he will do that every day so it’s bits like that that’s not quite relevant to him because he’s not bedbound.
The content on the FITNET-NHS platform did not seem to suit all participants, particularly younger adolescents or those more severely affected with cognitive difficulties such as problems concentrating and brain fog. Some participants and parents felt there was too much reading and too many questions to complete on the platform, ‘it’s like a lot of content to read through and then answer the questions’ (C10000080). They worried about keeping up with this alongside school and the new activity plans as part of treatment.
M1000384:So, yeah, it became a little bit tricky towards the end, keeping on top of her, you know, going through all the questions because it was quite a lot to read. We even tried me reading the questions and then I’d get to the end and she’d say “Can you read that again?” like her mind had gone; started wandering.
C1000117:I struggled with it, and then remembering it afterwards was quite hard as well, I’d have to read it a few times.
There were opposing views of the age-appropriateness of the FITNET-NHS platform content. One mother of an 11-year-old felt the language on the platform and questions were too technical and difficult to understand. An 11-year-old did not like reading and therefore his mother ‘… was having to do it or read it all to him’ (M1000041). By contrast, the mother of another 11-year-old felt it was ‘pitched at the right level’ (M1000040). A family that had withdrawn from treatment reported that there was too much reading and the mother was ‘nagging’ her child to log onto the platform. A few of the participants also talked about having to clarify understanding with their parents, ‘so quite often he’ll say to me “What does that mean?” or, and I have to reword it in a different way’. (M1000009).
M1000149:We think things weren’t worded very well on some of the questions and they certainly weren’t geared up for a 12-year-old, or even an 11-year-old is what she was at the time. We struggled understanding what they meant.
M1000040:I have to say I’ve been really impressed with the language that’s been used. I thought well certainly (child) whose at the younger end of the study age 11 I felt like it was pitched just right really for him to be able to – to understand it because there is a lot of information but I think the way it’s broken down and set out is really good.
Families had different ways of completing the treatment. Some went through the chapters together, for others, the child completed the chapters on their own and checked areas they were unsure about with parents, ‘The majority of it we don’t really discuss it, only when I don’t understand it’ (C1000029). One family described how discussing the treatment within the family had been a positive experience. Parents described how participants became more confident completing chapters on their own with time. Most parents felt comfortable with the therapist having a private dialogue with their child and the therapist often contacted them if they had any concerns, ‘I feel included where required. But also, I feel that (child) has got his own relationship with the [therapist] and that’s very strong’. (M1000002). Participants also described being happy that their parents could not read their messages to the therapist.
M1000034:Yeah we sort of did it together to start with didn’t we and then now as your confidence has grown with it you do your own now don’t you? You email [therapist] yourself.
M1000002:He’s been asking her [therapist] and been having totally private dialogue with her, I have totally private dialogue with her, it works really well. It feels weird as a parent but it absolutely works well (laughs).
On the other hand, one father described how ‘I don’t really know if you’re doing it or not’. (D1000115). One mother with a younger child (11 years old) completed the chapters on behalf of her son and felt face-to-face treatment would have been more beneficial as ‘if a parent’s telling them it all they don’t listen as much’ (M1000041) and the child is more likely to take a health professional seriously.
M1000273:No, he came up and I’d have to nag him, because you know what they’re like, “Go and do your FITNET-NHS update and all of that, answer any questions”.
Having contact and communication with a therapist was essential to all families. One parent described how this was more important than the FITNET-NHS platform content. Most participants were positive about their contact and relationship with the therapist. Participants described therapists as ‘supportive’, ‘lovely’, ‘nice’ and ‘friendly’. They felt it was beneficial to talk to someone ‘that is experienced with chronic fatigue and can give me advice on how to improve and I can ask questions’ (C1000064). They felt comfortable to ask therapists questions, clarify understanding, and confidence in communicating developed over time. The therapist helped participants develop ‘realistic goals’ and participants liked the positive feedback from therapists.
C1000002:In the beginning, I could sort of had to trust in whatever she [therapist] said, I didn’t know her that well. But as the weeks have gone by and we’ve had more emails, I feel more confident that I could message her with whatever at home, with problems. Yeah, I think it’s good and nice that I could have that capability just to ask her anything I guess.
Parents felt they got personal e-consultations from therapists who answered questions and were confident that they ‘got the measure of (child) really quickly’ (M100002). They described therapists as ‘understanding’, ‘brilliant’, ‘caring’ ‘on your side’, ‘you feel like they are really listening’. Therapists would often comment on the child’s progress and parents found this encouragement and affirmation empowering. Parents also talked about how the therapist worked with schools and gave advice on things they had not thought of before. However, some families still would have preferred more face-to-face contact. One child described how the e-consultations from the therapist were very practical about ‘what to do and how to do it’ but felt the therapist didn’t really understand ‘what you really struggle with’ (C1000117). One family who had struggled with understanding the content on the platform felt ‘it would have been better to be able to speak to someone rather than into e-mail the whole time’ (M1000149).
M1000040:I think the biggest thing for me has been [Therapist’s] sort of affirmation of you know, just encouragement really “You’re doing well, it’s great, well done” that’s been a tremendous boost and particularly then seeing (child) really improve in – in areas has been – has been great. So I wouldn’t say that not meeting face to face has been a disadvantage at all, I think the level of support through those messages has been excellent.
D1000064:I think personally (therapist) help and support not only through the platform but through helping get (child) special educational needs assessed has been absolutely brilliant. Just really the best it could be. We’re really deeply indebted to her for all her help so it’s on a personal side it’s been absolutely brilliant I think and I think (therapist) is very personable and a great enabler.
Most of the families were positive about their experience of the FITNET-NHS treatment. One patient interviewed felt they had recovered; most noticed a little to a lot of improvement and only two patients reported no improvement. Participants talked about a range of improvements including: building up stamina, doing more and having more energy, sleeping better, attending more school and having more positive thoughts. For many participants, the activity plan had helped them build up stamina to enable them to enjoy more activities with friends and days out. For one child, reducing their school timetable helped them attend school more.
C1000019:It’s quite nice the difference from where I would be tired from doing the usual thing to being able to do trips out and stuff and not being tired again.
Parents noticed that their child had returned to their old self, was happier, ‘more chatty’ (M1000009), ‘taking more interest in life’ (D1000034). For a few patients, treatment was better than expected. Parents of participants who had experienced improvement from treatment reflected that their participants had engaged and ‘taken it seriously’ (M1000040).
D1000064:It’s quite profound really … it’s made a huge difference and…at the same time (child’s) anxiety level has dropped considerably.
However, some participants only noticed small improvements such as better fitness and fewer bad days. One parent described following therapist advice to increase school, but they felt this led to a relapse.
C1000008:I don’t feel physically better, but it reassures me if that makes sense.
M1000041:… he’ll dip again and then be alright and then it’s just… so… yeah. I don’t know if it’s really had much of an effect to be honest.
Therapists’ views and experiences of delivering FITNET-NHS
Overall, therapists were positive about FITNET-NHS. They liked that it offered treatment to those without a service and felt it was going well and really working for some patients. Engagement was challenging for other patients. They talked about the benefits and challenges or treating patients online via the FITNET-NHS platform compared to seeing patients face to face and the. Online treatment required them to employ a different set of skills.
Therapists talked about several benefits of the online FITNET-NHS treatment platform including: easily accessible content, flexible treatment delivery by gradual chapter opening, firmer advice than could be provided face-to-face, and intensive e-consultations that provided a good picture of patients. Therapists felt the written information in platform treatment chapters was ‘clear and quite directive’ (HP70007). The examples and case studies were ‘relatable’ (HP70007) to patients and helped patients develop their own treatment plans (based on the suggestions) and take ownership of their treatment. Therapists were able to tailor treatment according to individual patient progress by flexibly opening treatment chapters and referring patients back to certain information as a reminder or if they were struggling: ‘we adjust it and tailor it to what they can manage’ (HP70007). The written medium provided a structure to guide families and provided direct treatment advice in black and white that therapists felt was sometimes hard to give face-to-face, ‘it is [the platform] really quite like if you don’t do this you’re staying ill. You have to do this you need to’ (HP70004).
They felt separate parental treatment chapters were ‘empowering’ for parents to know exactly what they should be doing with their child. Therapists also felt they gained more information from parental e-consultations as parents were able to be more honest about their child’s condition, ‘you can have more direct interaction with the parents [with FITNET-NHS] because they are separated out, as it were, and maybe the parents can be more honest than they would be if they were in the room with the child’ (HP70006). E-consultations additionally allowed therapists ‘much more time to think about what you’re saying and doing than you do when you’re seeing someone synchronously’ (HP70006) as well as time to seek advice from colleagues for more complex patients.
HP70006:… rather than me starting from telling them a school plan, I’m already looking at what they’ve worked out according to the examples and then I can go from there, saying, “that’s brilliant, you’ve come up with a great plan.”
HP70007:I think parents are particularly – find it hugely helpful and I think also because it’s all written down. I think it’s quite helpful for a parent to have this information. You know, they’re often saying to us things like, you know, it kind of gave me permission to know what I needed to do or not do; it was really clear and helpful and I think patients like it too.
FITNET-NHS was felt to be more intensive than face-to-face treatment as therapists had more contact with patients through weekly e-consultations, ‘it’s more a kind of a little and often approach I think with the more regular contact’ (HP70007). Information provided by patients through the platform as well as e-consultations enabled therapists to get a good sense of patients; ‘they feel quite individual in my mind’ (HP70004) and enabled them to provide tailored treatment advice. Therapists were able to suggest goals and treatment plans according to the lifestyles and interests of participants. Where therapists received detailed e-consultation replies from patients, they felt they could gain ‘a really good picture of some of them’ (HP70004). One therapist described how they had ‘really in-depth kind of conversations’ (HP70004) with some patients over weekly e-consultations allowing her to develop ‘a better relationship than with some of the face to face’ (HP70004). Therapists were able to make comparisons with their therapeutic relationships with other treatment approaches, whereas for participants, FITNET-NHS may be their only experience of treatment.
HP70004:I feel like I’ve got a really good picture of some of them and I’ve been able to be quite kind of specific. Some of it has really nice, I know exactly what they want to be doing, and we can think together about how they’re going to get there. And it feels very like very tailored to exactly what they want to do and when.
HP70006:I probably know quite a lot about her [patient’s] character, like I could really see from what she’s doing and saying that she’s got a real determination.
Therapists also discussed several challenges of treatment through an online platform including: inability to detect patient status using non-verbal communication, using creative techniques, misunderstandings and e-consultations taken less seriously. The ability to use verbal and non-verbal communication was an important part of therapeutic care that was missing for therapists delivering FITNET-NHS treatment. Therapists were unable to read body language or use their own non-verbal communication they would usually employ during face-to-face treatment. They described feeling better able to detect patient improvement/decline in face-to-face appointments and ‘what it is that they might be feeling about something’ (HP70006) based on patients’ non-verbal communication; ‘in a session you can pick up a bit better from body language you know if you think there’s an element that needs working on’ (HP70002). Therapists also mentioned being unable to use ‘playful’ body language and communication such as smiling and jokes to question patients on why they were not following treatment in a non-confrontational manner. Therapists reported that the platform restricted their use of creative skills, such as drawing, to help explain treatment to patients: ‘I draw a lot of diagrams, things like that that you can’t do online and share’ (HP70002).
Therapists felt the conversational nature of face-to-face treatment in person or over videocall enabled them to cover more in an appointment than was possible in an e-consultation: ‘if I’m face-to-face I can just say that to him [patient], and he would say “oh it’s this”. And if he didn’t I could still say, “no no no answer my question” [laughs]’ (HP70004). E-consultations added treatment delays when patients misunderstood content/instructions, because of the need to wait for the next e-consultation for the therapist to clarify and explain. Therapists also reported often facilitating conversations between family members during face-to-face appointments, ‘you can enable people to start communicating differently with each other’ (HP70006), and that this was out of their control during the FITNET-NHS treatment.
HP70007:…when you see somebody; you’ve got their face; you’ve got their body language and like you’ve got a sense of them from physically seeing them, so I think I’ve been quite keen to try and recreate that personal sense of who they are.
HP70006:You’re not having any kind of interaction with the person to know how they’re responding and what their non-verbals might be telling you if you have them in the room. I can see when a young person walks into a clinic room with me, I can see if they’re looking a bit better than they did last week or if they’re looking really bad or I can see what their intonation is when they’re saying something to me. I can see from their intonation, from their gesturing, for instance, what it is that they might be feeling about something and that’s really hard to interpret when all you’re getting is a message.
Therapists felt that some families did not take the FITNET-NHS treatment as seriously as face-to-face appointments: ‘I think people don’t see it like they see an appointment’ (HP70002). Where families would usually call to say they were unable to make a face-to-face appointment, therapists found that some FITNET-NHS patients would fail to reply to e-consultations: ‘I do find people don’t get back to you and I find that really odd’ (HP70002). They talked about finding it harder to convey their credibility in writing rather than during face-to-face appointments where they felt better able to demonstrate their competence/knowledge, ‘… you’re a faceless person’ (HP70006). Therapists recalled instances of patients being less respectful than they may have been in person, such as writing ‘I’m very bored of doing this’ (HP70006) on the platform. Writing e-consultations were also taken less seriously by clinical colleagues. Where a face-to-face appointment may be protected time, therapists were often interrupted and ‘disturbed’ (HP70006) by colleagues while constructing e-consultations.
HP70002:I think people don’t see it like they see an appointment, I don’t think they see it in the same way and so that’s why we have people that don’t message us.
HP70006:I’m not sure how you convey the credibility when you’re not a real person in the same way. Are you less credible because actually it’s just written down? You can’t really get the sense of how much this person believes it or how invested they are in the message.
Making notes to keep each patient in mind and careful construction of e-consultations using patient names, praise, personalisation and giving options were the different skills therapists had to develop to deliver FITNET-NHS. Therapists reported working harder to individualise patients in their mind and build rapport than with regular treatment. Some therapists made notes about patients throughout treatment to remember personal details such as hobbies: ‘because in chapter one they give you a lot of information about themselves, but it’s hard to keep that in mind if it’s just written down, so I’ve kind of got a page of like a summary of everybody’ (HP70002). One therapist had started drawing pictures on her patients notes in order to help remember and differentiate her patients. Remembering key information about patients (e.g. pets) helped therapists provide individualised treatment advice: ‘you want to be able to say you know “go out, spend some time with your dog as a fun thing to do”, but they might not have a dog you know, so those kind of things are quite important’. (HP70002).
The FITNET-NHS therapists had begun calling some families at the start of treatment to work out their current activity levels and felt this was beneficial in terms of getting to know the family and building rapport.
HP70007:…for some young people, if we need more information to work out their activity level, sometimes we need to have a brief call with them. That’s something that’s been sort of agreed, just to determine their activity level and there is something quite nice when you do, do that. I’ve only had to do it for one or two patients I think, but it’s quite nice just speaking to them on the phone...
Writing e-consultations took a lot longer than therapists had anticipated as they often required ‘rewording e-mails several times’ (HP70006) to avoid misunderstandings or coming across as negative or criticising. Therapists described misunderstandings that had occurred; ‘that patients have or misunderstandings that we [therapists] might have’ (HP70006) and having to work hard to convey information in a different way. One therapist felt it required a ‘higher level of therapist skill than seeing someone face to face’ (HP70006) to consider the nuances in written communication from and to, the patient. If the young person was not quite following treatment this posed the most challenge as it required the therapist to ensure they were being ‘empathic about her view whilst also suggesting something different’ (HP70002). Therapists needed to check a patient’s answers on the platform, diary entries and e-mails and construct a response that included the necessary praise and advice without being too long. They described using patient names more than in face-to-face interactions, using lots of praise and including significant information about patients in order to add more personalisation and warmth into e-consultations: ‘you’ve then got to add in bits about trying to make it more friendly and more personable’. (HP70002). One therapist described how they were ‘overly enthusiastic’ (HP70002) in order to come across as motivating and genuine. Therapists also aimed for e-consultations to be more collaborative and less directive to ensure patients ‘take ownership and to decide’ (HP70006) their treatment, for example providing several suggested options. Finally, therapists had to skilfully manage confidentiality between participants and parents: ‘that’s a bit challenging to know how much to share and what to say and whether to address things when they’re not being raised by one of the parties’ (HP70002).
HP70006:What’s been most surprising for me as a therapist is how long it takes you to write a decent message to someone. …you’ve got to be really careful about wording things to make sure that it’s not coming across in the wrong way.
HP70006:I use people’s names a lot more so if I’m writing a message to a young person, I’ll use their name a lot more than I normally would in conversation, again, I think just to build that sense of a bit of familiarity, thinking really carefully about how I open and close my emails. …Lots of praise for things they have done and are working on and noticing too, I guess, some of the things they’ve told me and remembering them, so if they’ve told me about a hobby a bit, remembering that kind of information about them, again, to help them know I’m not just a computer making a script, but I’m holding them in mind, trying to use the words they’ve used, those kinds of things.
All the therapists liked the ‘recovery’ model of FITNET-NHS and felt it offered ‘more’ than simply regulating. The ‘directive’ Dutch approach was helpful to say things therapists couldn’t but was often softened in practice. Therapists felt that the clear ‘recovery’ model of FITNET-NHS helped instil hope in patients. The FITNET-NHS treatment moved beyond regulating activity (as in Activity Management treatment) to then test recovery through deregulating activity and being more spontaneous, ‘we’re aiming for this not be affecting your life at all anymore’ (HP70002). One therapist reported being quite ‘nervous’ about implementing the final stages of the FITNET-NHS treatment where adolescents are required to deregulate their activity and break the routines they implemented during treatment. FITNET-NHS was also felt to offer that bit ‘more’ for those patients who may have had ME/CFS for a long time and developed unhelpful ways of thinking and become ‘stuck’. One therapist thought the principle from FITNET-NHS of ‘shifting attention away from fatigue’ was particularly valuable and had begun to utilise it in her face-to-face work: ‘it’s really made me think about the unhelpful beliefs about fatigue specifically and actually I’m doing more of that now in my face-to-face practice as well as a result of having had the FITNET-NHS training’ (HP70006).
FITNET-NHS focused on building up physical activity first rather than all activity in the Activity Management approach:
… the FITNET-NHS programme we’re trying to use that physical build up for people to change the way they view their fatigue, so the point of the physical activity build up in FITNET-NHS is for them to learn, “I can gradually do more”.
(HP70006)
Sleep advice was felt to be critical: ‘I think that’s true in face-to-face work too, but I think if you help people to get their sleep right at an early stage, that can really help everything else to fall into place’ (HP70006).
HP70002:I think the other element of FITNET-NHS that’s quite different from activity management is where you’re aiming for, so with FITNET-NHS it feels like it takes it to the next kind of step on whereas activity management is kind of about doing the sleep and regulating and building it up. Once someone’s on the road to building up, then it feels like, okay, we’re getting there, whereas FITNET-NHS kind of takes it to… are you actually recovered yet?
HP70006:… with some people where for whatever reason things have got a little bit more engrained, be it because they’ve got anxiety or depression or be it because they’ve got chronic fatigue for quite a long time, or be it because they’ve developed some really quite unhelpful ways of thinking about the fatigue, that you need something more and FITNET-NHS offers that something more. It offers that opportunity to address some of those other unhelpful cycles.
The FITNET-NHS treatment model was felt to be ‘directive’, ‘regimented’ and ‘strict’ compared to the collaborative training as a CBT therapist that some of the therapists were used to. It includes a specific walking plan and advice on remaining upright throughout the day: ‘FITNET-NHS programme are really strict from again a very early stage of chapter five about not lying down during the day and the rationale for that is about the orthostatic intolerance and symptoms that go alongside that’ (HP70006).
Some practical and cultural differences made translation of the original Dutch model challenging to implement in the UK. The suggested walking and cycling activities were difficult for some patients due to the ‘hilly’ terrain in England compared to the flat landscape in the Netherlands, ‘it is really hilly and so the walking and cycling suggestion is quite difficult for some normal people to do, because they’re like I can’t walk without walking up a massive hill’ (HP70002). The approach of categorising patients as ‘low active’ versus ‘relatively active’ was challenging for one therapist:
I’ve never been very good at trying to categorise people as relatively active or low active, because I think that conceptualisation is quite unique to the FITNET-NHS model and to the Dutch way of thinking about chronic fatigue syndrome more broadly.
(HP70006)
Therapists were modifying the original Dutch model slightly to work clinically in the NHS. They mentioned flexibly opening treatment chapters depending on the stage of the child and softening advice given on the platform or adjusting the treatment timelines if the patient was struggling, ‘we adjust it and tailor it to what they can manage’ (HP70007).
HP70006:…we’re trained to be very collaborative to work alongside people. We’re not experts who tell them the answers. We’re people who help them to think about how they can overcome their difficulties and in FITNET-NHS we had to be more directive for two reasons. One is the FITNET-NHS model is a bit more directive in saying things like, right, “you need to build a walking programme”, but even in that we do encourage people to make a plan, the examples in the platform and then they’re encouraged to make a plan of how they’ll do that.
HP70004:I think the platform can say things that we can’t say out loud. The platform can say in black and white and I think in capitals in some places, if you don’t get your sleep sorted you’re not going to get better, in places where it is really quite like if you don’t do this you’re staying ill. You have to do this you need to.
Therapists had received feedback from participants that the treatment was quite intense to start, with a lot of reading which may have caused disengagement in some, ‘They say it is a lot to do at the start.…They just send really horrible messages to me about how [laughs] bad it is’. (HP70004). Therapists suggested the use of audio buttons and videos and well as more images for younger participants, ‘So they can just play it and just listen to the information rather than read it’ (HP70004).
The treatment content was not always relevant, for example, the school chapter was not applicable during the school holidays or for older adolescents on study leave: ‘there is a school chapter that I want to open for a couple of them but they are on study leave’. (HP70004). The use of branching logic and pop up sections to avoid irrelevant content for patients was suggested.
Some therapists felt FITNET-NHS did not address mood specifically, ‘I don’t feel that FITNET-NHS addresses mood’ (HP70002). Therefore, if a patient had significant mood issues alongside ME/CFS it may be a significant barrier to treatment that is hard to detect and treat via FITNET-NHS. Therapists reported that: ‘it would be really nice to have a chapter on depression and a chapter on anxiety’ (HP70006).
Therapists described different types of engagement from patients: ‘people who are following it well, and doing well. Following it well and still struggling and then the people who just don’t seem to be really doing it’ (HP70004). Some patients were reading the platform, completing diaries, responding to e-consultations and following treatment advice. Other patients failed to complete the platform or respond to e-consultations. One young person had fed back to the therapist that she ‘doesn’t like talking about herself or doesn’t like people knowing lots about what she’s thinking’ (HP70002). One therapist described how two patients at exactly the same point on the FITNET-NHS platform had completely different reactions, one negative and one positive.
Therapists found it difficult to gain a sense of patients that replied with short messages and as a result the treatment relied more on the platform instructions rather than individualised therapist suggestions: ‘it’s hard if they send you a really short message because then you haven’t got much to go on’ (HP70007), ‘a bit more falling back on the platform’ (HP70004). Face-to-face appointments were felt to allow more of ‘a two-way conversation about what are the reasons that are stopping you from kind of getting on with this’ (HP70002). By contrast, therapists found it challenging to understand the barriers to treatment when patients failed to respond to messages.
HP70007:… if they’re not as motivated to log on every week and read all the stuff and email me back and it – and we’ve had a few people where it’s really difficult to kind of get started, but that’s the minority.
HP70002:…same week, same, exactly the same thing and one [patient] said you know this is making me think about my fatigue a lot more and it’s making her really stressed and actually she needs to focus on her exams and the other one [patient] said it has been great, you know she’s not focusing on her fatigue very much and she doesn’t get it much at all and you know it’s really great.
Some therapists reported experiencing better engagement and ‘warmth’ in messages from girls compared to boys. Boys took longer to warm up and build rapport and provided more concrete messages while girls shared more personal information and feelings. One therapist felt it was harder to elicit rich responses from boys than girls, who they felt provided lots of detail and moved through the treatment quicker. Age differences were also noted by some therapists. Younger participants seemed to struggle more with the programme and therapists had to work more with the parents to facilitate treatment, ‘parents kind of fill in quite a lot of the blanks’ (HP70004). Parents were an essential source of information for the participants not responding to e-consultations. One therapist had noticed a difference in engagement based on gender but not age. ‘Within my very small number, the girls are doing a bit more pro-active than the boys [ … ] No real difference in age that I’m aware of’ (HP70007).
HP70004:I think some of the boys just take a little bit longer to warm up. So there is a couple who are a bit tricky at first but now they’re good, they will talk to me. Like I say they are all still quite concrete. …most of the girls will share how they’re feeling, how difficult things are. Yes, then we can have a bit more of a relationship.
HP70006:Certainly, the younger ones seem to struggle more with the programme and we need to open chapters and do things slower than with the older ones. …Those people who are younger, we also do much more work with parents generally, I think, sending lots more emails to parents to help them facilitate it.
Therapists described FITNET-NHS as a ‘cure’ for some patients, others as ‘getting there’, however, not yet seeing a shift towards recovery for some at the time of interview. One therapist who had had a particularly good experience with the first patients commented: ‘So at first it was like, oh my goodness this is amazing, like wow’ (HP70004). Patients had reported improvements in their mood because they were able to do more. Therapists had noticed a shift in some patients who had stopped pushing and doing too much activity, ‘Yeah kind of an acceptance and a change of approach from young people’ (HP70007). They felt that FITNET-NHS was better than expected for some patients who had returned to school and part-time jobs. Patients who were investing in it appeared to therapists to be getting more out of treatment, ‘people who work harder at really following the principles get a lot out of it’ (HP70006). One therapist described finding it harder to help patients who were ‘relatively active’ to even out their activity. However, FITNET-NHS did not work for everyone. Some patients had postponed treatment and felt FITNET-NHS made them focus more on fatigue and added stress.
HP70004:I feel like FITNET-NHS has been the cure for these two girls. I just feel like, yes I’m shocked at how well it has gone, it’s amazing. There are others who are doing okay, they are getting there, they are seeing some improvement. And I feel like, yes it’s FITNET-NHS that has done it but something else could maybe have done it. So it’s good but they’re getting there, more slowly a bit more variable but it’s okay. Then others I’m thinking I’m not seeing a shift in you.
HP70002:… the one person who’s postponed, she said she really struggled with FITNET-NHS and that it made her focus on her fatigue and that really brought her down and made her more stressed…
Therapists reported that some families did not adhere to treatment as they were unwilling and this might be linked to how motivated they are and whether ‘it’s their idea to do it and their parents’ idea’ (HP70002). Some struggled to regulate their activity and/or external factors such as schoolwork and exams took priority. Some participants were overactive, continuously booming and busting and struggling to implement the FITNET-NHS treatment. One therapist commented that younger patients had space to change routines whereas older adolescents had the pressure of exams meaning they didn’t necessarily have the space for the FITNET-NHS treatment.
HP70004:I think some of the older ones are a bit more motivated because they want to get well, because they want to do better in their exams. But they’re also a bit like, “I am just about to do my exam I can’t focus on this other thing”. If they are managing okay enough, they don’t want to start mixing things up because they just want to get through.
HP70007:…the relatively active patients, you might have, you know, the boom and bust typically, but young people who were like really busy at school, load of extra curricula stuff; loads going on and it crashing and sometimes lots of different types of situations but often there are patients who are really reluctant to cut stuff down, or you know, kind of high achievers, really driven, so then it can be harder to be doing the regulating. Whereas with the low active it is much more a sense of sort of starting from a simpler place.
Participants’ views and experiences of Activity Management with a therapist via videocall
Table 21 summarises the main themes and subthemes from participants and their parents and therapists.
| Participants’ views and experiences | Therapists’ views and experiences |
|---|---|
Benefits of online treatment
|
Benefits of online treatment via videocall
|
Disadvantages of online treatment
|
Challenges of online treatment
|
Frequency and flexibility of contact with a therapist via videocall
|
Making up for lack of non-verbal communication- working harder to build rapport |
Activity Management treatment content and advice
|
Variable patient engagement
|
| Parental involvement | Parental involvement |
| Contact with a therapist as essential | |
| Treatment effectiveness | Treatment effectiveness
|
Overall, some families liked Activity Management treatment via videocall as they didn’t have to travel and valued speaking to a specialist ME/CFS therapist who gave them individualised advice. Other families still would have preferred to be seen face-to-face and found it difficult to build rapport over videocall. Families naturally talked about the benefits and disadvantages of online treatment, and their views on the structure, content, relationship with the therapist and treatment effectiveness are discussed below.
Families were generally positive about videocall as a method for receiving treatment as they didn’t have to travel and it was still ‘kind of like face-to-face’ (C1000020) and ‘the norm’ (M100093) for teenagers who regularly talk to their friends online. Videocalls allowed participants to engage even when feeling unwell. Remote treatment also reduced payback (increased fatigue and symptoms) from travel and concentrating in a new hospital environment: ‘I find myself a lot more tired if I’m in a new place’ (C1000072). Parents felt videocall was more natural for their child than for them and described it as a bit ‘odd’ and ‘strange’ (M1000093) as it was not what they were used to. They also felt it was beneficial in terms of reduced travel time, payback and less time missed form work and school and it ‘helps being in your home environment’ (M1000020). Some families felt videocall was ‘as good as being physically in a room with somebody’ (M1000107) as treatment for ME/CFS does not actually involve physical contact.
C1000071:Yeah Skype’s easy and I’ve no problem with it.
M1000072:I find it odd, just in that I’m used to face-to-face, and [inaudible 42:10] I’d never done Skype before, so it was a new thing for me, but that’s fine, but from the point of view of appointments and taking (child) to appointments, it was brilliant, because you haven’t got the journey time. You haven’t got having to take her out of school to go to an appointment, you know, and that would have reduced the school time. So, I think it’s worked really well for her condition to have a Skype appointment.
Talking to a therapist over video didn’t suit all patients and some would have preferred to be seen in person. Skype appeared to be easy to use once set up; however, technical problems were experienced such as connection failures and sound problems. Parents of shy participants, or those not wanting to talk/talking quietly, felt videocall was less suitable as it can be very structured with no opportunity to build rapport and have ‘banter’, ‘it is very clinical, there’s no, no personalities involved’ (M1000145). One parent whose child was severely affected withdrew from the trial as her child had been too severe to take part in the videocalls: ‘she [child] wasn’t well enough to talk to [the therapist] anyway’ (M1000039). Some parents thought their child may have taken more personal responsibility for their treatment with more face-to-face contact. An initial face-to-face meeting was suggested as an alternative with follow-up appointments over videocall. Families felt videocall was still preferable to phone calls, which can be ‘a bit strange cause you can’t see them’ (C1000107). However, some families said they would have preferred to see a doctor in person: ‘I would’ve preferred to have had it face-to-face just for the sense it makes it easier … I think it makes it easier to have a proper conversation’. (C1000107).
M1000037:I just don’t like Skype. Full stop. I do like to sit in a room with somebody…it was good ‘cause obviously travelling down, we physically couldn’t do it anyway. Doing it this way does actually work even though I don’t like it.
M1000093:I think if (child) had known that he was going to have to go and see somebody at some point he might have taken on a little bit more personal responsibility for it.
Overall videocalls were found to convenient; however, there were divergent views about how often these should occur. Arranging videocall appointments was found to be flexible as families were able to ‘choose’ a time. However, one family talked about how they were restricted to arranging appointments during 9 a.m.–5 p.m. working hours meaning they had to take time out of school/work, stating that evening appointments would have been helpful. There were mixed views about the time in between videocall appointments. Some families felt they were given the ‘tools’ and needed time to implement the changes. Others described feeling unsure if they were ‘doing it right’ (C1000107) and if they relapsed in between appointments they simply had to wait until the next videocall appointment. More frequent weekly contact or the ability to have a shorter therapist call/e-mail would allow them to ‘check in’ and correct any uncertainties. Some therapists had been flexible and allowed families to split appointments to allow for more contact following setbacks.
M1000093:Yeah it was probably about right. I’m trying to think. I mean it was probably a couple of months in between each call, and I would say it was about right, because actually they give you the tools and they say this is what you need to do.
C1000145:…one major call, like, every month or so, but then had, like, shorter calls just to check up on how things are going.
The diaries helped families understand what activity the young person was ‘actually doing’ (C1000020) and visually see and understand what changes were needed. They were able to track activity and rest, communicate with their therapist and see improvements. Some families found it difficult to remember to complete them every day and maintain completion. A digital fitness tracker was used by one parent after losing the paper diaries.
M1000031:I thought the chart was a really good way, a very visual way of (child) being able to see what he needs to do and cut down on or pick up on, if you see what I mean?
M1000100:I like the graph better on the FITBIT thing, because it actually shows how much resting activity and how much active and how many steps. And we’ve definitely seen an improvement since we’ve been monitoring that.
There were differing views about the advice provided during Activity Management treatment. Some patients felt it was individualised and helped them manage their activity; others struggled to follow it or felt it was nothing new.
The initial videocall appointment with a therapist helped parents and participants understand the difference between high- and low-energy activities. Parents felt they got ‘more backing’ (M1000100) and reported advice on changing bedtimes, sleep and reducing screen time to be most helpful and had the most impact on their child’s condition. Participants reported that the advice helped them structure their day and use their energy efficiently and had begun to even out their activity over the week to reduce boom and bust cycles. Parents felt they received useful tips on how to manage their child’s school attendance. Some therapists had been able to communicate directly with schools to advise on ‘special arrangements’, such as rest breaks for the young person, that families reported to be extremely helpful. The therapist was perceived as being experienced and understanding of in the condition and parents felt they received personalised advice based on their child’s interests: ‘So, the fact that she’s [therapist] got so much more experience with it, each time, she came up with some ideas based on [child’s] interests’. (M1000100). One parent described how the advice was more ‘practical’ and ‘precise’ than she had been expecting.
M1000072:The whole of the first appointment with (therapist) was hugely helpful, just everything she said was really helpful. The most helpful bit has been knowing that some of the activities (child) was doing that we thought were low-energy are actually high-energy.
M1000031:So (therapist) was really good at getting the stuff to the school to say (child) might need these special arrangements. So the study for us was a bit of a lifeline really, it has been, we can certainly see an improvement in (child) without a doubt. I don’t know, if we didn’t have the study available to us, I don’t know whether he would have got through his GCSEs.
Some of the families felt the advice was difficult to follow, didn’t take into account comorbid conditions and was missing psychological support. The advice was not clear to all families: ‘… I don’t know to move it up by how much and how often. So, kind of, it does feel a bit like you have the equipment then it’s just go out and do it and you’re not always clear on how to’ (C1000107). Not all families were following the treatment advice, particularly around reducing school. Some were too unwell and parents worried about ‘pushing’, others were reluctant to reduce activity, ‘It’s annoying that I have to cut down some stuff’ (C1000072) and parents found it hard to make sure their child followed the advice. One mother explained that her son’s ADHD medication interfered with his sleep and therefore made it difficult to follow the recommended Activity Management treatment, but there was ‘no one really dealing with chronic fatigue and ADHD together’ (M1000100). Some families acknowledged that not following the advice may have impacted on recovery, ‘But then you see, that could be our fault then couldn’t it because we’ve not done what we were told’ (M1000037). Another young person had found it hard to make the changes to his bedtime, medication, exercise and routine all at once: ‘… but it’s kind of hard to … you’ve been told to make all of these changes and it’s quite hard to kind of keep track of them all’ (C1000107). The fluctuating nature of the condition made it difficult to follow treatment for some: ‘It doesn’t take very much for (child) to have like a bad day and throws things out the window’ (M1000128). The ‘psychological’ aspect of treatment was missing from the advice for some families: ‘So, I think apart from just physical, a little bit about how to emotionally cope with being stuck in your house with no friends would help’ (M1000071). A few families felt the advice was ‘nothing new’ and one parent felt they didn’t get medical help: ‘I thought he was going to get medical help, but it’s, obviously it’s trial’ (M1000145).
M1000007:Originally, she [therapist] was saying maybe to miss a bit more of school, reduce the school, but we’d kind of worked ourselves up to get back to 100 percent. That was a little bit in conflict with what we’d already achieved.
M1000128:…it’s, it kind of feels that we’ve taken on all this advice but it is hard to implement it.
Most parents and participants attended videocall appointments together, some would have liked separate calls and some parents felt the treatment relied on them to implement it. Participants were happy with parents being present during videocall appointments as they often struggled with memory and parents could help fill the therapist in when they were tired. Some participants and parents wanted to have the ability to have a separate videocall alone so they could talk about things that might ‘upset’ each other or mental health issues. One family had calls both together and separately and felt both worked well. Parental involvement and ‘nagging’ were often taking place to encourage participants to adhere to treatment. Parents of older participants wanted them to take responsibility for their treatment: ‘at the moment I’m putting it in her hands’ (M1000120). A mother of a 16-year-old felt the treatment ‘relies very much, I think, on parents running it almost’ (M1000093) and would have liked more contact between the therapist and her child to reinforce the treatment.
M1000128:Yeah, I can try and remember things and I can jump in if (child’s) struggling or getting really tired.
C1000107:I mean it works okay. There’s points where it would be best to just do it individually. Like things just when you’re talking about say mental health and stuff, it can be easier just to do it one-on-one. But also like my mum tends to have a lot to say in the appointment, so it’s important to have her there.
Contact with a therapist was essential to all the families receiving the Activity Management treatment. Families were very positive about the therapists, describing them as: ‘nice’, ‘reassuring’, ‘approachable’, ‘excellent’ and ‘supportive’. Therapists were able to find ‘common ground’ with participants and some families described ‘having a laugh’ (M1000107) over videocall appointments. Therapists were felt to be ‘very good at explaining’ (M1000031) what participants needed to change as part of treatment, and participants felt they gained advice that was specific to them and could ask questions: ‘Well, she’s really good, she’s really nice and she is always able to answer any questions I have’ (C1000107). Parents felt supported as there had ‘always been somebody there if you needed somebody’ (M1000037) and reassured that they would improve. Parents additionally thought the therapist calls gave them the ‘backing’ to help reduce their child’s activity and their children were more likely to ‘listen’ to an independent health professional: ‘hearing it from somebody completely unrelated, you know, of course he’ll listen to that’. (M1000093). However, one parent described how her child felt obliged to tell the therapist he was fine when he was not.
C1000071:Yeah, she [therapist] actually gave ones [advice] that were very relevant to my situation or me specifically.
M1000093:I almost would have liked to have had a separate chat away from (child) to say actually look this is really what’s happening. (Child)’s telling you what he thinks you want to hear, but this is really what, what’s going on.
Most participants were positive about the Activity Management treatment. Some families had only just started the Activity Management treatment and participants had noticed ‘a bit of improvement’ (C1000072) in their health such as better sleep, doing more activities and attending more events but were often still tired or experiencing symptoms. Activity Management was helping them to better manage their ME/CFS, be aware of what causes them to feel worse and structure their activity. A few participants reported no change, ‘I still feel the same’. (C1000007), and one noticed an improvement but had recently crashed again.
Parents similarly reported that their children were coping better and had ‘more energy [ … ] eating more [ … ] feeling happier’ (M1000031) but were still ‘struggling’ (M1000100). One parent felt the Activity Management treatment was a ‘lifeline’ as it had enabled her child to ‘get through his GCSEs’ (M1000031). Other parents felt it was simply a ‘coping strategy not a cure’ (M1000120), helping them understand the illness, but felt the basic advice was ‘nothing new’ and ‘not enough’. Colds and overactivity caused relapse and acted as a barrier to recovery ‘if she gets that cold, that’s it her completely laid out for 2 weeks and we are back at square one’ (M1000120).
C1000020:It was really good. It sorted a lot of my sleep out. I used to sleep quite, [laughter] I say quite a lot. I used to sleep the majority of the day.
M1000120:That’s all it is, it’s some sort of coping strategy, whether that’s the CBT or – or the graded exercise. It isn’t a cure.
Therapists’ views and experiences of delivering Activity Management
Therapists overall felt that treatment via videocall had a good part to play, was better than telephone appointments, but would never be as good as face-to-face. Therapists acknowledged the benefits for families in terms of not having to travel and therefore not missing work and school. They felt NHS patients should have the option to be seen in person or remotely based on preference: ‘it’s not for everybody but I think it’s definitely – it should be an option I think’. (HP70009)
HP70009:It has, you know it’s never gonna be quite as good as face to face I don’t think but I think it’s definitely a good second place or a you know, a good alternative and it’s much better than being on the phone.
HP70005:I think it’s brilliant. It is potentially giving people opportunity who don’t have any input whatsoever.
Therapists felt videocall technology had a good part to play in treating young adolescents with ME/CFS and discussed a number of benefits including: patients more relaxed at home and able to leave the call and return if tired/upset, and greater insight into patients’ home life.
Hands-on physiotherapy work is not necessarily required for ME/CFS, ‘It’s quite rare that we do stretches or manual handling unless it’s a specific problem’ (HP70008) and often patients are too tired to do anything physical in an appointment, ‘the appointment itself is draining. So to try and get them to do physical exercise as well, even demonstration it’s too much’. (HP70005). Videocall appointments had also become a more routine part of usual care: ‘I’d actually say the amount of Skypes that I’m doing is increasing’ (HP70009). Therapists felt patients were often more relaxed at home, particularly as they did not have to travel. This enabled them to gain a better picture of the home environment, ‘people may bring in other family members because of it, so you get introduced to the cat and stuff’ (HP70005). Being in the home environment also allowed appointments to be quite flexible and patients to leave and come back if they became tired or upset; ‘I’ve had times where the young person’s left ‘cause they’ve got upset and then they come back in when they feel a bit better’ (HP70009).
One therapist found that for videocall sessions ‘attendance seems to be better than actual face-to-face’ (HP70009). Patients seemed to have embraced the technology and had been organised and e-mailed their diaries prior to their videocall appointments: ‘they’ll e-mail across their activity diaries or information sheets so we’ve got that information before clinic which can really help’ (HP70009). One therapist commented that videocall also worked particularly well for patients with Autism Spectrum Disorder as ‘it doesn’t change their routine too much and you know they don’t have to go into that busy environment’ (HP70009).
HP70005:And the other thing is, often people are different in Skype, than they are in a clinic environment. Because they are at their home, they are feeling more relaxed. They haven’t had to turnout and travel somewhere, alien environment, being uptight with the traffic. The poor child being exhausted with the travelling, so in many ways you can get a better view of exactly what’s going on for them at home.
HP70009:You end up doing sort of a Skype, like you do a slightly different consultation. I can’t really explain it but a slightly different consultation and maybe it’s a little bit more relaxed, I don’t know ‘cause you’re sitting there with Skype and their Skyping at home so maybe they feel – normally they drag a pet in so they’re sitting there with their dog [laugh] and you know, that kind of thing.
Overall therapists felt video calling was ‘second best’, the quality of communication could be disrupted by technical problems, therapists were unable to control if patients became distracted and the home was felt be less of safe space to discuss sensitive topics.
Therapists felt face-to-face treatment was still the ‘gold standard’ (HP70009); however, videocall had a ‘good part to play’ (HP70005), and ‘it’s definitely a good second-best’ (HP70008).
Physiotherapists felt that it could not replace the physical contact that is sometimes needed to assess patients or demonstrate exercises. Paper activity diaries were less practical as families had to have facilities to scan and e-mail copies to the therapist to review, or they would ‘just hold it up to the screen’ (HP70008). One therapist was using visual diagrams over videocall: ‘I had to come up with my own way so I’ve got visuals that I use’ (HP70008). Technical problems also made it harder for therapists to convey their expertise and they sometimes felt they were taken less seriously.
HP70005:But if they do have a particular problem with something then you can’t investigate that further, because they are not in front of you, so it doesn’t replace everything but I think it has a very good part to play.
HP70009:I think it’s [Skype] harder because it’s, you know, as a therapist you look at body language, you look at you know communication and sometimes if the quality of the picture’s not very good or the WIFI’s cutting out or so kind of technical issues sometimes or they can’t quite work the camera so there’s only half their face in the shot or you know, that kind of thing or sometimes you’re not quite sure whose in the room ‘cause you suddenly hear a voice and your like oh, there’s somebody else there and that can be a bit off putting sometimes so I think face to face is kind of almost like the gold standard.
The success of videocall appointments and the contact and quality of communication between the therapist and family was often dependent on the internet connection. Problems including screens freezing and intermittent audio would interrupt the flow of conversation. Therapists described ‘shouting over the computer’ and getting more to the point, rather than providing more detailed explanations. This was particularly problematic for patients who had hearing problems and therefore needed to lip read. Therapists had found it ‘frustrating’ but reported that the majority of families had been tolerant and accepting of the challenges. Even when therapists ensured they had good internet connection, they were unable to control the strength of connection in patient homes. Interrupting the flow of conversation was particularly detrimental when discussing sensitive topics: ‘they’re saying something quite sensitive and it’s cut out and you’re like pardon’ (HP70009).
HP70004:There are still times where their connection might not be very good and it breaks up and the camera freezes. It keeps doing that at the moment. So there are a few little niggles. In general I probably find Skype the difficult of the three [treatment approaches] I think. I don’t know why, because I think you know when you’re typing for FITNET-NHS, you can be quite warm and you can kind of... Yes, oh that is fab, lots of exclamation marks that kind of think. But you can do it with Skype, there is a bit of like, I’m shouting at you over a computer and you keep missing bits of what I’ve been saying.
HP70008:… you’re trying to have a conversation with someone and it’s frozen on your face and a really awkward facial expression and they’re trying to take you seriously... Or I’ve got a kid, a boy, who spends the whole time just playing with his hair on the screen.
Therapists were unable to control distractions in the home environment and some felt it was easier for some participants to disengage over videocall compared to a face-to-face appointment:
I think it’s maybe easier to opt out in the videocall. I think they can do it in certain ways that they’ll come off the screen a little bit and then come back in whereas in face to face, you can’t.
HP70009
Issues with confidentiality were also discussed, as it was not always clear to therapists who was in the room during a videocall appointment. Therapists reported that some ‘parents stay back out of the camera’ (HP70005) and one therapist had experienced a father contributing suddenly while off camera. Clinic rooms were felt to be a ‘more of a safe space to talk about things’ (HP70008). Therapists routinely ask parents to leave the room in standard face-to-face treatment. However, they were not always sure if the child felt the parent could still hear the conversation during a videocall appointment and therefore would be less likely to confide in them. One therapist felt a route to allow participants to raise confidential issues, for example through e-mail or telephone would be essential to address safeguarding issues.
HP70003:I would just assume that the two people sitting in front of the screen are the people there and then now and then you hear somebody chipping in, usually the dad chipping in from the other side. So I think I need to say more explicitly, “Who else is in the room with you?”
HP70009:… if somebody’s in the house maybe kind of that could overhear so that’s perhaps an issue that we may need to look at and I don’t know just maybe have an option for young people to say look normally we ask to see you on your own, if there’s anything you feel like you don’t…can’t say you can always email or you can phone later or yes.
Videocall made it harder for therapists to pick up on non-verbal communication such as how the patient held themselves, their reactions and engagement that indicated if they were tired, improving/declining or upset as well as family dynamics. Therapists felt it was harder to gauge if a patient was actively listening over videocall and if they were getting tired, bored or had wandered off, ‘if they look like they’re flagging then you can be like, ‘OK, well, let’s go away and have a 10-minute tea break’ (HP70008). They found it harder to use techniques such as pauses to encourage participants to talk over videocall: ‘That silence doesn’t have the same effect as it does in a room. You know when you go, “Let’s think about this,” and you don’t say anything until they say something, that doesn’t have the same effect over Skype’ (HP70008). Therapists described having to consciously make an effort to build rapport by looking at the camera and maintaining eye contact as videocall ‘loses a little bit of that intimacy’ (HP70008). One therapist felt treatment worked best when they had had one face-to-face meeting with a patient to start, then moved to remote follow-up appointments: ‘I think it’s worked best when perhaps I’ve seen somebody face-to-face and then gone to Skype because you had that initial meeting’ (HP70009).
HP70003:I felt very much that being in the room with them I would have felt more of a nonverbal conversation would have been there so I would have been able to feel the dynamic of what the girl and the mum were both thinking.
HP70007:…being really conscious to sort of look at the camera rather than at the screen where you can see them, but look at the camera so that they’re seeing eye contact and they’re feeling that sense of rapport. Try not to look down too much to write your notes and stuff, because if all you’ve got is that screen then it just makes you adapt really.
Therapists reported varying experiences of engagement from families to those who prepared well for the videocall appointment and followed the advice, to those who seemed to take it less seriously than a face-to-face appointment. One therapist found that families taking part in the study tended to be well prepared for the videocall appointments, ‘They really try and make the most of the sessions’ (HP70008). Another therapist noted that families taking part in the trial seemed more motivated than those in usual care and wondered if this was due to their previous ‘struggle’ and ‘time taken to get to this point’ (HP70009) due to the lack of available treatment. The set number of videocall sessions as part of the trial may also have played a role.
HP70009:I wonder whether because they only know that they’ve got a set number of sessions whether they’re a little bit more motivated to kind of go right this is all we’ve got, we’ve gotta try and do everything.
Some therapists had experience of families physically not engaging, not looking into the camera during appointments: ‘There were great issues on our first Skype call in as much as the son didn’t want to look into the camera and neither did mum’. (HP70005). They felt some families took the videocall appointments less seriously than face-to-face appointments and one therapist described a family taking the videocall from a car park. Therapists found some families were not willing to follow the advice: ‘some people are very adamant that they don’t want to give up school or they want to walk to school’. (HP70008).
HP70003:… the girl didn’t really look at me or the screen or anything, she was just sort of staring away to one side, I felt as though it was mum that was doing all the pushing really.
HP70008:“Why have I got to talk about this? I don’t want to talk about this.” So they’re not very respectful of you being, “This is a session. This is important that we are engaged with this.” Or someone’s been talking to me on the phone from a car park.
HP70003:… they really didn’t want to change anything else and they didn’t want to cut back on any of the other subjects that she was doing and that seemed to be a joint decision.
Some age differences were noted. Older adolescents seemed to like and engage better on videocall, ‘I think the older teenagers do pretty well with Skype ‘cause it’s technology and you know, they like it’ (HP70009). Some therapists found it slightly harder to engage younger participants on videocall and treatment often fell on the parents. A different method to engage younger adolescents was suggested, ‘they could engage by writing you know’ (HP70009). Another therapist felt there were no differences in terms of age.
HP70009:I’d definitely say it’s harder with the younger children on Skype. They tend to not engage so much, and I don’t…I don’t know whether that’s because they’re more severally affected the ones that I’ve seen or and that’s why we’re Skyping, but I would often say the younger one, it tends to be more parent based so I’ll perhaps see the child for 10 minutes or I’ve had one outside the study that I’ve never seen the young person in a Skype. They’ve never wanted to come on the screen.
HP70005:I don’t think I can distinguish any differences because of age. I think people are either comfortable with it or not so comfortable with it.
Therapists were most often seeing families together on videocalls and were also seeing them separately at times. They also found managing the ownership of the treatment between parent and child challenging. Therapists felt it was important for parents to be in videocall sessions, particularly for participants with cognitive impairment, as patients found it difficult to take in and retain information given during appointments. Some therapists were flexibly undertaking videocall appointments with the child or parent alone:
There are situations where I will give the opportunity for a child to be on their own talking to me. And equally if parents need time to talk to me individually as well, there may be something that shows it is necessary to offer that.
HP70005
One therapist described how a young person behaved differently when Skyping alone compared to Skyping with a parent present and did not find videocall sessions with a younger child alone as productive ‘she was sort of ten nine ten. So that was not as productive’ (HP70005). The responsibility for implementing treatment often fell on the parents and therapists found it difficult to shift ownership to participants: ‘Sometimes Activity Management is more for the mums and sometimes it’s hard to shift the control over to the kids’ (HP70008).
HP70008:Sometimes their attention might not be very good if their chronic fatigue’s very bad so it’s good for the parent to be there to take the information home with them, to carry it through so they retain it. Sometimes they have questions about school that maybe the kid hadn’t really... They have said but not really thought about in the session.
HP70005:I had a one to one, sorry a Skype appointment with an individual without their parents. And I found it very productive. He was really engaging in talking, things were going well. His perception that things were going well for him. Talking to mum at another time, then she has reported back that actually he doesn’t like Skype lessons very much. And things aren’t going as well as he says they were. […] But when he did Skype with his mum and he did Skype on his own, he was very different. So I’m wondering whether it’s more mum doesn’t like Skype.
Therapists had seen some patients recover, some patients manage the condition better, ‘They tend to not have as many crashes and they’re pacing themselves better!’ (HP70008) and some patients had been re-referred, ‘one went to the adult service, one’s come back’ (HP70009). One therapist commented: ‘I think it works for some people and I think it doesn’t work for other people’ (HP70009).
HP70008:Oh, yeah, there’s a kid who was only doing an hour at home of home tutoring and he’s managed to get all of his GCSEs …he’s managed to go outside for a little walk and he’s improved his walking. He’s able now to get into college to do an A-level in a college setting, so I think that’s quite a big improvement.
Participants were not always following the treatment and specific reasons why some patients failed to engage in treatment were identified such as one family wanting psychological help in particular and being disappointed when randomised to Activity Management: ‘I think they were quite disappointed when they were randomised to the Activity Management only. So it is very difficult to establish another appointment at the moment’. (HP70005). The particular challenge that therapists faced was trying to stop participants booming and busting and even out their activity. School exams often took precedence in a young person’s life and made it hard for them to focus on treatment. Therapists would often put the programme on hold.
HP70009:It’s the booming and busting I think the kids do mostly that I’ve seen so they tend to – they’ve got that little bit of energy and then they go for it and then they’ll do a full day at school or half day at school and then they’re in bed for two days so it’s just getting them to realise to kind of conserve – kind of rest and save a bit of their battery for the next day.
HP70005:… they are just about to take on their GCSEs. They’re only just managing. It is not unusual; I have had lots of young people at this time of year who that happens, and you get so far in and you think it’s not fair on them to put extra things extra plans extra pressure on them really. So I tend to give them advice on damage limitation over that period of time. And so the recovery programme is sort of put on hold.
Suggested future improvements to the trial treatments
Table 22 summarises suggested future improvements to the trial treatments if they were to be rolled out into standard care. These are additional to the smaller changes that were made during the trial (presented earlier).
| Suggested changes to treatments | Quote |
|---|---|
| FITNET-NHS: Tools to decrease the amount of reading: print button, audio and dictation buttons |
M1000149:‘You’re reading it online. There’s no print button. Actually, if it had a print button so we could print the jargon, that would be so much better because you’re trying to read it on a screen but your eyes get tired after a time when you’re looking at a screen. If we had written off the jargon to sit down on the sofa, because then you can make notes and you can make comments on the – but you couldn’t comment on anything.’ |
| M1000384:‘You know like sometimes on Google you can type in a word and there’s a little speaker at the top and you press it and it reads it. So, maybe a way of something like that and then it reads it to you, and then you could actually, similar to WhatsApp, press the button and then say your answer, rather than typing it out. It’s just all things that then make it easier for somebody that, you know, does have quite bad brain fog, or can’t concentrate for long periods of time. Does that make sense?’ | |
| FITNET-NHS: Branching logic to enable patients to skip irrelevant content or tailor content to ensure less content for younger patients or those with cognitive difficulties |
HP70007:‘I would want it to be a bit more varied in terms of how the text is presented. So I believe it did used to be a bit more interactive with like pop up screens or press this for more information pops up from what I know – which is little – I think that was taken to a patient group and they suggested that that was taken out. I think it’s quite a lot of text for them to go through, but then people manage, so … And I think it’s tricky ‘cause you’ve got quite a range of ages accessing it, so getting a one size fits all in terms of the interactiveness I think would be quite hard, ‘cause an 18 year old or a seventeen year old is not going to want the same as a 12 year old and that sort of thing. But that’s the only thing.’ |
| ACTIVITY MANAGEMENT: Consistent sleep advice between therapists | HP70008:‘We all seem to prescribe different amounts of sleep. The two paediatricians seem to be giving quite a lot and when I came, [name] is using what we call the [name] Clinic. So if you’re 13, 14, it varies as to how much sleep they should have.’ INT:‘For their age?’ |
| HP70008:‘For their age. Then when they get to 16, we’re looking at seven to eight hours’ sleep. I think with things like that it would be good to have everybody on the same page. I think there’s so much variability between patients. I do think there could be some more consistency between what each clinician says.’ HP70008: ‘You could benefit from having a shorter session more frequently.’ |
|
| ACTIVITY MANAGEMENT: Better handover to local team following treatment | HP70009: ‘The worry I have is the handover ‘cause a big part of the protocol I guess was to once you’re … you’ve completed your treatment, you hand … try and hand it over to the local service and they carry it on but that hasn’t happened. There isn’t … there doesn’t seem – I think that’s the whole point, there isn’t a local service there to pick it up and the GPs can’t really necessarily manage all of the activity management, you know they don’t have the resources.’ |
| BOTH TREATMENTS: One face to face appointment to start treatment | HP70007:‘So I know for some young people, if we need more information to work out their activity level, sometimes we need to have a brief call with them. That’s something that’s been sort of agreed, just to determine their activity level and there is something quite nice when you do, do that. I’ve only had to do it for one or two patients I think, but it’s quite nice just speaking to them on the phone..’ |
| BOTH TREATMENTS: Better working arrangements with schools | D1000064:‘How we get through the next part I don’t know because it will be the same techniques and ideas as the steps change and progress but because with reintegration to education because it involves so many different people and new people who aren’t aware. If you can get them up to speed, get the understanding in place that’s going to be a challenge. We’re hoping at college level it’s going to be better than it was at school because over two years at school it was hopeless. The people who were dealing with (name)’s education just refused to accept that there was anything other than an emotional issue which meant they didn’t have to deal with the consequences of their actions and determinations about how (name)’s education progressed. So, the experience at school was just very poor and experience of an education delivered through a virtual learning environment that was very poor because (name) was classed in with … basically put in with people who didn’t want to attend school.’ |
Chapter 8 Discussion
Implications for decision-makers
This study has shown that children and young people are more likely to have better physical function at 6 months and attend more school at both 6 and 12 months after receiving FITNET-NHS compared to Activity Management. However, at 12 months, physical function is similar in both FITNET-NHS and Activity Management. Our results suggest FITNET-NHS is unlikely to be cost-effective compared with Activity Management in the short term and therefore we would recommend further research is conducted on more cost-effective approaches to provide treatment.
Strengths and limitations
The main strengths of our study include the randomised allocation of the intervention, pragmatic evaluation of the interventions as delivered in an NHS service, the economic and qualitative evaluations, recruitment across the UK (improving generalisability), and good retention.
The main limitation is our inability to blind participants and their families to their intervention allocation, hence reporting bias may affect the patient reported primary outcome. Although we had good retention in terms of the primary outcome, half of our patients had some missing data over the 12-month follow-up, affecting the economic analysis in particular. We tried to overcome this by using primary care data, but this was not possible, and therefore we relied on parental report of primary care contacts.
A minority of participants scored highly on the baseline SF-36-PFS, suggesting that, ahead of treatment, some participants are not aware of how disabled they are by their illness. Consequently, the primary outcome will not be sensitive to any improvements and may suggest the participant is getting worse as they become more aware of how ME/CFS is affecting them.
We followed up participants for 12 months; however, it is possible that the impact (particularly of school attendance) would continue to be seen after 12 months. It is possible FITNET-NHS would become more cost-effective if its clinical benefits were maintained or even increased beyond 12 months.
Generalisability
This study is generalisable to children and young people aged 8–18 years who do not have access to specialist local ME/CFS care. We had few patients entering the trial from Scotland, Ireland and Wales, and therefore this study is not necessarily generalisable to them. The trial participants were unwell (157/281 participants were attending 40% or less school at recruitment); therefore, the results may not be generalisable to those with mild ME/CFS.
We recruited children and young people using the NICE 2007 definition for ME/CFS but collected sufficient data to ensure we can re-analyse our results using different criteria. We conducted a post hoc analysis with only participants (one participant was excluded as we did not have sufficient data) that would have received a diagnosis using the NICE 2021 definition and the results were consistent with our primary analysis.
Equality, diversity and inclusion
The 2021 UK Census shows that, nationally, about 30% of 11-to-17-year-olds are from ethnic minority backgrounds. 83 Of the FITNET-NHS participants, 9% were from ethnic minority backgrounds, this being consistent with the low level of children and young people from ethnic minorities who are referred to our service. This will in part be due to the study not recruiting from London, which is home to large ethnic minority communities, as there are specialist ME/CFS services locally at University College London Hospitals NHS Foundation Trust and at South London and Maudsley NHS Foundation Trust. The very limited data on the prevalence of ME/CFS in different ethnic groups do not suggest that the relatively low proportion of FITNET participants from ethnic minority backgrounds is a reflection of the disease’s epidemiology. 84 With so few participants from minority ethnic backgrounds, it is not clear from the present study if FITNET-NHS is effective for them; a study recruiting in London would be required to make specific conclusions about the effectiveness of FITNET-NHS in adolescents from ethnic minority backgrounds.
We wished to evaluate FITNET-NHS as a way to increase access to treatment among adolescents with ME/CFS and without a local specialist service. However, for those living in poverty, a lack of internet access (the ‘digital divide’) and a private space in which to speak to their therapist remain as significant obstacles to treatment delivered online. 85 Whilst very few adolescents referred to the present study were recorded as excluded because they lacked internet access, it is likely a greater number were not referred.
Interpretation
Effectiveness
This study is consistent with previous RCTs that have shown that CBT for fatigue, delivered face-to-face with children and young people with ME/CFS, results in improvements in physical function and school attendance. 8,20,22 However, our results were less effective than the Dutch FITNET study (N = 135 randomised to FITNET or usual care), or the Dutch implementation study (a large observational cohort of 244 adolescents who started FITNET). 8,23 The Dutch FITNET study used dichotomised outcomes at 6 months, and 85% of participants randomised to FITNET no longer had severe fatigue, 75% were attending full-time school and 78% had ‘normal physical function’. 23 This compares to a mean school attendance in our study of 52% at 6 months in the FITNET group. In the Dutch FITNET study, 66% were defined as having recovered if they had a fatigue score and physical function score within the normal range, and if they had attended 90% school within the last 2 weeks. This compares to our study where only 32% said (using the CGI) that they were very much better or much better at 6 months.
This difference could be due to differences in the participants, or differences in the way treatment was given within the study and in routine care. The participants in our study appeared to be more severely affected and had a higher rate of comorbid mental health problems compared to both the Dutch FITNET trial and the FITNET implementation study. At baseline, 50% of FITNET-NHS participants were attending only 2 days a week (40%) or less of school. This compares to a much higher mean school attendance (61%) in the implementation study. Participants in FITNET-NHS also had a much higher rate of comorbid mental health problems: 46% of our participants had depression that was clinically significant. However, in the Dutch FITNET study, only 14% scored more than 16 on the CDI (threshold for clinical depression is 20), suggesting a much lower rate of comorbid mental health problems.
Alternatively, the reduced effectiveness could be because the adherence rate for FITNET-NHS was much lower (only 37.4% of FITNET participants completed 80% or more sessions in FITNET-NHS), or because in FITNET-NHS we did not offer face-to-face assessments. Adherence rate is not reported in the Dutch FITNET trial or the implementation trial but is thought to be higher than in FITNET-NHS Whilst our qualitative data suggest that FITNET-NHS was acceptable to most patients, it was considered burdensome by some. Low retention rates in online interventions is well described, and our adherence is consistent with studies in adults. 86
We did not offer face-to-face consultations in FITNET-NHS as we were testing whether FITNET-NHS could be offered throughout the UK for those with no access to local services. This is a key difference to the original FITNET trial where participants were seen face-to-face at assessment. It is also very different to the FITNET implementation study where 102 (41.8%) had a least one face-to-face consultation, and of these, 41 (16.8%) had three face-to-face consultations. Our qualitative data suggest that some face-to-face appointments may improve engagement.
Comorbid anxiety and depression
Little is known about the impact depression and anxiety have on treatment outcomes for paediatric ME/CFS. Most RCTs have excluded participants with significant comorbid anxiety and depression and few (if any) have stratified by comorbid mental health problems. Our baseline results, which showed that participants in both FITNET-NHS and those allocated Activity Management, had worse physical function if they had comorbid depression and anxiety is consistent with observational cohorts. We did not show a difference in outcome between those with comorbid depression and anxiety and those without depression and anxiety; however, we did not have sufficient power for this analysis.
Using remote interventions for young people
During the pandemic, adolescents with ME/CFS were unable to access face-to-face assessments or treatment and FITNET-NHS provided a safe method for assessment. This type of approach is acceptable and feasible. Our qualitative analysis provides rich data on how to improve remote interventions for children and young people including using more audio data, branching logic to enable participants to skip irrelevant content. One face-to-face assessment was a recommendation from health professionals, which is consistent with the improved outcomes from FITNET and the FITNET implementation study. 8,23
Safety
In the FITNET implementation study, 3/244 (1.2%) of participants reported a clinically significant deterioration of fatigue severity and 10/244 (4.1%) in physical function. In the waiting list for face-to-face CBT, 2/35 (5.7%) had a clinically significant deterioration in fatigue. This is consistent with studies in adults, where the frequency of symptom deterioration varies from 2 to 12% in patients receiving CBT and from 7 to 17% in the control group. 87 In our study, 10% of participants reported being much worse or very much worse at 6 months using the CGI. Only 3 (2%) reported a worsening of the physical condition and 7 (5%) reported worsening and withdrawing from treatment. This is similar to the FITNET implementation study and to studies in adults.
Economic evaluation
Results from the primary analysis suggest FITNET-NHS is unlikely to be cost-effective compared to Activity Management within the first 12 months. Our findings were not sensitive to alternative costing methods; EQ-5D-Y value set; and assumptions around missing data. In all analyses, FITNET-NHS resulted in higher costs than Activity Management. This difference in costs was driven mostly by the greater amount of consultations offered through the FITNET-NHS programme.
In our primary analysis, even at a WTP threshold of up to £50,000 per additional QALY, the probability of FITNET-NHS being cost-effective compared to Activity Management never rose above 25%. More specifically, the shape of the CEAC indicated that FITNET-NHS was unlikely to cost less than Activity Management, though there is uncertainty as to whether FITNET-NHS is likely to result in a gain in QALYs. However, when we restricted the cost-effectiveness analysis to participants with comorbid anxiety/depression, the results were more favourable for FITNET-NHS. FITNET-NHS was designed to treat those with comorbid anxiety/depression as well as ME/CFS. However, the analysis was powered on the primary outcome, and so our subgroup analysis should be interpreted with care. Nonetheless, our subgroup analysis warrants further research, especially as children with comorbid anxiety/depression have been previously excluded from ME/CFS trials.
Just over two-thirds (n = 222, 70.70%) of our participants had some data missing over the 12-month follow-up. Excluding these participants from our analysis had the potential to bias our findings. The complete-case analysis resulted in higher QALYs for both groups compared to our MI results. This indicates that participants with missing data have worse utility scores. Similarly, baseline data indicated that participants with missing data had lower baseline utility scores, although this association was not statistically significant. Consequently, missingness was handling by using MI in order to include all patients in the primary analysis. This means we assumed that data were MAR, although we can’t exclude the possibility of bias if the data were in fact MNAR.
Fatigue In Teenagers on the interNET in the National Health Service was more than three times more intensive and costly than Activity Management. Both FITNET-NHS and Activity Management were primarily delivered by clinicians with a similar level of experience and salary band. It is possible FITNET-NHS could be delivered by CBT therapists with a lower salary band; however, this would only reduce the mean delivery costs by around £160 and would not reverse our primary conclusions. While FITNET-NHS appears to be a substantial investment compared to Activity Management, the cost per participant for FITNET-NHS is similar to costs reported for CBT treatments for adults with ME/CFS. 88–90
Both groups experienced a moderate improvement in utility, with the improvement being slightly higher in the FITNET-NHS group. This seems to be inconsistent with the improvement in physical function, fatigue and school attendance seen in the FITNET-NHS group. It is possible that the EQ-5D-Y questionnaire is insensitive in detecting improvements in health-related quality of life for children with ME/CFS. In particular, our results showed that few children reported severe problems in ‘mobility’ and ‘looking after yourself’ which created a ‘ceiling’ effect for these two domains.
This is the first study to investigate cost effectiveness for CBT for paediatric ME/CFS that we are aware of. There are few studies to compare our utility results to. Two explorative studies91,92 have reported the EQ-5D-3L to be insensitive to problems with ‘mobility’ and ‘looking after yourself’. An additional concern with the EQ-5D-Y is that it asks respondents to report on their health today, which raises the question of how appropriate the measure is for CFS/ME, a chronic condition where health fluctuates widely.
We are aware of one other ME/CFS trial50 in children where health-related quality of life was measured using the EQ-5D-Y. Compared to our trial, this was a smaller study, children had lower EQ-5D-Y scores at baseline and there was a larger difference between treatment arms at 12 months’ follow-up. Nonetheless, our results are not directly comparable to this trial since our valuation approach differed as it was informed by the latest literature on how to value the EQ-5D-Y.
As illustrated in the baseline utility scores, children with ME/CFS experience low health-related quality of life. Our within-trial economic evaluation makes a novel contribution to this important field of study. To our knowledge, just one previous study has assessed the cost-effectiveness of treatment options for children with CFS/ME and no studies have assessed cost-effectiveness for children with comorbid anxiety/depression. 93
Recommendations for future research
The FITNET-NHS intervention
Our qualitative data suggest children and young people may benefit if some of the sessions are delivered face-to-face. This is consistent with the Dutch implementation study where 42% had at least one face-to-face appointment. 23 Future trials need to investigate treatment delivery and whether it is possible to individualise the mode of delivery for participants so they can chose whether sessions are delivered face-to-face or online. Patients could be randomised to having or not having choice.
Randomised controlled trials of adults with ME/CFS have evaluated different approaches to delivering CBT interventions which may vary in their cost-effectiveness. 93 Further trials should investigate different approaches to the delivery of CBT in children and adolescents. It will be possible to learn from the delivery of CBT to children and adolescents with conditions other than ME/CFS, and from innovations in remote delivery necessitated during the pandemic.
The NHS context
Future trials should consider how to improve the patient pathway to get a clinical diagnosis before entering a national trial like FITNET-NHS. We were unable to recruit children and young people without a diagnosis. Accessing paediatric clinics involves a wait now of approximately 6–8 months throughout the UK, delaying access to treatment.
Whilst our motivation for evaluating CBT for ME/CFS delivered online was to improve access to specialist care, we need to consider ways of overcoming the ‘digital divide’ so that unsurmountable obstacles do not remain for large sections of society.
Conduct of research in the NHS
A sensitive, reliable and validated measure of outcome which is meaningful for patients is needed for studies of paediatric ME/CFS. 94 Ideally this would be part of core outcome and core measurement sets for intervention outcome in paediatric ME/CFS. 95
There is a growing evidence base supporting measures to improve participant retention in studies of adults. 96,97 However, it is likely that distinct approaches will be needed for children and adolescents because they don’t necessarily have the same level of autonomy as adults.
We spent considerable time and effort failing to get routine data from primary care. This needs to be improved for future trials. We were delayed in getting data from NHS Digital. This was a large cost pressure and delayed analysis. This needs to be improved for future trials.
Conclusions
Findings of this research suggest that children and young people who cannot access a local specialist service, are more likely to benefit from FITNET-NHS delivered as online CBT compared to Activity Management provided using video clinics. However, as FITNET-NHS is not cost-effective using the normal threshold to pay, we need to consider alternative treatment options that are less intensive.
Additional information
Contributions of authors
Esther Crawley (https://orcid.org/0000-0002-2521-0747) was chief investigator with oversight of all aspects of the trial and a Trial Group Management member; was involved in the conception and design of the work and the acquisition of funding, obtaining NHS Digital data, report writing; and reviewed and made an intellectual contribution to the report.
Emma Anderson (https://orcid.org/0000-0002-4639-9067) was involved in trial co-ordination, report writing, and reviewed and made an intellectual contribution to the report.
Madeleine Cochrane (https://orcid.org/0000-0003-1856-3293) was involved in health economics analysis, report writing, and reviewed and made an intellectual contribution to the report.
Beverly A Shirkey (https://orcid.org/0000-0002-4347-6784) was involved in statistical expertise and analysis, reporting to the Data Monitoring and Safety Committee; report writing; and reviewed and made an intellectual contribution to the report.
Roxanne Parslow (https://orcid.org/0000-0002-3612-7121) was involved in qualitative research and reviewed and made an intellectual contribution to the report.
William Hollingworth (https://orcid.org/0000-0002-0840-6254) was involved in the conception and design of the work and acquisition of funding, health economics analysis, obtaining NHS Digital data, report writing, and reviewed and made an intellectual contribution to the report.
Nicola Mills (https://orcid.org/0000-0002-2960-2940) was a Trial Management Group member; was involved in qualitative research and reviewed and made an intellectual contribution to the report.
Daisy Gaunt (https://orcid.org/0000-0002-1253-307X) was involved in the conception and design of the work and acquisition of funding; statistical expertise and analysis, reporting to the Data Monitoring and Safety Committee; and reviewed and made an intellectual contribution to the report.
Georgia Treneman-Evans (https://orcid.org/0000-0003-0707-6078) was involved in trial co-ordination, report writing, and reviewed and made an intellectual contribution to the report.
Manmita Rai (https://orcid.org/0000-0001-6232-2716) was involved in trial co-ordination, obtaining NHS Digital data, report writing, and reviewed and made an intellectual contribution to the report.
John Macleod (https://orcid.org/0000-0001-8202-1144) was a Trial Management Group member; was involved in the conception and design of the work and acquisition of funding, report writing, and made an intellectual contribution to the report.
David Kessler (https://orcid.org/0000-0001-5333-132X) was a Trial Management Group member; was involved in the conception and design of work and acquisition of funding, report writing, and reviewed and made an intellectual contribution to the report.
Kieren Pitts (https://orcid.org/0000-0002-0927-7677) was involved in FITNET-NHS web platform development and evaluation, and reviewed and made an intellectual contribution to the report.
Serena Cooper (https://orcid.org/0000-0003-4948-6235) was involved in FITNET-NHS web platform development and evaluation, and reviewed and made an intellectual contribution to the report.
Maria Loades (https://orcid.org/0000-0002-0839-3190) was a Trial Management Group member; and reviewed and made an intellectual contribution to the report.
Ammar Annaw (https://orcid.org/0000-0001-7626-7519) was involved in health economics analysis, and reviewed and made an intellectual contribution to the report.
Paul Stallard (https://orcid.org/0000-0001-8046-0784) was a Trial Management Group member; was involved in the conception and design of the work and acquisition of funding, and reviewed and made an intellectual contribution to the report.
Hans Knoop (https://orcid.org/0000-0001-7763-3517) was involved in the conception and design of the work and acquisition of funding, and reviewed and made an intellectual contribution to the report.
Elise Van de Putte (https://orcid.org/0000-0001-7232-3827) was a Trial Management Group member; was involved in the conception and design of the work and acquisition of funding, and reviewed and made an intellectual contribution to the report.
Sanne Nijhof (https://orcid.org/0000-0003-1538-5014) was a Trial Management Group member; was involved in the conception and design of the work and acquisition of funding, and reviewed and made an intellectual contribution to the report.
Gijs Bleijenberg (https://orcid.org/0000-0002-3822-1162) was involved in the conception and design of the work and acquisition of funding, and reviewed and made an intellectual contribution to the report.
Chris Metcalfe (https://orcid.org/0000-0001-8318-8907) was a Trial Management Group member; was involved in the conception and design of the work and acquisition of funding; statistical expertise and analysis, reporting to the Data Monitoring and Safety Committee; report writing; and reviewed and made an intellectual contribution to the report.
Acknowledgements
First, we would like to thank the 314 participants and their parents and carers who took part in this trial, as well as the additional families who were screened but did not participate.
We would like to thank the clinical team at the Bath Specialist ME/CFS Service for delivering the treatments, the research nurses who took care of all the patient enrolment processes and the administrative staff who supported the trial.
We would like to thank the members of the PPI group for giving their time and advice.
We also wish to thank the Trial Steering Committee and Data Safety and Monitoring Committee members for their oversight and support of the trial.
We would like to thank the Clinical Research Networks across England who have supported the study and for the collaboration of national general practitioner staff and paediatricians for referring their patients to the trial.
We would also like to thank Nicholas Christoforou who was the trial administrator for the study from September 2021 to July 2022, and Alison Horne who provided REDCap support throughout the study.
This study was designed and delivered in collaboration with the Bristol Trials Centre, a UK Clinical Research Collaboration-registered clinical trials unit, which is in receipt of NIHR CTU support funding.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives
You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
Given the nature of this dataset, access is controlled. All data will be available from https://data.bris.ac.uk/data/ following publication of the results, and any queries should be directed to the corresponding author. Access to data may be granted following review by the University of Bristol Data Access Committee (DAC) and completion of a data sharing agreement.
Ethics statement
The pilot and full RCT protocol and all associated documents were reviewed and approved by the South West-Frenchay Research Ethics Committee (REC) (reference 16/SW/0268), via Integrated Research Application System (IRAS) project number: 211202.
Information governance statement
The University of Bristol is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, The University of Bristol is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: www.bristol.ac.uk/secretary/data-protection/gdpr/data-protection-officer.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journal Library report publication page at https://doi.org/10.3310/VLRW6701.
Primary conflicts of interest: William Hollingworth was a member of the NIHR HTA Clinical Evaluation and Trials Committee until March 2021. Maria Loades declares the following: Personal funding from NIHR Doctoral Research Fellowship, DRF-2016-09-021, coapplicant on project (Wellcome Trust Seed Award in Science. ZAR2,060,512.50), visiting Lecturer at University of Exeter and external examiner at Trinity College, Dublin. Ammar Annaw declares funding from the NIHR. Hans Knoop and Gijs Bleijenberg received royalties from the publication and sale of a treatment manual for Cognitive Behaviour Therapy in ME/CFS in adults. Chris Metcalfe was co-director of the Bristol Randomised Trials Collaboration until August 2021, a UK Clinical Research Collaboration-registered trials unit in receipt of NIHR support funding. In addition, Metcalfe declares funding from the NIHR for a number of projects and sits on a number of data safety monitoring boards and study steering committees.
Publications
Baos S, Brigden A, Anderson E, Hollingworth W, Price S, Mills N, et al. Investigating the effectiveness and cost-effectiveness of FITNET-NHS (Fatigue In Teenagers on the interNET in the NHS) compared to Activity Management to treat paediatric chronic fatigue syndrome (CFS)/myalgic encephalomyelitis (ME): protocol for a randomised controlled trial. Trials 2018;19:136.
Anderson E, Gaunt D, Metcalfe C, Rai M, Hollingworth W, Mills N, et al. Investigating the effectiveness and cost-effectiveness of FITNET-NHS (Fatigue In Teenagers on the interNET in the NHS) compared to activity management to treat paediatric chronic fatigue syndrome (CFS)/myalgic encephalomyelitis (ME): amendment to the published protocol. Trials 2019;20:750.
Anderson E, Parslow R, Hollingworth W, Mills N, Beasant L, Gaunt D, et al. Recruiting adolescents with chronic fatigue syndrome/myalgic encephalomyelitis to internet-delivered therapy: internal pilot within a randomized controlled trial. J Med Internet Res 2020;22:e17768. https://doi.org/10.2196/17768
Loades ME, Stallard P, Morris R, Kessler D, Crawley E. Do adolescents with chronic fatigue syndrome (ME/CFS) and comorbid anxiety and/or depressive symptoms think differently to those who do not have comorbid psychopathology? J Affect Disord 2020;274:752–8.
Jones LS, Anderson E, Loades M, Barnes R, Crawley E. Can linguistic analysis be used to identify whether adolescents with a chronic illness are depressed? Clin Psychol Psychother 2020;27:179–92.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Lim E-J, Ahn Y-C, Jang E-S, Lee S-W, Lee S-H, Son C-G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med 2020;18:1-15.
- Royal College of P, Child H . Evidence Based Guideline for the Management of CFS ME (Chronic Fatigue Syndrome Myalgic Encephalopathy) in Children and Young People 2004.
- Baker R, Shaw B. Guidelines: Diagnosis and management of chronic fatigue syndrome or myalgic encephalomyelitis (or encephalopathy): Summary of NICE guidance. BMJ 2007;335:446-8. https://doi.org/10.1136/bmj.39302.509005.AE.
- National Institute for Health and Care Excellence (NICE) . Myalgic Encephalomyelitis (or Encephalopathy) Chronic Fatigue Syndrome: Diagnosis and Management 2021. www.nice.org.uk/guidance/ng206 (accessed 24 March 2023).
- Rangel L, Garralda ME, Levin M, Roberts H. The course of severe chronic fatigue syndrome in childhood. J R Soc Med 2000;93:129-34.
- Crawley E, Sterne JAC. Association between school absence and physical function in paediatric chronic fatigue syndrome/myalgic encephalopathy. Arch Dis Child 2009;94:752-6.
- Webb CM, Collin SM, Deave T, Haig-Ferguson A, Spatz A, Crawley E. What stops children with a chronic illness accessing health care: a mixed methods study in children with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME). BMC Health Serv Res 2011;11.
- Nijhof SL, Bleijenberg G, Uiterwaal CS, Kimpen JL, van de Putte EM. Effectiveness of internet-based cognitive behavioural treatment for adolescents with chronic fatigue syndrome (FITNET): a randomised controlled trial. Lancet 2012;379:1412-8.
- Cairns R, Hotopf M. A systematic review describing the prognosis of chronic fatigue syndrome. Occup Med (Oxf) 2005;55:20-31.
- Missen A, Hollingworth W, Eaton N, Crawley E. The financial and psychological impacts on mothers of children with chronic fatigue syndrome (CFS/ME). Child Care Health Dev 2012;38:505-12.
- White PD, Sharpe MC, Chalder T, DeCesare JC, Walwyn R, the P. Protocol for the PACE trial: A randomised controlled trial of adaptive pacing, cognitive behaviour therapy, and graded exercise as supplements to standardised specialist medical care versus standardised specialist medical care alone for patients with the chronic fatigue syndrome/myalgic encephalomyelitis or encephalopathy. BMC Neurol 2007;7.
- Whiting P, Bagnall A-M, Sowden AJ, Cornell JE, Mulrow CD, Ramírez G. Interventions for the treatment and management of chronic fatigue syndrome a systematic review. JAMA 2001;286:1360-8.
- Larun L, Brurberg KG, Odgaard‐Jensen J, Price JR. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev 2019;10.
- Chambers D, Bagnall A-M, Hempel S, Forbes C. Interventions for the treatment, management and rehabilitation of patients with chronic fatigue syndrome/myalgic encephalomyelitis: an updated systematic review. J R Soc Med 2006;99:506-20.
- Castell BD, Kazantzis N, Moss-Morris RE. Cognitive behavioral therapy and graded exercise for chronic fatigue syndrome: a meta‐analysis. Clin Psychol: Sci Pract 2011;18:311-24.
- White PD, Goldsmith KA, Johnson AL, Potts L, Walwyn R, DeCesare JC, et al. PACE trial management group . Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial. Lancet (London, England) 2011;377:823-36.
- Smith SN, Crawley E. Is there effective behavioural treatment for children with chronic fatigue syndrome/myalgic encephalomyelitis?. Arch Dis Child 2013;98:561-3.
- Moore Y, Anderson N, Crawley E. G358 A systematic review to identify the definitions of recovery for paediatric patients with chronic fatigue syndrome (cfs) or myalgic encephalomyelitis (me) used in studies since 1994. Arch Dis Child 2015;100:A146.3-A147.
- Knight SJ, Scheinberg A, Harvey AR. Interventions in pediatric chronic fatigue syndrome/myalgic encephalomyelitis: a systematic review. J Adolesc Health 2013;53:154-65.
- Stulemeijer M, de Jong LW, Fiselier TJ, Hoogveld SW, Bleijenberg G. Cognitive behaviour therapy for adolescents with chronic fatigue syndrome: randomised controlled trial. BMJ 2004;330.
- Al-Haggar MS, Al-Naggar ZA, Abdel-Salam MA. Biofeedback and cognitive behavioral therapy for Egyptian adolescents suffering from chronic fatigue syndrome. J Pediatr Neurol 2006;4:161-9.
- Chalder T, Deary V, Husain K, Walwyn R. Family-focused cognitive behaviour therapy versus psycho-education for chronic fatigue syndrome in 11-to 18-year-olds: a randomized controlled treatment trial. Psychol Med 2010;40:1269-79.
- Albers E, Nijhof LN, Berkelbach van der Sprenkel EE, van de Putte EM, Nijhof SL, Knoop H. Effectiveness of internet-based cognitive behavior therapy (Fatigue In Teenagers on the interNET) for adolescents with chronic fatigue syndrome in routine clinical care: observational study. J Med Internet Res 2021;23.
- Bould H, Collin SM, Lewis G, Rimes K, Crawley E. Depression in paediatric chronic fatigue syndrome. Arch Dis Child 2013;98:425-8.
- Crawley E, Hunt L, Stallard P. Anxiety in children with CFS/ME. Eur Child Adolesc Psychiatry 2009;18:683-9.
- Loades ME, Rimes KA, Ali S, Chalder T. Depressive symptoms in adolescents with chronic fatigue syndrome (CFS): Are rates higher than in controls and do depressive symptoms affect outcome?. Clin Child Psychol Psychiatry 2019;24:580-92.
- Prins J, Bleijenberg G, Rouweler EK, Van Der Meer J. Effect of psychiatric disorders on outcome of cognitive-behavioural therapy for chronic fatigue syndrome. Br J Psychiatry 2005;187:184-5.
- Kempke S, Goossens L, Luyten P, Bekaert P, Van Houdenhove B, Van Wambeke P. Predictors of outcome in a multi-component treatment program for chronic fatigue syndrome. J Affect Disord 2010;126:174-9.
- Tummers M, Knoop H, van Dam A, Bleijenberg G. Moderators of the treatment response to guided self-instruction for chronic fatigue syndrome. J Psychosom Res 2013;74:373-7.
- Loades ME, Stallard P, Morris R, Kessler D, Crawley E. Do adolescents with chronic fatigue syndrome (CFS/ME) and co-morbid anxiety and/or depressive symptoms think differently to those who do not have co-morbid psychopathology?. J Affect Disord 2020;274:752-8.
- Health NCCfM . E-Therapies Systematic Review for Children and Young People With Mental Health Problems 2014.
- Cervin M, Lundgren T. Technology-delivered cognitive-behavioral therapy for pediatric anxiety disorders: a meta-analysis of remission, posttreatment anxiety, and functioning. J Child Psychol Psychiatry 2022;63:7-18.
- Fisher E, Law E, Dudeney J, Eccleston C, Palermo TM. Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev 2019.
- Baos S, Brigden A, Anderson E, Hollingworth W, Price S, Mills N, et al. Investigating the effectiveness and cost-effectiveness of FITNET-NHS (Fatigue In Teenagers on the interNET in the NHS) compared to Activity Management to treat paediatric chronic fatigue syndrome (CFS)/myalgic encephalomyelitis (ME): protocol for a randomised controlled trial. Trials 2018;19:1-12.
- Anderson E, Gaunt D, Metcalfe C, Rai M, Hollingworth W, Mills N, et al. Investigating the effectiveness and cost-effectiveness of FITNET-NHS (Fatigue In Teenagers on the interNET in the NHS) compared to activity management to treat paediatric chronic fatigue syndrome (CFS)/myalgic encephalomyelitis (ME): amendment to the published protocol. Trials 2019;20:1-3.
- Esbjørn BH, Sømhovd MJ, Turnstedt C, Reinholdt-Dunne ML. Assessing the Revised Child Anxiety and Depression Scale (RCADS) in a national sample of Danish youth aged 8–16 years. PLOS ONE 2012;7.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-81.
- Chorpita BF, Moffitt CE, Gray J. Psychometric properties of the Revised Child Anxiety and Depression Scale in a clinical sample. Behav Res Ther 2005;43:309-22.
- Chorpita BF, Yim L, Moffitt C, Umemoto LA, Francis SE. Assessment of symptoms of DSM-IV anxiety and depression in children: a revised child anxiety and depression scale. Behav Res Ther 2000;38:835-55.
- Bruce F, Chorpita CE, Susan H. Spence. Revised Children’s Anxiety and Depression Scale: User’s Guide. 2015.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348.
- Ware Jr JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I Conceptual framework and item selection. Med Care 1992;30:473-83.
- Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. J Psychosom Res 1993;37:147-53.
- Beurskens AJ, Bültmann U, Kant I, Vercoulen JH, Bleijenberg G, Swaen GM. Fatigue among working people: validity of a questionnaire measure. Occup Environ Med 2000;57:353-7.
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short‐form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form‐36 bodily pain scale (SF‐36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP). Arthritis Care Res 2011;63:S240-52.
- Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4:28-37.
- Wille N, Badia X, Bonsel G, Burström K, Cavrini G, Devlin N, et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Qual Life Res 2010;19:875-6.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEcon 1993;4:353-65.
- Parslow R, Patel A, Beasant L, Haywood K, Johnson D, Crawley E. What matters to children with CFS/ME? A conceptual model as the first stage in developing a PROM. Arch Dis Child 2015;100:1141-7.
- Crawley EM, Gaunt DM, Garfield K, Hollingworth W, Sterne JAC, Beasant L, et al. Clinical and cost-effectiveness of the Lightning Process in addition to specialist medical care for paediatric chronic fatigue syndrome: randomised controlled trial. Arch Dis Child 2018;103:155-64.
- Crawley E, Mills N, Beasant L, Johnson D, Collin SM, Deans Z, et al. The feasibility and acceptability of conducting a trial of specialist medical care and the Lightning Process in children with chronic fatigue syndrome: feasibility randomized controlled trial (SMILE study). Trials 2013;14.
- Chalder T, Goldsmith KA, White PD, Sharpe M, Pickles AR. Rehabilitative therapies for chronic fatigue syndrome: a secondary mediation analysis of the PACE trial. Lancet Psychiatry 2015;2:141-52.
- Loades ME, Rimes K, Lievesley K, Ali S, Chalder T. Cognitive and behavioural responses to symptoms in adolescents with chronic fatigue syndrome: a case-control study nested within a cohort. Clin Child Psychol Psychiatry 2019;24:564-79.
- Brigden A, Parslow RM, Gaunt D, Collin SM, Jones A, Crawley E. Defining the minimally clinically important difference of the SF-36 physical function subscale for paediatric CFS/ME: triangulation using three different methods. Health Qual Life Out 2018;16.
- Metcalfe C, Crawley E. FITNET NHS Statistical Analysis Plan. University of Bristol; 2021.
- Groenwold RH, Donders ART, Roes KC, Harrell Jr FE, Moons KG. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol 2012;175:210-7.
- White IR, Carpenter J, Horton NJ. A mean score method for sensitivity analysis to departures from the missing at random assumption in randomised trials. Statistica Sinica 2018;28:1985-2003.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2020. Canterbury: Personal Social Services Research Unit; 2020.
- National Health Service . 2019/20/National/Cost/Collection/Data/Publication 2020. www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication/ (accessed 24 March 2023).
- Office for National Statistics . Annual Survey of Hours and Earnings (ASHE) 2020. www.ons.gov.uk/surveys/informationforbusinesses/businesssurveys/annualsurveyofhoursandearningsashe.
- Curtis LA. Unit Costs of Health and Social Care 2013. Personal Social Services Research Unit, University of Kent; 2013.
- Pope C, Turnbull J, Jones J, Prichard J, Rowsell A, Halford S. Has the NHS 111 urgent care telephone service been a success? Case study and secondary data analysis in England. BMJ Open 2017;7.
- National Health Service Business Services Authority . Prescription Cost Analysis – England 2020 21 2021. www.nhsbsa.nhs.uk/statistical-collections/prescription-cost-analysis-england/prescription-cost-analysis-england-202021 (accessed 24 March 2023).
- HM Revenue & Customs . Travel — Mileage and Fuel Rates and Allowances 2022. www.gov.uk/government/publications/rates-and-allowances-travel-mileage-and-fuel-allowances/travel-mileage-and-fuel-rates-and-allowances (accessed 24 March 2023).
- Kind P, Klose K, Gusi N, Olivares PR, Greiner W. Can adult weights be used to value child health states? Testing the influence of perspective in valuing EQ-5D-Y. Qual Life Res 2015;24:2519-39.
- Kreimeier S, Mott D, Ludwig K, Greiner W, Prevolnik Rupel V, Ramos-Goñi JM. EQ-5D-Y Value Set for Germany. PharmacoEcon 2022;40:217-29.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial‐based cost‐effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96.
- Hoch JS, Hay A, Isaranuwatchai W, Thavorn K, Leighl NB, Tu D, et al. Advantages of the net benefit regression framework for trial-based economic evaluations of cancer treatments: an example from the Canadian Cancer Trials Group CO 17 trial. BMC Cancer 2019;19:1-9.
- Leurent B, Gomes M, Faria R, Morris S, Grieve R, Carpenter JR. Sensitivity analysis for not-at-random missing data in trial-based cost-effectiveness analysis: a tutorial. PharmacoEcon 2018;36:889-901.
- Ramos-Goñi JM, Oppe M, Estévez-Carrillo A, Rivero-Arias O. IMPACT HTA HRQoL Group . Accounting for unobservable preference heterogeneity and evaluating alternative anchoring approaches to estimate country-specific EQ-5D-Y value sets: a case study using Spanish preference data. Value Health 2022;25:835-43.
- Donovan JL, Rooshenas L, Jepson M, Elliott D, Wade J, Avery K, et al. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI). Trials 2016;17.
- Donovan JL, Paramasivan S, de Salis I, Toerien M. Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014;15.
- Ritchie J, Lewis J, Nicholls CM, Ormston R. Qualitative Research Practice: A Guide for Social Science Students and Researchers. Sage; 2013.
- Pope C, Ziebland S, Mays N. Analysing qualitative data. BMJ 2000;320:114-6.
- Glaser BG, Holton J. Discovery of Grounded Theory. 1967.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101.
- Guest G, Bunce A, Johnson L. How many interviews are enough?: An experiment with data saturation and variability. Field Methods 2006;18:59-82.
- Anderson E, Parslow R, Hollingworth W, Mills N, Beasant L, Gaunt D, et al. Recruiting adolescents with chronic fatigue syndrome/myalgic encephalomyelitis to internet-delivered therapy: internal pilot within a randomized controlled trial. J Med Internet Res 2020;22.
- Centre for Academic Child Health . FITNET-NHS Study – Overview 2016. www.bristol.ac.uk/academic-child-health/research/research/cfsme/fitnet-nhs/fitnet-nhs/ (accessed 23 March 2023).
- Mills N, Blazeby JM, Hamdy FC, Neal DE, Campbell B, Wilson C, et al. Training recruiters to randomized trials to facilitate recruitment and informed consent by exploring patients’ treatment preferences. Trials 2014;15:1-13.
- Mills N, Donovan JL, Wade J, Hamdy FC, Neal DE, Lane JA. Exploring treatment preferences facilitated recruitment to randomized controlled trials. J Clin Epidemiol 2011;64:1127-36.
- Jones LS, Anderson E, Loades M, Barnes R, Crawley E. Can linguistic analysis be used to identify whether adolescents with a chronic illness are depressed?. Clin Psychol Psychother 2020;27:179-92.
- Office for National Statistics . Ethnic Group by Age and Sex, England and Wales: Census 2021 2023. www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/articles/ethnicgroupbyageandsexenglandandwales/latest (accessed 24 March).
- Lievesley K, Rimes KA, Chalder T. A review of the predisposing, precipitating and perpetuating factors in Chronic Fatigue Syndrome in children and adolescents. Clin Psychol Rev 2014;34:233-48.
- Aisbitt GM, Nolte T, Fonagy P. Editorial Perspective: the digital divide - inequalities in remote therapy for children and adolescents. Child Adolesc Ment Health 2023;28:105-7.
- Meyerowitz-Katz G, Ravi S, Arnolda L, Feng X, Maberly G, Astell-Burt T. Rates of attrition and dropout in app-based interventions for chronic disease: systematic review and meta-analysis. J Med Internet Res 2020;22.
- Heins MJ, Knoop H, Prins JB, Stulemeijer M, van der Meer JWM, Bleijenberg G. Possible detrimental effects of cognitive behaviour therapy for chronic fatigue syndrome. Psychother Psychosom 2010;79:249-56.
- McCrone P, Sharpe M, Chalder T, Knapp M, Johnson AL, Goldsmith KA, et al. Adaptive pacing, cognitive behaviour therapy, graded exercise, and specialist medical care for chronic fatigue syndrome: a cost-effectiveness analysis. PLOS ONE 2012;7.
- Vos-Vromans D, Evers S, Huijnen I, Köke A, Hitters M, Rijnders N, et al. Economic evaluation of multidisciplinary rehabilitation treatment versus cognitive behavioural therapy for patients with chronic fatigue syndrome: a randomized controlled trial. PLOS ONE 2017;12.
- Severens JL, Prins JB, van der Wilt GJ, van der Meer JW, Bleijenberg G. Cost-effectiveness of cognitive behaviour therapy for patients with chronic fatigue syndrome. QJM 2004;97:153-61.
- Myers C, Wilks D. Comparison of Euroqol EQ-5D and SF-36 in patients with chronic fatigue syndrome. Qual Life Res 1999;8:9-16.
- Spronk I, Polinder S, Bonsel G, Janssen M, Haagsma J. The relation between EQ-5D and fatigue in a Dutch general population sample: an explorative study. Health Qual Life Out 2021;19:1-11.
- Cochrane M, Mitchell E, Hollingworth W, Crawley E, Trépel D. Cost-effectiveness of interventions for chronic fatigue syndrome or Myalgic encephalomyelitis: a systematic review of economic evaluations. Appl Health Econ Health Policy 2021;19:473-86.
- Parslow RM, Shaw A, Haywood KL, Crawley E. Developing and pretesting a new patient reported outcome measure for paediatric Chronic Fatigue Syndrome/ Myalgic Encephalopathy (CFS/ME): cognitive interviews with children. J Patient Rep Outcomes 2019;3.
- Gargon E, Gurung B, Medley N, Altman DG, Blazeby JM, Clarke M, et al. Choosing important health outcomes for comparative effectiveness research: a systematic review. PLOS ONE 2014;9.
- Brueton VC, Stevenson F, Vale CL, Stenning SP, Tierney JF, Harding S, et al. Use of strategies to improve retention in primary care randomised trials: a qualitative study with in-depth interviews. BMJ Open 2014;4.
- Kearney A, Rosala-Hallas A, Bacon N, Daykin A, Shaw ARG, Lane AJ, et al. Reducing attrition within clinical trials: the communication of retention and withdrawal within patient information leaflets. PLOS ONE 2018;13.
- Schneider LH, Hadjistavropoulos HD, Faller YN. Internet-delivered cognitive behaviour therapy for depressive symptoms: an exploratory examination of therapist behaviours and their relationship to outcome and therapeutic alliance. Behav Cogn Psychother 2016;44:625-39.
- Paxling B, Lundgren S, Norman A, Almlov J, Carlbring P, Cuijpers P, et al. Therapist behaviours in internet-delivered cognitive behaviour therapy: analyses of e-mail correspondence in the treatment of generalized anxiety disorder. Behav Cogn Psychother 2013;41:280-89. https://doi.org/10.1017/s1352465812000240.
Appendix 1 Supplementary information about interventions
FITNET-NHS platform chapter headings
Chapter 1 – Getting acquainted
-
Section 1.0 Information for parents/carers
-
Section 1.1 Welcome to FITNET-NHS!
-
Section 1.2 Introduction
-
Section 1.3 Questions about your family
-
Section 1.4 Questions about school/studies
-
Section 1.5 What do you do during a regular day?
-
Section 1.6 Questions about your physical activity
-
Section 1.7 Questions about your friends
-
Section 1.8 Do you have any questions?
Chapter 2 – The treatment explained
-
Section 2.0 Information for parents/carers
-
Section 2.1 Introduction
-
Section 2.2 The aim of the treatment (1)
-
Section 2.3 The aim of the treatment (2)
-
Section 2.4 The aim of the treatment (3)
-
Section 2.5 CBT (1)
-
Section 2.6 CBT (2)
-
Section 2.7 CBT (3)
-
Section 2.8 CBT (4)
-
Section 2.9 CBT (5)
-
Section 2.10 CBT (6)
-
Section 2.11 CBT (7)
-
Section 2.12 CBT (8)
-
Section 2.13 The FITNET-NHS treatment (1)
-
Section 2.14 The duration of the treatment
-
Section 2.15 What do you have to do?
-
Section 2.16 Before you begin (1)
-
Section 2.17 Before you begin (2)
-
Section 2.18 Before you begin (3)
-
Section 2.19 Before you begin (4)
-
Section 2.20 Before you begin (5)
-
Section 2.21 Your first diary!
-
Section 2.22 Contact with your therapist
Chapter 3 – Your parents/carers
-
Section 3.0 Information for parents/carers
-
Section 3.1 Your parents/carers’ role in the treatment (1)
-
Section 3.2 Your parents/carers’ role in the treatment (2)
-
Section 3.3 Support of your parents/carers
-
Section 3.4 Examples of the role of parents/carers
-
Section 3.5 Not speaking about your fatigue
-
Section 3.6 Will your parents/carers know what you tell me?
-
Section 3.7 Contact with me
Chapter 4 – Your goals
-
Section 4.0 Information for parents/carers
-
Section 4.1 What are you working towards?
-
Section 4.2 What are your goals?
-
Section 4.3 Goal diary
-
Section 4.4 An example – Miranda
-
Section 4.5 Another example – Tom
-
Section 4.6 Changing your goal diary?
-
Section 4.7 Keep me updated
Chapter 5 – Your sleeping pattern
-
Section 5.0 Information for parents/carers
-
Section 5.1 Biological clock (1)
-
Section 5.2 Biological clock (2)
-
Section 5.3 Biological clock (3)
-
Section 5.4 Your sleep-wake rhythm
-
Section 5.5 Your sleep-wake rhythm: questions
-
Section 5.6 Your sleep-wake rhythm: answers
-
Section 5.7 A disrupted sleep-wake rhythm
-
Section 5.8 At set times?
-
Section 5.9 Do not lie down during the day!
-
Section 5.10 Homework
Chapter 6 – Helpful thoughts
-
Section 6.0 Information for parents/carers
-
Section 6.1 Introduction
-
Section 6.2 Unhelpful thoughts
-
Section 6.3 Your unhelpful thoughts about fatigue
-
Section 6.4 Examples of helpful thoughts
-
Section 6.5 Diary – helpful thoughts
-
Section 6.6 Other examples of helpful thoughts
-
Section 6.7 Homework
Chapter 7 – Taking your attention away from fatigue
-
Section 7.0 Information for parents/carers
-
Section 7.1 Explanation
-
Section 7.2 Not speaking about fatigue
-
Section 7.3 Not thinking of fatigue
-
Section 7.4 Homework
Chapter 8 – Build-up of physical activities
-
Section 8.0 Information for parents/carers
-
Section 8.1 Introduction
-
Section 8.2 Activity schedule, a stairway
-
Section 8.3 Instructions for building up your activity
-
Section 8.4 Help with the activity schedule
-
Section 8.5 Susan’s example
-
Section 8.6 Your activity schedule
-
Section 8.7 Get to work!
-
Section 8.8 After a week
-
Section 8.9 After a couple of weeks
-
Section 8.10 The next few weeks
Chapter 9 – Striking a balance
-
Section 9.0 Information for parents/carers
-
Section 9.1 Introduction
-
Section 9.2 Explaining striking a balance
-
Section 9.3 Avoid activities which produce ‘payback’
-
Section 9.4 Homework (1)
-
Section 9.5 Homework (2)
Chapter 10 – Build-up of physical activities II
-
Section 10.0 Information for parents/carers
-
Section 10.1 Introduction
-
Section 10.2 Activity schedule, a ladder
-
Section 10.3 Walking?
-
Section 10.4 Instructions for building up your level of physical activity
-
Section 10.5 Help with the activity schedule
-
Section 10.6 Susan’s example
-
Section 10.7 Your activity schedule
-
Section 10.8 Get to work!
-
Section 10.9 After a week
-
Section 10.10 After a couple of weeks
Chapter 11 – Problems during the build-up of activities
-
Section 11.0 Information for parents/carers
-
Section 11.1 Introduction
-
Section 11.2 I can’t manage to keep up
-
Section 11.3 Are you less motivated?
-
Section 11.4 Helpful thoughts
-
Section 11.5 Pep talk
-
Section 11.6 Can I build up more quickly?
-
Section 11.7 How can I combine this activity schedule with school?
-
Section 11.8 Any more questions?
Chapter 12 – Build-up of mental activities
-
Section 12.0 Information for parents/carers
-
Section 12.1 Introduction
-
Section 12.2 Questions about your mental activities
-
Section 12.3 Exercise for the build-up of mental activities
-
Section 12.4 Your personal schedule
-
Section 12.5 How is it coming along?
Chapter 13 – Your plan for school
-
Section 13.0 Information for parents/carers
-
Section 13.1 Introduction
-
Section 13.2 Your situation
-
Section 13.3 Your goals
-
Section 13.4 Your school plan
-
Section 13.5 Sharon and Tim’s Examples
-
Section 13.6 Discussions with people at school
-
Section 13.7 Executing the school plan
-
Section 13.8 Keeping a school diary
-
Section 13.9 Helpful thoughts for your school plan
-
Section 13.10 School trips
Chapter 14 – Social activities
-
Section 14.0 Information for parents/carers
-
Section 14.1 Introduction
-
Section 14.2 Your situation
-
Section 14.3 Homework about social activities
-
Section 14.4 Evaluation
Chapter 15 – You’re reaching your goals
-
Section 15.0 Information for parents/carers
-
Section 15.1 Introduction
-
Section 15.2 Which goals?
-
Section 15.3 Bob’s example
-
Section 15.4 Homework
Chapter 16 – Plan for work
-
Section 16.0 Information for parents/carers
-
Section 16.1 Introduction
-
Section 16.2 Your situation
-
Section 16.3 Your goals
-
Section 16.4 Your work plan
-
Section 16.5 John’s example
-
Section 16.6 Helpful thoughts for your work plan
-
Section 16.7 Speaking to your employer
-
Section 16.8 Executing the work plan
-
Section 16.9 Keeping a diary
Chapter 17 – Going out
-
Section 17.0 Information for parents/carers
-
Section 17.1 Introduction
-
Section 17.2 Homework – tips
-
Section 17.3 Homework – plan
-
Section 17.4 How did it go?
Chapter 18 – Are you still a ME/CFS patient?
-
Section 18.0 Information for parents/carers
-
Section 18.1 Introduction
-
Section 18.2 Could you say you are no longer a ME/CFS patient?
-
Section 18.3 What has helped you most?
-
Section 18.4 Hannah’s example
-
Section 18.5 What do you do when it’s not going well?
-
Section 18.6 Do you have any questions?
-
Section 18.7 Keeping it going
Chapter 19 – Follow-up
-
Section 19.0 3-month follow-up information for parents/carers
-
Section 19.1 3-month follow-up introduction
-
Section 19.2 3-month follow-up questions
-
Section 19.3 6-month follow-up information for parents/carers
-
Section 19.4 6-month follow-up introduction
-
Section 19.5 6-month follow-up questions
-
Section 19.6 Goodbye
Activity Management fidelity checklists
Baseline checklist
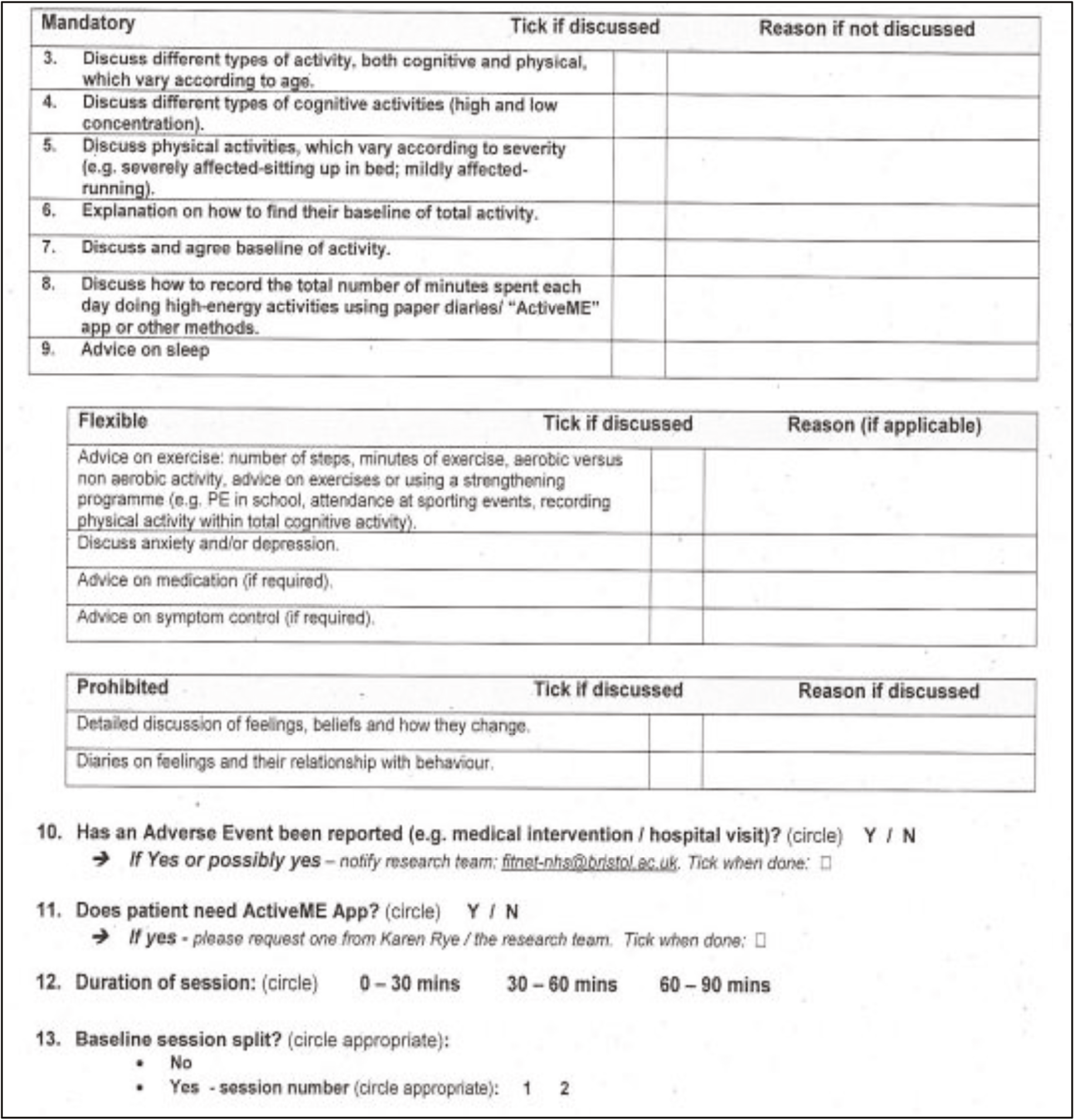
Follow-up checklist

Appendix 2 Accessing routinely collected data
NHS digital record linkage field variables
| HES ECDS variables | HES outpatient variables | HES admitted patient care variables |
|---|---|---|
| AGE_AT_ARRIVAL | ACTIVAGE Age at activity date | ACPDISP_N Augmented care period disposal |
| AGE_AT_CDS_ACTIVITY_DATE | ADMINCAT Administrative category | ACPDQIND_N Augmented care period data quality indicator |
| ARRIVAL_DATE | APPTAGE Age on day of appointment | ACPEND_N Augmented care period end date |
| ARRIVAL_MODE | APPTAGE_CALC Appointment Age - babies decimalised | ACPLOC_N Augmented care location |
| ARRIVAL_TIME | APPTDATE Appointment date | ACPN_N Augmented care period number |
| ATTENDANCE_CATEGORY | ATENTYPE Attendance type | ACPPLAN_N Augmented care period planned indicator |
| BIRTH_MONTH | ATTENDED Attended or did not attend | ACPOUT_N Augmented care period outcome indicator |
| BIRTH_YEAR | ATTENDID Attendance identifier | ACPSEQ ACP sequence number |
| CCG_FROM_GP_PRACTICE | ATTENDKEY Record identifier | ACPSOUR_N Augmented care period source |
| CCG_FROM_PATIENT_POSTCODE | ATTENDKEY_FLAG Attendance Key Flag | ACPSPEF_N Augmented care period speciality function code |
| CONCLUSION_TIME_SINCE_ARRIVAL | BABYAGE Age of Baby | ACPSTAR_N Augmented care period start date |
| DEPARTMENT_TYPE | CARERSI Carer support indicator | ACTIVAGE Age at activity date |
| DEPARTURE_DATE | CDSEXTDATE CDS extract date | ADM_CFL Admission date check flag |
| DEPARTURE_TIME | CDSUNIQUEID CDS unique identifier | ADMIAGE Age on admission |
| DEPARTURE_TIME_SINCE_ARRIVAL | CDSVERPROTID CDS protocol identifier | ADMIDATE Date of admission |
| DIAGNOSIS_FIRST | CHAPTER Primary diagnosis chapter | ADMIFLAG Admission episode flag |
| HEALTH_RESOURCE_GROUP | CSNUM Commissioning serial number | ADMIMETH Method of admission |
| INITIAL_ASSESSMENT_TIME_SINCE_ARRIVAL | DIAG_3_01 Primary diagnosis - 3 character | ADMINCAT Administrative category |
| NHS_NUMBER_IS_VALID | DIAG_3_CONCAT 3 character concatenated diagnosis | ADMINCATST Admin category at start of episode |
| NHS_NUMBER_STATUS_INDICATOR_CODE | DIAG_3_NN Secondary diagnoses - 3 character | ADMISORC Source of admission, |
| ORGANISATION_CODE_UBRN | DIAG_4_01 Primary diagnosis - 4 character | AEKEY Record identifier |
| PATIENT_POSTCODE_DISTRICT | DIAG_4_CONCAT 4 character concatenated diagnosis | BEDYEAR Bed days within the year |
| PDS_GENERAL_PRACTICE | DIAG_4_NN Secondary diagnoses - 4 character | CATEGORY Administrative and legal status of patient |
| PRACTICE_CODE_PATIENT_REGISTRATION | DIAG_COUNT Count of diagnoses | CAUSE Cause code |
| PROVIDER_CODE | DIAG_NN Diagnosis | CAUSE_3 Cause code - 3 characters |
| PROVIDER_POSTCODE_DISTRICT | DNADATE Last DNA or patient cancelled date | CAUSE_4 Cause code - 4 characters |
| TREATMENT_DATE | DOB_CFL Date of birth check flag - patient | CDSEXTDATE CDS extract date |
| TREATMENT_TIME | ENCRYPTED_HESID Encrypted HESID | CDSUNIQUEID CDS unique identifier |
| ETHNOS Ethnic category | CDSVERPROTID CDS protocol identifier | |
| ETHRAWL Ethnic category (audit version) | CDSVERSION CDS version number | |
| FIRSTATT First attendance | CHAPTER Primary diagnosis chapter | |
| FYEAR Financial Year | CLASSPAT Patient classification | |
| GPPRAC Code of GP practice | CSNUM Commissioning serial number | |
| GPPRACHA Health Authority area where patient’s GP is registered | DEPDAYS_N High-dependency care level | |
| GPPRACRO Regional office area where patient’s GP practice was registered | Diag_3_01 Primary Diagnosis - 3 characters | |
| GPPRPCT Primary Care Trust area where patient’s GP was registered | DIAG_3_CONCAT 3 character concatenated diagnosis | |
| GPPRSTHA Strategic health authority area where patient’s GP practice was registered | DIAG_3_NN Diagnosis - 3 characters | |
| HAR/PCTNHS NHS provided HA/PCT of residence | Diag_4_01 Primary Diagnosis - 4 characters | |
| HESID_ORIG Patient ID HES generated (original) | DIAG_4_CONCAT 4 character concatenated diagnosis | |
| HRGNHS Trust derived HRG value | DIAG_4_NN Diagnosis - 4 characters | |
| HRGNHSVN Version No. of Trust derived HRG | DIAG_NN All Diagnosis codes | |
| LOCCLASS Location class | DIS_CFL Discharge date check flag | |
| LOCTYPE Location type | DISDATE Date of discharge | |
| MAINSPEF Main specialty | DISDATE_UNCLN Date of discharge - Uncleaned | |
| MATCH_RANK MATCH_RANK | DISDEST Destination on discharge | |
| MYDOB Date of Birth - month and year | DISDEST_UNCLN Destination on discharge - uncleaned | |
| NEWNHSNO_CHECK NHS Number valid flag | DISFLAG Discharge episode flag | |
| NHSNOIND NHS number status indicator | DISMETH Method of discharge | |
| NODIAGS Number of Diagnoses | DISMETH_UNCLN Method of discharge - uncleaned | |
| NOPROCS Number of Procedures | DISREADYDATE Discharge ready date | |
| OPCS43 OPCS43 | DOB_CFL Date of birth check flag - patient | |
| OPERSTAT Operation status code | DOMPROC Trust derived dominant procedure | |
| OPERTN_3_01 Primary operation - 3 character | EARLDATOFF Earliest reasonable date offered | |
| OPERTN_3_NN Secondary operations - 3 character | ELEC_CFL Date of decision to admit check flag | |
| OPERTN_4_01 Primary operation - 4 character | ELECDATE Date of decision to admit | |
| OPERTN_4_CONCAT 4 character concatenated procedure | ELECDUR Waiting time | |
| OPERTN_4_NN All Operative procedure codes | ELECDUR_CALC Calculation of Elecdur | |
| OPERTN_NN Operative procedure | ELECDURD Waiting time - derived | |
| OUTCOME Outcome of attendance | ENCRYPTED_HESID Encrypted HESID | |
| PARTYEAR Year and month of data | ENDAGE Age at end of episode | |
| PCFOUND Postcode Found | EPIDUR Episode duration | |
| PCGORIG PCG - Origin of code | EPIE_CFL Episode end date check flag | |
| PCTCODE Primary care trust of responsibility | EPIEND Date episode ended | |
| PCTCODE_HIS PCTCODE_HIS | EPIKEY Record identifier | |
| PCTCODE02 Primary care trust of responsibility - historic | EPIORDER Episode order | |
| PCTCODE06 Primary care trust of responsibility - current | EPIS_CFL Episode start date check flag | |
| PCTNHS Primary care trust of responsibility - NHS | EPISTART Date episode started | |
| PCTORIG_HIS PCTORIG_HIS | EPISTAT Episode status | |
| PCTORIG02 Origin of primary care trust of responsibility - historic | EPITYPE Episode type | |
| PCTORIG06 Origin of primary care trust of responsibility - current | ETHNOS Ethnic category | |
| PGPPRAC Pseudonymised code of GP practice | ETHRAW Ethnic character (audit version) | |
| POSTDIST Postcode district of patient’s residence | ETHRAWL Ethnic category (audit version) | |
| PREFERER Pseudonymised referrer code | FAE Finished Admission Episode | |
| Primerecp | FAE_EMERGENCY Finished Admission Episode, emergency classification | |
| PRIORITY Priority type | FCE Finished Consultant Episode | |
| PROCODE Organisation code (code of provider) | FCEFLAG Finished consultant episode flag | |
| PROCODE3 Provider code (3 character) | FDE Finished In-Year Discharge Episode | |
| PROCODE5 Provider code (5 character) | FIRSTREG First regular day or night admission | |
| PROCODET Provider code | FYEAR Financial Year | |
| PROTYPE Provider type | GPPRAC Code of GP practice | |
| PURCODE Commissioner code | GPPRACHA Health Authority area where patient’s GP is registered | |
| PURSTHA Commissioner’s strategic health authority | GPPRACRO Regional Office area where patient’s GP was registered | |
| PURVAL Commissioner code status | GPPRPCT Primary Care Trust area where patient’s GP was registered | |
| REFSOURC Source of referral | GPPRSTHA Strategic Health Authority area where patient’s GP was registered | |
| REQDATE Referral request received date | HESID_ORIG Patient ID - HES generated (original) | |
| RESPCT_HIS RESPCT_HIS | HRG40 Healthcare resource group: version 3.1 | |
| RESSTHA_HIS RESSTHA_HIS | HRGLATE35 Healthcare resource group: version 3.1 | |
| SENDER | HRGNHS Trust derived HRG value | |
| SERVTYPE Service type requested | HRGNHSVN Version No. of Trust derived HRG | |
| SEX Sex of patient | IMD04 IMD Index of Multiple Deprivation | |
| STAFFTYP Medical staff type seeing patient | IMD04_DECILE IMD Decile Group | |
| STUDY_ID | IMD04C IMD Crime Domain | |
| SUBDATE Submission date | IMD04ED IMD Education Training and Skills Domain | |
| SUSHRG SUS generated HRG | IMD04EM IMD Employment Deprivation Domain | |
| SUSHRGVERS SUS generated HRG version number | IMD04HD IMD Health and Disability Domain | |
| SUSLDDATE SUS loaded staging date | IMD04HS IMD Barriers to Housing and Service Domain | |
| SUSRECID SUS record id | IMD04I IMD Income Domain | |
| SUSSPELLID SUS generated spell id | IMD04IA IMD Income affecting Adults Domain | |
| TRETSPEF Treatment specialty | IMD04IC IMD Income affecting Children Domain | |
| WAIT_IND Waiting calculation indicator | IMD04LE IMD Living Environment Domain | |
| WAITING Days waiting | IMD04RK IMD Overall Rank | |
| WAITWEEKS Waiting time weeks | INTDAYS_N Intensive care level days | |
| INTMANIG Intended management | ||
| INYRFLAG In Year flag | ||
| LEGLSTATST Legal status classification code at start of episode | ||
| MAINSPEF Main specialty | ||
| MATCH_RANK | ||
| MATCHID Patient identifier (HES generated) - basis of match | ||
| MYDOB Date of Birth - month and year | ||
| NEWNHSNO_CHECK NHS Number valid flag | ||
| NHSNOIND NHS number status indicator | ||
| NUMACP Number of augmented care periods within episode | ||
| OPCS43 OPCS4.3 version flag | ||
| OPDATE_NN Date of operation | ||
| OPERSTAT Operation status code | ||
| OPERTN_3_01 Primary Operative procedure codes 3 character | ||
| OPERTN_3_CONCAT 3 character concatenated procedure | ||
| OPERTN_3_NN All secondary Operative procedure codes - 3 character | ||
| OPERTN_4_01 Primary Operative procedure codes 4 character | ||
| OPERTN_4_CONCAT 4 character concatenated procedure | ||
| OPERTN_4_NN All secondary Operative procedure codes 4 character | ||
| OPERTN_COUNT Total number of procedures per episode | ||
| OPERTN_NN Primary Operative Procedure Codes | ||
| ORGPPPID Organisation code (patient pathway ID issuer) | ||
| ORGSUP_N Number of organ systems supported | ||
| PARTYEAR Year and month of data | ||
| PCFOUND Postcode Found | ||
| PCGCODE Primary care group | ||
| PCGORIG Origin of primary care group | ||
| PCONSULT Pseudonymised consultant team code | ||
| PCTCODE Primary care trust of responsibility | ||
| PCTCODE_HIS Primary Care Trust | ||
| PCTCODE02 Primary care trust of responsibility - historic | ||
| PCTCODE06 Primary care trust of responsibility - current | ||
| PCTNHS Primary care trust of responsibility - NHS | ||
| PCTORIG Origin of primary care trust of responsibility | ||
| PCTORIG_HIS PCTORIG_HIS | ||
| PCTORIG02 Origin of primary care trust of responsibility - historic | ||
| PCTORIG06 Origin of primary care trust of responsibility - current | ||
| POSOPDUR Post-operative duration | ||
| POSTDIST Postcode district of patient’s residence | ||
| PREFERER Pseudonymised referrer code | ||
| PREGGMP Pseudonymised code of patient’s registered or referring general medical practitioner | ||
| PREOPDUR Pre-operative duration | ||
| PRIMERCP CDS Prime Recipient Identity | ||
| PROCODE Organisation Code (code of provider) | ||
| PROCODE3 Provider code - 3 character | ||
| PROCODE5 Provider code - 5 character | ||
| PROCODET Provider code | ||
| PROTYPE Provider type, | ||
| PROVSPNO Hospital provider spell number | ||
| PROVSPNOPS Pseudonymised hospital provider spell number | ||
| PURCODE Commissioner code | ||
| PURRO Commissioner’s Regional Office | ||
| PURSTHA Commissioner’s Strategic Health Authority | ||
| PURVAL Commissioner code status | ||
| REFERORG Referring organisation code | ||
| RESSTHA_HIS RESSTHA_HIS | ||
| RTTPEREND RTT period end date | ||
| RTTPERSTART RTT period start date | ||
| RTTPERSTAT RTT period status | ||
| SENDER | ||
| SEX Sex of patient | ||
| SPELBGIN Beginning of spell | ||
| SPELDUR Duration of spell | ||
| SPELEND End of spell | ||
| STARTAGE Age at start of episode | ||
| STARTAGE_CALC Age of patients at start of episode, babies restated | ||
| STUDY_ID STUDY_ID | ||
| SUBDATE Submission date | ||
| SUSCOREHRG SUS generated Core Spell HRG | ||
| SUSHRG SUS generated HRG | ||
| SUSHRGVERS SUS generated HRG version number | ||
| SUSLDDATE SUS loaded staging date | ||
| SUSRECID SUS record id | ||
| SUSSPELLID SUS generated spell id | ||
| TRETSPEF Treatment specialty | ||
| WAITDAYS Duration of elective wait | ||
| WAITLIST Method of Admission - Waiting List | ||
| WARDSTRT Ward type at start of episode |
EMIS dummy general practice dataset extract screenshot
Timeline of FITNET-NHS obtaining NHS Digital Information
| 14 March 2019 | First enquiry sent to NHS Digital (NHSD) |
| 26 March 2019 | Received study registration number and assigned case officer (CO) who suggests changes |
| 04 April 2019 | Suggested changes presented to FITNET-NHS TMG |
| 15 April 2019 | Meeting with NHSD CO. Proposals accepted |
| 19 June 2019 | Ethical amendments sent to REC and HRA for changes to study documentation |
| 02 July 2019 | CO suggested further changes. FITNET-NHS replied that documentation was sufficiently clear |
| 10 July 2019 | Access to DARS granted by NHSD to start application |
| 04 November 2019 | DARS first draft submitted. Cost breakdown provided: £22,346. (Estimated cost provided in November 2016 was £5000) |
| 14 January 2020 | NHSD first draft response. Numerous changes to DARS application form suggested |
| 28 January 2020 | DARS second draft submitted. More changes suggested by NHSD |
| 04 February 2020 | DARS third draft submitted |
| 06 February 2020 | Response received from NHSD. FITNET-NHS asked to add more information to application |
| 01 April 2020 | DARS fourth draft submitted and request for final cost breakdown |
| 02 April 2020 | CO satisfied with application. Cost breakdown for final extraction: £16,512 inclusive of VAT |
| 23 March 2021 | Resubmitted DARS application 1 year after original as advised by CO |
| 15 April 2021 | Received confirmation from NHSD that application had been received |
| 16 April 2021 | New CO assigned to FITNET-NHS by NHSD |
| 25 May 2021 | Meeting between new CO and FITNET-NHS |
| 11 June 2021 | E-mail from CO saying application reviewed and it was a priority |
| 23 July 2021 | E-mail from another CO asking if we wished to proceed with application and delivery dates could not be guaranteed |
| 26 July 2021 | E-mail from FITNET-NHS confirming we had been advised to wait 1 year and saying we needed data by August 2021 |
| 27 July 2021 | E-mail from NHSD asking for details of the impact a delay in receiving the data would cause |
| 09 August 2021 | FITNET-NHS provided details of the impacts a delay would cause |
| 16 August 2021 | E-mail from NHSD saying they had lots of applications but they were preparing FITNET-NHS for a pre-IGARD review |
| 17 August 2021 | E-mail from CO asking for patient details before submitting for pre-IGARD review |
| 18 August 2021 | FITNET-NHS e-mailed to say patient data had already been discussed and submitted |
| 03 September 2021 | New NHSD CO assigned. Said more information was needed before IGARD submission |
| 21 September 2021 | E-mail from NHSD Senior Case Officer (SCO) saying cost was now £25,428 |
| 30 September 2021 | FITNET-NHS provided further details for NHSD application |
| 07 October 2021 | FITNET-NHS queried the progress of application |
| 07 October 2021 | E-mail from NHSD SCO saying cost was now £14,544 and asking for previously submitted documents |
| 12 October 2021 | FITNET-NHS provided requested documents and asked when IGARD will review the application |
| 13 October 2021 | E-mail from NHSD SCO saying application had been moved to pre-IGARD review |
| 19 October 2021 | E-mail from NHSD SCO saying pre-IGARD review had been completed and they had more questions before IGARD review |
| 26 October 2021 | FITNET-NHS provided the extra information requested |
| 28 October 2021 | E-mail from NHSD SCO saying extra information had been given to their Senior Management team and permission sought for agreement to be presented at their next IGARD meeting |
| 08 November 2021 | E-mail from FITNET-NHS asking for update on application |
| 08 November 2021 | E-mail from NHSD SCO saying she would present application to IGARD on 11 November 2021 |
| 12 November 2021 | E-mail from NHSD SCO saying IGARD were supportive of application but had suggested amendments |
| 17 November 2021 | E-mail from NHSD SCO saying extra information added to application and it was being moved forward for sign off by head of data access |
| 25 November 2021 | FITNET-NHS e-mailed NHSD to see if the head of data access had signed off application |
| 25 November 2021 | NHSD confirmed that sign-off happened on 25 November 2021. Confirmation that because of backlog, data would be sent at start of March 2022 |
| 30 November 2021 | FITNET-NHS contacted NHSD to put in a formal complaint over the delay in order to expediate data extraction |
| 01 December 02021 | E-mail from NHSD SCO to say complaint had been passed to Clinical Trials Head of Business Operations. And asking for cohort information |
| 07 December 2021 | FITNET-NHS management team met Head of Business Operations at NHSD |
| 21 December 2021 | After some technical issues the cohort data were successfully transferred by FITNET-NHS |
| 10 January 2022 | E-mail from NHSD Service Delivery Manager saying data would be sent on 14 January 2022 |
| 12 January 2022 | E-mail from NHSD Service Delivery Manager saying internal problems meant data would be delivered on 28 January 2022 |
| 28 January 2022 | E-mail from NHSD saying they were having problems with pseudo-identifiers/ECDS and would send an update by the end of February 2022. This did not apply to FITNET-NHS data |
| 25 February 2022 | NHSD sent the data. They were downloaded by FITNET-NHS on 28 February 2022 |
Overview of the DARS application process
Data access request service offers clinicians, researchers and commissioners the data required to help improve NHS services. Organisations and individuals wanting to use certain kinds of data related to their study requirements need to show they meet strict data governance standards by completing the NHS Digital DARS Application process. In order to start the application process and to request the data from NHS Digital, the Trial Coordinator (MR) and Health Economists identified the variables of interest for the record linkage and subsequently completed the following data governance requirements:
-
GDPR Legal basis for processing:
-
Article 6(1)(e) of the GDPR, that is our processing is necessary for the performance of a task carried out in the public interest. Research is a task that UoB performs in the public interest, as part of our core function as a university.
-
Article 9(2)(j) of the GDPR, that is our processing is necessary for research purposes or statistical purposes. This condition applies if we are applying appropriate protections to keep your data secure and safeguard your interests.
-
The UoB has eVM (encapsulated Virtual Machine) set-up where these data will be stored and analysed. The eVM can only be accessed by approved researchers at specific computers encrypted with security passcodes. The Trial Coordinator and the Health Economists team completed relevant Data Governance training, followed by the completion of necessary data safeguarding audit undertaken by the Trial Coordinator which received on time registration and approval of the FITNET-NHS Trial Data Sharing and Protection Toolkit (DSPT).
-
-
Fair processing information for patient data:
For identifiable data, key aspects to include around the fair and transparent processing of information would be typically a published privacy notice, which is published on our study website for participants and families:
-
Common Law Duty of Confidentiality:
The FITNET-NHS Trial was designed to keep participants informed by sending newsletters (the first batch of newsletters were sent in February 2020) and by updating the study website (https://www.bristol.ac.uk/academic-child-health/research/research/cfsme/fitnet-nhs/fitnet-nhs/). A Research Festival was organised in September 2020 which FITNET-NHS participants and their families were invited to attend. The event was attended by patients and families, researchers and clinicians’ teams from the University of Bristol, The Bath Royal United Hospital around the world, as well as members of the Patient Advisory Group (PAG). The purpose was to update the families and patients of any changes made to the trial and handling of their data.
The journey towards the submission of the DARS application process
On 14 March 2019, the FITNET-NHS Trial Coordinator (MR) sent the first application query to the NHS Digital enquiries team along with a summary of the study, the purpose for requesting record linkage and study documentation (protocol, consent forms and PILs). On 26 March 2019, The FITNET- NHS Trial received a study registration number and was also assigned a NHS Digital Case Officer (NHSD CO), whose role was to review and advice on aspects of the application process.
Upon reviewing the study documentation, the CO suggested to make amendments to the study documentation to comply with the NHS Digital requirements. The suggestions were considered and was presented to the TMG members on 04 April 2019. The concern raised by the TMG was about the impact of the changes (if made) to the data and consents to be collected from patients retrospectively. After gathering feedback from the TMG, the best possible solution identified were to change the study documentation wordings for prospective patients as per the NHS Digital guidelines, updating the study website with links to the HRA guidance and circulating newsletters informing the changes introduced to all patients recruited so far on to the study.
The proposal was agreed and approved by the NHSD CO on 15 April 2019 and on 19 June 2019, an ethical amendment was submitted to the REC and HRA for the changes made to the study documentation. Once the approvals from REC and HRA were on place, the amended documents were sent to the NHSD CO. However, on 02 July 2019, the NHSD CO asked the Trial Coordinator to make further changes to the Consent forms, to which the Trial Coordinator explained that those changes have been integrated in the PILs.
The FITNET-NHS Trial was finally granted access to DARS on 10 July 2019. With the advice gained from the Health Economists, the Trial Coordinator submitted the first draft of the DARS application on 04 November 2019. After carrying out substantive changes to the DARS application with repetitive corrections suggested by the NHSD CO, altogether, the Trial Coordinator submitted 4 different versions of the DARS application to NHS Digital in a span of 6 months and the complete process from enquiries to draft approval took 11 months (this excludes the time taken for the NHSD Independent Group Advising on the Release of Data (IGARD) panel to inform the outcome of the application process, which takes ~ 4 to 8 months). The final draft of the DARS application was approved on 02 April 2020.
In November 2016 NHS Digital provided a quote of £5000 for data linkage. When the DARS application was eventually signed off, on 25 November 2021, the cost had risen to £14,544. The data were sent by NHS Digital on 25 February 2022.
Data extraction from record linkage
Only data items relating to the purpose of the study were requested. No sensitive or identifiable data fields were requested. Details of the proposed data linkage were provided to trial participants in the PIL and data were requested from NHS Digital only for those participants who consented to this.
While considering data minimisation (as per NHS Digital requirements for processing sensitive patient data) we could not filter on diagnosis codes to minimise because:
-
Chronic fatigue is often underdiagnosed and healthcare contacts which are related to chronic fatigue may well be coded using a diverse range of diagnosis codes.
-
Diagnosis codes are very poorly recorded in HES OP and ED in particular.
EMIS contract set-up and impact of COVID-19
We started contacting the EMIS Health enquiries team in January 2019 and organised the first face-to-face meeting with the Head of EMIS Health in June 2019. We originally planned that the data sharing agreement (DSA) would be agreed with EMIS Health and EMIS general practices was in December 2019 and the first extraction of patient data was scheduled to be February 2020 (for patients who have completed their 12-month follow-up period), but the process was delayed due to a broken link within the EMIS Health system, which took 3 months to be restored. After the successful restoration of the link, we submitted the completed DSA to EMIS Health. However, due to the pandemic, the process was delayed further by 10 months as EMIS had to prioritise COVID-19 clinical trials undertaken by Public Health England (PHE).
In Nov 2020, we were informed that due to the impact of COVID-19 on the usage and release of data, a new system had been established within EMIS in April 2021, called ‘Intex Analytics’, which was designed to copy the live data where people will have immediate access to up-to-date data. We were advised to wait and carry out both the extractions in the new system to avoid dissimilarities in the extract formats.
Between September and December 2021, EMIS and the University of Bristol were in discussions to create the necessary contracts, however the data were not available. The data were going to be available in January 2022, but new difficulties over data access became apparent. In April 2022 we were told the data would be ready in June 2022, but it then became clear that it would be after June 2022 and the health economic analysis for this report would therefore not be possible. We therefore abandoned using EMIS data.
Appendix 3 Trial governance
Trial Steering Committee
The TSC comprised a patient and a parent, an independent chair, two clinicians and four methodologists. The TSC ensured milestones were realistic and achieved. The TSC was responsible for reviewing the internal pilot study and advising the NIHR HTA over implementation of trial stopping rules. The TSC met prior to the start of the trial and then annually. The TSC met formally seven times over the duration of the trial, on the following dates (as well as being consulted via e-mail at other time points):
-
21 July 2016
-
03 October 2017
-
28 November 2018
-
26 November 2019
-
10 February 2021
-
14 December 2021
-
12 May 2022.
Data Safety Monitoring Committee
The Data Safety and Monitoring Committee comprised 3 independent experts in ME/CFS, medical statistics and trials. The Data Safety and Monitoring Committee met at the start of the study, before a decision was made about continuing the trial and then at least annually. The Data Safety and Monitoring Committee had unblinded access to data. Data Safety and Monitoring Committee meetings were timed to provide reports to the TSC. The Data Safety and Monitoring Committee met 9 times over the duration of the trial, on the following dates (as well as being consulted via e-mail at other time points):
-
14 July 2016
-
05 September 2017
-
20 December 2017
-
10 October 2018
-
06 March 2019 (formal report provided and e-mail exchange rather than meeting)
-
02 October 2019
-
28 May 2020
-
03 March 2021
-
12 May 2022.
Trial Management Group
The TMG comprised the chief investigator, BTC statisticians, the research team and coapplicants (all authors of this report). The TMG, in collaboration with the BTC, was responsible for overall trial management, monitoring trial progress and quality, and ensuring that the study protocol was adhered to and that participants were safe. The TMG met every 2–6 weeks (as well as being consulted via e-mail at other time points).
Appendix 4 Recruitment graphs
FIGURE 7.
Target vs. actual referrals and recruits within the 12-month pilot phase. Note: six of the patients referred within the pilot phase were recruited later – five in November 2017; one in January 2018 (not depicted here as the trial was ongoing).
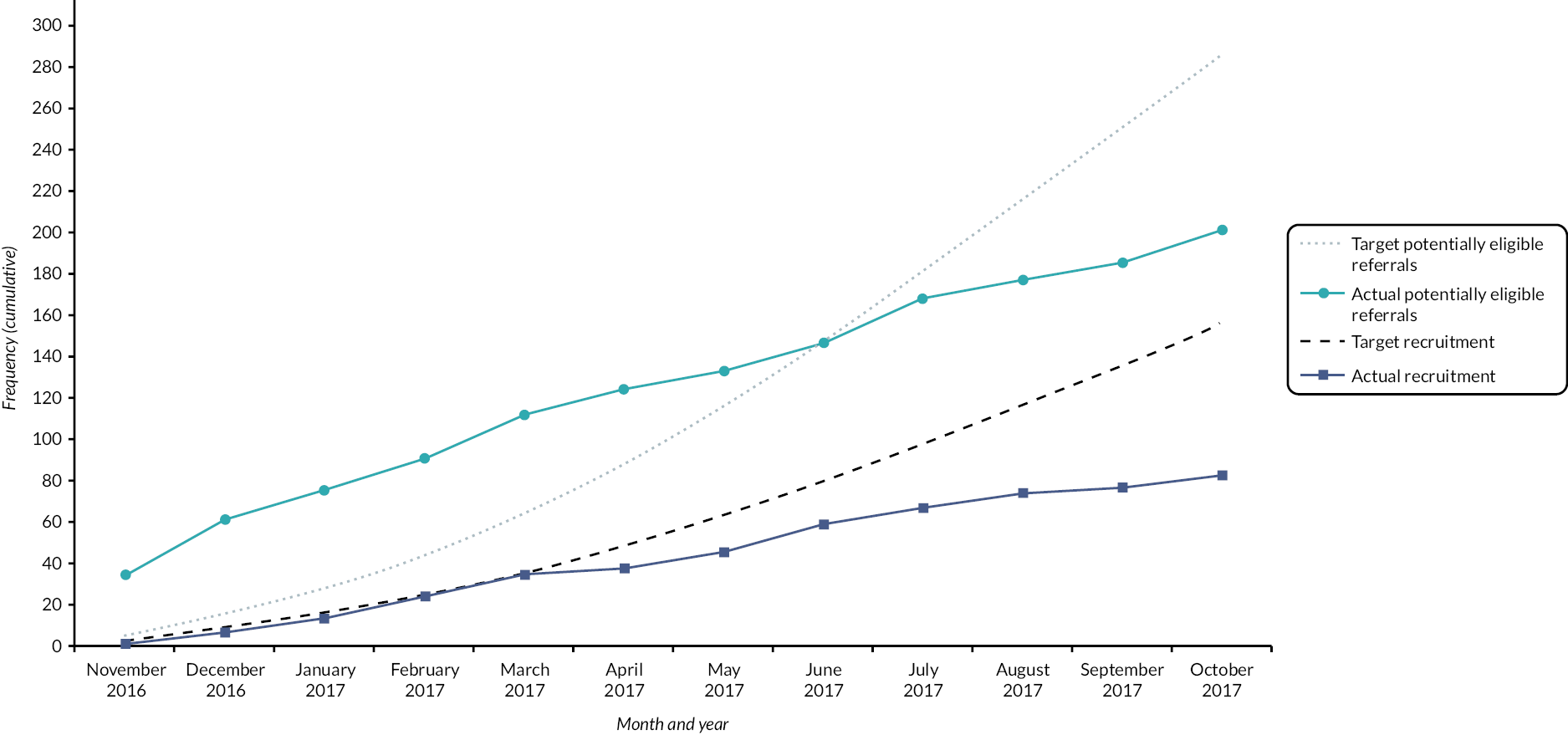
FIGURE 8.
Final referrals and recruits against revised target.
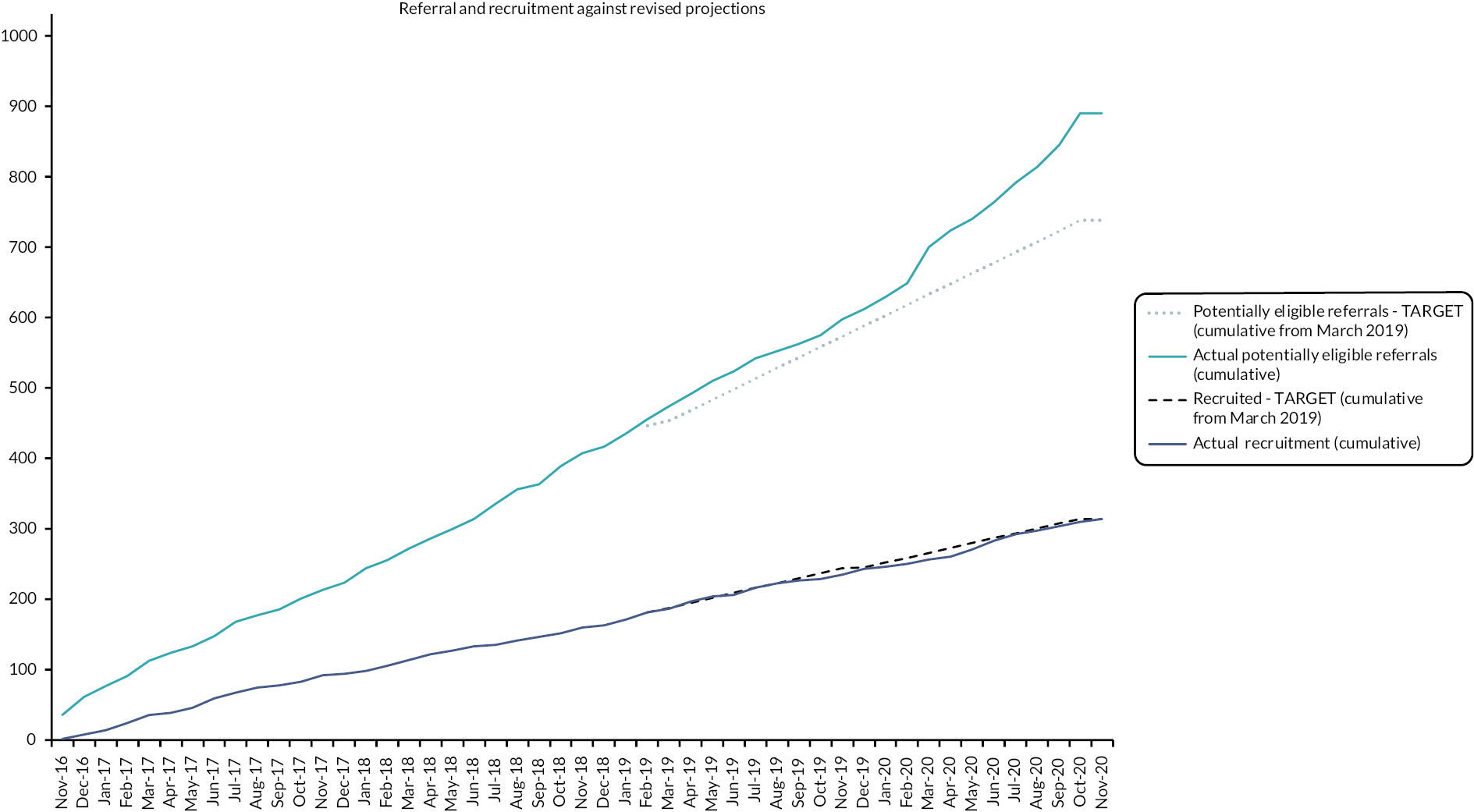
Appendix 5 Substantial amendments
Substantial amendment 1
SA 1 –APPROVED BY REC (19 October 2017) AND HRA (23 October 2017)
DETAILS OF SUBSTANTIAL AMENDMENT REQUEST SUBMITTED 01 August 2017
We are requesting two substantial amendments as follows:
-
We are requesting a change to the Activity Management treatment group. We propose to increase the number of treatment sessions offered from 3 to 6 sessions.
Reasons
-
- The average number of sessions offered by the Bath clinical team as standard (outside of the trial) is 4.8 follow-ups after a first appt (i.e. 5.8 total). The theoretical model is to see if FITNET-NHS is effective and cost-effective compared to a form of treatment as usual (TAU). Three Activity Management sessions is less than TAU.
-
- The FITNET Trial protocol does not stop Activity Management participants from re-referring to the service after three sessions for face-to-face treatment (outside of the trial).
-
- Feedback is that three is seen as not enough (from recruitment calls and treatment response). That is unacceptable for families that Activity Management is so brief, and we feel it is unethical to continue at three sessions for this reason.
-
- We think this change will help increase recruitment.
The TMG, TSC, Sponsor and funder (HTA) have all been consulted and approve of this plan.
For our research outcomes, we will conduct sensitivity analyses comparing those earlier participants receiving three Activity Management sessions with those who had six.
On receipt of ethical approval of this change, any new children and carers recruited thereafter will receive the new information leaflet and will sign the consent form (updated to confirm reading of the new version information leaflet). Any children already recruited and currently receiving three Activity Management treatment within the trial will be offered (via e-mail, telephone or Skype) the option to extend up to six sessions if they choose. This will form a part of the standard process of re-consenting to trial participation at each contact, but offering the revised treatment duration.
-
Our second requested amendment is to allow researchers to analyse the content of therapeutic e-mails sent/received within the FITNET-NHS platform.
Background
Previous research investigating the specific content of e-mails sent by the therapist within therapist-assisted internet-delivered CBT (ICBT) in adults with depression (Schneider et al. )98 has identified 11 distinct therapist behaviours such as task reinforcement, self-efficacy shaping, task prompting, alliance building, etc. This has also been done in adults with anxiety (Paxling et al. ). 99 However, it has not been done in an adolescent sample, nor in a ME/CFS (or other chronic illness) sample.
What we are requesting
Examining the content of the e-mails exchanged between the patients (participants) and the therapists will enable us to explore a variety of process issues in internet-delivered CBT with adolescents with ME/CFS. Potentially, we could explore therapist behaviours, patient behaviours, parent behaviours (in the e-mails they exchange with the therapists) and inter-relational patterns between the therapists and the patients/parents. This is important as it could help us to identify how to most effectively utilise an online therapy approach with this patient group. For example, it could help us to identify early warning signs of disengagement from therapy and what therapist strategies are most effective in terms of addressing this.
We are therefore requesting an amendment to the FITNET-NHS group (internet delivered CBT) of the FITNET-NHS study. Participants will not be required to do anything differently to the current study procedures, and the intervention will not change in any way. The amendment is that patients and their parents will be informed that the data from their e-mail exchanges with the therapists may be used in this way (PIL) and will be asked to give their explicit permission for this (Consent Forms).
Details of changes to key documents
IRAS form
A13. Activity Management – states provide up to three video calls. Now will be up to six.
A19. Total number of interventions/procedures. Activity Management delivery – states up to two follow up consultations. Now will be up to five.
Protocol
-
- Updated to show six Activity Management sessions in the following sections:
-
Section 1. Summary
-
Section 4.2, Figure 2
-
Section 4.6.1 [activity management (comparator)].
-
-
- Further minor amendments to protocol (detailed in ‘any other relevant information’ below).
Information sheets for participants
-
- Updated to show six Activity Management sessions.
-
- Updated to state that the content of therapeutic e-mails will be analysed.
-
- Further minor amendments to information leaflets.
Substantial amendment 2
SA 2 – APPROVED BY HRA AND REC (09 August 2018)
DETAILS OF SUBSTANTIAL AMENDMENT REQUEST SUBMITTED 21 June 2018
Our priority is to increase recruitment, and the key target is to increase the number of referrals from GP surgeries across the UK to the Bath Royal United Hospital. The main amendments to this aim are as follows.
-
Setting up PIC sites:
We have received feedback (via CRNs) that GP surgeries across the UK would like to be set up as PIC sites, which will involve a database search and mailout to families. We are focusing on large conglomerations of GP surgeries, or ‘superpractices’ where the set-up and patient mailout work will be worthwhile in terms of impact on recruitment. We will offer UK ‘superpractices’ the option of being set up as a PIC site. The PIC site work will involve:
-
- Database search to identify patients aged 11–17 years with recent diagnosis of ME/CFS or post-viral syndrome.
-
- GP approval of list of patients for mailing out invitation letter.
-
- Letter mailout (directly to the child if aged 16–17 years or to the parent/carer if aged 11–15 years) to invite families to consider the trial and ask them to make a GP appointment for a clinical referral to Bath Royal United Hospital if they are interested.
We are working with the Birmingham Modality to set up there in the first instance, so will be seeking approval to commence PIC site activity across the West Midlands. We are likely to add other superpractices across other areas of the UK in future, which we will submit as non-substantial amendments, in line with recent advice from a REC representative.
-
- See new GP mailout letters attached.
-
Patient flyers and posters.
We also have feedback from UK general practices (via our CRN contacts) that GPs would like patient flyers to hand to patients as well as patient-facing posters to display in their surgeries. We have produced a flyer, which can also be printed on a larger scale and displayed as a poster.
-
- See new patient flyer/poster attached.
Also attached for review:
-
- PIC site Schedule of Events.
-
- PIC site Statement of Activities.
CHANGES TO DETAILS IN ORIGINAL IRAS FORM:
A27-2. Will the identification of potential participants involve reviewing or screening the identifiable personal information of patients, service users or any other person?
ANSWER NOW = YES, VIA DATABASE SEARCH AND MAILOUT FROM PIC SITES (NHS SUPERPRACTICES ACROSS THE UK). ONLY NHS STAFF EMPLOYED AT PIC SITES WILL SCREEN THE IDENTIFIABLE INFORMATION OF THEIR OWN PATIENTS FOR INVITATION.
A28. Will any participants be recruited by publicity through posters, leaflets, adverts or websites?
ANSWER NOW = YES, AT NHS GENERAL PRACTITIONER SURGERIES ACROSS THE UK
A73-1. Will potential participants be identified through any organisations other than the research sites listed above?
ANSWER NOW = YES, UK NHS ORGANISATIONS (THOSE SET UP AS PIC SITES)
Substantial amendment 3
SA3 – APPROVED BY REC (26 June 2019) AND HRA (10 July 2019)
DETAILS OF SUBSTANTIAL AMENDMENT REQUEST SUBMITTED 07 June 2019
The main changes are:
-
Revision of sample size to 314 participants (instead of 734).
Recruitment has been slower than anticipated, so the sample size was recalculated for achieving our primary outcome (effectiveness and cost-effectiveness of FITNET NHS) with appropriate power instead of the original target which was based on power calculation to detect a secondary outcome (effectiveness for subgroup with comorbid mood disorders).
-
6-month extension to recruitment phase to enable us to realistically meet 314 participants.
-
- See amended and protocol (for both the above), plus revisions to PIL (for recruitment numbers) and PIC Site statement of activities (for revised end date for recruitment).
-
-
Expanding the PIC site database searches to include patients with read-codes (within the previous 2 years) of ‘Tired all the time (TATT)’.
This is in response to feedback from GPs from our first PIC site roll-out which identified far fewer patients to invite than expected. GPs reported that they tend to use ‘TATT’ rather than ME/CFS diagnostic codes, and that sending a letter to these patients would be beneficial. We gained advice from several GPs on the wording of the letter to these patients.
-
- See TATT GP letters.
-
-
The PIL and consent forms have been updated in line with advice for applying to NHS digital for data collection.
Minor changes:
A few further minor amendments have been made to the study protocol - these are clearly visible in the tracked changes document submitted, and are detailed in the ‘summary of changes’ section at the end of the protocol.
CHANGES FROM ORIGINAL IRAS FORM:
-
A3-1 Chief Investigator: Professor Esther Crawley has a different work address: 1–5 Whiteladies Road, and within Population Health Sciences (the School of Social and Community Medicine no longer exists and we have moved.
-
A4: Dr Birgit Whitman is no longer the contact on behalf of the sponsor. She has left and is replaced by Mr Adam.
-
Taylor. The best contact details for the University of Bristol Research and Enterprise/sponsor/sponsor representative is: research-governance@bristol.ac.uk; tel: +44 (0)117 42 83065.
-
A59: Sample size – NOW = 314.
-
A60: Sample size calculation – see revised protocol for updated sample size calculation.
-
A69: Planned end date: NOW = 01 May 2022. Total duration: NOW = 6 years.
Appendix 6 Additional statistical analyses
A note was made when parents reported helping their child to complete the primary outcome measure, this being the case for 16% of those completing the SF-36-PFS in the FITNET-NHS group and 15% in the Activity Management group (Table 23). The baseline measure indicated that parents were more likely to help with completion when their child was more disabled. There was little suggestion of an improvement in the Activity Management group, irrespective of whether the child completed the SF-36-PFS independently or with health. Whilst more improvement was observed among the FITNET-NHS participants completing the SF-36-PFS independently, the interaction test (p = 0.58) indicated this difference could have occurred by chance.
| FITNET-NHS | Activity Management | Difference in means (95% CI) | p-value | |
|---|---|---|---|---|
| Mean (SD), N | Mean (SD), N | |||
| Subgroup analysis | ||||
| Baseline assessment | ||||
| Child completed | 51.6 (20.8), 107 | 50.0 (22.6), 114 | ||
| Parent reports assisting completion | 42.6 (26.0), 21 | 38.8 (26.2), 21 | ||
| 6-month assessment | ||||
| Child completed | 63.6 (27.7), 107 | 52.8 (26.1), 117 | ||
| Parent reports assisting completion | 44.3 (34.1), 20 | 36.2 (24.8), 21 | ||
| Interaction effect | −4.2 (−19.1 to 10.6) | 0.58 | ||
FIGURE 9.
Individual changes from baseline to 6 months on the primary outcome measure.
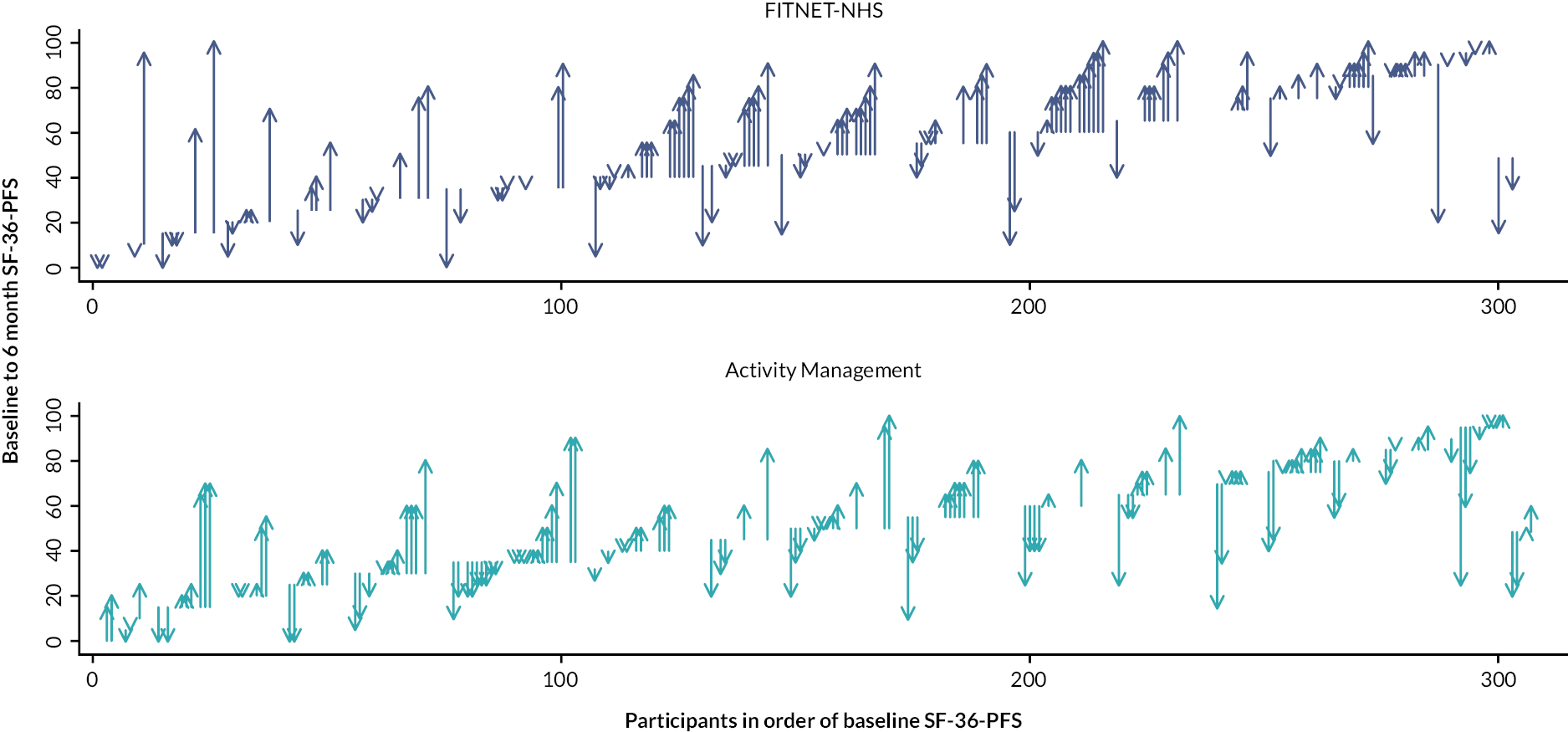
Appendix 7 Qualitative analysis results table with quotations
| Theme | Quotes |
|---|---|
| ADOLESCENTS’ AND PARENTS’ VIEWS AND EXPERIENCES OF TRIAL PARTICIPATION | |
| Previous treatment | M1000093: … I would be willing to try anything to see if we could get some improvement, because our local services were, were non-existent … |
| M1000034: … we would absolutely try anything because we weren’t getting any proper answers from the NHS and nobody really seemed to understand the condition … | |
| M1000107: … the waiting list is currently about nine months and so because of that she said “you might be eligible for this trial instead”. | |
| C1000008: … when the funding was cut, there was nothing I could do and it’s frustrating …
C1000002: I would say hydrotherapy and physiotherapy worked quite well. |
|
| M1000002: We had tried ourselves putting in schooling in different patterns and all sorts of things, it wasn’t that we didn’t have any guidance, but we didn’t have anything that was particularly relevant just to (child). It was all quite generic that we’d been able to find research-wise. | |
| Trial participation reasons | M1000002: (Child) felt quite excited when he realised he could get on with the trial, and I guess help others in the future.
M1000031: We liked the idea it was online so that we didn’t have to travel anywhere, any great distance at all, so that meant that it was easier for us. |
| M1000015: I think I was a little bit sceptical because the only previous experience I’d had of any online support was when I did um, a qualification, a small qualification online and I found it you know very impersonal. | |
| C1000029: I suppose just that after [treatment] I would feel really tired and that I couldn’t get it done, it wouldn’t maybe like make me feel even more tired … | |
| Participant preferences for trial treatments | C1000002: Whereas this I can just do whenever I fancy. I quite liked that when reading about FITNET I suppose.
M1000106: If (child’s) not coming out of someone who has major anxiety problems if she gets on the FITNET one would that be, it would seem that that would be less effective to help her … |
| Acceptability of patient information and recruitment process | C1000107: Yeah, I understood everything that was on the leaflets, it made sense.
C1000008: … if you randomly select it then everyone’s got an equal chance of each condition, if that makes sense. I wouldn’t say there’s a better way … |
| M1000039: Yeah I mean I think we chat – we had quite a long chat with the research nurse and she was very nice and she spoke to (child) and (child) sort of did talk back to her when we went over it all. | |
| M1000093: It was – there was quite a lot of reading, but that was good. I mean I don’t mind that. It was, it was quite difficult to get (child) to sit and concentrate because there was stuff for him to read as well I seem to recall. | |
| M1000002: The only thing I saw as missing, and is still missing, but there’s a very good reason why it’s missing, is you don’t really know how long it’s [treatment] going to take. | |
| M1000120: Brilliant yeah, no paperwork, not having to put it [consent form] in the post, I can do it on my phone [Laughs], yeah, we like that a lot. | |
| ADOLESCENTS’ AND PARENTS’ VIEWS AND EXPERIENCES OF FITNET-NHS | |
| Benefits of online treatment | M1000273: … if I had a thought of an evening I could just fire off an email, or if I had a query of a weekend I could just fire off an email, rather than have to write it down and wait for a specific appointment. I wouldn’t change anything about it, I don’t think. |
| M1000019: I was thinking more of not needing to take any more time off school which was a big thing for me ‘cause obviously she’d already missed quite a lot with the illness itself … | |
| C1000025: …sometimes I am embarrassed by myself, it is easier when you don’t always have to look at them and then it is over the phone or over email and it is okay like that. | |
| Disadvantages of online treatment | C1000008: … I don’t know, like, with someone telling you what to do, it teaches you how to cope rather than just text on the screen, I think.
C1000064: …maybe having one face to face session or meeting would be quite good … |
| C1000115: The message is always quite long so and it has lots of questions in it and when it comes to like the end, you don’t know which one to answer first or I don’t usually feel like answering like ten questions and then saying how everything’s going as well. It’s a lot of writing. | |
| M1000049: I sometimes feel that if (child) had a bad week I would have liked to have emailed her on the Sunday. And then I’ve got to wait 2 weeks. | |
| C1000149: It would be nice if they had so every, every like three, six weeks or something they called you and said how are you doing? Do you have any questions instead of doing it all over email? | |
| D1000034: They’ve [therapists] always replied haven’t they? [inaudible 12:07] Always friendly and always encouraging. But the thing is is that thing about actually connecting with people means something as well doesn’t it? | |
| Platform usability | C1000002: I think it’s really easy, everything’s quite easy to find. I don’t think there’s anything that I’ve struggled to find, or been irritated by. Every time I go on there I know exactly where I’m going, it’s quite easy to navigate. |
| C1000064: … with the diaries I think what was a bit difficult is how you can only edit the current day and the day before but if you’ve still got to fill in one from two days ago you wouldn’t be able to edit that or fill it in. | |
| Treatment content and advice | C1000008: Like, it gives you good examples of how fatigue seems to other people and how it genuinely is, how it makes you feel and everything. It’s got really good examples of that. |
| C1000009: … it’s given or telling me to do all different activities. Like, it gives me something to do instead of just sitting in the house or something. | |
| C1000015: … if you think like you’re tired, um like um, and that makes you even more tired, and yeah its like, I found that chapter very helpful. | |
| C1000040: … I found that all of these thought process stuff really helped me just seeing like don’t think about your fatigue when you don’t need to. Sometimes you can think ‘Oh I’ve got fatigue I’ll never get better,” and stuff like that so I think that helps definitely. | |
| C1000029: Yeah when I first started and I first got the cycling and the sleep routine, I was questioning it a bit but then actually that week I did log on and there was a bit where a section, are you feeling less motivated and it reassured that it was really normal to feel like this and that I will probably feel more tired and hearing that, on the weeks that I was starting to feel a bit drained from it, it really boosted me and I felt really positive about it again, knowing that its really normal to have felt a bit less motivated so like one day I was a bit down about it, but straight away it was fine. | |
| C1000002: I would say probably the treatment’s that’s helping me the most, are really the activities. I’m now up to two lots of 30 minutes of running a day, which there’s no way I’d be able to do that if you were to talk to me three months ago, so that certainly has helped me. I think the building up of the stamina certainly helps me go to London on Monday with my friends, and just do activities more, like regular activities. | |
| C1000025: I think it was useful, some of the stuff that was mentally helpful and helpful thoughts at being ill and also to not think about actually being ill, so I did find some of it helpful. | |
| Content challenging for younger children and those with cognitive difficulties | C1000029: Well there is a lot of reading, that’s all I really meant, so every week I have a lot of reading to do which kind of takes me a while when I have had a hard day at school with lots of lessons. I come home and my brain feels tired, so it takes me a while to understand it, if you know what I mean. It’s easy but for me it’s quite hard to get the gist of it, it takes me a couple of times to read it. |
| M1000149: I mean you’re given lots of flow diagrams and things with some really technical wording on for her to understand and she didn’t understand most of it and nor did we and you’re supposed to be able to read all the documentation first. We’re trying to sit with her. We’re trying to sit with her and talk to her about it but we didn’t understand it ourselves some of it, and we’re quite bright people but it was – the wording was just not, it was obviously done with a medical point of view not a member of the public point of view. | |
| M1000008: She’ll read a question out and say, “I don’t know what it’s asking me.” So it’s only something similar like that. | |
| M1000002: Okay, so yeah it is, it’s really easy but there’s a hell of a lot of text on there. If you’re not the most able person in the world at reading I think it would be quite intimidating, [pause] that would be my feedback. | |
| M1000117: That’s probably where a Skype would have probably come in better, instead of (child) having to read through it, because when he was poorly it was really hard for him to take in. | |
| Parental involvement | M1000040: … the trials been set up that we message individually rather than together again I think there’s been something very healthy about that and probably that’s because with CF there’s such a potentially unhealthy relationship going on cause it’s such an intense sort of condition there can be – potentially you’re kind of focussing on it and it can almost become well bigger that it needs to be … |
| Interviewer: So, what’s that (child)? Do you prefer that your dad can’t see what you’re saying to the therapist?
C1000115: Definitely. |
|
| Contact with a therapist as essential | C1000064: I’m finding it good. I think it’s been very beneficial just to be able to email and talk to someone that is experienced with chronic fatigue and can give me advice on how to improve and I can ask questions and … yeah. |
| C1000029: I have really liked it actually, I didn’t think I would because I didn’t think it would be personal, but it has been really really good. As I said before she doesn’t just ask me about it, she asks me about other things as well which I really like and really like supportive. Every week when I tell her how I have been getting on, she always has, says something like well done and that I am doing really well and its really nice to hear, … | |
| M1000117: It was quite helpful, but it was more helpful having the questions answered by the clinical psychologist, that was a lot more helpful.
C1000049: Yeah, yeah, no she’s [therapist] really nice and she always makes it a bit more personal. Sort of chats away, as if you are just chatting to her, rather than just a general message … |
|
| M1000002: She [therapist] was very open, very open with communication channels, and I felt utterly supported, I felt very much like she’s been brilliant with (child). She’s got the measure of (child) really quickly, as she would I guess with her experience and her background, and I feel very confident that she really understands him and is working well with him, and with me. | |
| D1000115: … I think she’s [the therapist] first class, I mean she’s really, really good. She responds, she answers questions, sorry?
C1000115: She’s very friendly as well. D1000115: Yeah friendly and gives the impression and understanding and knowing where we are at. |
|
| Treatment effectiveness | C1000040: … my energy has definitely gone up and yeah it’s helped a lot I have definitely noticed a difference.
M1000040: … (child) is going around singing so we know that when (child)’s happy and doing well and he hums away or sings and that has been a big, yeah a noticeable improvement which has been lovely actually. |
| C1000029: … before I started the treatment, I would never have expected to be able to do about half an hour on a bike twice a day, but now I am up to 27 minutes twice a day, so it’s really, it’s hard but it’s really really good.
INT: Um so overall would you say the treatments worked or not worked for you? |
|
| C1000015: Um, I think that it has worked very well.
C1000117: Yeah, I just find it hard to do it because I don’t really think it’s helping that much either. |
|
| C1000002: Okay, so before I took part in the study I was in a worse position than I am in now. I struggled with everyday activities, I still do now but to a lesser extent I would say. I was at school less, and my sleep was worse. | |
| M1000040: … I have to say I think (child) has been an absolute star, he really has taken it seriously and you know the benefits are there to see so it’s very encouraging.
M1000009: He’s more chatty. More chatty to us like where he’d like usually be sat struggling and like, you know…. not being able to get dressed. |
|
| THERAPISTS’ EXPERIENCES OF DELIVERING FITNET-NHS | |
| Benefits of online treatment:
Accessible content and flexible treatment delivery |
HP70007: But on FITNET-NHS – maybe because it’s separate, parents feel like “oh well she won’t want me to say this in front of her”, but you know, now that I can have a conversation, maybe I can share my reflections or ask my questions, more than if they were together in the room. |
| HP70006: I think as a therapist it’s really nice. Actually you get much more time to think about what you’re saying and doing than you do when you’re seeing someone synchronously, either on Skype or face to face. I’ve got to respond immediately and often I think I really shouldn’t have gone down that and maybe that wasn’t the right thing to do. | |
| HP70007: … we adjust it and tailor it to what they can manage, ‘cause it is quite intensive – particularly at the beginning – so that’s quite demanding for patients, so I think we’re just managing that by being more flexible with the timings when we need to. | |
| Develop a good ‘picture’ of patients and tailor treatment | HP70002: I was quite surprised actually that they do feel like you’ve got a therapeutic relationship, … |
| HP70006: The person I’m working with at the moment in FITNET-NHS, she really gets it and she gets that I need to know stuff about her so, the other day via email she said, “I’m sorry that this is such a long email but I think it’s important for you to know what my personality’s like,” and then she gave this really chatty couple of paragraphs about the things that are important to her and what kind of person she is, so really communicating about her values and she did that without a lot of questioning or prompting from me, … | |
| HP70004: I feel like I’ve got probably a better relationship than with some of the face to face. I think the how intense it is and the timeframes make a difference for that. Because even the face to face work, CBT we would see someone every two to three weeks and the messages are weekly at the start. So when they’re saying a lot and you’re saying a lot then yes, you feel like you can build up a really nice relationship. | |
| Challenges of online treatment:
Lack of verbal and non-verbal communication |
HP70004: I guess face to face work is some ways is a bit easier because you are there with someone. You can definitely build a relationship. Drawing stuff out and things like that
HP70002: I think you can fit more into a Skype session, so when you’re having a conversation you can probably say quite a lot more within an hour than you can within an email hour, … |
| HP70006: The difference is when you’ve got a mum or dad in the therapy room, or indeed even in the waiting room, you can actually have a possibility of having a conversation all together, where you can enable people to start communicating differently with each other. Although in the FITNET-NHS programme we really encourage people to talk to each other about what they’re doing in the FITNET-NHS programme, you’ve got no control over to what extent they’re doing that. | |
| HP70006: … in the therapy session I might be a bit confrontational with someone, but I might be able to do that in quite a playful way, but they can see if they can see my face that I’ve got a smile on my face and I’m giving them a bit of a wink and a nudge, “Do you think it might just be that actually a bit of you doesn’t want to be back in school and that’s what’s getting in the way here?” I can do that in a nice way but if I write that down, it could come across really quite critical, quite negative, like I’m not understanding them, so you’ve got to be really careful. | |
| E-consultations taken less seriously | HP70006: … you’re a faceless person, you’re not a person, you’re someone at the end of a computer message, I do think both children and parents in some instances have come about not being as respectful as they would be if you were in the room with them, so another example of a colleague’s case was a young person who filled something in on the platform just like writing, “I’m really bored of doing this.” It’s very unlikely that if that person had been sitting in the room with that therapist they’re gonna write, “I’m very bored of doing this,” but because you’re not a real person to them, actually, I think the social etiquette rules don’t necessarily apply the way they would when you’re in a room. |
| HP70006: The risk is that, because you’re just sitting in front of a computer and not synchronously interacting with someone, that actually you get disturbed by other people … | |
| A different set of clinical skills are required
Working harder to build a rapport |
HP70004: … the activity calls we were doing and still do sometimes, that was helpful just a couple of weeks in, being able to just actually speak to each other and hear that we are all people I think made a bit of a difference as well. |
| Careful construction of e-consultations | HP70002: I don’t know, I found that one [e-consultation] I had to kind of word quite carefully to make sure that I was being quite empathic about her view whilst also suggesting something different and yeah, it took a few weeks of doing, a couple of back and forth’s like that. |
| HP70006: If I can think of a couple of ways of doing it, I might suggest that to give them some ideas so I’m not just saying “this is what you need to do” or ‘this is what people find helpful but “it could be this and it could be this”, but building that ability for them to take ownership and to decide, “which one might fit with me.” | |
| HP70002: … I do find myself being quite like overly enthusiastic sometimes in what, you know to try and get across “that’s great, you’re doing really well” and I hope that comes across as genuine and sometimes you wonder whether it does. I think that’s probably what I spend a bit of my time wondering about is how to phrase something to make, to try and get that element across, you can’t just say what you think, you’ve then got to add in bits about trying to make it more friendly and more personable. | |
| Treatment approach and content
FITNET-NHS ‘recovery’ treatment model |
HP70006: I think the other thing that’s critical in FITNET-NHS is that from an early stage it says recovery is going to happen, really instilling hope in people, and I think again that’s nothing unique about FITNET-NHS in and of itself because we know that, for a therapeutic process to work, we have to instil hope, but I think FITNET-NHS’s very good about talking about recovery from the beginning and then you will recover and what are your goals and let’s set them as high as you want to because you’re gonna get there. |
| HP70002: … you’re actually kind of getting somebody to a point where they might see themselves as not having CFS anymore and I like the fact that that is the message that that’s what we’re aiming for, we’re not just aiming for you to do a little bit more school or a little bit more of this, we’re aiming for this not be affecting your life at all anymore, … | |
| HP70002: Yeah, because I don’t know how it’s going to go [deregulating] and I’m really conscious of trying not to let that anxiety of mine, that “oh my God what if they stay up late and then they have a big crash” and I try not to let that get in the way of me actually unlocking that with somebody … | |
| Cultural differences from original Dutch treatment model | HP70006: Well, it’s hard but on the other hand it’s great because the FITNET-NHS programme does enable quite a lot of flexibility in that so compared to some manualised treatments where you would just have to do things in a certain order, actually in FITNET-NHS I can choose which options I enact when, so what chapter do I open when? Do I use a chapter or not? It’s actually got loads of scope for using it flexibly according to what the young person needs. |
| HP70007: … we adjust it and tailor it to what they can manage, ‘cause it is quite intensive – particularly at the beginning – so that’s quite demanding for patients, so I think we’re just managing that by being more flexible with the timings when we need to. | |
| Variable patient engagement | HP70002: … some people have been more open and told me loads, other people haven’t at all … |
| HP70004: So they are actually completing the diaries, reading the platform, being thoughtful and following it through. I am seeing even better results than I expected to, which I shouldn’t be surprised but I am. Just how well it has worked for some people, I really like it. | |
| HP70002: It’s, I mean you get that, you get those same people in clinic face to face, but I guess you can have more of a conversation, a two way conversation about what are the reasons that are stopping you from kind of getting on with this or you know how you are going to do this and you can ask some of those questions in email messages, but then you have to wait for them to come back and if they’re not coming back to you anyway, then they are kind of not doing it, you don’t get anywhere with those questions. | |
| HP70004: … there would be just more detail from the older girls of, I really want to go to the gym because it’s really important to me and what do you think about this? And then my younger boys I get one line of “I did my exercise”, like that is the message for the week [laughs]. It is like okay, so for people like that it is really hard to draw things from them. | |
| HP70004: I haven’t got much of a flavour of them and it does seem to be the younger boy in particular. The older boys are a bit better and all of the girls are good. I don’t know if it’s a different way of communicating or because they know I am the girl or what, but all of the girls seem to just … I feel like I’ve been able to have a bit more warmth within the messages which has been really nice. | |
| HP70004: some of the older girls have got so much detail, we can move through the program quite quickly. I know where they are at, I know how it is going, I know what they are thinking and it moves quite fluidly. | |
| HP70002: I’ve got a couple where the parent and child are both really engaged and they’re both sending me messages, they are both like really keen for feedback and then I’ve got others where parents don’t get back at all, I don’t know if they’re bothering or if they’re reading it, I don’t know if they’re just getting on with it. I’ve got some where the parents are kind of more involved and the kid is not doing as much, so it’s quite variable really and I mean your emphasis is slightly different depending on the age of the child, supposed to be really the younger ones that you maybe have a bit more emphasis on the parent being a coach alongside you and put a bit more effort in to helping the parents with it and then when they’re older and you know it’s more on the young person themselves, so you maybe have less input from the parents and certainly a couple of my older ones, the parents not, don’t, one of them the parents not messaging at all … | |
| Treatment effectiveness | HP70004: … relatively actives just feel it is hard to get them to regulate. It’s hard to know when they have regulated. |
| HP70006: I think there’s a good group of people for whom it does work and I think it’s a case of how much they invest in it, they tend to get out of it for the people who work harder at really following the principles get a lot out of it. | |
| HP70002: … it seems to be the ones generally that are actually like quite busy and in that booming and busting that just can’t find the time to do the FITNET-NHS … | |
| ADOLESCENTS’ AND PARENTS’ EXPERIENCES OF ACTIVITY MANAGEMENT | |
| Benefits of online treatment | C1000128: I don’t know why but I tend to feel a bit more nervous over a call but it has been, it’s been okay. I don’t think it would be much different. |
| M1000093: Yeah. I mean, I, I, once again I have mixed feelings about it. I think for (child) it seems very natural. You know kids nowadays that’s how they communicate, so you know, for him it was kind of the norm. To me it felt a bit strange and struggling with technology was a bit of a pain each time … | |
| M1000100: I think – yes, he’s liked it because even when he’s poorly and he’s not felt like going up and getting outside he’s still been able to talk to her [therapist]. | |
| Disadvantages of online treatment | M1000145: Whereas it’s, “Hi, I’m your nurse, let’s get straight into it,” there’s no – it is very clinical, there’s no, no personalities involved, which I don’t know if that would have helped. Not helped sorry, because as I say, (therapist’s) really good, they do talk, but it’s not the same as knowing who you’re speaking to. |
| M1000039: … it wasn’t that easy to kind of hear and especially with (child) cause she’s quite quiet when she talks … | |
| Frequency and flexibility of contact with a therapist via Skype and paper diaries | M1000107: “We’ll split this session”. Which I thought was quite helpful actually. So, we booked that one a month on. I guess that we can assess whether that was a blip or whether, you know, we need to sort of go a little bit further back to square one again. |
| M1000107: There’s quite a lot of flexibility [inaudible 1:06:13.8] around how close the appointments can be. | |
| M1000093: I think sometimes it feels very, very bleak and it’s quite good to see actually you are, you are doing a little bit more than you were, and you know this is where you’re at now so that was quite good. | |
| Treatment content and advice | M1000072: So, it’s actually been useful talking to (therapist), because we have picked up quite a few more really useful tips for managing activity that we weren’t aware of, … |
| M1000093: But it just gave me a little bit more – what’s the word? Support to be able to say, (child) it’s not just me saying this, you know this is, this is based on evidence. It’s not just mum being a parent. | |
| M1000107: Then (therapist) sort of explained that that’s the thing to avoid, a kind of boom bust cycle. So, what she sort of managed to get us to do is to actually reduce the activity a little bit during the week but to try and push it up at the weekend so that it’s much more even. | |
| M1000100: … – trying different ADHD medicine, but they interfere with his sleep, and also make him drowsy in the day, which makes him less likely to do the activities he needs to do in the day, even if he could concentrate at school. | |
| C1000072: I think, when we first started doing the high and low activity, it was a bit like, I had a lot of things, like, coming up, so it was a bit like – I found it, like, really hard, to like, not do as much as I wanted to, … | |
| Did the treatment work? | M1000100: … there’s a definite improvement with him, it’s just he can do more and he’s still tired and he’s still struggling but he’s doing much – he wasn’t even managing the two-hour sessions with the tutors, where he more or less is now. |
| M1000031: Generally speaking he’s got more energy about him, he’s eating more as well, so he’s got his appetite back a little bit, which is good. I think just generally feeling happier in himself as well, which is good. | |
| M1000120: No. No, it’s helped me deal with the situation but no, it hasn’t – it hasn’t – it hasn’t changed her. | |
| M1000100: But it’s made him more aware, and it’s given me more backing to say, “right, you need to have no screens now”, … | |
| C1000145: For a while I was able to go – I was actually able to go to school, and I was able to do full days. But that gradually got less and less, and eventually it just came to nothing. | |
| M1000031: So the study for us was a bit of a lifeline really, it has been, we can certainly see an improvement in (child) without a doubt. I don’t know, if we didn’t have the study available to us, I don’t know whether he would have got through his GCSEs. | |
| C1000007: I don’t really think anything’s changed because I still feel the same. | |
| THERAPISTS’ EXPERIENCES OF DELIVERING ACTIVITY MANAGEMENT | |
| Overall opinion | HP70009: … I think some people really like it, it stops them travelling, they have less time off school or parents don’t have to travel so work so financially I think it’s actually a massive impact and clinically I’ve got used to it. |
| Benefits of online treatment via videocall (e.g. Skype) | HP70009: I think it’s a different relationship because you see their house and see what their like in the house so in some ways it’s a bit more of a – I don’t know it’s an insight to you know, if they’re sitting there in their pyjamas and it’s like two o’clock in the morning and their hair – you know and straightaway you can think things aren’t going that well but if they’re up and bouncing around the room, you’re like um you’re having a good day so it can … in some ways it can give you more information than if they come into clinic and you can kind of sense what homes like – if it’s quite a manic environment and they’ve got eight siblings running around and you can go okay it’s quite hard for you to get some rest or if it’s very different, you know so in some ways I think it’s a different – sometimes better insight but yes, I think you do definitely build up a rapport over time. |
| Challenges of online treatment | HP70009: Yes so naturally I probably would have a preference to see them face to face ‘cause I kind of get how they walk, how they come in, how they just hold themselves and their posture … |
| HP70008: … parent to leave because I’d like to talk to them [young person] by themselves. You can do that with Skype but you can’t guarantee that they’re not just hiding behind the door, and then they’re not going to confide in you. At least here, we know that if we say, “Can you go and stand outside?” or “Can you go and sit in the waiting room?” that there’s no way there’s any chance they could hear what’s going on, but you don’t know that with Skype. | |
| Making up for lack of non-verbal communication – working harder to build rapport | HP70008: Sometimes in clinic you’re able to pick up on things that might be a little bit more personal. You see that they’re very upset about something. |
| Variable patient engagement | HP70009: I think the heart of the difficulties is when they’re not maybe doing anything that you suggest and they’re still not getting better and you have that trickiness of no progression but actually they’re not following the advice and that can be looking at why they’re not doing that and the barriers and what’s stopping them and that can take a bit of time. |
| HP70008: So, yeah, engagement is difficult sometimes, but then I think, again, it depends on the person, but that’s why having a set amount of sessions is really helpful because you don’t get into that habit of just seeing someone and then them going, ‘Oh, I didn’t do it’. | |
| Parental involvement | HP70009: … I always give them the option that if there’s anything they want to talk about alone, we can try and do that if they want but they don’t seem to get up so much on Skype, but I don’t know. I don’t know why that is. |
| HP70005: I think we need to be careful, to make sure that through Skype or through clinic, that maybe we have parental consent, that we will see them individually. | |
| Treatment effectiveness | HP70009: … school is such a huge part so that sort of comes under activity and management. Just what their expectations are, how helpful school are and sometimes just changing that can actually make their lives you know, suddenly everything becomes a bit easier and then they do a lot better whereas if they’re really struggling at school that can cause kind of difficulties throughout really. |
List of abbreviations
- CAMHS
- Child and Adolescent Mental Health Services
- CBRSQ
- cognitive behavioural responses to symptoms questionnaire
- CBT
- cognitive–behavioural therapy
- CCG
- Clinical Commissioning Group
- CDC
- Centres for Disease Control and Prevention
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CIS
- checklist individual strength
- CNCEQ-R
- Children’s negative cognitive error questionnaire – revised
- CRN
- Clinical Research Network
- DARS
- data access request service
- DSA
- data-sharing agreement
- ECDS
- emergency care data set
- EMIS
- Egton Medical Information System
- EQ-5D-Y
- EuroQol-5 Dimensions Youth
- FAQ
- frequently asked questions
- FITNET
- Fatigue In Teenagers on the interNET
- FITNET-NHS
- Fatigue In Teenagers on the interNET in the National Health Service
- GET
- graded exercise therapy
- GP
- general practitioner
- HEAP
- health economics analysis plan
- HES
- hospital episode statistics
- HRA
- Health Research Authority
- HTA
- Health Technology Assessment
- IMD
- indices of multiple deprivation
- iNMB
- incremental net monetary benefit
- MAR
- missing at random
- MCAR
- missing completely at random
- MCID
- minimally clinically important difference
- MNAR
- missing not at random
- ME/CFS
- myalgic encephalomyelitis/chronic fatigue syndrome
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- PACE
- Pacing, graded Activity and Cognitive behavioural therapy, a randomised Evaluation trial
- PIC
- patient identification centre
- PIL
- patient information leaflet
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RCADS
- Revised Children’s Anxiety and Depression Scale
- REC
- Research Ethics Committee
- REDCap
- research electronic data capture
- RUQ
- resource use questionnaire
- SD
- standard deviation
- SF-36-PFS
- 36-item Short Form Health Survey Physical Function subscale
- SUR
- seemingly unrelated regression
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- WPAI:GH
- work productivity and activity impairment questionnaire general health
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/VLRW6701).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
